User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
AHA challenges diet doctor’s study alleging COVID vax risks
An abstract and poster presentation questioning the safety of mRNA-based COVID-19 vaccines, embraced by some and lambasted by others, has drawn an “expression of concern” from the American Heart Association, along with a bid for correction.
The abstract in question concludes that COVID vaccines “dramatically increase” levels of certain inflammatory biomarkers, and therefore, the 5-year risk of acute coronary syndromes (ACS), based on pre- and post-vaccination results of an obscure blood panel called the PULS Cardiac Test (GD Biosciences). The findings were presented at the AHA’s 2021 Scientific Sessionsas, an uncontrolled observational study of 566 patients in a preventive cardiology practice.
Some on social media have seized on the abstract as evidence of serious potential harm from the two available mRNA-based SARS-CoV-2 vaccines, BNT162b2 (Pfizer-BioNTech) and mRNA-1273 (Moderna). But others contend that the study’s described design and findings are specious and its conclusions overstated.
They also point to the notoriety of its one listed author, Steven R. Gundry, MD, who promotes his diet books and supplements as well as fringe, highly criticized theories about diet and disease on several websites, including drgundry.com. Dr. Gundry has not responded to requests for an interview.
Dr. Gundry’s abstract from the AHA Scientific Sessions 2021, available on the meeting’s program planner, was marked with an “expression of concern” by the AHA that is to stand “until a suitable correction is published, to indicate that the abstract in its current version may not be reliable.”
The expression of concern statement, also published online Nov. 24 in Circulation, says “potential errors in the abstract” were brought to the attention of the meeting planners. “Specifically, there are several typographical errors, there is no data in the abstract regarding myocardial T-cell infiltration, there are no statistical analyses for significance provided, and the author is not clear that only anecdotal data was used.”
The biomarker elevations on which the abstract’s conclusions are based included hepatocyte growth factor, “which serves as a marker for chemotaxis of T-cells into epithelium and cardiac tissue,” it states.
“The expression of concern about the abstract will remain in place until a correction is accepted and published” in Circulation, AHA spokesperson Suzanne Grant told this news organization by email.
“The specific data needed will be up to the abstract author to determine and supply,” she said, noting that Dr. Gundry “has been in communication with the journal throughout this process.”
Submitting researchers “must always attest to the validity of the abstract,” Ms. Grant said. “Abstracts are then curated by independent review panels, blinded to the identities of the abstract authors, and are considered based on the potential to add to the diversity of scientific issues and views discussed at the meeting.”
Regarding the AHA’s system for vetting abstracts vying for acceptance to the scientific sessions, she said it is not primarily intended to “evaluate scientific validity” and that the organization is “currently reviewing its existing abstract submission processes.”
A recent Reuters report reviews the controversy and provides links to criticisms of the study on social media.
A version of this article first appeared on Medscape.com.
An abstract and poster presentation questioning the safety of mRNA-based COVID-19 vaccines, embraced by some and lambasted by others, has drawn an “expression of concern” from the American Heart Association, along with a bid for correction.
The abstract in question concludes that COVID vaccines “dramatically increase” levels of certain inflammatory biomarkers, and therefore, the 5-year risk of acute coronary syndromes (ACS), based on pre- and post-vaccination results of an obscure blood panel called the PULS Cardiac Test (GD Biosciences). The findings were presented at the AHA’s 2021 Scientific Sessionsas, an uncontrolled observational study of 566 patients in a preventive cardiology practice.
Some on social media have seized on the abstract as evidence of serious potential harm from the two available mRNA-based SARS-CoV-2 vaccines, BNT162b2 (Pfizer-BioNTech) and mRNA-1273 (Moderna). But others contend that the study’s described design and findings are specious and its conclusions overstated.
They also point to the notoriety of its one listed author, Steven R. Gundry, MD, who promotes his diet books and supplements as well as fringe, highly criticized theories about diet and disease on several websites, including drgundry.com. Dr. Gundry has not responded to requests for an interview.
Dr. Gundry’s abstract from the AHA Scientific Sessions 2021, available on the meeting’s program planner, was marked with an “expression of concern” by the AHA that is to stand “until a suitable correction is published, to indicate that the abstract in its current version may not be reliable.”
The expression of concern statement, also published online Nov. 24 in Circulation, says “potential errors in the abstract” were brought to the attention of the meeting planners. “Specifically, there are several typographical errors, there is no data in the abstract regarding myocardial T-cell infiltration, there are no statistical analyses for significance provided, and the author is not clear that only anecdotal data was used.”
The biomarker elevations on which the abstract’s conclusions are based included hepatocyte growth factor, “which serves as a marker for chemotaxis of T-cells into epithelium and cardiac tissue,” it states.
“The expression of concern about the abstract will remain in place until a correction is accepted and published” in Circulation, AHA spokesperson Suzanne Grant told this news organization by email.
“The specific data needed will be up to the abstract author to determine and supply,” she said, noting that Dr. Gundry “has been in communication with the journal throughout this process.”
Submitting researchers “must always attest to the validity of the abstract,” Ms. Grant said. “Abstracts are then curated by independent review panels, blinded to the identities of the abstract authors, and are considered based on the potential to add to the diversity of scientific issues and views discussed at the meeting.”
Regarding the AHA’s system for vetting abstracts vying for acceptance to the scientific sessions, she said it is not primarily intended to “evaluate scientific validity” and that the organization is “currently reviewing its existing abstract submission processes.”
A recent Reuters report reviews the controversy and provides links to criticisms of the study on social media.
A version of this article first appeared on Medscape.com.
An abstract and poster presentation questioning the safety of mRNA-based COVID-19 vaccines, embraced by some and lambasted by others, has drawn an “expression of concern” from the American Heart Association, along with a bid for correction.
The abstract in question concludes that COVID vaccines “dramatically increase” levels of certain inflammatory biomarkers, and therefore, the 5-year risk of acute coronary syndromes (ACS), based on pre- and post-vaccination results of an obscure blood panel called the PULS Cardiac Test (GD Biosciences). The findings were presented at the AHA’s 2021 Scientific Sessionsas, an uncontrolled observational study of 566 patients in a preventive cardiology practice.
Some on social media have seized on the abstract as evidence of serious potential harm from the two available mRNA-based SARS-CoV-2 vaccines, BNT162b2 (Pfizer-BioNTech) and mRNA-1273 (Moderna). But others contend that the study’s described design and findings are specious and its conclusions overstated.
They also point to the notoriety of its one listed author, Steven R. Gundry, MD, who promotes his diet books and supplements as well as fringe, highly criticized theories about diet and disease on several websites, including drgundry.com. Dr. Gundry has not responded to requests for an interview.
Dr. Gundry’s abstract from the AHA Scientific Sessions 2021, available on the meeting’s program planner, was marked with an “expression of concern” by the AHA that is to stand “until a suitable correction is published, to indicate that the abstract in its current version may not be reliable.”
The expression of concern statement, also published online Nov. 24 in Circulation, says “potential errors in the abstract” were brought to the attention of the meeting planners. “Specifically, there are several typographical errors, there is no data in the abstract regarding myocardial T-cell infiltration, there are no statistical analyses for significance provided, and the author is not clear that only anecdotal data was used.”
The biomarker elevations on which the abstract’s conclusions are based included hepatocyte growth factor, “which serves as a marker for chemotaxis of T-cells into epithelium and cardiac tissue,” it states.
“The expression of concern about the abstract will remain in place until a correction is accepted and published” in Circulation, AHA spokesperson Suzanne Grant told this news organization by email.
“The specific data needed will be up to the abstract author to determine and supply,” she said, noting that Dr. Gundry “has been in communication with the journal throughout this process.”
Submitting researchers “must always attest to the validity of the abstract,” Ms. Grant said. “Abstracts are then curated by independent review panels, blinded to the identities of the abstract authors, and are considered based on the potential to add to the diversity of scientific issues and views discussed at the meeting.”
Regarding the AHA’s system for vetting abstracts vying for acceptance to the scientific sessions, she said it is not primarily intended to “evaluate scientific validity” and that the organization is “currently reviewing its existing abstract submission processes.”
A recent Reuters report reviews the controversy and provides links to criticisms of the study on social media.
A version of this article first appeared on Medscape.com.
Louisiana to require the COVID-19 vaccine for students
Louisiana Gov. John Bel Edwards says the state government plans to make the COVID-19 vaccine a required immunization for students 16 and older in the state’s public school system.
“I just think it’s really, really important to embrace the science and really it’s also important to not engage in misinformation,” said Gov. Edwards, a Democrat, according to The Advocate. “Absent some compelling reason, which I at present have not seen, I fully expect that we will be adding the vaccine to the schedule.”
Parents could opt out their children from the requirement with a letter from a medical provider or a simple signature in dissent, The Advocate reported. The new rule would go into effect at the start of the 2022 school year and at first would apply to students aged 16 and older.
Republican legislators voiced their opposition to the COVID-19 vaccine requirement at a hearing on Dec. 6, calling it unneeded and an example of governmental overreach.
“I believe the vaccine should be highly recommended but not mandated,” state Rep. Laurie Schlegel said, according to TV station WDSU.
State Sen. Cameron Henry of Metairie said he received “hundreds of emails” from parents asking him to prevent the rule from going into effect, WDSU said.
WDSU said the governor can overrule the committee if it rejects the proposed vaccine rule.
Louisiana State Health Officer Joseph Kanter, MD, testified on Dec. 6 that 18 children had died of COVID-19 in Louisiana and many others had become sick because of it.
“I can’t think of another disease on that childhood schedule that we’ve lost that many kids from. In my mind, it’s very much in the public interest. But it’s the family and the parents’ decision,” Dr. Kanter said.
The addition of the vaccine is being proposed by the Louisiana Department of Health, which has added other vaccines to the required list over the years. In 2015, the legislature added meningitis as a required shot with no controversy, The Advocate said.
A version of this article first appeared on WebMD.com.
Louisiana Gov. John Bel Edwards says the state government plans to make the COVID-19 vaccine a required immunization for students 16 and older in the state’s public school system.
“I just think it’s really, really important to embrace the science and really it’s also important to not engage in misinformation,” said Gov. Edwards, a Democrat, according to The Advocate. “Absent some compelling reason, which I at present have not seen, I fully expect that we will be adding the vaccine to the schedule.”
Parents could opt out their children from the requirement with a letter from a medical provider or a simple signature in dissent, The Advocate reported. The new rule would go into effect at the start of the 2022 school year and at first would apply to students aged 16 and older.
Republican legislators voiced their opposition to the COVID-19 vaccine requirement at a hearing on Dec. 6, calling it unneeded and an example of governmental overreach.
“I believe the vaccine should be highly recommended but not mandated,” state Rep. Laurie Schlegel said, according to TV station WDSU.
State Sen. Cameron Henry of Metairie said he received “hundreds of emails” from parents asking him to prevent the rule from going into effect, WDSU said.
WDSU said the governor can overrule the committee if it rejects the proposed vaccine rule.
Louisiana State Health Officer Joseph Kanter, MD, testified on Dec. 6 that 18 children had died of COVID-19 in Louisiana and many others had become sick because of it.
“I can’t think of another disease on that childhood schedule that we’ve lost that many kids from. In my mind, it’s very much in the public interest. But it’s the family and the parents’ decision,” Dr. Kanter said.
The addition of the vaccine is being proposed by the Louisiana Department of Health, which has added other vaccines to the required list over the years. In 2015, the legislature added meningitis as a required shot with no controversy, The Advocate said.
A version of this article first appeared on WebMD.com.
Louisiana Gov. John Bel Edwards says the state government plans to make the COVID-19 vaccine a required immunization for students 16 and older in the state’s public school system.
“I just think it’s really, really important to embrace the science and really it’s also important to not engage in misinformation,” said Gov. Edwards, a Democrat, according to The Advocate. “Absent some compelling reason, which I at present have not seen, I fully expect that we will be adding the vaccine to the schedule.”
Parents could opt out their children from the requirement with a letter from a medical provider or a simple signature in dissent, The Advocate reported. The new rule would go into effect at the start of the 2022 school year and at first would apply to students aged 16 and older.
Republican legislators voiced their opposition to the COVID-19 vaccine requirement at a hearing on Dec. 6, calling it unneeded and an example of governmental overreach.
“I believe the vaccine should be highly recommended but not mandated,” state Rep. Laurie Schlegel said, according to TV station WDSU.
State Sen. Cameron Henry of Metairie said he received “hundreds of emails” from parents asking him to prevent the rule from going into effect, WDSU said.
WDSU said the governor can overrule the committee if it rejects the proposed vaccine rule.
Louisiana State Health Officer Joseph Kanter, MD, testified on Dec. 6 that 18 children had died of COVID-19 in Louisiana and many others had become sick because of it.
“I can’t think of another disease on that childhood schedule that we’ve lost that many kids from. In my mind, it’s very much in the public interest. But it’s the family and the parents’ decision,” Dr. Kanter said.
The addition of the vaccine is being proposed by the Louisiana Department of Health, which has added other vaccines to the required list over the years. In 2015, the legislature added meningitis as a required shot with no controversy, The Advocate said.
A version of this article first appeared on WebMD.com.
CLL and COVID-19: Outcome trends and lessons learned
Retrospective but the data also highlight areas for further investigation, according to the researchers.
Specifically, “the data highlight opportunities for further investigation into optimal management of COVID-19, immune response after infection, and effective vaccination strategy for patients with CLL,” Lindsey E. Roeker, MD, a hematologic oncologist at Memorial Sloan Kettering Cancer Center, New York, and colleagues wrote in a Nov. 4, 2021, letter to the editor of Blood.
The researchers noted that recently reported COVID-19 case fatality rates from two large series of patients with CLL ranged from 31% to 33%, but trends over time were unclear.
“To understand change in outcomes over time, we present this follow-up study, which builds upon a previously reported cohort with extended follow up and addition of more recently diagnosed cases,” they wrote, explaining that “early data from a small series suggest that patients with CLL may not consistently generate anti–SARS-CoV-2 antibodies after infection.”
“This finding, along with previous reports of inadequate response to vaccines in patients with CLL, highlight significant questions regarding COVID-19 vaccine efficacy in this population,” they added.
Trends in outcomes
The review of outcomes in 374 CLL patients from 45 centers who were diagnosed with COVID-19 between Feb. 17, 2020, and Feb. 1, 2021, showed an overall case fatality rate (CFR) of 28%. Among the 278 patients (75%) admitted to the hospital, the CFR was 36%; among those not admitted, the CFR was 4.3%.
Independent predictors of poor survival were ages over 75 years (adjusted hazard ratio, 1.6) and Cumulative Illness Rating Scale–Geriatric (CIRS) scores greater than 6 (aHR, 1.6).
Updated data for 254 patients diagnosed from Feb. 17 to April 30, 2020, and 120 diagnosed from May 1, 2020, to Feb. 1, 2021, showed that more patients in the early versus later cohort were admitted to the hospital (85% vs. 55%) and more required ICU admission (32% vs. 11%).
The overall case fatality rates in the early and later cohorts were 35% and 11%, respectively (P < .001), and among those requiring hospitalization, the rates were 40% and 20% (P = .003).
“The proportion of hospitalized patients requiring ICU-level care was lower in the later cohort (37% vs. 29%), whereas the CFR remained high for the subset of patients who required ICU-level care (52% vs. 50%; P = .89),” the investigators wrote, noting that “[a] difference in management of BTKi[Bruton’s tyrosine kinase inhibitor]-treated patients was observed in the early versus the later cohort.”
“In the early cohort, 76% of patients receiving BTKi had their drug therapy suspended or discontinued. In the later cohort, only 20% of BTKi-treated patients had their therapy suspended or discontinued,” they added.
Univariate analyses showed significant associations between use of remdesivir and OS (HR, 0.48) and use of convalescent plasma and OS (HR, 0.50) in patients who were admitted, whereas admitted patients who received corticosteroids or hydroxychloroquine had an increased risk of death (HRs, 1.73 and 1.53, respectively).
“Corticosteroids were associated with increased risk of death when the data were adjusted for admission status (HR, 1.8) and the need for mechanical ventilation (HR, 2.0), although they were not significantly associated with survival when the data were adjusted for use of supplemental oxygen (HR, 1.4),” they wrote, also noting that admitted patients treated with corticosteroids in the later cohort did not experience an OS benefit (HR, 2.6).
The findings mirror population-based studies with decreasing CFR (35% in those diagnosed before May 1, 2020, versus 11% in those diagnosed after that date), they said, adding that “these trends suggest that patients in the later cohort experienced a less severe clinical course and that the observed difference in CFR over time may not just be due to more frequent testing and identification of less symptomatic patients.”
Of note, the outcomes observed for steroid-treated patients in the current cohort contrast with those from the RECOVERY trial as published in July 2020, which “may be an artifact of their use in patients with more severe disease,” they suggested.
They added that these data “are hypothesis generating and suggest that COVID-19 directed interventions, particularly immunomodulatory agents, require prospective study, specifically in immunocompromised populations.”
The investigators also noted that, consistent with a prior single-center study, 60% of patients with CLL developed positive anti–SARS-CoV-2 serology results after polymerase chain reaction diagnosis of COVID-19, adding further evidence of nonuniform antibody production after COVID-19 in patients with CLL.
Study is ongoing to gain understanding of the immune response to SARS-CoV-2 vaccination in patients with CLL, they said.
Changing the odds
In a related commentary also published in Blood, Yair Herishanu, MD, and Chava Perry, MD, PhD, of Tel Aviv Sourasky Medical Center called the reduction in mortality over time as reported by Dr. Roeker and colleagues “encouraging and intriguing.”
“One explanation is that the later cohort included a larger proportion of patients with mild symptoms who were diagnosed because of increased awareness of COVID-19 and more extensive screening to detect SARS-CoV-2 over time. That is supported by the lower hospitalization rates and lower rates of hospitalized patients requiring ICU care in the later cohort,” they wrote. “Another possibility is better patient management owing to increasing experience, expanding therapeutic options, and improved capacity of health systems to manage an influx of patients.”
The lower mortality in hospitalized patients over time may reflect better management of patients over time, but it also highlights the significance of “early introduction of various anti–COVID-19 therapies to prevent clinical deterioration to ICU-level care,” they added.
Also intriguing, according to Dr. Herishanu and Dr. Perry, was the finding of increased secondary infections and death rates among corticosteroid-treatment patients.
In the RECOVERY trial, the use of dexamethasone improved survival in patients hospitalized with COVID-19 who received respiratory support. Perhaps the impaired immune reactions in patients with CLL moderate the hyperinflammatory reactions to COVID-19, thus turning corticosteroids beneficial effects to somewhat redundant in this frail population,” they wrote.
Further, the finding that only 60% of patients with CLL seroconvert after the acute phase of SARS-CoV-2 infection suggests CLL patients may be at risk for reinfection, which “justifies vaccinating all patients with CLL who have recovered from COVID-19.”
“Likewise, patients with CLL may develop persistent COVID-19 infection,” they added, explaining that “prolonged shedding of infectious SARS-CoV-2 virus and within-host genomic evolution may eventually lead to emergence of new virus variants.”
Given the high risk of severe COVID-19 disease and impaired antibody-mediated immune response to the virus and its vaccine, a booster dose may be warranted in patients with CLL who fail to achieve seropositivity after 2 vaccine doses, they said.
The available data to date “call for early application of antiviral drugs, [monoclonal antibodies], and convalescent plasma as well as improved vaccination strategy, to improve the odds for patients with CLL confronting COVID-19,” they concluded, adding that large-scale prospective studies on the clinical disease course, outcomes, efficacy of treatments, and vaccination timing and schedule in patients with CLL and COVID-19 are still warranted.
The research was supported by a National Cancer Institute Cancer Center support grant. Dr. Roeker, Dr. Herishanu, and Dr. Perry reported having no financial disclosures.
Retrospective but the data also highlight areas for further investigation, according to the researchers.
Specifically, “the data highlight opportunities for further investigation into optimal management of COVID-19, immune response after infection, and effective vaccination strategy for patients with CLL,” Lindsey E. Roeker, MD, a hematologic oncologist at Memorial Sloan Kettering Cancer Center, New York, and colleagues wrote in a Nov. 4, 2021, letter to the editor of Blood.
The researchers noted that recently reported COVID-19 case fatality rates from two large series of patients with CLL ranged from 31% to 33%, but trends over time were unclear.
“To understand change in outcomes over time, we present this follow-up study, which builds upon a previously reported cohort with extended follow up and addition of more recently diagnosed cases,” they wrote, explaining that “early data from a small series suggest that patients with CLL may not consistently generate anti–SARS-CoV-2 antibodies after infection.”
“This finding, along with previous reports of inadequate response to vaccines in patients with CLL, highlight significant questions regarding COVID-19 vaccine efficacy in this population,” they added.
Trends in outcomes
The review of outcomes in 374 CLL patients from 45 centers who were diagnosed with COVID-19 between Feb. 17, 2020, and Feb. 1, 2021, showed an overall case fatality rate (CFR) of 28%. Among the 278 patients (75%) admitted to the hospital, the CFR was 36%; among those not admitted, the CFR was 4.3%.
Independent predictors of poor survival were ages over 75 years (adjusted hazard ratio, 1.6) and Cumulative Illness Rating Scale–Geriatric (CIRS) scores greater than 6 (aHR, 1.6).
Updated data for 254 patients diagnosed from Feb. 17 to April 30, 2020, and 120 diagnosed from May 1, 2020, to Feb. 1, 2021, showed that more patients in the early versus later cohort were admitted to the hospital (85% vs. 55%) and more required ICU admission (32% vs. 11%).
The overall case fatality rates in the early and later cohorts were 35% and 11%, respectively (P < .001), and among those requiring hospitalization, the rates were 40% and 20% (P = .003).
“The proportion of hospitalized patients requiring ICU-level care was lower in the later cohort (37% vs. 29%), whereas the CFR remained high for the subset of patients who required ICU-level care (52% vs. 50%; P = .89),” the investigators wrote, noting that “[a] difference in management of BTKi[Bruton’s tyrosine kinase inhibitor]-treated patients was observed in the early versus the later cohort.”
“In the early cohort, 76% of patients receiving BTKi had their drug therapy suspended or discontinued. In the later cohort, only 20% of BTKi-treated patients had their therapy suspended or discontinued,” they added.
Univariate analyses showed significant associations between use of remdesivir and OS (HR, 0.48) and use of convalescent plasma and OS (HR, 0.50) in patients who were admitted, whereas admitted patients who received corticosteroids or hydroxychloroquine had an increased risk of death (HRs, 1.73 and 1.53, respectively).
“Corticosteroids were associated with increased risk of death when the data were adjusted for admission status (HR, 1.8) and the need for mechanical ventilation (HR, 2.0), although they were not significantly associated with survival when the data were adjusted for use of supplemental oxygen (HR, 1.4),” they wrote, also noting that admitted patients treated with corticosteroids in the later cohort did not experience an OS benefit (HR, 2.6).
The findings mirror population-based studies with decreasing CFR (35% in those diagnosed before May 1, 2020, versus 11% in those diagnosed after that date), they said, adding that “these trends suggest that patients in the later cohort experienced a less severe clinical course and that the observed difference in CFR over time may not just be due to more frequent testing and identification of less symptomatic patients.”
Of note, the outcomes observed for steroid-treated patients in the current cohort contrast with those from the RECOVERY trial as published in July 2020, which “may be an artifact of their use in patients with more severe disease,” they suggested.
They added that these data “are hypothesis generating and suggest that COVID-19 directed interventions, particularly immunomodulatory agents, require prospective study, specifically in immunocompromised populations.”
The investigators also noted that, consistent with a prior single-center study, 60% of patients with CLL developed positive anti–SARS-CoV-2 serology results after polymerase chain reaction diagnosis of COVID-19, adding further evidence of nonuniform antibody production after COVID-19 in patients with CLL.
Study is ongoing to gain understanding of the immune response to SARS-CoV-2 vaccination in patients with CLL, they said.
Changing the odds
In a related commentary also published in Blood, Yair Herishanu, MD, and Chava Perry, MD, PhD, of Tel Aviv Sourasky Medical Center called the reduction in mortality over time as reported by Dr. Roeker and colleagues “encouraging and intriguing.”
“One explanation is that the later cohort included a larger proportion of patients with mild symptoms who were diagnosed because of increased awareness of COVID-19 and more extensive screening to detect SARS-CoV-2 over time. That is supported by the lower hospitalization rates and lower rates of hospitalized patients requiring ICU care in the later cohort,” they wrote. “Another possibility is better patient management owing to increasing experience, expanding therapeutic options, and improved capacity of health systems to manage an influx of patients.”
The lower mortality in hospitalized patients over time may reflect better management of patients over time, but it also highlights the significance of “early introduction of various anti–COVID-19 therapies to prevent clinical deterioration to ICU-level care,” they added.
Also intriguing, according to Dr. Herishanu and Dr. Perry, was the finding of increased secondary infections and death rates among corticosteroid-treatment patients.
In the RECOVERY trial, the use of dexamethasone improved survival in patients hospitalized with COVID-19 who received respiratory support. Perhaps the impaired immune reactions in patients with CLL moderate the hyperinflammatory reactions to COVID-19, thus turning corticosteroids beneficial effects to somewhat redundant in this frail population,” they wrote.
Further, the finding that only 60% of patients with CLL seroconvert after the acute phase of SARS-CoV-2 infection suggests CLL patients may be at risk for reinfection, which “justifies vaccinating all patients with CLL who have recovered from COVID-19.”
“Likewise, patients with CLL may develop persistent COVID-19 infection,” they added, explaining that “prolonged shedding of infectious SARS-CoV-2 virus and within-host genomic evolution may eventually lead to emergence of new virus variants.”
Given the high risk of severe COVID-19 disease and impaired antibody-mediated immune response to the virus and its vaccine, a booster dose may be warranted in patients with CLL who fail to achieve seropositivity after 2 vaccine doses, they said.
The available data to date “call for early application of antiviral drugs, [monoclonal antibodies], and convalescent plasma as well as improved vaccination strategy, to improve the odds for patients with CLL confronting COVID-19,” they concluded, adding that large-scale prospective studies on the clinical disease course, outcomes, efficacy of treatments, and vaccination timing and schedule in patients with CLL and COVID-19 are still warranted.
The research was supported by a National Cancer Institute Cancer Center support grant. Dr. Roeker, Dr. Herishanu, and Dr. Perry reported having no financial disclosures.
Retrospective but the data also highlight areas for further investigation, according to the researchers.
Specifically, “the data highlight opportunities for further investigation into optimal management of COVID-19, immune response after infection, and effective vaccination strategy for patients with CLL,” Lindsey E. Roeker, MD, a hematologic oncologist at Memorial Sloan Kettering Cancer Center, New York, and colleagues wrote in a Nov. 4, 2021, letter to the editor of Blood.
The researchers noted that recently reported COVID-19 case fatality rates from two large series of patients with CLL ranged from 31% to 33%, but trends over time were unclear.
“To understand change in outcomes over time, we present this follow-up study, which builds upon a previously reported cohort with extended follow up and addition of more recently diagnosed cases,” they wrote, explaining that “early data from a small series suggest that patients with CLL may not consistently generate anti–SARS-CoV-2 antibodies after infection.”
“This finding, along with previous reports of inadequate response to vaccines in patients with CLL, highlight significant questions regarding COVID-19 vaccine efficacy in this population,” they added.
Trends in outcomes
The review of outcomes in 374 CLL patients from 45 centers who were diagnosed with COVID-19 between Feb. 17, 2020, and Feb. 1, 2021, showed an overall case fatality rate (CFR) of 28%. Among the 278 patients (75%) admitted to the hospital, the CFR was 36%; among those not admitted, the CFR was 4.3%.
Independent predictors of poor survival were ages over 75 years (adjusted hazard ratio, 1.6) and Cumulative Illness Rating Scale–Geriatric (CIRS) scores greater than 6 (aHR, 1.6).
Updated data for 254 patients diagnosed from Feb. 17 to April 30, 2020, and 120 diagnosed from May 1, 2020, to Feb. 1, 2021, showed that more patients in the early versus later cohort were admitted to the hospital (85% vs. 55%) and more required ICU admission (32% vs. 11%).
The overall case fatality rates in the early and later cohorts were 35% and 11%, respectively (P < .001), and among those requiring hospitalization, the rates were 40% and 20% (P = .003).
“The proportion of hospitalized patients requiring ICU-level care was lower in the later cohort (37% vs. 29%), whereas the CFR remained high for the subset of patients who required ICU-level care (52% vs. 50%; P = .89),” the investigators wrote, noting that “[a] difference in management of BTKi[Bruton’s tyrosine kinase inhibitor]-treated patients was observed in the early versus the later cohort.”
“In the early cohort, 76% of patients receiving BTKi had their drug therapy suspended or discontinued. In the later cohort, only 20% of BTKi-treated patients had their therapy suspended or discontinued,” they added.
Univariate analyses showed significant associations between use of remdesivir and OS (HR, 0.48) and use of convalescent plasma and OS (HR, 0.50) in patients who were admitted, whereas admitted patients who received corticosteroids or hydroxychloroquine had an increased risk of death (HRs, 1.73 and 1.53, respectively).
“Corticosteroids were associated with increased risk of death when the data were adjusted for admission status (HR, 1.8) and the need for mechanical ventilation (HR, 2.0), although they were not significantly associated with survival when the data were adjusted for use of supplemental oxygen (HR, 1.4),” they wrote, also noting that admitted patients treated with corticosteroids in the later cohort did not experience an OS benefit (HR, 2.6).
The findings mirror population-based studies with decreasing CFR (35% in those diagnosed before May 1, 2020, versus 11% in those diagnosed after that date), they said, adding that “these trends suggest that patients in the later cohort experienced a less severe clinical course and that the observed difference in CFR over time may not just be due to more frequent testing and identification of less symptomatic patients.”
Of note, the outcomes observed for steroid-treated patients in the current cohort contrast with those from the RECOVERY trial as published in July 2020, which “may be an artifact of their use in patients with more severe disease,” they suggested.
They added that these data “are hypothesis generating and suggest that COVID-19 directed interventions, particularly immunomodulatory agents, require prospective study, specifically in immunocompromised populations.”
The investigators also noted that, consistent with a prior single-center study, 60% of patients with CLL developed positive anti–SARS-CoV-2 serology results after polymerase chain reaction diagnosis of COVID-19, adding further evidence of nonuniform antibody production after COVID-19 in patients with CLL.
Study is ongoing to gain understanding of the immune response to SARS-CoV-2 vaccination in patients with CLL, they said.
Changing the odds
In a related commentary also published in Blood, Yair Herishanu, MD, and Chava Perry, MD, PhD, of Tel Aviv Sourasky Medical Center called the reduction in mortality over time as reported by Dr. Roeker and colleagues “encouraging and intriguing.”
“One explanation is that the later cohort included a larger proportion of patients with mild symptoms who were diagnosed because of increased awareness of COVID-19 and more extensive screening to detect SARS-CoV-2 over time. That is supported by the lower hospitalization rates and lower rates of hospitalized patients requiring ICU care in the later cohort,” they wrote. “Another possibility is better patient management owing to increasing experience, expanding therapeutic options, and improved capacity of health systems to manage an influx of patients.”
The lower mortality in hospitalized patients over time may reflect better management of patients over time, but it also highlights the significance of “early introduction of various anti–COVID-19 therapies to prevent clinical deterioration to ICU-level care,” they added.
Also intriguing, according to Dr. Herishanu and Dr. Perry, was the finding of increased secondary infections and death rates among corticosteroid-treatment patients.
In the RECOVERY trial, the use of dexamethasone improved survival in patients hospitalized with COVID-19 who received respiratory support. Perhaps the impaired immune reactions in patients with CLL moderate the hyperinflammatory reactions to COVID-19, thus turning corticosteroids beneficial effects to somewhat redundant in this frail population,” they wrote.
Further, the finding that only 60% of patients with CLL seroconvert after the acute phase of SARS-CoV-2 infection suggests CLL patients may be at risk for reinfection, which “justifies vaccinating all patients with CLL who have recovered from COVID-19.”
“Likewise, patients with CLL may develop persistent COVID-19 infection,” they added, explaining that “prolonged shedding of infectious SARS-CoV-2 virus and within-host genomic evolution may eventually lead to emergence of new virus variants.”
Given the high risk of severe COVID-19 disease and impaired antibody-mediated immune response to the virus and its vaccine, a booster dose may be warranted in patients with CLL who fail to achieve seropositivity after 2 vaccine doses, they said.
The available data to date “call for early application of antiviral drugs, [monoclonal antibodies], and convalescent plasma as well as improved vaccination strategy, to improve the odds for patients with CLL confronting COVID-19,” they concluded, adding that large-scale prospective studies on the clinical disease course, outcomes, efficacy of treatments, and vaccination timing and schedule in patients with CLL and COVID-19 are still warranted.
The research was supported by a National Cancer Institute Cancer Center support grant. Dr. Roeker, Dr. Herishanu, and Dr. Perry reported having no financial disclosures.
FROM BLOOD
Is mindfulness key to helping physicians with mental health?
In 2011, the Mayo Clinic began surveying physicians about burnout and found 45% of physicians experienced at least one symptom, such as emotional exhaustion, finding work no longer meaningful, feelings of ineffectiveness, and depersonalizing patients. Associated manifestations can range from headache and insomnia to impaired memory and decreased attention.
Fast forward 10 years to the Medscape National Physician Burnout and Suicide Report, which found that a similar number of physicians (42%) feel burned out. The COVID-19 pandemic only added insult to injury. A Medscape survey that included nearly 5,000 U.S. physicians revealed that about two-thirds (64%) of them reported burnout had intensified during the crisis.
These elevated numbers are being labeled as “a public health crisis” for the impact widespread physician burnout could have on the health of the doctor and patient safety. The relatively consistent levels across the decade seem to suggest that, if health organizations are attempting to improve physician well-being, it doesn’t appear to be working, forcing doctors to find solutions for themselves.
Jill Wener, MD, considers herself part of the 45% burned out 10 years ago. She was working as an internist at Rush University Medical Center in Chicago, but the “existential reality of being a doctor in this world” was wearing on her. “Staying up with the literature, knowing that every day you’re going to go into work without knowing what you’re going to find, threats of lawsuits, the pressure of perfectionism,” Dr. Wener told this news organization. “By the time I hit burnout, everything made me feel like the world was crashing down on me.”
When Dr. Wener encountered someone who meditated twice a day, she was intrigued, even though the self-described “most Type-A, inside-the-box, nonspiritual type, anxious, linear-path doctor” didn’t think people like her could meditate. Dr. Wener is not alone in her hesitation to explore meditation as a means to help prevent burnout because the causes of burnout are primarily linked to external rather than internal factors. Issues including a loss of autonomy, the burden and distraction of electronic health records, and the intense pressure to comply with rules from the government are not things mindfulness can fix.
And because the sources of burnout are primarily environmental and inherent to the current medical system, the suggestion that physicians need to fix themselves with meditation can come as a slap in the face. However, when up against a system slow to change, mindfulness can provide physicians access to the one thing they can control: How they perceive and react to what’s in front of them.
At the recommendation of an acquaintance, Dr. Wener enrolled in a Vedic Meditation (also known as Conscious Health Meditation) course taught by Light Watkins, a well-known traveling instructor, author, and speaker. By the second meeting she was successfully practicing 20 minutes twice a day. This form of mediation traces its roots to the Vedas, ancient Indian texts (also the foundation for yoga), and uses a mantra to settle the mind, transitioning to an awake state of inner contentment.
Three weeks later, Dr. Wener’s daily crying jags ended as did her propensity for road rage. “I felt like I was on the cusp of something life-changing, I just didn’t understand it,” she recalled. “But I knew I was never going to give it up.”
Defining mindfulness
“Mindfulness is being able to be present in the moment that you’re in with acceptance of what it is and without judging it,” said Donna Rockwell, PsyD, a leading mindfulness meditation teacher. The practice of mindfulness is really meditation. Dr. Rockwell explained that the noise of our mind is most often focused on either the past or the future. “We’re either bemoaning something that happened earlier or we’re catastrophizing the future,” she said, which prevents us from being present in the moment.
Meditation allows you to notice when your mind has drifted from the present moment into the past or future. “You gently notice it, label it with a lot of self-compassion, and then bring your mind back by focusing on your breath – going out, going in – and the incoming stimuli through your five senses,” said Dr. Rockwell. “When you’re doing that, you can’t be in the past or future.”
Dr. Rockwell also pointed out that we constantly categorize incoming data of the moment as either “good for me or bad for me,” which gets in the way of simply being present for what you’re facing. “When you’re more fully present, you become more skillful and able to do what this moment is asking of you,” she said. Being mindful allows us to better navigate incoming stimuli, which could be a “code blue” in the ED or a patient who needs another 2 minutes during an office visit.
When Dr. Wener was burned out, she felt unable to adapt whenever something unexpected happened. “When you have no emotional reserves, everything feels like a big deal,” she said. “The meditation gave me what we call adaptation energy; it filled up my tank and kept me from feeling like I was going to lose it at 10 o’clock in the morning.”
Dr. Rockwell explained burnout as an overactive fight or flight response activated by the amygdala. It starts pumping cortisol, our pupils dilate, and our pores open. The prefrontal cortex is offline when we’re experiencing this physiological response because they both can’t be operational at the same time. “When we’re constantly in a ‘fight or flight’ response and don’t have any access to our prefrontal cortex, we are coming from a brain that is pumping cortisol and that leads to burnout,” said Dr. Rockwell.
“Any fight or flight response leaves a mark on your body,” Dr. Wener echoed. “When we go into our state of deep rest in the meditation practice, which is two to five times more restful than sleep, it heals those stress scars.”
Making time for mindfulness
Prescribing mindfulness for physicians is not new. Molecular biologist Jon Kabat-Zinn, PhD, developed Mindfulness-Based Stress Reduction (MBSR) in 1979, a practice that incorporates mindfulness exercises to help people become familiar with their behavior patterns in stressful situations. Thus, instead of reacting, they can respond with a clearer understanding of the circumstance. Dr. Kabat-Zinn initially targeted people with chronic health problems to help them cope with the effects of pain and the condition of their illness, but it has expanded to anyone experiencing challenges in their life, including physicians. A standard MBSR course runs 8 weeks, making it a commitment for most people.
Mindfulness training requires that physicians use what they already have so little of: time.
Dr. Wener was able to take a sabbatical, embarking on a 3-month trip to India to immerse herself in the study of Vedic Meditation. Upon her return, Dr. Wener took a position at Emory University, Atlanta, and has launched a number of CME-accredited meditation courses and retreats. Unlike Dr. Kabat-Zinn, her programs are by physicians and for physicians. She also created an online version of the meditation course to make it more accessible.
For these reasons, Kara Pepper, MD, an internist in outpatient primary care in Atlanta, was drawn to the meditation course. Dr. Pepper was 7 years into practice when she burned out. “The program dovetailed into my burnout recovery,” she said. “It allowed me space to separate myself from the thoughts I was having about work and just recognize them as just that – as thoughts.”
In the course, Dr. Wener teaches the REST Technique, which she says is different than mindfulness in that she encourages the mind to run rampant. “Trying to control the mind can feel very uncomfortable because we always have thoughts,” she says. “We can’t tell the mind to stop thinking just like we can’t tell the heart to stop beating.” Dr. Wener said the REST Technique lets “the mind swim downstream,” allowing the brain to go into a deep state of rest and start to heal from the scars caused by stress.
Dr. Pepper said the self-paced online course gave her all the tools she needed, and it was pragmatic and evidence based. “I didn’t feel ‘woo’ or like another gimmick,” she said. Pepper, who continues to practice medicine, became a life coach in 2019 to teach others the skills she uses daily.
An integrated strategy
In a review published in The American Journal of Medicine in 2019, Scott Yates, MD, MBA, from the Center for Executive Medicine in Plano, Tex., found that physicians who had adopted mediation and mindfulness training to decrease anxiety and perceived work stress only experienced modest benefits. In fact, Dr. Yates claims that there’s little data to suggest the long-term benefit of any particular stress management intervention in the prevention of burnout symptoms.
“The often-repeated goals of the Triple Aim [enhancing patient experience, improving population health, and reducing costs] may be unreachable until we recognize and address burnout in health care providers,” Dr. Yates wrote. He recommends adding a fourth goal to specifically address physician wellness, which certainly could include mindfulness training and meditation.
Burnout coach, trainer, and consultant Dike Drummond, MD, also professes that physician wellness must be added as the key fourth ingredient to improving health care. “Burnout is a dilemma, a balancing act,” he said. “It takes an integrated strategy.” The CEO and founder of TheHappyMD.com, Dr. Drummond’s integrated strategy to stop physician burnout has been taught to more than 40,000 physicians in 175 organizations, and one element of that strategy can be mindfulness training.
Dr. Drummond said he doesn’t use the word meditation “because that scares most people”; it takes a commitment and isn’t accessible for a lot of doctors. Instead, he coaches doctors to use a ‘single-breath’ technique to help them reset multiple times throughout the day. “I teach people how to breathe up to the top of their head and then down to the bottom of their feet,” Dr. Drummond said. He calls it the Squeegee Breath Technique because when they exhale, they “wipe away” anything that doesn’t need to be there right now. “If you happen to have a mindfulness practice like meditation, they work synergistically because the calmness you feel in your mediation is available to you at the bottom of these releasing breaths.”
Various studies and surveys provide great detail as to the “why” of physician burnout. And while mindfulness is not the sole answer, it’s something physicians can explore for themselves while health care as an industry looks for a more comprehensive solution.
“It’s not rocket science,” Dr. Drummond insisted. “You want a different result? You’re not satisfied with the way things are now and you want to feel different? You absolutely must do something different.”
A version of this article first appeared on Medscape.com.
In 2011, the Mayo Clinic began surveying physicians about burnout and found 45% of physicians experienced at least one symptom, such as emotional exhaustion, finding work no longer meaningful, feelings of ineffectiveness, and depersonalizing patients. Associated manifestations can range from headache and insomnia to impaired memory and decreased attention.
Fast forward 10 years to the Medscape National Physician Burnout and Suicide Report, which found that a similar number of physicians (42%) feel burned out. The COVID-19 pandemic only added insult to injury. A Medscape survey that included nearly 5,000 U.S. physicians revealed that about two-thirds (64%) of them reported burnout had intensified during the crisis.
These elevated numbers are being labeled as “a public health crisis” for the impact widespread physician burnout could have on the health of the doctor and patient safety. The relatively consistent levels across the decade seem to suggest that, if health organizations are attempting to improve physician well-being, it doesn’t appear to be working, forcing doctors to find solutions for themselves.
Jill Wener, MD, considers herself part of the 45% burned out 10 years ago. She was working as an internist at Rush University Medical Center in Chicago, but the “existential reality of being a doctor in this world” was wearing on her. “Staying up with the literature, knowing that every day you’re going to go into work without knowing what you’re going to find, threats of lawsuits, the pressure of perfectionism,” Dr. Wener told this news organization. “By the time I hit burnout, everything made me feel like the world was crashing down on me.”
When Dr. Wener encountered someone who meditated twice a day, she was intrigued, even though the self-described “most Type-A, inside-the-box, nonspiritual type, anxious, linear-path doctor” didn’t think people like her could meditate. Dr. Wener is not alone in her hesitation to explore meditation as a means to help prevent burnout because the causes of burnout are primarily linked to external rather than internal factors. Issues including a loss of autonomy, the burden and distraction of electronic health records, and the intense pressure to comply with rules from the government are not things mindfulness can fix.
And because the sources of burnout are primarily environmental and inherent to the current medical system, the suggestion that physicians need to fix themselves with meditation can come as a slap in the face. However, when up against a system slow to change, mindfulness can provide physicians access to the one thing they can control: How they perceive and react to what’s in front of them.
At the recommendation of an acquaintance, Dr. Wener enrolled in a Vedic Meditation (also known as Conscious Health Meditation) course taught by Light Watkins, a well-known traveling instructor, author, and speaker. By the second meeting she was successfully practicing 20 minutes twice a day. This form of mediation traces its roots to the Vedas, ancient Indian texts (also the foundation for yoga), and uses a mantra to settle the mind, transitioning to an awake state of inner contentment.
Three weeks later, Dr. Wener’s daily crying jags ended as did her propensity for road rage. “I felt like I was on the cusp of something life-changing, I just didn’t understand it,” she recalled. “But I knew I was never going to give it up.”
Defining mindfulness
“Mindfulness is being able to be present in the moment that you’re in with acceptance of what it is and without judging it,” said Donna Rockwell, PsyD, a leading mindfulness meditation teacher. The practice of mindfulness is really meditation. Dr. Rockwell explained that the noise of our mind is most often focused on either the past or the future. “We’re either bemoaning something that happened earlier or we’re catastrophizing the future,” she said, which prevents us from being present in the moment.
Meditation allows you to notice when your mind has drifted from the present moment into the past or future. “You gently notice it, label it with a lot of self-compassion, and then bring your mind back by focusing on your breath – going out, going in – and the incoming stimuli through your five senses,” said Dr. Rockwell. “When you’re doing that, you can’t be in the past or future.”
Dr. Rockwell also pointed out that we constantly categorize incoming data of the moment as either “good for me or bad for me,” which gets in the way of simply being present for what you’re facing. “When you’re more fully present, you become more skillful and able to do what this moment is asking of you,” she said. Being mindful allows us to better navigate incoming stimuli, which could be a “code blue” in the ED or a patient who needs another 2 minutes during an office visit.
When Dr. Wener was burned out, she felt unable to adapt whenever something unexpected happened. “When you have no emotional reserves, everything feels like a big deal,” she said. “The meditation gave me what we call adaptation energy; it filled up my tank and kept me from feeling like I was going to lose it at 10 o’clock in the morning.”
Dr. Rockwell explained burnout as an overactive fight or flight response activated by the amygdala. It starts pumping cortisol, our pupils dilate, and our pores open. The prefrontal cortex is offline when we’re experiencing this physiological response because they both can’t be operational at the same time. “When we’re constantly in a ‘fight or flight’ response and don’t have any access to our prefrontal cortex, we are coming from a brain that is pumping cortisol and that leads to burnout,” said Dr. Rockwell.
“Any fight or flight response leaves a mark on your body,” Dr. Wener echoed. “When we go into our state of deep rest in the meditation practice, which is two to five times more restful than sleep, it heals those stress scars.”
Making time for mindfulness
Prescribing mindfulness for physicians is not new. Molecular biologist Jon Kabat-Zinn, PhD, developed Mindfulness-Based Stress Reduction (MBSR) in 1979, a practice that incorporates mindfulness exercises to help people become familiar with their behavior patterns in stressful situations. Thus, instead of reacting, they can respond with a clearer understanding of the circumstance. Dr. Kabat-Zinn initially targeted people with chronic health problems to help them cope with the effects of pain and the condition of their illness, but it has expanded to anyone experiencing challenges in their life, including physicians. A standard MBSR course runs 8 weeks, making it a commitment for most people.
Mindfulness training requires that physicians use what they already have so little of: time.
Dr. Wener was able to take a sabbatical, embarking on a 3-month trip to India to immerse herself in the study of Vedic Meditation. Upon her return, Dr. Wener took a position at Emory University, Atlanta, and has launched a number of CME-accredited meditation courses and retreats. Unlike Dr. Kabat-Zinn, her programs are by physicians and for physicians. She also created an online version of the meditation course to make it more accessible.
For these reasons, Kara Pepper, MD, an internist in outpatient primary care in Atlanta, was drawn to the meditation course. Dr. Pepper was 7 years into practice when she burned out. “The program dovetailed into my burnout recovery,” she said. “It allowed me space to separate myself from the thoughts I was having about work and just recognize them as just that – as thoughts.”
In the course, Dr. Wener teaches the REST Technique, which she says is different than mindfulness in that she encourages the mind to run rampant. “Trying to control the mind can feel very uncomfortable because we always have thoughts,” she says. “We can’t tell the mind to stop thinking just like we can’t tell the heart to stop beating.” Dr. Wener said the REST Technique lets “the mind swim downstream,” allowing the brain to go into a deep state of rest and start to heal from the scars caused by stress.
Dr. Pepper said the self-paced online course gave her all the tools she needed, and it was pragmatic and evidence based. “I didn’t feel ‘woo’ or like another gimmick,” she said. Pepper, who continues to practice medicine, became a life coach in 2019 to teach others the skills she uses daily.
An integrated strategy
In a review published in The American Journal of Medicine in 2019, Scott Yates, MD, MBA, from the Center for Executive Medicine in Plano, Tex., found that physicians who had adopted mediation and mindfulness training to decrease anxiety and perceived work stress only experienced modest benefits. In fact, Dr. Yates claims that there’s little data to suggest the long-term benefit of any particular stress management intervention in the prevention of burnout symptoms.
“The often-repeated goals of the Triple Aim [enhancing patient experience, improving population health, and reducing costs] may be unreachable until we recognize and address burnout in health care providers,” Dr. Yates wrote. He recommends adding a fourth goal to specifically address physician wellness, which certainly could include mindfulness training and meditation.
Burnout coach, trainer, and consultant Dike Drummond, MD, also professes that physician wellness must be added as the key fourth ingredient to improving health care. “Burnout is a dilemma, a balancing act,” he said. “It takes an integrated strategy.” The CEO and founder of TheHappyMD.com, Dr. Drummond’s integrated strategy to stop physician burnout has been taught to more than 40,000 physicians in 175 organizations, and one element of that strategy can be mindfulness training.
Dr. Drummond said he doesn’t use the word meditation “because that scares most people”; it takes a commitment and isn’t accessible for a lot of doctors. Instead, he coaches doctors to use a ‘single-breath’ technique to help them reset multiple times throughout the day. “I teach people how to breathe up to the top of their head and then down to the bottom of their feet,” Dr. Drummond said. He calls it the Squeegee Breath Technique because when they exhale, they “wipe away” anything that doesn’t need to be there right now. “If you happen to have a mindfulness practice like meditation, they work synergistically because the calmness you feel in your mediation is available to you at the bottom of these releasing breaths.”
Various studies and surveys provide great detail as to the “why” of physician burnout. And while mindfulness is not the sole answer, it’s something physicians can explore for themselves while health care as an industry looks for a more comprehensive solution.
“It’s not rocket science,” Dr. Drummond insisted. “You want a different result? You’re not satisfied with the way things are now and you want to feel different? You absolutely must do something different.”
A version of this article first appeared on Medscape.com.
In 2011, the Mayo Clinic began surveying physicians about burnout and found 45% of physicians experienced at least one symptom, such as emotional exhaustion, finding work no longer meaningful, feelings of ineffectiveness, and depersonalizing patients. Associated manifestations can range from headache and insomnia to impaired memory and decreased attention.
Fast forward 10 years to the Medscape National Physician Burnout and Suicide Report, which found that a similar number of physicians (42%) feel burned out. The COVID-19 pandemic only added insult to injury. A Medscape survey that included nearly 5,000 U.S. physicians revealed that about two-thirds (64%) of them reported burnout had intensified during the crisis.
These elevated numbers are being labeled as “a public health crisis” for the impact widespread physician burnout could have on the health of the doctor and patient safety. The relatively consistent levels across the decade seem to suggest that, if health organizations are attempting to improve physician well-being, it doesn’t appear to be working, forcing doctors to find solutions for themselves.
Jill Wener, MD, considers herself part of the 45% burned out 10 years ago. She was working as an internist at Rush University Medical Center in Chicago, but the “existential reality of being a doctor in this world” was wearing on her. “Staying up with the literature, knowing that every day you’re going to go into work without knowing what you’re going to find, threats of lawsuits, the pressure of perfectionism,” Dr. Wener told this news organization. “By the time I hit burnout, everything made me feel like the world was crashing down on me.”
When Dr. Wener encountered someone who meditated twice a day, she was intrigued, even though the self-described “most Type-A, inside-the-box, nonspiritual type, anxious, linear-path doctor” didn’t think people like her could meditate. Dr. Wener is not alone in her hesitation to explore meditation as a means to help prevent burnout because the causes of burnout are primarily linked to external rather than internal factors. Issues including a loss of autonomy, the burden and distraction of electronic health records, and the intense pressure to comply with rules from the government are not things mindfulness can fix.
And because the sources of burnout are primarily environmental and inherent to the current medical system, the suggestion that physicians need to fix themselves with meditation can come as a slap in the face. However, when up against a system slow to change, mindfulness can provide physicians access to the one thing they can control: How they perceive and react to what’s in front of them.
At the recommendation of an acquaintance, Dr. Wener enrolled in a Vedic Meditation (also known as Conscious Health Meditation) course taught by Light Watkins, a well-known traveling instructor, author, and speaker. By the second meeting she was successfully practicing 20 minutes twice a day. This form of mediation traces its roots to the Vedas, ancient Indian texts (also the foundation for yoga), and uses a mantra to settle the mind, transitioning to an awake state of inner contentment.
Three weeks later, Dr. Wener’s daily crying jags ended as did her propensity for road rage. “I felt like I was on the cusp of something life-changing, I just didn’t understand it,” she recalled. “But I knew I was never going to give it up.”
Defining mindfulness
“Mindfulness is being able to be present in the moment that you’re in with acceptance of what it is and without judging it,” said Donna Rockwell, PsyD, a leading mindfulness meditation teacher. The practice of mindfulness is really meditation. Dr. Rockwell explained that the noise of our mind is most often focused on either the past or the future. “We’re either bemoaning something that happened earlier or we’re catastrophizing the future,” she said, which prevents us from being present in the moment.
Meditation allows you to notice when your mind has drifted from the present moment into the past or future. “You gently notice it, label it with a lot of self-compassion, and then bring your mind back by focusing on your breath – going out, going in – and the incoming stimuli through your five senses,” said Dr. Rockwell. “When you’re doing that, you can’t be in the past or future.”
Dr. Rockwell also pointed out that we constantly categorize incoming data of the moment as either “good for me or bad for me,” which gets in the way of simply being present for what you’re facing. “When you’re more fully present, you become more skillful and able to do what this moment is asking of you,” she said. Being mindful allows us to better navigate incoming stimuli, which could be a “code blue” in the ED or a patient who needs another 2 minutes during an office visit.
When Dr. Wener was burned out, she felt unable to adapt whenever something unexpected happened. “When you have no emotional reserves, everything feels like a big deal,” she said. “The meditation gave me what we call adaptation energy; it filled up my tank and kept me from feeling like I was going to lose it at 10 o’clock in the morning.”
Dr. Rockwell explained burnout as an overactive fight or flight response activated by the amygdala. It starts pumping cortisol, our pupils dilate, and our pores open. The prefrontal cortex is offline when we’re experiencing this physiological response because they both can’t be operational at the same time. “When we’re constantly in a ‘fight or flight’ response and don’t have any access to our prefrontal cortex, we are coming from a brain that is pumping cortisol and that leads to burnout,” said Dr. Rockwell.
“Any fight or flight response leaves a mark on your body,” Dr. Wener echoed. “When we go into our state of deep rest in the meditation practice, which is two to five times more restful than sleep, it heals those stress scars.”
Making time for mindfulness
Prescribing mindfulness for physicians is not new. Molecular biologist Jon Kabat-Zinn, PhD, developed Mindfulness-Based Stress Reduction (MBSR) in 1979, a practice that incorporates mindfulness exercises to help people become familiar with their behavior patterns in stressful situations. Thus, instead of reacting, they can respond with a clearer understanding of the circumstance. Dr. Kabat-Zinn initially targeted people with chronic health problems to help them cope with the effects of pain and the condition of their illness, but it has expanded to anyone experiencing challenges in their life, including physicians. A standard MBSR course runs 8 weeks, making it a commitment for most people.
Mindfulness training requires that physicians use what they already have so little of: time.
Dr. Wener was able to take a sabbatical, embarking on a 3-month trip to India to immerse herself in the study of Vedic Meditation. Upon her return, Dr. Wener took a position at Emory University, Atlanta, and has launched a number of CME-accredited meditation courses and retreats. Unlike Dr. Kabat-Zinn, her programs are by physicians and for physicians. She also created an online version of the meditation course to make it more accessible.
For these reasons, Kara Pepper, MD, an internist in outpatient primary care in Atlanta, was drawn to the meditation course. Dr. Pepper was 7 years into practice when she burned out. “The program dovetailed into my burnout recovery,” she said. “It allowed me space to separate myself from the thoughts I was having about work and just recognize them as just that – as thoughts.”
In the course, Dr. Wener teaches the REST Technique, which she says is different than mindfulness in that she encourages the mind to run rampant. “Trying to control the mind can feel very uncomfortable because we always have thoughts,” she says. “We can’t tell the mind to stop thinking just like we can’t tell the heart to stop beating.” Dr. Wener said the REST Technique lets “the mind swim downstream,” allowing the brain to go into a deep state of rest and start to heal from the scars caused by stress.
Dr. Pepper said the self-paced online course gave her all the tools she needed, and it was pragmatic and evidence based. “I didn’t feel ‘woo’ or like another gimmick,” she said. Pepper, who continues to practice medicine, became a life coach in 2019 to teach others the skills she uses daily.
An integrated strategy
In a review published in The American Journal of Medicine in 2019, Scott Yates, MD, MBA, from the Center for Executive Medicine in Plano, Tex., found that physicians who had adopted mediation and mindfulness training to decrease anxiety and perceived work stress only experienced modest benefits. In fact, Dr. Yates claims that there’s little data to suggest the long-term benefit of any particular stress management intervention in the prevention of burnout symptoms.
“The often-repeated goals of the Triple Aim [enhancing patient experience, improving population health, and reducing costs] may be unreachable until we recognize and address burnout in health care providers,” Dr. Yates wrote. He recommends adding a fourth goal to specifically address physician wellness, which certainly could include mindfulness training and meditation.
Burnout coach, trainer, and consultant Dike Drummond, MD, also professes that physician wellness must be added as the key fourth ingredient to improving health care. “Burnout is a dilemma, a balancing act,” he said. “It takes an integrated strategy.” The CEO and founder of TheHappyMD.com, Dr. Drummond’s integrated strategy to stop physician burnout has been taught to more than 40,000 physicians in 175 organizations, and one element of that strategy can be mindfulness training.
Dr. Drummond said he doesn’t use the word meditation “because that scares most people”; it takes a commitment and isn’t accessible for a lot of doctors. Instead, he coaches doctors to use a ‘single-breath’ technique to help them reset multiple times throughout the day. “I teach people how to breathe up to the top of their head and then down to the bottom of their feet,” Dr. Drummond said. He calls it the Squeegee Breath Technique because when they exhale, they “wipe away” anything that doesn’t need to be there right now. “If you happen to have a mindfulness practice like meditation, they work synergistically because the calmness you feel in your mediation is available to you at the bottom of these releasing breaths.”
Various studies and surveys provide great detail as to the “why” of physician burnout. And while mindfulness is not the sole answer, it’s something physicians can explore for themselves while health care as an industry looks for a more comprehensive solution.
“It’s not rocket science,” Dr. Drummond insisted. “You want a different result? You’re not satisfied with the way things are now and you want to feel different? You absolutely must do something different.”
A version of this article first appeared on Medscape.com.
Children and COVID-19: 7 million cases and still counting
Total COVID-19 cases in children surpassed the 7-million mark as new cases rose slightly after the previous week’s decline, according to the American Academy of Pediatrics and the Children’s Hospital Association.
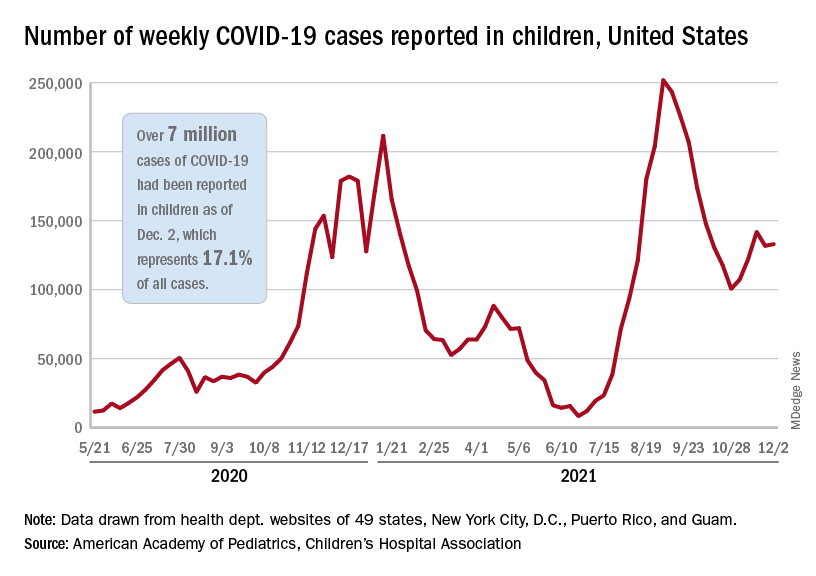
, the AAP and CHA said in their weekly COVID-19 report. New cases had dropped the previous week after 3 straight weeks of increases since late October.
The Centers for Disease Control and Prevention puts the total number of child COVID-19 cases at 6.2 million, but both estimates are based on all-age totals – 40 million for the CDC and 41 million for the AAP/CHA – that are well short of the CDC’s latest cumulative figure, which is now just over 49 million, so the actual figures are undoubtedly higher.
Meanwhile, the 1-month anniversary of 5- to 11-year-olds’ vaccine eligibility brought many completions: 923,000 received their second dose during the week ending Dec. 6, compared with 405,000 the previous week. About 16.9% (4.9 million) of children aged 5-11 have gotten at least one dose of the COVID-19 vaccine thus far, of whom almost 1.5 million children (5.1% of the age group) are now fully vaccinated, the CDC said on its COVID-19 Data Tracker.
The pace of vaccinations, however, is much lower for older children. Weekly numbers for all COVID-19 vaccinations, both first and second doses, dropped from 84,000 (Nov. 23-29) to 70,000 (Nov. 30 to Dec. 6), for those aged 12-17 years. In that group, 61.6% have received at least one dose and 51.8% are fully vaccinated, the CDC said.
The pace of vaccinations varies for younger children as well, when geography is considered. The AAP analyzed the CDC’s data and found that 42% of all 5- to 11-year-olds in Vermont had received at least one dose as of Dec. 1, followed by Massachusetts (33%), Maine (30%), and Rhode Island (28%). At the other end of the vaccination scale are Alabama, Louisiana, Mississippi, and West Virginia, all with 4%, the AAP reported.
As the United States puts 7 million children infected with COVID-19 in its rear view mirror, another milestone is looming ahead: The CDC’s current count of deaths in children is 974.
Total COVID-19 cases in children surpassed the 7-million mark as new cases rose slightly after the previous week’s decline, according to the American Academy of Pediatrics and the Children’s Hospital Association.

, the AAP and CHA said in their weekly COVID-19 report. New cases had dropped the previous week after 3 straight weeks of increases since late October.
The Centers for Disease Control and Prevention puts the total number of child COVID-19 cases at 6.2 million, but both estimates are based on all-age totals – 40 million for the CDC and 41 million for the AAP/CHA – that are well short of the CDC’s latest cumulative figure, which is now just over 49 million, so the actual figures are undoubtedly higher.
Meanwhile, the 1-month anniversary of 5- to 11-year-olds’ vaccine eligibility brought many completions: 923,000 received their second dose during the week ending Dec. 6, compared with 405,000 the previous week. About 16.9% (4.9 million) of children aged 5-11 have gotten at least one dose of the COVID-19 vaccine thus far, of whom almost 1.5 million children (5.1% of the age group) are now fully vaccinated, the CDC said on its COVID-19 Data Tracker.
The pace of vaccinations, however, is much lower for older children. Weekly numbers for all COVID-19 vaccinations, both first and second doses, dropped from 84,000 (Nov. 23-29) to 70,000 (Nov. 30 to Dec. 6), for those aged 12-17 years. In that group, 61.6% have received at least one dose and 51.8% are fully vaccinated, the CDC said.
The pace of vaccinations varies for younger children as well, when geography is considered. The AAP analyzed the CDC’s data and found that 42% of all 5- to 11-year-olds in Vermont had received at least one dose as of Dec. 1, followed by Massachusetts (33%), Maine (30%), and Rhode Island (28%). At the other end of the vaccination scale are Alabama, Louisiana, Mississippi, and West Virginia, all with 4%, the AAP reported.
As the United States puts 7 million children infected with COVID-19 in its rear view mirror, another milestone is looming ahead: The CDC’s current count of deaths in children is 974.
Total COVID-19 cases in children surpassed the 7-million mark as new cases rose slightly after the previous week’s decline, according to the American Academy of Pediatrics and the Children’s Hospital Association.

, the AAP and CHA said in their weekly COVID-19 report. New cases had dropped the previous week after 3 straight weeks of increases since late October.
The Centers for Disease Control and Prevention puts the total number of child COVID-19 cases at 6.2 million, but both estimates are based on all-age totals – 40 million for the CDC and 41 million for the AAP/CHA – that are well short of the CDC’s latest cumulative figure, which is now just over 49 million, so the actual figures are undoubtedly higher.
Meanwhile, the 1-month anniversary of 5- to 11-year-olds’ vaccine eligibility brought many completions: 923,000 received their second dose during the week ending Dec. 6, compared with 405,000 the previous week. About 16.9% (4.9 million) of children aged 5-11 have gotten at least one dose of the COVID-19 vaccine thus far, of whom almost 1.5 million children (5.1% of the age group) are now fully vaccinated, the CDC said on its COVID-19 Data Tracker.
The pace of vaccinations, however, is much lower for older children. Weekly numbers for all COVID-19 vaccinations, both first and second doses, dropped from 84,000 (Nov. 23-29) to 70,000 (Nov. 30 to Dec. 6), for those aged 12-17 years. In that group, 61.6% have received at least one dose and 51.8% are fully vaccinated, the CDC said.
The pace of vaccinations varies for younger children as well, when geography is considered. The AAP analyzed the CDC’s data and found that 42% of all 5- to 11-year-olds in Vermont had received at least one dose as of Dec. 1, followed by Massachusetts (33%), Maine (30%), and Rhode Island (28%). At the other end of the vaccination scale are Alabama, Louisiana, Mississippi, and West Virginia, all with 4%, the AAP reported.
As the United States puts 7 million children infected with COVID-19 in its rear view mirror, another milestone is looming ahead: The CDC’s current count of deaths in children is 974.
In (K)need of Help
ANSWER
The correct answer is bullous impetigo (choice “a”).
Had the source of the problem been MRSA (choice “b”), there would have been marked tenderness and swelling. Psoriasis vulgaris (choice “c”) was a possibility; however, it almost never manifests with blisters, it rarely comes on as quickly as this patient’s problem did, and it produces scale that is far thicker and more tenacious than that seen in bullous impetigo. Nummular eczema (choice “d”) does not manifest with blisters and would likely have caused itching.
DISCUSSION
Impetigo is one of many common skin diseases—other examples include granuloma annulare and lichen planus—that have bullous variants, which can make diagnosis challenging. Impetigo is easy to diagnose in its more common papulosquamous form. But it also can manifest with flaccid blisters that last only a short time, leaving the round, scaly lesions seen in this case.
Staphylococcus aureus, the organism responsible for bullous impetigo, elaborates serine proteases, which bind to and cleave desmoglein-1. This effectively destroys the connections between skin layers, creating a space that is quickly filled with serum. The level of this separation is typically subcorneal, which allows the formation of a very thin, friable roof for the bulla.
This modern version of impetigo is not considered dangerous, despite its association with staph aureus. In pre-antibiotic times, the predominant organism was streptococcal, some types of which could be nephritogenic—that is, capable of causing Bright disease or, as it is now known, acute post-streptococcal glomerulonephritis. Fortunately, this potentially fatal condition is only rarely seen in modern times.
Two items of note about this case: Bullous impetigo, contrary to what was seen in this patient, typically favors intertriginous areas. And a key factor is the history of atopy, which renders the patient more susceptible to skin infections of all kinds.
Treatment
The patient responded well to topical mupirocin ointment, applied three times a day. In rare instances, impetigo can require an oral antibiotic, such as cephalexin.
ANSWER
The correct answer is bullous impetigo (choice “a”).
Had the source of the problem been MRSA (choice “b”), there would have been marked tenderness and swelling. Psoriasis vulgaris (choice “c”) was a possibility; however, it almost never manifests with blisters, it rarely comes on as quickly as this patient’s problem did, and it produces scale that is far thicker and more tenacious than that seen in bullous impetigo. Nummular eczema (choice “d”) does not manifest with blisters and would likely have caused itching.
DISCUSSION
Impetigo is one of many common skin diseases—other examples include granuloma annulare and lichen planus—that have bullous variants, which can make diagnosis challenging. Impetigo is easy to diagnose in its more common papulosquamous form. But it also can manifest with flaccid blisters that last only a short time, leaving the round, scaly lesions seen in this case.
Staphylococcus aureus, the organism responsible for bullous impetigo, elaborates serine proteases, which bind to and cleave desmoglein-1. This effectively destroys the connections between skin layers, creating a space that is quickly filled with serum. The level of this separation is typically subcorneal, which allows the formation of a very thin, friable roof for the bulla.
This modern version of impetigo is not considered dangerous, despite its association with staph aureus. In pre-antibiotic times, the predominant organism was streptococcal, some types of which could be nephritogenic—that is, capable of causing Bright disease or, as it is now known, acute post-streptococcal glomerulonephritis. Fortunately, this potentially fatal condition is only rarely seen in modern times.
Two items of note about this case: Bullous impetigo, contrary to what was seen in this patient, typically favors intertriginous areas. And a key factor is the history of atopy, which renders the patient more susceptible to skin infections of all kinds.
Treatment
The patient responded well to topical mupirocin ointment, applied three times a day. In rare instances, impetigo can require an oral antibiotic, such as cephalexin.
ANSWER
The correct answer is bullous impetigo (choice “a”).
Had the source of the problem been MRSA (choice “b”), there would have been marked tenderness and swelling. Psoriasis vulgaris (choice “c”) was a possibility; however, it almost never manifests with blisters, it rarely comes on as quickly as this patient’s problem did, and it produces scale that is far thicker and more tenacious than that seen in bullous impetigo. Nummular eczema (choice “d”) does not manifest with blisters and would likely have caused itching.
DISCUSSION
Impetigo is one of many common skin diseases—other examples include granuloma annulare and lichen planus—that have bullous variants, which can make diagnosis challenging. Impetigo is easy to diagnose in its more common papulosquamous form. But it also can manifest with flaccid blisters that last only a short time, leaving the round, scaly lesions seen in this case.
Staphylococcus aureus, the organism responsible for bullous impetigo, elaborates serine proteases, which bind to and cleave desmoglein-1. This effectively destroys the connections between skin layers, creating a space that is quickly filled with serum. The level of this separation is typically subcorneal, which allows the formation of a very thin, friable roof for the bulla.
This modern version of impetigo is not considered dangerous, despite its association with staph aureus. In pre-antibiotic times, the predominant organism was streptococcal, some types of which could be nephritogenic—that is, capable of causing Bright disease or, as it is now known, acute post-streptococcal glomerulonephritis. Fortunately, this potentially fatal condition is only rarely seen in modern times.
Two items of note about this case: Bullous impetigo, contrary to what was seen in this patient, typically favors intertriginous areas. And a key factor is the history of atopy, which renders the patient more susceptible to skin infections of all kinds.
Treatment
The patient responded well to topical mupirocin ointment, applied three times a day. In rare instances, impetigo can require an oral antibiotic, such as cephalexin.
The parents of a 6-year-old boy were quite concerned about several lesions on the child’s left knee. The asymptomatic blisters had first appeared about 2 weeks prior. A topical steroid cream prescribed by the family’s primary care provider had not helped.
No one else in the family was similarly affected. They all were reportedly quite healthy, although all were atopic—prone to seasonal allergies and eczema.
Examination revealed at least 6 round, scaly, red lesions on the patient’s knee, ranging from 3 mm to 3 cm in diameter. According to the parents, these had first appeared as intact blisters. There was little to no tenderness or redness around the lesions, and there was no palpable adenopathy in the groin.
The child was in no distress and was afebrile.
Is it time to change the definition of ‘fully vaccinated’?
As more indoor venues require proof of vaccination for entrance and with winter — as well as omicron, a new COVID variant — looming,
It’s been more than six months since many Americans finished their vaccination course against COVID; statistically, their immunity is waning.
At the same time, cases of infections with the Omicron variant have been reported in at least 17 states, as of Dec. 6. Omicron is distinguished by at least 50 mutations, some of which appear to be associated with increased transmissibility. The World Health Organization dubbed it a variant of concern on Nov. 26.
The Centers for Disease Control and Prevention has recommended that everyone 18 and older get a COVID booster shot, revising its narrower guidance that only people 50 and up “should” get a shot while younger adults could choose whether or not to do so. Scientists assume the additional shots will offer significant protection from the new variant, though they do not know for certain how much.
Anthony Fauci, MD, chief medical adviser to President Joe Biden, during a White House press briefing was unequivocal in advising the public. “Get boosted now,” Dr. Fauci said, adding urgency to the current federal guidance. About a quarter of U.S. adults have received additional vaccine doses.
“The definition of ‘fully vaccinated’ has not changed. That’s, you know, after your second dose of a Pfizer or Moderna vaccine, after your single dose of a Johnson & Johnson vaccine,” said the CDC’s director, Dr. Rochelle Walensky, during a Nov. 30 White House briefing on COVID. “We are absolutely encouraging those who are eligible for a boost six months after those mRNA doses to get your boost. But we are not changing the definition of ‘fully vaccinated’ right now.” A booster is recommended two months after receiving the J&J shot.
But that, she noted, could change: “As that science evolves, we will look at whether we need to update our definition of ‘fully vaccinated.’”
Still, the Democratic governors of Connecticut and New Mexico are sending a different signal in their states, as are some countries — such as Israel, which arguably has been the most aggressive nation in its approach. Some scientists point out that many vaccines involve three doses over six months for robust long-term protection, such as the shot against hepatitis. So “fully vaccinated” may need to include shot No. 3 to be considered a full course.
“In my view, if you were vaccinated more than six months ago, you’re not fully vaccinated,” Connecticut Gov. Ned Lamont said Nov. 18 during a press briefing. He was encouraging everyone to get boosted at that time, even before the federal government authorized extra shots for everyone.
New Mexico Gov. Michelle Lujan Grisham had a similar response in mid-November, saying she defined “fully vaccinated” as receiving three shots of the mRNA type. She also opened up booster eligibility to all of her state residents before the CDC and Food and Drug Administration did.
What do the varying views on the evolving science mean for vaccine requirements imposed on travelers, or by schools or workplaces? And what about businesses that have required patrons to provide proof of vaccination?
Dr. Paul Offit, director of the Vaccine Education Center at the Children’s Hospital of Pennsylvania, said the CDC’s stronger recommendation for everyone to get boosted signals to him that a booster is now part of the vaccine regimen. Yet Dr. Offit, who is also a member of the FDA’s vaccine advisory committee, wrote a joint op-ed this week in which he and two other scientists argued that boosters were not yet needed for everyone and that healthy young people should wait to see whether an Omicron-specific booster might be needed.
“I think when the CDC said they are recommending a third dose, they just made the statement that this is a three-dose vaccine series,” Dr. Offit told KHN. “And, frankly, I think it’s going to throw a wrench into mandates.”
Yet to be determined is whether restaurants or other places of business will look more closely at vaccine cards for the booster.
Dr. Georges Benjamin, executive director of the American Public Health Association, said it’s too early to say. “For now, businesses should stay focused on current guidelines,” he said.
Dr. Marc Siegel, an associate professor of medicine at the George Washington School of Medicine and Health Sciences, in Washington, said the question of whether you are fully vaccinated with just two doses or need a booster is a question of semantics. COVID immunity level is the more important issue.
Dr. Siegel said he thinks more suitable terminology would be to call someone “appropriately” or “adequately” vaccinated against COVID rather than “fully” vaccinated, since it’s possible that more boosters could be needed in the future — making “full vaccination” a moving target.
But, as with so many aspects of the pandemic, ambiguity prevails — both in federal guidance on the definition of “fully vaccinated” and in entrance policies, which vary by state, school and business.
Right now, businesses don’t appear to be checking for boosters, but that could change. So, it may be wise to first check the requirements — lest patrons present a two-shot vaccine passport, only to be turned away as inadequately protected.
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
As more indoor venues require proof of vaccination for entrance and with winter — as well as omicron, a new COVID variant — looming,
It’s been more than six months since many Americans finished their vaccination course against COVID; statistically, their immunity is waning.
At the same time, cases of infections with the Omicron variant have been reported in at least 17 states, as of Dec. 6. Omicron is distinguished by at least 50 mutations, some of which appear to be associated with increased transmissibility. The World Health Organization dubbed it a variant of concern on Nov. 26.
The Centers for Disease Control and Prevention has recommended that everyone 18 and older get a COVID booster shot, revising its narrower guidance that only people 50 and up “should” get a shot while younger adults could choose whether or not to do so. Scientists assume the additional shots will offer significant protection from the new variant, though they do not know for certain how much.
Anthony Fauci, MD, chief medical adviser to President Joe Biden, during a White House press briefing was unequivocal in advising the public. “Get boosted now,” Dr. Fauci said, adding urgency to the current federal guidance. About a quarter of U.S. adults have received additional vaccine doses.
“The definition of ‘fully vaccinated’ has not changed. That’s, you know, after your second dose of a Pfizer or Moderna vaccine, after your single dose of a Johnson & Johnson vaccine,” said the CDC’s director, Dr. Rochelle Walensky, during a Nov. 30 White House briefing on COVID. “We are absolutely encouraging those who are eligible for a boost six months after those mRNA doses to get your boost. But we are not changing the definition of ‘fully vaccinated’ right now.” A booster is recommended two months after receiving the J&J shot.
But that, she noted, could change: “As that science evolves, we will look at whether we need to update our definition of ‘fully vaccinated.’”
Still, the Democratic governors of Connecticut and New Mexico are sending a different signal in their states, as are some countries — such as Israel, which arguably has been the most aggressive nation in its approach. Some scientists point out that many vaccines involve three doses over six months for robust long-term protection, such as the shot against hepatitis. So “fully vaccinated” may need to include shot No. 3 to be considered a full course.
“In my view, if you were vaccinated more than six months ago, you’re not fully vaccinated,” Connecticut Gov. Ned Lamont said Nov. 18 during a press briefing. He was encouraging everyone to get boosted at that time, even before the federal government authorized extra shots for everyone.
New Mexico Gov. Michelle Lujan Grisham had a similar response in mid-November, saying she defined “fully vaccinated” as receiving three shots of the mRNA type. She also opened up booster eligibility to all of her state residents before the CDC and Food and Drug Administration did.
What do the varying views on the evolving science mean for vaccine requirements imposed on travelers, or by schools or workplaces? And what about businesses that have required patrons to provide proof of vaccination?
Dr. Paul Offit, director of the Vaccine Education Center at the Children’s Hospital of Pennsylvania, said the CDC’s stronger recommendation for everyone to get boosted signals to him that a booster is now part of the vaccine regimen. Yet Dr. Offit, who is also a member of the FDA’s vaccine advisory committee, wrote a joint op-ed this week in which he and two other scientists argued that boosters were not yet needed for everyone and that healthy young people should wait to see whether an Omicron-specific booster might be needed.
“I think when the CDC said they are recommending a third dose, they just made the statement that this is a three-dose vaccine series,” Dr. Offit told KHN. “And, frankly, I think it’s going to throw a wrench into mandates.”
Yet to be determined is whether restaurants or other places of business will look more closely at vaccine cards for the booster.
Dr. Georges Benjamin, executive director of the American Public Health Association, said it’s too early to say. “For now, businesses should stay focused on current guidelines,” he said.
Dr. Marc Siegel, an associate professor of medicine at the George Washington School of Medicine and Health Sciences, in Washington, said the question of whether you are fully vaccinated with just two doses or need a booster is a question of semantics. COVID immunity level is the more important issue.
Dr. Siegel said he thinks more suitable terminology would be to call someone “appropriately” or “adequately” vaccinated against COVID rather than “fully” vaccinated, since it’s possible that more boosters could be needed in the future — making “full vaccination” a moving target.
But, as with so many aspects of the pandemic, ambiguity prevails — both in federal guidance on the definition of “fully vaccinated” and in entrance policies, which vary by state, school and business.
Right now, businesses don’t appear to be checking for boosters, but that could change. So, it may be wise to first check the requirements — lest patrons present a two-shot vaccine passport, only to be turned away as inadequately protected.
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
As more indoor venues require proof of vaccination for entrance and with winter — as well as omicron, a new COVID variant — looming,
It’s been more than six months since many Americans finished their vaccination course against COVID; statistically, their immunity is waning.
At the same time, cases of infections with the Omicron variant have been reported in at least 17 states, as of Dec. 6. Omicron is distinguished by at least 50 mutations, some of which appear to be associated with increased transmissibility. The World Health Organization dubbed it a variant of concern on Nov. 26.
The Centers for Disease Control and Prevention has recommended that everyone 18 and older get a COVID booster shot, revising its narrower guidance that only people 50 and up “should” get a shot while younger adults could choose whether or not to do so. Scientists assume the additional shots will offer significant protection from the new variant, though they do not know for certain how much.
Anthony Fauci, MD, chief medical adviser to President Joe Biden, during a White House press briefing was unequivocal in advising the public. “Get boosted now,” Dr. Fauci said, adding urgency to the current federal guidance. About a quarter of U.S. adults have received additional vaccine doses.
“The definition of ‘fully vaccinated’ has not changed. That’s, you know, after your second dose of a Pfizer or Moderna vaccine, after your single dose of a Johnson & Johnson vaccine,” said the CDC’s director, Dr. Rochelle Walensky, during a Nov. 30 White House briefing on COVID. “We are absolutely encouraging those who are eligible for a boost six months after those mRNA doses to get your boost. But we are not changing the definition of ‘fully vaccinated’ right now.” A booster is recommended two months after receiving the J&J shot.
But that, she noted, could change: “As that science evolves, we will look at whether we need to update our definition of ‘fully vaccinated.’”
Still, the Democratic governors of Connecticut and New Mexico are sending a different signal in their states, as are some countries — such as Israel, which arguably has been the most aggressive nation in its approach. Some scientists point out that many vaccines involve three doses over six months for robust long-term protection, such as the shot against hepatitis. So “fully vaccinated” may need to include shot No. 3 to be considered a full course.
“In my view, if you were vaccinated more than six months ago, you’re not fully vaccinated,” Connecticut Gov. Ned Lamont said Nov. 18 during a press briefing. He was encouraging everyone to get boosted at that time, even before the federal government authorized extra shots for everyone.
New Mexico Gov. Michelle Lujan Grisham had a similar response in mid-November, saying she defined “fully vaccinated” as receiving three shots of the mRNA type. She also opened up booster eligibility to all of her state residents before the CDC and Food and Drug Administration did.
What do the varying views on the evolving science mean for vaccine requirements imposed on travelers, or by schools or workplaces? And what about businesses that have required patrons to provide proof of vaccination?
Dr. Paul Offit, director of the Vaccine Education Center at the Children’s Hospital of Pennsylvania, said the CDC’s stronger recommendation for everyone to get boosted signals to him that a booster is now part of the vaccine regimen. Yet Dr. Offit, who is also a member of the FDA’s vaccine advisory committee, wrote a joint op-ed this week in which he and two other scientists argued that boosters were not yet needed for everyone and that healthy young people should wait to see whether an Omicron-specific booster might be needed.
“I think when the CDC said they are recommending a third dose, they just made the statement that this is a three-dose vaccine series,” Dr. Offit told KHN. “And, frankly, I think it’s going to throw a wrench into mandates.”
Yet to be determined is whether restaurants or other places of business will look more closely at vaccine cards for the booster.
Dr. Georges Benjamin, executive director of the American Public Health Association, said it’s too early to say. “For now, businesses should stay focused on current guidelines,” he said.
Dr. Marc Siegel, an associate professor of medicine at the George Washington School of Medicine and Health Sciences, in Washington, said the question of whether you are fully vaccinated with just two doses or need a booster is a question of semantics. COVID immunity level is the more important issue.
Dr. Siegel said he thinks more suitable terminology would be to call someone “appropriately” or “adequately” vaccinated against COVID rather than “fully” vaccinated, since it’s possible that more boosters could be needed in the future — making “full vaccination” a moving target.
But, as with so many aspects of the pandemic, ambiguity prevails — both in federal guidance on the definition of “fully vaccinated” and in entrance policies, which vary by state, school and business.
Right now, businesses don’t appear to be checking for boosters, but that could change. So, it may be wise to first check the requirements — lest patrons present a two-shot vaccine passport, only to be turned away as inadequately protected.
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Seven legal risks of promoting unproven COVID-19 treatments
The emergence of COVID-19 has given the medical world a bewildering array of prevention and treatment protocols. Some physicians are advocating treatments that have not been validated by sound scientific studies. This has already led to licensing issues and other disciplinary actions being taken against physicians, pharmacies, and other health care providers across the country.
Medical professionals try their very best to give sound advice to patients. A medical license does not, however, confer immunity from being misled.
The supporting “science” for alternative prevention and treatments may look legitimate, but these claims are often based on anecdotal evidence. Some studies involve small populations, some are meta-analyses of several small or single-case studies, and others are not properly designed, interpreted, or executed in line with U.S. research and requirements. Yet others have been conducted only in nonhuman analogues, such as frogs or mice.
Many people are refusing a vaccine that has been proven to be relatively safe and effective in numerous repeated and validated studies in the best medical centers across the globe – all in favor of less validated alternatives. This can have serious legal consequences.
The crux of the issue
This is not a question of a physician’s first amendment rights. Nor is it a question of advocating for a scientifically valid minority medical opinion. The point of this article is that promoting unproven products, preventives, treatments, and cures can have dire consequences for licensed medical professionals.
On July 29, 2021, the Federation of State Medical Boards’ Board of Directors released a statement in response to a dramatic increase in the dissemination of COVID-19 vaccine misinformation and disinformation by physicians and other health care professionals on social media platforms, online, and in the media. The statement reads as follows:
“Physicians who generate and spread COVID-19 vaccine misinformation or disinformation are risking disciplinary action by state medical boards, including the suspension or revocation of their medical license. Due to their specialized knowledge and training, licensed physicians possess a high degree of public trust and therefore have a powerful platform in society, whether they recognize it or not. They also have an ethical and professional responsibility to practice medicine in the best interests of their patients and must share information that is factual, scientifically grounded, and consensus-driven for the betterment of public health. Spreading inaccurate COVID-19 vaccine information contradicts that responsibility, threatens to further erode public trust in the medical profession, and puts all patients at risk.”
What are the legal consequences?
Medical malpractice
The first consequence to consider is professional liability or medical malpractice. This applies if a patient claims harm as a result of the health care practitioner’s recommendation of an unproven treatment, product, or protocol. For example, strongly discouraging vaccination can result in a wrongful death claim if the patient follows the doctor’s advice, chooses not to vaccinate, contracts COVID-19, and does not recover. Recommending or providing unproven approaches and unapproved treatments is arguably a violation of the standard of care.
The standard of care is grounded in evidence-based medicine: It is commonly defined as the degree of care and skill that would be used by the average physician, who is practicing in his or her relevant specialty, under the same or similar circumstances, given the generally accepted medical knowledge at the time in question.
By way of example, one can see why inhaling peroxide, drinking bleach, or even taking Food and Drug Administration–approved medications that have little or no proven efficacy in treating or preventing COVID-19 is not what the average physician would advocate for under the same or similar circumstances, considering available and commonly accepted medical knowledge. Recommending or providing such treatments can be a breach of the standard of care and can form the basis of a medical malpractice action if, in fact, compensable harm has occurred.
In addition, recommending unproven and unapproved COVID-19 preventives and treatments without appropriate informed consent from patients is arguably also a breach of the standard of care. The claim would be that the patient has not been appropriately informed of the all the known benefits, risks, costs, and other legally required information such as proven efficacy and reasonably available alternatives.
In any event, physicians can rest assured that if a patient is harmed as a result of any of these situations, they’ll probably be answering to someone in the legal system.
Professional licensing action
Regardless of whether there is a medical malpractice action, there is still the potential for a patient complaint to be filed with the state licensing authority on the basis of the same facts and grounds. This can result in an investigation or an administrative complaint against the license of the health care provider.
This is not a mere potential risk. Licensing investigations are underway across the country. Disciplinary licensing actions have already taken place. For example, a Washington Medical Commission panel suspended the license of a physician assistant (PA) on Oct. 12, 2021, after an allegation that his treatment of COVID-19 patients fell below the standard of care. The PA allegedly began a public campaign promoting ivermectin as a curative agent for COVID-19 and prescribed it without adequate examination to at least one person, with no evidence from reliable clinical studies that establish its efficacy in preventing or treating COVID-19.
In licensing claims, alleged violations of failing to comply with the standard of care are usually asserted. These claims may also cite violations of other state statutes that encompass such concepts as negligence; breach of the duty of due care; incompetence; lack of good moral character; and lack of ability to serve the public in a fair, honest, and open manner. A licensing complaint may include alleged violations of statutes that address prescribing protocols, reckless endangerment, failure to supervise, and other issues.
The filing of an administrative complaint is a different animal from a medical malpractice action – they are not even in the same system or branch of government. The focus is not just about what happened to the one patient who complained; it is about protection of the public.
The states’ power to put a clinician on probation, condition, limit, suspend, or revoke the clinician’s license, as well as issue other sanctions such as physician monitoring and fines), is profound. The discipline imposed can upend a clinician’s career and potentially end it entirely.
Administrative discipline determinations are usually available to the public and are required to be reported to all employers (current and future). These discipline determinations are also sent to the National Practitioner Data Bank, other professional clearinghouse organizations (such as the Federation of State Medical Boards), state offices, professional liability insurers, payers with whom the clinician contracts, accreditation and certification organizations, and the clinician’s patients.
Discipline determinations must be promptly reported to licensing agencies in other states where the clinician holds a license, and often results in “sister state” actions because discipline was issued against the clinician in another state. It must be disclosed every time a clinician applies for hospital privileges or new employment. It can result in de-participation from health care insurance programs and can affect board certification, recertification, or accreditation for care programs in which the clinician participates.
In sum, licensing actions can be much worse than medical malpractice judgments and can have longer-term consequences.
Peer review and affected privileges
Recommending, promoting, and providing unapproved or unproven treatments, cures, or preventives to patients may violate hospital/health system, practice group, or surgical center bylaws. This can trigger the peer review process, which serves to improve patient safety and the quality of care.
The peer review process may be commenced because of a concern about the clinician’s compliance with the standard of care; potential patient safety issues; ethical issues; and the clinician’s stability, credibility, or professional competence. Any hospital disciplinary penalty is generally reported to state licensing authorities, which can trigger a licensing investigation. If clinical privileges are affected for a period of more than 30 days, the organization must report the situation to the National Practitioner Data Bank.
Criminal charges
Depending on the facts, a physician or other health care professional could be charged with reckless endangerment, criminal negligence, or manslaughter. If the clinician was assisting someone else who profited from that clinician’s actions, then we can look to a variety of potential federal and state fraud charges as well.
Conviction of a fraud-related felony may also lead to federal health care program and Centers for Medicare & Medicaid Services (CMS) exclusion for several years, and then CMS preclusion that can be imposed for years beyond the conclusion of the statutorily required exclusion.
Breach of contract
Some practice groups or other organizational employers have provisions in employment contracts that treat discipline for this type of conduct as a breach of contract. Because of this, the clinician committing breach may be subject to liquidated damages clauses, forfeiture of monies (such as bonuses or other incentives or rewards), termination of employment, forced withdrawal from ownership status, and being sued for breach of contract to recover damages.
Reputation/credibility damage and the attendant consequences
In regard to hospitals and health care system practice groups, another risk is the loss of referrals and revenue. Local media may air or publish exposés. Such stories may widely publicize the media’s version of the facts – true or not. This can cause immediate reputation and credibility damage within the community and may adversely affect a clinician’s patient base. Any information that is publicly broadcast might attract the attention of licensing and law enforcement authorities and taint potential jurors.
Hospitals and health care systems may pull privileges; post on websites; make official statements about the termination of affiliation; or denounce the clinician’s behavior, conduct, and beliefs as being inconsistent with quality care and patient safety. This causes further damage to a physician’s reputation and credibility.
In a group practice, accusations of this sort, licensing discipline, medical malpractice liability, investigations, loss of privileges, and the other sequelae of this conduct can force the withdrawal of the clinician as a member or shareholder in multiprovider groups. Adverse effects on the financial bottom line, patient referrals, and patient volume and bad press are often the basis for voting a clinician out.
Violation of the COVID-19 Consumer Protection Act of 2020
For the duration of the COVID-19 public health emergency, the FTC COVID-19 Consumer Protection Act makes it unlawful for any person, partnership, or corporation (as those terms are defined broadly in the act) to engage in a deceptive act or practice in or affecting commerce associated with the treatment, cure, prevention, mitigation, or diagnosis of COVID-19 or a government benefit related to COVID-19.
The first enforcement action authorized by this act took place in April 2021 against a chiropractor who promised vitamin treatments and cures for COVID-19. The act provides that such a violation shall be treated as a violation of a rule defining an unfair or deceptive act or practice prescribed under the FTC Act.
Under the act, the FTC is authorized to prescribe “rules that define with specificity acts or practices which are unfair or deceptive acts or practices in or affecting commerce.” Deceptive practices are defined as involving a material representation, omission, or practice that is “likely to mislead a consumer acting reasonably in the circumstances.” An act or practice is unfair if it “causes or is likely to cause substantial injury to consumers which is not reasonably avoidable by consumers themselves and not outweighed by countervailing benefits to consumers or to competition.”
After an investigation, the FTC may initiate an enforcement action using either an administrative or judicial process if it has “reason to believe” that the law has been violated. Violations of some laws may result in injunctive relief or civil monetary penalties, which are adjusted annually for inflation.
In addition, many states have deceptive and unfair trade laws that can be enforced in regard to the recommendation, sale, or provision of unproven or unapproved COVID-19 treatments, cures, and preventives as well.
Conclusion
It is difficult even for intelligent, well-intentioned physicians to know precisely what to believe and what to advocate for in the middle of a pandemic. It seems as though new reports and recommendations for preventing and treating COVID-19 are surfacing on a weekly basis. By far, the safest approach for any medical clinician to take is to advocate for positions that are generally accepted in the medical and scientific community at the time advice is given.
Mr. Whitelaw disclosed no relevant financial relationships. Ms. Janeway disclosed various associations with the Michigan Association for Healthcare Quality and the Greater Houston Society for Healthcare Risk Management. A version of this article first appeared on Medscape.com.
The emergence of COVID-19 has given the medical world a bewildering array of prevention and treatment protocols. Some physicians are advocating treatments that have not been validated by sound scientific studies. This has already led to licensing issues and other disciplinary actions being taken against physicians, pharmacies, and other health care providers across the country.
Medical professionals try their very best to give sound advice to patients. A medical license does not, however, confer immunity from being misled.
The supporting “science” for alternative prevention and treatments may look legitimate, but these claims are often based on anecdotal evidence. Some studies involve small populations, some are meta-analyses of several small or single-case studies, and others are not properly designed, interpreted, or executed in line with U.S. research and requirements. Yet others have been conducted only in nonhuman analogues, such as frogs or mice.
Many people are refusing a vaccine that has been proven to be relatively safe and effective in numerous repeated and validated studies in the best medical centers across the globe – all in favor of less validated alternatives. This can have serious legal consequences.
The crux of the issue
This is not a question of a physician’s first amendment rights. Nor is it a question of advocating for a scientifically valid minority medical opinion. The point of this article is that promoting unproven products, preventives, treatments, and cures can have dire consequences for licensed medical professionals.
On July 29, 2021, the Federation of State Medical Boards’ Board of Directors released a statement in response to a dramatic increase in the dissemination of COVID-19 vaccine misinformation and disinformation by physicians and other health care professionals on social media platforms, online, and in the media. The statement reads as follows:
“Physicians who generate and spread COVID-19 vaccine misinformation or disinformation are risking disciplinary action by state medical boards, including the suspension or revocation of their medical license. Due to their specialized knowledge and training, licensed physicians possess a high degree of public trust and therefore have a powerful platform in society, whether they recognize it or not. They also have an ethical and professional responsibility to practice medicine in the best interests of their patients and must share information that is factual, scientifically grounded, and consensus-driven for the betterment of public health. Spreading inaccurate COVID-19 vaccine information contradicts that responsibility, threatens to further erode public trust in the medical profession, and puts all patients at risk.”
What are the legal consequences?
Medical malpractice
The first consequence to consider is professional liability or medical malpractice. This applies if a patient claims harm as a result of the health care practitioner’s recommendation of an unproven treatment, product, or protocol. For example, strongly discouraging vaccination can result in a wrongful death claim if the patient follows the doctor’s advice, chooses not to vaccinate, contracts COVID-19, and does not recover. Recommending or providing unproven approaches and unapproved treatments is arguably a violation of the standard of care.
The standard of care is grounded in evidence-based medicine: It is commonly defined as the degree of care and skill that would be used by the average physician, who is practicing in his or her relevant specialty, under the same or similar circumstances, given the generally accepted medical knowledge at the time in question.
By way of example, one can see why inhaling peroxide, drinking bleach, or even taking Food and Drug Administration–approved medications that have little or no proven efficacy in treating or preventing COVID-19 is not what the average physician would advocate for under the same or similar circumstances, considering available and commonly accepted medical knowledge. Recommending or providing such treatments can be a breach of the standard of care and can form the basis of a medical malpractice action if, in fact, compensable harm has occurred.
In addition, recommending unproven and unapproved COVID-19 preventives and treatments without appropriate informed consent from patients is arguably also a breach of the standard of care. The claim would be that the patient has not been appropriately informed of the all the known benefits, risks, costs, and other legally required information such as proven efficacy and reasonably available alternatives.
In any event, physicians can rest assured that if a patient is harmed as a result of any of these situations, they’ll probably be answering to someone in the legal system.
Professional licensing action
Regardless of whether there is a medical malpractice action, there is still the potential for a patient complaint to be filed with the state licensing authority on the basis of the same facts and grounds. This can result in an investigation or an administrative complaint against the license of the health care provider.
This is not a mere potential risk. Licensing investigations are underway across the country. Disciplinary licensing actions have already taken place. For example, a Washington Medical Commission panel suspended the license of a physician assistant (PA) on Oct. 12, 2021, after an allegation that his treatment of COVID-19 patients fell below the standard of care. The PA allegedly began a public campaign promoting ivermectin as a curative agent for COVID-19 and prescribed it without adequate examination to at least one person, with no evidence from reliable clinical studies that establish its efficacy in preventing or treating COVID-19.
In licensing claims, alleged violations of failing to comply with the standard of care are usually asserted. These claims may also cite violations of other state statutes that encompass such concepts as negligence; breach of the duty of due care; incompetence; lack of good moral character; and lack of ability to serve the public in a fair, honest, and open manner. A licensing complaint may include alleged violations of statutes that address prescribing protocols, reckless endangerment, failure to supervise, and other issues.
The filing of an administrative complaint is a different animal from a medical malpractice action – they are not even in the same system or branch of government. The focus is not just about what happened to the one patient who complained; it is about protection of the public.
The states’ power to put a clinician on probation, condition, limit, suspend, or revoke the clinician’s license, as well as issue other sanctions such as physician monitoring and fines), is profound. The discipline imposed can upend a clinician’s career and potentially end it entirely.
Administrative discipline determinations are usually available to the public and are required to be reported to all employers (current and future). These discipline determinations are also sent to the National Practitioner Data Bank, other professional clearinghouse organizations (such as the Federation of State Medical Boards), state offices, professional liability insurers, payers with whom the clinician contracts, accreditation and certification organizations, and the clinician’s patients.
Discipline determinations must be promptly reported to licensing agencies in other states where the clinician holds a license, and often results in “sister state” actions because discipline was issued against the clinician in another state. It must be disclosed every time a clinician applies for hospital privileges or new employment. It can result in de-participation from health care insurance programs and can affect board certification, recertification, or accreditation for care programs in which the clinician participates.
In sum, licensing actions can be much worse than medical malpractice judgments and can have longer-term consequences.
Peer review and affected privileges
Recommending, promoting, and providing unapproved or unproven treatments, cures, or preventives to patients may violate hospital/health system, practice group, or surgical center bylaws. This can trigger the peer review process, which serves to improve patient safety and the quality of care.
The peer review process may be commenced because of a concern about the clinician’s compliance with the standard of care; potential patient safety issues; ethical issues; and the clinician’s stability, credibility, or professional competence. Any hospital disciplinary penalty is generally reported to state licensing authorities, which can trigger a licensing investigation. If clinical privileges are affected for a period of more than 30 days, the organization must report the situation to the National Practitioner Data Bank.
Criminal charges
Depending on the facts, a physician or other health care professional could be charged with reckless endangerment, criminal negligence, or manslaughter. If the clinician was assisting someone else who profited from that clinician’s actions, then we can look to a variety of potential federal and state fraud charges as well.
Conviction of a fraud-related felony may also lead to federal health care program and Centers for Medicare & Medicaid Services (CMS) exclusion for several years, and then CMS preclusion that can be imposed for years beyond the conclusion of the statutorily required exclusion.
Breach of contract
Some practice groups or other organizational employers have provisions in employment contracts that treat discipline for this type of conduct as a breach of contract. Because of this, the clinician committing breach may be subject to liquidated damages clauses, forfeiture of monies (such as bonuses or other incentives or rewards), termination of employment, forced withdrawal from ownership status, and being sued for breach of contract to recover damages.
Reputation/credibility damage and the attendant consequences
In regard to hospitals and health care system practice groups, another risk is the loss of referrals and revenue. Local media may air or publish exposés. Such stories may widely publicize the media’s version of the facts – true or not. This can cause immediate reputation and credibility damage within the community and may adversely affect a clinician’s patient base. Any information that is publicly broadcast might attract the attention of licensing and law enforcement authorities and taint potential jurors.
Hospitals and health care systems may pull privileges; post on websites; make official statements about the termination of affiliation; or denounce the clinician’s behavior, conduct, and beliefs as being inconsistent with quality care and patient safety. This causes further damage to a physician’s reputation and credibility.
In a group practice, accusations of this sort, licensing discipline, medical malpractice liability, investigations, loss of privileges, and the other sequelae of this conduct can force the withdrawal of the clinician as a member or shareholder in multiprovider groups. Adverse effects on the financial bottom line, patient referrals, and patient volume and bad press are often the basis for voting a clinician out.
Violation of the COVID-19 Consumer Protection Act of 2020
For the duration of the COVID-19 public health emergency, the FTC COVID-19 Consumer Protection Act makes it unlawful for any person, partnership, or corporation (as those terms are defined broadly in the act) to engage in a deceptive act or practice in or affecting commerce associated with the treatment, cure, prevention, mitigation, or diagnosis of COVID-19 or a government benefit related to COVID-19.
The first enforcement action authorized by this act took place in April 2021 against a chiropractor who promised vitamin treatments and cures for COVID-19. The act provides that such a violation shall be treated as a violation of a rule defining an unfair or deceptive act or practice prescribed under the FTC Act.
Under the act, the FTC is authorized to prescribe “rules that define with specificity acts or practices which are unfair or deceptive acts or practices in or affecting commerce.” Deceptive practices are defined as involving a material representation, omission, or practice that is “likely to mislead a consumer acting reasonably in the circumstances.” An act or practice is unfair if it “causes or is likely to cause substantial injury to consumers which is not reasonably avoidable by consumers themselves and not outweighed by countervailing benefits to consumers or to competition.”
After an investigation, the FTC may initiate an enforcement action using either an administrative or judicial process if it has “reason to believe” that the law has been violated. Violations of some laws may result in injunctive relief or civil monetary penalties, which are adjusted annually for inflation.
In addition, many states have deceptive and unfair trade laws that can be enforced in regard to the recommendation, sale, or provision of unproven or unapproved COVID-19 treatments, cures, and preventives as well.
Conclusion
It is difficult even for intelligent, well-intentioned physicians to know precisely what to believe and what to advocate for in the middle of a pandemic. It seems as though new reports and recommendations for preventing and treating COVID-19 are surfacing on a weekly basis. By far, the safest approach for any medical clinician to take is to advocate for positions that are generally accepted in the medical and scientific community at the time advice is given.
Mr. Whitelaw disclosed no relevant financial relationships. Ms. Janeway disclosed various associations with the Michigan Association for Healthcare Quality and the Greater Houston Society for Healthcare Risk Management. A version of this article first appeared on Medscape.com.
The emergence of COVID-19 has given the medical world a bewildering array of prevention and treatment protocols. Some physicians are advocating treatments that have not been validated by sound scientific studies. This has already led to licensing issues and other disciplinary actions being taken against physicians, pharmacies, and other health care providers across the country.
Medical professionals try their very best to give sound advice to patients. A medical license does not, however, confer immunity from being misled.
The supporting “science” for alternative prevention and treatments may look legitimate, but these claims are often based on anecdotal evidence. Some studies involve small populations, some are meta-analyses of several small or single-case studies, and others are not properly designed, interpreted, or executed in line with U.S. research and requirements. Yet others have been conducted only in nonhuman analogues, such as frogs or mice.
Many people are refusing a vaccine that has been proven to be relatively safe and effective in numerous repeated and validated studies in the best medical centers across the globe – all in favor of less validated alternatives. This can have serious legal consequences.
The crux of the issue
This is not a question of a physician’s first amendment rights. Nor is it a question of advocating for a scientifically valid minority medical opinion. The point of this article is that promoting unproven products, preventives, treatments, and cures can have dire consequences for licensed medical professionals.
On July 29, 2021, the Federation of State Medical Boards’ Board of Directors released a statement in response to a dramatic increase in the dissemination of COVID-19 vaccine misinformation and disinformation by physicians and other health care professionals on social media platforms, online, and in the media. The statement reads as follows:
“Physicians who generate and spread COVID-19 vaccine misinformation or disinformation are risking disciplinary action by state medical boards, including the suspension or revocation of their medical license. Due to their specialized knowledge and training, licensed physicians possess a high degree of public trust and therefore have a powerful platform in society, whether they recognize it or not. They also have an ethical and professional responsibility to practice medicine in the best interests of their patients and must share information that is factual, scientifically grounded, and consensus-driven for the betterment of public health. Spreading inaccurate COVID-19 vaccine information contradicts that responsibility, threatens to further erode public trust in the medical profession, and puts all patients at risk.”
What are the legal consequences?
Medical malpractice
The first consequence to consider is professional liability or medical malpractice. This applies if a patient claims harm as a result of the health care practitioner’s recommendation of an unproven treatment, product, or protocol. For example, strongly discouraging vaccination can result in a wrongful death claim if the patient follows the doctor’s advice, chooses not to vaccinate, contracts COVID-19, and does not recover. Recommending or providing unproven approaches and unapproved treatments is arguably a violation of the standard of care.
The standard of care is grounded in evidence-based medicine: It is commonly defined as the degree of care and skill that would be used by the average physician, who is practicing in his or her relevant specialty, under the same or similar circumstances, given the generally accepted medical knowledge at the time in question.
By way of example, one can see why inhaling peroxide, drinking bleach, or even taking Food and Drug Administration–approved medications that have little or no proven efficacy in treating or preventing COVID-19 is not what the average physician would advocate for under the same or similar circumstances, considering available and commonly accepted medical knowledge. Recommending or providing such treatments can be a breach of the standard of care and can form the basis of a medical malpractice action if, in fact, compensable harm has occurred.
In addition, recommending unproven and unapproved COVID-19 preventives and treatments without appropriate informed consent from patients is arguably also a breach of the standard of care. The claim would be that the patient has not been appropriately informed of the all the known benefits, risks, costs, and other legally required information such as proven efficacy and reasonably available alternatives.
In any event, physicians can rest assured that if a patient is harmed as a result of any of these situations, they’ll probably be answering to someone in the legal system.
Professional licensing action
Regardless of whether there is a medical malpractice action, there is still the potential for a patient complaint to be filed with the state licensing authority on the basis of the same facts and grounds. This can result in an investigation or an administrative complaint against the license of the health care provider.
This is not a mere potential risk. Licensing investigations are underway across the country. Disciplinary licensing actions have already taken place. For example, a Washington Medical Commission panel suspended the license of a physician assistant (PA) on Oct. 12, 2021, after an allegation that his treatment of COVID-19 patients fell below the standard of care. The PA allegedly began a public campaign promoting ivermectin as a curative agent for COVID-19 and prescribed it without adequate examination to at least one person, with no evidence from reliable clinical studies that establish its efficacy in preventing or treating COVID-19.
In licensing claims, alleged violations of failing to comply with the standard of care are usually asserted. These claims may also cite violations of other state statutes that encompass such concepts as negligence; breach of the duty of due care; incompetence; lack of good moral character; and lack of ability to serve the public in a fair, honest, and open manner. A licensing complaint may include alleged violations of statutes that address prescribing protocols, reckless endangerment, failure to supervise, and other issues.
The filing of an administrative complaint is a different animal from a medical malpractice action – they are not even in the same system or branch of government. The focus is not just about what happened to the one patient who complained; it is about protection of the public.
The states’ power to put a clinician on probation, condition, limit, suspend, or revoke the clinician’s license, as well as issue other sanctions such as physician monitoring and fines), is profound. The discipline imposed can upend a clinician’s career and potentially end it entirely.
Administrative discipline determinations are usually available to the public and are required to be reported to all employers (current and future). These discipline determinations are also sent to the National Practitioner Data Bank, other professional clearinghouse organizations (such as the Federation of State Medical Boards), state offices, professional liability insurers, payers with whom the clinician contracts, accreditation and certification organizations, and the clinician’s patients.
Discipline determinations must be promptly reported to licensing agencies in other states where the clinician holds a license, and often results in “sister state” actions because discipline was issued against the clinician in another state. It must be disclosed every time a clinician applies for hospital privileges or new employment. It can result in de-participation from health care insurance programs and can affect board certification, recertification, or accreditation for care programs in which the clinician participates.
In sum, licensing actions can be much worse than medical malpractice judgments and can have longer-term consequences.
Peer review and affected privileges
Recommending, promoting, and providing unapproved or unproven treatments, cures, or preventives to patients may violate hospital/health system, practice group, or surgical center bylaws. This can trigger the peer review process, which serves to improve patient safety and the quality of care.
The peer review process may be commenced because of a concern about the clinician’s compliance with the standard of care; potential patient safety issues; ethical issues; and the clinician’s stability, credibility, or professional competence. Any hospital disciplinary penalty is generally reported to state licensing authorities, which can trigger a licensing investigation. If clinical privileges are affected for a period of more than 30 days, the organization must report the situation to the National Practitioner Data Bank.
Criminal charges
Depending on the facts, a physician or other health care professional could be charged with reckless endangerment, criminal negligence, or manslaughter. If the clinician was assisting someone else who profited from that clinician’s actions, then we can look to a variety of potential federal and state fraud charges as well.
Conviction of a fraud-related felony may also lead to federal health care program and Centers for Medicare & Medicaid Services (CMS) exclusion for several years, and then CMS preclusion that can be imposed for years beyond the conclusion of the statutorily required exclusion.
Breach of contract
Some practice groups or other organizational employers have provisions in employment contracts that treat discipline for this type of conduct as a breach of contract. Because of this, the clinician committing breach may be subject to liquidated damages clauses, forfeiture of monies (such as bonuses or other incentives or rewards), termination of employment, forced withdrawal from ownership status, and being sued for breach of contract to recover damages.
Reputation/credibility damage and the attendant consequences
In regard to hospitals and health care system practice groups, another risk is the loss of referrals and revenue. Local media may air or publish exposés. Such stories may widely publicize the media’s version of the facts – true or not. This can cause immediate reputation and credibility damage within the community and may adversely affect a clinician’s patient base. Any information that is publicly broadcast might attract the attention of licensing and law enforcement authorities and taint potential jurors.
Hospitals and health care systems may pull privileges; post on websites; make official statements about the termination of affiliation; or denounce the clinician’s behavior, conduct, and beliefs as being inconsistent with quality care and patient safety. This causes further damage to a physician’s reputation and credibility.
In a group practice, accusations of this sort, licensing discipline, medical malpractice liability, investigations, loss of privileges, and the other sequelae of this conduct can force the withdrawal of the clinician as a member or shareholder in multiprovider groups. Adverse effects on the financial bottom line, patient referrals, and patient volume and bad press are often the basis for voting a clinician out.
Violation of the COVID-19 Consumer Protection Act of 2020
For the duration of the COVID-19 public health emergency, the FTC COVID-19 Consumer Protection Act makes it unlawful for any person, partnership, or corporation (as those terms are defined broadly in the act) to engage in a deceptive act or practice in or affecting commerce associated with the treatment, cure, prevention, mitigation, or diagnosis of COVID-19 or a government benefit related to COVID-19.
The first enforcement action authorized by this act took place in April 2021 against a chiropractor who promised vitamin treatments and cures for COVID-19. The act provides that such a violation shall be treated as a violation of a rule defining an unfair or deceptive act or practice prescribed under the FTC Act.
Under the act, the FTC is authorized to prescribe “rules that define with specificity acts or practices which are unfair or deceptive acts or practices in or affecting commerce.” Deceptive practices are defined as involving a material representation, omission, or practice that is “likely to mislead a consumer acting reasonably in the circumstances.” An act or practice is unfair if it “causes or is likely to cause substantial injury to consumers which is not reasonably avoidable by consumers themselves and not outweighed by countervailing benefits to consumers or to competition.”
After an investigation, the FTC may initiate an enforcement action using either an administrative or judicial process if it has “reason to believe” that the law has been violated. Violations of some laws may result in injunctive relief or civil monetary penalties, which are adjusted annually for inflation.
In addition, many states have deceptive and unfair trade laws that can be enforced in regard to the recommendation, sale, or provision of unproven or unapproved COVID-19 treatments, cures, and preventives as well.
Conclusion
It is difficult even for intelligent, well-intentioned physicians to know precisely what to believe and what to advocate for in the middle of a pandemic. It seems as though new reports and recommendations for preventing and treating COVID-19 are surfacing on a weekly basis. By far, the safest approach for any medical clinician to take is to advocate for positions that are generally accepted in the medical and scientific community at the time advice is given.
Mr. Whitelaw disclosed no relevant financial relationships. Ms. Janeway disclosed various associations with the Michigan Association for Healthcare Quality and the Greater Houston Society for Healthcare Risk Management. A version of this article first appeared on Medscape.com.
Former nurse sentenced to 10 years in prison for sexual assault of incapacitated patient
A former nurse was sentenced to 10 years in prison for the sexual assault of an incapacitated woman in the long-term care facility where he worked as a licensed practical nurse.
according to a deal in which the former nurse pleaded guilty in September to sexual assault and vulnerable adult abuse. He will also be placed on probation for the rest of his life and will have to register as a sex offender.
The crime came to light in December 2018, when the patient, a 29-year-old woman who lived at Hacienda HealthCare, a private, nonprofit, long-term health care facility, was found giving birth when staff changed her clothes and bedding. The woman, who had lived at the Phoenix facility since she was 3 years old, is not in a coma but has severe cognitive disabilities resulting from seizures. She does not speak but has some ability to move and can respond to sounds and make facial gestures.
According to a 911 call made by staff members, no one knew she was pregnant until she went into labor. “One of the patients just had a baby, and we had no idea she was pregnant,” a nurse told responders on the frantic 911 call.
Police tested the DNA of all male employees at the facility and found the baby’s DNA matched that of Nathan Sutherland, who was the woman’s primary caregiver at the time of the assault. The child, a boy, is being cared for by the woman’s mother.
“It’s hard to imagine a more vulnerable adult than the victim in this case,” said Superior Court Judge Margaret LaBianca when she handed down the sentence, according to the Associated Press
Mr. Sutherland, 39, who surrendered his nursing license after his arrest, apologized to his victim at the sentencing, saying: “You didn’t deserve to be hurt no matter what was going on in my personal life and the demons I was fighting. I had no right to put you through that.”
In a statement to this news organization, Perry Petrilli, CEO of Hacienda HealthCare, said, “For nearly 3 years, our team has cooperated in every way possible with law enforcement and prosecutors in the Sutherland case. We are relieved that [Mr. Sutherland] will never again torment another innocent human being.” He added: “Our thoughts and hearts are with the victim, the victim’s family and loved ones. We hope this last chapter of the Sutherland case brings them all greater peace.”
In June, the court approved a $15 million settlement between the woman’s parents and Phillip Gear Jr., MD, the doctor who cared for the woman during the 26 years she was a patient at Hacienda Healthcare. Dr. Gear died in 2020, prior to the settlement agreement.
A version of this article first appeared on Medscape.com.
A former nurse was sentenced to 10 years in prison for the sexual assault of an incapacitated woman in the long-term care facility where he worked as a licensed practical nurse.
according to a deal in which the former nurse pleaded guilty in September to sexual assault and vulnerable adult abuse. He will also be placed on probation for the rest of his life and will have to register as a sex offender.
The crime came to light in December 2018, when the patient, a 29-year-old woman who lived at Hacienda HealthCare, a private, nonprofit, long-term health care facility, was found giving birth when staff changed her clothes and bedding. The woman, who had lived at the Phoenix facility since she was 3 years old, is not in a coma but has severe cognitive disabilities resulting from seizures. She does not speak but has some ability to move and can respond to sounds and make facial gestures.
According to a 911 call made by staff members, no one knew she was pregnant until she went into labor. “One of the patients just had a baby, and we had no idea she was pregnant,” a nurse told responders on the frantic 911 call.
Police tested the DNA of all male employees at the facility and found the baby’s DNA matched that of Nathan Sutherland, who was the woman’s primary caregiver at the time of the assault. The child, a boy, is being cared for by the woman’s mother.
“It’s hard to imagine a more vulnerable adult than the victim in this case,” said Superior Court Judge Margaret LaBianca when she handed down the sentence, according to the Associated Press
Mr. Sutherland, 39, who surrendered his nursing license after his arrest, apologized to his victim at the sentencing, saying: “You didn’t deserve to be hurt no matter what was going on in my personal life and the demons I was fighting. I had no right to put you through that.”
In a statement to this news organization, Perry Petrilli, CEO of Hacienda HealthCare, said, “For nearly 3 years, our team has cooperated in every way possible with law enforcement and prosecutors in the Sutherland case. We are relieved that [Mr. Sutherland] will never again torment another innocent human being.” He added: “Our thoughts and hearts are with the victim, the victim’s family and loved ones. We hope this last chapter of the Sutherland case brings them all greater peace.”
In June, the court approved a $15 million settlement between the woman’s parents and Phillip Gear Jr., MD, the doctor who cared for the woman during the 26 years she was a patient at Hacienda Healthcare. Dr. Gear died in 2020, prior to the settlement agreement.
A version of this article first appeared on Medscape.com.
A former nurse was sentenced to 10 years in prison for the sexual assault of an incapacitated woman in the long-term care facility where he worked as a licensed practical nurse.
according to a deal in which the former nurse pleaded guilty in September to sexual assault and vulnerable adult abuse. He will also be placed on probation for the rest of his life and will have to register as a sex offender.
The crime came to light in December 2018, when the patient, a 29-year-old woman who lived at Hacienda HealthCare, a private, nonprofit, long-term health care facility, was found giving birth when staff changed her clothes and bedding. The woman, who had lived at the Phoenix facility since she was 3 years old, is not in a coma but has severe cognitive disabilities resulting from seizures. She does not speak but has some ability to move and can respond to sounds and make facial gestures.
According to a 911 call made by staff members, no one knew she was pregnant until she went into labor. “One of the patients just had a baby, and we had no idea she was pregnant,” a nurse told responders on the frantic 911 call.
Police tested the DNA of all male employees at the facility and found the baby’s DNA matched that of Nathan Sutherland, who was the woman’s primary caregiver at the time of the assault. The child, a boy, is being cared for by the woman’s mother.
“It’s hard to imagine a more vulnerable adult than the victim in this case,” said Superior Court Judge Margaret LaBianca when she handed down the sentence, according to the Associated Press
Mr. Sutherland, 39, who surrendered his nursing license after his arrest, apologized to his victim at the sentencing, saying: “You didn’t deserve to be hurt no matter what was going on in my personal life and the demons I was fighting. I had no right to put you through that.”
In a statement to this news organization, Perry Petrilli, CEO of Hacienda HealthCare, said, “For nearly 3 years, our team has cooperated in every way possible with law enforcement and prosecutors in the Sutherland case. We are relieved that [Mr. Sutherland] will never again torment another innocent human being.” He added: “Our thoughts and hearts are with the victim, the victim’s family and loved ones. We hope this last chapter of the Sutherland case brings them all greater peace.”
In June, the court approved a $15 million settlement between the woman’s parents and Phillip Gear Jr., MD, the doctor who cared for the woman during the 26 years she was a patient at Hacienda Healthcare. Dr. Gear died in 2020, prior to the settlement agreement.
A version of this article first appeared on Medscape.com.
Cardiologist positive for Omicron after London conference
Elad Maor, MD, an interventional cardiologist at Sheba Medical Centre near Tel Aviv, posted on Twitter on Nov. 30: “What a mess! Came back from a conference in London. With a mask and 3 Pfizer vaccines I managed to get Omicron.”
Dr. Maor traveled to London on November 19 to attend the PCR London Valves 2021 conference held at the ExCeL Centre Nov. 21-23. He stayed four nights at a hotel in north London and took public transport to and from the ExCeL Centre in East London each day of the meeting. He returned to Israel on the evening of Nov. 23.
Dr. Maor, 45, who has received three doses of the Pfizer COVID-19 vaccine, had two PCR tests in the United Kingdom – on November 20 and 21 in line with travel requirements – and another PCR test upon arriving back in Israel in the early hours of Nov. 24. All three tests were negative.
He began experiencing symptoms within days and tested positive on Nov. 27. His symptoms have been mild so far, and he said he was feeling “better” at the time of his tweet on Nov. 30.
Dr. Maor believes he was infected during his trip to London. “The only reasonable explanation is that I got infected on the last day of the meeting – maybe at the airport, maybe at the meeting,” he told The Guardian newspaper.
Although his wife accompanied him to London, neither she nor any of his 3 children have experienced symptoms or tested positive for COVID-19. But Dr. Maor believes he has passed the infection to a 69-year-old colleague in Israel who has since tested positive for the Omicron variant. The colleague, who has also received three vaccine doses, is understood to have mild symptoms at present.
The case suggests that the Omicron variant of COVID-19 may have been circulating in the United Kingdom earlier than previously thought.
Implications for in-person conferences
It will also inevitably lead to questions about the safety of face-to-face conferences, which are only just starting to get underway again.
The PCR Valves 2021 meeting had more than 1,250 on-site attendees as well as 2,400 or more joining online, according to figures on its website. Dr. Maor said he did not have any issues with the conference organizers, who required proof of vaccination before entry. But he posted a photograph on his Twitter account of a crowded auditorium with many delegates not wearing masks.
The conference subsequently posted an announcement on its website alerting delegates that one of the attendees had tested positive for COVID-19 after returning to their home country. It reads: “Since the reported case comes less than a week after the end of PCR London Valves, we want to inform you so that you may decide the best course of action, for yourself, if any.” It does not mention that the case was the Omicron variant.
Patrick Jolly, strategic and market development director of the conference, commented: “As you may imagine, the health, safety and well-being of everyone who visited PCR London Valves was our number-one priority. All protocols mandated by the U.K. government were put in place. Anyone entering the congress center had to present a valid health pass and were requested to wear a mask. Hydro-alcoholic gel and masks were made readily available for all participants and disposal bins for used protective equipment were provided.”
Mr. Jolly also noted: “To date – more than 9 days after the end of PCR London Valves – we have had no report of any other case of participants testing positive who attended PCR London Valves.”
He said the EuroPCR organization believes that medical conferences are safe to be held in person.
“With the above sanitary requirements and protocols, and no complacency in their enforcement, we believe strongly that medical conferences can take place, as the benefits of in-person medical conferences are obvious for the concerned medical communities,” Mr. Jolly added.
But what about other meetings happening imminently and planning in-person attendance?
Eileen Murray, executive director of the American Epilepsy Society (AES), whose annual 5-day meeting starts today at Chicago’s McCormick Place Convention Center, said in an interview that the health, safety, and well-being of everyone attending is a priority.
“Vaccinations are required, with no exceptions, to anyone attending the in-person event,” Ms. Murray said. “AES is using the CLEAR HealthPass to verify identity and vaccination status for our attendees. No one who cannot verify identity and vaccination requirement will be permitted to attend the in-person event.”
She noted that masks will also be required except in limited circumstances when actively eating or drinking, or for a faculty member when actively presenting at a lecture or panel. “Anyone not adhering to the mask policy will be asked to leave the meeting and will be denied readmission to the meeting with no refund,” she said.
“These guidelines were developed in accordance with the latest public health guidance and AES will continue to follow that guidance as any updates are made with the emergence of the Omicron variant,” Ms. Murray added.
Also commenting on this issue, a spokesperson for the American Heart Association, which has its large annual international stroke meeting planned for in-person attendance in New Orleans in February, said: “As we have throughout the pandemic, the American Heart Association is closely monitoring conditions and following the guidance of the CDC as well as state and local health departments related to all in-person meetings.”
“Our upcoming International Stroke Conference, February 9-11, is planned as an in-person and digital experience which allows us the ultimate flexibility to address changing pandemic conditions. The health, safety, and well-being of our volunteers, members, and attendees from around the world remains our number-one priority,” the AHA spokesperson added.
But some COVID-19 experts are taking a more cautious view.
Rowland Kao, PhD, an expert in infectious disease dynamics at the University of Edinburgh, United Kingdom, expressed concern about such large in-person conferences.
“We know that the Omicron variant appears to be spreading rapidly, with a recent preprint also telling us that the reinfection rate appears to be higher in South Africa. Should this be borne out, then the evidence would support that our reliance on a combination of vaccine-induced and natural immunity may be compromised by the Omicron variant,” he commented.
“We already know that extended contact indoors provides an additional risk, and so large meetings of this type have the potential to create extended risks. Until we know the extent to which Omicron causes severe illness, we should be extra cautious about these high-risk settings,” Dr. Kao commented.
A version of this article first appeared on Medscape.com.
Elad Maor, MD, an interventional cardiologist at Sheba Medical Centre near Tel Aviv, posted on Twitter on Nov. 30: “What a mess! Came back from a conference in London. With a mask and 3 Pfizer vaccines I managed to get Omicron.”
Dr. Maor traveled to London on November 19 to attend the PCR London Valves 2021 conference held at the ExCeL Centre Nov. 21-23. He stayed four nights at a hotel in north London and took public transport to and from the ExCeL Centre in East London each day of the meeting. He returned to Israel on the evening of Nov. 23.
Dr. Maor, 45, who has received three doses of the Pfizer COVID-19 vaccine, had two PCR tests in the United Kingdom – on November 20 and 21 in line with travel requirements – and another PCR test upon arriving back in Israel in the early hours of Nov. 24. All three tests were negative.
He began experiencing symptoms within days and tested positive on Nov. 27. His symptoms have been mild so far, and he said he was feeling “better” at the time of his tweet on Nov. 30.
Dr. Maor believes he was infected during his trip to London. “The only reasonable explanation is that I got infected on the last day of the meeting – maybe at the airport, maybe at the meeting,” he told The Guardian newspaper.
Although his wife accompanied him to London, neither she nor any of his 3 children have experienced symptoms or tested positive for COVID-19. But Dr. Maor believes he has passed the infection to a 69-year-old colleague in Israel who has since tested positive for the Omicron variant. The colleague, who has also received three vaccine doses, is understood to have mild symptoms at present.
The case suggests that the Omicron variant of COVID-19 may have been circulating in the United Kingdom earlier than previously thought.
Implications for in-person conferences
It will also inevitably lead to questions about the safety of face-to-face conferences, which are only just starting to get underway again.
The PCR Valves 2021 meeting had more than 1,250 on-site attendees as well as 2,400 or more joining online, according to figures on its website. Dr. Maor said he did not have any issues with the conference organizers, who required proof of vaccination before entry. But he posted a photograph on his Twitter account of a crowded auditorium with many delegates not wearing masks.
The conference subsequently posted an announcement on its website alerting delegates that one of the attendees had tested positive for COVID-19 after returning to their home country. It reads: “Since the reported case comes less than a week after the end of PCR London Valves, we want to inform you so that you may decide the best course of action, for yourself, if any.” It does not mention that the case was the Omicron variant.
Patrick Jolly, strategic and market development director of the conference, commented: “As you may imagine, the health, safety and well-being of everyone who visited PCR London Valves was our number-one priority. All protocols mandated by the U.K. government were put in place. Anyone entering the congress center had to present a valid health pass and were requested to wear a mask. Hydro-alcoholic gel and masks were made readily available for all participants and disposal bins for used protective equipment were provided.”
Mr. Jolly also noted: “To date – more than 9 days after the end of PCR London Valves – we have had no report of any other case of participants testing positive who attended PCR London Valves.”
He said the EuroPCR organization believes that medical conferences are safe to be held in person.
“With the above sanitary requirements and protocols, and no complacency in their enforcement, we believe strongly that medical conferences can take place, as the benefits of in-person medical conferences are obvious for the concerned medical communities,” Mr. Jolly added.
But what about other meetings happening imminently and planning in-person attendance?
Eileen Murray, executive director of the American Epilepsy Society (AES), whose annual 5-day meeting starts today at Chicago’s McCormick Place Convention Center, said in an interview that the health, safety, and well-being of everyone attending is a priority.
“Vaccinations are required, with no exceptions, to anyone attending the in-person event,” Ms. Murray said. “AES is using the CLEAR HealthPass to verify identity and vaccination status for our attendees. No one who cannot verify identity and vaccination requirement will be permitted to attend the in-person event.”
She noted that masks will also be required except in limited circumstances when actively eating or drinking, or for a faculty member when actively presenting at a lecture or panel. “Anyone not adhering to the mask policy will be asked to leave the meeting and will be denied readmission to the meeting with no refund,” she said.
“These guidelines were developed in accordance with the latest public health guidance and AES will continue to follow that guidance as any updates are made with the emergence of the Omicron variant,” Ms. Murray added.
Also commenting on this issue, a spokesperson for the American Heart Association, which has its large annual international stroke meeting planned for in-person attendance in New Orleans in February, said: “As we have throughout the pandemic, the American Heart Association is closely monitoring conditions and following the guidance of the CDC as well as state and local health departments related to all in-person meetings.”
“Our upcoming International Stroke Conference, February 9-11, is planned as an in-person and digital experience which allows us the ultimate flexibility to address changing pandemic conditions. The health, safety, and well-being of our volunteers, members, and attendees from around the world remains our number-one priority,” the AHA spokesperson added.
But some COVID-19 experts are taking a more cautious view.
Rowland Kao, PhD, an expert in infectious disease dynamics at the University of Edinburgh, United Kingdom, expressed concern about such large in-person conferences.
“We know that the Omicron variant appears to be spreading rapidly, with a recent preprint also telling us that the reinfection rate appears to be higher in South Africa. Should this be borne out, then the evidence would support that our reliance on a combination of vaccine-induced and natural immunity may be compromised by the Omicron variant,” he commented.
“We already know that extended contact indoors provides an additional risk, and so large meetings of this type have the potential to create extended risks. Until we know the extent to which Omicron causes severe illness, we should be extra cautious about these high-risk settings,” Dr. Kao commented.
A version of this article first appeared on Medscape.com.
Elad Maor, MD, an interventional cardiologist at Sheba Medical Centre near Tel Aviv, posted on Twitter on Nov. 30: “What a mess! Came back from a conference in London. With a mask and 3 Pfizer vaccines I managed to get Omicron.”
Dr. Maor traveled to London on November 19 to attend the PCR London Valves 2021 conference held at the ExCeL Centre Nov. 21-23. He stayed four nights at a hotel in north London and took public transport to and from the ExCeL Centre in East London each day of the meeting. He returned to Israel on the evening of Nov. 23.
Dr. Maor, 45, who has received three doses of the Pfizer COVID-19 vaccine, had two PCR tests in the United Kingdom – on November 20 and 21 in line with travel requirements – and another PCR test upon arriving back in Israel in the early hours of Nov. 24. All three tests were negative.
He began experiencing symptoms within days and tested positive on Nov. 27. His symptoms have been mild so far, and he said he was feeling “better” at the time of his tweet on Nov. 30.
Dr. Maor believes he was infected during his trip to London. “The only reasonable explanation is that I got infected on the last day of the meeting – maybe at the airport, maybe at the meeting,” he told The Guardian newspaper.
Although his wife accompanied him to London, neither she nor any of his 3 children have experienced symptoms or tested positive for COVID-19. But Dr. Maor believes he has passed the infection to a 69-year-old colleague in Israel who has since tested positive for the Omicron variant. The colleague, who has also received three vaccine doses, is understood to have mild symptoms at present.
The case suggests that the Omicron variant of COVID-19 may have been circulating in the United Kingdom earlier than previously thought.
Implications for in-person conferences
It will also inevitably lead to questions about the safety of face-to-face conferences, which are only just starting to get underway again.
The PCR Valves 2021 meeting had more than 1,250 on-site attendees as well as 2,400 or more joining online, according to figures on its website. Dr. Maor said he did not have any issues with the conference organizers, who required proof of vaccination before entry. But he posted a photograph on his Twitter account of a crowded auditorium with many delegates not wearing masks.
The conference subsequently posted an announcement on its website alerting delegates that one of the attendees had tested positive for COVID-19 after returning to their home country. It reads: “Since the reported case comes less than a week after the end of PCR London Valves, we want to inform you so that you may decide the best course of action, for yourself, if any.” It does not mention that the case was the Omicron variant.
Patrick Jolly, strategic and market development director of the conference, commented: “As you may imagine, the health, safety and well-being of everyone who visited PCR London Valves was our number-one priority. All protocols mandated by the U.K. government were put in place. Anyone entering the congress center had to present a valid health pass and were requested to wear a mask. Hydro-alcoholic gel and masks were made readily available for all participants and disposal bins for used protective equipment were provided.”
Mr. Jolly also noted: “To date – more than 9 days after the end of PCR London Valves – we have had no report of any other case of participants testing positive who attended PCR London Valves.”
He said the EuroPCR organization believes that medical conferences are safe to be held in person.
“With the above sanitary requirements and protocols, and no complacency in their enforcement, we believe strongly that medical conferences can take place, as the benefits of in-person medical conferences are obvious for the concerned medical communities,” Mr. Jolly added.
But what about other meetings happening imminently and planning in-person attendance?
Eileen Murray, executive director of the American Epilepsy Society (AES), whose annual 5-day meeting starts today at Chicago’s McCormick Place Convention Center, said in an interview that the health, safety, and well-being of everyone attending is a priority.
“Vaccinations are required, with no exceptions, to anyone attending the in-person event,” Ms. Murray said. “AES is using the CLEAR HealthPass to verify identity and vaccination status for our attendees. No one who cannot verify identity and vaccination requirement will be permitted to attend the in-person event.”
She noted that masks will also be required except in limited circumstances when actively eating or drinking, or for a faculty member when actively presenting at a lecture or panel. “Anyone not adhering to the mask policy will be asked to leave the meeting and will be denied readmission to the meeting with no refund,” she said.
“These guidelines were developed in accordance with the latest public health guidance and AES will continue to follow that guidance as any updates are made with the emergence of the Omicron variant,” Ms. Murray added.
Also commenting on this issue, a spokesperson for the American Heart Association, which has its large annual international stroke meeting planned for in-person attendance in New Orleans in February, said: “As we have throughout the pandemic, the American Heart Association is closely monitoring conditions and following the guidance of the CDC as well as state and local health departments related to all in-person meetings.”
“Our upcoming International Stroke Conference, February 9-11, is planned as an in-person and digital experience which allows us the ultimate flexibility to address changing pandemic conditions. The health, safety, and well-being of our volunteers, members, and attendees from around the world remains our number-one priority,” the AHA spokesperson added.
But some COVID-19 experts are taking a more cautious view.
Rowland Kao, PhD, an expert in infectious disease dynamics at the University of Edinburgh, United Kingdom, expressed concern about such large in-person conferences.
“We know that the Omicron variant appears to be spreading rapidly, with a recent preprint also telling us that the reinfection rate appears to be higher in South Africa. Should this be borne out, then the evidence would support that our reliance on a combination of vaccine-induced and natural immunity may be compromised by the Omicron variant,” he commented.
“We already know that extended contact indoors provides an additional risk, and so large meetings of this type have the potential to create extended risks. Until we know the extent to which Omicron causes severe illness, we should be extra cautious about these high-risk settings,” Dr. Kao commented.
A version of this article first appeared on Medscape.com.