User login
Formerly Skin & Allergy News
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')]
The leading independent newspaper covering dermatology news and commentary.
12 state boards have disciplined docs for COVID misinformation
, according to a new survey from the Federation of State Medical Boards (FSMB).
The FSMB reports that in its 2021 annual survey two-thirds of its 71 member boards (which includes the United States, its territories, and Washington, DC) reported an increase in complaints about doctors spreading false or misleading information.
“The staggering number of state medical boards that have seen an increase in COVID-19 disinformation complaints is a sign of how widespread the issue has become,” said Humayun J. Chaudhry, DO, MACP, president and CEO of the FSMB, in a statement.
The FSMB board of directors warned physicians in July that they risked disciplinary action if they spread COVID-19 vaccine misinformation or disinformation.
The organization said 15 state boards have now adopted similar statements.
Dr. Chaudhry said the FSMB was “encouraged by the number of boards that have already taken action to combat COVID-19 disinformation by disciplining physicians who engage in that behavior and by reminding all physicians that their words and actions matter, and they should think twice before spreading disinformation that may harm patients.”
This news organization asked the FSMB for further comment on why more physicians have not been disciplined, but did not receive a response before publication.
Misinformation policies a new battleground
The FSMB and member board policies on COVID-19 around the country have become a new front in the war against mandates and restrictions.
The Tennessee Board of Medical Examiners voted just recently to remove its statement of policy against the spread of misinformation from its website after a Republican lawmaker allegedly threatened to dissolve the board.
The vote came just a few months after the board had approved the policy. The board did not rescind the policy, however, according to a report by the Associated Press.
In California, the president of the state’s medical board tweeted on December 8 about what she said was an incident of harassment by a group that has promoted “fake COVID-19 treatments.”Ms. Kristina Lawson said she observed four men sitting in front of her house in a truck. They flew a drone over her residence, and then followed her to work, parking nose-to-nose with her vehicle.
Ms. Lawson claimed that when she went to drive home the four men ambushed her in what was by then a dark parking garage. She said her “concern turned to terror” as they jumped out, cameras and recording equipment in hand.
The men told law enforcement called to the scene that they were just trying to interview her, according to a statement emailed by Ms. Lawson.
They had not made such a request to the California Medical Board.
Ms. Lawson tweeted that she would continue to volunteer for the board. “That means protecting Californians from bad doctors, and ensuring disinformation and misinformation do not detract from our work to protect patients and consumers,” she wrote.
The men who ambushed Ms. Larson allegedly identified themselves and were wearing clothing emblazoned with the logo of “America’s Frontline Doctors,” an organization that has trafficked in COVID-19 conspiracy theories and promoted unproven treatments like hydroxychloroquine and ivermectin, according to Time. It is led by Simone Gold, MD, who was arrested for breaching the U.S. Capitol on January 6.
Despite her activities, on November 30, the California Medical Board renewed Ms. Gold’s 2-year license to practice.
Who’s being disciplined, who’s not
Dr. Gold is not alone. An investigation by NPRin September found that 15 of 16 physicians who have spread false information in a high-profile manner have medical licenses in good standing.
Sherri Tenpenny, DO, who has claimed that COVID-19 vaccines magnetize people and “interface” with 5G cell phone towers, was able to renew her license with the Ohio State Medical Board on October 1, according to the Cincinnati Enquirer.
Some boards have acted. The Oregon Medical Board revoked the license of Steven LaTulippe, MD, and fined him $10,000 for spreading misinformation about masks and overprescribing opioids.
In August, Rhode Island’s Board of Medical Licensure suspended Mark Brody’s license for 5 years after finding that the doctor spread falsehoods about COVID-19 vaccines, according to board documents.
Maine physician Paul Gosselin, DO, is on temporary suspension until a February hearing, while the osteopathic board investigates his issuance of vaccine exemption letters and the promotion of unproven COVID-19 therapies.
The board found that Gosselin had “engaged in conduct that constitutes fraud or deceit,” according to official documents.
The Washington State Medical Board has opened an investigation into Ryan N. Cole, MD, a physician who has claimed that COVID vaccines are “fake,” and was appointed to a regional health board in Idaho in September, according to the Washington Post.
The Idaho Capital Sun reported that Dr. Cole claims he is licensed in 11 states, including Washington. The Idaho Medical Association has also filed a complaint about Dr. Cole with the Idaho Board of Medicine, according to the paper.
New FSMB guidance coming
The FSMB said it expects more disciplinary actions as investigations continue to unfold.
The organization is drafting a new policy document that will include further guidelines and recommendations for state medical boards “to help address the spread of disinformation,” it said. The final document would be released in April 2022.
In the meantime, some states, like Tennessee and others, are trying to find ways to counter the current policy — a development the FSMB called “troubling.”
“The FSMB strongly opposes any effort to restrict a board’s authority to evaluate the standard of care and assess risk for patient harm,” the organization said in its statement.
A version of this article was first published on Medscape.com.
, according to a new survey from the Federation of State Medical Boards (FSMB).
The FSMB reports that in its 2021 annual survey two-thirds of its 71 member boards (which includes the United States, its territories, and Washington, DC) reported an increase in complaints about doctors spreading false or misleading information.
“The staggering number of state medical boards that have seen an increase in COVID-19 disinformation complaints is a sign of how widespread the issue has become,” said Humayun J. Chaudhry, DO, MACP, president and CEO of the FSMB, in a statement.
The FSMB board of directors warned physicians in July that they risked disciplinary action if they spread COVID-19 vaccine misinformation or disinformation.
The organization said 15 state boards have now adopted similar statements.
Dr. Chaudhry said the FSMB was “encouraged by the number of boards that have already taken action to combat COVID-19 disinformation by disciplining physicians who engage in that behavior and by reminding all physicians that their words and actions matter, and they should think twice before spreading disinformation that may harm patients.”
This news organization asked the FSMB for further comment on why more physicians have not been disciplined, but did not receive a response before publication.
Misinformation policies a new battleground
The FSMB and member board policies on COVID-19 around the country have become a new front in the war against mandates and restrictions.
The Tennessee Board of Medical Examiners voted just recently to remove its statement of policy against the spread of misinformation from its website after a Republican lawmaker allegedly threatened to dissolve the board.
The vote came just a few months after the board had approved the policy. The board did not rescind the policy, however, according to a report by the Associated Press.
In California, the president of the state’s medical board tweeted on December 8 about what she said was an incident of harassment by a group that has promoted “fake COVID-19 treatments.”Ms. Kristina Lawson said she observed four men sitting in front of her house in a truck. They flew a drone over her residence, and then followed her to work, parking nose-to-nose with her vehicle.
Ms. Lawson claimed that when she went to drive home the four men ambushed her in what was by then a dark parking garage. She said her “concern turned to terror” as they jumped out, cameras and recording equipment in hand.
The men told law enforcement called to the scene that they were just trying to interview her, according to a statement emailed by Ms. Lawson.
They had not made such a request to the California Medical Board.
Ms. Lawson tweeted that she would continue to volunteer for the board. “That means protecting Californians from bad doctors, and ensuring disinformation and misinformation do not detract from our work to protect patients and consumers,” she wrote.
The men who ambushed Ms. Larson allegedly identified themselves and were wearing clothing emblazoned with the logo of “America’s Frontline Doctors,” an organization that has trafficked in COVID-19 conspiracy theories and promoted unproven treatments like hydroxychloroquine and ivermectin, according to Time. It is led by Simone Gold, MD, who was arrested for breaching the U.S. Capitol on January 6.
Despite her activities, on November 30, the California Medical Board renewed Ms. Gold’s 2-year license to practice.
Who’s being disciplined, who’s not
Dr. Gold is not alone. An investigation by NPRin September found that 15 of 16 physicians who have spread false information in a high-profile manner have medical licenses in good standing.
Sherri Tenpenny, DO, who has claimed that COVID-19 vaccines magnetize people and “interface” with 5G cell phone towers, was able to renew her license with the Ohio State Medical Board on October 1, according to the Cincinnati Enquirer.
Some boards have acted. The Oregon Medical Board revoked the license of Steven LaTulippe, MD, and fined him $10,000 for spreading misinformation about masks and overprescribing opioids.
In August, Rhode Island’s Board of Medical Licensure suspended Mark Brody’s license for 5 years after finding that the doctor spread falsehoods about COVID-19 vaccines, according to board documents.
Maine physician Paul Gosselin, DO, is on temporary suspension until a February hearing, while the osteopathic board investigates his issuance of vaccine exemption letters and the promotion of unproven COVID-19 therapies.
The board found that Gosselin had “engaged in conduct that constitutes fraud or deceit,” according to official documents.
The Washington State Medical Board has opened an investigation into Ryan N. Cole, MD, a physician who has claimed that COVID vaccines are “fake,” and was appointed to a regional health board in Idaho in September, according to the Washington Post.
The Idaho Capital Sun reported that Dr. Cole claims he is licensed in 11 states, including Washington. The Idaho Medical Association has also filed a complaint about Dr. Cole with the Idaho Board of Medicine, according to the paper.
New FSMB guidance coming
The FSMB said it expects more disciplinary actions as investigations continue to unfold.
The organization is drafting a new policy document that will include further guidelines and recommendations for state medical boards “to help address the spread of disinformation,” it said. The final document would be released in April 2022.
In the meantime, some states, like Tennessee and others, are trying to find ways to counter the current policy — a development the FSMB called “troubling.”
“The FSMB strongly opposes any effort to restrict a board’s authority to evaluate the standard of care and assess risk for patient harm,” the organization said in its statement.
A version of this article was first published on Medscape.com.
, according to a new survey from the Federation of State Medical Boards (FSMB).
The FSMB reports that in its 2021 annual survey two-thirds of its 71 member boards (which includes the United States, its territories, and Washington, DC) reported an increase in complaints about doctors spreading false or misleading information.
“The staggering number of state medical boards that have seen an increase in COVID-19 disinformation complaints is a sign of how widespread the issue has become,” said Humayun J. Chaudhry, DO, MACP, president and CEO of the FSMB, in a statement.
The FSMB board of directors warned physicians in July that they risked disciplinary action if they spread COVID-19 vaccine misinformation or disinformation.
The organization said 15 state boards have now adopted similar statements.
Dr. Chaudhry said the FSMB was “encouraged by the number of boards that have already taken action to combat COVID-19 disinformation by disciplining physicians who engage in that behavior and by reminding all physicians that their words and actions matter, and they should think twice before spreading disinformation that may harm patients.”
This news organization asked the FSMB for further comment on why more physicians have not been disciplined, but did not receive a response before publication.
Misinformation policies a new battleground
The FSMB and member board policies on COVID-19 around the country have become a new front in the war against mandates and restrictions.
The Tennessee Board of Medical Examiners voted just recently to remove its statement of policy against the spread of misinformation from its website after a Republican lawmaker allegedly threatened to dissolve the board.
The vote came just a few months after the board had approved the policy. The board did not rescind the policy, however, according to a report by the Associated Press.
In California, the president of the state’s medical board tweeted on December 8 about what she said was an incident of harassment by a group that has promoted “fake COVID-19 treatments.”Ms. Kristina Lawson said she observed four men sitting in front of her house in a truck. They flew a drone over her residence, and then followed her to work, parking nose-to-nose with her vehicle.
Ms. Lawson claimed that when she went to drive home the four men ambushed her in what was by then a dark parking garage. She said her “concern turned to terror” as they jumped out, cameras and recording equipment in hand.
The men told law enforcement called to the scene that they were just trying to interview her, according to a statement emailed by Ms. Lawson.
They had not made such a request to the California Medical Board.
Ms. Lawson tweeted that she would continue to volunteer for the board. “That means protecting Californians from bad doctors, and ensuring disinformation and misinformation do not detract from our work to protect patients and consumers,” she wrote.
The men who ambushed Ms. Larson allegedly identified themselves and were wearing clothing emblazoned with the logo of “America’s Frontline Doctors,” an organization that has trafficked in COVID-19 conspiracy theories and promoted unproven treatments like hydroxychloroquine and ivermectin, according to Time. It is led by Simone Gold, MD, who was arrested for breaching the U.S. Capitol on January 6.
Despite her activities, on November 30, the California Medical Board renewed Ms. Gold’s 2-year license to practice.
Who’s being disciplined, who’s not
Dr. Gold is not alone. An investigation by NPRin September found that 15 of 16 physicians who have spread false information in a high-profile manner have medical licenses in good standing.
Sherri Tenpenny, DO, who has claimed that COVID-19 vaccines magnetize people and “interface” with 5G cell phone towers, was able to renew her license with the Ohio State Medical Board on October 1, according to the Cincinnati Enquirer.
Some boards have acted. The Oregon Medical Board revoked the license of Steven LaTulippe, MD, and fined him $10,000 for spreading misinformation about masks and overprescribing opioids.
In August, Rhode Island’s Board of Medical Licensure suspended Mark Brody’s license for 5 years after finding that the doctor spread falsehoods about COVID-19 vaccines, according to board documents.
Maine physician Paul Gosselin, DO, is on temporary suspension until a February hearing, while the osteopathic board investigates his issuance of vaccine exemption letters and the promotion of unproven COVID-19 therapies.
The board found that Gosselin had “engaged in conduct that constitutes fraud or deceit,” according to official documents.
The Washington State Medical Board has opened an investigation into Ryan N. Cole, MD, a physician who has claimed that COVID vaccines are “fake,” and was appointed to a regional health board in Idaho in September, according to the Washington Post.
The Idaho Capital Sun reported that Dr. Cole claims he is licensed in 11 states, including Washington. The Idaho Medical Association has also filed a complaint about Dr. Cole with the Idaho Board of Medicine, according to the paper.
New FSMB guidance coming
The FSMB said it expects more disciplinary actions as investigations continue to unfold.
The organization is drafting a new policy document that will include further guidelines and recommendations for state medical boards “to help address the spread of disinformation,” it said. The final document would be released in April 2022.
In the meantime, some states, like Tennessee and others, are trying to find ways to counter the current policy — a development the FSMB called “troubling.”
“The FSMB strongly opposes any effort to restrict a board’s authority to evaluate the standard of care and assess risk for patient harm,” the organization said in its statement.
A version of this article was first published on Medscape.com.
Major COVID-19 case growth expected in coming weeks
by the PolicyLab at Children’s Hospital of Philadelphia.
Large metropolitan areas, especially those in the Northeast, are already seeing a major increase in cases following Thanksgiving, and that trend is expected to continue.
“Why? Simply stated, the large amount of Thanksgiving travel and gatherings undermined the nation’s pandemic footing and has elevated disease burden in areas of the country that were fortunate to have lower case rates before the holidays,” the forecasters wrote.
Case numbers in New York City are expected to double throughout December, the forecasters said. Similar growth could happen across Boston, Philadelphia, and Baltimore.
Overall, COVID-19 cases, hospitalizations, and deaths are rising across the United States but remain below levels seen during the summer and last winter’s surges, according to the New York Times. The increase is still being driven by the Delta variant, though it remains unclear how the Omicron variant, which has been detected in 27 states, could affect the trends in the coming weeks.
During the past week, the United States has reported an average of more than 120,000 new cases each day, the newspaper reported, which is an increase of 38% from two weeks ago.
The daily average of COVID-19 hospitalizations is around 64,000, which marks an increase of 22% from two weeks ago. More than 1,300 deaths are being reported each day, which is up 26%.
Numerous states are reporting double the cases from two weeks ago, stretching across the country from states in the Northeast such as Connecticut and Rhode Island to southern states such as North Carolina and Texas and western states such as California.
The Great Lakes region and the Northeast are seeing some of the most severe increases, the newspaper reported. New Hampshire leads the United States in recent cases per capita, and Maine has reported more cases in the past week than in any other seven-day period during the pandemic.
Michigan has the country’s highest hospitalization rate, and federal medical teams have been sent to the state to help with the surge in patients, according to The Detroit News. Michigan’s top public health officials described the surge as a “critical” and “deeply concerning” situation on Dec. 10, and they requested 200 more ventilators from the Strategic National Stockpile.
Indiana, Maine, and New York have also requested aid from the National Guard, according to USA Today. Health officials in those states urged residents to get vaccines or booster shots and wear masks in indoor public settings.
The Omicron variant can evade some vaccine protection, but booster shots can increase efficacy and offer more coverage, Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, said Dec. 12.
“If you want to be optimally protected, absolutely get a booster,” he said on ABC’s “This Week.”
In addition, New York Gov. Kathy Hochul has announced a statewide mask mandate, which will take effect Dec. 13. Masks will be required in all indoor public spaces and businesses, unless the location implements a vaccine requirement instead, the news outlet reported.
A version of this article first appeared on WebMD.com.
by the PolicyLab at Children’s Hospital of Philadelphia.
Large metropolitan areas, especially those in the Northeast, are already seeing a major increase in cases following Thanksgiving, and that trend is expected to continue.
“Why? Simply stated, the large amount of Thanksgiving travel and gatherings undermined the nation’s pandemic footing and has elevated disease burden in areas of the country that were fortunate to have lower case rates before the holidays,” the forecasters wrote.
Case numbers in New York City are expected to double throughout December, the forecasters said. Similar growth could happen across Boston, Philadelphia, and Baltimore.
Overall, COVID-19 cases, hospitalizations, and deaths are rising across the United States but remain below levels seen during the summer and last winter’s surges, according to the New York Times. The increase is still being driven by the Delta variant, though it remains unclear how the Omicron variant, which has been detected in 27 states, could affect the trends in the coming weeks.
During the past week, the United States has reported an average of more than 120,000 new cases each day, the newspaper reported, which is an increase of 38% from two weeks ago.
The daily average of COVID-19 hospitalizations is around 64,000, which marks an increase of 22% from two weeks ago. More than 1,300 deaths are being reported each day, which is up 26%.
Numerous states are reporting double the cases from two weeks ago, stretching across the country from states in the Northeast such as Connecticut and Rhode Island to southern states such as North Carolina and Texas and western states such as California.
The Great Lakes region and the Northeast are seeing some of the most severe increases, the newspaper reported. New Hampshire leads the United States in recent cases per capita, and Maine has reported more cases in the past week than in any other seven-day period during the pandemic.
Michigan has the country’s highest hospitalization rate, and federal medical teams have been sent to the state to help with the surge in patients, according to The Detroit News. Michigan’s top public health officials described the surge as a “critical” and “deeply concerning” situation on Dec. 10, and they requested 200 more ventilators from the Strategic National Stockpile.
Indiana, Maine, and New York have also requested aid from the National Guard, according to USA Today. Health officials in those states urged residents to get vaccines or booster shots and wear masks in indoor public settings.
The Omicron variant can evade some vaccine protection, but booster shots can increase efficacy and offer more coverage, Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, said Dec. 12.
“If you want to be optimally protected, absolutely get a booster,” he said on ABC’s “This Week.”
In addition, New York Gov. Kathy Hochul has announced a statewide mask mandate, which will take effect Dec. 13. Masks will be required in all indoor public spaces and businesses, unless the location implements a vaccine requirement instead, the news outlet reported.
A version of this article first appeared on WebMD.com.
by the PolicyLab at Children’s Hospital of Philadelphia.
Large metropolitan areas, especially those in the Northeast, are already seeing a major increase in cases following Thanksgiving, and that trend is expected to continue.
“Why? Simply stated, the large amount of Thanksgiving travel and gatherings undermined the nation’s pandemic footing and has elevated disease burden in areas of the country that were fortunate to have lower case rates before the holidays,” the forecasters wrote.
Case numbers in New York City are expected to double throughout December, the forecasters said. Similar growth could happen across Boston, Philadelphia, and Baltimore.
Overall, COVID-19 cases, hospitalizations, and deaths are rising across the United States but remain below levels seen during the summer and last winter’s surges, according to the New York Times. The increase is still being driven by the Delta variant, though it remains unclear how the Omicron variant, which has been detected in 27 states, could affect the trends in the coming weeks.
During the past week, the United States has reported an average of more than 120,000 new cases each day, the newspaper reported, which is an increase of 38% from two weeks ago.
The daily average of COVID-19 hospitalizations is around 64,000, which marks an increase of 22% from two weeks ago. More than 1,300 deaths are being reported each day, which is up 26%.
Numerous states are reporting double the cases from two weeks ago, stretching across the country from states in the Northeast such as Connecticut and Rhode Island to southern states such as North Carolina and Texas and western states such as California.
The Great Lakes region and the Northeast are seeing some of the most severe increases, the newspaper reported. New Hampshire leads the United States in recent cases per capita, and Maine has reported more cases in the past week than in any other seven-day period during the pandemic.
Michigan has the country’s highest hospitalization rate, and federal medical teams have been sent to the state to help with the surge in patients, according to The Detroit News. Michigan’s top public health officials described the surge as a “critical” and “deeply concerning” situation on Dec. 10, and they requested 200 more ventilators from the Strategic National Stockpile.
Indiana, Maine, and New York have also requested aid from the National Guard, according to USA Today. Health officials in those states urged residents to get vaccines or booster shots and wear masks in indoor public settings.
The Omicron variant can evade some vaccine protection, but booster shots can increase efficacy and offer more coverage, Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, said Dec. 12.
“If you want to be optimally protected, absolutely get a booster,” he said on ABC’s “This Week.”
In addition, New York Gov. Kathy Hochul has announced a statewide mask mandate, which will take effect Dec. 13. Masks will be required in all indoor public spaces and businesses, unless the location implements a vaccine requirement instead, the news outlet reported.
A version of this article first appeared on WebMD.com.
Abrocitinib approved for atopic dermatitis in Europe
who are candidates for systemic therapy, the manufacturer announced.
Approval by the European Commission was based on the results of studies that include four phase 3 clinical trials (JADE MONO-1, JADE-MONO-2, JADE COMPARE, JADE REGIMEN) and an ongoing open-label extension study (JADE EXTEND) in over 2,800 patients, according to the Pfizer press release announcing the approval. The approved doses are 100 and 200 mg a day; a 50-mg dose was approved for patients with moderate and severe renal impairment and “ certain patients receiving treatment with inhibitors of cytochrome P450 (CYP) 2C19,” the release said.
The approval follows a positive opinion by the Committee for Medicinal Products for Human Use of the European Medicines Agency supporting marketing authorization for treating AD, issued in October. It will be marketed as Cibinqo.
Abrocitinib is under review at the Food and Drug Administration. It was approved earlier in 2021 for treating AD in the United Kingdom, Japan, and Korea.
[email protected]
who are candidates for systemic therapy, the manufacturer announced.
Approval by the European Commission was based on the results of studies that include four phase 3 clinical trials (JADE MONO-1, JADE-MONO-2, JADE COMPARE, JADE REGIMEN) and an ongoing open-label extension study (JADE EXTEND) in over 2,800 patients, according to the Pfizer press release announcing the approval. The approved doses are 100 and 200 mg a day; a 50-mg dose was approved for patients with moderate and severe renal impairment and “ certain patients receiving treatment with inhibitors of cytochrome P450 (CYP) 2C19,” the release said.
The approval follows a positive opinion by the Committee for Medicinal Products for Human Use of the European Medicines Agency supporting marketing authorization for treating AD, issued in October. It will be marketed as Cibinqo.
Abrocitinib is under review at the Food and Drug Administration. It was approved earlier in 2021 for treating AD in the United Kingdom, Japan, and Korea.
[email protected]
who are candidates for systemic therapy, the manufacturer announced.
Approval by the European Commission was based on the results of studies that include four phase 3 clinical trials (JADE MONO-1, JADE-MONO-2, JADE COMPARE, JADE REGIMEN) and an ongoing open-label extension study (JADE EXTEND) in over 2,800 patients, according to the Pfizer press release announcing the approval. The approved doses are 100 and 200 mg a day; a 50-mg dose was approved for patients with moderate and severe renal impairment and “ certain patients receiving treatment with inhibitors of cytochrome P450 (CYP) 2C19,” the release said.
The approval follows a positive opinion by the Committee for Medicinal Products for Human Use of the European Medicines Agency supporting marketing authorization for treating AD, issued in October. It will be marketed as Cibinqo.
Abrocitinib is under review at the Food and Drug Administration. It was approved earlier in 2021 for treating AD in the United Kingdom, Japan, and Korea.
[email protected]
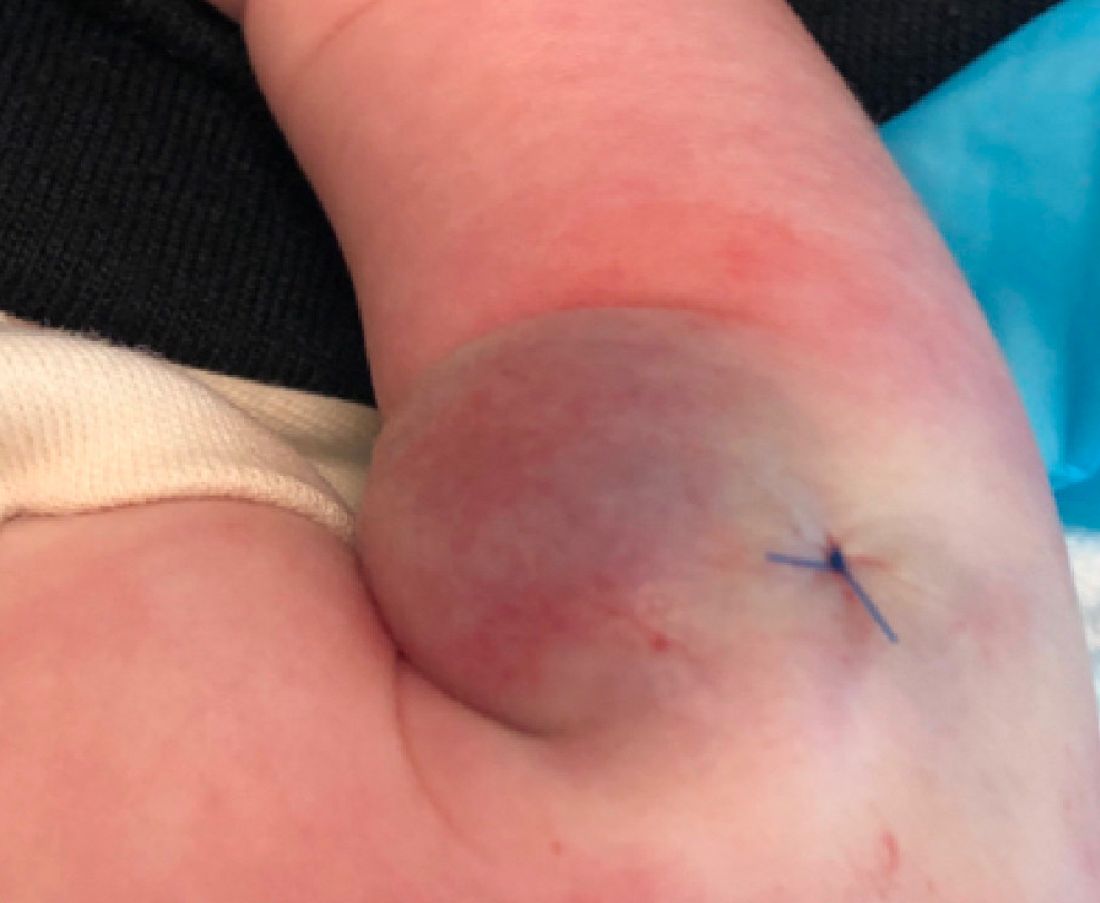
What is the diagnosis?
As the lesion was growing, getting more violaceous and indurated, a biopsy was performed. The biopsy showed multiple discrete lobules of dermal capillaries with slight extension into the superficial subcutis. Capillary lobules demonstrate the “cannonball-like” architecture often associated with tufted angioma, and some lobules showed bulging into adjacent thin-walled vessels. Spindled endothelial cells lining slit-like vessels were present in the mid dermis, although this comprises a minority of the lesion. The majority of the subcutis was uninvolved. The findings are overall most consistent with a tufted angioma.
Kaposiform hemangioendothelioma (KHE) has been considered given the presence of occasional slit-like vascular spaces; however, the lesion is predominantly superficial and therefore the lesion is best classified as tufted angioma. GLUT–1 staining was negative.
At the time of biopsy, blood work was ordered, which showed a normal complete blood count with normal number of platelets, slightly elevated D-dimer, and slightly low fibrinogen. Several repeat blood counts and coagulation tests once a week for a few weeks revealed no changes.
The patient was started on aspirin at a dose of 5 mg/kg per day. After a week on the medication the lesion was starting to get smaller and less red.
Tufted angiomas are a rare type of vascular tumor within the spectrum of kaposiform hemangioendotheliomas. Most cases present within the first year of life; some occur at birth. They usually present as papules, plaques, or erythematous, violaceous indurated nodules on the face, neck, trunk, and extremities. The lesions can also be present with hyperhidrosis and hypertrichosis. Clinically, the lesions will have to be differentiated from other vascular tumors such as infantile hemangiomas, congenital hemangiomas, and Kaposi’s sarcoma, as well as subcutaneous fat necrosis of the newborn, cellulitis, and nonaccidental trauma.
Pathogenesis of tufted angiomas is poorly understood. A recent case report found a somatic mutation on GNA14.This protein regulates Ras activity and modulates endothelial cell permeability and migration in response to FGF2 and VEGFA. The p.205L mutation causes activation of GNA14, which upregulates pERK-MAPK pathway, suggesting MAPK inhibition as a potential target for therapy. Clinically, tufted angioma can present in three patterns: uncomplicated tufted angioma (most common type); tufted angioma without thrombocytopenia but with chronic coagulopathy, as it was seen in our patient; and tufted angioma associated with Kasabach-Merritt phenomenon (KMP). KMP is characterized by thrombocytopenia in association with microangiopathic hemolytic anemia, consumptive coagulopathy, and enlarging vascular tumor. Treatment of uncomplicated tufted angioma will depend on symptomatology, size, and location of the lesion. Smaller lesions in noncosmetically sensitive areas can be treated with surgical excision. Cases that are not amenable to excision can be treated with aspirin. There are also reports of response to topical modalities including tacrolimus and timolol. For complicated cases associated with KMP, sirolimus, systemic corticosteroids, ticlopidine, interferon, or vincristine are recommended. Some lesions may demonstrate spontaneous regression.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego.
References
Cohen S et al. Dermatol Online J. 2019 Sep 15;25(9):13030/qt6pv254mc.
Lim YH et al. Pediatr Dermatol. 2019 Nov;36(6):963-4.
Prasuna A, Rao PN. Indian Dermatol Online J. 2015;6:266-8.
As the lesion was growing, getting more violaceous and indurated, a biopsy was performed. The biopsy showed multiple discrete lobules of dermal capillaries with slight extension into the superficial subcutis. Capillary lobules demonstrate the “cannonball-like” architecture often associated with tufted angioma, and some lobules showed bulging into adjacent thin-walled vessels. Spindled endothelial cells lining slit-like vessels were present in the mid dermis, although this comprises a minority of the lesion. The majority of the subcutis was uninvolved. The findings are overall most consistent with a tufted angioma.
Kaposiform hemangioendothelioma (KHE) has been considered given the presence of occasional slit-like vascular spaces; however, the lesion is predominantly superficial and therefore the lesion is best classified as tufted angioma. GLUT–1 staining was negative.
At the time of biopsy, blood work was ordered, which showed a normal complete blood count with normal number of platelets, slightly elevated D-dimer, and slightly low fibrinogen. Several repeat blood counts and coagulation tests once a week for a few weeks revealed no changes.
The patient was started on aspirin at a dose of 5 mg/kg per day. After a week on the medication the lesion was starting to get smaller and less red.
Tufted angiomas are a rare type of vascular tumor within the spectrum of kaposiform hemangioendotheliomas. Most cases present within the first year of life; some occur at birth. They usually present as papules, plaques, or erythematous, violaceous indurated nodules on the face, neck, trunk, and extremities. The lesions can also be present with hyperhidrosis and hypertrichosis. Clinically, the lesions will have to be differentiated from other vascular tumors such as infantile hemangiomas, congenital hemangiomas, and Kaposi’s sarcoma, as well as subcutaneous fat necrosis of the newborn, cellulitis, and nonaccidental trauma.
Pathogenesis of tufted angiomas is poorly understood. A recent case report found a somatic mutation on GNA14.This protein regulates Ras activity and modulates endothelial cell permeability and migration in response to FGF2 and VEGFA. The p.205L mutation causes activation of GNA14, which upregulates pERK-MAPK pathway, suggesting MAPK inhibition as a potential target for therapy. Clinically, tufted angioma can present in three patterns: uncomplicated tufted angioma (most common type); tufted angioma without thrombocytopenia but with chronic coagulopathy, as it was seen in our patient; and tufted angioma associated with Kasabach-Merritt phenomenon (KMP). KMP is characterized by thrombocytopenia in association with microangiopathic hemolytic anemia, consumptive coagulopathy, and enlarging vascular tumor. Treatment of uncomplicated tufted angioma will depend on symptomatology, size, and location of the lesion. Smaller lesions in noncosmetically sensitive areas can be treated with surgical excision. Cases that are not amenable to excision can be treated with aspirin. There are also reports of response to topical modalities including tacrolimus and timolol. For complicated cases associated with KMP, sirolimus, systemic corticosteroids, ticlopidine, interferon, or vincristine are recommended. Some lesions may demonstrate spontaneous regression.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego.
References
Cohen S et al. Dermatol Online J. 2019 Sep 15;25(9):13030/qt6pv254mc.
Lim YH et al. Pediatr Dermatol. 2019 Nov;36(6):963-4.
Prasuna A, Rao PN. Indian Dermatol Online J. 2015;6:266-8.
As the lesion was growing, getting more violaceous and indurated, a biopsy was performed. The biopsy showed multiple discrete lobules of dermal capillaries with slight extension into the superficial subcutis. Capillary lobules demonstrate the “cannonball-like” architecture often associated with tufted angioma, and some lobules showed bulging into adjacent thin-walled vessels. Spindled endothelial cells lining slit-like vessels were present in the mid dermis, although this comprises a minority of the lesion. The majority of the subcutis was uninvolved. The findings are overall most consistent with a tufted angioma.
Kaposiform hemangioendothelioma (KHE) has been considered given the presence of occasional slit-like vascular spaces; however, the lesion is predominantly superficial and therefore the lesion is best classified as tufted angioma. GLUT–1 staining was negative.
At the time of biopsy, blood work was ordered, which showed a normal complete blood count with normal number of platelets, slightly elevated D-dimer, and slightly low fibrinogen. Several repeat blood counts and coagulation tests once a week for a few weeks revealed no changes.
The patient was started on aspirin at a dose of 5 mg/kg per day. After a week on the medication the lesion was starting to get smaller and less red.
Tufted angiomas are a rare type of vascular tumor within the spectrum of kaposiform hemangioendotheliomas. Most cases present within the first year of life; some occur at birth. They usually present as papules, plaques, or erythematous, violaceous indurated nodules on the face, neck, trunk, and extremities. The lesions can also be present with hyperhidrosis and hypertrichosis. Clinically, the lesions will have to be differentiated from other vascular tumors such as infantile hemangiomas, congenital hemangiomas, and Kaposi’s sarcoma, as well as subcutaneous fat necrosis of the newborn, cellulitis, and nonaccidental trauma.
Pathogenesis of tufted angiomas is poorly understood. A recent case report found a somatic mutation on GNA14.This protein regulates Ras activity and modulates endothelial cell permeability and migration in response to FGF2 and VEGFA. The p.205L mutation causes activation of GNA14, which upregulates pERK-MAPK pathway, suggesting MAPK inhibition as a potential target for therapy. Clinically, tufted angioma can present in three patterns: uncomplicated tufted angioma (most common type); tufted angioma without thrombocytopenia but with chronic coagulopathy, as it was seen in our patient; and tufted angioma associated with Kasabach-Merritt phenomenon (KMP). KMP is characterized by thrombocytopenia in association with microangiopathic hemolytic anemia, consumptive coagulopathy, and enlarging vascular tumor. Treatment of uncomplicated tufted angioma will depend on symptomatology, size, and location of the lesion. Smaller lesions in noncosmetically sensitive areas can be treated with surgical excision. Cases that are not amenable to excision can be treated with aspirin. There are also reports of response to topical modalities including tacrolimus and timolol. For complicated cases associated with KMP, sirolimus, systemic corticosteroids, ticlopidine, interferon, or vincristine are recommended. Some lesions may demonstrate spontaneous regression.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego.
References
Cohen S et al. Dermatol Online J. 2019 Sep 15;25(9):13030/qt6pv254mc.
Lim YH et al. Pediatr Dermatol. 2019 Nov;36(6):963-4.
Prasuna A, Rao PN. Indian Dermatol Online J. 2015;6:266-8.
A 35-day-old female was referred to our pediatric dermatology clinic for evaluation of a red lesion on the right arm. The lesion presented at about 4 days of life as a red plaque (image 1 at 8 days of life).
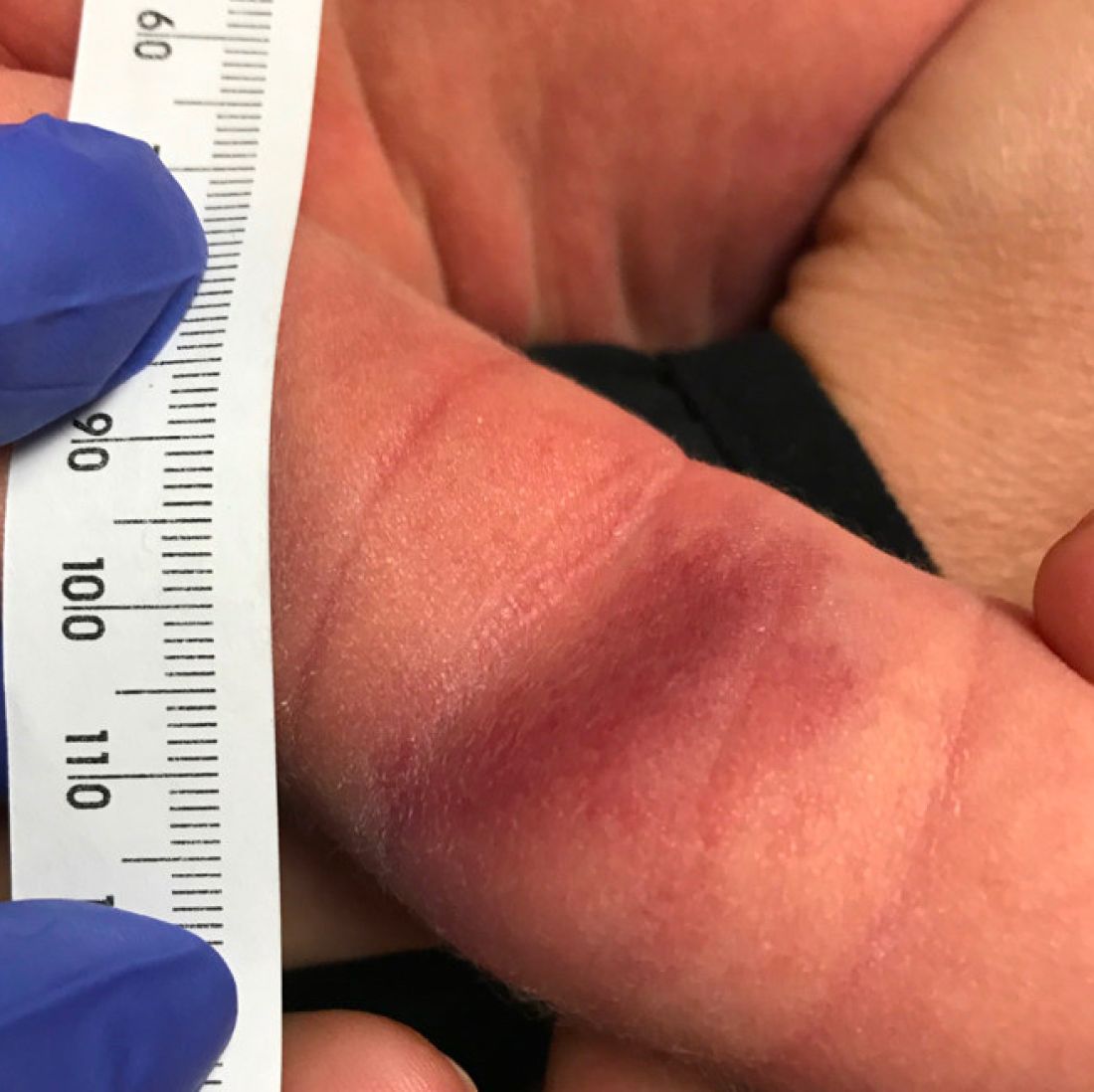
On the following days, the lesion started growing but it didn't seem to be tender or bothersome to the patient (image 2, at 35 days of life).
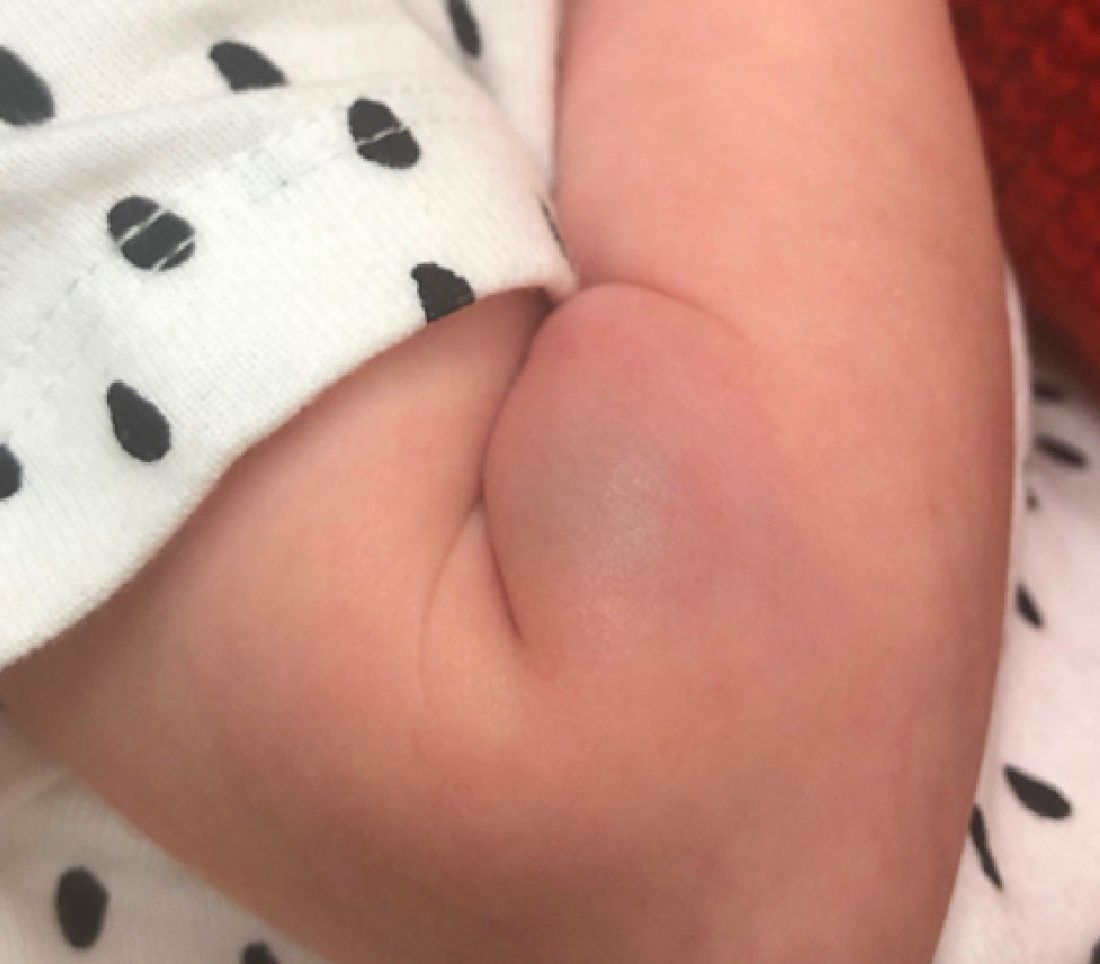
At a 2-week follow up the lesion was getting fuller and more violaceous. There was no history of fever and the lesion didn't appear tender to the touch.
She was born via normal spontaneous vaginal delivery. There were no complications and the mother received prenatal care.
On exam she had a red to violaceous nodule on the right arm (image 3 at 45 days of life).
Filler complications involving vascular necrosis, vision changes on the rise
analysis showed.
“The ASDS estimates that 1.6 million soft tissue filler procedures were performed in 2019, a 78% increase from 2012,” presenting author Michelle Xiong, a 4th-year student at Brown University, Providence, R.I., said during a virtual abstract session at the annual meeting of the American Society for Dermatologic Surgery. “The popularity of dermal fillers continues to increase. With that, there is increasing concern of possible associated adverse events. Most concerning are those related to vascular occlusion.”
Under the supervision of senior author Kachiu C. Lee, MD, MPH, of Main Line Center for Laser Surgery in Ardmore, Pa., Ms. Xiong and colleagues analyzed the Food and Drug Administration’s Manufacturer and User Facility Device Experience (MAUDE) database of medical device–related adverse event reports, to better understand and characterize dermal filler-related complications. They limited the analysis to adverse events involving injectable fillers from January 2014 to December 2020 and determined the number of complications by type per year and reviewed reports to identify injection site locations. Next, they used the binomial test to compare the proportion of complication categories from 2014 through 2016 and from 2017 through 2020.
In all, 5,994 reports were identified during the 7-year study period. To evaluate trends over time, the researchers estimated the rate of complications per 100 reports each year. While the absolute number of reports increased over time, the rate of adverse events per 100 reports decreased, suggesting an overall improvement in safety.
When the researchers focused on complications involving vascular occlusion, they found that vascular necrosis accounted for 3.5% of all complications, compared with vision changes (1.5% of all complications), and stroke (0.3% of all complications). When comparing the years 2014-2016 with 2017-2020, there was a significant increase in adverse events involving vascular necrosis (0.9%; P = .018) and vision changes (0.94%; P = .001), but no significant difference in the number of reports of stroke (-0.1%; P = .409). “This highlights that serious complications like necrosis and vision changes have increased over time,” Ms. Xiong said.
Overall, the three most common injection sites involving necrosis and vision changes were the cheek, the nose, and the nasolabial fold. The cheek was the most common site associated with stroke. “These findings are similar to those of previous studies, further emphasizing that the nose, nasolabial fold, and cheek are possibly challenging injection sites,” she said.
“In general, as the face is a highly vascular area with many anastomoses, it’s especially important to be aware of facial anatomy when injecting. In addition to awareness of anatomy, injection techniques can influence vascular complications. Unfortunately, the event narratives in the MAUDE database did not go into detail about the procedural technique.”
Ms. Xiong said that as the popularity of dermal fillers continues to grow, “it’s important for providers to understand the possible adverse events, both to better counsel patients and to improve safety management. The proportion of serious complications such as vascular necrosis and vision changes have increased from 2014 to 2020. This highlights an increased need for training to better understand facial anatomy and to emphasize practice techniques to minimize risk.”
Dr. Lee acknowledged certain limitations of the study, including that “submission of adverse events to the MAUDE database are not verified or standardized,” she told this news organization.
“With the ever-increasing popularity of fillers, it is not surprising that the absolute number of complications is rising, but it is also reassuring to see that the overall ratio of complications per hundred reports is down,” said Lawrence J. Green, MD, clinical professor of dermatology at George Washington University, Washington, who was asked to comment on the study. “I would be curious to know what proportion of filler complications are due to non–core practitioners compared to dermatologists and plastic surgeons.”
The researchers reported having no financial disclosures.
Dr. Green disclosed that he is a speaker, consultant, or investigator for numerous pharmaceutical companies.
analysis showed.
“The ASDS estimates that 1.6 million soft tissue filler procedures were performed in 2019, a 78% increase from 2012,” presenting author Michelle Xiong, a 4th-year student at Brown University, Providence, R.I., said during a virtual abstract session at the annual meeting of the American Society for Dermatologic Surgery. “The popularity of dermal fillers continues to increase. With that, there is increasing concern of possible associated adverse events. Most concerning are those related to vascular occlusion.”
Under the supervision of senior author Kachiu C. Lee, MD, MPH, of Main Line Center for Laser Surgery in Ardmore, Pa., Ms. Xiong and colleagues analyzed the Food and Drug Administration’s Manufacturer and User Facility Device Experience (MAUDE) database of medical device–related adverse event reports, to better understand and characterize dermal filler-related complications. They limited the analysis to adverse events involving injectable fillers from January 2014 to December 2020 and determined the number of complications by type per year and reviewed reports to identify injection site locations. Next, they used the binomial test to compare the proportion of complication categories from 2014 through 2016 and from 2017 through 2020.
In all, 5,994 reports were identified during the 7-year study period. To evaluate trends over time, the researchers estimated the rate of complications per 100 reports each year. While the absolute number of reports increased over time, the rate of adverse events per 100 reports decreased, suggesting an overall improvement in safety.
When the researchers focused on complications involving vascular occlusion, they found that vascular necrosis accounted for 3.5% of all complications, compared with vision changes (1.5% of all complications), and stroke (0.3% of all complications). When comparing the years 2014-2016 with 2017-2020, there was a significant increase in adverse events involving vascular necrosis (0.9%; P = .018) and vision changes (0.94%; P = .001), but no significant difference in the number of reports of stroke (-0.1%; P = .409). “This highlights that serious complications like necrosis and vision changes have increased over time,” Ms. Xiong said.
Overall, the three most common injection sites involving necrosis and vision changes were the cheek, the nose, and the nasolabial fold. The cheek was the most common site associated with stroke. “These findings are similar to those of previous studies, further emphasizing that the nose, nasolabial fold, and cheek are possibly challenging injection sites,” she said.
“In general, as the face is a highly vascular area with many anastomoses, it’s especially important to be aware of facial anatomy when injecting. In addition to awareness of anatomy, injection techniques can influence vascular complications. Unfortunately, the event narratives in the MAUDE database did not go into detail about the procedural technique.”
Ms. Xiong said that as the popularity of dermal fillers continues to grow, “it’s important for providers to understand the possible adverse events, both to better counsel patients and to improve safety management. The proportion of serious complications such as vascular necrosis and vision changes have increased from 2014 to 2020. This highlights an increased need for training to better understand facial anatomy and to emphasize practice techniques to minimize risk.”
Dr. Lee acknowledged certain limitations of the study, including that “submission of adverse events to the MAUDE database are not verified or standardized,” she told this news organization.
“With the ever-increasing popularity of fillers, it is not surprising that the absolute number of complications is rising, but it is also reassuring to see that the overall ratio of complications per hundred reports is down,” said Lawrence J. Green, MD, clinical professor of dermatology at George Washington University, Washington, who was asked to comment on the study. “I would be curious to know what proportion of filler complications are due to non–core practitioners compared to dermatologists and plastic surgeons.”
The researchers reported having no financial disclosures.
Dr. Green disclosed that he is a speaker, consultant, or investigator for numerous pharmaceutical companies.
analysis showed.
“The ASDS estimates that 1.6 million soft tissue filler procedures were performed in 2019, a 78% increase from 2012,” presenting author Michelle Xiong, a 4th-year student at Brown University, Providence, R.I., said during a virtual abstract session at the annual meeting of the American Society for Dermatologic Surgery. “The popularity of dermal fillers continues to increase. With that, there is increasing concern of possible associated adverse events. Most concerning are those related to vascular occlusion.”
Under the supervision of senior author Kachiu C. Lee, MD, MPH, of Main Line Center for Laser Surgery in Ardmore, Pa., Ms. Xiong and colleagues analyzed the Food and Drug Administration’s Manufacturer and User Facility Device Experience (MAUDE) database of medical device–related adverse event reports, to better understand and characterize dermal filler-related complications. They limited the analysis to adverse events involving injectable fillers from January 2014 to December 2020 and determined the number of complications by type per year and reviewed reports to identify injection site locations. Next, they used the binomial test to compare the proportion of complication categories from 2014 through 2016 and from 2017 through 2020.
In all, 5,994 reports were identified during the 7-year study period. To evaluate trends over time, the researchers estimated the rate of complications per 100 reports each year. While the absolute number of reports increased over time, the rate of adverse events per 100 reports decreased, suggesting an overall improvement in safety.
When the researchers focused on complications involving vascular occlusion, they found that vascular necrosis accounted for 3.5% of all complications, compared with vision changes (1.5% of all complications), and stroke (0.3% of all complications). When comparing the years 2014-2016 with 2017-2020, there was a significant increase in adverse events involving vascular necrosis (0.9%; P = .018) and vision changes (0.94%; P = .001), but no significant difference in the number of reports of stroke (-0.1%; P = .409). “This highlights that serious complications like necrosis and vision changes have increased over time,” Ms. Xiong said.
Overall, the three most common injection sites involving necrosis and vision changes were the cheek, the nose, and the nasolabial fold. The cheek was the most common site associated with stroke. “These findings are similar to those of previous studies, further emphasizing that the nose, nasolabial fold, and cheek are possibly challenging injection sites,” she said.
“In general, as the face is a highly vascular area with many anastomoses, it’s especially important to be aware of facial anatomy when injecting. In addition to awareness of anatomy, injection techniques can influence vascular complications. Unfortunately, the event narratives in the MAUDE database did not go into detail about the procedural technique.”
Ms. Xiong said that as the popularity of dermal fillers continues to grow, “it’s important for providers to understand the possible adverse events, both to better counsel patients and to improve safety management. The proportion of serious complications such as vascular necrosis and vision changes have increased from 2014 to 2020. This highlights an increased need for training to better understand facial anatomy and to emphasize practice techniques to minimize risk.”
Dr. Lee acknowledged certain limitations of the study, including that “submission of adverse events to the MAUDE database are not verified or standardized,” she told this news organization.
“With the ever-increasing popularity of fillers, it is not surprising that the absolute number of complications is rising, but it is also reassuring to see that the overall ratio of complications per hundred reports is down,” said Lawrence J. Green, MD, clinical professor of dermatology at George Washington University, Washington, who was asked to comment on the study. “I would be curious to know what proportion of filler complications are due to non–core practitioners compared to dermatologists and plastic surgeons.”
The researchers reported having no financial disclosures.
Dr. Green disclosed that he is a speaker, consultant, or investigator for numerous pharmaceutical companies.
FROM ASDS 2021
Omicron may require fourth vaccine dose, Pfizer says
, Pfizer officials said on Dec. 8.
The standard two doses may be less effective against the variant, the company announced earlier in the day, and a booster dose increases neutralizing antibodies.
But the timeline might need to be moved up for a fourth dose. Previously, Pfizer CEO Albert Bourla, PhD, said another dose might be needed about a year after a third shot. Now the company’s scientists believe that a fourth shot, which targets the Omicron variant, could be required sooner.
“With Omicron, we need to wait and see because we have very little information. We may need it faster,” Dr. Bourla said on CNBC’s Squawk Box.
“But for right now, the most important thing is that we have winter in front of us,” he said. “From a healthcare perspective, it is important to understand that we need to be well-protected to go through the winter.”
A third dose should provide protection throughout the winter, Dr. Bourla said. That may buy time until the early spring to develop new shots that target Omicron, which Pfizer could have ready by March, according to Bloomberg News.
As of the afternoon of Dec. 8, 43 people in 19 states had tested positive for the Omicron variant, according to The Associated Press. More than 75% had been vaccinated, and a third had had booster shots. About a third had traveled internationally.
Nearly all of them have had mild symptoms so far, the AP reported, with the most common symptoms being a cough, congestion, and fatigue. One person has been hospitalized, but no deaths have been reported so far.
The CDC is still trying to determine how the Omicron variant may affect the course of the pandemic and whether the strain is more contagious or causes more severe disease.
“What we generally know is the more mutations a variant has, the higher level you need your immunity to be,” Rochelle Walensky, MD, director of the CDC, told the AP.
“We want to make sure we bolster everybody’s immunity,” she said. “And that’s really what motivated the decision to expand our guidance [on boosters for all adults].”
The Omicron variant has been reported in 57 countries so far, World Health Organization officials reported Dec. 8, and they expect that number to continue growing.
“Certain features of Omicron, including its global spread and large number of mutations, suggest it could have a major impact on the course of the pandemic. Exactly what that impact will be is still difficult to know,” Tedros Adhanom Ghebreyesus, PhD, the World Health Organization’s director-general, said during a media briefing.
Several studies suggest that Omicron leads to a rapid increase in transmission, he said, though scientists are still trying to understand whether it can “outcompete Delta.” Data from South Africa also suggests a higher risk of reinfection with Omicron, though it appears to cause milder disease than Delta, he noted.
“Even though we still need answers to some crucial questions, we are not defenseless against Omicron or Delta,” he said. “The steps countries take today, and in the coming days and weeks, will determine how Omicron unfolds.”
A version of this article first appeared on WebMD.com.
, Pfizer officials said on Dec. 8.
The standard two doses may be less effective against the variant, the company announced earlier in the day, and a booster dose increases neutralizing antibodies.
But the timeline might need to be moved up for a fourth dose. Previously, Pfizer CEO Albert Bourla, PhD, said another dose might be needed about a year after a third shot. Now the company’s scientists believe that a fourth shot, which targets the Omicron variant, could be required sooner.
“With Omicron, we need to wait and see because we have very little information. We may need it faster,” Dr. Bourla said on CNBC’s Squawk Box.
“But for right now, the most important thing is that we have winter in front of us,” he said. “From a healthcare perspective, it is important to understand that we need to be well-protected to go through the winter.”
A third dose should provide protection throughout the winter, Dr. Bourla said. That may buy time until the early spring to develop new shots that target Omicron, which Pfizer could have ready by March, according to Bloomberg News.
As of the afternoon of Dec. 8, 43 people in 19 states had tested positive for the Omicron variant, according to The Associated Press. More than 75% had been vaccinated, and a third had had booster shots. About a third had traveled internationally.
Nearly all of them have had mild symptoms so far, the AP reported, with the most common symptoms being a cough, congestion, and fatigue. One person has been hospitalized, but no deaths have been reported so far.
The CDC is still trying to determine how the Omicron variant may affect the course of the pandemic and whether the strain is more contagious or causes more severe disease.
“What we generally know is the more mutations a variant has, the higher level you need your immunity to be,” Rochelle Walensky, MD, director of the CDC, told the AP.
“We want to make sure we bolster everybody’s immunity,” she said. “And that’s really what motivated the decision to expand our guidance [on boosters for all adults].”
The Omicron variant has been reported in 57 countries so far, World Health Organization officials reported Dec. 8, and they expect that number to continue growing.
“Certain features of Omicron, including its global spread and large number of mutations, suggest it could have a major impact on the course of the pandemic. Exactly what that impact will be is still difficult to know,” Tedros Adhanom Ghebreyesus, PhD, the World Health Organization’s director-general, said during a media briefing.
Several studies suggest that Omicron leads to a rapid increase in transmission, he said, though scientists are still trying to understand whether it can “outcompete Delta.” Data from South Africa also suggests a higher risk of reinfection with Omicron, though it appears to cause milder disease than Delta, he noted.
“Even though we still need answers to some crucial questions, we are not defenseless against Omicron or Delta,” he said. “The steps countries take today, and in the coming days and weeks, will determine how Omicron unfolds.”
A version of this article first appeared on WebMD.com.
, Pfizer officials said on Dec. 8.
The standard two doses may be less effective against the variant, the company announced earlier in the day, and a booster dose increases neutralizing antibodies.
But the timeline might need to be moved up for a fourth dose. Previously, Pfizer CEO Albert Bourla, PhD, said another dose might be needed about a year after a third shot. Now the company’s scientists believe that a fourth shot, which targets the Omicron variant, could be required sooner.
“With Omicron, we need to wait and see because we have very little information. We may need it faster,” Dr. Bourla said on CNBC’s Squawk Box.
“But for right now, the most important thing is that we have winter in front of us,” he said. “From a healthcare perspective, it is important to understand that we need to be well-protected to go through the winter.”
A third dose should provide protection throughout the winter, Dr. Bourla said. That may buy time until the early spring to develop new shots that target Omicron, which Pfizer could have ready by March, according to Bloomberg News.
As of the afternoon of Dec. 8, 43 people in 19 states had tested positive for the Omicron variant, according to The Associated Press. More than 75% had been vaccinated, and a third had had booster shots. About a third had traveled internationally.
Nearly all of them have had mild symptoms so far, the AP reported, with the most common symptoms being a cough, congestion, and fatigue. One person has been hospitalized, but no deaths have been reported so far.
The CDC is still trying to determine how the Omicron variant may affect the course of the pandemic and whether the strain is more contagious or causes more severe disease.
“What we generally know is the more mutations a variant has, the higher level you need your immunity to be,” Rochelle Walensky, MD, director of the CDC, told the AP.
“We want to make sure we bolster everybody’s immunity,” she said. “And that’s really what motivated the decision to expand our guidance [on boosters for all adults].”
The Omicron variant has been reported in 57 countries so far, World Health Organization officials reported Dec. 8, and they expect that number to continue growing.
“Certain features of Omicron, including its global spread and large number of mutations, suggest it could have a major impact on the course of the pandemic. Exactly what that impact will be is still difficult to know,” Tedros Adhanom Ghebreyesus, PhD, the World Health Organization’s director-general, said during a media briefing.
Several studies suggest that Omicron leads to a rapid increase in transmission, he said, though scientists are still trying to understand whether it can “outcompete Delta.” Data from South Africa also suggests a higher risk of reinfection with Omicron, though it appears to cause milder disease than Delta, he noted.
“Even though we still need answers to some crucial questions, we are not defenseless against Omicron or Delta,” he said. “The steps countries take today, and in the coming days and weeks, will determine how Omicron unfolds.”
A version of this article first appeared on WebMD.com.
Medical board stops warning docs against giving false COVID information
Under pressure from Republican state lawmakers, t
The board’s 7-3 vote on December 7 to delete the statement followed repeated threats by a powerful state House Republican to dissolve the board and appoint all new members if it did not immediately take it down.
The Tennessee board’s statement was a verbatim restatement of a warning to physicians issued by the Federation of State Medical Boards in July. The federation cited a “dramatic increase” in dissemination of misinformation and disinformation about the COVID-19 vaccine by physicians. It said that’s dangerous because physicians enjoy a high degree of public credibility.
Across the country, state medical licensing boards and state and national medical associations and specialty boards are struggling with how to respond to scientifically baseless public statements about COVID-19 by some physicians, which they say are increasing public confusion, political conflict, and preventable illnesses and deaths.
There have been only a small number of disciplinary actions by medical boards against physicians for spreading false COVID-19 information. Critics say the boards have been weak in responding to these dangerous violations of medical standards. As an example, they cite the State Medical Board of Ohio’s September renewal of the medical license of Sherri Tenpenny, DO, who had previously testified before Ohio lawmakers that COVID-19 vaccines magnetize their recipients and “interface” with cell phone towers.
“I’m not satisfied with what medical boards have done, and we are ramping up our efforts to press the boards to hold these physicians accountable,” said Nick Sawyer, MD, an emergency physician in Sacramento, Calif., who heads a group of healthcare professionals called No License for Disinformation.
Still, Tennessee board members insisted that the board’s policy of disciplining physicians who disseminate false information about COVID-19 vaccinations remains in effect, because state law empowers the board to take action against doctors whose unprofessional behavior endangers the public.
“COVID misinformation and disinformation has caused undue loss of life and jobs and other incalculable loss in our society,” said Melanie Blake, MD, MBA, a Chattanooga internist who’s president of the board. “Physicians have a responsibility to uphold their oath and put forward consensus-driven medical principles.”
But state Rep. John Ragan, the Republican co-chairman of the Joint Government Operations Committee, told the Tennessean newspaper that deleting the statement from the board’s website was equivalent to rescinding the policy. Ragan, who identifies himself as a business consultant and retired Air Force pilot, did not respond to a request for comment for this article.
Blake acknowledged that removing the statement from the board’s website has the potential to confuse Tennessee physicians. And the pressure from GOP lawmakers, who overwhelmingly control the Tennessee legislature, could discourage investigations and disciplinary actions against physicians who allegedly spread COVID-19 misinformation, she added. “It’s hard for me to answer whether this puts a chill on us,” she said.
In September, the Tennessee board, besides approving the general statement that physicians who spread COVID-19 disinformation could face licensure action, also directed the State Department of Health to prioritize investigations of physicians who spread outrageous claims. The board cited statements such as the vaccines are poisonous, cause infertility, contain microchips, or magnetize the body.
In response, the Tennessee General Assembly passed a bill in late October prohibiting the board from implementing any disciplinary process regarding the prescribing of “medication for COVID-19” without review and approval by Ragan’s committee. It’s not clear whether that language covers vaccines.
Last summer, in a similar move, Ragan threatened to dissolve the State Department of Health because its top vaccination official wrote a letter to medical providers explaining that state law allowed them to give COVID-19 vaccinations to minors older than 14 without parental consent. That official, Michelle Fiscus, MD, was fired in July.
Republican Sen. Richard Briggs, MD, a cardiothoracic surgeon who voted against the October legislation affecting COVID-related disciplinary actions, criticized his GOP colleagues’ interference in the medical board’s licensure decisions. “The mission of the board is to protect the health and safety of Tennessee citizens, and this was in complete conflict with that mission,” he said.
The Federation of State Medical Boards similarly condemned the Tennessee lawmakers’ moves. “The FSMB strongly opposes restricting a board’s authority to evaluate the standard of care and assess potential risk for patient harm,” a spokesman said. “Any interference, politically motivated or otherwise, is unhelpful and dangerous.”
But Arthur Caplan, PhD, a professor of bioethics at NYU School of Medicine, doubts that state medical boards are up to the task of policing disinformation spread by physicians. That’s because they ultimately are under the control of elected state officials, who may force the boards to base policy on ideology rather than science.
He said medical board members in Florida and another GOP-controlled state have told him they do not want to pursue disciplinary actions against physicians for COVID-19 misinformation for fear of political backlash.
Michele Heisler, MD, medical director of Physicians for Human Rights, agreed that the Tennessee situation highlights the looming political threat to the independence of state medical boards. She urged other medical organizations, particularly medical specialty boards, to step in.
“As a profession, we need to take a stance against this,” said Heisler, who’s a professor of internal medicine and public health at the University of Michigan. “Our credibility as physicians is at stake.”
A version of this article first appeared on Medscape.com.
Under pressure from Republican state lawmakers, t
The board’s 7-3 vote on December 7 to delete the statement followed repeated threats by a powerful state House Republican to dissolve the board and appoint all new members if it did not immediately take it down.
The Tennessee board’s statement was a verbatim restatement of a warning to physicians issued by the Federation of State Medical Boards in July. The federation cited a “dramatic increase” in dissemination of misinformation and disinformation about the COVID-19 vaccine by physicians. It said that’s dangerous because physicians enjoy a high degree of public credibility.
Across the country, state medical licensing boards and state and national medical associations and specialty boards are struggling with how to respond to scientifically baseless public statements about COVID-19 by some physicians, which they say are increasing public confusion, political conflict, and preventable illnesses and deaths.
There have been only a small number of disciplinary actions by medical boards against physicians for spreading false COVID-19 information. Critics say the boards have been weak in responding to these dangerous violations of medical standards. As an example, they cite the State Medical Board of Ohio’s September renewal of the medical license of Sherri Tenpenny, DO, who had previously testified before Ohio lawmakers that COVID-19 vaccines magnetize their recipients and “interface” with cell phone towers.
“I’m not satisfied with what medical boards have done, and we are ramping up our efforts to press the boards to hold these physicians accountable,” said Nick Sawyer, MD, an emergency physician in Sacramento, Calif., who heads a group of healthcare professionals called No License for Disinformation.
Still, Tennessee board members insisted that the board’s policy of disciplining physicians who disseminate false information about COVID-19 vaccinations remains in effect, because state law empowers the board to take action against doctors whose unprofessional behavior endangers the public.
“COVID misinformation and disinformation has caused undue loss of life and jobs and other incalculable loss in our society,” said Melanie Blake, MD, MBA, a Chattanooga internist who’s president of the board. “Physicians have a responsibility to uphold their oath and put forward consensus-driven medical principles.”
But state Rep. John Ragan, the Republican co-chairman of the Joint Government Operations Committee, told the Tennessean newspaper that deleting the statement from the board’s website was equivalent to rescinding the policy. Ragan, who identifies himself as a business consultant and retired Air Force pilot, did not respond to a request for comment for this article.
Blake acknowledged that removing the statement from the board’s website has the potential to confuse Tennessee physicians. And the pressure from GOP lawmakers, who overwhelmingly control the Tennessee legislature, could discourage investigations and disciplinary actions against physicians who allegedly spread COVID-19 misinformation, she added. “It’s hard for me to answer whether this puts a chill on us,” she said.
In September, the Tennessee board, besides approving the general statement that physicians who spread COVID-19 disinformation could face licensure action, also directed the State Department of Health to prioritize investigations of physicians who spread outrageous claims. The board cited statements such as the vaccines are poisonous, cause infertility, contain microchips, or magnetize the body.
In response, the Tennessee General Assembly passed a bill in late October prohibiting the board from implementing any disciplinary process regarding the prescribing of “medication for COVID-19” without review and approval by Ragan’s committee. It’s not clear whether that language covers vaccines.
Last summer, in a similar move, Ragan threatened to dissolve the State Department of Health because its top vaccination official wrote a letter to medical providers explaining that state law allowed them to give COVID-19 vaccinations to minors older than 14 without parental consent. That official, Michelle Fiscus, MD, was fired in July.
Republican Sen. Richard Briggs, MD, a cardiothoracic surgeon who voted against the October legislation affecting COVID-related disciplinary actions, criticized his GOP colleagues’ interference in the medical board’s licensure decisions. “The mission of the board is to protect the health and safety of Tennessee citizens, and this was in complete conflict with that mission,” he said.
The Federation of State Medical Boards similarly condemned the Tennessee lawmakers’ moves. “The FSMB strongly opposes restricting a board’s authority to evaluate the standard of care and assess potential risk for patient harm,” a spokesman said. “Any interference, politically motivated or otherwise, is unhelpful and dangerous.”
But Arthur Caplan, PhD, a professor of bioethics at NYU School of Medicine, doubts that state medical boards are up to the task of policing disinformation spread by physicians. That’s because they ultimately are under the control of elected state officials, who may force the boards to base policy on ideology rather than science.
He said medical board members in Florida and another GOP-controlled state have told him they do not want to pursue disciplinary actions against physicians for COVID-19 misinformation for fear of political backlash.
Michele Heisler, MD, medical director of Physicians for Human Rights, agreed that the Tennessee situation highlights the looming political threat to the independence of state medical boards. She urged other medical organizations, particularly medical specialty boards, to step in.
“As a profession, we need to take a stance against this,” said Heisler, who’s a professor of internal medicine and public health at the University of Michigan. “Our credibility as physicians is at stake.”
A version of this article first appeared on Medscape.com.
Under pressure from Republican state lawmakers, t
The board’s 7-3 vote on December 7 to delete the statement followed repeated threats by a powerful state House Republican to dissolve the board and appoint all new members if it did not immediately take it down.
The Tennessee board’s statement was a verbatim restatement of a warning to physicians issued by the Federation of State Medical Boards in July. The federation cited a “dramatic increase” in dissemination of misinformation and disinformation about the COVID-19 vaccine by physicians. It said that’s dangerous because physicians enjoy a high degree of public credibility.
Across the country, state medical licensing boards and state and national medical associations and specialty boards are struggling with how to respond to scientifically baseless public statements about COVID-19 by some physicians, which they say are increasing public confusion, political conflict, and preventable illnesses and deaths.
There have been only a small number of disciplinary actions by medical boards against physicians for spreading false COVID-19 information. Critics say the boards have been weak in responding to these dangerous violations of medical standards. As an example, they cite the State Medical Board of Ohio’s September renewal of the medical license of Sherri Tenpenny, DO, who had previously testified before Ohio lawmakers that COVID-19 vaccines magnetize their recipients and “interface” with cell phone towers.
“I’m not satisfied with what medical boards have done, and we are ramping up our efforts to press the boards to hold these physicians accountable,” said Nick Sawyer, MD, an emergency physician in Sacramento, Calif., who heads a group of healthcare professionals called No License for Disinformation.
Still, Tennessee board members insisted that the board’s policy of disciplining physicians who disseminate false information about COVID-19 vaccinations remains in effect, because state law empowers the board to take action against doctors whose unprofessional behavior endangers the public.
“COVID misinformation and disinformation has caused undue loss of life and jobs and other incalculable loss in our society,” said Melanie Blake, MD, MBA, a Chattanooga internist who’s president of the board. “Physicians have a responsibility to uphold their oath and put forward consensus-driven medical principles.”
But state Rep. John Ragan, the Republican co-chairman of the Joint Government Operations Committee, told the Tennessean newspaper that deleting the statement from the board’s website was equivalent to rescinding the policy. Ragan, who identifies himself as a business consultant and retired Air Force pilot, did not respond to a request for comment for this article.
Blake acknowledged that removing the statement from the board’s website has the potential to confuse Tennessee physicians. And the pressure from GOP lawmakers, who overwhelmingly control the Tennessee legislature, could discourage investigations and disciplinary actions against physicians who allegedly spread COVID-19 misinformation, she added. “It’s hard for me to answer whether this puts a chill on us,” she said.
In September, the Tennessee board, besides approving the general statement that physicians who spread COVID-19 disinformation could face licensure action, also directed the State Department of Health to prioritize investigations of physicians who spread outrageous claims. The board cited statements such as the vaccines are poisonous, cause infertility, contain microchips, or magnetize the body.
In response, the Tennessee General Assembly passed a bill in late October prohibiting the board from implementing any disciplinary process regarding the prescribing of “medication for COVID-19” without review and approval by Ragan’s committee. It’s not clear whether that language covers vaccines.
Last summer, in a similar move, Ragan threatened to dissolve the State Department of Health because its top vaccination official wrote a letter to medical providers explaining that state law allowed them to give COVID-19 vaccinations to minors older than 14 without parental consent. That official, Michelle Fiscus, MD, was fired in July.
Republican Sen. Richard Briggs, MD, a cardiothoracic surgeon who voted against the October legislation affecting COVID-related disciplinary actions, criticized his GOP colleagues’ interference in the medical board’s licensure decisions. “The mission of the board is to protect the health and safety of Tennessee citizens, and this was in complete conflict with that mission,” he said.
The Federation of State Medical Boards similarly condemned the Tennessee lawmakers’ moves. “The FSMB strongly opposes restricting a board’s authority to evaluate the standard of care and assess potential risk for patient harm,” a spokesman said. “Any interference, politically motivated or otherwise, is unhelpful and dangerous.”
But Arthur Caplan, PhD, a professor of bioethics at NYU School of Medicine, doubts that state medical boards are up to the task of policing disinformation spread by physicians. That’s because they ultimately are under the control of elected state officials, who may force the boards to base policy on ideology rather than science.
He said medical board members in Florida and another GOP-controlled state have told him they do not want to pursue disciplinary actions against physicians for COVID-19 misinformation for fear of political backlash.
Michele Heisler, MD, medical director of Physicians for Human Rights, agreed that the Tennessee situation highlights the looming political threat to the independence of state medical boards. She urged other medical organizations, particularly medical specialty boards, to step in.
“As a profession, we need to take a stance against this,” said Heisler, who’s a professor of internal medicine and public health at the University of Michigan. “Our credibility as physicians is at stake.”
A version of this article first appeared on Medscape.com.
AMA, hospital group sue federal government over surprise billing law
which tilts toward using prevailing rates paid for services.
The American Hospital Association and American Medical Association said they will ask the U.S. District Court for the District of Columbia to try to prevent implementation of certain provisions of new federal rules on surprise bills. This court is often a venue for fights over federal rules. Also joining the suit are Nevada-based Renown Health, UMass Memorial Health, and two physicians based in North Carolina, AHA and AMA said.
Federal agencies, including the Department of Health & Human Services, in September had unveiled the rule on surprise medical bills that will take effect Jan. 1.
Under this rule, a key benchmark for payment disputes would be the qualifying payment amount (QPA), which is pegged to median contracted rates. In the dispute-resolution process outlined in the rule, there is a presumption that the QPA is the appropriate out-of-network rate.
The rule allows for exceptions in which the independent mediating organization handling the payment dispute resolution has “credible information” as to why the QPA is materially different from the appropriate out-of-network rate.
In the view of the federal agencies that issued the rule, this approach “encourages predictable outcomes,” which likely would reduce the number of disputes that go through the resolution process while also “providing equitable and clear standards” for cases to appropriately deviate from QPA. HHS was joined in issuing the rule by the Treasury and Labor Departments and the Office of Personnel Management.
AMA and AHA disagree with their view, seeing this approach as a boon for insurers at the expense of physicians and hospitals.
In a press release, they said the rule’s approach to surprise billing would “all but ensure that hospitals, physicians, and other providers will routinely be undercompensated by commercial insurers, and patients will have fewer choices for access to in-network services.”
The rule is part of the implementation of a federal law passed in December 2020, known as the No Surprises Act. In their statement, AHA and AMA said their legal challenge would not prevent “core patient protections’’ of that law from moving forward.
“No patient should fear receiving a surprise medical bill,” Rick Pollack, AHA president and chief executive, said in the statement. “That is why hospitals and health systems supported the No Surprises Act to protect patients and keep them out of the middle of disputes between providers and insurers. Congress carefully crafted the law with a balanced, patient-friendly approach and it should be implemented as intended.”
AMA President Gerald E. Harmon, MD, added the approach used in the rule on surprise billing could create “an unsustainable situation for physicians.”
“Our legal challenge urges regulators to ensure there is a fair and meaningful process to resolve disputes between health care providers and insurance companies,” Dr. Harmon said.
AHA and AMA included with their statement a link to a November letter from more than 150 members of Congress, who also objected to the approach taken in designing the independent dispute-resolution (IDR) process.
“This directive establishes a de facto benchmark rate, making the median in-network rate the default factor considered in the IDR process. This approach is contrary to statute and could incentivize insurance companies to set artificially low payment rates, which would narrow provider networks and jeopardize patient access to care – the exact opposite of the goal of the law,” wrote the members of Congress, including Rep. Raul Ruiz, MD, a California Democrat, and Rep. Larry Bucshon, MD, an Indiana Republican.
A version of this article first appeared on Medscape.com.
which tilts toward using prevailing rates paid for services.
The American Hospital Association and American Medical Association said they will ask the U.S. District Court for the District of Columbia to try to prevent implementation of certain provisions of new federal rules on surprise bills. This court is often a venue for fights over federal rules. Also joining the suit are Nevada-based Renown Health, UMass Memorial Health, and two physicians based in North Carolina, AHA and AMA said.
Federal agencies, including the Department of Health & Human Services, in September had unveiled the rule on surprise medical bills that will take effect Jan. 1.
Under this rule, a key benchmark for payment disputes would be the qualifying payment amount (QPA), which is pegged to median contracted rates. In the dispute-resolution process outlined in the rule, there is a presumption that the QPA is the appropriate out-of-network rate.
The rule allows for exceptions in which the independent mediating organization handling the payment dispute resolution has “credible information” as to why the QPA is materially different from the appropriate out-of-network rate.
In the view of the federal agencies that issued the rule, this approach “encourages predictable outcomes,” which likely would reduce the number of disputes that go through the resolution process while also “providing equitable and clear standards” for cases to appropriately deviate from QPA. HHS was joined in issuing the rule by the Treasury and Labor Departments and the Office of Personnel Management.
AMA and AHA disagree with their view, seeing this approach as a boon for insurers at the expense of physicians and hospitals.
In a press release, they said the rule’s approach to surprise billing would “all but ensure that hospitals, physicians, and other providers will routinely be undercompensated by commercial insurers, and patients will have fewer choices for access to in-network services.”
The rule is part of the implementation of a federal law passed in December 2020, known as the No Surprises Act. In their statement, AHA and AMA said their legal challenge would not prevent “core patient protections’’ of that law from moving forward.
“No patient should fear receiving a surprise medical bill,” Rick Pollack, AHA president and chief executive, said in the statement. “That is why hospitals and health systems supported the No Surprises Act to protect patients and keep them out of the middle of disputes between providers and insurers. Congress carefully crafted the law with a balanced, patient-friendly approach and it should be implemented as intended.”
AMA President Gerald E. Harmon, MD, added the approach used in the rule on surprise billing could create “an unsustainable situation for physicians.”
“Our legal challenge urges regulators to ensure there is a fair and meaningful process to resolve disputes between health care providers and insurance companies,” Dr. Harmon said.
AHA and AMA included with their statement a link to a November letter from more than 150 members of Congress, who also objected to the approach taken in designing the independent dispute-resolution (IDR) process.
“This directive establishes a de facto benchmark rate, making the median in-network rate the default factor considered in the IDR process. This approach is contrary to statute and could incentivize insurance companies to set artificially low payment rates, which would narrow provider networks and jeopardize patient access to care – the exact opposite of the goal of the law,” wrote the members of Congress, including Rep. Raul Ruiz, MD, a California Democrat, and Rep. Larry Bucshon, MD, an Indiana Republican.
A version of this article first appeared on Medscape.com.
which tilts toward using prevailing rates paid for services.
The American Hospital Association and American Medical Association said they will ask the U.S. District Court for the District of Columbia to try to prevent implementation of certain provisions of new federal rules on surprise bills. This court is often a venue for fights over federal rules. Also joining the suit are Nevada-based Renown Health, UMass Memorial Health, and two physicians based in North Carolina, AHA and AMA said.
Federal agencies, including the Department of Health & Human Services, in September had unveiled the rule on surprise medical bills that will take effect Jan. 1.
Under this rule, a key benchmark for payment disputes would be the qualifying payment amount (QPA), which is pegged to median contracted rates. In the dispute-resolution process outlined in the rule, there is a presumption that the QPA is the appropriate out-of-network rate.
The rule allows for exceptions in which the independent mediating organization handling the payment dispute resolution has “credible information” as to why the QPA is materially different from the appropriate out-of-network rate.
In the view of the federal agencies that issued the rule, this approach “encourages predictable outcomes,” which likely would reduce the number of disputes that go through the resolution process while also “providing equitable and clear standards” for cases to appropriately deviate from QPA. HHS was joined in issuing the rule by the Treasury and Labor Departments and the Office of Personnel Management.
AMA and AHA disagree with their view, seeing this approach as a boon for insurers at the expense of physicians and hospitals.
In a press release, they said the rule’s approach to surprise billing would “all but ensure that hospitals, physicians, and other providers will routinely be undercompensated by commercial insurers, and patients will have fewer choices for access to in-network services.”
The rule is part of the implementation of a federal law passed in December 2020, known as the No Surprises Act. In their statement, AHA and AMA said their legal challenge would not prevent “core patient protections’’ of that law from moving forward.
“No patient should fear receiving a surprise medical bill,” Rick Pollack, AHA president and chief executive, said in the statement. “That is why hospitals and health systems supported the No Surprises Act to protect patients and keep them out of the middle of disputes between providers and insurers. Congress carefully crafted the law with a balanced, patient-friendly approach and it should be implemented as intended.”
AMA President Gerald E. Harmon, MD, added the approach used in the rule on surprise billing could create “an unsustainable situation for physicians.”
“Our legal challenge urges regulators to ensure there is a fair and meaningful process to resolve disputes between health care providers and insurance companies,” Dr. Harmon said.
AHA and AMA included with their statement a link to a November letter from more than 150 members of Congress, who also objected to the approach taken in designing the independent dispute-resolution (IDR) process.
“This directive establishes a de facto benchmark rate, making the median in-network rate the default factor considered in the IDR process. This approach is contrary to statute and could incentivize insurance companies to set artificially low payment rates, which would narrow provider networks and jeopardize patient access to care – the exact opposite of the goal of the law,” wrote the members of Congress, including Rep. Raul Ruiz, MD, a California Democrat, and Rep. Larry Bucshon, MD, an Indiana Republican.
A version of this article first appeared on Medscape.com.
Spam filter failure: Selling physician emails equals big $$
Despite the best efforts of my institution’s spam filter, I’ve realized that I spend at least 4 minutes every day of the week removing junk email from my in basket: EMR vendors, predatory journals trying to lure me into paying their outrageous publication fees, people who want to help me with my billing software (evidently that .edu extension hasn’t clicked for them yet), headhunters trying to fill specialty positions in other states, market researchers offering a gift card for 40 minutes filling out a survey.
If you do the math, 4 minutes daily is 1,460 minutes per year. That’s an entire day of my life lost each year to this useless nonsense, which I never agreed to receive in the first place. Now multiply that by the 22 million health care workers in the United States, or even just by the 985,000 licensed physicians in this country. Then factor in the $638 per hour in gross revenue generated by the average primary care physician, as a conservative, well-documented value.
By my reckoning, these bozos owe the United States alone over $15 billion in lost GDP each year.
So why don’t we shut it down!? The CAN-SPAM Act of 2003 attempted to at least mitigate the problem. It applies only to commercial entities (I know, I’d love to report some political groups, too). To avoid violating the law and risking fines of up to $16,000 per individual email, senders must:
- Not use misleading header info (including domain name and email address)
- Not use deceptive subject lines
- Clearly label the email as an ad
- Give an actual physical address of the sender
- Tell recipients how to opt out of future emails
- Honor opt-out requests within 10 business days
- Monitor the activities of any subcontractor sending email on their behalf
I can say with certainty that much of the trash in my inbox violates at least one of these. But that doesn’t matter if there is not an efficient way to report the violators and ensure that they’ll be tracked down. Hard enough if they live here, impossible if the email is routed from overseas, as much of it clearly is.
If you receive email in violation of the act, experts recommend that you write down the email address and the business name of the sender, fill out a complaint form on the Federal Trade Commission website, or send an email to [email protected], then send an email to your Internet service provider’s abuse desk. If you’re not working within a big institution like mine that has hot and cold running IT personnel that operate their own abuse prevention office, the address you’ll need is likely abuse@domain_name or postmaster@domain_name. Just hitting the spam button at the top of your browser/email software may do the trick. There’s more good advice at the FTC’s consumer spam page.
The answer came, ironically, to my email inbox in the form of one of those emails that did indeed violate the law.
I rolled my eyes and started into my reporting subroutine but then stopped cold. Just 1 second. If this person is selling lists of email addresses of conference attendees, somebody within the conference structure must be providing them. How is that legal? I have never agreed, in registering for a medical conference, to allow them to share my email address with anyone. To think that they are making money from that is extremely galling.
Vermont, at least, has enacted a law requiring companies that traffic in such email lists to register with the state. Although it has been in effect for 2 years, the jury is out regarding its efficacy. Our European counterparts are protected by the General Data Protection Regulation, which specifies that commercial email can be sent only to individuals who have explicitly opted into such mailings, and that purchased email lists are not compliant with the requirement.
Anybody have the inside scoop on this? Can we demand that our professional societies safeguard their attendee databases so this won’t happen? If they won’t, why am I paying big money to attend their conferences, only for them to make even more money at my expense?
Dr. Hitchcock is assistant professor, department of radiation oncology, at the University of Florida, Gainesville. She reported receiving research grant money from Merck. A version of this article first appeared on Medscape.com.
Despite the best efforts of my institution’s spam filter, I’ve realized that I spend at least 4 minutes every day of the week removing junk email from my in basket: EMR vendors, predatory journals trying to lure me into paying their outrageous publication fees, people who want to help me with my billing software (evidently that .edu extension hasn’t clicked for them yet), headhunters trying to fill specialty positions in other states, market researchers offering a gift card for 40 minutes filling out a survey.
If you do the math, 4 minutes daily is 1,460 minutes per year. That’s an entire day of my life lost each year to this useless nonsense, which I never agreed to receive in the first place. Now multiply that by the 22 million health care workers in the United States, or even just by the 985,000 licensed physicians in this country. Then factor in the $638 per hour in gross revenue generated by the average primary care physician, as a conservative, well-documented value.
By my reckoning, these bozos owe the United States alone over $15 billion in lost GDP each year.
So why don’t we shut it down!? The CAN-SPAM Act of 2003 attempted to at least mitigate the problem. It applies only to commercial entities (I know, I’d love to report some political groups, too). To avoid violating the law and risking fines of up to $16,000 per individual email, senders must:
- Not use misleading header info (including domain name and email address)
- Not use deceptive subject lines
- Clearly label the email as an ad
- Give an actual physical address of the sender
- Tell recipients how to opt out of future emails
- Honor opt-out requests within 10 business days
- Monitor the activities of any subcontractor sending email on their behalf
I can say with certainty that much of the trash in my inbox violates at least one of these. But that doesn’t matter if there is not an efficient way to report the violators and ensure that they’ll be tracked down. Hard enough if they live here, impossible if the email is routed from overseas, as much of it clearly is.
If you receive email in violation of the act, experts recommend that you write down the email address and the business name of the sender, fill out a complaint form on the Federal Trade Commission website, or send an email to [email protected], then send an email to your Internet service provider’s abuse desk. If you’re not working within a big institution like mine that has hot and cold running IT personnel that operate their own abuse prevention office, the address you’ll need is likely abuse@domain_name or postmaster@domain_name. Just hitting the spam button at the top of your browser/email software may do the trick. There’s more good advice at the FTC’s consumer spam page.
The answer came, ironically, to my email inbox in the form of one of those emails that did indeed violate the law.
I rolled my eyes and started into my reporting subroutine but then stopped cold. Just 1 second. If this person is selling lists of email addresses of conference attendees, somebody within the conference structure must be providing them. How is that legal? I have never agreed, in registering for a medical conference, to allow them to share my email address with anyone. To think that they are making money from that is extremely galling.
Vermont, at least, has enacted a law requiring companies that traffic in such email lists to register with the state. Although it has been in effect for 2 years, the jury is out regarding its efficacy. Our European counterparts are protected by the General Data Protection Regulation, which specifies that commercial email can be sent only to individuals who have explicitly opted into such mailings, and that purchased email lists are not compliant with the requirement.
Anybody have the inside scoop on this? Can we demand that our professional societies safeguard their attendee databases so this won’t happen? If they won’t, why am I paying big money to attend their conferences, only for them to make even more money at my expense?
Dr. Hitchcock is assistant professor, department of radiation oncology, at the University of Florida, Gainesville. She reported receiving research grant money from Merck. A version of this article first appeared on Medscape.com.
Despite the best efforts of my institution’s spam filter, I’ve realized that I spend at least 4 minutes every day of the week removing junk email from my in basket: EMR vendors, predatory journals trying to lure me into paying their outrageous publication fees, people who want to help me with my billing software (evidently that .edu extension hasn’t clicked for them yet), headhunters trying to fill specialty positions in other states, market researchers offering a gift card for 40 minutes filling out a survey.
If you do the math, 4 minutes daily is 1,460 minutes per year. That’s an entire day of my life lost each year to this useless nonsense, which I never agreed to receive in the first place. Now multiply that by the 22 million health care workers in the United States, or even just by the 985,000 licensed physicians in this country. Then factor in the $638 per hour in gross revenue generated by the average primary care physician, as a conservative, well-documented value.
By my reckoning, these bozos owe the United States alone over $15 billion in lost GDP each year.
So why don’t we shut it down!? The CAN-SPAM Act of 2003 attempted to at least mitigate the problem. It applies only to commercial entities (I know, I’d love to report some political groups, too). To avoid violating the law and risking fines of up to $16,000 per individual email, senders must:
- Not use misleading header info (including domain name and email address)
- Not use deceptive subject lines
- Clearly label the email as an ad
- Give an actual physical address of the sender
- Tell recipients how to opt out of future emails
- Honor opt-out requests within 10 business days
- Monitor the activities of any subcontractor sending email on their behalf
I can say with certainty that much of the trash in my inbox violates at least one of these. But that doesn’t matter if there is not an efficient way to report the violators and ensure that they’ll be tracked down. Hard enough if they live here, impossible if the email is routed from overseas, as much of it clearly is.
If you receive email in violation of the act, experts recommend that you write down the email address and the business name of the sender, fill out a complaint form on the Federal Trade Commission website, or send an email to [email protected], then send an email to your Internet service provider’s abuse desk. If you’re not working within a big institution like mine that has hot and cold running IT personnel that operate their own abuse prevention office, the address you’ll need is likely abuse@domain_name or postmaster@domain_name. Just hitting the spam button at the top of your browser/email software may do the trick. There’s more good advice at the FTC’s consumer spam page.
The answer came, ironically, to my email inbox in the form of one of those emails that did indeed violate the law.
I rolled my eyes and started into my reporting subroutine but then stopped cold. Just 1 second. If this person is selling lists of email addresses of conference attendees, somebody within the conference structure must be providing them. How is that legal? I have never agreed, in registering for a medical conference, to allow them to share my email address with anyone. To think that they are making money from that is extremely galling.
Vermont, at least, has enacted a law requiring companies that traffic in such email lists to register with the state. Although it has been in effect for 2 years, the jury is out regarding its efficacy. Our European counterparts are protected by the General Data Protection Regulation, which specifies that commercial email can be sent only to individuals who have explicitly opted into such mailings, and that purchased email lists are not compliant with the requirement.
Anybody have the inside scoop on this? Can we demand that our professional societies safeguard their attendee databases so this won’t happen? If they won’t, why am I paying big money to attend their conferences, only for them to make even more money at my expense?
Dr. Hitchcock is assistant professor, department of radiation oncology, at the University of Florida, Gainesville. She reported receiving research grant money from Merck. A version of this article first appeared on Medscape.com.
Closing your practice
“I might have to close my office,” a colleague wrote me recently. “I can’t find reliable medical assistants; no one good applies. Sad, but oh, well.”
A paucity of good employees is just one of many reasons given by physicians who have decided to close up shop. (See my recent column, “Finding Employees During a Pandemic”).
to address in order to ensure a smooth exit.
First, this cannot (and should not) be a hasty process. You will need at least a year to do it correctly, because there is a lot to do.
Once you have settled on a closing date, inform your attorney. If the firm you are using does not have experience in medical practice sales or closures, ask them to recommend one that does. You will need expert legal guidance during many of the steps that follow.
Next, review all of your contracts and leases. Most of them cannot be terminated at the drop of a hat. Facility and equipment leases may require a year’s notice, or even longer. Contracts with managed care, maintenance, cleaning, and hazardous waste disposal companies, and others such as answering services and website managers, should be reviewed to determine what sort of advance notice you will need to give.
Another step to take well in advance is to contact your malpractice insurance carrier. Most carriers have specific guidelines for when to notify your patients – and that notification will vary from carrier to carrier, state to state, and situation to situation. If you have a claims-made policy, you also need to inquire about the necessity of purchasing “tail” coverage, which will protect you in the event of a lawsuit after your practice has closed. Many carriers include tail coverage at no charge if you are retiring completely, but if you expect to do part-time, locum tenens, or volunteer medical work, you will need to pay for it.
Once you have the basics nailed down, notify your employees. You will want them to hear the news from you, not through the grapevine, and certainly not from your patients. You may be worried that some will quit, but keeping them in the dark will not prevent that, as they will find out soon enough. Besides, if you help them by assisting in finding them new employment, they will most likely help you by staying to the end.
At this point, you should also begin thinking about disposition of your patients’ records. You can’t just shred them, much as you might be tempted. Your attorney and malpractice carrier will guide you in how long they must be retained; 7-10 years is typical in many states, but it could be longer in yours. Unless you are selling part or all of your practice to another physician, you will have to designate someone else to be the legal custodian of the records and obtain a written custodial agreement from that person or organization.
Once that is arranged, you can notify your patients. Send them a letter or e-mail (or both) informing them of the date that you intend to close the practice. Let them know where their records will be kept, who to contact for a copy, and that their written consent will be required to obtain it. Some states also require that a notice be placed in the local newspaper or online, including the date of closure and how to request records.
This is also the time to inform all your third-party payers, including Medicare and Medicaid if applicable, any hospitals where you have privileges, and referring physicians. Notify any business concerns not notified already, such as utilities and other ancillary services. Your state medical board and the Drug Enforcement Agency will need to know as well. Contact a liquidator or used equipment dealer to arrange for disposal of any office equipment that has resale value. It is also a good time to decide how you will handle patient collections that trickle in after closing, and where mail should be forwarded.
As the closing date approaches, determine how to properly dispose of any medications you have on-hand. Your state may have requirements for disposal of controlled substances, and possibly for noncontrolled pharmaceuticals as well. Check your state’s controlled substances reporting system and other applicable regulators. Once the office is closed, don’t forget to shred any blank prescription pads and dissolve your corporation, if you have one.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
“I might have to close my office,” a colleague wrote me recently. “I can’t find reliable medical assistants; no one good applies. Sad, but oh, well.”
A paucity of good employees is just one of many reasons given by physicians who have decided to close up shop. (See my recent column, “Finding Employees During a Pandemic”).
to address in order to ensure a smooth exit.
First, this cannot (and should not) be a hasty process. You will need at least a year to do it correctly, because there is a lot to do.
Once you have settled on a closing date, inform your attorney. If the firm you are using does not have experience in medical practice sales or closures, ask them to recommend one that does. You will need expert legal guidance during many of the steps that follow.
Next, review all of your contracts and leases. Most of them cannot be terminated at the drop of a hat. Facility and equipment leases may require a year’s notice, or even longer. Contracts with managed care, maintenance, cleaning, and hazardous waste disposal companies, and others such as answering services and website managers, should be reviewed to determine what sort of advance notice you will need to give.
Another step to take well in advance is to contact your malpractice insurance carrier. Most carriers have specific guidelines for when to notify your patients – and that notification will vary from carrier to carrier, state to state, and situation to situation. If you have a claims-made policy, you also need to inquire about the necessity of purchasing “tail” coverage, which will protect you in the event of a lawsuit after your practice has closed. Many carriers include tail coverage at no charge if you are retiring completely, but if you expect to do part-time, locum tenens, or volunteer medical work, you will need to pay for it.
Once you have the basics nailed down, notify your employees. You will want them to hear the news from you, not through the grapevine, and certainly not from your patients. You may be worried that some will quit, but keeping them in the dark will not prevent that, as they will find out soon enough. Besides, if you help them by assisting in finding them new employment, they will most likely help you by staying to the end.
At this point, you should also begin thinking about disposition of your patients’ records. You can’t just shred them, much as you might be tempted. Your attorney and malpractice carrier will guide you in how long they must be retained; 7-10 years is typical in many states, but it could be longer in yours. Unless you are selling part or all of your practice to another physician, you will have to designate someone else to be the legal custodian of the records and obtain a written custodial agreement from that person or organization.
Once that is arranged, you can notify your patients. Send them a letter or e-mail (or both) informing them of the date that you intend to close the practice. Let them know where their records will be kept, who to contact for a copy, and that their written consent will be required to obtain it. Some states also require that a notice be placed in the local newspaper or online, including the date of closure and how to request records.
This is also the time to inform all your third-party payers, including Medicare and Medicaid if applicable, any hospitals where you have privileges, and referring physicians. Notify any business concerns not notified already, such as utilities and other ancillary services. Your state medical board and the Drug Enforcement Agency will need to know as well. Contact a liquidator or used equipment dealer to arrange for disposal of any office equipment that has resale value. It is also a good time to decide how you will handle patient collections that trickle in after closing, and where mail should be forwarded.
As the closing date approaches, determine how to properly dispose of any medications you have on-hand. Your state may have requirements for disposal of controlled substances, and possibly for noncontrolled pharmaceuticals as well. Check your state’s controlled substances reporting system and other applicable regulators. Once the office is closed, don’t forget to shred any blank prescription pads and dissolve your corporation, if you have one.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
“I might have to close my office,” a colleague wrote me recently. “I can’t find reliable medical assistants; no one good applies. Sad, but oh, well.”
A paucity of good employees is just one of many reasons given by physicians who have decided to close up shop. (See my recent column, “Finding Employees During a Pandemic”).
to address in order to ensure a smooth exit.
First, this cannot (and should not) be a hasty process. You will need at least a year to do it correctly, because there is a lot to do.
Once you have settled on a closing date, inform your attorney. If the firm you are using does not have experience in medical practice sales or closures, ask them to recommend one that does. You will need expert legal guidance during many of the steps that follow.
Next, review all of your contracts and leases. Most of them cannot be terminated at the drop of a hat. Facility and equipment leases may require a year’s notice, or even longer. Contracts with managed care, maintenance, cleaning, and hazardous waste disposal companies, and others such as answering services and website managers, should be reviewed to determine what sort of advance notice you will need to give.
Another step to take well in advance is to contact your malpractice insurance carrier. Most carriers have specific guidelines for when to notify your patients – and that notification will vary from carrier to carrier, state to state, and situation to situation. If you have a claims-made policy, you also need to inquire about the necessity of purchasing “tail” coverage, which will protect you in the event of a lawsuit after your practice has closed. Many carriers include tail coverage at no charge if you are retiring completely, but if you expect to do part-time, locum tenens, or volunteer medical work, you will need to pay for it.
Once you have the basics nailed down, notify your employees. You will want them to hear the news from you, not through the grapevine, and certainly not from your patients. You may be worried that some will quit, but keeping them in the dark will not prevent that, as they will find out soon enough. Besides, if you help them by assisting in finding them new employment, they will most likely help you by staying to the end.
At this point, you should also begin thinking about disposition of your patients’ records. You can’t just shred them, much as you might be tempted. Your attorney and malpractice carrier will guide you in how long they must be retained; 7-10 years is typical in many states, but it could be longer in yours. Unless you are selling part or all of your practice to another physician, you will have to designate someone else to be the legal custodian of the records and obtain a written custodial agreement from that person or organization.
Once that is arranged, you can notify your patients. Send them a letter or e-mail (or both) informing them of the date that you intend to close the practice. Let them know where their records will be kept, who to contact for a copy, and that their written consent will be required to obtain it. Some states also require that a notice be placed in the local newspaper or online, including the date of closure and how to request records.
This is also the time to inform all your third-party payers, including Medicare and Medicaid if applicable, any hospitals where you have privileges, and referring physicians. Notify any business concerns not notified already, such as utilities and other ancillary services. Your state medical board and the Drug Enforcement Agency will need to know as well. Contact a liquidator or used equipment dealer to arrange for disposal of any office equipment that has resale value. It is also a good time to decide how you will handle patient collections that trickle in after closing, and where mail should be forwarded.
As the closing date approaches, determine how to properly dispose of any medications you have on-hand. Your state may have requirements for disposal of controlled substances, and possibly for noncontrolled pharmaceuticals as well. Check your state’s controlled substances reporting system and other applicable regulators. Once the office is closed, don’t forget to shred any blank prescription pads and dissolve your corporation, if you have one.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].