User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
Updated ACG GERD guideline addresses increased scrutiny of PPI therapy
For the first time since 2013, the American College of Gastroenterology has issued updated evidence-based recommendations and practical guidance on the evaluation and management of gastroesophageal reflux disease (GERD), including pharmacologic, lifestyle, surgical, and endoscopic management.
Over the past 8 years, understanding of the varied presentations of GERD, enhancements in diagnostic testing, and approach to patient management have evolved, and there has been closer scrutiny of proton pump inhibitor (PPI) therapy and its potential side effects, the guideline authors said.
While PPIs remain the “medical treatment of choice” for GERD, multiple studies have raised questions about adverse events, they noted.
“We now know a lot more about PPI adverse events in the sense that we have another 8 years of experience” since the 2013 guideline, first author Philip O. Katz, MD, professor of medicine and director of motility laboratories at Weill Cornell Medicine, New York, added in an interview.
This update emphasizes the importance of making an accurate diagnosis and recommends PPI therapy “when patients really have GERD and being careful to use the lowest effective dose,” Dr. Katz said.
The guideline was published online Nov. 22, 2021, in the American Journal of Gastroenterology.
Benefits outweigh risks
The guideline suggests telling patients that PPIs are the most effective medical treatment for GERD.
Some studies have identified an association between the long-term use of PPIs and the development of several adverse conditions, including intestinal infections, pneumonia, stomach cancer, osteoporosis-related bone fractures, chronic kidney disease, deficiencies of certain vitamins and minerals, heart attacks, strokes, dementia, and early death.
Clinicians should emphasize, however, that these studies have flaws, are not considered definitive, and do not establish a cause-and-effect relationship between PPIs and the adverse conditions.
They should also emphasize to patients that high-quality studies have found that PPIs do not significantly raise the risk of any of these conditions except intestinal infections.
Patients should be told that, for the treatment of GERD, “gastroenterologists generally agree that the well-established benefits of PPIs far outweigh their theoretical risks.”
“Everything in this guideline makes sense,” Scott Gabbard, MD, gastroenterologist and section head, Center for Neurogastroenterology and Motility, Cleveland Clinic, who wasn’t involved in the guideline development, said in an interview.
“A PPI trial for anyone with typical GERD symptoms and having those who respond taper to the lowest effective dose is still the first line for anyone with GERD,” Dr. Gabbard said.
Making the diagnosis
As there is currently no gold standard for the diagnosis of GERD, diagnosis is based on a combination of symptoms, endoscopic evaluation of esophageal mucosa, reflux monitoring, and response to therapeutic intervention, the guideline says.
For patients with classic symptoms of heartburn and regurgitation with no alarm symptoms, the authors recommend an 8-week trial of empiric once-daily PPIs before a meal. If the patient responds, the guideline recommends attempting to discontinue the medication.
The guideline recommends diagnostic endoscopy after PPIs are stopped for 2-4 weeks in patients whose classic symptoms fail to respond adequately to the 8-week empiric PPI trial or in those whose symptoms return when PPIs are discontinued.
For patients with chest pain but no heartburn who have undergone an adequate evaluation to exclude heart disease, the guideline advises objective testing for GERD (endoscopy and/or reflux monitoring).
The use of barium swallow solely as a diagnostic test for GERD is not recommended.
Endoscopy should be the first test for evaluating patients presenting with dysphagia or other alarm symptoms, such as weight loss and gastrointestinal bleeding, as well as for patients with risk factors for Barrett’s esophagus.
For patients in whom the diagnosis of GERD is suspected but unclear and endoscopy fails to show objective evidence of GERD, the guidelines advise reflux monitoring off therapy to establish the diagnosis.
The guideline recommends against reflux monitoring off therapy solely as a diagnostic test for GERD in patients with known endoscopic evidence of Los Angeles grade C or D reflux esophagitis or in patients with long-segment Barrett’s esophagus.
High-resolution manometry solely as a diagnostic test for GERD is also not recommended.
Medical management of GERD
Recommendations for medical management of GERD include weight loss in patients who are overweight or obese, avoidance of meals within 2-3 hours of bedtime, avoidance of tobacco products and “trigger foods,” and elevation of the head of the bed for nighttime symptoms.
Treatment with a PPI is recommended over histamine2-receptor antagonists for healing and maintenance of healing of eosinophilic esophagitis. Taking a PPI 30-60 minutes prior to a meal rather than at bedtime is recommended.
“Use of the lowest effective PPI dose is recommended and logical but must be individualized,” the guideline states.
There is “conceptual rationale” for a trial of switching PPIs for patients who don’t respond to one PPI. However, switching more than once to another PPI “cannot be supported,” the guideline says.
Dr. Gabbard said the advice about switching PPIs in nonresponders is particularly helpful.
“In clinical practice, I see patients who try one PPI, and if it doesn’t work, their doctor puts them on another PPI, then another and another, until they get through five PPIs and gotten nowhere,” he said in an interview.
“This new guideline is very helpful in saying, if a patient has GERD symptoms that do not respond to a PPI, you can do one switch. But, if that doesn’t work, have a low threshold to perform pH testing to determine if the patient truly has reflux or not,” Dr. Gabbard said.
“Some studies have suggested that up to 75% of PPI nonresponders actually don’t have reflux. They have functional heartburn, which is not reflux and is treated without PPIs,” he noted.
One area of controversy relates to abrupt PPI discontinuation and potential rebound acid hypersecretion, resulting in increased reflux symptoms. While this has been found in healthy control patients, strong evidence for an increase in symptoms after abrupt PPI withdrawal is lacking.
The guideline makes “no definitive recommendation as to whether weaning or stopping PPIs cold turkey is a better approach, due to a lack of evidence,” Dr. Katz said in an interview.
For patients with GERD without erosive esophagitis or Barrett’s esophagus and whose symptoms resolve with PPI therapy, the guideline says an attempt should be made to discontinue PPI therapy or to switch to on-demand therapy in which a PPI is taken only when symptoms occur and is stopped when they are relieved.
For patients with Los Angeles grade C or D esophagitis, the recommendation is for maintenance PPI therapy indefinitely or antireflux surgery.
Dr. Gabbard said it’s “nice to have in writing from the ACG that patients with erosive esophagitis or Barrett’s esophagus – those who truly need a PPI – should be on indefinite PPI therapy, because the benefit of a PPI far outweighs the theoretical risks.”
The research had no financial support. Dr. Katz has served as consultant for Phathom Pharma and Medtronic, has received research support from Diversatek and royalties from UpToDate, and serves on the Medscape Gastroenterology advisory board. Dr. Gabbard disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
For the first time since 2013, the American College of Gastroenterology has issued updated evidence-based recommendations and practical guidance on the evaluation and management of gastroesophageal reflux disease (GERD), including pharmacologic, lifestyle, surgical, and endoscopic management.
Over the past 8 years, understanding of the varied presentations of GERD, enhancements in diagnostic testing, and approach to patient management have evolved, and there has been closer scrutiny of proton pump inhibitor (PPI) therapy and its potential side effects, the guideline authors said.
While PPIs remain the “medical treatment of choice” for GERD, multiple studies have raised questions about adverse events, they noted.
“We now know a lot more about PPI adverse events in the sense that we have another 8 years of experience” since the 2013 guideline, first author Philip O. Katz, MD, professor of medicine and director of motility laboratories at Weill Cornell Medicine, New York, added in an interview.
This update emphasizes the importance of making an accurate diagnosis and recommends PPI therapy “when patients really have GERD and being careful to use the lowest effective dose,” Dr. Katz said.
The guideline was published online Nov. 22, 2021, in the American Journal of Gastroenterology.
Benefits outweigh risks
The guideline suggests telling patients that PPIs are the most effective medical treatment for GERD.
Some studies have identified an association between the long-term use of PPIs and the development of several adverse conditions, including intestinal infections, pneumonia, stomach cancer, osteoporosis-related bone fractures, chronic kidney disease, deficiencies of certain vitamins and minerals, heart attacks, strokes, dementia, and early death.
Clinicians should emphasize, however, that these studies have flaws, are not considered definitive, and do not establish a cause-and-effect relationship between PPIs and the adverse conditions.
They should also emphasize to patients that high-quality studies have found that PPIs do not significantly raise the risk of any of these conditions except intestinal infections.
Patients should be told that, for the treatment of GERD, “gastroenterologists generally agree that the well-established benefits of PPIs far outweigh their theoretical risks.”
“Everything in this guideline makes sense,” Scott Gabbard, MD, gastroenterologist and section head, Center for Neurogastroenterology and Motility, Cleveland Clinic, who wasn’t involved in the guideline development, said in an interview.
“A PPI trial for anyone with typical GERD symptoms and having those who respond taper to the lowest effective dose is still the first line for anyone with GERD,” Dr. Gabbard said.
Making the diagnosis
As there is currently no gold standard for the diagnosis of GERD, diagnosis is based on a combination of symptoms, endoscopic evaluation of esophageal mucosa, reflux monitoring, and response to therapeutic intervention, the guideline says.
For patients with classic symptoms of heartburn and regurgitation with no alarm symptoms, the authors recommend an 8-week trial of empiric once-daily PPIs before a meal. If the patient responds, the guideline recommends attempting to discontinue the medication.
The guideline recommends diagnostic endoscopy after PPIs are stopped for 2-4 weeks in patients whose classic symptoms fail to respond adequately to the 8-week empiric PPI trial or in those whose symptoms return when PPIs are discontinued.
For patients with chest pain but no heartburn who have undergone an adequate evaluation to exclude heart disease, the guideline advises objective testing for GERD (endoscopy and/or reflux monitoring).
The use of barium swallow solely as a diagnostic test for GERD is not recommended.
Endoscopy should be the first test for evaluating patients presenting with dysphagia or other alarm symptoms, such as weight loss and gastrointestinal bleeding, as well as for patients with risk factors for Barrett’s esophagus.
For patients in whom the diagnosis of GERD is suspected but unclear and endoscopy fails to show objective evidence of GERD, the guidelines advise reflux monitoring off therapy to establish the diagnosis.
The guideline recommends against reflux monitoring off therapy solely as a diagnostic test for GERD in patients with known endoscopic evidence of Los Angeles grade C or D reflux esophagitis or in patients with long-segment Barrett’s esophagus.
High-resolution manometry solely as a diagnostic test for GERD is also not recommended.
Medical management of GERD
Recommendations for medical management of GERD include weight loss in patients who are overweight or obese, avoidance of meals within 2-3 hours of bedtime, avoidance of tobacco products and “trigger foods,” and elevation of the head of the bed for nighttime symptoms.
Treatment with a PPI is recommended over histamine2-receptor antagonists for healing and maintenance of healing of eosinophilic esophagitis. Taking a PPI 30-60 minutes prior to a meal rather than at bedtime is recommended.
“Use of the lowest effective PPI dose is recommended and logical but must be individualized,” the guideline states.
There is “conceptual rationale” for a trial of switching PPIs for patients who don’t respond to one PPI. However, switching more than once to another PPI “cannot be supported,” the guideline says.
Dr. Gabbard said the advice about switching PPIs in nonresponders is particularly helpful.
“In clinical practice, I see patients who try one PPI, and if it doesn’t work, their doctor puts them on another PPI, then another and another, until they get through five PPIs and gotten nowhere,” he said in an interview.
“This new guideline is very helpful in saying, if a patient has GERD symptoms that do not respond to a PPI, you can do one switch. But, if that doesn’t work, have a low threshold to perform pH testing to determine if the patient truly has reflux or not,” Dr. Gabbard said.
“Some studies have suggested that up to 75% of PPI nonresponders actually don’t have reflux. They have functional heartburn, which is not reflux and is treated without PPIs,” he noted.
One area of controversy relates to abrupt PPI discontinuation and potential rebound acid hypersecretion, resulting in increased reflux symptoms. While this has been found in healthy control patients, strong evidence for an increase in symptoms after abrupt PPI withdrawal is lacking.
The guideline makes “no definitive recommendation as to whether weaning or stopping PPIs cold turkey is a better approach, due to a lack of evidence,” Dr. Katz said in an interview.
For patients with GERD without erosive esophagitis or Barrett’s esophagus and whose symptoms resolve with PPI therapy, the guideline says an attempt should be made to discontinue PPI therapy or to switch to on-demand therapy in which a PPI is taken only when symptoms occur and is stopped when they are relieved.
For patients with Los Angeles grade C or D esophagitis, the recommendation is for maintenance PPI therapy indefinitely or antireflux surgery.
Dr. Gabbard said it’s “nice to have in writing from the ACG that patients with erosive esophagitis or Barrett’s esophagus – those who truly need a PPI – should be on indefinite PPI therapy, because the benefit of a PPI far outweighs the theoretical risks.”
The research had no financial support. Dr. Katz has served as consultant for Phathom Pharma and Medtronic, has received research support from Diversatek and royalties from UpToDate, and serves on the Medscape Gastroenterology advisory board. Dr. Gabbard disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
For the first time since 2013, the American College of Gastroenterology has issued updated evidence-based recommendations and practical guidance on the evaluation and management of gastroesophageal reflux disease (GERD), including pharmacologic, lifestyle, surgical, and endoscopic management.
Over the past 8 years, understanding of the varied presentations of GERD, enhancements in diagnostic testing, and approach to patient management have evolved, and there has been closer scrutiny of proton pump inhibitor (PPI) therapy and its potential side effects, the guideline authors said.
While PPIs remain the “medical treatment of choice” for GERD, multiple studies have raised questions about adverse events, they noted.
“We now know a lot more about PPI adverse events in the sense that we have another 8 years of experience” since the 2013 guideline, first author Philip O. Katz, MD, professor of medicine and director of motility laboratories at Weill Cornell Medicine, New York, added in an interview.
This update emphasizes the importance of making an accurate diagnosis and recommends PPI therapy “when patients really have GERD and being careful to use the lowest effective dose,” Dr. Katz said.
The guideline was published online Nov. 22, 2021, in the American Journal of Gastroenterology.
Benefits outweigh risks
The guideline suggests telling patients that PPIs are the most effective medical treatment for GERD.
Some studies have identified an association between the long-term use of PPIs and the development of several adverse conditions, including intestinal infections, pneumonia, stomach cancer, osteoporosis-related bone fractures, chronic kidney disease, deficiencies of certain vitamins and minerals, heart attacks, strokes, dementia, and early death.
Clinicians should emphasize, however, that these studies have flaws, are not considered definitive, and do not establish a cause-and-effect relationship between PPIs and the adverse conditions.
They should also emphasize to patients that high-quality studies have found that PPIs do not significantly raise the risk of any of these conditions except intestinal infections.
Patients should be told that, for the treatment of GERD, “gastroenterologists generally agree that the well-established benefits of PPIs far outweigh their theoretical risks.”
“Everything in this guideline makes sense,” Scott Gabbard, MD, gastroenterologist and section head, Center for Neurogastroenterology and Motility, Cleveland Clinic, who wasn’t involved in the guideline development, said in an interview.
“A PPI trial for anyone with typical GERD symptoms and having those who respond taper to the lowest effective dose is still the first line for anyone with GERD,” Dr. Gabbard said.
Making the diagnosis
As there is currently no gold standard for the diagnosis of GERD, diagnosis is based on a combination of symptoms, endoscopic evaluation of esophageal mucosa, reflux monitoring, and response to therapeutic intervention, the guideline says.
For patients with classic symptoms of heartburn and regurgitation with no alarm symptoms, the authors recommend an 8-week trial of empiric once-daily PPIs before a meal. If the patient responds, the guideline recommends attempting to discontinue the medication.
The guideline recommends diagnostic endoscopy after PPIs are stopped for 2-4 weeks in patients whose classic symptoms fail to respond adequately to the 8-week empiric PPI trial or in those whose symptoms return when PPIs are discontinued.
For patients with chest pain but no heartburn who have undergone an adequate evaluation to exclude heart disease, the guideline advises objective testing for GERD (endoscopy and/or reflux monitoring).
The use of barium swallow solely as a diagnostic test for GERD is not recommended.
Endoscopy should be the first test for evaluating patients presenting with dysphagia or other alarm symptoms, such as weight loss and gastrointestinal bleeding, as well as for patients with risk factors for Barrett’s esophagus.
For patients in whom the diagnosis of GERD is suspected but unclear and endoscopy fails to show objective evidence of GERD, the guidelines advise reflux monitoring off therapy to establish the diagnosis.
The guideline recommends against reflux monitoring off therapy solely as a diagnostic test for GERD in patients with known endoscopic evidence of Los Angeles grade C or D reflux esophagitis or in patients with long-segment Barrett’s esophagus.
High-resolution manometry solely as a diagnostic test for GERD is also not recommended.
Medical management of GERD
Recommendations for medical management of GERD include weight loss in patients who are overweight or obese, avoidance of meals within 2-3 hours of bedtime, avoidance of tobacco products and “trigger foods,” and elevation of the head of the bed for nighttime symptoms.
Treatment with a PPI is recommended over histamine2-receptor antagonists for healing and maintenance of healing of eosinophilic esophagitis. Taking a PPI 30-60 minutes prior to a meal rather than at bedtime is recommended.
“Use of the lowest effective PPI dose is recommended and logical but must be individualized,” the guideline states.
There is “conceptual rationale” for a trial of switching PPIs for patients who don’t respond to one PPI. However, switching more than once to another PPI “cannot be supported,” the guideline says.
Dr. Gabbard said the advice about switching PPIs in nonresponders is particularly helpful.
“In clinical practice, I see patients who try one PPI, and if it doesn’t work, their doctor puts them on another PPI, then another and another, until they get through five PPIs and gotten nowhere,” he said in an interview.
“This new guideline is very helpful in saying, if a patient has GERD symptoms that do not respond to a PPI, you can do one switch. But, if that doesn’t work, have a low threshold to perform pH testing to determine if the patient truly has reflux or not,” Dr. Gabbard said.
“Some studies have suggested that up to 75% of PPI nonresponders actually don’t have reflux. They have functional heartburn, which is not reflux and is treated without PPIs,” he noted.
One area of controversy relates to abrupt PPI discontinuation and potential rebound acid hypersecretion, resulting in increased reflux symptoms. While this has been found in healthy control patients, strong evidence for an increase in symptoms after abrupt PPI withdrawal is lacking.
The guideline makes “no definitive recommendation as to whether weaning or stopping PPIs cold turkey is a better approach, due to a lack of evidence,” Dr. Katz said in an interview.
For patients with GERD without erosive esophagitis or Barrett’s esophagus and whose symptoms resolve with PPI therapy, the guideline says an attempt should be made to discontinue PPI therapy or to switch to on-demand therapy in which a PPI is taken only when symptoms occur and is stopped when they are relieved.
For patients with Los Angeles grade C or D esophagitis, the recommendation is for maintenance PPI therapy indefinitely or antireflux surgery.
Dr. Gabbard said it’s “nice to have in writing from the ACG that patients with erosive esophagitis or Barrett’s esophagus – those who truly need a PPI – should be on indefinite PPI therapy, because the benefit of a PPI far outweighs the theoretical risks.”
The research had no financial support. Dr. Katz has served as consultant for Phathom Pharma and Medtronic, has received research support from Diversatek and royalties from UpToDate, and serves on the Medscape Gastroenterology advisory board. Dr. Gabbard disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE AMERICAN JOURNAL OF GASTROENTEROLOGY
Omicron may require fourth vaccine dose, Pfizer says
, Pfizer officials said on Dec. 8.
The standard two doses may be less effective against the variant, the company announced earlier in the day, and a booster dose increases neutralizing antibodies.
But the timeline might need to be moved up for a fourth dose. Previously, Pfizer CEO Albert Bourla, PhD, said another dose might be needed about a year after a third shot. Now the company’s scientists believe that a fourth shot, which targets the Omicron variant, could be required sooner.
“With Omicron, we need to wait and see because we have very little information. We may need it faster,” Dr. Bourla said on CNBC’s Squawk Box.
“But for right now, the most important thing is that we have winter in front of us,” he said. “From a healthcare perspective, it is important to understand that we need to be well-protected to go through the winter.”
A third dose should provide protection throughout the winter, Dr. Bourla said. That may buy time until the early spring to develop new shots that target Omicron, which Pfizer could have ready by March, according to Bloomberg News.
As of the afternoon of Dec. 8, 43 people in 19 states had tested positive for the Omicron variant, according to The Associated Press. More than 75% had been vaccinated, and a third had had booster shots. About a third had traveled internationally.
Nearly all of them have had mild symptoms so far, the AP reported, with the most common symptoms being a cough, congestion, and fatigue. One person has been hospitalized, but no deaths have been reported so far.
The CDC is still trying to determine how the Omicron variant may affect the course of the pandemic and whether the strain is more contagious or causes more severe disease.
“What we generally know is the more mutations a variant has, the higher level you need your immunity to be,” Rochelle Walensky, MD, director of the CDC, told the AP.
“We want to make sure we bolster everybody’s immunity,” she said. “And that’s really what motivated the decision to expand our guidance [on boosters for all adults].”
The Omicron variant has been reported in 57 countries so far, World Health Organization officials reported Dec. 8, and they expect that number to continue growing.
“Certain features of Omicron, including its global spread and large number of mutations, suggest it could have a major impact on the course of the pandemic. Exactly what that impact will be is still difficult to know,” Tedros Adhanom Ghebreyesus, PhD, the World Health Organization’s director-general, said during a media briefing.
Several studies suggest that Omicron leads to a rapid increase in transmission, he said, though scientists are still trying to understand whether it can “outcompete Delta.” Data from South Africa also suggests a higher risk of reinfection with Omicron, though it appears to cause milder disease than Delta, he noted.
“Even though we still need answers to some crucial questions, we are not defenseless against Omicron or Delta,” he said. “The steps countries take today, and in the coming days and weeks, will determine how Omicron unfolds.”
A version of this article first appeared on WebMD.com.
, Pfizer officials said on Dec. 8.
The standard two doses may be less effective against the variant, the company announced earlier in the day, and a booster dose increases neutralizing antibodies.
But the timeline might need to be moved up for a fourth dose. Previously, Pfizer CEO Albert Bourla, PhD, said another dose might be needed about a year after a third shot. Now the company’s scientists believe that a fourth shot, which targets the Omicron variant, could be required sooner.
“With Omicron, we need to wait and see because we have very little information. We may need it faster,” Dr. Bourla said on CNBC’s Squawk Box.
“But for right now, the most important thing is that we have winter in front of us,” he said. “From a healthcare perspective, it is important to understand that we need to be well-protected to go through the winter.”
A third dose should provide protection throughout the winter, Dr. Bourla said. That may buy time until the early spring to develop new shots that target Omicron, which Pfizer could have ready by March, according to Bloomberg News.
As of the afternoon of Dec. 8, 43 people in 19 states had tested positive for the Omicron variant, according to The Associated Press. More than 75% had been vaccinated, and a third had had booster shots. About a third had traveled internationally.
Nearly all of them have had mild symptoms so far, the AP reported, with the most common symptoms being a cough, congestion, and fatigue. One person has been hospitalized, but no deaths have been reported so far.
The CDC is still trying to determine how the Omicron variant may affect the course of the pandemic and whether the strain is more contagious or causes more severe disease.
“What we generally know is the more mutations a variant has, the higher level you need your immunity to be,” Rochelle Walensky, MD, director of the CDC, told the AP.
“We want to make sure we bolster everybody’s immunity,” she said. “And that’s really what motivated the decision to expand our guidance [on boosters for all adults].”
The Omicron variant has been reported in 57 countries so far, World Health Organization officials reported Dec. 8, and they expect that number to continue growing.
“Certain features of Omicron, including its global spread and large number of mutations, suggest it could have a major impact on the course of the pandemic. Exactly what that impact will be is still difficult to know,” Tedros Adhanom Ghebreyesus, PhD, the World Health Organization’s director-general, said during a media briefing.
Several studies suggest that Omicron leads to a rapid increase in transmission, he said, though scientists are still trying to understand whether it can “outcompete Delta.” Data from South Africa also suggests a higher risk of reinfection with Omicron, though it appears to cause milder disease than Delta, he noted.
“Even though we still need answers to some crucial questions, we are not defenseless against Omicron or Delta,” he said. “The steps countries take today, and in the coming days and weeks, will determine how Omicron unfolds.”
A version of this article first appeared on WebMD.com.
, Pfizer officials said on Dec. 8.
The standard two doses may be less effective against the variant, the company announced earlier in the day, and a booster dose increases neutralizing antibodies.
But the timeline might need to be moved up for a fourth dose. Previously, Pfizer CEO Albert Bourla, PhD, said another dose might be needed about a year after a third shot. Now the company’s scientists believe that a fourth shot, which targets the Omicron variant, could be required sooner.
“With Omicron, we need to wait and see because we have very little information. We may need it faster,” Dr. Bourla said on CNBC’s Squawk Box.
“But for right now, the most important thing is that we have winter in front of us,” he said. “From a healthcare perspective, it is important to understand that we need to be well-protected to go through the winter.”
A third dose should provide protection throughout the winter, Dr. Bourla said. That may buy time until the early spring to develop new shots that target Omicron, which Pfizer could have ready by March, according to Bloomberg News.
As of the afternoon of Dec. 8, 43 people in 19 states had tested positive for the Omicron variant, according to The Associated Press. More than 75% had been vaccinated, and a third had had booster shots. About a third had traveled internationally.
Nearly all of them have had mild symptoms so far, the AP reported, with the most common symptoms being a cough, congestion, and fatigue. One person has been hospitalized, but no deaths have been reported so far.
The CDC is still trying to determine how the Omicron variant may affect the course of the pandemic and whether the strain is more contagious or causes more severe disease.
“What we generally know is the more mutations a variant has, the higher level you need your immunity to be,” Rochelle Walensky, MD, director of the CDC, told the AP.
“We want to make sure we bolster everybody’s immunity,” she said. “And that’s really what motivated the decision to expand our guidance [on boosters for all adults].”
The Omicron variant has been reported in 57 countries so far, World Health Organization officials reported Dec. 8, and they expect that number to continue growing.
“Certain features of Omicron, including its global spread and large number of mutations, suggest it could have a major impact on the course of the pandemic. Exactly what that impact will be is still difficult to know,” Tedros Adhanom Ghebreyesus, PhD, the World Health Organization’s director-general, said during a media briefing.
Several studies suggest that Omicron leads to a rapid increase in transmission, he said, though scientists are still trying to understand whether it can “outcompete Delta.” Data from South Africa also suggests a higher risk of reinfection with Omicron, though it appears to cause milder disease than Delta, he noted.
“Even though we still need answers to some crucial questions, we are not defenseless against Omicron or Delta,” he said. “The steps countries take today, and in the coming days and weeks, will determine how Omicron unfolds.”
A version of this article first appeared on WebMD.com.
FDA authorizes Pfizer boosters for 16- and 17-year-olds
, clearing the way for millions of teenagers to get a third dose of vaccine starting 6 months after their second dose.
The FDA said it was basing its emergency authorization of boosters for 16- and 17-year-olds on data from 200 individuals who were 18-55 years of age when they received a booster dose. They are requiring Pfizer to collect data on safety in postauthorization studies.
“The FDA has determined that the benefits of a single booster dose of the Pfizer-BioNTech COVID-19 Vaccine or Comirnaty outweigh the risks of myocarditis and pericarditis in individuals 16 and 17 years of age to provide continued protection against COVID-19 and the associated serious consequences that can occur including hospitalization and death,” the agency said in a news release.
Israel has been giving booster doses of Pfizer’s vaccine to everyone 12 and up since late August. Data from that country show that myocarditis cases continue to be very rare, even in younger age groups, and are mild and temporary.
The authorization comes as the effectiveness of the current vaccines against the new Omicron variant has become a point of intense scientific inquiry.
Early studies suggest that booster doses may be necessary to keep Omicron at bay, at least until new variant-specific vaccines are ready next spring.
Current evidence suggests that the protection of the vaccines is holding up well against severe disease and death, at least with Delta and early iterations of the virus.
How well they will do against Omicron, and how severe Omicron infections may be for different age groups, remain open questions.
On Dec. 8, the World Health Organization urged countries not to wait for all the science to come in, but to act now to contain any potential threat.
The first pieces of evidence on Omicron suggest that it is highly contagious, perhaps even more than Delta, though early reports suggest symptoms caused by this version of the new coronavirus may be less severe than in previous waves. Experts have cautioned that the true severity of Omicron infections isn’t yet known, since the first cases have been detected in younger people, who tend to have milder COVID-19 symptoms than those of adults and seniors.
“Vaccination and getting a booster when eligible, along with other preventive measures like masking and avoiding large crowds and poorly ventilated spaces, remain our most effective methods for fighting COVID-19,” Acting FDA Commissioner Janet Woodcock, MD, said in a news release. “As people gather indoors with family and friends for the holidays, we can’t let up on all the preventive public health measures that we have been taking during the pandemic. With both the Delta and Omicron variants continuing to spread, vaccination remains the best protection against COVID-19.”
In mid-November, the FDA authorized boosters of the Pfizer vaccine for all individuals 18 and older, but the agency held off on expanding the use of boosters for younger age groups, partly because they have the highest risk of myocarditis, a very rare side effect.
Myocarditis cases seem to be temporary, with patients making a full recovery, though they need to be monitored in the hospital. The risk of myocarditis with a COVID-19 infection is many times higher than it is from a vaccine.
There have been little data to support the need for boosters in this age group, because children and teens tend to experience milder COVID-19 disease, though they are still at risk for post–COVID-19 complications such as long COVID and a delayed reaction to the virus called Post Acute Sequelae of SARS-CoV2 Infection among Children, or PAS-C.
All that changed with the arrival of Omicron.
A version of this article first appeared on WebMD.com.
, clearing the way for millions of teenagers to get a third dose of vaccine starting 6 months after their second dose.
The FDA said it was basing its emergency authorization of boosters for 16- and 17-year-olds on data from 200 individuals who were 18-55 years of age when they received a booster dose. They are requiring Pfizer to collect data on safety in postauthorization studies.
“The FDA has determined that the benefits of a single booster dose of the Pfizer-BioNTech COVID-19 Vaccine or Comirnaty outweigh the risks of myocarditis and pericarditis in individuals 16 and 17 years of age to provide continued protection against COVID-19 and the associated serious consequences that can occur including hospitalization and death,” the agency said in a news release.
Israel has been giving booster doses of Pfizer’s vaccine to everyone 12 and up since late August. Data from that country show that myocarditis cases continue to be very rare, even in younger age groups, and are mild and temporary.
The authorization comes as the effectiveness of the current vaccines against the new Omicron variant has become a point of intense scientific inquiry.
Early studies suggest that booster doses may be necessary to keep Omicron at bay, at least until new variant-specific vaccines are ready next spring.
Current evidence suggests that the protection of the vaccines is holding up well against severe disease and death, at least with Delta and early iterations of the virus.
How well they will do against Omicron, and how severe Omicron infections may be for different age groups, remain open questions.
On Dec. 8, the World Health Organization urged countries not to wait for all the science to come in, but to act now to contain any potential threat.
The first pieces of evidence on Omicron suggest that it is highly contagious, perhaps even more than Delta, though early reports suggest symptoms caused by this version of the new coronavirus may be less severe than in previous waves. Experts have cautioned that the true severity of Omicron infections isn’t yet known, since the first cases have been detected in younger people, who tend to have milder COVID-19 symptoms than those of adults and seniors.
“Vaccination and getting a booster when eligible, along with other preventive measures like masking and avoiding large crowds and poorly ventilated spaces, remain our most effective methods for fighting COVID-19,” Acting FDA Commissioner Janet Woodcock, MD, said in a news release. “As people gather indoors with family and friends for the holidays, we can’t let up on all the preventive public health measures that we have been taking during the pandemic. With both the Delta and Omicron variants continuing to spread, vaccination remains the best protection against COVID-19.”
In mid-November, the FDA authorized boosters of the Pfizer vaccine for all individuals 18 and older, but the agency held off on expanding the use of boosters for younger age groups, partly because they have the highest risk of myocarditis, a very rare side effect.
Myocarditis cases seem to be temporary, with patients making a full recovery, though they need to be monitored in the hospital. The risk of myocarditis with a COVID-19 infection is many times higher than it is from a vaccine.
There have been little data to support the need for boosters in this age group, because children and teens tend to experience milder COVID-19 disease, though they are still at risk for post–COVID-19 complications such as long COVID and a delayed reaction to the virus called Post Acute Sequelae of SARS-CoV2 Infection among Children, or PAS-C.
All that changed with the arrival of Omicron.
A version of this article first appeared on WebMD.com.
, clearing the way for millions of teenagers to get a third dose of vaccine starting 6 months after their second dose.
The FDA said it was basing its emergency authorization of boosters for 16- and 17-year-olds on data from 200 individuals who were 18-55 years of age when they received a booster dose. They are requiring Pfizer to collect data on safety in postauthorization studies.
“The FDA has determined that the benefits of a single booster dose of the Pfizer-BioNTech COVID-19 Vaccine or Comirnaty outweigh the risks of myocarditis and pericarditis in individuals 16 and 17 years of age to provide continued protection against COVID-19 and the associated serious consequences that can occur including hospitalization and death,” the agency said in a news release.
Israel has been giving booster doses of Pfizer’s vaccine to everyone 12 and up since late August. Data from that country show that myocarditis cases continue to be very rare, even in younger age groups, and are mild and temporary.
The authorization comes as the effectiveness of the current vaccines against the new Omicron variant has become a point of intense scientific inquiry.
Early studies suggest that booster doses may be necessary to keep Omicron at bay, at least until new variant-specific vaccines are ready next spring.
Current evidence suggests that the protection of the vaccines is holding up well against severe disease and death, at least with Delta and early iterations of the virus.
How well they will do against Omicron, and how severe Omicron infections may be for different age groups, remain open questions.
On Dec. 8, the World Health Organization urged countries not to wait for all the science to come in, but to act now to contain any potential threat.
The first pieces of evidence on Omicron suggest that it is highly contagious, perhaps even more than Delta, though early reports suggest symptoms caused by this version of the new coronavirus may be less severe than in previous waves. Experts have cautioned that the true severity of Omicron infections isn’t yet known, since the first cases have been detected in younger people, who tend to have milder COVID-19 symptoms than those of adults and seniors.
“Vaccination and getting a booster when eligible, along with other preventive measures like masking and avoiding large crowds and poorly ventilated spaces, remain our most effective methods for fighting COVID-19,” Acting FDA Commissioner Janet Woodcock, MD, said in a news release. “As people gather indoors with family and friends for the holidays, we can’t let up on all the preventive public health measures that we have been taking during the pandemic. With both the Delta and Omicron variants continuing to spread, vaccination remains the best protection against COVID-19.”
In mid-November, the FDA authorized boosters of the Pfizer vaccine for all individuals 18 and older, but the agency held off on expanding the use of boosters for younger age groups, partly because they have the highest risk of myocarditis, a very rare side effect.
Myocarditis cases seem to be temporary, with patients making a full recovery, though they need to be monitored in the hospital. The risk of myocarditis with a COVID-19 infection is many times higher than it is from a vaccine.
There have been little data to support the need for boosters in this age group, because children and teens tend to experience milder COVID-19 disease, though they are still at risk for post–COVID-19 complications such as long COVID and a delayed reaction to the virus called Post Acute Sequelae of SARS-CoV2 Infection among Children, or PAS-C.
All that changed with the arrival of Omicron.
A version of this article first appeared on WebMD.com.
AMA, hospital group sue federal government over surprise billing law
which tilts toward using prevailing rates paid for services.
The American Hospital Association and American Medical Association said they will ask the U.S. District Court for the District of Columbia to try to prevent implementation of certain provisions of new federal rules on surprise bills. This court is often a venue for fights over federal rules. Also joining the suit are Nevada-based Renown Health, UMass Memorial Health, and two physicians based in North Carolina, AHA and AMA said.
Federal agencies, including the Department of Health & Human Services, in September had unveiled the rule on surprise medical bills that will take effect Jan. 1.
Under this rule, a key benchmark for payment disputes would be the qualifying payment amount (QPA), which is pegged to median contracted rates. In the dispute-resolution process outlined in the rule, there is a presumption that the QPA is the appropriate out-of-network rate.
The rule allows for exceptions in which the independent mediating organization handling the payment dispute resolution has “credible information” as to why the QPA is materially different from the appropriate out-of-network rate.
In the view of the federal agencies that issued the rule, this approach “encourages predictable outcomes,” which likely would reduce the number of disputes that go through the resolution process while also “providing equitable and clear standards” for cases to appropriately deviate from QPA. HHS was joined in issuing the rule by the Treasury and Labor Departments and the Office of Personnel Management.
AMA and AHA disagree with their view, seeing this approach as a boon for insurers at the expense of physicians and hospitals.
In a press release, they said the rule’s approach to surprise billing would “all but ensure that hospitals, physicians, and other providers will routinely be undercompensated by commercial insurers, and patients will have fewer choices for access to in-network services.”
The rule is part of the implementation of a federal law passed in December 2020, known as the No Surprises Act. In their statement, AHA and AMA said their legal challenge would not prevent “core patient protections’’ of that law from moving forward.
“No patient should fear receiving a surprise medical bill,” Rick Pollack, AHA president and chief executive, said in the statement. “That is why hospitals and health systems supported the No Surprises Act to protect patients and keep them out of the middle of disputes between providers and insurers. Congress carefully crafted the law with a balanced, patient-friendly approach and it should be implemented as intended.”
AMA President Gerald E. Harmon, MD, added the approach used in the rule on surprise billing could create “an unsustainable situation for physicians.”
“Our legal challenge urges regulators to ensure there is a fair and meaningful process to resolve disputes between health care providers and insurance companies,” Dr. Harmon said.
AHA and AMA included with their statement a link to a November letter from more than 150 members of Congress, who also objected to the approach taken in designing the independent dispute-resolution (IDR) process.
“This directive establishes a de facto benchmark rate, making the median in-network rate the default factor considered in the IDR process. This approach is contrary to statute and could incentivize insurance companies to set artificially low payment rates, which would narrow provider networks and jeopardize patient access to care – the exact opposite of the goal of the law,” wrote the members of Congress, including Rep. Raul Ruiz, MD, a California Democrat, and Rep. Larry Bucshon, MD, an Indiana Republican.
A version of this article first appeared on Medscape.com.
which tilts toward using prevailing rates paid for services.
The American Hospital Association and American Medical Association said they will ask the U.S. District Court for the District of Columbia to try to prevent implementation of certain provisions of new federal rules on surprise bills. This court is often a venue for fights over federal rules. Also joining the suit are Nevada-based Renown Health, UMass Memorial Health, and two physicians based in North Carolina, AHA and AMA said.
Federal agencies, including the Department of Health & Human Services, in September had unveiled the rule on surprise medical bills that will take effect Jan. 1.
Under this rule, a key benchmark for payment disputes would be the qualifying payment amount (QPA), which is pegged to median contracted rates. In the dispute-resolution process outlined in the rule, there is a presumption that the QPA is the appropriate out-of-network rate.
The rule allows for exceptions in which the independent mediating organization handling the payment dispute resolution has “credible information” as to why the QPA is materially different from the appropriate out-of-network rate.
In the view of the federal agencies that issued the rule, this approach “encourages predictable outcomes,” which likely would reduce the number of disputes that go through the resolution process while also “providing equitable and clear standards” for cases to appropriately deviate from QPA. HHS was joined in issuing the rule by the Treasury and Labor Departments and the Office of Personnel Management.
AMA and AHA disagree with their view, seeing this approach as a boon for insurers at the expense of physicians and hospitals.
In a press release, they said the rule’s approach to surprise billing would “all but ensure that hospitals, physicians, and other providers will routinely be undercompensated by commercial insurers, and patients will have fewer choices for access to in-network services.”
The rule is part of the implementation of a federal law passed in December 2020, known as the No Surprises Act. In their statement, AHA and AMA said their legal challenge would not prevent “core patient protections’’ of that law from moving forward.
“No patient should fear receiving a surprise medical bill,” Rick Pollack, AHA president and chief executive, said in the statement. “That is why hospitals and health systems supported the No Surprises Act to protect patients and keep them out of the middle of disputes between providers and insurers. Congress carefully crafted the law with a balanced, patient-friendly approach and it should be implemented as intended.”
AMA President Gerald E. Harmon, MD, added the approach used in the rule on surprise billing could create “an unsustainable situation for physicians.”
“Our legal challenge urges regulators to ensure there is a fair and meaningful process to resolve disputes between health care providers and insurance companies,” Dr. Harmon said.
AHA and AMA included with their statement a link to a November letter from more than 150 members of Congress, who also objected to the approach taken in designing the independent dispute-resolution (IDR) process.
“This directive establishes a de facto benchmark rate, making the median in-network rate the default factor considered in the IDR process. This approach is contrary to statute and could incentivize insurance companies to set artificially low payment rates, which would narrow provider networks and jeopardize patient access to care – the exact opposite of the goal of the law,” wrote the members of Congress, including Rep. Raul Ruiz, MD, a California Democrat, and Rep. Larry Bucshon, MD, an Indiana Republican.
A version of this article first appeared on Medscape.com.
which tilts toward using prevailing rates paid for services.
The American Hospital Association and American Medical Association said they will ask the U.S. District Court for the District of Columbia to try to prevent implementation of certain provisions of new federal rules on surprise bills. This court is often a venue for fights over federal rules. Also joining the suit are Nevada-based Renown Health, UMass Memorial Health, and two physicians based in North Carolina, AHA and AMA said.
Federal agencies, including the Department of Health & Human Services, in September had unveiled the rule on surprise medical bills that will take effect Jan. 1.
Under this rule, a key benchmark for payment disputes would be the qualifying payment amount (QPA), which is pegged to median contracted rates. In the dispute-resolution process outlined in the rule, there is a presumption that the QPA is the appropriate out-of-network rate.
The rule allows for exceptions in which the independent mediating organization handling the payment dispute resolution has “credible information” as to why the QPA is materially different from the appropriate out-of-network rate.
In the view of the federal agencies that issued the rule, this approach “encourages predictable outcomes,” which likely would reduce the number of disputes that go through the resolution process while also “providing equitable and clear standards” for cases to appropriately deviate from QPA. HHS was joined in issuing the rule by the Treasury and Labor Departments and the Office of Personnel Management.
AMA and AHA disagree with their view, seeing this approach as a boon for insurers at the expense of physicians and hospitals.
In a press release, they said the rule’s approach to surprise billing would “all but ensure that hospitals, physicians, and other providers will routinely be undercompensated by commercial insurers, and patients will have fewer choices for access to in-network services.”
The rule is part of the implementation of a federal law passed in December 2020, known as the No Surprises Act. In their statement, AHA and AMA said their legal challenge would not prevent “core patient protections’’ of that law from moving forward.
“No patient should fear receiving a surprise medical bill,” Rick Pollack, AHA president and chief executive, said in the statement. “That is why hospitals and health systems supported the No Surprises Act to protect patients and keep them out of the middle of disputes between providers and insurers. Congress carefully crafted the law with a balanced, patient-friendly approach and it should be implemented as intended.”
AMA President Gerald E. Harmon, MD, added the approach used in the rule on surprise billing could create “an unsustainable situation for physicians.”
“Our legal challenge urges regulators to ensure there is a fair and meaningful process to resolve disputes between health care providers and insurance companies,” Dr. Harmon said.
AHA and AMA included with their statement a link to a November letter from more than 150 members of Congress, who also objected to the approach taken in designing the independent dispute-resolution (IDR) process.
“This directive establishes a de facto benchmark rate, making the median in-network rate the default factor considered in the IDR process. This approach is contrary to statute and could incentivize insurance companies to set artificially low payment rates, which would narrow provider networks and jeopardize patient access to care – the exact opposite of the goal of the law,” wrote the members of Congress, including Rep. Raul Ruiz, MD, a California Democrat, and Rep. Larry Bucshon, MD, an Indiana Republican.
A version of this article first appeared on Medscape.com.
Risk for severe COVID-19 and death plummets with Pfizer booster
Both studies were completed before the advent of the Omicron variant.
In one study that included data on more than 4 million patients, led by Yinon M. Bar-On, MSc, of the Weizmann Institute of Science in Rehovot, Israel, the rate of confirmed SARS-CoV-2 infection was lower in the booster group than in the nonbooster group by a factor of about 10.
This was true across all five age groups studied (range among the groups [starting with age 16], 9.0-17.2).
The risk for severe COVID-19 in the primary analysis decreased in the booster group by a factor of 17.9 (95% confidence interval, 15.1-21.2), among those aged 60 years or older. Risk for severe illness in those ages 40-59 was lower by a factor of 21.7 (95% CI, 10.6-44.2).
Among the 60 and older age group, risk for death was also reduced by a factor of 14.7 (95% CI, 10.0-21.4).
Researchers analyzed data for the period from July 30 to Oct. 10, 2021, from the Israel Ministry of Health database on 4.69 million people at least 16 years old who had received two Pfizer doses at least 5 months earlier.
In the main analysis, the researchers compared the rates of confirmed COVID-19, severe disease, and death among those who had gotten a booster at least 12 days earlier with the rates in a nonbooster group.
The authors wrote: “Booster vaccination programs may provide a way to control transmission without costly social-distancing measures and quarantines. Our findings provide evidence for the short-term effectiveness of the booster dose against the currently dominant Delta variant in persons 16 years of age or older.”
Death risk down by 90%
A second study, led by Ronen Arbel, PhD, with the community medical services division, Clalit Health Services (CHS), Tel Aviv, which included more than 800,000 participants, also found mortality risk was greatly reduced among those who received the booster compared with those who didn’t get the booster.
Participants aged 50 years or older who received a booster at least 5 months after a second Pfizer dose had 90% lower mortality risk because of COVID-19 than participants who did not get the booster.
The adjusted hazard ratio for death as a result of COVID-19 in the booster group, as compared with the nonbooster group, was 0.10 (95% CI, 0.07-0.14; P < .001). Of the 843,208 eligible participants, 758,118 (90%) received the booster during the 54-day study period.
The study included all CHS members who were aged 50 years or older on the study start date and had received two Pfizer doses at least 5 months earlier. CHS covers about 52% of the Israeli population and is the largest of four health care organizations in Israel that provide mandatory health care.
The authors noted that, although the study period was only 54 days (Aug. 6–Sept. 29), during that time “the incidence of COVID-19 in Israel was one of the highest in the world.”
The authors of both original articles pointed out that the studies are limited by short time periods and that longer-term studies are needed to see how the booster shots stand up to known and future variants, such as Omicron.
None of the authors involved in both studies reported relevant financial relationships.
A version of this article first appeared on Medscape.com.
Both studies were completed before the advent of the Omicron variant.
In one study that included data on more than 4 million patients, led by Yinon M. Bar-On, MSc, of the Weizmann Institute of Science in Rehovot, Israel, the rate of confirmed SARS-CoV-2 infection was lower in the booster group than in the nonbooster group by a factor of about 10.
This was true across all five age groups studied (range among the groups [starting with age 16], 9.0-17.2).
The risk for severe COVID-19 in the primary analysis decreased in the booster group by a factor of 17.9 (95% confidence interval, 15.1-21.2), among those aged 60 years or older. Risk for severe illness in those ages 40-59 was lower by a factor of 21.7 (95% CI, 10.6-44.2).
Among the 60 and older age group, risk for death was also reduced by a factor of 14.7 (95% CI, 10.0-21.4).
Researchers analyzed data for the period from July 30 to Oct. 10, 2021, from the Israel Ministry of Health database on 4.69 million people at least 16 years old who had received two Pfizer doses at least 5 months earlier.
In the main analysis, the researchers compared the rates of confirmed COVID-19, severe disease, and death among those who had gotten a booster at least 12 days earlier with the rates in a nonbooster group.
The authors wrote: “Booster vaccination programs may provide a way to control transmission without costly social-distancing measures and quarantines. Our findings provide evidence for the short-term effectiveness of the booster dose against the currently dominant Delta variant in persons 16 years of age or older.”
Death risk down by 90%
A second study, led by Ronen Arbel, PhD, with the community medical services division, Clalit Health Services (CHS), Tel Aviv, which included more than 800,000 participants, also found mortality risk was greatly reduced among those who received the booster compared with those who didn’t get the booster.
Participants aged 50 years or older who received a booster at least 5 months after a second Pfizer dose had 90% lower mortality risk because of COVID-19 than participants who did not get the booster.
The adjusted hazard ratio for death as a result of COVID-19 in the booster group, as compared with the nonbooster group, was 0.10 (95% CI, 0.07-0.14; P < .001). Of the 843,208 eligible participants, 758,118 (90%) received the booster during the 54-day study period.
The study included all CHS members who were aged 50 years or older on the study start date and had received two Pfizer doses at least 5 months earlier. CHS covers about 52% of the Israeli population and is the largest of four health care organizations in Israel that provide mandatory health care.
The authors noted that, although the study period was only 54 days (Aug. 6–Sept. 29), during that time “the incidence of COVID-19 in Israel was one of the highest in the world.”
The authors of both original articles pointed out that the studies are limited by short time periods and that longer-term studies are needed to see how the booster shots stand up to known and future variants, such as Omicron.
None of the authors involved in both studies reported relevant financial relationships.
A version of this article first appeared on Medscape.com.
Both studies were completed before the advent of the Omicron variant.
In one study that included data on more than 4 million patients, led by Yinon M. Bar-On, MSc, of the Weizmann Institute of Science in Rehovot, Israel, the rate of confirmed SARS-CoV-2 infection was lower in the booster group than in the nonbooster group by a factor of about 10.
This was true across all five age groups studied (range among the groups [starting with age 16], 9.0-17.2).
The risk for severe COVID-19 in the primary analysis decreased in the booster group by a factor of 17.9 (95% confidence interval, 15.1-21.2), among those aged 60 years or older. Risk for severe illness in those ages 40-59 was lower by a factor of 21.7 (95% CI, 10.6-44.2).
Among the 60 and older age group, risk for death was also reduced by a factor of 14.7 (95% CI, 10.0-21.4).
Researchers analyzed data for the period from July 30 to Oct. 10, 2021, from the Israel Ministry of Health database on 4.69 million people at least 16 years old who had received two Pfizer doses at least 5 months earlier.
In the main analysis, the researchers compared the rates of confirmed COVID-19, severe disease, and death among those who had gotten a booster at least 12 days earlier with the rates in a nonbooster group.
The authors wrote: “Booster vaccination programs may provide a way to control transmission without costly social-distancing measures and quarantines. Our findings provide evidence for the short-term effectiveness of the booster dose against the currently dominant Delta variant in persons 16 years of age or older.”
Death risk down by 90%
A second study, led by Ronen Arbel, PhD, with the community medical services division, Clalit Health Services (CHS), Tel Aviv, which included more than 800,000 participants, also found mortality risk was greatly reduced among those who received the booster compared with those who didn’t get the booster.
Participants aged 50 years or older who received a booster at least 5 months after a second Pfizer dose had 90% lower mortality risk because of COVID-19 than participants who did not get the booster.
The adjusted hazard ratio for death as a result of COVID-19 in the booster group, as compared with the nonbooster group, was 0.10 (95% CI, 0.07-0.14; P < .001). Of the 843,208 eligible participants, 758,118 (90%) received the booster during the 54-day study period.
The study included all CHS members who were aged 50 years or older on the study start date and had received two Pfizer doses at least 5 months earlier. CHS covers about 52% of the Israeli population and is the largest of four health care organizations in Israel that provide mandatory health care.
The authors noted that, although the study period was only 54 days (Aug. 6–Sept. 29), during that time “the incidence of COVID-19 in Israel was one of the highest in the world.”
The authors of both original articles pointed out that the studies are limited by short time periods and that longer-term studies are needed to see how the booster shots stand up to known and future variants, such as Omicron.
None of the authors involved in both studies reported relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
New HIV PrEP guidelines call for clinicians to talk to patients about HIV prevention meds
Starting Dec. 8, the Centers for Disease Control and Prevention recommends all clinicians talk to their sexually active adolescent and adult patients about HIV pre-exposure prophylaxis (PrEP) at least once and prescribe the prevention pills to anyone who asks for them, whether or not you understand their need for it.
“PrEP is a part of good primary care,” Demetre Daskalakis, MD, CDC’s director of the division of HIV/AIDS prevention, said in an interview. “Listening to people and what they need, as opposed to assessing what you think they need, is a seismic shift in how PrEP should be offered.”
The expanded recommendation comes as part of the 2021 update to the U.S. Public Health Service’s PrEP prescribing guidelines. It’s the third iteration since the Food and Drug Administration approved the first HIV prevention pill in 2012, and the first to include guidance on how to prescribe and monitor an injectable version of PrEP, which the FDA may approve as early as December 2021.
There are currently two pills, Truvada (emtricitabine/tenofovir disoproxil fumarate, Gilead Sciences and generic) and Descovy (emtricitabine/tenofovir alafenamide, Gilead Sciences). The pills have been found to be up to 99% effective in preventing HIV acquisition. The new injectable cabotegravir appears to be even more effective.
The broadened guidance is part of an effort from the country’s top health officials to expand PrEP prescribing from infectious disease specialists and sexual health clinics to health care professionals, including gynecologists, internal medicine physicians, and family practice clinicians. It appears to be necessary. In 2020, just 25% of the 1.2 million Americans who could benefit from PrEP were taking it, according to CDC data.
But those rates belie stark disparities in PrEP use by race and gender. The vast majority of those using PrEP are White Americans and men. About 66% of White Americans who could benefit from PrEP used it in 2020, and more than a quarter of the men who could benefit used it. By contrast, just 16% of Latinx people who could benefit had a prescription. And fewer than 1 in 10 Black Americans, who make up nearly half of those with indications for PrEP, had a prescription. The same was true for the women who could benefit.
Researchers and data from early PrEP demonstration projects have documented that clinicians are less likely to refer or prescribe the HIV prevention pills to Black people, especially the Black cisgender and transgender women and same-gender-loving men who bear the disproportionate burden of new cases in the United States, as well as fail to prescribe the medication to people who inject drugs.
Normalizing PrEP in primary care
When Courtney Sherman, DNP, APRN, first heard about PrEP in the early 2010s, she joked that her reaction was: “You’re ridiculous. You’re making that up. That’s not real.”
Ms. Sherman is now launching a tele-PrEP program from CAN Community Health, a nonprofit network of community health centers in southern Florida. The tele-PrEP program is meant to serve people in Florida and beyond, to increase access to the pill in areas with few health care professionals, or clinicians unwilling to prescribe it.
“When I go other places, I can’t do what I do for a living without getting some sort of bizarre comment or look,” she said. But the looks don’t just come from family, friends, or her children’s teachers. They come from colleagues, too. “What I’ve learned is that anybody – anybody – can be impacted [by HIV] and the illusion that ‘those people who live over there do things that me and my kind don’t do’ is just garbage.”
That’s the PrEP stigma that the universal PrEP counseling in the guidelines is meant to override, said Dr. Daskalakis. Going forward, he said that informing people about PrEP should be treated as normally as counseling people about smoking.
“You can change the blank: You talk to all adolescents and adults about not smoking,” he said. “This is: ‘Tell adolescents and adults about ways you can prevent HIV, and PrEP is one of them.’ ”
The guidelines also simplify for monitoring lab levels for the current daily pills, checking creatinine clearance levels twice a year in people older than age 50 and once a year in those younger than 50 taking the oral pills. Dr. Daskalakis said that should ease the burden of monitoring PrEP patients for health care professionals with busy caseloads.
It’s a move that drew praise from Shawnika Hull, PhD, assistant professor of health communications at Rutgers University, New Brunswick, N.J.. Dr. Hull’s recent data showed that clinicians who espoused more biased racial views were also less likely to prescribe PrEP to Black women who asked for it.
“Public health practitioners and scientists have been advocating for this as a strategy, as one way to address several ongoing barriers to PrEP specifically but also equity in PrEP,” said Dr. Hull. “This sort of universal provision of information is really an important strategy to try to undo some of the deeply intertwined barriers to uptake.”
‘Don’t grill them’
The updated guidelines keep the number and proportion of Americans who could benefit from PrEP the same: 1.2 million Americans, with nearly half of those Black. And the reasons people would qualify for PrEP remain the same: inconsistent condom use, sharing injection drug equipment, and a STI diagnosis in the last 6 months. There are also 57 jurisdictions, including seven rural states, where dating and having sex carries an increased risk of acquiring HIV because of high rates of untreated HIV in the community.
That’s why the other big change in the update is guidance to prescribe PrEP to whoever asks for it, whether the patient divulges their risk or not. Or as Dr. Daskalakis puts it: “If someone asks for PrEP, don’t grill them.”
There are lots of reasons that someone might ask for PrEP without divulging their risk behaviors, said Dr. Daskalakis, who was an infectious disease doctor in New York back in 2012 (and a member of the FDA committee) when the first pill for PrEP was approved. He said he’s seen this particularly with women who ask about it. Asking for PrEP ends up being an “ice breaker” to discussing the woman’s sexual and injection drug use history, which can then improve the kinds of tests and vaccinations clinicians suggest for her.
“So many women will open the door and say, ‘I want to do this,’ and not necessarily want to go into the details,” he said. “Now, will they go into the details later? Absolutely. That’s how you create trust and connection.”
A mandate and a guideline
Leisha McKinley-Beach, MPH, a member of the U.S. Women and PrEP Working Group, has been urging greater funding and mandates to expand PrEP to women since the first pill was approved. And still, Ms. McKinley-Beach said she recently met a woman who worked for a community group scheduling PrEP appointments for gay men. But the woman didn’t know that she, too, could take it.
The American Academy of Family Physicians recommends health care professionals offer PrEP to those who can benefit. The American College of Obstetricians and Gynecologists have a 2014 committee opinion stating that PrEP “may be a useful tool for women at highest risk of HIV acquisition.”
But the ACOG opinion is not a recommendation, stating that it “should not be construed as dictating an exclusive course of treatment or procedure to be followed.” Ms. McKinley-Beach said she hopes that the new CDC guidelines will prompt ACOG and other professional organizations to issue statements to include PrEP education in all health assessments. A spokesperson for ACOG said that the organization had not seen the new CDC guidelines and had no statement on them, but pointed out that the 2014 committee opinion is one of the “highest level of documents we produce.
“We have failed for nearly a decade to raise awareness that PrEP is also a prevention strategy for women,” Ms. McKinley-Beach said in an interview. “In many ways, we’re still back in 2012 as it relates to women.”
Dr. Hull reported having done previous research funded by Gilead Sciences and having received consulting fees from Gilead Sciences in 2018. Ms. McKinley-Beach reported receiving honoraria from ViiV Healthcare. Ms. Sherman and Dr. Daskalakis disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Starting Dec. 8, the Centers for Disease Control and Prevention recommends all clinicians talk to their sexually active adolescent and adult patients about HIV pre-exposure prophylaxis (PrEP) at least once and prescribe the prevention pills to anyone who asks for them, whether or not you understand their need for it.
“PrEP is a part of good primary care,” Demetre Daskalakis, MD, CDC’s director of the division of HIV/AIDS prevention, said in an interview. “Listening to people and what they need, as opposed to assessing what you think they need, is a seismic shift in how PrEP should be offered.”
The expanded recommendation comes as part of the 2021 update to the U.S. Public Health Service’s PrEP prescribing guidelines. It’s the third iteration since the Food and Drug Administration approved the first HIV prevention pill in 2012, and the first to include guidance on how to prescribe and monitor an injectable version of PrEP, which the FDA may approve as early as December 2021.
There are currently two pills, Truvada (emtricitabine/tenofovir disoproxil fumarate, Gilead Sciences and generic) and Descovy (emtricitabine/tenofovir alafenamide, Gilead Sciences). The pills have been found to be up to 99% effective in preventing HIV acquisition. The new injectable cabotegravir appears to be even more effective.
The broadened guidance is part of an effort from the country’s top health officials to expand PrEP prescribing from infectious disease specialists and sexual health clinics to health care professionals, including gynecologists, internal medicine physicians, and family practice clinicians. It appears to be necessary. In 2020, just 25% of the 1.2 million Americans who could benefit from PrEP were taking it, according to CDC data.
But those rates belie stark disparities in PrEP use by race and gender. The vast majority of those using PrEP are White Americans and men. About 66% of White Americans who could benefit from PrEP used it in 2020, and more than a quarter of the men who could benefit used it. By contrast, just 16% of Latinx people who could benefit had a prescription. And fewer than 1 in 10 Black Americans, who make up nearly half of those with indications for PrEP, had a prescription. The same was true for the women who could benefit.
Researchers and data from early PrEP demonstration projects have documented that clinicians are less likely to refer or prescribe the HIV prevention pills to Black people, especially the Black cisgender and transgender women and same-gender-loving men who bear the disproportionate burden of new cases in the United States, as well as fail to prescribe the medication to people who inject drugs.
Normalizing PrEP in primary care
When Courtney Sherman, DNP, APRN, first heard about PrEP in the early 2010s, she joked that her reaction was: “You’re ridiculous. You’re making that up. That’s not real.”
Ms. Sherman is now launching a tele-PrEP program from CAN Community Health, a nonprofit network of community health centers in southern Florida. The tele-PrEP program is meant to serve people in Florida and beyond, to increase access to the pill in areas with few health care professionals, or clinicians unwilling to prescribe it.
“When I go other places, I can’t do what I do for a living without getting some sort of bizarre comment or look,” she said. But the looks don’t just come from family, friends, or her children’s teachers. They come from colleagues, too. “What I’ve learned is that anybody – anybody – can be impacted [by HIV] and the illusion that ‘those people who live over there do things that me and my kind don’t do’ is just garbage.”
That’s the PrEP stigma that the universal PrEP counseling in the guidelines is meant to override, said Dr. Daskalakis. Going forward, he said that informing people about PrEP should be treated as normally as counseling people about smoking.
“You can change the blank: You talk to all adolescents and adults about not smoking,” he said. “This is: ‘Tell adolescents and adults about ways you can prevent HIV, and PrEP is one of them.’ ”
The guidelines also simplify for monitoring lab levels for the current daily pills, checking creatinine clearance levels twice a year in people older than age 50 and once a year in those younger than 50 taking the oral pills. Dr. Daskalakis said that should ease the burden of monitoring PrEP patients for health care professionals with busy caseloads.
It’s a move that drew praise from Shawnika Hull, PhD, assistant professor of health communications at Rutgers University, New Brunswick, N.J.. Dr. Hull’s recent data showed that clinicians who espoused more biased racial views were also less likely to prescribe PrEP to Black women who asked for it.
“Public health practitioners and scientists have been advocating for this as a strategy, as one way to address several ongoing barriers to PrEP specifically but also equity in PrEP,” said Dr. Hull. “This sort of universal provision of information is really an important strategy to try to undo some of the deeply intertwined barriers to uptake.”
‘Don’t grill them’
The updated guidelines keep the number and proportion of Americans who could benefit from PrEP the same: 1.2 million Americans, with nearly half of those Black. And the reasons people would qualify for PrEP remain the same: inconsistent condom use, sharing injection drug equipment, and a STI diagnosis in the last 6 months. There are also 57 jurisdictions, including seven rural states, where dating and having sex carries an increased risk of acquiring HIV because of high rates of untreated HIV in the community.
That’s why the other big change in the update is guidance to prescribe PrEP to whoever asks for it, whether the patient divulges their risk or not. Or as Dr. Daskalakis puts it: “If someone asks for PrEP, don’t grill them.”
There are lots of reasons that someone might ask for PrEP without divulging their risk behaviors, said Dr. Daskalakis, who was an infectious disease doctor in New York back in 2012 (and a member of the FDA committee) when the first pill for PrEP was approved. He said he’s seen this particularly with women who ask about it. Asking for PrEP ends up being an “ice breaker” to discussing the woman’s sexual and injection drug use history, which can then improve the kinds of tests and vaccinations clinicians suggest for her.
“So many women will open the door and say, ‘I want to do this,’ and not necessarily want to go into the details,” he said. “Now, will they go into the details later? Absolutely. That’s how you create trust and connection.”
A mandate and a guideline
Leisha McKinley-Beach, MPH, a member of the U.S. Women and PrEP Working Group, has been urging greater funding and mandates to expand PrEP to women since the first pill was approved. And still, Ms. McKinley-Beach said she recently met a woman who worked for a community group scheduling PrEP appointments for gay men. But the woman didn’t know that she, too, could take it.
The American Academy of Family Physicians recommends health care professionals offer PrEP to those who can benefit. The American College of Obstetricians and Gynecologists have a 2014 committee opinion stating that PrEP “may be a useful tool for women at highest risk of HIV acquisition.”
But the ACOG opinion is not a recommendation, stating that it “should not be construed as dictating an exclusive course of treatment or procedure to be followed.” Ms. McKinley-Beach said she hopes that the new CDC guidelines will prompt ACOG and other professional organizations to issue statements to include PrEP education in all health assessments. A spokesperson for ACOG said that the organization had not seen the new CDC guidelines and had no statement on them, but pointed out that the 2014 committee opinion is one of the “highest level of documents we produce.
“We have failed for nearly a decade to raise awareness that PrEP is also a prevention strategy for women,” Ms. McKinley-Beach said in an interview. “In many ways, we’re still back in 2012 as it relates to women.”
Dr. Hull reported having done previous research funded by Gilead Sciences and having received consulting fees from Gilead Sciences in 2018. Ms. McKinley-Beach reported receiving honoraria from ViiV Healthcare. Ms. Sherman and Dr. Daskalakis disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Starting Dec. 8, the Centers for Disease Control and Prevention recommends all clinicians talk to their sexually active adolescent and adult patients about HIV pre-exposure prophylaxis (PrEP) at least once and prescribe the prevention pills to anyone who asks for them, whether or not you understand their need for it.
“PrEP is a part of good primary care,” Demetre Daskalakis, MD, CDC’s director of the division of HIV/AIDS prevention, said in an interview. “Listening to people and what they need, as opposed to assessing what you think they need, is a seismic shift in how PrEP should be offered.”
The expanded recommendation comes as part of the 2021 update to the U.S. Public Health Service’s PrEP prescribing guidelines. It’s the third iteration since the Food and Drug Administration approved the first HIV prevention pill in 2012, and the first to include guidance on how to prescribe and monitor an injectable version of PrEP, which the FDA may approve as early as December 2021.
There are currently two pills, Truvada (emtricitabine/tenofovir disoproxil fumarate, Gilead Sciences and generic) and Descovy (emtricitabine/tenofovir alafenamide, Gilead Sciences). The pills have been found to be up to 99% effective in preventing HIV acquisition. The new injectable cabotegravir appears to be even more effective.
The broadened guidance is part of an effort from the country’s top health officials to expand PrEP prescribing from infectious disease specialists and sexual health clinics to health care professionals, including gynecologists, internal medicine physicians, and family practice clinicians. It appears to be necessary. In 2020, just 25% of the 1.2 million Americans who could benefit from PrEP were taking it, according to CDC data.
But those rates belie stark disparities in PrEP use by race and gender. The vast majority of those using PrEP are White Americans and men. About 66% of White Americans who could benefit from PrEP used it in 2020, and more than a quarter of the men who could benefit used it. By contrast, just 16% of Latinx people who could benefit had a prescription. And fewer than 1 in 10 Black Americans, who make up nearly half of those with indications for PrEP, had a prescription. The same was true for the women who could benefit.
Researchers and data from early PrEP demonstration projects have documented that clinicians are less likely to refer or prescribe the HIV prevention pills to Black people, especially the Black cisgender and transgender women and same-gender-loving men who bear the disproportionate burden of new cases in the United States, as well as fail to prescribe the medication to people who inject drugs.
Normalizing PrEP in primary care
When Courtney Sherman, DNP, APRN, first heard about PrEP in the early 2010s, she joked that her reaction was: “You’re ridiculous. You’re making that up. That’s not real.”
Ms. Sherman is now launching a tele-PrEP program from CAN Community Health, a nonprofit network of community health centers in southern Florida. The tele-PrEP program is meant to serve people in Florida and beyond, to increase access to the pill in areas with few health care professionals, or clinicians unwilling to prescribe it.
“When I go other places, I can’t do what I do for a living without getting some sort of bizarre comment or look,” she said. But the looks don’t just come from family, friends, or her children’s teachers. They come from colleagues, too. “What I’ve learned is that anybody – anybody – can be impacted [by HIV] and the illusion that ‘those people who live over there do things that me and my kind don’t do’ is just garbage.”
That’s the PrEP stigma that the universal PrEP counseling in the guidelines is meant to override, said Dr. Daskalakis. Going forward, he said that informing people about PrEP should be treated as normally as counseling people about smoking.
“You can change the blank: You talk to all adolescents and adults about not smoking,” he said. “This is: ‘Tell adolescents and adults about ways you can prevent HIV, and PrEP is one of them.’ ”
The guidelines also simplify for monitoring lab levels for the current daily pills, checking creatinine clearance levels twice a year in people older than age 50 and once a year in those younger than 50 taking the oral pills. Dr. Daskalakis said that should ease the burden of monitoring PrEP patients for health care professionals with busy caseloads.
It’s a move that drew praise from Shawnika Hull, PhD, assistant professor of health communications at Rutgers University, New Brunswick, N.J.. Dr. Hull’s recent data showed that clinicians who espoused more biased racial views were also less likely to prescribe PrEP to Black women who asked for it.
“Public health practitioners and scientists have been advocating for this as a strategy, as one way to address several ongoing barriers to PrEP specifically but also equity in PrEP,” said Dr. Hull. “This sort of universal provision of information is really an important strategy to try to undo some of the deeply intertwined barriers to uptake.”
‘Don’t grill them’
The updated guidelines keep the number and proportion of Americans who could benefit from PrEP the same: 1.2 million Americans, with nearly half of those Black. And the reasons people would qualify for PrEP remain the same: inconsistent condom use, sharing injection drug equipment, and a STI diagnosis in the last 6 months. There are also 57 jurisdictions, including seven rural states, where dating and having sex carries an increased risk of acquiring HIV because of high rates of untreated HIV in the community.
That’s why the other big change in the update is guidance to prescribe PrEP to whoever asks for it, whether the patient divulges their risk or not. Or as Dr. Daskalakis puts it: “If someone asks for PrEP, don’t grill them.”
There are lots of reasons that someone might ask for PrEP without divulging their risk behaviors, said Dr. Daskalakis, who was an infectious disease doctor in New York back in 2012 (and a member of the FDA committee) when the first pill for PrEP was approved. He said he’s seen this particularly with women who ask about it. Asking for PrEP ends up being an “ice breaker” to discussing the woman’s sexual and injection drug use history, which can then improve the kinds of tests and vaccinations clinicians suggest for her.
“So many women will open the door and say, ‘I want to do this,’ and not necessarily want to go into the details,” he said. “Now, will they go into the details later? Absolutely. That’s how you create trust and connection.”
A mandate and a guideline
Leisha McKinley-Beach, MPH, a member of the U.S. Women and PrEP Working Group, has been urging greater funding and mandates to expand PrEP to women since the first pill was approved. And still, Ms. McKinley-Beach said she recently met a woman who worked for a community group scheduling PrEP appointments for gay men. But the woman didn’t know that she, too, could take it.
The American Academy of Family Physicians recommends health care professionals offer PrEP to those who can benefit. The American College of Obstetricians and Gynecologists have a 2014 committee opinion stating that PrEP “may be a useful tool for women at highest risk of HIV acquisition.”
But the ACOG opinion is not a recommendation, stating that it “should not be construed as dictating an exclusive course of treatment or procedure to be followed.” Ms. McKinley-Beach said she hopes that the new CDC guidelines will prompt ACOG and other professional organizations to issue statements to include PrEP education in all health assessments. A spokesperson for ACOG said that the organization had not seen the new CDC guidelines and had no statement on them, but pointed out that the 2014 committee opinion is one of the “highest level of documents we produce.
“We have failed for nearly a decade to raise awareness that PrEP is also a prevention strategy for women,” Ms. McKinley-Beach said in an interview. “In many ways, we’re still back in 2012 as it relates to women.”
Dr. Hull reported having done previous research funded by Gilead Sciences and having received consulting fees from Gilead Sciences in 2018. Ms. McKinley-Beach reported receiving honoraria from ViiV Healthcare. Ms. Sherman and Dr. Daskalakis disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Vaccine protection drops against Omicron, making boosters crucial
A raft of new
The new studies, from teams of researchers in Germany, South Africa, Sweden, and the drug company Pfizer, showed 25 to 40-fold drops in the ability of antibodies created by two doses of the Pfizer-BioNTech vaccine to neutralize the virus.
But there seemed to be a bright spot in the studies too. The virus didn’t completely escape the immunity from the vaccines, and giving a third, booster dose appeared to restore antibodies to a level that’s been associated with protection against variants in the past.
“One of the silver linings of this pandemic so far is that mRNA vaccines manufactured based on the ancestral SARS-CoV-2 continue to work in the laboratory and, importantly, in real life against variant strains,” said Hana El Sahly, MD, professor of molecular virology and microbiology at Baylor College of Medicine in Houston. “The strains so far vary by their degree of being neutralized by the antibodies from these vaccines, but they are being neutralized nonetheless.”
Dr. El Sahly points out that the Beta variant was associated with a 10-fold drop in antibodies, but two doses of the vaccines still protected against it.
President Biden hailed the study results as good news.
“That Pfizer lab report came back saying that the expectation is that the existing vaccines protect against Omicron. But if you get the booster, you’re really in good shape. And so that’s very encouraging,” he said in a press briefing Dec. 8.
More research needed
Other scientists, however, stressed that these studies are from lab tests, and don’t necessarily reflect what will happen with Omicron in the real world. They cautioned about a worldwide push for boosters with so many countries still struggling to give first doses of vaccines.
Soumya Swaminathan, MD, chief scientist for the World Health Organization, stressed in a press briefing Dec. 8 that the results from the four studies varied widely, showing dips in neutralizing activity with Omicron that ranged from 5-fold to 40-fold.
The types of lab tests that were run were different, too, and involved small numbers of blood samples from patients.
She stressed that immunity depends not just on neutralizing antibodies, which act as a first line of defense when a virus invades, but also on B cells and T cells, and so far, tests show that these crucial components — which are important for preventing severe disease and death — had been less impacted than antibodies.
“So, I think it’s premature to conclude that this reduction in neutralizing activity would result in a significant reduction in vaccine effectiveness,” she said.
Whether or not these first-generation vaccines will be enough to stop Omicron, though, remains to be seen. A study of the Pfizer, Moderna, and AstraZeneca vaccines, led by German physician Sandra Ciesek, MD, who directs the Institute of Medical Virology at the University of Frankfurt, shows a booster didn’t appear to hold up well over time.
Dr. Ciesek and her team exposed Omicron viruses to the antibodies of volunteers who had been boosted with the Pfizer vaccine 3 months prior.
She also compared the results to what happened to those same 3-month antibody levels against Delta variant viruses. She found only a 25% neutralization of Omicron compared with a 95% neutralization of Delta. That represented about a 37-fold reduction in the ability of the antibodies to neutralize Omicron vs Delta.
“The data confirm that developing a vaccine adapted for Omicron makes sense,” she tweeted as part of a long thread she posted on her results.
Retool the vaccines?
Both Pfizer and Moderna are retooling their vaccines to better match them to the changes in the Omicron variant. In a press release, Pfizer said it could start deliveries of that updated vaccine by March, pending U.S. Food and Drug Administration authorization.
“What the booster really does in neutralizing Omicron right now, they don’t know, they have no idea,” said Peter Palese, PhD, chair of the department of microbiology at the Mount Sinai School of Medicine in New York City.
Dr. Palese said he was definitely concerned about a possible Omicron wave.
“There are four major sites on the spike protein targeted by antibodies from the vaccines, and all four sites have mutations,” he said. “All these important antigenic sites are changed.
“If Omicron becomes the new Delta, and the old vaccines really aren’t good enough, then we have to make new Omicron vaccines. Then we have to revaccinate everybody twice,” he said, and the costs could be staggering. “I am worried.”
Tedros Adhanom Ghebreyesus, PhD, director general of the WHO, urged countries to move quickly.
“Don’t wait. Act now,” he said, even before all the science is in hand. “All of us, every government, every individual should use all the tools we have right now,” to drive down transmission, increase testing and surveillance, and share scientific findings.
“We can prevent Omicron [from] becoming a global crisis right now,” he said.
A version of this article first appeared on Medscape.com.
A raft of new
The new studies, from teams of researchers in Germany, South Africa, Sweden, and the drug company Pfizer, showed 25 to 40-fold drops in the ability of antibodies created by two doses of the Pfizer-BioNTech vaccine to neutralize the virus.
But there seemed to be a bright spot in the studies too. The virus didn’t completely escape the immunity from the vaccines, and giving a third, booster dose appeared to restore antibodies to a level that’s been associated with protection against variants in the past.
“One of the silver linings of this pandemic so far is that mRNA vaccines manufactured based on the ancestral SARS-CoV-2 continue to work in the laboratory and, importantly, in real life against variant strains,” said Hana El Sahly, MD, professor of molecular virology and microbiology at Baylor College of Medicine in Houston. “The strains so far vary by their degree of being neutralized by the antibodies from these vaccines, but they are being neutralized nonetheless.”
Dr. El Sahly points out that the Beta variant was associated with a 10-fold drop in antibodies, but two doses of the vaccines still protected against it.
President Biden hailed the study results as good news.
“That Pfizer lab report came back saying that the expectation is that the existing vaccines protect against Omicron. But if you get the booster, you’re really in good shape. And so that’s very encouraging,” he said in a press briefing Dec. 8.
More research needed
Other scientists, however, stressed that these studies are from lab tests, and don’t necessarily reflect what will happen with Omicron in the real world. They cautioned about a worldwide push for boosters with so many countries still struggling to give first doses of vaccines.
Soumya Swaminathan, MD, chief scientist for the World Health Organization, stressed in a press briefing Dec. 8 that the results from the four studies varied widely, showing dips in neutralizing activity with Omicron that ranged from 5-fold to 40-fold.
The types of lab tests that were run were different, too, and involved small numbers of blood samples from patients.
She stressed that immunity depends not just on neutralizing antibodies, which act as a first line of defense when a virus invades, but also on B cells and T cells, and so far, tests show that these crucial components — which are important for preventing severe disease and death — had been less impacted than antibodies.
“So, I think it’s premature to conclude that this reduction in neutralizing activity would result in a significant reduction in vaccine effectiveness,” she said.
Whether or not these first-generation vaccines will be enough to stop Omicron, though, remains to be seen. A study of the Pfizer, Moderna, and AstraZeneca vaccines, led by German physician Sandra Ciesek, MD, who directs the Institute of Medical Virology at the University of Frankfurt, shows a booster didn’t appear to hold up well over time.
Dr. Ciesek and her team exposed Omicron viruses to the antibodies of volunteers who had been boosted with the Pfizer vaccine 3 months prior.
She also compared the results to what happened to those same 3-month antibody levels against Delta variant viruses. She found only a 25% neutralization of Omicron compared with a 95% neutralization of Delta. That represented about a 37-fold reduction in the ability of the antibodies to neutralize Omicron vs Delta.
“The data confirm that developing a vaccine adapted for Omicron makes sense,” she tweeted as part of a long thread she posted on her results.
Retool the vaccines?
Both Pfizer and Moderna are retooling their vaccines to better match them to the changes in the Omicron variant. In a press release, Pfizer said it could start deliveries of that updated vaccine by March, pending U.S. Food and Drug Administration authorization.
“What the booster really does in neutralizing Omicron right now, they don’t know, they have no idea,” said Peter Palese, PhD, chair of the department of microbiology at the Mount Sinai School of Medicine in New York City.
Dr. Palese said he was definitely concerned about a possible Omicron wave.
“There are four major sites on the spike protein targeted by antibodies from the vaccines, and all four sites have mutations,” he said. “All these important antigenic sites are changed.
“If Omicron becomes the new Delta, and the old vaccines really aren’t good enough, then we have to make new Omicron vaccines. Then we have to revaccinate everybody twice,” he said, and the costs could be staggering. “I am worried.”
Tedros Adhanom Ghebreyesus, PhD, director general of the WHO, urged countries to move quickly.
“Don’t wait. Act now,” he said, even before all the science is in hand. “All of us, every government, every individual should use all the tools we have right now,” to drive down transmission, increase testing and surveillance, and share scientific findings.
“We can prevent Omicron [from] becoming a global crisis right now,” he said.
A version of this article first appeared on Medscape.com.
A raft of new
The new studies, from teams of researchers in Germany, South Africa, Sweden, and the drug company Pfizer, showed 25 to 40-fold drops in the ability of antibodies created by two doses of the Pfizer-BioNTech vaccine to neutralize the virus.
But there seemed to be a bright spot in the studies too. The virus didn’t completely escape the immunity from the vaccines, and giving a third, booster dose appeared to restore antibodies to a level that’s been associated with protection against variants in the past.
“One of the silver linings of this pandemic so far is that mRNA vaccines manufactured based on the ancestral SARS-CoV-2 continue to work in the laboratory and, importantly, in real life against variant strains,” said Hana El Sahly, MD, professor of molecular virology and microbiology at Baylor College of Medicine in Houston. “The strains so far vary by their degree of being neutralized by the antibodies from these vaccines, but they are being neutralized nonetheless.”
Dr. El Sahly points out that the Beta variant was associated with a 10-fold drop in antibodies, but two doses of the vaccines still protected against it.
President Biden hailed the study results as good news.
“That Pfizer lab report came back saying that the expectation is that the existing vaccines protect against Omicron. But if you get the booster, you’re really in good shape. And so that’s very encouraging,” he said in a press briefing Dec. 8.
More research needed
Other scientists, however, stressed that these studies are from lab tests, and don’t necessarily reflect what will happen with Omicron in the real world. They cautioned about a worldwide push for boosters with so many countries still struggling to give first doses of vaccines.
Soumya Swaminathan, MD, chief scientist for the World Health Organization, stressed in a press briefing Dec. 8 that the results from the four studies varied widely, showing dips in neutralizing activity with Omicron that ranged from 5-fold to 40-fold.
The types of lab tests that were run were different, too, and involved small numbers of blood samples from patients.
She stressed that immunity depends not just on neutralizing antibodies, which act as a first line of defense when a virus invades, but also on B cells and T cells, and so far, tests show that these crucial components — which are important for preventing severe disease and death — had been less impacted than antibodies.
“So, I think it’s premature to conclude that this reduction in neutralizing activity would result in a significant reduction in vaccine effectiveness,” she said.
Whether or not these first-generation vaccines will be enough to stop Omicron, though, remains to be seen. A study of the Pfizer, Moderna, and AstraZeneca vaccines, led by German physician Sandra Ciesek, MD, who directs the Institute of Medical Virology at the University of Frankfurt, shows a booster didn’t appear to hold up well over time.
Dr. Ciesek and her team exposed Omicron viruses to the antibodies of volunteers who had been boosted with the Pfizer vaccine 3 months prior.
She also compared the results to what happened to those same 3-month antibody levels against Delta variant viruses. She found only a 25% neutralization of Omicron compared with a 95% neutralization of Delta. That represented about a 37-fold reduction in the ability of the antibodies to neutralize Omicron vs Delta.
“The data confirm that developing a vaccine adapted for Omicron makes sense,” she tweeted as part of a long thread she posted on her results.
Retool the vaccines?
Both Pfizer and Moderna are retooling their vaccines to better match them to the changes in the Omicron variant. In a press release, Pfizer said it could start deliveries of that updated vaccine by March, pending U.S. Food and Drug Administration authorization.
“What the booster really does in neutralizing Omicron right now, they don’t know, they have no idea,” said Peter Palese, PhD, chair of the department of microbiology at the Mount Sinai School of Medicine in New York City.
Dr. Palese said he was definitely concerned about a possible Omicron wave.
“There are four major sites on the spike protein targeted by antibodies from the vaccines, and all four sites have mutations,” he said. “All these important antigenic sites are changed.
“If Omicron becomes the new Delta, and the old vaccines really aren’t good enough, then we have to make new Omicron vaccines. Then we have to revaccinate everybody twice,” he said, and the costs could be staggering. “I am worried.”
Tedros Adhanom Ghebreyesus, PhD, director general of the WHO, urged countries to move quickly.
“Don’t wait. Act now,” he said, even before all the science is in hand. “All of us, every government, every individual should use all the tools we have right now,” to drive down transmission, increase testing and surveillance, and share scientific findings.
“We can prevent Omicron [from] becoming a global crisis right now,” he said.
A version of this article first appeared on Medscape.com.
A sun distributed rash

The photo distribution and annular quality of this patient’s rash, combined with his positive autoimmune work-up, led to a diagnosis of subacute cutaneous lupus erythematosus (SCLE), a nonscarring subtype of cutaneous lupus erythematosus.
SCLE is a chronic and relapsing condition that may manifest as either a papulosquamous or annular eruption.1 It most commonly affects areas of sun exposure such as the shoulders, upper back, and extensor surfaces of the arms. This disorder typically affects young or middle-aged women between the ages of 30 and 40 years.
The differential diagnosis of this eruption includes dermatomyositis, polymorphous light eruption, psoriasis, tinea corporis, and other photodermatoses. The etiology of SCLE is multifactorial and may include a genetic susceptibility in combination with environmental triggers that provoke an autoimmune response to sunlight.1 There is strong evidence linking drug-induced SCLE with proton pump inhibitors, anticonvulsants, beta-blockers, terbinafine, and immune modulators.2
As many as 70% of patients with SCLE have positive anti-Ro/SSA autoantibodies, and this is most often associated with Sjogren syndrome.1 Interestingly, SCLE patients often exhibit symptoms that overlap with Sjogren syndrome. Systemic involvement is rare in SCLE, and if present, these symptoms are usually limited to arthritis and myalgia.
Treatment of SCLE includes photo-protective behaviors, topical corticosteroids/calcineurin inhibitors, and systemic therapies such as hydroxychloroquine (first-line), methotrexate, and mycophenolate mofetil (second-line).2
Our patient was started on hydroxychloroquine 200 mg orally bid, with complete resolution of the lesions at his 2 month–follow-up appointment. This case emphasizes the importance of distinguishing SCLE from other subtypes of lupus erythematosus as the prognostic course and treatment varies between these conditions.
Photos courtesy of Kriti Mishra, MD. Text courtesy of Jaimie Lin, BS, Kriti Mishra, MD, Department of Dermatology, and Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
1. Okon LG, Werth VP. Cutaneous lupus erythematosus: diagnosis and treatment. Best Pract Res Clin Rheumatol. 2013;27:391-404. https://doi.org/10.1016/j.berh.2013.07.008
2. Jatwani S, Hearth Holmes MP. Subacute cutaneous lupus erythematosus. 2021. StatPearls. StatPearls Publishing; 2021.

The photo distribution and annular quality of this patient’s rash, combined with his positive autoimmune work-up, led to a diagnosis of subacute cutaneous lupus erythematosus (SCLE), a nonscarring subtype of cutaneous lupus erythematosus.
SCLE is a chronic and relapsing condition that may manifest as either a papulosquamous or annular eruption.1 It most commonly affects areas of sun exposure such as the shoulders, upper back, and extensor surfaces of the arms. This disorder typically affects young or middle-aged women between the ages of 30 and 40 years.
The differential diagnosis of this eruption includes dermatomyositis, polymorphous light eruption, psoriasis, tinea corporis, and other photodermatoses. The etiology of SCLE is multifactorial and may include a genetic susceptibility in combination with environmental triggers that provoke an autoimmune response to sunlight.1 There is strong evidence linking drug-induced SCLE with proton pump inhibitors, anticonvulsants, beta-blockers, terbinafine, and immune modulators.2
As many as 70% of patients with SCLE have positive anti-Ro/SSA autoantibodies, and this is most often associated with Sjogren syndrome.1 Interestingly, SCLE patients often exhibit symptoms that overlap with Sjogren syndrome. Systemic involvement is rare in SCLE, and if present, these symptoms are usually limited to arthritis and myalgia.
Treatment of SCLE includes photo-protective behaviors, topical corticosteroids/calcineurin inhibitors, and systemic therapies such as hydroxychloroquine (first-line), methotrexate, and mycophenolate mofetil (second-line).2
Our patient was started on hydroxychloroquine 200 mg orally bid, with complete resolution of the lesions at his 2 month–follow-up appointment. This case emphasizes the importance of distinguishing SCLE from other subtypes of lupus erythematosus as the prognostic course and treatment varies between these conditions.
Photos courtesy of Kriti Mishra, MD. Text courtesy of Jaimie Lin, BS, Kriti Mishra, MD, Department of Dermatology, and Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.

The photo distribution and annular quality of this patient’s rash, combined with his positive autoimmune work-up, led to a diagnosis of subacute cutaneous lupus erythematosus (SCLE), a nonscarring subtype of cutaneous lupus erythematosus.
SCLE is a chronic and relapsing condition that may manifest as either a papulosquamous or annular eruption.1 It most commonly affects areas of sun exposure such as the shoulders, upper back, and extensor surfaces of the arms. This disorder typically affects young or middle-aged women between the ages of 30 and 40 years.
The differential diagnosis of this eruption includes dermatomyositis, polymorphous light eruption, psoriasis, tinea corporis, and other photodermatoses. The etiology of SCLE is multifactorial and may include a genetic susceptibility in combination with environmental triggers that provoke an autoimmune response to sunlight.1 There is strong evidence linking drug-induced SCLE with proton pump inhibitors, anticonvulsants, beta-blockers, terbinafine, and immune modulators.2
As many as 70% of patients with SCLE have positive anti-Ro/SSA autoantibodies, and this is most often associated with Sjogren syndrome.1 Interestingly, SCLE patients often exhibit symptoms that overlap with Sjogren syndrome. Systemic involvement is rare in SCLE, and if present, these symptoms are usually limited to arthritis and myalgia.
Treatment of SCLE includes photo-protective behaviors, topical corticosteroids/calcineurin inhibitors, and systemic therapies such as hydroxychloroquine (first-line), methotrexate, and mycophenolate mofetil (second-line).2
Our patient was started on hydroxychloroquine 200 mg orally bid, with complete resolution of the lesions at his 2 month–follow-up appointment. This case emphasizes the importance of distinguishing SCLE from other subtypes of lupus erythematosus as the prognostic course and treatment varies between these conditions.
Photos courtesy of Kriti Mishra, MD. Text courtesy of Jaimie Lin, BS, Kriti Mishra, MD, Department of Dermatology, and Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
1. Okon LG, Werth VP. Cutaneous lupus erythematosus: diagnosis and treatment. Best Pract Res Clin Rheumatol. 2013;27:391-404. https://doi.org/10.1016/j.berh.2013.07.008
2. Jatwani S, Hearth Holmes MP. Subacute cutaneous lupus erythematosus. 2021. StatPearls. StatPearls Publishing; 2021.
1. Okon LG, Werth VP. Cutaneous lupus erythematosus: diagnosis and treatment. Best Pract Res Clin Rheumatol. 2013;27:391-404. https://doi.org/10.1016/j.berh.2013.07.008
2. Jatwani S, Hearth Holmes MP. Subacute cutaneous lupus erythematosus. 2021. StatPearls. StatPearls Publishing; 2021.
White ankle scars
A 42-year-old woman presented to our dermatology center with white scars on both of her ankles. She first noticed the lesions 2 years prior; they were initially erythematous and painful, even when she was at rest. Her past medical history included 3 spontaneous term miscarriages. She denied any prolonged standing or trauma.
On examination, atrophic porcelain-white stellate scars were visible with surrounding hyperpigmentation on the medial aspect of both ankles (FIGURE 1A & 1B). There were no tender erythematous nodules,
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Atrophie blanche
Atrophie blanche is a morphologic feature described as porcelain-white stellate scars with surrounding telangiectasia and hyperpigmentation. The lesions are typically found over the peri-malleolar region and are sequelae of healed erythematous and painful ulcers. The lesions arise from upper dermal, small vessel, thrombotic vasculopathy leading to ischemic rest pain; if left untreated, atrophic white scars eventually develop.
A sign of venous insufficiency or thrombotic vasculopathy
Atrophie blanche may develop following healing of an ulcer due to venous insufficiency or small vessel thrombotic vasculopathy.1 The incidence of thrombotic vasculopathy is 1:100,000 with a female predominance, and up to 50% of cases are associated with procoagulant conditions.2 Thrombotic vasculopathy can be due to an inherited or acquired thrombophilia.1
Causes of hereditary thrombophilia include Factor V Leiden/prothrombin mutations, anti-thrombin III/protein C/protein S deficiencies, dysfibrinogenemia, and hyperhomocysteinemia.
Acquired thrombophilia arises from underlying prothrombotic states associated with the Virchow triad: hypercoagulability, blood flow stasis, and endothelial injury. The use of oral contraceptives or hormone replacement therapy, presence of malignancy, and antiphospholipid syndrome (APS) are causes of acquired thrombophilia.2
Obtaining a careful history is crucial
Thorough history-taking and physical examination are required to determine the underlying cause of atrophie blanche.
Continue to: Chronic venous insufficiency
Chronic venous insufficiency is more likely in patients with a history of prolonged standing, obesity, or previous injury/surgery to leg veins. Physical examination would reveal hyperpigmentation, telangiectasia, varicose veins, pedal edema, and venous ulcers.3
Inherited thrombophilia may be at work in patients with a family history of arterial and venous thrombosis (eg, stroke, acute coronary syndrome, or deep vein thromboses).
Acquired thrombophilia should be suspected if there is a history of recurrent miscarriages or malignancy.4 Given our patient’s history of miscarriages, we ordered further lab work and found that she had elevated anticardiolipin levels (> 40 U/mL) fulfilling the revised Sapporo criteria5 for APS.
Thrombophilia or chronic venous insufficiency? In a patient with a history suggestive of thrombophilia, further work-up should be done before attributing atrophie blanche to healed venous ulcers from chronic venous insufficiency. A skin lesion biopsy could reveal classic changes of thrombotic vasculopathy subjacent to the ulcer, including intraluminal thrombosis, endothelial proliferation, and subintimal hyaline degeneration, as opposed to dermal changes consistent with venous stasis, such as increased siderophages, hemosiderin deposition, erythrocyte extravasation, dermal fibrosis, and adipocytic damage.
Differential diagnosis includes atrophic scarring
The differential diagnosis for hypopigmented atrophic macules and plaques over the lower limbs include atrophic scarring from previous trauma, guttate morphea, extra-genital lichen sclerosus, and tuberculoid leprosy.
Continue to: Atrophic scarring
Atrophic scarring occurs only after trauma.
Guttate morphea lesions are sclerotic and may be depressed.
Extra-genital lichen sclerosus is characterized by polygonal, shiny, ivory-white sclerotic lesions with or without follicular plugging.
Tuberculoid leprosy involves loss of nociception, hypotrichosis, and palpable thickened regional nerves (eg, great auricular, sural, or ulnar nerve).
Treatment requires long-term anticoagulation
Our patient had APS and the mainstay of treatment is long-term systemic anticoagulation along with attentive wound care.6 Warfarin is preferred over a direct oral anticoagulant as it is more effective in the prevention of recurrent thrombosis in patients with APS.7
Our patient was started on warfarin. Since APS may occur as a primary condition or in the setting of a systemic disease, such as systemic lupus erythematosus, she was referred to a rheumatologist.
1. Alavi A, Hafner J, Dutz JP, et al. Atrophie blanche: is it associated with venous disease or livedoid vasculopathy? Adv Skin Wound Care. 2014;27:518-24. doi: 10.1097/01.ASW.0000455098.98684.95
2. Di Giacomo TB, Hussein TP, Souza DG, et al. Frequency of thrombophilia determinant factors in patients with livedoid vasculopathy and treatment with anticoagulant drugs—a prospective study. J Eur Acad Dermatol Venereol. 2010;24:1340-1346. doi: 10.1111/j.1468-3083.2010.03646.x
3. Millan SB, Gan R, Townsend PE. Venous ulcers: diagnosis and treatment. Am Fam Physician. 2019;100:298-305.
4. Armstrong EM, Bellone JM, Hornsby LB, et al. Acquired thrombophilia. J Pharm Pract. 2014;27:234-242. doi: 10.1177/0897190014530424
5. Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost. 2006;4:295-306. doi: 10.1111/j.1538-7836.2006.01753.x
6. Stevens SM, Woller SC, Bauer KA, et al. Guidance for the evaluation and treatment of hereditary and acquired thrombophilia. J Thromb Thrombolysis. 2016;41:154-164. doi: 10.1007/s11239-015-1316-1
7. Cohen H, Hunt BJ, Efthymiou M, et al. Rivaroxaban versus warfarin to treat patients with thrombotic antiphospholipid syndrome, with or without systemic lupus erythematosus (RAPS): a randomised, controlled, open-label, phase 2/3, non-inferiority trial. Lancet Haematol. 2016;3:e426-e436. doi: 10.1016/S2352-3026(16)30079-5
A 42-year-old woman presented to our dermatology center with white scars on both of her ankles. She first noticed the lesions 2 years prior; they were initially erythematous and painful, even when she was at rest. Her past medical history included 3 spontaneous term miscarriages. She denied any prolonged standing or trauma.
On examination, atrophic porcelain-white stellate scars were visible with surrounding hyperpigmentation on the medial aspect of both ankles (FIGURE 1A & 1B). There were no tender erythematous nodules,

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Atrophie blanche
Atrophie blanche is a morphologic feature described as porcelain-white stellate scars with surrounding telangiectasia and hyperpigmentation. The lesions are typically found over the peri-malleolar region and are sequelae of healed erythematous and painful ulcers. The lesions arise from upper dermal, small vessel, thrombotic vasculopathy leading to ischemic rest pain; if left untreated, atrophic white scars eventually develop.
A sign of venous insufficiency or thrombotic vasculopathy
Atrophie blanche may develop following healing of an ulcer due to venous insufficiency or small vessel thrombotic vasculopathy.1 The incidence of thrombotic vasculopathy is 1:100,000 with a female predominance, and up to 50% of cases are associated with procoagulant conditions.2 Thrombotic vasculopathy can be due to an inherited or acquired thrombophilia.1
Causes of hereditary thrombophilia include Factor V Leiden/prothrombin mutations, anti-thrombin III/protein C/protein S deficiencies, dysfibrinogenemia, and hyperhomocysteinemia.
Acquired thrombophilia arises from underlying prothrombotic states associated with the Virchow triad: hypercoagulability, blood flow stasis, and endothelial injury. The use of oral contraceptives or hormone replacement therapy, presence of malignancy, and antiphospholipid syndrome (APS) are causes of acquired thrombophilia.2
Obtaining a careful history is crucial
Thorough history-taking and physical examination are required to determine the underlying cause of atrophie blanche.
Continue to: Chronic venous insufficiency
Chronic venous insufficiency is more likely in patients with a history of prolonged standing, obesity, or previous injury/surgery to leg veins. Physical examination would reveal hyperpigmentation, telangiectasia, varicose veins, pedal edema, and venous ulcers.3
Inherited thrombophilia may be at work in patients with a family history of arterial and venous thrombosis (eg, stroke, acute coronary syndrome, or deep vein thromboses).
Acquired thrombophilia should be suspected if there is a history of recurrent miscarriages or malignancy.4 Given our patient’s history of miscarriages, we ordered further lab work and found that she had elevated anticardiolipin levels (> 40 U/mL) fulfilling the revised Sapporo criteria5 for APS.
Thrombophilia or chronic venous insufficiency? In a patient with a history suggestive of thrombophilia, further work-up should be done before attributing atrophie blanche to healed venous ulcers from chronic venous insufficiency. A skin lesion biopsy could reveal classic changes of thrombotic vasculopathy subjacent to the ulcer, including intraluminal thrombosis, endothelial proliferation, and subintimal hyaline degeneration, as opposed to dermal changes consistent with venous stasis, such as increased siderophages, hemosiderin deposition, erythrocyte extravasation, dermal fibrosis, and adipocytic damage.
Differential diagnosis includes atrophic scarring
The differential diagnosis for hypopigmented atrophic macules and plaques over the lower limbs include atrophic scarring from previous trauma, guttate morphea, extra-genital lichen sclerosus, and tuberculoid leprosy.
Continue to: Atrophic scarring
Atrophic scarring occurs only after trauma.
Guttate morphea lesions are sclerotic and may be depressed.
Extra-genital lichen sclerosus is characterized by polygonal, shiny, ivory-white sclerotic lesions with or without follicular plugging.
Tuberculoid leprosy involves loss of nociception, hypotrichosis, and palpable thickened regional nerves (eg, great auricular, sural, or ulnar nerve).
Treatment requires long-term anticoagulation
Our patient had APS and the mainstay of treatment is long-term systemic anticoagulation along with attentive wound care.6 Warfarin is preferred over a direct oral anticoagulant as it is more effective in the prevention of recurrent thrombosis in patients with APS.7
Our patient was started on warfarin. Since APS may occur as a primary condition or in the setting of a systemic disease, such as systemic lupus erythematosus, she was referred to a rheumatologist.
A 42-year-old woman presented to our dermatology center with white scars on both of her ankles. She first noticed the lesions 2 years prior; they were initially erythematous and painful, even when she was at rest. Her past medical history included 3 spontaneous term miscarriages. She denied any prolonged standing or trauma.
On examination, atrophic porcelain-white stellate scars were visible with surrounding hyperpigmentation on the medial aspect of both ankles (FIGURE 1A & 1B). There were no tender erythematous nodules,

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Atrophie blanche
Atrophie blanche is a morphologic feature described as porcelain-white stellate scars with surrounding telangiectasia and hyperpigmentation. The lesions are typically found over the peri-malleolar region and are sequelae of healed erythematous and painful ulcers. The lesions arise from upper dermal, small vessel, thrombotic vasculopathy leading to ischemic rest pain; if left untreated, atrophic white scars eventually develop.
A sign of venous insufficiency or thrombotic vasculopathy
Atrophie blanche may develop following healing of an ulcer due to venous insufficiency or small vessel thrombotic vasculopathy.1 The incidence of thrombotic vasculopathy is 1:100,000 with a female predominance, and up to 50% of cases are associated with procoagulant conditions.2 Thrombotic vasculopathy can be due to an inherited or acquired thrombophilia.1
Causes of hereditary thrombophilia include Factor V Leiden/prothrombin mutations, anti-thrombin III/protein C/protein S deficiencies, dysfibrinogenemia, and hyperhomocysteinemia.
Acquired thrombophilia arises from underlying prothrombotic states associated with the Virchow triad: hypercoagulability, blood flow stasis, and endothelial injury. The use of oral contraceptives or hormone replacement therapy, presence of malignancy, and antiphospholipid syndrome (APS) are causes of acquired thrombophilia.2
Obtaining a careful history is crucial
Thorough history-taking and physical examination are required to determine the underlying cause of atrophie blanche.
Continue to: Chronic venous insufficiency
Chronic venous insufficiency is more likely in patients with a history of prolonged standing, obesity, or previous injury/surgery to leg veins. Physical examination would reveal hyperpigmentation, telangiectasia, varicose veins, pedal edema, and venous ulcers.3
Inherited thrombophilia may be at work in patients with a family history of arterial and venous thrombosis (eg, stroke, acute coronary syndrome, or deep vein thromboses).
Acquired thrombophilia should be suspected if there is a history of recurrent miscarriages or malignancy.4 Given our patient’s history of miscarriages, we ordered further lab work and found that she had elevated anticardiolipin levels (> 40 U/mL) fulfilling the revised Sapporo criteria5 for APS.
Thrombophilia or chronic venous insufficiency? In a patient with a history suggestive of thrombophilia, further work-up should be done before attributing atrophie blanche to healed venous ulcers from chronic venous insufficiency. A skin lesion biopsy could reveal classic changes of thrombotic vasculopathy subjacent to the ulcer, including intraluminal thrombosis, endothelial proliferation, and subintimal hyaline degeneration, as opposed to dermal changes consistent with venous stasis, such as increased siderophages, hemosiderin deposition, erythrocyte extravasation, dermal fibrosis, and adipocytic damage.
Differential diagnosis includes atrophic scarring
The differential diagnosis for hypopigmented atrophic macules and plaques over the lower limbs include atrophic scarring from previous trauma, guttate morphea, extra-genital lichen sclerosus, and tuberculoid leprosy.
Continue to: Atrophic scarring
Atrophic scarring occurs only after trauma.
Guttate morphea lesions are sclerotic and may be depressed.
Extra-genital lichen sclerosus is characterized by polygonal, shiny, ivory-white sclerotic lesions with or without follicular plugging.
Tuberculoid leprosy involves loss of nociception, hypotrichosis, and palpable thickened regional nerves (eg, great auricular, sural, or ulnar nerve).
Treatment requires long-term anticoagulation
Our patient had APS and the mainstay of treatment is long-term systemic anticoagulation along with attentive wound care.6 Warfarin is preferred over a direct oral anticoagulant as it is more effective in the prevention of recurrent thrombosis in patients with APS.7
Our patient was started on warfarin. Since APS may occur as a primary condition or in the setting of a systemic disease, such as systemic lupus erythematosus, she was referred to a rheumatologist.
1. Alavi A, Hafner J, Dutz JP, et al. Atrophie blanche: is it associated with venous disease or livedoid vasculopathy? Adv Skin Wound Care. 2014;27:518-24. doi: 10.1097/01.ASW.0000455098.98684.95
2. Di Giacomo TB, Hussein TP, Souza DG, et al. Frequency of thrombophilia determinant factors in patients with livedoid vasculopathy and treatment with anticoagulant drugs—a prospective study. J Eur Acad Dermatol Venereol. 2010;24:1340-1346. doi: 10.1111/j.1468-3083.2010.03646.x
3. Millan SB, Gan R, Townsend PE. Venous ulcers: diagnosis and treatment. Am Fam Physician. 2019;100:298-305.
4. Armstrong EM, Bellone JM, Hornsby LB, et al. Acquired thrombophilia. J Pharm Pract. 2014;27:234-242. doi: 10.1177/0897190014530424
5. Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost. 2006;4:295-306. doi: 10.1111/j.1538-7836.2006.01753.x
6. Stevens SM, Woller SC, Bauer KA, et al. Guidance for the evaluation and treatment of hereditary and acquired thrombophilia. J Thromb Thrombolysis. 2016;41:154-164. doi: 10.1007/s11239-015-1316-1
7. Cohen H, Hunt BJ, Efthymiou M, et al. Rivaroxaban versus warfarin to treat patients with thrombotic antiphospholipid syndrome, with or without systemic lupus erythematosus (RAPS): a randomised, controlled, open-label, phase 2/3, non-inferiority trial. Lancet Haematol. 2016;3:e426-e436. doi: 10.1016/S2352-3026(16)30079-5
1. Alavi A, Hafner J, Dutz JP, et al. Atrophie blanche: is it associated with venous disease or livedoid vasculopathy? Adv Skin Wound Care. 2014;27:518-24. doi: 10.1097/01.ASW.0000455098.98684.95
2. Di Giacomo TB, Hussein TP, Souza DG, et al. Frequency of thrombophilia determinant factors in patients with livedoid vasculopathy and treatment with anticoagulant drugs—a prospective study. J Eur Acad Dermatol Venereol. 2010;24:1340-1346. doi: 10.1111/j.1468-3083.2010.03646.x
3. Millan SB, Gan R, Townsend PE. Venous ulcers: diagnosis and treatment. Am Fam Physician. 2019;100:298-305.
4. Armstrong EM, Bellone JM, Hornsby LB, et al. Acquired thrombophilia. J Pharm Pract. 2014;27:234-242. doi: 10.1177/0897190014530424
5. Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost. 2006;4:295-306. doi: 10.1111/j.1538-7836.2006.01753.x
6. Stevens SM, Woller SC, Bauer KA, et al. Guidance for the evaluation and treatment of hereditary and acquired thrombophilia. J Thromb Thrombolysis. 2016;41:154-164. doi: 10.1007/s11239-015-1316-1
7. Cohen H, Hunt BJ, Efthymiou M, et al. Rivaroxaban versus warfarin to treat patients with thrombotic antiphospholipid syndrome, with or without systemic lupus erythematosus (RAPS): a randomised, controlled, open-label, phase 2/3, non-inferiority trial. Lancet Haematol. 2016;3:e426-e436. doi: 10.1016/S2352-3026(16)30079-5
Waxy fingers and skin tethering
A 73-year-old man with longstanding, poorly controlled type 1 diabetes (T1D) and worsening paresthesia presented to the dermatology clinic following a painless thermal burn of his fingertips from holding a hot cup of coffee. The patient’s paresthesia in a stocking-and-glove distribution was attributable to diabetes-associated polyneuropathy. Two years prior, he had been diagnosed with mildly symptomatic, diabetes-associated scleredema of his upper back and treated with topical corticosteroids.
Physical examination revealed tense bullae on the pads of all 5 digits of his right hand (FIGURE 1). Localized, waxy tightening of the skin was noted on all digits of both hands, along with mild tethering of thickened skin on the right palm.
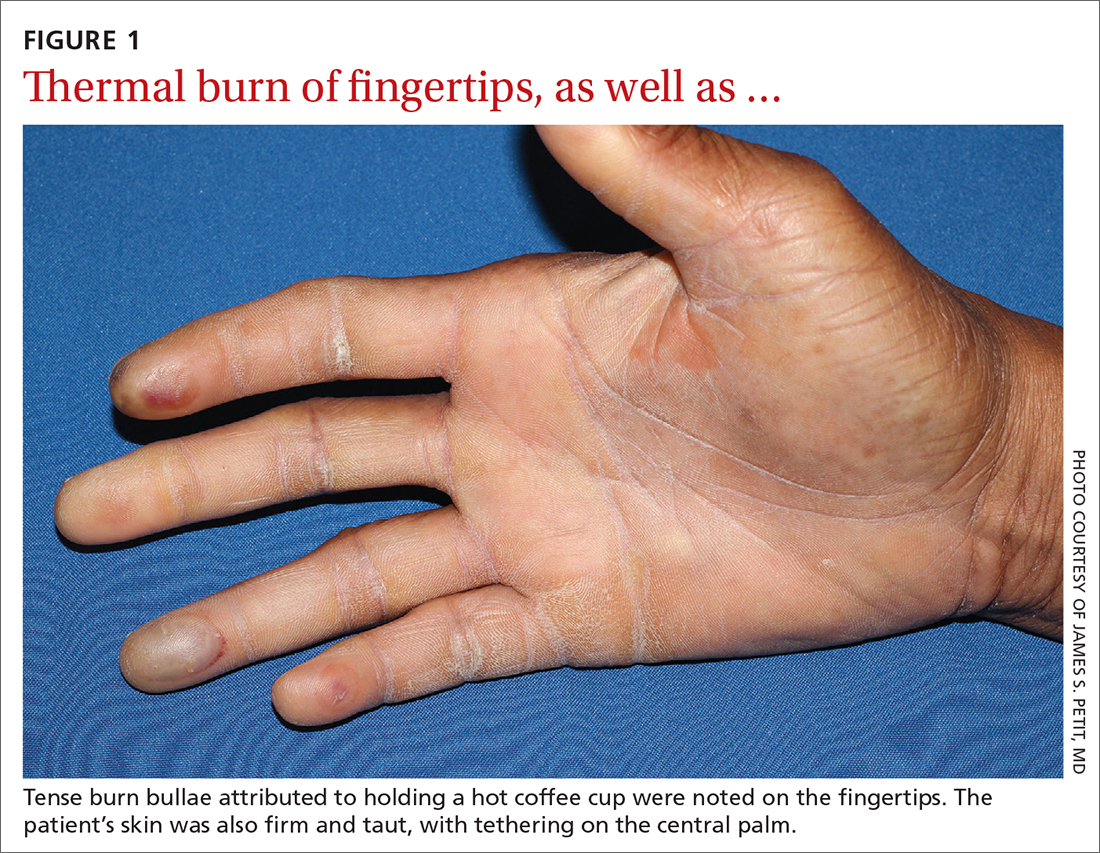
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Diabetic hand syndrome
Subtle, early signs of diabetic sclerodactyly and Dupuytren contracture (DC) were observed in the context of an existing diagnosis of T1D, leading to a diagnosis of diabetic hand syndrome.
Sclerodactyly, a thickening and tightening of the skin, is a characteristic component of limited and systemic sclerosis. Sclerodactyly is not commonly observed in association with type 1 and type 2 diabetes; however, when it does occur, it is typically found in patients who have had uncontrolled diabetes for some time.1-3 (In the context of diabetes, this skin manifestation is known as pseudoscleroderma and scleredema diabeticorum.) In 1 study of 238 patients with T1D, the prevalence of this diabetes manifestation was 39%, with a range of 10% to 50% also reported.3
Diabetic hand syndrome is an umbrella term for the constellation of debilitating fibroproliferative sequelae of the hand rendered by diabetes.3 In addition to diabetic sclerodactyly, diabetic hand syndrome includes limited joint mobility (LJM), or diabetic cheiroarthropathy, which typically manifests with either the “prayer sign” (the inability of the palms to obtain full approximation while the wrists are maximally flexed) or the “tabletop sign” (the inability of the palm to flatten completely against the surface of a table) (FIGURE 2).4,5 The prevalence of LJM has been reported to range from 8% to 50% of patients diagnosed with longstanding, uncontrolled diabetes.4
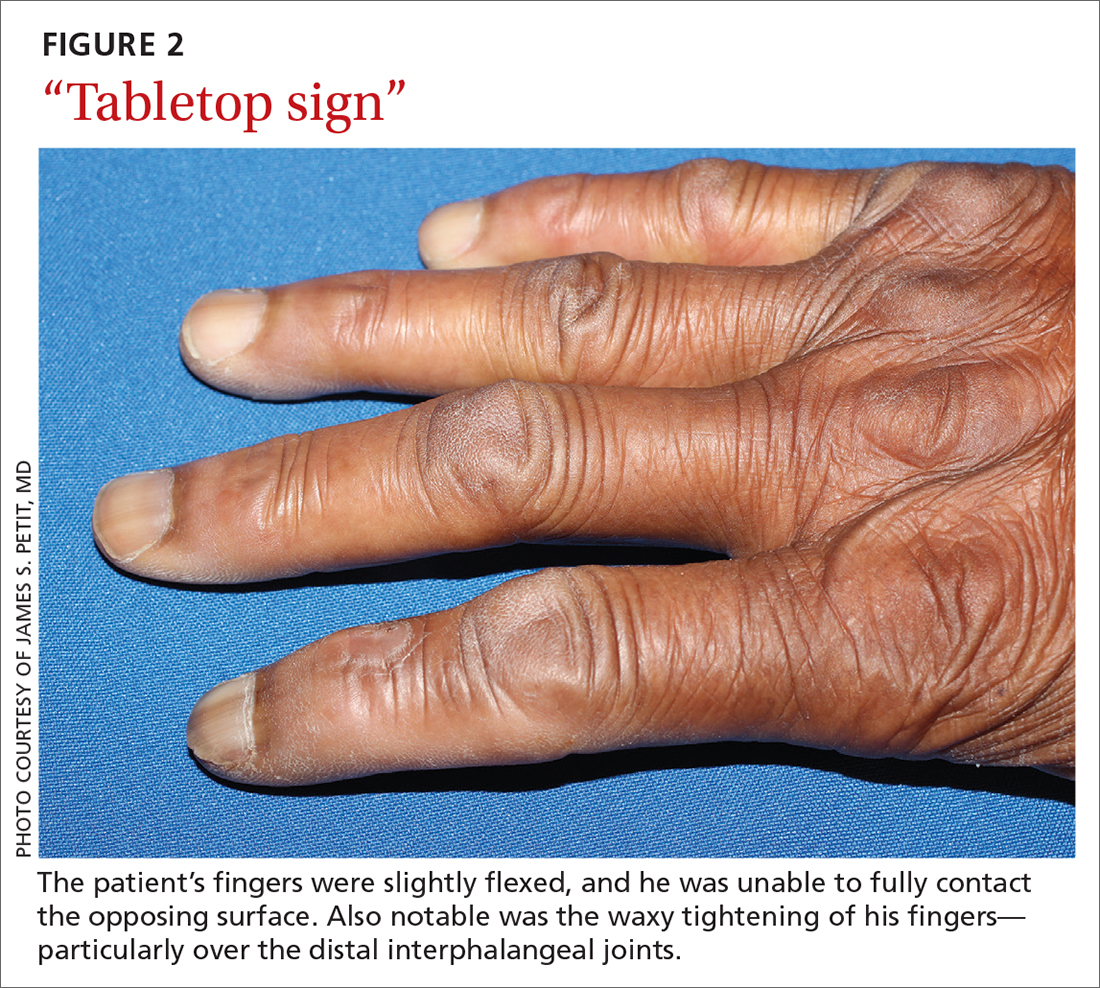
Other musculoskeletal abnormalities seen in this syndrome include: DC, often found clinically as a palpable palmar nodule that ultimately results in a flexion contracture of the affected finger; stenosing tenosynovitis, or trigger finger, in which a reproducible locking phenomenon occurs on flexion of a finger, typically in the first, third, and fourth digits; and carpal tunnel syndrome, a median nerve entrapment neuropathy that results in pain and/or paresthesia over the thumb, index, middle, and lateral half of the ring fingers.3-5
Secondary symptoms can signal long-term degenerative disease
Stocking-and-glove distribution polyneuropathy with deterioration of tactile sensation is a common sequela of diabetes, especially as disease severity progresses.2 Although the exact pathogenesis remains unclear, it has been proposed that both diabetic polyneuropathy and increased skin thickness occur secondary to long-term degenerative microvascular disease.
Continue to: Specifically, prolonged...
Specifically, prolonged hyperglycemia and secondary chronic inflammation set the stage for protein glycation, with formation of advanced glycation end products (AGEs). It is thought that these AGEs in cutaneous and connective tissues stiffen collagen, leading to scleroderma-like skin changes.2
These microvascular and fibroproliferative changes are also considered important contributors in the etiology of DC and trigger finger, ultimately leading to increased collagen deposition and fascial thickening.4,5 In addition, increased activation of the polyol pathway may occur secondary to hyperglycemia, resulting in increased intracellular water and cellular edema.5
The differential is comprisedof components of systemic disease
The differential diagnosis includes tropical diabetic hand, autoimmune-related scleroderma (also called systemic sclerosis), complex regional pain syndrome, and diabetic scleredema.
Tropical diabetic hand, a potentially dangerous infection, is generally found only in tropical regions and in the setting of injury.5,6
Autoimmune-related scleroderma may be diagnosed alongside other signs and symptoms of CREST: calcinosis, Raynaud phenomenon, esophageal dysmotility, sclerodactyly, and telangiectasia. In the absence of other signs and symptoms, and in the presence of uncontrolled diabetes, biopsy would be needed to definitively diagnose it. Clinically, diabetic hand can be distinguished with concurrent involvement of the upper back.
Continue to: Complex regional pain syndrome
Complex regional pain syndrome is characterized by chronic, disabling pain, swelling, and motor impairment that frequently affect the hand, often secondary to surgery or trauma.5,7 This diagnosis differs from the generally painless skin hardening of diabetic hand.
The co-existence of diabetic scleredema and diabetic sclerodactyly has been previously reported, although the onset of each condition is often temporally distinct.8 In contrast to diabetic sclerodactyly, the firm indurated skin characteristic of diabetic scleredema (which our patient had) initially involves the shoulders and neck and may progress over the trunk, including the upper back, typically sparing the distal extremities. Of note, the dermis in scleredema is thickened with marked deposition of mucopolysaccharide.9
Glycemic control is paramount
Studies of patients with diabetes who have thick, waxy skin and LJM have shown that tight glycemic control may reduce skin thickness and palmar fascia fibrosis.3,5,9 Thus, in this patient with poorly controlled T1D, diabetic sclerodactyly, early DC, and second-degree burns attributable to advanced polyneuropathy, tightened glycemic control is logical and warranted. Such control could potentially impact the trajectory and morbidity of skin and musculoskeletal manifestations in this broad-reaching disease.
Although there are limited treatments for mobility-related symptoms of diabetic hand syndrome, physiotherapy is recommended in more severe stages of disease to increase joint range of motion.4,5 More severe cases of DC and trigger finger have been successfully treated with topical steroids, corticosteroid injections, and surgery.4,5 Simply stated—and in line with compulsive foot care—the diabetic milieu necessitates clinicians’ close attention to the hands. Components of diabetic hand, LJM, DC, or trigger finger may indicate a need to screen not only for diabetes in a patient previously undiagnosed but also, importantly, for other sequelae of diabetes, including retinopathy.4,5
Our patient was treated with a moderate-potency topical steroid, triamcinolone 0.1% cream, and was advised to continue optimizing glycemic control with the aid of his primary care physician. It was unclear whether the patient improved with use, as he was lost to follow-up.
1. Yosipovitch G, Hodak E, Vardi P, et al. The prevalence of cutaneous manifestations in IDDM patients and their association with diabetes risk factors and microvascular complications. Diabetes Care. 1998;21:506-509. doi: 10.2337/diacare.21.4.506
2. Redmond CL, Bain GI, Laslett LL, et al. Deteriorating tactile sensation in patients with hand syndromes associated with diabetes: a two-year observational study. J Diabetes Complications. 2012;26:313-318. doi: 10.1016/j.jdiacomp.2012.04.009
3. Rosen J, Yosipovitch G. Skin manifestations of diabetes mellitus. In: Feingold KR, Anawalt B, Boyce A, et al, eds. Endotext. 2018. South Dartmouth, MA. Accessed November 30, 2021. www.ncbi.nlm.nih.gov/books/NBK481900/
4. Goyal A, Tiwari V, Gupta Y. Diabetic hand: a neglected complication of diabetes mellitus. Cureus. 2018;10:e2772. doi: 10.7759/cureus.2772
5. Papanas N, Maltezos E. The diabetic hand: a forgotten complication? J Diabetes Complications. 2010;24:154-162. doi: 10.1016/j.jdiacomp.2008.12.009
6. Gill GV, Famuyiwa OO, Rolfe M, et al. Tropical diabetic hand syndrome. Lancet. 1998;351:113-114. doi: 10.1016/S0140-6736(05)78146-0
7. Goh EL, Chidambaram S, Ma D. Complex regional pain syndrome: a recent update. Burns Trauma. 2017;5:2. doi: 10.1186/s41038-016-0066-4
8. Gruson LM, Franks A Jr. Scleredema and diabetic sclerodactyly. Dermatol Online J. 2005;11:3.
9. Shazzad MN, Azad AK, Abdal SJ, et al. Scleredema diabeticorum – a case report. Mymensingh Med J. 2015;24:606-609.
A 73-year-old man with longstanding, poorly controlled type 1 diabetes (T1D) and worsening paresthesia presented to the dermatology clinic following a painless thermal burn of his fingertips from holding a hot cup of coffee. The patient’s paresthesia in a stocking-and-glove distribution was attributable to diabetes-associated polyneuropathy. Two years prior, he had been diagnosed with mildly symptomatic, diabetes-associated scleredema of his upper back and treated with topical corticosteroids.
Physical examination revealed tense bullae on the pads of all 5 digits of his right hand (FIGURE 1). Localized, waxy tightening of the skin was noted on all digits of both hands, along with mild tethering of thickened skin on the right palm.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Diabetic hand syndrome
Subtle, early signs of diabetic sclerodactyly and Dupuytren contracture (DC) were observed in the context of an existing diagnosis of T1D, leading to a diagnosis of diabetic hand syndrome.
Sclerodactyly, a thickening and tightening of the skin, is a characteristic component of limited and systemic sclerosis. Sclerodactyly is not commonly observed in association with type 1 and type 2 diabetes; however, when it does occur, it is typically found in patients who have had uncontrolled diabetes for some time.1-3 (In the context of diabetes, this skin manifestation is known as pseudoscleroderma and scleredema diabeticorum.) In 1 study of 238 patients with T1D, the prevalence of this diabetes manifestation was 39%, with a range of 10% to 50% also reported.3
Diabetic hand syndrome is an umbrella term for the constellation of debilitating fibroproliferative sequelae of the hand rendered by diabetes.3 In addition to diabetic sclerodactyly, diabetic hand syndrome includes limited joint mobility (LJM), or diabetic cheiroarthropathy, which typically manifests with either the “prayer sign” (the inability of the palms to obtain full approximation while the wrists are maximally flexed) or the “tabletop sign” (the inability of the palm to flatten completely against the surface of a table) (FIGURE 2).4,5 The prevalence of LJM has been reported to range from 8% to 50% of patients diagnosed with longstanding, uncontrolled diabetes.4

Other musculoskeletal abnormalities seen in this syndrome include: DC, often found clinically as a palpable palmar nodule that ultimately results in a flexion contracture of the affected finger; stenosing tenosynovitis, or trigger finger, in which a reproducible locking phenomenon occurs on flexion of a finger, typically in the first, third, and fourth digits; and carpal tunnel syndrome, a median nerve entrapment neuropathy that results in pain and/or paresthesia over the thumb, index, middle, and lateral half of the ring fingers.3-5
Secondary symptoms can signal long-term degenerative disease
Stocking-and-glove distribution polyneuropathy with deterioration of tactile sensation is a common sequela of diabetes, especially as disease severity progresses.2 Although the exact pathogenesis remains unclear, it has been proposed that both diabetic polyneuropathy and increased skin thickness occur secondary to long-term degenerative microvascular disease.
Continue to: Specifically, prolonged...
Specifically, prolonged hyperglycemia and secondary chronic inflammation set the stage for protein glycation, with formation of advanced glycation end products (AGEs). It is thought that these AGEs in cutaneous and connective tissues stiffen collagen, leading to scleroderma-like skin changes.2
These microvascular and fibroproliferative changes are also considered important contributors in the etiology of DC and trigger finger, ultimately leading to increased collagen deposition and fascial thickening.4,5 In addition, increased activation of the polyol pathway may occur secondary to hyperglycemia, resulting in increased intracellular water and cellular edema.5
The differential is comprisedof components of systemic disease
The differential diagnosis includes tropical diabetic hand, autoimmune-related scleroderma (also called systemic sclerosis), complex regional pain syndrome, and diabetic scleredema.
Tropical diabetic hand, a potentially dangerous infection, is generally found only in tropical regions and in the setting of injury.5,6
Autoimmune-related scleroderma may be diagnosed alongside other signs and symptoms of CREST: calcinosis, Raynaud phenomenon, esophageal dysmotility, sclerodactyly, and telangiectasia. In the absence of other signs and symptoms, and in the presence of uncontrolled diabetes, biopsy would be needed to definitively diagnose it. Clinically, diabetic hand can be distinguished with concurrent involvement of the upper back.
Continue to: Complex regional pain syndrome
Complex regional pain syndrome is characterized by chronic, disabling pain, swelling, and motor impairment that frequently affect the hand, often secondary to surgery or trauma.5,7 This diagnosis differs from the generally painless skin hardening of diabetic hand.
The co-existence of diabetic scleredema and diabetic sclerodactyly has been previously reported, although the onset of each condition is often temporally distinct.8 In contrast to diabetic sclerodactyly, the firm indurated skin characteristic of diabetic scleredema (which our patient had) initially involves the shoulders and neck and may progress over the trunk, including the upper back, typically sparing the distal extremities. Of note, the dermis in scleredema is thickened with marked deposition of mucopolysaccharide.9
Glycemic control is paramount
Studies of patients with diabetes who have thick, waxy skin and LJM have shown that tight glycemic control may reduce skin thickness and palmar fascia fibrosis.3,5,9 Thus, in this patient with poorly controlled T1D, diabetic sclerodactyly, early DC, and second-degree burns attributable to advanced polyneuropathy, tightened glycemic control is logical and warranted. Such control could potentially impact the trajectory and morbidity of skin and musculoskeletal manifestations in this broad-reaching disease.
Although there are limited treatments for mobility-related symptoms of diabetic hand syndrome, physiotherapy is recommended in more severe stages of disease to increase joint range of motion.4,5 More severe cases of DC and trigger finger have been successfully treated with topical steroids, corticosteroid injections, and surgery.4,5 Simply stated—and in line with compulsive foot care—the diabetic milieu necessitates clinicians’ close attention to the hands. Components of diabetic hand, LJM, DC, or trigger finger may indicate a need to screen not only for diabetes in a patient previously undiagnosed but also, importantly, for other sequelae of diabetes, including retinopathy.4,5
Our patient was treated with a moderate-potency topical steroid, triamcinolone 0.1% cream, and was advised to continue optimizing glycemic control with the aid of his primary care physician. It was unclear whether the patient improved with use, as he was lost to follow-up.
A 73-year-old man with longstanding, poorly controlled type 1 diabetes (T1D) and worsening paresthesia presented to the dermatology clinic following a painless thermal burn of his fingertips from holding a hot cup of coffee. The patient’s paresthesia in a stocking-and-glove distribution was attributable to diabetes-associated polyneuropathy. Two years prior, he had been diagnosed with mildly symptomatic, diabetes-associated scleredema of his upper back and treated with topical corticosteroids.
Physical examination revealed tense bullae on the pads of all 5 digits of his right hand (FIGURE 1). Localized, waxy tightening of the skin was noted on all digits of both hands, along with mild tethering of thickened skin on the right palm.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Diabetic hand syndrome
Subtle, early signs of diabetic sclerodactyly and Dupuytren contracture (DC) were observed in the context of an existing diagnosis of T1D, leading to a diagnosis of diabetic hand syndrome.
Sclerodactyly, a thickening and tightening of the skin, is a characteristic component of limited and systemic sclerosis. Sclerodactyly is not commonly observed in association with type 1 and type 2 diabetes; however, when it does occur, it is typically found in patients who have had uncontrolled diabetes for some time.1-3 (In the context of diabetes, this skin manifestation is known as pseudoscleroderma and scleredema diabeticorum.) In 1 study of 238 patients with T1D, the prevalence of this diabetes manifestation was 39%, with a range of 10% to 50% also reported.3
Diabetic hand syndrome is an umbrella term for the constellation of debilitating fibroproliferative sequelae of the hand rendered by diabetes.3 In addition to diabetic sclerodactyly, diabetic hand syndrome includes limited joint mobility (LJM), or diabetic cheiroarthropathy, which typically manifests with either the “prayer sign” (the inability of the palms to obtain full approximation while the wrists are maximally flexed) or the “tabletop sign” (the inability of the palm to flatten completely against the surface of a table) (FIGURE 2).4,5 The prevalence of LJM has been reported to range from 8% to 50% of patients diagnosed with longstanding, uncontrolled diabetes.4

Other musculoskeletal abnormalities seen in this syndrome include: DC, often found clinically as a palpable palmar nodule that ultimately results in a flexion contracture of the affected finger; stenosing tenosynovitis, or trigger finger, in which a reproducible locking phenomenon occurs on flexion of a finger, typically in the first, third, and fourth digits; and carpal tunnel syndrome, a median nerve entrapment neuropathy that results in pain and/or paresthesia over the thumb, index, middle, and lateral half of the ring fingers.3-5
Secondary symptoms can signal long-term degenerative disease
Stocking-and-glove distribution polyneuropathy with deterioration of tactile sensation is a common sequela of diabetes, especially as disease severity progresses.2 Although the exact pathogenesis remains unclear, it has been proposed that both diabetic polyneuropathy and increased skin thickness occur secondary to long-term degenerative microvascular disease.
Continue to: Specifically, prolonged...
Specifically, prolonged hyperglycemia and secondary chronic inflammation set the stage for protein glycation, with formation of advanced glycation end products (AGEs). It is thought that these AGEs in cutaneous and connective tissues stiffen collagen, leading to scleroderma-like skin changes.2
These microvascular and fibroproliferative changes are also considered important contributors in the etiology of DC and trigger finger, ultimately leading to increased collagen deposition and fascial thickening.4,5 In addition, increased activation of the polyol pathway may occur secondary to hyperglycemia, resulting in increased intracellular water and cellular edema.5
The differential is comprisedof components of systemic disease
The differential diagnosis includes tropical diabetic hand, autoimmune-related scleroderma (also called systemic sclerosis), complex regional pain syndrome, and diabetic scleredema.
Tropical diabetic hand, a potentially dangerous infection, is generally found only in tropical regions and in the setting of injury.5,6
Autoimmune-related scleroderma may be diagnosed alongside other signs and symptoms of CREST: calcinosis, Raynaud phenomenon, esophageal dysmotility, sclerodactyly, and telangiectasia. In the absence of other signs and symptoms, and in the presence of uncontrolled diabetes, biopsy would be needed to definitively diagnose it. Clinically, diabetic hand can be distinguished with concurrent involvement of the upper back.
Continue to: Complex regional pain syndrome
Complex regional pain syndrome is characterized by chronic, disabling pain, swelling, and motor impairment that frequently affect the hand, often secondary to surgery or trauma.5,7 This diagnosis differs from the generally painless skin hardening of diabetic hand.
The co-existence of diabetic scleredema and diabetic sclerodactyly has been previously reported, although the onset of each condition is often temporally distinct.8 In contrast to diabetic sclerodactyly, the firm indurated skin characteristic of diabetic scleredema (which our patient had) initially involves the shoulders and neck and may progress over the trunk, including the upper back, typically sparing the distal extremities. Of note, the dermis in scleredema is thickened with marked deposition of mucopolysaccharide.9
Glycemic control is paramount
Studies of patients with diabetes who have thick, waxy skin and LJM have shown that tight glycemic control may reduce skin thickness and palmar fascia fibrosis.3,5,9 Thus, in this patient with poorly controlled T1D, diabetic sclerodactyly, early DC, and second-degree burns attributable to advanced polyneuropathy, tightened glycemic control is logical and warranted. Such control could potentially impact the trajectory and morbidity of skin and musculoskeletal manifestations in this broad-reaching disease.
Although there are limited treatments for mobility-related symptoms of diabetic hand syndrome, physiotherapy is recommended in more severe stages of disease to increase joint range of motion.4,5 More severe cases of DC and trigger finger have been successfully treated with topical steroids, corticosteroid injections, and surgery.4,5 Simply stated—and in line with compulsive foot care—the diabetic milieu necessitates clinicians’ close attention to the hands. Components of diabetic hand, LJM, DC, or trigger finger may indicate a need to screen not only for diabetes in a patient previously undiagnosed but also, importantly, for other sequelae of diabetes, including retinopathy.4,5
Our patient was treated with a moderate-potency topical steroid, triamcinolone 0.1% cream, and was advised to continue optimizing glycemic control with the aid of his primary care physician. It was unclear whether the patient improved with use, as he was lost to follow-up.
1. Yosipovitch G, Hodak E, Vardi P, et al. The prevalence of cutaneous manifestations in IDDM patients and their association with diabetes risk factors and microvascular complications. Diabetes Care. 1998;21:506-509. doi: 10.2337/diacare.21.4.506
2. Redmond CL, Bain GI, Laslett LL, et al. Deteriorating tactile sensation in patients with hand syndromes associated with diabetes: a two-year observational study. J Diabetes Complications. 2012;26:313-318. doi: 10.1016/j.jdiacomp.2012.04.009
3. Rosen J, Yosipovitch G. Skin manifestations of diabetes mellitus. In: Feingold KR, Anawalt B, Boyce A, et al, eds. Endotext. 2018. South Dartmouth, MA. Accessed November 30, 2021. www.ncbi.nlm.nih.gov/books/NBK481900/
4. Goyal A, Tiwari V, Gupta Y. Diabetic hand: a neglected complication of diabetes mellitus. Cureus. 2018;10:e2772. doi: 10.7759/cureus.2772
5. Papanas N, Maltezos E. The diabetic hand: a forgotten complication? J Diabetes Complications. 2010;24:154-162. doi: 10.1016/j.jdiacomp.2008.12.009
6. Gill GV, Famuyiwa OO, Rolfe M, et al. Tropical diabetic hand syndrome. Lancet. 1998;351:113-114. doi: 10.1016/S0140-6736(05)78146-0
7. Goh EL, Chidambaram S, Ma D. Complex regional pain syndrome: a recent update. Burns Trauma. 2017;5:2. doi: 10.1186/s41038-016-0066-4
8. Gruson LM, Franks A Jr. Scleredema and diabetic sclerodactyly. Dermatol Online J. 2005;11:3.
9. Shazzad MN, Azad AK, Abdal SJ, et al. Scleredema diabeticorum – a case report. Mymensingh Med J. 2015;24:606-609.
1. Yosipovitch G, Hodak E, Vardi P, et al. The prevalence of cutaneous manifestations in IDDM patients and their association with diabetes risk factors and microvascular complications. Diabetes Care. 1998;21:506-509. doi: 10.2337/diacare.21.4.506
2. Redmond CL, Bain GI, Laslett LL, et al. Deteriorating tactile sensation in patients with hand syndromes associated with diabetes: a two-year observational study. J Diabetes Complications. 2012;26:313-318. doi: 10.1016/j.jdiacomp.2012.04.009
3. Rosen J, Yosipovitch G. Skin manifestations of diabetes mellitus. In: Feingold KR, Anawalt B, Boyce A, et al, eds. Endotext. 2018. South Dartmouth, MA. Accessed November 30, 2021. www.ncbi.nlm.nih.gov/books/NBK481900/
4. Goyal A, Tiwari V, Gupta Y. Diabetic hand: a neglected complication of diabetes mellitus. Cureus. 2018;10:e2772. doi: 10.7759/cureus.2772
5. Papanas N, Maltezos E. The diabetic hand: a forgotten complication? J Diabetes Complications. 2010;24:154-162. doi: 10.1016/j.jdiacomp.2008.12.009
6. Gill GV, Famuyiwa OO, Rolfe M, et al. Tropical diabetic hand syndrome. Lancet. 1998;351:113-114. doi: 10.1016/S0140-6736(05)78146-0
7. Goh EL, Chidambaram S, Ma D. Complex regional pain syndrome: a recent update. Burns Trauma. 2017;5:2. doi: 10.1186/s41038-016-0066-4
8. Gruson LM, Franks A Jr. Scleredema and diabetic sclerodactyly. Dermatol Online J. 2005;11:3.
9. Shazzad MN, Azad AK, Abdal SJ, et al. Scleredema diabeticorum – a case report. Mymensingh Med J. 2015;24:606-609.