User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
What are the cardiorenal differences between type 1 and type 2 diabetes?
While type 2 diabetes is associated with a greater risk for cardiovascular events than type 1 diabetes, the latter is more associated with chronic kidney complications, according to data from a French observational study.
That’s not to say that type 1 diabetes isn’t also associated with poor heart health that is of concern, according to Denis Angoulvant, MD, of Tours (France) Regional University Hospital and Trousseau Hospital in Paris.
“The difference is that, in the middle or older ages, we suddenly see a surge of cardiovascular events in type 1 diabetic patients,” he said at the annual meeting of the European Association for the Study of Diabetes. “As a cardiologist, I must say that we are barely see these patients ahead of those complications, so we advocate that there’s a gap to be filled here to prevent these events in these patients.”
Few studies have looked at the comparative risks for cardiovascular and renal outcomes between patients with type 1 and type 2 diabetes, Dr. Angoulvant said, so the aim of the study he presented was to look at this in more detail.
Comparing cardiovascular and renal outcomes
Data from the French hospital discharge database (PMSI), which covers more than 98% of the country’s population, were used to find all adults with type 1 or type 2 diabetes who had at least 5 years of follow-up data starting from 2013.
Not surprisingly, there were eight times as many individuals with type 2 diabetes (425,207) than those with type 1 diabetes (50,623), and patients with type 2 diabetes tended to be older than those with type 1 diabetes (mean age, 68.6 vs. 61.4 years).
There were many significant differences between the two groups of patients in terms of clinical variables, such as patients with type 2 diabetes having more cardiovascular risk factors or preexisting heart problems, and those with type 1 diabetes more likely to have diabetic eye disease.
Indeed, Dr. Angoulvant pointed out that those with type 2 diabetes were significantly more likely (all P < .0001) than those with type 1 diabetes to have: hypertension (70.8% vs. 50.5%), heart failure (35.7% vs. 16.4%), valvular heart disease (7.2% vs. 3.5%), dilated cardiomyopathy (5.5% vs. 2.7%), coronary artery disease (27.6 vs. 18.6%), previous MI (3.0% vs. 2.4%), peripheral vascular disease (22.0% vs. 15.5%), and ischemic stroke (3.3 vs. 2.2%).
“Regarding more specific microvascular diabetic complications, we had a higher incidence of chronic kidney disease in type 2 diabetes patients [10.2% vs. 9.1%], but a higher incidence of diabetic retinopathy in type 1 diabetes patients [6.6% vs. 12.2%],” Dr. Angoulvant said.
Considering more than 2 million person-years of follow-up, the annual rates of MI, new-onset heart failure, ischemic stroke, and chronic kidney disease for the whole study population were respective 1.4%, 5.4%, 1.2%, and 3.4%. The annual rates for death from any cause was 9.7%, and for a cardiovascular reason was 2.4%.
Cardiovascular disease prevalence and event rates
The mean follow-up period was 4.3 years, and over this time the age- and sex-adjusted prevalence of cardiovascular disease was found to be highest in individuals with type 2 diabetes, especially after the age of 40 years.
Looking at the rates of different cardiovascular events showed that both younger (18-29 years) and older (60+ years) people with type 1 diabetes had a 1.2-fold higher risk for MI than similarly aged individuals with type 2 diabetes.
Furthermore, younger and older type 1 diabetes individuals had a 1.1- to 1.4-fold greater risk of new-onset heart failure than those with type 2 diabetes.
“Interestingly, regarding the incidence of ischemic stroke in our population, we found no significant difference between patients with type 1 diabetes, and patients with type 2 diabetes,” Dr. Angoulvant said.
Chronic kidney disease and risk for death
Chronic kidney disease was most common in individuals with type 1 diabetes who were aged between 18 and 69 years, with a greater prevalence also seen in those with type 2 diabetes only after age 80.
The risk of new chronic kidney disease was significantly increased in patients with type 1 diabetes, compared with patients with type 2 diabetes, with a 1.1- to 2.4-fold increase seen, first in individuals aged 18-49 years, and then again after the age of 60 years.
Dr. Angoulvant reported that the risk of dying from any cause was 1.1-fold higher in people with type 1 diabetes, compared with those with type 2 diabetes, but after the age of 60 years.
The risk of death from cardiovascular events was also increased in people with type 1 diabetes, but between the ages of 60 and 69 years.
Asked what his take-home message might be, Dr. Angoulvant stressed the importance of heart failure, in all patients with diabetes but particularly in those with type 1 diabetes.
“I think there is room for improvement in terms of assessing who is going to have heart failure, how to assess heart failure, and more importantly, how to prevent heart failure,” perhaps by “introducing those drugs that have shown tremendous benefit regarding hospitalization, such as [sodium-glucose transporter 2] inhibitors” in patients with type 1 diabetes ahead of the events, he said.
Dr. Angoulvant had no conflicts of interest to disclose.
While type 2 diabetes is associated with a greater risk for cardiovascular events than type 1 diabetes, the latter is more associated with chronic kidney complications, according to data from a French observational study.
That’s not to say that type 1 diabetes isn’t also associated with poor heart health that is of concern, according to Denis Angoulvant, MD, of Tours (France) Regional University Hospital and Trousseau Hospital in Paris.
“The difference is that, in the middle or older ages, we suddenly see a surge of cardiovascular events in type 1 diabetic patients,” he said at the annual meeting of the European Association for the Study of Diabetes. “As a cardiologist, I must say that we are barely see these patients ahead of those complications, so we advocate that there’s a gap to be filled here to prevent these events in these patients.”
Few studies have looked at the comparative risks for cardiovascular and renal outcomes between patients with type 1 and type 2 diabetes, Dr. Angoulvant said, so the aim of the study he presented was to look at this in more detail.
Comparing cardiovascular and renal outcomes
Data from the French hospital discharge database (PMSI), which covers more than 98% of the country’s population, were used to find all adults with type 1 or type 2 diabetes who had at least 5 years of follow-up data starting from 2013.
Not surprisingly, there were eight times as many individuals with type 2 diabetes (425,207) than those with type 1 diabetes (50,623), and patients with type 2 diabetes tended to be older than those with type 1 diabetes (mean age, 68.6 vs. 61.4 years).
There were many significant differences between the two groups of patients in terms of clinical variables, such as patients with type 2 diabetes having more cardiovascular risk factors or preexisting heart problems, and those with type 1 diabetes more likely to have diabetic eye disease.
Indeed, Dr. Angoulvant pointed out that those with type 2 diabetes were significantly more likely (all P < .0001) than those with type 1 diabetes to have: hypertension (70.8% vs. 50.5%), heart failure (35.7% vs. 16.4%), valvular heart disease (7.2% vs. 3.5%), dilated cardiomyopathy (5.5% vs. 2.7%), coronary artery disease (27.6 vs. 18.6%), previous MI (3.0% vs. 2.4%), peripheral vascular disease (22.0% vs. 15.5%), and ischemic stroke (3.3 vs. 2.2%).
“Regarding more specific microvascular diabetic complications, we had a higher incidence of chronic kidney disease in type 2 diabetes patients [10.2% vs. 9.1%], but a higher incidence of diabetic retinopathy in type 1 diabetes patients [6.6% vs. 12.2%],” Dr. Angoulvant said.
Considering more than 2 million person-years of follow-up, the annual rates of MI, new-onset heart failure, ischemic stroke, and chronic kidney disease for the whole study population were respective 1.4%, 5.4%, 1.2%, and 3.4%. The annual rates for death from any cause was 9.7%, and for a cardiovascular reason was 2.4%.
Cardiovascular disease prevalence and event rates
The mean follow-up period was 4.3 years, and over this time the age- and sex-adjusted prevalence of cardiovascular disease was found to be highest in individuals with type 2 diabetes, especially after the age of 40 years.
Looking at the rates of different cardiovascular events showed that both younger (18-29 years) and older (60+ years) people with type 1 diabetes had a 1.2-fold higher risk for MI than similarly aged individuals with type 2 diabetes.
Furthermore, younger and older type 1 diabetes individuals had a 1.1- to 1.4-fold greater risk of new-onset heart failure than those with type 2 diabetes.
“Interestingly, regarding the incidence of ischemic stroke in our population, we found no significant difference between patients with type 1 diabetes, and patients with type 2 diabetes,” Dr. Angoulvant said.
Chronic kidney disease and risk for death
Chronic kidney disease was most common in individuals with type 1 diabetes who were aged between 18 and 69 years, with a greater prevalence also seen in those with type 2 diabetes only after age 80.
The risk of new chronic kidney disease was significantly increased in patients with type 1 diabetes, compared with patients with type 2 diabetes, with a 1.1- to 2.4-fold increase seen, first in individuals aged 18-49 years, and then again after the age of 60 years.
Dr. Angoulvant reported that the risk of dying from any cause was 1.1-fold higher in people with type 1 diabetes, compared with those with type 2 diabetes, but after the age of 60 years.
The risk of death from cardiovascular events was also increased in people with type 1 diabetes, but between the ages of 60 and 69 years.
Asked what his take-home message might be, Dr. Angoulvant stressed the importance of heart failure, in all patients with diabetes but particularly in those with type 1 diabetes.
“I think there is room for improvement in terms of assessing who is going to have heart failure, how to assess heart failure, and more importantly, how to prevent heart failure,” perhaps by “introducing those drugs that have shown tremendous benefit regarding hospitalization, such as [sodium-glucose transporter 2] inhibitors” in patients with type 1 diabetes ahead of the events, he said.
Dr. Angoulvant had no conflicts of interest to disclose.
While type 2 diabetes is associated with a greater risk for cardiovascular events than type 1 diabetes, the latter is more associated with chronic kidney complications, according to data from a French observational study.
That’s not to say that type 1 diabetes isn’t also associated with poor heart health that is of concern, according to Denis Angoulvant, MD, of Tours (France) Regional University Hospital and Trousseau Hospital in Paris.
“The difference is that, in the middle or older ages, we suddenly see a surge of cardiovascular events in type 1 diabetic patients,” he said at the annual meeting of the European Association for the Study of Diabetes. “As a cardiologist, I must say that we are barely see these patients ahead of those complications, so we advocate that there’s a gap to be filled here to prevent these events in these patients.”
Few studies have looked at the comparative risks for cardiovascular and renal outcomes between patients with type 1 and type 2 diabetes, Dr. Angoulvant said, so the aim of the study he presented was to look at this in more detail.
Comparing cardiovascular and renal outcomes
Data from the French hospital discharge database (PMSI), which covers more than 98% of the country’s population, were used to find all adults with type 1 or type 2 diabetes who had at least 5 years of follow-up data starting from 2013.
Not surprisingly, there were eight times as many individuals with type 2 diabetes (425,207) than those with type 1 diabetes (50,623), and patients with type 2 diabetes tended to be older than those with type 1 diabetes (mean age, 68.6 vs. 61.4 years).
There were many significant differences between the two groups of patients in terms of clinical variables, such as patients with type 2 diabetes having more cardiovascular risk factors or preexisting heart problems, and those with type 1 diabetes more likely to have diabetic eye disease.
Indeed, Dr. Angoulvant pointed out that those with type 2 diabetes were significantly more likely (all P < .0001) than those with type 1 diabetes to have: hypertension (70.8% vs. 50.5%), heart failure (35.7% vs. 16.4%), valvular heart disease (7.2% vs. 3.5%), dilated cardiomyopathy (5.5% vs. 2.7%), coronary artery disease (27.6 vs. 18.6%), previous MI (3.0% vs. 2.4%), peripheral vascular disease (22.0% vs. 15.5%), and ischemic stroke (3.3 vs. 2.2%).
“Regarding more specific microvascular diabetic complications, we had a higher incidence of chronic kidney disease in type 2 diabetes patients [10.2% vs. 9.1%], but a higher incidence of diabetic retinopathy in type 1 diabetes patients [6.6% vs. 12.2%],” Dr. Angoulvant said.
Considering more than 2 million person-years of follow-up, the annual rates of MI, new-onset heart failure, ischemic stroke, and chronic kidney disease for the whole study population were respective 1.4%, 5.4%, 1.2%, and 3.4%. The annual rates for death from any cause was 9.7%, and for a cardiovascular reason was 2.4%.
Cardiovascular disease prevalence and event rates
The mean follow-up period was 4.3 years, and over this time the age- and sex-adjusted prevalence of cardiovascular disease was found to be highest in individuals with type 2 diabetes, especially after the age of 40 years.
Looking at the rates of different cardiovascular events showed that both younger (18-29 years) and older (60+ years) people with type 1 diabetes had a 1.2-fold higher risk for MI than similarly aged individuals with type 2 diabetes.
Furthermore, younger and older type 1 diabetes individuals had a 1.1- to 1.4-fold greater risk of new-onset heart failure than those with type 2 diabetes.
“Interestingly, regarding the incidence of ischemic stroke in our population, we found no significant difference between patients with type 1 diabetes, and patients with type 2 diabetes,” Dr. Angoulvant said.
Chronic kidney disease and risk for death
Chronic kidney disease was most common in individuals with type 1 diabetes who were aged between 18 and 69 years, with a greater prevalence also seen in those with type 2 diabetes only after age 80.
The risk of new chronic kidney disease was significantly increased in patients with type 1 diabetes, compared with patients with type 2 diabetes, with a 1.1- to 2.4-fold increase seen, first in individuals aged 18-49 years, and then again after the age of 60 years.
Dr. Angoulvant reported that the risk of dying from any cause was 1.1-fold higher in people with type 1 diabetes, compared with those with type 2 diabetes, but after the age of 60 years.
The risk of death from cardiovascular events was also increased in people with type 1 diabetes, but between the ages of 60 and 69 years.
Asked what his take-home message might be, Dr. Angoulvant stressed the importance of heart failure, in all patients with diabetes but particularly in those with type 1 diabetes.
“I think there is room for improvement in terms of assessing who is going to have heart failure, how to assess heart failure, and more importantly, how to prevent heart failure,” perhaps by “introducing those drugs that have shown tremendous benefit regarding hospitalization, such as [sodium-glucose transporter 2] inhibitors” in patients with type 1 diabetes ahead of the events, he said.
Dr. Angoulvant had no conflicts of interest to disclose.
FROM EASD 2021
My experience as a family medicine resident in 2021
I did not get a medical school graduation; I was one of the many thousands of newly graduated students who simply left their 4th-year rotation sites one chilly day in March 2020 and just never went back. My medical school education didn’t end with me walking triumphantly across the stage – a first-generation college student finally achieving the greatest dream in her life. Instead, it ended with a Zoom “graduation” and a cross-country move from Georgia to Pennsylvania amidst the greatest pandemic in recent memory. To say my impostor syndrome was bad would be an understatement.
Residency in the COVID-19-era
The joy and the draw to family medicine for me has always been the broad scope of conditions that we see and treat. From day 1, however, much of my residency has been devoted to one very small subset of patients – those with COVID-19. At one point, our hospital was so strained that our family medicine program had to run a second inpatient service alongside our usual five-resident service team just to provide care to everybody. Patients were in the hallways. The ER was packed to the gills. We were sleepless, terrified, unvaccinated, and desperate to help our patients survive a disease that was incompletely understood, with very few tools in our toolbox to combat it.
I distinctly remember sitting in the workroom with a coresident of mine, our faces seemingly permanently lined from wearing N95s all shift, and saying to him, “I worry I will be a bad family medicine physician. I worry I haven’t seen enough, other than COVID.” It was midway through my intern year; the days were short, so I was driving to and from the hospital in chilly darkness. My patients, like many around the country, were doing poorly. Vaccines seemed like a promise too good to be true. Worst of all: Those of us who were interns, who had no triumphant podium moment to end our medical school education, were suffering with an intense sense of impostor syndrome which was strengthened by every “there is nothing else we can offer your loved one at this time,” conversation we had. My apprehension about not having seen a wider breadth of medicine during my training is a sentiment still widely shared by COVID-era residents.
Luckily, my coresident was supportive.
“We’re going to be great family medicine physicians,” he said. “We’re learning the hard stuff – the bread and butter of FM – up-front. You’ll see.”
In some ways, I think he was right. Clinical skills, empathy, humility, and forging strong relationships are at the center of every family medicine physician’s heart; my generation has had to learn these skills early and under pressure. Sometimes, there are no answers. Sometimes, the best thing a family doctor can do for a patient is to hear them, understand them, and hold their hand.
‘We watched Cinderella together’
Shortly after that conversation with my coresident, I had a particular case which moved me. This gentleman with intellectual disability and COVID had been declining steadily since his admission to the hospital. He was isolated from everybody he knew and loved, but it did not dampen his spirits. He was cheerful to every person who entered his room, clad in their shrouds of PPE, which more often than not felt more like mourning garb than protective wear. I remember very little about this patient’s clinical picture – the COVID, the superimposed pneumonia, the repeated intubations. What I do remember is he loved the Disney classic, Cinderella. I knew this because I developed a very close relationship with his family during the course of his hospitalization. Amidst the torrential onslaught of patients, I made sure to call families every day – not because I wanted to, but because my mentors and attendings and coresidents had all drilled into me from day 1 that we are family medicine, and a large part of our role is to advocate for our patients, and to communicate with their loved ones. So I called. I learned a lot about him; his likes, his dislikes, his close bond with his siblings, and of course his lifelong love for Cinderella. On the last week of my ICU rotation, my patient passed peacefully. His nurse and I were bedside. We held his hand. We told him his family loved him. We watched Cinderella together on an iPad encased in protective plastic.
My next rotation was an outpatient one and it looked more like the “bread and butter” of family medicine. But as I whisked in and out of patient rooms, attending to patients with diabetes, with depression, with pain, I could not stop thinking about my hospitalized patients who my coresidents had assumed care of. Each exam room I entered, I rather morbidly thought “this patient could be next on our hospital service.” Without realizing it, I made more of an effort to get to know each patient holistically. I learned who they were as people. I found myself writing small, medically low-yield details in the chart: “Margaret loves to sing in her church choir;” “Katherine is a self-published author.”
I learned from my attendings. As I sat at the precepting table with them, observing their conversations about patients, their collective decades of experience were apparent.
“I’ve been seeing this patient every few weeks since I was a resident,” said one of my attendings.
“I don’t even see my parents that often,” I thought.
The depth of her relationship with, understanding of, and compassion for this patient struck me deeply. This was why I went into family medicine. My attending knew her patients; they were not faceless unknowns in a hospital gown to her. She would have known to play Cinderella for them in the end.
This is a unique time for trainees. We have been challenged, terrified, overwhelmed, and heartbroken. But at no point have we been isolated. We’ve had the generations of doctors before us to lead the way, to teach us the “hard stuff.” We’ve had senior residents to lean on, who have taken us aside and told us, “I can do the goals-of-care talk today, you need a break.” While the plague seems to have passed over our hospital for now, it has left behind a class of family medicine residents who are proud to carry on our specialty’s long tradition of compassionate, empathetic, lifelong care. “We care for all life stages, from cradle to grave,” says every family medicine physician.
My class, for better or for worse, has cared more often for patients in the twilight of their lives, and while it has been hard, I believe it has made us all better doctors. Now, when I hold a newborn in my arms for a well-child check, I am exceptionally grateful – for the opportunities I have been given, for new beginnings amidst so much sadness, and for the great privilege of being a family medicine physician.
Dr. Persampiere is a 2nd-year resident in the family medicine residency program at Abington (Pa.) Jefferson Health. You can contact her directly at [email protected] or via [email protected].
I did not get a medical school graduation; I was one of the many thousands of newly graduated students who simply left their 4th-year rotation sites one chilly day in March 2020 and just never went back. My medical school education didn’t end with me walking triumphantly across the stage – a first-generation college student finally achieving the greatest dream in her life. Instead, it ended with a Zoom “graduation” and a cross-country move from Georgia to Pennsylvania amidst the greatest pandemic in recent memory. To say my impostor syndrome was bad would be an understatement.
Residency in the COVID-19-era
The joy and the draw to family medicine for me has always been the broad scope of conditions that we see and treat. From day 1, however, much of my residency has been devoted to one very small subset of patients – those with COVID-19. At one point, our hospital was so strained that our family medicine program had to run a second inpatient service alongside our usual five-resident service team just to provide care to everybody. Patients were in the hallways. The ER was packed to the gills. We were sleepless, terrified, unvaccinated, and desperate to help our patients survive a disease that was incompletely understood, with very few tools in our toolbox to combat it.
I distinctly remember sitting in the workroom with a coresident of mine, our faces seemingly permanently lined from wearing N95s all shift, and saying to him, “I worry I will be a bad family medicine physician. I worry I haven’t seen enough, other than COVID.” It was midway through my intern year; the days were short, so I was driving to and from the hospital in chilly darkness. My patients, like many around the country, were doing poorly. Vaccines seemed like a promise too good to be true. Worst of all: Those of us who were interns, who had no triumphant podium moment to end our medical school education, were suffering with an intense sense of impostor syndrome which was strengthened by every “there is nothing else we can offer your loved one at this time,” conversation we had. My apprehension about not having seen a wider breadth of medicine during my training is a sentiment still widely shared by COVID-era residents.
Luckily, my coresident was supportive.
“We’re going to be great family medicine physicians,” he said. “We’re learning the hard stuff – the bread and butter of FM – up-front. You’ll see.”
In some ways, I think he was right. Clinical skills, empathy, humility, and forging strong relationships are at the center of every family medicine physician’s heart; my generation has had to learn these skills early and under pressure. Sometimes, there are no answers. Sometimes, the best thing a family doctor can do for a patient is to hear them, understand them, and hold their hand.
‘We watched Cinderella together’
Shortly after that conversation with my coresident, I had a particular case which moved me. This gentleman with intellectual disability and COVID had been declining steadily since his admission to the hospital. He was isolated from everybody he knew and loved, but it did not dampen his spirits. He was cheerful to every person who entered his room, clad in their shrouds of PPE, which more often than not felt more like mourning garb than protective wear. I remember very little about this patient’s clinical picture – the COVID, the superimposed pneumonia, the repeated intubations. What I do remember is he loved the Disney classic, Cinderella. I knew this because I developed a very close relationship with his family during the course of his hospitalization. Amidst the torrential onslaught of patients, I made sure to call families every day – not because I wanted to, but because my mentors and attendings and coresidents had all drilled into me from day 1 that we are family medicine, and a large part of our role is to advocate for our patients, and to communicate with their loved ones. So I called. I learned a lot about him; his likes, his dislikes, his close bond with his siblings, and of course his lifelong love for Cinderella. On the last week of my ICU rotation, my patient passed peacefully. His nurse and I were bedside. We held his hand. We told him his family loved him. We watched Cinderella together on an iPad encased in protective plastic.
My next rotation was an outpatient one and it looked more like the “bread and butter” of family medicine. But as I whisked in and out of patient rooms, attending to patients with diabetes, with depression, with pain, I could not stop thinking about my hospitalized patients who my coresidents had assumed care of. Each exam room I entered, I rather morbidly thought “this patient could be next on our hospital service.” Without realizing it, I made more of an effort to get to know each patient holistically. I learned who they were as people. I found myself writing small, medically low-yield details in the chart: “Margaret loves to sing in her church choir;” “Katherine is a self-published author.”
I learned from my attendings. As I sat at the precepting table with them, observing their conversations about patients, their collective decades of experience were apparent.
“I’ve been seeing this patient every few weeks since I was a resident,” said one of my attendings.
“I don’t even see my parents that often,” I thought.
The depth of her relationship with, understanding of, and compassion for this patient struck me deeply. This was why I went into family medicine. My attending knew her patients; they were not faceless unknowns in a hospital gown to her. She would have known to play Cinderella for them in the end.
This is a unique time for trainees. We have been challenged, terrified, overwhelmed, and heartbroken. But at no point have we been isolated. We’ve had the generations of doctors before us to lead the way, to teach us the “hard stuff.” We’ve had senior residents to lean on, who have taken us aside and told us, “I can do the goals-of-care talk today, you need a break.” While the plague seems to have passed over our hospital for now, it has left behind a class of family medicine residents who are proud to carry on our specialty’s long tradition of compassionate, empathetic, lifelong care. “We care for all life stages, from cradle to grave,” says every family medicine physician.
My class, for better or for worse, has cared more often for patients in the twilight of their lives, and while it has been hard, I believe it has made us all better doctors. Now, when I hold a newborn in my arms for a well-child check, I am exceptionally grateful – for the opportunities I have been given, for new beginnings amidst so much sadness, and for the great privilege of being a family medicine physician.
Dr. Persampiere is a 2nd-year resident in the family medicine residency program at Abington (Pa.) Jefferson Health. You can contact her directly at [email protected] or via [email protected].
I did not get a medical school graduation; I was one of the many thousands of newly graduated students who simply left their 4th-year rotation sites one chilly day in March 2020 and just never went back. My medical school education didn’t end with me walking triumphantly across the stage – a first-generation college student finally achieving the greatest dream in her life. Instead, it ended with a Zoom “graduation” and a cross-country move from Georgia to Pennsylvania amidst the greatest pandemic in recent memory. To say my impostor syndrome was bad would be an understatement.
Residency in the COVID-19-era
The joy and the draw to family medicine for me has always been the broad scope of conditions that we see and treat. From day 1, however, much of my residency has been devoted to one very small subset of patients – those with COVID-19. At one point, our hospital was so strained that our family medicine program had to run a second inpatient service alongside our usual five-resident service team just to provide care to everybody. Patients were in the hallways. The ER was packed to the gills. We were sleepless, terrified, unvaccinated, and desperate to help our patients survive a disease that was incompletely understood, with very few tools in our toolbox to combat it.
I distinctly remember sitting in the workroom with a coresident of mine, our faces seemingly permanently lined from wearing N95s all shift, and saying to him, “I worry I will be a bad family medicine physician. I worry I haven’t seen enough, other than COVID.” It was midway through my intern year; the days were short, so I was driving to and from the hospital in chilly darkness. My patients, like many around the country, were doing poorly. Vaccines seemed like a promise too good to be true. Worst of all: Those of us who were interns, who had no triumphant podium moment to end our medical school education, were suffering with an intense sense of impostor syndrome which was strengthened by every “there is nothing else we can offer your loved one at this time,” conversation we had. My apprehension about not having seen a wider breadth of medicine during my training is a sentiment still widely shared by COVID-era residents.
Luckily, my coresident was supportive.
“We’re going to be great family medicine physicians,” he said. “We’re learning the hard stuff – the bread and butter of FM – up-front. You’ll see.”
In some ways, I think he was right. Clinical skills, empathy, humility, and forging strong relationships are at the center of every family medicine physician’s heart; my generation has had to learn these skills early and under pressure. Sometimes, there are no answers. Sometimes, the best thing a family doctor can do for a patient is to hear them, understand them, and hold their hand.
‘We watched Cinderella together’
Shortly after that conversation with my coresident, I had a particular case which moved me. This gentleman with intellectual disability and COVID had been declining steadily since his admission to the hospital. He was isolated from everybody he knew and loved, but it did not dampen his spirits. He was cheerful to every person who entered his room, clad in their shrouds of PPE, which more often than not felt more like mourning garb than protective wear. I remember very little about this patient’s clinical picture – the COVID, the superimposed pneumonia, the repeated intubations. What I do remember is he loved the Disney classic, Cinderella. I knew this because I developed a very close relationship with his family during the course of his hospitalization. Amidst the torrential onslaught of patients, I made sure to call families every day – not because I wanted to, but because my mentors and attendings and coresidents had all drilled into me from day 1 that we are family medicine, and a large part of our role is to advocate for our patients, and to communicate with their loved ones. So I called. I learned a lot about him; his likes, his dislikes, his close bond with his siblings, and of course his lifelong love for Cinderella. On the last week of my ICU rotation, my patient passed peacefully. His nurse and I were bedside. We held his hand. We told him his family loved him. We watched Cinderella together on an iPad encased in protective plastic.
My next rotation was an outpatient one and it looked more like the “bread and butter” of family medicine. But as I whisked in and out of patient rooms, attending to patients with diabetes, with depression, with pain, I could not stop thinking about my hospitalized patients who my coresidents had assumed care of. Each exam room I entered, I rather morbidly thought “this patient could be next on our hospital service.” Without realizing it, I made more of an effort to get to know each patient holistically. I learned who they were as people. I found myself writing small, medically low-yield details in the chart: “Margaret loves to sing in her church choir;” “Katherine is a self-published author.”
I learned from my attendings. As I sat at the precepting table with them, observing their conversations about patients, their collective decades of experience were apparent.
“I’ve been seeing this patient every few weeks since I was a resident,” said one of my attendings.
“I don’t even see my parents that often,” I thought.
The depth of her relationship with, understanding of, and compassion for this patient struck me deeply. This was why I went into family medicine. My attending knew her patients; they were not faceless unknowns in a hospital gown to her. She would have known to play Cinderella for them in the end.
This is a unique time for trainees. We have been challenged, terrified, overwhelmed, and heartbroken. But at no point have we been isolated. We’ve had the generations of doctors before us to lead the way, to teach us the “hard stuff.” We’ve had senior residents to lean on, who have taken us aside and told us, “I can do the goals-of-care talk today, you need a break.” While the plague seems to have passed over our hospital for now, it has left behind a class of family medicine residents who are proud to carry on our specialty’s long tradition of compassionate, empathetic, lifelong care. “We care for all life stages, from cradle to grave,” says every family medicine physician.
My class, for better or for worse, has cared more often for patients in the twilight of their lives, and while it has been hard, I believe it has made us all better doctors. Now, when I hold a newborn in my arms for a well-child check, I am exceptionally grateful – for the opportunities I have been given, for new beginnings amidst so much sadness, and for the great privilege of being a family medicine physician.
Dr. Persampiere is a 2nd-year resident in the family medicine residency program at Abington (Pa.) Jefferson Health. You can contact her directly at [email protected] or via [email protected].
Antithrombotic therapy not warranted in COVID-19 outpatients
Antithrombotic therapy in clinically stable, nonhospitalized COVID-19 patients does not offer protection against adverse cardiovascular or pulmonary events, new randomized clinical trial results suggest.
Antithrombotic therapy has proven useful in acutely ill inpatients with COVID-19, but in this study, treatment with aspirin or apixaban (Eliquis) did not reduce the rate of all-cause mortality, symptomatic venous or arterial thromboembolism, myocardial infarction, stroke, or hospitalization for cardiovascular or pulmonary causes in patients ill with COVID-19 but who were not hospitalized.
“Among symptomatic, clinically stable outpatients with COVID-19, treatment with aspirin or apixaban compared with placebo did not reduce the rate of a composite clinical outcome,” the authors conclude. “However, the study was terminated after enrollment of 9% of participants because of a primary event rate lower than anticipated.”
The study, which was led by Jean M. Connors, MD, Brigham and Women’s Hospital, Boston, was published online October 11 in JAMA.
The ACTIV-4B Outpatient Thrombosis Prevention Trial was a randomized, adaptive, double-blind, placebo-controlled trial that sought to compare anticoagulant and antiplatelet therapy among 7,000 symptomatic but clinically stable outpatients with COVID-19.
The trial was conducted at 52 sites in the U.S. between Sept. 2020 and June 2021, with final follow-up this past August 5, and involved minimal face-to-face interactions with study participants.
Patients were randomized in a 1:1:1:1 ratio to aspirin (81 mg orally once daily; n = 164 patients), prophylactic-dose apixaban (2.5 mg orally twice daily; n = 165), therapeutic-dose apixaban (5 mg orally twice daily; n = 164), or placebo (n = 164) for 45 days.
The primary endpoint was a composite of all-cause mortality, symptomatic venous or arterial thromboembolism, myocardial infarction, stroke, or hospitalization for cardiovascular or pulmonary cause.
The trial was terminated early this past June by the independent data monitoring committee because of lower than anticipated event rates. At the time, just 657 symptomatic outpatients with COVID-19 had been enrolled.
The median age of the study participants was 54 years (Interquartile Range [IQR] 46-59); 59% were women.
The median time from diagnosis to randomization was 7 days, and the median time from randomization to initiation of study medications was 3 days.
The trial’s primary efficacy and safety analyses were restricted to patients who received at least one dose of trial medication, for a final number of 558 patients.
Among these patients, the primary endpoint occurred in 1 patient (0.7%) in the aspirin group, 1 patient (0.7%) in the 2.5 mg apixaban group, 2 patients (1.4%) in the 5-mg apixaban group, and 1 patient (0.7%) in the placebo group.
The researchers found that the absolute risk reductions compared with placebo for the primary outcome were 0.0% (95% confidence interval not calculable) in the aspirin group, 0.7% (95% confidence interval, -2.1% to 4.1%) in the prophylactic-dose apixaban group, and 1.4% (95% CI, -1.5% to 5%) in the therapeutic-dose apixaban group.
No major bleeding events were reported.
The absolute risk differences compared with placebo for clinically relevant nonmajor bleeding events were 2% (95% CI, -2.7% to 6.8%) in the aspirin group, 4.5% (95% CI, -0.7% to 10.2%) in the prophylactic-dose apixaban group, and 6.9% (95% CI, 1.4% to 12.9%) in the therapeutic-dose apixaban group.
Safety and efficacy results were similar in all randomly assigned patients.
The researchers speculated that a combination of two demographic shifts over time may have led to the lower than anticipated rate of events in ACTIV-4B.
“First, the threshold for hospital admission has markedly declined since the beginning of the pandemic, such that hospitalization is no longer limited almost exclusively to those with severe pulmonary distress likely to require mechanical ventilation,” they write. “As a result, the severity of illness among individuals with COVID-19 and destined for outpatient care has declined.”
“Second, at least within the U.S., where the trial was conducted, individuals currently being infected with SARS-CoV-2 tend to be younger and have fewer comorbidities when compared with individuals with incident infection at the onset of the pandemic,” they add.
Further, COVID-19 testing was quite limited early in the pandemic, they note, “and it is possible that the anticipated event rates based on data from registries available at that time were overestimated because the denominator (that is, the number of infected individuals overall) was essentially unknown.”
Robust evidence
“The ACTIV-4B trial is the first randomized trial to generate robust evidence about the effects of antithrombotic therapy in outpatients with COVID-19,” Otavio Berwanger, MD, PhD, director of the Academic Research Organization, Hospital Israelita Albert Einstein, Sao Paulo-SP, Brazil, told this news organization.
“It should be noted that this was a well-designed trial with low risk of bias. On the other hand, the main limitation is the low number of events and, consequently, the limited statistical power,” said Dr. Berwanger, who wrote an accompanying editorial.
The ACTIV-4B trial has immediate implications for clinical practice, he added.
“In this sense, considering the neutral results for major cardiopulmonary outcomes, the use of aspirin or apixaban for the management of outpatients with COVID-19 should not be recommended.”
ACTIV-4B also provides useful information for the steering committees of other ongoing trials of antithrombotic therapy for patients with COVID-19 who are not hospitalized, Dr. Berwanger added.
“In this sense, probably issues like statistical power, outcome choices, recruitment feasibility, and even futility would need to be revisited. And finally, lessons learned from the implementation of an innovative, pragmatic, and decentralized trial design represent an important legacy for future trials in cardiovascular diseases and other common conditions,” he said.
The study was funded by the National Institutes of Health, and the National Heart, Lung, and Blood Institute. Dr. Connors reports financial relationships with Bristol-Myers Squibb, Pfizer, Abbott, Alnylam, Takeda, Roche, and Sanofi. Dr. Berwanger reports financial relationships with AstraZeneca, Amgen, Servier, Bristol-Myers Squibb, Bayer, Novartis, Pfizer, and Boehringer Ingelheim.
A version of this article first appeared on Medscape.com.
Antithrombotic therapy in clinically stable, nonhospitalized COVID-19 patients does not offer protection against adverse cardiovascular or pulmonary events, new randomized clinical trial results suggest.
Antithrombotic therapy has proven useful in acutely ill inpatients with COVID-19, but in this study, treatment with aspirin or apixaban (Eliquis) did not reduce the rate of all-cause mortality, symptomatic venous or arterial thromboembolism, myocardial infarction, stroke, or hospitalization for cardiovascular or pulmonary causes in patients ill with COVID-19 but who were not hospitalized.
“Among symptomatic, clinically stable outpatients with COVID-19, treatment with aspirin or apixaban compared with placebo did not reduce the rate of a composite clinical outcome,” the authors conclude. “However, the study was terminated after enrollment of 9% of participants because of a primary event rate lower than anticipated.”
The study, which was led by Jean M. Connors, MD, Brigham and Women’s Hospital, Boston, was published online October 11 in JAMA.
The ACTIV-4B Outpatient Thrombosis Prevention Trial was a randomized, adaptive, double-blind, placebo-controlled trial that sought to compare anticoagulant and antiplatelet therapy among 7,000 symptomatic but clinically stable outpatients with COVID-19.
The trial was conducted at 52 sites in the U.S. between Sept. 2020 and June 2021, with final follow-up this past August 5, and involved minimal face-to-face interactions with study participants.
Patients were randomized in a 1:1:1:1 ratio to aspirin (81 mg orally once daily; n = 164 patients), prophylactic-dose apixaban (2.5 mg orally twice daily; n = 165), therapeutic-dose apixaban (5 mg orally twice daily; n = 164), or placebo (n = 164) for 45 days.
The primary endpoint was a composite of all-cause mortality, symptomatic venous or arterial thromboembolism, myocardial infarction, stroke, or hospitalization for cardiovascular or pulmonary cause.
The trial was terminated early this past June by the independent data monitoring committee because of lower than anticipated event rates. At the time, just 657 symptomatic outpatients with COVID-19 had been enrolled.
The median age of the study participants was 54 years (Interquartile Range [IQR] 46-59); 59% were women.
The median time from diagnosis to randomization was 7 days, and the median time from randomization to initiation of study medications was 3 days.
The trial’s primary efficacy and safety analyses were restricted to patients who received at least one dose of trial medication, for a final number of 558 patients.
Among these patients, the primary endpoint occurred in 1 patient (0.7%) in the aspirin group, 1 patient (0.7%) in the 2.5 mg apixaban group, 2 patients (1.4%) in the 5-mg apixaban group, and 1 patient (0.7%) in the placebo group.
The researchers found that the absolute risk reductions compared with placebo for the primary outcome were 0.0% (95% confidence interval not calculable) in the aspirin group, 0.7% (95% confidence interval, -2.1% to 4.1%) in the prophylactic-dose apixaban group, and 1.4% (95% CI, -1.5% to 5%) in the therapeutic-dose apixaban group.
No major bleeding events were reported.
The absolute risk differences compared with placebo for clinically relevant nonmajor bleeding events were 2% (95% CI, -2.7% to 6.8%) in the aspirin group, 4.5% (95% CI, -0.7% to 10.2%) in the prophylactic-dose apixaban group, and 6.9% (95% CI, 1.4% to 12.9%) in the therapeutic-dose apixaban group.
Safety and efficacy results were similar in all randomly assigned patients.
The researchers speculated that a combination of two demographic shifts over time may have led to the lower than anticipated rate of events in ACTIV-4B.
“First, the threshold for hospital admission has markedly declined since the beginning of the pandemic, such that hospitalization is no longer limited almost exclusively to those with severe pulmonary distress likely to require mechanical ventilation,” they write. “As a result, the severity of illness among individuals with COVID-19 and destined for outpatient care has declined.”
“Second, at least within the U.S., where the trial was conducted, individuals currently being infected with SARS-CoV-2 tend to be younger and have fewer comorbidities when compared with individuals with incident infection at the onset of the pandemic,” they add.
Further, COVID-19 testing was quite limited early in the pandemic, they note, “and it is possible that the anticipated event rates based on data from registries available at that time were overestimated because the denominator (that is, the number of infected individuals overall) was essentially unknown.”
Robust evidence
“The ACTIV-4B trial is the first randomized trial to generate robust evidence about the effects of antithrombotic therapy in outpatients with COVID-19,” Otavio Berwanger, MD, PhD, director of the Academic Research Organization, Hospital Israelita Albert Einstein, Sao Paulo-SP, Brazil, told this news organization.
“It should be noted that this was a well-designed trial with low risk of bias. On the other hand, the main limitation is the low number of events and, consequently, the limited statistical power,” said Dr. Berwanger, who wrote an accompanying editorial.
The ACTIV-4B trial has immediate implications for clinical practice, he added.
“In this sense, considering the neutral results for major cardiopulmonary outcomes, the use of aspirin or apixaban for the management of outpatients with COVID-19 should not be recommended.”
ACTIV-4B also provides useful information for the steering committees of other ongoing trials of antithrombotic therapy for patients with COVID-19 who are not hospitalized, Dr. Berwanger added.
“In this sense, probably issues like statistical power, outcome choices, recruitment feasibility, and even futility would need to be revisited. And finally, lessons learned from the implementation of an innovative, pragmatic, and decentralized trial design represent an important legacy for future trials in cardiovascular diseases and other common conditions,” he said.
The study was funded by the National Institutes of Health, and the National Heart, Lung, and Blood Institute. Dr. Connors reports financial relationships with Bristol-Myers Squibb, Pfizer, Abbott, Alnylam, Takeda, Roche, and Sanofi. Dr. Berwanger reports financial relationships with AstraZeneca, Amgen, Servier, Bristol-Myers Squibb, Bayer, Novartis, Pfizer, and Boehringer Ingelheim.
A version of this article first appeared on Medscape.com.
Antithrombotic therapy in clinically stable, nonhospitalized COVID-19 patients does not offer protection against adverse cardiovascular or pulmonary events, new randomized clinical trial results suggest.
Antithrombotic therapy has proven useful in acutely ill inpatients with COVID-19, but in this study, treatment with aspirin or apixaban (Eliquis) did not reduce the rate of all-cause mortality, symptomatic venous or arterial thromboembolism, myocardial infarction, stroke, or hospitalization for cardiovascular or pulmonary causes in patients ill with COVID-19 but who were not hospitalized.
“Among symptomatic, clinically stable outpatients with COVID-19, treatment with aspirin or apixaban compared with placebo did not reduce the rate of a composite clinical outcome,” the authors conclude. “However, the study was terminated after enrollment of 9% of participants because of a primary event rate lower than anticipated.”
The study, which was led by Jean M. Connors, MD, Brigham and Women’s Hospital, Boston, was published online October 11 in JAMA.
The ACTIV-4B Outpatient Thrombosis Prevention Trial was a randomized, adaptive, double-blind, placebo-controlled trial that sought to compare anticoagulant and antiplatelet therapy among 7,000 symptomatic but clinically stable outpatients with COVID-19.
The trial was conducted at 52 sites in the U.S. between Sept. 2020 and June 2021, with final follow-up this past August 5, and involved minimal face-to-face interactions with study participants.
Patients were randomized in a 1:1:1:1 ratio to aspirin (81 mg orally once daily; n = 164 patients), prophylactic-dose apixaban (2.5 mg orally twice daily; n = 165), therapeutic-dose apixaban (5 mg orally twice daily; n = 164), or placebo (n = 164) for 45 days.
The primary endpoint was a composite of all-cause mortality, symptomatic venous or arterial thromboembolism, myocardial infarction, stroke, or hospitalization for cardiovascular or pulmonary cause.
The trial was terminated early this past June by the independent data monitoring committee because of lower than anticipated event rates. At the time, just 657 symptomatic outpatients with COVID-19 had been enrolled.
The median age of the study participants was 54 years (Interquartile Range [IQR] 46-59); 59% were women.
The median time from diagnosis to randomization was 7 days, and the median time from randomization to initiation of study medications was 3 days.
The trial’s primary efficacy and safety analyses were restricted to patients who received at least one dose of trial medication, for a final number of 558 patients.
Among these patients, the primary endpoint occurred in 1 patient (0.7%) in the aspirin group, 1 patient (0.7%) in the 2.5 mg apixaban group, 2 patients (1.4%) in the 5-mg apixaban group, and 1 patient (0.7%) in the placebo group.
The researchers found that the absolute risk reductions compared with placebo for the primary outcome were 0.0% (95% confidence interval not calculable) in the aspirin group, 0.7% (95% confidence interval, -2.1% to 4.1%) in the prophylactic-dose apixaban group, and 1.4% (95% CI, -1.5% to 5%) in the therapeutic-dose apixaban group.
No major bleeding events were reported.
The absolute risk differences compared with placebo for clinically relevant nonmajor bleeding events were 2% (95% CI, -2.7% to 6.8%) in the aspirin group, 4.5% (95% CI, -0.7% to 10.2%) in the prophylactic-dose apixaban group, and 6.9% (95% CI, 1.4% to 12.9%) in the therapeutic-dose apixaban group.
Safety and efficacy results were similar in all randomly assigned patients.
The researchers speculated that a combination of two demographic shifts over time may have led to the lower than anticipated rate of events in ACTIV-4B.
“First, the threshold for hospital admission has markedly declined since the beginning of the pandemic, such that hospitalization is no longer limited almost exclusively to those with severe pulmonary distress likely to require mechanical ventilation,” they write. “As a result, the severity of illness among individuals with COVID-19 and destined for outpatient care has declined.”
“Second, at least within the U.S., where the trial was conducted, individuals currently being infected with SARS-CoV-2 tend to be younger and have fewer comorbidities when compared with individuals with incident infection at the onset of the pandemic,” they add.
Further, COVID-19 testing was quite limited early in the pandemic, they note, “and it is possible that the anticipated event rates based on data from registries available at that time were overestimated because the denominator (that is, the number of infected individuals overall) was essentially unknown.”
Robust evidence
“The ACTIV-4B trial is the first randomized trial to generate robust evidence about the effects of antithrombotic therapy in outpatients with COVID-19,” Otavio Berwanger, MD, PhD, director of the Academic Research Organization, Hospital Israelita Albert Einstein, Sao Paulo-SP, Brazil, told this news organization.
“It should be noted that this was a well-designed trial with low risk of bias. On the other hand, the main limitation is the low number of events and, consequently, the limited statistical power,” said Dr. Berwanger, who wrote an accompanying editorial.
The ACTIV-4B trial has immediate implications for clinical practice, he added.
“In this sense, considering the neutral results for major cardiopulmonary outcomes, the use of aspirin or apixaban for the management of outpatients with COVID-19 should not be recommended.”
ACTIV-4B also provides useful information for the steering committees of other ongoing trials of antithrombotic therapy for patients with COVID-19 who are not hospitalized, Dr. Berwanger added.
“In this sense, probably issues like statistical power, outcome choices, recruitment feasibility, and even futility would need to be revisited. And finally, lessons learned from the implementation of an innovative, pragmatic, and decentralized trial design represent an important legacy for future trials in cardiovascular diseases and other common conditions,” he said.
The study was funded by the National Institutes of Health, and the National Heart, Lung, and Blood Institute. Dr. Connors reports financial relationships with Bristol-Myers Squibb, Pfizer, Abbott, Alnylam, Takeda, Roche, and Sanofi. Dr. Berwanger reports financial relationships with AstraZeneca, Amgen, Servier, Bristol-Myers Squibb, Bayer, Novartis, Pfizer, and Boehringer Ingelheim.
A version of this article first appeared on Medscape.com.
Flesh-Colored Papule in the Nose of a Child
The Diagnosis: Striated Muscle Hamartoma
Histopathologic evaluation revealed a dome-shaped papule with a center composed of mature striated muscle bundles, vellus hairs, sebaceous lobules, and nerve twigs (Figure) consistent with a diagnosis of striated muscle hamartoma (SMH).
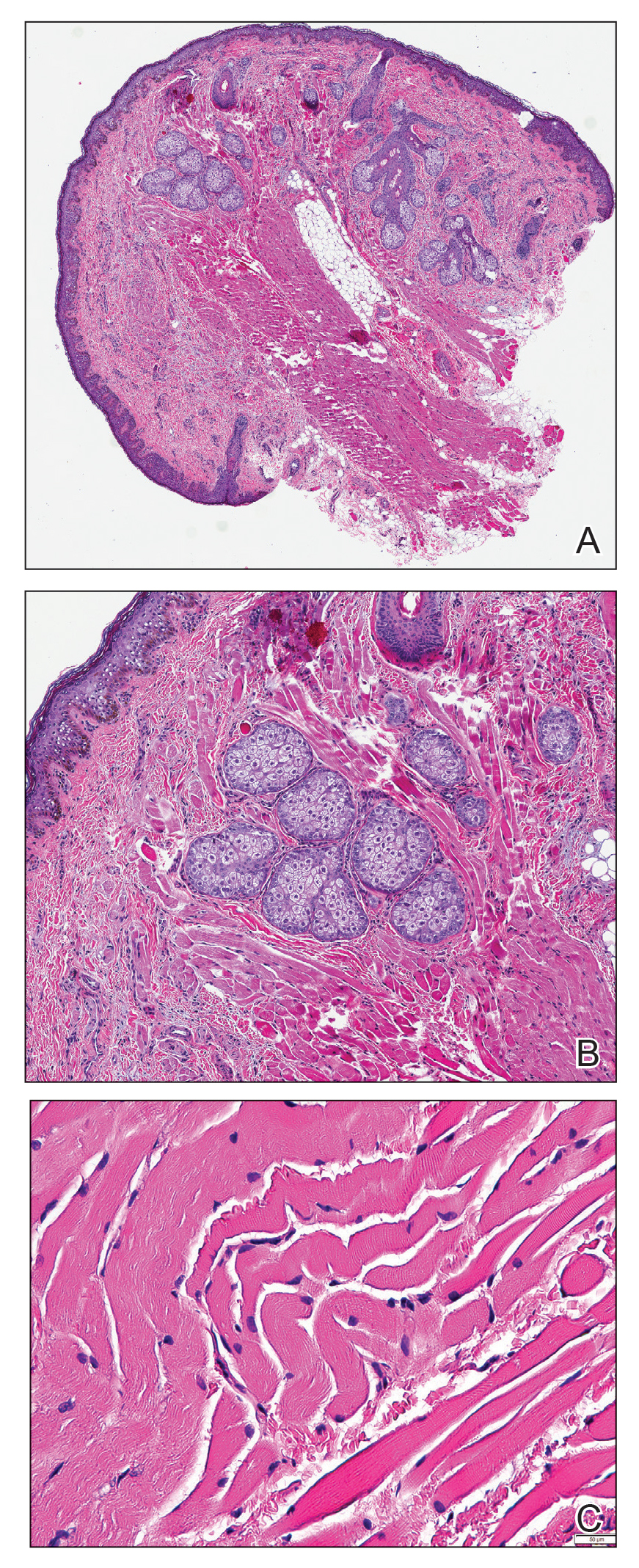
Striated muscle hamartoma was first described in 1986 by Hendrick et al1 with 2 cases in neonates. Biopsies of the lesions taken from the upper lip and sternum showed a characteristic histology consisting of dermal striated muscle fibers and nerve bundles in the central core of the papules associated with a marked number of adnexa. In 1989, the diagnosis of rhabdomyomatous mesenchymal hamartoma was described, which showed similar findings.2 Cases reported since these entities were discovered have used the terms striated muscle hamartoma and rhabdomyomatous mesenchymal hamartoma interchangeably.3
Most commonly found on the head and neck, SMH has now been observed in diverse locations including the sternum, hallux, vagina, and oral cavity.1-15 Many reported cases describe lesions around or in the nose.4,7,8 Multiple congenital anomalies have been described alongside SMH and may be associated with this entity including amniotic bands, cleft lip and palate, coloboma, and Delleman syndrome.1,3,4 Almost all of the lesions present as a sessile or pedunculated papule, polyp, nodule, or plaque measuring from 0.3 cm up to 4.9 cm and typically are present since birth.3,5,15 However, there are a few cases of lesions presenting in adults with no prior history.5,6,15
Microscopically, SMH is defined by a dermal lesion with a core comprised of mature skeletal muscle admixed with adipose tissue, adnexa, nerve bundles, and fibrovascular tissue.1 There are other entities that should be considered before making the diagnosis of SMH. Other hamartomas such as accessory tragus, connective tissue nevus, fibrous hamartoma of infancy, and nevus lipomatosis may present similarly; however, these lesions classically lack skeletal muscle. Benign triton tumors, or neuromuscular hamartomas, are rare lesions composed of skeletal muscle and abundant, intimately associated neural tissue. Neuromuscular hamartomas frequently involve large nerves.16 Rhabdomyomas also should be considered. Adult rhabdomyomas are composed of eosinophilic polygonal cells with granular cytoplasm and occasional cross-striations. Fetal rhabdomyomas have multiple histologic types and are defined by a variable myxoid stroma, eosinophilic spindled cells, and rhabdomyocytes in various stages of maturity. Genital rhabdomyomas histopathologically appear similar to fetal rhabdomyomas but are confined to the genital region. The skeletal muscle present in rhabdomyomas typically is less differentiated.17 TMature skeletal bundles should be a dominant component of the lesion before diagnosing SMH.
Typically presenting as congenital lesions in the head and neck region, papules with a dermal core of mature skeletal muscle associated with adnexa and nerve twigs should prompt consideration of a diagnosis of SMH or rhabdomyomatous mesenchymal hamartoma. These lesions are benign and usually are cured with complete excision.
- Hendrick SJ, Sanchez RL, Blackwell SJ, et al. Striated muscle hamartoma: description of two cases. Pediatr Dermatol. 1986;3:153-157.
- Mills AE. Rhabdomyomatous mesenchymal hamartoma of skin. Am J Dermatopathol. 1989;1:58-63.
- Rosenberg AS, Kirk J, Morgan MB. Rhabdomyomatous mesenchymal hamartoma: an unusual dermal entity with a report of two cases and a review of the literature. J Cutan Pathol. 2002;29:238-243.
- Sánchez RL, Raimer SS. Clinical and histologic features of striated muscle hamartoma: possible relationship to Delleman’s syndrome. J Cutan Pathol. 1994;21:40-46.
- Chang CP, Chen GS. Rhabdomyomatous mesenchymal hamartoma: a plaque-type variant in an adult. Kaohsiung J Med Sci. 2005;21:185-188.
- Harris MA, Dutton JJ, Proia AD. Striated muscle hamartoma of the eyelid in an adult woman. Ophthalmic Plast Reconstr Surg. 2008;24:492-494.
- Nakanishi H, Hashimoto I, Takiwaki H, et al. Striated muscle hamartoma of the nostril. J Dermatol. 1995;22:504-507.
- Farris PE, Manning S, Veatch F. Rhabdomyomatous mesenchymal hamartoma. Am J Dermatopathol. 1994;16:73-75.
- Grilli R, Escalonilla P, Soriano ML, et al. The so-called striated muscle hamartoma is a hamartoma of cutaneous adnexa and mesenchyme, but not of striated muscle. Acta Derm Venereol. 1998;78:390.
- Sampat K, Cheesman E, Siminas S. Perianal rhabdomyomatous mesenchymal hamartoma. Ann R Coll Surg Engl. 2017;99:E193-E195.
- Brinster NK, Farmer ER. Rhabdomyomatous mesenchymal hamartoma presenting on a digit. J Cutan Pathol. 2009;36:61-63.
- Han SH, Song HJ, Hong WK, et al. Rhabdomyomatous mesenchymal hamartoma of the vagina. Pediatr Dermatol. 2009;26:753-755.
- De la Sotta P, Salomone C, González S. Rhabdomyomatous (mesenchymal) hamartoma of the tongue: report of a case. J Oral Pathol Med. 2007;36:58-59.
- Magro G, Di Benedetto A, Sanges G, et al. Rhabdomyomatous mesenchymal hamartoma of oral cavity: an unusual location for such a rare lesion. Virchows Arch. 2005;446:346-347.
- Wang Y, Zhao H, Yue X, et al. Rhabdomyomatous mesenchymal hamartoma presenting as a big subcutaneous mass on the neck: a case report. J Med Case Rep. 2014;8:410.
- Amita K, Shankar SV, Nischal KC, et al. Benign triton tumor: a rare entity in head and neck region. Korean J Pathol. 2013;47:74-76.
- Walsh S, Hurt M. Cutaneous fetal rhabdomyoma: a case report and historical review of the literature. Am J Surg Pathol. 2008;32:485-491.
The Diagnosis: Striated Muscle Hamartoma
Histopathologic evaluation revealed a dome-shaped papule with a center composed of mature striated muscle bundles, vellus hairs, sebaceous lobules, and nerve twigs (Figure) consistent with a diagnosis of striated muscle hamartoma (SMH).

Striated muscle hamartoma was first described in 1986 by Hendrick et al1 with 2 cases in neonates. Biopsies of the lesions taken from the upper lip and sternum showed a characteristic histology consisting of dermal striated muscle fibers and nerve bundles in the central core of the papules associated with a marked number of adnexa. In 1989, the diagnosis of rhabdomyomatous mesenchymal hamartoma was described, which showed similar findings.2 Cases reported since these entities were discovered have used the terms striated muscle hamartoma and rhabdomyomatous mesenchymal hamartoma interchangeably.3
Most commonly found on the head and neck, SMH has now been observed in diverse locations including the sternum, hallux, vagina, and oral cavity.1-15 Many reported cases describe lesions around or in the nose.4,7,8 Multiple congenital anomalies have been described alongside SMH and may be associated with this entity including amniotic bands, cleft lip and palate, coloboma, and Delleman syndrome.1,3,4 Almost all of the lesions present as a sessile or pedunculated papule, polyp, nodule, or plaque measuring from 0.3 cm up to 4.9 cm and typically are present since birth.3,5,15 However, there are a few cases of lesions presenting in adults with no prior history.5,6,15
Microscopically, SMH is defined by a dermal lesion with a core comprised of mature skeletal muscle admixed with adipose tissue, adnexa, nerve bundles, and fibrovascular tissue.1 There are other entities that should be considered before making the diagnosis of SMH. Other hamartomas such as accessory tragus, connective tissue nevus, fibrous hamartoma of infancy, and nevus lipomatosis may present similarly; however, these lesions classically lack skeletal muscle. Benign triton tumors, or neuromuscular hamartomas, are rare lesions composed of skeletal muscle and abundant, intimately associated neural tissue. Neuromuscular hamartomas frequently involve large nerves.16 Rhabdomyomas also should be considered. Adult rhabdomyomas are composed of eosinophilic polygonal cells with granular cytoplasm and occasional cross-striations. Fetal rhabdomyomas have multiple histologic types and are defined by a variable myxoid stroma, eosinophilic spindled cells, and rhabdomyocytes in various stages of maturity. Genital rhabdomyomas histopathologically appear similar to fetal rhabdomyomas but are confined to the genital region. The skeletal muscle present in rhabdomyomas typically is less differentiated.17 TMature skeletal bundles should be a dominant component of the lesion before diagnosing SMH.
Typically presenting as congenital lesions in the head and neck region, papules with a dermal core of mature skeletal muscle associated with adnexa and nerve twigs should prompt consideration of a diagnosis of SMH or rhabdomyomatous mesenchymal hamartoma. These lesions are benign and usually are cured with complete excision.
The Diagnosis: Striated Muscle Hamartoma
Histopathologic evaluation revealed a dome-shaped papule with a center composed of mature striated muscle bundles, vellus hairs, sebaceous lobules, and nerve twigs (Figure) consistent with a diagnosis of striated muscle hamartoma (SMH).

Striated muscle hamartoma was first described in 1986 by Hendrick et al1 with 2 cases in neonates. Biopsies of the lesions taken from the upper lip and sternum showed a characteristic histology consisting of dermal striated muscle fibers and nerve bundles in the central core of the papules associated with a marked number of adnexa. In 1989, the diagnosis of rhabdomyomatous mesenchymal hamartoma was described, which showed similar findings.2 Cases reported since these entities were discovered have used the terms striated muscle hamartoma and rhabdomyomatous mesenchymal hamartoma interchangeably.3
Most commonly found on the head and neck, SMH has now been observed in diverse locations including the sternum, hallux, vagina, and oral cavity.1-15 Many reported cases describe lesions around or in the nose.4,7,8 Multiple congenital anomalies have been described alongside SMH and may be associated with this entity including amniotic bands, cleft lip and palate, coloboma, and Delleman syndrome.1,3,4 Almost all of the lesions present as a sessile or pedunculated papule, polyp, nodule, or plaque measuring from 0.3 cm up to 4.9 cm and typically are present since birth.3,5,15 However, there are a few cases of lesions presenting in adults with no prior history.5,6,15
Microscopically, SMH is defined by a dermal lesion with a core comprised of mature skeletal muscle admixed with adipose tissue, adnexa, nerve bundles, and fibrovascular tissue.1 There are other entities that should be considered before making the diagnosis of SMH. Other hamartomas such as accessory tragus, connective tissue nevus, fibrous hamartoma of infancy, and nevus lipomatosis may present similarly; however, these lesions classically lack skeletal muscle. Benign triton tumors, or neuromuscular hamartomas, are rare lesions composed of skeletal muscle and abundant, intimately associated neural tissue. Neuromuscular hamartomas frequently involve large nerves.16 Rhabdomyomas also should be considered. Adult rhabdomyomas are composed of eosinophilic polygonal cells with granular cytoplasm and occasional cross-striations. Fetal rhabdomyomas have multiple histologic types and are defined by a variable myxoid stroma, eosinophilic spindled cells, and rhabdomyocytes in various stages of maturity. Genital rhabdomyomas histopathologically appear similar to fetal rhabdomyomas but are confined to the genital region. The skeletal muscle present in rhabdomyomas typically is less differentiated.17 TMature skeletal bundles should be a dominant component of the lesion before diagnosing SMH.
Typically presenting as congenital lesions in the head and neck region, papules with a dermal core of mature skeletal muscle associated with adnexa and nerve twigs should prompt consideration of a diagnosis of SMH or rhabdomyomatous mesenchymal hamartoma. These lesions are benign and usually are cured with complete excision.
- Hendrick SJ, Sanchez RL, Blackwell SJ, et al. Striated muscle hamartoma: description of two cases. Pediatr Dermatol. 1986;3:153-157.
- Mills AE. Rhabdomyomatous mesenchymal hamartoma of skin. Am J Dermatopathol. 1989;1:58-63.
- Rosenberg AS, Kirk J, Morgan MB. Rhabdomyomatous mesenchymal hamartoma: an unusual dermal entity with a report of two cases and a review of the literature. J Cutan Pathol. 2002;29:238-243.
- Sánchez RL, Raimer SS. Clinical and histologic features of striated muscle hamartoma: possible relationship to Delleman’s syndrome. J Cutan Pathol. 1994;21:40-46.
- Chang CP, Chen GS. Rhabdomyomatous mesenchymal hamartoma: a plaque-type variant in an adult. Kaohsiung J Med Sci. 2005;21:185-188.
- Harris MA, Dutton JJ, Proia AD. Striated muscle hamartoma of the eyelid in an adult woman. Ophthalmic Plast Reconstr Surg. 2008;24:492-494.
- Nakanishi H, Hashimoto I, Takiwaki H, et al. Striated muscle hamartoma of the nostril. J Dermatol. 1995;22:504-507.
- Farris PE, Manning S, Veatch F. Rhabdomyomatous mesenchymal hamartoma. Am J Dermatopathol. 1994;16:73-75.
- Grilli R, Escalonilla P, Soriano ML, et al. The so-called striated muscle hamartoma is a hamartoma of cutaneous adnexa and mesenchyme, but not of striated muscle. Acta Derm Venereol. 1998;78:390.
- Sampat K, Cheesman E, Siminas S. Perianal rhabdomyomatous mesenchymal hamartoma. Ann R Coll Surg Engl. 2017;99:E193-E195.
- Brinster NK, Farmer ER. Rhabdomyomatous mesenchymal hamartoma presenting on a digit. J Cutan Pathol. 2009;36:61-63.
- Han SH, Song HJ, Hong WK, et al. Rhabdomyomatous mesenchymal hamartoma of the vagina. Pediatr Dermatol. 2009;26:753-755.
- De la Sotta P, Salomone C, González S. Rhabdomyomatous (mesenchymal) hamartoma of the tongue: report of a case. J Oral Pathol Med. 2007;36:58-59.
- Magro G, Di Benedetto A, Sanges G, et al. Rhabdomyomatous mesenchymal hamartoma of oral cavity: an unusual location for such a rare lesion. Virchows Arch. 2005;446:346-347.
- Wang Y, Zhao H, Yue X, et al. Rhabdomyomatous mesenchymal hamartoma presenting as a big subcutaneous mass on the neck: a case report. J Med Case Rep. 2014;8:410.
- Amita K, Shankar SV, Nischal KC, et al. Benign triton tumor: a rare entity in head and neck region. Korean J Pathol. 2013;47:74-76.
- Walsh S, Hurt M. Cutaneous fetal rhabdomyoma: a case report and historical review of the literature. Am J Surg Pathol. 2008;32:485-491.
- Hendrick SJ, Sanchez RL, Blackwell SJ, et al. Striated muscle hamartoma: description of two cases. Pediatr Dermatol. 1986;3:153-157.
- Mills AE. Rhabdomyomatous mesenchymal hamartoma of skin. Am J Dermatopathol. 1989;1:58-63.
- Rosenberg AS, Kirk J, Morgan MB. Rhabdomyomatous mesenchymal hamartoma: an unusual dermal entity with a report of two cases and a review of the literature. J Cutan Pathol. 2002;29:238-243.
- Sánchez RL, Raimer SS. Clinical and histologic features of striated muscle hamartoma: possible relationship to Delleman’s syndrome. J Cutan Pathol. 1994;21:40-46.
- Chang CP, Chen GS. Rhabdomyomatous mesenchymal hamartoma: a plaque-type variant in an adult. Kaohsiung J Med Sci. 2005;21:185-188.
- Harris MA, Dutton JJ, Proia AD. Striated muscle hamartoma of the eyelid in an adult woman. Ophthalmic Plast Reconstr Surg. 2008;24:492-494.
- Nakanishi H, Hashimoto I, Takiwaki H, et al. Striated muscle hamartoma of the nostril. J Dermatol. 1995;22:504-507.
- Farris PE, Manning S, Veatch F. Rhabdomyomatous mesenchymal hamartoma. Am J Dermatopathol. 1994;16:73-75.
- Grilli R, Escalonilla P, Soriano ML, et al. The so-called striated muscle hamartoma is a hamartoma of cutaneous adnexa and mesenchyme, but not of striated muscle. Acta Derm Venereol. 1998;78:390.
- Sampat K, Cheesman E, Siminas S. Perianal rhabdomyomatous mesenchymal hamartoma. Ann R Coll Surg Engl. 2017;99:E193-E195.
- Brinster NK, Farmer ER. Rhabdomyomatous mesenchymal hamartoma presenting on a digit. J Cutan Pathol. 2009;36:61-63.
- Han SH, Song HJ, Hong WK, et al. Rhabdomyomatous mesenchymal hamartoma of the vagina. Pediatr Dermatol. 2009;26:753-755.
- De la Sotta P, Salomone C, González S. Rhabdomyomatous (mesenchymal) hamartoma of the tongue: report of a case. J Oral Pathol Med. 2007;36:58-59.
- Magro G, Di Benedetto A, Sanges G, et al. Rhabdomyomatous mesenchymal hamartoma of oral cavity: an unusual location for such a rare lesion. Virchows Arch. 2005;446:346-347.
- Wang Y, Zhao H, Yue X, et al. Rhabdomyomatous mesenchymal hamartoma presenting as a big subcutaneous mass on the neck: a case report. J Med Case Rep. 2014;8:410.
- Amita K, Shankar SV, Nischal KC, et al. Benign triton tumor: a rare entity in head and neck region. Korean J Pathol. 2013;47:74-76.
- Walsh S, Hurt M. Cutaneous fetal rhabdomyoma: a case report and historical review of the literature. Am J Surg Pathol. 2008;32:485-491.
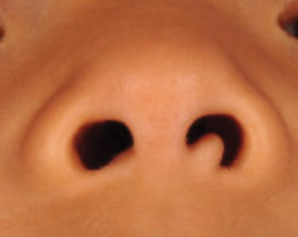
A 4-year-old girl presented to our clinic with an asymptomatic flesh-colored papule in the left nostril. The lesion had been present since birth and grew in relation to the patient with no rapid changes. There had been no pigmentation changes and no bleeding, pain, or itching. The patient’s birth and developmental history were normal. Physical examination revealed a singular, 10×5-mm, flesh-colored, pedunculated mass on the left nasal sill. There were no additional lesions present. An excisional biopsy was performed and submitted for pathologic diagnosis.
The male biological clock – How to tell the time
For decades, we have recognized the age-related natural decline in female fecundity (the ability to reproduce) after the age of 30 (Maturitas 1988;[Suppl]1:15-22). Advanced maternal age (AMA) has also been demonstrated to increase miscarriage and pregnancies with chromosomal abnormalities, presumably from the increased rate of oocyte aneuploidy. There has been a sixfold increase in the rate of first birth in women aged 35-39 years (NCHS Data Brief 2014;152:1-8). Consequently, over the last decade, women, often before they reach AMA, have turned to elective oocyte cryopreservation for fertility preservation.
Ovarian aging
Ovarian aging occurs through the decline in quality and quantity of oocytes. The former is a reflection of the woman’s chronologic age. Markers of female ovarian aging have been utilized, for the past 3 decades, most commonly by basal follicle stimulating hormone. Currently, to assess the quantity of ovarian follicles, antimüllerian hormone (AMH) and transvaginal ultrasound for ovarian antral follicle count (AFC) are the most accurate indicators (J Clin Endocrinol Metab 2004:89:2977-81). While ovarian age testing, particularly AMH, has been widely used to assess a woman’s “fertility potential,” it does not reflect her natural fecundity. In a prospective cohort study, AMH levels (ng/mL) divided into < 0.7, 0.7-8.4, and > 8.4, did not affect natural conception in women aged 30-44 who were divided into the categories of <35, 35-37, or 38-44 years (JAMA 2017;318:1367-76). Although AMH does reduce success with IVF, its main value is the inverse correlation when prescribing gonadotropin dosage for controlled ovarian stimulation.
Despite the familiarity with ovarian aging effects on fertility, the male biological clock remains less studied and understood. Over the last 4 decades, paternal age has increased an average of 3.5 years presumably due to delayed child rearing from professional or personal reasons, improved contraception as well as increased divorce, remarriage, and life expectancy (Hum Reprod. 2017;32:2110-6). Nevertheless, we have little data to definitively counsel men on the effects of advanced paternal age (APA) and no consensus on an actual defined age of designation. This month’s article will summarize the current literature on male age and its impact on fertility.
Testicular aging
Men older than 45 years require approximately five times longer to achieve a pregnancy as men less than 25 after adjustment for female age (Fertil Steril. 2003;79:1520-7). The most likely parameter to assess male fertility, other than pregnancy rates, would be the sperm. Sperm counts, beginning at age 41, may decline but concentrations have been shown to increase in older men apparently because of declining semen volume (Ageing Res Rev. 2015;19:22-33). Sperm motility, but not morphology, also declines while genetic alterations of sperm increase with age. The issue of chromosomal abnormalities in sperm from men of advanced age appears to be similar to that in the oocytes of women with AMA. Consequently, both sexes may contribute to embryo aneuploidy resulting in declining fertility and increasing miscarriage.
For all ages, studies have suggested that elevated male body mass index as well as alcohol consumption and cigarette smoking, including e-cigarettes, can lead to impaired sperm production (Hum Reprod Update 2013;19:221-31).
Fertility treatment outcomes
A mainstay of fertility treatment, particularly in men with mild to moderate impairments in semen parameters, is ovulation induction with intrauterine insemination. Male age has been shown to be a significant indicator for pregnancy rates, including those with normal semen parameters (J Obstet Gynaecol. 2011;31:420-3). Men above age 45 contributed to lower pregnancy rates and higher miscarriages during IUI treatment cycles (Reprod BioMed Online 2008;17:392-7).
During IVF cycles, the sperm of men with APA often undergo ICSI (intracytoplasmic sperm injection) due to higher fertilization rates compared with standard insemination. However, APA sperm appear to have lower fertilization rates and decreased embryo development to the blastocyst stage during cycles using donor oocytes, although pregnancy outcomes are inconsistent (Trans Androl Urol. 2019;8[Suppl 1]:S22-S30; Fertil Steril. 2008;90:97-103).
Perinatal and children’s health
The offspring from APA men appear to have higher rates of stillbirth, low birth weight, and preterm birth, as well as birth defects. Men older than 40-45 years have twice the risk of an autistic child and three times the risk of schizophrenia in their offspring (Transl Psychiatry 2017;7:e1019; Am J Psychiatry 2002;159:1528-33).
Conclusions
Most of the literature supports negative effects on sperm and reproduction from men with APA. The challenge in deciphering the true role of APA on fertility is that the partner is often of AMA. A consideration to avoid this effect would be sperm cryopreservation at a younger age, similar to the common trend among women. Preimplantation genetic testing of embryos from men with APA is also a potential option to reduce miscarriage and avoid a chromosomally abnormal pregnancy. Ethicists have pondered the impact of APA on parenthood and the detrimental effect of early paternal death on the child. Nevertheless, the effect of APA in reproduction is a vital area to study with the same fervor as AMA (Fertil Steril 2009;92:1772-5).
Dr. Trolice is director of Fertility CARE – The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando. He has no conflicts. Email him at [email protected].
For decades, we have recognized the age-related natural decline in female fecundity (the ability to reproduce) after the age of 30 (Maturitas 1988;[Suppl]1:15-22). Advanced maternal age (AMA) has also been demonstrated to increase miscarriage and pregnancies with chromosomal abnormalities, presumably from the increased rate of oocyte aneuploidy. There has been a sixfold increase in the rate of first birth in women aged 35-39 years (NCHS Data Brief 2014;152:1-8). Consequently, over the last decade, women, often before they reach AMA, have turned to elective oocyte cryopreservation for fertility preservation.
Ovarian aging
Ovarian aging occurs through the decline in quality and quantity of oocytes. The former is a reflection of the woman’s chronologic age. Markers of female ovarian aging have been utilized, for the past 3 decades, most commonly by basal follicle stimulating hormone. Currently, to assess the quantity of ovarian follicles, antimüllerian hormone (AMH) and transvaginal ultrasound for ovarian antral follicle count (AFC) are the most accurate indicators (J Clin Endocrinol Metab 2004:89:2977-81). While ovarian age testing, particularly AMH, has been widely used to assess a woman’s “fertility potential,” it does not reflect her natural fecundity. In a prospective cohort study, AMH levels (ng/mL) divided into < 0.7, 0.7-8.4, and > 8.4, did not affect natural conception in women aged 30-44 who were divided into the categories of <35, 35-37, or 38-44 years (JAMA 2017;318:1367-76). Although AMH does reduce success with IVF, its main value is the inverse correlation when prescribing gonadotropin dosage for controlled ovarian stimulation.
Despite the familiarity with ovarian aging effects on fertility, the male biological clock remains less studied and understood. Over the last 4 decades, paternal age has increased an average of 3.5 years presumably due to delayed child rearing from professional or personal reasons, improved contraception as well as increased divorce, remarriage, and life expectancy (Hum Reprod. 2017;32:2110-6). Nevertheless, we have little data to definitively counsel men on the effects of advanced paternal age (APA) and no consensus on an actual defined age of designation. This month’s article will summarize the current literature on male age and its impact on fertility.
Testicular aging
Men older than 45 years require approximately five times longer to achieve a pregnancy as men less than 25 after adjustment for female age (Fertil Steril. 2003;79:1520-7). The most likely parameter to assess male fertility, other than pregnancy rates, would be the sperm. Sperm counts, beginning at age 41, may decline but concentrations have been shown to increase in older men apparently because of declining semen volume (Ageing Res Rev. 2015;19:22-33). Sperm motility, but not morphology, also declines while genetic alterations of sperm increase with age. The issue of chromosomal abnormalities in sperm from men of advanced age appears to be similar to that in the oocytes of women with AMA. Consequently, both sexes may contribute to embryo aneuploidy resulting in declining fertility and increasing miscarriage.
For all ages, studies have suggested that elevated male body mass index as well as alcohol consumption and cigarette smoking, including e-cigarettes, can lead to impaired sperm production (Hum Reprod Update 2013;19:221-31).
Fertility treatment outcomes
A mainstay of fertility treatment, particularly in men with mild to moderate impairments in semen parameters, is ovulation induction with intrauterine insemination. Male age has been shown to be a significant indicator for pregnancy rates, including those with normal semen parameters (J Obstet Gynaecol. 2011;31:420-3). Men above age 45 contributed to lower pregnancy rates and higher miscarriages during IUI treatment cycles (Reprod BioMed Online 2008;17:392-7).
During IVF cycles, the sperm of men with APA often undergo ICSI (intracytoplasmic sperm injection) due to higher fertilization rates compared with standard insemination. However, APA sperm appear to have lower fertilization rates and decreased embryo development to the blastocyst stage during cycles using donor oocytes, although pregnancy outcomes are inconsistent (Trans Androl Urol. 2019;8[Suppl 1]:S22-S30; Fertil Steril. 2008;90:97-103).
Perinatal and children’s health
The offspring from APA men appear to have higher rates of stillbirth, low birth weight, and preterm birth, as well as birth defects. Men older than 40-45 years have twice the risk of an autistic child and three times the risk of schizophrenia in their offspring (Transl Psychiatry 2017;7:e1019; Am J Psychiatry 2002;159:1528-33).
Conclusions
Most of the literature supports negative effects on sperm and reproduction from men with APA. The challenge in deciphering the true role of APA on fertility is that the partner is often of AMA. A consideration to avoid this effect would be sperm cryopreservation at a younger age, similar to the common trend among women. Preimplantation genetic testing of embryos from men with APA is also a potential option to reduce miscarriage and avoid a chromosomally abnormal pregnancy. Ethicists have pondered the impact of APA on parenthood and the detrimental effect of early paternal death on the child. Nevertheless, the effect of APA in reproduction is a vital area to study with the same fervor as AMA (Fertil Steril 2009;92:1772-5).
Dr. Trolice is director of Fertility CARE – The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando. He has no conflicts. Email him at [email protected].
For decades, we have recognized the age-related natural decline in female fecundity (the ability to reproduce) after the age of 30 (Maturitas 1988;[Suppl]1:15-22). Advanced maternal age (AMA) has also been demonstrated to increase miscarriage and pregnancies with chromosomal abnormalities, presumably from the increased rate of oocyte aneuploidy. There has been a sixfold increase in the rate of first birth in women aged 35-39 years (NCHS Data Brief 2014;152:1-8). Consequently, over the last decade, women, often before they reach AMA, have turned to elective oocyte cryopreservation for fertility preservation.
Ovarian aging
Ovarian aging occurs through the decline in quality and quantity of oocytes. The former is a reflection of the woman’s chronologic age. Markers of female ovarian aging have been utilized, for the past 3 decades, most commonly by basal follicle stimulating hormone. Currently, to assess the quantity of ovarian follicles, antimüllerian hormone (AMH) and transvaginal ultrasound for ovarian antral follicle count (AFC) are the most accurate indicators (J Clin Endocrinol Metab 2004:89:2977-81). While ovarian age testing, particularly AMH, has been widely used to assess a woman’s “fertility potential,” it does not reflect her natural fecundity. In a prospective cohort study, AMH levels (ng/mL) divided into < 0.7, 0.7-8.4, and > 8.4, did not affect natural conception in women aged 30-44 who were divided into the categories of <35, 35-37, or 38-44 years (JAMA 2017;318:1367-76). Although AMH does reduce success with IVF, its main value is the inverse correlation when prescribing gonadotropin dosage for controlled ovarian stimulation.
Despite the familiarity with ovarian aging effects on fertility, the male biological clock remains less studied and understood. Over the last 4 decades, paternal age has increased an average of 3.5 years presumably due to delayed child rearing from professional or personal reasons, improved contraception as well as increased divorce, remarriage, and life expectancy (Hum Reprod. 2017;32:2110-6). Nevertheless, we have little data to definitively counsel men on the effects of advanced paternal age (APA) and no consensus on an actual defined age of designation. This month’s article will summarize the current literature on male age and its impact on fertility.
Testicular aging
Men older than 45 years require approximately five times longer to achieve a pregnancy as men less than 25 after adjustment for female age (Fertil Steril. 2003;79:1520-7). The most likely parameter to assess male fertility, other than pregnancy rates, would be the sperm. Sperm counts, beginning at age 41, may decline but concentrations have been shown to increase in older men apparently because of declining semen volume (Ageing Res Rev. 2015;19:22-33). Sperm motility, but not morphology, also declines while genetic alterations of sperm increase with age. The issue of chromosomal abnormalities in sperm from men of advanced age appears to be similar to that in the oocytes of women with AMA. Consequently, both sexes may contribute to embryo aneuploidy resulting in declining fertility and increasing miscarriage.
For all ages, studies have suggested that elevated male body mass index as well as alcohol consumption and cigarette smoking, including e-cigarettes, can lead to impaired sperm production (Hum Reprod Update 2013;19:221-31).
Fertility treatment outcomes
A mainstay of fertility treatment, particularly in men with mild to moderate impairments in semen parameters, is ovulation induction with intrauterine insemination. Male age has been shown to be a significant indicator for pregnancy rates, including those with normal semen parameters (J Obstet Gynaecol. 2011;31:420-3). Men above age 45 contributed to lower pregnancy rates and higher miscarriages during IUI treatment cycles (Reprod BioMed Online 2008;17:392-7).
During IVF cycles, the sperm of men with APA often undergo ICSI (intracytoplasmic sperm injection) due to higher fertilization rates compared with standard insemination. However, APA sperm appear to have lower fertilization rates and decreased embryo development to the blastocyst stage during cycles using donor oocytes, although pregnancy outcomes are inconsistent (Trans Androl Urol. 2019;8[Suppl 1]:S22-S30; Fertil Steril. 2008;90:97-103).
Perinatal and children’s health
The offspring from APA men appear to have higher rates of stillbirth, low birth weight, and preterm birth, as well as birth defects. Men older than 40-45 years have twice the risk of an autistic child and three times the risk of schizophrenia in their offspring (Transl Psychiatry 2017;7:e1019; Am J Psychiatry 2002;159:1528-33).
Conclusions
Most of the literature supports negative effects on sperm and reproduction from men with APA. The challenge in deciphering the true role of APA on fertility is that the partner is often of AMA. A consideration to avoid this effect would be sperm cryopreservation at a younger age, similar to the common trend among women. Preimplantation genetic testing of embryos from men with APA is also a potential option to reduce miscarriage and avoid a chromosomally abnormal pregnancy. Ethicists have pondered the impact of APA on parenthood and the detrimental effect of early paternal death on the child. Nevertheless, the effect of APA in reproduction is a vital area to study with the same fervor as AMA (Fertil Steril 2009;92:1772-5).
Dr. Trolice is director of Fertility CARE – The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando. He has no conflicts. Email him at [email protected].
COVID vaccination rates vary by zodiac sign
COVID-19 vaccination rates vary dramatically by astrological sign, with Leos at the top of the list and Scorpios at the bottom, according to The Salt Lake Tribune.
The Salt Lake County Health Department calculated the rates based on anonymous birth dates from the county’s vaccination data and then compared those figures to national estimates for the overall population represented by each sign.
“Now that Mercury is not in retrograde, we’re just going to leave this here … (and yes, this is based on data),” the Health Department wrote in a Twitter post on Tuesday.
“The COVID-19 vaccine is backed by science and is no way influenced by horoscopes,” the department continued. “But come on Scorpios!”
According to the graphic, 70% of those with the Leo sign are fully vaccinated, followed by Aquarius at 67%, and Aries and Sagittarius both at 59%. The other signs range from 58% to 50%, in descending order: Cancer, Taurus, Gemini, Libra, Pisces, Capricorn, and Virgo. Scorpio sits at the bottom of the list, with 46% fully vaccinated.
Notably, three of the top four signs are elemental fire signs, The Salt Lake Tribune noted.
“We are overachievers,” Jeff Eason, an Aries and the department’s bureau manager of population health and informatics, who did the analysis, told the newspaper.
The Health Department’s post sparked positive and negative feedback across social media, with some musing about their own sign’s inclinations and others scoffing at astrology altogether.
“What we’re really doing is finding new and different ways to keep our community talking about vaccination when there is significant message fatigue around this topic,” the department wrote in the comments.
The range of vaccination rates was startlingly wide, Mr. Eason told The Salt Lake Tribune. But he noted that the difference “could all come down to denominators.”
Each sign’s vaccination rate was ranked almost exactly inverse to its share of the overall population, the newspaper reported. Scorpios and Virgos make up 9.4% and 9.3% of the U.S. population, respectively, as compared with 7.1% for Leos and 6.3% for Aquarians.
If the 12 astrological signs were more evenly distributed in Salt Lake County than nationally, Mr. Eason said, the range of vaccinations rates wouldn’t be as wide as the analysis shows.
“Obviously, it’s not super scientific because we are talking astrology,” Nicholas Rupp, a spokesman for the health department and a vaccinated Scorpio, told the newspaper.
Still, health department officials wanted to do the analysis as a fun way to start conversations and promote vaccinations. About 59% of Salt Lake County residents are fully vaccinated, and about 54% of Utah residents are fully vaccinated.
“We do have message fatigue around vaccines,” Mr. Rupp said.
A version of this article first appeared on WebMD.com.
COVID-19 vaccination rates vary dramatically by astrological sign, with Leos at the top of the list and Scorpios at the bottom, according to The Salt Lake Tribune.
The Salt Lake County Health Department calculated the rates based on anonymous birth dates from the county’s vaccination data and then compared those figures to national estimates for the overall population represented by each sign.
“Now that Mercury is not in retrograde, we’re just going to leave this here … (and yes, this is based on data),” the Health Department wrote in a Twitter post on Tuesday.
“The COVID-19 vaccine is backed by science and is no way influenced by horoscopes,” the department continued. “But come on Scorpios!”
According to the graphic, 70% of those with the Leo sign are fully vaccinated, followed by Aquarius at 67%, and Aries and Sagittarius both at 59%. The other signs range from 58% to 50%, in descending order: Cancer, Taurus, Gemini, Libra, Pisces, Capricorn, and Virgo. Scorpio sits at the bottom of the list, with 46% fully vaccinated.
Notably, three of the top four signs are elemental fire signs, The Salt Lake Tribune noted.
“We are overachievers,” Jeff Eason, an Aries and the department’s bureau manager of population health and informatics, who did the analysis, told the newspaper.
The Health Department’s post sparked positive and negative feedback across social media, with some musing about their own sign’s inclinations and others scoffing at astrology altogether.
“What we’re really doing is finding new and different ways to keep our community talking about vaccination when there is significant message fatigue around this topic,” the department wrote in the comments.
The range of vaccination rates was startlingly wide, Mr. Eason told The Salt Lake Tribune. But he noted that the difference “could all come down to denominators.”
Each sign’s vaccination rate was ranked almost exactly inverse to its share of the overall population, the newspaper reported. Scorpios and Virgos make up 9.4% and 9.3% of the U.S. population, respectively, as compared with 7.1% for Leos and 6.3% for Aquarians.
If the 12 astrological signs were more evenly distributed in Salt Lake County than nationally, Mr. Eason said, the range of vaccinations rates wouldn’t be as wide as the analysis shows.
“Obviously, it’s not super scientific because we are talking astrology,” Nicholas Rupp, a spokesman for the health department and a vaccinated Scorpio, told the newspaper.
Still, health department officials wanted to do the analysis as a fun way to start conversations and promote vaccinations. About 59% of Salt Lake County residents are fully vaccinated, and about 54% of Utah residents are fully vaccinated.
“We do have message fatigue around vaccines,” Mr. Rupp said.
A version of this article first appeared on WebMD.com.
COVID-19 vaccination rates vary dramatically by astrological sign, with Leos at the top of the list and Scorpios at the bottom, according to The Salt Lake Tribune.
The Salt Lake County Health Department calculated the rates based on anonymous birth dates from the county’s vaccination data and then compared those figures to national estimates for the overall population represented by each sign.
“Now that Mercury is not in retrograde, we’re just going to leave this here … (and yes, this is based on data),” the Health Department wrote in a Twitter post on Tuesday.
“The COVID-19 vaccine is backed by science and is no way influenced by horoscopes,” the department continued. “But come on Scorpios!”
According to the graphic, 70% of those with the Leo sign are fully vaccinated, followed by Aquarius at 67%, and Aries and Sagittarius both at 59%. The other signs range from 58% to 50%, in descending order: Cancer, Taurus, Gemini, Libra, Pisces, Capricorn, and Virgo. Scorpio sits at the bottom of the list, with 46% fully vaccinated.
Notably, three of the top four signs are elemental fire signs, The Salt Lake Tribune noted.
“We are overachievers,” Jeff Eason, an Aries and the department’s bureau manager of population health and informatics, who did the analysis, told the newspaper.
The Health Department’s post sparked positive and negative feedback across social media, with some musing about their own sign’s inclinations and others scoffing at astrology altogether.
“What we’re really doing is finding new and different ways to keep our community talking about vaccination when there is significant message fatigue around this topic,” the department wrote in the comments.
The range of vaccination rates was startlingly wide, Mr. Eason told The Salt Lake Tribune. But he noted that the difference “could all come down to denominators.”
Each sign’s vaccination rate was ranked almost exactly inverse to its share of the overall population, the newspaper reported. Scorpios and Virgos make up 9.4% and 9.3% of the U.S. population, respectively, as compared with 7.1% for Leos and 6.3% for Aquarians.
If the 12 astrological signs were more evenly distributed in Salt Lake County than nationally, Mr. Eason said, the range of vaccinations rates wouldn’t be as wide as the analysis shows.
“Obviously, it’s not super scientific because we are talking astrology,” Nicholas Rupp, a spokesman for the health department and a vaccinated Scorpio, told the newspaper.
Still, health department officials wanted to do the analysis as a fun way to start conversations and promote vaccinations. About 59% of Salt Lake County residents are fully vaccinated, and about 54% of Utah residents are fully vaccinated.
“We do have message fatigue around vaccines,” Mr. Rupp said.
A version of this article first appeared on WebMD.com.
Better COVID-19 outcomes confirmed in TNF inhibitor users
Among patients with immune-mediated inflammatory diseases (IMIDs) who get COVID-19, the risk for hospitalization and death is lower if they are receiving tumor necrosis factor (TNF) inhibitor monotherapy, compared with receiving most other common drugs for these conditions, with or without TNF inhibitors, according to a study published in JAMA Network Open The only combination not associated with an increased risk for hospitalization or death was TNF inhibitor therapy with methotrexate.
“These findings support the continued use of TNF inhibitor monotherapy during the pandemic and warrant further research investigating the association of other biologic therapies with COVID-19 outcomes,” write Zara Izadi, MPharm, of the University of California, San Francisco, and her colleagues. “Treatment with TNF inhibitor combination therapy was associated with a more favorable safety profile when methotrexate rather than azathioprine/6-mercaptopurine was used, suggesting that clinicians would benefit from weighing the risks versus benefits of deescalating treatment or changing medications when a patient is receiving concomitant TNF inhibitors and azathioprine/6-mercaptopurine,” they write.
Findings mirror those seen in other settings
These findings are in line with what has been found in other settings, according to Joel M. Gelfand, MD, director of the psoriasis and phototherapy treatment center, vice chair of clinical research, and medical director of the dermatology clinical studies unit at the University of Pennsylvania, Philadelphia.
“In the beginning of the pandemic, there was concern about use of immune-modulating treatments, and many patients self-discontinued treatments like TNF inhibitors,” Dr. Gelfand, who was not involved in the study, told this news organization. “This has ultimately proved unnecessary and unfortunately resulted in harm to many patients due to flaring of their underlying disease.”
Dr. Gelfand emphasized the importance of vaccinating patients against COVID-19 as soon as possible and of getting a third dose for those who are already fully vaccinated with the Pfizer or Moderna shots, as recommended by the Centers for Disease Control and Prevention.
“I typically recommend this third dose be taken 6 months after the second dose,” Dr. Gelfand said. “The good news is that TNF inhibitors do not seem to meaningfully impact response to mRNA vaccines.”
Study details
The researchers analyzed data from three international registries of adults with rheumatic diseases, inflammatory bowel disease, and psoriasis who had COVID-19 between March 12, 2020, and Feb. 1, 2021. The registries included the Secure Epidemiology of Coronavirus Under Research Exclusion for Inflammatory Bowel Disease (SECURE-IBD) registry, the Psoriasis Patient Registry for Outcomes, Therapy and Epidemiology of COVID-19 Infection (PsoProtect), and the physician-reported registry from the Global Rheumatology Alliance (GRA).
The population included 6,077 patients from 74 countries. About half of the cohort (52.9%) were from Europe; more than half were women (58.6%). The average age was 48 years. A little over one-third of the patients (35.3%) had rheumatoid arthritis, 25.3% had Crohn’s disease, 12.5% had ulcerative colitis, 10.3% had spondyloarthritis, and 9.3% had psoriatic arthritis. Smaller percentages had psoriasis (4.9%), another type of arthritis or multiple types (1.7%), or another inflammatory bowel disease (0.6%).
One in five patients (21.3%) were hospitalized, and 3.1% died. The researchers compared outcomes for those who were receiving TNF inhibitor therapy alone to outcomes for those who were taking azathioprine/6-mercaptopurine therapy (alone or with a TNF inhibitor), methotrexate (alone or with a TNF inhibitor), and Janus kinase (JAK) inhibitors. They adjusted their analysis to account for active disease and common comorbidities, as well as geography and the period during the pandemic in which the person was admitted, because treatment regimens and hospitalization indications have varied over time.
All of the therapies except the combination of TNF inhibitors and methotrexate were associated with higher odds of hospitalization and death than TNF inhibitor monotherapy.
The researchers explored several possible explanations for the findings, including the possibility that high serum TNF concentrations may have been associated with more organ damage at the time of COVID-19 admission, owing to interaction with SARS-CoV-2–associated hyperinflammation.
“Therefore, blocking TNF could inhibit this detrimental immune response,” the authors write. “Multiple case series reporting favorable outcomes among patients receiving TNF inhibitor therapy support this assertion.”
Another possibility relates to the effects of taking non–TNF inhibitor medications for immunosuppression. The authors note that thiopurine medications are linked to a greater risk for opportunistic viral infections and that JAK inhibitors may reduce the body’s ability to clear the virus because of its suppression of innate immune response.
The authors also postulate that methotrexate may lower the likelihood of cytokine storm linked to COVID-19, even though methotrexate monotherapy was associated with poorer outcomes. “This association could mean that TNF inhibitor therapy is exerting a protective benefit or that methotrexate therapy is exerting a harmful consequence,” the authors write.
Caution needed in interpreting uncontrolled, registry-based data
The findings were not surprising to Stephen B. Hanauer, MD, medical director of the Digestive Health Center at Northwestern University, Chicago, who was not involved in the research.
“We’ve been monitoring IBD [inflammatory bowel disease] patients through the Secure registry similar to the rheumatologic and dermatologic societies and have not identified a signal of harm from any international groups,” Dr. Hanauer told this news organization. He noted that these registries also have not shown an increased risk for COVID-19 complications among patients receiving TNF inhibitors, antiadhesion therapies, or anti–IL12/23 inhibitors, compared with the general population not taking these therapies.
The study’s size and the diversity of patients strengthen its findings. However, the registries’ use of convenience sampling increases the potential for reporting bias, although the results remained similar after a sensitivity analysis. The study also lacked a control group, and the registries did not collect data uniformly.
“These are databases that rely on reporting from investigators and are not comprehensive prospective studies,” Dr. Hanauer noted as another study limitation.
Dr. Gelfand similarly advised caution in interpreting these findings, inasmuch as the study is a “collection of spontaneous reports” that should be viewed as hypothesis-generating rather than testing.
“Fortunately, more rigorous studies have been conducted, typically in large medical record systems, and have confirmed the hypothesis that TNF inhibitors are associated with a lower risk of poor COVID-19 outcomes, compared to other treatments,” Dr. Gelfand said.
Previous smaller studies similarly found better outcomes among patients taking TNF inhibitors, compared with other therapies, but their participants were predominantly from North America and Europe, noted Licio A. Velloso, MD, PhD, of the University of Campinas, in São Paulo, in an accompanying commentary.
On the basis of the findings of this study, “which included a much larger sample comprising distinct diseases and patients with a multitude of genetic backgrounds, the evidence in favor of the continued use of TNF inhibitor monotherapy for patients with IMIDs during the COVID-19 pandemic has become more substantial,” Dr. Velloso writes. “The finding that maintenance of TNF inhibitor monotherapy is associated with reductions in the risk of severe COVID-19 among patients with IMIDs offers new perspective that may guide health care professionals in the difficult decisions regarding therapeutic approaches among this specific group of patients.”
The research was funded by the American College of Rheumatology, the European Alliance of Associations for Rheumatology, the United Kingdom’s National Institute for Health Research Biomedical Research Center, and the Psoriasis Association. Many authors reported receiving grants and/or personal fees from a variety of pharmaceutical companies. Dr. Velloso has disclosed no relevant financial relationships. Dr. Hanauer has served as a consultant to companies that market TNF inhibitors. Dr. Gelfand has consulted for and received research grants from companies that market TNF inhibitors.
A version of this article first appeared on Medscape.com.
Among patients with immune-mediated inflammatory diseases (IMIDs) who get COVID-19, the risk for hospitalization and death is lower if they are receiving tumor necrosis factor (TNF) inhibitor monotherapy, compared with receiving most other common drugs for these conditions, with or without TNF inhibitors, according to a study published in JAMA Network Open The only combination not associated with an increased risk for hospitalization or death was TNF inhibitor therapy with methotrexate.
“These findings support the continued use of TNF inhibitor monotherapy during the pandemic and warrant further research investigating the association of other biologic therapies with COVID-19 outcomes,” write Zara Izadi, MPharm, of the University of California, San Francisco, and her colleagues. “Treatment with TNF inhibitor combination therapy was associated with a more favorable safety profile when methotrexate rather than azathioprine/6-mercaptopurine was used, suggesting that clinicians would benefit from weighing the risks versus benefits of deescalating treatment or changing medications when a patient is receiving concomitant TNF inhibitors and azathioprine/6-mercaptopurine,” they write.
Findings mirror those seen in other settings
These findings are in line with what has been found in other settings, according to Joel M. Gelfand, MD, director of the psoriasis and phototherapy treatment center, vice chair of clinical research, and medical director of the dermatology clinical studies unit at the University of Pennsylvania, Philadelphia.
“In the beginning of the pandemic, there was concern about use of immune-modulating treatments, and many patients self-discontinued treatments like TNF inhibitors,” Dr. Gelfand, who was not involved in the study, told this news organization. “This has ultimately proved unnecessary and unfortunately resulted in harm to many patients due to flaring of their underlying disease.”
Dr. Gelfand emphasized the importance of vaccinating patients against COVID-19 as soon as possible and of getting a third dose for those who are already fully vaccinated with the Pfizer or Moderna shots, as recommended by the Centers for Disease Control and Prevention.
“I typically recommend this third dose be taken 6 months after the second dose,” Dr. Gelfand said. “The good news is that TNF inhibitors do not seem to meaningfully impact response to mRNA vaccines.”
Study details
The researchers analyzed data from three international registries of adults with rheumatic diseases, inflammatory bowel disease, and psoriasis who had COVID-19 between March 12, 2020, and Feb. 1, 2021. The registries included the Secure Epidemiology of Coronavirus Under Research Exclusion for Inflammatory Bowel Disease (SECURE-IBD) registry, the Psoriasis Patient Registry for Outcomes, Therapy and Epidemiology of COVID-19 Infection (PsoProtect), and the physician-reported registry from the Global Rheumatology Alliance (GRA).
The population included 6,077 patients from 74 countries. About half of the cohort (52.9%) were from Europe; more than half were women (58.6%). The average age was 48 years. A little over one-third of the patients (35.3%) had rheumatoid arthritis, 25.3% had Crohn’s disease, 12.5% had ulcerative colitis, 10.3% had spondyloarthritis, and 9.3% had psoriatic arthritis. Smaller percentages had psoriasis (4.9%), another type of arthritis or multiple types (1.7%), or another inflammatory bowel disease (0.6%).
One in five patients (21.3%) were hospitalized, and 3.1% died. The researchers compared outcomes for those who were receiving TNF inhibitor therapy alone to outcomes for those who were taking azathioprine/6-mercaptopurine therapy (alone or with a TNF inhibitor), methotrexate (alone or with a TNF inhibitor), and Janus kinase (JAK) inhibitors. They adjusted their analysis to account for active disease and common comorbidities, as well as geography and the period during the pandemic in which the person was admitted, because treatment regimens and hospitalization indications have varied over time.
All of the therapies except the combination of TNF inhibitors and methotrexate were associated with higher odds of hospitalization and death than TNF inhibitor monotherapy.
The researchers explored several possible explanations for the findings, including the possibility that high serum TNF concentrations may have been associated with more organ damage at the time of COVID-19 admission, owing to interaction with SARS-CoV-2–associated hyperinflammation.
“Therefore, blocking TNF could inhibit this detrimental immune response,” the authors write. “Multiple case series reporting favorable outcomes among patients receiving TNF inhibitor therapy support this assertion.”
Another possibility relates to the effects of taking non–TNF inhibitor medications for immunosuppression. The authors note that thiopurine medications are linked to a greater risk for opportunistic viral infections and that JAK inhibitors may reduce the body’s ability to clear the virus because of its suppression of innate immune response.
The authors also postulate that methotrexate may lower the likelihood of cytokine storm linked to COVID-19, even though methotrexate monotherapy was associated with poorer outcomes. “This association could mean that TNF inhibitor therapy is exerting a protective benefit or that methotrexate therapy is exerting a harmful consequence,” the authors write.
Caution needed in interpreting uncontrolled, registry-based data
The findings were not surprising to Stephen B. Hanauer, MD, medical director of the Digestive Health Center at Northwestern University, Chicago, who was not involved in the research.
“We’ve been monitoring IBD [inflammatory bowel disease] patients through the Secure registry similar to the rheumatologic and dermatologic societies and have not identified a signal of harm from any international groups,” Dr. Hanauer told this news organization. He noted that these registries also have not shown an increased risk for COVID-19 complications among patients receiving TNF inhibitors, antiadhesion therapies, or anti–IL12/23 inhibitors, compared with the general population not taking these therapies.
The study’s size and the diversity of patients strengthen its findings. However, the registries’ use of convenience sampling increases the potential for reporting bias, although the results remained similar after a sensitivity analysis. The study also lacked a control group, and the registries did not collect data uniformly.
“These are databases that rely on reporting from investigators and are not comprehensive prospective studies,” Dr. Hanauer noted as another study limitation.
Dr. Gelfand similarly advised caution in interpreting these findings, inasmuch as the study is a “collection of spontaneous reports” that should be viewed as hypothesis-generating rather than testing.
“Fortunately, more rigorous studies have been conducted, typically in large medical record systems, and have confirmed the hypothesis that TNF inhibitors are associated with a lower risk of poor COVID-19 outcomes, compared to other treatments,” Dr. Gelfand said.
Previous smaller studies similarly found better outcomes among patients taking TNF inhibitors, compared with other therapies, but their participants were predominantly from North America and Europe, noted Licio A. Velloso, MD, PhD, of the University of Campinas, in São Paulo, in an accompanying commentary.
On the basis of the findings of this study, “which included a much larger sample comprising distinct diseases and patients with a multitude of genetic backgrounds, the evidence in favor of the continued use of TNF inhibitor monotherapy for patients with IMIDs during the COVID-19 pandemic has become more substantial,” Dr. Velloso writes. “The finding that maintenance of TNF inhibitor monotherapy is associated with reductions in the risk of severe COVID-19 among patients with IMIDs offers new perspective that may guide health care professionals in the difficult decisions regarding therapeutic approaches among this specific group of patients.”
The research was funded by the American College of Rheumatology, the European Alliance of Associations for Rheumatology, the United Kingdom’s National Institute for Health Research Biomedical Research Center, and the Psoriasis Association. Many authors reported receiving grants and/or personal fees from a variety of pharmaceutical companies. Dr. Velloso has disclosed no relevant financial relationships. Dr. Hanauer has served as a consultant to companies that market TNF inhibitors. Dr. Gelfand has consulted for and received research grants from companies that market TNF inhibitors.
A version of this article first appeared on Medscape.com.
Among patients with immune-mediated inflammatory diseases (IMIDs) who get COVID-19, the risk for hospitalization and death is lower if they are receiving tumor necrosis factor (TNF) inhibitor monotherapy, compared with receiving most other common drugs for these conditions, with or without TNF inhibitors, according to a study published in JAMA Network Open The only combination not associated with an increased risk for hospitalization or death was TNF inhibitor therapy with methotrexate.
“These findings support the continued use of TNF inhibitor monotherapy during the pandemic and warrant further research investigating the association of other biologic therapies with COVID-19 outcomes,” write Zara Izadi, MPharm, of the University of California, San Francisco, and her colleagues. “Treatment with TNF inhibitor combination therapy was associated with a more favorable safety profile when methotrexate rather than azathioprine/6-mercaptopurine was used, suggesting that clinicians would benefit from weighing the risks versus benefits of deescalating treatment or changing medications when a patient is receiving concomitant TNF inhibitors and azathioprine/6-mercaptopurine,” they write.
Findings mirror those seen in other settings
These findings are in line with what has been found in other settings, according to Joel M. Gelfand, MD, director of the psoriasis and phototherapy treatment center, vice chair of clinical research, and medical director of the dermatology clinical studies unit at the University of Pennsylvania, Philadelphia.
“In the beginning of the pandemic, there was concern about use of immune-modulating treatments, and many patients self-discontinued treatments like TNF inhibitors,” Dr. Gelfand, who was not involved in the study, told this news organization. “This has ultimately proved unnecessary and unfortunately resulted in harm to many patients due to flaring of their underlying disease.”
Dr. Gelfand emphasized the importance of vaccinating patients against COVID-19 as soon as possible and of getting a third dose for those who are already fully vaccinated with the Pfizer or Moderna shots, as recommended by the Centers for Disease Control and Prevention.
“I typically recommend this third dose be taken 6 months after the second dose,” Dr. Gelfand said. “The good news is that TNF inhibitors do not seem to meaningfully impact response to mRNA vaccines.”
Study details
The researchers analyzed data from three international registries of adults with rheumatic diseases, inflammatory bowel disease, and psoriasis who had COVID-19 between March 12, 2020, and Feb. 1, 2021. The registries included the Secure Epidemiology of Coronavirus Under Research Exclusion for Inflammatory Bowel Disease (SECURE-IBD) registry, the Psoriasis Patient Registry for Outcomes, Therapy and Epidemiology of COVID-19 Infection (PsoProtect), and the physician-reported registry from the Global Rheumatology Alliance (GRA).
The population included 6,077 patients from 74 countries. About half of the cohort (52.9%) were from Europe; more than half were women (58.6%). The average age was 48 years. A little over one-third of the patients (35.3%) had rheumatoid arthritis, 25.3% had Crohn’s disease, 12.5% had ulcerative colitis, 10.3% had spondyloarthritis, and 9.3% had psoriatic arthritis. Smaller percentages had psoriasis (4.9%), another type of arthritis or multiple types (1.7%), or another inflammatory bowel disease (0.6%).
One in five patients (21.3%) were hospitalized, and 3.1% died. The researchers compared outcomes for those who were receiving TNF inhibitor therapy alone to outcomes for those who were taking azathioprine/6-mercaptopurine therapy (alone or with a TNF inhibitor), methotrexate (alone or with a TNF inhibitor), and Janus kinase (JAK) inhibitors. They adjusted their analysis to account for active disease and common comorbidities, as well as geography and the period during the pandemic in which the person was admitted, because treatment regimens and hospitalization indications have varied over time.
All of the therapies except the combination of TNF inhibitors and methotrexate were associated with higher odds of hospitalization and death than TNF inhibitor monotherapy.
The researchers explored several possible explanations for the findings, including the possibility that high serum TNF concentrations may have been associated with more organ damage at the time of COVID-19 admission, owing to interaction with SARS-CoV-2–associated hyperinflammation.
“Therefore, blocking TNF could inhibit this detrimental immune response,” the authors write. “Multiple case series reporting favorable outcomes among patients receiving TNF inhibitor therapy support this assertion.”
Another possibility relates to the effects of taking non–TNF inhibitor medications for immunosuppression. The authors note that thiopurine medications are linked to a greater risk for opportunistic viral infections and that JAK inhibitors may reduce the body’s ability to clear the virus because of its suppression of innate immune response.
The authors also postulate that methotrexate may lower the likelihood of cytokine storm linked to COVID-19, even though methotrexate monotherapy was associated with poorer outcomes. “This association could mean that TNF inhibitor therapy is exerting a protective benefit or that methotrexate therapy is exerting a harmful consequence,” the authors write.
Caution needed in interpreting uncontrolled, registry-based data
The findings were not surprising to Stephen B. Hanauer, MD, medical director of the Digestive Health Center at Northwestern University, Chicago, who was not involved in the research.
“We’ve been monitoring IBD [inflammatory bowel disease] patients through the Secure registry similar to the rheumatologic and dermatologic societies and have not identified a signal of harm from any international groups,” Dr. Hanauer told this news organization. He noted that these registries also have not shown an increased risk for COVID-19 complications among patients receiving TNF inhibitors, antiadhesion therapies, or anti–IL12/23 inhibitors, compared with the general population not taking these therapies.
The study’s size and the diversity of patients strengthen its findings. However, the registries’ use of convenience sampling increases the potential for reporting bias, although the results remained similar after a sensitivity analysis. The study also lacked a control group, and the registries did not collect data uniformly.
“These are databases that rely on reporting from investigators and are not comprehensive prospective studies,” Dr. Hanauer noted as another study limitation.
Dr. Gelfand similarly advised caution in interpreting these findings, inasmuch as the study is a “collection of spontaneous reports” that should be viewed as hypothesis-generating rather than testing.
“Fortunately, more rigorous studies have been conducted, typically in large medical record systems, and have confirmed the hypothesis that TNF inhibitors are associated with a lower risk of poor COVID-19 outcomes, compared to other treatments,” Dr. Gelfand said.
Previous smaller studies similarly found better outcomes among patients taking TNF inhibitors, compared with other therapies, but their participants were predominantly from North America and Europe, noted Licio A. Velloso, MD, PhD, of the University of Campinas, in São Paulo, in an accompanying commentary.
On the basis of the findings of this study, “which included a much larger sample comprising distinct diseases and patients with a multitude of genetic backgrounds, the evidence in favor of the continued use of TNF inhibitor monotherapy for patients with IMIDs during the COVID-19 pandemic has become more substantial,” Dr. Velloso writes. “The finding that maintenance of TNF inhibitor monotherapy is associated with reductions in the risk of severe COVID-19 among patients with IMIDs offers new perspective that may guide health care professionals in the difficult decisions regarding therapeutic approaches among this specific group of patients.”
The research was funded by the American College of Rheumatology, the European Alliance of Associations for Rheumatology, the United Kingdom’s National Institute for Health Research Biomedical Research Center, and the Psoriasis Association. Many authors reported receiving grants and/or personal fees from a variety of pharmaceutical companies. Dr. Velloso has disclosed no relevant financial relationships. Dr. Hanauer has served as a consultant to companies that market TNF inhibitors. Dr. Gelfand has consulted for and received research grants from companies that market TNF inhibitors.
A version of this article first appeared on Medscape.com.
Drink up: Large study confirms coffee beneficial to liver health
Drinking more than three cups of caffeinated coffee a day is associated with less liver stiffness, according to an analysis of a nationally representative survey, which was recently published in Clinical Gastroenterology and Hepatology.
The study is likely the most rigorous look to date on the benefits of coffee on liver health in the U.S. It was based on data from the National Health and Nutrition Examination Survey (NHANES), in which participants were asked about what they eat and drink. Crucially, in 2017, NHANES began to include elastography (FibroScan), of participants’ liver stiffness, not because of suspected problems with the liver but as across-the-board evaluations of all participants.
“Because it’s an unselected population for FibroScan and because of the detail, the granularity, the richness of the information from the nutritional surveys that they do, this is the closest we’re ever going to get to a linkage between what people are eating or drinking and the health of their liver, absent a longitudinal study where we set out to follow people for many, many years,” said Elliot Tapper, MD, assistant professor of gastroenterology at the University of Michigan, Ann Arbor, and the study’s senior author.
Researchers examined data from about 4,500 patients who had participated in the NHANES study in 2017-2018. The participants were aged 20 years or older, with an average age of 48; 73% were overweight, about the national average.
The researchers found no association between coffee consumption and controlled attenuation parameter (CAP), a measure of fatty liver. But they found a link between coffee and liver stiffness.
Those who drank more than three cups of coffee daily had a liver stiffness measure (LSM) that was 0.9 kilopascals (kPa) lower than others (P = .03). Drinking more than three cups a day also was found to be protective against an LSM of 9.5 kPa or higher, the threshold for advanced liver fibrosis (OR, 0.4; P = .05). Decaffeinated coffee was not found to be associated with LSM.
Caffeine is an antagonist to adenosine receptors in the liver cell that, if blocked, stops the production of scar tissue, according to the researchers. But when they looked at estimated caffeine consumption, calculated through the detailed, trained interviews performed by nutritionists, there was no association with liver stiffness. That said, Dr. Tapper noted that this could be due to the imperfection of making those estimations.
“If we had to hypothesize about a mechanism, it would most likely be caffeine, and the reason we couldn’t see that here is because these are estimated milligrams of caffeine per coffee – but the way that we brew coffee, and the beans that we’re using, are so highly variable it just can’t be captured in this kind of database,” he said.
He said the data will be reassuring to clinicians who suggest coffee-drinking to patients.
“There are hepatologists around the world who are actively recommending coffee – they’ll feel empowered by these data,” he said. “I would still like to see more robust longitudinal data before I start spending our precious time counseling patients about coffee. There are many other data-driven interventions for the management of liver disease that we should be focusing our time on.”
Moreover, he said that the data will be important for patients who are particularly interested in natural remedies.
“For patients who are very interested in a natural supplement, to feel like they’re taking an active role in the health of their liver, I will tell them to avoid carbohydrates and increase their exercise – and that it is OK to add coffee to their daily routine.”
A study based on a UK database found that coffee was associated with protection against chronic liver disease, but the association was seen for both caffeinated and decaffeinated drinks, noted Nathan Davies, PhD, professor of biochemistry at the Institute of the Liver and Digestive Health at the University College London.
Dr. Davies, a registered nutritionist who has studied coffee’s effects on the liver, said that while including elastography in the Michigan study is interesting, it “does not necessarily by itself add greatly” to the evidence base.
The outcomes from both studies do suggest a positive effect for coffee, but he said it’s important to remember that liver disease develops over years and decades.
“Looking at a snapshot moment does not necessarily reflect an individual’s behavior during the onset and development of their condition,” he said. “As such, there are a number of behavioral and nutritional factors that could be contributing to the observed effect over a period of years.”
He pointed out that while different coffee and brewing types affect the amount of caffeine in a cup, all cups of coffee in this study were treated the same way. He noted there was no apparent dose-dependent effect, which would have been expected if there is an active ingredient that affects liver stiffness.
“In general, my advice is to improve diet, take more exercise, and reduce alcohol consumption, which is likely to be more effective in preventing liver disease – and its progression – than drinking an extra cup of coffee,” Dr. Davies said. “That being said, for patients at increased risk for liver disease who currently drink three cups or more of coffee daily, it may be prudent for them to continue because this level of consumption might be actively lowering their chances of developing more serious disease.”
Dr. Tapper has done consulting for Novartis, Axcella and Allergan, has served on advisory boards for Mallinckrodt, Bausch Health, Kaleido, and Novo Nordisk, and has unrestricted research grants from Gilead and Valeant. The remaining authors disclose no conflicts. Dr. Davies reported no relevant disclosures.
Drinking more than three cups of caffeinated coffee a day is associated with less liver stiffness, according to an analysis of a nationally representative survey, which was recently published in Clinical Gastroenterology and Hepatology.
The study is likely the most rigorous look to date on the benefits of coffee on liver health in the U.S. It was based on data from the National Health and Nutrition Examination Survey (NHANES), in which participants were asked about what they eat and drink. Crucially, in 2017, NHANES began to include elastography (FibroScan), of participants’ liver stiffness, not because of suspected problems with the liver but as across-the-board evaluations of all participants.
“Because it’s an unselected population for FibroScan and because of the detail, the granularity, the richness of the information from the nutritional surveys that they do, this is the closest we’re ever going to get to a linkage between what people are eating or drinking and the health of their liver, absent a longitudinal study where we set out to follow people for many, many years,” said Elliot Tapper, MD, assistant professor of gastroenterology at the University of Michigan, Ann Arbor, and the study’s senior author.
Researchers examined data from about 4,500 patients who had participated in the NHANES study in 2017-2018. The participants were aged 20 years or older, with an average age of 48; 73% were overweight, about the national average.
The researchers found no association between coffee consumption and controlled attenuation parameter (CAP), a measure of fatty liver. But they found a link between coffee and liver stiffness.
Those who drank more than three cups of coffee daily had a liver stiffness measure (LSM) that was 0.9 kilopascals (kPa) lower than others (P = .03). Drinking more than three cups a day also was found to be protective against an LSM of 9.5 kPa or higher, the threshold for advanced liver fibrosis (OR, 0.4; P = .05). Decaffeinated coffee was not found to be associated with LSM.
Caffeine is an antagonist to adenosine receptors in the liver cell that, if blocked, stops the production of scar tissue, according to the researchers. But when they looked at estimated caffeine consumption, calculated through the detailed, trained interviews performed by nutritionists, there was no association with liver stiffness. That said, Dr. Tapper noted that this could be due to the imperfection of making those estimations.
“If we had to hypothesize about a mechanism, it would most likely be caffeine, and the reason we couldn’t see that here is because these are estimated milligrams of caffeine per coffee – but the way that we brew coffee, and the beans that we’re using, are so highly variable it just can’t be captured in this kind of database,” he said.
He said the data will be reassuring to clinicians who suggest coffee-drinking to patients.
“There are hepatologists around the world who are actively recommending coffee – they’ll feel empowered by these data,” he said. “I would still like to see more robust longitudinal data before I start spending our precious time counseling patients about coffee. There are many other data-driven interventions for the management of liver disease that we should be focusing our time on.”
Moreover, he said that the data will be important for patients who are particularly interested in natural remedies.
“For patients who are very interested in a natural supplement, to feel like they’re taking an active role in the health of their liver, I will tell them to avoid carbohydrates and increase their exercise – and that it is OK to add coffee to their daily routine.”
A study based on a UK database found that coffee was associated with protection against chronic liver disease, but the association was seen for both caffeinated and decaffeinated drinks, noted Nathan Davies, PhD, professor of biochemistry at the Institute of the Liver and Digestive Health at the University College London.
Dr. Davies, a registered nutritionist who has studied coffee’s effects on the liver, said that while including elastography in the Michigan study is interesting, it “does not necessarily by itself add greatly” to the evidence base.
The outcomes from both studies do suggest a positive effect for coffee, but he said it’s important to remember that liver disease develops over years and decades.
“Looking at a snapshot moment does not necessarily reflect an individual’s behavior during the onset and development of their condition,” he said. “As such, there are a number of behavioral and nutritional factors that could be contributing to the observed effect over a period of years.”
He pointed out that while different coffee and brewing types affect the amount of caffeine in a cup, all cups of coffee in this study were treated the same way. He noted there was no apparent dose-dependent effect, which would have been expected if there is an active ingredient that affects liver stiffness.
“In general, my advice is to improve diet, take more exercise, and reduce alcohol consumption, which is likely to be more effective in preventing liver disease – and its progression – than drinking an extra cup of coffee,” Dr. Davies said. “That being said, for patients at increased risk for liver disease who currently drink three cups or more of coffee daily, it may be prudent for them to continue because this level of consumption might be actively lowering their chances of developing more serious disease.”
Dr. Tapper has done consulting for Novartis, Axcella and Allergan, has served on advisory boards for Mallinckrodt, Bausch Health, Kaleido, and Novo Nordisk, and has unrestricted research grants from Gilead and Valeant. The remaining authors disclose no conflicts. Dr. Davies reported no relevant disclosures.
Drinking more than three cups of caffeinated coffee a day is associated with less liver stiffness, according to an analysis of a nationally representative survey, which was recently published in Clinical Gastroenterology and Hepatology.
The study is likely the most rigorous look to date on the benefits of coffee on liver health in the U.S. It was based on data from the National Health and Nutrition Examination Survey (NHANES), in which participants were asked about what they eat and drink. Crucially, in 2017, NHANES began to include elastography (FibroScan), of participants’ liver stiffness, not because of suspected problems with the liver but as across-the-board evaluations of all participants.
“Because it’s an unselected population for FibroScan and because of the detail, the granularity, the richness of the information from the nutritional surveys that they do, this is the closest we’re ever going to get to a linkage between what people are eating or drinking and the health of their liver, absent a longitudinal study where we set out to follow people for many, many years,” said Elliot Tapper, MD, assistant professor of gastroenterology at the University of Michigan, Ann Arbor, and the study’s senior author.
Researchers examined data from about 4,500 patients who had participated in the NHANES study in 2017-2018. The participants were aged 20 years or older, with an average age of 48; 73% were overweight, about the national average.
The researchers found no association between coffee consumption and controlled attenuation parameter (CAP), a measure of fatty liver. But they found a link between coffee and liver stiffness.
Those who drank more than three cups of coffee daily had a liver stiffness measure (LSM) that was 0.9 kilopascals (kPa) lower than others (P = .03). Drinking more than three cups a day also was found to be protective against an LSM of 9.5 kPa or higher, the threshold for advanced liver fibrosis (OR, 0.4; P = .05). Decaffeinated coffee was not found to be associated with LSM.
Caffeine is an antagonist to adenosine receptors in the liver cell that, if blocked, stops the production of scar tissue, according to the researchers. But when they looked at estimated caffeine consumption, calculated through the detailed, trained interviews performed by nutritionists, there was no association with liver stiffness. That said, Dr. Tapper noted that this could be due to the imperfection of making those estimations.
“If we had to hypothesize about a mechanism, it would most likely be caffeine, and the reason we couldn’t see that here is because these are estimated milligrams of caffeine per coffee – but the way that we brew coffee, and the beans that we’re using, are so highly variable it just can’t be captured in this kind of database,” he said.
He said the data will be reassuring to clinicians who suggest coffee-drinking to patients.
“There are hepatologists around the world who are actively recommending coffee – they’ll feel empowered by these data,” he said. “I would still like to see more robust longitudinal data before I start spending our precious time counseling patients about coffee. There are many other data-driven interventions for the management of liver disease that we should be focusing our time on.”
Moreover, he said that the data will be important for patients who are particularly interested in natural remedies.
“For patients who are very interested in a natural supplement, to feel like they’re taking an active role in the health of their liver, I will tell them to avoid carbohydrates and increase their exercise – and that it is OK to add coffee to their daily routine.”
A study based on a UK database found that coffee was associated with protection against chronic liver disease, but the association was seen for both caffeinated and decaffeinated drinks, noted Nathan Davies, PhD, professor of biochemistry at the Institute of the Liver and Digestive Health at the University College London.
Dr. Davies, a registered nutritionist who has studied coffee’s effects on the liver, said that while including elastography in the Michigan study is interesting, it “does not necessarily by itself add greatly” to the evidence base.
The outcomes from both studies do suggest a positive effect for coffee, but he said it’s important to remember that liver disease develops over years and decades.
“Looking at a snapshot moment does not necessarily reflect an individual’s behavior during the onset and development of their condition,” he said. “As such, there are a number of behavioral and nutritional factors that could be contributing to the observed effect over a period of years.”
He pointed out that while different coffee and brewing types affect the amount of caffeine in a cup, all cups of coffee in this study were treated the same way. He noted there was no apparent dose-dependent effect, which would have been expected if there is an active ingredient that affects liver stiffness.
“In general, my advice is to improve diet, take more exercise, and reduce alcohol consumption, which is likely to be more effective in preventing liver disease – and its progression – than drinking an extra cup of coffee,” Dr. Davies said. “That being said, for patients at increased risk for liver disease who currently drink three cups or more of coffee daily, it may be prudent for them to continue because this level of consumption might be actively lowering their chances of developing more serious disease.”
Dr. Tapper has done consulting for Novartis, Axcella and Allergan, has served on advisory boards for Mallinckrodt, Bausch Health, Kaleido, and Novo Nordisk, and has unrestricted research grants from Gilead and Valeant. The remaining authors disclose no conflicts. Dr. Davies reported no relevant disclosures.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
USPSTF statement on aspirin: poor messaging at best
: “The USPSTF concludes with moderate certainty that initiating aspirin use for the primary prevention of CVD events in adults age 60 years or older has no net benefit.” I take no issue with the data and appreciate the efforts of the researchers, but at a minimum the public statement is incomplete. At most, it’s dangerously poor messaging.
As physicians, we understand how best to apply this information, but most laypeople, some at significant cardiovascular risk, closed their medicine cabinets this morning and left their aspirin bottle unopened on the shelf. Some of these patients have never spent an hour in the hospital for cardiac-related issues, but they have mitigated their risk for myocardial infarction by purposely poisoning their platelets daily with 81 mg of aspirin. And they should continue to do so.
Don’t forget the calcium score
Take, for instance, my patient Jack, who is typical of many patients I’ve seen throughout the years. Jack is 68 years old and has never had a cardiac event or a gastrointestinal bleed. His daily routine includes a walk, a statin, and a baby aspirin because his CT coronary artery calcium (CAC) score was 10,000 at age 58.
He first visited me 10 years ago because his father died of a myocardial infarction in his late 50s. Jack’s left ventricular ejection fraction is normal and his stress ECG shows 1-mm ST-segment depression at 8 minutes on a Bruce protocol stress test, without angina. Because Jack is well-educated and keeps up with the latest cardiology recommendations, he is precisely the type of patient who may be harmed by this new USPSTF statement by stopping his aspirin.
In October 2020, an analysis from the DALLAS Heart Study showed that persons with a CAC score greater than 100 had a higher cumulative incidence of bleeding and of atherosclerotic cardiovascular disease (ASCVD) events compared with those with no coronary calcium. After adjustment for clinical risk factors, the association between CAC and bleeding was attenuated and no longer statistically significant, whereas the relationship between CAC and ASCVD remained.
I asked one of the investigators, Amit Khera, MD, MSc, from UT Southwestern Medical Center, about the latest recommendations. He emphasized that both the American College of Cardiology/American Heart Association prevention guidelines and the USPSTF statement say that aspirin could still be considered among patients who are at higher risk for cardiovascular events. The USPSTF delineated this as a 10-year ASCVD risk greater than 10%.
Dr. Khera, who was an author of the 2019 guidelines, explained that the guideline committee purposely did not make specific recommendations as to what demarcated higher risk because the data were not clear at that time. Since then, a couple of papers, including the Dallas Heart Study analysis published in JAMA Cardiology, showed that patients at low bleeding risk with a calcium score above 100 may get a net benefit from aspirin. “Thus, in my patients who have a high calcium score and low bleeding risk, I do discuss the option to start or continue aspirin,” he said.
One size does not fit all
I watched ABC World News Tonight on Tuesday, October 12, and was immediately troubled about the coverage of the USPSTF statement. With viewership for the “Big Three” networks in the millions, the message to discontinue aspirin may have unintended consequences for many at-risk patients. The blood-thinning effects of a single dose of aspirin last about 10 days; it will be interesting to see if the rates of myocardial infarction increase over time. This could have been avoided with a better-worded statement – I’m concerned that the lack of nuance could spell big trouble for some.
In JAMA Cardiology, Dr. Khera and colleagues wrote that, “Aspirin use is not a one-size-fits-all therapy.” All physicians likely agree with that opinion. The USPSTF statement should have included the point that if you have a high CT coronary artery calcium score and a low bleeding risk, aspirin still fits very well even if you haven’t experienced a cardiac event. At a minimum, the USPSTF statement should have included the suggestion for patients to consult their physician for advice before discontinuing aspirin therapy.
I hope patients like Jack get the right message.
Melissa Walton-Shirley, MD, is a native Kentuckian who retired from full-time invasive cardiology.
A version of this article first appeared on Medscape.com.
: “The USPSTF concludes with moderate certainty that initiating aspirin use for the primary prevention of CVD events in adults age 60 years or older has no net benefit.” I take no issue with the data and appreciate the efforts of the researchers, but at a minimum the public statement is incomplete. At most, it’s dangerously poor messaging.
As physicians, we understand how best to apply this information, but most laypeople, some at significant cardiovascular risk, closed their medicine cabinets this morning and left their aspirin bottle unopened on the shelf. Some of these patients have never spent an hour in the hospital for cardiac-related issues, but they have mitigated their risk for myocardial infarction by purposely poisoning their platelets daily with 81 mg of aspirin. And they should continue to do so.
Don’t forget the calcium score
Take, for instance, my patient Jack, who is typical of many patients I’ve seen throughout the years. Jack is 68 years old and has never had a cardiac event or a gastrointestinal bleed. His daily routine includes a walk, a statin, and a baby aspirin because his CT coronary artery calcium (CAC) score was 10,000 at age 58.
He first visited me 10 years ago because his father died of a myocardial infarction in his late 50s. Jack’s left ventricular ejection fraction is normal and his stress ECG shows 1-mm ST-segment depression at 8 minutes on a Bruce protocol stress test, without angina. Because Jack is well-educated and keeps up with the latest cardiology recommendations, he is precisely the type of patient who may be harmed by this new USPSTF statement by stopping his aspirin.
In October 2020, an analysis from the DALLAS Heart Study showed that persons with a CAC score greater than 100 had a higher cumulative incidence of bleeding and of atherosclerotic cardiovascular disease (ASCVD) events compared with those with no coronary calcium. After adjustment for clinical risk factors, the association between CAC and bleeding was attenuated and no longer statistically significant, whereas the relationship between CAC and ASCVD remained.
I asked one of the investigators, Amit Khera, MD, MSc, from UT Southwestern Medical Center, about the latest recommendations. He emphasized that both the American College of Cardiology/American Heart Association prevention guidelines and the USPSTF statement say that aspirin could still be considered among patients who are at higher risk for cardiovascular events. The USPSTF delineated this as a 10-year ASCVD risk greater than 10%.
Dr. Khera, who was an author of the 2019 guidelines, explained that the guideline committee purposely did not make specific recommendations as to what demarcated higher risk because the data were not clear at that time. Since then, a couple of papers, including the Dallas Heart Study analysis published in JAMA Cardiology, showed that patients at low bleeding risk with a calcium score above 100 may get a net benefit from aspirin. “Thus, in my patients who have a high calcium score and low bleeding risk, I do discuss the option to start or continue aspirin,” he said.
One size does not fit all
I watched ABC World News Tonight on Tuesday, October 12, and was immediately troubled about the coverage of the USPSTF statement. With viewership for the “Big Three” networks in the millions, the message to discontinue aspirin may have unintended consequences for many at-risk patients. The blood-thinning effects of a single dose of aspirin last about 10 days; it will be interesting to see if the rates of myocardial infarction increase over time. This could have been avoided with a better-worded statement – I’m concerned that the lack of nuance could spell big trouble for some.
In JAMA Cardiology, Dr. Khera and colleagues wrote that, “Aspirin use is not a one-size-fits-all therapy.” All physicians likely agree with that opinion. The USPSTF statement should have included the point that if you have a high CT coronary artery calcium score and a low bleeding risk, aspirin still fits very well even if you haven’t experienced a cardiac event. At a minimum, the USPSTF statement should have included the suggestion for patients to consult their physician for advice before discontinuing aspirin therapy.
I hope patients like Jack get the right message.
Melissa Walton-Shirley, MD, is a native Kentuckian who retired from full-time invasive cardiology.
A version of this article first appeared on Medscape.com.
: “The USPSTF concludes with moderate certainty that initiating aspirin use for the primary prevention of CVD events in adults age 60 years or older has no net benefit.” I take no issue with the data and appreciate the efforts of the researchers, but at a minimum the public statement is incomplete. At most, it’s dangerously poor messaging.
As physicians, we understand how best to apply this information, but most laypeople, some at significant cardiovascular risk, closed their medicine cabinets this morning and left their aspirin bottle unopened on the shelf. Some of these patients have never spent an hour in the hospital for cardiac-related issues, but they have mitigated their risk for myocardial infarction by purposely poisoning their platelets daily with 81 mg of aspirin. And they should continue to do so.
Don’t forget the calcium score
Take, for instance, my patient Jack, who is typical of many patients I’ve seen throughout the years. Jack is 68 years old and has never had a cardiac event or a gastrointestinal bleed. His daily routine includes a walk, a statin, and a baby aspirin because his CT coronary artery calcium (CAC) score was 10,000 at age 58.
He first visited me 10 years ago because his father died of a myocardial infarction in his late 50s. Jack’s left ventricular ejection fraction is normal and his stress ECG shows 1-mm ST-segment depression at 8 minutes on a Bruce protocol stress test, without angina. Because Jack is well-educated and keeps up with the latest cardiology recommendations, he is precisely the type of patient who may be harmed by this new USPSTF statement by stopping his aspirin.
In October 2020, an analysis from the DALLAS Heart Study showed that persons with a CAC score greater than 100 had a higher cumulative incidence of bleeding and of atherosclerotic cardiovascular disease (ASCVD) events compared with those with no coronary calcium. After adjustment for clinical risk factors, the association between CAC and bleeding was attenuated and no longer statistically significant, whereas the relationship between CAC and ASCVD remained.
I asked one of the investigators, Amit Khera, MD, MSc, from UT Southwestern Medical Center, about the latest recommendations. He emphasized that both the American College of Cardiology/American Heart Association prevention guidelines and the USPSTF statement say that aspirin could still be considered among patients who are at higher risk for cardiovascular events. The USPSTF delineated this as a 10-year ASCVD risk greater than 10%.
Dr. Khera, who was an author of the 2019 guidelines, explained that the guideline committee purposely did not make specific recommendations as to what demarcated higher risk because the data were not clear at that time. Since then, a couple of papers, including the Dallas Heart Study analysis published in JAMA Cardiology, showed that patients at low bleeding risk with a calcium score above 100 may get a net benefit from aspirin. “Thus, in my patients who have a high calcium score and low bleeding risk, I do discuss the option to start or continue aspirin,” he said.
One size does not fit all
I watched ABC World News Tonight on Tuesday, October 12, and was immediately troubled about the coverage of the USPSTF statement. With viewership for the “Big Three” networks in the millions, the message to discontinue aspirin may have unintended consequences for many at-risk patients. The blood-thinning effects of a single dose of aspirin last about 10 days; it will be interesting to see if the rates of myocardial infarction increase over time. This could have been avoided with a better-worded statement – I’m concerned that the lack of nuance could spell big trouble for some.
In JAMA Cardiology, Dr. Khera and colleagues wrote that, “Aspirin use is not a one-size-fits-all therapy.” All physicians likely agree with that opinion. The USPSTF statement should have included the point that if you have a high CT coronary artery calcium score and a low bleeding risk, aspirin still fits very well even if you haven’t experienced a cardiac event. At a minimum, the USPSTF statement should have included the suggestion for patients to consult their physician for advice before discontinuing aspirin therapy.
I hope patients like Jack get the right message.
Melissa Walton-Shirley, MD, is a native Kentuckian who retired from full-time invasive cardiology.
A version of this article first appeared on Medscape.com.
CDC panel backs COVID-19 boosters for nearly all adults
Editor’s note: This story was updated with the CDC director’s endorsement.
Centers for Disease Control and Prevention (CDC) Director Rochelle Walensky, MD, has signed off on an advisory panel’s earlier unanimous vote to recommend boosters for the Moderna and Johnson and Johnson COVID vaccines.
The decision now means that millions of Americans are eligible to get a booster shot for either the Pfizer, Moderna, or J&J COVID vaccines.
“The evidence shows that all three COVID-19 vaccines authorized in the United States are safe – as demonstrated by the over 400 million vaccine doses already given. And, they are all highly effective in reducing the risk of severe disease, hospitalization, and death, even in the midst of the widely circulating Delta variant,” Dr. Walensky said in a CDC news release.
She also signed off on the panel’s suggestion that individuals can mix or match the booster from any one of the three available COVID-19 vaccines.
The Advisory Committee on Immunization Practices (ACIP) recommended in a late afternoon 15-0 vote that everyone over age 18 who are at least 2 months past their Johnson & Johnson vaccine should get a booster, an endorsement that affects an estimated 13 million Americans.
Those eligible for a booster at least 6 months after their last Moderna shot are the same groups who can get a Pfizer booster.
They are:
- Anyone over age 65.
- Those over age 18 with an underlying health condition that puts them at risk of severe COVID-19.
- Those over age 18 who may be at higher risk of a COVID-19 infection because they live or work in a risky setting.
These recommendations are in line with the Food and Drug Administration’s Oct. 20 authorization of the boosters, along with the ability to mix-and-match vaccines.
There are an estimated 47 million Pfizer recipients and 39 million people vaccinated with Moderna who are now eligible for a booster dose, according to data presented by the CDC.
Questions, concerns
Before voting, some committee members expressed discomfort in broadly recommending boosters, stressing that there is very little evidence supporting the need for boosters in people younger than age 50.
“I can’t say that I am comfortable that anybody under 50 – an otherwise healthy individual – needs a booster vaccine at this time with either Moderna or Pfizer,” said ACIP member Sarah Long, MD, professor of pediatrics at Drexel University in Philadelphia.
She said she would try to mitigate any potential harm by having some kind of age restriction on the otherwise worried well.
“We don’t usually have the vaccines [for] the worried well. We give it because we have a need that’s worth the risk, and there’s a burden of severity of disease,” Dr. Long said.
The evidence to date shows that all the vaccines authorized for use in the U.S. continue to protect people well against severe COVID-19 outcomes, including hospitalization and death.
But breakthrough infections are on the rise, especially for people who initially received the Johnson and Johnson one-dose vaccine.
On Oct. 21, Pfizer released data from a study of more than 10,000 fully vaccinated people. Half were randomly assigned to get a booster of their Comirnaty vaccine, the other half were given a placebo.
Over the ensuing 2.5 months, there were 5 COVID-19 cases in the boosted group, and 109 in the group that got a placebo.
The data were posted in a press release and have not yet been peer reviewed, but are the first to show clinical effectiveness of boosters at preventing COVID-19 infections.
Data recently considered by the FDA and CDC for booster doses come from studies that were mostly shorter and smaller. These studies looked at biomarkers of immunity like the concentration of antibodies in a person’s blood and the percentage of study participants who saw a boost to those antibodies.
The studies demonstrated that boosters indeed restore high levels of antibodies, but unlike the newest Pfizer data they were not able to show that these antibodies prevented COVID-19.
These studies also weren’t powered to pick up on any less common safety problems that might arise after another dose of the shots.
“Real world” recommendations
In the end, however, the panel felt it was more important to be permissive in allowing boosters so that individuals and their doctors could be free to make their own decisions.
“The decision made by the FDA and the ACIP recommendations, I think, reflects the real world. The public is going to do what they feel driven to do. This at least adds a scientific review of the currently available data,” said Jay Varkey, MD, an infectious disease physician and associate professor at Emory University in Atlanta, who was not involved in the ACIP’s deliberations.
Dr. Varkey said he would recommend that anyone who is younger than 65, and who has no underlying medical conditions such as diabetes or obesity, speak with their doctor about their individual benefits and risks before getting a booster.
The CDC is planning to release a detailed suite of clinical considerations to help people weigh the risks and benefits of getting a booster.
Safety updates presented at the meeting show that serious adverse events after vaccination are extremely rare, but in some cases, they may rise above the risk for those problems generally seen in the population.
Those rare events include the disabling autoimmune condition Guillain-Barré syndrome and the platelet disorder thrombosis with thrombocytopenia (TTS), which causes blood clots along with the risk of excess bleeding because of a low platelet count.
Both can occur after the J&J vaccine. Out of 15.3 million doses of the vaccine given to date, there have been 47 cases of TTS and five deaths. These events are more common in younger women.
The mRNA vaccines, such as those from Pfizer and Moderna, can cause heart inflammation called myocarditis or pericarditis. This side effect is more common in men 18-24 years old. The reported rate of myocarditis after vaccination is 39 cases for every 1 million doses.
In voting to permit boosters, committee member Wilbur Chen, MD, professor at the University of Maryland’s Center for Vaccine Development, said he hoped boosters wouldn’t give Americans false confidence.
Dr. Chen stressed that ending the pandemic would depend on “a multilayered approach” that includes masking, social distancing, avoiding large crowds indoors, and convincing more Americans to take their first doses of the vaccines.
“We’re not just going to vaccinate ourselves out of this situation,” Dr. Chen said.
A version of this article first appeared on WebMD.com.
Editor’s note: This story was updated with the CDC director’s endorsement.
Centers for Disease Control and Prevention (CDC) Director Rochelle Walensky, MD, has signed off on an advisory panel’s earlier unanimous vote to recommend boosters for the Moderna and Johnson and Johnson COVID vaccines.
The decision now means that millions of Americans are eligible to get a booster shot for either the Pfizer, Moderna, or J&J COVID vaccines.
“The evidence shows that all three COVID-19 vaccines authorized in the United States are safe – as demonstrated by the over 400 million vaccine doses already given. And, they are all highly effective in reducing the risk of severe disease, hospitalization, and death, even in the midst of the widely circulating Delta variant,” Dr. Walensky said in a CDC news release.
She also signed off on the panel’s suggestion that individuals can mix or match the booster from any one of the three available COVID-19 vaccines.
The Advisory Committee on Immunization Practices (ACIP) recommended in a late afternoon 15-0 vote that everyone over age 18 who are at least 2 months past their Johnson & Johnson vaccine should get a booster, an endorsement that affects an estimated 13 million Americans.
Those eligible for a booster at least 6 months after their last Moderna shot are the same groups who can get a Pfizer booster.
They are:
- Anyone over age 65.
- Those over age 18 with an underlying health condition that puts them at risk of severe COVID-19.
- Those over age 18 who may be at higher risk of a COVID-19 infection because they live or work in a risky setting.
These recommendations are in line with the Food and Drug Administration’s Oct. 20 authorization of the boosters, along with the ability to mix-and-match vaccines.
There are an estimated 47 million Pfizer recipients and 39 million people vaccinated with Moderna who are now eligible for a booster dose, according to data presented by the CDC.
Questions, concerns
Before voting, some committee members expressed discomfort in broadly recommending boosters, stressing that there is very little evidence supporting the need for boosters in people younger than age 50.
“I can’t say that I am comfortable that anybody under 50 – an otherwise healthy individual – needs a booster vaccine at this time with either Moderna or Pfizer,” said ACIP member Sarah Long, MD, professor of pediatrics at Drexel University in Philadelphia.
She said she would try to mitigate any potential harm by having some kind of age restriction on the otherwise worried well.
“We don’t usually have the vaccines [for] the worried well. We give it because we have a need that’s worth the risk, and there’s a burden of severity of disease,” Dr. Long said.
The evidence to date shows that all the vaccines authorized for use in the U.S. continue to protect people well against severe COVID-19 outcomes, including hospitalization and death.
But breakthrough infections are on the rise, especially for people who initially received the Johnson and Johnson one-dose vaccine.
On Oct. 21, Pfizer released data from a study of more than 10,000 fully vaccinated people. Half were randomly assigned to get a booster of their Comirnaty vaccine, the other half were given a placebo.
Over the ensuing 2.5 months, there were 5 COVID-19 cases in the boosted group, and 109 in the group that got a placebo.
The data were posted in a press release and have not yet been peer reviewed, but are the first to show clinical effectiveness of boosters at preventing COVID-19 infections.
Data recently considered by the FDA and CDC for booster doses come from studies that were mostly shorter and smaller. These studies looked at biomarkers of immunity like the concentration of antibodies in a person’s blood and the percentage of study participants who saw a boost to those antibodies.
The studies demonstrated that boosters indeed restore high levels of antibodies, but unlike the newest Pfizer data they were not able to show that these antibodies prevented COVID-19.
These studies also weren’t powered to pick up on any less common safety problems that might arise after another dose of the shots.
“Real world” recommendations
In the end, however, the panel felt it was more important to be permissive in allowing boosters so that individuals and their doctors could be free to make their own decisions.
“The decision made by the FDA and the ACIP recommendations, I think, reflects the real world. The public is going to do what they feel driven to do. This at least adds a scientific review of the currently available data,” said Jay Varkey, MD, an infectious disease physician and associate professor at Emory University in Atlanta, who was not involved in the ACIP’s deliberations.
Dr. Varkey said he would recommend that anyone who is younger than 65, and who has no underlying medical conditions such as diabetes or obesity, speak with their doctor about their individual benefits and risks before getting a booster.
The CDC is planning to release a detailed suite of clinical considerations to help people weigh the risks and benefits of getting a booster.
Safety updates presented at the meeting show that serious adverse events after vaccination are extremely rare, but in some cases, they may rise above the risk for those problems generally seen in the population.
Those rare events include the disabling autoimmune condition Guillain-Barré syndrome and the platelet disorder thrombosis with thrombocytopenia (TTS), which causes blood clots along with the risk of excess bleeding because of a low platelet count.
Both can occur after the J&J vaccine. Out of 15.3 million doses of the vaccine given to date, there have been 47 cases of TTS and five deaths. These events are more common in younger women.
The mRNA vaccines, such as those from Pfizer and Moderna, can cause heart inflammation called myocarditis or pericarditis. This side effect is more common in men 18-24 years old. The reported rate of myocarditis after vaccination is 39 cases for every 1 million doses.
In voting to permit boosters, committee member Wilbur Chen, MD, professor at the University of Maryland’s Center for Vaccine Development, said he hoped boosters wouldn’t give Americans false confidence.
Dr. Chen stressed that ending the pandemic would depend on “a multilayered approach” that includes masking, social distancing, avoiding large crowds indoors, and convincing more Americans to take their first doses of the vaccines.
“We’re not just going to vaccinate ourselves out of this situation,” Dr. Chen said.
A version of this article first appeared on WebMD.com.
Editor’s note: This story was updated with the CDC director’s endorsement.
Centers for Disease Control and Prevention (CDC) Director Rochelle Walensky, MD, has signed off on an advisory panel’s earlier unanimous vote to recommend boosters for the Moderna and Johnson and Johnson COVID vaccines.
The decision now means that millions of Americans are eligible to get a booster shot for either the Pfizer, Moderna, or J&J COVID vaccines.
“The evidence shows that all three COVID-19 vaccines authorized in the United States are safe – as demonstrated by the over 400 million vaccine doses already given. And, they are all highly effective in reducing the risk of severe disease, hospitalization, and death, even in the midst of the widely circulating Delta variant,” Dr. Walensky said in a CDC news release.
She also signed off on the panel’s suggestion that individuals can mix or match the booster from any one of the three available COVID-19 vaccines.
The Advisory Committee on Immunization Practices (ACIP) recommended in a late afternoon 15-0 vote that everyone over age 18 who are at least 2 months past their Johnson & Johnson vaccine should get a booster, an endorsement that affects an estimated 13 million Americans.
Those eligible for a booster at least 6 months after their last Moderna shot are the same groups who can get a Pfizer booster.
They are:
- Anyone over age 65.
- Those over age 18 with an underlying health condition that puts them at risk of severe COVID-19.
- Those over age 18 who may be at higher risk of a COVID-19 infection because they live or work in a risky setting.
These recommendations are in line with the Food and Drug Administration’s Oct. 20 authorization of the boosters, along with the ability to mix-and-match vaccines.
There are an estimated 47 million Pfizer recipients and 39 million people vaccinated with Moderna who are now eligible for a booster dose, according to data presented by the CDC.
Questions, concerns
Before voting, some committee members expressed discomfort in broadly recommending boosters, stressing that there is very little evidence supporting the need for boosters in people younger than age 50.
“I can’t say that I am comfortable that anybody under 50 – an otherwise healthy individual – needs a booster vaccine at this time with either Moderna or Pfizer,” said ACIP member Sarah Long, MD, professor of pediatrics at Drexel University in Philadelphia.
She said she would try to mitigate any potential harm by having some kind of age restriction on the otherwise worried well.
“We don’t usually have the vaccines [for] the worried well. We give it because we have a need that’s worth the risk, and there’s a burden of severity of disease,” Dr. Long said.
The evidence to date shows that all the vaccines authorized for use in the U.S. continue to protect people well against severe COVID-19 outcomes, including hospitalization and death.
But breakthrough infections are on the rise, especially for people who initially received the Johnson and Johnson one-dose vaccine.
On Oct. 21, Pfizer released data from a study of more than 10,000 fully vaccinated people. Half were randomly assigned to get a booster of their Comirnaty vaccine, the other half were given a placebo.
Over the ensuing 2.5 months, there were 5 COVID-19 cases in the boosted group, and 109 in the group that got a placebo.
The data were posted in a press release and have not yet been peer reviewed, but are the first to show clinical effectiveness of boosters at preventing COVID-19 infections.
Data recently considered by the FDA and CDC for booster doses come from studies that were mostly shorter and smaller. These studies looked at biomarkers of immunity like the concentration of antibodies in a person’s blood and the percentage of study participants who saw a boost to those antibodies.
The studies demonstrated that boosters indeed restore high levels of antibodies, but unlike the newest Pfizer data they were not able to show that these antibodies prevented COVID-19.
These studies also weren’t powered to pick up on any less common safety problems that might arise after another dose of the shots.
“Real world” recommendations
In the end, however, the panel felt it was more important to be permissive in allowing boosters so that individuals and their doctors could be free to make their own decisions.
“The decision made by the FDA and the ACIP recommendations, I think, reflects the real world. The public is going to do what they feel driven to do. This at least adds a scientific review of the currently available data,” said Jay Varkey, MD, an infectious disease physician and associate professor at Emory University in Atlanta, who was not involved in the ACIP’s deliberations.
Dr. Varkey said he would recommend that anyone who is younger than 65, and who has no underlying medical conditions such as diabetes or obesity, speak with their doctor about their individual benefits and risks before getting a booster.
The CDC is planning to release a detailed suite of clinical considerations to help people weigh the risks and benefits of getting a booster.
Safety updates presented at the meeting show that serious adverse events after vaccination are extremely rare, but in some cases, they may rise above the risk for those problems generally seen in the population.
Those rare events include the disabling autoimmune condition Guillain-Barré syndrome and the platelet disorder thrombosis with thrombocytopenia (TTS), which causes blood clots along with the risk of excess bleeding because of a low platelet count.
Both can occur after the J&J vaccine. Out of 15.3 million doses of the vaccine given to date, there have been 47 cases of TTS and five deaths. These events are more common in younger women.
The mRNA vaccines, such as those from Pfizer and Moderna, can cause heart inflammation called myocarditis or pericarditis. This side effect is more common in men 18-24 years old. The reported rate of myocarditis after vaccination is 39 cases for every 1 million doses.
In voting to permit boosters, committee member Wilbur Chen, MD, professor at the University of Maryland’s Center for Vaccine Development, said he hoped boosters wouldn’t give Americans false confidence.
Dr. Chen stressed that ending the pandemic would depend on “a multilayered approach” that includes masking, social distancing, avoiding large crowds indoors, and convincing more Americans to take their first doses of the vaccines.
“We’re not just going to vaccinate ourselves out of this situation,” Dr. Chen said.
A version of this article first appeared on WebMD.com.