User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
Corticosteroid bursts may increase risk of sepsis, GI bleeding in children
The adverse events are rare, and the risk attenuates in subsequent months, the analysis shows. Still, the study “provides evidence that corticosteroid bursts are not innocuous but may pose potentially serious health risks,” study author Tsung-Chieh Yao, MD, PhD, and colleagues said. “Clinicians prescribing corticosteroid bursts to children need to weigh the benefits against the risks of severe adverse events.”
The study, which was published online in JAMA Pediatrics, indicates that oral corticosteroids are “not a benign medication, which is something that we should have all along known,” commented Harold J. Farber, MD, MSPH, professor of pediatrics at Baylor College of Medicine and a pediatric pulmonologist at Texas Children’s Hospital, both in Houston.
While oral corticosteroids may be important for the treatment of asthma, inflammatory bowel disease, and rheumatoid arthritis, they often are overprescribed – a phenomenon that Dr. Farber and collaborators saw when they analyzed data from children with public health insurance in Texas.
The medication is “not uncommonly used for minor asthma exacerbations or minor respiratory symptoms, which do not require oral steroids,” said Dr. Farber, who was not involved with the study. “What this study tells us is to save it for when they are really needed,” such as to treat a severe asthma exacerbation.
Despite the risk of adverse events, oral corticosteroids remain an important medication, and clinicians should aim to strike “the right balance,” Dr. Farber said.
Prior research has shown that the long-term use of oral corticosteroids is associated with adverse events such as infections, glaucoma, hyperglycemia, cardiovascular diseases, and osteoporosis. In addition, data indicate that corticosteroid bursts are associated with GI bleeding and sepsis in adults. But few studies have looked at the risk of corticosteroid bursts in children, the researchers said.
To evaluate associations of corticosteroid bursts – defined as the use of oral corticosteroids for 14 days or less – with GI bleeding, sepsis, pneumonia, and glaucoma in children, Dr. Yao and colleagues analyzed data from the National Health Insurance Research Database in Taiwan between 2013 and 2017. Dr. Yao is affiliated with the division of allergy, asthma, and rheumatology in the department of pediatrics at Chang Gung Memorial Hospital in Taoyuan City, Taiwan.
Of more than 4.5 million children in the database, 42% received at least one corticosteroid burst, typically for acute respiratory tract infections and allergic diseases. The researchers focused on 1,064,587 children who received a single corticosteroid burst, and compared the incidence of adverse events before and after treatment using a self-controlled case series design. “Corticosteroid bursts were significantly associated with a 1.4- to 2.2-fold increase of GI bleeding, sepsis, and pneumonia, but not glaucoma, within the first month after initiation of corticosteroid therapy,” the investigators reported.
Incidence rate ratios in the 5-30 days after starting corticosteroid bursts were 1.41 for GI bleeding, 2.02 for sepsis, 2.19 for pneumonia, and 0.98 for glaucoma, compared with a pretreatment reference period.
The incidence rate per 1,000 person-years for GI bleeding was 2.48 with corticosteroid bursts, compared with 1.88 without corticosteroids. For sepsis, the rates with and without corticosteroids were 0.37 and 0.34, respectively. And for pneumonia, the rates were 25.74 versus 16.39.
Further research is needed to assess the validity of these findings, the authors noted. Because many children receive corticosteroid bursts worldwide, however, the “findings call for a careful reevaluation regarding the prudent use” of this treatment.
The study was supported by grants from the National Health Research Institutes; Ministry of Science and Technology of Taiwan; National Cheng Kung University, Tainan, Taiwan; Chang Gung Medical Foundation; and the National Institutes of Health. A coauthor disclosed grants from GlaxoSmithKline outside of the study.
The adverse events are rare, and the risk attenuates in subsequent months, the analysis shows. Still, the study “provides evidence that corticosteroid bursts are not innocuous but may pose potentially serious health risks,” study author Tsung-Chieh Yao, MD, PhD, and colleagues said. “Clinicians prescribing corticosteroid bursts to children need to weigh the benefits against the risks of severe adverse events.”
The study, which was published online in JAMA Pediatrics, indicates that oral corticosteroids are “not a benign medication, which is something that we should have all along known,” commented Harold J. Farber, MD, MSPH, professor of pediatrics at Baylor College of Medicine and a pediatric pulmonologist at Texas Children’s Hospital, both in Houston.
While oral corticosteroids may be important for the treatment of asthma, inflammatory bowel disease, and rheumatoid arthritis, they often are overprescribed – a phenomenon that Dr. Farber and collaborators saw when they analyzed data from children with public health insurance in Texas.
The medication is “not uncommonly used for minor asthma exacerbations or minor respiratory symptoms, which do not require oral steroids,” said Dr. Farber, who was not involved with the study. “What this study tells us is to save it for when they are really needed,” such as to treat a severe asthma exacerbation.
Despite the risk of adverse events, oral corticosteroids remain an important medication, and clinicians should aim to strike “the right balance,” Dr. Farber said.
Prior research has shown that the long-term use of oral corticosteroids is associated with adverse events such as infections, glaucoma, hyperglycemia, cardiovascular diseases, and osteoporosis. In addition, data indicate that corticosteroid bursts are associated with GI bleeding and sepsis in adults. But few studies have looked at the risk of corticosteroid bursts in children, the researchers said.
To evaluate associations of corticosteroid bursts – defined as the use of oral corticosteroids for 14 days or less – with GI bleeding, sepsis, pneumonia, and glaucoma in children, Dr. Yao and colleagues analyzed data from the National Health Insurance Research Database in Taiwan between 2013 and 2017. Dr. Yao is affiliated with the division of allergy, asthma, and rheumatology in the department of pediatrics at Chang Gung Memorial Hospital in Taoyuan City, Taiwan.
Of more than 4.5 million children in the database, 42% received at least one corticosteroid burst, typically for acute respiratory tract infections and allergic diseases. The researchers focused on 1,064,587 children who received a single corticosteroid burst, and compared the incidence of adverse events before and after treatment using a self-controlled case series design. “Corticosteroid bursts were significantly associated with a 1.4- to 2.2-fold increase of GI bleeding, sepsis, and pneumonia, but not glaucoma, within the first month after initiation of corticosteroid therapy,” the investigators reported.
Incidence rate ratios in the 5-30 days after starting corticosteroid bursts were 1.41 for GI bleeding, 2.02 for sepsis, 2.19 for pneumonia, and 0.98 for glaucoma, compared with a pretreatment reference period.
The incidence rate per 1,000 person-years for GI bleeding was 2.48 with corticosteroid bursts, compared with 1.88 without corticosteroids. For sepsis, the rates with and without corticosteroids were 0.37 and 0.34, respectively. And for pneumonia, the rates were 25.74 versus 16.39.
Further research is needed to assess the validity of these findings, the authors noted. Because many children receive corticosteroid bursts worldwide, however, the “findings call for a careful reevaluation regarding the prudent use” of this treatment.
The study was supported by grants from the National Health Research Institutes; Ministry of Science and Technology of Taiwan; National Cheng Kung University, Tainan, Taiwan; Chang Gung Medical Foundation; and the National Institutes of Health. A coauthor disclosed grants from GlaxoSmithKline outside of the study.
The adverse events are rare, and the risk attenuates in subsequent months, the analysis shows. Still, the study “provides evidence that corticosteroid bursts are not innocuous but may pose potentially serious health risks,” study author Tsung-Chieh Yao, MD, PhD, and colleagues said. “Clinicians prescribing corticosteroid bursts to children need to weigh the benefits against the risks of severe adverse events.”
The study, which was published online in JAMA Pediatrics, indicates that oral corticosteroids are “not a benign medication, which is something that we should have all along known,” commented Harold J. Farber, MD, MSPH, professor of pediatrics at Baylor College of Medicine and a pediatric pulmonologist at Texas Children’s Hospital, both in Houston.
While oral corticosteroids may be important for the treatment of asthma, inflammatory bowel disease, and rheumatoid arthritis, they often are overprescribed – a phenomenon that Dr. Farber and collaborators saw when they analyzed data from children with public health insurance in Texas.
The medication is “not uncommonly used for minor asthma exacerbations or minor respiratory symptoms, which do not require oral steroids,” said Dr. Farber, who was not involved with the study. “What this study tells us is to save it for when they are really needed,” such as to treat a severe asthma exacerbation.
Despite the risk of adverse events, oral corticosteroids remain an important medication, and clinicians should aim to strike “the right balance,” Dr. Farber said.
Prior research has shown that the long-term use of oral corticosteroids is associated with adverse events such as infections, glaucoma, hyperglycemia, cardiovascular diseases, and osteoporosis. In addition, data indicate that corticosteroid bursts are associated with GI bleeding and sepsis in adults. But few studies have looked at the risk of corticosteroid bursts in children, the researchers said.
To evaluate associations of corticosteroid bursts – defined as the use of oral corticosteroids for 14 days or less – with GI bleeding, sepsis, pneumonia, and glaucoma in children, Dr. Yao and colleagues analyzed data from the National Health Insurance Research Database in Taiwan between 2013 and 2017. Dr. Yao is affiliated with the division of allergy, asthma, and rheumatology in the department of pediatrics at Chang Gung Memorial Hospital in Taoyuan City, Taiwan.
Of more than 4.5 million children in the database, 42% received at least one corticosteroid burst, typically for acute respiratory tract infections and allergic diseases. The researchers focused on 1,064,587 children who received a single corticosteroid burst, and compared the incidence of adverse events before and after treatment using a self-controlled case series design. “Corticosteroid bursts were significantly associated with a 1.4- to 2.2-fold increase of GI bleeding, sepsis, and pneumonia, but not glaucoma, within the first month after initiation of corticosteroid therapy,” the investigators reported.
Incidence rate ratios in the 5-30 days after starting corticosteroid bursts were 1.41 for GI bleeding, 2.02 for sepsis, 2.19 for pneumonia, and 0.98 for glaucoma, compared with a pretreatment reference period.
The incidence rate per 1,000 person-years for GI bleeding was 2.48 with corticosteroid bursts, compared with 1.88 without corticosteroids. For sepsis, the rates with and without corticosteroids were 0.37 and 0.34, respectively. And for pneumonia, the rates were 25.74 versus 16.39.
Further research is needed to assess the validity of these findings, the authors noted. Because many children receive corticosteroid bursts worldwide, however, the “findings call for a careful reevaluation regarding the prudent use” of this treatment.
The study was supported by grants from the National Health Research Institutes; Ministry of Science and Technology of Taiwan; National Cheng Kung University, Tainan, Taiwan; Chang Gung Medical Foundation; and the National Institutes of Health. A coauthor disclosed grants from GlaxoSmithKline outside of the study.
FROM JAMA PEDIATRICS
HPV vaccination rates continue to climb among young adults in U.S.
Although vaccination rates against the human papillomavirus remain low for young adults across the United States, the number of self-reported HPV vaccinations among women and men aged between 18 and 21 years has markedly increased since 2010, according to new research findings.
The findings were published online April 27, 2021, as a research letter in JAMA.
In 2006, the Food and Drug Administration approved the HPV vaccine for the prevention of cervical cancer and genital warts in female patients. Three years later, the FDA approved the vaccine for the prevention of anogenital cancer and warts in male patients.
The Advisory Committee on Immunization Practices and the Centers for Disease Control and Prevention recommend two doses of the HPV vaccine for children aged 11-12 years. Adolescents and young adults may need three doses over the course of 6 months if they start their vaccine series on or following their 15th birthday.
For persons who have not previously received the HPV vaccine or who did not receive adequate doses, the HPV vaccine is recommended through age 26. Data on the rates of vaccination among young adults between 18 and 21 years of age in the United States are sparse, and it is not known how well vaccination programs are progressing in the country.
In the recently published JAMA research letter, investigators from the University of Michigan, Ann Arbor, examined data for the period 2010-2018 from the cross-sectional National Health Interview Survey. Respondents included in the analysis were aged 18-21 years. They were asked whether they had received the HPV vaccine before age 18 and at what age they had been vaccinated against the virus.
The researchers also assessed whether the respondents had received any HPV vaccine dose between the ages of 18 and 21 years. The findings were limited to self-reported vaccination status.
In total, 6,606 women and 6,038 men were included in the analysis. Approximately 42% of women and 16% of men said they had received at least one HPV vaccine dose at any age. The proportion of female patients who reported receiving an HPV vaccine dose significantly increased from 32% in 2010 to 55% in 2018 (P =.001). Similarly, among men, the percentage significantly increased from 2% in 2010 to 34% in 2018 (P <.001).
Approximately 4% of the female respondents and 3% of the male respondents reported that they had received an HPV vaccine between the ages of 18 and 21 years; 46% of women and 29% of men who received the vaccine between these ages completed the recommended vaccination series.
Findings from the study highlight the continual need for improving vaccination rates among vulnerable populations. Lead study author Michelle Chen, MD, MHS, a professor in the department of otolaryngology–head and neck surgery at the University of Michigan, explained in an interview that there are multiple barriers to HPV vaccination among young adults. “These barriers to vaccination among young adults primarily include cost, lack of knowledge and awareness, missed opportunities for vaccination, rapidly changing guidelines, and initial gender-based guidelines,” said Dr. Chen.
Clinicians play a large role in improving vaccination rates among young adults, who may lack awareness of the overall importance of inoculation against the potentially debilitating and deadly virus. Dr. Chen noted that clinicians can lead the way by increasing gender-inclusive awareness of HPV-associated diseases and HPV vaccination, by performing routine vaccine eligibility assessments for young adults regardless of sex, by developing robust reminder and recall strategies to improve series completion rates, and by offering patients resources regarding assistance programs to address cost barriers for uninsured patients.
“Young adult men are particularly vulnerable [to HPV], because they start to age out of pediatric health practices,” added Dr. Chen. “Thus, a multilevel gender-inclusive approach is needed to target clinicians, patients, parents, and community-based organizations.”
Gypsyamber D’Souza, PhD, professor of epidemiology at Johns Hopkins University, Baltimore, said in an interview that the initial uptake of HPV vaccination was slow in the United States but that progress has been made in recent years among persons in the targeted age range of 11-12 years. “However, catch-up vaccination has lagged behind, and sadly, we’re still seeing low uptake in those older ages that are still eligible and where we know there still is tremendous benefit,” she said.
Dr. D’Souza is a lead investigator in the MOUTH trial, which is currently enrolling patients. That trial will examine potential biomarkers for oropharyngeal cancer risk among people with known risk factors for HPV who came of age prior to the rollout of the vaccine.
She explained that many parents want their children to be vaccinated for HPV after they hear about the vaccine, but because the health care system in the United States is an “opt-in” system, rather than an “opt-out” one, parents need to actively seek out vaccination. Children then move toward adulthood without having received the recommended vaccine course. “There are individuals who did not get vaccinated at the ages of 11 and 12 and then forget to ask about it later, or the provider asks about it and the patients don’t have enough information,” Dr. D’Souza said.
She noted that one reason why HPV vaccination rates remain low among young adults is that the vaccine is not often kept in stock other than in pediatric clinics. “Because vaccines expire and clinics don’t have a lot of people in that age group getting vaccinated, they may not have it regularly in stock, making this one reason it might be hard for someone to get vaccinated.”
The HPV vaccine is not effective for clearing HPV once a patient acquires the infection, she added. “So young adulthood is a critical time where we have individuals who still can benefit from being vaccinated, but if we wait too long, they’ll age out of those ages where we see the highest efficacy.”
Ultimately, said Dr. D’Souza, clinicians need to catch people at multiple time points and work to remove barriers to vaccination, including letting patients know that HPV vaccination is covered by insurance. “There’s a lot of opportunity to prevent future cancers in young adults by having care providers for that age group talk about the vaccine and remember to offer it.”
A version of this article first appeared on Medscape.com.
Although vaccination rates against the human papillomavirus remain low for young adults across the United States, the number of self-reported HPV vaccinations among women and men aged between 18 and 21 years has markedly increased since 2010, according to new research findings.
The findings were published online April 27, 2021, as a research letter in JAMA.
In 2006, the Food and Drug Administration approved the HPV vaccine for the prevention of cervical cancer and genital warts in female patients. Three years later, the FDA approved the vaccine for the prevention of anogenital cancer and warts in male patients.
The Advisory Committee on Immunization Practices and the Centers for Disease Control and Prevention recommend two doses of the HPV vaccine for children aged 11-12 years. Adolescents and young adults may need three doses over the course of 6 months if they start their vaccine series on or following their 15th birthday.
For persons who have not previously received the HPV vaccine or who did not receive adequate doses, the HPV vaccine is recommended through age 26. Data on the rates of vaccination among young adults between 18 and 21 years of age in the United States are sparse, and it is not known how well vaccination programs are progressing in the country.
In the recently published JAMA research letter, investigators from the University of Michigan, Ann Arbor, examined data for the period 2010-2018 from the cross-sectional National Health Interview Survey. Respondents included in the analysis were aged 18-21 years. They were asked whether they had received the HPV vaccine before age 18 and at what age they had been vaccinated against the virus.
The researchers also assessed whether the respondents had received any HPV vaccine dose between the ages of 18 and 21 years. The findings were limited to self-reported vaccination status.
In total, 6,606 women and 6,038 men were included in the analysis. Approximately 42% of women and 16% of men said they had received at least one HPV vaccine dose at any age. The proportion of female patients who reported receiving an HPV vaccine dose significantly increased from 32% in 2010 to 55% in 2018 (P =.001). Similarly, among men, the percentage significantly increased from 2% in 2010 to 34% in 2018 (P <.001).
Approximately 4% of the female respondents and 3% of the male respondents reported that they had received an HPV vaccine between the ages of 18 and 21 years; 46% of women and 29% of men who received the vaccine between these ages completed the recommended vaccination series.
Findings from the study highlight the continual need for improving vaccination rates among vulnerable populations. Lead study author Michelle Chen, MD, MHS, a professor in the department of otolaryngology–head and neck surgery at the University of Michigan, explained in an interview that there are multiple barriers to HPV vaccination among young adults. “These barriers to vaccination among young adults primarily include cost, lack of knowledge and awareness, missed opportunities for vaccination, rapidly changing guidelines, and initial gender-based guidelines,” said Dr. Chen.
Clinicians play a large role in improving vaccination rates among young adults, who may lack awareness of the overall importance of inoculation against the potentially debilitating and deadly virus. Dr. Chen noted that clinicians can lead the way by increasing gender-inclusive awareness of HPV-associated diseases and HPV vaccination, by performing routine vaccine eligibility assessments for young adults regardless of sex, by developing robust reminder and recall strategies to improve series completion rates, and by offering patients resources regarding assistance programs to address cost barriers for uninsured patients.
“Young adult men are particularly vulnerable [to HPV], because they start to age out of pediatric health practices,” added Dr. Chen. “Thus, a multilevel gender-inclusive approach is needed to target clinicians, patients, parents, and community-based organizations.”
Gypsyamber D’Souza, PhD, professor of epidemiology at Johns Hopkins University, Baltimore, said in an interview that the initial uptake of HPV vaccination was slow in the United States but that progress has been made in recent years among persons in the targeted age range of 11-12 years. “However, catch-up vaccination has lagged behind, and sadly, we’re still seeing low uptake in those older ages that are still eligible and where we know there still is tremendous benefit,” she said.
Dr. D’Souza is a lead investigator in the MOUTH trial, which is currently enrolling patients. That trial will examine potential biomarkers for oropharyngeal cancer risk among people with known risk factors for HPV who came of age prior to the rollout of the vaccine.
She explained that many parents want their children to be vaccinated for HPV after they hear about the vaccine, but because the health care system in the United States is an “opt-in” system, rather than an “opt-out” one, parents need to actively seek out vaccination. Children then move toward adulthood without having received the recommended vaccine course. “There are individuals who did not get vaccinated at the ages of 11 and 12 and then forget to ask about it later, or the provider asks about it and the patients don’t have enough information,” Dr. D’Souza said.
She noted that one reason why HPV vaccination rates remain low among young adults is that the vaccine is not often kept in stock other than in pediatric clinics. “Because vaccines expire and clinics don’t have a lot of people in that age group getting vaccinated, they may not have it regularly in stock, making this one reason it might be hard for someone to get vaccinated.”
The HPV vaccine is not effective for clearing HPV once a patient acquires the infection, she added. “So young adulthood is a critical time where we have individuals who still can benefit from being vaccinated, but if we wait too long, they’ll age out of those ages where we see the highest efficacy.”
Ultimately, said Dr. D’Souza, clinicians need to catch people at multiple time points and work to remove barriers to vaccination, including letting patients know that HPV vaccination is covered by insurance. “There’s a lot of opportunity to prevent future cancers in young adults by having care providers for that age group talk about the vaccine and remember to offer it.”
A version of this article first appeared on Medscape.com.
Although vaccination rates against the human papillomavirus remain low for young adults across the United States, the number of self-reported HPV vaccinations among women and men aged between 18 and 21 years has markedly increased since 2010, according to new research findings.
The findings were published online April 27, 2021, as a research letter in JAMA.
In 2006, the Food and Drug Administration approved the HPV vaccine for the prevention of cervical cancer and genital warts in female patients. Three years later, the FDA approved the vaccine for the prevention of anogenital cancer and warts in male patients.
The Advisory Committee on Immunization Practices and the Centers for Disease Control and Prevention recommend two doses of the HPV vaccine for children aged 11-12 years. Adolescents and young adults may need three doses over the course of 6 months if they start their vaccine series on or following their 15th birthday.
For persons who have not previously received the HPV vaccine or who did not receive adequate doses, the HPV vaccine is recommended through age 26. Data on the rates of vaccination among young adults between 18 and 21 years of age in the United States are sparse, and it is not known how well vaccination programs are progressing in the country.
In the recently published JAMA research letter, investigators from the University of Michigan, Ann Arbor, examined data for the period 2010-2018 from the cross-sectional National Health Interview Survey. Respondents included in the analysis were aged 18-21 years. They were asked whether they had received the HPV vaccine before age 18 and at what age they had been vaccinated against the virus.
The researchers also assessed whether the respondents had received any HPV vaccine dose between the ages of 18 and 21 years. The findings were limited to self-reported vaccination status.
In total, 6,606 women and 6,038 men were included in the analysis. Approximately 42% of women and 16% of men said they had received at least one HPV vaccine dose at any age. The proportion of female patients who reported receiving an HPV vaccine dose significantly increased from 32% in 2010 to 55% in 2018 (P =.001). Similarly, among men, the percentage significantly increased from 2% in 2010 to 34% in 2018 (P <.001).
Approximately 4% of the female respondents and 3% of the male respondents reported that they had received an HPV vaccine between the ages of 18 and 21 years; 46% of women and 29% of men who received the vaccine between these ages completed the recommended vaccination series.
Findings from the study highlight the continual need for improving vaccination rates among vulnerable populations. Lead study author Michelle Chen, MD, MHS, a professor in the department of otolaryngology–head and neck surgery at the University of Michigan, explained in an interview that there are multiple barriers to HPV vaccination among young adults. “These barriers to vaccination among young adults primarily include cost, lack of knowledge and awareness, missed opportunities for vaccination, rapidly changing guidelines, and initial gender-based guidelines,” said Dr. Chen.
Clinicians play a large role in improving vaccination rates among young adults, who may lack awareness of the overall importance of inoculation against the potentially debilitating and deadly virus. Dr. Chen noted that clinicians can lead the way by increasing gender-inclusive awareness of HPV-associated diseases and HPV vaccination, by performing routine vaccine eligibility assessments for young adults regardless of sex, by developing robust reminder and recall strategies to improve series completion rates, and by offering patients resources regarding assistance programs to address cost barriers for uninsured patients.
“Young adult men are particularly vulnerable [to HPV], because they start to age out of pediatric health practices,” added Dr. Chen. “Thus, a multilevel gender-inclusive approach is needed to target clinicians, patients, parents, and community-based organizations.”
Gypsyamber D’Souza, PhD, professor of epidemiology at Johns Hopkins University, Baltimore, said in an interview that the initial uptake of HPV vaccination was slow in the United States but that progress has been made in recent years among persons in the targeted age range of 11-12 years. “However, catch-up vaccination has lagged behind, and sadly, we’re still seeing low uptake in those older ages that are still eligible and where we know there still is tremendous benefit,” she said.
Dr. D’Souza is a lead investigator in the MOUTH trial, which is currently enrolling patients. That trial will examine potential biomarkers for oropharyngeal cancer risk among people with known risk factors for HPV who came of age prior to the rollout of the vaccine.
She explained that many parents want their children to be vaccinated for HPV after they hear about the vaccine, but because the health care system in the United States is an “opt-in” system, rather than an “opt-out” one, parents need to actively seek out vaccination. Children then move toward adulthood without having received the recommended vaccine course. “There are individuals who did not get vaccinated at the ages of 11 and 12 and then forget to ask about it later, or the provider asks about it and the patients don’t have enough information,” Dr. D’Souza said.
She noted that one reason why HPV vaccination rates remain low among young adults is that the vaccine is not often kept in stock other than in pediatric clinics. “Because vaccines expire and clinics don’t have a lot of people in that age group getting vaccinated, they may not have it regularly in stock, making this one reason it might be hard for someone to get vaccinated.”
The HPV vaccine is not effective for clearing HPV once a patient acquires the infection, she added. “So young adulthood is a critical time where we have individuals who still can benefit from being vaccinated, but if we wait too long, they’ll age out of those ages where we see the highest efficacy.”
Ultimately, said Dr. D’Souza, clinicians need to catch people at multiple time points and work to remove barriers to vaccination, including letting patients know that HPV vaccination is covered by insurance. “There’s a lot of opportunity to prevent future cancers in young adults by having care providers for that age group talk about the vaccine and remember to offer it.”
A version of this article first appeared on Medscape.com.
FDA moves to ban menthol in cigarettes
The Food and Drug Administration said that within a year it will ban menthol in cigarettes and ban all flavors including menthol in cigars.
Menthol makes it easier to start smoking, and also enhances the effects of nicotine, making it more addictive and harder to quit, the FDA said in announcing its actions on Thursday.
Nineteen organizations – including the American Academy of Pediatrics, American Cancer Society, American College of Chest Physicians, American Medical Association, American Heart Association, and the National Medical Association – have pushed the FDA to ban menthol for years. The agency banned all flavors in cigarettes in 2009 but did not take any action against menthol. In 2013, the groups filed a petition demanding that the FDA ban menthol, too. The agency responded months later with a notice that it would start the process.
But it never took any action. Action on Smoking and Health and the African American Tobacco Control Leadership Council, later joined by the AMA and the NMA, sued in 2020 to compel the agency to do something. Now it has finally agreed to act.
The African American Tobacco Control Leadership Council welcomed the move but said the fight is not over and encouraged tobacco control activists to fight to ban menthol tobacco products at the local, state and federal level. “We know that this rule-making process could take years and we know that the tobacco industry will continue to do everything in their power to derail any attempt to remove their deadly products from the market,” Phillip Gardiner, MD, council cochair, said in a statement.
The AMA is urging the FDA to quickly implement the ban and remove the products “without further delay,” AMA President Susan R. Bailey, MD, said in a statement.
“FDA’s long-awaited decision to take action to eliminate menthol flavoring in cigarettes and all flavors in cigars ends a decades-long deference to the tobacco industry, which has repeatedly demonstrated its willingness to profit from products that result in death,” Lisa Lacasse, president of the American Cancer Society Cancer Action Network, said in her own statement.
Ms. Lacasse said banning menthol will help eliminate health disparities. She said 86% of Black people who smoke use menthol cigarettes, compared with 46% of Hispanic people who smoke, 39% of Asian people who smoke, and 29% of White people who smoke. “FDA’s actions today send a clear message that Big Tobacco’s strategy to profit off addicting Black communities will no longer be tolerated,” she said.
Not all groups are on board, however. The American Civil Liberties Union and several other organizations wrote to the country’s top health officials urging them to reconsider.
“Such a ban will trigger criminal penalties which will disproportionately impact people of color, as well as prioritize criminalization over public health and harm reduction,” the letter says. “A ban will also lead to unconstitutional policing and other negative interactions with local law enforcement.”
The letter calls the proposed ban “well intentioned,” but said any effort to reduce death and disease from tobacco “must avoid solutions that will create yet another reason for armed police to engage citizens on the street based on pretext or conduct that does not pose a threat to public safety.”
Instead of a ban, the organizations said, policy makers should consider increased education for adults and minors, stop-smoking programs, and increased funding for health centers in communities of color.
The Biden administration, however, pressed the point that banning menthol will bring many positives. Acting FDA Commissioner Janet Woodcock, MD said in a statement that banning menthol “will help significantly reduce youth initiation, increase the chances of smoking cessation among current smokers, and address health disparities experienced by communities of color, low-income populations, and LGBTQ-plus individuals, all of whom are far more likely to use these tobacco products.”
The FDA cited data showing that, in the first year or so after a ban goes into effect, an additional 923,000 smokers would quit, including 230,000 African Americans. Another study suggests that 633,000 deaths would be averted, including 237,000 Black Americans.
Dr. Woodcock added that, “armed with strong scientific evidence, and with full support from the [Biden] administration, we believe these actions will launch us on a trajectory toward ending tobacco-related disease and death in the U.S.”
The FDA estimates that 18.6 million Americans who are current smokers use menthol cigarettes, with a disproportionately high number being Black people. Menthol cigarette use among Black and Hispanic youth increased from 2011 to 2018, but declined for non-Hispanic White youth.
Flavored mass-produced cigars and cigarillos are disproportionately popular among youth, especially non-Hispanic Black high school students, who in 2020 reported past 30-day cigar smoking at levels twice as high as their White counterparts, said the FDA. Three-quarters of 12- to 17-year-olds reported they smoke cigars because they like the flavors. In 2020, more young people tried a cigar every day than tried a cigarette, reports the agency.
“This long-overdue decision will protect future generations of young people from nicotine addiction, especially Black children and communities, which have disproportionately suffered from menthol tobacco use due to targeted efforts from the tobacco industry,” Lee Savio Beers, MD, president of the American Academy of Pediatrics, said in a statement.
The FDA’s announcement “is only a first step that must be followed with urgent, comprehensive action to remove these flavored products from the market,” he said.
A version of this article first appeared on WebMD.com.
The Food and Drug Administration said that within a year it will ban menthol in cigarettes and ban all flavors including menthol in cigars.
Menthol makes it easier to start smoking, and also enhances the effects of nicotine, making it more addictive and harder to quit, the FDA said in announcing its actions on Thursday.
Nineteen organizations – including the American Academy of Pediatrics, American Cancer Society, American College of Chest Physicians, American Medical Association, American Heart Association, and the National Medical Association – have pushed the FDA to ban menthol for years. The agency banned all flavors in cigarettes in 2009 but did not take any action against menthol. In 2013, the groups filed a petition demanding that the FDA ban menthol, too. The agency responded months later with a notice that it would start the process.
But it never took any action. Action on Smoking and Health and the African American Tobacco Control Leadership Council, later joined by the AMA and the NMA, sued in 2020 to compel the agency to do something. Now it has finally agreed to act.
The African American Tobacco Control Leadership Council welcomed the move but said the fight is not over and encouraged tobacco control activists to fight to ban menthol tobacco products at the local, state and federal level. “We know that this rule-making process could take years and we know that the tobacco industry will continue to do everything in their power to derail any attempt to remove their deadly products from the market,” Phillip Gardiner, MD, council cochair, said in a statement.
The AMA is urging the FDA to quickly implement the ban and remove the products “without further delay,” AMA President Susan R. Bailey, MD, said in a statement.
“FDA’s long-awaited decision to take action to eliminate menthol flavoring in cigarettes and all flavors in cigars ends a decades-long deference to the tobacco industry, which has repeatedly demonstrated its willingness to profit from products that result in death,” Lisa Lacasse, president of the American Cancer Society Cancer Action Network, said in her own statement.
Ms. Lacasse said banning menthol will help eliminate health disparities. She said 86% of Black people who smoke use menthol cigarettes, compared with 46% of Hispanic people who smoke, 39% of Asian people who smoke, and 29% of White people who smoke. “FDA’s actions today send a clear message that Big Tobacco’s strategy to profit off addicting Black communities will no longer be tolerated,” she said.
Not all groups are on board, however. The American Civil Liberties Union and several other organizations wrote to the country’s top health officials urging them to reconsider.
“Such a ban will trigger criminal penalties which will disproportionately impact people of color, as well as prioritize criminalization over public health and harm reduction,” the letter says. “A ban will also lead to unconstitutional policing and other negative interactions with local law enforcement.”
The letter calls the proposed ban “well intentioned,” but said any effort to reduce death and disease from tobacco “must avoid solutions that will create yet another reason for armed police to engage citizens on the street based on pretext or conduct that does not pose a threat to public safety.”
Instead of a ban, the organizations said, policy makers should consider increased education for adults and minors, stop-smoking programs, and increased funding for health centers in communities of color.
The Biden administration, however, pressed the point that banning menthol will bring many positives. Acting FDA Commissioner Janet Woodcock, MD said in a statement that banning menthol “will help significantly reduce youth initiation, increase the chances of smoking cessation among current smokers, and address health disparities experienced by communities of color, low-income populations, and LGBTQ-plus individuals, all of whom are far more likely to use these tobacco products.”
The FDA cited data showing that, in the first year or so after a ban goes into effect, an additional 923,000 smokers would quit, including 230,000 African Americans. Another study suggests that 633,000 deaths would be averted, including 237,000 Black Americans.
Dr. Woodcock added that, “armed with strong scientific evidence, and with full support from the [Biden] administration, we believe these actions will launch us on a trajectory toward ending tobacco-related disease and death in the U.S.”
The FDA estimates that 18.6 million Americans who are current smokers use menthol cigarettes, with a disproportionately high number being Black people. Menthol cigarette use among Black and Hispanic youth increased from 2011 to 2018, but declined for non-Hispanic White youth.
Flavored mass-produced cigars and cigarillos are disproportionately popular among youth, especially non-Hispanic Black high school students, who in 2020 reported past 30-day cigar smoking at levels twice as high as their White counterparts, said the FDA. Three-quarters of 12- to 17-year-olds reported they smoke cigars because they like the flavors. In 2020, more young people tried a cigar every day than tried a cigarette, reports the agency.
“This long-overdue decision will protect future generations of young people from nicotine addiction, especially Black children and communities, which have disproportionately suffered from menthol tobacco use due to targeted efforts from the tobacco industry,” Lee Savio Beers, MD, president of the American Academy of Pediatrics, said in a statement.
The FDA’s announcement “is only a first step that must be followed with urgent, comprehensive action to remove these flavored products from the market,” he said.
A version of this article first appeared on WebMD.com.
The Food and Drug Administration said that within a year it will ban menthol in cigarettes and ban all flavors including menthol in cigars.
Menthol makes it easier to start smoking, and also enhances the effects of nicotine, making it more addictive and harder to quit, the FDA said in announcing its actions on Thursday.
Nineteen organizations – including the American Academy of Pediatrics, American Cancer Society, American College of Chest Physicians, American Medical Association, American Heart Association, and the National Medical Association – have pushed the FDA to ban menthol for years. The agency banned all flavors in cigarettes in 2009 but did not take any action against menthol. In 2013, the groups filed a petition demanding that the FDA ban menthol, too. The agency responded months later with a notice that it would start the process.
But it never took any action. Action on Smoking and Health and the African American Tobacco Control Leadership Council, later joined by the AMA and the NMA, sued in 2020 to compel the agency to do something. Now it has finally agreed to act.
The African American Tobacco Control Leadership Council welcomed the move but said the fight is not over and encouraged tobacco control activists to fight to ban menthol tobacco products at the local, state and federal level. “We know that this rule-making process could take years and we know that the tobacco industry will continue to do everything in their power to derail any attempt to remove their deadly products from the market,” Phillip Gardiner, MD, council cochair, said in a statement.
The AMA is urging the FDA to quickly implement the ban and remove the products “without further delay,” AMA President Susan R. Bailey, MD, said in a statement.
“FDA’s long-awaited decision to take action to eliminate menthol flavoring in cigarettes and all flavors in cigars ends a decades-long deference to the tobacco industry, which has repeatedly demonstrated its willingness to profit from products that result in death,” Lisa Lacasse, president of the American Cancer Society Cancer Action Network, said in her own statement.
Ms. Lacasse said banning menthol will help eliminate health disparities. She said 86% of Black people who smoke use menthol cigarettes, compared with 46% of Hispanic people who smoke, 39% of Asian people who smoke, and 29% of White people who smoke. “FDA’s actions today send a clear message that Big Tobacco’s strategy to profit off addicting Black communities will no longer be tolerated,” she said.
Not all groups are on board, however. The American Civil Liberties Union and several other organizations wrote to the country’s top health officials urging them to reconsider.
“Such a ban will trigger criminal penalties which will disproportionately impact people of color, as well as prioritize criminalization over public health and harm reduction,” the letter says. “A ban will also lead to unconstitutional policing and other negative interactions with local law enforcement.”
The letter calls the proposed ban “well intentioned,” but said any effort to reduce death and disease from tobacco “must avoid solutions that will create yet another reason for armed police to engage citizens on the street based on pretext or conduct that does not pose a threat to public safety.”
Instead of a ban, the organizations said, policy makers should consider increased education for adults and minors, stop-smoking programs, and increased funding for health centers in communities of color.
The Biden administration, however, pressed the point that banning menthol will bring many positives. Acting FDA Commissioner Janet Woodcock, MD said in a statement that banning menthol “will help significantly reduce youth initiation, increase the chances of smoking cessation among current smokers, and address health disparities experienced by communities of color, low-income populations, and LGBTQ-plus individuals, all of whom are far more likely to use these tobacco products.”
The FDA cited data showing that, in the first year or so after a ban goes into effect, an additional 923,000 smokers would quit, including 230,000 African Americans. Another study suggests that 633,000 deaths would be averted, including 237,000 Black Americans.
Dr. Woodcock added that, “armed with strong scientific evidence, and with full support from the [Biden] administration, we believe these actions will launch us on a trajectory toward ending tobacco-related disease and death in the U.S.”
The FDA estimates that 18.6 million Americans who are current smokers use menthol cigarettes, with a disproportionately high number being Black people. Menthol cigarette use among Black and Hispanic youth increased from 2011 to 2018, but declined for non-Hispanic White youth.
Flavored mass-produced cigars and cigarillos are disproportionately popular among youth, especially non-Hispanic Black high school students, who in 2020 reported past 30-day cigar smoking at levels twice as high as their White counterparts, said the FDA. Three-quarters of 12- to 17-year-olds reported they smoke cigars because they like the flavors. In 2020, more young people tried a cigar every day than tried a cigarette, reports the agency.
“This long-overdue decision will protect future generations of young people from nicotine addiction, especially Black children and communities, which have disproportionately suffered from menthol tobacco use due to targeted efforts from the tobacco industry,” Lee Savio Beers, MD, president of the American Academy of Pediatrics, said in a statement.
The FDA’s announcement “is only a first step that must be followed with urgent, comprehensive action to remove these flavored products from the market,” he said.
A version of this article first appeared on WebMD.com.
USPSTF reaffirms advice to screen all adults for hypertension
The U.S. Preventive Services Task Force continues to recommend that clinicians screen all adults aged 18 years and older for high blood pressure and that they confirm a diagnosis of hypertension with blood pressure measurements taken outside the office before starting treatment.
This grade A recommendation is consistent with the 2015 recommendation from the task force.
Hypertension affects approximately 45% of adults in the United States and is a major contributing risk factor for heart failure, myocardial infarction, stroke, and chronic kidney disease.
Using a reaffirmation deliberation process, the USPSTF concluded with high certainty that there was “substantial net benefit” from screening adults for hypertension in clinical office settings.
The reaffirmation recommendation clarifies that initial screening should be performed with office-based blood pressure measurement.
The task force found “convincing” evidence that screening for and treatment of hypertension detected in clinical office settings substantially reduces cardiovascular events and have few major harms.
To confirm a diagnosis of hypertension outside the office before starting treatment, ambulatory blood pressure monitoring or home blood pressure monitoring is recommended. Blood pressure measurements should be taken at the brachial artery with a validated and accurate device in a seated position after 5 minutes of rest.
Although evidence regarding optimal screening intervals is limited, the task force says “reasonable” options include screening for hypertension every year for adults aged 40 years or older and for adults who are at increased risk for hypertension, such as Black persons, persons with high-normal blood pressure, or those who are overweight or obese.
Screening less frequently (every 3-5 years) is appropriate for adults aged 18-39 years who are not at increased risk for hypertension and who have received a prior blood pressure reading that was in the normal range, said the task force, led by Alex Krist, MD, MPH, Virginia Commonwealth University, Richmond.
The recommendation and supporting evidence report were published online April 27, 2021, in JAMA.
‘Screening is just the first step’
In a JAMA editorial, Marwah Abdalla, MD, MPH, Columbia University Irving Medical Center, New York, and coauthors said the COVID-19 pandemic has demonstrated that “rapid and significant innovation in science, health care, and society is possible. Implementing the latest USPSTF recommendations will require widespread changes to how the health care system and other entities screen for hypertension.
“Yet screening is just the first step in a long road to controlling hypertension. Medicine and society need to implement a variety of interventions proven to be effective in controlling blood pressure at scale,” the editorialists said.
“Additionally, these efforts need to consider how to achieve success for all people. This will require working to address the roots of structural racism and reduce the racial disparities that increase hypertension-related morbidity and mortality for vulnerable populations,” they added.
“These changes will take innovation in how care delivery is provided at both the individual and population levels – lessons the health care system and society learned are achievable through the response to the COVID-19 pandemic,” Dr. Abdalla and colleagues concluded.
The USPSTF and Dr. Abdalla reported no relevant financial relationships. One editorialist reported receiving personal fees from Livongo and Cerner and grants from Bristol-Myers Squibb.
A version of this article first appeared on Medscape.com.
The U.S. Preventive Services Task Force continues to recommend that clinicians screen all adults aged 18 years and older for high blood pressure and that they confirm a diagnosis of hypertension with blood pressure measurements taken outside the office before starting treatment.
This grade A recommendation is consistent with the 2015 recommendation from the task force.
Hypertension affects approximately 45% of adults in the United States and is a major contributing risk factor for heart failure, myocardial infarction, stroke, and chronic kidney disease.
Using a reaffirmation deliberation process, the USPSTF concluded with high certainty that there was “substantial net benefit” from screening adults for hypertension in clinical office settings.
The reaffirmation recommendation clarifies that initial screening should be performed with office-based blood pressure measurement.
The task force found “convincing” evidence that screening for and treatment of hypertension detected in clinical office settings substantially reduces cardiovascular events and have few major harms.
To confirm a diagnosis of hypertension outside the office before starting treatment, ambulatory blood pressure monitoring or home blood pressure monitoring is recommended. Blood pressure measurements should be taken at the brachial artery with a validated and accurate device in a seated position after 5 minutes of rest.
Although evidence regarding optimal screening intervals is limited, the task force says “reasonable” options include screening for hypertension every year for adults aged 40 years or older and for adults who are at increased risk for hypertension, such as Black persons, persons with high-normal blood pressure, or those who are overweight or obese.
Screening less frequently (every 3-5 years) is appropriate for adults aged 18-39 years who are not at increased risk for hypertension and who have received a prior blood pressure reading that was in the normal range, said the task force, led by Alex Krist, MD, MPH, Virginia Commonwealth University, Richmond.
The recommendation and supporting evidence report were published online April 27, 2021, in JAMA.
‘Screening is just the first step’
In a JAMA editorial, Marwah Abdalla, MD, MPH, Columbia University Irving Medical Center, New York, and coauthors said the COVID-19 pandemic has demonstrated that “rapid and significant innovation in science, health care, and society is possible. Implementing the latest USPSTF recommendations will require widespread changes to how the health care system and other entities screen for hypertension.
“Yet screening is just the first step in a long road to controlling hypertension. Medicine and society need to implement a variety of interventions proven to be effective in controlling blood pressure at scale,” the editorialists said.
“Additionally, these efforts need to consider how to achieve success for all people. This will require working to address the roots of structural racism and reduce the racial disparities that increase hypertension-related morbidity and mortality for vulnerable populations,” they added.
“These changes will take innovation in how care delivery is provided at both the individual and population levels – lessons the health care system and society learned are achievable through the response to the COVID-19 pandemic,” Dr. Abdalla and colleagues concluded.
The USPSTF and Dr. Abdalla reported no relevant financial relationships. One editorialist reported receiving personal fees from Livongo and Cerner and grants from Bristol-Myers Squibb.
A version of this article first appeared on Medscape.com.
The U.S. Preventive Services Task Force continues to recommend that clinicians screen all adults aged 18 years and older for high blood pressure and that they confirm a diagnosis of hypertension with blood pressure measurements taken outside the office before starting treatment.
This grade A recommendation is consistent with the 2015 recommendation from the task force.
Hypertension affects approximately 45% of adults in the United States and is a major contributing risk factor for heart failure, myocardial infarction, stroke, and chronic kidney disease.
Using a reaffirmation deliberation process, the USPSTF concluded with high certainty that there was “substantial net benefit” from screening adults for hypertension in clinical office settings.
The reaffirmation recommendation clarifies that initial screening should be performed with office-based blood pressure measurement.
The task force found “convincing” evidence that screening for and treatment of hypertension detected in clinical office settings substantially reduces cardiovascular events and have few major harms.
To confirm a diagnosis of hypertension outside the office before starting treatment, ambulatory blood pressure monitoring or home blood pressure monitoring is recommended. Blood pressure measurements should be taken at the brachial artery with a validated and accurate device in a seated position after 5 minutes of rest.
Although evidence regarding optimal screening intervals is limited, the task force says “reasonable” options include screening for hypertension every year for adults aged 40 years or older and for adults who are at increased risk for hypertension, such as Black persons, persons with high-normal blood pressure, or those who are overweight or obese.
Screening less frequently (every 3-5 years) is appropriate for adults aged 18-39 years who are not at increased risk for hypertension and who have received a prior blood pressure reading that was in the normal range, said the task force, led by Alex Krist, MD, MPH, Virginia Commonwealth University, Richmond.
The recommendation and supporting evidence report were published online April 27, 2021, in JAMA.
‘Screening is just the first step’
In a JAMA editorial, Marwah Abdalla, MD, MPH, Columbia University Irving Medical Center, New York, and coauthors said the COVID-19 pandemic has demonstrated that “rapid and significant innovation in science, health care, and society is possible. Implementing the latest USPSTF recommendations will require widespread changes to how the health care system and other entities screen for hypertension.
“Yet screening is just the first step in a long road to controlling hypertension. Medicine and society need to implement a variety of interventions proven to be effective in controlling blood pressure at scale,” the editorialists said.
“Additionally, these efforts need to consider how to achieve success for all people. This will require working to address the roots of structural racism and reduce the racial disparities that increase hypertension-related morbidity and mortality for vulnerable populations,” they added.
“These changes will take innovation in how care delivery is provided at both the individual and population levels – lessons the health care system and society learned are achievable through the response to the COVID-19 pandemic,” Dr. Abdalla and colleagues concluded.
The USPSTF and Dr. Abdalla reported no relevant financial relationships. One editorialist reported receiving personal fees from Livongo and Cerner and grants from Bristol-Myers Squibb.
A version of this article first appeared on Medscape.com.
AHA statement flags CV risk of hormonal cancer therapies
Hormonal therapies for the treatment of hormone-dependent breast and prostate cancer could raise the risk for myocardial infarction and stroke, and patients need to be closely monitored to allow early detection and treatment of cardiovascular disease (CVD), the American Heart Association says in a new scientific statement.
“The statement provides data on the risks of each type of hormonal therapy so clinicians can use it as a guide to help manage cardiovascular risks during cancer treatment,” Tochi Okwuosa, DO, chair of the writing group, said in a news release.
“A team-based approach to patient care that includes the oncology team, cardiologist, primary care clinician, dietitian, endocrinologist, and other health care professionals as appropriate is needed to work with each patient to manage and reduce the increased risk of heart disease and strokes associated with hormonal therapy in breast and prostate cancer treatment,” said Dr. Okwuosa, director of cardio-oncology services, Rush University Medical Center, Chicago.
The scientific statement was published online April 26 in Circulation: Genomic and Precision Medicine.
Hormone-dependent cancers, such as prostate and breast cancer, are the most common noncutaneous cancers in the United States and around the world. As hormonal therapies have markedly improved survival in these patients, CVD has emerged as a leading cause illness and death.
The increased CVD burden might be explained by the increasing average age of cancer survivors, leading to higher rates of age-related CV risk factors and coronary artery disease.
The writing group reviewed existing evidence from observational studies and randomized controlled trials on the cardiovascular impact of anticancer hormonal therapies.
Among the key findings:
- In patients with breast cancer, has been shown to increase the risk for venous thromboembolic events, but to have somewhat protective to neutral effects on CVD risk burden and CVD events. Conversely, aromatase inhibitors have been shown to increase the risk for CVD risk factors and events, including MI and stroke.
- Androgen-deprivation therapy for prostate cancer appears to increase the risk for CV events, although gonadotrophin-releasing hormone (GnRH) antagonists are associated with a lower risk for CV events than are GnRH agonists. The oral antiandrogens appear to be associated with increased CVD risk as well, particularly when used for complete androgen blockade as combination GnRH/anti-androgen therapy.
- The duration of hormonal therapies has a significant impact on CVD risk; the longer patients receive hormonal therapy, the greater the risk. More research is needed to better define the risks associated with duration of treatment.
- The data are mixed on the impact of preexisting CV risk factors and CVD on CV events associated with hormonal therapy. Although the presence of baseline CV risk factors and CVD can increase CV events associated with aromatase inhibitors, it is not clear that tamoxifen does.
- Studies suggest that patients with prostate cancer and baseline CVD and CV risk factors have increased rates of CV events when treated with androgen-deprivation therapy.
- Although the prolonged use of some hormonal therapies worsens CV risk factors and , the effects of the duration of therapy on CV events are less clear.
The writing group noted that there are no definitive guidelines for the monitoring and management of hormonal therapy-related CVD risks.
The authors encourage clinicians to be alert for worsening CV problems in those with preexisting heart disease or risk factors, and to recognize that even patients without preexisting CV problems are at higher risk because of their exposure to hormonal therapies.
“For patients who have two or more cardiovascular risk factors, it is likely that referral to a cardiologist would be appropriate prior to beginning hormone treatment. For patients already receiving hormonal therapies, a discussion with the oncology team can help to determine if a cardiology referral is recommended,” Dr. Okwuosa said in the news release.
This scientific statement was prepared by the volunteer writing group on behalf of the AHA Cardio-Oncology Subcommittee of the Council on Clinical Cardiology and the Council on Genomic and Precision Medicine; the Council on Arteriosclerosis, Thrombosis, and Vascular Biology; and the Council on Cardiovascular Radiology and Intervention.
The research had no commercial funding. Dr. Okwuosa has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Hormonal therapies for the treatment of hormone-dependent breast and prostate cancer could raise the risk for myocardial infarction and stroke, and patients need to be closely monitored to allow early detection and treatment of cardiovascular disease (CVD), the American Heart Association says in a new scientific statement.
“The statement provides data on the risks of each type of hormonal therapy so clinicians can use it as a guide to help manage cardiovascular risks during cancer treatment,” Tochi Okwuosa, DO, chair of the writing group, said in a news release.
“A team-based approach to patient care that includes the oncology team, cardiologist, primary care clinician, dietitian, endocrinologist, and other health care professionals as appropriate is needed to work with each patient to manage and reduce the increased risk of heart disease and strokes associated with hormonal therapy in breast and prostate cancer treatment,” said Dr. Okwuosa, director of cardio-oncology services, Rush University Medical Center, Chicago.
The scientific statement was published online April 26 in Circulation: Genomic and Precision Medicine.
Hormone-dependent cancers, such as prostate and breast cancer, are the most common noncutaneous cancers in the United States and around the world. As hormonal therapies have markedly improved survival in these patients, CVD has emerged as a leading cause illness and death.
The increased CVD burden might be explained by the increasing average age of cancer survivors, leading to higher rates of age-related CV risk factors and coronary artery disease.
The writing group reviewed existing evidence from observational studies and randomized controlled trials on the cardiovascular impact of anticancer hormonal therapies.
Among the key findings:
- In patients with breast cancer, has been shown to increase the risk for venous thromboembolic events, but to have somewhat protective to neutral effects on CVD risk burden and CVD events. Conversely, aromatase inhibitors have been shown to increase the risk for CVD risk factors and events, including MI and stroke.
- Androgen-deprivation therapy for prostate cancer appears to increase the risk for CV events, although gonadotrophin-releasing hormone (GnRH) antagonists are associated with a lower risk for CV events than are GnRH agonists. The oral antiandrogens appear to be associated with increased CVD risk as well, particularly when used for complete androgen blockade as combination GnRH/anti-androgen therapy.
- The duration of hormonal therapies has a significant impact on CVD risk; the longer patients receive hormonal therapy, the greater the risk. More research is needed to better define the risks associated with duration of treatment.
- The data are mixed on the impact of preexisting CV risk factors and CVD on CV events associated with hormonal therapy. Although the presence of baseline CV risk factors and CVD can increase CV events associated with aromatase inhibitors, it is not clear that tamoxifen does.
- Studies suggest that patients with prostate cancer and baseline CVD and CV risk factors have increased rates of CV events when treated with androgen-deprivation therapy.
- Although the prolonged use of some hormonal therapies worsens CV risk factors and , the effects of the duration of therapy on CV events are less clear.
The writing group noted that there are no definitive guidelines for the monitoring and management of hormonal therapy-related CVD risks.
The authors encourage clinicians to be alert for worsening CV problems in those with preexisting heart disease or risk factors, and to recognize that even patients without preexisting CV problems are at higher risk because of their exposure to hormonal therapies.
“For patients who have two or more cardiovascular risk factors, it is likely that referral to a cardiologist would be appropriate prior to beginning hormone treatment. For patients already receiving hormonal therapies, a discussion with the oncology team can help to determine if a cardiology referral is recommended,” Dr. Okwuosa said in the news release.
This scientific statement was prepared by the volunteer writing group on behalf of the AHA Cardio-Oncology Subcommittee of the Council on Clinical Cardiology and the Council on Genomic and Precision Medicine; the Council on Arteriosclerosis, Thrombosis, and Vascular Biology; and the Council on Cardiovascular Radiology and Intervention.
The research had no commercial funding. Dr. Okwuosa has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Hormonal therapies for the treatment of hormone-dependent breast and prostate cancer could raise the risk for myocardial infarction and stroke, and patients need to be closely monitored to allow early detection and treatment of cardiovascular disease (CVD), the American Heart Association says in a new scientific statement.
“The statement provides data on the risks of each type of hormonal therapy so clinicians can use it as a guide to help manage cardiovascular risks during cancer treatment,” Tochi Okwuosa, DO, chair of the writing group, said in a news release.
“A team-based approach to patient care that includes the oncology team, cardiologist, primary care clinician, dietitian, endocrinologist, and other health care professionals as appropriate is needed to work with each patient to manage and reduce the increased risk of heart disease and strokes associated with hormonal therapy in breast and prostate cancer treatment,” said Dr. Okwuosa, director of cardio-oncology services, Rush University Medical Center, Chicago.
The scientific statement was published online April 26 in Circulation: Genomic and Precision Medicine.
Hormone-dependent cancers, such as prostate and breast cancer, are the most common noncutaneous cancers in the United States and around the world. As hormonal therapies have markedly improved survival in these patients, CVD has emerged as a leading cause illness and death.
The increased CVD burden might be explained by the increasing average age of cancer survivors, leading to higher rates of age-related CV risk factors and coronary artery disease.
The writing group reviewed existing evidence from observational studies and randomized controlled trials on the cardiovascular impact of anticancer hormonal therapies.
Among the key findings:
- In patients with breast cancer, has been shown to increase the risk for venous thromboembolic events, but to have somewhat protective to neutral effects on CVD risk burden and CVD events. Conversely, aromatase inhibitors have been shown to increase the risk for CVD risk factors and events, including MI and stroke.
- Androgen-deprivation therapy for prostate cancer appears to increase the risk for CV events, although gonadotrophin-releasing hormone (GnRH) antagonists are associated with a lower risk for CV events than are GnRH agonists. The oral antiandrogens appear to be associated with increased CVD risk as well, particularly when used for complete androgen blockade as combination GnRH/anti-androgen therapy.
- The duration of hormonal therapies has a significant impact on CVD risk; the longer patients receive hormonal therapy, the greater the risk. More research is needed to better define the risks associated with duration of treatment.
- The data are mixed on the impact of preexisting CV risk factors and CVD on CV events associated with hormonal therapy. Although the presence of baseline CV risk factors and CVD can increase CV events associated with aromatase inhibitors, it is not clear that tamoxifen does.
- Studies suggest that patients with prostate cancer and baseline CVD and CV risk factors have increased rates of CV events when treated with androgen-deprivation therapy.
- Although the prolonged use of some hormonal therapies worsens CV risk factors and , the effects of the duration of therapy on CV events are less clear.
The writing group noted that there are no definitive guidelines for the monitoring and management of hormonal therapy-related CVD risks.
The authors encourage clinicians to be alert for worsening CV problems in those with preexisting heart disease or risk factors, and to recognize that even patients without preexisting CV problems are at higher risk because of their exposure to hormonal therapies.
“For patients who have two or more cardiovascular risk factors, it is likely that referral to a cardiologist would be appropriate prior to beginning hormone treatment. For patients already receiving hormonal therapies, a discussion with the oncology team can help to determine if a cardiology referral is recommended,” Dr. Okwuosa said in the news release.
This scientific statement was prepared by the volunteer writing group on behalf of the AHA Cardio-Oncology Subcommittee of the Council on Clinical Cardiology and the Council on Genomic and Precision Medicine; the Council on Arteriosclerosis, Thrombosis, and Vascular Biology; and the Council on Cardiovascular Radiology and Intervention.
The research had no commercial funding. Dr. Okwuosa has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Pfizer and Moderna vaccines appear safe, effective during pregnancy
The Pfizer and Moderna COVID-19 vaccines appear to be safe in pregnant patients, according to preliminary findings published in the New England Journal of Medicine.
The Centers for Disease Control and Prevention have said pregnant people have an increased risk of being severely ill from COVID-19; however, this group was excluded from major clinical trials that led up to the current vaccine approvals.
But based on the new findings, Rochelle Walensky, MD, director of the CDC, announced during a White House COVID-19 briefing that the CDC recommends that pregnant people receive the COVID-19 vaccine.
The new study, which analyzed data between Dec. 14, 2020, and Feb. 28, 2021, from three federal databases, adds to a pool of limited data about the safety and efficacy of the vaccine in pregnant persons. Researchers did not include people who received the Johnson & Johnson vaccine because it received emergency use authorization on Feb. 27, just 1 day before they study’s cutoff.
“Our hope is that these initial data will be reassuring to pregnant people and their health care providers as well as the public, and contribute to increasing vaccination rates,” study author Christine Olson, MD, said in an interview. “While the data are preliminary and will continue to be analyzed as more reports become available, our findings are reassuring.”
For the study, Dr. Olson and colleagues analyzed v-safe survey data, data from those enrolled in the v-safe pregnancy registry, and Vaccine Adverse Event Reporting System (VAERS) reports.
Researchers found that 86% of pregnancies resulted in a live birth, 12.6 % resulted in spontaneous abortions, and 0.1% resulted in stillbirth. They also found that, among the live births, 9.4% were preterm, 3.2% of babies were small for their gestational age, and 2.2% had congenital anomalies.
Researchers also found that injection-site pain, fatigue, and headaches were reported more frequently in pregnant patients than among those who were not pregnant. Among VAERS reports, they found that 70% of adverse events were nonpregnancy specific. Nearly 30% involved pregnancy- or neonatal-specific adverse events. The most frequently reported pregnancy-related events were spontaneous abortions, followed by stillbirths, premature rupture of membranes and vaginal bleeding.
“I think the results are actually quite reassuring as the proportion of the pregnancy outcomes, such as pregnancy loss and health effects to the newborns, are really quite consistent with what we’d expect in the background rate of the population,” Dr. Walensky said in a podcast accompanying the study. “So this study adds to growing evidence confirming that pregnant people develop a robust immune response to COVID-19 vaccination without so far seeing any adverse events to the mom or the fetus.”
Researchers said limitations of the study include the accuracy of self-reported data, and there being limited information on other potential risk factors for adverse pregnancies and neonatal outcomes. They acknowledged that continuous monitoring is needed to look at maternal safety and pregnancy outcomes in earlier stages of pregnancy and during the preconception period.
David Jaspan, DO, chair of the department of obstetrics and gynecology at Einstein Medical Center, Philadelphia, who was not involved with the study, said in an interview that, despite the limitations, the study provides much-needed insight on the vaccine’s safety and efficacy in pregnant patients.
“In December we had no data for any pregnant patient,” Dr. Jaspan said. “And now just 4 short months later, this paper [has data from] at least had 35,000 people. We can’t answer every question, but we have more answers today than we had just 4 months ago.”
Dr. Olson hopes the present data is enough to help inform decision-making of pregnant patients and their health care providers when it comes to deciding to get the COVID-19 vaccination.
The study author and experts interviewed disclosed no relevant financial relationships.
The Pfizer and Moderna COVID-19 vaccines appear to be safe in pregnant patients, according to preliminary findings published in the New England Journal of Medicine.
The Centers for Disease Control and Prevention have said pregnant people have an increased risk of being severely ill from COVID-19; however, this group was excluded from major clinical trials that led up to the current vaccine approvals.
But based on the new findings, Rochelle Walensky, MD, director of the CDC, announced during a White House COVID-19 briefing that the CDC recommends that pregnant people receive the COVID-19 vaccine.
The new study, which analyzed data between Dec. 14, 2020, and Feb. 28, 2021, from three federal databases, adds to a pool of limited data about the safety and efficacy of the vaccine in pregnant persons. Researchers did not include people who received the Johnson & Johnson vaccine because it received emergency use authorization on Feb. 27, just 1 day before they study’s cutoff.
“Our hope is that these initial data will be reassuring to pregnant people and their health care providers as well as the public, and contribute to increasing vaccination rates,” study author Christine Olson, MD, said in an interview. “While the data are preliminary and will continue to be analyzed as more reports become available, our findings are reassuring.”
For the study, Dr. Olson and colleagues analyzed v-safe survey data, data from those enrolled in the v-safe pregnancy registry, and Vaccine Adverse Event Reporting System (VAERS) reports.
Researchers found that 86% of pregnancies resulted in a live birth, 12.6 % resulted in spontaneous abortions, and 0.1% resulted in stillbirth. They also found that, among the live births, 9.4% were preterm, 3.2% of babies were small for their gestational age, and 2.2% had congenital anomalies.
Researchers also found that injection-site pain, fatigue, and headaches were reported more frequently in pregnant patients than among those who were not pregnant. Among VAERS reports, they found that 70% of adverse events were nonpregnancy specific. Nearly 30% involved pregnancy- or neonatal-specific adverse events. The most frequently reported pregnancy-related events were spontaneous abortions, followed by stillbirths, premature rupture of membranes and vaginal bleeding.
“I think the results are actually quite reassuring as the proportion of the pregnancy outcomes, such as pregnancy loss and health effects to the newborns, are really quite consistent with what we’d expect in the background rate of the population,” Dr. Walensky said in a podcast accompanying the study. “So this study adds to growing evidence confirming that pregnant people develop a robust immune response to COVID-19 vaccination without so far seeing any adverse events to the mom or the fetus.”
Researchers said limitations of the study include the accuracy of self-reported data, and there being limited information on other potential risk factors for adverse pregnancies and neonatal outcomes. They acknowledged that continuous monitoring is needed to look at maternal safety and pregnancy outcomes in earlier stages of pregnancy and during the preconception period.
David Jaspan, DO, chair of the department of obstetrics and gynecology at Einstein Medical Center, Philadelphia, who was not involved with the study, said in an interview that, despite the limitations, the study provides much-needed insight on the vaccine’s safety and efficacy in pregnant patients.
“In December we had no data for any pregnant patient,” Dr. Jaspan said. “And now just 4 short months later, this paper [has data from] at least had 35,000 people. We can’t answer every question, but we have more answers today than we had just 4 months ago.”
Dr. Olson hopes the present data is enough to help inform decision-making of pregnant patients and their health care providers when it comes to deciding to get the COVID-19 vaccination.
The study author and experts interviewed disclosed no relevant financial relationships.
The Pfizer and Moderna COVID-19 vaccines appear to be safe in pregnant patients, according to preliminary findings published in the New England Journal of Medicine.
The Centers for Disease Control and Prevention have said pregnant people have an increased risk of being severely ill from COVID-19; however, this group was excluded from major clinical trials that led up to the current vaccine approvals.
But based on the new findings, Rochelle Walensky, MD, director of the CDC, announced during a White House COVID-19 briefing that the CDC recommends that pregnant people receive the COVID-19 vaccine.
The new study, which analyzed data between Dec. 14, 2020, and Feb. 28, 2021, from three federal databases, adds to a pool of limited data about the safety and efficacy of the vaccine in pregnant persons. Researchers did not include people who received the Johnson & Johnson vaccine because it received emergency use authorization on Feb. 27, just 1 day before they study’s cutoff.
“Our hope is that these initial data will be reassuring to pregnant people and their health care providers as well as the public, and contribute to increasing vaccination rates,” study author Christine Olson, MD, said in an interview. “While the data are preliminary and will continue to be analyzed as more reports become available, our findings are reassuring.”
For the study, Dr. Olson and colleagues analyzed v-safe survey data, data from those enrolled in the v-safe pregnancy registry, and Vaccine Adverse Event Reporting System (VAERS) reports.
Researchers found that 86% of pregnancies resulted in a live birth, 12.6 % resulted in spontaneous abortions, and 0.1% resulted in stillbirth. They also found that, among the live births, 9.4% were preterm, 3.2% of babies were small for their gestational age, and 2.2% had congenital anomalies.
Researchers also found that injection-site pain, fatigue, and headaches were reported more frequently in pregnant patients than among those who were not pregnant. Among VAERS reports, they found that 70% of adverse events were nonpregnancy specific. Nearly 30% involved pregnancy- or neonatal-specific adverse events. The most frequently reported pregnancy-related events were spontaneous abortions, followed by stillbirths, premature rupture of membranes and vaginal bleeding.
“I think the results are actually quite reassuring as the proportion of the pregnancy outcomes, such as pregnancy loss and health effects to the newborns, are really quite consistent with what we’d expect in the background rate of the population,” Dr. Walensky said in a podcast accompanying the study. “So this study adds to growing evidence confirming that pregnant people develop a robust immune response to COVID-19 vaccination without so far seeing any adverse events to the mom or the fetus.”
Researchers said limitations of the study include the accuracy of self-reported data, and there being limited information on other potential risk factors for adverse pregnancies and neonatal outcomes. They acknowledged that continuous monitoring is needed to look at maternal safety and pregnancy outcomes in earlier stages of pregnancy and during the preconception period.
David Jaspan, DO, chair of the department of obstetrics and gynecology at Einstein Medical Center, Philadelphia, who was not involved with the study, said in an interview that, despite the limitations, the study provides much-needed insight on the vaccine’s safety and efficacy in pregnant patients.
“In December we had no data for any pregnant patient,” Dr. Jaspan said. “And now just 4 short months later, this paper [has data from] at least had 35,000 people. We can’t answer every question, but we have more answers today than we had just 4 months ago.”
Dr. Olson hopes the present data is enough to help inform decision-making of pregnant patients and their health care providers when it comes to deciding to get the COVID-19 vaccination.
The study author and experts interviewed disclosed no relevant financial relationships.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Half of patients in hospital for COVID-19 get acute kidney injury
in two independent, European case series presented recently at the International Society of Nephrology: 2021 World Congress. Many of the cases progressed to more severe, stage 3 AKI. Factors linked with incident AKI in the two reports included use of mechanical ventilation, vasopressors, or diuretics, and elevations in inflammatory markers.
The new findings confirm several U.S. reports published during the past year. In those reports, roughly a third of patients hospitalized for COVID-19 developed AKI during their hospital stay, said Jay L. Koyner, MD, during another renal conference, the National Kidney Foundation 2021 Spring Clinical Meetings.
Experience has shown it’s bad news when hospitalized COVID-19 patients develop AKI, which can prove fatal or can lead to the development or worsening of chronic kidney disease (CKD), which in some cases rapidly progresses to end-stage disease.
COVID-19 giving nephrologists an opportunity to improve AKI care
“COVID is giving us an opportunity to do a better job of taking care of patients who develop AKI, which is something that nephrologists have not often excelled at doing,” said Dr. Koyner, professor and director of the nephrology ICU at the University of Chicago.
“Many studies will look at how we can manage COVID-19 patients better after they develop AKI, because I suspect a large number of these patients will wind up with CKD,” Dr. Koyner said during his talk.
He cited several lessons from reports of AKI that occurs in patients hospitalized for COVID-19:
- Preexisting CKD, , and severe COVID-19 appear to be risk factors for developing COVID-related AKI.
- Patients who develop AKI during acutely severe COVID-19 may have slightly worse outcomes than patients without COVID-19 who develop AKI.
- Certain genetic susceptibilities may play a role in developing COVID-19–related AKI.
- Routine follow-up of AKI is generally inadequate and is not standardized, whether AKI develops while ill with COVID-19 or in other settings.
The most encouraging AKI takeaway from COVID-19’s first year is that its incidence among patients hospitalized with COVID-19 appears to have dropped from very high rates early on, possibly because of more routine use of steroids for critically ill patients with COVID-19 and a reduction in the use of ventilators, Dr. Koyner suggested.
In-hospital diuretic treatment links with AKI
One of the World Congress of Nephrology reports involved 1,248 patients admitted with confirmed COVID-19 at two tertiary care hospitals in London during March–May 2020. The average age of the patients was 69 years, 59% were men, and 17% had CKD at admission, as determined on the basis of estimated glomerular filtration rate <60 mL/min per 1.73 m2.
During hospitalization, 487 patients (39%) developed AKI, including 175 (14%) with stage 3 AKI and 109 (9%) who required renal replacement therapy (dialysis or kidney transplant). The incidence of AKI peaked 5 weeks after COVID-19 admission, Paul Jewell and associates from King’s College Hospital, London, reported in a poster.
Multivariate analysis identified several demographic and clinical variables that were significantly linked with an increased risk of developing AKI: male sex (which boosted risk by 55%), Black race (79% higher risk), CKD at admission (triple the risk), being hypertensive on admission (73% higher risk), and being administered diuretics during hospitalization (69% higher risk).
The findings of a risk linked with diuretic use “supports the cautious use of diuretics in patients hospitalized with COVID-19, especially in the presence of background renal impairment,” the authors said.
For patients with incident AKI, the 30-day mortality rate was significantly increased; mortality was 59% higher among patients who developed stage 1 AKI and was roughly triple among patients who developed stage 2 or 3 AKI.
Second report links ventilation, vasopressors with worse AKI
A separate report from clinicians at Charité Hospital, Berlin, retrospectively analyzed 223 patients admitted with symptomatic COVID-19 to three Charité sites during March–June 2020. During hospitalization, 117 patients (52%) developed AKI, including 70 (31%) with stage 3 disease; 67 (30%) required renal replacement therapy. Half the patients with stage 3 AKI required ICU admission.
Compared with patients with less severe AKI, patients who developed stage 3 AKI were more often male, older, and had a higher body mass index.
In a multivariate model, compared with patients who developed less severe AKI, those who developed stage 3 disease also were significantly more likely to have received mechanical ventilation or vasopressor drugs and were more likely to have increased levels of leukocytes or procalcitonin, two inflammatory markers, Jan-Hendrink B. Hardenburg, MD, a Charité nephrologist, and associates reported in a poster at the meeting.
Mechanical ventilation was linked with a sixfold higher rate of stage 3 AKI, and treatment with vasopressor drugs was linked with a threefold higher rate. Elevations in procalcitonin or leukocyte levels were linked with about 60% increases in rates of stage 3 AKI. For both of these risk factors, temporal relationships were tighter; increases in both values appeared just before onset of stage 3 disease.
Dr. Joyner has been a speaker on behalf of NXStage Medical; a consultant to Astute Medical, Baxter, Mallinckrodt, Pfizer, and Sphingotec; and he has received research funding from Astute, Bioporto, NxStage, and Satellite Healthcare. Mr. Jewell and Dr. Hardenburg disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
in two independent, European case series presented recently at the International Society of Nephrology: 2021 World Congress. Many of the cases progressed to more severe, stage 3 AKI. Factors linked with incident AKI in the two reports included use of mechanical ventilation, vasopressors, or diuretics, and elevations in inflammatory markers.
The new findings confirm several U.S. reports published during the past year. In those reports, roughly a third of patients hospitalized for COVID-19 developed AKI during their hospital stay, said Jay L. Koyner, MD, during another renal conference, the National Kidney Foundation 2021 Spring Clinical Meetings.
Experience has shown it’s bad news when hospitalized COVID-19 patients develop AKI, which can prove fatal or can lead to the development or worsening of chronic kidney disease (CKD), which in some cases rapidly progresses to end-stage disease.
COVID-19 giving nephrologists an opportunity to improve AKI care
“COVID is giving us an opportunity to do a better job of taking care of patients who develop AKI, which is something that nephrologists have not often excelled at doing,” said Dr. Koyner, professor and director of the nephrology ICU at the University of Chicago.
“Many studies will look at how we can manage COVID-19 patients better after they develop AKI, because I suspect a large number of these patients will wind up with CKD,” Dr. Koyner said during his talk.
He cited several lessons from reports of AKI that occurs in patients hospitalized for COVID-19:
- Preexisting CKD, , and severe COVID-19 appear to be risk factors for developing COVID-related AKI.
- Patients who develop AKI during acutely severe COVID-19 may have slightly worse outcomes than patients without COVID-19 who develop AKI.
- Certain genetic susceptibilities may play a role in developing COVID-19–related AKI.
- Routine follow-up of AKI is generally inadequate and is not standardized, whether AKI develops while ill with COVID-19 or in other settings.
The most encouraging AKI takeaway from COVID-19’s first year is that its incidence among patients hospitalized with COVID-19 appears to have dropped from very high rates early on, possibly because of more routine use of steroids for critically ill patients with COVID-19 and a reduction in the use of ventilators, Dr. Koyner suggested.
In-hospital diuretic treatment links with AKI
One of the World Congress of Nephrology reports involved 1,248 patients admitted with confirmed COVID-19 at two tertiary care hospitals in London during March–May 2020. The average age of the patients was 69 years, 59% were men, and 17% had CKD at admission, as determined on the basis of estimated glomerular filtration rate <60 mL/min per 1.73 m2.
During hospitalization, 487 patients (39%) developed AKI, including 175 (14%) with stage 3 AKI and 109 (9%) who required renal replacement therapy (dialysis or kidney transplant). The incidence of AKI peaked 5 weeks after COVID-19 admission, Paul Jewell and associates from King’s College Hospital, London, reported in a poster.
Multivariate analysis identified several demographic and clinical variables that were significantly linked with an increased risk of developing AKI: male sex (which boosted risk by 55%), Black race (79% higher risk), CKD at admission (triple the risk), being hypertensive on admission (73% higher risk), and being administered diuretics during hospitalization (69% higher risk).
The findings of a risk linked with diuretic use “supports the cautious use of diuretics in patients hospitalized with COVID-19, especially in the presence of background renal impairment,” the authors said.
For patients with incident AKI, the 30-day mortality rate was significantly increased; mortality was 59% higher among patients who developed stage 1 AKI and was roughly triple among patients who developed stage 2 or 3 AKI.
Second report links ventilation, vasopressors with worse AKI
A separate report from clinicians at Charité Hospital, Berlin, retrospectively analyzed 223 patients admitted with symptomatic COVID-19 to three Charité sites during March–June 2020. During hospitalization, 117 patients (52%) developed AKI, including 70 (31%) with stage 3 disease; 67 (30%) required renal replacement therapy. Half the patients with stage 3 AKI required ICU admission.
Compared with patients with less severe AKI, patients who developed stage 3 AKI were more often male, older, and had a higher body mass index.
In a multivariate model, compared with patients who developed less severe AKI, those who developed stage 3 disease also were significantly more likely to have received mechanical ventilation or vasopressor drugs and were more likely to have increased levels of leukocytes or procalcitonin, two inflammatory markers, Jan-Hendrink B. Hardenburg, MD, a Charité nephrologist, and associates reported in a poster at the meeting.
Mechanical ventilation was linked with a sixfold higher rate of stage 3 AKI, and treatment with vasopressor drugs was linked with a threefold higher rate. Elevations in procalcitonin or leukocyte levels were linked with about 60% increases in rates of stage 3 AKI. For both of these risk factors, temporal relationships were tighter; increases in both values appeared just before onset of stage 3 disease.
Dr. Joyner has been a speaker on behalf of NXStage Medical; a consultant to Astute Medical, Baxter, Mallinckrodt, Pfizer, and Sphingotec; and he has received research funding from Astute, Bioporto, NxStage, and Satellite Healthcare. Mr. Jewell and Dr. Hardenburg disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
in two independent, European case series presented recently at the International Society of Nephrology: 2021 World Congress. Many of the cases progressed to more severe, stage 3 AKI. Factors linked with incident AKI in the two reports included use of mechanical ventilation, vasopressors, or diuretics, and elevations in inflammatory markers.
The new findings confirm several U.S. reports published during the past year. In those reports, roughly a third of patients hospitalized for COVID-19 developed AKI during their hospital stay, said Jay L. Koyner, MD, during another renal conference, the National Kidney Foundation 2021 Spring Clinical Meetings.
Experience has shown it’s bad news when hospitalized COVID-19 patients develop AKI, which can prove fatal or can lead to the development or worsening of chronic kidney disease (CKD), which in some cases rapidly progresses to end-stage disease.
COVID-19 giving nephrologists an opportunity to improve AKI care
“COVID is giving us an opportunity to do a better job of taking care of patients who develop AKI, which is something that nephrologists have not often excelled at doing,” said Dr. Koyner, professor and director of the nephrology ICU at the University of Chicago.
“Many studies will look at how we can manage COVID-19 patients better after they develop AKI, because I suspect a large number of these patients will wind up with CKD,” Dr. Koyner said during his talk.
He cited several lessons from reports of AKI that occurs in patients hospitalized for COVID-19:
- Preexisting CKD, , and severe COVID-19 appear to be risk factors for developing COVID-related AKI.
- Patients who develop AKI during acutely severe COVID-19 may have slightly worse outcomes than patients without COVID-19 who develop AKI.
- Certain genetic susceptibilities may play a role in developing COVID-19–related AKI.
- Routine follow-up of AKI is generally inadequate and is not standardized, whether AKI develops while ill with COVID-19 or in other settings.
The most encouraging AKI takeaway from COVID-19’s first year is that its incidence among patients hospitalized with COVID-19 appears to have dropped from very high rates early on, possibly because of more routine use of steroids for critically ill patients with COVID-19 and a reduction in the use of ventilators, Dr. Koyner suggested.
In-hospital diuretic treatment links with AKI
One of the World Congress of Nephrology reports involved 1,248 patients admitted with confirmed COVID-19 at two tertiary care hospitals in London during March–May 2020. The average age of the patients was 69 years, 59% were men, and 17% had CKD at admission, as determined on the basis of estimated glomerular filtration rate <60 mL/min per 1.73 m2.
During hospitalization, 487 patients (39%) developed AKI, including 175 (14%) with stage 3 AKI and 109 (9%) who required renal replacement therapy (dialysis or kidney transplant). The incidence of AKI peaked 5 weeks after COVID-19 admission, Paul Jewell and associates from King’s College Hospital, London, reported in a poster.
Multivariate analysis identified several demographic and clinical variables that were significantly linked with an increased risk of developing AKI: male sex (which boosted risk by 55%), Black race (79% higher risk), CKD at admission (triple the risk), being hypertensive on admission (73% higher risk), and being administered diuretics during hospitalization (69% higher risk).
The findings of a risk linked with diuretic use “supports the cautious use of diuretics in patients hospitalized with COVID-19, especially in the presence of background renal impairment,” the authors said.
For patients with incident AKI, the 30-day mortality rate was significantly increased; mortality was 59% higher among patients who developed stage 1 AKI and was roughly triple among patients who developed stage 2 or 3 AKI.
Second report links ventilation, vasopressors with worse AKI
A separate report from clinicians at Charité Hospital, Berlin, retrospectively analyzed 223 patients admitted with symptomatic COVID-19 to three Charité sites during March–June 2020. During hospitalization, 117 patients (52%) developed AKI, including 70 (31%) with stage 3 disease; 67 (30%) required renal replacement therapy. Half the patients with stage 3 AKI required ICU admission.
Compared with patients with less severe AKI, patients who developed stage 3 AKI were more often male, older, and had a higher body mass index.
In a multivariate model, compared with patients who developed less severe AKI, those who developed stage 3 disease also were significantly more likely to have received mechanical ventilation or vasopressor drugs and were more likely to have increased levels of leukocytes or procalcitonin, two inflammatory markers, Jan-Hendrink B. Hardenburg, MD, a Charité nephrologist, and associates reported in a poster at the meeting.
Mechanical ventilation was linked with a sixfold higher rate of stage 3 AKI, and treatment with vasopressor drugs was linked with a threefold higher rate. Elevations in procalcitonin or leukocyte levels were linked with about 60% increases in rates of stage 3 AKI. For both of these risk factors, temporal relationships were tighter; increases in both values appeared just before onset of stage 3 disease.
Dr. Joyner has been a speaker on behalf of NXStage Medical; a consultant to Astute Medical, Baxter, Mallinckrodt, Pfizer, and Sphingotec; and he has received research funding from Astute, Bioporto, NxStage, and Satellite Healthcare. Mr. Jewell and Dr. Hardenburg disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Dark Brown Hyperkeratotic Nodule on the Back
The Diagnosis: Seborrheic Keratosis-like Melanoma
Seborrheic keratosis (SK) is a benign neoplasm commonly encountered on the skin and frequently diagnosed by clinical examination alone. Seborrheic keratosis-like melanomas are melanomas that clinically or dermatoscopically resemble SKs and thus can be challenging to accurately diagnose. Melanomas can have a hyperkeratotic or verrucous appearance1-3 and can even exhibit dermatoscopic and microscopic features that are found in SKs such as comedolike openings and milialike cysts as well as acanthosis and pseudohorn cysts, respectively.2
In our patient, histopathology revealed SK-like architecture with hyperorthokeratosis, papillomatosis, pseudohorn cyst formation, and basaloid acanthosis (Figure). However, within the lesion was an asymmetric proliferation of nested atypical melanocytes with melanin pigment production. The atypical melanocytes filled and expanded papillomatous projections without notable pagetoid growth and extended into the dermis. There was a background congenital nevus component. These findings were diagnostic of invasive malignant melanoma, extending to a Breslow depth of 5.5 mm. A follow-up sentinel lymph node biopsy was negative for metastatic melanoma. The clinical and histologic findings did not show melanoma in the surrounding skin to suggest colonization of an SK by an adjacent melanoma. The clinical history of a long-standing lesion in conjunction with a congenital nevus component on histology favored a diagnosis of melanoma arising in association with a congenital nevus with an SK-like architecture rather than arising in a preexisting SK or de novo melanoma.
Because our patient did not have multiple widespread SKs and reported rapid growth in the lesion in the last 6 months, there was concern for a malignant neoplasm. However, in patients with numerous SKs or areas of chronically sun-damaged skin, it can be difficult to identify suspicious lesions. It is important for clinicians to remain aware of SK-like melanomas and have a lower threshold for biopsy of any changing or symptomatic lesion that clinically resembles an SK. In our case, the history of change and the markedly different clinical appearance of the lesion in comparison to our patient's SKs prompted the biopsy. Criteria have been proposed to help differentiate these entities under dermoscopy, with melanoma showing the presence of the blue-black sign, pigment network, pseudopods or streaks, and/or the blue-white veil.4
Cutaneous metastases classically present as dermal nodules, plaques, or ulcers.5,6 A rare pigmented case of metastatic breast adenocarcinoma clinically mimicking melanoma has been reported.7 There is limited literature on the dermoscopic features of cutaneous metastases, but it appears that polymorphic vascular patterns are most common.5,8 The possibility of a metastatic melanoma involving an SK is a theoretical consideration, but there was no prior history of melanoma in our patient, and the histologic findings were consistent with primary melanoma. There was no histologic evidence of pigmented metastatic breast carcinoma or metastatic lung carcinoma.
Pigmented malignant hidroacanthoma simplex and pigmented porocarcinomas are rare malignant sweat gland tumors.9-11 Their benign counterparts are the more commonly encountered hidroacanthoma simplex (intraepidermal poroma) and poroma. Pigmented malignant hidroacanthoma simplex has been reported to clinically mimic an irritated SK.10 The histopathology of our case did not have features of malignant hidroacanthoma simplex or porocarcinoma. Pigmented squamous cell carcinoma is an uncommon variant of squamous cell carcinoma, and histopathology would reveal proliferation of atypical keratinocytes.12
- Saggini A, Cota C, Lora V, et al. Uncommon histopathological variants of malignant melanoma. part 2. Am J Dermatopathol. 2019;41:321-342.
- Klebanov N, Gunasekera N, Lin WM, et al. The clinical spectrum of cutaneous melanoma morphology. J Am Acad Dermatol. 2019;80:178-188.
- Tran PT, Truong AK, Munday W, et al. Verrucous melanoma masquerading as a seborrheic keratosis. Dermatol Online J. 2019;25:13030/qt1m07k7fm.
- Carrera C, Segura S, Aguilera P. Dermoscopic clues for diagnosing melanomas that resemble seborrheic keratosis. JAMA Dermatol. 2017;153:544-551.
- Strickley JD, Jenson AB, Jung JY. Cutaneous metastasis. Hematol Oncol Clin North Am. 2019;33:173-197.
- Chernoff KA, Marghoob AA, Lacouture ME. Dermoscopic findings in cutaneous metastases. JAMA Dermatol. 2014;150:429-433.
- Marti N, Molina I, Monteagudo C, et al. Cutaneous metastasis of breast carcinoma mimicking malignant melanoma in scalp. Dermatol Online J. 2008;14:12.
- Kelati A, Gallouj S. Dermoscopy of skin metastases from breast cancer: two case reports. J Med Case Rep. 2018;12:273.
- Ishida M, Hotta M, Kushima R, et al. A case of porocarcinoma arising in pigmented hidroacanthoma simplex with multiple lymph node, liver and bone metastases. J Cutan Pathol. 2011;38:227-231.
- Lee JY, Lin MH. Pigmented malignant hidroacanthoma simplex mimicking irritated seborrheic keratosis. J Cutan Pathol. 2006;33:705-708.
- Ueo T, Kashima K, Daa T, et al. Porocarcinoma arising in pigmented hidroacanthoma simplex. Am J Dermatopathol. 2005;27:500-503.
- Motta de Morais P, Schettini A, Rocha J, et al. Pigmented squamous cell carcinoma: case report and importance of differential diagnosis. An Bras Dermatol. 2018;93:96-98.
The Diagnosis: Seborrheic Keratosis-like Melanoma
Seborrheic keratosis (SK) is a benign neoplasm commonly encountered on the skin and frequently diagnosed by clinical examination alone. Seborrheic keratosis-like melanomas are melanomas that clinically or dermatoscopically resemble SKs and thus can be challenging to accurately diagnose. Melanomas can have a hyperkeratotic or verrucous appearance1-3 and can even exhibit dermatoscopic and microscopic features that are found in SKs such as comedolike openings and milialike cysts as well as acanthosis and pseudohorn cysts, respectively.2
In our patient, histopathology revealed SK-like architecture with hyperorthokeratosis, papillomatosis, pseudohorn cyst formation, and basaloid acanthosis (Figure). However, within the lesion was an asymmetric proliferation of nested atypical melanocytes with melanin pigment production. The atypical melanocytes filled and expanded papillomatous projections without notable pagetoid growth and extended into the dermis. There was a background congenital nevus component. These findings were diagnostic of invasive malignant melanoma, extending to a Breslow depth of 5.5 mm. A follow-up sentinel lymph node biopsy was negative for metastatic melanoma. The clinical and histologic findings did not show melanoma in the surrounding skin to suggest colonization of an SK by an adjacent melanoma. The clinical history of a long-standing lesion in conjunction with a congenital nevus component on histology favored a diagnosis of melanoma arising in association with a congenital nevus with an SK-like architecture rather than arising in a preexisting SK or de novo melanoma.

Because our patient did not have multiple widespread SKs and reported rapid growth in the lesion in the last 6 months, there was concern for a malignant neoplasm. However, in patients with numerous SKs or areas of chronically sun-damaged skin, it can be difficult to identify suspicious lesions. It is important for clinicians to remain aware of SK-like melanomas and have a lower threshold for biopsy of any changing or symptomatic lesion that clinically resembles an SK. In our case, the history of change and the markedly different clinical appearance of the lesion in comparison to our patient's SKs prompted the biopsy. Criteria have been proposed to help differentiate these entities under dermoscopy, with melanoma showing the presence of the blue-black sign, pigment network, pseudopods or streaks, and/or the blue-white veil.4
Cutaneous metastases classically present as dermal nodules, plaques, or ulcers.5,6 A rare pigmented case of metastatic breast adenocarcinoma clinically mimicking melanoma has been reported.7 There is limited literature on the dermoscopic features of cutaneous metastases, but it appears that polymorphic vascular patterns are most common.5,8 The possibility of a metastatic melanoma involving an SK is a theoretical consideration, but there was no prior history of melanoma in our patient, and the histologic findings were consistent with primary melanoma. There was no histologic evidence of pigmented metastatic breast carcinoma or metastatic lung carcinoma.
Pigmented malignant hidroacanthoma simplex and pigmented porocarcinomas are rare malignant sweat gland tumors.9-11 Their benign counterparts are the more commonly encountered hidroacanthoma simplex (intraepidermal poroma) and poroma. Pigmented malignant hidroacanthoma simplex has been reported to clinically mimic an irritated SK.10 The histopathology of our case did not have features of malignant hidroacanthoma simplex or porocarcinoma. Pigmented squamous cell carcinoma is an uncommon variant of squamous cell carcinoma, and histopathology would reveal proliferation of atypical keratinocytes.12
The Diagnosis: Seborrheic Keratosis-like Melanoma
Seborrheic keratosis (SK) is a benign neoplasm commonly encountered on the skin and frequently diagnosed by clinical examination alone. Seborrheic keratosis-like melanomas are melanomas that clinically or dermatoscopically resemble SKs and thus can be challenging to accurately diagnose. Melanomas can have a hyperkeratotic or verrucous appearance1-3 and can even exhibit dermatoscopic and microscopic features that are found in SKs such as comedolike openings and milialike cysts as well as acanthosis and pseudohorn cysts, respectively.2
In our patient, histopathology revealed SK-like architecture with hyperorthokeratosis, papillomatosis, pseudohorn cyst formation, and basaloid acanthosis (Figure). However, within the lesion was an asymmetric proliferation of nested atypical melanocytes with melanin pigment production. The atypical melanocytes filled and expanded papillomatous projections without notable pagetoid growth and extended into the dermis. There was a background congenital nevus component. These findings were diagnostic of invasive malignant melanoma, extending to a Breslow depth of 5.5 mm. A follow-up sentinel lymph node biopsy was negative for metastatic melanoma. The clinical and histologic findings did not show melanoma in the surrounding skin to suggest colonization of an SK by an adjacent melanoma. The clinical history of a long-standing lesion in conjunction with a congenital nevus component on histology favored a diagnosis of melanoma arising in association with a congenital nevus with an SK-like architecture rather than arising in a preexisting SK or de novo melanoma.

Because our patient did not have multiple widespread SKs and reported rapid growth in the lesion in the last 6 months, there was concern for a malignant neoplasm. However, in patients with numerous SKs or areas of chronically sun-damaged skin, it can be difficult to identify suspicious lesions. It is important for clinicians to remain aware of SK-like melanomas and have a lower threshold for biopsy of any changing or symptomatic lesion that clinically resembles an SK. In our case, the history of change and the markedly different clinical appearance of the lesion in comparison to our patient's SKs prompted the biopsy. Criteria have been proposed to help differentiate these entities under dermoscopy, with melanoma showing the presence of the blue-black sign, pigment network, pseudopods or streaks, and/or the blue-white veil.4
Cutaneous metastases classically present as dermal nodules, plaques, or ulcers.5,6 A rare pigmented case of metastatic breast adenocarcinoma clinically mimicking melanoma has been reported.7 There is limited literature on the dermoscopic features of cutaneous metastases, but it appears that polymorphic vascular patterns are most common.5,8 The possibility of a metastatic melanoma involving an SK is a theoretical consideration, but there was no prior history of melanoma in our patient, and the histologic findings were consistent with primary melanoma. There was no histologic evidence of pigmented metastatic breast carcinoma or metastatic lung carcinoma.
Pigmented malignant hidroacanthoma simplex and pigmented porocarcinomas are rare malignant sweat gland tumors.9-11 Their benign counterparts are the more commonly encountered hidroacanthoma simplex (intraepidermal poroma) and poroma. Pigmented malignant hidroacanthoma simplex has been reported to clinically mimic an irritated SK.10 The histopathology of our case did not have features of malignant hidroacanthoma simplex or porocarcinoma. Pigmented squamous cell carcinoma is an uncommon variant of squamous cell carcinoma, and histopathology would reveal proliferation of atypical keratinocytes.12
- Saggini A, Cota C, Lora V, et al. Uncommon histopathological variants of malignant melanoma. part 2. Am J Dermatopathol. 2019;41:321-342.
- Klebanov N, Gunasekera N, Lin WM, et al. The clinical spectrum of cutaneous melanoma morphology. J Am Acad Dermatol. 2019;80:178-188.
- Tran PT, Truong AK, Munday W, et al. Verrucous melanoma masquerading as a seborrheic keratosis. Dermatol Online J. 2019;25:13030/qt1m07k7fm.
- Carrera C, Segura S, Aguilera P. Dermoscopic clues for diagnosing melanomas that resemble seborrheic keratosis. JAMA Dermatol. 2017;153:544-551.
- Strickley JD, Jenson AB, Jung JY. Cutaneous metastasis. Hematol Oncol Clin North Am. 2019;33:173-197.
- Chernoff KA, Marghoob AA, Lacouture ME. Dermoscopic findings in cutaneous metastases. JAMA Dermatol. 2014;150:429-433.
- Marti N, Molina I, Monteagudo C, et al. Cutaneous metastasis of breast carcinoma mimicking malignant melanoma in scalp. Dermatol Online J. 2008;14:12.
- Kelati A, Gallouj S. Dermoscopy of skin metastases from breast cancer: two case reports. J Med Case Rep. 2018;12:273.
- Ishida M, Hotta M, Kushima R, et al. A case of porocarcinoma arising in pigmented hidroacanthoma simplex with multiple lymph node, liver and bone metastases. J Cutan Pathol. 2011;38:227-231.
- Lee JY, Lin MH. Pigmented malignant hidroacanthoma simplex mimicking irritated seborrheic keratosis. J Cutan Pathol. 2006;33:705-708.
- Ueo T, Kashima K, Daa T, et al. Porocarcinoma arising in pigmented hidroacanthoma simplex. Am J Dermatopathol. 2005;27:500-503.
- Motta de Morais P, Schettini A, Rocha J, et al. Pigmented squamous cell carcinoma: case report and importance of differential diagnosis. An Bras Dermatol. 2018;93:96-98.
- Saggini A, Cota C, Lora V, et al. Uncommon histopathological variants of malignant melanoma. part 2. Am J Dermatopathol. 2019;41:321-342.
- Klebanov N, Gunasekera N, Lin WM, et al. The clinical spectrum of cutaneous melanoma morphology. J Am Acad Dermatol. 2019;80:178-188.
- Tran PT, Truong AK, Munday W, et al. Verrucous melanoma masquerading as a seborrheic keratosis. Dermatol Online J. 2019;25:13030/qt1m07k7fm.
- Carrera C, Segura S, Aguilera P. Dermoscopic clues for diagnosing melanomas that resemble seborrheic keratosis. JAMA Dermatol. 2017;153:544-551.
- Strickley JD, Jenson AB, Jung JY. Cutaneous metastasis. Hematol Oncol Clin North Am. 2019;33:173-197.
- Chernoff KA, Marghoob AA, Lacouture ME. Dermoscopic findings in cutaneous metastases. JAMA Dermatol. 2014;150:429-433.
- Marti N, Molina I, Monteagudo C, et al. Cutaneous metastasis of breast carcinoma mimicking malignant melanoma in scalp. Dermatol Online J. 2008;14:12.
- Kelati A, Gallouj S. Dermoscopy of skin metastases from breast cancer: two case reports. J Med Case Rep. 2018;12:273.
- Ishida M, Hotta M, Kushima R, et al. A case of porocarcinoma arising in pigmented hidroacanthoma simplex with multiple lymph node, liver and bone metastases. J Cutan Pathol. 2011;38:227-231.
- Lee JY, Lin MH. Pigmented malignant hidroacanthoma simplex mimicking irritated seborrheic keratosis. J Cutan Pathol. 2006;33:705-708.
- Ueo T, Kashima K, Daa T, et al. Porocarcinoma arising in pigmented hidroacanthoma simplex. Am J Dermatopathol. 2005;27:500-503.
- Motta de Morais P, Schettini A, Rocha J, et al. Pigmented squamous cell carcinoma: case report and importance of differential diagnosis. An Bras Dermatol. 2018;93:96-98.
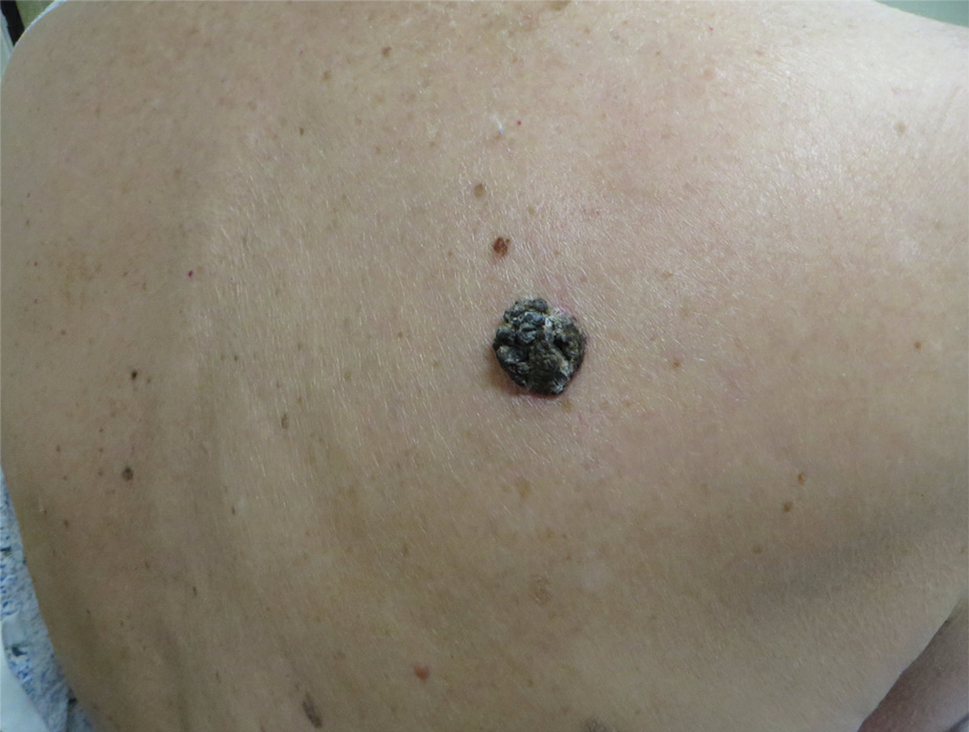
A 71-year-old woman presented with a persistent asymptomatic lesion on the right upper back that had recently increased in size and changed in color, shape, and texture. The lesion had been present for many years. Physical examination revealed a 1.5-cm, dark brown, hyperkeratotic nodule with no identifiable pigment network on dermatoscopy. The patient had no personal history of melanoma but did have a history of stage I non–small cell lung cancer. A review of systems was noncontributory. A shave biopsy of the lesion was performed.
Promising data on once-weekly insulin, phase 3 study ongoing
Two new phase 2 studies show encouraging findings with the investigational once-weekly basal insulin analogue icodec (Novo Nordisk) for people with type 2 diabetes who require insulin.
Insulin icodec works by reversibly binding to albumin, which slows the release of the active insulin analogue. It has a half-life of about 1 week. The glucose-lowering effect is distributed nearly evenly over the course of that week.
Ildiko Lingvay, MD, of the University of Texas Southwestern Medical Center, Dallas, who is an author of both new articles, said: “A weekly insulin is a game changer that will decrease the treatment burden for patients while also improving compliance.”
She noted that these studies demonstrate optimal approaches to initiating treatment with icodec and serve “as the steppingstones for a large phase 3 clinical trial program that is currently ongoing ... which is designed to evaluate the efficacy of once-weekly insulin administration in patients with either type 1 or type 2 diabetes.”
Another advantage of the formulation, Dr. Lingvay pointed out in a press release from her institution, is that it could decrease the burden on caregivers of patients with diabetes who require insulin.
“For example, for patients who need help injecting, those living in long-term care facilities, and those with memory problems, a once-weekly insulin will facilitate treatment and decrease the burden on the care providers,” she explained.
Titration balances glycemic control with hypoglycemic risk reduction
The first phase 2 trial, published online April 19, 2021, in Diabetes Care, was an open-label, 16-week, treat-to-target study that involved 205 insulin-naive adults with type 2 diabetes whose hemoglobin A1c levels were 7%-10% while using oral glucose-lowering medications.
They were randomly assigned to one of three once-weekly icodec titration groups:
- Group A – Fasting glucose target of 80-130 mg/dL with adjustments ±21 units/wk
- Group B – Fasting glucose target of 80-130 mg/dL with ±28 units/wk
- Group C – Fasting glucose target of 70-108 mg/dL, adjusting by ±28 units/wk or to once-daily glargine U100 with a fasting glucose target of 80-130 mg/dL with adjustments of ±4 units/d
The percentage of time in the ideal glucose range of 70-180 mg/dL, assessed by continuous glucose monitoring during weeks 15-16, improved from baseline levels of 57.0%, 55.2%, 51.0% for groups A, B, and C, respectively, and from 55.3% for glargine to 76.6%, 83.0%, 80.9%, and 75.9%, respectively.
There were no unexpected safety problems. Hypoglycemia episodes of glucose levels <54 mg/dL occurred in 0.05, 0.15, 0.38, and 0.00 per patient-year for the four groups, respectively. None were severe (i.e., required assistance).
The titration for patients in group A (80-130 mg/dL, ±21 units/wk) yielded the best balance between glycemic control and risk for hypoglycemia, Dr. Lingvay and colleagues said.
Use of loading dose when switching to icodec improves time in range
In the other phase 2 trial, also published online April 19 in Diabetes Care, Harpreet S. Bajaj, MD, of Mount Sinai Hospital, Toronto, and colleagues, with Dr. Lingvay as a coauthor, examined two methods of switching to icodec. This multicenter, open-label, treat-to-target study included 154 patients with A1c levels of 7-10% who were already receiving basal insulin daily and at least one oral glucose-lowering medication.
Patients were randomly assigned to one of three treatment approaches: a 100% loading dose of icodec (only the first dose was doubled), no loading dose, or once-daily glargine.
The primary endpoint was time in range (70-180 mg/dL) during weeks 15 and 16. This was achieved with 72.9% of patients receiving the icodec loading dose, 66.0% of patients receiving icodec without the loading dose, and 65.0% of patients receiving daily glargine. The difference between the icodec loading-dose method and glargine was significant, Dr. Bajaj and colleagues reported.
The mean A1c level was reduced from an overall average of 7.9% at baseline to 7.1% in the icodec loading dose group and to 7.4% in both the no-loading-dose and glargine groups.
Rates of adverse events and hypoglycemic episodes did not differ significantly among the groups.
Previous phase 2 data showing that the efficacy and safety of icodec were comparable with that of once-daily insulin glargine U100 in 247 insulin-naive patients with type 2 diabetes were published in November 2020 in the New England Journal of Medicine and were presented at the European Association for the Study of Diabetes (EASD) 2020 Annual Meeting, as reported by this news organization.
Both studies were funded by Novo Nordisk. Dr. Lingvey has received research funding, advisory/consulting fees, or other support from Novo Nordisk, Eli Lilly, Sanofi, AstraZeneca, Boehringer Ingelheim, Janssen, Intercept, Intarcia, Target RWE, Merck, Pfizer, Novartis, GI Dynamics, Mylan, Mannkind, Valeritas, Bayer, and Zealand Pharma. Dr. Bajaj has received speaking fees from AstraZeneca, Eli Lilly, Janssen Pharmaceuticals, Merck, and Novo Nordisk and research funding paid to LMC Healthcare for serving as principal investigator on clinical trials from Amgen, AstraZeneca Boehringer Ingelheim, Ceapro Inc, Eli Lilly, Gilead Sciences, Janssen Pharmaceuticals, Kowa Pharmaceuticals, Madrigal Pharmaceuticals, Merck, Novo Nordisk, Sanofi, and Tricida.
A version of this article first appeared on Medscape.com.
Two new phase 2 studies show encouraging findings with the investigational once-weekly basal insulin analogue icodec (Novo Nordisk) for people with type 2 diabetes who require insulin.
Insulin icodec works by reversibly binding to albumin, which slows the release of the active insulin analogue. It has a half-life of about 1 week. The glucose-lowering effect is distributed nearly evenly over the course of that week.
Ildiko Lingvay, MD, of the University of Texas Southwestern Medical Center, Dallas, who is an author of both new articles, said: “A weekly insulin is a game changer that will decrease the treatment burden for patients while also improving compliance.”
She noted that these studies demonstrate optimal approaches to initiating treatment with icodec and serve “as the steppingstones for a large phase 3 clinical trial program that is currently ongoing ... which is designed to evaluate the efficacy of once-weekly insulin administration in patients with either type 1 or type 2 diabetes.”
Another advantage of the formulation, Dr. Lingvay pointed out in a press release from her institution, is that it could decrease the burden on caregivers of patients with diabetes who require insulin.
“For example, for patients who need help injecting, those living in long-term care facilities, and those with memory problems, a once-weekly insulin will facilitate treatment and decrease the burden on the care providers,” she explained.
Titration balances glycemic control with hypoglycemic risk reduction
The first phase 2 trial, published online April 19, 2021, in Diabetes Care, was an open-label, 16-week, treat-to-target study that involved 205 insulin-naive adults with type 2 diabetes whose hemoglobin A1c levels were 7%-10% while using oral glucose-lowering medications.
They were randomly assigned to one of three once-weekly icodec titration groups:
- Group A – Fasting glucose target of 80-130 mg/dL with adjustments ±21 units/wk
- Group B – Fasting glucose target of 80-130 mg/dL with ±28 units/wk
- Group C – Fasting glucose target of 70-108 mg/dL, adjusting by ±28 units/wk or to once-daily glargine U100 with a fasting glucose target of 80-130 mg/dL with adjustments of ±4 units/d
The percentage of time in the ideal glucose range of 70-180 mg/dL, assessed by continuous glucose monitoring during weeks 15-16, improved from baseline levels of 57.0%, 55.2%, 51.0% for groups A, B, and C, respectively, and from 55.3% for glargine to 76.6%, 83.0%, 80.9%, and 75.9%, respectively.
There were no unexpected safety problems. Hypoglycemia episodes of glucose levels <54 mg/dL occurred in 0.05, 0.15, 0.38, and 0.00 per patient-year for the four groups, respectively. None were severe (i.e., required assistance).
The titration for patients in group A (80-130 mg/dL, ±21 units/wk) yielded the best balance between glycemic control and risk for hypoglycemia, Dr. Lingvay and colleagues said.
Use of loading dose when switching to icodec improves time in range
In the other phase 2 trial, also published online April 19 in Diabetes Care, Harpreet S. Bajaj, MD, of Mount Sinai Hospital, Toronto, and colleagues, with Dr. Lingvay as a coauthor, examined two methods of switching to icodec. This multicenter, open-label, treat-to-target study included 154 patients with A1c levels of 7-10% who were already receiving basal insulin daily and at least one oral glucose-lowering medication.
Patients were randomly assigned to one of three treatment approaches: a 100% loading dose of icodec (only the first dose was doubled), no loading dose, or once-daily glargine.
The primary endpoint was time in range (70-180 mg/dL) during weeks 15 and 16. This was achieved with 72.9% of patients receiving the icodec loading dose, 66.0% of patients receiving icodec without the loading dose, and 65.0% of patients receiving daily glargine. The difference between the icodec loading-dose method and glargine was significant, Dr. Bajaj and colleagues reported.
The mean A1c level was reduced from an overall average of 7.9% at baseline to 7.1% in the icodec loading dose group and to 7.4% in both the no-loading-dose and glargine groups.
Rates of adverse events and hypoglycemic episodes did not differ significantly among the groups.
Previous phase 2 data showing that the efficacy and safety of icodec were comparable with that of once-daily insulin glargine U100 in 247 insulin-naive patients with type 2 diabetes were published in November 2020 in the New England Journal of Medicine and were presented at the European Association for the Study of Diabetes (EASD) 2020 Annual Meeting, as reported by this news organization.
Both studies were funded by Novo Nordisk. Dr. Lingvey has received research funding, advisory/consulting fees, or other support from Novo Nordisk, Eli Lilly, Sanofi, AstraZeneca, Boehringer Ingelheim, Janssen, Intercept, Intarcia, Target RWE, Merck, Pfizer, Novartis, GI Dynamics, Mylan, Mannkind, Valeritas, Bayer, and Zealand Pharma. Dr. Bajaj has received speaking fees from AstraZeneca, Eli Lilly, Janssen Pharmaceuticals, Merck, and Novo Nordisk and research funding paid to LMC Healthcare for serving as principal investigator on clinical trials from Amgen, AstraZeneca Boehringer Ingelheim, Ceapro Inc, Eli Lilly, Gilead Sciences, Janssen Pharmaceuticals, Kowa Pharmaceuticals, Madrigal Pharmaceuticals, Merck, Novo Nordisk, Sanofi, and Tricida.
A version of this article first appeared on Medscape.com.
Two new phase 2 studies show encouraging findings with the investigational once-weekly basal insulin analogue icodec (Novo Nordisk) for people with type 2 diabetes who require insulin.
Insulin icodec works by reversibly binding to albumin, which slows the release of the active insulin analogue. It has a half-life of about 1 week. The glucose-lowering effect is distributed nearly evenly over the course of that week.
Ildiko Lingvay, MD, of the University of Texas Southwestern Medical Center, Dallas, who is an author of both new articles, said: “A weekly insulin is a game changer that will decrease the treatment burden for patients while also improving compliance.”
She noted that these studies demonstrate optimal approaches to initiating treatment with icodec and serve “as the steppingstones for a large phase 3 clinical trial program that is currently ongoing ... which is designed to evaluate the efficacy of once-weekly insulin administration in patients with either type 1 or type 2 diabetes.”
Another advantage of the formulation, Dr. Lingvay pointed out in a press release from her institution, is that it could decrease the burden on caregivers of patients with diabetes who require insulin.
“For example, for patients who need help injecting, those living in long-term care facilities, and those with memory problems, a once-weekly insulin will facilitate treatment and decrease the burden on the care providers,” she explained.
Titration balances glycemic control with hypoglycemic risk reduction
The first phase 2 trial, published online April 19, 2021, in Diabetes Care, was an open-label, 16-week, treat-to-target study that involved 205 insulin-naive adults with type 2 diabetes whose hemoglobin A1c levels were 7%-10% while using oral glucose-lowering medications.
They were randomly assigned to one of three once-weekly icodec titration groups:
- Group A – Fasting glucose target of 80-130 mg/dL with adjustments ±21 units/wk
- Group B – Fasting glucose target of 80-130 mg/dL with ±28 units/wk
- Group C – Fasting glucose target of 70-108 mg/dL, adjusting by ±28 units/wk or to once-daily glargine U100 with a fasting glucose target of 80-130 mg/dL with adjustments of ±4 units/d
The percentage of time in the ideal glucose range of 70-180 mg/dL, assessed by continuous glucose monitoring during weeks 15-16, improved from baseline levels of 57.0%, 55.2%, 51.0% for groups A, B, and C, respectively, and from 55.3% for glargine to 76.6%, 83.0%, 80.9%, and 75.9%, respectively.
There were no unexpected safety problems. Hypoglycemia episodes of glucose levels <54 mg/dL occurred in 0.05, 0.15, 0.38, and 0.00 per patient-year for the four groups, respectively. None were severe (i.e., required assistance).
The titration for patients in group A (80-130 mg/dL, ±21 units/wk) yielded the best balance between glycemic control and risk for hypoglycemia, Dr. Lingvay and colleagues said.
Use of loading dose when switching to icodec improves time in range
In the other phase 2 trial, also published online April 19 in Diabetes Care, Harpreet S. Bajaj, MD, of Mount Sinai Hospital, Toronto, and colleagues, with Dr. Lingvay as a coauthor, examined two methods of switching to icodec. This multicenter, open-label, treat-to-target study included 154 patients with A1c levels of 7-10% who were already receiving basal insulin daily and at least one oral glucose-lowering medication.
Patients were randomly assigned to one of three treatment approaches: a 100% loading dose of icodec (only the first dose was doubled), no loading dose, or once-daily glargine.
The primary endpoint was time in range (70-180 mg/dL) during weeks 15 and 16. This was achieved with 72.9% of patients receiving the icodec loading dose, 66.0% of patients receiving icodec without the loading dose, and 65.0% of patients receiving daily glargine. The difference between the icodec loading-dose method and glargine was significant, Dr. Bajaj and colleagues reported.
The mean A1c level was reduced from an overall average of 7.9% at baseline to 7.1% in the icodec loading dose group and to 7.4% in both the no-loading-dose and glargine groups.
Rates of adverse events and hypoglycemic episodes did not differ significantly among the groups.
Previous phase 2 data showing that the efficacy and safety of icodec were comparable with that of once-daily insulin glargine U100 in 247 insulin-naive patients with type 2 diabetes were published in November 2020 in the New England Journal of Medicine and were presented at the European Association for the Study of Diabetes (EASD) 2020 Annual Meeting, as reported by this news organization.
Both studies were funded by Novo Nordisk. Dr. Lingvey has received research funding, advisory/consulting fees, or other support from Novo Nordisk, Eli Lilly, Sanofi, AstraZeneca, Boehringer Ingelheim, Janssen, Intercept, Intarcia, Target RWE, Merck, Pfizer, Novartis, GI Dynamics, Mylan, Mannkind, Valeritas, Bayer, and Zealand Pharma. Dr. Bajaj has received speaking fees from AstraZeneca, Eli Lilly, Janssen Pharmaceuticals, Merck, and Novo Nordisk and research funding paid to LMC Healthcare for serving as principal investigator on clinical trials from Amgen, AstraZeneca Boehringer Ingelheim, Ceapro Inc, Eli Lilly, Gilead Sciences, Janssen Pharmaceuticals, Kowa Pharmaceuticals, Madrigal Pharmaceuticals, Merck, Novo Nordisk, Sanofi, and Tricida.
A version of this article first appeared on Medscape.com.
How does fragmented care affect IBD outcomes?
Poor continuity of care may lead to worse outcomes among patients with active inflammatory bowel disease (IBD), according to data from more than 20,000 veterans.
Even in the Veterans Health Administration health care system, which “may provide the ideal environment for care coordination,” patients with active IBD had “substantial variation” in dispersion of care, leading to more frequent surgical interventions, corticosteroid use, and hospitalizations, reported lead author Shirley Cohen-Mekelburg, MD, MS, of the University of Michigan, Ann Arbor, and colleagues.
“Health care in the United States is marked by substantial fragmentation, with patients pursuing and receiving care from multiple clinicians, often at different institutions,” the investigators wrote in JAMA Network Open. “Fragmented care has been associated with poor chronic disease outcomes, higher health care use, duplication in testing, and increased costs of care.”
In the VHA, these issues prompted creation of the Patient Aligned Care Team (PACT), a medical home model in which primary care physicians coordinate clinical teams of specialists and other health care practitioners. But coordination can be challenging with chronic medical conditions like IBD, according to Dr. Cohen-Mekelburg and colleagues.
“High-quality care for IBD includes not only disease-specific management of symptoms but also disease-specific preventive care, such as immunizations and cancer screening, to prevent associated adverse outcomes,” the investigators wrote. “Identifying which physician is responsible for managing each aspect of care requires some degree of coordination and makes patients with IBD vulnerable to care fragmentation.”
Worse outcomes tied to poor first-year continuity
To evaluate care fragmentation within the VHA, the investigators identified 20,079 veterans with IBD who had at least one outpatient encounter with the system between the beginning of 2002 and the end of 2014. Continuity of care (COC) was calculated with the Bice-Boxerman COC index, which measures how much a patient’s care is connected with a distinct physician. The investigators used the first year COC as the primary independent variable.
In the first year of care, the median COC index was 0.24 (interquartile range, 0.13-0.46). The investigators noted that this figure was lower than reported by previous studies involving patients with several other chronic conditions, including IBD.
After controlling for covariates and adjusting for facility-related clustering, the investigators found a lower COC index in the first year was associated with a higher rate of worse outcomes in the subsequent 2 years, including surgical interventions (adjusted hazard ratio, 1.72; 95% confidence interval, 1.43-2.07), hospitalizations (aHR, 1.25; 95% CI, 1.06-1.47), and outpatient flares requiring corticosteroids (aHR, 1.11; 95% CI, 1.01-1.22). Conversely, improving COC index score by 0.1 reduced risk of outpatient flare (aHR, 0.69; 95%CI, 0.58-0.82), hospitalization (aHR, 0.57; 95%CI, 0.41-0.79), and surgical intervention (aHR, 0.25; 95% CI, 0.16-0.38).
Further analyses showed that the relationship between lower COC and worse outcomes carried across measures such as baseline use of an immunomodulator or biological agent, as well as subgroups such as patients with nonsevere IBD and nonsurgical patients.
Among those treated by a VHA gastroenterologist, a lower level of COC was associated with a higher rate of surgical interventions, but not hospitalizations or outpatient flares. Physician-specific COC index scores were highest for primary care providers (0.54), followed by gastroenterologists (0.25) and surgeons (0.17). However, lower physician-specific COC scores did not translate to worse IBD outcomes.
“The level of COC among patients with IBD in the present VHA cohort was ... lower than the values described in previous studies of veterans in the VHA system, including a study of VHA-Medicare dual enrollees who were especially prone to fragmented care because of their ability to seek care both inside and outside of the VHA system,” the investigators wrote, referring to a 2018 study. “The difference in COC among patients with IBD vs. patients without IBD is likely multifactorial and may be associated with confusion about physician accountability and lack of focus on coordination in IBD multidisciplinary care. Patients with IBD require care by primary care providers, gastroenterologists, and surgeons, but the delineation of responsibility by physician is often unclear.”
‘Better care, not just more care,’ is needed
“These outcomes cannot be improved with a more robust treatment armamentarium alone,” according to Jason K. Hou, MD, MS, AGAF, FACG, interim chief of gastroenterology and hepatology at Michael E. DeBakey VA Medical Center and associate professor of medicine at Baylor College of Medicine, Houston, who cowrote a simultaneously published editorial, which was also authored by David I. Fudman, MD.
“Examples exist of improving care coordination and outcomes through patient-aligned care teams in primary care and medical specialty homes for IBD,” Dr. Hou said in an interview. “However, significant barriers to widespread implementation remain.”
Dr. Hou offered several possible approaches to overcome these barriers.
“We need improved methods to identify and follow high-risk patients most likely to have complications and health care utilization,” he said. “We need an investment by payers and health care systems on care coordination so the identified high-risk patients can receive timely testing, referral, and treatment. These changes require reevaluation of how the health care system incentivizes health care to provide better care, not just more care.”
The investigators reported grants from the U.S. Department of Veterans Affairs and the National Institutes of Health and financial relationships with AbbVie, UCB, and Takeda. Dr. Hou reported no conflicts of interest.
Poor continuity of care may lead to worse outcomes among patients with active inflammatory bowel disease (IBD), according to data from more than 20,000 veterans.
Even in the Veterans Health Administration health care system, which “may provide the ideal environment for care coordination,” patients with active IBD had “substantial variation” in dispersion of care, leading to more frequent surgical interventions, corticosteroid use, and hospitalizations, reported lead author Shirley Cohen-Mekelburg, MD, MS, of the University of Michigan, Ann Arbor, and colleagues.
“Health care in the United States is marked by substantial fragmentation, with patients pursuing and receiving care from multiple clinicians, often at different institutions,” the investigators wrote in JAMA Network Open. “Fragmented care has been associated with poor chronic disease outcomes, higher health care use, duplication in testing, and increased costs of care.”
In the VHA, these issues prompted creation of the Patient Aligned Care Team (PACT), a medical home model in which primary care physicians coordinate clinical teams of specialists and other health care practitioners. But coordination can be challenging with chronic medical conditions like IBD, according to Dr. Cohen-Mekelburg and colleagues.
“High-quality care for IBD includes not only disease-specific management of symptoms but also disease-specific preventive care, such as immunizations and cancer screening, to prevent associated adverse outcomes,” the investigators wrote. “Identifying which physician is responsible for managing each aspect of care requires some degree of coordination and makes patients with IBD vulnerable to care fragmentation.”
Worse outcomes tied to poor first-year continuity
To evaluate care fragmentation within the VHA, the investigators identified 20,079 veterans with IBD who had at least one outpatient encounter with the system between the beginning of 2002 and the end of 2014. Continuity of care (COC) was calculated with the Bice-Boxerman COC index, which measures how much a patient’s care is connected with a distinct physician. The investigators used the first year COC as the primary independent variable.
In the first year of care, the median COC index was 0.24 (interquartile range, 0.13-0.46). The investigators noted that this figure was lower than reported by previous studies involving patients with several other chronic conditions, including IBD.
After controlling for covariates and adjusting for facility-related clustering, the investigators found a lower COC index in the first year was associated with a higher rate of worse outcomes in the subsequent 2 years, including surgical interventions (adjusted hazard ratio, 1.72; 95% confidence interval, 1.43-2.07), hospitalizations (aHR, 1.25; 95% CI, 1.06-1.47), and outpatient flares requiring corticosteroids (aHR, 1.11; 95% CI, 1.01-1.22). Conversely, improving COC index score by 0.1 reduced risk of outpatient flare (aHR, 0.69; 95%CI, 0.58-0.82), hospitalization (aHR, 0.57; 95%CI, 0.41-0.79), and surgical intervention (aHR, 0.25; 95% CI, 0.16-0.38).
Further analyses showed that the relationship between lower COC and worse outcomes carried across measures such as baseline use of an immunomodulator or biological agent, as well as subgroups such as patients with nonsevere IBD and nonsurgical patients.
Among those treated by a VHA gastroenterologist, a lower level of COC was associated with a higher rate of surgical interventions, but not hospitalizations or outpatient flares. Physician-specific COC index scores were highest for primary care providers (0.54), followed by gastroenterologists (0.25) and surgeons (0.17). However, lower physician-specific COC scores did not translate to worse IBD outcomes.
“The level of COC among patients with IBD in the present VHA cohort was ... lower than the values described in previous studies of veterans in the VHA system, including a study of VHA-Medicare dual enrollees who were especially prone to fragmented care because of their ability to seek care both inside and outside of the VHA system,” the investigators wrote, referring to a 2018 study. “The difference in COC among patients with IBD vs. patients without IBD is likely multifactorial and may be associated with confusion about physician accountability and lack of focus on coordination in IBD multidisciplinary care. Patients with IBD require care by primary care providers, gastroenterologists, and surgeons, but the delineation of responsibility by physician is often unclear.”
‘Better care, not just more care,’ is needed
“These outcomes cannot be improved with a more robust treatment armamentarium alone,” according to Jason K. Hou, MD, MS, AGAF, FACG, interim chief of gastroenterology and hepatology at Michael E. DeBakey VA Medical Center and associate professor of medicine at Baylor College of Medicine, Houston, who cowrote a simultaneously published editorial, which was also authored by David I. Fudman, MD.
“Examples exist of improving care coordination and outcomes through patient-aligned care teams in primary care and medical specialty homes for IBD,” Dr. Hou said in an interview. “However, significant barriers to widespread implementation remain.”
Dr. Hou offered several possible approaches to overcome these barriers.
“We need improved methods to identify and follow high-risk patients most likely to have complications and health care utilization,” he said. “We need an investment by payers and health care systems on care coordination so the identified high-risk patients can receive timely testing, referral, and treatment. These changes require reevaluation of how the health care system incentivizes health care to provide better care, not just more care.”
The investigators reported grants from the U.S. Department of Veterans Affairs and the National Institutes of Health and financial relationships with AbbVie, UCB, and Takeda. Dr. Hou reported no conflicts of interest.
Poor continuity of care may lead to worse outcomes among patients with active inflammatory bowel disease (IBD), according to data from more than 20,000 veterans.
Even in the Veterans Health Administration health care system, which “may provide the ideal environment for care coordination,” patients with active IBD had “substantial variation” in dispersion of care, leading to more frequent surgical interventions, corticosteroid use, and hospitalizations, reported lead author Shirley Cohen-Mekelburg, MD, MS, of the University of Michigan, Ann Arbor, and colleagues.
“Health care in the United States is marked by substantial fragmentation, with patients pursuing and receiving care from multiple clinicians, often at different institutions,” the investigators wrote in JAMA Network Open. “Fragmented care has been associated with poor chronic disease outcomes, higher health care use, duplication in testing, and increased costs of care.”
In the VHA, these issues prompted creation of the Patient Aligned Care Team (PACT), a medical home model in which primary care physicians coordinate clinical teams of specialists and other health care practitioners. But coordination can be challenging with chronic medical conditions like IBD, according to Dr. Cohen-Mekelburg and colleagues.
“High-quality care for IBD includes not only disease-specific management of symptoms but also disease-specific preventive care, such as immunizations and cancer screening, to prevent associated adverse outcomes,” the investigators wrote. “Identifying which physician is responsible for managing each aspect of care requires some degree of coordination and makes patients with IBD vulnerable to care fragmentation.”
Worse outcomes tied to poor first-year continuity
To evaluate care fragmentation within the VHA, the investigators identified 20,079 veterans with IBD who had at least one outpatient encounter with the system between the beginning of 2002 and the end of 2014. Continuity of care (COC) was calculated with the Bice-Boxerman COC index, which measures how much a patient’s care is connected with a distinct physician. The investigators used the first year COC as the primary independent variable.
In the first year of care, the median COC index was 0.24 (interquartile range, 0.13-0.46). The investigators noted that this figure was lower than reported by previous studies involving patients with several other chronic conditions, including IBD.
After controlling for covariates and adjusting for facility-related clustering, the investigators found a lower COC index in the first year was associated with a higher rate of worse outcomes in the subsequent 2 years, including surgical interventions (adjusted hazard ratio, 1.72; 95% confidence interval, 1.43-2.07), hospitalizations (aHR, 1.25; 95% CI, 1.06-1.47), and outpatient flares requiring corticosteroids (aHR, 1.11; 95% CI, 1.01-1.22). Conversely, improving COC index score by 0.1 reduced risk of outpatient flare (aHR, 0.69; 95%CI, 0.58-0.82), hospitalization (aHR, 0.57; 95%CI, 0.41-0.79), and surgical intervention (aHR, 0.25; 95% CI, 0.16-0.38).
Further analyses showed that the relationship between lower COC and worse outcomes carried across measures such as baseline use of an immunomodulator or biological agent, as well as subgroups such as patients with nonsevere IBD and nonsurgical patients.
Among those treated by a VHA gastroenterologist, a lower level of COC was associated with a higher rate of surgical interventions, but not hospitalizations or outpatient flares. Physician-specific COC index scores were highest for primary care providers (0.54), followed by gastroenterologists (0.25) and surgeons (0.17). However, lower physician-specific COC scores did not translate to worse IBD outcomes.
“The level of COC among patients with IBD in the present VHA cohort was ... lower than the values described in previous studies of veterans in the VHA system, including a study of VHA-Medicare dual enrollees who were especially prone to fragmented care because of their ability to seek care both inside and outside of the VHA system,” the investigators wrote, referring to a 2018 study. “The difference in COC among patients with IBD vs. patients without IBD is likely multifactorial and may be associated with confusion about physician accountability and lack of focus on coordination in IBD multidisciplinary care. Patients with IBD require care by primary care providers, gastroenterologists, and surgeons, but the delineation of responsibility by physician is often unclear.”
‘Better care, not just more care,’ is needed
“These outcomes cannot be improved with a more robust treatment armamentarium alone,” according to Jason K. Hou, MD, MS, AGAF, FACG, interim chief of gastroenterology and hepatology at Michael E. DeBakey VA Medical Center and associate professor of medicine at Baylor College of Medicine, Houston, who cowrote a simultaneously published editorial, which was also authored by David I. Fudman, MD.
“Examples exist of improving care coordination and outcomes through patient-aligned care teams in primary care and medical specialty homes for IBD,” Dr. Hou said in an interview. “However, significant barriers to widespread implementation remain.”
Dr. Hou offered several possible approaches to overcome these barriers.
“We need improved methods to identify and follow high-risk patients most likely to have complications and health care utilization,” he said. “We need an investment by payers and health care systems on care coordination so the identified high-risk patients can receive timely testing, referral, and treatment. These changes require reevaluation of how the health care system incentivizes health care to provide better care, not just more care.”
The investigators reported grants from the U.S. Department of Veterans Affairs and the National Institutes of Health and financial relationships with AbbVie, UCB, and Takeda. Dr. Hou reported no conflicts of interest.
FROM JAMA NETWORK OPEN