User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
Appendicitis: Up-front antibiotics OK in select patients
a comprehensive review of the literature suggests.
“I think this is a wonderful thing that we have for our patients now, because think about the patient who had a heart attack yesterday and has appendicitis today – you don’t want to operate on that patient – so this gives us a wonderful option in an environment where sometimes surgery is just bad timing,” Theodore Pappas, MD, professor of surgery, Duke University, Durham, N.C., told this news organization.
“It’s not that every 25-year-old who comes in should get antibiotics instead of surgery. It’s really better to say that this gives us flexibility for patients who we may not want to operate on immediately, and now we have a great option,” he stressed.
The study was published Dec. 14, 2021, in JAMA.
Acute appendicitis is the most common abdominal surgical emergency in the world, as the authors pointed out.
“We think it’s going to be 60%-70% of patients who are good candidates for consideration of antibiotics,” they speculated.
Current evidence
The review summarizes current evidence regarding the diagnosis and management of acute appendicitis based on a total of 71 articles including 10 systematic reviews, 9 meta-analyses, and 11 practice guidelines. “Appendicitis is classified as uncomplicated or complicated,” the authors explained. Uncomplicated appendicitis is acute appendicitis in the absence of clinical or radiographic signs of perforation.
In contrast, complicated appendicitis is when there is appendiceal rupture with subsequent abscess of phlegmon formation, the definitive diagnosis of which can be confirmed by CT scan. “In cases of diagnostic uncertainty imaging should be performed,” investigators cautioned – usually with ultrasound and CT scans.
If uncomplicated appendicitis is confirmed, three different guidelines now support the role of an antibiotics-first approach, including guidelines from the American Association for Surgery of Trauma. For this group of patients, empirical broad-spectrum antibiotic coverage that can be transitioned to outpatient treatment is commonly used. For example, patients may be initially treated with intravenous ertapenem monotherapy or intravenous cephalosporin plus metronidazole, then on discharge put on oral fluoroquinolones plus metronidazole.
Antibiotics that cover streptococci, nonresistant Enterobacteriaceae, and the anaerobes are usually adequate, they added. “The recommended duration of antibiotics is 10 days,” they noted. In most of the clinical trials comparing antibiotics first to surgery, the primary endpoint was treatment failure at 1 year, in other words, recurrence of symptoms during that year-long period. Across a number of clinical trials, that recurrence rate ranged from a low of 15% to a high of 41%.
In contrast, recurrence rarely occurs after surgical appendectomy. Early treatment failure, defined as clinical deterioration or lack of clinical improvement within 24-72 hours following initiation of antibiotics, is much less likely to occur, with a reported rate of between 8% and 12% of patients. The only long-term follow-up of an antibiotics-first approach in uncomplicated appendicitis was done in the Appendicitis Acuta (APPAC) trial, where at 5 years, the recurrence rate of acute appendicitis was 39% (95% confidence interval, 33.1%-45.3%) in patients initially treated with antibiotics alone.
Typically, there have been no differences in the length of hospital stay in most of the clinical trials reviewed. As Dr. Pappas explained, following a standard appendectomy, patients are typically sent home within 24 hours of undergoing surgery. On the other hand, if treated with intravenous antibiotics first, patients are usually admitted overnight then switched to oral antibiotics on discharge – suggesting that there is little difference in the time spent in hospital between the two groups.
However, there are groups of patients who predictably will not do well on antibiotics first, he cautioned. For example, patients who present with a high fever, shaking and chills, and severe abdominal pain do not have a mild case of appendicitis. Neither do patients who may not look sick but on CT scan, they have a hard piece of stool jammed into the end of the appendix that’s causing the blockage: These patients are also more likely to fail antibiotics, Dr. Pappas added.
“There is also a group of patients who have a much more dilated appendix with some fluid around it,” he noted, “and these patients are less likely to be managed with antibiotics successfully as well.” Lastly, though not part of this review and for whom an antibiotics-first protocol has long been in place, there is a subset of patients who have a perforated appendix, and that perforation has been walled off in a pocket of pus.
“These patients are treated with an antibiotic first because if you operate on them, it’s a mess, whereas if patients are reasonably stable, you can drain the abscess and then put them on antibiotics, and then you can decide 6-8 weeks later if you are going to take the appendix out,” Dr. Pappas said, adding: “Most of the time, what should be happening is the surgeon should consult with the patient and then they can weigh in – here are the options and here’s what I recommend.
“But patients will pick what they pick, and surgery is a very compelling argument: It’s laparoscopic surgery, patients are home in 24 hours, and the complication rate [and the recurrence rate] are incredibly low, so you have to think through all sorts of issues and when you come to a certain conclusion, it has to make a lot of sense to the patient,” Dr. Pappas emphasized.
Asked to comment on the findings, Ram Nirula, MD, D. Rees and Eleanor T. Jensen Presidential Chair in Surgery, University of Utah, Salt Lake City, noted that, as with all things in medicine, nothing is 100%.
“There are times where antibiotics for uncomplicated appendicitis may be appropriate, and times where appendectomy is most appropriate,” he said in an interview. Most of the evidence now shows that the risk of treatment failure following nonoperative management for uncomplicated appendicitis is significant, ranging from 15% to 40%, as Dr. Nirula reaffirmed.
A more recent randomized controlled trial from the CODA collaborative found that quality of life was similar for patients who got up-front antibiotics as for those who got surgery at 30 days, but the failure rate was high, particularly for those with appendicolith (what review authors would have classified as complicated appendicitis).
Moreover, when looking at this subset of patients, quality of life and patient satisfaction in the antibiotic treatment group were lower than it was for surgical controls, as Dr. Nirula also pointed out. While length of hospital stay was similar, overall health care resource utilization was higher in the antibiotic group. “So, if it were me, I would want my appendix removed at this stage in my life, however, for those who are poor surgical candidates, I would favor antibiotics,” Dr. Nirula stressed. He added that the presence of an appendicolith makes the argument for surgery more compelling, although he would still try antibiotics in patients with an appendicolith who are poor surgical candidates.
Dr. Pappas reported serving as a paid consultant for Transenterix. Dr. Nirula disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
a comprehensive review of the literature suggests.
“I think this is a wonderful thing that we have for our patients now, because think about the patient who had a heart attack yesterday and has appendicitis today – you don’t want to operate on that patient – so this gives us a wonderful option in an environment where sometimes surgery is just bad timing,” Theodore Pappas, MD, professor of surgery, Duke University, Durham, N.C., told this news organization.
“It’s not that every 25-year-old who comes in should get antibiotics instead of surgery. It’s really better to say that this gives us flexibility for patients who we may not want to operate on immediately, and now we have a great option,” he stressed.
The study was published Dec. 14, 2021, in JAMA.
Acute appendicitis is the most common abdominal surgical emergency in the world, as the authors pointed out.
“We think it’s going to be 60%-70% of patients who are good candidates for consideration of antibiotics,” they speculated.
Current evidence
The review summarizes current evidence regarding the diagnosis and management of acute appendicitis based on a total of 71 articles including 10 systematic reviews, 9 meta-analyses, and 11 practice guidelines. “Appendicitis is classified as uncomplicated or complicated,” the authors explained. Uncomplicated appendicitis is acute appendicitis in the absence of clinical or radiographic signs of perforation.
In contrast, complicated appendicitis is when there is appendiceal rupture with subsequent abscess of phlegmon formation, the definitive diagnosis of which can be confirmed by CT scan. “In cases of diagnostic uncertainty imaging should be performed,” investigators cautioned – usually with ultrasound and CT scans.
If uncomplicated appendicitis is confirmed, three different guidelines now support the role of an antibiotics-first approach, including guidelines from the American Association for Surgery of Trauma. For this group of patients, empirical broad-spectrum antibiotic coverage that can be transitioned to outpatient treatment is commonly used. For example, patients may be initially treated with intravenous ertapenem monotherapy or intravenous cephalosporin plus metronidazole, then on discharge put on oral fluoroquinolones plus metronidazole.
Antibiotics that cover streptococci, nonresistant Enterobacteriaceae, and the anaerobes are usually adequate, they added. “The recommended duration of antibiotics is 10 days,” they noted. In most of the clinical trials comparing antibiotics first to surgery, the primary endpoint was treatment failure at 1 year, in other words, recurrence of symptoms during that year-long period. Across a number of clinical trials, that recurrence rate ranged from a low of 15% to a high of 41%.
In contrast, recurrence rarely occurs after surgical appendectomy. Early treatment failure, defined as clinical deterioration or lack of clinical improvement within 24-72 hours following initiation of antibiotics, is much less likely to occur, with a reported rate of between 8% and 12% of patients. The only long-term follow-up of an antibiotics-first approach in uncomplicated appendicitis was done in the Appendicitis Acuta (APPAC) trial, where at 5 years, the recurrence rate of acute appendicitis was 39% (95% confidence interval, 33.1%-45.3%) in patients initially treated with antibiotics alone.
Typically, there have been no differences in the length of hospital stay in most of the clinical trials reviewed. As Dr. Pappas explained, following a standard appendectomy, patients are typically sent home within 24 hours of undergoing surgery. On the other hand, if treated with intravenous antibiotics first, patients are usually admitted overnight then switched to oral antibiotics on discharge – suggesting that there is little difference in the time spent in hospital between the two groups.
However, there are groups of patients who predictably will not do well on antibiotics first, he cautioned. For example, patients who present with a high fever, shaking and chills, and severe abdominal pain do not have a mild case of appendicitis. Neither do patients who may not look sick but on CT scan, they have a hard piece of stool jammed into the end of the appendix that’s causing the blockage: These patients are also more likely to fail antibiotics, Dr. Pappas added.
“There is also a group of patients who have a much more dilated appendix with some fluid around it,” he noted, “and these patients are less likely to be managed with antibiotics successfully as well.” Lastly, though not part of this review and for whom an antibiotics-first protocol has long been in place, there is a subset of patients who have a perforated appendix, and that perforation has been walled off in a pocket of pus.
“These patients are treated with an antibiotic first because if you operate on them, it’s a mess, whereas if patients are reasonably stable, you can drain the abscess and then put them on antibiotics, and then you can decide 6-8 weeks later if you are going to take the appendix out,” Dr. Pappas said, adding: “Most of the time, what should be happening is the surgeon should consult with the patient and then they can weigh in – here are the options and here’s what I recommend.
“But patients will pick what they pick, and surgery is a very compelling argument: It’s laparoscopic surgery, patients are home in 24 hours, and the complication rate [and the recurrence rate] are incredibly low, so you have to think through all sorts of issues and when you come to a certain conclusion, it has to make a lot of sense to the patient,” Dr. Pappas emphasized.
Asked to comment on the findings, Ram Nirula, MD, D. Rees and Eleanor T. Jensen Presidential Chair in Surgery, University of Utah, Salt Lake City, noted that, as with all things in medicine, nothing is 100%.
“There are times where antibiotics for uncomplicated appendicitis may be appropriate, and times where appendectomy is most appropriate,” he said in an interview. Most of the evidence now shows that the risk of treatment failure following nonoperative management for uncomplicated appendicitis is significant, ranging from 15% to 40%, as Dr. Nirula reaffirmed.
A more recent randomized controlled trial from the CODA collaborative found that quality of life was similar for patients who got up-front antibiotics as for those who got surgery at 30 days, but the failure rate was high, particularly for those with appendicolith (what review authors would have classified as complicated appendicitis).
Moreover, when looking at this subset of patients, quality of life and patient satisfaction in the antibiotic treatment group were lower than it was for surgical controls, as Dr. Nirula also pointed out. While length of hospital stay was similar, overall health care resource utilization was higher in the antibiotic group. “So, if it were me, I would want my appendix removed at this stage in my life, however, for those who are poor surgical candidates, I would favor antibiotics,” Dr. Nirula stressed. He added that the presence of an appendicolith makes the argument for surgery more compelling, although he would still try antibiotics in patients with an appendicolith who are poor surgical candidates.
Dr. Pappas reported serving as a paid consultant for Transenterix. Dr. Nirula disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
a comprehensive review of the literature suggests.
“I think this is a wonderful thing that we have for our patients now, because think about the patient who had a heart attack yesterday and has appendicitis today – you don’t want to operate on that patient – so this gives us a wonderful option in an environment where sometimes surgery is just bad timing,” Theodore Pappas, MD, professor of surgery, Duke University, Durham, N.C., told this news organization.
“It’s not that every 25-year-old who comes in should get antibiotics instead of surgery. It’s really better to say that this gives us flexibility for patients who we may not want to operate on immediately, and now we have a great option,” he stressed.
The study was published Dec. 14, 2021, in JAMA.
Acute appendicitis is the most common abdominal surgical emergency in the world, as the authors pointed out.
“We think it’s going to be 60%-70% of patients who are good candidates for consideration of antibiotics,” they speculated.
Current evidence
The review summarizes current evidence regarding the diagnosis and management of acute appendicitis based on a total of 71 articles including 10 systematic reviews, 9 meta-analyses, and 11 practice guidelines. “Appendicitis is classified as uncomplicated or complicated,” the authors explained. Uncomplicated appendicitis is acute appendicitis in the absence of clinical or radiographic signs of perforation.
In contrast, complicated appendicitis is when there is appendiceal rupture with subsequent abscess of phlegmon formation, the definitive diagnosis of which can be confirmed by CT scan. “In cases of diagnostic uncertainty imaging should be performed,” investigators cautioned – usually with ultrasound and CT scans.
If uncomplicated appendicitis is confirmed, three different guidelines now support the role of an antibiotics-first approach, including guidelines from the American Association for Surgery of Trauma. For this group of patients, empirical broad-spectrum antibiotic coverage that can be transitioned to outpatient treatment is commonly used. For example, patients may be initially treated with intravenous ertapenem monotherapy or intravenous cephalosporin plus metronidazole, then on discharge put on oral fluoroquinolones plus metronidazole.
Antibiotics that cover streptococci, nonresistant Enterobacteriaceae, and the anaerobes are usually adequate, they added. “The recommended duration of antibiotics is 10 days,” they noted. In most of the clinical trials comparing antibiotics first to surgery, the primary endpoint was treatment failure at 1 year, in other words, recurrence of symptoms during that year-long period. Across a number of clinical trials, that recurrence rate ranged from a low of 15% to a high of 41%.
In contrast, recurrence rarely occurs after surgical appendectomy. Early treatment failure, defined as clinical deterioration or lack of clinical improvement within 24-72 hours following initiation of antibiotics, is much less likely to occur, with a reported rate of between 8% and 12% of patients. The only long-term follow-up of an antibiotics-first approach in uncomplicated appendicitis was done in the Appendicitis Acuta (APPAC) trial, where at 5 years, the recurrence rate of acute appendicitis was 39% (95% confidence interval, 33.1%-45.3%) in patients initially treated with antibiotics alone.
Typically, there have been no differences in the length of hospital stay in most of the clinical trials reviewed. As Dr. Pappas explained, following a standard appendectomy, patients are typically sent home within 24 hours of undergoing surgery. On the other hand, if treated with intravenous antibiotics first, patients are usually admitted overnight then switched to oral antibiotics on discharge – suggesting that there is little difference in the time spent in hospital between the two groups.
However, there are groups of patients who predictably will not do well on antibiotics first, he cautioned. For example, patients who present with a high fever, shaking and chills, and severe abdominal pain do not have a mild case of appendicitis. Neither do patients who may not look sick but on CT scan, they have a hard piece of stool jammed into the end of the appendix that’s causing the blockage: These patients are also more likely to fail antibiotics, Dr. Pappas added.
“There is also a group of patients who have a much more dilated appendix with some fluid around it,” he noted, “and these patients are less likely to be managed with antibiotics successfully as well.” Lastly, though not part of this review and for whom an antibiotics-first protocol has long been in place, there is a subset of patients who have a perforated appendix, and that perforation has been walled off in a pocket of pus.
“These patients are treated with an antibiotic first because if you operate on them, it’s a mess, whereas if patients are reasonably stable, you can drain the abscess and then put them on antibiotics, and then you can decide 6-8 weeks later if you are going to take the appendix out,” Dr. Pappas said, adding: “Most of the time, what should be happening is the surgeon should consult with the patient and then they can weigh in – here are the options and here’s what I recommend.
“But patients will pick what they pick, and surgery is a very compelling argument: It’s laparoscopic surgery, patients are home in 24 hours, and the complication rate [and the recurrence rate] are incredibly low, so you have to think through all sorts of issues and when you come to a certain conclusion, it has to make a lot of sense to the patient,” Dr. Pappas emphasized.
Asked to comment on the findings, Ram Nirula, MD, D. Rees and Eleanor T. Jensen Presidential Chair in Surgery, University of Utah, Salt Lake City, noted that, as with all things in medicine, nothing is 100%.
“There are times where antibiotics for uncomplicated appendicitis may be appropriate, and times where appendectomy is most appropriate,” he said in an interview. Most of the evidence now shows that the risk of treatment failure following nonoperative management for uncomplicated appendicitis is significant, ranging from 15% to 40%, as Dr. Nirula reaffirmed.
A more recent randomized controlled trial from the CODA collaborative found that quality of life was similar for patients who got up-front antibiotics as for those who got surgery at 30 days, but the failure rate was high, particularly for those with appendicolith (what review authors would have classified as complicated appendicitis).
Moreover, when looking at this subset of patients, quality of life and patient satisfaction in the antibiotic treatment group were lower than it was for surgical controls, as Dr. Nirula also pointed out. While length of hospital stay was similar, overall health care resource utilization was higher in the antibiotic group. “So, if it were me, I would want my appendix removed at this stage in my life, however, for those who are poor surgical candidates, I would favor antibiotics,” Dr. Nirula stressed. He added that the presence of an appendicolith makes the argument for surgery more compelling, although he would still try antibiotics in patients with an appendicolith who are poor surgical candidates.
Dr. Pappas reported serving as a paid consultant for Transenterix. Dr. Nirula disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA
Children and COVID: Weekly cases resume their climb
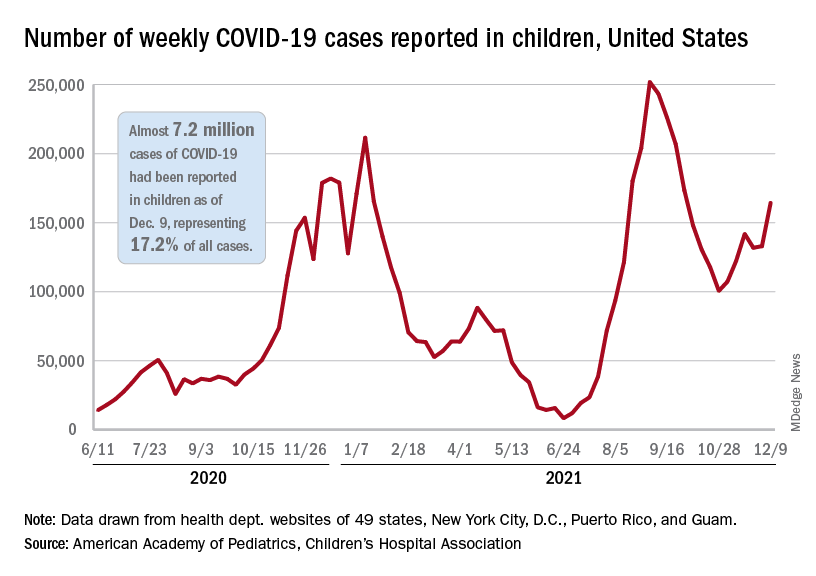
After a brief lull in activity, weekly COVID-19 cases in children returned to the upward trend that began in early November, based on data from the American Academy of Pediatrics and the Children’s Hospital Association.
according to the Centers for Disease Control and Prevention.
New COVID-19 cases were up by 23.5% for the week of Dec. 3-9, after a 2-week period that saw a drop and then just a slight increase, the AAP and CHA said in their latest weekly COVID report. There were 164,000 new cases from Dec. 3 to Dec. 9 in 46 states (Alabama, Nebraska, and Texas stopped reporting over the summer of 2021 and New York has never reported by age), the District of Columbia, New York City, Puerto Rico, and Guam.
The increase occurred across all four regions of the country, but the largest share came in the Midwest, with over 65,000 new cases, followed by the West (just over 35,000), the Northeast (just under 35,000), and the South (close to 28,000), the AAP/CHA data show.
The 7.2 million cumulative cases in children as of Dec. 9 represent 17.2% of all cases reported in the United States since the start of the pandemic, with available state reports showing that proportion ranges from 12.3% in Florida to 26.1% in Vermont. Alaska has the highest incidence of COVID at 19,000 cases per 100,000 children, and Hawaii has the lowest (5,300 per 100,000) among the states currently reporting, the AAP and CHA said.
State reporting on vaccinations shows that 37% of children aged 5-11 years in Massachusetts have received at least one dose, the highest of any state, while West Virginia is lowest at just 4%. The highest vaccination rate for children aged 12-17 goes to Massachusetts at 84%, with Wyoming lowest at 37%, the AAP said in a separate report.
Nationally, new vaccinations fell by a third during the week of Dec. 7-13, compared with the previous week, with the largest decline (34.7%) coming from the 5- to 11-year-olds, who still represented the majority (almost 84%) of the 430,000 new child vaccinations received, according to the CDC’s COVID Data Tracker. Corresponding declines for the last week were 27.5% for 12- to 15-year-olds and 22.7% for those aged 16-17.
Altogether, 21.2 million children aged 5-17 had received at least one dose and 16.0 million were fully vaccinated as of Dec. 13. By age group, 19.2% of children aged 5-11 years have gotten at least one dose and 9.6% are fully vaccinated, compared with 62.1% and 52.3%, respectively, among children aged 12-17, the CDC said.
After a brief lull in activity, weekly COVID-19 cases in children returned to the upward trend that began in early November, based on data from the American Academy of Pediatrics and the Children’s Hospital Association.

according to the Centers for Disease Control and Prevention.
New COVID-19 cases were up by 23.5% for the week of Dec. 3-9, after a 2-week period that saw a drop and then just a slight increase, the AAP and CHA said in their latest weekly COVID report. There were 164,000 new cases from Dec. 3 to Dec. 9 in 46 states (Alabama, Nebraska, and Texas stopped reporting over the summer of 2021 and New York has never reported by age), the District of Columbia, New York City, Puerto Rico, and Guam.
The increase occurred across all four regions of the country, but the largest share came in the Midwest, with over 65,000 new cases, followed by the West (just over 35,000), the Northeast (just under 35,000), and the South (close to 28,000), the AAP/CHA data show.
The 7.2 million cumulative cases in children as of Dec. 9 represent 17.2% of all cases reported in the United States since the start of the pandemic, with available state reports showing that proportion ranges from 12.3% in Florida to 26.1% in Vermont. Alaska has the highest incidence of COVID at 19,000 cases per 100,000 children, and Hawaii has the lowest (5,300 per 100,000) among the states currently reporting, the AAP and CHA said.
State reporting on vaccinations shows that 37% of children aged 5-11 years in Massachusetts have received at least one dose, the highest of any state, while West Virginia is lowest at just 4%. The highest vaccination rate for children aged 12-17 goes to Massachusetts at 84%, with Wyoming lowest at 37%, the AAP said in a separate report.
Nationally, new vaccinations fell by a third during the week of Dec. 7-13, compared with the previous week, with the largest decline (34.7%) coming from the 5- to 11-year-olds, who still represented the majority (almost 84%) of the 430,000 new child vaccinations received, according to the CDC’s COVID Data Tracker. Corresponding declines for the last week were 27.5% for 12- to 15-year-olds and 22.7% for those aged 16-17.
Altogether, 21.2 million children aged 5-17 had received at least one dose and 16.0 million were fully vaccinated as of Dec. 13. By age group, 19.2% of children aged 5-11 years have gotten at least one dose and 9.6% are fully vaccinated, compared with 62.1% and 52.3%, respectively, among children aged 12-17, the CDC said.
After a brief lull in activity, weekly COVID-19 cases in children returned to the upward trend that began in early November, based on data from the American Academy of Pediatrics and the Children’s Hospital Association.

according to the Centers for Disease Control and Prevention.
New COVID-19 cases were up by 23.5% for the week of Dec. 3-9, after a 2-week period that saw a drop and then just a slight increase, the AAP and CHA said in their latest weekly COVID report. There were 164,000 new cases from Dec. 3 to Dec. 9 in 46 states (Alabama, Nebraska, and Texas stopped reporting over the summer of 2021 and New York has never reported by age), the District of Columbia, New York City, Puerto Rico, and Guam.
The increase occurred across all four regions of the country, but the largest share came in the Midwest, with over 65,000 new cases, followed by the West (just over 35,000), the Northeast (just under 35,000), and the South (close to 28,000), the AAP/CHA data show.
The 7.2 million cumulative cases in children as of Dec. 9 represent 17.2% of all cases reported in the United States since the start of the pandemic, with available state reports showing that proportion ranges from 12.3% in Florida to 26.1% in Vermont. Alaska has the highest incidence of COVID at 19,000 cases per 100,000 children, and Hawaii has the lowest (5,300 per 100,000) among the states currently reporting, the AAP and CHA said.
State reporting on vaccinations shows that 37% of children aged 5-11 years in Massachusetts have received at least one dose, the highest of any state, while West Virginia is lowest at just 4%. The highest vaccination rate for children aged 12-17 goes to Massachusetts at 84%, with Wyoming lowest at 37%, the AAP said in a separate report.
Nationally, new vaccinations fell by a third during the week of Dec. 7-13, compared with the previous week, with the largest decline (34.7%) coming from the 5- to 11-year-olds, who still represented the majority (almost 84%) of the 430,000 new child vaccinations received, according to the CDC’s COVID Data Tracker. Corresponding declines for the last week were 27.5% for 12- to 15-year-olds and 22.7% for those aged 16-17.
Altogether, 21.2 million children aged 5-17 had received at least one dose and 16.0 million were fully vaccinated as of Dec. 13. By age group, 19.2% of children aged 5-11 years have gotten at least one dose and 9.6% are fully vaccinated, compared with 62.1% and 52.3%, respectively, among children aged 12-17, the CDC said.
Unrestricted prescribing of mifepristone: Safe and effective, says study
Abortion rates remained stable and adverse events were rare after removal of mifepristone prescribing restrictions in Canada, a new study shows.
“Our study is a signal to other countries that restrictions are not necessary to ensure patient safety,” senior author Wendy V. Norman, MD, professor in the department of family practice at the University of British Columbia, Vancouver, said in a press release.
“This is the strongest evidence yet that it is safe to provide the abortion pill like most other prescriptions – meaning any doctor or nurse practitioner can prescribe, any pharmacist can dispense, and patients can take the pills if, when, and where they choose,” said lead author Laura Schummers, ScD, a postdoctoral fellow in the same department.
The findings “add to the accumulating evidence that removing restrictions from medication abortion is safe, effective, and improves access,” agreed Eve Espey, MD, professor and chair of the department of obstetrics and gynecology at the University of New Mexico, Albuquerque, who was not part of the research team. “This is additional confirmation that it is safe for patients to receive abortion care medications in the ‘normal’ fashion, through a prescription available at a pharmacy,” she said in an interview.
The study, published in the New England Journal of Medicine, compared medical abortion use, safety, and effectiveness in the province of Ontario before the Canadian availability of mifepristone and after it became available without restrictions that are similar to the Risk Evaluation and Mitigation Strategy (REMS) restrictions in place for mifepristone in the United States.
Using linked administrative health data, the researchers created a population-based cohort of all Ontario residents aged 12-49 years who had received abortion services during the study period. In total, 195,183 abortions were performed in the period before mifepristone was approved (January 2012–December 2016), and 84,032 were performed after it was made available without restrictions (Nov. 7, 2017, through March 15, 2020). The vast majority of these abortions (89.3%) were surgical, with about 10% being medically induced, the authors reported.
The study found that, while the overall abortion rate declined over the study period (from 11.9 to 11.3 per 1,000 female residents), the proportion of medical abortions jumped sharply from 2.2% to 31.4%, and the rate of second-trimester abortions declined from 5.5% of all abortions to 5.1%.
Abortion safety outcomes within 6 weeks of abortion remained stable over the two study periods. This included severe adverse events (0.03% vs. 0.04%) such as blood transfusions, abdominal surgery, admission to an ICU, or sepsis during an abortion-related hospitalization; and complications (0.74% vs. 0.69%,) such as genital tract or pelvic infection, hemorrhage, embolism, shock, renal failure, damage to pelvic organs or tissues, and venous complications among other things.
There were slight declines in overall abortion effectiveness, but ongoing pregnancy rates “remained infrequent,” the authors noted. While there was a modest rise in the rates of subsequent uterine evacuation (from 1.0% to 2.2%), and ongoing intrauterine pregnancy continuing until delivery (from 0.03% to 0.08%), the rate of ectopic pregnancy diagnosed within 6 weeks after the abortion date remained stable (from 0.15% to 0.22%).
Canada was the first country in the world to remove all supplemental restrictions on the dispensing and administration of mifepristone, according to the press release. And while professional organizations have called for the removal of such restrictions “because they impede access to abortion services without improving safety,” high-quality data on this are lacking, they added.
The study’s finding are consistent with existing U.S. and U.K. data showing Food and Drug Administration REMS restrictions requiring abortion care medications to be dispensed in a clinic by a certified provider “are unnecessary and create obstacles to early abortion access,” said Dr. Espey. “For clinicians and patients in the U.S., it’s important to note that the increasing number of legislative restrictions on abortion, including medication abortion, are non–evidence based. Politically motivated false claims of safety concerns are countered by this study and others conducted during the pandemic when both the U.S. and U.K. removed REMS-type restrictions. These studies show that receiving abortion care through usual pharmacy channels and through telemedicine is safe, effective, and reduces barriers to care.”
Dr. Norman reported receiving grants from the Canadian Institutes of Health Research, providing expert witness services to the government of Ontario and Office of the Attorney General, and serving on the board of directors of the Society of Family Planning. No other researchers reported conflicts of interest. Dr. Espey reported no conflicts of interest. The Canadian Institutes of Health Research and the Women’s Health Research Institute with the support of ICES (formerly known as the Institute for Clinical Evaluative Sciences).
Abortion rates remained stable and adverse events were rare after removal of mifepristone prescribing restrictions in Canada, a new study shows.
“Our study is a signal to other countries that restrictions are not necessary to ensure patient safety,” senior author Wendy V. Norman, MD, professor in the department of family practice at the University of British Columbia, Vancouver, said in a press release.
“This is the strongest evidence yet that it is safe to provide the abortion pill like most other prescriptions – meaning any doctor or nurse practitioner can prescribe, any pharmacist can dispense, and patients can take the pills if, when, and where they choose,” said lead author Laura Schummers, ScD, a postdoctoral fellow in the same department.
The findings “add to the accumulating evidence that removing restrictions from medication abortion is safe, effective, and improves access,” agreed Eve Espey, MD, professor and chair of the department of obstetrics and gynecology at the University of New Mexico, Albuquerque, who was not part of the research team. “This is additional confirmation that it is safe for patients to receive abortion care medications in the ‘normal’ fashion, through a prescription available at a pharmacy,” she said in an interview.
The study, published in the New England Journal of Medicine, compared medical abortion use, safety, and effectiveness in the province of Ontario before the Canadian availability of mifepristone and after it became available without restrictions that are similar to the Risk Evaluation and Mitigation Strategy (REMS) restrictions in place for mifepristone in the United States.
Using linked administrative health data, the researchers created a population-based cohort of all Ontario residents aged 12-49 years who had received abortion services during the study period. In total, 195,183 abortions were performed in the period before mifepristone was approved (January 2012–December 2016), and 84,032 were performed after it was made available without restrictions (Nov. 7, 2017, through March 15, 2020). The vast majority of these abortions (89.3%) were surgical, with about 10% being medically induced, the authors reported.
The study found that, while the overall abortion rate declined over the study period (from 11.9 to 11.3 per 1,000 female residents), the proportion of medical abortions jumped sharply from 2.2% to 31.4%, and the rate of second-trimester abortions declined from 5.5% of all abortions to 5.1%.
Abortion safety outcomes within 6 weeks of abortion remained stable over the two study periods. This included severe adverse events (0.03% vs. 0.04%) such as blood transfusions, abdominal surgery, admission to an ICU, or sepsis during an abortion-related hospitalization; and complications (0.74% vs. 0.69%,) such as genital tract or pelvic infection, hemorrhage, embolism, shock, renal failure, damage to pelvic organs or tissues, and venous complications among other things.
There were slight declines in overall abortion effectiveness, but ongoing pregnancy rates “remained infrequent,” the authors noted. While there was a modest rise in the rates of subsequent uterine evacuation (from 1.0% to 2.2%), and ongoing intrauterine pregnancy continuing until delivery (from 0.03% to 0.08%), the rate of ectopic pregnancy diagnosed within 6 weeks after the abortion date remained stable (from 0.15% to 0.22%).
Canada was the first country in the world to remove all supplemental restrictions on the dispensing and administration of mifepristone, according to the press release. And while professional organizations have called for the removal of such restrictions “because they impede access to abortion services without improving safety,” high-quality data on this are lacking, they added.
The study’s finding are consistent with existing U.S. and U.K. data showing Food and Drug Administration REMS restrictions requiring abortion care medications to be dispensed in a clinic by a certified provider “are unnecessary and create obstacles to early abortion access,” said Dr. Espey. “For clinicians and patients in the U.S., it’s important to note that the increasing number of legislative restrictions on abortion, including medication abortion, are non–evidence based. Politically motivated false claims of safety concerns are countered by this study and others conducted during the pandemic when both the U.S. and U.K. removed REMS-type restrictions. These studies show that receiving abortion care through usual pharmacy channels and through telemedicine is safe, effective, and reduces barriers to care.”
Dr. Norman reported receiving grants from the Canadian Institutes of Health Research, providing expert witness services to the government of Ontario and Office of the Attorney General, and serving on the board of directors of the Society of Family Planning. No other researchers reported conflicts of interest. Dr. Espey reported no conflicts of interest. The Canadian Institutes of Health Research and the Women’s Health Research Institute with the support of ICES (formerly known as the Institute for Clinical Evaluative Sciences).
Abortion rates remained stable and adverse events were rare after removal of mifepristone prescribing restrictions in Canada, a new study shows.
“Our study is a signal to other countries that restrictions are not necessary to ensure patient safety,” senior author Wendy V. Norman, MD, professor in the department of family practice at the University of British Columbia, Vancouver, said in a press release.
“This is the strongest evidence yet that it is safe to provide the abortion pill like most other prescriptions – meaning any doctor or nurse practitioner can prescribe, any pharmacist can dispense, and patients can take the pills if, when, and where they choose,” said lead author Laura Schummers, ScD, a postdoctoral fellow in the same department.
The findings “add to the accumulating evidence that removing restrictions from medication abortion is safe, effective, and improves access,” agreed Eve Espey, MD, professor and chair of the department of obstetrics and gynecology at the University of New Mexico, Albuquerque, who was not part of the research team. “This is additional confirmation that it is safe for patients to receive abortion care medications in the ‘normal’ fashion, through a prescription available at a pharmacy,” she said in an interview.
The study, published in the New England Journal of Medicine, compared medical abortion use, safety, and effectiveness in the province of Ontario before the Canadian availability of mifepristone and after it became available without restrictions that are similar to the Risk Evaluation and Mitigation Strategy (REMS) restrictions in place for mifepristone in the United States.
Using linked administrative health data, the researchers created a population-based cohort of all Ontario residents aged 12-49 years who had received abortion services during the study period. In total, 195,183 abortions were performed in the period before mifepristone was approved (January 2012–December 2016), and 84,032 were performed after it was made available without restrictions (Nov. 7, 2017, through March 15, 2020). The vast majority of these abortions (89.3%) were surgical, with about 10% being medically induced, the authors reported.
The study found that, while the overall abortion rate declined over the study period (from 11.9 to 11.3 per 1,000 female residents), the proportion of medical abortions jumped sharply from 2.2% to 31.4%, and the rate of second-trimester abortions declined from 5.5% of all abortions to 5.1%.
Abortion safety outcomes within 6 weeks of abortion remained stable over the two study periods. This included severe adverse events (0.03% vs. 0.04%) such as blood transfusions, abdominal surgery, admission to an ICU, or sepsis during an abortion-related hospitalization; and complications (0.74% vs. 0.69%,) such as genital tract or pelvic infection, hemorrhage, embolism, shock, renal failure, damage to pelvic organs or tissues, and venous complications among other things.
There were slight declines in overall abortion effectiveness, but ongoing pregnancy rates “remained infrequent,” the authors noted. While there was a modest rise in the rates of subsequent uterine evacuation (from 1.0% to 2.2%), and ongoing intrauterine pregnancy continuing until delivery (from 0.03% to 0.08%), the rate of ectopic pregnancy diagnosed within 6 weeks after the abortion date remained stable (from 0.15% to 0.22%).
Canada was the first country in the world to remove all supplemental restrictions on the dispensing and administration of mifepristone, according to the press release. And while professional organizations have called for the removal of such restrictions “because they impede access to abortion services without improving safety,” high-quality data on this are lacking, they added.
The study’s finding are consistent with existing U.S. and U.K. data showing Food and Drug Administration REMS restrictions requiring abortion care medications to be dispensed in a clinic by a certified provider “are unnecessary and create obstacles to early abortion access,” said Dr. Espey. “For clinicians and patients in the U.S., it’s important to note that the increasing number of legislative restrictions on abortion, including medication abortion, are non–evidence based. Politically motivated false claims of safety concerns are countered by this study and others conducted during the pandemic when both the U.S. and U.K. removed REMS-type restrictions. These studies show that receiving abortion care through usual pharmacy channels and through telemedicine is safe, effective, and reduces barriers to care.”
Dr. Norman reported receiving grants from the Canadian Institutes of Health Research, providing expert witness services to the government of Ontario and Office of the Attorney General, and serving on the board of directors of the Society of Family Planning. No other researchers reported conflicts of interest. Dr. Espey reported no conflicts of interest. The Canadian Institutes of Health Research and the Women’s Health Research Institute with the support of ICES (formerly known as the Institute for Clinical Evaluative Sciences).
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Epilepsy linked to 1.5-fold higher COVID-19 mortality in hospital
according to a new study presented at the annual meeting of the American Epilepsy Society. While the findings are preliminary and not yet adjusted for various confounders, the authors say they are a warning sign that patients with epilepsy may face higher risks.
“These findings suggest that epilepsy may be a pre-existing condition that places patients at increased risk for death if hospitalized with a COVID-19 infection. It may offer neurologists guidance when counseling patients on critical preventative measures such as masking, social distancing, and most importantly, vaccination,” lead author Claire Ufongene, a student at Icahn School of Medicine at Mount Sinai, New York, said in an interview.
According to Ms. Ufongene, there’s sparse data about COVID-19 outcomes in patients with epilepsy, although she highlighted a 2021 meta-analysis of 13 studies that found a higher risk of severity (odds ratio, 1.69; 95% confidence interval, 1.11-2.59, P = .010) and mortality (OR, 1.71; 95% CI, 1.14-2.56, P = .010).
For the new study, researchers retrospectively tracked identified 334 patients with epilepsy and COVID-19 and 9,499 other patients with COVID-19 from March 15, 2020, to May 17, 2021. All were treated at hospitals within the New York–based Icahn School of Medicine at Mount Sinai.
The groups of patients with and without epilepsy were similar in some ways: 45% and 46%, respectively, were female (P = .674), and their ages were similar (average, 62 years and 65 years, respectively; P = .02). Racial makeup was also similar (non-Hispanic groups made up 27.8% of those with epilepsy and 24.5% of those without; the difference was not statistically significant).
“In addition, more of those with epilepsy were English speaking [83.2% vs. 77.9%] and had Medicaid insurance [50.9% vs. 38.9%], while fewer of those with epilepsy had private insurance [16.2% vs. 25.5%] or were Spanish speaking [14.0% vs. 9.3%],” study coauthor Nathalie Jette, MD, MSc, a neurologist at Icahn School of Medicine at Mount Sinai, said in an interview.
In terms of outcomes, patients with epilepsy were much more likely to need ventilator support (37.7% vs. 14.3%; P < .001), to be admitted to the ICU (39.2% vs. 17.7%; P < .001), and to die in the hospital (29.6% vs. 19.9%; P < .001).
“Most patients we follow in our practices with epilepsy who experienced COVID-19 in general have had symptoms similar to the general population,” Dr. Jette said. “There are rare instances where COVID-19 can result in an exacerbation of seizures in some with pre-existing epilepsy. This is not surprising as infections in particular can decrease the seizure threshold and result in breakthrough seizures in people living with epilepsy.”
Loss of seizure control
How might epilepsy be related to worse outcomes in COVID-19? Andrew Wilner, MD, a neurologist and internist at University of Tennessee Health Science Center, Memphis, who’s familiar with the study findings, said COVID-19 itself may not worsen epilepsy. “Evidence to suggest that COVID-19 directly affects the central nervous system is extremely limited. As such, one would not expect that a COVID-19 infection would cause epilepsy or exacerbate epilepsy,” he said. “However, patients with epilepsy who suffer from infections may be predisposed to decreased seizure control. Consequently, it would not be surprising if patients with epilepsy who also had COVID-19 had loss of seizure control and even status epilepticus, which could adversely affect their hospital course. However, there are no data on this potential phenomenon.”
Dr. Wilner suspected that comorbidities explain the higher mortality in patients with epilepsy. “The findings are probably most useful in that they call attention to the fact that epilepsy patients are more vulnerable to a host of comorbidities and resultant poorer outcomes due to any acute illness.”
As for treatment, Dr. Wilner urged colleagues to make sure that hospitalized patients with epilepsy “continue to receive their antiepileptic medications, which they may no longer be able to take orally. They may need to be switched temporarily to an intravenous formulation.”
In an interview, Selim Benbadis, MD, a neurologist from the University of South Florida, Tampa, suggested that antiseizure medications may play a role in the COVID-19 disease course because they can reduce the efficacy of other medications, although he noted that drug treatments for COVID-19 were limited early on. He recommended that neurologists “avoid old enzyme-inducing seizure medications, as is generally recommended.”
No study funding is reported. The study authors and Dr. Benbadis reported no relevant disclosures. Dr. Wilner is a medical adviser for the epilepsy disease management program for CVS/Health.
according to a new study presented at the annual meeting of the American Epilepsy Society. While the findings are preliminary and not yet adjusted for various confounders, the authors say they are a warning sign that patients with epilepsy may face higher risks.
“These findings suggest that epilepsy may be a pre-existing condition that places patients at increased risk for death if hospitalized with a COVID-19 infection. It may offer neurologists guidance when counseling patients on critical preventative measures such as masking, social distancing, and most importantly, vaccination,” lead author Claire Ufongene, a student at Icahn School of Medicine at Mount Sinai, New York, said in an interview.
According to Ms. Ufongene, there’s sparse data about COVID-19 outcomes in patients with epilepsy, although she highlighted a 2021 meta-analysis of 13 studies that found a higher risk of severity (odds ratio, 1.69; 95% confidence interval, 1.11-2.59, P = .010) and mortality (OR, 1.71; 95% CI, 1.14-2.56, P = .010).
For the new study, researchers retrospectively tracked identified 334 patients with epilepsy and COVID-19 and 9,499 other patients with COVID-19 from March 15, 2020, to May 17, 2021. All were treated at hospitals within the New York–based Icahn School of Medicine at Mount Sinai.
The groups of patients with and without epilepsy were similar in some ways: 45% and 46%, respectively, were female (P = .674), and their ages were similar (average, 62 years and 65 years, respectively; P = .02). Racial makeup was also similar (non-Hispanic groups made up 27.8% of those with epilepsy and 24.5% of those without; the difference was not statistically significant).
“In addition, more of those with epilepsy were English speaking [83.2% vs. 77.9%] and had Medicaid insurance [50.9% vs. 38.9%], while fewer of those with epilepsy had private insurance [16.2% vs. 25.5%] or were Spanish speaking [14.0% vs. 9.3%],” study coauthor Nathalie Jette, MD, MSc, a neurologist at Icahn School of Medicine at Mount Sinai, said in an interview.
In terms of outcomes, patients with epilepsy were much more likely to need ventilator support (37.7% vs. 14.3%; P < .001), to be admitted to the ICU (39.2% vs. 17.7%; P < .001), and to die in the hospital (29.6% vs. 19.9%; P < .001).
“Most patients we follow in our practices with epilepsy who experienced COVID-19 in general have had symptoms similar to the general population,” Dr. Jette said. “There are rare instances where COVID-19 can result in an exacerbation of seizures in some with pre-existing epilepsy. This is not surprising as infections in particular can decrease the seizure threshold and result in breakthrough seizures in people living with epilepsy.”
Loss of seizure control
How might epilepsy be related to worse outcomes in COVID-19? Andrew Wilner, MD, a neurologist and internist at University of Tennessee Health Science Center, Memphis, who’s familiar with the study findings, said COVID-19 itself may not worsen epilepsy. “Evidence to suggest that COVID-19 directly affects the central nervous system is extremely limited. As such, one would not expect that a COVID-19 infection would cause epilepsy or exacerbate epilepsy,” he said. “However, patients with epilepsy who suffer from infections may be predisposed to decreased seizure control. Consequently, it would not be surprising if patients with epilepsy who also had COVID-19 had loss of seizure control and even status epilepticus, which could adversely affect their hospital course. However, there are no data on this potential phenomenon.”
Dr. Wilner suspected that comorbidities explain the higher mortality in patients with epilepsy. “The findings are probably most useful in that they call attention to the fact that epilepsy patients are more vulnerable to a host of comorbidities and resultant poorer outcomes due to any acute illness.”
As for treatment, Dr. Wilner urged colleagues to make sure that hospitalized patients with epilepsy “continue to receive their antiepileptic medications, which they may no longer be able to take orally. They may need to be switched temporarily to an intravenous formulation.”
In an interview, Selim Benbadis, MD, a neurologist from the University of South Florida, Tampa, suggested that antiseizure medications may play a role in the COVID-19 disease course because they can reduce the efficacy of other medications, although he noted that drug treatments for COVID-19 were limited early on. He recommended that neurologists “avoid old enzyme-inducing seizure medications, as is generally recommended.”
No study funding is reported. The study authors and Dr. Benbadis reported no relevant disclosures. Dr. Wilner is a medical adviser for the epilepsy disease management program for CVS/Health.
according to a new study presented at the annual meeting of the American Epilepsy Society. While the findings are preliminary and not yet adjusted for various confounders, the authors say they are a warning sign that patients with epilepsy may face higher risks.
“These findings suggest that epilepsy may be a pre-existing condition that places patients at increased risk for death if hospitalized with a COVID-19 infection. It may offer neurologists guidance when counseling patients on critical preventative measures such as masking, social distancing, and most importantly, vaccination,” lead author Claire Ufongene, a student at Icahn School of Medicine at Mount Sinai, New York, said in an interview.
According to Ms. Ufongene, there’s sparse data about COVID-19 outcomes in patients with epilepsy, although she highlighted a 2021 meta-analysis of 13 studies that found a higher risk of severity (odds ratio, 1.69; 95% confidence interval, 1.11-2.59, P = .010) and mortality (OR, 1.71; 95% CI, 1.14-2.56, P = .010).
For the new study, researchers retrospectively tracked identified 334 patients with epilepsy and COVID-19 and 9,499 other patients with COVID-19 from March 15, 2020, to May 17, 2021. All were treated at hospitals within the New York–based Icahn School of Medicine at Mount Sinai.
The groups of patients with and without epilepsy were similar in some ways: 45% and 46%, respectively, were female (P = .674), and their ages were similar (average, 62 years and 65 years, respectively; P = .02). Racial makeup was also similar (non-Hispanic groups made up 27.8% of those with epilepsy and 24.5% of those without; the difference was not statistically significant).
“In addition, more of those with epilepsy were English speaking [83.2% vs. 77.9%] and had Medicaid insurance [50.9% vs. 38.9%], while fewer of those with epilepsy had private insurance [16.2% vs. 25.5%] or were Spanish speaking [14.0% vs. 9.3%],” study coauthor Nathalie Jette, MD, MSc, a neurologist at Icahn School of Medicine at Mount Sinai, said in an interview.
In terms of outcomes, patients with epilepsy were much more likely to need ventilator support (37.7% vs. 14.3%; P < .001), to be admitted to the ICU (39.2% vs. 17.7%; P < .001), and to die in the hospital (29.6% vs. 19.9%; P < .001).
“Most patients we follow in our practices with epilepsy who experienced COVID-19 in general have had symptoms similar to the general population,” Dr. Jette said. “There are rare instances where COVID-19 can result in an exacerbation of seizures in some with pre-existing epilepsy. This is not surprising as infections in particular can decrease the seizure threshold and result in breakthrough seizures in people living with epilepsy.”
Loss of seizure control
How might epilepsy be related to worse outcomes in COVID-19? Andrew Wilner, MD, a neurologist and internist at University of Tennessee Health Science Center, Memphis, who’s familiar with the study findings, said COVID-19 itself may not worsen epilepsy. “Evidence to suggest that COVID-19 directly affects the central nervous system is extremely limited. As such, one would not expect that a COVID-19 infection would cause epilepsy or exacerbate epilepsy,” he said. “However, patients with epilepsy who suffer from infections may be predisposed to decreased seizure control. Consequently, it would not be surprising if patients with epilepsy who also had COVID-19 had loss of seizure control and even status epilepticus, which could adversely affect their hospital course. However, there are no data on this potential phenomenon.”
Dr. Wilner suspected that comorbidities explain the higher mortality in patients with epilepsy. “The findings are probably most useful in that they call attention to the fact that epilepsy patients are more vulnerable to a host of comorbidities and resultant poorer outcomes due to any acute illness.”
As for treatment, Dr. Wilner urged colleagues to make sure that hospitalized patients with epilepsy “continue to receive their antiepileptic medications, which they may no longer be able to take orally. They may need to be switched temporarily to an intravenous formulation.”
In an interview, Selim Benbadis, MD, a neurologist from the University of South Florida, Tampa, suggested that antiseizure medications may play a role in the COVID-19 disease course because they can reduce the efficacy of other medications, although he noted that drug treatments for COVID-19 were limited early on. He recommended that neurologists “avoid old enzyme-inducing seizure medications, as is generally recommended.”
No study funding is reported. The study authors and Dr. Benbadis reported no relevant disclosures. Dr. Wilner is a medical adviser for the epilepsy disease management program for CVS/Health.
FROM AES 2021
Vegetative Plaques on the Face
THE DIAGNOSIS: Vegetative Majocchi Granuloma
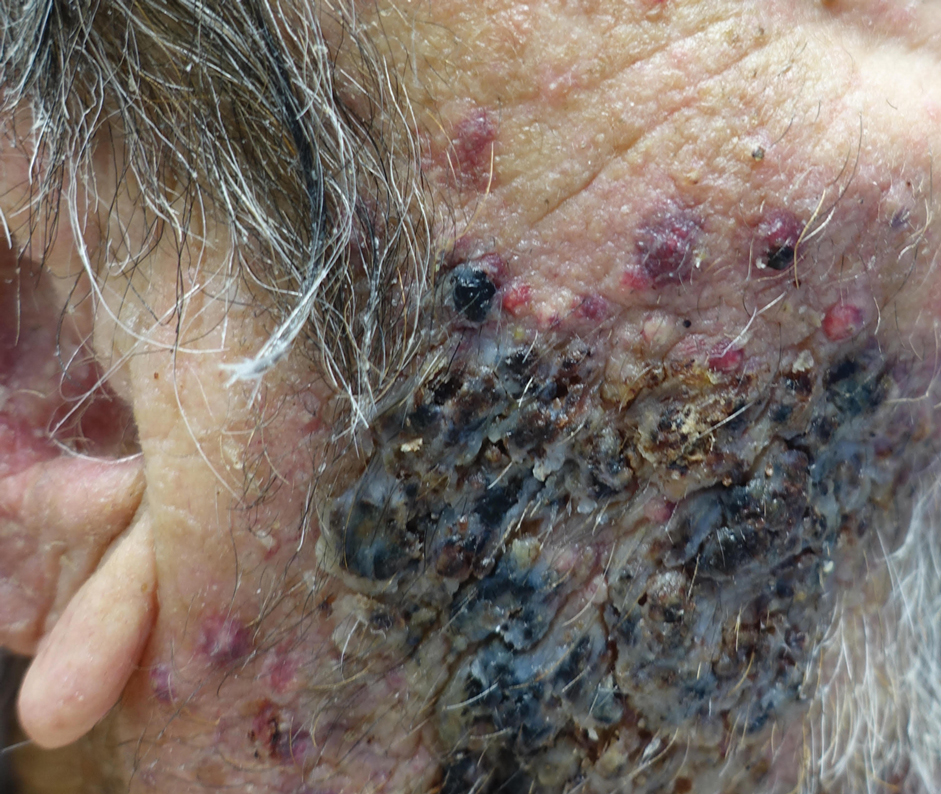
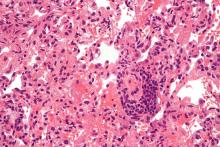
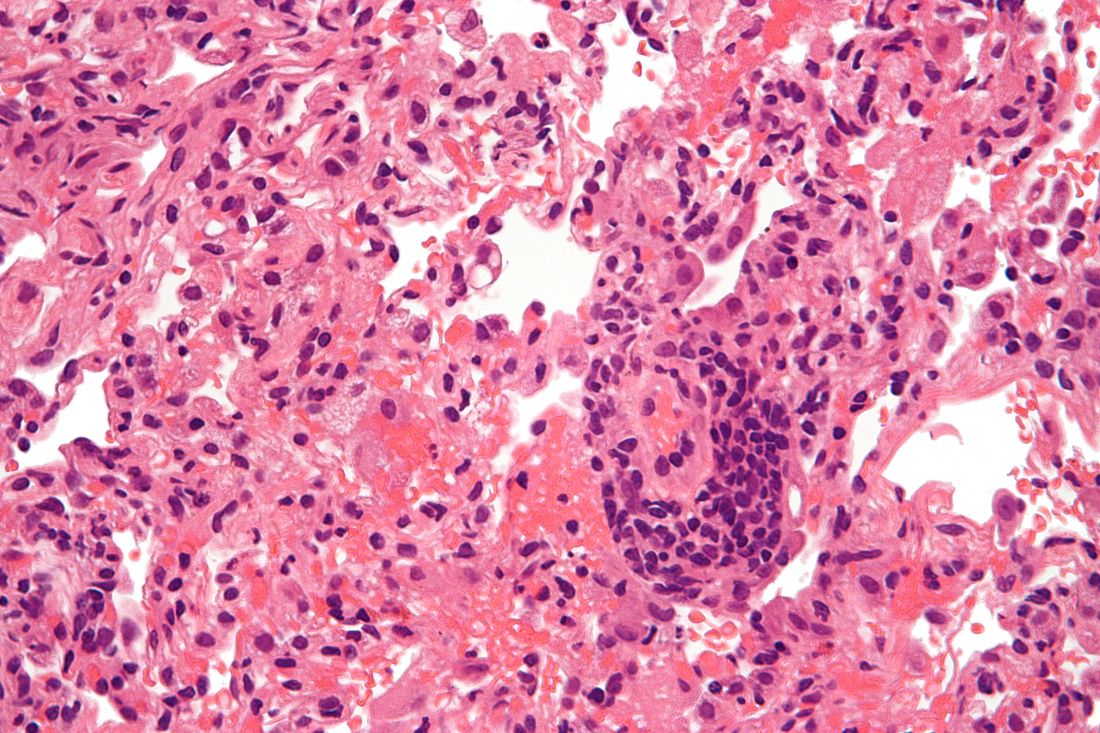
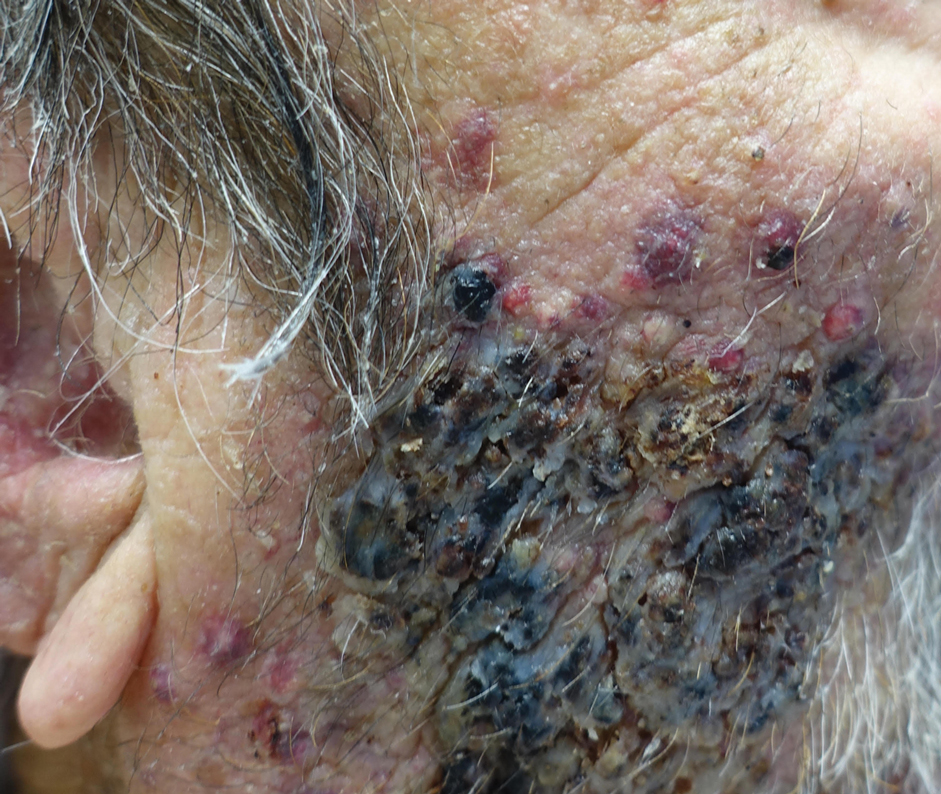
A biopsy and tissue culture showed acute dermal inflammation with granulomatous features and numerous fungal hyphae within the stratum corneum (Figure 1A), which were confirmed on GrocottGomori methenamine-silver staining (Figure 1B). Gram and Fite stains were negative for bacteria. A tissue culture speciated Trichophyton rubrum, which led to a diagnosis of deep dermatophyte infection (Majocchi granuloma) with a highly unusual clinical presentation of vegetative plaques. Predisposing factors included treatment with topical corticosteroids and possibly poor health and nutritional status at baseline. Our patient was treated with fluconazole 200 mg daily for 6 weeks, with near resolution of lesions at 3-week follow-up (Figure 2).
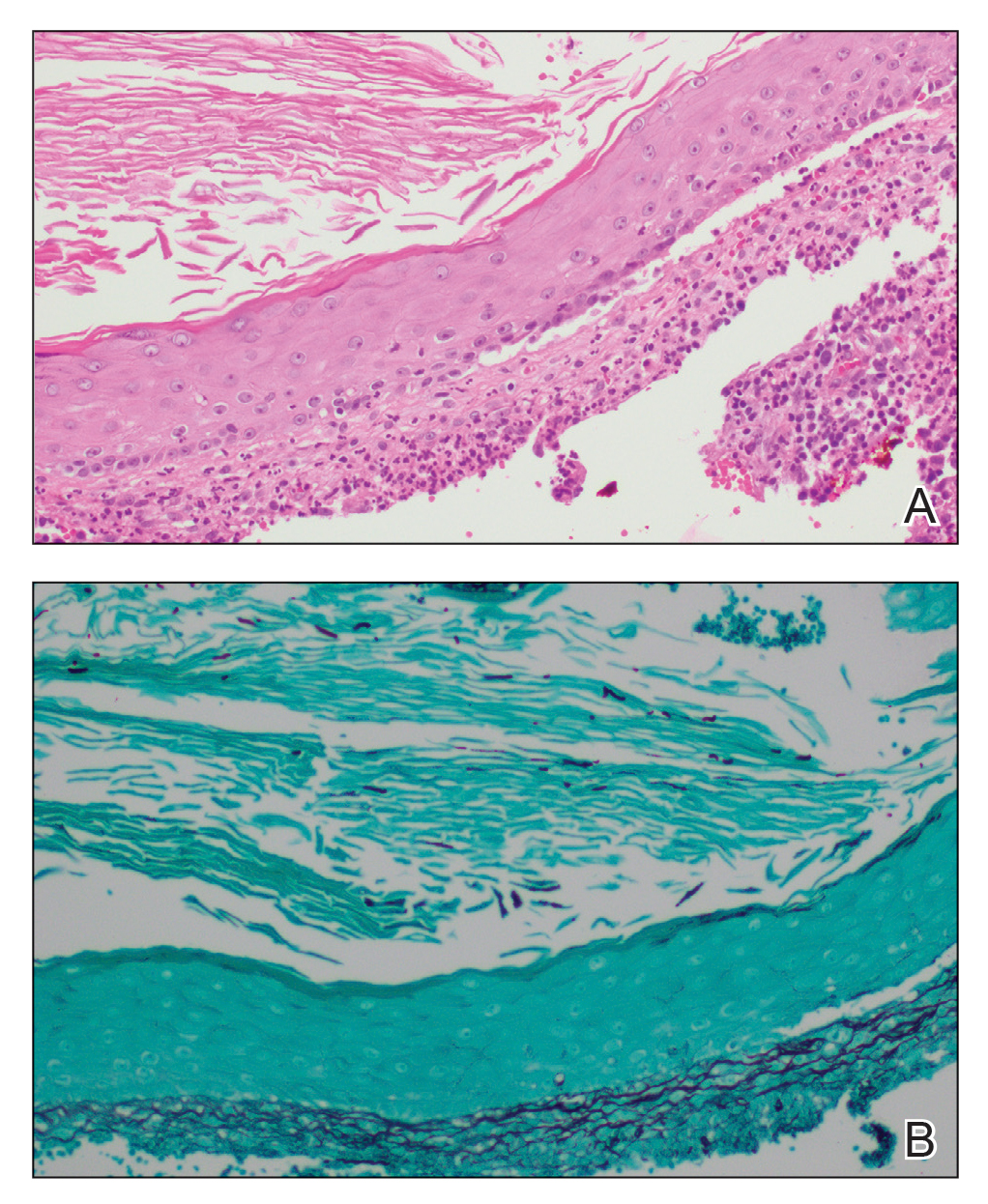
Dermatophytes are a common cause of superficial skin infections. The classic morphology consists of an annular scaly plaque; however, a wide variety of presentations have been observed (eg, verrucous, vesicular, pustular, granulomatous). Therefore, dermatophyte infections often mimic other dermatologic conditions, including atopic dermatitis, rosacea, psoriasis, bacterial abscess, erythema gyratum repens, lupus, granuloma annulare, cutaneous lymphoma, Hailey-Hailey disease, scarring alopecia, and syphilis.1
Notably, when dermatophytes grow downward along hair follicles causing deeper infection, disruption of the follicular wall can lead to an excessive inflammatory response with granulomatous features.2 Risk factors include cutaneous trauma, long-standing infection, immunocompromise, and treatment with topical corticosteroids.3 This disease evolution clinically appears as a nodule or infiltrated plaque, often without scale. The most well-known example is a kerion on the scalp. Elsewhere on the body, lesions often are termed Majocchi granulomas.2
Vegetative plaques, as seen in our patient, are a highly unusual morphology for deep tinea infection. Guanziroli et al4 reported a case of vegetative lesions on the forearm of a 67-year-old immunocompromised man that were successfully treated with a 3-month course of oral terbinafine after Trichophyton verrucosum was isolated. Skorepova et al5 reported a case of pyoderma vegetans triggered by recurrent Trichophyton mentagrophytes on the dorsal hands of a 64-year-old man with immunoglobulin deficiency of unknown etiology. The lesions were successfully treated with a prolonged course of doxycycline, topical triamcinolone, and intravenous immunoglobulin following 2 initial courses of terbinafine.
The differential diagnosis for vegetative lesions includes pemphigus vegetans, a vegetative variant of pyoderma gangrenosum; halogenoderma; and a variety of infections, including dimorphic fungi (histoplasmosis, blastomycosis), blastomycosislike pyoderma (bacterial), and candidiasis.6 These conditions usually can be distinguished based on histopathology. Clinically, pemphigus vegetans presents with pustules and vegetative lesions, as in our patient, but usually is more diffuse and favors the intertriginous areas. Histology likely would reveal foci of acantholysis and eosinophils. Vegetative pyoderma gangrenosum favors the trunk, particularly in sites of surgical trauma. In our patient, no lesions were present near the abdominal surgical sites, and there was no antecedent cribriform ulceration. Halogenoderma was a strong initial consideration given the localization, presence of large pustules, and history of numerous contrast computed tomography studies; however, our patient’s iodine levels were normal. Infectious etiologies including dimorphic fungi and blastomycosislike pyoderma generally are not restricted to the head and neck, and tissue culture helps exclude them. Vegetative lesions may occur in the setting of other infections, and tissue culture may be necessary to differentiate them if histopathology is not suggestive.
Deep dermatophyte infections require treatment with oral antifungals, as topicals do not penetrate adequately into the hair follicles. Exact regimens vary, but generally oral terbinafine or an oral azole (except ketoconazole) is administered for 2 to 6 weeks, with immunocompromise necessitating longer courses.
We present a rare case of vegetative Majocchi granuloma secondary to T rubrum infection. A dermatophyte infection should be included in the differential for vegetative lesions, especially in dense hair-bearing areas such as the beard. Treatment generally is straightforward with oral antifungals.
- Atzori L, Pau M, Aste N, et al. Dermatophyte infections mimicking other skin diseases: a 154-person case survey of tinea atypica in the district of Cagliari (Italy). Int J Dermatol. 2012;51:410-415.
- Ilkit M, Durdu M, Karakas M. Majocchi’s granuloma: a symptom complex caused by fungal pathogens. Med Mycol. 2012;50:449-457.
- Jevremovic L, Ilijin I, Kostic K, et al. Pyoderma vegetans—a case report. Serbian J Dermatol Venereol. 2017;9:22-28.
- Guanziroli E, Pavia G, Guttadauro A, et al. Deep dermatophytosis caused by Trichophyton verrucosum in an immunosuppressed patient: successful outcome with terbinafine. Mycopathologia. 2019;184:543-545.
- Skorepová M, Stuchlík D. Chronic pyoderma vegetans triggered by Trichophyton mentagrophytes. Mycoses. 2006;49:143-144.
- Reinholz M, Hermans C, Dietrich A, et al. A case of cutaneous vegetating candidiasis in a patient with keratitis-ichthyosis-deafness syndrome. J Eur Acad Dermatol Venereol. 2016;30:537-539.
THE DIAGNOSIS: Vegetative Majocchi Granuloma
A biopsy and tissue culture showed acute dermal inflammation with granulomatous features and numerous fungal hyphae within the stratum corneum (Figure 1A), which were confirmed on GrocottGomori methenamine-silver staining (Figure 1B). Gram and Fite stains were negative for bacteria. A tissue culture speciated Trichophyton rubrum, which led to a diagnosis of deep dermatophyte infection (Majocchi granuloma) with a highly unusual clinical presentation of vegetative plaques. Predisposing factors included treatment with topical corticosteroids and possibly poor health and nutritional status at baseline. Our patient was treated with fluconazole 200 mg daily for 6 weeks, with near resolution of lesions at 3-week follow-up (Figure 2).


Dermatophytes are a common cause of superficial skin infections. The classic morphology consists of an annular scaly plaque; however, a wide variety of presentations have been observed (eg, verrucous, vesicular, pustular, granulomatous). Therefore, dermatophyte infections often mimic other dermatologic conditions, including atopic dermatitis, rosacea, psoriasis, bacterial abscess, erythema gyratum repens, lupus, granuloma annulare, cutaneous lymphoma, Hailey-Hailey disease, scarring alopecia, and syphilis.1
Notably, when dermatophytes grow downward along hair follicles causing deeper infection, disruption of the follicular wall can lead to an excessive inflammatory response with granulomatous features.2 Risk factors include cutaneous trauma, long-standing infection, immunocompromise, and treatment with topical corticosteroids.3 This disease evolution clinically appears as a nodule or infiltrated plaque, often without scale. The most well-known example is a kerion on the scalp. Elsewhere on the body, lesions often are termed Majocchi granulomas.2
Vegetative plaques, as seen in our patient, are a highly unusual morphology for deep tinea infection. Guanziroli et al4 reported a case of vegetative lesions on the forearm of a 67-year-old immunocompromised man that were successfully treated with a 3-month course of oral terbinafine after Trichophyton verrucosum was isolated. Skorepova et al5 reported a case of pyoderma vegetans triggered by recurrent Trichophyton mentagrophytes on the dorsal hands of a 64-year-old man with immunoglobulin deficiency of unknown etiology. The lesions were successfully treated with a prolonged course of doxycycline, topical triamcinolone, and intravenous immunoglobulin following 2 initial courses of terbinafine.
The differential diagnosis for vegetative lesions includes pemphigus vegetans, a vegetative variant of pyoderma gangrenosum; halogenoderma; and a variety of infections, including dimorphic fungi (histoplasmosis, blastomycosis), blastomycosislike pyoderma (bacterial), and candidiasis.6 These conditions usually can be distinguished based on histopathology. Clinically, pemphigus vegetans presents with pustules and vegetative lesions, as in our patient, but usually is more diffuse and favors the intertriginous areas. Histology likely would reveal foci of acantholysis and eosinophils. Vegetative pyoderma gangrenosum favors the trunk, particularly in sites of surgical trauma. In our patient, no lesions were present near the abdominal surgical sites, and there was no antecedent cribriform ulceration. Halogenoderma was a strong initial consideration given the localization, presence of large pustules, and history of numerous contrast computed tomography studies; however, our patient’s iodine levels were normal. Infectious etiologies including dimorphic fungi and blastomycosislike pyoderma generally are not restricted to the head and neck, and tissue culture helps exclude them. Vegetative lesions may occur in the setting of other infections, and tissue culture may be necessary to differentiate them if histopathology is not suggestive.
Deep dermatophyte infections require treatment with oral antifungals, as topicals do not penetrate adequately into the hair follicles. Exact regimens vary, but generally oral terbinafine or an oral azole (except ketoconazole) is administered for 2 to 6 weeks, with immunocompromise necessitating longer courses.
We present a rare case of vegetative Majocchi granuloma secondary to T rubrum infection. A dermatophyte infection should be included in the differential for vegetative lesions, especially in dense hair-bearing areas such as the beard. Treatment generally is straightforward with oral antifungals.
THE DIAGNOSIS: Vegetative Majocchi Granuloma
A biopsy and tissue culture showed acute dermal inflammation with granulomatous features and numerous fungal hyphae within the stratum corneum (Figure 1A), which were confirmed on GrocottGomori methenamine-silver staining (Figure 1B). Gram and Fite stains were negative for bacteria. A tissue culture speciated Trichophyton rubrum, which led to a diagnosis of deep dermatophyte infection (Majocchi granuloma) with a highly unusual clinical presentation of vegetative plaques. Predisposing factors included treatment with topical corticosteroids and possibly poor health and nutritional status at baseline. Our patient was treated with fluconazole 200 mg daily for 6 weeks, with near resolution of lesions at 3-week follow-up (Figure 2).


Dermatophytes are a common cause of superficial skin infections. The classic morphology consists of an annular scaly plaque; however, a wide variety of presentations have been observed (eg, verrucous, vesicular, pustular, granulomatous). Therefore, dermatophyte infections often mimic other dermatologic conditions, including atopic dermatitis, rosacea, psoriasis, bacterial abscess, erythema gyratum repens, lupus, granuloma annulare, cutaneous lymphoma, Hailey-Hailey disease, scarring alopecia, and syphilis.1
Notably, when dermatophytes grow downward along hair follicles causing deeper infection, disruption of the follicular wall can lead to an excessive inflammatory response with granulomatous features.2 Risk factors include cutaneous trauma, long-standing infection, immunocompromise, and treatment with topical corticosteroids.3 This disease evolution clinically appears as a nodule or infiltrated plaque, often without scale. The most well-known example is a kerion on the scalp. Elsewhere on the body, lesions often are termed Majocchi granulomas.2
Vegetative plaques, as seen in our patient, are a highly unusual morphology for deep tinea infection. Guanziroli et al4 reported a case of vegetative lesions on the forearm of a 67-year-old immunocompromised man that were successfully treated with a 3-month course of oral terbinafine after Trichophyton verrucosum was isolated. Skorepova et al5 reported a case of pyoderma vegetans triggered by recurrent Trichophyton mentagrophytes on the dorsal hands of a 64-year-old man with immunoglobulin deficiency of unknown etiology. The lesions were successfully treated with a prolonged course of doxycycline, topical triamcinolone, and intravenous immunoglobulin following 2 initial courses of terbinafine.
The differential diagnosis for vegetative lesions includes pemphigus vegetans, a vegetative variant of pyoderma gangrenosum; halogenoderma; and a variety of infections, including dimorphic fungi (histoplasmosis, blastomycosis), blastomycosislike pyoderma (bacterial), and candidiasis.6 These conditions usually can be distinguished based on histopathology. Clinically, pemphigus vegetans presents with pustules and vegetative lesions, as in our patient, but usually is more diffuse and favors the intertriginous areas. Histology likely would reveal foci of acantholysis and eosinophils. Vegetative pyoderma gangrenosum favors the trunk, particularly in sites of surgical trauma. In our patient, no lesions were present near the abdominal surgical sites, and there was no antecedent cribriform ulceration. Halogenoderma was a strong initial consideration given the localization, presence of large pustules, and history of numerous contrast computed tomography studies; however, our patient’s iodine levels were normal. Infectious etiologies including dimorphic fungi and blastomycosislike pyoderma generally are not restricted to the head and neck, and tissue culture helps exclude them. Vegetative lesions may occur in the setting of other infections, and tissue culture may be necessary to differentiate them if histopathology is not suggestive.
Deep dermatophyte infections require treatment with oral antifungals, as topicals do not penetrate adequately into the hair follicles. Exact regimens vary, but generally oral terbinafine or an oral azole (except ketoconazole) is administered for 2 to 6 weeks, with immunocompromise necessitating longer courses.
We present a rare case of vegetative Majocchi granuloma secondary to T rubrum infection. A dermatophyte infection should be included in the differential for vegetative lesions, especially in dense hair-bearing areas such as the beard. Treatment generally is straightforward with oral antifungals.
- Atzori L, Pau M, Aste N, et al. Dermatophyte infections mimicking other skin diseases: a 154-person case survey of tinea atypica in the district of Cagliari (Italy). Int J Dermatol. 2012;51:410-415.
- Ilkit M, Durdu M, Karakas M. Majocchi’s granuloma: a symptom complex caused by fungal pathogens. Med Mycol. 2012;50:449-457.
- Jevremovic L, Ilijin I, Kostic K, et al. Pyoderma vegetans—a case report. Serbian J Dermatol Venereol. 2017;9:22-28.
- Guanziroli E, Pavia G, Guttadauro A, et al. Deep dermatophytosis caused by Trichophyton verrucosum in an immunosuppressed patient: successful outcome with terbinafine. Mycopathologia. 2019;184:543-545.
- Skorepová M, Stuchlík D. Chronic pyoderma vegetans triggered by Trichophyton mentagrophytes. Mycoses. 2006;49:143-144.
- Reinholz M, Hermans C, Dietrich A, et al. A case of cutaneous vegetating candidiasis in a patient with keratitis-ichthyosis-deafness syndrome. J Eur Acad Dermatol Venereol. 2016;30:537-539.
- Atzori L, Pau M, Aste N, et al. Dermatophyte infections mimicking other skin diseases: a 154-person case survey of tinea atypica in the district of Cagliari (Italy). Int J Dermatol. 2012;51:410-415.
- Ilkit M, Durdu M, Karakas M. Majocchi’s granuloma: a symptom complex caused by fungal pathogens. Med Mycol. 2012;50:449-457.
- Jevremovic L, Ilijin I, Kostic K, et al. Pyoderma vegetans—a case report. Serbian J Dermatol Venereol. 2017;9:22-28.
- Guanziroli E, Pavia G, Guttadauro A, et al. Deep dermatophytosis caused by Trichophyton verrucosum in an immunosuppressed patient: successful outcome with terbinafine. Mycopathologia. 2019;184:543-545.
- Skorepová M, Stuchlík D. Chronic pyoderma vegetans triggered by Trichophyton mentagrophytes. Mycoses. 2006;49:143-144.
- Reinholz M, Hermans C, Dietrich A, et al. A case of cutaneous vegetating candidiasis in a patient with keratitis-ichthyosis-deafness syndrome. J Eur Acad Dermatol Venereol. 2016;30:537-539.
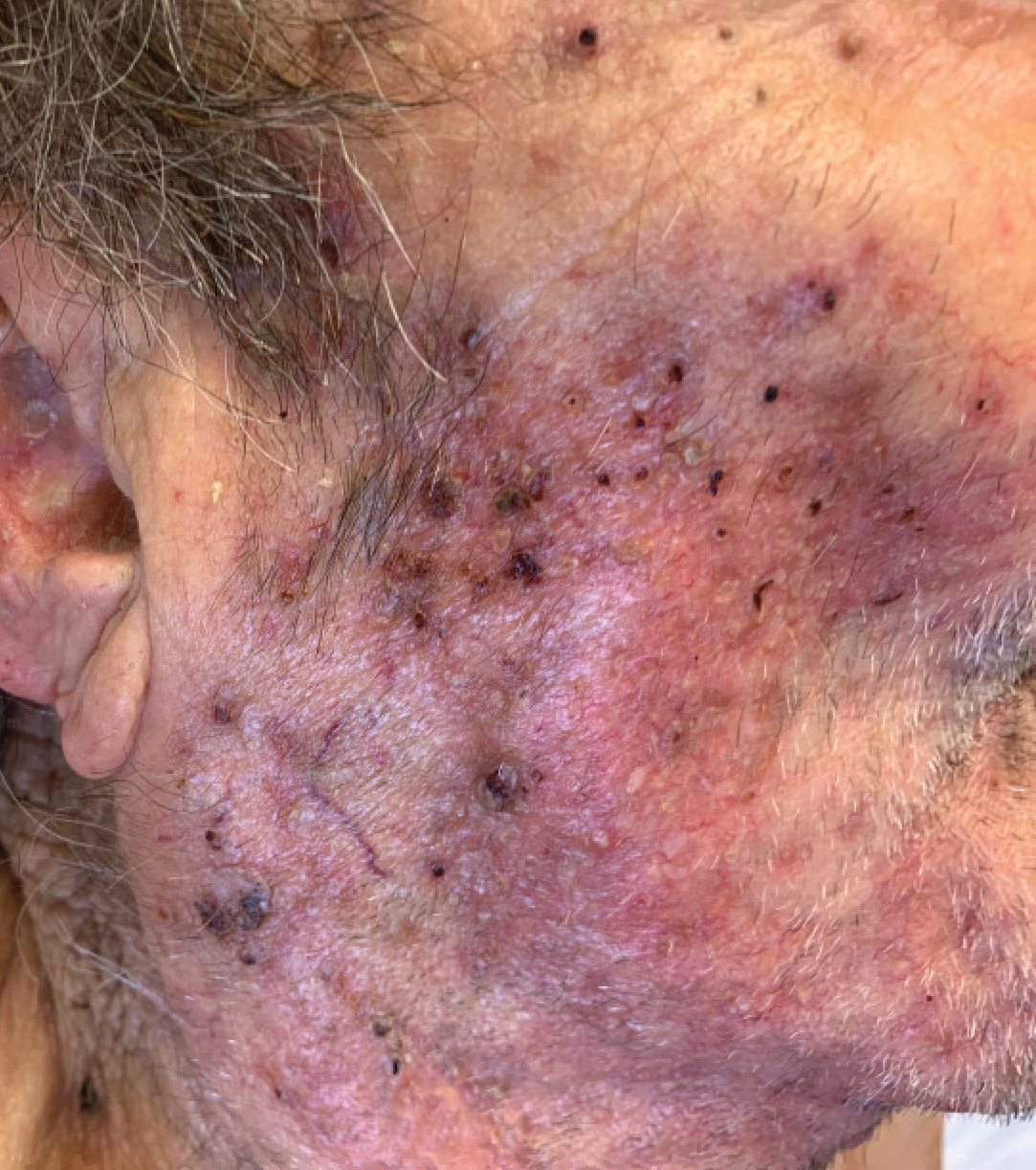
An 86-year-old man was admitted to the hospital for sigmoid colon perforation secondary to ischemic colitis. His medical history consisted of sequelae from atherosclerotic vascular disease. He had no known personal or family history of skin disease. His bowel perforation was surgically repaired, and his clinical status was stabilized, enabling transfer to a transitional care hospital. His course was complicated by delayed healing of the midline abdominal surgical wounds, leading to multiple computed tomography studies with iodinated contrast. One week following arrival at the transitional care hospital, he was noted to have a pustular rash on the face. He was empirically treated with topical steroids, mupirocin, and sulfacetamide. The rash did not improve, and the appearance changed, at which point dermatology was consulted. On evaluation, the patient was afebrile with a normal white blood cell count. Physical examination revealed gray-brown, moist, vegetative plaques on the cheeks with a few large pustules as well as similar-appearing lesions on the neck and upper chest. Attempted removal of a portion of the plaque left an erosion.
Booster recommendations for pregnant women, teens, and other groups explained
These recommendations have been widened because of the continued emergence of new variants of the virus and the wane of protection over time for both vaccinations and previous disease.
The new recommendations take away some of the questions surrounding eligibility for booster vaccinations while potentially leaving some additional questions. All in all, they provide flexibility for individuals to help protect themselves against the COVID-19 virus, as many are considering celebrating the holidays with friends and family.
The first item that has become clear is that all individuals over 18 are now not only eligible for a booster vaccination a certain time after they have completed their series, but have a recommendation for one.1
But what about a fourth dose? There is a possibility that some patients should be receiving one. For those who require a three-dose series due to a condition that makes them immunocompromised, they should receive their booster vaccination six months after completion of the three-dose series. This distinction may cause confusion for some, but is important for those immunocompromised.
Boosters in women who are pregnant
The recommendations also include specific comments about individuals who are pregnant. Although initial studies did not include pregnant individuals, there has been increasing real world data that vaccination against COVID, including booster vaccinations, is safe and recommended. As pregnancy increases the risk of severe disease if infected by COVID-19, both the CDC and the American College of Obstetricians and Gynecologists,2 along with other specialty organizations, such as the Royal College of Obstetricians and Gynaecologists, recommend vaccinations for pregnant individuals.
The CDC goes on to describe that there is no evidence of vaccination increasing the risk of infertility. The vaccine protects the pregnant individual and also provides protection to the baby once born. The same is true of breastfeeding individuals.3
I hope that this information allows physicians to feel comfortable recommending vaccinations and boosters to those who are pregnant and breast feeding.
Expanded recommendations for those aged 16-17 years
Recently, the CDC also expanded booster recommendations to include those aged 16-17 years, 6 months after completing their vaccine series.
Those under 18 are currently only able to receive the Pfizer-BioNtech vaccine. This new guidance has left some parents wondering if there will also be approval for booster vaccinations soon for those aged 12-16 who are approaching or have reached six months past the initial vaccine.1
Booster brand for those over 18 years?
Although the recommendation has been simplified for all over age 18 years, there is still a decision to be made about which vaccine to use as the booster.
The recommendations allow individuals to decide which brand of vaccine they would like to have as a booster. They may choose to be vaccinated with the same vaccine they originally received or with a different vaccine. This vaccine flexibility may cause confusion, but ultimately is a good thing as it allows individuals to receive whatever vaccine is available and most convenient. This also allows individuals who have been vaccinated outside of the United States by a different brand of vaccine to also receive a booster vaccination with one of the options available here.
Take home message
Overall, the expansion of booster recommendations will help everyone avoid severe disease from COVID-19 infections. Physicians now have more clarity on who should be receiving these vaccines. Along with testing, masking, and appropriate distancing, these recommendations should help prevent severe disease and death from COVID-19.
Dr. Wheat is a family physician at Erie Family Health Center in Chicago. She is program director of Northwestern’s McGaw Family Medicine residency program, also in Chicago. Dr. Wheat serves on the editorial advisory board of Family Practice News. You can contact her at [email protected].
References
1. COVID-19 Vaccine Booster Shots. Centers for Disease Control and Prevention. 2021 Dec 9.
2. COVID-19 Vaccines and Pregnancy: Conversation Guide. American College of Obstetricians and Gynecologists. 2021 November.
3. COVID-19 Vaccines While Pregnant or Breastfeeding. Centers for Disease Control and Prevention. 2021 Dec 6.
These recommendations have been widened because of the continued emergence of new variants of the virus and the wane of protection over time for both vaccinations and previous disease.
The new recommendations take away some of the questions surrounding eligibility for booster vaccinations while potentially leaving some additional questions. All in all, they provide flexibility for individuals to help protect themselves against the COVID-19 virus, as many are considering celebrating the holidays with friends and family.
The first item that has become clear is that all individuals over 18 are now not only eligible for a booster vaccination a certain time after they have completed their series, but have a recommendation for one.1
But what about a fourth dose? There is a possibility that some patients should be receiving one. For those who require a three-dose series due to a condition that makes them immunocompromised, they should receive their booster vaccination six months after completion of the three-dose series. This distinction may cause confusion for some, but is important for those immunocompromised.
Boosters in women who are pregnant
The recommendations also include specific comments about individuals who are pregnant. Although initial studies did not include pregnant individuals, there has been increasing real world data that vaccination against COVID, including booster vaccinations, is safe and recommended. As pregnancy increases the risk of severe disease if infected by COVID-19, both the CDC and the American College of Obstetricians and Gynecologists,2 along with other specialty organizations, such as the Royal College of Obstetricians and Gynaecologists, recommend vaccinations for pregnant individuals.
The CDC goes on to describe that there is no evidence of vaccination increasing the risk of infertility. The vaccine protects the pregnant individual and also provides protection to the baby once born. The same is true of breastfeeding individuals.3
I hope that this information allows physicians to feel comfortable recommending vaccinations and boosters to those who are pregnant and breast feeding.
Expanded recommendations for those aged 16-17 years
Recently, the CDC also expanded booster recommendations to include those aged 16-17 years, 6 months after completing their vaccine series.
Those under 18 are currently only able to receive the Pfizer-BioNtech vaccine. This new guidance has left some parents wondering if there will also be approval for booster vaccinations soon for those aged 12-16 who are approaching or have reached six months past the initial vaccine.1
Booster brand for those over 18 years?
Although the recommendation has been simplified for all over age 18 years, there is still a decision to be made about which vaccine to use as the booster.
The recommendations allow individuals to decide which brand of vaccine they would like to have as a booster. They may choose to be vaccinated with the same vaccine they originally received or with a different vaccine. This vaccine flexibility may cause confusion, but ultimately is a good thing as it allows individuals to receive whatever vaccine is available and most convenient. This also allows individuals who have been vaccinated outside of the United States by a different brand of vaccine to also receive a booster vaccination with one of the options available here.
Take home message
Overall, the expansion of booster recommendations will help everyone avoid severe disease from COVID-19 infections. Physicians now have more clarity on who should be receiving these vaccines. Along with testing, masking, and appropriate distancing, these recommendations should help prevent severe disease and death from COVID-19.
Dr. Wheat is a family physician at Erie Family Health Center in Chicago. She is program director of Northwestern’s McGaw Family Medicine residency program, also in Chicago. Dr. Wheat serves on the editorial advisory board of Family Practice News. You can contact her at [email protected].
References
1. COVID-19 Vaccine Booster Shots. Centers for Disease Control and Prevention. 2021 Dec 9.
2. COVID-19 Vaccines and Pregnancy: Conversation Guide. American College of Obstetricians and Gynecologists. 2021 November.
3. COVID-19 Vaccines While Pregnant or Breastfeeding. Centers for Disease Control and Prevention. 2021 Dec 6.
These recommendations have been widened because of the continued emergence of new variants of the virus and the wane of protection over time for both vaccinations and previous disease.
The new recommendations take away some of the questions surrounding eligibility for booster vaccinations while potentially leaving some additional questions. All in all, they provide flexibility for individuals to help protect themselves against the COVID-19 virus, as many are considering celebrating the holidays with friends and family.
The first item that has become clear is that all individuals over 18 are now not only eligible for a booster vaccination a certain time after they have completed their series, but have a recommendation for one.1
But what about a fourth dose? There is a possibility that some patients should be receiving one. For those who require a three-dose series due to a condition that makes them immunocompromised, they should receive their booster vaccination six months after completion of the three-dose series. This distinction may cause confusion for some, but is important for those immunocompromised.
Boosters in women who are pregnant
The recommendations also include specific comments about individuals who are pregnant. Although initial studies did not include pregnant individuals, there has been increasing real world data that vaccination against COVID, including booster vaccinations, is safe and recommended. As pregnancy increases the risk of severe disease if infected by COVID-19, both the CDC and the American College of Obstetricians and Gynecologists,2 along with other specialty organizations, such as the Royal College of Obstetricians and Gynaecologists, recommend vaccinations for pregnant individuals.
The CDC goes on to describe that there is no evidence of vaccination increasing the risk of infertility. The vaccine protects the pregnant individual and also provides protection to the baby once born. The same is true of breastfeeding individuals.3
I hope that this information allows physicians to feel comfortable recommending vaccinations and boosters to those who are pregnant and breast feeding.
Expanded recommendations for those aged 16-17 years
Recently, the CDC also expanded booster recommendations to include those aged 16-17 years, 6 months after completing their vaccine series.
Those under 18 are currently only able to receive the Pfizer-BioNtech vaccine. This new guidance has left some parents wondering if there will also be approval for booster vaccinations soon for those aged 12-16 who are approaching or have reached six months past the initial vaccine.1
Booster brand for those over 18 years?
Although the recommendation has been simplified for all over age 18 years, there is still a decision to be made about which vaccine to use as the booster.
The recommendations allow individuals to decide which brand of vaccine they would like to have as a booster. They may choose to be vaccinated with the same vaccine they originally received or with a different vaccine. This vaccine flexibility may cause confusion, but ultimately is a good thing as it allows individuals to receive whatever vaccine is available and most convenient. This also allows individuals who have been vaccinated outside of the United States by a different brand of vaccine to also receive a booster vaccination with one of the options available here.
Take home message
Overall, the expansion of booster recommendations will help everyone avoid severe disease from COVID-19 infections. Physicians now have more clarity on who should be receiving these vaccines. Along with testing, masking, and appropriate distancing, these recommendations should help prevent severe disease and death from COVID-19.
Dr. Wheat is a family physician at Erie Family Health Center in Chicago. She is program director of Northwestern’s McGaw Family Medicine residency program, also in Chicago. Dr. Wheat serves on the editorial advisory board of Family Practice News. You can contact her at [email protected].
References
1. COVID-19 Vaccine Booster Shots. Centers for Disease Control and Prevention. 2021 Dec 9.
2. COVID-19 Vaccines and Pregnancy: Conversation Guide. American College of Obstetricians and Gynecologists. 2021 November.
3. COVID-19 Vaccines While Pregnant or Breastfeeding. Centers for Disease Control and Prevention. 2021 Dec 6.
12 state boards have disciplined docs for COVID misinformation
, according to a new survey from the Federation of State Medical Boards (FSMB).
The FSMB reports that in its 2021 annual survey two-thirds of its 71 member boards (which includes the United States, its territories, and Washington, DC) reported an increase in complaints about doctors spreading false or misleading information.
“The staggering number of state medical boards that have seen an increase in COVID-19 disinformation complaints is a sign of how widespread the issue has become,” said Humayun J. Chaudhry, DO, MACP, president and CEO of the FSMB, in a statement.
The FSMB board of directors warned physicians in July that they risked disciplinary action if they spread COVID-19 vaccine misinformation or disinformation.
The organization said 15 state boards have now adopted similar statements.
Dr. Chaudhry said the FSMB was “encouraged by the number of boards that have already taken action to combat COVID-19 disinformation by disciplining physicians who engage in that behavior and by reminding all physicians that their words and actions matter, and they should think twice before spreading disinformation that may harm patients.”
This news organization asked the FSMB for further comment on why more physicians have not been disciplined, but did not receive a response before publication.
Misinformation policies a new battleground
The FSMB and member board policies on COVID-19 around the country have become a new front in the war against mandates and restrictions.
The Tennessee Board of Medical Examiners voted just recently to remove its statement of policy against the spread of misinformation from its website after a Republican lawmaker allegedly threatened to dissolve the board.
The vote came just a few months after the board had approved the policy. The board did not rescind the policy, however, according to a report by the Associated Press.
In California, the president of the state’s medical board tweeted on December 8 about what she said was an incident of harassment by a group that has promoted “fake COVID-19 treatments.”Ms. Kristina Lawson said she observed four men sitting in front of her house in a truck. They flew a drone over her residence, and then followed her to work, parking nose-to-nose with her vehicle.
Ms. Lawson claimed that when she went to drive home the four men ambushed her in what was by then a dark parking garage. She said her “concern turned to terror” as they jumped out, cameras and recording equipment in hand.
The men told law enforcement called to the scene that they were just trying to interview her, according to a statement emailed by Ms. Lawson.
They had not made such a request to the California Medical Board.
Ms. Lawson tweeted that she would continue to volunteer for the board. “That means protecting Californians from bad doctors, and ensuring disinformation and misinformation do not detract from our work to protect patients and consumers,” she wrote.
The men who ambushed Ms. Larson allegedly identified themselves and were wearing clothing emblazoned with the logo of “America’s Frontline Doctors,” an organization that has trafficked in COVID-19 conspiracy theories and promoted unproven treatments like hydroxychloroquine and ivermectin, according to Time. It is led by Simone Gold, MD, who was arrested for breaching the U.S. Capitol on January 6.
Despite her activities, on November 30, the California Medical Board renewed Ms. Gold’s 2-year license to practice.
Who’s being disciplined, who’s not
Dr. Gold is not alone. An investigation by NPRin September found that 15 of 16 physicians who have spread false information in a high-profile manner have medical licenses in good standing.
Sherri Tenpenny, DO, who has claimed that COVID-19 vaccines magnetize people and “interface” with 5G cell phone towers, was able to renew her license with the Ohio State Medical Board on October 1, according to the Cincinnati Enquirer.
Some boards have acted. The Oregon Medical Board revoked the license of Steven LaTulippe, MD, and fined him $10,000 for spreading misinformation about masks and overprescribing opioids.
In August, Rhode Island’s Board of Medical Licensure suspended Mark Brody’s license for 5 years after finding that the doctor spread falsehoods about COVID-19 vaccines, according to board documents.
Maine physician Paul Gosselin, DO, is on temporary suspension until a February hearing, while the osteopathic board investigates his issuance of vaccine exemption letters and the promotion of unproven COVID-19 therapies.
The board found that Gosselin had “engaged in conduct that constitutes fraud or deceit,” according to official documents.
The Washington State Medical Board has opened an investigation into Ryan N. Cole, MD, a physician who has claimed that COVID vaccines are “fake,” and was appointed to a regional health board in Idaho in September, according to the Washington Post.
The Idaho Capital Sun reported that Dr. Cole claims he is licensed in 11 states, including Washington. The Idaho Medical Association has also filed a complaint about Dr. Cole with the Idaho Board of Medicine, according to the paper.
New FSMB guidance coming
The FSMB said it expects more disciplinary actions as investigations continue to unfold.
The organization is drafting a new policy document that will include further guidelines and recommendations for state medical boards “to help address the spread of disinformation,” it said. The final document would be released in April 2022.
In the meantime, some states, like Tennessee and others, are trying to find ways to counter the current policy — a development the FSMB called “troubling.”
“The FSMB strongly opposes any effort to restrict a board’s authority to evaluate the standard of care and assess risk for patient harm,” the organization said in its statement.
A version of this article was first published on Medscape.com.
, according to a new survey from the Federation of State Medical Boards (FSMB).
The FSMB reports that in its 2021 annual survey two-thirds of its 71 member boards (which includes the United States, its territories, and Washington, DC) reported an increase in complaints about doctors spreading false or misleading information.
“The staggering number of state medical boards that have seen an increase in COVID-19 disinformation complaints is a sign of how widespread the issue has become,” said Humayun J. Chaudhry, DO, MACP, president and CEO of the FSMB, in a statement.
The FSMB board of directors warned physicians in July that they risked disciplinary action if they spread COVID-19 vaccine misinformation or disinformation.
The organization said 15 state boards have now adopted similar statements.
Dr. Chaudhry said the FSMB was “encouraged by the number of boards that have already taken action to combat COVID-19 disinformation by disciplining physicians who engage in that behavior and by reminding all physicians that their words and actions matter, and they should think twice before spreading disinformation that may harm patients.”
This news organization asked the FSMB for further comment on why more physicians have not been disciplined, but did not receive a response before publication.
Misinformation policies a new battleground
The FSMB and member board policies on COVID-19 around the country have become a new front in the war against mandates and restrictions.
The Tennessee Board of Medical Examiners voted just recently to remove its statement of policy against the spread of misinformation from its website after a Republican lawmaker allegedly threatened to dissolve the board.
The vote came just a few months after the board had approved the policy. The board did not rescind the policy, however, according to a report by the Associated Press.
In California, the president of the state’s medical board tweeted on December 8 about what she said was an incident of harassment by a group that has promoted “fake COVID-19 treatments.”Ms. Kristina Lawson said she observed four men sitting in front of her house in a truck. They flew a drone over her residence, and then followed her to work, parking nose-to-nose with her vehicle.
Ms. Lawson claimed that when she went to drive home the four men ambushed her in what was by then a dark parking garage. She said her “concern turned to terror” as they jumped out, cameras and recording equipment in hand.
The men told law enforcement called to the scene that they were just trying to interview her, according to a statement emailed by Ms. Lawson.
They had not made such a request to the California Medical Board.
Ms. Lawson tweeted that she would continue to volunteer for the board. “That means protecting Californians from bad doctors, and ensuring disinformation and misinformation do not detract from our work to protect patients and consumers,” she wrote.
The men who ambushed Ms. Larson allegedly identified themselves and were wearing clothing emblazoned with the logo of “America’s Frontline Doctors,” an organization that has trafficked in COVID-19 conspiracy theories and promoted unproven treatments like hydroxychloroquine and ivermectin, according to Time. It is led by Simone Gold, MD, who was arrested for breaching the U.S. Capitol on January 6.
Despite her activities, on November 30, the California Medical Board renewed Ms. Gold’s 2-year license to practice.
Who’s being disciplined, who’s not
Dr. Gold is not alone. An investigation by NPRin September found that 15 of 16 physicians who have spread false information in a high-profile manner have medical licenses in good standing.
Sherri Tenpenny, DO, who has claimed that COVID-19 vaccines magnetize people and “interface” with 5G cell phone towers, was able to renew her license with the Ohio State Medical Board on October 1, according to the Cincinnati Enquirer.
Some boards have acted. The Oregon Medical Board revoked the license of Steven LaTulippe, MD, and fined him $10,000 for spreading misinformation about masks and overprescribing opioids.
In August, Rhode Island’s Board of Medical Licensure suspended Mark Brody’s license for 5 years after finding that the doctor spread falsehoods about COVID-19 vaccines, according to board documents.
Maine physician Paul Gosselin, DO, is on temporary suspension until a February hearing, while the osteopathic board investigates his issuance of vaccine exemption letters and the promotion of unproven COVID-19 therapies.
The board found that Gosselin had “engaged in conduct that constitutes fraud or deceit,” according to official documents.
The Washington State Medical Board has opened an investigation into Ryan N. Cole, MD, a physician who has claimed that COVID vaccines are “fake,” and was appointed to a regional health board in Idaho in September, according to the Washington Post.
The Idaho Capital Sun reported that Dr. Cole claims he is licensed in 11 states, including Washington. The Idaho Medical Association has also filed a complaint about Dr. Cole with the Idaho Board of Medicine, according to the paper.
New FSMB guidance coming
The FSMB said it expects more disciplinary actions as investigations continue to unfold.
The organization is drafting a new policy document that will include further guidelines and recommendations for state medical boards “to help address the spread of disinformation,” it said. The final document would be released in April 2022.
In the meantime, some states, like Tennessee and others, are trying to find ways to counter the current policy — a development the FSMB called “troubling.”
“The FSMB strongly opposes any effort to restrict a board’s authority to evaluate the standard of care and assess risk for patient harm,” the organization said in its statement.
A version of this article was first published on Medscape.com.
, according to a new survey from the Federation of State Medical Boards (FSMB).
The FSMB reports that in its 2021 annual survey two-thirds of its 71 member boards (which includes the United States, its territories, and Washington, DC) reported an increase in complaints about doctors spreading false or misleading information.
“The staggering number of state medical boards that have seen an increase in COVID-19 disinformation complaints is a sign of how widespread the issue has become,” said Humayun J. Chaudhry, DO, MACP, president and CEO of the FSMB, in a statement.
The FSMB board of directors warned physicians in July that they risked disciplinary action if they spread COVID-19 vaccine misinformation or disinformation.
The organization said 15 state boards have now adopted similar statements.
Dr. Chaudhry said the FSMB was “encouraged by the number of boards that have already taken action to combat COVID-19 disinformation by disciplining physicians who engage in that behavior and by reminding all physicians that their words and actions matter, and they should think twice before spreading disinformation that may harm patients.”
This news organization asked the FSMB for further comment on why more physicians have not been disciplined, but did not receive a response before publication.
Misinformation policies a new battleground
The FSMB and member board policies on COVID-19 around the country have become a new front in the war against mandates and restrictions.
The Tennessee Board of Medical Examiners voted just recently to remove its statement of policy against the spread of misinformation from its website after a Republican lawmaker allegedly threatened to dissolve the board.
The vote came just a few months after the board had approved the policy. The board did not rescind the policy, however, according to a report by the Associated Press.
In California, the president of the state’s medical board tweeted on December 8 about what she said was an incident of harassment by a group that has promoted “fake COVID-19 treatments.”Ms. Kristina Lawson said she observed four men sitting in front of her house in a truck. They flew a drone over her residence, and then followed her to work, parking nose-to-nose with her vehicle.
Ms. Lawson claimed that when she went to drive home the four men ambushed her in what was by then a dark parking garage. She said her “concern turned to terror” as they jumped out, cameras and recording equipment in hand.
The men told law enforcement called to the scene that they were just trying to interview her, according to a statement emailed by Ms. Lawson.
They had not made such a request to the California Medical Board.
Ms. Lawson tweeted that she would continue to volunteer for the board. “That means protecting Californians from bad doctors, and ensuring disinformation and misinformation do not detract from our work to protect patients and consumers,” she wrote.
The men who ambushed Ms. Larson allegedly identified themselves and were wearing clothing emblazoned with the logo of “America’s Frontline Doctors,” an organization that has trafficked in COVID-19 conspiracy theories and promoted unproven treatments like hydroxychloroquine and ivermectin, according to Time. It is led by Simone Gold, MD, who was arrested for breaching the U.S. Capitol on January 6.
Despite her activities, on November 30, the California Medical Board renewed Ms. Gold’s 2-year license to practice.
Who’s being disciplined, who’s not
Dr. Gold is not alone. An investigation by NPRin September found that 15 of 16 physicians who have spread false information in a high-profile manner have medical licenses in good standing.
Sherri Tenpenny, DO, who has claimed that COVID-19 vaccines magnetize people and “interface” with 5G cell phone towers, was able to renew her license with the Ohio State Medical Board on October 1, according to the Cincinnati Enquirer.
Some boards have acted. The Oregon Medical Board revoked the license of Steven LaTulippe, MD, and fined him $10,000 for spreading misinformation about masks and overprescribing opioids.
In August, Rhode Island’s Board of Medical Licensure suspended Mark Brody’s license for 5 years after finding that the doctor spread falsehoods about COVID-19 vaccines, according to board documents.
Maine physician Paul Gosselin, DO, is on temporary suspension until a February hearing, while the osteopathic board investigates his issuance of vaccine exemption letters and the promotion of unproven COVID-19 therapies.
The board found that Gosselin had “engaged in conduct that constitutes fraud or deceit,” according to official documents.
The Washington State Medical Board has opened an investigation into Ryan N. Cole, MD, a physician who has claimed that COVID vaccines are “fake,” and was appointed to a regional health board in Idaho in September, according to the Washington Post.
The Idaho Capital Sun reported that Dr. Cole claims he is licensed in 11 states, including Washington. The Idaho Medical Association has also filed a complaint about Dr. Cole with the Idaho Board of Medicine, according to the paper.
New FSMB guidance coming
The FSMB said it expects more disciplinary actions as investigations continue to unfold.
The organization is drafting a new policy document that will include further guidelines and recommendations for state medical boards “to help address the spread of disinformation,” it said. The final document would be released in April 2022.
In the meantime, some states, like Tennessee and others, are trying to find ways to counter the current policy — a development the FSMB called “troubling.”
“The FSMB strongly opposes any effort to restrict a board’s authority to evaluate the standard of care and assess risk for patient harm,” the organization said in its statement.
A version of this article was first published on Medscape.com.
Lung transplantation in the era of COVID-19: New issues and paradigms
Data is sparse thus far, but there is concern in lung transplant medicine about the long-term risk of chronic lung allograft dysfunction (CLAD) and a potentially shortened longevity of transplanted lungs in recipients who become ill with COVID-19.
“My fear is that we’re potentially sitting on this iceberg worth of people who, come 6 months or a year from [the acute phase of] their COVID illness, will in fact have earlier and progressive, chronic rejection,” said Cameron R. Wolfe, MBBS, MPH, associate professor of medicine in transplant infectious disease at Duke University, Durham, N.C.
Lower respiratory viral infections have long been concerning for lung transplant recipients given their propensity to cause scarring, a decline in lung function, and a heightened risk of allograft rejection. Time will tell whether lung transplant recipients who survive COVID-19 follow a similar path, or one that is worse, he said.
Short-term data
Outcomes beyond hospitalization and acute illness for lung transplant recipients affected by COVID-19 have been reported in the literature by only a few lung transplant programs. These reports – as well as anecdotal experiences being informally shared among transplant programs – have raised the specter of more severe dysfunction following the acute phase and more early CLAD, said Tathagat Narula, MD, assistant professor of medicine at the Mayo Medical School, Rochester, Minn., and a consultant in lung transplantation at the Mayo Clinic’s Jacksonville program.
“The available data cover only 3-6 months out. We don’t know what will happen in the next 6 months and beyond,” Dr. Narula said in an interview.
The risks of COVID-19 in already-transplanted patients and issues relating to the inadequate antibody responses to vaccination are just some of the challenges of lung transplant medicine in the era of SARS-CoV-2. “COVID-19,” said Dr. Narula, “has completely changed the way we practice lung transplant medicine – the way we’re looking both at our recipients and our donors.”
Potential donors are being evaluated with lower respiratory SARS-CoV-2 testing and an abundance of caution. And patients with severe COVID-19 affecting their own lungs are roundly expected to drive up lung transplant volume in the near future. “The whole paradigm has changed,” Dr. Narula said.
Post-acute trajectories
A chart review study published in October by the lung transplant team at the University of Texas Southwestern Medical Center, Dallas, covered 44 consecutive survivors at a median follow-up of 4.5 months from hospital discharge or acute illness (the survival rate was 83.3%). Patients had significantly impaired functional status, and 18 of the 44 (40.9%) had a significant and persistent loss of forced vital capacity or forced expiratory volume in 1 second (>10% from pre–COVID-19 baseline).
Three patients met the criteria for new CLAD after COVID-19 infection, with all three classified as restrictive allograft syndrome (RAS) phenotype.
Moreover, the majority of COVID-19 survivors who had CT chest scans (22 of 28) showed persistent parenchymal opacities – a finding that, regardless of symptomatology, suggests persistent allograft injury, said Amit Banga, MD, associate professor of medicine and medical director of the ex vivo lung perfusion program in UT Southwestern’s lung transplant program.
“The implication is that there may be long-term consequences of COVID-19, perhaps related to some degree of ongoing inflammation and damage,” said Dr. Banga, a coauthor of the postinfection outcomes paper.
The UT Southwestern lung transplant program, which normally performs 60-80 transplants a year, began routine CT scanning 4-5 months into the pandemic, after “stumbling into a few patients who had no symptoms indicative of COVID pneumonia and no changes on an x-ray but significant involvement on a CT,” he said.
Without routine scanning in the general population of COVID-19 patients, Dr. Banga noted, “we’re limited in convincingly saying that COVID is uniquely doing this to lung transplant recipients.” Nor can they conclude that SARS-CoV-2 is unique from other respiratory viruses such as respiratory syncytial virus (RSV) in this regard. (The program has added CT scanning to its protocol for lung transplant recipients afflicted with other respiratory viruses to learn more.)
However, in the big picture, COVID-19 has proven to be far worse for lung transplant recipients than illness with other respiratory viruses, including RSV. “Patients have more frequent and greater loss of lung function, and worse debility from the acute illness,” Dr. Banga said.
“The cornerstones of treatment of both these viruses are very similar, but both the in-hospital course and the postdischarge outcomes are significantly different.”
In an initial paper published in September 2021, Dr. Banga and colleagues compared their first 25 lung transplant patients testing positive for SARS-CoV-2 with a historical cohort of 36 patients with RSV treated during 2016-2018.
Patients with COVID-19 had significantly worse morbidity and mortality, including worse postinfection lung function loss, functional decline, and 3-month survival.
More time, he said, will shed light on the risks of CLAD and the long-term potential for recovery of lung function. Currently, at UT Southwestern, it appears that patients who survive acute illness and the “first 3-6 months after COVID-19, when we’re seeing all the postinfection morbidity, may [enter] a period of stability,” Dr. Banga said.
Overall, he said, patients in their initial cohort are “holding steady” without unusual morbidity, readmissions, or “other setbacks to their allografts.”
At the Mayo Clinic in Jacksonville, which normally performs 40-50 lung transplants a year, transplant physicians have similarly observed significant declines in lung function beyond the acute phase of COVID-19. “Anecdotally, we’re seeing that some patients are beginning to recover some of their lung function, while others have not,” said Dr. Narula. “And we don’t have predictors as to who will progress to CLAD. It’s a big knowledge gap.”
Dr. Narula noted that patients with restrictive allograft syndrome, such as those reported by the UT Southwestern team, “have scarring of the lung and a much worse prognosis than the obstructive type of chronic rejection.” Whether there’s a role for antifibrotic therapy is a question worthy of research.
In UT Southwestern’s analysis, persistently lower absolute lymphocyte counts (< 600/dL) and higher ferritin levels (>150 ng/mL) at the time of hospital discharge were independently associated with significant lung function loss. This finding, reported in their October paper, has helped guide their management practices, Dr. Banga said.
“Persistently elevated ferritin may indicate ongoing inflammation at the allograft level,” he said. “We now send [such patients] home on a longer course of oral corticosteroids.”
At the front end of care for infected lung transplant recipients, Dr. Banga said that his team and physicians at other lung transplant programs are holding the cell-cycle inhibitor component of patients’ maintenance immunosuppression therapy (commonly mycophenolate or azathioprine) once infection is diagnosed to maximize chances of a better outcome.
“There may be variation on how long [the regimens are adjusted],” he said. “We changed our duration from 4 weeks to 2 due to patients developing a rebound worsening in the third and fourth week of acute illness.”
There is significant variation from institution to institution in how viral infections are managed in lung transplant recipients, he and Dr. Narula said. “Our numbers are so small in lung transplant, and we don’t have standardized protocols – it’s one of the biggest challenges in our field,” said Dr. Narula.
Vaccination issues, evaluation of donors
Whether or not immunosuppression regimens should be adjusted prior to vaccination is a controversial question, but is “an absolutely valid one” and is currently being studied in at least one National Institutes of Health–funded trial involving solid organ transplant recipients, said Dr. Wolfe.
“Some have jumped to the conclusion [based on some earlier data] that they should reduce immunosuppression regimens for everyone at the time of vaccination ... but I don’t know the answer yet,” he said. “Balancing staying rejection free with potentially gaining more immune response is complicated ... and it may depend on where the pandemic is going in your area and other factors.”
Reductions aside, Dr. Wolfe tells lung transplant recipients that, based on his approximation of a number of different studies in solid organ transplant recipients, approximately 40%-50% of patients who are immunized with two doses of the COVID-19 mRNA vaccines will develop meaningful antibody levels – and that this rises to 50%-60% after a third dose.
It is difficult to glean from available studies the level of vaccine response for lung transplant recipients specifically. But given that their level of maintenance immunosuppression is higher than for recipients of other donor organs, “as a broad sweep, lung transplant recipients tend to be lower in the pecking order of response,” he said.
Still, “there’s a lot to gain,” he said, pointing to a recent study from the Morbidity and Mortality Weekly Report (2021 Nov 5. doi: 10.15585/mmwr.mm7044e3) showing that effectiveness of mRNA vaccination against COVID-19–associated hospitalization was 77% among immunocompromised adults (compared with 90% in immunocompetent adults).
“This is good vindication to keep vaccinating,” he said, “and perhaps speaks to how difficult it is to assess the vaccine response [through measurement of antibody only].”
Neither Duke University’s transplant program, which performed 100-120 lung transplants a year pre-COVID, nor the programs at UT Southwestern or the Mayo Clinic in Jacksonville require that solid organ transplant candidates be vaccinated against SARS-CoV-2 in order to receive transplants, as some other transplant programs have done. (When asked about the issue, Dr. Banga and Dr. Narula each said that they have had no or little trouble convincing patients awaiting lung transplants of the need for COVID-19 vaccination.)
In an August statement, the American Society of Transplantation recommended vaccination for all solid organ transplant recipients, preferably prior to transplantation, and said that it “support[s] the development of institutional policies regarding pretransplant vaccination.”
The Society is not tracking centers’ vaccination policies. But Kaiser Health News reported in October that a growing number of transplant programs, such as UCHealth in Denver and UW Medicine in Seattle, have decided to either bar patients who refuse to be vaccinated from receiving transplants or give them lower priority on waitlists.
Potential lung donors, meanwhile, must be evaluated with lower respiratory COVID-19 testing, with results available prior to transplantation, according to policy developed by the Organ Procurement and Transplantation Network and effective in May 2021. The policy followed three published cases of donor-derived COVID-19 in lung transplant recipients, said Dr. Wolfe, who wrote about use of COVID-positive donors in an editorial published in October.
In each case, the donor had a negative COVID-19 nasopharyngeal swab at the time of organ procurement but was later found to have the virus on bronchoalveolar lavage, he said.
(The use of other organs from COVID-positive donors is appearing thus far to be safe, Dr. Wolfe noted. In the editorial, he references 13 cases of solid organ transplantation from SARS-CoV-2–infected donors into noninfected recipients; none of the 13 transplant recipients developed COVID-19).
Some questions remain, such as how many lower respiratory tests should be run, and how donors should be evaluated in cases of discordant results. Dr. Banga shared the case of a donor with one positive lower respiratory test result followed by two negative results. After internal debate, and consideration of potential false positives and other issues, the team at UT Southwestern decided to decline the donor, Dr. Banga said.
Other programs are likely making similar, appropriately cautious decisions, said Dr. Wolfe. “There’s no way in real-time donor evaluation to know whether the positive test is active virus that could infect the recipient and replicate ... or whether it’s [picking up] inactive or dead fragments of virus that was there several weeks ago. Our tests don’t differentiate that.”
Transplants in COVID-19 patients
Decision-making about lung transplant candidacy among patients with COVID-19 acute respiratory distress syndrome is complex and in need of a new paradigm.
“Some of these patients have the potential to recover, and they’re going to recover way later than what we’re used to,” said Dr. Banga. “We can’t extrapolate for COVID ARDS what we’ve learned for any other virus-related ARDS.”
Dr. Narula also has recently seen at least one COVID-19 patient on ECMO and under evaluation for transplantation recover. “We do not want to transplant too early,” he said, noting that there is consensus that lung transplant should be pursued only when the damage is deemed irreversible clinically and radiologically in the best judgment of the team. Still, “for many of these patients the only exit route will be lung transplants. For the next 12-24 months, a significant proportion of our lung transplant patients will have had post-COVID–related lung damage.”
As of October 2021, 233 lung transplants had been performed in the United States in recipients whose primary diagnosis was reported as COVID related, said Anne Paschke, media relations specialist with the United Network for Organ Sharing.
Dr. Banga, Dr. Wolfe, and Dr. Narula reported that they have no relevant disclosures.
Data is sparse thus far, but there is concern in lung transplant medicine about the long-term risk of chronic lung allograft dysfunction (CLAD) and a potentially shortened longevity of transplanted lungs in recipients who become ill with COVID-19.
“My fear is that we’re potentially sitting on this iceberg worth of people who, come 6 months or a year from [the acute phase of] their COVID illness, will in fact have earlier and progressive, chronic rejection,” said Cameron R. Wolfe, MBBS, MPH, associate professor of medicine in transplant infectious disease at Duke University, Durham, N.C.
Lower respiratory viral infections have long been concerning for lung transplant recipients given their propensity to cause scarring, a decline in lung function, and a heightened risk of allograft rejection. Time will tell whether lung transplant recipients who survive COVID-19 follow a similar path, or one that is worse, he said.
Short-term data
Outcomes beyond hospitalization and acute illness for lung transplant recipients affected by COVID-19 have been reported in the literature by only a few lung transplant programs. These reports – as well as anecdotal experiences being informally shared among transplant programs – have raised the specter of more severe dysfunction following the acute phase and more early CLAD, said Tathagat Narula, MD, assistant professor of medicine at the Mayo Medical School, Rochester, Minn., and a consultant in lung transplantation at the Mayo Clinic’s Jacksonville program.
“The available data cover only 3-6 months out. We don’t know what will happen in the next 6 months and beyond,” Dr. Narula said in an interview.
The risks of COVID-19 in already-transplanted patients and issues relating to the inadequate antibody responses to vaccination are just some of the challenges of lung transplant medicine in the era of SARS-CoV-2. “COVID-19,” said Dr. Narula, “has completely changed the way we practice lung transplant medicine – the way we’re looking both at our recipients and our donors.”
Potential donors are being evaluated with lower respiratory SARS-CoV-2 testing and an abundance of caution. And patients with severe COVID-19 affecting their own lungs are roundly expected to drive up lung transplant volume in the near future. “The whole paradigm has changed,” Dr. Narula said.
Post-acute trajectories
A chart review study published in October by the lung transplant team at the University of Texas Southwestern Medical Center, Dallas, covered 44 consecutive survivors at a median follow-up of 4.5 months from hospital discharge or acute illness (the survival rate was 83.3%). Patients had significantly impaired functional status, and 18 of the 44 (40.9%) had a significant and persistent loss of forced vital capacity or forced expiratory volume in 1 second (>10% from pre–COVID-19 baseline).
Three patients met the criteria for new CLAD after COVID-19 infection, with all three classified as restrictive allograft syndrome (RAS) phenotype.
Moreover, the majority of COVID-19 survivors who had CT chest scans (22 of 28) showed persistent parenchymal opacities – a finding that, regardless of symptomatology, suggests persistent allograft injury, said Amit Banga, MD, associate professor of medicine and medical director of the ex vivo lung perfusion program in UT Southwestern’s lung transplant program.
“The implication is that there may be long-term consequences of COVID-19, perhaps related to some degree of ongoing inflammation and damage,” said Dr. Banga, a coauthor of the postinfection outcomes paper.
The UT Southwestern lung transplant program, which normally performs 60-80 transplants a year, began routine CT scanning 4-5 months into the pandemic, after “stumbling into a few patients who had no symptoms indicative of COVID pneumonia and no changes on an x-ray but significant involvement on a CT,” he said.
Without routine scanning in the general population of COVID-19 patients, Dr. Banga noted, “we’re limited in convincingly saying that COVID is uniquely doing this to lung transplant recipients.” Nor can they conclude that SARS-CoV-2 is unique from other respiratory viruses such as respiratory syncytial virus (RSV) in this regard. (The program has added CT scanning to its protocol for lung transplant recipients afflicted with other respiratory viruses to learn more.)
However, in the big picture, COVID-19 has proven to be far worse for lung transplant recipients than illness with other respiratory viruses, including RSV. “Patients have more frequent and greater loss of lung function, and worse debility from the acute illness,” Dr. Banga said.
“The cornerstones of treatment of both these viruses are very similar, but both the in-hospital course and the postdischarge outcomes are significantly different.”
In an initial paper published in September 2021, Dr. Banga and colleagues compared their first 25 lung transplant patients testing positive for SARS-CoV-2 with a historical cohort of 36 patients with RSV treated during 2016-2018.
Patients with COVID-19 had significantly worse morbidity and mortality, including worse postinfection lung function loss, functional decline, and 3-month survival.
More time, he said, will shed light on the risks of CLAD and the long-term potential for recovery of lung function. Currently, at UT Southwestern, it appears that patients who survive acute illness and the “first 3-6 months after COVID-19, when we’re seeing all the postinfection morbidity, may [enter] a period of stability,” Dr. Banga said.
Overall, he said, patients in their initial cohort are “holding steady” without unusual morbidity, readmissions, or “other setbacks to their allografts.”
At the Mayo Clinic in Jacksonville, which normally performs 40-50 lung transplants a year, transplant physicians have similarly observed significant declines in lung function beyond the acute phase of COVID-19. “Anecdotally, we’re seeing that some patients are beginning to recover some of their lung function, while others have not,” said Dr. Narula. “And we don’t have predictors as to who will progress to CLAD. It’s a big knowledge gap.”
Dr. Narula noted that patients with restrictive allograft syndrome, such as those reported by the UT Southwestern team, “have scarring of the lung and a much worse prognosis than the obstructive type of chronic rejection.” Whether there’s a role for antifibrotic therapy is a question worthy of research.
In UT Southwestern’s analysis, persistently lower absolute lymphocyte counts (< 600/dL) and higher ferritin levels (>150 ng/mL) at the time of hospital discharge were independently associated with significant lung function loss. This finding, reported in their October paper, has helped guide their management practices, Dr. Banga said.
“Persistently elevated ferritin may indicate ongoing inflammation at the allograft level,” he said. “We now send [such patients] home on a longer course of oral corticosteroids.”
At the front end of care for infected lung transplant recipients, Dr. Banga said that his team and physicians at other lung transplant programs are holding the cell-cycle inhibitor component of patients’ maintenance immunosuppression therapy (commonly mycophenolate or azathioprine) once infection is diagnosed to maximize chances of a better outcome.
“There may be variation on how long [the regimens are adjusted],” he said. “We changed our duration from 4 weeks to 2 due to patients developing a rebound worsening in the third and fourth week of acute illness.”
There is significant variation from institution to institution in how viral infections are managed in lung transplant recipients, he and Dr. Narula said. “Our numbers are so small in lung transplant, and we don’t have standardized protocols – it’s one of the biggest challenges in our field,” said Dr. Narula.
Vaccination issues, evaluation of donors
Whether or not immunosuppression regimens should be adjusted prior to vaccination is a controversial question, but is “an absolutely valid one” and is currently being studied in at least one National Institutes of Health–funded trial involving solid organ transplant recipients, said Dr. Wolfe.
“Some have jumped to the conclusion [based on some earlier data] that they should reduce immunosuppression regimens for everyone at the time of vaccination ... but I don’t know the answer yet,” he said. “Balancing staying rejection free with potentially gaining more immune response is complicated ... and it may depend on where the pandemic is going in your area and other factors.”
Reductions aside, Dr. Wolfe tells lung transplant recipients that, based on his approximation of a number of different studies in solid organ transplant recipients, approximately 40%-50% of patients who are immunized with two doses of the COVID-19 mRNA vaccines will develop meaningful antibody levels – and that this rises to 50%-60% after a third dose.
It is difficult to glean from available studies the level of vaccine response for lung transplant recipients specifically. But given that their level of maintenance immunosuppression is higher than for recipients of other donor organs, “as a broad sweep, lung transplant recipients tend to be lower in the pecking order of response,” he said.
Still, “there’s a lot to gain,” he said, pointing to a recent study from the Morbidity and Mortality Weekly Report (2021 Nov 5. doi: 10.15585/mmwr.mm7044e3) showing that effectiveness of mRNA vaccination against COVID-19–associated hospitalization was 77% among immunocompromised adults (compared with 90% in immunocompetent adults).
“This is good vindication to keep vaccinating,” he said, “and perhaps speaks to how difficult it is to assess the vaccine response [through measurement of antibody only].”
Neither Duke University’s transplant program, which performed 100-120 lung transplants a year pre-COVID, nor the programs at UT Southwestern or the Mayo Clinic in Jacksonville require that solid organ transplant candidates be vaccinated against SARS-CoV-2 in order to receive transplants, as some other transplant programs have done. (When asked about the issue, Dr. Banga and Dr. Narula each said that they have had no or little trouble convincing patients awaiting lung transplants of the need for COVID-19 vaccination.)
In an August statement, the American Society of Transplantation recommended vaccination for all solid organ transplant recipients, preferably prior to transplantation, and said that it “support[s] the development of institutional policies regarding pretransplant vaccination.”
The Society is not tracking centers’ vaccination policies. But Kaiser Health News reported in October that a growing number of transplant programs, such as UCHealth in Denver and UW Medicine in Seattle, have decided to either bar patients who refuse to be vaccinated from receiving transplants or give them lower priority on waitlists.
Potential lung donors, meanwhile, must be evaluated with lower respiratory COVID-19 testing, with results available prior to transplantation, according to policy developed by the Organ Procurement and Transplantation Network and effective in May 2021. The policy followed three published cases of donor-derived COVID-19 in lung transplant recipients, said Dr. Wolfe, who wrote about use of COVID-positive donors in an editorial published in October.
In each case, the donor had a negative COVID-19 nasopharyngeal swab at the time of organ procurement but was later found to have the virus on bronchoalveolar lavage, he said.
(The use of other organs from COVID-positive donors is appearing thus far to be safe, Dr. Wolfe noted. In the editorial, he references 13 cases of solid organ transplantation from SARS-CoV-2–infected donors into noninfected recipients; none of the 13 transplant recipients developed COVID-19).
Some questions remain, such as how many lower respiratory tests should be run, and how donors should be evaluated in cases of discordant results. Dr. Banga shared the case of a donor with one positive lower respiratory test result followed by two negative results. After internal debate, and consideration of potential false positives and other issues, the team at UT Southwestern decided to decline the donor, Dr. Banga said.
Other programs are likely making similar, appropriately cautious decisions, said Dr. Wolfe. “There’s no way in real-time donor evaluation to know whether the positive test is active virus that could infect the recipient and replicate ... or whether it’s [picking up] inactive or dead fragments of virus that was there several weeks ago. Our tests don’t differentiate that.”
Transplants in COVID-19 patients
Decision-making about lung transplant candidacy among patients with COVID-19 acute respiratory distress syndrome is complex and in need of a new paradigm.
“Some of these patients have the potential to recover, and they’re going to recover way later than what we’re used to,” said Dr. Banga. “We can’t extrapolate for COVID ARDS what we’ve learned for any other virus-related ARDS.”
Dr. Narula also has recently seen at least one COVID-19 patient on ECMO and under evaluation for transplantation recover. “We do not want to transplant too early,” he said, noting that there is consensus that lung transplant should be pursued only when the damage is deemed irreversible clinically and radiologically in the best judgment of the team. Still, “for many of these patients the only exit route will be lung transplants. For the next 12-24 months, a significant proportion of our lung transplant patients will have had post-COVID–related lung damage.”
As of October 2021, 233 lung transplants had been performed in the United States in recipients whose primary diagnosis was reported as COVID related, said Anne Paschke, media relations specialist with the United Network for Organ Sharing.
Dr. Banga, Dr. Wolfe, and Dr. Narula reported that they have no relevant disclosures.
Data is sparse thus far, but there is concern in lung transplant medicine about the long-term risk of chronic lung allograft dysfunction (CLAD) and a potentially shortened longevity of transplanted lungs in recipients who become ill with COVID-19.
“My fear is that we’re potentially sitting on this iceberg worth of people who, come 6 months or a year from [the acute phase of] their COVID illness, will in fact have earlier and progressive, chronic rejection,” said Cameron R. Wolfe, MBBS, MPH, associate professor of medicine in transplant infectious disease at Duke University, Durham, N.C.
Lower respiratory viral infections have long been concerning for lung transplant recipients given their propensity to cause scarring, a decline in lung function, and a heightened risk of allograft rejection. Time will tell whether lung transplant recipients who survive COVID-19 follow a similar path, or one that is worse, he said.
Short-term data
Outcomes beyond hospitalization and acute illness for lung transplant recipients affected by COVID-19 have been reported in the literature by only a few lung transplant programs. These reports – as well as anecdotal experiences being informally shared among transplant programs – have raised the specter of more severe dysfunction following the acute phase and more early CLAD, said Tathagat Narula, MD, assistant professor of medicine at the Mayo Medical School, Rochester, Minn., and a consultant in lung transplantation at the Mayo Clinic’s Jacksonville program.
“The available data cover only 3-6 months out. We don’t know what will happen in the next 6 months and beyond,” Dr. Narula said in an interview.
The risks of COVID-19 in already-transplanted patients and issues relating to the inadequate antibody responses to vaccination are just some of the challenges of lung transplant medicine in the era of SARS-CoV-2. “COVID-19,” said Dr. Narula, “has completely changed the way we practice lung transplant medicine – the way we’re looking both at our recipients and our donors.”
Potential donors are being evaluated with lower respiratory SARS-CoV-2 testing and an abundance of caution. And patients with severe COVID-19 affecting their own lungs are roundly expected to drive up lung transplant volume in the near future. “The whole paradigm has changed,” Dr. Narula said.
Post-acute trajectories
A chart review study published in October by the lung transplant team at the University of Texas Southwestern Medical Center, Dallas, covered 44 consecutive survivors at a median follow-up of 4.5 months from hospital discharge or acute illness (the survival rate was 83.3%). Patients had significantly impaired functional status, and 18 of the 44 (40.9%) had a significant and persistent loss of forced vital capacity or forced expiratory volume in 1 second (>10% from pre–COVID-19 baseline).
Three patients met the criteria for new CLAD after COVID-19 infection, with all three classified as restrictive allograft syndrome (RAS) phenotype.
Moreover, the majority of COVID-19 survivors who had CT chest scans (22 of 28) showed persistent parenchymal opacities – a finding that, regardless of symptomatology, suggests persistent allograft injury, said Amit Banga, MD, associate professor of medicine and medical director of the ex vivo lung perfusion program in UT Southwestern’s lung transplant program.
“The implication is that there may be long-term consequences of COVID-19, perhaps related to some degree of ongoing inflammation and damage,” said Dr. Banga, a coauthor of the postinfection outcomes paper.
The UT Southwestern lung transplant program, which normally performs 60-80 transplants a year, began routine CT scanning 4-5 months into the pandemic, after “stumbling into a few patients who had no symptoms indicative of COVID pneumonia and no changes on an x-ray but significant involvement on a CT,” he said.
Without routine scanning in the general population of COVID-19 patients, Dr. Banga noted, “we’re limited in convincingly saying that COVID is uniquely doing this to lung transplant recipients.” Nor can they conclude that SARS-CoV-2 is unique from other respiratory viruses such as respiratory syncytial virus (RSV) in this regard. (The program has added CT scanning to its protocol for lung transplant recipients afflicted with other respiratory viruses to learn more.)
However, in the big picture, COVID-19 has proven to be far worse for lung transplant recipients than illness with other respiratory viruses, including RSV. “Patients have more frequent and greater loss of lung function, and worse debility from the acute illness,” Dr. Banga said.
“The cornerstones of treatment of both these viruses are very similar, but both the in-hospital course and the postdischarge outcomes are significantly different.”
In an initial paper published in September 2021, Dr. Banga and colleagues compared their first 25 lung transplant patients testing positive for SARS-CoV-2 with a historical cohort of 36 patients with RSV treated during 2016-2018.
Patients with COVID-19 had significantly worse morbidity and mortality, including worse postinfection lung function loss, functional decline, and 3-month survival.
More time, he said, will shed light on the risks of CLAD and the long-term potential for recovery of lung function. Currently, at UT Southwestern, it appears that patients who survive acute illness and the “first 3-6 months after COVID-19, when we’re seeing all the postinfection morbidity, may [enter] a period of stability,” Dr. Banga said.
Overall, he said, patients in their initial cohort are “holding steady” without unusual morbidity, readmissions, or “other setbacks to their allografts.”
At the Mayo Clinic in Jacksonville, which normally performs 40-50 lung transplants a year, transplant physicians have similarly observed significant declines in lung function beyond the acute phase of COVID-19. “Anecdotally, we’re seeing that some patients are beginning to recover some of their lung function, while others have not,” said Dr. Narula. “And we don’t have predictors as to who will progress to CLAD. It’s a big knowledge gap.”
Dr. Narula noted that patients with restrictive allograft syndrome, such as those reported by the UT Southwestern team, “have scarring of the lung and a much worse prognosis than the obstructive type of chronic rejection.” Whether there’s a role for antifibrotic therapy is a question worthy of research.
In UT Southwestern’s analysis, persistently lower absolute lymphocyte counts (< 600/dL) and higher ferritin levels (>150 ng/mL) at the time of hospital discharge were independently associated with significant lung function loss. This finding, reported in their October paper, has helped guide their management practices, Dr. Banga said.
“Persistently elevated ferritin may indicate ongoing inflammation at the allograft level,” he said. “We now send [such patients] home on a longer course of oral corticosteroids.”
At the front end of care for infected lung transplant recipients, Dr. Banga said that his team and physicians at other lung transplant programs are holding the cell-cycle inhibitor component of patients’ maintenance immunosuppression therapy (commonly mycophenolate or azathioprine) once infection is diagnosed to maximize chances of a better outcome.
“There may be variation on how long [the regimens are adjusted],” he said. “We changed our duration from 4 weeks to 2 due to patients developing a rebound worsening in the third and fourth week of acute illness.”
There is significant variation from institution to institution in how viral infections are managed in lung transplant recipients, he and Dr. Narula said. “Our numbers are so small in lung transplant, and we don’t have standardized protocols – it’s one of the biggest challenges in our field,” said Dr. Narula.
Vaccination issues, evaluation of donors
Whether or not immunosuppression regimens should be adjusted prior to vaccination is a controversial question, but is “an absolutely valid one” and is currently being studied in at least one National Institutes of Health–funded trial involving solid organ transplant recipients, said Dr. Wolfe.
“Some have jumped to the conclusion [based on some earlier data] that they should reduce immunosuppression regimens for everyone at the time of vaccination ... but I don’t know the answer yet,” he said. “Balancing staying rejection free with potentially gaining more immune response is complicated ... and it may depend on where the pandemic is going in your area and other factors.”
Reductions aside, Dr. Wolfe tells lung transplant recipients that, based on his approximation of a number of different studies in solid organ transplant recipients, approximately 40%-50% of patients who are immunized with two doses of the COVID-19 mRNA vaccines will develop meaningful antibody levels – and that this rises to 50%-60% after a third dose.
It is difficult to glean from available studies the level of vaccine response for lung transplant recipients specifically. But given that their level of maintenance immunosuppression is higher than for recipients of other donor organs, “as a broad sweep, lung transplant recipients tend to be lower in the pecking order of response,” he said.
Still, “there’s a lot to gain,” he said, pointing to a recent study from the Morbidity and Mortality Weekly Report (2021 Nov 5. doi: 10.15585/mmwr.mm7044e3) showing that effectiveness of mRNA vaccination against COVID-19–associated hospitalization was 77% among immunocompromised adults (compared with 90% in immunocompetent adults).
“This is good vindication to keep vaccinating,” he said, “and perhaps speaks to how difficult it is to assess the vaccine response [through measurement of antibody only].”
Neither Duke University’s transplant program, which performed 100-120 lung transplants a year pre-COVID, nor the programs at UT Southwestern or the Mayo Clinic in Jacksonville require that solid organ transplant candidates be vaccinated against SARS-CoV-2 in order to receive transplants, as some other transplant programs have done. (When asked about the issue, Dr. Banga and Dr. Narula each said that they have had no or little trouble convincing patients awaiting lung transplants of the need for COVID-19 vaccination.)
In an August statement, the American Society of Transplantation recommended vaccination for all solid organ transplant recipients, preferably prior to transplantation, and said that it “support[s] the development of institutional policies regarding pretransplant vaccination.”
The Society is not tracking centers’ vaccination policies. But Kaiser Health News reported in October that a growing number of transplant programs, such as UCHealth in Denver and UW Medicine in Seattle, have decided to either bar patients who refuse to be vaccinated from receiving transplants or give them lower priority on waitlists.
Potential lung donors, meanwhile, must be evaluated with lower respiratory COVID-19 testing, with results available prior to transplantation, according to policy developed by the Organ Procurement and Transplantation Network and effective in May 2021. The policy followed three published cases of donor-derived COVID-19 in lung transplant recipients, said Dr. Wolfe, who wrote about use of COVID-positive donors in an editorial published in October.
In each case, the donor had a negative COVID-19 nasopharyngeal swab at the time of organ procurement but was later found to have the virus on bronchoalveolar lavage, he said.
(The use of other organs from COVID-positive donors is appearing thus far to be safe, Dr. Wolfe noted. In the editorial, he references 13 cases of solid organ transplantation from SARS-CoV-2–infected donors into noninfected recipients; none of the 13 transplant recipients developed COVID-19).
Some questions remain, such as how many lower respiratory tests should be run, and how donors should be evaluated in cases of discordant results. Dr. Banga shared the case of a donor with one positive lower respiratory test result followed by two negative results. After internal debate, and consideration of potential false positives and other issues, the team at UT Southwestern decided to decline the donor, Dr. Banga said.
Other programs are likely making similar, appropriately cautious decisions, said Dr. Wolfe. “There’s no way in real-time donor evaluation to know whether the positive test is active virus that could infect the recipient and replicate ... or whether it’s [picking up] inactive or dead fragments of virus that was there several weeks ago. Our tests don’t differentiate that.”
Transplants in COVID-19 patients
Decision-making about lung transplant candidacy among patients with COVID-19 acute respiratory distress syndrome is complex and in need of a new paradigm.
“Some of these patients have the potential to recover, and they’re going to recover way later than what we’re used to,” said Dr. Banga. “We can’t extrapolate for COVID ARDS what we’ve learned for any other virus-related ARDS.”
Dr. Narula also has recently seen at least one COVID-19 patient on ECMO and under evaluation for transplantation recover. “We do not want to transplant too early,” he said, noting that there is consensus that lung transplant should be pursued only when the damage is deemed irreversible clinically and radiologically in the best judgment of the team. Still, “for many of these patients the only exit route will be lung transplants. For the next 12-24 months, a significant proportion of our lung transplant patients will have had post-COVID–related lung damage.”
As of October 2021, 233 lung transplants had been performed in the United States in recipients whose primary diagnosis was reported as COVID related, said Anne Paschke, media relations specialist with the United Network for Organ Sharing.
Dr. Banga, Dr. Wolfe, and Dr. Narula reported that they have no relevant disclosures.
Major COVID-19 case growth expected in coming weeks
by the PolicyLab at Children’s Hospital of Philadelphia.
Large metropolitan areas, especially those in the Northeast, are already seeing a major increase in cases following Thanksgiving, and that trend is expected to continue.
“Why? Simply stated, the large amount of Thanksgiving travel and gatherings undermined the nation’s pandemic footing and has elevated disease burden in areas of the country that were fortunate to have lower case rates before the holidays,” the forecasters wrote.
Case numbers in New York City are expected to double throughout December, the forecasters said. Similar growth could happen across Boston, Philadelphia, and Baltimore.
Overall, COVID-19 cases, hospitalizations, and deaths are rising across the United States but remain below levels seen during the summer and last winter’s surges, according to the New York Times. The increase is still being driven by the Delta variant, though it remains unclear how the Omicron variant, which has been detected in 27 states, could affect the trends in the coming weeks.
During the past week, the United States has reported an average of more than 120,000 new cases each day, the newspaper reported, which is an increase of 38% from two weeks ago.
The daily average of COVID-19 hospitalizations is around 64,000, which marks an increase of 22% from two weeks ago. More than 1,300 deaths are being reported each day, which is up 26%.
Numerous states are reporting double the cases from two weeks ago, stretching across the country from states in the Northeast such as Connecticut and Rhode Island to southern states such as North Carolina and Texas and western states such as California.
The Great Lakes region and the Northeast are seeing some of the most severe increases, the newspaper reported. New Hampshire leads the United States in recent cases per capita, and Maine has reported more cases in the past week than in any other seven-day period during the pandemic.
Michigan has the country’s highest hospitalization rate, and federal medical teams have been sent to the state to help with the surge in patients, according to The Detroit News. Michigan’s top public health officials described the surge as a “critical” and “deeply concerning” situation on Dec. 10, and they requested 200 more ventilators from the Strategic National Stockpile.
Indiana, Maine, and New York have also requested aid from the National Guard, according to USA Today. Health officials in those states urged residents to get vaccines or booster shots and wear masks in indoor public settings.
The Omicron variant can evade some vaccine protection, but booster shots can increase efficacy and offer more coverage, Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, said Dec. 12.
“If you want to be optimally protected, absolutely get a booster,” he said on ABC’s “This Week.”
In addition, New York Gov. Kathy Hochul has announced a statewide mask mandate, which will take effect Dec. 13. Masks will be required in all indoor public spaces and businesses, unless the location implements a vaccine requirement instead, the news outlet reported.
A version of this article first appeared on WebMD.com.
by the PolicyLab at Children’s Hospital of Philadelphia.
Large metropolitan areas, especially those in the Northeast, are already seeing a major increase in cases following Thanksgiving, and that trend is expected to continue.
“Why? Simply stated, the large amount of Thanksgiving travel and gatherings undermined the nation’s pandemic footing and has elevated disease burden in areas of the country that were fortunate to have lower case rates before the holidays,” the forecasters wrote.
Case numbers in New York City are expected to double throughout December, the forecasters said. Similar growth could happen across Boston, Philadelphia, and Baltimore.
Overall, COVID-19 cases, hospitalizations, and deaths are rising across the United States but remain below levels seen during the summer and last winter’s surges, according to the New York Times. The increase is still being driven by the Delta variant, though it remains unclear how the Omicron variant, which has been detected in 27 states, could affect the trends in the coming weeks.
During the past week, the United States has reported an average of more than 120,000 new cases each day, the newspaper reported, which is an increase of 38% from two weeks ago.
The daily average of COVID-19 hospitalizations is around 64,000, which marks an increase of 22% from two weeks ago. More than 1,300 deaths are being reported each day, which is up 26%.
Numerous states are reporting double the cases from two weeks ago, stretching across the country from states in the Northeast such as Connecticut and Rhode Island to southern states such as North Carolina and Texas and western states such as California.
The Great Lakes region and the Northeast are seeing some of the most severe increases, the newspaper reported. New Hampshire leads the United States in recent cases per capita, and Maine has reported more cases in the past week than in any other seven-day period during the pandemic.
Michigan has the country’s highest hospitalization rate, and federal medical teams have been sent to the state to help with the surge in patients, according to The Detroit News. Michigan’s top public health officials described the surge as a “critical” and “deeply concerning” situation on Dec. 10, and they requested 200 more ventilators from the Strategic National Stockpile.
Indiana, Maine, and New York have also requested aid from the National Guard, according to USA Today. Health officials in those states urged residents to get vaccines or booster shots and wear masks in indoor public settings.
The Omicron variant can evade some vaccine protection, but booster shots can increase efficacy and offer more coverage, Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, said Dec. 12.
“If you want to be optimally protected, absolutely get a booster,” he said on ABC’s “This Week.”
In addition, New York Gov. Kathy Hochul has announced a statewide mask mandate, which will take effect Dec. 13. Masks will be required in all indoor public spaces and businesses, unless the location implements a vaccine requirement instead, the news outlet reported.
A version of this article first appeared on WebMD.com.
by the PolicyLab at Children’s Hospital of Philadelphia.
Large metropolitan areas, especially those in the Northeast, are already seeing a major increase in cases following Thanksgiving, and that trend is expected to continue.
“Why? Simply stated, the large amount of Thanksgiving travel and gatherings undermined the nation’s pandemic footing and has elevated disease burden in areas of the country that were fortunate to have lower case rates before the holidays,” the forecasters wrote.
Case numbers in New York City are expected to double throughout December, the forecasters said. Similar growth could happen across Boston, Philadelphia, and Baltimore.
Overall, COVID-19 cases, hospitalizations, and deaths are rising across the United States but remain below levels seen during the summer and last winter’s surges, according to the New York Times. The increase is still being driven by the Delta variant, though it remains unclear how the Omicron variant, which has been detected in 27 states, could affect the trends in the coming weeks.
During the past week, the United States has reported an average of more than 120,000 new cases each day, the newspaper reported, which is an increase of 38% from two weeks ago.
The daily average of COVID-19 hospitalizations is around 64,000, which marks an increase of 22% from two weeks ago. More than 1,300 deaths are being reported each day, which is up 26%.
Numerous states are reporting double the cases from two weeks ago, stretching across the country from states in the Northeast such as Connecticut and Rhode Island to southern states such as North Carolina and Texas and western states such as California.
The Great Lakes region and the Northeast are seeing some of the most severe increases, the newspaper reported. New Hampshire leads the United States in recent cases per capita, and Maine has reported more cases in the past week than in any other seven-day period during the pandemic.
Michigan has the country’s highest hospitalization rate, and federal medical teams have been sent to the state to help with the surge in patients, according to The Detroit News. Michigan’s top public health officials described the surge as a “critical” and “deeply concerning” situation on Dec. 10, and they requested 200 more ventilators from the Strategic National Stockpile.
Indiana, Maine, and New York have also requested aid from the National Guard, according to USA Today. Health officials in those states urged residents to get vaccines or booster shots and wear masks in indoor public settings.
The Omicron variant can evade some vaccine protection, but booster shots can increase efficacy and offer more coverage, Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, said Dec. 12.
“If you want to be optimally protected, absolutely get a booster,” he said on ABC’s “This Week.”
In addition, New York Gov. Kathy Hochul has announced a statewide mask mandate, which will take effect Dec. 13. Masks will be required in all indoor public spaces and businesses, unless the location implements a vaccine requirement instead, the news outlet reported.
A version of this article first appeared on WebMD.com.
FIB-4 could ID liver risk in primary care
Fibrosis-4 index (FIB-4) scores are strongly associated with severe liver disease outcomes in a primary care population, both in patients with known chronic liver disease and those without known CLD. The result could help identify patients with CLD before their condition becomes severe.
FIB-4 has previously shown utility in predicting the risk of advanced fibrosis in patients with viral hepatitis B and C, nonalcoholic fatty liver disease (NAFLD), nonalcoholic steatohepatitis (NASH), and alcohol-related liver disease.
“This is really important in primary care because FIB-4 is easy to calculate. Its inputs are accessible, and it is inexpensive, often taking advantage of labs that we’ve ordered anyway. And if we can use it to find advanced fibrosis, it will be critically important because we know that advanced fibrosis is associated with severe liver outcomes – these are going to be patients that we need to make sure are in touch with our hepatology colleagues,” said Andrew Schreiner, MD, during a presentation of the results at the annual meeting of the American Association for the Study of Liver Diseases. Dr. Schreiner is general internist at the Medical University of South Carolina, Charleston.
He also noted that FIB-4 is playing an important role in the assessment of NAFLD and NASH. Many newer algorithms to manage NAFLD in the primary care setting rely on FIB-4, but that application is limited because NAFLD is underdiagnosed according to administrative database studies, which found rates of about 2%-5% despite the fact that estimates put it at having a prevalence of 25%-30% in the U.S. population.
To determine if FIB-4 scores could assist in identifying primary care patients at risk of severe outcomes, including cirrhosis, hepatocellular carcinoma, and liver transplant, the researchers conducted a retrospective analysis of primary care electronic health care data from 20,556 patients between 2007 and 2018 who were seen at their institution. Participants had ALT and AST values less than 500 IU/L, as well as a platelet count within two months preceding or on the day of the liver enzyme tests. They excluded individuals with known chronic or severe liver disease.
65% of patients were female, 45% were Black, and the mean BMI was 29.8 kg/m2. 64% of participants were ranked as low risk (FIB-4 ≤1.3), 29% with undetermined risk (1.3-2.67), and 7% with high risk (>2.67).
The population had more liver risk than expected. “[It is] a distribution that certainly may have more high risk and indeterminant risks than we would have anticipated, but we have seen this in external studies,” said Dr. Schreiner.
Over a mean follow-up period of 8.2 years, 11% were diagnosed with CLD: 2.3% developed NAFLD, 8.2% another CLD, and 0.5% had NAFLD and another CLD. About 4% developed a severe CLD. A severe liver outcome occurred in 2.2% of those who had been classified as FIB-4 low risk, 4.2% classified as indeterminate risk, and 20.8% of those classified as high risk.
“Troublingly,” said Dr. Schreiner, 49% of those who went on to develop a severe liver outcome had no CLD diagnosis before it occurred. “This is a tremendous opportunity to improve diagnosis in this setting.”
After adjustment for race, gender, marital status, smoking history, BMI, and various comorbidities, the researchers found a higher risk of severe liver disease associated with indeterminate FIB-4 risk score (hazard ratio, 1.62; 95% confidence interval, 1.36-1.92) and a high FIB-4 risk score (HR, 6.64; 95% CI, 5.58-7.90), compared with those with a low FIB-4 risk score. The same was true for individual liver diseases, including NAFLD (indeterminate HR, 1.88; 95% CI, 0.99-3.60; high HR, 7.32; 95% CI, 3.44-15.58), other liver diagnosis (indeterminate HR, 2.65; 95% CI, 1.93-3.63; high HR, 11.39; 95% CI, 8.53-15.20), and NAFLD plus another liver disease (intermediate HR, 2.53; 95% CI, 0.79-8.12; high HR, 6.89; 95% CI, 1.82-26.14).
Dr. Schreiner conceded that the study may not be generalizable, since FIB-4 was not designed for use in general populations, and it was conducted at a single center.
During the question-and-answer session after the talk, Dr. Schreiner was asked if the majority of the 49% who had a severe liver outcome without previous liver disease had NAFLD. He said that was the team’s hypothesis, and they are in the process of examining that data, but a significant number appear to be alcohol related. “For us in the primary care setting, it’s just another opportunity to emphasize that we have to do a better job getting exposure histories, and alcohol histories in particular, and finding ways to document those in ways that we can make diagnoses for patients and for our hepatology colleagues,” said Dr. Schreiner.
Comoderator Kathleen Corey, MD, asked Dr. Schreiner if he had any concerns about false positives from FIB-4 screening, and whether that could lead to overtreatment. “We’ve seen other screening tests leading to patient distress and overutilization of resources. How do you think we might be able to mitigate that?” asked Dr. Corey, who is an assistant professor of medicine at Harvard Medical School and director of the Fatty Liver Clinic at Massachusetts General Hospital, both in Boston.
Dr. Schreiner underscored the need for more physician education about FIB-4, both its potential and its pitfalls, since many primary care providers don’t use it or even know about it. “FIB-4 is very popular in the hepatology literature, but in primary care, we don’t talk about it as often. So I think educational efforts about its possible utility, about some of the drawbacks, or some of the things that might lead to inappropriately positive results – like advanced age, for those of us who see patients 60 and older. Those are really important considerations both for the patient and the provider for management of expectations and concerns. I’m worried too about application in our younger cohorts. The explosion of NAFLD in adolescence, and the likelihood that we might get a false negative in maybe a 28-year-old who might have problematic disease, is a concern as well,” said Dr. Schreiner.
Dr. Schreiner has no relevant financial disclosures. Dr. Corey has been on an advisory committee or review panel for Bristol-Myers Squibb, Novo Nordisk, and Gilead. She has consulted for Novo Nordisk and received research support from BMS, Boehringer Ingelheim, Novartis, and Boehringer Ingelheim.
Fibrosis-4 index (FIB-4) scores are strongly associated with severe liver disease outcomes in a primary care population, both in patients with known chronic liver disease and those without known CLD. The result could help identify patients with CLD before their condition becomes severe.
FIB-4 has previously shown utility in predicting the risk of advanced fibrosis in patients with viral hepatitis B and C, nonalcoholic fatty liver disease (NAFLD), nonalcoholic steatohepatitis (NASH), and alcohol-related liver disease.
“This is really important in primary care because FIB-4 is easy to calculate. Its inputs are accessible, and it is inexpensive, often taking advantage of labs that we’ve ordered anyway. And if we can use it to find advanced fibrosis, it will be critically important because we know that advanced fibrosis is associated with severe liver outcomes – these are going to be patients that we need to make sure are in touch with our hepatology colleagues,” said Andrew Schreiner, MD, during a presentation of the results at the annual meeting of the American Association for the Study of Liver Diseases. Dr. Schreiner is general internist at the Medical University of South Carolina, Charleston.
He also noted that FIB-4 is playing an important role in the assessment of NAFLD and NASH. Many newer algorithms to manage NAFLD in the primary care setting rely on FIB-4, but that application is limited because NAFLD is underdiagnosed according to administrative database studies, which found rates of about 2%-5% despite the fact that estimates put it at having a prevalence of 25%-30% in the U.S. population.
To determine if FIB-4 scores could assist in identifying primary care patients at risk of severe outcomes, including cirrhosis, hepatocellular carcinoma, and liver transplant, the researchers conducted a retrospective analysis of primary care electronic health care data from 20,556 patients between 2007 and 2018 who were seen at their institution. Participants had ALT and AST values less than 500 IU/L, as well as a platelet count within two months preceding or on the day of the liver enzyme tests. They excluded individuals with known chronic or severe liver disease.
65% of patients were female, 45% were Black, and the mean BMI was 29.8 kg/m2. 64% of participants were ranked as low risk (FIB-4 ≤1.3), 29% with undetermined risk (1.3-2.67), and 7% with high risk (>2.67).
The population had more liver risk than expected. “[It is] a distribution that certainly may have more high risk and indeterminant risks than we would have anticipated, but we have seen this in external studies,” said Dr. Schreiner.
Over a mean follow-up period of 8.2 years, 11% were diagnosed with CLD: 2.3% developed NAFLD, 8.2% another CLD, and 0.5% had NAFLD and another CLD. About 4% developed a severe CLD. A severe liver outcome occurred in 2.2% of those who had been classified as FIB-4 low risk, 4.2% classified as indeterminate risk, and 20.8% of those classified as high risk.
“Troublingly,” said Dr. Schreiner, 49% of those who went on to develop a severe liver outcome had no CLD diagnosis before it occurred. “This is a tremendous opportunity to improve diagnosis in this setting.”
After adjustment for race, gender, marital status, smoking history, BMI, and various comorbidities, the researchers found a higher risk of severe liver disease associated with indeterminate FIB-4 risk score (hazard ratio, 1.62; 95% confidence interval, 1.36-1.92) and a high FIB-4 risk score (HR, 6.64; 95% CI, 5.58-7.90), compared with those with a low FIB-4 risk score. The same was true for individual liver diseases, including NAFLD (indeterminate HR, 1.88; 95% CI, 0.99-3.60; high HR, 7.32; 95% CI, 3.44-15.58), other liver diagnosis (indeterminate HR, 2.65; 95% CI, 1.93-3.63; high HR, 11.39; 95% CI, 8.53-15.20), and NAFLD plus another liver disease (intermediate HR, 2.53; 95% CI, 0.79-8.12; high HR, 6.89; 95% CI, 1.82-26.14).
Dr. Schreiner conceded that the study may not be generalizable, since FIB-4 was not designed for use in general populations, and it was conducted at a single center.
During the question-and-answer session after the talk, Dr. Schreiner was asked if the majority of the 49% who had a severe liver outcome without previous liver disease had NAFLD. He said that was the team’s hypothesis, and they are in the process of examining that data, but a significant number appear to be alcohol related. “For us in the primary care setting, it’s just another opportunity to emphasize that we have to do a better job getting exposure histories, and alcohol histories in particular, and finding ways to document those in ways that we can make diagnoses for patients and for our hepatology colleagues,” said Dr. Schreiner.
Comoderator Kathleen Corey, MD, asked Dr. Schreiner if he had any concerns about false positives from FIB-4 screening, and whether that could lead to overtreatment. “We’ve seen other screening tests leading to patient distress and overutilization of resources. How do you think we might be able to mitigate that?” asked Dr. Corey, who is an assistant professor of medicine at Harvard Medical School and director of the Fatty Liver Clinic at Massachusetts General Hospital, both in Boston.
Dr. Schreiner underscored the need for more physician education about FIB-4, both its potential and its pitfalls, since many primary care providers don’t use it or even know about it. “FIB-4 is very popular in the hepatology literature, but in primary care, we don’t talk about it as often. So I think educational efforts about its possible utility, about some of the drawbacks, or some of the things that might lead to inappropriately positive results – like advanced age, for those of us who see patients 60 and older. Those are really important considerations both for the patient and the provider for management of expectations and concerns. I’m worried too about application in our younger cohorts. The explosion of NAFLD in adolescence, and the likelihood that we might get a false negative in maybe a 28-year-old who might have problematic disease, is a concern as well,” said Dr. Schreiner.
Dr. Schreiner has no relevant financial disclosures. Dr. Corey has been on an advisory committee or review panel for Bristol-Myers Squibb, Novo Nordisk, and Gilead. She has consulted for Novo Nordisk and received research support from BMS, Boehringer Ingelheim, Novartis, and Boehringer Ingelheim.
Fibrosis-4 index (FIB-4) scores are strongly associated with severe liver disease outcomes in a primary care population, both in patients with known chronic liver disease and those without known CLD. The result could help identify patients with CLD before their condition becomes severe.
FIB-4 has previously shown utility in predicting the risk of advanced fibrosis in patients with viral hepatitis B and C, nonalcoholic fatty liver disease (NAFLD), nonalcoholic steatohepatitis (NASH), and alcohol-related liver disease.
“This is really important in primary care because FIB-4 is easy to calculate. Its inputs are accessible, and it is inexpensive, often taking advantage of labs that we’ve ordered anyway. And if we can use it to find advanced fibrosis, it will be critically important because we know that advanced fibrosis is associated with severe liver outcomes – these are going to be patients that we need to make sure are in touch with our hepatology colleagues,” said Andrew Schreiner, MD, during a presentation of the results at the annual meeting of the American Association for the Study of Liver Diseases. Dr. Schreiner is general internist at the Medical University of South Carolina, Charleston.
He also noted that FIB-4 is playing an important role in the assessment of NAFLD and NASH. Many newer algorithms to manage NAFLD in the primary care setting rely on FIB-4, but that application is limited because NAFLD is underdiagnosed according to administrative database studies, which found rates of about 2%-5% despite the fact that estimates put it at having a prevalence of 25%-30% in the U.S. population.
To determine if FIB-4 scores could assist in identifying primary care patients at risk of severe outcomes, including cirrhosis, hepatocellular carcinoma, and liver transplant, the researchers conducted a retrospective analysis of primary care electronic health care data from 20,556 patients between 2007 and 2018 who were seen at their institution. Participants had ALT and AST values less than 500 IU/L, as well as a platelet count within two months preceding or on the day of the liver enzyme tests. They excluded individuals with known chronic or severe liver disease.
65% of patients were female, 45% were Black, and the mean BMI was 29.8 kg/m2. 64% of participants were ranked as low risk (FIB-4 ≤1.3), 29% with undetermined risk (1.3-2.67), and 7% with high risk (>2.67).
The population had more liver risk than expected. “[It is] a distribution that certainly may have more high risk and indeterminant risks than we would have anticipated, but we have seen this in external studies,” said Dr. Schreiner.
Over a mean follow-up period of 8.2 years, 11% were diagnosed with CLD: 2.3% developed NAFLD, 8.2% another CLD, and 0.5% had NAFLD and another CLD. About 4% developed a severe CLD. A severe liver outcome occurred in 2.2% of those who had been classified as FIB-4 low risk, 4.2% classified as indeterminate risk, and 20.8% of those classified as high risk.
“Troublingly,” said Dr. Schreiner, 49% of those who went on to develop a severe liver outcome had no CLD diagnosis before it occurred. “This is a tremendous opportunity to improve diagnosis in this setting.”
After adjustment for race, gender, marital status, smoking history, BMI, and various comorbidities, the researchers found a higher risk of severe liver disease associated with indeterminate FIB-4 risk score (hazard ratio, 1.62; 95% confidence interval, 1.36-1.92) and a high FIB-4 risk score (HR, 6.64; 95% CI, 5.58-7.90), compared with those with a low FIB-4 risk score. The same was true for individual liver diseases, including NAFLD (indeterminate HR, 1.88; 95% CI, 0.99-3.60; high HR, 7.32; 95% CI, 3.44-15.58), other liver diagnosis (indeterminate HR, 2.65; 95% CI, 1.93-3.63; high HR, 11.39; 95% CI, 8.53-15.20), and NAFLD plus another liver disease (intermediate HR, 2.53; 95% CI, 0.79-8.12; high HR, 6.89; 95% CI, 1.82-26.14).
Dr. Schreiner conceded that the study may not be generalizable, since FIB-4 was not designed for use in general populations, and it was conducted at a single center.
During the question-and-answer session after the talk, Dr. Schreiner was asked if the majority of the 49% who had a severe liver outcome without previous liver disease had NAFLD. He said that was the team’s hypothesis, and they are in the process of examining that data, but a significant number appear to be alcohol related. “For us in the primary care setting, it’s just another opportunity to emphasize that we have to do a better job getting exposure histories, and alcohol histories in particular, and finding ways to document those in ways that we can make diagnoses for patients and for our hepatology colleagues,” said Dr. Schreiner.
Comoderator Kathleen Corey, MD, asked Dr. Schreiner if he had any concerns about false positives from FIB-4 screening, and whether that could lead to overtreatment. “We’ve seen other screening tests leading to patient distress and overutilization of resources. How do you think we might be able to mitigate that?” asked Dr. Corey, who is an assistant professor of medicine at Harvard Medical School and director of the Fatty Liver Clinic at Massachusetts General Hospital, both in Boston.
Dr. Schreiner underscored the need for more physician education about FIB-4, both its potential and its pitfalls, since many primary care providers don’t use it or even know about it. “FIB-4 is very popular in the hepatology literature, but in primary care, we don’t talk about it as often. So I think educational efforts about its possible utility, about some of the drawbacks, or some of the things that might lead to inappropriately positive results – like advanced age, for those of us who see patients 60 and older. Those are really important considerations both for the patient and the provider for management of expectations and concerns. I’m worried too about application in our younger cohorts. The explosion of NAFLD in adolescence, and the likelihood that we might get a false negative in maybe a 28-year-old who might have problematic disease, is a concern as well,” said Dr. Schreiner.
Dr. Schreiner has no relevant financial disclosures. Dr. Corey has been on an advisory committee or review panel for Bristol-Myers Squibb, Novo Nordisk, and Gilead. She has consulted for Novo Nordisk and received research support from BMS, Boehringer Ingelheim, Novartis, and Boehringer Ingelheim.
FROM THE LIVER MEETING