User login
Formerly Skin & Allergy News
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')]
The leading independent newspaper covering dermatology news and commentary.
Global melanoma incidence high and on the rise
Even by cautious calculations,
An estimated 325,000 people worldwide received a new diagnosis of cutaneous melanoma in 2020, and if present trends continue, the incidence of new cases is predicted to increase by about 50% in 2040, with melanoma deaths expected to rise by almost 70%, Melina Arnold, PhD, from the Cancer Surveillance Branch of the International Agency for Research on Cancer in Lyon, France, and colleagues reported.
“Melanoma is the most lethal form of skin cancer; this epidemiological assessment found a heavy public health and economic burden, and our projections suggest that it will remain so in the coming decades,” they wrote in a study published online in JAMA Dermatology.
In an accompanying editorial, Mavis Obeng-Kusi, MPharm and Ivo Abraham, PhD from the Center for Health Outcomes and PharmacoEconomic Research at the University of Arizona, Tucson, commented that the findings are “sobering,” but may substantially underestimate the gravity of the problem in low- and middle-income countries (LMIC).
“The study by Arnold et al. brings to the fore a public health concern that requires global attention and initiates conversations particularly related to LMIC settings, where the incidence and mortality of melanoma is thought to be minimal and for which preventive measures may be insufficient,” they wrote.
Down Under nations lead
Dr. Arnold and colleagues looked at data on age-standardized melanoma incidence and mortality rates per 100,000 person-years (PY) by country, each of 20 world regions as defined by the United Nations, and according to the UN’s four-tier Human Development Index, which stratifies countries into low-, medium-, high-, and very high–income categories.
As noted previously, the researchers estimated that there were 325,000 new melanoma cases worldwide in 2020 (174,000 cases in males and 151,000 in females). There were 57,000 estimated melanoma deaths the same year (32,000 in males and 25,000 in females.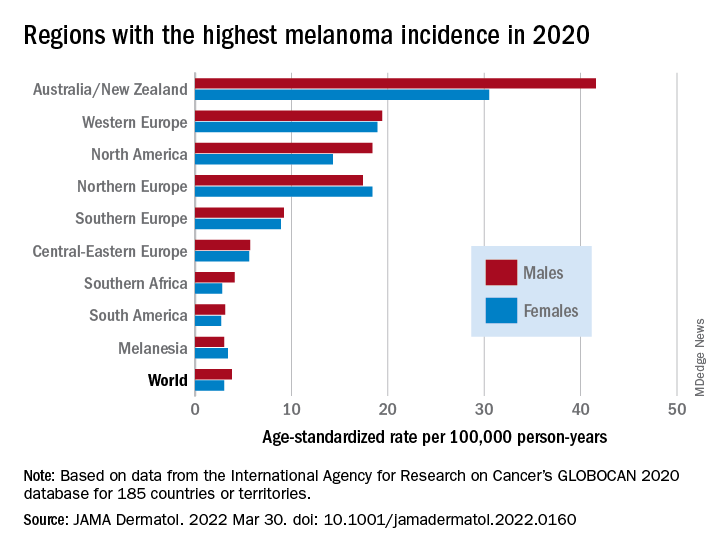
The highest incidence rates were seen in Australia and New Zealand, at 42 per 100,000 PY among males and 31 per 100,000 PY in females, followed by Western Europe with 19 per 100,000 PY in both males and females, North America with 18 and 14 cases per 100,000 PY in males and females respectively, and Northern Europe, with 17 per 100,000 PY in males, and 18 per 100,000 PY in females.
In contrast, in most African and Asian countries melanoma was rare, with rates commonly less than 1 per 100,000 PY, the investigators noted.
The melanoma mortality rate was highest in New Zealand, at 5 per 100,000 PY. Mortality rates worldwide varied less widely than incidence rates. In most other regions of the world, mortality rates were “much lower,” ranging between 0.2-1.0 per 100,000 PY, they wrote.
The authors estimated that, if 2020 rates remain stable, the global burden from melanoma in 2040 will increase to approximately 510,000 new cases and 96,000 deaths.
Public health efforts needed
In their editorial, Ms. Obeng-Kusi and Dr. Abraham pointed out that the study was hampered by the limited availability of cancer data from LMICs, leading the authors to estimate incidence and mortality rates based on proxy data, such as statistical modeling or averaged rates from neighboring countries.
They emphasized the need for going beyond the statistics: “Specific to cutaneous melanoma data, what is most important globally, knowing the exact numbers of cases and deaths or understanding the order of magnitude of the present and future epidemiology? No doubt the latter. Melanoma can be treated more easily if caught at earlier stages.”
Projections such as those provided by Dr. Arnold and colleagues could help to raise awareness of the importance of decreasing exposure to UV radiation, which accounts for three-fourths of all incident melanomas, the editorialists said.
The study was funded in part by a grant to coauthor Anna E. Cust, PhD, MPH. Dr. Cust reported receiving a fellowship from the Australian National Health and Medical Research Council outside the submitted work. Dr. Arnold had no conflicts of interested to disclose. Dr. Abraham reported financial relationships with various entities. Ms. Obeng-Kusi had no disclosures.
Even by cautious calculations,
An estimated 325,000 people worldwide received a new diagnosis of cutaneous melanoma in 2020, and if present trends continue, the incidence of new cases is predicted to increase by about 50% in 2040, with melanoma deaths expected to rise by almost 70%, Melina Arnold, PhD, from the Cancer Surveillance Branch of the International Agency for Research on Cancer in Lyon, France, and colleagues reported.
“Melanoma is the most lethal form of skin cancer; this epidemiological assessment found a heavy public health and economic burden, and our projections suggest that it will remain so in the coming decades,” they wrote in a study published online in JAMA Dermatology.
In an accompanying editorial, Mavis Obeng-Kusi, MPharm and Ivo Abraham, PhD from the Center for Health Outcomes and PharmacoEconomic Research at the University of Arizona, Tucson, commented that the findings are “sobering,” but may substantially underestimate the gravity of the problem in low- and middle-income countries (LMIC).
“The study by Arnold et al. brings to the fore a public health concern that requires global attention and initiates conversations particularly related to LMIC settings, where the incidence and mortality of melanoma is thought to be minimal and for which preventive measures may be insufficient,” they wrote.
Down Under nations lead
Dr. Arnold and colleagues looked at data on age-standardized melanoma incidence and mortality rates per 100,000 person-years (PY) by country, each of 20 world regions as defined by the United Nations, and according to the UN’s four-tier Human Development Index, which stratifies countries into low-, medium-, high-, and very high–income categories.
As noted previously, the researchers estimated that there were 325,000 new melanoma cases worldwide in 2020 (174,000 cases in males and 151,000 in females). There were 57,000 estimated melanoma deaths the same year (32,000 in males and 25,000 in females.
The highest incidence rates were seen in Australia and New Zealand, at 42 per 100,000 PY among males and 31 per 100,000 PY in females, followed by Western Europe with 19 per 100,000 PY in both males and females, North America with 18 and 14 cases per 100,000 PY in males and females respectively, and Northern Europe, with 17 per 100,000 PY in males, and 18 per 100,000 PY in females.
In contrast, in most African and Asian countries melanoma was rare, with rates commonly less than 1 per 100,000 PY, the investigators noted.
The melanoma mortality rate was highest in New Zealand, at 5 per 100,000 PY. Mortality rates worldwide varied less widely than incidence rates. In most other regions of the world, mortality rates were “much lower,” ranging between 0.2-1.0 per 100,000 PY, they wrote.
The authors estimated that, if 2020 rates remain stable, the global burden from melanoma in 2040 will increase to approximately 510,000 new cases and 96,000 deaths.
Public health efforts needed
In their editorial, Ms. Obeng-Kusi and Dr. Abraham pointed out that the study was hampered by the limited availability of cancer data from LMICs, leading the authors to estimate incidence and mortality rates based on proxy data, such as statistical modeling or averaged rates from neighboring countries.
They emphasized the need for going beyond the statistics: “Specific to cutaneous melanoma data, what is most important globally, knowing the exact numbers of cases and deaths or understanding the order of magnitude of the present and future epidemiology? No doubt the latter. Melanoma can be treated more easily if caught at earlier stages.”
Projections such as those provided by Dr. Arnold and colleagues could help to raise awareness of the importance of decreasing exposure to UV radiation, which accounts for three-fourths of all incident melanomas, the editorialists said.
The study was funded in part by a grant to coauthor Anna E. Cust, PhD, MPH. Dr. Cust reported receiving a fellowship from the Australian National Health and Medical Research Council outside the submitted work. Dr. Arnold had no conflicts of interested to disclose. Dr. Abraham reported financial relationships with various entities. Ms. Obeng-Kusi had no disclosures.
Even by cautious calculations,
An estimated 325,000 people worldwide received a new diagnosis of cutaneous melanoma in 2020, and if present trends continue, the incidence of new cases is predicted to increase by about 50% in 2040, with melanoma deaths expected to rise by almost 70%, Melina Arnold, PhD, from the Cancer Surveillance Branch of the International Agency for Research on Cancer in Lyon, France, and colleagues reported.
“Melanoma is the most lethal form of skin cancer; this epidemiological assessment found a heavy public health and economic burden, and our projections suggest that it will remain so in the coming decades,” they wrote in a study published online in JAMA Dermatology.
In an accompanying editorial, Mavis Obeng-Kusi, MPharm and Ivo Abraham, PhD from the Center for Health Outcomes and PharmacoEconomic Research at the University of Arizona, Tucson, commented that the findings are “sobering,” but may substantially underestimate the gravity of the problem in low- and middle-income countries (LMIC).
“The study by Arnold et al. brings to the fore a public health concern that requires global attention and initiates conversations particularly related to LMIC settings, where the incidence and mortality of melanoma is thought to be minimal and for which preventive measures may be insufficient,” they wrote.
Down Under nations lead
Dr. Arnold and colleagues looked at data on age-standardized melanoma incidence and mortality rates per 100,000 person-years (PY) by country, each of 20 world regions as defined by the United Nations, and according to the UN’s four-tier Human Development Index, which stratifies countries into low-, medium-, high-, and very high–income categories.
As noted previously, the researchers estimated that there were 325,000 new melanoma cases worldwide in 2020 (174,000 cases in males and 151,000 in females). There were 57,000 estimated melanoma deaths the same year (32,000 in males and 25,000 in females.
The highest incidence rates were seen in Australia and New Zealand, at 42 per 100,000 PY among males and 31 per 100,000 PY in females, followed by Western Europe with 19 per 100,000 PY in both males and females, North America with 18 and 14 cases per 100,000 PY in males and females respectively, and Northern Europe, with 17 per 100,000 PY in males, and 18 per 100,000 PY in females.
In contrast, in most African and Asian countries melanoma was rare, with rates commonly less than 1 per 100,000 PY, the investigators noted.
The melanoma mortality rate was highest in New Zealand, at 5 per 100,000 PY. Mortality rates worldwide varied less widely than incidence rates. In most other regions of the world, mortality rates were “much lower,” ranging between 0.2-1.0 per 100,000 PY, they wrote.
The authors estimated that, if 2020 rates remain stable, the global burden from melanoma in 2040 will increase to approximately 510,000 new cases and 96,000 deaths.
Public health efforts needed
In their editorial, Ms. Obeng-Kusi and Dr. Abraham pointed out that the study was hampered by the limited availability of cancer data from LMICs, leading the authors to estimate incidence and mortality rates based on proxy data, such as statistical modeling or averaged rates from neighboring countries.
They emphasized the need for going beyond the statistics: “Specific to cutaneous melanoma data, what is most important globally, knowing the exact numbers of cases and deaths or understanding the order of magnitude of the present and future epidemiology? No doubt the latter. Melanoma can be treated more easily if caught at earlier stages.”
Projections such as those provided by Dr. Arnold and colleagues could help to raise awareness of the importance of decreasing exposure to UV radiation, which accounts for three-fourths of all incident melanomas, the editorialists said.
The study was funded in part by a grant to coauthor Anna E. Cust, PhD, MPH. Dr. Cust reported receiving a fellowship from the Australian National Health and Medical Research Council outside the submitted work. Dr. Arnold had no conflicts of interested to disclose. Dr. Abraham reported financial relationships with various entities. Ms. Obeng-Kusi had no disclosures.
FROM JAMA DERMATOLOGY
We all struggle with the unwritten rules of medical culture
There is a two-lane bridge in my town. It is quaint and picturesque, and when we first moved here, I would gaze out at the water as I drove, letting my mind wander along with the seagulls drifting alongside the car. Until one day, crossing back over, I passed a school bus stopped in the other lane, and instead of waving back, the driver gave me such a fierce look of disapproval I felt like I’d been to the principal’s office. What had I done?
I started paying more attention to the pattern of the other cars on the bridge. Although it appeared to be a standard two-lane width, the lanes weren’t quite wide enough if a school bus or large truck needed to cross at the same time as a car coming from the opposite direction. They had to wait until the other lane was clear. It was an unwritten rule of the town that if you saw a school bus on the other side, you stopped your car and yielded the bridge to the bus. It took me weeks to figure this out. When I did, I felt like I finally belonged in the community. Before, I’d been an outsider.
This got me thinking about culture. Every place has its unwritten rules, whether a community or a workplace. But how do we know the culture of a place? It’s pretty much impossible until we experience it for ourselves.
When I did figure out the bridge, I had a little bit of anger, to be honest. How was I supposed to know about the lanes? There weren’t any signs. Geez.
Now, when I approach the bridge, I don’t even think about it. I know what to do if I see a bus coming.
But sometimes I remember that time of confusion before I deciphered the unwritten rule. I still have a twinge of guilt for having done something wrong, even though it hadn’t been my fault.
It reminded me of a memory from medical training. I was an MS4, and my ER rotation was in a busy county hospital with a level I trauma center. To say that the place was chaotic would be an understatement.
On the first morning, I was shown the chart rack (yes, this was back in the day of paper charts). Charts were placed in the order that patients arrived. Med students and residents were to take a chart in chronological order, go triage and assess the patient, and then find an attending. Once finished, you put the chart back on the rack and picked up the next one. This was the extent of my orientation to the ER.
The days and weeks of the rotation flew by. It was a busy and exciting time. By the end of the month, I’d come to feel a part of the team.
Until one day, after finishing discharging a patient, an attending asked me, “Where’s the billing sheet?”
I had no idea what she was talking about. No one had ever shown me a billing sheet. But by this point, as an MS4, I knew well that if an attending asked you something you didn’t know the answer to, you shouldn’t just say that you didn’t know. You should try to figure out if you could at least approximate an answer first.
As I scrambled in my mind to figure out what she was asking me, she took one look at the apprehension in my eyes and asked again, raising her voice, “You haven’t been doing the billing sheets?”
I thought back to the first day of the rotation. The cursory 30-second orientation. Chart rack. Take one. See the patient. Put it back. See the next patient. Nothing about billing sheets.
“No,” I said. “No one ever told me about – ”
But the attending didn’t care that I hadn’t been instructed on the billing sheets. She ripped into me, yelling about how she couldn’t believe I’d been working there the entire month and was not doing the billing sheets. She showed me what they were and where they were supposed to be going and, in front of the whole staff, treated me like not only the biggest idiot she’d ever worked with but that the hospital had ever seen.
As she berated me, I thought about all the patients I’d seen that month. All the billing sheets I hadn’t placed in the pile. All the attendings who hadn’t gotten credit for the patients they’d staffed with me.
But how could I have known? I wanted to ask. How could I have known if nobody showed me or told me?
It was like the bridge. I was in a new environment and somehow expected to know the rules without anyone telling me; and when I didn’t know, people treated me like I’d done it the wrong way on purpose.
I didn’t end up saying anything more to that attending. What could I have said? She had already unleashed a mountain of her pent-up anger at me.
What I did decide in that moment was that I would never be an attending like that.
Like the bridge, this memory years later can still make me feel guilt and shame for doing something wrong. Even though it wasn’t my fault.
I was thinking about this recently with the Match. Thousands of freshly graduated medical students embarking on their new positions as interns in teaching hospitals across the country.
If someone treats you poorly for not knowing something, you are not an idiot. You’ve worked incredibly hard to get where you are, and you deserve to be there.
For attendings and more senior trainees, remember what it was like to be starting in a new place. We all make mistakes, and often it’s simply because of a lack of information.
Trainees shouldn’t have to suffer and be made to feel like outsiders until they figure out the unwritten rules of the place. They belong.
Dr. Lycette is medical director of Providence Oncology and Hematology Care Clinic, Seaside, Ore. She disclosed no relevant conflicts of interest. A version of this article first appeared on Medscape.com.
There is a two-lane bridge in my town. It is quaint and picturesque, and when we first moved here, I would gaze out at the water as I drove, letting my mind wander along with the seagulls drifting alongside the car. Until one day, crossing back over, I passed a school bus stopped in the other lane, and instead of waving back, the driver gave me such a fierce look of disapproval I felt like I’d been to the principal’s office. What had I done?
I started paying more attention to the pattern of the other cars on the bridge. Although it appeared to be a standard two-lane width, the lanes weren’t quite wide enough if a school bus or large truck needed to cross at the same time as a car coming from the opposite direction. They had to wait until the other lane was clear. It was an unwritten rule of the town that if you saw a school bus on the other side, you stopped your car and yielded the bridge to the bus. It took me weeks to figure this out. When I did, I felt like I finally belonged in the community. Before, I’d been an outsider.
This got me thinking about culture. Every place has its unwritten rules, whether a community or a workplace. But how do we know the culture of a place? It’s pretty much impossible until we experience it for ourselves.
When I did figure out the bridge, I had a little bit of anger, to be honest. How was I supposed to know about the lanes? There weren’t any signs. Geez.
Now, when I approach the bridge, I don’t even think about it. I know what to do if I see a bus coming.
But sometimes I remember that time of confusion before I deciphered the unwritten rule. I still have a twinge of guilt for having done something wrong, even though it hadn’t been my fault.
It reminded me of a memory from medical training. I was an MS4, and my ER rotation was in a busy county hospital with a level I trauma center. To say that the place was chaotic would be an understatement.
On the first morning, I was shown the chart rack (yes, this was back in the day of paper charts). Charts were placed in the order that patients arrived. Med students and residents were to take a chart in chronological order, go triage and assess the patient, and then find an attending. Once finished, you put the chart back on the rack and picked up the next one. This was the extent of my orientation to the ER.
The days and weeks of the rotation flew by. It was a busy and exciting time. By the end of the month, I’d come to feel a part of the team.
Until one day, after finishing discharging a patient, an attending asked me, “Where’s the billing sheet?”
I had no idea what she was talking about. No one had ever shown me a billing sheet. But by this point, as an MS4, I knew well that if an attending asked you something you didn’t know the answer to, you shouldn’t just say that you didn’t know. You should try to figure out if you could at least approximate an answer first.
As I scrambled in my mind to figure out what she was asking me, she took one look at the apprehension in my eyes and asked again, raising her voice, “You haven’t been doing the billing sheets?”
I thought back to the first day of the rotation. The cursory 30-second orientation. Chart rack. Take one. See the patient. Put it back. See the next patient. Nothing about billing sheets.
“No,” I said. “No one ever told me about – ”
But the attending didn’t care that I hadn’t been instructed on the billing sheets. She ripped into me, yelling about how she couldn’t believe I’d been working there the entire month and was not doing the billing sheets. She showed me what they were and where they were supposed to be going and, in front of the whole staff, treated me like not only the biggest idiot she’d ever worked with but that the hospital had ever seen.
As she berated me, I thought about all the patients I’d seen that month. All the billing sheets I hadn’t placed in the pile. All the attendings who hadn’t gotten credit for the patients they’d staffed with me.
But how could I have known? I wanted to ask. How could I have known if nobody showed me or told me?
It was like the bridge. I was in a new environment and somehow expected to know the rules without anyone telling me; and when I didn’t know, people treated me like I’d done it the wrong way on purpose.
I didn’t end up saying anything more to that attending. What could I have said? She had already unleashed a mountain of her pent-up anger at me.
What I did decide in that moment was that I would never be an attending like that.
Like the bridge, this memory years later can still make me feel guilt and shame for doing something wrong. Even though it wasn’t my fault.
I was thinking about this recently with the Match. Thousands of freshly graduated medical students embarking on their new positions as interns in teaching hospitals across the country.
If someone treats you poorly for not knowing something, you are not an idiot. You’ve worked incredibly hard to get where you are, and you deserve to be there.
For attendings and more senior trainees, remember what it was like to be starting in a new place. We all make mistakes, and often it’s simply because of a lack of information.
Trainees shouldn’t have to suffer and be made to feel like outsiders until they figure out the unwritten rules of the place. They belong.
Dr. Lycette is medical director of Providence Oncology and Hematology Care Clinic, Seaside, Ore. She disclosed no relevant conflicts of interest. A version of this article first appeared on Medscape.com.
There is a two-lane bridge in my town. It is quaint and picturesque, and when we first moved here, I would gaze out at the water as I drove, letting my mind wander along with the seagulls drifting alongside the car. Until one day, crossing back over, I passed a school bus stopped in the other lane, and instead of waving back, the driver gave me such a fierce look of disapproval I felt like I’d been to the principal’s office. What had I done?
I started paying more attention to the pattern of the other cars on the bridge. Although it appeared to be a standard two-lane width, the lanes weren’t quite wide enough if a school bus or large truck needed to cross at the same time as a car coming from the opposite direction. They had to wait until the other lane was clear. It was an unwritten rule of the town that if you saw a school bus on the other side, you stopped your car and yielded the bridge to the bus. It took me weeks to figure this out. When I did, I felt like I finally belonged in the community. Before, I’d been an outsider.
This got me thinking about culture. Every place has its unwritten rules, whether a community or a workplace. But how do we know the culture of a place? It’s pretty much impossible until we experience it for ourselves.
When I did figure out the bridge, I had a little bit of anger, to be honest. How was I supposed to know about the lanes? There weren’t any signs. Geez.
Now, when I approach the bridge, I don’t even think about it. I know what to do if I see a bus coming.
But sometimes I remember that time of confusion before I deciphered the unwritten rule. I still have a twinge of guilt for having done something wrong, even though it hadn’t been my fault.
It reminded me of a memory from medical training. I was an MS4, and my ER rotation was in a busy county hospital with a level I trauma center. To say that the place was chaotic would be an understatement.
On the first morning, I was shown the chart rack (yes, this was back in the day of paper charts). Charts were placed in the order that patients arrived. Med students and residents were to take a chart in chronological order, go triage and assess the patient, and then find an attending. Once finished, you put the chart back on the rack and picked up the next one. This was the extent of my orientation to the ER.
The days and weeks of the rotation flew by. It was a busy and exciting time. By the end of the month, I’d come to feel a part of the team.
Until one day, after finishing discharging a patient, an attending asked me, “Where’s the billing sheet?”
I had no idea what she was talking about. No one had ever shown me a billing sheet. But by this point, as an MS4, I knew well that if an attending asked you something you didn’t know the answer to, you shouldn’t just say that you didn’t know. You should try to figure out if you could at least approximate an answer first.
As I scrambled in my mind to figure out what she was asking me, she took one look at the apprehension in my eyes and asked again, raising her voice, “You haven’t been doing the billing sheets?”
I thought back to the first day of the rotation. The cursory 30-second orientation. Chart rack. Take one. See the patient. Put it back. See the next patient. Nothing about billing sheets.
“No,” I said. “No one ever told me about – ”
But the attending didn’t care that I hadn’t been instructed on the billing sheets. She ripped into me, yelling about how she couldn’t believe I’d been working there the entire month and was not doing the billing sheets. She showed me what they were and where they were supposed to be going and, in front of the whole staff, treated me like not only the biggest idiot she’d ever worked with but that the hospital had ever seen.
As she berated me, I thought about all the patients I’d seen that month. All the billing sheets I hadn’t placed in the pile. All the attendings who hadn’t gotten credit for the patients they’d staffed with me.
But how could I have known? I wanted to ask. How could I have known if nobody showed me or told me?
It was like the bridge. I was in a new environment and somehow expected to know the rules without anyone telling me; and when I didn’t know, people treated me like I’d done it the wrong way on purpose.
I didn’t end up saying anything more to that attending. What could I have said? She had already unleashed a mountain of her pent-up anger at me.
What I did decide in that moment was that I would never be an attending like that.
Like the bridge, this memory years later can still make me feel guilt and shame for doing something wrong. Even though it wasn’t my fault.
I was thinking about this recently with the Match. Thousands of freshly graduated medical students embarking on their new positions as interns in teaching hospitals across the country.
If someone treats you poorly for not knowing something, you are not an idiot. You’ve worked incredibly hard to get where you are, and you deserve to be there.
For attendings and more senior trainees, remember what it was like to be starting in a new place. We all make mistakes, and often it’s simply because of a lack of information.
Trainees shouldn’t have to suffer and be made to feel like outsiders until they figure out the unwritten rules of the place. They belong.
Dr. Lycette is medical director of Providence Oncology and Hematology Care Clinic, Seaside, Ore. She disclosed no relevant conflicts of interest. A version of this article first appeared on Medscape.com.
IV gentamicin improves junctional epidermolysis bullosa in children
Intravenous (JEB) caused by nonsense variants.
The newly generated structural protein persisted during the 3-month randomized clinical trial and was associated with significant wound closure – with no signs of ototoxic effects, nephrotoxic effects, or anti–laminin 332 autoantibody induction, investigators recently reported in JAMA Dermatology.
JEB is a rare, autosomal recessive disorder caused mainly by nonsense variants (i.e., mutations) in the LAMA3, LAMB3, or LAMC2 genes that encode laminin, resulting in widespread blisters and erosions of the skin. Current treatment is limited to supportive management and palliative care, and children with its severe subtype are likely to die within the first year of life.
“With data indicating a robust response to short-term gentamicin treatment and the marked stability of laminin 332, we envision that gentamicin could be delivered as a short-term pulse therapy every 2-3 months for patients with JEB caused by nonsense variants,” the researchers wrote.
Of the five patients, ages 3 months to 10 years, three received 7.5 mg/kg IV gentamicin daily for 14 days, and two received 10 mg/kg daily for 24 days at the University of Southern California, Los Angeles.
All had confirmed nonsense variants in LAMA3 or LAMB3 in one or two alleles, and all had minimal laminin 332 expression at baseline as determined by immunofluorescence. After treatment, each of the children had increased, sustained expression of laminin 332.
The researchers monitored three open wounds in each patient. By 1 month, seven of nine wounds in those receiving the lower-dose therapy and all of the wounds in those receiving the higher-dose therapy showed at least 50% closure. By 3 months, eight of nine wounds in the lower-dose group, and all wounds in the higher-dose group showed greater than 85% closure.
In an interview, senior investigators Mei Chen, PhD, professor of dermatology, and David T. Woodley, MD, professor and chair of dermatology, both at USC, emphasized laminin’s long half-life.“Once these skin structural proteins are generated at the dermal-epidermal junction, they are long-lasting structures, which means the therapy can be pulsed rather than continuously delivered, which can obviate some of the known side effects of the medication,” Dr. Woodley said.
Gentamicin, an aminoglycoside, works as a “read-through therapy,” inducing ribosomal read-through of premature termination codons (PTCs) caused by nonsense mutations. The read-through allows translation to proceed and full-length proteins to be generated.
Gentamicin read-through therapy is also being investigated for recessive dystrophic epidermolysis bullosa (RDEB) attributable to nonsense mutations. The culprit mutations in this form of EB occur in a gene that encodes collagen type VII alpha 1, which, like laminin, is responsible for dermal-epidermal adherence. A clinical trial of intravenous gentamicin for RDEB is ongoing at USC, Dr. Chen said.
EBS-MD case report
It may also have a role in treating epidermolysis bullosa simplex with muscular dystrophy (EBS-MD), according to investigators in Madrid. Their case report, published in JAMA Dermatology, details how two 14-day courses of infused gentamicin therapy were followed by re-expression of plectin in the skin for 4-5 months and mild improvement in symptoms in one patient, a woman in her 30s, with a homozygous nonsense variant in PLEC1.
In an editorial accompanying the two reports, Anna L. Bruckner, MD, MSCS, professor of dermatology, University of Colorado at Denver, Aurora, and colleagues expressed cautious optimism and said that additional research on the feasibility, possible cumulative toxic effects, risk of microbial resistance, and overall clinical relevance is needed.
Still, the “investigators should be applauded for taking advantage of a readily available systemic treatment to target cutaneous and extracutaneous symptoms of patients who have very limited treatment options at this time,” they wrote. While all forms of EB are considered orphan disorders, JEB and EBS-MD have received less research attention than RDEB.
The JEB study evaluated patients with clinical assessments/quality of life surveys and with a validated clinical score that considers skin and mucosae – the Epidermolysis Bullosa Disease Activity and Scarring Index (EBDASI). There were small positive changes in EBDASI scores, but data were incomplete and therefore difficult to interpret.
A “noteworthy” finding, the authors wrote, were improvements in emotions and functioning in two of the children who were eligible given their older ages for assessment with the Skindex-16 quality-of-life survey. The improvements suggest “potential psychosocial benefits” of the gentamicin therapy.
The JEB study was supported in part by grants from the EB Research Partnership and EB Medical Research Foundation and an award from the Congressionally Directed Medical Research Program. In addition to the grants, Dr. Woodley and Dr. Chen reported receiving personal fees from Phoenix Tissue Repair outside of the submitted work. For the EBS-MD case report, the authors reported no disclosures. Dr. Bruckner, corresponding author of the editorial, reported grants from several companies outside the submitted work.
Intravenous (JEB) caused by nonsense variants.
The newly generated structural protein persisted during the 3-month randomized clinical trial and was associated with significant wound closure – with no signs of ototoxic effects, nephrotoxic effects, or anti–laminin 332 autoantibody induction, investigators recently reported in JAMA Dermatology.
JEB is a rare, autosomal recessive disorder caused mainly by nonsense variants (i.e., mutations) in the LAMA3, LAMB3, or LAMC2 genes that encode laminin, resulting in widespread blisters and erosions of the skin. Current treatment is limited to supportive management and palliative care, and children with its severe subtype are likely to die within the first year of life.
“With data indicating a robust response to short-term gentamicin treatment and the marked stability of laminin 332, we envision that gentamicin could be delivered as a short-term pulse therapy every 2-3 months for patients with JEB caused by nonsense variants,” the researchers wrote.
Of the five patients, ages 3 months to 10 years, three received 7.5 mg/kg IV gentamicin daily for 14 days, and two received 10 mg/kg daily for 24 days at the University of Southern California, Los Angeles.
All had confirmed nonsense variants in LAMA3 or LAMB3 in one or two alleles, and all had minimal laminin 332 expression at baseline as determined by immunofluorescence. After treatment, each of the children had increased, sustained expression of laminin 332.
The researchers monitored three open wounds in each patient. By 1 month, seven of nine wounds in those receiving the lower-dose therapy and all of the wounds in those receiving the higher-dose therapy showed at least 50% closure. By 3 months, eight of nine wounds in the lower-dose group, and all wounds in the higher-dose group showed greater than 85% closure.
In an interview, senior investigators Mei Chen, PhD, professor of dermatology, and David T. Woodley, MD, professor and chair of dermatology, both at USC, emphasized laminin’s long half-life.“Once these skin structural proteins are generated at the dermal-epidermal junction, they are long-lasting structures, which means the therapy can be pulsed rather than continuously delivered, which can obviate some of the known side effects of the medication,” Dr. Woodley said.
Gentamicin, an aminoglycoside, works as a “read-through therapy,” inducing ribosomal read-through of premature termination codons (PTCs) caused by nonsense mutations. The read-through allows translation to proceed and full-length proteins to be generated.
Gentamicin read-through therapy is also being investigated for recessive dystrophic epidermolysis bullosa (RDEB) attributable to nonsense mutations. The culprit mutations in this form of EB occur in a gene that encodes collagen type VII alpha 1, which, like laminin, is responsible for dermal-epidermal adherence. A clinical trial of intravenous gentamicin for RDEB is ongoing at USC, Dr. Chen said.
EBS-MD case report
It may also have a role in treating epidermolysis bullosa simplex with muscular dystrophy (EBS-MD), according to investigators in Madrid. Their case report, published in JAMA Dermatology, details how two 14-day courses of infused gentamicin therapy were followed by re-expression of plectin in the skin for 4-5 months and mild improvement in symptoms in one patient, a woman in her 30s, with a homozygous nonsense variant in PLEC1.
In an editorial accompanying the two reports, Anna L. Bruckner, MD, MSCS, professor of dermatology, University of Colorado at Denver, Aurora, and colleagues expressed cautious optimism and said that additional research on the feasibility, possible cumulative toxic effects, risk of microbial resistance, and overall clinical relevance is needed.
Still, the “investigators should be applauded for taking advantage of a readily available systemic treatment to target cutaneous and extracutaneous symptoms of patients who have very limited treatment options at this time,” they wrote. While all forms of EB are considered orphan disorders, JEB and EBS-MD have received less research attention than RDEB.
The JEB study evaluated patients with clinical assessments/quality of life surveys and with a validated clinical score that considers skin and mucosae – the Epidermolysis Bullosa Disease Activity and Scarring Index (EBDASI). There were small positive changes in EBDASI scores, but data were incomplete and therefore difficult to interpret.
A “noteworthy” finding, the authors wrote, were improvements in emotions and functioning in two of the children who were eligible given their older ages for assessment with the Skindex-16 quality-of-life survey. The improvements suggest “potential psychosocial benefits” of the gentamicin therapy.
The JEB study was supported in part by grants from the EB Research Partnership and EB Medical Research Foundation and an award from the Congressionally Directed Medical Research Program. In addition to the grants, Dr. Woodley and Dr. Chen reported receiving personal fees from Phoenix Tissue Repair outside of the submitted work. For the EBS-MD case report, the authors reported no disclosures. Dr. Bruckner, corresponding author of the editorial, reported grants from several companies outside the submitted work.
Intravenous (JEB) caused by nonsense variants.
The newly generated structural protein persisted during the 3-month randomized clinical trial and was associated with significant wound closure – with no signs of ototoxic effects, nephrotoxic effects, or anti–laminin 332 autoantibody induction, investigators recently reported in JAMA Dermatology.
JEB is a rare, autosomal recessive disorder caused mainly by nonsense variants (i.e., mutations) in the LAMA3, LAMB3, or LAMC2 genes that encode laminin, resulting in widespread blisters and erosions of the skin. Current treatment is limited to supportive management and palliative care, and children with its severe subtype are likely to die within the first year of life.
“With data indicating a robust response to short-term gentamicin treatment and the marked stability of laminin 332, we envision that gentamicin could be delivered as a short-term pulse therapy every 2-3 months for patients with JEB caused by nonsense variants,” the researchers wrote.
Of the five patients, ages 3 months to 10 years, three received 7.5 mg/kg IV gentamicin daily for 14 days, and two received 10 mg/kg daily for 24 days at the University of Southern California, Los Angeles.
All had confirmed nonsense variants in LAMA3 or LAMB3 in one or two alleles, and all had minimal laminin 332 expression at baseline as determined by immunofluorescence. After treatment, each of the children had increased, sustained expression of laminin 332.
The researchers monitored three open wounds in each patient. By 1 month, seven of nine wounds in those receiving the lower-dose therapy and all of the wounds in those receiving the higher-dose therapy showed at least 50% closure. By 3 months, eight of nine wounds in the lower-dose group, and all wounds in the higher-dose group showed greater than 85% closure.
In an interview, senior investigators Mei Chen, PhD, professor of dermatology, and David T. Woodley, MD, professor and chair of dermatology, both at USC, emphasized laminin’s long half-life.“Once these skin structural proteins are generated at the dermal-epidermal junction, they are long-lasting structures, which means the therapy can be pulsed rather than continuously delivered, which can obviate some of the known side effects of the medication,” Dr. Woodley said.
Gentamicin, an aminoglycoside, works as a “read-through therapy,” inducing ribosomal read-through of premature termination codons (PTCs) caused by nonsense mutations. The read-through allows translation to proceed and full-length proteins to be generated.
Gentamicin read-through therapy is also being investigated for recessive dystrophic epidermolysis bullosa (RDEB) attributable to nonsense mutations. The culprit mutations in this form of EB occur in a gene that encodes collagen type VII alpha 1, which, like laminin, is responsible for dermal-epidermal adherence. A clinical trial of intravenous gentamicin for RDEB is ongoing at USC, Dr. Chen said.
EBS-MD case report
It may also have a role in treating epidermolysis bullosa simplex with muscular dystrophy (EBS-MD), according to investigators in Madrid. Their case report, published in JAMA Dermatology, details how two 14-day courses of infused gentamicin therapy were followed by re-expression of plectin in the skin for 4-5 months and mild improvement in symptoms in one patient, a woman in her 30s, with a homozygous nonsense variant in PLEC1.
In an editorial accompanying the two reports, Anna L. Bruckner, MD, MSCS, professor of dermatology, University of Colorado at Denver, Aurora, and colleagues expressed cautious optimism and said that additional research on the feasibility, possible cumulative toxic effects, risk of microbial resistance, and overall clinical relevance is needed.
Still, the “investigators should be applauded for taking advantage of a readily available systemic treatment to target cutaneous and extracutaneous symptoms of patients who have very limited treatment options at this time,” they wrote. While all forms of EB are considered orphan disorders, JEB and EBS-MD have received less research attention than RDEB.
The JEB study evaluated patients with clinical assessments/quality of life surveys and with a validated clinical score that considers skin and mucosae – the Epidermolysis Bullosa Disease Activity and Scarring Index (EBDASI). There were small positive changes in EBDASI scores, but data were incomplete and therefore difficult to interpret.
A “noteworthy” finding, the authors wrote, were improvements in emotions and functioning in two of the children who were eligible given their older ages for assessment with the Skindex-16 quality-of-life survey. The improvements suggest “potential psychosocial benefits” of the gentamicin therapy.
The JEB study was supported in part by grants from the EB Research Partnership and EB Medical Research Foundation and an award from the Congressionally Directed Medical Research Program. In addition to the grants, Dr. Woodley and Dr. Chen reported receiving personal fees from Phoenix Tissue Repair outside of the submitted work. For the EBS-MD case report, the authors reported no disclosures. Dr. Bruckner, corresponding author of the editorial, reported grants from several companies outside the submitted work.
FROM JAMA DERMATOLOGY
Ivermectin doesn’t help treat COVID-19, large study finds
according to results from a large clinical trial published in the New England Journal of Medicine.
The findings pretty much rule out the drug as a treatment for COVID-19, the study authors wrote.
“There’s really no sign of any benefit,” David Boulware, MD, one of the coauthors and an infectious disease specialist at the University of Minnesota, Minneapolis, told the New York Times.
The researchers shared a summary of the results in August 2021 during an online presentation hosted by the National Institutes of Health. The full data hadn’t been published until now.
“Now that people can dive into the details and the data, hopefully that will steer the majority of doctors away from ivermectin toward other therapies,” Dr. Boulware said.
In the trial, the research team compared more than 1,350 people infected with the coronavirus in Brazil who received either ivermectin or a placebo as treatment.
Between March and August 2021, 679 patients received a daily dose of ivermectin over the course of 3 days. The researchers found that ivermectin didn’t reduce the risk that people with COVID-19 would be hospitalized or go to an ED within 28 days after treatment.
In addition, the researchers looked at particular groups to understand if some patients benefited for some reason, such as taking ivermectin sooner after testing positive for COVID-19. But those who took the drug during the first 3 days after a positive coronavirus test ended up doing worse than those in the placebo group. The drug also didn’t help patients recover sooner.
The researchers found “no important effects” of treatment with ivermectin on the number of days people spent in the hospital, the number of days hospitalized people needed mechanical ventilation, or the risk of death.
Ivermectin has become a controversial focal point during the pandemic.
For decades, the drug has been widely used to treat parasitic infections. At the beginning of the pandemic, researchers checked thousands of existing drugs against the coronavirus to determine if a potential treatment already existed. Laboratory experiments on cells suggested that ivermectin might work, the New York Times reported.
But some researchers noted that the experiments worked because a high concentration of ivermectin was used, a much higher dose than would be safe for people. Despite the concerns, some doctors began prescribing ivermectin to patients. After receiving reports of people who needed medical attention, particularly after using formulations intended for livestock, the Food and Drug Administration issued a warning that the drug wasn’t approved to be used for COVID-19.
Researchers around the world have done small clinical trials to understand whether ivermectin treats COVID-19, the newspaper reported. At the end of 2020, Andrew Hill, MD, a virologist at the University of Liverpool in England, reviewed the results from 23 trials and concluded that the drug could lower the risk of death from COVID-19. He published the results in July 2021, but later reports found that many of the studies were flawed, and at least one was fraudulent.
Dr. Hill retracted his original study and began another analysis, which was published in January 2022. In this review, he and his colleagues focused on studies that were least likely to be biased. They found that ivermectin was not helpful.
Recently, Dr. Hill and associates ran another analysis using the new data from the Brazil trial, and once again they saw no benefit.
Several clinical trials are still testing ivermectin as a treatment, the New York Times reported, with results expected in upcoming months. After reviewing the data from the Brazil trial, which tested ivermectin and a variety of other drugs against COVID-19, some infectious disease experts say they’ll likely see more of the same – that ivermectin doesn’t help people with COVID-19.
“I welcome the results of the other clinical trials and will view them with an open mind,” Paul Sax, MD, an infectious disease expert at Brigham and Women’s Hospital, Boston, who has been watching the data on the drug throughout the pandemic, told the New York Times.
“But at some point, it will become a waste of resources to continue studying an unpromising approach,” he said.
A version of this article first appeared on WebMD.com.
according to results from a large clinical trial published in the New England Journal of Medicine.
The findings pretty much rule out the drug as a treatment for COVID-19, the study authors wrote.
“There’s really no sign of any benefit,” David Boulware, MD, one of the coauthors and an infectious disease specialist at the University of Minnesota, Minneapolis, told the New York Times.
The researchers shared a summary of the results in August 2021 during an online presentation hosted by the National Institutes of Health. The full data hadn’t been published until now.
“Now that people can dive into the details and the data, hopefully that will steer the majority of doctors away from ivermectin toward other therapies,” Dr. Boulware said.
In the trial, the research team compared more than 1,350 people infected with the coronavirus in Brazil who received either ivermectin or a placebo as treatment.
Between March and August 2021, 679 patients received a daily dose of ivermectin over the course of 3 days. The researchers found that ivermectin didn’t reduce the risk that people with COVID-19 would be hospitalized or go to an ED within 28 days after treatment.
In addition, the researchers looked at particular groups to understand if some patients benefited for some reason, such as taking ivermectin sooner after testing positive for COVID-19. But those who took the drug during the first 3 days after a positive coronavirus test ended up doing worse than those in the placebo group. The drug also didn’t help patients recover sooner.
The researchers found “no important effects” of treatment with ivermectin on the number of days people spent in the hospital, the number of days hospitalized people needed mechanical ventilation, or the risk of death.
Ivermectin has become a controversial focal point during the pandemic.
For decades, the drug has been widely used to treat parasitic infections. At the beginning of the pandemic, researchers checked thousands of existing drugs against the coronavirus to determine if a potential treatment already existed. Laboratory experiments on cells suggested that ivermectin might work, the New York Times reported.
But some researchers noted that the experiments worked because a high concentration of ivermectin was used, a much higher dose than would be safe for people. Despite the concerns, some doctors began prescribing ivermectin to patients. After receiving reports of people who needed medical attention, particularly after using formulations intended for livestock, the Food and Drug Administration issued a warning that the drug wasn’t approved to be used for COVID-19.
Researchers around the world have done small clinical trials to understand whether ivermectin treats COVID-19, the newspaper reported. At the end of 2020, Andrew Hill, MD, a virologist at the University of Liverpool in England, reviewed the results from 23 trials and concluded that the drug could lower the risk of death from COVID-19. He published the results in July 2021, but later reports found that many of the studies were flawed, and at least one was fraudulent.
Dr. Hill retracted his original study and began another analysis, which was published in January 2022. In this review, he and his colleagues focused on studies that were least likely to be biased. They found that ivermectin was not helpful.
Recently, Dr. Hill and associates ran another analysis using the new data from the Brazil trial, and once again they saw no benefit.
Several clinical trials are still testing ivermectin as a treatment, the New York Times reported, with results expected in upcoming months. After reviewing the data from the Brazil trial, which tested ivermectin and a variety of other drugs against COVID-19, some infectious disease experts say they’ll likely see more of the same – that ivermectin doesn’t help people with COVID-19.
“I welcome the results of the other clinical trials and will view them with an open mind,” Paul Sax, MD, an infectious disease expert at Brigham and Women’s Hospital, Boston, who has been watching the data on the drug throughout the pandemic, told the New York Times.
“But at some point, it will become a waste of resources to continue studying an unpromising approach,” he said.
A version of this article first appeared on WebMD.com.
according to results from a large clinical trial published in the New England Journal of Medicine.
The findings pretty much rule out the drug as a treatment for COVID-19, the study authors wrote.
“There’s really no sign of any benefit,” David Boulware, MD, one of the coauthors and an infectious disease specialist at the University of Minnesota, Minneapolis, told the New York Times.
The researchers shared a summary of the results in August 2021 during an online presentation hosted by the National Institutes of Health. The full data hadn’t been published until now.
“Now that people can dive into the details and the data, hopefully that will steer the majority of doctors away from ivermectin toward other therapies,” Dr. Boulware said.
In the trial, the research team compared more than 1,350 people infected with the coronavirus in Brazil who received either ivermectin or a placebo as treatment.
Between March and August 2021, 679 patients received a daily dose of ivermectin over the course of 3 days. The researchers found that ivermectin didn’t reduce the risk that people with COVID-19 would be hospitalized or go to an ED within 28 days after treatment.
In addition, the researchers looked at particular groups to understand if some patients benefited for some reason, such as taking ivermectin sooner after testing positive for COVID-19. But those who took the drug during the first 3 days after a positive coronavirus test ended up doing worse than those in the placebo group. The drug also didn’t help patients recover sooner.
The researchers found “no important effects” of treatment with ivermectin on the number of days people spent in the hospital, the number of days hospitalized people needed mechanical ventilation, or the risk of death.
Ivermectin has become a controversial focal point during the pandemic.
For decades, the drug has been widely used to treat parasitic infections. At the beginning of the pandemic, researchers checked thousands of existing drugs against the coronavirus to determine if a potential treatment already existed. Laboratory experiments on cells suggested that ivermectin might work, the New York Times reported.
But some researchers noted that the experiments worked because a high concentration of ivermectin was used, a much higher dose than would be safe for people. Despite the concerns, some doctors began prescribing ivermectin to patients. After receiving reports of people who needed medical attention, particularly after using formulations intended for livestock, the Food and Drug Administration issued a warning that the drug wasn’t approved to be used for COVID-19.
Researchers around the world have done small clinical trials to understand whether ivermectin treats COVID-19, the newspaper reported. At the end of 2020, Andrew Hill, MD, a virologist at the University of Liverpool in England, reviewed the results from 23 trials and concluded that the drug could lower the risk of death from COVID-19. He published the results in July 2021, but later reports found that many of the studies were flawed, and at least one was fraudulent.
Dr. Hill retracted his original study and began another analysis, which was published in January 2022. In this review, he and his colleagues focused on studies that were least likely to be biased. They found that ivermectin was not helpful.
Recently, Dr. Hill and associates ran another analysis using the new data from the Brazil trial, and once again they saw no benefit.
Several clinical trials are still testing ivermectin as a treatment, the New York Times reported, with results expected in upcoming months. After reviewing the data from the Brazil trial, which tested ivermectin and a variety of other drugs against COVID-19, some infectious disease experts say they’ll likely see more of the same – that ivermectin doesn’t help people with COVID-19.
“I welcome the results of the other clinical trials and will view them with an open mind,” Paul Sax, MD, an infectious disease expert at Brigham and Women’s Hospital, Boston, who has been watching the data on the drug throughout the pandemic, told the New York Times.
“But at some point, it will become a waste of resources to continue studying an unpromising approach,” he said.
A version of this article first appeared on WebMD.com.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
For pemphigus, rituximab is first line, expert says
BOSTON – . This drug is more rapidly effective, more likely to provide sustained remission, better tolerated, and lowers health care costs, according to an expert summary at the annual meeting of the American Academy of Dermatology.
With rituximab “we are not only able to offer better efficacy, earlier and longer remissions, less side effects, less risk of relapse after a response, but it is actually cheaper,” reported Erin X. Wei, MD, director of the Bullous Diseases Clinic at Brigham and Women’s Hospital, Boston.
There are many treatments that reduce the inflammatory component of pemphigus. Corticosteroids, doxycycline, mycophenolate mofetil, azathioprine, and methotrexate are among those options commonly considered in the early control of this rare and potentially fatal autoimmune blistering disease of the skin, mouth, and other tissues.
Not all of these options have been compared directly in controlled trials, but Dr. Wei indicated that the preponderance of evidence is now on the side of rituximab as a first-line choice. For example, in the multicenter Ritux 3 trial, which compared a tapered regimen of prednisone alone to rituximab combined with a shorter and lower-dose prednisone taper in patients with pemphigus, complete response rates off therapy at 2 years were 89% in the rituximab group versus 34% in the group that received prednisone alone.
“This was quite a remarkable difference,” said Dr. Wei, who noted that remissions overall occurred faster in the rituximab group and were more durable once achieved.
No other treatment option has demonstrated this degree of relative benefit over corticosteroids, according to Dr. Wei. She said there is evidence that mycophenolate mofetil acts more rapidly, but it has not been shown to be superior for sustained complete response. Nor has azathioprine provided a clear advantage over steroids. There are no well-conducted comparisons of methotrexate and prednisone, according to Dr. Wei, assistant professor at Harvard Medical School, Boston.
Corticosteroids, doxycycline, and immunomodulators have been characterized as mainstays of early treatment in pemphigus, but Dr. Wei argued that the evidence supports starting with the most effective therapy first. There are many advantages to suppressing disease activity “as soon as possible” after diagnosis.
Early control “is associated with a more sustained remission, lower overall steroid use, and better quality of life,” said Dr. Wei, listing the hazards of starting with less effective therapy, and explaining why she has moved to rituximab as a first-line choice. According to her, there are data to support these advantages.
“Several studies have observed that rituximab, within the first 6 months of disease onset, is associated with a higher rate of complete response and a longer duration of complete response,” Dr. Wei said.
Intravenous immunoglobulin (IVIG) therapy is effective in many patients but less reliable, and it has other disadvantages relative to rituximab as a first-line therapy.
“IVIG in pemphigus works quickly when it works, but it is more expensive and it is more of an ongoing therapy relative to rituximab,” said Dr. Wei, referring to the lower likelihood of IVIG to provide sustained remissions.
The price of rituximab is high relative to prednisone or other immunomodulators, but management costs are ultimately reduced because of better disease control, according to Dr. Wei. She cited a Canadian study published several years ago in which health care costs in the 6 months prior to rituximab were compared to costs over 6 months after it was initiated.
In this cohort of 89 patients with pemphigus or pemphigoid, the average cost per patient for 6 months of care prior to starting rituximab was $42,231 in Canadian dollars. After treatment was started, the cost fell to $29,423, a 30% reduction, over the next 6 months.
“It takes rituximab up to 3 months or sometimes even longer to achieve its greatest benefit, making these results even more impressive,” Dr. Wei said.
The activity of rituximab to suppress autoreactive B-cells can be monitored with antidesmoglein autoantibody levels and by measuring CD20-positive cell percentages. Unlike severity of disease at baseline, which Dr. Wei said is not a reliable predictor of relapse risk, these can guide steroid tapering.
“If the patient is not making new autoantibodies, then tapering steroids can be considered safe,” Dr. Wei said.
One small case series cited by Dr. Wei has suggested that rituximab might be effectively employed as a maintenance therapy for pemphigus. The maintenance treatment, which initially consisted of 1 g of rituximab every 6 months, was evaluated in 11 patients with a history of severe and frequent relapses.
In this group, rituximab was first employed to achieve a complete response. The maintenance was initiated when patients were in remission. In some patients, the maintenance dose interval was extended to once every 12 months over time. During a mean follow-up of 4 years, all 11 patients remained in complete remission.
“This was a remarkable result,” said Dr. Wei, who noted that there were no serious adverse events associated with rituximab maintenance over this period. This cannot be considered a routine strategy without a large patient experience, according to Dr. Wei, but it does provide another piece of evidence that rituximab is effective and well tolerated.
There are no guidelines from a major organization that establish an evidence-based treatment algorithm for pemphigus, but Dr. Wei is not alone in considering early initiation of the most effective therapy as the best approach to sustained control.
“I agree that rituximab is a good first-line option for pemphigus patients,” said Kara Heelan, MBBCh, MD, a consultant dermatologist at the Royal Marsden and Lister Hospital, London. She was the first author of the cost-effectiveness study that Dr. Wei cited. The study was published when she was an associate in the division of dermatology at the University of Toronto.
By calling rituximab “a good” option rather than a potential standard, Dr. Heelan appeared to be more circumspect than Dr. Wei about its central role in the care of pemphigus, but she did agree in an interview that this agent “has been shown to be cost-effective.” In her study, this was an advantage attributed to relative efficacy and safety that reduced use of health care resources.
A version of this article first appeared on Medscape.com.
BOSTON – . This drug is more rapidly effective, more likely to provide sustained remission, better tolerated, and lowers health care costs, according to an expert summary at the annual meeting of the American Academy of Dermatology.
With rituximab “we are not only able to offer better efficacy, earlier and longer remissions, less side effects, less risk of relapse after a response, but it is actually cheaper,” reported Erin X. Wei, MD, director of the Bullous Diseases Clinic at Brigham and Women’s Hospital, Boston.
There are many treatments that reduce the inflammatory component of pemphigus. Corticosteroids, doxycycline, mycophenolate mofetil, azathioprine, and methotrexate are among those options commonly considered in the early control of this rare and potentially fatal autoimmune blistering disease of the skin, mouth, and other tissues.
Not all of these options have been compared directly in controlled trials, but Dr. Wei indicated that the preponderance of evidence is now on the side of rituximab as a first-line choice. For example, in the multicenter Ritux 3 trial, which compared a tapered regimen of prednisone alone to rituximab combined with a shorter and lower-dose prednisone taper in patients with pemphigus, complete response rates off therapy at 2 years were 89% in the rituximab group versus 34% in the group that received prednisone alone.
“This was quite a remarkable difference,” said Dr. Wei, who noted that remissions overall occurred faster in the rituximab group and were more durable once achieved.
No other treatment option has demonstrated this degree of relative benefit over corticosteroids, according to Dr. Wei. She said there is evidence that mycophenolate mofetil acts more rapidly, but it has not been shown to be superior for sustained complete response. Nor has azathioprine provided a clear advantage over steroids. There are no well-conducted comparisons of methotrexate and prednisone, according to Dr. Wei, assistant professor at Harvard Medical School, Boston.
Corticosteroids, doxycycline, and immunomodulators have been characterized as mainstays of early treatment in pemphigus, but Dr. Wei argued that the evidence supports starting with the most effective therapy first. There are many advantages to suppressing disease activity “as soon as possible” after diagnosis.
Early control “is associated with a more sustained remission, lower overall steroid use, and better quality of life,” said Dr. Wei, listing the hazards of starting with less effective therapy, and explaining why she has moved to rituximab as a first-line choice. According to her, there are data to support these advantages.
“Several studies have observed that rituximab, within the first 6 months of disease onset, is associated with a higher rate of complete response and a longer duration of complete response,” Dr. Wei said.
Intravenous immunoglobulin (IVIG) therapy is effective in many patients but less reliable, and it has other disadvantages relative to rituximab as a first-line therapy.
“IVIG in pemphigus works quickly when it works, but it is more expensive and it is more of an ongoing therapy relative to rituximab,” said Dr. Wei, referring to the lower likelihood of IVIG to provide sustained remissions.
The price of rituximab is high relative to prednisone or other immunomodulators, but management costs are ultimately reduced because of better disease control, according to Dr. Wei. She cited a Canadian study published several years ago in which health care costs in the 6 months prior to rituximab were compared to costs over 6 months after it was initiated.
In this cohort of 89 patients with pemphigus or pemphigoid, the average cost per patient for 6 months of care prior to starting rituximab was $42,231 in Canadian dollars. After treatment was started, the cost fell to $29,423, a 30% reduction, over the next 6 months.
“It takes rituximab up to 3 months or sometimes even longer to achieve its greatest benefit, making these results even more impressive,” Dr. Wei said.
The activity of rituximab to suppress autoreactive B-cells can be monitored with antidesmoglein autoantibody levels and by measuring CD20-positive cell percentages. Unlike severity of disease at baseline, which Dr. Wei said is not a reliable predictor of relapse risk, these can guide steroid tapering.
“If the patient is not making new autoantibodies, then tapering steroids can be considered safe,” Dr. Wei said.
One small case series cited by Dr. Wei has suggested that rituximab might be effectively employed as a maintenance therapy for pemphigus. The maintenance treatment, which initially consisted of 1 g of rituximab every 6 months, was evaluated in 11 patients with a history of severe and frequent relapses.
In this group, rituximab was first employed to achieve a complete response. The maintenance was initiated when patients were in remission. In some patients, the maintenance dose interval was extended to once every 12 months over time. During a mean follow-up of 4 years, all 11 patients remained in complete remission.
“This was a remarkable result,” said Dr. Wei, who noted that there were no serious adverse events associated with rituximab maintenance over this period. This cannot be considered a routine strategy without a large patient experience, according to Dr. Wei, but it does provide another piece of evidence that rituximab is effective and well tolerated.
There are no guidelines from a major organization that establish an evidence-based treatment algorithm for pemphigus, but Dr. Wei is not alone in considering early initiation of the most effective therapy as the best approach to sustained control.
“I agree that rituximab is a good first-line option for pemphigus patients,” said Kara Heelan, MBBCh, MD, a consultant dermatologist at the Royal Marsden and Lister Hospital, London. She was the first author of the cost-effectiveness study that Dr. Wei cited. The study was published when she was an associate in the division of dermatology at the University of Toronto.
By calling rituximab “a good” option rather than a potential standard, Dr. Heelan appeared to be more circumspect than Dr. Wei about its central role in the care of pemphigus, but she did agree in an interview that this agent “has been shown to be cost-effective.” In her study, this was an advantage attributed to relative efficacy and safety that reduced use of health care resources.
A version of this article first appeared on Medscape.com.
BOSTON – . This drug is more rapidly effective, more likely to provide sustained remission, better tolerated, and lowers health care costs, according to an expert summary at the annual meeting of the American Academy of Dermatology.
With rituximab “we are not only able to offer better efficacy, earlier and longer remissions, less side effects, less risk of relapse after a response, but it is actually cheaper,” reported Erin X. Wei, MD, director of the Bullous Diseases Clinic at Brigham and Women’s Hospital, Boston.
There are many treatments that reduce the inflammatory component of pemphigus. Corticosteroids, doxycycline, mycophenolate mofetil, azathioprine, and methotrexate are among those options commonly considered in the early control of this rare and potentially fatal autoimmune blistering disease of the skin, mouth, and other tissues.
Not all of these options have been compared directly in controlled trials, but Dr. Wei indicated that the preponderance of evidence is now on the side of rituximab as a first-line choice. For example, in the multicenter Ritux 3 trial, which compared a tapered regimen of prednisone alone to rituximab combined with a shorter and lower-dose prednisone taper in patients with pemphigus, complete response rates off therapy at 2 years were 89% in the rituximab group versus 34% in the group that received prednisone alone.
“This was quite a remarkable difference,” said Dr. Wei, who noted that remissions overall occurred faster in the rituximab group and were more durable once achieved.
No other treatment option has demonstrated this degree of relative benefit over corticosteroids, according to Dr. Wei. She said there is evidence that mycophenolate mofetil acts more rapidly, but it has not been shown to be superior for sustained complete response. Nor has azathioprine provided a clear advantage over steroids. There are no well-conducted comparisons of methotrexate and prednisone, according to Dr. Wei, assistant professor at Harvard Medical School, Boston.
Corticosteroids, doxycycline, and immunomodulators have been characterized as mainstays of early treatment in pemphigus, but Dr. Wei argued that the evidence supports starting with the most effective therapy first. There are many advantages to suppressing disease activity “as soon as possible” after diagnosis.
Early control “is associated with a more sustained remission, lower overall steroid use, and better quality of life,” said Dr. Wei, listing the hazards of starting with less effective therapy, and explaining why she has moved to rituximab as a first-line choice. According to her, there are data to support these advantages.
“Several studies have observed that rituximab, within the first 6 months of disease onset, is associated with a higher rate of complete response and a longer duration of complete response,” Dr. Wei said.
Intravenous immunoglobulin (IVIG) therapy is effective in many patients but less reliable, and it has other disadvantages relative to rituximab as a first-line therapy.
“IVIG in pemphigus works quickly when it works, but it is more expensive and it is more of an ongoing therapy relative to rituximab,” said Dr. Wei, referring to the lower likelihood of IVIG to provide sustained remissions.
The price of rituximab is high relative to prednisone or other immunomodulators, but management costs are ultimately reduced because of better disease control, according to Dr. Wei. She cited a Canadian study published several years ago in which health care costs in the 6 months prior to rituximab were compared to costs over 6 months after it was initiated.
In this cohort of 89 patients with pemphigus or pemphigoid, the average cost per patient for 6 months of care prior to starting rituximab was $42,231 in Canadian dollars. After treatment was started, the cost fell to $29,423, a 30% reduction, over the next 6 months.
“It takes rituximab up to 3 months or sometimes even longer to achieve its greatest benefit, making these results even more impressive,” Dr. Wei said.
The activity of rituximab to suppress autoreactive B-cells can be monitored with antidesmoglein autoantibody levels and by measuring CD20-positive cell percentages. Unlike severity of disease at baseline, which Dr. Wei said is not a reliable predictor of relapse risk, these can guide steroid tapering.
“If the patient is not making new autoantibodies, then tapering steroids can be considered safe,” Dr. Wei said.
One small case series cited by Dr. Wei has suggested that rituximab might be effectively employed as a maintenance therapy for pemphigus. The maintenance treatment, which initially consisted of 1 g of rituximab every 6 months, was evaluated in 11 patients with a history of severe and frequent relapses.
In this group, rituximab was first employed to achieve a complete response. The maintenance was initiated when patients were in remission. In some patients, the maintenance dose interval was extended to once every 12 months over time. During a mean follow-up of 4 years, all 11 patients remained in complete remission.
“This was a remarkable result,” said Dr. Wei, who noted that there were no serious adverse events associated with rituximab maintenance over this period. This cannot be considered a routine strategy without a large patient experience, according to Dr. Wei, but it does provide another piece of evidence that rituximab is effective and well tolerated.
There are no guidelines from a major organization that establish an evidence-based treatment algorithm for pemphigus, but Dr. Wei is not alone in considering early initiation of the most effective therapy as the best approach to sustained control.
“I agree that rituximab is a good first-line option for pemphigus patients,” said Kara Heelan, MBBCh, MD, a consultant dermatologist at the Royal Marsden and Lister Hospital, London. She was the first author of the cost-effectiveness study that Dr. Wei cited. The study was published when she was an associate in the division of dermatology at the University of Toronto.
By calling rituximab “a good” option rather than a potential standard, Dr. Heelan appeared to be more circumspect than Dr. Wei about its central role in the care of pemphigus, but she did agree in an interview that this agent “has been shown to be cost-effective.” In her study, this was an advantage attributed to relative efficacy and safety that reduced use of health care resources.
A version of this article first appeared on Medscape.com.
AT AAD 2022
Dupilumab treats itch and clears lesions in prurigo nodularis patients
BOSTON – of treatment, in a phase 3 trial presented at the American Academy of Dermatology 2022 Annual Meeting.
There are currently no Food and Drug Administration–approved systemic therapies for PN. Although several treatments for the disease are used off label for the condition, such as ultraviolet light therapy and immunosuppressive agents, moderate to severe PN is usually difficult to control, noted Gil Yosipovitch, MD, director of the Miami Itch Center at the University of Miami Miller School of Medicine, Florida. He led the research and presented the findings at the conference.
“Many dermatologists feel very uncomfortable dealing with these patients because they suffer from chronicity, they are miserable, and previously, the drugs didn’t work well,” Dr. Yosipovitch told this news organization. The results from this trial “are very promising,” he said. “It opens a new field of treatment for itchy conditions.”
The trial, named LIBERTY-PN PRIME2, enrolled patients aged 18-80 who had been living with PN for at least 3 months. Patients had at least 20 lesions at baseline as well as severe itch, defined as a score of 7 or greater on the Worst Itch Numerical Rating Scale (WI-NRS). The scale ranges from 0 (no itch) to 10 (worst itch imaginable). Participants also had a history of treatment failure with medium to super-potent topical corticosteroids (TCSs), or treatment with TCSs was not medically advisable for them.
The randomized, double-blinded study enrolled 160 adults with PN. Of those, 78 were assigned to the treatment arm and received a 600-mg loading dose of dupilumab, administered subcutaneously, followed by 300-mg doses every 2 weeks for 24 weeks; 82 patients were allocated to receive placebo.
During the study, 25 patients in the placebo arm discontinued treatment. In the treatment arm, one patient was not treated and two discontinued treatment due to lack of efficacy.
The primary endpoint of the study was a reduction of at least 4 points on the WI-NRS at 12 weeks. Secondary endpoints included at least a 4-point WI-NRS reduction at 24 weeks and clear to nearly clear skin, defined as having a score of 0 or 1 on the Investigator’s Global Assessment PN-Stage (IGN PN-S). The scale ranges from 0 (clear) to 4 (severe).
At 12 weeks, 37.2% of patients given dupilumab reported a reduction of at least 4 points in WI-NRS, compared with 22.0% of patients given placebo (P = .0216). By 24 weeks, 57.7% of adults who received dupilumab achieved a greater than or equal to 4-point reduction in WI-NRS, compared with 19.5% of those who received placebo (P < .0001). Additionally, 44.9% of participants in the treatment arm achieved a score of 0 or 1 on the IGA PN-S, compared with 15.9% of those in the placebo arm (P < .0001).
Forty-four participants who received dupilumab (57.1%) and 42 participants who received placebo (51.2%) reported at least one treatment-emergent adverse event (TEAE) during the study, though none of these events were serious. The most common TEAE in the study was headache, occurring in five patients taking placebo and four patients receiving dupilumab. In the dupilumab group, there were five cases of herpes virus infection, four non-herpes skin infections, and three cases of conjunctivitis. In the placebo group, seven non-herpes skin infections were reported.
Sanofi and Regeneron, who jointly developed dupilumab, plan to file for regulatory approval for dupilumab for PN “around the world” in the first half of this year, according to a press release.
“It’s great news and a step in the right direction,” Sarina Elmariah, MD, PhD, a dermatologist at Massachusetts General Hospital and instructor of dermatology at Harvard Medical School, both in Boston, told this news organization. She was not involved with the research.
“We’re finally starting to shed light on this condition and its pathogenesis,” she said. She noted that other potential therapeutics for PN are also in development. “It’s reflective of the fact that we are making strides in this area.”
Sanofi and Regeneron Pharmaceuticals sponsored the LIBERTY-PN PRIME2 trial. Dr. Yosipovitch has reported financial relationships with Bellus Health, Eli Lilly, Galderma, GSK, Kiniksa Pharmaceuticals, LEO Pharma, Novartis, Pfizer, Regeneron, Sanofi, and Trevi Therapeutics. Dr. Elmariah is on the advisory boards of Sanofi, Galderma, and Trevi Therapeutics.
A version of this article first appeared on Medscape.com.
BOSTON – of treatment, in a phase 3 trial presented at the American Academy of Dermatology 2022 Annual Meeting.
There are currently no Food and Drug Administration–approved systemic therapies for PN. Although several treatments for the disease are used off label for the condition, such as ultraviolet light therapy and immunosuppressive agents, moderate to severe PN is usually difficult to control, noted Gil Yosipovitch, MD, director of the Miami Itch Center at the University of Miami Miller School of Medicine, Florida. He led the research and presented the findings at the conference.
“Many dermatologists feel very uncomfortable dealing with these patients because they suffer from chronicity, they are miserable, and previously, the drugs didn’t work well,” Dr. Yosipovitch told this news organization. The results from this trial “are very promising,” he said. “It opens a new field of treatment for itchy conditions.”
The trial, named LIBERTY-PN PRIME2, enrolled patients aged 18-80 who had been living with PN for at least 3 months. Patients had at least 20 lesions at baseline as well as severe itch, defined as a score of 7 or greater on the Worst Itch Numerical Rating Scale (WI-NRS). The scale ranges from 0 (no itch) to 10 (worst itch imaginable). Participants also had a history of treatment failure with medium to super-potent topical corticosteroids (TCSs), or treatment with TCSs was not medically advisable for them.
The randomized, double-blinded study enrolled 160 adults with PN. Of those, 78 were assigned to the treatment arm and received a 600-mg loading dose of dupilumab, administered subcutaneously, followed by 300-mg doses every 2 weeks for 24 weeks; 82 patients were allocated to receive placebo.
During the study, 25 patients in the placebo arm discontinued treatment. In the treatment arm, one patient was not treated and two discontinued treatment due to lack of efficacy.
The primary endpoint of the study was a reduction of at least 4 points on the WI-NRS at 12 weeks. Secondary endpoints included at least a 4-point WI-NRS reduction at 24 weeks and clear to nearly clear skin, defined as having a score of 0 or 1 on the Investigator’s Global Assessment PN-Stage (IGN PN-S). The scale ranges from 0 (clear) to 4 (severe).
At 12 weeks, 37.2% of patients given dupilumab reported a reduction of at least 4 points in WI-NRS, compared with 22.0% of patients given placebo (P = .0216). By 24 weeks, 57.7% of adults who received dupilumab achieved a greater than or equal to 4-point reduction in WI-NRS, compared with 19.5% of those who received placebo (P < .0001). Additionally, 44.9% of participants in the treatment arm achieved a score of 0 or 1 on the IGA PN-S, compared with 15.9% of those in the placebo arm (P < .0001).
Forty-four participants who received dupilumab (57.1%) and 42 participants who received placebo (51.2%) reported at least one treatment-emergent adverse event (TEAE) during the study, though none of these events were serious. The most common TEAE in the study was headache, occurring in five patients taking placebo and four patients receiving dupilumab. In the dupilumab group, there were five cases of herpes virus infection, four non-herpes skin infections, and three cases of conjunctivitis. In the placebo group, seven non-herpes skin infections were reported.
Sanofi and Regeneron, who jointly developed dupilumab, plan to file for regulatory approval for dupilumab for PN “around the world” in the first half of this year, according to a press release.
“It’s great news and a step in the right direction,” Sarina Elmariah, MD, PhD, a dermatologist at Massachusetts General Hospital and instructor of dermatology at Harvard Medical School, both in Boston, told this news organization. She was not involved with the research.
“We’re finally starting to shed light on this condition and its pathogenesis,” she said. She noted that other potential therapeutics for PN are also in development. “It’s reflective of the fact that we are making strides in this area.”
Sanofi and Regeneron Pharmaceuticals sponsored the LIBERTY-PN PRIME2 trial. Dr. Yosipovitch has reported financial relationships with Bellus Health, Eli Lilly, Galderma, GSK, Kiniksa Pharmaceuticals, LEO Pharma, Novartis, Pfizer, Regeneron, Sanofi, and Trevi Therapeutics. Dr. Elmariah is on the advisory boards of Sanofi, Galderma, and Trevi Therapeutics.
A version of this article first appeared on Medscape.com.
BOSTON – of treatment, in a phase 3 trial presented at the American Academy of Dermatology 2022 Annual Meeting.
There are currently no Food and Drug Administration–approved systemic therapies for PN. Although several treatments for the disease are used off label for the condition, such as ultraviolet light therapy and immunosuppressive agents, moderate to severe PN is usually difficult to control, noted Gil Yosipovitch, MD, director of the Miami Itch Center at the University of Miami Miller School of Medicine, Florida. He led the research and presented the findings at the conference.
“Many dermatologists feel very uncomfortable dealing with these patients because they suffer from chronicity, they are miserable, and previously, the drugs didn’t work well,” Dr. Yosipovitch told this news organization. The results from this trial “are very promising,” he said. “It opens a new field of treatment for itchy conditions.”
The trial, named LIBERTY-PN PRIME2, enrolled patients aged 18-80 who had been living with PN for at least 3 months. Patients had at least 20 lesions at baseline as well as severe itch, defined as a score of 7 or greater on the Worst Itch Numerical Rating Scale (WI-NRS). The scale ranges from 0 (no itch) to 10 (worst itch imaginable). Participants also had a history of treatment failure with medium to super-potent topical corticosteroids (TCSs), or treatment with TCSs was not medically advisable for them.
The randomized, double-blinded study enrolled 160 adults with PN. Of those, 78 were assigned to the treatment arm and received a 600-mg loading dose of dupilumab, administered subcutaneously, followed by 300-mg doses every 2 weeks for 24 weeks; 82 patients were allocated to receive placebo.
During the study, 25 patients in the placebo arm discontinued treatment. In the treatment arm, one patient was not treated and two discontinued treatment due to lack of efficacy.
The primary endpoint of the study was a reduction of at least 4 points on the WI-NRS at 12 weeks. Secondary endpoints included at least a 4-point WI-NRS reduction at 24 weeks and clear to nearly clear skin, defined as having a score of 0 or 1 on the Investigator’s Global Assessment PN-Stage (IGN PN-S). The scale ranges from 0 (clear) to 4 (severe).
At 12 weeks, 37.2% of patients given dupilumab reported a reduction of at least 4 points in WI-NRS, compared with 22.0% of patients given placebo (P = .0216). By 24 weeks, 57.7% of adults who received dupilumab achieved a greater than or equal to 4-point reduction in WI-NRS, compared with 19.5% of those who received placebo (P < .0001). Additionally, 44.9% of participants in the treatment arm achieved a score of 0 or 1 on the IGA PN-S, compared with 15.9% of those in the placebo arm (P < .0001).
Forty-four participants who received dupilumab (57.1%) and 42 participants who received placebo (51.2%) reported at least one treatment-emergent adverse event (TEAE) during the study, though none of these events were serious. The most common TEAE in the study was headache, occurring in five patients taking placebo and four patients receiving dupilumab. In the dupilumab group, there were five cases of herpes virus infection, four non-herpes skin infections, and three cases of conjunctivitis. In the placebo group, seven non-herpes skin infections were reported.
Sanofi and Regeneron, who jointly developed dupilumab, plan to file for regulatory approval for dupilumab for PN “around the world” in the first half of this year, according to a press release.
“It’s great news and a step in the right direction,” Sarina Elmariah, MD, PhD, a dermatologist at Massachusetts General Hospital and instructor of dermatology at Harvard Medical School, both in Boston, told this news organization. She was not involved with the research.
“We’re finally starting to shed light on this condition and its pathogenesis,” she said. She noted that other potential therapeutics for PN are also in development. “It’s reflective of the fact that we are making strides in this area.”
Sanofi and Regeneron Pharmaceuticals sponsored the LIBERTY-PN PRIME2 trial. Dr. Yosipovitch has reported financial relationships with Bellus Health, Eli Lilly, Galderma, GSK, Kiniksa Pharmaceuticals, LEO Pharma, Novartis, Pfizer, Regeneron, Sanofi, and Trevi Therapeutics. Dr. Elmariah is on the advisory boards of Sanofi, Galderma, and Trevi Therapeutics.
A version of this article first appeared on Medscape.com.
AT AAD 2022
Skin reactions to first COVID-19 vaccine don’t justify forgoing second dose
BOSTON – Requests for a according to an analysis of several large sets of data presented at the annual meeting of the American Academy of Dermatology.
According to the data, “there are no serious adverse consequences from these cutaneous reactions,” said Esther Freeman, MD, PhD, director of Global Health Dermatology, Massachusetts General Hospital, Boston.
This is important because the risk of vaccine hesitancy goes up dramatically in patients who experience reactions to the first vaccine dose, according to follow-up of more than 50,000 employees vaccinated in the Mass General Brigham Healthcare System (MGBHS). According to Dr. Freeman, there was almost a fourfold increase in the rate of second-dose refusals for those with cutaneous reactions and a more than fourfold increase in those who developed angioedema.
Before the data were available, skin reactions were a source of concern among dermatologists and others involved in monitoring vaccine-related adverse events. Injection site reactions (ISRs) are associated with essentially every injectable vaccine, so these were expected, but a small proportion of patients developed large red plaques in the injection arm 7-8 days after the inoculation.
“These delayed reactions caused a lot of initial panic,” said Dr. Freeman, who counted herself among those alarmed about what the reactions might signify. “Was this cellulitis? Would the next dose cause anaphylaxis? We were concerned.”
This concern dissipated with the availability of more data. In a global registry that has so far captured more than 1,000 cutaneous reactions from 52 participating countries, it appears that about 2% of patients have a cutaneous reaction other than an ISR after the first dose. All resolve with minimal skin care or no treatment.
After the second dose, the proportion is lower. If there is a reaction, it typically occurs earlier and resolves more quickly.
“What we have learned is that fewer than half of patients who had a reaction to the first dose have a reaction to the second, and those who did have a reaction had a milder course,” said Dr. Freeman.
These data are “incredibly reassuring” on many levels, she explained. In addition, it allows clinicians to confidently explain to patients that there are no serious sequelae from the rashes, whether immediate or delayed, from the available COVID-19 vaccines.
“Every skin reaction I have seen is something we can treat through,” she added, noting that most reactions resolve with little or no supportive care. Following skin reactions, particularly the delayed lesions, it is not uncommon for patients to refuse a second shot. Some request a medical waiver to avoid further vaccine exposure. According to Dr. Freeman, this is unwarranted.
“I have granted exactly zero waivers,” she said. She explains to patients that these reactions have not been predictive of serious events, such as anaphylaxis. Although the trigger of the hypersensitivity reaction remains unknown, there is no evidence of serious consequences.
Delayed skin reactions are more commonly associated with the Moderna than the Pfizer vaccine. One notable difference between these vaccines is the greater content of mRNA in the Moderna formulation, but Freeman said that this is only one potential hypothesis for higher frequency of reactions to this version of the vaccine.
Patients with a history of allergic disease are more likely to develop a reaction but not significantly more likely to have a reaction that is more difficult to manage, according to Kimberly G. Blumenthal, MD, quality and safety officer for allergy, and codirector of the clinical epidemiology program in the division of rheumatology, allergy, and immunology at Mass General.
Anaphylaxis has been associated with COVD-19 vaccines just as it has with essentially every injectable vaccine, Dr. Blumenthal said during the same session. But the risk is very low, and it stays low even among those with a history of severe hypersensitivity reactions in the past.
Among the data collected from more than 52,000 vaccinated MGBHS employees, 0.9% had a history of severe allergic reaction to a prior vaccine. Of these, 11.6% had an allergic reaction to the COVID-19 vaccine. This was more than twice the 4.6% rate of allergic reactions among employees without a history of allergic reactions, but serious consequences were rare in both groups.
Of those with a reaction to the first dose, all but 2.4% took a subsequent dose. Again, serious reactions were exceedingly rare. These serious reactions did include anaphylaxis and hospitalization in 3% of patients, but there were no fatalities and all resolved.
The absence of serious sequelae from a reaction to a COVID-19 vaccine must be considered within the context of the benefit, which includes protection from death and hospitalization from the virus, according to Dr. Blumenthal. Citing the evidence that first-shot reactions are a source of vaccine hesitancy, she agreed that it is important to educate patients about relative risks.
“Even in our own cohort of MGBHS employees, we have people, including those who had been provaccine in the past, become hesitant,” commented Dr. Blumenthal, who said there are data from the Kaiser Permanente System showing similar vaccine reluctance following a first-shot reaction.
After more than 500 million doses of the Moderna and Pfizer vaccines had been administered worldwide, there was not a single reported death from anaphylaxis. Although Dr. Blumenthal said that an unconfirmed death of this type had been recently reported, she emphasized that this single death, if valid, is dwarfed by the lives saved with vaccination.
Asked about her strategy for counseling patients with vaccine hesitancy, Dr. Freeman said the body of safety data is large and compelling. There is overwhelming evidence of a favorable benefit-to-risk ratio overall and among those with a first-shot reaction.
“I can reassure them on the basis of the data,” Dr. Freeman said in an interview. “Less than half will have a reaction to the second shot and even if they do have a reaction, it is likely to be less severe.”
Although the main message is that vaccination is potentially lifesaving and far outweighs any risks, Freeman specifically gives this message to those hesitant to take a second shot after a first-shot reaction: “I can get you through it.”
Dr. Freeman encouraged health care professionals to report cases of COVID-19 vaccine–related dermatologic side effects to the American Academy of Dermatology / International League of Dermatologic Societies COVID-19 dermatology registry. Dermatologic manifestations of COVID-19 can also be reported to the registry.
Dr. Freeman disclosed receiving grants/research funding from the International League of Dermatologic Societies and from the National Institutes of Health. Dr. Blumenthal disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
BOSTON – Requests for a according to an analysis of several large sets of data presented at the annual meeting of the American Academy of Dermatology.
According to the data, “there are no serious adverse consequences from these cutaneous reactions,” said Esther Freeman, MD, PhD, director of Global Health Dermatology, Massachusetts General Hospital, Boston.
This is important because the risk of vaccine hesitancy goes up dramatically in patients who experience reactions to the first vaccine dose, according to follow-up of more than 50,000 employees vaccinated in the Mass General Brigham Healthcare System (MGBHS). According to Dr. Freeman, there was almost a fourfold increase in the rate of second-dose refusals for those with cutaneous reactions and a more than fourfold increase in those who developed angioedema.
Before the data were available, skin reactions were a source of concern among dermatologists and others involved in monitoring vaccine-related adverse events. Injection site reactions (ISRs) are associated with essentially every injectable vaccine, so these were expected, but a small proportion of patients developed large red plaques in the injection arm 7-8 days after the inoculation.
“These delayed reactions caused a lot of initial panic,” said Dr. Freeman, who counted herself among those alarmed about what the reactions might signify. “Was this cellulitis? Would the next dose cause anaphylaxis? We were concerned.”
This concern dissipated with the availability of more data. In a global registry that has so far captured more than 1,000 cutaneous reactions from 52 participating countries, it appears that about 2% of patients have a cutaneous reaction other than an ISR after the first dose. All resolve with minimal skin care or no treatment.
After the second dose, the proportion is lower. If there is a reaction, it typically occurs earlier and resolves more quickly.
“What we have learned is that fewer than half of patients who had a reaction to the first dose have a reaction to the second, and those who did have a reaction had a milder course,” said Dr. Freeman.
These data are “incredibly reassuring” on many levels, she explained. In addition, it allows clinicians to confidently explain to patients that there are no serious sequelae from the rashes, whether immediate or delayed, from the available COVID-19 vaccines.
“Every skin reaction I have seen is something we can treat through,” she added, noting that most reactions resolve with little or no supportive care. Following skin reactions, particularly the delayed lesions, it is not uncommon for patients to refuse a second shot. Some request a medical waiver to avoid further vaccine exposure. According to Dr. Freeman, this is unwarranted.
“I have granted exactly zero waivers,” she said. She explains to patients that these reactions have not been predictive of serious events, such as anaphylaxis. Although the trigger of the hypersensitivity reaction remains unknown, there is no evidence of serious consequences.
Delayed skin reactions are more commonly associated with the Moderna than the Pfizer vaccine. One notable difference between these vaccines is the greater content of mRNA in the Moderna formulation, but Freeman said that this is only one potential hypothesis for higher frequency of reactions to this version of the vaccine.
Patients with a history of allergic disease are more likely to develop a reaction but not significantly more likely to have a reaction that is more difficult to manage, according to Kimberly G. Blumenthal, MD, quality and safety officer for allergy, and codirector of the clinical epidemiology program in the division of rheumatology, allergy, and immunology at Mass General.
Anaphylaxis has been associated with COVD-19 vaccines just as it has with essentially every injectable vaccine, Dr. Blumenthal said during the same session. But the risk is very low, and it stays low even among those with a history of severe hypersensitivity reactions in the past.
Among the data collected from more than 52,000 vaccinated MGBHS employees, 0.9% had a history of severe allergic reaction to a prior vaccine. Of these, 11.6% had an allergic reaction to the COVID-19 vaccine. This was more than twice the 4.6% rate of allergic reactions among employees without a history of allergic reactions, but serious consequences were rare in both groups.
Of those with a reaction to the first dose, all but 2.4% took a subsequent dose. Again, serious reactions were exceedingly rare. These serious reactions did include anaphylaxis and hospitalization in 3% of patients, but there were no fatalities and all resolved.
The absence of serious sequelae from a reaction to a COVID-19 vaccine must be considered within the context of the benefit, which includes protection from death and hospitalization from the virus, according to Dr. Blumenthal. Citing the evidence that first-shot reactions are a source of vaccine hesitancy, she agreed that it is important to educate patients about relative risks.
“Even in our own cohort of MGBHS employees, we have people, including those who had been provaccine in the past, become hesitant,” commented Dr. Blumenthal, who said there are data from the Kaiser Permanente System showing similar vaccine reluctance following a first-shot reaction.
After more than 500 million doses of the Moderna and Pfizer vaccines had been administered worldwide, there was not a single reported death from anaphylaxis. Although Dr. Blumenthal said that an unconfirmed death of this type had been recently reported, she emphasized that this single death, if valid, is dwarfed by the lives saved with vaccination.
Asked about her strategy for counseling patients with vaccine hesitancy, Dr. Freeman said the body of safety data is large and compelling. There is overwhelming evidence of a favorable benefit-to-risk ratio overall and among those with a first-shot reaction.
“I can reassure them on the basis of the data,” Dr. Freeman said in an interview. “Less than half will have a reaction to the second shot and even if they do have a reaction, it is likely to be less severe.”
Although the main message is that vaccination is potentially lifesaving and far outweighs any risks, Freeman specifically gives this message to those hesitant to take a second shot after a first-shot reaction: “I can get you through it.”
Dr. Freeman encouraged health care professionals to report cases of COVID-19 vaccine–related dermatologic side effects to the American Academy of Dermatology / International League of Dermatologic Societies COVID-19 dermatology registry. Dermatologic manifestations of COVID-19 can also be reported to the registry.
Dr. Freeman disclosed receiving grants/research funding from the International League of Dermatologic Societies and from the National Institutes of Health. Dr. Blumenthal disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
BOSTON – Requests for a according to an analysis of several large sets of data presented at the annual meeting of the American Academy of Dermatology.
According to the data, “there are no serious adverse consequences from these cutaneous reactions,” said Esther Freeman, MD, PhD, director of Global Health Dermatology, Massachusetts General Hospital, Boston.
This is important because the risk of vaccine hesitancy goes up dramatically in patients who experience reactions to the first vaccine dose, according to follow-up of more than 50,000 employees vaccinated in the Mass General Brigham Healthcare System (MGBHS). According to Dr. Freeman, there was almost a fourfold increase in the rate of second-dose refusals for those with cutaneous reactions and a more than fourfold increase in those who developed angioedema.
Before the data were available, skin reactions were a source of concern among dermatologists and others involved in monitoring vaccine-related adverse events. Injection site reactions (ISRs) are associated with essentially every injectable vaccine, so these were expected, but a small proportion of patients developed large red plaques in the injection arm 7-8 days after the inoculation.
“These delayed reactions caused a lot of initial panic,” said Dr. Freeman, who counted herself among those alarmed about what the reactions might signify. “Was this cellulitis? Would the next dose cause anaphylaxis? We were concerned.”
This concern dissipated with the availability of more data. In a global registry that has so far captured more than 1,000 cutaneous reactions from 52 participating countries, it appears that about 2% of patients have a cutaneous reaction other than an ISR after the first dose. All resolve with minimal skin care or no treatment.
After the second dose, the proportion is lower. If there is a reaction, it typically occurs earlier and resolves more quickly.
“What we have learned is that fewer than half of patients who had a reaction to the first dose have a reaction to the second, and those who did have a reaction had a milder course,” said Dr. Freeman.
These data are “incredibly reassuring” on many levels, she explained. In addition, it allows clinicians to confidently explain to patients that there are no serious sequelae from the rashes, whether immediate or delayed, from the available COVID-19 vaccines.
“Every skin reaction I have seen is something we can treat through,” she added, noting that most reactions resolve with little or no supportive care. Following skin reactions, particularly the delayed lesions, it is not uncommon for patients to refuse a second shot. Some request a medical waiver to avoid further vaccine exposure. According to Dr. Freeman, this is unwarranted.
“I have granted exactly zero waivers,” she said. She explains to patients that these reactions have not been predictive of serious events, such as anaphylaxis. Although the trigger of the hypersensitivity reaction remains unknown, there is no evidence of serious consequences.
Delayed skin reactions are more commonly associated with the Moderna than the Pfizer vaccine. One notable difference between these vaccines is the greater content of mRNA in the Moderna formulation, but Freeman said that this is only one potential hypothesis for higher frequency of reactions to this version of the vaccine.
Patients with a history of allergic disease are more likely to develop a reaction but not significantly more likely to have a reaction that is more difficult to manage, according to Kimberly G. Blumenthal, MD, quality and safety officer for allergy, and codirector of the clinical epidemiology program in the division of rheumatology, allergy, and immunology at Mass General.
Anaphylaxis has been associated with COVD-19 vaccines just as it has with essentially every injectable vaccine, Dr. Blumenthal said during the same session. But the risk is very low, and it stays low even among those with a history of severe hypersensitivity reactions in the past.
Among the data collected from more than 52,000 vaccinated MGBHS employees, 0.9% had a history of severe allergic reaction to a prior vaccine. Of these, 11.6% had an allergic reaction to the COVID-19 vaccine. This was more than twice the 4.6% rate of allergic reactions among employees without a history of allergic reactions, but serious consequences were rare in both groups.
Of those with a reaction to the first dose, all but 2.4% took a subsequent dose. Again, serious reactions were exceedingly rare. These serious reactions did include anaphylaxis and hospitalization in 3% of patients, but there were no fatalities and all resolved.
The absence of serious sequelae from a reaction to a COVID-19 vaccine must be considered within the context of the benefit, which includes protection from death and hospitalization from the virus, according to Dr. Blumenthal. Citing the evidence that first-shot reactions are a source of vaccine hesitancy, she agreed that it is important to educate patients about relative risks.
“Even in our own cohort of MGBHS employees, we have people, including those who had been provaccine in the past, become hesitant,” commented Dr. Blumenthal, who said there are data from the Kaiser Permanente System showing similar vaccine reluctance following a first-shot reaction.
After more than 500 million doses of the Moderna and Pfizer vaccines had been administered worldwide, there was not a single reported death from anaphylaxis. Although Dr. Blumenthal said that an unconfirmed death of this type had been recently reported, she emphasized that this single death, if valid, is dwarfed by the lives saved with vaccination.
Asked about her strategy for counseling patients with vaccine hesitancy, Dr. Freeman said the body of safety data is large and compelling. There is overwhelming evidence of a favorable benefit-to-risk ratio overall and among those with a first-shot reaction.
“I can reassure them on the basis of the data,” Dr. Freeman said in an interview. “Less than half will have a reaction to the second shot and even if they do have a reaction, it is likely to be less severe.”
Although the main message is that vaccination is potentially lifesaving and far outweighs any risks, Freeman specifically gives this message to those hesitant to take a second shot after a first-shot reaction: “I can get you through it.”
Dr. Freeman encouraged health care professionals to report cases of COVID-19 vaccine–related dermatologic side effects to the American Academy of Dermatology / International League of Dermatologic Societies COVID-19 dermatology registry. Dermatologic manifestations of COVID-19 can also be reported to the registry.
Dr. Freeman disclosed receiving grants/research funding from the International League of Dermatologic Societies and from the National Institutes of Health. Dr. Blumenthal disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT AAD 2022
Fingers take the fight to COVID-19
Pointing a finger at COVID-19
The battle against COVID-19 is seemingly never ending. It’s been 2 years and still we struggle against the virus. But now, a new hero rises against the eternal menace, a powerful weapon against this scourge of humanity. And that weapon? Finger length.
Before you break out the sad trombone, hear us out. One of the big questions around COVID-19 is the role testosterone plays in its severity: Does low testosterone increase or decrease the odds of contracting severe COVID-19? To help answer that question, English researchers have published a study analyzing finger length ratios in both COVID-19 patients and a healthy control group. That seems random, but high testosterone in the womb leads to longer ring fingers in adulthood, while high estrogen leads to longer index fingers.
According to the researchers, those who had significant left hand–right hand differences in the ratio between the second and fourth digits, as well as the third and fifth digits, were significantly more likely to have severe COVID-19 compared with those with more even ratios. Those with “feminized” short little fingers were also at risk. Those large ratio differences indicate low testosterone and high estrogen, which may explain why elderly men are at such high risk for severe COVID-19. Testosterone naturally falls off as men get older.
The results add credence to clinical trials looking to use testosterone-boosting drugs against COVID-19, the researchers said. It also gives credence to LOTME’s brand-new 12-step finger strength fitness routine and our branded finger weights. Now just $19.95! It’s the bargain of the century! Boost your testosterone naturally and protect yourself from COVID-19! We promise it’s not a scam.
Some emergencies need a superhero
Last week, we learned about the most boring person in the world. This week just happens to be opposite week, so we’re looking at a candidate for the most interesting person. Someone who can swoop down from the sky to save the injured and helpless. Someone who can go where helicopters fear to tread. Someone with jet engines for arms. Superhero-type stuff.
The Great North Air Ambulance Service (GNAAS), a charitable organization located in the United Kingdom, recently announced that one of its members has completed training on the Gravity Industries Jet Suit. The suit “has two engines on each arm and a larger engine on the back [that] provide up to 317 pounds of thrust,” Interesting Engineering explained.
GNAAS is putting the suit into operation in England’s Lake District National Park, which includes mountainous terrain that is not very hospitable to helicopter landings. A paramedic using the suit can reach hikers stranded on mountainsides much faster than rescuers who have to run or hike from the nearest helicopter landing site.
“Everyone looks at the wow factor and the fact we are the world’s first jet suit paramedics, but for us, it’s about delivering patient care,” GNAAS’ Andy Mawson told Interesting Engineering. Sounds like superhero-speak to us.
So if you’re in the Lake District and have taken a bit of a tumble, you can call a superhero on your cell phone or you can use this to summon one.
Why we’re rejecting food as medicine
Humans have been using food to treat ailments much longer than we’ve had the advances of modern medicine. So why have we rejected its worth in our treatment processes? And what can be done to change that? The Center for Food as Medicine and the Hunter College NYC Food Policy Center just released a 335-page report that answers those questions.
First, the why: Meals in health care settings are not medically designed to help with the specific needs of the patient. Produce-prescription and nutrition-incentive programs don’t have the government funds to fully support them. And a lot of medical schools don’t even require students to take a basic nutrition course. So there’s a lack of knowledge and a disconnect between health care providers and food as a resource.
Then there’s a lack of trust in the food industry and their validity. Social media uses food as a means of promoting “pseudoscientific alternative medicine” or spreading false info, pushing away legitimate providers. The food industry has had its fingers in food science studies and an almost mafia-esque chokehold on American dietary guidelines. No wonder food for medicine is getting the boot!
To change the situation, the report offers 10 key recommendations on how to advance the idea of incorporating food into medicine for treatment and prevention. They include boosting the funding for research, making hospitals more food-as-medicine focused, expanding federal programs, and improving public awareness on the role nutrition can play in medical treatment or prevention.
So maybe instead of rejecting food outright, we should be looking a little deeper at how we can use it to our advantage. Just a thought: Ice cream as an antidepressant.
Being rude is a good thing, apparently
If you’ve ever been called argumentative, stubborn, or unpleasant, then this LOTME is for you. Researchers at the University of Geneva have found that people who are more stubborn and hate to conform have brains that are more protected against Alzheimer’s disease. That type of personality seems to preserve the part of the brain that usually deteriorates as we grow older.
The original hypothesis that personality may have a protective effect against brain degeneration led the investigators to conduct cognitive and personality assessments of 65 elderly participants over a 5-year period. Researchers have been attempting to create vaccines to protect against Alzheimer’s disease, but these new findings offer a nonbiological way to help.
“For a long time, the brain is able to compensate by activating alternative networks; when the first clinical signs appear, however, it is unfortunately often too late. The identification of early biomarkers is therefore essential for … effective disease management,” lead author Panteleimon Giannakopoulos, MD, said in a Study Finds report.
You may be wondering how people with more agreeable and less confrontational personalities can seek help. Well, researchers are working on that, too. It’s a complex situation, but as always, we’re rooting for you, science!
At least now you can take solace in the fact that your elderly next-door neighbor who yells at you for stepping on his lawn is probably more protected against Alzheimer’s disease.
Pointing a finger at COVID-19
The battle against COVID-19 is seemingly never ending. It’s been 2 years and still we struggle against the virus. But now, a new hero rises against the eternal menace, a powerful weapon against this scourge of humanity. And that weapon? Finger length.
Before you break out the sad trombone, hear us out. One of the big questions around COVID-19 is the role testosterone plays in its severity: Does low testosterone increase or decrease the odds of contracting severe COVID-19? To help answer that question, English researchers have published a study analyzing finger length ratios in both COVID-19 patients and a healthy control group. That seems random, but high testosterone in the womb leads to longer ring fingers in adulthood, while high estrogen leads to longer index fingers.
According to the researchers, those who had significant left hand–right hand differences in the ratio between the second and fourth digits, as well as the third and fifth digits, were significantly more likely to have severe COVID-19 compared with those with more even ratios. Those with “feminized” short little fingers were also at risk. Those large ratio differences indicate low testosterone and high estrogen, which may explain why elderly men are at such high risk for severe COVID-19. Testosterone naturally falls off as men get older.
The results add credence to clinical trials looking to use testosterone-boosting drugs against COVID-19, the researchers said. It also gives credence to LOTME’s brand-new 12-step finger strength fitness routine and our branded finger weights. Now just $19.95! It’s the bargain of the century! Boost your testosterone naturally and protect yourself from COVID-19! We promise it’s not a scam.
Some emergencies need a superhero
Last week, we learned about the most boring person in the world. This week just happens to be opposite week, so we’re looking at a candidate for the most interesting person. Someone who can swoop down from the sky to save the injured and helpless. Someone who can go where helicopters fear to tread. Someone with jet engines for arms. Superhero-type stuff.
The Great North Air Ambulance Service (GNAAS), a charitable organization located in the United Kingdom, recently announced that one of its members has completed training on the Gravity Industries Jet Suit. The suit “has two engines on each arm and a larger engine on the back [that] provide up to 317 pounds of thrust,” Interesting Engineering explained.
GNAAS is putting the suit into operation in England’s Lake District National Park, which includes mountainous terrain that is not very hospitable to helicopter landings. A paramedic using the suit can reach hikers stranded on mountainsides much faster than rescuers who have to run or hike from the nearest helicopter landing site.
“Everyone looks at the wow factor and the fact we are the world’s first jet suit paramedics, but for us, it’s about delivering patient care,” GNAAS’ Andy Mawson told Interesting Engineering. Sounds like superhero-speak to us.
So if you’re in the Lake District and have taken a bit of a tumble, you can call a superhero on your cell phone or you can use this to summon one.
Why we’re rejecting food as medicine
Humans have been using food to treat ailments much longer than we’ve had the advances of modern medicine. So why have we rejected its worth in our treatment processes? And what can be done to change that? The Center for Food as Medicine and the Hunter College NYC Food Policy Center just released a 335-page report that answers those questions.
First, the why: Meals in health care settings are not medically designed to help with the specific needs of the patient. Produce-prescription and nutrition-incentive programs don’t have the government funds to fully support them. And a lot of medical schools don’t even require students to take a basic nutrition course. So there’s a lack of knowledge and a disconnect between health care providers and food as a resource.
Then there’s a lack of trust in the food industry and their validity. Social media uses food as a means of promoting “pseudoscientific alternative medicine” or spreading false info, pushing away legitimate providers. The food industry has had its fingers in food science studies and an almost mafia-esque chokehold on American dietary guidelines. No wonder food for medicine is getting the boot!
To change the situation, the report offers 10 key recommendations on how to advance the idea of incorporating food into medicine for treatment and prevention. They include boosting the funding for research, making hospitals more food-as-medicine focused, expanding federal programs, and improving public awareness on the role nutrition can play in medical treatment or prevention.
So maybe instead of rejecting food outright, we should be looking a little deeper at how we can use it to our advantage. Just a thought: Ice cream as an antidepressant.
Being rude is a good thing, apparently
If you’ve ever been called argumentative, stubborn, or unpleasant, then this LOTME is for you. Researchers at the University of Geneva have found that people who are more stubborn and hate to conform have brains that are more protected against Alzheimer’s disease. That type of personality seems to preserve the part of the brain that usually deteriorates as we grow older.
The original hypothesis that personality may have a protective effect against brain degeneration led the investigators to conduct cognitive and personality assessments of 65 elderly participants over a 5-year period. Researchers have been attempting to create vaccines to protect against Alzheimer’s disease, but these new findings offer a nonbiological way to help.
“For a long time, the brain is able to compensate by activating alternative networks; when the first clinical signs appear, however, it is unfortunately often too late. The identification of early biomarkers is therefore essential for … effective disease management,” lead author Panteleimon Giannakopoulos, MD, said in a Study Finds report.
You may be wondering how people with more agreeable and less confrontational personalities can seek help. Well, researchers are working on that, too. It’s a complex situation, but as always, we’re rooting for you, science!
At least now you can take solace in the fact that your elderly next-door neighbor who yells at you for stepping on his lawn is probably more protected against Alzheimer’s disease.
Pointing a finger at COVID-19
The battle against COVID-19 is seemingly never ending. It’s been 2 years and still we struggle against the virus. But now, a new hero rises against the eternal menace, a powerful weapon against this scourge of humanity. And that weapon? Finger length.
Before you break out the sad trombone, hear us out. One of the big questions around COVID-19 is the role testosterone plays in its severity: Does low testosterone increase or decrease the odds of contracting severe COVID-19? To help answer that question, English researchers have published a study analyzing finger length ratios in both COVID-19 patients and a healthy control group. That seems random, but high testosterone in the womb leads to longer ring fingers in adulthood, while high estrogen leads to longer index fingers.
According to the researchers, those who had significant left hand–right hand differences in the ratio between the second and fourth digits, as well as the third and fifth digits, were significantly more likely to have severe COVID-19 compared with those with more even ratios. Those with “feminized” short little fingers were also at risk. Those large ratio differences indicate low testosterone and high estrogen, which may explain why elderly men are at such high risk for severe COVID-19. Testosterone naturally falls off as men get older.
The results add credence to clinical trials looking to use testosterone-boosting drugs against COVID-19, the researchers said. It also gives credence to LOTME’s brand-new 12-step finger strength fitness routine and our branded finger weights. Now just $19.95! It’s the bargain of the century! Boost your testosterone naturally and protect yourself from COVID-19! We promise it’s not a scam.
Some emergencies need a superhero
Last week, we learned about the most boring person in the world. This week just happens to be opposite week, so we’re looking at a candidate for the most interesting person. Someone who can swoop down from the sky to save the injured and helpless. Someone who can go where helicopters fear to tread. Someone with jet engines for arms. Superhero-type stuff.
The Great North Air Ambulance Service (GNAAS), a charitable organization located in the United Kingdom, recently announced that one of its members has completed training on the Gravity Industries Jet Suit. The suit “has two engines on each arm and a larger engine on the back [that] provide up to 317 pounds of thrust,” Interesting Engineering explained.
GNAAS is putting the suit into operation in England’s Lake District National Park, which includes mountainous terrain that is not very hospitable to helicopter landings. A paramedic using the suit can reach hikers stranded on mountainsides much faster than rescuers who have to run or hike from the nearest helicopter landing site.
“Everyone looks at the wow factor and the fact we are the world’s first jet suit paramedics, but for us, it’s about delivering patient care,” GNAAS’ Andy Mawson told Interesting Engineering. Sounds like superhero-speak to us.
So if you’re in the Lake District and have taken a bit of a tumble, you can call a superhero on your cell phone or you can use this to summon one.
Why we’re rejecting food as medicine
Humans have been using food to treat ailments much longer than we’ve had the advances of modern medicine. So why have we rejected its worth in our treatment processes? And what can be done to change that? The Center for Food as Medicine and the Hunter College NYC Food Policy Center just released a 335-page report that answers those questions.
First, the why: Meals in health care settings are not medically designed to help with the specific needs of the patient. Produce-prescription and nutrition-incentive programs don’t have the government funds to fully support them. And a lot of medical schools don’t even require students to take a basic nutrition course. So there’s a lack of knowledge and a disconnect between health care providers and food as a resource.
Then there’s a lack of trust in the food industry and their validity. Social media uses food as a means of promoting “pseudoscientific alternative medicine” or spreading false info, pushing away legitimate providers. The food industry has had its fingers in food science studies and an almost mafia-esque chokehold on American dietary guidelines. No wonder food for medicine is getting the boot!
To change the situation, the report offers 10 key recommendations on how to advance the idea of incorporating food into medicine for treatment and prevention. They include boosting the funding for research, making hospitals more food-as-medicine focused, expanding federal programs, and improving public awareness on the role nutrition can play in medical treatment or prevention.
So maybe instead of rejecting food outright, we should be looking a little deeper at how we can use it to our advantage. Just a thought: Ice cream as an antidepressant.
Being rude is a good thing, apparently
If you’ve ever been called argumentative, stubborn, or unpleasant, then this LOTME is for you. Researchers at the University of Geneva have found that people who are more stubborn and hate to conform have brains that are more protected against Alzheimer’s disease. That type of personality seems to preserve the part of the brain that usually deteriorates as we grow older.
The original hypothesis that personality may have a protective effect against brain degeneration led the investigators to conduct cognitive and personality assessments of 65 elderly participants over a 5-year period. Researchers have been attempting to create vaccines to protect against Alzheimer’s disease, but these new findings offer a nonbiological way to help.
“For a long time, the brain is able to compensate by activating alternative networks; when the first clinical signs appear, however, it is unfortunately often too late. The identification of early biomarkers is therefore essential for … effective disease management,” lead author Panteleimon Giannakopoulos, MD, said in a Study Finds report.
You may be wondering how people with more agreeable and less confrontational personalities can seek help. Well, researchers are working on that, too. It’s a complex situation, but as always, we’re rooting for you, science!
At least now you can take solace in the fact that your elderly next-door neighbor who yells at you for stepping on his lawn is probably more protected against Alzheimer’s disease.
Does evidence support benefits of omega-3 fatty acids?
Dietary supplements that contain omega-3 fatty acids have been widely consumed for years. Researchers have been investigating the benefits of such preparations for cardiovascular, neurologic, and psychological conditions. A recently published study on omega-3 fatty acids and depression inspired neurologist Hans-Christoph Diener, MD, PhD, of the Institute for Epidemiology at the University Duisburg-Essen (Germany), to examine scientific publications concerning omega-3 fatty acids or fish-oil capsules in more detail.
Prevention of depression
Dr. Diener told the story of how he stumbled upon an interesting article in JAMA in December 2021. It was about a placebo-controlled study that investigated whether omega-3 fatty acids can prevent incident depression.
As the study authors reported, treatment with omega-3 preparations in adults aged 50 years or older without clinically relevant symptoms of depression at study initiation was associated with a small but statistically significant increase in the risk for depression or clinically relevant symptoms of depression. There was no difference in mood scale value, however, over a median follow-up of 5.3 years. According to the study authors, these results did not support the administration of omega-3 preparations for the prevention of depression.
This study was, as Dr. Diener said, somewhat negative, but it did arouse his interest in questions such as what biological effects omega-3 fatty acids have and what is known “about this topic with regard to neurology,” he said. When reviewing the literature, he noticed that there “were association studies, i.e., studies that describe that the intake of omega-3 fatty acids may possibly be associated with a lower risk of certain diseases.”
Beginning with the Inuit
It all started “with observations of the Inuit [population] in Greenland and Alaska after World War II, because it was remarked upon that these people ate a lot of fish and seal meat and had a very low incidence of cardiovascular diseases.” Over the years, a large number of association studies have been published, which may have encouraged the assumption that omega-3 fatty acids have positive health effects on various conditions, such as cardiovascular diseases, hyperlipidemia, type 2 diabetes, various malignancies, cognitive impairments, Alzheimer’s disease, depression and anxiety disorders, heart failure, slipped disks, ADHD, symptoms of menopause, rheumatoid arthritis, asthma, periodontitis, epilepsy, chemotherapy tolerance, premenstrual syndrome, and nonalcoholic fatty liver disease.
Dr. Diener believes that the problem is that these are association studies. But association does not mean that there is a causal relationship.
Disappointing study results
On the contrary, the results from the randomized placebo-controlled studies are truly frustrating, according to the neurologist. A meta-analysis of the use of omega-3 fatty acids in cardiovascular diseases included 86 studies with over 162,000 patients. According to Dr. Diener, it did not reveal any benefit for overall and cardiovascular mortality, nor any benefit for the reduction of myocardial infarction and stroke.
The results did indicate a trend, however, for reduced mortality in coronary heart disease. Even so, the number needed to treat for this was 334, which means that 334 people would have to take omega-3 fatty acids for years to prevent one fatal cardiac event.
Aside from this study, Dr. Diener found six studies on Alzheimer’s disease and three studies on dementia with patient populations between 600 and 800. In these studies, too, a positive effect of omega-3 fatty acids could not be identified. Then he discovered another 31 placebo-controlled studies of omega-3 fatty acids for the treatment or prevention of depression and anxiety disorder. Despite including 50,000 patients, these studies also did not show any positive effect.
“I see a significant discrepancy between the promotion of omega-3 fatty acids, whether it’s on television, in the ‘yellow’ [journalism] press, or in advertisements, and the actual scientific evidence,” said Dr. Diener. “At least from a neurological perspective, there is no evidence that omega-3 fatty acids have any benefit. This is true for strokes, dementia, Alzheimer’s disease, depression, and anxiety disorders.”
Potential adverse effects
Omega-3 fatty acids also have potentially adverse effects. The VITAL Rhythm study recently provided evidence that, depending on the dose, preparations with omega-3 fatty acids may increase the risk for atrial fibrillation. As the authors wrote, the results do not support taking omega-3 fatty acids to prevent atrial fibrillation.
In 2019, the global market for omega-3 fatty acids reached a value of $4.1 billion. This value is expected to double by 2025, according to a comment by Gregory Curfman, MD, deputy editor of JAMA and lecturer in health care policy at Harvard Medical School, Boston.
As Dr. Curfman wrote, this impressive amount of expenditure shows how beloved these products are and how strongly many people believe that omega-3 fatty acids are beneficial for their health. It is therefore important to know the potential risks of such preparations. One such example for this would be the risk for atrial fibrillation.
According to Dr. Curfman, in the last 2 years, four randomized clinical studies have provided data on the risk for atrial fibrillation associated with omega-3 fatty acids. In the STRENGTH study, 13,078 high-risk patients with cardiovascular diseases were randomly assigned to one of two groups. The subjects received either a high dose (4 g/day) of a combination of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) or corn oil. After a median of 42 months, there was no significant difference between the two groups in the primary composite cardiovascular endpoint, but more frequent atrial fibrillation in the omega-3 fatty acid group, compared with the corn oil group (2.2% vs. 1.3%; hazard ratio, 1.69; 95% confidence interval, 1.29-2.21; P < .001).
In the REDUCE-IT study, 8179 subjects were randomly assigned to a high dose (4 g/day, as in STRENGTH) of an omega-3 fatty acid preparation consisting of a purified EPA (icosapent ethyl) or mineral oil. After a median observation period of 4.9 years, icosapent ethyl was associated with a relative reduction of the primary composite cardiovascular endpoint by 25%, compared with mineral oil. As in the STRENGTH study, this study found that the risk for atrial fibrillation associated with omega-3 fatty acids, compared with mineral oil, was significantly higher (5.3% vs. 3.9%; P = .003).
In a third study (OMEMI), as Dr. Curfman reported, 1027 elderly patients who had recently had a myocardial infarction were randomly assigned to receive either a median dose of 1.8 g/day of omega-3 fatty acids (a combination of EPA and DHA) or corn oil. After 2 years, there was no significant difference between the two groups in primary composite cardiovascular endpoints, but 7.2% of the patients taking omega-3 fatty acids developed atrial fibrillation. In the corn oil group, this proportion was 4% (HR, 1.84; 95% CI, 0.98-3.45; P = .06).
The data from the four studies together indicate a potential dose-dependent risk for atrial fibrillation associated with omega-3 fatty acids, according to Dr. Curfman. At a dose of 4.0 g/day, there is a highly significant risk increase (almost double). With a median dose of 1.8 g/day, the risk increase (HR, 1.84) did not reach statistical significance. At a daily standard dose of 840 mg/day, an increase in risk could not be determined.
Dr. Curfman’s recommendation is that patients who take, or want to take, preparations with omega-3 fatty acids be informed of the potential development of arrhythmia at higher dosages. These patients also should undergo cardiological monitoring.
A version of this article first appeared on Medscape.com.
Dietary supplements that contain omega-3 fatty acids have been widely consumed for years. Researchers have been investigating the benefits of such preparations for cardiovascular, neurologic, and psychological conditions. A recently published study on omega-3 fatty acids and depression inspired neurologist Hans-Christoph Diener, MD, PhD, of the Institute for Epidemiology at the University Duisburg-Essen (Germany), to examine scientific publications concerning omega-3 fatty acids or fish-oil capsules in more detail.
Prevention of depression
Dr. Diener told the story of how he stumbled upon an interesting article in JAMA in December 2021. It was about a placebo-controlled study that investigated whether omega-3 fatty acids can prevent incident depression.
As the study authors reported, treatment with omega-3 preparations in adults aged 50 years or older without clinically relevant symptoms of depression at study initiation was associated with a small but statistically significant increase in the risk for depression or clinically relevant symptoms of depression. There was no difference in mood scale value, however, over a median follow-up of 5.3 years. According to the study authors, these results did not support the administration of omega-3 preparations for the prevention of depression.
This study was, as Dr. Diener said, somewhat negative, but it did arouse his interest in questions such as what biological effects omega-3 fatty acids have and what is known “about this topic with regard to neurology,” he said. When reviewing the literature, he noticed that there “were association studies, i.e., studies that describe that the intake of omega-3 fatty acids may possibly be associated with a lower risk of certain diseases.”
Beginning with the Inuit
It all started “with observations of the Inuit [population] in Greenland and Alaska after World War II, because it was remarked upon that these people ate a lot of fish and seal meat and had a very low incidence of cardiovascular diseases.” Over the years, a large number of association studies have been published, which may have encouraged the assumption that omega-3 fatty acids have positive health effects on various conditions, such as cardiovascular diseases, hyperlipidemia, type 2 diabetes, various malignancies, cognitive impairments, Alzheimer’s disease, depression and anxiety disorders, heart failure, slipped disks, ADHD, symptoms of menopause, rheumatoid arthritis, asthma, periodontitis, epilepsy, chemotherapy tolerance, premenstrual syndrome, and nonalcoholic fatty liver disease.
Dr. Diener believes that the problem is that these are association studies. But association does not mean that there is a causal relationship.
Disappointing study results
On the contrary, the results from the randomized placebo-controlled studies are truly frustrating, according to the neurologist. A meta-analysis of the use of omega-3 fatty acids in cardiovascular diseases included 86 studies with over 162,000 patients. According to Dr. Diener, it did not reveal any benefit for overall and cardiovascular mortality, nor any benefit for the reduction of myocardial infarction and stroke.
The results did indicate a trend, however, for reduced mortality in coronary heart disease. Even so, the number needed to treat for this was 334, which means that 334 people would have to take omega-3 fatty acids for years to prevent one fatal cardiac event.
Aside from this study, Dr. Diener found six studies on Alzheimer’s disease and three studies on dementia with patient populations between 600 and 800. In these studies, too, a positive effect of omega-3 fatty acids could not be identified. Then he discovered another 31 placebo-controlled studies of omega-3 fatty acids for the treatment or prevention of depression and anxiety disorder. Despite including 50,000 patients, these studies also did not show any positive effect.
“I see a significant discrepancy between the promotion of omega-3 fatty acids, whether it’s on television, in the ‘yellow’ [journalism] press, or in advertisements, and the actual scientific evidence,” said Dr. Diener. “At least from a neurological perspective, there is no evidence that omega-3 fatty acids have any benefit. This is true for strokes, dementia, Alzheimer’s disease, depression, and anxiety disorders.”
Potential adverse effects
Omega-3 fatty acids also have potentially adverse effects. The VITAL Rhythm study recently provided evidence that, depending on the dose, preparations with omega-3 fatty acids may increase the risk for atrial fibrillation. As the authors wrote, the results do not support taking omega-3 fatty acids to prevent atrial fibrillation.
In 2019, the global market for omega-3 fatty acids reached a value of $4.1 billion. This value is expected to double by 2025, according to a comment by Gregory Curfman, MD, deputy editor of JAMA and lecturer in health care policy at Harvard Medical School, Boston.
As Dr. Curfman wrote, this impressive amount of expenditure shows how beloved these products are and how strongly many people believe that omega-3 fatty acids are beneficial for their health. It is therefore important to know the potential risks of such preparations. One such example for this would be the risk for atrial fibrillation.
According to Dr. Curfman, in the last 2 years, four randomized clinical studies have provided data on the risk for atrial fibrillation associated with omega-3 fatty acids. In the STRENGTH study, 13,078 high-risk patients with cardiovascular diseases were randomly assigned to one of two groups. The subjects received either a high dose (4 g/day) of a combination of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) or corn oil. After a median of 42 months, there was no significant difference between the two groups in the primary composite cardiovascular endpoint, but more frequent atrial fibrillation in the omega-3 fatty acid group, compared with the corn oil group (2.2% vs. 1.3%; hazard ratio, 1.69; 95% confidence interval, 1.29-2.21; P < .001).
In the REDUCE-IT study, 8179 subjects were randomly assigned to a high dose (4 g/day, as in STRENGTH) of an omega-3 fatty acid preparation consisting of a purified EPA (icosapent ethyl) or mineral oil. After a median observation period of 4.9 years, icosapent ethyl was associated with a relative reduction of the primary composite cardiovascular endpoint by 25%, compared with mineral oil. As in the STRENGTH study, this study found that the risk for atrial fibrillation associated with omega-3 fatty acids, compared with mineral oil, was significantly higher (5.3% vs. 3.9%; P = .003).
In a third study (OMEMI), as Dr. Curfman reported, 1027 elderly patients who had recently had a myocardial infarction were randomly assigned to receive either a median dose of 1.8 g/day of omega-3 fatty acids (a combination of EPA and DHA) or corn oil. After 2 years, there was no significant difference between the two groups in primary composite cardiovascular endpoints, but 7.2% of the patients taking omega-3 fatty acids developed atrial fibrillation. In the corn oil group, this proportion was 4% (HR, 1.84; 95% CI, 0.98-3.45; P = .06).
The data from the four studies together indicate a potential dose-dependent risk for atrial fibrillation associated with omega-3 fatty acids, according to Dr. Curfman. At a dose of 4.0 g/day, there is a highly significant risk increase (almost double). With a median dose of 1.8 g/day, the risk increase (HR, 1.84) did not reach statistical significance. At a daily standard dose of 840 mg/day, an increase in risk could not be determined.
Dr. Curfman’s recommendation is that patients who take, or want to take, preparations with omega-3 fatty acids be informed of the potential development of arrhythmia at higher dosages. These patients also should undergo cardiological monitoring.
A version of this article first appeared on Medscape.com.
Dietary supplements that contain omega-3 fatty acids have been widely consumed for years. Researchers have been investigating the benefits of such preparations for cardiovascular, neurologic, and psychological conditions. A recently published study on omega-3 fatty acids and depression inspired neurologist Hans-Christoph Diener, MD, PhD, of the Institute for Epidemiology at the University Duisburg-Essen (Germany), to examine scientific publications concerning omega-3 fatty acids or fish-oil capsules in more detail.
Prevention of depression
Dr. Diener told the story of how he stumbled upon an interesting article in JAMA in December 2021. It was about a placebo-controlled study that investigated whether omega-3 fatty acids can prevent incident depression.
As the study authors reported, treatment with omega-3 preparations in adults aged 50 years or older without clinically relevant symptoms of depression at study initiation was associated with a small but statistically significant increase in the risk for depression or clinically relevant symptoms of depression. There was no difference in mood scale value, however, over a median follow-up of 5.3 years. According to the study authors, these results did not support the administration of omega-3 preparations for the prevention of depression.
This study was, as Dr. Diener said, somewhat negative, but it did arouse his interest in questions such as what biological effects omega-3 fatty acids have and what is known “about this topic with regard to neurology,” he said. When reviewing the literature, he noticed that there “were association studies, i.e., studies that describe that the intake of omega-3 fatty acids may possibly be associated with a lower risk of certain diseases.”
Beginning with the Inuit
It all started “with observations of the Inuit [population] in Greenland and Alaska after World War II, because it was remarked upon that these people ate a lot of fish and seal meat and had a very low incidence of cardiovascular diseases.” Over the years, a large number of association studies have been published, which may have encouraged the assumption that omega-3 fatty acids have positive health effects on various conditions, such as cardiovascular diseases, hyperlipidemia, type 2 diabetes, various malignancies, cognitive impairments, Alzheimer’s disease, depression and anxiety disorders, heart failure, slipped disks, ADHD, symptoms of menopause, rheumatoid arthritis, asthma, periodontitis, epilepsy, chemotherapy tolerance, premenstrual syndrome, and nonalcoholic fatty liver disease.
Dr. Diener believes that the problem is that these are association studies. But association does not mean that there is a causal relationship.
Disappointing study results
On the contrary, the results from the randomized placebo-controlled studies are truly frustrating, according to the neurologist. A meta-analysis of the use of omega-3 fatty acids in cardiovascular diseases included 86 studies with over 162,000 patients. According to Dr. Diener, it did not reveal any benefit for overall and cardiovascular mortality, nor any benefit for the reduction of myocardial infarction and stroke.
The results did indicate a trend, however, for reduced mortality in coronary heart disease. Even so, the number needed to treat for this was 334, which means that 334 people would have to take omega-3 fatty acids for years to prevent one fatal cardiac event.
Aside from this study, Dr. Diener found six studies on Alzheimer’s disease and three studies on dementia with patient populations between 600 and 800. In these studies, too, a positive effect of omega-3 fatty acids could not be identified. Then he discovered another 31 placebo-controlled studies of omega-3 fatty acids for the treatment or prevention of depression and anxiety disorder. Despite including 50,000 patients, these studies also did not show any positive effect.
“I see a significant discrepancy between the promotion of omega-3 fatty acids, whether it’s on television, in the ‘yellow’ [journalism] press, or in advertisements, and the actual scientific evidence,” said Dr. Diener. “At least from a neurological perspective, there is no evidence that omega-3 fatty acids have any benefit. This is true for strokes, dementia, Alzheimer’s disease, depression, and anxiety disorders.”
Potential adverse effects
Omega-3 fatty acids also have potentially adverse effects. The VITAL Rhythm study recently provided evidence that, depending on the dose, preparations with omega-3 fatty acids may increase the risk for atrial fibrillation. As the authors wrote, the results do not support taking omega-3 fatty acids to prevent atrial fibrillation.
In 2019, the global market for omega-3 fatty acids reached a value of $4.1 billion. This value is expected to double by 2025, according to a comment by Gregory Curfman, MD, deputy editor of JAMA and lecturer in health care policy at Harvard Medical School, Boston.
As Dr. Curfman wrote, this impressive amount of expenditure shows how beloved these products are and how strongly many people believe that omega-3 fatty acids are beneficial for their health. It is therefore important to know the potential risks of such preparations. One such example for this would be the risk for atrial fibrillation.
According to Dr. Curfman, in the last 2 years, four randomized clinical studies have provided data on the risk for atrial fibrillation associated with omega-3 fatty acids. In the STRENGTH study, 13,078 high-risk patients with cardiovascular diseases were randomly assigned to one of two groups. The subjects received either a high dose (4 g/day) of a combination of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) or corn oil. After a median of 42 months, there was no significant difference between the two groups in the primary composite cardiovascular endpoint, but more frequent atrial fibrillation in the omega-3 fatty acid group, compared with the corn oil group (2.2% vs. 1.3%; hazard ratio, 1.69; 95% confidence interval, 1.29-2.21; P < .001).
In the REDUCE-IT study, 8179 subjects were randomly assigned to a high dose (4 g/day, as in STRENGTH) of an omega-3 fatty acid preparation consisting of a purified EPA (icosapent ethyl) or mineral oil. After a median observation period of 4.9 years, icosapent ethyl was associated with a relative reduction of the primary composite cardiovascular endpoint by 25%, compared with mineral oil. As in the STRENGTH study, this study found that the risk for atrial fibrillation associated with omega-3 fatty acids, compared with mineral oil, was significantly higher (5.3% vs. 3.9%; P = .003).
In a third study (OMEMI), as Dr. Curfman reported, 1027 elderly patients who had recently had a myocardial infarction were randomly assigned to receive either a median dose of 1.8 g/day of omega-3 fatty acids (a combination of EPA and DHA) or corn oil. After 2 years, there was no significant difference between the two groups in primary composite cardiovascular endpoints, but 7.2% of the patients taking omega-3 fatty acids developed atrial fibrillation. In the corn oil group, this proportion was 4% (HR, 1.84; 95% CI, 0.98-3.45; P = .06).
The data from the four studies together indicate a potential dose-dependent risk for atrial fibrillation associated with omega-3 fatty acids, according to Dr. Curfman. At a dose of 4.0 g/day, there is a highly significant risk increase (almost double). With a median dose of 1.8 g/day, the risk increase (HR, 1.84) did not reach statistical significance. At a daily standard dose of 840 mg/day, an increase in risk could not be determined.
Dr. Curfman’s recommendation is that patients who take, or want to take, preparations with omega-3 fatty acids be informed of the potential development of arrhythmia at higher dosages. These patients also should undergo cardiological monitoring.
A version of this article first appeared on Medscape.com.
Melanoma increasing, but is this overdiagnosis?
Melanoma has been increasing in incidence in the United States over the last few decades, but is this a true increase? Or is this a case of overdiagnosis, fueled by screening?
A new study argues the case for overdiagnosis.
commented lead author lead author Adewole Adamson, MD, an assistant professor of internal medicine, division of dermatology, at the University of Texas at Austin.
He posted this conclusion on Twitter after the study was published in JAMA Dermatology.
“The discrepancies in incidence and mortality trends found in this cohort study suggest considerable overdiagnosis of melanoma occurring among White patients in the U.S.,” the authors concluded.
They estimated that an estimated 59% of White women and 60% of White men with melanoma were overdiagnosed in 2014.
These results are similar to those from a recent study from Australia, which used a different method of assessing overdiagnosis. Those findings estimated that 54%-58% of melanoma cases represented overdiagnosis in Australia, Dr. Adamson noted.
“Our estimates shed light on the HUGE scope of this problem in the United States that we need to address,” Dr. Adamson commented on Twitter. “Calls for screening for melanoma in the general public will only push these numbers higher, and make patients out of healthy people.”
“Screening the general population for melanoma has never been shown to save lives and likely is responsible for the increase in melanoma overdiagnosis,” Dr. Adamson said in an interview. “Screening average- and/or low-risk patients is of low value and the harms may outweigh the theoretical benefits.”
Screening programs should be directed to those who may derive the most benefit. “Screening should be limited to high-risk patients such as older White men, patients with a lot of atypical nevi, heavy sun exposure, fair skin, and red hair,” he said. “Just like for other cancers, such as breast, prostate, and colorectal, there should be clear guidelines as to which populations to screen, as well as when to start and when to stop screening.”
Overdiagnosis is defined as the diagnosis of cancer that would never have caused any symptoms or problems in a patient’s lifetime. But therein lies the problem, explained Dr. Adamson. “Because we do not know which early, screen-detected skin cancers would be destined to progress, we are obligated to treat all of them.” There is evidence to suggest that melanoma in situ is not an obligate precursor lesion to invasive melanoma, similar to the situation in which not all ductal carcinoma in situ leads to invasive breast cancer. “It is possible that less aggressive management strategies could be the subject of future studies,” he said.
Patients out of healthy people
For their study, Dr. Adamson and colleagues compared rates of melanoma among White and Black patients. Melanoma is much less common among Black individuals, and they are also less likely to be screened. Additionally, screening rates among Black patients have remained more or less the same over the last decades, whereas screening has increased in White patients.
The team used trends in mortality as a result of melanoma in Black patients as a marker for improvements in medical care. From this, they estimated the expected mortality trends in White patients if medical care had not improved. This served as a marker for the change in true cancer occurrence. Overdiagnosis was calculated as the difference between observed incidence and estimated true cancer occurrence.
The incidence of melanoma rose dramatically among White patients from 1975 to 2014, increasing about fourfold in White women (incidence rate ratio, 4.01) and sixfold in White men (IRR, 5.97).
At the same time, there was much smaller increase (of less than 25%) in the incidence of melanoma in both Black women and Black men.
In that time period, melanoma-related mortality decreased approximately 25% in Black women and men; it remained stable in White women, but increased almost 50% in White men.
Had medical care not improved, estimated mortality would have increased 60% in White women and more than doubled in White men, the authors assert.
Guidelines needed
“Recognizing and addressing overdiagnosis is important,” said Anthony J. Olszanski, MD, RPh, associate professor, department of hematology/oncology at Fox Chase Cancer Center, Philadelphia, who was approached for comment on the paper.
That said, Dr. Olszanski noted that this particular study has important limitations. “It is, by nature, a retrospective study using data from the [Surveillance, Epidemiology, and End Results] database registry, limited to patients only in the U.S., and uses a control group of Black patients to estimate overdiagnosis in White patients. These important factors can certainly influence their findings. However, the paper also notes that White men have realized a true increase in diagnosis, backed by a notable increase in mortality.”
The findings should and do raise a number of provocative questions, Dr. Olszanski emphasized. “Should we curtail public screening? Should we mandate revised guidelines for biopsies or pathologic diagnosis?
“As a medical oncologist,” he continued, “I treat patients who clearly do not have benign disease and so it is easy for me to be biased toward aggressive screening. However, it is my opinion that we should develop guidelines aimed at lessening this apparent overdiagnosis.”
These guidelines should be based on prospective studies and would better define which lesions are most suspect and should be biopsied, which are rational for ongoing surveillance, and what pathologic features are most consistent with melanoma, he noted. “We also need to continue to educate the public, as all too often I see the patient who ignored a lesion that was changing over time. A changing lesion requires medical attention. Importantly, we likewise need to improve our commitment in educating the public about the risks of excessive ultraviolet radiation exposure and how to avoid it, as prevention continues to be a most prudent course.”
Screening catches disease early
Another expert approached for comment emphasized that identifying melanomas early on may prevent the need for aggressive therapy. “Many primary melanomas in the U.S. are diagnosed now at an early stage and are cured with surgery, and that hardly constitutes overdiagnosis,” said Jeffrey S. Weber, MD, PhD, deputy director of the Perlmutter Cancer Center and codirector of the melanoma research program, New York University Langone Health.
“In addition, the death rate from melanoma is likely decreased due to the advent of more effective therapies for metastatic disease, and the increasing use of adjuvant immune and targeted therapies that are highly effective at preventing relapse and undoubtedly at prolonging survival, but they have been approved only since 2017-2018,” he added.
This study was supported in part by the Robert Wood Johnson Foundation. Dr. Adamson and Dr. Olszanski disclosed no relevant financial relationships. Dr. Weber disclosed relationships with numerous pharmaceutical companies and holds equity in CytoMx, Biond, Neximmune, and Immunimax.
A version of this article first appeared on Medscape.com.
Melanoma has been increasing in incidence in the United States over the last few decades, but is this a true increase? Or is this a case of overdiagnosis, fueled by screening?
A new study argues the case for overdiagnosis.
commented lead author lead author Adewole Adamson, MD, an assistant professor of internal medicine, division of dermatology, at the University of Texas at Austin.
He posted this conclusion on Twitter after the study was published in JAMA Dermatology.
“The discrepancies in incidence and mortality trends found in this cohort study suggest considerable overdiagnosis of melanoma occurring among White patients in the U.S.,” the authors concluded.
They estimated that an estimated 59% of White women and 60% of White men with melanoma were overdiagnosed in 2014.
These results are similar to those from a recent study from Australia, which used a different method of assessing overdiagnosis. Those findings estimated that 54%-58% of melanoma cases represented overdiagnosis in Australia, Dr. Adamson noted.
“Our estimates shed light on the HUGE scope of this problem in the United States that we need to address,” Dr. Adamson commented on Twitter. “Calls for screening for melanoma in the general public will only push these numbers higher, and make patients out of healthy people.”
“Screening the general population for melanoma has never been shown to save lives and likely is responsible for the increase in melanoma overdiagnosis,” Dr. Adamson said in an interview. “Screening average- and/or low-risk patients is of low value and the harms may outweigh the theoretical benefits.”
Screening programs should be directed to those who may derive the most benefit. “Screening should be limited to high-risk patients such as older White men, patients with a lot of atypical nevi, heavy sun exposure, fair skin, and red hair,” he said. “Just like for other cancers, such as breast, prostate, and colorectal, there should be clear guidelines as to which populations to screen, as well as when to start and when to stop screening.”
Overdiagnosis is defined as the diagnosis of cancer that would never have caused any symptoms or problems in a patient’s lifetime. But therein lies the problem, explained Dr. Adamson. “Because we do not know which early, screen-detected skin cancers would be destined to progress, we are obligated to treat all of them.” There is evidence to suggest that melanoma in situ is not an obligate precursor lesion to invasive melanoma, similar to the situation in which not all ductal carcinoma in situ leads to invasive breast cancer. “It is possible that less aggressive management strategies could be the subject of future studies,” he said.
Patients out of healthy people
For their study, Dr. Adamson and colleagues compared rates of melanoma among White and Black patients. Melanoma is much less common among Black individuals, and they are also less likely to be screened. Additionally, screening rates among Black patients have remained more or less the same over the last decades, whereas screening has increased in White patients.
The team used trends in mortality as a result of melanoma in Black patients as a marker for improvements in medical care. From this, they estimated the expected mortality trends in White patients if medical care had not improved. This served as a marker for the change in true cancer occurrence. Overdiagnosis was calculated as the difference between observed incidence and estimated true cancer occurrence.
The incidence of melanoma rose dramatically among White patients from 1975 to 2014, increasing about fourfold in White women (incidence rate ratio, 4.01) and sixfold in White men (IRR, 5.97).
At the same time, there was much smaller increase (of less than 25%) in the incidence of melanoma in both Black women and Black men.
In that time period, melanoma-related mortality decreased approximately 25% in Black women and men; it remained stable in White women, but increased almost 50% in White men.
Had medical care not improved, estimated mortality would have increased 60% in White women and more than doubled in White men, the authors assert.
Guidelines needed
“Recognizing and addressing overdiagnosis is important,” said Anthony J. Olszanski, MD, RPh, associate professor, department of hematology/oncology at Fox Chase Cancer Center, Philadelphia, who was approached for comment on the paper.
That said, Dr. Olszanski noted that this particular study has important limitations. “It is, by nature, a retrospective study using data from the [Surveillance, Epidemiology, and End Results] database registry, limited to patients only in the U.S., and uses a control group of Black patients to estimate overdiagnosis in White patients. These important factors can certainly influence their findings. However, the paper also notes that White men have realized a true increase in diagnosis, backed by a notable increase in mortality.”
The findings should and do raise a number of provocative questions, Dr. Olszanski emphasized. “Should we curtail public screening? Should we mandate revised guidelines for biopsies or pathologic diagnosis?
“As a medical oncologist,” he continued, “I treat patients who clearly do not have benign disease and so it is easy for me to be biased toward aggressive screening. However, it is my opinion that we should develop guidelines aimed at lessening this apparent overdiagnosis.”
These guidelines should be based on prospective studies and would better define which lesions are most suspect and should be biopsied, which are rational for ongoing surveillance, and what pathologic features are most consistent with melanoma, he noted. “We also need to continue to educate the public, as all too often I see the patient who ignored a lesion that was changing over time. A changing lesion requires medical attention. Importantly, we likewise need to improve our commitment in educating the public about the risks of excessive ultraviolet radiation exposure and how to avoid it, as prevention continues to be a most prudent course.”
Screening catches disease early
Another expert approached for comment emphasized that identifying melanomas early on may prevent the need for aggressive therapy. “Many primary melanomas in the U.S. are diagnosed now at an early stage and are cured with surgery, and that hardly constitutes overdiagnosis,” said Jeffrey S. Weber, MD, PhD, deputy director of the Perlmutter Cancer Center and codirector of the melanoma research program, New York University Langone Health.
“In addition, the death rate from melanoma is likely decreased due to the advent of more effective therapies for metastatic disease, and the increasing use of adjuvant immune and targeted therapies that are highly effective at preventing relapse and undoubtedly at prolonging survival, but they have been approved only since 2017-2018,” he added.
This study was supported in part by the Robert Wood Johnson Foundation. Dr. Adamson and Dr. Olszanski disclosed no relevant financial relationships. Dr. Weber disclosed relationships with numerous pharmaceutical companies and holds equity in CytoMx, Biond, Neximmune, and Immunimax.
A version of this article first appeared on Medscape.com.
Melanoma has been increasing in incidence in the United States over the last few decades, but is this a true increase? Or is this a case of overdiagnosis, fueled by screening?
A new study argues the case for overdiagnosis.
commented lead author lead author Adewole Adamson, MD, an assistant professor of internal medicine, division of dermatology, at the University of Texas at Austin.
He posted this conclusion on Twitter after the study was published in JAMA Dermatology.
“The discrepancies in incidence and mortality trends found in this cohort study suggest considerable overdiagnosis of melanoma occurring among White patients in the U.S.,” the authors concluded.
They estimated that an estimated 59% of White women and 60% of White men with melanoma were overdiagnosed in 2014.
These results are similar to those from a recent study from Australia, which used a different method of assessing overdiagnosis. Those findings estimated that 54%-58% of melanoma cases represented overdiagnosis in Australia, Dr. Adamson noted.
“Our estimates shed light on the HUGE scope of this problem in the United States that we need to address,” Dr. Adamson commented on Twitter. “Calls for screening for melanoma in the general public will only push these numbers higher, and make patients out of healthy people.”
“Screening the general population for melanoma has never been shown to save lives and likely is responsible for the increase in melanoma overdiagnosis,” Dr. Adamson said in an interview. “Screening average- and/or low-risk patients is of low value and the harms may outweigh the theoretical benefits.”
Screening programs should be directed to those who may derive the most benefit. “Screening should be limited to high-risk patients such as older White men, patients with a lot of atypical nevi, heavy sun exposure, fair skin, and red hair,” he said. “Just like for other cancers, such as breast, prostate, and colorectal, there should be clear guidelines as to which populations to screen, as well as when to start and when to stop screening.”
Overdiagnosis is defined as the diagnosis of cancer that would never have caused any symptoms or problems in a patient’s lifetime. But therein lies the problem, explained Dr. Adamson. “Because we do not know which early, screen-detected skin cancers would be destined to progress, we are obligated to treat all of them.” There is evidence to suggest that melanoma in situ is not an obligate precursor lesion to invasive melanoma, similar to the situation in which not all ductal carcinoma in situ leads to invasive breast cancer. “It is possible that less aggressive management strategies could be the subject of future studies,” he said.
Patients out of healthy people
For their study, Dr. Adamson and colleagues compared rates of melanoma among White and Black patients. Melanoma is much less common among Black individuals, and they are also less likely to be screened. Additionally, screening rates among Black patients have remained more or less the same over the last decades, whereas screening has increased in White patients.
The team used trends in mortality as a result of melanoma in Black patients as a marker for improvements in medical care. From this, they estimated the expected mortality trends in White patients if medical care had not improved. This served as a marker for the change in true cancer occurrence. Overdiagnosis was calculated as the difference between observed incidence and estimated true cancer occurrence.
The incidence of melanoma rose dramatically among White patients from 1975 to 2014, increasing about fourfold in White women (incidence rate ratio, 4.01) and sixfold in White men (IRR, 5.97).
At the same time, there was much smaller increase (of less than 25%) in the incidence of melanoma in both Black women and Black men.
In that time period, melanoma-related mortality decreased approximately 25% in Black women and men; it remained stable in White women, but increased almost 50% in White men.
Had medical care not improved, estimated mortality would have increased 60% in White women and more than doubled in White men, the authors assert.
Guidelines needed
“Recognizing and addressing overdiagnosis is important,” said Anthony J. Olszanski, MD, RPh, associate professor, department of hematology/oncology at Fox Chase Cancer Center, Philadelphia, who was approached for comment on the paper.
That said, Dr. Olszanski noted that this particular study has important limitations. “It is, by nature, a retrospective study using data from the [Surveillance, Epidemiology, and End Results] database registry, limited to patients only in the U.S., and uses a control group of Black patients to estimate overdiagnosis in White patients. These important factors can certainly influence their findings. However, the paper also notes that White men have realized a true increase in diagnosis, backed by a notable increase in mortality.”
The findings should and do raise a number of provocative questions, Dr. Olszanski emphasized. “Should we curtail public screening? Should we mandate revised guidelines for biopsies or pathologic diagnosis?
“As a medical oncologist,” he continued, “I treat patients who clearly do not have benign disease and so it is easy for me to be biased toward aggressive screening. However, it is my opinion that we should develop guidelines aimed at lessening this apparent overdiagnosis.”
These guidelines should be based on prospective studies and would better define which lesions are most suspect and should be biopsied, which are rational for ongoing surveillance, and what pathologic features are most consistent with melanoma, he noted. “We also need to continue to educate the public, as all too often I see the patient who ignored a lesion that was changing over time. A changing lesion requires medical attention. Importantly, we likewise need to improve our commitment in educating the public about the risks of excessive ultraviolet radiation exposure and how to avoid it, as prevention continues to be a most prudent course.”
Screening catches disease early
Another expert approached for comment emphasized that identifying melanomas early on may prevent the need for aggressive therapy. “Many primary melanomas in the U.S. are diagnosed now at an early stage and are cured with surgery, and that hardly constitutes overdiagnosis,” said Jeffrey S. Weber, MD, PhD, deputy director of the Perlmutter Cancer Center and codirector of the melanoma research program, New York University Langone Health.
“In addition, the death rate from melanoma is likely decreased due to the advent of more effective therapies for metastatic disease, and the increasing use of adjuvant immune and targeted therapies that are highly effective at preventing relapse and undoubtedly at prolonging survival, but they have been approved only since 2017-2018,” he added.
This study was supported in part by the Robert Wood Johnson Foundation. Dr. Adamson and Dr. Olszanski disclosed no relevant financial relationships. Dr. Weber disclosed relationships with numerous pharmaceutical companies and holds equity in CytoMx, Biond, Neximmune, and Immunimax.
A version of this article first appeared on Medscape.com.
FROM JAMA DERMATOLOGY







