User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
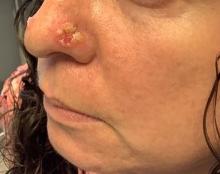
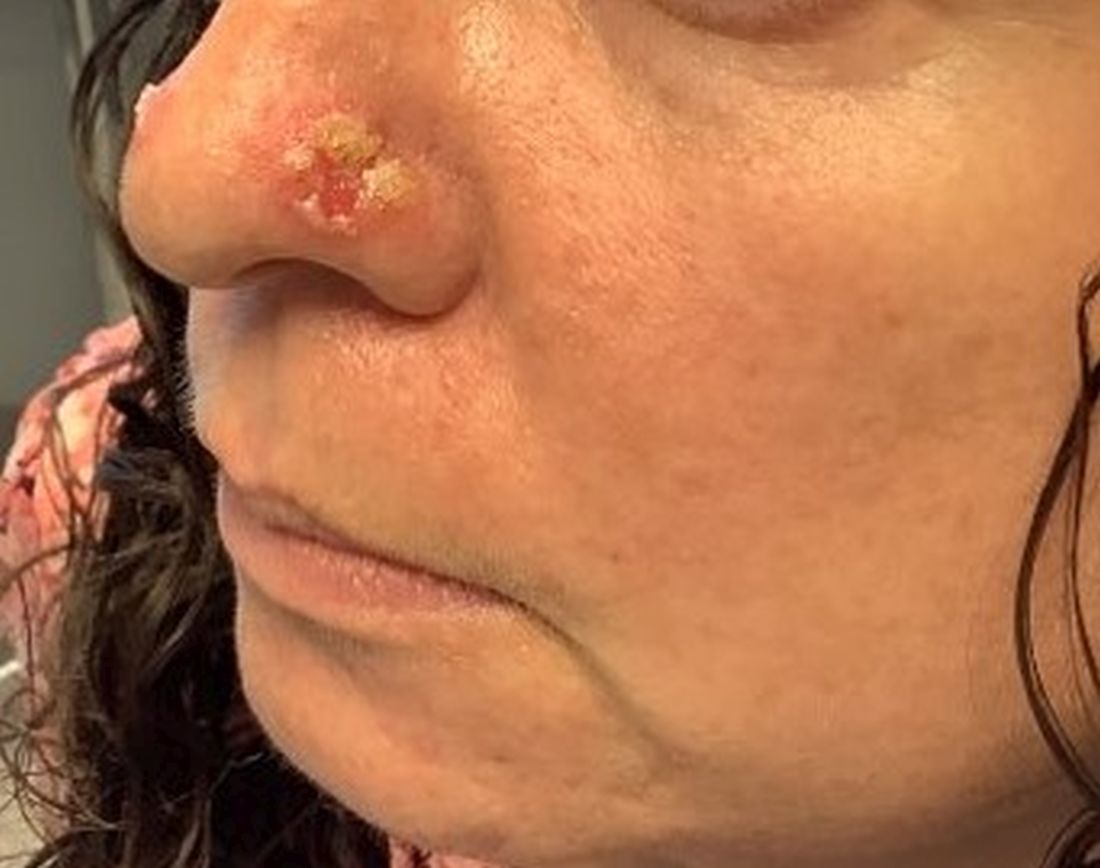
A 45-year-old White woman with no significant medical history presented with a 1-month history of lesions on the nose and right cheek
Cultures for bacteria, varicella zoster virus, herpes simplex virus, and mpox virus were all negative. A biopsy revealed suprabasilar acantholysis with follicular involvement in association with blister formation and inflammation. Direct immunofluorescence was positive for suprabasilar IgG and C3 deposition, consistent with pemphigus vulgaris (PV).
. There is likely a genetic predisposition. Medications that may induce pemphigus include penicillamine, nifedipine, or captopril.
Clinically, flaccid blistering lesions are present that may be cutaneous and/or mucosal. Bullae can progress to erosions and crusting, which then heal with pigment alteration but not scarring. The most commonly affected sites are the mouth, intertriginous areas, face, and neck. Mucosal lesions may involve the lips, esophagus, conjunctiva, and genitals.
Biopsy for histology and direct immunofluorescence is important in distinguishing between PV and other blistering disorders. Up to 75% of patients with active disease also have a positive indirect immunofluorescence with circulating IgG.
Treatment is generally immunosuppressive. Systemic therapy usually begins with prednisone and then is transitioned to a steroid-sparing agent such as mycophenolate mofetil. Other steroid-sparing agents include azathioprine, methotrexate, cyclophosphamide, and intravenous immunoglobulin. Secondary infections are possible and should be treated. Topical therapies aimed at reducing pain, especially in mucosal lesions, can be beneficial.
This case and the photos are from Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
Cultures for bacteria, varicella zoster virus, herpes simplex virus, and mpox virus were all negative. A biopsy revealed suprabasilar acantholysis with follicular involvement in association with blister formation and inflammation. Direct immunofluorescence was positive for suprabasilar IgG and C3 deposition, consistent with pemphigus vulgaris (PV).
. There is likely a genetic predisposition. Medications that may induce pemphigus include penicillamine, nifedipine, or captopril.
Clinically, flaccid blistering lesions are present that may be cutaneous and/or mucosal. Bullae can progress to erosions and crusting, which then heal with pigment alteration but not scarring. The most commonly affected sites are the mouth, intertriginous areas, face, and neck. Mucosal lesions may involve the lips, esophagus, conjunctiva, and genitals.
Biopsy for histology and direct immunofluorescence is important in distinguishing between PV and other blistering disorders. Up to 75% of patients with active disease also have a positive indirect immunofluorescence with circulating IgG.
Treatment is generally immunosuppressive. Systemic therapy usually begins with prednisone and then is transitioned to a steroid-sparing agent such as mycophenolate mofetil. Other steroid-sparing agents include azathioprine, methotrexate, cyclophosphamide, and intravenous immunoglobulin. Secondary infections are possible and should be treated. Topical therapies aimed at reducing pain, especially in mucosal lesions, can be beneficial.
This case and the photos are from Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
Cultures for bacteria, varicella zoster virus, herpes simplex virus, and mpox virus were all negative. A biopsy revealed suprabasilar acantholysis with follicular involvement in association with blister formation and inflammation. Direct immunofluorescence was positive for suprabasilar IgG and C3 deposition, consistent with pemphigus vulgaris (PV).
. There is likely a genetic predisposition. Medications that may induce pemphigus include penicillamine, nifedipine, or captopril.
Clinically, flaccid blistering lesions are present that may be cutaneous and/or mucosal. Bullae can progress to erosions and crusting, which then heal with pigment alteration but not scarring. The most commonly affected sites are the mouth, intertriginous areas, face, and neck. Mucosal lesions may involve the lips, esophagus, conjunctiva, and genitals.
Biopsy for histology and direct immunofluorescence is important in distinguishing between PV and other blistering disorders. Up to 75% of patients with active disease also have a positive indirect immunofluorescence with circulating IgG.
Treatment is generally immunosuppressive. Systemic therapy usually begins with prednisone and then is transitioned to a steroid-sparing agent such as mycophenolate mofetil. Other steroid-sparing agents include azathioprine, methotrexate, cyclophosphamide, and intravenous immunoglobulin. Secondary infections are possible and should be treated. Topical therapies aimed at reducing pain, especially in mucosal lesions, can be beneficial.
This case and the photos are from Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
Cigna accused of using AI, not doctors, to deny claims: Lawsuit
and forcing providers to bill patients in full.
In a complaint filed recently in California’s eastern district court, plaintiffs and Cigna health plan members Suzanne Kisting-Leung and Ayesha Smiley and their attorneys say that Cigna violates state insurance regulations by failing to conduct a “thorough, fair, and objective” review of their and other members’ claims.
The lawsuit says that, instead, Cigna relies on an algorithm, PxDx, to review and frequently deny medically necessary claims. According to court records, the system allows Cigna’s doctors to “instantly reject claims on medical grounds without ever opening patient files.” With use of the system, the average claims processing time is 1.2 seconds.
Cigna says it uses technology to verify coding on standard, low-cost procedures and to expedite physician reimbursement. In a statement to CBS News, the company called the lawsuit “highly questionable.”
The case highlights growing concerns about AI and its ability to replace humans for tasks and interactions in health care, business, and beyond. Public advocacy law firm Clarkson, which is representing the plaintiffs, has previously sued tech giants Google and ChatGPT creator OpenAI for harvesting Internet users’ personal and professional data to train their AI systems.
According to the complaint, Cigna denied the plaintiffs medically necessary tests, including blood work to screen for vitamin D deficiency and ultrasounds for patients suspected of having ovarian cancer. The plaintiffs’ attempts to appeal were unfruitful, and they were forced to pay out of pocket.
The plaintiff’s attorneys argue that the claims do not undergo more detailed reviews by physicians and employees, as mandated by California insurance laws, and that Cigna benefits by saving on labor costs.
Clarkson is demanding a jury trial and has asked the court to certify the Cigna case as a federal class action, potentially allowing the insurer’s other 2 million health plan members in California to join the lawsuit.
I. Glenn Cohen, JD, deputy dean and professor at Harvard Law School, Cambridge, Mass., said in an interview that this is the first lawsuit he’s aware of in which AI was involved in denying health insurance claims and that it is probably an uphill battle for the plaintiffs.
“In the last 25 years, the U.S. Supreme Court’s decisions have made getting a class action approved more difficult. If allowed to go forward as a class action, which Cigna is likely to vigorously oppose, then the pressure on Cigna to settle the case becomes enormous,” he said.
The allegations come after a recent deep dive by the nonprofit ProPublica uncovered similar claim denial issues. One physician who worked for Cigna told the nonprofit that he and other company doctors essentially rubber-stamped the denials in batches, which took “all of 10 seconds to do 50 at a time.”
In 2022, the American Medical Association and two state physician groups joined another class action against Cigna stemming from allegations that the insurer’s intermediary, Multiplan, intentionally underpaid medical claims. And in March, Cigna’s pharmacy benefit manager, Express Scripts, was accused of conspiring with other PBMs to drive up prescription drug prices for Ohio consumers, violating state antitrust laws.
Mr. Cohen said he expects Cigna to push back in court about the California class size, which the plaintiff’s attorneys hope will encompass all Cigna health plan members in the state.
“The injury is primarily to those whose claims were denied by AI, presumably a much smaller set of individuals and harder to identify,” said Mr. Cohen.
A version of this article first appeared on Medscape.com.
and forcing providers to bill patients in full.
In a complaint filed recently in California’s eastern district court, plaintiffs and Cigna health plan members Suzanne Kisting-Leung and Ayesha Smiley and their attorneys say that Cigna violates state insurance regulations by failing to conduct a “thorough, fair, and objective” review of their and other members’ claims.
The lawsuit says that, instead, Cigna relies on an algorithm, PxDx, to review and frequently deny medically necessary claims. According to court records, the system allows Cigna’s doctors to “instantly reject claims on medical grounds without ever opening patient files.” With use of the system, the average claims processing time is 1.2 seconds.
Cigna says it uses technology to verify coding on standard, low-cost procedures and to expedite physician reimbursement. In a statement to CBS News, the company called the lawsuit “highly questionable.”
The case highlights growing concerns about AI and its ability to replace humans for tasks and interactions in health care, business, and beyond. Public advocacy law firm Clarkson, which is representing the plaintiffs, has previously sued tech giants Google and ChatGPT creator OpenAI for harvesting Internet users’ personal and professional data to train their AI systems.
According to the complaint, Cigna denied the plaintiffs medically necessary tests, including blood work to screen for vitamin D deficiency and ultrasounds for patients suspected of having ovarian cancer. The plaintiffs’ attempts to appeal were unfruitful, and they were forced to pay out of pocket.
The plaintiff’s attorneys argue that the claims do not undergo more detailed reviews by physicians and employees, as mandated by California insurance laws, and that Cigna benefits by saving on labor costs.
Clarkson is demanding a jury trial and has asked the court to certify the Cigna case as a federal class action, potentially allowing the insurer’s other 2 million health plan members in California to join the lawsuit.
I. Glenn Cohen, JD, deputy dean and professor at Harvard Law School, Cambridge, Mass., said in an interview that this is the first lawsuit he’s aware of in which AI was involved in denying health insurance claims and that it is probably an uphill battle for the plaintiffs.
“In the last 25 years, the U.S. Supreme Court’s decisions have made getting a class action approved more difficult. If allowed to go forward as a class action, which Cigna is likely to vigorously oppose, then the pressure on Cigna to settle the case becomes enormous,” he said.
The allegations come after a recent deep dive by the nonprofit ProPublica uncovered similar claim denial issues. One physician who worked for Cigna told the nonprofit that he and other company doctors essentially rubber-stamped the denials in batches, which took “all of 10 seconds to do 50 at a time.”
In 2022, the American Medical Association and two state physician groups joined another class action against Cigna stemming from allegations that the insurer’s intermediary, Multiplan, intentionally underpaid medical claims. And in March, Cigna’s pharmacy benefit manager, Express Scripts, was accused of conspiring with other PBMs to drive up prescription drug prices for Ohio consumers, violating state antitrust laws.
Mr. Cohen said he expects Cigna to push back in court about the California class size, which the plaintiff’s attorneys hope will encompass all Cigna health plan members in the state.
“The injury is primarily to those whose claims were denied by AI, presumably a much smaller set of individuals and harder to identify,” said Mr. Cohen.
A version of this article first appeared on Medscape.com.
and forcing providers to bill patients in full.
In a complaint filed recently in California’s eastern district court, plaintiffs and Cigna health plan members Suzanne Kisting-Leung and Ayesha Smiley and their attorneys say that Cigna violates state insurance regulations by failing to conduct a “thorough, fair, and objective” review of their and other members’ claims.
The lawsuit says that, instead, Cigna relies on an algorithm, PxDx, to review and frequently deny medically necessary claims. According to court records, the system allows Cigna’s doctors to “instantly reject claims on medical grounds without ever opening patient files.” With use of the system, the average claims processing time is 1.2 seconds.
Cigna says it uses technology to verify coding on standard, low-cost procedures and to expedite physician reimbursement. In a statement to CBS News, the company called the lawsuit “highly questionable.”
The case highlights growing concerns about AI and its ability to replace humans for tasks and interactions in health care, business, and beyond. Public advocacy law firm Clarkson, which is representing the plaintiffs, has previously sued tech giants Google and ChatGPT creator OpenAI for harvesting Internet users’ personal and professional data to train their AI systems.
According to the complaint, Cigna denied the plaintiffs medically necessary tests, including blood work to screen for vitamin D deficiency and ultrasounds for patients suspected of having ovarian cancer. The plaintiffs’ attempts to appeal were unfruitful, and they were forced to pay out of pocket.
The plaintiff’s attorneys argue that the claims do not undergo more detailed reviews by physicians and employees, as mandated by California insurance laws, and that Cigna benefits by saving on labor costs.
Clarkson is demanding a jury trial and has asked the court to certify the Cigna case as a federal class action, potentially allowing the insurer’s other 2 million health plan members in California to join the lawsuit.
I. Glenn Cohen, JD, deputy dean and professor at Harvard Law School, Cambridge, Mass., said in an interview that this is the first lawsuit he’s aware of in which AI was involved in denying health insurance claims and that it is probably an uphill battle for the plaintiffs.
“In the last 25 years, the U.S. Supreme Court’s decisions have made getting a class action approved more difficult. If allowed to go forward as a class action, which Cigna is likely to vigorously oppose, then the pressure on Cigna to settle the case becomes enormous,” he said.
The allegations come after a recent deep dive by the nonprofit ProPublica uncovered similar claim denial issues. One physician who worked for Cigna told the nonprofit that he and other company doctors essentially rubber-stamped the denials in batches, which took “all of 10 seconds to do 50 at a time.”
In 2022, the American Medical Association and two state physician groups joined another class action against Cigna stemming from allegations that the insurer’s intermediary, Multiplan, intentionally underpaid medical claims. And in March, Cigna’s pharmacy benefit manager, Express Scripts, was accused of conspiring with other PBMs to drive up prescription drug prices for Ohio consumers, violating state antitrust laws.
Mr. Cohen said he expects Cigna to push back in court about the California class size, which the plaintiff’s attorneys hope will encompass all Cigna health plan members in the state.
“The injury is primarily to those whose claims were denied by AI, presumably a much smaller set of individuals and harder to identify,” said Mr. Cohen.
A version of this article first appeared on Medscape.com.
Black women weigh emerging risks of ‘creamy crack’ hair straighteners
Deanna Denham Hughes was stunned when she was diagnosed with ovarian cancer in 2022. She was only 32. She had no family history of cancer, and tests found no genetic link. Ms. Hughes wondered why she, an otherwise healthy Black mother of two, would develop a malignancy known as a “silent killer.”
After emergency surgery to remove the mass, along with her ovaries, uterus, fallopian tubes, and appendix, Ms. Hughes said, she saw an Instagram post in which a woman with uterine cancer linked her condition to chemical hair straighteners.
“I almost fell over,” she said from her home in Smyrna, Ga.
When Ms. Hughes was about 4, her mother began applying a chemical straightener, or relaxer, to her hair every 6-8 weeks. “It burned, and it smelled awful,” Ms. Hughes recalled. “But it was just part of our routine to ‘deal with my hair.’ ”
The routine continued until she went to college and met other Black women who wore their hair naturally. Soon, Ms. Hughes quit relaxers.
Social and economic pressures have long compelled Black girls and women to straighten their hair to conform to Eurocentric beauty standards. But chemical straighteners are stinky and costly and sometimes cause painful scalp burns. Mounting evidence now shows they could be a health hazard.
Relaxers can contain carcinogens, such as formaldehyde-releasing agents, phthalates, and other endocrine-disrupting compounds, according to National Institutes of Health studies. The compounds can mimic the body’s hormones and have been linked to breast, uterine, and ovarian cancers, studies show.
African American women’s often frequent and lifelong application of chemical relaxers to their hair and scalp might explain why hormone-related cancers kill disproportionately more Black than White women, say researchers and cancer doctors.
“What’s in these products is harmful,” said Tamarra James-Todd, PhD, an epidemiology professor at Harvard T.H. Chan School of Public Health, Boston, who has studied straightening products for the past 20 years.
She believes manufacturers, policymakers, and physicians should warn consumers that relaxers might cause cancer and other health problems.
But regulators have been slow to act, physicians have been reluctant to take up the cause, and racism continues to dictate fashion standards that make it tough for women to quit relaxers, products so addictive they’re known as “creamy crack.”
Michelle Obama straightened her hair when Barack Obama served as president because she believed Americans were “not ready” to see her in braids, the former first lady said after leaving the White House. The U.S. military still prohibited popular Black hairstyles such as dreadlocks and twists while the nation’s first Black president was in office.
California in 2019 became the first of nearly two dozen states to ban race-based hair discrimination. Last year, the U.S. House of Representatives passed similar legislation, known as the CROWN Act, for Creating a Respectful and Open World for Natural Hair. But the bill failed in the Senate.
The need for legislation underscores the challenges Black girls and women face at school and in the workplace.
“You have to pick your struggles,” said Atlanta-based surgical oncologist Ryland J. Gore, MD. She informs her breast cancer patients about the increased cancer risk from relaxers. Despite her knowledge, however, Dr. Gore continues to use chemical straighteners on her own hair, as she has since she was about 7 years old.
“Your hair tells a story,” she said.
In conversations with patients, Dr. Gore sometimes talks about how African American women once wove messages into their braids about the route to take on the Underground Railroad as they sought freedom from slavery.
“It’s just a deep discussion,” one that touches on culture, history, and research into current hairstyling practices, she said. “The data is out there. So patients should be warned, and then they can make a decision.”
The first hint of a connection between hair products and health issues surfaced in the 1990s. Doctors began seeing signs of sexual maturation in Black babies and young girls who developed breasts and pubic hair after using shampoo containing estrogen or placental extract. When the girls stopped using the shampoo, the hair and breast development receded, according to a study published in the journal Clinical Pediatrics in 1998.
Since then, Dr. James-Todd and other researchers have linked chemicals in hair products to a variety of health issues more prevalent among Black women – from early puberty to preterm birth, obesity, and diabetes.
In recent years, researchers have focused on a possible connection between ingredients in chemical relaxers and hormone-related cancers, like the one Ms. Hughes developed, which tend to be more aggressive and deadly in Black women.
A 2017 study found White women who used chemical relaxers were nearly twice as likely to develop breast cancer as those who did not use them. Because the vast majority of the Black study participants used relaxers, researchers could not effectively test the association in Black women, said lead author Adana Llanos, PhD, associate professor of epidemiology at Columbia University’s Mailman School of Public Health, New York.
Researchers did test it in 2020.
The so-called Sister Study, a landmark National Institute of Environmental Health Sciences investigation into the causes of breast cancer and related diseases, followed 50,000 U.S. women whose sisters had been diagnosed with breast cancer and who were cancer-free when they enrolled. Regardless of race, women who reported using relaxers in the prior year were 18% more likely to be diagnosed with breast cancer. Those who used relaxers at least every 5-8 weeks had a 31% higher breast cancer risk.
Nearly 75% of the Black sisters used relaxers in the prior year, compared with 3% of the non-Hispanic White sisters. Three-quarters of Black women self-reported using the straighteners as adolescents, and frequent use of chemical straighteners during adolescence raised the risk of premenopausal breast cancer, a 2021 NIH-funded study in the International Journal of Cancer found.
Another 2021 analysis of the Sister Study data showed sisters who self-reported that they frequently used relaxers or pressing products doubled their ovarian cancer risk. In 2022, another study found frequent use more than doubled uterine cancer risk.
After researchers discovered the link with uterine cancer, some called for policy changes and other measures to reduce exposure to chemical relaxers.
“It is time to intervene,” Dr. Llanos and her colleagues wrote in a Journal of the National Cancer Institute editorial accompanying the uterine cancer analysis. While acknowledging the need for more research, they issued a “call for action.”
No one can say that using permanent hair straighteners will give you cancer, Dr. Llanos said in an interview. “That’s not how cancer works,” she said, noting that some smokers never develop lung cancer, despite tobacco use being a known risk factor.
The body of research linking hair straighteners and cancer is more limited, said Dr. Llanos, who quit using chemical relaxers 15 years ago. But, she asked rhetorically, “Do we need to do the research for 50 more years to know that chemical relaxers are harmful?”
Charlotte R. Gamble, MD, a gynecological oncologist whose Washington, D.C., practice includes Black women with uterine and ovarian cancer, said she and her colleagues see the uterine cancer study findings as worthy of further exploration – but not yet worthy of discussion with patients.
“The jury’s out for me personally,” she said. “There’s so much more data that’s needed.”
Meanwhile, Dr. James-Todd and other researchers believe they have built a solid body of evidence.
“There are enough things we do know to begin taking action, developing interventions, providing useful information to clinicians and patients and the general public,” said Traci N. Bethea, PhD, assistant professor in the Office of Minority Health and Health Disparities Research at Georgetown University.
Responsibility for regulating personal-care products, including chemical hair straighteners and hair dyes – which also have been linked to hormone-related cancers – lies with the Food and Drug Administration. But the FDA does not subject personal-care products to the same approval process it uses for food and drugs. The FDA restricts only 11 categories of chemicals used in cosmetics, while concerns about health effects have prompted the European Union to restrict the use of at least 2,400 substances.
In March, Reps. Ayanna Pressley (D-Mass.) and Shontel Brown (D-Ohio) asked the FDA to investigate the potential health threat posed by chemical relaxers. An FDA representative said the agency would look into it.
Natural hairstyles are enjoying a resurgence among Black girls and women, but many continue to rely on the creamy crack, said Dede Teteh, DrPH, assistant professor of public health at Chapman University, Irvine, Calif.
She had her first straightening perm at 8 and has struggled to withdraw from relaxers as an adult, said Dr. Teteh, who now wears locs. Not long ago, she considered chemically straightening her hair for an academic job interview because she didn’t want her hair to “be a hindrance” when she appeared before White professors.
Dr. Teteh led “The Cost of Beauty,” a hair-health research project published in 2017. She and her team interviewed 91 Black women in Southern California. Some became “combative” at the idea of quitting relaxers and claimed “everything can cause cancer.”
Their reactions speak to the challenges Black women face in America, Dr. Teteh said.
“It’s not that people do not want to hear the information related to their health,” she said. “But they want people to share the information in a way that it’s really empathetic to the plight of being Black here in the United States.”
KFF Health News is a national newsroom that produces in-depth journalism about health issues and is one of the core operating programs at KFF – the independent source for health policy research, polling, and journalism.
Deanna Denham Hughes was stunned when she was diagnosed with ovarian cancer in 2022. She was only 32. She had no family history of cancer, and tests found no genetic link. Ms. Hughes wondered why she, an otherwise healthy Black mother of two, would develop a malignancy known as a “silent killer.”
After emergency surgery to remove the mass, along with her ovaries, uterus, fallopian tubes, and appendix, Ms. Hughes said, she saw an Instagram post in which a woman with uterine cancer linked her condition to chemical hair straighteners.
“I almost fell over,” she said from her home in Smyrna, Ga.
When Ms. Hughes was about 4, her mother began applying a chemical straightener, or relaxer, to her hair every 6-8 weeks. “It burned, and it smelled awful,” Ms. Hughes recalled. “But it was just part of our routine to ‘deal with my hair.’ ”
The routine continued until she went to college and met other Black women who wore their hair naturally. Soon, Ms. Hughes quit relaxers.
Social and economic pressures have long compelled Black girls and women to straighten their hair to conform to Eurocentric beauty standards. But chemical straighteners are stinky and costly and sometimes cause painful scalp burns. Mounting evidence now shows they could be a health hazard.
Relaxers can contain carcinogens, such as formaldehyde-releasing agents, phthalates, and other endocrine-disrupting compounds, according to National Institutes of Health studies. The compounds can mimic the body’s hormones and have been linked to breast, uterine, and ovarian cancers, studies show.
African American women’s often frequent and lifelong application of chemical relaxers to their hair and scalp might explain why hormone-related cancers kill disproportionately more Black than White women, say researchers and cancer doctors.
“What’s in these products is harmful,” said Tamarra James-Todd, PhD, an epidemiology professor at Harvard T.H. Chan School of Public Health, Boston, who has studied straightening products for the past 20 years.
She believes manufacturers, policymakers, and physicians should warn consumers that relaxers might cause cancer and other health problems.
But regulators have been slow to act, physicians have been reluctant to take up the cause, and racism continues to dictate fashion standards that make it tough for women to quit relaxers, products so addictive they’re known as “creamy crack.”
Michelle Obama straightened her hair when Barack Obama served as president because she believed Americans were “not ready” to see her in braids, the former first lady said after leaving the White House. The U.S. military still prohibited popular Black hairstyles such as dreadlocks and twists while the nation’s first Black president was in office.
California in 2019 became the first of nearly two dozen states to ban race-based hair discrimination. Last year, the U.S. House of Representatives passed similar legislation, known as the CROWN Act, for Creating a Respectful and Open World for Natural Hair. But the bill failed in the Senate.
The need for legislation underscores the challenges Black girls and women face at school and in the workplace.
“You have to pick your struggles,” said Atlanta-based surgical oncologist Ryland J. Gore, MD. She informs her breast cancer patients about the increased cancer risk from relaxers. Despite her knowledge, however, Dr. Gore continues to use chemical straighteners on her own hair, as she has since she was about 7 years old.
“Your hair tells a story,” she said.
In conversations with patients, Dr. Gore sometimes talks about how African American women once wove messages into their braids about the route to take on the Underground Railroad as they sought freedom from slavery.
“It’s just a deep discussion,” one that touches on culture, history, and research into current hairstyling practices, she said. “The data is out there. So patients should be warned, and then they can make a decision.”
The first hint of a connection between hair products and health issues surfaced in the 1990s. Doctors began seeing signs of sexual maturation in Black babies and young girls who developed breasts and pubic hair after using shampoo containing estrogen or placental extract. When the girls stopped using the shampoo, the hair and breast development receded, according to a study published in the journal Clinical Pediatrics in 1998.
Since then, Dr. James-Todd and other researchers have linked chemicals in hair products to a variety of health issues more prevalent among Black women – from early puberty to preterm birth, obesity, and diabetes.
In recent years, researchers have focused on a possible connection between ingredients in chemical relaxers and hormone-related cancers, like the one Ms. Hughes developed, which tend to be more aggressive and deadly in Black women.
A 2017 study found White women who used chemical relaxers were nearly twice as likely to develop breast cancer as those who did not use them. Because the vast majority of the Black study participants used relaxers, researchers could not effectively test the association in Black women, said lead author Adana Llanos, PhD, associate professor of epidemiology at Columbia University’s Mailman School of Public Health, New York.
Researchers did test it in 2020.
The so-called Sister Study, a landmark National Institute of Environmental Health Sciences investigation into the causes of breast cancer and related diseases, followed 50,000 U.S. women whose sisters had been diagnosed with breast cancer and who were cancer-free when they enrolled. Regardless of race, women who reported using relaxers in the prior year were 18% more likely to be diagnosed with breast cancer. Those who used relaxers at least every 5-8 weeks had a 31% higher breast cancer risk.
Nearly 75% of the Black sisters used relaxers in the prior year, compared with 3% of the non-Hispanic White sisters. Three-quarters of Black women self-reported using the straighteners as adolescents, and frequent use of chemical straighteners during adolescence raised the risk of premenopausal breast cancer, a 2021 NIH-funded study in the International Journal of Cancer found.
Another 2021 analysis of the Sister Study data showed sisters who self-reported that they frequently used relaxers or pressing products doubled their ovarian cancer risk. In 2022, another study found frequent use more than doubled uterine cancer risk.
After researchers discovered the link with uterine cancer, some called for policy changes and other measures to reduce exposure to chemical relaxers.
“It is time to intervene,” Dr. Llanos and her colleagues wrote in a Journal of the National Cancer Institute editorial accompanying the uterine cancer analysis. While acknowledging the need for more research, they issued a “call for action.”
No one can say that using permanent hair straighteners will give you cancer, Dr. Llanos said in an interview. “That’s not how cancer works,” she said, noting that some smokers never develop lung cancer, despite tobacco use being a known risk factor.
The body of research linking hair straighteners and cancer is more limited, said Dr. Llanos, who quit using chemical relaxers 15 years ago. But, she asked rhetorically, “Do we need to do the research for 50 more years to know that chemical relaxers are harmful?”
Charlotte R. Gamble, MD, a gynecological oncologist whose Washington, D.C., practice includes Black women with uterine and ovarian cancer, said she and her colleagues see the uterine cancer study findings as worthy of further exploration – but not yet worthy of discussion with patients.
“The jury’s out for me personally,” she said. “There’s so much more data that’s needed.”
Meanwhile, Dr. James-Todd and other researchers believe they have built a solid body of evidence.
“There are enough things we do know to begin taking action, developing interventions, providing useful information to clinicians and patients and the general public,” said Traci N. Bethea, PhD, assistant professor in the Office of Minority Health and Health Disparities Research at Georgetown University.
Responsibility for regulating personal-care products, including chemical hair straighteners and hair dyes – which also have been linked to hormone-related cancers – lies with the Food and Drug Administration. But the FDA does not subject personal-care products to the same approval process it uses for food and drugs. The FDA restricts only 11 categories of chemicals used in cosmetics, while concerns about health effects have prompted the European Union to restrict the use of at least 2,400 substances.
In March, Reps. Ayanna Pressley (D-Mass.) and Shontel Brown (D-Ohio) asked the FDA to investigate the potential health threat posed by chemical relaxers. An FDA representative said the agency would look into it.
Natural hairstyles are enjoying a resurgence among Black girls and women, but many continue to rely on the creamy crack, said Dede Teteh, DrPH, assistant professor of public health at Chapman University, Irvine, Calif.
She had her first straightening perm at 8 and has struggled to withdraw from relaxers as an adult, said Dr. Teteh, who now wears locs. Not long ago, she considered chemically straightening her hair for an academic job interview because she didn’t want her hair to “be a hindrance” when she appeared before White professors.
Dr. Teteh led “The Cost of Beauty,” a hair-health research project published in 2017. She and her team interviewed 91 Black women in Southern California. Some became “combative” at the idea of quitting relaxers and claimed “everything can cause cancer.”
Their reactions speak to the challenges Black women face in America, Dr. Teteh said.
“It’s not that people do not want to hear the information related to their health,” she said. “But they want people to share the information in a way that it’s really empathetic to the plight of being Black here in the United States.”
KFF Health News is a national newsroom that produces in-depth journalism about health issues and is one of the core operating programs at KFF – the independent source for health policy research, polling, and journalism.
Deanna Denham Hughes was stunned when she was diagnosed with ovarian cancer in 2022. She was only 32. She had no family history of cancer, and tests found no genetic link. Ms. Hughes wondered why she, an otherwise healthy Black mother of two, would develop a malignancy known as a “silent killer.”
After emergency surgery to remove the mass, along with her ovaries, uterus, fallopian tubes, and appendix, Ms. Hughes said, she saw an Instagram post in which a woman with uterine cancer linked her condition to chemical hair straighteners.
“I almost fell over,” she said from her home in Smyrna, Ga.
When Ms. Hughes was about 4, her mother began applying a chemical straightener, or relaxer, to her hair every 6-8 weeks. “It burned, and it smelled awful,” Ms. Hughes recalled. “But it was just part of our routine to ‘deal with my hair.’ ”
The routine continued until she went to college and met other Black women who wore their hair naturally. Soon, Ms. Hughes quit relaxers.
Social and economic pressures have long compelled Black girls and women to straighten their hair to conform to Eurocentric beauty standards. But chemical straighteners are stinky and costly and sometimes cause painful scalp burns. Mounting evidence now shows they could be a health hazard.
Relaxers can contain carcinogens, such as formaldehyde-releasing agents, phthalates, and other endocrine-disrupting compounds, according to National Institutes of Health studies. The compounds can mimic the body’s hormones and have been linked to breast, uterine, and ovarian cancers, studies show.
African American women’s often frequent and lifelong application of chemical relaxers to their hair and scalp might explain why hormone-related cancers kill disproportionately more Black than White women, say researchers and cancer doctors.
“What’s in these products is harmful,” said Tamarra James-Todd, PhD, an epidemiology professor at Harvard T.H. Chan School of Public Health, Boston, who has studied straightening products for the past 20 years.
She believes manufacturers, policymakers, and physicians should warn consumers that relaxers might cause cancer and other health problems.
But regulators have been slow to act, physicians have been reluctant to take up the cause, and racism continues to dictate fashion standards that make it tough for women to quit relaxers, products so addictive they’re known as “creamy crack.”
Michelle Obama straightened her hair when Barack Obama served as president because she believed Americans were “not ready” to see her in braids, the former first lady said after leaving the White House. The U.S. military still prohibited popular Black hairstyles such as dreadlocks and twists while the nation’s first Black president was in office.
California in 2019 became the first of nearly two dozen states to ban race-based hair discrimination. Last year, the U.S. House of Representatives passed similar legislation, known as the CROWN Act, for Creating a Respectful and Open World for Natural Hair. But the bill failed in the Senate.
The need for legislation underscores the challenges Black girls and women face at school and in the workplace.
“You have to pick your struggles,” said Atlanta-based surgical oncologist Ryland J. Gore, MD. She informs her breast cancer patients about the increased cancer risk from relaxers. Despite her knowledge, however, Dr. Gore continues to use chemical straighteners on her own hair, as she has since she was about 7 years old.
“Your hair tells a story,” she said.
In conversations with patients, Dr. Gore sometimes talks about how African American women once wove messages into their braids about the route to take on the Underground Railroad as they sought freedom from slavery.
“It’s just a deep discussion,” one that touches on culture, history, and research into current hairstyling practices, she said. “The data is out there. So patients should be warned, and then they can make a decision.”
The first hint of a connection between hair products and health issues surfaced in the 1990s. Doctors began seeing signs of sexual maturation in Black babies and young girls who developed breasts and pubic hair after using shampoo containing estrogen or placental extract. When the girls stopped using the shampoo, the hair and breast development receded, according to a study published in the journal Clinical Pediatrics in 1998.
Since then, Dr. James-Todd and other researchers have linked chemicals in hair products to a variety of health issues more prevalent among Black women – from early puberty to preterm birth, obesity, and diabetes.
In recent years, researchers have focused on a possible connection between ingredients in chemical relaxers and hormone-related cancers, like the one Ms. Hughes developed, which tend to be more aggressive and deadly in Black women.
A 2017 study found White women who used chemical relaxers were nearly twice as likely to develop breast cancer as those who did not use them. Because the vast majority of the Black study participants used relaxers, researchers could not effectively test the association in Black women, said lead author Adana Llanos, PhD, associate professor of epidemiology at Columbia University’s Mailman School of Public Health, New York.
Researchers did test it in 2020.
The so-called Sister Study, a landmark National Institute of Environmental Health Sciences investigation into the causes of breast cancer and related diseases, followed 50,000 U.S. women whose sisters had been diagnosed with breast cancer and who were cancer-free when they enrolled. Regardless of race, women who reported using relaxers in the prior year were 18% more likely to be diagnosed with breast cancer. Those who used relaxers at least every 5-8 weeks had a 31% higher breast cancer risk.
Nearly 75% of the Black sisters used relaxers in the prior year, compared with 3% of the non-Hispanic White sisters. Three-quarters of Black women self-reported using the straighteners as adolescents, and frequent use of chemical straighteners during adolescence raised the risk of premenopausal breast cancer, a 2021 NIH-funded study in the International Journal of Cancer found.
Another 2021 analysis of the Sister Study data showed sisters who self-reported that they frequently used relaxers or pressing products doubled their ovarian cancer risk. In 2022, another study found frequent use more than doubled uterine cancer risk.
After researchers discovered the link with uterine cancer, some called for policy changes and other measures to reduce exposure to chemical relaxers.
“It is time to intervene,” Dr. Llanos and her colleagues wrote in a Journal of the National Cancer Institute editorial accompanying the uterine cancer analysis. While acknowledging the need for more research, they issued a “call for action.”
No one can say that using permanent hair straighteners will give you cancer, Dr. Llanos said in an interview. “That’s not how cancer works,” she said, noting that some smokers never develop lung cancer, despite tobacco use being a known risk factor.
The body of research linking hair straighteners and cancer is more limited, said Dr. Llanos, who quit using chemical relaxers 15 years ago. But, she asked rhetorically, “Do we need to do the research for 50 more years to know that chemical relaxers are harmful?”
Charlotte R. Gamble, MD, a gynecological oncologist whose Washington, D.C., practice includes Black women with uterine and ovarian cancer, said she and her colleagues see the uterine cancer study findings as worthy of further exploration – but not yet worthy of discussion with patients.
“The jury’s out for me personally,” she said. “There’s so much more data that’s needed.”
Meanwhile, Dr. James-Todd and other researchers believe they have built a solid body of evidence.
“There are enough things we do know to begin taking action, developing interventions, providing useful information to clinicians and patients and the general public,” said Traci N. Bethea, PhD, assistant professor in the Office of Minority Health and Health Disparities Research at Georgetown University.
Responsibility for regulating personal-care products, including chemical hair straighteners and hair dyes – which also have been linked to hormone-related cancers – lies with the Food and Drug Administration. But the FDA does not subject personal-care products to the same approval process it uses for food and drugs. The FDA restricts only 11 categories of chemicals used in cosmetics, while concerns about health effects have prompted the European Union to restrict the use of at least 2,400 substances.
In March, Reps. Ayanna Pressley (D-Mass.) and Shontel Brown (D-Ohio) asked the FDA to investigate the potential health threat posed by chemical relaxers. An FDA representative said the agency would look into it.
Natural hairstyles are enjoying a resurgence among Black girls and women, but many continue to rely on the creamy crack, said Dede Teteh, DrPH, assistant professor of public health at Chapman University, Irvine, Calif.
She had her first straightening perm at 8 and has struggled to withdraw from relaxers as an adult, said Dr. Teteh, who now wears locs. Not long ago, she considered chemically straightening her hair for an academic job interview because she didn’t want her hair to “be a hindrance” when she appeared before White professors.
Dr. Teteh led “The Cost of Beauty,” a hair-health research project published in 2017. She and her team interviewed 91 Black women in Southern California. Some became “combative” at the idea of quitting relaxers and claimed “everything can cause cancer.”
Their reactions speak to the challenges Black women face in America, Dr. Teteh said.
“It’s not that people do not want to hear the information related to their health,” she said. “But they want people to share the information in a way that it’s really empathetic to the plight of being Black here in the United States.”
KFF Health News is a national newsroom that produces in-depth journalism about health issues and is one of the core operating programs at KFF – the independent source for health policy research, polling, and journalism.
Many users of skin-lightening product unaware of risks
, a recent cross-sectional survey suggests.
Skin lightening – which uses chemicals to lighten dark areas of skin or to generally lighten skin tone – poses a health risk from potentially unsafe formulations, the authors write in the International Journal of Women’s Dermatology.
Skin lightening is “influenced by colorism, the system of inequality that affords opportunities and privileges to lighter-skinned individuals across racial/ethnic groups,” they add. “Women, in particular, are vulnerable as media and popular culture propagate beauty standards that lighter skin can elevate physical appearance and social acceptance.”
“It is important to recognize that the primary motivator for skin lightening is most often dermatological disease but that, less frequently, it can be colorism,” senior study author Roopal V. Kundu, MD, professor of dermatology and founding director of the Northwestern Center for Ethnic Skin and Hair at Northwestern University, Chicago, said in an email interview.
Skin lightening is a growing, multibillion-dollar, largely unregulated, global industry. Rates have been estimated at 27% in South Africa, 40% in China and South Korea, 77% in Nigeria, but U.S. rates are unknown.
To investigate skin-lightening habits and the role colorism plays in skin-lightening practices in the United States, Dr. Kundu and her colleagues sent an online survey to 578 adults with darker skin who participated in ResearchMatch, a national health registry supported by the National Institutes of Health that connects volunteers with research studies they choose to take part in.
Of the 455 people who completed the 19-item anonymous questionnaire, 238 (52.3%) identified as Black or African American, 83 (18.2%) as Asian, 84 (18.5%) as multiracial, 31 (6.8%) as Hispanic, 14 (3.1%) as American Indian or Alaska Native, and 5 (1.1%) as other. Overall, 364 (80.0%) were women.
The survey asked about demographics, colorism attitudes, skin tone satisfaction, and skin-lightening product use. To assess colorism attitudes, the researchers asked respondents to rate six colorism statements on a Likert scale of 1 (strongly disagree) to 5 (strongly agree). The statements included “Lighter skin tone increases one’s self-esteem,” and “Lighter skin tone increases one’s chance of having a romantic relationship or getting married.” The researchers also asked them to rate their skin satisfaction levels on a Likert scale from 1 (very unsatisfied) to 5 (very satisfied).
Used mostly to treat skin conditions
Despite a lack of medical input, about three-quarters of people who used skin-lightening products reported using them for medical conditions, and around one-quarter used them for general lightening, the researchers report.
Of all respondents, 97 (21.3%) reported using skin-lightening agents. Of them, 71 (73.2%) used them to treat a skin condition such as acne, melasma, or postinflammatory hyperpigmentation, and 26 (26.8% of skin-lightening product users; 5.7% of all respondents) used them for generalized skin lightening.
The 97 users mostly obtained skin-lightening products from chain pharmacy and grocery stores, and also from community beauty stores, abroad, online, and medical providers, while two made them at home.
Skin-lightening product use did not differ with age, gender, race or ethnicity, education level, or immigration status.
Only 22 (22.7%) of the product users consulted a medical provider before using the products, and only 14 (14.4%) received skin-lightening products from medical providers.
In addition, 44 respondents (45.4%) could not identify the active ingredient in their skin-lightening products, but 34 (35.1%) reported using hydroquinone-based products. Other reported active ingredients included ascorbic acid, glycolic acid, salicylic acid, niacinamide, steroids, and mercury.
The face (86 people or 88.7%) and neck (37 or 38.1%) were the most common application sites.
Skin-lightening users were more likely to report that lighter skin was more beautiful and that it increased self-esteem and romantic prospects (P < .001 for all).
Elma Baron, MD, professor of dermatology at Case Western Reserve University, Cleveland, advised doctors to remind patients to consult a dermatologist before they use skin-lightening agents. “A dermatologist can evaluate whether there is a true indication for skin-lightening agents and explain the benefits, risks, and limitations of common skin-lightening formulations.
“When dealing with hyperpigmentation, clinicians should remember that ultraviolet light is a potent stimulus for melanogenesis,” added Dr. Baron by email. She was not involved in the study. “Wearing hats and other sun-protective clothing, using sunscreen, and avoiding sunlight during peak hours must always be emphasized.”
Amy J. McMichael, MD, professor of dermatology at Wake Forest University, Winston-Salem, N.C., often sees patients who try products based on persuasive advertising, not scientific benefit, she said by email.
“The findings are important, because many primary care providers and dermatologists do not realize that patients will use skin-lightening agents simply to provide a glow and in an attempt to attain complexion blending,” added Dr. McMichael, also not involved in the study.
She encouraged doctors to understand what motivates their patients to use skin-lightening agents, so they can effectively communicate what works and what does not work for their condition, as well as inform them about potential risks.
Strengths of the study, Dr. McMichael said, are the number of people surveyed and the inclusion of colorism data not typically gathered in studies of skin-lightening product use. Limitations include whether the reported conditions were what people actually had, and that, with over 50% of respondents being Black, the results may not be generalizable to other groups.
“Colorism is complex,” Dr. Kundu noted. “Dermatologists need to recognize how colorism impacts their patients, so they can provide them with culturally mindful care and deter them from using potentially harmful products.”
Illegal products may still be available
Dr. McMichael would like to know how many of these patients used products containing > 4%-strength hydroquinone, because they “can be dangerous, and patients don’t understand how these higher-strength medications can damage the skin.”
“Following the Coronavirus Aid, Relief, and Economic Security [CARES] Act of 2020, over-the-counter hydroquinone sales were prohibited in the U.S.,” the authors write. In 2022, the Food and Drug Administration issued warning letters to 12 companies that sold products containing unsafe concentrations of hydroquinone, because of concerns about swelling, rashes, and discoloration. Hydroquinone has also been linked with skin cancer.
“However, this study demonstrates that consumers in the U.S. may still have access to hydroquinone formulations,” the authors caution.
At its Skin Facts! Resources website, the FDA warns about potentially harmful over-the-counter skin-lightening products containing hydroquinone or mercury and recommends using only prescribed products. The information site was created by the FDA Office of Minority Health and Health Equity.
The study authors, Dr. Baron, and Dr. McMichael report no relevant financial relationships. The study did not receive external funding. All experts commented by email.
, a recent cross-sectional survey suggests.
Skin lightening – which uses chemicals to lighten dark areas of skin or to generally lighten skin tone – poses a health risk from potentially unsafe formulations, the authors write in the International Journal of Women’s Dermatology.
Skin lightening is “influenced by colorism, the system of inequality that affords opportunities and privileges to lighter-skinned individuals across racial/ethnic groups,” they add. “Women, in particular, are vulnerable as media and popular culture propagate beauty standards that lighter skin can elevate physical appearance and social acceptance.”
“It is important to recognize that the primary motivator for skin lightening is most often dermatological disease but that, less frequently, it can be colorism,” senior study author Roopal V. Kundu, MD, professor of dermatology and founding director of the Northwestern Center for Ethnic Skin and Hair at Northwestern University, Chicago, said in an email interview.
Skin lightening is a growing, multibillion-dollar, largely unregulated, global industry. Rates have been estimated at 27% in South Africa, 40% in China and South Korea, 77% in Nigeria, but U.S. rates are unknown.
To investigate skin-lightening habits and the role colorism plays in skin-lightening practices in the United States, Dr. Kundu and her colleagues sent an online survey to 578 adults with darker skin who participated in ResearchMatch, a national health registry supported by the National Institutes of Health that connects volunteers with research studies they choose to take part in.
Of the 455 people who completed the 19-item anonymous questionnaire, 238 (52.3%) identified as Black or African American, 83 (18.2%) as Asian, 84 (18.5%) as multiracial, 31 (6.8%) as Hispanic, 14 (3.1%) as American Indian or Alaska Native, and 5 (1.1%) as other. Overall, 364 (80.0%) were women.
The survey asked about demographics, colorism attitudes, skin tone satisfaction, and skin-lightening product use. To assess colorism attitudes, the researchers asked respondents to rate six colorism statements on a Likert scale of 1 (strongly disagree) to 5 (strongly agree). The statements included “Lighter skin tone increases one’s self-esteem,” and “Lighter skin tone increases one’s chance of having a romantic relationship or getting married.” The researchers also asked them to rate their skin satisfaction levels on a Likert scale from 1 (very unsatisfied) to 5 (very satisfied).
Used mostly to treat skin conditions
Despite a lack of medical input, about three-quarters of people who used skin-lightening products reported using them for medical conditions, and around one-quarter used them for general lightening, the researchers report.
Of all respondents, 97 (21.3%) reported using skin-lightening agents. Of them, 71 (73.2%) used them to treat a skin condition such as acne, melasma, or postinflammatory hyperpigmentation, and 26 (26.8% of skin-lightening product users; 5.7% of all respondents) used them for generalized skin lightening.
The 97 users mostly obtained skin-lightening products from chain pharmacy and grocery stores, and also from community beauty stores, abroad, online, and medical providers, while two made them at home.
Skin-lightening product use did not differ with age, gender, race or ethnicity, education level, or immigration status.
Only 22 (22.7%) of the product users consulted a medical provider before using the products, and only 14 (14.4%) received skin-lightening products from medical providers.
In addition, 44 respondents (45.4%) could not identify the active ingredient in their skin-lightening products, but 34 (35.1%) reported using hydroquinone-based products. Other reported active ingredients included ascorbic acid, glycolic acid, salicylic acid, niacinamide, steroids, and mercury.
The face (86 people or 88.7%) and neck (37 or 38.1%) were the most common application sites.
Skin-lightening users were more likely to report that lighter skin was more beautiful and that it increased self-esteem and romantic prospects (P < .001 for all).
Elma Baron, MD, professor of dermatology at Case Western Reserve University, Cleveland, advised doctors to remind patients to consult a dermatologist before they use skin-lightening agents. “A dermatologist can evaluate whether there is a true indication for skin-lightening agents and explain the benefits, risks, and limitations of common skin-lightening formulations.
“When dealing with hyperpigmentation, clinicians should remember that ultraviolet light is a potent stimulus for melanogenesis,” added Dr. Baron by email. She was not involved in the study. “Wearing hats and other sun-protective clothing, using sunscreen, and avoiding sunlight during peak hours must always be emphasized.”
Amy J. McMichael, MD, professor of dermatology at Wake Forest University, Winston-Salem, N.C., often sees patients who try products based on persuasive advertising, not scientific benefit, she said by email.
“The findings are important, because many primary care providers and dermatologists do not realize that patients will use skin-lightening agents simply to provide a glow and in an attempt to attain complexion blending,” added Dr. McMichael, also not involved in the study.
She encouraged doctors to understand what motivates their patients to use skin-lightening agents, so they can effectively communicate what works and what does not work for their condition, as well as inform them about potential risks.
Strengths of the study, Dr. McMichael said, are the number of people surveyed and the inclusion of colorism data not typically gathered in studies of skin-lightening product use. Limitations include whether the reported conditions were what people actually had, and that, with over 50% of respondents being Black, the results may not be generalizable to other groups.
“Colorism is complex,” Dr. Kundu noted. “Dermatologists need to recognize how colorism impacts their patients, so they can provide them with culturally mindful care and deter them from using potentially harmful products.”
Illegal products may still be available
Dr. McMichael would like to know how many of these patients used products containing > 4%-strength hydroquinone, because they “can be dangerous, and patients don’t understand how these higher-strength medications can damage the skin.”
“Following the Coronavirus Aid, Relief, and Economic Security [CARES] Act of 2020, over-the-counter hydroquinone sales were prohibited in the U.S.,” the authors write. In 2022, the Food and Drug Administration issued warning letters to 12 companies that sold products containing unsafe concentrations of hydroquinone, because of concerns about swelling, rashes, and discoloration. Hydroquinone has also been linked with skin cancer.
“However, this study demonstrates that consumers in the U.S. may still have access to hydroquinone formulations,” the authors caution.
At its Skin Facts! Resources website, the FDA warns about potentially harmful over-the-counter skin-lightening products containing hydroquinone or mercury and recommends using only prescribed products. The information site was created by the FDA Office of Minority Health and Health Equity.
The study authors, Dr. Baron, and Dr. McMichael report no relevant financial relationships. The study did not receive external funding. All experts commented by email.
, a recent cross-sectional survey suggests.
Skin lightening – which uses chemicals to lighten dark areas of skin or to generally lighten skin tone – poses a health risk from potentially unsafe formulations, the authors write in the International Journal of Women’s Dermatology.
Skin lightening is “influenced by colorism, the system of inequality that affords opportunities and privileges to lighter-skinned individuals across racial/ethnic groups,” they add. “Women, in particular, are vulnerable as media and popular culture propagate beauty standards that lighter skin can elevate physical appearance and social acceptance.”
“It is important to recognize that the primary motivator for skin lightening is most often dermatological disease but that, less frequently, it can be colorism,” senior study author Roopal V. Kundu, MD, professor of dermatology and founding director of the Northwestern Center for Ethnic Skin and Hair at Northwestern University, Chicago, said in an email interview.
Skin lightening is a growing, multibillion-dollar, largely unregulated, global industry. Rates have been estimated at 27% in South Africa, 40% in China and South Korea, 77% in Nigeria, but U.S. rates are unknown.
To investigate skin-lightening habits and the role colorism plays in skin-lightening practices in the United States, Dr. Kundu and her colleagues sent an online survey to 578 adults with darker skin who participated in ResearchMatch, a national health registry supported by the National Institutes of Health that connects volunteers with research studies they choose to take part in.
Of the 455 people who completed the 19-item anonymous questionnaire, 238 (52.3%) identified as Black or African American, 83 (18.2%) as Asian, 84 (18.5%) as multiracial, 31 (6.8%) as Hispanic, 14 (3.1%) as American Indian or Alaska Native, and 5 (1.1%) as other. Overall, 364 (80.0%) were women.
The survey asked about demographics, colorism attitudes, skin tone satisfaction, and skin-lightening product use. To assess colorism attitudes, the researchers asked respondents to rate six colorism statements on a Likert scale of 1 (strongly disagree) to 5 (strongly agree). The statements included “Lighter skin tone increases one’s self-esteem,” and “Lighter skin tone increases one’s chance of having a romantic relationship or getting married.” The researchers also asked them to rate their skin satisfaction levels on a Likert scale from 1 (very unsatisfied) to 5 (very satisfied).
Used mostly to treat skin conditions
Despite a lack of medical input, about three-quarters of people who used skin-lightening products reported using them for medical conditions, and around one-quarter used them for general lightening, the researchers report.
Of all respondents, 97 (21.3%) reported using skin-lightening agents. Of them, 71 (73.2%) used them to treat a skin condition such as acne, melasma, or postinflammatory hyperpigmentation, and 26 (26.8% of skin-lightening product users; 5.7% of all respondents) used them for generalized skin lightening.
The 97 users mostly obtained skin-lightening products from chain pharmacy and grocery stores, and also from community beauty stores, abroad, online, and medical providers, while two made them at home.
Skin-lightening product use did not differ with age, gender, race or ethnicity, education level, or immigration status.
Only 22 (22.7%) of the product users consulted a medical provider before using the products, and only 14 (14.4%) received skin-lightening products from medical providers.
In addition, 44 respondents (45.4%) could not identify the active ingredient in their skin-lightening products, but 34 (35.1%) reported using hydroquinone-based products. Other reported active ingredients included ascorbic acid, glycolic acid, salicylic acid, niacinamide, steroids, and mercury.
The face (86 people or 88.7%) and neck (37 or 38.1%) were the most common application sites.
Skin-lightening users were more likely to report that lighter skin was more beautiful and that it increased self-esteem and romantic prospects (P < .001 for all).
Elma Baron, MD, professor of dermatology at Case Western Reserve University, Cleveland, advised doctors to remind patients to consult a dermatologist before they use skin-lightening agents. “A dermatologist can evaluate whether there is a true indication for skin-lightening agents and explain the benefits, risks, and limitations of common skin-lightening formulations.
“When dealing with hyperpigmentation, clinicians should remember that ultraviolet light is a potent stimulus for melanogenesis,” added Dr. Baron by email. She was not involved in the study. “Wearing hats and other sun-protective clothing, using sunscreen, and avoiding sunlight during peak hours must always be emphasized.”
Amy J. McMichael, MD, professor of dermatology at Wake Forest University, Winston-Salem, N.C., often sees patients who try products based on persuasive advertising, not scientific benefit, she said by email.
“The findings are important, because many primary care providers and dermatologists do not realize that patients will use skin-lightening agents simply to provide a glow and in an attempt to attain complexion blending,” added Dr. McMichael, also not involved in the study.
She encouraged doctors to understand what motivates their patients to use skin-lightening agents, so they can effectively communicate what works and what does not work for their condition, as well as inform them about potential risks.
Strengths of the study, Dr. McMichael said, are the number of people surveyed and the inclusion of colorism data not typically gathered in studies of skin-lightening product use. Limitations include whether the reported conditions were what people actually had, and that, with over 50% of respondents being Black, the results may not be generalizable to other groups.
“Colorism is complex,” Dr. Kundu noted. “Dermatologists need to recognize how colorism impacts their patients, so they can provide them with culturally mindful care and deter them from using potentially harmful products.”
Illegal products may still be available
Dr. McMichael would like to know how many of these patients used products containing > 4%-strength hydroquinone, because they “can be dangerous, and patients don’t understand how these higher-strength medications can damage the skin.”
“Following the Coronavirus Aid, Relief, and Economic Security [CARES] Act of 2020, over-the-counter hydroquinone sales were prohibited in the U.S.,” the authors write. In 2022, the Food and Drug Administration issued warning letters to 12 companies that sold products containing unsafe concentrations of hydroquinone, because of concerns about swelling, rashes, and discoloration. Hydroquinone has also been linked with skin cancer.
“However, this study demonstrates that consumers in the U.S. may still have access to hydroquinone formulations,” the authors caution.
At its Skin Facts! Resources website, the FDA warns about potentially harmful over-the-counter skin-lightening products containing hydroquinone or mercury and recommends using only prescribed products. The information site was created by the FDA Office of Minority Health and Health Equity.
The study authors, Dr. Baron, and Dr. McMichael report no relevant financial relationships. The study did not receive external funding. All experts commented by email.
FROM THE INTERNATIONAL JOURNAL OF WOMEN’S DERMATOLOGY
Autoantibodies could help predict cancer risk in scleroderma
TOPLINE:
METHODOLOGY:
- Included patients from the Johns Hopkins Scleroderma Center Research Registry and the University of Pittsburgh Scleroderma Center, Pittsburgh.
- A total of 676 patients with scleroderma and a history of cancer were compared with 687 control patients with scleroderma but without a history of cancer.
- Serum tested via line blot and enzyme-linked immunosorbent assay for an array of scleroderma autoantibodies.
- Examined association between autoantibodies and overall cancer risk.
TAKEAWAYS:
- Anti-POLR3 and monospecific anti-Ro52 were associated with significantly increased overall cancer risk.
- Anti-centromere and anti-U1RNP were associated with a decreased cancer risk.
- These associations remained when looking specifically at cancer-associated scleroderma.
- Patients positive for anti-Ro52 in combination with either anti-U1RNP or anti-Th/To had a decreased risk of cancer, compared with those who had anti-Ro52 alone.
IN PRACTICE:
This study is too preliminary to have practice application.
SOURCE:
Ji Soo Kim, PhD, of John Hopkins University, Baltimore, was the first author of the study, published in Arthritis & Rheumatology on July 24, 2023. Fellow Johns Hopkins researchers Livia Casciola-Rosen, PhD, and Ami A. Shah, MD, were joint senior authors.
DISCLOSURES:
The study was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, the Donald B. and Dorothy L. Stabler Foundation, the Jerome L. Greene Foundation, the Chresanthe Staurulakis Memorial Discovery Fund, the Martha McCrory Professorship, and the Johns Hopkins inHealth initiative. The authors disclosed the following patents or patent applications: Autoimmune Antigens and Cancer, Materials and Methods for Assessing Cancer Risk and Treating Cancer.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Included patients from the Johns Hopkins Scleroderma Center Research Registry and the University of Pittsburgh Scleroderma Center, Pittsburgh.
- A total of 676 patients with scleroderma and a history of cancer were compared with 687 control patients with scleroderma but without a history of cancer.
- Serum tested via line blot and enzyme-linked immunosorbent assay for an array of scleroderma autoantibodies.
- Examined association between autoantibodies and overall cancer risk.
TAKEAWAYS:
- Anti-POLR3 and monospecific anti-Ro52 were associated with significantly increased overall cancer risk.
- Anti-centromere and anti-U1RNP were associated with a decreased cancer risk.
- These associations remained when looking specifically at cancer-associated scleroderma.
- Patients positive for anti-Ro52 in combination with either anti-U1RNP or anti-Th/To had a decreased risk of cancer, compared with those who had anti-Ro52 alone.
IN PRACTICE:
This study is too preliminary to have practice application.
SOURCE:
Ji Soo Kim, PhD, of John Hopkins University, Baltimore, was the first author of the study, published in Arthritis & Rheumatology on July 24, 2023. Fellow Johns Hopkins researchers Livia Casciola-Rosen, PhD, and Ami A. Shah, MD, were joint senior authors.
DISCLOSURES:
The study was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, the Donald B. and Dorothy L. Stabler Foundation, the Jerome L. Greene Foundation, the Chresanthe Staurulakis Memorial Discovery Fund, the Martha McCrory Professorship, and the Johns Hopkins inHealth initiative. The authors disclosed the following patents or patent applications: Autoimmune Antigens and Cancer, Materials and Methods for Assessing Cancer Risk and Treating Cancer.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Included patients from the Johns Hopkins Scleroderma Center Research Registry and the University of Pittsburgh Scleroderma Center, Pittsburgh.
- A total of 676 patients with scleroderma and a history of cancer were compared with 687 control patients with scleroderma but without a history of cancer.
- Serum tested via line blot and enzyme-linked immunosorbent assay for an array of scleroderma autoantibodies.
- Examined association between autoantibodies and overall cancer risk.
TAKEAWAYS:
- Anti-POLR3 and monospecific anti-Ro52 were associated with significantly increased overall cancer risk.
- Anti-centromere and anti-U1RNP were associated with a decreased cancer risk.
- These associations remained when looking specifically at cancer-associated scleroderma.
- Patients positive for anti-Ro52 in combination with either anti-U1RNP or anti-Th/To had a decreased risk of cancer, compared with those who had anti-Ro52 alone.
IN PRACTICE:
This study is too preliminary to have practice application.
SOURCE:
Ji Soo Kim, PhD, of John Hopkins University, Baltimore, was the first author of the study, published in Arthritis & Rheumatology on July 24, 2023. Fellow Johns Hopkins researchers Livia Casciola-Rosen, PhD, and Ami A. Shah, MD, were joint senior authors.
DISCLOSURES:
The study was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, the Donald B. and Dorothy L. Stabler Foundation, the Jerome L. Greene Foundation, the Chresanthe Staurulakis Memorial Discovery Fund, the Martha McCrory Professorship, and the Johns Hopkins inHealth initiative. The authors disclosed the following patents or patent applications: Autoimmune Antigens and Cancer, Materials and Methods for Assessing Cancer Risk and Treating Cancer.
A version of this article appeared on Medscape.com.
FROM ARTHRITIS & RHEUMATOLOGY
Medical students are skipping class lectures: Does it matter?
New technologies, including online lectures and guided-lesson websites, along with alternative teaching methods, such as the flipped classroom model, in which med students complete before-class assignments and participate in group projects, are helping to train future physicians for their medical careers.
So though students may not be attending in-person lectures like they did in the past, proponents of online learning say the education students receive and the subsequent care they deliver remains the same.
The Association of American Medical Colleges’ most recent annual survey of 2nd-year medical students found that 25% “almost never” attended their in-person lectures in 2022. The figure has steadily improved since 2020 but mirrors what AAMC recorded in 2017.
“The pandemic may have exacerbated the trend, but it’s a long-standing issue,” said Katherine McOwen, senior director of educational and student affairs at AAMC. She said in an interview that she’s witnessed the pattern for 24 years in her work with medical schools.
“I know it sounds alarming that students aren’t attending lectures. But that doesn’t mean they’re not learning,” said Ahmed Ahmed, MD, MPP, MSc, a recent graduate of Harvard Medical School and now a resident at Brigham and Women’s Hospital, Boston.
Today’s generation of medical students grew up in the age of technology. They are comfortable in front of the screen, so it makes sense for them to learn certain aspects of medical sciences and public health in the same way, Dr. Ahmed told this news organization.
Dr. Ahmed said that at Harvard he participated in one or two case-based classes per week that followed a flipped classroom model, which allows students to study topics on their own before discussing in a lecture format as a group. “We had to come up with a diagnostic plan and walk through the case slide by slide,” he said. “It got us to think like a clinician.”
The flipped classroom allows students to study at their own pace using their preferred learning style, leading to more collaboration in the classroom and between students, according to a 2022 article on the “new standard in medical education” published in Trends in Anaesthesia & Critical Care.
Students use online education tools to complete pre-class assignments such as watching short videos, listening to podcasts, or reading journal articles. In-class time can then be used to cement and create connections through discussions, interactive exercises, group learning, and case studies, the article stated.
Benefits of the flipped classroom include student satisfaction, learner motivation, and faculty interest in learning new teaching methods, according to the article: “Students are performing at least as well as those who attended traditional lectures, while some studies in select health care settings show increased retention in flipped classroom settings.”
Another study on the flipped classroom, published in 2018 in BMC Medical Education found that the teaching method was superior to traditional classrooms for health professions education. Researchers focused specifically on flipped classrooms that provided prerecorded videos to students.
Molly Cooke, MD, director of education for global health sciences at the University of California, San Francisco, School of Medicine, said that the school no longer requires attendance at lectures. “Personally, my position is that medical students are very busy people and make, by and large, rational decisions about how to spend their time. As learning and retention from 50-minute lectures has been shown for decades to be poor, I think it’s perfectly reasonable to watch lectures on their own time.”
Dr. Ahmed agrees. “By our standards, the old model is archaic. It’s passive, and instead we should be encouraging lifelong, self-directed learning.”
To that end, Dr. Ahmed and his fellow students also relied heavily during medical school on secondary educational sources such as Boards and Beyond and Sketchy. “There’s an entire community of medical school students across the country using them,” Dr. Ahmed explained. “You can learn what you need in a tenth of the time of lectures.”
Today lectures only provide a portion of the information delivered to students, Dr. McGowen said. “They also learn in small groups, in problem-solving sessions, and in clinical experiences, all of which make up the meat of their education.”
The purpose of medical school is to prepare students for residency, she added. “Medical school education is very different from other types of education. Students are examined in a variety of ways before they move on to residency and ultimately, practice.”
For example, every student must pass the three-part United States Medical Licensing Examination. Students complete the first two parts in medical school and the third part during residency. “The tests represent a combination of everything students have learned, from lectures, clinical time, and in self-directed learning,” Dr. McGowen said.
Post pandemic, the tools and styles of learning in medical education have changed, and they are likely to continue to evolve along with students and technology, according to the 2022 article on the flipped classroom. “The future of medical education will continue to move in ways that embrace digital technology, as this is what digital native learners are increasingly expecting for their health care education,” states the article.
A version of this article first appeared on Medscape.com.
New technologies, including online lectures and guided-lesson websites, along with alternative teaching methods, such as the flipped classroom model, in which med students complete before-class assignments and participate in group projects, are helping to train future physicians for their medical careers.
So though students may not be attending in-person lectures like they did in the past, proponents of online learning say the education students receive and the subsequent care they deliver remains the same.
The Association of American Medical Colleges’ most recent annual survey of 2nd-year medical students found that 25% “almost never” attended their in-person lectures in 2022. The figure has steadily improved since 2020 but mirrors what AAMC recorded in 2017.
“The pandemic may have exacerbated the trend, but it’s a long-standing issue,” said Katherine McOwen, senior director of educational and student affairs at AAMC. She said in an interview that she’s witnessed the pattern for 24 years in her work with medical schools.
“I know it sounds alarming that students aren’t attending lectures. But that doesn’t mean they’re not learning,” said Ahmed Ahmed, MD, MPP, MSc, a recent graduate of Harvard Medical School and now a resident at Brigham and Women’s Hospital, Boston.
Today’s generation of medical students grew up in the age of technology. They are comfortable in front of the screen, so it makes sense for them to learn certain aspects of medical sciences and public health in the same way, Dr. Ahmed told this news organization.
Dr. Ahmed said that at Harvard he participated in one or two case-based classes per week that followed a flipped classroom model, which allows students to study topics on their own before discussing in a lecture format as a group. “We had to come up with a diagnostic plan and walk through the case slide by slide,” he said. “It got us to think like a clinician.”
The flipped classroom allows students to study at their own pace using their preferred learning style, leading to more collaboration in the classroom and between students, according to a 2022 article on the “new standard in medical education” published in Trends in Anaesthesia & Critical Care.
Students use online education tools to complete pre-class assignments such as watching short videos, listening to podcasts, or reading journal articles. In-class time can then be used to cement and create connections through discussions, interactive exercises, group learning, and case studies, the article stated.
Benefits of the flipped classroom include student satisfaction, learner motivation, and faculty interest in learning new teaching methods, according to the article: “Students are performing at least as well as those who attended traditional lectures, while some studies in select health care settings show increased retention in flipped classroom settings.”
Another study on the flipped classroom, published in 2018 in BMC Medical Education found that the teaching method was superior to traditional classrooms for health professions education. Researchers focused specifically on flipped classrooms that provided prerecorded videos to students.
Molly Cooke, MD, director of education for global health sciences at the University of California, San Francisco, School of Medicine, said that the school no longer requires attendance at lectures. “Personally, my position is that medical students are very busy people and make, by and large, rational decisions about how to spend their time. As learning and retention from 50-minute lectures has been shown for decades to be poor, I think it’s perfectly reasonable to watch lectures on their own time.”
Dr. Ahmed agrees. “By our standards, the old model is archaic. It’s passive, and instead we should be encouraging lifelong, self-directed learning.”
To that end, Dr. Ahmed and his fellow students also relied heavily during medical school on secondary educational sources such as Boards and Beyond and Sketchy. “There’s an entire community of medical school students across the country using them,” Dr. Ahmed explained. “You can learn what you need in a tenth of the time of lectures.”
Today lectures only provide a portion of the information delivered to students, Dr. McGowen said. “They also learn in small groups, in problem-solving sessions, and in clinical experiences, all of which make up the meat of their education.”
The purpose of medical school is to prepare students for residency, she added. “Medical school education is very different from other types of education. Students are examined in a variety of ways before they move on to residency and ultimately, practice.”
For example, every student must pass the three-part United States Medical Licensing Examination. Students complete the first two parts in medical school and the third part during residency. “The tests represent a combination of everything students have learned, from lectures, clinical time, and in self-directed learning,” Dr. McGowen said.
Post pandemic, the tools and styles of learning in medical education have changed, and they are likely to continue to evolve along with students and technology, according to the 2022 article on the flipped classroom. “The future of medical education will continue to move in ways that embrace digital technology, as this is what digital native learners are increasingly expecting for their health care education,” states the article.
A version of this article first appeared on Medscape.com.
New technologies, including online lectures and guided-lesson websites, along with alternative teaching methods, such as the flipped classroom model, in which med students complete before-class assignments and participate in group projects, are helping to train future physicians for their medical careers.
So though students may not be attending in-person lectures like they did in the past, proponents of online learning say the education students receive and the subsequent care they deliver remains the same.
The Association of American Medical Colleges’ most recent annual survey of 2nd-year medical students found that 25% “almost never” attended their in-person lectures in 2022. The figure has steadily improved since 2020 but mirrors what AAMC recorded in 2017.
“The pandemic may have exacerbated the trend, but it’s a long-standing issue,” said Katherine McOwen, senior director of educational and student affairs at AAMC. She said in an interview that she’s witnessed the pattern for 24 years in her work with medical schools.
“I know it sounds alarming that students aren’t attending lectures. But that doesn’t mean they’re not learning,” said Ahmed Ahmed, MD, MPP, MSc, a recent graduate of Harvard Medical School and now a resident at Brigham and Women’s Hospital, Boston.
Today’s generation of medical students grew up in the age of technology. They are comfortable in front of the screen, so it makes sense for them to learn certain aspects of medical sciences and public health in the same way, Dr. Ahmed told this news organization.
Dr. Ahmed said that at Harvard he participated in one or two case-based classes per week that followed a flipped classroom model, which allows students to study topics on their own before discussing in a lecture format as a group. “We had to come up with a diagnostic plan and walk through the case slide by slide,” he said. “It got us to think like a clinician.”
The flipped classroom allows students to study at their own pace using their preferred learning style, leading to more collaboration in the classroom and between students, according to a 2022 article on the “new standard in medical education” published in Trends in Anaesthesia & Critical Care.
Students use online education tools to complete pre-class assignments such as watching short videos, listening to podcasts, or reading journal articles. In-class time can then be used to cement and create connections through discussions, interactive exercises, group learning, and case studies, the article stated.
Benefits of the flipped classroom include student satisfaction, learner motivation, and faculty interest in learning new teaching methods, according to the article: “Students are performing at least as well as those who attended traditional lectures, while some studies in select health care settings show increased retention in flipped classroom settings.”
Another study on the flipped classroom, published in 2018 in BMC Medical Education found that the teaching method was superior to traditional classrooms for health professions education. Researchers focused specifically on flipped classrooms that provided prerecorded videos to students.
Molly Cooke, MD, director of education for global health sciences at the University of California, San Francisco, School of Medicine, said that the school no longer requires attendance at lectures. “Personally, my position is that medical students are very busy people and make, by and large, rational decisions about how to spend their time. As learning and retention from 50-minute lectures has been shown for decades to be poor, I think it’s perfectly reasonable to watch lectures on their own time.”
Dr. Ahmed agrees. “By our standards, the old model is archaic. It’s passive, and instead we should be encouraging lifelong, self-directed learning.”
To that end, Dr. Ahmed and his fellow students also relied heavily during medical school on secondary educational sources such as Boards and Beyond and Sketchy. “There’s an entire community of medical school students across the country using them,” Dr. Ahmed explained. “You can learn what you need in a tenth of the time of lectures.”
Today lectures only provide a portion of the information delivered to students, Dr. McGowen said. “They also learn in small groups, in problem-solving sessions, and in clinical experiences, all of which make up the meat of their education.”
The purpose of medical school is to prepare students for residency, she added. “Medical school education is very different from other types of education. Students are examined in a variety of ways before they move on to residency and ultimately, practice.”
For example, every student must pass the three-part United States Medical Licensing Examination. Students complete the first two parts in medical school and the third part during residency. “The tests represent a combination of everything students have learned, from lectures, clinical time, and in self-directed learning,” Dr. McGowen said.
Post pandemic, the tools and styles of learning in medical education have changed, and they are likely to continue to evolve along with students and technology, according to the 2022 article on the flipped classroom. “The future of medical education will continue to move in ways that embrace digital technology, as this is what digital native learners are increasingly expecting for their health care education,” states the article.
A version of this article first appeared on Medscape.com.
Evaluation of Micrographic Surgery and Dermatologic Oncology Fellowship Program Websites
To the Editor:
Micrographic surgery and dermatologic oncology (MSDO) is a highly competitive subspecialty fellowship in dermatology. Prospective applicants often depend on the Internet to obtain pertinent information about fellowship programs to navigate the application process. An up-to-date and comprehensive fellowship website has the potential to be advantageous for both applicants and programs—applicants can more readily identify programs that align with their goals and values, and programs can effectively attract compatible applicants. These advantages are increasingly relevant with the virtual application process that has become essential considering the COVID-19 pandemic. At the height of the COVID-19 pandemic in 2020, we sought to evaluate the comprehensiveness of the content of Accreditation Council for Graduate Medical Education (ACGME) MSDO fellowship program websites to identify possible areas for improvement.
We obtained a list of all ACGME MSDO fellowships from the ACGME website (https://www.acgme.org/) and verified it against the list of MSDO programs in FREIDA, the American Medical Association residency and fellowship database (https://freida.ama-assn.org/). All programs without a website were excluded from further analysis. All data collection from currently accessible fellowship websites and evaluation occurred in April 2020.
The remaining MSDO fellowship program websites were evaluated using 25 criteria distributed among 5 domains: education/research, clinical training, program information, application process, and incentives. These criteria were determined based on earlier studies that similarly evaluated the website content of fellowship programs with inclusion of information that was considered valuable in the appraisal of fellowship programs.1,2 Criteria were further refined by direct consideration of relevance and importance to MSDO fellowship applicants (eg, inclusion of case volume, exclusion of call schedule).
Each criterion was independently assessed by 2 investigators (J.Y.C. and S.J.E.S.). A third investigator (J.R.P.) then independently evaluated those 2 assessments for agreement. Where disagreement was discovered, the third evaluator (J.R.P.) provided a final appraisal. Cohen’s kappa (κ) was conducted to evaluate for concordance between the 2 primary website evaluators. We found there to be substantial agreement between the reviewers within the education/research (κ [SD]=0.772 [0.077]), clinical training (κ [SD]=0.740 [0.051]), application process (κ [SD]=0.726 [0.103]), and incentives domains (κ [SD]=0.730 [0.110]). There was moderate agreement (κ [SD]=0.603 [0.128]) between the reviewers within the program information domain.
We identified 77 active MSDO fellowship programs. Sixty of those 77 programs (77.9%) had a dedicated fellowship website that was readily accessible. Most programs that had a dedicated fellowship website had a core or affiliated residency program (49/60 [81.7%]).
Websites that we evaluated fulfilled a mean (SD) of 9.37 (4.17) of the 25 identified criteria. Only 13 of 60 (21.7%) websites fulfilled more than 50% of evaluated criteria.
There was no statistical difference in the number of criteria fulfilled based on whether the fellowship program had a core or affiliated residency program.
Upon reviewing website accessibility directly from FREIDA, only 5 of 60 programs (8.3%) provided applicants with a link directly to their fellowship page (Table). Most programs (41 [68.3%]) provided a link to the dermatology department website, not to the specific fellowship program page, thus requiring a multistep process to find the fellowship-specific page. The remaining programs had an inaccessible (4 [6.7%]) or absent (10 [16.7%]) link on FREIDA, though a fellowship website could be identified by an Internet search of the program name.
The domain most fulfilled was program information with an average of 51.1% of programs satisfying the criteria, whereas the incentives domain was least fulfilled with an average of only 20.8% of programs satisfying the criteria. Across the various criteria, websites more often included a description of the program (58 [96.6%]), mentioned accreditation (53 [88.3%]), and provided case descriptions (48 [80.0%]). They less often reported information regarding a fellow’s call responsibility (3 [5%]); evaluation criteria (5 [8.3%]); and rotation schedule or options (6 [10.0%]).
The highest number of criteria fulfilled by a single program was 19 (76%). The lowest number of criteria met was 2 (8%). These findings suggest a large variation in comprehensiveness across fellowship websites.
Our research suggests that many current MSDO fellowship programs have room to maximize the information provided to applicants through their websites, which is particularly relevant following the COVID-19 pandemic, as the value of providing comprehensive and transparent information through an online platform is greater than ever. Given the ongoing desire to limit travel, virtual methods for navigating the application process have been readily used, including online videoconferencing for interviews and virtual program visits. This scenario has placed applicants in a challenging situation—their ability to directly evaluate their compatibility with a given program has been limited.3
Earlier studies that analyzed rheumatology fellowship recruitment during the COVID-19 pandemic found that programs may have more difficulty highlighting the strengths of their institution (eg, clinical facilities, professional opportunities, educational environment).4 An updated and comprehensive fellowship website was recommended4 as a key part in facing these new challenges. On the other hand, given the large number of applicants each year for fellowship positions in any given program, we acknowledge the potential benefit programs may obtain from limiting electronic information that is readily accessible to all applicants, as doing so may encourage applicants to communicate directly with a program and allow programs to identify candidates who are more interested.
In light of the movement to a more virtual-friendly and technology-driven fellowship application process, we identified 25 content areas that fellowships may want to include on their websites so that potential applicants can be well informed about the program before submitting an application and scheduling an interview. Efforts to improve accessibility and maximize the content of these websites may help programs attract compatible candidates, improve transparency, and guide applicants throughout the application process.
- Lu F, Vijayasarathi A, Murray N, et al. Evaluation of pediatric radiology fellowship website content in USA and Canada. Curr Prob Diagn Radiol. 2021;50:151-155. doi:10.1067/j.cpradiol.2020.01.007
- Cantrell CK, Bergstresser SL, Schuh AC, et al. Accessibility and content of abdominal transplant fellowship program websites in the United States. J Surg Res. 2018;232:271-274. doi:10.1016/j.jss.2018.06.052
- Nesemeier BR, Lebo NL, Schmalbach CE, et al. Impact of the COVID-19 global pandemic on the otolaryngology fellowship application process. Otolaryngol Head Neck Surg. 2020;163:712-713. doi:10.1177/0194599820934370
- Kilian A, Dua AB, Bolster MB, et al. Rheumatology fellowship recruitment in 2020: benefits, challenges, and adaptations. Arthritis Care Res (Hoboken). 2021;73:459-461. doi:10.1002/acr.24445
To the Editor:
Micrographic surgery and dermatologic oncology (MSDO) is a highly competitive subspecialty fellowship in dermatology. Prospective applicants often depend on the Internet to obtain pertinent information about fellowship programs to navigate the application process. An up-to-date and comprehensive fellowship website has the potential to be advantageous for both applicants and programs—applicants can more readily identify programs that align with their goals and values, and programs can effectively attract compatible applicants. These advantages are increasingly relevant with the virtual application process that has become essential considering the COVID-19 pandemic. At the height of the COVID-19 pandemic in 2020, we sought to evaluate the comprehensiveness of the content of Accreditation Council for Graduate Medical Education (ACGME) MSDO fellowship program websites to identify possible areas for improvement.
We obtained a list of all ACGME MSDO fellowships from the ACGME website (https://www.acgme.org/) and verified it against the list of MSDO programs in FREIDA, the American Medical Association residency and fellowship database (https://freida.ama-assn.org/). All programs without a website were excluded from further analysis. All data collection from currently accessible fellowship websites and evaluation occurred in April 2020.
The remaining MSDO fellowship program websites were evaluated using 25 criteria distributed among 5 domains: education/research, clinical training, program information, application process, and incentives. These criteria were determined based on earlier studies that similarly evaluated the website content of fellowship programs with inclusion of information that was considered valuable in the appraisal of fellowship programs.1,2 Criteria were further refined by direct consideration of relevance and importance to MSDO fellowship applicants (eg, inclusion of case volume, exclusion of call schedule).
Each criterion was independently assessed by 2 investigators (J.Y.C. and S.J.E.S.). A third investigator (J.R.P.) then independently evaluated those 2 assessments for agreement. Where disagreement was discovered, the third evaluator (J.R.P.) provided a final appraisal. Cohen’s kappa (κ) was conducted to evaluate for concordance between the 2 primary website evaluators. We found there to be substantial agreement between the reviewers within the education/research (κ [SD]=0.772 [0.077]), clinical training (κ [SD]=0.740 [0.051]), application process (κ [SD]=0.726 [0.103]), and incentives domains (κ [SD]=0.730 [0.110]). There was moderate agreement (κ [SD]=0.603 [0.128]) between the reviewers within the program information domain.
We identified 77 active MSDO fellowship programs. Sixty of those 77 programs (77.9%) had a dedicated fellowship website that was readily accessible. Most programs that had a dedicated fellowship website had a core or affiliated residency program (49/60 [81.7%]).
Websites that we evaluated fulfilled a mean (SD) of 9.37 (4.17) of the 25 identified criteria. Only 13 of 60 (21.7%) websites fulfilled more than 50% of evaluated criteria.
There was no statistical difference in the number of criteria fulfilled based on whether the fellowship program had a core or affiliated residency program.
Upon reviewing website accessibility directly from FREIDA, only 5 of 60 programs (8.3%) provided applicants with a link directly to their fellowship page (Table). Most programs (41 [68.3%]) provided a link to the dermatology department website, not to the specific fellowship program page, thus requiring a multistep process to find the fellowship-specific page. The remaining programs had an inaccessible (4 [6.7%]) or absent (10 [16.7%]) link on FREIDA, though a fellowship website could be identified by an Internet search of the program name.

The domain most fulfilled was program information with an average of 51.1% of programs satisfying the criteria, whereas the incentives domain was least fulfilled with an average of only 20.8% of programs satisfying the criteria. Across the various criteria, websites more often included a description of the program (58 [96.6%]), mentioned accreditation (53 [88.3%]), and provided case descriptions (48 [80.0%]). They less often reported information regarding a fellow’s call responsibility (3 [5%]); evaluation criteria (5 [8.3%]); and rotation schedule or options (6 [10.0%]).
The highest number of criteria fulfilled by a single program was 19 (76%). The lowest number of criteria met was 2 (8%). These findings suggest a large variation in comprehensiveness across fellowship websites.
Our research suggests that many current MSDO fellowship programs have room to maximize the information provided to applicants through their websites, which is particularly relevant following the COVID-19 pandemic, as the value of providing comprehensive and transparent information through an online platform is greater than ever. Given the ongoing desire to limit travel, virtual methods for navigating the application process have been readily used, including online videoconferencing for interviews and virtual program visits. This scenario has placed applicants in a challenging situation—their ability to directly evaluate their compatibility with a given program has been limited.3
Earlier studies that analyzed rheumatology fellowship recruitment during the COVID-19 pandemic found that programs may have more difficulty highlighting the strengths of their institution (eg, clinical facilities, professional opportunities, educational environment).4 An updated and comprehensive fellowship website was recommended4 as a key part in facing these new challenges. On the other hand, given the large number of applicants each year for fellowship positions in any given program, we acknowledge the potential benefit programs may obtain from limiting electronic information that is readily accessible to all applicants, as doing so may encourage applicants to communicate directly with a program and allow programs to identify candidates who are more interested.
In light of the movement to a more virtual-friendly and technology-driven fellowship application process, we identified 25 content areas that fellowships may want to include on their websites so that potential applicants can be well informed about the program before submitting an application and scheduling an interview. Efforts to improve accessibility and maximize the content of these websites may help programs attract compatible candidates, improve transparency, and guide applicants throughout the application process.
To the Editor:
Micrographic surgery and dermatologic oncology (MSDO) is a highly competitive subspecialty fellowship in dermatology. Prospective applicants often depend on the Internet to obtain pertinent information about fellowship programs to navigate the application process. An up-to-date and comprehensive fellowship website has the potential to be advantageous for both applicants and programs—applicants can more readily identify programs that align with their goals and values, and programs can effectively attract compatible applicants. These advantages are increasingly relevant with the virtual application process that has become essential considering the COVID-19 pandemic. At the height of the COVID-19 pandemic in 2020, we sought to evaluate the comprehensiveness of the content of Accreditation Council for Graduate Medical Education (ACGME) MSDO fellowship program websites to identify possible areas for improvement.
We obtained a list of all ACGME MSDO fellowships from the ACGME website (https://www.acgme.org/) and verified it against the list of MSDO programs in FREIDA, the American Medical Association residency and fellowship database (https://freida.ama-assn.org/). All programs without a website were excluded from further analysis. All data collection from currently accessible fellowship websites and evaluation occurred in April 2020.
The remaining MSDO fellowship program websites were evaluated using 25 criteria distributed among 5 domains: education/research, clinical training, program information, application process, and incentives. These criteria were determined based on earlier studies that similarly evaluated the website content of fellowship programs with inclusion of information that was considered valuable in the appraisal of fellowship programs.1,2 Criteria were further refined by direct consideration of relevance and importance to MSDO fellowship applicants (eg, inclusion of case volume, exclusion of call schedule).
Each criterion was independently assessed by 2 investigators (J.Y.C. and S.J.E.S.). A third investigator (J.R.P.) then independently evaluated those 2 assessments for agreement. Where disagreement was discovered, the third evaluator (J.R.P.) provided a final appraisal. Cohen’s kappa (κ) was conducted to evaluate for concordance between the 2 primary website evaluators. We found there to be substantial agreement between the reviewers within the education/research (κ [SD]=0.772 [0.077]), clinical training (κ [SD]=0.740 [0.051]), application process (κ [SD]=0.726 [0.103]), and incentives domains (κ [SD]=0.730 [0.110]). There was moderate agreement (κ [SD]=0.603 [0.128]) between the reviewers within the program information domain.
We identified 77 active MSDO fellowship programs. Sixty of those 77 programs (77.9%) had a dedicated fellowship website that was readily accessible. Most programs that had a dedicated fellowship website had a core or affiliated residency program (49/60 [81.7%]).
Websites that we evaluated fulfilled a mean (SD) of 9.37 (4.17) of the 25 identified criteria. Only 13 of 60 (21.7%) websites fulfilled more than 50% of evaluated criteria.
There was no statistical difference in the number of criteria fulfilled based on whether the fellowship program had a core or affiliated residency program.
Upon reviewing website accessibility directly from FREIDA, only 5 of 60 programs (8.3%) provided applicants with a link directly to their fellowship page (Table). Most programs (41 [68.3%]) provided a link to the dermatology department website, not to the specific fellowship program page, thus requiring a multistep process to find the fellowship-specific page. The remaining programs had an inaccessible (4 [6.7%]) or absent (10 [16.7%]) link on FREIDA, though a fellowship website could be identified by an Internet search of the program name.

The domain most fulfilled was program information with an average of 51.1% of programs satisfying the criteria, whereas the incentives domain was least fulfilled with an average of only 20.8% of programs satisfying the criteria. Across the various criteria, websites more often included a description of the program (58 [96.6%]), mentioned accreditation (53 [88.3%]), and provided case descriptions (48 [80.0%]). They less often reported information regarding a fellow’s call responsibility (3 [5%]); evaluation criteria (5 [8.3%]); and rotation schedule or options (6 [10.0%]).
The highest number of criteria fulfilled by a single program was 19 (76%). The lowest number of criteria met was 2 (8%). These findings suggest a large variation in comprehensiveness across fellowship websites.
Our research suggests that many current MSDO fellowship programs have room to maximize the information provided to applicants through their websites, which is particularly relevant following the COVID-19 pandemic, as the value of providing comprehensive and transparent information through an online platform is greater than ever. Given the ongoing desire to limit travel, virtual methods for navigating the application process have been readily used, including online videoconferencing for interviews and virtual program visits. This scenario has placed applicants in a challenging situation—their ability to directly evaluate their compatibility with a given program has been limited.3
Earlier studies that analyzed rheumatology fellowship recruitment during the COVID-19 pandemic found that programs may have more difficulty highlighting the strengths of their institution (eg, clinical facilities, professional opportunities, educational environment).4 An updated and comprehensive fellowship website was recommended4 as a key part in facing these new challenges. On the other hand, given the large number of applicants each year for fellowship positions in any given program, we acknowledge the potential benefit programs may obtain from limiting electronic information that is readily accessible to all applicants, as doing so may encourage applicants to communicate directly with a program and allow programs to identify candidates who are more interested.
In light of the movement to a more virtual-friendly and technology-driven fellowship application process, we identified 25 content areas that fellowships may want to include on their websites so that potential applicants can be well informed about the program before submitting an application and scheduling an interview. Efforts to improve accessibility and maximize the content of these websites may help programs attract compatible candidates, improve transparency, and guide applicants throughout the application process.
- Lu F, Vijayasarathi A, Murray N, et al. Evaluation of pediatric radiology fellowship website content in USA and Canada. Curr Prob Diagn Radiol. 2021;50:151-155. doi:10.1067/j.cpradiol.2020.01.007
- Cantrell CK, Bergstresser SL, Schuh AC, et al. Accessibility and content of abdominal transplant fellowship program websites in the United States. J Surg Res. 2018;232:271-274. doi:10.1016/j.jss.2018.06.052
- Nesemeier BR, Lebo NL, Schmalbach CE, et al. Impact of the COVID-19 global pandemic on the otolaryngology fellowship application process. Otolaryngol Head Neck Surg. 2020;163:712-713. doi:10.1177/0194599820934370
- Kilian A, Dua AB, Bolster MB, et al. Rheumatology fellowship recruitment in 2020: benefits, challenges, and adaptations. Arthritis Care Res (Hoboken). 2021;73:459-461. doi:10.1002/acr.24445
- Lu F, Vijayasarathi A, Murray N, et al. Evaluation of pediatric radiology fellowship website content in USA and Canada. Curr Prob Diagn Radiol. 2021;50:151-155. doi:10.1067/j.cpradiol.2020.01.007
- Cantrell CK, Bergstresser SL, Schuh AC, et al. Accessibility and content of abdominal transplant fellowship program websites in the United States. J Surg Res. 2018;232:271-274. doi:10.1016/j.jss.2018.06.052
- Nesemeier BR, Lebo NL, Schmalbach CE, et al. Impact of the COVID-19 global pandemic on the otolaryngology fellowship application process. Otolaryngol Head Neck Surg. 2020;163:712-713. doi:10.1177/0194599820934370
- Kilian A, Dua AB, Bolster MB, et al. Rheumatology fellowship recruitment in 2020: benefits, challenges, and adaptations. Arthritis Care Res (Hoboken). 2021;73:459-461. doi:10.1002/acr.24445
Practice Points
- With the COVID-19 pandemic and the movement to a virtual fellowship application process, fellowship program websites that are comprehensive and accessible may help programs attract compatible candidates, improve transparency, and guide applicants through the application process.
- There is variation in the content of current micrographic surgery and dermatologic oncology fellowship program websites and areas upon which programs may seek to augment their website content to better reflect program strengths while attracting competitive candidates best suited for their program.
Economic Burden and Quality of Life of Patients With Moderate to Severe Atopic Dermatitis in a Tertiary Care Hospital in Helsinki, Finland: A Survey-Based Study
Atopic dermatitis (AD) is a common inflammatory skin disease that may severely decrease quality of life (QOL) and lead to psychiatric comorbidities.1-3 Prior studies have indicated that AD causes a substantial economic burden, and disease severity has been proportionally linked to medical costs.4,5 Results of a multicenter cost-of-illness study from Germany estimated that a relapse of AD costs approximately €123 (US $136). The authors calculated the average annual cost of AD per patient to be €1425 (US $1580), whereas it is €956 (US $1060) in moderate disease and €2068 (US $2293) in severe disease (direct and indirect medical costs included).6 An observational cohort study from the Netherlands found that total direct cost per patient-year (PPY) was €4401 (US $4879) for patients with controlled AD vs €6993 (US $7756) for patients with uncontrolled AD.7
In a retrospective survey-based study, it was estimated that the annual cost of AD in Canada was approximately CAD $1.4 billion. The cost per patient varied from CAD $282 to CAD $1242 depending on disease severity.8 In another retrospective cohort study from the Netherlands, the average direct medical cost per patient with AD seeing a general practitioner was US $71 during follow-up in primary care. If the patient needed specialist consultation, the cost increased to an average of US $186.9
We aimed to assess the direct and indirect medical costs in adult patients with moderate to severe AD who attended a tertiary health care center in Finland. In addition, we evaluated the impact of AD on QOL in this patient cohort.
Methods
Study Design—Patients with AD who were treated at the Department of Dermatology and Allergology, Helsinki University Hospital, Finland, between February 2018 and December 2019 were randomly selected to participate in our survey study. All participants provided written informed consent. In Finland, patients with mild AD generally are treated in primary health care centers, and only patients with moderate to severe AD are referred to specialists and tertiary care centers. Patients were excluded if they were younger than 18 years, had AD confined to the hands, or reported the presence of other concomitant skin diseases that were being treated with topical or systemic therapies. The protocol for the study was approved by the local ethics committee of the University of Helsinki.
Questionnaire and Analysis of Disease Severity—The survey included the medical history, signs of atopy, former treatment(s) for AD, skin infections, visits to dermatologists or general practitioners, questions on mental health and hospitalization, and absence from work due to AD in the last 12 months. Disease severity was evaluated using the patient-oriented Rajka & Langeland eczema severity score and Patient Oriented Eczema Measure (POEM).10,11 The impact on QOL was evaluated by the Dermatology Life Quality Index (DLQI).12
Medication Costs—The cost of prescription drugs was based on data from the Finnish national electronic prescription center. In Finland, all prescriptions are made electronically in the database. We analyzed all topical medications (eg, topical corticosteroids [TCSs], topical calcineurin inhibitors [TCIs], and emollients) and systemic medicaments (eg, antibiotics, antihistamines, cyclosporine, methotrexate, and corticosteroids) prescribed for the treatment of AD. In Finland, dupilumab was introduced for the treatment of severe AD in early 2019, and patients receiving dupilumab were excluded from the study. Over-the-counter medications were not included. The costs for laboratory testing were estimations based on the standard monitoring protocols of the Helsinki University Hospital. All costs were based on the Finnish price level standard for the year 2019.
Inpatient/Outpatient Visits and Sick Leave Due to AD—The number of inpatient and outpatient visits due to AD in the last 12 months was evaluated. Outpatient specialist consultations or nurse appointments at Helsinki University Hospital were verified from electronic patient records. In addition, inpatient treatment and phototherapy sessions were calculated from the database.
We assessed the number of sick leave days from work or educational activities during the last year. All costs of transportation for doctors’ appointments, laboratory monitoring, and phototherapy treatments were summed together to estimate the total transportation cost. Visits to nurse and inpatient visits were not included in the total transportation cost because patients often were hospitalized directly after consultation visits, and nurse appointments often were combined with inpatient and outpatient visits. To calculate the total transportation cost, we used a rate of €0.43 per kilometer measured from the patients’ home addresses, which was the official compensation rate of the Finnish Tax Administration for 2019.13
Statistical Analysis—Statistical analyses were performed using SPSS Statistics 25 (IBM). Descriptive analyses were used to describe baseline characteristics and to evaluate the mean costs of AD. The patients were divided into 2 groups according to POEM: (1) controlled AD (patients with clear skin or only mild AD; POEM score 0–7) and (2) uncontrolled AD (patients with moderate to very severe AD; POEM score 8–28). The Mann-Whitney U statistic was used to evaluate differences between the study groups.
Results
Patient Characteristics—One hundred sixty-seven patients answered the survey, of which 69 (41.3%) were males and 98 (58.7%) were females. There were 16 patients with controlled AD and 148 patients with uncontrolled AD. Three patients did not answer to POEM and were excluded. The baseline characteristics are presented in Table 1 and include self-reported symptoms related to atopy.
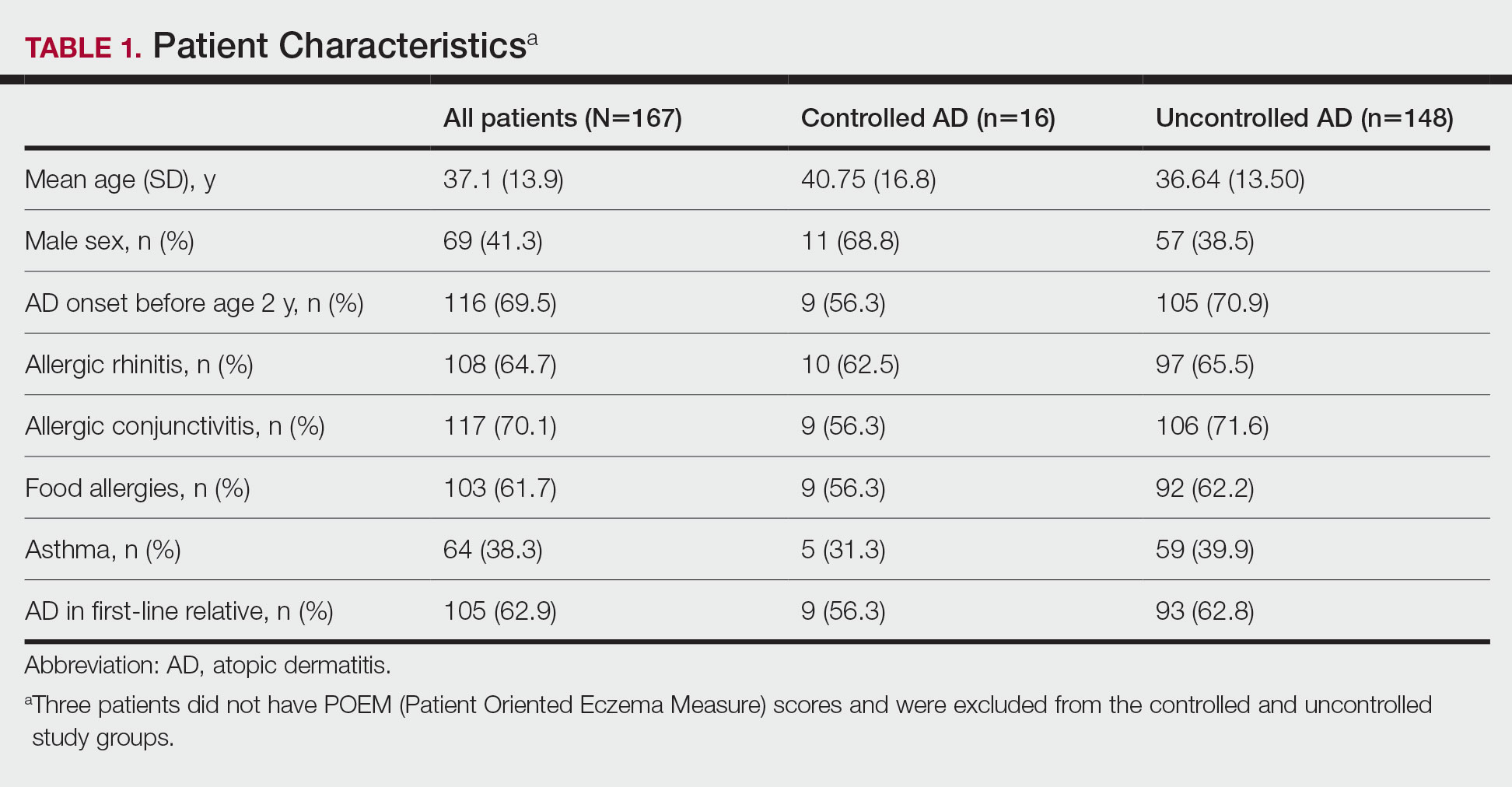
The most-used topical treatments were TCSs (n=155; 92.8%) and emollients (n=166; 99.4%). One hundred sixteen (69.5%) patients had used TCIs. The median amount of TCSs used was 300 g/y vs 30 g/y for TCIs (range, 0-5160 g/y) and 1200 g/y for emollients.
Fifteen (9.0%) patients had been hospitalized for AD in the last year. The mean (SD) length of hospitalization was 6.5 (2.8) days. Thirty-four (20.4%) patients received UVB phototherapy. Thirty-four (20.4%) patients were treated with at least 1 antibiotic course for secondary AD infection. Thirty-six (21.6%) patients needed at least 1 oral corticosteroid course for the treatment of an AD flare.
Fifteen (9.0%) patients reported a diagnosed psychiatric illness, and 17 (10.2%) patients were using prescription drugs for psychiatric illness. Forty-nine (29.3%) patients reported anxiety or depression often or very often, 54 (32.3%) patients reported sometimes, 33 (19.8%) patients reported rarely, and only 30 (18.0%) patients reported none.
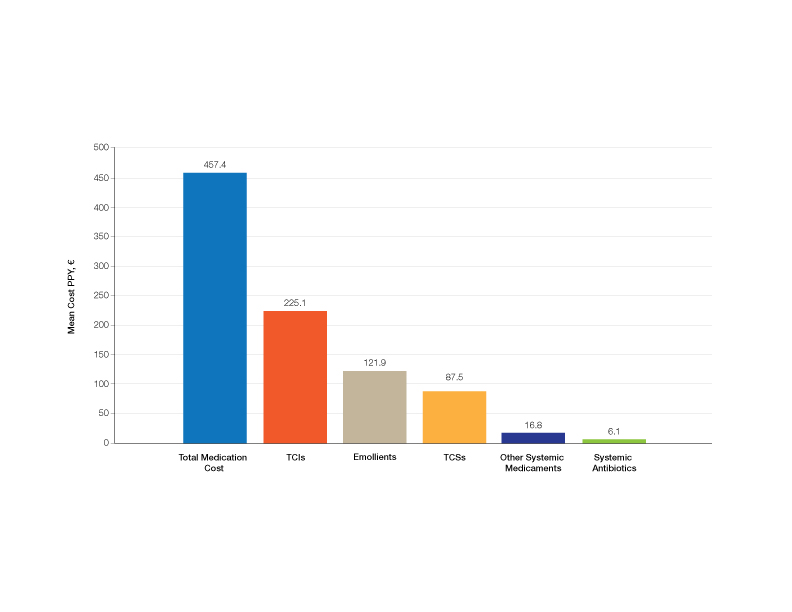
Medication Costs—Mean medication cost PPY was €457.40 (US $507.34)(Figure 1 and Table 2). On average, one patient spent €87.50 (US $97.05) for TCSs, €121.90 (US $135.21) for emollients, and €225.10 (US $249.68) for TCIs. The average cost PPY for antibiotics was €6.10 (US $6.77). Other systemic treatments, including (US $18.65). Seventeen patients (10.2%) were on methotrexate therapy for AD in the last year, and 1 patient also used cyclosporine. The costs for laboratory monitoring in these patients were included in the direct cost calculations. The mean cost PPY of laboratory monitoring in the whole study cohort was €6.60 (US $7.32). In patients with systemic immunosuppressive therapy, the mean cost PPY for laboratory monitoring was €65.00 (US $72.09). Five patients had been tested for contact dermatitis; the costs of patch tests or other diagnostic tests were not included.
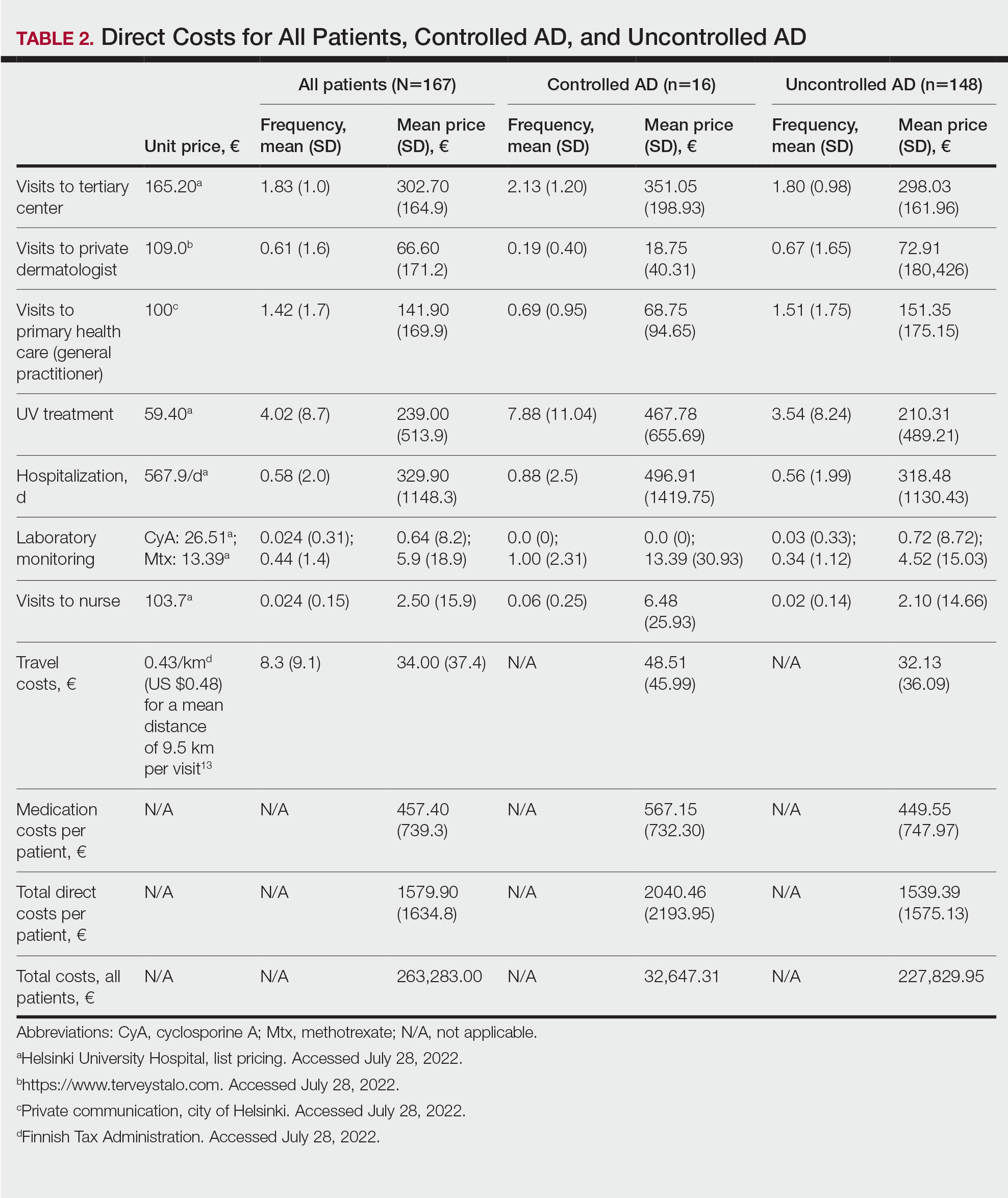
Visits to Health Care Providers—In the last year, patients had an average of 1.83 dermatologist consultations in the tertiary center (Table 2). In addition, the mean number of visits to private dermatologists was 0.61 and 1.42 visits to general practitioners. The mean cost of physician visits was €302.70 (US $335.75) in the tertiary center, €66.60 (US $73.87) in the private sector, and €141.90 (US $157.39) in primary health care. In total, the average cost of doctors’ appointments PPY was €506.30 (US $561.57). The mean estimated distance traveled per visit was 9.5 km.
The mean cost PPY of inpatient treatments was €329.90 (US $365.92) and €239.00 (US $265.09) for UV phototherapy. Only 4 patients had visited a nurse in the last year, with an average cost PPY of €2.50 (US $2.78).
In total, the cost PPY for health care provider visits was €1084.20, which included specialist consultations in a tertiary center and private sector, visits in primary health care, inpatient treatments, UV phototherapy sessions, nurse appointments in a tertiary center, and laboratory monitoring. The average transportation cost PPY was €34.00 (US $37.71). The mean number of visits to health care providers was 8.3 per year. Altogether, the direct cost PPY in the study cohort was €1580.60 (US $1752.39)(Table 2 and Figure 2).

Comparison of Medical Costs in Controlled vs Uncontrolled AD—In the controlled AD group (POEM score <8), the mean medication cost PPY was €567.15 (US $629.13), and the mean total direct cost PPY was €2040.46 (US $2263.24). In the uncontrolled AD group (POEM score ≥8), the mean medication cost PPY was €449.55 (US $498.63), and the mean total direct cost PPY was €1539.39 (US $1707.36)(Table 2). The comparisons of the study groups—controlled vs uncontrolled AD—showed no significant differences regarding medication costs PPY (P=.305, Mann-Whitney U statistic) and total direct costs PPY (P=.361, Mann-Whitney U statistic)(Figure 3). Thus, the distribution of medical costs was similar across all categories of the POEM score.
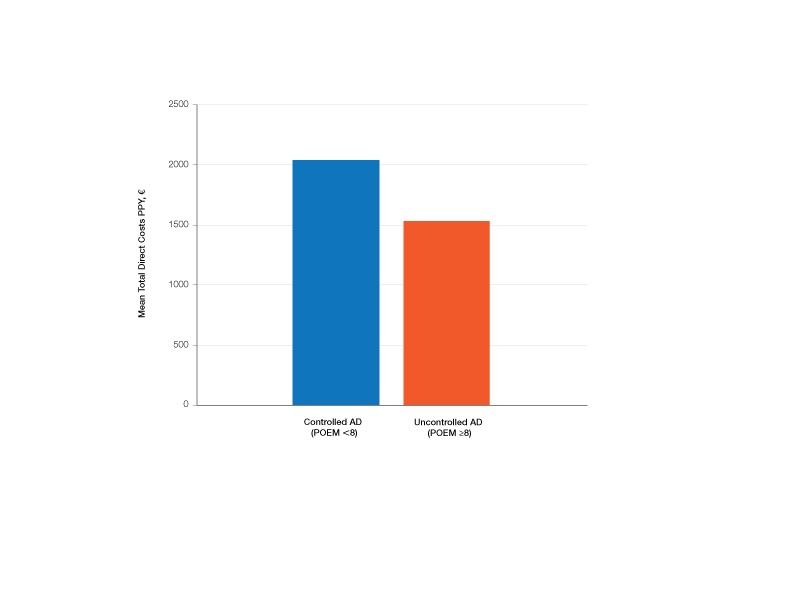
AD Severity and QOL—The mean (SD) POEM score in the study cohort was 17.9 (6.9). Sixteen (9.6%) patients had clear to almost clear skin or mild AD (POEM score 0–7). Forty-two (25.1%) patients had moderate AD (POEM score 8–16). Most of the patients (106; 63.5%) had severe or very severe AD (POEM score 17–28). According to the Rajka & Langeland score, 5 (3.0%) patients had mild disease (score 34), 81 (48.5%) patients had moderate disease (score 5–7), and 81 (48.5%) patients had severe disease (score 8–9). Eighty-one (48.5%) patients answered that AD affects their lives greatly, and 58 (34.7%) patients answered that it affects their lives extremely. Twenty-five (15.0%) patients answered that AD affects their everyday life to some extent, and only 2 (1.2%) patients answered that AD had little or no effect.
The mean (SD) DLQI was 13 (7.2). Based on the DLQI, 31 (18.6%) patients answered that AD had no effect or only a small effect on QOL (DLQI 0–5). In 36 (21.6%) patients, AD had a moderate effect on QOL (DLQI 6–10). The QOL impact was large (DLQI 11–20) and very large (DLQI 21–30) in 67 (40.1%) and 33 (19.8%) patients, respectively.
There was no significant difference in the impact of disease severity (POEM score) on the decrease of QOL (severe or very severe disease; P=.305, Mann-Whitney U statistic).
Absence From Work or Studies—At the study inclusion, 12 (7.2%) patients were not working or studying. Of the remaining 155 patients, 73 (47.1%) reported absence from work or educational activities due to AD in the last 12 months. The mean (SD) length of absence was 11.6 (10.2) days.
Comment
In this survey-based study of Finnish patients with moderate to severe AD, we observed that AD creates a substantial economic burden14 and negative impact on everyday life and QOL. According to DLQI, AD had a large or very large effect on most of the patients’ (59.9%) lives, and 90.2% of the included patients had self-reported moderate to very severe symptoms (POEM score 8–28). Our observations can partly be explained by characteristics of the Finnish health care system, in which patients with moderate to severe AD mainly are referred to specialist consultation. In the investigated cohort, many patients had used antibiotics (20.4%) and/or oral corticosteroids (21.6%) in the last year for the treatment of AD, which might indicate inadequate treatment of AD in the Finnish health care system.
Motivating patients to remain compliant is one of the main challenges in AD therapy.15 Fear of adverse effects from TCSs is common among patients and may cause poor treatment adherence.16 In a prospective study from the United Kingdom, the use of emollients in moderate to severe AD was considerably lower than AD guidelines recommend—approximately 10 g/d on average in adult patients. The median use of TCSs was between 35 and 38 g/mo.17 In our Finnish patient cohort, the amount of topical treatments was even lower, with a median use of emollients of 3.3 g/d and median use of TCSs of 25 g/mo. In another study from Denmark (N=322), 31% of patients with AD did not redeem their topical prescription medicaments, indicating poor adherence to topical treatment.18
It has been demonstrated that most of the patients’ habituation (tachyphylaxis) to TCSs is due to poor adherence instead of physiologic changes in tissue corticosteroid receptors.19,20 Treatment adherence may be increased by scheduling early follow-up visits and providing adequate therapeutic patient education,21 which requires major efforts by the health care system and a financial investment.
Inadequate treatment will lead to more frequent disease flares and subsequently increase the medical costs for the patients and the health care system.22 In our Finnish patient cohort, a large part of direct treatment costs was due to inpatient treatment (Figure 2) even though only a small proportion of patients had been hospitalized. The patients were frequently young and otherwise in good general health, and they did not necessarily need continuous inpatient treatment and monitoring. In Finland, it will be necessary to develop more cost-effective treatment regimens for patients with AD with severe and frequent flares. Many patients would benefit from subsequent and regular sessions of topical treatment in an outpatient setting. In addition, the prevention of flares in moderate to severe AD will decrease medical costs.23
The mean medication cost PPY was €457.40 (US $507.34), and mean total direct cost PPY was €1579.90 (US $1752.40), which indicates that AD causes a major economic burden to Finnish patients and to the Finnish health care system (Figures 1 and 2).24 We did not observe significant differences between controlled and uncontrolled AD medical costs in our patient cohort (Figure 3), which may have been due to the relatively small sample size of only 16 patients in the controlled AD group. All patients attending the tertiary care hospital had moderate to severe AD, so it is likely that the patients with lower POEM scores had better-controlled disease. The POEM score estimates the grade of AD in the last 7 days, but based on the relapsing course of the disease, the grading score may differ substantially during the year in the same patient depending on the timing.25,26
Topical calcineurin inhibitors comprised almost half of the medication costs (Figure 1), which may be caused by their higher prices compared with TCSs in Finland. In the beginning of 2019, a 50% less expensive biosimilar of tacrolimus ointment 0.1% was introduced to the Finnish market, which might decrease future treatment costs of TCIs. However, availability problems in both topical tacrolimus products were seen throughout 2019, which also may have affected the results in our study cohort. The median use of TCIs was unexpectedly low (only 30 g/y), which may be explained by different application habits. The use of large TCI amounts in some patients may have elevated mean costs.27
In the Finnish public health care system, 40% of the cost for prescription medication and emollients is reimbursed after an initial deductible of €50. Emollients are reimbursed up to an amount of 1500 g/mo. Therefore, patients mostly acquired emollients as prescription medicine and not over-the-counter. Nonprescription medicaments were not included in our study, so the actual costs of topical treatment may have been higher.28
In our cohort, 61.7% of the patients reported food allergies, and 70.1% reported allergic conjunctivitis. However, the study included only questionnaire-based data, and many of these patients probably had symptoms not associated with IgE-mediated allergies. The high prevalence indicates a substantial concomitant burden of more than skin symptoms in patients with AD.29 Nine percent of patients reported a diagnosed psychiatric disorder, and 29.3% had self-reported anxiety or depression often or very often in the last year. Based on these findings, there may be high percentages of undiagnosed psychiatric comorbidities such as depression and anxiety disorders in patients with moderate to severe AD in Finland.30 An important limitation of our study was that the patient data were based on a voluntary and anonymous survey and that depression and anxiety were addressed solely by a single question. In addition, the response rate cannot be analyzed correctly, and the demographics of the survey responders likely will differ substantially from all patients with AD at the university hospital.
Atopic dermatitis had a substantial effect on QOL in our patient cohort. Inadequate treatment of AD is known to negatively affect patient QOL and may lead to hospitalization or frequent oral corticosteroid courses.31,32 In most cases, structured patient education and early follow-up visits may improve patient adherence to treatment and should be considered as an integral part of AD treatment.33 In the investigated Finnish tertiary care hospital, a structured patient education system unfortunately was still lacking, though it has been proven effective elsewhere.34 In addition, patient-centred educational programs are recommended in European guidelines for the treatment of AD.35
Medical costs of AD may increase in the future as new treatments with higher direct costs, such as dupilumab, are introduced. Eichenfeld et al36 analyzed electronic health plan claims in patients with AD with newly introduced systemic therapies and phototherapies after the availability of dupilumab in the United States (March 2017). Mean annualized total cost in all patients was $20,722; the highest in the dupilumab group with $36,505. Compared to our data, the total costs are much higher, but these are likely to rise in Finland in the future if a substantial amount (eg, 1%–5%) of patients will be on advanced therapies, including dupilumab. If advanced therapies will be introduced more broadly in Finland (eg, in the treatment of moderate AD [10%–20% of patients]), they will represent a major direct cost to the health care system. Zimmermann et al37 showed in a cost-utility analysis that dupilumab improves health outcomes but with additional direct costs, and it is likely more cost-effective in patients with severe AD. Conversely, more efficient treatments may improve severe AD, reduce the need for hospitalization and recurrent doctors’ appointments as well as absence from work, and improve patient QOL,38 consequently decreasing indirect medical costs and disease burden. Ariëns et al39 showed in a recent registry-based study that dupilumab treatment induces a notable rise in work productivity and reduction of associated costs in patients with difficult-to-treat AD.
Conclusion
We aimed to analyze the economic burden of AD in Finland before the introduction of dupilumab. It will be interesting to see what the introduction of dupilumab and other novel systemic therapies have on total economic burden and medical costs. Most patients with AD in Finland can achieve disease control with topical treatments, but it is important to efficiently manage the patients who require additional supportive measures and specialist consultations, which may be challenging in the primary health care system because of the relapsing and remitting nature of the disease.
- Nutten S. Atopic dermatitis: global epidemiology and risk factors. Ann Nutr Metab. 2015;66(suppl 1):8-16.
- Eichenfield LF, Tom WL, Chamlin SL, et al. Guidelines of care for the management of atopic dermatitis: section 1. diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol. 2014;70:338-351.
- Yang EJ, Beck KM, Sekhon S, et al. The impact of pediatric atopic dermatitis on families: a review. Pediatr Dermatol. 2019;36:66-71.
- Eckert L, Gupta S, Amand C, et al. Impact of atopic dermatitis on health-related quality of life and productivity in adults in the United States: an analysis using the National Health and Wellness Survey. J Am Acad Dermatol. 2017;77:274-279.
- Drucker AM, Wang AR, Li WQ, et al. The burden of atopic dermatitis: summary of a report for the National Eczema Association. J Invest Dermatol. 2017;137:26-30.
- Ehlken B, Möhrenschlager M, Kugland B, et al. Cost-of-illness study in patients suffering from atopic eczema in Germany. Der Hautarzt. 2006;56:1144-1151.
- Ariëns LFM, van Nimwegen KJM, Shams M, et al. Economic burden of adult patients with moderate to severe atopic dermatitis indicated for systemic treatment. Acta Derm Venereol. 2019;99:762-768.
- Barbeau M, Bpharm HL. Burden of atopic dermatitis in Canada. Int J Dermatol. 2006;45:31-36.
- Verboom P, Hakkaart‐Van Roijen L, Sturkenboom M, et al. The cost of atopic dermatitis in the Netherlands: an international comparison. Br J Dermatol. 2002;147:716-724.
- Gånemo A, Svensson Å, Svedman C, et al. Usefulness of Rajka & Langeland eczema severity score in clinical practice. Acta Derm Venereol. 2016;96:521-524.
- Charman CR, Venn AJ, Williams HC. The Patient-Oriented Eczema Measure: development and initial validation of a new tool for measuring atopic eczema severity from the patients’ perspective. Arch Dermatol. 2004;140:1513-1519.
- Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI): a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19:210-216.
- Rehunen A, Reissell E, Honkatukia J, et al. Social and health services: regional changes in need, use and production and future options. Accessed July 20, 2023. http://urn.fi/URN:ISBN:978-952-287-294-4
- Reed B, Blaiss MS. The burden of atopic dermatitis. Allergy Asthma Proc. 2018;39:406-410.
- Koszorú K, Borza J, Gulácsi L, et al. Quality of life in patients with atopic dermatitis. Cutis. 2019;104:174-177.
- Li AW, Yin ES, Antaya RJ. Topical corticosteroid phobia in atopic dermatitis: a systematic review. JAMA Dermatol. 2017;153:1036-1042.
- Choi J, Dawe R, Ibbotson S, et al. Quantitative analysis of topical treatments in atopic dermatitis: unexpectedly low use of emollients and strong correlation of topical corticosteroid use both with depression and concurrent asthma. Br J Dermatol. 2020;182:1017-1025.
- Storm A, Andersen SE, Benfeldt E, et al. One in 3 prescriptions are never redeemed: primary nonadherence in an outpatient clinic. J Am Acad Dermatol. 2008;59:27-33.
- Okwundu N, Cardwell LA, Cline A, et al. Topical corticosteroids for treatment-resistant atopic dermatitis. Cutis. 2018;102:205-209.
- Eicher L, Knop M, Aszodi N, et al. A systematic review of factors influencing treatment adherence in chronic inflammatory skin disease—strategies for optimizing treatment outcome. J Eur Acad Dermatol Venereol. 2019;33:2253-2263.
- Heratizadeh A, Werfel T, Wollenberg A, et al; Arbeitsgemeinschaft Neurodermitisschulung für Erwachsene (ARNE) Study Group. Effects of structured patient education in adults with atopic dermatitis: multicenter randomized controlled trial. J Allergy Clin Immunol. 2017;140:845-853.
- Dierick BJH, van der Molen T, Flokstra-de Blok BMJ, et al. Burden and socioeconomics of asthma, allergic rhinitis, atopic dermatitis and food allergy. Expert Rev Pharmacoecon Outcomes Res. 2020;20:437-453.
- Olsson M, Bajpai R, Yew YW, et al. Associations between health-related quality of life and health care costs among children with atopic dermatitis and their caregivers: a cross-sectional study. Pediatr Dermatol. 2020;37:284-293.
- Bruin-Weller M, Pink AE, Patrizi A, et al. Disease burden and treatment history among adults with atopic dermatitis receiving systemic therapy: baseline characteristics of participants on the EUROSTAD prospective observational study. J Dermatolog Treat. 2021;32:164-173.
- Silverberg JI, Lei D, Yousaf M, et al. Comparison of Patient-Oriented Eczema Measure and Patient-Oriented Scoring Atopic Dermatitis vs Eczema Area and Severity Index and other measures of atopic dermatitis: a validation study. Ann Allergy Asthma Immunol. 2020;125:78-83.
- Kido-Nakahara M, Nakahara T, Yasukochi Y, et al. Patient-oriented eczema measure score: a useful tool for web-based surveys in patients with atopic dermatitis. Acta Derm Venereol. 2020;47:924-925.
- Komura Y, Kogure T, Kawahara K, et al. Economic assessment of actual prescription of drugs for treatment of atopic dermatitis: differences between dermatology and pediatrics in large-scale receipt data. J Dermatol. 2018;45:165-174.
- Thompson AM, Chan A, Torabi M, et al. Eczema moisturizers: allergenic potential, marketing claims, and costs. Dermatol Ther. 2020;33:E14228.
- Egeberg A, Andersen YM, Gislason GH, et al. Prevalence of comorbidity and associated risk factors in adults with atopic dermatitis. Allergy. 2017;72:783-791.
- Kauppi S, Jokelainen J, Timonen M, et al. Adult patients with atopic eczema have a high burden of psychiatric disease: a Finnish nationwide registry study. Acta Derm Venereol. 2019;99:647-651.
- Ali F, Vyas J, Finlay AY. Counting the burden: atopic dermatitis and health-related quality of life. Acta Derm Venereol. 2020;100:adv00161.
- Birdi G, Cooke R, Knibb RC. Impact of atopic dermatitis on quality of life in adults: a systematic review and meta-analysis. Int J Dermatol. 2020;59:E75-E91.
- Gabes M, Tischer C, Apfelbacher C; quality of life working group of the Harmonising Outcome Measures for Eczema (HOME) initiative. Measurement properties of quality-of-life outcome measures for children and adults with eczema: an updated systematic review. Pediatr Allergy Immunol. 2020;31:66-77.
- Staab D, Diepgen TL, Fartasch M, et al. Age related, structured educational programmes for the management of atopic dermatitis in children and adolescents: multicentre, randomised controlled trial. BMJ. 2006;332:933-938.
- Wollenberg A, Barbarot S, Bieber T, et al; European Dermatology Forum (EDF), the European Academy of Dermatology and Venereology (EADV), the European Academy of Allergy and Clinical Immunology (EAACI), the European Task Force on Atopic Dermatitis (ETFAD), European Federation of Allergy and Airways Diseases Patients’ Associations (EFA), the European Society for Dermatology and Psychiatry (ESDaP), the European Society of Pediatric Dermatology (ESPD), Global Allergy and Asthma European Network (GA2LEN) and the European Union of Medical Specialists (UEMS). Consensus-based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: part II. J Eur Acad Dermatol Venereol. 2018;32:850-878.
- Eichenfield LF, DiBonaventura M, Xenakis J, et al. Costs and treatment patterns among patients with atopic dermatitis using advanced therapies in the United States: analysis of a retrospective claims database. Dermatol Ther (Heidelb). 2020;10:791-806.
- Zimmermann M, Rind D, Chapman R, et al. Economic evaluation of dupilumab for moderate-to-severe atopic dermatitis: a cost-utility analysis. J Drugs Dermatol. 2018;17:750-756.
- Mata E, Loh TY, Ludwig C, et al. Pharmacy costs of systemic and topical medications for atopic dermatitis. J Dermatolog Treat. 2019;12:1-3.
- Ariëns LFM, Bakker DS, Spekhorst LS, et al. Rapid and sustained effect of dupilumab on work productivity in patients with difficult-to-treat atopic dermatitis: results from the Dutch BioDay Registry. Acta Derm Venereol. 2021;19;101:adv00573.
Atopic dermatitis (AD) is a common inflammatory skin disease that may severely decrease quality of life (QOL) and lead to psychiatric comorbidities.1-3 Prior studies have indicated that AD causes a substantial economic burden, and disease severity has been proportionally linked to medical costs.4,5 Results of a multicenter cost-of-illness study from Germany estimated that a relapse of AD costs approximately €123 (US $136). The authors calculated the average annual cost of AD per patient to be €1425 (US $1580), whereas it is €956 (US $1060) in moderate disease and €2068 (US $2293) in severe disease (direct and indirect medical costs included).6 An observational cohort study from the Netherlands found that total direct cost per patient-year (PPY) was €4401 (US $4879) for patients with controlled AD vs €6993 (US $7756) for patients with uncontrolled AD.7
In a retrospective survey-based study, it was estimated that the annual cost of AD in Canada was approximately CAD $1.4 billion. The cost per patient varied from CAD $282 to CAD $1242 depending on disease severity.8 In another retrospective cohort study from the Netherlands, the average direct medical cost per patient with AD seeing a general practitioner was US $71 during follow-up in primary care. If the patient needed specialist consultation, the cost increased to an average of US $186.9
We aimed to assess the direct and indirect medical costs in adult patients with moderate to severe AD who attended a tertiary health care center in Finland. In addition, we evaluated the impact of AD on QOL in this patient cohort.
Methods
Study Design—Patients with AD who were treated at the Department of Dermatology and Allergology, Helsinki University Hospital, Finland, between February 2018 and December 2019 were randomly selected to participate in our survey study. All participants provided written informed consent. In Finland, patients with mild AD generally are treated in primary health care centers, and only patients with moderate to severe AD are referred to specialists and tertiary care centers. Patients were excluded if they were younger than 18 years, had AD confined to the hands, or reported the presence of other concomitant skin diseases that were being treated with topical or systemic therapies. The protocol for the study was approved by the local ethics committee of the University of Helsinki.
Questionnaire and Analysis of Disease Severity—The survey included the medical history, signs of atopy, former treatment(s) for AD, skin infections, visits to dermatologists or general practitioners, questions on mental health and hospitalization, and absence from work due to AD in the last 12 months. Disease severity was evaluated using the patient-oriented Rajka & Langeland eczema severity score and Patient Oriented Eczema Measure (POEM).10,11 The impact on QOL was evaluated by the Dermatology Life Quality Index (DLQI).12
Medication Costs—The cost of prescription drugs was based on data from the Finnish national electronic prescription center. In Finland, all prescriptions are made electronically in the database. We analyzed all topical medications (eg, topical corticosteroids [TCSs], topical calcineurin inhibitors [TCIs], and emollients) and systemic medicaments (eg, antibiotics, antihistamines, cyclosporine, methotrexate, and corticosteroids) prescribed for the treatment of AD. In Finland, dupilumab was introduced for the treatment of severe AD in early 2019, and patients receiving dupilumab were excluded from the study. Over-the-counter medications were not included. The costs for laboratory testing were estimations based on the standard monitoring protocols of the Helsinki University Hospital. All costs were based on the Finnish price level standard for the year 2019.
Inpatient/Outpatient Visits and Sick Leave Due to AD—The number of inpatient and outpatient visits due to AD in the last 12 months was evaluated. Outpatient specialist consultations or nurse appointments at Helsinki University Hospital were verified from electronic patient records. In addition, inpatient treatment and phototherapy sessions were calculated from the database.
We assessed the number of sick leave days from work or educational activities during the last year. All costs of transportation for doctors’ appointments, laboratory monitoring, and phototherapy treatments were summed together to estimate the total transportation cost. Visits to nurse and inpatient visits were not included in the total transportation cost because patients often were hospitalized directly after consultation visits, and nurse appointments often were combined with inpatient and outpatient visits. To calculate the total transportation cost, we used a rate of €0.43 per kilometer measured from the patients’ home addresses, which was the official compensation rate of the Finnish Tax Administration for 2019.13
Statistical Analysis—Statistical analyses were performed using SPSS Statistics 25 (IBM). Descriptive analyses were used to describe baseline characteristics and to evaluate the mean costs of AD. The patients were divided into 2 groups according to POEM: (1) controlled AD (patients with clear skin or only mild AD; POEM score 0–7) and (2) uncontrolled AD (patients with moderate to very severe AD; POEM score 8–28). The Mann-Whitney U statistic was used to evaluate differences between the study groups.
Results
Patient Characteristics—One hundred sixty-seven patients answered the survey, of which 69 (41.3%) were males and 98 (58.7%) were females. There were 16 patients with controlled AD and 148 patients with uncontrolled AD. Three patients did not answer to POEM and were excluded. The baseline characteristics are presented in Table 1 and include self-reported symptoms related to atopy.

The most-used topical treatments were TCSs (n=155; 92.8%) and emollients (n=166; 99.4%). One hundred sixteen (69.5%) patients had used TCIs. The median amount of TCSs used was 300 g/y vs 30 g/y for TCIs (range, 0-5160 g/y) and 1200 g/y for emollients.
Fifteen (9.0%) patients had been hospitalized for AD in the last year. The mean (SD) length of hospitalization was 6.5 (2.8) days. Thirty-four (20.4%) patients received UVB phototherapy. Thirty-four (20.4%) patients were treated with at least 1 antibiotic course for secondary AD infection. Thirty-six (21.6%) patients needed at least 1 oral corticosteroid course for the treatment of an AD flare.
Fifteen (9.0%) patients reported a diagnosed psychiatric illness, and 17 (10.2%) patients were using prescription drugs for psychiatric illness. Forty-nine (29.3%) patients reported anxiety or depression often or very often, 54 (32.3%) patients reported sometimes, 33 (19.8%) patients reported rarely, and only 30 (18.0%) patients reported none.

Medication Costs—Mean medication cost PPY was €457.40 (US $507.34)(Figure 1 and Table 2). On average, one patient spent €87.50 (US $97.05) for TCSs, €121.90 (US $135.21) for emollients, and €225.10 (US $249.68) for TCIs. The average cost PPY for antibiotics was €6.10 (US $6.77). Other systemic treatments, including (US $18.65). Seventeen patients (10.2%) were on methotrexate therapy for AD in the last year, and 1 patient also used cyclosporine. The costs for laboratory monitoring in these patients were included in the direct cost calculations. The mean cost PPY of laboratory monitoring in the whole study cohort was €6.60 (US $7.32). In patients with systemic immunosuppressive therapy, the mean cost PPY for laboratory monitoring was €65.00 (US $72.09). Five patients had been tested for contact dermatitis; the costs of patch tests or other diagnostic tests were not included.

Visits to Health Care Providers—In the last year, patients had an average of 1.83 dermatologist consultations in the tertiary center (Table 2). In addition, the mean number of visits to private dermatologists was 0.61 and 1.42 visits to general practitioners. The mean cost of physician visits was €302.70 (US $335.75) in the tertiary center, €66.60 (US $73.87) in the private sector, and €141.90 (US $157.39) in primary health care. In total, the average cost of doctors’ appointments PPY was €506.30 (US $561.57). The mean estimated distance traveled per visit was 9.5 km.
The mean cost PPY of inpatient treatments was €329.90 (US $365.92) and €239.00 (US $265.09) for UV phototherapy. Only 4 patients had visited a nurse in the last year, with an average cost PPY of €2.50 (US $2.78).
In total, the cost PPY for health care provider visits was €1084.20, which included specialist consultations in a tertiary center and private sector, visits in primary health care, inpatient treatments, UV phototherapy sessions, nurse appointments in a tertiary center, and laboratory monitoring. The average transportation cost PPY was €34.00 (US $37.71). The mean number of visits to health care providers was 8.3 per year. Altogether, the direct cost PPY in the study cohort was €1580.60 (US $1752.39)(Table 2 and Figure 2).

Comparison of Medical Costs in Controlled vs Uncontrolled AD—In the controlled AD group (POEM score <8), the mean medication cost PPY was €567.15 (US $629.13), and the mean total direct cost PPY was €2040.46 (US $2263.24). In the uncontrolled AD group (POEM score ≥8), the mean medication cost PPY was €449.55 (US $498.63), and the mean total direct cost PPY was €1539.39 (US $1707.36)(Table 2). The comparisons of the study groups—controlled vs uncontrolled AD—showed no significant differences regarding medication costs PPY (P=.305, Mann-Whitney U statistic) and total direct costs PPY (P=.361, Mann-Whitney U statistic)(Figure 3). Thus, the distribution of medical costs was similar across all categories of the POEM score.

AD Severity and QOL—The mean (SD) POEM score in the study cohort was 17.9 (6.9). Sixteen (9.6%) patients had clear to almost clear skin or mild AD (POEM score 0–7). Forty-two (25.1%) patients had moderate AD (POEM score 8–16). Most of the patients (106; 63.5%) had severe or very severe AD (POEM score 17–28). According to the Rajka & Langeland score, 5 (3.0%) patients had mild disease (score 34), 81 (48.5%) patients had moderate disease (score 5–7), and 81 (48.5%) patients had severe disease (score 8–9). Eighty-one (48.5%) patients answered that AD affects their lives greatly, and 58 (34.7%) patients answered that it affects their lives extremely. Twenty-five (15.0%) patients answered that AD affects their everyday life to some extent, and only 2 (1.2%) patients answered that AD had little or no effect.
The mean (SD) DLQI was 13 (7.2). Based on the DLQI, 31 (18.6%) patients answered that AD had no effect or only a small effect on QOL (DLQI 0–5). In 36 (21.6%) patients, AD had a moderate effect on QOL (DLQI 6–10). The QOL impact was large (DLQI 11–20) and very large (DLQI 21–30) in 67 (40.1%) and 33 (19.8%) patients, respectively.
There was no significant difference in the impact of disease severity (POEM score) on the decrease of QOL (severe or very severe disease; P=.305, Mann-Whitney U statistic).
Absence From Work or Studies—At the study inclusion, 12 (7.2%) patients were not working or studying. Of the remaining 155 patients, 73 (47.1%) reported absence from work or educational activities due to AD in the last 12 months. The mean (SD) length of absence was 11.6 (10.2) days.
Comment
In this survey-based study of Finnish patients with moderate to severe AD, we observed that AD creates a substantial economic burden14 and negative impact on everyday life and QOL. According to DLQI, AD had a large or very large effect on most of the patients’ (59.9%) lives, and 90.2% of the included patients had self-reported moderate to very severe symptoms (POEM score 8–28). Our observations can partly be explained by characteristics of the Finnish health care system, in which patients with moderate to severe AD mainly are referred to specialist consultation. In the investigated cohort, many patients had used antibiotics (20.4%) and/or oral corticosteroids (21.6%) in the last year for the treatment of AD, which might indicate inadequate treatment of AD in the Finnish health care system.
Motivating patients to remain compliant is one of the main challenges in AD therapy.15 Fear of adverse effects from TCSs is common among patients and may cause poor treatment adherence.16 In a prospective study from the United Kingdom, the use of emollients in moderate to severe AD was considerably lower than AD guidelines recommend—approximately 10 g/d on average in adult patients. The median use of TCSs was between 35 and 38 g/mo.17 In our Finnish patient cohort, the amount of topical treatments was even lower, with a median use of emollients of 3.3 g/d and median use of TCSs of 25 g/mo. In another study from Denmark (N=322), 31% of patients with AD did not redeem their topical prescription medicaments, indicating poor adherence to topical treatment.18
It has been demonstrated that most of the patients’ habituation (tachyphylaxis) to TCSs is due to poor adherence instead of physiologic changes in tissue corticosteroid receptors.19,20 Treatment adherence may be increased by scheduling early follow-up visits and providing adequate therapeutic patient education,21 which requires major efforts by the health care system and a financial investment.
Inadequate treatment will lead to more frequent disease flares and subsequently increase the medical costs for the patients and the health care system.22 In our Finnish patient cohort, a large part of direct treatment costs was due to inpatient treatment (Figure 2) even though only a small proportion of patients had been hospitalized. The patients were frequently young and otherwise in good general health, and they did not necessarily need continuous inpatient treatment and monitoring. In Finland, it will be necessary to develop more cost-effective treatment regimens for patients with AD with severe and frequent flares. Many patients would benefit from subsequent and regular sessions of topical treatment in an outpatient setting. In addition, the prevention of flares in moderate to severe AD will decrease medical costs.23
The mean medication cost PPY was €457.40 (US $507.34), and mean total direct cost PPY was €1579.90 (US $1752.40), which indicates that AD causes a major economic burden to Finnish patients and to the Finnish health care system (Figures 1 and 2).24 We did not observe significant differences between controlled and uncontrolled AD medical costs in our patient cohort (Figure 3), which may have been due to the relatively small sample size of only 16 patients in the controlled AD group. All patients attending the tertiary care hospital had moderate to severe AD, so it is likely that the patients with lower POEM scores had better-controlled disease. The POEM score estimates the grade of AD in the last 7 days, but based on the relapsing course of the disease, the grading score may differ substantially during the year in the same patient depending on the timing.25,26
Topical calcineurin inhibitors comprised almost half of the medication costs (Figure 1), which may be caused by their higher prices compared with TCSs in Finland. In the beginning of 2019, a 50% less expensive biosimilar of tacrolimus ointment 0.1% was introduced to the Finnish market, which might decrease future treatment costs of TCIs. However, availability problems in both topical tacrolimus products were seen throughout 2019, which also may have affected the results in our study cohort. The median use of TCIs was unexpectedly low (only 30 g/y), which may be explained by different application habits. The use of large TCI amounts in some patients may have elevated mean costs.27
In the Finnish public health care system, 40% of the cost for prescription medication and emollients is reimbursed after an initial deductible of €50. Emollients are reimbursed up to an amount of 1500 g/mo. Therefore, patients mostly acquired emollients as prescription medicine and not over-the-counter. Nonprescription medicaments were not included in our study, so the actual costs of topical treatment may have been higher.28
In our cohort, 61.7% of the patients reported food allergies, and 70.1% reported allergic conjunctivitis. However, the study included only questionnaire-based data, and many of these patients probably had symptoms not associated with IgE-mediated allergies. The high prevalence indicates a substantial concomitant burden of more than skin symptoms in patients with AD.29 Nine percent of patients reported a diagnosed psychiatric disorder, and 29.3% had self-reported anxiety or depression often or very often in the last year. Based on these findings, there may be high percentages of undiagnosed psychiatric comorbidities such as depression and anxiety disorders in patients with moderate to severe AD in Finland.30 An important limitation of our study was that the patient data were based on a voluntary and anonymous survey and that depression and anxiety were addressed solely by a single question. In addition, the response rate cannot be analyzed correctly, and the demographics of the survey responders likely will differ substantially from all patients with AD at the university hospital.
Atopic dermatitis had a substantial effect on QOL in our patient cohort. Inadequate treatment of AD is known to negatively affect patient QOL and may lead to hospitalization or frequent oral corticosteroid courses.31,32 In most cases, structured patient education and early follow-up visits may improve patient adherence to treatment and should be considered as an integral part of AD treatment.33 In the investigated Finnish tertiary care hospital, a structured patient education system unfortunately was still lacking, though it has been proven effective elsewhere.34 In addition, patient-centred educational programs are recommended in European guidelines for the treatment of AD.35
Medical costs of AD may increase in the future as new treatments with higher direct costs, such as dupilumab, are introduced. Eichenfeld et al36 analyzed electronic health plan claims in patients with AD with newly introduced systemic therapies and phototherapies after the availability of dupilumab in the United States (March 2017). Mean annualized total cost in all patients was $20,722; the highest in the dupilumab group with $36,505. Compared to our data, the total costs are much higher, but these are likely to rise in Finland in the future if a substantial amount (eg, 1%–5%) of patients will be on advanced therapies, including dupilumab. If advanced therapies will be introduced more broadly in Finland (eg, in the treatment of moderate AD [10%–20% of patients]), they will represent a major direct cost to the health care system. Zimmermann et al37 showed in a cost-utility analysis that dupilumab improves health outcomes but with additional direct costs, and it is likely more cost-effective in patients with severe AD. Conversely, more efficient treatments may improve severe AD, reduce the need for hospitalization and recurrent doctors’ appointments as well as absence from work, and improve patient QOL,38 consequently decreasing indirect medical costs and disease burden. Ariëns et al39 showed in a recent registry-based study that dupilumab treatment induces a notable rise in work productivity and reduction of associated costs in patients with difficult-to-treat AD.
Conclusion
We aimed to analyze the economic burden of AD in Finland before the introduction of dupilumab. It will be interesting to see what the introduction of dupilumab and other novel systemic therapies have on total economic burden and medical costs. Most patients with AD in Finland can achieve disease control with topical treatments, but it is important to efficiently manage the patients who require additional supportive measures and specialist consultations, which may be challenging in the primary health care system because of the relapsing and remitting nature of the disease.
Atopic dermatitis (AD) is a common inflammatory skin disease that may severely decrease quality of life (QOL) and lead to psychiatric comorbidities.1-3 Prior studies have indicated that AD causes a substantial economic burden, and disease severity has been proportionally linked to medical costs.4,5 Results of a multicenter cost-of-illness study from Germany estimated that a relapse of AD costs approximately €123 (US $136). The authors calculated the average annual cost of AD per patient to be €1425 (US $1580), whereas it is €956 (US $1060) in moderate disease and €2068 (US $2293) in severe disease (direct and indirect medical costs included).6 An observational cohort study from the Netherlands found that total direct cost per patient-year (PPY) was €4401 (US $4879) for patients with controlled AD vs €6993 (US $7756) for patients with uncontrolled AD.7
In a retrospective survey-based study, it was estimated that the annual cost of AD in Canada was approximately CAD $1.4 billion. The cost per patient varied from CAD $282 to CAD $1242 depending on disease severity.8 In another retrospective cohort study from the Netherlands, the average direct medical cost per patient with AD seeing a general practitioner was US $71 during follow-up in primary care. If the patient needed specialist consultation, the cost increased to an average of US $186.9
We aimed to assess the direct and indirect medical costs in adult patients with moderate to severe AD who attended a tertiary health care center in Finland. In addition, we evaluated the impact of AD on QOL in this patient cohort.
Methods
Study Design—Patients with AD who were treated at the Department of Dermatology and Allergology, Helsinki University Hospital, Finland, between February 2018 and December 2019 were randomly selected to participate in our survey study. All participants provided written informed consent. In Finland, patients with mild AD generally are treated in primary health care centers, and only patients with moderate to severe AD are referred to specialists and tertiary care centers. Patients were excluded if they were younger than 18 years, had AD confined to the hands, or reported the presence of other concomitant skin diseases that were being treated with topical or systemic therapies. The protocol for the study was approved by the local ethics committee of the University of Helsinki.
Questionnaire and Analysis of Disease Severity—The survey included the medical history, signs of atopy, former treatment(s) for AD, skin infections, visits to dermatologists or general practitioners, questions on mental health and hospitalization, and absence from work due to AD in the last 12 months. Disease severity was evaluated using the patient-oriented Rajka & Langeland eczema severity score and Patient Oriented Eczema Measure (POEM).10,11 The impact on QOL was evaluated by the Dermatology Life Quality Index (DLQI).12
Medication Costs—The cost of prescription drugs was based on data from the Finnish national electronic prescription center. In Finland, all prescriptions are made electronically in the database. We analyzed all topical medications (eg, topical corticosteroids [TCSs], topical calcineurin inhibitors [TCIs], and emollients) and systemic medicaments (eg, antibiotics, antihistamines, cyclosporine, methotrexate, and corticosteroids) prescribed for the treatment of AD. In Finland, dupilumab was introduced for the treatment of severe AD in early 2019, and patients receiving dupilumab were excluded from the study. Over-the-counter medications were not included. The costs for laboratory testing were estimations based on the standard monitoring protocols of the Helsinki University Hospital. All costs were based on the Finnish price level standard for the year 2019.
Inpatient/Outpatient Visits and Sick Leave Due to AD—The number of inpatient and outpatient visits due to AD in the last 12 months was evaluated. Outpatient specialist consultations or nurse appointments at Helsinki University Hospital were verified from electronic patient records. In addition, inpatient treatment and phototherapy sessions were calculated from the database.
We assessed the number of sick leave days from work or educational activities during the last year. All costs of transportation for doctors’ appointments, laboratory monitoring, and phototherapy treatments were summed together to estimate the total transportation cost. Visits to nurse and inpatient visits were not included in the total transportation cost because patients often were hospitalized directly after consultation visits, and nurse appointments often were combined with inpatient and outpatient visits. To calculate the total transportation cost, we used a rate of €0.43 per kilometer measured from the patients’ home addresses, which was the official compensation rate of the Finnish Tax Administration for 2019.13
Statistical Analysis—Statistical analyses were performed using SPSS Statistics 25 (IBM). Descriptive analyses were used to describe baseline characteristics and to evaluate the mean costs of AD. The patients were divided into 2 groups according to POEM: (1) controlled AD (patients with clear skin or only mild AD; POEM score 0–7) and (2) uncontrolled AD (patients with moderate to very severe AD; POEM score 8–28). The Mann-Whitney U statistic was used to evaluate differences between the study groups.
Results
Patient Characteristics—One hundred sixty-seven patients answered the survey, of which 69 (41.3%) were males and 98 (58.7%) were females. There were 16 patients with controlled AD and 148 patients with uncontrolled AD. Three patients did not answer to POEM and were excluded. The baseline characteristics are presented in Table 1 and include self-reported symptoms related to atopy.

The most-used topical treatments were TCSs (n=155; 92.8%) and emollients (n=166; 99.4%). One hundred sixteen (69.5%) patients had used TCIs. The median amount of TCSs used was 300 g/y vs 30 g/y for TCIs (range, 0-5160 g/y) and 1200 g/y for emollients.
Fifteen (9.0%) patients had been hospitalized for AD in the last year. The mean (SD) length of hospitalization was 6.5 (2.8) days. Thirty-four (20.4%) patients received UVB phototherapy. Thirty-four (20.4%) patients were treated with at least 1 antibiotic course for secondary AD infection. Thirty-six (21.6%) patients needed at least 1 oral corticosteroid course for the treatment of an AD flare.
Fifteen (9.0%) patients reported a diagnosed psychiatric illness, and 17 (10.2%) patients were using prescription drugs for psychiatric illness. Forty-nine (29.3%) patients reported anxiety or depression often or very often, 54 (32.3%) patients reported sometimes, 33 (19.8%) patients reported rarely, and only 30 (18.0%) patients reported none.

Medication Costs—Mean medication cost PPY was €457.40 (US $507.34)(Figure 1 and Table 2). On average, one patient spent €87.50 (US $97.05) for TCSs, €121.90 (US $135.21) for emollients, and €225.10 (US $249.68) for TCIs. The average cost PPY for antibiotics was €6.10 (US $6.77). Other systemic treatments, including (US $18.65). Seventeen patients (10.2%) were on methotrexate therapy for AD in the last year, and 1 patient also used cyclosporine. The costs for laboratory monitoring in these patients were included in the direct cost calculations. The mean cost PPY of laboratory monitoring in the whole study cohort was €6.60 (US $7.32). In patients with systemic immunosuppressive therapy, the mean cost PPY for laboratory monitoring was €65.00 (US $72.09). Five patients had been tested for contact dermatitis; the costs of patch tests or other diagnostic tests were not included.

Visits to Health Care Providers—In the last year, patients had an average of 1.83 dermatologist consultations in the tertiary center (Table 2). In addition, the mean number of visits to private dermatologists was 0.61 and 1.42 visits to general practitioners. The mean cost of physician visits was €302.70 (US $335.75) in the tertiary center, €66.60 (US $73.87) in the private sector, and €141.90 (US $157.39) in primary health care. In total, the average cost of doctors’ appointments PPY was €506.30 (US $561.57). The mean estimated distance traveled per visit was 9.5 km.
The mean cost PPY of inpatient treatments was €329.90 (US $365.92) and €239.00 (US $265.09) for UV phototherapy. Only 4 patients had visited a nurse in the last year, with an average cost PPY of €2.50 (US $2.78).
In total, the cost PPY for health care provider visits was €1084.20, which included specialist consultations in a tertiary center and private sector, visits in primary health care, inpatient treatments, UV phototherapy sessions, nurse appointments in a tertiary center, and laboratory monitoring. The average transportation cost PPY was €34.00 (US $37.71). The mean number of visits to health care providers was 8.3 per year. Altogether, the direct cost PPY in the study cohort was €1580.60 (US $1752.39)(Table 2 and Figure 2).

Comparison of Medical Costs in Controlled vs Uncontrolled AD—In the controlled AD group (POEM score <8), the mean medication cost PPY was €567.15 (US $629.13), and the mean total direct cost PPY was €2040.46 (US $2263.24). In the uncontrolled AD group (POEM score ≥8), the mean medication cost PPY was €449.55 (US $498.63), and the mean total direct cost PPY was €1539.39 (US $1707.36)(Table 2). The comparisons of the study groups—controlled vs uncontrolled AD—showed no significant differences regarding medication costs PPY (P=.305, Mann-Whitney U statistic) and total direct costs PPY (P=.361, Mann-Whitney U statistic)(Figure 3). Thus, the distribution of medical costs was similar across all categories of the POEM score.

AD Severity and QOL—The mean (SD) POEM score in the study cohort was 17.9 (6.9). Sixteen (9.6%) patients had clear to almost clear skin or mild AD (POEM score 0–7). Forty-two (25.1%) patients had moderate AD (POEM score 8–16). Most of the patients (106; 63.5%) had severe or very severe AD (POEM score 17–28). According to the Rajka & Langeland score, 5 (3.0%) patients had mild disease (score 34), 81 (48.5%) patients had moderate disease (score 5–7), and 81 (48.5%) patients had severe disease (score 8–9). Eighty-one (48.5%) patients answered that AD affects their lives greatly, and 58 (34.7%) patients answered that it affects their lives extremely. Twenty-five (15.0%) patients answered that AD affects their everyday life to some extent, and only 2 (1.2%) patients answered that AD had little or no effect.
The mean (SD) DLQI was 13 (7.2). Based on the DLQI, 31 (18.6%) patients answered that AD had no effect or only a small effect on QOL (DLQI 0–5). In 36 (21.6%) patients, AD had a moderate effect on QOL (DLQI 6–10). The QOL impact was large (DLQI 11–20) and very large (DLQI 21–30) in 67 (40.1%) and 33 (19.8%) patients, respectively.
There was no significant difference in the impact of disease severity (POEM score) on the decrease of QOL (severe or very severe disease; P=.305, Mann-Whitney U statistic).
Absence From Work or Studies—At the study inclusion, 12 (7.2%) patients were not working or studying. Of the remaining 155 patients, 73 (47.1%) reported absence from work or educational activities due to AD in the last 12 months. The mean (SD) length of absence was 11.6 (10.2) days.
Comment
In this survey-based study of Finnish patients with moderate to severe AD, we observed that AD creates a substantial economic burden14 and negative impact on everyday life and QOL. According to DLQI, AD had a large or very large effect on most of the patients’ (59.9%) lives, and 90.2% of the included patients had self-reported moderate to very severe symptoms (POEM score 8–28). Our observations can partly be explained by characteristics of the Finnish health care system, in which patients with moderate to severe AD mainly are referred to specialist consultation. In the investigated cohort, many patients had used antibiotics (20.4%) and/or oral corticosteroids (21.6%) in the last year for the treatment of AD, which might indicate inadequate treatment of AD in the Finnish health care system.
Motivating patients to remain compliant is one of the main challenges in AD therapy.15 Fear of adverse effects from TCSs is common among patients and may cause poor treatment adherence.16 In a prospective study from the United Kingdom, the use of emollients in moderate to severe AD was considerably lower than AD guidelines recommend—approximately 10 g/d on average in adult patients. The median use of TCSs was between 35 and 38 g/mo.17 In our Finnish patient cohort, the amount of topical treatments was even lower, with a median use of emollients of 3.3 g/d and median use of TCSs of 25 g/mo. In another study from Denmark (N=322), 31% of patients with AD did not redeem their topical prescription medicaments, indicating poor adherence to topical treatment.18
It has been demonstrated that most of the patients’ habituation (tachyphylaxis) to TCSs is due to poor adherence instead of physiologic changes in tissue corticosteroid receptors.19,20 Treatment adherence may be increased by scheduling early follow-up visits and providing adequate therapeutic patient education,21 which requires major efforts by the health care system and a financial investment.
Inadequate treatment will lead to more frequent disease flares and subsequently increase the medical costs for the patients and the health care system.22 In our Finnish patient cohort, a large part of direct treatment costs was due to inpatient treatment (Figure 2) even though only a small proportion of patients had been hospitalized. The patients were frequently young and otherwise in good general health, and they did not necessarily need continuous inpatient treatment and monitoring. In Finland, it will be necessary to develop more cost-effective treatment regimens for patients with AD with severe and frequent flares. Many patients would benefit from subsequent and regular sessions of topical treatment in an outpatient setting. In addition, the prevention of flares in moderate to severe AD will decrease medical costs.23
The mean medication cost PPY was €457.40 (US $507.34), and mean total direct cost PPY was €1579.90 (US $1752.40), which indicates that AD causes a major economic burden to Finnish patients and to the Finnish health care system (Figures 1 and 2).24 We did not observe significant differences between controlled and uncontrolled AD medical costs in our patient cohort (Figure 3), which may have been due to the relatively small sample size of only 16 patients in the controlled AD group. All patients attending the tertiary care hospital had moderate to severe AD, so it is likely that the patients with lower POEM scores had better-controlled disease. The POEM score estimates the grade of AD in the last 7 days, but based on the relapsing course of the disease, the grading score may differ substantially during the year in the same patient depending on the timing.25,26
Topical calcineurin inhibitors comprised almost half of the medication costs (Figure 1), which may be caused by their higher prices compared with TCSs in Finland. In the beginning of 2019, a 50% less expensive biosimilar of tacrolimus ointment 0.1% was introduced to the Finnish market, which might decrease future treatment costs of TCIs. However, availability problems in both topical tacrolimus products were seen throughout 2019, which also may have affected the results in our study cohort. The median use of TCIs was unexpectedly low (only 30 g/y), which may be explained by different application habits. The use of large TCI amounts in some patients may have elevated mean costs.27
In the Finnish public health care system, 40% of the cost for prescription medication and emollients is reimbursed after an initial deductible of €50. Emollients are reimbursed up to an amount of 1500 g/mo. Therefore, patients mostly acquired emollients as prescription medicine and not over-the-counter. Nonprescription medicaments were not included in our study, so the actual costs of topical treatment may have been higher.28
In our cohort, 61.7% of the patients reported food allergies, and 70.1% reported allergic conjunctivitis. However, the study included only questionnaire-based data, and many of these patients probably had symptoms not associated with IgE-mediated allergies. The high prevalence indicates a substantial concomitant burden of more than skin symptoms in patients with AD.29 Nine percent of patients reported a diagnosed psychiatric disorder, and 29.3% had self-reported anxiety or depression often or very often in the last year. Based on these findings, there may be high percentages of undiagnosed psychiatric comorbidities such as depression and anxiety disorders in patients with moderate to severe AD in Finland.30 An important limitation of our study was that the patient data were based on a voluntary and anonymous survey and that depression and anxiety were addressed solely by a single question. In addition, the response rate cannot be analyzed correctly, and the demographics of the survey responders likely will differ substantially from all patients with AD at the university hospital.
Atopic dermatitis had a substantial effect on QOL in our patient cohort. Inadequate treatment of AD is known to negatively affect patient QOL and may lead to hospitalization or frequent oral corticosteroid courses.31,32 In most cases, structured patient education and early follow-up visits may improve patient adherence to treatment and should be considered as an integral part of AD treatment.33 In the investigated Finnish tertiary care hospital, a structured patient education system unfortunately was still lacking, though it has been proven effective elsewhere.34 In addition, patient-centred educational programs are recommended in European guidelines for the treatment of AD.35
Medical costs of AD may increase in the future as new treatments with higher direct costs, such as dupilumab, are introduced. Eichenfeld et al36 analyzed electronic health plan claims in patients with AD with newly introduced systemic therapies and phototherapies after the availability of dupilumab in the United States (March 2017). Mean annualized total cost in all patients was $20,722; the highest in the dupilumab group with $36,505. Compared to our data, the total costs are much higher, but these are likely to rise in Finland in the future if a substantial amount (eg, 1%–5%) of patients will be on advanced therapies, including dupilumab. If advanced therapies will be introduced more broadly in Finland (eg, in the treatment of moderate AD [10%–20% of patients]), they will represent a major direct cost to the health care system. Zimmermann et al37 showed in a cost-utility analysis that dupilumab improves health outcomes but with additional direct costs, and it is likely more cost-effective in patients with severe AD. Conversely, more efficient treatments may improve severe AD, reduce the need for hospitalization and recurrent doctors’ appointments as well as absence from work, and improve patient QOL,38 consequently decreasing indirect medical costs and disease burden. Ariëns et al39 showed in a recent registry-based study that dupilumab treatment induces a notable rise in work productivity and reduction of associated costs in patients with difficult-to-treat AD.
Conclusion
We aimed to analyze the economic burden of AD in Finland before the introduction of dupilumab. It will be interesting to see what the introduction of dupilumab and other novel systemic therapies have on total economic burden and medical costs. Most patients with AD in Finland can achieve disease control with topical treatments, but it is important to efficiently manage the patients who require additional supportive measures and specialist consultations, which may be challenging in the primary health care system because of the relapsing and remitting nature of the disease.
- Nutten S. Atopic dermatitis: global epidemiology and risk factors. Ann Nutr Metab. 2015;66(suppl 1):8-16.
- Eichenfield LF, Tom WL, Chamlin SL, et al. Guidelines of care for the management of atopic dermatitis: section 1. diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol. 2014;70:338-351.
- Yang EJ, Beck KM, Sekhon S, et al. The impact of pediatric atopic dermatitis on families: a review. Pediatr Dermatol. 2019;36:66-71.
- Eckert L, Gupta S, Amand C, et al. Impact of atopic dermatitis on health-related quality of life and productivity in adults in the United States: an analysis using the National Health and Wellness Survey. J Am Acad Dermatol. 2017;77:274-279.
- Drucker AM, Wang AR, Li WQ, et al. The burden of atopic dermatitis: summary of a report for the National Eczema Association. J Invest Dermatol. 2017;137:26-30.
- Ehlken B, Möhrenschlager M, Kugland B, et al. Cost-of-illness study in patients suffering from atopic eczema in Germany. Der Hautarzt. 2006;56:1144-1151.
- Ariëns LFM, van Nimwegen KJM, Shams M, et al. Economic burden of adult patients with moderate to severe atopic dermatitis indicated for systemic treatment. Acta Derm Venereol. 2019;99:762-768.
- Barbeau M, Bpharm HL. Burden of atopic dermatitis in Canada. Int J Dermatol. 2006;45:31-36.
- Verboom P, Hakkaart‐Van Roijen L, Sturkenboom M, et al. The cost of atopic dermatitis in the Netherlands: an international comparison. Br J Dermatol. 2002;147:716-724.
- Gånemo A, Svensson Å, Svedman C, et al. Usefulness of Rajka & Langeland eczema severity score in clinical practice. Acta Derm Venereol. 2016;96:521-524.
- Charman CR, Venn AJ, Williams HC. The Patient-Oriented Eczema Measure: development and initial validation of a new tool for measuring atopic eczema severity from the patients’ perspective. Arch Dermatol. 2004;140:1513-1519.
- Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI): a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19:210-216.
- Rehunen A, Reissell E, Honkatukia J, et al. Social and health services: regional changes in need, use and production and future options. Accessed July 20, 2023. http://urn.fi/URN:ISBN:978-952-287-294-4
- Reed B, Blaiss MS. The burden of atopic dermatitis. Allergy Asthma Proc. 2018;39:406-410.
- Koszorú K, Borza J, Gulácsi L, et al. Quality of life in patients with atopic dermatitis. Cutis. 2019;104:174-177.
- Li AW, Yin ES, Antaya RJ. Topical corticosteroid phobia in atopic dermatitis: a systematic review. JAMA Dermatol. 2017;153:1036-1042.
- Choi J, Dawe R, Ibbotson S, et al. Quantitative analysis of topical treatments in atopic dermatitis: unexpectedly low use of emollients and strong correlation of topical corticosteroid use both with depression and concurrent asthma. Br J Dermatol. 2020;182:1017-1025.
- Storm A, Andersen SE, Benfeldt E, et al. One in 3 prescriptions are never redeemed: primary nonadherence in an outpatient clinic. J Am Acad Dermatol. 2008;59:27-33.
- Okwundu N, Cardwell LA, Cline A, et al. Topical corticosteroids for treatment-resistant atopic dermatitis. Cutis. 2018;102:205-209.
- Eicher L, Knop M, Aszodi N, et al. A systematic review of factors influencing treatment adherence in chronic inflammatory skin disease—strategies for optimizing treatment outcome. J Eur Acad Dermatol Venereol. 2019;33:2253-2263.
- Heratizadeh A, Werfel T, Wollenberg A, et al; Arbeitsgemeinschaft Neurodermitisschulung für Erwachsene (ARNE) Study Group. Effects of structured patient education in adults with atopic dermatitis: multicenter randomized controlled trial. J Allergy Clin Immunol. 2017;140:845-853.
- Dierick BJH, van der Molen T, Flokstra-de Blok BMJ, et al. Burden and socioeconomics of asthma, allergic rhinitis, atopic dermatitis and food allergy. Expert Rev Pharmacoecon Outcomes Res. 2020;20:437-453.
- Olsson M, Bajpai R, Yew YW, et al. Associations between health-related quality of life and health care costs among children with atopic dermatitis and their caregivers: a cross-sectional study. Pediatr Dermatol. 2020;37:284-293.
- Bruin-Weller M, Pink AE, Patrizi A, et al. Disease burden and treatment history among adults with atopic dermatitis receiving systemic therapy: baseline characteristics of participants on the EUROSTAD prospective observational study. J Dermatolog Treat. 2021;32:164-173.
- Silverberg JI, Lei D, Yousaf M, et al. Comparison of Patient-Oriented Eczema Measure and Patient-Oriented Scoring Atopic Dermatitis vs Eczema Area and Severity Index and other measures of atopic dermatitis: a validation study. Ann Allergy Asthma Immunol. 2020;125:78-83.
- Kido-Nakahara M, Nakahara T, Yasukochi Y, et al. Patient-oriented eczema measure score: a useful tool for web-based surveys in patients with atopic dermatitis. Acta Derm Venereol. 2020;47:924-925.
- Komura Y, Kogure T, Kawahara K, et al. Economic assessment of actual prescription of drugs for treatment of atopic dermatitis: differences between dermatology and pediatrics in large-scale receipt data. J Dermatol. 2018;45:165-174.
- Thompson AM, Chan A, Torabi M, et al. Eczema moisturizers: allergenic potential, marketing claims, and costs. Dermatol Ther. 2020;33:E14228.
- Egeberg A, Andersen YM, Gislason GH, et al. Prevalence of comorbidity and associated risk factors in adults with atopic dermatitis. Allergy. 2017;72:783-791.
- Kauppi S, Jokelainen J, Timonen M, et al. Adult patients with atopic eczema have a high burden of psychiatric disease: a Finnish nationwide registry study. Acta Derm Venereol. 2019;99:647-651.
- Ali F, Vyas J, Finlay AY. Counting the burden: atopic dermatitis and health-related quality of life. Acta Derm Venereol. 2020;100:adv00161.
- Birdi G, Cooke R, Knibb RC. Impact of atopic dermatitis on quality of life in adults: a systematic review and meta-analysis. Int J Dermatol. 2020;59:E75-E91.
- Gabes M, Tischer C, Apfelbacher C; quality of life working group of the Harmonising Outcome Measures for Eczema (HOME) initiative. Measurement properties of quality-of-life outcome measures for children and adults with eczema: an updated systematic review. Pediatr Allergy Immunol. 2020;31:66-77.
- Staab D, Diepgen TL, Fartasch M, et al. Age related, structured educational programmes for the management of atopic dermatitis in children and adolescents: multicentre, randomised controlled trial. BMJ. 2006;332:933-938.
- Wollenberg A, Barbarot S, Bieber T, et al; European Dermatology Forum (EDF), the European Academy of Dermatology and Venereology (EADV), the European Academy of Allergy and Clinical Immunology (EAACI), the European Task Force on Atopic Dermatitis (ETFAD), European Federation of Allergy and Airways Diseases Patients’ Associations (EFA), the European Society for Dermatology and Psychiatry (ESDaP), the European Society of Pediatric Dermatology (ESPD), Global Allergy and Asthma European Network (GA2LEN) and the European Union of Medical Specialists (UEMS). Consensus-based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: part II. J Eur Acad Dermatol Venereol. 2018;32:850-878.
- Eichenfield LF, DiBonaventura M, Xenakis J, et al. Costs and treatment patterns among patients with atopic dermatitis using advanced therapies in the United States: analysis of a retrospective claims database. Dermatol Ther (Heidelb). 2020;10:791-806.
- Zimmermann M, Rind D, Chapman R, et al. Economic evaluation of dupilumab for moderate-to-severe atopic dermatitis: a cost-utility analysis. J Drugs Dermatol. 2018;17:750-756.
- Mata E, Loh TY, Ludwig C, et al. Pharmacy costs of systemic and topical medications for atopic dermatitis. J Dermatolog Treat. 2019;12:1-3.
- Ariëns LFM, Bakker DS, Spekhorst LS, et al. Rapid and sustained effect of dupilumab on work productivity in patients with difficult-to-treat atopic dermatitis: results from the Dutch BioDay Registry. Acta Derm Venereol. 2021;19;101:adv00573.
- Nutten S. Atopic dermatitis: global epidemiology and risk factors. Ann Nutr Metab. 2015;66(suppl 1):8-16.
- Eichenfield LF, Tom WL, Chamlin SL, et al. Guidelines of care for the management of atopic dermatitis: section 1. diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol. 2014;70:338-351.
- Yang EJ, Beck KM, Sekhon S, et al. The impact of pediatric atopic dermatitis on families: a review. Pediatr Dermatol. 2019;36:66-71.
- Eckert L, Gupta S, Amand C, et al. Impact of atopic dermatitis on health-related quality of life and productivity in adults in the United States: an analysis using the National Health and Wellness Survey. J Am Acad Dermatol. 2017;77:274-279.
- Drucker AM, Wang AR, Li WQ, et al. The burden of atopic dermatitis: summary of a report for the National Eczema Association. J Invest Dermatol. 2017;137:26-30.
- Ehlken B, Möhrenschlager M, Kugland B, et al. Cost-of-illness study in patients suffering from atopic eczema in Germany. Der Hautarzt. 2006;56:1144-1151.
- Ariëns LFM, van Nimwegen KJM, Shams M, et al. Economic burden of adult patients with moderate to severe atopic dermatitis indicated for systemic treatment. Acta Derm Venereol. 2019;99:762-768.
- Barbeau M, Bpharm HL. Burden of atopic dermatitis in Canada. Int J Dermatol. 2006;45:31-36.
- Verboom P, Hakkaart‐Van Roijen L, Sturkenboom M, et al. The cost of atopic dermatitis in the Netherlands: an international comparison. Br J Dermatol. 2002;147:716-724.
- Gånemo A, Svensson Å, Svedman C, et al. Usefulness of Rajka & Langeland eczema severity score in clinical practice. Acta Derm Venereol. 2016;96:521-524.
- Charman CR, Venn AJ, Williams HC. The Patient-Oriented Eczema Measure: development and initial validation of a new tool for measuring atopic eczema severity from the patients’ perspective. Arch Dermatol. 2004;140:1513-1519.
- Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI): a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19:210-216.
- Rehunen A, Reissell E, Honkatukia J, et al. Social and health services: regional changes in need, use and production and future options. Accessed July 20, 2023. http://urn.fi/URN:ISBN:978-952-287-294-4
- Reed B, Blaiss MS. The burden of atopic dermatitis. Allergy Asthma Proc. 2018;39:406-410.
- Koszorú K, Borza J, Gulácsi L, et al. Quality of life in patients with atopic dermatitis. Cutis. 2019;104:174-177.
- Li AW, Yin ES, Antaya RJ. Topical corticosteroid phobia in atopic dermatitis: a systematic review. JAMA Dermatol. 2017;153:1036-1042.
- Choi J, Dawe R, Ibbotson S, et al. Quantitative analysis of topical treatments in atopic dermatitis: unexpectedly low use of emollients and strong correlation of topical corticosteroid use both with depression and concurrent asthma. Br J Dermatol. 2020;182:1017-1025.
- Storm A, Andersen SE, Benfeldt E, et al. One in 3 prescriptions are never redeemed: primary nonadherence in an outpatient clinic. J Am Acad Dermatol. 2008;59:27-33.
- Okwundu N, Cardwell LA, Cline A, et al. Topical corticosteroids for treatment-resistant atopic dermatitis. Cutis. 2018;102:205-209.
- Eicher L, Knop M, Aszodi N, et al. A systematic review of factors influencing treatment adherence in chronic inflammatory skin disease—strategies for optimizing treatment outcome. J Eur Acad Dermatol Venereol. 2019;33:2253-2263.
- Heratizadeh A, Werfel T, Wollenberg A, et al; Arbeitsgemeinschaft Neurodermitisschulung für Erwachsene (ARNE) Study Group. Effects of structured patient education in adults with atopic dermatitis: multicenter randomized controlled trial. J Allergy Clin Immunol. 2017;140:845-853.
- Dierick BJH, van der Molen T, Flokstra-de Blok BMJ, et al. Burden and socioeconomics of asthma, allergic rhinitis, atopic dermatitis and food allergy. Expert Rev Pharmacoecon Outcomes Res. 2020;20:437-453.
- Olsson M, Bajpai R, Yew YW, et al. Associations between health-related quality of life and health care costs among children with atopic dermatitis and their caregivers: a cross-sectional study. Pediatr Dermatol. 2020;37:284-293.
- Bruin-Weller M, Pink AE, Patrizi A, et al. Disease burden and treatment history among adults with atopic dermatitis receiving systemic therapy: baseline characteristics of participants on the EUROSTAD prospective observational study. J Dermatolog Treat. 2021;32:164-173.
- Silverberg JI, Lei D, Yousaf M, et al. Comparison of Patient-Oriented Eczema Measure and Patient-Oriented Scoring Atopic Dermatitis vs Eczema Area and Severity Index and other measures of atopic dermatitis: a validation study. Ann Allergy Asthma Immunol. 2020;125:78-83.
- Kido-Nakahara M, Nakahara T, Yasukochi Y, et al. Patient-oriented eczema measure score: a useful tool for web-based surveys in patients with atopic dermatitis. Acta Derm Venereol. 2020;47:924-925.
- Komura Y, Kogure T, Kawahara K, et al. Economic assessment of actual prescription of drugs for treatment of atopic dermatitis: differences between dermatology and pediatrics in large-scale receipt data. J Dermatol. 2018;45:165-174.
- Thompson AM, Chan A, Torabi M, et al. Eczema moisturizers: allergenic potential, marketing claims, and costs. Dermatol Ther. 2020;33:E14228.
- Egeberg A, Andersen YM, Gislason GH, et al. Prevalence of comorbidity and associated risk factors in adults with atopic dermatitis. Allergy. 2017;72:783-791.
- Kauppi S, Jokelainen J, Timonen M, et al. Adult patients with atopic eczema have a high burden of psychiatric disease: a Finnish nationwide registry study. Acta Derm Venereol. 2019;99:647-651.
- Ali F, Vyas J, Finlay AY. Counting the burden: atopic dermatitis and health-related quality of life. Acta Derm Venereol. 2020;100:adv00161.
- Birdi G, Cooke R, Knibb RC. Impact of atopic dermatitis on quality of life in adults: a systematic review and meta-analysis. Int J Dermatol. 2020;59:E75-E91.
- Gabes M, Tischer C, Apfelbacher C; quality of life working group of the Harmonising Outcome Measures for Eczema (HOME) initiative. Measurement properties of quality-of-life outcome measures for children and adults with eczema: an updated systematic review. Pediatr Allergy Immunol. 2020;31:66-77.
- Staab D, Diepgen TL, Fartasch M, et al. Age related, structured educational programmes for the management of atopic dermatitis in children and adolescents: multicentre, randomised controlled trial. BMJ. 2006;332:933-938.
- Wollenberg A, Barbarot S, Bieber T, et al; European Dermatology Forum (EDF), the European Academy of Dermatology and Venereology (EADV), the European Academy of Allergy and Clinical Immunology (EAACI), the European Task Force on Atopic Dermatitis (ETFAD), European Federation of Allergy and Airways Diseases Patients’ Associations (EFA), the European Society for Dermatology and Psychiatry (ESDaP), the European Society of Pediatric Dermatology (ESPD), Global Allergy and Asthma European Network (GA2LEN) and the European Union of Medical Specialists (UEMS). Consensus-based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: part II. J Eur Acad Dermatol Venereol. 2018;32:850-878.
- Eichenfield LF, DiBonaventura M, Xenakis J, et al. Costs and treatment patterns among patients with atopic dermatitis using advanced therapies in the United States: analysis of a retrospective claims database. Dermatol Ther (Heidelb). 2020;10:791-806.
- Zimmermann M, Rind D, Chapman R, et al. Economic evaluation of dupilumab for moderate-to-severe atopic dermatitis: a cost-utility analysis. J Drugs Dermatol. 2018;17:750-756.
- Mata E, Loh TY, Ludwig C, et al. Pharmacy costs of systemic and topical medications for atopic dermatitis. J Dermatolog Treat. 2019;12:1-3.
- Ariëns LFM, Bakker DS, Spekhorst LS, et al. Rapid and sustained effect of dupilumab on work productivity in patients with difficult-to-treat atopic dermatitis: results from the Dutch BioDay Registry. Acta Derm Venereol. 2021;19;101:adv00573.
Practice Points
- Atopic dermatitis (AD) causes a substantial economic burden.
- Atopic dermatitis profoundly affects quality of life and is associated with psychiatric comorbidities. With effective treatments, AD-associated comorbidities may be decreased and the economic burden for the patient and health care system reduced.
Imaging Tools for Noninvasive Hair Assessment
New imaging tools along with adaptations to existing technologies have been emerging in recent years, with the potential to improve hair diagnostics and treatment monitoring. We provide an overview of 4 noninvasive hair imaging technologies: global photography, trichoscopy, reflectance confocal microscopy (RCM), and optical coherence tomography (OCT). For each instrument, we discuss current and future applications in clinical practice and research along with advantages and disadvantages.
Global Photography
Global photography allows for the analysis of hair growth, volume, distribution, and density through serial standardized photographs.1 Global photography was first introduced for hair growth studies in 1987 and soon after was used for hair and scalp assessments in finasteride clinical trials.2
Hair Assessment—Washed, dried, and combed hair, without hair product, are required for accurate imaging; wet conditions increase reflection and promote hair clumping, thus revealing more scalp and depicting the patient as having less hair.1 Headshots are taken from short distances and use stereotactic positioning devices to create 4 global views: vertex, midline, frontal, and temporal.3 Stereotactic positioning involves fixing the patient’s chin and forehead as well as mounting the camera and flash device to ensure proper magnification. These adjustments ensure lighting remains consistent throughout consecutive study visits.4 Various grading scales are available for use in hair growth clinical studies to increase objectivity in the analysis of serial global photographs. A blinded evaluator should assess the before and after photographs to limit experimenter bias. Global photography often is combined with quantitative software analysis for improved detection of hair changes.1
Advancements—Growing interest in improving global photography has resulted in various application-based, artificial intelligence (AI)–mediated tools to simplify photograph collection and analysis. For instance, new hair analysis software utilizes AI algorithms to account for facial features in determining the optimal angle for capturing global photographs (Figure 1), which simplifies the generation of global photography images through smartphone applications and obviates the need for additional stereotactic positioning equipment.5,6

Limitations—Clinicians should be aware of global photography’s requirements for consistency in lighting, camera settings, film, and image processing, which can limit the accuracy of hair assessment over time if not replicated correctly.7,8 Emerging global photography software has helped to overcome some of these limitations.
Global photography is less precise when a patient’s hair loss is less than 50%, as it is difficult to discern subtle hair changes. Thus, global photography provides limited utility in assessing minimal to moderate hair loss.9 Currently, global photography largely functions as an adjunct tool for other hair analysis methods rather than as a stand-alone tool.
Trichoscopy
Trichoscopy (also known as dermoscopy of the hair and scalp) may be performed with a manual dermoscope (with 10× magnification) or a digital videodermatoscope (up to 1000× magnification).10-12 Unlike global photography, trichoscopy provides a detailed structural analysis of hair shafts, follicular openings, and perifollicular and interfollicular areas.13 Kinoshita-Ise and Sachdeva13 provided an in-depth, updated review of trichoscopy terminology with their definitions and associated conditions (with prevalence), which should be referenced when performing trichoscopic examination.
Hair Assessment—Trichoscopic assessment begins with inspection of follicular openings (also referred to as “dots”), which vary in color depending on the material filling them—degrading keratinocytes, keratin, sebaceous debris, melanin, or fractured hairs.13 The structure of hair shafts also is examined, showing broken hairs, short vellus hairs, and comma hairs, among others. Perifollicular areas are examined for scale, erythema, blue-gray dots, and whitish halos. Interfollicular areas are examined for pigment pattern as well as vascularization, which often presents in a looping configuration under dermoscopy. A combination of dot colorization, hair shaft structure, and perifollicular and interfollicular findings inform diagnostic algorithms of hair and scalp conditions. For example, central centrifugal cicatricial alopecia, the most common alopecia seen in Black women, has been associated with a combination of honeycomb pigment pattern, perifollicular whitish halo, pinpoint white dots, white patches, and perifollicular erythema.13
Advantages—Perhaps the most useful feature of trichoscopy is its ability to translate visualized features into simple diagnostic algorithms. For instance, if the clinician has diagnosed the patient with noncicatricial alopecia, they would next focus on dot colors. With black dots, the next step would be to determine whether the hairs are tapered or coiled, and so on. This systematic approach enables the clinician to narrow possible diagnoses.2 An additional advantage of trichoscopy is that it examines large surface areas noninvasively as compared to hair-pull tests and scalp biopsy.14,15 Trichoscopy allows temporal comparisons of the same area for disease and treatment monitoring with more diagnostic detail than global photography.16 Trichoscopy also is useful in selecting biopsy locations by discerning and avoiding areas of scar tissue.17
Limitations—Diagnosis via the trichoscopy algorithm is limiting because it is not comprehensive of all hair and scalp disease.18 Additionally, many pathologies exhibit overlapping follicular and interfollicular patterning. For example, almost all subtypes of scarring alopecia present with hair loss and scarred follicles once they have progressed to advanced stages. Further studies should identify more specific patterns of hair and scalp pathologies, which could then be incorporated into a diagnostic algorithm.13
Advancements—The advent of hair analysis software has expanded the role of videodermoscopy by rapidly quantifying hair growth parameters such as hair count, follicular density, and follicular diameter, as well as interfollicular distances (Figure 2).14,17 Vellus and terminal hairs are differentiated according to their thickness and length.17 Moreover, the software can analyze the same area of the scalp over time by either virtual tattoos, semipermanent markings, or precise location measurements, increasing intra- and interclass correlation. The rate of hair growth, hair shedding, and parameters of anagen and telogen hairs can be studied by a method termed phototrichogram whereby a transitional area of hair loss and normal hair growth is identified and trimmed to less than 1 mm from the skin surface.19 A baseline photograph is taken using videodermoscopy. After approximately 3 days, the identical region is photographed and compared with the initial image to observe changes in the hair. Software programs can distinguish the growing hair as anagen and nongrowing hair as telogen, calculating the anagen-to-telogen ratio as well as hair growth rate, which are essential measurements in hair research and clinical studies. Software programs have replaced laborious and time-consuming manual hair counts and have rapidly grown in popularity in evaluating patterned hair loss.
Reflectance Confocal Microscopy
Reflectance confocal microscopy is a noninvasive imaging tool that visualizes skin and its appendages at near-histologic resolution (lateral resolution of 0.5–1 μm). It produces grayscale horizontal images that can be taken at levels ranging from the stratum corneum to the superficial papillary dermis, corresponding to a depth of approximately 100 to 150 µm. Thus, a hair follicle can be imaged starting from the follicular ostia down to the reachable papillary dermis (Figure 3).20 Image contrast is provided by differences in the size and refractive indices of cellular organelles.21,22 There are 2 commercially available RCM devices: VivaScope 1500 and VivaScope 3000 (Caliber Imaging & Diagnostics, Inc).
VivaScope 1500, a wide-probe microscope, requires the attachment of a plastic window to the desired imaging area. The plastic window is lined with medical adhesive tape to prevent movement during imaging. The adhesive tape can pull on hair upon removal, which is not ideal for patients with existing hair loss. Additionally, the image quality of VivaSope 1500 is best in flat areas and areas where hair is shaved.20,23,24 Despite these disadvantages, VivaScope 1500 has successfully shown utility in research studies, which suggests that these obstacles can be overcome by experienced users. The handheld VivaScope 3000 is ergonomically designed and suitable for curved surfaces such as the scalp, with the advantage of not requiring any adhesive. However, the images acquired from the VivaScope 3000 cover a smaller surface area.
Structures Visualized—Structures distinguished with RCM include keratinocytes, melanocytes, inflammatory cells, hair follicles, hair shafts, adnexal infundibular epithelium, blood vessels, fibroblasts, and collagen.23 Real-time visualization of blood flow also can be seen.
Applications of RCM—Reflectance confocal microscopy has been used to study scalp discoid lupus, lichen planopilaris, frontal fibrosing alopecia, folliculitis decalvans, chemotherapy-induced alopecia (CIA), alopecia areata, and androgenetic alopecia. Diagnostic RCM criteria for such alopecias have been developed based on their correspondence to histopathology. An RCM study of classic lichen planopilaris and frontal fibrosing alopecia identified features of epidermal disarray, infundibular hyperkeratosis, inflammatory cells, pigment incontinence, perifollicular fibrosis, bandlike scarring, melanophages in the dermis, dilated blood vessels, basal layer vacuolar degeneration, and necrotic keratinocytes.25 Pigment incontinence in the superficial epidermis, perifollicular lichenoid inflammation, and hyperkeratosis were characteristic RCM features of early-stage lichen planopilaris, while perifollicular fibrosis and dilated blood vessels were characteristic RCM features of late-stage disease. The ability of RCM features to distinguish different stages of lichen planopilaris shows its potential in treating early disease and preventing irreversible hair loss.
Differentiating between scarring and nonscarring alopecia also is possible through RCM. The presence of periadnexal, epidermal, and dermal inflammatory cells, in addition to periadnexal sclerosis, are defining RCM features of scarring alopecia.26 These features are absent in nonscarring alopecias. Reflectance confocal microscopy additionally has been shown to be useful in the treatment monitoring of lichen planopilaris and discoid lupus erythematosus.20 Independent reviewers, blinded to the patients’ identities, were able to characterize and follow features of these scarring alopecias by RCM. The assessed RCM features were comparable to those observed by histopathologic evaluation: epidermal disarray, spongiosis, exocytosis of inflammatory cells in the epidermis, interface dermatitis, peri- and intra-adnexal infiltration of inflammatory cells, dilated vessels in the dermis, dermal infiltration of inflammatory cells and melanophages, and dermal sclerosis. A reduction in inflammatory cells across multiple skin layers and at the level of the adnexal epithelium correlated with clinical response to treatment. Reflectance confocal microscopy also was able to detect recurrence of inflammation in cases where treatment had been interrupted before clinical signs of disease recurrence were evident. The authors thus concluded that RCM’s sensitivity can guide timing of treatment and avoid delays in starting or restarting treatment.20
Reflectance confocal microscopy also has served as a learning tool for new subclinical understandings of alopecia. In a study of CIA, the disease was found to be a dynamic process that could be categorized into 4 distinct phases distinguishable by combined confocal and dermoscopic features. This study also identified a new feature observable on RCM images—a CIA dot—defined as a dilated follicular infundibulum containing mashed, malted, nonhomogeneous material and normal or fragmented hair. This dot is thought to represent the initial microscopic sign of direct toxicity of chemotherapy on the hair follicle. Chemotherapy-induced alopecia dots persist throughout chemotherapy and subsequently disappear after chemotherapy ends.27
Limitations and Advantages—Currently, subtypes of cicatricial alopecias cannot be characterized on RCM because inflammatory cell types are not distinguished from each other (eg, eosinophils vs neutrophils). Another limitation of RCM is the loss of resolution below the superficial papillary dermis (a depth of approximately 150 µm); thus, deeper structures, such as the hair bulb, cannot be visualized.
Unlike global photography and trichoscopy, which are low-cost methods, RCM is much more costly, ranging upwards of several thousand dollars, and it may require additional technical support fees, making it less accessible for clinical practice. However, RCM imaging continues to be recommended as an intermediate step between trichoscopy and histology for the diagnosis and management of hair disease.26 If a biopsy is required, RCM can aid in the selection of a biopsy site, as areas with active inflammation are more informative than atrophic and fibrosed areas.23 The role of RCM in trichoscopy can be expanded by designing a more cost-effective and ergonomically suited scope for hair and scalp assessment.
Optical Coherence Tomography
Optical coherence tomography is a noninvasive handheld device that emits low-power infrared light to visualize the skin and adnexal structures. Optical coherence tomography relies on the principle of interferometry to detect phase differences in optical backscattering at varying tissue depths.28,29 It allows visualization up to 2 mm, which is 2 to 5 times deeper than RCM.36 Unlike RCM, which has cellular resolution, OCT has an axial resolution of 3 to 15 μm, which allows only for the detection of structural boundaries.30 There are various OCT modalities that differ in lateral and axial resolutions and maximum depth. Commercial software is available that measures changes in vascular density by depth, epidermal thickness, skin surface texture, and optical attenuation—the latter being an indirect measurement of collagen density and skin hydration.
Structures Visualized—Hair follicles can be well distinguished on OCT images, and as such, OCT is recognized as a diagnostic tool in trichology (Figure 4).31 Follicular openings, interfollicular collagen, and outlines of the hair shafts are visible; however, detailed components of the follicular unit cannot be visualized by OCT. Keratin hyperrefractivity identifies the hair shaft. Additionally, the hair matrix is denoted by a slightly granular texture in the dermis. Dynamic OCT produces colorized images that visualize blood flow within vessels.
Applications of OCT—Optical coherence tomography is utilized in investigative trichology because it provides highly reproducible measurements of hair shaft diameters, cross-sectional surface areas, and form factor, which is a surrogate parameter for hair shape. The cross-section of hair shafts provides insight into local metabolism and perifollicular inflammation. Cross-sections of hair shafts in areas of alopecia areata were found to be smaller than cross-sections in the unaffected scalp within the same individual.32 Follicular density can be manually quantified on OCT images, but there also is promise for automated quantification. A recent study by Urban et al33 described training a convolutional neural network to automatically count hair as well as hair-bearing and non–hair-bearing follicles in OCT scans. These investigators also were able to color-code hair according to height, resulting in the creation of a “height” map.
Optical coherence tomography has furthered our understanding of the pathophysiology of cicatricial and nonscarring alopecias. Vazquez-Herrera et al34 assessed the inflammatory and cicatricial stages of frontal fibrosing alopecia by OCT imaging. Inflammatory hairlines, which are seen in the early stages of frontal fibrosing alopecia, exhibited a thickened dermis, irregular distribution of collagen, and increased vascularity in both the superficial and deep dermal layers compared to cicatricial and healthy scalp. Conversely, late-stage cicatricial areas exhibited a thin dermis and collagen that appeared in a hyperreflective, concentric, onion-shaped pattern around remnant follicular openings. Vascular flow was reduced in the superficial dermis of a cicatricial scalp but increased in the deep dermal layers compared with a healthy scalp. The attenuation coefficients of these disease stages also were assessed. The attenuation coefficient of the inflammatory hairline was higher compared with normal skin, likely as a reflection of inflammatory infiltrate and edema, whereas the attenuation coefficient of cicatricial scalp was lower compared with normal skin, likely reflecting the reduced water content of atrophic skin.34 This differentiation of early- and late-stage cicatricial alopecias has implications for early treatment and improved prognosis. Additionally, there is potential for OCT to assist in the differentiation of alopecia subtypes, as it can measure the epidermal thickness and follicular density and was previously used to compare scarring and nonscarring alopecia.35
Advantages and Limitations—Similar to RCM, OCT may be cost prohibitive for some clinicians. In addition, OCT cannot visualize the follicular unit in cellular detail. However, the extent of OCT’s capabilities may not be fully realized. Dynamic OCT is a new angiographic type of OCT that shows potential in monitoring early subclinical responses to novel alopecia therapies, such as platelet-rich plasminogen, which is hypothesized to stimulate hair growth through angiogenesis. Additionally, OCT may improve outcomes of hair transplantation procedures by allowing for visualization of the subcutaneous angle of hair follicles. Blind extraction of hair follicles in follicular unit extraction procedures can result in inadvertent transection and damage to the hair follicle; OCT could help identify good candidates for follicular unit extraction, such as patients with hair follicles in parallel arrangement, who are predicted to have better results.36
Conclusion
The field of trichology will continue to evolve with the emergence of noninvasive imaging technologies that diagnose hair disease in early stages and enable treatment monitoring with quantification of hair parameters. As discussed in this review, global photography, trichoscopy, RCM, and OCT have furthered our understanding of alopecia pathophysiology and provided objective methods of treatment evaluation. The capabilities of these tools will continue to expand with advancements in add-on software and AI algorithms.
- Canfield D. Photographic documentation of hair growth in androgenetic alopecia. Dermatol Clin. 1996;14:713-721.
- Peytavi U, Hillmann K, Guarrera M. Hair growth assessment techniques. In: Peytavi U, Hillmann K, Guarrera M, eds. Hair Growth and Disorders. 4th ed. Springer; 2008:140-144.
- Chamberlain AJ, Dawber RP. Methods of evaluating hair growth. Australas J Dermatol. 2003;44:10-18.
- Dhurat R, Saraogi P. Hair evaluation methods: merits and demerits. Int J Trichology. 2009;1:108-119.
- Kaufman KD, Olsen EA, Whiting D, et al. Finasteride in the treatment of men with androgenetic alopecia. J Am Acad Dermatol. 1998;39:578-579.
- Capily Institute. Artificial intelligence (A.I.) powered hair growth tracking. Accessed July 31, 2023. https://tss-aesthetics.com/capily-hair-tracking-syst
- Dinh Q, Sinclair R. Female pattern hair loss: current treatment concepts. Clin Interv Aging. 2007;2:189-199.
- Dhurat R, Saraogi P. Hair evaluation methods: merits and demerits. Int J Trichology. 2009;1:108-119.
- Wikramanayake TC, Mauro LM, Tabas IA, et al. Cross-section trichometry: a clinical tool for assessing the progression and treatment response of alopecia. Int J Trichology. 2012;4:259-264.
- Alessandrini A, Bruni F, Piraccini BM, et al. Common causes of hair loss—clinical manifestations, trichoscopy and therapy. J Eur Acad Dermatol Venereol. 2021;35:629-640.
- Ashique K, Kaliyadan F. Clinical photography for trichology practice: tips and tricks. Int J Trichology. 2011;3:7-13.
- Rudnicka L, Olszewska M, Rakowska A, et al. Trichoscopy: a new method for diagnosing hair loss. J Drugs Dermatol. 2008;7:651-654.
- Kinoshita-Ise M, Sachdeva M. Update on trichoscopy: integration of the terminology by systematic approach and a proposal of a diagnostic flowchart. J Dermatol. 2022;49:4-18. doi:10.1111/1346-8138.16233
- Van Neste D, Trüeb RM. Critical study of hair growth analysis with computer-assisted methods. J Eur Acad Dermatol Venereol. 2006;20:578-583.
- Romero J, Grimalt R. Trichoscopy: essentials for the dermatologist. World J Dermatol. 2015;4:63-68.
- Trichoscopy: a new frontier for the diagnosis of hair diseases. Exp Rev Dermatol. 2012;7:429-437.
- Lee B, Chan J, Monselise A, et al. Assessment of hair density and caliber in Caucasian and Asian female subjects with female pattern hair loss by using the Folliscope. J Am Acad Dermatol. 2012;66:166-167.
- Inui S. Trichoscopy for common hair loss diseases: algorithmic method for diagnosis. J Dermatol. 2010;38:71-75.
- Dhurat R. Phototrichogram. Indian J Dermatol Venereol Leprol. 2006;72:242-244.
- Agozzino M, Tosti A, Barbieri L, et al. Confocal microscopic features of scarring alopecia: preliminary report. Br J Dermatol. 2011;165:534-540.
- Kuck M, Schanzer S, Ulrich M, et al. Analysis of the efficiency of hair removal by different optical methods: comparison of Trichoscan, reflectance confocal microscopy, and optical coherence tomography. J Biomed Opt. 2012;17:101504.
- Levine A, Markowitz O. Introduction to reflectance confocal microscopy and its use in clinical practice. JAAD Case Rep. 2018;4:1014-1023.
- Agozzino M, Ardigò M. Scalp confocal microscopy. In: Humbert P, Maibach H, Fanian F, et al, eds. Agache’s Measuring the Skin: Non-invasive Investigations, Physiology, Normal Constants. 2nd ed. Springer International Publishing; 2016:311-326.
- Rudnicka L, Olszewska M, Rakowska A. In vivo reflectance confocal microscopy: usefulness for diagnosing hair diseases. J Dermatol Case Rep. 2008;2:55-59.
- Kurzeja M, Czuwara J, Walecka I, et al. Features of classic lichen planopilaris and frontal fibrosing alopecia in reflectance confocal microscopy: a preliminary study. Skin Res Technol. 2021;27:266-271.
- Ardigò M, Agozzino M, Franceschini C, et al. Reflectance confocal microscopy for scarring and non-scarring alopecia real-time assessment. Arch Dermatol Res. 2016;308:309-318.
- Franceschini C, Garelli V, Persechino F, et al. Dermoscopy and confocal microscopy for different chemotherapy-induced alopecia (CIA) phases characterization: preliminary study. Skin Res Technol. 2020;26:269-276.
- Martinez-Velasco MA, Perper M, Maddy AJ, et al. In vitro determination of Mexican Mestizo hair shaft diameter using optical coherence tomography. Skin Res Technol. 2018;24;274-277.
- Srivastava R, Manfredini M, Rao BK. Noninvasive imaging tools in dermatology. Cutis. 2019;104:108-113.
- Wan B, Ganier C, Du-Harpur X, et al. Applications and future directions for optical coherence tomography in dermatology. Br J Dermatol. 2021;184:1014-1022.
- Blume-Peytavi U, Vieten J, Knuttel A et al. Optical coherent tomography (OCT): a new method for online-measurement of hair shaft thickness. J Dtsch Dermatol Ges. 2004;2:546.
- Garcia Bartels N, Jahnke I, Patzelt A, et al. Hair shaft abnormalities in alopecia areata evaluated by optical coherence tomography. Skin Res Technol. 2011;17:201-205.
- Urban G, Feil N, Csuka E, et al. Combining deep learning with optical coherence tomography imaging to determine scalp hair and follicle counts. Lasers Surg Med. 2021;53:171-178.
- Vazquez-Herrera NE, Eber AE, Martinez-Velasco MA, et al. Optical coherence tomography for the investigation of frontal fibrosing alopecia. J Eur Acad Dermatol Venereol. 2018;32:318-322.
- Ekelem C, Feil N, Csuka E, et al. Optical coherence tomography in the evaluation of the scalp and hair: common features and clinical utility. Lasers Surg Med. 2021;53:129-140.
- Schicho K, Seemann R, Binder M, et al. Optical coherence tomography for planning of follicular unit extraction. Dermatol Surg. 2015;41:358-363.
New imaging tools along with adaptations to existing technologies have been emerging in recent years, with the potential to improve hair diagnostics and treatment monitoring. We provide an overview of 4 noninvasive hair imaging technologies: global photography, trichoscopy, reflectance confocal microscopy (RCM), and optical coherence tomography (OCT). For each instrument, we discuss current and future applications in clinical practice and research along with advantages and disadvantages.
Global Photography
Global photography allows for the analysis of hair growth, volume, distribution, and density through serial standardized photographs.1 Global photography was first introduced for hair growth studies in 1987 and soon after was used for hair and scalp assessments in finasteride clinical trials.2
Hair Assessment—Washed, dried, and combed hair, without hair product, are required for accurate imaging; wet conditions increase reflection and promote hair clumping, thus revealing more scalp and depicting the patient as having less hair.1 Headshots are taken from short distances and use stereotactic positioning devices to create 4 global views: vertex, midline, frontal, and temporal.3 Stereotactic positioning involves fixing the patient’s chin and forehead as well as mounting the camera and flash device to ensure proper magnification. These adjustments ensure lighting remains consistent throughout consecutive study visits.4 Various grading scales are available for use in hair growth clinical studies to increase objectivity in the analysis of serial global photographs. A blinded evaluator should assess the before and after photographs to limit experimenter bias. Global photography often is combined with quantitative software analysis for improved detection of hair changes.1
Advancements—Growing interest in improving global photography has resulted in various application-based, artificial intelligence (AI)–mediated tools to simplify photograph collection and analysis. For instance, new hair analysis software utilizes AI algorithms to account for facial features in determining the optimal angle for capturing global photographs (Figure 1), which simplifies the generation of global photography images through smartphone applications and obviates the need for additional stereotactic positioning equipment.5,6

Limitations—Clinicians should be aware of global photography’s requirements for consistency in lighting, camera settings, film, and image processing, which can limit the accuracy of hair assessment over time if not replicated correctly.7,8 Emerging global photography software has helped to overcome some of these limitations.
Global photography is less precise when a patient’s hair loss is less than 50%, as it is difficult to discern subtle hair changes. Thus, global photography provides limited utility in assessing minimal to moderate hair loss.9 Currently, global photography largely functions as an adjunct tool for other hair analysis methods rather than as a stand-alone tool.
Trichoscopy
Trichoscopy (also known as dermoscopy of the hair and scalp) may be performed with a manual dermoscope (with 10× magnification) or a digital videodermatoscope (up to 1000× magnification).10-12 Unlike global photography, trichoscopy provides a detailed structural analysis of hair shafts, follicular openings, and perifollicular and interfollicular areas.13 Kinoshita-Ise and Sachdeva13 provided an in-depth, updated review of trichoscopy terminology with their definitions and associated conditions (with prevalence), which should be referenced when performing trichoscopic examination.
Hair Assessment—Trichoscopic assessment begins with inspection of follicular openings (also referred to as “dots”), which vary in color depending on the material filling them—degrading keratinocytes, keratin, sebaceous debris, melanin, or fractured hairs.13 The structure of hair shafts also is examined, showing broken hairs, short vellus hairs, and comma hairs, among others. Perifollicular areas are examined for scale, erythema, blue-gray dots, and whitish halos. Interfollicular areas are examined for pigment pattern as well as vascularization, which often presents in a looping configuration under dermoscopy. A combination of dot colorization, hair shaft structure, and perifollicular and interfollicular findings inform diagnostic algorithms of hair and scalp conditions. For example, central centrifugal cicatricial alopecia, the most common alopecia seen in Black women, has been associated with a combination of honeycomb pigment pattern, perifollicular whitish halo, pinpoint white dots, white patches, and perifollicular erythema.13
Advantages—Perhaps the most useful feature of trichoscopy is its ability to translate visualized features into simple diagnostic algorithms. For instance, if the clinician has diagnosed the patient with noncicatricial alopecia, they would next focus on dot colors. With black dots, the next step would be to determine whether the hairs are tapered or coiled, and so on. This systematic approach enables the clinician to narrow possible diagnoses.2 An additional advantage of trichoscopy is that it examines large surface areas noninvasively as compared to hair-pull tests and scalp biopsy.14,15 Trichoscopy allows temporal comparisons of the same area for disease and treatment monitoring with more diagnostic detail than global photography.16 Trichoscopy also is useful in selecting biopsy locations by discerning and avoiding areas of scar tissue.17
Limitations—Diagnosis via the trichoscopy algorithm is limiting because it is not comprehensive of all hair and scalp disease.18 Additionally, many pathologies exhibit overlapping follicular and interfollicular patterning. For example, almost all subtypes of scarring alopecia present with hair loss and scarred follicles once they have progressed to advanced stages. Further studies should identify more specific patterns of hair and scalp pathologies, which could then be incorporated into a diagnostic algorithm.13
Advancements—The advent of hair analysis software has expanded the role of videodermoscopy by rapidly quantifying hair growth parameters such as hair count, follicular density, and follicular diameter, as well as interfollicular distances (Figure 2).14,17 Vellus and terminal hairs are differentiated according to their thickness and length.17 Moreover, the software can analyze the same area of the scalp over time by either virtual tattoos, semipermanent markings, or precise location measurements, increasing intra- and interclass correlation. The rate of hair growth, hair shedding, and parameters of anagen and telogen hairs can be studied by a method termed phototrichogram whereby a transitional area of hair loss and normal hair growth is identified and trimmed to less than 1 mm from the skin surface.19 A baseline photograph is taken using videodermoscopy. After approximately 3 days, the identical region is photographed and compared with the initial image to observe changes in the hair. Software programs can distinguish the growing hair as anagen and nongrowing hair as telogen, calculating the anagen-to-telogen ratio as well as hair growth rate, which are essential measurements in hair research and clinical studies. Software programs have replaced laborious and time-consuming manual hair counts and have rapidly grown in popularity in evaluating patterned hair loss.

Reflectance Confocal Microscopy
Reflectance confocal microscopy is a noninvasive imaging tool that visualizes skin and its appendages at near-histologic resolution (lateral resolution of 0.5–1 μm). It produces grayscale horizontal images that can be taken at levels ranging from the stratum corneum to the superficial papillary dermis, corresponding to a depth of approximately 100 to 150 µm. Thus, a hair follicle can be imaged starting from the follicular ostia down to the reachable papillary dermis (Figure 3).20 Image contrast is provided by differences in the size and refractive indices of cellular organelles.21,22 There are 2 commercially available RCM devices: VivaScope 1500 and VivaScope 3000 (Caliber Imaging & Diagnostics, Inc).

VivaScope 1500, a wide-probe microscope, requires the attachment of a plastic window to the desired imaging area. The plastic window is lined with medical adhesive tape to prevent movement during imaging. The adhesive tape can pull on hair upon removal, which is not ideal for patients with existing hair loss. Additionally, the image quality of VivaSope 1500 is best in flat areas and areas where hair is shaved.20,23,24 Despite these disadvantages, VivaScope 1500 has successfully shown utility in research studies, which suggests that these obstacles can be overcome by experienced users. The handheld VivaScope 3000 is ergonomically designed and suitable for curved surfaces such as the scalp, with the advantage of not requiring any adhesive. However, the images acquired from the VivaScope 3000 cover a smaller surface area.
Structures Visualized—Structures distinguished with RCM include keratinocytes, melanocytes, inflammatory cells, hair follicles, hair shafts, adnexal infundibular epithelium, blood vessels, fibroblasts, and collagen.23 Real-time visualization of blood flow also can be seen.
Applications of RCM—Reflectance confocal microscopy has been used to study scalp discoid lupus, lichen planopilaris, frontal fibrosing alopecia, folliculitis decalvans, chemotherapy-induced alopecia (CIA), alopecia areata, and androgenetic alopecia. Diagnostic RCM criteria for such alopecias have been developed based on their correspondence to histopathology. An RCM study of classic lichen planopilaris and frontal fibrosing alopecia identified features of epidermal disarray, infundibular hyperkeratosis, inflammatory cells, pigment incontinence, perifollicular fibrosis, bandlike scarring, melanophages in the dermis, dilated blood vessels, basal layer vacuolar degeneration, and necrotic keratinocytes.25 Pigment incontinence in the superficial epidermis, perifollicular lichenoid inflammation, and hyperkeratosis were characteristic RCM features of early-stage lichen planopilaris, while perifollicular fibrosis and dilated blood vessels were characteristic RCM features of late-stage disease. The ability of RCM features to distinguish different stages of lichen planopilaris shows its potential in treating early disease and preventing irreversible hair loss.
Differentiating between scarring and nonscarring alopecia also is possible through RCM. The presence of periadnexal, epidermal, and dermal inflammatory cells, in addition to periadnexal sclerosis, are defining RCM features of scarring alopecia.26 These features are absent in nonscarring alopecias. Reflectance confocal microscopy additionally has been shown to be useful in the treatment monitoring of lichen planopilaris and discoid lupus erythematosus.20 Independent reviewers, blinded to the patients’ identities, were able to characterize and follow features of these scarring alopecias by RCM. The assessed RCM features were comparable to those observed by histopathologic evaluation: epidermal disarray, spongiosis, exocytosis of inflammatory cells in the epidermis, interface dermatitis, peri- and intra-adnexal infiltration of inflammatory cells, dilated vessels in the dermis, dermal infiltration of inflammatory cells and melanophages, and dermal sclerosis. A reduction in inflammatory cells across multiple skin layers and at the level of the adnexal epithelium correlated with clinical response to treatment. Reflectance confocal microscopy also was able to detect recurrence of inflammation in cases where treatment had been interrupted before clinical signs of disease recurrence were evident. The authors thus concluded that RCM’s sensitivity can guide timing of treatment and avoid delays in starting or restarting treatment.20
Reflectance confocal microscopy also has served as a learning tool for new subclinical understandings of alopecia. In a study of CIA, the disease was found to be a dynamic process that could be categorized into 4 distinct phases distinguishable by combined confocal and dermoscopic features. This study also identified a new feature observable on RCM images—a CIA dot—defined as a dilated follicular infundibulum containing mashed, malted, nonhomogeneous material and normal or fragmented hair. This dot is thought to represent the initial microscopic sign of direct toxicity of chemotherapy on the hair follicle. Chemotherapy-induced alopecia dots persist throughout chemotherapy and subsequently disappear after chemotherapy ends.27
Limitations and Advantages—Currently, subtypes of cicatricial alopecias cannot be characterized on RCM because inflammatory cell types are not distinguished from each other (eg, eosinophils vs neutrophils). Another limitation of RCM is the loss of resolution below the superficial papillary dermis (a depth of approximately 150 µm); thus, deeper structures, such as the hair bulb, cannot be visualized.
Unlike global photography and trichoscopy, which are low-cost methods, RCM is much more costly, ranging upwards of several thousand dollars, and it may require additional technical support fees, making it less accessible for clinical practice. However, RCM imaging continues to be recommended as an intermediate step between trichoscopy and histology for the diagnosis and management of hair disease.26 If a biopsy is required, RCM can aid in the selection of a biopsy site, as areas with active inflammation are more informative than atrophic and fibrosed areas.23 The role of RCM in trichoscopy can be expanded by designing a more cost-effective and ergonomically suited scope for hair and scalp assessment.
Optical Coherence Tomography
Optical coherence tomography is a noninvasive handheld device that emits low-power infrared light to visualize the skin and adnexal structures. Optical coherence tomography relies on the principle of interferometry to detect phase differences in optical backscattering at varying tissue depths.28,29 It allows visualization up to 2 mm, which is 2 to 5 times deeper than RCM.36 Unlike RCM, which has cellular resolution, OCT has an axial resolution of 3 to 15 μm, which allows only for the detection of structural boundaries.30 There are various OCT modalities that differ in lateral and axial resolutions and maximum depth. Commercial software is available that measures changes in vascular density by depth, epidermal thickness, skin surface texture, and optical attenuation—the latter being an indirect measurement of collagen density and skin hydration.
Structures Visualized—Hair follicles can be well distinguished on OCT images, and as such, OCT is recognized as a diagnostic tool in trichology (Figure 4).31 Follicular openings, interfollicular collagen, and outlines of the hair shafts are visible; however, detailed components of the follicular unit cannot be visualized by OCT. Keratin hyperrefractivity identifies the hair shaft. Additionally, the hair matrix is denoted by a slightly granular texture in the dermis. Dynamic OCT produces colorized images that visualize blood flow within vessels.

Applications of OCT—Optical coherence tomography is utilized in investigative trichology because it provides highly reproducible measurements of hair shaft diameters, cross-sectional surface areas, and form factor, which is a surrogate parameter for hair shape. The cross-section of hair shafts provides insight into local metabolism and perifollicular inflammation. Cross-sections of hair shafts in areas of alopecia areata were found to be smaller than cross-sections in the unaffected scalp within the same individual.32 Follicular density can be manually quantified on OCT images, but there also is promise for automated quantification. A recent study by Urban et al33 described training a convolutional neural network to automatically count hair as well as hair-bearing and non–hair-bearing follicles in OCT scans. These investigators also were able to color-code hair according to height, resulting in the creation of a “height” map.
Optical coherence tomography has furthered our understanding of the pathophysiology of cicatricial and nonscarring alopecias. Vazquez-Herrera et al34 assessed the inflammatory and cicatricial stages of frontal fibrosing alopecia by OCT imaging. Inflammatory hairlines, which are seen in the early stages of frontal fibrosing alopecia, exhibited a thickened dermis, irregular distribution of collagen, and increased vascularity in both the superficial and deep dermal layers compared to cicatricial and healthy scalp. Conversely, late-stage cicatricial areas exhibited a thin dermis and collagen that appeared in a hyperreflective, concentric, onion-shaped pattern around remnant follicular openings. Vascular flow was reduced in the superficial dermis of a cicatricial scalp but increased in the deep dermal layers compared with a healthy scalp. The attenuation coefficients of these disease stages also were assessed. The attenuation coefficient of the inflammatory hairline was higher compared with normal skin, likely as a reflection of inflammatory infiltrate and edema, whereas the attenuation coefficient of cicatricial scalp was lower compared with normal skin, likely reflecting the reduced water content of atrophic skin.34 This differentiation of early- and late-stage cicatricial alopecias has implications for early treatment and improved prognosis. Additionally, there is potential for OCT to assist in the differentiation of alopecia subtypes, as it can measure the epidermal thickness and follicular density and was previously used to compare scarring and nonscarring alopecia.35
Advantages and Limitations—Similar to RCM, OCT may be cost prohibitive for some clinicians. In addition, OCT cannot visualize the follicular unit in cellular detail. However, the extent of OCT’s capabilities may not be fully realized. Dynamic OCT is a new angiographic type of OCT that shows potential in monitoring early subclinical responses to novel alopecia therapies, such as platelet-rich plasminogen, which is hypothesized to stimulate hair growth through angiogenesis. Additionally, OCT may improve outcomes of hair transplantation procedures by allowing for visualization of the subcutaneous angle of hair follicles. Blind extraction of hair follicles in follicular unit extraction procedures can result in inadvertent transection and damage to the hair follicle; OCT could help identify good candidates for follicular unit extraction, such as patients with hair follicles in parallel arrangement, who are predicted to have better results.36
Conclusion
The field of trichology will continue to evolve with the emergence of noninvasive imaging technologies that diagnose hair disease in early stages and enable treatment monitoring with quantification of hair parameters. As discussed in this review, global photography, trichoscopy, RCM, and OCT have furthered our understanding of alopecia pathophysiology and provided objective methods of treatment evaluation. The capabilities of these tools will continue to expand with advancements in add-on software and AI algorithms.
New imaging tools along with adaptations to existing technologies have been emerging in recent years, with the potential to improve hair diagnostics and treatment monitoring. We provide an overview of 4 noninvasive hair imaging technologies: global photography, trichoscopy, reflectance confocal microscopy (RCM), and optical coherence tomography (OCT). For each instrument, we discuss current and future applications in clinical practice and research along with advantages and disadvantages.
Global Photography
Global photography allows for the analysis of hair growth, volume, distribution, and density through serial standardized photographs.1 Global photography was first introduced for hair growth studies in 1987 and soon after was used for hair and scalp assessments in finasteride clinical trials.2
Hair Assessment—Washed, dried, and combed hair, without hair product, are required for accurate imaging; wet conditions increase reflection and promote hair clumping, thus revealing more scalp and depicting the patient as having less hair.1 Headshots are taken from short distances and use stereotactic positioning devices to create 4 global views: vertex, midline, frontal, and temporal.3 Stereotactic positioning involves fixing the patient’s chin and forehead as well as mounting the camera and flash device to ensure proper magnification. These adjustments ensure lighting remains consistent throughout consecutive study visits.4 Various grading scales are available for use in hair growth clinical studies to increase objectivity in the analysis of serial global photographs. A blinded evaluator should assess the before and after photographs to limit experimenter bias. Global photography often is combined with quantitative software analysis for improved detection of hair changes.1
Advancements—Growing interest in improving global photography has resulted in various application-based, artificial intelligence (AI)–mediated tools to simplify photograph collection and analysis. For instance, new hair analysis software utilizes AI algorithms to account for facial features in determining the optimal angle for capturing global photographs (Figure 1), which simplifies the generation of global photography images through smartphone applications and obviates the need for additional stereotactic positioning equipment.5,6

Limitations—Clinicians should be aware of global photography’s requirements for consistency in lighting, camera settings, film, and image processing, which can limit the accuracy of hair assessment over time if not replicated correctly.7,8 Emerging global photography software has helped to overcome some of these limitations.
Global photography is less precise when a patient’s hair loss is less than 50%, as it is difficult to discern subtle hair changes. Thus, global photography provides limited utility in assessing minimal to moderate hair loss.9 Currently, global photography largely functions as an adjunct tool for other hair analysis methods rather than as a stand-alone tool.
Trichoscopy
Trichoscopy (also known as dermoscopy of the hair and scalp) may be performed with a manual dermoscope (with 10× magnification) or a digital videodermatoscope (up to 1000× magnification).10-12 Unlike global photography, trichoscopy provides a detailed structural analysis of hair shafts, follicular openings, and perifollicular and interfollicular areas.13 Kinoshita-Ise and Sachdeva13 provided an in-depth, updated review of trichoscopy terminology with their definitions and associated conditions (with prevalence), which should be referenced when performing trichoscopic examination.
Hair Assessment—Trichoscopic assessment begins with inspection of follicular openings (also referred to as “dots”), which vary in color depending on the material filling them—degrading keratinocytes, keratin, sebaceous debris, melanin, or fractured hairs.13 The structure of hair shafts also is examined, showing broken hairs, short vellus hairs, and comma hairs, among others. Perifollicular areas are examined for scale, erythema, blue-gray dots, and whitish halos. Interfollicular areas are examined for pigment pattern as well as vascularization, which often presents in a looping configuration under dermoscopy. A combination of dot colorization, hair shaft structure, and perifollicular and interfollicular findings inform diagnostic algorithms of hair and scalp conditions. For example, central centrifugal cicatricial alopecia, the most common alopecia seen in Black women, has been associated with a combination of honeycomb pigment pattern, perifollicular whitish halo, pinpoint white dots, white patches, and perifollicular erythema.13
Advantages—Perhaps the most useful feature of trichoscopy is its ability to translate visualized features into simple diagnostic algorithms. For instance, if the clinician has diagnosed the patient with noncicatricial alopecia, they would next focus on dot colors. With black dots, the next step would be to determine whether the hairs are tapered or coiled, and so on. This systematic approach enables the clinician to narrow possible diagnoses.2 An additional advantage of trichoscopy is that it examines large surface areas noninvasively as compared to hair-pull tests and scalp biopsy.14,15 Trichoscopy allows temporal comparisons of the same area for disease and treatment monitoring with more diagnostic detail than global photography.16 Trichoscopy also is useful in selecting biopsy locations by discerning and avoiding areas of scar tissue.17
Limitations—Diagnosis via the trichoscopy algorithm is limiting because it is not comprehensive of all hair and scalp disease.18 Additionally, many pathologies exhibit overlapping follicular and interfollicular patterning. For example, almost all subtypes of scarring alopecia present with hair loss and scarred follicles once they have progressed to advanced stages. Further studies should identify more specific patterns of hair and scalp pathologies, which could then be incorporated into a diagnostic algorithm.13
Advancements—The advent of hair analysis software has expanded the role of videodermoscopy by rapidly quantifying hair growth parameters such as hair count, follicular density, and follicular diameter, as well as interfollicular distances (Figure 2).14,17 Vellus and terminal hairs are differentiated according to their thickness and length.17 Moreover, the software can analyze the same area of the scalp over time by either virtual tattoos, semipermanent markings, or precise location measurements, increasing intra- and interclass correlation. The rate of hair growth, hair shedding, and parameters of anagen and telogen hairs can be studied by a method termed phototrichogram whereby a transitional area of hair loss and normal hair growth is identified and trimmed to less than 1 mm from the skin surface.19 A baseline photograph is taken using videodermoscopy. After approximately 3 days, the identical region is photographed and compared with the initial image to observe changes in the hair. Software programs can distinguish the growing hair as anagen and nongrowing hair as telogen, calculating the anagen-to-telogen ratio as well as hair growth rate, which are essential measurements in hair research and clinical studies. Software programs have replaced laborious and time-consuming manual hair counts and have rapidly grown in popularity in evaluating patterned hair loss.

Reflectance Confocal Microscopy
Reflectance confocal microscopy is a noninvasive imaging tool that visualizes skin and its appendages at near-histologic resolution (lateral resolution of 0.5–1 μm). It produces grayscale horizontal images that can be taken at levels ranging from the stratum corneum to the superficial papillary dermis, corresponding to a depth of approximately 100 to 150 µm. Thus, a hair follicle can be imaged starting from the follicular ostia down to the reachable papillary dermis (Figure 3).20 Image contrast is provided by differences in the size and refractive indices of cellular organelles.21,22 There are 2 commercially available RCM devices: VivaScope 1500 and VivaScope 3000 (Caliber Imaging & Diagnostics, Inc).

VivaScope 1500, a wide-probe microscope, requires the attachment of a plastic window to the desired imaging area. The plastic window is lined with medical adhesive tape to prevent movement during imaging. The adhesive tape can pull on hair upon removal, which is not ideal for patients with existing hair loss. Additionally, the image quality of VivaSope 1500 is best in flat areas and areas where hair is shaved.20,23,24 Despite these disadvantages, VivaScope 1500 has successfully shown utility in research studies, which suggests that these obstacles can be overcome by experienced users. The handheld VivaScope 3000 is ergonomically designed and suitable for curved surfaces such as the scalp, with the advantage of not requiring any adhesive. However, the images acquired from the VivaScope 3000 cover a smaller surface area.
Structures Visualized—Structures distinguished with RCM include keratinocytes, melanocytes, inflammatory cells, hair follicles, hair shafts, adnexal infundibular epithelium, blood vessels, fibroblasts, and collagen.23 Real-time visualization of blood flow also can be seen.
Applications of RCM—Reflectance confocal microscopy has been used to study scalp discoid lupus, lichen planopilaris, frontal fibrosing alopecia, folliculitis decalvans, chemotherapy-induced alopecia (CIA), alopecia areata, and androgenetic alopecia. Diagnostic RCM criteria for such alopecias have been developed based on their correspondence to histopathology. An RCM study of classic lichen planopilaris and frontal fibrosing alopecia identified features of epidermal disarray, infundibular hyperkeratosis, inflammatory cells, pigment incontinence, perifollicular fibrosis, bandlike scarring, melanophages in the dermis, dilated blood vessels, basal layer vacuolar degeneration, and necrotic keratinocytes.25 Pigment incontinence in the superficial epidermis, perifollicular lichenoid inflammation, and hyperkeratosis were characteristic RCM features of early-stage lichen planopilaris, while perifollicular fibrosis and dilated blood vessels were characteristic RCM features of late-stage disease. The ability of RCM features to distinguish different stages of lichen planopilaris shows its potential in treating early disease and preventing irreversible hair loss.
Differentiating between scarring and nonscarring alopecia also is possible through RCM. The presence of periadnexal, epidermal, and dermal inflammatory cells, in addition to periadnexal sclerosis, are defining RCM features of scarring alopecia.26 These features are absent in nonscarring alopecias. Reflectance confocal microscopy additionally has been shown to be useful in the treatment monitoring of lichen planopilaris and discoid lupus erythematosus.20 Independent reviewers, blinded to the patients’ identities, were able to characterize and follow features of these scarring alopecias by RCM. The assessed RCM features were comparable to those observed by histopathologic evaluation: epidermal disarray, spongiosis, exocytosis of inflammatory cells in the epidermis, interface dermatitis, peri- and intra-adnexal infiltration of inflammatory cells, dilated vessels in the dermis, dermal infiltration of inflammatory cells and melanophages, and dermal sclerosis. A reduction in inflammatory cells across multiple skin layers and at the level of the adnexal epithelium correlated with clinical response to treatment. Reflectance confocal microscopy also was able to detect recurrence of inflammation in cases where treatment had been interrupted before clinical signs of disease recurrence were evident. The authors thus concluded that RCM’s sensitivity can guide timing of treatment and avoid delays in starting or restarting treatment.20
Reflectance confocal microscopy also has served as a learning tool for new subclinical understandings of alopecia. In a study of CIA, the disease was found to be a dynamic process that could be categorized into 4 distinct phases distinguishable by combined confocal and dermoscopic features. This study also identified a new feature observable on RCM images—a CIA dot—defined as a dilated follicular infundibulum containing mashed, malted, nonhomogeneous material and normal or fragmented hair. This dot is thought to represent the initial microscopic sign of direct toxicity of chemotherapy on the hair follicle. Chemotherapy-induced alopecia dots persist throughout chemotherapy and subsequently disappear after chemotherapy ends.27
Limitations and Advantages—Currently, subtypes of cicatricial alopecias cannot be characterized on RCM because inflammatory cell types are not distinguished from each other (eg, eosinophils vs neutrophils). Another limitation of RCM is the loss of resolution below the superficial papillary dermis (a depth of approximately 150 µm); thus, deeper structures, such as the hair bulb, cannot be visualized.
Unlike global photography and trichoscopy, which are low-cost methods, RCM is much more costly, ranging upwards of several thousand dollars, and it may require additional technical support fees, making it less accessible for clinical practice. However, RCM imaging continues to be recommended as an intermediate step between trichoscopy and histology for the diagnosis and management of hair disease.26 If a biopsy is required, RCM can aid in the selection of a biopsy site, as areas with active inflammation are more informative than atrophic and fibrosed areas.23 The role of RCM in trichoscopy can be expanded by designing a more cost-effective and ergonomically suited scope for hair and scalp assessment.
Optical Coherence Tomography
Optical coherence tomography is a noninvasive handheld device that emits low-power infrared light to visualize the skin and adnexal structures. Optical coherence tomography relies on the principle of interferometry to detect phase differences in optical backscattering at varying tissue depths.28,29 It allows visualization up to 2 mm, which is 2 to 5 times deeper than RCM.36 Unlike RCM, which has cellular resolution, OCT has an axial resolution of 3 to 15 μm, which allows only for the detection of structural boundaries.30 There are various OCT modalities that differ in lateral and axial resolutions and maximum depth. Commercial software is available that measures changes in vascular density by depth, epidermal thickness, skin surface texture, and optical attenuation—the latter being an indirect measurement of collagen density and skin hydration.
Structures Visualized—Hair follicles can be well distinguished on OCT images, and as such, OCT is recognized as a diagnostic tool in trichology (Figure 4).31 Follicular openings, interfollicular collagen, and outlines of the hair shafts are visible; however, detailed components of the follicular unit cannot be visualized by OCT. Keratin hyperrefractivity identifies the hair shaft. Additionally, the hair matrix is denoted by a slightly granular texture in the dermis. Dynamic OCT produces colorized images that visualize blood flow within vessels.

Applications of OCT—Optical coherence tomography is utilized in investigative trichology because it provides highly reproducible measurements of hair shaft diameters, cross-sectional surface areas, and form factor, which is a surrogate parameter for hair shape. The cross-section of hair shafts provides insight into local metabolism and perifollicular inflammation. Cross-sections of hair shafts in areas of alopecia areata were found to be smaller than cross-sections in the unaffected scalp within the same individual.32 Follicular density can be manually quantified on OCT images, but there also is promise for automated quantification. A recent study by Urban et al33 described training a convolutional neural network to automatically count hair as well as hair-bearing and non–hair-bearing follicles in OCT scans. These investigators also were able to color-code hair according to height, resulting in the creation of a “height” map.
Optical coherence tomography has furthered our understanding of the pathophysiology of cicatricial and nonscarring alopecias. Vazquez-Herrera et al34 assessed the inflammatory and cicatricial stages of frontal fibrosing alopecia by OCT imaging. Inflammatory hairlines, which are seen in the early stages of frontal fibrosing alopecia, exhibited a thickened dermis, irregular distribution of collagen, and increased vascularity in both the superficial and deep dermal layers compared to cicatricial and healthy scalp. Conversely, late-stage cicatricial areas exhibited a thin dermis and collagen that appeared in a hyperreflective, concentric, onion-shaped pattern around remnant follicular openings. Vascular flow was reduced in the superficial dermis of a cicatricial scalp but increased in the deep dermal layers compared with a healthy scalp. The attenuation coefficients of these disease stages also were assessed. The attenuation coefficient of the inflammatory hairline was higher compared with normal skin, likely as a reflection of inflammatory infiltrate and edema, whereas the attenuation coefficient of cicatricial scalp was lower compared with normal skin, likely reflecting the reduced water content of atrophic skin.34 This differentiation of early- and late-stage cicatricial alopecias has implications for early treatment and improved prognosis. Additionally, there is potential for OCT to assist in the differentiation of alopecia subtypes, as it can measure the epidermal thickness and follicular density and was previously used to compare scarring and nonscarring alopecia.35
Advantages and Limitations—Similar to RCM, OCT may be cost prohibitive for some clinicians. In addition, OCT cannot visualize the follicular unit in cellular detail. However, the extent of OCT’s capabilities may not be fully realized. Dynamic OCT is a new angiographic type of OCT that shows potential in monitoring early subclinical responses to novel alopecia therapies, such as platelet-rich plasminogen, which is hypothesized to stimulate hair growth through angiogenesis. Additionally, OCT may improve outcomes of hair transplantation procedures by allowing for visualization of the subcutaneous angle of hair follicles. Blind extraction of hair follicles in follicular unit extraction procedures can result in inadvertent transection and damage to the hair follicle; OCT could help identify good candidates for follicular unit extraction, such as patients with hair follicles in parallel arrangement, who are predicted to have better results.36
Conclusion
The field of trichology will continue to evolve with the emergence of noninvasive imaging technologies that diagnose hair disease in early stages and enable treatment monitoring with quantification of hair parameters. As discussed in this review, global photography, trichoscopy, RCM, and OCT have furthered our understanding of alopecia pathophysiology and provided objective methods of treatment evaluation. The capabilities of these tools will continue to expand with advancements in add-on software and AI algorithms.
- Canfield D. Photographic documentation of hair growth in androgenetic alopecia. Dermatol Clin. 1996;14:713-721.
- Peytavi U, Hillmann K, Guarrera M. Hair growth assessment techniques. In: Peytavi U, Hillmann K, Guarrera M, eds. Hair Growth and Disorders. 4th ed. Springer; 2008:140-144.
- Chamberlain AJ, Dawber RP. Methods of evaluating hair growth. Australas J Dermatol. 2003;44:10-18.
- Dhurat R, Saraogi P. Hair evaluation methods: merits and demerits. Int J Trichology. 2009;1:108-119.
- Kaufman KD, Olsen EA, Whiting D, et al. Finasteride in the treatment of men with androgenetic alopecia. J Am Acad Dermatol. 1998;39:578-579.
- Capily Institute. Artificial intelligence (A.I.) powered hair growth tracking. Accessed July 31, 2023. https://tss-aesthetics.com/capily-hair-tracking-syst
- Dinh Q, Sinclair R. Female pattern hair loss: current treatment concepts. Clin Interv Aging. 2007;2:189-199.
- Dhurat R, Saraogi P. Hair evaluation methods: merits and demerits. Int J Trichology. 2009;1:108-119.
- Wikramanayake TC, Mauro LM, Tabas IA, et al. Cross-section trichometry: a clinical tool for assessing the progression and treatment response of alopecia. Int J Trichology. 2012;4:259-264.
- Alessandrini A, Bruni F, Piraccini BM, et al. Common causes of hair loss—clinical manifestations, trichoscopy and therapy. J Eur Acad Dermatol Venereol. 2021;35:629-640.
- Ashique K, Kaliyadan F. Clinical photography for trichology practice: tips and tricks. Int J Trichology. 2011;3:7-13.
- Rudnicka L, Olszewska M, Rakowska A, et al. Trichoscopy: a new method for diagnosing hair loss. J Drugs Dermatol. 2008;7:651-654.
- Kinoshita-Ise M, Sachdeva M. Update on trichoscopy: integration of the terminology by systematic approach and a proposal of a diagnostic flowchart. J Dermatol. 2022;49:4-18. doi:10.1111/1346-8138.16233
- Van Neste D, Trüeb RM. Critical study of hair growth analysis with computer-assisted methods. J Eur Acad Dermatol Venereol. 2006;20:578-583.
- Romero J, Grimalt R. Trichoscopy: essentials for the dermatologist. World J Dermatol. 2015;4:63-68.
- Trichoscopy: a new frontier for the diagnosis of hair diseases. Exp Rev Dermatol. 2012;7:429-437.
- Lee B, Chan J, Monselise A, et al. Assessment of hair density and caliber in Caucasian and Asian female subjects with female pattern hair loss by using the Folliscope. J Am Acad Dermatol. 2012;66:166-167.
- Inui S. Trichoscopy for common hair loss diseases: algorithmic method for diagnosis. J Dermatol. 2010;38:71-75.
- Dhurat R. Phototrichogram. Indian J Dermatol Venereol Leprol. 2006;72:242-244.
- Agozzino M, Tosti A, Barbieri L, et al. Confocal microscopic features of scarring alopecia: preliminary report. Br J Dermatol. 2011;165:534-540.
- Kuck M, Schanzer S, Ulrich M, et al. Analysis of the efficiency of hair removal by different optical methods: comparison of Trichoscan, reflectance confocal microscopy, and optical coherence tomography. J Biomed Opt. 2012;17:101504.
- Levine A, Markowitz O. Introduction to reflectance confocal microscopy and its use in clinical practice. JAAD Case Rep. 2018;4:1014-1023.
- Agozzino M, Ardigò M. Scalp confocal microscopy. In: Humbert P, Maibach H, Fanian F, et al, eds. Agache’s Measuring the Skin: Non-invasive Investigations, Physiology, Normal Constants. 2nd ed. Springer International Publishing; 2016:311-326.
- Rudnicka L, Olszewska M, Rakowska A. In vivo reflectance confocal microscopy: usefulness for diagnosing hair diseases. J Dermatol Case Rep. 2008;2:55-59.
- Kurzeja M, Czuwara J, Walecka I, et al. Features of classic lichen planopilaris and frontal fibrosing alopecia in reflectance confocal microscopy: a preliminary study. Skin Res Technol. 2021;27:266-271.
- Ardigò M, Agozzino M, Franceschini C, et al. Reflectance confocal microscopy for scarring and non-scarring alopecia real-time assessment. Arch Dermatol Res. 2016;308:309-318.
- Franceschini C, Garelli V, Persechino F, et al. Dermoscopy and confocal microscopy for different chemotherapy-induced alopecia (CIA) phases characterization: preliminary study. Skin Res Technol. 2020;26:269-276.
- Martinez-Velasco MA, Perper M, Maddy AJ, et al. In vitro determination of Mexican Mestizo hair shaft diameter using optical coherence tomography. Skin Res Technol. 2018;24;274-277.
- Srivastava R, Manfredini M, Rao BK. Noninvasive imaging tools in dermatology. Cutis. 2019;104:108-113.
- Wan B, Ganier C, Du-Harpur X, et al. Applications and future directions for optical coherence tomography in dermatology. Br J Dermatol. 2021;184:1014-1022.
- Blume-Peytavi U, Vieten J, Knuttel A et al. Optical coherent tomography (OCT): a new method for online-measurement of hair shaft thickness. J Dtsch Dermatol Ges. 2004;2:546.
- Garcia Bartels N, Jahnke I, Patzelt A, et al. Hair shaft abnormalities in alopecia areata evaluated by optical coherence tomography. Skin Res Technol. 2011;17:201-205.
- Urban G, Feil N, Csuka E, et al. Combining deep learning with optical coherence tomography imaging to determine scalp hair and follicle counts. Lasers Surg Med. 2021;53:171-178.
- Vazquez-Herrera NE, Eber AE, Martinez-Velasco MA, et al. Optical coherence tomography for the investigation of frontal fibrosing alopecia. J Eur Acad Dermatol Venereol. 2018;32:318-322.
- Ekelem C, Feil N, Csuka E, et al. Optical coherence tomography in the evaluation of the scalp and hair: common features and clinical utility. Lasers Surg Med. 2021;53:129-140.
- Schicho K, Seemann R, Binder M, et al. Optical coherence tomography for planning of follicular unit extraction. Dermatol Surg. 2015;41:358-363.
- Canfield D. Photographic documentation of hair growth in androgenetic alopecia. Dermatol Clin. 1996;14:713-721.
- Peytavi U, Hillmann K, Guarrera M. Hair growth assessment techniques. In: Peytavi U, Hillmann K, Guarrera M, eds. Hair Growth and Disorders. 4th ed. Springer; 2008:140-144.
- Chamberlain AJ, Dawber RP. Methods of evaluating hair growth. Australas J Dermatol. 2003;44:10-18.
- Dhurat R, Saraogi P. Hair evaluation methods: merits and demerits. Int J Trichology. 2009;1:108-119.
- Kaufman KD, Olsen EA, Whiting D, et al. Finasteride in the treatment of men with androgenetic alopecia. J Am Acad Dermatol. 1998;39:578-579.
- Capily Institute. Artificial intelligence (A.I.) powered hair growth tracking. Accessed July 31, 2023. https://tss-aesthetics.com/capily-hair-tracking-syst
- Dinh Q, Sinclair R. Female pattern hair loss: current treatment concepts. Clin Interv Aging. 2007;2:189-199.
- Dhurat R, Saraogi P. Hair evaluation methods: merits and demerits. Int J Trichology. 2009;1:108-119.
- Wikramanayake TC, Mauro LM, Tabas IA, et al. Cross-section trichometry: a clinical tool for assessing the progression and treatment response of alopecia. Int J Trichology. 2012;4:259-264.
- Alessandrini A, Bruni F, Piraccini BM, et al. Common causes of hair loss—clinical manifestations, trichoscopy and therapy. J Eur Acad Dermatol Venereol. 2021;35:629-640.
- Ashique K, Kaliyadan F. Clinical photography for trichology practice: tips and tricks. Int J Trichology. 2011;3:7-13.
- Rudnicka L, Olszewska M, Rakowska A, et al. Trichoscopy: a new method for diagnosing hair loss. J Drugs Dermatol. 2008;7:651-654.
- Kinoshita-Ise M, Sachdeva M. Update on trichoscopy: integration of the terminology by systematic approach and a proposal of a diagnostic flowchart. J Dermatol. 2022;49:4-18. doi:10.1111/1346-8138.16233
- Van Neste D, Trüeb RM. Critical study of hair growth analysis with computer-assisted methods. J Eur Acad Dermatol Venereol. 2006;20:578-583.
- Romero J, Grimalt R. Trichoscopy: essentials for the dermatologist. World J Dermatol. 2015;4:63-68.
- Trichoscopy: a new frontier for the diagnosis of hair diseases. Exp Rev Dermatol. 2012;7:429-437.
- Lee B, Chan J, Monselise A, et al. Assessment of hair density and caliber in Caucasian and Asian female subjects with female pattern hair loss by using the Folliscope. J Am Acad Dermatol. 2012;66:166-167.
- Inui S. Trichoscopy for common hair loss diseases: algorithmic method for diagnosis. J Dermatol. 2010;38:71-75.
- Dhurat R. Phototrichogram. Indian J Dermatol Venereol Leprol. 2006;72:242-244.
- Agozzino M, Tosti A, Barbieri L, et al. Confocal microscopic features of scarring alopecia: preliminary report. Br J Dermatol. 2011;165:534-540.
- Kuck M, Schanzer S, Ulrich M, et al. Analysis of the efficiency of hair removal by different optical methods: comparison of Trichoscan, reflectance confocal microscopy, and optical coherence tomography. J Biomed Opt. 2012;17:101504.
- Levine A, Markowitz O. Introduction to reflectance confocal microscopy and its use in clinical practice. JAAD Case Rep. 2018;4:1014-1023.
- Agozzino M, Ardigò M. Scalp confocal microscopy. In: Humbert P, Maibach H, Fanian F, et al, eds. Agache’s Measuring the Skin: Non-invasive Investigations, Physiology, Normal Constants. 2nd ed. Springer International Publishing; 2016:311-326.
- Rudnicka L, Olszewska M, Rakowska A. In vivo reflectance confocal microscopy: usefulness for diagnosing hair diseases. J Dermatol Case Rep. 2008;2:55-59.
- Kurzeja M, Czuwara J, Walecka I, et al. Features of classic lichen planopilaris and frontal fibrosing alopecia in reflectance confocal microscopy: a preliminary study. Skin Res Technol. 2021;27:266-271.
- Ardigò M, Agozzino M, Franceschini C, et al. Reflectance confocal microscopy for scarring and non-scarring alopecia real-time assessment. Arch Dermatol Res. 2016;308:309-318.
- Franceschini C, Garelli V, Persechino F, et al. Dermoscopy and confocal microscopy for different chemotherapy-induced alopecia (CIA) phases characterization: preliminary study. Skin Res Technol. 2020;26:269-276.
- Martinez-Velasco MA, Perper M, Maddy AJ, et al. In vitro determination of Mexican Mestizo hair shaft diameter using optical coherence tomography. Skin Res Technol. 2018;24;274-277.
- Srivastava R, Manfredini M, Rao BK. Noninvasive imaging tools in dermatology. Cutis. 2019;104:108-113.
- Wan B, Ganier C, Du-Harpur X, et al. Applications and future directions for optical coherence tomography in dermatology. Br J Dermatol. 2021;184:1014-1022.
- Blume-Peytavi U, Vieten J, Knuttel A et al. Optical coherent tomography (OCT): a new method for online-measurement of hair shaft thickness. J Dtsch Dermatol Ges. 2004;2:546.
- Garcia Bartels N, Jahnke I, Patzelt A, et al. Hair shaft abnormalities in alopecia areata evaluated by optical coherence tomography. Skin Res Technol. 2011;17:201-205.
- Urban G, Feil N, Csuka E, et al. Combining deep learning with optical coherence tomography imaging to determine scalp hair and follicle counts. Lasers Surg Med. 2021;53:171-178.
- Vazquez-Herrera NE, Eber AE, Martinez-Velasco MA, et al. Optical coherence tomography for the investigation of frontal fibrosing alopecia. J Eur Acad Dermatol Venereol. 2018;32:318-322.
- Ekelem C, Feil N, Csuka E, et al. Optical coherence tomography in the evaluation of the scalp and hair: common features and clinical utility. Lasers Surg Med. 2021;53:129-140.
- Schicho K, Seemann R, Binder M, et al. Optical coherence tomography for planning of follicular unit extraction. Dermatol Surg. 2015;41:358-363.
Practice Points
- Reflectance confocal microscopy (RCM) imaging can be taken at levels from the stratum corneum to the papillary dermis and can be used to study scalp discoid lupus, lichen planopilaris, frontal fibrosing alopecia, alopecia areata, and androgenetic alopecia.
- Because of its ability to distinguish different stages of disease, RCM can be recommended as an intermediate step between trichoscopy and histology for the diagnosis and management of hair disease.
- Optical coherence tomography has the potential to monitor early subclinical responses to alopecia therapies while also improving hair transplantation outcomes by allowing for visualization of the subcutaneous angle of hair follicles.
- Software development paired with trichoscopy has the ability to quantify hair growth parameters such as hair count, density, and diameter.
Long COVID disability court battles just ‘tip of iceberg’
At least 30 lawsuits have been filed seeking legal resolution of disability insurance claims, according to searches of court records. In addition, the Social Security Administration said it has received about 52,000 disability claims tied to SARS-CoV-2 infections, which represents 1% of all applications.
But legal experts say those cases may not reflect the total number of cases that have gone to court. They note many claims are initially dismissed and are not appealed by claimants.
“With this system, they deny two-thirds of initial applications, then people who appeal get denied almost 90% of the time, and then they can appeal before a judge,” said Kevin LaPorte, a Social Security disability attorney at LaPorte Law Firm in Oakland, Calif. “What happens next doesn’t have a lot of precedent because long COVID is a mass disabling event, and we haven’t seen that many of these cases get all the way through the legal system yet.”
As a result, the exact number of long COVID disability claims and the number of these cases going to court isn’t clear, he said.
“It can take a year or more for cases to get to court, and even longer to reach resolution,” Mr. LaPorte added. “I suspect the few cases we’ve heard about at this point are going to be the tip of the iceberg.”
The process is convoluted and can drag on for months with multiple denials and appeals along the way. Many disabled workers find their only recourse is to take insurers to court.
Long COVID patients typically apply for disability benefits through private insurance or Social Security. But the process can drag on for months, so many find their only recourse is to take insurers to court, according to legal experts.
But even in the courts, many encounter delays and hurdles to resolution.
In one of the first federal lawsuits involving long COVID disability benefits, William Abrams, a trial and appellate attorney and active marathon runner, sued Unum Life Insurance seeking long-term disability income. Symptoms included extreme fatigue, brain fog, decreased attention and concentration, and nearly daily fevers, causing him to stop working in April 2020.
His diagnosis wasn’t definitive. Three doctors said he had long COVID, and four said he had chronic fatigue syndrome. Unum cited this inconsistency as a rationale for rejecting his claim. But the court sided with Mr. Abrams, granting him disability income. The court concluded: “Unum may be correct that [the plaintiff] has not been correctly diagnosed. But that does not mean he is not sick. If [the plaintiff’s] complaints, and [the doctor’s] assessments, are to be believed, [the plaintiff] cannot focus for more than a few minutes at a time, making it impossible for [the plaintiff] to perform the varied and complex tasks his job requires.”
Unum said in an emailed statement that the company doesn’t comment on specific claims as a matter of policy, adding that its total payouts for disability claims from March 2020 to February 2022 were 35% higher than prepandemic levels. “In general, disability and leave claims connected to COVID-19 have been primarily short-term events with the majority of claimants recovering prior to completing the normal qualification period for long-term disability insurance,” Unum said.
Mr. Abrams prevailed in part because he had detailed documentation of the numerous impairments that eventually required him to stop work, said Michelle Roberts of Roberts Disability Law in Oakland, Calif.
He submitted videos of himself taking his temperature to prove he had almost daily fevers, according to court records. He underwent neuropsychological testing, which found learning deficiencies and memory deficits.
Mr. Abrams also submitted statements from a colleague who worked with him on a complex technology patent case involving radiofrequency identification. Before he got COVID, Mr. Abrams “had the analytical ability, legal acumen, and mental energy to attack that learning curve and get up to speed very rapidly,” according to court records.
“The court focused on credulity.” Ms. Roberts said. “There was all this work to be done to show this person was high functioning and ran marathons and worked in an intense, high-pressure occupation but then couldn’t do anything after long COVID.”
Documentation was also crucial in another early federal long COVID disability lawsuit that was filed in 2022 on behalf of Wendy Haut, an educational software sales representative in California who turned to the courts seeking disability income through her company’s employee benefits plan.
Several of Ms. Haut’s doctors documented a detailed list of long COVID symptoms, including “profound fatigue and extreme cognitive difficulties,” that they said prevented her from working as a sales representative or doing any other type of job. A settlement agreement in June 2022 required Reliance Standard Life Insurance to pay Ms. Haut long-term disability benefits, including previously unpaid benefits, according to a report by the advocacy group Pandemic Patients.
Representatives of Reliance Standard didn’t respond to a request for comment.
The growing number of workers being sidelined by long COVID makes more claims and more court cases likely. Right now, an estimated 16 million working-age Americans aged 18-65 years have long COVID, and as many as 4 million of them can’t work, according to a July 2023 Census Bureau report.
Uncertainty about the volume of claims in the pipeline is part of what’s driving some insurers to fight long COVID claims, Ms. Roberts said. Another factor is the lack of clarity around how many years people with long COVID may be out of work, particularly if they’re in their 30s or 40s and might be seeking disability income until they reach retirement age.
“Doctors are not always saying that this person will be permanently disabled,” Ms. Roberts said. “If this person doesn’t get better and they’re disabled until retirement age, this could be a payout in the high six or seven figures if a person is very young and was a very high earner.”
Insurance companies routinely deny claims that can’t be backed up with objective measures, such as specific lab test results or clear findings from a physical exam. But there are steps that can increase the odds of a successful claim for long COVID disability benefits, according to New York–based law firm Hiller.
For starters, patients can document COVID test results, and if testing wasn’t conducted, patients can detail the specific symptoms that led to this diagnosis, Hiller advises. Then patients can keep a daily symptom log at home that run lists all of the specific symptoms that occur at different times during the day and night to help establish a pattern of disability. These logs should provide specific details about every job duty patients have and exactly how specific symptoms of long COVID interfere with these duties.
Even though objective testing is hard to come by for long COVID, people should undergo all the tests they can that may help document the frequency or severity of specific symptoms that make it impossible to carry on with business as usual at work, Hiller advises. This may include neuropsychological testing to document brain fog, a cardiopulmonary exercise test to demonstrate chronic fatigue and the inability to exercise, or a tilt table test to measure dizziness.
Seeking a doctor’s diagnosis can be key to collecting disability payments, in or out of court.
All of this puts a lot of pressure on doctors and patients to build strong cases, said Jonathan Whiteson, MD, codirector of the NYU Langone Health post-COVID care program in New York. “Many physicians are not familiar with the disability benefit paperwork, and so this is a challenge for the doctors to know how to complete and to build the time into their highly scheduled days to take the time needed to complete.
“It’s also challenging because most of the disability benefit forms are ‘generic’ and do not ask specific questions about COVID disability,” Dr. Whiteson added. “It can be like trying to drive a square peg into a round hole.”
Still, when it comes to long COVID, completing disability paperwork is increasingly becoming part of standard care, along with managing medication, rehabilitation therapies, and lifestyle changes to navigate daily life with this illness, Dr. Whiteson noted.
Monica Verduzco-Gutierrez, MD, chair of rehabilitation medicine and director of the Post-COVID-19 Recovery Clinic at the University of Texas Health Science Center, San Antonio, agreed with this assessment.
“I have done letter upon letter of appeal to disability insurance companies,” she said.
Some doctors, however, are reluctant to step up in such cases, in part because no standard diagnostic guidelines exist for long COVID and because it can be frustrating.
“This is the work that is not paid and causes burnout in physicians,” Dr. Verduzco-Gutierrez said. “The paperwork, the fighting with insurance companies, the resubmission of forms for disability all to get what your patient needs – and then it gets denied.
“We will keep doing this because our patients need this disability income in order to live their lives and to afford what they need for recovery,” said Dr. Verduzco-Gutierrez. “But at some point something has to change because this isn’t sustainable.”
A version of this article appeared on Medscape.com.
At least 30 lawsuits have been filed seeking legal resolution of disability insurance claims, according to searches of court records. In addition, the Social Security Administration said it has received about 52,000 disability claims tied to SARS-CoV-2 infections, which represents 1% of all applications.
But legal experts say those cases may not reflect the total number of cases that have gone to court. They note many claims are initially dismissed and are not appealed by claimants.
“With this system, they deny two-thirds of initial applications, then people who appeal get denied almost 90% of the time, and then they can appeal before a judge,” said Kevin LaPorte, a Social Security disability attorney at LaPorte Law Firm in Oakland, Calif. “What happens next doesn’t have a lot of precedent because long COVID is a mass disabling event, and we haven’t seen that many of these cases get all the way through the legal system yet.”
As a result, the exact number of long COVID disability claims and the number of these cases going to court isn’t clear, he said.
“It can take a year or more for cases to get to court, and even longer to reach resolution,” Mr. LaPorte added. “I suspect the few cases we’ve heard about at this point are going to be the tip of the iceberg.”
The process is convoluted and can drag on for months with multiple denials and appeals along the way. Many disabled workers find their only recourse is to take insurers to court.
Long COVID patients typically apply for disability benefits through private insurance or Social Security. But the process can drag on for months, so many find their only recourse is to take insurers to court, according to legal experts.
But even in the courts, many encounter delays and hurdles to resolution.
In one of the first federal lawsuits involving long COVID disability benefits, William Abrams, a trial and appellate attorney and active marathon runner, sued Unum Life Insurance seeking long-term disability income. Symptoms included extreme fatigue, brain fog, decreased attention and concentration, and nearly daily fevers, causing him to stop working in April 2020.
His diagnosis wasn’t definitive. Three doctors said he had long COVID, and four said he had chronic fatigue syndrome. Unum cited this inconsistency as a rationale for rejecting his claim. But the court sided with Mr. Abrams, granting him disability income. The court concluded: “Unum may be correct that [the plaintiff] has not been correctly diagnosed. But that does not mean he is not sick. If [the plaintiff’s] complaints, and [the doctor’s] assessments, are to be believed, [the plaintiff] cannot focus for more than a few minutes at a time, making it impossible for [the plaintiff] to perform the varied and complex tasks his job requires.”
Unum said in an emailed statement that the company doesn’t comment on specific claims as a matter of policy, adding that its total payouts for disability claims from March 2020 to February 2022 were 35% higher than prepandemic levels. “In general, disability and leave claims connected to COVID-19 have been primarily short-term events with the majority of claimants recovering prior to completing the normal qualification period for long-term disability insurance,” Unum said.
Mr. Abrams prevailed in part because he had detailed documentation of the numerous impairments that eventually required him to stop work, said Michelle Roberts of Roberts Disability Law in Oakland, Calif.
He submitted videos of himself taking his temperature to prove he had almost daily fevers, according to court records. He underwent neuropsychological testing, which found learning deficiencies and memory deficits.
Mr. Abrams also submitted statements from a colleague who worked with him on a complex technology patent case involving radiofrequency identification. Before he got COVID, Mr. Abrams “had the analytical ability, legal acumen, and mental energy to attack that learning curve and get up to speed very rapidly,” according to court records.
“The court focused on credulity.” Ms. Roberts said. “There was all this work to be done to show this person was high functioning and ran marathons and worked in an intense, high-pressure occupation but then couldn’t do anything after long COVID.”
Documentation was also crucial in another early federal long COVID disability lawsuit that was filed in 2022 on behalf of Wendy Haut, an educational software sales representative in California who turned to the courts seeking disability income through her company’s employee benefits plan.
Several of Ms. Haut’s doctors documented a detailed list of long COVID symptoms, including “profound fatigue and extreme cognitive difficulties,” that they said prevented her from working as a sales representative or doing any other type of job. A settlement agreement in June 2022 required Reliance Standard Life Insurance to pay Ms. Haut long-term disability benefits, including previously unpaid benefits, according to a report by the advocacy group Pandemic Patients.
Representatives of Reliance Standard didn’t respond to a request for comment.
The growing number of workers being sidelined by long COVID makes more claims and more court cases likely. Right now, an estimated 16 million working-age Americans aged 18-65 years have long COVID, and as many as 4 million of them can’t work, according to a July 2023 Census Bureau report.
Uncertainty about the volume of claims in the pipeline is part of what’s driving some insurers to fight long COVID claims, Ms. Roberts said. Another factor is the lack of clarity around how many years people with long COVID may be out of work, particularly if they’re in their 30s or 40s and might be seeking disability income until they reach retirement age.
“Doctors are not always saying that this person will be permanently disabled,” Ms. Roberts said. “If this person doesn’t get better and they’re disabled until retirement age, this could be a payout in the high six or seven figures if a person is very young and was a very high earner.”
Insurance companies routinely deny claims that can’t be backed up with objective measures, such as specific lab test results or clear findings from a physical exam. But there are steps that can increase the odds of a successful claim for long COVID disability benefits, according to New York–based law firm Hiller.
For starters, patients can document COVID test results, and if testing wasn’t conducted, patients can detail the specific symptoms that led to this diagnosis, Hiller advises. Then patients can keep a daily symptom log at home that run lists all of the specific symptoms that occur at different times during the day and night to help establish a pattern of disability. These logs should provide specific details about every job duty patients have and exactly how specific symptoms of long COVID interfere with these duties.
Even though objective testing is hard to come by for long COVID, people should undergo all the tests they can that may help document the frequency or severity of specific symptoms that make it impossible to carry on with business as usual at work, Hiller advises. This may include neuropsychological testing to document brain fog, a cardiopulmonary exercise test to demonstrate chronic fatigue and the inability to exercise, or a tilt table test to measure dizziness.
Seeking a doctor’s diagnosis can be key to collecting disability payments, in or out of court.
All of this puts a lot of pressure on doctors and patients to build strong cases, said Jonathan Whiteson, MD, codirector of the NYU Langone Health post-COVID care program in New York. “Many physicians are not familiar with the disability benefit paperwork, and so this is a challenge for the doctors to know how to complete and to build the time into their highly scheduled days to take the time needed to complete.
“It’s also challenging because most of the disability benefit forms are ‘generic’ and do not ask specific questions about COVID disability,” Dr. Whiteson added. “It can be like trying to drive a square peg into a round hole.”
Still, when it comes to long COVID, completing disability paperwork is increasingly becoming part of standard care, along with managing medication, rehabilitation therapies, and lifestyle changes to navigate daily life with this illness, Dr. Whiteson noted.
Monica Verduzco-Gutierrez, MD, chair of rehabilitation medicine and director of the Post-COVID-19 Recovery Clinic at the University of Texas Health Science Center, San Antonio, agreed with this assessment.
“I have done letter upon letter of appeal to disability insurance companies,” she said.
Some doctors, however, are reluctant to step up in such cases, in part because no standard diagnostic guidelines exist for long COVID and because it can be frustrating.
“This is the work that is not paid and causes burnout in physicians,” Dr. Verduzco-Gutierrez said. “The paperwork, the fighting with insurance companies, the resubmission of forms for disability all to get what your patient needs – and then it gets denied.
“We will keep doing this because our patients need this disability income in order to live their lives and to afford what they need for recovery,” said Dr. Verduzco-Gutierrez. “But at some point something has to change because this isn’t sustainable.”
A version of this article appeared on Medscape.com.
At least 30 lawsuits have been filed seeking legal resolution of disability insurance claims, according to searches of court records. In addition, the Social Security Administration said it has received about 52,000 disability claims tied to SARS-CoV-2 infections, which represents 1% of all applications.
But legal experts say those cases may not reflect the total number of cases that have gone to court. They note many claims are initially dismissed and are not appealed by claimants.
“With this system, they deny two-thirds of initial applications, then people who appeal get denied almost 90% of the time, and then they can appeal before a judge,” said Kevin LaPorte, a Social Security disability attorney at LaPorte Law Firm in Oakland, Calif. “What happens next doesn’t have a lot of precedent because long COVID is a mass disabling event, and we haven’t seen that many of these cases get all the way through the legal system yet.”
As a result, the exact number of long COVID disability claims and the number of these cases going to court isn’t clear, he said.
“It can take a year or more for cases to get to court, and even longer to reach resolution,” Mr. LaPorte added. “I suspect the few cases we’ve heard about at this point are going to be the tip of the iceberg.”
The process is convoluted and can drag on for months with multiple denials and appeals along the way. Many disabled workers find their only recourse is to take insurers to court.
Long COVID patients typically apply for disability benefits through private insurance or Social Security. But the process can drag on for months, so many find their only recourse is to take insurers to court, according to legal experts.
But even in the courts, many encounter delays and hurdles to resolution.
In one of the first federal lawsuits involving long COVID disability benefits, William Abrams, a trial and appellate attorney and active marathon runner, sued Unum Life Insurance seeking long-term disability income. Symptoms included extreme fatigue, brain fog, decreased attention and concentration, and nearly daily fevers, causing him to stop working in April 2020.
His diagnosis wasn’t definitive. Three doctors said he had long COVID, and four said he had chronic fatigue syndrome. Unum cited this inconsistency as a rationale for rejecting his claim. But the court sided with Mr. Abrams, granting him disability income. The court concluded: “Unum may be correct that [the plaintiff] has not been correctly diagnosed. But that does not mean he is not sick. If [the plaintiff’s] complaints, and [the doctor’s] assessments, are to be believed, [the plaintiff] cannot focus for more than a few minutes at a time, making it impossible for [the plaintiff] to perform the varied and complex tasks his job requires.”
Unum said in an emailed statement that the company doesn’t comment on specific claims as a matter of policy, adding that its total payouts for disability claims from March 2020 to February 2022 were 35% higher than prepandemic levels. “In general, disability and leave claims connected to COVID-19 have been primarily short-term events with the majority of claimants recovering prior to completing the normal qualification period for long-term disability insurance,” Unum said.
Mr. Abrams prevailed in part because he had detailed documentation of the numerous impairments that eventually required him to stop work, said Michelle Roberts of Roberts Disability Law in Oakland, Calif.
He submitted videos of himself taking his temperature to prove he had almost daily fevers, according to court records. He underwent neuropsychological testing, which found learning deficiencies and memory deficits.
Mr. Abrams also submitted statements from a colleague who worked with him on a complex technology patent case involving radiofrequency identification. Before he got COVID, Mr. Abrams “had the analytical ability, legal acumen, and mental energy to attack that learning curve and get up to speed very rapidly,” according to court records.
“The court focused on credulity.” Ms. Roberts said. “There was all this work to be done to show this person was high functioning and ran marathons and worked in an intense, high-pressure occupation but then couldn’t do anything after long COVID.”
Documentation was also crucial in another early federal long COVID disability lawsuit that was filed in 2022 on behalf of Wendy Haut, an educational software sales representative in California who turned to the courts seeking disability income through her company’s employee benefits plan.
Several of Ms. Haut’s doctors documented a detailed list of long COVID symptoms, including “profound fatigue and extreme cognitive difficulties,” that they said prevented her from working as a sales representative or doing any other type of job. A settlement agreement in June 2022 required Reliance Standard Life Insurance to pay Ms. Haut long-term disability benefits, including previously unpaid benefits, according to a report by the advocacy group Pandemic Patients.
Representatives of Reliance Standard didn’t respond to a request for comment.
The growing number of workers being sidelined by long COVID makes more claims and more court cases likely. Right now, an estimated 16 million working-age Americans aged 18-65 years have long COVID, and as many as 4 million of them can’t work, according to a July 2023 Census Bureau report.
Uncertainty about the volume of claims in the pipeline is part of what’s driving some insurers to fight long COVID claims, Ms. Roberts said. Another factor is the lack of clarity around how many years people with long COVID may be out of work, particularly if they’re in their 30s or 40s and might be seeking disability income until they reach retirement age.
“Doctors are not always saying that this person will be permanently disabled,” Ms. Roberts said. “If this person doesn’t get better and they’re disabled until retirement age, this could be a payout in the high six or seven figures if a person is very young and was a very high earner.”
Insurance companies routinely deny claims that can’t be backed up with objective measures, such as specific lab test results or clear findings from a physical exam. But there are steps that can increase the odds of a successful claim for long COVID disability benefits, according to New York–based law firm Hiller.
For starters, patients can document COVID test results, and if testing wasn’t conducted, patients can detail the specific symptoms that led to this diagnosis, Hiller advises. Then patients can keep a daily symptom log at home that run lists all of the specific symptoms that occur at different times during the day and night to help establish a pattern of disability. These logs should provide specific details about every job duty patients have and exactly how specific symptoms of long COVID interfere with these duties.
Even though objective testing is hard to come by for long COVID, people should undergo all the tests they can that may help document the frequency or severity of specific symptoms that make it impossible to carry on with business as usual at work, Hiller advises. This may include neuropsychological testing to document brain fog, a cardiopulmonary exercise test to demonstrate chronic fatigue and the inability to exercise, or a tilt table test to measure dizziness.
Seeking a doctor’s diagnosis can be key to collecting disability payments, in or out of court.
All of this puts a lot of pressure on doctors and patients to build strong cases, said Jonathan Whiteson, MD, codirector of the NYU Langone Health post-COVID care program in New York. “Many physicians are not familiar with the disability benefit paperwork, and so this is a challenge for the doctors to know how to complete and to build the time into their highly scheduled days to take the time needed to complete.
“It’s also challenging because most of the disability benefit forms are ‘generic’ and do not ask specific questions about COVID disability,” Dr. Whiteson added. “It can be like trying to drive a square peg into a round hole.”
Still, when it comes to long COVID, completing disability paperwork is increasingly becoming part of standard care, along with managing medication, rehabilitation therapies, and lifestyle changes to navigate daily life with this illness, Dr. Whiteson noted.
Monica Verduzco-Gutierrez, MD, chair of rehabilitation medicine and director of the Post-COVID-19 Recovery Clinic at the University of Texas Health Science Center, San Antonio, agreed with this assessment.
“I have done letter upon letter of appeal to disability insurance companies,” she said.
Some doctors, however, are reluctant to step up in such cases, in part because no standard diagnostic guidelines exist for long COVID and because it can be frustrating.
“This is the work that is not paid and causes burnout in physicians,” Dr. Verduzco-Gutierrez said. “The paperwork, the fighting with insurance companies, the resubmission of forms for disability all to get what your patient needs – and then it gets denied.
“We will keep doing this because our patients need this disability income in order to live their lives and to afford what they need for recovery,” said Dr. Verduzco-Gutierrez. “But at some point something has to change because this isn’t sustainable.”
A version of this article appeared on Medscape.com.