User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
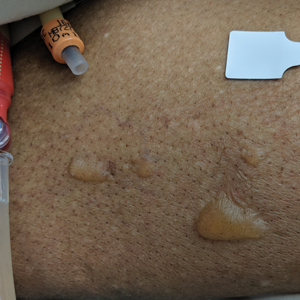
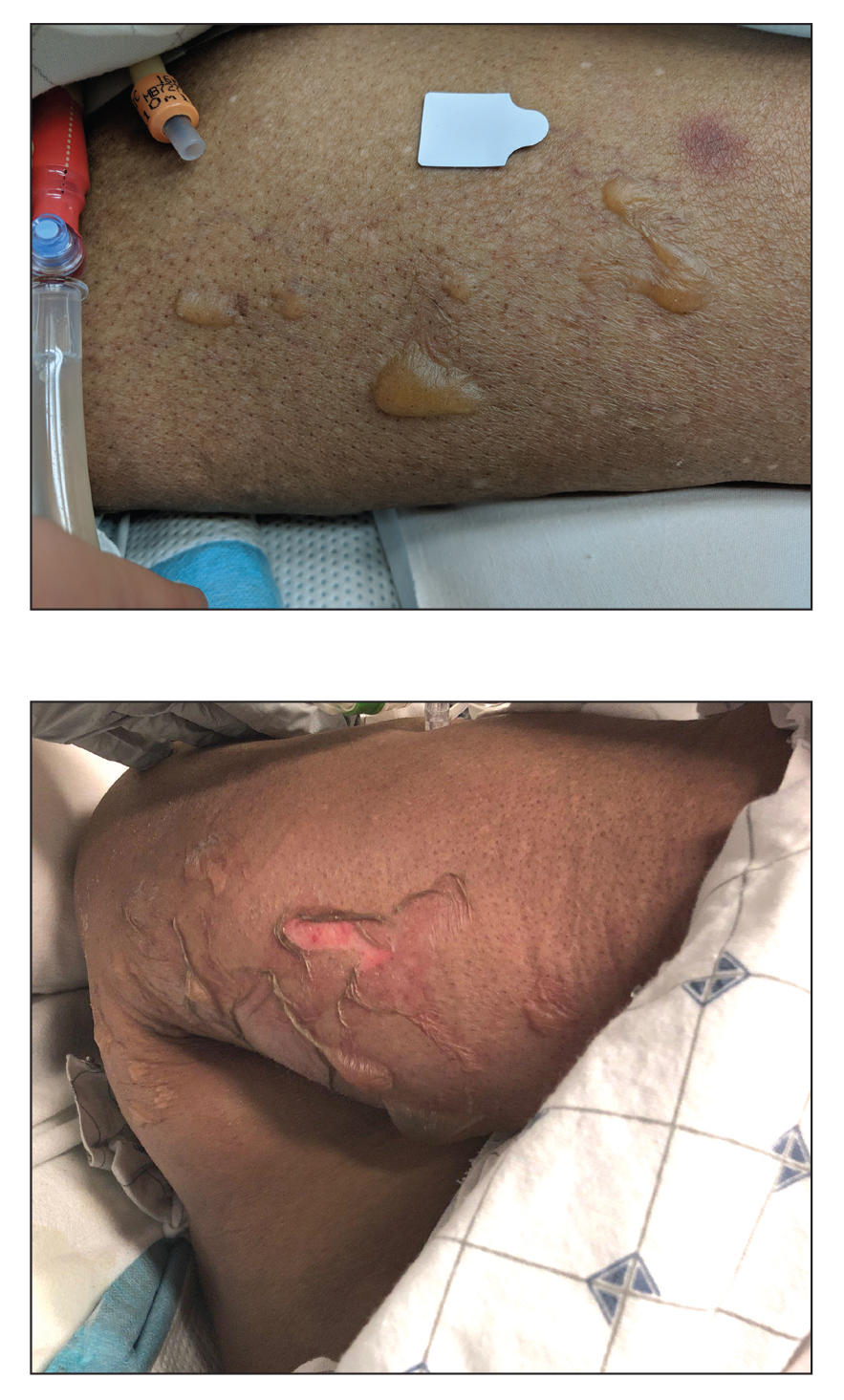
Blisters in a Comatose Elderly Woman
The Diagnosis: Coma Blisters
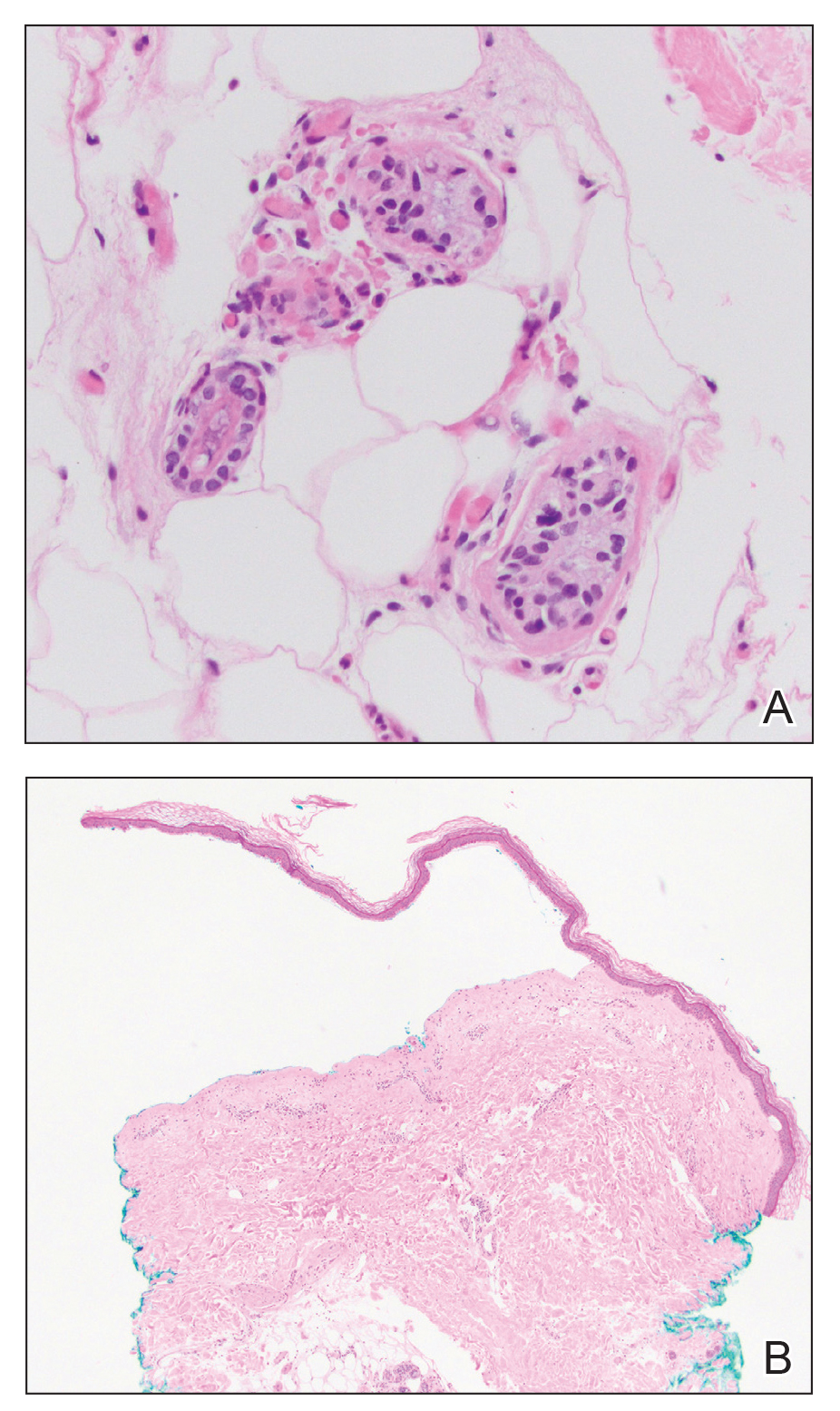
Histologic examination revealed pauci-inflammatory subepidermal blisters with swelling of eccrine cells, signaling impending gland necrosis (Figure). Direct immunofluorescence testing on perilesional skin was negative. These findings would be inconsistent for diagnoses of edema blisters (most commonly seen in patients with an acute exacerbation of chronic lower extremity edema), friction blisters (intraepidermal blisters seen on histopathology), and bullous pemphigoid (linear IgG and/or C3 staining along the basement membrane zone on direct immunofluorescence testing is characteristic). Although eccrine gland alterations have been seen in toxic epidermal necrolysis,1 the mucous membranes are involved in more than 90% of cases, making the diagnosis less likely. Furthermore, interface changes including prominent keratinocyte necrosis were not seen on histology.
Given the localized nature of the lesions in our patient and negative direct immunofluorescence studies, a diagnosis of coma blisters was made. Gentle wound care practices to the areas of denuded skin were implemented with complete resolution. The patient’s condition gradually improved, and she was extubated and discharged home.
Coma blisters are self-limited bullous lesions that have been reported in comatose patients as early as 1812 when Napoleon’s surgeon first noticed cutaneous blisters in comatose French soldiers being treated for carbon monoxide intoxication.2 Since then, barbiturate overdose has remained the most common association, but coma blisters have occurred in the absence of specific drug exposures. Clinically, erythematous or violaceous plaques typically appear within 24 hours of drug ingestion, and progression to large tense bullae usually occurs within 48 to 72 hours of unconsciousness.3 They characteristically occur in pressure-dependent areas, but reports have shown lesions in non–pressure-dependent areas, including the penis and mouth.1,4 Spontaneous resolution within 1 to 2 weeks is typical.5
The underlying pathogenesis remains controversial, as multiple mechanisms have been suggested, but clear causal evidence is lacking. The original proposition that direct effects of drug toxicity caused the cutaneous observations was later refuted after similar bullous lesions with eccrine gland necrosis were reported in comatose patients with neurologic conditions.6 It is largely accepted that pressure-induced local ischemia—proportional to the duration and amount of pressure—leads to tissue injury and is critical to the pathogenesis. During periods of ischemia, the most metabolically active tissues will undergo necrosis first; however, in eccrine glands, the earliest and most severe damage does not seem to occur in the most metabolically active cells.7 Additionally, this would not provide a viable explanation for coma blisters with eccrine gland necrosis developing in variable non–pressuredependent areas.
Moreover, drug- and non–drug-induced coma blisters can appear identically, but specific histopathologic differences have been reported. The most notable markers of non–drug-induced coma blisters are the absence of an inflammatory infiltrate in the epidermis and the presence of thrombosis in dermal vessels.8 Demonstration of necrotic changes in the secretory portion of the eccrine gland is considered the histopathologic hallmark for drug-induced coma blisters, but other findings can include subepidermal or intraepidermal bullae; perivascular infiltrates; and focal necrosis of the epidermis, dermis, subcutis, or epidermal appendages.6 Arteriolar wall necrosis and dermal inflammatory infiltrates also have been observed.7
Benzodiazepines have been widely prescribed and abused since their development, and overdose is much more common today than with barbiturates.9 Coma blisters rarely have been documented in the setting of isolated benzodiazepine overdose, and of the few cases, only one report implicated lorazepam as the causative agent.4,7 The characteristic finding of eccrine gland necrosis consistently was seen in our patient. This case not only emphasizes the need for greater awareness of the association between benzodiazepine overdose and coma blisters but also the importance of clinical context when considering diagnoses. It is essential to note that coma blisters themselves are nonspecific, and the diagnosis of drug-induced coma blisters warrants confirmatory toxicologic analysis.
- Ferreli C, Sulica VI, Aste N, et al. Drug-induced sweat gland necrosis in a non-comatose patient: a case presentation. J Eur Acad Dermatol Venereol. 2003;17:443-445.
- Larrey DJ. Memoires de Chirurgie Militaire et Campagnes. Smith and Buisson; 1812.
- Agarwal A, Bansal M, Conner K. Coma blisters with hypoxemic respiratory failure. Dermatol Online J. 2012;18:10.
- Varma AJ, Fisher BK, Sarin MK. Diazepam-induced coma with bullae and eccrine sweat gland necrosis. Arch Intern Med. 1977;137:1207-1210.
- Rocha J, Pereira T, Ventura F, et al. Coma blisters. Case Rep Dermatol. 2009;1:66-70.
- Arndt KA, Mihm MC, Parrish JA. Bullae: a cutaneous sign of a variety of neurologic diseases. J Invest Dermatol. 1973;60:312-320.
- Sánchez Yus E, Requena L, Simón P. Histopathology of cutaneous changes in drug-induced coma. Am J Dermatopathol. 1993;15:208-216.
- Kato N, Ueno H, Mimura M. Histopathology of cutaneous changes in non-drug-induced coma. Am J Dermatopathol. 1996;18:344-350.
- Kang M, Ghassemzadeh S. Benzodiazepine Toxicity. StatPearls Publishing; 2018.
The Diagnosis: Coma Blisters
Histologic examination revealed pauci-inflammatory subepidermal blisters with swelling of eccrine cells, signaling impending gland necrosis (Figure). Direct immunofluorescence testing on perilesional skin was negative. These findings would be inconsistent for diagnoses of edema blisters (most commonly seen in patients with an acute exacerbation of chronic lower extremity edema), friction blisters (intraepidermal blisters seen on histopathology), and bullous pemphigoid (linear IgG and/or C3 staining along the basement membrane zone on direct immunofluorescence testing is characteristic). Although eccrine gland alterations have been seen in toxic epidermal necrolysis,1 the mucous membranes are involved in more than 90% of cases, making the diagnosis less likely. Furthermore, interface changes including prominent keratinocyte necrosis were not seen on histology.

Given the localized nature of the lesions in our patient and negative direct immunofluorescence studies, a diagnosis of coma blisters was made. Gentle wound care practices to the areas of denuded skin were implemented with complete resolution. The patient’s condition gradually improved, and she was extubated and discharged home.
Coma blisters are self-limited bullous lesions that have been reported in comatose patients as early as 1812 when Napoleon’s surgeon first noticed cutaneous blisters in comatose French soldiers being treated for carbon monoxide intoxication.2 Since then, barbiturate overdose has remained the most common association, but coma blisters have occurred in the absence of specific drug exposures. Clinically, erythematous or violaceous plaques typically appear within 24 hours of drug ingestion, and progression to large tense bullae usually occurs within 48 to 72 hours of unconsciousness.3 They characteristically occur in pressure-dependent areas, but reports have shown lesions in non–pressure-dependent areas, including the penis and mouth.1,4 Spontaneous resolution within 1 to 2 weeks is typical.5
The underlying pathogenesis remains controversial, as multiple mechanisms have been suggested, but clear causal evidence is lacking. The original proposition that direct effects of drug toxicity caused the cutaneous observations was later refuted after similar bullous lesions with eccrine gland necrosis were reported in comatose patients with neurologic conditions.6 It is largely accepted that pressure-induced local ischemia—proportional to the duration and amount of pressure—leads to tissue injury and is critical to the pathogenesis. During periods of ischemia, the most metabolically active tissues will undergo necrosis first; however, in eccrine glands, the earliest and most severe damage does not seem to occur in the most metabolically active cells.7 Additionally, this would not provide a viable explanation for coma blisters with eccrine gland necrosis developing in variable non–pressuredependent areas.
Moreover, drug- and non–drug-induced coma blisters can appear identically, but specific histopathologic differences have been reported. The most notable markers of non–drug-induced coma blisters are the absence of an inflammatory infiltrate in the epidermis and the presence of thrombosis in dermal vessels.8 Demonstration of necrotic changes in the secretory portion of the eccrine gland is considered the histopathologic hallmark for drug-induced coma blisters, but other findings can include subepidermal or intraepidermal bullae; perivascular infiltrates; and focal necrosis of the epidermis, dermis, subcutis, or epidermal appendages.6 Arteriolar wall necrosis and dermal inflammatory infiltrates also have been observed.7
Benzodiazepines have been widely prescribed and abused since their development, and overdose is much more common today than with barbiturates.9 Coma blisters rarely have been documented in the setting of isolated benzodiazepine overdose, and of the few cases, only one report implicated lorazepam as the causative agent.4,7 The characteristic finding of eccrine gland necrosis consistently was seen in our patient. This case not only emphasizes the need for greater awareness of the association between benzodiazepine overdose and coma blisters but also the importance of clinical context when considering diagnoses. It is essential to note that coma blisters themselves are nonspecific, and the diagnosis of drug-induced coma blisters warrants confirmatory toxicologic analysis.
The Diagnosis: Coma Blisters
Histologic examination revealed pauci-inflammatory subepidermal blisters with swelling of eccrine cells, signaling impending gland necrosis (Figure). Direct immunofluorescence testing on perilesional skin was negative. These findings would be inconsistent for diagnoses of edema blisters (most commonly seen in patients with an acute exacerbation of chronic lower extremity edema), friction blisters (intraepidermal blisters seen on histopathology), and bullous pemphigoid (linear IgG and/or C3 staining along the basement membrane zone on direct immunofluorescence testing is characteristic). Although eccrine gland alterations have been seen in toxic epidermal necrolysis,1 the mucous membranes are involved in more than 90% of cases, making the diagnosis less likely. Furthermore, interface changes including prominent keratinocyte necrosis were not seen on histology.

Given the localized nature of the lesions in our patient and negative direct immunofluorescence studies, a diagnosis of coma blisters was made. Gentle wound care practices to the areas of denuded skin were implemented with complete resolution. The patient’s condition gradually improved, and she was extubated and discharged home.
Coma blisters are self-limited bullous lesions that have been reported in comatose patients as early as 1812 when Napoleon’s surgeon first noticed cutaneous blisters in comatose French soldiers being treated for carbon monoxide intoxication.2 Since then, barbiturate overdose has remained the most common association, but coma blisters have occurred in the absence of specific drug exposures. Clinically, erythematous or violaceous plaques typically appear within 24 hours of drug ingestion, and progression to large tense bullae usually occurs within 48 to 72 hours of unconsciousness.3 They characteristically occur in pressure-dependent areas, but reports have shown lesions in non–pressure-dependent areas, including the penis and mouth.1,4 Spontaneous resolution within 1 to 2 weeks is typical.5
The underlying pathogenesis remains controversial, as multiple mechanisms have been suggested, but clear causal evidence is lacking. The original proposition that direct effects of drug toxicity caused the cutaneous observations was later refuted after similar bullous lesions with eccrine gland necrosis were reported in comatose patients with neurologic conditions.6 It is largely accepted that pressure-induced local ischemia—proportional to the duration and amount of pressure—leads to tissue injury and is critical to the pathogenesis. During periods of ischemia, the most metabolically active tissues will undergo necrosis first; however, in eccrine glands, the earliest and most severe damage does not seem to occur in the most metabolically active cells.7 Additionally, this would not provide a viable explanation for coma blisters with eccrine gland necrosis developing in variable non–pressuredependent areas.
Moreover, drug- and non–drug-induced coma blisters can appear identically, but specific histopathologic differences have been reported. The most notable markers of non–drug-induced coma blisters are the absence of an inflammatory infiltrate in the epidermis and the presence of thrombosis in dermal vessels.8 Demonstration of necrotic changes in the secretory portion of the eccrine gland is considered the histopathologic hallmark for drug-induced coma blisters, but other findings can include subepidermal or intraepidermal bullae; perivascular infiltrates; and focal necrosis of the epidermis, dermis, subcutis, or epidermal appendages.6 Arteriolar wall necrosis and dermal inflammatory infiltrates also have been observed.7
Benzodiazepines have been widely prescribed and abused since their development, and overdose is much more common today than with barbiturates.9 Coma blisters rarely have been documented in the setting of isolated benzodiazepine overdose, and of the few cases, only one report implicated lorazepam as the causative agent.4,7 The characteristic finding of eccrine gland necrosis consistently was seen in our patient. This case not only emphasizes the need for greater awareness of the association between benzodiazepine overdose and coma blisters but also the importance of clinical context when considering diagnoses. It is essential to note that coma blisters themselves are nonspecific, and the diagnosis of drug-induced coma blisters warrants confirmatory toxicologic analysis.
- Ferreli C, Sulica VI, Aste N, et al. Drug-induced sweat gland necrosis in a non-comatose patient: a case presentation. J Eur Acad Dermatol Venereol. 2003;17:443-445.
- Larrey DJ. Memoires de Chirurgie Militaire et Campagnes. Smith and Buisson; 1812.
- Agarwal A, Bansal M, Conner K. Coma blisters with hypoxemic respiratory failure. Dermatol Online J. 2012;18:10.
- Varma AJ, Fisher BK, Sarin MK. Diazepam-induced coma with bullae and eccrine sweat gland necrosis. Arch Intern Med. 1977;137:1207-1210.
- Rocha J, Pereira T, Ventura F, et al. Coma blisters. Case Rep Dermatol. 2009;1:66-70.
- Arndt KA, Mihm MC, Parrish JA. Bullae: a cutaneous sign of a variety of neurologic diseases. J Invest Dermatol. 1973;60:312-320.
- Sánchez Yus E, Requena L, Simón P. Histopathology of cutaneous changes in drug-induced coma. Am J Dermatopathol. 1993;15:208-216.
- Kato N, Ueno H, Mimura M. Histopathology of cutaneous changes in non-drug-induced coma. Am J Dermatopathol. 1996;18:344-350.
- Kang M, Ghassemzadeh S. Benzodiazepine Toxicity. StatPearls Publishing; 2018.
- Ferreli C, Sulica VI, Aste N, et al. Drug-induced sweat gland necrosis in a non-comatose patient: a case presentation. J Eur Acad Dermatol Venereol. 2003;17:443-445.
- Larrey DJ. Memoires de Chirurgie Militaire et Campagnes. Smith and Buisson; 1812.
- Agarwal A, Bansal M, Conner K. Coma blisters with hypoxemic respiratory failure. Dermatol Online J. 2012;18:10.
- Varma AJ, Fisher BK, Sarin MK. Diazepam-induced coma with bullae and eccrine sweat gland necrosis. Arch Intern Med. 1977;137:1207-1210.
- Rocha J, Pereira T, Ventura F, et al. Coma blisters. Case Rep Dermatol. 2009;1:66-70.
- Arndt KA, Mihm MC, Parrish JA. Bullae: a cutaneous sign of a variety of neurologic diseases. J Invest Dermatol. 1973;60:312-320.
- Sánchez Yus E, Requena L, Simón P. Histopathology of cutaneous changes in drug-induced coma. Am J Dermatopathol. 1993;15:208-216.
- Kato N, Ueno H, Mimura M. Histopathology of cutaneous changes in non-drug-induced coma. Am J Dermatopathol. 1996;18:344-350.
- Kang M, Ghassemzadeh S. Benzodiazepine Toxicity. StatPearls Publishing; 2018.
An 82-year-old woman presented to the emergency department after her daughter found her unconscious in the bathroom laying on her right side. Her medical history was notable for hypertension and asthma for which she was on losartan, furosemide, diltiazem, and albuterol. She recently had been prescribed lorazepam for insomnia and had started taking the medication 2 days prior. She underwent intubation and was noted to have flaccid, fluid-filled bullae on the right thigh (top) along with large areas of desquamation on the right lateral arm (bottom) with minimal surrounding erythema. There was no mucous membrane involvement. Urine toxicology was positive for benzodiazepines and negative for all other drugs, including barbiturates.
Children and COVID: U.S. sees almost 1 million new cases
Another record week for COVID-19 brought almost 1 million new cases to the children of the United States, according to new data from the American Academy of Pediatrics and the Children’s Hospital Association.
The pre-Omicron high for new cases in a week – 252,000 during the Delta surge of the late summer and early fall – has been surpassed each of the last 3 weeks and now stands at 981,000 (Jan. 7-13), according to the AAP/CHA weekly COVID-19 report. Over the 3-week stretch from Dec. 17 to Jan. 13, weekly cases increased by 394%.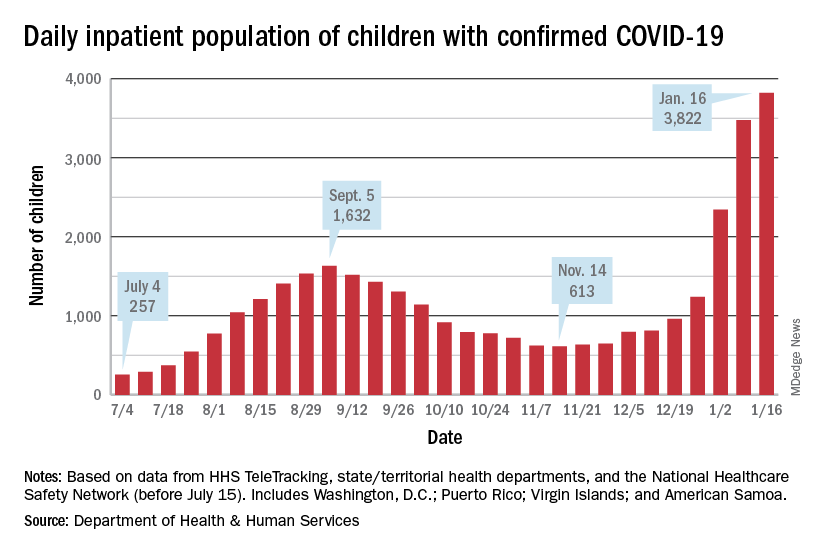
Hospitalizations also climbed to new heights, as daily admissions reached 1.23 per 100,000 children on Jan. 14, an increase of 547% since Nov. 30, when the rate was 0.19 per 100,000. Before Omicron, the highest rate for children was 0.47 per 100,000, based on data from the Centers for Disease Control and Prevention.
The inpatient population count, meanwhile, has followed suit. On Jan. 16, there were 3,822 children hospitalized in pediatric inpatient beds with laboratory-confirmed COVID-19, which is 523% higher than the 613 children who were hospitalized on Nov. 14, according to the Department of Health & Human Services. In the last week, though, the population was up by just 10%.
The one thing that has not surged in the last few weeks is vaccination. Among children aged 5-11 years, the weekly count of those who have received at least one dose dropped by 34% over the last 5 weeks, falling from 527,000 for Dec.11-17 to 347,000 during Jan. 8-14, the CDC said on the COVID Data Tracker, which also noted that just 18.4% of this age group is fully vaccinated.
The situation was reversed in children aged 12-15, who were up by 36% over that same time, but their numbers were much smaller: 78,000 for the week of Dec. 11-17 and 106,000 for Jan. 8-14. Those aged 16-17 were up by just 4% over that 5-week span, the CDC data show.
Over the course of the entire pandemic, almost 9.5 million cases of COVID-19 in children have been reported, and children represent 17.8% of all cases reported in 49 states (excluding New York but including New York City), the District of Columbia, Puerto Rico, and Guam, the AAP and CHA said in their report.
Three states (Alabama, Nebraska, and Texas) stopped public reporting over the summer, but many states count individuals up to age 19 as children, and others (South Carolina, Tennessee, and West Virginia) go up to age 20, the AAP and CHA noted. The CDC, by comparison, puts the number of cases for those aged 0-17 at 8.3 million, but that estimate is based on only 51 million of the nearly 67 million U.S. cases as of Jan. 18.
Another record week for COVID-19 brought almost 1 million new cases to the children of the United States, according to new data from the American Academy of Pediatrics and the Children’s Hospital Association.
The pre-Omicron high for new cases in a week – 252,000 during the Delta surge of the late summer and early fall – has been surpassed each of the last 3 weeks and now stands at 981,000 (Jan. 7-13), according to the AAP/CHA weekly COVID-19 report. Over the 3-week stretch from Dec. 17 to Jan. 13, weekly cases increased by 394%.
Hospitalizations also climbed to new heights, as daily admissions reached 1.23 per 100,000 children on Jan. 14, an increase of 547% since Nov. 30, when the rate was 0.19 per 100,000. Before Omicron, the highest rate for children was 0.47 per 100,000, based on data from the Centers for Disease Control and Prevention.
The inpatient population count, meanwhile, has followed suit. On Jan. 16, there were 3,822 children hospitalized in pediatric inpatient beds with laboratory-confirmed COVID-19, which is 523% higher than the 613 children who were hospitalized on Nov. 14, according to the Department of Health & Human Services. In the last week, though, the population was up by just 10%.
The one thing that has not surged in the last few weeks is vaccination. Among children aged 5-11 years, the weekly count of those who have received at least one dose dropped by 34% over the last 5 weeks, falling from 527,000 for Dec.11-17 to 347,000 during Jan. 8-14, the CDC said on the COVID Data Tracker, which also noted that just 18.4% of this age group is fully vaccinated.
The situation was reversed in children aged 12-15, who were up by 36% over that same time, but their numbers were much smaller: 78,000 for the week of Dec. 11-17 and 106,000 for Jan. 8-14. Those aged 16-17 were up by just 4% over that 5-week span, the CDC data show.
Over the course of the entire pandemic, almost 9.5 million cases of COVID-19 in children have been reported, and children represent 17.8% of all cases reported in 49 states (excluding New York but including New York City), the District of Columbia, Puerto Rico, and Guam, the AAP and CHA said in their report.
Three states (Alabama, Nebraska, and Texas) stopped public reporting over the summer, but many states count individuals up to age 19 as children, and others (South Carolina, Tennessee, and West Virginia) go up to age 20, the AAP and CHA noted. The CDC, by comparison, puts the number of cases for those aged 0-17 at 8.3 million, but that estimate is based on only 51 million of the nearly 67 million U.S. cases as of Jan. 18.
Another record week for COVID-19 brought almost 1 million new cases to the children of the United States, according to new data from the American Academy of Pediatrics and the Children’s Hospital Association.
The pre-Omicron high for new cases in a week – 252,000 during the Delta surge of the late summer and early fall – has been surpassed each of the last 3 weeks and now stands at 981,000 (Jan. 7-13), according to the AAP/CHA weekly COVID-19 report. Over the 3-week stretch from Dec. 17 to Jan. 13, weekly cases increased by 394%.
Hospitalizations also climbed to new heights, as daily admissions reached 1.23 per 100,000 children on Jan. 14, an increase of 547% since Nov. 30, when the rate was 0.19 per 100,000. Before Omicron, the highest rate for children was 0.47 per 100,000, based on data from the Centers for Disease Control and Prevention.
The inpatient population count, meanwhile, has followed suit. On Jan. 16, there were 3,822 children hospitalized in pediatric inpatient beds with laboratory-confirmed COVID-19, which is 523% higher than the 613 children who were hospitalized on Nov. 14, according to the Department of Health & Human Services. In the last week, though, the population was up by just 10%.
The one thing that has not surged in the last few weeks is vaccination. Among children aged 5-11 years, the weekly count of those who have received at least one dose dropped by 34% over the last 5 weeks, falling from 527,000 for Dec.11-17 to 347,000 during Jan. 8-14, the CDC said on the COVID Data Tracker, which also noted that just 18.4% of this age group is fully vaccinated.
The situation was reversed in children aged 12-15, who were up by 36% over that same time, but their numbers were much smaller: 78,000 for the week of Dec. 11-17 and 106,000 for Jan. 8-14. Those aged 16-17 were up by just 4% over that 5-week span, the CDC data show.
Over the course of the entire pandemic, almost 9.5 million cases of COVID-19 in children have been reported, and children represent 17.8% of all cases reported in 49 states (excluding New York but including New York City), the District of Columbia, Puerto Rico, and Guam, the AAP and CHA said in their report.
Three states (Alabama, Nebraska, and Texas) stopped public reporting over the summer, but many states count individuals up to age 19 as children, and others (South Carolina, Tennessee, and West Virginia) go up to age 20, the AAP and CHA noted. The CDC, by comparison, puts the number of cases for those aged 0-17 at 8.3 million, but that estimate is based on only 51 million of the nearly 67 million U.S. cases as of Jan. 18.
Negative home COVID test no ‘free pass’ for kids, study finds
With the country looking increasingly to rapid testing as an off-ramp from the COVID-19 pandemic, a new study shows that the performance of the tests in children falls below standards set by regulatory agencies in the United States and elsewhere for diagnostic accuracy.
Experts said the findings, from a meta-analysis by researchers in the United Kingdom and Germany, underscore that, while a positive result on a rapid test is almost certainly an indicator of infection, negative results often are unreliable and can lead to a false sense of security.
“Real-life performance of current antigen tests for professional use in pediatric populations is below the minimum performance criteria set by WHO, the United States Food and Drug Administration, or the Medicines and Healthcare products Regulatory Agency (U.K.),” according to Naomi Fujita-Rohwerder, PhD, a research associate at the Cologne-based German Institute for Quality and Efficiency in Health Care (IQWiG), and her colleagues, whose study appears in BMJ Evidence-Based Medicine.
The researchers said that the study suggests that performance of rapid testing in a pediatric population is comparable to that in adults. However, they said they could not identify any studies investigating self-testing in children, which also could affect test performance.
Egon Ozer, MD, PhD, director of the center for pathogen genomics and microbial evolution at Northwestern University in Chicago, said the finding that specificity was high but sensitivity was middling “suggests that we should be very careful about interpreting negative antigen test results in children and recognize that there is a fair amount of uncertainty in the tests in this situation.”
Researchers from IQWiG, which examines the advantages and disadvantages of medical interventions, and the University of Manchester (England), conducted the systematic review and meta-analysis, which they described as the first of its kind to evaluate the diagnostic accuracy of rapid point-of-care tests for current SARS-CoV-2 infections in children.
They compiled information from 17 studies with a total 6,355 participants. They compared all antigen tests to reverse-transcription polymerase chain reaction (PCR). The studies compared eight antigen tests from six different brands. The rapid antigen tests, available from pharmacies and online stores, are widely used for self-testing in schools and testing toddlers before kindergarten.
The pooled diagnostic sensitivity of antigen tests was 64.2% and specificity was 99.1%.
Dr. Ozer noted that the analysis “was not able to address important outstanding questions such as the likelihood of transmitting infection with a false-negative antigen test versus a true-negative antigen test or how much repeated testing can increase the sensitivity.”
“In Europe, we don’t know how most tests perform in real life,” Dr. Fujita-Rohwerder said. “And even in countries like the United States, where market access is more stringent, we don’t know whether self-testing performed by children or sample collection in toddlers by laypersons has a significant impact on the diagnostic accuracy. Also, diagnostic accuracy estimates reported in our study may not apply to the current omicron or future variants of SARS-CoV-2 or vaccinated children. Hopefully, these essential gaps in the evidence will get addressed soon.”
Dr. Ozer said one takeaway from this study is negative antigen tests should not be considered a “free pass” in children, especially if the child is symptomatic, has been recently exposed to COVID-19, or is planning to spend time with individuals with conditions that place them at high risk for complications of COVID-19 infection. “In such cases, consider getting PCR testing or at least performing a repeat antigen test 36-48 hours after the first negative,” he said.
Dr. Fujita-Rohwerder said the low diagnostic sensitivity may affect the use of the tests. The gaps in evidence her group found in their study point to research needed to support evidence-based decision-making. “In particular, evidence is needed on real-life performance of tests in schools, self-testing performed by children, and kindergarten, [particularly] sample collection in toddlers by laypersons,” she said.
However, she stressed, testing is only a single measure. “Effectively reducing the spread of SARS-CoV-2 during the current pandemic requires multilayered mitigation measures,” she said. “Rapid testing represents one single layer. It can have its use at the population level, even though the sensitivity of antigen tests is lower than expected. However, antigen-based rapid testing is not a magic bullet: If your kid tests negative, do not disregard other mitigation measures.”
Edward Campbell, PhD, a virologist at Loyola University of Chicago, who serves on the board of LaGrange Elementary School District 102 outside Chicago, said the findings were unsurprising.
“This study generally looks consistent with what is known for adults. These rapid antigen tests are less sensitive than other tests,” said Dr. Campbell, who also runs a testing company for private schools in the Chicago area using reverse transcription-loop-mediated isothermal amplification technology. Even so, he said, “These tests are still effective at identifying people who are infectious to some degree. Never miss an opportunity to test.”
Dr. Fujita-Rohwerder disclosed no relevant financial conflicts of interest. Dr. Campbell owns Safeguard Surveillance.
With the country looking increasingly to rapid testing as an off-ramp from the COVID-19 pandemic, a new study shows that the performance of the tests in children falls below standards set by regulatory agencies in the United States and elsewhere for diagnostic accuracy.
Experts said the findings, from a meta-analysis by researchers in the United Kingdom and Germany, underscore that, while a positive result on a rapid test is almost certainly an indicator of infection, negative results often are unreliable and can lead to a false sense of security.
“Real-life performance of current antigen tests for professional use in pediatric populations is below the minimum performance criteria set by WHO, the United States Food and Drug Administration, or the Medicines and Healthcare products Regulatory Agency (U.K.),” according to Naomi Fujita-Rohwerder, PhD, a research associate at the Cologne-based German Institute for Quality and Efficiency in Health Care (IQWiG), and her colleagues, whose study appears in BMJ Evidence-Based Medicine.
The researchers said that the study suggests that performance of rapid testing in a pediatric population is comparable to that in adults. However, they said they could not identify any studies investigating self-testing in children, which also could affect test performance.
Egon Ozer, MD, PhD, director of the center for pathogen genomics and microbial evolution at Northwestern University in Chicago, said the finding that specificity was high but sensitivity was middling “suggests that we should be very careful about interpreting negative antigen test results in children and recognize that there is a fair amount of uncertainty in the tests in this situation.”
Researchers from IQWiG, which examines the advantages and disadvantages of medical interventions, and the University of Manchester (England), conducted the systematic review and meta-analysis, which they described as the first of its kind to evaluate the diagnostic accuracy of rapid point-of-care tests for current SARS-CoV-2 infections in children.
They compiled information from 17 studies with a total 6,355 participants. They compared all antigen tests to reverse-transcription polymerase chain reaction (PCR). The studies compared eight antigen tests from six different brands. The rapid antigen tests, available from pharmacies and online stores, are widely used for self-testing in schools and testing toddlers before kindergarten.
The pooled diagnostic sensitivity of antigen tests was 64.2% and specificity was 99.1%.
Dr. Ozer noted that the analysis “was not able to address important outstanding questions such as the likelihood of transmitting infection with a false-negative antigen test versus a true-negative antigen test or how much repeated testing can increase the sensitivity.”
“In Europe, we don’t know how most tests perform in real life,” Dr. Fujita-Rohwerder said. “And even in countries like the United States, where market access is more stringent, we don’t know whether self-testing performed by children or sample collection in toddlers by laypersons has a significant impact on the diagnostic accuracy. Also, diagnostic accuracy estimates reported in our study may not apply to the current omicron or future variants of SARS-CoV-2 or vaccinated children. Hopefully, these essential gaps in the evidence will get addressed soon.”
Dr. Ozer said one takeaway from this study is negative antigen tests should not be considered a “free pass” in children, especially if the child is symptomatic, has been recently exposed to COVID-19, or is planning to spend time with individuals with conditions that place them at high risk for complications of COVID-19 infection. “In such cases, consider getting PCR testing or at least performing a repeat antigen test 36-48 hours after the first negative,” he said.
Dr. Fujita-Rohwerder said the low diagnostic sensitivity may affect the use of the tests. The gaps in evidence her group found in their study point to research needed to support evidence-based decision-making. “In particular, evidence is needed on real-life performance of tests in schools, self-testing performed by children, and kindergarten, [particularly] sample collection in toddlers by laypersons,” she said.
However, she stressed, testing is only a single measure. “Effectively reducing the spread of SARS-CoV-2 during the current pandemic requires multilayered mitigation measures,” she said. “Rapid testing represents one single layer. It can have its use at the population level, even though the sensitivity of antigen tests is lower than expected. However, antigen-based rapid testing is not a magic bullet: If your kid tests negative, do not disregard other mitigation measures.”
Edward Campbell, PhD, a virologist at Loyola University of Chicago, who serves on the board of LaGrange Elementary School District 102 outside Chicago, said the findings were unsurprising.
“This study generally looks consistent with what is known for adults. These rapid antigen tests are less sensitive than other tests,” said Dr. Campbell, who also runs a testing company for private schools in the Chicago area using reverse transcription-loop-mediated isothermal amplification technology. Even so, he said, “These tests are still effective at identifying people who are infectious to some degree. Never miss an opportunity to test.”
Dr. Fujita-Rohwerder disclosed no relevant financial conflicts of interest. Dr. Campbell owns Safeguard Surveillance.
With the country looking increasingly to rapid testing as an off-ramp from the COVID-19 pandemic, a new study shows that the performance of the tests in children falls below standards set by regulatory agencies in the United States and elsewhere for diagnostic accuracy.
Experts said the findings, from a meta-analysis by researchers in the United Kingdom and Germany, underscore that, while a positive result on a rapid test is almost certainly an indicator of infection, negative results often are unreliable and can lead to a false sense of security.
“Real-life performance of current antigen tests for professional use in pediatric populations is below the minimum performance criteria set by WHO, the United States Food and Drug Administration, or the Medicines and Healthcare products Regulatory Agency (U.K.),” according to Naomi Fujita-Rohwerder, PhD, a research associate at the Cologne-based German Institute for Quality and Efficiency in Health Care (IQWiG), and her colleagues, whose study appears in BMJ Evidence-Based Medicine.
The researchers said that the study suggests that performance of rapid testing in a pediatric population is comparable to that in adults. However, they said they could not identify any studies investigating self-testing in children, which also could affect test performance.
Egon Ozer, MD, PhD, director of the center for pathogen genomics and microbial evolution at Northwestern University in Chicago, said the finding that specificity was high but sensitivity was middling “suggests that we should be very careful about interpreting negative antigen test results in children and recognize that there is a fair amount of uncertainty in the tests in this situation.”
Researchers from IQWiG, which examines the advantages and disadvantages of medical interventions, and the University of Manchester (England), conducted the systematic review and meta-analysis, which they described as the first of its kind to evaluate the diagnostic accuracy of rapid point-of-care tests for current SARS-CoV-2 infections in children.
They compiled information from 17 studies with a total 6,355 participants. They compared all antigen tests to reverse-transcription polymerase chain reaction (PCR). The studies compared eight antigen tests from six different brands. The rapid antigen tests, available from pharmacies and online stores, are widely used for self-testing in schools and testing toddlers before kindergarten.
The pooled diagnostic sensitivity of antigen tests was 64.2% and specificity was 99.1%.
Dr. Ozer noted that the analysis “was not able to address important outstanding questions such as the likelihood of transmitting infection with a false-negative antigen test versus a true-negative antigen test or how much repeated testing can increase the sensitivity.”
“In Europe, we don’t know how most tests perform in real life,” Dr. Fujita-Rohwerder said. “And even in countries like the United States, where market access is more stringent, we don’t know whether self-testing performed by children or sample collection in toddlers by laypersons has a significant impact on the diagnostic accuracy. Also, diagnostic accuracy estimates reported in our study may not apply to the current omicron or future variants of SARS-CoV-2 or vaccinated children. Hopefully, these essential gaps in the evidence will get addressed soon.”
Dr. Ozer said one takeaway from this study is negative antigen tests should not be considered a “free pass” in children, especially if the child is symptomatic, has been recently exposed to COVID-19, or is planning to spend time with individuals with conditions that place them at high risk for complications of COVID-19 infection. “In such cases, consider getting PCR testing or at least performing a repeat antigen test 36-48 hours after the first negative,” he said.
Dr. Fujita-Rohwerder said the low diagnostic sensitivity may affect the use of the tests. The gaps in evidence her group found in their study point to research needed to support evidence-based decision-making. “In particular, evidence is needed on real-life performance of tests in schools, self-testing performed by children, and kindergarten, [particularly] sample collection in toddlers by laypersons,” she said.
However, she stressed, testing is only a single measure. “Effectively reducing the spread of SARS-CoV-2 during the current pandemic requires multilayered mitigation measures,” she said. “Rapid testing represents one single layer. It can have its use at the population level, even though the sensitivity of antigen tests is lower than expected. However, antigen-based rapid testing is not a magic bullet: If your kid tests negative, do not disregard other mitigation measures.”
Edward Campbell, PhD, a virologist at Loyola University of Chicago, who serves on the board of LaGrange Elementary School District 102 outside Chicago, said the findings were unsurprising.
“This study generally looks consistent with what is known for adults. These rapid antigen tests are less sensitive than other tests,” said Dr. Campbell, who also runs a testing company for private schools in the Chicago area using reverse transcription-loop-mediated isothermal amplification technology. Even so, he said, “These tests are still effective at identifying people who are infectious to some degree. Never miss an opportunity to test.”
Dr. Fujita-Rohwerder disclosed no relevant financial conflicts of interest. Dr. Campbell owns Safeguard Surveillance.
BMJ EVIDENCE-BASED MEDICINE
Feds’ website for free at-home COVID tests launches day early
The Biden administration’s new no-cost, at-home testing program launched Jan. 18, a day ahead of schedule.
The administration said 500 million tests are available to be delivered to homes across the country. This accounts for half of the president’s recent pledge to purchase 1 billion free at-home COVID-19 tests to distribute to the American public.
On a Jan. 14 call with reporters, senior White House officials offered some details about the new program.
Here’s what we know so far.
How do I order my free tests?
Americans can visit COVIDtests.gov to order their rapid at-home tests. You can also order directly from the U.S. Postal Service website. After you order, you’ll receive a confirmation email that promises to send tracking information once your order ships.
What information do I need to order the tests?
You only need your name and home mailing address.
There is also an option to provide your email address to get updates on the status of your order.
What if someone needs help ordering the tests?
There will be a free call-in line for people needing more help, including those having trouble accessing the internet, according to White House officials.
What tests will be available?
There are nine at-home tests available through FDA emergency use authorization. According to the Frequently Asked Questions section of COVIDtests.gov, "You will not be able to choose the brand you order as part of this program.”
How long will it take to get the tests once I order them?
Tests are expected to ship 7 to 12 days after you order them.
But White House officials say that the time frame will likely shorten as the program gains steam.
How many can I order?
There’s a limit of four tests per residential mailing address.
For larger families, White House officials suggest trying other free testing options, like visiting COVID-19 testing sites or your local health center.
Is this a one-time opportunity?
The White House doesn’t say, but officials did mention that if you run out of your four free tests, there are many other ways to access free at-home tests, such as COVID-19 testing sites, pharmacies, and community health centers.
The free tests available through COVIDtests.gov are in addition to an estimated 375 million at-home rapid tests on the market in the U.S. this month.
When should people use a rapid at-home test?
The CDC and experts with other public health groups agree that Americans should consider using at-home rapid tests in the following situations:
- If they begin to have symptoms consistent with COVID-19;
- At least 5 days after close contact with someone who has COVID;
- If someone is indoors with a group of people who are at risk of severe disease or are unvaccinated.
Are at-home rapid tests accurate?
The U.S. Department of Health and Human Services and other federal officials confirmed through studies that all tests distributed through this program can detect the Omicron variant. These agencies also confirmed that their performance is consistent with the FDA’s emergency use authorization.
Is the website designed to handle high demand?
After the original website to sign up for health insurance under the Affordable Care Act crashed repeatedly at launch, the government says it has prepared for high demand for ordering at-home rapid tests.
The U.S. Digital Service (USDS), an organization founded after Healthcare.gov, has partnered with the Postal Service to plan for the launch.
The Postal Service has expanded its staffing, similar to what’s done during the holidays.
All orders in the continental United States will be shipped through first-class mail, with shipments to Alaska, Hawaii, U.S. territories, and military and overseas addresses sent through priority mail.
A version of this article first appeared on WebMD.com.
The Biden administration’s new no-cost, at-home testing program launched Jan. 18, a day ahead of schedule.
The administration said 500 million tests are available to be delivered to homes across the country. This accounts for half of the president’s recent pledge to purchase 1 billion free at-home COVID-19 tests to distribute to the American public.
On a Jan. 14 call with reporters, senior White House officials offered some details about the new program.
Here’s what we know so far.
How do I order my free tests?
Americans can visit COVIDtests.gov to order their rapid at-home tests. You can also order directly from the U.S. Postal Service website. After you order, you’ll receive a confirmation email that promises to send tracking information once your order ships.
What information do I need to order the tests?
You only need your name and home mailing address.
There is also an option to provide your email address to get updates on the status of your order.
What if someone needs help ordering the tests?
There will be a free call-in line for people needing more help, including those having trouble accessing the internet, according to White House officials.
What tests will be available?
There are nine at-home tests available through FDA emergency use authorization. According to the Frequently Asked Questions section of COVIDtests.gov, "You will not be able to choose the brand you order as part of this program.”
How long will it take to get the tests once I order them?
Tests are expected to ship 7 to 12 days after you order them.
But White House officials say that the time frame will likely shorten as the program gains steam.
How many can I order?
There’s a limit of four tests per residential mailing address.
For larger families, White House officials suggest trying other free testing options, like visiting COVID-19 testing sites or your local health center.
Is this a one-time opportunity?
The White House doesn’t say, but officials did mention that if you run out of your four free tests, there are many other ways to access free at-home tests, such as COVID-19 testing sites, pharmacies, and community health centers.
The free tests available through COVIDtests.gov are in addition to an estimated 375 million at-home rapid tests on the market in the U.S. this month.
When should people use a rapid at-home test?
The CDC and experts with other public health groups agree that Americans should consider using at-home rapid tests in the following situations:
- If they begin to have symptoms consistent with COVID-19;
- At least 5 days after close contact with someone who has COVID;
- If someone is indoors with a group of people who are at risk of severe disease or are unvaccinated.
Are at-home rapid tests accurate?
The U.S. Department of Health and Human Services and other federal officials confirmed through studies that all tests distributed through this program can detect the Omicron variant. These agencies also confirmed that their performance is consistent with the FDA’s emergency use authorization.
Is the website designed to handle high demand?
After the original website to sign up for health insurance under the Affordable Care Act crashed repeatedly at launch, the government says it has prepared for high demand for ordering at-home rapid tests.
The U.S. Digital Service (USDS), an organization founded after Healthcare.gov, has partnered with the Postal Service to plan for the launch.
The Postal Service has expanded its staffing, similar to what’s done during the holidays.
All orders in the continental United States will be shipped through first-class mail, with shipments to Alaska, Hawaii, U.S. territories, and military and overseas addresses sent through priority mail.
A version of this article first appeared on WebMD.com.
The Biden administration’s new no-cost, at-home testing program launched Jan. 18, a day ahead of schedule.
The administration said 500 million tests are available to be delivered to homes across the country. This accounts for half of the president’s recent pledge to purchase 1 billion free at-home COVID-19 tests to distribute to the American public.
On a Jan. 14 call with reporters, senior White House officials offered some details about the new program.
Here’s what we know so far.
How do I order my free tests?
Americans can visit COVIDtests.gov to order their rapid at-home tests. You can also order directly from the U.S. Postal Service website. After you order, you’ll receive a confirmation email that promises to send tracking information once your order ships.
What information do I need to order the tests?
You only need your name and home mailing address.
There is also an option to provide your email address to get updates on the status of your order.
What if someone needs help ordering the tests?
There will be a free call-in line for people needing more help, including those having trouble accessing the internet, according to White House officials.
What tests will be available?
There are nine at-home tests available through FDA emergency use authorization. According to the Frequently Asked Questions section of COVIDtests.gov, "You will not be able to choose the brand you order as part of this program.”
How long will it take to get the tests once I order them?
Tests are expected to ship 7 to 12 days after you order them.
But White House officials say that the time frame will likely shorten as the program gains steam.
How many can I order?
There’s a limit of four tests per residential mailing address.
For larger families, White House officials suggest trying other free testing options, like visiting COVID-19 testing sites or your local health center.
Is this a one-time opportunity?
The White House doesn’t say, but officials did mention that if you run out of your four free tests, there are many other ways to access free at-home tests, such as COVID-19 testing sites, pharmacies, and community health centers.
The free tests available through COVIDtests.gov are in addition to an estimated 375 million at-home rapid tests on the market in the U.S. this month.
When should people use a rapid at-home test?
The CDC and experts with other public health groups agree that Americans should consider using at-home rapid tests in the following situations:
- If they begin to have symptoms consistent with COVID-19;
- At least 5 days after close contact with someone who has COVID;
- If someone is indoors with a group of people who are at risk of severe disease or are unvaccinated.
Are at-home rapid tests accurate?
The U.S. Department of Health and Human Services and other federal officials confirmed through studies that all tests distributed through this program can detect the Omicron variant. These agencies also confirmed that their performance is consistent with the FDA’s emergency use authorization.
Is the website designed to handle high demand?
After the original website to sign up for health insurance under the Affordable Care Act crashed repeatedly at launch, the government says it has prepared for high demand for ordering at-home rapid tests.
The U.S. Digital Service (USDS), an organization founded after Healthcare.gov, has partnered with the Postal Service to plan for the launch.
The Postal Service has expanded its staffing, similar to what’s done during the holidays.
All orders in the continental United States will be shipped through first-class mail, with shipments to Alaska, Hawaii, U.S. territories, and military and overseas addresses sent through priority mail.
A version of this article first appeared on WebMD.com.
Federal website for free COVID-19 tests opens Jan. 19
The tests will ship within 7 to 12 days after being ordered, senior officials from President Joe Biden’s administration said Jan. 14. The U.S. Postal Service will handle the shipping and delivery through first-class mail.
People will input their name and mailing address on the website and can share an email address to receive updates on the order, according to NPR. People won’t need to pay shipping costs or enter a credit card number to order tests, according to the website’s homepage.
The website will be offered in both English and Spanish. The Biden administration will also set up a phone number so those without internet access can place orders.
Officials didn’t share a specific time that the website will open, according to he New York Times — simply that it will go live sometime on Jan. 19. Each household will be limited to ordering four tests.
Starting Jan. 15, people with private insurance were able to seek reimbursement for tests they purchase on their own. At the same time, some insurers have said it could take weeks to set up a system for smooth reimbursement, the newspaper reported.
Last week’s announcement is the latest step in the president’s pledge to get coronavirus tests to Americans. In December, Biden said his administration would purchase 500 million tests and distribute them to Americans for free. On Jan. 13, he announced that the administration would buy another 500 million tests, bringing the total to 1 billion.
So far, the administration has signed contracts to produce 420 million tests, the newspaper reported. With the website opening this week and the lag in shipping, the tests will likely arrive by the end of January at the earliest, which could be after the peak of the current coronavirus surge in some parts of the country.
At-home tests have been in high demand, with some pharmacies, retailers, and websites reporting no stock in recent weeks. People have lined up at community testing sites for hours to get tested as the national average of daily cases has climbed above 800,000 last week.
Some consumers have also been confused about how or when to use at-home tests. On Jan. 14, Biden administration officials said that people should use rapid tests for three reasons:
- If they begin to experience COVID-19 symptoms;
- When it has been five or more days after being exposed to someone who tests positive;
- If they are gathering indoors with a high-risk person and want to check if they are negative.
A version of this article first appeared on WebMD.com.
The tests will ship within 7 to 12 days after being ordered, senior officials from President Joe Biden’s administration said Jan. 14. The U.S. Postal Service will handle the shipping and delivery through first-class mail.
People will input their name and mailing address on the website and can share an email address to receive updates on the order, according to NPR. People won’t need to pay shipping costs or enter a credit card number to order tests, according to the website’s homepage.
The website will be offered in both English and Spanish. The Biden administration will also set up a phone number so those without internet access can place orders.
Officials didn’t share a specific time that the website will open, according to he New York Times — simply that it will go live sometime on Jan. 19. Each household will be limited to ordering four tests.
Starting Jan. 15, people with private insurance were able to seek reimbursement for tests they purchase on their own. At the same time, some insurers have said it could take weeks to set up a system for smooth reimbursement, the newspaper reported.
Last week’s announcement is the latest step in the president’s pledge to get coronavirus tests to Americans. In December, Biden said his administration would purchase 500 million tests and distribute them to Americans for free. On Jan. 13, he announced that the administration would buy another 500 million tests, bringing the total to 1 billion.
So far, the administration has signed contracts to produce 420 million tests, the newspaper reported. With the website opening this week and the lag in shipping, the tests will likely arrive by the end of January at the earliest, which could be after the peak of the current coronavirus surge in some parts of the country.
At-home tests have been in high demand, with some pharmacies, retailers, and websites reporting no stock in recent weeks. People have lined up at community testing sites for hours to get tested as the national average of daily cases has climbed above 800,000 last week.
Some consumers have also been confused about how or when to use at-home tests. On Jan. 14, Biden administration officials said that people should use rapid tests for three reasons:
- If they begin to experience COVID-19 symptoms;
- When it has been five or more days after being exposed to someone who tests positive;
- If they are gathering indoors with a high-risk person and want to check if they are negative.
A version of this article first appeared on WebMD.com.
The tests will ship within 7 to 12 days after being ordered, senior officials from President Joe Biden’s administration said Jan. 14. The U.S. Postal Service will handle the shipping and delivery through first-class mail.
People will input their name and mailing address on the website and can share an email address to receive updates on the order, according to NPR. People won’t need to pay shipping costs or enter a credit card number to order tests, according to the website’s homepage.
The website will be offered in both English and Spanish. The Biden administration will also set up a phone number so those without internet access can place orders.
Officials didn’t share a specific time that the website will open, according to he New York Times — simply that it will go live sometime on Jan. 19. Each household will be limited to ordering four tests.
Starting Jan. 15, people with private insurance were able to seek reimbursement for tests they purchase on their own. At the same time, some insurers have said it could take weeks to set up a system for smooth reimbursement, the newspaper reported.
Last week’s announcement is the latest step in the president’s pledge to get coronavirus tests to Americans. In December, Biden said his administration would purchase 500 million tests and distribute them to Americans for free. On Jan. 13, he announced that the administration would buy another 500 million tests, bringing the total to 1 billion.
So far, the administration has signed contracts to produce 420 million tests, the newspaper reported. With the website opening this week and the lag in shipping, the tests will likely arrive by the end of January at the earliest, which could be after the peak of the current coronavirus surge in some parts of the country.
At-home tests have been in high demand, with some pharmacies, retailers, and websites reporting no stock in recent weeks. People have lined up at community testing sites for hours to get tested as the national average of daily cases has climbed above 800,000 last week.
Some consumers have also been confused about how or when to use at-home tests. On Jan. 14, Biden administration officials said that people should use rapid tests for three reasons:
- If they begin to experience COVID-19 symptoms;
- When it has been five or more days after being exposed to someone who tests positive;
- If they are gathering indoors with a high-risk person and want to check if they are negative.
A version of this article first appeared on WebMD.com.
ACP advocates outpatient treatment of uncomplicated diverticulitis
The estimated prevalence of acute colonic diverticulitis in the United States appears to be on the rise, wrote Amir Qaseem, MD, and members of the ACP Clinical Guidelines Committee. “Approximately 200,000 hospitalizations for acute left-sided colonic diverticulitis occur in the United States each year, with annual costs of more than $8 billion. Timely and correct diagnosis of acute left-sided colonic diverticulitis is essential for the selection of the most appropriate management options.”
Diverticulitis is becoming increasingly common in patients treated by internal medicine physicians, according to the ACP, and the new clinical guidelines specify a course of treatment focused on outpatient management and minimal medications.
The guidelines, published in Annals of Internal Medicine, were based on a systematic review of evidence from studies published between Jan. 1, 1990, and June 1, 2020. Notably, right-sided diverticulitis was excluded because it is rare in Western countries and involves a different natural history and management options, the authors wrote.
In the guidelines, uncomplicated diverticulitis refers to localized inflammation, and complicated diverticulitis refers to “inflammation associated with an abscess, a phlegmon, a fistula, an obstruction, bleeding, or perforation.”
Guidance on diagnosis and management
In the first guideline, “Diagnosis and Management of Acute Left-Sided Colonic Diverticulitis”, the authors provided three recommendations. First, they recommended that clinicians use abdominal CT imaging in cases of diagnostic uncertainty for patients with suspected acute left-sided colonic diverticulitis. The evidence showed that abdominal CT was associated with appropriate management in patients with suspected acute left-sided colonic diverticulitis, and that misdiagnosis with CT was rare.
Second, the authors of this guidance recommended management of most patients with acute left-sided colonic diverticulitis in an outpatient setting. Evidence showed that the risk for elective surgery and for recurrence were not significantly different based on inpatient or outpatient management.
The third recommendation advised clinicians to manage most patients without antibiotics. This recommendation was based on data showing no significant difference in quality of life at 3, 6, 12, or 24 months; no difference in diverticulitis-related complications; and no difference in the need for surgery in patients treated with antibiotics and those not treated with antibiotics.
All three recommendations are conditional, with low-certainty evidence, according to the authors.
Colonoscopy for diagnostic evaluation and interventions
In the second guideline, “Colonoscopy for Diagnostic Evaluation and Interventions to Prevent Recurrence After Acute Left-Sided Colonic Diverticulitis, the authors advised clinicians to refer patients for a colonoscopy after an initial episode of complicated left-sided colonic diverticulitis if they have not had a recent colonoscopy.
Although acute diverticulitis is usually uncomplicated, approximately 12% of cases are considered complicated, and these patients may have a higher prevalence of colorectal cancer, the authors noted. This recommendation was conditional, with low-certainty evidence. Additional diagnostic colonoscopy is not needed for patients who are up to date on recommended colorectal cancer screening, according to this guideline.
A second recommendation, given as a strong recommendation with high-certainty evidence, advised against using mesalamine to prevent recurrent diverticulitis. Evidence showed that use of mesalamine at doses ranging from 1.2 g/day to 4.8 g/day made no difference in recurrent diverticulitis risk compared with placebo. Mesalamine has no demonstrated clinical benefits, and has been associated with epigastric pain, nausea, diarrhea, dizziness, rash, and renal and hepatic impairment, the authors wrote.
The third recommendation advised the discussion of elective surgery with patients with a history of uncomplicated diverticulitis that persists or recurs frequently. Surgery also may be an option for patients with complicated diverticulitis, according to the guideline. However, “this recommendation does not apply to patients with uncomplicated diverticulitis that is not persistent or frequently recurring,” the authors wrote.
The decision to pursue elective surgery should be informed and personalized according to potential benefits, harms, costs, and patient preferences, they said. This recommendation is conditional, with low-certainty evidence.
This new guideline was designed “to guide care based on the best available evidence and may not apply to all patients or individual clinical situations,” the authors emphasized. “It should not be used as a replacement for a clinician’s judgment.”
Update confirms best practices
“Concerns about inappropriate antimicrobial therapy use and the delay in seeking preventative care such as a colonoscopy have led to poorer outcomes for patients,” ACP president George Abraham, MD, said in an interview. These concerns about a lack of antimicrobial stewardship and of care not being representative of ‘high value care’ “supported the need to reinforce best practices.”
Although most clinicians are aware of the nature of the recommendations in their own clinical practices, “a systematic review helped confirm and codify best practice that everyone can confidently incorporate into their daily decision-making,” Dr. Abraham said.
Compared with previous guidelines, “the single biggest difference is the fact that antimicrobial therapy is not indicated in mild, uncomplicated diverticulitis; we hope this will lead to lesser and more judicious antimicrobial prescribing,” Dr. Abraham emphasized.
Like all guidelines, the current guidelines are meant to be advisory, not mandatory; “they do not replace good clinical judgment and individual patient care decision-making,” Dr. Abraham said. “These guidelines are useful when they are widely read by clinicians, including physicians and advanced practice clinicians, and incorporated into their daily practice.”
Curbing antibiotic use
It is important for clinicians to recognize that uncomplicated diverticulitis in selected patients does not require initial antibiotics, David A. Johnson, MD, chief of gastroenterology at Eastern Virginia School of Medicine, Norfolk, said in an interview. “This paradigm shift began with the AGA guidelines in 2015, and was more recently updated with the 2021 best practice recommendations,” first published in Gastroenterology.
“I was surprised to see this current guideline not mentioning that, if antibiotics are to be used, that amoxicillin-clavulanate alone should be favored over combination of fluoroquinolones and metronidazole,” Dr. Johnson noted. “Furthermore, the U.S. Food and Drug Administration has advised that fluoroquinolones should be reserved for conditions with no alternative treatment options.”
“The initial management approach for the AGA guidelines and best practice are comparable with these most recent ACP recommendations,” said Dr. Johnson. However, “I would suggest that clinicians treating diverticulitis also review the AGA best practice recommendations, which build out important other important points for diverticulitis management including timeframes for colonoscopy, strong effect of genetics, dietary effects, recurrence rates, and the role of surgery.”
As for research gaps, “further data on cost savings would be helpful,” as savings may be likely with significant reduction without antibiotics and imaging in select patients, Dr. Johnson said. “Cost savings and risk reduction of adverse implications of antibiotic and radiation risks should be included in these analyses.”
The guidelines were based on systematic reviews conducted by the Evidence-based Practice Center at Brown University, Providence, R.I., funded by the Agency for Healthcare Research and Quality. The development of the guidelines was supported by the ACP operating budget. The authors, Dr. Abraham, and Dr. Johnson had no financial conflicts to disclose.
The estimated prevalence of acute colonic diverticulitis in the United States appears to be on the rise, wrote Amir Qaseem, MD, and members of the ACP Clinical Guidelines Committee. “Approximately 200,000 hospitalizations for acute left-sided colonic diverticulitis occur in the United States each year, with annual costs of more than $8 billion. Timely and correct diagnosis of acute left-sided colonic diverticulitis is essential for the selection of the most appropriate management options.”
Diverticulitis is becoming increasingly common in patients treated by internal medicine physicians, according to the ACP, and the new clinical guidelines specify a course of treatment focused on outpatient management and minimal medications.
The guidelines, published in Annals of Internal Medicine, were based on a systematic review of evidence from studies published between Jan. 1, 1990, and June 1, 2020. Notably, right-sided diverticulitis was excluded because it is rare in Western countries and involves a different natural history and management options, the authors wrote.
In the guidelines, uncomplicated diverticulitis refers to localized inflammation, and complicated diverticulitis refers to “inflammation associated with an abscess, a phlegmon, a fistula, an obstruction, bleeding, or perforation.”
Guidance on diagnosis and management
In the first guideline, “Diagnosis and Management of Acute Left-Sided Colonic Diverticulitis”, the authors provided three recommendations. First, they recommended that clinicians use abdominal CT imaging in cases of diagnostic uncertainty for patients with suspected acute left-sided colonic diverticulitis. The evidence showed that abdominal CT was associated with appropriate management in patients with suspected acute left-sided colonic diverticulitis, and that misdiagnosis with CT was rare.
Second, the authors of this guidance recommended management of most patients with acute left-sided colonic diverticulitis in an outpatient setting. Evidence showed that the risk for elective surgery and for recurrence were not significantly different based on inpatient or outpatient management.
The third recommendation advised clinicians to manage most patients without antibiotics. This recommendation was based on data showing no significant difference in quality of life at 3, 6, 12, or 24 months; no difference in diverticulitis-related complications; and no difference in the need for surgery in patients treated with antibiotics and those not treated with antibiotics.
All three recommendations are conditional, with low-certainty evidence, according to the authors.
Colonoscopy for diagnostic evaluation and interventions
In the second guideline, “Colonoscopy for Diagnostic Evaluation and Interventions to Prevent Recurrence After Acute Left-Sided Colonic Diverticulitis, the authors advised clinicians to refer patients for a colonoscopy after an initial episode of complicated left-sided colonic diverticulitis if they have not had a recent colonoscopy.
Although acute diverticulitis is usually uncomplicated, approximately 12% of cases are considered complicated, and these patients may have a higher prevalence of colorectal cancer, the authors noted. This recommendation was conditional, with low-certainty evidence. Additional diagnostic colonoscopy is not needed for patients who are up to date on recommended colorectal cancer screening, according to this guideline.
A second recommendation, given as a strong recommendation with high-certainty evidence, advised against using mesalamine to prevent recurrent diverticulitis. Evidence showed that use of mesalamine at doses ranging from 1.2 g/day to 4.8 g/day made no difference in recurrent diverticulitis risk compared with placebo. Mesalamine has no demonstrated clinical benefits, and has been associated with epigastric pain, nausea, diarrhea, dizziness, rash, and renal and hepatic impairment, the authors wrote.
The third recommendation advised the discussion of elective surgery with patients with a history of uncomplicated diverticulitis that persists or recurs frequently. Surgery also may be an option for patients with complicated diverticulitis, according to the guideline. However, “this recommendation does not apply to patients with uncomplicated diverticulitis that is not persistent or frequently recurring,” the authors wrote.
The decision to pursue elective surgery should be informed and personalized according to potential benefits, harms, costs, and patient preferences, they said. This recommendation is conditional, with low-certainty evidence.
This new guideline was designed “to guide care based on the best available evidence and may not apply to all patients or individual clinical situations,” the authors emphasized. “It should not be used as a replacement for a clinician’s judgment.”
Update confirms best practices
“Concerns about inappropriate antimicrobial therapy use and the delay in seeking preventative care such as a colonoscopy have led to poorer outcomes for patients,” ACP president George Abraham, MD, said in an interview. These concerns about a lack of antimicrobial stewardship and of care not being representative of ‘high value care’ “supported the need to reinforce best practices.”
Although most clinicians are aware of the nature of the recommendations in their own clinical practices, “a systematic review helped confirm and codify best practice that everyone can confidently incorporate into their daily decision-making,” Dr. Abraham said.
Compared with previous guidelines, “the single biggest difference is the fact that antimicrobial therapy is not indicated in mild, uncomplicated diverticulitis; we hope this will lead to lesser and more judicious antimicrobial prescribing,” Dr. Abraham emphasized.
Like all guidelines, the current guidelines are meant to be advisory, not mandatory; “they do not replace good clinical judgment and individual patient care decision-making,” Dr. Abraham said. “These guidelines are useful when they are widely read by clinicians, including physicians and advanced practice clinicians, and incorporated into their daily practice.”
Curbing antibiotic use
It is important for clinicians to recognize that uncomplicated diverticulitis in selected patients does not require initial antibiotics, David A. Johnson, MD, chief of gastroenterology at Eastern Virginia School of Medicine, Norfolk, said in an interview. “This paradigm shift began with the AGA guidelines in 2015, and was more recently updated with the 2021 best practice recommendations,” first published in Gastroenterology.
“I was surprised to see this current guideline not mentioning that, if antibiotics are to be used, that amoxicillin-clavulanate alone should be favored over combination of fluoroquinolones and metronidazole,” Dr. Johnson noted. “Furthermore, the U.S. Food and Drug Administration has advised that fluoroquinolones should be reserved for conditions with no alternative treatment options.”
“The initial management approach for the AGA guidelines and best practice are comparable with these most recent ACP recommendations,” said Dr. Johnson. However, “I would suggest that clinicians treating diverticulitis also review the AGA best practice recommendations, which build out important other important points for diverticulitis management including timeframes for colonoscopy, strong effect of genetics, dietary effects, recurrence rates, and the role of surgery.”
As for research gaps, “further data on cost savings would be helpful,” as savings may be likely with significant reduction without antibiotics and imaging in select patients, Dr. Johnson said. “Cost savings and risk reduction of adverse implications of antibiotic and radiation risks should be included in these analyses.”
The guidelines were based on systematic reviews conducted by the Evidence-based Practice Center at Brown University, Providence, R.I., funded by the Agency for Healthcare Research and Quality. The development of the guidelines was supported by the ACP operating budget. The authors, Dr. Abraham, and Dr. Johnson had no financial conflicts to disclose.
The estimated prevalence of acute colonic diverticulitis in the United States appears to be on the rise, wrote Amir Qaseem, MD, and members of the ACP Clinical Guidelines Committee. “Approximately 200,000 hospitalizations for acute left-sided colonic diverticulitis occur in the United States each year, with annual costs of more than $8 billion. Timely and correct diagnosis of acute left-sided colonic diverticulitis is essential for the selection of the most appropriate management options.”
Diverticulitis is becoming increasingly common in patients treated by internal medicine physicians, according to the ACP, and the new clinical guidelines specify a course of treatment focused on outpatient management and minimal medications.
The guidelines, published in Annals of Internal Medicine, were based on a systematic review of evidence from studies published between Jan. 1, 1990, and June 1, 2020. Notably, right-sided diverticulitis was excluded because it is rare in Western countries and involves a different natural history and management options, the authors wrote.
In the guidelines, uncomplicated diverticulitis refers to localized inflammation, and complicated diverticulitis refers to “inflammation associated with an abscess, a phlegmon, a fistula, an obstruction, bleeding, or perforation.”
Guidance on diagnosis and management
In the first guideline, “Diagnosis and Management of Acute Left-Sided Colonic Diverticulitis”, the authors provided three recommendations. First, they recommended that clinicians use abdominal CT imaging in cases of diagnostic uncertainty for patients with suspected acute left-sided colonic diverticulitis. The evidence showed that abdominal CT was associated with appropriate management in patients with suspected acute left-sided colonic diverticulitis, and that misdiagnosis with CT was rare.
Second, the authors of this guidance recommended management of most patients with acute left-sided colonic diverticulitis in an outpatient setting. Evidence showed that the risk for elective surgery and for recurrence were not significantly different based on inpatient or outpatient management.
The third recommendation advised clinicians to manage most patients without antibiotics. This recommendation was based on data showing no significant difference in quality of life at 3, 6, 12, or 24 months; no difference in diverticulitis-related complications; and no difference in the need for surgery in patients treated with antibiotics and those not treated with antibiotics.
All three recommendations are conditional, with low-certainty evidence, according to the authors.
Colonoscopy for diagnostic evaluation and interventions
In the second guideline, “Colonoscopy for Diagnostic Evaluation and Interventions to Prevent Recurrence After Acute Left-Sided Colonic Diverticulitis, the authors advised clinicians to refer patients for a colonoscopy after an initial episode of complicated left-sided colonic diverticulitis if they have not had a recent colonoscopy.
Although acute diverticulitis is usually uncomplicated, approximately 12% of cases are considered complicated, and these patients may have a higher prevalence of colorectal cancer, the authors noted. This recommendation was conditional, with low-certainty evidence. Additional diagnostic colonoscopy is not needed for patients who are up to date on recommended colorectal cancer screening, according to this guideline.
A second recommendation, given as a strong recommendation with high-certainty evidence, advised against using mesalamine to prevent recurrent diverticulitis. Evidence showed that use of mesalamine at doses ranging from 1.2 g/day to 4.8 g/day made no difference in recurrent diverticulitis risk compared with placebo. Mesalamine has no demonstrated clinical benefits, and has been associated with epigastric pain, nausea, diarrhea, dizziness, rash, and renal and hepatic impairment, the authors wrote.
The third recommendation advised the discussion of elective surgery with patients with a history of uncomplicated diverticulitis that persists or recurs frequently. Surgery also may be an option for patients with complicated diverticulitis, according to the guideline. However, “this recommendation does not apply to patients with uncomplicated diverticulitis that is not persistent or frequently recurring,” the authors wrote.
The decision to pursue elective surgery should be informed and personalized according to potential benefits, harms, costs, and patient preferences, they said. This recommendation is conditional, with low-certainty evidence.
This new guideline was designed “to guide care based on the best available evidence and may not apply to all patients or individual clinical situations,” the authors emphasized. “It should not be used as a replacement for a clinician’s judgment.”
Update confirms best practices
“Concerns about inappropriate antimicrobial therapy use and the delay in seeking preventative care such as a colonoscopy have led to poorer outcomes for patients,” ACP president George Abraham, MD, said in an interview. These concerns about a lack of antimicrobial stewardship and of care not being representative of ‘high value care’ “supported the need to reinforce best practices.”
Although most clinicians are aware of the nature of the recommendations in their own clinical practices, “a systematic review helped confirm and codify best practice that everyone can confidently incorporate into their daily decision-making,” Dr. Abraham said.
Compared with previous guidelines, “the single biggest difference is the fact that antimicrobial therapy is not indicated in mild, uncomplicated diverticulitis; we hope this will lead to lesser and more judicious antimicrobial prescribing,” Dr. Abraham emphasized.
Like all guidelines, the current guidelines are meant to be advisory, not mandatory; “they do not replace good clinical judgment and individual patient care decision-making,” Dr. Abraham said. “These guidelines are useful when they are widely read by clinicians, including physicians and advanced practice clinicians, and incorporated into their daily practice.”
Curbing antibiotic use
It is important for clinicians to recognize that uncomplicated diverticulitis in selected patients does not require initial antibiotics, David A. Johnson, MD, chief of gastroenterology at Eastern Virginia School of Medicine, Norfolk, said in an interview. “This paradigm shift began with the AGA guidelines in 2015, and was more recently updated with the 2021 best practice recommendations,” first published in Gastroenterology.
“I was surprised to see this current guideline not mentioning that, if antibiotics are to be used, that amoxicillin-clavulanate alone should be favored over combination of fluoroquinolones and metronidazole,” Dr. Johnson noted. “Furthermore, the U.S. Food and Drug Administration has advised that fluoroquinolones should be reserved for conditions with no alternative treatment options.”
“The initial management approach for the AGA guidelines and best practice are comparable with these most recent ACP recommendations,” said Dr. Johnson. However, “I would suggest that clinicians treating diverticulitis also review the AGA best practice recommendations, which build out important other important points for diverticulitis management including timeframes for colonoscopy, strong effect of genetics, dietary effects, recurrence rates, and the role of surgery.”
As for research gaps, “further data on cost savings would be helpful,” as savings may be likely with significant reduction without antibiotics and imaging in select patients, Dr. Johnson said. “Cost savings and risk reduction of adverse implications of antibiotic and radiation risks should be included in these analyses.”
The guidelines were based on systematic reviews conducted by the Evidence-based Practice Center at Brown University, Providence, R.I., funded by the Agency for Healthcare Research and Quality. The development of the guidelines was supported by the ACP operating budget. The authors, Dr. Abraham, and Dr. Johnson had no financial conflicts to disclose.
FROM ANNALS OF INTERNAL MEDICINE
Docs pen open letter to support Fauci against partisan ‘attacks’
“We deplore the personal attacks on Dr. Fauci. The criticism is inaccurate, unscientific, ill-founded in the facts and, increasingly, motivated by partisan politics,” reads the letter of support, initiated by Ezekiel Emanuel, MD, and signed by almost 300 scientists and public health and medical professionals, including Nobel Laureates, a former Republican senator, and leadership of medical societies and institutions.
Dr. Fauci has led the National Institute for Allergy and Infectious Diseases since 1984 and serves as President Biden’s top medical advisor on the pandemic.
“Dr. Anthony Fauci has served the U.S.A. with wisdom and integrity for nearly 40 years. Through HIV, Ebola, and now COVID, he has unswervingly served the United States guiding the country to very successful outcomes. He has our unreserved respect and trust as a scientist and a national leader,” the letter reads.
Dr. Fauci has repeatedly faced harsh criticism from congressional Republicans, especially Sen. Rand Paul (R-Ky.) and Sen. Roger Marshall (R-Kan.).
At a particularly contentious congressional hearing earlier this week on the federal government’s response to Omicron, Dr. Fauci fought back, telling Sen. Marshall, “You’re so misinformed, it’s extraordinary.”
Dr. Fauci, who has received death threats and harassment of his family, told Sen. Rand that his “completely untrue” statements and rhetoric “kindles the crazies out there.”
‘Sagacious counsel’
The personal attacks on Dr. Fauci are a “distraction from what should be the national focus – working together to finally overcome a pandemic that is killing about 500,000 people a year. We are grateful for Dr. Fauci’s dedication and tireless efforts to help the country through this pandemic and other health crises,” the letter reads.
“Throughout the COVID-19 pandemic, Dr. Fauci has provided the American political leadership and the public with sagacious counsel in these most difficult of times. His advice has been as well informed as data and the rapidly evolving circumstances allowed,” it states.
“Importantly,” Dr. Fauci has given his advice with “humility, being clear about what we know and what is unknown, but requires judgment. He has consistently emphasized the importance of mask-wearing, social distancing, and vaccination. These are standard and necessary public health measures that we all support,” the letter states.
“We are grateful that Dr. Fauci has consistently stated the science in a way that represents the facts as they emerge, without unwarranted speculation.”
“Sadly, in these politically polarized times where misinformation contaminates the United States’ response to the pandemic, routine public health measures have become unnecessarily controversial, undermining the effectiveness of our country’s response,” the letter reads.
A version of this article first appeared on Medscape.com.
“We deplore the personal attacks on Dr. Fauci. The criticism is inaccurate, unscientific, ill-founded in the facts and, increasingly, motivated by partisan politics,” reads the letter of support, initiated by Ezekiel Emanuel, MD, and signed by almost 300 scientists and public health and medical professionals, including Nobel Laureates, a former Republican senator, and leadership of medical societies and institutions.
Dr. Fauci has led the National Institute for Allergy and Infectious Diseases since 1984 and serves as President Biden’s top medical advisor on the pandemic.
“Dr. Anthony Fauci has served the U.S.A. with wisdom and integrity for nearly 40 years. Through HIV, Ebola, and now COVID, he has unswervingly served the United States guiding the country to very successful outcomes. He has our unreserved respect and trust as a scientist and a national leader,” the letter reads.
Dr. Fauci has repeatedly faced harsh criticism from congressional Republicans, especially Sen. Rand Paul (R-Ky.) and Sen. Roger Marshall (R-Kan.).
At a particularly contentious congressional hearing earlier this week on the federal government’s response to Omicron, Dr. Fauci fought back, telling Sen. Marshall, “You’re so misinformed, it’s extraordinary.”
Dr. Fauci, who has received death threats and harassment of his family, told Sen. Rand that his “completely untrue” statements and rhetoric “kindles the crazies out there.”
‘Sagacious counsel’
The personal attacks on Dr. Fauci are a “distraction from what should be the national focus – working together to finally overcome a pandemic that is killing about 500,000 people a year. We are grateful for Dr. Fauci’s dedication and tireless efforts to help the country through this pandemic and other health crises,” the letter reads.
“Throughout the COVID-19 pandemic, Dr. Fauci has provided the American political leadership and the public with sagacious counsel in these most difficult of times. His advice has been as well informed as data and the rapidly evolving circumstances allowed,” it states.
“Importantly,” Dr. Fauci has given his advice with “humility, being clear about what we know and what is unknown, but requires judgment. He has consistently emphasized the importance of mask-wearing, social distancing, and vaccination. These are standard and necessary public health measures that we all support,” the letter states.
“We are grateful that Dr. Fauci has consistently stated the science in a way that represents the facts as they emerge, without unwarranted speculation.”
“Sadly, in these politically polarized times where misinformation contaminates the United States’ response to the pandemic, routine public health measures have become unnecessarily controversial, undermining the effectiveness of our country’s response,” the letter reads.
A version of this article first appeared on Medscape.com.
“We deplore the personal attacks on Dr. Fauci. The criticism is inaccurate, unscientific, ill-founded in the facts and, increasingly, motivated by partisan politics,” reads the letter of support, initiated by Ezekiel Emanuel, MD, and signed by almost 300 scientists and public health and medical professionals, including Nobel Laureates, a former Republican senator, and leadership of medical societies and institutions.
Dr. Fauci has led the National Institute for Allergy and Infectious Diseases since 1984 and serves as President Biden’s top medical advisor on the pandemic.
“Dr. Anthony Fauci has served the U.S.A. with wisdom and integrity for nearly 40 years. Through HIV, Ebola, and now COVID, he has unswervingly served the United States guiding the country to very successful outcomes. He has our unreserved respect and trust as a scientist and a national leader,” the letter reads.
Dr. Fauci has repeatedly faced harsh criticism from congressional Republicans, especially Sen. Rand Paul (R-Ky.) and Sen. Roger Marshall (R-Kan.).
At a particularly contentious congressional hearing earlier this week on the federal government’s response to Omicron, Dr. Fauci fought back, telling Sen. Marshall, “You’re so misinformed, it’s extraordinary.”
Dr. Fauci, who has received death threats and harassment of his family, told Sen. Rand that his “completely untrue” statements and rhetoric “kindles the crazies out there.”
‘Sagacious counsel’
The personal attacks on Dr. Fauci are a “distraction from what should be the national focus – working together to finally overcome a pandemic that is killing about 500,000 people a year. We are grateful for Dr. Fauci’s dedication and tireless efforts to help the country through this pandemic and other health crises,” the letter reads.
“Throughout the COVID-19 pandemic, Dr. Fauci has provided the American political leadership and the public with sagacious counsel in these most difficult of times. His advice has been as well informed as data and the rapidly evolving circumstances allowed,” it states.
“Importantly,” Dr. Fauci has given his advice with “humility, being clear about what we know and what is unknown, but requires judgment. He has consistently emphasized the importance of mask-wearing, social distancing, and vaccination. These are standard and necessary public health measures that we all support,” the letter states.
“We are grateful that Dr. Fauci has consistently stated the science in a way that represents the facts as they emerge, without unwarranted speculation.”
“Sadly, in these politically polarized times where misinformation contaminates the United States’ response to the pandemic, routine public health measures have become unnecessarily controversial, undermining the effectiveness of our country’s response,” the letter reads.
A version of this article first appeared on Medscape.com.
Program targets preschoolers to promote heart health
Creators of a pilot program that educates preschoolers about good heart health have validated a template for successful early childhood intervention that, they claim, provides a pathway for translating scientific evidence into the community and classroom for educational purposes to encourage long-lasting lifestyle changes.
That validation supports the creators' plans to take the program into more schools.
They reported key lessons in crafting the program, known as the SI! Program (for Salud Integral-Comprehensive Health), online in the Journal of the American College of Cardiology.
“This is a research-based program that uses randomized clinical trial evidence with implementation strategies to design educational health promotion programs,” senior author Valentin Fuster, MD, PhD, founder and trustees chairman of the Foundation for Science, Health, and Education (SHE) based in Barcelona, under whose aegis the SI! Program was implemented, said in an interview. Dr. Fuster is also director of Mount Sinai Heart and physician-in-chief at Mount Sinai Hospital in New York, and general director of the National Center for Cardiovascular Investigation (CNIC) in Madrid, Spain’s equivalent of the National Heart, Lung, and Blood Institute.
“There are specific times in a child’s life when improvements can be made to enhance long-term cardiovascular health status,” said Rodrigo Fernández-Jiménez, MD, PhD, group leader of the cardiovascular health and imaging lab at CNIC and study coauthor. “Our review, and previous studies, suggest that 4-5 years of age is the most favorable time to start a school-based intervention focused on healthy habits.”
A key piece of the SI! Program used a Sesame Street character, known as Dr. Ruster, a Muppet based on Dr. Fuster, to introduce and convey most messages and activities to the preschool children. The program also used a heart-shaped mascot named “Cardio” to teach about healthy behaviors. Other components include video segments, a colorful storybook, an interactive board game, flash cards, and a teacher’s guide. The activities and messages were tailored based on the country in which the program was implemented.
A decade of experience
The review evaluated 10 years of experience with the preschool-based program, drawing upon cluster-randomized clinical trials of the program in three countries with different socioeconomic conditions: Colombia, Spain, and the United States. The studies randomized schools to receive the SI! Program for 4 months or to a control group and included more than 3,800 children from 50 schools, along with their parents or caregivers and teachers. The studies found significant increases in preschoolers’ knowledge, attitudes, and habits toward healthy eating and living an active lifestyle. Now, the SI! Program is expanding into more than 250 schools in Spain and more than 40 schools in all five boroughs of New York City.
“This is a multidimensional program,” Dr. Fuster said. The review identified five stages for implementing the program: dissemination; adoption; implementation; evaluation; and institutionalization.
Dissemination involves three substages for intervention: components, design, and strategy. With regard to the components, said Dr. Fuster, “We’re targeting children to educate them in four topics: how the body works; nutritional and dietary requirements; physical activity; and the need to control emotions – to say no in the future when they’re confronted with alcohol, drugs, and tobacco.”
Design involved a multidisciplinary team of experts to develop the intervention, Dr. Fuster said. The strategy itself enlists parents and teachers in the implementation, but goes beyond that. “This is a community,” Dr. Fuster said. Hence, the school environment and classroom itself are also engaged to support the message of the four topics.
Dr. Fuster said future research should look at knowledge, attitude, and habits and biological outcomes in children who’ve been in the SI! Program when they reach adolescence. “Our hypothesis is that we can do this in older children, but when they reach age 10 we want to reintervene in them,” Dr. Fuster said. “Humans need reintervention. Our findings don’t get into sustainability.” He added that further research should also identify socioeconomic factors that influence child health.
Expanding the program across the New York City’s five boroughs “offers a unique opportunity to explore which socioeconomic factors, at both the family and borough level, and may eventually affect children’s health, how they are implicated in the intervention’s effectiveness, and how they can be addressed to reduce the gap in health inequalities,” he said.
Karalyn Kinsella, MD, a pediatrician affiliated with Yale New Haven (Conn.) Medical Center, noted the program’s multidimensional nature is an important element. “I think what is so important about this intervention is that it is not one single intervention but a curriculum that takes a significant amount of time (up to 50 hours) that allows for repetition of the information, which allows it to become remembered,” she said in an interview. “I also think incorporating families in the intervention is key as that is where change often has to happen.”
While she said the program may provide a template for a mental health curriculum, she added, “My concern is that teachers are already feeling overwhelmed and this may be viewed as another burden.”
The American Heart Association provided funding for the study in the United States. Dr Fernández-Jiménez has received funding from the Fondo de Investigación Sanitaria–Instituto de Salud Carlos III, which is cofunded by the European Regional Development Fund/European Social Fund. Dr. Fuster and Dr. Kinsella have no relevant disclosures.
Creators of a pilot program that educates preschoolers about good heart health have validated a template for successful early childhood intervention that, they claim, provides a pathway for translating scientific evidence into the community and classroom for educational purposes to encourage long-lasting lifestyle changes.
That validation supports the creators' plans to take the program into more schools.
They reported key lessons in crafting the program, known as the SI! Program (for Salud Integral-Comprehensive Health), online in the Journal of the American College of Cardiology.
“This is a research-based program that uses randomized clinical trial evidence with implementation strategies to design educational health promotion programs,” senior author Valentin Fuster, MD, PhD, founder and trustees chairman of the Foundation for Science, Health, and Education (SHE) based in Barcelona, under whose aegis the SI! Program was implemented, said in an interview. Dr. Fuster is also director of Mount Sinai Heart and physician-in-chief at Mount Sinai Hospital in New York, and general director of the National Center for Cardiovascular Investigation (CNIC) in Madrid, Spain’s equivalent of the National Heart, Lung, and Blood Institute.
“There are specific times in a child’s life when improvements can be made to enhance long-term cardiovascular health status,” said Rodrigo Fernández-Jiménez, MD, PhD, group leader of the cardiovascular health and imaging lab at CNIC and study coauthor. “Our review, and previous studies, suggest that 4-5 years of age is the most favorable time to start a school-based intervention focused on healthy habits.”
A key piece of the SI! Program used a Sesame Street character, known as Dr. Ruster, a Muppet based on Dr. Fuster, to introduce and convey most messages and activities to the preschool children. The program also used a heart-shaped mascot named “Cardio” to teach about healthy behaviors. Other components include video segments, a colorful storybook, an interactive board game, flash cards, and a teacher’s guide. The activities and messages were tailored based on the country in which the program was implemented.
A decade of experience
The review evaluated 10 years of experience with the preschool-based program, drawing upon cluster-randomized clinical trials of the program in three countries with different socioeconomic conditions: Colombia, Spain, and the United States. The studies randomized schools to receive the SI! Program for 4 months or to a control group and included more than 3,800 children from 50 schools, along with their parents or caregivers and teachers. The studies found significant increases in preschoolers’ knowledge, attitudes, and habits toward healthy eating and living an active lifestyle. Now, the SI! Program is expanding into more than 250 schools in Spain and more than 40 schools in all five boroughs of New York City.
“This is a multidimensional program,” Dr. Fuster said. The review identified five stages for implementing the program: dissemination; adoption; implementation; evaluation; and institutionalization.
Dissemination involves three substages for intervention: components, design, and strategy. With regard to the components, said Dr. Fuster, “We’re targeting children to educate them in four topics: how the body works; nutritional and dietary requirements; physical activity; and the need to control emotions – to say no in the future when they’re confronted with alcohol, drugs, and tobacco.”
Design involved a multidisciplinary team of experts to develop the intervention, Dr. Fuster said. The strategy itself enlists parents and teachers in the implementation, but goes beyond that. “This is a community,” Dr. Fuster said. Hence, the school environment and classroom itself are also engaged to support the message of the four topics.
Dr. Fuster said future research should look at knowledge, attitude, and habits and biological outcomes in children who’ve been in the SI! Program when they reach adolescence. “Our hypothesis is that we can do this in older children, but when they reach age 10 we want to reintervene in them,” Dr. Fuster said. “Humans need reintervention. Our findings don’t get into sustainability.” He added that further research should also identify socioeconomic factors that influence child health.
Expanding the program across the New York City’s five boroughs “offers a unique opportunity to explore which socioeconomic factors, at both the family and borough level, and may eventually affect children’s health, how they are implicated in the intervention’s effectiveness, and how they can be addressed to reduce the gap in health inequalities,” he said.
Karalyn Kinsella, MD, a pediatrician affiliated with Yale New Haven (Conn.) Medical Center, noted the program’s multidimensional nature is an important element. “I think what is so important about this intervention is that it is not one single intervention but a curriculum that takes a significant amount of time (up to 50 hours) that allows for repetition of the information, which allows it to become remembered,” she said in an interview. “I also think incorporating families in the intervention is key as that is where change often has to happen.”
While she said the program may provide a template for a mental health curriculum, she added, “My concern is that teachers are already feeling overwhelmed and this may be viewed as another burden.”
The American Heart Association provided funding for the study in the United States. Dr Fernández-Jiménez has received funding from the Fondo de Investigación Sanitaria–Instituto de Salud Carlos III, which is cofunded by the European Regional Development Fund/European Social Fund. Dr. Fuster and Dr. Kinsella have no relevant disclosures.
Creators of a pilot program that educates preschoolers about good heart health have validated a template for successful early childhood intervention that, they claim, provides a pathway for translating scientific evidence into the community and classroom for educational purposes to encourage long-lasting lifestyle changes.
That validation supports the creators' plans to take the program into more schools.
They reported key lessons in crafting the program, known as the SI! Program (for Salud Integral-Comprehensive Health), online in the Journal of the American College of Cardiology.
“This is a research-based program that uses randomized clinical trial evidence with implementation strategies to design educational health promotion programs,” senior author Valentin Fuster, MD, PhD, founder and trustees chairman of the Foundation for Science, Health, and Education (SHE) based in Barcelona, under whose aegis the SI! Program was implemented, said in an interview. Dr. Fuster is also director of Mount Sinai Heart and physician-in-chief at Mount Sinai Hospital in New York, and general director of the National Center for Cardiovascular Investigation (CNIC) in Madrid, Spain’s equivalent of the National Heart, Lung, and Blood Institute.
“There are specific times in a child’s life when improvements can be made to enhance long-term cardiovascular health status,” said Rodrigo Fernández-Jiménez, MD, PhD, group leader of the cardiovascular health and imaging lab at CNIC and study coauthor. “Our review, and previous studies, suggest that 4-5 years of age is the most favorable time to start a school-based intervention focused on healthy habits.”
A key piece of the SI! Program used a Sesame Street character, known as Dr. Ruster, a Muppet based on Dr. Fuster, to introduce and convey most messages and activities to the preschool children. The program also used a heart-shaped mascot named “Cardio” to teach about healthy behaviors. Other components include video segments, a colorful storybook, an interactive board game, flash cards, and a teacher’s guide. The activities and messages were tailored based on the country in which the program was implemented.
A decade of experience
The review evaluated 10 years of experience with the preschool-based program, drawing upon cluster-randomized clinical trials of the program in three countries with different socioeconomic conditions: Colombia, Spain, and the United States. The studies randomized schools to receive the SI! Program for 4 months or to a control group and included more than 3,800 children from 50 schools, along with their parents or caregivers and teachers. The studies found significant increases in preschoolers’ knowledge, attitudes, and habits toward healthy eating and living an active lifestyle. Now, the SI! Program is expanding into more than 250 schools in Spain and more than 40 schools in all five boroughs of New York City.
“This is a multidimensional program,” Dr. Fuster said. The review identified five stages for implementing the program: dissemination; adoption; implementation; evaluation; and institutionalization.
Dissemination involves three substages for intervention: components, design, and strategy. With regard to the components, said Dr. Fuster, “We’re targeting children to educate them in four topics: how the body works; nutritional and dietary requirements; physical activity; and the need to control emotions – to say no in the future when they’re confronted with alcohol, drugs, and tobacco.”
Design involved a multidisciplinary team of experts to develop the intervention, Dr. Fuster said. The strategy itself enlists parents and teachers in the implementation, but goes beyond that. “This is a community,” Dr. Fuster said. Hence, the school environment and classroom itself are also engaged to support the message of the four topics.
Dr. Fuster said future research should look at knowledge, attitude, and habits and biological outcomes in children who’ve been in the SI! Program when they reach adolescence. “Our hypothesis is that we can do this in older children, but when they reach age 10 we want to reintervene in them,” Dr. Fuster said. “Humans need reintervention. Our findings don’t get into sustainability.” He added that further research should also identify socioeconomic factors that influence child health.
Expanding the program across the New York City’s five boroughs “offers a unique opportunity to explore which socioeconomic factors, at both the family and borough level, and may eventually affect children’s health, how they are implicated in the intervention’s effectiveness, and how they can be addressed to reduce the gap in health inequalities,” he said.
Karalyn Kinsella, MD, a pediatrician affiliated with Yale New Haven (Conn.) Medical Center, noted the program’s multidimensional nature is an important element. “I think what is so important about this intervention is that it is not one single intervention but a curriculum that takes a significant amount of time (up to 50 hours) that allows for repetition of the information, which allows it to become remembered,” she said in an interview. “I also think incorporating families in the intervention is key as that is where change often has to happen.”
While she said the program may provide a template for a mental health curriculum, she added, “My concern is that teachers are already feeling overwhelmed and this may be viewed as another burden.”
The American Heart Association provided funding for the study in the United States. Dr Fernández-Jiménez has received funding from the Fondo de Investigación Sanitaria–Instituto de Salud Carlos III, which is cofunded by the European Regional Development Fund/European Social Fund. Dr. Fuster and Dr. Kinsella have no relevant disclosures.
FROM JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
How safe is a drug holiday from bisphosphonates for osteoporosis?
Researchers found a small but greater risk of a hip fracture after 2 years of taking a “drug holiday” – stopping therapy – after long-term (≥3-year) use of one bisphosphonate, risedronate, versus another, alendronate.
The risk of a hip fracture after stopping either of these oral bisphosphonate osteoporosis drugs was similar until 2 years, suggesting that patients who take a drug holiday from risedronate should be revaluated before 2 years.
These top-line findings from a propensity-matched cohort study of older patients in Ontario, Canada, were reported at the annual American Society of Bone and Mineral Research (ASBMR) last fall.
The full study, led by Kaleen N. Hayes, PharmD, PhD, Brown University School of Public Health, Providence, R.I., was published online in the Annals of Internal Medicine.
“We emphasize that our results do not indicate that alendronate therapy should be preferred over risedronate therapy,” the researchers stress, as several real-world studies found a similar risk of fractures while patients were receiving either drug.
“The decision to initiate alendronate or risedronate therapy [the two most commonly prescribed bisphosphonates] is driven by the prescriber,” they note, adding that some patients may prefer risedronate because it is available as a monthly dose or a weekly delayed-release formula that does not require fasting.
“We found little difference in the association between risedronate versus alendronate drug holidays and hip fractures until approximately 2 years of not receiving therapy,” Dr. Hayes and colleagues summarize.
Over 3 years, risedronate drug holidays were associated with an 18% relative and 0.6% absolute increased risk for hip fracture compared with alendronate drug holidays.
“To further inform clinical decision-making on drug holidays,” they conclude, “future research should examine when to start and restart osteoporosis therapy on the basis of initial length and type of treatment, patient characteristics, and relative risk for hip fractures versus [atypical femoral fracture].”
Hip fracture risk with risedronate vs. alendronate drug holiday
Long-term bisphosphonate use is associated with a rare risk of osteonecrosis of the jaw or atypical femoral fractures. At the same time, bisphosphonates continue to have a therapeutic effect after therapy is discontinued.
Guidelines recommend that patients at low risk of fracture should therefore have a “drug holiday” after 3 to 5 years of bisphosphonate use and be reassessed 2 to 3 years later, largely based on the Fracture Intervention Trial Long-Term Extension (FLEX) study of alendronate. But risedronate has a shorter half-life, so it may provide shorter residual fracture protection.
Using Ontario administrative data, Dr. Hayes and associates identified more than 60,000 patients who were over aged 65, had received at least 3 years of continuous alendronate or risedronate, and had a subsequent 3-year drug holiday between 2000 and 2020.
They excluded patients who had a fracture or entered a nursing home within 120 days of starting a drug holiday who may have stopped the bisphosphonate due to declining health rather than a drug holiday.
Roughly half (55%) had been taking risedronate and 45% had been taking alendronate.
Using propensity scores, the researchers matched 25,077 patients who had been taking risedronate with an equal number who had been taking alendronate.
Most of the patients were women (82%) and were White.
They started the drug holiday when they were on average 81 years old, after taking the bisphosphonate for 5.9 years on average.
During the 3-year drug holiday, 915 of the 50,154 patients had hip fractures.
This was equivalent to 12.4 hip fractures per 1,000 patients per year during a risedronate holiday and 10.6 hip fractures per 1,000 patients per year during an alendronate holiday (hazard ratio, 1.18).
The risk of hip fracture was not significantly higher at 1 year (HR, 1.03) or at 2 years of a risedronate holiday versus an alendronate holiday (HR, 1.14).
However, the risk of a hip fracture was significantly higher at 2 to 3 years of a risedronate holiday than after an alendronate holiday (HR, 1.34).
There was no significant difference in the risk of any osteoporotic fracture overall (including hip, vertebrae, pelvis, ribs, forearm), however, during a 3-year risedronate versus alendronate drug holiday (HR, 1.07).
The research was supported by the Canadian Institutes of Health Research and Institute for Clinical Evaluative Sciences. Dr. Hayes was supported by a CIHR doctoral research award. The authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Researchers found a small but greater risk of a hip fracture after 2 years of taking a “drug holiday” – stopping therapy – after long-term (≥3-year) use of one bisphosphonate, risedronate, versus another, alendronate.
The risk of a hip fracture after stopping either of these oral bisphosphonate osteoporosis drugs was similar until 2 years, suggesting that patients who take a drug holiday from risedronate should be revaluated before 2 years.
These top-line findings from a propensity-matched cohort study of older patients in Ontario, Canada, were reported at the annual American Society of Bone and Mineral Research (ASBMR) last fall.
The full study, led by Kaleen N. Hayes, PharmD, PhD, Brown University School of Public Health, Providence, R.I., was published online in the Annals of Internal Medicine.
“We emphasize that our results do not indicate that alendronate therapy should be preferred over risedronate therapy,” the researchers stress, as several real-world studies found a similar risk of fractures while patients were receiving either drug.
“The decision to initiate alendronate or risedronate therapy [the two most commonly prescribed bisphosphonates] is driven by the prescriber,” they note, adding that some patients may prefer risedronate because it is available as a monthly dose or a weekly delayed-release formula that does not require fasting.
“We found little difference in the association between risedronate versus alendronate drug holidays and hip fractures until approximately 2 years of not receiving therapy,” Dr. Hayes and colleagues summarize.
Over 3 years, risedronate drug holidays were associated with an 18% relative and 0.6% absolute increased risk for hip fracture compared with alendronate drug holidays.
“To further inform clinical decision-making on drug holidays,” they conclude, “future research should examine when to start and restart osteoporosis therapy on the basis of initial length and type of treatment, patient characteristics, and relative risk for hip fractures versus [atypical femoral fracture].”
Hip fracture risk with risedronate vs. alendronate drug holiday
Long-term bisphosphonate use is associated with a rare risk of osteonecrosis of the jaw or atypical femoral fractures. At the same time, bisphosphonates continue to have a therapeutic effect after therapy is discontinued.
Guidelines recommend that patients at low risk of fracture should therefore have a “drug holiday” after 3 to 5 years of bisphosphonate use and be reassessed 2 to 3 years later, largely based on the Fracture Intervention Trial Long-Term Extension (FLEX) study of alendronate. But risedronate has a shorter half-life, so it may provide shorter residual fracture protection.
Using Ontario administrative data, Dr. Hayes and associates identified more than 60,000 patients who were over aged 65, had received at least 3 years of continuous alendronate or risedronate, and had a subsequent 3-year drug holiday between 2000 and 2020.
They excluded patients who had a fracture or entered a nursing home within 120 days of starting a drug holiday who may have stopped the bisphosphonate due to declining health rather than a drug holiday.
Roughly half (55%) had been taking risedronate and 45% had been taking alendronate.
Using propensity scores, the researchers matched 25,077 patients who had been taking risedronate with an equal number who had been taking alendronate.
Most of the patients were women (82%) and were White.
They started the drug holiday when they were on average 81 years old, after taking the bisphosphonate for 5.9 years on average.
During the 3-year drug holiday, 915 of the 50,154 patients had hip fractures.
This was equivalent to 12.4 hip fractures per 1,000 patients per year during a risedronate holiday and 10.6 hip fractures per 1,000 patients per year during an alendronate holiday (hazard ratio, 1.18).
The risk of hip fracture was not significantly higher at 1 year (HR, 1.03) or at 2 years of a risedronate holiday versus an alendronate holiday (HR, 1.14).
However, the risk of a hip fracture was significantly higher at 2 to 3 years of a risedronate holiday than after an alendronate holiday (HR, 1.34).
There was no significant difference in the risk of any osteoporotic fracture overall (including hip, vertebrae, pelvis, ribs, forearm), however, during a 3-year risedronate versus alendronate drug holiday (HR, 1.07).
The research was supported by the Canadian Institutes of Health Research and Institute for Clinical Evaluative Sciences. Dr. Hayes was supported by a CIHR doctoral research award. The authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Researchers found a small but greater risk of a hip fracture after 2 years of taking a “drug holiday” – stopping therapy – after long-term (≥3-year) use of one bisphosphonate, risedronate, versus another, alendronate.
The risk of a hip fracture after stopping either of these oral bisphosphonate osteoporosis drugs was similar until 2 years, suggesting that patients who take a drug holiday from risedronate should be revaluated before 2 years.
These top-line findings from a propensity-matched cohort study of older patients in Ontario, Canada, were reported at the annual American Society of Bone and Mineral Research (ASBMR) last fall.
The full study, led by Kaleen N. Hayes, PharmD, PhD, Brown University School of Public Health, Providence, R.I., was published online in the Annals of Internal Medicine.
“We emphasize that our results do not indicate that alendronate therapy should be preferred over risedronate therapy,” the researchers stress, as several real-world studies found a similar risk of fractures while patients were receiving either drug.
“The decision to initiate alendronate or risedronate therapy [the two most commonly prescribed bisphosphonates] is driven by the prescriber,” they note, adding that some patients may prefer risedronate because it is available as a monthly dose or a weekly delayed-release formula that does not require fasting.
“We found little difference in the association between risedronate versus alendronate drug holidays and hip fractures until approximately 2 years of not receiving therapy,” Dr. Hayes and colleagues summarize.
Over 3 years, risedronate drug holidays were associated with an 18% relative and 0.6% absolute increased risk for hip fracture compared with alendronate drug holidays.
“To further inform clinical decision-making on drug holidays,” they conclude, “future research should examine when to start and restart osteoporosis therapy on the basis of initial length and type of treatment, patient characteristics, and relative risk for hip fractures versus [atypical femoral fracture].”
Hip fracture risk with risedronate vs. alendronate drug holiday
Long-term bisphosphonate use is associated with a rare risk of osteonecrosis of the jaw or atypical femoral fractures. At the same time, bisphosphonates continue to have a therapeutic effect after therapy is discontinued.
Guidelines recommend that patients at low risk of fracture should therefore have a “drug holiday” after 3 to 5 years of bisphosphonate use and be reassessed 2 to 3 years later, largely based on the Fracture Intervention Trial Long-Term Extension (FLEX) study of alendronate. But risedronate has a shorter half-life, so it may provide shorter residual fracture protection.
Using Ontario administrative data, Dr. Hayes and associates identified more than 60,000 patients who were over aged 65, had received at least 3 years of continuous alendronate or risedronate, and had a subsequent 3-year drug holiday between 2000 and 2020.
They excluded patients who had a fracture or entered a nursing home within 120 days of starting a drug holiday who may have stopped the bisphosphonate due to declining health rather than a drug holiday.
Roughly half (55%) had been taking risedronate and 45% had been taking alendronate.
Using propensity scores, the researchers matched 25,077 patients who had been taking risedronate with an equal number who had been taking alendronate.
Most of the patients were women (82%) and were White.
They started the drug holiday when they were on average 81 years old, after taking the bisphosphonate for 5.9 years on average.
During the 3-year drug holiday, 915 of the 50,154 patients had hip fractures.
This was equivalent to 12.4 hip fractures per 1,000 patients per year during a risedronate holiday and 10.6 hip fractures per 1,000 patients per year during an alendronate holiday (hazard ratio, 1.18).
The risk of hip fracture was not significantly higher at 1 year (HR, 1.03) or at 2 years of a risedronate holiday versus an alendronate holiday (HR, 1.14).
However, the risk of a hip fracture was significantly higher at 2 to 3 years of a risedronate holiday than after an alendronate holiday (HR, 1.34).
There was no significant difference in the risk of any osteoporotic fracture overall (including hip, vertebrae, pelvis, ribs, forearm), however, during a 3-year risedronate versus alendronate drug holiday (HR, 1.07).
The research was supported by the Canadian Institutes of Health Research and Institute for Clinical Evaluative Sciences. Dr. Hayes was supported by a CIHR doctoral research award. The authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Cervical cancer screening rates on the decline in the U.S.
The number of women screened for cervical cancer in the United States declined between 2005 and 2019 with lack of knowledge about the need for screening being cited as the most common reason for not receiving up-to-date screening. These are the results of a population-based, cross-sectional study conducted by the U.S. Preventive Services Task Force and were published online in JAMA Network Open.
“The fact that this reason increased over time across most sociodemographic groups suggests a need for interventions targeting screening awareness for all women,” lead author Ryan Suk, PhD, MS, from the University of Texas Health Science Center, Houston, and colleagues wrote.
Between 2005 and 2019, the researchers evaluated data from 20,557 women (weighted, 113.1 million women) included in the U.S. National Health Interview Survey. The cohort included women aged 21-65 years without previous hysterectomy and included data on sociodemographic factors such as race, ethnicity, sexual orientation, health insurance type, and rurality of residence.
Dr. Suk and colleagues found that the proportion of women without current screening increased from 2005 to 2019 (from 14.4% to 23.0%; P < .001) and that a higher proportion of those women were in the 21- to 29-year age group (weighted, 29.1%), compared with women in the 30- to 65-year age group (weighted, 21.1%; P < .001). Regardless of age, not knowing that screening was indicated was the most common reason cited for not having up-to-date screening.
Sociodemographic factors influence on rates and reasons for overdue screening
Based on weighted population estimates, 6.1% of women included were Asian, 17.2% were Hispanic, 13.1% were non-Hispanic Black, 61% were non-Hispanic White, and 2.7% were other races and/or ethnicities.
Dr. Suk and colleagues found that Asian women had the highest rates of overdue screening, compared with non-Hispanic White women, who had the lowest rates (weighted, 31.4% vs. 20.1%, respectively). The authors also found that reasons for overdue screening varied by sociodemographic factors. For example, while both Asian and Hispanic women cited lack of knowledge as a barrier to routine screening, Asian women were more likely to also report lack of recommendation from a health care professional as a barrier while Hispanic women were more likely to also report lack of access as a barrier to timely screening.
Over the 14-year study period, higher rates of overdue screening were also noted among those identifying as LGBTQ+ versus heterosexual (32.0% vs. 22.2%; P < .001), those with no insurance versus private insurance (41.7% vs. 18.1%; P < .001), and those living in rural versus urban areas (26.2% vs. 22.6%; P = .04).
For the study, guideline-concordant, up-to-date screening in 2005 was defined as screening every 3 years for women aged 21-65 years based on USPSTF guidelines and clinical recommendations. For 2019, up-to-date screening was defined as screening every 3 years with a Papanicolaou (Pap smear) test alone for women aged 21-29 years and screening every 3 years with a Pap smear alone or every 5 years with high-risk human papillomavirus testing or cotesting for women aged 30-65 years.
Dr. Suk and colleagues suggested that guideline updates over the study period could have led to uncertainty regarding appropriate timing and recommended screening intervals, which in turn, may have played a role in decreased cancer screening recommendations.
“Studies have suggested that changing guidelines may produce an increase in both overscreening and underscreening but those already at higher risk of cervical cancer may be most susceptible to underscreening,” wrote the authors.
In an interview, Ruchi Garg, MD, from Mid Atlantic Gynecologic Oncology and Pelvic Surgery Associates, Fairfax, Va., commented: “I think it has been hard to keep up with the guidelines changing so frequently. Furthermore it’s not clearly delineated (or at least there seems to be confusion or extrapolation) that the guidelines are just for Pap smear and that it doesn’t translate into a well woman checkup/pelvic exam; [however], if physicians continue to tell the patients to come in every year, then there won’t be so much underscreening since the physicians/providers will be able to keep track of when the Pap smears need to get done.”
Similar to the study authors, Dr. Garg also suggested that community lectures and public health announcements, particularly when guidelines are updated, will be helpful in enhancing patient education and reducing the rate of this preventable cancer.
The study authors and commentator disclosed no relevant financial relationships.
The number of women screened for cervical cancer in the United States declined between 2005 and 2019 with lack of knowledge about the need for screening being cited as the most common reason for not receiving up-to-date screening. These are the results of a population-based, cross-sectional study conducted by the U.S. Preventive Services Task Force and were published online in JAMA Network Open.
“The fact that this reason increased over time across most sociodemographic groups suggests a need for interventions targeting screening awareness for all women,” lead author Ryan Suk, PhD, MS, from the University of Texas Health Science Center, Houston, and colleagues wrote.
Between 2005 and 2019, the researchers evaluated data from 20,557 women (weighted, 113.1 million women) included in the U.S. National Health Interview Survey. The cohort included women aged 21-65 years without previous hysterectomy and included data on sociodemographic factors such as race, ethnicity, sexual orientation, health insurance type, and rurality of residence.
Dr. Suk and colleagues found that the proportion of women without current screening increased from 2005 to 2019 (from 14.4% to 23.0%; P < .001) and that a higher proportion of those women were in the 21- to 29-year age group (weighted, 29.1%), compared with women in the 30- to 65-year age group (weighted, 21.1%; P < .001). Regardless of age, not knowing that screening was indicated was the most common reason cited for not having up-to-date screening.
Sociodemographic factors influence on rates and reasons for overdue screening
Based on weighted population estimates, 6.1% of women included were Asian, 17.2% were Hispanic, 13.1% were non-Hispanic Black, 61% were non-Hispanic White, and 2.7% were other races and/or ethnicities.
Dr. Suk and colleagues found that Asian women had the highest rates of overdue screening, compared with non-Hispanic White women, who had the lowest rates (weighted, 31.4% vs. 20.1%, respectively). The authors also found that reasons for overdue screening varied by sociodemographic factors. For example, while both Asian and Hispanic women cited lack of knowledge as a barrier to routine screening, Asian women were more likely to also report lack of recommendation from a health care professional as a barrier while Hispanic women were more likely to also report lack of access as a barrier to timely screening.
Over the 14-year study period, higher rates of overdue screening were also noted among those identifying as LGBTQ+ versus heterosexual (32.0% vs. 22.2%; P < .001), those with no insurance versus private insurance (41.7% vs. 18.1%; P < .001), and those living in rural versus urban areas (26.2% vs. 22.6%; P = .04).
For the study, guideline-concordant, up-to-date screening in 2005 was defined as screening every 3 years for women aged 21-65 years based on USPSTF guidelines and clinical recommendations. For 2019, up-to-date screening was defined as screening every 3 years with a Papanicolaou (Pap smear) test alone for women aged 21-29 years and screening every 3 years with a Pap smear alone or every 5 years with high-risk human papillomavirus testing or cotesting for women aged 30-65 years.
Dr. Suk and colleagues suggested that guideline updates over the study period could have led to uncertainty regarding appropriate timing and recommended screening intervals, which in turn, may have played a role in decreased cancer screening recommendations.
“Studies have suggested that changing guidelines may produce an increase in both overscreening and underscreening but those already at higher risk of cervical cancer may be most susceptible to underscreening,” wrote the authors.
In an interview, Ruchi Garg, MD, from Mid Atlantic Gynecologic Oncology and Pelvic Surgery Associates, Fairfax, Va., commented: “I think it has been hard to keep up with the guidelines changing so frequently. Furthermore it’s not clearly delineated (or at least there seems to be confusion or extrapolation) that the guidelines are just for Pap smear and that it doesn’t translate into a well woman checkup/pelvic exam; [however], if physicians continue to tell the patients to come in every year, then there won’t be so much underscreening since the physicians/providers will be able to keep track of when the Pap smears need to get done.”
Similar to the study authors, Dr. Garg also suggested that community lectures and public health announcements, particularly when guidelines are updated, will be helpful in enhancing patient education and reducing the rate of this preventable cancer.
The study authors and commentator disclosed no relevant financial relationships.
The number of women screened for cervical cancer in the United States declined between 2005 and 2019 with lack of knowledge about the need for screening being cited as the most common reason for not receiving up-to-date screening. These are the results of a population-based, cross-sectional study conducted by the U.S. Preventive Services Task Force and were published online in JAMA Network Open.
“The fact that this reason increased over time across most sociodemographic groups suggests a need for interventions targeting screening awareness for all women,” lead author Ryan Suk, PhD, MS, from the University of Texas Health Science Center, Houston, and colleagues wrote.
Between 2005 and 2019, the researchers evaluated data from 20,557 women (weighted, 113.1 million women) included in the U.S. National Health Interview Survey. The cohort included women aged 21-65 years without previous hysterectomy and included data on sociodemographic factors such as race, ethnicity, sexual orientation, health insurance type, and rurality of residence.
Dr. Suk and colleagues found that the proportion of women without current screening increased from 2005 to 2019 (from 14.4% to 23.0%; P < .001) and that a higher proportion of those women were in the 21- to 29-year age group (weighted, 29.1%), compared with women in the 30- to 65-year age group (weighted, 21.1%; P < .001). Regardless of age, not knowing that screening was indicated was the most common reason cited for not having up-to-date screening.
Sociodemographic factors influence on rates and reasons for overdue screening
Based on weighted population estimates, 6.1% of women included were Asian, 17.2% were Hispanic, 13.1% were non-Hispanic Black, 61% were non-Hispanic White, and 2.7% were other races and/or ethnicities.
Dr. Suk and colleagues found that Asian women had the highest rates of overdue screening, compared with non-Hispanic White women, who had the lowest rates (weighted, 31.4% vs. 20.1%, respectively). The authors also found that reasons for overdue screening varied by sociodemographic factors. For example, while both Asian and Hispanic women cited lack of knowledge as a barrier to routine screening, Asian women were more likely to also report lack of recommendation from a health care professional as a barrier while Hispanic women were more likely to also report lack of access as a barrier to timely screening.
Over the 14-year study period, higher rates of overdue screening were also noted among those identifying as LGBTQ+ versus heterosexual (32.0% vs. 22.2%; P < .001), those with no insurance versus private insurance (41.7% vs. 18.1%; P < .001), and those living in rural versus urban areas (26.2% vs. 22.6%; P = .04).
For the study, guideline-concordant, up-to-date screening in 2005 was defined as screening every 3 years for women aged 21-65 years based on USPSTF guidelines and clinical recommendations. For 2019, up-to-date screening was defined as screening every 3 years with a Papanicolaou (Pap smear) test alone for women aged 21-29 years and screening every 3 years with a Pap smear alone or every 5 years with high-risk human papillomavirus testing or cotesting for women aged 30-65 years.
Dr. Suk and colleagues suggested that guideline updates over the study period could have led to uncertainty regarding appropriate timing and recommended screening intervals, which in turn, may have played a role in decreased cancer screening recommendations.
“Studies have suggested that changing guidelines may produce an increase in both overscreening and underscreening but those already at higher risk of cervical cancer may be most susceptible to underscreening,” wrote the authors.
In an interview, Ruchi Garg, MD, from Mid Atlantic Gynecologic Oncology and Pelvic Surgery Associates, Fairfax, Va., commented: “I think it has been hard to keep up with the guidelines changing so frequently. Furthermore it’s not clearly delineated (or at least there seems to be confusion or extrapolation) that the guidelines are just for Pap smear and that it doesn’t translate into a well woman checkup/pelvic exam; [however], if physicians continue to tell the patients to come in every year, then there won’t be so much underscreening since the physicians/providers will be able to keep track of when the Pap smears need to get done.”
Similar to the study authors, Dr. Garg also suggested that community lectures and public health announcements, particularly when guidelines are updated, will be helpful in enhancing patient education and reducing the rate of this preventable cancer.
The study authors and commentator disclosed no relevant financial relationships.
FROM JAMA NETWORK OPEN