User login
Welcome to Current Psychiatry, a leading source of information, online and in print, for practitioners of psychiatry and its related subspecialties, including addiction psychiatry, child and adolescent psychiatry, and geriatric psychiatry. This Web site contains evidence-based reviews of the prevention, diagnosis, and treatment of mental illness and psychological disorders; case reports; updates on psychopharmacology; news about the specialty of psychiatry; pearls for practice; and other topics of interest and use to this audience.
Dear Drupal User: You're seeing this because you're logged in to Drupal, and not redirected to MDedge.com/psychiatry.
Depression
adolescent depression
adolescent major depressive disorder
adolescent schizophrenia
adolescent with major depressive disorder
animals
autism
baby
brexpiprazole
child
child bipolar
child depression
child schizophrenia
children with bipolar disorder
children with depression
children with major depressive disorder
compulsive behaviors
cure
elderly bipolar
elderly depression
elderly major depressive disorder
elderly schizophrenia
elderly with dementia
first break
first episode
gambling
gaming
geriatric depression
geriatric major depressive disorder
geriatric schizophrenia
infant
kid
major depressive disorder
major depressive disorder in adolescents
major depressive disorder in children
parenting
pediatric
pediatric bipolar
pediatric depression
pediatric major depressive disorder
pediatric schizophrenia
pregnancy
pregnant
rexulti
skin care
teen
wine
section[contains(@class, 'nav-hidden')]
footer[@id='footer']
div[contains(@class, 'pane-pub-article-current-psychiatry')]
div[contains(@class, 'pane-pub-home-current-psychiatry')]
div[contains(@class, 'pane-pub-topic-current-psychiatry')]
div[contains(@class, 'panel-panel-inner')]
div[contains(@class, 'pane-node-field-article-topics')]
section[contains(@class, 'footer-nav-section-wrapper')]
How should you use the lab to monitor patients taking a mood stabilizer?
Ms. W, age 27, presents with a chief concern of “depression.” She describes a history of several hypomanic episodes as well as the current depressive episode, prompting a bipolar II disorder diagnosis. She is naïve to all psychotropics. You plan to initiate a mood-stabilizing agent. What would you include in your initial workup before starting treatment and how would you monitor her as she continues treatment?
Mood stabilizers are employed to treat bipolar spectrum disorders (bipolar I, bipolar II, and cyclothymic disorder) and schizoaffective disorder, bipolar type. Some evidence suggests that mood stabilizers also can be used for treatment-resistant depressive disorders and borderline personality disorder.1 Mood stabilizers include lithium, valproate, carbamazepine, oxcarbazepine, and lamotrigine.2-5
This review focuses on applications and monitoring of mood stabilizers for bipolar I and II disorders. We also will briefly review atypical antipsychotics because they also are used to treat bipolar spectrum disorders (see the September 2013 issue of Current Psychiatry at CurrentPsychiatry.com for a more detailed article on monitoring of antipsychotics).6
There are several well-researched guidelines used to guide clinical practice.2-5 Many guidelines recommend baseline and routine monitoring parameters based on the characteristics of the agent used. However, the International Society for Bipolar Disorders (ISBD) guidelines highlight the importance of monitoring medical comorbidities, which are common among patients with bipolar disorder and can affect pharmacotherapy and clinical outcomes. These recommendations are similar to metabolic monitoring guidelines for antipsychotics.5
Reviews of therapeutic monitoring show that only one-third to one-half of patien
taking a mood stabilizer are appropriately monitored. Poor adherence to guideline recommendations often is observed because of patients’ lack of insight or medication adherence and because psychiatric care generally is segregated from other medical care.7-9
Baseline testing
The ISBD guidelines recommend an initial workup for all patients that includes:
• waist circumference or body mass index (BMI), or both
• blood pressure
• complete blood count (CBC)
• electrolytes
• blood urea nitrogen (BUN) and creatinine
• liver function tests (LFTs)
• fasting glucose
• fasting lipid profile.
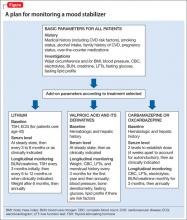
In addition, medical history, cigarette smoking status, alcohol intake, and family history of cardiovascular disease, cerebrovascular disease, hypertension, dyslipidemia, and diabetes mellitus should be documented. Rule out pregnancy in women of childbearing potential.2 The Figure describes monitoring parameters based on selected agent.
Agent-specific monitoring
Lithium. Patients beginning lithium therapy should undergo thyroid function testing and, for patients age >40, ECG monitoring. Educate patients about potential side effects of lithium, signs and symptoms of lithium toxicity, and the importance of avoiding dehydration. Adding or changing certain medications could elevate the serum lithium level (eg, diuretics, angiotensin-converting enzyme [ACE]-inhibitors, nonsteroidal anti-inflammatory drugs [NSAIDs], COX-2 inhibitors).
Lithium can cause weight gain and adverse effects in several organ systems, including:
• gastrointestinal (GI) (nausea, vomiting, abdominal pain, loss of appetite, diarrhea)
• renal (nephrogenic diabetes insipidus, tubulointerstitial renal disease)
• neurologic (tremors, cognitive dulling, raised intracranial pressure)
• endocrine (thyroid and parathyroid dysfunction)
• cardiac (benign electrocardiographic changes, conduction abnormalities)
• dermatologic (acne, psoriasis, hair loss)
• hematologic (benign leukocytosis).
Lithium has a narrow therapeutic index (0.5 to 1.2 mEq/L), which means that small changes in the serum level can result in therapeutic inefficacy or toxicity. Lithium toxicity can cause irreversible organ damage or death. Serum lithium levels, symptomatic response, emergence and evolution of adverse drug reactions (ADRs), and the recognition of patient risk factors for toxicity can help guide dosing. From a safety monitoring viewpoint, lithium toxicity, renal and endocrine adverse effects, and potential drug interactions are foremost concerns.
Lithium usually is started at a low, divided dosages to minimize side effects, and titrated according to response. Check lithium levels before and after each dose increase. Serum levels reach steady state 5 days after dosage adjustment, but might need to be checked sooner if a rapid increase is necessary, such as when treating acute mania, or if you suspect toxicity.
If the patient has renal insufficiency, it may take longer for the lithium to reach steady state; therefore, delaying a blood level beyond 5 days may be necessary to gauge a true steady state. Also, anytime a medication that interferes with lithium renal elimination, such as diuretics, ACE inhibitors, NSAIDs, COX-2 inhibitors, is added or the dosage is changed, a new lithium level will need to be obtained to reassess the level in 5 days, assuming adequate renal function. In general, renal function and thyroid function should be evaluated once or twice during the first 6 months of lithium treatment.
Subsequently, renal and thyroid function can be checked every 6 months to 1 year in stable patients or when clinically indicated. Check a patient’s weight after 6 months of therapy, then at least annually.2
Valproic acid (VPA) and its derivatives. The most important initial monitoring for VPA therapy includes LFTs and CBC. Before initiating VPA treatment, take a medical history, with special attention to hepatic, hematologic, and bleeding abnormalities. Therapeutic blood monitoring can be conducted once steady state is achieved and as clinically necessary thereafter.
VPA can be administered at an initial starting dosage of 20 to 30 mg/kg/d in inpatients. In outpatients it is given in low, divided doses or as once-daily dosing using an extended-release formulation to minimize GI and neurologic toxicity and titrated every few days. Target serum level is 50 to 125 μg/mL.
Side effects of VPA include GI distress (eg, anorexia, nausea, dyspepsia, vomiting, diarrhea), hematologic effects (reversible leukopenia, thrombocytopenia), hair loss, weight gain, tremor, hepatic effects (benign LFT elevations, hepatotoxicity), osteoporosis, and sedation. Patients with prior or current hepatic disease may be at greater risk for hepatotoxicity. There is an association between VPA and polycystic ovarian syndrome. Rare, idiosyncratic, but potentially fatal adverse events with valproate include irreversible hepatic failure, hemorrhagic pancreatitis, and agranulocytosis.
Older monitoring standards indicated taking LFTs and CBC every 6 months and serum VPA level as clinically indicated. According to ISBD guidelines, weight, CBC, LFTs, and menstrual history should be monitored every 3 months for the first year and then annually; blood pressure, bone status (densitometry), fasting glucose, and fasting lipids should be monitored only in patients with related risk factors. Routine ammonia levels are not recommended but might be indicated if a patient has sudden mental status changes or change in condition.2
Carbamazepine and oxcarbazepine. The most important initial monitoring for carbamazepine therapy includes LFTs, renal function, electrolytes, and CBC. Before treatment, take a medical history, with special emphasis on history of blood dyscrasias or liver disease. After initiating carbamazepine, CBC, LFTs, electrolytes, and renal function should be done monthly for 3 months, then repeated annually.
Carbamazepine is a substrate and an inducer of the cytochrome P450 (CYP) system, so it can reduce levels of many other drugs including other antiepileptics, warfarin, and oral contraceptives. Serum level of carbamazepine can be measured at trough after 5 days, with a target level of 4 to 12 μg/mL. Two levels should be drawn, 4 weeks apart, to establish therapeutic dosage secondary to autoinduction of the CYP450 system.2
As many as one-half of patients experience side effects with carbamazepine. The most common side effects include fatigue, nausea, and neurologic symptoms (diplopia, blurred vision, and ataxia). Less frequent side effects include skin rashes, leukopenia, liver enzyme elevations, thrombocytopenia, hyponatremia, and hypo-osmolality. Rare, potentially fatal side effects include agranulocytosis, aplastic anemia, thrombocytopenia, hepatic failure, and exfoliative dermatitis (eg, Stevens-Johnson syndrome).
Patients of Asian descent who are taking carbamazepine should undergo genetic testing for the HLA-B*1502 enzyme because persons with this allele are at higher risk of developing Stevens-Johnson syndrome. Also, patients should be educated about the signs and symptoms of these rare adverse reactions so that medical treatment is not delayed should these adverse events present.
Lamotrigine does not require further laboratory monitoring beyond the initial recommended workup. The most important variables to consider are interactions with other medications (especially other antiepileptics, such as VPA and carbamazepine) and observing for rash. Titration takes several weeks to minimize risk of developing a rash.2 Similar to carbamazepine, the patient should be educated on the signs and symptoms of exfoliative dermatitis (eg, Stevens-Johnson syndrome) so that medical treatment is sought out should this reaction occur.
Atypical antipsychotics. Baseline workup includes the general monitoring parameters described above. Atypical antipsychotics have a lower incidence of extrapyramidal side effects than typical antipsychotics, but are associated with an increased risk of metabolic complications. Other major ADRs to consider are cardiac effects and hyperprolactinemia; clinicians should therefore inquire about a personal or family history of cardiac problems, including congenital long QT syndrome. Patients should be screened for any medications that can prolong the QTc interval or interact with the metabolism of medications known to cause QTc prolongation.
Measure weight monthly for the first 3 months, then every 3 months to monitor for metabolic side effects during ongoing treatment. Obtain blood pressure and fasting glucose every 3 months for the first year, then annually. Repeat a fasting lipid profile 3 months after initiating treatment, then annually. Cardiac effects and prolactin levels can be monitored as needed if clinically indicated.2
CASE CONTINUED
You discuss with Ms. W choices of a mood stabilizing agent to treat her bipolar II disorder; she agrees to start lithium. Before initiating treatment, you obtain her weight (and calculate her BMI), blood pressure, CBC, electrolyte levels, BUN and creatinine levels, liver function tests, fasting glucose, fasting lipid profile, and thyroid panel. You also review her medical history, lifestyle factors (cigarette smoking status, alcohol intake), and family history. A urine pregnancy screen is negative. The pharmacist assists in screening for potential drug-drug interactions, including over-the-counter medications that Ms. W occasionally takes as needed. She is counseled on the use of NSAIDS because these drugs can increase the lithium level.
Ms. W tolerates and responds well to lithium. No further dosing recommendations are made, based on clinical response. You measure her weight at 6 months, then annually. Renal function and thyroid function are monitored at 3 and 6 months after lithium is initiated, and then annually. One year after starting lithium, she continues to tolerate the medication and has minimal metabolic side effects.
Related Resources
• McInnis MG. Lithium for bipolar disorder: A re-emerging treatment for mood instability. Current Psychiatry. 2014; 13(6):38-44.
• Stahl SM. Stahl’s illustrated mood stabilizers. New York, NY: Cambridge University Press; 2009.
Drug Brand Names
Carbamazepine • Tegretol Valproic acid • Depacon, Depakote
Lamotrigine • Lamictal Warfarin • Coumadin
Lithium • Lithobid, Eskalith
Oxcarbazepine • Trileptal
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Maglione M, Ruelaz Maher A, Hu J, et al. Off-label use of atypical antipsychotics: an update. Comparative Effectiveness Review No. 43. Rockville, MD: Agency for Healthcare Research and Quality; 2011. http://www.effectivehealthcare.ahrq.gov/ehc/products/150/778/CER43_Off-LabelAntipsychotics_20110928.pdf. Published September 2011. Accessed June 6, 2014.
2. American Psychiatric Association. Practice guideline for the treatment of patients with bipolar disorder (revision). Am J Psychiatry. 2002;159(suppl 4):1-50.
3. Ng F, Mammen OK, Wilting I, et al; International Society for Bipolar Disorders. The International Society for Bipolar Disorders (ISBD) consensus guidelines for the safety monitoring of bipolar disorder treatments. Bipolar Disord. 2009;11(6):559-595.
4. National Institute for Health and Clinical Excellence. Bipolar disorder (CG38). The management of bipolar disorder in adults, children and adolescents, in primary and secondary care. http://www.nice.org.uk/CG038. Updated February 13, 2014. Accessed June 6, 2014.
5. Yatham LN, Kennedy SH, O’Donovan C, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) guidelines for the management of patients with bipolar disorder: update 2007. Bipolar Disord. 2006;8(6):721-739.
6. Zeier K, Connell R, Resch W, et al. Recommendations for lab monitoring of atypical antipsychotics. Current Psychiatry. 2013; 12(9):51-54.
7. Krishnan KR. Psychiatric and medical comorbidities of bipolar disorder. Psychosom Med. 2005;67(1):1-8.
8. Kilbourne AM, Post EP, Bauer MS, et al. Therapeutic drug and cardiovascular disease risk monitoring in patients with bipolar disorder. J Affect Disord. 2007;102(1-3):145-151.
9. Marcus SC, Olfson M, Pincus HA, et al. Therapeutic drug monitoring of mood stabilizers in Medicaid patients with bipolar disorder. Am J Psychiatry. 1999;156(7):1014-1018.
Ms. W, age 27, presents with a chief concern of “depression.” She describes a history of several hypomanic episodes as well as the current depressive episode, prompting a bipolar II disorder diagnosis. She is naïve to all psychotropics. You plan to initiate a mood-stabilizing agent. What would you include in your initial workup before starting treatment and how would you monitor her as she continues treatment?
Mood stabilizers are employed to treat bipolar spectrum disorders (bipolar I, bipolar II, and cyclothymic disorder) and schizoaffective disorder, bipolar type. Some evidence suggests that mood stabilizers also can be used for treatment-resistant depressive disorders and borderline personality disorder.1 Mood stabilizers include lithium, valproate, carbamazepine, oxcarbazepine, and lamotrigine.2-5
This review focuses on applications and monitoring of mood stabilizers for bipolar I and II disorders. We also will briefly review atypical antipsychotics because they also are used to treat bipolar spectrum disorders (see the September 2013 issue of Current Psychiatry at CurrentPsychiatry.com for a more detailed article on monitoring of antipsychotics).6
There are several well-researched guidelines used to guide clinical practice.2-5 Many guidelines recommend baseline and routine monitoring parameters based on the characteristics of the agent used. However, the International Society for Bipolar Disorders (ISBD) guidelines highlight the importance of monitoring medical comorbidities, which are common among patients with bipolar disorder and can affect pharmacotherapy and clinical outcomes. These recommendations are similar to metabolic monitoring guidelines for antipsychotics.5
Reviews of therapeutic monitoring show that only one-third to one-half of patien
taking a mood stabilizer are appropriately monitored. Poor adherence to guideline recommendations often is observed because of patients’ lack of insight or medication adherence and because psychiatric care generally is segregated from other medical care.7-9
Baseline testing
The ISBD guidelines recommend an initial workup for all patients that includes:
• waist circumference or body mass index (BMI), or both
• blood pressure
• complete blood count (CBC)
• electrolytes
• blood urea nitrogen (BUN) and creatinine
• liver function tests (LFTs)
• fasting glucose
• fasting lipid profile.
In addition, medical history, cigarette smoking status, alcohol intake, and family history of cardiovascular disease, cerebrovascular disease, hypertension, dyslipidemia, and diabetes mellitus should be documented. Rule out pregnancy in women of childbearing potential.2 The Figure describes monitoring parameters based on selected agent.
Agent-specific monitoring
Lithium. Patients beginning lithium therapy should undergo thyroid function testing and, for patients age >40, ECG monitoring. Educate patients about potential side effects of lithium, signs and symptoms of lithium toxicity, and the importance of avoiding dehydration. Adding or changing certain medications could elevate the serum lithium level (eg, diuretics, angiotensin-converting enzyme [ACE]-inhibitors, nonsteroidal anti-inflammatory drugs [NSAIDs], COX-2 inhibitors).
Lithium can cause weight gain and adverse effects in several organ systems, including:
• gastrointestinal (GI) (nausea, vomiting, abdominal pain, loss of appetite, diarrhea)
• renal (nephrogenic diabetes insipidus, tubulointerstitial renal disease)
• neurologic (tremors, cognitive dulling, raised intracranial pressure)
• endocrine (thyroid and parathyroid dysfunction)
• cardiac (benign electrocardiographic changes, conduction abnormalities)
• dermatologic (acne, psoriasis, hair loss)
• hematologic (benign leukocytosis).
Lithium has a narrow therapeutic index (0.5 to 1.2 mEq/L), which means that small changes in the serum level can result in therapeutic inefficacy or toxicity. Lithium toxicity can cause irreversible organ damage or death. Serum lithium levels, symptomatic response, emergence and evolution of adverse drug reactions (ADRs), and the recognition of patient risk factors for toxicity can help guide dosing. From a safety monitoring viewpoint, lithium toxicity, renal and endocrine adverse effects, and potential drug interactions are foremost concerns.
Lithium usually is started at a low, divided dosages to minimize side effects, and titrated according to response. Check lithium levels before and after each dose increase. Serum levels reach steady state 5 days after dosage adjustment, but might need to be checked sooner if a rapid increase is necessary, such as when treating acute mania, or if you suspect toxicity.
If the patient has renal insufficiency, it may take longer for the lithium to reach steady state; therefore, delaying a blood level beyond 5 days may be necessary to gauge a true steady state. Also, anytime a medication that interferes with lithium renal elimination, such as diuretics, ACE inhibitors, NSAIDs, COX-2 inhibitors, is added or the dosage is changed, a new lithium level will need to be obtained to reassess the level in 5 days, assuming adequate renal function. In general, renal function and thyroid function should be evaluated once or twice during the first 6 months of lithium treatment.
Subsequently, renal and thyroid function can be checked every 6 months to 1 year in stable patients or when clinically indicated. Check a patient’s weight after 6 months of therapy, then at least annually.2
Valproic acid (VPA) and its derivatives. The most important initial monitoring for VPA therapy includes LFTs and CBC. Before initiating VPA treatment, take a medical history, with special attention to hepatic, hematologic, and bleeding abnormalities. Therapeutic blood monitoring can be conducted once steady state is achieved and as clinically necessary thereafter.
VPA can be administered at an initial starting dosage of 20 to 30 mg/kg/d in inpatients. In outpatients it is given in low, divided doses or as once-daily dosing using an extended-release formulation to minimize GI and neurologic toxicity and titrated every few days. Target serum level is 50 to 125 μg/mL.
Side effects of VPA include GI distress (eg, anorexia, nausea, dyspepsia, vomiting, diarrhea), hematologic effects (reversible leukopenia, thrombocytopenia), hair loss, weight gain, tremor, hepatic effects (benign LFT elevations, hepatotoxicity), osteoporosis, and sedation. Patients with prior or current hepatic disease may be at greater risk for hepatotoxicity. There is an association between VPA and polycystic ovarian syndrome. Rare, idiosyncratic, but potentially fatal adverse events with valproate include irreversible hepatic failure, hemorrhagic pancreatitis, and agranulocytosis.
Older monitoring standards indicated taking LFTs and CBC every 6 months and serum VPA level as clinically indicated. According to ISBD guidelines, weight, CBC, LFTs, and menstrual history should be monitored every 3 months for the first year and then annually; blood pressure, bone status (densitometry), fasting glucose, and fasting lipids should be monitored only in patients with related risk factors. Routine ammonia levels are not recommended but might be indicated if a patient has sudden mental status changes or change in condition.2
Carbamazepine and oxcarbazepine. The most important initial monitoring for carbamazepine therapy includes LFTs, renal function, electrolytes, and CBC. Before treatment, take a medical history, with special emphasis on history of blood dyscrasias or liver disease. After initiating carbamazepine, CBC, LFTs, electrolytes, and renal function should be done monthly for 3 months, then repeated annually.
Carbamazepine is a substrate and an inducer of the cytochrome P450 (CYP) system, so it can reduce levels of many other drugs including other antiepileptics, warfarin, and oral contraceptives. Serum level of carbamazepine can be measured at trough after 5 days, with a target level of 4 to 12 μg/mL. Two levels should be drawn, 4 weeks apart, to establish therapeutic dosage secondary to autoinduction of the CYP450 system.2
As many as one-half of patients experience side effects with carbamazepine. The most common side effects include fatigue, nausea, and neurologic symptoms (diplopia, blurred vision, and ataxia). Less frequent side effects include skin rashes, leukopenia, liver enzyme elevations, thrombocytopenia, hyponatremia, and hypo-osmolality. Rare, potentially fatal side effects include agranulocytosis, aplastic anemia, thrombocytopenia, hepatic failure, and exfoliative dermatitis (eg, Stevens-Johnson syndrome).
Patients of Asian descent who are taking carbamazepine should undergo genetic testing for the HLA-B*1502 enzyme because persons with this allele are at higher risk of developing Stevens-Johnson syndrome. Also, patients should be educated about the signs and symptoms of these rare adverse reactions so that medical treatment is not delayed should these adverse events present.
Lamotrigine does not require further laboratory monitoring beyond the initial recommended workup. The most important variables to consider are interactions with other medications (especially other antiepileptics, such as VPA and carbamazepine) and observing for rash. Titration takes several weeks to minimize risk of developing a rash.2 Similar to carbamazepine, the patient should be educated on the signs and symptoms of exfoliative dermatitis (eg, Stevens-Johnson syndrome) so that medical treatment is sought out should this reaction occur.
Atypical antipsychotics. Baseline workup includes the general monitoring parameters described above. Atypical antipsychotics have a lower incidence of extrapyramidal side effects than typical antipsychotics, but are associated with an increased risk of metabolic complications. Other major ADRs to consider are cardiac effects and hyperprolactinemia; clinicians should therefore inquire about a personal or family history of cardiac problems, including congenital long QT syndrome. Patients should be screened for any medications that can prolong the QTc interval or interact with the metabolism of medications known to cause QTc prolongation.
Measure weight monthly for the first 3 months, then every 3 months to monitor for metabolic side effects during ongoing treatment. Obtain blood pressure and fasting glucose every 3 months for the first year, then annually. Repeat a fasting lipid profile 3 months after initiating treatment, then annually. Cardiac effects and prolactin levels can be monitored as needed if clinically indicated.2
CASE CONTINUED
You discuss with Ms. W choices of a mood stabilizing agent to treat her bipolar II disorder; she agrees to start lithium. Before initiating treatment, you obtain her weight (and calculate her BMI), blood pressure, CBC, electrolyte levels, BUN and creatinine levels, liver function tests, fasting glucose, fasting lipid profile, and thyroid panel. You also review her medical history, lifestyle factors (cigarette smoking status, alcohol intake), and family history. A urine pregnancy screen is negative. The pharmacist assists in screening for potential drug-drug interactions, including over-the-counter medications that Ms. W occasionally takes as needed. She is counseled on the use of NSAIDS because these drugs can increase the lithium level.
Ms. W tolerates and responds well to lithium. No further dosing recommendations are made, based on clinical response. You measure her weight at 6 months, then annually. Renal function and thyroid function are monitored at 3 and 6 months after lithium is initiated, and then annually. One year after starting lithium, she continues to tolerate the medication and has minimal metabolic side effects.
Related Resources
• McInnis MG. Lithium for bipolar disorder: A re-emerging treatment for mood instability. Current Psychiatry. 2014; 13(6):38-44.
• Stahl SM. Stahl’s illustrated mood stabilizers. New York, NY: Cambridge University Press; 2009.
Drug Brand Names
Carbamazepine • Tegretol Valproic acid • Depacon, Depakote
Lamotrigine • Lamictal Warfarin • Coumadin
Lithium • Lithobid, Eskalith
Oxcarbazepine • Trileptal
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Ms. W, age 27, presents with a chief concern of “depression.” She describes a history of several hypomanic episodes as well as the current depressive episode, prompting a bipolar II disorder diagnosis. She is naïve to all psychotropics. You plan to initiate a mood-stabilizing agent. What would you include in your initial workup before starting treatment and how would you monitor her as she continues treatment?
Mood stabilizers are employed to treat bipolar spectrum disorders (bipolar I, bipolar II, and cyclothymic disorder) and schizoaffective disorder, bipolar type. Some evidence suggests that mood stabilizers also can be used for treatment-resistant depressive disorders and borderline personality disorder.1 Mood stabilizers include lithium, valproate, carbamazepine, oxcarbazepine, and lamotrigine.2-5
This review focuses on applications and monitoring of mood stabilizers for bipolar I and II disorders. We also will briefly review atypical antipsychotics because they also are used to treat bipolar spectrum disorders (see the September 2013 issue of Current Psychiatry at CurrentPsychiatry.com for a more detailed article on monitoring of antipsychotics).6
There are several well-researched guidelines used to guide clinical practice.2-5 Many guidelines recommend baseline and routine monitoring parameters based on the characteristics of the agent used. However, the International Society for Bipolar Disorders (ISBD) guidelines highlight the importance of monitoring medical comorbidities, which are common among patients with bipolar disorder and can affect pharmacotherapy and clinical outcomes. These recommendations are similar to metabolic monitoring guidelines for antipsychotics.5
Reviews of therapeutic monitoring show that only one-third to one-half of patien
taking a mood stabilizer are appropriately monitored. Poor adherence to guideline recommendations often is observed because of patients’ lack of insight or medication adherence and because psychiatric care generally is segregated from other medical care.7-9
Baseline testing
The ISBD guidelines recommend an initial workup for all patients that includes:
• waist circumference or body mass index (BMI), or both
• blood pressure
• complete blood count (CBC)
• electrolytes
• blood urea nitrogen (BUN) and creatinine
• liver function tests (LFTs)
• fasting glucose
• fasting lipid profile.
In addition, medical history, cigarette smoking status, alcohol intake, and family history of cardiovascular disease, cerebrovascular disease, hypertension, dyslipidemia, and diabetes mellitus should be documented. Rule out pregnancy in women of childbearing potential.2 The Figure describes monitoring parameters based on selected agent.
Agent-specific monitoring
Lithium. Patients beginning lithium therapy should undergo thyroid function testing and, for patients age >40, ECG monitoring. Educate patients about potential side effects of lithium, signs and symptoms of lithium toxicity, and the importance of avoiding dehydration. Adding or changing certain medications could elevate the serum lithium level (eg, diuretics, angiotensin-converting enzyme [ACE]-inhibitors, nonsteroidal anti-inflammatory drugs [NSAIDs], COX-2 inhibitors).
Lithium can cause weight gain and adverse effects in several organ systems, including:
• gastrointestinal (GI) (nausea, vomiting, abdominal pain, loss of appetite, diarrhea)
• renal (nephrogenic diabetes insipidus, tubulointerstitial renal disease)
• neurologic (tremors, cognitive dulling, raised intracranial pressure)
• endocrine (thyroid and parathyroid dysfunction)
• cardiac (benign electrocardiographic changes, conduction abnormalities)
• dermatologic (acne, psoriasis, hair loss)
• hematologic (benign leukocytosis).
Lithium has a narrow therapeutic index (0.5 to 1.2 mEq/L), which means that small changes in the serum level can result in therapeutic inefficacy or toxicity. Lithium toxicity can cause irreversible organ damage or death. Serum lithium levels, symptomatic response, emergence and evolution of adverse drug reactions (ADRs), and the recognition of patient risk factors for toxicity can help guide dosing. From a safety monitoring viewpoint, lithium toxicity, renal and endocrine adverse effects, and potential drug interactions are foremost concerns.
Lithium usually is started at a low, divided dosages to minimize side effects, and titrated according to response. Check lithium levels before and after each dose increase. Serum levels reach steady state 5 days after dosage adjustment, but might need to be checked sooner if a rapid increase is necessary, such as when treating acute mania, or if you suspect toxicity.
If the patient has renal insufficiency, it may take longer for the lithium to reach steady state; therefore, delaying a blood level beyond 5 days may be necessary to gauge a true steady state. Also, anytime a medication that interferes with lithium renal elimination, such as diuretics, ACE inhibitors, NSAIDs, COX-2 inhibitors, is added or the dosage is changed, a new lithium level will need to be obtained to reassess the level in 5 days, assuming adequate renal function. In general, renal function and thyroid function should be evaluated once or twice during the first 6 months of lithium treatment.
Subsequently, renal and thyroid function can be checked every 6 months to 1 year in stable patients or when clinically indicated. Check a patient’s weight after 6 months of therapy, then at least annually.2
Valproic acid (VPA) and its derivatives. The most important initial monitoring for VPA therapy includes LFTs and CBC. Before initiating VPA treatment, take a medical history, with special attention to hepatic, hematologic, and bleeding abnormalities. Therapeutic blood monitoring can be conducted once steady state is achieved and as clinically necessary thereafter.
VPA can be administered at an initial starting dosage of 20 to 30 mg/kg/d in inpatients. In outpatients it is given in low, divided doses or as once-daily dosing using an extended-release formulation to minimize GI and neurologic toxicity and titrated every few days. Target serum level is 50 to 125 μg/mL.
Side effects of VPA include GI distress (eg, anorexia, nausea, dyspepsia, vomiting, diarrhea), hematologic effects (reversible leukopenia, thrombocytopenia), hair loss, weight gain, tremor, hepatic effects (benign LFT elevations, hepatotoxicity), osteoporosis, and sedation. Patients with prior or current hepatic disease may be at greater risk for hepatotoxicity. There is an association between VPA and polycystic ovarian syndrome. Rare, idiosyncratic, but potentially fatal adverse events with valproate include irreversible hepatic failure, hemorrhagic pancreatitis, and agranulocytosis.
Older monitoring standards indicated taking LFTs and CBC every 6 months and serum VPA level as clinically indicated. According to ISBD guidelines, weight, CBC, LFTs, and menstrual history should be monitored every 3 months for the first year and then annually; blood pressure, bone status (densitometry), fasting glucose, and fasting lipids should be monitored only in patients with related risk factors. Routine ammonia levels are not recommended but might be indicated if a patient has sudden mental status changes or change in condition.2
Carbamazepine and oxcarbazepine. The most important initial monitoring for carbamazepine therapy includes LFTs, renal function, electrolytes, and CBC. Before treatment, take a medical history, with special emphasis on history of blood dyscrasias or liver disease. After initiating carbamazepine, CBC, LFTs, electrolytes, and renal function should be done monthly for 3 months, then repeated annually.
Carbamazepine is a substrate and an inducer of the cytochrome P450 (CYP) system, so it can reduce levels of many other drugs including other antiepileptics, warfarin, and oral contraceptives. Serum level of carbamazepine can be measured at trough after 5 days, with a target level of 4 to 12 μg/mL. Two levels should be drawn, 4 weeks apart, to establish therapeutic dosage secondary to autoinduction of the CYP450 system.2
As many as one-half of patients experience side effects with carbamazepine. The most common side effects include fatigue, nausea, and neurologic symptoms (diplopia, blurred vision, and ataxia). Less frequent side effects include skin rashes, leukopenia, liver enzyme elevations, thrombocytopenia, hyponatremia, and hypo-osmolality. Rare, potentially fatal side effects include agranulocytosis, aplastic anemia, thrombocytopenia, hepatic failure, and exfoliative dermatitis (eg, Stevens-Johnson syndrome).
Patients of Asian descent who are taking carbamazepine should undergo genetic testing for the HLA-B*1502 enzyme because persons with this allele are at higher risk of developing Stevens-Johnson syndrome. Also, patients should be educated about the signs and symptoms of these rare adverse reactions so that medical treatment is not delayed should these adverse events present.
Lamotrigine does not require further laboratory monitoring beyond the initial recommended workup. The most important variables to consider are interactions with other medications (especially other antiepileptics, such as VPA and carbamazepine) and observing for rash. Titration takes several weeks to minimize risk of developing a rash.2 Similar to carbamazepine, the patient should be educated on the signs and symptoms of exfoliative dermatitis (eg, Stevens-Johnson syndrome) so that medical treatment is sought out should this reaction occur.
Atypical antipsychotics. Baseline workup includes the general monitoring parameters described above. Atypical antipsychotics have a lower incidence of extrapyramidal side effects than typical antipsychotics, but are associated with an increased risk of metabolic complications. Other major ADRs to consider are cardiac effects and hyperprolactinemia; clinicians should therefore inquire about a personal or family history of cardiac problems, including congenital long QT syndrome. Patients should be screened for any medications that can prolong the QTc interval or interact with the metabolism of medications known to cause QTc prolongation.
Measure weight monthly for the first 3 months, then every 3 months to monitor for metabolic side effects during ongoing treatment. Obtain blood pressure and fasting glucose every 3 months for the first year, then annually. Repeat a fasting lipid profile 3 months after initiating treatment, then annually. Cardiac effects and prolactin levels can be monitored as needed if clinically indicated.2
CASE CONTINUED
You discuss with Ms. W choices of a mood stabilizing agent to treat her bipolar II disorder; she agrees to start lithium. Before initiating treatment, you obtain her weight (and calculate her BMI), blood pressure, CBC, electrolyte levels, BUN and creatinine levels, liver function tests, fasting glucose, fasting lipid profile, and thyroid panel. You also review her medical history, lifestyle factors (cigarette smoking status, alcohol intake), and family history. A urine pregnancy screen is negative. The pharmacist assists in screening for potential drug-drug interactions, including over-the-counter medications that Ms. W occasionally takes as needed. She is counseled on the use of NSAIDS because these drugs can increase the lithium level.
Ms. W tolerates and responds well to lithium. No further dosing recommendations are made, based on clinical response. You measure her weight at 6 months, then annually. Renal function and thyroid function are monitored at 3 and 6 months after lithium is initiated, and then annually. One year after starting lithium, she continues to tolerate the medication and has minimal metabolic side effects.
Related Resources
• McInnis MG. Lithium for bipolar disorder: A re-emerging treatment for mood instability. Current Psychiatry. 2014; 13(6):38-44.
• Stahl SM. Stahl’s illustrated mood stabilizers. New York, NY: Cambridge University Press; 2009.
Drug Brand Names
Carbamazepine • Tegretol Valproic acid • Depacon, Depakote
Lamotrigine • Lamictal Warfarin • Coumadin
Lithium • Lithobid, Eskalith
Oxcarbazepine • Trileptal
Disclosure
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Maglione M, Ruelaz Maher A, Hu J, et al. Off-label use of atypical antipsychotics: an update. Comparative Effectiveness Review No. 43. Rockville, MD: Agency for Healthcare Research and Quality; 2011. http://www.effectivehealthcare.ahrq.gov/ehc/products/150/778/CER43_Off-LabelAntipsychotics_20110928.pdf. Published September 2011. Accessed June 6, 2014.
2. American Psychiatric Association. Practice guideline for the treatment of patients with bipolar disorder (revision). Am J Psychiatry. 2002;159(suppl 4):1-50.
3. Ng F, Mammen OK, Wilting I, et al; International Society for Bipolar Disorders. The International Society for Bipolar Disorders (ISBD) consensus guidelines for the safety monitoring of bipolar disorder treatments. Bipolar Disord. 2009;11(6):559-595.
4. National Institute for Health and Clinical Excellence. Bipolar disorder (CG38). The management of bipolar disorder in adults, children and adolescents, in primary and secondary care. http://www.nice.org.uk/CG038. Updated February 13, 2014. Accessed June 6, 2014.
5. Yatham LN, Kennedy SH, O’Donovan C, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) guidelines for the management of patients with bipolar disorder: update 2007. Bipolar Disord. 2006;8(6):721-739.
6. Zeier K, Connell R, Resch W, et al. Recommendations for lab monitoring of atypical antipsychotics. Current Psychiatry. 2013; 12(9):51-54.
7. Krishnan KR. Psychiatric and medical comorbidities of bipolar disorder. Psychosom Med. 2005;67(1):1-8.
8. Kilbourne AM, Post EP, Bauer MS, et al. Therapeutic drug and cardiovascular disease risk monitoring in patients with bipolar disorder. J Affect Disord. 2007;102(1-3):145-151.
9. Marcus SC, Olfson M, Pincus HA, et al. Therapeutic drug monitoring of mood stabilizers in Medicaid patients with bipolar disorder. Am J Psychiatry. 1999;156(7):1014-1018.
1. Maglione M, Ruelaz Maher A, Hu J, et al. Off-label use of atypical antipsychotics: an update. Comparative Effectiveness Review No. 43. Rockville, MD: Agency for Healthcare Research and Quality; 2011. http://www.effectivehealthcare.ahrq.gov/ehc/products/150/778/CER43_Off-LabelAntipsychotics_20110928.pdf. Published September 2011. Accessed June 6, 2014.
2. American Psychiatric Association. Practice guideline for the treatment of patients with bipolar disorder (revision). Am J Psychiatry. 2002;159(suppl 4):1-50.
3. Ng F, Mammen OK, Wilting I, et al; International Society for Bipolar Disorders. The International Society for Bipolar Disorders (ISBD) consensus guidelines for the safety monitoring of bipolar disorder treatments. Bipolar Disord. 2009;11(6):559-595.
4. National Institute for Health and Clinical Excellence. Bipolar disorder (CG38). The management of bipolar disorder in adults, children and adolescents, in primary and secondary care. http://www.nice.org.uk/CG038. Updated February 13, 2014. Accessed June 6, 2014.
5. Yatham LN, Kennedy SH, O’Donovan C, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) guidelines for the management of patients with bipolar disorder: update 2007. Bipolar Disord. 2006;8(6):721-739.
6. Zeier K, Connell R, Resch W, et al. Recommendations for lab monitoring of atypical antipsychotics. Current Psychiatry. 2013; 12(9):51-54.
7. Krishnan KR. Psychiatric and medical comorbidities of bipolar disorder. Psychosom Med. 2005;67(1):1-8.
8. Kilbourne AM, Post EP, Bauer MS, et al. Therapeutic drug and cardiovascular disease risk monitoring in patients with bipolar disorder. J Affect Disord. 2007;102(1-3):145-151.
9. Marcus SC, Olfson M, Pincus HA, et al. Therapeutic drug monitoring of mood stabilizers in Medicaid patients with bipolar disorder. Am J Psychiatry. 1999;156(7):1014-1018.
Post-World War II psychiatry: 70 years of momentous change
A large percentage of psychiatrists practicing today are Boomers, and have experienced the tumultuous change in their profession since the end of World War II. At a recent Grand Rounds presentation in the Department of Neurology & Psychiatry at Saint Louis University, participants examined major changes and paradigm shifts that have reshaped psychiatry since 1946. The audience, which included me, contributed historical observations to the list of those changes and shifts, which I’ve classified here for your benefit, whether or not you are a Boomer.
Medical advances
Consider these discoveries and developments:
• Penicillin in 1947, which led to a reduction in cases of psychosis caused by tertiary syphilis, a disease that accounted for 10% to 15% of state hospital admissions.
• Lithium in 1948, the first pharmaceutical treatment for mania.
• Chlorpromazine, the first antipsychotic drug, in 1952, launching the psychopharmacology era and ending lifetime institutional sequestration as the only “treatment” for serious mental disorders.
• Monoamine oxidase inhibitors in 1959, from observations that iproniazid, a drug used in tuberculosis sanitariums, improved the mood of tuberculosis patients. This was the first pharmacotherapy for depression, which had been treated with electroconvulsive therapy (ECT), developed in the 1930s.
• Tricyclic antidepressants, starting with imipramine in the late 1950s, during attempts to synthesize additional phenothiazine antipsychotics.
• Diazepam, introduced in 1963 for its anti-anxiety effects, became the most widely used drug in the world over the next 2 decades.
• Pre-frontal lobotomy to treat severe psychiatric disorders. The neurosurgeon-inventor of this so-called medical advance won the 1949 Nobel Prize for Medicine or Physiology. The procedure was rapidly discredited after the development of antipsychotic drugs.
• Fluoxetine, the first selective serotonin reuptake inhibitor, in 1987, revolutionized the treatment of depression, especially in primary care settings.
• Clozapine, as an effective treatment for refractory and suicidal schizophrenia, and the spawning of second-generation antipsychotics. These newer agents shifted focus from neurologic adverse effects (extrapyramidal symptoms, tardive dyskinesia) to cardio-metabolic side effects (obesity, diabetes, dyslipidemia, and hypertension).
Changes to the landscape of health care
Three noteworthy developments made the list:
• The Community Mental Health Act of 1963, signed into law by President John F. Kennedy, revolutionized psychiatric care by shifting delivery of care from inpatient, hospital-based facilities to outpatient, clinic-based centers. There are now close to 800 community mental health centers in the United States, where care is dominated by non-physician mental health providers—in contrast to the era of state hospitals, during which physicians and nurses provided care for mentally ill patients.
• Deinstitutionalization. This move-ment gathered momentum in the 1970s and 1980s, leading to closing of the majority of state hospitals, with tragic consequences for the seriously mentally ill—including early demise, homelessness, substance abuse, and incarceration. In fact, the large percentage of mentally ill people in U.S. jails and prisons, instead of in a hospital, represents what has been labeled trans-institutionalization (see my March 2008 editorial, “Bring back the asylums?,” available at CurrentPsychiatry.com).
• Managed care, emerging in the late 1980s and early 1990s, caused a seismic disturbance in the delivery of, and reimbursement for, psychiatric care. The result was a significant decline in access to, and quality of, care—especially the so-called carve-out model that reduced payment for psychiatric care even more drastically than for general medical care. Under managed care, the priority became saving money, rather than saving lives. Average hospital stay for patients who had a psychiatric disorder, which was years in the pre-pharmacotherapy era, and weeks or months after that, shrunk to a few days under managed care.
Changes in professional direction
Two major shifts in the complexion of the specialty were identified:
• The decline of psychoanalysis, which had dominated psychiatry for decades (the 1940s through the 1970s), was a major shift in the conceptualization, training, and delivery of care in psychiatry. The rise of biological psychiatry and the medical model of psychiatric brain disorders, as well as the emergence of evidence-based (and briefer) psychotherapies (eg, cognitive-behavioral therapy, dialectical behavior therapy, and interpersonal therapy), gradually replaced the Freudian model of mental illness.
As a result, it became no longer necessary to be a certified psychoanalyst to be named chair of a department of psychiatry. The impact of this change on psychiatric training has been profound, because medical management by psychiatrists superseded psychotherapy— given the brief hospitalization that is required and the diminishing coverage for psychotherapy by insurers.
• Delegation of psychosocial treatments to non-psychiatrists. The unintended consequences of psychiatrists’ change of focus to 1) consultation on medical/surgical patients and 2) the medical evaluation, diagnosis, and pharmacotherapy of mental disorders led to the so-called “dual treatment model” for the most seriously mentally ill patients: The physician provides medical management and non-physician mental health professionals provide counseling, psychosocial therapy, and rehabilitation.
Disruptive breakthroughs
Several are notable:
• National Institute of Mental Health (NIMH). Establishment of NIMH in April 1949 was a major step toward funding research into psychiatric disorders. Billions of dollars have been invested to generate knowledge about the causes, treatment, course, and prevention of mental illness. No other country has spent as much on psychiatric research. It would have been nearly impossible to discover what we know today without the work of NIMH.
• Neuroscience. The meteoric rise of neuroscience from the 1960s to the present has had a dramatic effect, transforming old psychiatry and the study and therapy of the mind to a focus on the brain-mind continuum and the prospects of brain repair and neuroplasticity. Psychiatry is now regarded as a clinical neuroscience specialty of brain disorders that manifest as changes in thought, affect, mood, cognition, and behavior.
• Brain imaging. Techniques developed since the 1970s—the veritable alphabet soup of CT, PET, SPECT, MRI, MRS, fMRI, and DTI— has revolutionized understanding of brain structure and function in all psychiatric disorders but especially in psychotic and mood disorders.
• Molecular genetics. Advances over the past 2 decades have shed unprecedented light on the complex genetics of psychiatric disorders. It is becoming apparent that most psychiatric disorders are caused via gene-by-environment interaction; etiology is therefore a consequence of genetic and non-genetic variables. Risk genes, copy number variants, and de novo mutations are being discovered almost weekly, and progress in epigenetics holds promise for preventing medical disorders, including psychiatric illness.
• Neuromodulation. Advances represent an important paradigm shift, from pharmacotherapy to brain stimulation. Several techniques have been approved by the FDA, including transcranial magnetic stimulation, vagus nerve stimulation, and deep brain stimulation, to supplement, and perhaps eventually supplant, ECT.
Legal intrusiveness
No other medical specialty has been subject to laws governing clinical practice as psychiatry has been. Progressive intrusion of laws (ostensibly, enacted to protect the civil rights of “the disabled”) ends up hurting patients who refuse admission and then often harm themselves or others or decline urgent treatment, which can be associated with loss of brain tissue in acute psychotic, manic, and depressed states. No legal shackles apply to treating unconscious stroke patients, delirious geriatric patients, or semiconscious myocardial infarction patients when they are admitted to a hospital.
Distortions of the anti-psychiatry movement
The antipsychiatry movement preceded the Baby Boomer era by a century but has continued unabated. The movement gained momentum and became more defamatory after release of the movie One Flew Over the Cuckoo’s Nest in 1975, which portrayed psychiatry in a purely negative light. Despite progress in public understanding of psychiatry, and tangible improvements in practice, the stigma of mental illness persists. Media portrayals, including motion pictures, continue to distort the good that psychiatrists do for their patients.
Gender and sexuality
• Gender distribution of psychiatrists. A major shift occurred over the past 7 decades, reflecting the same pattern that has been documented in other medical specialties. At least one-half of psychiatry residents are now women—a welcome change from the pre-1946 era, when nearly all psychiatrists were men. This demographic transformation has had an impact on the dynamics of psychiatric practice.
• Acceptance and depathologization of homosexuality. Until 1974, homosexuality was considered a psychiatric disorder, and many homosexual persons sought treatment. That year, membership of the American Psychiatric Association voted to remove homosexuality from DSM-II and to no longer regard it as a behavioral abnormality. This was a huge step toward de-pathologizing same-sex orientation and love, and might have been the major impetus for the progressive social acceptance of gay, lesbian, and transgendered people over the past 4 decades.
The DSM paradigm shift in psychiatric diagnosis
• DSM-III. Perhaps the most radical change in the diagnostic criteria of psychiatric disorders occurred in 1980, with introduction of DSM-III to replace DSM-I and DSM-II, which were absurdly vague, unreliable, and with poor validity.
The move toward more operational and reliable diagnostic requirements began with the Research Diagnostic Criteria, developed by the Department of Psychiatry at Washington University in St. Louis. DSM-III represented a complete paradigm shift in psychiatric diagnosis. Subsequent editions maintained the same methodology, with relatively modest changes. The field expects continued evolution in DSM diagnostic criteria, including the future inclusion of biomarkers, based on sound, controlled studies.
• Recognizing PTSD. Develop-ment of posttraumatic stress disorder (PTSD) as a diagnostic entity, and its inclusion in DSM-III, were major changes in psychiatric nosology. At last, the old terms—shell shock, battle fatigue, neurasthenia—were legitimized as a recognizable syndrome secondary to major life trauma, including war and rape. That legitimacy has instigated substantial clinical and research interest in identifying how serious psychopathology can be triggered by life events.
Pharmaceutical industry debacle
Few industries have fallen so far from grace in the eyes of psychiatric professionals and the public as the manufacturers of psychotropic drugs.
At the dawn of the psychopharmacology era (the 1950s, 1960s, and 1970s) pharmaceutical companies were respected and regarded by physicians and patients as a vital partner in health care for their discovery and development of medications to treat psychiatric disorders. That image was tarnished in the 1990s, however, with the approval and release of several atypical antipsychotics. Cutthroat competition, questionable publication methods, concealment of negative findings, and excessive spending on marketing, including FDA-approved educational programs for clinicians on efficacy, safety, and dosing, all contributed to escalating cynicism about the industry. Academic faculty who received research grants to conduct FDA-required clinical trials of new agents were painted with the same brush.
Disclosure of potential conflict of interest became a mandatory procedure at universities and for NIMH grant applicants and journal publishers. Class-action lawsuits against companies that manufacture second-generation antipsychotics—filed for lack of transparency about metabolic side effects—exacerbated the intensity of criticism and condemnation.
Although new drug development has become measurably more rigorous and ethical because of self-regulation, combined with vigorous government scrutiny and regulation, demonization of the pharmaceutical industry remains unabated. That might be the reason why several major pharmaceutical companies have abandoned research and development of psychotropic drugs. That is likely to impede progress in psychopharmacotherapeutics; after all, no other private or government entity develops drugs for patients who have a psychiatric illness. The need for such agents is great: There is no FDA-indicated drug for the majority of DSM-5 diagnoses.
We entrust the future to next generations
Momentous events have transformed psychiatry during the lifespan of Baby Boomers like me and many of you. Because the cohort of 80 million Baby Boomers has comprised a significant percentage of the nation’s scientists, media representatives, members of the American Psychiatric Association, academicians, and community leaders over the past few decades, it is conceivable that the Baby Boomer generation helped trigger most of the transformative changes in psychiatry we have seen over the past 70 years.
I can only wonder: What direction will psychiatry take in the age of Generation X, Generation Y, and the Millennials? Only this is certain: Psychiatry will continue to evolve— long after Baby Boomers are gone.
A large percentage of psychiatrists practicing today are Boomers, and have experienced the tumultuous change in their profession since the end of World War II. At a recent Grand Rounds presentation in the Department of Neurology & Psychiatry at Saint Louis University, participants examined major changes and paradigm shifts that have reshaped psychiatry since 1946. The audience, which included me, contributed historical observations to the list of those changes and shifts, which I’ve classified here for your benefit, whether or not you are a Boomer.
Medical advances
Consider these discoveries and developments:
• Penicillin in 1947, which led to a reduction in cases of psychosis caused by tertiary syphilis, a disease that accounted for 10% to 15% of state hospital admissions.
• Lithium in 1948, the first pharmaceutical treatment for mania.
• Chlorpromazine, the first antipsychotic drug, in 1952, launching the psychopharmacology era and ending lifetime institutional sequestration as the only “treatment” for serious mental disorders.
• Monoamine oxidase inhibitors in 1959, from observations that iproniazid, a drug used in tuberculosis sanitariums, improved the mood of tuberculosis patients. This was the first pharmacotherapy for depression, which had been treated with electroconvulsive therapy (ECT), developed in the 1930s.
• Tricyclic antidepressants, starting with imipramine in the late 1950s, during attempts to synthesize additional phenothiazine antipsychotics.
• Diazepam, introduced in 1963 for its anti-anxiety effects, became the most widely used drug in the world over the next 2 decades.
• Pre-frontal lobotomy to treat severe psychiatric disorders. The neurosurgeon-inventor of this so-called medical advance won the 1949 Nobel Prize for Medicine or Physiology. The procedure was rapidly discredited after the development of antipsychotic drugs.
• Fluoxetine, the first selective serotonin reuptake inhibitor, in 1987, revolutionized the treatment of depression, especially in primary care settings.
• Clozapine, as an effective treatment for refractory and suicidal schizophrenia, and the spawning of second-generation antipsychotics. These newer agents shifted focus from neurologic adverse effects (extrapyramidal symptoms, tardive dyskinesia) to cardio-metabolic side effects (obesity, diabetes, dyslipidemia, and hypertension).
Changes to the landscape of health care
Three noteworthy developments made the list:
• The Community Mental Health Act of 1963, signed into law by President John F. Kennedy, revolutionized psychiatric care by shifting delivery of care from inpatient, hospital-based facilities to outpatient, clinic-based centers. There are now close to 800 community mental health centers in the United States, where care is dominated by non-physician mental health providers—in contrast to the era of state hospitals, during which physicians and nurses provided care for mentally ill patients.
• Deinstitutionalization. This move-ment gathered momentum in the 1970s and 1980s, leading to closing of the majority of state hospitals, with tragic consequences for the seriously mentally ill—including early demise, homelessness, substance abuse, and incarceration. In fact, the large percentage of mentally ill people in U.S. jails and prisons, instead of in a hospital, represents what has been labeled trans-institutionalization (see my March 2008 editorial, “Bring back the asylums?,” available at CurrentPsychiatry.com).
• Managed care, emerging in the late 1980s and early 1990s, caused a seismic disturbance in the delivery of, and reimbursement for, psychiatric care. The result was a significant decline in access to, and quality of, care—especially the so-called carve-out model that reduced payment for psychiatric care even more drastically than for general medical care. Under managed care, the priority became saving money, rather than saving lives. Average hospital stay for patients who had a psychiatric disorder, which was years in the pre-pharmacotherapy era, and weeks or months after that, shrunk to a few days under managed care.
Changes in professional direction
Two major shifts in the complexion of the specialty were identified:
• The decline of psychoanalysis, which had dominated psychiatry for decades (the 1940s through the 1970s), was a major shift in the conceptualization, training, and delivery of care in psychiatry. The rise of biological psychiatry and the medical model of psychiatric brain disorders, as well as the emergence of evidence-based (and briefer) psychotherapies (eg, cognitive-behavioral therapy, dialectical behavior therapy, and interpersonal therapy), gradually replaced the Freudian model of mental illness.
As a result, it became no longer necessary to be a certified psychoanalyst to be named chair of a department of psychiatry. The impact of this change on psychiatric training has been profound, because medical management by psychiatrists superseded psychotherapy— given the brief hospitalization that is required and the diminishing coverage for psychotherapy by insurers.
• Delegation of psychosocial treatments to non-psychiatrists. The unintended consequences of psychiatrists’ change of focus to 1) consultation on medical/surgical patients and 2) the medical evaluation, diagnosis, and pharmacotherapy of mental disorders led to the so-called “dual treatment model” for the most seriously mentally ill patients: The physician provides medical management and non-physician mental health professionals provide counseling, psychosocial therapy, and rehabilitation.
Disruptive breakthroughs
Several are notable:
• National Institute of Mental Health (NIMH). Establishment of NIMH in April 1949 was a major step toward funding research into psychiatric disorders. Billions of dollars have been invested to generate knowledge about the causes, treatment, course, and prevention of mental illness. No other country has spent as much on psychiatric research. It would have been nearly impossible to discover what we know today without the work of NIMH.
• Neuroscience. The meteoric rise of neuroscience from the 1960s to the present has had a dramatic effect, transforming old psychiatry and the study and therapy of the mind to a focus on the brain-mind continuum and the prospects of brain repair and neuroplasticity. Psychiatry is now regarded as a clinical neuroscience specialty of brain disorders that manifest as changes in thought, affect, mood, cognition, and behavior.
• Brain imaging. Techniques developed since the 1970s—the veritable alphabet soup of CT, PET, SPECT, MRI, MRS, fMRI, and DTI— has revolutionized understanding of brain structure and function in all psychiatric disorders but especially in psychotic and mood disorders.
• Molecular genetics. Advances over the past 2 decades have shed unprecedented light on the complex genetics of psychiatric disorders. It is becoming apparent that most psychiatric disorders are caused via gene-by-environment interaction; etiology is therefore a consequence of genetic and non-genetic variables. Risk genes, copy number variants, and de novo mutations are being discovered almost weekly, and progress in epigenetics holds promise for preventing medical disorders, including psychiatric illness.
• Neuromodulation. Advances represent an important paradigm shift, from pharmacotherapy to brain stimulation. Several techniques have been approved by the FDA, including transcranial magnetic stimulation, vagus nerve stimulation, and deep brain stimulation, to supplement, and perhaps eventually supplant, ECT.
Legal intrusiveness
No other medical specialty has been subject to laws governing clinical practice as psychiatry has been. Progressive intrusion of laws (ostensibly, enacted to protect the civil rights of “the disabled”) ends up hurting patients who refuse admission and then often harm themselves or others or decline urgent treatment, which can be associated with loss of brain tissue in acute psychotic, manic, and depressed states. No legal shackles apply to treating unconscious stroke patients, delirious geriatric patients, or semiconscious myocardial infarction patients when they are admitted to a hospital.
Distortions of the anti-psychiatry movement
The antipsychiatry movement preceded the Baby Boomer era by a century but has continued unabated. The movement gained momentum and became more defamatory after release of the movie One Flew Over the Cuckoo’s Nest in 1975, which portrayed psychiatry in a purely negative light. Despite progress in public understanding of psychiatry, and tangible improvements in practice, the stigma of mental illness persists. Media portrayals, including motion pictures, continue to distort the good that psychiatrists do for their patients.
Gender and sexuality
• Gender distribution of psychiatrists. A major shift occurred over the past 7 decades, reflecting the same pattern that has been documented in other medical specialties. At least one-half of psychiatry residents are now women—a welcome change from the pre-1946 era, when nearly all psychiatrists were men. This demographic transformation has had an impact on the dynamics of psychiatric practice.
• Acceptance and depathologization of homosexuality. Until 1974, homosexuality was considered a psychiatric disorder, and many homosexual persons sought treatment. That year, membership of the American Psychiatric Association voted to remove homosexuality from DSM-II and to no longer regard it as a behavioral abnormality. This was a huge step toward de-pathologizing same-sex orientation and love, and might have been the major impetus for the progressive social acceptance of gay, lesbian, and transgendered people over the past 4 decades.
The DSM paradigm shift in psychiatric diagnosis
• DSM-III. Perhaps the most radical change in the diagnostic criteria of psychiatric disorders occurred in 1980, with introduction of DSM-III to replace DSM-I and DSM-II, which were absurdly vague, unreliable, and with poor validity.
The move toward more operational and reliable diagnostic requirements began with the Research Diagnostic Criteria, developed by the Department of Psychiatry at Washington University in St. Louis. DSM-III represented a complete paradigm shift in psychiatric diagnosis. Subsequent editions maintained the same methodology, with relatively modest changes. The field expects continued evolution in DSM diagnostic criteria, including the future inclusion of biomarkers, based on sound, controlled studies.
• Recognizing PTSD. Develop-ment of posttraumatic stress disorder (PTSD) as a diagnostic entity, and its inclusion in DSM-III, were major changes in psychiatric nosology. At last, the old terms—shell shock, battle fatigue, neurasthenia—were legitimized as a recognizable syndrome secondary to major life trauma, including war and rape. That legitimacy has instigated substantial clinical and research interest in identifying how serious psychopathology can be triggered by life events.
Pharmaceutical industry debacle
Few industries have fallen so far from grace in the eyes of psychiatric professionals and the public as the manufacturers of psychotropic drugs.
At the dawn of the psychopharmacology era (the 1950s, 1960s, and 1970s) pharmaceutical companies were respected and regarded by physicians and patients as a vital partner in health care for their discovery and development of medications to treat psychiatric disorders. That image was tarnished in the 1990s, however, with the approval and release of several atypical antipsychotics. Cutthroat competition, questionable publication methods, concealment of negative findings, and excessive spending on marketing, including FDA-approved educational programs for clinicians on efficacy, safety, and dosing, all contributed to escalating cynicism about the industry. Academic faculty who received research grants to conduct FDA-required clinical trials of new agents were painted with the same brush.
Disclosure of potential conflict of interest became a mandatory procedure at universities and for NIMH grant applicants and journal publishers. Class-action lawsuits against companies that manufacture second-generation antipsychotics—filed for lack of transparency about metabolic side effects—exacerbated the intensity of criticism and condemnation.
Although new drug development has become measurably more rigorous and ethical because of self-regulation, combined with vigorous government scrutiny and regulation, demonization of the pharmaceutical industry remains unabated. That might be the reason why several major pharmaceutical companies have abandoned research and development of psychotropic drugs. That is likely to impede progress in psychopharmacotherapeutics; after all, no other private or government entity develops drugs for patients who have a psychiatric illness. The need for such agents is great: There is no FDA-indicated drug for the majority of DSM-5 diagnoses.
We entrust the future to next generations
Momentous events have transformed psychiatry during the lifespan of Baby Boomers like me and many of you. Because the cohort of 80 million Baby Boomers has comprised a significant percentage of the nation’s scientists, media representatives, members of the American Psychiatric Association, academicians, and community leaders over the past few decades, it is conceivable that the Baby Boomer generation helped trigger most of the transformative changes in psychiatry we have seen over the past 70 years.
I can only wonder: What direction will psychiatry take in the age of Generation X, Generation Y, and the Millennials? Only this is certain: Psychiatry will continue to evolve— long after Baby Boomers are gone.
A large percentage of psychiatrists practicing today are Boomers, and have experienced the tumultuous change in their profession since the end of World War II. At a recent Grand Rounds presentation in the Department of Neurology & Psychiatry at Saint Louis University, participants examined major changes and paradigm shifts that have reshaped psychiatry since 1946. The audience, which included me, contributed historical observations to the list of those changes and shifts, which I’ve classified here for your benefit, whether or not you are a Boomer.
Medical advances
Consider these discoveries and developments:
• Penicillin in 1947, which led to a reduction in cases of psychosis caused by tertiary syphilis, a disease that accounted for 10% to 15% of state hospital admissions.
• Lithium in 1948, the first pharmaceutical treatment for mania.
• Chlorpromazine, the first antipsychotic drug, in 1952, launching the psychopharmacology era and ending lifetime institutional sequestration as the only “treatment” for serious mental disorders.
• Monoamine oxidase inhibitors in 1959, from observations that iproniazid, a drug used in tuberculosis sanitariums, improved the mood of tuberculosis patients. This was the first pharmacotherapy for depression, which had been treated with electroconvulsive therapy (ECT), developed in the 1930s.
• Tricyclic antidepressants, starting with imipramine in the late 1950s, during attempts to synthesize additional phenothiazine antipsychotics.
• Diazepam, introduced in 1963 for its anti-anxiety effects, became the most widely used drug in the world over the next 2 decades.
• Pre-frontal lobotomy to treat severe psychiatric disorders. The neurosurgeon-inventor of this so-called medical advance won the 1949 Nobel Prize for Medicine or Physiology. The procedure was rapidly discredited after the development of antipsychotic drugs.
• Fluoxetine, the first selective serotonin reuptake inhibitor, in 1987, revolutionized the treatment of depression, especially in primary care settings.
• Clozapine, as an effective treatment for refractory and suicidal schizophrenia, and the spawning of second-generation antipsychotics. These newer agents shifted focus from neurologic adverse effects (extrapyramidal symptoms, tardive dyskinesia) to cardio-metabolic side effects (obesity, diabetes, dyslipidemia, and hypertension).
Changes to the landscape of health care
Three noteworthy developments made the list:
• The Community Mental Health Act of 1963, signed into law by President John F. Kennedy, revolutionized psychiatric care by shifting delivery of care from inpatient, hospital-based facilities to outpatient, clinic-based centers. There are now close to 800 community mental health centers in the United States, where care is dominated by non-physician mental health providers—in contrast to the era of state hospitals, during which physicians and nurses provided care for mentally ill patients.
• Deinstitutionalization. This move-ment gathered momentum in the 1970s and 1980s, leading to closing of the majority of state hospitals, with tragic consequences for the seriously mentally ill—including early demise, homelessness, substance abuse, and incarceration. In fact, the large percentage of mentally ill people in U.S. jails and prisons, instead of in a hospital, represents what has been labeled trans-institutionalization (see my March 2008 editorial, “Bring back the asylums?,” available at CurrentPsychiatry.com).
• Managed care, emerging in the late 1980s and early 1990s, caused a seismic disturbance in the delivery of, and reimbursement for, psychiatric care. The result was a significant decline in access to, and quality of, care—especially the so-called carve-out model that reduced payment for psychiatric care even more drastically than for general medical care. Under managed care, the priority became saving money, rather than saving lives. Average hospital stay for patients who had a psychiatric disorder, which was years in the pre-pharmacotherapy era, and weeks or months after that, shrunk to a few days under managed care.
Changes in professional direction
Two major shifts in the complexion of the specialty were identified:
• The decline of psychoanalysis, which had dominated psychiatry for decades (the 1940s through the 1970s), was a major shift in the conceptualization, training, and delivery of care in psychiatry. The rise of biological psychiatry and the medical model of psychiatric brain disorders, as well as the emergence of evidence-based (and briefer) psychotherapies (eg, cognitive-behavioral therapy, dialectical behavior therapy, and interpersonal therapy), gradually replaced the Freudian model of mental illness.
As a result, it became no longer necessary to be a certified psychoanalyst to be named chair of a department of psychiatry. The impact of this change on psychiatric training has been profound, because medical management by psychiatrists superseded psychotherapy— given the brief hospitalization that is required and the diminishing coverage for psychotherapy by insurers.
• Delegation of psychosocial treatments to non-psychiatrists. The unintended consequences of psychiatrists’ change of focus to 1) consultation on medical/surgical patients and 2) the medical evaluation, diagnosis, and pharmacotherapy of mental disorders led to the so-called “dual treatment model” for the most seriously mentally ill patients: The physician provides medical management and non-physician mental health professionals provide counseling, psychosocial therapy, and rehabilitation.
Disruptive breakthroughs
Several are notable:
• National Institute of Mental Health (NIMH). Establishment of NIMH in April 1949 was a major step toward funding research into psychiatric disorders. Billions of dollars have been invested to generate knowledge about the causes, treatment, course, and prevention of mental illness. No other country has spent as much on psychiatric research. It would have been nearly impossible to discover what we know today without the work of NIMH.
• Neuroscience. The meteoric rise of neuroscience from the 1960s to the present has had a dramatic effect, transforming old psychiatry and the study and therapy of the mind to a focus on the brain-mind continuum and the prospects of brain repair and neuroplasticity. Psychiatry is now regarded as a clinical neuroscience specialty of brain disorders that manifest as changes in thought, affect, mood, cognition, and behavior.
• Brain imaging. Techniques developed since the 1970s—the veritable alphabet soup of CT, PET, SPECT, MRI, MRS, fMRI, and DTI— has revolutionized understanding of brain structure and function in all psychiatric disorders but especially in psychotic and mood disorders.
• Molecular genetics. Advances over the past 2 decades have shed unprecedented light on the complex genetics of psychiatric disorders. It is becoming apparent that most psychiatric disorders are caused via gene-by-environment interaction; etiology is therefore a consequence of genetic and non-genetic variables. Risk genes, copy number variants, and de novo mutations are being discovered almost weekly, and progress in epigenetics holds promise for preventing medical disorders, including psychiatric illness.
• Neuromodulation. Advances represent an important paradigm shift, from pharmacotherapy to brain stimulation. Several techniques have been approved by the FDA, including transcranial magnetic stimulation, vagus nerve stimulation, and deep brain stimulation, to supplement, and perhaps eventually supplant, ECT.
Legal intrusiveness
No other medical specialty has been subject to laws governing clinical practice as psychiatry has been. Progressive intrusion of laws (ostensibly, enacted to protect the civil rights of “the disabled”) ends up hurting patients who refuse admission and then often harm themselves or others or decline urgent treatment, which can be associated with loss of brain tissue in acute psychotic, manic, and depressed states. No legal shackles apply to treating unconscious stroke patients, delirious geriatric patients, or semiconscious myocardial infarction patients when they are admitted to a hospital.
Distortions of the anti-psychiatry movement
The antipsychiatry movement preceded the Baby Boomer era by a century but has continued unabated. The movement gained momentum and became more defamatory after release of the movie One Flew Over the Cuckoo’s Nest in 1975, which portrayed psychiatry in a purely negative light. Despite progress in public understanding of psychiatry, and tangible improvements in practice, the stigma of mental illness persists. Media portrayals, including motion pictures, continue to distort the good that psychiatrists do for their patients.
Gender and sexuality
• Gender distribution of psychiatrists. A major shift occurred over the past 7 decades, reflecting the same pattern that has been documented in other medical specialties. At least one-half of psychiatry residents are now women—a welcome change from the pre-1946 era, when nearly all psychiatrists were men. This demographic transformation has had an impact on the dynamics of psychiatric practice.
• Acceptance and depathologization of homosexuality. Until 1974, homosexuality was considered a psychiatric disorder, and many homosexual persons sought treatment. That year, membership of the American Psychiatric Association voted to remove homosexuality from DSM-II and to no longer regard it as a behavioral abnormality. This was a huge step toward de-pathologizing same-sex orientation and love, and might have been the major impetus for the progressive social acceptance of gay, lesbian, and transgendered people over the past 4 decades.
The DSM paradigm shift in psychiatric diagnosis
• DSM-III. Perhaps the most radical change in the diagnostic criteria of psychiatric disorders occurred in 1980, with introduction of DSM-III to replace DSM-I and DSM-II, which were absurdly vague, unreliable, and with poor validity.
The move toward more operational and reliable diagnostic requirements began with the Research Diagnostic Criteria, developed by the Department of Psychiatry at Washington University in St. Louis. DSM-III represented a complete paradigm shift in psychiatric diagnosis. Subsequent editions maintained the same methodology, with relatively modest changes. The field expects continued evolution in DSM diagnostic criteria, including the future inclusion of biomarkers, based on sound, controlled studies.
• Recognizing PTSD. Develop-ment of posttraumatic stress disorder (PTSD) as a diagnostic entity, and its inclusion in DSM-III, were major changes in psychiatric nosology. At last, the old terms—shell shock, battle fatigue, neurasthenia—were legitimized as a recognizable syndrome secondary to major life trauma, including war and rape. That legitimacy has instigated substantial clinical and research interest in identifying how serious psychopathology can be triggered by life events.
Pharmaceutical industry debacle
Few industries have fallen so far from grace in the eyes of psychiatric professionals and the public as the manufacturers of psychotropic drugs.
At the dawn of the psychopharmacology era (the 1950s, 1960s, and 1970s) pharmaceutical companies were respected and regarded by physicians and patients as a vital partner in health care for their discovery and development of medications to treat psychiatric disorders. That image was tarnished in the 1990s, however, with the approval and release of several atypical antipsychotics. Cutthroat competition, questionable publication methods, concealment of negative findings, and excessive spending on marketing, including FDA-approved educational programs for clinicians on efficacy, safety, and dosing, all contributed to escalating cynicism about the industry. Academic faculty who received research grants to conduct FDA-required clinical trials of new agents were painted with the same brush.
Disclosure of potential conflict of interest became a mandatory procedure at universities and for NIMH grant applicants and journal publishers. Class-action lawsuits against companies that manufacture second-generation antipsychotics—filed for lack of transparency about metabolic side effects—exacerbated the intensity of criticism and condemnation.
Although new drug development has become measurably more rigorous and ethical because of self-regulation, combined with vigorous government scrutiny and regulation, demonization of the pharmaceutical industry remains unabated. That might be the reason why several major pharmaceutical companies have abandoned research and development of psychotropic drugs. That is likely to impede progress in psychopharmacotherapeutics; after all, no other private or government entity develops drugs for patients who have a psychiatric illness. The need for such agents is great: There is no FDA-indicated drug for the majority of DSM-5 diagnoses.
We entrust the future to next generations
Momentous events have transformed psychiatry during the lifespan of Baby Boomers like me and many of you. Because the cohort of 80 million Baby Boomers has comprised a significant percentage of the nation’s scientists, media representatives, members of the American Psychiatric Association, academicians, and community leaders over the past few decades, it is conceivable that the Baby Boomer generation helped trigger most of the transformative changes in psychiatry we have seen over the past 70 years.
I can only wonder: What direction will psychiatry take in the age of Generation X, Generation Y, and the Millennials? Only this is certain: Psychiatry will continue to evolve— long after Baby Boomers are gone.
Interactive Journal Review on the Treatment of Bipolar Depression
Dr. Henry Nasrallah and Dr. Joseph Calabrese highlight pivotal data from 2 randomized, double-blind, placebo-controlled, short-term clinical studies, published in the February 2014 issue of The American Journal of Psychiatry, that established the efficacy and safety of a treatment option approved for patients with major depressive episodes associated with bipolar I disorder.
Watch the video here:
Dr. Henry Nasrallah and Dr. Joseph Calabrese highlight pivotal data from 2 randomized, double-blind, placebo-controlled, short-term clinical studies, published in the February 2014 issue of The American Journal of Psychiatry, that established the efficacy and safety of a treatment option approved for patients with major depressive episodes associated with bipolar I disorder.
Watch the video here:
Dr. Henry Nasrallah and Dr. Joseph Calabrese highlight pivotal data from 2 randomized, double-blind, placebo-controlled, short-term clinical studies, published in the February 2014 issue of The American Journal of Psychiatry, that established the efficacy and safety of a treatment option approved for patients with major depressive episodes associated with bipolar I disorder.
Watch the video here:
Clozapine trials
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Lithium for bipolar disorder
Frontotemporal dementia and its variants: What to look for
Frontotemporal dementia (FTD) is a neurologic disease that affects the frontal and the temporal lobes of the cerebral cortex.1 This disorder is observed most often in people between age 45 to 65, but also can manifest in younger or older persons.1 The cause varies among a range of pathologies affecting the anterior portions of the brain.2
Presentations
FTD presents with changes in personality, social skills, ability to concentrate, motivation, reasoning, and language abnormality.3 Memory loss is less prominent in this condition compared with other dementias; therefore, identification may be a diagnostic challenge. FTD can be misdiagnosed as a psychiatric illness or not recognized because social symptoms dominate over cognitive dysfunction. As the disease progresses, patients may become increasingly unable to plan or organize activities of daily living, behave appropriately, and react normally in social interactions.1
FTD has 3 diagnostic variants1-4:
Behavioral variant. Known as Pick disease or the “frontal variant,”1,2 this type of FTD manifests as changes in personality, improper behavior in social settings, personal neglect, or impulsivity, such as shoplifting or hypersexuality.
Primary progressive aphasia. Two types of language dysfunction are observed in FTD:
• Semantic dementia (SD)3: Left-sided SD presents with “meaningless speech” or “word substitutions” (eg, “chair” instead of “table”). Right-sided SD, however, is characterized by forgetting the faces of familiar people or objects.
• Primary nonfluent aphasia3: Language fluency is compromised. Persons with such language dysfunction cannot produce words easily, and their speech is stumbling and nonfluent.
FTD with motor neuron disease.4 The most common type of motor neuron disease associated with FTD is amyotrophic lateral sclerosis. Afflicted patients exhibit muscle weakness, spasms, and rigidity. This leads to difficulty in swallowing or breathing because the diaphragm and pharynx are paralyzed. Other diseases associated with FTD include corticobasal degeneration and progressive supranuclear palsy.
Diagnosis
In DSM-5, FTD has been renamed “frontotemporal lobar degeneration” under the category of “Major and Mild Neurocognitive Disorders.”5 The workup begins with a history, physical examination, and mental status assessment. Physical signs can include frontal-release, primitive reflexes. Early in the disease course, a palmomental reflex often is observed; later, as disease progress, the rooting reflex or palmar grasp may become apparent.1,5
Diagnosing FTD requires recognizing its symptoms and ruling out conditions such as Alzheimer’s disease, depression, and schizophrenia.6 Laboratory studies may help identify other conditions. Brain imaging, such as MRI, can depict frontotemporal pathology and rule in or exclude other diseases.3,5
Psychometric testing can evaluate memory or cognitive ability, which might be unremarkable during the initial phases of FTD.4 Further psychological assessments may provide objective verification of frontal lobe deficiencies in social skills or activities of daily living.3 Positron emission tomography and single-photon emission computed tomography may demonstrate areas of decreased activity or hypoperfusion in frontal and temporal lobes.7
Interventions
Treatment of FTD is limited to symptomatic therapy8; there are no specific, approved countermeasures available. Comorbid conditions, such as diabetes mellitus or hypertension, should be treated medically. Social interventions such as day care, increased supervision, and emotional support from the family can be effective adjuvants.2
Disclosures
The authors report no financial relationship whose products are mentioned in this article or with manufacturers of competing products.
1. Snowden JS, Neary D, Mann DM. Frontotemporal dementia. Br J Psychiatry. 2002;180:140-143.
2. Frontotemporal degeneration. The Association for Frontotemporal Degeneration. http://www.theaftd.org/ frontotemporal-degeneration/ftd-overview. Accessed April 24, 2014.
3. Neary D, Snowden JS, Gustafson L, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology. 1998;51(6):1546-1554.
4. Clark CM, Forman MS. Frontotemporal lobar degeneration with motor neuron disease: a clinical and pathological spectrum. Arch Neurol. 2006;63(4):489-490.
5. Diagnostic and statistical manual of mental disorders. 5th ed. Washington, DC: American Psychiatric Association; 2013:614-618.
6. Frontotemporal dementia diagnosis. UCSF Medical Center. http://www.ucsfhealth.org/conditions/frontotemporal_ dementia/diagnosis.html. Accessed April 24, 2014.
7. McMurtray AM, Chen AK, Shapira JS, et al. Variations in regional SPECT hypoperfusion and clinical features in frontotemporal dementia. Neurology. 2006;66(4):517-522.
8. Miller BL, Lee SE. Frontotemporal dementia: treatment. Up To Date. http://www.uptodate.com/contents/frontotemporal-dementia-treatment?source=search_result&search=frontote mporal+dementia+treatment&selectedTitle=1~150. Updated December 30, 2013. Accessed April 24, 2014.
Frontotemporal dementia (FTD) is a neurologic disease that affects the frontal and the temporal lobes of the cerebral cortex.1 This disorder is observed most often in people between age 45 to 65, but also can manifest in younger or older persons.1 The cause varies among a range of pathologies affecting the anterior portions of the brain.2
Presentations
FTD presents with changes in personality, social skills, ability to concentrate, motivation, reasoning, and language abnormality.3 Memory loss is less prominent in this condition compared with other dementias; therefore, identification may be a diagnostic challenge. FTD can be misdiagnosed as a psychiatric illness or not recognized because social symptoms dominate over cognitive dysfunction. As the disease progresses, patients may become increasingly unable to plan or organize activities of daily living, behave appropriately, and react normally in social interactions.1
FTD has 3 diagnostic variants1-4:
Behavioral variant. Known as Pick disease or the “frontal variant,”1,2 this type of FTD manifests as changes in personality, improper behavior in social settings, personal neglect, or impulsivity, such as shoplifting or hypersexuality.
Primary progressive aphasia. Two types of language dysfunction are observed in FTD:
• Semantic dementia (SD)3: Left-sided SD presents with “meaningless speech” or “word substitutions” (eg, “chair” instead of “table”). Right-sided SD, however, is characterized by forgetting the faces of familiar people or objects.
• Primary nonfluent aphasia3: Language fluency is compromised. Persons with such language dysfunction cannot produce words easily, and their speech is stumbling and nonfluent.
FTD with motor neuron disease.4 The most common type of motor neuron disease associated with FTD is amyotrophic lateral sclerosis. Afflicted patients exhibit muscle weakness, spasms, and rigidity. This leads to difficulty in swallowing or breathing because the diaphragm and pharynx are paralyzed. Other diseases associated with FTD include corticobasal degeneration and progressive supranuclear palsy.
Diagnosis
In DSM-5, FTD has been renamed “frontotemporal lobar degeneration” under the category of “Major and Mild Neurocognitive Disorders.”5 The workup begins with a history, physical examination, and mental status assessment. Physical signs can include frontal-release, primitive reflexes. Early in the disease course, a palmomental reflex often is observed; later, as disease progress, the rooting reflex or palmar grasp may become apparent.1,5
Diagnosing FTD requires recognizing its symptoms and ruling out conditions such as Alzheimer’s disease, depression, and schizophrenia.6 Laboratory studies may help identify other conditions. Brain imaging, such as MRI, can depict frontotemporal pathology and rule in or exclude other diseases.3,5
Psychometric testing can evaluate memory or cognitive ability, which might be unremarkable during the initial phases of FTD.4 Further psychological assessments may provide objective verification of frontal lobe deficiencies in social skills or activities of daily living.3 Positron emission tomography and single-photon emission computed tomography may demonstrate areas of decreased activity or hypoperfusion in frontal and temporal lobes.7
Interventions
Treatment of FTD is limited to symptomatic therapy8; there are no specific, approved countermeasures available. Comorbid conditions, such as diabetes mellitus or hypertension, should be treated medically. Social interventions such as day care, increased supervision, and emotional support from the family can be effective adjuvants.2
Disclosures
The authors report no financial relationship whose products are mentioned in this article or with manufacturers of competing products.
Frontotemporal dementia (FTD) is a neurologic disease that affects the frontal and the temporal lobes of the cerebral cortex.1 This disorder is observed most often in people between age 45 to 65, but also can manifest in younger or older persons.1 The cause varies among a range of pathologies affecting the anterior portions of the brain.2
Presentations
FTD presents with changes in personality, social skills, ability to concentrate, motivation, reasoning, and language abnormality.3 Memory loss is less prominent in this condition compared with other dementias; therefore, identification may be a diagnostic challenge. FTD can be misdiagnosed as a psychiatric illness or not recognized because social symptoms dominate over cognitive dysfunction. As the disease progresses, patients may become increasingly unable to plan or organize activities of daily living, behave appropriately, and react normally in social interactions.1
FTD has 3 diagnostic variants1-4:
Behavioral variant. Known as Pick disease or the “frontal variant,”1,2 this type of FTD manifests as changes in personality, improper behavior in social settings, personal neglect, or impulsivity, such as shoplifting or hypersexuality.
Primary progressive aphasia. Two types of language dysfunction are observed in FTD:
• Semantic dementia (SD)3: Left-sided SD presents with “meaningless speech” or “word substitutions” (eg, “chair” instead of “table”). Right-sided SD, however, is characterized by forgetting the faces of familiar people or objects.
• Primary nonfluent aphasia3: Language fluency is compromised. Persons with such language dysfunction cannot produce words easily, and their speech is stumbling and nonfluent.
FTD with motor neuron disease.4 The most common type of motor neuron disease associated with FTD is amyotrophic lateral sclerosis. Afflicted patients exhibit muscle weakness, spasms, and rigidity. This leads to difficulty in swallowing or breathing because the diaphragm and pharynx are paralyzed. Other diseases associated with FTD include corticobasal degeneration and progressive supranuclear palsy.
Diagnosis
In DSM-5, FTD has been renamed “frontotemporal lobar degeneration” under the category of “Major and Mild Neurocognitive Disorders.”5 The workup begins with a history, physical examination, and mental status assessment. Physical signs can include frontal-release, primitive reflexes. Early in the disease course, a palmomental reflex often is observed; later, as disease progress, the rooting reflex or palmar grasp may become apparent.1,5
Diagnosing FTD requires recognizing its symptoms and ruling out conditions such as Alzheimer’s disease, depression, and schizophrenia.6 Laboratory studies may help identify other conditions. Brain imaging, such as MRI, can depict frontotemporal pathology and rule in or exclude other diseases.3,5
Psychometric testing can evaluate memory or cognitive ability, which might be unremarkable during the initial phases of FTD.4 Further psychological assessments may provide objective verification of frontal lobe deficiencies in social skills or activities of daily living.3 Positron emission tomography and single-photon emission computed tomography may demonstrate areas of decreased activity or hypoperfusion in frontal and temporal lobes.7
Interventions
Treatment of FTD is limited to symptomatic therapy8; there are no specific, approved countermeasures available. Comorbid conditions, such as diabetes mellitus or hypertension, should be treated medically. Social interventions such as day care, increased supervision, and emotional support from the family can be effective adjuvants.2
Disclosures
The authors report no financial relationship whose products are mentioned in this article or with manufacturers of competing products.
1. Snowden JS, Neary D, Mann DM. Frontotemporal dementia. Br J Psychiatry. 2002;180:140-143.
2. Frontotemporal degeneration. The Association for Frontotemporal Degeneration. http://www.theaftd.org/ frontotemporal-degeneration/ftd-overview. Accessed April 24, 2014.
3. Neary D, Snowden JS, Gustafson L, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology. 1998;51(6):1546-1554.
4. Clark CM, Forman MS. Frontotemporal lobar degeneration with motor neuron disease: a clinical and pathological spectrum. Arch Neurol. 2006;63(4):489-490.
5. Diagnostic and statistical manual of mental disorders. 5th ed. Washington, DC: American Psychiatric Association; 2013:614-618.
6. Frontotemporal dementia diagnosis. UCSF Medical Center. http://www.ucsfhealth.org/conditions/frontotemporal_ dementia/diagnosis.html. Accessed April 24, 2014.
7. McMurtray AM, Chen AK, Shapira JS, et al. Variations in regional SPECT hypoperfusion and clinical features in frontotemporal dementia. Neurology. 2006;66(4):517-522.
8. Miller BL, Lee SE. Frontotemporal dementia: treatment. Up To Date. http://www.uptodate.com/contents/frontotemporal-dementia-treatment?source=search_result&search=frontote mporal+dementia+treatment&selectedTitle=1~150. Updated December 30, 2013. Accessed April 24, 2014.
1. Snowden JS, Neary D, Mann DM. Frontotemporal dementia. Br J Psychiatry. 2002;180:140-143.
2. Frontotemporal degeneration. The Association for Frontotemporal Degeneration. http://www.theaftd.org/ frontotemporal-degeneration/ftd-overview. Accessed April 24, 2014.
3. Neary D, Snowden JS, Gustafson L, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology. 1998;51(6):1546-1554.
4. Clark CM, Forman MS. Frontotemporal lobar degeneration with motor neuron disease: a clinical and pathological spectrum. Arch Neurol. 2006;63(4):489-490.
5. Diagnostic and statistical manual of mental disorders. 5th ed. Washington, DC: American Psychiatric Association; 2013:614-618.
6. Frontotemporal dementia diagnosis. UCSF Medical Center. http://www.ucsfhealth.org/conditions/frontotemporal_ dementia/diagnosis.html. Accessed April 24, 2014.
7. McMurtray AM, Chen AK, Shapira JS, et al. Variations in regional SPECT hypoperfusion and clinical features in frontotemporal dementia. Neurology. 2006;66(4):517-522.
8. Miller BL, Lee SE. Frontotemporal dementia: treatment. Up To Date. http://www.uptodate.com/contents/frontotemporal-dementia-treatment?source=search_result&search=frontote mporal+dementia+treatment&selectedTitle=1~150. Updated December 30, 2013. Accessed April 24, 2014.
Clozapine: Talking about risks, benefits, and alternatives with patients
Clozapine is a life-saving medication for many patients with schizophrenia, including those who have a schizophrenia spectrum disorder with suicidality or treatment-resistant disease, but clinicians’ discomfort with managing its risk profile has led to it being underutilized. Clinicians who are prepared to discuss the risks and benefits of clozapine—and alternatives, including no treatment—with patients may encounter less reluctance when they recommend a time-limited trial of the drug.
Risks
Clinicians need to be aware of both 1) serious adverse effects that can occur when clozapine needs to be interrupted or discontinued (Table)1 and 2) common side effects associated with continued use that can be managed without stopping the drug.2 Common side effects that patients may experience as treatment is initiated include sedation, orthostatic hypotension, constipation, drooling, tachycardia, and metabolic side effects such as weight gain, diabetes, and hyperlipidemia, which are problematic in the long term.
Reassure patients that frequent monitoring of metabolic metrics (including baseline HbA1C, lipid panel, waist circumference, and body mass index, as well as weight monitoring at each visit and metabolic laboratory monitoring every 3 to 6 months thereafter) should be expected, along with early intervention (eg, adding metformin) as appropriate. Constipation is common and can lead to serious, large bowel ileus. Ask about drooling, which can be treated by reducing the dosage or adding glycopyrrolate.
Extrapyramidal symptoms (EPS) including parkinsonism, dystonia, akathisia are uncommon (clozapine was the first “atypical” antipsychotic for this reason), but neuroleptic malignant syndrome (NMS) can occur. Although tardive dyskinesia (TD) is a small risk, clozapine will improve established TD in many patients once they are switched to clozapine. Blood dyscrasias include granulocytopenia and the rare risk of agranulocytosis which are monitored by means of a prescribing registry. Myocarditis and pancreatitis are likely idiosyncratic immune-related side effects that are unique to clozapine among antipsychotics. Other dangerous side effects include a dosage-related risk of seizure, severe hyperglycemia, and diabetic ketoacidosis.
Benefits
Clozapine is FDA-approved for treatment-resistant schizophrenia and for schizophrenia spectrum disorders with recurrent suicidality. Clozapine can be the best antipsychotic for patients who are sensitive to EPS and for those with TD. Antipsychotic efficacy often can be determined in a 2 to 3 month time-limited trial, although, in practice, you might need to wait 6 to 12 months to observe how well clozapine’s benefits have accrued.
Alternatives
Not using the most effective antipsychotic, or using no antipsychotic when one is indicated, often results in unstable psychiatric illness, which increases the risk of adverse outcomes (eg, suicide, accidents). Unstable psychiatric disease also complicates treatment of medical problems. An 11-year follow-up study in Finland of patients with schizophrenia showed a lower all-cause mortality with clozapine than with other antipsychotics, all of which collectively were associated with lower mortality compared with no antipsychotic use.3 Clozapine also is associated with the lowest discontinuation rate of any antipsychotic, which suggests that patients perceive its risk-benefit ratio favorably. Last, patients who might benefit from clozapine, but do not receive it, often will receive polypharmacy, which poses its own risks.
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Nielsen J, Correll CU, Manu P, et al. Termination of clozapine treatment due to medical reasons: when is it warranted and how can it be avoided? J Clin Psychiatry. 2013;74(6): 603-613.
2. Goldberg JF, Ernst CL. Managing the side effects of psychotropic medications. Arlington, VA: American Psychiatric Publishing; 2012.
3. Tiihonen J, Löngqvist J, Wahlbeck K, et al. 11-year follow-up of mortality in patients with schizophrenia: a population-based cohort study (FIN11 study). Lancet. 2009; 374(9690):620-627.
Clozapine is a life-saving medication for many patients with schizophrenia, including those who have a schizophrenia spectrum disorder with suicidality or treatment-resistant disease, but clinicians’ discomfort with managing its risk profile has led to it being underutilized. Clinicians who are prepared to discuss the risks and benefits of clozapine—and alternatives, including no treatment—with patients may encounter less reluctance when they recommend a time-limited trial of the drug.
Risks
Clinicians need to be aware of both 1) serious adverse effects that can occur when clozapine needs to be interrupted or discontinued (Table)1 and 2) common side effects associated with continued use that can be managed without stopping the drug.2 Common side effects that patients may experience as treatment is initiated include sedation, orthostatic hypotension, constipation, drooling, tachycardia, and metabolic side effects such as weight gain, diabetes, and hyperlipidemia, which are problematic in the long term.
Reassure patients that frequent monitoring of metabolic metrics (including baseline HbA1C, lipid panel, waist circumference, and body mass index, as well as weight monitoring at each visit and metabolic laboratory monitoring every 3 to 6 months thereafter) should be expected, along with early intervention (eg, adding metformin) as appropriate. Constipation is common and can lead to serious, large bowel ileus. Ask about drooling, which can be treated by reducing the dosage or adding glycopyrrolate.
Extrapyramidal symptoms (EPS) including parkinsonism, dystonia, akathisia are uncommon (clozapine was the first “atypical” antipsychotic for this reason), but neuroleptic malignant syndrome (NMS) can occur. Although tardive dyskinesia (TD) is a small risk, clozapine will improve established TD in many patients once they are switched to clozapine. Blood dyscrasias include granulocytopenia and the rare risk of agranulocytosis which are monitored by means of a prescribing registry. Myocarditis and pancreatitis are likely idiosyncratic immune-related side effects that are unique to clozapine among antipsychotics. Other dangerous side effects include a dosage-related risk of seizure, severe hyperglycemia, and diabetic ketoacidosis.
Benefits
Clozapine is FDA-approved for treatment-resistant schizophrenia and for schizophrenia spectrum disorders with recurrent suicidality. Clozapine can be the best antipsychotic for patients who are sensitive to EPS and for those with TD. Antipsychotic efficacy often can be determined in a 2 to 3 month time-limited trial, although, in practice, you might need to wait 6 to 12 months to observe how well clozapine’s benefits have accrued.
Alternatives
Not using the most effective antipsychotic, or using no antipsychotic when one is indicated, often results in unstable psychiatric illness, which increases the risk of adverse outcomes (eg, suicide, accidents). Unstable psychiatric disease also complicates treatment of medical problems. An 11-year follow-up study in Finland of patients with schizophrenia showed a lower all-cause mortality with clozapine than with other antipsychotics, all of which collectively were associated with lower mortality compared with no antipsychotic use.3 Clozapine also is associated with the lowest discontinuation rate of any antipsychotic, which suggests that patients perceive its risk-benefit ratio favorably. Last, patients who might benefit from clozapine, but do not receive it, often will receive polypharmacy, which poses its own risks.
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Clozapine is a life-saving medication for many patients with schizophrenia, including those who have a schizophrenia spectrum disorder with suicidality or treatment-resistant disease, but clinicians’ discomfort with managing its risk profile has led to it being underutilized. Clinicians who are prepared to discuss the risks and benefits of clozapine—and alternatives, including no treatment—with patients may encounter less reluctance when they recommend a time-limited trial of the drug.
Risks
Clinicians need to be aware of both 1) serious adverse effects that can occur when clozapine needs to be interrupted or discontinued (Table)1 and 2) common side effects associated with continued use that can be managed without stopping the drug.2 Common side effects that patients may experience as treatment is initiated include sedation, orthostatic hypotension, constipation, drooling, tachycardia, and metabolic side effects such as weight gain, diabetes, and hyperlipidemia, which are problematic in the long term.
Reassure patients that frequent monitoring of metabolic metrics (including baseline HbA1C, lipid panel, waist circumference, and body mass index, as well as weight monitoring at each visit and metabolic laboratory monitoring every 3 to 6 months thereafter) should be expected, along with early intervention (eg, adding metformin) as appropriate. Constipation is common and can lead to serious, large bowel ileus. Ask about drooling, which can be treated by reducing the dosage or adding glycopyrrolate.
Extrapyramidal symptoms (EPS) including parkinsonism, dystonia, akathisia are uncommon (clozapine was the first “atypical” antipsychotic for this reason), but neuroleptic malignant syndrome (NMS) can occur. Although tardive dyskinesia (TD) is a small risk, clozapine will improve established TD in many patients once they are switched to clozapine. Blood dyscrasias include granulocytopenia and the rare risk of agranulocytosis which are monitored by means of a prescribing registry. Myocarditis and pancreatitis are likely idiosyncratic immune-related side effects that are unique to clozapine among antipsychotics. Other dangerous side effects include a dosage-related risk of seizure, severe hyperglycemia, and diabetic ketoacidosis.
Benefits
Clozapine is FDA-approved for treatment-resistant schizophrenia and for schizophrenia spectrum disorders with recurrent suicidality. Clozapine can be the best antipsychotic for patients who are sensitive to EPS and for those with TD. Antipsychotic efficacy often can be determined in a 2 to 3 month time-limited trial, although, in practice, you might need to wait 6 to 12 months to observe how well clozapine’s benefits have accrued.
Alternatives
Not using the most effective antipsychotic, or using no antipsychotic when one is indicated, often results in unstable psychiatric illness, which increases the risk of adverse outcomes (eg, suicide, accidents). Unstable psychiatric disease also complicates treatment of medical problems. An 11-year follow-up study in Finland of patients with schizophrenia showed a lower all-cause mortality with clozapine than with other antipsychotics, all of which collectively were associated with lower mortality compared with no antipsychotic use.3 Clozapine also is associated with the lowest discontinuation rate of any antipsychotic, which suggests that patients perceive its risk-benefit ratio favorably. Last, patients who might benefit from clozapine, but do not receive it, often will receive polypharmacy, which poses its own risks.
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Nielsen J, Correll CU, Manu P, et al. Termination of clozapine treatment due to medical reasons: when is it warranted and how can it be avoided? J Clin Psychiatry. 2013;74(6): 603-613.
2. Goldberg JF, Ernst CL. Managing the side effects of psychotropic medications. Arlington, VA: American Psychiatric Publishing; 2012.
3. Tiihonen J, Löngqvist J, Wahlbeck K, et al. 11-year follow-up of mortality in patients with schizophrenia: a population-based cohort study (FIN11 study). Lancet. 2009; 374(9690):620-627.
1. Nielsen J, Correll CU, Manu P, et al. Termination of clozapine treatment due to medical reasons: when is it warranted and how can it be avoided? J Clin Psychiatry. 2013;74(6): 603-613.
2. Goldberg JF, Ernst CL. Managing the side effects of psychotropic medications. Arlington, VA: American Psychiatric Publishing; 2012.
3. Tiihonen J, Löngqvist J, Wahlbeck K, et al. 11-year follow-up of mortality in patients with schizophrenia: a population-based cohort study (FIN11 study). Lancet. 2009; 374(9690):620-627.
Aggressive and delusional about his alien origins, but refusing treatment
CASE Alien thoughts
Mr. C, age 23, is admitted to an intermediate-security facility because of unmanageable aggression. He is not charged with a crime and his legal status is admission by guardian. He is taking haloperidol decanoate, 300 mg IM every 28 days, and divalproex sodium, 1500 mg/d, but he continues to experience auditory hallucinations and the delusion that he is an alien.
Mr. C is given a primary diagnosis of chronic undifferentiated schizophrenia. He is started on risperidone tablets, 3 mg/d, and then switched to risperidone orally disintegrating tablets, titrated to 8 mg/d, to ensure compliance. Later, he receives separate trials of high-dose quetiapine (up to 1200 mg/d) and olanzapine orally disintegrating tablets (up to 30 mg/d). Lithium, 1200 mg/d, sertraline, 100 mg/d, and long-acting propranolol, 120 mg/d, were added at various periods of his treatment.
He continues to experience hallucinations and delusions, is intermittently aggressive, is not engaged in the treatment program, and needs prompting for basic hygiene. Several times, we discuss with Mr. C using clozapine, but he refuses, mainly because of weekly blood draws.
How would you proceed with Mr. C’s care?
a) consider electroconvulsive therapy
b) order aripiprazole and an omega-3 fish oil supplement
c) consider involuntary clozapine therapy and lab testing
The author’s observations
Schizophrenia remains a chronic and often refractory illness. Patients suffer from intrusive hallucinations; social and self-care deficits; cognitive impairment; and increased risk of violence, suicide, and premature death from medical causes. Pharmacotherapy is the mainstay of treatment, supplemented by individual and group therapies, psychosocial rehabilitation, housing assistance, and income support. Antipsychotics are fundamental and clozapine has been established as the most effective antipsychotic in the Clinical Antipsychotic Trials for Intervention Effectiveness (CATIE) study,1 but it remains underutilized.2
In 2008, clozapine accounted for only 4.4% of antipsychotic prescriptions in the United States.3 In our state forensic facility, only 10% of patients on an antipsychotic received clozapine in 2011. Despite the CATIE trial, there were no significant increases in clozapine prescribing after the results were published4 and patients often experience a substantial delay before clozapine is initiated.5 In the last several years, we have looked at methods to increase clozapine use in our hospital and have described some of our experiences. Despite enthusiasm for, and good experience with, clozapine, barriers limit the use of this medication (Table 1). One significant barrier is patient acceptance. Although most of our patients taking an atypical antipsychotic will accept a blood draws every 6 months for metabolic monitoring, many will reject clozapine because of the initial weekly blood draw. Other patients will reject a trial of clozapine because of fears of serious adverse reactions.
Clinicians may be reluctant to initiate clozapine treatment because of increased time demands to obtain and document informed consent, complete initial paperwork, initiate a clozapine titration protocol, and order laboratory work. Clinicians also may fear more serious adverse reactions with clozapine such as agranulocytosis, acute diabetes, severe constipation, and myocarditis. With close monitoring, however, these outcomes can be avoided, and clozapine therapy can decrease mortality.6 With the increasing availability and decreasing cost of genetic analysis, in the near future we may be able to better predict clozapine responders and the risk of agranulocytosis before initiating clozapine.7,8
Overcoming barriers
When initiating clozapine, it is helpful to reduce barriers to treatment. One strategy to improve patient acceptance of blood testing is to use fingerstick hematology profiles rather than the typical venipuncture technique. The Micros 60 analyzer can provide a complete blood count and granulocyte count from a blood specimen collected in a mini capillary tube.
National clozapine registries accept results derived from this method of blood analysis. Using preprinted medication and treatment orders can ease the paperwork burden for the psychiatrist. To help ensure safe use of clozapine, clinical pharmacists can help interface with the clozapine registry (see this article at CurrentPsychiatry. com for a list of clozapine registry Web sites), assist with monitoring laboratory and medication orders, and anticipate drug interactions and side effects. Staff members directly involved in the patient’s care can try to improve the patient’s insight of his (her) illness. Nursing staff can provide medication education.
Many efforts have been made to educate medical staff to reduce adverse effects and improve patients’ experience with clozapine. Employing agents such as polyethylene glycol, desmopressin, terazosin, and topiramate can help to manage adverse effects of clozapine such as constipation, nocturnal enuresis, drooling, and weight gain, respectively. Lithium can help boost a low neutrophil count9; a lithium level >0.4 mEq/L may be needed to achieve this response. Although generally well tolerated, adding lithium can increase the risk of seizures with clozapine. A final hurdle has been the dilemma of an unwilling, but obviously ill and suffering, patient who has failed several medication trials and other therapeutic interventions.
TREATMENT Involuntary clozapine
Mr. C continues to believe that he is an alien. He also thinks he is involved in a mission for God. He has physically assaulted staff on occasion. Overall, his mood shows no persistent abnormality and his sleep and appetite are normal. Family history reveals that Mr. C’s brother has schizophrenia. Because of Mr. C’s refractory illness, we seek the guardian’s consent for a trial of clozapine and ask for permission to give backup medication and lab testing involuntarily if necessary.
We obtain informed consent and orders are written. Mr. C refuses the first 2 doses of clozapine (12.5 mg at bedtime) and receives a backup order of IM olanzapine, 5 mg. He initially refuses baseline and 1-week hematology profiles, which then are obtained involuntarily by manual hold. Subsequently, Mr. C no longer refused medication or lab tests. His clozapine dosage is titrated to 400 mg/d, guided by clinical response and plasma level.
The authors’ observations
We work in a public forensic psychiatry facility, where the average length of stay is 680 days. In a public psychiatry facility there may be pressure to reduce the length of stay by moving patients to a less restrictive setting and thereby reducing the overall census. Many patients at our facility likely would benefit from clozapine. In an effort to provide this important therapy to patients who refuse it despite refractory symptoms, chronic hospitalization, and dangerous behaviors, we have developed an option of involuntary clozapine administration. When efforts to convince the patient to agree to clozapine treatment fail, approval for the involuntary administration of medication and laboratory testing can be requested.
Involuntary clozapine treatment may be an important option for patients who have a guardian (as do approximately one-half of patients at our facility). It also might be an option for patients who have a court order or other legal document approving a trial of involuntary clozapine. When seeking approval from a guardian, explain the benefits and risks of treatment. Some guardians are public administrators, such as elected officials who serve as conservators and guardians, and may be familiar with clozapine and successes with other patients, and quickly support the request. In other cases, the guardian is a family member and might require more education and time to make a decision.
After obtaining approval from a guardian, inform the patient of the plan to initiate clozapine, with the goal of gradually reducing some or most of the other psychotropics. Describe to your patient why weekly hematology profiles are necessary. In collaboration with the treatment team, a convenient time is scheduled for the baseline lab draw. If lab results meet the baseline requirements, clozapine is initiated, usually using the orally disintegrating formulation. The patient is informed about the lab results, medication orders, and potential side effects. If the patient refuses medication, an IM backup of another atypical antipsychotic may be ordered in place of the missed clozapine dose, after obtaining the guardian’s permission. Employing physical restraint such as a manual hold to obtain laboratory testing or to administer medication triggers restraint and seclusion policies.
How do you ensure compliance with clozapine therapy in an unwilling patient?
a) mouth check
b) medication watch (sitting in a public area for 30 minutes after a dose)
c) dissolving clozapine tablets
d) monitoring therapy with clozapine/norclozapine plasma levels
The authors’ observations
At times we have instituted all of the methods noted in Table 2. We have most often used dissolving tablets and plasma monitoring.
OUTCOME Improvement, transfer
Mr. C gradually improves over 6 months. The voices, delusions, and aggression resolve. He remains mildly disorganized and has poor insight, with unrealistic goals. Approximately 3 years after admission and 1 year after clozapine was initiated, Mr. C is transferred to a minimum-security facility.
The authors’ observations
Overall, our experience has been successful with the approach we have described. Patients often do not resist the treatment plan once they see our commitment to their well-being. When they do resist, it has been only for 1 to 3 doses of medication, and 1 or 2 blood draws. Of 6 recent cases under this protocol, we have discharged 3; 1 is approaching discharge; 1 has had minimal improvement to date; and 1 required discontinuation because of neutropenia. We recommend considering involuntary clozapine therapy for refractory patients who have a poor prognosis.
Bottom Line
Clozapine is an underutilized treatment for refractory schizophrenia, often because of patient refusal. In a case presentation format we review the barriers to clozapine therapy. We discuss clinical and legal issues for administering clozapine to an unwilling patient.
Related Resources
• Hill M, Freundenrich O. Clozapine: key discussion points for prescribers. Clin Schizophr Relat Psychoses. 2013;6(4):177-185.
• Nielsen J, Correll C, Manu P, et al. Termination of clozapine treatment due to medical reasons: when is it warranted and how can it be avoided? J Clin Psychiatry. 2013;74(6):603-613.
Drug Brand Names
Aripiprazole • Abilify
Polyethylene glycol • MiraLax
Clozapine • Clozaril, FazaClo
ropranolol • Inderal LA
Desmopressin • DDAVP
Quetiapine • Seroquel
Divalproex sodium • Depakote
Risperidone • Risperdal
Haloperidol • Haldol
Sertraline • Zoloft
Lithium • Eskalith, Lithobid
Terazosin • Hytrin
Olanzapine • Zyprexa
Topiramate • Topamax
1. McEvoy JP, Lieberman JA, Stroup TS, et al; CATIE Investigators. Effectiveness of clozapine versus olanzapine, quetiapine, and risperidone in patients with chronic schizophrenia who did not respond to prior atypical antipsychotic treatment. Am J Psychiatry. 2006;163(4): 600-610.
2. Stroup TS, Lieberman JA, McEvoy JP, et al; CATIE Investigators. Results of phase 3 of the CATIE schizophrenia trial. Schizophr Res. 2009;107(1):1-12.
3. Meltzer HY. Clozapine: balancing safety with superior antipsychotic efficacy. Clin Schizophr Relat Psychoses. 2012;6(3):134-144.
4. Berkowitz RL, Patel U, Ni Q, et al. The impact of the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) on prescribing practices: an analysis of data from a large midwestern state. J Clin Psychiatry. 2012;73(4):498-503.
5. Howes OD, Vergunst F, Gee S, et al. Adherence to treatment guidelines in clinical practice: study of antipsychotic treatment prior to clozapine initiation. Br J Psychiatry. 2012;201(6):481-485.
6. Tiihonen J, Lönnqvist J, Wahlbeck K, et al. 11-year follow-up of mortality in patients with schizophrenia: a population-based cohort study (FIN11 study). Lancet. 2009;374(9690):620-627.
7. Arranz MJ, Munro J, Birkett J, et al. Pharmacogenetic prediction of clozapine response. Lancet. 2000;355(9215): 1615-1616.
8. Athanasiou MC, Dettling M, Cascorbi I, et al. Candidate gene analysis identifies a polymorphism on HLA-DQB1 associated with clozapine-induced agranulocytosis. J Clin Psychiatry. 2011;72(4):458-463.
9. Paton C, Esop R. Managing clozapine-induced neutropenia with lithium. Psychiatric Bulletin. 2005;29(5):186-188.
CASE Alien thoughts
Mr. C, age 23, is admitted to an intermediate-security facility because of unmanageable aggression. He is not charged with a crime and his legal status is admission by guardian. He is taking haloperidol decanoate, 300 mg IM every 28 days, and divalproex sodium, 1500 mg/d, but he continues to experience auditory hallucinations and the delusion that he is an alien.
Mr. C is given a primary diagnosis of chronic undifferentiated schizophrenia. He is started on risperidone tablets, 3 mg/d, and then switched to risperidone orally disintegrating tablets, titrated to 8 mg/d, to ensure compliance. Later, he receives separate trials of high-dose quetiapine (up to 1200 mg/d) and olanzapine orally disintegrating tablets (up to 30 mg/d). Lithium, 1200 mg/d, sertraline, 100 mg/d, and long-acting propranolol, 120 mg/d, were added at various periods of his treatment.
He continues to experience hallucinations and delusions, is intermittently aggressive, is not engaged in the treatment program, and needs prompting for basic hygiene. Several times, we discuss with Mr. C using clozapine, but he refuses, mainly because of weekly blood draws.
How would you proceed with Mr. C’s care?
a) consider electroconvulsive therapy
b) order aripiprazole and an omega-3 fish oil supplement
c) consider involuntary clozapine therapy and lab testing
The author’s observations
Schizophrenia remains a chronic and often refractory illness. Patients suffer from intrusive hallucinations; social and self-care deficits; cognitive impairment; and increased risk of violence, suicide, and premature death from medical causes. Pharmacotherapy is the mainstay of treatment, supplemented by individual and group therapies, psychosocial rehabilitation, housing assistance, and income support. Antipsychotics are fundamental and clozapine has been established as the most effective antipsychotic in the Clinical Antipsychotic Trials for Intervention Effectiveness (CATIE) study,1 but it remains underutilized.2
In 2008, clozapine accounted for only 4.4% of antipsychotic prescriptions in the United States.3 In our state forensic facility, only 10% of patients on an antipsychotic received clozapine in 2011. Despite the CATIE trial, there were no significant increases in clozapine prescribing after the results were published4 and patients often experience a substantial delay before clozapine is initiated.5 In the last several years, we have looked at methods to increase clozapine use in our hospital and have described some of our experiences. Despite enthusiasm for, and good experience with, clozapine, barriers limit the use of this medication (Table 1). One significant barrier is patient acceptance. Although most of our patients taking an atypical antipsychotic will accept a blood draws every 6 months for metabolic monitoring, many will reject clozapine because of the initial weekly blood draw. Other patients will reject a trial of clozapine because of fears of serious adverse reactions.
Clinicians may be reluctant to initiate clozapine treatment because of increased time demands to obtain and document informed consent, complete initial paperwork, initiate a clozapine titration protocol, and order laboratory work. Clinicians also may fear more serious adverse reactions with clozapine such as agranulocytosis, acute diabetes, severe constipation, and myocarditis. With close monitoring, however, these outcomes can be avoided, and clozapine therapy can decrease mortality.6 With the increasing availability and decreasing cost of genetic analysis, in the near future we may be able to better predict clozapine responders and the risk of agranulocytosis before initiating clozapine.7,8
Overcoming barriers
When initiating clozapine, it is helpful to reduce barriers to treatment. One strategy to improve patient acceptance of blood testing is to use fingerstick hematology profiles rather than the typical venipuncture technique. The Micros 60 analyzer can provide a complete blood count and granulocyte count from a blood specimen collected in a mini capillary tube.
National clozapine registries accept results derived from this method of blood analysis. Using preprinted medication and treatment orders can ease the paperwork burden for the psychiatrist. To help ensure safe use of clozapine, clinical pharmacists can help interface with the clozapine registry (see this article at CurrentPsychiatry. com for a list of clozapine registry Web sites), assist with monitoring laboratory and medication orders, and anticipate drug interactions and side effects. Staff members directly involved in the patient’s care can try to improve the patient’s insight of his (her) illness. Nursing staff can provide medication education.
Many efforts have been made to educate medical staff to reduce adverse effects and improve patients’ experience with clozapine. Employing agents such as polyethylene glycol, desmopressin, terazosin, and topiramate can help to manage adverse effects of clozapine such as constipation, nocturnal enuresis, drooling, and weight gain, respectively. Lithium can help boost a low neutrophil count9; a lithium level >0.4 mEq/L may be needed to achieve this response. Although generally well tolerated, adding lithium can increase the risk of seizures with clozapine. A final hurdle has been the dilemma of an unwilling, but obviously ill and suffering, patient who has failed several medication trials and other therapeutic interventions.
TREATMENT Involuntary clozapine
Mr. C continues to believe that he is an alien. He also thinks he is involved in a mission for God. He has physically assaulted staff on occasion. Overall, his mood shows no persistent abnormality and his sleep and appetite are normal. Family history reveals that Mr. C’s brother has schizophrenia. Because of Mr. C’s refractory illness, we seek the guardian’s consent for a trial of clozapine and ask for permission to give backup medication and lab testing involuntarily if necessary.
We obtain informed consent and orders are written. Mr. C refuses the first 2 doses of clozapine (12.5 mg at bedtime) and receives a backup order of IM olanzapine, 5 mg. He initially refuses baseline and 1-week hematology profiles, which then are obtained involuntarily by manual hold. Subsequently, Mr. C no longer refused medication or lab tests. His clozapine dosage is titrated to 400 mg/d, guided by clinical response and plasma level.
The authors’ observations
We work in a public forensic psychiatry facility, where the average length of stay is 680 days. In a public psychiatry facility there may be pressure to reduce the length of stay by moving patients to a less restrictive setting and thereby reducing the overall census. Many patients at our facility likely would benefit from clozapine. In an effort to provide this important therapy to patients who refuse it despite refractory symptoms, chronic hospitalization, and dangerous behaviors, we have developed an option of involuntary clozapine administration. When efforts to convince the patient to agree to clozapine treatment fail, approval for the involuntary administration of medication and laboratory testing can be requested.
Involuntary clozapine treatment may be an important option for patients who have a guardian (as do approximately one-half of patients at our facility). It also might be an option for patients who have a court order or other legal document approving a trial of involuntary clozapine. When seeking approval from a guardian, explain the benefits and risks of treatment. Some guardians are public administrators, such as elected officials who serve as conservators and guardians, and may be familiar with clozapine and successes with other patients, and quickly support the request. In other cases, the guardian is a family member and might require more education and time to make a decision.
After obtaining approval from a guardian, inform the patient of the plan to initiate clozapine, with the goal of gradually reducing some or most of the other psychotropics. Describe to your patient why weekly hematology profiles are necessary. In collaboration with the treatment team, a convenient time is scheduled for the baseline lab draw. If lab results meet the baseline requirements, clozapine is initiated, usually using the orally disintegrating formulation. The patient is informed about the lab results, medication orders, and potential side effects. If the patient refuses medication, an IM backup of another atypical antipsychotic may be ordered in place of the missed clozapine dose, after obtaining the guardian’s permission. Employing physical restraint such as a manual hold to obtain laboratory testing or to administer medication triggers restraint and seclusion policies.
How do you ensure compliance with clozapine therapy in an unwilling patient?
a) mouth check
b) medication watch (sitting in a public area for 30 minutes after a dose)
c) dissolving clozapine tablets
d) monitoring therapy with clozapine/norclozapine plasma levels
The authors’ observations
At times we have instituted all of the methods noted in Table 2. We have most often used dissolving tablets and plasma monitoring.
OUTCOME Improvement, transfer
Mr. C gradually improves over 6 months. The voices, delusions, and aggression resolve. He remains mildly disorganized and has poor insight, with unrealistic goals. Approximately 3 years after admission and 1 year after clozapine was initiated, Mr. C is transferred to a minimum-security facility.
The authors’ observations
Overall, our experience has been successful with the approach we have described. Patients often do not resist the treatment plan once they see our commitment to their well-being. When they do resist, it has been only for 1 to 3 doses of medication, and 1 or 2 blood draws. Of 6 recent cases under this protocol, we have discharged 3; 1 is approaching discharge; 1 has had minimal improvement to date; and 1 required discontinuation because of neutropenia. We recommend considering involuntary clozapine therapy for refractory patients who have a poor prognosis.
Bottom Line
Clozapine is an underutilized treatment for refractory schizophrenia, often because of patient refusal. In a case presentation format we review the barriers to clozapine therapy. We discuss clinical and legal issues for administering clozapine to an unwilling patient.
Related Resources
• Hill M, Freundenrich O. Clozapine: key discussion points for prescribers. Clin Schizophr Relat Psychoses. 2013;6(4):177-185.
• Nielsen J, Correll C, Manu P, et al. Termination of clozapine treatment due to medical reasons: when is it warranted and how can it be avoided? J Clin Psychiatry. 2013;74(6):603-613.
Drug Brand Names
Aripiprazole • Abilify
Polyethylene glycol • MiraLax
Clozapine • Clozaril, FazaClo
ropranolol • Inderal LA
Desmopressin • DDAVP
Quetiapine • Seroquel
Divalproex sodium • Depakote
Risperidone • Risperdal
Haloperidol • Haldol
Sertraline • Zoloft
Lithium • Eskalith, Lithobid
Terazosin • Hytrin
Olanzapine • Zyprexa
Topiramate • Topamax
CASE Alien thoughts
Mr. C, age 23, is admitted to an intermediate-security facility because of unmanageable aggression. He is not charged with a crime and his legal status is admission by guardian. He is taking haloperidol decanoate, 300 mg IM every 28 days, and divalproex sodium, 1500 mg/d, but he continues to experience auditory hallucinations and the delusion that he is an alien.
Mr. C is given a primary diagnosis of chronic undifferentiated schizophrenia. He is started on risperidone tablets, 3 mg/d, and then switched to risperidone orally disintegrating tablets, titrated to 8 mg/d, to ensure compliance. Later, he receives separate trials of high-dose quetiapine (up to 1200 mg/d) and olanzapine orally disintegrating tablets (up to 30 mg/d). Lithium, 1200 mg/d, sertraline, 100 mg/d, and long-acting propranolol, 120 mg/d, were added at various periods of his treatment.
He continues to experience hallucinations and delusions, is intermittently aggressive, is not engaged in the treatment program, and needs prompting for basic hygiene. Several times, we discuss with Mr. C using clozapine, but he refuses, mainly because of weekly blood draws.
How would you proceed with Mr. C’s care?
a) consider electroconvulsive therapy
b) order aripiprazole and an omega-3 fish oil supplement
c) consider involuntary clozapine therapy and lab testing
The author’s observations
Schizophrenia remains a chronic and often refractory illness. Patients suffer from intrusive hallucinations; social and self-care deficits; cognitive impairment; and increased risk of violence, suicide, and premature death from medical causes. Pharmacotherapy is the mainstay of treatment, supplemented by individual and group therapies, psychosocial rehabilitation, housing assistance, and income support. Antipsychotics are fundamental and clozapine has been established as the most effective antipsychotic in the Clinical Antipsychotic Trials for Intervention Effectiveness (CATIE) study,1 but it remains underutilized.2
In 2008, clozapine accounted for only 4.4% of antipsychotic prescriptions in the United States.3 In our state forensic facility, only 10% of patients on an antipsychotic received clozapine in 2011. Despite the CATIE trial, there were no significant increases in clozapine prescribing after the results were published4 and patients often experience a substantial delay before clozapine is initiated.5 In the last several years, we have looked at methods to increase clozapine use in our hospital and have described some of our experiences. Despite enthusiasm for, and good experience with, clozapine, barriers limit the use of this medication (Table 1). One significant barrier is patient acceptance. Although most of our patients taking an atypical antipsychotic will accept a blood draws every 6 months for metabolic monitoring, many will reject clozapine because of the initial weekly blood draw. Other patients will reject a trial of clozapine because of fears of serious adverse reactions.
Clinicians may be reluctant to initiate clozapine treatment because of increased time demands to obtain and document informed consent, complete initial paperwork, initiate a clozapine titration protocol, and order laboratory work. Clinicians also may fear more serious adverse reactions with clozapine such as agranulocytosis, acute diabetes, severe constipation, and myocarditis. With close monitoring, however, these outcomes can be avoided, and clozapine therapy can decrease mortality.6 With the increasing availability and decreasing cost of genetic analysis, in the near future we may be able to better predict clozapine responders and the risk of agranulocytosis before initiating clozapine.7,8
Overcoming barriers
When initiating clozapine, it is helpful to reduce barriers to treatment. One strategy to improve patient acceptance of blood testing is to use fingerstick hematology profiles rather than the typical venipuncture technique. The Micros 60 analyzer can provide a complete blood count and granulocyte count from a blood specimen collected in a mini capillary tube.
National clozapine registries accept results derived from this method of blood analysis. Using preprinted medication and treatment orders can ease the paperwork burden for the psychiatrist. To help ensure safe use of clozapine, clinical pharmacists can help interface with the clozapine registry (see this article at CurrentPsychiatry. com for a list of clozapine registry Web sites), assist with monitoring laboratory and medication orders, and anticipate drug interactions and side effects. Staff members directly involved in the patient’s care can try to improve the patient’s insight of his (her) illness. Nursing staff can provide medication education.
Many efforts have been made to educate medical staff to reduce adverse effects and improve patients’ experience with clozapine. Employing agents such as polyethylene glycol, desmopressin, terazosin, and topiramate can help to manage adverse effects of clozapine such as constipation, nocturnal enuresis, drooling, and weight gain, respectively. Lithium can help boost a low neutrophil count9; a lithium level >0.4 mEq/L may be needed to achieve this response. Although generally well tolerated, adding lithium can increase the risk of seizures with clozapine. A final hurdle has been the dilemma of an unwilling, but obviously ill and suffering, patient who has failed several medication trials and other therapeutic interventions.
TREATMENT Involuntary clozapine
Mr. C continues to believe that he is an alien. He also thinks he is involved in a mission for God. He has physically assaulted staff on occasion. Overall, his mood shows no persistent abnormality and his sleep and appetite are normal. Family history reveals that Mr. C’s brother has schizophrenia. Because of Mr. C’s refractory illness, we seek the guardian’s consent for a trial of clozapine and ask for permission to give backup medication and lab testing involuntarily if necessary.
We obtain informed consent and orders are written. Mr. C refuses the first 2 doses of clozapine (12.5 mg at bedtime) and receives a backup order of IM olanzapine, 5 mg. He initially refuses baseline and 1-week hematology profiles, which then are obtained involuntarily by manual hold. Subsequently, Mr. C no longer refused medication or lab tests. His clozapine dosage is titrated to 400 mg/d, guided by clinical response and plasma level.
The authors’ observations
We work in a public forensic psychiatry facility, where the average length of stay is 680 days. In a public psychiatry facility there may be pressure to reduce the length of stay by moving patients to a less restrictive setting and thereby reducing the overall census. Many patients at our facility likely would benefit from clozapine. In an effort to provide this important therapy to patients who refuse it despite refractory symptoms, chronic hospitalization, and dangerous behaviors, we have developed an option of involuntary clozapine administration. When efforts to convince the patient to agree to clozapine treatment fail, approval for the involuntary administration of medication and laboratory testing can be requested.
Involuntary clozapine treatment may be an important option for patients who have a guardian (as do approximately one-half of patients at our facility). It also might be an option for patients who have a court order or other legal document approving a trial of involuntary clozapine. When seeking approval from a guardian, explain the benefits and risks of treatment. Some guardians are public administrators, such as elected officials who serve as conservators and guardians, and may be familiar with clozapine and successes with other patients, and quickly support the request. In other cases, the guardian is a family member and might require more education and time to make a decision.
After obtaining approval from a guardian, inform the patient of the plan to initiate clozapine, with the goal of gradually reducing some or most of the other psychotropics. Describe to your patient why weekly hematology profiles are necessary. In collaboration with the treatment team, a convenient time is scheduled for the baseline lab draw. If lab results meet the baseline requirements, clozapine is initiated, usually using the orally disintegrating formulation. The patient is informed about the lab results, medication orders, and potential side effects. If the patient refuses medication, an IM backup of another atypical antipsychotic may be ordered in place of the missed clozapine dose, after obtaining the guardian’s permission. Employing physical restraint such as a manual hold to obtain laboratory testing or to administer medication triggers restraint and seclusion policies.
How do you ensure compliance with clozapine therapy in an unwilling patient?
a) mouth check
b) medication watch (sitting in a public area for 30 minutes after a dose)
c) dissolving clozapine tablets
d) monitoring therapy with clozapine/norclozapine plasma levels
The authors’ observations
At times we have instituted all of the methods noted in Table 2. We have most often used dissolving tablets and plasma monitoring.
OUTCOME Improvement, transfer
Mr. C gradually improves over 6 months. The voices, delusions, and aggression resolve. He remains mildly disorganized and has poor insight, with unrealistic goals. Approximately 3 years after admission and 1 year after clozapine was initiated, Mr. C is transferred to a minimum-security facility.
The authors’ observations
Overall, our experience has been successful with the approach we have described. Patients often do not resist the treatment plan once they see our commitment to their well-being. When they do resist, it has been only for 1 to 3 doses of medication, and 1 or 2 blood draws. Of 6 recent cases under this protocol, we have discharged 3; 1 is approaching discharge; 1 has had minimal improvement to date; and 1 required discontinuation because of neutropenia. We recommend considering involuntary clozapine therapy for refractory patients who have a poor prognosis.
Bottom Line
Clozapine is an underutilized treatment for refractory schizophrenia, often because of patient refusal. In a case presentation format we review the barriers to clozapine therapy. We discuss clinical and legal issues for administering clozapine to an unwilling patient.
Related Resources
• Hill M, Freundenrich O. Clozapine: key discussion points for prescribers. Clin Schizophr Relat Psychoses. 2013;6(4):177-185.
• Nielsen J, Correll C, Manu P, et al. Termination of clozapine treatment due to medical reasons: when is it warranted and how can it be avoided? J Clin Psychiatry. 2013;74(6):603-613.
Drug Brand Names
Aripiprazole • Abilify
Polyethylene glycol • MiraLax
Clozapine • Clozaril, FazaClo
ropranolol • Inderal LA
Desmopressin • DDAVP
Quetiapine • Seroquel
Divalproex sodium • Depakote
Risperidone • Risperdal
Haloperidol • Haldol
Sertraline • Zoloft
Lithium • Eskalith, Lithobid
Terazosin • Hytrin
Olanzapine • Zyprexa
Topiramate • Topamax
1. McEvoy JP, Lieberman JA, Stroup TS, et al; CATIE Investigators. Effectiveness of clozapine versus olanzapine, quetiapine, and risperidone in patients with chronic schizophrenia who did not respond to prior atypical antipsychotic treatment. Am J Psychiatry. 2006;163(4): 600-610.
2. Stroup TS, Lieberman JA, McEvoy JP, et al; CATIE Investigators. Results of phase 3 of the CATIE schizophrenia trial. Schizophr Res. 2009;107(1):1-12.
3. Meltzer HY. Clozapine: balancing safety with superior antipsychotic efficacy. Clin Schizophr Relat Psychoses. 2012;6(3):134-144.
4. Berkowitz RL, Patel U, Ni Q, et al. The impact of the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) on prescribing practices: an analysis of data from a large midwestern state. J Clin Psychiatry. 2012;73(4):498-503.
5. Howes OD, Vergunst F, Gee S, et al. Adherence to treatment guidelines in clinical practice: study of antipsychotic treatment prior to clozapine initiation. Br J Psychiatry. 2012;201(6):481-485.
6. Tiihonen J, Lönnqvist J, Wahlbeck K, et al. 11-year follow-up of mortality in patients with schizophrenia: a population-based cohort study (FIN11 study). Lancet. 2009;374(9690):620-627.
7. Arranz MJ, Munro J, Birkett J, et al. Pharmacogenetic prediction of clozapine response. Lancet. 2000;355(9215): 1615-1616.
8. Athanasiou MC, Dettling M, Cascorbi I, et al. Candidate gene analysis identifies a polymorphism on HLA-DQB1 associated with clozapine-induced agranulocytosis. J Clin Psychiatry. 2011;72(4):458-463.
9. Paton C, Esop R. Managing clozapine-induced neutropenia with lithium. Psychiatric Bulletin. 2005;29(5):186-188.
1. McEvoy JP, Lieberman JA, Stroup TS, et al; CATIE Investigators. Effectiveness of clozapine versus olanzapine, quetiapine, and risperidone in patients with chronic schizophrenia who did not respond to prior atypical antipsychotic treatment. Am J Psychiatry. 2006;163(4): 600-610.
2. Stroup TS, Lieberman JA, McEvoy JP, et al; CATIE Investigators. Results of phase 3 of the CATIE schizophrenia trial. Schizophr Res. 2009;107(1):1-12.
3. Meltzer HY. Clozapine: balancing safety with superior antipsychotic efficacy. Clin Schizophr Relat Psychoses. 2012;6(3):134-144.
4. Berkowitz RL, Patel U, Ni Q, et al. The impact of the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) on prescribing practices: an analysis of data from a large midwestern state. J Clin Psychiatry. 2012;73(4):498-503.
5. Howes OD, Vergunst F, Gee S, et al. Adherence to treatment guidelines in clinical practice: study of antipsychotic treatment prior to clozapine initiation. Br J Psychiatry. 2012;201(6):481-485.
6. Tiihonen J, Lönnqvist J, Wahlbeck K, et al. 11-year follow-up of mortality in patients with schizophrenia: a population-based cohort study (FIN11 study). Lancet. 2009;374(9690):620-627.
7. Arranz MJ, Munro J, Birkett J, et al. Pharmacogenetic prediction of clozapine response. Lancet. 2000;355(9215): 1615-1616.
8. Athanasiou MC, Dettling M, Cascorbi I, et al. Candidate gene analysis identifies a polymorphism on HLA-DQB1 associated with clozapine-induced agranulocytosis. J Clin Psychiatry. 2011;72(4):458-463.
9. Paton C, Esop R. Managing clozapine-induced neutropenia with lithium. Psychiatric Bulletin. 2005;29(5):186-188.
Using CBT effectively for treating depression and anxiety
Fewer than 20% of people seeking help for depression and anxiety disorders receive cognitive-behavioral therapy (CBT), the most established evidence-based psychotherapeutic treatment.1 Efforts are being made to increase access to CBT,2 but a substantial barrier remains: therapist training is a strong predictor of treatment outcome, and many therapists offering CBT services are not sufficiently trained to deliver multiple manual-based interventions with adequate fidelity to the model. Proposed solutions to this barrier include:
• abbreviated versions of CBT training for practitioners in primary care and community settings
• culturally adapted CBT training for community health workers3
• Internet-based CBT and telemedicine (telephone and video conferencing)2
• mobile phone applications that use text messaging, social support, and physiological monitoring as adjuncts to clinical practice or stand-alone interventions.4
New models of CBT also are emerging, including transdiagnostic CBT and metacognitive approaches (mindfulness-based cognitive therapy and acceptance and commitment therapy), and several new foci for exposure therapy.
In light of these ongoing modulations, this article is intended to help clinicians make informed decisions about CBT when selecting treatment for patients with depressive and anxiety disorders (Box5 ). We review the evidence of CBT’s efficacy for acute-phase treatment and relapse prevention; explain the common elements considered essential to CBT practice; describe CBT adaptations for specific anxiety disorders; and provide an overview of recent advances in conceptualizing and adapting CBT.
Efficacy for mood and anxiety disorders
Depression. Dozens of randomized controlled trials (RCT) and other studies support CBT’s efficacy in treating major depressive disorder (MDD). For acute treatment:
• CBT is more effective in producing remission when compared with no treatment, treatment as usual, or nonspecific psychotherapy.
• For mild to moderate depression, CBT is equivalent to antidepressant medication in terms of response and remission rates.
• Combining antidepressant therapy with CBT increases treatment adherence.6
Less well known may be that a successful response to CBT in the acute phase may have a protective effect against depression recurrences. A 2013 meta-analysis that totaled 506 individuals with depressive disorders found a trend toward significantly lower relapse rates when CBT was discontinued after acute therapy, compared with antidepressant therapy that continued beyond the acute phase.7
Anxiety. Among psychotherapies, CBT’s superior efficacy for anxiety disorders is well-established. CBT and its specific-disorder adaptations are considered first-line treatment.8
CBT’s essential elements
CBT focuses on distorted cognitions about the self, the world, and the future, and on behaviors that lead to or maintain symptoms.
Cognitive interventions seek to identify thoughts and beliefs that trigger emotional and behavioral reactions. A person with social anxiety disorder, for example, might believe that people will notice if he makes even a minor social mistake and then reject him, which will make him feel worthless. CBT can help him subject these beliefs to rational analysis and develop more adaptive beliefs, such as: “It is not certain that I will behave so badly that people would notice, but if that happened, the likelihood of being outright rejected is probably low. If—in the worst-case scenario—I was rejected, I am not worthless; I’m just a fallible human being.”
CBT’s behavioral component can be conceptualized as behavioral activation (BA), a structured approach to help the patient:
• increase behaviors and experiences that are rewarding
• overcome barriers to engaging in these new behaviors
• and decrease behaviors that maintain symptoms.
BA can be a useful intervention for individuals with depression characterized by lack of engagement or capacity for pleasurable experiences. During pregnancy and the postpartum period, for example, a woman undergoes physical, social, and environmental changes that might gradually deprive her of sources of pleasure and other reinforcing activities. BA would focus on developing creative solutions to regain access to or create new opportunities for rewarding experiences and to avoid behaviors (such as social withdrawal or physical activity restriction) that perpetuate depressed mood.
Common elements. Cognitive and behavioral interventions focus on problem solving, individualized case conceptualization (Figure 1), and collaborative empiricism.9
Individualized case conceptualization lays the foundation for the course of CBT, and may be thought of as a map for therapy. Case conceptualization brings in several domains of assessment including symptoms and diagnosis, the patient’s strengths, formative experiences (including biopsychosocial aspects), contextual factors, and cognitive factors that influence diagnosis and treatment, such as automatic thoughts or schemas. The case formulation leads to a working hypothesis about the optimal course and focus of CBT.
Collaborative empiricism is the way in which the patient and therapist work together to continually refine this working hypothesis. The pair works together to investigate the hypotheses and all aspects of the therapeutic relationship.
Although no specific technique defines CBT, a common practice is to educate a person about interrelationships between behaviors/activities, thoughts, and mood. A mood activity log (Figure 2) can illuminate links between moods and activities and be useful with targeting interventions. For a person with social anxiety, for example, a mood activity log could assist in developing a hierarchy of feared social situations and avoidance intensity. Systematic exposure therapy would follow, beginning with the least frightening/intense situation, accompanied by teaching new coping skills (such as relaxation strategies).
CBT adaptations for anxiety disorders
Elements of CBT have been adapted for a variety of anxiety disorders, based on specific symptoms and features (Table).10-15
Panic disorder. Panic control treatment is considered the first-line intervention for panic disorder’s defining features: spontaneous panic attacks, worry about future occurrence of attacks, and perceived catastrophic consequences (such as heart attack, fainting).10 This CBT adaptation includes:
• patient education about the nature of panic
• breathing retraining to foster exposure to feared bodily sensations and avoided activities and places
• cognitive restructuring of danger-related thoughts (such as “I’m going to faint,” or “It would be catastrophic if I did”).
Obsessive-compulsive disorder. Exposure and response prevention (ERP) is the first-line treatment for obsessive-compulsive disorder (OCD).11 In traditional therapist-guided ERP, patients expose themselves to perceived contaminants while refraining from inappropriate compulsive behaviors (such as hand washing).
Cognitive interventions also can be an effective treatment of obsessions, without patients having to engage in exposure to their horrific thoughts and images.16 Consider, for example, a new mother who upon seeing the kitchen knife has the intrusive thought, “What if I stabbed my baby?” Instead of the traditional exposure approach for OCD (ie, having her vividly imagine stabbing her baby until her anxiety level subsided), the cognitive intervention would be to educate her about the normalcy of intrusive thoughts, particularly in the postpartum period.
Generalized anxiety disorder. CBT for generalized anxiety disorder (GAD) targets patients’ overestimation of the likelihood of negative events and the belief that these events, should they occur, would be catastrophic and render them unable to cope.12
Motivational interviewing (MI) appears to be a useful adjunct to precede traditional CBT, particularly for severe worriers.17 MI attempts to help individuals with GAD recognize their ambivalence about giving up worry. This technique acknowledges and validates perceived benefits of worry (eg, “It helps me prepare for the worst, so I won’t be emotionally devastated if it happens”), but also explores how worry is destructive.
Emerging CBT models for anxiety disorders
Metacognitive treatment. Evidence, such as presented by Dobson,18 suggests that the field of CBT is shifting towards a metacognitive model of change and treatment. A metacognitive approach goes beyond changing thinking and emphasizes thoughts about thoughts and experiences. Examples include mindfulness-based cognitive therapy (MBCT) and acceptance and commitment therapy (ACT).
MBCT typically consists of an 8-week program of 2-hour sessions each week and 1 full-day retreat. MBCT is modeled after Kabat-Zinn’s widely disseminated and empirically supported mindfulness based stress reduction course.19 MBCT was developed as a relapse prevention program for patients who had recovered from depression. Unlike traditional cognitive therapy for depression that targets changing the content of automatic thoughts and core beliefs, in MBCT patients are aware of negative automatic thoughts and find ways to change their relationship with these thoughts, learning that thoughts are not facts. This process mainly is carried out by practicing mindfulness meditation exercises. Importantly, MBCT goes beyond mindful acceptance of negative thoughts and teaches patients mindful acceptance of all internal experiences.
A fundamental difference between ACT and traditional CBT is the approach to cognitions.20 Although CBT focuses on changing the content of maladaptive thoughts, such as “I am a worthless person,” ACT focuses on changing the function of thoughts. ACT strives to help patients to accept their internal experiences—whether unwanted thoughts, feelings, bodily sensations, or memories—while committing themselves to pursuing their life goals and values. Strategies aim to help patients step back from their thoughts and observe them as just thoughts. The patient who thinks, “I am worthless” would be instructed to practice saying “I am having the thought I am worthless.” Therefore the thought no longer controls the person’s behavior.
These approaches train the patient to keenly observe distressing thoughts and experiences—not necessarily with the goal of changing them but to accept them and act in a way that is consistent with his (her) goals and values. A meta-analysis of 39 studies found mindfulness-based therapy effective in improving symptoms in participants with anxiety and mood disorders.21 Similarly, ACT has demonstrated efficacy with mixed anxiety disorders.22
Transdiagnostic CBT. Recent research18 suggests that mood and anxiety disorders may have more commonalities than differences in underlying biological and psychological traits. Because the symptoms of anxiety and depressive disorders tend to overlap, and their rate of comorbidity may be as high as 55%,23 so-called transdiagnostic treatments have been developed. Transdiagnostic treatments target impairing symptoms that cut across different diagnoses. For example, patients with depression, anxiety, or substance abuse might share a common difficulty with regulating and coping with negative emotions.
In a preliminary comparison trial,24 46 patients with social anxiety disorder, panic disorder, or GAD were randomly assigned to transdiagnostic CBT (n = 23) or diagnosis-specific CBT (n = 23). Treatments were based on widely used manuals and offered in 2-hour group sessions across 12 weeks. Transdiagnostic CBT was found to be as effective as specific CBT protocols in terms of symptom improvement. Participants attended an average of 8.46 sessions, with similar attendance in each protocol. Fourteen participants (30%) discontinued treatment, similar to attrition rates reported in other trials of transdiagnostic and diagnosis-specific CBT.
Transdiagnostic treatments may facilitate the dissemination of empirically supported treatments because therapists would not be required to have training and supervision to competency in delivering multiple manuals for specific anxiety disorders. This could be attractive to busy practitioners with limited time to learn new treatments.
Bottom Line
Efficacy of cognitive-behavioral therapy (CBT) for depression and anxiety is well established. Although no specific technique defines CBT, a common practice is to educate an individual about interrelationships between behaviors/activities, thoughts, and mood. CBT techniques can be customized to treat specific anxiety disorders, such as panic disorder, obsessive-compulsive disorder, and generalized anxiety disorder.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Collins K, Westra H, Dozois D, et al. Gaps in accessing treatment for anxiety and depressions: challenges for the delivery of care. Clin Psychol Rev. 2004;24(5):583-616.
2. Foa EB, Gillihan SJ, Bryant RA. Challenges and successes in dissemination of evidence-based treatments for posttraumatic stress: lessons learned from prolonged exposure therapy for PTSD. Psychological Science in the Public Interest. 2013;14(2):65-111.
3. Rahman A, Malik A, Sikander S, et al. Cognitive behaviour therapy-based intervention by community health workers for mothers with depression and their infants in rural Pakistan: a cluster-randomised controlled trial. Lancet. 2008;372(9642):902-909.
4. Aguilera A, Muench F. There’s an app for that: information technology applications for cognitive behavioral practitioners. Behavior Therapist. 2012;35(4):65-73.
5. Dimidjian S, Hollon SD, Dobson KS, et al. Randomized trial of behavioral activation, cognitive therapy, and antidepressant medication in the acute treatment of adults with major depression. J Consult Clin Psychol. 2006; 74(4):658-670.
6. Hollon SD, Jarrett RB, Nierenberg AA, et al. Psychotherapy and medication in the treatment of adult and geriatric depression: which monotherapy or combined treatment? J Clin Psychiatry. 2005;66(4):455-468.
7. Cuijpers P, Hollon SD, van Straten A, et al. Does cognitive behaviour therapy have an enduring effect that is superior to keeping patients on continuation pharmacotherapy? A meta-analysis. BMJ Open. 2013;3(4):1-8.
8. Stewart R, Chambless D. Cognitive-behavioral therapy for adult anxiety disorders in clinical practice: a meta-analysis of effectiveness studies. J Consult Clin Psychol. 2009;77(4): 595-606.
9. Wright JH, Basco MR, Thase M. Learning cognitive behavior therapy: an illustrated guide. Arlington, VA: American Psychiatric Publishing; 2006.
10. Barlow DH, Craske MG. Mastery of your anxiety and panic. 4th ed. New York, NY: Oxford University Press, Inc.; 2007.
11. Foa EB, Yadin E, Lichner TK. Exposure and response prevention for obsessive-compulsive disorder: therapist guide. New York, NY: Oxford University Press, Inc.; 2012.
12. Dugas MJ, Robichaud M. Cognitive-behavioral treatment for generalized anxiety disorder. New York, NY: Routledge; 2007.
13. Zlomke K, Davis TE. One-session treatment of specific phobias: a detailed description and review of treatment efficacy. Behav Ther. 2008;39(3):207-223.
14. Foa EB, Hembree E, Rothbaum B. Prolonged exposure therapy for PTSD: emotional processing of traumatic experiences. Therapist guide. New York, NY: Oxford University Press, Inc.; 2007.
15. Resick PA, Schnicke MK. Cognitive processing therapy for rape victims. London, United Kingdom: Sage Publications; 1996.
16. Whittal ML, Robichaud M, Woody SR. Cognitive treatment of obsessions: enhancing dissemination with video components. Cognitive and Behavioral Practice. 2010;17(1):1-8.
17. Westra H, Arkowitz H, Dozois D. Adding a motivational interviewing pretreatment to cognitive behavioral therapy for generalized anxiety disorder: a preliminary randomized controlled trial. J Anxiety Disord. 2009;23(2): 1106-1117.
18. Dobson KS. The science of CBT: toward a metacognitive model of change? Behav Ther. 2013;44(2):224-227.
19. Kabat-Zinn J. Full catastrophe living. Using the wisdom of your body and mind to face stress, pain, and illness. Revised edition. New York, NY: Bantam Books; 2013.
20. Hayes SC, Strosahl KD. Acceptance and commitment therapy. The process and practice of mindful change. 2nd ed. New York, NY: The Guilford Press; 2012.
21. Hofmann S, Sawyer A, Witt A, et al. The effect of mindfulness-based therapy on anxiety and depression: a meta-analytic review. J Consult Clin Psychol. 2010;78(2): 169-183.
22. Arch J, Eifert G, Davies C, et al. Randomized clinical trial of cognitive behavioral therapy (CBT) versus acceptance and commitment therapy (ACT) for mixed anxiety disorders. J Consult Clin Psychol. 2012;80(5):750-765.
23. Brown TA, Campbell LA, Lehman CL, et al. Current and lifetime comorbidity of the DSM-IV anxiety and mood disorders in a large clinical sample. J Abnorm Psychol. 2001;110(4):585-599.
24. Norton P, Barrera T. Transdiagnostic versus diagnosis-specific CBT for anxiety disorders: a preliminary randomized controlled noninferiority trial. Depress Anxiety. 2012;29(10):874-882.
Fewer than 20% of people seeking help for depression and anxiety disorders receive cognitive-behavioral therapy (CBT), the most established evidence-based psychotherapeutic treatment.1 Efforts are being made to increase access to CBT,2 but a substantial barrier remains: therapist training is a strong predictor of treatment outcome, and many therapists offering CBT services are not sufficiently trained to deliver multiple manual-based interventions with adequate fidelity to the model. Proposed solutions to this barrier include:
• abbreviated versions of CBT training for practitioners in primary care and community settings
• culturally adapted CBT training for community health workers3
• Internet-based CBT and telemedicine (telephone and video conferencing)2
• mobile phone applications that use text messaging, social support, and physiological monitoring as adjuncts to clinical practice or stand-alone interventions.4
New models of CBT also are emerging, including transdiagnostic CBT and metacognitive approaches (mindfulness-based cognitive therapy and acceptance and commitment therapy), and several new foci for exposure therapy.
In light of these ongoing modulations, this article is intended to help clinicians make informed decisions about CBT when selecting treatment for patients with depressive and anxiety disorders (Box5 ). We review the evidence of CBT’s efficacy for acute-phase treatment and relapse prevention; explain the common elements considered essential to CBT practice; describe CBT adaptations for specific anxiety disorders; and provide an overview of recent advances in conceptualizing and adapting CBT.
Efficacy for mood and anxiety disorders
Depression. Dozens of randomized controlled trials (RCT) and other studies support CBT’s efficacy in treating major depressive disorder (MDD). For acute treatment:
• CBT is more effective in producing remission when compared with no treatment, treatment as usual, or nonspecific psychotherapy.
• For mild to moderate depression, CBT is equivalent to antidepressant medication in terms of response and remission rates.
• Combining antidepressant therapy with CBT increases treatment adherence.6
Less well known may be that a successful response to CBT in the acute phase may have a protective effect against depression recurrences. A 2013 meta-analysis that totaled 506 individuals with depressive disorders found a trend toward significantly lower relapse rates when CBT was discontinued after acute therapy, compared with antidepressant therapy that continued beyond the acute phase.7
Anxiety. Among psychotherapies, CBT’s superior efficacy for anxiety disorders is well-established. CBT and its specific-disorder adaptations are considered first-line treatment.8
CBT’s essential elements
CBT focuses on distorted cognitions about the self, the world, and the future, and on behaviors that lead to or maintain symptoms.
Cognitive interventions seek to identify thoughts and beliefs that trigger emotional and behavioral reactions. A person with social anxiety disorder, for example, might believe that people will notice if he makes even a minor social mistake and then reject him, which will make him feel worthless. CBT can help him subject these beliefs to rational analysis and develop more adaptive beliefs, such as: “It is not certain that I will behave so badly that people would notice, but if that happened, the likelihood of being outright rejected is probably low. If—in the worst-case scenario—I was rejected, I am not worthless; I’m just a fallible human being.”
CBT’s behavioral component can be conceptualized as behavioral activation (BA), a structured approach to help the patient:
• increase behaviors and experiences that are rewarding
• overcome barriers to engaging in these new behaviors
• and decrease behaviors that maintain symptoms.
BA can be a useful intervention for individuals with depression characterized by lack of engagement or capacity for pleasurable experiences. During pregnancy and the postpartum period, for example, a woman undergoes physical, social, and environmental changes that might gradually deprive her of sources of pleasure and other reinforcing activities. BA would focus on developing creative solutions to regain access to or create new opportunities for rewarding experiences and to avoid behaviors (such as social withdrawal or physical activity restriction) that perpetuate depressed mood.
Common elements. Cognitive and behavioral interventions focus on problem solving, individualized case conceptualization (Figure 1), and collaborative empiricism.9
Individualized case conceptualization lays the foundation for the course of CBT, and may be thought of as a map for therapy. Case conceptualization brings in several domains of assessment including symptoms and diagnosis, the patient’s strengths, formative experiences (including biopsychosocial aspects), contextual factors, and cognitive factors that influence diagnosis and treatment, such as automatic thoughts or schemas. The case formulation leads to a working hypothesis about the optimal course and focus of CBT.
Collaborative empiricism is the way in which the patient and therapist work together to continually refine this working hypothesis. The pair works together to investigate the hypotheses and all aspects of the therapeutic relationship.
Although no specific technique defines CBT, a common practice is to educate a person about interrelationships between behaviors/activities, thoughts, and mood. A mood activity log (Figure 2) can illuminate links between moods and activities and be useful with targeting interventions. For a person with social anxiety, for example, a mood activity log could assist in developing a hierarchy of feared social situations and avoidance intensity. Systematic exposure therapy would follow, beginning with the least frightening/intense situation, accompanied by teaching new coping skills (such as relaxation strategies).
CBT adaptations for anxiety disorders
Elements of CBT have been adapted for a variety of anxiety disorders, based on specific symptoms and features (Table).10-15
Panic disorder. Panic control treatment is considered the first-line intervention for panic disorder’s defining features: spontaneous panic attacks, worry about future occurrence of attacks, and perceived catastrophic consequences (such as heart attack, fainting).10 This CBT adaptation includes:
• patient education about the nature of panic
• breathing retraining to foster exposure to feared bodily sensations and avoided activities and places
• cognitive restructuring of danger-related thoughts (such as “I’m going to faint,” or “It would be catastrophic if I did”).
Obsessive-compulsive disorder. Exposure and response prevention (ERP) is the first-line treatment for obsessive-compulsive disorder (OCD).11 In traditional therapist-guided ERP, patients expose themselves to perceived contaminants while refraining from inappropriate compulsive behaviors (such as hand washing).
Cognitive interventions also can be an effective treatment of obsessions, without patients having to engage in exposure to their horrific thoughts and images.16 Consider, for example, a new mother who upon seeing the kitchen knife has the intrusive thought, “What if I stabbed my baby?” Instead of the traditional exposure approach for OCD (ie, having her vividly imagine stabbing her baby until her anxiety level subsided), the cognitive intervention would be to educate her about the normalcy of intrusive thoughts, particularly in the postpartum period.
Generalized anxiety disorder. CBT for generalized anxiety disorder (GAD) targets patients’ overestimation of the likelihood of negative events and the belief that these events, should they occur, would be catastrophic and render them unable to cope.12
Motivational interviewing (MI) appears to be a useful adjunct to precede traditional CBT, particularly for severe worriers.17 MI attempts to help individuals with GAD recognize their ambivalence about giving up worry. This technique acknowledges and validates perceived benefits of worry (eg, “It helps me prepare for the worst, so I won’t be emotionally devastated if it happens”), but also explores how worry is destructive.
Emerging CBT models for anxiety disorders
Metacognitive treatment. Evidence, such as presented by Dobson,18 suggests that the field of CBT is shifting towards a metacognitive model of change and treatment. A metacognitive approach goes beyond changing thinking and emphasizes thoughts about thoughts and experiences. Examples include mindfulness-based cognitive therapy (MBCT) and acceptance and commitment therapy (ACT).
MBCT typically consists of an 8-week program of 2-hour sessions each week and 1 full-day retreat. MBCT is modeled after Kabat-Zinn’s widely disseminated and empirically supported mindfulness based stress reduction course.19 MBCT was developed as a relapse prevention program for patients who had recovered from depression. Unlike traditional cognitive therapy for depression that targets changing the content of automatic thoughts and core beliefs, in MBCT patients are aware of negative automatic thoughts and find ways to change their relationship with these thoughts, learning that thoughts are not facts. This process mainly is carried out by practicing mindfulness meditation exercises. Importantly, MBCT goes beyond mindful acceptance of negative thoughts and teaches patients mindful acceptance of all internal experiences.
A fundamental difference between ACT and traditional CBT is the approach to cognitions.20 Although CBT focuses on changing the content of maladaptive thoughts, such as “I am a worthless person,” ACT focuses on changing the function of thoughts. ACT strives to help patients to accept their internal experiences—whether unwanted thoughts, feelings, bodily sensations, or memories—while committing themselves to pursuing their life goals and values. Strategies aim to help patients step back from their thoughts and observe them as just thoughts. The patient who thinks, “I am worthless” would be instructed to practice saying “I am having the thought I am worthless.” Therefore the thought no longer controls the person’s behavior.
These approaches train the patient to keenly observe distressing thoughts and experiences—not necessarily with the goal of changing them but to accept them and act in a way that is consistent with his (her) goals and values. A meta-analysis of 39 studies found mindfulness-based therapy effective in improving symptoms in participants with anxiety and mood disorders.21 Similarly, ACT has demonstrated efficacy with mixed anxiety disorders.22
Transdiagnostic CBT. Recent research18 suggests that mood and anxiety disorders may have more commonalities than differences in underlying biological and psychological traits. Because the symptoms of anxiety and depressive disorders tend to overlap, and their rate of comorbidity may be as high as 55%,23 so-called transdiagnostic treatments have been developed. Transdiagnostic treatments target impairing symptoms that cut across different diagnoses. For example, patients with depression, anxiety, or substance abuse might share a common difficulty with regulating and coping with negative emotions.
In a preliminary comparison trial,24 46 patients with social anxiety disorder, panic disorder, or GAD were randomly assigned to transdiagnostic CBT (n = 23) or diagnosis-specific CBT (n = 23). Treatments were based on widely used manuals and offered in 2-hour group sessions across 12 weeks. Transdiagnostic CBT was found to be as effective as specific CBT protocols in terms of symptom improvement. Participants attended an average of 8.46 sessions, with similar attendance in each protocol. Fourteen participants (30%) discontinued treatment, similar to attrition rates reported in other trials of transdiagnostic and diagnosis-specific CBT.
Transdiagnostic treatments may facilitate the dissemination of empirically supported treatments because therapists would not be required to have training and supervision to competency in delivering multiple manuals for specific anxiety disorders. This could be attractive to busy practitioners with limited time to learn new treatments.
Bottom Line
Efficacy of cognitive-behavioral therapy (CBT) for depression and anxiety is well established. Although no specific technique defines CBT, a common practice is to educate an individual about interrelationships between behaviors/activities, thoughts, and mood. CBT techniques can be customized to treat specific anxiety disorders, such as panic disorder, obsessive-compulsive disorder, and generalized anxiety disorder.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Fewer than 20% of people seeking help for depression and anxiety disorders receive cognitive-behavioral therapy (CBT), the most established evidence-based psychotherapeutic treatment.1 Efforts are being made to increase access to CBT,2 but a substantial barrier remains: therapist training is a strong predictor of treatment outcome, and many therapists offering CBT services are not sufficiently trained to deliver multiple manual-based interventions with adequate fidelity to the model. Proposed solutions to this barrier include:
• abbreviated versions of CBT training for practitioners in primary care and community settings
• culturally adapted CBT training for community health workers3
• Internet-based CBT and telemedicine (telephone and video conferencing)2
• mobile phone applications that use text messaging, social support, and physiological monitoring as adjuncts to clinical practice or stand-alone interventions.4
New models of CBT also are emerging, including transdiagnostic CBT and metacognitive approaches (mindfulness-based cognitive therapy and acceptance and commitment therapy), and several new foci for exposure therapy.
In light of these ongoing modulations, this article is intended to help clinicians make informed decisions about CBT when selecting treatment for patients with depressive and anxiety disorders (Box5 ). We review the evidence of CBT’s efficacy for acute-phase treatment and relapse prevention; explain the common elements considered essential to CBT practice; describe CBT adaptations for specific anxiety disorders; and provide an overview of recent advances in conceptualizing and adapting CBT.
Efficacy for mood and anxiety disorders
Depression. Dozens of randomized controlled trials (RCT) and other studies support CBT’s efficacy in treating major depressive disorder (MDD). For acute treatment:
• CBT is more effective in producing remission when compared with no treatment, treatment as usual, or nonspecific psychotherapy.
• For mild to moderate depression, CBT is equivalent to antidepressant medication in terms of response and remission rates.
• Combining antidepressant therapy with CBT increases treatment adherence.6
Less well known may be that a successful response to CBT in the acute phase may have a protective effect against depression recurrences. A 2013 meta-analysis that totaled 506 individuals with depressive disorders found a trend toward significantly lower relapse rates when CBT was discontinued after acute therapy, compared with antidepressant therapy that continued beyond the acute phase.7
Anxiety. Among psychotherapies, CBT’s superior efficacy for anxiety disorders is well-established. CBT and its specific-disorder adaptations are considered first-line treatment.8
CBT’s essential elements
CBT focuses on distorted cognitions about the self, the world, and the future, and on behaviors that lead to or maintain symptoms.
Cognitive interventions seek to identify thoughts and beliefs that trigger emotional and behavioral reactions. A person with social anxiety disorder, for example, might believe that people will notice if he makes even a minor social mistake and then reject him, which will make him feel worthless. CBT can help him subject these beliefs to rational analysis and develop more adaptive beliefs, such as: “It is not certain that I will behave so badly that people would notice, but if that happened, the likelihood of being outright rejected is probably low. If—in the worst-case scenario—I was rejected, I am not worthless; I’m just a fallible human being.”
CBT’s behavioral component can be conceptualized as behavioral activation (BA), a structured approach to help the patient:
• increase behaviors and experiences that are rewarding
• overcome barriers to engaging in these new behaviors
• and decrease behaviors that maintain symptoms.
BA can be a useful intervention for individuals with depression characterized by lack of engagement or capacity for pleasurable experiences. During pregnancy and the postpartum period, for example, a woman undergoes physical, social, and environmental changes that might gradually deprive her of sources of pleasure and other reinforcing activities. BA would focus on developing creative solutions to regain access to or create new opportunities for rewarding experiences and to avoid behaviors (such as social withdrawal or physical activity restriction) that perpetuate depressed mood.
Common elements. Cognitive and behavioral interventions focus on problem solving, individualized case conceptualization (Figure 1), and collaborative empiricism.9
Individualized case conceptualization lays the foundation for the course of CBT, and may be thought of as a map for therapy. Case conceptualization brings in several domains of assessment including symptoms and diagnosis, the patient’s strengths, formative experiences (including biopsychosocial aspects), contextual factors, and cognitive factors that influence diagnosis and treatment, such as automatic thoughts or schemas. The case formulation leads to a working hypothesis about the optimal course and focus of CBT.
Collaborative empiricism is the way in which the patient and therapist work together to continually refine this working hypothesis. The pair works together to investigate the hypotheses and all aspects of the therapeutic relationship.
Although no specific technique defines CBT, a common practice is to educate a person about interrelationships between behaviors/activities, thoughts, and mood. A mood activity log (Figure 2) can illuminate links between moods and activities and be useful with targeting interventions. For a person with social anxiety, for example, a mood activity log could assist in developing a hierarchy of feared social situations and avoidance intensity. Systematic exposure therapy would follow, beginning with the least frightening/intense situation, accompanied by teaching new coping skills (such as relaxation strategies).
CBT adaptations for anxiety disorders
Elements of CBT have been adapted for a variety of anxiety disorders, based on specific symptoms and features (Table).10-15
Panic disorder. Panic control treatment is considered the first-line intervention for panic disorder’s defining features: spontaneous panic attacks, worry about future occurrence of attacks, and perceived catastrophic consequences (such as heart attack, fainting).10 This CBT adaptation includes:
• patient education about the nature of panic
• breathing retraining to foster exposure to feared bodily sensations and avoided activities and places
• cognitive restructuring of danger-related thoughts (such as “I’m going to faint,” or “It would be catastrophic if I did”).
Obsessive-compulsive disorder. Exposure and response prevention (ERP) is the first-line treatment for obsessive-compulsive disorder (OCD).11 In traditional therapist-guided ERP, patients expose themselves to perceived contaminants while refraining from inappropriate compulsive behaviors (such as hand washing).
Cognitive interventions also can be an effective treatment of obsessions, without patients having to engage in exposure to their horrific thoughts and images.16 Consider, for example, a new mother who upon seeing the kitchen knife has the intrusive thought, “What if I stabbed my baby?” Instead of the traditional exposure approach for OCD (ie, having her vividly imagine stabbing her baby until her anxiety level subsided), the cognitive intervention would be to educate her about the normalcy of intrusive thoughts, particularly in the postpartum period.
Generalized anxiety disorder. CBT for generalized anxiety disorder (GAD) targets patients’ overestimation of the likelihood of negative events and the belief that these events, should they occur, would be catastrophic and render them unable to cope.12
Motivational interviewing (MI) appears to be a useful adjunct to precede traditional CBT, particularly for severe worriers.17 MI attempts to help individuals with GAD recognize their ambivalence about giving up worry. This technique acknowledges and validates perceived benefits of worry (eg, “It helps me prepare for the worst, so I won’t be emotionally devastated if it happens”), but also explores how worry is destructive.
Emerging CBT models for anxiety disorders
Metacognitive treatment. Evidence, such as presented by Dobson,18 suggests that the field of CBT is shifting towards a metacognitive model of change and treatment. A metacognitive approach goes beyond changing thinking and emphasizes thoughts about thoughts and experiences. Examples include mindfulness-based cognitive therapy (MBCT) and acceptance and commitment therapy (ACT).
MBCT typically consists of an 8-week program of 2-hour sessions each week and 1 full-day retreat. MBCT is modeled after Kabat-Zinn’s widely disseminated and empirically supported mindfulness based stress reduction course.19 MBCT was developed as a relapse prevention program for patients who had recovered from depression. Unlike traditional cognitive therapy for depression that targets changing the content of automatic thoughts and core beliefs, in MBCT patients are aware of negative automatic thoughts and find ways to change their relationship with these thoughts, learning that thoughts are not facts. This process mainly is carried out by practicing mindfulness meditation exercises. Importantly, MBCT goes beyond mindful acceptance of negative thoughts and teaches patients mindful acceptance of all internal experiences.
A fundamental difference between ACT and traditional CBT is the approach to cognitions.20 Although CBT focuses on changing the content of maladaptive thoughts, such as “I am a worthless person,” ACT focuses on changing the function of thoughts. ACT strives to help patients to accept their internal experiences—whether unwanted thoughts, feelings, bodily sensations, or memories—while committing themselves to pursuing their life goals and values. Strategies aim to help patients step back from their thoughts and observe them as just thoughts. The patient who thinks, “I am worthless” would be instructed to practice saying “I am having the thought I am worthless.” Therefore the thought no longer controls the person’s behavior.
These approaches train the patient to keenly observe distressing thoughts and experiences—not necessarily with the goal of changing them but to accept them and act in a way that is consistent with his (her) goals and values. A meta-analysis of 39 studies found mindfulness-based therapy effective in improving symptoms in participants with anxiety and mood disorders.21 Similarly, ACT has demonstrated efficacy with mixed anxiety disorders.22
Transdiagnostic CBT. Recent research18 suggests that mood and anxiety disorders may have more commonalities than differences in underlying biological and psychological traits. Because the symptoms of anxiety and depressive disorders tend to overlap, and their rate of comorbidity may be as high as 55%,23 so-called transdiagnostic treatments have been developed. Transdiagnostic treatments target impairing symptoms that cut across different diagnoses. For example, patients with depression, anxiety, or substance abuse might share a common difficulty with regulating and coping with negative emotions.
In a preliminary comparison trial,24 46 patients with social anxiety disorder, panic disorder, or GAD were randomly assigned to transdiagnostic CBT (n = 23) or diagnosis-specific CBT (n = 23). Treatments were based on widely used manuals and offered in 2-hour group sessions across 12 weeks. Transdiagnostic CBT was found to be as effective as specific CBT protocols in terms of symptom improvement. Participants attended an average of 8.46 sessions, with similar attendance in each protocol. Fourteen participants (30%) discontinued treatment, similar to attrition rates reported in other trials of transdiagnostic and diagnosis-specific CBT.
Transdiagnostic treatments may facilitate the dissemination of empirically supported treatments because therapists would not be required to have training and supervision to competency in delivering multiple manuals for specific anxiety disorders. This could be attractive to busy practitioners with limited time to learn new treatments.
Bottom Line
Efficacy of cognitive-behavioral therapy (CBT) for depression and anxiety is well established. Although no specific technique defines CBT, a common practice is to educate an individual about interrelationships between behaviors/activities, thoughts, and mood. CBT techniques can be customized to treat specific anxiety disorders, such as panic disorder, obsessive-compulsive disorder, and generalized anxiety disorder.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Collins K, Westra H, Dozois D, et al. Gaps in accessing treatment for anxiety and depressions: challenges for the delivery of care. Clin Psychol Rev. 2004;24(5):583-616.
2. Foa EB, Gillihan SJ, Bryant RA. Challenges and successes in dissemination of evidence-based treatments for posttraumatic stress: lessons learned from prolonged exposure therapy for PTSD. Psychological Science in the Public Interest. 2013;14(2):65-111.
3. Rahman A, Malik A, Sikander S, et al. Cognitive behaviour therapy-based intervention by community health workers for mothers with depression and their infants in rural Pakistan: a cluster-randomised controlled trial. Lancet. 2008;372(9642):902-909.
4. Aguilera A, Muench F. There’s an app for that: information technology applications for cognitive behavioral practitioners. Behavior Therapist. 2012;35(4):65-73.
5. Dimidjian S, Hollon SD, Dobson KS, et al. Randomized trial of behavioral activation, cognitive therapy, and antidepressant medication in the acute treatment of adults with major depression. J Consult Clin Psychol. 2006; 74(4):658-670.
6. Hollon SD, Jarrett RB, Nierenberg AA, et al. Psychotherapy and medication in the treatment of adult and geriatric depression: which monotherapy or combined treatment? J Clin Psychiatry. 2005;66(4):455-468.
7. Cuijpers P, Hollon SD, van Straten A, et al. Does cognitive behaviour therapy have an enduring effect that is superior to keeping patients on continuation pharmacotherapy? A meta-analysis. BMJ Open. 2013;3(4):1-8.
8. Stewart R, Chambless D. Cognitive-behavioral therapy for adult anxiety disorders in clinical practice: a meta-analysis of effectiveness studies. J Consult Clin Psychol. 2009;77(4): 595-606.
9. Wright JH, Basco MR, Thase M. Learning cognitive behavior therapy: an illustrated guide. Arlington, VA: American Psychiatric Publishing; 2006.
10. Barlow DH, Craske MG. Mastery of your anxiety and panic. 4th ed. New York, NY: Oxford University Press, Inc.; 2007.
11. Foa EB, Yadin E, Lichner TK. Exposure and response prevention for obsessive-compulsive disorder: therapist guide. New York, NY: Oxford University Press, Inc.; 2012.
12. Dugas MJ, Robichaud M. Cognitive-behavioral treatment for generalized anxiety disorder. New York, NY: Routledge; 2007.
13. Zlomke K, Davis TE. One-session treatment of specific phobias: a detailed description and review of treatment efficacy. Behav Ther. 2008;39(3):207-223.
14. Foa EB, Hembree E, Rothbaum B. Prolonged exposure therapy for PTSD: emotional processing of traumatic experiences. Therapist guide. New York, NY: Oxford University Press, Inc.; 2007.
15. Resick PA, Schnicke MK. Cognitive processing therapy for rape victims. London, United Kingdom: Sage Publications; 1996.
16. Whittal ML, Robichaud M, Woody SR. Cognitive treatment of obsessions: enhancing dissemination with video components. Cognitive and Behavioral Practice. 2010;17(1):1-8.
17. Westra H, Arkowitz H, Dozois D. Adding a motivational interviewing pretreatment to cognitive behavioral therapy for generalized anxiety disorder: a preliminary randomized controlled trial. J Anxiety Disord. 2009;23(2): 1106-1117.
18. Dobson KS. The science of CBT: toward a metacognitive model of change? Behav Ther. 2013;44(2):224-227.
19. Kabat-Zinn J. Full catastrophe living. Using the wisdom of your body and mind to face stress, pain, and illness. Revised edition. New York, NY: Bantam Books; 2013.
20. Hayes SC, Strosahl KD. Acceptance and commitment therapy. The process and practice of mindful change. 2nd ed. New York, NY: The Guilford Press; 2012.
21. Hofmann S, Sawyer A, Witt A, et al. The effect of mindfulness-based therapy on anxiety and depression: a meta-analytic review. J Consult Clin Psychol. 2010;78(2): 169-183.
22. Arch J, Eifert G, Davies C, et al. Randomized clinical trial of cognitive behavioral therapy (CBT) versus acceptance and commitment therapy (ACT) for mixed anxiety disorders. J Consult Clin Psychol. 2012;80(5):750-765.
23. Brown TA, Campbell LA, Lehman CL, et al. Current and lifetime comorbidity of the DSM-IV anxiety and mood disorders in a large clinical sample. J Abnorm Psychol. 2001;110(4):585-599.
24. Norton P, Barrera T. Transdiagnostic versus diagnosis-specific CBT for anxiety disorders: a preliminary randomized controlled noninferiority trial. Depress Anxiety. 2012;29(10):874-882.
1. Collins K, Westra H, Dozois D, et al. Gaps in accessing treatment for anxiety and depressions: challenges for the delivery of care. Clin Psychol Rev. 2004;24(5):583-616.
2. Foa EB, Gillihan SJ, Bryant RA. Challenges and successes in dissemination of evidence-based treatments for posttraumatic stress: lessons learned from prolonged exposure therapy for PTSD. Psychological Science in the Public Interest. 2013;14(2):65-111.
3. Rahman A, Malik A, Sikander S, et al. Cognitive behaviour therapy-based intervention by community health workers for mothers with depression and their infants in rural Pakistan: a cluster-randomised controlled trial. Lancet. 2008;372(9642):902-909.
4. Aguilera A, Muench F. There’s an app for that: information technology applications for cognitive behavioral practitioners. Behavior Therapist. 2012;35(4):65-73.
5. Dimidjian S, Hollon SD, Dobson KS, et al. Randomized trial of behavioral activation, cognitive therapy, and antidepressant medication in the acute treatment of adults with major depression. J Consult Clin Psychol. 2006; 74(4):658-670.
6. Hollon SD, Jarrett RB, Nierenberg AA, et al. Psychotherapy and medication in the treatment of adult and geriatric depression: which monotherapy or combined treatment? J Clin Psychiatry. 2005;66(4):455-468.
7. Cuijpers P, Hollon SD, van Straten A, et al. Does cognitive behaviour therapy have an enduring effect that is superior to keeping patients on continuation pharmacotherapy? A meta-analysis. BMJ Open. 2013;3(4):1-8.
8. Stewart R, Chambless D. Cognitive-behavioral therapy for adult anxiety disorders in clinical practice: a meta-analysis of effectiveness studies. J Consult Clin Psychol. 2009;77(4): 595-606.
9. Wright JH, Basco MR, Thase M. Learning cognitive behavior therapy: an illustrated guide. Arlington, VA: American Psychiatric Publishing; 2006.
10. Barlow DH, Craske MG. Mastery of your anxiety and panic. 4th ed. New York, NY: Oxford University Press, Inc.; 2007.
11. Foa EB, Yadin E, Lichner TK. Exposure and response prevention for obsessive-compulsive disorder: therapist guide. New York, NY: Oxford University Press, Inc.; 2012.
12. Dugas MJ, Robichaud M. Cognitive-behavioral treatment for generalized anxiety disorder. New York, NY: Routledge; 2007.
13. Zlomke K, Davis TE. One-session treatment of specific phobias: a detailed description and review of treatment efficacy. Behav Ther. 2008;39(3):207-223.
14. Foa EB, Hembree E, Rothbaum B. Prolonged exposure therapy for PTSD: emotional processing of traumatic experiences. Therapist guide. New York, NY: Oxford University Press, Inc.; 2007.
15. Resick PA, Schnicke MK. Cognitive processing therapy for rape victims. London, United Kingdom: Sage Publications; 1996.
16. Whittal ML, Robichaud M, Woody SR. Cognitive treatment of obsessions: enhancing dissemination with video components. Cognitive and Behavioral Practice. 2010;17(1):1-8.
17. Westra H, Arkowitz H, Dozois D. Adding a motivational interviewing pretreatment to cognitive behavioral therapy for generalized anxiety disorder: a preliminary randomized controlled trial. J Anxiety Disord. 2009;23(2): 1106-1117.
18. Dobson KS. The science of CBT: toward a metacognitive model of change? Behav Ther. 2013;44(2):224-227.
19. Kabat-Zinn J. Full catastrophe living. Using the wisdom of your body and mind to face stress, pain, and illness. Revised edition. New York, NY: Bantam Books; 2013.
20. Hayes SC, Strosahl KD. Acceptance and commitment therapy. The process and practice of mindful change. 2nd ed. New York, NY: The Guilford Press; 2012.
21. Hofmann S, Sawyer A, Witt A, et al. The effect of mindfulness-based therapy on anxiety and depression: a meta-analytic review. J Consult Clin Psychol. 2010;78(2): 169-183.
22. Arch J, Eifert G, Davies C, et al. Randomized clinical trial of cognitive behavioral therapy (CBT) versus acceptance and commitment therapy (ACT) for mixed anxiety disorders. J Consult Clin Psychol. 2012;80(5):750-765.
23. Brown TA, Campbell LA, Lehman CL, et al. Current and lifetime comorbidity of the DSM-IV anxiety and mood disorders in a large clinical sample. J Abnorm Psychol. 2001;110(4):585-599.
24. Norton P, Barrera T. Transdiagnostic versus diagnosis-specific CBT for anxiety disorders: a preliminary randomized controlled noninferiority trial. Depress Anxiety. 2012;29(10):874-882.
Do biomarkers for Alzheimer’s disease have utility in everyday practice?
Guidelines for diagnosing Alzheimer’s disease (AD) are undergoing the first major changes since they were developed 30 years ago. The National Institute on Aging (NIA) and the Alzheimer’s Association (AA) have established workgroups to revise guidelines that were written in 1984.1
One of the major changes to these new guidelines is mention of research on biomarkers for diagnosing and monitoring progression of dementia in AD. This is an exciting and provocative development, but the questions practitioners who diagnose and treat AD should be asking are whether such biomarkers have utility in clinical practice today, or whether their application is a distant promise of continuing research.
Principles put forward in the guidelines
The new AD guidelines set forth in 3 major papers by the workgroups created by the NIA and AA include a change in nomenclature of AD.2 The workgroups have sought to define AD with specific stages that include:
• a preclinical/prodromal phase, in which the pathophysiology responsible for future cognitive changes is ongoing but lacks clinical manifestations3
• mild cognitive impairment, now considered a distinct entity from dementia and diagnosed when a person has early signs of AD; manifestations of impaired cognition in early disease are not significant enough to affect daily functioning.4
These newly formulated stages of AD rely on clinical judgment, and AD remains a clinical diagnosis. However, the new diagnostic guidelines include the use of biomarkers to measure disease progression.
Biomarkers of normal biologic function and pathology
The Biomarkers Definitions Working Group defines a biomarker as:
… a characteristic that is objectively measured and evaluated as an indicator of normal biologic processes, pathogenic processes, or pharmacologic responses to a therapeutic intervention.5
These characteristics include imaging studies and body fluids, such as serum and cerebrospinal fluid (CSF).
In AD, biomarkers are meant to measure the pathogenic processes of:
• accumulation and deposition of amyloid β _protein (Aβ42) plaques
• neuronal degeneration characterized by an increase in phosphorylated tau protein and neurofibrillary tangles.6
The purpose of these biomarkers is to identify ongoing disease and help the clinician stage patients who display a spectrum of symptoms.
Four classes of biomarkers (Table7)have been identified for use in the diagnosis of, and research on, AD:
• neuroimaging
• CSF
• serum
• genetic markers.
Neuroimaging
The basic purpose of CT and MRI of the head in the workup of cognitive impairment is to rule out a lesion in the brain, such as a tumor or hemorrhage, as the cause of, or contributor to, the impairment. Several neuroimaging studies are available to aid in diagnosing AD and distinguishing it from other causes of dementia, including:
• Fludeoxyglucose (FDG) positron-emission tomography (PET) scanning
• MRI
• Florbetapir F 18 Injection for PET.
FDG PET identifies areas of the brain in which glucose metabolism is decreased. This finding is thought to represent synaptic dysfunction.8 The true clinical utility of FDG PET appears to be as an aid in distinguishing cases of AD from frontotemporal dementia, by identifying regions of metabolic dysfunction.9 (Note: Medicare will reimburse for FDG PET only if 1) the patient has met diagnostic criteria for both AD and frontotemporal dementia for at least 6 months and 2) the cause of symptoms is uncertain.10)
FDG PET also can be useful in patients with mild cognitive impairment by identifying hypometabolism in the temporal and parietal regions of the brain years before clinical AD develops.In addition to FDG, 2 other imaging probes—Pittsburgh compound and 2-(1-{6-[(2-[fluorine-18]fluoroethyl)(methyl) amino]-2-naphthyl}-ethylidene) malononitrile (more commonly, FDDNP)—have been used with PET as research tools to demonstrate evidence of AD.11
MRI has been used to measure hippocampal atrophy and cortical thinning that occurs as a patient progresses from normal cognitive function or mild cognitive impairment to full dementia.5 The degree of atrophy has not been well correlated with the degree of functional impairment.
Florbetapir F 18 Injection was approved by the FDA in October 2013, under the brand name AMYViD, for measuring the quantity of Aβ42 deposition in the brain. When injected, this radiopharmaceutical binds to Aβ42 and can be detected on PET.12 Use criteria for AMYViD PET recently were developed13; the technique is indicated as an additional diagnostic tool for ruling out AD.
A negative AMYViD scan indicates sparse or no Aβ42 plaques, and is inconsistent with AD. However, a positive AMYViD scan does not establish a diagnosis of AD or other cognitive disorder.14 This lack of specificity decreases the potential utility of the scan in clinical practice.
Use of AMYViD PET in general practice also is constrained by cost, which varies by location, based on the fee for the PET scan ($1,000 to $3,000)15; to that, add the cost of a dose of AMYViD ($1,600, wholesale).16 The technique is not reimbursable, and the total out-of-pocket expense can be as much as $5,000—making an AMYViD PET prohibitive.
Cerebrospinal fluid markers
CSF biomarkers used in the evaluation of AD are Aβ42, t-tau protein, and p-tau protein.6,17 It is generally thought that the level of Aβ42 in CSF decreases in AD—indicative of Aβ42 being deposited in the brain.8 Tau proteins are elevated in CSF as neurons are destroyed. P-tau is associated with the neurofibrillary tangles of AD; its presence in CSF is thought to represent an increase in those tangles. The combination of a low level of Aβ42 and an elevated level of p-tau in CSF is considered the signature CSF biomarker of AD.6
Serum markers
The search for reliable serum biomarkers of AD is the area of greatest research interest because a blood test is a less invasive form of screening. Regrettably, the utility of serum biomarkers for clinical practice has not been established.
Aβ42 can be measured in serum, but levels do not correlate well with CSF levels.18 Other serum markers that have been evaluated for clinical utility include measures of lipid metabolism, oxidation, and inflammation. With none of these is there clear correlation between the level of protein and AD.18
Fourth front: Genetics
Several alleles are associated with AD. Mutations in amyloid precursor protein, presenilin 1, and presenilin 2 have been shown to cause a change in the processing of Aβ42 and thus lead to AD.19 These mutations are inherited in an autosomal-dominant fashion and are detected in early-onset (age <65) AD.
Mutations in apolipoprotein 4-β4 also has been the subject of much research; this allele usually is associated with increased risk of the more common, later-onset AD.20 Some evidence suggests that apolipoprotein 4-β4 carriers who develop AD might be at risk of earlier onset of symptoms, compared to noncarriers,21 but the clinical significance of that increased risk has not been established.
What utility do biomarkers have?
As we said at the beginning of this article, the question that clinicians should be asking is: “What is the current clinical utility of these sophisticated biomarkers and genetic testing?”
The answer is “little utility.” Diagnosing AD is a clinical enterprise, with, as we’ve outlined, specific and narrow exceptions.
Recently, researchers demonstrated biomarker evidence of AD before symptom onset in patients who have known autosomal-dominant gene mutations for AD.19 There is no evidence, however, that these biomarkers are useful for screening the general population to identify people who 1) are at risk of, or who have, AD and 2) do not have AD.
That being said, CSF and imaging biomarkers of AD are being used in clinical settings in some European countries to aid investigation of cognitive decline.
In conclusion
Here are key points to take away from this discussion of biomarkers of AD:
• The utility of these biomarkers today is in research—although some of them might, on occasion, be useful to distinguish dementia caused by AD from other dementias.
• The ultimate goal of research is to uncover a serum biomarker that can identify patients in the preclinical/prodromal stage of AD, so that disease-modifying therapies and preventive measures can be initiated before symptoms manifest.
• Science is a long way from making this goal a reality, but recent changes in the diagnostic criteria for AD will encourage research in this area of study.
Bottom Line
Researchers are working to uncover biomarkers that will identify patients in the preclinical or prodromal stage of Alzheimer’s disease, but diagnosis remains clinical. Recent changes to diagnostic criteria will encourage research in this area.
Related Resources
• Blennow K, Dubois B, Fagan AM, et al. Clinical utility of cerebrospinal fluid biomarkers in the diagnosis of early Alzheimer’s disease [published online May 5, 2014]. Alzheimers Dement. doi: 10.1016/j.jalz.2014.02.004.
• Chase A. Alzheimer disease: Advances in imaging of AD biomarkers could aid early diagnosis. Nat Rev Neurol. 2014;10(5):239.
• De Riva V, Galloni E, Marcon M, et al. Analysis of combined CSF biomarkers in AD diagnosis. Clin Lab. 2014;60(4):629-634.
• Kristofikova Z, Ricny J, Kolarova M, et al. Interactions between amyloid-β and tau in cerebrospinal fluid of people with mild cognitive impairment and Alzheimer’s disease [published online March 26, 2014]. J Alzheimers Dis. doi: 10.3233/ JAD-132393.
Drug Brand Name
Florbetapir F 18 Injection • AMYViD
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Jack CR Jr, Albert MS, Knopman DS, et al. Introduction to the recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011; 7(3):257-262.
2. McKhann GM, Knopman DS. Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging- Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):263-269.
3. Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):280-292.
4. Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging- Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011; 7(3):270-279.
5. Cummings JL. Biomarkers in Alzheimer’s disease– perspectives for the future. US Neurology. 2010;6(1):23-27.
6. Sperling R, Keith J. Biomarkers of Alzheimer disease: current and future applications to diagnostic criteria. Continuum (Minneap Minn). 2013;19(2 Dementia):325-338.
7. Craig-Shapiro R, Fagan AM, Holtzman DM. Biomarkers of Alzheimer’s disease. Neurobiol Dis. 2009;35(2):128-140.
8. Jack CR Jr, Knopman DS, Jagust WJ, et al. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol. 2010;9(1):119-128.
9. Foster NL, Heidebrink JL, Clark CM, et al. FDG-PET improves accuracy in distinguishing frontotemporal dementia and Alzheimer’s disease. Brain. 2007;130(pt 10):2616-2635.
10. National Coverage Determination (NCD) for FDG PET for Dementia and Neurodegenerative Diseases (220.6.13). Centers for Medicare and Medicaid Services. http://www. cms.gov/medicare-coverage-database/details/ncd-details. aspx?NCDId=288&ncdver=3&bc=BAABAAAAAAAA&. Accessed May 9, 2014.
11. Small GW, Bookheimer SY, Thompson PM, et al. Current and future uses of neuroimaging for cognitively impaired patients. Lancet Neurol. 2008;7(2):161-172. 12. Clark CM, Schneider JA, Bedell BJ, et al. Use of florbetapir- PET for imaging beta-amyloid pathology. JAMA. 2011;305(3): 275-283.
13. Johnson KA, Minoshima S, Bohnen NI, et al. Update on appropriate use criteria for amyloid PET imaging: dementia experts, mild cognitive impairment, and education. Amyloid Imaging Task Force of the Alzheimer’s Association and Society for Nuclear Medicine and Molecular Imaging. Alzheimers Dement. 2013;9(4):e106-e109.
14. AMYViD [package insert]. Indianapolis, IN: Eli Lilly & Co; 2012.
15. First guidelines published for brain amyloid imaging in Alzheimer’s. Alzheimer’s Association. http://www.alz.org/ news_and_events_60578.asp. Published January 28, 2013. Accessed May 9, 2014.
16. Zakaib GD. FDA approves Amyvid for clinical use. Alzforum. http://www.alzforum.org/news/research-news/ fda-approves-amyvid-clinical-use. Published April 9, 2012. Accessed May 16, 2014.
17. Skillbäck T, Zetterberg H, Blennow K, et al. Cerebrospinal fluid biomarkers for Alzheimer disease and subcortical axonal damage in 5,542 clinical samples. Alzheimers Res Ther. 2013;5(5):47.
18. Irizarry MC. Biomarkers of Alzheimer disease in plasma. NeuroRx. 2004;1(2):226-234.
19. Bateman RJ, Xiong C, Benzinger TL, et al. Clinical and biomarker changes in dominantly inherited Alzheimer’s disease. N Engl J Med. 2012;367(9):795-804.
20. Bertram L, McQueen MB, Mullin K, et al. Systematic meta-analyses of Alzheimer disease genetic association studies: the AlzGene database. Nature Genetics. 2007;39(1):17-23.
21. Strittmatter WJ, Roses AD. Apolipoprotein E and Alzheimer’s disease. Annu Rev Neurosci. 1996;19:53-77.
Guidelines for diagnosing Alzheimer’s disease (AD) are undergoing the first major changes since they were developed 30 years ago. The National Institute on Aging (NIA) and the Alzheimer’s Association (AA) have established workgroups to revise guidelines that were written in 1984.1
One of the major changes to these new guidelines is mention of research on biomarkers for diagnosing and monitoring progression of dementia in AD. This is an exciting and provocative development, but the questions practitioners who diagnose and treat AD should be asking are whether such biomarkers have utility in clinical practice today, or whether their application is a distant promise of continuing research.
Principles put forward in the guidelines
The new AD guidelines set forth in 3 major papers by the workgroups created by the NIA and AA include a change in nomenclature of AD.2 The workgroups have sought to define AD with specific stages that include:
• a preclinical/prodromal phase, in which the pathophysiology responsible for future cognitive changes is ongoing but lacks clinical manifestations3
• mild cognitive impairment, now considered a distinct entity from dementia and diagnosed when a person has early signs of AD; manifestations of impaired cognition in early disease are not significant enough to affect daily functioning.4
These newly formulated stages of AD rely on clinical judgment, and AD remains a clinical diagnosis. However, the new diagnostic guidelines include the use of biomarkers to measure disease progression.
Biomarkers of normal biologic function and pathology
The Biomarkers Definitions Working Group defines a biomarker as:
… a characteristic that is objectively measured and evaluated as an indicator of normal biologic processes, pathogenic processes, or pharmacologic responses to a therapeutic intervention.5
These characteristics include imaging studies and body fluids, such as serum and cerebrospinal fluid (CSF).
In AD, biomarkers are meant to measure the pathogenic processes of:
• accumulation and deposition of amyloid β _protein (Aβ42) plaques
• neuronal degeneration characterized by an increase in phosphorylated tau protein and neurofibrillary tangles.6
The purpose of these biomarkers is to identify ongoing disease and help the clinician stage patients who display a spectrum of symptoms.
Four classes of biomarkers (Table7)have been identified for use in the diagnosis of, and research on, AD:
• neuroimaging
• CSF
• serum
• genetic markers.
Neuroimaging
The basic purpose of CT and MRI of the head in the workup of cognitive impairment is to rule out a lesion in the brain, such as a tumor or hemorrhage, as the cause of, or contributor to, the impairment. Several neuroimaging studies are available to aid in diagnosing AD and distinguishing it from other causes of dementia, including:
• Fludeoxyglucose (FDG) positron-emission tomography (PET) scanning
• MRI
• Florbetapir F 18 Injection for PET.
FDG PET identifies areas of the brain in which glucose metabolism is decreased. This finding is thought to represent synaptic dysfunction.8 The true clinical utility of FDG PET appears to be as an aid in distinguishing cases of AD from frontotemporal dementia, by identifying regions of metabolic dysfunction.9 (Note: Medicare will reimburse for FDG PET only if 1) the patient has met diagnostic criteria for both AD and frontotemporal dementia for at least 6 months and 2) the cause of symptoms is uncertain.10)
FDG PET also can be useful in patients with mild cognitive impairment by identifying hypometabolism in the temporal and parietal regions of the brain years before clinical AD develops.In addition to FDG, 2 other imaging probes—Pittsburgh compound and 2-(1-{6-[(2-[fluorine-18]fluoroethyl)(methyl) amino]-2-naphthyl}-ethylidene) malononitrile (more commonly, FDDNP)—have been used with PET as research tools to demonstrate evidence of AD.11
MRI has been used to measure hippocampal atrophy and cortical thinning that occurs as a patient progresses from normal cognitive function or mild cognitive impairment to full dementia.5 The degree of atrophy has not been well correlated with the degree of functional impairment.
Florbetapir F 18 Injection was approved by the FDA in October 2013, under the brand name AMYViD, for measuring the quantity of Aβ42 deposition in the brain. When injected, this radiopharmaceutical binds to Aβ42 and can be detected on PET.12 Use criteria for AMYViD PET recently were developed13; the technique is indicated as an additional diagnostic tool for ruling out AD.
A negative AMYViD scan indicates sparse or no Aβ42 plaques, and is inconsistent with AD. However, a positive AMYViD scan does not establish a diagnosis of AD or other cognitive disorder.14 This lack of specificity decreases the potential utility of the scan in clinical practice.
Use of AMYViD PET in general practice also is constrained by cost, which varies by location, based on the fee for the PET scan ($1,000 to $3,000)15; to that, add the cost of a dose of AMYViD ($1,600, wholesale).16 The technique is not reimbursable, and the total out-of-pocket expense can be as much as $5,000—making an AMYViD PET prohibitive.
Cerebrospinal fluid markers
CSF biomarkers used in the evaluation of AD are Aβ42, t-tau protein, and p-tau protein.6,17 It is generally thought that the level of Aβ42 in CSF decreases in AD—indicative of Aβ42 being deposited in the brain.8 Tau proteins are elevated in CSF as neurons are destroyed. P-tau is associated with the neurofibrillary tangles of AD; its presence in CSF is thought to represent an increase in those tangles. The combination of a low level of Aβ42 and an elevated level of p-tau in CSF is considered the signature CSF biomarker of AD.6
Serum markers
The search for reliable serum biomarkers of AD is the area of greatest research interest because a blood test is a less invasive form of screening. Regrettably, the utility of serum biomarkers for clinical practice has not been established.
Aβ42 can be measured in serum, but levels do not correlate well with CSF levels.18 Other serum markers that have been evaluated for clinical utility include measures of lipid metabolism, oxidation, and inflammation. With none of these is there clear correlation between the level of protein and AD.18
Fourth front: Genetics
Several alleles are associated with AD. Mutations in amyloid precursor protein, presenilin 1, and presenilin 2 have been shown to cause a change in the processing of Aβ42 and thus lead to AD.19 These mutations are inherited in an autosomal-dominant fashion and are detected in early-onset (age <65) AD.
Mutations in apolipoprotein 4-β4 also has been the subject of much research; this allele usually is associated with increased risk of the more common, later-onset AD.20 Some evidence suggests that apolipoprotein 4-β4 carriers who develop AD might be at risk of earlier onset of symptoms, compared to noncarriers,21 but the clinical significance of that increased risk has not been established.
What utility do biomarkers have?
As we said at the beginning of this article, the question that clinicians should be asking is: “What is the current clinical utility of these sophisticated biomarkers and genetic testing?”
The answer is “little utility.” Diagnosing AD is a clinical enterprise, with, as we’ve outlined, specific and narrow exceptions.
Recently, researchers demonstrated biomarker evidence of AD before symptom onset in patients who have known autosomal-dominant gene mutations for AD.19 There is no evidence, however, that these biomarkers are useful for screening the general population to identify people who 1) are at risk of, or who have, AD and 2) do not have AD.
That being said, CSF and imaging biomarkers of AD are being used in clinical settings in some European countries to aid investigation of cognitive decline.
In conclusion
Here are key points to take away from this discussion of biomarkers of AD:
• The utility of these biomarkers today is in research—although some of them might, on occasion, be useful to distinguish dementia caused by AD from other dementias.
• The ultimate goal of research is to uncover a serum biomarker that can identify patients in the preclinical/prodromal stage of AD, so that disease-modifying therapies and preventive measures can be initiated before symptoms manifest.
• Science is a long way from making this goal a reality, but recent changes in the diagnostic criteria for AD will encourage research in this area of study.
Bottom Line
Researchers are working to uncover biomarkers that will identify patients in the preclinical or prodromal stage of Alzheimer’s disease, but diagnosis remains clinical. Recent changes to diagnostic criteria will encourage research in this area.
Related Resources
• Blennow K, Dubois B, Fagan AM, et al. Clinical utility of cerebrospinal fluid biomarkers in the diagnosis of early Alzheimer’s disease [published online May 5, 2014]. Alzheimers Dement. doi: 10.1016/j.jalz.2014.02.004.
• Chase A. Alzheimer disease: Advances in imaging of AD biomarkers could aid early diagnosis. Nat Rev Neurol. 2014;10(5):239.
• De Riva V, Galloni E, Marcon M, et al. Analysis of combined CSF biomarkers in AD diagnosis. Clin Lab. 2014;60(4):629-634.
• Kristofikova Z, Ricny J, Kolarova M, et al. Interactions between amyloid-β and tau in cerebrospinal fluid of people with mild cognitive impairment and Alzheimer’s disease [published online March 26, 2014]. J Alzheimers Dis. doi: 10.3233/ JAD-132393.
Drug Brand Name
Florbetapir F 18 Injection • AMYViD
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Guidelines for diagnosing Alzheimer’s disease (AD) are undergoing the first major changes since they were developed 30 years ago. The National Institute on Aging (NIA) and the Alzheimer’s Association (AA) have established workgroups to revise guidelines that were written in 1984.1
One of the major changes to these new guidelines is mention of research on biomarkers for diagnosing and monitoring progression of dementia in AD. This is an exciting and provocative development, but the questions practitioners who diagnose and treat AD should be asking are whether such biomarkers have utility in clinical practice today, or whether their application is a distant promise of continuing research.
Principles put forward in the guidelines
The new AD guidelines set forth in 3 major papers by the workgroups created by the NIA and AA include a change in nomenclature of AD.2 The workgroups have sought to define AD with specific stages that include:
• a preclinical/prodromal phase, in which the pathophysiology responsible for future cognitive changes is ongoing but lacks clinical manifestations3
• mild cognitive impairment, now considered a distinct entity from dementia and diagnosed when a person has early signs of AD; manifestations of impaired cognition in early disease are not significant enough to affect daily functioning.4
These newly formulated stages of AD rely on clinical judgment, and AD remains a clinical diagnosis. However, the new diagnostic guidelines include the use of biomarkers to measure disease progression.
Biomarkers of normal biologic function and pathology
The Biomarkers Definitions Working Group defines a biomarker as:
… a characteristic that is objectively measured and evaluated as an indicator of normal biologic processes, pathogenic processes, or pharmacologic responses to a therapeutic intervention.5
These characteristics include imaging studies and body fluids, such as serum and cerebrospinal fluid (CSF).
In AD, biomarkers are meant to measure the pathogenic processes of:
• accumulation and deposition of amyloid β _protein (Aβ42) plaques
• neuronal degeneration characterized by an increase in phosphorylated tau protein and neurofibrillary tangles.6
The purpose of these biomarkers is to identify ongoing disease and help the clinician stage patients who display a spectrum of symptoms.
Four classes of biomarkers (Table7)have been identified for use in the diagnosis of, and research on, AD:
• neuroimaging
• CSF
• serum
• genetic markers.
Neuroimaging
The basic purpose of CT and MRI of the head in the workup of cognitive impairment is to rule out a lesion in the brain, such as a tumor or hemorrhage, as the cause of, or contributor to, the impairment. Several neuroimaging studies are available to aid in diagnosing AD and distinguishing it from other causes of dementia, including:
• Fludeoxyglucose (FDG) positron-emission tomography (PET) scanning
• MRI
• Florbetapir F 18 Injection for PET.
FDG PET identifies areas of the brain in which glucose metabolism is decreased. This finding is thought to represent synaptic dysfunction.8 The true clinical utility of FDG PET appears to be as an aid in distinguishing cases of AD from frontotemporal dementia, by identifying regions of metabolic dysfunction.9 (Note: Medicare will reimburse for FDG PET only if 1) the patient has met diagnostic criteria for both AD and frontotemporal dementia for at least 6 months and 2) the cause of symptoms is uncertain.10)
FDG PET also can be useful in patients with mild cognitive impairment by identifying hypometabolism in the temporal and parietal regions of the brain years before clinical AD develops.In addition to FDG, 2 other imaging probes—Pittsburgh compound and 2-(1-{6-[(2-[fluorine-18]fluoroethyl)(methyl) amino]-2-naphthyl}-ethylidene) malononitrile (more commonly, FDDNP)—have been used with PET as research tools to demonstrate evidence of AD.11
MRI has been used to measure hippocampal atrophy and cortical thinning that occurs as a patient progresses from normal cognitive function or mild cognitive impairment to full dementia.5 The degree of atrophy has not been well correlated with the degree of functional impairment.
Florbetapir F 18 Injection was approved by the FDA in October 2013, under the brand name AMYViD, for measuring the quantity of Aβ42 deposition in the brain. When injected, this radiopharmaceutical binds to Aβ42 and can be detected on PET.12 Use criteria for AMYViD PET recently were developed13; the technique is indicated as an additional diagnostic tool for ruling out AD.
A negative AMYViD scan indicates sparse or no Aβ42 plaques, and is inconsistent with AD. However, a positive AMYViD scan does not establish a diagnosis of AD or other cognitive disorder.14 This lack of specificity decreases the potential utility of the scan in clinical practice.
Use of AMYViD PET in general practice also is constrained by cost, which varies by location, based on the fee for the PET scan ($1,000 to $3,000)15; to that, add the cost of a dose of AMYViD ($1,600, wholesale).16 The technique is not reimbursable, and the total out-of-pocket expense can be as much as $5,000—making an AMYViD PET prohibitive.
Cerebrospinal fluid markers
CSF biomarkers used in the evaluation of AD are Aβ42, t-tau protein, and p-tau protein.6,17 It is generally thought that the level of Aβ42 in CSF decreases in AD—indicative of Aβ42 being deposited in the brain.8 Tau proteins are elevated in CSF as neurons are destroyed. P-tau is associated with the neurofibrillary tangles of AD; its presence in CSF is thought to represent an increase in those tangles. The combination of a low level of Aβ42 and an elevated level of p-tau in CSF is considered the signature CSF biomarker of AD.6
Serum markers
The search for reliable serum biomarkers of AD is the area of greatest research interest because a blood test is a less invasive form of screening. Regrettably, the utility of serum biomarkers for clinical practice has not been established.
Aβ42 can be measured in serum, but levels do not correlate well with CSF levels.18 Other serum markers that have been evaluated for clinical utility include measures of lipid metabolism, oxidation, and inflammation. With none of these is there clear correlation between the level of protein and AD.18
Fourth front: Genetics
Several alleles are associated with AD. Mutations in amyloid precursor protein, presenilin 1, and presenilin 2 have been shown to cause a change in the processing of Aβ42 and thus lead to AD.19 These mutations are inherited in an autosomal-dominant fashion and are detected in early-onset (age <65) AD.
Mutations in apolipoprotein 4-β4 also has been the subject of much research; this allele usually is associated with increased risk of the more common, later-onset AD.20 Some evidence suggests that apolipoprotein 4-β4 carriers who develop AD might be at risk of earlier onset of symptoms, compared to noncarriers,21 but the clinical significance of that increased risk has not been established.
What utility do biomarkers have?
As we said at the beginning of this article, the question that clinicians should be asking is: “What is the current clinical utility of these sophisticated biomarkers and genetic testing?”
The answer is “little utility.” Diagnosing AD is a clinical enterprise, with, as we’ve outlined, specific and narrow exceptions.
Recently, researchers demonstrated biomarker evidence of AD before symptom onset in patients who have known autosomal-dominant gene mutations for AD.19 There is no evidence, however, that these biomarkers are useful for screening the general population to identify people who 1) are at risk of, or who have, AD and 2) do not have AD.
That being said, CSF and imaging biomarkers of AD are being used in clinical settings in some European countries to aid investigation of cognitive decline.
In conclusion
Here are key points to take away from this discussion of biomarkers of AD:
• The utility of these biomarkers today is in research—although some of them might, on occasion, be useful to distinguish dementia caused by AD from other dementias.
• The ultimate goal of research is to uncover a serum biomarker that can identify patients in the preclinical/prodromal stage of AD, so that disease-modifying therapies and preventive measures can be initiated before symptoms manifest.
• Science is a long way from making this goal a reality, but recent changes in the diagnostic criteria for AD will encourage research in this area of study.
Bottom Line
Researchers are working to uncover biomarkers that will identify patients in the preclinical or prodromal stage of Alzheimer’s disease, but diagnosis remains clinical. Recent changes to diagnostic criteria will encourage research in this area.
Related Resources
• Blennow K, Dubois B, Fagan AM, et al. Clinical utility of cerebrospinal fluid biomarkers in the diagnosis of early Alzheimer’s disease [published online May 5, 2014]. Alzheimers Dement. doi: 10.1016/j.jalz.2014.02.004.
• Chase A. Alzheimer disease: Advances in imaging of AD biomarkers could aid early diagnosis. Nat Rev Neurol. 2014;10(5):239.
• De Riva V, Galloni E, Marcon M, et al. Analysis of combined CSF biomarkers in AD diagnosis. Clin Lab. 2014;60(4):629-634.
• Kristofikova Z, Ricny J, Kolarova M, et al. Interactions between amyloid-β and tau in cerebrospinal fluid of people with mild cognitive impairment and Alzheimer’s disease [published online March 26, 2014]. J Alzheimers Dis. doi: 10.3233/ JAD-132393.
Drug Brand Name
Florbetapir F 18 Injection • AMYViD
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Jack CR Jr, Albert MS, Knopman DS, et al. Introduction to the recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011; 7(3):257-262.
2. McKhann GM, Knopman DS. Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging- Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):263-269.
3. Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):280-292.
4. Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging- Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011; 7(3):270-279.
5. Cummings JL. Biomarkers in Alzheimer’s disease– perspectives for the future. US Neurology. 2010;6(1):23-27.
6. Sperling R, Keith J. Biomarkers of Alzheimer disease: current and future applications to diagnostic criteria. Continuum (Minneap Minn). 2013;19(2 Dementia):325-338.
7. Craig-Shapiro R, Fagan AM, Holtzman DM. Biomarkers of Alzheimer’s disease. Neurobiol Dis. 2009;35(2):128-140.
8. Jack CR Jr, Knopman DS, Jagust WJ, et al. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol. 2010;9(1):119-128.
9. Foster NL, Heidebrink JL, Clark CM, et al. FDG-PET improves accuracy in distinguishing frontotemporal dementia and Alzheimer’s disease. Brain. 2007;130(pt 10):2616-2635.
10. National Coverage Determination (NCD) for FDG PET for Dementia and Neurodegenerative Diseases (220.6.13). Centers for Medicare and Medicaid Services. http://www. cms.gov/medicare-coverage-database/details/ncd-details. aspx?NCDId=288&ncdver=3&bc=BAABAAAAAAAA&. Accessed May 9, 2014.
11. Small GW, Bookheimer SY, Thompson PM, et al. Current and future uses of neuroimaging for cognitively impaired patients. Lancet Neurol. 2008;7(2):161-172. 12. Clark CM, Schneider JA, Bedell BJ, et al. Use of florbetapir- PET for imaging beta-amyloid pathology. JAMA. 2011;305(3): 275-283.
13. Johnson KA, Minoshima S, Bohnen NI, et al. Update on appropriate use criteria for amyloid PET imaging: dementia experts, mild cognitive impairment, and education. Amyloid Imaging Task Force of the Alzheimer’s Association and Society for Nuclear Medicine and Molecular Imaging. Alzheimers Dement. 2013;9(4):e106-e109.
14. AMYViD [package insert]. Indianapolis, IN: Eli Lilly & Co; 2012.
15. First guidelines published for brain amyloid imaging in Alzheimer’s. Alzheimer’s Association. http://www.alz.org/ news_and_events_60578.asp. Published January 28, 2013. Accessed May 9, 2014.
16. Zakaib GD. FDA approves Amyvid for clinical use. Alzforum. http://www.alzforum.org/news/research-news/ fda-approves-amyvid-clinical-use. Published April 9, 2012. Accessed May 16, 2014.
17. Skillbäck T, Zetterberg H, Blennow K, et al. Cerebrospinal fluid biomarkers for Alzheimer disease and subcortical axonal damage in 5,542 clinical samples. Alzheimers Res Ther. 2013;5(5):47.
18. Irizarry MC. Biomarkers of Alzheimer disease in plasma. NeuroRx. 2004;1(2):226-234.
19. Bateman RJ, Xiong C, Benzinger TL, et al. Clinical and biomarker changes in dominantly inherited Alzheimer’s disease. N Engl J Med. 2012;367(9):795-804.
20. Bertram L, McQueen MB, Mullin K, et al. Systematic meta-analyses of Alzheimer disease genetic association studies: the AlzGene database. Nature Genetics. 2007;39(1):17-23.
21. Strittmatter WJ, Roses AD. Apolipoprotein E and Alzheimer’s disease. Annu Rev Neurosci. 1996;19:53-77.
1. Jack CR Jr, Albert MS, Knopman DS, et al. Introduction to the recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011; 7(3):257-262.
2. McKhann GM, Knopman DS. Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging- Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):263-269.
3. Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):280-292.
4. Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging- Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011; 7(3):270-279.
5. Cummings JL. Biomarkers in Alzheimer’s disease– perspectives for the future. US Neurology. 2010;6(1):23-27.
6. Sperling R, Keith J. Biomarkers of Alzheimer disease: current and future applications to diagnostic criteria. Continuum (Minneap Minn). 2013;19(2 Dementia):325-338.
7. Craig-Shapiro R, Fagan AM, Holtzman DM. Biomarkers of Alzheimer’s disease. Neurobiol Dis. 2009;35(2):128-140.
8. Jack CR Jr, Knopman DS, Jagust WJ, et al. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol. 2010;9(1):119-128.
9. Foster NL, Heidebrink JL, Clark CM, et al. FDG-PET improves accuracy in distinguishing frontotemporal dementia and Alzheimer’s disease. Brain. 2007;130(pt 10):2616-2635.
10. National Coverage Determination (NCD) for FDG PET for Dementia and Neurodegenerative Diseases (220.6.13). Centers for Medicare and Medicaid Services. http://www. cms.gov/medicare-coverage-database/details/ncd-details. aspx?NCDId=288&ncdver=3&bc=BAABAAAAAAAA&. Accessed May 9, 2014.
11. Small GW, Bookheimer SY, Thompson PM, et al. Current and future uses of neuroimaging for cognitively impaired patients. Lancet Neurol. 2008;7(2):161-172. 12. Clark CM, Schneider JA, Bedell BJ, et al. Use of florbetapir- PET for imaging beta-amyloid pathology. JAMA. 2011;305(3): 275-283.
13. Johnson KA, Minoshima S, Bohnen NI, et al. Update on appropriate use criteria for amyloid PET imaging: dementia experts, mild cognitive impairment, and education. Amyloid Imaging Task Force of the Alzheimer’s Association and Society for Nuclear Medicine and Molecular Imaging. Alzheimers Dement. 2013;9(4):e106-e109.
14. AMYViD [package insert]. Indianapolis, IN: Eli Lilly & Co; 2012.
15. First guidelines published for brain amyloid imaging in Alzheimer’s. Alzheimer’s Association. http://www.alz.org/ news_and_events_60578.asp. Published January 28, 2013. Accessed May 9, 2014.
16. Zakaib GD. FDA approves Amyvid for clinical use. Alzforum. http://www.alzforum.org/news/research-news/ fda-approves-amyvid-clinical-use. Published April 9, 2012. Accessed May 16, 2014.
17. Skillbäck T, Zetterberg H, Blennow K, et al. Cerebrospinal fluid biomarkers for Alzheimer disease and subcortical axonal damage in 5,542 clinical samples. Alzheimers Res Ther. 2013;5(5):47.
18. Irizarry MC. Biomarkers of Alzheimer disease in plasma. NeuroRx. 2004;1(2):226-234.
19. Bateman RJ, Xiong C, Benzinger TL, et al. Clinical and biomarker changes in dominantly inherited Alzheimer’s disease. N Engl J Med. 2012;367(9):795-804.
20. Bertram L, McQueen MB, Mullin K, et al. Systematic meta-analyses of Alzheimer disease genetic association studies: the AlzGene database. Nature Genetics. 2007;39(1):17-23.
21. Strittmatter WJ, Roses AD. Apolipoprotein E and Alzheimer’s disease. Annu Rev Neurosci. 1996;19:53-77.