User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Acne Vulgaris
THE COMPARISON
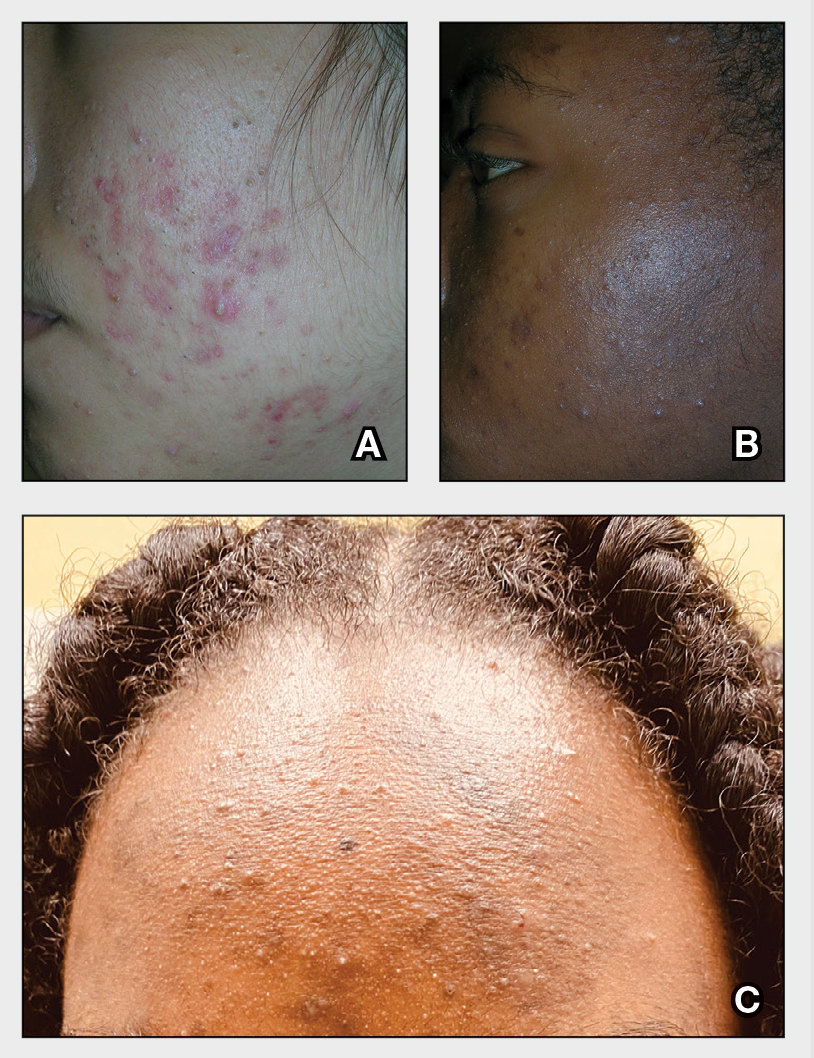
A A 27-year-old Hispanic woman with comedonal and inflammatory acne. Erythema is prominent around the inflammatory lesions. Note the pustule on the cheek surrounded by pink color.
B A teenaged Black boy with acne papules and pustules on the face. There are comedones, hyperpigmented macules, and pustules on the cheek.
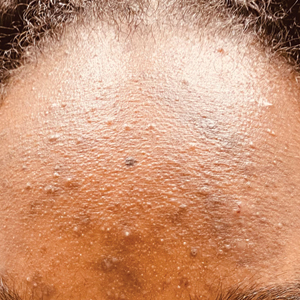
C A teenaged Black girl with pomade acne. The patient used various hair care products, which obstructed the pilosebaceous units on the forehead.
Epidemiology
Acne is a leading dermatologic condition in individuals with skin of color in the United States.1
Key clinical features in people with darker skin tones include:
- erythematous or hyperpigmented papules or comedones
- hyperpigmented macules and postinflammatory hyperpigmentation (PIH)
- increased risk for keloidal scars.2
Worth noting
- Patients with darker skin tones may be more concerned with the dark marks (also referred to as scars or manchas in Spanish) than the acne itself. This PIH may be viewed by patients as the major problem.
- Acne medications such as azelaic acid and some retinoids (when applied appropriately) can treat both acne and PIH.3
- Irritation from topical acne medications, including retinoid dermatitis, may lead to more PIH. Using noncomedogenic moisturizers and applying medication appropriately (ie, a pea-sized amount of topical retinoid per application) may help limit irritation.4,5
- One type of acne seen more commonly, although not exclusively, in Black patients is pomade acne, which principally appears on the forehead and is associated with use of hair care and styling products (Figure, C).
Health disparity highlight
Disparities in access to health care exist for those with dermatologic concerns. According to one study, African American (28.5%) and Hispanic patients (23.9%) were less likely to be seen by a dermatologist solely for the diagnosis of a dermatologic condition compared to Asian and Pacific Islander patients (36.7%) or White patients (43.2%).1
Noting that isotretinoin is the most potent systemic therapy for severe cystic acne vulgaris, Bell et al6 reported that Black patients had lower odds of receiving isotretinoin compared to White patients. Hispanic patients had lower odds of receiving a topical retinoid, tretinoin, than non-Hispanic patients.6
- Davis SA, Narahari S, Feldman SR, et al. Top dermatologic conditions in patients of color: an analysis of nationally representative data. J Drugs Dermatol. 2012;11:466-473.
- Alexis AF, Woolery-Lloyd H, Williams K, et al. Racial/ethnic variations in acne: implications for treatment and skin care recommendations for acne patients with skin of color. J Drugs Dermatol. 2021;20:716-725.
- Woolery-Lloyd HC, Keri J, Doig S. Retinoids and azelaic acid to treat acne and hyperpigmentation in skin of color. J Drugs Dermatol. 2013;12:434-437.
- Grayson C, Heath C. Tips for addressing common conditions affecting pediatric and adolescent patients with skin of color [published online March 2, 2021]. Pediatr Dermatol. doi:10.1111/pde.14525
- Alexis AD, Harper JC, Stein Gold L, et al. Treating acne in patients with skin of color. Semin Cutan Med Surg. 2018;37(suppl 3):S71-S73.
- Bell MA, Whang KA, Thomas J, et al. Racial and ethnic disparities in access to emerging and frontline therapies in common dermatological conditions: a cross-sectional study. J Natl Med Assoc. 2020;112:650-653.

THE COMPARISON
A A 27-year-old Hispanic woman with comedonal and inflammatory acne. Erythema is prominent around the inflammatory lesions. Note the pustule on the cheek surrounded by pink color.
B A teenaged Black boy with acne papules and pustules on the face. There are comedones, hyperpigmented macules, and pustules on the cheek.
C A teenaged Black girl with pomade acne. The patient used various hair care products, which obstructed the pilosebaceous units on the forehead.
Epidemiology
Acne is a leading dermatologic condition in individuals with skin of color in the United States.1
Key clinical features in people with darker skin tones include:
- erythematous or hyperpigmented papules or comedones
- hyperpigmented macules and postinflammatory hyperpigmentation (PIH)
- increased risk for keloidal scars.2
Worth noting
- Patients with darker skin tones may be more concerned with the dark marks (also referred to as scars or manchas in Spanish) than the acne itself. This PIH may be viewed by patients as the major problem.
- Acne medications such as azelaic acid and some retinoids (when applied appropriately) can treat both acne and PIH.3
- Irritation from topical acne medications, including retinoid dermatitis, may lead to more PIH. Using noncomedogenic moisturizers and applying medication appropriately (ie, a pea-sized amount of topical retinoid per application) may help limit irritation.4,5
- One type of acne seen more commonly, although not exclusively, in Black patients is pomade acne, which principally appears on the forehead and is associated with use of hair care and styling products (Figure, C).
Health disparity highlight
Disparities in access to health care exist for those with dermatologic concerns. According to one study, African American (28.5%) and Hispanic patients (23.9%) were less likely to be seen by a dermatologist solely for the diagnosis of a dermatologic condition compared to Asian and Pacific Islander patients (36.7%) or White patients (43.2%).1
Noting that isotretinoin is the most potent systemic therapy for severe cystic acne vulgaris, Bell et al6 reported that Black patients had lower odds of receiving isotretinoin compared to White patients. Hispanic patients had lower odds of receiving a topical retinoid, tretinoin, than non-Hispanic patients.6

THE COMPARISON
A A 27-year-old Hispanic woman with comedonal and inflammatory acne. Erythema is prominent around the inflammatory lesions. Note the pustule on the cheek surrounded by pink color.
B A teenaged Black boy with acne papules and pustules on the face. There are comedones, hyperpigmented macules, and pustules on the cheek.
C A teenaged Black girl with pomade acne. The patient used various hair care products, which obstructed the pilosebaceous units on the forehead.
Epidemiology
Acne is a leading dermatologic condition in individuals with skin of color in the United States.1
Key clinical features in people with darker skin tones include:
- erythematous or hyperpigmented papules or comedones
- hyperpigmented macules and postinflammatory hyperpigmentation (PIH)
- increased risk for keloidal scars.2
Worth noting
- Patients with darker skin tones may be more concerned with the dark marks (also referred to as scars or manchas in Spanish) than the acne itself. This PIH may be viewed by patients as the major problem.
- Acne medications such as azelaic acid and some retinoids (when applied appropriately) can treat both acne and PIH.3
- Irritation from topical acne medications, including retinoid dermatitis, may lead to more PIH. Using noncomedogenic moisturizers and applying medication appropriately (ie, a pea-sized amount of topical retinoid per application) may help limit irritation.4,5
- One type of acne seen more commonly, although not exclusively, in Black patients is pomade acne, which principally appears on the forehead and is associated with use of hair care and styling products (Figure, C).
Health disparity highlight
Disparities in access to health care exist for those with dermatologic concerns. According to one study, African American (28.5%) and Hispanic patients (23.9%) were less likely to be seen by a dermatologist solely for the diagnosis of a dermatologic condition compared to Asian and Pacific Islander patients (36.7%) or White patients (43.2%).1
Noting that isotretinoin is the most potent systemic therapy for severe cystic acne vulgaris, Bell et al6 reported that Black patients had lower odds of receiving isotretinoin compared to White patients. Hispanic patients had lower odds of receiving a topical retinoid, tretinoin, than non-Hispanic patients.6
- Davis SA, Narahari S, Feldman SR, et al. Top dermatologic conditions in patients of color: an analysis of nationally representative data. J Drugs Dermatol. 2012;11:466-473.
- Alexis AF, Woolery-Lloyd H, Williams K, et al. Racial/ethnic variations in acne: implications for treatment and skin care recommendations for acne patients with skin of color. J Drugs Dermatol. 2021;20:716-725.
- Woolery-Lloyd HC, Keri J, Doig S. Retinoids and azelaic acid to treat acne and hyperpigmentation in skin of color. J Drugs Dermatol. 2013;12:434-437.
- Grayson C, Heath C. Tips for addressing common conditions affecting pediatric and adolescent patients with skin of color [published online March 2, 2021]. Pediatr Dermatol. doi:10.1111/pde.14525
- Alexis AD, Harper JC, Stein Gold L, et al. Treating acne in patients with skin of color. Semin Cutan Med Surg. 2018;37(suppl 3):S71-S73.
- Bell MA, Whang KA, Thomas J, et al. Racial and ethnic disparities in access to emerging and frontline therapies in common dermatological conditions: a cross-sectional study. J Natl Med Assoc. 2020;112:650-653.
- Davis SA, Narahari S, Feldman SR, et al. Top dermatologic conditions in patients of color: an analysis of nationally representative data. J Drugs Dermatol. 2012;11:466-473.
- Alexis AF, Woolery-Lloyd H, Williams K, et al. Racial/ethnic variations in acne: implications for treatment and skin care recommendations for acne patients with skin of color. J Drugs Dermatol. 2021;20:716-725.
- Woolery-Lloyd HC, Keri J, Doig S. Retinoids and azelaic acid to treat acne and hyperpigmentation in skin of color. J Drugs Dermatol. 2013;12:434-437.
- Grayson C, Heath C. Tips for addressing common conditions affecting pediatric and adolescent patients with skin of color [published online March 2, 2021]. Pediatr Dermatol. doi:10.1111/pde.14525
- Alexis AD, Harper JC, Stein Gold L, et al. Treating acne in patients with skin of color. Semin Cutan Med Surg. 2018;37(suppl 3):S71-S73.
- Bell MA, Whang KA, Thomas J, et al. Racial and ethnic disparities in access to emerging and frontline therapies in common dermatological conditions: a cross-sectional study. J Natl Med Assoc. 2020;112:650-653.
Vetiver: More than a pleasant aroma?
An important ingredient in the contemporary perfume and cosmetics industries, vetiver, is the only grass cultivated throughout the world to retain its essential oil, which contains sesquiterpene alcohols and hydrocarbons.1-3 Field and glasshouse studies have revealed that vetiver grass can tolerate extreme variations in climate well, including protracted drought, floods, submergence, temperature, and soils high in acidity, alkalinity, and various heavy metals. Its heartiness may explain its continuing or even increasing use in fragrances and other products pertinent to skin health as humanity strives to adapt to climate change.4 In a 2017 review of various commercial essential oils as antimicrobial therapy for cutaneous disorders, Orchard and van Vuuren identified vetiver as warranting particular attention for its capacity to confer broad benefits to the skin in addressing acne, cuts, eczema, oiliness, sores, wounds, and aging skin.5 The focus of this column will be the dermatologic potential of vetiver.
Chemical constituents
Vetiver is thought to be one of the most complex of the essential oils owing to the hundreds of sesquiterpene derivatives with large structural diversity that contribute to its composition. 3
In a 2012 analysis of the components of South Indian vetiver oils, Mallavarapu et al. found an abundance of sesquiterpenes and oxygenated sesquiterpenes with cedrane, bisabolane, eudesmane, eremophilane, and zizaane skeletons. The primary constituents identified in the four oils evaluated included eudesma-4,6-diene (delta-selinene) + beta-vetispirene (3.9%-6.1%), beta-vetivenene (0.9%-9.4%), 13-nor-trans-eudesma-4(15),7-dien-11-one + amorph-4-en-10-ol (5.0%-6.4%), trans-eudesma-4(15),7-dien-12-ol (vetiselinenol) + (E)-opposita-4(15),7(11)-dien-12-ol (3.7%-5.9%), eremophila-1 (10),11-dien-2alpha-ol (nootkatol) + ziza-6(13)-en-12-ol (khusimol) (16.1%-19.2%), and eremophila-1(10),7(11)-dien-2alpha-ol (isonootkatol) + (E)-eremophila-1(10),7(11)-12-ol (isovalencenol) (5.6%-6.9%).6
Antimicrobial activity
In 2012, Saikia et al. assessed the antimycobacterial activity of Vetiveria zizanioides against Mycobacterium tuberculosis H(37)Rv and H(37)Ra strains. Their results showed that ethanolic extracts and hexane fractions displayed robust antimycobacterial properties, buttressing the traditional medical uses of the plant, as well as consideration of this agent as a modern antituberculosis agent.7
Two years later, Dos Santos et al. showed that Vetiveria zizanioides roots grown in Brazil exhibited notable antimicrobial effects against various pathogenic organisms.8In 2017, Burger et al. showed that vetiver essential oil primarily contributes its scent to cosmetic formulations but also displayed antimicrobial activity against Gram-positive bacterial strains, as well as one strain of Candida glabrata. They suggest that vetiver should be considered for its antimicrobial capacity as an added bonus to cosmetic formulations.2
In a 2018 study to ascertain the antimicrobial activity of 247 essential oil combinations against five reference strains of wound pathogens, Orchard et al. found that 26 combinations exhibited extensive antimicrobial activity. Sandalwood and vetiver were found to contribute most to antimicrobial function when used in combination. The investigators concluded that such combinations warrant consideration for wound therapy.9
Antiacne activity
In 2018, Orchard et al. conducted another study of the efficacy of commercial essential oil combinations against the two pathogens responsible for acne, Propionibacterium acnes and Staphlyococcus epidermidis. They investigated 408 combinations, of which 167 exhibited notable antimicrobial activity. They observed that the combination with the lowest minimum inhibitory concentration value against P. acnes and S. epidermidis was vetiver and cinnamon bark.10 This usage points to the potential of vetiver use as an antiacne ingredient.
Safety
The Scientific Committee on Consumer Safety (SCCS) offered a final opinion on the safety of the fragrance ingredient acetylated vetiver oil in 2019, declaring its use with 1% alpha-tocopherol in cosmetic leave-on and rinse-off products safe at proposed concentration levels. They noted that acetylated vetiver oil has been used for several years without provoking contact allergies.11
Conclusion
Much more research is necessary to determine just what kind of a role this perfumery powerhouse can play in dermatology.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at [email protected].
References
1. Del Giudice L et al. Environ Microbiol. 2008 Oct;10(10):2824-41.
2. Burger P et al. Medicines (Basel). 2017 Jun 16;4(2):41.
3. Belhassen E et al. Chem Biodivers. 2014 Nov;11(11):1821–42.
4. Danh LT et al. Int J Phytoremediation. 2009 Oct-Dec;11(8):664–91.
5. Orchard A and van Vuuren S. Evid Based Complement Alternat Med. 2017;2017:4517971.
6. Mallavarapu GR et al. Nat Prod Commun. 2012 Feb;7(2):223–5.
7. Saikia D et al. Complement Ther Med. 2012 Dec;20(6):434–6.
8. Dos Santos DS et al. Acta Pharm. 2014 Dec;64(4):495-501.
9. Orchard A et al. Chem Biodivers. 2018 Dec;15(12):e1800405.
10. Orchard A et al. Int J Cosmet Sci. 2018 Mar 24. [Epub ahead of print].
11. SCCS members & External experts. Regul Toxicol Pharmacol. 2019 Oct;107:104389.
An important ingredient in the contemporary perfume and cosmetics industries, vetiver, is the only grass cultivated throughout the world to retain its essential oil, which contains sesquiterpene alcohols and hydrocarbons.1-3 Field and glasshouse studies have revealed that vetiver grass can tolerate extreme variations in climate well, including protracted drought, floods, submergence, temperature, and soils high in acidity, alkalinity, and various heavy metals. Its heartiness may explain its continuing or even increasing use in fragrances and other products pertinent to skin health as humanity strives to adapt to climate change.4 In a 2017 review of various commercial essential oils as antimicrobial therapy for cutaneous disorders, Orchard and van Vuuren identified vetiver as warranting particular attention for its capacity to confer broad benefits to the skin in addressing acne, cuts, eczema, oiliness, sores, wounds, and aging skin.5 The focus of this column will be the dermatologic potential of vetiver.
Chemical constituents
Vetiver is thought to be one of the most complex of the essential oils owing to the hundreds of sesquiterpene derivatives with large structural diversity that contribute to its composition. 3
In a 2012 analysis of the components of South Indian vetiver oils, Mallavarapu et al. found an abundance of sesquiterpenes and oxygenated sesquiterpenes with cedrane, bisabolane, eudesmane, eremophilane, and zizaane skeletons. The primary constituents identified in the four oils evaluated included eudesma-4,6-diene (delta-selinene) + beta-vetispirene (3.9%-6.1%), beta-vetivenene (0.9%-9.4%), 13-nor-trans-eudesma-4(15),7-dien-11-one + amorph-4-en-10-ol (5.0%-6.4%), trans-eudesma-4(15),7-dien-12-ol (vetiselinenol) + (E)-opposita-4(15),7(11)-dien-12-ol (3.7%-5.9%), eremophila-1 (10),11-dien-2alpha-ol (nootkatol) + ziza-6(13)-en-12-ol (khusimol) (16.1%-19.2%), and eremophila-1(10),7(11)-dien-2alpha-ol (isonootkatol) + (E)-eremophila-1(10),7(11)-12-ol (isovalencenol) (5.6%-6.9%).6
Antimicrobial activity
In 2012, Saikia et al. assessed the antimycobacterial activity of Vetiveria zizanioides against Mycobacterium tuberculosis H(37)Rv and H(37)Ra strains. Their results showed that ethanolic extracts and hexane fractions displayed robust antimycobacterial properties, buttressing the traditional medical uses of the plant, as well as consideration of this agent as a modern antituberculosis agent.7
Two years later, Dos Santos et al. showed that Vetiveria zizanioides roots grown in Brazil exhibited notable antimicrobial effects against various pathogenic organisms.8In 2017, Burger et al. showed that vetiver essential oil primarily contributes its scent to cosmetic formulations but also displayed antimicrobial activity against Gram-positive bacterial strains, as well as one strain of Candida glabrata. They suggest that vetiver should be considered for its antimicrobial capacity as an added bonus to cosmetic formulations.2
In a 2018 study to ascertain the antimicrobial activity of 247 essential oil combinations against five reference strains of wound pathogens, Orchard et al. found that 26 combinations exhibited extensive antimicrobial activity. Sandalwood and vetiver were found to contribute most to antimicrobial function when used in combination. The investigators concluded that such combinations warrant consideration for wound therapy.9
Antiacne activity
In 2018, Orchard et al. conducted another study of the efficacy of commercial essential oil combinations against the two pathogens responsible for acne, Propionibacterium acnes and Staphlyococcus epidermidis. They investigated 408 combinations, of which 167 exhibited notable antimicrobial activity. They observed that the combination with the lowest minimum inhibitory concentration value against P. acnes and S. epidermidis was vetiver and cinnamon bark.10 This usage points to the potential of vetiver use as an antiacne ingredient.
Safety
The Scientific Committee on Consumer Safety (SCCS) offered a final opinion on the safety of the fragrance ingredient acetylated vetiver oil in 2019, declaring its use with 1% alpha-tocopherol in cosmetic leave-on and rinse-off products safe at proposed concentration levels. They noted that acetylated vetiver oil has been used for several years without provoking contact allergies.11
Conclusion
Much more research is necessary to determine just what kind of a role this perfumery powerhouse can play in dermatology.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at [email protected].
References
1. Del Giudice L et al. Environ Microbiol. 2008 Oct;10(10):2824-41.
2. Burger P et al. Medicines (Basel). 2017 Jun 16;4(2):41.
3. Belhassen E et al. Chem Biodivers. 2014 Nov;11(11):1821–42.
4. Danh LT et al. Int J Phytoremediation. 2009 Oct-Dec;11(8):664–91.
5. Orchard A and van Vuuren S. Evid Based Complement Alternat Med. 2017;2017:4517971.
6. Mallavarapu GR et al. Nat Prod Commun. 2012 Feb;7(2):223–5.
7. Saikia D et al. Complement Ther Med. 2012 Dec;20(6):434–6.
8. Dos Santos DS et al. Acta Pharm. 2014 Dec;64(4):495-501.
9. Orchard A et al. Chem Biodivers. 2018 Dec;15(12):e1800405.
10. Orchard A et al. Int J Cosmet Sci. 2018 Mar 24. [Epub ahead of print].
11. SCCS members & External experts. Regul Toxicol Pharmacol. 2019 Oct;107:104389.
An important ingredient in the contemporary perfume and cosmetics industries, vetiver, is the only grass cultivated throughout the world to retain its essential oil, which contains sesquiterpene alcohols and hydrocarbons.1-3 Field and glasshouse studies have revealed that vetiver grass can tolerate extreme variations in climate well, including protracted drought, floods, submergence, temperature, and soils high in acidity, alkalinity, and various heavy metals. Its heartiness may explain its continuing or even increasing use in fragrances and other products pertinent to skin health as humanity strives to adapt to climate change.4 In a 2017 review of various commercial essential oils as antimicrobial therapy for cutaneous disorders, Orchard and van Vuuren identified vetiver as warranting particular attention for its capacity to confer broad benefits to the skin in addressing acne, cuts, eczema, oiliness, sores, wounds, and aging skin.5 The focus of this column will be the dermatologic potential of vetiver.
Chemical constituents
Vetiver is thought to be one of the most complex of the essential oils owing to the hundreds of sesquiterpene derivatives with large structural diversity that contribute to its composition. 3
In a 2012 analysis of the components of South Indian vetiver oils, Mallavarapu et al. found an abundance of sesquiterpenes and oxygenated sesquiterpenes with cedrane, bisabolane, eudesmane, eremophilane, and zizaane skeletons. The primary constituents identified in the four oils evaluated included eudesma-4,6-diene (delta-selinene) + beta-vetispirene (3.9%-6.1%), beta-vetivenene (0.9%-9.4%), 13-nor-trans-eudesma-4(15),7-dien-11-one + amorph-4-en-10-ol (5.0%-6.4%), trans-eudesma-4(15),7-dien-12-ol (vetiselinenol) + (E)-opposita-4(15),7(11)-dien-12-ol (3.7%-5.9%), eremophila-1 (10),11-dien-2alpha-ol (nootkatol) + ziza-6(13)-en-12-ol (khusimol) (16.1%-19.2%), and eremophila-1(10),7(11)-dien-2alpha-ol (isonootkatol) + (E)-eremophila-1(10),7(11)-12-ol (isovalencenol) (5.6%-6.9%).6
Antimicrobial activity
In 2012, Saikia et al. assessed the antimycobacterial activity of Vetiveria zizanioides against Mycobacterium tuberculosis H(37)Rv and H(37)Ra strains. Their results showed that ethanolic extracts and hexane fractions displayed robust antimycobacterial properties, buttressing the traditional medical uses of the plant, as well as consideration of this agent as a modern antituberculosis agent.7
Two years later, Dos Santos et al. showed that Vetiveria zizanioides roots grown in Brazil exhibited notable antimicrobial effects against various pathogenic organisms.8In 2017, Burger et al. showed that vetiver essential oil primarily contributes its scent to cosmetic formulations but also displayed antimicrobial activity against Gram-positive bacterial strains, as well as one strain of Candida glabrata. They suggest that vetiver should be considered for its antimicrobial capacity as an added bonus to cosmetic formulations.2
In a 2018 study to ascertain the antimicrobial activity of 247 essential oil combinations against five reference strains of wound pathogens, Orchard et al. found that 26 combinations exhibited extensive antimicrobial activity. Sandalwood and vetiver were found to contribute most to antimicrobial function when used in combination. The investigators concluded that such combinations warrant consideration for wound therapy.9
Antiacne activity
In 2018, Orchard et al. conducted another study of the efficacy of commercial essential oil combinations against the two pathogens responsible for acne, Propionibacterium acnes and Staphlyococcus epidermidis. They investigated 408 combinations, of which 167 exhibited notable antimicrobial activity. They observed that the combination with the lowest minimum inhibitory concentration value against P. acnes and S. epidermidis was vetiver and cinnamon bark.10 This usage points to the potential of vetiver use as an antiacne ingredient.
Safety
The Scientific Committee on Consumer Safety (SCCS) offered a final opinion on the safety of the fragrance ingredient acetylated vetiver oil in 2019, declaring its use with 1% alpha-tocopherol in cosmetic leave-on and rinse-off products safe at proposed concentration levels. They noted that acetylated vetiver oil has been used for several years without provoking contact allergies.11
Conclusion
Much more research is necessary to determine just what kind of a role this perfumery powerhouse can play in dermatology.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at [email protected].
References
1. Del Giudice L et al. Environ Microbiol. 2008 Oct;10(10):2824-41.
2. Burger P et al. Medicines (Basel). 2017 Jun 16;4(2):41.
3. Belhassen E et al. Chem Biodivers. 2014 Nov;11(11):1821–42.
4. Danh LT et al. Int J Phytoremediation. 2009 Oct-Dec;11(8):664–91.
5. Orchard A and van Vuuren S. Evid Based Complement Alternat Med. 2017;2017:4517971.
6. Mallavarapu GR et al. Nat Prod Commun. 2012 Feb;7(2):223–5.
7. Saikia D et al. Complement Ther Med. 2012 Dec;20(6):434–6.
8. Dos Santos DS et al. Acta Pharm. 2014 Dec;64(4):495-501.
9. Orchard A et al. Chem Biodivers. 2018 Dec;15(12):e1800405.
10. Orchard A et al. Int J Cosmet Sci. 2018 Mar 24. [Epub ahead of print].
11. SCCS members & External experts. Regul Toxicol Pharmacol. 2019 Oct;107:104389.
‘Empathy fatigue’ in clinicians rises with latest COVID-19 surge
Heidi Erickson, MD, is tired. As a pulmonary and critical care physician at Hennepin Healthcare in Minneapolis, she has been providing care for patients with COVID-19 since the start of the pandemic.
It was exhausting from the beginning, as she and her colleagues scrambled to understand how to deal with this new disease. But lately, she has noticed a different kind of exhaustion arising from the knowledge that with vaccines widely available, the latest surge was preventable.
Her intensive care unit is currently as full as it has ever been with COVID-19 patients, many of them young adults and most of them unvaccinated. After the recent death of one patient, an unvaccinated man with teenage children, she had to face his family’s questions about why ivermectin, an antiparasitic medication that was falsely promoted as a COVID-19 treatment, was not administered.
“I’m fatigued because I’m working more than ever, but more people don’t have to die,” Dr. Erickson said in an interview . “It’s been very hard physically, mentally, emotionally.”
Amid yet another surge in COVID-19 cases around the United States, clinicians are speaking out about their growing frustration with this preventable crisis.
Some are using the terms “empathy fatigue” and “compassion fatigue” – a sense that they are losing empathy for unvaccinated individuals who are fueling the pandemic.
Dr. Erickson says she is frustrated not by individual patients but by a system that has allowed disinformation to proliferate. Experts say these types of feelings fit into a widespread pattern of physician burnout that has taken a new turn at this stage of the pandemic.
Paradoxical choices
Empathy is a cornerstone of what clinicians do, and the ability to understand and share a patient’s feelings is an essential skill for providing effective care, says Kaz Nelson, MD, a psychiatrist at the University of Minnesota, Minneapolis.
Practitioners face paradoxical situations all the time, she notes. These include individuals who break bones and go skydiving again, people who have high cholesterol but continue to eat fried foods, and those with advanced lung cancer who continue to smoke.
To treat patients with compassion, practitioners learn to set aside judgment by acknowledging the complexity of human behavior. They may lament the addictive nature of nicotine and advertising that targets children, for example, while still listening and caring.
Empathy requires high-level brain function, but as stress levels rise, brain function that drives empathy tends to shut down. It’s a survival mechanism, Dr. Nelson says.
When health care workers feel overwhelmed, trapped, or threatened by patients demanding unproven treatments or by ICUs with more patients than ventilators, they may experience a fight-or-flight response that makes them defensive, frustrated, angry, or uncaring, notes Mona Masood, DO, a Philadelphia-area psychiatrist and founder of Physician Support Line, a free mental health hotline for doctors.
Some clinicians have taken to Twitter and other social media platforms to post about these types of experiences.
These feelings, which have been brewing for months, have been exacerbated by the complexity of the current situation. Clinicians see a disconnect between what is and what could be, Dr. Nelson notes.
“Prior to vaccines, there weren’t other options, and so we had toxic stress and we had fatigue, but we could still maintain little bits of empathy by saying, ‘You know, people didn’t choose to get infected, and we are in a pandemic.’ We could kind of hate the virus. Now with access to vaccines, that last connection to empathy is removed for many people,” she says.
Self-preservation vs. empathy
Compassion fatigue or empathy fatigue is just one reaction to feeling completely maxed out and overstressed, Dr. Nelson says. Anger at society, such as what Dr. Erickson experienced, is another response.
Practitioners may also feel as if they are just going through the motions of their job, or they might disassociate, ceasing to feel that their patients are human. Plenty of doctors and nurses have cried in their cars after shifts and have posted tearful videos on social media.
Early in the pandemic, Dr. Masood says, physicians who called the support hotline expressed sadness and grief. Now, she had her colleagues hear frustration and anger, along with guilt and shame for having feelings they believe they shouldn’t be having, especially toward patients. They may feel unprofessional or worse – unworthy of being physicians, she says.
One recent caller to the hotline was a long-time ICU physician who had been told so many times by patients that ivermectin was the only medicine that would cure them that he began to doubt himself, says Dr. Masood. This caller needed to be reassured by another physician that he was doing the right thing.
Another emergency department physician told Dr. Masood about a young child who had arrived at the hospital with COVID-19 symptoms. When asked whether the family had been exposed to anyone with COVID-19, the child’s parent lied so that they could be triaged faster.
The physician, who needed to step away from the situation, reached out to Dr. Masood to express her frustration so that she wouldn’t “let it out” on the patient.
“It’s hard to have empathy for people who, for all intents and purposes, are very self-centered,” Dr. Masood says. “We’re at a place where we’re having to choose between self-preservation and empathy.”
How to cope
To help practitioners cope, Dr. Masood offers words that describe what they’re experiencing. She often hears clinicians say things such as, “This is a type of burnout that I feel to my bones,” or “This makes me want to quit,” or “I feel like I’m at the end of my rope.”
She encourages them to consider the terms “empathy fatigue,” and “moral injury” in order to reconcile how their sense of responsibility to take care of people is compromised by factors outside of their control.
It is not shameful to acknowledge that they experience emotions, including difficult ones such as frustration, anger, sadness, and anxiety, Dr. Masood adds.
Being frustrated with a patient doesn’t make someone a bad doctor, and admitting those emotions is the first step toward dealing with them, she says.
before they cause a sense of callousness or other consequences that become harder to heal from as time goes on.
“We’re trained to just go, go, go and sometimes not pause and check in,” she says. Clinicians who open up are likely to find they are not the only ones feeling tired or frustrated right now, she adds.
“Connect with peers and colleagues, because chances are, they can relate,” Dr. Nelson says.
A version of this article first appeared on Medscape.com.
Heidi Erickson, MD, is tired. As a pulmonary and critical care physician at Hennepin Healthcare in Minneapolis, she has been providing care for patients with COVID-19 since the start of the pandemic.
It was exhausting from the beginning, as she and her colleagues scrambled to understand how to deal with this new disease. But lately, she has noticed a different kind of exhaustion arising from the knowledge that with vaccines widely available, the latest surge was preventable.
Her intensive care unit is currently as full as it has ever been with COVID-19 patients, many of them young adults and most of them unvaccinated. After the recent death of one patient, an unvaccinated man with teenage children, she had to face his family’s questions about why ivermectin, an antiparasitic medication that was falsely promoted as a COVID-19 treatment, was not administered.
“I’m fatigued because I’m working more than ever, but more people don’t have to die,” Dr. Erickson said in an interview . “It’s been very hard physically, mentally, emotionally.”
Amid yet another surge in COVID-19 cases around the United States, clinicians are speaking out about their growing frustration with this preventable crisis.
Some are using the terms “empathy fatigue” and “compassion fatigue” – a sense that they are losing empathy for unvaccinated individuals who are fueling the pandemic.
Dr. Erickson says she is frustrated not by individual patients but by a system that has allowed disinformation to proliferate. Experts say these types of feelings fit into a widespread pattern of physician burnout that has taken a new turn at this stage of the pandemic.
Paradoxical choices
Empathy is a cornerstone of what clinicians do, and the ability to understand and share a patient’s feelings is an essential skill for providing effective care, says Kaz Nelson, MD, a psychiatrist at the University of Minnesota, Minneapolis.
Practitioners face paradoxical situations all the time, she notes. These include individuals who break bones and go skydiving again, people who have high cholesterol but continue to eat fried foods, and those with advanced lung cancer who continue to smoke.
To treat patients with compassion, practitioners learn to set aside judgment by acknowledging the complexity of human behavior. They may lament the addictive nature of nicotine and advertising that targets children, for example, while still listening and caring.
Empathy requires high-level brain function, but as stress levels rise, brain function that drives empathy tends to shut down. It’s a survival mechanism, Dr. Nelson says.
When health care workers feel overwhelmed, trapped, or threatened by patients demanding unproven treatments or by ICUs with more patients than ventilators, they may experience a fight-or-flight response that makes them defensive, frustrated, angry, or uncaring, notes Mona Masood, DO, a Philadelphia-area psychiatrist and founder of Physician Support Line, a free mental health hotline for doctors.
Some clinicians have taken to Twitter and other social media platforms to post about these types of experiences.
These feelings, which have been brewing for months, have been exacerbated by the complexity of the current situation. Clinicians see a disconnect between what is and what could be, Dr. Nelson notes.
“Prior to vaccines, there weren’t other options, and so we had toxic stress and we had fatigue, but we could still maintain little bits of empathy by saying, ‘You know, people didn’t choose to get infected, and we are in a pandemic.’ We could kind of hate the virus. Now with access to vaccines, that last connection to empathy is removed for many people,” she says.
Self-preservation vs. empathy
Compassion fatigue or empathy fatigue is just one reaction to feeling completely maxed out and overstressed, Dr. Nelson says. Anger at society, such as what Dr. Erickson experienced, is another response.
Practitioners may also feel as if they are just going through the motions of their job, or they might disassociate, ceasing to feel that their patients are human. Plenty of doctors and nurses have cried in their cars after shifts and have posted tearful videos on social media.
Early in the pandemic, Dr. Masood says, physicians who called the support hotline expressed sadness and grief. Now, she had her colleagues hear frustration and anger, along with guilt and shame for having feelings they believe they shouldn’t be having, especially toward patients. They may feel unprofessional or worse – unworthy of being physicians, she says.
One recent caller to the hotline was a long-time ICU physician who had been told so many times by patients that ivermectin was the only medicine that would cure them that he began to doubt himself, says Dr. Masood. This caller needed to be reassured by another physician that he was doing the right thing.
Another emergency department physician told Dr. Masood about a young child who had arrived at the hospital with COVID-19 symptoms. When asked whether the family had been exposed to anyone with COVID-19, the child’s parent lied so that they could be triaged faster.
The physician, who needed to step away from the situation, reached out to Dr. Masood to express her frustration so that she wouldn’t “let it out” on the patient.
“It’s hard to have empathy for people who, for all intents and purposes, are very self-centered,” Dr. Masood says. “We’re at a place where we’re having to choose between self-preservation and empathy.”
How to cope
To help practitioners cope, Dr. Masood offers words that describe what they’re experiencing. She often hears clinicians say things such as, “This is a type of burnout that I feel to my bones,” or “This makes me want to quit,” or “I feel like I’m at the end of my rope.”
She encourages them to consider the terms “empathy fatigue,” and “moral injury” in order to reconcile how their sense of responsibility to take care of people is compromised by factors outside of their control.
It is not shameful to acknowledge that they experience emotions, including difficult ones such as frustration, anger, sadness, and anxiety, Dr. Masood adds.
Being frustrated with a patient doesn’t make someone a bad doctor, and admitting those emotions is the first step toward dealing with them, she says.
before they cause a sense of callousness or other consequences that become harder to heal from as time goes on.
“We’re trained to just go, go, go and sometimes not pause and check in,” she says. Clinicians who open up are likely to find they are not the only ones feeling tired or frustrated right now, she adds.
“Connect with peers and colleagues, because chances are, they can relate,” Dr. Nelson says.
A version of this article first appeared on Medscape.com.
Heidi Erickson, MD, is tired. As a pulmonary and critical care physician at Hennepin Healthcare in Minneapolis, she has been providing care for patients with COVID-19 since the start of the pandemic.
It was exhausting from the beginning, as she and her colleagues scrambled to understand how to deal with this new disease. But lately, she has noticed a different kind of exhaustion arising from the knowledge that with vaccines widely available, the latest surge was preventable.
Her intensive care unit is currently as full as it has ever been with COVID-19 patients, many of them young adults and most of them unvaccinated. After the recent death of one patient, an unvaccinated man with teenage children, she had to face his family’s questions about why ivermectin, an antiparasitic medication that was falsely promoted as a COVID-19 treatment, was not administered.
“I’m fatigued because I’m working more than ever, but more people don’t have to die,” Dr. Erickson said in an interview . “It’s been very hard physically, mentally, emotionally.”
Amid yet another surge in COVID-19 cases around the United States, clinicians are speaking out about their growing frustration with this preventable crisis.
Some are using the terms “empathy fatigue” and “compassion fatigue” – a sense that they are losing empathy for unvaccinated individuals who are fueling the pandemic.
Dr. Erickson says she is frustrated not by individual patients but by a system that has allowed disinformation to proliferate. Experts say these types of feelings fit into a widespread pattern of physician burnout that has taken a new turn at this stage of the pandemic.
Paradoxical choices
Empathy is a cornerstone of what clinicians do, and the ability to understand and share a patient’s feelings is an essential skill for providing effective care, says Kaz Nelson, MD, a psychiatrist at the University of Minnesota, Minneapolis.
Practitioners face paradoxical situations all the time, she notes. These include individuals who break bones and go skydiving again, people who have high cholesterol but continue to eat fried foods, and those with advanced lung cancer who continue to smoke.
To treat patients with compassion, practitioners learn to set aside judgment by acknowledging the complexity of human behavior. They may lament the addictive nature of nicotine and advertising that targets children, for example, while still listening and caring.
Empathy requires high-level brain function, but as stress levels rise, brain function that drives empathy tends to shut down. It’s a survival mechanism, Dr. Nelson says.
When health care workers feel overwhelmed, trapped, or threatened by patients demanding unproven treatments or by ICUs with more patients than ventilators, they may experience a fight-or-flight response that makes them defensive, frustrated, angry, or uncaring, notes Mona Masood, DO, a Philadelphia-area psychiatrist and founder of Physician Support Line, a free mental health hotline for doctors.
Some clinicians have taken to Twitter and other social media platforms to post about these types of experiences.
These feelings, which have been brewing for months, have been exacerbated by the complexity of the current situation. Clinicians see a disconnect between what is and what could be, Dr. Nelson notes.
“Prior to vaccines, there weren’t other options, and so we had toxic stress and we had fatigue, but we could still maintain little bits of empathy by saying, ‘You know, people didn’t choose to get infected, and we are in a pandemic.’ We could kind of hate the virus. Now with access to vaccines, that last connection to empathy is removed for many people,” she says.
Self-preservation vs. empathy
Compassion fatigue or empathy fatigue is just one reaction to feeling completely maxed out and overstressed, Dr. Nelson says. Anger at society, such as what Dr. Erickson experienced, is another response.
Practitioners may also feel as if they are just going through the motions of their job, or they might disassociate, ceasing to feel that their patients are human. Plenty of doctors and nurses have cried in their cars after shifts and have posted tearful videos on social media.
Early in the pandemic, Dr. Masood says, physicians who called the support hotline expressed sadness and grief. Now, she had her colleagues hear frustration and anger, along with guilt and shame for having feelings they believe they shouldn’t be having, especially toward patients. They may feel unprofessional or worse – unworthy of being physicians, she says.
One recent caller to the hotline was a long-time ICU physician who had been told so many times by patients that ivermectin was the only medicine that would cure them that he began to doubt himself, says Dr. Masood. This caller needed to be reassured by another physician that he was doing the right thing.
Another emergency department physician told Dr. Masood about a young child who had arrived at the hospital with COVID-19 symptoms. When asked whether the family had been exposed to anyone with COVID-19, the child’s parent lied so that they could be triaged faster.
The physician, who needed to step away from the situation, reached out to Dr. Masood to express her frustration so that she wouldn’t “let it out” on the patient.
“It’s hard to have empathy for people who, for all intents and purposes, are very self-centered,” Dr. Masood says. “We’re at a place where we’re having to choose between self-preservation and empathy.”
How to cope
To help practitioners cope, Dr. Masood offers words that describe what they’re experiencing. She often hears clinicians say things such as, “This is a type of burnout that I feel to my bones,” or “This makes me want to quit,” or “I feel like I’m at the end of my rope.”
She encourages them to consider the terms “empathy fatigue,” and “moral injury” in order to reconcile how their sense of responsibility to take care of people is compromised by factors outside of their control.
It is not shameful to acknowledge that they experience emotions, including difficult ones such as frustration, anger, sadness, and anxiety, Dr. Masood adds.
Being frustrated with a patient doesn’t make someone a bad doctor, and admitting those emotions is the first step toward dealing with them, she says.
before they cause a sense of callousness or other consequences that become harder to heal from as time goes on.
“We’re trained to just go, go, go and sometimes not pause and check in,” she says. Clinicians who open up are likely to find they are not the only ones feeling tired or frustrated right now, she adds.
“Connect with peers and colleagues, because chances are, they can relate,” Dr. Nelson says.
A version of this article first appeared on Medscape.com.
Finding employees during a pandemic
.
My own office is prime example: I have had job listings for both front- and back-office positions posted on all the major job boards and other employment portals for months, with a disappointing response. Of the few who do respond, many, incredibly, do not show up for their interviews!
It turns out that this is a widespread problem, and not just in medicine. A recent survey by the National Federation of Independent Business found that 42% of business owners, in all walks of life, had job openings that could not be filled, a record high. Over 90% of those hiring reported few or no qualified applicants and an increase in interview no-shows.
Clearly, this is a huge obstacle to growth – and even to conducting normal operations – for my practice and many others.
Reasons for the situation vary, but a big one has been the unfortunate fact that many open job positions actually pay less than the expanded unemployment benefits that many people have received under the March 2020 CARES Act. By one estimate, almost 70% of unemployed workers have been collecting more on unemployment than they earned while working. The CARES benefits expired in early September, but many potential workers continue to receive payments through a newer FEMA program, and some states have their own ongoing benefit programs.
Other reasons have been offered: Some candidates are unvaccinated (an immediate deal-breaker in my office), and some working parents continue to face a lack of childcare or in-person schooling for their children. Some applicants – regardless of vaccination status – have said they are hesitant to work in a medical office setting and risk getting COVID-19, despite all the precautions we have in place. Others have said they are waiting until the job market improves.
There are no easy solutions to this complicated problem, but here are a few suggestions culled from my research and conversations with HR professionals and others.
One obvious option is to offer higher wages, and perhaps even signing bonuses. “Whenever anyone says they can’t find the workers they need,” a consultant told me, “they are really saying they can’t find them at the wages they want to pay.” There are limits to the wages and benefits a private office with a very finite salary budget can offer, of course – but a few higher-paid employees may be preferable to no new workers at all.
For job candidates who fear COVID-19 exposure, assure them that their health and safety is a priority by spelling out the procedures your office is following (social distancing, reduced patient capacity, interaction barriers, face masks, avoidance of handshakes, enhanced cleaning procedures, symptom questionnaires, temperature checks, etc.) to minimize the risk of exposure.
You also may need to rework your interview process. In the Zoom era, most preliminary interviews can be conducted remotely. For on-site interviews, explain how you’re maintaining a safe interview environment by applying the same office safety policies to interactions with interviewees.
If a promising candidate doesn’t show up for an interview, the applicant could be making a token effort to obtain a job in order to perpetuate unemployment payments, but don’t jump to that conclusion. There may be extenuating circumstances, such as an emergency, illness, or traffic issues. Also, consider the possibility that it was your fault. If you waited too long to schedule the interview, another office could have lured them away. Or you may not have adequately explained your COVID-19 exposure safeguards. At the very least, a drawn-out process or a lack of transparency can make applicants apprehensive about accepting a job with you, particularly if other employers are pursuing them.
To counter the shortsighted appeal of collecting unemployment benefits, it may help to highlight the long-term growth opportunities available at your office. Consider outlining typical career tracks, or providing specific examples of how people have advanced their careers at your facility. I frequently cite the example of my current office manager, who began as an assistant receptionist almost 30 years ago.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
.
My own office is prime example: I have had job listings for both front- and back-office positions posted on all the major job boards and other employment portals for months, with a disappointing response. Of the few who do respond, many, incredibly, do not show up for their interviews!
It turns out that this is a widespread problem, and not just in medicine. A recent survey by the National Federation of Independent Business found that 42% of business owners, in all walks of life, had job openings that could not be filled, a record high. Over 90% of those hiring reported few or no qualified applicants and an increase in interview no-shows.
Clearly, this is a huge obstacle to growth – and even to conducting normal operations – for my practice and many others.
Reasons for the situation vary, but a big one has been the unfortunate fact that many open job positions actually pay less than the expanded unemployment benefits that many people have received under the March 2020 CARES Act. By one estimate, almost 70% of unemployed workers have been collecting more on unemployment than they earned while working. The CARES benefits expired in early September, but many potential workers continue to receive payments through a newer FEMA program, and some states have their own ongoing benefit programs.
Other reasons have been offered: Some candidates are unvaccinated (an immediate deal-breaker in my office), and some working parents continue to face a lack of childcare or in-person schooling for their children. Some applicants – regardless of vaccination status – have said they are hesitant to work in a medical office setting and risk getting COVID-19, despite all the precautions we have in place. Others have said they are waiting until the job market improves.
There are no easy solutions to this complicated problem, but here are a few suggestions culled from my research and conversations with HR professionals and others.
One obvious option is to offer higher wages, and perhaps even signing bonuses. “Whenever anyone says they can’t find the workers they need,” a consultant told me, “they are really saying they can’t find them at the wages they want to pay.” There are limits to the wages and benefits a private office with a very finite salary budget can offer, of course – but a few higher-paid employees may be preferable to no new workers at all.
For job candidates who fear COVID-19 exposure, assure them that their health and safety is a priority by spelling out the procedures your office is following (social distancing, reduced patient capacity, interaction barriers, face masks, avoidance of handshakes, enhanced cleaning procedures, symptom questionnaires, temperature checks, etc.) to minimize the risk of exposure.
You also may need to rework your interview process. In the Zoom era, most preliminary interviews can be conducted remotely. For on-site interviews, explain how you’re maintaining a safe interview environment by applying the same office safety policies to interactions with interviewees.
If a promising candidate doesn’t show up for an interview, the applicant could be making a token effort to obtain a job in order to perpetuate unemployment payments, but don’t jump to that conclusion. There may be extenuating circumstances, such as an emergency, illness, or traffic issues. Also, consider the possibility that it was your fault. If you waited too long to schedule the interview, another office could have lured them away. Or you may not have adequately explained your COVID-19 exposure safeguards. At the very least, a drawn-out process or a lack of transparency can make applicants apprehensive about accepting a job with you, particularly if other employers are pursuing them.
To counter the shortsighted appeal of collecting unemployment benefits, it may help to highlight the long-term growth opportunities available at your office. Consider outlining typical career tracks, or providing specific examples of how people have advanced their careers at your facility. I frequently cite the example of my current office manager, who began as an assistant receptionist almost 30 years ago.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
.
My own office is prime example: I have had job listings for both front- and back-office positions posted on all the major job boards and other employment portals for months, with a disappointing response. Of the few who do respond, many, incredibly, do not show up for their interviews!
It turns out that this is a widespread problem, and not just in medicine. A recent survey by the National Federation of Independent Business found that 42% of business owners, in all walks of life, had job openings that could not be filled, a record high. Over 90% of those hiring reported few or no qualified applicants and an increase in interview no-shows.
Clearly, this is a huge obstacle to growth – and even to conducting normal operations – for my practice and many others.
Reasons for the situation vary, but a big one has been the unfortunate fact that many open job positions actually pay less than the expanded unemployment benefits that many people have received under the March 2020 CARES Act. By one estimate, almost 70% of unemployed workers have been collecting more on unemployment than they earned while working. The CARES benefits expired in early September, but many potential workers continue to receive payments through a newer FEMA program, and some states have their own ongoing benefit programs.
Other reasons have been offered: Some candidates are unvaccinated (an immediate deal-breaker in my office), and some working parents continue to face a lack of childcare or in-person schooling for their children. Some applicants – regardless of vaccination status – have said they are hesitant to work in a medical office setting and risk getting COVID-19, despite all the precautions we have in place. Others have said they are waiting until the job market improves.
There are no easy solutions to this complicated problem, but here are a few suggestions culled from my research and conversations with HR professionals and others.
One obvious option is to offer higher wages, and perhaps even signing bonuses. “Whenever anyone says they can’t find the workers they need,” a consultant told me, “they are really saying they can’t find them at the wages they want to pay.” There are limits to the wages and benefits a private office with a very finite salary budget can offer, of course – but a few higher-paid employees may be preferable to no new workers at all.
For job candidates who fear COVID-19 exposure, assure them that their health and safety is a priority by spelling out the procedures your office is following (social distancing, reduced patient capacity, interaction barriers, face masks, avoidance of handshakes, enhanced cleaning procedures, symptom questionnaires, temperature checks, etc.) to minimize the risk of exposure.
You also may need to rework your interview process. In the Zoom era, most preliminary interviews can be conducted remotely. For on-site interviews, explain how you’re maintaining a safe interview environment by applying the same office safety policies to interactions with interviewees.
If a promising candidate doesn’t show up for an interview, the applicant could be making a token effort to obtain a job in order to perpetuate unemployment payments, but don’t jump to that conclusion. There may be extenuating circumstances, such as an emergency, illness, or traffic issues. Also, consider the possibility that it was your fault. If you waited too long to schedule the interview, another office could have lured them away. Or you may not have adequately explained your COVID-19 exposure safeguards. At the very least, a drawn-out process or a lack of transparency can make applicants apprehensive about accepting a job with you, particularly if other employers are pursuing them.
To counter the shortsighted appeal of collecting unemployment benefits, it may help to highlight the long-term growth opportunities available at your office. Consider outlining typical career tracks, or providing specific examples of how people have advanced their careers at your facility. I frequently cite the example of my current office manager, who began as an assistant receptionist almost 30 years ago.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
Skin ulcers can pose tricky diagnostic challenges
In the clinical opinion of Alex G. Ortega-Loayza, MD, MCR, few absolutes drive the initial assessment of patients who present with skin ulcers.
The causes can be neoplastic, infectious, inflammatory, vasculopathic, external, and genetic. “Sometimes they can be of mixed etiology, which make them even more complicated to heal,” Dr. Ortega-Loayza, of the department of dermatology at Oregon Health & Science University, Portland, said during the annual meeting of the Pacific Dermatologic Association.
In a study published in 2019, he and his colleagues at four academic hospitals evaluated characteristics and diagnoses of ulcers in 274 patients with skin ulcers in inpatient dermatology consultation services between July 2015 and July 2018. Most primary teams requesting the consultation (93%) were from nonsurgical specialties. The median age of these patients was 54 years, 45% were male, and 50% had lower-extremity ulcers. Nearly two-thirds of the ulcers (62%) were chronic in nature, while the remaining 38% were acute. The skin ulcer was the chief reason for admission in 49% of cases and 66% were admitted through the ED. In addition, 11% had a superinfected skin ulcer.
The top three etiologies rendered by dermatologists after assessing these patients were pyoderma gangrenosum (17%), infection (13%), and exogenous causes (12%); another 12% remained diagnostically inconclusive after consultation. Diagnostic agreements between the primary team requesting the consultation and the dermatologist were poor to modest.
These data highlights the role of the dermatologists in the workup of skin ulcers of unknown etiology.
“The diagnosis of skin ulcers can be challenging,” Dr. Ortega-Loayza said. “Subjective factors playing a role in the diagnosis of skin ulcers include the type of level of training/experience you’ve had and general awareness and education about skin ulcers.” In addition, there is also a lack of gold-standard diagnostic criteria for atypical/inflammatory ulcers and a lack of specificity of ancillary testing, such as for pyoderma gangrenosum.
Dr. Ortega-Loayza’s basic workup is based on the review of systems and the patient’s comorbidities. Blood work may include CBC, comprehensive metabolic panel, erythrocyte sedimentation rate/C-reactive protein, glucose-6-phosphate dehydrogenase, albumin/prealbumin, autoimmune panels, and hypercoagulable panels. He may order a skin biopsy with H&E staining and microbiological studies, superficial bacterial wound cultures, and vascular studies, such as ankle brachial index (ABI) and chronic venous reflux tests, and Doppler ultrasound, and he might consider an angiogram for certain type of ulcers. Additional imaging studies may include x-ray, CT scan, and/or MRI.
The four key factors to control in patients with skin ulcers, he continued, include effective management of edema (such as compression garments depending on the results of the vascular studies); infection (with topical/oral antibiotics and debridement); the wound microenvironment (with wound dressings), and pain (mainly with nonopioids). “In my practice, we tend to do multilayered compression,” he said. “This can be two- or four-layer. I do light compression if the patient has peripheral arterial disease. I always bring in the patient 2 days later to check on them, or do a telehealth visit, to make sure they are not developing any worsening of the ulcers.”
Infections can be managed with topical antimicrobials such as metronidazole 1% gel and cadexomer iodine. “Iodine can also help dry the wound when you need to do so,” said Dr. Ortega-Loayza, who directs a pyoderma gangrenosum clinic at OHSU. “Debridement can be done with a curette or with commercially available enzymatic products such as Collagenase, PluroGel, and MediHoney.”
When the ulcer is in an active phase (characterized by significant amount of drainage and erythema), he uses one or more of the following products to control the wound microenvironment: zinc oxide, an antimicrobial dressing, a hyperabsorbent dressing, an abdominal pad, and compression.
During the healing phase, with evidence of re-epithelization, he tends to use more foam dressings and continues with compression. His preferred options for managing pain associated with ulcers are medications to control neuropathic pain including initially gabapentin (100 mg-300 mg at bedtime), pregabalin (75 mg twice a day), or duloxetine (extended release, 30 mg once a day). All of these medications can be titrated up based on patients’ needs. Foam dressings with ibuprofen can also provide comfort, he said.
Dr. Ortega-Loayza also provided a few clinical pearls highlighting the role and utility of interleukin-23 inhibitors in the management of patients with pyoderma gangrenosum, oral vitamin K in patients with calciphylaxis, and stanozolol for lipodermatosclerosis. He is also leading the first open-label trial testing a Janus kinase inhibitor – baricitinib – as a treatment for patients with pyoderma gangrenosum.
Dr. Ortega-Loayza disclosed that he is a consultant to Genentech and Guidepoint and is a member of the advisory board for Bristol-Myers Squibb, Boehringer Ingelheim, and Janssen. He also has received research support from Lilly.
In the clinical opinion of Alex G. Ortega-Loayza, MD, MCR, few absolutes drive the initial assessment of patients who present with skin ulcers.
The causes can be neoplastic, infectious, inflammatory, vasculopathic, external, and genetic. “Sometimes they can be of mixed etiology, which make them even more complicated to heal,” Dr. Ortega-Loayza, of the department of dermatology at Oregon Health & Science University, Portland, said during the annual meeting of the Pacific Dermatologic Association.
In a study published in 2019, he and his colleagues at four academic hospitals evaluated characteristics and diagnoses of ulcers in 274 patients with skin ulcers in inpatient dermatology consultation services between July 2015 and July 2018. Most primary teams requesting the consultation (93%) were from nonsurgical specialties. The median age of these patients was 54 years, 45% were male, and 50% had lower-extremity ulcers. Nearly two-thirds of the ulcers (62%) were chronic in nature, while the remaining 38% were acute. The skin ulcer was the chief reason for admission in 49% of cases and 66% were admitted through the ED. In addition, 11% had a superinfected skin ulcer.
The top three etiologies rendered by dermatologists after assessing these patients were pyoderma gangrenosum (17%), infection (13%), and exogenous causes (12%); another 12% remained diagnostically inconclusive after consultation. Diagnostic agreements between the primary team requesting the consultation and the dermatologist were poor to modest.
These data highlights the role of the dermatologists in the workup of skin ulcers of unknown etiology.
“The diagnosis of skin ulcers can be challenging,” Dr. Ortega-Loayza said. “Subjective factors playing a role in the diagnosis of skin ulcers include the type of level of training/experience you’ve had and general awareness and education about skin ulcers.” In addition, there is also a lack of gold-standard diagnostic criteria for atypical/inflammatory ulcers and a lack of specificity of ancillary testing, such as for pyoderma gangrenosum.
Dr. Ortega-Loayza’s basic workup is based on the review of systems and the patient’s comorbidities. Blood work may include CBC, comprehensive metabolic panel, erythrocyte sedimentation rate/C-reactive protein, glucose-6-phosphate dehydrogenase, albumin/prealbumin, autoimmune panels, and hypercoagulable panels. He may order a skin biopsy with H&E staining and microbiological studies, superficial bacterial wound cultures, and vascular studies, such as ankle brachial index (ABI) and chronic venous reflux tests, and Doppler ultrasound, and he might consider an angiogram for certain type of ulcers. Additional imaging studies may include x-ray, CT scan, and/or MRI.
The four key factors to control in patients with skin ulcers, he continued, include effective management of edema (such as compression garments depending on the results of the vascular studies); infection (with topical/oral antibiotics and debridement); the wound microenvironment (with wound dressings), and pain (mainly with nonopioids). “In my practice, we tend to do multilayered compression,” he said. “This can be two- or four-layer. I do light compression if the patient has peripheral arterial disease. I always bring in the patient 2 days later to check on them, or do a telehealth visit, to make sure they are not developing any worsening of the ulcers.”
Infections can be managed with topical antimicrobials such as metronidazole 1% gel and cadexomer iodine. “Iodine can also help dry the wound when you need to do so,” said Dr. Ortega-Loayza, who directs a pyoderma gangrenosum clinic at OHSU. “Debridement can be done with a curette or with commercially available enzymatic products such as Collagenase, PluroGel, and MediHoney.”
When the ulcer is in an active phase (characterized by significant amount of drainage and erythema), he uses one or more of the following products to control the wound microenvironment: zinc oxide, an antimicrobial dressing, a hyperabsorbent dressing, an abdominal pad, and compression.
During the healing phase, with evidence of re-epithelization, he tends to use more foam dressings and continues with compression. His preferred options for managing pain associated with ulcers are medications to control neuropathic pain including initially gabapentin (100 mg-300 mg at bedtime), pregabalin (75 mg twice a day), or duloxetine (extended release, 30 mg once a day). All of these medications can be titrated up based on patients’ needs. Foam dressings with ibuprofen can also provide comfort, he said.
Dr. Ortega-Loayza also provided a few clinical pearls highlighting the role and utility of interleukin-23 inhibitors in the management of patients with pyoderma gangrenosum, oral vitamin K in patients with calciphylaxis, and stanozolol for lipodermatosclerosis. He is also leading the first open-label trial testing a Janus kinase inhibitor – baricitinib – as a treatment for patients with pyoderma gangrenosum.
Dr. Ortega-Loayza disclosed that he is a consultant to Genentech and Guidepoint and is a member of the advisory board for Bristol-Myers Squibb, Boehringer Ingelheim, and Janssen. He also has received research support from Lilly.
In the clinical opinion of Alex G. Ortega-Loayza, MD, MCR, few absolutes drive the initial assessment of patients who present with skin ulcers.
The causes can be neoplastic, infectious, inflammatory, vasculopathic, external, and genetic. “Sometimes they can be of mixed etiology, which make them even more complicated to heal,” Dr. Ortega-Loayza, of the department of dermatology at Oregon Health & Science University, Portland, said during the annual meeting of the Pacific Dermatologic Association.
In a study published in 2019, he and his colleagues at four academic hospitals evaluated characteristics and diagnoses of ulcers in 274 patients with skin ulcers in inpatient dermatology consultation services between July 2015 and July 2018. Most primary teams requesting the consultation (93%) were from nonsurgical specialties. The median age of these patients was 54 years, 45% were male, and 50% had lower-extremity ulcers. Nearly two-thirds of the ulcers (62%) were chronic in nature, while the remaining 38% were acute. The skin ulcer was the chief reason for admission in 49% of cases and 66% were admitted through the ED. In addition, 11% had a superinfected skin ulcer.
The top three etiologies rendered by dermatologists after assessing these patients were pyoderma gangrenosum (17%), infection (13%), and exogenous causes (12%); another 12% remained diagnostically inconclusive after consultation. Diagnostic agreements between the primary team requesting the consultation and the dermatologist were poor to modest.
These data highlights the role of the dermatologists in the workup of skin ulcers of unknown etiology.
“The diagnosis of skin ulcers can be challenging,” Dr. Ortega-Loayza said. “Subjective factors playing a role in the diagnosis of skin ulcers include the type of level of training/experience you’ve had and general awareness and education about skin ulcers.” In addition, there is also a lack of gold-standard diagnostic criteria for atypical/inflammatory ulcers and a lack of specificity of ancillary testing, such as for pyoderma gangrenosum.
Dr. Ortega-Loayza’s basic workup is based on the review of systems and the patient’s comorbidities. Blood work may include CBC, comprehensive metabolic panel, erythrocyte sedimentation rate/C-reactive protein, glucose-6-phosphate dehydrogenase, albumin/prealbumin, autoimmune panels, and hypercoagulable panels. He may order a skin biopsy with H&E staining and microbiological studies, superficial bacterial wound cultures, and vascular studies, such as ankle brachial index (ABI) and chronic venous reflux tests, and Doppler ultrasound, and he might consider an angiogram for certain type of ulcers. Additional imaging studies may include x-ray, CT scan, and/or MRI.
The four key factors to control in patients with skin ulcers, he continued, include effective management of edema (such as compression garments depending on the results of the vascular studies); infection (with topical/oral antibiotics and debridement); the wound microenvironment (with wound dressings), and pain (mainly with nonopioids). “In my practice, we tend to do multilayered compression,” he said. “This can be two- or four-layer. I do light compression if the patient has peripheral arterial disease. I always bring in the patient 2 days later to check on them, or do a telehealth visit, to make sure they are not developing any worsening of the ulcers.”
Infections can be managed with topical antimicrobials such as metronidazole 1% gel and cadexomer iodine. “Iodine can also help dry the wound when you need to do so,” said Dr. Ortega-Loayza, who directs a pyoderma gangrenosum clinic at OHSU. “Debridement can be done with a curette or with commercially available enzymatic products such as Collagenase, PluroGel, and MediHoney.”
When the ulcer is in an active phase (characterized by significant amount of drainage and erythema), he uses one or more of the following products to control the wound microenvironment: zinc oxide, an antimicrobial dressing, a hyperabsorbent dressing, an abdominal pad, and compression.
During the healing phase, with evidence of re-epithelization, he tends to use more foam dressings and continues with compression. His preferred options for managing pain associated with ulcers are medications to control neuropathic pain including initially gabapentin (100 mg-300 mg at bedtime), pregabalin (75 mg twice a day), or duloxetine (extended release, 30 mg once a day). All of these medications can be titrated up based on patients’ needs. Foam dressings with ibuprofen can also provide comfort, he said.
Dr. Ortega-Loayza also provided a few clinical pearls highlighting the role and utility of interleukin-23 inhibitors in the management of patients with pyoderma gangrenosum, oral vitamin K in patients with calciphylaxis, and stanozolol for lipodermatosclerosis. He is also leading the first open-label trial testing a Janus kinase inhibitor – baricitinib – as a treatment for patients with pyoderma gangrenosum.
Dr. Ortega-Loayza disclosed that he is a consultant to Genentech and Guidepoint and is a member of the advisory board for Bristol-Myers Squibb, Boehringer Ingelheim, and Janssen. He also has received research support from Lilly.
FROM PDA 2021
Eurocentric standards of beauty are no longer dominant, experts agree
Addressing current standards of beauty at the Skin of Color Update 2021, dermatologists speaking about attitudes within four ethnic groups recounted a similar story: .
This change is relevant to dermatologists consulting with patients for cosmetic procedures. Four dermatologists who recounted the types of procedures their patients are requesting each reported that more patients are seeking cosmetic enhancements that accentuate rather than modify ethnic features.
Lips in Black, Asian, and Arab ethnic groups are just one example.
“Where several years ago, the conversation was really about lip reductions – how we can deemphasize the lip – I am now seeing lots of women of color coming in to ask about lip augmentation, looking to highlight their lips as a point of beauty,” reported Michelle Henry, MD, a dermatologist who practices in New York City.
She is not alone. Others participating on the same panel spoke of a growing interest among their patients to maintain or even emphasize the same ethnic features – including but not limited to lip shape and size that they were once anxious to modify.
In Asian patients, “the goal is not to Westernize,” agreed Annie Chiu, MD, a dermatologist who practices in North Redondo Beach, Calif. For lips, she spoke of the “50-50 ratio” of upper and lower lip symmetry that is consistent with a traditional Asian characteristic.
Like Dr. Henry, Dr. Chiu said that many requests for cosmetic work now involve accentuating Asian features, such as the oval shape of the face, rather than steps to modify this shape. This is a relatively recent change.
“I am finding that more of my patients want to improve the esthetic balance to optimize the appearance within their own ethnicity,” she said.
In the United Arab Emirates (UAE), Hassan Galadari, MD, an American-trained physician who is assistant professor of dermatology at the UAE University in Dubai, recently conducted a poll of his patients. In order of importance, full lips came after wide eyes, a straight nose, and a sharp jaw line. Full cheeks and a round face completed a list that diverges from the California-blond prototype.
Although Angelina Jolie was selected over several Lebanese actresses as a first choice for an icon of beauty in this same poll, Dr. Galadari pointed out that this actress has many of the features, including wide eyes, a straight nose, and full lips, that are consistent with traditional features of Arab beauty.
Perceptions of beauty are not just changing within ethnic groups but reflected in mass culture. Dr. Henry pointed to a published comparison of the “World’s Most Beautiful” list from People magazine in 2017 relative to 1990. Of the 50 celebrities on the list in 1990, 88% were Fitzpatrick skin types I-III. Only 12% were types IV-VI, which increased to almost 30% of the 135 celebrities on the list in 2017 (P = .01). In 1990, just one celebrity (2%) was of mixed race, which increased to 10.4% in 2017.
Among Hispanic women, the changes in attitude are perhaps best captured among younger relative to older patients requesting cosmetic work, according to Maritza I. Perez, MD, professor of dermatology, University of Connecticut, Farmington. She said that her younger patients are less likely to seek rhinoplasty and blepharoplasty relative to her older patients, a reflection perhaps of comfort with their natural looks.
However, “the celebration of Latinas as beautiful, seductive, and sexual is hardly new,” she said, indicating that younger Hispanic patients are probably not driven to modify their ethnic features because they are already widely admired. “Six of the 10 women crowned Miss Universe in the last decade were from Latin American countries,” she noted.
The general willingness of patients within ethnic groups and society as a whole to see ethnic features as admirable and attractive was generally regarded by all the panelists as a positive development.
Dr. Henry, who said she was “encouraged” by such trends as “the natural hair movement” and diminishing interest among her darker patients in lightening skin pigment, said, “I definitely see a change among my patients in regard to their goals.”
For clinicians offering consults to patients seeking cosmetic work, Dr. Henry recommended being aware and sensitive to this evolution in order to offer appropriate care.
Dr. Chiu, emphasizing the pride that many of her patients take in their Asian features, made the same recommendation. She credited globalization and social media for attitudes that have allowed an embrace of what are now far more inclusive standards of beauty.
Dr. Henry reports financial relationships with Allergan and Merz. Dr. Chiu has financial relationships with AbbVie, Cynosure, Merz, Revance, and Solta. Dr. Galadari reports financial relationships with nine pharmaceutical companies, including Allergan, Merz, Revance, and Fillmed Laboratories. Dr. Perez reports no relevant conflicts of interest.
Addressing current standards of beauty at the Skin of Color Update 2021, dermatologists speaking about attitudes within four ethnic groups recounted a similar story: .
This change is relevant to dermatologists consulting with patients for cosmetic procedures. Four dermatologists who recounted the types of procedures their patients are requesting each reported that more patients are seeking cosmetic enhancements that accentuate rather than modify ethnic features.
Lips in Black, Asian, and Arab ethnic groups are just one example.
“Where several years ago, the conversation was really about lip reductions – how we can deemphasize the lip – I am now seeing lots of women of color coming in to ask about lip augmentation, looking to highlight their lips as a point of beauty,” reported Michelle Henry, MD, a dermatologist who practices in New York City.
She is not alone. Others participating on the same panel spoke of a growing interest among their patients to maintain or even emphasize the same ethnic features – including but not limited to lip shape and size that they were once anxious to modify.
In Asian patients, “the goal is not to Westernize,” agreed Annie Chiu, MD, a dermatologist who practices in North Redondo Beach, Calif. For lips, she spoke of the “50-50 ratio” of upper and lower lip symmetry that is consistent with a traditional Asian characteristic.
Like Dr. Henry, Dr. Chiu said that many requests for cosmetic work now involve accentuating Asian features, such as the oval shape of the face, rather than steps to modify this shape. This is a relatively recent change.
“I am finding that more of my patients want to improve the esthetic balance to optimize the appearance within their own ethnicity,” she said.
In the United Arab Emirates (UAE), Hassan Galadari, MD, an American-trained physician who is assistant professor of dermatology at the UAE University in Dubai, recently conducted a poll of his patients. In order of importance, full lips came after wide eyes, a straight nose, and a sharp jaw line. Full cheeks and a round face completed a list that diverges from the California-blond prototype.
Although Angelina Jolie was selected over several Lebanese actresses as a first choice for an icon of beauty in this same poll, Dr. Galadari pointed out that this actress has many of the features, including wide eyes, a straight nose, and full lips, that are consistent with traditional features of Arab beauty.
Perceptions of beauty are not just changing within ethnic groups but reflected in mass culture. Dr. Henry pointed to a published comparison of the “World’s Most Beautiful” list from People magazine in 2017 relative to 1990. Of the 50 celebrities on the list in 1990, 88% were Fitzpatrick skin types I-III. Only 12% were types IV-VI, which increased to almost 30% of the 135 celebrities on the list in 2017 (P = .01). In 1990, just one celebrity (2%) was of mixed race, which increased to 10.4% in 2017.
Among Hispanic women, the changes in attitude are perhaps best captured among younger relative to older patients requesting cosmetic work, according to Maritza I. Perez, MD, professor of dermatology, University of Connecticut, Farmington. She said that her younger patients are less likely to seek rhinoplasty and blepharoplasty relative to her older patients, a reflection perhaps of comfort with their natural looks.
However, “the celebration of Latinas as beautiful, seductive, and sexual is hardly new,” she said, indicating that younger Hispanic patients are probably not driven to modify their ethnic features because they are already widely admired. “Six of the 10 women crowned Miss Universe in the last decade were from Latin American countries,” she noted.
The general willingness of patients within ethnic groups and society as a whole to see ethnic features as admirable and attractive was generally regarded by all the panelists as a positive development.
Dr. Henry, who said she was “encouraged” by such trends as “the natural hair movement” and diminishing interest among her darker patients in lightening skin pigment, said, “I definitely see a change among my patients in regard to their goals.”
For clinicians offering consults to patients seeking cosmetic work, Dr. Henry recommended being aware and sensitive to this evolution in order to offer appropriate care.
Dr. Chiu, emphasizing the pride that many of her patients take in their Asian features, made the same recommendation. She credited globalization and social media for attitudes that have allowed an embrace of what are now far more inclusive standards of beauty.
Dr. Henry reports financial relationships with Allergan and Merz. Dr. Chiu has financial relationships with AbbVie, Cynosure, Merz, Revance, and Solta. Dr. Galadari reports financial relationships with nine pharmaceutical companies, including Allergan, Merz, Revance, and Fillmed Laboratories. Dr. Perez reports no relevant conflicts of interest.
Addressing current standards of beauty at the Skin of Color Update 2021, dermatologists speaking about attitudes within four ethnic groups recounted a similar story: .
This change is relevant to dermatologists consulting with patients for cosmetic procedures. Four dermatologists who recounted the types of procedures their patients are requesting each reported that more patients are seeking cosmetic enhancements that accentuate rather than modify ethnic features.
Lips in Black, Asian, and Arab ethnic groups are just one example.
“Where several years ago, the conversation was really about lip reductions – how we can deemphasize the lip – I am now seeing lots of women of color coming in to ask about lip augmentation, looking to highlight their lips as a point of beauty,” reported Michelle Henry, MD, a dermatologist who practices in New York City.
She is not alone. Others participating on the same panel spoke of a growing interest among their patients to maintain or even emphasize the same ethnic features – including but not limited to lip shape and size that they were once anxious to modify.
In Asian patients, “the goal is not to Westernize,” agreed Annie Chiu, MD, a dermatologist who practices in North Redondo Beach, Calif. For lips, she spoke of the “50-50 ratio” of upper and lower lip symmetry that is consistent with a traditional Asian characteristic.
Like Dr. Henry, Dr. Chiu said that many requests for cosmetic work now involve accentuating Asian features, such as the oval shape of the face, rather than steps to modify this shape. This is a relatively recent change.
“I am finding that more of my patients want to improve the esthetic balance to optimize the appearance within their own ethnicity,” she said.
In the United Arab Emirates (UAE), Hassan Galadari, MD, an American-trained physician who is assistant professor of dermatology at the UAE University in Dubai, recently conducted a poll of his patients. In order of importance, full lips came after wide eyes, a straight nose, and a sharp jaw line. Full cheeks and a round face completed a list that diverges from the California-blond prototype.
Although Angelina Jolie was selected over several Lebanese actresses as a first choice for an icon of beauty in this same poll, Dr. Galadari pointed out that this actress has many of the features, including wide eyes, a straight nose, and full lips, that are consistent with traditional features of Arab beauty.
Perceptions of beauty are not just changing within ethnic groups but reflected in mass culture. Dr. Henry pointed to a published comparison of the “World’s Most Beautiful” list from People magazine in 2017 relative to 1990. Of the 50 celebrities on the list in 1990, 88% were Fitzpatrick skin types I-III. Only 12% were types IV-VI, which increased to almost 30% of the 135 celebrities on the list in 2017 (P = .01). In 1990, just one celebrity (2%) was of mixed race, which increased to 10.4% in 2017.
Among Hispanic women, the changes in attitude are perhaps best captured among younger relative to older patients requesting cosmetic work, according to Maritza I. Perez, MD, professor of dermatology, University of Connecticut, Farmington. She said that her younger patients are less likely to seek rhinoplasty and blepharoplasty relative to her older patients, a reflection perhaps of comfort with their natural looks.
However, “the celebration of Latinas as beautiful, seductive, and sexual is hardly new,” she said, indicating that younger Hispanic patients are probably not driven to modify their ethnic features because they are already widely admired. “Six of the 10 women crowned Miss Universe in the last decade were from Latin American countries,” she noted.
The general willingness of patients within ethnic groups and society as a whole to see ethnic features as admirable and attractive was generally regarded by all the panelists as a positive development.
Dr. Henry, who said she was “encouraged” by such trends as “the natural hair movement” and diminishing interest among her darker patients in lightening skin pigment, said, “I definitely see a change among my patients in regard to their goals.”
For clinicians offering consults to patients seeking cosmetic work, Dr. Henry recommended being aware and sensitive to this evolution in order to offer appropriate care.
Dr. Chiu, emphasizing the pride that many of her patients take in their Asian features, made the same recommendation. She credited globalization and social media for attitudes that have allowed an embrace of what are now far more inclusive standards of beauty.
Dr. Henry reports financial relationships with Allergan and Merz. Dr. Chiu has financial relationships with AbbVie, Cynosure, Merz, Revance, and Solta. Dr. Galadari reports financial relationships with nine pharmaceutical companies, including Allergan, Merz, Revance, and Fillmed Laboratories. Dr. Perez reports no relevant conflicts of interest.
FROM SOC 2021
Severe skin reactions with enfortumab vedotin
The cases came to light during routine surveillance, say staff from the division of pharmacovigilance of the Food and Drug Administration in a research letter published online Sept. 8, 2021, in JAMA Dermatology.
Eight cases of serious skin reactions characterized as SJS/TEN were identified from the FDA’s Adverse Event Reporting System (FAERS). In five of these cases, the diagnosis of SJS/TEN was confirmed by a dermatologist and/or biopsy findings.
The median time to onset of SJS/TEN was 11 days (range, 9-21 days) from the start of treatment.
In the eight cases, serious outcomes were reported. In four cases, deaths that were attributed to SJS/TEN occurred. “Other serious outcomes included admission to the burn unit in four cases,” the researchers wrote.
First-in-class agent
Enfortumab vedotin is a first-in-class agent directed against cell adhesion molecule nectin-4, which is located on the surface of cells and is highly expressed in bladder cancer. The product is an antibody conjugate, in which the antibody directs the product to these cells and then releases the cytoxic monomethyl auristantin E. It is administered intravenously.
The product was granted accelerated approval by the FDA in December 2019. This approval was based on response data from the EV-201 study, a phase 2 clinical trial that involved 125 patients with locally advanced or metastatic urothelial cancer who received prior treatment with a PD-1 or PD-L1 inhibitor and platinum-based chemotherapy.
The results were presented in June 2019 at the annual meeting of the American Society of Clinical Oncology. The overall response rate was 44%; 12% of patients achieved a complete response, and 32% had a partial response. The median duration of response was 7.6 months.
At the meeting, Daniel P. Petrylak, MD, professor of medicine (medical oncology) and urology at Yale Cancer Center, New Haven, Conn., noted that there is a “high unmet need” among patients with advanced and metastatic urothelial cancer. There has been a flurry of new drug approvals for this disease. Five immune checkpoint inhibitor drugs have been approved in recent years. Most patients (75%-80%) experience disease progression after receiving immunotherapy.
Enfortumab vedotin is the “first novel therapeutic to demonstrate substantial clinical activity” in patients whose disease has progressed after platinum chemotherapy and immunotherapies, commented Dr. Petrylak.
At the time, maculopapular rash of grade 3 or higher was reported in 4% of the cohort. That was the only serious dermatologic adverse event noted.
Clinically significant findings
The cases of severe skin reactions now being reported come from postmarketing surveillance, noted the authors, led by Michelle Nadeau Nguyen, PharmD, BCOP, BCPS. They reviewed data from FAERS, PubMed, and Embase from Dec. 18, 2019, the date the product was approved, to Oct. 7, 2020.
Other than the eight cases reported to FAERS, no additional cases were identified from PubMed or Embase.
The authors noted that, because cases of SJS/TEN are rare but serious, these well-documented postmarketing reports are clinically significant. “Moreover, we find the rapid accumulation of cases over an approximate 12-month marketing period a concerning observation,” they wrote.
The rate at which these reactions were reported is higher than would be expected, they commented.
The annual incidence of locally advanced urothelial cancer, the disease most likely to be treated with this drug, is around 12,494-40,000 cases per year in the United States. The expected incidence rate of SJS/TEN is about 1-7 cases per 1,000,000 patients. The team calculated from the reports that, among patients who received enfortumab vedotin, the rate was 20 cases per 1,000,000 patients.
This reporting rate is likely to be underestimated, inasmuch as underreporting is known to be a limitation of spontaneous reporting systems such as FAERS, the authors noted.
The mechanism for toxic skin effects with enfortumab vedotin is as yet unknown, but it may be related to the inhibitory effects of the drug on nectin-4 expression, they suggest. Nectin-4 is expressed by epithelial tissues, including skin.
Dr. Nguyen and colleagues noted that, on approval, the U.S. prescribing information for the drug noted that skin reactions were seen in 55% of patients in clinical trials.
The prescribing information was recently revised to include SJS/TEN and to recommend permanent discontinuance of the drug if cases of SJS/TEN are suspected.
“This revision is intended to increase clinicians’ awareness of the risk for SJS/TEN and mitigate serious outcomes by improving the likelihood of early identification and intervention,” they added.
The authors also encouraged continued reporting of adverse events with enfortumab vedotin to the FDA via the MedWatch portal.
The authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The cases came to light during routine surveillance, say staff from the division of pharmacovigilance of the Food and Drug Administration in a research letter published online Sept. 8, 2021, in JAMA Dermatology.
Eight cases of serious skin reactions characterized as SJS/TEN were identified from the FDA’s Adverse Event Reporting System (FAERS). In five of these cases, the diagnosis of SJS/TEN was confirmed by a dermatologist and/or biopsy findings.
The median time to onset of SJS/TEN was 11 days (range, 9-21 days) from the start of treatment.
In the eight cases, serious outcomes were reported. In four cases, deaths that were attributed to SJS/TEN occurred. “Other serious outcomes included admission to the burn unit in four cases,” the researchers wrote.
First-in-class agent
Enfortumab vedotin is a first-in-class agent directed against cell adhesion molecule nectin-4, which is located on the surface of cells and is highly expressed in bladder cancer. The product is an antibody conjugate, in which the antibody directs the product to these cells and then releases the cytoxic monomethyl auristantin E. It is administered intravenously.
The product was granted accelerated approval by the FDA in December 2019. This approval was based on response data from the EV-201 study, a phase 2 clinical trial that involved 125 patients with locally advanced or metastatic urothelial cancer who received prior treatment with a PD-1 or PD-L1 inhibitor and platinum-based chemotherapy.
The results were presented in June 2019 at the annual meeting of the American Society of Clinical Oncology. The overall response rate was 44%; 12% of patients achieved a complete response, and 32% had a partial response. The median duration of response was 7.6 months.
At the meeting, Daniel P. Petrylak, MD, professor of medicine (medical oncology) and urology at Yale Cancer Center, New Haven, Conn., noted that there is a “high unmet need” among patients with advanced and metastatic urothelial cancer. There has been a flurry of new drug approvals for this disease. Five immune checkpoint inhibitor drugs have been approved in recent years. Most patients (75%-80%) experience disease progression after receiving immunotherapy.
Enfortumab vedotin is the “first novel therapeutic to demonstrate substantial clinical activity” in patients whose disease has progressed after platinum chemotherapy and immunotherapies, commented Dr. Petrylak.
At the time, maculopapular rash of grade 3 or higher was reported in 4% of the cohort. That was the only serious dermatologic adverse event noted.
Clinically significant findings
The cases of severe skin reactions now being reported come from postmarketing surveillance, noted the authors, led by Michelle Nadeau Nguyen, PharmD, BCOP, BCPS. They reviewed data from FAERS, PubMed, and Embase from Dec. 18, 2019, the date the product was approved, to Oct. 7, 2020.
Other than the eight cases reported to FAERS, no additional cases were identified from PubMed or Embase.
The authors noted that, because cases of SJS/TEN are rare but serious, these well-documented postmarketing reports are clinically significant. “Moreover, we find the rapid accumulation of cases over an approximate 12-month marketing period a concerning observation,” they wrote.
The rate at which these reactions were reported is higher than would be expected, they commented.
The annual incidence of locally advanced urothelial cancer, the disease most likely to be treated with this drug, is around 12,494-40,000 cases per year in the United States. The expected incidence rate of SJS/TEN is about 1-7 cases per 1,000,000 patients. The team calculated from the reports that, among patients who received enfortumab vedotin, the rate was 20 cases per 1,000,000 patients.
This reporting rate is likely to be underestimated, inasmuch as underreporting is known to be a limitation of spontaneous reporting systems such as FAERS, the authors noted.
The mechanism for toxic skin effects with enfortumab vedotin is as yet unknown, but it may be related to the inhibitory effects of the drug on nectin-4 expression, they suggest. Nectin-4 is expressed by epithelial tissues, including skin.
Dr. Nguyen and colleagues noted that, on approval, the U.S. prescribing information for the drug noted that skin reactions were seen in 55% of patients in clinical trials.
The prescribing information was recently revised to include SJS/TEN and to recommend permanent discontinuance of the drug if cases of SJS/TEN are suspected.
“This revision is intended to increase clinicians’ awareness of the risk for SJS/TEN and mitigate serious outcomes by improving the likelihood of early identification and intervention,” they added.
The authors also encouraged continued reporting of adverse events with enfortumab vedotin to the FDA via the MedWatch portal.
The authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The cases came to light during routine surveillance, say staff from the division of pharmacovigilance of the Food and Drug Administration in a research letter published online Sept. 8, 2021, in JAMA Dermatology.
Eight cases of serious skin reactions characterized as SJS/TEN were identified from the FDA’s Adverse Event Reporting System (FAERS). In five of these cases, the diagnosis of SJS/TEN was confirmed by a dermatologist and/or biopsy findings.
The median time to onset of SJS/TEN was 11 days (range, 9-21 days) from the start of treatment.
In the eight cases, serious outcomes were reported. In four cases, deaths that were attributed to SJS/TEN occurred. “Other serious outcomes included admission to the burn unit in four cases,” the researchers wrote.
First-in-class agent
Enfortumab vedotin is a first-in-class agent directed against cell adhesion molecule nectin-4, which is located on the surface of cells and is highly expressed in bladder cancer. The product is an antibody conjugate, in which the antibody directs the product to these cells and then releases the cytoxic monomethyl auristantin E. It is administered intravenously.
The product was granted accelerated approval by the FDA in December 2019. This approval was based on response data from the EV-201 study, a phase 2 clinical trial that involved 125 patients with locally advanced or metastatic urothelial cancer who received prior treatment with a PD-1 or PD-L1 inhibitor and platinum-based chemotherapy.
The results were presented in June 2019 at the annual meeting of the American Society of Clinical Oncology. The overall response rate was 44%; 12% of patients achieved a complete response, and 32% had a partial response. The median duration of response was 7.6 months.
At the meeting, Daniel P. Petrylak, MD, professor of medicine (medical oncology) and urology at Yale Cancer Center, New Haven, Conn., noted that there is a “high unmet need” among patients with advanced and metastatic urothelial cancer. There has been a flurry of new drug approvals for this disease. Five immune checkpoint inhibitor drugs have been approved in recent years. Most patients (75%-80%) experience disease progression after receiving immunotherapy.
Enfortumab vedotin is the “first novel therapeutic to demonstrate substantial clinical activity” in patients whose disease has progressed after platinum chemotherapy and immunotherapies, commented Dr. Petrylak.
At the time, maculopapular rash of grade 3 or higher was reported in 4% of the cohort. That was the only serious dermatologic adverse event noted.
Clinically significant findings
The cases of severe skin reactions now being reported come from postmarketing surveillance, noted the authors, led by Michelle Nadeau Nguyen, PharmD, BCOP, BCPS. They reviewed data from FAERS, PubMed, and Embase from Dec. 18, 2019, the date the product was approved, to Oct. 7, 2020.
Other than the eight cases reported to FAERS, no additional cases were identified from PubMed or Embase.
The authors noted that, because cases of SJS/TEN are rare but serious, these well-documented postmarketing reports are clinically significant. “Moreover, we find the rapid accumulation of cases over an approximate 12-month marketing period a concerning observation,” they wrote.
The rate at which these reactions were reported is higher than would be expected, they commented.
The annual incidence of locally advanced urothelial cancer, the disease most likely to be treated with this drug, is around 12,494-40,000 cases per year in the United States. The expected incidence rate of SJS/TEN is about 1-7 cases per 1,000,000 patients. The team calculated from the reports that, among patients who received enfortumab vedotin, the rate was 20 cases per 1,000,000 patients.
This reporting rate is likely to be underestimated, inasmuch as underreporting is known to be a limitation of spontaneous reporting systems such as FAERS, the authors noted.
The mechanism for toxic skin effects with enfortumab vedotin is as yet unknown, but it may be related to the inhibitory effects of the drug on nectin-4 expression, they suggest. Nectin-4 is expressed by epithelial tissues, including skin.
Dr. Nguyen and colleagues noted that, on approval, the U.S. prescribing information for the drug noted that skin reactions were seen in 55% of patients in clinical trials.
The prescribing information was recently revised to include SJS/TEN and to recommend permanent discontinuance of the drug if cases of SJS/TEN are suspected.
“This revision is intended to increase clinicians’ awareness of the risk for SJS/TEN and mitigate serious outcomes by improving the likelihood of early identification and intervention,” they added.
The authors also encouraged continued reporting of adverse events with enfortumab vedotin to the FDA via the MedWatch portal.
The authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
New Moderna vaccine data ‘support’ booster shot after 8 months
Moderna has released new data that it said support the argument for COVID-19 booster shots – specifically showing that people who received a first shot of their mRNA vaccine a median of 13 months ago are more likely to experience a breakthrough infection compared to individuals who received a first shot a median of 8 months ago.
The findings come from the ongoing phase 3 COVE clinical trial, the results of which the Food and Drug Administration considered in granting emergency use authorization for the vaccine. In the initial stage of the trial, people were randomly assigned to receive the company’s mRNA vaccine or placebo.
according to the analysis of the open-label extension of the study during which placebo participants could cross over and get immunized as well.
The updated COVE trial data show that 88 breakthrough cases of COVID-19 occurred among 11,431 participants vaccinated between December 2020 and March 2021 (49.0 cases per 1,000 person-years).
In contrast, there were 162 breakthrough cases among 14,746 people vaccinated between July and October 2020 (77.1 cases per 1,000 person-years).
The breakthrough infections include 19 severe cases. Although not statically different, there was a trend toward fewer severe cases among the more recently vaccinated, at a rate of 3.3 per 1,000 person-years, compared with 6.2 per 1,000 person-years in the group vaccinated in 2020
The findings were posted as a preprint to the medRxiv server and have not yet been peer reviewed.
“The increased risk of breakthrough infections in COVE study participants who were vaccinated last year compared to more recently illustrates the impact of waning immunity and supports the need for a booster to maintain high levels of protection,” Moderna CEO Stéphane Bancel said in a company statement.
An FDA advisory committee is meeting Sept. 17 to look at the available evidence on boosters to help the agency decide whether the additional shots are warranted.
There is still a lot of debate in the medical community about the need for boosters. U.S. physicians and nurses are divided about the need for them and about how the country should prioritize its vaccine supplies, according to a Medscape poll of more than 1,700 clinicians that collected responses from Aug. 25 to Sept. 6, 2020.
The research was funded by Moderna, and also supported by the Office of the Assistant Secretary for Preparedness and Response, Biomedical Advanced Research and Development Authority, and by the National Institute of Allergy and Infectious Diseases.
A version of this article first appeared on Medscape.com.
Moderna has released new data that it said support the argument for COVID-19 booster shots – specifically showing that people who received a first shot of their mRNA vaccine a median of 13 months ago are more likely to experience a breakthrough infection compared to individuals who received a first shot a median of 8 months ago.
The findings come from the ongoing phase 3 COVE clinical trial, the results of which the Food and Drug Administration considered in granting emergency use authorization for the vaccine. In the initial stage of the trial, people were randomly assigned to receive the company’s mRNA vaccine or placebo.
according to the analysis of the open-label extension of the study during which placebo participants could cross over and get immunized as well.
The updated COVE trial data show that 88 breakthrough cases of COVID-19 occurred among 11,431 participants vaccinated between December 2020 and March 2021 (49.0 cases per 1,000 person-years).
In contrast, there were 162 breakthrough cases among 14,746 people vaccinated between July and October 2020 (77.1 cases per 1,000 person-years).
The breakthrough infections include 19 severe cases. Although not statically different, there was a trend toward fewer severe cases among the more recently vaccinated, at a rate of 3.3 per 1,000 person-years, compared with 6.2 per 1,000 person-years in the group vaccinated in 2020
The findings were posted as a preprint to the medRxiv server and have not yet been peer reviewed.
“The increased risk of breakthrough infections in COVE study participants who were vaccinated last year compared to more recently illustrates the impact of waning immunity and supports the need for a booster to maintain high levels of protection,” Moderna CEO Stéphane Bancel said in a company statement.
An FDA advisory committee is meeting Sept. 17 to look at the available evidence on boosters to help the agency decide whether the additional shots are warranted.
There is still a lot of debate in the medical community about the need for boosters. U.S. physicians and nurses are divided about the need for them and about how the country should prioritize its vaccine supplies, according to a Medscape poll of more than 1,700 clinicians that collected responses from Aug. 25 to Sept. 6, 2020.
The research was funded by Moderna, and also supported by the Office of the Assistant Secretary for Preparedness and Response, Biomedical Advanced Research and Development Authority, and by the National Institute of Allergy and Infectious Diseases.
A version of this article first appeared on Medscape.com.
Moderna has released new data that it said support the argument for COVID-19 booster shots – specifically showing that people who received a first shot of their mRNA vaccine a median of 13 months ago are more likely to experience a breakthrough infection compared to individuals who received a first shot a median of 8 months ago.
The findings come from the ongoing phase 3 COVE clinical trial, the results of which the Food and Drug Administration considered in granting emergency use authorization for the vaccine. In the initial stage of the trial, people were randomly assigned to receive the company’s mRNA vaccine or placebo.
according to the analysis of the open-label extension of the study during which placebo participants could cross over and get immunized as well.
The updated COVE trial data show that 88 breakthrough cases of COVID-19 occurred among 11,431 participants vaccinated between December 2020 and March 2021 (49.0 cases per 1,000 person-years).
In contrast, there were 162 breakthrough cases among 14,746 people vaccinated between July and October 2020 (77.1 cases per 1,000 person-years).
The breakthrough infections include 19 severe cases. Although not statically different, there was a trend toward fewer severe cases among the more recently vaccinated, at a rate of 3.3 per 1,000 person-years, compared with 6.2 per 1,000 person-years in the group vaccinated in 2020
The findings were posted as a preprint to the medRxiv server and have not yet been peer reviewed.
“The increased risk of breakthrough infections in COVE study participants who were vaccinated last year compared to more recently illustrates the impact of waning immunity and supports the need for a booster to maintain high levels of protection,” Moderna CEO Stéphane Bancel said in a company statement.
An FDA advisory committee is meeting Sept. 17 to look at the available evidence on boosters to help the agency decide whether the additional shots are warranted.
There is still a lot of debate in the medical community about the need for boosters. U.S. physicians and nurses are divided about the need for them and about how the country should prioritize its vaccine supplies, according to a Medscape poll of more than 1,700 clinicians that collected responses from Aug. 25 to Sept. 6, 2020.
The research was funded by Moderna, and also supported by the Office of the Assistant Secretary for Preparedness and Response, Biomedical Advanced Research and Development Authority, and by the National Institute of Allergy and Infectious Diseases.
A version of this article first appeared on Medscape.com.
When the juggling act becomes impossible
Objectivity is tough, but essential: a critical part of patient care, allowing you to make appropriate decisions based on facts and circumstances, not emotions. We’re supposed to be compassionate Vulcans – able to logically weigh possibilities and treatment options under pressure, and at the same time exhibit empathy and sensitivity.
For the most part, all of us become very good at this juggling act. But we’re only human, and once the ability to do that with a given person is lost, it’s gone for good.
Have you ever lost objectivity with a patient? I have. Generally it involves the patient being so difficult, unpleasant, or dislikable that it exceeds my ability to remain impartial and pragmatic in their care.
I don’t know any physician it hasn’t happened to. And when it does, ending the doctor-patient relationship is the only effective answer.
It’s never easy sending that letter, telling someone that they need to seek care elsewhere, and often the specific reason is harder to define. In patients who are overtly rude or noncompliant it’s easy. But often a loss in objectivity is from something less tangible, such as the vagaries of personal chemistry.
I try to get along with all my patients. I really do. That’s part of the job. But sometimes, for whatever reason, it’s just an impossible task. Too many conflicts and differences of opinion over treatments, tests, diagnosis, what they read on Facebook … whatever.
Regardless of cause, professionalism requires that it be the end of the road. If I can’t objectively weigh a patient’s symptoms and treatment options, then I’m not going to be able to do my very best for them. And my very best is what every patient deserves.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Objectivity is tough, but essential: a critical part of patient care, allowing you to make appropriate decisions based on facts and circumstances, not emotions. We’re supposed to be compassionate Vulcans – able to logically weigh possibilities and treatment options under pressure, and at the same time exhibit empathy and sensitivity.
For the most part, all of us become very good at this juggling act. But we’re only human, and once the ability to do that with a given person is lost, it’s gone for good.
Have you ever lost objectivity with a patient? I have. Generally it involves the patient being so difficult, unpleasant, or dislikable that it exceeds my ability to remain impartial and pragmatic in their care.
I don’t know any physician it hasn’t happened to. And when it does, ending the doctor-patient relationship is the only effective answer.
It’s never easy sending that letter, telling someone that they need to seek care elsewhere, and often the specific reason is harder to define. In patients who are overtly rude or noncompliant it’s easy. But often a loss in objectivity is from something less tangible, such as the vagaries of personal chemistry.
I try to get along with all my patients. I really do. That’s part of the job. But sometimes, for whatever reason, it’s just an impossible task. Too many conflicts and differences of opinion over treatments, tests, diagnosis, what they read on Facebook … whatever.
Regardless of cause, professionalism requires that it be the end of the road. If I can’t objectively weigh a patient’s symptoms and treatment options, then I’m not going to be able to do my very best for them. And my very best is what every patient deserves.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Objectivity is tough, but essential: a critical part of patient care, allowing you to make appropriate decisions based on facts and circumstances, not emotions. We’re supposed to be compassionate Vulcans – able to logically weigh possibilities and treatment options under pressure, and at the same time exhibit empathy and sensitivity.
For the most part, all of us become very good at this juggling act. But we’re only human, and once the ability to do that with a given person is lost, it’s gone for good.
Have you ever lost objectivity with a patient? I have. Generally it involves the patient being so difficult, unpleasant, or dislikable that it exceeds my ability to remain impartial and pragmatic in their care.
I don’t know any physician it hasn’t happened to. And when it does, ending the doctor-patient relationship is the only effective answer.
It’s never easy sending that letter, telling someone that they need to seek care elsewhere, and often the specific reason is harder to define. In patients who are overtly rude or noncompliant it’s easy. But often a loss in objectivity is from something less tangible, such as the vagaries of personal chemistry.
I try to get along with all my patients. I really do. That’s part of the job. But sometimes, for whatever reason, it’s just an impossible task. Too many conflicts and differences of opinion over treatments, tests, diagnosis, what they read on Facebook … whatever.
Regardless of cause, professionalism requires that it be the end of the road. If I can’t objectively weigh a patient’s symptoms and treatment options, then I’m not going to be able to do my very best for them. And my very best is what every patient deserves.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
COVID vaccine preprint study prompts Twitter outrage
A preprint study finding that the Pfizer-BioNTech mRNA COVID vaccine is associated with an increased risk for cardiac adverse events in teenage boys has elicited a firestorm on Twitter. Although some people issued thoughtful critiques, others lobbed insults against the authors, and still others accused them of either being antivaccine or stoking the fires of the vaccine skeptic movement.
The controversy began soon after the study was posted online September 8 on medRxiv. The authors conclude that for boys, the risk for a cardiac adverse event or hospitalization after the second dose of the Pfizer mRNA vaccine was “considerably higher” than the 120-day risk for hospitalization for COVID-19, “even at times of peak disease prevalence.” This was especially true for those aged 12 to 15 years and even those with no underlying health conditions.
The conclusion – as well as the paper’s source, the Vaccine Adverse Event Reporting System (VAERS), and its methodology, modeled after the Centers for Disease Control and Prevention assessment of the database – did not sit well with many.
“Your methodology hugely overestimates risk, which many commentators who are specialists in the field have highlighted,” tweeted Deepti Gurdasani, senior lecturer in epidemiology at Queen Mary University of London. “Why make this claim when you must know it’s wrong?”
“The authors don’t know what they are doing and they are following their own ideology,” tweeted Boback Ziaeian, MD, PhD, assistant professor of medicine at the University of California, Los Angeles, in the cardiology division. Dr. Ziaeian also tweeted, “I believe the CDC is doing honest work and not dredging slop like you are.”
“Holy shit. Truly terrible methods in that paper,” tweeted Michael Mina, MD, PhD, an epidemiologist and immunologist at the Harvard School of Public Health, Boston, more bluntly.
Some pointed out that VAERS is often used by vaccine skeptics to spread misinformation. “‘Dumpster diving’ describes studies using #VAERS by authors (almost always antivaxxers) who don’t understand its limitations,” tweeted David Gorski, MD, PhD, the editor of Science-Based Medicine, who says in his Twitter bio that he “exposes quackery.”
Added Dr. Gorski: “Doctors fell into this trap with their study suggesting #CovidVaccine is more dangerous to children than #COVID19.”
Dr. Gorski said he did not think that the authors were antivaccine. But, he tweeted, “I’d argue that at least one of the authors (Stevenson) is grossly unqualified to analyze the data. Mandrola? Marginal. The other two *might* be qualified in public health/epi, but they clearly either had no clue about #VAERS limitations or didn’t take them seriously enough.”
Two of the authors, John Mandrola, MD, a cardiac electrophysiologist who is also a columnist for Medscape, and Tracy Beth Hoeg, MD, PhD, an epidemiologist and sports medicine specialist, told this news organization that their estimates are not definitive, owing to the nature of the VAERS database.
“I want to emphasize that our signal is hypothesis-generating,” said Dr. Mandrola. “There’s obviously more research that needs to be done.”
“I don’t think it should be used to establish a for-certain rate,” said Dr. Hoeg, about the study. “It’s not a perfect way of establishing what the rate of cardiac adverse events was, but it gives you an estimate, and generally with VAERS, it’s a significant underestimate.”
Both Dr. Hoeg and Dr. Mandrola said their analysis showed enough of a signal that it warranted a rush to publish. “We felt that it was super time-sensitive,” Dr. Mandrola said.
Vaccine risks versus COVID harm
The authors searched the VAERS system for children aged 12 to 17 years who had received one or two doses of an mRNA vaccine and had symptoms of myocarditis, pericarditis, myopericarditis, or chest pain, and also troponin levels available in the lab data.
Of the 257 patients they examined, 211 had peak troponin values available for analysis. All but one received the Pfizer vaccine. Results were stratified by age and sex.
The authors found that the rates of cardiac adverse events (CAEs) after dose 1 were 12.0 per million for 12- to 15-year-old boys and 8.2 per million for 16- and 17-year-old boys, compared with 0.0 per million and 2.0 per million for girls the same ages.
The estimates for the 12- to 15-year-old boys were 22% to 150% higher than what the CDC had previously reported.
After the second dose, the rate of CAEs for boys 12 to 15 years was 162.2 per million (143% to 280% higher than the CDC estimate) and for boys 16 and 17 years, it was 94.0 per million, or 30% to 40% higher than CDC estimate.
Dr. Mandrola said he and his colleagues found potentially more cases by using slightly broader search terms than those employed by the CDC but agreed with some critics that a limitation was that they did not call the reporting physicians, as is typical with CDC follow-up on VAERS reports.
The authors point to troponin levels as valid indicators of myocardial damage. Peak troponin levels exceeded 2 ng/mL in 71% of the 12- to 15-year-olds and 82% of 16- and 17-year-olds.
The study shows that for boys 12 to 15 years with no comorbidities, the risk for a CAE after the second dose would be 22.8 times higher than the risk for hospitalization for COVID-19 during periods of low disease burden, 6.0 times higher during periods of moderate transmission, and 4.3 times higher during periods of high transmission.
The authors acknowledge in the paper that their analysis “does not take into account any benefits the vaccine provides against transmission to others, long-term COVID-19 disease risk, or protection from nonsevere COVID-19 symptoms.”
Both Dr. Mandrola and Dr. Hoeg told this news organization that they are currently recalculating their estimates because of the rising numbers of pediatric hospitalizations from the Delta variant surge.
Paper rejected by journals
Dr. Hoeg said in an interview that the paper went through peer-review at three journals but was rejected by all three, for reasons that were not made clear.
She and the other authors incorporated the reviewers’ feedback at each turn and included all of their suggestions in the paper that was ultimately uploaded to medRxiv, said Dr. Hoeg.
They decided to put it out as a preprint after the U.S. Food and Drug Administration issued its data and then a warning on June 25 about myocarditis with use of the Pfizer vaccine in children 12 to 15 years of age.
The preprint study was picked up by some media outlets, including The Telegraph and The Guardian newspapers, and tweeted out by vaccine skeptics like Robert W. Malone, MD.
Rep. Marjorie Taylor Greene (R-Georgia), an outspoken vaccine skeptic, tweeted out the Guardian story saying that the findings mean “there is every reason to stop the covid vaccine mandates.”
Dr. Gorski noted in tweets and in a blog post that one of the paper’s coauthors, Josh Stevenson, is part of Rational Ground, a group that supports the Great Barrington Declaration and is against lockdowns and mask mandates.
Mr. Stevenson did not disclose his affiliation in the paper, and Dr. Hoeg said in an interview that she was unaware of the group and Mr. Stevenson’s association with it and that she did not have the impression that he was altering the data to show any bias.
Both Dr. Mandrola and Dr. Hoeg said they are provaccine and that they were dismayed to find their work being used to support any agenda. “It’s very frustrating,” said Dr. Hoeg, adding that she understands that “when you publish research on a controversial topic, people are going to take it and use it for their agendas.”
Some on Twitter blamed the open and free-wheeling nature of preprints.
Harlan Krumholz, MD, SM, the Harold H. Hines, junior professor of medicine and public health at Yale University, New Haven, Conn., which oversees medRxiv, tweeted, “Do you get that the discussion about the preprint is exactly the purpose of #preprints. So that way when someone claims something, you can look at the source and experts can comment.”
But Dr. Ziaeian tweeted back, “Preprints like this one can be weaponized to stir anti-vaccine lies and damage public health.”
In turn, the Yale physician replied, “Unfortunately these days, almost anything can be weaponized, distorted, misunderstood.” Dr. Krumholz added: “There is no question that this preprint is worthy of deep vetting and discussion. But there is a #preprint artifact to examine.”
Measured support
Some clinicians signaled their support for open debate and the preprint’s findings.
“I’ve been very critical of preprints that are too quickly disseminated in the media, and this one is no exception,” tweeted Walid Gellad, MD, MPH, associate professor of medicine at the University of Pittsburgh. “On the other hand, I think the vitriol directed at these authors is wrong,” he added.
“Like it or not, the issue of myocarditis in kids is an issue. Other countries have made vaccination decisions because of this issue, not because they’re driven by some ideology,” he tweeted.
Dr. Gellad also notes that the FDA has estimated the risk could be as high as one in 5,000 and that the preprint numbers could actually be underestimates.
In a long thread, Frank Han, MD, an adult congenital and pediatric cardiologist at the University of Illinois, tweets that relying on the VAERS reports might be faulty and that advanced cardiac imaging – guided by strict criteria – is the best way to determine myocarditis. And, he tweeted, “Physician review of VAERS reports really matters.”
Dr. Han concluded that vaccination “trades in a significant risk with a much smaller risk. That’s what counts in the end.”
In a response, Dr. Mandrola called Han’s tweets “reasoned criticism of our analysis.” He adds that his and Dr. Hoeg’s study have limits, but “our point is not to avoid protecting kids, but how to do so most safely.”
Both Dr. Mandrola and Dr. Hoeg said they welcomed critiques, but they felt blindsided by the vehemence of some of the Twitter debate.
“Some of the vitriol was surprising,” Dr. Mandrola said. “I kind of have this naive notion that people would assume that we’re not bad people,” he added.
However, Dr. Mandrola is known on Twitter for sometimes being highly critical of other researchers’ work, referring to some studies as “howlers,” and has in the past called out others for citing those papers.
Dr. Hoeg said she found critiques about weaknesses in the methods to be helpful. But she said many tweets were “attacking us as people, or not really attacking anything about our study, but just attacking the finding,” which does not help anyone “figure out what we should do about the safety signal or how we can research it further.”
Said Dr. Mandrola: “Why would we just ignore that and go forward with two-shot vaccination as a mandate when other countries are looking at other strategies?”
He noted that the United Kingdom has announced that children 12 to 15 years of age should receive just one shot of the mRNA vaccines instead of two because of the risk for myocarditis. Sixteen- to 18-year-olds have already been advised to get only one dose.
A version of this article first appeared on Medscape.com.
A preprint study finding that the Pfizer-BioNTech mRNA COVID vaccine is associated with an increased risk for cardiac adverse events in teenage boys has elicited a firestorm on Twitter. Although some people issued thoughtful critiques, others lobbed insults against the authors, and still others accused them of either being antivaccine or stoking the fires of the vaccine skeptic movement.
The controversy began soon after the study was posted online September 8 on medRxiv. The authors conclude that for boys, the risk for a cardiac adverse event or hospitalization after the second dose of the Pfizer mRNA vaccine was “considerably higher” than the 120-day risk for hospitalization for COVID-19, “even at times of peak disease prevalence.” This was especially true for those aged 12 to 15 years and even those with no underlying health conditions.
The conclusion – as well as the paper’s source, the Vaccine Adverse Event Reporting System (VAERS), and its methodology, modeled after the Centers for Disease Control and Prevention assessment of the database – did not sit well with many.
“Your methodology hugely overestimates risk, which many commentators who are specialists in the field have highlighted,” tweeted Deepti Gurdasani, senior lecturer in epidemiology at Queen Mary University of London. “Why make this claim when you must know it’s wrong?”
“The authors don’t know what they are doing and they are following their own ideology,” tweeted Boback Ziaeian, MD, PhD, assistant professor of medicine at the University of California, Los Angeles, in the cardiology division. Dr. Ziaeian also tweeted, “I believe the CDC is doing honest work and not dredging slop like you are.”
“Holy shit. Truly terrible methods in that paper,” tweeted Michael Mina, MD, PhD, an epidemiologist and immunologist at the Harvard School of Public Health, Boston, more bluntly.
Some pointed out that VAERS is often used by vaccine skeptics to spread misinformation. “‘Dumpster diving’ describes studies using #VAERS by authors (almost always antivaxxers) who don’t understand its limitations,” tweeted David Gorski, MD, PhD, the editor of Science-Based Medicine, who says in his Twitter bio that he “exposes quackery.”
Added Dr. Gorski: “Doctors fell into this trap with their study suggesting #CovidVaccine is more dangerous to children than #COVID19.”
Dr. Gorski said he did not think that the authors were antivaccine. But, he tweeted, “I’d argue that at least one of the authors (Stevenson) is grossly unqualified to analyze the data. Mandrola? Marginal. The other two *might* be qualified in public health/epi, but they clearly either had no clue about #VAERS limitations or didn’t take them seriously enough.”
Two of the authors, John Mandrola, MD, a cardiac electrophysiologist who is also a columnist for Medscape, and Tracy Beth Hoeg, MD, PhD, an epidemiologist and sports medicine specialist, told this news organization that their estimates are not definitive, owing to the nature of the VAERS database.
“I want to emphasize that our signal is hypothesis-generating,” said Dr. Mandrola. “There’s obviously more research that needs to be done.”
“I don’t think it should be used to establish a for-certain rate,” said Dr. Hoeg, about the study. “It’s not a perfect way of establishing what the rate of cardiac adverse events was, but it gives you an estimate, and generally with VAERS, it’s a significant underestimate.”
Both Dr. Hoeg and Dr. Mandrola said their analysis showed enough of a signal that it warranted a rush to publish. “We felt that it was super time-sensitive,” Dr. Mandrola said.
Vaccine risks versus COVID harm
The authors searched the VAERS system for children aged 12 to 17 years who had received one or two doses of an mRNA vaccine and had symptoms of myocarditis, pericarditis, myopericarditis, or chest pain, and also troponin levels available in the lab data.
Of the 257 patients they examined, 211 had peak troponin values available for analysis. All but one received the Pfizer vaccine. Results were stratified by age and sex.
The authors found that the rates of cardiac adverse events (CAEs) after dose 1 were 12.0 per million for 12- to 15-year-old boys and 8.2 per million for 16- and 17-year-old boys, compared with 0.0 per million and 2.0 per million for girls the same ages.
The estimates for the 12- to 15-year-old boys were 22% to 150% higher than what the CDC had previously reported.
After the second dose, the rate of CAEs for boys 12 to 15 years was 162.2 per million (143% to 280% higher than the CDC estimate) and for boys 16 and 17 years, it was 94.0 per million, or 30% to 40% higher than CDC estimate.
Dr. Mandrola said he and his colleagues found potentially more cases by using slightly broader search terms than those employed by the CDC but agreed with some critics that a limitation was that they did not call the reporting physicians, as is typical with CDC follow-up on VAERS reports.
The authors point to troponin levels as valid indicators of myocardial damage. Peak troponin levels exceeded 2 ng/mL in 71% of the 12- to 15-year-olds and 82% of 16- and 17-year-olds.
The study shows that for boys 12 to 15 years with no comorbidities, the risk for a CAE after the second dose would be 22.8 times higher than the risk for hospitalization for COVID-19 during periods of low disease burden, 6.0 times higher during periods of moderate transmission, and 4.3 times higher during periods of high transmission.
The authors acknowledge in the paper that their analysis “does not take into account any benefits the vaccine provides against transmission to others, long-term COVID-19 disease risk, or protection from nonsevere COVID-19 symptoms.”
Both Dr. Mandrola and Dr. Hoeg told this news organization that they are currently recalculating their estimates because of the rising numbers of pediatric hospitalizations from the Delta variant surge.
Paper rejected by journals
Dr. Hoeg said in an interview that the paper went through peer-review at three journals but was rejected by all three, for reasons that were not made clear.
She and the other authors incorporated the reviewers’ feedback at each turn and included all of their suggestions in the paper that was ultimately uploaded to medRxiv, said Dr. Hoeg.
They decided to put it out as a preprint after the U.S. Food and Drug Administration issued its data and then a warning on June 25 about myocarditis with use of the Pfizer vaccine in children 12 to 15 years of age.
The preprint study was picked up by some media outlets, including The Telegraph and The Guardian newspapers, and tweeted out by vaccine skeptics like Robert W. Malone, MD.
Rep. Marjorie Taylor Greene (R-Georgia), an outspoken vaccine skeptic, tweeted out the Guardian story saying that the findings mean “there is every reason to stop the covid vaccine mandates.”
Dr. Gorski noted in tweets and in a blog post that one of the paper’s coauthors, Josh Stevenson, is part of Rational Ground, a group that supports the Great Barrington Declaration and is against lockdowns and mask mandates.
Mr. Stevenson did not disclose his affiliation in the paper, and Dr. Hoeg said in an interview that she was unaware of the group and Mr. Stevenson’s association with it and that she did not have the impression that he was altering the data to show any bias.
Both Dr. Mandrola and Dr. Hoeg said they are provaccine and that they were dismayed to find their work being used to support any agenda. “It’s very frustrating,” said Dr. Hoeg, adding that she understands that “when you publish research on a controversial topic, people are going to take it and use it for their agendas.”
Some on Twitter blamed the open and free-wheeling nature of preprints.
Harlan Krumholz, MD, SM, the Harold H. Hines, junior professor of medicine and public health at Yale University, New Haven, Conn., which oversees medRxiv, tweeted, “Do you get that the discussion about the preprint is exactly the purpose of #preprints. So that way when someone claims something, you can look at the source and experts can comment.”
But Dr. Ziaeian tweeted back, “Preprints like this one can be weaponized to stir anti-vaccine lies and damage public health.”
In turn, the Yale physician replied, “Unfortunately these days, almost anything can be weaponized, distorted, misunderstood.” Dr. Krumholz added: “There is no question that this preprint is worthy of deep vetting and discussion. But there is a #preprint artifact to examine.”
Measured support
Some clinicians signaled their support for open debate and the preprint’s findings.
“I’ve been very critical of preprints that are too quickly disseminated in the media, and this one is no exception,” tweeted Walid Gellad, MD, MPH, associate professor of medicine at the University of Pittsburgh. “On the other hand, I think the vitriol directed at these authors is wrong,” he added.
“Like it or not, the issue of myocarditis in kids is an issue. Other countries have made vaccination decisions because of this issue, not because they’re driven by some ideology,” he tweeted.
Dr. Gellad also notes that the FDA has estimated the risk could be as high as one in 5,000 and that the preprint numbers could actually be underestimates.
In a long thread, Frank Han, MD, an adult congenital and pediatric cardiologist at the University of Illinois, tweets that relying on the VAERS reports might be faulty and that advanced cardiac imaging – guided by strict criteria – is the best way to determine myocarditis. And, he tweeted, “Physician review of VAERS reports really matters.”
Dr. Han concluded that vaccination “trades in a significant risk with a much smaller risk. That’s what counts in the end.”
In a response, Dr. Mandrola called Han’s tweets “reasoned criticism of our analysis.” He adds that his and Dr. Hoeg’s study have limits, but “our point is not to avoid protecting kids, but how to do so most safely.”
Both Dr. Mandrola and Dr. Hoeg said they welcomed critiques, but they felt blindsided by the vehemence of some of the Twitter debate.
“Some of the vitriol was surprising,” Dr. Mandrola said. “I kind of have this naive notion that people would assume that we’re not bad people,” he added.
However, Dr. Mandrola is known on Twitter for sometimes being highly critical of other researchers’ work, referring to some studies as “howlers,” and has in the past called out others for citing those papers.
Dr. Hoeg said she found critiques about weaknesses in the methods to be helpful. But she said many tweets were “attacking us as people, or not really attacking anything about our study, but just attacking the finding,” which does not help anyone “figure out what we should do about the safety signal or how we can research it further.”
Said Dr. Mandrola: “Why would we just ignore that and go forward with two-shot vaccination as a mandate when other countries are looking at other strategies?”
He noted that the United Kingdom has announced that children 12 to 15 years of age should receive just one shot of the mRNA vaccines instead of two because of the risk for myocarditis. Sixteen- to 18-year-olds have already been advised to get only one dose.
A version of this article first appeared on Medscape.com.
A preprint study finding that the Pfizer-BioNTech mRNA COVID vaccine is associated with an increased risk for cardiac adverse events in teenage boys has elicited a firestorm on Twitter. Although some people issued thoughtful critiques, others lobbed insults against the authors, and still others accused them of either being antivaccine or stoking the fires of the vaccine skeptic movement.
The controversy began soon after the study was posted online September 8 on medRxiv. The authors conclude that for boys, the risk for a cardiac adverse event or hospitalization after the second dose of the Pfizer mRNA vaccine was “considerably higher” than the 120-day risk for hospitalization for COVID-19, “even at times of peak disease prevalence.” This was especially true for those aged 12 to 15 years and even those with no underlying health conditions.
The conclusion – as well as the paper’s source, the Vaccine Adverse Event Reporting System (VAERS), and its methodology, modeled after the Centers for Disease Control and Prevention assessment of the database – did not sit well with many.
“Your methodology hugely overestimates risk, which many commentators who are specialists in the field have highlighted,” tweeted Deepti Gurdasani, senior lecturer in epidemiology at Queen Mary University of London. “Why make this claim when you must know it’s wrong?”
“The authors don’t know what they are doing and they are following their own ideology,” tweeted Boback Ziaeian, MD, PhD, assistant professor of medicine at the University of California, Los Angeles, in the cardiology division. Dr. Ziaeian also tweeted, “I believe the CDC is doing honest work and not dredging slop like you are.”
“Holy shit. Truly terrible methods in that paper,” tweeted Michael Mina, MD, PhD, an epidemiologist and immunologist at the Harvard School of Public Health, Boston, more bluntly.
Some pointed out that VAERS is often used by vaccine skeptics to spread misinformation. “‘Dumpster diving’ describes studies using #VAERS by authors (almost always antivaxxers) who don’t understand its limitations,” tweeted David Gorski, MD, PhD, the editor of Science-Based Medicine, who says in his Twitter bio that he “exposes quackery.”
Added Dr. Gorski: “Doctors fell into this trap with their study suggesting #CovidVaccine is more dangerous to children than #COVID19.”
Dr. Gorski said he did not think that the authors were antivaccine. But, he tweeted, “I’d argue that at least one of the authors (Stevenson) is grossly unqualified to analyze the data. Mandrola? Marginal. The other two *might* be qualified in public health/epi, but they clearly either had no clue about #VAERS limitations or didn’t take them seriously enough.”
Two of the authors, John Mandrola, MD, a cardiac electrophysiologist who is also a columnist for Medscape, and Tracy Beth Hoeg, MD, PhD, an epidemiologist and sports medicine specialist, told this news organization that their estimates are not definitive, owing to the nature of the VAERS database.
“I want to emphasize that our signal is hypothesis-generating,” said Dr. Mandrola. “There’s obviously more research that needs to be done.”
“I don’t think it should be used to establish a for-certain rate,” said Dr. Hoeg, about the study. “It’s not a perfect way of establishing what the rate of cardiac adverse events was, but it gives you an estimate, and generally with VAERS, it’s a significant underestimate.”
Both Dr. Hoeg and Dr. Mandrola said their analysis showed enough of a signal that it warranted a rush to publish. “We felt that it was super time-sensitive,” Dr. Mandrola said.
Vaccine risks versus COVID harm
The authors searched the VAERS system for children aged 12 to 17 years who had received one or two doses of an mRNA vaccine and had symptoms of myocarditis, pericarditis, myopericarditis, or chest pain, and also troponin levels available in the lab data.
Of the 257 patients they examined, 211 had peak troponin values available for analysis. All but one received the Pfizer vaccine. Results were stratified by age and sex.
The authors found that the rates of cardiac adverse events (CAEs) after dose 1 were 12.0 per million for 12- to 15-year-old boys and 8.2 per million for 16- and 17-year-old boys, compared with 0.0 per million and 2.0 per million for girls the same ages.
The estimates for the 12- to 15-year-old boys were 22% to 150% higher than what the CDC had previously reported.
After the second dose, the rate of CAEs for boys 12 to 15 years was 162.2 per million (143% to 280% higher than the CDC estimate) and for boys 16 and 17 years, it was 94.0 per million, or 30% to 40% higher than CDC estimate.
Dr. Mandrola said he and his colleagues found potentially more cases by using slightly broader search terms than those employed by the CDC but agreed with some critics that a limitation was that they did not call the reporting physicians, as is typical with CDC follow-up on VAERS reports.
The authors point to troponin levels as valid indicators of myocardial damage. Peak troponin levels exceeded 2 ng/mL in 71% of the 12- to 15-year-olds and 82% of 16- and 17-year-olds.
The study shows that for boys 12 to 15 years with no comorbidities, the risk for a CAE after the second dose would be 22.8 times higher than the risk for hospitalization for COVID-19 during periods of low disease burden, 6.0 times higher during periods of moderate transmission, and 4.3 times higher during periods of high transmission.
The authors acknowledge in the paper that their analysis “does not take into account any benefits the vaccine provides against transmission to others, long-term COVID-19 disease risk, or protection from nonsevere COVID-19 symptoms.”
Both Dr. Mandrola and Dr. Hoeg told this news organization that they are currently recalculating their estimates because of the rising numbers of pediatric hospitalizations from the Delta variant surge.
Paper rejected by journals
Dr. Hoeg said in an interview that the paper went through peer-review at three journals but was rejected by all three, for reasons that were not made clear.
She and the other authors incorporated the reviewers’ feedback at each turn and included all of their suggestions in the paper that was ultimately uploaded to medRxiv, said Dr. Hoeg.
They decided to put it out as a preprint after the U.S. Food and Drug Administration issued its data and then a warning on June 25 about myocarditis with use of the Pfizer vaccine in children 12 to 15 years of age.
The preprint study was picked up by some media outlets, including The Telegraph and The Guardian newspapers, and tweeted out by vaccine skeptics like Robert W. Malone, MD.
Rep. Marjorie Taylor Greene (R-Georgia), an outspoken vaccine skeptic, tweeted out the Guardian story saying that the findings mean “there is every reason to stop the covid vaccine mandates.”
Dr. Gorski noted in tweets and in a blog post that one of the paper’s coauthors, Josh Stevenson, is part of Rational Ground, a group that supports the Great Barrington Declaration and is against lockdowns and mask mandates.
Mr. Stevenson did not disclose his affiliation in the paper, and Dr. Hoeg said in an interview that she was unaware of the group and Mr. Stevenson’s association with it and that she did not have the impression that he was altering the data to show any bias.
Both Dr. Mandrola and Dr. Hoeg said they are provaccine and that they were dismayed to find their work being used to support any agenda. “It’s very frustrating,” said Dr. Hoeg, adding that she understands that “when you publish research on a controversial topic, people are going to take it and use it for their agendas.”
Some on Twitter blamed the open and free-wheeling nature of preprints.
Harlan Krumholz, MD, SM, the Harold H. Hines, junior professor of medicine and public health at Yale University, New Haven, Conn., which oversees medRxiv, tweeted, “Do you get that the discussion about the preprint is exactly the purpose of #preprints. So that way when someone claims something, you can look at the source and experts can comment.”
But Dr. Ziaeian tweeted back, “Preprints like this one can be weaponized to stir anti-vaccine lies and damage public health.”
In turn, the Yale physician replied, “Unfortunately these days, almost anything can be weaponized, distorted, misunderstood.” Dr. Krumholz added: “There is no question that this preprint is worthy of deep vetting and discussion. But there is a #preprint artifact to examine.”
Measured support
Some clinicians signaled their support for open debate and the preprint’s findings.
“I’ve been very critical of preprints that are too quickly disseminated in the media, and this one is no exception,” tweeted Walid Gellad, MD, MPH, associate professor of medicine at the University of Pittsburgh. “On the other hand, I think the vitriol directed at these authors is wrong,” he added.
“Like it or not, the issue of myocarditis in kids is an issue. Other countries have made vaccination decisions because of this issue, not because they’re driven by some ideology,” he tweeted.
Dr. Gellad also notes that the FDA has estimated the risk could be as high as one in 5,000 and that the preprint numbers could actually be underestimates.
In a long thread, Frank Han, MD, an adult congenital and pediatric cardiologist at the University of Illinois, tweets that relying on the VAERS reports might be faulty and that advanced cardiac imaging – guided by strict criteria – is the best way to determine myocarditis. And, he tweeted, “Physician review of VAERS reports really matters.”
Dr. Han concluded that vaccination “trades in a significant risk with a much smaller risk. That’s what counts in the end.”
In a response, Dr. Mandrola called Han’s tweets “reasoned criticism of our analysis.” He adds that his and Dr. Hoeg’s study have limits, but “our point is not to avoid protecting kids, but how to do so most safely.”
Both Dr. Mandrola and Dr. Hoeg said they welcomed critiques, but they felt blindsided by the vehemence of some of the Twitter debate.
“Some of the vitriol was surprising,” Dr. Mandrola said. “I kind of have this naive notion that people would assume that we’re not bad people,” he added.
However, Dr. Mandrola is known on Twitter for sometimes being highly critical of other researchers’ work, referring to some studies as “howlers,” and has in the past called out others for citing those papers.
Dr. Hoeg said she found critiques about weaknesses in the methods to be helpful. But she said many tweets were “attacking us as people, or not really attacking anything about our study, but just attacking the finding,” which does not help anyone “figure out what we should do about the safety signal or how we can research it further.”
Said Dr. Mandrola: “Why would we just ignore that and go forward with two-shot vaccination as a mandate when other countries are looking at other strategies?”
He noted that the United Kingdom has announced that children 12 to 15 years of age should receive just one shot of the mRNA vaccines instead of two because of the risk for myocarditis. Sixteen- to 18-year-olds have already been advised to get only one dose.
A version of this article first appeared on Medscape.com.