User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
Nondiabetes hospitalization is wrong time to up diabetes meds
“Short-term hospitalization [for reasons other than diabetes] may not be the time to intervene in long-term diabetes management,” researchers conclude.
They found that, in a national cohort of older almost entirely male veterans with non–insulin-treated type 2 diabetes who were hospitalized for non–diabetes-related common medical conditions, intensified diabetes treatment on hospital discharge was linked to an increased risk of severe hypoglycemia in the immediate postdischarge period.
However, diabetes treatment intensification – that is, receiving a prescription for a new or higher dose of diabetes medicine – was not associated with decreased risks of severe hyperglycemia or with improved glycemic (hemoglobin A1c) control at 30 days or 1 year, according to study results, published in JAMA Network Open.
“We didn’t see a reduction in diabetes emergencies in more intensively treated patients,” lead investigator Timothy S. Anderson, MD, said in an interview.
Also, importantly, there was a low rate of persistence with the new treatment. “Half of the patients were no longer taking these [intensified diabetes medicines] at 1 year, which tells me that context is key,” he pointed out. “If a patient is in the hospital for diabetes [unlike the patients in this study], I think it makes a lot of sense to modify and adjust their regimen to try to help them right then and there.”
The overall risk of severe hyperglycemia or severe hypoglycemia was pretty small in the overall cohort, Dr. Anderson noted, “but we do put people at risk of leaving the hospital and ending up back in the hospital with low blood sugar when we intensify medications, and there’s not necessarily a good signal to suggest that it’s all that urgent to change these medicines.”
Instead, the “safer path” may be to make recommendations to the patient’s outpatient physician and also inform the patient – for example, “We saw some concerns about your diabetes while you were in the hospital, and this is really something that should be looked at when you’re recovered and feeling better from the rest of your health standpoint” – rather than making a diabetes medication change while the person is acutely ill or recovering from illness, said Dr. Anderson, from Beth Israel Deaconess Medical Center and Harvard Medical School, Boston.
The researchers also found an “unexpected” significant decrease in 30-day mortality in the patients with intensified diabetes treatment, which was probably because of confounding that was not accounted for, Dr. Anderson speculated, since clinical trials have consistently shown that benefits from diabetes medications take a longer time to show an effect.
‘Important study,’ but lacked newer meds
This is an “important” study for primary care and in-hospital physicians that shows that “hospitalization is really not the time and the place” to intensify diabetes medication, Rozalina G. McCoy, MD, coauthor of an invited commentary, told this news organization in an interview.
“While overcoming treatment inertia is important, [it should be] done appropriately, so that we don’t overtreat patients,” Dr. McCoy, of the Mayo Clinic in Rochester, Minn., stressed.
The very low rate of persistence of taking intensified medications is a major finding, she agreed. Hospitalized patients “are not in their usual state of health, so if we make long-term treatment decisions based on their acute abnormal situation, that may not be appropriate.”
However, patients with high A1c may benefit from a change at hospital discharge rather than when they see their primary care provider, with the caveat that they need close follow-up as an outpatient.
The study emphasizes the “need for longitudinal patient care rather than episodic patches,” according to Dr. McCoy.
For example, a patient who is hospitalized for a chronic obstructive pulmonary disease or asthma exacerbation may be receiving steroids that cause high blood glucose levels but as soon as they’re done with their steroid course, blood glucose will decrease, so the “need for close outpatient follow-up is very important.”
One limitation of the current work is that an earlier study in the same population by the research group showed that 49% of patients whose treatment regimens were intensified had limited life expectancy or were at or below their A1c goal, so they would not have benefited from the stepped-up treatment, she noted.
Another limitation is that the findings cannot be generalized to women or younger patients, or to patients treated with glucagonlike peptide 1 (GLP-1)–receptor agonists or sodium-glucose cotransporter 2 (SGLT2) inhibitors.
The study patients were seen in the U.S. Veterans Health Administration health system when these newer agents were not used. Three-quarters of patients received intensified treatment with sulfonylurea or insulin, and only one patient received a new GLP-1–receptor agonist.
Ideally, Dr. McCoy said, patients should have been prescribed a GLP-1–receptor agonist if they had atherosclerotic cardiovascular disease or kidney disease, or an SGLT2 inhibitor if they had kidney disease or heart failure, which may have led to different results, and would need to be determined in further study.
Dr. Anderson agreed that “SGLT2 inhibitors and GLP1 agonists are broadly much safer than the older diabetes medicines, at least when it comes to risk of hypoglycemia, and may have more clear benefits in heart disease and mortality. So I would not want to extrapolate our findings to those new classes,” he said. “A similar set of studies would need to be done.”
Study rationale and findings
Hospitalized older adults with diabetes commonly have transiently elevated blood glucose levels that might lead clinicians to discharge them from hospital with a prescription for more intensive diabetes medications than they were on before they were hospitalized, but it is not clear if these diabetes medication changes would improve outcomes.
To investigate this, the researchers analyzed data from patients with diabetes who were 65 and older and hospitalized for common medical conditions in VHA hospitals during January 2011–September 2016, and then discharged to the community.
They excluded patients who were hospitalized for things that require immediate change in diabetes treatment and patients who were using insulin before their hospitalization (because instructions to modify insulin dosing frequently don’t have a new prescription).
The researchers identified 28,198 adults with diabetes who were not on insulin and were hospitalized in the VHA health system for heart failure (18%), coronary artery disease (13%), chronic obstructive pulmonary disease (10%), pneumonia (9.6%), and urinary tract infection (7.5%), and less often and not in decreasing order, for acute coronary syndrome, arrhythmia, asthma, chest pain, conduction disorders, heart valve disorders, sepsis, skin infection, stroke, and transient ischemic attack.
Of these patients, 2,768 patients (9.8%) received diabetes medication intensification, and the researchers matched 2,648 of these patients with an equal number of patients who did not receive this treatment intensification.
The patients in each group had a mean age of 73 and 98.5% were male; 78% were White.
They had a mean A1c of 7.9%. Most were receiving sulfonylurea (43%) or metformin (39%), and few were receiving thiazolidinediones (4.1%), alpha-glucosidase inhibitors (2.7%), dipeptidyl peptidase 4 inhibitors (2.0%), or other types of diabetes drugs (0.1%).
Of the 2,768 patients who received intensified diabetes medication, most received a prescription for insulin (51%) or sulfonylurea (23%).
In the propensity-matched cohort, patients with intensified diabetes medication had a higher rate of severe hypoglycemia at 30 days (1% vs. 0.5%), which translated into a significant twofold higher risk (hazard ratio, 2.17).
The rates of severe hypoglycemia at 1 year were similar in both groups (3.1% and 2.9%).
The incidence of severe hyperglycemia was the same in both groups at 30 days (0.3%) and 1 year (1.3%).
In secondary outcomes, at 1 year, 48% of new oral diabetes medications and 39% of new insulin prescriptions were no longer being filled.
Overall, patients who were discharged with intensified diabetes medication were significantly less likely to die within 30 days than the other patients (1.3% vs. 2.4%; HR, 0.55).
However, this mortality benefit was found only in the subgroup of 2,524 patients who had uncontrolled diabetes when they were admitted to hospital (A1c >7.5%; mean A1c, 9.1%), and not in the propensity-matched subgroup of 2,672 patients who had controlled diabetes then (A1c up to 7.5%; mean A1c, 6.8%).
There was no significant difference in 1-year mortality in patients with versus without intensified treatment (15.8% vs. 17.8%).
There were also no significant between-group difference in rates of hospital readmission at 30 days (roughly 17%) or 1 year (roughly 51%).
The decreases in mean A1c from hospital discharge to 1 year later were also the same in both groups (going from 7.9% to 7.7%).
The study was funded by grants from the National Institute on Aging and the American College of Cardiology. Dr. Anderson has no relevant financial disclosures. Dr. McCoy reported receiving grants from the National Institute of Diabetes and Digestive and Kidney Diseases, AARP, and the Patient-Centered Outcomes Research Institute outside the submitted work. The disclosures of the other authors and the editorial coauthor are available with the article and commentary.
“Short-term hospitalization [for reasons other than diabetes] may not be the time to intervene in long-term diabetes management,” researchers conclude.
They found that, in a national cohort of older almost entirely male veterans with non–insulin-treated type 2 diabetes who were hospitalized for non–diabetes-related common medical conditions, intensified diabetes treatment on hospital discharge was linked to an increased risk of severe hypoglycemia in the immediate postdischarge period.
However, diabetes treatment intensification – that is, receiving a prescription for a new or higher dose of diabetes medicine – was not associated with decreased risks of severe hyperglycemia or with improved glycemic (hemoglobin A1c) control at 30 days or 1 year, according to study results, published in JAMA Network Open.
“We didn’t see a reduction in diabetes emergencies in more intensively treated patients,” lead investigator Timothy S. Anderson, MD, said in an interview.
Also, importantly, there was a low rate of persistence with the new treatment. “Half of the patients were no longer taking these [intensified diabetes medicines] at 1 year, which tells me that context is key,” he pointed out. “If a patient is in the hospital for diabetes [unlike the patients in this study], I think it makes a lot of sense to modify and adjust their regimen to try to help them right then and there.”
The overall risk of severe hyperglycemia or severe hypoglycemia was pretty small in the overall cohort, Dr. Anderson noted, “but we do put people at risk of leaving the hospital and ending up back in the hospital with low blood sugar when we intensify medications, and there’s not necessarily a good signal to suggest that it’s all that urgent to change these medicines.”
Instead, the “safer path” may be to make recommendations to the patient’s outpatient physician and also inform the patient – for example, “We saw some concerns about your diabetes while you were in the hospital, and this is really something that should be looked at when you’re recovered and feeling better from the rest of your health standpoint” – rather than making a diabetes medication change while the person is acutely ill or recovering from illness, said Dr. Anderson, from Beth Israel Deaconess Medical Center and Harvard Medical School, Boston.
The researchers also found an “unexpected” significant decrease in 30-day mortality in the patients with intensified diabetes treatment, which was probably because of confounding that was not accounted for, Dr. Anderson speculated, since clinical trials have consistently shown that benefits from diabetes medications take a longer time to show an effect.
‘Important study,’ but lacked newer meds
This is an “important” study for primary care and in-hospital physicians that shows that “hospitalization is really not the time and the place” to intensify diabetes medication, Rozalina G. McCoy, MD, coauthor of an invited commentary, told this news organization in an interview.
“While overcoming treatment inertia is important, [it should be] done appropriately, so that we don’t overtreat patients,” Dr. McCoy, of the Mayo Clinic in Rochester, Minn., stressed.
The very low rate of persistence of taking intensified medications is a major finding, she agreed. Hospitalized patients “are not in their usual state of health, so if we make long-term treatment decisions based on their acute abnormal situation, that may not be appropriate.”
However, patients with high A1c may benefit from a change at hospital discharge rather than when they see their primary care provider, with the caveat that they need close follow-up as an outpatient.
The study emphasizes the “need for longitudinal patient care rather than episodic patches,” according to Dr. McCoy.
For example, a patient who is hospitalized for a chronic obstructive pulmonary disease or asthma exacerbation may be receiving steroids that cause high blood glucose levels but as soon as they’re done with their steroid course, blood glucose will decrease, so the “need for close outpatient follow-up is very important.”
One limitation of the current work is that an earlier study in the same population by the research group showed that 49% of patients whose treatment regimens were intensified had limited life expectancy or were at or below their A1c goal, so they would not have benefited from the stepped-up treatment, she noted.
Another limitation is that the findings cannot be generalized to women or younger patients, or to patients treated with glucagonlike peptide 1 (GLP-1)–receptor agonists or sodium-glucose cotransporter 2 (SGLT2) inhibitors.
The study patients were seen in the U.S. Veterans Health Administration health system when these newer agents were not used. Three-quarters of patients received intensified treatment with sulfonylurea or insulin, and only one patient received a new GLP-1–receptor agonist.
Ideally, Dr. McCoy said, patients should have been prescribed a GLP-1–receptor agonist if they had atherosclerotic cardiovascular disease or kidney disease, or an SGLT2 inhibitor if they had kidney disease or heart failure, which may have led to different results, and would need to be determined in further study.
Dr. Anderson agreed that “SGLT2 inhibitors and GLP1 agonists are broadly much safer than the older diabetes medicines, at least when it comes to risk of hypoglycemia, and may have more clear benefits in heart disease and mortality. So I would not want to extrapolate our findings to those new classes,” he said. “A similar set of studies would need to be done.”
Study rationale and findings
Hospitalized older adults with diabetes commonly have transiently elevated blood glucose levels that might lead clinicians to discharge them from hospital with a prescription for more intensive diabetes medications than they were on before they were hospitalized, but it is not clear if these diabetes medication changes would improve outcomes.
To investigate this, the researchers analyzed data from patients with diabetes who were 65 and older and hospitalized for common medical conditions in VHA hospitals during January 2011–September 2016, and then discharged to the community.
They excluded patients who were hospitalized for things that require immediate change in diabetes treatment and patients who were using insulin before their hospitalization (because instructions to modify insulin dosing frequently don’t have a new prescription).
The researchers identified 28,198 adults with diabetes who were not on insulin and were hospitalized in the VHA health system for heart failure (18%), coronary artery disease (13%), chronic obstructive pulmonary disease (10%), pneumonia (9.6%), and urinary tract infection (7.5%), and less often and not in decreasing order, for acute coronary syndrome, arrhythmia, asthma, chest pain, conduction disorders, heart valve disorders, sepsis, skin infection, stroke, and transient ischemic attack.
Of these patients, 2,768 patients (9.8%) received diabetes medication intensification, and the researchers matched 2,648 of these patients with an equal number of patients who did not receive this treatment intensification.
The patients in each group had a mean age of 73 and 98.5% were male; 78% were White.
They had a mean A1c of 7.9%. Most were receiving sulfonylurea (43%) or metformin (39%), and few were receiving thiazolidinediones (4.1%), alpha-glucosidase inhibitors (2.7%), dipeptidyl peptidase 4 inhibitors (2.0%), or other types of diabetes drugs (0.1%).
Of the 2,768 patients who received intensified diabetes medication, most received a prescription for insulin (51%) or sulfonylurea (23%).
In the propensity-matched cohort, patients with intensified diabetes medication had a higher rate of severe hypoglycemia at 30 days (1% vs. 0.5%), which translated into a significant twofold higher risk (hazard ratio, 2.17).
The rates of severe hypoglycemia at 1 year were similar in both groups (3.1% and 2.9%).
The incidence of severe hyperglycemia was the same in both groups at 30 days (0.3%) and 1 year (1.3%).
In secondary outcomes, at 1 year, 48% of new oral diabetes medications and 39% of new insulin prescriptions were no longer being filled.
Overall, patients who were discharged with intensified diabetes medication were significantly less likely to die within 30 days than the other patients (1.3% vs. 2.4%; HR, 0.55).
However, this mortality benefit was found only in the subgroup of 2,524 patients who had uncontrolled diabetes when they were admitted to hospital (A1c >7.5%; mean A1c, 9.1%), and not in the propensity-matched subgroup of 2,672 patients who had controlled diabetes then (A1c up to 7.5%; mean A1c, 6.8%).
There was no significant difference in 1-year mortality in patients with versus without intensified treatment (15.8% vs. 17.8%).
There were also no significant between-group difference in rates of hospital readmission at 30 days (roughly 17%) or 1 year (roughly 51%).
The decreases in mean A1c from hospital discharge to 1 year later were also the same in both groups (going from 7.9% to 7.7%).
The study was funded by grants from the National Institute on Aging and the American College of Cardiology. Dr. Anderson has no relevant financial disclosures. Dr. McCoy reported receiving grants from the National Institute of Diabetes and Digestive and Kidney Diseases, AARP, and the Patient-Centered Outcomes Research Institute outside the submitted work. The disclosures of the other authors and the editorial coauthor are available with the article and commentary.
“Short-term hospitalization [for reasons other than diabetes] may not be the time to intervene in long-term diabetes management,” researchers conclude.
They found that, in a national cohort of older almost entirely male veterans with non–insulin-treated type 2 diabetes who were hospitalized for non–diabetes-related common medical conditions, intensified diabetes treatment on hospital discharge was linked to an increased risk of severe hypoglycemia in the immediate postdischarge period.
However, diabetes treatment intensification – that is, receiving a prescription for a new or higher dose of diabetes medicine – was not associated with decreased risks of severe hyperglycemia or with improved glycemic (hemoglobin A1c) control at 30 days or 1 year, according to study results, published in JAMA Network Open.
“We didn’t see a reduction in diabetes emergencies in more intensively treated patients,” lead investigator Timothy S. Anderson, MD, said in an interview.
Also, importantly, there was a low rate of persistence with the new treatment. “Half of the patients were no longer taking these [intensified diabetes medicines] at 1 year, which tells me that context is key,” he pointed out. “If a patient is in the hospital for diabetes [unlike the patients in this study], I think it makes a lot of sense to modify and adjust their regimen to try to help them right then and there.”
The overall risk of severe hyperglycemia or severe hypoglycemia was pretty small in the overall cohort, Dr. Anderson noted, “but we do put people at risk of leaving the hospital and ending up back in the hospital with low blood sugar when we intensify medications, and there’s not necessarily a good signal to suggest that it’s all that urgent to change these medicines.”
Instead, the “safer path” may be to make recommendations to the patient’s outpatient physician and also inform the patient – for example, “We saw some concerns about your diabetes while you were in the hospital, and this is really something that should be looked at when you’re recovered and feeling better from the rest of your health standpoint” – rather than making a diabetes medication change while the person is acutely ill or recovering from illness, said Dr. Anderson, from Beth Israel Deaconess Medical Center and Harvard Medical School, Boston.
The researchers also found an “unexpected” significant decrease in 30-day mortality in the patients with intensified diabetes treatment, which was probably because of confounding that was not accounted for, Dr. Anderson speculated, since clinical trials have consistently shown that benefits from diabetes medications take a longer time to show an effect.
‘Important study,’ but lacked newer meds
This is an “important” study for primary care and in-hospital physicians that shows that “hospitalization is really not the time and the place” to intensify diabetes medication, Rozalina G. McCoy, MD, coauthor of an invited commentary, told this news organization in an interview.
“While overcoming treatment inertia is important, [it should be] done appropriately, so that we don’t overtreat patients,” Dr. McCoy, of the Mayo Clinic in Rochester, Minn., stressed.
The very low rate of persistence of taking intensified medications is a major finding, she agreed. Hospitalized patients “are not in their usual state of health, so if we make long-term treatment decisions based on their acute abnormal situation, that may not be appropriate.”
However, patients with high A1c may benefit from a change at hospital discharge rather than when they see their primary care provider, with the caveat that they need close follow-up as an outpatient.
The study emphasizes the “need for longitudinal patient care rather than episodic patches,” according to Dr. McCoy.
For example, a patient who is hospitalized for a chronic obstructive pulmonary disease or asthma exacerbation may be receiving steroids that cause high blood glucose levels but as soon as they’re done with their steroid course, blood glucose will decrease, so the “need for close outpatient follow-up is very important.”
One limitation of the current work is that an earlier study in the same population by the research group showed that 49% of patients whose treatment regimens were intensified had limited life expectancy or were at or below their A1c goal, so they would not have benefited from the stepped-up treatment, she noted.
Another limitation is that the findings cannot be generalized to women or younger patients, or to patients treated with glucagonlike peptide 1 (GLP-1)–receptor agonists or sodium-glucose cotransporter 2 (SGLT2) inhibitors.
The study patients were seen in the U.S. Veterans Health Administration health system when these newer agents were not used. Three-quarters of patients received intensified treatment with sulfonylurea or insulin, and only one patient received a new GLP-1–receptor agonist.
Ideally, Dr. McCoy said, patients should have been prescribed a GLP-1–receptor agonist if they had atherosclerotic cardiovascular disease or kidney disease, or an SGLT2 inhibitor if they had kidney disease or heart failure, which may have led to different results, and would need to be determined in further study.
Dr. Anderson agreed that “SGLT2 inhibitors and GLP1 agonists are broadly much safer than the older diabetes medicines, at least when it comes to risk of hypoglycemia, and may have more clear benefits in heart disease and mortality. So I would not want to extrapolate our findings to those new classes,” he said. “A similar set of studies would need to be done.”
Study rationale and findings
Hospitalized older adults with diabetes commonly have transiently elevated blood glucose levels that might lead clinicians to discharge them from hospital with a prescription for more intensive diabetes medications than they were on before they were hospitalized, but it is not clear if these diabetes medication changes would improve outcomes.
To investigate this, the researchers analyzed data from patients with diabetes who were 65 and older and hospitalized for common medical conditions in VHA hospitals during January 2011–September 2016, and then discharged to the community.
They excluded patients who were hospitalized for things that require immediate change in diabetes treatment and patients who were using insulin before their hospitalization (because instructions to modify insulin dosing frequently don’t have a new prescription).
The researchers identified 28,198 adults with diabetes who were not on insulin and were hospitalized in the VHA health system for heart failure (18%), coronary artery disease (13%), chronic obstructive pulmonary disease (10%), pneumonia (9.6%), and urinary tract infection (7.5%), and less often and not in decreasing order, for acute coronary syndrome, arrhythmia, asthma, chest pain, conduction disorders, heart valve disorders, sepsis, skin infection, stroke, and transient ischemic attack.
Of these patients, 2,768 patients (9.8%) received diabetes medication intensification, and the researchers matched 2,648 of these patients with an equal number of patients who did not receive this treatment intensification.
The patients in each group had a mean age of 73 and 98.5% were male; 78% were White.
They had a mean A1c of 7.9%. Most were receiving sulfonylurea (43%) or metformin (39%), and few were receiving thiazolidinediones (4.1%), alpha-glucosidase inhibitors (2.7%), dipeptidyl peptidase 4 inhibitors (2.0%), or other types of diabetes drugs (0.1%).
Of the 2,768 patients who received intensified diabetes medication, most received a prescription for insulin (51%) or sulfonylurea (23%).
In the propensity-matched cohort, patients with intensified diabetes medication had a higher rate of severe hypoglycemia at 30 days (1% vs. 0.5%), which translated into a significant twofold higher risk (hazard ratio, 2.17).
The rates of severe hypoglycemia at 1 year were similar in both groups (3.1% and 2.9%).
The incidence of severe hyperglycemia was the same in both groups at 30 days (0.3%) and 1 year (1.3%).
In secondary outcomes, at 1 year, 48% of new oral diabetes medications and 39% of new insulin prescriptions were no longer being filled.
Overall, patients who were discharged with intensified diabetes medication were significantly less likely to die within 30 days than the other patients (1.3% vs. 2.4%; HR, 0.55).
However, this mortality benefit was found only in the subgroup of 2,524 patients who had uncontrolled diabetes when they were admitted to hospital (A1c >7.5%; mean A1c, 9.1%), and not in the propensity-matched subgroup of 2,672 patients who had controlled diabetes then (A1c up to 7.5%; mean A1c, 6.8%).
There was no significant difference in 1-year mortality in patients with versus without intensified treatment (15.8% vs. 17.8%).
There were also no significant between-group difference in rates of hospital readmission at 30 days (roughly 17%) or 1 year (roughly 51%).
The decreases in mean A1c from hospital discharge to 1 year later were also the same in both groups (going from 7.9% to 7.7%).
The study was funded by grants from the National Institute on Aging and the American College of Cardiology. Dr. Anderson has no relevant financial disclosures. Dr. McCoy reported receiving grants from the National Institute of Diabetes and Digestive and Kidney Diseases, AARP, and the Patient-Centered Outcomes Research Institute outside the submitted work. The disclosures of the other authors and the editorial coauthor are available with the article and commentary.
FROM JAMA NETWORK OPEN
Cervical cancer mortality stagnates despite screening
Approximately 12,000 new cases of cervical cancer are diagnosed in women in the United States each year, based on data from the Centers for Disease Control and Prevention, said B.J. Rimel, MD, of Cedars-Sinai Medical Center, Los Angeles, in a presentation at the virtual Advancing NIH Research on the Health of Women conference sponsored by the National Institutes of Health.
Despite increased cervical cancer prevention and screening efforts, the incidence of, and mortality from, cervical cancer has remained stable for the past 2 decades, said Dr. Rimel.
Cervical cancer is the only cancer that can be prevented by vaccination, Dr. Rimel noted. It is essential to identify the women who are dying from cervical cancer, as well as who gets screened, who gets vaccinated, and who ends up in clinical trials, she said.
Novel agents for treating cervical cancer suggest that improvement in stagnant mortality rates is possible, said Dr. Rimel. She noted recent studies of cemiplimab, tisotumab vedotin, and a combination therapy involving pembrolizumab and platinum/paclitaxel, with and without bevacizumab.
Dr. Rimel suggested several opportunities to improve the identification and treatment of cervical cancer: Treat it like a rare disease; address structural racism through clinical trials; create opportunities for low–socioeconomic status patients to be involved in research; and develop solutions according to location (urban vs. rural), she said.
Compared with other cancers, cervical cancer is relatively rare in the United States, Dr. Rimel said. However, “It is important that those with cervical cancer can get treated and get healed from the disease,” she said. To better identify the women with cervical cancer who need treatment and to get them into clinical trials, she suggested using strategies employed by rare disease groups, such as seeking out patient support groups and registries.
Significant racial and ethnic disparities persist in cervical cancer, Dr. Rimel emphasized. Data from the CDC show that Black and Hispanic women in the United States are diagnosed with cervical cancer more frequently than women of other races and ethnicities and are less likely to survive.
“Reimagine cervical cancer as a disease of patients who are historically underrepresented due to race, language, poverty, and location,” she said.
Improving equity in cervical cancer care involves structural and trial-specific issues, said Dr. Rimel. Structural issues start with addressing how women enter into the health care system, she said. Consider where women receive care, and whether women have the opportunity to be vaccinated, and later screened, she said. Consider barriers to cervical cancer trials in centers with larger underserved populations, not only cost or insurance, but also issues of language and trust between patients and health care providers, she noted.
To improve the equity of cervical cancer clinical trials, consider potential barriers to enrollment, she added.
“Low English fluency is a barrier to trial enrollment,” said Dr. Rimel. In-person translation is essential for consent to participate in a trial, and “clinical trial budgets must reflect this requirement,” she added. Patient-reported outcomes need to be in the patient’s preferred language, “this includes online content,” Dr. Rimel said.
Dr. Rimel presented other strategies for clinical trial designs to improve equity.
“Compensate patients for their travel, or provide them with tech to allow for off-site monitoring,” she proposed. Patients of lower socioeconomic status in rural and urban areas have different barriers to enrollment, but virtual visits might be an option for those able to access the Internet when given a device. For others, smaller trial sites closer to home, combined with compensation for travel or missed work, might create more opportunities to participate, Dr. Rimel said. Finally, researchers should consider potential roles for smaller or broader studies that involve less travel and testing that would be feasible for more patients who might not otherwise participate in a clinical trial, she concluded.
Dr. Rimel had no financial conflicts to disclose.
Approximately 12,000 new cases of cervical cancer are diagnosed in women in the United States each year, based on data from the Centers for Disease Control and Prevention, said B.J. Rimel, MD, of Cedars-Sinai Medical Center, Los Angeles, in a presentation at the virtual Advancing NIH Research on the Health of Women conference sponsored by the National Institutes of Health.
Despite increased cervical cancer prevention and screening efforts, the incidence of, and mortality from, cervical cancer has remained stable for the past 2 decades, said Dr. Rimel.
Cervical cancer is the only cancer that can be prevented by vaccination, Dr. Rimel noted. It is essential to identify the women who are dying from cervical cancer, as well as who gets screened, who gets vaccinated, and who ends up in clinical trials, she said.
Novel agents for treating cervical cancer suggest that improvement in stagnant mortality rates is possible, said Dr. Rimel. She noted recent studies of cemiplimab, tisotumab vedotin, and a combination therapy involving pembrolizumab and platinum/paclitaxel, with and without bevacizumab.
Dr. Rimel suggested several opportunities to improve the identification and treatment of cervical cancer: Treat it like a rare disease; address structural racism through clinical trials; create opportunities for low–socioeconomic status patients to be involved in research; and develop solutions according to location (urban vs. rural), she said.
Compared with other cancers, cervical cancer is relatively rare in the United States, Dr. Rimel said. However, “It is important that those with cervical cancer can get treated and get healed from the disease,” she said. To better identify the women with cervical cancer who need treatment and to get them into clinical trials, she suggested using strategies employed by rare disease groups, such as seeking out patient support groups and registries.
Significant racial and ethnic disparities persist in cervical cancer, Dr. Rimel emphasized. Data from the CDC show that Black and Hispanic women in the United States are diagnosed with cervical cancer more frequently than women of other races and ethnicities and are less likely to survive.
“Reimagine cervical cancer as a disease of patients who are historically underrepresented due to race, language, poverty, and location,” she said.
Improving equity in cervical cancer care involves structural and trial-specific issues, said Dr. Rimel. Structural issues start with addressing how women enter into the health care system, she said. Consider where women receive care, and whether women have the opportunity to be vaccinated, and later screened, she said. Consider barriers to cervical cancer trials in centers with larger underserved populations, not only cost or insurance, but also issues of language and trust between patients and health care providers, she noted.
To improve the equity of cervical cancer clinical trials, consider potential barriers to enrollment, she added.
“Low English fluency is a barrier to trial enrollment,” said Dr. Rimel. In-person translation is essential for consent to participate in a trial, and “clinical trial budgets must reflect this requirement,” she added. Patient-reported outcomes need to be in the patient’s preferred language, “this includes online content,” Dr. Rimel said.
Dr. Rimel presented other strategies for clinical trial designs to improve equity.
“Compensate patients for their travel, or provide them with tech to allow for off-site monitoring,” she proposed. Patients of lower socioeconomic status in rural and urban areas have different barriers to enrollment, but virtual visits might be an option for those able to access the Internet when given a device. For others, smaller trial sites closer to home, combined with compensation for travel or missed work, might create more opportunities to participate, Dr. Rimel said. Finally, researchers should consider potential roles for smaller or broader studies that involve less travel and testing that would be feasible for more patients who might not otherwise participate in a clinical trial, she concluded.
Dr. Rimel had no financial conflicts to disclose.
Approximately 12,000 new cases of cervical cancer are diagnosed in women in the United States each year, based on data from the Centers for Disease Control and Prevention, said B.J. Rimel, MD, of Cedars-Sinai Medical Center, Los Angeles, in a presentation at the virtual Advancing NIH Research on the Health of Women conference sponsored by the National Institutes of Health.
Despite increased cervical cancer prevention and screening efforts, the incidence of, and mortality from, cervical cancer has remained stable for the past 2 decades, said Dr. Rimel.
Cervical cancer is the only cancer that can be prevented by vaccination, Dr. Rimel noted. It is essential to identify the women who are dying from cervical cancer, as well as who gets screened, who gets vaccinated, and who ends up in clinical trials, she said.
Novel agents for treating cervical cancer suggest that improvement in stagnant mortality rates is possible, said Dr. Rimel. She noted recent studies of cemiplimab, tisotumab vedotin, and a combination therapy involving pembrolizumab and platinum/paclitaxel, with and without bevacizumab.
Dr. Rimel suggested several opportunities to improve the identification and treatment of cervical cancer: Treat it like a rare disease; address structural racism through clinical trials; create opportunities for low–socioeconomic status patients to be involved in research; and develop solutions according to location (urban vs. rural), she said.
Compared with other cancers, cervical cancer is relatively rare in the United States, Dr. Rimel said. However, “It is important that those with cervical cancer can get treated and get healed from the disease,” she said. To better identify the women with cervical cancer who need treatment and to get them into clinical trials, she suggested using strategies employed by rare disease groups, such as seeking out patient support groups and registries.
Significant racial and ethnic disparities persist in cervical cancer, Dr. Rimel emphasized. Data from the CDC show that Black and Hispanic women in the United States are diagnosed with cervical cancer more frequently than women of other races and ethnicities and are less likely to survive.
“Reimagine cervical cancer as a disease of patients who are historically underrepresented due to race, language, poverty, and location,” she said.
Improving equity in cervical cancer care involves structural and trial-specific issues, said Dr. Rimel. Structural issues start with addressing how women enter into the health care system, she said. Consider where women receive care, and whether women have the opportunity to be vaccinated, and later screened, she said. Consider barriers to cervical cancer trials in centers with larger underserved populations, not only cost or insurance, but also issues of language and trust between patients and health care providers, she noted.
To improve the equity of cervical cancer clinical trials, consider potential barriers to enrollment, she added.
“Low English fluency is a barrier to trial enrollment,” said Dr. Rimel. In-person translation is essential for consent to participate in a trial, and “clinical trial budgets must reflect this requirement,” she added. Patient-reported outcomes need to be in the patient’s preferred language, “this includes online content,” Dr. Rimel said.
Dr. Rimel presented other strategies for clinical trial designs to improve equity.
“Compensate patients for their travel, or provide them with tech to allow for off-site monitoring,” she proposed. Patients of lower socioeconomic status in rural and urban areas have different barriers to enrollment, but virtual visits might be an option for those able to access the Internet when given a device. For others, smaller trial sites closer to home, combined with compensation for travel or missed work, might create more opportunities to participate, Dr. Rimel said. Finally, researchers should consider potential roles for smaller or broader studies that involve less travel and testing that would be feasible for more patients who might not otherwise participate in a clinical trial, she concluded.
Dr. Rimel had no financial conflicts to disclose.
FROM ADVANCING NIH RESEARCH ON THE HEALTH OF WOMEN
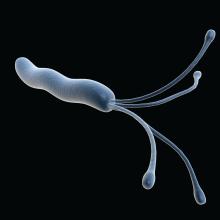
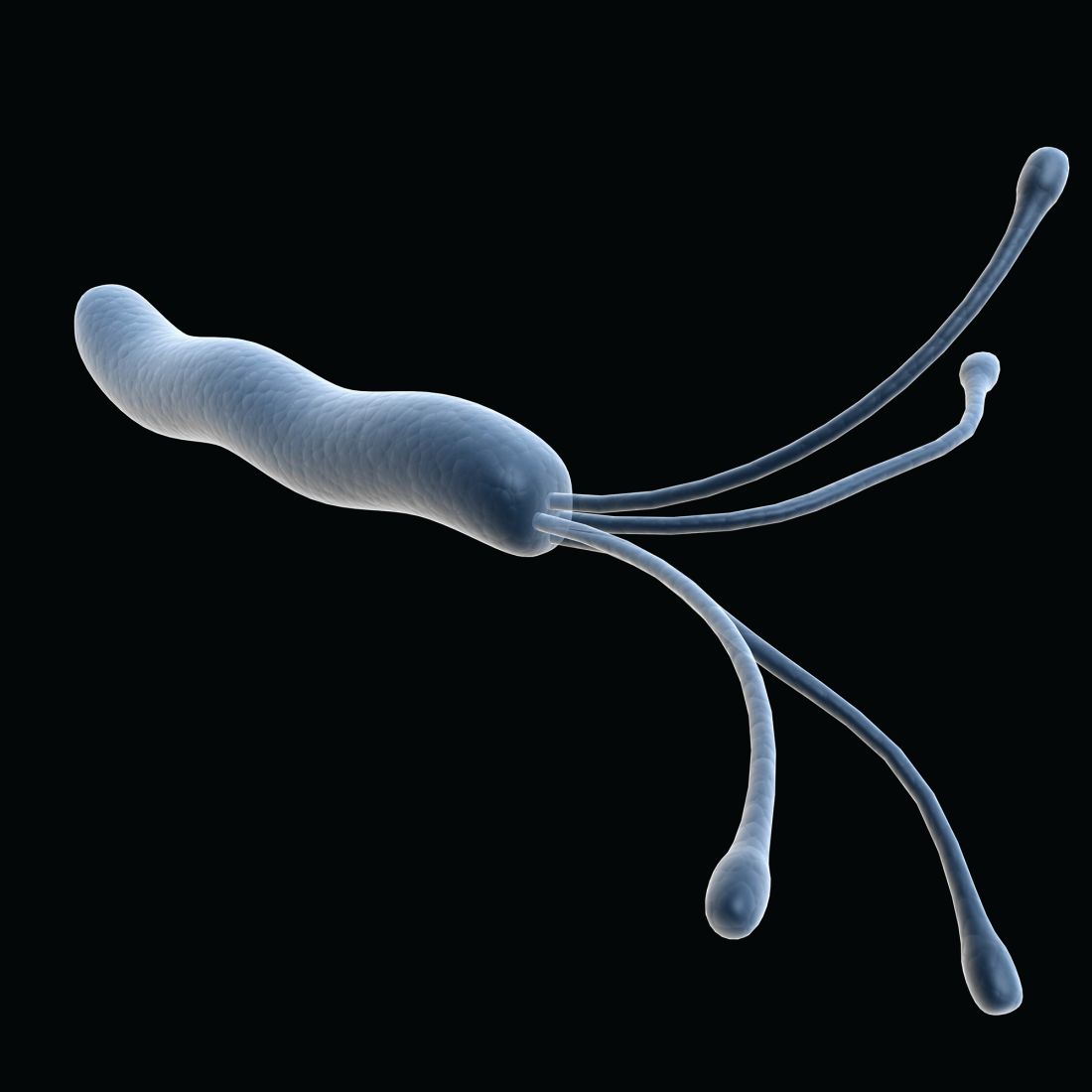
Stool samples meet gastric biopsies for H. pylori antibiotic resistance testing
Using stool samples to test for Helicobacter pylori antibiotic resistance provides highly similar results to those of gastric biopsy samples, which suggests that stool testing may be a safer, more convenient, and more cost-effective option, according to investigators.
Head-to-head testing for resistance-associated mutations using next-generation sequencing (NGS) showed 92% concordance between the two sample types, with 100% technical success among polymerase chain reaction (PCR)–positive stool samples, lead author Steven Moss, MD, of Brown University, Providence, R.I., and colleagues reported.
“H. pylori eradication rates have declined largely due to rising antimicrobial resistance worldwide,” Dr. Moss said at the annual meeting of the American College of Gastroenterology. “There is therefore a need for rapid, accurate, reliable antibiotic resistance testing.”
According to Dr. Moss, molecular resistance testing of gastric biopsies yields similar results to culture-based testing of gastric biopsies, but endoscopic sample collection remains inconvenient and relatively costly, so “it is not commonly performed in many GI practices.
“Whether reliable resistance testing by NGS is possible from stool samples remains unclear,” Dr. Moss said.
To explore this possibility, Dr. Moss and colleagues recruited 262 patients scheduled for upper endoscopy at four sites in the United States. From each patient, two gastric biopsies were taken, and within 2 weeks of the procedure, prior to starting anti–H. pylori therapy, one stool sample was collected.
For gastric biopsy samples, H. pylori positivity was confirmed by PCR, whereas positivity in stool samples was confirmed by both fecal antigen testing and PCR. After confirmation, NGS was conducted, with screening for resistance-associated mutations to six commonly used antibiotics: clarithromycin, levofloxacin, metronidazole, tetracycline, amoxicillin, and rifabutin.
Out of 262 patients, 73 tested positive for H. pylori via stool testing; however, 2 of these patients had inadequate gastric DNA for analysis, leaving 71 patients in the evaluable dataset. Within this group, samples from 50 patients (70.4%) had at least one resistance-association mutation.
Among all 71 individuals, 65 patients (91.5%) had fully concordant results between the two sample types. In four out of the six discordant cases, there was only one difference in antibiotic-associated mutations. Concordance ranged from 89% for metronidazole mutations to 100% for tetracycline, amoxicillin, and rifabutin mutations.
“It is now possible to rapidly obtain susceptibility data without endoscopy,” Dr. Moss concluded. “Using NGS to determine H. pylori antibiotic resistance using stool obviates the cost, inconvenience, and risks of endoscopy resistance profiling.”
Dr. Moss noted that the cost of the stool-based test, through study sponsor American Molecular Laboratories, is about $450, and that the company is “working with various insurance companies to try to get [the test] reimbursed.”
For cases of H. pylori infection without resistance testing results, Dr. Moss recommended first-line treatment with quadruple bismuth–based therapy; however, he noted that “most gastroenterologists, in all kinds of practice, are not measuring their eradication success rate ... so it’s really difficult to know if your best guess is really the appropriate treatment.”
According to Lukasz Kwapisz, MD, of Baylor College of Medicine, Houston, the concordance results are “encouraging,” and suggest that stool-based testing “could be much easier for the patient and the clinician” to find ways to eradicate H. pylori infection.
Dr. Kwapisz predicted that it will take additional successful studies, as well as real-world data, to convert clinicians to the new approach. He suggested that the transition may be gradual, like the adoption of fecal calprotectin testing.
“I don’t know if it’s one singular defining study that will tell you: ‘Okay, we all have to use this [stool-based resistance testing],’ ” he said. “It kind of happens over time – over a 2- or 3-year stretch, I would think, with positive results.”
The study was supported by American Molecular Labs. The investigators disclosed additional relationships with Takeda, Phathom, and Redhill. Dr. Kwapisz reported no conflicts of interest.
Using stool samples to test for Helicobacter pylori antibiotic resistance provides highly similar results to those of gastric biopsy samples, which suggests that stool testing may be a safer, more convenient, and more cost-effective option, according to investigators.
Head-to-head testing for resistance-associated mutations using next-generation sequencing (NGS) showed 92% concordance between the two sample types, with 100% technical success among polymerase chain reaction (PCR)–positive stool samples, lead author Steven Moss, MD, of Brown University, Providence, R.I., and colleagues reported.
“H. pylori eradication rates have declined largely due to rising antimicrobial resistance worldwide,” Dr. Moss said at the annual meeting of the American College of Gastroenterology. “There is therefore a need for rapid, accurate, reliable antibiotic resistance testing.”
According to Dr. Moss, molecular resistance testing of gastric biopsies yields similar results to culture-based testing of gastric biopsies, but endoscopic sample collection remains inconvenient and relatively costly, so “it is not commonly performed in many GI practices.
“Whether reliable resistance testing by NGS is possible from stool samples remains unclear,” Dr. Moss said.
To explore this possibility, Dr. Moss and colleagues recruited 262 patients scheduled for upper endoscopy at four sites in the United States. From each patient, two gastric biopsies were taken, and within 2 weeks of the procedure, prior to starting anti–H. pylori therapy, one stool sample was collected.
For gastric biopsy samples, H. pylori positivity was confirmed by PCR, whereas positivity in stool samples was confirmed by both fecal antigen testing and PCR. After confirmation, NGS was conducted, with screening for resistance-associated mutations to six commonly used antibiotics: clarithromycin, levofloxacin, metronidazole, tetracycline, amoxicillin, and rifabutin.
Out of 262 patients, 73 tested positive for H. pylori via stool testing; however, 2 of these patients had inadequate gastric DNA for analysis, leaving 71 patients in the evaluable dataset. Within this group, samples from 50 patients (70.4%) had at least one resistance-association mutation.
Among all 71 individuals, 65 patients (91.5%) had fully concordant results between the two sample types. In four out of the six discordant cases, there was only one difference in antibiotic-associated mutations. Concordance ranged from 89% for metronidazole mutations to 100% for tetracycline, amoxicillin, and rifabutin mutations.
“It is now possible to rapidly obtain susceptibility data without endoscopy,” Dr. Moss concluded. “Using NGS to determine H. pylori antibiotic resistance using stool obviates the cost, inconvenience, and risks of endoscopy resistance profiling.”
Dr. Moss noted that the cost of the stool-based test, through study sponsor American Molecular Laboratories, is about $450, and that the company is “working with various insurance companies to try to get [the test] reimbursed.”
For cases of H. pylori infection without resistance testing results, Dr. Moss recommended first-line treatment with quadruple bismuth–based therapy; however, he noted that “most gastroenterologists, in all kinds of practice, are not measuring their eradication success rate ... so it’s really difficult to know if your best guess is really the appropriate treatment.”
According to Lukasz Kwapisz, MD, of Baylor College of Medicine, Houston, the concordance results are “encouraging,” and suggest that stool-based testing “could be much easier for the patient and the clinician” to find ways to eradicate H. pylori infection.
Dr. Kwapisz predicted that it will take additional successful studies, as well as real-world data, to convert clinicians to the new approach. He suggested that the transition may be gradual, like the adoption of fecal calprotectin testing.
“I don’t know if it’s one singular defining study that will tell you: ‘Okay, we all have to use this [stool-based resistance testing],’ ” he said. “It kind of happens over time – over a 2- or 3-year stretch, I would think, with positive results.”
The study was supported by American Molecular Labs. The investigators disclosed additional relationships with Takeda, Phathom, and Redhill. Dr. Kwapisz reported no conflicts of interest.
Using stool samples to test for Helicobacter pylori antibiotic resistance provides highly similar results to those of gastric biopsy samples, which suggests that stool testing may be a safer, more convenient, and more cost-effective option, according to investigators.
Head-to-head testing for resistance-associated mutations using next-generation sequencing (NGS) showed 92% concordance between the two sample types, with 100% technical success among polymerase chain reaction (PCR)–positive stool samples, lead author Steven Moss, MD, of Brown University, Providence, R.I., and colleagues reported.
“H. pylori eradication rates have declined largely due to rising antimicrobial resistance worldwide,” Dr. Moss said at the annual meeting of the American College of Gastroenterology. “There is therefore a need for rapid, accurate, reliable antibiotic resistance testing.”
According to Dr. Moss, molecular resistance testing of gastric biopsies yields similar results to culture-based testing of gastric biopsies, but endoscopic sample collection remains inconvenient and relatively costly, so “it is not commonly performed in many GI practices.
“Whether reliable resistance testing by NGS is possible from stool samples remains unclear,” Dr. Moss said.
To explore this possibility, Dr. Moss and colleagues recruited 262 patients scheduled for upper endoscopy at four sites in the United States. From each patient, two gastric biopsies were taken, and within 2 weeks of the procedure, prior to starting anti–H. pylori therapy, one stool sample was collected.
For gastric biopsy samples, H. pylori positivity was confirmed by PCR, whereas positivity in stool samples was confirmed by both fecal antigen testing and PCR. After confirmation, NGS was conducted, with screening for resistance-associated mutations to six commonly used antibiotics: clarithromycin, levofloxacin, metronidazole, tetracycline, amoxicillin, and rifabutin.
Out of 262 patients, 73 tested positive for H. pylori via stool testing; however, 2 of these patients had inadequate gastric DNA for analysis, leaving 71 patients in the evaluable dataset. Within this group, samples from 50 patients (70.4%) had at least one resistance-association mutation.
Among all 71 individuals, 65 patients (91.5%) had fully concordant results between the two sample types. In four out of the six discordant cases, there was only one difference in antibiotic-associated mutations. Concordance ranged from 89% for metronidazole mutations to 100% for tetracycline, amoxicillin, and rifabutin mutations.
“It is now possible to rapidly obtain susceptibility data without endoscopy,” Dr. Moss concluded. “Using NGS to determine H. pylori antibiotic resistance using stool obviates the cost, inconvenience, and risks of endoscopy resistance profiling.”
Dr. Moss noted that the cost of the stool-based test, through study sponsor American Molecular Laboratories, is about $450, and that the company is “working with various insurance companies to try to get [the test] reimbursed.”
For cases of H. pylori infection without resistance testing results, Dr. Moss recommended first-line treatment with quadruple bismuth–based therapy; however, he noted that “most gastroenterologists, in all kinds of practice, are not measuring their eradication success rate ... so it’s really difficult to know if your best guess is really the appropriate treatment.”
According to Lukasz Kwapisz, MD, of Baylor College of Medicine, Houston, the concordance results are “encouraging,” and suggest that stool-based testing “could be much easier for the patient and the clinician” to find ways to eradicate H. pylori infection.
Dr. Kwapisz predicted that it will take additional successful studies, as well as real-world data, to convert clinicians to the new approach. He suggested that the transition may be gradual, like the adoption of fecal calprotectin testing.
“I don’t know if it’s one singular defining study that will tell you: ‘Okay, we all have to use this [stool-based resistance testing],’ ” he said. “It kind of happens over time – over a 2- or 3-year stretch, I would think, with positive results.”
The study was supported by American Molecular Labs. The investigators disclosed additional relationships with Takeda, Phathom, and Redhill. Dr. Kwapisz reported no conflicts of interest.
FROM ACG 2021
Ohio records more deaths than births for first time
In 2020, around 143,661 Ohioans died and 129,313 Ohioans were born, according to The Columbus Dispatch. The trend appears to have continued so far this year, with 107,462 deaths and 100,781 births reported to date.
Deaths haven’t surpassed births in the 112 years since the state began compiling data in 1909, the newspaper reported. The state’s birth rate has been declining for years while the number of deaths has risen, though data shows that the COVID-19 pandemic accelerated the shift.
“It doesn’t surprise me at all,” Joseph Gastaldo, MD, the medical director of infectious diseases for OhioHealth, told the newspaper.
Ohio’s birth rate fell by 4% in 2020, which defied public expectations about a pandemic birth boom. In addition, the state reported 13,927 COVID-19 deaths throughout the year.
“It’s COVID, clearly,” he noted.
Alabama also recorded more deaths than births for the first time last year, according to The New York Times. The state reported 64,714 deaths and 57,641 births in 2020.
“Our state literally shrunk in 2020,” Scott Harris, MD, the state health officer for Alabama, said at a news conference in September.
The state had never recorded a gap that large, even during World War I, World War II, and the 1918 flu pandemic, he said. Alabama has kept records on the numbers since 1900.
“We’ve never had a time when deaths exceeded births,” Dr. Harris said.
In fact, about half of U.S. states reported death rates higher than birth rates in 2020, according to a recent study from researchers at the University of New Hampshire. In 2019, only five states --Maine, New Hampshire, Rhode Island, Vermont, and West Virginia -- reported more deaths than births.
In 2020, the United States reported a record of nearly 3.4 million deaths, which was 18% more than in 2019, the researchers found. COVID-19 was the primary reason for the increase in deaths, accounting for about 11% of total deaths. Meanwhile, births dropped by 4% to about 3.6 million.
The surplus of births over deaths added 229,000 people to the U.S. population in 2020, as compared to 892,000 in 2019, which means the country’s population growth slowed last year. The decline, paired with lower immigration rates during the pandemic, led to the smallest annual percentage population gain in at least 100 years.
“Deaths will likely exceed births again in many states in 2021,” Kenneth Johnson, PhD, a senior demographer and study author, wrote in a statement.
“How large or protracted these fertility declines and mortality increases will be remains to be seen, but they have already dramatically reduced population growth in the United States,” he said.
A version of this article first appeared on WebMD.com.
In 2020, around 143,661 Ohioans died and 129,313 Ohioans were born, according to The Columbus Dispatch. The trend appears to have continued so far this year, with 107,462 deaths and 100,781 births reported to date.
Deaths haven’t surpassed births in the 112 years since the state began compiling data in 1909, the newspaper reported. The state’s birth rate has been declining for years while the number of deaths has risen, though data shows that the COVID-19 pandemic accelerated the shift.
“It doesn’t surprise me at all,” Joseph Gastaldo, MD, the medical director of infectious diseases for OhioHealth, told the newspaper.
Ohio’s birth rate fell by 4% in 2020, which defied public expectations about a pandemic birth boom. In addition, the state reported 13,927 COVID-19 deaths throughout the year.
“It’s COVID, clearly,” he noted.
Alabama also recorded more deaths than births for the first time last year, according to The New York Times. The state reported 64,714 deaths and 57,641 births in 2020.
“Our state literally shrunk in 2020,” Scott Harris, MD, the state health officer for Alabama, said at a news conference in September.
The state had never recorded a gap that large, even during World War I, World War II, and the 1918 flu pandemic, he said. Alabama has kept records on the numbers since 1900.
“We’ve never had a time when deaths exceeded births,” Dr. Harris said.
In fact, about half of U.S. states reported death rates higher than birth rates in 2020, according to a recent study from researchers at the University of New Hampshire. In 2019, only five states --Maine, New Hampshire, Rhode Island, Vermont, and West Virginia -- reported more deaths than births.
In 2020, the United States reported a record of nearly 3.4 million deaths, which was 18% more than in 2019, the researchers found. COVID-19 was the primary reason for the increase in deaths, accounting for about 11% of total deaths. Meanwhile, births dropped by 4% to about 3.6 million.
The surplus of births over deaths added 229,000 people to the U.S. population in 2020, as compared to 892,000 in 2019, which means the country’s population growth slowed last year. The decline, paired with lower immigration rates during the pandemic, led to the smallest annual percentage population gain in at least 100 years.
“Deaths will likely exceed births again in many states in 2021,” Kenneth Johnson, PhD, a senior demographer and study author, wrote in a statement.
“How large or protracted these fertility declines and mortality increases will be remains to be seen, but they have already dramatically reduced population growth in the United States,” he said.
A version of this article first appeared on WebMD.com.
In 2020, around 143,661 Ohioans died and 129,313 Ohioans were born, according to The Columbus Dispatch. The trend appears to have continued so far this year, with 107,462 deaths and 100,781 births reported to date.
Deaths haven’t surpassed births in the 112 years since the state began compiling data in 1909, the newspaper reported. The state’s birth rate has been declining for years while the number of deaths has risen, though data shows that the COVID-19 pandemic accelerated the shift.
“It doesn’t surprise me at all,” Joseph Gastaldo, MD, the medical director of infectious diseases for OhioHealth, told the newspaper.
Ohio’s birth rate fell by 4% in 2020, which defied public expectations about a pandemic birth boom. In addition, the state reported 13,927 COVID-19 deaths throughout the year.
“It’s COVID, clearly,” he noted.
Alabama also recorded more deaths than births for the first time last year, according to The New York Times. The state reported 64,714 deaths and 57,641 births in 2020.
“Our state literally shrunk in 2020,” Scott Harris, MD, the state health officer for Alabama, said at a news conference in September.
The state had never recorded a gap that large, even during World War I, World War II, and the 1918 flu pandemic, he said. Alabama has kept records on the numbers since 1900.
“We’ve never had a time when deaths exceeded births,” Dr. Harris said.
In fact, about half of U.S. states reported death rates higher than birth rates in 2020, according to a recent study from researchers at the University of New Hampshire. In 2019, only five states --Maine, New Hampshire, Rhode Island, Vermont, and West Virginia -- reported more deaths than births.
In 2020, the United States reported a record of nearly 3.4 million deaths, which was 18% more than in 2019, the researchers found. COVID-19 was the primary reason for the increase in deaths, accounting for about 11% of total deaths. Meanwhile, births dropped by 4% to about 3.6 million.
The surplus of births over deaths added 229,000 people to the U.S. population in 2020, as compared to 892,000 in 2019, which means the country’s population growth slowed last year. The decline, paired with lower immigration rates during the pandemic, led to the smallest annual percentage population gain in at least 100 years.
“Deaths will likely exceed births again in many states in 2021,” Kenneth Johnson, PhD, a senior demographer and study author, wrote in a statement.
“How large or protracted these fertility declines and mortality increases will be remains to be seen, but they have already dramatically reduced population growth in the United States,” he said.
A version of this article first appeared on WebMD.com.
Unvaccinated pregnant women have more severe COVID
An increasing number of people who are unvaccinated and pregnant are being hospitalized for COVID-19, report investigators who saw hospital admissions double in a single year.
“With the surge, we had expected to begin treating patients who developed severe or critical illness again in pregnancy,” says Emily Adhikari, MD, from the University of Texas Southwestern Medical Center in Dallas. “But we did not expect the level of respiratory illness that we began to see in our patients. That was a surprise and an alarming finding that we felt was really important to get out there.”
The researchers followed more than 1,500 pregnant women diagnosed with COVID-19 who received care from Parkland Health and Hospital System in Dallas County, one of the nation’s busiest for deliveries. After the emergence of the Delta variant, the number of pregnant women hospitalized with COVID-19 more than doubled over the previous year.
And 82 pregnant women went on to develop severe or critical COVID, they report in their study, published online in the American Journal of Obstetrics and Gynecology. All but 1 of these patients were unvaccinated, 10 needed a ventilator, and two died.
The proportion of cases that were critical was about 5% in 2020. However, in April 2021, even though the number of total cases remained low, the number of severe illnesses started to rise. After the Delta variant became dominant, both the number and severity of cases increased, and after August 2021, more than 25% of pregnant people diagnosed with COVID-19 required hospitalization.
Hospitalizations Double
“We need to focus and really act urgently to recommend vaccination in pregnancy because that is the primary prevention tool that we have,” says Dr. Adhikari. “We do not have a proven cure for this illness, and that is important to know.”
These findings, which focus on a vulnerable population, are especially important given the elevated prevalence of COVID-19 in pregnant people of lower economic status, said Lissette Tanner, MD, MPH, from Emory University in Atlanta, who was not involved with the study.
“There are higher rates of hospitalization and death among Black, Hispanic, and Native American communities,” she reported. “It is essential to know how the virus is affecting those most affected and often most disadvantaged to deal with the pandemic.”
Vaccination rates are low in this population; just 19.2% of pregnant women receive at least one dose during pregnancy, according to the CDC. But pregnancy confers a higher risk for severe COVID-19 illness and for adverse outcomes, such as preterm birth and stillbirth.
Of the 665 people in the study cohort who were pregnant or had given birth when the vaccines were available, only 21.4% received at least one dose of a COVID-19 vaccine.
Given the increased risk for COVID-19 during pregnancy, the American College of Obstetricians and Gynecologists, the Society for Maternal-Fetal Medicine, and the CDC recommend vaccination for people who are pregnant, breastfeeding, or trying to get pregnant.
According to ACOG, pregnant women who are fully vaccinated can follow the same guidelines as everyone else who is fully vaccinated; however, to prevent breakthrough infections, they might want to continue wearing a mask. ACOG also recommends that those not fully vaccinated follow physical-distancing guidelines and limit contact with people as much as possible to avoid infection.
A version of this article first appeared on WebMD.com.
An increasing number of people who are unvaccinated and pregnant are being hospitalized for COVID-19, report investigators who saw hospital admissions double in a single year.
“With the surge, we had expected to begin treating patients who developed severe or critical illness again in pregnancy,” says Emily Adhikari, MD, from the University of Texas Southwestern Medical Center in Dallas. “But we did not expect the level of respiratory illness that we began to see in our patients. That was a surprise and an alarming finding that we felt was really important to get out there.”
The researchers followed more than 1,500 pregnant women diagnosed with COVID-19 who received care from Parkland Health and Hospital System in Dallas County, one of the nation’s busiest for deliveries. After the emergence of the Delta variant, the number of pregnant women hospitalized with COVID-19 more than doubled over the previous year.
And 82 pregnant women went on to develop severe or critical COVID, they report in their study, published online in the American Journal of Obstetrics and Gynecology. All but 1 of these patients were unvaccinated, 10 needed a ventilator, and two died.
The proportion of cases that were critical was about 5% in 2020. However, in April 2021, even though the number of total cases remained low, the number of severe illnesses started to rise. After the Delta variant became dominant, both the number and severity of cases increased, and after August 2021, more than 25% of pregnant people diagnosed with COVID-19 required hospitalization.
Hospitalizations Double
“We need to focus and really act urgently to recommend vaccination in pregnancy because that is the primary prevention tool that we have,” says Dr. Adhikari. “We do not have a proven cure for this illness, and that is important to know.”
These findings, which focus on a vulnerable population, are especially important given the elevated prevalence of COVID-19 in pregnant people of lower economic status, said Lissette Tanner, MD, MPH, from Emory University in Atlanta, who was not involved with the study.
“There are higher rates of hospitalization and death among Black, Hispanic, and Native American communities,” she reported. “It is essential to know how the virus is affecting those most affected and often most disadvantaged to deal with the pandemic.”
Vaccination rates are low in this population; just 19.2% of pregnant women receive at least one dose during pregnancy, according to the CDC. But pregnancy confers a higher risk for severe COVID-19 illness and for adverse outcomes, such as preterm birth and stillbirth.
Of the 665 people in the study cohort who were pregnant or had given birth when the vaccines were available, only 21.4% received at least one dose of a COVID-19 vaccine.
Given the increased risk for COVID-19 during pregnancy, the American College of Obstetricians and Gynecologists, the Society for Maternal-Fetal Medicine, and the CDC recommend vaccination for people who are pregnant, breastfeeding, or trying to get pregnant.
According to ACOG, pregnant women who are fully vaccinated can follow the same guidelines as everyone else who is fully vaccinated; however, to prevent breakthrough infections, they might want to continue wearing a mask. ACOG also recommends that those not fully vaccinated follow physical-distancing guidelines and limit contact with people as much as possible to avoid infection.
A version of this article first appeared on WebMD.com.
An increasing number of people who are unvaccinated and pregnant are being hospitalized for COVID-19, report investigators who saw hospital admissions double in a single year.
“With the surge, we had expected to begin treating patients who developed severe or critical illness again in pregnancy,” says Emily Adhikari, MD, from the University of Texas Southwestern Medical Center in Dallas. “But we did not expect the level of respiratory illness that we began to see in our patients. That was a surprise and an alarming finding that we felt was really important to get out there.”
The researchers followed more than 1,500 pregnant women diagnosed with COVID-19 who received care from Parkland Health and Hospital System in Dallas County, one of the nation’s busiest for deliveries. After the emergence of the Delta variant, the number of pregnant women hospitalized with COVID-19 more than doubled over the previous year.
And 82 pregnant women went on to develop severe or critical COVID, they report in their study, published online in the American Journal of Obstetrics and Gynecology. All but 1 of these patients were unvaccinated, 10 needed a ventilator, and two died.
The proportion of cases that were critical was about 5% in 2020. However, in April 2021, even though the number of total cases remained low, the number of severe illnesses started to rise. After the Delta variant became dominant, both the number and severity of cases increased, and after August 2021, more than 25% of pregnant people diagnosed with COVID-19 required hospitalization.
Hospitalizations Double
“We need to focus and really act urgently to recommend vaccination in pregnancy because that is the primary prevention tool that we have,” says Dr. Adhikari. “We do not have a proven cure for this illness, and that is important to know.”
These findings, which focus on a vulnerable population, are especially important given the elevated prevalence of COVID-19 in pregnant people of lower economic status, said Lissette Tanner, MD, MPH, from Emory University in Atlanta, who was not involved with the study.
“There are higher rates of hospitalization and death among Black, Hispanic, and Native American communities,” she reported. “It is essential to know how the virus is affecting those most affected and often most disadvantaged to deal with the pandemic.”
Vaccination rates are low in this population; just 19.2% of pregnant women receive at least one dose during pregnancy, according to the CDC. But pregnancy confers a higher risk for severe COVID-19 illness and for adverse outcomes, such as preterm birth and stillbirth.
Of the 665 people in the study cohort who were pregnant or had given birth when the vaccines were available, only 21.4% received at least one dose of a COVID-19 vaccine.
Given the increased risk for COVID-19 during pregnancy, the American College of Obstetricians and Gynecologists, the Society for Maternal-Fetal Medicine, and the CDC recommend vaccination for people who are pregnant, breastfeeding, or trying to get pregnant.
According to ACOG, pregnant women who are fully vaccinated can follow the same guidelines as everyone else who is fully vaccinated; however, to prevent breakthrough infections, they might want to continue wearing a mask. ACOG also recommends that those not fully vaccinated follow physical-distancing guidelines and limit contact with people as much as possible to avoid infection.
A version of this article first appeared on WebMD.com.
FDA panel votes to approve Pfizer’s vaccine for children
Seventeen of the 18 members of the Vaccines and Related Biological Products Advisory Committee (VRBPAC) on Oct. 26 voted to recommend the 10-microgram shot for kids, which is one-third the dose given to adults.
One member, Michael Kurilla, MD, director of the division of clinical innovation at the National Institutes of Health, Bethesda, Md., abstained from voting.
If the FDA follows the recommendation, as it typically does, and issues an Emergency Use Authorization for the vaccine, the shots could be available within days.
After the FDA’s final decision, the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices will meet to make specific recommendations for its use. The CDC committee must stick closely to the conditions for use spelled out in the EUA, so their recommendations are likely to be similar to those made by the FDA. Their next meeting is scheduled for Nov. 2 and 3.
In the end, some on the panel felt uneasy with their decision.
“I voted yes primarily because I wanted to make sure that children who really need this vaccine, the Black and brown children of our country, get the vaccine,” said James Hildreth, MD, PhD, president and CEO of Meharry Medical College in Nashville.
“But to be honest, the best way to protect the health of some children will be to do nothing because they will be just fine,” he said.
Others said they were surprised by how difficult the decision had been.
“This is a much tougher one than we had expected going into it,” said committee member Eric Rubin, MD, editor and chief of the New England Journal of Medicine, during the FDA advisory committee’s meeting.
Ahead of the vote, the committee heard presentations outlining the expected benefits of vaccinating children along with potential risks.
“Children have been greatly impacted by the pandemic,” said Fiona Havers, MD, a medical officer with the CDC in Atlanta who reviewed the epidemiology of COVID-19 in kids.
In the second year of the pandemic, as more seniors have been vaccinated against the virus, COVID cases have largely shifted from older to younger age groups.
So far, there have been more than 1.9 million COVID-19 cases in children ages 5 through 11 in the United States.. Cases in kids saw a big jump in July and August with summer travel, schools reopening, and the dominance of the Delta variant.
And those are just the cases reported to the CDC. Regular testing of anonymous blood samples collected at sites across the United States indicates that 6 times as many kids have had COVID than what is reflected in official counts.
Last winter, blood sample testing showed about 13% of children had antibodies against the virus, suggesting they’d been infected. By this summer, that number had risen to 42%.
That figure clearly made an impression on many members of the committee who asked the FDA’s vaccine reviewers if they had tried to account for immunity from past infections in their modeling. They had not.
Some felt that even with a highly effective vaccine — new data presented by Pfizer showed the children’s dose was 90% effective at preventing symptomatic infections in kids — caution was warranted as much is still unknown about myocarditis, a rare side effect of the mRNA vaccines.
Myocarditis has been more common in younger age groups. It usually goes away over time but requires hospital care. It’s not known if myocarditis could have lingering effects for those who experience it.
There were no cases of myocarditis seen in Pfizer’s studies of the vaccine in children, and no other serious events were seen. Vaccine side effects reported in the Pfizer studies were mostly mild and included fatigue, headache, and pain at the injection site.
“We think we have optimized the immune response and minimized our reactions,” said William Gruber, MD, senior vice president vaccine research and clinical development at Pfizer.
But the studies didn’t include enough participants to pick up rare, but serious adverse events like myocarditis.
“We’re worried about a side effect that we can’t measure yet, but it’s probably real, and we see a benefit that isn’t the same as it is in older age groups,” said Dr. Rubin.
Benefits vs. risks
FDA modeled the benefits and risks for children under a variety of scenarios. The benefits of the vaccines to children very much depend on the amount of transmission in the community.
When transmission is high, the benefits to children — in terms of infections, hospitalizations, ICU admissions — clearly outweigh its risks.
But when COVID-19 rates are low in the community, as they were in June, FDA analysts predicted the vaccines might send more children to the hospital for myocarditis than the virus would.
The FDA noted that kids who are hospitalized for myocarditis tend not to be as ill as children with COVID-19, however.
“If the trends continue the way they are going, the emergency for children is not what we might think it would be. That was my concern,” Dr. Hildreth said.
But others warned against complacency.
“Thinking that this is going to be the end of the wave permanently may be a little overly optimistic,” said committee chairman Arnold Monto, MD, a professor of public health and epidemiology at the University of Michigan, Ann Arbor.
The majority of COVID-19 cases in children are mild. Only about 1% of kids are hospitalized for their infections, according to CDC data. But the rates of hospitalizations in kids are about 3 times higher for people of color — including Blacks, Hispanics, and Native Americans, as compared to Whites and Asian Americans.
Since the start of the pandemic, 94 children ages 5 to 11 have died, making it the eighth leading cause of death for kids this age last year.
More than 5,200 children have developed a delayed complication from their infections called Multi-System Inflammatory Syndrome (MIS-C).
MIS-C can be severe and require hospital care and can lead to myocarditis. Children ages 5 to 11 are the age group at greatest risk for this complication.
Kids can also get long COVID. There’s not a lot of data on how often this happens, though it appears to be less frequent in children than in adults.
But a survey in the United Kingdom found that 7%-8% of kids have symptoms from their infections that last longer than 12 weeks, Dr. Havers said. Symptoms that can linger for kids include fatigue, cough, muscle and joint pain, headaches, and insomnia.
More than 1 million children have been impacted by school closures so far this year, and quarantines have had lasting impacts on learning, social development, and mental health.
Even though kids aren’t usually COVID superspreaders, they can still pass the infection on to others.
“What is clear is that secondary transmission from children, both to other children and to adults, does occur,” Dr. Havers said.
For that reason, they can continue the spread of the virus and give it opportunities to mutate and become more dangerous.
Safety monitoring to continue
Some committee members referenced thousands of letters they had received within the past few days urging them to vote against the vaccine.
Jay Portnoy, MD, a professor of pediatrics at Children’s Mercy Hospital in Kansas City, Mo., said he had personally received about 4,000 emails.
“But I feel like I need to also represent the consumers, the parents that I see every day in the clinic who are terrified of sending their children to school because they’re not protected against COVID,” he said, explaining his vote to recommend authorization.
“Our kids are going to be dealing with this virus for many years to come. It’s going to come repeatedly. Getting this vaccine is just the first step that they can take to protect themselves from having bad outcomes,” Dr. Portnoy said.
Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, reminded members of the committee that there were several government surveillance systems in place to catch any potential safety issues in near real time.
“I really appreciate very much the concern here. The safety monitoring of this vaccine will continue,” Dr. Marks said. “I do view this as one of our greatest responsibilities.”
“I really am so grateful that we had this discussion and voted to approve,” said Capt. Amanda Cohn, MD, chief medical officer at the National Center for Immunization and Respiratory Diseases.
“I think the benefits in this age group really are super important even if they are lower than for other age groups.”
This article was updated 10/27/21.
A version of this article first appeared on WebMD.com.
Seventeen of the 18 members of the Vaccines and Related Biological Products Advisory Committee (VRBPAC) on Oct. 26 voted to recommend the 10-microgram shot for kids, which is one-third the dose given to adults.
One member, Michael Kurilla, MD, director of the division of clinical innovation at the National Institutes of Health, Bethesda, Md., abstained from voting.
If the FDA follows the recommendation, as it typically does, and issues an Emergency Use Authorization for the vaccine, the shots could be available within days.
After the FDA’s final decision, the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices will meet to make specific recommendations for its use. The CDC committee must stick closely to the conditions for use spelled out in the EUA, so their recommendations are likely to be similar to those made by the FDA. Their next meeting is scheduled for Nov. 2 and 3.
In the end, some on the panel felt uneasy with their decision.
“I voted yes primarily because I wanted to make sure that children who really need this vaccine, the Black and brown children of our country, get the vaccine,” said James Hildreth, MD, PhD, president and CEO of Meharry Medical College in Nashville.
“But to be honest, the best way to protect the health of some children will be to do nothing because they will be just fine,” he said.
Others said they were surprised by how difficult the decision had been.
“This is a much tougher one than we had expected going into it,” said committee member Eric Rubin, MD, editor and chief of the New England Journal of Medicine, during the FDA advisory committee’s meeting.
Ahead of the vote, the committee heard presentations outlining the expected benefits of vaccinating children along with potential risks.
“Children have been greatly impacted by the pandemic,” said Fiona Havers, MD, a medical officer with the CDC in Atlanta who reviewed the epidemiology of COVID-19 in kids.
In the second year of the pandemic, as more seniors have been vaccinated against the virus, COVID cases have largely shifted from older to younger age groups.
So far, there have been more than 1.9 million COVID-19 cases in children ages 5 through 11 in the United States.. Cases in kids saw a big jump in July and August with summer travel, schools reopening, and the dominance of the Delta variant.
And those are just the cases reported to the CDC. Regular testing of anonymous blood samples collected at sites across the United States indicates that 6 times as many kids have had COVID than what is reflected in official counts.
Last winter, blood sample testing showed about 13% of children had antibodies against the virus, suggesting they’d been infected. By this summer, that number had risen to 42%.
That figure clearly made an impression on many members of the committee who asked the FDA’s vaccine reviewers if they had tried to account for immunity from past infections in their modeling. They had not.
Some felt that even with a highly effective vaccine — new data presented by Pfizer showed the children’s dose was 90% effective at preventing symptomatic infections in kids — caution was warranted as much is still unknown about myocarditis, a rare side effect of the mRNA vaccines.
Myocarditis has been more common in younger age groups. It usually goes away over time but requires hospital care. It’s not known if myocarditis could have lingering effects for those who experience it.
There were no cases of myocarditis seen in Pfizer’s studies of the vaccine in children, and no other serious events were seen. Vaccine side effects reported in the Pfizer studies were mostly mild and included fatigue, headache, and pain at the injection site.
“We think we have optimized the immune response and minimized our reactions,” said William Gruber, MD, senior vice president vaccine research and clinical development at Pfizer.
But the studies didn’t include enough participants to pick up rare, but serious adverse events like myocarditis.
“We’re worried about a side effect that we can’t measure yet, but it’s probably real, and we see a benefit that isn’t the same as it is in older age groups,” said Dr. Rubin.
Benefits vs. risks
FDA modeled the benefits and risks for children under a variety of scenarios. The benefits of the vaccines to children very much depend on the amount of transmission in the community.
When transmission is high, the benefits to children — in terms of infections, hospitalizations, ICU admissions — clearly outweigh its risks.
But when COVID-19 rates are low in the community, as they were in June, FDA analysts predicted the vaccines might send more children to the hospital for myocarditis than the virus would.
The FDA noted that kids who are hospitalized for myocarditis tend not to be as ill as children with COVID-19, however.
“If the trends continue the way they are going, the emergency for children is not what we might think it would be. That was my concern,” Dr. Hildreth said.
But others warned against complacency.
“Thinking that this is going to be the end of the wave permanently may be a little overly optimistic,” said committee chairman Arnold Monto, MD, a professor of public health and epidemiology at the University of Michigan, Ann Arbor.
The majority of COVID-19 cases in children are mild. Only about 1% of kids are hospitalized for their infections, according to CDC data. But the rates of hospitalizations in kids are about 3 times higher for people of color — including Blacks, Hispanics, and Native Americans, as compared to Whites and Asian Americans.
Since the start of the pandemic, 94 children ages 5 to 11 have died, making it the eighth leading cause of death for kids this age last year.
More than 5,200 children have developed a delayed complication from their infections called Multi-System Inflammatory Syndrome (MIS-C).
MIS-C can be severe and require hospital care and can lead to myocarditis. Children ages 5 to 11 are the age group at greatest risk for this complication.
Kids can also get long COVID. There’s not a lot of data on how often this happens, though it appears to be less frequent in children than in adults.
But a survey in the United Kingdom found that 7%-8% of kids have symptoms from their infections that last longer than 12 weeks, Dr. Havers said. Symptoms that can linger for kids include fatigue, cough, muscle and joint pain, headaches, and insomnia.
More than 1 million children have been impacted by school closures so far this year, and quarantines have had lasting impacts on learning, social development, and mental health.
Even though kids aren’t usually COVID superspreaders, they can still pass the infection on to others.
“What is clear is that secondary transmission from children, both to other children and to adults, does occur,” Dr. Havers said.
For that reason, they can continue the spread of the virus and give it opportunities to mutate and become more dangerous.
Safety monitoring to continue
Some committee members referenced thousands of letters they had received within the past few days urging them to vote against the vaccine.
Jay Portnoy, MD, a professor of pediatrics at Children’s Mercy Hospital in Kansas City, Mo., said he had personally received about 4,000 emails.
“But I feel like I need to also represent the consumers, the parents that I see every day in the clinic who are terrified of sending their children to school because they’re not protected against COVID,” he said, explaining his vote to recommend authorization.
“Our kids are going to be dealing with this virus for many years to come. It’s going to come repeatedly. Getting this vaccine is just the first step that they can take to protect themselves from having bad outcomes,” Dr. Portnoy said.
Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, reminded members of the committee that there were several government surveillance systems in place to catch any potential safety issues in near real time.
“I really appreciate very much the concern here. The safety monitoring of this vaccine will continue,” Dr. Marks said. “I do view this as one of our greatest responsibilities.”
“I really am so grateful that we had this discussion and voted to approve,” said Capt. Amanda Cohn, MD, chief medical officer at the National Center for Immunization and Respiratory Diseases.
“I think the benefits in this age group really are super important even if they are lower than for other age groups.”
This article was updated 10/27/21.
A version of this article first appeared on WebMD.com.
Seventeen of the 18 members of the Vaccines and Related Biological Products Advisory Committee (VRBPAC) on Oct. 26 voted to recommend the 10-microgram shot for kids, which is one-third the dose given to adults.
One member, Michael Kurilla, MD, director of the division of clinical innovation at the National Institutes of Health, Bethesda, Md., abstained from voting.
If the FDA follows the recommendation, as it typically does, and issues an Emergency Use Authorization for the vaccine, the shots could be available within days.
After the FDA’s final decision, the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices will meet to make specific recommendations for its use. The CDC committee must stick closely to the conditions for use spelled out in the EUA, so their recommendations are likely to be similar to those made by the FDA. Their next meeting is scheduled for Nov. 2 and 3.
In the end, some on the panel felt uneasy with their decision.
“I voted yes primarily because I wanted to make sure that children who really need this vaccine, the Black and brown children of our country, get the vaccine,” said James Hildreth, MD, PhD, president and CEO of Meharry Medical College in Nashville.
“But to be honest, the best way to protect the health of some children will be to do nothing because they will be just fine,” he said.
Others said they were surprised by how difficult the decision had been.
“This is a much tougher one than we had expected going into it,” said committee member Eric Rubin, MD, editor and chief of the New England Journal of Medicine, during the FDA advisory committee’s meeting.
Ahead of the vote, the committee heard presentations outlining the expected benefits of vaccinating children along with potential risks.
“Children have been greatly impacted by the pandemic,” said Fiona Havers, MD, a medical officer with the CDC in Atlanta who reviewed the epidemiology of COVID-19 in kids.
In the second year of the pandemic, as more seniors have been vaccinated against the virus, COVID cases have largely shifted from older to younger age groups.
So far, there have been more than 1.9 million COVID-19 cases in children ages 5 through 11 in the United States.. Cases in kids saw a big jump in July and August with summer travel, schools reopening, and the dominance of the Delta variant.
And those are just the cases reported to the CDC. Regular testing of anonymous blood samples collected at sites across the United States indicates that 6 times as many kids have had COVID than what is reflected in official counts.
Last winter, blood sample testing showed about 13% of children had antibodies against the virus, suggesting they’d been infected. By this summer, that number had risen to 42%.
That figure clearly made an impression on many members of the committee who asked the FDA’s vaccine reviewers if they had tried to account for immunity from past infections in their modeling. They had not.
Some felt that even with a highly effective vaccine — new data presented by Pfizer showed the children’s dose was 90% effective at preventing symptomatic infections in kids — caution was warranted as much is still unknown about myocarditis, a rare side effect of the mRNA vaccines.
Myocarditis has been more common in younger age groups. It usually goes away over time but requires hospital care. It’s not known if myocarditis could have lingering effects for those who experience it.
There were no cases of myocarditis seen in Pfizer’s studies of the vaccine in children, and no other serious events were seen. Vaccine side effects reported in the Pfizer studies were mostly mild and included fatigue, headache, and pain at the injection site.
“We think we have optimized the immune response and minimized our reactions,” said William Gruber, MD, senior vice president vaccine research and clinical development at Pfizer.
But the studies didn’t include enough participants to pick up rare, but serious adverse events like myocarditis.
“We’re worried about a side effect that we can’t measure yet, but it’s probably real, and we see a benefit that isn’t the same as it is in older age groups,” said Dr. Rubin.
Benefits vs. risks
FDA modeled the benefits and risks for children under a variety of scenarios. The benefits of the vaccines to children very much depend on the amount of transmission in the community.
When transmission is high, the benefits to children — in terms of infections, hospitalizations, ICU admissions — clearly outweigh its risks.
But when COVID-19 rates are low in the community, as they were in June, FDA analysts predicted the vaccines might send more children to the hospital for myocarditis than the virus would.
The FDA noted that kids who are hospitalized for myocarditis tend not to be as ill as children with COVID-19, however.
“If the trends continue the way they are going, the emergency for children is not what we might think it would be. That was my concern,” Dr. Hildreth said.
But others warned against complacency.
“Thinking that this is going to be the end of the wave permanently may be a little overly optimistic,” said committee chairman Arnold Monto, MD, a professor of public health and epidemiology at the University of Michigan, Ann Arbor.
The majority of COVID-19 cases in children are mild. Only about 1% of kids are hospitalized for their infections, according to CDC data. But the rates of hospitalizations in kids are about 3 times higher for people of color — including Blacks, Hispanics, and Native Americans, as compared to Whites and Asian Americans.
Since the start of the pandemic, 94 children ages 5 to 11 have died, making it the eighth leading cause of death for kids this age last year.
More than 5,200 children have developed a delayed complication from their infections called Multi-System Inflammatory Syndrome (MIS-C).
MIS-C can be severe and require hospital care and can lead to myocarditis. Children ages 5 to 11 are the age group at greatest risk for this complication.
Kids can also get long COVID. There’s not a lot of data on how often this happens, though it appears to be less frequent in children than in adults.
But a survey in the United Kingdom found that 7%-8% of kids have symptoms from their infections that last longer than 12 weeks, Dr. Havers said. Symptoms that can linger for kids include fatigue, cough, muscle and joint pain, headaches, and insomnia.
More than 1 million children have been impacted by school closures so far this year, and quarantines have had lasting impacts on learning, social development, and mental health.
Even though kids aren’t usually COVID superspreaders, they can still pass the infection on to others.
“What is clear is that secondary transmission from children, both to other children and to adults, does occur,” Dr. Havers said.
For that reason, they can continue the spread of the virus and give it opportunities to mutate and become more dangerous.
Safety monitoring to continue
Some committee members referenced thousands of letters they had received within the past few days urging them to vote against the vaccine.
Jay Portnoy, MD, a professor of pediatrics at Children’s Mercy Hospital in Kansas City, Mo., said he had personally received about 4,000 emails.
“But I feel like I need to also represent the consumers, the parents that I see every day in the clinic who are terrified of sending their children to school because they’re not protected against COVID,” he said, explaining his vote to recommend authorization.
“Our kids are going to be dealing with this virus for many years to come. It’s going to come repeatedly. Getting this vaccine is just the first step that they can take to protect themselves from having bad outcomes,” Dr. Portnoy said.
Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, reminded members of the committee that there were several government surveillance systems in place to catch any potential safety issues in near real time.
“I really appreciate very much the concern here. The safety monitoring of this vaccine will continue,” Dr. Marks said. “I do view this as one of our greatest responsibilities.”
“I really am so grateful that we had this discussion and voted to approve,” said Capt. Amanda Cohn, MD, chief medical officer at the National Center for Immunization and Respiratory Diseases.
“I think the benefits in this age group really are super important even if they are lower than for other age groups.”
This article was updated 10/27/21.
A version of this article first appeared on WebMD.com.
SURPASS-4: ‘Twincretin’ tirzepatide surpasses insulin glargine in pivotal trial
, which compared the investigational agent to insulin glargine for treatment of type 2 diabetes. The study comprised 1,995 randomized patients with inadequately controlled type 2 diabetes and high cardiovascular disease risk.
Positive results for tirzepatide from SURPASS-4, the fifth and final registration trial for the drug, as well as in the other four studies, tee up the agent for a planned approval submission to the Food and Drug Administration by the end of 2021.
SURPASS-4 differed from the four other pivotal trials not only in its comparator agent, but also by being the longest of the five and the only one that, by design, enrolled exclusively patients with either established cardiovascular disease or high risk for the disease.
The new results “provide initial support for glycemic control [by tirzepatide] being sustained for more than 1 year,” wrote Stefano Del Prato, MD, and associates in their published report in The Lancet.
Despite the trial’s primary endpoint of change in hemoglobin A1c after 52 weeks on treatment, the study continued for another year and had a median time on treatment of 85 weeks, with 7% of enrolled patients remaining on treatment for the maximum on-treatment follow-up of 104 weeks.
Potent glycemic control
The primary endpoint showed that treatment with tirzepatide produced an average incremental reduction in A1c of 0.99% among 328 patients treated with a 10 mg weekly subcutaneous dosage compared with the 1,000 patients who received insulin glargine (Basaglar, Lantus, Toujeo), and an average 1.14% incremental reduction in A1c among 338 patients on a 15-mg dosage once weekly, reported Dr. Del Prato, professor and chief of the section of diabetes at the University of Pisa (Italy).
This met the prespecified criteria for noninferiority of tirzepatide to insulin glargine for reduction of A1c, the study’s primary objective, and also met the study’s prespecified definition of superiority, both statistically significant results. The study also tested a weekly tirzepatide dosage of 5 mg that was significantly superior to insulin glargine for glycemic control.
“The magnitude of A1c reduction and the proportions of patients reaching glycemic targets appear to be larger than in similar studies in which GLP-1 [glucagon-like peptide–1] receptor agonists have been compared with glargine,” the investigators wrote in their report.
The A1c effect of tirzepatide seen across all five SURPASS trials “surpasses what we’ve seen with other [glycemia control] drugs, with the possible exception of insulin,” said Jan W. Eriksson, MD, PhD, professor of clinical diabetes and metabolism at Uppsala (Sweden) University.
The results also showed several other clinically meaningful benefits from tirzepatide treatment. A composite outcome of reduction of A1c to less than 7% with no weight gain and no clinically significant documented symptomatic or severe hypoglycemia occurred in 74%-88% of patients in the three tirzepatide arms compared with 13% of patients treated with insulin glargine. After 52 weeks on treatment, body weight fell by an average of 8%, 11%, and 13% from baseline in the three tirzepatide treatment arms in a dose-dependent way, while weight rose by an average of 2% among those who received insulin glargine. Weight reduction of at least 10% occurred in 36%-66% of patients treated with tirzepatide, compared with 2% on treatment with insulin glargine.
SURPASS-4 was not run as a blinded study because of differences in administration of the comparator agents.
Safety appears similar to GLP-1 receptor agonists
The safety profile of tirzepatide in SURPASS-4, as it was in all of the other four trials in the SURPASS series, was consistent with previously reported safety of agents in the GLP-1 receptor agonist class, said Dr. Del Prato. It was an expected finding as tirzepatide combines activity as a GLP-1 receptor agonist with activity as a glucose-dependent insulinotropic polypeptide (GIP) receptor agonist in a single molecule.
The most common adverse effects were gastrointestinal, including diarrhea, nausea, decreased appetite, and vomiting. Most of these effects were mild or moderate, and they occurred most often during dose escalation of tirzepatide in the first 24 weeks on treatment.
The GIP receptor agonist effect of tirzepatide may diminish the nausea experienced by patients as a result of the drug’s GLP-1 receptor agonist action, Dr. Eriksson, designated discussant for the SURPASS trials, said during a session Sept. 30 at the virtual annual meeting of the European Association for the Study of Diabetes (EASD).
Clinically significant or severe hypoglycemia occurred in 8% of all patients on tirzepatide, with no apparent dose relationship, about half the rate of the patients treated with insulin glargine. Notably, the hypoglycemia episodes among patients treated with tirzepatide clustered almost entirely in the subgroup of patients who also took a sulfonylurea agent during the study. (SURPASS-4 allowed enrolled patients to be on their background antidiabetes regimen throughout the study, and at baseline 95% were taking metformin, 54% were on a sulfonylurea, and about a quarter were on a sodium-glucose cotransporter-2 inhibitor.)
“I would advise not using tirzepatide with insulin or with a sulfonylurea,” Dr. Eriksson said. Aside from this risk for hypoglycemia when tirzepatide is used concurrently with certain other antidiabetes drugs, the SURPASS trials have shown “no other important safety signals,” Dr. Eriksson added.
Cardiovascular safety
All enrolled patients had either known coronary, cerebrovascular, or peripheral arterial disease or were at high risk for having one or more of these conditions because they were at least 50 years old with a history of either chronic kidney disease with depressed glomerular filtration or heart failure.
During complete follow-up, the composite rate of cardiovascular death, MI, stroke, or hospitalization for unstable angina was numerically less in the patients who received tirzepatide, 5%, than in those on insulin glargine, 6%, a 26% relative risk reduction that did not achieve significance. The rate of total mortality was 3% in the tirzepatide group and 4% among those on glargine, a 30% relative risk reduction that was not significant.
The cardiovascular disease outcomes “suggest that tirzepatide is safe from a cardiovascular perspective,” Dr. Del Prato said when he presented the SURPASS-4 results during the virtual annual meeting of the EASD. However, a much larger cardiovascular outcomes trial of tirzepatide, SURPASS-CVOT, with more than 12,000 randomized patients and using a GLP-1 receptor agonist as the comparator, is now in progress, with a report on the findings expected in 2025.
Overall, results from all five SURPASS trials of tirzepatide have shown that the drug is “effective and safe in people with type 2 diabetes, providing stringent glycemic control and additional metabolic benefits including weight reduction and an improvement in other cardiometabolic markers,” said Melanie J. Davies, MD, professor of diabetes medicine at the University of Leicester, England.
Looking forward to when tirzepatide will be available for routine use, Dr. Eriksson positioned it near-term as part of a dual or triple regimen, especially for patients with type 2 diabetes who are obese or have uncontrolled hyperglycemia, renal impairment, high cardiovascular disease risk, or high risk for clinically significant or severe hypoglycemia.
A role for tirzepatide as a first-line agent is currently “more speculative,” he added, with more data needed on cardiovascular outcomes, long-term safety, and cost effectiveness.
The existing evidence base for tirzepatide shows “very promising efficacy” for weight loss and glucose lowering with “reassuring safety and tolerability,” and is a “very important addition to current options,” although the long-term safety of chronic tirzepatide treatment remains unproven, he said.
Dr. Eriksson called the drug’s glycemic control “strong and durable” based on the entire SURPASS program, with a “major” weight loss effect. He also suggested that while the adverse effect profile of tirzepatide appears similar to the GLP-1 receptor agonists, the incidence of gastrointestinal adverse events may be lower with tirzepatide.
SURPASS-4 and the other SURPASS trials were funded by Lilly, the company developing tirzepatide. Dr. Del Prato has ties with Lilly, Applied Therapeutics, AstraZeneca, Boehringer Ingelheim, Merck Sharpe and Dohme, Novartis, Novo Nordisk, and Sanofi. Dr. Davies has ties with Lilly, AstraZeneca, Boehringer Ingelheim, Janssen, Merck Sharp & Dohme, Novo Nordisk, Sanofi-Aventis, Servier, Gilead Sciences, Napp Pharmaceuticals, Mitsubishi Tanabe, and Takeda. Dr. Eriksson has ties with AstraZeneca, Ilya Pharma, Merck Sharp & Dohme, and Novo Nordisk.
, which compared the investigational agent to insulin glargine for treatment of type 2 diabetes. The study comprised 1,995 randomized patients with inadequately controlled type 2 diabetes and high cardiovascular disease risk.
Positive results for tirzepatide from SURPASS-4, the fifth and final registration trial for the drug, as well as in the other four studies, tee up the agent for a planned approval submission to the Food and Drug Administration by the end of 2021.
SURPASS-4 differed from the four other pivotal trials not only in its comparator agent, but also by being the longest of the five and the only one that, by design, enrolled exclusively patients with either established cardiovascular disease or high risk for the disease.
The new results “provide initial support for glycemic control [by tirzepatide] being sustained for more than 1 year,” wrote Stefano Del Prato, MD, and associates in their published report in The Lancet.
Despite the trial’s primary endpoint of change in hemoglobin A1c after 52 weeks on treatment, the study continued for another year and had a median time on treatment of 85 weeks, with 7% of enrolled patients remaining on treatment for the maximum on-treatment follow-up of 104 weeks.
Potent glycemic control
The primary endpoint showed that treatment with tirzepatide produced an average incremental reduction in A1c of 0.99% among 328 patients treated with a 10 mg weekly subcutaneous dosage compared with the 1,000 patients who received insulin glargine (Basaglar, Lantus, Toujeo), and an average 1.14% incremental reduction in A1c among 338 patients on a 15-mg dosage once weekly, reported Dr. Del Prato, professor and chief of the section of diabetes at the University of Pisa (Italy).
This met the prespecified criteria for noninferiority of tirzepatide to insulin glargine for reduction of A1c, the study’s primary objective, and also met the study’s prespecified definition of superiority, both statistically significant results. The study also tested a weekly tirzepatide dosage of 5 mg that was significantly superior to insulin glargine for glycemic control.
“The magnitude of A1c reduction and the proportions of patients reaching glycemic targets appear to be larger than in similar studies in which GLP-1 [glucagon-like peptide–1] receptor agonists have been compared with glargine,” the investigators wrote in their report.
The A1c effect of tirzepatide seen across all five SURPASS trials “surpasses what we’ve seen with other [glycemia control] drugs, with the possible exception of insulin,” said Jan W. Eriksson, MD, PhD, professor of clinical diabetes and metabolism at Uppsala (Sweden) University.
The results also showed several other clinically meaningful benefits from tirzepatide treatment. A composite outcome of reduction of A1c to less than 7% with no weight gain and no clinically significant documented symptomatic or severe hypoglycemia occurred in 74%-88% of patients in the three tirzepatide arms compared with 13% of patients treated with insulin glargine. After 52 weeks on treatment, body weight fell by an average of 8%, 11%, and 13% from baseline in the three tirzepatide treatment arms in a dose-dependent way, while weight rose by an average of 2% among those who received insulin glargine. Weight reduction of at least 10% occurred in 36%-66% of patients treated with tirzepatide, compared with 2% on treatment with insulin glargine.
SURPASS-4 was not run as a blinded study because of differences in administration of the comparator agents.
Safety appears similar to GLP-1 receptor agonists
The safety profile of tirzepatide in SURPASS-4, as it was in all of the other four trials in the SURPASS series, was consistent with previously reported safety of agents in the GLP-1 receptor agonist class, said Dr. Del Prato. It was an expected finding as tirzepatide combines activity as a GLP-1 receptor agonist with activity as a glucose-dependent insulinotropic polypeptide (GIP) receptor agonist in a single molecule.
The most common adverse effects were gastrointestinal, including diarrhea, nausea, decreased appetite, and vomiting. Most of these effects were mild or moderate, and they occurred most often during dose escalation of tirzepatide in the first 24 weeks on treatment.
The GIP receptor agonist effect of tirzepatide may diminish the nausea experienced by patients as a result of the drug’s GLP-1 receptor agonist action, Dr. Eriksson, designated discussant for the SURPASS trials, said during a session Sept. 30 at the virtual annual meeting of the European Association for the Study of Diabetes (EASD).
Clinically significant or severe hypoglycemia occurred in 8% of all patients on tirzepatide, with no apparent dose relationship, about half the rate of the patients treated with insulin glargine. Notably, the hypoglycemia episodes among patients treated with tirzepatide clustered almost entirely in the subgroup of patients who also took a sulfonylurea agent during the study. (SURPASS-4 allowed enrolled patients to be on their background antidiabetes regimen throughout the study, and at baseline 95% were taking metformin, 54% were on a sulfonylurea, and about a quarter were on a sodium-glucose cotransporter-2 inhibitor.)
“I would advise not using tirzepatide with insulin or with a sulfonylurea,” Dr. Eriksson said. Aside from this risk for hypoglycemia when tirzepatide is used concurrently with certain other antidiabetes drugs, the SURPASS trials have shown “no other important safety signals,” Dr. Eriksson added.
Cardiovascular safety
All enrolled patients had either known coronary, cerebrovascular, or peripheral arterial disease or were at high risk for having one or more of these conditions because they were at least 50 years old with a history of either chronic kidney disease with depressed glomerular filtration or heart failure.
During complete follow-up, the composite rate of cardiovascular death, MI, stroke, or hospitalization for unstable angina was numerically less in the patients who received tirzepatide, 5%, than in those on insulin glargine, 6%, a 26% relative risk reduction that did not achieve significance. The rate of total mortality was 3% in the tirzepatide group and 4% among those on glargine, a 30% relative risk reduction that was not significant.
The cardiovascular disease outcomes “suggest that tirzepatide is safe from a cardiovascular perspective,” Dr. Del Prato said when he presented the SURPASS-4 results during the virtual annual meeting of the EASD. However, a much larger cardiovascular outcomes trial of tirzepatide, SURPASS-CVOT, with more than 12,000 randomized patients and using a GLP-1 receptor agonist as the comparator, is now in progress, with a report on the findings expected in 2025.
Overall, results from all five SURPASS trials of tirzepatide have shown that the drug is “effective and safe in people with type 2 diabetes, providing stringent glycemic control and additional metabolic benefits including weight reduction and an improvement in other cardiometabolic markers,” said Melanie J. Davies, MD, professor of diabetes medicine at the University of Leicester, England.
Looking forward to when tirzepatide will be available for routine use, Dr. Eriksson positioned it near-term as part of a dual or triple regimen, especially for patients with type 2 diabetes who are obese or have uncontrolled hyperglycemia, renal impairment, high cardiovascular disease risk, or high risk for clinically significant or severe hypoglycemia.
A role for tirzepatide as a first-line agent is currently “more speculative,” he added, with more data needed on cardiovascular outcomes, long-term safety, and cost effectiveness.
The existing evidence base for tirzepatide shows “very promising efficacy” for weight loss and glucose lowering with “reassuring safety and tolerability,” and is a “very important addition to current options,” although the long-term safety of chronic tirzepatide treatment remains unproven, he said.
Dr. Eriksson called the drug’s glycemic control “strong and durable” based on the entire SURPASS program, with a “major” weight loss effect. He also suggested that while the adverse effect profile of tirzepatide appears similar to the GLP-1 receptor agonists, the incidence of gastrointestinal adverse events may be lower with tirzepatide.
SURPASS-4 and the other SURPASS trials were funded by Lilly, the company developing tirzepatide. Dr. Del Prato has ties with Lilly, Applied Therapeutics, AstraZeneca, Boehringer Ingelheim, Merck Sharpe and Dohme, Novartis, Novo Nordisk, and Sanofi. Dr. Davies has ties with Lilly, AstraZeneca, Boehringer Ingelheim, Janssen, Merck Sharp & Dohme, Novo Nordisk, Sanofi-Aventis, Servier, Gilead Sciences, Napp Pharmaceuticals, Mitsubishi Tanabe, and Takeda. Dr. Eriksson has ties with AstraZeneca, Ilya Pharma, Merck Sharp & Dohme, and Novo Nordisk.
, which compared the investigational agent to insulin glargine for treatment of type 2 diabetes. The study comprised 1,995 randomized patients with inadequately controlled type 2 diabetes and high cardiovascular disease risk.
Positive results for tirzepatide from SURPASS-4, the fifth and final registration trial for the drug, as well as in the other four studies, tee up the agent for a planned approval submission to the Food and Drug Administration by the end of 2021.
SURPASS-4 differed from the four other pivotal trials not only in its comparator agent, but also by being the longest of the five and the only one that, by design, enrolled exclusively patients with either established cardiovascular disease or high risk for the disease.
The new results “provide initial support for glycemic control [by tirzepatide] being sustained for more than 1 year,” wrote Stefano Del Prato, MD, and associates in their published report in The Lancet.
Despite the trial’s primary endpoint of change in hemoglobin A1c after 52 weeks on treatment, the study continued for another year and had a median time on treatment of 85 weeks, with 7% of enrolled patients remaining on treatment for the maximum on-treatment follow-up of 104 weeks.
Potent glycemic control
The primary endpoint showed that treatment with tirzepatide produced an average incremental reduction in A1c of 0.99% among 328 patients treated with a 10 mg weekly subcutaneous dosage compared with the 1,000 patients who received insulin glargine (Basaglar, Lantus, Toujeo), and an average 1.14% incremental reduction in A1c among 338 patients on a 15-mg dosage once weekly, reported Dr. Del Prato, professor and chief of the section of diabetes at the University of Pisa (Italy).
This met the prespecified criteria for noninferiority of tirzepatide to insulin glargine for reduction of A1c, the study’s primary objective, and also met the study’s prespecified definition of superiority, both statistically significant results. The study also tested a weekly tirzepatide dosage of 5 mg that was significantly superior to insulin glargine for glycemic control.
“The magnitude of A1c reduction and the proportions of patients reaching glycemic targets appear to be larger than in similar studies in which GLP-1 [glucagon-like peptide–1] receptor agonists have been compared with glargine,” the investigators wrote in their report.
The A1c effect of tirzepatide seen across all five SURPASS trials “surpasses what we’ve seen with other [glycemia control] drugs, with the possible exception of insulin,” said Jan W. Eriksson, MD, PhD, professor of clinical diabetes and metabolism at Uppsala (Sweden) University.
The results also showed several other clinically meaningful benefits from tirzepatide treatment. A composite outcome of reduction of A1c to less than 7% with no weight gain and no clinically significant documented symptomatic or severe hypoglycemia occurred in 74%-88% of patients in the three tirzepatide arms compared with 13% of patients treated with insulin glargine. After 52 weeks on treatment, body weight fell by an average of 8%, 11%, and 13% from baseline in the three tirzepatide treatment arms in a dose-dependent way, while weight rose by an average of 2% among those who received insulin glargine. Weight reduction of at least 10% occurred in 36%-66% of patients treated with tirzepatide, compared with 2% on treatment with insulin glargine.
SURPASS-4 was not run as a blinded study because of differences in administration of the comparator agents.
Safety appears similar to GLP-1 receptor agonists
The safety profile of tirzepatide in SURPASS-4, as it was in all of the other four trials in the SURPASS series, was consistent with previously reported safety of agents in the GLP-1 receptor agonist class, said Dr. Del Prato. It was an expected finding as tirzepatide combines activity as a GLP-1 receptor agonist with activity as a glucose-dependent insulinotropic polypeptide (GIP) receptor agonist in a single molecule.
The most common adverse effects were gastrointestinal, including diarrhea, nausea, decreased appetite, and vomiting. Most of these effects were mild or moderate, and they occurred most often during dose escalation of tirzepatide in the first 24 weeks on treatment.
The GIP receptor agonist effect of tirzepatide may diminish the nausea experienced by patients as a result of the drug’s GLP-1 receptor agonist action, Dr. Eriksson, designated discussant for the SURPASS trials, said during a session Sept. 30 at the virtual annual meeting of the European Association for the Study of Diabetes (EASD).
Clinically significant or severe hypoglycemia occurred in 8% of all patients on tirzepatide, with no apparent dose relationship, about half the rate of the patients treated with insulin glargine. Notably, the hypoglycemia episodes among patients treated with tirzepatide clustered almost entirely in the subgroup of patients who also took a sulfonylurea agent during the study. (SURPASS-4 allowed enrolled patients to be on their background antidiabetes regimen throughout the study, and at baseline 95% were taking metformin, 54% were on a sulfonylurea, and about a quarter were on a sodium-glucose cotransporter-2 inhibitor.)
“I would advise not using tirzepatide with insulin or with a sulfonylurea,” Dr. Eriksson said. Aside from this risk for hypoglycemia when tirzepatide is used concurrently with certain other antidiabetes drugs, the SURPASS trials have shown “no other important safety signals,” Dr. Eriksson added.
Cardiovascular safety
All enrolled patients had either known coronary, cerebrovascular, or peripheral arterial disease or were at high risk for having one or more of these conditions because they were at least 50 years old with a history of either chronic kidney disease with depressed glomerular filtration or heart failure.
During complete follow-up, the composite rate of cardiovascular death, MI, stroke, or hospitalization for unstable angina was numerically less in the patients who received tirzepatide, 5%, than in those on insulin glargine, 6%, a 26% relative risk reduction that did not achieve significance. The rate of total mortality was 3% in the tirzepatide group and 4% among those on glargine, a 30% relative risk reduction that was not significant.
The cardiovascular disease outcomes “suggest that tirzepatide is safe from a cardiovascular perspective,” Dr. Del Prato said when he presented the SURPASS-4 results during the virtual annual meeting of the EASD. However, a much larger cardiovascular outcomes trial of tirzepatide, SURPASS-CVOT, with more than 12,000 randomized patients and using a GLP-1 receptor agonist as the comparator, is now in progress, with a report on the findings expected in 2025.
Overall, results from all five SURPASS trials of tirzepatide have shown that the drug is “effective and safe in people with type 2 diabetes, providing stringent glycemic control and additional metabolic benefits including weight reduction and an improvement in other cardiometabolic markers,” said Melanie J. Davies, MD, professor of diabetes medicine at the University of Leicester, England.
Looking forward to when tirzepatide will be available for routine use, Dr. Eriksson positioned it near-term as part of a dual or triple regimen, especially for patients with type 2 diabetes who are obese or have uncontrolled hyperglycemia, renal impairment, high cardiovascular disease risk, or high risk for clinically significant or severe hypoglycemia.
A role for tirzepatide as a first-line agent is currently “more speculative,” he added, with more data needed on cardiovascular outcomes, long-term safety, and cost effectiveness.
The existing evidence base for tirzepatide shows “very promising efficacy” for weight loss and glucose lowering with “reassuring safety and tolerability,” and is a “very important addition to current options,” although the long-term safety of chronic tirzepatide treatment remains unproven, he said.
Dr. Eriksson called the drug’s glycemic control “strong and durable” based on the entire SURPASS program, with a “major” weight loss effect. He also suggested that while the adverse effect profile of tirzepatide appears similar to the GLP-1 receptor agonists, the incidence of gastrointestinal adverse events may be lower with tirzepatide.
SURPASS-4 and the other SURPASS trials were funded by Lilly, the company developing tirzepatide. Dr. Del Prato has ties with Lilly, Applied Therapeutics, AstraZeneca, Boehringer Ingelheim, Merck Sharpe and Dohme, Novartis, Novo Nordisk, and Sanofi. Dr. Davies has ties with Lilly, AstraZeneca, Boehringer Ingelheim, Janssen, Merck Sharp & Dohme, Novo Nordisk, Sanofi-Aventis, Servier, Gilead Sciences, Napp Pharmaceuticals, Mitsubishi Tanabe, and Takeda. Dr. Eriksson has ties with AstraZeneca, Ilya Pharma, Merck Sharp & Dohme, and Novo Nordisk.
FROM THE LANCET
Researchers parse which patients with T2D need SGLT2 inhibition
Agents that form the sodium-glucose cotransporter 2 inhibitor class – including canagliflozin (Invokana), dapagliflozin (Farxiga), and empagliflozin (Jardiance) – have show remarkably consistent cardiovascular efficacy and safety for treating patients with heart failure, chronic kidney disease, and higher-risk patients with type 2 diabetes.
But despite an essential role now established for drugs in the SGLT2 inhibitor class for patients with heart failure with reduced ejection fraction, progressive renal dysfunction, or – most recently – patients with heart failure with preserved ejection fraction, the scope may be less clear when using these agents in patients with type 2 diabetes because they fall across a broad spectrum of risk for cardiorenal disease.
“What makes patients with type 2 diabetes distinct from other patients in whom SGLT2 inhibitors have been studied, such as patients with heart failure, is that they have a much wider spectrum of risk. Low-risk patients with type 2 diabetes were not included in the SGLT2 inhibitor trials. Defining risk in patients with type 2 diabetes has the potential to inform prioritization” for treatment with an SGLT2 inhibitor, explained David D. Berg, MD, who has led one effort to develop risk scores that can risk-stratify patients with type 2 diabetes based on their vulnerability to incident heart failure and hospitalization for these episodes,
The hefty cost for these drugs, with retail prices that run over $6,000 annually for the most widely used and most potent agents in the class, has spurred researchers to try to find cost-effective ways to identify patients with type 2 diabetes who stand to benefit most from taking an SGLT2 inhibitor.
‘Cost must be considered’
“Cost must be considered, and at this point it’s probably more responsible on a societal level to advise using SGLT2 inhibitors mainly in patients [with type 2 diabetes] with compelling indications,” said Silvio Inzucchi, MD, professor and director of the Yale Medicine Diabetes Center in New Haven, Conn. Dr. Inzucchi added, however, that “I can easily foresee a day when these agents are considered foundational therapy for all patients with type 2 diabetes, after they go generic and cost is not a major issue. I’m starting to lean toward this very simplified approach, but the costs are prohibitive at this time.”
“If the SGLT2 inhibitors were available at a low cost, I’d argue that they should be used in all patients with type 2 diabetes who have no contraindications or tolerability issues; but we live in a world where they are not yet low cost,” agreed Mikhail N. Kosiborod, MD, a cardiologist and codirector of the Cardiometabolic Center of Excellence at Saint Luke’s Mid-America Heart Institute in Kansas City, Mo.
“We can’t give SGLT2 inhibitors to everyone with type 2 diabetes right now because that would be too costly; these agents are so expensive. You start by targeting the patients with the highest risk” for incident heart failure, said Ambarish Pandey, MD, a cardiologist at the University of Texas Southwestern Medical Center, Dallas.
The spotlight the SGLT2 inhibitor class has received, based on its unexpectedly potent efficacy in cutting rates of acute heart failure episodes in patients with type 2 diabetes, has also sharply raised the profile of this complication of type 2 diabetes, an outcome that until recently many clinicians had largely ignored, overshadowed by a focus on adverse outcomes from atherosclerotic cardiovascular disease such as MIs and strokes.
“Results from the SGLT2 inhibitor trials have reignited interest in the relationship between type 2 diabetes and heart failure and have started to shift the mindset of clinicians toward thinking about reducing both atherothrombotic risk and heart failure risk in patients with type 2 diabetes,” said Dr. Berg, a cardiologist at Brigham and Women’s Hospital in Boston.
“Prior to the SGLT2 inhibitor trials, heart failure was on the radar of diabetes clinicians only as something to watch for as a potential side effect of certain glucose-lowering therapies. Now that there are therapies that can lower heart failure hospitalization, it’s made us think more about heart failure, how common it is in patients with type 2 diabetes, and what can we do to lower this risk,” commented Alice Y.Y. Cheng, MD, a diabetes specialist at the University of Toronto.
Banking on biomarkers
Risk scores for assessing the likelihood of people developing incident heart failure date back more than a decade. More recent efforts have focused on patients with type 2 diabetes, starting with scores that relied entirely on clinical markers of risk such as prior heart failure, established coronary artery disease, and chronic kidney disease. Reports of two of these validated scores appeared in 2019, one from a team led by Dr. Berg and associates in 2019, and a second score developed by Dr. Pandey and associates.
More recently, both research teams behind these two scores validated newer versions that further refined assessment of patients with diabetes by including biomarkers of incipient heart failure, such as N-terminal of the prohormone brain natriuretic peptide (NT-proBNP). The UT Southwestern group’s biomarker-based score relies on levels of NT-proBNP as well as on levels of high sensitivity troponin T (hsTnT) and C-reactive protein, plus ECG-based assessment of left ventricular hypertrophy to assess risk for incident heart failure. Developers reported in 2021 that this biomarker score could account for 74% (C-statistic) of the 5-year risk for heart failure among patients with diabetes.
The biomarker-based score devised by Dr. Berg and associates, relies on NT-proBNP, hsTnT, and a history of heart failure to predict the risk for a future hospitalization for heart failure. They reported in Diabetes Care that in validation testing this score accounted for 84% of the risk.
“I’m hopeful that both our original clinically-based risk score and our new biomarker-based score will be endorsed by professional society guidelines. The intent of the biomarker-based score is not to replace the clinical one,” Dr. Berg stressed in an interview. But he acknowledged that it uses biomarker values that currently are not routinely collected in U.S. practice. Biomarkers like NT-proBNP “are highly associated with future heart failure risk, but are not yet routinely assessed,” he said. Because of this, “widespread adoption of the [biomarker] risk tool will require some education.”
It may also require some sort of preliminary screening to determine the appropriateness of using it in a specific patient because of the relative expense of a test for NT-proBNP.
A Texas two-step process
“We can’t perform a [NT-proBNP] test on every patient with type 2 diabetes because cost is a huge barrier,” with a U.K. price of roughly £28 (about $40) per test, commented Naveed Sattar, MD, PhD, professor of metabolic medicine at the University of Glasgow. “NT-proBNP is the best biomarker by far to predict risk” for heart failure,” but “it’s too expensive. It’s not going to happen in everyone,” he said in an interview. He suggested taking a two-step approach to identify patients to test for NT-proBNP based on clinical measures like blood pressure, weight and height, lipid levels and renal function and the presence of suggestive symptoms like dyspnea, fatigue, and peripheral edema, an argument he recently spelled out in detail in an editorial he coauthored.
“More work is needed to define which patients would usefully have cardiac biomarkers measured,” Dr. Sattar wrote with his associate.
Two-step is the approach used in routine practice by clinicians at UT Southwestern Medical Center. “We screen all patients with type 2 diabetes and no diagnosed heart failure who are not already on an SGLT2 inhibitor” using their 2019 screening tool, called the WATCH-DM Risk score, said Dr. Pandey. Patients flagged at high risk by their clinical score receive an SGLT2 inhibitor (presuming no contraindications). The remaining patients with low or intermediate risk may then undergo biomarker-based assessment to find additional patients who warrant SGLT2 inhibitor treatment, he said in an interview.
Often, a record of the most important biomarker, NT-proBNP, is already in the patient’s record and less than a year old, in which case clinicians use that value. An NT-proBNP level of at least 125 pg/mL indicates increased risk in people with a body mass index of less than 30 kg/m2, while for those with higher body mass indexes clinicians at Southwestern apply a threshold for higher risk of at least 100 pg/mL.
In addition to starting those patients on an SGLT2 inhibitor, the Southwestern protocol calls for intensified efforts at weight loss and improved fitness to further lower incident heart failure risk, and they are also considering targeting treatment with a glucagonlike peptide–1 receptor agonist to these patients as well. They have a research protocol in place, called WATCH-DM, that will prospectively assess the efficacy of this strategy.
Despite the cost, others also believe that the time is right for biomarker-based tests to boost access to the benefits that treatment with SGLT2 inhibitors can give patients with type 2 diabetes.
“In theory it’s reasonable” to use a risk score like the recent one reported by Dr. Berg and coauthors, said Vanita R. Aroda, MD, an endocrinologist and director of diabetes clinical research at Brigham and Women’s Hospital in Boston. “We need to pay attention to heart failure as an outcome and use risk stratification” to decide which patients with type 2 diabetes but without established cardiovascular disease warrant treatment with an SGLT2 inhibitor, she said in an interview. “Given the data, we need more concrete recommendations” from medical societies on how to reasonably use biomarkers and imaging to identify patients with type 2 diabetes who are at increased risk for heart failure and hence would benefit from treatment. “This should be of high interest to guidelines committees,” she added.
The earlier version of Dr. Berg’s score, based exclusively on clinical observations and conventional measures like estimated glomerular filtration rate and urinary creatinine to albumin ratio, had overlap with established criteria for starting treatment with an SGLT2 inhibitor, such as the presence of chronic kidney disease, she noted. “A biomarker-based score may provide the additional level of discrimination needed to characterize risk and potential benefit.”
Asymptomatic diabetic cardiomyopathy
Dr. Aroda and several coauthors recently published a review that describes a subset of patients with type 2 diabetes who might get picked up by intensified screening for heart failure risk: those with asymptomatic diabetic cardiomyopathy, a clinical state that they said represents patients with stage B heart failure based on the new Universal Definition and Classification of Heart Failure. Until recently, these patients with type 2 diabetes and asymptomatic cardiomyopathy have mostly gone unrecognized.
A recent report from Dr. Pandey and associates reviewed records from 2,900 U.S. patients with diabetes and no symptoms who had been included in any of three cohort studies and found echocardiographic evidence of early-stage cardiomyopathy in as many as two-thirds. In an editorial about this report, Dr. Aroda and coauthors called these patients a potential “window of opportunity for prevention and treatment of heart failure.”
“There is evidence of structural cardiac changes that progress through the stages of heart failure,” and starting treatment with an SGLT2 inhibitor during an earlier stage can potentially slow or prevent this progression and thereby limit future functional decline, Dr. Aroda said.
Dr. Sattar agreed. Type 2 diabetes appears to help cause “fluid derangements” and abnormal hemodynamics that produces cardiac stress, changes in heart structure, and adverse remodeling of the heart, a process that “some call cardiomyopathy,” which is exacerbated by other pathologic forces that are also often present in these patients such as obesity and hypertension. SGLT2 inhibitors can help these patients by producing “reverse remodeling of the heart.”
“This process was neglected because for many years our focus was on ischemic heart disease in patients with type 2 diabetes. It was there in plain sight, but we were missing it,” explained Dr. Sattar. Having agents from the SGLT2 inhibitor class “has allowed us to better understand this mechanism.”
The SGLT2 inhibitors are “absolutely the driving reason” why the diabetes–heart failure link has become so important, said Dr. Inzucchi. Having drugs that reduce heart failure risk provided clinicians with a tool that has “changed our mindset.”
“Heart failure prevention has been largely neglected in patients with type 2 diabetes. Reprioritizing heart failure prevention to first and foremost among patients with type 2 diabetes is long overdue,” commented Gregg C. Fonarow, MD, professor and chief of cardiology at the University of California, Los Angeles.
Clinicians don’t like risk scores
Will systematic screening for heart failure risk in selected patients with type 2 diabetes take hold, and with it expanded and better-targeted use of SGLT2 inhibitors?
“I hope so,” said Dr. Kosiborod, but one challenge is that “for the most part clinicians don’t like using risk scores.” Only a few have ever been widely incorporated into practice; mostly they become tools for research. Plus, SGLT2 inhibitor uptake has in general been slow to catch on, which Dr. Kosiborod blames primarily on clinical inertia, a pervasive issue that has also hampered optimal use of drugs as commonplace as statins, ACE inhibitors, and angiotensin-receptor blockers.
“Given the avalanche of positive data, uptake of SGLT2 inhibitors will continue to improve and accelerate; but unfortunately, unless something dramatic happens we’ll likely see their continued underuse for several more years,” he predicted. “Designing better systems of care that prioritize prevention are absolutely needed to improve implementation of effective therapies, including SGLT2 inhibitors.”
Despite their underuse the SGLT2 inhibitor class has, in just 6 years since results from the EMPA-REG OUTCOME trial came out and launched the current treatment era, transformed thinking about the risk that heart failure poses to patients with type 2 diabetes and the need to manage this risk.
“I thank the SGLT2 inhibitors for raising awareness of heart failure risk in patients with diabetes,” and for giving clinicians a new way to mitigate this risk, said Dr. Cheng.
Dr. Berg has been a consultant to AstraZeneca, and received research grant support to his institution from AstraZeneca and Pfizer. Dr. Cheng has received personal fees from multiple pharmaceutical companies. Dr. Kosiborod has been an adviser and consultant to multiple pharmaceutical companies; has received research grants from AstraZeneca and Boehringer Ingelheim; and has received other research support from AstraZeneca. Dr. Pandey has been an adviser to Roche Diagnostics; has received nonfinancial support from Pfizer and Merck; and has received research support from Gilead Sciences, Myovista, and Applied Therapeutics. Dr. Sattar has received consulting honoraria from multiple pharmaceutical companies, and has received grant support from Boehringer Ingelheim, Roche Diagnostics, and Novartis. Dr. Aroda has been a consultant for several pharmaceutical companies; has a spouse employed with Janssen; and has received research support (institutional contracts) from multiple pharmaceutical companies. Dr. Fonarow has been a consultant to several pharmaceutical companies.
Agents that form the sodium-glucose cotransporter 2 inhibitor class – including canagliflozin (Invokana), dapagliflozin (Farxiga), and empagliflozin (Jardiance) – have show remarkably consistent cardiovascular efficacy and safety for treating patients with heart failure, chronic kidney disease, and higher-risk patients with type 2 diabetes.
But despite an essential role now established for drugs in the SGLT2 inhibitor class for patients with heart failure with reduced ejection fraction, progressive renal dysfunction, or – most recently – patients with heart failure with preserved ejection fraction, the scope may be less clear when using these agents in patients with type 2 diabetes because they fall across a broad spectrum of risk for cardiorenal disease.
“What makes patients with type 2 diabetes distinct from other patients in whom SGLT2 inhibitors have been studied, such as patients with heart failure, is that they have a much wider spectrum of risk. Low-risk patients with type 2 diabetes were not included in the SGLT2 inhibitor trials. Defining risk in patients with type 2 diabetes has the potential to inform prioritization” for treatment with an SGLT2 inhibitor, explained David D. Berg, MD, who has led one effort to develop risk scores that can risk-stratify patients with type 2 diabetes based on their vulnerability to incident heart failure and hospitalization for these episodes,
The hefty cost for these drugs, with retail prices that run over $6,000 annually for the most widely used and most potent agents in the class, has spurred researchers to try to find cost-effective ways to identify patients with type 2 diabetes who stand to benefit most from taking an SGLT2 inhibitor.
‘Cost must be considered’
“Cost must be considered, and at this point it’s probably more responsible on a societal level to advise using SGLT2 inhibitors mainly in patients [with type 2 diabetes] with compelling indications,” said Silvio Inzucchi, MD, professor and director of the Yale Medicine Diabetes Center in New Haven, Conn. Dr. Inzucchi added, however, that “I can easily foresee a day when these agents are considered foundational therapy for all patients with type 2 diabetes, after they go generic and cost is not a major issue. I’m starting to lean toward this very simplified approach, but the costs are prohibitive at this time.”
“If the SGLT2 inhibitors were available at a low cost, I’d argue that they should be used in all patients with type 2 diabetes who have no contraindications or tolerability issues; but we live in a world where they are not yet low cost,” agreed Mikhail N. Kosiborod, MD, a cardiologist and codirector of the Cardiometabolic Center of Excellence at Saint Luke’s Mid-America Heart Institute in Kansas City, Mo.
“We can’t give SGLT2 inhibitors to everyone with type 2 diabetes right now because that would be too costly; these agents are so expensive. You start by targeting the patients with the highest risk” for incident heart failure, said Ambarish Pandey, MD, a cardiologist at the University of Texas Southwestern Medical Center, Dallas.
The spotlight the SGLT2 inhibitor class has received, based on its unexpectedly potent efficacy in cutting rates of acute heart failure episodes in patients with type 2 diabetes, has also sharply raised the profile of this complication of type 2 diabetes, an outcome that until recently many clinicians had largely ignored, overshadowed by a focus on adverse outcomes from atherosclerotic cardiovascular disease such as MIs and strokes.
“Results from the SGLT2 inhibitor trials have reignited interest in the relationship between type 2 diabetes and heart failure and have started to shift the mindset of clinicians toward thinking about reducing both atherothrombotic risk and heart failure risk in patients with type 2 diabetes,” said Dr. Berg, a cardiologist at Brigham and Women’s Hospital in Boston.
“Prior to the SGLT2 inhibitor trials, heart failure was on the radar of diabetes clinicians only as something to watch for as a potential side effect of certain glucose-lowering therapies. Now that there are therapies that can lower heart failure hospitalization, it’s made us think more about heart failure, how common it is in patients with type 2 diabetes, and what can we do to lower this risk,” commented Alice Y.Y. Cheng, MD, a diabetes specialist at the University of Toronto.
Banking on biomarkers
Risk scores for assessing the likelihood of people developing incident heart failure date back more than a decade. More recent efforts have focused on patients with type 2 diabetes, starting with scores that relied entirely on clinical markers of risk such as prior heart failure, established coronary artery disease, and chronic kidney disease. Reports of two of these validated scores appeared in 2019, one from a team led by Dr. Berg and associates in 2019, and a second score developed by Dr. Pandey and associates.
More recently, both research teams behind these two scores validated newer versions that further refined assessment of patients with diabetes by including biomarkers of incipient heart failure, such as N-terminal of the prohormone brain natriuretic peptide (NT-proBNP). The UT Southwestern group’s biomarker-based score relies on levels of NT-proBNP as well as on levels of high sensitivity troponin T (hsTnT) and C-reactive protein, plus ECG-based assessment of left ventricular hypertrophy to assess risk for incident heart failure. Developers reported in 2021 that this biomarker score could account for 74% (C-statistic) of the 5-year risk for heart failure among patients with diabetes.
The biomarker-based score devised by Dr. Berg and associates, relies on NT-proBNP, hsTnT, and a history of heart failure to predict the risk for a future hospitalization for heart failure. They reported in Diabetes Care that in validation testing this score accounted for 84% of the risk.
“I’m hopeful that both our original clinically-based risk score and our new biomarker-based score will be endorsed by professional society guidelines. The intent of the biomarker-based score is not to replace the clinical one,” Dr. Berg stressed in an interview. But he acknowledged that it uses biomarker values that currently are not routinely collected in U.S. practice. Biomarkers like NT-proBNP “are highly associated with future heart failure risk, but are not yet routinely assessed,” he said. Because of this, “widespread adoption of the [biomarker] risk tool will require some education.”
It may also require some sort of preliminary screening to determine the appropriateness of using it in a specific patient because of the relative expense of a test for NT-proBNP.
A Texas two-step process
“We can’t perform a [NT-proBNP] test on every patient with type 2 diabetes because cost is a huge barrier,” with a U.K. price of roughly £28 (about $40) per test, commented Naveed Sattar, MD, PhD, professor of metabolic medicine at the University of Glasgow. “NT-proBNP is the best biomarker by far to predict risk” for heart failure,” but “it’s too expensive. It’s not going to happen in everyone,” he said in an interview. He suggested taking a two-step approach to identify patients to test for NT-proBNP based on clinical measures like blood pressure, weight and height, lipid levels and renal function and the presence of suggestive symptoms like dyspnea, fatigue, and peripheral edema, an argument he recently spelled out in detail in an editorial he coauthored.
“More work is needed to define which patients would usefully have cardiac biomarkers measured,” Dr. Sattar wrote with his associate.
Two-step is the approach used in routine practice by clinicians at UT Southwestern Medical Center. “We screen all patients with type 2 diabetes and no diagnosed heart failure who are not already on an SGLT2 inhibitor” using their 2019 screening tool, called the WATCH-DM Risk score, said Dr. Pandey. Patients flagged at high risk by their clinical score receive an SGLT2 inhibitor (presuming no contraindications). The remaining patients with low or intermediate risk may then undergo biomarker-based assessment to find additional patients who warrant SGLT2 inhibitor treatment, he said in an interview.
Often, a record of the most important biomarker, NT-proBNP, is already in the patient’s record and less than a year old, in which case clinicians use that value. An NT-proBNP level of at least 125 pg/mL indicates increased risk in people with a body mass index of less than 30 kg/m2, while for those with higher body mass indexes clinicians at Southwestern apply a threshold for higher risk of at least 100 pg/mL.
In addition to starting those patients on an SGLT2 inhibitor, the Southwestern protocol calls for intensified efforts at weight loss and improved fitness to further lower incident heart failure risk, and they are also considering targeting treatment with a glucagonlike peptide–1 receptor agonist to these patients as well. They have a research protocol in place, called WATCH-DM, that will prospectively assess the efficacy of this strategy.
Despite the cost, others also believe that the time is right for biomarker-based tests to boost access to the benefits that treatment with SGLT2 inhibitors can give patients with type 2 diabetes.
“In theory it’s reasonable” to use a risk score like the recent one reported by Dr. Berg and coauthors, said Vanita R. Aroda, MD, an endocrinologist and director of diabetes clinical research at Brigham and Women’s Hospital in Boston. “We need to pay attention to heart failure as an outcome and use risk stratification” to decide which patients with type 2 diabetes but without established cardiovascular disease warrant treatment with an SGLT2 inhibitor, she said in an interview. “Given the data, we need more concrete recommendations” from medical societies on how to reasonably use biomarkers and imaging to identify patients with type 2 diabetes who are at increased risk for heart failure and hence would benefit from treatment. “This should be of high interest to guidelines committees,” she added.
The earlier version of Dr. Berg’s score, based exclusively on clinical observations and conventional measures like estimated glomerular filtration rate and urinary creatinine to albumin ratio, had overlap with established criteria for starting treatment with an SGLT2 inhibitor, such as the presence of chronic kidney disease, she noted. “A biomarker-based score may provide the additional level of discrimination needed to characterize risk and potential benefit.”
Asymptomatic diabetic cardiomyopathy
Dr. Aroda and several coauthors recently published a review that describes a subset of patients with type 2 diabetes who might get picked up by intensified screening for heart failure risk: those with asymptomatic diabetic cardiomyopathy, a clinical state that they said represents patients with stage B heart failure based on the new Universal Definition and Classification of Heart Failure. Until recently, these patients with type 2 diabetes and asymptomatic cardiomyopathy have mostly gone unrecognized.
A recent report from Dr. Pandey and associates reviewed records from 2,900 U.S. patients with diabetes and no symptoms who had been included in any of three cohort studies and found echocardiographic evidence of early-stage cardiomyopathy in as many as two-thirds. In an editorial about this report, Dr. Aroda and coauthors called these patients a potential “window of opportunity for prevention and treatment of heart failure.”
“There is evidence of structural cardiac changes that progress through the stages of heart failure,” and starting treatment with an SGLT2 inhibitor during an earlier stage can potentially slow or prevent this progression and thereby limit future functional decline, Dr. Aroda said.
Dr. Sattar agreed. Type 2 diabetes appears to help cause “fluid derangements” and abnormal hemodynamics that produces cardiac stress, changes in heart structure, and adverse remodeling of the heart, a process that “some call cardiomyopathy,” which is exacerbated by other pathologic forces that are also often present in these patients such as obesity and hypertension. SGLT2 inhibitors can help these patients by producing “reverse remodeling of the heart.”
“This process was neglected because for many years our focus was on ischemic heart disease in patients with type 2 diabetes. It was there in plain sight, but we were missing it,” explained Dr. Sattar. Having agents from the SGLT2 inhibitor class “has allowed us to better understand this mechanism.”
The SGLT2 inhibitors are “absolutely the driving reason” why the diabetes–heart failure link has become so important, said Dr. Inzucchi. Having drugs that reduce heart failure risk provided clinicians with a tool that has “changed our mindset.”
“Heart failure prevention has been largely neglected in patients with type 2 diabetes. Reprioritizing heart failure prevention to first and foremost among patients with type 2 diabetes is long overdue,” commented Gregg C. Fonarow, MD, professor and chief of cardiology at the University of California, Los Angeles.
Clinicians don’t like risk scores
Will systematic screening for heart failure risk in selected patients with type 2 diabetes take hold, and with it expanded and better-targeted use of SGLT2 inhibitors?
“I hope so,” said Dr. Kosiborod, but one challenge is that “for the most part clinicians don’t like using risk scores.” Only a few have ever been widely incorporated into practice; mostly they become tools for research. Plus, SGLT2 inhibitor uptake has in general been slow to catch on, which Dr. Kosiborod blames primarily on clinical inertia, a pervasive issue that has also hampered optimal use of drugs as commonplace as statins, ACE inhibitors, and angiotensin-receptor blockers.
“Given the avalanche of positive data, uptake of SGLT2 inhibitors will continue to improve and accelerate; but unfortunately, unless something dramatic happens we’ll likely see their continued underuse for several more years,” he predicted. “Designing better systems of care that prioritize prevention are absolutely needed to improve implementation of effective therapies, including SGLT2 inhibitors.”
Despite their underuse the SGLT2 inhibitor class has, in just 6 years since results from the EMPA-REG OUTCOME trial came out and launched the current treatment era, transformed thinking about the risk that heart failure poses to patients with type 2 diabetes and the need to manage this risk.
“I thank the SGLT2 inhibitors for raising awareness of heart failure risk in patients with diabetes,” and for giving clinicians a new way to mitigate this risk, said Dr. Cheng.
Dr. Berg has been a consultant to AstraZeneca, and received research grant support to his institution from AstraZeneca and Pfizer. Dr. Cheng has received personal fees from multiple pharmaceutical companies. Dr. Kosiborod has been an adviser and consultant to multiple pharmaceutical companies; has received research grants from AstraZeneca and Boehringer Ingelheim; and has received other research support from AstraZeneca. Dr. Pandey has been an adviser to Roche Diagnostics; has received nonfinancial support from Pfizer and Merck; and has received research support from Gilead Sciences, Myovista, and Applied Therapeutics. Dr. Sattar has received consulting honoraria from multiple pharmaceutical companies, and has received grant support from Boehringer Ingelheim, Roche Diagnostics, and Novartis. Dr. Aroda has been a consultant for several pharmaceutical companies; has a spouse employed with Janssen; and has received research support (institutional contracts) from multiple pharmaceutical companies. Dr. Fonarow has been a consultant to several pharmaceutical companies.
Agents that form the sodium-glucose cotransporter 2 inhibitor class – including canagliflozin (Invokana), dapagliflozin (Farxiga), and empagliflozin (Jardiance) – have show remarkably consistent cardiovascular efficacy and safety for treating patients with heart failure, chronic kidney disease, and higher-risk patients with type 2 diabetes.
But despite an essential role now established for drugs in the SGLT2 inhibitor class for patients with heart failure with reduced ejection fraction, progressive renal dysfunction, or – most recently – patients with heart failure with preserved ejection fraction, the scope may be less clear when using these agents in patients with type 2 diabetes because they fall across a broad spectrum of risk for cardiorenal disease.
“What makes patients with type 2 diabetes distinct from other patients in whom SGLT2 inhibitors have been studied, such as patients with heart failure, is that they have a much wider spectrum of risk. Low-risk patients with type 2 diabetes were not included in the SGLT2 inhibitor trials. Defining risk in patients with type 2 diabetes has the potential to inform prioritization” for treatment with an SGLT2 inhibitor, explained David D. Berg, MD, who has led one effort to develop risk scores that can risk-stratify patients with type 2 diabetes based on their vulnerability to incident heart failure and hospitalization for these episodes,
The hefty cost for these drugs, with retail prices that run over $6,000 annually for the most widely used and most potent agents in the class, has spurred researchers to try to find cost-effective ways to identify patients with type 2 diabetes who stand to benefit most from taking an SGLT2 inhibitor.
‘Cost must be considered’
“Cost must be considered, and at this point it’s probably more responsible on a societal level to advise using SGLT2 inhibitors mainly in patients [with type 2 diabetes] with compelling indications,” said Silvio Inzucchi, MD, professor and director of the Yale Medicine Diabetes Center in New Haven, Conn. Dr. Inzucchi added, however, that “I can easily foresee a day when these agents are considered foundational therapy for all patients with type 2 diabetes, after they go generic and cost is not a major issue. I’m starting to lean toward this very simplified approach, but the costs are prohibitive at this time.”
“If the SGLT2 inhibitors were available at a low cost, I’d argue that they should be used in all patients with type 2 diabetes who have no contraindications or tolerability issues; but we live in a world where they are not yet low cost,” agreed Mikhail N. Kosiborod, MD, a cardiologist and codirector of the Cardiometabolic Center of Excellence at Saint Luke’s Mid-America Heart Institute in Kansas City, Mo.
“We can’t give SGLT2 inhibitors to everyone with type 2 diabetes right now because that would be too costly; these agents are so expensive. You start by targeting the patients with the highest risk” for incident heart failure, said Ambarish Pandey, MD, a cardiologist at the University of Texas Southwestern Medical Center, Dallas.
The spotlight the SGLT2 inhibitor class has received, based on its unexpectedly potent efficacy in cutting rates of acute heart failure episodes in patients with type 2 diabetes, has also sharply raised the profile of this complication of type 2 diabetes, an outcome that until recently many clinicians had largely ignored, overshadowed by a focus on adverse outcomes from atherosclerotic cardiovascular disease such as MIs and strokes.
“Results from the SGLT2 inhibitor trials have reignited interest in the relationship between type 2 diabetes and heart failure and have started to shift the mindset of clinicians toward thinking about reducing both atherothrombotic risk and heart failure risk in patients with type 2 diabetes,” said Dr. Berg, a cardiologist at Brigham and Women’s Hospital in Boston.
“Prior to the SGLT2 inhibitor trials, heart failure was on the radar of diabetes clinicians only as something to watch for as a potential side effect of certain glucose-lowering therapies. Now that there are therapies that can lower heart failure hospitalization, it’s made us think more about heart failure, how common it is in patients with type 2 diabetes, and what can we do to lower this risk,” commented Alice Y.Y. Cheng, MD, a diabetes specialist at the University of Toronto.
Banking on biomarkers
Risk scores for assessing the likelihood of people developing incident heart failure date back more than a decade. More recent efforts have focused on patients with type 2 diabetes, starting with scores that relied entirely on clinical markers of risk such as prior heart failure, established coronary artery disease, and chronic kidney disease. Reports of two of these validated scores appeared in 2019, one from a team led by Dr. Berg and associates in 2019, and a second score developed by Dr. Pandey and associates.
More recently, both research teams behind these two scores validated newer versions that further refined assessment of patients with diabetes by including biomarkers of incipient heart failure, such as N-terminal of the prohormone brain natriuretic peptide (NT-proBNP). The UT Southwestern group’s biomarker-based score relies on levels of NT-proBNP as well as on levels of high sensitivity troponin T (hsTnT) and C-reactive protein, plus ECG-based assessment of left ventricular hypertrophy to assess risk for incident heart failure. Developers reported in 2021 that this biomarker score could account for 74% (C-statistic) of the 5-year risk for heart failure among patients with diabetes.
The biomarker-based score devised by Dr. Berg and associates, relies on NT-proBNP, hsTnT, and a history of heart failure to predict the risk for a future hospitalization for heart failure. They reported in Diabetes Care that in validation testing this score accounted for 84% of the risk.
“I’m hopeful that both our original clinically-based risk score and our new biomarker-based score will be endorsed by professional society guidelines. The intent of the biomarker-based score is not to replace the clinical one,” Dr. Berg stressed in an interview. But he acknowledged that it uses biomarker values that currently are not routinely collected in U.S. practice. Biomarkers like NT-proBNP “are highly associated with future heart failure risk, but are not yet routinely assessed,” he said. Because of this, “widespread adoption of the [biomarker] risk tool will require some education.”
It may also require some sort of preliminary screening to determine the appropriateness of using it in a specific patient because of the relative expense of a test for NT-proBNP.
A Texas two-step process
“We can’t perform a [NT-proBNP] test on every patient with type 2 diabetes because cost is a huge barrier,” with a U.K. price of roughly £28 (about $40) per test, commented Naveed Sattar, MD, PhD, professor of metabolic medicine at the University of Glasgow. “NT-proBNP is the best biomarker by far to predict risk” for heart failure,” but “it’s too expensive. It’s not going to happen in everyone,” he said in an interview. He suggested taking a two-step approach to identify patients to test for NT-proBNP based on clinical measures like blood pressure, weight and height, lipid levels and renal function and the presence of suggestive symptoms like dyspnea, fatigue, and peripheral edema, an argument he recently spelled out in detail in an editorial he coauthored.
“More work is needed to define which patients would usefully have cardiac biomarkers measured,” Dr. Sattar wrote with his associate.
Two-step is the approach used in routine practice by clinicians at UT Southwestern Medical Center. “We screen all patients with type 2 diabetes and no diagnosed heart failure who are not already on an SGLT2 inhibitor” using their 2019 screening tool, called the WATCH-DM Risk score, said Dr. Pandey. Patients flagged at high risk by their clinical score receive an SGLT2 inhibitor (presuming no contraindications). The remaining patients with low or intermediate risk may then undergo biomarker-based assessment to find additional patients who warrant SGLT2 inhibitor treatment, he said in an interview.
Often, a record of the most important biomarker, NT-proBNP, is already in the patient’s record and less than a year old, in which case clinicians use that value. An NT-proBNP level of at least 125 pg/mL indicates increased risk in people with a body mass index of less than 30 kg/m2, while for those with higher body mass indexes clinicians at Southwestern apply a threshold for higher risk of at least 100 pg/mL.
In addition to starting those patients on an SGLT2 inhibitor, the Southwestern protocol calls for intensified efforts at weight loss and improved fitness to further lower incident heart failure risk, and they are also considering targeting treatment with a glucagonlike peptide–1 receptor agonist to these patients as well. They have a research protocol in place, called WATCH-DM, that will prospectively assess the efficacy of this strategy.
Despite the cost, others also believe that the time is right for biomarker-based tests to boost access to the benefits that treatment with SGLT2 inhibitors can give patients with type 2 diabetes.
“In theory it’s reasonable” to use a risk score like the recent one reported by Dr. Berg and coauthors, said Vanita R. Aroda, MD, an endocrinologist and director of diabetes clinical research at Brigham and Women’s Hospital in Boston. “We need to pay attention to heart failure as an outcome and use risk stratification” to decide which patients with type 2 diabetes but without established cardiovascular disease warrant treatment with an SGLT2 inhibitor, she said in an interview. “Given the data, we need more concrete recommendations” from medical societies on how to reasonably use biomarkers and imaging to identify patients with type 2 diabetes who are at increased risk for heart failure and hence would benefit from treatment. “This should be of high interest to guidelines committees,” she added.
The earlier version of Dr. Berg’s score, based exclusively on clinical observations and conventional measures like estimated glomerular filtration rate and urinary creatinine to albumin ratio, had overlap with established criteria for starting treatment with an SGLT2 inhibitor, such as the presence of chronic kidney disease, she noted. “A biomarker-based score may provide the additional level of discrimination needed to characterize risk and potential benefit.”
Asymptomatic diabetic cardiomyopathy
Dr. Aroda and several coauthors recently published a review that describes a subset of patients with type 2 diabetes who might get picked up by intensified screening for heart failure risk: those with asymptomatic diabetic cardiomyopathy, a clinical state that they said represents patients with stage B heart failure based on the new Universal Definition and Classification of Heart Failure. Until recently, these patients with type 2 diabetes and asymptomatic cardiomyopathy have mostly gone unrecognized.
A recent report from Dr. Pandey and associates reviewed records from 2,900 U.S. patients with diabetes and no symptoms who had been included in any of three cohort studies and found echocardiographic evidence of early-stage cardiomyopathy in as many as two-thirds. In an editorial about this report, Dr. Aroda and coauthors called these patients a potential “window of opportunity for prevention and treatment of heart failure.”
“There is evidence of structural cardiac changes that progress through the stages of heart failure,” and starting treatment with an SGLT2 inhibitor during an earlier stage can potentially slow or prevent this progression and thereby limit future functional decline, Dr. Aroda said.
Dr. Sattar agreed. Type 2 diabetes appears to help cause “fluid derangements” and abnormal hemodynamics that produces cardiac stress, changes in heart structure, and adverse remodeling of the heart, a process that “some call cardiomyopathy,” which is exacerbated by other pathologic forces that are also often present in these patients such as obesity and hypertension. SGLT2 inhibitors can help these patients by producing “reverse remodeling of the heart.”
“This process was neglected because for many years our focus was on ischemic heart disease in patients with type 2 diabetes. It was there in plain sight, but we were missing it,” explained Dr. Sattar. Having agents from the SGLT2 inhibitor class “has allowed us to better understand this mechanism.”
The SGLT2 inhibitors are “absolutely the driving reason” why the diabetes–heart failure link has become so important, said Dr. Inzucchi. Having drugs that reduce heart failure risk provided clinicians with a tool that has “changed our mindset.”
“Heart failure prevention has been largely neglected in patients with type 2 diabetes. Reprioritizing heart failure prevention to first and foremost among patients with type 2 diabetes is long overdue,” commented Gregg C. Fonarow, MD, professor and chief of cardiology at the University of California, Los Angeles.
Clinicians don’t like risk scores
Will systematic screening for heart failure risk in selected patients with type 2 diabetes take hold, and with it expanded and better-targeted use of SGLT2 inhibitors?
“I hope so,” said Dr. Kosiborod, but one challenge is that “for the most part clinicians don’t like using risk scores.” Only a few have ever been widely incorporated into practice; mostly they become tools for research. Plus, SGLT2 inhibitor uptake has in general been slow to catch on, which Dr. Kosiborod blames primarily on clinical inertia, a pervasive issue that has also hampered optimal use of drugs as commonplace as statins, ACE inhibitors, and angiotensin-receptor blockers.
“Given the avalanche of positive data, uptake of SGLT2 inhibitors will continue to improve and accelerate; but unfortunately, unless something dramatic happens we’ll likely see their continued underuse for several more years,” he predicted. “Designing better systems of care that prioritize prevention are absolutely needed to improve implementation of effective therapies, including SGLT2 inhibitors.”
Despite their underuse the SGLT2 inhibitor class has, in just 6 years since results from the EMPA-REG OUTCOME trial came out and launched the current treatment era, transformed thinking about the risk that heart failure poses to patients with type 2 diabetes and the need to manage this risk.
“I thank the SGLT2 inhibitors for raising awareness of heart failure risk in patients with diabetes,” and for giving clinicians a new way to mitigate this risk, said Dr. Cheng.
Dr. Berg has been a consultant to AstraZeneca, and received research grant support to his institution from AstraZeneca and Pfizer. Dr. Cheng has received personal fees from multiple pharmaceutical companies. Dr. Kosiborod has been an adviser and consultant to multiple pharmaceutical companies; has received research grants from AstraZeneca and Boehringer Ingelheim; and has received other research support from AstraZeneca. Dr. Pandey has been an adviser to Roche Diagnostics; has received nonfinancial support from Pfizer and Merck; and has received research support from Gilead Sciences, Myovista, and Applied Therapeutics. Dr. Sattar has received consulting honoraria from multiple pharmaceutical companies, and has received grant support from Boehringer Ingelheim, Roche Diagnostics, and Novartis. Dr. Aroda has been a consultant for several pharmaceutical companies; has a spouse employed with Janssen; and has received research support (institutional contracts) from multiple pharmaceutical companies. Dr. Fonarow has been a consultant to several pharmaceutical companies.
FROM DIABETES CARE
Pandemic exacerbates primary care practices’ financial struggles
according to experts and the results of recent surveys by the Primary Care Collaborative (PCC).
Fewer than 30% (26.4%) of primary care clinicians report that their practices are financially healthy, according to the latest results from a periodic survey by the PCC. An earlier survey by the PCC suggests clinicians’ confidence in the financial viability of their practices has significantly declined since last year, when compared with the new survey’s results. When the older survey was taken between Sept. 4 and Sept. 8 of 2020, only 35% of primary care clinicians said that revenue and pay were significantly lower than they were before the pandemic.
Submissions to the new PCC survey were collected between Aug. 13 and Aug. 17 of 2021 and included 1,263 respondents from 49 states, the District of Columbia, and two territories. The PCC and the Larry A. Green Center have been regularly surveying primary care clinicians to better understand the impact of COVID-19 throughout the pandemic.
PCC President and CEO Ann Greiner said in an interview that the drop over a year follows a trend.
Though primary care faced struggles before the pandemic, the COVID-19 effect has been striking and cumulative, she noted.
“[Primary care practices] were healthier prepandemic,” said Ms. Greiner. “The precipitous drop in revenue when stay-at-home orders went into effect had a very big effect though pay structure and lack of investment in primary care was a problem long before COVID-19.”
COVID-19 has exacerbated all that ails primary care, and has increased fears of viability of primary care offices, she said.
Ms. Greiner pointed to a report from Health Affairs, that projected in 2020 that primary care would lose $65,000 in revenue per full-time physician by the end of the year for a total shortfall of $15 billion, following steep drops in office visits and fees for services from March to May, 2020.
In July of this year, she said, PCC’s survey found that, “Four in 10 clinicians worry that primary care will be gone in 5 years and one-fifth of respondents expect to leave the profession within the next three.”
The July PCC survey also showed that 13% of primary care clinicians said they have discussed selling their practice and cite high-level burnout/exhaustion as a main challenge for the next 6 months.
Robert L. Phillips, MD, a Virginia-based physician who oversees research for the American Board of Family Medicine, said, “Practices in our national primary care practice registry (PRIME) saw visit volumes drop 40% in the 2-3 months around the start of the pandemic and had not seen them return to normal as of June of this year. This means most remain financially underwater.”
End to paycheck protection hurt practices
Conrad L. Flick, MD, managing partner of Family Medical Associates in Raleigh, N.C., said the end of the federal Paycheck Protection Program (PPP) at the end of 2020 caused further distress to primary care and could also help explain the drop in healthy practices that PCC’s survey from last year suggested.
“Many of us who struggled financially as the pandemic hit last year were really worried. PPP certainly shored that up for a lot of us. But now it’s no longer here,” he said.
Dr. Flick said his 10-clinician independent practice is financially sound and he credits that to having the PPP loan, shared savings from an accountable care organization, and holding some profit over from last year to this year.
His practice had to cut two nurse practitioners this year when volume did not return to prepandemic levels.
“The PPP loan let us keep [those NPs] employed through spring, but we were hoping the volume would come back. Come spring this year the volume hasn’t come back, and we couldn’t afford to keep the office at full staff,” he said.
The way primary care physicians are paid is what makes them so vulnerable in a pandemic, he explained.
“Our revenue is purely based on how many people I can get through my office at a given period of time. We don’t have ways to generate revenue and build a cushion.”
Family physician L. Allen Dobson, MD, said the survey results may have become even more grim in the last year, because primary care practices, especially small practices, have not recovered from the 2020 losses and effects have snowballed.
Even though primary care offices have largely reopened and many patients have returned to in-person visits, he said, physicians are dealing with uncertainties of COVID-19 surges and variants and are having trouble recruiting and maintaining staff.
Revenue that should have come to primary care practices in testing and distributing vaccines instead went elsewhere to larger vaccination sites and retail clinics, noted Dr. Dobson, who is chair of the board of managers of Community Care Physicians Network in Mount Pleasant, N.C., which provides assistance with administrative tasks to small and solo primary care practices.
COVID-19 brought ‘accelerated change’
COVID-19 brought “an accelerated change,” in decreasing revenue, Dr. Dobson said.
Small primary care practices have followed the rules of changing to electronic health records, getting patient-centered medical home certification, and documenting quality improvement measures, but they have not reaped the financial benefits from these changes, he explained.
A report commissioned by the Physician Advocacy Institute found that the pandemic accelerated a long national trend of hospitals and corporate entities acquiring physician practices and employing physicians.
From January 2019 to January 2021, these entities acquired 20,900 additional physician practices and 48,000 additional physicians left independent practice for employment by hospital systems or other corporate entities.
Further straining practices is a thinning workforce, with 21% or respondents to the most recent PCC survey having said they were unable to hire clinicians for open positions and 54% saying they are unable to hire staff for open positions.
One respondent to the PCC survey from Utah said, “We need more support. It’s a moral injury to have our pay cut and be severely understaffed. Most of the burden of educating patients and getting them vaccinated has fallen to primary care and we are already overwhelmed with taking care of patients with worsening mental and physical health.”
According to Bruce Landon, MD, MBA, professor of health care policy at the Harvard Medical School’s Center for Primary Care, Boston, another source of financial strain for primary care practices is that they are having difficulty attracting doctors, nurses, and administrators.
These practices often need to increase pay for those positions to recruit people, and they are leaving many positions unfilled, Dr. Landon explained.
Plus, COVID-19 introduced costs for personal protective equipment (PPE) and cleaning products, and those expenses generally have not been reimbursed, Dr. Landon said.
Uncertainty around telemedicine
A new risk for primary care is a decline in telemedicine payments at a time when practices are still relying on telemedicine for revenue.
In the most recent PCC report, 40% of clinicians said they use telemedicine for at least a fifth of all office visits.
Even though most practices have reopened there’s still a fair amount of telemedicine and that will continue, Dr. Landon said in an interview.
In March of 2020, the Centers for Medicare & Medicaid Services lifted restrictions and that helped physicians with getting reimbursed for the services as they would office visits. But some commercial payers are starting to back off full payment for telemedicine, Dr. Landon noted.
“At some point the feds will probably start to do that with Medicare. I think that’s a mistake. [Telemedicine] has been one of the silver linings of this cloud of the pandemic,” he said.
If prepandemic payment regulations are restored, 41% of clinicians said, in the most recent PCC survey, that they worry their practices will no longer be able to support telemedicine.
Possible safety nets
Dr. Landon said that one thing that’s also clear is that some form of primary care capitation payment is necessary, at least for some of the work in primary care.
The practices that had capitation as part of payment were the ones who were most easily able to handle the pandemic because they didn’t see the immediate drop in revenue that fee-for-service practices saw, he noted.
“If we have a next pandemic, having a steady revenue stream to support primary care is really important and having a different way to pay for primary care is probably the best way to do that,” he said. “These longer-term strategies are going to be really crucial if we want to have a primary care system 10 years from now.”
Ms. Greiner, Dr. Flick, Dr. Phillips, Dr. Dobson, and Dr. Landon report no relevant financial relationships.
according to experts and the results of recent surveys by the Primary Care Collaborative (PCC).
Fewer than 30% (26.4%) of primary care clinicians report that their practices are financially healthy, according to the latest results from a periodic survey by the PCC. An earlier survey by the PCC suggests clinicians’ confidence in the financial viability of their practices has significantly declined since last year, when compared with the new survey’s results. When the older survey was taken between Sept. 4 and Sept. 8 of 2020, only 35% of primary care clinicians said that revenue and pay were significantly lower than they were before the pandemic.
Submissions to the new PCC survey were collected between Aug. 13 and Aug. 17 of 2021 and included 1,263 respondents from 49 states, the District of Columbia, and two territories. The PCC and the Larry A. Green Center have been regularly surveying primary care clinicians to better understand the impact of COVID-19 throughout the pandemic.
PCC President and CEO Ann Greiner said in an interview that the drop over a year follows a trend.
Though primary care faced struggles before the pandemic, the COVID-19 effect has been striking and cumulative, she noted.
“[Primary care practices] were healthier prepandemic,” said Ms. Greiner. “The precipitous drop in revenue when stay-at-home orders went into effect had a very big effect though pay structure and lack of investment in primary care was a problem long before COVID-19.”
COVID-19 has exacerbated all that ails primary care, and has increased fears of viability of primary care offices, she said.
Ms. Greiner pointed to a report from Health Affairs, that projected in 2020 that primary care would lose $65,000 in revenue per full-time physician by the end of the year for a total shortfall of $15 billion, following steep drops in office visits and fees for services from March to May, 2020.
In July of this year, she said, PCC’s survey found that, “Four in 10 clinicians worry that primary care will be gone in 5 years and one-fifth of respondents expect to leave the profession within the next three.”
The July PCC survey also showed that 13% of primary care clinicians said they have discussed selling their practice and cite high-level burnout/exhaustion as a main challenge for the next 6 months.
Robert L. Phillips, MD, a Virginia-based physician who oversees research for the American Board of Family Medicine, said, “Practices in our national primary care practice registry (PRIME) saw visit volumes drop 40% in the 2-3 months around the start of the pandemic and had not seen them return to normal as of June of this year. This means most remain financially underwater.”
End to paycheck protection hurt practices
Conrad L. Flick, MD, managing partner of Family Medical Associates in Raleigh, N.C., said the end of the federal Paycheck Protection Program (PPP) at the end of 2020 caused further distress to primary care and could also help explain the drop in healthy practices that PCC’s survey from last year suggested.
“Many of us who struggled financially as the pandemic hit last year were really worried. PPP certainly shored that up for a lot of us. But now it’s no longer here,” he said.
Dr. Flick said his 10-clinician independent practice is financially sound and he credits that to having the PPP loan, shared savings from an accountable care organization, and holding some profit over from last year to this year.
His practice had to cut two nurse practitioners this year when volume did not return to prepandemic levels.
“The PPP loan let us keep [those NPs] employed through spring, but we were hoping the volume would come back. Come spring this year the volume hasn’t come back, and we couldn’t afford to keep the office at full staff,” he said.
The way primary care physicians are paid is what makes them so vulnerable in a pandemic, he explained.
“Our revenue is purely based on how many people I can get through my office at a given period of time. We don’t have ways to generate revenue and build a cushion.”
Family physician L. Allen Dobson, MD, said the survey results may have become even more grim in the last year, because primary care practices, especially small practices, have not recovered from the 2020 losses and effects have snowballed.
Even though primary care offices have largely reopened and many patients have returned to in-person visits, he said, physicians are dealing with uncertainties of COVID-19 surges and variants and are having trouble recruiting and maintaining staff.
Revenue that should have come to primary care practices in testing and distributing vaccines instead went elsewhere to larger vaccination sites and retail clinics, noted Dr. Dobson, who is chair of the board of managers of Community Care Physicians Network in Mount Pleasant, N.C., which provides assistance with administrative tasks to small and solo primary care practices.
COVID-19 brought ‘accelerated change’
COVID-19 brought “an accelerated change,” in decreasing revenue, Dr. Dobson said.
Small primary care practices have followed the rules of changing to electronic health records, getting patient-centered medical home certification, and documenting quality improvement measures, but they have not reaped the financial benefits from these changes, he explained.
A report commissioned by the Physician Advocacy Institute found that the pandemic accelerated a long national trend of hospitals and corporate entities acquiring physician practices and employing physicians.
From January 2019 to January 2021, these entities acquired 20,900 additional physician practices and 48,000 additional physicians left independent practice for employment by hospital systems or other corporate entities.
Further straining practices is a thinning workforce, with 21% or respondents to the most recent PCC survey having said they were unable to hire clinicians for open positions and 54% saying they are unable to hire staff for open positions.
One respondent to the PCC survey from Utah said, “We need more support. It’s a moral injury to have our pay cut and be severely understaffed. Most of the burden of educating patients and getting them vaccinated has fallen to primary care and we are already overwhelmed with taking care of patients with worsening mental and physical health.”
According to Bruce Landon, MD, MBA, professor of health care policy at the Harvard Medical School’s Center for Primary Care, Boston, another source of financial strain for primary care practices is that they are having difficulty attracting doctors, nurses, and administrators.
These practices often need to increase pay for those positions to recruit people, and they are leaving many positions unfilled, Dr. Landon explained.
Plus, COVID-19 introduced costs for personal protective equipment (PPE) and cleaning products, and those expenses generally have not been reimbursed, Dr. Landon said.
Uncertainty around telemedicine
A new risk for primary care is a decline in telemedicine payments at a time when practices are still relying on telemedicine for revenue.
In the most recent PCC report, 40% of clinicians said they use telemedicine for at least a fifth of all office visits.
Even though most practices have reopened there’s still a fair amount of telemedicine and that will continue, Dr. Landon said in an interview.
In March of 2020, the Centers for Medicare & Medicaid Services lifted restrictions and that helped physicians with getting reimbursed for the services as they would office visits. But some commercial payers are starting to back off full payment for telemedicine, Dr. Landon noted.
“At some point the feds will probably start to do that with Medicare. I think that’s a mistake. [Telemedicine] has been one of the silver linings of this cloud of the pandemic,” he said.
If prepandemic payment regulations are restored, 41% of clinicians said, in the most recent PCC survey, that they worry their practices will no longer be able to support telemedicine.
Possible safety nets
Dr. Landon said that one thing that’s also clear is that some form of primary care capitation payment is necessary, at least for some of the work in primary care.
The practices that had capitation as part of payment were the ones who were most easily able to handle the pandemic because they didn’t see the immediate drop in revenue that fee-for-service practices saw, he noted.
“If we have a next pandemic, having a steady revenue stream to support primary care is really important and having a different way to pay for primary care is probably the best way to do that,” he said. “These longer-term strategies are going to be really crucial if we want to have a primary care system 10 years from now.”
Ms. Greiner, Dr. Flick, Dr. Phillips, Dr. Dobson, and Dr. Landon report no relevant financial relationships.
according to experts and the results of recent surveys by the Primary Care Collaborative (PCC).
Fewer than 30% (26.4%) of primary care clinicians report that their practices are financially healthy, according to the latest results from a periodic survey by the PCC. An earlier survey by the PCC suggests clinicians’ confidence in the financial viability of their practices has significantly declined since last year, when compared with the new survey’s results. When the older survey was taken between Sept. 4 and Sept. 8 of 2020, only 35% of primary care clinicians said that revenue and pay were significantly lower than they were before the pandemic.
Submissions to the new PCC survey were collected between Aug. 13 and Aug. 17 of 2021 and included 1,263 respondents from 49 states, the District of Columbia, and two territories. The PCC and the Larry A. Green Center have been regularly surveying primary care clinicians to better understand the impact of COVID-19 throughout the pandemic.
PCC President and CEO Ann Greiner said in an interview that the drop over a year follows a trend.
Though primary care faced struggles before the pandemic, the COVID-19 effect has been striking and cumulative, she noted.
“[Primary care practices] were healthier prepandemic,” said Ms. Greiner. “The precipitous drop in revenue when stay-at-home orders went into effect had a very big effect though pay structure and lack of investment in primary care was a problem long before COVID-19.”
COVID-19 has exacerbated all that ails primary care, and has increased fears of viability of primary care offices, she said.
Ms. Greiner pointed to a report from Health Affairs, that projected in 2020 that primary care would lose $65,000 in revenue per full-time physician by the end of the year for a total shortfall of $15 billion, following steep drops in office visits and fees for services from March to May, 2020.
In July of this year, she said, PCC’s survey found that, “Four in 10 clinicians worry that primary care will be gone in 5 years and one-fifth of respondents expect to leave the profession within the next three.”
The July PCC survey also showed that 13% of primary care clinicians said they have discussed selling their practice and cite high-level burnout/exhaustion as a main challenge for the next 6 months.
Robert L. Phillips, MD, a Virginia-based physician who oversees research for the American Board of Family Medicine, said, “Practices in our national primary care practice registry (PRIME) saw visit volumes drop 40% in the 2-3 months around the start of the pandemic and had not seen them return to normal as of June of this year. This means most remain financially underwater.”
End to paycheck protection hurt practices
Conrad L. Flick, MD, managing partner of Family Medical Associates in Raleigh, N.C., said the end of the federal Paycheck Protection Program (PPP) at the end of 2020 caused further distress to primary care and could also help explain the drop in healthy practices that PCC’s survey from last year suggested.
“Many of us who struggled financially as the pandemic hit last year were really worried. PPP certainly shored that up for a lot of us. But now it’s no longer here,” he said.
Dr. Flick said his 10-clinician independent practice is financially sound and he credits that to having the PPP loan, shared savings from an accountable care organization, and holding some profit over from last year to this year.
His practice had to cut two nurse practitioners this year when volume did not return to prepandemic levels.
“The PPP loan let us keep [those NPs] employed through spring, but we were hoping the volume would come back. Come spring this year the volume hasn’t come back, and we couldn’t afford to keep the office at full staff,” he said.
The way primary care physicians are paid is what makes them so vulnerable in a pandemic, he explained.
“Our revenue is purely based on how many people I can get through my office at a given period of time. We don’t have ways to generate revenue and build a cushion.”
Family physician L. Allen Dobson, MD, said the survey results may have become even more grim in the last year, because primary care practices, especially small practices, have not recovered from the 2020 losses and effects have snowballed.
Even though primary care offices have largely reopened and many patients have returned to in-person visits, he said, physicians are dealing with uncertainties of COVID-19 surges and variants and are having trouble recruiting and maintaining staff.
Revenue that should have come to primary care practices in testing and distributing vaccines instead went elsewhere to larger vaccination sites and retail clinics, noted Dr. Dobson, who is chair of the board of managers of Community Care Physicians Network in Mount Pleasant, N.C., which provides assistance with administrative tasks to small and solo primary care practices.
COVID-19 brought ‘accelerated change’
COVID-19 brought “an accelerated change,” in decreasing revenue, Dr. Dobson said.
Small primary care practices have followed the rules of changing to electronic health records, getting patient-centered medical home certification, and documenting quality improvement measures, but they have not reaped the financial benefits from these changes, he explained.
A report commissioned by the Physician Advocacy Institute found that the pandemic accelerated a long national trend of hospitals and corporate entities acquiring physician practices and employing physicians.
From January 2019 to January 2021, these entities acquired 20,900 additional physician practices and 48,000 additional physicians left independent practice for employment by hospital systems or other corporate entities.
Further straining practices is a thinning workforce, with 21% or respondents to the most recent PCC survey having said they were unable to hire clinicians for open positions and 54% saying they are unable to hire staff for open positions.
One respondent to the PCC survey from Utah said, “We need more support. It’s a moral injury to have our pay cut and be severely understaffed. Most of the burden of educating patients and getting them vaccinated has fallen to primary care and we are already overwhelmed with taking care of patients with worsening mental and physical health.”
According to Bruce Landon, MD, MBA, professor of health care policy at the Harvard Medical School’s Center for Primary Care, Boston, another source of financial strain for primary care practices is that they are having difficulty attracting doctors, nurses, and administrators.
These practices often need to increase pay for those positions to recruit people, and they are leaving many positions unfilled, Dr. Landon explained.
Plus, COVID-19 introduced costs for personal protective equipment (PPE) and cleaning products, and those expenses generally have not been reimbursed, Dr. Landon said.
Uncertainty around telemedicine
A new risk for primary care is a decline in telemedicine payments at a time when practices are still relying on telemedicine for revenue.
In the most recent PCC report, 40% of clinicians said they use telemedicine for at least a fifth of all office visits.
Even though most practices have reopened there’s still a fair amount of telemedicine and that will continue, Dr. Landon said in an interview.
In March of 2020, the Centers for Medicare & Medicaid Services lifted restrictions and that helped physicians with getting reimbursed for the services as they would office visits. But some commercial payers are starting to back off full payment for telemedicine, Dr. Landon noted.
“At some point the feds will probably start to do that with Medicare. I think that’s a mistake. [Telemedicine] has been one of the silver linings of this cloud of the pandemic,” he said.
If prepandemic payment regulations are restored, 41% of clinicians said, in the most recent PCC survey, that they worry their practices will no longer be able to support telemedicine.
Possible safety nets
Dr. Landon said that one thing that’s also clear is that some form of primary care capitation payment is necessary, at least for some of the work in primary care.
The practices that had capitation as part of payment were the ones who were most easily able to handle the pandemic because they didn’t see the immediate drop in revenue that fee-for-service practices saw, he noted.
“If we have a next pandemic, having a steady revenue stream to support primary care is really important and having a different way to pay for primary care is probably the best way to do that,” he said. “These longer-term strategies are going to be really crucial if we want to have a primary care system 10 years from now.”
Ms. Greiner, Dr. Flick, Dr. Phillips, Dr. Dobson, and Dr. Landon report no relevant financial relationships.
Unvaccinated people likely to catch COVID repeatedly
according to a recent study published in The Lancet Microbe.
Since COVID-19 hasn’t existed for long enough to perform a long-term study, researchers at Yale University and the University of North Carolina at Charlotte looked at reinfection data for six other human-infecting coronaviruses, including SARS and MERS.
“Reinfection can reasonably happen in three months or less,” Jeffrey Townsend, PhD, lead study author and a biostatistics professor at the Yale School of Public Health, said in a statement.
“Therefore, those who have been naturally infected should get vaccinated,” he said. “Previous infection alone can offer very little long-term protection against subsequent infections.”
The research team looked at post-infection data for six coronaviruses between 1984-2020 and found reinfection ranged from 128 days to 28 years. They calculated that reinfection with COVID-19 would likely occur between 3 months to 5 years after peak antibody response, with an average of 16 months. This is less than half the duration seen for other coronaviruses that circulate among humans.
The risk of COVID-19 reinfection is about 5% at three months, which jumps to 50% after 17 months, the research team found. Reinfection could become increasingly common as immunity wanes and new variants develop, they said.
“We tend to think about immunity as being immune or not immune. Our study cautions that we instead should be more focused on the risk of reinfection through time,” Alex Dornburg, PhD, senior study author and assistant professor of bioinformatics and genomics at UNC, said in the statement.
“As new variants arise, previous immune responses become less effective at combating the virus,” he said. “Those who were naturally infected early in the pandemic are increasingly likely to become reinfected in the near future.”
Study estimates are based on average times of declining immunity across different coronaviruses, the researchers told the Yale Daily News. At the individual level, people have different levels of immunity, which can provide shorter or longer duration of protection based on immune status, immunity within a community, age, underlying health conditions, environmental exposure, and other factors.
The research team said that preventive health measures and global distribution of vaccines will be “critical” in minimizing reinfection and COVID-19 deaths. In areas with low vaccination rates, for instance, unvaccinated people should continue safety practices such as social distancing, wearing masks, and proper indoor ventilation to avoid reinfection.
“We need to be very aware of the fact that this disease is likely to be circulating over the long term and that we don’t have this long-term immunity that many people seem to be hoping to rely on in order to protect them from disease,” Dr. Townsend told the newspaper.
A version of this article first appeared on WebMD.com.
according to a recent study published in The Lancet Microbe.
Since COVID-19 hasn’t existed for long enough to perform a long-term study, researchers at Yale University and the University of North Carolina at Charlotte looked at reinfection data for six other human-infecting coronaviruses, including SARS and MERS.
“Reinfection can reasonably happen in three months or less,” Jeffrey Townsend, PhD, lead study author and a biostatistics professor at the Yale School of Public Health, said in a statement.
“Therefore, those who have been naturally infected should get vaccinated,” he said. “Previous infection alone can offer very little long-term protection against subsequent infections.”
The research team looked at post-infection data for six coronaviruses between 1984-2020 and found reinfection ranged from 128 days to 28 years. They calculated that reinfection with COVID-19 would likely occur between 3 months to 5 years after peak antibody response, with an average of 16 months. This is less than half the duration seen for other coronaviruses that circulate among humans.
The risk of COVID-19 reinfection is about 5% at three months, which jumps to 50% after 17 months, the research team found. Reinfection could become increasingly common as immunity wanes and new variants develop, they said.
“We tend to think about immunity as being immune or not immune. Our study cautions that we instead should be more focused on the risk of reinfection through time,” Alex Dornburg, PhD, senior study author and assistant professor of bioinformatics and genomics at UNC, said in the statement.
“As new variants arise, previous immune responses become less effective at combating the virus,” he said. “Those who were naturally infected early in the pandemic are increasingly likely to become reinfected in the near future.”
Study estimates are based on average times of declining immunity across different coronaviruses, the researchers told the Yale Daily News. At the individual level, people have different levels of immunity, which can provide shorter or longer duration of protection based on immune status, immunity within a community, age, underlying health conditions, environmental exposure, and other factors.
The research team said that preventive health measures and global distribution of vaccines will be “critical” in minimizing reinfection and COVID-19 deaths. In areas with low vaccination rates, for instance, unvaccinated people should continue safety practices such as social distancing, wearing masks, and proper indoor ventilation to avoid reinfection.
“We need to be very aware of the fact that this disease is likely to be circulating over the long term and that we don’t have this long-term immunity that many people seem to be hoping to rely on in order to protect them from disease,” Dr. Townsend told the newspaper.
A version of this article first appeared on WebMD.com.
according to a recent study published in The Lancet Microbe.
Since COVID-19 hasn’t existed for long enough to perform a long-term study, researchers at Yale University and the University of North Carolina at Charlotte looked at reinfection data for six other human-infecting coronaviruses, including SARS and MERS.
“Reinfection can reasonably happen in three months or less,” Jeffrey Townsend, PhD, lead study author and a biostatistics professor at the Yale School of Public Health, said in a statement.
“Therefore, those who have been naturally infected should get vaccinated,” he said. “Previous infection alone can offer very little long-term protection against subsequent infections.”
The research team looked at post-infection data for six coronaviruses between 1984-2020 and found reinfection ranged from 128 days to 28 years. They calculated that reinfection with COVID-19 would likely occur between 3 months to 5 years after peak antibody response, with an average of 16 months. This is less than half the duration seen for other coronaviruses that circulate among humans.
The risk of COVID-19 reinfection is about 5% at three months, which jumps to 50% after 17 months, the research team found. Reinfection could become increasingly common as immunity wanes and new variants develop, they said.
“We tend to think about immunity as being immune or not immune. Our study cautions that we instead should be more focused on the risk of reinfection through time,” Alex Dornburg, PhD, senior study author and assistant professor of bioinformatics and genomics at UNC, said in the statement.
“As new variants arise, previous immune responses become less effective at combating the virus,” he said. “Those who were naturally infected early in the pandemic are increasingly likely to become reinfected in the near future.”
Study estimates are based on average times of declining immunity across different coronaviruses, the researchers told the Yale Daily News. At the individual level, people have different levels of immunity, which can provide shorter or longer duration of protection based on immune status, immunity within a community, age, underlying health conditions, environmental exposure, and other factors.
The research team said that preventive health measures and global distribution of vaccines will be “critical” in minimizing reinfection and COVID-19 deaths. In areas with low vaccination rates, for instance, unvaccinated people should continue safety practices such as social distancing, wearing masks, and proper indoor ventilation to avoid reinfection.
“We need to be very aware of the fact that this disease is likely to be circulating over the long term and that we don’t have this long-term immunity that many people seem to be hoping to rely on in order to protect them from disease,” Dr. Townsend told the newspaper.
A version of this article first appeared on WebMD.com.