User login
Welcome to Current Psychiatry, a leading source of information, online and in print, for practitioners of psychiatry and its related subspecialties, including addiction psychiatry, child and adolescent psychiatry, and geriatric psychiatry. This Web site contains evidence-based reviews of the prevention, diagnosis, and treatment of mental illness and psychological disorders; case reports; updates on psychopharmacology; news about the specialty of psychiatry; pearls for practice; and other topics of interest and use to this audience.
Dear Drupal User: You're seeing this because you're logged in to Drupal, and not redirected to MDedge.com/psychiatry.
Depression
adolescent depression
adolescent major depressive disorder
adolescent schizophrenia
adolescent with major depressive disorder
animals
autism
baby
brexpiprazole
child
child bipolar
child depression
child schizophrenia
children with bipolar disorder
children with depression
children with major depressive disorder
compulsive behaviors
cure
elderly bipolar
elderly depression
elderly major depressive disorder
elderly schizophrenia
elderly with dementia
first break
first episode
gambling
gaming
geriatric depression
geriatric major depressive disorder
geriatric schizophrenia
infant
kid
major depressive disorder
major depressive disorder in adolescents
major depressive disorder in children
parenting
pediatric
pediatric bipolar
pediatric depression
pediatric major depressive disorder
pediatric schizophrenia
pregnancy
pregnant
rexulti
skin care
teen
wine
section[contains(@class, 'nav-hidden')]
footer[@id='footer']
div[contains(@class, 'pane-pub-article-current-psychiatry')]
div[contains(@class, 'pane-pub-home-current-psychiatry')]
div[contains(@class, 'pane-pub-topic-current-psychiatry')]
div[contains(@class, 'panel-panel-inner')]
div[contains(@class, 'pane-node-field-article-topics')]
section[contains(@class, 'footer-nav-section-wrapper')]
10 Recent paradigm shifts in the neurobiology and treatment of depression
Nowhere is that change in landscape more apparent than in major depression, the No. 1 disabling condition in all of medicine, according to the World Health Organization. The past decade has generated at least 10 paradigm shifts in the neurobiology and pharmacotherapeutics of depression.
Clinging to simplistic tradition
Most contemporary clinicians continue to practice the traditional model of depression, which is based on the assumption that depression is caused by a deficiency of monoamines: serotonin (5-HT) and norepinephrine (NE). The entire antidepressant armamentarium approved for use by the FDA was designed according to the amine deficiency hypothesis. Depressed patients uniformly receive reuptake inhibitors of 5-HT and NE, but few achieve full remission, as the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study showed.1
As scientific paradigm shifts infiltrate clinical practice, however, the tired notion of “chemical imbalance” will yield to more complex and evidence-based models.
Usually, it would be remarkable to witness a single paradigm shift in the understanding of a brain disorder. Imagine the disruptive impact of multiple scientific shifts within the past decade! Consider the following departures from the old dogma about the simplistic old explanation of depression.
1. From neurotransmitters to neuroplasticity
For half a century, our field tenaciously held to the monoamine theory, which posits that depression is caused by a deficiency of 5-HT or NE, or both. All antidepressants in use today were developed to increase brain monoamines by inhibiting their reuptake at the synaptic cleft. Now, research points to other causes:
• impaired neuroplasticity
• a decrement of neurogenesis
• synaptic deficits
• decreased neurotrophins (such as brain-derived neurotrophic factor)
• dendritic pathology.2,3
2. From ‘chemical imbalance’ to neuroinflammation
The simplistic notion that depression is a chemical imbalance, so to speak, in the brain is giving way to rapidly emerging evidence that depression is associated with neuroinflammation.4
Pro-inflammatory cytokines are elevated in the plasma of depressed patients, and subside when the acute episode is treated. Current antidepressants actually have anti-inflammatory effects that have gone unrecognized.5 A meta-analysis of the use of anti-inflammatory agents (such as nonsteroidal anti-inflammatory drugs and aspirin) in depression shows promising efficacy.6 Some inflammatory markers, such as C-reactive protein, already have been reported to predict response to some antidepressants, but not to others.7
3. From 5-HT and NE pathways to glutamate NMDA receptors
Recent landmark studies8 have, taken together, demonstrated that a single IV dose of the N-methyl-d-aspartate (NMDA) receptor antagonist ketamine (a psychotogenic drug of abuse FDA-approved only as an anesthetic) can produce clinical improvement of severe depression and even full remission for several days. Such studies demonstrate that the old dogma of 5-HT and NE deficiency might not be a valid overarching hypothesis of the depression syndrome.
Long-term maintenance studies of ketamine to document its safety and continued efficacy need to be conducted. The mechanism of action of ketamine is believed to be a rapid trigger for enhancing neuroplasticity.
4. From oral to parenteral administration
Several studies have been published showing the efficacy of IV or intranasal administration of new agents for depression. Ketamine studies, for example, were conducted using an IV infusion of a 150-mg dose over 1 hour. Other IV studies used the anticholinergic scopolamine.9
Intranasal ketamine also has been shown to be clinically efficacious.10 Inhalable nitrous oxide (laughing gas, an NMDA antagonist) recently was reported to improve depression as well.11
It is possible that parenteral administration of antidepressant agents may exert a different neurobiological effect and provide a more rapid response than oral medication.
5. From delayed efficacy (weeks) to immediate onset (1 or 2 hours)
The widely entrenched notion that depression takes several weeks to improve with an antidepressant has collapsed with emerging evidence that symptoms of the disorder (even suicidal ideation) can be reversed within 1 or 2 hours.12 IV ketamine isn’t the only example; IV scopolamine,9 inhalable nitrous oxide,11 and overnight sleep deprivation13 also exert a rapid therapeutic effect. This is a major rethinking of how quickly the curtain of severe depression can be lifted, and is great news for patients and their family.
6. From psychological symptoms to cortical or subcortical changes
Depression traditionally has been recognized as a clinical syndrome of sadness, self-deprecation, cognitive dulling, and vegetative symptoms. In recent studies, however, researchers report that low hippocampus volume14 in healthy young girls predicts future depression. Patients with unremitting depression have been reported to have an abnormally shaped hippocampus.15
In addition, gray-matter volume in the subgenual anterior cingulate (Brodmann area 24) is hypoplastic in depressed persons,16 making that area a target for deep-brain stimulation (DBS). Brain morphological changes such as a hypoplastic hippocampus might become useful biomarkers for identifying persons at risk of severe depression, and might become a useful adjunctive biomarker for making a clinical diagnosis.
7. From healing the mind to repairing the brain
It is well-established that depression is associated with loss of dendritic spines and arborizations, loss of synapses, and diminishment of glial cells, especially in the hippocampus17 and anterior cingulate.18 Treating depression, whether pharmaceutical or somatic, involves reversing these changes by increasing neurotrophic factors, enhancing neurogenesis and gliogenesis, and restoring synaptic and dendritic health and cell survival in the hippocampus and frontal cortex.19,20 Treating depression involves brain repair, which is reflected, ultimately, in healing the mind.
8. From pharmacotherapy to neuromodulation
Although drugs remain the predominant treatment modality for depression, there is palpable escalation in the use of neuromodulation methods.
The oldest of these neuromodulatory techniques is electroconvulsive therapy, an excellent treatment for severe depression (and one that enhances hippocampal neurogenesis). In addition, several novel neuromodulation methods have been approved (transcranial magnetic stimulation and vagus nerve stimulation) or are in development (transcranial direct-current stimulation, cranial electrotherapy stimulation, and DBS).21 These somatic approaches to treating the brain directly to alleviate depression target regions involved in depression and reduce the needless risks associated with exposing other organ systems to a drug.
9. From monotherapy to combination therapy
The use of combination therapy for depression has escalated with FDA approval of adjunctive use of atypical antipsychotics in unipolar and bipolar depression. In addition, the landmark STAR*D study1 demonstrated the value of augmentation therapy with a second antidepressant when 1 agent fails. Other controlled studies have shown that combining 2 antidepressants is superior to administering 1.22
Just as other serious medical disorders—such as cancer and hypertension—are treated with 2 or 3 medications, severe depression might require a similar strategy. The field gradually is adopting that approach.
10. From cortical folds to wrinkles on the face
Last, a new (and unexpected) paradigm shift recently emerged, which is genuinely intriguing—even baffling. Using placebo-controlled designs, several researchers have reported significant, persistent improvement of depressive symptoms after injection of onabotulinumtoxinA in the corrugator muscles of the glabellar region of the face, where the omega sign often appears in a depressed person.23,24
The longest of the studies25 was 6 months; investigators reported that improvement continued even after the effect of the botulinum toxin on the omega sign wore off. The proposed mechanism of action is the facial feedback hypothesis, which suggests that, biologically, facial expression has an impact on one’s emotional state.
Big payoffs coming from research in neuroscience
These 10 paradigm shifts in a single psychiatric syndrome are emblematic of exciting clinical and research advances in our field. Like all syndromes, depression is associated with multiple genetic and environmental causes; it isn’t surprising that myriad treatment approaches are emerging.
The days of clinging to monolithic, serendipity-generated models surely are over. Evidence-based psychiatric brain research is shattering aging dogmas that have, for decades, stifled innovation in psychiatric therapeutics that is now moving in novel directions.
Take note, however, that the only paradigm shift that matters to depressed patients is the one that transcends mere control of their symptoms and restores their wellness, functional capacity, and quality of life. With the explosive momentum of neuroscience discovery, psychiatry is, at last, poised to deliver—in splendid, even seismic, fashion.
1. Trivedi MH, Fava M, Wisniewski SR, et al. Medication augmentation after the failure of SSRIs for depression. N Eng J Med. 2006;354(12):1243-1252.
2. Serafini G, Hayley S, Pompili M, et al. Hippocampal neurogenesis, neurotrophic factors and depression: possible therapeutic targets [published online November 30, 2014]? CNS Neurol Disord Drug Targets. doi: 10.2174/1871527313666141130223723.
3. Duman RS, Aghajanian GK. Synaptic dysfunction in depression: potential therapeutic targets. Science. 2012;338(6103):68-72.
4. Iwata M, Ota KT, Duman RS. The inflammasome: pathways linking psychological stress, depression, and systemic illnesses. Brain Behav Immun. 2013;31:105-114.
5. Sacre S, Medghalichi M, Gregory B, et al. Fluoxetine and citalopram exhibit potent anti-inflammatory activity in human and murine models of rheumatoid arthritis and inhibit toll-like receptors. Arthritis Rheum. 2010;62(3):683-693.
6. Köhler O, Benros ME, Nordentoft M, et al. Effect of anti-inflammatory treatment on depression, depressive symptoms, and adverse effects: a systematic review and meta-analysis of randomized clinical trials. JAMA Psychiatry. 2014;71(12): 1381-1391.
7. Uher R, Tansey KE, Dew T, et al. An inflammatory biomarker as a differential predictor of outcome of depression treatment with escitalopram and nortiptyline. Am J Psychiatry. 2014;171(14):1278-1286.
8. Abdallah CG, Sanacora G, Duman RS, et al. Ketamine and rapid-acting antidepressants: a window into a new neurobiology for mood disorder therapeutics [published online October 17, 2014]. Annual Rev Med. doi: 10.1146/annurev-med-053013-062946.
9. Furey ML, Khanna A, Hoffman EM, et al. Scopolamine produces larger antidepressant and antianxiety effects in women than in men. Neuropsychopharmacology. 2010;35(12):2479-2488.
10. Lapidus KA, Levitch CF, Perez AM, et al. A randomized controlled trial of intranasal ketamine in major depressive disorder. Biol Psychiatry. 2014; 76(12):970-976.
11. Nagele P, Duma A, Kopec M, et al. Nitrous oxide for treatment-resistant major depression: a proof-of-concept trial [published December 14, 2014]. Biol Psychiatry. doi: http://dx.doi.org/10.1016/j.biopsych.2014.11.016.
12. Köhler O, Benros ME, Nordentoft M, et al. Effect of anti-inflammatory treatment on depression, depressive symptoms, and adverse effects: a systematic review and meta-analysis of randomized clinical trials. JAMA Psychiatry. 2014;71(12): 1381-1391.
13. Bunney BG, Bunney WE. Mechanisms of rapid antidepressant effects of sleep deprivation therapy: clock genes and circadian rhythms. Biol Psychiatry. 2013;73(12):1164-1171.
14. Chen MC, Hamilton JP, Gotlib IH. Decreased hippocampal volume in healthy girls at risk for depression. Arch Gen Psychiatry. 2010;67(3):270-276.
15. Tae WS, Kim SS, Lee KU, et al. Hippocampal shape deformation in female patients with unremitting major depressive disorder. AJNR Am J Neuroradiol. 2011;32(4):671-676.
16. Hamani C, Mayberg H, Synder B, et al. Deep brain stimulation of the subcallosal cingulate gyrus for depression: anatomical location of active contacts in clinical responders and a suggested guideline for targeting. J Neurosurg. 2009;111(6):1209-1215.
17. Sheline YI, Gado MH, Kraemer HC. Untreated depression and hippocampal volume loss. Am J Psychiatry. 2003;160(8):1516-1518.
18. Redlich R, Almeoda JJ, Grotegerd D, et al. Brain morphometric biomarkers distinguishing unipolar and bipolar depression. A voxel-based morphometry-pattern classification approach. JAMA Psychiatry. 2014;71(11):1222-1230.
19. Mendez-David I, Hen R, Gardier AM, et al. Adult hippocampal neurogenesis: an actor in the antidepressant-like action. Ann Pharm Fr. 2013;71(3):143-149.
20. Serafini G. Neuroplasticity and major depression, the role of modern antidepressant drugs. World J Psychiatry. 2012;2(3):49-57.
21. Rosa MA, Lisanby SH. Somatic treatments for mood disorders. Neuropsychopharmacology. 2012;37(1):102-116.
22. Blier P, Ward HE, Tremblay P, et al. Combination of antidepressant medications from treatment initiation for major depressive disorder: a double-blind randomized study. Am J Psychiatry. 2010;167(3):281-288.
23. Wollmer MA, de Boer C, Kalak N, et al. Facing depression with botulinum toxin: a randomized controlled trial. J Psychiatr Res. 2012;46(5):574-581.
24. Finzi E, Rosenthal NE. Treatment of depression with onabotulinumtoxinA: a randomized, double-blind, placebo controlled trial. J Psychiatr Res. 2014;52:1-6.
25. Magid M, Reichenberg JS, Poth PE, et al. Treatment of major depressive disorder using botulinum toxin A: a 24-week randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2014;75(8):837-844.
Nowhere is that change in landscape more apparent than in major depression, the No. 1 disabling condition in all of medicine, according to the World Health Organization. The past decade has generated at least 10 paradigm shifts in the neurobiology and pharmacotherapeutics of depression.
Clinging to simplistic tradition
Most contemporary clinicians continue to practice the traditional model of depression, which is based on the assumption that depression is caused by a deficiency of monoamines: serotonin (5-HT) and norepinephrine (NE). The entire antidepressant armamentarium approved for use by the FDA was designed according to the amine deficiency hypothesis. Depressed patients uniformly receive reuptake inhibitors of 5-HT and NE, but few achieve full remission, as the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study showed.1
As scientific paradigm shifts infiltrate clinical practice, however, the tired notion of “chemical imbalance” will yield to more complex and evidence-based models.
Usually, it would be remarkable to witness a single paradigm shift in the understanding of a brain disorder. Imagine the disruptive impact of multiple scientific shifts within the past decade! Consider the following departures from the old dogma about the simplistic old explanation of depression.
1. From neurotransmitters to neuroplasticity
For half a century, our field tenaciously held to the monoamine theory, which posits that depression is caused by a deficiency of 5-HT or NE, or both. All antidepressants in use today were developed to increase brain monoamines by inhibiting their reuptake at the synaptic cleft. Now, research points to other causes:
• impaired neuroplasticity
• a decrement of neurogenesis
• synaptic deficits
• decreased neurotrophins (such as brain-derived neurotrophic factor)
• dendritic pathology.2,3
2. From ‘chemical imbalance’ to neuroinflammation
The simplistic notion that depression is a chemical imbalance, so to speak, in the brain is giving way to rapidly emerging evidence that depression is associated with neuroinflammation.4
Pro-inflammatory cytokines are elevated in the plasma of depressed patients, and subside when the acute episode is treated. Current antidepressants actually have anti-inflammatory effects that have gone unrecognized.5 A meta-analysis of the use of anti-inflammatory agents (such as nonsteroidal anti-inflammatory drugs and aspirin) in depression shows promising efficacy.6 Some inflammatory markers, such as C-reactive protein, already have been reported to predict response to some antidepressants, but not to others.7
3. From 5-HT and NE pathways to glutamate NMDA receptors
Recent landmark studies8 have, taken together, demonstrated that a single IV dose of the N-methyl-d-aspartate (NMDA) receptor antagonist ketamine (a psychotogenic drug of abuse FDA-approved only as an anesthetic) can produce clinical improvement of severe depression and even full remission for several days. Such studies demonstrate that the old dogma of 5-HT and NE deficiency might not be a valid overarching hypothesis of the depression syndrome.
Long-term maintenance studies of ketamine to document its safety and continued efficacy need to be conducted. The mechanism of action of ketamine is believed to be a rapid trigger for enhancing neuroplasticity.
4. From oral to parenteral administration
Several studies have been published showing the efficacy of IV or intranasal administration of new agents for depression. Ketamine studies, for example, were conducted using an IV infusion of a 150-mg dose over 1 hour. Other IV studies used the anticholinergic scopolamine.9
Intranasal ketamine also has been shown to be clinically efficacious.10 Inhalable nitrous oxide (laughing gas, an NMDA antagonist) recently was reported to improve depression as well.11
It is possible that parenteral administration of antidepressant agents may exert a different neurobiological effect and provide a more rapid response than oral medication.
5. From delayed efficacy (weeks) to immediate onset (1 or 2 hours)
The widely entrenched notion that depression takes several weeks to improve with an antidepressant has collapsed with emerging evidence that symptoms of the disorder (even suicidal ideation) can be reversed within 1 or 2 hours.12 IV ketamine isn’t the only example; IV scopolamine,9 inhalable nitrous oxide,11 and overnight sleep deprivation13 also exert a rapid therapeutic effect. This is a major rethinking of how quickly the curtain of severe depression can be lifted, and is great news for patients and their family.
6. From psychological symptoms to cortical or subcortical changes
Depression traditionally has been recognized as a clinical syndrome of sadness, self-deprecation, cognitive dulling, and vegetative symptoms. In recent studies, however, researchers report that low hippocampus volume14 in healthy young girls predicts future depression. Patients with unremitting depression have been reported to have an abnormally shaped hippocampus.15
In addition, gray-matter volume in the subgenual anterior cingulate (Brodmann area 24) is hypoplastic in depressed persons,16 making that area a target for deep-brain stimulation (DBS). Brain morphological changes such as a hypoplastic hippocampus might become useful biomarkers for identifying persons at risk of severe depression, and might become a useful adjunctive biomarker for making a clinical diagnosis.
7. From healing the mind to repairing the brain
It is well-established that depression is associated with loss of dendritic spines and arborizations, loss of synapses, and diminishment of glial cells, especially in the hippocampus17 and anterior cingulate.18 Treating depression, whether pharmaceutical or somatic, involves reversing these changes by increasing neurotrophic factors, enhancing neurogenesis and gliogenesis, and restoring synaptic and dendritic health and cell survival in the hippocampus and frontal cortex.19,20 Treating depression involves brain repair, which is reflected, ultimately, in healing the mind.
8. From pharmacotherapy to neuromodulation
Although drugs remain the predominant treatment modality for depression, there is palpable escalation in the use of neuromodulation methods.
The oldest of these neuromodulatory techniques is electroconvulsive therapy, an excellent treatment for severe depression (and one that enhances hippocampal neurogenesis). In addition, several novel neuromodulation methods have been approved (transcranial magnetic stimulation and vagus nerve stimulation) or are in development (transcranial direct-current stimulation, cranial electrotherapy stimulation, and DBS).21 These somatic approaches to treating the brain directly to alleviate depression target regions involved in depression and reduce the needless risks associated with exposing other organ systems to a drug.
9. From monotherapy to combination therapy
The use of combination therapy for depression has escalated with FDA approval of adjunctive use of atypical antipsychotics in unipolar and bipolar depression. In addition, the landmark STAR*D study1 demonstrated the value of augmentation therapy with a second antidepressant when 1 agent fails. Other controlled studies have shown that combining 2 antidepressants is superior to administering 1.22
Just as other serious medical disorders—such as cancer and hypertension—are treated with 2 or 3 medications, severe depression might require a similar strategy. The field gradually is adopting that approach.
10. From cortical folds to wrinkles on the face
Last, a new (and unexpected) paradigm shift recently emerged, which is genuinely intriguing—even baffling. Using placebo-controlled designs, several researchers have reported significant, persistent improvement of depressive symptoms after injection of onabotulinumtoxinA in the corrugator muscles of the glabellar region of the face, where the omega sign often appears in a depressed person.23,24
The longest of the studies25 was 6 months; investigators reported that improvement continued even after the effect of the botulinum toxin on the omega sign wore off. The proposed mechanism of action is the facial feedback hypothesis, which suggests that, biologically, facial expression has an impact on one’s emotional state.
Big payoffs coming from research in neuroscience
These 10 paradigm shifts in a single psychiatric syndrome are emblematic of exciting clinical and research advances in our field. Like all syndromes, depression is associated with multiple genetic and environmental causes; it isn’t surprising that myriad treatment approaches are emerging.
The days of clinging to monolithic, serendipity-generated models surely are over. Evidence-based psychiatric brain research is shattering aging dogmas that have, for decades, stifled innovation in psychiatric therapeutics that is now moving in novel directions.
Take note, however, that the only paradigm shift that matters to depressed patients is the one that transcends mere control of their symptoms and restores their wellness, functional capacity, and quality of life. With the explosive momentum of neuroscience discovery, psychiatry is, at last, poised to deliver—in splendid, even seismic, fashion.
Nowhere is that change in landscape more apparent than in major depression, the No. 1 disabling condition in all of medicine, according to the World Health Organization. The past decade has generated at least 10 paradigm shifts in the neurobiology and pharmacotherapeutics of depression.
Clinging to simplistic tradition
Most contemporary clinicians continue to practice the traditional model of depression, which is based on the assumption that depression is caused by a deficiency of monoamines: serotonin (5-HT) and norepinephrine (NE). The entire antidepressant armamentarium approved for use by the FDA was designed according to the amine deficiency hypothesis. Depressed patients uniformly receive reuptake inhibitors of 5-HT and NE, but few achieve full remission, as the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study showed.1
As scientific paradigm shifts infiltrate clinical practice, however, the tired notion of “chemical imbalance” will yield to more complex and evidence-based models.
Usually, it would be remarkable to witness a single paradigm shift in the understanding of a brain disorder. Imagine the disruptive impact of multiple scientific shifts within the past decade! Consider the following departures from the old dogma about the simplistic old explanation of depression.
1. From neurotransmitters to neuroplasticity
For half a century, our field tenaciously held to the monoamine theory, which posits that depression is caused by a deficiency of 5-HT or NE, or both. All antidepressants in use today were developed to increase brain monoamines by inhibiting their reuptake at the synaptic cleft. Now, research points to other causes:
• impaired neuroplasticity
• a decrement of neurogenesis
• synaptic deficits
• decreased neurotrophins (such as brain-derived neurotrophic factor)
• dendritic pathology.2,3
2. From ‘chemical imbalance’ to neuroinflammation
The simplistic notion that depression is a chemical imbalance, so to speak, in the brain is giving way to rapidly emerging evidence that depression is associated with neuroinflammation.4
Pro-inflammatory cytokines are elevated in the plasma of depressed patients, and subside when the acute episode is treated. Current antidepressants actually have anti-inflammatory effects that have gone unrecognized.5 A meta-analysis of the use of anti-inflammatory agents (such as nonsteroidal anti-inflammatory drugs and aspirin) in depression shows promising efficacy.6 Some inflammatory markers, such as C-reactive protein, already have been reported to predict response to some antidepressants, but not to others.7
3. From 5-HT and NE pathways to glutamate NMDA receptors
Recent landmark studies8 have, taken together, demonstrated that a single IV dose of the N-methyl-d-aspartate (NMDA) receptor antagonist ketamine (a psychotogenic drug of abuse FDA-approved only as an anesthetic) can produce clinical improvement of severe depression and even full remission for several days. Such studies demonstrate that the old dogma of 5-HT and NE deficiency might not be a valid overarching hypothesis of the depression syndrome.
Long-term maintenance studies of ketamine to document its safety and continued efficacy need to be conducted. The mechanism of action of ketamine is believed to be a rapid trigger for enhancing neuroplasticity.
4. From oral to parenteral administration
Several studies have been published showing the efficacy of IV or intranasal administration of new agents for depression. Ketamine studies, for example, were conducted using an IV infusion of a 150-mg dose over 1 hour. Other IV studies used the anticholinergic scopolamine.9
Intranasal ketamine also has been shown to be clinically efficacious.10 Inhalable nitrous oxide (laughing gas, an NMDA antagonist) recently was reported to improve depression as well.11
It is possible that parenteral administration of antidepressant agents may exert a different neurobiological effect and provide a more rapid response than oral medication.
5. From delayed efficacy (weeks) to immediate onset (1 or 2 hours)
The widely entrenched notion that depression takes several weeks to improve with an antidepressant has collapsed with emerging evidence that symptoms of the disorder (even suicidal ideation) can be reversed within 1 or 2 hours.12 IV ketamine isn’t the only example; IV scopolamine,9 inhalable nitrous oxide,11 and overnight sleep deprivation13 also exert a rapid therapeutic effect. This is a major rethinking of how quickly the curtain of severe depression can be lifted, and is great news for patients and their family.
6. From psychological symptoms to cortical or subcortical changes
Depression traditionally has been recognized as a clinical syndrome of sadness, self-deprecation, cognitive dulling, and vegetative symptoms. In recent studies, however, researchers report that low hippocampus volume14 in healthy young girls predicts future depression. Patients with unremitting depression have been reported to have an abnormally shaped hippocampus.15
In addition, gray-matter volume in the subgenual anterior cingulate (Brodmann area 24) is hypoplastic in depressed persons,16 making that area a target for deep-brain stimulation (DBS). Brain morphological changes such as a hypoplastic hippocampus might become useful biomarkers for identifying persons at risk of severe depression, and might become a useful adjunctive biomarker for making a clinical diagnosis.
7. From healing the mind to repairing the brain
It is well-established that depression is associated with loss of dendritic spines and arborizations, loss of synapses, and diminishment of glial cells, especially in the hippocampus17 and anterior cingulate.18 Treating depression, whether pharmaceutical or somatic, involves reversing these changes by increasing neurotrophic factors, enhancing neurogenesis and gliogenesis, and restoring synaptic and dendritic health and cell survival in the hippocampus and frontal cortex.19,20 Treating depression involves brain repair, which is reflected, ultimately, in healing the mind.
8. From pharmacotherapy to neuromodulation
Although drugs remain the predominant treatment modality for depression, there is palpable escalation in the use of neuromodulation methods.
The oldest of these neuromodulatory techniques is electroconvulsive therapy, an excellent treatment for severe depression (and one that enhances hippocampal neurogenesis). In addition, several novel neuromodulation methods have been approved (transcranial magnetic stimulation and vagus nerve stimulation) or are in development (transcranial direct-current stimulation, cranial electrotherapy stimulation, and DBS).21 These somatic approaches to treating the brain directly to alleviate depression target regions involved in depression and reduce the needless risks associated with exposing other organ systems to a drug.
9. From monotherapy to combination therapy
The use of combination therapy for depression has escalated with FDA approval of adjunctive use of atypical antipsychotics in unipolar and bipolar depression. In addition, the landmark STAR*D study1 demonstrated the value of augmentation therapy with a second antidepressant when 1 agent fails. Other controlled studies have shown that combining 2 antidepressants is superior to administering 1.22
Just as other serious medical disorders—such as cancer and hypertension—are treated with 2 or 3 medications, severe depression might require a similar strategy. The field gradually is adopting that approach.
10. From cortical folds to wrinkles on the face
Last, a new (and unexpected) paradigm shift recently emerged, which is genuinely intriguing—even baffling. Using placebo-controlled designs, several researchers have reported significant, persistent improvement of depressive symptoms after injection of onabotulinumtoxinA in the corrugator muscles of the glabellar region of the face, where the omega sign often appears in a depressed person.23,24
The longest of the studies25 was 6 months; investigators reported that improvement continued even after the effect of the botulinum toxin on the omega sign wore off. The proposed mechanism of action is the facial feedback hypothesis, which suggests that, biologically, facial expression has an impact on one’s emotional state.
Big payoffs coming from research in neuroscience
These 10 paradigm shifts in a single psychiatric syndrome are emblematic of exciting clinical and research advances in our field. Like all syndromes, depression is associated with multiple genetic and environmental causes; it isn’t surprising that myriad treatment approaches are emerging.
The days of clinging to monolithic, serendipity-generated models surely are over. Evidence-based psychiatric brain research is shattering aging dogmas that have, for decades, stifled innovation in psychiatric therapeutics that is now moving in novel directions.
Take note, however, that the only paradigm shift that matters to depressed patients is the one that transcends mere control of their symptoms and restores their wellness, functional capacity, and quality of life. With the explosive momentum of neuroscience discovery, psychiatry is, at last, poised to deliver—in splendid, even seismic, fashion.
1. Trivedi MH, Fava M, Wisniewski SR, et al. Medication augmentation after the failure of SSRIs for depression. N Eng J Med. 2006;354(12):1243-1252.
2. Serafini G, Hayley S, Pompili M, et al. Hippocampal neurogenesis, neurotrophic factors and depression: possible therapeutic targets [published online November 30, 2014]? CNS Neurol Disord Drug Targets. doi: 10.2174/1871527313666141130223723.
3. Duman RS, Aghajanian GK. Synaptic dysfunction in depression: potential therapeutic targets. Science. 2012;338(6103):68-72.
4. Iwata M, Ota KT, Duman RS. The inflammasome: pathways linking psychological stress, depression, and systemic illnesses. Brain Behav Immun. 2013;31:105-114.
5. Sacre S, Medghalichi M, Gregory B, et al. Fluoxetine and citalopram exhibit potent anti-inflammatory activity in human and murine models of rheumatoid arthritis and inhibit toll-like receptors. Arthritis Rheum. 2010;62(3):683-693.
6. Köhler O, Benros ME, Nordentoft M, et al. Effect of anti-inflammatory treatment on depression, depressive symptoms, and adverse effects: a systematic review and meta-analysis of randomized clinical trials. JAMA Psychiatry. 2014;71(12): 1381-1391.
7. Uher R, Tansey KE, Dew T, et al. An inflammatory biomarker as a differential predictor of outcome of depression treatment with escitalopram and nortiptyline. Am J Psychiatry. 2014;171(14):1278-1286.
8. Abdallah CG, Sanacora G, Duman RS, et al. Ketamine and rapid-acting antidepressants: a window into a new neurobiology for mood disorder therapeutics [published online October 17, 2014]. Annual Rev Med. doi: 10.1146/annurev-med-053013-062946.
9. Furey ML, Khanna A, Hoffman EM, et al. Scopolamine produces larger antidepressant and antianxiety effects in women than in men. Neuropsychopharmacology. 2010;35(12):2479-2488.
10. Lapidus KA, Levitch CF, Perez AM, et al. A randomized controlled trial of intranasal ketamine in major depressive disorder. Biol Psychiatry. 2014; 76(12):970-976.
11. Nagele P, Duma A, Kopec M, et al. Nitrous oxide for treatment-resistant major depression: a proof-of-concept trial [published December 14, 2014]. Biol Psychiatry. doi: http://dx.doi.org/10.1016/j.biopsych.2014.11.016.
12. Köhler O, Benros ME, Nordentoft M, et al. Effect of anti-inflammatory treatment on depression, depressive symptoms, and adverse effects: a systematic review and meta-analysis of randomized clinical trials. JAMA Psychiatry. 2014;71(12): 1381-1391.
13. Bunney BG, Bunney WE. Mechanisms of rapid antidepressant effects of sleep deprivation therapy: clock genes and circadian rhythms. Biol Psychiatry. 2013;73(12):1164-1171.
14. Chen MC, Hamilton JP, Gotlib IH. Decreased hippocampal volume in healthy girls at risk for depression. Arch Gen Psychiatry. 2010;67(3):270-276.
15. Tae WS, Kim SS, Lee KU, et al. Hippocampal shape deformation in female patients with unremitting major depressive disorder. AJNR Am J Neuroradiol. 2011;32(4):671-676.
16. Hamani C, Mayberg H, Synder B, et al. Deep brain stimulation of the subcallosal cingulate gyrus for depression: anatomical location of active contacts in clinical responders and a suggested guideline for targeting. J Neurosurg. 2009;111(6):1209-1215.
17. Sheline YI, Gado MH, Kraemer HC. Untreated depression and hippocampal volume loss. Am J Psychiatry. 2003;160(8):1516-1518.
18. Redlich R, Almeoda JJ, Grotegerd D, et al. Brain morphometric biomarkers distinguishing unipolar and bipolar depression. A voxel-based morphometry-pattern classification approach. JAMA Psychiatry. 2014;71(11):1222-1230.
19. Mendez-David I, Hen R, Gardier AM, et al. Adult hippocampal neurogenesis: an actor in the antidepressant-like action. Ann Pharm Fr. 2013;71(3):143-149.
20. Serafini G. Neuroplasticity and major depression, the role of modern antidepressant drugs. World J Psychiatry. 2012;2(3):49-57.
21. Rosa MA, Lisanby SH. Somatic treatments for mood disorders. Neuropsychopharmacology. 2012;37(1):102-116.
22. Blier P, Ward HE, Tremblay P, et al. Combination of antidepressant medications from treatment initiation for major depressive disorder: a double-blind randomized study. Am J Psychiatry. 2010;167(3):281-288.
23. Wollmer MA, de Boer C, Kalak N, et al. Facing depression with botulinum toxin: a randomized controlled trial. J Psychiatr Res. 2012;46(5):574-581.
24. Finzi E, Rosenthal NE. Treatment of depression with onabotulinumtoxinA: a randomized, double-blind, placebo controlled trial. J Psychiatr Res. 2014;52:1-6.
25. Magid M, Reichenberg JS, Poth PE, et al. Treatment of major depressive disorder using botulinum toxin A: a 24-week randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2014;75(8):837-844.
1. Trivedi MH, Fava M, Wisniewski SR, et al. Medication augmentation after the failure of SSRIs for depression. N Eng J Med. 2006;354(12):1243-1252.
2. Serafini G, Hayley S, Pompili M, et al. Hippocampal neurogenesis, neurotrophic factors and depression: possible therapeutic targets [published online November 30, 2014]? CNS Neurol Disord Drug Targets. doi: 10.2174/1871527313666141130223723.
3. Duman RS, Aghajanian GK. Synaptic dysfunction in depression: potential therapeutic targets. Science. 2012;338(6103):68-72.
4. Iwata M, Ota KT, Duman RS. The inflammasome: pathways linking psychological stress, depression, and systemic illnesses. Brain Behav Immun. 2013;31:105-114.
5. Sacre S, Medghalichi M, Gregory B, et al. Fluoxetine and citalopram exhibit potent anti-inflammatory activity in human and murine models of rheumatoid arthritis and inhibit toll-like receptors. Arthritis Rheum. 2010;62(3):683-693.
6. Köhler O, Benros ME, Nordentoft M, et al. Effect of anti-inflammatory treatment on depression, depressive symptoms, and adverse effects: a systematic review and meta-analysis of randomized clinical trials. JAMA Psychiatry. 2014;71(12): 1381-1391.
7. Uher R, Tansey KE, Dew T, et al. An inflammatory biomarker as a differential predictor of outcome of depression treatment with escitalopram and nortiptyline. Am J Psychiatry. 2014;171(14):1278-1286.
8. Abdallah CG, Sanacora G, Duman RS, et al. Ketamine and rapid-acting antidepressants: a window into a new neurobiology for mood disorder therapeutics [published online October 17, 2014]. Annual Rev Med. doi: 10.1146/annurev-med-053013-062946.
9. Furey ML, Khanna A, Hoffman EM, et al. Scopolamine produces larger antidepressant and antianxiety effects in women than in men. Neuropsychopharmacology. 2010;35(12):2479-2488.
10. Lapidus KA, Levitch CF, Perez AM, et al. A randomized controlled trial of intranasal ketamine in major depressive disorder. Biol Psychiatry. 2014; 76(12):970-976.
11. Nagele P, Duma A, Kopec M, et al. Nitrous oxide for treatment-resistant major depression: a proof-of-concept trial [published December 14, 2014]. Biol Psychiatry. doi: http://dx.doi.org/10.1016/j.biopsych.2014.11.016.
12. Köhler O, Benros ME, Nordentoft M, et al. Effect of anti-inflammatory treatment on depression, depressive symptoms, and adverse effects: a systematic review and meta-analysis of randomized clinical trials. JAMA Psychiatry. 2014;71(12): 1381-1391.
13. Bunney BG, Bunney WE. Mechanisms of rapid antidepressant effects of sleep deprivation therapy: clock genes and circadian rhythms. Biol Psychiatry. 2013;73(12):1164-1171.
14. Chen MC, Hamilton JP, Gotlib IH. Decreased hippocampal volume in healthy girls at risk for depression. Arch Gen Psychiatry. 2010;67(3):270-276.
15. Tae WS, Kim SS, Lee KU, et al. Hippocampal shape deformation in female patients with unremitting major depressive disorder. AJNR Am J Neuroradiol. 2011;32(4):671-676.
16. Hamani C, Mayberg H, Synder B, et al. Deep brain stimulation of the subcallosal cingulate gyrus for depression: anatomical location of active contacts in clinical responders and a suggested guideline for targeting. J Neurosurg. 2009;111(6):1209-1215.
17. Sheline YI, Gado MH, Kraemer HC. Untreated depression and hippocampal volume loss. Am J Psychiatry. 2003;160(8):1516-1518.
18. Redlich R, Almeoda JJ, Grotegerd D, et al. Brain morphometric biomarkers distinguishing unipolar and bipolar depression. A voxel-based morphometry-pattern classification approach. JAMA Psychiatry. 2014;71(11):1222-1230.
19. Mendez-David I, Hen R, Gardier AM, et al. Adult hippocampal neurogenesis: an actor in the antidepressant-like action. Ann Pharm Fr. 2013;71(3):143-149.
20. Serafini G. Neuroplasticity and major depression, the role of modern antidepressant drugs. World J Psychiatry. 2012;2(3):49-57.
21. Rosa MA, Lisanby SH. Somatic treatments for mood disorders. Neuropsychopharmacology. 2012;37(1):102-116.
22. Blier P, Ward HE, Tremblay P, et al. Combination of antidepressant medications from treatment initiation for major depressive disorder: a double-blind randomized study. Am J Psychiatry. 2010;167(3):281-288.
23. Wollmer MA, de Boer C, Kalak N, et al. Facing depression with botulinum toxin: a randomized controlled trial. J Psychiatr Res. 2012;46(5):574-581.
24. Finzi E, Rosenthal NE. Treatment of depression with onabotulinumtoxinA: a randomized, double-blind, placebo controlled trial. J Psychiatr Res. 2014;52:1-6.
25. Magid M, Reichenberg JS, Poth PE, et al. Treatment of major depressive disorder using botulinum toxin A: a 24-week randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2014;75(8):837-844.
Beware of methylmercury during pregnancy!
Dr. Henry A. Nasrallah is correct that wild salmon is a good choice for pregnant women who want to boost intake of omega-3 fatty acids (Current Psychiatry, Comments & Controversies, December 2014; pg 33 [http://bit.ly/1wQoXdP]). The main concern about fish intake during pregnancy is exposure to methylmercury, and much of this concern is derived from the tragic results of epic mercury poisonings of food sources in the past.
The FDA advises that pregnant women and children avoid eating shark, tilefish, king mackerel, and swordfish because these fish have a relatively high level of mercury.1 Fish that are low in methyl-mercury include salmon and canned light tuna. (More information is available at http://www.fda.gov/Food/ResourcesForYou/HealthEducators/ucm083324.htm.)
Although wild fish tend to be higher in omega-3 fatty acids than farm-raised fish, farmed fish can be an excellent source of omega-3 fatty acids. This is analogous to eating farm-produced livestock vs free-range, grass-fed livestock: Animals in their natural environment eat healthier and have more omega-3 fatty acids, whereas farmed livestock generally eat cheap and less healthy feed. Because wild fish can be pricey, it’s important that women understand that farm-raised fish are a good source of protein and other nutrients such as omega-3 fatty acids.
Research has been inconclusive regarding the antidepressant benefits of omega-3 fatty acids, with some, but not all, studies demonstrating an add-on benefit of omega-3 fatty acid supplements for mood disorders. However, several epidemiological studies have reported that the low quality of dietary intake of omega-3 fatty acids is associated with psychiatric illness, and fish and seafood are sources of essential fatty acids and other nutrients.2
1. Food safety for moms-to-be: while you’re pregnant–methylmercury. U.S. Food and Drug Administration. http://www.fda.gov/Food/ ResourcesForYou/HealthEducators/ucm083324. htm. Updated October 30, 2014. Accessed January 5, 2015.
2. Quirk SE, Williams LJ, O’Neil A, et al. The association between diet quality, dietary patterns and depression in adults: a systematic review. BMC Psychiatry. 2013;13:175.
Dr. Henry A. Nasrallah is correct that wild salmon is a good choice for pregnant women who want to boost intake of omega-3 fatty acids (Current Psychiatry, Comments & Controversies, December 2014; pg 33 [http://bit.ly/1wQoXdP]). The main concern about fish intake during pregnancy is exposure to methylmercury, and much of this concern is derived from the tragic results of epic mercury poisonings of food sources in the past.
The FDA advises that pregnant women and children avoid eating shark, tilefish, king mackerel, and swordfish because these fish have a relatively high level of mercury.1 Fish that are low in methyl-mercury include salmon and canned light tuna. (More information is available at http://www.fda.gov/Food/ResourcesForYou/HealthEducators/ucm083324.htm.)
Although wild fish tend to be higher in omega-3 fatty acids than farm-raised fish, farmed fish can be an excellent source of omega-3 fatty acids. This is analogous to eating farm-produced livestock vs free-range, grass-fed livestock: Animals in their natural environment eat healthier and have more omega-3 fatty acids, whereas farmed livestock generally eat cheap and less healthy feed. Because wild fish can be pricey, it’s important that women understand that farm-raised fish are a good source of protein and other nutrients such as omega-3 fatty acids.
Research has been inconclusive regarding the antidepressant benefits of omega-3 fatty acids, with some, but not all, studies demonstrating an add-on benefit of omega-3 fatty acid supplements for mood disorders. However, several epidemiological studies have reported that the low quality of dietary intake of omega-3 fatty acids is associated with psychiatric illness, and fish and seafood are sources of essential fatty acids and other nutrients.2
Dr. Henry A. Nasrallah is correct that wild salmon is a good choice for pregnant women who want to boost intake of omega-3 fatty acids (Current Psychiatry, Comments & Controversies, December 2014; pg 33 [http://bit.ly/1wQoXdP]). The main concern about fish intake during pregnancy is exposure to methylmercury, and much of this concern is derived from the tragic results of epic mercury poisonings of food sources in the past.
The FDA advises that pregnant women and children avoid eating shark, tilefish, king mackerel, and swordfish because these fish have a relatively high level of mercury.1 Fish that are low in methyl-mercury include salmon and canned light tuna. (More information is available at http://www.fda.gov/Food/ResourcesForYou/HealthEducators/ucm083324.htm.)
Although wild fish tend to be higher in omega-3 fatty acids than farm-raised fish, farmed fish can be an excellent source of omega-3 fatty acids. This is analogous to eating farm-produced livestock vs free-range, grass-fed livestock: Animals in their natural environment eat healthier and have more omega-3 fatty acids, whereas farmed livestock generally eat cheap and less healthy feed. Because wild fish can be pricey, it’s important that women understand that farm-raised fish are a good source of protein and other nutrients such as omega-3 fatty acids.
Research has been inconclusive regarding the antidepressant benefits of omega-3 fatty acids, with some, but not all, studies demonstrating an add-on benefit of omega-3 fatty acid supplements for mood disorders. However, several epidemiological studies have reported that the low quality of dietary intake of omega-3 fatty acids is associated with psychiatric illness, and fish and seafood are sources of essential fatty acids and other nutrients.2
1. Food safety for moms-to-be: while you’re pregnant–methylmercury. U.S. Food and Drug Administration. http://www.fda.gov/Food/ ResourcesForYou/HealthEducators/ucm083324. htm. Updated October 30, 2014. Accessed January 5, 2015.
2. Quirk SE, Williams LJ, O’Neil A, et al. The association between diet quality, dietary patterns and depression in adults: a systematic review. BMC Psychiatry. 2013;13:175.
1. Food safety for moms-to-be: while you’re pregnant–methylmercury. U.S. Food and Drug Administration. http://www.fda.gov/Food/ ResourcesForYou/HealthEducators/ucm083324. htm. Updated October 30, 2014. Accessed January 5, 2015.
2. Quirk SE, Williams LJ, O’Neil A, et al. The association between diet quality, dietary patterns and depression in adults: a systematic review. BMC Psychiatry. 2013;13:175.
More on insomnia disorders in older patients
Regarding Drs. Irene S. Hong’s and Jeffrey R. Bishop’s article, “Sedative-hypnotics for sleepless geriatric patients: Choose wisely” (Current Psychiatry, 2014;13(10):36-39, 46-50, 52 [http://bit.ly/1ApmcoO]), which undertook a comprehensive review of current therapies for insomnia in geriatric patients, here are 3 clarifications.
• I want to reinforce the latest thinking about the nature and pathophysiology of insomnia. DSM-5 classifies insomnia as a disorder, not as a symptom of other problems; the concept of “secondary insomnia” is rejected in DSM-5. Insomnia typically is seen as comorbid with other medical and psychiatric disorders. Often, insomnia predates the comorbid disorder (eg, depression), but rarely is it resolved by treating the comorbid condition.
• Good clinical practice, therefore, requires treating the comorbid condition and the insomnia each directly.
• The insomnia disorder manifests itself, in part, by a report of difficulty falling asleep or staying asleep. The authors use the example of sleep-onset insomnia as typical in older adults. However, sleep maintenance and early morning awakenings are the most common symptoms among geriatric insomnia patients.
• The authors mention only in passing an important medication for sleep maintenance in adults and in the geriatric patient specifically: doxepin. Low-dose doxepin, at 3 mg (for the geriatric patient) and 6 mg, is FDA-approved as a nonscheduled hypnotic for sleep maintenance insomnia. This formulationa is the only hypnotic classified as safe for geriatric patients in the 2012 Beers Criteria Update of the American Geriatrics Society.1 Unlike higher dosages of doxepin, the action of low-dose doxepin is, essentially, selective H1 antagonism.
aSold as Silenor.
Reference
1. American Geriatrics Society 2012 Beers Criteria Update Expert Panel. American Geriatrics Society updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2012;60(4):616-631.
Regarding Drs. Irene S. Hong’s and Jeffrey R. Bishop’s article, “Sedative-hypnotics for sleepless geriatric patients: Choose wisely” (Current Psychiatry, 2014;13(10):36-39, 46-50, 52 [http://bit.ly/1ApmcoO]), which undertook a comprehensive review of current therapies for insomnia in geriatric patients, here are 3 clarifications.
• I want to reinforce the latest thinking about the nature and pathophysiology of insomnia. DSM-5 classifies insomnia as a disorder, not as a symptom of other problems; the concept of “secondary insomnia” is rejected in DSM-5. Insomnia typically is seen as comorbid with other medical and psychiatric disorders. Often, insomnia predates the comorbid disorder (eg, depression), but rarely is it resolved by treating the comorbid condition.
• Good clinical practice, therefore, requires treating the comorbid condition and the insomnia each directly.
• The insomnia disorder manifests itself, in part, by a report of difficulty falling asleep or staying asleep. The authors use the example of sleep-onset insomnia as typical in older adults. However, sleep maintenance and early morning awakenings are the most common symptoms among geriatric insomnia patients.
• The authors mention only in passing an important medication for sleep maintenance in adults and in the geriatric patient specifically: doxepin. Low-dose doxepin, at 3 mg (for the geriatric patient) and 6 mg, is FDA-approved as a nonscheduled hypnotic for sleep maintenance insomnia. This formulationa is the only hypnotic classified as safe for geriatric patients in the 2012 Beers Criteria Update of the American Geriatrics Society.1 Unlike higher dosages of doxepin, the action of low-dose doxepin is, essentially, selective H1 antagonism.
aSold as Silenor.
Regarding Drs. Irene S. Hong’s and Jeffrey R. Bishop’s article, “Sedative-hypnotics for sleepless geriatric patients: Choose wisely” (Current Psychiatry, 2014;13(10):36-39, 46-50, 52 [http://bit.ly/1ApmcoO]), which undertook a comprehensive review of current therapies for insomnia in geriatric patients, here are 3 clarifications.
• I want to reinforce the latest thinking about the nature and pathophysiology of insomnia. DSM-5 classifies insomnia as a disorder, not as a symptom of other problems; the concept of “secondary insomnia” is rejected in DSM-5. Insomnia typically is seen as comorbid with other medical and psychiatric disorders. Often, insomnia predates the comorbid disorder (eg, depression), but rarely is it resolved by treating the comorbid condition.
• Good clinical practice, therefore, requires treating the comorbid condition and the insomnia each directly.
• The insomnia disorder manifests itself, in part, by a report of difficulty falling asleep or staying asleep. The authors use the example of sleep-onset insomnia as typical in older adults. However, sleep maintenance and early morning awakenings are the most common symptoms among geriatric insomnia patients.
• The authors mention only in passing an important medication for sleep maintenance in adults and in the geriatric patient specifically: doxepin. Low-dose doxepin, at 3 mg (for the geriatric patient) and 6 mg, is FDA-approved as a nonscheduled hypnotic for sleep maintenance insomnia. This formulationa is the only hypnotic classified as safe for geriatric patients in the 2012 Beers Criteria Update of the American Geriatrics Society.1 Unlike higher dosages of doxepin, the action of low-dose doxepin is, essentially, selective H1 antagonism.
aSold as Silenor.
Reference
1. American Geriatrics Society 2012 Beers Criteria Update Expert Panel. American Geriatrics Society updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2012;60(4):616-631.
Reference
1. American Geriatrics Society 2012 Beers Criteria Update Expert Panel. American Geriatrics Society updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2012;60(4):616-631.
Should the use of ‘endorse’ be endorsed in writing in psychiatry?
The word “endorse” often appears in the medical literature and is heard in oral presentations; psychiatrists use the term to mean that a person is reporting psychiatric symptoms or problems. However, such usage may be a stylistic catachresis—one that has the potential for misinterpretation or misunderstanding.
Finding ‘endorse’ in the psychiatric literature
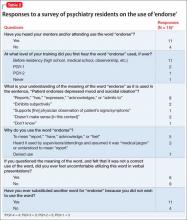
We conducted a literature search to identify instances of “endorse” in scholarly articles published in psychiatric journals between January 1, 2012, and November 25, 2013. Table 11-14 shows examples of typical uses of “endorse” in recent publications.
Even when “endorse” is used as a synonym for “report” or “describe,” use of the word in that context can seem out of place. We could not find any rationale in the medical literature for using “endorse” as a synonym for “report” or “describe.”
The definition of “endorse” in Merriam-Webster15 and Oxford Dictionaries16 includes:
• inscribing or signing a legal document, check, or bill
• approving or recommending an idea, product, or candidate.
We believe that using “endorse” in a psychiatric context could create confusion among medical trainees and professionals who are familiar with the correct meanings of the word.
Survey: Some residents use ‘endorse’ in oral presentations
We asked residents in the Department of Psychiatry at Drexel University College of Medicine to respond to a questionnaire regarding their understanding of the use of “endorse.” Their responses are summarized in Table 2.
What is wrong with using ‘endorse’? Except when “endorse” describes a patient formally affixing her (his) signature to a document for the purpose of (1) certification or (2) giving or showing one’s support for a cause, we think that use of the word in psychiatry is not in keeping with its formal, accepted definition. Furthermore, residents’ responses to our survey suggest that there is the danger of causing confusion in using the word“endorse” when “report” or “describe” is meant.
For example, if a patient “endorses” antisocial behavior, is she stating that she feels justified in exhibiting such behavior? Do students who “endorse” drug use approve of drug use? Another example: Youth who “endorse” gang membership have merely confirmed that they belonged to a gang at some time.
The intended meaning of “endorse” in these examples is probably closer to “admit” or “acknowledge.” The patient replies “yes” when the physician asks if she uses drugs or has had behavior problems; she is not necessarily recommending or approving these behaviors.
Usage is shifting. In the past, “complain” was common medical parlance for a patient’s report of symptoms or other health-related problems. In fact, medical, surgical, and psychiatric evaluations still begin with a “chief complaint” section. It’s possible that, because “complaint” might suggest that the patient is whining, the word fell out of favor in the medical lexicon and was replaced in the scholarly literature by the construction “the patient reports….”
Avoid jargon. Employ accurate terminology
We propose that “endorse,” like “complain,” is a cant of psychiatrists. We recommend that, when describing a patient’s statement or report of symptoms or experiences, practitioners should avoid “endorse” and write or say “report,” “express,” “exhibit,” or similar words. Using accurate terminology and avoiding imprecise or misleading jargon is not only linguistically appropriate but also can help avoid misunderstanding and improve patient care.
Acknowledgment
Diana Winters, Academic Publishing Services, Drexel University College of Medicine, provided editorial assistance in preparing the manuscript of this article and offered comment on the use of medical jargon.
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Strauss GP, Gold JM. A new perspective on anhedonia in schizophrenia. Am J Psychiatry. 2012;169(4):364-373.
2. Thomas JJ, Weigel TJ, Lawton RK, et al. Cognitive-behavioral treatment of body image disturbance in a congenitally blind patient with anorexia nervosa. Am J Psychiatry. 2012;169(1):16-20.
3. Purcell B, Heisel MJ, Speice J, et al. Family connectedness moderates the association between living alone and suicide ideation in a clinical sample of adults 50 years and older. Am J Geriatr Psychiatry. 2012;20(8):717-723.
4. Dakin EK, Areán P. Patient perspectives on the benefits of psychotherapy for late-life depression. Am J Geriatr Psychiatry. 2013;21(2):155-163.
5. Mezuk B, Lohman M, Dumenci, L, et al. Are depression and frailty overlapping syndromes in mid- and late-life? A latent variable analysis. Am J Geriatr Psychiatry. 2013;21(6):560-569.
6. Boindala NS, Tucker P, Trautman RP. “Culture and psychiatry”: a course for second-year psychiatry residents. Acad Psychiatry. 2013;37(1):46-50.
7. Henry A, Kisicki MD, Varley C. Efficacy and safety of antidepressant drug treatment in children and adolescents. Mol Psychiatry. 2012;17(12):1186-1193.
8. Rodriguez CI, Kegeles LS, Levinson A, et al. A randomized controlled crossover trial of ketamine in obsessive-compulsive disorder (abstract W7). Neuropsychopharmacology. 2012;38:S317.
9. Whelan R, Garavan H. Fractionating the impulsivity construct in adolescence. Neuropsychopharmacology. 2013;38(1):250-251.
10. Rück C, Larsson KJ, Mataix-Cols D. Predictors of medium and long-term outcome following capsulotomy for obsessive-compulsive disorder: one site may not fit all. Eur Neuropsychopharmacol. 2012;22(6):406-414.
11. Mausbach BT, Chattillion EA, Roepke SK, et al. A comparison of psychosocial outcomes in elderly Alzheimer caregivers and noncaregivers. Am J Geriatr Psychiatry. 2013;21(1):5-13.
12. Peavy GM, Salmon DP, Edland SD, et al. Neuropsychiatric features of frontal lobe dysfunction in autopsy-confirmed patients with lewy bodies and “pure” Alzheimer disease. Am J Geriatr Psychiatry. 2013;21(6):509-519.
13. Coid JW, Ullrich S, Keers R, et al. Gang membership, violence, and psychiatric morbidity. Am J Psychiatry. 2013;170(9):985-993.
14. Choi D, Tolova V, Socha E, et al. Substance use and attitudes on professional conduct among medical students: a single-institution study. Acad Psychiatry. 2013;37(3):191-195.
15. Endorse. Merriam-Webster. http://www.merriam-webster. com/dictionary/endorse. Accessed January 14, 2014.
16. Endorse. Oxford Dictionaries. http://www.oxforddictionaries. com/definition/english/endorse. Accessed January 14, 2014.
The word “endorse” often appears in the medical literature and is heard in oral presentations; psychiatrists use the term to mean that a person is reporting psychiatric symptoms or problems. However, such usage may be a stylistic catachresis—one that has the potential for misinterpretation or misunderstanding.
Finding ‘endorse’ in the psychiatric literature
We conducted a literature search to identify instances of “endorse” in scholarly articles published in psychiatric journals between January 1, 2012, and November 25, 2013. Table 11-14 shows examples of typical uses of “endorse” in recent publications.
Even when “endorse” is used as a synonym for “report” or “describe,” use of the word in that context can seem out of place. We could not find any rationale in the medical literature for using “endorse” as a synonym for “report” or “describe.”
The definition of “endorse” in Merriam-Webster15 and Oxford Dictionaries16 includes:
• inscribing or signing a legal document, check, or bill
• approving or recommending an idea, product, or candidate.
We believe that using “endorse” in a psychiatric context could create confusion among medical trainees and professionals who are familiar with the correct meanings of the word.
Survey: Some residents use ‘endorse’ in oral presentations
We asked residents in the Department of Psychiatry at Drexel University College of Medicine to respond to a questionnaire regarding their understanding of the use of “endorse.” Their responses are summarized in Table 2.
What is wrong with using ‘endorse’? Except when “endorse” describes a patient formally affixing her (his) signature to a document for the purpose of (1) certification or (2) giving or showing one’s support for a cause, we think that use of the word in psychiatry is not in keeping with its formal, accepted definition. Furthermore, residents’ responses to our survey suggest that there is the danger of causing confusion in using the word“endorse” when “report” or “describe” is meant.
For example, if a patient “endorses” antisocial behavior, is she stating that she feels justified in exhibiting such behavior? Do students who “endorse” drug use approve of drug use? Another example: Youth who “endorse” gang membership have merely confirmed that they belonged to a gang at some time.
The intended meaning of “endorse” in these examples is probably closer to “admit” or “acknowledge.” The patient replies “yes” when the physician asks if she uses drugs or has had behavior problems; she is not necessarily recommending or approving these behaviors.
Usage is shifting. In the past, “complain” was common medical parlance for a patient’s report of symptoms or other health-related problems. In fact, medical, surgical, and psychiatric evaluations still begin with a “chief complaint” section. It’s possible that, because “complaint” might suggest that the patient is whining, the word fell out of favor in the medical lexicon and was replaced in the scholarly literature by the construction “the patient reports….”
Avoid jargon. Employ accurate terminology
We propose that “endorse,” like “complain,” is a cant of psychiatrists. We recommend that, when describing a patient’s statement or report of symptoms or experiences, practitioners should avoid “endorse” and write or say “report,” “express,” “exhibit,” or similar words. Using accurate terminology and avoiding imprecise or misleading jargon is not only linguistically appropriate but also can help avoid misunderstanding and improve patient care.
Acknowledgment
Diana Winters, Academic Publishing Services, Drexel University College of Medicine, provided editorial assistance in preparing the manuscript of this article and offered comment on the use of medical jargon.
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
The word “endorse” often appears in the medical literature and is heard in oral presentations; psychiatrists use the term to mean that a person is reporting psychiatric symptoms or problems. However, such usage may be a stylistic catachresis—one that has the potential for misinterpretation or misunderstanding.
Finding ‘endorse’ in the psychiatric literature
We conducted a literature search to identify instances of “endorse” in scholarly articles published in psychiatric journals between January 1, 2012, and November 25, 2013. Table 11-14 shows examples of typical uses of “endorse” in recent publications.
Even when “endorse” is used as a synonym for “report” or “describe,” use of the word in that context can seem out of place. We could not find any rationale in the medical literature for using “endorse” as a synonym for “report” or “describe.”
The definition of “endorse” in Merriam-Webster15 and Oxford Dictionaries16 includes:
• inscribing or signing a legal document, check, or bill
• approving or recommending an idea, product, or candidate.
We believe that using “endorse” in a psychiatric context could create confusion among medical trainees and professionals who are familiar with the correct meanings of the word.
Survey: Some residents use ‘endorse’ in oral presentations
We asked residents in the Department of Psychiatry at Drexel University College of Medicine to respond to a questionnaire regarding their understanding of the use of “endorse.” Their responses are summarized in Table 2.
What is wrong with using ‘endorse’? Except when “endorse” describes a patient formally affixing her (his) signature to a document for the purpose of (1) certification or (2) giving or showing one’s support for a cause, we think that use of the word in psychiatry is not in keeping with its formal, accepted definition. Furthermore, residents’ responses to our survey suggest that there is the danger of causing confusion in using the word“endorse” when “report” or “describe” is meant.
For example, if a patient “endorses” antisocial behavior, is she stating that she feels justified in exhibiting such behavior? Do students who “endorse” drug use approve of drug use? Another example: Youth who “endorse” gang membership have merely confirmed that they belonged to a gang at some time.
The intended meaning of “endorse” in these examples is probably closer to “admit” or “acknowledge.” The patient replies “yes” when the physician asks if she uses drugs or has had behavior problems; she is not necessarily recommending or approving these behaviors.
Usage is shifting. In the past, “complain” was common medical parlance for a patient’s report of symptoms or other health-related problems. In fact, medical, surgical, and psychiatric evaluations still begin with a “chief complaint” section. It’s possible that, because “complaint” might suggest that the patient is whining, the word fell out of favor in the medical lexicon and was replaced in the scholarly literature by the construction “the patient reports….”
Avoid jargon. Employ accurate terminology
We propose that “endorse,” like “complain,” is a cant of psychiatrists. We recommend that, when describing a patient’s statement or report of symptoms or experiences, practitioners should avoid “endorse” and write or say “report,” “express,” “exhibit,” or similar words. Using accurate terminology and avoiding imprecise or misleading jargon is not only linguistically appropriate but also can help avoid misunderstanding and improve patient care.
Acknowledgment
Diana Winters, Academic Publishing Services, Drexel University College of Medicine, provided editorial assistance in preparing the manuscript of this article and offered comment on the use of medical jargon.
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Strauss GP, Gold JM. A new perspective on anhedonia in schizophrenia. Am J Psychiatry. 2012;169(4):364-373.
2. Thomas JJ, Weigel TJ, Lawton RK, et al. Cognitive-behavioral treatment of body image disturbance in a congenitally blind patient with anorexia nervosa. Am J Psychiatry. 2012;169(1):16-20.
3. Purcell B, Heisel MJ, Speice J, et al. Family connectedness moderates the association between living alone and suicide ideation in a clinical sample of adults 50 years and older. Am J Geriatr Psychiatry. 2012;20(8):717-723.
4. Dakin EK, Areán P. Patient perspectives on the benefits of psychotherapy for late-life depression. Am J Geriatr Psychiatry. 2013;21(2):155-163.
5. Mezuk B, Lohman M, Dumenci, L, et al. Are depression and frailty overlapping syndromes in mid- and late-life? A latent variable analysis. Am J Geriatr Psychiatry. 2013;21(6):560-569.
6. Boindala NS, Tucker P, Trautman RP. “Culture and psychiatry”: a course for second-year psychiatry residents. Acad Psychiatry. 2013;37(1):46-50.
7. Henry A, Kisicki MD, Varley C. Efficacy and safety of antidepressant drug treatment in children and adolescents. Mol Psychiatry. 2012;17(12):1186-1193.
8. Rodriguez CI, Kegeles LS, Levinson A, et al. A randomized controlled crossover trial of ketamine in obsessive-compulsive disorder (abstract W7). Neuropsychopharmacology. 2012;38:S317.
9. Whelan R, Garavan H. Fractionating the impulsivity construct in adolescence. Neuropsychopharmacology. 2013;38(1):250-251.
10. Rück C, Larsson KJ, Mataix-Cols D. Predictors of medium and long-term outcome following capsulotomy for obsessive-compulsive disorder: one site may not fit all. Eur Neuropsychopharmacol. 2012;22(6):406-414.
11. Mausbach BT, Chattillion EA, Roepke SK, et al. A comparison of psychosocial outcomes in elderly Alzheimer caregivers and noncaregivers. Am J Geriatr Psychiatry. 2013;21(1):5-13.
12. Peavy GM, Salmon DP, Edland SD, et al. Neuropsychiatric features of frontal lobe dysfunction in autopsy-confirmed patients with lewy bodies and “pure” Alzheimer disease. Am J Geriatr Psychiatry. 2013;21(6):509-519.
13. Coid JW, Ullrich S, Keers R, et al. Gang membership, violence, and psychiatric morbidity. Am J Psychiatry. 2013;170(9):985-993.
14. Choi D, Tolova V, Socha E, et al. Substance use and attitudes on professional conduct among medical students: a single-institution study. Acad Psychiatry. 2013;37(3):191-195.
15. Endorse. Merriam-Webster. http://www.merriam-webster. com/dictionary/endorse. Accessed January 14, 2014.
16. Endorse. Oxford Dictionaries. http://www.oxforddictionaries. com/definition/english/endorse. Accessed January 14, 2014.
1. Strauss GP, Gold JM. A new perspective on anhedonia in schizophrenia. Am J Psychiatry. 2012;169(4):364-373.
2. Thomas JJ, Weigel TJ, Lawton RK, et al. Cognitive-behavioral treatment of body image disturbance in a congenitally blind patient with anorexia nervosa. Am J Psychiatry. 2012;169(1):16-20.
3. Purcell B, Heisel MJ, Speice J, et al. Family connectedness moderates the association between living alone and suicide ideation in a clinical sample of adults 50 years and older. Am J Geriatr Psychiatry. 2012;20(8):717-723.
4. Dakin EK, Areán P. Patient perspectives on the benefits of psychotherapy for late-life depression. Am J Geriatr Psychiatry. 2013;21(2):155-163.
5. Mezuk B, Lohman M, Dumenci, L, et al. Are depression and frailty overlapping syndromes in mid- and late-life? A latent variable analysis. Am J Geriatr Psychiatry. 2013;21(6):560-569.
6. Boindala NS, Tucker P, Trautman RP. “Culture and psychiatry”: a course for second-year psychiatry residents. Acad Psychiatry. 2013;37(1):46-50.
7. Henry A, Kisicki MD, Varley C. Efficacy and safety of antidepressant drug treatment in children and adolescents. Mol Psychiatry. 2012;17(12):1186-1193.
8. Rodriguez CI, Kegeles LS, Levinson A, et al. A randomized controlled crossover trial of ketamine in obsessive-compulsive disorder (abstract W7). Neuropsychopharmacology. 2012;38:S317.
9. Whelan R, Garavan H. Fractionating the impulsivity construct in adolescence. Neuropsychopharmacology. 2013;38(1):250-251.
10. Rück C, Larsson KJ, Mataix-Cols D. Predictors of medium and long-term outcome following capsulotomy for obsessive-compulsive disorder: one site may not fit all. Eur Neuropsychopharmacol. 2012;22(6):406-414.
11. Mausbach BT, Chattillion EA, Roepke SK, et al. A comparison of psychosocial outcomes in elderly Alzheimer caregivers and noncaregivers. Am J Geriatr Psychiatry. 2013;21(1):5-13.
12. Peavy GM, Salmon DP, Edland SD, et al. Neuropsychiatric features of frontal lobe dysfunction in autopsy-confirmed patients with lewy bodies and “pure” Alzheimer disease. Am J Geriatr Psychiatry. 2013;21(6):509-519.
13. Coid JW, Ullrich S, Keers R, et al. Gang membership, violence, and psychiatric morbidity. Am J Psychiatry. 2013;170(9):985-993.
14. Choi D, Tolova V, Socha E, et al. Substance use and attitudes on professional conduct among medical students: a single-institution study. Acad Psychiatry. 2013;37(3):191-195.
15. Endorse. Merriam-Webster. http://www.merriam-webster. com/dictionary/endorse. Accessed January 14, 2014.
16. Endorse. Oxford Dictionaries. http://www.oxforddictionaries. com/definition/english/endorse. Accessed January 14, 2014.
‘Acting out’ or pathological?
Mental illness during pregnancy
Consider a mandibular positioning device to alleviate sleep-disordered breathing
Snoring, snorting, gasping, and obstructive sleep apnea are caused by collapse of the pharyngeal airway during sleep.1 Pathophysiology includes a combination of anatomical and physiological variables.1 Common anatomical predisposing conditions include abnormalities of pharyngeal, lingual, and dental arches; physiological concerns are advancing age, male sex, obesity, use of sedatives, body positioning, and reduced muscle tone during rapid eye movement sleep. Coexistence of anatomic and physiological elements can produce significant narrowing of the upper airway.
Comorbidities include vascular, metabolic, and psychiatric conditions. As many as one-third of people with symptoms of sleep apnea report depressed mood; approximately 10% of these patients meet criteria for moderate or severe depression.2
In short, sleep-disordered breathing has a globally negative effect on mental health.
When should you consider obtaining a sleep apnea study?
Refer patients for a sleep study when snoring, snorting, gasping, or pauses in breathing occur during sleep, or in the case of daytime sleepiness, fatigue, or unrefreshing sleep that cannot be explained by another medical or psychiatric illness.2 A sleep specialist can determine the most appropriate intervention for sleep-disordered breathing.
An apneic event is characterized by complete cessation of airflow; hypopnea is a partially compromised airway. In either event, at least a 3% decrease in oxygen saturation occurs for at least 10 seconds.3 A diagnosis of obstructive sleep apnea or hypopnea is required when polysomnography reveals either of:
• ≥5 episodes of apnea or hypopnea, or both, per hour of sleep, with symptoms of a rhythmic breathing disturbance or daytime sleepiness or fatigue
• ≥15 episodes of apnea or hypopnea, or both, per hour of sleep, regardless of accompanying symptoms.2
What are the treatment options?
• Continuous positive airway pressure (CPAP) machines.
• Surgical procedures include adeno-tonsillectomy in children and surgical maxilla-mandibular advancement or palatal implants for adults.
• A novel implantable electrical stimulation device stimulates the hypoglossal nerve, which activates the genioglossus muscle, thus moving the tongue forward to open the airway.
• An anterior mandibular positioning (AMP) device increases the diameter of the retroglossal space by preventing posterior movement of the mandible and tongue, thereby limiting encroachment on the airway diameter and reducing the potential for collapse.1-4
When should you recommend an AMP device?
Consider recommending an AMP device to treat sleep-disordered breathing when (1) lifestyle changes, such as sleep hygiene, weight loss, and stopping sedatives, do not work and (2) a CPAP machine or a surgical procedure is contraindicated or has been ineffective.1 An AMP device can minimize snoring and relieve airway obstruction, especially in patients with supine position-related apnea.4 To keep the airway open in non-supine position-related cases, an AMP device might be indicated in addition to CPAP delivered nasally.1
This plastic oral appliance is either a 1- or 2-piece design, and looks and is sized similarly to an athletic mouth-protection guard or an oral anti-bruxism tooth-protection appliance. It is affixed to the mandible and maxillary arches by clasps (Figure).
An AMP device often is most beneficial for supine-dependent sleep apnea patients and those with loud snoring, without sleep apnea.4 Response is best in young adults and in patients who have a low body mass index, are free of sedatives, and have appropriate cephalometrics of the oral, dental, or pharyngeal anatomy. Improved sleep architecture, continuous sleep with less snoring, and increased daytime alertness are observed in patients who respond to an AMP device.
An AMP device is contraindicated when the device cannot be affixed to the dental arches and in some patients with an anatomical or pain-related temporomandibular joint disorder.5 The device is easy to use, noninvasive, readily accessible, and less expensive than alternatives.3
How can you help maintain treatment adherence?
AMP devices can induce adverse effects, including dental pain or discomfort through orthodontic alterations; patient reports and follow-up can yield detection and device adjustments can alleviate such problems. Adherence generally is good, with complaints usually limited to minor tooth discomfort, occlusive changes, and increased or decreased salivation.5 In our clinical experience, many patients find these devices comfortable and easy to use, but might complain of feeling awkward when wearing them.
Changes in occlusion can occur during long-term treatment with an AMP device. Proper fitting is essential to facilitate a more open airway and the ability to speak and drink fluids, and to maintain safety, even if vomiting occurs while the device is in place.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Epstein LJ, Kristo D, Strollo PJ, et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5(3):263-276.
2. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
3. de Britto Teixeira AO, Abi-Ramia LB, de Oliveira Almeida MA. Treatment of obstructive sleep apnea with oral appliances. Prog Orthod. 2013;14:10.
4. Marklund M, Stenlund H, Franklin K. Mandibular advancement devices in 630 men and women with obstructive sleep apnea and snoring: tolerability and predictors of treatment success. Chest. 2004;125(4):1270-1278.
5. Ferguson KA, Cartwright R, Rogers R, et al. Oral appliances for snoring and obstructive sleep apnea: a review. Sleep. 2006;29(2):244-262.
Snoring, snorting, gasping, and obstructive sleep apnea are caused by collapse of the pharyngeal airway during sleep.1 Pathophysiology includes a combination of anatomical and physiological variables.1 Common anatomical predisposing conditions include abnormalities of pharyngeal, lingual, and dental arches; physiological concerns are advancing age, male sex, obesity, use of sedatives, body positioning, and reduced muscle tone during rapid eye movement sleep. Coexistence of anatomic and physiological elements can produce significant narrowing of the upper airway.
Comorbidities include vascular, metabolic, and psychiatric conditions. As many as one-third of people with symptoms of sleep apnea report depressed mood; approximately 10% of these patients meet criteria for moderate or severe depression.2
In short, sleep-disordered breathing has a globally negative effect on mental health.
When should you consider obtaining a sleep apnea study?
Refer patients for a sleep study when snoring, snorting, gasping, or pauses in breathing occur during sleep, or in the case of daytime sleepiness, fatigue, or unrefreshing sleep that cannot be explained by another medical or psychiatric illness.2 A sleep specialist can determine the most appropriate intervention for sleep-disordered breathing.
An apneic event is characterized by complete cessation of airflow; hypopnea is a partially compromised airway. In either event, at least a 3% decrease in oxygen saturation occurs for at least 10 seconds.3 A diagnosis of obstructive sleep apnea or hypopnea is required when polysomnography reveals either of:
• ≥5 episodes of apnea or hypopnea, or both, per hour of sleep, with symptoms of a rhythmic breathing disturbance or daytime sleepiness or fatigue
• ≥15 episodes of apnea or hypopnea, or both, per hour of sleep, regardless of accompanying symptoms.2
What are the treatment options?
• Continuous positive airway pressure (CPAP) machines.
• Surgical procedures include adeno-tonsillectomy in children and surgical maxilla-mandibular advancement or palatal implants for adults.
• A novel implantable electrical stimulation device stimulates the hypoglossal nerve, which activates the genioglossus muscle, thus moving the tongue forward to open the airway.
• An anterior mandibular positioning (AMP) device increases the diameter of the retroglossal space by preventing posterior movement of the mandible and tongue, thereby limiting encroachment on the airway diameter and reducing the potential for collapse.1-4
When should you recommend an AMP device?
Consider recommending an AMP device to treat sleep-disordered breathing when (1) lifestyle changes, such as sleep hygiene, weight loss, and stopping sedatives, do not work and (2) a CPAP machine or a surgical procedure is contraindicated or has been ineffective.1 An AMP device can minimize snoring and relieve airway obstruction, especially in patients with supine position-related apnea.4 To keep the airway open in non-supine position-related cases, an AMP device might be indicated in addition to CPAP delivered nasally.1
This plastic oral appliance is either a 1- or 2-piece design, and looks and is sized similarly to an athletic mouth-protection guard or an oral anti-bruxism tooth-protection appliance. It is affixed to the mandible and maxillary arches by clasps (Figure).
An AMP device often is most beneficial for supine-dependent sleep apnea patients and those with loud snoring, without sleep apnea.4 Response is best in young adults and in patients who have a low body mass index, are free of sedatives, and have appropriate cephalometrics of the oral, dental, or pharyngeal anatomy. Improved sleep architecture, continuous sleep with less snoring, and increased daytime alertness are observed in patients who respond to an AMP device.
An AMP device is contraindicated when the device cannot be affixed to the dental arches and in some patients with an anatomical or pain-related temporomandibular joint disorder.5 The device is easy to use, noninvasive, readily accessible, and less expensive than alternatives.3
How can you help maintain treatment adherence?
AMP devices can induce adverse effects, including dental pain or discomfort through orthodontic alterations; patient reports and follow-up can yield detection and device adjustments can alleviate such problems. Adherence generally is good, with complaints usually limited to minor tooth discomfort, occlusive changes, and increased or decreased salivation.5 In our clinical experience, many patients find these devices comfortable and easy to use, but might complain of feeling awkward when wearing them.
Changes in occlusion can occur during long-term treatment with an AMP device. Proper fitting is essential to facilitate a more open airway and the ability to speak and drink fluids, and to maintain safety, even if vomiting occurs while the device is in place.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Snoring, snorting, gasping, and obstructive sleep apnea are caused by collapse of the pharyngeal airway during sleep.1 Pathophysiology includes a combination of anatomical and physiological variables.1 Common anatomical predisposing conditions include abnormalities of pharyngeal, lingual, and dental arches; physiological concerns are advancing age, male sex, obesity, use of sedatives, body positioning, and reduced muscle tone during rapid eye movement sleep. Coexistence of anatomic and physiological elements can produce significant narrowing of the upper airway.
Comorbidities include vascular, metabolic, and psychiatric conditions. As many as one-third of people with symptoms of sleep apnea report depressed mood; approximately 10% of these patients meet criteria for moderate or severe depression.2
In short, sleep-disordered breathing has a globally negative effect on mental health.
When should you consider obtaining a sleep apnea study?
Refer patients for a sleep study when snoring, snorting, gasping, or pauses in breathing occur during sleep, or in the case of daytime sleepiness, fatigue, or unrefreshing sleep that cannot be explained by another medical or psychiatric illness.2 A sleep specialist can determine the most appropriate intervention for sleep-disordered breathing.
An apneic event is characterized by complete cessation of airflow; hypopnea is a partially compromised airway. In either event, at least a 3% decrease in oxygen saturation occurs for at least 10 seconds.3 A diagnosis of obstructive sleep apnea or hypopnea is required when polysomnography reveals either of:
• ≥5 episodes of apnea or hypopnea, or both, per hour of sleep, with symptoms of a rhythmic breathing disturbance or daytime sleepiness or fatigue
• ≥15 episodes of apnea or hypopnea, or both, per hour of sleep, regardless of accompanying symptoms.2
What are the treatment options?
• Continuous positive airway pressure (CPAP) machines.
• Surgical procedures include adeno-tonsillectomy in children and surgical maxilla-mandibular advancement or palatal implants for adults.
• A novel implantable electrical stimulation device stimulates the hypoglossal nerve, which activates the genioglossus muscle, thus moving the tongue forward to open the airway.
• An anterior mandibular positioning (AMP) device increases the diameter of the retroglossal space by preventing posterior movement of the mandible and tongue, thereby limiting encroachment on the airway diameter and reducing the potential for collapse.1-4
When should you recommend an AMP device?
Consider recommending an AMP device to treat sleep-disordered breathing when (1) lifestyle changes, such as sleep hygiene, weight loss, and stopping sedatives, do not work and (2) a CPAP machine or a surgical procedure is contraindicated or has been ineffective.1 An AMP device can minimize snoring and relieve airway obstruction, especially in patients with supine position-related apnea.4 To keep the airway open in non-supine position-related cases, an AMP device might be indicated in addition to CPAP delivered nasally.1
This plastic oral appliance is either a 1- or 2-piece design, and looks and is sized similarly to an athletic mouth-protection guard or an oral anti-bruxism tooth-protection appliance. It is affixed to the mandible and maxillary arches by clasps (Figure).
An AMP device often is most beneficial for supine-dependent sleep apnea patients and those with loud snoring, without sleep apnea.4 Response is best in young adults and in patients who have a low body mass index, are free of sedatives, and have appropriate cephalometrics of the oral, dental, or pharyngeal anatomy. Improved sleep architecture, continuous sleep with less snoring, and increased daytime alertness are observed in patients who respond to an AMP device.
An AMP device is contraindicated when the device cannot be affixed to the dental arches and in some patients with an anatomical or pain-related temporomandibular joint disorder.5 The device is easy to use, noninvasive, readily accessible, and less expensive than alternatives.3
How can you help maintain treatment adherence?
AMP devices can induce adverse effects, including dental pain or discomfort through orthodontic alterations; patient reports and follow-up can yield detection and device adjustments can alleviate such problems. Adherence generally is good, with complaints usually limited to minor tooth discomfort, occlusive changes, and increased or decreased salivation.5 In our clinical experience, many patients find these devices comfortable and easy to use, but might complain of feeling awkward when wearing them.
Changes in occlusion can occur during long-term treatment with an AMP device. Proper fitting is essential to facilitate a more open airway and the ability to speak and drink fluids, and to maintain safety, even if vomiting occurs while the device is in place.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Epstein LJ, Kristo D, Strollo PJ, et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5(3):263-276.
2. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
3. de Britto Teixeira AO, Abi-Ramia LB, de Oliveira Almeida MA. Treatment of obstructive sleep apnea with oral appliances. Prog Orthod. 2013;14:10.
4. Marklund M, Stenlund H, Franklin K. Mandibular advancement devices in 630 men and women with obstructive sleep apnea and snoring: tolerability and predictors of treatment success. Chest. 2004;125(4):1270-1278.
5. Ferguson KA, Cartwright R, Rogers R, et al. Oral appliances for snoring and obstructive sleep apnea: a review. Sleep. 2006;29(2):244-262.
1. Epstein LJ, Kristo D, Strollo PJ, et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5(3):263-276.
2. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
3. de Britto Teixeira AO, Abi-Ramia LB, de Oliveira Almeida MA. Treatment of obstructive sleep apnea with oral appliances. Prog Orthod. 2013;14:10.
4. Marklund M, Stenlund H, Franklin K. Mandibular advancement devices in 630 men and women with obstructive sleep apnea and snoring: tolerability and predictors of treatment success. Chest. 2004;125(4):1270-1278.
5. Ferguson KA, Cartwright R, Rogers R, et al. Oral appliances for snoring and obstructive sleep apnea: a review. Sleep. 2006;29(2):244-262.
Young, pregnant, ataxic—and jilted
CASE Difficulty walking
Ms. M, age 15, is a pregnant, Spanish-speaking Guatemalan woman who is brought to obstetrics triage in a large academic medical center at 35 weeks gestational age. She complains of dizziness, tinnitus, left orbital headache, and difficulty walking.
The neurology service finds profound truncal ataxia, astasia-abasia, and buckling of the knees; a normal brain and spine MRI are not consistent with a neurologic etiology. Otolaryngology service evaluates Ms. M to rule out a cholesteatoma and suggests a head CT and endoscopy, which are normal.
Ms. M’s symptoms resolve after 3 days, although the gait disturbances persist. When no clear cause is found for her difficulty walking, the psychiatry service is consulted to evaluate whether an underlying psychiatric disorder is contributing to symptoms.
What could be causing Ms. M’s symptoms?
a) malingering
b) factitious disorder
c) undiagnosed neurologic disorder
d) conversion disorder
The authors’ observations
Women are vulnerable to a variety of psychiatric illnesses during pregnancy1 that have deleterious effects on mother, baby, and family.2-6 Although there is a burgeoning literature on affective and anxiety disorders occurring in pregnancy, there is a dearth of information about somatoform disorders.
HISTORY Abandonment
Ms. M reports that, although her boyfriend deserted her after learning about the unexpected pregnancy, she will welcome the baby and looks forward to motherhood. She seems unaware of the responsibilities of being a mother.
Ms. M acknowledges a history of depression and self-harm a few years earlier, yet says she feels better now and thinks that psychiatric care is unnecessary. Because she does not endorse a history of trauma or symptoms suggesting an affective, anxiety, or psychotic illness, the psychiatrist does not recommend treatment with psychotropic medication.
At age 5, Ms. M’s parents sent her to the United States with her aunt, hoping that she would have a better life than she would have had in Guatemala. Her aunt reports that Ms. M initially had difficulty adjusting to life in the United States without her parents, yet she has made substantial strides over the years and is now quite accustomed to the country. Her aunt describes Ms. M as an independent high school student who earns good grades.
During the interview, the psychiatrist observes that Ms. M exhibits childlike mannerisms, including sleeping with stuffed toys and coloring in Disney books with crayons. She also is indifferent to her gait difficulty, pregnancy, and psychosocial stressors. Her affect is inconsistent with the content of her speech and she is alexithymic.
Ms. M’s aunt reports that her niece is becoming more dependent on her, which is not consistent with her baseline. Her aunt also notes that several years earlier, Ms. M’s nephew was diagnosed with a cholesteatoma after he presented with similar symptoms.
The combination of (1) Ms. M’s clinical presentation, which was causing her significant impairment in her social functioning, (2) the incompatibility of symptoms with any recognized neurologic and medical disease, and (3) prior family experience with cholesteatoma leads the consulting psychiatrist to suspect conversion disorder. Ms. M’s alexithymia, indifference to her symptoms, and recent abandonment by the baby’s father also support a conversion disorder diagnosis.
From a psychodynamic perspective, the ataxia appears to be her way of protecting herself from the abandonment she is experiencing by being left again to “stand alone” by her boyfriend as she had been when her parents sent her to the United States. Her regressive behavior could be her way of securing her aunt’s love and support.
The authors’ observations
This is the first case of psychogenic gait disturbance during pregnancy described in the literature. Authors have reported on pseudotoxemia,7 hyperemesis gravidarum,8 and pesudocyesis,9 yet there is a paucity of information on psychogenic gait disturbance during pregnancy. Ms. M’s case elucidates many of the clinical quandaries that occur when managing psychiatric illness—and, more specifically, conversion disorder— during pregnancy. Many women are hesitant to seek psychiatric treatment during pregnancy because of shame, stigma, and fear of loss of personal or parental rights10,11; it is not surprising that emotionally distressed women communicate their feelings or troubled thoughts through physical symptoms.
Likely diagnosis
Conversion disorder is the presence of neurologic symptoms in the absence of a neurologic diagnosis that fully explains those symptoms. Conversion disorder, previously known as hysteria, is called functional neurologic symptom disorder in DSM-5 (Box).12 Symptoms are not feigned; instead, they represent “conversion” of emotional distress into neurologic symptoms.13,14 Although misdiagnosing conversion disorder in patients with true neurologic disease is uncommon, clinicians often are uncomfortable making the diagnosis until all medical causes have been ruled out.14 It is not always possible to find a psychological explanation for conversion disorder, but a history of childhood abuse, particularly sexual abuse, could play a role.14
Because of the variety of presentations, clinicians in all specialties should be familiar with somatoform disorders; this is especially important in obstetrics and gynecology because women are more likely than men to develop these disorders.15 It is important to consider that Ms. M is a teenager and somatoform disorders can present differently in adults. The diagnostic process should include a diligent somatic workup and a personal and social history to identify the patient’s developmental tasks, stressors, and coping style.15
How would you treat Ms. M?
a) destigmatize psychiatric illness and provide psychoeducation regarding treatment benefits
b) identify and treat any comorbid psychiatric disorders
c) maintain a proactive and multidisciplinary approach that includes assessment of psychosocial stressors and psychodynamic factors, particularly those related to the pregnancy
d) all of the above
TREATMENT Close follow-up
The psychiatrist recommends continued close psychiatric follow-up as well as multidisciplinary involvement, including physical therapy, neurology, and obstetrics.
Ms. M initially is resistant to psychiatric follow-up because she says that “people on the street” told her that, if she saw a psychiatrist, her baby would be taken away. After the psychiatrist explains that it is unlikely her baby would be taken away, Ms. M immediately appears relieved, smiles, and readily agrees to outpatient psychotherapy.
Over the next 24 hours, she continues to work with a physical therapist and her gait significantly improves. She is discharged home 2 days later with a walking aid (Zimmer frame) for assistance.
Four days later, however, Ms. M is readmitted with worsening ataxia. Her aunt reports that, at home, Ms. M’s regressed behaviors are worsening; she is sleeping in bed with her and had several episodes of enuresis at home.
Ms. M continues to deny psychiatric symptoms or anxiety about the delivery. Although she shows some improvement when working with physical therapists, they note that Ms. M is still unable to ambulate or stand on her own. The psychiatrist is increasingly concerned about her regressed behavior and continued ataxia.
A family meeting is held and the psychiatrist and social worker educate Ms. M and her aunt about conversion disorder, including how some emotionally distressed women communicate their feelings or troubled thoughts through physical symptoms and how that may apply to Ms. M. During the meeting, the team also destigmatizes psychiatric illness and treatment and provides psychoeducation regarding its benefits. The psychiatrist and social worker also provide a psychodynamic interpretation that her ataxia could be a way of protecting herself against the abandonment she is experiencing by being left to “stand alone” by her boyfriend— as she had been when her parents sent her to the United States, and that her behavior could be her way of securing her aunt’s love and support.Ms. M and her aunt both readily agree with this interpretation. The aunt notes that her niece is more anxious about motherhood than she acknowledges and is concerned that Ms. M expects her to be the primary caregiver for the baby. Those present note that Ms. M is becoming increasingly dependent on her aunt, and that it is important for her to retain her independence, especially once she becomes a mother.
Ms. M immediately begins to display more affect; she smiles and reports feeling relieved. Similar to the previous admission, her gait significantly improves over the next 2 days and she is discharged home with a walking aid.
The authors’ observations
A broad differential diagnosis and early multidisciplinary involvement might facilitate earlier diagnosis and treatment.16 Assessment of psychosocial stressors in the patient’s personal and family life, including circumstances around the pregnancy and the meaning of motherhood, as well as investigation of what the patient may gain from the sick role, are paramount. In Ms. M’s case, cultural background, separation from her parents at a young age, and recent abandonment by her boyfriend have contributed to her inability to “stand alone,” which manifested as ataxia. Young age, regressed behavior, and her minimization of stressors also point to her difficulty acknowledging and coping with psychosocial stressors.
Successful delivery of the diagnosis is key to treatment success. After building a therapeutic alliance, a multidisciplinary discussion should take place that allows the patient to understand the diagnosis and treatment plan.17,18 The patient and family should be reassured that the fetus is healthy and all organic causes of symptoms have been investigated.17 Although management of conversion disorder during pregnancy is similar to that in non-pregnant women, several additional avenues of investigation should be considered:
• Explore the psychodynamic basis of the disorder and the role of the pregnancy and motherhood.
• Identify any comorbid psychiatric disorders, particularly those specific to pregnancy or the postpartum period.
• Because of the shame and stigma associated with seeking psychiatric treatment during pregnancy,10,11 it is imperative to destigmatize treatment and provide psychoeducation regarding its benefits.
A treatment plan can then be developed that involves psychotherapy, psychoeducation, stress management, and, when appropriate, pharmacotherapy.17
Providing psychoeducation about postpartum depression and other perinatal psychiatric illness could be beneficial. Physical therapy often is culturally acceptable and can help re-establish healthy patterns of motor function.19 Ms. M’s gait showed some improvement with physical therapy as part of the multidisciplinary approach, which also should include a thorough medical workup. Appropriate psychiatric treatment can help patients give up the sick role and return to their previous level of functioning.17
Maintain close communication with the outpatient perinatal care team as they monitor the patient’s parenting capacity. The outpatient perinatal care team also should engage pregnant or postpartum women in prioritizing their emotional well-being and encourage outpatient mental health treatment. Despite a dearth of data on the regressive symptoms and prognosis for future pregnancies, it is important to monitor maternal capacity and discuss the possibility of symptom recurrence.
OUTCOME Healthy baby
Three days later, Ms. M returns in labor with improved gait yet still using a walking aid. She has a normal vaginal delivery of a healthy baby boy at 37 weeks’ gestational age.
After the birth, Ms. M reports feeling well and enjoying motherhood, and denies psychiatric symptoms. She is ambulating without assistance within hours of delivery. This spontaneous resolution of symptoms could have been because of the psychodynamically oriented multidisciplinary approach to her care, which may have helped her realize that she did not have to “stand alone” as she embarked on motherhood.
Before being discharged home, Ms. M and her aunt meet with the inpatient obstetric social worker to assess Ms. M’s ability to care for the baby and discuss the importance of continued emotional support. The social worker does not contact the Department of Children and Families because Ms. M is walking independently and not endorsing or exhibiting regressive behaviors. Ms. M also reports that she will ask her aunt to take care of the baby should ataxia recur. Her aunt reassures the social workers that she will encourage Ms. M to attend outpatient psychotherapy and will contact the social worker if she becomes concerned about Ms. M’s or the baby’s well-being.
During her postpartum obstetric visit, Ms. M is walking independently and does not exhibit or endorse neurologic symptoms. The social worker provides psychoeducation about the importance of outpatient psychotherapy and schedules an initial appointment; Ms. M does not attend outpatient psychotherapy after discharge.
Bottom Line
Consider conversion disorder in obstetric patients who present with ataxia without a neurologic cause. Management involves a proactive and multidisciplinary approach that includes a thorough medical workup and assessment of psychosocial stressors and psychodynamic factors, particularly those related to the pregnancy. Early identification and delivery of the diagnosis, destigmatization, and provision of appropriate psychiatric treatment can facilitate treatment success.
Disclosures
Dr. Byatt has received grant funding/support for this project from the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant KL2TR000160. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Dr. Toor reports no financial relationships with any company whose products are mentioned in this article or manufacturers of competing products.
1. Vesga-Lopez O, Blanco C, Keyes K, et al. Psychiatric disorders in pregnant and postpartum women in the United States. Arch Gen Psychiatry. 2008;65(7):805-815.
2. Britton HL, Gronwaldt V, Britton JR. Maternal postpartum behaviors and mother-infant relationship during the first year of life. J Pediatr. 2001;138(6):905-909.
3. Deave T, Heron J, Evans J, et al. The impact of maternal depression in pregnancy on early child development. BJOG. 2008;115(8):1043-1051.
4. Paulson JF, Keefe HA, Leiferman JA. Early parental depression and child language development. J Child Psychol Psychiatry. 2009;50(3):254-262.
5. Zuckerman B, Amaro H, Bauchner H, et al. Depressive symptoms during pregnancy: relationship to poor health behaviors. Am J Obstet Gynecol. 1989;160(5 pt 1):1107-1111.
6. Forman DR, O’Hara MW, Stuart S, et al. Effective treatment for postpartum depression is not sufficient to improve the developing mother-child relationship. Dev Psychopathol. 2007;19(2):585-602.
7. Brady WJ Jr, Huff JS. Pseudotoxemia: new onset psychogenic seizure in third trimester pregnancy. J Emerg Med. 1997;15(6):815-820.
8. el-Mallakh RS, Liebowitz NR, Hale MS. Hyperemesis gravidarum as conversion disorder. J Nerv Ment Dis. 1990; 178(10):655-659.
9. Paulman PM, Sadat A. Pseudocyesis. J Fam Pract. 1990;30(5):575-576.
10. Dennis CL, Chung-Lee L. Postpartum depression help-seeking barriers and maternal treatment p: a qualitative systematic review. Birth. 2006;33(4):323-331.
11. Byatt N, Simas TA, Lundquist RS, et al. Strategies for improving perinatal depression treatment in North American outpatient obstetric settings. J Psychosom Obstetr Gynaecol. 2012;33(4):143-161.
12. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
13. Feinstein A. Conversion disorder: advances in our understanding. CMAJ. 2011;183(8):915-920.
14. Nicholson TR, Stone J, Kanaan RA. Conversion disorder: a problematic diagnosis. J Neurol Neurosurg Psychiatry. 2011;82(11):1267-1273.
15. Bitzer J. Somatization disorders in obstetrics and gynecology. Arch Womens Mental health, 2003;6(2):99-107.
16. Smith HE, Rynning RE, Okafor C, et al. Evaluation of neurologic deficit without apparent cause: the importance of a multidisciplinary approach. J Spinal Cord Med. 2007;30(5):509-517.
17. Hinson VK, Haren WB. Psychogenic movement disorders. Lancet Neurol. 2006;5(8):695-700.
18. Oyama O, Paltoo C, Greengold J. Somatoform disorders. Am Fam Physician. 2007;76(9):1333-1338.
19. Ness D. Physical therapy management for conversion disorder: case series. J Neurol Phys Ther. 2007;31(1):30-39.
CASE Difficulty walking
Ms. M, age 15, is a pregnant, Spanish-speaking Guatemalan woman who is brought to obstetrics triage in a large academic medical center at 35 weeks gestational age. She complains of dizziness, tinnitus, left orbital headache, and difficulty walking.
The neurology service finds profound truncal ataxia, astasia-abasia, and buckling of the knees; a normal brain and spine MRI are not consistent with a neurologic etiology. Otolaryngology service evaluates Ms. M to rule out a cholesteatoma and suggests a head CT and endoscopy, which are normal.
Ms. M’s symptoms resolve after 3 days, although the gait disturbances persist. When no clear cause is found for her difficulty walking, the psychiatry service is consulted to evaluate whether an underlying psychiatric disorder is contributing to symptoms.
What could be causing Ms. M’s symptoms?
a) malingering
b) factitious disorder
c) undiagnosed neurologic disorder
d) conversion disorder
The authors’ observations
Women are vulnerable to a variety of psychiatric illnesses during pregnancy1 that have deleterious effects on mother, baby, and family.2-6 Although there is a burgeoning literature on affective and anxiety disorders occurring in pregnancy, there is a dearth of information about somatoform disorders.
HISTORY Abandonment
Ms. M reports that, although her boyfriend deserted her after learning about the unexpected pregnancy, she will welcome the baby and looks forward to motherhood. She seems unaware of the responsibilities of being a mother.
Ms. M acknowledges a history of depression and self-harm a few years earlier, yet says she feels better now and thinks that psychiatric care is unnecessary. Because she does not endorse a history of trauma or symptoms suggesting an affective, anxiety, or psychotic illness, the psychiatrist does not recommend treatment with psychotropic medication.
At age 5, Ms. M’s parents sent her to the United States with her aunt, hoping that she would have a better life than she would have had in Guatemala. Her aunt reports that Ms. M initially had difficulty adjusting to life in the United States without her parents, yet she has made substantial strides over the years and is now quite accustomed to the country. Her aunt describes Ms. M as an independent high school student who earns good grades.
During the interview, the psychiatrist observes that Ms. M exhibits childlike mannerisms, including sleeping with stuffed toys and coloring in Disney books with crayons. She also is indifferent to her gait difficulty, pregnancy, and psychosocial stressors. Her affect is inconsistent with the content of her speech and she is alexithymic.
Ms. M’s aunt reports that her niece is becoming more dependent on her, which is not consistent with her baseline. Her aunt also notes that several years earlier, Ms. M’s nephew was diagnosed with a cholesteatoma after he presented with similar symptoms.
The combination of (1) Ms. M’s clinical presentation, which was causing her significant impairment in her social functioning, (2) the incompatibility of symptoms with any recognized neurologic and medical disease, and (3) prior family experience with cholesteatoma leads the consulting psychiatrist to suspect conversion disorder. Ms. M’s alexithymia, indifference to her symptoms, and recent abandonment by the baby’s father also support a conversion disorder diagnosis.
From a psychodynamic perspective, the ataxia appears to be her way of protecting herself from the abandonment she is experiencing by being left again to “stand alone” by her boyfriend as she had been when her parents sent her to the United States. Her regressive behavior could be her way of securing her aunt’s love and support.
The authors’ observations
This is the first case of psychogenic gait disturbance during pregnancy described in the literature. Authors have reported on pseudotoxemia,7 hyperemesis gravidarum,8 and pesudocyesis,9 yet there is a paucity of information on psychogenic gait disturbance during pregnancy. Ms. M’s case elucidates many of the clinical quandaries that occur when managing psychiatric illness—and, more specifically, conversion disorder— during pregnancy. Many women are hesitant to seek psychiatric treatment during pregnancy because of shame, stigma, and fear of loss of personal or parental rights10,11; it is not surprising that emotionally distressed women communicate their feelings or troubled thoughts through physical symptoms.
Likely diagnosis
Conversion disorder is the presence of neurologic symptoms in the absence of a neurologic diagnosis that fully explains those symptoms. Conversion disorder, previously known as hysteria, is called functional neurologic symptom disorder in DSM-5 (Box).12 Symptoms are not feigned; instead, they represent “conversion” of emotional distress into neurologic symptoms.13,14 Although misdiagnosing conversion disorder in patients with true neurologic disease is uncommon, clinicians often are uncomfortable making the diagnosis until all medical causes have been ruled out.14 It is not always possible to find a psychological explanation for conversion disorder, but a history of childhood abuse, particularly sexual abuse, could play a role.14
Because of the variety of presentations, clinicians in all specialties should be familiar with somatoform disorders; this is especially important in obstetrics and gynecology because women are more likely than men to develop these disorders.15 It is important to consider that Ms. M is a teenager and somatoform disorders can present differently in adults. The diagnostic process should include a diligent somatic workup and a personal and social history to identify the patient’s developmental tasks, stressors, and coping style.15
How would you treat Ms. M?
a) destigmatize psychiatric illness and provide psychoeducation regarding treatment benefits
b) identify and treat any comorbid psychiatric disorders
c) maintain a proactive and multidisciplinary approach that includes assessment of psychosocial stressors and psychodynamic factors, particularly those related to the pregnancy
d) all of the above
TREATMENT Close follow-up
The psychiatrist recommends continued close psychiatric follow-up as well as multidisciplinary involvement, including physical therapy, neurology, and obstetrics.
Ms. M initially is resistant to psychiatric follow-up because she says that “people on the street” told her that, if she saw a psychiatrist, her baby would be taken away. After the psychiatrist explains that it is unlikely her baby would be taken away, Ms. M immediately appears relieved, smiles, and readily agrees to outpatient psychotherapy.
Over the next 24 hours, she continues to work with a physical therapist and her gait significantly improves. She is discharged home 2 days later with a walking aid (Zimmer frame) for assistance.
Four days later, however, Ms. M is readmitted with worsening ataxia. Her aunt reports that, at home, Ms. M’s regressed behaviors are worsening; she is sleeping in bed with her and had several episodes of enuresis at home.
Ms. M continues to deny psychiatric symptoms or anxiety about the delivery. Although she shows some improvement when working with physical therapists, they note that Ms. M is still unable to ambulate or stand on her own. The psychiatrist is increasingly concerned about her regressed behavior and continued ataxia.
A family meeting is held and the psychiatrist and social worker educate Ms. M and her aunt about conversion disorder, including how some emotionally distressed women communicate their feelings or troubled thoughts through physical symptoms and how that may apply to Ms. M. During the meeting, the team also destigmatizes psychiatric illness and treatment and provides psychoeducation regarding its benefits. The psychiatrist and social worker also provide a psychodynamic interpretation that her ataxia could be a way of protecting herself against the abandonment she is experiencing by being left to “stand alone” by her boyfriend— as she had been when her parents sent her to the United States, and that her behavior could be her way of securing her aunt’s love and support.Ms. M and her aunt both readily agree with this interpretation. The aunt notes that her niece is more anxious about motherhood than she acknowledges and is concerned that Ms. M expects her to be the primary caregiver for the baby. Those present note that Ms. M is becoming increasingly dependent on her aunt, and that it is important for her to retain her independence, especially once she becomes a mother.
Ms. M immediately begins to display more affect; she smiles and reports feeling relieved. Similar to the previous admission, her gait significantly improves over the next 2 days and she is discharged home with a walking aid.
The authors’ observations
A broad differential diagnosis and early multidisciplinary involvement might facilitate earlier diagnosis and treatment.16 Assessment of psychosocial stressors in the patient’s personal and family life, including circumstances around the pregnancy and the meaning of motherhood, as well as investigation of what the patient may gain from the sick role, are paramount. In Ms. M’s case, cultural background, separation from her parents at a young age, and recent abandonment by her boyfriend have contributed to her inability to “stand alone,” which manifested as ataxia. Young age, regressed behavior, and her minimization of stressors also point to her difficulty acknowledging and coping with psychosocial stressors.
Successful delivery of the diagnosis is key to treatment success. After building a therapeutic alliance, a multidisciplinary discussion should take place that allows the patient to understand the diagnosis and treatment plan.17,18 The patient and family should be reassured that the fetus is healthy and all organic causes of symptoms have been investigated.17 Although management of conversion disorder during pregnancy is similar to that in non-pregnant women, several additional avenues of investigation should be considered:
• Explore the psychodynamic basis of the disorder and the role of the pregnancy and motherhood.
• Identify any comorbid psychiatric disorders, particularly those specific to pregnancy or the postpartum period.
• Because of the shame and stigma associated with seeking psychiatric treatment during pregnancy,10,11 it is imperative to destigmatize treatment and provide psychoeducation regarding its benefits.
A treatment plan can then be developed that involves psychotherapy, psychoeducation, stress management, and, when appropriate, pharmacotherapy.17
Providing psychoeducation about postpartum depression and other perinatal psychiatric illness could be beneficial. Physical therapy often is culturally acceptable and can help re-establish healthy patterns of motor function.19 Ms. M’s gait showed some improvement with physical therapy as part of the multidisciplinary approach, which also should include a thorough medical workup. Appropriate psychiatric treatment can help patients give up the sick role and return to their previous level of functioning.17
Maintain close communication with the outpatient perinatal care team as they monitor the patient’s parenting capacity. The outpatient perinatal care team also should engage pregnant or postpartum women in prioritizing their emotional well-being and encourage outpatient mental health treatment. Despite a dearth of data on the regressive symptoms and prognosis for future pregnancies, it is important to monitor maternal capacity and discuss the possibility of symptom recurrence.
OUTCOME Healthy baby
Three days later, Ms. M returns in labor with improved gait yet still using a walking aid. She has a normal vaginal delivery of a healthy baby boy at 37 weeks’ gestational age.
After the birth, Ms. M reports feeling well and enjoying motherhood, and denies psychiatric symptoms. She is ambulating without assistance within hours of delivery. This spontaneous resolution of symptoms could have been because of the psychodynamically oriented multidisciplinary approach to her care, which may have helped her realize that she did not have to “stand alone” as she embarked on motherhood.
Before being discharged home, Ms. M and her aunt meet with the inpatient obstetric social worker to assess Ms. M’s ability to care for the baby and discuss the importance of continued emotional support. The social worker does not contact the Department of Children and Families because Ms. M is walking independently and not endorsing or exhibiting regressive behaviors. Ms. M also reports that she will ask her aunt to take care of the baby should ataxia recur. Her aunt reassures the social workers that she will encourage Ms. M to attend outpatient psychotherapy and will contact the social worker if she becomes concerned about Ms. M’s or the baby’s well-being.
During her postpartum obstetric visit, Ms. M is walking independently and does not exhibit or endorse neurologic symptoms. The social worker provides psychoeducation about the importance of outpatient psychotherapy and schedules an initial appointment; Ms. M does not attend outpatient psychotherapy after discharge.
Bottom Line
Consider conversion disorder in obstetric patients who present with ataxia without a neurologic cause. Management involves a proactive and multidisciplinary approach that includes a thorough medical workup and assessment of psychosocial stressors and psychodynamic factors, particularly those related to the pregnancy. Early identification and delivery of the diagnosis, destigmatization, and provision of appropriate psychiatric treatment can facilitate treatment success.
Disclosures
Dr. Byatt has received grant funding/support for this project from the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant KL2TR000160. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Dr. Toor reports no financial relationships with any company whose products are mentioned in this article or manufacturers of competing products.
CASE Difficulty walking
Ms. M, age 15, is a pregnant, Spanish-speaking Guatemalan woman who is brought to obstetrics triage in a large academic medical center at 35 weeks gestational age. She complains of dizziness, tinnitus, left orbital headache, and difficulty walking.
The neurology service finds profound truncal ataxia, astasia-abasia, and buckling of the knees; a normal brain and spine MRI are not consistent with a neurologic etiology. Otolaryngology service evaluates Ms. M to rule out a cholesteatoma and suggests a head CT and endoscopy, which are normal.
Ms. M’s symptoms resolve after 3 days, although the gait disturbances persist. When no clear cause is found for her difficulty walking, the psychiatry service is consulted to evaluate whether an underlying psychiatric disorder is contributing to symptoms.
What could be causing Ms. M’s symptoms?
a) malingering
b) factitious disorder
c) undiagnosed neurologic disorder
d) conversion disorder
The authors’ observations
Women are vulnerable to a variety of psychiatric illnesses during pregnancy1 that have deleterious effects on mother, baby, and family.2-6 Although there is a burgeoning literature on affective and anxiety disorders occurring in pregnancy, there is a dearth of information about somatoform disorders.
HISTORY Abandonment
Ms. M reports that, although her boyfriend deserted her after learning about the unexpected pregnancy, she will welcome the baby and looks forward to motherhood. She seems unaware of the responsibilities of being a mother.
Ms. M acknowledges a history of depression and self-harm a few years earlier, yet says she feels better now and thinks that psychiatric care is unnecessary. Because she does not endorse a history of trauma or symptoms suggesting an affective, anxiety, or psychotic illness, the psychiatrist does not recommend treatment with psychotropic medication.
At age 5, Ms. M’s parents sent her to the United States with her aunt, hoping that she would have a better life than she would have had in Guatemala. Her aunt reports that Ms. M initially had difficulty adjusting to life in the United States without her parents, yet she has made substantial strides over the years and is now quite accustomed to the country. Her aunt describes Ms. M as an independent high school student who earns good grades.
During the interview, the psychiatrist observes that Ms. M exhibits childlike mannerisms, including sleeping with stuffed toys and coloring in Disney books with crayons. She also is indifferent to her gait difficulty, pregnancy, and psychosocial stressors. Her affect is inconsistent with the content of her speech and she is alexithymic.
Ms. M’s aunt reports that her niece is becoming more dependent on her, which is not consistent with her baseline. Her aunt also notes that several years earlier, Ms. M’s nephew was diagnosed with a cholesteatoma after he presented with similar symptoms.
The combination of (1) Ms. M’s clinical presentation, which was causing her significant impairment in her social functioning, (2) the incompatibility of symptoms with any recognized neurologic and medical disease, and (3) prior family experience with cholesteatoma leads the consulting psychiatrist to suspect conversion disorder. Ms. M’s alexithymia, indifference to her symptoms, and recent abandonment by the baby’s father also support a conversion disorder diagnosis.
From a psychodynamic perspective, the ataxia appears to be her way of protecting herself from the abandonment she is experiencing by being left again to “stand alone” by her boyfriend as she had been when her parents sent her to the United States. Her regressive behavior could be her way of securing her aunt’s love and support.
The authors’ observations
This is the first case of psychogenic gait disturbance during pregnancy described in the literature. Authors have reported on pseudotoxemia,7 hyperemesis gravidarum,8 and pesudocyesis,9 yet there is a paucity of information on psychogenic gait disturbance during pregnancy. Ms. M’s case elucidates many of the clinical quandaries that occur when managing psychiatric illness—and, more specifically, conversion disorder— during pregnancy. Many women are hesitant to seek psychiatric treatment during pregnancy because of shame, stigma, and fear of loss of personal or parental rights10,11; it is not surprising that emotionally distressed women communicate their feelings or troubled thoughts through physical symptoms.
Likely diagnosis
Conversion disorder is the presence of neurologic symptoms in the absence of a neurologic diagnosis that fully explains those symptoms. Conversion disorder, previously known as hysteria, is called functional neurologic symptom disorder in DSM-5 (Box).12 Symptoms are not feigned; instead, they represent “conversion” of emotional distress into neurologic symptoms.13,14 Although misdiagnosing conversion disorder in patients with true neurologic disease is uncommon, clinicians often are uncomfortable making the diagnosis until all medical causes have been ruled out.14 It is not always possible to find a psychological explanation for conversion disorder, but a history of childhood abuse, particularly sexual abuse, could play a role.14
Because of the variety of presentations, clinicians in all specialties should be familiar with somatoform disorders; this is especially important in obstetrics and gynecology because women are more likely than men to develop these disorders.15 It is important to consider that Ms. M is a teenager and somatoform disorders can present differently in adults. The diagnostic process should include a diligent somatic workup and a personal and social history to identify the patient’s developmental tasks, stressors, and coping style.15
How would you treat Ms. M?
a) destigmatize psychiatric illness and provide psychoeducation regarding treatment benefits
b) identify and treat any comorbid psychiatric disorders
c) maintain a proactive and multidisciplinary approach that includes assessment of psychosocial stressors and psychodynamic factors, particularly those related to the pregnancy
d) all of the above
TREATMENT Close follow-up
The psychiatrist recommends continued close psychiatric follow-up as well as multidisciplinary involvement, including physical therapy, neurology, and obstetrics.
Ms. M initially is resistant to psychiatric follow-up because she says that “people on the street” told her that, if she saw a psychiatrist, her baby would be taken away. After the psychiatrist explains that it is unlikely her baby would be taken away, Ms. M immediately appears relieved, smiles, and readily agrees to outpatient psychotherapy.
Over the next 24 hours, she continues to work with a physical therapist and her gait significantly improves. She is discharged home 2 days later with a walking aid (Zimmer frame) for assistance.
Four days later, however, Ms. M is readmitted with worsening ataxia. Her aunt reports that, at home, Ms. M’s regressed behaviors are worsening; she is sleeping in bed with her and had several episodes of enuresis at home.
Ms. M continues to deny psychiatric symptoms or anxiety about the delivery. Although she shows some improvement when working with physical therapists, they note that Ms. M is still unable to ambulate or stand on her own. The psychiatrist is increasingly concerned about her regressed behavior and continued ataxia.
A family meeting is held and the psychiatrist and social worker educate Ms. M and her aunt about conversion disorder, including how some emotionally distressed women communicate their feelings or troubled thoughts through physical symptoms and how that may apply to Ms. M. During the meeting, the team also destigmatizes psychiatric illness and treatment and provides psychoeducation regarding its benefits. The psychiatrist and social worker also provide a psychodynamic interpretation that her ataxia could be a way of protecting herself against the abandonment she is experiencing by being left to “stand alone” by her boyfriend— as she had been when her parents sent her to the United States, and that her behavior could be her way of securing her aunt’s love and support.Ms. M and her aunt both readily agree with this interpretation. The aunt notes that her niece is more anxious about motherhood than she acknowledges and is concerned that Ms. M expects her to be the primary caregiver for the baby. Those present note that Ms. M is becoming increasingly dependent on her aunt, and that it is important for her to retain her independence, especially once she becomes a mother.
Ms. M immediately begins to display more affect; she smiles and reports feeling relieved. Similar to the previous admission, her gait significantly improves over the next 2 days and she is discharged home with a walking aid.
The authors’ observations
A broad differential diagnosis and early multidisciplinary involvement might facilitate earlier diagnosis and treatment.16 Assessment of psychosocial stressors in the patient’s personal and family life, including circumstances around the pregnancy and the meaning of motherhood, as well as investigation of what the patient may gain from the sick role, are paramount. In Ms. M’s case, cultural background, separation from her parents at a young age, and recent abandonment by her boyfriend have contributed to her inability to “stand alone,” which manifested as ataxia. Young age, regressed behavior, and her minimization of stressors also point to her difficulty acknowledging and coping with psychosocial stressors.
Successful delivery of the diagnosis is key to treatment success. After building a therapeutic alliance, a multidisciplinary discussion should take place that allows the patient to understand the diagnosis and treatment plan.17,18 The patient and family should be reassured that the fetus is healthy and all organic causes of symptoms have been investigated.17 Although management of conversion disorder during pregnancy is similar to that in non-pregnant women, several additional avenues of investigation should be considered:
• Explore the psychodynamic basis of the disorder and the role of the pregnancy and motherhood.
• Identify any comorbid psychiatric disorders, particularly those specific to pregnancy or the postpartum period.
• Because of the shame and stigma associated with seeking psychiatric treatment during pregnancy,10,11 it is imperative to destigmatize treatment and provide psychoeducation regarding its benefits.
A treatment plan can then be developed that involves psychotherapy, psychoeducation, stress management, and, when appropriate, pharmacotherapy.17
Providing psychoeducation about postpartum depression and other perinatal psychiatric illness could be beneficial. Physical therapy often is culturally acceptable and can help re-establish healthy patterns of motor function.19 Ms. M’s gait showed some improvement with physical therapy as part of the multidisciplinary approach, which also should include a thorough medical workup. Appropriate psychiatric treatment can help patients give up the sick role and return to their previous level of functioning.17
Maintain close communication with the outpatient perinatal care team as they monitor the patient’s parenting capacity. The outpatient perinatal care team also should engage pregnant or postpartum women in prioritizing their emotional well-being and encourage outpatient mental health treatment. Despite a dearth of data on the regressive symptoms and prognosis for future pregnancies, it is important to monitor maternal capacity and discuss the possibility of symptom recurrence.
OUTCOME Healthy baby
Three days later, Ms. M returns in labor with improved gait yet still using a walking aid. She has a normal vaginal delivery of a healthy baby boy at 37 weeks’ gestational age.
After the birth, Ms. M reports feeling well and enjoying motherhood, and denies psychiatric symptoms. She is ambulating without assistance within hours of delivery. This spontaneous resolution of symptoms could have been because of the psychodynamically oriented multidisciplinary approach to her care, which may have helped her realize that she did not have to “stand alone” as she embarked on motherhood.
Before being discharged home, Ms. M and her aunt meet with the inpatient obstetric social worker to assess Ms. M’s ability to care for the baby and discuss the importance of continued emotional support. The social worker does not contact the Department of Children and Families because Ms. M is walking independently and not endorsing or exhibiting regressive behaviors. Ms. M also reports that she will ask her aunt to take care of the baby should ataxia recur. Her aunt reassures the social workers that she will encourage Ms. M to attend outpatient psychotherapy and will contact the social worker if she becomes concerned about Ms. M’s or the baby’s well-being.
During her postpartum obstetric visit, Ms. M is walking independently and does not exhibit or endorse neurologic symptoms. The social worker provides psychoeducation about the importance of outpatient psychotherapy and schedules an initial appointment; Ms. M does not attend outpatient psychotherapy after discharge.
Bottom Line
Consider conversion disorder in obstetric patients who present with ataxia without a neurologic cause. Management involves a proactive and multidisciplinary approach that includes a thorough medical workup and assessment of psychosocial stressors and psychodynamic factors, particularly those related to the pregnancy. Early identification and delivery of the diagnosis, destigmatization, and provision of appropriate psychiatric treatment can facilitate treatment success.
Disclosures
Dr. Byatt has received grant funding/support for this project from the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant KL2TR000160. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Dr. Toor reports no financial relationships with any company whose products are mentioned in this article or manufacturers of competing products.
1. Vesga-Lopez O, Blanco C, Keyes K, et al. Psychiatric disorders in pregnant and postpartum women in the United States. Arch Gen Psychiatry. 2008;65(7):805-815.
2. Britton HL, Gronwaldt V, Britton JR. Maternal postpartum behaviors and mother-infant relationship during the first year of life. J Pediatr. 2001;138(6):905-909.
3. Deave T, Heron J, Evans J, et al. The impact of maternal depression in pregnancy on early child development. BJOG. 2008;115(8):1043-1051.
4. Paulson JF, Keefe HA, Leiferman JA. Early parental depression and child language development. J Child Psychol Psychiatry. 2009;50(3):254-262.
5. Zuckerman B, Amaro H, Bauchner H, et al. Depressive symptoms during pregnancy: relationship to poor health behaviors. Am J Obstet Gynecol. 1989;160(5 pt 1):1107-1111.
6. Forman DR, O’Hara MW, Stuart S, et al. Effective treatment for postpartum depression is not sufficient to improve the developing mother-child relationship. Dev Psychopathol. 2007;19(2):585-602.
7. Brady WJ Jr, Huff JS. Pseudotoxemia: new onset psychogenic seizure in third trimester pregnancy. J Emerg Med. 1997;15(6):815-820.
8. el-Mallakh RS, Liebowitz NR, Hale MS. Hyperemesis gravidarum as conversion disorder. J Nerv Ment Dis. 1990; 178(10):655-659.
9. Paulman PM, Sadat A. Pseudocyesis. J Fam Pract. 1990;30(5):575-576.
10. Dennis CL, Chung-Lee L. Postpartum depression help-seeking barriers and maternal treatment p: a qualitative systematic review. Birth. 2006;33(4):323-331.
11. Byatt N, Simas TA, Lundquist RS, et al. Strategies for improving perinatal depression treatment in North American outpatient obstetric settings. J Psychosom Obstetr Gynaecol. 2012;33(4):143-161.
12. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
13. Feinstein A. Conversion disorder: advances in our understanding. CMAJ. 2011;183(8):915-920.
14. Nicholson TR, Stone J, Kanaan RA. Conversion disorder: a problematic diagnosis. J Neurol Neurosurg Psychiatry. 2011;82(11):1267-1273.
15. Bitzer J. Somatization disorders in obstetrics and gynecology. Arch Womens Mental health, 2003;6(2):99-107.
16. Smith HE, Rynning RE, Okafor C, et al. Evaluation of neurologic deficit without apparent cause: the importance of a multidisciplinary approach. J Spinal Cord Med. 2007;30(5):509-517.
17. Hinson VK, Haren WB. Psychogenic movement disorders. Lancet Neurol. 2006;5(8):695-700.
18. Oyama O, Paltoo C, Greengold J. Somatoform disorders. Am Fam Physician. 2007;76(9):1333-1338.
19. Ness D. Physical therapy management for conversion disorder: case series. J Neurol Phys Ther. 2007;31(1):30-39.
1. Vesga-Lopez O, Blanco C, Keyes K, et al. Psychiatric disorders in pregnant and postpartum women in the United States. Arch Gen Psychiatry. 2008;65(7):805-815.
2. Britton HL, Gronwaldt V, Britton JR. Maternal postpartum behaviors and mother-infant relationship during the first year of life. J Pediatr. 2001;138(6):905-909.
3. Deave T, Heron J, Evans J, et al. The impact of maternal depression in pregnancy on early child development. BJOG. 2008;115(8):1043-1051.
4. Paulson JF, Keefe HA, Leiferman JA. Early parental depression and child language development. J Child Psychol Psychiatry. 2009;50(3):254-262.
5. Zuckerman B, Amaro H, Bauchner H, et al. Depressive symptoms during pregnancy: relationship to poor health behaviors. Am J Obstet Gynecol. 1989;160(5 pt 1):1107-1111.
6. Forman DR, O’Hara MW, Stuart S, et al. Effective treatment for postpartum depression is not sufficient to improve the developing mother-child relationship. Dev Psychopathol. 2007;19(2):585-602.
7. Brady WJ Jr, Huff JS. Pseudotoxemia: new onset psychogenic seizure in third trimester pregnancy. J Emerg Med. 1997;15(6):815-820.
8. el-Mallakh RS, Liebowitz NR, Hale MS. Hyperemesis gravidarum as conversion disorder. J Nerv Ment Dis. 1990; 178(10):655-659.
9. Paulman PM, Sadat A. Pseudocyesis. J Fam Pract. 1990;30(5):575-576.
10. Dennis CL, Chung-Lee L. Postpartum depression help-seeking barriers and maternal treatment p: a qualitative systematic review. Birth. 2006;33(4):323-331.
11. Byatt N, Simas TA, Lundquist RS, et al. Strategies for improving perinatal depression treatment in North American outpatient obstetric settings. J Psychosom Obstetr Gynaecol. 2012;33(4):143-161.
12. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
13. Feinstein A. Conversion disorder: advances in our understanding. CMAJ. 2011;183(8):915-920.
14. Nicholson TR, Stone J, Kanaan RA. Conversion disorder: a problematic diagnosis. J Neurol Neurosurg Psychiatry. 2011;82(11):1267-1273.
15. Bitzer J. Somatization disorders in obstetrics and gynecology. Arch Womens Mental health, 2003;6(2):99-107.
16. Smith HE, Rynning RE, Okafor C, et al. Evaluation of neurologic deficit without apparent cause: the importance of a multidisciplinary approach. J Spinal Cord Med. 2007;30(5):509-517.
17. Hinson VK, Haren WB. Psychogenic movement disorders. Lancet Neurol. 2006;5(8):695-700.
18. Oyama O, Paltoo C, Greengold J. Somatoform disorders. Am Fam Physician. 2007;76(9):1333-1338.
19. Ness D. Physical therapy management for conversion disorder: case series. J Neurol Phys Ther. 2007;31(1):30-39.
Have you RULED O2uT medical illness in the presumptive psychiatric patient?
What a practitioner might identify and report as “psychiatric” symptoms or signs cannot always be explained in terms of psychological stress or a psychiatric disorder. In fact, a range of medical1 and neurologic2 illnesses can manifest in ways that appear psychiatric in nature. Common examples are sleep and thyroid disorders; deficiencies of vitamin D, folate, and B12; Parkinson’s disease; and anti-N-methyl-d-aspartate receptor autoimmune encephalitis.
People who have a medical illness with what appear to be psychiatric manifestations often elude identification and diagnosis because they do not visit a health care provider for any of several reasons, including difficulty obtaining health insurance. Instead, they might seek care in an emergency room (ER).
When such patients present for evaluation, it is easy—especially in a fast-paced ER—to miss the underlying cause of their illness. Some are then treated on the assumption that their diagnosis is psychiatric, while their medical illness goes unidentified.3
We propose a mnemonic, RULED O2uT, as a reminder in the ER (and any other setting) of the need to rule out physical illness before treating a patient for a psychiatric disorder. To demonstrate how the work-up of a patient whose medical illness was obscured by psychiatric signs and symptoms could benefit from applying RULED O2uT, we also present a case.
CASE REPORT
A man with a medical illness who presented with psychiatric symptoms
Mr. Z, in his late 40s, is brought to the ER by his sister for evaluation of depressed mood of 6 to 8 months’ duration. He has no psychiatric history.
On evaluation, Mr. Z does not remember an event or stressors that could have triggered depression. He describes complete loss of motivation for activities of daily living, such as personal grooming. He has stopped leaving the house and meeting friends and family members.
Mr. Z’s sister is concerned for his well-being because he has been living without heat and electricity, which were disconnected for nonpayment. Mr. Z reveals that he has not seen his primary care physician “for 20 or 25 years,” although he recently sought care in the ER of another hospital because of mild gait instability for several months.
Mr. Z has a blunted affect, with linear and goal-directed thought processes. He denies suicidal ideation. Laboratory testing, including a comprehensive metabolic panel, complete blood count, and urine toxicology and urinalysis, are negative.
A non-contrast CT scan of the head reveals foci of low attenuation in the left frontal corona radiata. Follow-up MRI of the brain, with and without contrast, shows extensive supratentorial and infratentorial demyelinating lesions consistent with multiple sclerosis (MS). Several cerebral lesions in the white matter are consistent with active demyelination.
Mr. Z is admitted to the neurology service and started on methylprednisolone for MS. The psychiatry consultation-liaison team prescribes sertraline, 50 mg titrated to 100 mg, for depression.
Detailed history means better overall evaluation
Mr. Z presented to the busy ER with psychiatric symptoms. It was easy to make a diagnosis of depression and refer him to the outpatient psychiatrist. However, a detailed history provided pertinent information about Mr. Z such as no regular medical check-ups, no family history of mental illness, and gait disturbance in absence of physical injury. This enabled the physicians to conduct a thorough evaluation including a neurologic examination, laboratory tests, and imaging of the brain.
The 8 components of RULED O2uT
Rx interactions. Review medications that the patient is taking or recently stopped taking, to rule out drug−drug interactions and adverse effects.
Unusual presentation. Be mindful of any unusual presentation. For example, sudden onset of psychiatric symptoms with seizures or hypersensitivity to the sun with depression or psychosis.
Labs. Obtain appropriate blood work, including:
• comprehensive metabolic panel
• complete blood count
• thyroid-stimulating hormone (myxedema, thyrotoxicosis)
• delta-aminolevulinic acid and porphobilinogen (acute intermittent porphyria)
• antinuclear antibody (systemic lupus erythematosus)
• B12 level
• fluorescent treponemal antibody absorption test (neurosyphilis)
• serum ceruloplasmin and copper (Wilson’s disease).
Examination. Perform a thorough examination, including a proper neurological exam. This is especially important when you see signs, or the patient reports symptoms, that cannot be explained by depression alone. An abnormality or change in gait, for example, might be a consequence of injury or a manifestation of MS, stroke, or Parkinson’s disease. Additional testing, such as CT of the head or lumbar puncture, might be appropriate to supplement or clarify findings of the exam. Mr. Z’s neurologic exam revealed weakness in his left leg with variability in reflexes.
Drugs. Ensure that a patient presenting with new-onset psychosis is not taking dopaminergic medications or steroids and, based on results of a toxicology screen, is not under the influence of stimulants or hallucinogens.
Onset and Office. Determine:
• the time since onset of symptoms; this is crucial to differentiate psychiatric disorders and ruling out a nonpsychiatric cause of the patient’s presentation
• if the patient gets a regular medical check-up with her (his) primary care physician.
Thorough history. Obtain a thorough history so that you have a clear picture of the patient’s current situation; this includes medical history and family history of neurologic and psychiatric disorders and substance abuse.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Rahul R, Pieters T. An unusual psychiatric presentation of polycythaemia ‘Difficulties lie in our habits of thought rather than in the nature of things’ Andre Tardieu. BMJ Case Rep. 2013. pii: bcr2012008215. doi: 10.1136/bcr-2012-008215.
2. Butler C, Zeman AZ. Neurological syndromes which can be mistaken for psychiatric conditions. J Neurol Neurosurg Psychiatry. 2005;76(suppl 1):i31-i38.
3. Roie EV, Labarque V, Renard M, et al. Obsessive-compulsive behavior as presenting symptom of primary antiphospholipid syndrome. Psychosom Med. 2013;75(3):326-330.
What a practitioner might identify and report as “psychiatric” symptoms or signs cannot always be explained in terms of psychological stress or a psychiatric disorder. In fact, a range of medical1 and neurologic2 illnesses can manifest in ways that appear psychiatric in nature. Common examples are sleep and thyroid disorders; deficiencies of vitamin D, folate, and B12; Parkinson’s disease; and anti-N-methyl-d-aspartate receptor autoimmune encephalitis.
People who have a medical illness with what appear to be psychiatric manifestations often elude identification and diagnosis because they do not visit a health care provider for any of several reasons, including difficulty obtaining health insurance. Instead, they might seek care in an emergency room (ER).
When such patients present for evaluation, it is easy—especially in a fast-paced ER—to miss the underlying cause of their illness. Some are then treated on the assumption that their diagnosis is psychiatric, while their medical illness goes unidentified.3
We propose a mnemonic, RULED O2uT, as a reminder in the ER (and any other setting) of the need to rule out physical illness before treating a patient for a psychiatric disorder. To demonstrate how the work-up of a patient whose medical illness was obscured by psychiatric signs and symptoms could benefit from applying RULED O2uT, we also present a case.
CASE REPORT
A man with a medical illness who presented with psychiatric symptoms
Mr. Z, in his late 40s, is brought to the ER by his sister for evaluation of depressed mood of 6 to 8 months’ duration. He has no psychiatric history.
On evaluation, Mr. Z does not remember an event or stressors that could have triggered depression. He describes complete loss of motivation for activities of daily living, such as personal grooming. He has stopped leaving the house and meeting friends and family members.
Mr. Z’s sister is concerned for his well-being because he has been living without heat and electricity, which were disconnected for nonpayment. Mr. Z reveals that he has not seen his primary care physician “for 20 or 25 years,” although he recently sought care in the ER of another hospital because of mild gait instability for several months.
Mr. Z has a blunted affect, with linear and goal-directed thought processes. He denies suicidal ideation. Laboratory testing, including a comprehensive metabolic panel, complete blood count, and urine toxicology and urinalysis, are negative.
A non-contrast CT scan of the head reveals foci of low attenuation in the left frontal corona radiata. Follow-up MRI of the brain, with and without contrast, shows extensive supratentorial and infratentorial demyelinating lesions consistent with multiple sclerosis (MS). Several cerebral lesions in the white matter are consistent with active demyelination.
Mr. Z is admitted to the neurology service and started on methylprednisolone for MS. The psychiatry consultation-liaison team prescribes sertraline, 50 mg titrated to 100 mg, for depression.
Detailed history means better overall evaluation
Mr. Z presented to the busy ER with psychiatric symptoms. It was easy to make a diagnosis of depression and refer him to the outpatient psychiatrist. However, a detailed history provided pertinent information about Mr. Z such as no regular medical check-ups, no family history of mental illness, and gait disturbance in absence of physical injury. This enabled the physicians to conduct a thorough evaluation including a neurologic examination, laboratory tests, and imaging of the brain.
The 8 components of RULED O2uT
Rx interactions. Review medications that the patient is taking or recently stopped taking, to rule out drug−drug interactions and adverse effects.
Unusual presentation. Be mindful of any unusual presentation. For example, sudden onset of psychiatric symptoms with seizures or hypersensitivity to the sun with depression or psychosis.
Labs. Obtain appropriate blood work, including:
• comprehensive metabolic panel
• complete blood count
• thyroid-stimulating hormone (myxedema, thyrotoxicosis)
• delta-aminolevulinic acid and porphobilinogen (acute intermittent porphyria)
• antinuclear antibody (systemic lupus erythematosus)
• B12 level
• fluorescent treponemal antibody absorption test (neurosyphilis)
• serum ceruloplasmin and copper (Wilson’s disease).
Examination. Perform a thorough examination, including a proper neurological exam. This is especially important when you see signs, or the patient reports symptoms, that cannot be explained by depression alone. An abnormality or change in gait, for example, might be a consequence of injury or a manifestation of MS, stroke, or Parkinson’s disease. Additional testing, such as CT of the head or lumbar puncture, might be appropriate to supplement or clarify findings of the exam. Mr. Z’s neurologic exam revealed weakness in his left leg with variability in reflexes.
Drugs. Ensure that a patient presenting with new-onset psychosis is not taking dopaminergic medications or steroids and, based on results of a toxicology screen, is not under the influence of stimulants or hallucinogens.
Onset and Office. Determine:
• the time since onset of symptoms; this is crucial to differentiate psychiatric disorders and ruling out a nonpsychiatric cause of the patient’s presentation
• if the patient gets a regular medical check-up with her (his) primary care physician.
Thorough history. Obtain a thorough history so that you have a clear picture of the patient’s current situation; this includes medical history and family history of neurologic and psychiatric disorders and substance abuse.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
What a practitioner might identify and report as “psychiatric” symptoms or signs cannot always be explained in terms of psychological stress or a psychiatric disorder. In fact, a range of medical1 and neurologic2 illnesses can manifest in ways that appear psychiatric in nature. Common examples are sleep and thyroid disorders; deficiencies of vitamin D, folate, and B12; Parkinson’s disease; and anti-N-methyl-d-aspartate receptor autoimmune encephalitis.
People who have a medical illness with what appear to be psychiatric manifestations often elude identification and diagnosis because they do not visit a health care provider for any of several reasons, including difficulty obtaining health insurance. Instead, they might seek care in an emergency room (ER).
When such patients present for evaluation, it is easy—especially in a fast-paced ER—to miss the underlying cause of their illness. Some are then treated on the assumption that their diagnosis is psychiatric, while their medical illness goes unidentified.3
We propose a mnemonic, RULED O2uT, as a reminder in the ER (and any other setting) of the need to rule out physical illness before treating a patient for a psychiatric disorder. To demonstrate how the work-up of a patient whose medical illness was obscured by psychiatric signs and symptoms could benefit from applying RULED O2uT, we also present a case.
CASE REPORT
A man with a medical illness who presented with psychiatric symptoms
Mr. Z, in his late 40s, is brought to the ER by his sister for evaluation of depressed mood of 6 to 8 months’ duration. He has no psychiatric history.
On evaluation, Mr. Z does not remember an event or stressors that could have triggered depression. He describes complete loss of motivation for activities of daily living, such as personal grooming. He has stopped leaving the house and meeting friends and family members.
Mr. Z’s sister is concerned for his well-being because he has been living without heat and electricity, which were disconnected for nonpayment. Mr. Z reveals that he has not seen his primary care physician “for 20 or 25 years,” although he recently sought care in the ER of another hospital because of mild gait instability for several months.
Mr. Z has a blunted affect, with linear and goal-directed thought processes. He denies suicidal ideation. Laboratory testing, including a comprehensive metabolic panel, complete blood count, and urine toxicology and urinalysis, are negative.
A non-contrast CT scan of the head reveals foci of low attenuation in the left frontal corona radiata. Follow-up MRI of the brain, with and without contrast, shows extensive supratentorial and infratentorial demyelinating lesions consistent with multiple sclerosis (MS). Several cerebral lesions in the white matter are consistent with active demyelination.
Mr. Z is admitted to the neurology service and started on methylprednisolone for MS. The psychiatry consultation-liaison team prescribes sertraline, 50 mg titrated to 100 mg, for depression.
Detailed history means better overall evaluation
Mr. Z presented to the busy ER with psychiatric symptoms. It was easy to make a diagnosis of depression and refer him to the outpatient psychiatrist. However, a detailed history provided pertinent information about Mr. Z such as no regular medical check-ups, no family history of mental illness, and gait disturbance in absence of physical injury. This enabled the physicians to conduct a thorough evaluation including a neurologic examination, laboratory tests, and imaging of the brain.
The 8 components of RULED O2uT
Rx interactions. Review medications that the patient is taking or recently stopped taking, to rule out drug−drug interactions and adverse effects.
Unusual presentation. Be mindful of any unusual presentation. For example, sudden onset of psychiatric symptoms with seizures or hypersensitivity to the sun with depression or psychosis.
Labs. Obtain appropriate blood work, including:
• comprehensive metabolic panel
• complete blood count
• thyroid-stimulating hormone (myxedema, thyrotoxicosis)
• delta-aminolevulinic acid and porphobilinogen (acute intermittent porphyria)
• antinuclear antibody (systemic lupus erythematosus)
• B12 level
• fluorescent treponemal antibody absorption test (neurosyphilis)
• serum ceruloplasmin and copper (Wilson’s disease).
Examination. Perform a thorough examination, including a proper neurological exam. This is especially important when you see signs, or the patient reports symptoms, that cannot be explained by depression alone. An abnormality or change in gait, for example, might be a consequence of injury or a manifestation of MS, stroke, or Parkinson’s disease. Additional testing, such as CT of the head or lumbar puncture, might be appropriate to supplement or clarify findings of the exam. Mr. Z’s neurologic exam revealed weakness in his left leg with variability in reflexes.
Drugs. Ensure that a patient presenting with new-onset psychosis is not taking dopaminergic medications or steroids and, based on results of a toxicology screen, is not under the influence of stimulants or hallucinogens.
Onset and Office. Determine:
• the time since onset of symptoms; this is crucial to differentiate psychiatric disorders and ruling out a nonpsychiatric cause of the patient’s presentation
• if the patient gets a regular medical check-up with her (his) primary care physician.
Thorough history. Obtain a thorough history so that you have a clear picture of the patient’s current situation; this includes medical history and family history of neurologic and psychiatric disorders and substance abuse.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Rahul R, Pieters T. An unusual psychiatric presentation of polycythaemia ‘Difficulties lie in our habits of thought rather than in the nature of things’ Andre Tardieu. BMJ Case Rep. 2013. pii: bcr2012008215. doi: 10.1136/bcr-2012-008215.
2. Butler C, Zeman AZ. Neurological syndromes which can be mistaken for psychiatric conditions. J Neurol Neurosurg Psychiatry. 2005;76(suppl 1):i31-i38.
3. Roie EV, Labarque V, Renard M, et al. Obsessive-compulsive behavior as presenting symptom of primary antiphospholipid syndrome. Psychosom Med. 2013;75(3):326-330.
1. Rahul R, Pieters T. An unusual psychiatric presentation of polycythaemia ‘Difficulties lie in our habits of thought rather than in the nature of things’ Andre Tardieu. BMJ Case Rep. 2013. pii: bcr2012008215. doi: 10.1136/bcr-2012-008215.
2. Butler C, Zeman AZ. Neurological syndromes which can be mistaken for psychiatric conditions. J Neurol Neurosurg Psychiatry. 2005;76(suppl 1):i31-i38.
3. Roie EV, Labarque V, Renard M, et al. Obsessive-compulsive behavior as presenting symptom of primary antiphospholipid syndrome. Psychosom Med. 2013;75(3):326-330.
Second of 2 parts: The mysteries of psychiatry maintenance of certification, further unraveled
To recap what I discussed in Part 1 of this article (December 2014): As part of a trend across all medical specialty boards, the American Board of Psychiatry and Neurology (ABPN) instituted a recertification process for all new general psychiatry certifications, starting October 1, 1994.1 In 2000, the specialties that comprise the American Board of Medical Specialties (ABMS) agreed to develop a comprehensive maintenance of certification (MOC) process to demonstrate ongoing learning and competency beyond what can be captured by a recertification examination. All ABMS member boards now use a 4-part process for recertification.
A great deal of professional and personal importance has been attached to maintaining one’s general and subspecialty certifications. To that end, the 2 parts of this article highlight current ABPN MOC requirements and provide resources for understanding, tracking, and completing the self-assessment (SA) and performance-in-practice (PIP) components.
In this installment, I examine 3 components of MOC:
• continuing medical education (CME), including SA requirements
• improvement in medical practice (PIP)
• continuous maintenance of certification (C-MOC)
In addition to this review, all physicians who are subject to MOC should download and read the 20-page revised MOC Program booklet v. 2.1 (May 2014).2
Continuing medical education
The CME requirement is clear: All diplomate physicians must accrue, on average, 30 Category-1 CME credits a year; the CME must be relevant to the specialty or subspecialty in which the diplomate practices.3 For physicians who hold >1 ABPN certificates, the total CME requirement is the same; CME credits can be applied across each specialty and subspecialty.
The May 2014 MOC revision states that, for physicians who certified or recertified between 2005 and 2011 and who applied for the 2015 examination in 2014, the required CME credit total is 270.2 For all subsequent years of certification or recertification, including 2012, diplomates are enrolled in C-MOC, which is described below.2
To even out the accrual of CME credits across the prior 10 years, ABPN mandates that, for diplomates who certified or recertified between 2005 and 2011, one hundred fifty of the CME credits be accrued in the 5 years before they apply for the examination. Diplomates in C-MOC should accrue, on average, 30 CME credits a year in each of the 3-year blocks (ie, 90 units in each block).2
Self-assessment
SA is a specific form of CME that is designed to provide comprehensive test-based feedback on knowledge acquired, to enhance the learning process.4 SA CME feedback must include:
• the correct answer to each test question
• recommended literature resources for each question
• performance compared to peers on each question.
Given the structured nature of SA activities, beginning January 1, 2014, one must use only ABPN-approved SA products (see Related Resources for a list of APBN-approved SA products).5
Table 1 and Table 2 outline SA requirements for, respectively, physicians who certified or recertified from 2005 through 2011, and those who certified or recertified in 2012 (and later). The SA requirement increases after 2011 to 24 credits in each 3-year block (8 credits a year, on average).2 Multiple SA activities can be used to fulfill the credit requirement of each 3-year block.
Note: Credits accrued by performing SA activities count toward the CME credit total.
Improvement in medical practice, or PIP
Physicians who are active clinically must complete PIP modules. Each module comprises peer or patient feedback plus a clinical aspect. The May 2014 MOC revision simplified the feedback process to mandate peer or patient feedback—but not both, as required previously.2 For the feedback PIP module, the physician selects 5 peers or patients to complete review forms, examines the results, and creates a plan of improvement. An exception to this “rule of 5” applies to diplomates who have a supervisor capable of evaluating all general competencies, defined below.
Related Resources provides a link to ABPN-created forms.
Within 24 months, but not sooner than 1 month, 5 peers or patients (or 1 applicable supervisor) are selected to complete review forms; changes in practice are noted. The same peers or patients might be selected for a second review. As noted in Table 1 and Table 2, the number of PIP modules is fewer for physicians who certified or recertified between 2005 and 2011; from 2012 onward, 1 PIP clinical module is required in each 3-year block.2
There are 6 ABPN-approved feedback module options, of which the diplomate must choose 1 in any given block2:
• 5 patient surveys
• 5 peer evaluations of general competenciesa
• 5 resident evaluations of general competenciesa
• 360° evaluation of general competencies,a with 5 respondents
• institutional peer review of general competencies,a with 5 respondents
• 1 supervisor evaluation of general competencies.a
aGeneral competencies include patient care; practice-based learning and improvement; professionalism; medical knowledge; interpersonal and communication skills; and system-based practices.
Although many institutions have a quality improvement (QI) program, that program must be approved by the Multi-Specialty MOC Portfolio Approval Program sponsored by ABMS for a clinician to receive credit for 1 PIP clinical module. If the approved QI program includes patient or peer feedback (eg, a survey), the diplo mate can receive credit for 1 PIP feedback module.2
For the clinical PIP module, the physician selects 5 charts for review and examines them based on criteria found in an ABPN-approved (starting in 2014) PIP product. (Related Resources provides a link to this list.) After reviewing the initial 5 charts, a plan for improvement is created. Within 24 months, but no sooner than 1 month, 5 charts are again selected and reviewed, and changes in practice are noted. The same charts can be selected for the second review.
As noted in Table 1 and Table 2, the number of PIP modules is fewer for those who initially certified or recertified between 2005 and 2011; from 2012 onward, 1 PIP clinical module is required in each 3-year block.2
The C-MOC process
Physicians who certified or recertified in 2012, or who will certify or recertify after that year, are enrolled automatically in C-MOC.6,7 The purpose of C-MOC is to keep diplomates on track to fulfill the higher level of SA requirements that began with this group; this is done by mandating use of the ABPN Physician Folios system. As shown in Table 2, there is no longer a 10-year cycle; instead, there are continuous 3-year stages, within which each diplomate must accrue 90 CME credits (on average, 30 credits a year), 24 SA credits (on average, 8 a year), 1 PIP clinical module, and 1 PIP feedback module.6,7
The first 3-year block of C-MOC requirements will be waived for physicians who complete Accreditation Council on Graduate Medical Education–accredited or ABPN-approved subspecialty training in 2012 or later—if they pass the corresponding ABPN subspecialty examination during the first 3-year block of enrollment in C-MOC.2 For diplomates enrolled in C-MOC, failure to track progress of each 3-year block, via the ABPN Physician Folios system, has significant consequences: Those who do not complete the first stage of the program by the end of 3 years will be listed on the ABPN Web site as “certified— not meeting MOC requirements.” Those who do not complete 2 stages by the end of 6 years will be listed as “not certified.”2
Cognitive exam still in place. The only remnant of the old 10-year cycle is the requirement to pass the cognitive examination every 10 years, although the exam can be taken earlier if the diplomate wishes. If all requirements are met and one does not sit for, or fails, the exam, the ABPN Web site will report the diplomate as “not meeting MOC requirements.” One can retake the exam within 1 year of the failed or missed exam, but a subsequent failure or missed exam will result in being listed as “not certified.”2
Fee structure. Instead of a single fee paid at the time of the exam(s), physicians in the C-MOC program pay an annual fee that covers participation in ABPN Physician Folios and 1 exam in a 10-year period. Fewer than 10 years of participation, or applying for a combined examination (for diplomates who hold multiple certifications), requires an additional fee.7
Bottom Line
Maintenance of certification (MOC) is manageable, although it requires you to be familiar with its various elements. Those elements include continuing medical education (CME requirements); the additional self-assessment component of CME; performance-in-practice modules; and continuous maintenance of certification. The MOC program booklet of the American Board of Psychiatry and Neurology provides all necessary details.
Disclosure
Dr. Meyer reports no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Faulkner LR, Tivnan PW, Winstead DK, et al. The ABPN Maintenance of Certification Program for psychiatrists: past history, current status, and future directions. Acad Psychiatry. 2008;32(3):241-248.
2. Maintenance of Certification Program. American Board of Psychiatry and Neurology Inc. http://www.abpn.com/ downloads/moc/moc_web_doc.pdf. Published May 2014. Accessed August 25, 2014.
3. Faulkner LR, Vondrak PA. Frequently asked questions about maintenance of certification (MOC). J Clin Psychiatry. 2010;71(5):632-633.
4. Ebert MH, Faulkner L, Stubbe DE, et al. Maintenance of certification in psychiatry. J Clin Psychiatry. 2009;70(10):e39.
5. Approved MOC Products. American Board of Psychiatry and Neurology Inc. http://www.abpn.com/moc_products. asp. Accessed August 25, 2014.
6. Continuous MOC (C-MOC). American Board of Psychiatry and Neurology Inc. http://www.abpn.com/downloads/ moc/ContinuousCertificationApproach_0311.pdf. Accessed August 25, 2014.
7. C-MOC Program Overview. American Board of Psychiatry and Neurology Inc. http://www.abpn.com/downloads/ moc/moc-handouts-CMOC-051314.pdf. Published May 13, 2014. Accessed August 25, 2014.
To recap what I discussed in Part 1 of this article (December 2014): As part of a trend across all medical specialty boards, the American Board of Psychiatry and Neurology (ABPN) instituted a recertification process for all new general psychiatry certifications, starting October 1, 1994.1 In 2000, the specialties that comprise the American Board of Medical Specialties (ABMS) agreed to develop a comprehensive maintenance of certification (MOC) process to demonstrate ongoing learning and competency beyond what can be captured by a recertification examination. All ABMS member boards now use a 4-part process for recertification.
A great deal of professional and personal importance has been attached to maintaining one’s general and subspecialty certifications. To that end, the 2 parts of this article highlight current ABPN MOC requirements and provide resources for understanding, tracking, and completing the self-assessment (SA) and performance-in-practice (PIP) components.
In this installment, I examine 3 components of MOC:
• continuing medical education (CME), including SA requirements
• improvement in medical practice (PIP)
• continuous maintenance of certification (C-MOC)
In addition to this review, all physicians who are subject to MOC should download and read the 20-page revised MOC Program booklet v. 2.1 (May 2014).2
Continuing medical education
The CME requirement is clear: All diplomate physicians must accrue, on average, 30 Category-1 CME credits a year; the CME must be relevant to the specialty or subspecialty in which the diplomate practices.3 For physicians who hold >1 ABPN certificates, the total CME requirement is the same; CME credits can be applied across each specialty and subspecialty.
The May 2014 MOC revision states that, for physicians who certified or recertified between 2005 and 2011 and who applied for the 2015 examination in 2014, the required CME credit total is 270.2 For all subsequent years of certification or recertification, including 2012, diplomates are enrolled in C-MOC, which is described below.2
To even out the accrual of CME credits across the prior 10 years, ABPN mandates that, for diplomates who certified or recertified between 2005 and 2011, one hundred fifty of the CME credits be accrued in the 5 years before they apply for the examination. Diplomates in C-MOC should accrue, on average, 30 CME credits a year in each of the 3-year blocks (ie, 90 units in each block).2
Self-assessment
SA is a specific form of CME that is designed to provide comprehensive test-based feedback on knowledge acquired, to enhance the learning process.4 SA CME feedback must include:
• the correct answer to each test question
• recommended literature resources for each question
• performance compared to peers on each question.
Given the structured nature of SA activities, beginning January 1, 2014, one must use only ABPN-approved SA products (see Related Resources for a list of APBN-approved SA products).5
Table 1 and Table 2 outline SA requirements for, respectively, physicians who certified or recertified from 2005 through 2011, and those who certified or recertified in 2012 (and later). The SA requirement increases after 2011 to 24 credits in each 3-year block (8 credits a year, on average).2 Multiple SA activities can be used to fulfill the credit requirement of each 3-year block.
Note: Credits accrued by performing SA activities count toward the CME credit total.
Improvement in medical practice, or PIP
Physicians who are active clinically must complete PIP modules. Each module comprises peer or patient feedback plus a clinical aspect. The May 2014 MOC revision simplified the feedback process to mandate peer or patient feedback—but not both, as required previously.2 For the feedback PIP module, the physician selects 5 peers or patients to complete review forms, examines the results, and creates a plan of improvement. An exception to this “rule of 5” applies to diplomates who have a supervisor capable of evaluating all general competencies, defined below.
Related Resources provides a link to ABPN-created forms.
Within 24 months, but not sooner than 1 month, 5 peers or patients (or 1 applicable supervisor) are selected to complete review forms; changes in practice are noted. The same peers or patients might be selected for a second review. As noted in Table 1 and Table 2, the number of PIP modules is fewer for physicians who certified or recertified between 2005 and 2011; from 2012 onward, 1 PIP clinical module is required in each 3-year block.2
There are 6 ABPN-approved feedback module options, of which the diplomate must choose 1 in any given block2:
• 5 patient surveys
• 5 peer evaluations of general competenciesa
• 5 resident evaluations of general competenciesa
• 360° evaluation of general competencies,a with 5 respondents
• institutional peer review of general competencies,a with 5 respondents
• 1 supervisor evaluation of general competencies.a
aGeneral competencies include patient care; practice-based learning and improvement; professionalism; medical knowledge; interpersonal and communication skills; and system-based practices.
Although many institutions have a quality improvement (QI) program, that program must be approved by the Multi-Specialty MOC Portfolio Approval Program sponsored by ABMS for a clinician to receive credit for 1 PIP clinical module. If the approved QI program includes patient or peer feedback (eg, a survey), the diplo mate can receive credit for 1 PIP feedback module.2
For the clinical PIP module, the physician selects 5 charts for review and examines them based on criteria found in an ABPN-approved (starting in 2014) PIP product. (Related Resources provides a link to this list.) After reviewing the initial 5 charts, a plan for improvement is created. Within 24 months, but no sooner than 1 month, 5 charts are again selected and reviewed, and changes in practice are noted. The same charts can be selected for the second review.
As noted in Table 1 and Table 2, the number of PIP modules is fewer for those who initially certified or recertified between 2005 and 2011; from 2012 onward, 1 PIP clinical module is required in each 3-year block.2
The C-MOC process
Physicians who certified or recertified in 2012, or who will certify or recertify after that year, are enrolled automatically in C-MOC.6,7 The purpose of C-MOC is to keep diplomates on track to fulfill the higher level of SA requirements that began with this group; this is done by mandating use of the ABPN Physician Folios system. As shown in Table 2, there is no longer a 10-year cycle; instead, there are continuous 3-year stages, within which each diplomate must accrue 90 CME credits (on average, 30 credits a year), 24 SA credits (on average, 8 a year), 1 PIP clinical module, and 1 PIP feedback module.6,7
The first 3-year block of C-MOC requirements will be waived for physicians who complete Accreditation Council on Graduate Medical Education–accredited or ABPN-approved subspecialty training in 2012 or later—if they pass the corresponding ABPN subspecialty examination during the first 3-year block of enrollment in C-MOC.2 For diplomates enrolled in C-MOC, failure to track progress of each 3-year block, via the ABPN Physician Folios system, has significant consequences: Those who do not complete the first stage of the program by the end of 3 years will be listed on the ABPN Web site as “certified— not meeting MOC requirements.” Those who do not complete 2 stages by the end of 6 years will be listed as “not certified.”2
Cognitive exam still in place. The only remnant of the old 10-year cycle is the requirement to pass the cognitive examination every 10 years, although the exam can be taken earlier if the diplomate wishes. If all requirements are met and one does not sit for, or fails, the exam, the ABPN Web site will report the diplomate as “not meeting MOC requirements.” One can retake the exam within 1 year of the failed or missed exam, but a subsequent failure or missed exam will result in being listed as “not certified.”2
Fee structure. Instead of a single fee paid at the time of the exam(s), physicians in the C-MOC program pay an annual fee that covers participation in ABPN Physician Folios and 1 exam in a 10-year period. Fewer than 10 years of participation, or applying for a combined examination (for diplomates who hold multiple certifications), requires an additional fee.7
Bottom Line
Maintenance of certification (MOC) is manageable, although it requires you to be familiar with its various elements. Those elements include continuing medical education (CME requirements); the additional self-assessment component of CME; performance-in-practice modules; and continuous maintenance of certification. The MOC program booklet of the American Board of Psychiatry and Neurology provides all necessary details.
Disclosure
Dr. Meyer reports no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
To recap what I discussed in Part 1 of this article (December 2014): As part of a trend across all medical specialty boards, the American Board of Psychiatry and Neurology (ABPN) instituted a recertification process for all new general psychiatry certifications, starting October 1, 1994.1 In 2000, the specialties that comprise the American Board of Medical Specialties (ABMS) agreed to develop a comprehensive maintenance of certification (MOC) process to demonstrate ongoing learning and competency beyond what can be captured by a recertification examination. All ABMS member boards now use a 4-part process for recertification.
A great deal of professional and personal importance has been attached to maintaining one’s general and subspecialty certifications. To that end, the 2 parts of this article highlight current ABPN MOC requirements and provide resources for understanding, tracking, and completing the self-assessment (SA) and performance-in-practice (PIP) components.
In this installment, I examine 3 components of MOC:
• continuing medical education (CME), including SA requirements
• improvement in medical practice (PIP)
• continuous maintenance of certification (C-MOC)
In addition to this review, all physicians who are subject to MOC should download and read the 20-page revised MOC Program booklet v. 2.1 (May 2014).2
Continuing medical education
The CME requirement is clear: All diplomate physicians must accrue, on average, 30 Category-1 CME credits a year; the CME must be relevant to the specialty or subspecialty in which the diplomate practices.3 For physicians who hold >1 ABPN certificates, the total CME requirement is the same; CME credits can be applied across each specialty and subspecialty.
The May 2014 MOC revision states that, for physicians who certified or recertified between 2005 and 2011 and who applied for the 2015 examination in 2014, the required CME credit total is 270.2 For all subsequent years of certification or recertification, including 2012, diplomates are enrolled in C-MOC, which is described below.2
To even out the accrual of CME credits across the prior 10 years, ABPN mandates that, for diplomates who certified or recertified between 2005 and 2011, one hundred fifty of the CME credits be accrued in the 5 years before they apply for the examination. Diplomates in C-MOC should accrue, on average, 30 CME credits a year in each of the 3-year blocks (ie, 90 units in each block).2
Self-assessment
SA is a specific form of CME that is designed to provide comprehensive test-based feedback on knowledge acquired, to enhance the learning process.4 SA CME feedback must include:
• the correct answer to each test question
• recommended literature resources for each question
• performance compared to peers on each question.
Given the structured nature of SA activities, beginning January 1, 2014, one must use only ABPN-approved SA products (see Related Resources for a list of APBN-approved SA products).5
Table 1 and Table 2 outline SA requirements for, respectively, physicians who certified or recertified from 2005 through 2011, and those who certified or recertified in 2012 (and later). The SA requirement increases after 2011 to 24 credits in each 3-year block (8 credits a year, on average).2 Multiple SA activities can be used to fulfill the credit requirement of each 3-year block.
Note: Credits accrued by performing SA activities count toward the CME credit total.
Improvement in medical practice, or PIP
Physicians who are active clinically must complete PIP modules. Each module comprises peer or patient feedback plus a clinical aspect. The May 2014 MOC revision simplified the feedback process to mandate peer or patient feedback—but not both, as required previously.2 For the feedback PIP module, the physician selects 5 peers or patients to complete review forms, examines the results, and creates a plan of improvement. An exception to this “rule of 5” applies to diplomates who have a supervisor capable of evaluating all general competencies, defined below.
Related Resources provides a link to ABPN-created forms.
Within 24 months, but not sooner than 1 month, 5 peers or patients (or 1 applicable supervisor) are selected to complete review forms; changes in practice are noted. The same peers or patients might be selected for a second review. As noted in Table 1 and Table 2, the number of PIP modules is fewer for physicians who certified or recertified between 2005 and 2011; from 2012 onward, 1 PIP clinical module is required in each 3-year block.2
There are 6 ABPN-approved feedback module options, of which the diplomate must choose 1 in any given block2:
• 5 patient surveys
• 5 peer evaluations of general competenciesa
• 5 resident evaluations of general competenciesa
• 360° evaluation of general competencies,a with 5 respondents
• institutional peer review of general competencies,a with 5 respondents
• 1 supervisor evaluation of general competencies.a
aGeneral competencies include patient care; practice-based learning and improvement; professionalism; medical knowledge; interpersonal and communication skills; and system-based practices.
Although many institutions have a quality improvement (QI) program, that program must be approved by the Multi-Specialty MOC Portfolio Approval Program sponsored by ABMS for a clinician to receive credit for 1 PIP clinical module. If the approved QI program includes patient or peer feedback (eg, a survey), the diplo mate can receive credit for 1 PIP feedback module.2
For the clinical PIP module, the physician selects 5 charts for review and examines them based on criteria found in an ABPN-approved (starting in 2014) PIP product. (Related Resources provides a link to this list.) After reviewing the initial 5 charts, a plan for improvement is created. Within 24 months, but no sooner than 1 month, 5 charts are again selected and reviewed, and changes in practice are noted. The same charts can be selected for the second review.
As noted in Table 1 and Table 2, the number of PIP modules is fewer for those who initially certified or recertified between 2005 and 2011; from 2012 onward, 1 PIP clinical module is required in each 3-year block.2
The C-MOC process
Physicians who certified or recertified in 2012, or who will certify or recertify after that year, are enrolled automatically in C-MOC.6,7 The purpose of C-MOC is to keep diplomates on track to fulfill the higher level of SA requirements that began with this group; this is done by mandating use of the ABPN Physician Folios system. As shown in Table 2, there is no longer a 10-year cycle; instead, there are continuous 3-year stages, within which each diplomate must accrue 90 CME credits (on average, 30 credits a year), 24 SA credits (on average, 8 a year), 1 PIP clinical module, and 1 PIP feedback module.6,7
The first 3-year block of C-MOC requirements will be waived for physicians who complete Accreditation Council on Graduate Medical Education–accredited or ABPN-approved subspecialty training in 2012 or later—if they pass the corresponding ABPN subspecialty examination during the first 3-year block of enrollment in C-MOC.2 For diplomates enrolled in C-MOC, failure to track progress of each 3-year block, via the ABPN Physician Folios system, has significant consequences: Those who do not complete the first stage of the program by the end of 3 years will be listed on the ABPN Web site as “certified— not meeting MOC requirements.” Those who do not complete 2 stages by the end of 6 years will be listed as “not certified.”2
Cognitive exam still in place. The only remnant of the old 10-year cycle is the requirement to pass the cognitive examination every 10 years, although the exam can be taken earlier if the diplomate wishes. If all requirements are met and one does not sit for, or fails, the exam, the ABPN Web site will report the diplomate as “not meeting MOC requirements.” One can retake the exam within 1 year of the failed or missed exam, but a subsequent failure or missed exam will result in being listed as “not certified.”2
Fee structure. Instead of a single fee paid at the time of the exam(s), physicians in the C-MOC program pay an annual fee that covers participation in ABPN Physician Folios and 1 exam in a 10-year period. Fewer than 10 years of participation, or applying for a combined examination (for diplomates who hold multiple certifications), requires an additional fee.7
Bottom Line
Maintenance of certification (MOC) is manageable, although it requires you to be familiar with its various elements. Those elements include continuing medical education (CME requirements); the additional self-assessment component of CME; performance-in-practice modules; and continuous maintenance of certification. The MOC program booklet of the American Board of Psychiatry and Neurology provides all necessary details.
Disclosure
Dr. Meyer reports no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Faulkner LR, Tivnan PW, Winstead DK, et al. The ABPN Maintenance of Certification Program for psychiatrists: past history, current status, and future directions. Acad Psychiatry. 2008;32(3):241-248.
2. Maintenance of Certification Program. American Board of Psychiatry and Neurology Inc. http://www.abpn.com/ downloads/moc/moc_web_doc.pdf. Published May 2014. Accessed August 25, 2014.
3. Faulkner LR, Vondrak PA. Frequently asked questions about maintenance of certification (MOC). J Clin Psychiatry. 2010;71(5):632-633.
4. Ebert MH, Faulkner L, Stubbe DE, et al. Maintenance of certification in psychiatry. J Clin Psychiatry. 2009;70(10):e39.
5. Approved MOC Products. American Board of Psychiatry and Neurology Inc. http://www.abpn.com/moc_products. asp. Accessed August 25, 2014.
6. Continuous MOC (C-MOC). American Board of Psychiatry and Neurology Inc. http://www.abpn.com/downloads/ moc/ContinuousCertificationApproach_0311.pdf. Accessed August 25, 2014.
7. C-MOC Program Overview. American Board of Psychiatry and Neurology Inc. http://www.abpn.com/downloads/ moc/moc-handouts-CMOC-051314.pdf. Published May 13, 2014. Accessed August 25, 2014.
1. Faulkner LR, Tivnan PW, Winstead DK, et al. The ABPN Maintenance of Certification Program for psychiatrists: past history, current status, and future directions. Acad Psychiatry. 2008;32(3):241-248.
2. Maintenance of Certification Program. American Board of Psychiatry and Neurology Inc. http://www.abpn.com/ downloads/moc/moc_web_doc.pdf. Published May 2014. Accessed August 25, 2014.
3. Faulkner LR, Vondrak PA. Frequently asked questions about maintenance of certification (MOC). J Clin Psychiatry. 2010;71(5):632-633.
4. Ebert MH, Faulkner L, Stubbe DE, et al. Maintenance of certification in psychiatry. J Clin Psychiatry. 2009;70(10):e39.
5. Approved MOC Products. American Board of Psychiatry and Neurology Inc. http://www.abpn.com/moc_products. asp. Accessed August 25, 2014.
6. Continuous MOC (C-MOC). American Board of Psychiatry and Neurology Inc. http://www.abpn.com/downloads/ moc/ContinuousCertificationApproach_0311.pdf. Accessed August 25, 2014.
7. C-MOC Program Overview. American Board of Psychiatry and Neurology Inc. http://www.abpn.com/downloads/ moc/moc-handouts-CMOC-051314.pdf. Published May 13, 2014. Accessed August 25, 2014.