User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
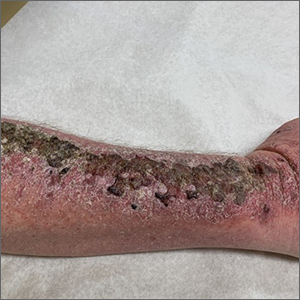
Full results of anal cancer study point to barriers to care
Reports based on a press release in October 2021 suggested it, but now the full data tell the story:
“We now show, for the first time, that treatment of anal HSIL is effective in reducing the incidence of anal cancer,” said Joel Palefsky, MD, lead investigator of the Anal Cancer/HSIL Outcomes Research (ANCHOR) study and founder/director of the Anal Neoplasia Clinic at the University of California, San Francisco. “These data should be included in an overall assessment for inclusion of screening for and treating HSIL as standard of care in people living with HIV.”
Dr. Palefsky presented the full results in a special session at the Conference on Retroviruses and Opportunistic Infections, which drew excitement, gratitude, and relief from both researchers and clinicians, who flocked to the session.
But it’s not just people with HIV who will benefit from this research. Dr. Palefsky suggested that the findings should also be considered as guides for other people at high-risk for anal cancer, such as people who are immunocompromised for other reasons, including those with lupus, ulcerative colitis, Crohn’s disease, or cisgender women who have had vulvar or cervical cancer or precancer.
“If we can show efficacy in the most challenging group of all, which are people living with HIV, we think the results can be as good, if not even better, in the other groups at high risk of anal cancer,” Dr. Palefsky said.
But to serve anyone – whether living with HIV or not – infrastructure, algorithms, and workforce training are going be needed to meet the currently unserved people through use of high-resolution anoscopy (HRA) and other screening technology, he said.
Dr. Palefsy and colleagues screened 10,723 people living with HIV being served at 15 clinics nationwide. More than half, 52.2%, had anal HSIL – 53.3% of the cisgender men living with HIV in the trial, 45.8% of the cis women living with HIV, and a full 62.5% of transgender participants.
Those 4,446 participants were split evenly between the treatment arm and the control arm. Those in the treatment arm received treatment for HSIL on their first study visit via one of five options: hyfrecation, office-based electrocautery ablation, infrared coagulation, topical 5-fluorouracil cream, or topical imiquimod. Then, every 6 months after that, they came in for HRA, blood tests, anal Pap smears, and biopsies to check for any lingering or new HSIL. If clinicians found such cells, they received treatment again. If biopsies still showed HSIL and clinician and participant were worried about cancer, they could come in as frequently as every 3 months and receive treatment each time.
The active-monitoring control group still received an anal Pap smear, blood tests, biopsy, and HRA every 6 months – a level of care that is currently not mandated anywhere for people living with HIV, Dr. Palefsky said in an interview. They were also able to come in for more frequent monitoring (every 3 months) if clinicians were worried about cancer.
“Those in both arms would have been getting more attention than if they had not participated in the study,” he said.
In addition, during screening, researchers found that cancer was already present in 17 other people, who skipped the study to go right to treatment.
Participants reflected the demographics of the HIV epidemic in the United States. They were older (median age, 51 years), mostly gay (78%), and cisgender male (80%). Close to half, 42%, were Black, and 16% were Latinx. In addition, cisgender women made up 16% of the participants and transgender people, and nonbinary individuals accounted for more than 3% of the participants. In addition, one in three smoked.
The vast majority of participants had well-controlled HIV and healthy immune systems, though half in each arm had a history of AIDS, defined as lowest-ever CD4 immune cell counts below 200. Today, more than 80% of participants had undetectable viral loads, defined as a viral load less than 50 copies/mL, and another 7% had HIV viral loads below 200. In total, 9.3% in the treatment arm and 10.9% in the control arm had HIV viral loads higher than that. At time of enrollment, CD4 counts were above 600 in each group, indicating healthy immune systems.
Although all participants were there because they had anal HSIL, more than 1 in 10 – 13% – had abnormal cells so extensive that they covered more than half of the anal canal or the perianal region.
Once everyone was enrolled, researchers began monitoring and treatment, looking specifically for 31 cases of cancer – a number the team had determined that they’d need in order to draw any conclusions. Dr. Palefsky didn’t have to wait long. They were still trying to enroll the last 1,000 participants to have the power necessary to reach that number when the cancer diagnoses came in.
Dr. Palefsky told this news organization that the reason for that is unclear. It could be that some of those cases would have resolved on their own, and so the swiftness with which they reached the required number of cancer cases belies their seriousness. It could also be that the particular people who enrolled in this trial were engaging in behaviors that put them at even higher risk for anal cancer than the population of people living with HIV in the United States.
Or it could be that symptom-based screening is missing a lot of cancers that currently go untreated.
“So perhaps we will be seeing an increase in anal cancer reported in the future compared to the currently reported rates,” he said. “We don’t really know.”
Regardless of the reason for the speed to cases of cancer, the results were definitive: Nine participants were diagnosed with invasive anal cancer in the treatment arm, while 21 were diagnosed with invasive anal cancer in the control arm. That’s a 57% reduction in cancer occurrence between the arms. Or, to put it another way, the rate of anal cancer among people in the treatment arm was 173 per 100,000 people-years. In the active monitoring arm, it was 402 per 100,000 person-years. For context, the overall rate of anal cancer among people living with HIV is 50 per 100,000 person-years. The rate in the general U.S. population is 8 per 100,000 people-years.
The experimental treatment was such a definitive success that the investigators stopped the trial and shifted all participants in the control arm to treatment.
‘We have to build’
Before Dr. Palefsky was even done presenting the data, clinicians, people living with HIV, and experts at the session were already brainstorming as to how to get these results into practice.
“These data are what we have long needed to fuel some action on this important problem, including medical cost reimbursement through insurance and increasing the number of persons trained and capable in anal cancer screening,” John Brooks, MD, head of the epidemiology research team at the Centers for Disease Control and Prevention’s division of HIV/AIDS prevention, wrote in the virtual chat.
Jeff Taylor, a member of the ANCHOR advisory board and a person living with HIV who participated in one of the first azidothymidine trials in the late 1980s, responded quickly.
“What kind of advocacy from researchers, HIV clinicians and [people living with HIV] is needed to get this on treatment guidelines, HRA providers trained and certified, and payors to cover this so [people living with HIV] actually have access to lifesaving screening and [treatment]?” Mr. Taylor asked.
It’s a serious challenge. David Malebranche, MD, an Atlanta-based internal medicine physician who specializes in sexual health and HIV, commented in an interview. When he saw the initial press release last year on the ANCHOR findings, his first reaction was: “Thank god. We finally have some data to show what we’ve been trying to get people to do” all along.
But then he wondered, who is going to perform these tests? It’s a fair question. Currently, the wait for an HRA is 6-12 months in many parts of the country. And Dr. Malebranche can’t imagine this being added to his already full plate as a primary care provider.
“If you tell a primary care provider now that they have to do a rectal Pap smear, that’s going to be a problem while you’re also asking them to screen each patient for depression, anxiety, domestic abuse, intimate partner violence, all the healthcare maintenance and all the other screening tests – and then you deal with not only the urgent complaint but then all the complex medical issues on top of that – in a 15-minute or 10-minute visit,” he said.
Now that we have these data, he said, “we have to build.”
Dr. Palefsky agreed. Very few centers have enough people skilled at performing HRAs to meet the current demand, and it’s not realistic to expect clinicians to perform an HRA every 6 months like the study team did. There need to be algorithms put in place to help practitioners figure out who among their patients living with HIV could benefit from this increased screening, as well as biomarkers to identify HSIL progression and regression without the use of HRA, Dr. Palefsky told attendees. And more clinicians need to be recruited and trained to read HRAs, which can be difficult for the untrained eye to decipher.
Dr. Malebranche added another, more fundamental thing that needs to be built. Dr. Malebranche has worked in HIV clinics where the majority of his patients qualify for insurance under the Ryan White Program and get their medications through the AIDS Drug Assistance Program. While Ryan White programs can provide critical wraparound care, Dr. Malebranche has had to refer out for something like an HRA or cancer treatment. But the people who only access care through such programs may not have coverage with the clinics that perform HRA or that treat cancer. And that’s if they can even find someone to see them.
“If I live in a state like Georgia, which doesn’t have Medicaid expansion and we have people who are uninsured, where do you send them?” Dr. Malebranche asked. “This isn’t theoretical. I ran into this problem when I was working at the AIDS Healthcare Foundation last year. ... This is a call for infrastructure.”
The study was funded by the National Cancer Institute. Dr. Brooks reported no relevant financial relationships. Dr. Palefsky has received consultant fees from Merck, Vir Biotechnology, Virion Therapeutics, and Antiva Bioscience, as well as speaker fees from Merck. Dr. Malebranche has received consulting and advising fees from ViiV Healthcare.
A version of this article first appeared on Medscape.com.
Reports based on a press release in October 2021 suggested it, but now the full data tell the story:
“We now show, for the first time, that treatment of anal HSIL is effective in reducing the incidence of anal cancer,” said Joel Palefsky, MD, lead investigator of the Anal Cancer/HSIL Outcomes Research (ANCHOR) study and founder/director of the Anal Neoplasia Clinic at the University of California, San Francisco. “These data should be included in an overall assessment for inclusion of screening for and treating HSIL as standard of care in people living with HIV.”
Dr. Palefsky presented the full results in a special session at the Conference on Retroviruses and Opportunistic Infections, which drew excitement, gratitude, and relief from both researchers and clinicians, who flocked to the session.
But it’s not just people with HIV who will benefit from this research. Dr. Palefsky suggested that the findings should also be considered as guides for other people at high-risk for anal cancer, such as people who are immunocompromised for other reasons, including those with lupus, ulcerative colitis, Crohn’s disease, or cisgender women who have had vulvar or cervical cancer or precancer.
“If we can show efficacy in the most challenging group of all, which are people living with HIV, we think the results can be as good, if not even better, in the other groups at high risk of anal cancer,” Dr. Palefsky said.
But to serve anyone – whether living with HIV or not – infrastructure, algorithms, and workforce training are going be needed to meet the currently unserved people through use of high-resolution anoscopy (HRA) and other screening technology, he said.
Dr. Palefsy and colleagues screened 10,723 people living with HIV being served at 15 clinics nationwide. More than half, 52.2%, had anal HSIL – 53.3% of the cisgender men living with HIV in the trial, 45.8% of the cis women living with HIV, and a full 62.5% of transgender participants.
Those 4,446 participants were split evenly between the treatment arm and the control arm. Those in the treatment arm received treatment for HSIL on their first study visit via one of five options: hyfrecation, office-based electrocautery ablation, infrared coagulation, topical 5-fluorouracil cream, or topical imiquimod. Then, every 6 months after that, they came in for HRA, blood tests, anal Pap smears, and biopsies to check for any lingering or new HSIL. If clinicians found such cells, they received treatment again. If biopsies still showed HSIL and clinician and participant were worried about cancer, they could come in as frequently as every 3 months and receive treatment each time.
The active-monitoring control group still received an anal Pap smear, blood tests, biopsy, and HRA every 6 months – a level of care that is currently not mandated anywhere for people living with HIV, Dr. Palefsky said in an interview. They were also able to come in for more frequent monitoring (every 3 months) if clinicians were worried about cancer.
“Those in both arms would have been getting more attention than if they had not participated in the study,” he said.
In addition, during screening, researchers found that cancer was already present in 17 other people, who skipped the study to go right to treatment.
Participants reflected the demographics of the HIV epidemic in the United States. They were older (median age, 51 years), mostly gay (78%), and cisgender male (80%). Close to half, 42%, were Black, and 16% were Latinx. In addition, cisgender women made up 16% of the participants and transgender people, and nonbinary individuals accounted for more than 3% of the participants. In addition, one in three smoked.
The vast majority of participants had well-controlled HIV and healthy immune systems, though half in each arm had a history of AIDS, defined as lowest-ever CD4 immune cell counts below 200. Today, more than 80% of participants had undetectable viral loads, defined as a viral load less than 50 copies/mL, and another 7% had HIV viral loads below 200. In total, 9.3% in the treatment arm and 10.9% in the control arm had HIV viral loads higher than that. At time of enrollment, CD4 counts were above 600 in each group, indicating healthy immune systems.
Although all participants were there because they had anal HSIL, more than 1 in 10 – 13% – had abnormal cells so extensive that they covered more than half of the anal canal or the perianal region.
Once everyone was enrolled, researchers began monitoring and treatment, looking specifically for 31 cases of cancer – a number the team had determined that they’d need in order to draw any conclusions. Dr. Palefsky didn’t have to wait long. They were still trying to enroll the last 1,000 participants to have the power necessary to reach that number when the cancer diagnoses came in.
Dr. Palefsky told this news organization that the reason for that is unclear. It could be that some of those cases would have resolved on their own, and so the swiftness with which they reached the required number of cancer cases belies their seriousness. It could also be that the particular people who enrolled in this trial were engaging in behaviors that put them at even higher risk for anal cancer than the population of people living with HIV in the United States.
Or it could be that symptom-based screening is missing a lot of cancers that currently go untreated.
“So perhaps we will be seeing an increase in anal cancer reported in the future compared to the currently reported rates,” he said. “We don’t really know.”
Regardless of the reason for the speed to cases of cancer, the results were definitive: Nine participants were diagnosed with invasive anal cancer in the treatment arm, while 21 were diagnosed with invasive anal cancer in the control arm. That’s a 57% reduction in cancer occurrence between the arms. Or, to put it another way, the rate of anal cancer among people in the treatment arm was 173 per 100,000 people-years. In the active monitoring arm, it was 402 per 100,000 person-years. For context, the overall rate of anal cancer among people living with HIV is 50 per 100,000 person-years. The rate in the general U.S. population is 8 per 100,000 people-years.
The experimental treatment was such a definitive success that the investigators stopped the trial and shifted all participants in the control arm to treatment.
‘We have to build’
Before Dr. Palefsky was even done presenting the data, clinicians, people living with HIV, and experts at the session were already brainstorming as to how to get these results into practice.
“These data are what we have long needed to fuel some action on this important problem, including medical cost reimbursement through insurance and increasing the number of persons trained and capable in anal cancer screening,” John Brooks, MD, head of the epidemiology research team at the Centers for Disease Control and Prevention’s division of HIV/AIDS prevention, wrote in the virtual chat.
Jeff Taylor, a member of the ANCHOR advisory board and a person living with HIV who participated in one of the first azidothymidine trials in the late 1980s, responded quickly.
“What kind of advocacy from researchers, HIV clinicians and [people living with HIV] is needed to get this on treatment guidelines, HRA providers trained and certified, and payors to cover this so [people living with HIV] actually have access to lifesaving screening and [treatment]?” Mr. Taylor asked.
It’s a serious challenge. David Malebranche, MD, an Atlanta-based internal medicine physician who specializes in sexual health and HIV, commented in an interview. When he saw the initial press release last year on the ANCHOR findings, his first reaction was: “Thank god. We finally have some data to show what we’ve been trying to get people to do” all along.
But then he wondered, who is going to perform these tests? It’s a fair question. Currently, the wait for an HRA is 6-12 months in many parts of the country. And Dr. Malebranche can’t imagine this being added to his already full plate as a primary care provider.
“If you tell a primary care provider now that they have to do a rectal Pap smear, that’s going to be a problem while you’re also asking them to screen each patient for depression, anxiety, domestic abuse, intimate partner violence, all the healthcare maintenance and all the other screening tests – and then you deal with not only the urgent complaint but then all the complex medical issues on top of that – in a 15-minute or 10-minute visit,” he said.
Now that we have these data, he said, “we have to build.”
Dr. Palefsky agreed. Very few centers have enough people skilled at performing HRAs to meet the current demand, and it’s not realistic to expect clinicians to perform an HRA every 6 months like the study team did. There need to be algorithms put in place to help practitioners figure out who among their patients living with HIV could benefit from this increased screening, as well as biomarkers to identify HSIL progression and regression without the use of HRA, Dr. Palefsky told attendees. And more clinicians need to be recruited and trained to read HRAs, which can be difficult for the untrained eye to decipher.
Dr. Malebranche added another, more fundamental thing that needs to be built. Dr. Malebranche has worked in HIV clinics where the majority of his patients qualify for insurance under the Ryan White Program and get their medications through the AIDS Drug Assistance Program. While Ryan White programs can provide critical wraparound care, Dr. Malebranche has had to refer out for something like an HRA or cancer treatment. But the people who only access care through such programs may not have coverage with the clinics that perform HRA or that treat cancer. And that’s if they can even find someone to see them.
“If I live in a state like Georgia, which doesn’t have Medicaid expansion and we have people who are uninsured, where do you send them?” Dr. Malebranche asked. “This isn’t theoretical. I ran into this problem when I was working at the AIDS Healthcare Foundation last year. ... This is a call for infrastructure.”
The study was funded by the National Cancer Institute. Dr. Brooks reported no relevant financial relationships. Dr. Palefsky has received consultant fees from Merck, Vir Biotechnology, Virion Therapeutics, and Antiva Bioscience, as well as speaker fees from Merck. Dr. Malebranche has received consulting and advising fees from ViiV Healthcare.
A version of this article first appeared on Medscape.com.
Reports based on a press release in October 2021 suggested it, but now the full data tell the story:
“We now show, for the first time, that treatment of anal HSIL is effective in reducing the incidence of anal cancer,” said Joel Palefsky, MD, lead investigator of the Anal Cancer/HSIL Outcomes Research (ANCHOR) study and founder/director of the Anal Neoplasia Clinic at the University of California, San Francisco. “These data should be included in an overall assessment for inclusion of screening for and treating HSIL as standard of care in people living with HIV.”
Dr. Palefsky presented the full results in a special session at the Conference on Retroviruses and Opportunistic Infections, which drew excitement, gratitude, and relief from both researchers and clinicians, who flocked to the session.
But it’s not just people with HIV who will benefit from this research. Dr. Palefsky suggested that the findings should also be considered as guides for other people at high-risk for anal cancer, such as people who are immunocompromised for other reasons, including those with lupus, ulcerative colitis, Crohn’s disease, or cisgender women who have had vulvar or cervical cancer or precancer.
“If we can show efficacy in the most challenging group of all, which are people living with HIV, we think the results can be as good, if not even better, in the other groups at high risk of anal cancer,” Dr. Palefsky said.
But to serve anyone – whether living with HIV or not – infrastructure, algorithms, and workforce training are going be needed to meet the currently unserved people through use of high-resolution anoscopy (HRA) and other screening technology, he said.
Dr. Palefsy and colleagues screened 10,723 people living with HIV being served at 15 clinics nationwide. More than half, 52.2%, had anal HSIL – 53.3% of the cisgender men living with HIV in the trial, 45.8% of the cis women living with HIV, and a full 62.5% of transgender participants.
Those 4,446 participants were split evenly between the treatment arm and the control arm. Those in the treatment arm received treatment for HSIL on their first study visit via one of five options: hyfrecation, office-based electrocautery ablation, infrared coagulation, topical 5-fluorouracil cream, or topical imiquimod. Then, every 6 months after that, they came in for HRA, blood tests, anal Pap smears, and biopsies to check for any lingering or new HSIL. If clinicians found such cells, they received treatment again. If biopsies still showed HSIL and clinician and participant were worried about cancer, they could come in as frequently as every 3 months and receive treatment each time.
The active-monitoring control group still received an anal Pap smear, blood tests, biopsy, and HRA every 6 months – a level of care that is currently not mandated anywhere for people living with HIV, Dr. Palefsky said in an interview. They were also able to come in for more frequent monitoring (every 3 months) if clinicians were worried about cancer.
“Those in both arms would have been getting more attention than if they had not participated in the study,” he said.
In addition, during screening, researchers found that cancer was already present in 17 other people, who skipped the study to go right to treatment.
Participants reflected the demographics of the HIV epidemic in the United States. They were older (median age, 51 years), mostly gay (78%), and cisgender male (80%). Close to half, 42%, were Black, and 16% were Latinx. In addition, cisgender women made up 16% of the participants and transgender people, and nonbinary individuals accounted for more than 3% of the participants. In addition, one in three smoked.
The vast majority of participants had well-controlled HIV and healthy immune systems, though half in each arm had a history of AIDS, defined as lowest-ever CD4 immune cell counts below 200. Today, more than 80% of participants had undetectable viral loads, defined as a viral load less than 50 copies/mL, and another 7% had HIV viral loads below 200. In total, 9.3% in the treatment arm and 10.9% in the control arm had HIV viral loads higher than that. At time of enrollment, CD4 counts were above 600 in each group, indicating healthy immune systems.
Although all participants were there because they had anal HSIL, more than 1 in 10 – 13% – had abnormal cells so extensive that they covered more than half of the anal canal or the perianal region.
Once everyone was enrolled, researchers began monitoring and treatment, looking specifically for 31 cases of cancer – a number the team had determined that they’d need in order to draw any conclusions. Dr. Palefsky didn’t have to wait long. They were still trying to enroll the last 1,000 participants to have the power necessary to reach that number when the cancer diagnoses came in.
Dr. Palefsky told this news organization that the reason for that is unclear. It could be that some of those cases would have resolved on their own, and so the swiftness with which they reached the required number of cancer cases belies their seriousness. It could also be that the particular people who enrolled in this trial were engaging in behaviors that put them at even higher risk for anal cancer than the population of people living with HIV in the United States.
Or it could be that symptom-based screening is missing a lot of cancers that currently go untreated.
“So perhaps we will be seeing an increase in anal cancer reported in the future compared to the currently reported rates,” he said. “We don’t really know.”
Regardless of the reason for the speed to cases of cancer, the results were definitive: Nine participants were diagnosed with invasive anal cancer in the treatment arm, while 21 were diagnosed with invasive anal cancer in the control arm. That’s a 57% reduction in cancer occurrence between the arms. Or, to put it another way, the rate of anal cancer among people in the treatment arm was 173 per 100,000 people-years. In the active monitoring arm, it was 402 per 100,000 person-years. For context, the overall rate of anal cancer among people living with HIV is 50 per 100,000 person-years. The rate in the general U.S. population is 8 per 100,000 people-years.
The experimental treatment was such a definitive success that the investigators stopped the trial and shifted all participants in the control arm to treatment.
‘We have to build’
Before Dr. Palefsky was even done presenting the data, clinicians, people living with HIV, and experts at the session were already brainstorming as to how to get these results into practice.
“These data are what we have long needed to fuel some action on this important problem, including medical cost reimbursement through insurance and increasing the number of persons trained and capable in anal cancer screening,” John Brooks, MD, head of the epidemiology research team at the Centers for Disease Control and Prevention’s division of HIV/AIDS prevention, wrote in the virtual chat.
Jeff Taylor, a member of the ANCHOR advisory board and a person living with HIV who participated in one of the first azidothymidine trials in the late 1980s, responded quickly.
“What kind of advocacy from researchers, HIV clinicians and [people living with HIV] is needed to get this on treatment guidelines, HRA providers trained and certified, and payors to cover this so [people living with HIV] actually have access to lifesaving screening and [treatment]?” Mr. Taylor asked.
It’s a serious challenge. David Malebranche, MD, an Atlanta-based internal medicine physician who specializes in sexual health and HIV, commented in an interview. When he saw the initial press release last year on the ANCHOR findings, his first reaction was: “Thank god. We finally have some data to show what we’ve been trying to get people to do” all along.
But then he wondered, who is going to perform these tests? It’s a fair question. Currently, the wait for an HRA is 6-12 months in many parts of the country. And Dr. Malebranche can’t imagine this being added to his already full plate as a primary care provider.
“If you tell a primary care provider now that they have to do a rectal Pap smear, that’s going to be a problem while you’re also asking them to screen each patient for depression, anxiety, domestic abuse, intimate partner violence, all the healthcare maintenance and all the other screening tests – and then you deal with not only the urgent complaint but then all the complex medical issues on top of that – in a 15-minute or 10-minute visit,” he said.
Now that we have these data, he said, “we have to build.”
Dr. Palefsky agreed. Very few centers have enough people skilled at performing HRAs to meet the current demand, and it’s not realistic to expect clinicians to perform an HRA every 6 months like the study team did. There need to be algorithms put in place to help practitioners figure out who among their patients living with HIV could benefit from this increased screening, as well as biomarkers to identify HSIL progression and regression without the use of HRA, Dr. Palefsky told attendees. And more clinicians need to be recruited and trained to read HRAs, which can be difficult for the untrained eye to decipher.
Dr. Malebranche added another, more fundamental thing that needs to be built. Dr. Malebranche has worked in HIV clinics where the majority of his patients qualify for insurance under the Ryan White Program and get their medications through the AIDS Drug Assistance Program. While Ryan White programs can provide critical wraparound care, Dr. Malebranche has had to refer out for something like an HRA or cancer treatment. But the people who only access care through such programs may not have coverage with the clinics that perform HRA or that treat cancer. And that’s if they can even find someone to see them.
“If I live in a state like Georgia, which doesn’t have Medicaid expansion and we have people who are uninsured, where do you send them?” Dr. Malebranche asked. “This isn’t theoretical. I ran into this problem when I was working at the AIDS Healthcare Foundation last year. ... This is a call for infrastructure.”
The study was funded by the National Cancer Institute. Dr. Brooks reported no relevant financial relationships. Dr. Palefsky has received consultant fees from Merck, Vir Biotechnology, Virion Therapeutics, and Antiva Bioscience, as well as speaker fees from Merck. Dr. Malebranche has received consulting and advising fees from ViiV Healthcare.
A version of this article first appeared on Medscape.com.
FROM CROI 2022
Third transplant patient cured of HIV marks important firsts
that has plagued the world for decades.
But while this case is certainly cause for celebration, experts involved in the effort say we are still a long way from a universal cure.
Researcher Yvonne Bryson, MD, chief of pediatric infectious diseases at the University of California, Los Angeles, told those attending the Conference on Retroviruses and Opportunistic Infections that this case is special. The patient was a woman living with HIV who is multiracial. The previous two patients were men: one white, one Latinx.
The woman in this case was given transplants of stem cells and umbilical cord blood to treat leukemia. The treatment not only sent her cancer into remission, but her HIV as well.
The success of this case suggests that cord stem cell transplants should be considered to produce remission and cure for those with HIV who also have cancers and other diseases, the researchers said.
While the news was met with excitement in the scientific community, the approach will not be available universally, since the transplants were all done to treat cancers in the three HIV-infected patients. Overall, Dr. Bryson estimates that about 50 people per year may benefit from this procedure.
Even so, other experts say the approach could provide insight into other ways to find cures. And Dr. Bryson says it opens up options for more diverse populations.
“A bone marrow transplant is not a viable large-scale strategy for curing HIV, but it does present a proof of concept that HIV can be cured,” said Sharon Lewin, MD, president-elect of the International AIDS Society. “It also further strengthens using gene therapy as a viable strategy for an HIV cure.”
The woman needed a stem cell transplant after being diagnosed with leukemia. The stem cell transplant technique used was also novel, Dr. Bryson said. The medical team used a combination of adult stem cells from a relative’s blood and umbilical cord blood from a cord-blood bank that had a rare mutation that makes the immune system resistant to HIV.
In the previous two cases of HIV cures after transplants, both patients were treated with stem cell transplants, with the same mutation, but from bone marrow transplants, a more difficult procedure. And no cord blood was used for those.
The combination of adult cells and cord-blood cells proved to be the ticket to success. Using the adult cells provides a kind of bridge that helps until the cord blood takes over, the researchers said. By day 100 after the transplant, Dr. Bryson said, the woman basically had a new immune system.
HIV remained undetectable in T cells and in bone marrow. And 37 months after the transplant, the woman stopped taking the antiretroviral treatment commonly given to treat HIV infection.
‘’She is currently clinically well,” Dr. Bryson said. Her cancer is in remission.
Case histories: Three patients
The woman, who is middle-aged, has requested privacy, asking that neither her age nor other details be released. But the researchers did provide some background on her medical history and her route back to health. She was diagnosed with HIV in 2013 and began treatment with antiretroviral therapy (ART). Four years after her HIV diagnosis, she developed high-risk acute myelogenous leukemia. The transplant was done to treat that.
Her recovery was much less bumpy than that of the previous two patients, the researchers said. She left the hospital 17 days after the transplant. She didn’t have serious complications like the first two, who developed a condition that occurs when donor bone marrow or stem cells attack the recipient.
“This case also suggests that it’s the transplant of HIV-resistant cells that was key to achieving a cure here,” said Dr. Lewin. The first patient who had HIV remission after a stem cell transplant, a White man, stayed in remission for 12 years and was termed cured. But he died of leukemia in September 2020. The other, a Latinx man, has been in remission for more than 30 months.
HIV statistics, ethnic/racial burdens
In the United States, about 1.2 million people have HIV, according to HIV.gov. Thirteen percent of those who have it do not know they have it. In 2019, 34,800 new infections were diagnosed.
Certain ethnic and racial groups are more affected by HIV than others, given their proportions in the U.S. population, federal statistics suggest. In 2019, for instance, African Americans were 13% of the U.S. population but 40% of those with HIV. Hispanics/Latinx represented 18.5% of the total population but 25% of those diagnosed with HIV.
Disparities also affect women unequally, with Black women disproportionately affected, compared to women of other ethnic and racial groups. Annual HIV infections remained stable overall among Black women from 2015 to 2019, but the rate of new HIV infections among Black women is 11 times that of White women and 4 times that of Latinx, according to federal statistics.
Expert perspective, reactions
Vincent Marconi, MD, professor of infectious diseases at Emory University, Atlanta, whose research focuses on disparities in HIV treatment responses, called the news “an exciting development for the cure agenda. This is the first woman to have been cured for at least 14 months, and they used cord blood, which could allow for potentially less toxic regimens and fewer adverse effects.”
Although the approach, meant to be used to treat the cancers, will not be widely available, he said that ‘’it does provide insight into somewhat related alternative models of cure involving gene therapy.”
Meanwhile, Dr. Marconi and other researchers are focusing on the concept of long-term HIV remission if a cure is not possible. Among the strategies under study are gene editing and immune-based treatments. HIV remission is generally defined as having an HIV viral load that is not detectable after stopping treatment.
A version of this article first appeared on WebMD.com.
that has plagued the world for decades.
But while this case is certainly cause for celebration, experts involved in the effort say we are still a long way from a universal cure.
Researcher Yvonne Bryson, MD, chief of pediatric infectious diseases at the University of California, Los Angeles, told those attending the Conference on Retroviruses and Opportunistic Infections that this case is special. The patient was a woman living with HIV who is multiracial. The previous two patients were men: one white, one Latinx.
The woman in this case was given transplants of stem cells and umbilical cord blood to treat leukemia. The treatment not only sent her cancer into remission, but her HIV as well.
The success of this case suggests that cord stem cell transplants should be considered to produce remission and cure for those with HIV who also have cancers and other diseases, the researchers said.
While the news was met with excitement in the scientific community, the approach will not be available universally, since the transplants were all done to treat cancers in the three HIV-infected patients. Overall, Dr. Bryson estimates that about 50 people per year may benefit from this procedure.
Even so, other experts say the approach could provide insight into other ways to find cures. And Dr. Bryson says it opens up options for more diverse populations.
“A bone marrow transplant is not a viable large-scale strategy for curing HIV, but it does present a proof of concept that HIV can be cured,” said Sharon Lewin, MD, president-elect of the International AIDS Society. “It also further strengthens using gene therapy as a viable strategy for an HIV cure.”
The woman needed a stem cell transplant after being diagnosed with leukemia. The stem cell transplant technique used was also novel, Dr. Bryson said. The medical team used a combination of adult stem cells from a relative’s blood and umbilical cord blood from a cord-blood bank that had a rare mutation that makes the immune system resistant to HIV.
In the previous two cases of HIV cures after transplants, both patients were treated with stem cell transplants, with the same mutation, but from bone marrow transplants, a more difficult procedure. And no cord blood was used for those.
The combination of adult cells and cord-blood cells proved to be the ticket to success. Using the adult cells provides a kind of bridge that helps until the cord blood takes over, the researchers said. By day 100 after the transplant, Dr. Bryson said, the woman basically had a new immune system.
HIV remained undetectable in T cells and in bone marrow. And 37 months after the transplant, the woman stopped taking the antiretroviral treatment commonly given to treat HIV infection.
‘’She is currently clinically well,” Dr. Bryson said. Her cancer is in remission.
Case histories: Three patients
The woman, who is middle-aged, has requested privacy, asking that neither her age nor other details be released. But the researchers did provide some background on her medical history and her route back to health. She was diagnosed with HIV in 2013 and began treatment with antiretroviral therapy (ART). Four years after her HIV diagnosis, she developed high-risk acute myelogenous leukemia. The transplant was done to treat that.
Her recovery was much less bumpy than that of the previous two patients, the researchers said. She left the hospital 17 days after the transplant. She didn’t have serious complications like the first two, who developed a condition that occurs when donor bone marrow or stem cells attack the recipient.
“This case also suggests that it’s the transplant of HIV-resistant cells that was key to achieving a cure here,” said Dr. Lewin. The first patient who had HIV remission after a stem cell transplant, a White man, stayed in remission for 12 years and was termed cured. But he died of leukemia in September 2020. The other, a Latinx man, has been in remission for more than 30 months.
HIV statistics, ethnic/racial burdens
In the United States, about 1.2 million people have HIV, according to HIV.gov. Thirteen percent of those who have it do not know they have it. In 2019, 34,800 new infections were diagnosed.
Certain ethnic and racial groups are more affected by HIV than others, given their proportions in the U.S. population, federal statistics suggest. In 2019, for instance, African Americans were 13% of the U.S. population but 40% of those with HIV. Hispanics/Latinx represented 18.5% of the total population but 25% of those diagnosed with HIV.
Disparities also affect women unequally, with Black women disproportionately affected, compared to women of other ethnic and racial groups. Annual HIV infections remained stable overall among Black women from 2015 to 2019, but the rate of new HIV infections among Black women is 11 times that of White women and 4 times that of Latinx, according to federal statistics.
Expert perspective, reactions
Vincent Marconi, MD, professor of infectious diseases at Emory University, Atlanta, whose research focuses on disparities in HIV treatment responses, called the news “an exciting development for the cure agenda. This is the first woman to have been cured for at least 14 months, and they used cord blood, which could allow for potentially less toxic regimens and fewer adverse effects.”
Although the approach, meant to be used to treat the cancers, will not be widely available, he said that ‘’it does provide insight into somewhat related alternative models of cure involving gene therapy.”
Meanwhile, Dr. Marconi and other researchers are focusing on the concept of long-term HIV remission if a cure is not possible. Among the strategies under study are gene editing and immune-based treatments. HIV remission is generally defined as having an HIV viral load that is not detectable after stopping treatment.
A version of this article first appeared on WebMD.com.
that has plagued the world for decades.
But while this case is certainly cause for celebration, experts involved in the effort say we are still a long way from a universal cure.
Researcher Yvonne Bryson, MD, chief of pediatric infectious diseases at the University of California, Los Angeles, told those attending the Conference on Retroviruses and Opportunistic Infections that this case is special. The patient was a woman living with HIV who is multiracial. The previous two patients were men: one white, one Latinx.
The woman in this case was given transplants of stem cells and umbilical cord blood to treat leukemia. The treatment not only sent her cancer into remission, but her HIV as well.
The success of this case suggests that cord stem cell transplants should be considered to produce remission and cure for those with HIV who also have cancers and other diseases, the researchers said.
While the news was met with excitement in the scientific community, the approach will not be available universally, since the transplants were all done to treat cancers in the three HIV-infected patients. Overall, Dr. Bryson estimates that about 50 people per year may benefit from this procedure.
Even so, other experts say the approach could provide insight into other ways to find cures. And Dr. Bryson says it opens up options for more diverse populations.
“A bone marrow transplant is not a viable large-scale strategy for curing HIV, but it does present a proof of concept that HIV can be cured,” said Sharon Lewin, MD, president-elect of the International AIDS Society. “It also further strengthens using gene therapy as a viable strategy for an HIV cure.”
The woman needed a stem cell transplant after being diagnosed with leukemia. The stem cell transplant technique used was also novel, Dr. Bryson said. The medical team used a combination of adult stem cells from a relative’s blood and umbilical cord blood from a cord-blood bank that had a rare mutation that makes the immune system resistant to HIV.
In the previous two cases of HIV cures after transplants, both patients were treated with stem cell transplants, with the same mutation, but from bone marrow transplants, a more difficult procedure. And no cord blood was used for those.
The combination of adult cells and cord-blood cells proved to be the ticket to success. Using the adult cells provides a kind of bridge that helps until the cord blood takes over, the researchers said. By day 100 after the transplant, Dr. Bryson said, the woman basically had a new immune system.
HIV remained undetectable in T cells and in bone marrow. And 37 months after the transplant, the woman stopped taking the antiretroviral treatment commonly given to treat HIV infection.
‘’She is currently clinically well,” Dr. Bryson said. Her cancer is in remission.
Case histories: Three patients
The woman, who is middle-aged, has requested privacy, asking that neither her age nor other details be released. But the researchers did provide some background on her medical history and her route back to health. She was diagnosed with HIV in 2013 and began treatment with antiretroviral therapy (ART). Four years after her HIV diagnosis, she developed high-risk acute myelogenous leukemia. The transplant was done to treat that.
Her recovery was much less bumpy than that of the previous two patients, the researchers said. She left the hospital 17 days after the transplant. She didn’t have serious complications like the first two, who developed a condition that occurs when donor bone marrow or stem cells attack the recipient.
“This case also suggests that it’s the transplant of HIV-resistant cells that was key to achieving a cure here,” said Dr. Lewin. The first patient who had HIV remission after a stem cell transplant, a White man, stayed in remission for 12 years and was termed cured. But he died of leukemia in September 2020. The other, a Latinx man, has been in remission for more than 30 months.
HIV statistics, ethnic/racial burdens
In the United States, about 1.2 million people have HIV, according to HIV.gov. Thirteen percent of those who have it do not know they have it. In 2019, 34,800 new infections were diagnosed.
Certain ethnic and racial groups are more affected by HIV than others, given their proportions in the U.S. population, federal statistics suggest. In 2019, for instance, African Americans were 13% of the U.S. population but 40% of those with HIV. Hispanics/Latinx represented 18.5% of the total population but 25% of those diagnosed with HIV.
Disparities also affect women unequally, with Black women disproportionately affected, compared to women of other ethnic and racial groups. Annual HIV infections remained stable overall among Black women from 2015 to 2019, but the rate of new HIV infections among Black women is 11 times that of White women and 4 times that of Latinx, according to federal statistics.
Expert perspective, reactions
Vincent Marconi, MD, professor of infectious diseases at Emory University, Atlanta, whose research focuses on disparities in HIV treatment responses, called the news “an exciting development for the cure agenda. This is the first woman to have been cured for at least 14 months, and they used cord blood, which could allow for potentially less toxic regimens and fewer adverse effects.”
Although the approach, meant to be used to treat the cancers, will not be widely available, he said that ‘’it does provide insight into somewhat related alternative models of cure involving gene therapy.”
Meanwhile, Dr. Marconi and other researchers are focusing on the concept of long-term HIV remission if a cure is not possible. Among the strategies under study are gene editing and immune-based treatments. HIV remission is generally defined as having an HIV viral load that is not detectable after stopping treatment.
A version of this article first appeared on WebMD.com.
FROM CROI 22
Full-press therapy rare in diabetes with ASCVD
A high percentage of people with type 2 diabetes also have atherosclerotic cardiovascular disease (ASCVD), but fewer than 1 in 20 get the triumvirate of evidence-based medications – drugs to lower cholesterol, blood pressure, and glucose levels – that can mitigate the dominant health risks they face, a large multicenter cohort study reported.
The cohort consisted of 324,706 patients with diabetes and ASCVD in the National Patient-Centered Clinical Research Network in 2018.
Senior study author Christopher B. Granger, MD, said in an interview that the findings represent “a shocking underuse of treatments proven to improve outcomes in this high-risk population.” For example, he noted that high-intensity statins are “inexpensive, well tolerated, and highly effective, but the fact that they’re only used in 26.8% of this population is really an indictment and embarrassment for our health-care system.”
The study analyzed prescriptions of high-intensity statins to lower cholesterol, ACE inhibitors or angiotensin-receptor blockers (ARBs) for blood pressure, and SGLT2 inhibitors or GLP-1 receptor agonists for hyperglycemia in a population with both diabetes and ASCVD.
This study amplifies the perceived treatment gap in cardiovascular risk reduction in persons with diabetes,” Paul S. Jellinger, MD, of the Center for Diabetes and Endocrine Care in Hollywood, Fla., said in an interview. “The unfortunate treatment deficiency documented among 325,000 patients in 12 health systems is carefully quantitated and the message is loud, clear, and simple: There is gross underutilization of agents – ACE inhibitors and ARBs, SGLT-2 inhibitors, GLP-1 receptor agonists, and high-intensity statins – with definitively proven ASCVD benefit.”
In the cohort population, 44% were women and 56% were men; 18.2% were black and 12.8% were Latinx. In terms of care patterns for the 205,885 patients who had specialized visit data from the year before the study, the most (74.8%) saw a primary care physician, while only 8.7% visited an endocrinologist and 26.4% saw a cardiologist.
In terms of the prescriptions they received, 58.6% were on a statin, with less than half on a high-intensity statin; 45.5% were on either an ACE inhibitor or ARB, 3.9% received a GLP-1 receptor agonist, and 2.8% were taking a SGLT2 inhibitor.
The investigators pointed out that figure of 58.6% for patients who got a statin was significantly lower than the 74.6% reported in a study of a database of commercially insured patients, but was more in line with findings a 2018 study of patients with diabetes and ASCVD.
Only 4.8% of patients got all three types of therapies, and a high percentage (42.6%) didn’t get any prescription for the three major risk factors.
Overcoming barriers to prescriptions
The study noted that more work needs to be done to overcome the barriers to more widespread use of these therapies in patients with both diabetes and ASCVD.
Specifically with SGLT2 inhibitors and GLP-1 receptor agonists, cost was more likely to be a barrier than with the other drug groups, but that didn’t explain the low levels of high-intensity statin prescriptions, said Dr. Granger of Duke University, Durham, N.C.
The first barrier he mentioned is what he called “clinical inertia.” He said: “I’m a cardiologist who cares for these patients in my clinic each week, and there are so many different things that we need to be trying to achieve with the brief time we have with each patient in our clinic setting that people tend to miss the opportunity.”
The cost barrier, especially with the glucose-lowering therapies, can be overcome with clinic and health care system programs that aid patients in getting discounted drugs, he noted.
Other barriers Dr. Granger pointed out are lack of education – “So many people think that people with previous muscle aches can’t take a high-intensity statin, and we know that’s not true” – and misinformation, which he called “the more nefarious issue.”
He said, “Part of the problem is that misinformation travels much faster than accurate information. There’s so much out there about statins being toxic, which is just not true.”
Fragmentation of the U.S. health care system and the lack of feedback on quality measures, and physicians deferring decisions on glucose-lowering therapy to endocrinologists also pose barriers to more widespread use of evidence-based therapies in patients with diabetes and ASCVD, Dr. Granger said.
“This is a call to action,” Dr. Granger said. “By clearly describing these gaps, we hope that people will see this as an important opportunity to improve care not only at the level of individual providers, but even more importantly at the level of health systems.”
Dr. Jellinger said the “dismal results” of the study serve as a “wake-up call,” adding that “my own perception among my colleagues, along with the data referred to in this article, point to definitely higher usage among commercially insured patients. However, even in more enriched populations the message is not having its full impact. We have remarkable agents for our patients with diabetes that can make a real impact in diabetes-related morbidity and mortality. Our twofold goal should be to aggressively educate a broad slate of health care professionals and, of course, make patient access easy and affordable without ‘prior authorization.’ ”
The study noted the need to bring the prescribing patterns for patients with both diabetes and ASCVD more in line with evidence-based guidelines. To that end, said Dr. Granger, the researchers are moving ahead on a randomized study of a quality improvement project involving about 45 U.S. cardiology clinics using a feedback loop to apply more consistent prescribing patterns for the three therapy groups. “Hopefully a year from now we’ll have a lot more information about this problem,” Dr. Granger added.
Boehringer Ingelheim and Lilly funded the study. Dr. Granger reported financial relationships with Boehringer Ingelheim, Bristol-Myers Squibb, Janssen, Pfizer, Medtronic, Akros Pharma, Apple, AstraZeneca, Daichi-Sankyo, Novartis, AbbVie, Bayer, Boston Scientific, CeleCor, Correvio, Espero, Merck, Novo Nordisk, Rhoshan Pharmaceuticals, and Roche Diagnostics. Dr. Jellinger is on speaker’s bureaus for Esperion and Amgen.
A high percentage of people with type 2 diabetes also have atherosclerotic cardiovascular disease (ASCVD), but fewer than 1 in 20 get the triumvirate of evidence-based medications – drugs to lower cholesterol, blood pressure, and glucose levels – that can mitigate the dominant health risks they face, a large multicenter cohort study reported.
The cohort consisted of 324,706 patients with diabetes and ASCVD in the National Patient-Centered Clinical Research Network in 2018.
Senior study author Christopher B. Granger, MD, said in an interview that the findings represent “a shocking underuse of treatments proven to improve outcomes in this high-risk population.” For example, he noted that high-intensity statins are “inexpensive, well tolerated, and highly effective, but the fact that they’re only used in 26.8% of this population is really an indictment and embarrassment for our health-care system.”
The study analyzed prescriptions of high-intensity statins to lower cholesterol, ACE inhibitors or angiotensin-receptor blockers (ARBs) for blood pressure, and SGLT2 inhibitors or GLP-1 receptor agonists for hyperglycemia in a population with both diabetes and ASCVD.
This study amplifies the perceived treatment gap in cardiovascular risk reduction in persons with diabetes,” Paul S. Jellinger, MD, of the Center for Diabetes and Endocrine Care in Hollywood, Fla., said in an interview. “The unfortunate treatment deficiency documented among 325,000 patients in 12 health systems is carefully quantitated and the message is loud, clear, and simple: There is gross underutilization of agents – ACE inhibitors and ARBs, SGLT-2 inhibitors, GLP-1 receptor agonists, and high-intensity statins – with definitively proven ASCVD benefit.”
In the cohort population, 44% were women and 56% were men; 18.2% were black and 12.8% were Latinx. In terms of care patterns for the 205,885 patients who had specialized visit data from the year before the study, the most (74.8%) saw a primary care physician, while only 8.7% visited an endocrinologist and 26.4% saw a cardiologist.
In terms of the prescriptions they received, 58.6% were on a statin, with less than half on a high-intensity statin; 45.5% were on either an ACE inhibitor or ARB, 3.9% received a GLP-1 receptor agonist, and 2.8% were taking a SGLT2 inhibitor.
The investigators pointed out that figure of 58.6% for patients who got a statin was significantly lower than the 74.6% reported in a study of a database of commercially insured patients, but was more in line with findings a 2018 study of patients with diabetes and ASCVD.
Only 4.8% of patients got all three types of therapies, and a high percentage (42.6%) didn’t get any prescription for the three major risk factors.
Overcoming barriers to prescriptions
The study noted that more work needs to be done to overcome the barriers to more widespread use of these therapies in patients with both diabetes and ASCVD.
Specifically with SGLT2 inhibitors and GLP-1 receptor agonists, cost was more likely to be a barrier than with the other drug groups, but that didn’t explain the low levels of high-intensity statin prescriptions, said Dr. Granger of Duke University, Durham, N.C.
The first barrier he mentioned is what he called “clinical inertia.” He said: “I’m a cardiologist who cares for these patients in my clinic each week, and there are so many different things that we need to be trying to achieve with the brief time we have with each patient in our clinic setting that people tend to miss the opportunity.”
The cost barrier, especially with the glucose-lowering therapies, can be overcome with clinic and health care system programs that aid patients in getting discounted drugs, he noted.
Other barriers Dr. Granger pointed out are lack of education – “So many people think that people with previous muscle aches can’t take a high-intensity statin, and we know that’s not true” – and misinformation, which he called “the more nefarious issue.”
He said, “Part of the problem is that misinformation travels much faster than accurate information. There’s so much out there about statins being toxic, which is just not true.”
Fragmentation of the U.S. health care system and the lack of feedback on quality measures, and physicians deferring decisions on glucose-lowering therapy to endocrinologists also pose barriers to more widespread use of evidence-based therapies in patients with diabetes and ASCVD, Dr. Granger said.
“This is a call to action,” Dr. Granger said. “By clearly describing these gaps, we hope that people will see this as an important opportunity to improve care not only at the level of individual providers, but even more importantly at the level of health systems.”
Dr. Jellinger said the “dismal results” of the study serve as a “wake-up call,” adding that “my own perception among my colleagues, along with the data referred to in this article, point to definitely higher usage among commercially insured patients. However, even in more enriched populations the message is not having its full impact. We have remarkable agents for our patients with diabetes that can make a real impact in diabetes-related morbidity and mortality. Our twofold goal should be to aggressively educate a broad slate of health care professionals and, of course, make patient access easy and affordable without ‘prior authorization.’ ”
The study noted the need to bring the prescribing patterns for patients with both diabetes and ASCVD more in line with evidence-based guidelines. To that end, said Dr. Granger, the researchers are moving ahead on a randomized study of a quality improvement project involving about 45 U.S. cardiology clinics using a feedback loop to apply more consistent prescribing patterns for the three therapy groups. “Hopefully a year from now we’ll have a lot more information about this problem,” Dr. Granger added.
Boehringer Ingelheim and Lilly funded the study. Dr. Granger reported financial relationships with Boehringer Ingelheim, Bristol-Myers Squibb, Janssen, Pfizer, Medtronic, Akros Pharma, Apple, AstraZeneca, Daichi-Sankyo, Novartis, AbbVie, Bayer, Boston Scientific, CeleCor, Correvio, Espero, Merck, Novo Nordisk, Rhoshan Pharmaceuticals, and Roche Diagnostics. Dr. Jellinger is on speaker’s bureaus for Esperion and Amgen.
A high percentage of people with type 2 diabetes also have atherosclerotic cardiovascular disease (ASCVD), but fewer than 1 in 20 get the triumvirate of evidence-based medications – drugs to lower cholesterol, blood pressure, and glucose levels – that can mitigate the dominant health risks they face, a large multicenter cohort study reported.
The cohort consisted of 324,706 patients with diabetes and ASCVD in the National Patient-Centered Clinical Research Network in 2018.
Senior study author Christopher B. Granger, MD, said in an interview that the findings represent “a shocking underuse of treatments proven to improve outcomes in this high-risk population.” For example, he noted that high-intensity statins are “inexpensive, well tolerated, and highly effective, but the fact that they’re only used in 26.8% of this population is really an indictment and embarrassment for our health-care system.”
The study analyzed prescriptions of high-intensity statins to lower cholesterol, ACE inhibitors or angiotensin-receptor blockers (ARBs) for blood pressure, and SGLT2 inhibitors or GLP-1 receptor agonists for hyperglycemia in a population with both diabetes and ASCVD.
This study amplifies the perceived treatment gap in cardiovascular risk reduction in persons with diabetes,” Paul S. Jellinger, MD, of the Center for Diabetes and Endocrine Care in Hollywood, Fla., said in an interview. “The unfortunate treatment deficiency documented among 325,000 patients in 12 health systems is carefully quantitated and the message is loud, clear, and simple: There is gross underutilization of agents – ACE inhibitors and ARBs, SGLT-2 inhibitors, GLP-1 receptor agonists, and high-intensity statins – with definitively proven ASCVD benefit.”
In the cohort population, 44% were women and 56% were men; 18.2% were black and 12.8% were Latinx. In terms of care patterns for the 205,885 patients who had specialized visit data from the year before the study, the most (74.8%) saw a primary care physician, while only 8.7% visited an endocrinologist and 26.4% saw a cardiologist.
In terms of the prescriptions they received, 58.6% were on a statin, with less than half on a high-intensity statin; 45.5% were on either an ACE inhibitor or ARB, 3.9% received a GLP-1 receptor agonist, and 2.8% were taking a SGLT2 inhibitor.
The investigators pointed out that figure of 58.6% for patients who got a statin was significantly lower than the 74.6% reported in a study of a database of commercially insured patients, but was more in line with findings a 2018 study of patients with diabetes and ASCVD.
Only 4.8% of patients got all three types of therapies, and a high percentage (42.6%) didn’t get any prescription for the three major risk factors.
Overcoming barriers to prescriptions
The study noted that more work needs to be done to overcome the barriers to more widespread use of these therapies in patients with both diabetes and ASCVD.
Specifically with SGLT2 inhibitors and GLP-1 receptor agonists, cost was more likely to be a barrier than with the other drug groups, but that didn’t explain the low levels of high-intensity statin prescriptions, said Dr. Granger of Duke University, Durham, N.C.
The first barrier he mentioned is what he called “clinical inertia.” He said: “I’m a cardiologist who cares for these patients in my clinic each week, and there are so many different things that we need to be trying to achieve with the brief time we have with each patient in our clinic setting that people tend to miss the opportunity.”
The cost barrier, especially with the glucose-lowering therapies, can be overcome with clinic and health care system programs that aid patients in getting discounted drugs, he noted.
Other barriers Dr. Granger pointed out are lack of education – “So many people think that people with previous muscle aches can’t take a high-intensity statin, and we know that’s not true” – and misinformation, which he called “the more nefarious issue.”
He said, “Part of the problem is that misinformation travels much faster than accurate information. There’s so much out there about statins being toxic, which is just not true.”
Fragmentation of the U.S. health care system and the lack of feedback on quality measures, and physicians deferring decisions on glucose-lowering therapy to endocrinologists also pose barriers to more widespread use of evidence-based therapies in patients with diabetes and ASCVD, Dr. Granger said.
“This is a call to action,” Dr. Granger said. “By clearly describing these gaps, we hope that people will see this as an important opportunity to improve care not only at the level of individual providers, but even more importantly at the level of health systems.”
Dr. Jellinger said the “dismal results” of the study serve as a “wake-up call,” adding that “my own perception among my colleagues, along with the data referred to in this article, point to definitely higher usage among commercially insured patients. However, even in more enriched populations the message is not having its full impact. We have remarkable agents for our patients with diabetes that can make a real impact in diabetes-related morbidity and mortality. Our twofold goal should be to aggressively educate a broad slate of health care professionals and, of course, make patient access easy and affordable without ‘prior authorization.’ ”
The study noted the need to bring the prescribing patterns for patients with both diabetes and ASCVD more in line with evidence-based guidelines. To that end, said Dr. Granger, the researchers are moving ahead on a randomized study of a quality improvement project involving about 45 U.S. cardiology clinics using a feedback loop to apply more consistent prescribing patterns for the three therapy groups. “Hopefully a year from now we’ll have a lot more information about this problem,” Dr. Granger added.
Boehringer Ingelheim and Lilly funded the study. Dr. Granger reported financial relationships with Boehringer Ingelheim, Bristol-Myers Squibb, Janssen, Pfizer, Medtronic, Akros Pharma, Apple, AstraZeneca, Daichi-Sankyo, Novartis, AbbVie, Bayer, Boston Scientific, CeleCor, Correvio, Espero, Merck, Novo Nordisk, Rhoshan Pharmaceuticals, and Roche Diagnostics. Dr. Jellinger is on speaker’s bureaus for Esperion and Amgen.
FROM JAMA OPEN NETWORK
Long COVID is real and consists of these conditions – or does it?
Loss of smell. Fatigue. Mental health challenges. Difficulty breathing and other lower respiratory diseases. Fluid and electrolyte disorders. Cardiac dysrhythmia and other nonspecific chest pains. Trouble with urination. Diabetes?
Statistically, . The data, presented at the Conference on Retroviruses and Opportunistic Infections, can be used to guide diagnoses of long COVID, and may be the guide soon at Kaiser Permanente offices, Michael Horberg, MD, executive director of research, community benefit, and Medicaid strategy at the Mid-Atlantic Permanente Research Institute, said in an interview.
“There are some real conditions you could ask about” if you were evaluating a patient who believes they have PASC, Dr. Horberg said. “And there are real conditions that are symptoms patients have but they don’t fit the PASC diagnosis.”
That list is likely to evolve as specific symptoms emerge with new variants, he said. And there’s also the nationwide Researching COVID to Enhance Recovery (RECOVER) trial being conducted by the National Institutes of Health (NIH). Dr. Horberg is withholding judgment on diabetes, though, until more data come in.
During the global pandemic, Dr. Horberg, an HIV physician by training, found himself writing policies and guidelines for Kaiser’s Mid-Atlantic States (KPMAS) COVID response. Not long after that, the reports of symptoms that have come to be called long COVID started to come in. But they were “a mishmash of things” – everything from binge eating to the skin condition vitiligo to cranial nerve impairment, along with the more common complaints like fever, insomnia, and shortness of breath.
So Dr. Horberg looked back through KPMAS patient charts and found 28,118 members who had received a positive SARS-CoV-2 PCR test result in 2020. Then he matched them 3:1 with 70,293 members who didn’t have a positive PCR. The majority were women, nearly half were younger than 50, more than 40% were Black, and 24.5% were Latinx. The majority met clinical definitions of overweight or obese and many had other chronic illnesses, including diabetes (18.7% in the COVID-positive group), chronic kidney disease (3%) and cancer (2.6%). Rates of chronic illnesses were similar between arms.
Then they went back to 4 years before each positive PCR test and looked for all the illnesses before COVID, all those that emerged within 30 days of COVID diagnosis and those illnesses that emerged between 1 and 3 months after diagnosis.
From that search, they found 15 symptoms that were more common among people who’d had COVID. In addition to the symptoms listed above, those included abdominal pain, other nervous system disorders, dizziness or vertigo, and nausea and vomiting. Then they looked at whether each patient had experienced those symptoms in the 4 years before COVID to see if they were, in fact, new diagnoses.
More than 1 in 10
About one in four people who’d had COVID reported symptoms they thought might be long COVID, but through the analysis, they found that only 13% actually developed new conditions that could be categorized as long COVID.
“When you start controlling for all those chronic conditions, a lot of symptoms fall out,” Dr. Horberg told this news organization. “Plus, when you start comparing to the COVID-negative population, especially in the first 30 days of your positive diagnosis, actually, the COVID-negative patients have essentially almost the same amount, sometimes more.”
For instance, in the first month after diagnosis, though people with COVID reported anxiety symptoms after their diagnoses, people who’d never had COVID were coming in even more often with that symptom. And although gastrointestinal disorders were common in people who’d had COVID, they were just as likely in people who had not. Nausea and vomiting were actually 19% more common in people without COVID than in those with it. And people without COVID were nearly twice as likely to develop nutritional and endocrine disorders.
In the longer run, people who’d had COVID were 25% more likely to develop dysrhythmias, 20% more likely to develop diabetes, 60% more likely to develop fatigue, 21% more likely to develop genitourinary conditions, 39% more likely to develop chest pains, and a full 3.88 times more likely to develop trouble with olfaction.
And although people who’d had COVID were numerically 5% more likely to develop both abdominal pain and vertigo, 4% more likely to develop nervous system disorders, and 1% more likely to develop anxiety disorders longer term, none of those reached statistical significance.
The only diagnosis that doesn’t make sense to Dr. Horberg is diabetes.
“At this point I don’t think it’s been fully explained,” Dr. Horberg said. “I don’t think COVID is affecting the pancreas. But I do think that these are people who probably sought medical care, who hadn’t been seeking medical care and that the findings of diabetes were incidental diagnoses.”
Still, Dr. Horberg isn’t saying never on that. “As they say, more research is needed,” he added.
Ready to define long COVID?
As an intensive care unit physician and pulmonologist, Michael Risbano, MD, assistant professor of medicine at the University of Pittsburgh, has seen a lot of COVID. As the co-manager of the medical system’s post-COVID clinic, he’s also seen a lot of people coming in for help with what could be long COVID. When he saw the data from Dr. Horberg’s presentation, at first it seemed to confirm what he’d already known. But then he looked further.
“Well, this is actually making sense,” Dr. Risbano thought. At his clinic, it’s been an ongoing challenge to tease out what symptoms existed before COVID. Unlike Kaiser, the University of Pittsburgh Medical Center is not a closed system.
“We know some people who tend to get sick [with COVID] have some underlying medical issues already,” Dr. Risbano said in an interview. “But we don’t always have a good baseline as to what they were like beforehand, so we don’t always know what’s changed.”
He said the study design here, though retrospective and based on chart review rather than prospective observation, starts to put symptoms into the larger context of a patient’s life. And the diabetes association really stood out to him. He recalled one patient who, when she was admitted to the ICU, had a hemoglobin A1c that was totally normal. But when that patient returned a few months later, her blood sugar had skyrocketed.
“It was sky-high, like 13, and she was in diabetic ketoacidosis,” he said. “I know that’s an N of 1, but my wife is a dietitian and a case manager, and she’s having a lot of people coming in with a new diagnosis of diabetes.”
Still, he said he’s not sure that the conditions the study identified should be the basis for a definition of long COVID.
“I don’t know if you can come up with a definition out of this,” he said. “But I think this is at least helpful in telling us what disease states are different pre- and post-COVID, and what sorts of diagnoses clinicians should look for when a patient comes in after having a COVID diagnosis.”
Dr. Horberg and Dr. Risbano have disclosed no relevant financial relationships. The study was funded by the National Institute of Allergy and Infectious Diseases at the National Institutes of Health.
A version of this article first appeared on Medscape.com.
Loss of smell. Fatigue. Mental health challenges. Difficulty breathing and other lower respiratory diseases. Fluid and electrolyte disorders. Cardiac dysrhythmia and other nonspecific chest pains. Trouble with urination. Diabetes?
Statistically, . The data, presented at the Conference on Retroviruses and Opportunistic Infections, can be used to guide diagnoses of long COVID, and may be the guide soon at Kaiser Permanente offices, Michael Horberg, MD, executive director of research, community benefit, and Medicaid strategy at the Mid-Atlantic Permanente Research Institute, said in an interview.
“There are some real conditions you could ask about” if you were evaluating a patient who believes they have PASC, Dr. Horberg said. “And there are real conditions that are symptoms patients have but they don’t fit the PASC diagnosis.”
That list is likely to evolve as specific symptoms emerge with new variants, he said. And there’s also the nationwide Researching COVID to Enhance Recovery (RECOVER) trial being conducted by the National Institutes of Health (NIH). Dr. Horberg is withholding judgment on diabetes, though, until more data come in.
During the global pandemic, Dr. Horberg, an HIV physician by training, found himself writing policies and guidelines for Kaiser’s Mid-Atlantic States (KPMAS) COVID response. Not long after that, the reports of symptoms that have come to be called long COVID started to come in. But they were “a mishmash of things” – everything from binge eating to the skin condition vitiligo to cranial nerve impairment, along with the more common complaints like fever, insomnia, and shortness of breath.
So Dr. Horberg looked back through KPMAS patient charts and found 28,118 members who had received a positive SARS-CoV-2 PCR test result in 2020. Then he matched them 3:1 with 70,293 members who didn’t have a positive PCR. The majority were women, nearly half were younger than 50, more than 40% were Black, and 24.5% were Latinx. The majority met clinical definitions of overweight or obese and many had other chronic illnesses, including diabetes (18.7% in the COVID-positive group), chronic kidney disease (3%) and cancer (2.6%). Rates of chronic illnesses were similar between arms.
Then they went back to 4 years before each positive PCR test and looked for all the illnesses before COVID, all those that emerged within 30 days of COVID diagnosis and those illnesses that emerged between 1 and 3 months after diagnosis.
From that search, they found 15 symptoms that were more common among people who’d had COVID. In addition to the symptoms listed above, those included abdominal pain, other nervous system disorders, dizziness or vertigo, and nausea and vomiting. Then they looked at whether each patient had experienced those symptoms in the 4 years before COVID to see if they were, in fact, new diagnoses.
More than 1 in 10
About one in four people who’d had COVID reported symptoms they thought might be long COVID, but through the analysis, they found that only 13% actually developed new conditions that could be categorized as long COVID.
“When you start controlling for all those chronic conditions, a lot of symptoms fall out,” Dr. Horberg told this news organization. “Plus, when you start comparing to the COVID-negative population, especially in the first 30 days of your positive diagnosis, actually, the COVID-negative patients have essentially almost the same amount, sometimes more.”
For instance, in the first month after diagnosis, though people with COVID reported anxiety symptoms after their diagnoses, people who’d never had COVID were coming in even more often with that symptom. And although gastrointestinal disorders were common in people who’d had COVID, they were just as likely in people who had not. Nausea and vomiting were actually 19% more common in people without COVID than in those with it. And people without COVID were nearly twice as likely to develop nutritional and endocrine disorders.
In the longer run, people who’d had COVID were 25% more likely to develop dysrhythmias, 20% more likely to develop diabetes, 60% more likely to develop fatigue, 21% more likely to develop genitourinary conditions, 39% more likely to develop chest pains, and a full 3.88 times more likely to develop trouble with olfaction.
And although people who’d had COVID were numerically 5% more likely to develop both abdominal pain and vertigo, 4% more likely to develop nervous system disorders, and 1% more likely to develop anxiety disorders longer term, none of those reached statistical significance.
The only diagnosis that doesn’t make sense to Dr. Horberg is diabetes.
“At this point I don’t think it’s been fully explained,” Dr. Horberg said. “I don’t think COVID is affecting the pancreas. But I do think that these are people who probably sought medical care, who hadn’t been seeking medical care and that the findings of diabetes were incidental diagnoses.”
Still, Dr. Horberg isn’t saying never on that. “As they say, more research is needed,” he added.
Ready to define long COVID?
As an intensive care unit physician and pulmonologist, Michael Risbano, MD, assistant professor of medicine at the University of Pittsburgh, has seen a lot of COVID. As the co-manager of the medical system’s post-COVID clinic, he’s also seen a lot of people coming in for help with what could be long COVID. When he saw the data from Dr. Horberg’s presentation, at first it seemed to confirm what he’d already known. But then he looked further.
“Well, this is actually making sense,” Dr. Risbano thought. At his clinic, it’s been an ongoing challenge to tease out what symptoms existed before COVID. Unlike Kaiser, the University of Pittsburgh Medical Center is not a closed system.
“We know some people who tend to get sick [with COVID] have some underlying medical issues already,” Dr. Risbano said in an interview. “But we don’t always have a good baseline as to what they were like beforehand, so we don’t always know what’s changed.”
He said the study design here, though retrospective and based on chart review rather than prospective observation, starts to put symptoms into the larger context of a patient’s life. And the diabetes association really stood out to him. He recalled one patient who, when she was admitted to the ICU, had a hemoglobin A1c that was totally normal. But when that patient returned a few months later, her blood sugar had skyrocketed.
“It was sky-high, like 13, and she was in diabetic ketoacidosis,” he said. “I know that’s an N of 1, but my wife is a dietitian and a case manager, and she’s having a lot of people coming in with a new diagnosis of diabetes.”
Still, he said he’s not sure that the conditions the study identified should be the basis for a definition of long COVID.
“I don’t know if you can come up with a definition out of this,” he said. “But I think this is at least helpful in telling us what disease states are different pre- and post-COVID, and what sorts of diagnoses clinicians should look for when a patient comes in after having a COVID diagnosis.”
Dr. Horberg and Dr. Risbano have disclosed no relevant financial relationships. The study was funded by the National Institute of Allergy and Infectious Diseases at the National Institutes of Health.
A version of this article first appeared on Medscape.com.
Loss of smell. Fatigue. Mental health challenges. Difficulty breathing and other lower respiratory diseases. Fluid and electrolyte disorders. Cardiac dysrhythmia and other nonspecific chest pains. Trouble with urination. Diabetes?
Statistically, . The data, presented at the Conference on Retroviruses and Opportunistic Infections, can be used to guide diagnoses of long COVID, and may be the guide soon at Kaiser Permanente offices, Michael Horberg, MD, executive director of research, community benefit, and Medicaid strategy at the Mid-Atlantic Permanente Research Institute, said in an interview.
“There are some real conditions you could ask about” if you were evaluating a patient who believes they have PASC, Dr. Horberg said. “And there are real conditions that are symptoms patients have but they don’t fit the PASC diagnosis.”
That list is likely to evolve as specific symptoms emerge with new variants, he said. And there’s also the nationwide Researching COVID to Enhance Recovery (RECOVER) trial being conducted by the National Institutes of Health (NIH). Dr. Horberg is withholding judgment on diabetes, though, until more data come in.
During the global pandemic, Dr. Horberg, an HIV physician by training, found himself writing policies and guidelines for Kaiser’s Mid-Atlantic States (KPMAS) COVID response. Not long after that, the reports of symptoms that have come to be called long COVID started to come in. But they were “a mishmash of things” – everything from binge eating to the skin condition vitiligo to cranial nerve impairment, along with the more common complaints like fever, insomnia, and shortness of breath.
So Dr. Horberg looked back through KPMAS patient charts and found 28,118 members who had received a positive SARS-CoV-2 PCR test result in 2020. Then he matched them 3:1 with 70,293 members who didn’t have a positive PCR. The majority were women, nearly half were younger than 50, more than 40% were Black, and 24.5% were Latinx. The majority met clinical definitions of overweight or obese and many had other chronic illnesses, including diabetes (18.7% in the COVID-positive group), chronic kidney disease (3%) and cancer (2.6%). Rates of chronic illnesses were similar between arms.
Then they went back to 4 years before each positive PCR test and looked for all the illnesses before COVID, all those that emerged within 30 days of COVID diagnosis and those illnesses that emerged between 1 and 3 months after diagnosis.
From that search, they found 15 symptoms that were more common among people who’d had COVID. In addition to the symptoms listed above, those included abdominal pain, other nervous system disorders, dizziness or vertigo, and nausea and vomiting. Then they looked at whether each patient had experienced those symptoms in the 4 years before COVID to see if they were, in fact, new diagnoses.
More than 1 in 10
About one in four people who’d had COVID reported symptoms they thought might be long COVID, but through the analysis, they found that only 13% actually developed new conditions that could be categorized as long COVID.
“When you start controlling for all those chronic conditions, a lot of symptoms fall out,” Dr. Horberg told this news organization. “Plus, when you start comparing to the COVID-negative population, especially in the first 30 days of your positive diagnosis, actually, the COVID-negative patients have essentially almost the same amount, sometimes more.”
For instance, in the first month after diagnosis, though people with COVID reported anxiety symptoms after their diagnoses, people who’d never had COVID were coming in even more often with that symptom. And although gastrointestinal disorders were common in people who’d had COVID, they were just as likely in people who had not. Nausea and vomiting were actually 19% more common in people without COVID than in those with it. And people without COVID were nearly twice as likely to develop nutritional and endocrine disorders.
In the longer run, people who’d had COVID were 25% more likely to develop dysrhythmias, 20% more likely to develop diabetes, 60% more likely to develop fatigue, 21% more likely to develop genitourinary conditions, 39% more likely to develop chest pains, and a full 3.88 times more likely to develop trouble with olfaction.
And although people who’d had COVID were numerically 5% more likely to develop both abdominal pain and vertigo, 4% more likely to develop nervous system disorders, and 1% more likely to develop anxiety disorders longer term, none of those reached statistical significance.
The only diagnosis that doesn’t make sense to Dr. Horberg is diabetes.
“At this point I don’t think it’s been fully explained,” Dr. Horberg said. “I don’t think COVID is affecting the pancreas. But I do think that these are people who probably sought medical care, who hadn’t been seeking medical care and that the findings of diabetes were incidental diagnoses.”
Still, Dr. Horberg isn’t saying never on that. “As they say, more research is needed,” he added.
Ready to define long COVID?
As an intensive care unit physician and pulmonologist, Michael Risbano, MD, assistant professor of medicine at the University of Pittsburgh, has seen a lot of COVID. As the co-manager of the medical system’s post-COVID clinic, he’s also seen a lot of people coming in for help with what could be long COVID. When he saw the data from Dr. Horberg’s presentation, at first it seemed to confirm what he’d already known. But then he looked further.
“Well, this is actually making sense,” Dr. Risbano thought. At his clinic, it’s been an ongoing challenge to tease out what symptoms existed before COVID. Unlike Kaiser, the University of Pittsburgh Medical Center is not a closed system.
“We know some people who tend to get sick [with COVID] have some underlying medical issues already,” Dr. Risbano said in an interview. “But we don’t always have a good baseline as to what they were like beforehand, so we don’t always know what’s changed.”
He said the study design here, though retrospective and based on chart review rather than prospective observation, starts to put symptoms into the larger context of a patient’s life. And the diabetes association really stood out to him. He recalled one patient who, when she was admitted to the ICU, had a hemoglobin A1c that was totally normal. But when that patient returned a few months later, her blood sugar had skyrocketed.
“It was sky-high, like 13, and she was in diabetic ketoacidosis,” he said. “I know that’s an N of 1, but my wife is a dietitian and a case manager, and she’s having a lot of people coming in with a new diagnosis of diabetes.”
Still, he said he’s not sure that the conditions the study identified should be the basis for a definition of long COVID.
“I don’t know if you can come up with a definition out of this,” he said. “But I think this is at least helpful in telling us what disease states are different pre- and post-COVID, and what sorts of diagnoses clinicians should look for when a patient comes in after having a COVID diagnosis.”
Dr. Horberg and Dr. Risbano have disclosed no relevant financial relationships. The study was funded by the National Institute of Allergy and Infectious Diseases at the National Institutes of Health.
A version of this article first appeared on Medscape.com.
FROM CROI 2022
AAP approves CDC’s child/adolescent vax schedule for 2022
In a policy statement published online Feb. 17 in Pediatrics, the AAP said the updated recommendations differ little from those released last year by the Advisory Committee on Immunization Practices of the Centers for Disease Control and Prevention.
“The only significant change this year was to add the dengue vaccine to the schedule,” Sean T. O’Leary, MD, MPH, vice chair of the AAP’s 2021-2022 Committee on Infectious Diseases and a coauthor of the statement, told this news organization. “But that is really only relevant for children living in endemic areas, primarily Puerto Rico but some other smaller U.S .territories as well.”
Dengue fever also is endemic in American Samoa and the U.S. Virgin Islands.
Notably, a new section has been added on routine recommendations for use of the Dengvaxia vaccine.
The 2022 policy statement addresses regular immunization of children from birth to 18 years and catch-up vaccination for those aged 4 months to 18 years. In addition to the AAP, multiple complementary physician and nurse organizations have approved the updates. The ACIP schedule is revised annually to reflect current recommendations on vaccines licensed by the U.S. Food and Drug Administration.
Most of the other changes this year involve minor updates to clarify language or improve usability. “CDC and AAP are always working to make the schedule as user-friendly as possible, with improvements made every year,” Dr. O’Leary, professor of pediatric infectious diseases at the University of Colorado at Denver, Aurora, said.
In terms of physician acceptance, he added, “I don’t think any of the changes would be considered controversial.”
Among other updates and clarifications:
- For Haemophilus influenzae type b (Hib) vaccination, the text now includes recommendations for the hexavalent Vaxelis vaccine (diphtheria, tetanus, pertussis, polio, Hib, and hepatitis B) for both routine and catch-up vaccination.
- For hepatitis A, the relevant note has been updated to clarify the age for routine vaccination.
- For human papillomavirus (HPV), the note now clarifies when an HPV series is complete with no additional dose recommended.
- The special situations section has been amended to specify which persons with immunocompromising conditions such as HIV should receive three doses of HPV vaccine regardless of age at initial vaccination.
- For measles, mumps, and rubella, routine vaccination now includes recommendations on the combination measles, mumps, rubella, and varicella vaccine.
- For meningococcal serogroup A, C, W, and Y vaccines, the augmented text explains when these can be simultaneously administered with serogroup B meningococcal vaccines, preferably at different anatomic sites. The language for the dosing schedule for Menveo vaccination in infants also has been clarified.
- In the catch-up immunization schedule for late-starting children aged 4 months to 18 years, the text on Hib has been changed so that the minimum interval between dose two and dose three now refers to Vaxelis, while reference to the discontinued Comvax (Hib-Hep B) vaccine has been removed.
As in other years, graphic changes have been made to table coloration and layout to improve accessibility. And as before, the 2022 childhood and adolescent immunization schedule has been updated to ensure consistency between its format and that of the 2022 adult immunization schedules.
The AAP committee stressed that clinically significant adverse events after immunization should be reported to the Vaccine Adverse Event Reporting System.
The full 2022 schedule can be found on the CDC’s website.
A version of this article first appeared on Medscape.com.
In a policy statement published online Feb. 17 in Pediatrics, the AAP said the updated recommendations differ little from those released last year by the Advisory Committee on Immunization Practices of the Centers for Disease Control and Prevention.
“The only significant change this year was to add the dengue vaccine to the schedule,” Sean T. O’Leary, MD, MPH, vice chair of the AAP’s 2021-2022 Committee on Infectious Diseases and a coauthor of the statement, told this news organization. “But that is really only relevant for children living in endemic areas, primarily Puerto Rico but some other smaller U.S .territories as well.”
Dengue fever also is endemic in American Samoa and the U.S. Virgin Islands.
Notably, a new section has been added on routine recommendations for use of the Dengvaxia vaccine.
The 2022 policy statement addresses regular immunization of children from birth to 18 years and catch-up vaccination for those aged 4 months to 18 years. In addition to the AAP, multiple complementary physician and nurse organizations have approved the updates. The ACIP schedule is revised annually to reflect current recommendations on vaccines licensed by the U.S. Food and Drug Administration.
Most of the other changes this year involve minor updates to clarify language or improve usability. “CDC and AAP are always working to make the schedule as user-friendly as possible, with improvements made every year,” Dr. O’Leary, professor of pediatric infectious diseases at the University of Colorado at Denver, Aurora, said.
In terms of physician acceptance, he added, “I don’t think any of the changes would be considered controversial.”
Among other updates and clarifications:
- For Haemophilus influenzae type b (Hib) vaccination, the text now includes recommendations for the hexavalent Vaxelis vaccine (diphtheria, tetanus, pertussis, polio, Hib, and hepatitis B) for both routine and catch-up vaccination.
- For hepatitis A, the relevant note has been updated to clarify the age for routine vaccination.
- For human papillomavirus (HPV), the note now clarifies when an HPV series is complete with no additional dose recommended.
- The special situations section has been amended to specify which persons with immunocompromising conditions such as HIV should receive three doses of HPV vaccine regardless of age at initial vaccination.
- For measles, mumps, and rubella, routine vaccination now includes recommendations on the combination measles, mumps, rubella, and varicella vaccine.
- For meningococcal serogroup A, C, W, and Y vaccines, the augmented text explains when these can be simultaneously administered with serogroup B meningococcal vaccines, preferably at different anatomic sites. The language for the dosing schedule for Menveo vaccination in infants also has been clarified.
- In the catch-up immunization schedule for late-starting children aged 4 months to 18 years, the text on Hib has been changed so that the minimum interval between dose two and dose three now refers to Vaxelis, while reference to the discontinued Comvax (Hib-Hep B) vaccine has been removed.
As in other years, graphic changes have been made to table coloration and layout to improve accessibility. And as before, the 2022 childhood and adolescent immunization schedule has been updated to ensure consistency between its format and that of the 2022 adult immunization schedules.
The AAP committee stressed that clinically significant adverse events after immunization should be reported to the Vaccine Adverse Event Reporting System.
The full 2022 schedule can be found on the CDC’s website.
A version of this article first appeared on Medscape.com.
In a policy statement published online Feb. 17 in Pediatrics, the AAP said the updated recommendations differ little from those released last year by the Advisory Committee on Immunization Practices of the Centers for Disease Control and Prevention.
“The only significant change this year was to add the dengue vaccine to the schedule,” Sean T. O’Leary, MD, MPH, vice chair of the AAP’s 2021-2022 Committee on Infectious Diseases and a coauthor of the statement, told this news organization. “But that is really only relevant for children living in endemic areas, primarily Puerto Rico but some other smaller U.S .territories as well.”
Dengue fever also is endemic in American Samoa and the U.S. Virgin Islands.
Notably, a new section has been added on routine recommendations for use of the Dengvaxia vaccine.
The 2022 policy statement addresses regular immunization of children from birth to 18 years and catch-up vaccination for those aged 4 months to 18 years. In addition to the AAP, multiple complementary physician and nurse organizations have approved the updates. The ACIP schedule is revised annually to reflect current recommendations on vaccines licensed by the U.S. Food and Drug Administration.
Most of the other changes this year involve minor updates to clarify language or improve usability. “CDC and AAP are always working to make the schedule as user-friendly as possible, with improvements made every year,” Dr. O’Leary, professor of pediatric infectious diseases at the University of Colorado at Denver, Aurora, said.
In terms of physician acceptance, he added, “I don’t think any of the changes would be considered controversial.”
Among other updates and clarifications:
- For Haemophilus influenzae type b (Hib) vaccination, the text now includes recommendations for the hexavalent Vaxelis vaccine (diphtheria, tetanus, pertussis, polio, Hib, and hepatitis B) for both routine and catch-up vaccination.
- For hepatitis A, the relevant note has been updated to clarify the age for routine vaccination.
- For human papillomavirus (HPV), the note now clarifies when an HPV series is complete with no additional dose recommended.
- The special situations section has been amended to specify which persons with immunocompromising conditions such as HIV should receive three doses of HPV vaccine regardless of age at initial vaccination.
- For measles, mumps, and rubella, routine vaccination now includes recommendations on the combination measles, mumps, rubella, and varicella vaccine.
- For meningococcal serogroup A, C, W, and Y vaccines, the augmented text explains when these can be simultaneously administered with serogroup B meningococcal vaccines, preferably at different anatomic sites. The language for the dosing schedule for Menveo vaccination in infants also has been clarified.
- In the catch-up immunization schedule for late-starting children aged 4 months to 18 years, the text on Hib has been changed so that the minimum interval between dose two and dose three now refers to Vaxelis, while reference to the discontinued Comvax (Hib-Hep B) vaccine has been removed.
As in other years, graphic changes have been made to table coloration and layout to improve accessibility. And as before, the 2022 childhood and adolescent immunization schedule has been updated to ensure consistency between its format and that of the 2022 adult immunization schedules.
The AAP committee stressed that clinically significant adverse events after immunization should be reported to the Vaccine Adverse Event Reporting System.
The full 2022 schedule can be found on the CDC’s website.
A version of this article first appeared on Medscape.com.
Thirty-seven percent of COVID-19 patients lose sense of taste, study says
, according to a new study.
Many COVID-19 patients report losing their sense of taste as well as their sense of smell, but scientists have been skeptical because the two senses are closely related and it was relatively rare for people to lose their taste sense before the COVID pandemic, says the Monell Chemical Senses Center, a nonprofit research institute in Philadelphia.
But a new Monell Center analysis found that 37% – or about four in every 10 -- of COVID-19 patients actually did lose their sense of taste and that “reports of taste loss are in fact genuine and distinguishable from smell loss.”
Taste dysfunction can be total taste loss, partial taste loss, and taste distortion. It’s an “underrated” symptom that could help doctors better treat COVID patients, the Monell Center said in a news release.
“It is time to turn to the tongue” to learn why taste is affected and to start on how to reverse or repair the loss, said Mackenzie Hannum, PhD, an author of the report and a postdoctoral fellow in the lab of Danielle Reed, PhD.
Researchers looked at data regarding 138,785 COVID patients from 241 studies that assessed taste loss and were published between May 15, 2020, and June 1, 2021. Of those patients, 32,918 said they had some form of taste loss. Further, female patients were more likely than males to lose their sense of taste, and people 36-50 years old had the highest rate of taste loss.
The information came from self-reports and direct reports.
“Self-reports are more subjective and can be in the form of questionnaires, interviews, health records, for example,” Dr. Hannum said. “On the other hand, direct measures of taste are more objective. They are conducted using testing kits that contain various sweet, salty, and sometimes bitter and sour solutions given to participants via drops, strips, or sprays.”
Though self-reports were subjective, they proved just as good as direct reports at detecting taste loss, the study said.
“Here self-reports are backed up by direct measures, proving that loss of taste is a real, distinct symptom of COVID-19 that is not to be confused with smell loss,” said study co-author Vicente Ramirez, a visiting scientist at Monell and a doctoral student at the University of California, Merced.
A version of this article first appeared on WebMD.com.
, according to a new study.
Many COVID-19 patients report losing their sense of taste as well as their sense of smell, but scientists have been skeptical because the two senses are closely related and it was relatively rare for people to lose their taste sense before the COVID pandemic, says the Monell Chemical Senses Center, a nonprofit research institute in Philadelphia.
But a new Monell Center analysis found that 37% – or about four in every 10 -- of COVID-19 patients actually did lose their sense of taste and that “reports of taste loss are in fact genuine and distinguishable from smell loss.”
Taste dysfunction can be total taste loss, partial taste loss, and taste distortion. It’s an “underrated” symptom that could help doctors better treat COVID patients, the Monell Center said in a news release.
“It is time to turn to the tongue” to learn why taste is affected and to start on how to reverse or repair the loss, said Mackenzie Hannum, PhD, an author of the report and a postdoctoral fellow in the lab of Danielle Reed, PhD.
Researchers looked at data regarding 138,785 COVID patients from 241 studies that assessed taste loss and were published between May 15, 2020, and June 1, 2021. Of those patients, 32,918 said they had some form of taste loss. Further, female patients were more likely than males to lose their sense of taste, and people 36-50 years old had the highest rate of taste loss.
The information came from self-reports and direct reports.
“Self-reports are more subjective and can be in the form of questionnaires, interviews, health records, for example,” Dr. Hannum said. “On the other hand, direct measures of taste are more objective. They are conducted using testing kits that contain various sweet, salty, and sometimes bitter and sour solutions given to participants via drops, strips, or sprays.”
Though self-reports were subjective, they proved just as good as direct reports at detecting taste loss, the study said.
“Here self-reports are backed up by direct measures, proving that loss of taste is a real, distinct symptom of COVID-19 that is not to be confused with smell loss,” said study co-author Vicente Ramirez, a visiting scientist at Monell and a doctoral student at the University of California, Merced.
A version of this article first appeared on WebMD.com.
, according to a new study.
Many COVID-19 patients report losing their sense of taste as well as their sense of smell, but scientists have been skeptical because the two senses are closely related and it was relatively rare for people to lose their taste sense before the COVID pandemic, says the Monell Chemical Senses Center, a nonprofit research institute in Philadelphia.
But a new Monell Center analysis found that 37% – or about four in every 10 -- of COVID-19 patients actually did lose their sense of taste and that “reports of taste loss are in fact genuine and distinguishable from smell loss.”
Taste dysfunction can be total taste loss, partial taste loss, and taste distortion. It’s an “underrated” symptom that could help doctors better treat COVID patients, the Monell Center said in a news release.
“It is time to turn to the tongue” to learn why taste is affected and to start on how to reverse or repair the loss, said Mackenzie Hannum, PhD, an author of the report and a postdoctoral fellow in the lab of Danielle Reed, PhD.
Researchers looked at data regarding 138,785 COVID patients from 241 studies that assessed taste loss and were published between May 15, 2020, and June 1, 2021. Of those patients, 32,918 said they had some form of taste loss. Further, female patients were more likely than males to lose their sense of taste, and people 36-50 years old had the highest rate of taste loss.
The information came from self-reports and direct reports.
“Self-reports are more subjective and can be in the form of questionnaires, interviews, health records, for example,” Dr. Hannum said. “On the other hand, direct measures of taste are more objective. They are conducted using testing kits that contain various sweet, salty, and sometimes bitter and sour solutions given to participants via drops, strips, or sprays.”
Though self-reports were subjective, they proved just as good as direct reports at detecting taste loss, the study said.
“Here self-reports are backed up by direct measures, proving that loss of taste is a real, distinct symptom of COVID-19 that is not to be confused with smell loss,” said study co-author Vicente Ramirez, a visiting scientist at Monell and a doctoral student at the University of California, Merced.
A version of this article first appeared on WebMD.com.
FROM CHEMICAL SENSES
Babies better protected from COVID if mother vaccinated during pregnancy: study
In a first of its kind study, researchers found women who received two mRNA COVID vaccine doses during pregnancy were 61% less likely to have a baby hospitalized for COVID-19 during the first 6 months of life.
In addition, two doses of the Pfizer/BioNTech or Moderna COVID vaccine later in a pregnancy were linked to an even higher level of protection, 80%, compared with 32% when given before 20 weeks’ gestation.
This finding suggests a greater transfer of maternal antibodies closer to birth, but more research is needed, cautioned senior study author Manish Patel, MD, during a Tuesday media telebriefing held by the Centers for Disease Control and Prevention.
Unanswered questions include how the babies got infected or if there is any protection afforded to babies for women vaccinated before pregnancy.
“We cannot be sure about the source of the infection,” said Dr. Patel, a medical epidemiologist with the CDC COVID-19 Emergency Response Team.
Dana Meaney-Delman, MD, MPH, agreed, but added that “perinatal transmission of the virus is very rare” with SARS-CoV-2. She is a practicing obstetrician and gynecologist and chief of the CDC Infant Outcomes Monitoring Research and Prevention Branch.
The study numbers were too small to show if a booster shot during pregnancy or breastfeeding could provide even greater protection for babies, Dr. Patel said.
The early release study was published online Feb. 15 in the CDC’s Morbidity and Mortality Weekly Report (MMWR).
Many previous studies looking at COVID-19 immunization during pregnancy focused on maternal health and “have clearly shown that receiving an mRNA COVID-19 vaccine during pregnancy reduces the risk for severe illness,” Dr. Meaney-Delman said.
Some dual protection suggested
Now there is evidence for a potential benefit to babies as well when a pregnant woman gets vaccinated. The study “provides real-world evidence that getting COVID-19 vaccination during pregnancy might help protect infants less than 6 months [of age],” Dr. Meaney-Delman said.
“These findings continue to emphasize the importance of COVID-19 vaccination during pregnancy to protect people who are pregnant and also to protect their babies,” she said.
Dr. Patel and colleagues studied 379 infants younger than 6 months hospitalized between July 1, 2021 and Jan. 17 of this year. Delta and then the Omicron variant predominated during this time.
The infants were admitted to one of 20 children’s hospitals in 17 states. The researchers compared 176 infants admitted with a positive COVID-19 PCR test to another 203 infants with a negative PCR test who served as controls.
Half as many mothers of infants admitted with COVID-19 were vaccinated during pregnancy, 16%, versus 32% of mothers of the control infants.
Vaccination with two doses of mRNA vaccine during pregnancy was 61% effective (95% confidence interval, 31%-78%) at preventing hospitalization among these infants. Because the study was epidemiological, the lower risk was an association, not a cause-and-effect finding, Dr. Patel said.
Babies admitted to the hospital positive for COVID-19 were more likely to be non-Hispanic Black, 18%, versus 9% of control group babies; and more likely to be Hispanic, 34% versus 28%, respectively.
A total 24% of infants with COVID-19 were admitted to the ICU, including the baby of an unvaccinated mother who required extracorporeal membrane oxygenation (ECMO). Another baby of an unvaccinated mother was the only infant death during the study.
Maternal vaccination trends
A reporter pointed out that COVID-19 vaccination rates tend to be low among pregnant women. “So there is some exciting news,” Dr. Meaney-Delman said, referring to a steady increase in the percentages of pregnant women in the U.S. choosing to get vaccinated, according to the CDC Data Tracker website.
“The numbers are encouraging, [but] they’re not quite where we need them to be, and they do differ by race and ethnicity,” she added.
A version of this article first appeared on Medscape.com.
In a first of its kind study, researchers found women who received two mRNA COVID vaccine doses during pregnancy were 61% less likely to have a baby hospitalized for COVID-19 during the first 6 months of life.
In addition, two doses of the Pfizer/BioNTech or Moderna COVID vaccine later in a pregnancy were linked to an even higher level of protection, 80%, compared with 32% when given before 20 weeks’ gestation.
This finding suggests a greater transfer of maternal antibodies closer to birth, but more research is needed, cautioned senior study author Manish Patel, MD, during a Tuesday media telebriefing held by the Centers for Disease Control and Prevention.
Unanswered questions include how the babies got infected or if there is any protection afforded to babies for women vaccinated before pregnancy.
“We cannot be sure about the source of the infection,” said Dr. Patel, a medical epidemiologist with the CDC COVID-19 Emergency Response Team.
Dana Meaney-Delman, MD, MPH, agreed, but added that “perinatal transmission of the virus is very rare” with SARS-CoV-2. She is a practicing obstetrician and gynecologist and chief of the CDC Infant Outcomes Monitoring Research and Prevention Branch.
The study numbers were too small to show if a booster shot during pregnancy or breastfeeding could provide even greater protection for babies, Dr. Patel said.
The early release study was published online Feb. 15 in the CDC’s Morbidity and Mortality Weekly Report (MMWR).
Many previous studies looking at COVID-19 immunization during pregnancy focused on maternal health and “have clearly shown that receiving an mRNA COVID-19 vaccine during pregnancy reduces the risk for severe illness,” Dr. Meaney-Delman said.
Some dual protection suggested
Now there is evidence for a potential benefit to babies as well when a pregnant woman gets vaccinated. The study “provides real-world evidence that getting COVID-19 vaccination during pregnancy might help protect infants less than 6 months [of age],” Dr. Meaney-Delman said.
“These findings continue to emphasize the importance of COVID-19 vaccination during pregnancy to protect people who are pregnant and also to protect their babies,” she said.
Dr. Patel and colleagues studied 379 infants younger than 6 months hospitalized between July 1, 2021 and Jan. 17 of this year. Delta and then the Omicron variant predominated during this time.
The infants were admitted to one of 20 children’s hospitals in 17 states. The researchers compared 176 infants admitted with a positive COVID-19 PCR test to another 203 infants with a negative PCR test who served as controls.
Half as many mothers of infants admitted with COVID-19 were vaccinated during pregnancy, 16%, versus 32% of mothers of the control infants.
Vaccination with two doses of mRNA vaccine during pregnancy was 61% effective (95% confidence interval, 31%-78%) at preventing hospitalization among these infants. Because the study was epidemiological, the lower risk was an association, not a cause-and-effect finding, Dr. Patel said.
Babies admitted to the hospital positive for COVID-19 were more likely to be non-Hispanic Black, 18%, versus 9% of control group babies; and more likely to be Hispanic, 34% versus 28%, respectively.
A total 24% of infants with COVID-19 were admitted to the ICU, including the baby of an unvaccinated mother who required extracorporeal membrane oxygenation (ECMO). Another baby of an unvaccinated mother was the only infant death during the study.
Maternal vaccination trends
A reporter pointed out that COVID-19 vaccination rates tend to be low among pregnant women. “So there is some exciting news,” Dr. Meaney-Delman said, referring to a steady increase in the percentages of pregnant women in the U.S. choosing to get vaccinated, according to the CDC Data Tracker website.
“The numbers are encouraging, [but] they’re not quite where we need them to be, and they do differ by race and ethnicity,” she added.
A version of this article first appeared on Medscape.com.
In a first of its kind study, researchers found women who received two mRNA COVID vaccine doses during pregnancy were 61% less likely to have a baby hospitalized for COVID-19 during the first 6 months of life.
In addition, two doses of the Pfizer/BioNTech or Moderna COVID vaccine later in a pregnancy were linked to an even higher level of protection, 80%, compared with 32% when given before 20 weeks’ gestation.
This finding suggests a greater transfer of maternal antibodies closer to birth, but more research is needed, cautioned senior study author Manish Patel, MD, during a Tuesday media telebriefing held by the Centers for Disease Control and Prevention.
Unanswered questions include how the babies got infected or if there is any protection afforded to babies for women vaccinated before pregnancy.
“We cannot be sure about the source of the infection,” said Dr. Patel, a medical epidemiologist with the CDC COVID-19 Emergency Response Team.
Dana Meaney-Delman, MD, MPH, agreed, but added that “perinatal transmission of the virus is very rare” with SARS-CoV-2. She is a practicing obstetrician and gynecologist and chief of the CDC Infant Outcomes Monitoring Research and Prevention Branch.
The study numbers were too small to show if a booster shot during pregnancy or breastfeeding could provide even greater protection for babies, Dr. Patel said.
The early release study was published online Feb. 15 in the CDC’s Morbidity and Mortality Weekly Report (MMWR).
Many previous studies looking at COVID-19 immunization during pregnancy focused on maternal health and “have clearly shown that receiving an mRNA COVID-19 vaccine during pregnancy reduces the risk for severe illness,” Dr. Meaney-Delman said.
Some dual protection suggested
Now there is evidence for a potential benefit to babies as well when a pregnant woman gets vaccinated. The study “provides real-world evidence that getting COVID-19 vaccination during pregnancy might help protect infants less than 6 months [of age],” Dr. Meaney-Delman said.
“These findings continue to emphasize the importance of COVID-19 vaccination during pregnancy to protect people who are pregnant and also to protect their babies,” she said.
Dr. Patel and colleagues studied 379 infants younger than 6 months hospitalized between July 1, 2021 and Jan. 17 of this year. Delta and then the Omicron variant predominated during this time.
The infants were admitted to one of 20 children’s hospitals in 17 states. The researchers compared 176 infants admitted with a positive COVID-19 PCR test to another 203 infants with a negative PCR test who served as controls.
Half as many mothers of infants admitted with COVID-19 were vaccinated during pregnancy, 16%, versus 32% of mothers of the control infants.
Vaccination with two doses of mRNA vaccine during pregnancy was 61% effective (95% confidence interval, 31%-78%) at preventing hospitalization among these infants. Because the study was epidemiological, the lower risk was an association, not a cause-and-effect finding, Dr. Patel said.
Babies admitted to the hospital positive for COVID-19 were more likely to be non-Hispanic Black, 18%, versus 9% of control group babies; and more likely to be Hispanic, 34% versus 28%, respectively.
A total 24% of infants with COVID-19 were admitted to the ICU, including the baby of an unvaccinated mother who required extracorporeal membrane oxygenation (ECMO). Another baby of an unvaccinated mother was the only infant death during the study.
Maternal vaccination trends
A reporter pointed out that COVID-19 vaccination rates tend to be low among pregnant women. “So there is some exciting news,” Dr. Meaney-Delman said, referring to a steady increase in the percentages of pregnant women in the U.S. choosing to get vaccinated, according to the CDC Data Tracker website.
“The numbers are encouraging, [but] they’re not quite where we need them to be, and they do differ by race and ethnicity,” she added.
A version of this article first appeared on Medscape.com.
CDC preparing to update mask guidance
, CDC Director Rochelle P. Walensky, MD, said on Feb. 16.
“As we consider future metrics, which will be updated soon, we recognize the importance of not just cases … but critically, medically severe disease that leads to hospitalizations,” Dr. Walensky said at a White House news briefing. “We must consider hospital capacity as an additional important barometer.”
She later added, “We are looking at an overview of much of our guidance, and masking in all settings will be a part of that.”
Coronavirus cases continue to drop nationwide. This week’s 7-day daily average of cases is 147,000, a decrease of 40%. Hospitalizations have dropped 28% to 9,500, and daily deaths are 2,200, a decrease of 9%.
“Omicron cases are declining, and we are all cautiously optimistic about the trajectory we’re on,” Dr. Walensky said. “Things are moving in the right direction, but we want to remain vigilant to do all we can so this trajectory continues.”
Dr. Walensky said public masking remains especially important if someone is symptomatic or not feeling well, or if there has been a COVID-19 exposure. Those who are within 10 days of being diagnosed with the virus should also remain masked in public.
“We all share the same goal: to get to a point where COVID-19 is no longer disrupting our daily lives. A time when it won’t be a constant crisis,” Dr. Walensky said. “Moving from this pandemic will be a process led by science and epidemiological trends, and one that relies on the powerful tools we already have.”
A version of this article first appeared on WebMD.com.
, CDC Director Rochelle P. Walensky, MD, said on Feb. 16.
“As we consider future metrics, which will be updated soon, we recognize the importance of not just cases … but critically, medically severe disease that leads to hospitalizations,” Dr. Walensky said at a White House news briefing. “We must consider hospital capacity as an additional important barometer.”
She later added, “We are looking at an overview of much of our guidance, and masking in all settings will be a part of that.”
Coronavirus cases continue to drop nationwide. This week’s 7-day daily average of cases is 147,000, a decrease of 40%. Hospitalizations have dropped 28% to 9,500, and daily deaths are 2,200, a decrease of 9%.
“Omicron cases are declining, and we are all cautiously optimistic about the trajectory we’re on,” Dr. Walensky said. “Things are moving in the right direction, but we want to remain vigilant to do all we can so this trajectory continues.”
Dr. Walensky said public masking remains especially important if someone is symptomatic or not feeling well, or if there has been a COVID-19 exposure. Those who are within 10 days of being diagnosed with the virus should also remain masked in public.
“We all share the same goal: to get to a point where COVID-19 is no longer disrupting our daily lives. A time when it won’t be a constant crisis,” Dr. Walensky said. “Moving from this pandemic will be a process led by science and epidemiological trends, and one that relies on the powerful tools we already have.”
A version of this article first appeared on WebMD.com.
, CDC Director Rochelle P. Walensky, MD, said on Feb. 16.
“As we consider future metrics, which will be updated soon, we recognize the importance of not just cases … but critically, medically severe disease that leads to hospitalizations,” Dr. Walensky said at a White House news briefing. “We must consider hospital capacity as an additional important barometer.”
She later added, “We are looking at an overview of much of our guidance, and masking in all settings will be a part of that.”
Coronavirus cases continue to drop nationwide. This week’s 7-day daily average of cases is 147,000, a decrease of 40%. Hospitalizations have dropped 28% to 9,500, and daily deaths are 2,200, a decrease of 9%.
“Omicron cases are declining, and we are all cautiously optimistic about the trajectory we’re on,” Dr. Walensky said. “Things are moving in the right direction, but we want to remain vigilant to do all we can so this trajectory continues.”
Dr. Walensky said public masking remains especially important if someone is symptomatic or not feeling well, or if there has been a COVID-19 exposure. Those who are within 10 days of being diagnosed with the virus should also remain masked in public.
“We all share the same goal: to get to a point where COVID-19 is no longer disrupting our daily lives. A time when it won’t be a constant crisis,” Dr. Walensky said. “Moving from this pandemic will be a process led by science and epidemiological trends, and one that relies on the powerful tools we already have.”
A version of this article first appeared on WebMD.com.
Right arm rash
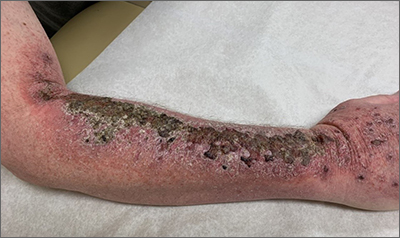
Common skin reactions to treatment with topical 5-FU for actinic keratosis include erythema, ulceration, and burning. In this case, however, the skin disruption opened the door to secondary impetigo.
Secondary skin infections are a known risk of treatment with topical 5-FU. The agent inhibits thymidylate synthetase, an enzyme involved in the synthesis and repair of DNA. The inhibition of this enzyme can lead to skin disruption with erosion, desquamation, and the risk of superimposed skin infections, as was seen with this patient.1
Impetigo is a common skin infection affecting the superficial layers of the epidermis and is most commonly caused by gram-positive bacteria, such as Staphylococcus aureus or Streptococcus pyogenes.2 Secondary impetigo, also known as impetiginization, is an infection of previously disrupted skin due to eczema, trauma, insect bites, and other conditions. This contrasts with primary impetigo, which results from a direct bacterial invasion of intact healthy skin. While impetigo predominantly affects children between the ages of 2 and 5 years, people of any age can be affected.2 Impetigo characteristically manifests with painful erosions, classically covered by honey-colored crusts. Thin-walled vesicles often appear and subsequently rupture.
Treatment options for impetigo include both topical and systemic antibiotics. Topical therapy is preferred for patients with limited skin involvement, while systemic therapy is indicated for patients with numerous lesions. Mupirocin and retapamulin are first-line topical treatments. Systemic antibiotic therapy should provide coverage for both S. aureus and streptococcal infections; cephalexin and dicloxacillin are preferred. Doxycycline, trimethoprim-sulfamethoxazole, or clindamycin can be used if methicillin-resistant Staphylococcus aureus is suspected.3
This patient was advised to try warm soaks (to reduce the crusting) and to follow that with the application of white petrolatum bid. The patient was also prescribed doxycycline 100 mg orally bid for 10 days. At the 1-month follow-up, there was some residual erythema, but the impetigo and crusting had resolved. The actinic keratoses had resolved, as well.
Image courtesy of Daniel Stulberg, MD. Text courtesy of Tess Pei Lemon, BA, University of New Mexico School of Medicine and Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque
1. Chughtai K, Gupta R, Upadhaya S, et al. Topical 5-Fluorouracil associated skin reaction. Oxf Med Case Rep. 2017;2017(8):omx043. doi:10.1093/omcr/omx043
2. Hartman-Adams H, Banvard C, Juckett G. Impetigo: diagnosis and treatment. Am Fam Physician. 2014;90:229-235.
3. Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis. 2014;59:147-159. doi:10.1093/cid/ciu296

Common skin reactions to treatment with topical 5-FU for actinic keratosis include erythema, ulceration, and burning. In this case, however, the skin disruption opened the door to secondary impetigo.
Secondary skin infections are a known risk of treatment with topical 5-FU. The agent inhibits thymidylate synthetase, an enzyme involved in the synthesis and repair of DNA. The inhibition of this enzyme can lead to skin disruption with erosion, desquamation, and the risk of superimposed skin infections, as was seen with this patient.1
Impetigo is a common skin infection affecting the superficial layers of the epidermis and is most commonly caused by gram-positive bacteria, such as Staphylococcus aureus or Streptococcus pyogenes.2 Secondary impetigo, also known as impetiginization, is an infection of previously disrupted skin due to eczema, trauma, insect bites, and other conditions. This contrasts with primary impetigo, which results from a direct bacterial invasion of intact healthy skin. While impetigo predominantly affects children between the ages of 2 and 5 years, people of any age can be affected.2 Impetigo characteristically manifests with painful erosions, classically covered by honey-colored crusts. Thin-walled vesicles often appear and subsequently rupture.
Treatment options for impetigo include both topical and systemic antibiotics. Topical therapy is preferred for patients with limited skin involvement, while systemic therapy is indicated for patients with numerous lesions. Mupirocin and retapamulin are first-line topical treatments. Systemic antibiotic therapy should provide coverage for both S. aureus and streptococcal infections; cephalexin and dicloxacillin are preferred. Doxycycline, trimethoprim-sulfamethoxazole, or clindamycin can be used if methicillin-resistant Staphylococcus aureus is suspected.3
This patient was advised to try warm soaks (to reduce the crusting) and to follow that with the application of white petrolatum bid. The patient was also prescribed doxycycline 100 mg orally bid for 10 days. At the 1-month follow-up, there was some residual erythema, but the impetigo and crusting had resolved. The actinic keratoses had resolved, as well.
Image courtesy of Daniel Stulberg, MD. Text courtesy of Tess Pei Lemon, BA, University of New Mexico School of Medicine and Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque

Common skin reactions to treatment with topical 5-FU for actinic keratosis include erythema, ulceration, and burning. In this case, however, the skin disruption opened the door to secondary impetigo.
Secondary skin infections are a known risk of treatment with topical 5-FU. The agent inhibits thymidylate synthetase, an enzyme involved in the synthesis and repair of DNA. The inhibition of this enzyme can lead to skin disruption with erosion, desquamation, and the risk of superimposed skin infections, as was seen with this patient.1
Impetigo is a common skin infection affecting the superficial layers of the epidermis and is most commonly caused by gram-positive bacteria, such as Staphylococcus aureus or Streptococcus pyogenes.2 Secondary impetigo, also known as impetiginization, is an infection of previously disrupted skin due to eczema, trauma, insect bites, and other conditions. This contrasts with primary impetigo, which results from a direct bacterial invasion of intact healthy skin. While impetigo predominantly affects children between the ages of 2 and 5 years, people of any age can be affected.2 Impetigo characteristically manifests with painful erosions, classically covered by honey-colored crusts. Thin-walled vesicles often appear and subsequently rupture.
Treatment options for impetigo include both topical and systemic antibiotics. Topical therapy is preferred for patients with limited skin involvement, while systemic therapy is indicated for patients with numerous lesions. Mupirocin and retapamulin are first-line topical treatments. Systemic antibiotic therapy should provide coverage for both S. aureus and streptococcal infections; cephalexin and dicloxacillin are preferred. Doxycycline, trimethoprim-sulfamethoxazole, or clindamycin can be used if methicillin-resistant Staphylococcus aureus is suspected.3
This patient was advised to try warm soaks (to reduce the crusting) and to follow that with the application of white petrolatum bid. The patient was also prescribed doxycycline 100 mg orally bid for 10 days. At the 1-month follow-up, there was some residual erythema, but the impetigo and crusting had resolved. The actinic keratoses had resolved, as well.
Image courtesy of Daniel Stulberg, MD. Text courtesy of Tess Pei Lemon, BA, University of New Mexico School of Medicine and Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque
1. Chughtai K, Gupta R, Upadhaya S, et al. Topical 5-Fluorouracil associated skin reaction. Oxf Med Case Rep. 2017;2017(8):omx043. doi:10.1093/omcr/omx043
2. Hartman-Adams H, Banvard C, Juckett G. Impetigo: diagnosis and treatment. Am Fam Physician. 2014;90:229-235.
3. Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis. 2014;59:147-159. doi:10.1093/cid/ciu296
1. Chughtai K, Gupta R, Upadhaya S, et al. Topical 5-Fluorouracil associated skin reaction. Oxf Med Case Rep. 2017;2017(8):omx043. doi:10.1093/omcr/omx043
2. Hartman-Adams H, Banvard C, Juckett G. Impetigo: diagnosis and treatment. Am Fam Physician. 2014;90:229-235.
3. Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis. 2014;59:147-159. doi:10.1093/cid/ciu296
Stroke risk is highest right after COVID infection
, new research shows.
The study among Medicare beneficiaries with COVID-19 also showed that stroke risk is higher for relatively young older adults, those aged 65 to 74 years, and those without a history of stroke.
The study highlights the impact COVID-19 has on the cardiovascular system, said study author Quanhe Yang, PhD, senior scientist, Division for Heart Disease and Stroke Prevention, Centers for Disease Control and Prevention, Atlanta.
“Clinicians and patients should understand that stroke might be one of the very important clinical consequences of COVID-19.”
The study was presented during the hybrid International Stroke Conference held in New Orleans and online. The meeting was presented by the American Stroke Association, a division of the American Heart Association.
Stroke is the fifth leading cause of death in the U.S. As an increasing number of people become infected with COVID-19, “it’s important to determine if there’s a relationship between COVID and the risk of stroke,” said Dr. Yang.
Findings from prior research examining the link between stroke and COVID-19 have been inconsistent, he noted. Some studies found an association while others did not, and in still others, the association was not as strong as expected.
Many factors may contribute to these inconsistent findings, said Dr. Yang, including differences in study design, inclusion criteria, comparison groups, sample sizes, and countries where the research was carried out. Dr. Yang pointed out that many of these studies were done in the early stages of the pandemic or didn’t include older adults, the population most at risk for stroke.
The current study included 19,553 Medicare beneficiaries aged 65 years and older diagnosed with COVID-19 and hospitalized with acute ischemic stroke. The median age at diagnosis of COVID-19 was 80.5 years, 57.5% were women, and more than 75% were non-Hispanic Whites.
To ensure the stroke occurred after a COVID infection, researchers used a self-controlled case series study design, a “within person” comparison between the risk period and the control period.
They divided the study period (Jan. 1, 2019 to Feb. 28, 2021) into the exposure or stroke risk periods after the COVID diagnosis (0-3 days; 4-7 days; 8-15 days; and 15-28 days) and control periods.
Strokes that occurred 7 days before or 28 days after a COVID diagnosis served as a control period. “Any stroke that occurred outside the risk window is in the control period,” explained Dr. Yang.
He added that the control period provides a baseline. “Without COVID-19, this is what I would expect” in terms of the number of strokes.
To estimate the incidence rate ratio (IRR), investigators compared the incidence of acute ischemic stroke in the various risk periods with control periods.
The IRR was 10.97 (95% confidence interval, 10.30-11.68) at 0-3 days. The risk then quickly declined but stayed higher than the control period. The IRRs were: 1.59 (95% CI, 1.35-1.87) at 4-7 days; 1.23 (95% CI, 1.07-1.41) at 8-14 days; and 1.06 (95% CI, 0.95-1.18) at 15-28 days.
The temporary increase in stroke risk early after an infection isn’t novel; the pattern has been observed with influenza, respiratory infections, and shingles, said Dr. Yang. “But COVID-19 appears to be particularly risky.”
Although the mechanism driving the early increased stroke risk isn’t fully understood, it’s likely tied to an “exaggerated inflammatory response,” said Dr. Yang. This can trigger the cascade of events setting the stage for a stroke – a hypercoagulation state leading to the formation of blood clots that then block arteries to the brain, he said.
It’s also possible the infection directly affects endothelial cells, leading to rupture of plaque, again blocking arteries and raising stroke risks, added Dr. Yang.
The association was stronger among younger beneficiaries, aged 65 to 74 years, compared with those 85 years and older, a finding Dr. Yang said was somewhat surprising. But he noted other studies have found stroke patients with COVID are younger than stroke patients without COVID – by some 5 to 6 years.
“If COVID-19 disproportionately affects younger patients, that may explain the stronger association,” said Dr. Yang. “Stroke risk increases tremendously with age, so if you’re a younger age, your baseline stroke risk is lower.”
The association was also stronger among beneficiaries without a history of stroke. Again, this could be related to the stronger association among younger patients who are less likely to have suffered a stroke. The association was largely consistent across sex and race/ethnicities.
Dr. Yang stressed that the findings need to be confirmed with further studies.
The study was carried out before widespread use of vaccinations in the U.S. Once those data are available, Dr. Yang and his colleagues plan to determine if vaccinations modify the association between COVID-19 and stroke risk.
The new results contribute to the mounting evidence that a COVID-19 infection “can actually affect multiple human organs structurally or functionally in addition to the impact on [the] respiratory system,” said Dr. Yang.
Some dates of COVID-19 diagnoses may be incorrect due to limited test availability, particularly early in the pandemic. Another limitation of the study was possible misclassification from the use of Medicare real-time preliminary claims.
In a provided statement, Louise D. McCullough, MD, PhD, chair of the ISC 2022 and professor and chair of neurology, McGovern Medical School, University of Texas Health Science Center at Houston, noted that the study focused on older adults because it was examining Medicare beneficiaries.
“But everyone is likely at risk for stroke after COVID,” she said. “Any infection is linked to stroke risk, probably because any infection will cause inflammation, and inflammation can cause clots or thrombus, which is the cause of stroke.”
There was no outside funding for the study. No relevant conflicts of interest were disclosed.
A version of this article first appeared on Medscape.com.
, new research shows.
The study among Medicare beneficiaries with COVID-19 also showed that stroke risk is higher for relatively young older adults, those aged 65 to 74 years, and those without a history of stroke.
The study highlights the impact COVID-19 has on the cardiovascular system, said study author Quanhe Yang, PhD, senior scientist, Division for Heart Disease and Stroke Prevention, Centers for Disease Control and Prevention, Atlanta.
“Clinicians and patients should understand that stroke might be one of the very important clinical consequences of COVID-19.”
The study was presented during the hybrid International Stroke Conference held in New Orleans and online. The meeting was presented by the American Stroke Association, a division of the American Heart Association.
Stroke is the fifth leading cause of death in the U.S. As an increasing number of people become infected with COVID-19, “it’s important to determine if there’s a relationship between COVID and the risk of stroke,” said Dr. Yang.
Findings from prior research examining the link between stroke and COVID-19 have been inconsistent, he noted. Some studies found an association while others did not, and in still others, the association was not as strong as expected.
Many factors may contribute to these inconsistent findings, said Dr. Yang, including differences in study design, inclusion criteria, comparison groups, sample sizes, and countries where the research was carried out. Dr. Yang pointed out that many of these studies were done in the early stages of the pandemic or didn’t include older adults, the population most at risk for stroke.
The current study included 19,553 Medicare beneficiaries aged 65 years and older diagnosed with COVID-19 and hospitalized with acute ischemic stroke. The median age at diagnosis of COVID-19 was 80.5 years, 57.5% were women, and more than 75% were non-Hispanic Whites.
To ensure the stroke occurred after a COVID infection, researchers used a self-controlled case series study design, a “within person” comparison between the risk period and the control period.
They divided the study period (Jan. 1, 2019 to Feb. 28, 2021) into the exposure or stroke risk periods after the COVID diagnosis (0-3 days; 4-7 days; 8-15 days; and 15-28 days) and control periods.
Strokes that occurred 7 days before or 28 days after a COVID diagnosis served as a control period. “Any stroke that occurred outside the risk window is in the control period,” explained Dr. Yang.
He added that the control period provides a baseline. “Without COVID-19, this is what I would expect” in terms of the number of strokes.
To estimate the incidence rate ratio (IRR), investigators compared the incidence of acute ischemic stroke in the various risk periods with control periods.
The IRR was 10.97 (95% confidence interval, 10.30-11.68) at 0-3 days. The risk then quickly declined but stayed higher than the control period. The IRRs were: 1.59 (95% CI, 1.35-1.87) at 4-7 days; 1.23 (95% CI, 1.07-1.41) at 8-14 days; and 1.06 (95% CI, 0.95-1.18) at 15-28 days.
The temporary increase in stroke risk early after an infection isn’t novel; the pattern has been observed with influenza, respiratory infections, and shingles, said Dr. Yang. “But COVID-19 appears to be particularly risky.”
Although the mechanism driving the early increased stroke risk isn’t fully understood, it’s likely tied to an “exaggerated inflammatory response,” said Dr. Yang. This can trigger the cascade of events setting the stage for a stroke – a hypercoagulation state leading to the formation of blood clots that then block arteries to the brain, he said.
It’s also possible the infection directly affects endothelial cells, leading to rupture of plaque, again blocking arteries and raising stroke risks, added Dr. Yang.
The association was stronger among younger beneficiaries, aged 65 to 74 years, compared with those 85 years and older, a finding Dr. Yang said was somewhat surprising. But he noted other studies have found stroke patients with COVID are younger than stroke patients without COVID – by some 5 to 6 years.
“If COVID-19 disproportionately affects younger patients, that may explain the stronger association,” said Dr. Yang. “Stroke risk increases tremendously with age, so if you’re a younger age, your baseline stroke risk is lower.”
The association was also stronger among beneficiaries without a history of stroke. Again, this could be related to the stronger association among younger patients who are less likely to have suffered a stroke. The association was largely consistent across sex and race/ethnicities.
Dr. Yang stressed that the findings need to be confirmed with further studies.
The study was carried out before widespread use of vaccinations in the U.S. Once those data are available, Dr. Yang and his colleagues plan to determine if vaccinations modify the association between COVID-19 and stroke risk.
The new results contribute to the mounting evidence that a COVID-19 infection “can actually affect multiple human organs structurally or functionally in addition to the impact on [the] respiratory system,” said Dr. Yang.
Some dates of COVID-19 diagnoses may be incorrect due to limited test availability, particularly early in the pandemic. Another limitation of the study was possible misclassification from the use of Medicare real-time preliminary claims.
In a provided statement, Louise D. McCullough, MD, PhD, chair of the ISC 2022 and professor and chair of neurology, McGovern Medical School, University of Texas Health Science Center at Houston, noted that the study focused on older adults because it was examining Medicare beneficiaries.
“But everyone is likely at risk for stroke after COVID,” she said. “Any infection is linked to stroke risk, probably because any infection will cause inflammation, and inflammation can cause clots or thrombus, which is the cause of stroke.”
There was no outside funding for the study. No relevant conflicts of interest were disclosed.
A version of this article first appeared on Medscape.com.
, new research shows.
The study among Medicare beneficiaries with COVID-19 also showed that stroke risk is higher for relatively young older adults, those aged 65 to 74 years, and those without a history of stroke.
The study highlights the impact COVID-19 has on the cardiovascular system, said study author Quanhe Yang, PhD, senior scientist, Division for Heart Disease and Stroke Prevention, Centers for Disease Control and Prevention, Atlanta.
“Clinicians and patients should understand that stroke might be one of the very important clinical consequences of COVID-19.”
The study was presented during the hybrid International Stroke Conference held in New Orleans and online. The meeting was presented by the American Stroke Association, a division of the American Heart Association.
Stroke is the fifth leading cause of death in the U.S. As an increasing number of people become infected with COVID-19, “it’s important to determine if there’s a relationship between COVID and the risk of stroke,” said Dr. Yang.
Findings from prior research examining the link between stroke and COVID-19 have been inconsistent, he noted. Some studies found an association while others did not, and in still others, the association was not as strong as expected.
Many factors may contribute to these inconsistent findings, said Dr. Yang, including differences in study design, inclusion criteria, comparison groups, sample sizes, and countries where the research was carried out. Dr. Yang pointed out that many of these studies were done in the early stages of the pandemic or didn’t include older adults, the population most at risk for stroke.
The current study included 19,553 Medicare beneficiaries aged 65 years and older diagnosed with COVID-19 and hospitalized with acute ischemic stroke. The median age at diagnosis of COVID-19 was 80.5 years, 57.5% were women, and more than 75% were non-Hispanic Whites.
To ensure the stroke occurred after a COVID infection, researchers used a self-controlled case series study design, a “within person” comparison between the risk period and the control period.
They divided the study period (Jan. 1, 2019 to Feb. 28, 2021) into the exposure or stroke risk periods after the COVID diagnosis (0-3 days; 4-7 days; 8-15 days; and 15-28 days) and control periods.
Strokes that occurred 7 days before or 28 days after a COVID diagnosis served as a control period. “Any stroke that occurred outside the risk window is in the control period,” explained Dr. Yang.
He added that the control period provides a baseline. “Without COVID-19, this is what I would expect” in terms of the number of strokes.
To estimate the incidence rate ratio (IRR), investigators compared the incidence of acute ischemic stroke in the various risk periods with control periods.
The IRR was 10.97 (95% confidence interval, 10.30-11.68) at 0-3 days. The risk then quickly declined but stayed higher than the control period. The IRRs were: 1.59 (95% CI, 1.35-1.87) at 4-7 days; 1.23 (95% CI, 1.07-1.41) at 8-14 days; and 1.06 (95% CI, 0.95-1.18) at 15-28 days.
The temporary increase in stroke risk early after an infection isn’t novel; the pattern has been observed with influenza, respiratory infections, and shingles, said Dr. Yang. “But COVID-19 appears to be particularly risky.”
Although the mechanism driving the early increased stroke risk isn’t fully understood, it’s likely tied to an “exaggerated inflammatory response,” said Dr. Yang. This can trigger the cascade of events setting the stage for a stroke – a hypercoagulation state leading to the formation of blood clots that then block arteries to the brain, he said.
It’s also possible the infection directly affects endothelial cells, leading to rupture of plaque, again blocking arteries and raising stroke risks, added Dr. Yang.
The association was stronger among younger beneficiaries, aged 65 to 74 years, compared with those 85 years and older, a finding Dr. Yang said was somewhat surprising. But he noted other studies have found stroke patients with COVID are younger than stroke patients without COVID – by some 5 to 6 years.
“If COVID-19 disproportionately affects younger patients, that may explain the stronger association,” said Dr. Yang. “Stroke risk increases tremendously with age, so if you’re a younger age, your baseline stroke risk is lower.”
The association was also stronger among beneficiaries without a history of stroke. Again, this could be related to the stronger association among younger patients who are less likely to have suffered a stroke. The association was largely consistent across sex and race/ethnicities.
Dr. Yang stressed that the findings need to be confirmed with further studies.
The study was carried out before widespread use of vaccinations in the U.S. Once those data are available, Dr. Yang and his colleagues plan to determine if vaccinations modify the association between COVID-19 and stroke risk.
The new results contribute to the mounting evidence that a COVID-19 infection “can actually affect multiple human organs structurally or functionally in addition to the impact on [the] respiratory system,” said Dr. Yang.
Some dates of COVID-19 diagnoses may be incorrect due to limited test availability, particularly early in the pandemic. Another limitation of the study was possible misclassification from the use of Medicare real-time preliminary claims.
In a provided statement, Louise D. McCullough, MD, PhD, chair of the ISC 2022 and professor and chair of neurology, McGovern Medical School, University of Texas Health Science Center at Houston, noted that the study focused on older adults because it was examining Medicare beneficiaries.
“But everyone is likely at risk for stroke after COVID,” she said. “Any infection is linked to stroke risk, probably because any infection will cause inflammation, and inflammation can cause clots or thrombus, which is the cause of stroke.”
There was no outside funding for the study. No relevant conflicts of interest were disclosed.
A version of this article first appeared on Medscape.com.
FROM ISC 2022