User login
Formerly Skin & Allergy News
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')]
The leading independent newspaper covering dermatology news and commentary.
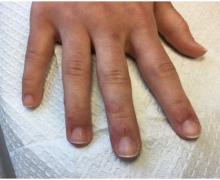
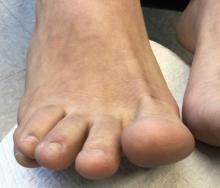
How does atopic dermatitis present in skin of color?
“We see very heterogenous and broad clinical presentations across the diverse patient populations that we see,” Andrew F. Alexis, MD, MPH, said at the Revolutionizing Atopic Dermatitis symposium. “Some of these differences might be related to population variations in skin barrier function, immunologic factors, genetic factors, and environmental factors, which all interplay to produce variations in the clinical presentation and overall impact of AD. Many nongenetic factors also contribute to differences that we see, including some socioeconomic and other factors that feed into health disparities.”
Dr. Alexis, professor of clinical dermatology at Weill Cornell Medicine, New York, discussed four main clinical features of AD in skin of color.
Erythema is less visible because it is masked by pigment
“There can be some masking of the redness and alteration of that color such that it doesn’t look bright red as it would in the background of lightly pigmented skin,” Dr. Alexis said. “Instead, the [AD lesions] have shades of grayish-red or grayish-brown or reddish-brown. It’s important to recognize this clinical presentation and look carefully and assess the patient – not just visually but with palpation and take into consideration symptomatology so that you don’t fall into the trap of calling an AD lesion postinflammatory hyperpigmentation. It’s also helpful to isolate the islands of normal or nonlesional skin and contrast that with the areas of lesional skin, to get a sense of how active and inflamed the areas are. Palpation really helps to appreciate the elevation of the lesions that are involved.”
Follicular accentuation
Morphological variants common in skin of color include the follicular variant or micropapular variant of AD. “You might just see a collection of papules that are 1-2 mm in size and pruritic and in typical sites of predilection [for] eczema,” he said. Prurigo nodularis–like lesions or prurigo nodularis in association with AD are also seen more frequently in skin of color.
Lichenification
The lichenoid variant of AD is characterized by a violaceous hue and other features that resemble lichen planus and has been reported to be more common in individuals of African descent. A prospective study of about 1,000 patients with AD seen over 2 years at a dermatology clinic in southeastern Nigeria found that 54% of patients had papular lichenoid lesions. In addition, 51% had elevated blood eosinophil counts, especially those with severe disease.
Dr. Alexis added that psoriasiform features have been reported in studies of East Asian populations with AD. These plaques may be more well demarcated and have clinical and histologic features that resemble psoriasis.
Dyspigmentation
One common feature across the spectrum of patients with skin of color “is the risk of longstanding pigmentary sequelae in the form of hyperpigmentation or hypopigmentation,” said Dr. Alexis, who is also vice chair for diversity and inclusion for the department of dermatology at Weill Cornell Medicine. “In very severe longstanding areas with chronic excoriation to the point of breaking of the skin, eroding of the skin, causing permanent damage to the melanocytes, dyspigmentation that resembles vitiligo can be seen. We can also see hypopigmentation as a consequence of topical corticosteroids, particularly those that are class I or class II and are used for prolonged periods of time.”
Dr. Alexis noted that delays in treatment and undertreatment can contribute to a higher risk of pigmentary and other long-term sequelae. “New therapies show promise in improving outcomes in AD patients with skin of color. When it comes to therapeutic responses, there are some post hoc studies that have investigated potential differences in safety and efficacy of the agents that have been recently approved. We clearly need more data to better understand if there are potential racial or ethnic differences.”
Dr. Alexis reported no relevant financial relationships.
Commentary by Lawrence F. Eichenfield, MD
Atopic dermatitis (AD) is highly heterogenous, with tremendous variations in extent, qualities of eczema, symptom complex, and physical presentation. Prior studies have reported disparities of care delivered to racial and ethnic minorities in the United States, as well as higher susceptibility to AD and odds of persistent disease into adulthood from child-onset AD. Recognizing some differences in presentation of AD in patients with skin of color is important as we select our therapeutic interventions, including assessing new treatments being added to our armamentarium. Erythema may be harder to notice in darker skin, but attempting to blanch the skin with pressure can help to assess the color and inflammation. Appreciating lichenoid changes, including papular and “micropapular” AD, and psoriasiform-like thickening in certain patients (reportedly more common in East Asian populations) are important as well. And dyspigmentation is an important aspect of the disease presentation and patient and parental concern, given both hypopigmentaton and hyperpigmentation commonly seen over the course of AD.
Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children's Hospital-San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. He disclosed that he has served as an investigator and/or consultant to AbbVie, Lilly, Pfizer, Regeneron, Sanofi-Genzyme, and Verrica.
A version of this article first appeared on Medscape.com.
This article was updated 6/18/22.
“We see very heterogenous and broad clinical presentations across the diverse patient populations that we see,” Andrew F. Alexis, MD, MPH, said at the Revolutionizing Atopic Dermatitis symposium. “Some of these differences might be related to population variations in skin barrier function, immunologic factors, genetic factors, and environmental factors, which all interplay to produce variations in the clinical presentation and overall impact of AD. Many nongenetic factors also contribute to differences that we see, including some socioeconomic and other factors that feed into health disparities.”
Dr. Alexis, professor of clinical dermatology at Weill Cornell Medicine, New York, discussed four main clinical features of AD in skin of color.
Erythema is less visible because it is masked by pigment
“There can be some masking of the redness and alteration of that color such that it doesn’t look bright red as it would in the background of lightly pigmented skin,” Dr. Alexis said. “Instead, the [AD lesions] have shades of grayish-red or grayish-brown or reddish-brown. It’s important to recognize this clinical presentation and look carefully and assess the patient – not just visually but with palpation and take into consideration symptomatology so that you don’t fall into the trap of calling an AD lesion postinflammatory hyperpigmentation. It’s also helpful to isolate the islands of normal or nonlesional skin and contrast that with the areas of lesional skin, to get a sense of how active and inflamed the areas are. Palpation really helps to appreciate the elevation of the lesions that are involved.”
Follicular accentuation
Morphological variants common in skin of color include the follicular variant or micropapular variant of AD. “You might just see a collection of papules that are 1-2 mm in size and pruritic and in typical sites of predilection [for] eczema,” he said. Prurigo nodularis–like lesions or prurigo nodularis in association with AD are also seen more frequently in skin of color.
Lichenification
The lichenoid variant of AD is characterized by a violaceous hue and other features that resemble lichen planus and has been reported to be more common in individuals of African descent. A prospective study of about 1,000 patients with AD seen over 2 years at a dermatology clinic in southeastern Nigeria found that 54% of patients had papular lichenoid lesions. In addition, 51% had elevated blood eosinophil counts, especially those with severe disease.
Dr. Alexis added that psoriasiform features have been reported in studies of East Asian populations with AD. These plaques may be more well demarcated and have clinical and histologic features that resemble psoriasis.
Dyspigmentation
One common feature across the spectrum of patients with skin of color “is the risk of longstanding pigmentary sequelae in the form of hyperpigmentation or hypopigmentation,” said Dr. Alexis, who is also vice chair for diversity and inclusion for the department of dermatology at Weill Cornell Medicine. “In very severe longstanding areas with chronic excoriation to the point of breaking of the skin, eroding of the skin, causing permanent damage to the melanocytes, dyspigmentation that resembles vitiligo can be seen. We can also see hypopigmentation as a consequence of topical corticosteroids, particularly those that are class I or class II and are used for prolonged periods of time.”
Dr. Alexis noted that delays in treatment and undertreatment can contribute to a higher risk of pigmentary and other long-term sequelae. “New therapies show promise in improving outcomes in AD patients with skin of color. When it comes to therapeutic responses, there are some post hoc studies that have investigated potential differences in safety and efficacy of the agents that have been recently approved. We clearly need more data to better understand if there are potential racial or ethnic differences.”
Dr. Alexis reported no relevant financial relationships.
Commentary by Lawrence F. Eichenfield, MD
Atopic dermatitis (AD) is highly heterogenous, with tremendous variations in extent, qualities of eczema, symptom complex, and physical presentation. Prior studies have reported disparities of care delivered to racial and ethnic minorities in the United States, as well as higher susceptibility to AD and odds of persistent disease into adulthood from child-onset AD. Recognizing some differences in presentation of AD in patients with skin of color is important as we select our therapeutic interventions, including assessing new treatments being added to our armamentarium. Erythema may be harder to notice in darker skin, but attempting to blanch the skin with pressure can help to assess the color and inflammation. Appreciating lichenoid changes, including papular and “micropapular” AD, and psoriasiform-like thickening in certain patients (reportedly more common in East Asian populations) are important as well. And dyspigmentation is an important aspect of the disease presentation and patient and parental concern, given both hypopigmentaton and hyperpigmentation commonly seen over the course of AD.
Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children's Hospital-San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. He disclosed that he has served as an investigator and/or consultant to AbbVie, Lilly, Pfizer, Regeneron, Sanofi-Genzyme, and Verrica.
A version of this article first appeared on Medscape.com.
This article was updated 6/18/22.
“We see very heterogenous and broad clinical presentations across the diverse patient populations that we see,” Andrew F. Alexis, MD, MPH, said at the Revolutionizing Atopic Dermatitis symposium. “Some of these differences might be related to population variations in skin barrier function, immunologic factors, genetic factors, and environmental factors, which all interplay to produce variations in the clinical presentation and overall impact of AD. Many nongenetic factors also contribute to differences that we see, including some socioeconomic and other factors that feed into health disparities.”
Dr. Alexis, professor of clinical dermatology at Weill Cornell Medicine, New York, discussed four main clinical features of AD in skin of color.
Erythema is less visible because it is masked by pigment
“There can be some masking of the redness and alteration of that color such that it doesn’t look bright red as it would in the background of lightly pigmented skin,” Dr. Alexis said. “Instead, the [AD lesions] have shades of grayish-red or grayish-brown or reddish-brown. It’s important to recognize this clinical presentation and look carefully and assess the patient – not just visually but with palpation and take into consideration symptomatology so that you don’t fall into the trap of calling an AD lesion postinflammatory hyperpigmentation. It’s also helpful to isolate the islands of normal or nonlesional skin and contrast that with the areas of lesional skin, to get a sense of how active and inflamed the areas are. Palpation really helps to appreciate the elevation of the lesions that are involved.”
Follicular accentuation
Morphological variants common in skin of color include the follicular variant or micropapular variant of AD. “You might just see a collection of papules that are 1-2 mm in size and pruritic and in typical sites of predilection [for] eczema,” he said. Prurigo nodularis–like lesions or prurigo nodularis in association with AD are also seen more frequently in skin of color.
Lichenification
The lichenoid variant of AD is characterized by a violaceous hue and other features that resemble lichen planus and has been reported to be more common in individuals of African descent. A prospective study of about 1,000 patients with AD seen over 2 years at a dermatology clinic in southeastern Nigeria found that 54% of patients had papular lichenoid lesions. In addition, 51% had elevated blood eosinophil counts, especially those with severe disease.
Dr. Alexis added that psoriasiform features have been reported in studies of East Asian populations with AD. These plaques may be more well demarcated and have clinical and histologic features that resemble psoriasis.
Dyspigmentation
One common feature across the spectrum of patients with skin of color “is the risk of longstanding pigmentary sequelae in the form of hyperpigmentation or hypopigmentation,” said Dr. Alexis, who is also vice chair for diversity and inclusion for the department of dermatology at Weill Cornell Medicine. “In very severe longstanding areas with chronic excoriation to the point of breaking of the skin, eroding of the skin, causing permanent damage to the melanocytes, dyspigmentation that resembles vitiligo can be seen. We can also see hypopigmentation as a consequence of topical corticosteroids, particularly those that are class I or class II and are used for prolonged periods of time.”
Dr. Alexis noted that delays in treatment and undertreatment can contribute to a higher risk of pigmentary and other long-term sequelae. “New therapies show promise in improving outcomes in AD patients with skin of color. When it comes to therapeutic responses, there are some post hoc studies that have investigated potential differences in safety and efficacy of the agents that have been recently approved. We clearly need more data to better understand if there are potential racial or ethnic differences.”
Dr. Alexis reported no relevant financial relationships.
Commentary by Lawrence F. Eichenfield, MD
Atopic dermatitis (AD) is highly heterogenous, with tremendous variations in extent, qualities of eczema, symptom complex, and physical presentation. Prior studies have reported disparities of care delivered to racial and ethnic minorities in the United States, as well as higher susceptibility to AD and odds of persistent disease into adulthood from child-onset AD. Recognizing some differences in presentation of AD in patients with skin of color is important as we select our therapeutic interventions, including assessing new treatments being added to our armamentarium. Erythema may be harder to notice in darker skin, but attempting to blanch the skin with pressure can help to assess the color and inflammation. Appreciating lichenoid changes, including papular and “micropapular” AD, and psoriasiform-like thickening in certain patients (reportedly more common in East Asian populations) are important as well. And dyspigmentation is an important aspect of the disease presentation and patient and parental concern, given both hypopigmentaton and hyperpigmentation commonly seen over the course of AD.
Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children's Hospital-San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. He disclosed that he has served as an investigator and/or consultant to AbbVie, Lilly, Pfizer, Regeneron, Sanofi-Genzyme, and Verrica.
A version of this article first appeared on Medscape.com.
This article was updated 6/18/22.
FROM REVOLUTIONIZING AD 2021
Abrocitinib efficacy dose-dependent, similar across AD age groups
and was comparable in patients aged 51 years and older, results from a post hoc analysis of four trials showed.
Abrocitinib (Cibinqo) is an oral, once-daily, Janus kinase 1 selective inhibitor that has shown good efficacy and safety as monotherapy or combined with topical therapy for treatment of patients with moderate to severe AD. The agent was approved in mid-December in Europe for the treatment of moderate to severe AD in adults who are candidates for systemic therapy and is currently under review by the Food and Drug Administration.
“We know that responses to, and adverse events associated with, systemic therapies may vary among patients of different ages,” Andrew F. Alexis, MD, MPH, said during a late-breaking abstract session at the Revolutionizing Atopic Dermatitis virtual symposium. “The efficacy and safety of abrocitinib monotherapy were previously evaluated in adolescent and adult subpopulations from controlled clinical trials in patients with moderate to severe AD. The objective of the current study was to assess the impact of age on short-term responses to abrocitinib treatment in patients with moderate to severe AD.”
Dr. Alexis, professor of clinical dermatology at Weill Cornell Medicine, New York, and colleagues performed a post hoc analysis across four randomized, double-blind studies that was stratified by age group: 12-17 years, 18-40 years, 41-50 years, and 51 years and older. Efficacy data were assessed separately for patients in the monotherapy pool and in the JADE COMPARE trial. The monotherapy pool included patients from one phase 2b study and two phase 3 studies who received abrocitinib 200 mg, abrocitinib 100 mg, or placebo monotherapy for 12 weeks (JADE-MONO-1 and JADE-MONO-2).
The JADE COMPARE pool included patients who received abrocitinib 200 mg, abrocitinib 100 mg, or placebo, plus medicated topical therapy for 16 weeks. Data from patients in all four trials were pooled for the analysis of treatment-emergent adverse events. Efficacy points analyzed were the Investigator Global Assessment (IGA) score of 0/1 (clear or almost clear), a 75% reduction from baseline in the Eczema Area and Severity Index (EASI-75), or Peak Pruritus Numeric Rating Scale score (PP-NRS4) at week 12 for the monotherapy pool and at week 16 for COMPARE.
In the monotherapy pool, the proportions of patients ages 12-17 years, 18-40 years, 41-50 years, and 51 years and older who achieved an IGA 0/1 response at 12 weeks were 31.3%, 40.2%, 43.8%, and 50.8% (abrocitinib 200 mg); 22%, 23.7%, 22.4%, and 40.8% (abrocitinib 100 mg); and 8.7%, 8%, 3.3%, and 10% (placebo).
In JADE COMPARE, the proportions of patients aged 18-40 years, 41-50 years, and 51 years and older who achieved an IGA 0/1 response were 50%, 53.2%, and 34.8% (abrocitinib 200 mg); 36.9%, 37.1%, and 26.1% (abrocitinib 100 mg); and 12%, 11.8%, and 16.7% (placebo) at 16 weeks. Similar trends were observed for EASI-75 and PP-NRS4 responses at 12 weeks.
Across all age groups, the most common treatment-emergent adverse events were infections/infestations and gastrointestinal effects; most cases were mild or moderate. Nausea was more frequent in the two younger age groups and was dose related: For abrocitinib 200 mg and abrocitinib 100 mg, respectively, the rates of nausea were 18.8% and 7.8% in patients aged 12-17 years; 17.1% and 6.4% in patients aged 18-40 years; and 7.1% and 3.3% in patients aged 51 and older.
“Efficacy responses in patients 51 years of age and older were comparable to those in other age groups,” concluded Dr. Alexis, vice chair for diversity and inclusion in the department of dermatology at Weill Cornell. “The safety profile was consistent across age ranges and was similar to that reported previously.”
The investigators found that treatment response to abrocitinib “in the absence or presence of medicated topical therapy was fairly consistent across age groups, showed similar dose-dependency, and importantly, did not show reduced efficacy in older adults as measured by lesional severity, extent, and itch at 4 months,” said Raj Chovatiya, MD, PhD, assistant professor of dermatology at Northwestern University, Chicago, who was asked to comment on the study.
“Furthermore, the safety profile was consistent across all adults, though notably, nausea was more common among younger age groups, highlighting an area of future investigation,” he added. “Overall, these data show that abrocitinib is associated with similar short-term responses across adulthood and underscore the importance of the JAK-STAT pathway in the underlying pathophysiology of AD in different age groups. It will be interesting to see how these data reflect the real-world setting with both short- and long-term outcomes in a heterogeneous patient population.”
In the interview, Dr. Chovatiya said, “the next frontier in personalized therapy for AD involves deeper clinical phenotyping of our patients and a better understanding of how efficacy and safety vary across patient groups.” For example, he noted, “AD in earlier versus later adulthood may be associated with different clinical signs, symptoms, comorbidities, and other measures of patient burden, and thus, may be associated with different treatment responses to systemic therapy.”
Dr. Alexis disclosed that he has served as an adviser to, or has received consulting fees from, Leo, Galderma, Pfizer, Sanofi-Regeneron, Dermavant, Beiersdorf, Valeant, L’Oréal, BMS, Bausch Health, UCB, Vyne, Arcutis, Janssen, Allergan, Almirall, AbbVie, Sol-Gel, and Amgen.
Dr. Chovatiya disclosed that he is a consultant to, a speaker for, and/or a member of the advisory board for AbbVie, Arena, Arcutis, Incyte, Pfizer, Regeneron, and Sanofi-Genzyme.
A version of this article first appeared on Medscape.com.
and was comparable in patients aged 51 years and older, results from a post hoc analysis of four trials showed.
Abrocitinib (Cibinqo) is an oral, once-daily, Janus kinase 1 selective inhibitor that has shown good efficacy and safety as monotherapy or combined with topical therapy for treatment of patients with moderate to severe AD. The agent was approved in mid-December in Europe for the treatment of moderate to severe AD in adults who are candidates for systemic therapy and is currently under review by the Food and Drug Administration.
“We know that responses to, and adverse events associated with, systemic therapies may vary among patients of different ages,” Andrew F. Alexis, MD, MPH, said during a late-breaking abstract session at the Revolutionizing Atopic Dermatitis virtual symposium. “The efficacy and safety of abrocitinib monotherapy were previously evaluated in adolescent and adult subpopulations from controlled clinical trials in patients with moderate to severe AD. The objective of the current study was to assess the impact of age on short-term responses to abrocitinib treatment in patients with moderate to severe AD.”
Dr. Alexis, professor of clinical dermatology at Weill Cornell Medicine, New York, and colleagues performed a post hoc analysis across four randomized, double-blind studies that was stratified by age group: 12-17 years, 18-40 years, 41-50 years, and 51 years and older. Efficacy data were assessed separately for patients in the monotherapy pool and in the JADE COMPARE trial. The monotherapy pool included patients from one phase 2b study and two phase 3 studies who received abrocitinib 200 mg, abrocitinib 100 mg, or placebo monotherapy for 12 weeks (JADE-MONO-1 and JADE-MONO-2).
The JADE COMPARE pool included patients who received abrocitinib 200 mg, abrocitinib 100 mg, or placebo, plus medicated topical therapy for 16 weeks. Data from patients in all four trials were pooled for the analysis of treatment-emergent adverse events. Efficacy points analyzed were the Investigator Global Assessment (IGA) score of 0/1 (clear or almost clear), a 75% reduction from baseline in the Eczema Area and Severity Index (EASI-75), or Peak Pruritus Numeric Rating Scale score (PP-NRS4) at week 12 for the monotherapy pool and at week 16 for COMPARE.
In the monotherapy pool, the proportions of patients ages 12-17 years, 18-40 years, 41-50 years, and 51 years and older who achieved an IGA 0/1 response at 12 weeks were 31.3%, 40.2%, 43.8%, and 50.8% (abrocitinib 200 mg); 22%, 23.7%, 22.4%, and 40.8% (abrocitinib 100 mg); and 8.7%, 8%, 3.3%, and 10% (placebo).
In JADE COMPARE, the proportions of patients aged 18-40 years, 41-50 years, and 51 years and older who achieved an IGA 0/1 response were 50%, 53.2%, and 34.8% (abrocitinib 200 mg); 36.9%, 37.1%, and 26.1% (abrocitinib 100 mg); and 12%, 11.8%, and 16.7% (placebo) at 16 weeks. Similar trends were observed for EASI-75 and PP-NRS4 responses at 12 weeks.
Across all age groups, the most common treatment-emergent adverse events were infections/infestations and gastrointestinal effects; most cases were mild or moderate. Nausea was more frequent in the two younger age groups and was dose related: For abrocitinib 200 mg and abrocitinib 100 mg, respectively, the rates of nausea were 18.8% and 7.8% in patients aged 12-17 years; 17.1% and 6.4% in patients aged 18-40 years; and 7.1% and 3.3% in patients aged 51 and older.
“Efficacy responses in patients 51 years of age and older were comparable to those in other age groups,” concluded Dr. Alexis, vice chair for diversity and inclusion in the department of dermatology at Weill Cornell. “The safety profile was consistent across age ranges and was similar to that reported previously.”
The investigators found that treatment response to abrocitinib “in the absence or presence of medicated topical therapy was fairly consistent across age groups, showed similar dose-dependency, and importantly, did not show reduced efficacy in older adults as measured by lesional severity, extent, and itch at 4 months,” said Raj Chovatiya, MD, PhD, assistant professor of dermatology at Northwestern University, Chicago, who was asked to comment on the study.
“Furthermore, the safety profile was consistent across all adults, though notably, nausea was more common among younger age groups, highlighting an area of future investigation,” he added. “Overall, these data show that abrocitinib is associated with similar short-term responses across adulthood and underscore the importance of the JAK-STAT pathway in the underlying pathophysiology of AD in different age groups. It will be interesting to see how these data reflect the real-world setting with both short- and long-term outcomes in a heterogeneous patient population.”
In the interview, Dr. Chovatiya said, “the next frontier in personalized therapy for AD involves deeper clinical phenotyping of our patients and a better understanding of how efficacy and safety vary across patient groups.” For example, he noted, “AD in earlier versus later adulthood may be associated with different clinical signs, symptoms, comorbidities, and other measures of patient burden, and thus, may be associated with different treatment responses to systemic therapy.”
Dr. Alexis disclosed that he has served as an adviser to, or has received consulting fees from, Leo, Galderma, Pfizer, Sanofi-Regeneron, Dermavant, Beiersdorf, Valeant, L’Oréal, BMS, Bausch Health, UCB, Vyne, Arcutis, Janssen, Allergan, Almirall, AbbVie, Sol-Gel, and Amgen.
Dr. Chovatiya disclosed that he is a consultant to, a speaker for, and/or a member of the advisory board for AbbVie, Arena, Arcutis, Incyte, Pfizer, Regeneron, and Sanofi-Genzyme.
A version of this article first appeared on Medscape.com.
and was comparable in patients aged 51 years and older, results from a post hoc analysis of four trials showed.
Abrocitinib (Cibinqo) is an oral, once-daily, Janus kinase 1 selective inhibitor that has shown good efficacy and safety as monotherapy or combined with topical therapy for treatment of patients with moderate to severe AD. The agent was approved in mid-December in Europe for the treatment of moderate to severe AD in adults who are candidates for systemic therapy and is currently under review by the Food and Drug Administration.
“We know that responses to, and adverse events associated with, systemic therapies may vary among patients of different ages,” Andrew F. Alexis, MD, MPH, said during a late-breaking abstract session at the Revolutionizing Atopic Dermatitis virtual symposium. “The efficacy and safety of abrocitinib monotherapy were previously evaluated in adolescent and adult subpopulations from controlled clinical trials in patients with moderate to severe AD. The objective of the current study was to assess the impact of age on short-term responses to abrocitinib treatment in patients with moderate to severe AD.”
Dr. Alexis, professor of clinical dermatology at Weill Cornell Medicine, New York, and colleagues performed a post hoc analysis across four randomized, double-blind studies that was stratified by age group: 12-17 years, 18-40 years, 41-50 years, and 51 years and older. Efficacy data were assessed separately for patients in the monotherapy pool and in the JADE COMPARE trial. The monotherapy pool included patients from one phase 2b study and two phase 3 studies who received abrocitinib 200 mg, abrocitinib 100 mg, or placebo monotherapy for 12 weeks (JADE-MONO-1 and JADE-MONO-2).
The JADE COMPARE pool included patients who received abrocitinib 200 mg, abrocitinib 100 mg, or placebo, plus medicated topical therapy for 16 weeks. Data from patients in all four trials were pooled for the analysis of treatment-emergent adverse events. Efficacy points analyzed were the Investigator Global Assessment (IGA) score of 0/1 (clear or almost clear), a 75% reduction from baseline in the Eczema Area and Severity Index (EASI-75), or Peak Pruritus Numeric Rating Scale score (PP-NRS4) at week 12 for the monotherapy pool and at week 16 for COMPARE.
In the monotherapy pool, the proportions of patients ages 12-17 years, 18-40 years, 41-50 years, and 51 years and older who achieved an IGA 0/1 response at 12 weeks were 31.3%, 40.2%, 43.8%, and 50.8% (abrocitinib 200 mg); 22%, 23.7%, 22.4%, and 40.8% (abrocitinib 100 mg); and 8.7%, 8%, 3.3%, and 10% (placebo).
In JADE COMPARE, the proportions of patients aged 18-40 years, 41-50 years, and 51 years and older who achieved an IGA 0/1 response were 50%, 53.2%, and 34.8% (abrocitinib 200 mg); 36.9%, 37.1%, and 26.1% (abrocitinib 100 mg); and 12%, 11.8%, and 16.7% (placebo) at 16 weeks. Similar trends were observed for EASI-75 and PP-NRS4 responses at 12 weeks.
Across all age groups, the most common treatment-emergent adverse events were infections/infestations and gastrointestinal effects; most cases were mild or moderate. Nausea was more frequent in the two younger age groups and was dose related: For abrocitinib 200 mg and abrocitinib 100 mg, respectively, the rates of nausea were 18.8% and 7.8% in patients aged 12-17 years; 17.1% and 6.4% in patients aged 18-40 years; and 7.1% and 3.3% in patients aged 51 and older.
“Efficacy responses in patients 51 years of age and older were comparable to those in other age groups,” concluded Dr. Alexis, vice chair for diversity and inclusion in the department of dermatology at Weill Cornell. “The safety profile was consistent across age ranges and was similar to that reported previously.”
The investigators found that treatment response to abrocitinib “in the absence or presence of medicated topical therapy was fairly consistent across age groups, showed similar dose-dependency, and importantly, did not show reduced efficacy in older adults as measured by lesional severity, extent, and itch at 4 months,” said Raj Chovatiya, MD, PhD, assistant professor of dermatology at Northwestern University, Chicago, who was asked to comment on the study.
“Furthermore, the safety profile was consistent across all adults, though notably, nausea was more common among younger age groups, highlighting an area of future investigation,” he added. “Overall, these data show that abrocitinib is associated with similar short-term responses across adulthood and underscore the importance of the JAK-STAT pathway in the underlying pathophysiology of AD in different age groups. It will be interesting to see how these data reflect the real-world setting with both short- and long-term outcomes in a heterogeneous patient population.”
In the interview, Dr. Chovatiya said, “the next frontier in personalized therapy for AD involves deeper clinical phenotyping of our patients and a better understanding of how efficacy and safety vary across patient groups.” For example, he noted, “AD in earlier versus later adulthood may be associated with different clinical signs, symptoms, comorbidities, and other measures of patient burden, and thus, may be associated with different treatment responses to systemic therapy.”
Dr. Alexis disclosed that he has served as an adviser to, or has received consulting fees from, Leo, Galderma, Pfizer, Sanofi-Regeneron, Dermavant, Beiersdorf, Valeant, L’Oréal, BMS, Bausch Health, UCB, Vyne, Arcutis, Janssen, Allergan, Almirall, AbbVie, Sol-Gel, and Amgen.
Dr. Chovatiya disclosed that he is a consultant to, a speaker for, and/or a member of the advisory board for AbbVie, Arena, Arcutis, Incyte, Pfizer, Regeneron, and Sanofi-Genzyme.
A version of this article first appeared on Medscape.com.
FROM REVOLUTIONIZING AD 2021
CRP elevated in adults with AD and sleep disturbance
and mortality, results from a large cohort analysis showed.
“The implications of these findings are vast,” presenting author Varsha Parthasarathy said during a late-breaking abstract session at the Revolutionizing Atopic Dermatitis virtual symposium. “Poor sleep quality is known to be associated with increased inflammatory markers such as IL-6, IL-17, and CRP, so it is interesting to see this reflected in AD patients with versus without sleep disturbance. Additionally, we know that CRP is a driver of inflammation and is strongly associated with cardiovascular complications such as heart attack and stroke. Therefore, CRP may be a useful prognostic marker in AD patients with sleep disturbances.”
To examine the comorbidity burden of sleep disorders in AD patients and associate findings with inflammatory CRP and cardiovascular comorbidities, Mr. Parthasarathy, a medical student and itch fellow in the department of dermatology at the Johns Hopkins University School of Medicine, Baltimore, and colleagues drew from TriNetX, a health care network of approximately 73 million de-identified medical records in 53 organizations. The years of study were 2015 to 2021. The researchers limited the analysis to adults with at least two instances of International Classification of Diseases, Tenth Revision (ICD-10) code L28 for AD, to capture a population with true AD. Controls were adults without AD who presented for general checkup and were matched to AD patients by age, race, and sex.
The study population consisted of 120,480 AD patients and matched controls. Their mean age was 36 years, 61% were female, and 26% were Black. Compared with controls, AD patients had an increased risk of developing general sleep disorders over the 6-year period (relative risk, 1.10), as well as obstructive sleep apnea (RR, 1.13), insomnia (RR, 1.10), hypersomnia (RR, 1.24), sleep-related movement disorders (RR, 1.36), restless legs syndrome (RR, 1.25), sleep deprivation (RR, 1.36), and unspecified sleep disorders (RR, 1.22).
To examine the association of sleep disturbance with the inflammatory biomarker CRP, the researchers measured CRP levels between these patient groups. They found a substantially higher CRP in AD patients compared with controls (21.2 mg/L vs. 7.6 mg/L, respectively; P < .0001). This finding “is suggestive of a higher level of inflammation in these patients,” Mr. Parthasarathy said. Interestingly, he added, they also found a higher CRP level in AD patients with sleep disturbances compared to AD patients without sleep disturbances (23.3 vs. 20.6 mg/L; P = .02), “also pointing to a higher inflammatory burden in AD patients whose sleep was affected.”
Compared to matched AD patients without sleep disorders, AD patients with sleep disorders were more likely to develop obesity (RR, 2.65), hyperlipidemia (RR, 2.18), type 2 diabetes (RR, 2.45), metabolic syndrome (RR, 4.16), atherosclerosis (RR, 2.42), peripheral vascular disease (RR, 2.47), stroke (RR, 2.37), venous thromboembolism (RR, 2.93), and mortality (hazard ratio, 1.24).
“There is a consequence of not treating patients with atopic dermatitis, especially those patients with sleep disturbance,” the study’s primary author, Shawn G. Kwatra, MD, associate professor of dermatology at Johns Hopkins, told this news organization. “Chronic inflammation can lead to the development of comorbidities, so it is important to offer patients early treatment to reduce their overall inflammation.” He said that he was most surprised by the degree of increased inflammation in the blood of AD as compared to healthy controls. “This likely plays a part in the development of several comorbidities,” he said.
Mr. Parthasarathy acknowledged certain limitations of the study, including the inability to infer causal relationships, as uncontrolled factors may be present. “Additionally, sampling of only patients that have had medical encounters limits the generalizability of the findings,” she said. “However, findings in this large cohort study suggest that clinicians should seek to identify sleep disorders in AD patients and screen for cardiac comorbidities secondary to inflammation in this patient population.”
“There is increased data to suggest that adults with AD, particularly those with more severe disease, may be at an increased risk of cardiovascular disease and the results from [this study] further support the concept of AD as systemic disease,” said Zelma C. Chiesa Fuxench, MD, MSCE, assistant professor of dermatology at the University of Pennsylvania, Philadelphia, who was asked to comment on the study. She cited the large population-based, retrospective design and use of two instances of ICD codes for AD to confirm diagnosis as key strengths of the research. “However, it is unclear if for each patient CRP levels were measured at one single timepoint,” Dr. Chiesa Fuxench said. “For future studies, it would be interesting to see if these levels fluctuate with time and if persistently elevated levels are associated with worse cardiovascular outcomes in this population. More data is needed to better understand the relationship better atopic dermatitis disease severity, impact on sleep, and how this relates to increased systemic inflammation and worse cardiovascular outcomes in this population.”
Dr. Kwatra disclosed support by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number K23AR077073-01A1 and previous funding by the Dermatology Foundation and Skin of Color Society. Dr. Kwatra is also an advisory board member/consultant for AbbVie, Celldex Therapeutics, Galderma, Incyte Corporation, Johnson & Johnson, Novartis Pharmaceuticals Corporation, Pfizer, Regeneron Pharmaceuticals, Sanofi, and Kiniksa Pharmaceuticals and has served as an investigator for Galderma, Pfizer, and Sanofi. Dr. Chiesa Fuxench disclosed research grants from several pharmaceutical companies for work related to AD. She has also served as a consultant for the Asthma and Allergy Foundation of America, National Eczema Association, AbbVie, Incyte Corporation, and Pfizer.
A version of this article first appeared on Medscape.com.
and mortality, results from a large cohort analysis showed.
“The implications of these findings are vast,” presenting author Varsha Parthasarathy said during a late-breaking abstract session at the Revolutionizing Atopic Dermatitis virtual symposium. “Poor sleep quality is known to be associated with increased inflammatory markers such as IL-6, IL-17, and CRP, so it is interesting to see this reflected in AD patients with versus without sleep disturbance. Additionally, we know that CRP is a driver of inflammation and is strongly associated with cardiovascular complications such as heart attack and stroke. Therefore, CRP may be a useful prognostic marker in AD patients with sleep disturbances.”
To examine the comorbidity burden of sleep disorders in AD patients and associate findings with inflammatory CRP and cardiovascular comorbidities, Mr. Parthasarathy, a medical student and itch fellow in the department of dermatology at the Johns Hopkins University School of Medicine, Baltimore, and colleagues drew from TriNetX, a health care network of approximately 73 million de-identified medical records in 53 organizations. The years of study were 2015 to 2021. The researchers limited the analysis to adults with at least two instances of International Classification of Diseases, Tenth Revision (ICD-10) code L28 for AD, to capture a population with true AD. Controls were adults without AD who presented for general checkup and were matched to AD patients by age, race, and sex.
The study population consisted of 120,480 AD patients and matched controls. Their mean age was 36 years, 61% were female, and 26% were Black. Compared with controls, AD patients had an increased risk of developing general sleep disorders over the 6-year period (relative risk, 1.10), as well as obstructive sleep apnea (RR, 1.13), insomnia (RR, 1.10), hypersomnia (RR, 1.24), sleep-related movement disorders (RR, 1.36), restless legs syndrome (RR, 1.25), sleep deprivation (RR, 1.36), and unspecified sleep disorders (RR, 1.22).
To examine the association of sleep disturbance with the inflammatory biomarker CRP, the researchers measured CRP levels between these patient groups. They found a substantially higher CRP in AD patients compared with controls (21.2 mg/L vs. 7.6 mg/L, respectively; P < .0001). This finding “is suggestive of a higher level of inflammation in these patients,” Mr. Parthasarathy said. Interestingly, he added, they also found a higher CRP level in AD patients with sleep disturbances compared to AD patients without sleep disturbances (23.3 vs. 20.6 mg/L; P = .02), “also pointing to a higher inflammatory burden in AD patients whose sleep was affected.”
Compared to matched AD patients without sleep disorders, AD patients with sleep disorders were more likely to develop obesity (RR, 2.65), hyperlipidemia (RR, 2.18), type 2 diabetes (RR, 2.45), metabolic syndrome (RR, 4.16), atherosclerosis (RR, 2.42), peripheral vascular disease (RR, 2.47), stroke (RR, 2.37), venous thromboembolism (RR, 2.93), and mortality (hazard ratio, 1.24).
“There is a consequence of not treating patients with atopic dermatitis, especially those patients with sleep disturbance,” the study’s primary author, Shawn G. Kwatra, MD, associate professor of dermatology at Johns Hopkins, told this news organization. “Chronic inflammation can lead to the development of comorbidities, so it is important to offer patients early treatment to reduce their overall inflammation.” He said that he was most surprised by the degree of increased inflammation in the blood of AD as compared to healthy controls. “This likely plays a part in the development of several comorbidities,” he said.
Mr. Parthasarathy acknowledged certain limitations of the study, including the inability to infer causal relationships, as uncontrolled factors may be present. “Additionally, sampling of only patients that have had medical encounters limits the generalizability of the findings,” she said. “However, findings in this large cohort study suggest that clinicians should seek to identify sleep disorders in AD patients and screen for cardiac comorbidities secondary to inflammation in this patient population.”
“There is increased data to suggest that adults with AD, particularly those with more severe disease, may be at an increased risk of cardiovascular disease and the results from [this study] further support the concept of AD as systemic disease,” said Zelma C. Chiesa Fuxench, MD, MSCE, assistant professor of dermatology at the University of Pennsylvania, Philadelphia, who was asked to comment on the study. She cited the large population-based, retrospective design and use of two instances of ICD codes for AD to confirm diagnosis as key strengths of the research. “However, it is unclear if for each patient CRP levels were measured at one single timepoint,” Dr. Chiesa Fuxench said. “For future studies, it would be interesting to see if these levels fluctuate with time and if persistently elevated levels are associated with worse cardiovascular outcomes in this population. More data is needed to better understand the relationship better atopic dermatitis disease severity, impact on sleep, and how this relates to increased systemic inflammation and worse cardiovascular outcomes in this population.”
Dr. Kwatra disclosed support by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number K23AR077073-01A1 and previous funding by the Dermatology Foundation and Skin of Color Society. Dr. Kwatra is also an advisory board member/consultant for AbbVie, Celldex Therapeutics, Galderma, Incyte Corporation, Johnson & Johnson, Novartis Pharmaceuticals Corporation, Pfizer, Regeneron Pharmaceuticals, Sanofi, and Kiniksa Pharmaceuticals and has served as an investigator for Galderma, Pfizer, and Sanofi. Dr. Chiesa Fuxench disclosed research grants from several pharmaceutical companies for work related to AD. She has also served as a consultant for the Asthma and Allergy Foundation of America, National Eczema Association, AbbVie, Incyte Corporation, and Pfizer.
A version of this article first appeared on Medscape.com.
and mortality, results from a large cohort analysis showed.
“The implications of these findings are vast,” presenting author Varsha Parthasarathy said during a late-breaking abstract session at the Revolutionizing Atopic Dermatitis virtual symposium. “Poor sleep quality is known to be associated with increased inflammatory markers such as IL-6, IL-17, and CRP, so it is interesting to see this reflected in AD patients with versus without sleep disturbance. Additionally, we know that CRP is a driver of inflammation and is strongly associated with cardiovascular complications such as heart attack and stroke. Therefore, CRP may be a useful prognostic marker in AD patients with sleep disturbances.”
To examine the comorbidity burden of sleep disorders in AD patients and associate findings with inflammatory CRP and cardiovascular comorbidities, Mr. Parthasarathy, a medical student and itch fellow in the department of dermatology at the Johns Hopkins University School of Medicine, Baltimore, and colleagues drew from TriNetX, a health care network of approximately 73 million de-identified medical records in 53 organizations. The years of study were 2015 to 2021. The researchers limited the analysis to adults with at least two instances of International Classification of Diseases, Tenth Revision (ICD-10) code L28 for AD, to capture a population with true AD. Controls were adults without AD who presented for general checkup and were matched to AD patients by age, race, and sex.
The study population consisted of 120,480 AD patients and matched controls. Their mean age was 36 years, 61% were female, and 26% were Black. Compared with controls, AD patients had an increased risk of developing general sleep disorders over the 6-year period (relative risk, 1.10), as well as obstructive sleep apnea (RR, 1.13), insomnia (RR, 1.10), hypersomnia (RR, 1.24), sleep-related movement disorders (RR, 1.36), restless legs syndrome (RR, 1.25), sleep deprivation (RR, 1.36), and unspecified sleep disorders (RR, 1.22).
To examine the association of sleep disturbance with the inflammatory biomarker CRP, the researchers measured CRP levels between these patient groups. They found a substantially higher CRP in AD patients compared with controls (21.2 mg/L vs. 7.6 mg/L, respectively; P < .0001). This finding “is suggestive of a higher level of inflammation in these patients,” Mr. Parthasarathy said. Interestingly, he added, they also found a higher CRP level in AD patients with sleep disturbances compared to AD patients without sleep disturbances (23.3 vs. 20.6 mg/L; P = .02), “also pointing to a higher inflammatory burden in AD patients whose sleep was affected.”
Compared to matched AD patients without sleep disorders, AD patients with sleep disorders were more likely to develop obesity (RR, 2.65), hyperlipidemia (RR, 2.18), type 2 diabetes (RR, 2.45), metabolic syndrome (RR, 4.16), atherosclerosis (RR, 2.42), peripheral vascular disease (RR, 2.47), stroke (RR, 2.37), venous thromboembolism (RR, 2.93), and mortality (hazard ratio, 1.24).
“There is a consequence of not treating patients with atopic dermatitis, especially those patients with sleep disturbance,” the study’s primary author, Shawn G. Kwatra, MD, associate professor of dermatology at Johns Hopkins, told this news organization. “Chronic inflammation can lead to the development of comorbidities, so it is important to offer patients early treatment to reduce their overall inflammation.” He said that he was most surprised by the degree of increased inflammation in the blood of AD as compared to healthy controls. “This likely plays a part in the development of several comorbidities,” he said.
Mr. Parthasarathy acknowledged certain limitations of the study, including the inability to infer causal relationships, as uncontrolled factors may be present. “Additionally, sampling of only patients that have had medical encounters limits the generalizability of the findings,” she said. “However, findings in this large cohort study suggest that clinicians should seek to identify sleep disorders in AD patients and screen for cardiac comorbidities secondary to inflammation in this patient population.”
“There is increased data to suggest that adults with AD, particularly those with more severe disease, may be at an increased risk of cardiovascular disease and the results from [this study] further support the concept of AD as systemic disease,” said Zelma C. Chiesa Fuxench, MD, MSCE, assistant professor of dermatology at the University of Pennsylvania, Philadelphia, who was asked to comment on the study. She cited the large population-based, retrospective design and use of two instances of ICD codes for AD to confirm diagnosis as key strengths of the research. “However, it is unclear if for each patient CRP levels were measured at one single timepoint,” Dr. Chiesa Fuxench said. “For future studies, it would be interesting to see if these levels fluctuate with time and if persistently elevated levels are associated with worse cardiovascular outcomes in this population. More data is needed to better understand the relationship better atopic dermatitis disease severity, impact on sleep, and how this relates to increased systemic inflammation and worse cardiovascular outcomes in this population.”
Dr. Kwatra disclosed support by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number K23AR077073-01A1 and previous funding by the Dermatology Foundation and Skin of Color Society. Dr. Kwatra is also an advisory board member/consultant for AbbVie, Celldex Therapeutics, Galderma, Incyte Corporation, Johnson & Johnson, Novartis Pharmaceuticals Corporation, Pfizer, Regeneron Pharmaceuticals, Sanofi, and Kiniksa Pharmaceuticals and has served as an investigator for Galderma, Pfizer, and Sanofi. Dr. Chiesa Fuxench disclosed research grants from several pharmaceutical companies for work related to AD. She has also served as a consultant for the Asthma and Allergy Foundation of America, National Eczema Association, AbbVie, Incyte Corporation, and Pfizer.
A version of this article first appeared on Medscape.com.
Upadacitinib (Rinvoq) gains psoriatic arthritis as second FDA-approved indication
upadacitinib (Rinvoq) for adults with psoriatic arthritis who had an inadequate response or intolerance to one or more anti-tumor necrosis factor drugs, manufacturer AbbVie announced December 14.
The approval is the second indication given by the agency for the selective Janus kinase (JAK) inhibitor upadacitinib, which was previously approved for rheumatoid arthritis (RA) in 2019.
Upadacitinib 15 mg is also approved by the European Commission for adults with RA, psoriatic arthritis, and ankylosing spondylitis. The European Commission also approved the drug for moderate to severe atopic dermatitis at both 15- and 30-mg doses for adults and at 15 mg for adolescents.
The approval is based on two phase 3 trials, SELECT-PsA 1 and SELECT-PsA 2, which together randomized more than 2,300 patients with psoriatic arthritis. In the trials, significantly more patients who took upadacitinib 15 mg met their primary endpoint of 20% improvement in American College of Rheumatology response criteria (ACR20) at week 12 (71% in SELECT-PsA 1 and 57% in SELECT-PsA 2) vs placebo (36% and 24%, respectively). Both trials also included treatment arms for upadacitinib at 30 mg, but the FDA approved only the 15-mg dose.
In the announcement, AbbVie noted that significantly higher percentages of patients treated with upadacitinib 15 mg in the SELECT-PSA 1 and 2 trials, respectively, met ACR50 (38% and 32%) and ACR70 (16% and 9%) criteria than did patients on placebo (13% and 5% for ACR50 and 2% and 1% for ACR70). Symptoms of dactylitis and enthesitis improved with upadacitinib for patients who had them at baseline.
The trials’ 12-week results also indicated that upadacitinib significantly improved physical function relative to placebo at baseline, based on the Health Assessment Questionnaire-Disability Index, as well as fatigue, according to Functional Assessment of Chronic Illness Therapy – Fatigue (FACIT-F) scores. Skin manifestations also improved during the trial, but upadacitinib has not been studied for treating plaque psoriasis.
AbbVie reported that the safety results of upadacitinib in the trials were consistent with the results seen in patients with rheumatoid arthritis, and during the trials’ 24-week placebo-controlled period, the most common adverse events reported with upadacitinib were upper respiratory tract infection and blood creatine phosphokinase elevations.
Upadacitinib comes with a boxed warning that was formally placed on the drug’s label this month after data from a postmarketing trial of the JAK inhibitor tofacitinib (Xeljanz and Xeljanz XR) in patients with RA aged 50 years and older with at least one cardiovascular risk factor showed numerically higher risks for all-cause mortality; lymphoma and other malignancies; major adverse cardiovascular events (cardiovascular death, myocardial infarction, and stroke); and thrombosis, including deep venous thrombosis, pulmonary embolism, and arterial thrombosis.
Upadacitinib also carries a boxed warning for an elevated risk of serious infection leading to hospitalization or death. In the SELECT-PsA 1 and 2 trials overall, rates of herpes zoster and herpes simplex were 1.1% and 1.4% with upadacitinib, compared with 0.8% and 1.3% with placebo.
Phase 3 trials of upadacitinib in RA, atopic dermatitis, psoriatic arthritis, axial spondyloarthritis, Crohn’s disease, ulcerative colitis, giant cell arteritis, and Takayasu arteritis are ongoing, according to AbbVie.
A version of this article first appeared on Medscape.com.
upadacitinib (Rinvoq) for adults with psoriatic arthritis who had an inadequate response or intolerance to one or more anti-tumor necrosis factor drugs, manufacturer AbbVie announced December 14.
The approval is the second indication given by the agency for the selective Janus kinase (JAK) inhibitor upadacitinib, which was previously approved for rheumatoid arthritis (RA) in 2019.
Upadacitinib 15 mg is also approved by the European Commission for adults with RA, psoriatic arthritis, and ankylosing spondylitis. The European Commission also approved the drug for moderate to severe atopic dermatitis at both 15- and 30-mg doses for adults and at 15 mg for adolescents.
The approval is based on two phase 3 trials, SELECT-PsA 1 and SELECT-PsA 2, which together randomized more than 2,300 patients with psoriatic arthritis. In the trials, significantly more patients who took upadacitinib 15 mg met their primary endpoint of 20% improvement in American College of Rheumatology response criteria (ACR20) at week 12 (71% in SELECT-PsA 1 and 57% in SELECT-PsA 2) vs placebo (36% and 24%, respectively). Both trials also included treatment arms for upadacitinib at 30 mg, but the FDA approved only the 15-mg dose.
In the announcement, AbbVie noted that significantly higher percentages of patients treated with upadacitinib 15 mg in the SELECT-PSA 1 and 2 trials, respectively, met ACR50 (38% and 32%) and ACR70 (16% and 9%) criteria than did patients on placebo (13% and 5% for ACR50 and 2% and 1% for ACR70). Symptoms of dactylitis and enthesitis improved with upadacitinib for patients who had them at baseline.
The trials’ 12-week results also indicated that upadacitinib significantly improved physical function relative to placebo at baseline, based on the Health Assessment Questionnaire-Disability Index, as well as fatigue, according to Functional Assessment of Chronic Illness Therapy – Fatigue (FACIT-F) scores. Skin manifestations also improved during the trial, but upadacitinib has not been studied for treating plaque psoriasis.
AbbVie reported that the safety results of upadacitinib in the trials were consistent with the results seen in patients with rheumatoid arthritis, and during the trials’ 24-week placebo-controlled period, the most common adverse events reported with upadacitinib were upper respiratory tract infection and blood creatine phosphokinase elevations.
Upadacitinib comes with a boxed warning that was formally placed on the drug’s label this month after data from a postmarketing trial of the JAK inhibitor tofacitinib (Xeljanz and Xeljanz XR) in patients with RA aged 50 years and older with at least one cardiovascular risk factor showed numerically higher risks for all-cause mortality; lymphoma and other malignancies; major adverse cardiovascular events (cardiovascular death, myocardial infarction, and stroke); and thrombosis, including deep venous thrombosis, pulmonary embolism, and arterial thrombosis.
Upadacitinib also carries a boxed warning for an elevated risk of serious infection leading to hospitalization or death. In the SELECT-PsA 1 and 2 trials overall, rates of herpes zoster and herpes simplex were 1.1% and 1.4% with upadacitinib, compared with 0.8% and 1.3% with placebo.
Phase 3 trials of upadacitinib in RA, atopic dermatitis, psoriatic arthritis, axial spondyloarthritis, Crohn’s disease, ulcerative colitis, giant cell arteritis, and Takayasu arteritis are ongoing, according to AbbVie.
A version of this article first appeared on Medscape.com.
upadacitinib (Rinvoq) for adults with psoriatic arthritis who had an inadequate response or intolerance to one or more anti-tumor necrosis factor drugs, manufacturer AbbVie announced December 14.
The approval is the second indication given by the agency for the selective Janus kinase (JAK) inhibitor upadacitinib, which was previously approved for rheumatoid arthritis (RA) in 2019.
Upadacitinib 15 mg is also approved by the European Commission for adults with RA, psoriatic arthritis, and ankylosing spondylitis. The European Commission also approved the drug for moderate to severe atopic dermatitis at both 15- and 30-mg doses for adults and at 15 mg for adolescents.
The approval is based on two phase 3 trials, SELECT-PsA 1 and SELECT-PsA 2, which together randomized more than 2,300 patients with psoriatic arthritis. In the trials, significantly more patients who took upadacitinib 15 mg met their primary endpoint of 20% improvement in American College of Rheumatology response criteria (ACR20) at week 12 (71% in SELECT-PsA 1 and 57% in SELECT-PsA 2) vs placebo (36% and 24%, respectively). Both trials also included treatment arms for upadacitinib at 30 mg, but the FDA approved only the 15-mg dose.
In the announcement, AbbVie noted that significantly higher percentages of patients treated with upadacitinib 15 mg in the SELECT-PSA 1 and 2 trials, respectively, met ACR50 (38% and 32%) and ACR70 (16% and 9%) criteria than did patients on placebo (13% and 5% for ACR50 and 2% and 1% for ACR70). Symptoms of dactylitis and enthesitis improved with upadacitinib for patients who had them at baseline.
The trials’ 12-week results also indicated that upadacitinib significantly improved physical function relative to placebo at baseline, based on the Health Assessment Questionnaire-Disability Index, as well as fatigue, according to Functional Assessment of Chronic Illness Therapy – Fatigue (FACIT-F) scores. Skin manifestations also improved during the trial, but upadacitinib has not been studied for treating plaque psoriasis.
AbbVie reported that the safety results of upadacitinib in the trials were consistent with the results seen in patients with rheumatoid arthritis, and during the trials’ 24-week placebo-controlled period, the most common adverse events reported with upadacitinib were upper respiratory tract infection and blood creatine phosphokinase elevations.
Upadacitinib comes with a boxed warning that was formally placed on the drug’s label this month after data from a postmarketing trial of the JAK inhibitor tofacitinib (Xeljanz and Xeljanz XR) in patients with RA aged 50 years and older with at least one cardiovascular risk factor showed numerically higher risks for all-cause mortality; lymphoma and other malignancies; major adverse cardiovascular events (cardiovascular death, myocardial infarction, and stroke); and thrombosis, including deep venous thrombosis, pulmonary embolism, and arterial thrombosis.
Upadacitinib also carries a boxed warning for an elevated risk of serious infection leading to hospitalization or death. In the SELECT-PsA 1 and 2 trials overall, rates of herpes zoster and herpes simplex were 1.1% and 1.4% with upadacitinib, compared with 0.8% and 1.3% with placebo.
Phase 3 trials of upadacitinib in RA, atopic dermatitis, psoriatic arthritis, axial spondyloarthritis, Crohn’s disease, ulcerative colitis, giant cell arteritis, and Takayasu arteritis are ongoing, according to AbbVie.
A version of this article first appeared on Medscape.com.
COVID-19 asymptomatic infection rate remains high
Based on data from a meta-analysis of 95 studies that included nearly 30,000,000 individuals, the pooled percentage of asymptomatic COVID-19 infections was 0.25% in the tested population and 40.5% among confirmed cases.
, wrote Qiuyue Ma, PhD, and colleagues of Peking University, Beijing.
In a study published in JAMA Network Open the researchers identified 44 cross-sectional studies, 41 cohort studies, seven case series, and three case series on transmission studies. A total of 74 studies were conducted in developed countries, including those in Europe, North America, and Asia. Approximately one-third (37) of the studies were conducted among health care workers or in-hospital patients, 17 among nursing home staff or residents, and 14 among community residents. In addition, 13 studies involved pregnant women, eight involved air or cruise ship travelers, and six involved close contacts of individuals with confirmed infections.
The meta-analysis included 29,776,306 tested individuals; 11,516 of them had asymptomatic infections.
Overall, the pooled percentage of asymptomatic infections among the tested population was 0.25%. In an analysis of different study populations, the percentage was higher in nursing home residents or staff (4.52%), air or cruise ship travelers (2.02%), and pregnant women (2.34%), compared against the pooled percentage.
The pooled percentage of asymptomatic infections among the confirmed population was 40.50%, and this percentage was higher in pregnant women (54.11%), air or cruise ship travelers (52.91%), and nursing home residents or staff (47.53%).
The pooled percentage in the tested population was higher than the overall percentage when the mean age of the study population was 60 years or older (3.69%). By contrast, in the confirmed population, the pooled percentage was higher than the overall percentage when the study population was younger than 20 years (60.2%) or aged 20 to 39 years (49.5%).
The researchers noted in their discussion that the varying percentage of asymptomatic individuals according to community prevalence might impact the heterogeneity of the included studies. They also noted the high number of studies conducted in nursing home populations, groups in which asymptomatic individuals were more likely to be tested.
The study findings were limited by several factors, including the potential for missed studies that were not published at the time of the meta-analysis, as well as the exclusion of studies written in Chinese, the researchers noted. Other limitations included lack of follow-up on presymptomatic and covert infections, and the focus on specific populations, factors that may limit the degree to which the results can be generalized.
However, the results highlight the need to screen for asymptomatic infections, especially in countries where COVID-19 has been better controlled, the researchers said. Management strategies for asymptomatic infections, when identified, should include isolation and contact tracing similar to strategies used with confirmed cases, they added.
More testing needed to catch cases early
“During the initial phase of [the] COVID-19 pandemic, testing was not widely available in the United States or the rest of the world,” Setu Patolia, MD, of Saint Louis University School of Medicine, Missouri, said in an interview. Much of the world still lacks access to COVID-19 testing, and early in the pandemic only severely symptomatic patients were tested, he said. “With new variants, particularly the Omicron variant, which may have mild or minimally symptomatic disease, asymptomatic carriers play an important role in propagation of the pandemic,” he explained. “It is important to know the asymptomatic carrier rate among the general population for the future control of [the] pandemic,” he added.
Dr. Patolia said he was surprised by the study finding that one in 400 people in the general population could be asymptomatic carriers of COVID-19.
“Also, nursing home patients are more at risk of complications of COVID, and I expected that they would have a higher rate of symptomatic disease as compared to [the] general population,” said Dr. Patolia. He was also surprised by the high rate of asymptomatic infections in travelers.
“Physicians should be more aware about the asymptomatic carrier rate, particularly in travelers and nursing home patients,” he noted. “Travelers carry high risk of transferring infection from one region to another region of the world, and physicians should advise them to get tested despite the absence of symptoms,” Dr. Patolia emphasized. “Similarly, once any nursing home patient has been diagnosed with COVID-19, physicians should be more careful with the rest of the nursing home patients and test them despite the absence of the symptoms,” he added.
Dr. Patolia also recommended that pregnant women wear masks to help prevent disease transmission when visiting a doctor’s office or labor unit.
Looking ahead, there is a need for cheaper at-home testing kits so that all vulnerable populations can be tested fast and frequently, Dr. Patolia said.
The study was supported by the National Natural Science Foundation of China. The researchers had no financial conflicts to disclose. Dr. Patolia has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Based on data from a meta-analysis of 95 studies that included nearly 30,000,000 individuals, the pooled percentage of asymptomatic COVID-19 infections was 0.25% in the tested population and 40.5% among confirmed cases.
, wrote Qiuyue Ma, PhD, and colleagues of Peking University, Beijing.
In a study published in JAMA Network Open the researchers identified 44 cross-sectional studies, 41 cohort studies, seven case series, and three case series on transmission studies. A total of 74 studies were conducted in developed countries, including those in Europe, North America, and Asia. Approximately one-third (37) of the studies were conducted among health care workers or in-hospital patients, 17 among nursing home staff or residents, and 14 among community residents. In addition, 13 studies involved pregnant women, eight involved air or cruise ship travelers, and six involved close contacts of individuals with confirmed infections.
The meta-analysis included 29,776,306 tested individuals; 11,516 of them had asymptomatic infections.
Overall, the pooled percentage of asymptomatic infections among the tested population was 0.25%. In an analysis of different study populations, the percentage was higher in nursing home residents or staff (4.52%), air or cruise ship travelers (2.02%), and pregnant women (2.34%), compared against the pooled percentage.
The pooled percentage of asymptomatic infections among the confirmed population was 40.50%, and this percentage was higher in pregnant women (54.11%), air or cruise ship travelers (52.91%), and nursing home residents or staff (47.53%).
The pooled percentage in the tested population was higher than the overall percentage when the mean age of the study population was 60 years or older (3.69%). By contrast, in the confirmed population, the pooled percentage was higher than the overall percentage when the study population was younger than 20 years (60.2%) or aged 20 to 39 years (49.5%).
The researchers noted in their discussion that the varying percentage of asymptomatic individuals according to community prevalence might impact the heterogeneity of the included studies. They also noted the high number of studies conducted in nursing home populations, groups in which asymptomatic individuals were more likely to be tested.
The study findings were limited by several factors, including the potential for missed studies that were not published at the time of the meta-analysis, as well as the exclusion of studies written in Chinese, the researchers noted. Other limitations included lack of follow-up on presymptomatic and covert infections, and the focus on specific populations, factors that may limit the degree to which the results can be generalized.
However, the results highlight the need to screen for asymptomatic infections, especially in countries where COVID-19 has been better controlled, the researchers said. Management strategies for asymptomatic infections, when identified, should include isolation and contact tracing similar to strategies used with confirmed cases, they added.
More testing needed to catch cases early
“During the initial phase of [the] COVID-19 pandemic, testing was not widely available in the United States or the rest of the world,” Setu Patolia, MD, of Saint Louis University School of Medicine, Missouri, said in an interview. Much of the world still lacks access to COVID-19 testing, and early in the pandemic only severely symptomatic patients were tested, he said. “With new variants, particularly the Omicron variant, which may have mild or minimally symptomatic disease, asymptomatic carriers play an important role in propagation of the pandemic,” he explained. “It is important to know the asymptomatic carrier rate among the general population for the future control of [the] pandemic,” he added.
Dr. Patolia said he was surprised by the study finding that one in 400 people in the general population could be asymptomatic carriers of COVID-19.
“Also, nursing home patients are more at risk of complications of COVID, and I expected that they would have a higher rate of symptomatic disease as compared to [the] general population,” said Dr. Patolia. He was also surprised by the high rate of asymptomatic infections in travelers.
“Physicians should be more aware about the asymptomatic carrier rate, particularly in travelers and nursing home patients,” he noted. “Travelers carry high risk of transferring infection from one region to another region of the world, and physicians should advise them to get tested despite the absence of symptoms,” Dr. Patolia emphasized. “Similarly, once any nursing home patient has been diagnosed with COVID-19, physicians should be more careful with the rest of the nursing home patients and test them despite the absence of the symptoms,” he added.
Dr. Patolia also recommended that pregnant women wear masks to help prevent disease transmission when visiting a doctor’s office or labor unit.
Looking ahead, there is a need for cheaper at-home testing kits so that all vulnerable populations can be tested fast and frequently, Dr. Patolia said.
The study was supported by the National Natural Science Foundation of China. The researchers had no financial conflicts to disclose. Dr. Patolia has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Based on data from a meta-analysis of 95 studies that included nearly 30,000,000 individuals, the pooled percentage of asymptomatic COVID-19 infections was 0.25% in the tested population and 40.5% among confirmed cases.
, wrote Qiuyue Ma, PhD, and colleagues of Peking University, Beijing.
In a study published in JAMA Network Open the researchers identified 44 cross-sectional studies, 41 cohort studies, seven case series, and three case series on transmission studies. A total of 74 studies were conducted in developed countries, including those in Europe, North America, and Asia. Approximately one-third (37) of the studies were conducted among health care workers or in-hospital patients, 17 among nursing home staff or residents, and 14 among community residents. In addition, 13 studies involved pregnant women, eight involved air or cruise ship travelers, and six involved close contacts of individuals with confirmed infections.
The meta-analysis included 29,776,306 tested individuals; 11,516 of them had asymptomatic infections.
Overall, the pooled percentage of asymptomatic infections among the tested population was 0.25%. In an analysis of different study populations, the percentage was higher in nursing home residents or staff (4.52%), air or cruise ship travelers (2.02%), and pregnant women (2.34%), compared against the pooled percentage.
The pooled percentage of asymptomatic infections among the confirmed population was 40.50%, and this percentage was higher in pregnant women (54.11%), air or cruise ship travelers (52.91%), and nursing home residents or staff (47.53%).
The pooled percentage in the tested population was higher than the overall percentage when the mean age of the study population was 60 years or older (3.69%). By contrast, in the confirmed population, the pooled percentage was higher than the overall percentage when the study population was younger than 20 years (60.2%) or aged 20 to 39 years (49.5%).
The researchers noted in their discussion that the varying percentage of asymptomatic individuals according to community prevalence might impact the heterogeneity of the included studies. They also noted the high number of studies conducted in nursing home populations, groups in which asymptomatic individuals were more likely to be tested.
The study findings were limited by several factors, including the potential for missed studies that were not published at the time of the meta-analysis, as well as the exclusion of studies written in Chinese, the researchers noted. Other limitations included lack of follow-up on presymptomatic and covert infections, and the focus on specific populations, factors that may limit the degree to which the results can be generalized.
However, the results highlight the need to screen for asymptomatic infections, especially in countries where COVID-19 has been better controlled, the researchers said. Management strategies for asymptomatic infections, when identified, should include isolation and contact tracing similar to strategies used with confirmed cases, they added.
More testing needed to catch cases early
“During the initial phase of [the] COVID-19 pandemic, testing was not widely available in the United States or the rest of the world,” Setu Patolia, MD, of Saint Louis University School of Medicine, Missouri, said in an interview. Much of the world still lacks access to COVID-19 testing, and early in the pandemic only severely symptomatic patients were tested, he said. “With new variants, particularly the Omicron variant, which may have mild or minimally symptomatic disease, asymptomatic carriers play an important role in propagation of the pandemic,” he explained. “It is important to know the asymptomatic carrier rate among the general population for the future control of [the] pandemic,” he added.
Dr. Patolia said he was surprised by the study finding that one in 400 people in the general population could be asymptomatic carriers of COVID-19.
“Also, nursing home patients are more at risk of complications of COVID, and I expected that they would have a higher rate of symptomatic disease as compared to [the] general population,” said Dr. Patolia. He was also surprised by the high rate of asymptomatic infections in travelers.
“Physicians should be more aware about the asymptomatic carrier rate, particularly in travelers and nursing home patients,” he noted. “Travelers carry high risk of transferring infection from one region to another region of the world, and physicians should advise them to get tested despite the absence of symptoms,” Dr. Patolia emphasized. “Similarly, once any nursing home patient has been diagnosed with COVID-19, physicians should be more careful with the rest of the nursing home patients and test them despite the absence of the symptoms,” he added.
Dr. Patolia also recommended that pregnant women wear masks to help prevent disease transmission when visiting a doctor’s office or labor unit.
Looking ahead, there is a need for cheaper at-home testing kits so that all vulnerable populations can be tested fast and frequently, Dr. Patolia said.
The study was supported by the National Natural Science Foundation of China. The researchers had no financial conflicts to disclose. Dr. Patolia has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Is it OK to just be satisfied?
It is possible to talk to a patient for a brief moment and just know if he or she is a satisficer or a maximizer. A “satisficer” when presented with treatment options will invariably say: “I’ll do whatever you say, Doctor.” A “maximizer,” in contrast, would like a printed copy of treatment choices, then would seek a second opinion before ultimately buying an UpToDate subscription to research treatments for him or herself.
.
This notion that we have tendencies toward maximizing or satisficing is thanks to Nobel Memorial Prize winner and all-around smart guy, Herbert A. Simon, PhD. Dr. Simon recognized that, although each person might be expected to make optimal decisions to benefit himself or herself, this is practically impossible. To do so would require an infinite amount of time and energy. He found therefore that we actually exhibit “bounded rationality;” that is, we make the best decision given the limits of time, the price of acquiring information, and even our cognitive abilities. The amount of effort we give to make a decision also depends on the situation: You might be very invested in choosing the right spouse, but not at all invested in choosing soup or salad. (Although, we all have friends who are: “Um, is there any thyme in the soup?”)
You’ll certainly recognize that people have different set points on the spectrum between being a satisficer, one who will take the first option that meets a standard, and a maximizer, one who will seek and accept only the best, even if choosing is at great cost. There are risks and benefits of each. In getting the best job, maximizers might be more successful, but satisficers seem to be happier.
How much this extends into other spheres of life is unclear. It is clear, though, that the work of choosing can come at a cost.
The psychologist Barry Schwartz, PhD, believes that, in general, having more choices leads to more anxiety, not more contentment. For example, which Christmas tree lot would you rather visit: One with hundreds of trees of half a dozen varieties? Or one with just a few trees each of Balsam and Douglas Firs? Dr. Schwartz would argue that you might waste an entire afternoon in the first lot only to bring it home and have remorse when you realize it’s a little lopsided. Or let’s say your child applied to all the Ivy League and Public Ivy schools and also threw in all the top liberal arts colleges. The anxiety of selecting the best and the terror that the “best one” might not choose him or her could be overwhelming. A key lesson is that more in life is by chance than we realize, including how straight your tree is and who gets into Princeton this year. Yet, our expectation that things will work out perfectly if only we maximize is ubiquitous. That confidence in our ability to choose correctly is, however, unwarranted. Better to do your best and know that your tree will be festive and there are many colleges which would lead to a happy life than to fret in choosing and then suffer from dashed expectations. Sometimes good enough is good enough.
Being a satisficer or maximizer is probably somewhat fixed, a personality trait, like being extroverted or conscientious. Yet, having insight can be helpful. If choosing a restaurant in Manhattan becomes an actual project for you with spreadsheets and your own statistical analysis, then go for it! Just know that if that process causes you angst and apprehension, then there is another way. Go to Eleven Madison Park, just because I say so. You might have the best dinner of your life or maybe not. At least by not choosing you’ll have the gift of time to spend picking out a tree instead.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
It is possible to talk to a patient for a brief moment and just know if he or she is a satisficer or a maximizer. A “satisficer” when presented with treatment options will invariably say: “I’ll do whatever you say, Doctor.” A “maximizer,” in contrast, would like a printed copy of treatment choices, then would seek a second opinion before ultimately buying an UpToDate subscription to research treatments for him or herself.
.
This notion that we have tendencies toward maximizing or satisficing is thanks to Nobel Memorial Prize winner and all-around smart guy, Herbert A. Simon, PhD. Dr. Simon recognized that, although each person might be expected to make optimal decisions to benefit himself or herself, this is practically impossible. To do so would require an infinite amount of time and energy. He found therefore that we actually exhibit “bounded rationality;” that is, we make the best decision given the limits of time, the price of acquiring information, and even our cognitive abilities. The amount of effort we give to make a decision also depends on the situation: You might be very invested in choosing the right spouse, but not at all invested in choosing soup or salad. (Although, we all have friends who are: “Um, is there any thyme in the soup?”)
You’ll certainly recognize that people have different set points on the spectrum between being a satisficer, one who will take the first option that meets a standard, and a maximizer, one who will seek and accept only the best, even if choosing is at great cost. There are risks and benefits of each. In getting the best job, maximizers might be more successful, but satisficers seem to be happier.
How much this extends into other spheres of life is unclear. It is clear, though, that the work of choosing can come at a cost.
The psychologist Barry Schwartz, PhD, believes that, in general, having more choices leads to more anxiety, not more contentment. For example, which Christmas tree lot would you rather visit: One with hundreds of trees of half a dozen varieties? Or one with just a few trees each of Balsam and Douglas Firs? Dr. Schwartz would argue that you might waste an entire afternoon in the first lot only to bring it home and have remorse when you realize it’s a little lopsided. Or let’s say your child applied to all the Ivy League and Public Ivy schools and also threw in all the top liberal arts colleges. The anxiety of selecting the best and the terror that the “best one” might not choose him or her could be overwhelming. A key lesson is that more in life is by chance than we realize, including how straight your tree is and who gets into Princeton this year. Yet, our expectation that things will work out perfectly if only we maximize is ubiquitous. That confidence in our ability to choose correctly is, however, unwarranted. Better to do your best and know that your tree will be festive and there are many colleges which would lead to a happy life than to fret in choosing and then suffer from dashed expectations. Sometimes good enough is good enough.
Being a satisficer or maximizer is probably somewhat fixed, a personality trait, like being extroverted or conscientious. Yet, having insight can be helpful. If choosing a restaurant in Manhattan becomes an actual project for you with spreadsheets and your own statistical analysis, then go for it! Just know that if that process causes you angst and apprehension, then there is another way. Go to Eleven Madison Park, just because I say so. You might have the best dinner of your life or maybe not. At least by not choosing you’ll have the gift of time to spend picking out a tree instead.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
It is possible to talk to a patient for a brief moment and just know if he or she is a satisficer or a maximizer. A “satisficer” when presented with treatment options will invariably say: “I’ll do whatever you say, Doctor.” A “maximizer,” in contrast, would like a printed copy of treatment choices, then would seek a second opinion before ultimately buying an UpToDate subscription to research treatments for him or herself.
.
This notion that we have tendencies toward maximizing or satisficing is thanks to Nobel Memorial Prize winner and all-around smart guy, Herbert A. Simon, PhD. Dr. Simon recognized that, although each person might be expected to make optimal decisions to benefit himself or herself, this is practically impossible. To do so would require an infinite amount of time and energy. He found therefore that we actually exhibit “bounded rationality;” that is, we make the best decision given the limits of time, the price of acquiring information, and even our cognitive abilities. The amount of effort we give to make a decision also depends on the situation: You might be very invested in choosing the right spouse, but not at all invested in choosing soup or salad. (Although, we all have friends who are: “Um, is there any thyme in the soup?”)
You’ll certainly recognize that people have different set points on the spectrum between being a satisficer, one who will take the first option that meets a standard, and a maximizer, one who will seek and accept only the best, even if choosing is at great cost. There are risks and benefits of each. In getting the best job, maximizers might be more successful, but satisficers seem to be happier.
How much this extends into other spheres of life is unclear. It is clear, though, that the work of choosing can come at a cost.
The psychologist Barry Schwartz, PhD, believes that, in general, having more choices leads to more anxiety, not more contentment. For example, which Christmas tree lot would you rather visit: One with hundreds of trees of half a dozen varieties? Or one with just a few trees each of Balsam and Douglas Firs? Dr. Schwartz would argue that you might waste an entire afternoon in the first lot only to bring it home and have remorse when you realize it’s a little lopsided. Or let’s say your child applied to all the Ivy League and Public Ivy schools and also threw in all the top liberal arts colleges. The anxiety of selecting the best and the terror that the “best one” might not choose him or her could be overwhelming. A key lesson is that more in life is by chance than we realize, including how straight your tree is and who gets into Princeton this year. Yet, our expectation that things will work out perfectly if only we maximize is ubiquitous. That confidence in our ability to choose correctly is, however, unwarranted. Better to do your best and know that your tree will be festive and there are many colleges which would lead to a happy life than to fret in choosing and then suffer from dashed expectations. Sometimes good enough is good enough.
Being a satisficer or maximizer is probably somewhat fixed, a personality trait, like being extroverted or conscientious. Yet, having insight can be helpful. If choosing a restaurant in Manhattan becomes an actual project for you with spreadsheets and your own statistical analysis, then go for it! Just know that if that process causes you angst and apprehension, then there is another way. Go to Eleven Madison Park, just because I say so. You might have the best dinner of your life or maybe not. At least by not choosing you’ll have the gift of time to spend picking out a tree instead.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
Physician gender pay gap isn’t news; health inequity is rampant
A recent study examined projected career earnings between the genders in a largely community-based physician population, finding a difference of about $2 million in career earnings. That a gender pay gap exists in medicine is not news – but the manner in which this study was done, the investigators’ ability to control for a number of confounding variables, and the size of the study group (over 80,000) are newsworthy.
Some of the key findings include that gender pay gaps start with your first job, and you never close the gap, even as you gain experience and efficiency. Also, the more highly remunerated your specialty, the larger the gap. The gender pay gap joins a growing list of inequities within health care. Although physician compensation is not the most important, given that nearly all physicians are well-paid, and we have much more significant inequities that lead to direct patient harm, the reasons for this discrepancy warrant further consideration.
When I was first being educated about social inequity as part of work in social determinants of health, I made the error of using “inequality” and “inequity” interchangeably. The subtle yet important difference between the two terms was quickly described to me. Inequality is a gastroenterologist getting paid more money to do a colonoscopy than a family physician. Inequity is a female gastroenterologist getting paid less than a male gastroenterologist. Global Health Europe boldly identifies that “inequity is the result of failure.” In looking at the inequity inherent in the gender pay gap, I consider what failed and why.
I’m currently making a major career change, leaving an executive leadership position to return to full-time clinical practice. There is a significant pay decrease that will accompany this change because I am in a primary care specialty. Beyond that, I am considering two employment contracts from different systems to do a similar clinical role.
One of the questions my husband asked was which will pay more over the long run. This is difficult to discern because the compensation formula each health system uses is different, even though they are based on standard national benchmarking data. It is possible that women, in general, are like I am and look for factors other than compensation to make a job decision – assuming, like I do, that it will be close enough to not matter or is generally fair. In fact, while compensation is most certainly a consideration for me, once I determined that it was likely to be in the same ballpark, I stopped comparing. Even as the sole breadwinner in our family, I take this (probably faulty) approach.
It’s time to reconsider how we pay physicians
Women may be more likely to gloss over compensation details that men evaluate and negotiate carefully. To change this, women must first take responsibility for being an active, informed, and engaged part of compensation negotiations. In addition, employers who value gender pay equity must negotiate in good faith, keeping in mind the well-described vulnerabilities in discussions about pay. Finally, male and female mentors and leaders should actively coach female physicians on how to approach these conversations with confidence and skill.
In primary care, female physicians spend, on average, about 15% more time with their patients during a visit. Despite spending as much time in clinic seeing patients per week, they see fewer patients, thereby generating less revenue. For compensation plans that are based on productivity, the extra time spent costs money. In this case, it costs the female physicians lost compensation.
The way in which women are more likely to practice medicine, which includes the amount of time they spend with patients, may affect clinical outcomes without directly increasing productivity. A 2017 study demonstrated that elderly patients had lower rates of mortality and readmission when cared for by a female rather than a male physician. These findings require health systems to critically evaluate what compensation plans value and to promote an appropriate balance between quality of care, quantity of care, and style of care.
Although I’ve seen gender pay inequity as blatant as two different salaries for physicians doing the same work – one male and one female – I think this is uncommon. Like many forms of inequity, the outputs are often related to a failed system rather than solely a series of individual failures. Making compensation formulas gender-blind is an important step – but it is only the first step, not the last. Recognizing that the structure of a compensation formula may be biased toward a style of medical practice more likely to be espoused by one gender is necessary as well.
The data, including the findings of this recent study, clearly identify the gender pay gap that exists in medicine, as it does in many other fields, and that it is not explainable solely by differences in specialties, work hours, family status, or title.
To address the inequity, it is imperative that women engage with employers and leaders to both understand and develop skills around effective and appropriate compensation negotiation. Recognizing that compensation plans, especially those built on productivity models, may fail to place adequate value on gender-specific practice styles.
Jennifer Frank is a family physician, physician leader, wife, and mother in Northeast Wisconsin.
A version of this article first appeared on Medscape.com.
A recent study examined projected career earnings between the genders in a largely community-based physician population, finding a difference of about $2 million in career earnings. That a gender pay gap exists in medicine is not news – but the manner in which this study was done, the investigators’ ability to control for a number of confounding variables, and the size of the study group (over 80,000) are newsworthy.
Some of the key findings include that gender pay gaps start with your first job, and you never close the gap, even as you gain experience and efficiency. Also, the more highly remunerated your specialty, the larger the gap. The gender pay gap joins a growing list of inequities within health care. Although physician compensation is not the most important, given that nearly all physicians are well-paid, and we have much more significant inequities that lead to direct patient harm, the reasons for this discrepancy warrant further consideration.
When I was first being educated about social inequity as part of work in social determinants of health, I made the error of using “inequality” and “inequity” interchangeably. The subtle yet important difference between the two terms was quickly described to me. Inequality is a gastroenterologist getting paid more money to do a colonoscopy than a family physician. Inequity is a female gastroenterologist getting paid less than a male gastroenterologist. Global Health Europe boldly identifies that “inequity is the result of failure.” In looking at the inequity inherent in the gender pay gap, I consider what failed and why.
I’m currently making a major career change, leaving an executive leadership position to return to full-time clinical practice. There is a significant pay decrease that will accompany this change because I am in a primary care specialty. Beyond that, I am considering two employment contracts from different systems to do a similar clinical role.
One of the questions my husband asked was which will pay more over the long run. This is difficult to discern because the compensation formula each health system uses is different, even though they are based on standard national benchmarking data. It is possible that women, in general, are like I am and look for factors other than compensation to make a job decision – assuming, like I do, that it will be close enough to not matter or is generally fair. In fact, while compensation is most certainly a consideration for me, once I determined that it was likely to be in the same ballpark, I stopped comparing. Even as the sole breadwinner in our family, I take this (probably faulty) approach.
It’s time to reconsider how we pay physicians
Women may be more likely to gloss over compensation details that men evaluate and negotiate carefully. To change this, women must first take responsibility for being an active, informed, and engaged part of compensation negotiations. In addition, employers who value gender pay equity must negotiate in good faith, keeping in mind the well-described vulnerabilities in discussions about pay. Finally, male and female mentors and leaders should actively coach female physicians on how to approach these conversations with confidence and skill.
In primary care, female physicians spend, on average, about 15% more time with their patients during a visit. Despite spending as much time in clinic seeing patients per week, they see fewer patients, thereby generating less revenue. For compensation plans that are based on productivity, the extra time spent costs money. In this case, it costs the female physicians lost compensation.
The way in which women are more likely to practice medicine, which includes the amount of time they spend with patients, may affect clinical outcomes without directly increasing productivity. A 2017 study demonstrated that elderly patients had lower rates of mortality and readmission when cared for by a female rather than a male physician. These findings require health systems to critically evaluate what compensation plans value and to promote an appropriate balance between quality of care, quantity of care, and style of care.
Although I’ve seen gender pay inequity as blatant as two different salaries for physicians doing the same work – one male and one female – I think this is uncommon. Like many forms of inequity, the outputs are often related to a failed system rather than solely a series of individual failures. Making compensation formulas gender-blind is an important step – but it is only the first step, not the last. Recognizing that the structure of a compensation formula may be biased toward a style of medical practice more likely to be espoused by one gender is necessary as well.
The data, including the findings of this recent study, clearly identify the gender pay gap that exists in medicine, as it does in many other fields, and that it is not explainable solely by differences in specialties, work hours, family status, or title.
To address the inequity, it is imperative that women engage with employers and leaders to both understand and develop skills around effective and appropriate compensation negotiation. Recognizing that compensation plans, especially those built on productivity models, may fail to place adequate value on gender-specific practice styles.
Jennifer Frank is a family physician, physician leader, wife, and mother in Northeast Wisconsin.
A version of this article first appeared on Medscape.com.
A recent study examined projected career earnings between the genders in a largely community-based physician population, finding a difference of about $2 million in career earnings. That a gender pay gap exists in medicine is not news – but the manner in which this study was done, the investigators’ ability to control for a number of confounding variables, and the size of the study group (over 80,000) are newsworthy.
Some of the key findings include that gender pay gaps start with your first job, and you never close the gap, even as you gain experience and efficiency. Also, the more highly remunerated your specialty, the larger the gap. The gender pay gap joins a growing list of inequities within health care. Although physician compensation is not the most important, given that nearly all physicians are well-paid, and we have much more significant inequities that lead to direct patient harm, the reasons for this discrepancy warrant further consideration.
When I was first being educated about social inequity as part of work in social determinants of health, I made the error of using “inequality” and “inequity” interchangeably. The subtle yet important difference between the two terms was quickly described to me. Inequality is a gastroenterologist getting paid more money to do a colonoscopy than a family physician. Inequity is a female gastroenterologist getting paid less than a male gastroenterologist. Global Health Europe boldly identifies that “inequity is the result of failure.” In looking at the inequity inherent in the gender pay gap, I consider what failed and why.
I’m currently making a major career change, leaving an executive leadership position to return to full-time clinical practice. There is a significant pay decrease that will accompany this change because I am in a primary care specialty. Beyond that, I am considering two employment contracts from different systems to do a similar clinical role.
One of the questions my husband asked was which will pay more over the long run. This is difficult to discern because the compensation formula each health system uses is different, even though they are based on standard national benchmarking data. It is possible that women, in general, are like I am and look for factors other than compensation to make a job decision – assuming, like I do, that it will be close enough to not matter or is generally fair. In fact, while compensation is most certainly a consideration for me, once I determined that it was likely to be in the same ballpark, I stopped comparing. Even as the sole breadwinner in our family, I take this (probably faulty) approach.
It’s time to reconsider how we pay physicians
Women may be more likely to gloss over compensation details that men evaluate and negotiate carefully. To change this, women must first take responsibility for being an active, informed, and engaged part of compensation negotiations. In addition, employers who value gender pay equity must negotiate in good faith, keeping in mind the well-described vulnerabilities in discussions about pay. Finally, male and female mentors and leaders should actively coach female physicians on how to approach these conversations with confidence and skill.
In primary care, female physicians spend, on average, about 15% more time with their patients during a visit. Despite spending as much time in clinic seeing patients per week, they see fewer patients, thereby generating less revenue. For compensation plans that are based on productivity, the extra time spent costs money. In this case, it costs the female physicians lost compensation.
The way in which women are more likely to practice medicine, which includes the amount of time they spend with patients, may affect clinical outcomes without directly increasing productivity. A 2017 study demonstrated that elderly patients had lower rates of mortality and readmission when cared for by a female rather than a male physician. These findings require health systems to critically evaluate what compensation plans value and to promote an appropriate balance between quality of care, quantity of care, and style of care.
Although I’ve seen gender pay inequity as blatant as two different salaries for physicians doing the same work – one male and one female – I think this is uncommon. Like many forms of inequity, the outputs are often related to a failed system rather than solely a series of individual failures. Making compensation formulas gender-blind is an important step – but it is only the first step, not the last. Recognizing that the structure of a compensation formula may be biased toward a style of medical practice more likely to be espoused by one gender is necessary as well.
The data, including the findings of this recent study, clearly identify the gender pay gap that exists in medicine, as it does in many other fields, and that it is not explainable solely by differences in specialties, work hours, family status, or title.
To address the inequity, it is imperative that women engage with employers and leaders to both understand and develop skills around effective and appropriate compensation negotiation. Recognizing that compensation plans, especially those built on productivity models, may fail to place adequate value on gender-specific practice styles.
Jennifer Frank is a family physician, physician leader, wife, and mother in Northeast Wisconsin.
A version of this article first appeared on Medscape.com.
Acid series: Azelaic acid
However, it has many positive qualities, including being gentle enough to use daily and is safe to use in pregnancy. It is antibacterial, comedolytic, keratolytic, and has antioxidant activity. Unfortunately, in the last decade the formulations of azelaic acid have not been changed considerably. The 20% cream, 15% gel, and 15% foam vehicles are often too irritating and drying to be used in the population it is intended for: those with rosacea, or with inflamed or sensitive skin.
Azelaic acid is a dicarboxylic acid produced by Pityrosporum ovale. It inhibits the synthesis of cellular proteins and is bactericidal against Propionibacterium acnes and Staphylococcus epidermidis. Azelaic acid is both keratolytic and comedolytic by decreasing keratohyalin granules and reducing filaggrin in the epidermis. It not only scavenges free oxygen radicals, thereby reducing inflammation, but is also a tyrosinase inhibitor – making it a safe, non–hydroquinone-based alternative to skin lightening.
Azelaic acid has little toxicity, it is ingested regularly as it is found in wheat, barley, and rye. Topical side effects are usually mild and can subside with increased use. The most common side effects include erythema, local stinging, pruritus, scaling, and a burning sensation. It is considered safe in pregnancy and a great alternative to medications for acne in pregnant or nursing patients.
The largest constraint with azelaic acid preparations on the market – and most likely the reason it has not been more widely used for acne, rosacea, antiaging, and hyperpigmentation – is the formulation. The foam and gel preparations are irritating and difficult to use on dry or sensitive skin. The 20% cream preparations are slightly better tolerated; however, in vitro skin-penetration studies have shown that cutaneous penetration of azelaic acid is greater after application of a 15% gel (aqueous-based vehicle) and 15% foam (hydrophilic oil-in-water emulsion) as compared with the 20% cream formulations.
In my clinical experience, azelaic acid can only be used in rosacea patients with oily or nonsensitive skin. The majority of my rosacea patients cannot tolerate the burning sensation, albeit transient and mild. Acne patients who do not have dry skin and pregnant patients with mild acne are a great population for integrating azelaic acid into an acne regimen. I also use azelaic acid as an alternative for mild melasma and lentigines in patients who are tapering off hydroquinone or cannot use hydroquinone. In the future, we need better, creamier, nonirritating formulations to be developed and more studies of higher concentrations of this acid for both prescription/patient at-home use, as well as more elegant in-office localized peel systems using azelaic acid.
Dr. Talakoub and Dr. Wesley are cocontributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. Write to them at [email protected]. They had no relevant disclosures.
References
Fitton A and Goa KL. Drugs. 1991 May;41(5):780-98.
Del Rosso JQ. J Clin Aesthet Dermatol. 2017 Mar;10(3):37-40.
Breathnach AC et al. Clin Dermatol. Apr-Jun 1989;7(2):106-19.
However, it has many positive qualities, including being gentle enough to use daily and is safe to use in pregnancy. It is antibacterial, comedolytic, keratolytic, and has antioxidant activity. Unfortunately, in the last decade the formulations of azelaic acid have not been changed considerably. The 20% cream, 15% gel, and 15% foam vehicles are often too irritating and drying to be used in the population it is intended for: those with rosacea, or with inflamed or sensitive skin.
Azelaic acid is a dicarboxylic acid produced by Pityrosporum ovale. It inhibits the synthesis of cellular proteins and is bactericidal against Propionibacterium acnes and Staphylococcus epidermidis. Azelaic acid is both keratolytic and comedolytic by decreasing keratohyalin granules and reducing filaggrin in the epidermis. It not only scavenges free oxygen radicals, thereby reducing inflammation, but is also a tyrosinase inhibitor – making it a safe, non–hydroquinone-based alternative to skin lightening.
Azelaic acid has little toxicity, it is ingested regularly as it is found in wheat, barley, and rye. Topical side effects are usually mild and can subside with increased use. The most common side effects include erythema, local stinging, pruritus, scaling, and a burning sensation. It is considered safe in pregnancy and a great alternative to medications for acne in pregnant or nursing patients.
The largest constraint with azelaic acid preparations on the market – and most likely the reason it has not been more widely used for acne, rosacea, antiaging, and hyperpigmentation – is the formulation. The foam and gel preparations are irritating and difficult to use on dry or sensitive skin. The 20% cream preparations are slightly better tolerated; however, in vitro skin-penetration studies have shown that cutaneous penetration of azelaic acid is greater after application of a 15% gel (aqueous-based vehicle) and 15% foam (hydrophilic oil-in-water emulsion) as compared with the 20% cream formulations.
In my clinical experience, azelaic acid can only be used in rosacea patients with oily or nonsensitive skin. The majority of my rosacea patients cannot tolerate the burning sensation, albeit transient and mild. Acne patients who do not have dry skin and pregnant patients with mild acne are a great population for integrating azelaic acid into an acne regimen. I also use azelaic acid as an alternative for mild melasma and lentigines in patients who are tapering off hydroquinone or cannot use hydroquinone. In the future, we need better, creamier, nonirritating formulations to be developed and more studies of higher concentrations of this acid for both prescription/patient at-home use, as well as more elegant in-office localized peel systems using azelaic acid.
Dr. Talakoub and Dr. Wesley are cocontributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. Write to them at [email protected]. They had no relevant disclosures.
References
Fitton A and Goa KL. Drugs. 1991 May;41(5):780-98.
Del Rosso JQ. J Clin Aesthet Dermatol. 2017 Mar;10(3):37-40.
Breathnach AC et al. Clin Dermatol. Apr-Jun 1989;7(2):106-19.
However, it has many positive qualities, including being gentle enough to use daily and is safe to use in pregnancy. It is antibacterial, comedolytic, keratolytic, and has antioxidant activity. Unfortunately, in the last decade the formulations of azelaic acid have not been changed considerably. The 20% cream, 15% gel, and 15% foam vehicles are often too irritating and drying to be used in the population it is intended for: those with rosacea, or with inflamed or sensitive skin.
Azelaic acid is a dicarboxylic acid produced by Pityrosporum ovale. It inhibits the synthesis of cellular proteins and is bactericidal against Propionibacterium acnes and Staphylococcus epidermidis. Azelaic acid is both keratolytic and comedolytic by decreasing keratohyalin granules and reducing filaggrin in the epidermis. It not only scavenges free oxygen radicals, thereby reducing inflammation, but is also a tyrosinase inhibitor – making it a safe, non–hydroquinone-based alternative to skin lightening.
Azelaic acid has little toxicity, it is ingested regularly as it is found in wheat, barley, and rye. Topical side effects are usually mild and can subside with increased use. The most common side effects include erythema, local stinging, pruritus, scaling, and a burning sensation. It is considered safe in pregnancy and a great alternative to medications for acne in pregnant or nursing patients.
The largest constraint with azelaic acid preparations on the market – and most likely the reason it has not been more widely used for acne, rosacea, antiaging, and hyperpigmentation – is the formulation. The foam and gel preparations are irritating and difficult to use on dry or sensitive skin. The 20% cream preparations are slightly better tolerated; however, in vitro skin-penetration studies have shown that cutaneous penetration of azelaic acid is greater after application of a 15% gel (aqueous-based vehicle) and 15% foam (hydrophilic oil-in-water emulsion) as compared with the 20% cream formulations.
In my clinical experience, azelaic acid can only be used in rosacea patients with oily or nonsensitive skin. The majority of my rosacea patients cannot tolerate the burning sensation, albeit transient and mild. Acne patients who do not have dry skin and pregnant patients with mild acne are a great population for integrating azelaic acid into an acne regimen. I also use azelaic acid as an alternative for mild melasma and lentigines in patients who are tapering off hydroquinone or cannot use hydroquinone. In the future, we need better, creamier, nonirritating formulations to be developed and more studies of higher concentrations of this acid for both prescription/patient at-home use, as well as more elegant in-office localized peel systems using azelaic acid.
Dr. Talakoub and Dr. Wesley are cocontributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. Write to them at [email protected]. They had no relevant disclosures.
References
Fitton A and Goa KL. Drugs. 1991 May;41(5):780-98.
Del Rosso JQ. J Clin Aesthet Dermatol. 2017 Mar;10(3):37-40.
Breathnach AC et al. Clin Dermatol. Apr-Jun 1989;7(2):106-19.
Moisturizers and skin barrier repair
There are dozens of skin care products that claim to repair the barrier that do not have the science or ingredient content to back them up.
Does a skin barrier repair moisturizer really repair?
First, let’s briefly review what the skin barrier is. The stratum corneum (SC), the most superficial layer of the epidermis, averages approximately 15-cell layers in thickness.1,2 The keratinocytes reside there in a pattern resembling a brick wall. The “mortar” is composed of the lipid contents extruded from the lamellar granules. This protective barrier functions to prevent transepidermal water loss (TEWL) and entry of allergens, irritants, and pathogens into deeper layers of the skin. This column will focus briefly on the structure and function of the skin barrier and the barrier repair technologies that use synthetic lipids such as myristoyl-palmitoyl and myristyl/palmityl-oxo-stearamide/arachamide MEA.
Structure of the skin barrier
SC keratinocytes are surrounded by lamella made from lipid bilayers. The lipids have hydrophilic heads and hydrophobic tails; the bilayer arises when the hydrophobic tails face the center and the hydrophilic heads face out of the bilayer. This formation yields a disc-shaped hydrophobic lamellar center. There are actually several of these lamellar layers between keratinocytes.
The naturally occurring primary lipids of the bilayer lamellae are made up of an equal ratio of ceramides, cholesterol, and free fatty acid. Arranged in a 1:1:1 ratio, they fit together like pieces of a puzzle to achieve skin barrier homeostasis. The shape and size of these puzzle pieces is critical. An incorrect shape results in a hole in the skin barrier resulting in dehydration, inflammation, and sensitivity.
Ceramides
Ceramides are a complex family of lipids (sphingolipids – a sphingoid base and a fatty acid) involved in cell, as well as barrier, homeostasis and water-holding capacity. In fact, they are known to play a crucial role in cell proliferation, differentiation, and apoptosis.3 There are at least 16 types of naturally occurring ceramides. For years, they have been included in barrier repair moisturizers. They are difficult to work with in moisturizers for several reasons:
- Ceramides are abundant in brain tissue and the ceramides used in moisturizers in the past were derived from bovine brain tissue. Prior to the emergence of bovine spongiform encephalopathy (mad cow disease), many ceramides in skin-care products were animal derived, which made them expensive and undesirable.
- Ceramides in skin care that are made from plant sources are referred to as phyto-derived ceramides. Although they share a similar structure with ceramides that occur in human skin, there are differences in chain length, hydroxylation pattern, and the degree of unsaturation that lead to structural diversity.4 The shape of ceramides is critical for a strong skin barrier because the lipids in the skin barrier must fit together like puzzle pieces to form a water-tight barrier. Natural sources of ceramides include rice, wheat, potato, konjac, and maize. Standardization of ceramide shape and structure makes using phyto-derived ceramides in skin care products challenging.
- Ceramides, because of their waxy consistency, require heat during the mixing process of skin care product manufacturing. This heat can make other ingredients inactive in the skin care formulation. (Ceramides are typically added early in the formulation process, and the heat-sensitive ones are added later.)
- Many forms of ceramides are unstable in the product manufacturing and bottling processes.
- Skin penetration of ceramides depends on the shape and size of ceramides.
Synthetic ceramides have been developed to make ceramides safe, affordable, and more easily formulated into moisturizers. These formulations synthesized in the lab are sometimes called pseudoceramides because they are structurally different compounds that mimic the activity of ceramides. They are developed to be less expensive to manufacture, safer than those derived from animals, and easier to formulate, and they can be made into the specific shape of the ceramide puzzle piece.
Ceramides in skin care
The naturally occurring intercellular lipids of the SC are composed of approximately equal proportions of ceramides, cholesterol, and fatty acids (referred to in this article as the “three barrier lipids” for simplicity).5-9 Alterations in any of these three barrier lipids or their regulatory enzymes result in impairments in the function of the epidermal barrier. Therefore, any synthetic ceramide must mimic the shape of natural ceramides, or the three barrier lipids in the moisturizer must mimic the shape of the entire bilayer lamella. Unfortunately, most barrier repair moisturizers do not meet these criteria and are not true barrier repair moisturizers.
How do you know if a moisturizer repairs the skin barrier?
Clinical tests such as measuring transepidermal water loss (TEWL) with a Tewameter are usually done to support the barrier repair claim. However, occlusive ingredients like oils can lower TEWL without affecting the barrier. In fact, we believe that sebum on the skin can make an impaired barrier and result in normal TEWL even when the barrier is impaired. So, just because a product improved TEWL does not necessarily mean that it repairs the barrier.
One way to test the ability of a moisturizer to repair the barrier is to look at a structural analysis of the moisturizer to see if it forms the requisite bilayer lamellar shape. An easy way to do this testing is to look for the cross pattern under a cross polarized microscope. The cross pattern is known as optical anisotropy. 8
The best barrier repair creams
Optimal barrier repair creams either feature a 1:1:1 ratio of epidermal lipids or form a cross structure when viewed with a cross-polarized microscope.8 There are several categories of barrier repair moisturizers that meet these criteria.
Barrier repair creams with a 1:1:1 ratio of lipids:
Peter Elias, MD, holds the patent on barrier repair moisturizer technology that has a 1:1:1 ratio. His well-established technology is used in a prescription barrier repair cream called EpiCeram® which is approved by the Food and Drug Administration to treat eczema. There are no other moisturizers that I know of that contain this 1:1:1 lipid ratio.
There is a barrier repair cream on the market that contains a 2:4:2 ratio of lipids based on a study that showed that this ratio is effective in older skin with an impaired barrier. It is unknown if this moisturizer forms a cross pattern.
Barrier repair creams that demonstrate a cross pattern:
Multilamellar emulsion (MLE) technology: This barrier repair technology, invented in South Korea, contains the synthetic pseudoceramide called myristyl/palmityl-oxo-stearamide/arachamide MEA (C34H67NO3/C36H71NO3/C38H75NO3), or the pseudoceramide myristoyl-palmitoyl-oxostearamide-arachamide MEA.
In a 2019 pilot study by Ye and colleagues, the investigators treated 33 older volunteers twice daily for 30 days with approximately 3 mL of an emollient containing MLE technology. In addition, 30 untreated older subjects and 11 young volunteers served as controls. The investigators found that the topically applied barrier repair emollient significantly improved barrier function, as well as stratum corneum hydration. Circulating levels of the important, age-related plasma cytokines interleukin-1 beta and IL-6 were found to have normalized, while tumor necrosis factor–alpha decreased markedly. The investigators suggested that repair of the skin barrier might diminish circulating proinflammatory cytokine levels (such as amyloid A) in aged humans, potentially mitigating the development of chronic inflammatory conditions.10
MLE technology has also been shown to improve childhood atopic dermatitis and prevent steroid atrophy.11,12 The consistent use of MLE technology in moisturizers has been shown to alleviate inflammatory factors in the blood and is believed to lessen systemic inflammation.10
Physiologic (PSL) lipid repair technology: This technology was invented by one of the South Korean researchers who helped develop MLE technology. It contains pseudoceramides, fatty acids, and cholesterol. The figure of the cross pattern above, as seen under the cross polarized microscope, is an image taken of this PSL lipid repair technology.
Conclusion
Do not believe that a moisturizer repairs the barrier just because it says so on the label. Three of the most popular body moisturizes used to treat eczema do not actually have the proper formula to repair the barrier. Unfortunately, there are dozens of skin care products that claim to repair the barrier that do not have the science or ingredient content to back them up. To restore the skin barrier to a healthy condition, it is imperative that the barrier repair moisturizers that you are recommending for patients have the correct 1:1:1 ratio of epidermal lipids or contain bilayer lamella that mimic the natural multilamellar layers and display the cross pattern under a cross-polarized microscope.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions, a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at [email protected].
References
1. Christophers E and Kligman AM. J Invest Dermatol. 1964;42:407-9.
2. Blair C. Br J Dermatol. 1968;80(7):430-6.
3. Morita O et al. Food Chem Toxicol. 2009 Apr;47(4):681-6.
4. Tessema E N et al. Skin pharmacology and physiology. 2017;30(3):115-38.
5. Coderch L et al. Am J Clin Dermatol. 2003;4(2):107-29.
6. Man MQ et al. Arch Dermatol. 1993;129(6):728-38.
7. Man MQ M et al. J Invest Dermatol. 1996 May;106(5):1096-101.
8. Park BD et al. J Invest Dermatol. 2003;121(4):794-801.
9. Proksch E and Jensen J. Skin as an organ of protection, in “Fitzpatrick’s Dermatology in General Medicine,” 7th ed. New York: McGraw-Hill, 2008, pp. 383-95.
10. Ye L et al. J Eur Acad Dermatol Venereol. 2019;33(11):2197-201.
11. Lee EJ et al. Ann Dermatol. 2003;15(4):133-8.
12. Ahn SK et al. J Dermatol. 2006;33(2):80-90.
There are dozens of skin care products that claim to repair the barrier that do not have the science or ingredient content to back them up.
Does a skin barrier repair moisturizer really repair?
First, let’s briefly review what the skin barrier is. The stratum corneum (SC), the most superficial layer of the epidermis, averages approximately 15-cell layers in thickness.1,2 The keratinocytes reside there in a pattern resembling a brick wall. The “mortar” is composed of the lipid contents extruded from the lamellar granules. This protective barrier functions to prevent transepidermal water loss (TEWL) and entry of allergens, irritants, and pathogens into deeper layers of the skin. This column will focus briefly on the structure and function of the skin barrier and the barrier repair technologies that use synthetic lipids such as myristoyl-palmitoyl and myristyl/palmityl-oxo-stearamide/arachamide MEA.
Structure of the skin barrier
SC keratinocytes are surrounded by lamella made from lipid bilayers. The lipids have hydrophilic heads and hydrophobic tails; the bilayer arises when the hydrophobic tails face the center and the hydrophilic heads face out of the bilayer. This formation yields a disc-shaped hydrophobic lamellar center. There are actually several of these lamellar layers between keratinocytes.

The naturally occurring primary lipids of the bilayer lamellae are made up of an equal ratio of ceramides, cholesterol, and free fatty acid. Arranged in a 1:1:1 ratio, they fit together like pieces of a puzzle to achieve skin barrier homeostasis. The shape and size of these puzzle pieces is critical. An incorrect shape results in a hole in the skin barrier resulting in dehydration, inflammation, and sensitivity.
Ceramides
Ceramides are a complex family of lipids (sphingolipids – a sphingoid base and a fatty acid) involved in cell, as well as barrier, homeostasis and water-holding capacity. In fact, they are known to play a crucial role in cell proliferation, differentiation, and apoptosis.3 There are at least 16 types of naturally occurring ceramides. For years, they have been included in barrier repair moisturizers. They are difficult to work with in moisturizers for several reasons:
- Ceramides are abundant in brain tissue and the ceramides used in moisturizers in the past were derived from bovine brain tissue. Prior to the emergence of bovine spongiform encephalopathy (mad cow disease), many ceramides in skin-care products were animal derived, which made them expensive and undesirable.
- Ceramides in skin care that are made from plant sources are referred to as phyto-derived ceramides. Although they share a similar structure with ceramides that occur in human skin, there are differences in chain length, hydroxylation pattern, and the degree of unsaturation that lead to structural diversity.4 The shape of ceramides is critical for a strong skin barrier because the lipids in the skin barrier must fit together like puzzle pieces to form a water-tight barrier. Natural sources of ceramides include rice, wheat, potato, konjac, and maize. Standardization of ceramide shape and structure makes using phyto-derived ceramides in skin care products challenging.
- Ceramides, because of their waxy consistency, require heat during the mixing process of skin care product manufacturing. This heat can make other ingredients inactive in the skin care formulation. (Ceramides are typically added early in the formulation process, and the heat-sensitive ones are added later.)
- Many forms of ceramides are unstable in the product manufacturing and bottling processes.
- Skin penetration of ceramides depends on the shape and size of ceramides.
Synthetic ceramides have been developed to make ceramides safe, affordable, and more easily formulated into moisturizers. These formulations synthesized in the lab are sometimes called pseudoceramides because they are structurally different compounds that mimic the activity of ceramides. They are developed to be less expensive to manufacture, safer than those derived from animals, and easier to formulate, and they can be made into the specific shape of the ceramide puzzle piece.
Ceramides in skin care
The naturally occurring intercellular lipids of the SC are composed of approximately equal proportions of ceramides, cholesterol, and fatty acids (referred to in this article as the “three barrier lipids” for simplicity).5-9 Alterations in any of these three barrier lipids or their regulatory enzymes result in impairments in the function of the epidermal barrier. Therefore, any synthetic ceramide must mimic the shape of natural ceramides, or the three barrier lipids in the moisturizer must mimic the shape of the entire bilayer lamella. Unfortunately, most barrier repair moisturizers do not meet these criteria and are not true barrier repair moisturizers.
How do you know if a moisturizer repairs the skin barrier?
Clinical tests such as measuring transepidermal water loss (TEWL) with a Tewameter are usually done to support the barrier repair claim. However, occlusive ingredients like oils can lower TEWL without affecting the barrier. In fact, we believe that sebum on the skin can make an impaired barrier and result in normal TEWL even when the barrier is impaired. So, just because a product improved TEWL does not necessarily mean that it repairs the barrier.
One way to test the ability of a moisturizer to repair the barrier is to look at a structural analysis of the moisturizer to see if it forms the requisite bilayer lamellar shape. An easy way to do this testing is to look for the cross pattern under a cross polarized microscope. The cross pattern is known as optical anisotropy. 8
The best barrier repair creams
Optimal barrier repair creams either feature a 1:1:1 ratio of epidermal lipids or form a cross structure when viewed with a cross-polarized microscope.8 There are several categories of barrier repair moisturizers that meet these criteria.
Barrier repair creams with a 1:1:1 ratio of lipids:
Peter Elias, MD, holds the patent on barrier repair moisturizer technology that has a 1:1:1 ratio. His well-established technology is used in a prescription barrier repair cream called EpiCeram® which is approved by the Food and Drug Administration to treat eczema. There are no other moisturizers that I know of that contain this 1:1:1 lipid ratio.
There is a barrier repair cream on the market that contains a 2:4:2 ratio of lipids based on a study that showed that this ratio is effective in older skin with an impaired barrier. It is unknown if this moisturizer forms a cross pattern.
Barrier repair creams that demonstrate a cross pattern:
Multilamellar emulsion (MLE) technology: This barrier repair technology, invented in South Korea, contains the synthetic pseudoceramide called myristyl/palmityl-oxo-stearamide/arachamide MEA (C34H67NO3/C36H71NO3/C38H75NO3), or the pseudoceramide myristoyl-palmitoyl-oxostearamide-arachamide MEA.
In a 2019 pilot study by Ye and colleagues, the investigators treated 33 older volunteers twice daily for 30 days with approximately 3 mL of an emollient containing MLE technology. In addition, 30 untreated older subjects and 11 young volunteers served as controls. The investigators found that the topically applied barrier repair emollient significantly improved barrier function, as well as stratum corneum hydration. Circulating levels of the important, age-related plasma cytokines interleukin-1 beta and IL-6 were found to have normalized, while tumor necrosis factor–alpha decreased markedly. The investigators suggested that repair of the skin barrier might diminish circulating proinflammatory cytokine levels (such as amyloid A) in aged humans, potentially mitigating the development of chronic inflammatory conditions.10
MLE technology has also been shown to improve childhood atopic dermatitis and prevent steroid atrophy.11,12 The consistent use of MLE technology in moisturizers has been shown to alleviate inflammatory factors in the blood and is believed to lessen systemic inflammation.10
Physiologic (PSL) lipid repair technology: This technology was invented by one of the South Korean researchers who helped develop MLE technology. It contains pseudoceramides, fatty acids, and cholesterol. The figure of the cross pattern above, as seen under the cross polarized microscope, is an image taken of this PSL lipid repair technology.
Conclusion
Do not believe that a moisturizer repairs the barrier just because it says so on the label. Three of the most popular body moisturizes used to treat eczema do not actually have the proper formula to repair the barrier. Unfortunately, there are dozens of skin care products that claim to repair the barrier that do not have the science or ingredient content to back them up. To restore the skin barrier to a healthy condition, it is imperative that the barrier repair moisturizers that you are recommending for patients have the correct 1:1:1 ratio of epidermal lipids or contain bilayer lamella that mimic the natural multilamellar layers and display the cross pattern under a cross-polarized microscope.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions, a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at [email protected].
References
1. Christophers E and Kligman AM. J Invest Dermatol. 1964;42:407-9.
2. Blair C. Br J Dermatol. 1968;80(7):430-6.
3. Morita O et al. Food Chem Toxicol. 2009 Apr;47(4):681-6.
4. Tessema E N et al. Skin pharmacology and physiology. 2017;30(3):115-38.
5. Coderch L et al. Am J Clin Dermatol. 2003;4(2):107-29.
6. Man MQ et al. Arch Dermatol. 1993;129(6):728-38.
7. Man MQ M et al. J Invest Dermatol. 1996 May;106(5):1096-101.
8. Park BD et al. J Invest Dermatol. 2003;121(4):794-801.
9. Proksch E and Jensen J. Skin as an organ of protection, in “Fitzpatrick’s Dermatology in General Medicine,” 7th ed. New York: McGraw-Hill, 2008, pp. 383-95.
10. Ye L et al. J Eur Acad Dermatol Venereol. 2019;33(11):2197-201.
11. Lee EJ et al. Ann Dermatol. 2003;15(4):133-8.
12. Ahn SK et al. J Dermatol. 2006;33(2):80-90.
There are dozens of skin care products that claim to repair the barrier that do not have the science or ingredient content to back them up.
Does a skin barrier repair moisturizer really repair?
First, let’s briefly review what the skin barrier is. The stratum corneum (SC), the most superficial layer of the epidermis, averages approximately 15-cell layers in thickness.1,2 The keratinocytes reside there in a pattern resembling a brick wall. The “mortar” is composed of the lipid contents extruded from the lamellar granules. This protective barrier functions to prevent transepidermal water loss (TEWL) and entry of allergens, irritants, and pathogens into deeper layers of the skin. This column will focus briefly on the structure and function of the skin barrier and the barrier repair technologies that use synthetic lipids such as myristoyl-palmitoyl and myristyl/palmityl-oxo-stearamide/arachamide MEA.
Structure of the skin barrier
SC keratinocytes are surrounded by lamella made from lipid bilayers. The lipids have hydrophilic heads and hydrophobic tails; the bilayer arises when the hydrophobic tails face the center and the hydrophilic heads face out of the bilayer. This formation yields a disc-shaped hydrophobic lamellar center. There are actually several of these lamellar layers between keratinocytes.

The naturally occurring primary lipids of the bilayer lamellae are made up of an equal ratio of ceramides, cholesterol, and free fatty acid. Arranged in a 1:1:1 ratio, they fit together like pieces of a puzzle to achieve skin barrier homeostasis. The shape and size of these puzzle pieces is critical. An incorrect shape results in a hole in the skin barrier resulting in dehydration, inflammation, and sensitivity.
Ceramides
Ceramides are a complex family of lipids (sphingolipids – a sphingoid base and a fatty acid) involved in cell, as well as barrier, homeostasis and water-holding capacity. In fact, they are known to play a crucial role in cell proliferation, differentiation, and apoptosis.3 There are at least 16 types of naturally occurring ceramides. For years, they have been included in barrier repair moisturizers. They are difficult to work with in moisturizers for several reasons:
- Ceramides are abundant in brain tissue and the ceramides used in moisturizers in the past were derived from bovine brain tissue. Prior to the emergence of bovine spongiform encephalopathy (mad cow disease), many ceramides in skin-care products were animal derived, which made them expensive and undesirable.
- Ceramides in skin care that are made from plant sources are referred to as phyto-derived ceramides. Although they share a similar structure with ceramides that occur in human skin, there are differences in chain length, hydroxylation pattern, and the degree of unsaturation that lead to structural diversity.4 The shape of ceramides is critical for a strong skin barrier because the lipids in the skin barrier must fit together like puzzle pieces to form a water-tight barrier. Natural sources of ceramides include rice, wheat, potato, konjac, and maize. Standardization of ceramide shape and structure makes using phyto-derived ceramides in skin care products challenging.
- Ceramides, because of their waxy consistency, require heat during the mixing process of skin care product manufacturing. This heat can make other ingredients inactive in the skin care formulation. (Ceramides are typically added early in the formulation process, and the heat-sensitive ones are added later.)
- Many forms of ceramides are unstable in the product manufacturing and bottling processes.
- Skin penetration of ceramides depends on the shape and size of ceramides.
Synthetic ceramides have been developed to make ceramides safe, affordable, and more easily formulated into moisturizers. These formulations synthesized in the lab are sometimes called pseudoceramides because they are structurally different compounds that mimic the activity of ceramides. They are developed to be less expensive to manufacture, safer than those derived from animals, and easier to formulate, and they can be made into the specific shape of the ceramide puzzle piece.
Ceramides in skin care
The naturally occurring intercellular lipids of the SC are composed of approximately equal proportions of ceramides, cholesterol, and fatty acids (referred to in this article as the “three barrier lipids” for simplicity).5-9 Alterations in any of these three barrier lipids or their regulatory enzymes result in impairments in the function of the epidermal barrier. Therefore, any synthetic ceramide must mimic the shape of natural ceramides, or the three barrier lipids in the moisturizer must mimic the shape of the entire bilayer lamella. Unfortunately, most barrier repair moisturizers do not meet these criteria and are not true barrier repair moisturizers.
How do you know if a moisturizer repairs the skin barrier?
Clinical tests such as measuring transepidermal water loss (TEWL) with a Tewameter are usually done to support the barrier repair claim. However, occlusive ingredients like oils can lower TEWL without affecting the barrier. In fact, we believe that sebum on the skin can make an impaired barrier and result in normal TEWL even when the barrier is impaired. So, just because a product improved TEWL does not necessarily mean that it repairs the barrier.
One way to test the ability of a moisturizer to repair the barrier is to look at a structural analysis of the moisturizer to see if it forms the requisite bilayer lamellar shape. An easy way to do this testing is to look for the cross pattern under a cross polarized microscope. The cross pattern is known as optical anisotropy. 8
The best barrier repair creams
Optimal barrier repair creams either feature a 1:1:1 ratio of epidermal lipids or form a cross structure when viewed with a cross-polarized microscope.8 There are several categories of barrier repair moisturizers that meet these criteria.
Barrier repair creams with a 1:1:1 ratio of lipids:
Peter Elias, MD, holds the patent on barrier repair moisturizer technology that has a 1:1:1 ratio. His well-established technology is used in a prescription barrier repair cream called EpiCeram® which is approved by the Food and Drug Administration to treat eczema. There are no other moisturizers that I know of that contain this 1:1:1 lipid ratio.
There is a barrier repair cream on the market that contains a 2:4:2 ratio of lipids based on a study that showed that this ratio is effective in older skin with an impaired barrier. It is unknown if this moisturizer forms a cross pattern.
Barrier repair creams that demonstrate a cross pattern:
Multilamellar emulsion (MLE) technology: This barrier repair technology, invented in South Korea, contains the synthetic pseudoceramide called myristyl/palmityl-oxo-stearamide/arachamide MEA (C34H67NO3/C36H71NO3/C38H75NO3), or the pseudoceramide myristoyl-palmitoyl-oxostearamide-arachamide MEA.
In a 2019 pilot study by Ye and colleagues, the investigators treated 33 older volunteers twice daily for 30 days with approximately 3 mL of an emollient containing MLE technology. In addition, 30 untreated older subjects and 11 young volunteers served as controls. The investigators found that the topically applied barrier repair emollient significantly improved barrier function, as well as stratum corneum hydration. Circulating levels of the important, age-related plasma cytokines interleukin-1 beta and IL-6 were found to have normalized, while tumor necrosis factor–alpha decreased markedly. The investigators suggested that repair of the skin barrier might diminish circulating proinflammatory cytokine levels (such as amyloid A) in aged humans, potentially mitigating the development of chronic inflammatory conditions.10
MLE technology has also been shown to improve childhood atopic dermatitis and prevent steroid atrophy.11,12 The consistent use of MLE technology in moisturizers has been shown to alleviate inflammatory factors in the blood and is believed to lessen systemic inflammation.10
Physiologic (PSL) lipid repair technology: This technology was invented by one of the South Korean researchers who helped develop MLE technology. It contains pseudoceramides, fatty acids, and cholesterol. The figure of the cross pattern above, as seen under the cross polarized microscope, is an image taken of this PSL lipid repair technology.
Conclusion
Do not believe that a moisturizer repairs the barrier just because it says so on the label. Three of the most popular body moisturizes used to treat eczema do not actually have the proper formula to repair the barrier. Unfortunately, there are dozens of skin care products that claim to repair the barrier that do not have the science or ingredient content to back them up. To restore the skin barrier to a healthy condition, it is imperative that the barrier repair moisturizers that you are recommending for patients have the correct 1:1:1 ratio of epidermal lipids or contain bilayer lamella that mimic the natural multilamellar layers and display the cross pattern under a cross-polarized microscope.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions, a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at [email protected].
References
1. Christophers E and Kligman AM. J Invest Dermatol. 1964;42:407-9.
2. Blair C. Br J Dermatol. 1968;80(7):430-6.
3. Morita O et al. Food Chem Toxicol. 2009 Apr;47(4):681-6.
4. Tessema E N et al. Skin pharmacology and physiology. 2017;30(3):115-38.
5. Coderch L et al. Am J Clin Dermatol. 2003;4(2):107-29.
6. Man MQ et al. Arch Dermatol. 1993;129(6):728-38.
7. Man MQ M et al. J Invest Dermatol. 1996 May;106(5):1096-101.
8. Park BD et al. J Invest Dermatol. 2003;121(4):794-801.
9. Proksch E and Jensen J. Skin as an organ of protection, in “Fitzpatrick’s Dermatology in General Medicine,” 7th ed. New York: McGraw-Hill, 2008, pp. 383-95.
10. Ye L et al. J Eur Acad Dermatol Venereol. 2019;33(11):2197-201.
11. Lee EJ et al. Ann Dermatol. 2003;15(4):133-8.
12. Ahn SK et al. J Dermatol. 2006;33(2):80-90.
A 22-year-old presented with erythematous papules on her fingers and toes
than men. Clinically, distal extremities such as toes, fingertips and heels, as well as the rims of the ears or nose develop erythematous to purple plaques. Lesions may be painful or pruritic. Over time, lesions may develop atrophy and resemble those of discoid lupus. While the pathogenesis is unknown, exposure to cold or wet environments can precipitate lesions.
Histopathology reveals a deep and superficial lymphocytic infiltrate with perieccrine involvement and fibrin deposition in vessels. Dermal edema is often present. Direct immunofluorescence shows an interface dermatitis positive for IgM, IgA, and C3.
The Mayo Clinic developed diagnostic criteria for diagnosing chilblains lupus. Two major criteria are acral skin lesions induced by cold exposure and evidence of lupus erythematosus in skin lesions (histopathologically or by direct immunofluorescence). Three minor criteria are the coexistence of systemic lupus erythematosus or discoid lupus erythematosus, response to antilupus treatment, and negative cryoglobulin and cold agglutinin studies.
Chilblains, or perniosis, has a similar clinical presentation to chilblain lupus erythematosus. However, serologic evidence of lupus, such as a positive antinuclear antibody (ANA), will be absent. Lupus pernio (Besnier-Tenneson syndrome) is a form of sarcoidosis that tends to favor the nose. These lesions are not precipitated by cold. It can be differentiated on histology. “COVID toes” is an entity described during the coronavirus pandemic, during which dermatologists noted pernio-like lesions in patients testing positive for coronavirus.
The patient’s labs revealed a positive ANA at 1:320 in a nucleolar speckled pattern, elevated double-stranded DNA, low C3 and C4 levels, elevated cardiolipin IgM Ab, and elevated sedimentation rate. COVID-19 antigen testing and COVID-19 antibodies were negative. A serum protein electrophoresis was negative. Cryoglobulins were negative.
Treatment includes protection from cold. Smoking cessation should be discussed. Topical steroids and topical calcineurin inhibitors are first-line treatments for mild disease. Antimalarials, such as hydroxychloroquine can be helpful. Systemic calcium channel blockers, systemic steroids, mycophenolate mofetil, and tacrolimus have all been reported as treatments. This patient responded well to hydroxychloroquine and topical steroids with full resolution of lesions.
This case was submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
Su WP et al. Cutis. 1994 Dec;54(6):395-9.
Werth V and Newman S. Chilblain lupus (SLE pernio). Dermatology Advisor. 2017.
than men. Clinically, distal extremities such as toes, fingertips and heels, as well as the rims of the ears or nose develop erythematous to purple plaques. Lesions may be painful or pruritic. Over time, lesions may develop atrophy and resemble those of discoid lupus. While the pathogenesis is unknown, exposure to cold or wet environments can precipitate lesions.
Histopathology reveals a deep and superficial lymphocytic infiltrate with perieccrine involvement and fibrin deposition in vessels. Dermal edema is often present. Direct immunofluorescence shows an interface dermatitis positive for IgM, IgA, and C3.
The Mayo Clinic developed diagnostic criteria for diagnosing chilblains lupus. Two major criteria are acral skin lesions induced by cold exposure and evidence of lupus erythematosus in skin lesions (histopathologically or by direct immunofluorescence). Three minor criteria are the coexistence of systemic lupus erythematosus or discoid lupus erythematosus, response to antilupus treatment, and negative cryoglobulin and cold agglutinin studies.
Chilblains, or perniosis, has a similar clinical presentation to chilblain lupus erythematosus. However, serologic evidence of lupus, such as a positive antinuclear antibody (ANA), will be absent. Lupus pernio (Besnier-Tenneson syndrome) is a form of sarcoidosis that tends to favor the nose. These lesions are not precipitated by cold. It can be differentiated on histology. “COVID toes” is an entity described during the coronavirus pandemic, during which dermatologists noted pernio-like lesions in patients testing positive for coronavirus.
The patient’s labs revealed a positive ANA at 1:320 in a nucleolar speckled pattern, elevated double-stranded DNA, low C3 and C4 levels, elevated cardiolipin IgM Ab, and elevated sedimentation rate. COVID-19 antigen testing and COVID-19 antibodies were negative. A serum protein electrophoresis was negative. Cryoglobulins were negative.
Treatment includes protection from cold. Smoking cessation should be discussed. Topical steroids and topical calcineurin inhibitors are first-line treatments for mild disease. Antimalarials, such as hydroxychloroquine can be helpful. Systemic calcium channel blockers, systemic steroids, mycophenolate mofetil, and tacrolimus have all been reported as treatments. This patient responded well to hydroxychloroquine and topical steroids with full resolution of lesions.
This case was submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
Su WP et al. Cutis. 1994 Dec;54(6):395-9.
Werth V and Newman S. Chilblain lupus (SLE pernio). Dermatology Advisor. 2017.
than men. Clinically, distal extremities such as toes, fingertips and heels, as well as the rims of the ears or nose develop erythematous to purple plaques. Lesions may be painful or pruritic. Over time, lesions may develop atrophy and resemble those of discoid lupus. While the pathogenesis is unknown, exposure to cold or wet environments can precipitate lesions.
Histopathology reveals a deep and superficial lymphocytic infiltrate with perieccrine involvement and fibrin deposition in vessels. Dermal edema is often present. Direct immunofluorescence shows an interface dermatitis positive for IgM, IgA, and C3.
The Mayo Clinic developed diagnostic criteria for diagnosing chilblains lupus. Two major criteria are acral skin lesions induced by cold exposure and evidence of lupus erythematosus in skin lesions (histopathologically or by direct immunofluorescence). Three minor criteria are the coexistence of systemic lupus erythematosus or discoid lupus erythematosus, response to antilupus treatment, and negative cryoglobulin and cold agglutinin studies.
Chilblains, or perniosis, has a similar clinical presentation to chilblain lupus erythematosus. However, serologic evidence of lupus, such as a positive antinuclear antibody (ANA), will be absent. Lupus pernio (Besnier-Tenneson syndrome) is a form of sarcoidosis that tends to favor the nose. These lesions are not precipitated by cold. It can be differentiated on histology. “COVID toes” is an entity described during the coronavirus pandemic, during which dermatologists noted pernio-like lesions in patients testing positive for coronavirus.
The patient’s labs revealed a positive ANA at 1:320 in a nucleolar speckled pattern, elevated double-stranded DNA, low C3 and C4 levels, elevated cardiolipin IgM Ab, and elevated sedimentation rate. COVID-19 antigen testing and COVID-19 antibodies were negative. A serum protein electrophoresis was negative. Cryoglobulins were negative.
Treatment includes protection from cold. Smoking cessation should be discussed. Topical steroids and topical calcineurin inhibitors are first-line treatments for mild disease. Antimalarials, such as hydroxychloroquine can be helpful. Systemic calcium channel blockers, systemic steroids, mycophenolate mofetil, and tacrolimus have all been reported as treatments. This patient responded well to hydroxychloroquine and topical steroids with full resolution of lesions.
This case was submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
Su WP et al. Cutis. 1994 Dec;54(6):395-9.
Werth V and Newman S. Chilblain lupus (SLE pernio). Dermatology Advisor. 2017.