User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Expert shares vulvovaginal candidiasis treatment pearls
that was approved in June 2021, Aruna Venkatesan, MD, recommends.
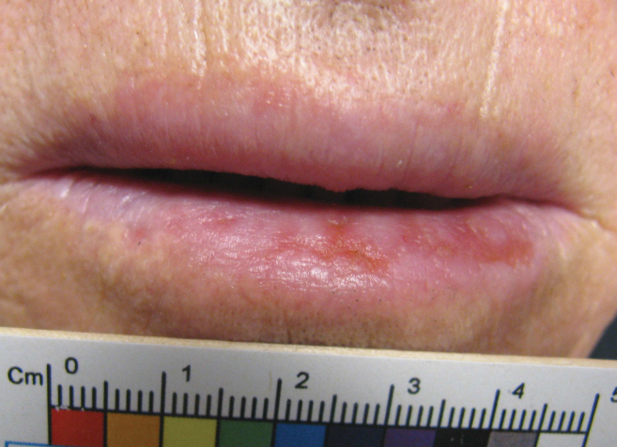
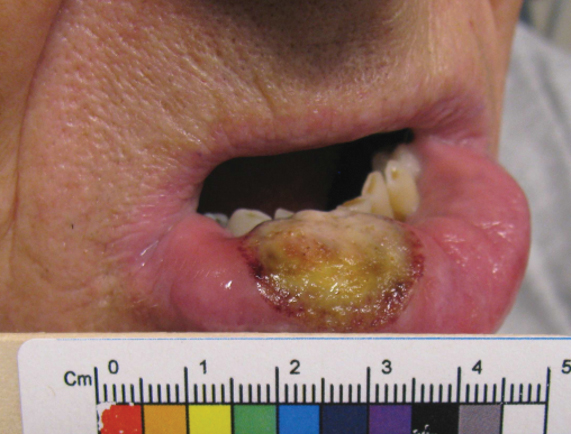
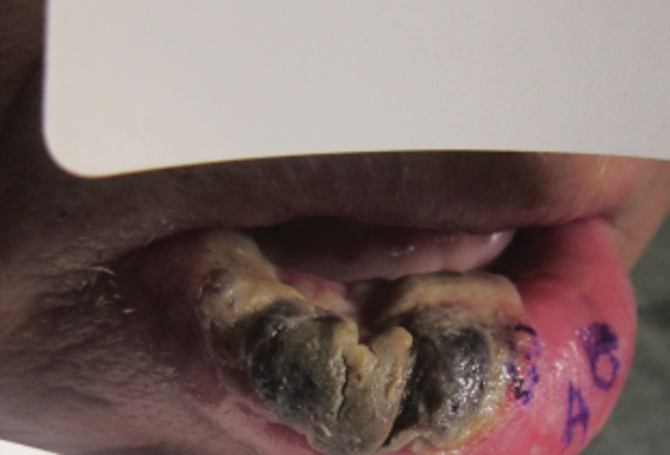
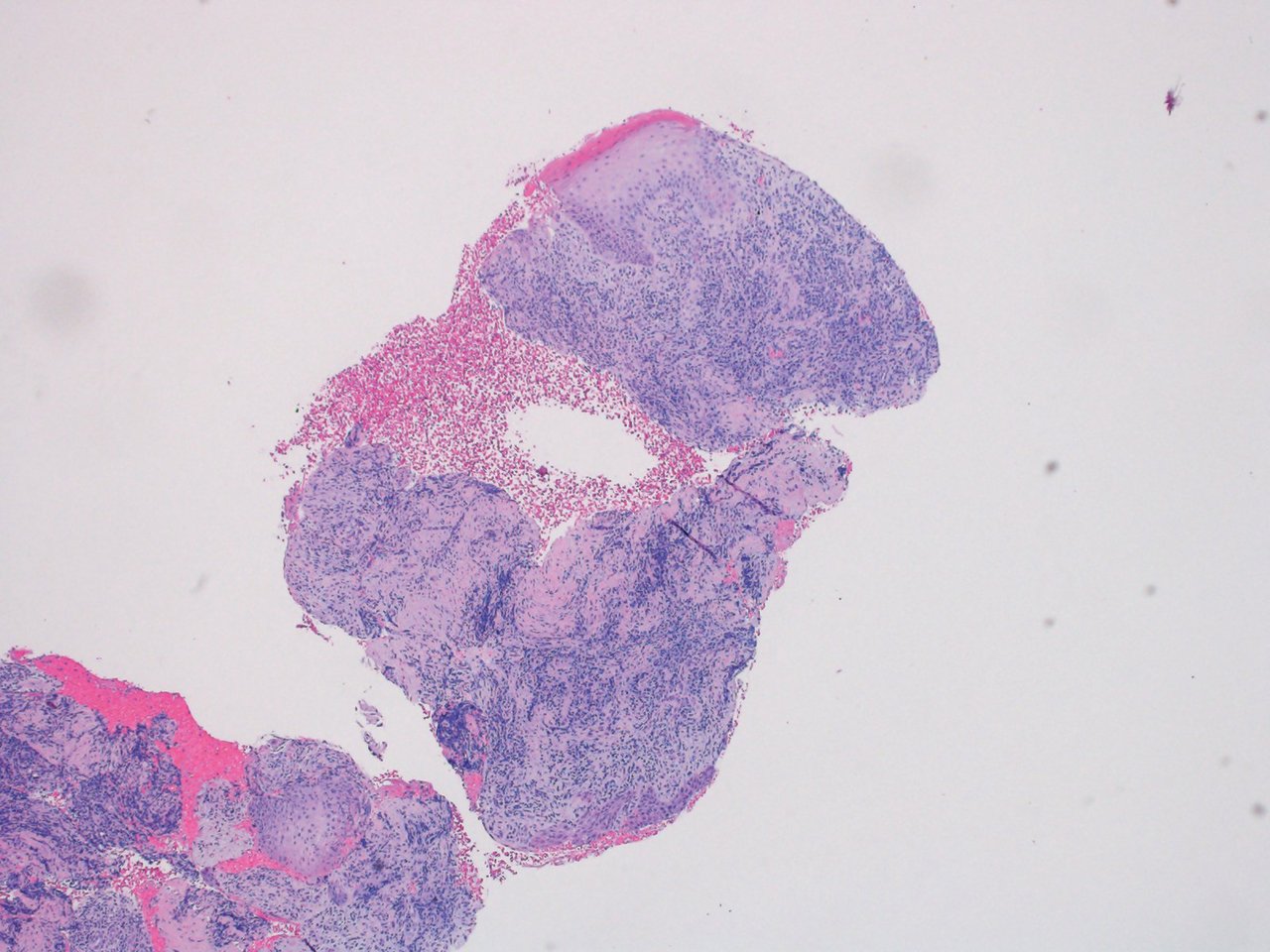
“Ibrexafungerp, an inhibitor of beta (1-3)–glucan synthase, is important for many reasons,” Dr. Venkatesan, chief of dermatology and director of the genital dermatology clinic at Santa Clara Valley Medical Center, San Jose, Calif., said during the annual meeting of the Pacific Dermatologic Association. “It’s one of the few drugs that can be used to treat Candida glabrata when C. glabrata is resistant to azoles and echinocandins. As the second-most common Candida species after C. albicans, C. glabrata is more common in immunosuppressed patients and it can cause mucosal and invasive disease, so ibrexafungerp is a welcome addition to our treatment armamentarium,” said Dr. Venkatesan, clinical professor of dermatology (affiliated) at Stanford (Calif.) Hospital and Clinics, adding that that vulvovaginal candidiasis can be tricky to diagnose. “In medical school, we learned that yeast infection in a woman presents as white, curd-like discharge, but that’s actually a minority of patients.”
For a patient who is being treated with topical steroids or estrogen for a genital condition, but is experiencing worsening itch, redness, or thick white discharge, she recommends performing a KOH exam.
“Instead of using a 15-blade scalpel, as we are used to performing on the skin for tinea, take a sterile [cotton swab], and swab the affected area. You can then apply it to a slide and perform a KOH exam as you normally would. Then look for yeast elements under the microscope. I also find it helpful to send for fungal culture to get speciation, especially in someone who’s not responding to therapy. This is because non-albicans yeast can be more resistant to azoles and require a different treatment plan.”
Often, patients with vulvovaginal candidiasis who present to her clinic are referred from an ob.gyn. and other general practitioners because they have failed a topical or oral azole. “I tend to avoid the topicals,” said Dr. Venkatesan, who is also president-elect of the North American chapter of the International Society for the Study of Vulvovaginal Disease. “If the culture shows C. albicans, I usually treat with oral fluconazole, 150 mg or 200 mg once, and consider repeat weekly dosing. Many patients come to me because they have recurrent refractory disease, so giving it once weekly for 6-8 weeks while they work on their potential risk factors such as diabetic blood sugar control is sensible.”
Non-albicans yeast can be resistant to azoles. If the fungal culture shows C. glabrata in such patients, “consider a course of intravaginal boric acid suppositories,” she advised. “These used to be difficult to give patients, because you would either have to send the prescription to a compounding pharmacy, or have the patients buy the capsules and boric acid crystals separately and make them themselves. That always made me nervous because of the chance of errors. The safety and the concern of taking it by mouth is an issue.” But now, intravaginal boric acid suppositories are available on Amazon and other web sites, and are relatively affordable, she said, adding, “just make sure the patient doesn’t take it by mouth as this is very toxic.”
Dr. Venkatesan reported having no financial disclosures.
that was approved in June 2021, Aruna Venkatesan, MD, recommends.
“Ibrexafungerp, an inhibitor of beta (1-3)–glucan synthase, is important for many reasons,” Dr. Venkatesan, chief of dermatology and director of the genital dermatology clinic at Santa Clara Valley Medical Center, San Jose, Calif., said during the annual meeting of the Pacific Dermatologic Association. “It’s one of the few drugs that can be used to treat Candida glabrata when C. glabrata is resistant to azoles and echinocandins. As the second-most common Candida species after C. albicans, C. glabrata is more common in immunosuppressed patients and it can cause mucosal and invasive disease, so ibrexafungerp is a welcome addition to our treatment armamentarium,” said Dr. Venkatesan, clinical professor of dermatology (affiliated) at Stanford (Calif.) Hospital and Clinics, adding that that vulvovaginal candidiasis can be tricky to diagnose. “In medical school, we learned that yeast infection in a woman presents as white, curd-like discharge, but that’s actually a minority of patients.”
For a patient who is being treated with topical steroids or estrogen for a genital condition, but is experiencing worsening itch, redness, or thick white discharge, she recommends performing a KOH exam.
“Instead of using a 15-blade scalpel, as we are used to performing on the skin for tinea, take a sterile [cotton swab], and swab the affected area. You can then apply it to a slide and perform a KOH exam as you normally would. Then look for yeast elements under the microscope. I also find it helpful to send for fungal culture to get speciation, especially in someone who’s not responding to therapy. This is because non-albicans yeast can be more resistant to azoles and require a different treatment plan.”
Often, patients with vulvovaginal candidiasis who present to her clinic are referred from an ob.gyn. and other general practitioners because they have failed a topical or oral azole. “I tend to avoid the topicals,” said Dr. Venkatesan, who is also president-elect of the North American chapter of the International Society for the Study of Vulvovaginal Disease. “If the culture shows C. albicans, I usually treat with oral fluconazole, 150 mg or 200 mg once, and consider repeat weekly dosing. Many patients come to me because they have recurrent refractory disease, so giving it once weekly for 6-8 weeks while they work on their potential risk factors such as diabetic blood sugar control is sensible.”
Non-albicans yeast can be resistant to azoles. If the fungal culture shows C. glabrata in such patients, “consider a course of intravaginal boric acid suppositories,” she advised. “These used to be difficult to give patients, because you would either have to send the prescription to a compounding pharmacy, or have the patients buy the capsules and boric acid crystals separately and make them themselves. That always made me nervous because of the chance of errors. The safety and the concern of taking it by mouth is an issue.” But now, intravaginal boric acid suppositories are available on Amazon and other web sites, and are relatively affordable, she said, adding, “just make sure the patient doesn’t take it by mouth as this is very toxic.”
Dr. Venkatesan reported having no financial disclosures.
that was approved in June 2021, Aruna Venkatesan, MD, recommends.
“Ibrexafungerp, an inhibitor of beta (1-3)–glucan synthase, is important for many reasons,” Dr. Venkatesan, chief of dermatology and director of the genital dermatology clinic at Santa Clara Valley Medical Center, San Jose, Calif., said during the annual meeting of the Pacific Dermatologic Association. “It’s one of the few drugs that can be used to treat Candida glabrata when C. glabrata is resistant to azoles and echinocandins. As the second-most common Candida species after C. albicans, C. glabrata is more common in immunosuppressed patients and it can cause mucosal and invasive disease, so ibrexafungerp is a welcome addition to our treatment armamentarium,” said Dr. Venkatesan, clinical professor of dermatology (affiliated) at Stanford (Calif.) Hospital and Clinics, adding that that vulvovaginal candidiasis can be tricky to diagnose. “In medical school, we learned that yeast infection in a woman presents as white, curd-like discharge, but that’s actually a minority of patients.”
For a patient who is being treated with topical steroids or estrogen for a genital condition, but is experiencing worsening itch, redness, or thick white discharge, she recommends performing a KOH exam.
“Instead of using a 15-blade scalpel, as we are used to performing on the skin for tinea, take a sterile [cotton swab], and swab the affected area. You can then apply it to a slide and perform a KOH exam as you normally would. Then look for yeast elements under the microscope. I also find it helpful to send for fungal culture to get speciation, especially in someone who’s not responding to therapy. This is because non-albicans yeast can be more resistant to azoles and require a different treatment plan.”
Often, patients with vulvovaginal candidiasis who present to her clinic are referred from an ob.gyn. and other general practitioners because they have failed a topical or oral azole. “I tend to avoid the topicals,” said Dr. Venkatesan, who is also president-elect of the North American chapter of the International Society for the Study of Vulvovaginal Disease. “If the culture shows C. albicans, I usually treat with oral fluconazole, 150 mg or 200 mg once, and consider repeat weekly dosing. Many patients come to me because they have recurrent refractory disease, so giving it once weekly for 6-8 weeks while they work on their potential risk factors such as diabetic blood sugar control is sensible.”
Non-albicans yeast can be resistant to azoles. If the fungal culture shows C. glabrata in such patients, “consider a course of intravaginal boric acid suppositories,” she advised. “These used to be difficult to give patients, because you would either have to send the prescription to a compounding pharmacy, or have the patients buy the capsules and boric acid crystals separately and make them themselves. That always made me nervous because of the chance of errors. The safety and the concern of taking it by mouth is an issue.” But now, intravaginal boric acid suppositories are available on Amazon and other web sites, and are relatively affordable, she said, adding, “just make sure the patient doesn’t take it by mouth as this is very toxic.”
Dr. Venkatesan reported having no financial disclosures.
FROM PDA 2021
Ask about itch and joint pain in pediatric psoriasis patients, expert advises
During the annual meeting of the Society for Pediatric Dermatology, Amy S. Paller, MD, MS, marveled on the remarkable advances in the treatment of inflammatory skin disorders during the past 2 decades.
“We’ve come a long way, from disease features being red, thick, and scaly and being treated with nonspecific therapy like topical steroids, keratolytics, and tar, to understanding disease pathogenesis and finding new targeted therapies for inflammatory skin disorders in children,” said Dr. Paller, professor and chair of the department of dermatology at Northwestern University, Chicago. “There are now studies moving forward with gene correction, gene replacement, the gene product replaced, or pathway inhibition to prevent the effects of genetic change.”
Technology is leading the way in generating new therapeutic advances, she continued, beyond traditional “omics” to lipidomics, metabolomics, glycomics, and kinomics. “This has enabled us to find new genetic disorders and their causes, to look at changes in gene expression patterns, and to look at changes in protein expression patterns that give us clues as to how to move forward with better therapy,” she said. “When we’re talking about new insights into pathogenesis-based therapy, we’re talking largely about understanding the pathways that lead to either inflammation or promoting cell proliferation and abnormal differentiation.”
Treating pediatric psoriasis
. “First of all, ask about itch and pain with these patients,” she advised. “Interviews have shown that 61% of children experience some itch, 39% have pain or stinging, and in the ixekizumab trials, 72% had what’s considered meaningful itch, with at least 4 out of 10 (mean intensity 5.3) on the itch numeric rating scale. Little is known about the itch associated with psoriasis and its underlying cause – unrelated to the IL-4/IL-13 pathway activation of atopic dermatitis – but it’s worth asking about. I find that itch of the scalp is especially a problem in psoriasis.”
Physicians should also ask pediatric psoriasis patients about joint pain, because about 1% of them have psoriatic arthritis, which is much less common than in adults, “but important to find and manage,” she added. Dr. Paller recommends the new R-JET rapid joint exam technique, which is accompanied by a three-question survey and body diagram that facilitates identification of true arthritis, “so you can know how quickly to refer”.
Several studies have described an increased risk of metabolic syndrome in adolescents with pediatric psoriasis and now in prepubertal children with the disease. In a recent study of 60 consecutive prepubertal children with psoriasis, 70% of whom had mild disease, 40% were overweight or obese, 53% had central obesity, 27% had high levels of the HOMA-IR (homeostasis model assessment of insulin resistance) despite generally normal levels of fasting glucose, and 30% met criteria for metabolic syndrome.
“This really struck me because our AAD [American Academy of Dermatology] guidelines did not recommend screening for type 2 diabetes in prepubertal children, even if overweight, because the risk is so small,” Dr. Paller said. “This report suggests that we may need to reconsider this recommendation in prepubertal children with psoriasis.”
Meanwhile, the number of medications approved by the Food and Drug Administration and the European Medicines Agency for children with psoriasis who are 6 years of age and above continues to expand, including tumor necrosis factor (TNF) inhibitors, interleukin (IL)-23 inhibitors, and IL-17 inhibitors. Most children can now achieve a PASI 90 within 12 weeks with the IL-23 inhibitor ustekinumab and the IL-17 inhibitors ixekizumab and secukinumab, Dr. Paller said.
In the ixekizumab trial, there are head-to-head comparison data in a European arm that involved the use of etanercept, she said. “What’s most noticeable is the significant difference in those who were able to achieve PASI 90 or above with this IL-17 inhibitor, versus etanercept,” which she added, raises the question of whether aiming for a PASI 75 is adequate, "or should we strive for PASI 90?” A pediatric psoriasis study published in 2020 found that the greatest improvement in quality of life was associated with a PASI 90 and use of systemic treatments.
Looking forward, phase 3 clinical trials are underway in pediatric patients with moderate to severe psoriasis for guselkumab, tildrakizumab, risankizumab, certolizumab, bimekizumab, and brodalumab. “The cost of all of these biologics is high, however. I remind everyone that we still have methotrexate,” she said. “The risk of side effects with our low-dose methotrexate treatment for psoriasis remains low, but methotrexate doesn’t hit these [high] PASI numbers and it’s much slower in its onset than biologics.”
Dr. Paller disclosed that she is a consultant to and/or an investigator for AbbVie, Arena, Bausch, Bristol Myers Squibb, Dermavant, Eli Lilly, Incyte, Forte, LEO Pharma, LifeMax, Pfizer, RAPT Therapeutics, Regeneron, and Sanofi.
Commentary by Robert Sidbury, MD, MPH
Dr. Paller reminds us of some essential features of pediatric psoriasis:
• It can hurt. Ask your patients if it does.
• It can itch. Look for excoriations, especially in the scalp.
• It is often associated with metabolic syndrome, so check relevant biometrics and labs, and consider coincident insulin resistance.
• Our traditional clinical trial target of PASI75, or a 75% reduction in body surface area involvement, is just not good enough. Studies have shown that the most meaningful quality-of-life gains come at PASI90 or above.
• With our newer biologics, such as IL-12/23 blockers (for instance, ustekinumab) and IL-17 blockers (for example, ixekizumab and secukinumab), PASI90 and better is a reasonable expectation, not a pipe dream.
Dr. Sidbury is chief of dermatology at Seattle Children's Hospital and professor, department of pediatrics, University of Washington, Seattle. He is a site principal investigator for dupilumab trials, for which the hospital has a contract with Regeneron.
This article was updated 6/16/22.
During the annual meeting of the Society for Pediatric Dermatology, Amy S. Paller, MD, MS, marveled on the remarkable advances in the treatment of inflammatory skin disorders during the past 2 decades.
“We’ve come a long way, from disease features being red, thick, and scaly and being treated with nonspecific therapy like topical steroids, keratolytics, and tar, to understanding disease pathogenesis and finding new targeted therapies for inflammatory skin disorders in children,” said Dr. Paller, professor and chair of the department of dermatology at Northwestern University, Chicago. “There are now studies moving forward with gene correction, gene replacement, the gene product replaced, or pathway inhibition to prevent the effects of genetic change.”
Technology is leading the way in generating new therapeutic advances, she continued, beyond traditional “omics” to lipidomics, metabolomics, glycomics, and kinomics. “This has enabled us to find new genetic disorders and their causes, to look at changes in gene expression patterns, and to look at changes in protein expression patterns that give us clues as to how to move forward with better therapy,” she said. “When we’re talking about new insights into pathogenesis-based therapy, we’re talking largely about understanding the pathways that lead to either inflammation or promoting cell proliferation and abnormal differentiation.”
Treating pediatric psoriasis
. “First of all, ask about itch and pain with these patients,” she advised. “Interviews have shown that 61% of children experience some itch, 39% have pain or stinging, and in the ixekizumab trials, 72% had what’s considered meaningful itch, with at least 4 out of 10 (mean intensity 5.3) on the itch numeric rating scale. Little is known about the itch associated with psoriasis and its underlying cause – unrelated to the IL-4/IL-13 pathway activation of atopic dermatitis – but it’s worth asking about. I find that itch of the scalp is especially a problem in psoriasis.”
Physicians should also ask pediatric psoriasis patients about joint pain, because about 1% of them have psoriatic arthritis, which is much less common than in adults, “but important to find and manage,” she added. Dr. Paller recommends the new R-JET rapid joint exam technique, which is accompanied by a three-question survey and body diagram that facilitates identification of true arthritis, “so you can know how quickly to refer”.
Several studies have described an increased risk of metabolic syndrome in adolescents with pediatric psoriasis and now in prepubertal children with the disease. In a recent study of 60 consecutive prepubertal children with psoriasis, 70% of whom had mild disease, 40% were overweight or obese, 53% had central obesity, 27% had high levels of the HOMA-IR (homeostasis model assessment of insulin resistance) despite generally normal levels of fasting glucose, and 30% met criteria for metabolic syndrome.
“This really struck me because our AAD [American Academy of Dermatology] guidelines did not recommend screening for type 2 diabetes in prepubertal children, even if overweight, because the risk is so small,” Dr. Paller said. “This report suggests that we may need to reconsider this recommendation in prepubertal children with psoriasis.”
Meanwhile, the number of medications approved by the Food and Drug Administration and the European Medicines Agency for children with psoriasis who are 6 years of age and above continues to expand, including tumor necrosis factor (TNF) inhibitors, interleukin (IL)-23 inhibitors, and IL-17 inhibitors. Most children can now achieve a PASI 90 within 12 weeks with the IL-23 inhibitor ustekinumab and the IL-17 inhibitors ixekizumab and secukinumab, Dr. Paller said.
In the ixekizumab trial, there are head-to-head comparison data in a European arm that involved the use of etanercept, she said. “What’s most noticeable is the significant difference in those who were able to achieve PASI 90 or above with this IL-17 inhibitor, versus etanercept,” which she added, raises the question of whether aiming for a PASI 75 is adequate, "or should we strive for PASI 90?” A pediatric psoriasis study published in 2020 found that the greatest improvement in quality of life was associated with a PASI 90 and use of systemic treatments.
Looking forward, phase 3 clinical trials are underway in pediatric patients with moderate to severe psoriasis for guselkumab, tildrakizumab, risankizumab, certolizumab, bimekizumab, and brodalumab. “The cost of all of these biologics is high, however. I remind everyone that we still have methotrexate,” she said. “The risk of side effects with our low-dose methotrexate treatment for psoriasis remains low, but methotrexate doesn’t hit these [high] PASI numbers and it’s much slower in its onset than biologics.”
Dr. Paller disclosed that she is a consultant to and/or an investigator for AbbVie, Arena, Bausch, Bristol Myers Squibb, Dermavant, Eli Lilly, Incyte, Forte, LEO Pharma, LifeMax, Pfizer, RAPT Therapeutics, Regeneron, and Sanofi.
Commentary by Robert Sidbury, MD, MPH
Dr. Paller reminds us of some essential features of pediatric psoriasis:
• It can hurt. Ask your patients if it does.
• It can itch. Look for excoriations, especially in the scalp.
• It is often associated with metabolic syndrome, so check relevant biometrics and labs, and consider coincident insulin resistance.
• Our traditional clinical trial target of PASI75, or a 75% reduction in body surface area involvement, is just not good enough. Studies have shown that the most meaningful quality-of-life gains come at PASI90 or above.
• With our newer biologics, such as IL-12/23 blockers (for instance, ustekinumab) and IL-17 blockers (for example, ixekizumab and secukinumab), PASI90 and better is a reasonable expectation, not a pipe dream.
Dr. Sidbury is chief of dermatology at Seattle Children's Hospital and professor, department of pediatrics, University of Washington, Seattle. He is a site principal investigator for dupilumab trials, for which the hospital has a contract with Regeneron.
This article was updated 6/16/22.
During the annual meeting of the Society for Pediatric Dermatology, Amy S. Paller, MD, MS, marveled on the remarkable advances in the treatment of inflammatory skin disorders during the past 2 decades.
“We’ve come a long way, from disease features being red, thick, and scaly and being treated with nonspecific therapy like topical steroids, keratolytics, and tar, to understanding disease pathogenesis and finding new targeted therapies for inflammatory skin disorders in children,” said Dr. Paller, professor and chair of the department of dermatology at Northwestern University, Chicago. “There are now studies moving forward with gene correction, gene replacement, the gene product replaced, or pathway inhibition to prevent the effects of genetic change.”
Technology is leading the way in generating new therapeutic advances, she continued, beyond traditional “omics” to lipidomics, metabolomics, glycomics, and kinomics. “This has enabled us to find new genetic disorders and their causes, to look at changes in gene expression patterns, and to look at changes in protein expression patterns that give us clues as to how to move forward with better therapy,” she said. “When we’re talking about new insights into pathogenesis-based therapy, we’re talking largely about understanding the pathways that lead to either inflammation or promoting cell proliferation and abnormal differentiation.”
Treating pediatric psoriasis
. “First of all, ask about itch and pain with these patients,” she advised. “Interviews have shown that 61% of children experience some itch, 39% have pain or stinging, and in the ixekizumab trials, 72% had what’s considered meaningful itch, with at least 4 out of 10 (mean intensity 5.3) on the itch numeric rating scale. Little is known about the itch associated with psoriasis and its underlying cause – unrelated to the IL-4/IL-13 pathway activation of atopic dermatitis – but it’s worth asking about. I find that itch of the scalp is especially a problem in psoriasis.”
Physicians should also ask pediatric psoriasis patients about joint pain, because about 1% of them have psoriatic arthritis, which is much less common than in adults, “but important to find and manage,” she added. Dr. Paller recommends the new R-JET rapid joint exam technique, which is accompanied by a three-question survey and body diagram that facilitates identification of true arthritis, “so you can know how quickly to refer”.
Several studies have described an increased risk of metabolic syndrome in adolescents with pediatric psoriasis and now in prepubertal children with the disease. In a recent study of 60 consecutive prepubertal children with psoriasis, 70% of whom had mild disease, 40% were overweight or obese, 53% had central obesity, 27% had high levels of the HOMA-IR (homeostasis model assessment of insulin resistance) despite generally normal levels of fasting glucose, and 30% met criteria for metabolic syndrome.
“This really struck me because our AAD [American Academy of Dermatology] guidelines did not recommend screening for type 2 diabetes in prepubertal children, even if overweight, because the risk is so small,” Dr. Paller said. “This report suggests that we may need to reconsider this recommendation in prepubertal children with psoriasis.”
Meanwhile, the number of medications approved by the Food and Drug Administration and the European Medicines Agency for children with psoriasis who are 6 years of age and above continues to expand, including tumor necrosis factor (TNF) inhibitors, interleukin (IL)-23 inhibitors, and IL-17 inhibitors. Most children can now achieve a PASI 90 within 12 weeks with the IL-23 inhibitor ustekinumab and the IL-17 inhibitors ixekizumab and secukinumab, Dr. Paller said.
In the ixekizumab trial, there are head-to-head comparison data in a European arm that involved the use of etanercept, she said. “What’s most noticeable is the significant difference in those who were able to achieve PASI 90 or above with this IL-17 inhibitor, versus etanercept,” which she added, raises the question of whether aiming for a PASI 75 is adequate, "or should we strive for PASI 90?” A pediatric psoriasis study published in 2020 found that the greatest improvement in quality of life was associated with a PASI 90 and use of systemic treatments.
Looking forward, phase 3 clinical trials are underway in pediatric patients with moderate to severe psoriasis for guselkumab, tildrakizumab, risankizumab, certolizumab, bimekizumab, and brodalumab. “The cost of all of these biologics is high, however. I remind everyone that we still have methotrexate,” she said. “The risk of side effects with our low-dose methotrexate treatment for psoriasis remains low, but methotrexate doesn’t hit these [high] PASI numbers and it’s much slower in its onset than biologics.”
Dr. Paller disclosed that she is a consultant to and/or an investigator for AbbVie, Arena, Bausch, Bristol Myers Squibb, Dermavant, Eli Lilly, Incyte, Forte, LEO Pharma, LifeMax, Pfizer, RAPT Therapeutics, Regeneron, and Sanofi.
Commentary by Robert Sidbury, MD, MPH
Dr. Paller reminds us of some essential features of pediatric psoriasis:
• It can hurt. Ask your patients if it does.
• It can itch. Look for excoriations, especially in the scalp.
• It is often associated with metabolic syndrome, so check relevant biometrics and labs, and consider coincident insulin resistance.
• Our traditional clinical trial target of PASI75, or a 75% reduction in body surface area involvement, is just not good enough. Studies have shown that the most meaningful quality-of-life gains come at PASI90 or above.
• With our newer biologics, such as IL-12/23 blockers (for instance, ustekinumab) and IL-17 blockers (for example, ixekizumab and secukinumab), PASI90 and better is a reasonable expectation, not a pipe dream.
Dr. Sidbury is chief of dermatology at Seattle Children's Hospital and professor, department of pediatrics, University of Washington, Seattle. He is a site principal investigator for dupilumab trials, for which the hospital has a contract with Regeneron.
This article was updated 6/16/22.
FROM SPD 2021
COVID-19 linked to baby bust in high-income countries
In an assessment of the pandemic’s early effects, Arnstein Aassve, PhD, and colleagues found a significant COVID-19–related decline in crude birth rates (CBRs) in 7 of 22 high-income countries, particularly in Southwestern Europe.
Dr. Aassve, an economist at the Carlo F. Dondena Center for Research on Social Dynamics and Public Policy at the Università Commerciale Luigi Bocconi, Milan, and colleagues report the results in an article published online August 30 in the Proceedings of the National Academy of Sciences.
Defining the start of the COVID-19 pandemic as February 2020, the study identifies strong declines in Italy (-9.1%), Hungary (-8.5%), Spain (-8.4%), and Portugal (-6.6%) beyond those predicted by past trends. In the United States, CBRs fell by 7.1% relative to 2019 for births occurring in Nov. and Dec. 2020 following conceptions in February and March of that year.
Significant declines in CBR also occurred in Belgium, Austria, and Singapore.
A year-to-year comparison of the mean for monthly CBRs per 1,000 population before and during the pandemic suggests a negative difference for all countries studied except for Denmark, Finland, Germany, and the Netherlands, Dr. Aassve and colleagues write. These findings may have policy implications for childcare, housing, and the labor market.
The Milan researchers compared monthly vital statistics data on live births from the international Human Fertility Database for the period of Jan. 2016 to March 2021. These figures reflect conceptions carried to term between April 2015 and June 2020. The 22 countries in the analysis represent 37% of the total reported COVID-19 cases and 34% of deaths worldwide.
The study findings align with surveys on “fertility intentions” collected early in the first COVID-19 wave in Germany, France, Spain, and the United Kingdom. These surveys indicated that 73% of people who were planning pregnancies in 2020 either decided to delay the pregnancy or they abandoned their plans.
“The popular media speculated that the lockdown would lead to a baby boom, as couples spent more time together,” Dr. Aassve told this news organization. “There’s very little evidence of this when you look to previous disasters and shocks, and the first data suggest more of an immediate collapse than a boom. But as you also see from the paper, the collapse is not seen everywhere.” Other current studies suggest the fertility drop is immediate but temporary, says Dr. Aassve, who is also a professor of demography.
Interestingly, Dr. Aassve and colleagues found that CBRs were relatively stable in Northern Europe. The authors point to supportive social and family policies in that region that might have reduced the effect of the pandemic on births. “These factors are likely to affect CBRs in the subsequent pandemic waves,” they write. They call for future studies to assess the full population implications of the pandemic, the moderating impact of policy interventions, and the nexus between short- and long-run effects in relation to the various waves of the COVID-19 pandemic.
Rebounds
Some regions have already reported a rebound from the COVID-19 fertility trough. Molly J. Stout, MD, director of maternal fetal medicine at the University of Michigan, Ann Arbor, and colleagues used electronic medical records to predict a surge in births after the initial decline.
“The surge we’ve seen at the end of this summer is exceeding the usual annual birth rate, as predicted,” she said in an interview. “But I think there’ll be a return to normal after this transient escalation. I don’t think birth rates will stay elevated above the normal because the birth surge is a temporary response to an event, although there will likely be regional differences.”
Looking ahead, Dr. Stout, who was not involved in Dr. Aassve’s analysis, is not certain how a fourth pandemic wave might ultimately modify a couple’s overall family size. But the toll the health crisis has taken on working women who have been forced to withdraw from the economy because of a lack of childcare points to a societal need that should be addressed.
According to Philip N. Cohen, PhD, a professor of sociology at the University of Maryland, College Park, who’s been tracking fertility trends since the onset of the COVID-19 emergency, the pandemic has combined a health crisis with an economic crisis, along with “the additional factor of social distancing and isolation, which all contributed to the decline in birth rates. Some people changed their plans to hold off on having children, while others didn’t get pregnant because they weren’t socializing and meeting people as much.”
Dr. Cohen, who was not involved in the study by Dr. Aassve and associates, said his provisional data show that although in many places, birth rates have rebounded more or less to prepandemic levels after a nadir around Jan. 2021, some areas of the United States still show substantially lower rates, including California, Hawaii, and Oregon.
As to the duration of the pandemic effect, Dr. Aassve cautions that his group’s estimates refer to the first wave only. “We then have the second, third, and currently the fourth wave. We can’t be sure about the impact of these waves on fertility since the data are not there yet, but I’d be surprised if they didn’t continue to have an impact on fertility rates,” he said.
Dr. Cohen agreed: “Some people who delayed childbearing will make up the delay. However, whenever there’s a delay, there’s inevitably some portion of the decline that’s not recouped.”
As for the wider effect across the world, Dr. Aassve said his team’s figures derive from high-income countries where data are readily available. For middle- and low-income countries, fewer data exist, and the quality of those data is not as good.
The lessons from this and other upheavals teach us that unforeseen shocks almost always have a negative impact on fertility, says Dr. Aassve. “[B]ut these effects may be separate from existing declining trends. The issue here is that those overall declining trends may be driven by other factors. In contrast, the shock of the pandemic is short-lived, and we may return to normal rather quickly. But if the pandemic also impacts other societal structures, such as the occupational and industrial sectors, then the pandemic might exacerbate the negative trend.”
The study was supported by funding from the European Research Council for funding under the European Union’s Horizon 2020 Research and Innovation Programme. The study authors, Dr. Stout, and Dr. Cohen have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In an assessment of the pandemic’s early effects, Arnstein Aassve, PhD, and colleagues found a significant COVID-19–related decline in crude birth rates (CBRs) in 7 of 22 high-income countries, particularly in Southwestern Europe.
Dr. Aassve, an economist at the Carlo F. Dondena Center for Research on Social Dynamics and Public Policy at the Università Commerciale Luigi Bocconi, Milan, and colleagues report the results in an article published online August 30 in the Proceedings of the National Academy of Sciences.
Defining the start of the COVID-19 pandemic as February 2020, the study identifies strong declines in Italy (-9.1%), Hungary (-8.5%), Spain (-8.4%), and Portugal (-6.6%) beyond those predicted by past trends. In the United States, CBRs fell by 7.1% relative to 2019 for births occurring in Nov. and Dec. 2020 following conceptions in February and March of that year.
Significant declines in CBR also occurred in Belgium, Austria, and Singapore.
A year-to-year comparison of the mean for monthly CBRs per 1,000 population before and during the pandemic suggests a negative difference for all countries studied except for Denmark, Finland, Germany, and the Netherlands, Dr. Aassve and colleagues write. These findings may have policy implications for childcare, housing, and the labor market.
The Milan researchers compared monthly vital statistics data on live births from the international Human Fertility Database for the period of Jan. 2016 to March 2021. These figures reflect conceptions carried to term between April 2015 and June 2020. The 22 countries in the analysis represent 37% of the total reported COVID-19 cases and 34% of deaths worldwide.
The study findings align with surveys on “fertility intentions” collected early in the first COVID-19 wave in Germany, France, Spain, and the United Kingdom. These surveys indicated that 73% of people who were planning pregnancies in 2020 either decided to delay the pregnancy or they abandoned their plans.
“The popular media speculated that the lockdown would lead to a baby boom, as couples spent more time together,” Dr. Aassve told this news organization. “There’s very little evidence of this when you look to previous disasters and shocks, and the first data suggest more of an immediate collapse than a boom. But as you also see from the paper, the collapse is not seen everywhere.” Other current studies suggest the fertility drop is immediate but temporary, says Dr. Aassve, who is also a professor of demography.
Interestingly, Dr. Aassve and colleagues found that CBRs were relatively stable in Northern Europe. The authors point to supportive social and family policies in that region that might have reduced the effect of the pandemic on births. “These factors are likely to affect CBRs in the subsequent pandemic waves,” they write. They call for future studies to assess the full population implications of the pandemic, the moderating impact of policy interventions, and the nexus between short- and long-run effects in relation to the various waves of the COVID-19 pandemic.
Rebounds
Some regions have already reported a rebound from the COVID-19 fertility trough. Molly J. Stout, MD, director of maternal fetal medicine at the University of Michigan, Ann Arbor, and colleagues used electronic medical records to predict a surge in births after the initial decline.
“The surge we’ve seen at the end of this summer is exceeding the usual annual birth rate, as predicted,” she said in an interview. “But I think there’ll be a return to normal after this transient escalation. I don’t think birth rates will stay elevated above the normal because the birth surge is a temporary response to an event, although there will likely be regional differences.”
Looking ahead, Dr. Stout, who was not involved in Dr. Aassve’s analysis, is not certain how a fourth pandemic wave might ultimately modify a couple’s overall family size. But the toll the health crisis has taken on working women who have been forced to withdraw from the economy because of a lack of childcare points to a societal need that should be addressed.
According to Philip N. Cohen, PhD, a professor of sociology at the University of Maryland, College Park, who’s been tracking fertility trends since the onset of the COVID-19 emergency, the pandemic has combined a health crisis with an economic crisis, along with “the additional factor of social distancing and isolation, which all contributed to the decline in birth rates. Some people changed their plans to hold off on having children, while others didn’t get pregnant because they weren’t socializing and meeting people as much.”
Dr. Cohen, who was not involved in the study by Dr. Aassve and associates, said his provisional data show that although in many places, birth rates have rebounded more or less to prepandemic levels after a nadir around Jan. 2021, some areas of the United States still show substantially lower rates, including California, Hawaii, and Oregon.
As to the duration of the pandemic effect, Dr. Aassve cautions that his group’s estimates refer to the first wave only. “We then have the second, third, and currently the fourth wave. We can’t be sure about the impact of these waves on fertility since the data are not there yet, but I’d be surprised if they didn’t continue to have an impact on fertility rates,” he said.
Dr. Cohen agreed: “Some people who delayed childbearing will make up the delay. However, whenever there’s a delay, there’s inevitably some portion of the decline that’s not recouped.”
As for the wider effect across the world, Dr. Aassve said his team’s figures derive from high-income countries where data are readily available. For middle- and low-income countries, fewer data exist, and the quality of those data is not as good.
The lessons from this and other upheavals teach us that unforeseen shocks almost always have a negative impact on fertility, says Dr. Aassve. “[B]ut these effects may be separate from existing declining trends. The issue here is that those overall declining trends may be driven by other factors. In contrast, the shock of the pandemic is short-lived, and we may return to normal rather quickly. But if the pandemic also impacts other societal structures, such as the occupational and industrial sectors, then the pandemic might exacerbate the negative trend.”
The study was supported by funding from the European Research Council for funding under the European Union’s Horizon 2020 Research and Innovation Programme. The study authors, Dr. Stout, and Dr. Cohen have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In an assessment of the pandemic’s early effects, Arnstein Aassve, PhD, and colleagues found a significant COVID-19–related decline in crude birth rates (CBRs) in 7 of 22 high-income countries, particularly in Southwestern Europe.
Dr. Aassve, an economist at the Carlo F. Dondena Center for Research on Social Dynamics and Public Policy at the Università Commerciale Luigi Bocconi, Milan, and colleagues report the results in an article published online August 30 in the Proceedings of the National Academy of Sciences.
Defining the start of the COVID-19 pandemic as February 2020, the study identifies strong declines in Italy (-9.1%), Hungary (-8.5%), Spain (-8.4%), and Portugal (-6.6%) beyond those predicted by past trends. In the United States, CBRs fell by 7.1% relative to 2019 for births occurring in Nov. and Dec. 2020 following conceptions in February and March of that year.
Significant declines in CBR also occurred in Belgium, Austria, and Singapore.
A year-to-year comparison of the mean for monthly CBRs per 1,000 population before and during the pandemic suggests a negative difference for all countries studied except for Denmark, Finland, Germany, and the Netherlands, Dr. Aassve and colleagues write. These findings may have policy implications for childcare, housing, and the labor market.
The Milan researchers compared monthly vital statistics data on live births from the international Human Fertility Database for the period of Jan. 2016 to March 2021. These figures reflect conceptions carried to term between April 2015 and June 2020. The 22 countries in the analysis represent 37% of the total reported COVID-19 cases and 34% of deaths worldwide.
The study findings align with surveys on “fertility intentions” collected early in the first COVID-19 wave in Germany, France, Spain, and the United Kingdom. These surveys indicated that 73% of people who were planning pregnancies in 2020 either decided to delay the pregnancy or they abandoned their plans.
“The popular media speculated that the lockdown would lead to a baby boom, as couples spent more time together,” Dr. Aassve told this news organization. “There’s very little evidence of this when you look to previous disasters and shocks, and the first data suggest more of an immediate collapse than a boom. But as you also see from the paper, the collapse is not seen everywhere.” Other current studies suggest the fertility drop is immediate but temporary, says Dr. Aassve, who is also a professor of demography.
Interestingly, Dr. Aassve and colleagues found that CBRs were relatively stable in Northern Europe. The authors point to supportive social and family policies in that region that might have reduced the effect of the pandemic on births. “These factors are likely to affect CBRs in the subsequent pandemic waves,” they write. They call for future studies to assess the full population implications of the pandemic, the moderating impact of policy interventions, and the nexus between short- and long-run effects in relation to the various waves of the COVID-19 pandemic.
Rebounds
Some regions have already reported a rebound from the COVID-19 fertility trough. Molly J. Stout, MD, director of maternal fetal medicine at the University of Michigan, Ann Arbor, and colleagues used electronic medical records to predict a surge in births after the initial decline.
“The surge we’ve seen at the end of this summer is exceeding the usual annual birth rate, as predicted,” she said in an interview. “But I think there’ll be a return to normal after this transient escalation. I don’t think birth rates will stay elevated above the normal because the birth surge is a temporary response to an event, although there will likely be regional differences.”
Looking ahead, Dr. Stout, who was not involved in Dr. Aassve’s analysis, is not certain how a fourth pandemic wave might ultimately modify a couple’s overall family size. But the toll the health crisis has taken on working women who have been forced to withdraw from the economy because of a lack of childcare points to a societal need that should be addressed.
According to Philip N. Cohen, PhD, a professor of sociology at the University of Maryland, College Park, who’s been tracking fertility trends since the onset of the COVID-19 emergency, the pandemic has combined a health crisis with an economic crisis, along with “the additional factor of social distancing and isolation, which all contributed to the decline in birth rates. Some people changed their plans to hold off on having children, while others didn’t get pregnant because they weren’t socializing and meeting people as much.”
Dr. Cohen, who was not involved in the study by Dr. Aassve and associates, said his provisional data show that although in many places, birth rates have rebounded more or less to prepandemic levels after a nadir around Jan. 2021, some areas of the United States still show substantially lower rates, including California, Hawaii, and Oregon.
As to the duration of the pandemic effect, Dr. Aassve cautions that his group’s estimates refer to the first wave only. “We then have the second, third, and currently the fourth wave. We can’t be sure about the impact of these waves on fertility since the data are not there yet, but I’d be surprised if they didn’t continue to have an impact on fertility rates,” he said.
Dr. Cohen agreed: “Some people who delayed childbearing will make up the delay. However, whenever there’s a delay, there’s inevitably some portion of the decline that’s not recouped.”
As for the wider effect across the world, Dr. Aassve said his team’s figures derive from high-income countries where data are readily available. For middle- and low-income countries, fewer data exist, and the quality of those data is not as good.
The lessons from this and other upheavals teach us that unforeseen shocks almost always have a negative impact on fertility, says Dr. Aassve. “[B]ut these effects may be separate from existing declining trends. The issue here is that those overall declining trends may be driven by other factors. In contrast, the shock of the pandemic is short-lived, and we may return to normal rather quickly. But if the pandemic also impacts other societal structures, such as the occupational and industrial sectors, then the pandemic might exacerbate the negative trend.”
The study was supported by funding from the European Research Council for funding under the European Union’s Horizon 2020 Research and Innovation Programme. The study authors, Dr. Stout, and Dr. Cohen have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Three JAK inhibitors get boxed warnings, modified indications
The arthritis and ulcerative colitis medicine tofacitinib (Xeljanz, Xeljanz XR) poses an increased risk of serious cardiac events such as heart attack or stroke, cancer, blood clots, and death, the Food and Drug Administration announced Sept 1.
Manufacturers of this drug along with other Janus kinase (JAK) inhibitors baricitinib (Olumiant) and upadacitinib (Rinvoq) must update their boxed warnings to include information about these health risks. The FDA made the determination after new study data from Pfizer, which manufacturers Xeljanz, found an association between a lower dose of Xeljanz and increased risk of blood clots and death.
“Recommendations for healthcare professionals will include consideration of the benefits and risks for the individual patient prior to initiating or continuing therapy,” the agency stated.
The FDA is limiting all approved uses of these three medications to patients who have not responded well to tumor necrosis factor (TNF) blockers to ensure their benefits outweigh their risks. Tofacitinib is indicated for rheumatoid arthritis, psoriatic arthritis, ulcerative colitis, and polyarticular course juvenile idiopathic arthritis. Baricitinib and upadacitinib are approved only for RA. The FDA included baricitinib and upadacitinib in the warning because of the similar properties they share with tofacitinib, even though they haven’t been studied as extensively.
“We believe this update will bring important clarity for healthcare plans on the risk/benefit profile of Xeljanz, which is a medicine informed by more clinical data than any other JAK inhibitor,” Pfizer said in a statement.
Investigators for the ORAL Surveillance trial compared two doses of tofacitinib (5 mg twice daily and 10 mg twice daily) with TNF blockers in patients with rheumatoid arthritis who were aged 50 years or older with at least one additional cardiovascular risk factor.
For both dose regimens of tofacitinib, they found an increased risk of major adverse cardiovascular events, malignancies, thrombosis, and death compared with the TNF blocker regimen. In addition, rates of lung cancers and lymphomas were higher with tofacitinib. In trial data released earlier this year, Pfizer revealed that the tofacitinib group had a much higher incidence of adjudicated malignancies compared with the TNF blocker group (1.13 vs. 0.77 per 100 person-years; hazard ratio, 1.48; 95% confidence interval, 1.04-2.09).
Impact on clinical practice
Physicians treating patients who have rheumatoid arthritis with tofacitinib may initially decrease prescriptions following the FDA’s drug safety communication, said Daniel E. Furst, MD, professor of medicine (emeritus) at the University of California, Los Angeles, adjunct professor at the University of Washington, Seattle, and a research professor at the University of Florence (Italy) – particularly those with a principal mechanism of action slightly different from that of tofacitinib, he added.
“Tofacitinib is principally a JAK 1,3 inhibitor at usual concentrations, whereas upadacitinib and baricitinib are JAK 1,2 inhibitors. Thus, I speculate that the tofacitinib prescriptions will go down more than the upadacitinib and baricitinib prescriptions,” he said in an interview.
Some patients may also be worried about taking tofacitinib, particularly those with previous events or predisposing conditions, Dr. Furst noted.
“First and foremost, I think we need to actually look at the data in a publication rather than just an FDA statement before making huge changes in our practice,” he advised.
“I am looking forward to the data finally being published ... It’s interesting that the full data still isn’t really out there beyond the press releases and an abstract. I think there’s a lot more to learn about how these drugs work and who is really at risk for harmful events,” said Alexis R. Ogdie, MD, MSCE, associate professor of medicine and epidemiology at the University of Pennsylvania, Philadelphia.
Pfizer’s data also may be affecting FDA approvals of other JAK inhibitors. This past summer, AbbVie and Eli Lilly stated that the FDA’s ongoing assessment of the safety trial was delaying the agency’s decisions about expanding use of their respective drugs upadacitinib and baricitinib.
“I think many rheumatologists have already taken this information in, and begun to incorporate it into their discussions with their patients” since it has been over a year since the first public release of information about the ORAL Surveillance trial, said Arthur Kavanaugh, MD, professor of medicine at the University of California, San Diego. “I don’t know that it will affect the approvals, but it will impact their labels.”
Wariness to prescribing tofacitinib may be lower for patients younger than those in the ORAL Surveillance trial without additional cardiovascular risk factors who are taking tofacitinib for non-RA indications, said gastroenterologist Miguel Regueiro, MD.
“The JAK inhibitor warning by the FDA is an important consideration for any prescriber or patient. The risk of cardiovascular disease and venous thromboembolism with this class of medicine appears higher in older rheumatoid arthritis patients with underlying cardiovascular disease. While the warning applies to all JAK inhibitors and likely the newer selective JAK inhibitors to come, we need to weigh the risk and benefit based on the indication for prescribing,” said Dr. Regueiro, chair of the Digestive Disease and Surgery Institute and of the department of gastroenterology, hepatology and nutrition at the Cleveland Clinic in Ohio.
“I do think that there will be a heightened awareness and wariness for older RA patients and for the prescribers. However, for inflammatory bowel disease (and other non-RA indications), it does not appear that the risk for cardiovascular disease and VTE are significantly increased. To that end, in my own practice, I still use tofacitinib for ulcerative colitis and will do the same for the selective JAK inhibitors to come for IBD. Of course, as with any medication, we need to have discussions with our patients, alert them to potential side effects and have an open line of communication for any questions or concerns.”
Gastroenterologist Stephen Hanauer, MD, professor of medicine at Northwestern University, Chicago, thought that while patients with RA have many other treatment options besides JAK inhibitors, fewer options available to patients with IBD “may motivate the use of oral [sphingosine-1-phosphate receptor modulator] agents such as ozanimod, although IBD patients are younger and [have fewer] MACE risk factors than RA patients, so absolute risk is very small in the ulcerative colitis population.”
Pfizer’s data may be affecting FDA approvals of other JAK inhibitors. This past summer, AbbVie and Eli Lilly stated that the FDA’s ongoing assessment of the safety trial was delaying the agency’s decisions about expanding use of their respective drugs upadacitinib and baricitinib.
The agency’s decision corroborates an earlier 2019 warning about the increased risk of blood clots and of death in patients with ulcerative colitis taking 10 mg tofacitinib twice daily.
The FDA said that two other JAK inhibitors, ruxolitinib (Jakafi) and fedratinib (Inrebic), are not indicated for the treatment of arthritis and other inflammatory conditions, and so are not a part of the updates being required.
Baricitinib, abrocitinib, and upadacitinib are currently under FDA review for treating atopic dermatitis (AD); a topical formulation of the JAK1/2 inhibitor ruxolitinib is under review for treating AD. Reviews for all 4 have been extended. In September 2020, baricitinib was approved for treating moderate to severe AD in Europe, at a dose of 4 mg once a day, with recommendations that the dose can be reduced to 2 mg once a day when the disease is under control, and that the dose may need to be reduced in patients with impaired kidney function, those with an increased risk of infections, and those older than aged 75 years.
In an interview, Jacob Thyssen, MD, PhD, professor of dermatology at the University of Copenhagen, said that in the EU, there has been “extensive education” about cardiovascular risks with baricitinib “and it is my impression that payers and dermatologists in Europe are confident that it is safe to use in AD.” In addition, there has been an emphasis on the differences in cardiovascular risk factors between RA and AD patients, “given that the latter group is generally young and lean.” In the United States, he added, it will be interesting to see which doses of the JAK inhibitors will be approved for AD.
Dr. Thyssen disclosed that he is a speaker, advisory board member and/or investigator for Regeneron, Sanofi-Genzyme, Eli Lilly, Pfizer, LEO Pharma, AbbVie, and Almirall.
*This story was updated 9/3/21 and 9/6/2021.
A version of this article first appeared on Medscape.com.
The arthritis and ulcerative colitis medicine tofacitinib (Xeljanz, Xeljanz XR) poses an increased risk of serious cardiac events such as heart attack or stroke, cancer, blood clots, and death, the Food and Drug Administration announced Sept 1.
Manufacturers of this drug along with other Janus kinase (JAK) inhibitors baricitinib (Olumiant) and upadacitinib (Rinvoq) must update their boxed warnings to include information about these health risks. The FDA made the determination after new study data from Pfizer, which manufacturers Xeljanz, found an association between a lower dose of Xeljanz and increased risk of blood clots and death.
“Recommendations for healthcare professionals will include consideration of the benefits and risks for the individual patient prior to initiating or continuing therapy,” the agency stated.
The FDA is limiting all approved uses of these three medications to patients who have not responded well to tumor necrosis factor (TNF) blockers to ensure their benefits outweigh their risks. Tofacitinib is indicated for rheumatoid arthritis, psoriatic arthritis, ulcerative colitis, and polyarticular course juvenile idiopathic arthritis. Baricitinib and upadacitinib are approved only for RA. The FDA included baricitinib and upadacitinib in the warning because of the similar properties they share with tofacitinib, even though they haven’t been studied as extensively.
“We believe this update will bring important clarity for healthcare plans on the risk/benefit profile of Xeljanz, which is a medicine informed by more clinical data than any other JAK inhibitor,” Pfizer said in a statement.
Investigators for the ORAL Surveillance trial compared two doses of tofacitinib (5 mg twice daily and 10 mg twice daily) with TNF blockers in patients with rheumatoid arthritis who were aged 50 years or older with at least one additional cardiovascular risk factor.
For both dose regimens of tofacitinib, they found an increased risk of major adverse cardiovascular events, malignancies, thrombosis, and death compared with the TNF blocker regimen. In addition, rates of lung cancers and lymphomas were higher with tofacitinib. In trial data released earlier this year, Pfizer revealed that the tofacitinib group had a much higher incidence of adjudicated malignancies compared with the TNF blocker group (1.13 vs. 0.77 per 100 person-years; hazard ratio, 1.48; 95% confidence interval, 1.04-2.09).
Impact on clinical practice
Physicians treating patients who have rheumatoid arthritis with tofacitinib may initially decrease prescriptions following the FDA’s drug safety communication, said Daniel E. Furst, MD, professor of medicine (emeritus) at the University of California, Los Angeles, adjunct professor at the University of Washington, Seattle, and a research professor at the University of Florence (Italy) – particularly those with a principal mechanism of action slightly different from that of tofacitinib, he added.
“Tofacitinib is principally a JAK 1,3 inhibitor at usual concentrations, whereas upadacitinib and baricitinib are JAK 1,2 inhibitors. Thus, I speculate that the tofacitinib prescriptions will go down more than the upadacitinib and baricitinib prescriptions,” he said in an interview.
Some patients may also be worried about taking tofacitinib, particularly those with previous events or predisposing conditions, Dr. Furst noted.
“First and foremost, I think we need to actually look at the data in a publication rather than just an FDA statement before making huge changes in our practice,” he advised.
“I am looking forward to the data finally being published ... It’s interesting that the full data still isn’t really out there beyond the press releases and an abstract. I think there’s a lot more to learn about how these drugs work and who is really at risk for harmful events,” said Alexis R. Ogdie, MD, MSCE, associate professor of medicine and epidemiology at the University of Pennsylvania, Philadelphia.
Pfizer’s data also may be affecting FDA approvals of other JAK inhibitors. This past summer, AbbVie and Eli Lilly stated that the FDA’s ongoing assessment of the safety trial was delaying the agency’s decisions about expanding use of their respective drugs upadacitinib and baricitinib.
“I think many rheumatologists have already taken this information in, and begun to incorporate it into their discussions with their patients” since it has been over a year since the first public release of information about the ORAL Surveillance trial, said Arthur Kavanaugh, MD, professor of medicine at the University of California, San Diego. “I don’t know that it will affect the approvals, but it will impact their labels.”
Wariness to prescribing tofacitinib may be lower for patients younger than those in the ORAL Surveillance trial without additional cardiovascular risk factors who are taking tofacitinib for non-RA indications, said gastroenterologist Miguel Regueiro, MD.
“The JAK inhibitor warning by the FDA is an important consideration for any prescriber or patient. The risk of cardiovascular disease and venous thromboembolism with this class of medicine appears higher in older rheumatoid arthritis patients with underlying cardiovascular disease. While the warning applies to all JAK inhibitors and likely the newer selective JAK inhibitors to come, we need to weigh the risk and benefit based on the indication for prescribing,” said Dr. Regueiro, chair of the Digestive Disease and Surgery Institute and of the department of gastroenterology, hepatology and nutrition at the Cleveland Clinic in Ohio.
“I do think that there will be a heightened awareness and wariness for older RA patients and for the prescribers. However, for inflammatory bowel disease (and other non-RA indications), it does not appear that the risk for cardiovascular disease and VTE are significantly increased. To that end, in my own practice, I still use tofacitinib for ulcerative colitis and will do the same for the selective JAK inhibitors to come for IBD. Of course, as with any medication, we need to have discussions with our patients, alert them to potential side effects and have an open line of communication for any questions or concerns.”
Gastroenterologist Stephen Hanauer, MD, professor of medicine at Northwestern University, Chicago, thought that while patients with RA have many other treatment options besides JAK inhibitors, fewer options available to patients with IBD “may motivate the use of oral [sphingosine-1-phosphate receptor modulator] agents such as ozanimod, although IBD patients are younger and [have fewer] MACE risk factors than RA patients, so absolute risk is very small in the ulcerative colitis population.”
Pfizer’s data may be affecting FDA approvals of other JAK inhibitors. This past summer, AbbVie and Eli Lilly stated that the FDA’s ongoing assessment of the safety trial was delaying the agency’s decisions about expanding use of their respective drugs upadacitinib and baricitinib.
The agency’s decision corroborates an earlier 2019 warning about the increased risk of blood clots and of death in patients with ulcerative colitis taking 10 mg tofacitinib twice daily.
The FDA said that two other JAK inhibitors, ruxolitinib (Jakafi) and fedratinib (Inrebic), are not indicated for the treatment of arthritis and other inflammatory conditions, and so are not a part of the updates being required.
Baricitinib, abrocitinib, and upadacitinib are currently under FDA review for treating atopic dermatitis (AD); a topical formulation of the JAK1/2 inhibitor ruxolitinib is under review for treating AD. Reviews for all 4 have been extended. In September 2020, baricitinib was approved for treating moderate to severe AD in Europe, at a dose of 4 mg once a day, with recommendations that the dose can be reduced to 2 mg once a day when the disease is under control, and that the dose may need to be reduced in patients with impaired kidney function, those with an increased risk of infections, and those older than aged 75 years.
In an interview, Jacob Thyssen, MD, PhD, professor of dermatology at the University of Copenhagen, said that in the EU, there has been “extensive education” about cardiovascular risks with baricitinib “and it is my impression that payers and dermatologists in Europe are confident that it is safe to use in AD.” In addition, there has been an emphasis on the differences in cardiovascular risk factors between RA and AD patients, “given that the latter group is generally young and lean.” In the United States, he added, it will be interesting to see which doses of the JAK inhibitors will be approved for AD.
Dr. Thyssen disclosed that he is a speaker, advisory board member and/or investigator for Regeneron, Sanofi-Genzyme, Eli Lilly, Pfizer, LEO Pharma, AbbVie, and Almirall.
*This story was updated 9/3/21 and 9/6/2021.
A version of this article first appeared on Medscape.com.
The arthritis and ulcerative colitis medicine tofacitinib (Xeljanz, Xeljanz XR) poses an increased risk of serious cardiac events such as heart attack or stroke, cancer, blood clots, and death, the Food and Drug Administration announced Sept 1.
Manufacturers of this drug along with other Janus kinase (JAK) inhibitors baricitinib (Olumiant) and upadacitinib (Rinvoq) must update their boxed warnings to include information about these health risks. The FDA made the determination after new study data from Pfizer, which manufacturers Xeljanz, found an association between a lower dose of Xeljanz and increased risk of blood clots and death.
“Recommendations for healthcare professionals will include consideration of the benefits and risks for the individual patient prior to initiating or continuing therapy,” the agency stated.
The FDA is limiting all approved uses of these three medications to patients who have not responded well to tumor necrosis factor (TNF) blockers to ensure their benefits outweigh their risks. Tofacitinib is indicated for rheumatoid arthritis, psoriatic arthritis, ulcerative colitis, and polyarticular course juvenile idiopathic arthritis. Baricitinib and upadacitinib are approved only for RA. The FDA included baricitinib and upadacitinib in the warning because of the similar properties they share with tofacitinib, even though they haven’t been studied as extensively.
“We believe this update will bring important clarity for healthcare plans on the risk/benefit profile of Xeljanz, which is a medicine informed by more clinical data than any other JAK inhibitor,” Pfizer said in a statement.
Investigators for the ORAL Surveillance trial compared two doses of tofacitinib (5 mg twice daily and 10 mg twice daily) with TNF blockers in patients with rheumatoid arthritis who were aged 50 years or older with at least one additional cardiovascular risk factor.
For both dose regimens of tofacitinib, they found an increased risk of major adverse cardiovascular events, malignancies, thrombosis, and death compared with the TNF blocker regimen. In addition, rates of lung cancers and lymphomas were higher with tofacitinib. In trial data released earlier this year, Pfizer revealed that the tofacitinib group had a much higher incidence of adjudicated malignancies compared with the TNF blocker group (1.13 vs. 0.77 per 100 person-years; hazard ratio, 1.48; 95% confidence interval, 1.04-2.09).
Impact on clinical practice
Physicians treating patients who have rheumatoid arthritis with tofacitinib may initially decrease prescriptions following the FDA’s drug safety communication, said Daniel E. Furst, MD, professor of medicine (emeritus) at the University of California, Los Angeles, adjunct professor at the University of Washington, Seattle, and a research professor at the University of Florence (Italy) – particularly those with a principal mechanism of action slightly different from that of tofacitinib, he added.
“Tofacitinib is principally a JAK 1,3 inhibitor at usual concentrations, whereas upadacitinib and baricitinib are JAK 1,2 inhibitors. Thus, I speculate that the tofacitinib prescriptions will go down more than the upadacitinib and baricitinib prescriptions,” he said in an interview.
Some patients may also be worried about taking tofacitinib, particularly those with previous events or predisposing conditions, Dr. Furst noted.
“First and foremost, I think we need to actually look at the data in a publication rather than just an FDA statement before making huge changes in our practice,” he advised.
“I am looking forward to the data finally being published ... It’s interesting that the full data still isn’t really out there beyond the press releases and an abstract. I think there’s a lot more to learn about how these drugs work and who is really at risk for harmful events,” said Alexis R. Ogdie, MD, MSCE, associate professor of medicine and epidemiology at the University of Pennsylvania, Philadelphia.
Pfizer’s data also may be affecting FDA approvals of other JAK inhibitors. This past summer, AbbVie and Eli Lilly stated that the FDA’s ongoing assessment of the safety trial was delaying the agency’s decisions about expanding use of their respective drugs upadacitinib and baricitinib.
“I think many rheumatologists have already taken this information in, and begun to incorporate it into their discussions with their patients” since it has been over a year since the first public release of information about the ORAL Surveillance trial, said Arthur Kavanaugh, MD, professor of medicine at the University of California, San Diego. “I don’t know that it will affect the approvals, but it will impact their labels.”
Wariness to prescribing tofacitinib may be lower for patients younger than those in the ORAL Surveillance trial without additional cardiovascular risk factors who are taking tofacitinib for non-RA indications, said gastroenterologist Miguel Regueiro, MD.
“The JAK inhibitor warning by the FDA is an important consideration for any prescriber or patient. The risk of cardiovascular disease and venous thromboembolism with this class of medicine appears higher in older rheumatoid arthritis patients with underlying cardiovascular disease. While the warning applies to all JAK inhibitors and likely the newer selective JAK inhibitors to come, we need to weigh the risk and benefit based on the indication for prescribing,” said Dr. Regueiro, chair of the Digestive Disease and Surgery Institute and of the department of gastroenterology, hepatology and nutrition at the Cleveland Clinic in Ohio.
“I do think that there will be a heightened awareness and wariness for older RA patients and for the prescribers. However, for inflammatory bowel disease (and other non-RA indications), it does not appear that the risk for cardiovascular disease and VTE are significantly increased. To that end, in my own practice, I still use tofacitinib for ulcerative colitis and will do the same for the selective JAK inhibitors to come for IBD. Of course, as with any medication, we need to have discussions with our patients, alert them to potential side effects and have an open line of communication for any questions or concerns.”
Gastroenterologist Stephen Hanauer, MD, professor of medicine at Northwestern University, Chicago, thought that while patients with RA have many other treatment options besides JAK inhibitors, fewer options available to patients with IBD “may motivate the use of oral [sphingosine-1-phosphate receptor modulator] agents such as ozanimod, although IBD patients are younger and [have fewer] MACE risk factors than RA patients, so absolute risk is very small in the ulcerative colitis population.”
Pfizer’s data may be affecting FDA approvals of other JAK inhibitors. This past summer, AbbVie and Eli Lilly stated that the FDA’s ongoing assessment of the safety trial was delaying the agency’s decisions about expanding use of their respective drugs upadacitinib and baricitinib.
The agency’s decision corroborates an earlier 2019 warning about the increased risk of blood clots and of death in patients with ulcerative colitis taking 10 mg tofacitinib twice daily.
The FDA said that two other JAK inhibitors, ruxolitinib (Jakafi) and fedratinib (Inrebic), are not indicated for the treatment of arthritis and other inflammatory conditions, and so are not a part of the updates being required.
Baricitinib, abrocitinib, and upadacitinib are currently under FDA review for treating atopic dermatitis (AD); a topical formulation of the JAK1/2 inhibitor ruxolitinib is under review for treating AD. Reviews for all 4 have been extended. In September 2020, baricitinib was approved for treating moderate to severe AD in Europe, at a dose of 4 mg once a day, with recommendations that the dose can be reduced to 2 mg once a day when the disease is under control, and that the dose may need to be reduced in patients with impaired kidney function, those with an increased risk of infections, and those older than aged 75 years.
In an interview, Jacob Thyssen, MD, PhD, professor of dermatology at the University of Copenhagen, said that in the EU, there has been “extensive education” about cardiovascular risks with baricitinib “and it is my impression that payers and dermatologists in Europe are confident that it is safe to use in AD.” In addition, there has been an emphasis on the differences in cardiovascular risk factors between RA and AD patients, “given that the latter group is generally young and lean.” In the United States, he added, it will be interesting to see which doses of the JAK inhibitors will be approved for AD.
Dr. Thyssen disclosed that he is a speaker, advisory board member and/or investigator for Regeneron, Sanofi-Genzyme, Eli Lilly, Pfizer, LEO Pharma, AbbVie, and Almirall.
*This story was updated 9/3/21 and 9/6/2021.
A version of this article first appeared on Medscape.com.
Exercising to lose weight is not for every ‘body’
Exercising to lose weight is not for every ‘body’
This first item comes from the “You’ve got to be kidding” section of LOTME’s supersecret topics-of-interest file.
Investigators at the Shenzhen Institute of Advanced Technology of the Chinese Academy of Sciences and the University of Roehampton noticed that some people who enrolled in exercise programs to lose weight did just the opposite: they gained weight.
Being scientists, they decided to look at the effects of energy expenditure and how those effects varied among individuals. The likely culprit in this case, they determined, is something called compensatory mechanisms. One such mechanism involves eating more food because exercise stimulates appetite, and another might reduce energy expenditure on other components like resting metabolism so that the exercise is, in effect, less costly.
A look at the numbers shows how compensatory mechanisms worked in the study population of 1,750 adults. Among individuals with the highest BMI, 51% of the calories burned during activity translated into calories burned at the end of the day. For those with normal BMI, however, 72% of calories burned during activity were reflected in total expenditure.
“People living with obesity cut back their resting metabolism when they are more active. The result is that for every calorie they spend on exercise they save about half a calorie on resting,” the investigators explained.
In other words, some bodies will, unconsciously, work against the conscious effort of exercising to lose weight. Thank you very much, compensatory mechanisms, for the boundarylessness exhibited in exceeding your job description.
When it comes to the mix, walnuts go nuts
When it comes to mixed nuts, walnuts get no love. But we may be able to give you a reason to not pick them out: Your arteries.
Participants in a recent study who ate about a half-cup of walnuts every day for 2 years saw a drop in their low-density lipoprotein (LDL) cholesterol. The number and quality of LDL particles in healthy older adults also improved. How? Good ol’ omega-3 fatty acids.
Omega-3 is found in many foods linked to lower risks of heart disease, lower cholesterol levels, and lower blood sugar levels, but the one thing that makes the walnut a front runner for Miss Super Food 2021 is their ability to improve the quality of LDL particles.
“LDL particles come in various sizes [and] research has shown that small, dense LDL particles are more often associated with atherosclerosis, the plaque or fatty deposits that build up in the arteries,” Emilio Ros, MD, PhD, of the Hospital Clínic of Barcelona and the study’s senior investigator, said in a written statement.
The 708 participants, aged 63-79 years and mostly women, were divided into two groups: One received the walnut diet and the other did not. After 2 years, the walnut group had lower LDL levels by an average of 4.3 mg/dL. Total cholesterol was reduced by an average of 8.5 mg/dL. Also, their total LDL particle count was 4.3% lower and small LDL particles were down by 6.1%.
So instead of picking the walnuts out of the mix, try to find it in your heart to appreciate them. Your body already does.
Begun, the clone war has
Well, not quite yet, Master Yoda, but perhaps one day soon, if a study from Japan into the uncanny valley of the usage of cloned humanlike faces in robotics and artificial intelligence, published in PLOS One, is to be believed.
The study consisted of a number of six smaller experiments in which participants judged a series of images based on subjective eeriness, emotional valence, and realism. The images included people with the same cloned face; people with different faces; dogs; identical twins, triplets, quadruplets, etc.; and cloned animated characters. In the sixth experiment, the photos were the same as in the second (six cloned faces, six different faces, and a single face) but participants also answered the Disgust Scale–Revised to accurately analyze disgust sensitivity.
The results of all these experiments were quite clear: People found the cloned faces far creepier than the varied or single face, an effect the researchers called clone devaluation. Notably, this effect only applied to realistic human faces; most people didn’t find the cloned dogs or cloned animated characters creepy. However, those who did were more likely to find the human clones eerie on the Disgust Scale.
The authors noted that future robotics technology needs to be carefully considered to avoid the uncanny valley and this clone devaluation effect, which is a very good point. The last thing we need is a few million robots with identical faces getting angry at us and pulling a Terminator/Order 66 combo. We’re already in a viral apocalypse; we don’t need a robot one on top of that.
Congratulations to our new favorite reader
The winner of last week’s inaugural Pandemic Pandemonium comes to us from Tiffanie Roe. By getting her entry in first, just ahead of the flood of responses we received – and by flood we mean a very slow and very quickly repaired drip – Ms. Roe puts the gold medal for COVID-related insanity around the necks of Australian magpies, who may start attacking people wearing face masks during “swooping season” because the birds don’t recognize them.
Exercising to lose weight is not for every ‘body’
This first item comes from the “You’ve got to be kidding” section of LOTME’s supersecret topics-of-interest file.
Investigators at the Shenzhen Institute of Advanced Technology of the Chinese Academy of Sciences and the University of Roehampton noticed that some people who enrolled in exercise programs to lose weight did just the opposite: they gained weight.
Being scientists, they decided to look at the effects of energy expenditure and how those effects varied among individuals. The likely culprit in this case, they determined, is something called compensatory mechanisms. One such mechanism involves eating more food because exercise stimulates appetite, and another might reduce energy expenditure on other components like resting metabolism so that the exercise is, in effect, less costly.
A look at the numbers shows how compensatory mechanisms worked in the study population of 1,750 adults. Among individuals with the highest BMI, 51% of the calories burned during activity translated into calories burned at the end of the day. For those with normal BMI, however, 72% of calories burned during activity were reflected in total expenditure.
“People living with obesity cut back their resting metabolism when they are more active. The result is that for every calorie they spend on exercise they save about half a calorie on resting,” the investigators explained.
In other words, some bodies will, unconsciously, work against the conscious effort of exercising to lose weight. Thank you very much, compensatory mechanisms, for the boundarylessness exhibited in exceeding your job description.
When it comes to the mix, walnuts go nuts
When it comes to mixed nuts, walnuts get no love. But we may be able to give you a reason to not pick them out: Your arteries.
Participants in a recent study who ate about a half-cup of walnuts every day for 2 years saw a drop in their low-density lipoprotein (LDL) cholesterol. The number and quality of LDL particles in healthy older adults also improved. How? Good ol’ omega-3 fatty acids.
Omega-3 is found in many foods linked to lower risks of heart disease, lower cholesterol levels, and lower blood sugar levels, but the one thing that makes the walnut a front runner for Miss Super Food 2021 is their ability to improve the quality of LDL particles.
“LDL particles come in various sizes [and] research has shown that small, dense LDL particles are more often associated with atherosclerosis, the plaque or fatty deposits that build up in the arteries,” Emilio Ros, MD, PhD, of the Hospital Clínic of Barcelona and the study’s senior investigator, said in a written statement.
The 708 participants, aged 63-79 years and mostly women, were divided into two groups: One received the walnut diet and the other did not. After 2 years, the walnut group had lower LDL levels by an average of 4.3 mg/dL. Total cholesterol was reduced by an average of 8.5 mg/dL. Also, their total LDL particle count was 4.3% lower and small LDL particles were down by 6.1%.
So instead of picking the walnuts out of the mix, try to find it in your heart to appreciate them. Your body already does.
Begun, the clone war has
Well, not quite yet, Master Yoda, but perhaps one day soon, if a study from Japan into the uncanny valley of the usage of cloned humanlike faces in robotics and artificial intelligence, published in PLOS One, is to be believed.
The study consisted of a number of six smaller experiments in which participants judged a series of images based on subjective eeriness, emotional valence, and realism. The images included people with the same cloned face; people with different faces; dogs; identical twins, triplets, quadruplets, etc.; and cloned animated characters. In the sixth experiment, the photos were the same as in the second (six cloned faces, six different faces, and a single face) but participants also answered the Disgust Scale–Revised to accurately analyze disgust sensitivity.
The results of all these experiments were quite clear: People found the cloned faces far creepier than the varied or single face, an effect the researchers called clone devaluation. Notably, this effect only applied to realistic human faces; most people didn’t find the cloned dogs or cloned animated characters creepy. However, those who did were more likely to find the human clones eerie on the Disgust Scale.
The authors noted that future robotics technology needs to be carefully considered to avoid the uncanny valley and this clone devaluation effect, which is a very good point. The last thing we need is a few million robots with identical faces getting angry at us and pulling a Terminator/Order 66 combo. We’re already in a viral apocalypse; we don’t need a robot one on top of that.
Congratulations to our new favorite reader
The winner of last week’s inaugural Pandemic Pandemonium comes to us from Tiffanie Roe. By getting her entry in first, just ahead of the flood of responses we received – and by flood we mean a very slow and very quickly repaired drip – Ms. Roe puts the gold medal for COVID-related insanity around the necks of Australian magpies, who may start attacking people wearing face masks during “swooping season” because the birds don’t recognize them.
Exercising to lose weight is not for every ‘body’
This first item comes from the “You’ve got to be kidding” section of LOTME’s supersecret topics-of-interest file.
Investigators at the Shenzhen Institute of Advanced Technology of the Chinese Academy of Sciences and the University of Roehampton noticed that some people who enrolled in exercise programs to lose weight did just the opposite: they gained weight.
Being scientists, they decided to look at the effects of energy expenditure and how those effects varied among individuals. The likely culprit in this case, they determined, is something called compensatory mechanisms. One such mechanism involves eating more food because exercise stimulates appetite, and another might reduce energy expenditure on other components like resting metabolism so that the exercise is, in effect, less costly.
A look at the numbers shows how compensatory mechanisms worked in the study population of 1,750 adults. Among individuals with the highest BMI, 51% of the calories burned during activity translated into calories burned at the end of the day. For those with normal BMI, however, 72% of calories burned during activity were reflected in total expenditure.
“People living with obesity cut back their resting metabolism when they are more active. The result is that for every calorie they spend on exercise they save about half a calorie on resting,” the investigators explained.
In other words, some bodies will, unconsciously, work against the conscious effort of exercising to lose weight. Thank you very much, compensatory mechanisms, for the boundarylessness exhibited in exceeding your job description.
When it comes to the mix, walnuts go nuts
When it comes to mixed nuts, walnuts get no love. But we may be able to give you a reason to not pick them out: Your arteries.
Participants in a recent study who ate about a half-cup of walnuts every day for 2 years saw a drop in their low-density lipoprotein (LDL) cholesterol. The number and quality of LDL particles in healthy older adults also improved. How? Good ol’ omega-3 fatty acids.
Omega-3 is found in many foods linked to lower risks of heart disease, lower cholesterol levels, and lower blood sugar levels, but the one thing that makes the walnut a front runner for Miss Super Food 2021 is their ability to improve the quality of LDL particles.
“LDL particles come in various sizes [and] research has shown that small, dense LDL particles are more often associated with atherosclerosis, the plaque or fatty deposits that build up in the arteries,” Emilio Ros, MD, PhD, of the Hospital Clínic of Barcelona and the study’s senior investigator, said in a written statement.
The 708 participants, aged 63-79 years and mostly women, were divided into two groups: One received the walnut diet and the other did not. After 2 years, the walnut group had lower LDL levels by an average of 4.3 mg/dL. Total cholesterol was reduced by an average of 8.5 mg/dL. Also, their total LDL particle count was 4.3% lower and small LDL particles were down by 6.1%.
So instead of picking the walnuts out of the mix, try to find it in your heart to appreciate them. Your body already does.
Begun, the clone war has
Well, not quite yet, Master Yoda, but perhaps one day soon, if a study from Japan into the uncanny valley of the usage of cloned humanlike faces in robotics and artificial intelligence, published in PLOS One, is to be believed.
The study consisted of a number of six smaller experiments in which participants judged a series of images based on subjective eeriness, emotional valence, and realism. The images included people with the same cloned face; people with different faces; dogs; identical twins, triplets, quadruplets, etc.; and cloned animated characters. In the sixth experiment, the photos were the same as in the second (six cloned faces, six different faces, and a single face) but participants also answered the Disgust Scale–Revised to accurately analyze disgust sensitivity.
The results of all these experiments were quite clear: People found the cloned faces far creepier than the varied or single face, an effect the researchers called clone devaluation. Notably, this effect only applied to realistic human faces; most people didn’t find the cloned dogs or cloned animated characters creepy. However, those who did were more likely to find the human clones eerie on the Disgust Scale.
The authors noted that future robotics technology needs to be carefully considered to avoid the uncanny valley and this clone devaluation effect, which is a very good point. The last thing we need is a few million robots with identical faces getting angry at us and pulling a Terminator/Order 66 combo. We’re already in a viral apocalypse; we don’t need a robot one on top of that.
Congratulations to our new favorite reader
The winner of last week’s inaugural Pandemic Pandemonium comes to us from Tiffanie Roe. By getting her entry in first, just ahead of the flood of responses we received – and by flood we mean a very slow and very quickly repaired drip – Ms. Roe puts the gold medal for COVID-related insanity around the necks of Australian magpies, who may start attacking people wearing face masks during “swooping season” because the birds don’t recognize them.
Even highly allergic adults unlikely to react to COVID-19 vaccine
published Aug. 31, 2021, in JAMA Network Open. Symptoms resolved in a few hours with medication, and no patients required hospitalization.
Risk for allergic reaction has been one of several obstacles in global vaccination efforts, the authors, led by Nancy Agmon-Levin, MD, of the Sheba Medical Center, Ramat Gan, Israel, wrote. Clinical trials for the Moderna and Pfizer-BioNTech COVID-19 vaccines excluded individuals with allergies to any component of the vaccine or with previous allergies to other vaccines. Early reports of anaphylaxis in reaction to the vaccines caused concern among patients and practitioners. Soon after, the Centers for Disease Control and Prevention and other authorities released guidance on preparing for allergic reactions. “Despite these recommendations, uncertainty remains, particularly among patients with a history of anaphylaxis and/or multiple allergies,” the authors added.
In response to early concerns, the Sheba Medical Center opened a COVID-19 referral center to address safety questions and to conduct assessments of allergy risk for the Pfizer-BioNTech vaccine, the first COVID-19 vaccine approved in Israel. From Dec. 27, 2020, to Feb. 22, 2021, the referral center assessed 8,102 patients with allergies. Those who were not clearly at low risk filled out a questionnaire about prior allergic or anaphylactic reactions to drugs or vaccines, other allergies, and other relevant medical history. Patients were considered to be at high risk for allergic reactions if they met at least one of the following criteria: previous anaphylactic reaction to any drug or vaccine, multiple drug allergies, multiple other allergies, and mast cell disorders. Individuals were also classified as high risk if their health care practitioner deferred vaccination because of allergy concerns.
Nearly 95% of the cohort (7,668 individuals) were classified as low risk and received both Pfizer vaccine doses at standard immunization sites and underwent 30 minutes of observation after immunization. Although the study did not follow these lower-risk patients, “no serious allergic reactions were reported back to our referral center by patients or their general practitioner after immunization in the regular settings,” the authors wrote.
Five patients were considered ineligible for immunization because of known sensitivity to polyethylene glycol or multiple anaphylactic reactions to different injectable drugs, following recommendations from the Ministry of Health of Israel at the time. The remaining 429 individuals were deemed high risk and underwent observation for 2 hours from a dedicated allergy team after immunization. For these high-risk patients, both vaccine doses were administered in the same setting. Patients also reported any adverse reactions in the 21 days between the first and second dose.
Women made up most of the high-risk cohort (70.9%). The average age of participants was 52 years. Of the high-risk individuals, 63.2% reported prior anaphylaxis, 32.9% had multiple drug allergies, and 30.3% had multiple other allergies.
During the first 2 hours following immunization, nine individuals (2.1%), all women, experienced allergic reactions. Six individuals (1.4%) experienced minor reactions, including skin flushing, tongue or uvula swelling, or a cough that resolved with antihistamine treatment during the observation period. Three patients (0.7%) had anaphylactic reactions that occurred 10 to 20 minutes after injection. All three patients experienced significant bronchospasm, skin eruption, itching, and shortness of breath. Two patients experienced angioedema, and one patient had gastrointestinal symptoms. They were treated with adrenaline, antihistamines, and an inhaled bronchodilator. All symptoms resolved within 2-6 hours, and no patient required hospitalization.
In the days following vaccination, patients commonly reported pain at the injection site, fatigue, muscle pain, and headache; 14.7% of patients reported skin eruption, itching, or urticaria.
As of Feb. 22, 2021, 218 patients from this highly allergic cohort received their second dose of the vaccine. Four patients (1.8%) had mild allergic reactions. All four developed flushing, and one patient also developed a cough that resolved with antihistamine treatment. Three of these patients had experienced mild allergic reactions to the first dose and were premedicated for the second dose. One patient only reacted to the second dose.
The findings should be “very reassuring” to individuals hesitant to receive the vaccine, Elizabeth Phillips, MD, the director of the Center for Drug Safety and Immunology at Vanderbilt University Medical Center, Nashville, Tenn., said in an interview. She was not involved with the research and wrote an invited commentary on the study. “The rates of anaphylaxis and allergic reactions are truly quite low,” she said. Although about 2% of the high-risk group developed allergic reactions to immunization, the overall percentage for the entire cohort would be much lower.
The study did not investigate specific risk factors for and mechanisms of allergic reactions to COVID-19 vaccines, Dr. Phillips said, which is a study limitation that the authors also acknowledge. The National Institute for Allergy and Infectious Diseases is currently trying to answer some of these questions with a multisite, randomized, double-blinded study. The study is intended to help understand why people have these allergic reactions, Dr. Phillips added. Vanderbilt is one of the sites for the study.
While researchers continue to hunt for answers, the algorithm developed by the authors provides “a great strategy to get people that are at higher risk vaccinated in a monitored setting,” she said. The results show that “people should not be avoiding vaccination because of a history of anaphylaxis.”
Dr. Phillips has received institutional grants from the National Institutes of Health and the National Health and Medical Research Council; royalties from UpToDate and Lexicomp; and consulting fees from Janssen, Vertex, Biocryst, and Regeneron.
A version of this article first appeared on Medscape.com.
published Aug. 31, 2021, in JAMA Network Open. Symptoms resolved in a few hours with medication, and no patients required hospitalization.
Risk for allergic reaction has been one of several obstacles in global vaccination efforts, the authors, led by Nancy Agmon-Levin, MD, of the Sheba Medical Center, Ramat Gan, Israel, wrote. Clinical trials for the Moderna and Pfizer-BioNTech COVID-19 vaccines excluded individuals with allergies to any component of the vaccine or with previous allergies to other vaccines. Early reports of anaphylaxis in reaction to the vaccines caused concern among patients and practitioners. Soon after, the Centers for Disease Control and Prevention and other authorities released guidance on preparing for allergic reactions. “Despite these recommendations, uncertainty remains, particularly among patients with a history of anaphylaxis and/or multiple allergies,” the authors added.
In response to early concerns, the Sheba Medical Center opened a COVID-19 referral center to address safety questions and to conduct assessments of allergy risk for the Pfizer-BioNTech vaccine, the first COVID-19 vaccine approved in Israel. From Dec. 27, 2020, to Feb. 22, 2021, the referral center assessed 8,102 patients with allergies. Those who were not clearly at low risk filled out a questionnaire about prior allergic or anaphylactic reactions to drugs or vaccines, other allergies, and other relevant medical history. Patients were considered to be at high risk for allergic reactions if they met at least one of the following criteria: previous anaphylactic reaction to any drug or vaccine, multiple drug allergies, multiple other allergies, and mast cell disorders. Individuals were also classified as high risk if their health care practitioner deferred vaccination because of allergy concerns.
Nearly 95% of the cohort (7,668 individuals) were classified as low risk and received both Pfizer vaccine doses at standard immunization sites and underwent 30 minutes of observation after immunization. Although the study did not follow these lower-risk patients, “no serious allergic reactions were reported back to our referral center by patients or their general practitioner after immunization in the regular settings,” the authors wrote.
Five patients were considered ineligible for immunization because of known sensitivity to polyethylene glycol or multiple anaphylactic reactions to different injectable drugs, following recommendations from the Ministry of Health of Israel at the time. The remaining 429 individuals were deemed high risk and underwent observation for 2 hours from a dedicated allergy team after immunization. For these high-risk patients, both vaccine doses were administered in the same setting. Patients also reported any adverse reactions in the 21 days between the first and second dose.
Women made up most of the high-risk cohort (70.9%). The average age of participants was 52 years. Of the high-risk individuals, 63.2% reported prior anaphylaxis, 32.9% had multiple drug allergies, and 30.3% had multiple other allergies.
During the first 2 hours following immunization, nine individuals (2.1%), all women, experienced allergic reactions. Six individuals (1.4%) experienced minor reactions, including skin flushing, tongue or uvula swelling, or a cough that resolved with antihistamine treatment during the observation period. Three patients (0.7%) had anaphylactic reactions that occurred 10 to 20 minutes after injection. All three patients experienced significant bronchospasm, skin eruption, itching, and shortness of breath. Two patients experienced angioedema, and one patient had gastrointestinal symptoms. They were treated with adrenaline, antihistamines, and an inhaled bronchodilator. All symptoms resolved within 2-6 hours, and no patient required hospitalization.
In the days following vaccination, patients commonly reported pain at the injection site, fatigue, muscle pain, and headache; 14.7% of patients reported skin eruption, itching, or urticaria.
As of Feb. 22, 2021, 218 patients from this highly allergic cohort received their second dose of the vaccine. Four patients (1.8%) had mild allergic reactions. All four developed flushing, and one patient also developed a cough that resolved with antihistamine treatment. Three of these patients had experienced mild allergic reactions to the first dose and were premedicated for the second dose. One patient only reacted to the second dose.
The findings should be “very reassuring” to individuals hesitant to receive the vaccine, Elizabeth Phillips, MD, the director of the Center for Drug Safety and Immunology at Vanderbilt University Medical Center, Nashville, Tenn., said in an interview. She was not involved with the research and wrote an invited commentary on the study. “The rates of anaphylaxis and allergic reactions are truly quite low,” she said. Although about 2% of the high-risk group developed allergic reactions to immunization, the overall percentage for the entire cohort would be much lower.
The study did not investigate specific risk factors for and mechanisms of allergic reactions to COVID-19 vaccines, Dr. Phillips said, which is a study limitation that the authors also acknowledge. The National Institute for Allergy and Infectious Diseases is currently trying to answer some of these questions with a multisite, randomized, double-blinded study. The study is intended to help understand why people have these allergic reactions, Dr. Phillips added. Vanderbilt is one of the sites for the study.
While researchers continue to hunt for answers, the algorithm developed by the authors provides “a great strategy to get people that are at higher risk vaccinated in a monitored setting,” she said. The results show that “people should not be avoiding vaccination because of a history of anaphylaxis.”
Dr. Phillips has received institutional grants from the National Institutes of Health and the National Health and Medical Research Council; royalties from UpToDate and Lexicomp; and consulting fees from Janssen, Vertex, Biocryst, and Regeneron.
A version of this article first appeared on Medscape.com.
published Aug. 31, 2021, in JAMA Network Open. Symptoms resolved in a few hours with medication, and no patients required hospitalization.
Risk for allergic reaction has been one of several obstacles in global vaccination efforts, the authors, led by Nancy Agmon-Levin, MD, of the Sheba Medical Center, Ramat Gan, Israel, wrote. Clinical trials for the Moderna and Pfizer-BioNTech COVID-19 vaccines excluded individuals with allergies to any component of the vaccine or with previous allergies to other vaccines. Early reports of anaphylaxis in reaction to the vaccines caused concern among patients and practitioners. Soon after, the Centers for Disease Control and Prevention and other authorities released guidance on preparing for allergic reactions. “Despite these recommendations, uncertainty remains, particularly among patients with a history of anaphylaxis and/or multiple allergies,” the authors added.
In response to early concerns, the Sheba Medical Center opened a COVID-19 referral center to address safety questions and to conduct assessments of allergy risk for the Pfizer-BioNTech vaccine, the first COVID-19 vaccine approved in Israel. From Dec. 27, 2020, to Feb. 22, 2021, the referral center assessed 8,102 patients with allergies. Those who were not clearly at low risk filled out a questionnaire about prior allergic or anaphylactic reactions to drugs or vaccines, other allergies, and other relevant medical history. Patients were considered to be at high risk for allergic reactions if they met at least one of the following criteria: previous anaphylactic reaction to any drug or vaccine, multiple drug allergies, multiple other allergies, and mast cell disorders. Individuals were also classified as high risk if their health care practitioner deferred vaccination because of allergy concerns.
Nearly 95% of the cohort (7,668 individuals) were classified as low risk and received both Pfizer vaccine doses at standard immunization sites and underwent 30 minutes of observation after immunization. Although the study did not follow these lower-risk patients, “no serious allergic reactions were reported back to our referral center by patients or their general practitioner after immunization in the regular settings,” the authors wrote.
Five patients were considered ineligible for immunization because of known sensitivity to polyethylene glycol or multiple anaphylactic reactions to different injectable drugs, following recommendations from the Ministry of Health of Israel at the time. The remaining 429 individuals were deemed high risk and underwent observation for 2 hours from a dedicated allergy team after immunization. For these high-risk patients, both vaccine doses were administered in the same setting. Patients also reported any adverse reactions in the 21 days between the first and second dose.
Women made up most of the high-risk cohort (70.9%). The average age of participants was 52 years. Of the high-risk individuals, 63.2% reported prior anaphylaxis, 32.9% had multiple drug allergies, and 30.3% had multiple other allergies.
During the first 2 hours following immunization, nine individuals (2.1%), all women, experienced allergic reactions. Six individuals (1.4%) experienced minor reactions, including skin flushing, tongue or uvula swelling, or a cough that resolved with antihistamine treatment during the observation period. Three patients (0.7%) had anaphylactic reactions that occurred 10 to 20 minutes after injection. All three patients experienced significant bronchospasm, skin eruption, itching, and shortness of breath. Two patients experienced angioedema, and one patient had gastrointestinal symptoms. They were treated with adrenaline, antihistamines, and an inhaled bronchodilator. All symptoms resolved within 2-6 hours, and no patient required hospitalization.
In the days following vaccination, patients commonly reported pain at the injection site, fatigue, muscle pain, and headache; 14.7% of patients reported skin eruption, itching, or urticaria.
As of Feb. 22, 2021, 218 patients from this highly allergic cohort received their second dose of the vaccine. Four patients (1.8%) had mild allergic reactions. All four developed flushing, and one patient also developed a cough that resolved with antihistamine treatment. Three of these patients had experienced mild allergic reactions to the first dose and were premedicated for the second dose. One patient only reacted to the second dose.
The findings should be “very reassuring” to individuals hesitant to receive the vaccine, Elizabeth Phillips, MD, the director of the Center for Drug Safety and Immunology at Vanderbilt University Medical Center, Nashville, Tenn., said in an interview. She was not involved with the research and wrote an invited commentary on the study. “The rates of anaphylaxis and allergic reactions are truly quite low,” she said. Although about 2% of the high-risk group developed allergic reactions to immunization, the overall percentage for the entire cohort would be much lower.
The study did not investigate specific risk factors for and mechanisms of allergic reactions to COVID-19 vaccines, Dr. Phillips said, which is a study limitation that the authors also acknowledge. The National Institute for Allergy and Infectious Diseases is currently trying to answer some of these questions with a multisite, randomized, double-blinded study. The study is intended to help understand why people have these allergic reactions, Dr. Phillips added. Vanderbilt is one of the sites for the study.
While researchers continue to hunt for answers, the algorithm developed by the authors provides “a great strategy to get people that are at higher risk vaccinated in a monitored setting,” she said. The results show that “people should not be avoiding vaccination because of a history of anaphylaxis.”
Dr. Phillips has received institutional grants from the National Institutes of Health and the National Health and Medical Research Council; royalties from UpToDate and Lexicomp; and consulting fees from Janssen, Vertex, Biocryst, and Regeneron.
A version of this article first appeared on Medscape.com.
COVID-clogged ICUs ‘terrify’ those with chronic or emergency illness
Jessica Gosnell, MD, 41, from Portland, Oregon, lives daily with the knowledge that her rare disease — a form of hereditary angioedema — could cause a sudden, severe swelling in her throat that could require quick intubation and land her in an intensive care unit (ICU) for days.
“I’ve been hospitalized for throat swells three times in the last year,” she said in an interview.
Dr. Gosnell no longer practices medicine because of a combination of illnesses, but lives with her husband, Andrew, and two young children, and said they are all “terrified” she will have to go to the hospital amid a COVID-19 surge that had shrunk the number of available ICU beds to 152 from 780 in Oregon as of Aug. 30. Thirty percent of the beds are in use for patients with COVID-19.
She said her life depends on being near hospitals that have ICUs and having access to highly specialized medications, one of which can cost up to $50,000 for the rescue dose.
Her fear has her “literally living bedbound.” In addition to hereditary angioedema, she has Ehlers-Danlos syndrome, which weakens connective tissue. She wears a cervical collar 24/7 to keep from tearing tissues, as any tissue injury can trigger a swell.
Patients worry there won’t be room
As ICU beds in most states are filling with COVID-19 patients as the Delta variant spreads, fears are rising among people like Dr. Gosnell, who have chronic conditions and diseases with unpredictable emergency visits, who worry that if they need emergency care there won’t be room.
As of Aug. 30, in the United States, 79% of ICU beds nationally were in use, 30% of them for COVID-19 patients, according to the U.S. Department of Health and Human Services.
In individual states, the picture is dire. Alabama has fewer than 10% of its ICU beds open across the entire state. In Florida, 93% of ICU beds are filled, 53% of them with COVID patients. In Louisiana, 87% of beds were already in use, 45% of them with COVID patients, just as category 4 hurricane Ida smashed into the coastline on Aug. 29.
News reports have told of people transported and airlifted as hospitals reach capacity.
In Bellville, Tex., U.S. Army veteran Daniel Wilkinson needed advanced care for gallstone pancreatitis that normally would take 30 minutes to treat, his Bellville doctor, Hasan Kakli, MD, told CBS News.
Mr. Wilkinson’s house was three doors from Bellville Hospital, but the hospital was not equipped to treat the condition. Calls to other hospitals found the same answer: no empty ICU beds. After a 7-hour wait on a stretcher, he was airlifted to a Veterans Affairs hospital in Houston, but it was too late. He died on August 22 at age 46.
Dr. Kakli said, “I’ve never lost a patient with this diagnosis. Ever. I’m scared that the next patient I see is someone that I can’t get to where they need to get to. We are playing musical chairs with 100 people and 10 chairs. When the music stops, what happens?”
Also in Texas in August, Joe Valdez, who was shot six times as an unlucky bystander in a domestic dispute, waited for more than a week for surgery at Ben Taub Hospital in Houston, which was over capacity with COVID patients, the Washington Post reported.
Others with chronic diseases fear needing emergency services or even entering a hospital for regular care with the COVID surge.
Nicole Seefeldt, 44, from Easton, Penn., who had a double-lung transplant in 2016, said that she hasn’t been able to see her lung transplant specialists in Philadelphia — an hour-and-a-half drive — for almost 2 years because of fear of contracting COVID. Before the pandemic, she made the trip almost weekly.
“I protect my lungs like they’re children,” she said.
She relies on her local hospital for care, but has put off some needed care, such as a colonoscopy, and has relied on telemedicine because she wants to limit her hospital exposure.
Ms. Seefeldt now faces an eventual kidney transplant, as her kidney function has been reduced to 20%. In the meantime, she worries she will need emergency care for either her lungs or kidneys.
“For those of us who are chronically ill or disabled, what if we have an emergency that is not COVID-related? Are we going to be able to get a bed? Are we going to be able to get treatment? It’s not just COVID patients who come to the [emergency room],” she said.
A pandemic problem
Paul E. Casey, MD, MBA, chief medical officer at Rush University Medical Center in Chicago, said that high vaccination rates in Chicago have helped Rush continue to accommodate both non-COVID and COVID patients in the emergency department.
Though the hospital treated a large volume of COVID patients, “The vast majority of people we see and did see through the pandemic were non-COVID patents,” he said.
Dr. Casey said that in the first wave the hospital noticed a concerning drop in patients coming in for strokes and heart attacks — “things we knew hadn’t gone away.”
And the data backs it up. Over the course of the pandemic, the Centers for Disease Control and Prevention’s National Health Interview Survey found that the percentage of Americans who reported seeing a doctor or health professional fell from 85% at the end of 2019 to about 80% in the first three months of 2021. The survey did not differentiate between in-person visits and telehealth appointments.
Medical practices and patients themselves postponed elective procedures and delayed routine visits during the early months of the crisis.
Patients also reported staying away from hospitals’ emergency departments throughout the pandemic. At the end of 2019, 22% of respondents reported visiting an emergency department in the past year. That dropped to 17% by the end of 2020, and was at 17.7% in the first 3 months of 2021.
Dr. Casey said that, in his hospital’s case, clear messaging became very important to assure patients it was safe to come back. And the message is still critical.
“We want to be loud and clear that patients should continue to seek care for those conditions,” Dr. Casey said. “Deferring healthcare only comes with the long-term sequelae of disease left untreated so we want people to be as proactive in seeking care as they always would be.”
In some cases, fears of entering emergency rooms because of excess patients and risk for infection are keeping some patients from seeking necessary care for minor injuries.
Jim Rickert, MD, an orthopedic surgeon with Indiana University Health in Bloomington, said that some of his patients have expressed fears of coming into the hospital for fractures.
Some patients, particularly elderly patients, he said, are having falls and fractures and wearing slings or braces at home rather than going into the hospital for injuries that need immediate attention.
Bones start healing incorrectly, Dr. Rickert said, and the correction becomes much more difficult.
Plea for vaccinations
Dr. Gosnell made a plea posted on her neighborhood news forum for people to get COVID vaccinations.
“It seems to me it’s easy for other people who are not in bodies like mine to take health for granted,” she said. “But there are a lot of us who live in very fragile bodies and our entire life is at the intersection of us and getting healthcare treatment. Small complications to getting treatment can be life altering.”
Dr. Gosnell, Ms. Seefeldt, Dr. Casey, and Dr. Rickert reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Jessica Gosnell, MD, 41, from Portland, Oregon, lives daily with the knowledge that her rare disease — a form of hereditary angioedema — could cause a sudden, severe swelling in her throat that could require quick intubation and land her in an intensive care unit (ICU) for days.
“I’ve been hospitalized for throat swells three times in the last year,” she said in an interview.
Dr. Gosnell no longer practices medicine because of a combination of illnesses, but lives with her husband, Andrew, and two young children, and said they are all “terrified” she will have to go to the hospital amid a COVID-19 surge that had shrunk the number of available ICU beds to 152 from 780 in Oregon as of Aug. 30. Thirty percent of the beds are in use for patients with COVID-19.
She said her life depends on being near hospitals that have ICUs and having access to highly specialized medications, one of which can cost up to $50,000 for the rescue dose.
Her fear has her “literally living bedbound.” In addition to hereditary angioedema, she has Ehlers-Danlos syndrome, which weakens connective tissue. She wears a cervical collar 24/7 to keep from tearing tissues, as any tissue injury can trigger a swell.
Patients worry there won’t be room
As ICU beds in most states are filling with COVID-19 patients as the Delta variant spreads, fears are rising among people like Dr. Gosnell, who have chronic conditions and diseases with unpredictable emergency visits, who worry that if they need emergency care there won’t be room.
As of Aug. 30, in the United States, 79% of ICU beds nationally were in use, 30% of them for COVID-19 patients, according to the U.S. Department of Health and Human Services.
In individual states, the picture is dire. Alabama has fewer than 10% of its ICU beds open across the entire state. In Florida, 93% of ICU beds are filled, 53% of them with COVID patients. In Louisiana, 87% of beds were already in use, 45% of them with COVID patients, just as category 4 hurricane Ida smashed into the coastline on Aug. 29.
News reports have told of people transported and airlifted as hospitals reach capacity.
In Bellville, Tex., U.S. Army veteran Daniel Wilkinson needed advanced care for gallstone pancreatitis that normally would take 30 minutes to treat, his Bellville doctor, Hasan Kakli, MD, told CBS News.
Mr. Wilkinson’s house was three doors from Bellville Hospital, but the hospital was not equipped to treat the condition. Calls to other hospitals found the same answer: no empty ICU beds. After a 7-hour wait on a stretcher, he was airlifted to a Veterans Affairs hospital in Houston, but it was too late. He died on August 22 at age 46.
Dr. Kakli said, “I’ve never lost a patient with this diagnosis. Ever. I’m scared that the next patient I see is someone that I can’t get to where they need to get to. We are playing musical chairs with 100 people and 10 chairs. When the music stops, what happens?”
Also in Texas in August, Joe Valdez, who was shot six times as an unlucky bystander in a domestic dispute, waited for more than a week for surgery at Ben Taub Hospital in Houston, which was over capacity with COVID patients, the Washington Post reported.
Others with chronic diseases fear needing emergency services or even entering a hospital for regular care with the COVID surge.
Nicole Seefeldt, 44, from Easton, Penn., who had a double-lung transplant in 2016, said that she hasn’t been able to see her lung transplant specialists in Philadelphia — an hour-and-a-half drive — for almost 2 years because of fear of contracting COVID. Before the pandemic, she made the trip almost weekly.
“I protect my lungs like they’re children,” she said.
She relies on her local hospital for care, but has put off some needed care, such as a colonoscopy, and has relied on telemedicine because she wants to limit her hospital exposure.
Ms. Seefeldt now faces an eventual kidney transplant, as her kidney function has been reduced to 20%. In the meantime, she worries she will need emergency care for either her lungs or kidneys.
“For those of us who are chronically ill or disabled, what if we have an emergency that is not COVID-related? Are we going to be able to get a bed? Are we going to be able to get treatment? It’s not just COVID patients who come to the [emergency room],” she said.
A pandemic problem
Paul E. Casey, MD, MBA, chief medical officer at Rush University Medical Center in Chicago, said that high vaccination rates in Chicago have helped Rush continue to accommodate both non-COVID and COVID patients in the emergency department.
Though the hospital treated a large volume of COVID patients, “The vast majority of people we see and did see through the pandemic were non-COVID patents,” he said.
Dr. Casey said that in the first wave the hospital noticed a concerning drop in patients coming in for strokes and heart attacks — “things we knew hadn’t gone away.”
And the data backs it up. Over the course of the pandemic, the Centers for Disease Control and Prevention’s National Health Interview Survey found that the percentage of Americans who reported seeing a doctor or health professional fell from 85% at the end of 2019 to about 80% in the first three months of 2021. The survey did not differentiate between in-person visits and telehealth appointments.
Medical practices and patients themselves postponed elective procedures and delayed routine visits during the early months of the crisis.
Patients also reported staying away from hospitals’ emergency departments throughout the pandemic. At the end of 2019, 22% of respondents reported visiting an emergency department in the past year. That dropped to 17% by the end of 2020, and was at 17.7% in the first 3 months of 2021.
Dr. Casey said that, in his hospital’s case, clear messaging became very important to assure patients it was safe to come back. And the message is still critical.
“We want to be loud and clear that patients should continue to seek care for those conditions,” Dr. Casey said. “Deferring healthcare only comes with the long-term sequelae of disease left untreated so we want people to be as proactive in seeking care as they always would be.”
In some cases, fears of entering emergency rooms because of excess patients and risk for infection are keeping some patients from seeking necessary care for minor injuries.
Jim Rickert, MD, an orthopedic surgeon with Indiana University Health in Bloomington, said that some of his patients have expressed fears of coming into the hospital for fractures.
Some patients, particularly elderly patients, he said, are having falls and fractures and wearing slings or braces at home rather than going into the hospital for injuries that need immediate attention.
Bones start healing incorrectly, Dr. Rickert said, and the correction becomes much more difficult.
Plea for vaccinations
Dr. Gosnell made a plea posted on her neighborhood news forum for people to get COVID vaccinations.
“It seems to me it’s easy for other people who are not in bodies like mine to take health for granted,” she said. “But there are a lot of us who live in very fragile bodies and our entire life is at the intersection of us and getting healthcare treatment. Small complications to getting treatment can be life altering.”
Dr. Gosnell, Ms. Seefeldt, Dr. Casey, and Dr. Rickert reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Jessica Gosnell, MD, 41, from Portland, Oregon, lives daily with the knowledge that her rare disease — a form of hereditary angioedema — could cause a sudden, severe swelling in her throat that could require quick intubation and land her in an intensive care unit (ICU) for days.
“I’ve been hospitalized for throat swells three times in the last year,” she said in an interview.
Dr. Gosnell no longer practices medicine because of a combination of illnesses, but lives with her husband, Andrew, and two young children, and said they are all “terrified” she will have to go to the hospital amid a COVID-19 surge that had shrunk the number of available ICU beds to 152 from 780 in Oregon as of Aug. 30. Thirty percent of the beds are in use for patients with COVID-19.
She said her life depends on being near hospitals that have ICUs and having access to highly specialized medications, one of which can cost up to $50,000 for the rescue dose.
Her fear has her “literally living bedbound.” In addition to hereditary angioedema, she has Ehlers-Danlos syndrome, which weakens connective tissue. She wears a cervical collar 24/7 to keep from tearing tissues, as any tissue injury can trigger a swell.
Patients worry there won’t be room
As ICU beds in most states are filling with COVID-19 patients as the Delta variant spreads, fears are rising among people like Dr. Gosnell, who have chronic conditions and diseases with unpredictable emergency visits, who worry that if they need emergency care there won’t be room.
As of Aug. 30, in the United States, 79% of ICU beds nationally were in use, 30% of them for COVID-19 patients, according to the U.S. Department of Health and Human Services.
In individual states, the picture is dire. Alabama has fewer than 10% of its ICU beds open across the entire state. In Florida, 93% of ICU beds are filled, 53% of them with COVID patients. In Louisiana, 87% of beds were already in use, 45% of them with COVID patients, just as category 4 hurricane Ida smashed into the coastline on Aug. 29.
News reports have told of people transported and airlifted as hospitals reach capacity.
In Bellville, Tex., U.S. Army veteran Daniel Wilkinson needed advanced care for gallstone pancreatitis that normally would take 30 minutes to treat, his Bellville doctor, Hasan Kakli, MD, told CBS News.
Mr. Wilkinson’s house was three doors from Bellville Hospital, but the hospital was not equipped to treat the condition. Calls to other hospitals found the same answer: no empty ICU beds. After a 7-hour wait on a stretcher, he was airlifted to a Veterans Affairs hospital in Houston, but it was too late. He died on August 22 at age 46.
Dr. Kakli said, “I’ve never lost a patient with this diagnosis. Ever. I’m scared that the next patient I see is someone that I can’t get to where they need to get to. We are playing musical chairs with 100 people and 10 chairs. When the music stops, what happens?”
Also in Texas in August, Joe Valdez, who was shot six times as an unlucky bystander in a domestic dispute, waited for more than a week for surgery at Ben Taub Hospital in Houston, which was over capacity with COVID patients, the Washington Post reported.
Others with chronic diseases fear needing emergency services or even entering a hospital for regular care with the COVID surge.
Nicole Seefeldt, 44, from Easton, Penn., who had a double-lung transplant in 2016, said that she hasn’t been able to see her lung transplant specialists in Philadelphia — an hour-and-a-half drive — for almost 2 years because of fear of contracting COVID. Before the pandemic, she made the trip almost weekly.
“I protect my lungs like they’re children,” she said.
She relies on her local hospital for care, but has put off some needed care, such as a colonoscopy, and has relied on telemedicine because she wants to limit her hospital exposure.
Ms. Seefeldt now faces an eventual kidney transplant, as her kidney function has been reduced to 20%. In the meantime, she worries she will need emergency care for either her lungs or kidneys.
“For those of us who are chronically ill or disabled, what if we have an emergency that is not COVID-related? Are we going to be able to get a bed? Are we going to be able to get treatment? It’s not just COVID patients who come to the [emergency room],” she said.
A pandemic problem
Paul E. Casey, MD, MBA, chief medical officer at Rush University Medical Center in Chicago, said that high vaccination rates in Chicago have helped Rush continue to accommodate both non-COVID and COVID patients in the emergency department.
Though the hospital treated a large volume of COVID patients, “The vast majority of people we see and did see through the pandemic were non-COVID patents,” he said.
Dr. Casey said that in the first wave the hospital noticed a concerning drop in patients coming in for strokes and heart attacks — “things we knew hadn’t gone away.”
And the data backs it up. Over the course of the pandemic, the Centers for Disease Control and Prevention’s National Health Interview Survey found that the percentage of Americans who reported seeing a doctor or health professional fell from 85% at the end of 2019 to about 80% in the first three months of 2021. The survey did not differentiate between in-person visits and telehealth appointments.
Medical practices and patients themselves postponed elective procedures and delayed routine visits during the early months of the crisis.
Patients also reported staying away from hospitals’ emergency departments throughout the pandemic. At the end of 2019, 22% of respondents reported visiting an emergency department in the past year. That dropped to 17% by the end of 2020, and was at 17.7% in the first 3 months of 2021.
Dr. Casey said that, in his hospital’s case, clear messaging became very important to assure patients it was safe to come back. And the message is still critical.
“We want to be loud and clear that patients should continue to seek care for those conditions,” Dr. Casey said. “Deferring healthcare only comes with the long-term sequelae of disease left untreated so we want people to be as proactive in seeking care as they always would be.”
In some cases, fears of entering emergency rooms because of excess patients and risk for infection are keeping some patients from seeking necessary care for minor injuries.
Jim Rickert, MD, an orthopedic surgeon with Indiana University Health in Bloomington, said that some of his patients have expressed fears of coming into the hospital for fractures.
Some patients, particularly elderly patients, he said, are having falls and fractures and wearing slings or braces at home rather than going into the hospital for injuries that need immediate attention.
Bones start healing incorrectly, Dr. Rickert said, and the correction becomes much more difficult.
Plea for vaccinations
Dr. Gosnell made a plea posted on her neighborhood news forum for people to get COVID vaccinations.
“It seems to me it’s easy for other people who are not in bodies like mine to take health for granted,” she said. “But there are a lot of us who live in very fragile bodies and our entire life is at the intersection of us and getting healthcare treatment. Small complications to getting treatment can be life altering.”
Dr. Gosnell, Ms. Seefeldt, Dr. Casey, and Dr. Rickert reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A Severe Presentation of Plasma Cell Cheilitis
Plasma cell cheilitis (PCC), also known as plasmocytosis circumorificialis and plasmocytosis mucosae,1 is a poorly understood, uncommon inflammatory condition characterized by dense infiltration of mature plasma cells in the mucosal dermis of the lip.2-5 The etiology of PCC is unknown but is thought to be a reactive immune process triggered by infection, mechanical friction, trauma, or solar damage.1,5,6
The most common presentation of PCC is a slowly evolving, red-brown patch or plaque on the lower lip in older individuals.2,3,5,7 Secondary changes with disease progression can include erosion, ulceration, fissures, edema, bleeding, or crusting.5 The diagnosis of PCC is challenging because it can mimic neoplastic, infectious, and inflammatory conditions.8,9
Treatment strategies for PCC described in the literature vary, as does therapeutic response. Resolution of PCC has been documented after systemic steroids, intralesional steroids, systemic griseofulvin, and topical calcineurin inhibitors, among other agents.6,7,10-16
We present the case of a patient with a lip lesion who ultimately was diagnosed with PCC after it progressed to an advanced necrotic stage.
Case Report
An 80-year-old male veteran of the Armed Services initially presented to our institution via teledermatology with redness and crusting of the lower lip (Figure 1). He had a history of myelodysplastic syndrome and anemia requiring iron transfusion. The process appeared to be consistent with actinic cheilitis vs squamous cell carcinoma. In-person dermatology consultation was recommended; however, the patient did not follow through with that appointment.
Five months later, additional photographs of the lesion were taken by the patient's primary care physician and sent through teledermatology, revealing progression to an erythematous, yellow-crusted erosion (Figure 2). The medical record indicated that a punch biopsy performed by the patient’s primary care physician showed hyperkeratosis and fungal organisms on periodic acid–Schiff staining. He subsequently applied ketoconazole and terbinafine cream to the lower lip without improvement. Prompt in-person evaluation by dermatology was again recommended.
Ten days later, the patient was seen in our dermatology clinic, at which point his condition had rapidly progressed. The lower lip displayed a 3.0×2.5-cm, yellow and black, crusted, ulcerated plaque (Figure 3). He reported severe burning and pain of the lip as well as spontaneous bleeding. He had lost approximately 10 pounds over the last month due to poor oral intake. A second punch biopsy showed benign mucosa with extensive ulceration and formation of full-thickness granulation tissue. No fungi or bacteria were identified.
Consultation and Histologic Analysis
Dermatopathology was consulted and recommended a third punch biopsy for additional testing. A repeat biopsy demonstrated ulceration with lateral elements of retained epidermis and a dense submucosal chronic inflammatory infiltrate comprising plasma cells and lymphocytes (Figures 4 and 5). Immunohistochemical staining demonstrated a mixed inflammatory infiltrate with CD3+ T cells and CD20+ B cells. In situ hybridization studies demonstrated numerous lambda-positive and kappa-positive plasma cells without chain restriction. Periodic acid–Schiff with diastase and Grocott-Gomori methenamine-silver staining demonstrated no fungi. Findings were interpreted to be most consistent with a diagnosis of PCC.
Treatment and Follow-up
The patient was treated with clobetasol ointment 0.05% twice daily for 6 weeks and topical lidocaine as needed for pain. At 6-week follow-up, he displayed substantial improvement, with normal-appearing lips and complete resolution of symptoms.
Comment
The diagnosis and management of PCC is difficult because the condition is uncommon (though its true incidence is unknown) and the presentation is nonspecific, invoking a wide differential diagnosis. In the literature, PCC presents as a slowly progressive, red-brown patch or plaque on the lower lip in older individuals.2,3,5,7 The lesion can progress to become eroded, ulcerated, fissured, or edematous.5
Differential Diagnosis
The clinical differential diagnosis of PCC is broad and includes inflammatory, infectious, and neoplastic causes, such as actinic cheilitis, allergic contact cheilitis, exfoliative cheilitis, granulomatous cheilitis, lichen planus, candidiasis, syphilis, and squamous cell carcinoma of the lip.7,9 The histologic differential diagnosis includes allergic contact cheilitis, secondary syphilis, actinic cheilitis, squamous cell carcinoma, cheilitis granulomatosa, and plasmacytoma.17-19
Histopathology
On biopsy, PCC usually is characterized by plasma cells in a bandlike pattern in the upper submucosa or even more diffusely throughout the submucosa.20 In earlier studies, polyclonality of plasma cells with kappa and lambda light chains has been demonstrated5; in this case, such polyclonality militated against a plasma cell dyscrasia. There have been reports of a various number of eosinophils in PCC,5,20 but eosinophils were not a prominent feature in our case.
Treatment
As reported in the literature, treatment of PCC has been attempted using a broad range of strategies; however, the optimal regimen has yet to be elucidated.15 Numerous therapies, including excision, radiation, electrocauterization, cryotherapy, steroids, systemic griseofulvin, topical fusidic acid, and topical calcineurin inhibitors, have yielded variable success.6,7,10-16
The success of topical corticosteroids, as demonstrated in our case, has been unpredictable; the reported response has ranged from complete resolution to failure.9 This variability is thought to be related to epithelial width and the degree of acanthosis, with ulcerative lesions demonstrating a superior response to topical corticosteroids.9
Conclusion
Our case highlights the challenges of diagnosing and managing PCC, especially through teledermatology. Initial photographs of the lesion (Figure 1) that were submitted demonstrated a nonspecific erosion, which was concerning for any of several infectious, inflammatory, and malignant causes. Prompt in-person evaluation was warranted; regrettably, the patient’s condition worsened rapidly in the 10 days it took for him to be seen in-person by dermatology.
Furthermore, this case necessitated 3 separate biopsies because the pathology on the first 2 biopsies initially was equivocal, demonstrating ulceration and granulation tissue. The diagnosis was finally made after a third biopsy was recommended by a dermatopathologist, who eventually identified a bandlike distribution of polyclonal plasma cells in the upper submucosa, consistent with a diagnosis of PCC. Our patient’s final disease presentation (Figure 3) was exuberant and may represent the end point of untreated PCC.
- Senol M, Ozcan A, Aydin NE, et al. Intertriginous plasmacytosis with plasmoacanthoma: report of a typical case and review of the literature. Int J Dermatol. 2008;47:265-268. doi:10.1111/j.1365-4632.2008.03385.x
- Rocha N, Mota F, Horta M, et al. Plasma cell cheilitis. J Eur Acad Dermatol Venereol. 2004;18:96-98. doi:10.1111/j.1468-3083.2004.00791.x
- Farrier JN, Perkins CS. Plasma cell cheilitis. Br J Oral Maxillofac Surg. 2008;46:679-680. doi:10.1016/j.bjoms.2008.03.009
- Baughman RD, Berger P, Pringle WM. Plasma cell cheilitis. Arch Dermatol. 1974;110:725-726.
- Lee JY, Kim KH, Hahm JE, et al. Plasma cell cheilitis: a clinicopathological and immunohistochemical study of 13 cases. Ann Dermatol. 2017;29:536-542. doi:10.5021/ad.2017.29.5.536
- da Cunha Filho RR, Tochetto LB, Tochetto BB, et al. “Angular” plasma cell cheilitis. Dermatol Online J. 2014;20:doj_21759.
- Yang JH, Lee UH, Jang SJ, et al. Plasma cell cheilitis treated with intralesional injection of corticosteroids. J Dermatol. 2005;32:987-990. doi:10.1111/j.1346-8138.2005.tb00887.x
- Solomon LW, Wein RO, Rosenwald I, et al. Plasma cell mucositis of the oral cavity: report of a case and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;106:853-860. doi:10.1016/j.tripleo.2008.08.016
- Dos Santos HT, Cunha JLS, Santana LAM, et al. Plasma cell cheilitis: the diagnosis of a disorder mimicking lip cancer. Autops Case Rep. 2019;9:e2018075. doi:10.4322/acr.2018.075
- Fujimura T, Furudate S, Ishibashi M, et al. Successful treatment of plasmacytosis circumorificialis with topical tacrolimus: two case reports and an immunohistochemical study. Case Rep Dermatol. 2013;5:79-83. doi:10.1159/000350184
- Tamaki K, Osada A, Tsukamoto K, et al. Treatment of plasma cell cheilitis with griseofulvin. J Am Acad Dermatol. 1994;30:789-790. doi:10.1016/s0190-9622(08)81515-0
- Choi JW, Choi M, Cho KH. Successful treatment of plasma cell cheilitis with topical calcineurin inhibitors. J Dermatol. 2009;36:669-671. doi:10.1111/j.1346-8138.2009.00733.x
- Hanami Y, Motoki Y, Yamamoto T. Successful treatment of plasma cell cheilitis with topical tacrolimus: report of two cases. Dermatol Online J. 2011;17:6.
- Jin SP, Cho KH, Huh CH. Plasma cell cheilitis, successfully treated with topical 0.03% tacrolimus ointment. J Dermatolog Treat. 2010;21:130-132. doi:10.1080/09546630903200620
- Tseng JT-P, Cheng C-J, Lee W-R, et al. Plasma-cell cheilitis: successful treatment with intralesional injections of corticosteroids. Clin Exp Dermatol. 2009;34:174-177. doi:10.1111/j.1365-2230.2008.02765.x
- Yoshimura K, Nakano S, Tsuruta D, et al. Successful treatment with 308-nm monochromatic excimer light and subsequent tacrolimus 0.03% ointment in refractory plasma cell cheilitis. J Dermatol. 2013;40:471-474. doi:10.1111/1346-8138.12152
- Fujimura Y, Natsuga K, Abe R, et al. Plasma cell cheilitis extending beyond vermillion border. J Dermatol. 2015;42:935-936. doi:10.1111/1346-8138.12985
- White JW Jr, Olsen KD, Banks PM. Plasma cell orificial mucositis. report of a case and review of the literature. Arch Dermatol. 1986;122:1321-1324. doi:10.1001/archderm.122.11.1321
- Román CC, Yuste CM, Gonzalez MA, et al. Plasma cell gingivitis. Cutis. 2002;69:41-45.
- Choe HC, Park HJ, Oh ST, et al. Clinicopathologic study of 8 patients with plasma cell cheilitis. Korean J Dermatol. 2003;41:174-178.
Plasma cell cheilitis (PCC), also known as plasmocytosis circumorificialis and plasmocytosis mucosae,1 is a poorly understood, uncommon inflammatory condition characterized by dense infiltration of mature plasma cells in the mucosal dermis of the lip.2-5 The etiology of PCC is unknown but is thought to be a reactive immune process triggered by infection, mechanical friction, trauma, or solar damage.1,5,6
The most common presentation of PCC is a slowly evolving, red-brown patch or plaque on the lower lip in older individuals.2,3,5,7 Secondary changes with disease progression can include erosion, ulceration, fissures, edema, bleeding, or crusting.5 The diagnosis of PCC is challenging because it can mimic neoplastic, infectious, and inflammatory conditions.8,9
Treatment strategies for PCC described in the literature vary, as does therapeutic response. Resolution of PCC has been documented after systemic steroids, intralesional steroids, systemic griseofulvin, and topical calcineurin inhibitors, among other agents.6,7,10-16
We present the case of a patient with a lip lesion who ultimately was diagnosed with PCC after it progressed to an advanced necrotic stage.
Case Report
An 80-year-old male veteran of the Armed Services initially presented to our institution via teledermatology with redness and crusting of the lower lip (Figure 1). He had a history of myelodysplastic syndrome and anemia requiring iron transfusion. The process appeared to be consistent with actinic cheilitis vs squamous cell carcinoma. In-person dermatology consultation was recommended; however, the patient did not follow through with that appointment.
Five months later, additional photographs of the lesion were taken by the patient's primary care physician and sent through teledermatology, revealing progression to an erythematous, yellow-crusted erosion (Figure 2). The medical record indicated that a punch biopsy performed by the patient’s primary care physician showed hyperkeratosis and fungal organisms on periodic acid–Schiff staining. He subsequently applied ketoconazole and terbinafine cream to the lower lip without improvement. Prompt in-person evaluation by dermatology was again recommended.
Ten days later, the patient was seen in our dermatology clinic, at which point his condition had rapidly progressed. The lower lip displayed a 3.0×2.5-cm, yellow and black, crusted, ulcerated plaque (Figure 3). He reported severe burning and pain of the lip as well as spontaneous bleeding. He had lost approximately 10 pounds over the last month due to poor oral intake. A second punch biopsy showed benign mucosa with extensive ulceration and formation of full-thickness granulation tissue. No fungi or bacteria were identified.
Consultation and Histologic Analysis
Dermatopathology was consulted and recommended a third punch biopsy for additional testing. A repeat biopsy demonstrated ulceration with lateral elements of retained epidermis and a dense submucosal chronic inflammatory infiltrate comprising plasma cells and lymphocytes (Figures 4 and 5). Immunohistochemical staining demonstrated a mixed inflammatory infiltrate with CD3+ T cells and CD20+ B cells. In situ hybridization studies demonstrated numerous lambda-positive and kappa-positive plasma cells without chain restriction. Periodic acid–Schiff with diastase and Grocott-Gomori methenamine-silver staining demonstrated no fungi. Findings were interpreted to be most consistent with a diagnosis of PCC.
Treatment and Follow-up
The patient was treated with clobetasol ointment 0.05% twice daily for 6 weeks and topical lidocaine as needed for pain. At 6-week follow-up, he displayed substantial improvement, with normal-appearing lips and complete resolution of symptoms.
Comment
The diagnosis and management of PCC is difficult because the condition is uncommon (though its true incidence is unknown) and the presentation is nonspecific, invoking a wide differential diagnosis. In the literature, PCC presents as a slowly progressive, red-brown patch or plaque on the lower lip in older individuals.2,3,5,7 The lesion can progress to become eroded, ulcerated, fissured, or edematous.5
Differential Diagnosis
The clinical differential diagnosis of PCC is broad and includes inflammatory, infectious, and neoplastic causes, such as actinic cheilitis, allergic contact cheilitis, exfoliative cheilitis, granulomatous cheilitis, lichen planus, candidiasis, syphilis, and squamous cell carcinoma of the lip.7,9 The histologic differential diagnosis includes allergic contact cheilitis, secondary syphilis, actinic cheilitis, squamous cell carcinoma, cheilitis granulomatosa, and plasmacytoma.17-19
Histopathology
On biopsy, PCC usually is characterized by plasma cells in a bandlike pattern in the upper submucosa or even more diffusely throughout the submucosa.20 In earlier studies, polyclonality of plasma cells with kappa and lambda light chains has been demonstrated5; in this case, such polyclonality militated against a plasma cell dyscrasia. There have been reports of a various number of eosinophils in PCC,5,20 but eosinophils were not a prominent feature in our case.
Treatment
As reported in the literature, treatment of PCC has been attempted using a broad range of strategies; however, the optimal regimen has yet to be elucidated.15 Numerous therapies, including excision, radiation, electrocauterization, cryotherapy, steroids, systemic griseofulvin, topical fusidic acid, and topical calcineurin inhibitors, have yielded variable success.6,7,10-16
The success of topical corticosteroids, as demonstrated in our case, has been unpredictable; the reported response has ranged from complete resolution to failure.9 This variability is thought to be related to epithelial width and the degree of acanthosis, with ulcerative lesions demonstrating a superior response to topical corticosteroids.9
Conclusion
Our case highlights the challenges of diagnosing and managing PCC, especially through teledermatology. Initial photographs of the lesion (Figure 1) that were submitted demonstrated a nonspecific erosion, which was concerning for any of several infectious, inflammatory, and malignant causes. Prompt in-person evaluation was warranted; regrettably, the patient’s condition worsened rapidly in the 10 days it took for him to be seen in-person by dermatology.
Furthermore, this case necessitated 3 separate biopsies because the pathology on the first 2 biopsies initially was equivocal, demonstrating ulceration and granulation tissue. The diagnosis was finally made after a third biopsy was recommended by a dermatopathologist, who eventually identified a bandlike distribution of polyclonal plasma cells in the upper submucosa, consistent with a diagnosis of PCC. Our patient’s final disease presentation (Figure 3) was exuberant and may represent the end point of untreated PCC.
Plasma cell cheilitis (PCC), also known as plasmocytosis circumorificialis and plasmocytosis mucosae,1 is a poorly understood, uncommon inflammatory condition characterized by dense infiltration of mature plasma cells in the mucosal dermis of the lip.2-5 The etiology of PCC is unknown but is thought to be a reactive immune process triggered by infection, mechanical friction, trauma, or solar damage.1,5,6
The most common presentation of PCC is a slowly evolving, red-brown patch or plaque on the lower lip in older individuals.2,3,5,7 Secondary changes with disease progression can include erosion, ulceration, fissures, edema, bleeding, or crusting.5 The diagnosis of PCC is challenging because it can mimic neoplastic, infectious, and inflammatory conditions.8,9
Treatment strategies for PCC described in the literature vary, as does therapeutic response. Resolution of PCC has been documented after systemic steroids, intralesional steroids, systemic griseofulvin, and topical calcineurin inhibitors, among other agents.6,7,10-16
We present the case of a patient with a lip lesion who ultimately was diagnosed with PCC after it progressed to an advanced necrotic stage.
Case Report
An 80-year-old male veteran of the Armed Services initially presented to our institution via teledermatology with redness and crusting of the lower lip (Figure 1). He had a history of myelodysplastic syndrome and anemia requiring iron transfusion. The process appeared to be consistent with actinic cheilitis vs squamous cell carcinoma. In-person dermatology consultation was recommended; however, the patient did not follow through with that appointment.
Five months later, additional photographs of the lesion were taken by the patient's primary care physician and sent through teledermatology, revealing progression to an erythematous, yellow-crusted erosion (Figure 2). The medical record indicated that a punch biopsy performed by the patient’s primary care physician showed hyperkeratosis and fungal organisms on periodic acid–Schiff staining. He subsequently applied ketoconazole and terbinafine cream to the lower lip without improvement. Prompt in-person evaluation by dermatology was again recommended.
Ten days later, the patient was seen in our dermatology clinic, at which point his condition had rapidly progressed. The lower lip displayed a 3.0×2.5-cm, yellow and black, crusted, ulcerated plaque (Figure 3). He reported severe burning and pain of the lip as well as spontaneous bleeding. He had lost approximately 10 pounds over the last month due to poor oral intake. A second punch biopsy showed benign mucosa with extensive ulceration and formation of full-thickness granulation tissue. No fungi or bacteria were identified.
Consultation and Histologic Analysis
Dermatopathology was consulted and recommended a third punch biopsy for additional testing. A repeat biopsy demonstrated ulceration with lateral elements of retained epidermis and a dense submucosal chronic inflammatory infiltrate comprising plasma cells and lymphocytes (Figures 4 and 5). Immunohistochemical staining demonstrated a mixed inflammatory infiltrate with CD3+ T cells and CD20+ B cells. In situ hybridization studies demonstrated numerous lambda-positive and kappa-positive plasma cells without chain restriction. Periodic acid–Schiff with diastase and Grocott-Gomori methenamine-silver staining demonstrated no fungi. Findings were interpreted to be most consistent with a diagnosis of PCC.
Treatment and Follow-up
The patient was treated with clobetasol ointment 0.05% twice daily for 6 weeks and topical lidocaine as needed for pain. At 6-week follow-up, he displayed substantial improvement, with normal-appearing lips and complete resolution of symptoms.
Comment
The diagnosis and management of PCC is difficult because the condition is uncommon (though its true incidence is unknown) and the presentation is nonspecific, invoking a wide differential diagnosis. In the literature, PCC presents as a slowly progressive, red-brown patch or plaque on the lower lip in older individuals.2,3,5,7 The lesion can progress to become eroded, ulcerated, fissured, or edematous.5
Differential Diagnosis
The clinical differential diagnosis of PCC is broad and includes inflammatory, infectious, and neoplastic causes, such as actinic cheilitis, allergic contact cheilitis, exfoliative cheilitis, granulomatous cheilitis, lichen planus, candidiasis, syphilis, and squamous cell carcinoma of the lip.7,9 The histologic differential diagnosis includes allergic contact cheilitis, secondary syphilis, actinic cheilitis, squamous cell carcinoma, cheilitis granulomatosa, and plasmacytoma.17-19
Histopathology
On biopsy, PCC usually is characterized by plasma cells in a bandlike pattern in the upper submucosa or even more diffusely throughout the submucosa.20 In earlier studies, polyclonality of plasma cells with kappa and lambda light chains has been demonstrated5; in this case, such polyclonality militated against a plasma cell dyscrasia. There have been reports of a various number of eosinophils in PCC,5,20 but eosinophils were not a prominent feature in our case.
Treatment
As reported in the literature, treatment of PCC has been attempted using a broad range of strategies; however, the optimal regimen has yet to be elucidated.15 Numerous therapies, including excision, radiation, electrocauterization, cryotherapy, steroids, systemic griseofulvin, topical fusidic acid, and topical calcineurin inhibitors, have yielded variable success.6,7,10-16
The success of topical corticosteroids, as demonstrated in our case, has been unpredictable; the reported response has ranged from complete resolution to failure.9 This variability is thought to be related to epithelial width and the degree of acanthosis, with ulcerative lesions demonstrating a superior response to topical corticosteroids.9
Conclusion
Our case highlights the challenges of diagnosing and managing PCC, especially through teledermatology. Initial photographs of the lesion (Figure 1) that were submitted demonstrated a nonspecific erosion, which was concerning for any of several infectious, inflammatory, and malignant causes. Prompt in-person evaluation was warranted; regrettably, the patient’s condition worsened rapidly in the 10 days it took for him to be seen in-person by dermatology.
Furthermore, this case necessitated 3 separate biopsies because the pathology on the first 2 biopsies initially was equivocal, demonstrating ulceration and granulation tissue. The diagnosis was finally made after a third biopsy was recommended by a dermatopathologist, who eventually identified a bandlike distribution of polyclonal plasma cells in the upper submucosa, consistent with a diagnosis of PCC. Our patient’s final disease presentation (Figure 3) was exuberant and may represent the end point of untreated PCC.
- Senol M, Ozcan A, Aydin NE, et al. Intertriginous plasmacytosis with plasmoacanthoma: report of a typical case and review of the literature. Int J Dermatol. 2008;47:265-268. doi:10.1111/j.1365-4632.2008.03385.x
- Rocha N, Mota F, Horta M, et al. Plasma cell cheilitis. J Eur Acad Dermatol Venereol. 2004;18:96-98. doi:10.1111/j.1468-3083.2004.00791.x
- Farrier JN, Perkins CS. Plasma cell cheilitis. Br J Oral Maxillofac Surg. 2008;46:679-680. doi:10.1016/j.bjoms.2008.03.009
- Baughman RD, Berger P, Pringle WM. Plasma cell cheilitis. Arch Dermatol. 1974;110:725-726.
- Lee JY, Kim KH, Hahm JE, et al. Plasma cell cheilitis: a clinicopathological and immunohistochemical study of 13 cases. Ann Dermatol. 2017;29:536-542. doi:10.5021/ad.2017.29.5.536
- da Cunha Filho RR, Tochetto LB, Tochetto BB, et al. “Angular” plasma cell cheilitis. Dermatol Online J. 2014;20:doj_21759.
- Yang JH, Lee UH, Jang SJ, et al. Plasma cell cheilitis treated with intralesional injection of corticosteroids. J Dermatol. 2005;32:987-990. doi:10.1111/j.1346-8138.2005.tb00887.x
- Solomon LW, Wein RO, Rosenwald I, et al. Plasma cell mucositis of the oral cavity: report of a case and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;106:853-860. doi:10.1016/j.tripleo.2008.08.016
- Dos Santos HT, Cunha JLS, Santana LAM, et al. Plasma cell cheilitis: the diagnosis of a disorder mimicking lip cancer. Autops Case Rep. 2019;9:e2018075. doi:10.4322/acr.2018.075
- Fujimura T, Furudate S, Ishibashi M, et al. Successful treatment of plasmacytosis circumorificialis with topical tacrolimus: two case reports and an immunohistochemical study. Case Rep Dermatol. 2013;5:79-83. doi:10.1159/000350184
- Tamaki K, Osada A, Tsukamoto K, et al. Treatment of plasma cell cheilitis with griseofulvin. J Am Acad Dermatol. 1994;30:789-790. doi:10.1016/s0190-9622(08)81515-0
- Choi JW, Choi M, Cho KH. Successful treatment of plasma cell cheilitis with topical calcineurin inhibitors. J Dermatol. 2009;36:669-671. doi:10.1111/j.1346-8138.2009.00733.x
- Hanami Y, Motoki Y, Yamamoto T. Successful treatment of plasma cell cheilitis with topical tacrolimus: report of two cases. Dermatol Online J. 2011;17:6.
- Jin SP, Cho KH, Huh CH. Plasma cell cheilitis, successfully treated with topical 0.03% tacrolimus ointment. J Dermatolog Treat. 2010;21:130-132. doi:10.1080/09546630903200620
- Tseng JT-P, Cheng C-J, Lee W-R, et al. Plasma-cell cheilitis: successful treatment with intralesional injections of corticosteroids. Clin Exp Dermatol. 2009;34:174-177. doi:10.1111/j.1365-2230.2008.02765.x
- Yoshimura K, Nakano S, Tsuruta D, et al. Successful treatment with 308-nm monochromatic excimer light and subsequent tacrolimus 0.03% ointment in refractory plasma cell cheilitis. J Dermatol. 2013;40:471-474. doi:10.1111/1346-8138.12152
- Fujimura Y, Natsuga K, Abe R, et al. Plasma cell cheilitis extending beyond vermillion border. J Dermatol. 2015;42:935-936. doi:10.1111/1346-8138.12985
- White JW Jr, Olsen KD, Banks PM. Plasma cell orificial mucositis. report of a case and review of the literature. Arch Dermatol. 1986;122:1321-1324. doi:10.1001/archderm.122.11.1321
- Román CC, Yuste CM, Gonzalez MA, et al. Plasma cell gingivitis. Cutis. 2002;69:41-45.
- Choe HC, Park HJ, Oh ST, et al. Clinicopathologic study of 8 patients with plasma cell cheilitis. Korean J Dermatol. 2003;41:174-178.
- Senol M, Ozcan A, Aydin NE, et al. Intertriginous plasmacytosis with plasmoacanthoma: report of a typical case and review of the literature. Int J Dermatol. 2008;47:265-268. doi:10.1111/j.1365-4632.2008.03385.x
- Rocha N, Mota F, Horta M, et al. Plasma cell cheilitis. J Eur Acad Dermatol Venereol. 2004;18:96-98. doi:10.1111/j.1468-3083.2004.00791.x
- Farrier JN, Perkins CS. Plasma cell cheilitis. Br J Oral Maxillofac Surg. 2008;46:679-680. doi:10.1016/j.bjoms.2008.03.009
- Baughman RD, Berger P, Pringle WM. Plasma cell cheilitis. Arch Dermatol. 1974;110:725-726.
- Lee JY, Kim KH, Hahm JE, et al. Plasma cell cheilitis: a clinicopathological and immunohistochemical study of 13 cases. Ann Dermatol. 2017;29:536-542. doi:10.5021/ad.2017.29.5.536
- da Cunha Filho RR, Tochetto LB, Tochetto BB, et al. “Angular” plasma cell cheilitis. Dermatol Online J. 2014;20:doj_21759.
- Yang JH, Lee UH, Jang SJ, et al. Plasma cell cheilitis treated with intralesional injection of corticosteroids. J Dermatol. 2005;32:987-990. doi:10.1111/j.1346-8138.2005.tb00887.x
- Solomon LW, Wein RO, Rosenwald I, et al. Plasma cell mucositis of the oral cavity: report of a case and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;106:853-860. doi:10.1016/j.tripleo.2008.08.016
- Dos Santos HT, Cunha JLS, Santana LAM, et al. Plasma cell cheilitis: the diagnosis of a disorder mimicking lip cancer. Autops Case Rep. 2019;9:e2018075. doi:10.4322/acr.2018.075
- Fujimura T, Furudate S, Ishibashi M, et al. Successful treatment of plasmacytosis circumorificialis with topical tacrolimus: two case reports and an immunohistochemical study. Case Rep Dermatol. 2013;5:79-83. doi:10.1159/000350184
- Tamaki K, Osada A, Tsukamoto K, et al. Treatment of plasma cell cheilitis with griseofulvin. J Am Acad Dermatol. 1994;30:789-790. doi:10.1016/s0190-9622(08)81515-0
- Choi JW, Choi M, Cho KH. Successful treatment of plasma cell cheilitis with topical calcineurin inhibitors. J Dermatol. 2009;36:669-671. doi:10.1111/j.1346-8138.2009.00733.x
- Hanami Y, Motoki Y, Yamamoto T. Successful treatment of plasma cell cheilitis with topical tacrolimus: report of two cases. Dermatol Online J. 2011;17:6.
- Jin SP, Cho KH, Huh CH. Plasma cell cheilitis, successfully treated with topical 0.03% tacrolimus ointment. J Dermatolog Treat. 2010;21:130-132. doi:10.1080/09546630903200620
- Tseng JT-P, Cheng C-J, Lee W-R, et al. Plasma-cell cheilitis: successful treatment with intralesional injections of corticosteroids. Clin Exp Dermatol. 2009;34:174-177. doi:10.1111/j.1365-2230.2008.02765.x
- Yoshimura K, Nakano S, Tsuruta D, et al. Successful treatment with 308-nm monochromatic excimer light and subsequent tacrolimus 0.03% ointment in refractory plasma cell cheilitis. J Dermatol. 2013;40:471-474. doi:10.1111/1346-8138.12152
- Fujimura Y, Natsuga K, Abe R, et al. Plasma cell cheilitis extending beyond vermillion border. J Dermatol. 2015;42:935-936. doi:10.1111/1346-8138.12985
- White JW Jr, Olsen KD, Banks PM. Plasma cell orificial mucositis. report of a case and review of the literature. Arch Dermatol. 1986;122:1321-1324. doi:10.1001/archderm.122.11.1321
- Román CC, Yuste CM, Gonzalez MA, et al. Plasma cell gingivitis. Cutis. 2002;69:41-45.
- Choe HC, Park HJ, Oh ST, et al. Clinicopathologic study of 8 patients with plasma cell cheilitis. Korean J Dermatol. 2003;41:174-178.
PRACTICE POINTS
- Plasma cell cheilitis (PCC) is a benign condition that affects the lower lip in older individuals, presenting as a nonspecific, red-brown patch or plaque that can progress slowly to erosions and edema.
- Our patient with PCC experienced full resolution of symptoms with application of a class I topical corticosteroid.
Verruca Vulgaris Arising Within the Red Portion of a Multicolored Tattoo
To the Editor:
The art of tattooing continues to gain popularity in the 21st century, albeit with accompanying hazards.1 Reported adverse reactions to tattoos include infections, tumors, and hypersensitivity and granulomatous reactions.2 Various infectious agents may involve tattoos, including human papillomavirus (HPV), molluscum contagiosum, herpes simplex virus, hepatitis C virus, tuberculoid and nontuberculoid mycobacteria, and Staphylococcus aureus.2 Verruca vulgaris infrequently has been reported to develop in tattoos.3,4 Previously reported cases of verruca in tattoos suggest a predilection for blue or black pigment.1-5 We report a case of verruca vulgaris occurring within the red-inked areas of a tattoo that first appeared approximately 18 years after the initial tattoo placement.
A 44-year-old woman presented with erythema, induration, and irritation of a tattoo on the left leg of 2 years’ duration. The tattoo initially was inscribed more than 20 years prior. The patient had a history of type 2 diabetes mellitus and chronic obstructive pulmonary disease. She reported no prior trauma to the area, prior rash or irritation, or similar changes to her other tattoos, including those with red ink. The affected tattoo was inscribed at a separate time from the other tattoos. Physical examination of the irritated tattoo revealed hyperkeratotic papules with firm scaling in the zone of dermal red pigment (Figure 1). Notable nodularity or deep induration was not present. The clinical differential diagnosis included a hypersensitivity reaction to red tattoo ink, sarcoidosis, and an infectious process, such as an atypical mycobacterial infection. A punch biopsy demonstrated papillomatous epidermal hyperplasia with hyperkeratosis, focal parakeratosis, and frequent vacuolization of keratinocytes with enlarged keratohyalin granules, diagnostic of verruca vulgaris (Figure 2). Of note, the patient did not have clinically apparent viral warts elsewhere on physical examination. The patient was successfully managedwith a combination of 2 treatments of intralesional Candida antigen and 3 treatments of cryotherapy with resolution of most lesions over the course of 8 months. Over the following several months, the patient applied topical salicylic acid, which led to the resolution of the remaining lesions. The verrucae had not recurred 19 months after the initial presentation.
The development of verruca vulgaris within a tattoo may occur secondary to various mechanisms of HPV inoculation, including introduction of the virus through contaminated ink, the tattoo artist’s saliva, autoinoculation, or koebnerization of a pre-existing verruca vulgaris.4 Local immune system dysregulation secondary to tattoo ink also has been proposed as a mechanism for HPV infection in this setting.1,5 The contents of darker tattoo pigments may promote formation of reactive oxygen species inducing local immunocompromise.5
The pathogenic mechanism was elusive in our patient. Although the localization of verruca vulgaris to the zones of red pigment may be merely coincidental, this phenomenon raised suspicion for direct inoculation via contaminated red ink. The patient’s other red ink–containing tattoos that were inscribed separately were spared, compatible with contamination of the red ink used for the affected tattoo. However, the delayed onset of nearly 2 decades was exceptional, given the shorter previously reported latencies ranging from months to 10 years.4 Autoinoculation or koebnerization is plausible, though greater involvement of nonred pigments would be expected as well as a briefer latency. Finally, the possibility of local immune dysregulation seemed feasible, given the slow evolution of the lesions largely restricted to one pigment type.
We report a case of verruca vulgaris within the red area of a multicolored tattoo that occurred approximately 18 years after tattoo placement. This case highlights a rare presentation of an infectious agent that may complicate tattoos. Both predilection for red pigment rather than black or blue pigment and the long latency period raised interesting questions regarding pathogenesis. Confirmatory biopsy enables effective management of this tattoo complication.
- Huynh TN, Jackson JD, Brodell RT. Tattoo and vaccination sites: possible nest for opportunistic infections, tumors, and dysimmune reactions. Clin Dermatol. 2014;32:678-684.
- Wenzel SM, Rittmann I, Landthaler M, et al. Adverse reactions after tattooing: review of the literature and comparison to results of a survey. Dermatology. 2013;226:138-147.
- Trefzer U, Schmollack K, Stockfleth E, et al. Verrucae in a multicolored decorative tattoo. J Am Acad Dermatol. 2004;50:478-479.
- Wanat KA, Tyring S, Rady P, et al. Human papillomavirus type 27 associated with multiple verruca within a tattoo: report of a case and review of the literature. Int J Dermatol. 2014;53:882-884.
- Ramey K, Ibrahim J, Brodell RT. Verruca localization predominately in black tattoo ink: a retrospective case series. J Eur Acad Dermatol Venereol. 2016;30:E34-E36.
To the Editor:
The art of tattooing continues to gain popularity in the 21st century, albeit with accompanying hazards.1 Reported adverse reactions to tattoos include infections, tumors, and hypersensitivity and granulomatous reactions.2 Various infectious agents may involve tattoos, including human papillomavirus (HPV), molluscum contagiosum, herpes simplex virus, hepatitis C virus, tuberculoid and nontuberculoid mycobacteria, and Staphylococcus aureus.2 Verruca vulgaris infrequently has been reported to develop in tattoos.3,4 Previously reported cases of verruca in tattoos suggest a predilection for blue or black pigment.1-5 We report a case of verruca vulgaris occurring within the red-inked areas of a tattoo that first appeared approximately 18 years after the initial tattoo placement.
A 44-year-old woman presented with erythema, induration, and irritation of a tattoo on the left leg of 2 years’ duration. The tattoo initially was inscribed more than 20 years prior. The patient had a history of type 2 diabetes mellitus and chronic obstructive pulmonary disease. She reported no prior trauma to the area, prior rash or irritation, or similar changes to her other tattoos, including those with red ink. The affected tattoo was inscribed at a separate time from the other tattoos. Physical examination of the irritated tattoo revealed hyperkeratotic papules with firm scaling in the zone of dermal red pigment (Figure 1). Notable nodularity or deep induration was not present. The clinical differential diagnosis included a hypersensitivity reaction to red tattoo ink, sarcoidosis, and an infectious process, such as an atypical mycobacterial infection. A punch biopsy demonstrated papillomatous epidermal hyperplasia with hyperkeratosis, focal parakeratosis, and frequent vacuolization of keratinocytes with enlarged keratohyalin granules, diagnostic of verruca vulgaris (Figure 2). Of note, the patient did not have clinically apparent viral warts elsewhere on physical examination. The patient was successfully managedwith a combination of 2 treatments of intralesional Candida antigen and 3 treatments of cryotherapy with resolution of most lesions over the course of 8 months. Over the following several months, the patient applied topical salicylic acid, which led to the resolution of the remaining lesions. The verrucae had not recurred 19 months after the initial presentation.
The development of verruca vulgaris within a tattoo may occur secondary to various mechanisms of HPV inoculation, including introduction of the virus through contaminated ink, the tattoo artist’s saliva, autoinoculation, or koebnerization of a pre-existing verruca vulgaris.4 Local immune system dysregulation secondary to tattoo ink also has been proposed as a mechanism for HPV infection in this setting.1,5 The contents of darker tattoo pigments may promote formation of reactive oxygen species inducing local immunocompromise.5
The pathogenic mechanism was elusive in our patient. Although the localization of verruca vulgaris to the zones of red pigment may be merely coincidental, this phenomenon raised suspicion for direct inoculation via contaminated red ink. The patient’s other red ink–containing tattoos that were inscribed separately were spared, compatible with contamination of the red ink used for the affected tattoo. However, the delayed onset of nearly 2 decades was exceptional, given the shorter previously reported latencies ranging from months to 10 years.4 Autoinoculation or koebnerization is plausible, though greater involvement of nonred pigments would be expected as well as a briefer latency. Finally, the possibility of local immune dysregulation seemed feasible, given the slow evolution of the lesions largely restricted to one pigment type.
We report a case of verruca vulgaris within the red area of a multicolored tattoo that occurred approximately 18 years after tattoo placement. This case highlights a rare presentation of an infectious agent that may complicate tattoos. Both predilection for red pigment rather than black or blue pigment and the long latency period raised interesting questions regarding pathogenesis. Confirmatory biopsy enables effective management of this tattoo complication.
To the Editor:
The art of tattooing continues to gain popularity in the 21st century, albeit with accompanying hazards.1 Reported adverse reactions to tattoos include infections, tumors, and hypersensitivity and granulomatous reactions.2 Various infectious agents may involve tattoos, including human papillomavirus (HPV), molluscum contagiosum, herpes simplex virus, hepatitis C virus, tuberculoid and nontuberculoid mycobacteria, and Staphylococcus aureus.2 Verruca vulgaris infrequently has been reported to develop in tattoos.3,4 Previously reported cases of verruca in tattoos suggest a predilection for blue or black pigment.1-5 We report a case of verruca vulgaris occurring within the red-inked areas of a tattoo that first appeared approximately 18 years after the initial tattoo placement.
A 44-year-old woman presented with erythema, induration, and irritation of a tattoo on the left leg of 2 years’ duration. The tattoo initially was inscribed more than 20 years prior. The patient had a history of type 2 diabetes mellitus and chronic obstructive pulmonary disease. She reported no prior trauma to the area, prior rash or irritation, or similar changes to her other tattoos, including those with red ink. The affected tattoo was inscribed at a separate time from the other tattoos. Physical examination of the irritated tattoo revealed hyperkeratotic papules with firm scaling in the zone of dermal red pigment (Figure 1). Notable nodularity or deep induration was not present. The clinical differential diagnosis included a hypersensitivity reaction to red tattoo ink, sarcoidosis, and an infectious process, such as an atypical mycobacterial infection. A punch biopsy demonstrated papillomatous epidermal hyperplasia with hyperkeratosis, focal parakeratosis, and frequent vacuolization of keratinocytes with enlarged keratohyalin granules, diagnostic of verruca vulgaris (Figure 2). Of note, the patient did not have clinically apparent viral warts elsewhere on physical examination. The patient was successfully managedwith a combination of 2 treatments of intralesional Candida antigen and 3 treatments of cryotherapy with resolution of most lesions over the course of 8 months. Over the following several months, the patient applied topical salicylic acid, which led to the resolution of the remaining lesions. The verrucae had not recurred 19 months after the initial presentation.
The development of verruca vulgaris within a tattoo may occur secondary to various mechanisms of HPV inoculation, including introduction of the virus through contaminated ink, the tattoo artist’s saliva, autoinoculation, or koebnerization of a pre-existing verruca vulgaris.4 Local immune system dysregulation secondary to tattoo ink also has been proposed as a mechanism for HPV infection in this setting.1,5 The contents of darker tattoo pigments may promote formation of reactive oxygen species inducing local immunocompromise.5
The pathogenic mechanism was elusive in our patient. Although the localization of verruca vulgaris to the zones of red pigment may be merely coincidental, this phenomenon raised suspicion for direct inoculation via contaminated red ink. The patient’s other red ink–containing tattoos that were inscribed separately were spared, compatible with contamination of the red ink used for the affected tattoo. However, the delayed onset of nearly 2 decades was exceptional, given the shorter previously reported latencies ranging from months to 10 years.4 Autoinoculation or koebnerization is plausible, though greater involvement of nonred pigments would be expected as well as a briefer latency. Finally, the possibility of local immune dysregulation seemed feasible, given the slow evolution of the lesions largely restricted to one pigment type.
We report a case of verruca vulgaris within the red area of a multicolored tattoo that occurred approximately 18 years after tattoo placement. This case highlights a rare presentation of an infectious agent that may complicate tattoos. Both predilection for red pigment rather than black or blue pigment and the long latency period raised interesting questions regarding pathogenesis. Confirmatory biopsy enables effective management of this tattoo complication.
- Huynh TN, Jackson JD, Brodell RT. Tattoo and vaccination sites: possible nest for opportunistic infections, tumors, and dysimmune reactions. Clin Dermatol. 2014;32:678-684.
- Wenzel SM, Rittmann I, Landthaler M, et al. Adverse reactions after tattooing: review of the literature and comparison to results of a survey. Dermatology. 2013;226:138-147.
- Trefzer U, Schmollack K, Stockfleth E, et al. Verrucae in a multicolored decorative tattoo. J Am Acad Dermatol. 2004;50:478-479.
- Wanat KA, Tyring S, Rady P, et al. Human papillomavirus type 27 associated with multiple verruca within a tattoo: report of a case and review of the literature. Int J Dermatol. 2014;53:882-884.
- Ramey K, Ibrahim J, Brodell RT. Verruca localization predominately in black tattoo ink: a retrospective case series. J Eur Acad Dermatol Venereol. 2016;30:E34-E36.
- Huynh TN, Jackson JD, Brodell RT. Tattoo and vaccination sites: possible nest for opportunistic infections, tumors, and dysimmune reactions. Clin Dermatol. 2014;32:678-684.
- Wenzel SM, Rittmann I, Landthaler M, et al. Adverse reactions after tattooing: review of the literature and comparison to results of a survey. Dermatology. 2013;226:138-147.
- Trefzer U, Schmollack K, Stockfleth E, et al. Verrucae in a multicolored decorative tattoo. J Am Acad Dermatol. 2004;50:478-479.
- Wanat KA, Tyring S, Rady P, et al. Human papillomavirus type 27 associated with multiple verruca within a tattoo: report of a case and review of the literature. Int J Dermatol. 2014;53:882-884.
- Ramey K, Ibrahim J, Brodell RT. Verruca localization predominately in black tattoo ink: a retrospective case series. J Eur Acad Dermatol Venereol. 2016;30:E34-E36.
Practice Points
- Various adverse reactions and infectious agents may involve tattoos.
- Verruca vulgaris may affect tattoos in a color-restricted manner and demonstrate latency of many years after tattoo placement.
- Timely diagnosis of the tattoo-involving process, confirmed by biopsy, allows for appropriate management.
Pediatric-Onset Refractory Lupus Erythematosus Panniculitis Treated With Rituximab
To the Editor:
Lupus erythematosus panniculitis (LEP) is rare in the pediatric population. It can be difficult to manage, as patients may not respond to conventional treatments including hydroxychloroquine and prednisone. We report the use of rituximab in the treatment of a 20-year-old woman with LEP of the face, legs, and arms that was refractory to standard treatments. She also had a history of hemophagocytic lymphohistiocytosis (HLH). Further studies are warranted to determine the role of rituximab in the treatment of pediatric patients with LEP.
A 20-year-old woman with history of LEP and HLH initially presented with migratory violaceous nodules on the face 16 years prior to the current presentation. A skin biopsy 3 years after that initial presentation suggested a diagnosis of cutaneous lupus erythematosus. Six years later, numerous asymptomatic lesions appeared on the legs, predominantly on the calves; she was successfully treated with hydroxychloroquine and high-dose prednisone. Four years prior to the current presentation, a febrile illness prompted discontinuation of hydroxychloroquine and hospitalization, where she was first was diagnosed with HLH; she achieved remission with cyclosporine. At the current presentation, she continued to have persistent violaceous lesions on the face, lower arms, and legs with underlying nodularity (Figure 1). Skin biopsies revealed LEP and were less suggestive of HLH. She was restarted on hydroxychloroquine, which did not adequately control the disease. Rheumatologic workup was only notable for an antinuclear antibody titer of 1:80 (reference range, <1:80) in a speckled pattern.
Due to the refractory nature of her condition, continued lesion development despite standard treatment, and concerns of possible scarring, we considered a trial of rituximab. Because HLH and LEP can mimic subcutaneous T-cell lymphoma, another skin biopsy was performed, which revealed a deep dermal and subcutaneous lymphohistiocytic infiltrate composed of predominantly CD3+ T cells with a mixed population of CD4+ and CD8+ cells (Figure 2). There was no evidence of transformation into lymphoma. Pathologic findings were most compatible with LEP rather than an HLH-associated panniculitis due to the lack of definitive phagocytosis. She received rituximab using body surface area–based dosing at 375 mg/m2. CD19 levels decreased to undetectable levels after the first dose. Rituximab was dosed based on clinical response; she tolerated treatment well and experienced considerable improvement in the number of lesions following completion of 4 doses at weeks 0, 1, 5, and 7 (Figure 3). She developed a flare at 7 months and improved again after another dose of rituximab.
Lupus erythematosus panniculitis is a rare variant of lupus erythematosus with an average age of presentation between 30 and 60 years.1 In children, LEP presents as recurrent subcutaneous nodules and plaques, commonly involving the face and upper arms.1,2 Long-term sequelae include local swelling and skin atrophy.3 Conventional treatment options for pediatric patients include hydroxychloroquine and corticosteroids.1 Management can be challenging due to the lack of response to conventional treatments as well as the chronic progressive nature of LEP.2 In refractory cases, cyclosporine, azathioprine, sulfones, thalidomide, mycophenolate mofetil, and cyclophosphamide are alternative treatment options.1-4
Rituximab, a chimeric monoclonal antibody targeting B-cell surface marker CD20, results in depletion of mature B cells. Use of rituximab for LEP has been described in multiple case reports involving an 8-year-old boy, 22-year-old girl, and 2 middle-aged women.2-4 In addition, a recently published case series of 4 patients with childhood-onset refractory LEP described improvement of disease activity with rituximab.5 It is important to rule out subcutaneous T-cell lymphoma before treatment with rituximab, as its histopathology can closely resemble that seen in LEP and HLH-associated cytophagic histiocytic panniculitis.1,6
Rituximab may be an effective treatment option in pediatric patients with refractory LEP. Larger studies on the use of rituximab in the pediatric population are necessary.
- Weingartner JS, Zedek DC, Burkhart CN, et al. Lupus erythematosus panniculitis in children: report of three cases and review of previously reported cases. Pediatr Dermatol. 2011;29:169-176.
- Moreno-Suárez F, Pulpillo-Ruiz Á. Rituximab for the treatment of lupus erythematosus panniculitis. Dermatol Ther. 2013;26:415-418.
- Guissa VR, Trudes G, Jesus AA, et al. Lupus erythematosus panniculitis in children and adolescents. Acta Reumatol Port. 2012;37:82-85.
- Mcardle A, Baker JF. A case of “refractory” lupus erythematosus profundus responsive to rituximab. Clin Rheumatol. 2009;28:745-746.
- Correll CK, Miller DD, Maguiness SM. Treatment of childhood-onset lupus erythematosus panniculitis with rituximab. JAMA Dermatol. 2020;156:566-569.
- Aronson IK, Worobec SM. Cytophagic histiocytic panniculitis and hemophagocytic lymphohistiocytosis: an overview. Dermatol Ther. 2010;23:389-402.
To the Editor:
Lupus erythematosus panniculitis (LEP) is rare in the pediatric population. It can be difficult to manage, as patients may not respond to conventional treatments including hydroxychloroquine and prednisone. We report the use of rituximab in the treatment of a 20-year-old woman with LEP of the face, legs, and arms that was refractory to standard treatments. She also had a history of hemophagocytic lymphohistiocytosis (HLH). Further studies are warranted to determine the role of rituximab in the treatment of pediatric patients with LEP.
A 20-year-old woman with history of LEP and HLH initially presented with migratory violaceous nodules on the face 16 years prior to the current presentation. A skin biopsy 3 years after that initial presentation suggested a diagnosis of cutaneous lupus erythematosus. Six years later, numerous asymptomatic lesions appeared on the legs, predominantly on the calves; she was successfully treated with hydroxychloroquine and high-dose prednisone. Four years prior to the current presentation, a febrile illness prompted discontinuation of hydroxychloroquine and hospitalization, where she was first was diagnosed with HLH; she achieved remission with cyclosporine. At the current presentation, she continued to have persistent violaceous lesions on the face, lower arms, and legs with underlying nodularity (Figure 1). Skin biopsies revealed LEP and were less suggestive of HLH. She was restarted on hydroxychloroquine, which did not adequately control the disease. Rheumatologic workup was only notable for an antinuclear antibody titer of 1:80 (reference range, <1:80) in a speckled pattern.
Due to the refractory nature of her condition, continued lesion development despite standard treatment, and concerns of possible scarring, we considered a trial of rituximab. Because HLH and LEP can mimic subcutaneous T-cell lymphoma, another skin biopsy was performed, which revealed a deep dermal and subcutaneous lymphohistiocytic infiltrate composed of predominantly CD3+ T cells with a mixed population of CD4+ and CD8+ cells (Figure 2). There was no evidence of transformation into lymphoma. Pathologic findings were most compatible with LEP rather than an HLH-associated panniculitis due to the lack of definitive phagocytosis. She received rituximab using body surface area–based dosing at 375 mg/m2. CD19 levels decreased to undetectable levels after the first dose. Rituximab was dosed based on clinical response; she tolerated treatment well and experienced considerable improvement in the number of lesions following completion of 4 doses at weeks 0, 1, 5, and 7 (Figure 3). She developed a flare at 7 months and improved again after another dose of rituximab.
Lupus erythematosus panniculitis is a rare variant of lupus erythematosus with an average age of presentation between 30 and 60 years.1 In children, LEP presents as recurrent subcutaneous nodules and plaques, commonly involving the face and upper arms.1,2 Long-term sequelae include local swelling and skin atrophy.3 Conventional treatment options for pediatric patients include hydroxychloroquine and corticosteroids.1 Management can be challenging due to the lack of response to conventional treatments as well as the chronic progressive nature of LEP.2 In refractory cases, cyclosporine, azathioprine, sulfones, thalidomide, mycophenolate mofetil, and cyclophosphamide are alternative treatment options.1-4
Rituximab, a chimeric monoclonal antibody targeting B-cell surface marker CD20, results in depletion of mature B cells. Use of rituximab for LEP has been described in multiple case reports involving an 8-year-old boy, 22-year-old girl, and 2 middle-aged women.2-4 In addition, a recently published case series of 4 patients with childhood-onset refractory LEP described improvement of disease activity with rituximab.5 It is important to rule out subcutaneous T-cell lymphoma before treatment with rituximab, as its histopathology can closely resemble that seen in LEP and HLH-associated cytophagic histiocytic panniculitis.1,6
Rituximab may be an effective treatment option in pediatric patients with refractory LEP. Larger studies on the use of rituximab in the pediatric population are necessary.
To the Editor:
Lupus erythematosus panniculitis (LEP) is rare in the pediatric population. It can be difficult to manage, as patients may not respond to conventional treatments including hydroxychloroquine and prednisone. We report the use of rituximab in the treatment of a 20-year-old woman with LEP of the face, legs, and arms that was refractory to standard treatments. She also had a history of hemophagocytic lymphohistiocytosis (HLH). Further studies are warranted to determine the role of rituximab in the treatment of pediatric patients with LEP.
A 20-year-old woman with history of LEP and HLH initially presented with migratory violaceous nodules on the face 16 years prior to the current presentation. A skin biopsy 3 years after that initial presentation suggested a diagnosis of cutaneous lupus erythematosus. Six years later, numerous asymptomatic lesions appeared on the legs, predominantly on the calves; she was successfully treated with hydroxychloroquine and high-dose prednisone. Four years prior to the current presentation, a febrile illness prompted discontinuation of hydroxychloroquine and hospitalization, where she was first was diagnosed with HLH; she achieved remission with cyclosporine. At the current presentation, she continued to have persistent violaceous lesions on the face, lower arms, and legs with underlying nodularity (Figure 1). Skin biopsies revealed LEP and were less suggestive of HLH. She was restarted on hydroxychloroquine, which did not adequately control the disease. Rheumatologic workup was only notable for an antinuclear antibody titer of 1:80 (reference range, <1:80) in a speckled pattern.
Due to the refractory nature of her condition, continued lesion development despite standard treatment, and concerns of possible scarring, we considered a trial of rituximab. Because HLH and LEP can mimic subcutaneous T-cell lymphoma, another skin biopsy was performed, which revealed a deep dermal and subcutaneous lymphohistiocytic infiltrate composed of predominantly CD3+ T cells with a mixed population of CD4+ and CD8+ cells (Figure 2). There was no evidence of transformation into lymphoma. Pathologic findings were most compatible with LEP rather than an HLH-associated panniculitis due to the lack of definitive phagocytosis. She received rituximab using body surface area–based dosing at 375 mg/m2. CD19 levels decreased to undetectable levels after the first dose. Rituximab was dosed based on clinical response; she tolerated treatment well and experienced considerable improvement in the number of lesions following completion of 4 doses at weeks 0, 1, 5, and 7 (Figure 3). She developed a flare at 7 months and improved again after another dose of rituximab.
Lupus erythematosus panniculitis is a rare variant of lupus erythematosus with an average age of presentation between 30 and 60 years.1 In children, LEP presents as recurrent subcutaneous nodules and plaques, commonly involving the face and upper arms.1,2 Long-term sequelae include local swelling and skin atrophy.3 Conventional treatment options for pediatric patients include hydroxychloroquine and corticosteroids.1 Management can be challenging due to the lack of response to conventional treatments as well as the chronic progressive nature of LEP.2 In refractory cases, cyclosporine, azathioprine, sulfones, thalidomide, mycophenolate mofetil, and cyclophosphamide are alternative treatment options.1-4
Rituximab, a chimeric monoclonal antibody targeting B-cell surface marker CD20, results in depletion of mature B cells. Use of rituximab for LEP has been described in multiple case reports involving an 8-year-old boy, 22-year-old girl, and 2 middle-aged women.2-4 In addition, a recently published case series of 4 patients with childhood-onset refractory LEP described improvement of disease activity with rituximab.5 It is important to rule out subcutaneous T-cell lymphoma before treatment with rituximab, as its histopathology can closely resemble that seen in LEP and HLH-associated cytophagic histiocytic panniculitis.1,6
Rituximab may be an effective treatment option in pediatric patients with refractory LEP. Larger studies on the use of rituximab in the pediatric population are necessary.
- Weingartner JS, Zedek DC, Burkhart CN, et al. Lupus erythematosus panniculitis in children: report of three cases and review of previously reported cases. Pediatr Dermatol. 2011;29:169-176.
- Moreno-Suárez F, Pulpillo-Ruiz Á. Rituximab for the treatment of lupus erythematosus panniculitis. Dermatol Ther. 2013;26:415-418.
- Guissa VR, Trudes G, Jesus AA, et al. Lupus erythematosus panniculitis in children and adolescents. Acta Reumatol Port. 2012;37:82-85.
- Mcardle A, Baker JF. A case of “refractory” lupus erythematosus profundus responsive to rituximab. Clin Rheumatol. 2009;28:745-746.
- Correll CK, Miller DD, Maguiness SM. Treatment of childhood-onset lupus erythematosus panniculitis with rituximab. JAMA Dermatol. 2020;156:566-569.
- Aronson IK, Worobec SM. Cytophagic histiocytic panniculitis and hemophagocytic lymphohistiocytosis: an overview. Dermatol Ther. 2010;23:389-402.
- Weingartner JS, Zedek DC, Burkhart CN, et al. Lupus erythematosus panniculitis in children: report of three cases and review of previously reported cases. Pediatr Dermatol. 2011;29:169-176.
- Moreno-Suárez F, Pulpillo-Ruiz Á. Rituximab for the treatment of lupus erythematosus panniculitis. Dermatol Ther. 2013;26:415-418.
- Guissa VR, Trudes G, Jesus AA, et al. Lupus erythematosus panniculitis in children and adolescents. Acta Reumatol Port. 2012;37:82-85.
- Mcardle A, Baker JF. A case of “refractory” lupus erythematosus profundus responsive to rituximab. Clin Rheumatol. 2009;28:745-746.
- Correll CK, Miller DD, Maguiness SM. Treatment of childhood-onset lupus erythematosus panniculitis with rituximab. JAMA Dermatol. 2020;156:566-569.
- Aronson IK, Worobec SM. Cytophagic histiocytic panniculitis and hemophagocytic lymphohistiocytosis: an overview. Dermatol Ther. 2010;23:389-402.
Practice Points
- Lupus erythematosus panniculitis (LEP) is rare in the pediatric population and often is difficult to treat.
- Rituximab can be an effective treatment option for refractory LEP.
- Before the initiation of rituximab, a biopsy is warranted to rule out subcutaneous T-cell lymphoma, which can mimic LEP and hemophagocytic lymphohistiocytosis–associated panniculitis.