User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
Two studies detail the dangers of COVID in pregnancy
Two new studies show how COVID-19 threatens the health of pregnant people and their newborn infants.
A study conducted in Scotland showed that unvaccinated pregnant people who got COVID were much more likely to have a stillborn infant or one that dies in the first 28 days. The study also found that pregnant women infected with COVID died or needed hospitalization at a much higher rate than vaccinated women who got pregnant.
The University of Edinburgh and Public Health Scotland studied national data in 88,000 pregnancies between Dec. 2020 and Oct. 2021, according to the study published in Nature Medicine.
Overall, 77.4% of infections, 90.9% of COVID-related hospitalizations, and 98% of critical care cases occurred in the unvaccinated people, as did all newborn deaths.
The study said 2,364 babies were born to women infected with COVID, with 2,353 live births. Eleven babies were stillborn and eight live-born babies died within 28 days. Of the live births, 241 were premature.
The problems were more likely if the infection occurred 28 days or less before the delivery date, the researchers said.
The authors said the low vaccination rate among pregnant people was a problem. Only 32% of people giving birth in Oct. 2021 were fully vaccinated, while 77% of the Scottish female population aged 18-44 was fully vaccinated.
“Vaccine hesitancy in pregnancy thus requires addressing, especially in light of new recommendations for booster vaccination administration 3 months after the initial vaccination course to help protect against new variants such as Omicron,” the authors wrote. “Addressing low vaccine uptake rates in pregnant women is imperative to protect the health of women and babies in the ongoing pandemic.”
Vaccinated women who were pregnant had complication rates that were about the same for all pregnant women, the study shows.
The second study, published in The Lancet, found that women who got COVID while pregnant in five Western U.S. states were more likely to have premature births, low birth weights, and stillbirths, even when the COVID cases are mild.
The Institute for Systems Biology researchers in Seattle studied data for women who gave birth in Alaska, California, Montana, Oregon, or Washington from March 5, 2020, to July 4, 2021. About 18,000 of them were tested for COVID, with 882 testing positive. Of the positive tests, 85 came in the first trimester, 226 in the second trimester, and 571 in the third semester. None of the pregnant women had been vaccinated at the time they were infected.
Most of the birth problems occurred with first and second trimester infections, the study noted, and problems occurred even if the pregnant person didn’t have respiratory complications, a major COVID symptom.
“Pregnant people are at an increased risk of adverse outcomes following SARS-CoV-2 infection, even when maternal COVID-19 is less severe, and they may benefit from increased monitoring following infection,” Jennifer Hadlock, MD, an author of the paper, said in a news release.
The study also pointed out continuing inequities in health care, with most of the positive cases occurring among young, non-White people with Medicaid and high body mass index.
A version of this article first appeared on WebMD.com.
Two new studies show how COVID-19 threatens the health of pregnant people and their newborn infants.
A study conducted in Scotland showed that unvaccinated pregnant people who got COVID were much more likely to have a stillborn infant or one that dies in the first 28 days. The study also found that pregnant women infected with COVID died or needed hospitalization at a much higher rate than vaccinated women who got pregnant.
The University of Edinburgh and Public Health Scotland studied national data in 88,000 pregnancies between Dec. 2020 and Oct. 2021, according to the study published in Nature Medicine.
Overall, 77.4% of infections, 90.9% of COVID-related hospitalizations, and 98% of critical care cases occurred in the unvaccinated people, as did all newborn deaths.
The study said 2,364 babies were born to women infected with COVID, with 2,353 live births. Eleven babies were stillborn and eight live-born babies died within 28 days. Of the live births, 241 were premature.
The problems were more likely if the infection occurred 28 days or less before the delivery date, the researchers said.
The authors said the low vaccination rate among pregnant people was a problem. Only 32% of people giving birth in Oct. 2021 were fully vaccinated, while 77% of the Scottish female population aged 18-44 was fully vaccinated.
“Vaccine hesitancy in pregnancy thus requires addressing, especially in light of new recommendations for booster vaccination administration 3 months after the initial vaccination course to help protect against new variants such as Omicron,” the authors wrote. “Addressing low vaccine uptake rates in pregnant women is imperative to protect the health of women and babies in the ongoing pandemic.”
Vaccinated women who were pregnant had complication rates that were about the same for all pregnant women, the study shows.
The second study, published in The Lancet, found that women who got COVID while pregnant in five Western U.S. states were more likely to have premature births, low birth weights, and stillbirths, even when the COVID cases are mild.
The Institute for Systems Biology researchers in Seattle studied data for women who gave birth in Alaska, California, Montana, Oregon, or Washington from March 5, 2020, to July 4, 2021. About 18,000 of them were tested for COVID, with 882 testing positive. Of the positive tests, 85 came in the first trimester, 226 in the second trimester, and 571 in the third semester. None of the pregnant women had been vaccinated at the time they were infected.
Most of the birth problems occurred with first and second trimester infections, the study noted, and problems occurred even if the pregnant person didn’t have respiratory complications, a major COVID symptom.
“Pregnant people are at an increased risk of adverse outcomes following SARS-CoV-2 infection, even when maternal COVID-19 is less severe, and they may benefit from increased monitoring following infection,” Jennifer Hadlock, MD, an author of the paper, said in a news release.
The study also pointed out continuing inequities in health care, with most of the positive cases occurring among young, non-White people with Medicaid and high body mass index.
A version of this article first appeared on WebMD.com.
Two new studies show how COVID-19 threatens the health of pregnant people and their newborn infants.
A study conducted in Scotland showed that unvaccinated pregnant people who got COVID were much more likely to have a stillborn infant or one that dies in the first 28 days. The study also found that pregnant women infected with COVID died or needed hospitalization at a much higher rate than vaccinated women who got pregnant.
The University of Edinburgh and Public Health Scotland studied national data in 88,000 pregnancies between Dec. 2020 and Oct. 2021, according to the study published in Nature Medicine.
Overall, 77.4% of infections, 90.9% of COVID-related hospitalizations, and 98% of critical care cases occurred in the unvaccinated people, as did all newborn deaths.
The study said 2,364 babies were born to women infected with COVID, with 2,353 live births. Eleven babies were stillborn and eight live-born babies died within 28 days. Of the live births, 241 were premature.
The problems were more likely if the infection occurred 28 days or less before the delivery date, the researchers said.
The authors said the low vaccination rate among pregnant people was a problem. Only 32% of people giving birth in Oct. 2021 were fully vaccinated, while 77% of the Scottish female population aged 18-44 was fully vaccinated.
“Vaccine hesitancy in pregnancy thus requires addressing, especially in light of new recommendations for booster vaccination administration 3 months after the initial vaccination course to help protect against new variants such as Omicron,” the authors wrote. “Addressing low vaccine uptake rates in pregnant women is imperative to protect the health of women and babies in the ongoing pandemic.”
Vaccinated women who were pregnant had complication rates that were about the same for all pregnant women, the study shows.
The second study, published in The Lancet, found that women who got COVID while pregnant in five Western U.S. states were more likely to have premature births, low birth weights, and stillbirths, even when the COVID cases are mild.
The Institute for Systems Biology researchers in Seattle studied data for women who gave birth in Alaska, California, Montana, Oregon, or Washington from March 5, 2020, to July 4, 2021. About 18,000 of them were tested for COVID, with 882 testing positive. Of the positive tests, 85 came in the first trimester, 226 in the second trimester, and 571 in the third semester. None of the pregnant women had been vaccinated at the time they were infected.
Most of the birth problems occurred with first and second trimester infections, the study noted, and problems occurred even if the pregnant person didn’t have respiratory complications, a major COVID symptom.
“Pregnant people are at an increased risk of adverse outcomes following SARS-CoV-2 infection, even when maternal COVID-19 is less severe, and they may benefit from increased monitoring following infection,” Jennifer Hadlock, MD, an author of the paper, said in a news release.
The study also pointed out continuing inequities in health care, with most of the positive cases occurring among young, non-White people with Medicaid and high body mass index.
A version of this article first appeared on WebMD.com.
COVID at 2 years: Preparing for a different ‘normal’
Two years into the COVID-19 pandemic, the United States is still breaking records in hospital overcrowding and new cases.
The United States is logging nearly 800,000 cases a day, hospitals are starting to fray, and deaths have topped 850,000. Schools oscillate from remote to in-person learning, polarizing communities.
The vaccines are lifesaving for many, yet frustration mounts as the numbers of unvaccinated people in this country stays relatively stagnant (63% in the United States are fully vaccinated) and other parts of the world have seen hardly a single dose. Africa has the slowest vaccination rate among continents, with only 14% of the population receiving one shot, according to the New York Times tracker.
Yet
Effective vaccines and treatments that can keep people out of the hospital were developed at an astounding pace, and advances in tracking and testing – in both access and effectiveness – are starting to pay off.
Some experts say it’s possible that the raging Omicron surge will slow by late spring, providing some relief and maybe shifting the pandemic to a slower-burning endemic.
But other experts caution to keep our guard up, saying it’s time to settle into a “new normal” and upend the strategy for fighting COVID-19.
Time to change COVID thinking
Three former members of the Biden-Harris Transition COVID-19 Advisory Board wrote recently in JAMA that COVID-19 has now become one of the many viral respiratory diseases that health care providers and patients will manage each year.
The group of experts from the University of Pennsylvania, University of Minnesota, and New York University write that “many of the measures to reduce transmission of SARS-CoV-2 (for example, ventilation) will also reduce transmission of other respiratory viruses. Thus, policy makers should retire previous public health categorizations, including deaths from pneumonia and influenza or pneumonia, influenza, and COVID-19, and focus on a new category: the aggregate risk of all respiratory virus infections.”
Other experts, including Amesh Adalja, MD, a senior scholar at the Johns Hopkins Center for Health Security, Baltimore, have said it’s been clear since the early days of SARS-CoV-2 that we must learn to live with the virus because it “will be ever present for the remaining history of our species.”
But that doesn’t mean the virus will always have the upper hand. Although the United States has been reaching record numbers of hospitalizations in January, these hospitalizations differ from those of last year – marked by fewer extreme lifesaving measures, fewer deaths, and shorter hospital stays – caused in part by medical and therapeutic advances and in part to the nature of the Omicron variant itself.
One sign of progress, Dr. Adalja said, will be the widespread decoupling of cases from hospitalizations, something that has already happened in countries such as the United Kingdom.
“That’s a reflection of how well they have vaccinated their high-risk population and how poorly we have vaccinated our high-risk population,” he said.
Omicron will bump up natural immunity
Dr. Adalja said though the numbers of unvaccinated in the United States appear to be stuck, Omicron’s sweep will make the difference, leaving behind more natural immunity in the population.
Currently, hospitals are struggling with staffing concerns as a “direct result” of too many unvaccinated people, he said.
Andrew Badley, MD, an infectious diseases specialist at Mayo Clinic in Rochester, Minn., and director of the clinic’s COVID-19 Task Force, said the good news with Omicron is that nearly all people it infects will recover.
Over time, when the body sees foreign antigens repeatedly, the quantity and quality of the antibodies the immune system produces increase and the body becomes better at fighting disease.
So “a large amount of the population will have recovered and have a degree of immunity,” Dr. Badley said.
His optimism is tempered by his belief that “it’s going to get worse before it gets better.”
But Dr. Badley still predicts a turnaround. “We’ll see a downturn in COVID in late spring or early summer,” and well into the second quarter of 2022, “we’ll see a reemergence of control.”
Right now, with Omicron, one infected person is infecting three to five others, he said. The hope is that it will eventually reach one-to-one endemic levels.
As for the threat of new variants, Badley said, “it’s not predictable whether they will be stronger or weaker.”
Masks may be around for years
Many experts predict that masks will continue to be part of the national wardrobe for the foreseeable future.
“We will continue to see new cases for years and years to come. Some will respond to that with masks in public places for a very long time. I personally will do so,” Dr. Badley said.
Two mindsets: Inside/outside the hospital
Emily Landon, MD, an infectious disease doctor and the executive medical director of infection prevention and control at University of Chicago Medicine, told this news organization she views the pandemic from two different vantage points.
As a health care provider, she sees her hospital, like others worldwide, overwhelmed. Supplies of a major weapon to help prevent hospitalization, the monoclonal antibody sotrovimab, are running out. Dr. Landon said she has been calling other hospitals to see if they have supplies and, if so, whether Omicron patients can transfer there.
Bottom line: The things they relied on a month ago to keep people out of the hospital are no longer there, she said.
Meanwhile, “We have more COVID patients than we have ever had,” Dr. Landon said.
Last year, UChicago hit a high of 170 people hospitalized with COVID. This year, so far, the peak was 270.
Dr. Landon said she is frustrated when she leaves that overburdened world inside the hospital for the outside world, where people wear no masks or ineffective face coverings and gather unsafely. Although some of that behavior reflects an intention to flout the advice of medical experts, some is caused in part, she said, by the lack of a clear national health strategy and garbled communication from those in charge of public safety.
Americans are deciding for themselves, on an a la carte basis, whether to wear a mask or get tested or travel, and school districts decide individually when it’s time to go virtual.
“People are exhausted from having to do a risk-benefit analysis for every single activity they, their friends, their kids want to participate in,” she said.
U.S. behind in several areas
Despite our self-image as the global leader in science and medicine, the United States stumbled badly in its response to the pandemic, with grave consequences both at home and abroad, experts say.
In a recent commentary in JAMA, Lawrence Gostin, JD, from Georgetown University, Washington, and Jennifer Nuzzo, DrPH, at Johns Hopkins University, Baltimore, pointed to several critical shortfalls in the nation’s efforts to control the disease.
One such shortfall is public trust.
This news organization reported in June 2021 that a poll of its readers found that 44% said their trust in the CDC had waned during the pandemic, and 33% said their trust in the FDA had eroded as well.
Health care providers who responded to the poll lost trust as well. About half of the doctors and nurses who responded said they disagreed with the FDA’s decision-making during the pandemic. Nearly 60% of doctors and 65% of nurses said they disagreed with the CDC’s overall pandemic guidance.
Lack of trust can make people resist vaccines and efforts to fight the virus, the authors wrote.
“This will become really relevant when we have ample supply of Pfizer’s antiviral medication,” Mr. Gostin, who directs the O’Neill Institute for National and Global Health Law at Georgetown, told this news organization. “The next phase of the pandemic is not to link testing to contact tracing, because we’re way past that, but to link testing to treatment.”
Lack of regional manufacturing of products is also thwarting global progress.
“It is extraordinarily important that our pharmaceutical industry transfer technology in a pandemic,” Mr. Gostin said. “The most glaring failure to do that is the mRNA vaccines. We’ve got this enormously effective vaccine and the two manufacturers – Pfizer and Moderna – are refusing to share the technology with producers in other countries. That keeps coming back to haunt us.”
Another problem: When the vaccines are shared with other countries, they are being delivered close to the date they expire or arriving at a shipyards without warning, so even some of the doses that get delivered are going to waste, Mr. Gostin said.
“It’s one of the greatest moral failures of my lifetime,” he said.
Also a failure is the “jaw-dropping” state of testing 2 years into the pandemic, he said, as people continue to pay high prices for tests or endure long lines.
The U.S. government updated its calculations and ordered 1 billion tests for the general public. The COVIDtests.gov website to order the free tests is now live.
It’s a step in the right direction. Mr. Gostin and Dr. Nuzzo wrote that there is every reason to expect future epidemics that are as serious or more serious than COVID.
“Failure to address clearly observed weaknesses in the COVID-19 response will have preventable adverse health, social, and economic consequences when the next novel outbreak occurs,” they wrote.
A version of this article first appeared on WebMD.com.
Two years into the COVID-19 pandemic, the United States is still breaking records in hospital overcrowding and new cases.
The United States is logging nearly 800,000 cases a day, hospitals are starting to fray, and deaths have topped 850,000. Schools oscillate from remote to in-person learning, polarizing communities.
The vaccines are lifesaving for many, yet frustration mounts as the numbers of unvaccinated people in this country stays relatively stagnant (63% in the United States are fully vaccinated) and other parts of the world have seen hardly a single dose. Africa has the slowest vaccination rate among continents, with only 14% of the population receiving one shot, according to the New York Times tracker.
Yet
Effective vaccines and treatments that can keep people out of the hospital were developed at an astounding pace, and advances in tracking and testing – in both access and effectiveness – are starting to pay off.
Some experts say it’s possible that the raging Omicron surge will slow by late spring, providing some relief and maybe shifting the pandemic to a slower-burning endemic.
But other experts caution to keep our guard up, saying it’s time to settle into a “new normal” and upend the strategy for fighting COVID-19.
Time to change COVID thinking
Three former members of the Biden-Harris Transition COVID-19 Advisory Board wrote recently in JAMA that COVID-19 has now become one of the many viral respiratory diseases that health care providers and patients will manage each year.
The group of experts from the University of Pennsylvania, University of Minnesota, and New York University write that “many of the measures to reduce transmission of SARS-CoV-2 (for example, ventilation) will also reduce transmission of other respiratory viruses. Thus, policy makers should retire previous public health categorizations, including deaths from pneumonia and influenza or pneumonia, influenza, and COVID-19, and focus on a new category: the aggregate risk of all respiratory virus infections.”
Other experts, including Amesh Adalja, MD, a senior scholar at the Johns Hopkins Center for Health Security, Baltimore, have said it’s been clear since the early days of SARS-CoV-2 that we must learn to live with the virus because it “will be ever present for the remaining history of our species.”
But that doesn’t mean the virus will always have the upper hand. Although the United States has been reaching record numbers of hospitalizations in January, these hospitalizations differ from those of last year – marked by fewer extreme lifesaving measures, fewer deaths, and shorter hospital stays – caused in part by medical and therapeutic advances and in part to the nature of the Omicron variant itself.
One sign of progress, Dr. Adalja said, will be the widespread decoupling of cases from hospitalizations, something that has already happened in countries such as the United Kingdom.
“That’s a reflection of how well they have vaccinated their high-risk population and how poorly we have vaccinated our high-risk population,” he said.
Omicron will bump up natural immunity
Dr. Adalja said though the numbers of unvaccinated in the United States appear to be stuck, Omicron’s sweep will make the difference, leaving behind more natural immunity in the population.
Currently, hospitals are struggling with staffing concerns as a “direct result” of too many unvaccinated people, he said.
Andrew Badley, MD, an infectious diseases specialist at Mayo Clinic in Rochester, Minn., and director of the clinic’s COVID-19 Task Force, said the good news with Omicron is that nearly all people it infects will recover.
Over time, when the body sees foreign antigens repeatedly, the quantity and quality of the antibodies the immune system produces increase and the body becomes better at fighting disease.
So “a large amount of the population will have recovered and have a degree of immunity,” Dr. Badley said.
His optimism is tempered by his belief that “it’s going to get worse before it gets better.”
But Dr. Badley still predicts a turnaround. “We’ll see a downturn in COVID in late spring or early summer,” and well into the second quarter of 2022, “we’ll see a reemergence of control.”
Right now, with Omicron, one infected person is infecting three to five others, he said. The hope is that it will eventually reach one-to-one endemic levels.
As for the threat of new variants, Badley said, “it’s not predictable whether they will be stronger or weaker.”
Masks may be around for years
Many experts predict that masks will continue to be part of the national wardrobe for the foreseeable future.
“We will continue to see new cases for years and years to come. Some will respond to that with masks in public places for a very long time. I personally will do so,” Dr. Badley said.
Two mindsets: Inside/outside the hospital
Emily Landon, MD, an infectious disease doctor and the executive medical director of infection prevention and control at University of Chicago Medicine, told this news organization she views the pandemic from two different vantage points.
As a health care provider, she sees her hospital, like others worldwide, overwhelmed. Supplies of a major weapon to help prevent hospitalization, the monoclonal antibody sotrovimab, are running out. Dr. Landon said she has been calling other hospitals to see if they have supplies and, if so, whether Omicron patients can transfer there.
Bottom line: The things they relied on a month ago to keep people out of the hospital are no longer there, she said.
Meanwhile, “We have more COVID patients than we have ever had,” Dr. Landon said.
Last year, UChicago hit a high of 170 people hospitalized with COVID. This year, so far, the peak was 270.
Dr. Landon said she is frustrated when she leaves that overburdened world inside the hospital for the outside world, where people wear no masks or ineffective face coverings and gather unsafely. Although some of that behavior reflects an intention to flout the advice of medical experts, some is caused in part, she said, by the lack of a clear national health strategy and garbled communication from those in charge of public safety.
Americans are deciding for themselves, on an a la carte basis, whether to wear a mask or get tested or travel, and school districts decide individually when it’s time to go virtual.
“People are exhausted from having to do a risk-benefit analysis for every single activity they, their friends, their kids want to participate in,” she said.
U.S. behind in several areas
Despite our self-image as the global leader in science and medicine, the United States stumbled badly in its response to the pandemic, with grave consequences both at home and abroad, experts say.
In a recent commentary in JAMA, Lawrence Gostin, JD, from Georgetown University, Washington, and Jennifer Nuzzo, DrPH, at Johns Hopkins University, Baltimore, pointed to several critical shortfalls in the nation’s efforts to control the disease.
One such shortfall is public trust.
This news organization reported in June 2021 that a poll of its readers found that 44% said their trust in the CDC had waned during the pandemic, and 33% said their trust in the FDA had eroded as well.
Health care providers who responded to the poll lost trust as well. About half of the doctors and nurses who responded said they disagreed with the FDA’s decision-making during the pandemic. Nearly 60% of doctors and 65% of nurses said they disagreed with the CDC’s overall pandemic guidance.
Lack of trust can make people resist vaccines and efforts to fight the virus, the authors wrote.
“This will become really relevant when we have ample supply of Pfizer’s antiviral medication,” Mr. Gostin, who directs the O’Neill Institute for National and Global Health Law at Georgetown, told this news organization. “The next phase of the pandemic is not to link testing to contact tracing, because we’re way past that, but to link testing to treatment.”
Lack of regional manufacturing of products is also thwarting global progress.
“It is extraordinarily important that our pharmaceutical industry transfer technology in a pandemic,” Mr. Gostin said. “The most glaring failure to do that is the mRNA vaccines. We’ve got this enormously effective vaccine and the two manufacturers – Pfizer and Moderna – are refusing to share the technology with producers in other countries. That keeps coming back to haunt us.”
Another problem: When the vaccines are shared with other countries, they are being delivered close to the date they expire or arriving at a shipyards without warning, so even some of the doses that get delivered are going to waste, Mr. Gostin said.
“It’s one of the greatest moral failures of my lifetime,” he said.
Also a failure is the “jaw-dropping” state of testing 2 years into the pandemic, he said, as people continue to pay high prices for tests or endure long lines.
The U.S. government updated its calculations and ordered 1 billion tests for the general public. The COVIDtests.gov website to order the free tests is now live.
It’s a step in the right direction. Mr. Gostin and Dr. Nuzzo wrote that there is every reason to expect future epidemics that are as serious or more serious than COVID.
“Failure to address clearly observed weaknesses in the COVID-19 response will have preventable adverse health, social, and economic consequences when the next novel outbreak occurs,” they wrote.

A version of this article first appeared on WebMD.com.
Two years into the COVID-19 pandemic, the United States is still breaking records in hospital overcrowding and new cases.
The United States is logging nearly 800,000 cases a day, hospitals are starting to fray, and deaths have topped 850,000. Schools oscillate from remote to in-person learning, polarizing communities.
The vaccines are lifesaving for many, yet frustration mounts as the numbers of unvaccinated people in this country stays relatively stagnant (63% in the United States are fully vaccinated) and other parts of the world have seen hardly a single dose. Africa has the slowest vaccination rate among continents, with only 14% of the population receiving one shot, according to the New York Times tracker.
Yet
Effective vaccines and treatments that can keep people out of the hospital were developed at an astounding pace, and advances in tracking and testing – in both access and effectiveness – are starting to pay off.
Some experts say it’s possible that the raging Omicron surge will slow by late spring, providing some relief and maybe shifting the pandemic to a slower-burning endemic.
But other experts caution to keep our guard up, saying it’s time to settle into a “new normal” and upend the strategy for fighting COVID-19.
Time to change COVID thinking
Three former members of the Biden-Harris Transition COVID-19 Advisory Board wrote recently in JAMA that COVID-19 has now become one of the many viral respiratory diseases that health care providers and patients will manage each year.
The group of experts from the University of Pennsylvania, University of Minnesota, and New York University write that “many of the measures to reduce transmission of SARS-CoV-2 (for example, ventilation) will also reduce transmission of other respiratory viruses. Thus, policy makers should retire previous public health categorizations, including deaths from pneumonia and influenza or pneumonia, influenza, and COVID-19, and focus on a new category: the aggregate risk of all respiratory virus infections.”
Other experts, including Amesh Adalja, MD, a senior scholar at the Johns Hopkins Center for Health Security, Baltimore, have said it’s been clear since the early days of SARS-CoV-2 that we must learn to live with the virus because it “will be ever present for the remaining history of our species.”
But that doesn’t mean the virus will always have the upper hand. Although the United States has been reaching record numbers of hospitalizations in January, these hospitalizations differ from those of last year – marked by fewer extreme lifesaving measures, fewer deaths, and shorter hospital stays – caused in part by medical and therapeutic advances and in part to the nature of the Omicron variant itself.
One sign of progress, Dr. Adalja said, will be the widespread decoupling of cases from hospitalizations, something that has already happened in countries such as the United Kingdom.
“That’s a reflection of how well they have vaccinated their high-risk population and how poorly we have vaccinated our high-risk population,” he said.
Omicron will bump up natural immunity
Dr. Adalja said though the numbers of unvaccinated in the United States appear to be stuck, Omicron’s sweep will make the difference, leaving behind more natural immunity in the population.
Currently, hospitals are struggling with staffing concerns as a “direct result” of too many unvaccinated people, he said.
Andrew Badley, MD, an infectious diseases specialist at Mayo Clinic in Rochester, Minn., and director of the clinic’s COVID-19 Task Force, said the good news with Omicron is that nearly all people it infects will recover.
Over time, when the body sees foreign antigens repeatedly, the quantity and quality of the antibodies the immune system produces increase and the body becomes better at fighting disease.
So “a large amount of the population will have recovered and have a degree of immunity,” Dr. Badley said.
His optimism is tempered by his belief that “it’s going to get worse before it gets better.”
But Dr. Badley still predicts a turnaround. “We’ll see a downturn in COVID in late spring or early summer,” and well into the second quarter of 2022, “we’ll see a reemergence of control.”
Right now, with Omicron, one infected person is infecting three to five others, he said. The hope is that it will eventually reach one-to-one endemic levels.
As for the threat of new variants, Badley said, “it’s not predictable whether they will be stronger or weaker.”
Masks may be around for years
Many experts predict that masks will continue to be part of the national wardrobe for the foreseeable future.
“We will continue to see new cases for years and years to come. Some will respond to that with masks in public places for a very long time. I personally will do so,” Dr. Badley said.
Two mindsets: Inside/outside the hospital
Emily Landon, MD, an infectious disease doctor and the executive medical director of infection prevention and control at University of Chicago Medicine, told this news organization she views the pandemic from two different vantage points.
As a health care provider, she sees her hospital, like others worldwide, overwhelmed. Supplies of a major weapon to help prevent hospitalization, the monoclonal antibody sotrovimab, are running out. Dr. Landon said she has been calling other hospitals to see if they have supplies and, if so, whether Omicron patients can transfer there.
Bottom line: The things they relied on a month ago to keep people out of the hospital are no longer there, she said.
Meanwhile, “We have more COVID patients than we have ever had,” Dr. Landon said.
Last year, UChicago hit a high of 170 people hospitalized with COVID. This year, so far, the peak was 270.
Dr. Landon said she is frustrated when she leaves that overburdened world inside the hospital for the outside world, where people wear no masks or ineffective face coverings and gather unsafely. Although some of that behavior reflects an intention to flout the advice of medical experts, some is caused in part, she said, by the lack of a clear national health strategy and garbled communication from those in charge of public safety.
Americans are deciding for themselves, on an a la carte basis, whether to wear a mask or get tested or travel, and school districts decide individually when it’s time to go virtual.
“People are exhausted from having to do a risk-benefit analysis for every single activity they, their friends, their kids want to participate in,” she said.
U.S. behind in several areas
Despite our self-image as the global leader in science and medicine, the United States stumbled badly in its response to the pandemic, with grave consequences both at home and abroad, experts say.
In a recent commentary in JAMA, Lawrence Gostin, JD, from Georgetown University, Washington, and Jennifer Nuzzo, DrPH, at Johns Hopkins University, Baltimore, pointed to several critical shortfalls in the nation’s efforts to control the disease.
One such shortfall is public trust.
This news organization reported in June 2021 that a poll of its readers found that 44% said their trust in the CDC had waned during the pandemic, and 33% said their trust in the FDA had eroded as well.
Health care providers who responded to the poll lost trust as well. About half of the doctors and nurses who responded said they disagreed with the FDA’s decision-making during the pandemic. Nearly 60% of doctors and 65% of nurses said they disagreed with the CDC’s overall pandemic guidance.
Lack of trust can make people resist vaccines and efforts to fight the virus, the authors wrote.
“This will become really relevant when we have ample supply of Pfizer’s antiviral medication,” Mr. Gostin, who directs the O’Neill Institute for National and Global Health Law at Georgetown, told this news organization. “The next phase of the pandemic is not to link testing to contact tracing, because we’re way past that, but to link testing to treatment.”
Lack of regional manufacturing of products is also thwarting global progress.
“It is extraordinarily important that our pharmaceutical industry transfer technology in a pandemic,” Mr. Gostin said. “The most glaring failure to do that is the mRNA vaccines. We’ve got this enormously effective vaccine and the two manufacturers – Pfizer and Moderna – are refusing to share the technology with producers in other countries. That keeps coming back to haunt us.”
Another problem: When the vaccines are shared with other countries, they are being delivered close to the date they expire or arriving at a shipyards without warning, so even some of the doses that get delivered are going to waste, Mr. Gostin said.
“It’s one of the greatest moral failures of my lifetime,” he said.
Also a failure is the “jaw-dropping” state of testing 2 years into the pandemic, he said, as people continue to pay high prices for tests or endure long lines.
The U.S. government updated its calculations and ordered 1 billion tests for the general public. The COVIDtests.gov website to order the free tests is now live.
It’s a step in the right direction. Mr. Gostin and Dr. Nuzzo wrote that there is every reason to expect future epidemics that are as serious or more serious than COVID.
“Failure to address clearly observed weaknesses in the COVID-19 response will have preventable adverse health, social, and economic consequences when the next novel outbreak occurs,” they wrote.

A version of this article first appeared on WebMD.com.
Antibiotic choices for inpatients with SSTIs vary by race
– in a national cross-sectional study involving over 1,000 patients in 91 hospitals.
The potential racial disparity in management of SSTI was detected after data were adjusted for penicillin allergy history and for MRSA colonization/infection. The data were also adjusted for hospital day (since admission) in order to control for the administration of more empiric therapy early on.
Clindamycin, a beta-lactam alternative, is not recommended as an SSTI treatment given its frequent dosing requirements and high potential for adverse events including Clostridioides difficile infection (DCI). “Clindamycin is an option but it’s considered inferior. ... It covers MRSA but it shouldn’t be a go-to for skin and soft-tissue infections,” said senior author Kimberly Blumenthal, MD, MSc, assistant professor of medicine at Harvard University, and an allergist, immunologist, and drug allergy and epidemiology researcher at Massachusetts General Hospital, both in Boston.
Cefazolin, on the other hand, does not cover MRSA but is “a guideline-recommended first-line antibiotic for cellulitis SSTI in the hospital,” she said in an interview.
The findings, recently published in JAMA Network Open, offer a valuable portrait of the antibiotics being prescribed in the inpatient setting for SSTIs. Vancomycin, which typically is reserved for MRSA, was the most commonly prescribed antibiotic, regardless of race. Piperacillin-tazobactam, a beta-lactam, was the second most commonly prescribed antibiotic, again regardless of race.
Intravenously administered cefazolin was used in 13% of White inpatients versus 5% of Black inpatients. After controlling for kidney disease, diabetes, and ICU location (in addition to hospital day, penicillin allergy history, and MRSA), White inpatients had an increased likelihood of being prescribed cefazolin (adjusted odds ratio, 2.82; 95% confidence interval, 1.41-5.63) and a decreased likelihood of clindamycin use (aOR, 0.54; 95% CI, 0.30-0.96), compared with Black inpatients.
The investigators utilized the Acute Care Hospital Groups network within Vizient, a member-driven health care performance improvement company, to collect data for the study. Most of the hospitals (91%) that submitted data on adult inpatients with cellulitis or SSTIs (without other infections) were in urban settings and 9% were in rural settings; 60% were community hospitals and 40% were academic medical centers. The researchers accounted for “clustering by hospital” – such as the use of internal guidelines – in their methodology.
Differential management and prescribing practices associated with race and ethnicity have been demonstrated for cardiovascular disease and other chronic problems, but “to see such racial differences play out in acute care is striking,” Utibe R. Essien, MD, MPH, assistant professor of medicine at the University of Pittsburgh and a core investigator with the Center for Health Equity Research and Promotion at the Veterans Affairs Pittsburgh Healthcare System, said in an interview.
“In acute care, we tend to practice pretty similarly across the board ... so the findings give me pause,” said Dr. Essien, an internist and a coauthor of the study, who also works with the University of Pittsburgh’s Center for Pharmaceutical Policy and Prescribing.
Also notable was the prevalence of historical penicillin allergy documented in the dataset: 23% in Black inpatients and 18% in White inpatients with SSTI. It’s a surprisingly high prevalence overall, Dr. Blumenthal said, and the racial difference was surprising because penicillin allergy has been commonly described in the literature as being more common in the White population.
Even though penicillin allergy was controlled for in the study, “given that historical penicillin allergies are associated with increased clindamycin use and risk of CDI, but are often disproved with formal testing, racial disparities in penicillin allergy documentation and assessment require additional study,” she and her coauthors wrote.
Ideally, Dr. Blumenthal said, all inpatients would have access to allergy consultations or testing or some sort of infrastructure for assessing a history of penicillin allergy. At Mass General, allergy consults and challenge doses of beta-lactams (also called graded challenges) are frequently employed.
The study did not collect data on income, educational level, and other structural vulnerability factors. More research is needed to better understand “what’s going on in acute care settings and what the potential drivers of disparities may be,” said Dr. Essien, who co-authored a recent JAMA editorial on “achieving pharmacoequity” to reduce health disparities.
“If guidelines suggest that medication A is the ideal and optimal treatment, we really have to do our best to ensure that every patient, regardless of race or ethnicity, can get that treatment,” he said.
In the study, race was extracted from the medical record and may not have been correctly assigned, the authors noted. “Other race” was not specified in the dataset, and Hispanic ethnicity was not captured. The number of individuals identified as Asian and other races was small, prompting the researchers to focus on antibiotic use in Black and White patients (224 and 854 patients, respectively).
Dr. Blumenthal and Dr. Essien both reported that they had no relevant disclosures. The study was supported with National Institutes of Health grants and the Massachusetts General Hospital department of medicine transformative scholar program.
– in a national cross-sectional study involving over 1,000 patients in 91 hospitals.
The potential racial disparity in management of SSTI was detected after data were adjusted for penicillin allergy history and for MRSA colonization/infection. The data were also adjusted for hospital day (since admission) in order to control for the administration of more empiric therapy early on.
Clindamycin, a beta-lactam alternative, is not recommended as an SSTI treatment given its frequent dosing requirements and high potential for adverse events including Clostridioides difficile infection (DCI). “Clindamycin is an option but it’s considered inferior. ... It covers MRSA but it shouldn’t be a go-to for skin and soft-tissue infections,” said senior author Kimberly Blumenthal, MD, MSc, assistant professor of medicine at Harvard University, and an allergist, immunologist, and drug allergy and epidemiology researcher at Massachusetts General Hospital, both in Boston.
Cefazolin, on the other hand, does not cover MRSA but is “a guideline-recommended first-line antibiotic for cellulitis SSTI in the hospital,” she said in an interview.
The findings, recently published in JAMA Network Open, offer a valuable portrait of the antibiotics being prescribed in the inpatient setting for SSTIs. Vancomycin, which typically is reserved for MRSA, was the most commonly prescribed antibiotic, regardless of race. Piperacillin-tazobactam, a beta-lactam, was the second most commonly prescribed antibiotic, again regardless of race.
Intravenously administered cefazolin was used in 13% of White inpatients versus 5% of Black inpatients. After controlling for kidney disease, diabetes, and ICU location (in addition to hospital day, penicillin allergy history, and MRSA), White inpatients had an increased likelihood of being prescribed cefazolin (adjusted odds ratio, 2.82; 95% confidence interval, 1.41-5.63) and a decreased likelihood of clindamycin use (aOR, 0.54; 95% CI, 0.30-0.96), compared with Black inpatients.
The investigators utilized the Acute Care Hospital Groups network within Vizient, a member-driven health care performance improvement company, to collect data for the study. Most of the hospitals (91%) that submitted data on adult inpatients with cellulitis or SSTIs (without other infections) were in urban settings and 9% were in rural settings; 60% were community hospitals and 40% were academic medical centers. The researchers accounted for “clustering by hospital” – such as the use of internal guidelines – in their methodology.
Differential management and prescribing practices associated with race and ethnicity have been demonstrated for cardiovascular disease and other chronic problems, but “to see such racial differences play out in acute care is striking,” Utibe R. Essien, MD, MPH, assistant professor of medicine at the University of Pittsburgh and a core investigator with the Center for Health Equity Research and Promotion at the Veterans Affairs Pittsburgh Healthcare System, said in an interview.
“In acute care, we tend to practice pretty similarly across the board ... so the findings give me pause,” said Dr. Essien, an internist and a coauthor of the study, who also works with the University of Pittsburgh’s Center for Pharmaceutical Policy and Prescribing.
Also notable was the prevalence of historical penicillin allergy documented in the dataset: 23% in Black inpatients and 18% in White inpatients with SSTI. It’s a surprisingly high prevalence overall, Dr. Blumenthal said, and the racial difference was surprising because penicillin allergy has been commonly described in the literature as being more common in the White population.
Even though penicillin allergy was controlled for in the study, “given that historical penicillin allergies are associated with increased clindamycin use and risk of CDI, but are often disproved with formal testing, racial disparities in penicillin allergy documentation and assessment require additional study,” she and her coauthors wrote.
Ideally, Dr. Blumenthal said, all inpatients would have access to allergy consultations or testing or some sort of infrastructure for assessing a history of penicillin allergy. At Mass General, allergy consults and challenge doses of beta-lactams (also called graded challenges) are frequently employed.
The study did not collect data on income, educational level, and other structural vulnerability factors. More research is needed to better understand “what’s going on in acute care settings and what the potential drivers of disparities may be,” said Dr. Essien, who co-authored a recent JAMA editorial on “achieving pharmacoequity” to reduce health disparities.
“If guidelines suggest that medication A is the ideal and optimal treatment, we really have to do our best to ensure that every patient, regardless of race or ethnicity, can get that treatment,” he said.
In the study, race was extracted from the medical record and may not have been correctly assigned, the authors noted. “Other race” was not specified in the dataset, and Hispanic ethnicity was not captured. The number of individuals identified as Asian and other races was small, prompting the researchers to focus on antibiotic use in Black and White patients (224 and 854 patients, respectively).
Dr. Blumenthal and Dr. Essien both reported that they had no relevant disclosures. The study was supported with National Institutes of Health grants and the Massachusetts General Hospital department of medicine transformative scholar program.
– in a national cross-sectional study involving over 1,000 patients in 91 hospitals.
The potential racial disparity in management of SSTI was detected after data were adjusted for penicillin allergy history and for MRSA colonization/infection. The data were also adjusted for hospital day (since admission) in order to control for the administration of more empiric therapy early on.
Clindamycin, a beta-lactam alternative, is not recommended as an SSTI treatment given its frequent dosing requirements and high potential for adverse events including Clostridioides difficile infection (DCI). “Clindamycin is an option but it’s considered inferior. ... It covers MRSA but it shouldn’t be a go-to for skin and soft-tissue infections,” said senior author Kimberly Blumenthal, MD, MSc, assistant professor of medicine at Harvard University, and an allergist, immunologist, and drug allergy and epidemiology researcher at Massachusetts General Hospital, both in Boston.
Cefazolin, on the other hand, does not cover MRSA but is “a guideline-recommended first-line antibiotic for cellulitis SSTI in the hospital,” she said in an interview.
The findings, recently published in JAMA Network Open, offer a valuable portrait of the antibiotics being prescribed in the inpatient setting for SSTIs. Vancomycin, which typically is reserved for MRSA, was the most commonly prescribed antibiotic, regardless of race. Piperacillin-tazobactam, a beta-lactam, was the second most commonly prescribed antibiotic, again regardless of race.
Intravenously administered cefazolin was used in 13% of White inpatients versus 5% of Black inpatients. After controlling for kidney disease, diabetes, and ICU location (in addition to hospital day, penicillin allergy history, and MRSA), White inpatients had an increased likelihood of being prescribed cefazolin (adjusted odds ratio, 2.82; 95% confidence interval, 1.41-5.63) and a decreased likelihood of clindamycin use (aOR, 0.54; 95% CI, 0.30-0.96), compared with Black inpatients.
The investigators utilized the Acute Care Hospital Groups network within Vizient, a member-driven health care performance improvement company, to collect data for the study. Most of the hospitals (91%) that submitted data on adult inpatients with cellulitis or SSTIs (without other infections) were in urban settings and 9% were in rural settings; 60% were community hospitals and 40% were academic medical centers. The researchers accounted for “clustering by hospital” – such as the use of internal guidelines – in their methodology.
Differential management and prescribing practices associated with race and ethnicity have been demonstrated for cardiovascular disease and other chronic problems, but “to see such racial differences play out in acute care is striking,” Utibe R. Essien, MD, MPH, assistant professor of medicine at the University of Pittsburgh and a core investigator with the Center for Health Equity Research and Promotion at the Veterans Affairs Pittsburgh Healthcare System, said in an interview.
“In acute care, we tend to practice pretty similarly across the board ... so the findings give me pause,” said Dr. Essien, an internist and a coauthor of the study, who also works with the University of Pittsburgh’s Center for Pharmaceutical Policy and Prescribing.
Also notable was the prevalence of historical penicillin allergy documented in the dataset: 23% in Black inpatients and 18% in White inpatients with SSTI. It’s a surprisingly high prevalence overall, Dr. Blumenthal said, and the racial difference was surprising because penicillin allergy has been commonly described in the literature as being more common in the White population.
Even though penicillin allergy was controlled for in the study, “given that historical penicillin allergies are associated with increased clindamycin use and risk of CDI, but are often disproved with formal testing, racial disparities in penicillin allergy documentation and assessment require additional study,” she and her coauthors wrote.
Ideally, Dr. Blumenthal said, all inpatients would have access to allergy consultations or testing or some sort of infrastructure for assessing a history of penicillin allergy. At Mass General, allergy consults and challenge doses of beta-lactams (also called graded challenges) are frequently employed.
The study did not collect data on income, educational level, and other structural vulnerability factors. More research is needed to better understand “what’s going on in acute care settings and what the potential drivers of disparities may be,” said Dr. Essien, who co-authored a recent JAMA editorial on “achieving pharmacoequity” to reduce health disparities.
“If guidelines suggest that medication A is the ideal and optimal treatment, we really have to do our best to ensure that every patient, regardless of race or ethnicity, can get that treatment,” he said.
In the study, race was extracted from the medical record and may not have been correctly assigned, the authors noted. “Other race” was not specified in the dataset, and Hispanic ethnicity was not captured. The number of individuals identified as Asian and other races was small, prompting the researchers to focus on antibiotic use in Black and White patients (224 and 854 patients, respectively).
Dr. Blumenthal and Dr. Essien both reported that they had no relevant disclosures. The study was supported with National Institutes of Health grants and the Massachusetts General Hospital department of medicine transformative scholar program.
FROM JAMA NETWORK OPEN
Dark plaque on back of ear
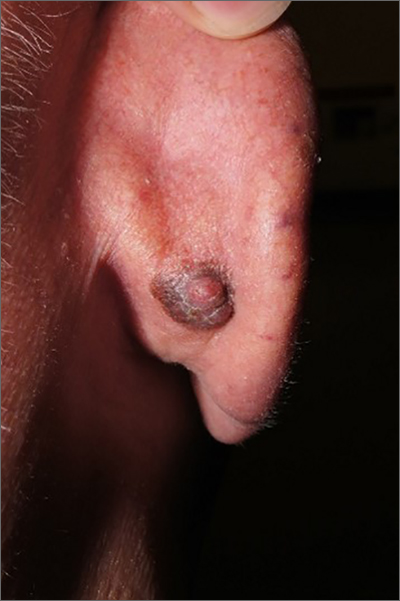
Dermoscopic findings were consistent with a melanocytic lesion and a scoop shave biopsy revealed a 2.7 mm thick nodular melanoma.
Melanoma is the most lethal skin cancer in the United States. The likelihood of metastatic spread to lymph nodes statistically increases beyond a probability of 5% when patients have primary lesions thicker than 0.8 mm.1 Thus, for patients with tumors thicker than 0.8 mm, or some other high-risk features such as high mitotic index, a sentinel lymph node biopsy (SLNB) is recommended. This patient underwent wide local excision and reconstruction of his ear. An SLNB was also performed and the results were negative.
The patient returned for a complete skin exam every 3 months. Ten months after the excision, he presented with episodes of headache and confusion. Magnetic resonance imaging revealed metastasis to the brain; a biopsy confirmed that it was melanoma. Two months later, after attempts at resection of the brain metastasis, the patient died.
This case demonstrates that patients with thick melanoma are at continued risk for recurrence and poor outcomes; they benefit from close surveillance and work-up of unusual symptoms that might suggest metastases. Phase 3 trials are currently underway to consider the use of adjuvant therapy in patients with advanced stage II melanoma who, on average, have worse outcomes than patients with early-stage III disease.2
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).
1. NCCN Guidelines Version 1.2022 Melanoma: Cutaneous. National Comprehensive Cancer Network. December 3, 2021. Accessed January 4, 2022. https://www.nccn.org/professionals/physician_gls/pdf/cutaneous_melanoma.pdf
2. Poklepovic AS, Luke JJ. Considering adjuvant therapy for stage II melanoma. Cancer. 2020;126:1166-1174. doi: 10.1002/cncr.32585

Dermoscopic findings were consistent with a melanocytic lesion and a scoop shave biopsy revealed a 2.7 mm thick nodular melanoma.
Melanoma is the most lethal skin cancer in the United States. The likelihood of metastatic spread to lymph nodes statistically increases beyond a probability of 5% when patients have primary lesions thicker than 0.8 mm.1 Thus, for patients with tumors thicker than 0.8 mm, or some other high-risk features such as high mitotic index, a sentinel lymph node biopsy (SLNB) is recommended. This patient underwent wide local excision and reconstruction of his ear. An SLNB was also performed and the results were negative.
The patient returned for a complete skin exam every 3 months. Ten months after the excision, he presented with episodes of headache and confusion. Magnetic resonance imaging revealed metastasis to the brain; a biopsy confirmed that it was melanoma. Two months later, after attempts at resection of the brain metastasis, the patient died.
This case demonstrates that patients with thick melanoma are at continued risk for recurrence and poor outcomes; they benefit from close surveillance and work-up of unusual symptoms that might suggest metastases. Phase 3 trials are currently underway to consider the use of adjuvant therapy in patients with advanced stage II melanoma who, on average, have worse outcomes than patients with early-stage III disease.2
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).

Dermoscopic findings were consistent with a melanocytic lesion and a scoop shave biopsy revealed a 2.7 mm thick nodular melanoma.
Melanoma is the most lethal skin cancer in the United States. The likelihood of metastatic spread to lymph nodes statistically increases beyond a probability of 5% when patients have primary lesions thicker than 0.8 mm.1 Thus, for patients with tumors thicker than 0.8 mm, or some other high-risk features such as high mitotic index, a sentinel lymph node biopsy (SLNB) is recommended. This patient underwent wide local excision and reconstruction of his ear. An SLNB was also performed and the results were negative.
The patient returned for a complete skin exam every 3 months. Ten months after the excision, he presented with episodes of headache and confusion. Magnetic resonance imaging revealed metastasis to the brain; a biopsy confirmed that it was melanoma. Two months later, after attempts at resection of the brain metastasis, the patient died.
This case demonstrates that patients with thick melanoma are at continued risk for recurrence and poor outcomes; they benefit from close surveillance and work-up of unusual symptoms that might suggest metastases. Phase 3 trials are currently underway to consider the use of adjuvant therapy in patients with advanced stage II melanoma who, on average, have worse outcomes than patients with early-stage III disease.2
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).
1. NCCN Guidelines Version 1.2022 Melanoma: Cutaneous. National Comprehensive Cancer Network. December 3, 2021. Accessed January 4, 2022. https://www.nccn.org/professionals/physician_gls/pdf/cutaneous_melanoma.pdf
2. Poklepovic AS, Luke JJ. Considering adjuvant therapy for stage II melanoma. Cancer. 2020;126:1166-1174. doi: 10.1002/cncr.32585
1. NCCN Guidelines Version 1.2022 Melanoma: Cutaneous. National Comprehensive Cancer Network. December 3, 2021. Accessed January 4, 2022. https://www.nccn.org/professionals/physician_gls/pdf/cutaneous_melanoma.pdf
2. Poklepovic AS, Luke JJ. Considering adjuvant therapy for stage II melanoma. Cancer. 2020;126:1166-1174. doi: 10.1002/cncr.32585
Severe outcomes increased in youth hospitalized after positive COVID-19 test
Approximately 3% of youth who tested positive for COVID-19 in an emergency department setting had severe outcomes after 2 weeks, but this risk was 0.5% among those not admitted to the hospital, based on data from more than 3,000 individuals aged 18 and younger.
In the early stages of the COVID-19 pandemic, youth younger than 18 years accounted for fewer than 5% of reported cases, but now account for approximately 25% of positive cases, wrote Anna L. Funk, PhD, of the University of Calgary, Alberta, Canada, and colleagues.
However, the risk of severe outcomes of youth with COVID-19 remains poorly understood and data from large studies are lacking, they noted.
In a prospective cohort study published in JAMA Network Open, the researchers reviewed data from 3,221 children and adolescents who were tested for COVID-19 at one of 41 emergency departments in 10 countries including Argentina, Australia, Canada, Costa Rica, Italy, New Zealand, Paraguay, Singapore, Spain, and the United States between March 2020 and June 2021. Positive infections were confirmed by polymerase chain reaction (PCR) testing. At 14 days’ follow-up after a positive test, 735 patients (22.8%), were hospitalized, 107 (3.3%) had severe outcomes, and 4 (0.12%) had died. Severe outcomes were significantly more likely in children aged 5-10 years and 10-18 years vs. less than 1 year (odds ratios, 1.60 and 2.39, respectively), and in children with a self-reported chronic illness (OR, 2.34) or a prior episode of pneumonia (OR, 3.15).
Severe outcomes were more likely in patients who presented with symptoms that started 4-7 days before seeking care, compared with those whose symptoms started 0-3 days before seeking care (OR, 2.22).
The researchers also reviewed data from a subgroup of 2,510 individuals who were discharged home from the ED after initial testing. At 14 days’ follow-up, 50 of these patients (2.0%) were hospitalized and 12 (0.5%) had severe outcomes. In addition, the researchers found that the risk of severe outcomes among hospitalized COVID-19–positive youth was nearly four times higher, compared with hospitalized youth who tested negative for COVID-19 (risk difference, 3.9%).
Previous retrospective studies of severe outcomes in children and adolescents with COVID-19 have yielded varying results, in part because of the variation in study populations, the researchers noted in their discussion of the findings. “Our study population provides a risk estimate for youths brought for ED care.” Therefore, “Our lower estimate of severe disease likely reflects our stringent definition, which required the occurrence of complications or specific invasive interventions,” they said.
The study limitations included the potential overestimation of the risk of severe outcomes because patients were recruited in the ED, the researchers noted. Other limitations included variation in regional case definitions, screening criteria, and testing capacity among different sites and time periods. “Thus, 5% of our SARS-CoV-2–positive participants were asymptomatic – most of whom were tested as they were positive contacts of known cases or as part of routine screening procedures,” they said. The findings also are not generalizable to all community EDs and did not account for variants, they added.
However, the results were strengthened by the ability to compare outcomes for children with positive tests to similar children with negative tests, and add to the literature showing an increased risk of severe outcomes for those hospitalized with positive tests, the researchers concluded.
Data may inform clinical decisions
“The data [in the current study] are concerning for severe outcomes for children even prior to the Omicron strain,” said Margaret Thew, DNP, FP-BC, of Children’s Wisconsin-Milwaukee Hospital, in an interview. “Presently, the number of children infected with the Omicron strain is much higher and hospitalizations among children are at their highest since COVID-19 began,” she said. “For medical providers caring for this population, the study sheds light on pediatric patients who may be at higher risk of severe illness when they become infected with COVID-19,” she added.
“I was surprised by how high the number of pediatric patients hospitalized (22%) and the percentage (3%) with severe disease were during this time,” given that the timeline for these data preceded the spread of the Omicron strain, said Ms. Thew. “The risk of prior pneumonia was quite surprising. I do not recall seeing prior pneumonia as a risk factor for more severe COVID-19 with children or adults,” she added.
The take-home messaging for clinicians caring for children and adolescents is the added knowledge of the risk factors for severe outcomes from COVID-19, including the 10-18 age range, chronic illness, prior pneumonia, and longer symptom duration before seeking care in the ED, Ms. Thew emphasized.
However, additional research is needed on the impact of the new strains of COVID-19 on pediatric and adolescent hospitalizations, Ms. Thew said. Research also is needed on the other illnesses that have resulted from COVID-19, including illness requiring antibiotic use or medical interventions or treatments, and on the risk of combined COVID-19 and influenza viruses, she noted.
The study was supported by the Canadian Institutes of Health Research, Alberta Innovates, the Alberta Health Services University of Calgary Clinical Research Fund, the Alberta Children’s Hospital Research Institute, the COVID-19 Research Accelerator Funding Track (CRAFT) Program at the University of California, Davis, and the Cincinnati Children’s Hospital Medical Center Division of Emergency Medicine Small Grants Program. Lead author Dr. Funk was supported by the University of Calgary Eyes-High Post-Doctoral Research Fund, but had no financial conflicts to disclose. Ms. Thew had no financial conflicts to disclose and serves on the Editorial Advisory Board of Pediatric News.
Approximately 3% of youth who tested positive for COVID-19 in an emergency department setting had severe outcomes after 2 weeks, but this risk was 0.5% among those not admitted to the hospital, based on data from more than 3,000 individuals aged 18 and younger.
In the early stages of the COVID-19 pandemic, youth younger than 18 years accounted for fewer than 5% of reported cases, but now account for approximately 25% of positive cases, wrote Anna L. Funk, PhD, of the University of Calgary, Alberta, Canada, and colleagues.
However, the risk of severe outcomes of youth with COVID-19 remains poorly understood and data from large studies are lacking, they noted.
In a prospective cohort study published in JAMA Network Open, the researchers reviewed data from 3,221 children and adolescents who were tested for COVID-19 at one of 41 emergency departments in 10 countries including Argentina, Australia, Canada, Costa Rica, Italy, New Zealand, Paraguay, Singapore, Spain, and the United States between March 2020 and June 2021. Positive infections were confirmed by polymerase chain reaction (PCR) testing. At 14 days’ follow-up after a positive test, 735 patients (22.8%), were hospitalized, 107 (3.3%) had severe outcomes, and 4 (0.12%) had died. Severe outcomes were significantly more likely in children aged 5-10 years and 10-18 years vs. less than 1 year (odds ratios, 1.60 and 2.39, respectively), and in children with a self-reported chronic illness (OR, 2.34) or a prior episode of pneumonia (OR, 3.15).
Severe outcomes were more likely in patients who presented with symptoms that started 4-7 days before seeking care, compared with those whose symptoms started 0-3 days before seeking care (OR, 2.22).
The researchers also reviewed data from a subgroup of 2,510 individuals who were discharged home from the ED after initial testing. At 14 days’ follow-up, 50 of these patients (2.0%) were hospitalized and 12 (0.5%) had severe outcomes. In addition, the researchers found that the risk of severe outcomes among hospitalized COVID-19–positive youth was nearly four times higher, compared with hospitalized youth who tested negative for COVID-19 (risk difference, 3.9%).
Previous retrospective studies of severe outcomes in children and adolescents with COVID-19 have yielded varying results, in part because of the variation in study populations, the researchers noted in their discussion of the findings. “Our study population provides a risk estimate for youths brought for ED care.” Therefore, “Our lower estimate of severe disease likely reflects our stringent definition, which required the occurrence of complications or specific invasive interventions,” they said.
The study limitations included the potential overestimation of the risk of severe outcomes because patients were recruited in the ED, the researchers noted. Other limitations included variation in regional case definitions, screening criteria, and testing capacity among different sites and time periods. “Thus, 5% of our SARS-CoV-2–positive participants were asymptomatic – most of whom were tested as they were positive contacts of known cases or as part of routine screening procedures,” they said. The findings also are not generalizable to all community EDs and did not account for variants, they added.
However, the results were strengthened by the ability to compare outcomes for children with positive tests to similar children with negative tests, and add to the literature showing an increased risk of severe outcomes for those hospitalized with positive tests, the researchers concluded.
Data may inform clinical decisions
“The data [in the current study] are concerning for severe outcomes for children even prior to the Omicron strain,” said Margaret Thew, DNP, FP-BC, of Children’s Wisconsin-Milwaukee Hospital, in an interview. “Presently, the number of children infected with the Omicron strain is much higher and hospitalizations among children are at their highest since COVID-19 began,” she said. “For medical providers caring for this population, the study sheds light on pediatric patients who may be at higher risk of severe illness when they become infected with COVID-19,” she added.
“I was surprised by how high the number of pediatric patients hospitalized (22%) and the percentage (3%) with severe disease were during this time,” given that the timeline for these data preceded the spread of the Omicron strain, said Ms. Thew. “The risk of prior pneumonia was quite surprising. I do not recall seeing prior pneumonia as a risk factor for more severe COVID-19 with children or adults,” she added.
The take-home messaging for clinicians caring for children and adolescents is the added knowledge of the risk factors for severe outcomes from COVID-19, including the 10-18 age range, chronic illness, prior pneumonia, and longer symptom duration before seeking care in the ED, Ms. Thew emphasized.
However, additional research is needed on the impact of the new strains of COVID-19 on pediatric and adolescent hospitalizations, Ms. Thew said. Research also is needed on the other illnesses that have resulted from COVID-19, including illness requiring antibiotic use or medical interventions or treatments, and on the risk of combined COVID-19 and influenza viruses, she noted.
The study was supported by the Canadian Institutes of Health Research, Alberta Innovates, the Alberta Health Services University of Calgary Clinical Research Fund, the Alberta Children’s Hospital Research Institute, the COVID-19 Research Accelerator Funding Track (CRAFT) Program at the University of California, Davis, and the Cincinnati Children’s Hospital Medical Center Division of Emergency Medicine Small Grants Program. Lead author Dr. Funk was supported by the University of Calgary Eyes-High Post-Doctoral Research Fund, but had no financial conflicts to disclose. Ms. Thew had no financial conflicts to disclose and serves on the Editorial Advisory Board of Pediatric News.
Approximately 3% of youth who tested positive for COVID-19 in an emergency department setting had severe outcomes after 2 weeks, but this risk was 0.5% among those not admitted to the hospital, based on data from more than 3,000 individuals aged 18 and younger.
In the early stages of the COVID-19 pandemic, youth younger than 18 years accounted for fewer than 5% of reported cases, but now account for approximately 25% of positive cases, wrote Anna L. Funk, PhD, of the University of Calgary, Alberta, Canada, and colleagues.
However, the risk of severe outcomes of youth with COVID-19 remains poorly understood and data from large studies are lacking, they noted.
In a prospective cohort study published in JAMA Network Open, the researchers reviewed data from 3,221 children and adolescents who were tested for COVID-19 at one of 41 emergency departments in 10 countries including Argentina, Australia, Canada, Costa Rica, Italy, New Zealand, Paraguay, Singapore, Spain, and the United States between March 2020 and June 2021. Positive infections were confirmed by polymerase chain reaction (PCR) testing. At 14 days’ follow-up after a positive test, 735 patients (22.8%), were hospitalized, 107 (3.3%) had severe outcomes, and 4 (0.12%) had died. Severe outcomes were significantly more likely in children aged 5-10 years and 10-18 years vs. less than 1 year (odds ratios, 1.60 and 2.39, respectively), and in children with a self-reported chronic illness (OR, 2.34) or a prior episode of pneumonia (OR, 3.15).
Severe outcomes were more likely in patients who presented with symptoms that started 4-7 days before seeking care, compared with those whose symptoms started 0-3 days before seeking care (OR, 2.22).
The researchers also reviewed data from a subgroup of 2,510 individuals who were discharged home from the ED after initial testing. At 14 days’ follow-up, 50 of these patients (2.0%) were hospitalized and 12 (0.5%) had severe outcomes. In addition, the researchers found that the risk of severe outcomes among hospitalized COVID-19–positive youth was nearly four times higher, compared with hospitalized youth who tested negative for COVID-19 (risk difference, 3.9%).
Previous retrospective studies of severe outcomes in children and adolescents with COVID-19 have yielded varying results, in part because of the variation in study populations, the researchers noted in their discussion of the findings. “Our study population provides a risk estimate for youths brought for ED care.” Therefore, “Our lower estimate of severe disease likely reflects our stringent definition, which required the occurrence of complications or specific invasive interventions,” they said.
The study limitations included the potential overestimation of the risk of severe outcomes because patients were recruited in the ED, the researchers noted. Other limitations included variation in regional case definitions, screening criteria, and testing capacity among different sites and time periods. “Thus, 5% of our SARS-CoV-2–positive participants were asymptomatic – most of whom were tested as they were positive contacts of known cases or as part of routine screening procedures,” they said. The findings also are not generalizable to all community EDs and did not account for variants, they added.
However, the results were strengthened by the ability to compare outcomes for children with positive tests to similar children with negative tests, and add to the literature showing an increased risk of severe outcomes for those hospitalized with positive tests, the researchers concluded.
Data may inform clinical decisions
“The data [in the current study] are concerning for severe outcomes for children even prior to the Omicron strain,” said Margaret Thew, DNP, FP-BC, of Children’s Wisconsin-Milwaukee Hospital, in an interview. “Presently, the number of children infected with the Omicron strain is much higher and hospitalizations among children are at their highest since COVID-19 began,” she said. “For medical providers caring for this population, the study sheds light on pediatric patients who may be at higher risk of severe illness when they become infected with COVID-19,” she added.
“I was surprised by how high the number of pediatric patients hospitalized (22%) and the percentage (3%) with severe disease were during this time,” given that the timeline for these data preceded the spread of the Omicron strain, said Ms. Thew. “The risk of prior pneumonia was quite surprising. I do not recall seeing prior pneumonia as a risk factor for more severe COVID-19 with children or adults,” she added.
The take-home messaging for clinicians caring for children and adolescents is the added knowledge of the risk factors for severe outcomes from COVID-19, including the 10-18 age range, chronic illness, prior pneumonia, and longer symptom duration before seeking care in the ED, Ms. Thew emphasized.
However, additional research is needed on the impact of the new strains of COVID-19 on pediatric and adolescent hospitalizations, Ms. Thew said. Research also is needed on the other illnesses that have resulted from COVID-19, including illness requiring antibiotic use or medical interventions or treatments, and on the risk of combined COVID-19 and influenza viruses, she noted.
The study was supported by the Canadian Institutes of Health Research, Alberta Innovates, the Alberta Health Services University of Calgary Clinical Research Fund, the Alberta Children’s Hospital Research Institute, the COVID-19 Research Accelerator Funding Track (CRAFT) Program at the University of California, Davis, and the Cincinnati Children’s Hospital Medical Center Division of Emergency Medicine Small Grants Program. Lead author Dr. Funk was supported by the University of Calgary Eyes-High Post-Doctoral Research Fund, but had no financial conflicts to disclose. Ms. Thew had no financial conflicts to disclose and serves on the Editorial Advisory Board of Pediatric News.
FROM JAMA NETWORK OPEN
Pediatric community-acquired pneumonia: 5 days of antibiotics better than 10 days
The evidence is in: and had the added benefit of a lower risk of inducing antibiotic resistance, according to the randomized, controlled SCOUT-CAP trial.
“Several studies have shown shorter antibiotic courses to be non-inferior to the standard treatment strategy, but in our study, we show that a shortened 5-day course of therapy was superior to standard therapy because the short course achieved similar outcomes with fewer days of antibiotics,” Derek Williams, MD, MPH, Vanderbilt University Medical Center, Nashville, Tenn., said in an email.
“These data are immediately applicable to frontline clinicians, and we hope this study will shift the paradigm towards more judicious treatment approaches for childhood pneumonia, resulting in care that is safer and more effective,” he added.
The study was published online Jan. 18 in JAMA Pediatrics.
Uncomplicated CAP
The study enrolled children aged 6 months to 71 months diagnosed with uncomplicated CAP who demonstrated early clinical improvement in response to 5 days of antibiotic treatment. Participants were prescribed either amoxicillin, amoxicillin and clavulanate, or cefdinir according to standard of care and were randomized on day 6 to another 5 days of their initially prescribed antibiotic course or to placebo.
“Those assessed on day 6 were eligible only if they had not yet received a dose of antibiotic therapy on that day,” the authors write. The primary endpoint was end-of-treatment response, adjusted for the duration of antibiotic risk as assessed by RADAR. As the authors explain, RADAR is a composite endpoint that ranks each child’s clinical response, resolution of symptoms, and antibiotic-associated adverse effects (AEs) in an ordinal desirability of outcome ranking, or DOOR.
“There were no differences between strategies in the DOOR or in its individual components,” Dr. Williams and colleagues point out. A total of 380 children took part in the study. The mean age of participants was 35.7 months, and half were male.
Over 90% of children randomized to active therapy were prescribed amoxicillin. “Fewer than 10% of children in either strategy had an inadequate clinical response,” the authors report.
However, the 5-day antibiotic strategy had a 69% (95% CI, 63%-75%) probability of children achieving a more desirable RADAR outcome compared with the standard, 10-day course, as assessed either on days 6 to 10 at outcome assessment visit one (OAV1) or at OAV2 on days 19 to 25.
There were also no significant differences between the two groups in the percentage of participants with persistent symptoms at either assessment point, they note. At assessment visit one, 40% of children assigned to the short-course strategy and 37% of children assigned to the 10-day strategy reported an antibiotic-related AE, most of which were mild.
Resistome analysis
Some 171 children were included in a resistome analysis in which throat swabs were collected between study days 19 and 25 to quantify antibiotic resistance genes in oropharyngeal flora. The total number of resistance genes per prokaryotic cell (RGPC) was significantly lower in children treated with antibiotics for 5 days compared with children who were treated for 10 days.
Specifically, the median number of total RGPC was 1.17 (95% CI, 0.35-2.43) for the short-course strategy and 1.33 (95% CI, 0.46-11.08) for the standard-course strategy (P = .01). Similarly, the median number of β-lactamase RGPC was 0.55 (0.18-1.24) for the short-course strategy and 0.60 (0.21-2.45) for the standard-course strategy (P = .03).
“Providing the shortest duration of antibiotics necessary to effectively treat an infection is a central tenet of antimicrobial stewardship and a convenient and cost-effective strategy for caregivers,” the authors observe. For example, reducing treatment from 10 to 5 days for outpatient CAP could reduce the number of days spent on antibiotics by up to 7.5 million days in the U.S. each year.
“If we can safely reduce antibiotic exposure, we can minimize antibiotic side effects while also helping to slow antibiotic resistance,” Dr. Williams pointed out.
Fewer days of having to give their child repeated doses of antibiotics is also more convenient for families, he added.
Asked to comment on the study, David Greenberg, MD, professor of pediatrics and infectious diseases, Ben Gurion University of the Negev, Israel, explained that the length of antibiotic therapy as recommended by various guidelines is more or less arbitrary, some infections being excepted.
“There have been no studies evaluating the recommendation for a 100-day treatment course, and it’s kind of a joke because if you look at the treatment of just about any infection, it’s either for 7 days or 14 days or even 20 days because it’s easy to calculate – it’s not that anybody proved that treatment of whatever infection it is should last this long,” he told this news organization.
Moreover, adherence to a shorter antibiotic course is much better than it is to a longer course. If, for example, physicians tell a mother to take two bottles of antibiotics for a treatment course of 10 days, she’ll finish the first bottle which is good for 5 days and, because the child is fine, “she forgets about the second bottle,” Dr. Greenberg said.
In one of the first studies to compare a short versus long course of antibiotic therapy in uncomplicated CAP in young children, Dr. Greenberg and colleagues initially compared a 3-day course of high-dose amoxicillin to a 10-day course of the same treatment, but the 3-day course was associated with an unacceptable failure rate. (At the time, the World Health Organization was recommending a 3-day course of antibiotics for the treatment of uncomplicated CAP in children.)
They stopped the study and then initiated a second study in which they compared a 5-day course of the same antibiotic to a 10-day course and found the 5-day course was comparable to the 10-day course in terms of clinical cure rates. As a result of his study, Dr. Greenberg has long since prescribed a 5-day course of antibiotics for his own patients.
“Five days is good,” he affirmed. “And if patients start a 10-day course of an antibiotic for, say, a urinary tract infection and a subsequent culture comes back negative, they don’t have to finish the antibiotics either.” Dr. Greenberg said.
Dr. Williams said he has no financial ties to industry. Dr. Greenberg said he has served as a consultant for Pfizer, Merck, Johnson & Johnson, and AstraZeneca. He is also a founder of the company Beyond Air.
A version of this article first appeared on Medscape.com.
The evidence is in: and had the added benefit of a lower risk of inducing antibiotic resistance, according to the randomized, controlled SCOUT-CAP trial.
“Several studies have shown shorter antibiotic courses to be non-inferior to the standard treatment strategy, but in our study, we show that a shortened 5-day course of therapy was superior to standard therapy because the short course achieved similar outcomes with fewer days of antibiotics,” Derek Williams, MD, MPH, Vanderbilt University Medical Center, Nashville, Tenn., said in an email.
“These data are immediately applicable to frontline clinicians, and we hope this study will shift the paradigm towards more judicious treatment approaches for childhood pneumonia, resulting in care that is safer and more effective,” he added.
The study was published online Jan. 18 in JAMA Pediatrics.
Uncomplicated CAP
The study enrolled children aged 6 months to 71 months diagnosed with uncomplicated CAP who demonstrated early clinical improvement in response to 5 days of antibiotic treatment. Participants were prescribed either amoxicillin, amoxicillin and clavulanate, or cefdinir according to standard of care and were randomized on day 6 to another 5 days of their initially prescribed antibiotic course or to placebo.
“Those assessed on day 6 were eligible only if they had not yet received a dose of antibiotic therapy on that day,” the authors write. The primary endpoint was end-of-treatment response, adjusted for the duration of antibiotic risk as assessed by RADAR. As the authors explain, RADAR is a composite endpoint that ranks each child’s clinical response, resolution of symptoms, and antibiotic-associated adverse effects (AEs) in an ordinal desirability of outcome ranking, or DOOR.
“There were no differences between strategies in the DOOR or in its individual components,” Dr. Williams and colleagues point out. A total of 380 children took part in the study. The mean age of participants was 35.7 months, and half were male.
Over 90% of children randomized to active therapy were prescribed amoxicillin. “Fewer than 10% of children in either strategy had an inadequate clinical response,” the authors report.
However, the 5-day antibiotic strategy had a 69% (95% CI, 63%-75%) probability of children achieving a more desirable RADAR outcome compared with the standard, 10-day course, as assessed either on days 6 to 10 at outcome assessment visit one (OAV1) or at OAV2 on days 19 to 25.
There were also no significant differences between the two groups in the percentage of participants with persistent symptoms at either assessment point, they note. At assessment visit one, 40% of children assigned to the short-course strategy and 37% of children assigned to the 10-day strategy reported an antibiotic-related AE, most of which were mild.
Resistome analysis
Some 171 children were included in a resistome analysis in which throat swabs were collected between study days 19 and 25 to quantify antibiotic resistance genes in oropharyngeal flora. The total number of resistance genes per prokaryotic cell (RGPC) was significantly lower in children treated with antibiotics for 5 days compared with children who were treated for 10 days.
Specifically, the median number of total RGPC was 1.17 (95% CI, 0.35-2.43) for the short-course strategy and 1.33 (95% CI, 0.46-11.08) for the standard-course strategy (P = .01). Similarly, the median number of β-lactamase RGPC was 0.55 (0.18-1.24) for the short-course strategy and 0.60 (0.21-2.45) for the standard-course strategy (P = .03).
“Providing the shortest duration of antibiotics necessary to effectively treat an infection is a central tenet of antimicrobial stewardship and a convenient and cost-effective strategy for caregivers,” the authors observe. For example, reducing treatment from 10 to 5 days for outpatient CAP could reduce the number of days spent on antibiotics by up to 7.5 million days in the U.S. each year.
“If we can safely reduce antibiotic exposure, we can minimize antibiotic side effects while also helping to slow antibiotic resistance,” Dr. Williams pointed out.
Fewer days of having to give their child repeated doses of antibiotics is also more convenient for families, he added.
Asked to comment on the study, David Greenberg, MD, professor of pediatrics and infectious diseases, Ben Gurion University of the Negev, Israel, explained that the length of antibiotic therapy as recommended by various guidelines is more or less arbitrary, some infections being excepted.
“There have been no studies evaluating the recommendation for a 100-day treatment course, and it’s kind of a joke because if you look at the treatment of just about any infection, it’s either for 7 days or 14 days or even 20 days because it’s easy to calculate – it’s not that anybody proved that treatment of whatever infection it is should last this long,” he told this news organization.
Moreover, adherence to a shorter antibiotic course is much better than it is to a longer course. If, for example, physicians tell a mother to take two bottles of antibiotics for a treatment course of 10 days, she’ll finish the first bottle which is good for 5 days and, because the child is fine, “she forgets about the second bottle,” Dr. Greenberg said.
In one of the first studies to compare a short versus long course of antibiotic therapy in uncomplicated CAP in young children, Dr. Greenberg and colleagues initially compared a 3-day course of high-dose amoxicillin to a 10-day course of the same treatment, but the 3-day course was associated with an unacceptable failure rate. (At the time, the World Health Organization was recommending a 3-day course of antibiotics for the treatment of uncomplicated CAP in children.)
They stopped the study and then initiated a second study in which they compared a 5-day course of the same antibiotic to a 10-day course and found the 5-day course was comparable to the 10-day course in terms of clinical cure rates. As a result of his study, Dr. Greenberg has long since prescribed a 5-day course of antibiotics for his own patients.
“Five days is good,” he affirmed. “And if patients start a 10-day course of an antibiotic for, say, a urinary tract infection and a subsequent culture comes back negative, they don’t have to finish the antibiotics either.” Dr. Greenberg said.
Dr. Williams said he has no financial ties to industry. Dr. Greenberg said he has served as a consultant for Pfizer, Merck, Johnson & Johnson, and AstraZeneca. He is also a founder of the company Beyond Air.
A version of this article first appeared on Medscape.com.
The evidence is in: and had the added benefit of a lower risk of inducing antibiotic resistance, according to the randomized, controlled SCOUT-CAP trial.
“Several studies have shown shorter antibiotic courses to be non-inferior to the standard treatment strategy, but in our study, we show that a shortened 5-day course of therapy was superior to standard therapy because the short course achieved similar outcomes with fewer days of antibiotics,” Derek Williams, MD, MPH, Vanderbilt University Medical Center, Nashville, Tenn., said in an email.
“These data are immediately applicable to frontline clinicians, and we hope this study will shift the paradigm towards more judicious treatment approaches for childhood pneumonia, resulting in care that is safer and more effective,” he added.
The study was published online Jan. 18 in JAMA Pediatrics.
Uncomplicated CAP
The study enrolled children aged 6 months to 71 months diagnosed with uncomplicated CAP who demonstrated early clinical improvement in response to 5 days of antibiotic treatment. Participants were prescribed either amoxicillin, amoxicillin and clavulanate, or cefdinir according to standard of care and were randomized on day 6 to another 5 days of their initially prescribed antibiotic course or to placebo.
“Those assessed on day 6 were eligible only if they had not yet received a dose of antibiotic therapy on that day,” the authors write. The primary endpoint was end-of-treatment response, adjusted for the duration of antibiotic risk as assessed by RADAR. As the authors explain, RADAR is a composite endpoint that ranks each child’s clinical response, resolution of symptoms, and antibiotic-associated adverse effects (AEs) in an ordinal desirability of outcome ranking, or DOOR.
“There were no differences between strategies in the DOOR or in its individual components,” Dr. Williams and colleagues point out. A total of 380 children took part in the study. The mean age of participants was 35.7 months, and half were male.
Over 90% of children randomized to active therapy were prescribed amoxicillin. “Fewer than 10% of children in either strategy had an inadequate clinical response,” the authors report.
However, the 5-day antibiotic strategy had a 69% (95% CI, 63%-75%) probability of children achieving a more desirable RADAR outcome compared with the standard, 10-day course, as assessed either on days 6 to 10 at outcome assessment visit one (OAV1) or at OAV2 on days 19 to 25.
There were also no significant differences between the two groups in the percentage of participants with persistent symptoms at either assessment point, they note. At assessment visit one, 40% of children assigned to the short-course strategy and 37% of children assigned to the 10-day strategy reported an antibiotic-related AE, most of which were mild.
Resistome analysis
Some 171 children were included in a resistome analysis in which throat swabs were collected between study days 19 and 25 to quantify antibiotic resistance genes in oropharyngeal flora. The total number of resistance genes per prokaryotic cell (RGPC) was significantly lower in children treated with antibiotics for 5 days compared with children who were treated for 10 days.
Specifically, the median number of total RGPC was 1.17 (95% CI, 0.35-2.43) for the short-course strategy and 1.33 (95% CI, 0.46-11.08) for the standard-course strategy (P = .01). Similarly, the median number of β-lactamase RGPC was 0.55 (0.18-1.24) for the short-course strategy and 0.60 (0.21-2.45) for the standard-course strategy (P = .03).
“Providing the shortest duration of antibiotics necessary to effectively treat an infection is a central tenet of antimicrobial stewardship and a convenient and cost-effective strategy for caregivers,” the authors observe. For example, reducing treatment from 10 to 5 days for outpatient CAP could reduce the number of days spent on antibiotics by up to 7.5 million days in the U.S. each year.
“If we can safely reduce antibiotic exposure, we can minimize antibiotic side effects while also helping to slow antibiotic resistance,” Dr. Williams pointed out.
Fewer days of having to give their child repeated doses of antibiotics is also more convenient for families, he added.
Asked to comment on the study, David Greenberg, MD, professor of pediatrics and infectious diseases, Ben Gurion University of the Negev, Israel, explained that the length of antibiotic therapy as recommended by various guidelines is more or less arbitrary, some infections being excepted.
“There have been no studies evaluating the recommendation for a 100-day treatment course, and it’s kind of a joke because if you look at the treatment of just about any infection, it’s either for 7 days or 14 days or even 20 days because it’s easy to calculate – it’s not that anybody proved that treatment of whatever infection it is should last this long,” he told this news organization.
Moreover, adherence to a shorter antibiotic course is much better than it is to a longer course. If, for example, physicians tell a mother to take two bottles of antibiotics for a treatment course of 10 days, she’ll finish the first bottle which is good for 5 days and, because the child is fine, “she forgets about the second bottle,” Dr. Greenberg said.
In one of the first studies to compare a short versus long course of antibiotic therapy in uncomplicated CAP in young children, Dr. Greenberg and colleagues initially compared a 3-day course of high-dose amoxicillin to a 10-day course of the same treatment, but the 3-day course was associated with an unacceptable failure rate. (At the time, the World Health Organization was recommending a 3-day course of antibiotics for the treatment of uncomplicated CAP in children.)
They stopped the study and then initiated a second study in which they compared a 5-day course of the same antibiotic to a 10-day course and found the 5-day course was comparable to the 10-day course in terms of clinical cure rates. As a result of his study, Dr. Greenberg has long since prescribed a 5-day course of antibiotics for his own patients.
“Five days is good,” he affirmed. “And if patients start a 10-day course of an antibiotic for, say, a urinary tract infection and a subsequent culture comes back negative, they don’t have to finish the antibiotics either.” Dr. Greenberg said.
Dr. Williams said he has no financial ties to industry. Dr. Greenberg said he has served as a consultant for Pfizer, Merck, Johnson & Johnson, and AstraZeneca. He is also a founder of the company Beyond Air.
A version of this article first appeared on Medscape.com.
Cardiac function normalizes by 3 months in MIS-C in study
While 80%-85% of children with multisystem inflammatory syndrome have cardiovascular involvement, “lack of knowledge about the short-term consequences of MIS-C has led to uncertainty among physicians in making recommendations about follow-up,” Daisuke Matsubara, MD, PhD, and colleagues wrote in their paper, which was published in the Journal of the American Heart Association.
Dr. Matsubara, of the department of pediatrics at the Children’s Hospital of Philadelphia, and colleagues examined cardiac outcomes among 60 patients aged 18 years or under admitted to two Philadelphia hospitals with MIS-C between April 2020 and January 2021. They compared those with outcomes in 60 age-matched healthy children who had undergone echocardiography for a range of non–COVID-related conditions such as chest pain or syncope.
The study used echocardiography, MRI, biochemistry, and functional and clinical parameters to assess the degree of change and damage to the heart at 3 months after admission.
When the patients first presented to a hospital, 42 had biochemical signs of myocardial injury, such as elevated brain-type natriuretic peptide and troponin levels. However, most patients’ symptoms were no longer present by the time they were discharged from hospital.
The researchers found that 81% of patients who presented with myocardial injury had lost the left atrial contraction phase. This dropped to 51% during the subacute phase, then 30% at 1 month. By 3-4 months, all patients achieved normal left atrial contraction phase.
At 1 month after admission, all MIS-C patients had significant signs of cardiac strain, compared with controls, including changes to global longitudinal strain, global circumferential strain, circumferential early diastolic strain rate, and right ventricular free wall longitudinal strain.
Parameters of strain normalized by 3 months
All parameters of strain had normalized, compared with controls, by 3 months. In the case of global longitudinal strain and left atrial strain, the median time to normalization was 6 days. For left ventricular ejection fraction the median time to normalization was 8 days and for right ventricular free wall longitudinal strain it was 9 days.
A small difference persisted with global longitudinal strain, but the authors said the difference was within the range of normal published values and not clinically relevant. The dysfunction appeared to be spread evenly across the heart rather than varying between segments, they noted.
“Deformation analysis could detect subtle myocardial changes; therefore, our study suggests the absence of persistent subclinical myocardial dysfunction after 3-4 months,” Dr. Matsubara said in an interview.
Four patients experienced small coronary aneurysms during the acute phase of MIS-C, but all had resolved within 2 months and none experienced any further lesions.
Among the 14 patients who underwent cardiac MRI at presentation, 2 had evidence of myocardial edema and fibrosis during the subacute phase of illness, despite having normal left ventricular systolic function and conventional echocardiography.
At follow-up, only one patient had residual edema; this individual had no evidence of fibrosis and had normal systolic function.
Study provides reassurance, but longer follow-up needed
Commenting on the study, pediatric cardiologist Devyani Chowdhury, MD, director of Cardiology Care for Children in Lancaster, Pa., said that overall it provided reassurance that most children do recover from MIS-C – and fits with her own clinical experience of the condition – but cautioned that longer-term follow-up was still needed.
“Three months is really not long term for a child,” Dr. Chowdhury said in an interview. “I’ve had a couple of patients whose MRIs have not normalized even after 1 year.”
Dr. Chowdhury also noted that it was a relatively small sample size, and it was also not yet possible to work out what host factors might play a role in increasing the risk of longer-term effects of MIS-C.
“I think it is a disease in evolution and we have to give it time, but in the very short term at least these kids are not dying, they are recovering, going home, and returning to activity and the heart is getting better,” she said.
The study authors suggested their findings could provide an evidence base for recommendations on when children with MIS-C can return to sports and physical activity, given that current consensus statements on the issue treat MIS-C as being equivalent to myocarditis in adults.
Dr. Matsubara noted that the cardiac outcomes of MIS-C were very different from those in COVID-19–affected adults, where echocardiography and MRI show longer-term evidence of myocardial impairments.
“This finding is also different from that of adult COVID-19, where the high troponin is reported to be the prognostic factor,” he said, suggesting this could explain different mechanisms of myocardial injury between MIS-C and COVID-19 myocarditis.
One author was supported by the National Institutes of Health. No conflicts of interest were declared.
While 80%-85% of children with multisystem inflammatory syndrome have cardiovascular involvement, “lack of knowledge about the short-term consequences of MIS-C has led to uncertainty among physicians in making recommendations about follow-up,” Daisuke Matsubara, MD, PhD, and colleagues wrote in their paper, which was published in the Journal of the American Heart Association.
Dr. Matsubara, of the department of pediatrics at the Children’s Hospital of Philadelphia, and colleagues examined cardiac outcomes among 60 patients aged 18 years or under admitted to two Philadelphia hospitals with MIS-C between April 2020 and January 2021. They compared those with outcomes in 60 age-matched healthy children who had undergone echocardiography for a range of non–COVID-related conditions such as chest pain or syncope.
The study used echocardiography, MRI, biochemistry, and functional and clinical parameters to assess the degree of change and damage to the heart at 3 months after admission.
When the patients first presented to a hospital, 42 had biochemical signs of myocardial injury, such as elevated brain-type natriuretic peptide and troponin levels. However, most patients’ symptoms were no longer present by the time they were discharged from hospital.
The researchers found that 81% of patients who presented with myocardial injury had lost the left atrial contraction phase. This dropped to 51% during the subacute phase, then 30% at 1 month. By 3-4 months, all patients achieved normal left atrial contraction phase.
At 1 month after admission, all MIS-C patients had significant signs of cardiac strain, compared with controls, including changes to global longitudinal strain, global circumferential strain, circumferential early diastolic strain rate, and right ventricular free wall longitudinal strain.
Parameters of strain normalized by 3 months
All parameters of strain had normalized, compared with controls, by 3 months. In the case of global longitudinal strain and left atrial strain, the median time to normalization was 6 days. For left ventricular ejection fraction the median time to normalization was 8 days and for right ventricular free wall longitudinal strain it was 9 days.
A small difference persisted with global longitudinal strain, but the authors said the difference was within the range of normal published values and not clinically relevant. The dysfunction appeared to be spread evenly across the heart rather than varying between segments, they noted.
“Deformation analysis could detect subtle myocardial changes; therefore, our study suggests the absence of persistent subclinical myocardial dysfunction after 3-4 months,” Dr. Matsubara said in an interview.
Four patients experienced small coronary aneurysms during the acute phase of MIS-C, but all had resolved within 2 months and none experienced any further lesions.
Among the 14 patients who underwent cardiac MRI at presentation, 2 had evidence of myocardial edema and fibrosis during the subacute phase of illness, despite having normal left ventricular systolic function and conventional echocardiography.
At follow-up, only one patient had residual edema; this individual had no evidence of fibrosis and had normal systolic function.
Study provides reassurance, but longer follow-up needed
Commenting on the study, pediatric cardiologist Devyani Chowdhury, MD, director of Cardiology Care for Children in Lancaster, Pa., said that overall it provided reassurance that most children do recover from MIS-C – and fits with her own clinical experience of the condition – but cautioned that longer-term follow-up was still needed.
“Three months is really not long term for a child,” Dr. Chowdhury said in an interview. “I’ve had a couple of patients whose MRIs have not normalized even after 1 year.”
Dr. Chowdhury also noted that it was a relatively small sample size, and it was also not yet possible to work out what host factors might play a role in increasing the risk of longer-term effects of MIS-C.
“I think it is a disease in evolution and we have to give it time, but in the very short term at least these kids are not dying, they are recovering, going home, and returning to activity and the heart is getting better,” she said.
The study authors suggested their findings could provide an evidence base for recommendations on when children with MIS-C can return to sports and physical activity, given that current consensus statements on the issue treat MIS-C as being equivalent to myocarditis in adults.
Dr. Matsubara noted that the cardiac outcomes of MIS-C were very different from those in COVID-19–affected adults, where echocardiography and MRI show longer-term evidence of myocardial impairments.
“This finding is also different from that of adult COVID-19, where the high troponin is reported to be the prognostic factor,” he said, suggesting this could explain different mechanisms of myocardial injury between MIS-C and COVID-19 myocarditis.
One author was supported by the National Institutes of Health. No conflicts of interest were declared.
While 80%-85% of children with multisystem inflammatory syndrome have cardiovascular involvement, “lack of knowledge about the short-term consequences of MIS-C has led to uncertainty among physicians in making recommendations about follow-up,” Daisuke Matsubara, MD, PhD, and colleagues wrote in their paper, which was published in the Journal of the American Heart Association.
Dr. Matsubara, of the department of pediatrics at the Children’s Hospital of Philadelphia, and colleagues examined cardiac outcomes among 60 patients aged 18 years or under admitted to two Philadelphia hospitals with MIS-C between April 2020 and January 2021. They compared those with outcomes in 60 age-matched healthy children who had undergone echocardiography for a range of non–COVID-related conditions such as chest pain or syncope.
The study used echocardiography, MRI, biochemistry, and functional and clinical parameters to assess the degree of change and damage to the heart at 3 months after admission.
When the patients first presented to a hospital, 42 had biochemical signs of myocardial injury, such as elevated brain-type natriuretic peptide and troponin levels. However, most patients’ symptoms were no longer present by the time they were discharged from hospital.
The researchers found that 81% of patients who presented with myocardial injury had lost the left atrial contraction phase. This dropped to 51% during the subacute phase, then 30% at 1 month. By 3-4 months, all patients achieved normal left atrial contraction phase.
At 1 month after admission, all MIS-C patients had significant signs of cardiac strain, compared with controls, including changes to global longitudinal strain, global circumferential strain, circumferential early diastolic strain rate, and right ventricular free wall longitudinal strain.
Parameters of strain normalized by 3 months
All parameters of strain had normalized, compared with controls, by 3 months. In the case of global longitudinal strain and left atrial strain, the median time to normalization was 6 days. For left ventricular ejection fraction the median time to normalization was 8 days and for right ventricular free wall longitudinal strain it was 9 days.
A small difference persisted with global longitudinal strain, but the authors said the difference was within the range of normal published values and not clinically relevant. The dysfunction appeared to be spread evenly across the heart rather than varying between segments, they noted.
“Deformation analysis could detect subtle myocardial changes; therefore, our study suggests the absence of persistent subclinical myocardial dysfunction after 3-4 months,” Dr. Matsubara said in an interview.
Four patients experienced small coronary aneurysms during the acute phase of MIS-C, but all had resolved within 2 months and none experienced any further lesions.
Among the 14 patients who underwent cardiac MRI at presentation, 2 had evidence of myocardial edema and fibrosis during the subacute phase of illness, despite having normal left ventricular systolic function and conventional echocardiography.
At follow-up, only one patient had residual edema; this individual had no evidence of fibrosis and had normal systolic function.
Study provides reassurance, but longer follow-up needed
Commenting on the study, pediatric cardiologist Devyani Chowdhury, MD, director of Cardiology Care for Children in Lancaster, Pa., said that overall it provided reassurance that most children do recover from MIS-C – and fits with her own clinical experience of the condition – but cautioned that longer-term follow-up was still needed.
“Three months is really not long term for a child,” Dr. Chowdhury said in an interview. “I’ve had a couple of patients whose MRIs have not normalized even after 1 year.”
Dr. Chowdhury also noted that it was a relatively small sample size, and it was also not yet possible to work out what host factors might play a role in increasing the risk of longer-term effects of MIS-C.
“I think it is a disease in evolution and we have to give it time, but in the very short term at least these kids are not dying, they are recovering, going home, and returning to activity and the heart is getting better,” she said.
The study authors suggested their findings could provide an evidence base for recommendations on when children with MIS-C can return to sports and physical activity, given that current consensus statements on the issue treat MIS-C as being equivalent to myocarditis in adults.
Dr. Matsubara noted that the cardiac outcomes of MIS-C were very different from those in COVID-19–affected adults, where echocardiography and MRI show longer-term evidence of myocardial impairments.
“This finding is also different from that of adult COVID-19, where the high troponin is reported to be the prognostic factor,” he said, suggesting this could explain different mechanisms of myocardial injury between MIS-C and COVID-19 myocarditis.
One author was supported by the National Institutes of Health. No conflicts of interest were declared.
FROM THE JOURNAL OF THE AMERICAN HEART ASSOCIATION
Mental health problems in kids linked with school closures
Behavior problems, anxiety, and depression in youths were associated with these individuals participating in remote schooling during broader social lockdowns in a new study.
The systematic review, which was published in JAMA Pediatrics on Jan. 18, 2022, was based on data from 36 studies from 11 countries on mental health, physical health, and well-being in children and adolescents aged 0-18 years. The total population included 79,781 children and 18,028 parents or caregivers. The studies reflected the first wave of pandemic school closures and lockdowns from February to July 2020, with the duration of school closure ranging from 1 week to 3 months.
“There are strong theoretical reasons to suggest that school closures may have contributed to a considerable proportion of the harms identified here, particularly mental health harms, through reduction in social contacts with peers and teachers,” Russell Viner, PhD, of UCL Great Ormond St Institute of Child Health, London, and colleagues wrote in their paper.
The researchers included 9 longitudinal pre-post studies, 5 cohort studies, 21 cross-sectional studies, and 1 modeling study in their analysis. Overall, approximately one-third of the studies (36%) were considered high quality, and approximately two-thirds (64%) of the studies were published in journals. Twenty-five of the reports analyzed focused on mental health and well-being.
Schools provide not only education, but also services including meals, health care, and health supplies. Schools also serve as a safety net and source of social support for children, the researchers noted.
The losses children may have experienced during school closures occurred during a time when more than 167,000 children younger than 18 years lost a parent or caregiver to COVID-19, according to a recent report titled “Hidden Pain” by researchers at the University of Pennsylvania, Nemours Children’s Health, and the COVID Collaborative. Although not addressed in the current study, school closures would prevent bereaved children from receiving social-emotional support from friends and teachers. This crisis of loss also prompted the American Academy of Pediatrics to issue a National State of Emergency in Children’s Mental Health in October 2021.
New study results
These studies identified associations between school closures during broader lockdowns and increased emotional and behavioral problems, as well as increased restlessness and inattention. Across these studies, 18%-60% of children and adolescents scored higher than the risk thresholds for diagnoses of distress, especially depressive symptoms and anxiety.
Although two studies showed no significant association with suicide in response to school closures during lockdowns, three studies suggested increased use of screen time, two studies reported increased social media use, and six studies reported lower levels of physical activity.
Three studies of child abuse showed decreases in notifications during lockdowns, likely driven by lack of referrals from schools, the authors noted. A total of 10 studies on sleep and 5 studies on diet showed inconsistent evidence of harm during the specific period of school closures and social lockdowns.
“The contrast of rises in distress with decreases in presentations suggests that there was an escalation of unmet mental health need during lockdowns in already vulnerable children and adolescents,” the researchers wrote. “More troubling still is evidence of a reduction in the ability of the health and social care systems to protect children in many countries, as shown by the large falls in child protection referrals seen in high-quality cohort studies.”
‘Study presents concrete assessments rather than speculation’
“Concerns have been widely expressed in the lay media and beyond that school closures could negatively impact the mental and physical health of children and adolescents,” M. Susan Jay, MD, of the Medical College of Wisconsin, Milwaukee, said in an interview. “The authors presented a narrative synthesis summarizing available evidence for the first wave of COVID-19 on school closures during the broader social lockdown occurring during this period.”
The “importance” of this research is that “it is not a single convenience sample study, but a systematic review from 11 countries including the United States, United Kingdom, China, and Turkey, among others, and that the quality of the information was graded,” Dr. Jay said. “Although not a meta-analysis, the study presents concrete assessments rather than speculation and overviews its limitations so that the clinician can weigh this information. Importantly, the authors excluded closure of schools with transmission of infection.
“Clearly, school lockdowns as a measure of controlling infectious disease needs balance with potential of negative health behaviors in children and adolescents. Ongoing prospective longitudinal studies are needed as sequential waves of the pandemic continue,” she emphasized.
“Clinically, this study highlights the need for clinicians to consider [asking] about the impact of school closures and remote versus hybrid versus in-person education [as part of their] patients and families question inventory,” Dr. Jay said. “Also, the use of depression inventories can be offered to youth to assess their mental health state at a visit, either via telemedicine or in person, and ideally at sequential visits for a more in-depth assessment.”
Schools play key role in social and emotional development
“It was important to conduct this study now, because this current time is unprecedented,” Peter L. Loper Jr., MD, of the University of South Carolina, Columbia, said in an interview. “We know based on evolutionary biology, anthropology, and developmental psychology, among other disciplines, that meaningful interpersonal interactions embedded in the context of community are vital to supporting human well-being.
“In our current time, the primary framework of community for our children is the school setting; it is the predominant space where they engage in the interpersonal interactions necessary for developing resilience, their sense of purpose, belonging, and fidelity,” he emphasized.
“Rarely in the course of human existence have kids been removed from the broader context of community to this extent and for this duration,” Dr. Loper said. “This study capitalizes on this unprecedented moment to begin to further understand how compromises in our sociocultural infrastructure of community, like school closures and lockdowns, may manifest as mental health problems in children and adolescents. More importantly, it contributes to the exploration of potential unintended consequences of our current infection control measures so we can adapt to support the overall well-being of our children in this ‘new normal.’ ”
Dr. Loper added that he was not surprised by the new study’s findings.
“We were already seeing a decline in pediatric mental health and overall well-being in the years preceding COVID-19 because of the ‘isolation epidemic’ involving many of the factors that this study explored,” he said. “I think this review further illustrates the vital necessity of community to support the health and well-being of humans, and specifically children and adolescents.”
From a clinical standpoint, “we need to be intentional and consistent in balancing infection control measures with our kids’ fundamental psychosocial needs,” Dr. Loper said.
“We need to recognize that, when children and adolescents are isolated from community, their fundamental psychosocial needs go unmet,” he emphasized. “If children and adolescents cannot access the meaningful interpersonal interactions necessary for resilience, then they cannot overcome or navigate distress. They will exhibit the avoidance and withdrawal behaviors that accumulate to manifest as adverse mental health symptoms like anxiety and depression.
“Additional research is needed to further explore how compromises in the psychosocial infrastructure of community manifest as downstream symptom indicators such as anxiety and depression,” which are often manifestations of unmet needs, Dr. Loper said.
Limitations and strengths, according to authors
The findings were limited by several factors, including a lack of examination of school closures’ effects on mental health independent of broader social lockdowns, according to the researchers. Other limitations included the authors potentially having missed studies, inclusion of cross-sectional studies with relatively weak evidence, potential bias from studies using parent reports, and a focus on the first COVID-19 wave, during which many school closures were of limited duration. Also, the researchers said they did not include studies focused on particular groups, such as children with learning difficulties or autism.
The use of large databases from education as well as health care in studies analyzed were strengths of the new research, they said. The investigators received no outside funding for their study. The researchers, Dr. Jay, and Dr. Loper had no financial conflicts to disclose. Dr. Jay serves on the editorial advisory board of Pediatric News.
Behavior problems, anxiety, and depression in youths were associated with these individuals participating in remote schooling during broader social lockdowns in a new study.
The systematic review, which was published in JAMA Pediatrics on Jan. 18, 2022, was based on data from 36 studies from 11 countries on mental health, physical health, and well-being in children and adolescents aged 0-18 years. The total population included 79,781 children and 18,028 parents or caregivers. The studies reflected the first wave of pandemic school closures and lockdowns from February to July 2020, with the duration of school closure ranging from 1 week to 3 months.
“There are strong theoretical reasons to suggest that school closures may have contributed to a considerable proportion of the harms identified here, particularly mental health harms, through reduction in social contacts with peers and teachers,” Russell Viner, PhD, of UCL Great Ormond St Institute of Child Health, London, and colleagues wrote in their paper.
The researchers included 9 longitudinal pre-post studies, 5 cohort studies, 21 cross-sectional studies, and 1 modeling study in their analysis. Overall, approximately one-third of the studies (36%) were considered high quality, and approximately two-thirds (64%) of the studies were published in journals. Twenty-five of the reports analyzed focused on mental health and well-being.
Schools provide not only education, but also services including meals, health care, and health supplies. Schools also serve as a safety net and source of social support for children, the researchers noted.
The losses children may have experienced during school closures occurred during a time when more than 167,000 children younger than 18 years lost a parent or caregiver to COVID-19, according to a recent report titled “Hidden Pain” by researchers at the University of Pennsylvania, Nemours Children’s Health, and the COVID Collaborative. Although not addressed in the current study, school closures would prevent bereaved children from receiving social-emotional support from friends and teachers. This crisis of loss also prompted the American Academy of Pediatrics to issue a National State of Emergency in Children’s Mental Health in October 2021.
New study results
These studies identified associations between school closures during broader lockdowns and increased emotional and behavioral problems, as well as increased restlessness and inattention. Across these studies, 18%-60% of children and adolescents scored higher than the risk thresholds for diagnoses of distress, especially depressive symptoms and anxiety.
Although two studies showed no significant association with suicide in response to school closures during lockdowns, three studies suggested increased use of screen time, two studies reported increased social media use, and six studies reported lower levels of physical activity.
Three studies of child abuse showed decreases in notifications during lockdowns, likely driven by lack of referrals from schools, the authors noted. A total of 10 studies on sleep and 5 studies on diet showed inconsistent evidence of harm during the specific period of school closures and social lockdowns.
“The contrast of rises in distress with decreases in presentations suggests that there was an escalation of unmet mental health need during lockdowns in already vulnerable children and adolescents,” the researchers wrote. “More troubling still is evidence of a reduction in the ability of the health and social care systems to protect children in many countries, as shown by the large falls in child protection referrals seen in high-quality cohort studies.”
‘Study presents concrete assessments rather than speculation’
“Concerns have been widely expressed in the lay media and beyond that school closures could negatively impact the mental and physical health of children and adolescents,” M. Susan Jay, MD, of the Medical College of Wisconsin, Milwaukee, said in an interview. “The authors presented a narrative synthesis summarizing available evidence for the first wave of COVID-19 on school closures during the broader social lockdown occurring during this period.”
The “importance” of this research is that “it is not a single convenience sample study, but a systematic review from 11 countries including the United States, United Kingdom, China, and Turkey, among others, and that the quality of the information was graded,” Dr. Jay said. “Although not a meta-analysis, the study presents concrete assessments rather than speculation and overviews its limitations so that the clinician can weigh this information. Importantly, the authors excluded closure of schools with transmission of infection.
“Clearly, school lockdowns as a measure of controlling infectious disease needs balance with potential of negative health behaviors in children and adolescents. Ongoing prospective longitudinal studies are needed as sequential waves of the pandemic continue,” she emphasized.
“Clinically, this study highlights the need for clinicians to consider [asking] about the impact of school closures and remote versus hybrid versus in-person education [as part of their] patients and families question inventory,” Dr. Jay said. “Also, the use of depression inventories can be offered to youth to assess their mental health state at a visit, either via telemedicine or in person, and ideally at sequential visits for a more in-depth assessment.”
Schools play key role in social and emotional development
“It was important to conduct this study now, because this current time is unprecedented,” Peter L. Loper Jr., MD, of the University of South Carolina, Columbia, said in an interview. “We know based on evolutionary biology, anthropology, and developmental psychology, among other disciplines, that meaningful interpersonal interactions embedded in the context of community are vital to supporting human well-being.
“In our current time, the primary framework of community for our children is the school setting; it is the predominant space where they engage in the interpersonal interactions necessary for developing resilience, their sense of purpose, belonging, and fidelity,” he emphasized.
“Rarely in the course of human existence have kids been removed from the broader context of community to this extent and for this duration,” Dr. Loper said. “This study capitalizes on this unprecedented moment to begin to further understand how compromises in our sociocultural infrastructure of community, like school closures and lockdowns, may manifest as mental health problems in children and adolescents. More importantly, it contributes to the exploration of potential unintended consequences of our current infection control measures so we can adapt to support the overall well-being of our children in this ‘new normal.’ ”
Dr. Loper added that he was not surprised by the new study’s findings.
“We were already seeing a decline in pediatric mental health and overall well-being in the years preceding COVID-19 because of the ‘isolation epidemic’ involving many of the factors that this study explored,” he said. “I think this review further illustrates the vital necessity of community to support the health and well-being of humans, and specifically children and adolescents.”
From a clinical standpoint, “we need to be intentional and consistent in balancing infection control measures with our kids’ fundamental psychosocial needs,” Dr. Loper said.
“We need to recognize that, when children and adolescents are isolated from community, their fundamental psychosocial needs go unmet,” he emphasized. “If children and adolescents cannot access the meaningful interpersonal interactions necessary for resilience, then they cannot overcome or navigate distress. They will exhibit the avoidance and withdrawal behaviors that accumulate to manifest as adverse mental health symptoms like anxiety and depression.
“Additional research is needed to further explore how compromises in the psychosocial infrastructure of community manifest as downstream symptom indicators such as anxiety and depression,” which are often manifestations of unmet needs, Dr. Loper said.
Limitations and strengths, according to authors
The findings were limited by several factors, including a lack of examination of school closures’ effects on mental health independent of broader social lockdowns, according to the researchers. Other limitations included the authors potentially having missed studies, inclusion of cross-sectional studies with relatively weak evidence, potential bias from studies using parent reports, and a focus on the first COVID-19 wave, during which many school closures were of limited duration. Also, the researchers said they did not include studies focused on particular groups, such as children with learning difficulties or autism.
The use of large databases from education as well as health care in studies analyzed were strengths of the new research, they said. The investigators received no outside funding for their study. The researchers, Dr. Jay, and Dr. Loper had no financial conflicts to disclose. Dr. Jay serves on the editorial advisory board of Pediatric News.
Behavior problems, anxiety, and depression in youths were associated with these individuals participating in remote schooling during broader social lockdowns in a new study.
The systematic review, which was published in JAMA Pediatrics on Jan. 18, 2022, was based on data from 36 studies from 11 countries on mental health, physical health, and well-being in children and adolescents aged 0-18 years. The total population included 79,781 children and 18,028 parents or caregivers. The studies reflected the first wave of pandemic school closures and lockdowns from February to July 2020, with the duration of school closure ranging from 1 week to 3 months.
“There are strong theoretical reasons to suggest that school closures may have contributed to a considerable proportion of the harms identified here, particularly mental health harms, through reduction in social contacts with peers and teachers,” Russell Viner, PhD, of UCL Great Ormond St Institute of Child Health, London, and colleagues wrote in their paper.
The researchers included 9 longitudinal pre-post studies, 5 cohort studies, 21 cross-sectional studies, and 1 modeling study in their analysis. Overall, approximately one-third of the studies (36%) were considered high quality, and approximately two-thirds (64%) of the studies were published in journals. Twenty-five of the reports analyzed focused on mental health and well-being.
Schools provide not only education, but also services including meals, health care, and health supplies. Schools also serve as a safety net and source of social support for children, the researchers noted.
The losses children may have experienced during school closures occurred during a time when more than 167,000 children younger than 18 years lost a parent or caregiver to COVID-19, according to a recent report titled “Hidden Pain” by researchers at the University of Pennsylvania, Nemours Children’s Health, and the COVID Collaborative. Although not addressed in the current study, school closures would prevent bereaved children from receiving social-emotional support from friends and teachers. This crisis of loss also prompted the American Academy of Pediatrics to issue a National State of Emergency in Children’s Mental Health in October 2021.
New study results
These studies identified associations between school closures during broader lockdowns and increased emotional and behavioral problems, as well as increased restlessness and inattention. Across these studies, 18%-60% of children and adolescents scored higher than the risk thresholds for diagnoses of distress, especially depressive symptoms and anxiety.
Although two studies showed no significant association with suicide in response to school closures during lockdowns, three studies suggested increased use of screen time, two studies reported increased social media use, and six studies reported lower levels of physical activity.
Three studies of child abuse showed decreases in notifications during lockdowns, likely driven by lack of referrals from schools, the authors noted. A total of 10 studies on sleep and 5 studies on diet showed inconsistent evidence of harm during the specific period of school closures and social lockdowns.
“The contrast of rises in distress with decreases in presentations suggests that there was an escalation of unmet mental health need during lockdowns in already vulnerable children and adolescents,” the researchers wrote. “More troubling still is evidence of a reduction in the ability of the health and social care systems to protect children in many countries, as shown by the large falls in child protection referrals seen in high-quality cohort studies.”
‘Study presents concrete assessments rather than speculation’
“Concerns have been widely expressed in the lay media and beyond that school closures could negatively impact the mental and physical health of children and adolescents,” M. Susan Jay, MD, of the Medical College of Wisconsin, Milwaukee, said in an interview. “The authors presented a narrative synthesis summarizing available evidence for the first wave of COVID-19 on school closures during the broader social lockdown occurring during this period.”
The “importance” of this research is that “it is not a single convenience sample study, but a systematic review from 11 countries including the United States, United Kingdom, China, and Turkey, among others, and that the quality of the information was graded,” Dr. Jay said. “Although not a meta-analysis, the study presents concrete assessments rather than speculation and overviews its limitations so that the clinician can weigh this information. Importantly, the authors excluded closure of schools with transmission of infection.
“Clearly, school lockdowns as a measure of controlling infectious disease needs balance with potential of negative health behaviors in children and adolescents. Ongoing prospective longitudinal studies are needed as sequential waves of the pandemic continue,” she emphasized.
“Clinically, this study highlights the need for clinicians to consider [asking] about the impact of school closures and remote versus hybrid versus in-person education [as part of their] patients and families question inventory,” Dr. Jay said. “Also, the use of depression inventories can be offered to youth to assess their mental health state at a visit, either via telemedicine or in person, and ideally at sequential visits for a more in-depth assessment.”
Schools play key role in social and emotional development
“It was important to conduct this study now, because this current time is unprecedented,” Peter L. Loper Jr., MD, of the University of South Carolina, Columbia, said in an interview. “We know based on evolutionary biology, anthropology, and developmental psychology, among other disciplines, that meaningful interpersonal interactions embedded in the context of community are vital to supporting human well-being.
“In our current time, the primary framework of community for our children is the school setting; it is the predominant space where they engage in the interpersonal interactions necessary for developing resilience, their sense of purpose, belonging, and fidelity,” he emphasized.
“Rarely in the course of human existence have kids been removed from the broader context of community to this extent and for this duration,” Dr. Loper said. “This study capitalizes on this unprecedented moment to begin to further understand how compromises in our sociocultural infrastructure of community, like school closures and lockdowns, may manifest as mental health problems in children and adolescents. More importantly, it contributes to the exploration of potential unintended consequences of our current infection control measures so we can adapt to support the overall well-being of our children in this ‘new normal.’ ”
Dr. Loper added that he was not surprised by the new study’s findings.
“We were already seeing a decline in pediatric mental health and overall well-being in the years preceding COVID-19 because of the ‘isolation epidemic’ involving many of the factors that this study explored,” he said. “I think this review further illustrates the vital necessity of community to support the health and well-being of humans, and specifically children and adolescents.”
From a clinical standpoint, “we need to be intentional and consistent in balancing infection control measures with our kids’ fundamental psychosocial needs,” Dr. Loper said.
“We need to recognize that, when children and adolescents are isolated from community, their fundamental psychosocial needs go unmet,” he emphasized. “If children and adolescents cannot access the meaningful interpersonal interactions necessary for resilience, then they cannot overcome or navigate distress. They will exhibit the avoidance and withdrawal behaviors that accumulate to manifest as adverse mental health symptoms like anxiety and depression.
“Additional research is needed to further explore how compromises in the psychosocial infrastructure of community manifest as downstream symptom indicators such as anxiety and depression,” which are often manifestations of unmet needs, Dr. Loper said.
Limitations and strengths, according to authors
The findings were limited by several factors, including a lack of examination of school closures’ effects on mental health independent of broader social lockdowns, according to the researchers. Other limitations included the authors potentially having missed studies, inclusion of cross-sectional studies with relatively weak evidence, potential bias from studies using parent reports, and a focus on the first COVID-19 wave, during which many school closures were of limited duration. Also, the researchers said they did not include studies focused on particular groups, such as children with learning difficulties or autism.
The use of large databases from education as well as health care in studies analyzed were strengths of the new research, they said. The investigators received no outside funding for their study. The researchers, Dr. Jay, and Dr. Loper had no financial conflicts to disclose. Dr. Jay serves on the editorial advisory board of Pediatric News.
FROM JAMA PEDIATRICS
Study finds genetic factor for COVID smell and taste loss
, according to a new study published in the journal Nature Genetics
The finding could eventually help the 1.6 million people in the United States who still can’t smell or have had a change in their ability to smell more than 6 months after getting the coronavirus. The exact cause related to COVID-19 is still unknown, but researchers believe it could be because of damage in a part of the nose called the olfactory epithelium.
“How we get from infection to smell loss remains unclear,” Justin Turner, MD, an associate professor of otolaryngology at Vanderbilt University, Nashville, Tenn., told NBC News. Dr. Turner was not part of the research team.
“Early data suggest that supporting cells of the olfactory epithelium are the ones mostly being infected by the virus, and presumably this leads to the death of the neurons themselves,” he said. “But we don’t really, really know why and when that happens, and why it seems to preferentially happen in certain individuals.”
Researchers at 23andMe, a genomics and biotechnology company, did the study as part of a larger COVID-19 project, which includes people in the United States and the United Kingdom. They analyzed data from nearly 70,000 people who took online surveys after receiving a positive coronavirus test. Among those, 68% reported a loss of smell or taste as a symptom.
The study team compared the genetic differences between those who lost their sense of smell and taste and those who didn’t. They found that a location near two olfactory genes – UGT2A1 and UGT2A2 – is associated with COVID-19 loss of smell and taste. The genetic risk factor makes it 11% more likely for a person with COVID-19 to lose their sense of smell or taste.
The research team also found that women were 11% more likely than men to report a loss of smell and taste. About 73% of those who reported a loss of smell and taste were ages 26-35.
The researchers aren’t sure how the genes are involved, though they suspect that infected cells could lead to smell loss. Typically, the genes are expressed in tissue inside the nose involved with smell and play a role in processing things that have an odor. To use the findings, researchers need to learn more about the genes, how they are expressed, and what their functions are, NBC News reported.
The findings could help lead to treatments. Other research has shown that the loss of taste and smell is related to a “failure to protect the sensory cells of the nose and tongue from viral infection,” Danielle Reed, PhD, associate director of the Monell Chemical Senses Center in Philadelphia, told NBC News. She was not part of the research team but studies person-to-person differences in the loss of these senses because of COVID-19.
“This study suggests a different direction,” she said. “The pathways that break down the chemicals that cause taste and smell in the first place might be over or underactive, reducing or distorting the ability to taste and smell.”
A version of this article first appeared on WebMD.com.
, according to a new study published in the journal Nature Genetics
The finding could eventually help the 1.6 million people in the United States who still can’t smell or have had a change in their ability to smell more than 6 months after getting the coronavirus. The exact cause related to COVID-19 is still unknown, but researchers believe it could be because of damage in a part of the nose called the olfactory epithelium.
“How we get from infection to smell loss remains unclear,” Justin Turner, MD, an associate professor of otolaryngology at Vanderbilt University, Nashville, Tenn., told NBC News. Dr. Turner was not part of the research team.
“Early data suggest that supporting cells of the olfactory epithelium are the ones mostly being infected by the virus, and presumably this leads to the death of the neurons themselves,” he said. “But we don’t really, really know why and when that happens, and why it seems to preferentially happen in certain individuals.”
Researchers at 23andMe, a genomics and biotechnology company, did the study as part of a larger COVID-19 project, which includes people in the United States and the United Kingdom. They analyzed data from nearly 70,000 people who took online surveys after receiving a positive coronavirus test. Among those, 68% reported a loss of smell or taste as a symptom.
The study team compared the genetic differences between those who lost their sense of smell and taste and those who didn’t. They found that a location near two olfactory genes – UGT2A1 and UGT2A2 – is associated with COVID-19 loss of smell and taste. The genetic risk factor makes it 11% more likely for a person with COVID-19 to lose their sense of smell or taste.
The research team also found that women were 11% more likely than men to report a loss of smell and taste. About 73% of those who reported a loss of smell and taste were ages 26-35.
The researchers aren’t sure how the genes are involved, though they suspect that infected cells could lead to smell loss. Typically, the genes are expressed in tissue inside the nose involved with smell and play a role in processing things that have an odor. To use the findings, researchers need to learn more about the genes, how they are expressed, and what their functions are, NBC News reported.
The findings could help lead to treatments. Other research has shown that the loss of taste and smell is related to a “failure to protect the sensory cells of the nose and tongue from viral infection,” Danielle Reed, PhD, associate director of the Monell Chemical Senses Center in Philadelphia, told NBC News. She was not part of the research team but studies person-to-person differences in the loss of these senses because of COVID-19.
“This study suggests a different direction,” she said. “The pathways that break down the chemicals that cause taste and smell in the first place might be over or underactive, reducing or distorting the ability to taste and smell.”
A version of this article first appeared on WebMD.com.
, according to a new study published in the journal Nature Genetics
The finding could eventually help the 1.6 million people in the United States who still can’t smell or have had a change in their ability to smell more than 6 months after getting the coronavirus. The exact cause related to COVID-19 is still unknown, but researchers believe it could be because of damage in a part of the nose called the olfactory epithelium.
“How we get from infection to smell loss remains unclear,” Justin Turner, MD, an associate professor of otolaryngology at Vanderbilt University, Nashville, Tenn., told NBC News. Dr. Turner was not part of the research team.
“Early data suggest that supporting cells of the olfactory epithelium are the ones mostly being infected by the virus, and presumably this leads to the death of the neurons themselves,” he said. “But we don’t really, really know why and when that happens, and why it seems to preferentially happen in certain individuals.”
Researchers at 23andMe, a genomics and biotechnology company, did the study as part of a larger COVID-19 project, which includes people in the United States and the United Kingdom. They analyzed data from nearly 70,000 people who took online surveys after receiving a positive coronavirus test. Among those, 68% reported a loss of smell or taste as a symptom.
The study team compared the genetic differences between those who lost their sense of smell and taste and those who didn’t. They found that a location near two olfactory genes – UGT2A1 and UGT2A2 – is associated with COVID-19 loss of smell and taste. The genetic risk factor makes it 11% more likely for a person with COVID-19 to lose their sense of smell or taste.
The research team also found that women were 11% more likely than men to report a loss of smell and taste. About 73% of those who reported a loss of smell and taste were ages 26-35.
The researchers aren’t sure how the genes are involved, though they suspect that infected cells could lead to smell loss. Typically, the genes are expressed in tissue inside the nose involved with smell and play a role in processing things that have an odor. To use the findings, researchers need to learn more about the genes, how they are expressed, and what their functions are, NBC News reported.
The findings could help lead to treatments. Other research has shown that the loss of taste and smell is related to a “failure to protect the sensory cells of the nose and tongue from viral infection,” Danielle Reed, PhD, associate director of the Monell Chemical Senses Center in Philadelphia, told NBC News. She was not part of the research team but studies person-to-person differences in the loss of these senses because of COVID-19.
“This study suggests a different direction,” she said. “The pathways that break down the chemicals that cause taste and smell in the first place might be over or underactive, reducing or distorting the ability to taste and smell.”
A version of this article first appeared on WebMD.com.
FROM NATURE GENETICS
Fourth vaccine shot less effective against Omicron, Israeli study says
, according to new research at an Israeli hospital.
The preliminary results, released on Jan. 17, challenge the idea of giving a second booster dose to slow the spread of the coronavirus, according to USA Today.
“Despite increased antibody levels, the fourth vaccine only offers a partial defense against the virus,” Gili Regev-Yochay, MD, director of the hospital’s infection prevention and control units, told reporters.
“The vaccines, which were more effective against previous variants, offer less protection versus Omicron,” she said.
In a clinical trial, 274 medical workers at Sheba Medical Center near Tel Aviv received a fourth vaccine dose in December – 154 got the Pfizer vaccine and 120 got the Moderna vaccine – after previously getting three Pfizer shots.
Both groups received a boost in antibodies that was “slightly higher” than after the third shot, Dr. Regev-Yochay said. But when compared with a control group that didn’t receive the fourth dose, the extra boost didn’t prevent the spread of Omicron.
“We see many infected with Omicron who received the fourth dose,” Dr. Regev-Yochay said. “Granted, a bit less than in the control group, but still a lot of infections.”
Some public health officials in Israel say the campaign for fourth doses is still worthwhile, according to The Times of Israel. The vaccine still works well against the Alpha and Delta variants, Dr. Regev-Yochay said, and a fourth shot should go to older adults and those who face higher risks for severe COVID-19.
Hours after releasing the preliminary results, Sheba Medical Center published a statement calling for “continuing the vaccination drive for risk groups at this time, even though the vaccine doesn’t provide optimal protection against getting infected with the variant.” News outlets reported that the hospital was pressured into issuing the statement after Israel’s Health Ministry didn’t like the release of the early study results, The Times of Israel reported.
The second booster “returns the level of antibodies to what it was at the beginning of the third booster,” Nachman Ash, MD, director of Israel’s Health Ministry, told Channel 13 TV in Israel, according to The Associated Press.
“That has great importance, especially among the older population,” he said.
As of Sunday, more than 500,000 people in Israel had received fourth doses since the country began offering them last month to medical workers, immunocompromised patients, and people ages 60 years and older, the AP reported. At the same time, the country has faced a recent coronavirus surge that has led to record-breaking numbers of cases and rising hospitalizations.
On Tuesday, the Israeli government said it would shorten the mandatory quarantine period from 7 days to 5 days, the AP reported.
“This decision will enable us to continue safeguarding public health on the one hand and to keep the economy going at this time on the other, even though it is difficult, so that we can get through this wave safely,” Prime Minister Naftali Bennett said.
A version of this article first appeared on WebMD.com.
, according to new research at an Israeli hospital.
The preliminary results, released on Jan. 17, challenge the idea of giving a second booster dose to slow the spread of the coronavirus, according to USA Today.
“Despite increased antibody levels, the fourth vaccine only offers a partial defense against the virus,” Gili Regev-Yochay, MD, director of the hospital’s infection prevention and control units, told reporters.
“The vaccines, which were more effective against previous variants, offer less protection versus Omicron,” she said.
In a clinical trial, 274 medical workers at Sheba Medical Center near Tel Aviv received a fourth vaccine dose in December – 154 got the Pfizer vaccine and 120 got the Moderna vaccine – after previously getting three Pfizer shots.
Both groups received a boost in antibodies that was “slightly higher” than after the third shot, Dr. Regev-Yochay said. But when compared with a control group that didn’t receive the fourth dose, the extra boost didn’t prevent the spread of Omicron.
“We see many infected with Omicron who received the fourth dose,” Dr. Regev-Yochay said. “Granted, a bit less than in the control group, but still a lot of infections.”
Some public health officials in Israel say the campaign for fourth doses is still worthwhile, according to The Times of Israel. The vaccine still works well against the Alpha and Delta variants, Dr. Regev-Yochay said, and a fourth shot should go to older adults and those who face higher risks for severe COVID-19.
Hours after releasing the preliminary results, Sheba Medical Center published a statement calling for “continuing the vaccination drive for risk groups at this time, even though the vaccine doesn’t provide optimal protection against getting infected with the variant.” News outlets reported that the hospital was pressured into issuing the statement after Israel’s Health Ministry didn’t like the release of the early study results, The Times of Israel reported.
The second booster “returns the level of antibodies to what it was at the beginning of the third booster,” Nachman Ash, MD, director of Israel’s Health Ministry, told Channel 13 TV in Israel, according to The Associated Press.
“That has great importance, especially among the older population,” he said.
As of Sunday, more than 500,000 people in Israel had received fourth doses since the country began offering them last month to medical workers, immunocompromised patients, and people ages 60 years and older, the AP reported. At the same time, the country has faced a recent coronavirus surge that has led to record-breaking numbers of cases and rising hospitalizations.
On Tuesday, the Israeli government said it would shorten the mandatory quarantine period from 7 days to 5 days, the AP reported.
“This decision will enable us to continue safeguarding public health on the one hand and to keep the economy going at this time on the other, even though it is difficult, so that we can get through this wave safely,” Prime Minister Naftali Bennett said.
A version of this article first appeared on WebMD.com.
, according to new research at an Israeli hospital.
The preliminary results, released on Jan. 17, challenge the idea of giving a second booster dose to slow the spread of the coronavirus, according to USA Today.
“Despite increased antibody levels, the fourth vaccine only offers a partial defense against the virus,” Gili Regev-Yochay, MD, director of the hospital’s infection prevention and control units, told reporters.
“The vaccines, which were more effective against previous variants, offer less protection versus Omicron,” she said.
In a clinical trial, 274 medical workers at Sheba Medical Center near Tel Aviv received a fourth vaccine dose in December – 154 got the Pfizer vaccine and 120 got the Moderna vaccine – after previously getting three Pfizer shots.
Both groups received a boost in antibodies that was “slightly higher” than after the third shot, Dr. Regev-Yochay said. But when compared with a control group that didn’t receive the fourth dose, the extra boost didn’t prevent the spread of Omicron.
“We see many infected with Omicron who received the fourth dose,” Dr. Regev-Yochay said. “Granted, a bit less than in the control group, but still a lot of infections.”
Some public health officials in Israel say the campaign for fourth doses is still worthwhile, according to The Times of Israel. The vaccine still works well against the Alpha and Delta variants, Dr. Regev-Yochay said, and a fourth shot should go to older adults and those who face higher risks for severe COVID-19.
Hours after releasing the preliminary results, Sheba Medical Center published a statement calling for “continuing the vaccination drive for risk groups at this time, even though the vaccine doesn’t provide optimal protection against getting infected with the variant.” News outlets reported that the hospital was pressured into issuing the statement after Israel’s Health Ministry didn’t like the release of the early study results, The Times of Israel reported.
The second booster “returns the level of antibodies to what it was at the beginning of the third booster,” Nachman Ash, MD, director of Israel’s Health Ministry, told Channel 13 TV in Israel, according to The Associated Press.
“That has great importance, especially among the older population,” he said.
As of Sunday, more than 500,000 people in Israel had received fourth doses since the country began offering them last month to medical workers, immunocompromised patients, and people ages 60 years and older, the AP reported. At the same time, the country has faced a recent coronavirus surge that has led to record-breaking numbers of cases and rising hospitalizations.
On Tuesday, the Israeli government said it would shorten the mandatory quarantine period from 7 days to 5 days, the AP reported.
“This decision will enable us to continue safeguarding public health on the one hand and to keep the economy going at this time on the other, even though it is difficult, so that we can get through this wave safely,” Prime Minister Naftali Bennett said.
A version of this article first appeared on WebMD.com.