User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
Making the Rounds to Diagnosis
ANSWER
The correct answer is granuloma annulare (GA; choice “d”).
DISCUSSION
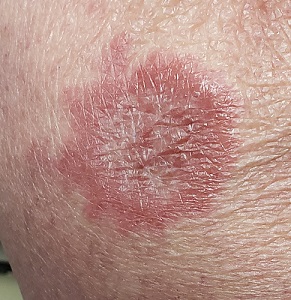
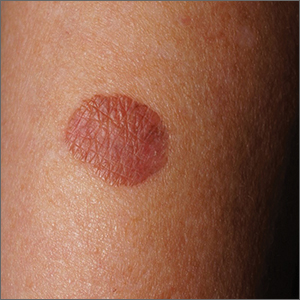
The biopsy showed rings of dermal epithelioid histiocytes surrounding a core of central mucin, configured in rows (the latter conferring the diagnostic term “palisaded” granulomas). Both the pathology results and the morphology of the lesions—color, shape, etc—were classic for GA. What was somewhat unusual about this case was the size and number of lesions, which are typically fewer and smaller with GA.
GA is seldom much of a problem and has no connection to serious disease. But it can, as this case illustrates, mimic some rather worrisome conditions. Had this been sarcoidosis (choice “a”), there would be no central “delling” (concavity), and the biopsy would have shown necrotic (caseating) nonpalisading granulomas.
Cutaneous T-cell lymphoma (CTCL; choice “b”) can manifest with plaques, but there wouldn’t be any delling and the shapes would not be so consistently round. CTCL will eventually give rise to palpable adenopathy.
Lupus profundus (choice “c”) also manifests with deep plaques, without delling. It is a truly rare variant of lupus, which would have been detected on biopsy.
Concern about these differential items is what drove the decision to perform full-thickness punch biopsies as opposed to taking a simple shave sample.
TREATMENT
The most common treatment for GA is intralesional steroid injection (eg, 10 mg/cc triamcinolone) of large lesions. Oral medications such as methotrexate or pentoxifylline have been used with some success. Often, the condition is self-limiting.
ANSWER
The correct answer is granuloma annulare (GA; choice “d”).
DISCUSSION
The biopsy showed rings of dermal epithelioid histiocytes surrounding a core of central mucin, configured in rows (the latter conferring the diagnostic term “palisaded” granulomas). Both the pathology results and the morphology of the lesions—color, shape, etc—were classic for GA. What was somewhat unusual about this case was the size and number of lesions, which are typically fewer and smaller with GA.
GA is seldom much of a problem and has no connection to serious disease. But it can, as this case illustrates, mimic some rather worrisome conditions. Had this been sarcoidosis (choice “a”), there would be no central “delling” (concavity), and the biopsy would have shown necrotic (caseating) nonpalisading granulomas.
Cutaneous T-cell lymphoma (CTCL; choice “b”) can manifest with plaques, but there wouldn’t be any delling and the shapes would not be so consistently round. CTCL will eventually give rise to palpable adenopathy.
Lupus profundus (choice “c”) also manifests with deep plaques, without delling. It is a truly rare variant of lupus, which would have been detected on biopsy.
Concern about these differential items is what drove the decision to perform full-thickness punch biopsies as opposed to taking a simple shave sample.
TREATMENT
The most common treatment for GA is intralesional steroid injection (eg, 10 mg/cc triamcinolone) of large lesions. Oral medications such as methotrexate or pentoxifylline have been used with some success. Often, the condition is self-limiting.
ANSWER
The correct answer is granuloma annulare (GA; choice “d”).
DISCUSSION
The biopsy showed rings of dermal epithelioid histiocytes surrounding a core of central mucin, configured in rows (the latter conferring the diagnostic term “palisaded” granulomas). Both the pathology results and the morphology of the lesions—color, shape, etc—were classic for GA. What was somewhat unusual about this case was the size and number of lesions, which are typically fewer and smaller with GA.
GA is seldom much of a problem and has no connection to serious disease. But it can, as this case illustrates, mimic some rather worrisome conditions. Had this been sarcoidosis (choice “a”), there would be no central “delling” (concavity), and the biopsy would have shown necrotic (caseating) nonpalisading granulomas.
Cutaneous T-cell lymphoma (CTCL; choice “b”) can manifest with plaques, but there wouldn’t be any delling and the shapes would not be so consistently round. CTCL will eventually give rise to palpable adenopathy.
Lupus profundus (choice “c”) also manifests with deep plaques, without delling. It is a truly rare variant of lupus, which would have been detected on biopsy.
Concern about these differential items is what drove the decision to perform full-thickness punch biopsies as opposed to taking a simple shave sample.
TREATMENT
The most common treatment for GA is intralesional steroid injection (eg, 10 mg/cc triamcinolone) of large lesions. Oral medications such as methotrexate or pentoxifylline have been used with some success. Often, the condition is self-limiting.
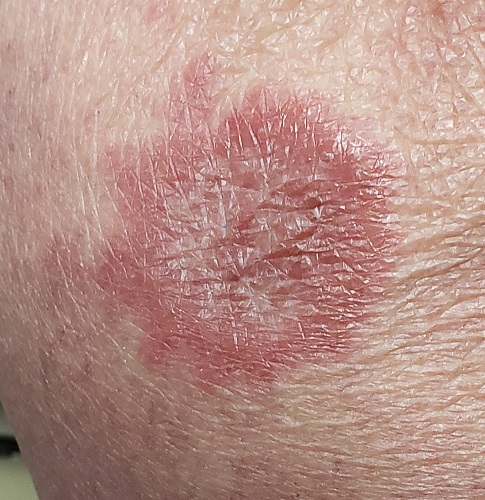
A 60-year-old woman is feeling fine—no fever, arthralgia, or malaise—but the sudden appearance of lesions on her extremities unsettles her to the point that she can think of little else. Visits to her primary care provider, the emergency department, several urgent care clinics, and a naturopath yield a consistent diagnosis: ringworm. Yet none of the topical or oral antifungal medications she is prescribed make the slightest difference. Thus, she finally agrees to consult a dermatologist.
The patient reports decent health, although she was a heavy smoker for years before quitting 2 years ago. She recently tested negative for diabetes.
History taking reveals no sources from which she could have acquired a fungal infection. No one else at home is similarly affected.
About 10 lesions, mostly located on the patient’s extremities, are examined. All are very similar in appearance: round, brownish-red intradermal plaques with no epidermal component (eg, scale, broken skin). The lesions vary from 6 mm to 4 cm in diameter. The centers of most lesions are slightly concave, with well-defined raised margins. On palpation, there is neither increased warmth nor tenderness. No nodes can be palpated in the groin, axillae, or epitrochlear locations.
A deep punch biopsy is performed on a thigh lesion, with the specimen submitted for pathologic examination.
Nutritional support may be lifesaving in heart failure
Personalized nutritional support for adults hospitalized with chronic heart failure and deemed to be at high nutritional risk reduced the risk of death or adverse cardiovascular events, compared with standard hospital food, new research indicates.
The Swiss EFFORT trial focused on patients with chronic heart failure and high risk of malnutrition defined by low body mass index, weight loss, and low food intake upon hospital admission.
“This high-risk group of chronic heart failure patients showed a significant improvement in mortality over 30 and 180 days, as well as other clinical outcomes, when individualized nutritional support interventions were offered to patients,” Philipp Schuetz, MD, MPH, Kantonsspital Aarau, Switzerland, said in an interview.
“While monitoring the nutritional status should be done also in outpatient settings by [general practitioners], malnutrition screening upon hospital admission may help to identify high-risk patients with high risk for nutritional status deterioration during the hospital stay who will benefit from nutritional assessment and treatment,” said Dr. Schuetz.
The study was published online May 3 in the Journal of the American College of Cardiology.
It’s not all about salt
The findings are based on a prespecified secondary analysis of outcomes in 645 patients (median age, 78.8 years, 52% men) hospitalized with chronic heart failure who participated in the open-label EFFORT study.
One-third of patients were hospitalized for acute decompensated heart failure and two-thirds had chronic heart failure and other acute medical illnesses requiring hospitalization.
All patients were at risk of malnutrition based on a Nutritional Risk Screening (NRS) score of 3 points or higher. They were randomly allocated 1:1 to individualized nutritional support to reach energy, protein, and micronutrient goals or usual hospital food (control group).
By 30 days, 27 of 321 patients (8.4%) receiving nutritional support had died compared with 48 of 324 patients (14.8%) in the control group (adjusted odds ratio [OR]: 0.44; 95% confidence interval, 0.26-0.75; P = .002)
Patients with high nutritional risk (NRS >4 points) showed the most benefit from nutritional support.
Compared with patients with moderate nutritional risk scores (NRS score 3-4), those with high nutritional risk (NRS >4) had a highly significant 65% increased mortality risk over 180 days.
The individual component of the NRS with the strongest association with mortality was low food intake in the week before hospitalization.
Patients who received nutritional support in the hospital also had a lower risk for major cardiovascular events at 30 days (17.4% vs. 26.9%; OR, 0.50; 95% CI, 0.34-0.75; P = .001).
“Historically, cardiologists and internists caring for patients with heart failure have mainly focused on salt-restrictive diets to reduce blood volume and thus optimize heart function. Yet, reduction of salt intake has not been shown to effectively improve clinical outcome but may, on the contrary, increase the risk of malnutrition as low-salt diets are often not tasty,” Dr. Schuetz said.
“Our data suggest that we should move our focus away from salt-restrictive diets to high-protein diets to cover individual nutritional goals in this high-risk group of patients, which includes screening, assessment, and nutritional support by dietitians,” Dr. Schuetz said.
In a linked editorial, Sheldon Gottlieb, MD, Johns Hopkins University, Baltimore, said there has been “relatively little attention” paid to the role of diet in heart failure other than recommending reduced salt intake.
In fact, in the 2021 American College of Cardiology expert consensus recommendations for optimizing heart failure treatment, roughly five words are devoted to diet and exercise and there is no mention of nutrition assessment by a dietitian, he points out.
“This study adds another tile to the still-fragmentary mosaic picture of the patient with heart failure at nutritional risk who might benefit from nutritional support,” Dr. Dr. Gottlieb wrote.
“ ‘Good medical care’ dictates that all hospitalized patients deserve to have a standardized nutritional assessment; the challenge remains: how to determine which patient with heart failure at nutritional risk will benefit by medical nutrition therapy,” Dr. Gottlieb said.
The Swiss National Science Foundation and the Research Council of the Kantonsspital Aarau provided funding for the trial. Dr. Schuetz’s institution has previously received unrestricted grant money unrelated to this project from Nestle Health Science and Abbott Nutrition. Dr. Gottlieb owns a federal trademark for the “Greens, Beans, and Leans” diet, and has a pending federal trademark for “FLOATS”: flax + oats cereal.
A version of this article first appeared on Medscape.com.
Personalized nutritional support for adults hospitalized with chronic heart failure and deemed to be at high nutritional risk reduced the risk of death or adverse cardiovascular events, compared with standard hospital food, new research indicates.
The Swiss EFFORT trial focused on patients with chronic heart failure and high risk of malnutrition defined by low body mass index, weight loss, and low food intake upon hospital admission.
“This high-risk group of chronic heart failure patients showed a significant improvement in mortality over 30 and 180 days, as well as other clinical outcomes, when individualized nutritional support interventions were offered to patients,” Philipp Schuetz, MD, MPH, Kantonsspital Aarau, Switzerland, said in an interview.
“While monitoring the nutritional status should be done also in outpatient settings by [general practitioners], malnutrition screening upon hospital admission may help to identify high-risk patients with high risk for nutritional status deterioration during the hospital stay who will benefit from nutritional assessment and treatment,” said Dr. Schuetz.
The study was published online May 3 in the Journal of the American College of Cardiology.
It’s not all about salt
The findings are based on a prespecified secondary analysis of outcomes in 645 patients (median age, 78.8 years, 52% men) hospitalized with chronic heart failure who participated in the open-label EFFORT study.
One-third of patients were hospitalized for acute decompensated heart failure and two-thirds had chronic heart failure and other acute medical illnesses requiring hospitalization.
All patients were at risk of malnutrition based on a Nutritional Risk Screening (NRS) score of 3 points or higher. They were randomly allocated 1:1 to individualized nutritional support to reach energy, protein, and micronutrient goals or usual hospital food (control group).
By 30 days, 27 of 321 patients (8.4%) receiving nutritional support had died compared with 48 of 324 patients (14.8%) in the control group (adjusted odds ratio [OR]: 0.44; 95% confidence interval, 0.26-0.75; P = .002)
Patients with high nutritional risk (NRS >4 points) showed the most benefit from nutritional support.
Compared with patients with moderate nutritional risk scores (NRS score 3-4), those with high nutritional risk (NRS >4) had a highly significant 65% increased mortality risk over 180 days.
The individual component of the NRS with the strongest association with mortality was low food intake in the week before hospitalization.
Patients who received nutritional support in the hospital also had a lower risk for major cardiovascular events at 30 days (17.4% vs. 26.9%; OR, 0.50; 95% CI, 0.34-0.75; P = .001).
“Historically, cardiologists and internists caring for patients with heart failure have mainly focused on salt-restrictive diets to reduce blood volume and thus optimize heart function. Yet, reduction of salt intake has not been shown to effectively improve clinical outcome but may, on the contrary, increase the risk of malnutrition as low-salt diets are often not tasty,” Dr. Schuetz said.
“Our data suggest that we should move our focus away from salt-restrictive diets to high-protein diets to cover individual nutritional goals in this high-risk group of patients, which includes screening, assessment, and nutritional support by dietitians,” Dr. Schuetz said.
In a linked editorial, Sheldon Gottlieb, MD, Johns Hopkins University, Baltimore, said there has been “relatively little attention” paid to the role of diet in heart failure other than recommending reduced salt intake.
In fact, in the 2021 American College of Cardiology expert consensus recommendations for optimizing heart failure treatment, roughly five words are devoted to diet and exercise and there is no mention of nutrition assessment by a dietitian, he points out.
“This study adds another tile to the still-fragmentary mosaic picture of the patient with heart failure at nutritional risk who might benefit from nutritional support,” Dr. Dr. Gottlieb wrote.
“ ‘Good medical care’ dictates that all hospitalized patients deserve to have a standardized nutritional assessment; the challenge remains: how to determine which patient with heart failure at nutritional risk will benefit by medical nutrition therapy,” Dr. Gottlieb said.
The Swiss National Science Foundation and the Research Council of the Kantonsspital Aarau provided funding for the trial. Dr. Schuetz’s institution has previously received unrestricted grant money unrelated to this project from Nestle Health Science and Abbott Nutrition. Dr. Gottlieb owns a federal trademark for the “Greens, Beans, and Leans” diet, and has a pending federal trademark for “FLOATS”: flax + oats cereal.
A version of this article first appeared on Medscape.com.
Personalized nutritional support for adults hospitalized with chronic heart failure and deemed to be at high nutritional risk reduced the risk of death or adverse cardiovascular events, compared with standard hospital food, new research indicates.
The Swiss EFFORT trial focused on patients with chronic heart failure and high risk of malnutrition defined by low body mass index, weight loss, and low food intake upon hospital admission.
“This high-risk group of chronic heart failure patients showed a significant improvement in mortality over 30 and 180 days, as well as other clinical outcomes, when individualized nutritional support interventions were offered to patients,” Philipp Schuetz, MD, MPH, Kantonsspital Aarau, Switzerland, said in an interview.
“While monitoring the nutritional status should be done also in outpatient settings by [general practitioners], malnutrition screening upon hospital admission may help to identify high-risk patients with high risk for nutritional status deterioration during the hospital stay who will benefit from nutritional assessment and treatment,” said Dr. Schuetz.
The study was published online May 3 in the Journal of the American College of Cardiology.
It’s not all about salt
The findings are based on a prespecified secondary analysis of outcomes in 645 patients (median age, 78.8 years, 52% men) hospitalized with chronic heart failure who participated in the open-label EFFORT study.
One-third of patients were hospitalized for acute decompensated heart failure and two-thirds had chronic heart failure and other acute medical illnesses requiring hospitalization.
All patients were at risk of malnutrition based on a Nutritional Risk Screening (NRS) score of 3 points or higher. They were randomly allocated 1:1 to individualized nutritional support to reach energy, protein, and micronutrient goals or usual hospital food (control group).
By 30 days, 27 of 321 patients (8.4%) receiving nutritional support had died compared with 48 of 324 patients (14.8%) in the control group (adjusted odds ratio [OR]: 0.44; 95% confidence interval, 0.26-0.75; P = .002)
Patients with high nutritional risk (NRS >4 points) showed the most benefit from nutritional support.
Compared with patients with moderate nutritional risk scores (NRS score 3-4), those with high nutritional risk (NRS >4) had a highly significant 65% increased mortality risk over 180 days.
The individual component of the NRS with the strongest association with mortality was low food intake in the week before hospitalization.
Patients who received nutritional support in the hospital also had a lower risk for major cardiovascular events at 30 days (17.4% vs. 26.9%; OR, 0.50; 95% CI, 0.34-0.75; P = .001).
“Historically, cardiologists and internists caring for patients with heart failure have mainly focused on salt-restrictive diets to reduce blood volume and thus optimize heart function. Yet, reduction of salt intake has not been shown to effectively improve clinical outcome but may, on the contrary, increase the risk of malnutrition as low-salt diets are often not tasty,” Dr. Schuetz said.
“Our data suggest that we should move our focus away from salt-restrictive diets to high-protein diets to cover individual nutritional goals in this high-risk group of patients, which includes screening, assessment, and nutritional support by dietitians,” Dr. Schuetz said.
In a linked editorial, Sheldon Gottlieb, MD, Johns Hopkins University, Baltimore, said there has been “relatively little attention” paid to the role of diet in heart failure other than recommending reduced salt intake.
In fact, in the 2021 American College of Cardiology expert consensus recommendations for optimizing heart failure treatment, roughly five words are devoted to diet and exercise and there is no mention of nutrition assessment by a dietitian, he points out.
“This study adds another tile to the still-fragmentary mosaic picture of the patient with heart failure at nutritional risk who might benefit from nutritional support,” Dr. Dr. Gottlieb wrote.
“ ‘Good medical care’ dictates that all hospitalized patients deserve to have a standardized nutritional assessment; the challenge remains: how to determine which patient with heart failure at nutritional risk will benefit by medical nutrition therapy,” Dr. Gottlieb said.
The Swiss National Science Foundation and the Research Council of the Kantonsspital Aarau provided funding for the trial. Dr. Schuetz’s institution has previously received unrestricted grant money unrelated to this project from Nestle Health Science and Abbott Nutrition. Dr. Gottlieb owns a federal trademark for the “Greens, Beans, and Leans” diet, and has a pending federal trademark for “FLOATS”: flax + oats cereal.
A version of this article first appeared on Medscape.com.
Hypertension worsened by commonly used prescription meds
Nearly half of these American adults had hypertension, and in this subgroup, 18.5% reported using a prescription drug known to increase blood pressure. The most widely used class of agents with this effect was antidepressants, used by 8.7%; followed by nonsteroidal anti-inflammatory drugs (NSAIDs), used by 6.5%; steroids, 1.9%; estrogens, 1.7%; and several other agents each used by fewer than 1% of the study cohort, John Vitarello, MD, said during a press briefing on reports from the upcoming annual scientific sessions of the American College of Cardiology.
He and his associates estimated that this use of prescription drugs known to raise blood pressure could be what stands in the way of some 560,000-2.2 million Americans from having their hypertension under control, depending on the exact blood pressure impact that various pressure-increasing drugs have and presuming that half of those on blood pressure increasing agents could stop them and switch to alternative agents, said Dr. Vitarello, a researcher at Beth Israel Deaconess Medical Center in Boston.
He also highlighted that the study assessed only prescription drugs and did not examine OTC drug use, which may be especially relevant for the many people who regularly take NSAIDs.
“Clinicians should review the prescription and OTC drug use of patients with hypertension and consider stopping drugs that increase blood pressure or switching the patient to alternatives” that are blood pressure neutral, Dr. Vitarello said during the briefing. He cautioned that maintaining hypertensive patients on agents that raise their blood pressure can result in “prescribing cascades” where taking drugs that boost blood pressure results in need for intensified antihypertensive treatment.
An opportunity for NSAID alternatives
“This study hopefully raises awareness that there is a very high use of medications that increase blood pressure, and use of OTC agents could increase the rate even higher” said Eugene Yang, MD, a cardiologist and codirector of the Cardiovascular Wellness and Prevention Program of the University of Washington, Seattle. Substituting for certain antidepressant agents may often not be realistic, but an opportunity exists for reducing NSAID use, a class also linked with an increased risk for bleeding and other adverse effects, Dr. Yang said during the briefing. Minimizing use of NSAIDs including ibuprofen and naproxen use “is something to think about,” he suggested.
“The effect of NSAIDs on blood pressure is not well studied and can vary from person to person” noted Dr. Vitarello, who added that higher NSAID dosages and more prolonged use likely increase the risk for an adverse effect on blood pressure. One reasonable option is to encourage patients to use an alternative class of pain reliever such as acetaminophen.
It remains “a challenge” to discern differences in adverse blood pressure effects, and in all adverse cardiovascular effects among different NSAIDs, said Dr. Yang. Results from “some studies show that certain NSAIDs may be safer, but other studies did not. We need to be very careful using NSAIDs because, on average, they increase blood pressure by about 3 mm Hg. We need to be mindful to try to prescribe alternative agents, like acetaminophen.”
A decade of data from NHANES
The analysis run by Dr. Vitarello and associates used data from 27,599 American adults included in the NHANES during 2009-2018, and focused on the 44% who either had an average blood pressure measurement of at least 130/80 mm Hg or reported having ever been told by a clinician that they had hypertension. The NHANES assessments included the prescription medications taken by each participant. The prevalence of using at least one prescription drug known to raise blood pressure was 24% among women and 14% among men, and 4% of those with hypertension were on two or more pressure-increasing agents.
The researchers based their identification of pressure-increasing prescription drugs on the list included in the 2017 guideline for managing high blood pressure from the American College of Cardiology and American Heart Association. This list specifies that the antidepressants that raise blood pressure are the monoamine oxidase inhibitors, serotonin norepinephrine reuptake inhibitors, and tricyclic antidepressants.
Dr. Vitarello and Dr. Yang had no disclosures.
Nearly half of these American adults had hypertension, and in this subgroup, 18.5% reported using a prescription drug known to increase blood pressure. The most widely used class of agents with this effect was antidepressants, used by 8.7%; followed by nonsteroidal anti-inflammatory drugs (NSAIDs), used by 6.5%; steroids, 1.9%; estrogens, 1.7%; and several other agents each used by fewer than 1% of the study cohort, John Vitarello, MD, said during a press briefing on reports from the upcoming annual scientific sessions of the American College of Cardiology.
He and his associates estimated that this use of prescription drugs known to raise blood pressure could be what stands in the way of some 560,000-2.2 million Americans from having their hypertension under control, depending on the exact blood pressure impact that various pressure-increasing drugs have and presuming that half of those on blood pressure increasing agents could stop them and switch to alternative agents, said Dr. Vitarello, a researcher at Beth Israel Deaconess Medical Center in Boston.
He also highlighted that the study assessed only prescription drugs and did not examine OTC drug use, which may be especially relevant for the many people who regularly take NSAIDs.
“Clinicians should review the prescription and OTC drug use of patients with hypertension and consider stopping drugs that increase blood pressure or switching the patient to alternatives” that are blood pressure neutral, Dr. Vitarello said during the briefing. He cautioned that maintaining hypertensive patients on agents that raise their blood pressure can result in “prescribing cascades” where taking drugs that boost blood pressure results in need for intensified antihypertensive treatment.
An opportunity for NSAID alternatives
“This study hopefully raises awareness that there is a very high use of medications that increase blood pressure, and use of OTC agents could increase the rate even higher” said Eugene Yang, MD, a cardiologist and codirector of the Cardiovascular Wellness and Prevention Program of the University of Washington, Seattle. Substituting for certain antidepressant agents may often not be realistic, but an opportunity exists for reducing NSAID use, a class also linked with an increased risk for bleeding and other adverse effects, Dr. Yang said during the briefing. Minimizing use of NSAIDs including ibuprofen and naproxen use “is something to think about,” he suggested.
“The effect of NSAIDs on blood pressure is not well studied and can vary from person to person” noted Dr. Vitarello, who added that higher NSAID dosages and more prolonged use likely increase the risk for an adverse effect on blood pressure. One reasonable option is to encourage patients to use an alternative class of pain reliever such as acetaminophen.
It remains “a challenge” to discern differences in adverse blood pressure effects, and in all adverse cardiovascular effects among different NSAIDs, said Dr. Yang. Results from “some studies show that certain NSAIDs may be safer, but other studies did not. We need to be very careful using NSAIDs because, on average, they increase blood pressure by about 3 mm Hg. We need to be mindful to try to prescribe alternative agents, like acetaminophen.”
A decade of data from NHANES
The analysis run by Dr. Vitarello and associates used data from 27,599 American adults included in the NHANES during 2009-2018, and focused on the 44% who either had an average blood pressure measurement of at least 130/80 mm Hg or reported having ever been told by a clinician that they had hypertension. The NHANES assessments included the prescription medications taken by each participant. The prevalence of using at least one prescription drug known to raise blood pressure was 24% among women and 14% among men, and 4% of those with hypertension were on two or more pressure-increasing agents.
The researchers based their identification of pressure-increasing prescription drugs on the list included in the 2017 guideline for managing high blood pressure from the American College of Cardiology and American Heart Association. This list specifies that the antidepressants that raise blood pressure are the monoamine oxidase inhibitors, serotonin norepinephrine reuptake inhibitors, and tricyclic antidepressants.
Dr. Vitarello and Dr. Yang had no disclosures.
Nearly half of these American adults had hypertension, and in this subgroup, 18.5% reported using a prescription drug known to increase blood pressure. The most widely used class of agents with this effect was antidepressants, used by 8.7%; followed by nonsteroidal anti-inflammatory drugs (NSAIDs), used by 6.5%; steroids, 1.9%; estrogens, 1.7%; and several other agents each used by fewer than 1% of the study cohort, John Vitarello, MD, said during a press briefing on reports from the upcoming annual scientific sessions of the American College of Cardiology.
He and his associates estimated that this use of prescription drugs known to raise blood pressure could be what stands in the way of some 560,000-2.2 million Americans from having their hypertension under control, depending on the exact blood pressure impact that various pressure-increasing drugs have and presuming that half of those on blood pressure increasing agents could stop them and switch to alternative agents, said Dr. Vitarello, a researcher at Beth Israel Deaconess Medical Center in Boston.
He also highlighted that the study assessed only prescription drugs and did not examine OTC drug use, which may be especially relevant for the many people who regularly take NSAIDs.
“Clinicians should review the prescription and OTC drug use of patients with hypertension and consider stopping drugs that increase blood pressure or switching the patient to alternatives” that are blood pressure neutral, Dr. Vitarello said during the briefing. He cautioned that maintaining hypertensive patients on agents that raise their blood pressure can result in “prescribing cascades” where taking drugs that boost blood pressure results in need for intensified antihypertensive treatment.
An opportunity for NSAID alternatives
“This study hopefully raises awareness that there is a very high use of medications that increase blood pressure, and use of OTC agents could increase the rate even higher” said Eugene Yang, MD, a cardiologist and codirector of the Cardiovascular Wellness and Prevention Program of the University of Washington, Seattle. Substituting for certain antidepressant agents may often not be realistic, but an opportunity exists for reducing NSAID use, a class also linked with an increased risk for bleeding and other adverse effects, Dr. Yang said during the briefing. Minimizing use of NSAIDs including ibuprofen and naproxen use “is something to think about,” he suggested.
“The effect of NSAIDs on blood pressure is not well studied and can vary from person to person” noted Dr. Vitarello, who added that higher NSAID dosages and more prolonged use likely increase the risk for an adverse effect on blood pressure. One reasonable option is to encourage patients to use an alternative class of pain reliever such as acetaminophen.
It remains “a challenge” to discern differences in adverse blood pressure effects, and in all adverse cardiovascular effects among different NSAIDs, said Dr. Yang. Results from “some studies show that certain NSAIDs may be safer, but other studies did not. We need to be very careful using NSAIDs because, on average, they increase blood pressure by about 3 mm Hg. We need to be mindful to try to prescribe alternative agents, like acetaminophen.”
A decade of data from NHANES
The analysis run by Dr. Vitarello and associates used data from 27,599 American adults included in the NHANES during 2009-2018, and focused on the 44% who either had an average blood pressure measurement of at least 130/80 mm Hg or reported having ever been told by a clinician that they had hypertension. The NHANES assessments included the prescription medications taken by each participant. The prevalence of using at least one prescription drug known to raise blood pressure was 24% among women and 14% among men, and 4% of those with hypertension were on two or more pressure-increasing agents.
The researchers based their identification of pressure-increasing prescription drugs on the list included in the 2017 guideline for managing high blood pressure from the American College of Cardiology and American Heart Association. This list specifies that the antidepressants that raise blood pressure are the monoamine oxidase inhibitors, serotonin norepinephrine reuptake inhibitors, and tricyclic antidepressants.
Dr. Vitarello and Dr. Yang had no disclosures.
FROM ACC 2021
Exercise plus liraglutide better for maintaining weight loss than either strategy alone
For persons with obesity who lost a substantial amount of weight on a low-calorie diet, the combination of exercise and medication significantly improved weight-loss maintenance, and more so than either strategy alone, according to results of a randomized, head-to-head trial.
A year after starting moderate to vigorous exercise coupled with liraglutide treatment, study participants had a weight loss 9.5 kg more than those who received placebo and usual activity, study results show.
Reductions in both weight and fat loss seen with exercise and liraglutide was roughly twice as much as what was achieved at 1 year with the strategies of liraglutide or exercise alone, according to authors of the study, which appears in the New England Journal of Medicine .
Although the findings may not apply to those who can’t or won’t perform moderate to vigorous exercise, the intervention in this study was nevertheless feasible in this group of persons with obesity who had a very low level of fitness, according to the authors.
Hope for healthy weight loss maintenance
Investigator Signe S. Torekov, PhD, said in an interview that these results provide hope that more-intensive exercise regimens, with or without medication, can be useful and well accepted among individuals struggling with obesity.
“When we started our study, we were told, ‘you are never going to have people with obesity exercising that much, and for that long’ – but people were actually very happy about the exercise,” said Dr. Torekov, a professor in the department of biomedical sciences at the University of Copenhagen.
“If you actually set up a program where people are monitored and you have a feedback system, then exercise is an excellent component in obesity treatment that should be much more actively used – not only for its weight-lowering component, but also for improving health and quality of life,” she said in an interview.
Weight-management specialist John D. Clark, MD, PhD, said results of this study can be used to help inform patients about how successful different strategies incorporating exercise and medication may be following initial weight loss.
“When patients plateau on a consistent, calorie-restricted dietary plan, we can educate them and manage expectations about what options may be available to them after their initial weight loss,” said Dr. Clark, of the University of Texas, Dallas.
“If the patient’s goal specifically is weight loss at all costs, then I may suggest, ‘let’s consider liraglutide or liraglutide in combination with exercise,’ ” he said in an interview. “Exercise improves body composition, even if it may not on its own be as successful in the next phase of their weight-loss journey, as shown in this study.”
Obesity and weight-loss challenges
Although it’s not uncommon for obese patients to lose a large amount of weight, keeping the weight off is frequently a challenge unless the patient follows a structured weight maintenance program, according to Dr. Torekov and coauthors.
The rapid weight regain seen in many obese patients could be a result of reductions in total energy expenditure or increased appetite. Exercise is one strategy to sustain weight loss, though according to the authors, very few studies have looked at exercise in isolation to quantify its contribution to maintenance.
Accordingly, the present study sought to determine whether exercise, medication, or the combination thereof works best to keep weight off.
The study incorporated liraglutide, a GLP-1 receptor agonist indicated for chronic weight management, along with a reduced-calorie diet and increased physical activity, in adults with elevated body mass index and at least one weight-related comorbidity.
The investigator-initiated phase 3 trial included 215 adults with a body mass index of 32-43. Individuals with type 2 diabetes were excluded. All participants followed an 8-week, low-calorie diet comprising 800 calories per day.
Participants who lost 5% or more of their body weight were then randomized to 1 year of exercise plus liraglutide, exercise plus placebo, usual activity plus liraglutide, or usual activity plus placebo.
The exercise program – which was structured but flexible, according to investigators – included group exercise sessions that incorporated 30 minutes of indoor cycling and 15 minutes of circuit training 2 days each week. Participants wore heart rate monitors during exercise to make sure they reached targets for moderate to vigorous intensity.
Instructors trained in exercise physiology planned and monitored individualized exercise programs for each participant in the exercise-medication or exercise-only arms of the study.
Participants in all groups attended 12 one-on-one consultations where body weight was measured and dietetic support was provided.
Weight loss with exercise and medication
Out of 215 individuals enrolled in the study, 195 lost at least 5% of body weight and continued on to the randomized portion, the investigators reported. During the diet phase, they lost a mean of 13.1 kg, translating into a 12% mean reduction in body weight.
The mean frequency of exercise was 2.4 times per week in the exercise-plus-medication group and 2.5 times per week in the exercise-only group. About one-third of the exercise took place in the group sessions, and there was no difference in relative intensity between group and individual exercise regimens, the investigators said.
Individuals in the exercise plus medication group continued to lose more weight, such that, at the end of 1 year, the weight loss decreased even further, by a mean of –3.4 kg. By contrast, weight increased by a mean of 6.1 kg for the placebo group, adding up to a treatment difference of –9.5 kg (95% confidence interval, –13.1 to –5.9; P < .001), according to the report.
That treatment effect was also seen, but more muted, in the exercise- and liraglutide-only groups, at –4.1 kg and –6.8 kg, respectively.
A significant treatment effect was observed for exercise plus liraglutide, compared with exercise alone, at –5.4 kg (P = .004), while the treatment effect for the combination versus liraglutide alone was not significant at –2.7 kg (P = .13), the data show.
Body-fat reduction at 52 weeks was –3.9 percentage points for exercise plus liraglutide as compared with placebo, or roughly twice the reductions seen in the exercise- and liraglutide-alone groups, the investigators said, adding that the combination preserved lean mass.
Reductions in hemoglobin A1c, which are generally thought to reduce diabetes risk, were reduced in both the liraglutide and liraglutide-exercise combination group, according to their report.
The research was supported in part by grants from the Novo Nordisk Foundation.
For persons with obesity who lost a substantial amount of weight on a low-calorie diet, the combination of exercise and medication significantly improved weight-loss maintenance, and more so than either strategy alone, according to results of a randomized, head-to-head trial.
A year after starting moderate to vigorous exercise coupled with liraglutide treatment, study participants had a weight loss 9.5 kg more than those who received placebo and usual activity, study results show.
Reductions in both weight and fat loss seen with exercise and liraglutide was roughly twice as much as what was achieved at 1 year with the strategies of liraglutide or exercise alone, according to authors of the study, which appears in the New England Journal of Medicine .
Although the findings may not apply to those who can’t or won’t perform moderate to vigorous exercise, the intervention in this study was nevertheless feasible in this group of persons with obesity who had a very low level of fitness, according to the authors.
Hope for healthy weight loss maintenance
Investigator Signe S. Torekov, PhD, said in an interview that these results provide hope that more-intensive exercise regimens, with or without medication, can be useful and well accepted among individuals struggling with obesity.
“When we started our study, we were told, ‘you are never going to have people with obesity exercising that much, and for that long’ – but people were actually very happy about the exercise,” said Dr. Torekov, a professor in the department of biomedical sciences at the University of Copenhagen.
“If you actually set up a program where people are monitored and you have a feedback system, then exercise is an excellent component in obesity treatment that should be much more actively used – not only for its weight-lowering component, but also for improving health and quality of life,” she said in an interview.
Weight-management specialist John D. Clark, MD, PhD, said results of this study can be used to help inform patients about how successful different strategies incorporating exercise and medication may be following initial weight loss.
“When patients plateau on a consistent, calorie-restricted dietary plan, we can educate them and manage expectations about what options may be available to them after their initial weight loss,” said Dr. Clark, of the University of Texas, Dallas.
“If the patient’s goal specifically is weight loss at all costs, then I may suggest, ‘let’s consider liraglutide or liraglutide in combination with exercise,’ ” he said in an interview. “Exercise improves body composition, even if it may not on its own be as successful in the next phase of their weight-loss journey, as shown in this study.”
Obesity and weight-loss challenges
Although it’s not uncommon for obese patients to lose a large amount of weight, keeping the weight off is frequently a challenge unless the patient follows a structured weight maintenance program, according to Dr. Torekov and coauthors.
The rapid weight regain seen in many obese patients could be a result of reductions in total energy expenditure or increased appetite. Exercise is one strategy to sustain weight loss, though according to the authors, very few studies have looked at exercise in isolation to quantify its contribution to maintenance.
Accordingly, the present study sought to determine whether exercise, medication, or the combination thereof works best to keep weight off.
The study incorporated liraglutide, a GLP-1 receptor agonist indicated for chronic weight management, along with a reduced-calorie diet and increased physical activity, in adults with elevated body mass index and at least one weight-related comorbidity.
The investigator-initiated phase 3 trial included 215 adults with a body mass index of 32-43. Individuals with type 2 diabetes were excluded. All participants followed an 8-week, low-calorie diet comprising 800 calories per day.
Participants who lost 5% or more of their body weight were then randomized to 1 year of exercise plus liraglutide, exercise plus placebo, usual activity plus liraglutide, or usual activity plus placebo.
The exercise program – which was structured but flexible, according to investigators – included group exercise sessions that incorporated 30 minutes of indoor cycling and 15 minutes of circuit training 2 days each week. Participants wore heart rate monitors during exercise to make sure they reached targets for moderate to vigorous intensity.
Instructors trained in exercise physiology planned and monitored individualized exercise programs for each participant in the exercise-medication or exercise-only arms of the study.
Participants in all groups attended 12 one-on-one consultations where body weight was measured and dietetic support was provided.
Weight loss with exercise and medication
Out of 215 individuals enrolled in the study, 195 lost at least 5% of body weight and continued on to the randomized portion, the investigators reported. During the diet phase, they lost a mean of 13.1 kg, translating into a 12% mean reduction in body weight.
The mean frequency of exercise was 2.4 times per week in the exercise-plus-medication group and 2.5 times per week in the exercise-only group. About one-third of the exercise took place in the group sessions, and there was no difference in relative intensity between group and individual exercise regimens, the investigators said.
Individuals in the exercise plus medication group continued to lose more weight, such that, at the end of 1 year, the weight loss decreased even further, by a mean of –3.4 kg. By contrast, weight increased by a mean of 6.1 kg for the placebo group, adding up to a treatment difference of –9.5 kg (95% confidence interval, –13.1 to –5.9; P < .001), according to the report.
That treatment effect was also seen, but more muted, in the exercise- and liraglutide-only groups, at –4.1 kg and –6.8 kg, respectively.
A significant treatment effect was observed for exercise plus liraglutide, compared with exercise alone, at –5.4 kg (P = .004), while the treatment effect for the combination versus liraglutide alone was not significant at –2.7 kg (P = .13), the data show.
Body-fat reduction at 52 weeks was –3.9 percentage points for exercise plus liraglutide as compared with placebo, or roughly twice the reductions seen in the exercise- and liraglutide-alone groups, the investigators said, adding that the combination preserved lean mass.
Reductions in hemoglobin A1c, which are generally thought to reduce diabetes risk, were reduced in both the liraglutide and liraglutide-exercise combination group, according to their report.
The research was supported in part by grants from the Novo Nordisk Foundation.
For persons with obesity who lost a substantial amount of weight on a low-calorie diet, the combination of exercise and medication significantly improved weight-loss maintenance, and more so than either strategy alone, according to results of a randomized, head-to-head trial.
A year after starting moderate to vigorous exercise coupled with liraglutide treatment, study participants had a weight loss 9.5 kg more than those who received placebo and usual activity, study results show.
Reductions in both weight and fat loss seen with exercise and liraglutide was roughly twice as much as what was achieved at 1 year with the strategies of liraglutide or exercise alone, according to authors of the study, which appears in the New England Journal of Medicine .
Although the findings may not apply to those who can’t or won’t perform moderate to vigorous exercise, the intervention in this study was nevertheless feasible in this group of persons with obesity who had a very low level of fitness, according to the authors.
Hope for healthy weight loss maintenance
Investigator Signe S. Torekov, PhD, said in an interview that these results provide hope that more-intensive exercise regimens, with or without medication, can be useful and well accepted among individuals struggling with obesity.
“When we started our study, we were told, ‘you are never going to have people with obesity exercising that much, and for that long’ – but people were actually very happy about the exercise,” said Dr. Torekov, a professor in the department of biomedical sciences at the University of Copenhagen.
“If you actually set up a program where people are monitored and you have a feedback system, then exercise is an excellent component in obesity treatment that should be much more actively used – not only for its weight-lowering component, but also for improving health and quality of life,” she said in an interview.
Weight-management specialist John D. Clark, MD, PhD, said results of this study can be used to help inform patients about how successful different strategies incorporating exercise and medication may be following initial weight loss.
“When patients plateau on a consistent, calorie-restricted dietary plan, we can educate them and manage expectations about what options may be available to them after their initial weight loss,” said Dr. Clark, of the University of Texas, Dallas.
“If the patient’s goal specifically is weight loss at all costs, then I may suggest, ‘let’s consider liraglutide or liraglutide in combination with exercise,’ ” he said in an interview. “Exercise improves body composition, even if it may not on its own be as successful in the next phase of their weight-loss journey, as shown in this study.”
Obesity and weight-loss challenges
Although it’s not uncommon for obese patients to lose a large amount of weight, keeping the weight off is frequently a challenge unless the patient follows a structured weight maintenance program, according to Dr. Torekov and coauthors.
The rapid weight regain seen in many obese patients could be a result of reductions in total energy expenditure or increased appetite. Exercise is one strategy to sustain weight loss, though according to the authors, very few studies have looked at exercise in isolation to quantify its contribution to maintenance.
Accordingly, the present study sought to determine whether exercise, medication, or the combination thereof works best to keep weight off.
The study incorporated liraglutide, a GLP-1 receptor agonist indicated for chronic weight management, along with a reduced-calorie diet and increased physical activity, in adults with elevated body mass index and at least one weight-related comorbidity.
The investigator-initiated phase 3 trial included 215 adults with a body mass index of 32-43. Individuals with type 2 diabetes were excluded. All participants followed an 8-week, low-calorie diet comprising 800 calories per day.
Participants who lost 5% or more of their body weight were then randomized to 1 year of exercise plus liraglutide, exercise plus placebo, usual activity plus liraglutide, or usual activity plus placebo.
The exercise program – which was structured but flexible, according to investigators – included group exercise sessions that incorporated 30 minutes of indoor cycling and 15 minutes of circuit training 2 days each week. Participants wore heart rate monitors during exercise to make sure they reached targets for moderate to vigorous intensity.
Instructors trained in exercise physiology planned and monitored individualized exercise programs for each participant in the exercise-medication or exercise-only arms of the study.
Participants in all groups attended 12 one-on-one consultations where body weight was measured and dietetic support was provided.
Weight loss with exercise and medication
Out of 215 individuals enrolled in the study, 195 lost at least 5% of body weight and continued on to the randomized portion, the investigators reported. During the diet phase, they lost a mean of 13.1 kg, translating into a 12% mean reduction in body weight.
The mean frequency of exercise was 2.4 times per week in the exercise-plus-medication group and 2.5 times per week in the exercise-only group. About one-third of the exercise took place in the group sessions, and there was no difference in relative intensity between group and individual exercise regimens, the investigators said.
Individuals in the exercise plus medication group continued to lose more weight, such that, at the end of 1 year, the weight loss decreased even further, by a mean of –3.4 kg. By contrast, weight increased by a mean of 6.1 kg for the placebo group, adding up to a treatment difference of –9.5 kg (95% confidence interval, –13.1 to –5.9; P < .001), according to the report.
That treatment effect was also seen, but more muted, in the exercise- and liraglutide-only groups, at –4.1 kg and –6.8 kg, respectively.
A significant treatment effect was observed for exercise plus liraglutide, compared with exercise alone, at –5.4 kg (P = .004), while the treatment effect for the combination versus liraglutide alone was not significant at –2.7 kg (P = .13), the data show.
Body-fat reduction at 52 weeks was –3.9 percentage points for exercise plus liraglutide as compared with placebo, or roughly twice the reductions seen in the exercise- and liraglutide-alone groups, the investigators said, adding that the combination preserved lean mass.
Reductions in hemoglobin A1c, which are generally thought to reduce diabetes risk, were reduced in both the liraglutide and liraglutide-exercise combination group, according to their report.
The research was supported in part by grants from the Novo Nordisk Foundation.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Increasing salt intake proves beneficial in POTS
For patients with postural tachycardia syndrome (POTS), dietary sodium intake can be increased more confidently, suggests the first study to yield solid evidence to support this treatment strategy.
The results showed that high dietary sodium intake can lower plasma norepinephrine levels and ameliorate standing and orthostatic tachycardia for patients with POTS.
“These results suggest that increasing dietary salt is a good rationale for treatment of this condition, and this study gives reassurance we are doing the right thing for POTS patients by increasing their sodium intake,” senior author Satish R. Raj, MD, said in an interview.
The study, with lead author Emily M. Garland, PhD, was published online April 26, 2021, in the Journal of the American College of Cardiology.
Dr. Raj, who is professor of cardiac science at the University of Calgary (Alta.), explained that POTS includes a spectrum of disorders that affect the automatic nervous system, which regulates heart rate and blood pressure.
“It is a disorder of orthostatic intolerance – patients feel better when they lie down. It differs from orthostatic hypotension in that, when a POTS patient stands up, the blood pressure does not necessarily drop, but the heart rate increases excessively.”
Although it is normal for the heart rate to increase somewhat on standing, among patients with POTS, the heart rate increases excessively. The condition is defined as an orthostatic heart rate increase of at least 30 beats/min (or 40 beats/min among individuals aged 12-19 years ) in the absence of orthostatic hypotension.
The disorder is characterized by a range of symptoms, including lightheadedness, shortness of breath, palpitations, and exertional intolerance, that are worse when in an upright position. Patients also experience chronic fatigue and perceived cognitive impairment, Dr. Raj noted.
The typical demographic for POTS is young women; the condition often starts during the teenage years.
Patients often have low blood volume, so one approach to treatment is to increase the intake of salt and water so as to increase blood volume.
“This is one of the mainstays of treatment, but it has never really been properly studied,” Dr. Raj commented. Increasing salt intake “is an unusual message from a cardiologist, and there have been concerns that we are making recommendations against traditional advice, so we urgently need evidence to support this recommendation.”
The current crossover study enrolled 14 patients with POTS and 13 healthy control persons who, over a period of 6 days, underwent treatment with a low-sodium diet (10 mEq sodium per day) or a high-sodium diet (300 mEq sodium per day).
Supine and standing heart rate, blood pressure, serum aldosterone level, plasma renin activity, blood volume, and plasma norepinephrine and epinephrine levels were measured.
Results showed that, among the POTS patients, the high-sodium diet reduced upright heart rate and the change in heart rate on standing, compared with the low-sodium diet.
Heart rate increased by 46 beats/min with the high-sodium diet versus 60 beats/min with the low-sodium diet.
Total blood volume and plasma volume increased, and standing norepinephrine levels decreased with the high-sodium diet, compared with the low-sodium diet.
However, upright heart rate, change in heart rate, and upright norepinephrine levels remained higher among POTS patients than among control persons receiving the high-sodium diet.
There was a nonsignificant trend for a lower symptom burden score among the POTS patients who received the high-sodium diet in comparison with those taking the low-sodium diet. Scores for mental confusion, palpitations, lightheadedness, and headache trending downward on the high-sodium diet.
“We found that high levels of dietary salt did what we hoped, with increased blood volume and reduced norepinephrine levels on standing and reduced excessive increase in heart rate. While it didn’t completely normalize heart rate, this was reduced significantly,” Dr. Raj said.
Another observation from the study was that the increased salt intake seemed to be beneficial across the whole spectrum of patients.
“There are some patients who have very high levels of sympathetic activation, and there have been anecdotal reports that increasing salt may not work so well in this group,” he said. “In this study, we didn’t differentiate, but average norepinephrine levels were very high, and many patients would be considered to be hyperadrenergic. Our results suggest this treatment will help these patients too.”
He noted that sodium intake was increased in this study just through diet. “We had a special metabolic kitchen. In clinical practice, we advise patients to add regular table salt to their food, and we only use salt tablets when they cannot tolerate so much salt in their diet.”
Recognizing that there may be concerns about hypertension with long-term use of such a treatment, Dr. Raj said there were no signs of an increase in blood pressure in this study. “But this should be considered a short-term therapy for the time being, and patients need to be reassessed every few years as their physiology changes.”
The authors estimated that POTS affects up to 1% of the population. Because there is no diagnostic code for the condition at present, all incidence data are estimates.
Dr. Raj pointed out that potentially a lot of people are affected, but there is little recognition of the condition among patients and physicians.
“Many family doctors are unaware of POTS,” he noted. “Patients often have to research their symptoms themselves and inform their doctor of the condition. Many patients wait years and often see many different doctors before getting a correct diagnosis.”
He explained that patients with POTS are often diagnosed as having a psychiatric illness. “They are mainly young women with palpitations, heart pounding, shakiness, which is often labeled as anxiety.”
Dr. Raj urged clinicians to consider POTS if patients have symptoms that are worse when standing up. The diagnosis is confirmed if their blood pressure doesn’t fall when standing up but their heart rate increases by at least 30 beats/min.
He noted that not enough specialists treat this condition, so family doctors need to be able to diagnose and initiate treatment. If more aggressive treatment is required, patients can be referred to a specialist.
“One of the problems is that this condition pans across different medical specialties. No one field owns it, so it tends to get ignored. But there are clinicians who are interested in POTS, and the key is finding one of these,” he said.
“We have finally established that this high-sodium diet works as treatment for POTS,” he concluded. “We have been using it for some time, but now we have evidence for its use across the whole spectrum of patients.”
In an accompanying editorial (J Am Coll Cardiol. 2021 May 4;77[17]:2185-2186), Blair P. Grubb, MD, University of Toledo (Ohio) Medical Center, wrote that this “superb study by Garland et al. helps better establish our understanding of the pathophysiologic process taking place in POTS while at the same time providing good evidence for the augmentation of dietary sodium as one of the cornerstones of treatment.”
He added that the field needs more such studies “in our quest to better understand POTS and to elaborate therapeutic modalities to help those suffering from this debilitating illness.”
The study was supported in part by the National Heart, Lung, and Blood Institute; the National Center for Advancing Translational Sciences; and the Vanderbilt Hormone and Analytical Services Core. Dr. Raj has served as a consultant for Lundbeck NA and Theravance; has served as chair of the data safety and monitoring board for Arena Pharmaceuticals and as Cardiac Arrhythmia Network of Canada network investigator; and has served on the medical advisory board of Dysautonomia International and PoTS UK, both without financial compensation.
A version of this article first appeared on Medscape.com.
For patients with postural tachycardia syndrome (POTS), dietary sodium intake can be increased more confidently, suggests the first study to yield solid evidence to support this treatment strategy.
The results showed that high dietary sodium intake can lower plasma norepinephrine levels and ameliorate standing and orthostatic tachycardia for patients with POTS.
“These results suggest that increasing dietary salt is a good rationale for treatment of this condition, and this study gives reassurance we are doing the right thing for POTS patients by increasing their sodium intake,” senior author Satish R. Raj, MD, said in an interview.
The study, with lead author Emily M. Garland, PhD, was published online April 26, 2021, in the Journal of the American College of Cardiology.
Dr. Raj, who is professor of cardiac science at the University of Calgary (Alta.), explained that POTS includes a spectrum of disorders that affect the automatic nervous system, which regulates heart rate and blood pressure.
“It is a disorder of orthostatic intolerance – patients feel better when they lie down. It differs from orthostatic hypotension in that, when a POTS patient stands up, the blood pressure does not necessarily drop, but the heart rate increases excessively.”
Although it is normal for the heart rate to increase somewhat on standing, among patients with POTS, the heart rate increases excessively. The condition is defined as an orthostatic heart rate increase of at least 30 beats/min (or 40 beats/min among individuals aged 12-19 years ) in the absence of orthostatic hypotension.
The disorder is characterized by a range of symptoms, including lightheadedness, shortness of breath, palpitations, and exertional intolerance, that are worse when in an upright position. Patients also experience chronic fatigue and perceived cognitive impairment, Dr. Raj noted.
The typical demographic for POTS is young women; the condition often starts during the teenage years.
Patients often have low blood volume, so one approach to treatment is to increase the intake of salt and water so as to increase blood volume.
“This is one of the mainstays of treatment, but it has never really been properly studied,” Dr. Raj commented. Increasing salt intake “is an unusual message from a cardiologist, and there have been concerns that we are making recommendations against traditional advice, so we urgently need evidence to support this recommendation.”
The current crossover study enrolled 14 patients with POTS and 13 healthy control persons who, over a period of 6 days, underwent treatment with a low-sodium diet (10 mEq sodium per day) or a high-sodium diet (300 mEq sodium per day).
Supine and standing heart rate, blood pressure, serum aldosterone level, plasma renin activity, blood volume, and plasma norepinephrine and epinephrine levels were measured.
Results showed that, among the POTS patients, the high-sodium diet reduced upright heart rate and the change in heart rate on standing, compared with the low-sodium diet.
Heart rate increased by 46 beats/min with the high-sodium diet versus 60 beats/min with the low-sodium diet.
Total blood volume and plasma volume increased, and standing norepinephrine levels decreased with the high-sodium diet, compared with the low-sodium diet.
However, upright heart rate, change in heart rate, and upright norepinephrine levels remained higher among POTS patients than among control persons receiving the high-sodium diet.
There was a nonsignificant trend for a lower symptom burden score among the POTS patients who received the high-sodium diet in comparison with those taking the low-sodium diet. Scores for mental confusion, palpitations, lightheadedness, and headache trending downward on the high-sodium diet.
“We found that high levels of dietary salt did what we hoped, with increased blood volume and reduced norepinephrine levels on standing and reduced excessive increase in heart rate. While it didn’t completely normalize heart rate, this was reduced significantly,” Dr. Raj said.
Another observation from the study was that the increased salt intake seemed to be beneficial across the whole spectrum of patients.
“There are some patients who have very high levels of sympathetic activation, and there have been anecdotal reports that increasing salt may not work so well in this group,” he said. “In this study, we didn’t differentiate, but average norepinephrine levels were very high, and many patients would be considered to be hyperadrenergic. Our results suggest this treatment will help these patients too.”
He noted that sodium intake was increased in this study just through diet. “We had a special metabolic kitchen. In clinical practice, we advise patients to add regular table salt to their food, and we only use salt tablets when they cannot tolerate so much salt in their diet.”
Recognizing that there may be concerns about hypertension with long-term use of such a treatment, Dr. Raj said there were no signs of an increase in blood pressure in this study. “But this should be considered a short-term therapy for the time being, and patients need to be reassessed every few years as their physiology changes.”
The authors estimated that POTS affects up to 1% of the population. Because there is no diagnostic code for the condition at present, all incidence data are estimates.
Dr. Raj pointed out that potentially a lot of people are affected, but there is little recognition of the condition among patients and physicians.
“Many family doctors are unaware of POTS,” he noted. “Patients often have to research their symptoms themselves and inform their doctor of the condition. Many patients wait years and often see many different doctors before getting a correct diagnosis.”
He explained that patients with POTS are often diagnosed as having a psychiatric illness. “They are mainly young women with palpitations, heart pounding, shakiness, which is often labeled as anxiety.”
Dr. Raj urged clinicians to consider POTS if patients have symptoms that are worse when standing up. The diagnosis is confirmed if their blood pressure doesn’t fall when standing up but their heart rate increases by at least 30 beats/min.
He noted that not enough specialists treat this condition, so family doctors need to be able to diagnose and initiate treatment. If more aggressive treatment is required, patients can be referred to a specialist.
“One of the problems is that this condition pans across different medical specialties. No one field owns it, so it tends to get ignored. But there are clinicians who are interested in POTS, and the key is finding one of these,” he said.
“We have finally established that this high-sodium diet works as treatment for POTS,” he concluded. “We have been using it for some time, but now we have evidence for its use across the whole spectrum of patients.”
In an accompanying editorial (J Am Coll Cardiol. 2021 May 4;77[17]:2185-2186), Blair P. Grubb, MD, University of Toledo (Ohio) Medical Center, wrote that this “superb study by Garland et al. helps better establish our understanding of the pathophysiologic process taking place in POTS while at the same time providing good evidence for the augmentation of dietary sodium as one of the cornerstones of treatment.”
He added that the field needs more such studies “in our quest to better understand POTS and to elaborate therapeutic modalities to help those suffering from this debilitating illness.”
The study was supported in part by the National Heart, Lung, and Blood Institute; the National Center for Advancing Translational Sciences; and the Vanderbilt Hormone and Analytical Services Core. Dr. Raj has served as a consultant for Lundbeck NA and Theravance; has served as chair of the data safety and monitoring board for Arena Pharmaceuticals and as Cardiac Arrhythmia Network of Canada network investigator; and has served on the medical advisory board of Dysautonomia International and PoTS UK, both without financial compensation.
A version of this article first appeared on Medscape.com.
For patients with postural tachycardia syndrome (POTS), dietary sodium intake can be increased more confidently, suggests the first study to yield solid evidence to support this treatment strategy.
The results showed that high dietary sodium intake can lower plasma norepinephrine levels and ameliorate standing and orthostatic tachycardia for patients with POTS.
“These results suggest that increasing dietary salt is a good rationale for treatment of this condition, and this study gives reassurance we are doing the right thing for POTS patients by increasing their sodium intake,” senior author Satish R. Raj, MD, said in an interview.
The study, with lead author Emily M. Garland, PhD, was published online April 26, 2021, in the Journal of the American College of Cardiology.
Dr. Raj, who is professor of cardiac science at the University of Calgary (Alta.), explained that POTS includes a spectrum of disorders that affect the automatic nervous system, which regulates heart rate and blood pressure.
“It is a disorder of orthostatic intolerance – patients feel better when they lie down. It differs from orthostatic hypotension in that, when a POTS patient stands up, the blood pressure does not necessarily drop, but the heart rate increases excessively.”
Although it is normal for the heart rate to increase somewhat on standing, among patients with POTS, the heart rate increases excessively. The condition is defined as an orthostatic heart rate increase of at least 30 beats/min (or 40 beats/min among individuals aged 12-19 years ) in the absence of orthostatic hypotension.
The disorder is characterized by a range of symptoms, including lightheadedness, shortness of breath, palpitations, and exertional intolerance, that are worse when in an upright position. Patients also experience chronic fatigue and perceived cognitive impairment, Dr. Raj noted.
The typical demographic for POTS is young women; the condition often starts during the teenage years.
Patients often have low blood volume, so one approach to treatment is to increase the intake of salt and water so as to increase blood volume.
“This is one of the mainstays of treatment, but it has never really been properly studied,” Dr. Raj commented. Increasing salt intake “is an unusual message from a cardiologist, and there have been concerns that we are making recommendations against traditional advice, so we urgently need evidence to support this recommendation.”
The current crossover study enrolled 14 patients with POTS and 13 healthy control persons who, over a period of 6 days, underwent treatment with a low-sodium diet (10 mEq sodium per day) or a high-sodium diet (300 mEq sodium per day).
Supine and standing heart rate, blood pressure, serum aldosterone level, plasma renin activity, blood volume, and plasma norepinephrine and epinephrine levels were measured.
Results showed that, among the POTS patients, the high-sodium diet reduced upright heart rate and the change in heart rate on standing, compared with the low-sodium diet.
Heart rate increased by 46 beats/min with the high-sodium diet versus 60 beats/min with the low-sodium diet.
Total blood volume and plasma volume increased, and standing norepinephrine levels decreased with the high-sodium diet, compared with the low-sodium diet.
However, upright heart rate, change in heart rate, and upright norepinephrine levels remained higher among POTS patients than among control persons receiving the high-sodium diet.
There was a nonsignificant trend for a lower symptom burden score among the POTS patients who received the high-sodium diet in comparison with those taking the low-sodium diet. Scores for mental confusion, palpitations, lightheadedness, and headache trending downward on the high-sodium diet.
“We found that high levels of dietary salt did what we hoped, with increased blood volume and reduced norepinephrine levels on standing and reduced excessive increase in heart rate. While it didn’t completely normalize heart rate, this was reduced significantly,” Dr. Raj said.
Another observation from the study was that the increased salt intake seemed to be beneficial across the whole spectrum of patients.
“There are some patients who have very high levels of sympathetic activation, and there have been anecdotal reports that increasing salt may not work so well in this group,” he said. “In this study, we didn’t differentiate, but average norepinephrine levels were very high, and many patients would be considered to be hyperadrenergic. Our results suggest this treatment will help these patients too.”
He noted that sodium intake was increased in this study just through diet. “We had a special metabolic kitchen. In clinical practice, we advise patients to add regular table salt to their food, and we only use salt tablets when they cannot tolerate so much salt in their diet.”
Recognizing that there may be concerns about hypertension with long-term use of such a treatment, Dr. Raj said there were no signs of an increase in blood pressure in this study. “But this should be considered a short-term therapy for the time being, and patients need to be reassessed every few years as their physiology changes.”
The authors estimated that POTS affects up to 1% of the population. Because there is no diagnostic code for the condition at present, all incidence data are estimates.
Dr. Raj pointed out that potentially a lot of people are affected, but there is little recognition of the condition among patients and physicians.
“Many family doctors are unaware of POTS,” he noted. “Patients often have to research their symptoms themselves and inform their doctor of the condition. Many patients wait years and often see many different doctors before getting a correct diagnosis.”
He explained that patients with POTS are often diagnosed as having a psychiatric illness. “They are mainly young women with palpitations, heart pounding, shakiness, which is often labeled as anxiety.”
Dr. Raj urged clinicians to consider POTS if patients have symptoms that are worse when standing up. The diagnosis is confirmed if their blood pressure doesn’t fall when standing up but their heart rate increases by at least 30 beats/min.
He noted that not enough specialists treat this condition, so family doctors need to be able to diagnose and initiate treatment. If more aggressive treatment is required, patients can be referred to a specialist.
“One of the problems is that this condition pans across different medical specialties. No one field owns it, so it tends to get ignored. But there are clinicians who are interested in POTS, and the key is finding one of these,” he said.
“We have finally established that this high-sodium diet works as treatment for POTS,” he concluded. “We have been using it for some time, but now we have evidence for its use across the whole spectrum of patients.”
In an accompanying editorial (J Am Coll Cardiol. 2021 May 4;77[17]:2185-2186), Blair P. Grubb, MD, University of Toledo (Ohio) Medical Center, wrote that this “superb study by Garland et al. helps better establish our understanding of the pathophysiologic process taking place in POTS while at the same time providing good evidence for the augmentation of dietary sodium as one of the cornerstones of treatment.”
He added that the field needs more such studies “in our quest to better understand POTS and to elaborate therapeutic modalities to help those suffering from this debilitating illness.”
The study was supported in part by the National Heart, Lung, and Blood Institute; the National Center for Advancing Translational Sciences; and the Vanderbilt Hormone and Analytical Services Core. Dr. Raj has served as a consultant for Lundbeck NA and Theravance; has served as chair of the data safety and monitoring board for Arena Pharmaceuticals and as Cardiac Arrhythmia Network of Canada network investigator; and has served on the medical advisory board of Dysautonomia International and PoTS UK, both without financial compensation.
A version of this article first appeared on Medscape.com.
Brown plaque on the arm
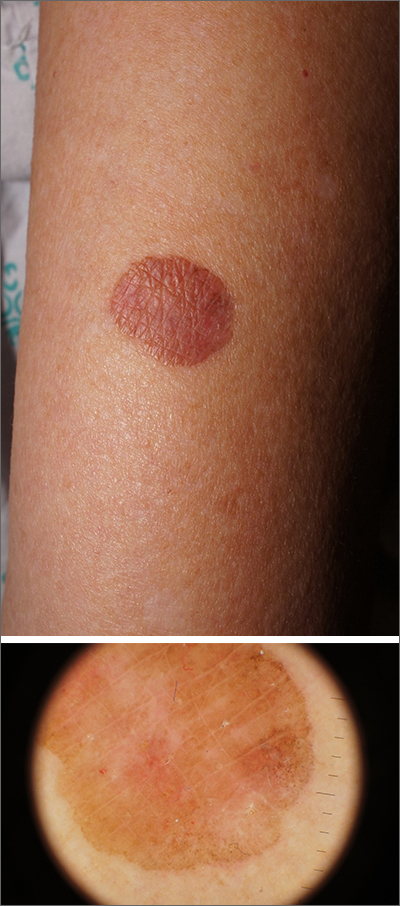
Due to its size, 2 shave biopsies targeting the most concerning portions of the lesion were performed; the results were consistent with a lichenoid keratosis (LK), also known as lichen planus-like keratosis.
LK is a benign solitary lesion that mimics basal cell carcinoma, squamous cell carcinoma, and superficial spreading or amelanotic melanoma.1 One theory suggests that LK is a solar lentigo or actinic keratosis undergoing attack from the immune system. Lesions most often manifest as a pink, gray, or brown macule to thin papule on the trunk or extremities. Itching or mild pain may be present. Dermoscopy can help distinguish an LK from malignancy but overlapping features of fine dark regression structures (called peppering, as seen in this case) should prompt further evaluation.
LKs are great mimics and biopsy is key to distinguishing them from cancer. In this case, shave biopsies were performed in the thickest and most characteristic portions of the lesion. Punch or incisional biopsies also would have been appropriate, but any result would have been a partial result. If the result had come back as an atypical melanocytic lesion, a complete excision would have been necessary to make sure the pathology reflected the entirety of the lesion.
Armed with the knowledge that the LK was benign, the patient in this case was scheduled for a follow-up visit for cryotherapy to remove the residual lesion.
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).
1. Maor D, Ondhia C, Yu LL, et al. Lichenoid keratosis is frequently misdiagnosed as basal cell carcinoma. Clin Exp Dermatol. 2017;42:663-666. doi: 10.1111/ced.13178

Due to its size, 2 shave biopsies targeting the most concerning portions of the lesion were performed; the results were consistent with a lichenoid keratosis (LK), also known as lichen planus-like keratosis.
LK is a benign solitary lesion that mimics basal cell carcinoma, squamous cell carcinoma, and superficial spreading or amelanotic melanoma.1 One theory suggests that LK is a solar lentigo or actinic keratosis undergoing attack from the immune system. Lesions most often manifest as a pink, gray, or brown macule to thin papule on the trunk or extremities. Itching or mild pain may be present. Dermoscopy can help distinguish an LK from malignancy but overlapping features of fine dark regression structures (called peppering, as seen in this case) should prompt further evaluation.
LKs are great mimics and biopsy is key to distinguishing them from cancer. In this case, shave biopsies were performed in the thickest and most characteristic portions of the lesion. Punch or incisional biopsies also would have been appropriate, but any result would have been a partial result. If the result had come back as an atypical melanocytic lesion, a complete excision would have been necessary to make sure the pathology reflected the entirety of the lesion.
Armed with the knowledge that the LK was benign, the patient in this case was scheduled for a follow-up visit for cryotherapy to remove the residual lesion.
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).

Due to its size, 2 shave biopsies targeting the most concerning portions of the lesion were performed; the results were consistent with a lichenoid keratosis (LK), also known as lichen planus-like keratosis.
LK is a benign solitary lesion that mimics basal cell carcinoma, squamous cell carcinoma, and superficial spreading or amelanotic melanoma.1 One theory suggests that LK is a solar lentigo or actinic keratosis undergoing attack from the immune system. Lesions most often manifest as a pink, gray, or brown macule to thin papule on the trunk or extremities. Itching or mild pain may be present. Dermoscopy can help distinguish an LK from malignancy but overlapping features of fine dark regression structures (called peppering, as seen in this case) should prompt further evaluation.
LKs are great mimics and biopsy is key to distinguishing them from cancer. In this case, shave biopsies were performed in the thickest and most characteristic portions of the lesion. Punch or incisional biopsies also would have been appropriate, but any result would have been a partial result. If the result had come back as an atypical melanocytic lesion, a complete excision would have been necessary to make sure the pathology reflected the entirety of the lesion.
Armed with the knowledge that the LK was benign, the patient in this case was scheduled for a follow-up visit for cryotherapy to remove the residual lesion.
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).
1. Maor D, Ondhia C, Yu LL, et al. Lichenoid keratosis is frequently misdiagnosed as basal cell carcinoma. Clin Exp Dermatol. 2017;42:663-666. doi: 10.1111/ced.13178
1. Maor D, Ondhia C, Yu LL, et al. Lichenoid keratosis is frequently misdiagnosed as basal cell carcinoma. Clin Exp Dermatol. 2017;42:663-666. doi: 10.1111/ced.13178
Look beyond liver biopsy for NAFLD diagnosis
Nonalcoholic fatty liver disease (NAFLD) was present in approximately two-thirds of patients who did not undergo a liver biopsy. These patients were more likely to be non-White and older, as well as have normal ALT levels, which shows potential gaps in knowledge about this population.
Data from studies of patients diagnosed with NAFLD that require biopsy among their inclusion criteria may be subject to selection and detection bias, wrote A. Sidney Barritt, MD, of the University of North Carolina at Chapel Hill, and colleagues. The researchers sought to compare characteristics of patients with NAFLD who were diagnosed using clinical criteria and those diagnosed via liver biopsy.
In a study published in Hepatology Communications, the researchers reviewed data from TARGET-NASH, a longitudinal, observational cohort study designed to follow patients with NAFLD in clinical practice to provide data on the effectiveness of treatments.
“TARGET-NASH represents a large cohort of NAFLD patients from multiple sites and can provide us with real world information on progression of disease in patients with NAFLD and particular risk factors that may be clinically relevant,” Zachary Henry, MD, MS, of the division of gastroenterology & hepatology at the University of Virginia Health System in Charlottesville, said in an interview. “This is one of the first studies from this database, and as time goes on, we will see more large-population data like this to answer specific questions for NAFLD patient.”
Surprising findings
The researchers included 3,474 patients aged 18 years and older who were enrolled in the TARGET-NASH study between Aug. 1, 2016, and March 4, 2019. The study participants were classified according to severity of liver disease: nonalcoholic fatty liver (30%), nonalcoholic steatohepatitis (37%), and NAFLD cirrhosis (33%).
A total of 766 patients were diagnosed with NASH based on clinical criteria without biopsy, and all met the criteria for abnormal ALT and steatosis based on imaging. In addition, these patients had at least one secondary diagnostic criteria: body mass index greater than 30 kg/m2 (74%), type 2 diabetes (42%), and dyslipidemia (54%). Significant independent predictors of liver biopsy included younger age, White race, female gender, diabetes, and elevated levels of ALT.
Elevated ALT increased the odds of liver biopsy by 14% per 10-point rise, according to the study. A machine learning model showed that non-White patients with ALT less than 69 IU/mL had a 6% chance of liver biopsy. By comparison, White patients had a 21% chance of biopsy with ALT between 29 IU/mL and 69 IU/mL that dropped to 10% if the ALT was less than 29 IU/mL.
However, ALT remains a “suboptimal surrogate” for disease severity, the researchers noted. “How a normal ALT is defined and how a normal ALT range may vary across different laboratories may play a role in its utility as a diagnostic tool as well.”
Dr. Henry was surprised by this finding: “With the advent of noninvasive measures of fibrosis, such as the NAFLD fibrosis score, Fibrosis-4, and transient elastography, I thought these would have a more significant role in that decision as opposed to ALT levels.”
Notably, mental health diagnoses accounted for nearly half (49%) of comorbid conditions, followed by cardiovascular disease (19%), and osteoarthritis (10%). The prevalence of these conditions emphasizes the challenges of managing patients with NAFLD with diet and exercise alone because mental and physical problems may impede progress, the researchers wrote.
The study findings were limited by several factors including the inability to determine health care provider intent, as well as undocumented factors related to patients and providers that might influence a biopsy decision, such as assessment of disease severity, the researchers noted. In addition, they noted that the mostly White study population treated in specialty settings might not generalize to other populations or primary care.
However, the findings are strengthened by the large study population and real-world setting, the researchers emphasized. “These data provide context for the selection bias that may be present in many registries and randomized, controlled trials of therapies for NAFLD, where biopsy is required for inclusion,” and show potential knowledge gaps about the patient population less likely to undergo biopsy.
Knowledge gaps and implications
The study is important because of the need to identify patient factors that predict histologic versus clinical diagnosis of NAFLD as the number of patient registries and clinical trials for NAFLD increase, Bubu Banini, MD, of Yale University, New Haven, Conn., said in an interview. “This information helps to elucidate selection and ascertainment bias and place findings from NAFLD registries and clinical trials into context.”
Dr. Banini said that some of the findings were to be expected, while others were not.
“Historically, males and non-Whites are less likely to participate in registries and clinical trials, compared to females and Whites. However, I was surprised to find that these discrepancies further paralleled the likelihood of undergoing liver biopsy even among those who chose to participate. In addition, while mental health disorders (such as anxiety and depression) are a fairly prevalent comorbidity in patients with NAFLD, I was surprised to find that NAFLD patients with mental health disorders were more likely to undergo liver biopsy compared to those without these disorders. I would have expected the reverse,” he noted.
“These findings highlight the gaps in knowledge regarding the impact of demographic and psychosocial factors on choice and assess to care among patients with NAFLD, and the need for further studies to address these gaps,” she emphasized.
“A number of [studies] such as TARGET-NASH are doing away with the requirement for liver biopsy for participation; hence, it is less likely that selection bias related to liver biopsy would be a problem in these [studies] if clinical diagnosis is considered as a surrogate for histological diagnosis,” Dr. Banini added.
“On the contrary, many NAFLD clinical trials require liver biopsy for inclusion.” As nicely demonstrated in the current study, “this inclusion criterion may introduce selection bias,” she said. “Awareness of potential biases would hopefully inform the design and recruitment strategy for registries and clinical trials in order to overcome these issues.”
“I think the results of this study may actually point to a larger issue within medicine in general, which is a difference in care provided to minority communities,” Dr. Henry said. “Whether this is intentional, related to unconscious bias on the part of providers, or related to a significant mistrust between minority communities and their health care providers is unclear but certainly needs to be addressed.”
He noted that the purpose of TARGET-NASH is to enroll all patients with NAFLD regardless of biopsy. “Over time, as we have more data on these patients, we will have a better understanding of both diagnostic and therapeutic decisions in patients with NAFLD.”
The study was supported by Target RWE, sponsor of the TARGET-NASH study. TARGET-NASH is a collaboration of academic and community investigators and the pharmaceutical industry. Lead author Dr. Barritt had no financial conflicts to disclose, but many study coauthors disclosed relationships with multiple pharmaceutical companies, including those involved in the TARGET-NASH study. Dr. Banini currently serves on the NASH advisory board for Boehringer Ingelheim. Dr. Henry reported no disclosures, although his institution is one of the enrollment sites for TARGET-NASH.
Nonalcoholic fatty liver disease (NAFLD) was present in approximately two-thirds of patients who did not undergo a liver biopsy. These patients were more likely to be non-White and older, as well as have normal ALT levels, which shows potential gaps in knowledge about this population.
Data from studies of patients diagnosed with NAFLD that require biopsy among their inclusion criteria may be subject to selection and detection bias, wrote A. Sidney Barritt, MD, of the University of North Carolina at Chapel Hill, and colleagues. The researchers sought to compare characteristics of patients with NAFLD who were diagnosed using clinical criteria and those diagnosed via liver biopsy.
In a study published in Hepatology Communications, the researchers reviewed data from TARGET-NASH, a longitudinal, observational cohort study designed to follow patients with NAFLD in clinical practice to provide data on the effectiveness of treatments.
“TARGET-NASH represents a large cohort of NAFLD patients from multiple sites and can provide us with real world information on progression of disease in patients with NAFLD and particular risk factors that may be clinically relevant,” Zachary Henry, MD, MS, of the division of gastroenterology & hepatology at the University of Virginia Health System in Charlottesville, said in an interview. “This is one of the first studies from this database, and as time goes on, we will see more large-population data like this to answer specific questions for NAFLD patient.”
Surprising findings
The researchers included 3,474 patients aged 18 years and older who were enrolled in the TARGET-NASH study between Aug. 1, 2016, and March 4, 2019. The study participants were classified according to severity of liver disease: nonalcoholic fatty liver (30%), nonalcoholic steatohepatitis (37%), and NAFLD cirrhosis (33%).
A total of 766 patients were diagnosed with NASH based on clinical criteria without biopsy, and all met the criteria for abnormal ALT and steatosis based on imaging. In addition, these patients had at least one secondary diagnostic criteria: body mass index greater than 30 kg/m2 (74%), type 2 diabetes (42%), and dyslipidemia (54%). Significant independent predictors of liver biopsy included younger age, White race, female gender, diabetes, and elevated levels of ALT.
Elevated ALT increased the odds of liver biopsy by 14% per 10-point rise, according to the study. A machine learning model showed that non-White patients with ALT less than 69 IU/mL had a 6% chance of liver biopsy. By comparison, White patients had a 21% chance of biopsy with ALT between 29 IU/mL and 69 IU/mL that dropped to 10% if the ALT was less than 29 IU/mL.
However, ALT remains a “suboptimal surrogate” for disease severity, the researchers noted. “How a normal ALT is defined and how a normal ALT range may vary across different laboratories may play a role in its utility as a diagnostic tool as well.”
Dr. Henry was surprised by this finding: “With the advent of noninvasive measures of fibrosis, such as the NAFLD fibrosis score, Fibrosis-4, and transient elastography, I thought these would have a more significant role in that decision as opposed to ALT levels.”
Notably, mental health diagnoses accounted for nearly half (49%) of comorbid conditions, followed by cardiovascular disease (19%), and osteoarthritis (10%). The prevalence of these conditions emphasizes the challenges of managing patients with NAFLD with diet and exercise alone because mental and physical problems may impede progress, the researchers wrote.
The study findings were limited by several factors including the inability to determine health care provider intent, as well as undocumented factors related to patients and providers that might influence a biopsy decision, such as assessment of disease severity, the researchers noted. In addition, they noted that the mostly White study population treated in specialty settings might not generalize to other populations or primary care.
However, the findings are strengthened by the large study population and real-world setting, the researchers emphasized. “These data provide context for the selection bias that may be present in many registries and randomized, controlled trials of therapies for NAFLD, where biopsy is required for inclusion,” and show potential knowledge gaps about the patient population less likely to undergo biopsy.
Knowledge gaps and implications
The study is important because of the need to identify patient factors that predict histologic versus clinical diagnosis of NAFLD as the number of patient registries and clinical trials for NAFLD increase, Bubu Banini, MD, of Yale University, New Haven, Conn., said in an interview. “This information helps to elucidate selection and ascertainment bias and place findings from NAFLD registries and clinical trials into context.”
Dr. Banini said that some of the findings were to be expected, while others were not.
“Historically, males and non-Whites are less likely to participate in registries and clinical trials, compared to females and Whites. However, I was surprised to find that these discrepancies further paralleled the likelihood of undergoing liver biopsy even among those who chose to participate. In addition, while mental health disorders (such as anxiety and depression) are a fairly prevalent comorbidity in patients with NAFLD, I was surprised to find that NAFLD patients with mental health disorders were more likely to undergo liver biopsy compared to those without these disorders. I would have expected the reverse,” he noted.
“These findings highlight the gaps in knowledge regarding the impact of demographic and psychosocial factors on choice and assess to care among patients with NAFLD, and the need for further studies to address these gaps,” she emphasized.
“A number of [studies] such as TARGET-NASH are doing away with the requirement for liver biopsy for participation; hence, it is less likely that selection bias related to liver biopsy would be a problem in these [studies] if clinical diagnosis is considered as a surrogate for histological diagnosis,” Dr. Banini added.
“On the contrary, many NAFLD clinical trials require liver biopsy for inclusion.” As nicely demonstrated in the current study, “this inclusion criterion may introduce selection bias,” she said. “Awareness of potential biases would hopefully inform the design and recruitment strategy for registries and clinical trials in order to overcome these issues.”
“I think the results of this study may actually point to a larger issue within medicine in general, which is a difference in care provided to minority communities,” Dr. Henry said. “Whether this is intentional, related to unconscious bias on the part of providers, or related to a significant mistrust between minority communities and their health care providers is unclear but certainly needs to be addressed.”
He noted that the purpose of TARGET-NASH is to enroll all patients with NAFLD regardless of biopsy. “Over time, as we have more data on these patients, we will have a better understanding of both diagnostic and therapeutic decisions in patients with NAFLD.”
The study was supported by Target RWE, sponsor of the TARGET-NASH study. TARGET-NASH is a collaboration of academic and community investigators and the pharmaceutical industry. Lead author Dr. Barritt had no financial conflicts to disclose, but many study coauthors disclosed relationships with multiple pharmaceutical companies, including those involved in the TARGET-NASH study. Dr. Banini currently serves on the NASH advisory board for Boehringer Ingelheim. Dr. Henry reported no disclosures, although his institution is one of the enrollment sites for TARGET-NASH.
Nonalcoholic fatty liver disease (NAFLD) was present in approximately two-thirds of patients who did not undergo a liver biopsy. These patients were more likely to be non-White and older, as well as have normal ALT levels, which shows potential gaps in knowledge about this population.
Data from studies of patients diagnosed with NAFLD that require biopsy among their inclusion criteria may be subject to selection and detection bias, wrote A. Sidney Barritt, MD, of the University of North Carolina at Chapel Hill, and colleagues. The researchers sought to compare characteristics of patients with NAFLD who were diagnosed using clinical criteria and those diagnosed via liver biopsy.
In a study published in Hepatology Communications, the researchers reviewed data from TARGET-NASH, a longitudinal, observational cohort study designed to follow patients with NAFLD in clinical practice to provide data on the effectiveness of treatments.
“TARGET-NASH represents a large cohort of NAFLD patients from multiple sites and can provide us with real world information on progression of disease in patients with NAFLD and particular risk factors that may be clinically relevant,” Zachary Henry, MD, MS, of the division of gastroenterology & hepatology at the University of Virginia Health System in Charlottesville, said in an interview. “This is one of the first studies from this database, and as time goes on, we will see more large-population data like this to answer specific questions for NAFLD patient.”
Surprising findings
The researchers included 3,474 patients aged 18 years and older who were enrolled in the TARGET-NASH study between Aug. 1, 2016, and March 4, 2019. The study participants were classified according to severity of liver disease: nonalcoholic fatty liver (30%), nonalcoholic steatohepatitis (37%), and NAFLD cirrhosis (33%).
A total of 766 patients were diagnosed with NASH based on clinical criteria without biopsy, and all met the criteria for abnormal ALT and steatosis based on imaging. In addition, these patients had at least one secondary diagnostic criteria: body mass index greater than 30 kg/m2 (74%), type 2 diabetes (42%), and dyslipidemia (54%). Significant independent predictors of liver biopsy included younger age, White race, female gender, diabetes, and elevated levels of ALT.
Elevated ALT increased the odds of liver biopsy by 14% per 10-point rise, according to the study. A machine learning model showed that non-White patients with ALT less than 69 IU/mL had a 6% chance of liver biopsy. By comparison, White patients had a 21% chance of biopsy with ALT between 29 IU/mL and 69 IU/mL that dropped to 10% if the ALT was less than 29 IU/mL.
However, ALT remains a “suboptimal surrogate” for disease severity, the researchers noted. “How a normal ALT is defined and how a normal ALT range may vary across different laboratories may play a role in its utility as a diagnostic tool as well.”
Dr. Henry was surprised by this finding: “With the advent of noninvasive measures of fibrosis, such as the NAFLD fibrosis score, Fibrosis-4, and transient elastography, I thought these would have a more significant role in that decision as opposed to ALT levels.”
Notably, mental health diagnoses accounted for nearly half (49%) of comorbid conditions, followed by cardiovascular disease (19%), and osteoarthritis (10%). The prevalence of these conditions emphasizes the challenges of managing patients with NAFLD with diet and exercise alone because mental and physical problems may impede progress, the researchers wrote.
The study findings were limited by several factors including the inability to determine health care provider intent, as well as undocumented factors related to patients and providers that might influence a biopsy decision, such as assessment of disease severity, the researchers noted. In addition, they noted that the mostly White study population treated in specialty settings might not generalize to other populations or primary care.
However, the findings are strengthened by the large study population and real-world setting, the researchers emphasized. “These data provide context for the selection bias that may be present in many registries and randomized, controlled trials of therapies for NAFLD, where biopsy is required for inclusion,” and show potential knowledge gaps about the patient population less likely to undergo biopsy.
Knowledge gaps and implications
The study is important because of the need to identify patient factors that predict histologic versus clinical diagnosis of NAFLD as the number of patient registries and clinical trials for NAFLD increase, Bubu Banini, MD, of Yale University, New Haven, Conn., said in an interview. “This information helps to elucidate selection and ascertainment bias and place findings from NAFLD registries and clinical trials into context.”
Dr. Banini said that some of the findings were to be expected, while others were not.
“Historically, males and non-Whites are less likely to participate in registries and clinical trials, compared to females and Whites. However, I was surprised to find that these discrepancies further paralleled the likelihood of undergoing liver biopsy even among those who chose to participate. In addition, while mental health disorders (such as anxiety and depression) are a fairly prevalent comorbidity in patients with NAFLD, I was surprised to find that NAFLD patients with mental health disorders were more likely to undergo liver biopsy compared to those without these disorders. I would have expected the reverse,” he noted.
“These findings highlight the gaps in knowledge regarding the impact of demographic and psychosocial factors on choice and assess to care among patients with NAFLD, and the need for further studies to address these gaps,” she emphasized.
“A number of [studies] such as TARGET-NASH are doing away with the requirement for liver biopsy for participation; hence, it is less likely that selection bias related to liver biopsy would be a problem in these [studies] if clinical diagnosis is considered as a surrogate for histological diagnosis,” Dr. Banini added.
“On the contrary, many NAFLD clinical trials require liver biopsy for inclusion.” As nicely demonstrated in the current study, “this inclusion criterion may introduce selection bias,” she said. “Awareness of potential biases would hopefully inform the design and recruitment strategy for registries and clinical trials in order to overcome these issues.”
“I think the results of this study may actually point to a larger issue within medicine in general, which is a difference in care provided to minority communities,” Dr. Henry said. “Whether this is intentional, related to unconscious bias on the part of providers, or related to a significant mistrust between minority communities and their health care providers is unclear but certainly needs to be addressed.”
He noted that the purpose of TARGET-NASH is to enroll all patients with NAFLD regardless of biopsy. “Over time, as we have more data on these patients, we will have a better understanding of both diagnostic and therapeutic decisions in patients with NAFLD.”
The study was supported by Target RWE, sponsor of the TARGET-NASH study. TARGET-NASH is a collaboration of academic and community investigators and the pharmaceutical industry. Lead author Dr. Barritt had no financial conflicts to disclose, but many study coauthors disclosed relationships with multiple pharmaceutical companies, including those involved in the TARGET-NASH study. Dr. Banini currently serves on the NASH advisory board for Boehringer Ingelheim. Dr. Henry reported no disclosures, although his institution is one of the enrollment sites for TARGET-NASH.
FROM HEPATOLOGY COMMUNICATIONS
Endoscopic device could expand treatment for GERD, reduce PPI use
In patients with proton pump inhibitor (PPI)–dependent gastroesophageal reflux disease (GERD), a procedure known as endoscopic full-thickness plication (EFTP) – performed with the novel GERD-X device – improved both symptoms and quality of life, compared with a sham procedure. It also had few side effects and a short procedure time, according to a new randomized, controlled trial.
“It seems like it is a quick, easy-to-use procedure,” Gyanprakash A. Ketwaroo, MD, MSc, who is an assistant professor of medicine at Baylor College of Medicine, Houston, commented in an interview. Dr. Ketwaroo was not involved in the study.
“Even though the objective measures were not as good as perhaps you had hoped, the subjective outcomes were good. [And] it seems that it may have more of a long-term benefit, compared to some of the other endoscopic procedures. But that wasn’t a [primary] outcome of the study, and we still need more long-term studies to figure that out,” he added.
The research, led by Rakesh Kalapala, MD, DNB, and D. Nageshwar Reddy, MD, FACG, of the Asian Institute of Gastroenterology, Hyderabad, India, appeared in Gut.
The fact that the EFTP procedure is relatively simple could reduce cost and ease the learning curve, which in turn could broaden accessibility if more gastroenterologists are trained on it.
“There are not many gastroenterologists who offer endoscopic approaches to GERD therapy, so increasing that cohort [could] potentially have a huge impact given the number of patients who have GERD in this country, and especially given the rising and persistent concern over long-term use of PPIs,” said Dr. Ketwaroo.
Addressing the drawbacks of long-term PPI use
Although PPIs are the most effective medical therapy for GERD, there are concerns that long-term use could increase the risk of acute and chronic kidney disease, hypomagnesaemia, Clostridioides difficile infection, and osteoporotic fractures. Surgical antireflux interventions are effective but may lead to dysphagia, bloating, and diarrhea.
EFTP applies transmural sutures to the gastroesophageal junction to strengthen the valvular mechanism, which reduces reflux. While the preponderance of published evidence supports the Esophyx device (EndoGastric Solutions), which has a 70% efficacy rate and few adverse events in one analysis, it requires advanced training and general anesthesia and takes 45-100 minutes.
“Endoscopic fundoplication is a minimally invasive antireflux therapy in patients with PPI dependence who refuse surgery; however, the majority of the endoscopic devices are cumbersome to use and robust data on their long-term efficacy are lacking,” Dr. Kalapala and colleagues noted in their new paper.
In 2014, the German company G-Surg introduced a novel endoscopic plication device called GERD-X. A prospective, single-arm study had shown efficacy in both patients taking PPIs and those with refractory GERD.
A closer look at the device
To bolster that evidence, Dr. Kalapala and colleagues conducted this new single-center, randomized, sham-controlled trial with 70 enrollees with PPI-dependent GERD, of which 70% had nonerosive reflux disease (mean DeMeester score, 18.9). The median participant age was 36 years, and 71.4% were male. The average procedure time was 17.4 minutes.
Of the subjects in the treatment group, 65.7% achieved at least a 50% improvement in GERD health-related quality of life (GERD-HRQL) after 3 months, compared with 2.9% in the sham group (P < .001). The median percentage improvement in GERD-HRQL score was higher in the treatment group at 6 months (81.4% vs. 8.0%; P < .001) and at 12 months (92.3% vs. 9.1%; P < .001). Similar improvements were seen at 6 months and 12 months in heartburn symptom score (75.0% vs. 13.0% and 89.7% vs. 15.4%, respectively; P < .001 for both) and regurgitation symptom score (96.2% vs. 6.9% and 100% vs. 3.4%, respectively; P < .001 for both). At 12 months, 62.8% of the treatment group no longer took PPIs, compared with 11.4% of the sham group (P < .001).
Objective measures of improvement were more modest. The treatment arm trended toward a reduction in esophageal acid exposure from baseline at 3 and 12 months, but the difference was not statistically significant. The median percentage of time with esophageal pH below 4 and the DeMeester score were similar between the groups at 3 and 12 months. The researchers also noted trends toward fewer reflux events in 24 hours in the treatment group at 6 months (P = .072) and 12 months (P = .051).
The treatment group had fewer non–acid reflux episodes at 12 months versus baseline (P = .038), but there was no difference in the median number of acid reflux episodes in 24 hours.
“Our study found endoscopic full-thickness fundoplication, using a novel device, was safe and significantly improved GERD-related quality of life and severity of reflux symptoms at short and long terms, compared with a sham procedure,” wrote the authors.
“This endoluminal procedure with a short operating time and very few side effects is a promising alternative option to surgery in appropriately selected group of patients, who may not want to continue PPI long term,” they concluded.
The authors of the study disclosed no external funding. Dr. Ketwaroo has no relevant financial disclosures, although he is on the editorial advisory board for GI & Hepatology News.
This article was updated May 6, 2021.
In patients with proton pump inhibitor (PPI)–dependent gastroesophageal reflux disease (GERD), a procedure known as endoscopic full-thickness plication (EFTP) – performed with the novel GERD-X device – improved both symptoms and quality of life, compared with a sham procedure. It also had few side effects and a short procedure time, according to a new randomized, controlled trial.
“It seems like it is a quick, easy-to-use procedure,” Gyanprakash A. Ketwaroo, MD, MSc, who is an assistant professor of medicine at Baylor College of Medicine, Houston, commented in an interview. Dr. Ketwaroo was not involved in the study.
“Even though the objective measures were not as good as perhaps you had hoped, the subjective outcomes were good. [And] it seems that it may have more of a long-term benefit, compared to some of the other endoscopic procedures. But that wasn’t a [primary] outcome of the study, and we still need more long-term studies to figure that out,” he added.
The research, led by Rakesh Kalapala, MD, DNB, and D. Nageshwar Reddy, MD, FACG, of the Asian Institute of Gastroenterology, Hyderabad, India, appeared in Gut.
The fact that the EFTP procedure is relatively simple could reduce cost and ease the learning curve, which in turn could broaden accessibility if more gastroenterologists are trained on it.
“There are not many gastroenterologists who offer endoscopic approaches to GERD therapy, so increasing that cohort [could] potentially have a huge impact given the number of patients who have GERD in this country, and especially given the rising and persistent concern over long-term use of PPIs,” said Dr. Ketwaroo.
Addressing the drawbacks of long-term PPI use
Although PPIs are the most effective medical therapy for GERD, there are concerns that long-term use could increase the risk of acute and chronic kidney disease, hypomagnesaemia, Clostridioides difficile infection, and osteoporotic fractures. Surgical antireflux interventions are effective but may lead to dysphagia, bloating, and diarrhea.
EFTP applies transmural sutures to the gastroesophageal junction to strengthen the valvular mechanism, which reduces reflux. While the preponderance of published evidence supports the Esophyx device (EndoGastric Solutions), which has a 70% efficacy rate and few adverse events in one analysis, it requires advanced training and general anesthesia and takes 45-100 minutes.
“Endoscopic fundoplication is a minimally invasive antireflux therapy in patients with PPI dependence who refuse surgery; however, the majority of the endoscopic devices are cumbersome to use and robust data on their long-term efficacy are lacking,” Dr. Kalapala and colleagues noted in their new paper.
In 2014, the German company G-Surg introduced a novel endoscopic plication device called GERD-X. A prospective, single-arm study had shown efficacy in both patients taking PPIs and those with refractory GERD.
A closer look at the device
To bolster that evidence, Dr. Kalapala and colleagues conducted this new single-center, randomized, sham-controlled trial with 70 enrollees with PPI-dependent GERD, of which 70% had nonerosive reflux disease (mean DeMeester score, 18.9). The median participant age was 36 years, and 71.4% were male. The average procedure time was 17.4 minutes.
Of the subjects in the treatment group, 65.7% achieved at least a 50% improvement in GERD health-related quality of life (GERD-HRQL) after 3 months, compared with 2.9% in the sham group (P < .001). The median percentage improvement in GERD-HRQL score was higher in the treatment group at 6 months (81.4% vs. 8.0%; P < .001) and at 12 months (92.3% vs. 9.1%; P < .001). Similar improvements were seen at 6 months and 12 months in heartburn symptom score (75.0% vs. 13.0% and 89.7% vs. 15.4%, respectively; P < .001 for both) and regurgitation symptom score (96.2% vs. 6.9% and 100% vs. 3.4%, respectively; P < .001 for both). At 12 months, 62.8% of the treatment group no longer took PPIs, compared with 11.4% of the sham group (P < .001).
Objective measures of improvement were more modest. The treatment arm trended toward a reduction in esophageal acid exposure from baseline at 3 and 12 months, but the difference was not statistically significant. The median percentage of time with esophageal pH below 4 and the DeMeester score were similar between the groups at 3 and 12 months. The researchers also noted trends toward fewer reflux events in 24 hours in the treatment group at 6 months (P = .072) and 12 months (P = .051).
The treatment group had fewer non–acid reflux episodes at 12 months versus baseline (P = .038), but there was no difference in the median number of acid reflux episodes in 24 hours.
“Our study found endoscopic full-thickness fundoplication, using a novel device, was safe and significantly improved GERD-related quality of life and severity of reflux symptoms at short and long terms, compared with a sham procedure,” wrote the authors.
“This endoluminal procedure with a short operating time and very few side effects is a promising alternative option to surgery in appropriately selected group of patients, who may not want to continue PPI long term,” they concluded.
The authors of the study disclosed no external funding. Dr. Ketwaroo has no relevant financial disclosures, although he is on the editorial advisory board for GI & Hepatology News.
This article was updated May 6, 2021.
In patients with proton pump inhibitor (PPI)–dependent gastroesophageal reflux disease (GERD), a procedure known as endoscopic full-thickness plication (EFTP) – performed with the novel GERD-X device – improved both symptoms and quality of life, compared with a sham procedure. It also had few side effects and a short procedure time, according to a new randomized, controlled trial.
“It seems like it is a quick, easy-to-use procedure,” Gyanprakash A. Ketwaroo, MD, MSc, who is an assistant professor of medicine at Baylor College of Medicine, Houston, commented in an interview. Dr. Ketwaroo was not involved in the study.
“Even though the objective measures were not as good as perhaps you had hoped, the subjective outcomes were good. [And] it seems that it may have more of a long-term benefit, compared to some of the other endoscopic procedures. But that wasn’t a [primary] outcome of the study, and we still need more long-term studies to figure that out,” he added.
The research, led by Rakesh Kalapala, MD, DNB, and D. Nageshwar Reddy, MD, FACG, of the Asian Institute of Gastroenterology, Hyderabad, India, appeared in Gut.
The fact that the EFTP procedure is relatively simple could reduce cost and ease the learning curve, which in turn could broaden accessibility if more gastroenterologists are trained on it.
“There are not many gastroenterologists who offer endoscopic approaches to GERD therapy, so increasing that cohort [could] potentially have a huge impact given the number of patients who have GERD in this country, and especially given the rising and persistent concern over long-term use of PPIs,” said Dr. Ketwaroo.
Addressing the drawbacks of long-term PPI use
Although PPIs are the most effective medical therapy for GERD, there are concerns that long-term use could increase the risk of acute and chronic kidney disease, hypomagnesaemia, Clostridioides difficile infection, and osteoporotic fractures. Surgical antireflux interventions are effective but may lead to dysphagia, bloating, and diarrhea.
EFTP applies transmural sutures to the gastroesophageal junction to strengthen the valvular mechanism, which reduces reflux. While the preponderance of published evidence supports the Esophyx device (EndoGastric Solutions), which has a 70% efficacy rate and few adverse events in one analysis, it requires advanced training and general anesthesia and takes 45-100 minutes.
“Endoscopic fundoplication is a minimally invasive antireflux therapy in patients with PPI dependence who refuse surgery; however, the majority of the endoscopic devices are cumbersome to use and robust data on their long-term efficacy are lacking,” Dr. Kalapala and colleagues noted in their new paper.
In 2014, the German company G-Surg introduced a novel endoscopic plication device called GERD-X. A prospective, single-arm study had shown efficacy in both patients taking PPIs and those with refractory GERD.
A closer look at the device
To bolster that evidence, Dr. Kalapala and colleagues conducted this new single-center, randomized, sham-controlled trial with 70 enrollees with PPI-dependent GERD, of which 70% had nonerosive reflux disease (mean DeMeester score, 18.9). The median participant age was 36 years, and 71.4% were male. The average procedure time was 17.4 minutes.
Of the subjects in the treatment group, 65.7% achieved at least a 50% improvement in GERD health-related quality of life (GERD-HRQL) after 3 months, compared with 2.9% in the sham group (P < .001). The median percentage improvement in GERD-HRQL score was higher in the treatment group at 6 months (81.4% vs. 8.0%; P < .001) and at 12 months (92.3% vs. 9.1%; P < .001). Similar improvements were seen at 6 months and 12 months in heartburn symptom score (75.0% vs. 13.0% and 89.7% vs. 15.4%, respectively; P < .001 for both) and regurgitation symptom score (96.2% vs. 6.9% and 100% vs. 3.4%, respectively; P < .001 for both). At 12 months, 62.8% of the treatment group no longer took PPIs, compared with 11.4% of the sham group (P < .001).
Objective measures of improvement were more modest. The treatment arm trended toward a reduction in esophageal acid exposure from baseline at 3 and 12 months, but the difference was not statistically significant. The median percentage of time with esophageal pH below 4 and the DeMeester score were similar between the groups at 3 and 12 months. The researchers also noted trends toward fewer reflux events in 24 hours in the treatment group at 6 months (P = .072) and 12 months (P = .051).
The treatment group had fewer non–acid reflux episodes at 12 months versus baseline (P = .038), but there was no difference in the median number of acid reflux episodes in 24 hours.
“Our study found endoscopic full-thickness fundoplication, using a novel device, was safe and significantly improved GERD-related quality of life and severity of reflux symptoms at short and long terms, compared with a sham procedure,” wrote the authors.
“This endoluminal procedure with a short operating time and very few side effects is a promising alternative option to surgery in appropriately selected group of patients, who may not want to continue PPI long term,” they concluded.
The authors of the study disclosed no external funding. Dr. Ketwaroo has no relevant financial disclosures, although he is on the editorial advisory board for GI & Hepatology News.
This article was updated May 6, 2021.
FROM GUT
Breast cancer survivors have specific gynecological needs
Sexual dysfunction is a common problem among breast cancer survivors, but it’s also an issue inadequately addressed by either ob.gyns. or hematologists and oncologists, according to Erin Keyser, MD, the program director of the San Antonio Uniformed Services Health Education Consortium. Dr. Keyser discussed management of sexual dysfunction and a variety of other issues frequently faced by women who have survived breast cancer at the at the 2021 virtual meeting of the American College of Obstetricians and Gynecologists.
“Despite the fact that no specialty is better qualified to render care for this consequence of cancer treatments, many obstetrician-gynecologists feel uncomfortable or ill-equipped to address sexual pain in women affected by cancer,” Dr. Keyser quoted from a 2016 article in Obstetrics & Gynecology about the sexual health of women affected by cancer. As a breast cancer survivor herself, Dr. Keyser said hematologists and oncologists are even less equipped to discuss sexual health, “so oftentimes patients get punted between their hem-onc and their gyn,” with each telling the patient to ask the other specialist.
“There’s plenty of data in chronic health disease that maintaining sexual function for women is an indicator of the overall quality of life and that many women really don’t want to bring this up,” Dr. Keyser told attendees, so the onus is on the ob.gyn. to bring it up.
The effects of breast cancer treatment can impact women’s body image, fertility, menopause, sexual function, osteoporosis, and cardiovascular disease, but the bulk of Dr. Keyser’s talk focused on sexual health and bilateral salpingo-oophorectomy (BSO).
Lauren Streicher, MD, a clinical professor of obstetrics and gynecology at Northwestern University, Chicago, thought Dr. Keyser’s talk was useful for the general gynecologist but had some concerns about a few parts.
“She gave a very thoughtful analysis of whether someone should have their ovaries removed or not in a breast cancer diagnosis, ” Dr. Streicher said in an interview. “I would have liked to hear more about the consequences of an early menopause in women in terms of heart health, bone health, and cognitive function.”
Dr. Keyser noted that her talk pertained mostly to survivors of estrogen receptor (ER)–positive breast cancer since that population tends to struggle most with side effects of treatment. The most common medications used in this population are tamoxifen and aromatase inhibitors – such as anastrazole, letrozole, and exemestane – and these medications can affect management of different concerns.
Current guidance on ovarian removal
For women with a BRCA mutation, ACOG clinical guidance already exists regarding BSO. For other women, the complementary TEXT and SOFT trials changed the management of breast cancer treatment in premenopausal women, Dr. Keyser said.
Before these trials, postmenopausal hormone receptor–positive women began aromatase inhibitors and premenopausal HR-positive women began tamoxifen. These trials found that premenopausal women with HR-positive early breast cancer were less likely to experience recurrence when receiving adjuvant treatment with exemestane plus ovarian suppression compared to tamoxifen plus ovarian suppression. Ovarian suppression was achieved by either GnRH agonist injections, surgical removal of the ovaries, or radiation therapy to the ovaries.
The side effects of these treatments included hot flushes (92%), depression (87%), musculoskeletal symptoms (89%), vaginal dryness (52%), decreased libido (45%), dyspareunia (31%), osteoporosis (39%), insomnia (58%), and fatigue (61%). These are all quality of life concerns, Dr. Keyser said, and these findings raise questions about the consequences of long-term ovarian suppression. Findings from the Nurses’ Health Study showed that BSO before age 47.5 years resulted in lower mortality from ovarian cancer and breast cancer but was linked in women under 50 to increased all-cause mortality and mortality from coronary heart disease, lung cancer, and colorectal cancer, compared with ovarian conservation. Further, 74% of women who undergo risk-reducing BSO experience sexual dysfunction.
The bottom line, Dr. Keyser said, is that “premature removal of ovaries is not completely benign.” Her own recommendation is to follow ACOG guidance for women with BRCA mutations and, for women aged under 35 years, use ovarian suppression for 5-10 years, after which ovarian function may resume along with improved quality of life. In women aged over 40, remove ovaries since, after 5-10 years of treatment, there’s likely no benefit of retaining ovaries.
Addressing sexual health
Dyspareunia affects up to 45% of cancer survivors, Dr. Keyser said, and multiple treatment options exist for breast cancer survivors. The therapies she discussed included lubricants, moisturizers, local vaginal estrogen, DHEA, ospemifene, and CO2 laser therapy.
Though Dr. Keyser briefly touched on vaginal lubricants and moisturizers, Dr. Streicher was disappointed that Dr. Keyser did not clearly define and differentiate between lubricants and moisturizers or mention hyaluronic acid products. Dr. Streicher also disagreed with the way Dr. Keyser represented the benefits of coconut oil as a lubricant. “Oils are not condom compatible and are known to potentially increase the risk of infection, and not just from poor handwashing,” Dr. Streicher said.
Small retrospective studies support the safety of topical vaginal estrogen in breast cancer survivors, Dr. Keyser said, and the 10-mcg Vagifem tablet and vaginal estradiol ring appear to have the lowest systemic absorption. ACOG guidance recommends that women taking aromatase inhibitors who don’t respond to nonhormonal approaches may benefit from switching temporarily to tamoxifen with vaginal estrogen and then returning to aromatase inhibitors. However, Dr. Keyser said there’s plenty of data to support using vaginal estrogen in patients taking aromatase inhibitors.
“I do feel that it’s safe for patients, whether they’re on tamoxifen or aromatase inhibitors, to take vaginal estrogen,” Dr. Keyser said. “I usually stick with the estradiol vaginal ring or the estradiol tablet, and I base that on a patient’s comfort with placing and removing a ring.” She also, instead of asking the patient’s hematologist-oncologist, simply notifies them of the treatment since most hematologist-oncologists are less familiar with the data.
Another effective option is vaginal DHEA/prasterone, which can significantly improve sexual desire, arousal, pain, and overall sexual function. Although breast cancer patients were included in early studies on DHEA, Intrarosa manufacturers excluded breast cancer patients in their Food and Drug Administration application, resulting in a package stating that “use of exogenous estrogen is contraindicated in women with a known or suspected history of breast cancer” and that “Intrarosa has not been studied in women with a history of breast cancer.” While that’s true for Intrarosa specifically, DHEA has been studied in breast cancer patients, Dr. Keyser said, so she expects to see more research in this area.
Ospemifene is another option for improving vulvovaginal atrophy but cannot be taken at the same time as tamoxifen. It has similar chemopreventive effects as tamoxifen in rat studies, but it’s not as effective. It’s a reasonable option in women with refractory genitourinary syndrome of menopause (GSM) who have completed their 5-10 years of adjuvant therapy and have no history of venous thromboembolism.
Dr. Keyser said CO2 laser therapy is still being studied for treating GSM, and current data have shown benefits for dyspareunia and vaginal dryness without documented harms. There have now been randomized, controlled trials; however, since it’s not FDA approved, it’s not covered by insurance and costs approximately $5,000 for three treatments.
Dr. Streicher was glad to see Dr. Keyser’s discussion of the safety and types of local vaginal estrogen, “although she neglected to mention the 4-mcg vaginal suppository, Imvexxy, which has the lowest systemic absorption,” Dr. Streicher said. Dr. Streicher also felt the inclusion of DHEA/prasterone and ospemifene were also important, especially since the latter is “underutilized in breast cancer patients.”
The information provided on CO2 laser therapy, however, was problematic, Dr. Streicher said, given that long-term and randomized, controlled studies have now been published. Dr. Streicher also noted that two of the devices listed on the presentation slide, Thermiva and Voltiva, are radiofrequency, not laser devices.
Aside from these treatment options, the most consistent predictor of satisfying sexual experiences in women with breast cancer is the quality of their relationships, Dr. Keyser said, so couples counseling is recommended, and treatments in general are more effective with regularly sexual activity.
In discussing nonhormonal options for treating vasomotor symptoms, Dr. Keyser recommended venlafaxine, gabapentin, and low-dose paroxetine (though SSRIs and tamoxifen are contraindicated since they may reduce tamoxifen’s efficacy).
These are all off label, Dr. Streicher said it was important to note, and she would have liked to have seen a mention of the development of KNdy neurokinin disrupters along with a more in-depth discussion about which lifestyle modifications and botanicals have been shown in randomized, controlled trials to mitigate vasomotor symptoms.
Dr. Keyser wrapped up with a few additional notes and takeaways:
- The only safe reversible long-term option for contraception in HR-positive breast cancer survivors is the Paraguard IUD.
- It’s important to discuss fertility with breast cancer patients and survivors since a majority report unmet needs in this area.
- Patients taking tamoxifen need to be sure to report any vaginal spotting or bleeding since it increases risk of endometrial cancer in postmenopausal women.
- Screen for depression and anxiety.
- Ask women about sexual health and hot flashes.
- Ensure that they’re getting bone screening.
- A recommended resource is Living Beyond Breast Cancer.
Dr. Keyser had no disclosures. Dr. Streicher has consulted for Astellas Pharma and Church & Dwight, and she owns investments in InControl Medical and Sermonix Pharmaceuticals.
Sexual dysfunction is a common problem among breast cancer survivors, but it’s also an issue inadequately addressed by either ob.gyns. or hematologists and oncologists, according to Erin Keyser, MD, the program director of the San Antonio Uniformed Services Health Education Consortium. Dr. Keyser discussed management of sexual dysfunction and a variety of other issues frequently faced by women who have survived breast cancer at the at the 2021 virtual meeting of the American College of Obstetricians and Gynecologists.
“Despite the fact that no specialty is better qualified to render care for this consequence of cancer treatments, many obstetrician-gynecologists feel uncomfortable or ill-equipped to address sexual pain in women affected by cancer,” Dr. Keyser quoted from a 2016 article in Obstetrics & Gynecology about the sexual health of women affected by cancer. As a breast cancer survivor herself, Dr. Keyser said hematologists and oncologists are even less equipped to discuss sexual health, “so oftentimes patients get punted between their hem-onc and their gyn,” with each telling the patient to ask the other specialist.
“There’s plenty of data in chronic health disease that maintaining sexual function for women is an indicator of the overall quality of life and that many women really don’t want to bring this up,” Dr. Keyser told attendees, so the onus is on the ob.gyn. to bring it up.
The effects of breast cancer treatment can impact women’s body image, fertility, menopause, sexual function, osteoporosis, and cardiovascular disease, but the bulk of Dr. Keyser’s talk focused on sexual health and bilateral salpingo-oophorectomy (BSO).
Lauren Streicher, MD, a clinical professor of obstetrics and gynecology at Northwestern University, Chicago, thought Dr. Keyser’s talk was useful for the general gynecologist but had some concerns about a few parts.
“She gave a very thoughtful analysis of whether someone should have their ovaries removed or not in a breast cancer diagnosis, ” Dr. Streicher said in an interview. “I would have liked to hear more about the consequences of an early menopause in women in terms of heart health, bone health, and cognitive function.”
Dr. Keyser noted that her talk pertained mostly to survivors of estrogen receptor (ER)–positive breast cancer since that population tends to struggle most with side effects of treatment. The most common medications used in this population are tamoxifen and aromatase inhibitors – such as anastrazole, letrozole, and exemestane – and these medications can affect management of different concerns.
Current guidance on ovarian removal
For women with a BRCA mutation, ACOG clinical guidance already exists regarding BSO. For other women, the complementary TEXT and SOFT trials changed the management of breast cancer treatment in premenopausal women, Dr. Keyser said.
Before these trials, postmenopausal hormone receptor–positive women began aromatase inhibitors and premenopausal HR-positive women began tamoxifen. These trials found that premenopausal women with HR-positive early breast cancer were less likely to experience recurrence when receiving adjuvant treatment with exemestane plus ovarian suppression compared to tamoxifen plus ovarian suppression. Ovarian suppression was achieved by either GnRH agonist injections, surgical removal of the ovaries, or radiation therapy to the ovaries.
The side effects of these treatments included hot flushes (92%), depression (87%), musculoskeletal symptoms (89%), vaginal dryness (52%), decreased libido (45%), dyspareunia (31%), osteoporosis (39%), insomnia (58%), and fatigue (61%). These are all quality of life concerns, Dr. Keyser said, and these findings raise questions about the consequences of long-term ovarian suppression. Findings from the Nurses’ Health Study showed that BSO before age 47.5 years resulted in lower mortality from ovarian cancer and breast cancer but was linked in women under 50 to increased all-cause mortality and mortality from coronary heart disease, lung cancer, and colorectal cancer, compared with ovarian conservation. Further, 74% of women who undergo risk-reducing BSO experience sexual dysfunction.
The bottom line, Dr. Keyser said, is that “premature removal of ovaries is not completely benign.” Her own recommendation is to follow ACOG guidance for women with BRCA mutations and, for women aged under 35 years, use ovarian suppression for 5-10 years, after which ovarian function may resume along with improved quality of life. In women aged over 40, remove ovaries since, after 5-10 years of treatment, there’s likely no benefit of retaining ovaries.
Addressing sexual health
Dyspareunia affects up to 45% of cancer survivors, Dr. Keyser said, and multiple treatment options exist for breast cancer survivors. The therapies she discussed included lubricants, moisturizers, local vaginal estrogen, DHEA, ospemifene, and CO2 laser therapy.
Though Dr. Keyser briefly touched on vaginal lubricants and moisturizers, Dr. Streicher was disappointed that Dr. Keyser did not clearly define and differentiate between lubricants and moisturizers or mention hyaluronic acid products. Dr. Streicher also disagreed with the way Dr. Keyser represented the benefits of coconut oil as a lubricant. “Oils are not condom compatible and are known to potentially increase the risk of infection, and not just from poor handwashing,” Dr. Streicher said.
Small retrospective studies support the safety of topical vaginal estrogen in breast cancer survivors, Dr. Keyser said, and the 10-mcg Vagifem tablet and vaginal estradiol ring appear to have the lowest systemic absorption. ACOG guidance recommends that women taking aromatase inhibitors who don’t respond to nonhormonal approaches may benefit from switching temporarily to tamoxifen with vaginal estrogen and then returning to aromatase inhibitors. However, Dr. Keyser said there’s plenty of data to support using vaginal estrogen in patients taking aromatase inhibitors.
“I do feel that it’s safe for patients, whether they’re on tamoxifen or aromatase inhibitors, to take vaginal estrogen,” Dr. Keyser said. “I usually stick with the estradiol vaginal ring or the estradiol tablet, and I base that on a patient’s comfort with placing and removing a ring.” She also, instead of asking the patient’s hematologist-oncologist, simply notifies them of the treatment since most hematologist-oncologists are less familiar with the data.
Another effective option is vaginal DHEA/prasterone, which can significantly improve sexual desire, arousal, pain, and overall sexual function. Although breast cancer patients were included in early studies on DHEA, Intrarosa manufacturers excluded breast cancer patients in their Food and Drug Administration application, resulting in a package stating that “use of exogenous estrogen is contraindicated in women with a known or suspected history of breast cancer” and that “Intrarosa has not been studied in women with a history of breast cancer.” While that’s true for Intrarosa specifically, DHEA has been studied in breast cancer patients, Dr. Keyser said, so she expects to see more research in this area.
Ospemifene is another option for improving vulvovaginal atrophy but cannot be taken at the same time as tamoxifen. It has similar chemopreventive effects as tamoxifen in rat studies, but it’s not as effective. It’s a reasonable option in women with refractory genitourinary syndrome of menopause (GSM) who have completed their 5-10 years of adjuvant therapy and have no history of venous thromboembolism.
Dr. Keyser said CO2 laser therapy is still being studied for treating GSM, and current data have shown benefits for dyspareunia and vaginal dryness without documented harms. There have now been randomized, controlled trials; however, since it’s not FDA approved, it’s not covered by insurance and costs approximately $5,000 for three treatments.
Dr. Streicher was glad to see Dr. Keyser’s discussion of the safety and types of local vaginal estrogen, “although she neglected to mention the 4-mcg vaginal suppository, Imvexxy, which has the lowest systemic absorption,” Dr. Streicher said. Dr. Streicher also felt the inclusion of DHEA/prasterone and ospemifene were also important, especially since the latter is “underutilized in breast cancer patients.”
The information provided on CO2 laser therapy, however, was problematic, Dr. Streicher said, given that long-term and randomized, controlled studies have now been published. Dr. Streicher also noted that two of the devices listed on the presentation slide, Thermiva and Voltiva, are radiofrequency, not laser devices.
Aside from these treatment options, the most consistent predictor of satisfying sexual experiences in women with breast cancer is the quality of their relationships, Dr. Keyser said, so couples counseling is recommended, and treatments in general are more effective with regularly sexual activity.
In discussing nonhormonal options for treating vasomotor symptoms, Dr. Keyser recommended venlafaxine, gabapentin, and low-dose paroxetine (though SSRIs and tamoxifen are contraindicated since they may reduce tamoxifen’s efficacy).
These are all off label, Dr. Streicher said it was important to note, and she would have liked to have seen a mention of the development of KNdy neurokinin disrupters along with a more in-depth discussion about which lifestyle modifications and botanicals have been shown in randomized, controlled trials to mitigate vasomotor symptoms.
Dr. Keyser wrapped up with a few additional notes and takeaways:
- The only safe reversible long-term option for contraception in HR-positive breast cancer survivors is the Paraguard IUD.
- It’s important to discuss fertility with breast cancer patients and survivors since a majority report unmet needs in this area.
- Patients taking tamoxifen need to be sure to report any vaginal spotting or bleeding since it increases risk of endometrial cancer in postmenopausal women.
- Screen for depression and anxiety.
- Ask women about sexual health and hot flashes.
- Ensure that they’re getting bone screening.
- A recommended resource is Living Beyond Breast Cancer.
Dr. Keyser had no disclosures. Dr. Streicher has consulted for Astellas Pharma and Church & Dwight, and she owns investments in InControl Medical and Sermonix Pharmaceuticals.
Sexual dysfunction is a common problem among breast cancer survivors, but it’s also an issue inadequately addressed by either ob.gyns. or hematologists and oncologists, according to Erin Keyser, MD, the program director of the San Antonio Uniformed Services Health Education Consortium. Dr. Keyser discussed management of sexual dysfunction and a variety of other issues frequently faced by women who have survived breast cancer at the at the 2021 virtual meeting of the American College of Obstetricians and Gynecologists.
“Despite the fact that no specialty is better qualified to render care for this consequence of cancer treatments, many obstetrician-gynecologists feel uncomfortable or ill-equipped to address sexual pain in women affected by cancer,” Dr. Keyser quoted from a 2016 article in Obstetrics & Gynecology about the sexual health of women affected by cancer. As a breast cancer survivor herself, Dr. Keyser said hematologists and oncologists are even less equipped to discuss sexual health, “so oftentimes patients get punted between their hem-onc and their gyn,” with each telling the patient to ask the other specialist.
“There’s plenty of data in chronic health disease that maintaining sexual function for women is an indicator of the overall quality of life and that many women really don’t want to bring this up,” Dr. Keyser told attendees, so the onus is on the ob.gyn. to bring it up.
The effects of breast cancer treatment can impact women’s body image, fertility, menopause, sexual function, osteoporosis, and cardiovascular disease, but the bulk of Dr. Keyser’s talk focused on sexual health and bilateral salpingo-oophorectomy (BSO).
Lauren Streicher, MD, a clinical professor of obstetrics and gynecology at Northwestern University, Chicago, thought Dr. Keyser’s talk was useful for the general gynecologist but had some concerns about a few parts.
“She gave a very thoughtful analysis of whether someone should have their ovaries removed or not in a breast cancer diagnosis, ” Dr. Streicher said in an interview. “I would have liked to hear more about the consequences of an early menopause in women in terms of heart health, bone health, and cognitive function.”
Dr. Keyser noted that her talk pertained mostly to survivors of estrogen receptor (ER)–positive breast cancer since that population tends to struggle most with side effects of treatment. The most common medications used in this population are tamoxifen and aromatase inhibitors – such as anastrazole, letrozole, and exemestane – and these medications can affect management of different concerns.
Current guidance on ovarian removal
For women with a BRCA mutation, ACOG clinical guidance already exists regarding BSO. For other women, the complementary TEXT and SOFT trials changed the management of breast cancer treatment in premenopausal women, Dr. Keyser said.
Before these trials, postmenopausal hormone receptor–positive women began aromatase inhibitors and premenopausal HR-positive women began tamoxifen. These trials found that premenopausal women with HR-positive early breast cancer were less likely to experience recurrence when receiving adjuvant treatment with exemestane plus ovarian suppression compared to tamoxifen plus ovarian suppression. Ovarian suppression was achieved by either GnRH agonist injections, surgical removal of the ovaries, or radiation therapy to the ovaries.
The side effects of these treatments included hot flushes (92%), depression (87%), musculoskeletal symptoms (89%), vaginal dryness (52%), decreased libido (45%), dyspareunia (31%), osteoporosis (39%), insomnia (58%), and fatigue (61%). These are all quality of life concerns, Dr. Keyser said, and these findings raise questions about the consequences of long-term ovarian suppression. Findings from the Nurses’ Health Study showed that BSO before age 47.5 years resulted in lower mortality from ovarian cancer and breast cancer but was linked in women under 50 to increased all-cause mortality and mortality from coronary heart disease, lung cancer, and colorectal cancer, compared with ovarian conservation. Further, 74% of women who undergo risk-reducing BSO experience sexual dysfunction.
The bottom line, Dr. Keyser said, is that “premature removal of ovaries is not completely benign.” Her own recommendation is to follow ACOG guidance for women with BRCA mutations and, for women aged under 35 years, use ovarian suppression for 5-10 years, after which ovarian function may resume along with improved quality of life. In women aged over 40, remove ovaries since, after 5-10 years of treatment, there’s likely no benefit of retaining ovaries.
Addressing sexual health
Dyspareunia affects up to 45% of cancer survivors, Dr. Keyser said, and multiple treatment options exist for breast cancer survivors. The therapies she discussed included lubricants, moisturizers, local vaginal estrogen, DHEA, ospemifene, and CO2 laser therapy.
Though Dr. Keyser briefly touched on vaginal lubricants and moisturizers, Dr. Streicher was disappointed that Dr. Keyser did not clearly define and differentiate between lubricants and moisturizers or mention hyaluronic acid products. Dr. Streicher also disagreed with the way Dr. Keyser represented the benefits of coconut oil as a lubricant. “Oils are not condom compatible and are known to potentially increase the risk of infection, and not just from poor handwashing,” Dr. Streicher said.
Small retrospective studies support the safety of topical vaginal estrogen in breast cancer survivors, Dr. Keyser said, and the 10-mcg Vagifem tablet and vaginal estradiol ring appear to have the lowest systemic absorption. ACOG guidance recommends that women taking aromatase inhibitors who don’t respond to nonhormonal approaches may benefit from switching temporarily to tamoxifen with vaginal estrogen and then returning to aromatase inhibitors. However, Dr. Keyser said there’s plenty of data to support using vaginal estrogen in patients taking aromatase inhibitors.
“I do feel that it’s safe for patients, whether they’re on tamoxifen or aromatase inhibitors, to take vaginal estrogen,” Dr. Keyser said. “I usually stick with the estradiol vaginal ring or the estradiol tablet, and I base that on a patient’s comfort with placing and removing a ring.” She also, instead of asking the patient’s hematologist-oncologist, simply notifies them of the treatment since most hematologist-oncologists are less familiar with the data.
Another effective option is vaginal DHEA/prasterone, which can significantly improve sexual desire, arousal, pain, and overall sexual function. Although breast cancer patients were included in early studies on DHEA, Intrarosa manufacturers excluded breast cancer patients in their Food and Drug Administration application, resulting in a package stating that “use of exogenous estrogen is contraindicated in women with a known or suspected history of breast cancer” and that “Intrarosa has not been studied in women with a history of breast cancer.” While that’s true for Intrarosa specifically, DHEA has been studied in breast cancer patients, Dr. Keyser said, so she expects to see more research in this area.
Ospemifene is another option for improving vulvovaginal atrophy but cannot be taken at the same time as tamoxifen. It has similar chemopreventive effects as tamoxifen in rat studies, but it’s not as effective. It’s a reasonable option in women with refractory genitourinary syndrome of menopause (GSM) who have completed their 5-10 years of adjuvant therapy and have no history of venous thromboembolism.
Dr. Keyser said CO2 laser therapy is still being studied for treating GSM, and current data have shown benefits for dyspareunia and vaginal dryness without documented harms. There have now been randomized, controlled trials; however, since it’s not FDA approved, it’s not covered by insurance and costs approximately $5,000 for three treatments.
Dr. Streicher was glad to see Dr. Keyser’s discussion of the safety and types of local vaginal estrogen, “although she neglected to mention the 4-mcg vaginal suppository, Imvexxy, which has the lowest systemic absorption,” Dr. Streicher said. Dr. Streicher also felt the inclusion of DHEA/prasterone and ospemifene were also important, especially since the latter is “underutilized in breast cancer patients.”
The information provided on CO2 laser therapy, however, was problematic, Dr. Streicher said, given that long-term and randomized, controlled studies have now been published. Dr. Streicher also noted that two of the devices listed on the presentation slide, Thermiva and Voltiva, are radiofrequency, not laser devices.
Aside from these treatment options, the most consistent predictor of satisfying sexual experiences in women with breast cancer is the quality of their relationships, Dr. Keyser said, so couples counseling is recommended, and treatments in general are more effective with regularly sexual activity.
In discussing nonhormonal options for treating vasomotor symptoms, Dr. Keyser recommended venlafaxine, gabapentin, and low-dose paroxetine (though SSRIs and tamoxifen are contraindicated since they may reduce tamoxifen’s efficacy).
These are all off label, Dr. Streicher said it was important to note, and she would have liked to have seen a mention of the development of KNdy neurokinin disrupters along with a more in-depth discussion about which lifestyle modifications and botanicals have been shown in randomized, controlled trials to mitigate vasomotor symptoms.
Dr. Keyser wrapped up with a few additional notes and takeaways:
- The only safe reversible long-term option for contraception in HR-positive breast cancer survivors is the Paraguard IUD.
- It’s important to discuss fertility with breast cancer patients and survivors since a majority report unmet needs in this area.
- Patients taking tamoxifen need to be sure to report any vaginal spotting or bleeding since it increases risk of endometrial cancer in postmenopausal women.
- Screen for depression and anxiety.
- Ask women about sexual health and hot flashes.
- Ensure that they’re getting bone screening.
- A recommended resource is Living Beyond Breast Cancer.
Dr. Keyser had no disclosures. Dr. Streicher has consulted for Astellas Pharma and Church & Dwight, and she owns investments in InControl Medical and Sermonix Pharmaceuticals.
FROM ACOG 2021
FDA set to okay Pfizer vaccine in younger teens
The Food and Drug Administration could expand the use of the Pfizer COVID-19 vaccine to teens early next week, The New York Times and CNN reported, both citing unnamed officials familiar with the agency’s plans.
In late March, Pfizer submitted data to the FDA showing its mRNA vaccine was 100% effective at preventing COVID-19 infection in children ages 12 to 15. Their vaccine is already authorized for use teens and adults ages 16 and older.
The move would make about 17 million more Americans eligible for vaccination and would be a major step toward getting both adolescents and teens back into classrooms full time by next fall.
“Across the globe, we are longing for a normal life. This is especially true for our children. The initial results we have seen in the adolescent studies suggest that children are particularly well protected by vaccination, which is very encouraging given the trends we have seen in recent weeks regarding the spread of the B.1.1.7 U.K. variant,” Ugur Sahin, CEO and co-founder of Pfizer partner BioNTech, said in a March 31 press release.
Getting schools fully reopened for in-person learning has been a goal of both the Trump and Biden administrations, but it has been tricky to pull off, as some parents and teachers have been reluctant to return to classrooms with so much uncertainty about the risk and the role of children in spreading the virus.
A recent study of roughly 150,000 school-aged children in Israel found that while kids under age 10 were unlikely to catch or spread the virus as they reentered classrooms. Older children, though, were a different story. The study found that children ages 10-19 had risks of catching the virus that were as high as adults ages 20-60.
The risk for severe illness and death from COVID-19 rises with age.
Children and teens are at relatively low risk from severe outcomes after a COVID-19 infection compared to adults, but they can catch it and some will get really sick with it, especially if they have an underlying health condition, like obesity or asthma that makes them more vulnerable.
Beyond the initial infection, children can get a rare late complication called MIS-C, that while treatable, can be severe and requires hospitalization. Emerging reports also suggest there are some kids that become long haulers in much the same way adults do, dealing with lingering problems for months after they first get sick.
As new variants of the coronavirus circulate in the United States, some states have seen big increases in the number of children and teens with COVID. In Michigan, for example, which recently dealt with a spring surge of cases dominated by the B.1.1.7 variant, cases in children and teens quadrupled in April compared to February.
Beyond individual protection, vaccinating children and teens has been seen as important to achieving strong community protection, or herd immunity, against the new coronavirus.
If the FDA expands the authorization for the Pfizer vaccine, the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices will likely meet to review data on the safety and efficacy of the vaccine. The committee may then vote on new recommendations for use of the vaccine in the United States.
Not everyone agrees with the idea that American adolescents, who are at relatively low risk of bad outcomes, could get access to COVID vaccines ahead of vulnerable essential workers and seniors in other parts of the world that are still fighting the pandemic with little access to vaccines.
A version of this article first appeared on WebMD.com.
The Food and Drug Administration could expand the use of the Pfizer COVID-19 vaccine to teens early next week, The New York Times and CNN reported, both citing unnamed officials familiar with the agency’s plans.
In late March, Pfizer submitted data to the FDA showing its mRNA vaccine was 100% effective at preventing COVID-19 infection in children ages 12 to 15. Their vaccine is already authorized for use teens and adults ages 16 and older.
The move would make about 17 million more Americans eligible for vaccination and would be a major step toward getting both adolescents and teens back into classrooms full time by next fall.
“Across the globe, we are longing for a normal life. This is especially true for our children. The initial results we have seen in the adolescent studies suggest that children are particularly well protected by vaccination, which is very encouraging given the trends we have seen in recent weeks regarding the spread of the B.1.1.7 U.K. variant,” Ugur Sahin, CEO and co-founder of Pfizer partner BioNTech, said in a March 31 press release.
Getting schools fully reopened for in-person learning has been a goal of both the Trump and Biden administrations, but it has been tricky to pull off, as some parents and teachers have been reluctant to return to classrooms with so much uncertainty about the risk and the role of children in spreading the virus.
A recent study of roughly 150,000 school-aged children in Israel found that while kids under age 10 were unlikely to catch or spread the virus as they reentered classrooms. Older children, though, were a different story. The study found that children ages 10-19 had risks of catching the virus that were as high as adults ages 20-60.
The risk for severe illness and death from COVID-19 rises with age.
Children and teens are at relatively low risk from severe outcomes after a COVID-19 infection compared to adults, but they can catch it and some will get really sick with it, especially if they have an underlying health condition, like obesity or asthma that makes them more vulnerable.
Beyond the initial infection, children can get a rare late complication called MIS-C, that while treatable, can be severe and requires hospitalization. Emerging reports also suggest there are some kids that become long haulers in much the same way adults do, dealing with lingering problems for months after they first get sick.
As new variants of the coronavirus circulate in the United States, some states have seen big increases in the number of children and teens with COVID. In Michigan, for example, which recently dealt with a spring surge of cases dominated by the B.1.1.7 variant, cases in children and teens quadrupled in April compared to February.
Beyond individual protection, vaccinating children and teens has been seen as important to achieving strong community protection, or herd immunity, against the new coronavirus.
If the FDA expands the authorization for the Pfizer vaccine, the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices will likely meet to review data on the safety and efficacy of the vaccine. The committee may then vote on new recommendations for use of the vaccine in the United States.
Not everyone agrees with the idea that American adolescents, who are at relatively low risk of bad outcomes, could get access to COVID vaccines ahead of vulnerable essential workers and seniors in other parts of the world that are still fighting the pandemic with little access to vaccines.
A version of this article first appeared on WebMD.com.
The Food and Drug Administration could expand the use of the Pfizer COVID-19 vaccine to teens early next week, The New York Times and CNN reported, both citing unnamed officials familiar with the agency’s plans.
In late March, Pfizer submitted data to the FDA showing its mRNA vaccine was 100% effective at preventing COVID-19 infection in children ages 12 to 15. Their vaccine is already authorized for use teens and adults ages 16 and older.
The move would make about 17 million more Americans eligible for vaccination and would be a major step toward getting both adolescents and teens back into classrooms full time by next fall.
“Across the globe, we are longing for a normal life. This is especially true for our children. The initial results we have seen in the adolescent studies suggest that children are particularly well protected by vaccination, which is very encouraging given the trends we have seen in recent weeks regarding the spread of the B.1.1.7 U.K. variant,” Ugur Sahin, CEO and co-founder of Pfizer partner BioNTech, said in a March 31 press release.
Getting schools fully reopened for in-person learning has been a goal of both the Trump and Biden administrations, but it has been tricky to pull off, as some parents and teachers have been reluctant to return to classrooms with so much uncertainty about the risk and the role of children in spreading the virus.
A recent study of roughly 150,000 school-aged children in Israel found that while kids under age 10 were unlikely to catch or spread the virus as they reentered classrooms. Older children, though, were a different story. The study found that children ages 10-19 had risks of catching the virus that were as high as adults ages 20-60.
The risk for severe illness and death from COVID-19 rises with age.
Children and teens are at relatively low risk from severe outcomes after a COVID-19 infection compared to adults, but they can catch it and some will get really sick with it, especially if they have an underlying health condition, like obesity or asthma that makes them more vulnerable.
Beyond the initial infection, children can get a rare late complication called MIS-C, that while treatable, can be severe and requires hospitalization. Emerging reports also suggest there are some kids that become long haulers in much the same way adults do, dealing with lingering problems for months after they first get sick.
As new variants of the coronavirus circulate in the United States, some states have seen big increases in the number of children and teens with COVID. In Michigan, for example, which recently dealt with a spring surge of cases dominated by the B.1.1.7 variant, cases in children and teens quadrupled in April compared to February.
Beyond individual protection, vaccinating children and teens has been seen as important to achieving strong community protection, or herd immunity, against the new coronavirus.
If the FDA expands the authorization for the Pfizer vaccine, the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices will likely meet to review data on the safety and efficacy of the vaccine. The committee may then vote on new recommendations for use of the vaccine in the United States.
Not everyone agrees with the idea that American adolescents, who are at relatively low risk of bad outcomes, could get access to COVID vaccines ahead of vulnerable essential workers and seniors in other parts of the world that are still fighting the pandemic with little access to vaccines.
A version of this article first appeared on WebMD.com.