User login
Formerly Skin & Allergy News
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')]
The leading independent newspaper covering dermatology news and commentary.
Wrong-site surgery doc says he can’t be sued
A neurosurgeon who operated on the wrong side of his patient’s spine claims he can’t be sued because of a federal law that protects health care professionals during a public health emergency, according to a report by KSDK, an NBC-affiliated television station in St. Louis.
Natalie Avilez, who lives in Missouri with her husband and five children, had been suffering from intense back pain. At some point in the recent past (the story doesn’t identify precisely when), she was referred to Fangxiang Chen, MD, a neurosurgeon affiliated with Mercy Hospital and Mercy Hospital South, in St. Louis. Ms. Avilez reportedly claims that Dr. Chen told her that an “easy” surgery – a hemilaminectomy – could relieve her back pain.
Something went wrong during the procedure, however. Dr. Chen ended up operating on the left side of Avilez’s spine instead of the right side, where he had initially diagnosed disk-related pressure. Dr. Chen realized his mistake while his patient was under anesthesia but couldn’t remedy it.
As the patient awakened, Dr. Chen asked her to authorize an immediate right-side surgery, but, as Ms. Avilez told the TV station, her “charge nurse would not let him get authorization because I wasn’t fully awake.” In the recovery room afterward, Dr. Chen explained what had happened to his patient, who permitted him to redo the surgery the following day.
But the redo didn’t remedy Ms. Avilez’s pain; in fact, the second surgery made things worse. “I’m always in constant pain,” she said. “I kind of feel like I would have been better off not even doing it at all.”
In January of this year, Ms. Avilez filed a medical malpractice suit against Dr. Chen and Mercy. But the neurosurgeon made a surprising claim:
Initially passed in 2005, PREP was intended to shield doctors and other licensed health care professionals from liability during a public health emergency except in cases of willful misconduct. On March 17, 2020, then–Health and Human Services Secretary Alex Azar invoked the PREP Act “for activities related to medical countermeasures against COVID-19.”
But could this declaration – which has since been amended multiple times – shield a physician from a claim of wrong-site surgery?
Ms. Avilez’s attorney, Morgan Murphy, doesn’t think so. “Obviously, we are not claiming that COVID had anything to do with the fact that Dr. Chen operated on the incorrect side of Natalie’s spine. It is a fairly straightforward situation. A doctor should never perform the incorrect surgery, period.”
Other observers are less certain that the Chen defense won’t hold. It’s true the PREP Act doesn’t protect doctors against claims of willful or intentional misconduct, says Deidre Gilbert, who leads a national medical malpractice patient-advocacy group. But such claims are, she quickly adds, very difficult to prove, never more so than during a pandemic.
Several states, including Missouri, have passed or are considering additional measures to protect health care professionals against the expected wave of COVID-related claims. (One estimate places the number of those claims at almost 6,000 as of February 2021.) “We want to make sure that there is a heightened standard for holding somebody liable in ... COVID transmission cases,” said the sponsor of the proposed Show-Me State legislation.
As for Ms. Avilez, she feels lucky that she’s not even worse off than she is now. She worries, though, about other patients who are less fortunate and who are told that the pandemic protects their health care professionals from liability. “That’s just not fair,” she says.
Hidden beliefs about people of color raise liability risks
Clinicians’ “implicit bias” can exacerbate medical disparities and also malpractice claims, a story in the Dayton Daily News reports.
The story’s authors cite La Fleur Small, PhD, a medical sociologist at Wayne State University, in Detroit, who sees “implicit bias” as a set of “unconscious associations and judgments” that affect social behavior, causing people to act in ways that are often contrary to their perceived value system. In the medical profession, such thinking can have unintended consequences, especially for people of color.
Implicit bias can erode the physician-patient relationship, which in turn can make a malpractice suit more likely should an adverse event occur. Studies reported in recent years in the AMA Journal of Ethics, for instance, found that poor communication was a factor in almost three-quarters of closed claims. Other studies have revealed that, of patients seeking legal advice following a medical mishap, more than half cited a poor doctor-patient relationship as a contributing factor in their decision.
To remedy things, it would be helpful to boost the number of doctors of color, at least to the point that it more closely reflects the percentage in the general population, say experts. Currently, although Black and Hispanic persons constitute 13.4% and 18.5%, respectively, of the overall U.S. population, they make up only 5.0% and 5.8% of active physicians. (As of 2018, 56.2% of all physicians were White and 17.2% were Asian, according to data from the Association of American Medical Colleges.)
Father of impaired baby seeks mega damages
An Oregon man whose son sustained permanent neurologic injuries during childbirth has sued the hospital where the 2017 delivery took place, as reported in The Astorian.
In the suit on behalf of his son, Wesley Humphries claims that Columbia Memorial Hospital in Astoria, Oregon, failed to monitor the baby’s heart rate and other aspects of the labor and delivery. As a consequence, the baby needed to be transferred to Oregon Health and Science University Hospital in Portland, approximately 100 miles away, for emergency treatment. Doctors there diagnosed the child as having hypoxic ischemic encephalopathy, which his lawyers say resulted in cerebral palsy, among other neurologic conditions.
Because of his son’s permanent impairment, Mr. Humphries is seeking significant damages: more than $45 million in medical, custodial, and life-care expenses and $65 million in noneconomic damages. Should his claim prove successful, the payout would mark one of the largest awards – if not the largest award – in Oregon State history. The hospital has declined to comment.
At press time, a trial date hadn’t been set.
A version of this article first appeared on Medscape.com.
A neurosurgeon who operated on the wrong side of his patient’s spine claims he can’t be sued because of a federal law that protects health care professionals during a public health emergency, according to a report by KSDK, an NBC-affiliated television station in St. Louis.
Natalie Avilez, who lives in Missouri with her husband and five children, had been suffering from intense back pain. At some point in the recent past (the story doesn’t identify precisely when), she was referred to Fangxiang Chen, MD, a neurosurgeon affiliated with Mercy Hospital and Mercy Hospital South, in St. Louis. Ms. Avilez reportedly claims that Dr. Chen told her that an “easy” surgery – a hemilaminectomy – could relieve her back pain.
Something went wrong during the procedure, however. Dr. Chen ended up operating on the left side of Avilez’s spine instead of the right side, where he had initially diagnosed disk-related pressure. Dr. Chen realized his mistake while his patient was under anesthesia but couldn’t remedy it.
As the patient awakened, Dr. Chen asked her to authorize an immediate right-side surgery, but, as Ms. Avilez told the TV station, her “charge nurse would not let him get authorization because I wasn’t fully awake.” In the recovery room afterward, Dr. Chen explained what had happened to his patient, who permitted him to redo the surgery the following day.
But the redo didn’t remedy Ms. Avilez’s pain; in fact, the second surgery made things worse. “I’m always in constant pain,” she said. “I kind of feel like I would have been better off not even doing it at all.”
In January of this year, Ms. Avilez filed a medical malpractice suit against Dr. Chen and Mercy. But the neurosurgeon made a surprising claim:
Initially passed in 2005, PREP was intended to shield doctors and other licensed health care professionals from liability during a public health emergency except in cases of willful misconduct. On March 17, 2020, then–Health and Human Services Secretary Alex Azar invoked the PREP Act “for activities related to medical countermeasures against COVID-19.”
But could this declaration – which has since been amended multiple times – shield a physician from a claim of wrong-site surgery?
Ms. Avilez’s attorney, Morgan Murphy, doesn’t think so. “Obviously, we are not claiming that COVID had anything to do with the fact that Dr. Chen operated on the incorrect side of Natalie’s spine. It is a fairly straightforward situation. A doctor should never perform the incorrect surgery, period.”
Other observers are less certain that the Chen defense won’t hold. It’s true the PREP Act doesn’t protect doctors against claims of willful or intentional misconduct, says Deidre Gilbert, who leads a national medical malpractice patient-advocacy group. But such claims are, she quickly adds, very difficult to prove, never more so than during a pandemic.
Several states, including Missouri, have passed or are considering additional measures to protect health care professionals against the expected wave of COVID-related claims. (One estimate places the number of those claims at almost 6,000 as of February 2021.) “We want to make sure that there is a heightened standard for holding somebody liable in ... COVID transmission cases,” said the sponsor of the proposed Show-Me State legislation.
As for Ms. Avilez, she feels lucky that she’s not even worse off than she is now. She worries, though, about other patients who are less fortunate and who are told that the pandemic protects their health care professionals from liability. “That’s just not fair,” she says.
Hidden beliefs about people of color raise liability risks
Clinicians’ “implicit bias” can exacerbate medical disparities and also malpractice claims, a story in the Dayton Daily News reports.
The story’s authors cite La Fleur Small, PhD, a medical sociologist at Wayne State University, in Detroit, who sees “implicit bias” as a set of “unconscious associations and judgments” that affect social behavior, causing people to act in ways that are often contrary to their perceived value system. In the medical profession, such thinking can have unintended consequences, especially for people of color.
Implicit bias can erode the physician-patient relationship, which in turn can make a malpractice suit more likely should an adverse event occur. Studies reported in recent years in the AMA Journal of Ethics, for instance, found that poor communication was a factor in almost three-quarters of closed claims. Other studies have revealed that, of patients seeking legal advice following a medical mishap, more than half cited a poor doctor-patient relationship as a contributing factor in their decision.
To remedy things, it would be helpful to boost the number of doctors of color, at least to the point that it more closely reflects the percentage in the general population, say experts. Currently, although Black and Hispanic persons constitute 13.4% and 18.5%, respectively, of the overall U.S. population, they make up only 5.0% and 5.8% of active physicians. (As of 2018, 56.2% of all physicians were White and 17.2% were Asian, according to data from the Association of American Medical Colleges.)
Father of impaired baby seeks mega damages
An Oregon man whose son sustained permanent neurologic injuries during childbirth has sued the hospital where the 2017 delivery took place, as reported in The Astorian.
In the suit on behalf of his son, Wesley Humphries claims that Columbia Memorial Hospital in Astoria, Oregon, failed to monitor the baby’s heart rate and other aspects of the labor and delivery. As a consequence, the baby needed to be transferred to Oregon Health and Science University Hospital in Portland, approximately 100 miles away, for emergency treatment. Doctors there diagnosed the child as having hypoxic ischemic encephalopathy, which his lawyers say resulted in cerebral palsy, among other neurologic conditions.
Because of his son’s permanent impairment, Mr. Humphries is seeking significant damages: more than $45 million in medical, custodial, and life-care expenses and $65 million in noneconomic damages. Should his claim prove successful, the payout would mark one of the largest awards – if not the largest award – in Oregon State history. The hospital has declined to comment.
At press time, a trial date hadn’t been set.
A version of this article first appeared on Medscape.com.
A neurosurgeon who operated on the wrong side of his patient’s spine claims he can’t be sued because of a federal law that protects health care professionals during a public health emergency, according to a report by KSDK, an NBC-affiliated television station in St. Louis.
Natalie Avilez, who lives in Missouri with her husband and five children, had been suffering from intense back pain. At some point in the recent past (the story doesn’t identify precisely when), she was referred to Fangxiang Chen, MD, a neurosurgeon affiliated with Mercy Hospital and Mercy Hospital South, in St. Louis. Ms. Avilez reportedly claims that Dr. Chen told her that an “easy” surgery – a hemilaminectomy – could relieve her back pain.
Something went wrong during the procedure, however. Dr. Chen ended up operating on the left side of Avilez’s spine instead of the right side, where he had initially diagnosed disk-related pressure. Dr. Chen realized his mistake while his patient was under anesthesia but couldn’t remedy it.
As the patient awakened, Dr. Chen asked her to authorize an immediate right-side surgery, but, as Ms. Avilez told the TV station, her “charge nurse would not let him get authorization because I wasn’t fully awake.” In the recovery room afterward, Dr. Chen explained what had happened to his patient, who permitted him to redo the surgery the following day.
But the redo didn’t remedy Ms. Avilez’s pain; in fact, the second surgery made things worse. “I’m always in constant pain,” she said. “I kind of feel like I would have been better off not even doing it at all.”
In January of this year, Ms. Avilez filed a medical malpractice suit against Dr. Chen and Mercy. But the neurosurgeon made a surprising claim:
Initially passed in 2005, PREP was intended to shield doctors and other licensed health care professionals from liability during a public health emergency except in cases of willful misconduct. On March 17, 2020, then–Health and Human Services Secretary Alex Azar invoked the PREP Act “for activities related to medical countermeasures against COVID-19.”
But could this declaration – which has since been amended multiple times – shield a physician from a claim of wrong-site surgery?
Ms. Avilez’s attorney, Morgan Murphy, doesn’t think so. “Obviously, we are not claiming that COVID had anything to do with the fact that Dr. Chen operated on the incorrect side of Natalie’s spine. It is a fairly straightforward situation. A doctor should never perform the incorrect surgery, period.”
Other observers are less certain that the Chen defense won’t hold. It’s true the PREP Act doesn’t protect doctors against claims of willful or intentional misconduct, says Deidre Gilbert, who leads a national medical malpractice patient-advocacy group. But such claims are, she quickly adds, very difficult to prove, never more so than during a pandemic.
Several states, including Missouri, have passed or are considering additional measures to protect health care professionals against the expected wave of COVID-related claims. (One estimate places the number of those claims at almost 6,000 as of February 2021.) “We want to make sure that there is a heightened standard for holding somebody liable in ... COVID transmission cases,” said the sponsor of the proposed Show-Me State legislation.
As for Ms. Avilez, she feels lucky that she’s not even worse off than she is now. She worries, though, about other patients who are less fortunate and who are told that the pandemic protects their health care professionals from liability. “That’s just not fair,” she says.
Hidden beliefs about people of color raise liability risks
Clinicians’ “implicit bias” can exacerbate medical disparities and also malpractice claims, a story in the Dayton Daily News reports.
The story’s authors cite La Fleur Small, PhD, a medical sociologist at Wayne State University, in Detroit, who sees “implicit bias” as a set of “unconscious associations and judgments” that affect social behavior, causing people to act in ways that are often contrary to their perceived value system. In the medical profession, such thinking can have unintended consequences, especially for people of color.
Implicit bias can erode the physician-patient relationship, which in turn can make a malpractice suit more likely should an adverse event occur. Studies reported in recent years in the AMA Journal of Ethics, for instance, found that poor communication was a factor in almost three-quarters of closed claims. Other studies have revealed that, of patients seeking legal advice following a medical mishap, more than half cited a poor doctor-patient relationship as a contributing factor in their decision.
To remedy things, it would be helpful to boost the number of doctors of color, at least to the point that it more closely reflects the percentage in the general population, say experts. Currently, although Black and Hispanic persons constitute 13.4% and 18.5%, respectively, of the overall U.S. population, they make up only 5.0% and 5.8% of active physicians. (As of 2018, 56.2% of all physicians were White and 17.2% were Asian, according to data from the Association of American Medical Colleges.)
Father of impaired baby seeks mega damages
An Oregon man whose son sustained permanent neurologic injuries during childbirth has sued the hospital where the 2017 delivery took place, as reported in The Astorian.
In the suit on behalf of his son, Wesley Humphries claims that Columbia Memorial Hospital in Astoria, Oregon, failed to monitor the baby’s heart rate and other aspects of the labor and delivery. As a consequence, the baby needed to be transferred to Oregon Health and Science University Hospital in Portland, approximately 100 miles away, for emergency treatment. Doctors there diagnosed the child as having hypoxic ischemic encephalopathy, which his lawyers say resulted in cerebral palsy, among other neurologic conditions.
Because of his son’s permanent impairment, Mr. Humphries is seeking significant damages: more than $45 million in medical, custodial, and life-care expenses and $65 million in noneconomic damages. Should his claim prove successful, the payout would mark one of the largest awards – if not the largest award – in Oregon State history. The hospital has declined to comment.
At press time, a trial date hadn’t been set.
A version of this article first appeared on Medscape.com.
Rate of cutaneous toxicities from ICIs may be lower than previously reported
A , according to research presented at the annual meeting of the Society for Investigative Dermatology, held virtually.
What’s more, many of the cutaneous immune-related adverse events (irAEs) from immune checkpoint inhibitors (ICIs) observed in the study may be unreported in clinical trial settings and by providers, according to one of the investigators, Yevgeniy Semenov, MD, MA, a dermatologist at Massachusetts General Hospital, Boston.
“Most cutaneous irAEs are low grade and might go unreported outside of clinical trial settings, as patients might not seek medical care, or when they do, providers might not report them in patient charts. As a result, the diagnoses identified in this study likely represent the most clinically relevant cutaneous events in the ICI population,” said Dr. Semenov, who presented the results at the meeting.
In the study, he said that one of the first issues he and his colleagues encountered was how to classify cutaneous irAEs, as they “can vary widely in morphology and severity.” Immune-related adverse events from ICIs are a “unique constellation of inflammatory toxicities,” affecting nearly every organ system, and may require treatment with immunosuppressive agents that can impact the effectiveness of the ICI. The matter is further complicated by a “lack of definitional standards of what constitutes a cutaneous immune-related adverse event, which greatly limits the research in this area,” Dr. Semenov said. There is also potential for misdiagnosis of irAEs as cutaneous eruptions occurring in patients receiving ICI therapy because of failure to account for the presence of skin disease at baseline, he pointed out.
Dr. Semenov noted that more than 40 cutaneous eruptions have been associated with ICI treatment. “Much of the observational data on cutaneous immune-related adverse events has been riddled with case reports and case series of cutaneous events that happen to be occurring in the setting of ICI therapy. These lack rigorous control groups and often associate events with little to no relationship to the actual ICI, which may have instead occurred in the setting of a competing medication,” he explained.
Real-world data
The researchers thus sought to identify the real-world incidence of cutaneous irAEs with population-level data. Using data from a national claims insurance database from January 2011 through 2019, they compared 8,637 of patients with cancer, treated with an ICI (who had not been treated with other cancer treatments within 6 months of starting an ICI) with 8,637 patients with cancer who were not treated with an ICI, matched for demographics, primary cancer type, and Charlson Comorbidity Index (CCI) score.
In both groups, the mean age of the patients was 67.5 years, 59.2% were men, and 93% had a severe CCI score. The most common cancer types were lung cancer (40%), melanoma (26.6%), and renal cell carcinoma (12.3%). The median follow-up time was 1.9 years, and the median treatment duration was 2.0 years.
Dr. Semenov and colleagues selected 42 dermatoses reported in the literature to evaluate and found an overall incidence of 25% within 2 years of starting ICI therapy. Of those 42 dermatoses, there were 10 with a significantly higher incidence among patients receiving ICIs, compared with controls: drug eruption or other nonspecific eruption (4.2%; incidence rate ratio, 5.00), bullous pemphigoid (0.3%; IRR, 4.91), maculopapular eruption (0.9%; IRR, 4.75), vitiligo (0.7%; IRR, 3.79), Grover’s disease (0.2%; IRR, 3.43), rash and other nonspecific eruption (9.0%; IRR, 2.34), mucositis (1.5%; IRR, 2.33), pruritus (4.8%; IRR, 1.92), lichen planus (0.5%; IRR, 1.75), and erythroderma (1.1%; IRR, 1.70).
After adjusting for a baseline history of squamous cell carcinoma and actinic keratosis, the researchers found that both were significantly less likely in patients receiving ICIs.
A delay in presentation of any cutaneous irAE after starting ICI therapy was also observed (a median of 16.1 weeks), which Dr. Semenov noted was longer than the 5 weeks reported in clinical trials. This delay in presentation increased to a median of 37.5 weeks for the 10 dermatoses with a significantly higher incidence among patients receiving ICIs, with 17.6% of patients presenting in the first month, 63.1% presenting by 6 months, and 84.6% presenting by 1 year.
Use of immunosuppressive treatment
The researchers also examined use of systemic immunosuppression for treating cutaneous toxicities, defined as “a new prescription for systemic glucocorticoids greater than 10 mg per day, prednisone equivalent, or nonsteroidal systemic immunosuppression,” administered within 7 days of the diagnosis of the cutaneous event. They found that 5% of patients overall received systemic immunosuppressive treatment within 7 days of a cutaneous event, which was “at the higher end of what was reported in clinical trials for the treatment of cutaneous toxicities,” Dr. Semenov noted.
“This is likely the result of the delays in diagnosis in nonclinical trial settings ... allowing more time for these events to progress to a higher grade. Also, there may be a greater willingness by providers to initiate systemic immunosuppression due to less stringent treatment protocols in real-world clinical settings,” he said.
Using a multivariable risk prediction model for cutaneous toxicities, the researchers identified use of ipilimumab, a CTLA-4-blocking antibody, as having a protective effect for not developing a cutaneous irAE, compared with the PD-1 blocker pembrolizumab (odds ratio, 0.78; 95% confidence interval, 0.62-0.98; P < .01). But combination ICI therapy (OR, 1.53; 95% CI, 1.25-1.88; P < .001), a melanoma diagnosis (OR, 2.47; 95% CI, 2.11-2.89; P < .001), and a renal cell carcinoma diagnosis (OR, 1.65; 95% CI, 1.36-2.00; P < .001) were found to be risk factors for developing cutaneous irAEs.
“The protective effect of ipilimumab identified in the study is interesting, as historically ipilimumab has been more likely to cause cutaneous toxicities,” Dr. Semenov said. “However, we believe that the majority of this association is mediated by the melanoma, for which ipilimumab was primarily used since its introduction. Independent of this relationship, it seems to be less likely to cause cutaneous toxicity than PD-1 inhibition, according to this data.”
Based on their findings, he said, “dermatologists can utilize this information to facilitate evaluations of high-risk patients so they can take steps to prevent progression to more severe toxicities and reduce reliance or systemic immunosuppression.”
The 25% real-world incidence of cutaneous irAEs observed in the study, Dr. Semenov said, is “somewhat lower than previous clinical trial estimates of over one-third of patients presenting with cutaneous toxicities” but he added that previous estimates were based primarily on studies of patients with melanoma.
That some patients delayed presentation with these conditions “should revise clinicians’ understanding of when to expect patients to present with these toxicities, and not to rule out a delayed onset of symptoms as being unrelated to immunotherapy,” Dr. Semenov said.
Most cutaneous irAEs are ‘manageable’
In an interview, Naiara Braghiroli, MD, PhD, a dermatologist at Baptist Health’s Miami Cancer Institute, Plantation, Fla., who was not an investigator in the study, noted that over the last decade, ICIs have “revolutionized the treatment of metastatic melanoma” and, more recently, the treatment of nonmelanoma skin cancers, with regard to survival rates and side effects.
She said that the results of the study show that “most of the cutaneous side effects are manageable with very few exceptions, like the cutaneous bullous disorders and rarely, more serious reactions [such as] Stevens-Johnson syndrome.”
The majority of the side effects are treatable “and when well controlled, the patient can have a good quality of life” during treatment, she added.
For future research, Dr. Braghiroli noted, it would be interesting to know more about whether the development of any specific cutaneous reaction associated with ICIs “is associated with a higher chance of good antitumor response,” as seen with other anticancer therapies such as epidermal growth factor receptor inhibitors.
Dr. Semenov and Dr. Braghiroli report having no relevant financial disclosures.
A , according to research presented at the annual meeting of the Society for Investigative Dermatology, held virtually.
What’s more, many of the cutaneous immune-related adverse events (irAEs) from immune checkpoint inhibitors (ICIs) observed in the study may be unreported in clinical trial settings and by providers, according to one of the investigators, Yevgeniy Semenov, MD, MA, a dermatologist at Massachusetts General Hospital, Boston.
“Most cutaneous irAEs are low grade and might go unreported outside of clinical trial settings, as patients might not seek medical care, or when they do, providers might not report them in patient charts. As a result, the diagnoses identified in this study likely represent the most clinically relevant cutaneous events in the ICI population,” said Dr. Semenov, who presented the results at the meeting.
In the study, he said that one of the first issues he and his colleagues encountered was how to classify cutaneous irAEs, as they “can vary widely in morphology and severity.” Immune-related adverse events from ICIs are a “unique constellation of inflammatory toxicities,” affecting nearly every organ system, and may require treatment with immunosuppressive agents that can impact the effectiveness of the ICI. The matter is further complicated by a “lack of definitional standards of what constitutes a cutaneous immune-related adverse event, which greatly limits the research in this area,” Dr. Semenov said. There is also potential for misdiagnosis of irAEs as cutaneous eruptions occurring in patients receiving ICI therapy because of failure to account for the presence of skin disease at baseline, he pointed out.
Dr. Semenov noted that more than 40 cutaneous eruptions have been associated with ICI treatment. “Much of the observational data on cutaneous immune-related adverse events has been riddled with case reports and case series of cutaneous events that happen to be occurring in the setting of ICI therapy. These lack rigorous control groups and often associate events with little to no relationship to the actual ICI, which may have instead occurred in the setting of a competing medication,” he explained.
Real-world data
The researchers thus sought to identify the real-world incidence of cutaneous irAEs with population-level data. Using data from a national claims insurance database from January 2011 through 2019, they compared 8,637 of patients with cancer, treated with an ICI (who had not been treated with other cancer treatments within 6 months of starting an ICI) with 8,637 patients with cancer who were not treated with an ICI, matched for demographics, primary cancer type, and Charlson Comorbidity Index (CCI) score.
In both groups, the mean age of the patients was 67.5 years, 59.2% were men, and 93% had a severe CCI score. The most common cancer types were lung cancer (40%), melanoma (26.6%), and renal cell carcinoma (12.3%). The median follow-up time was 1.9 years, and the median treatment duration was 2.0 years.
Dr. Semenov and colleagues selected 42 dermatoses reported in the literature to evaluate and found an overall incidence of 25% within 2 years of starting ICI therapy. Of those 42 dermatoses, there were 10 with a significantly higher incidence among patients receiving ICIs, compared with controls: drug eruption or other nonspecific eruption (4.2%; incidence rate ratio, 5.00), bullous pemphigoid (0.3%; IRR, 4.91), maculopapular eruption (0.9%; IRR, 4.75), vitiligo (0.7%; IRR, 3.79), Grover’s disease (0.2%; IRR, 3.43), rash and other nonspecific eruption (9.0%; IRR, 2.34), mucositis (1.5%; IRR, 2.33), pruritus (4.8%; IRR, 1.92), lichen planus (0.5%; IRR, 1.75), and erythroderma (1.1%; IRR, 1.70).
After adjusting for a baseline history of squamous cell carcinoma and actinic keratosis, the researchers found that both were significantly less likely in patients receiving ICIs.
A delay in presentation of any cutaneous irAE after starting ICI therapy was also observed (a median of 16.1 weeks), which Dr. Semenov noted was longer than the 5 weeks reported in clinical trials. This delay in presentation increased to a median of 37.5 weeks for the 10 dermatoses with a significantly higher incidence among patients receiving ICIs, with 17.6% of patients presenting in the first month, 63.1% presenting by 6 months, and 84.6% presenting by 1 year.
Use of immunosuppressive treatment
The researchers also examined use of systemic immunosuppression for treating cutaneous toxicities, defined as “a new prescription for systemic glucocorticoids greater than 10 mg per day, prednisone equivalent, or nonsteroidal systemic immunosuppression,” administered within 7 days of the diagnosis of the cutaneous event. They found that 5% of patients overall received systemic immunosuppressive treatment within 7 days of a cutaneous event, which was “at the higher end of what was reported in clinical trials for the treatment of cutaneous toxicities,” Dr. Semenov noted.
“This is likely the result of the delays in diagnosis in nonclinical trial settings ... allowing more time for these events to progress to a higher grade. Also, there may be a greater willingness by providers to initiate systemic immunosuppression due to less stringent treatment protocols in real-world clinical settings,” he said.
Using a multivariable risk prediction model for cutaneous toxicities, the researchers identified use of ipilimumab, a CTLA-4-blocking antibody, as having a protective effect for not developing a cutaneous irAE, compared with the PD-1 blocker pembrolizumab (odds ratio, 0.78; 95% confidence interval, 0.62-0.98; P < .01). But combination ICI therapy (OR, 1.53; 95% CI, 1.25-1.88; P < .001), a melanoma diagnosis (OR, 2.47; 95% CI, 2.11-2.89; P < .001), and a renal cell carcinoma diagnosis (OR, 1.65; 95% CI, 1.36-2.00; P < .001) were found to be risk factors for developing cutaneous irAEs.
“The protective effect of ipilimumab identified in the study is interesting, as historically ipilimumab has been more likely to cause cutaneous toxicities,” Dr. Semenov said. “However, we believe that the majority of this association is mediated by the melanoma, for which ipilimumab was primarily used since its introduction. Independent of this relationship, it seems to be less likely to cause cutaneous toxicity than PD-1 inhibition, according to this data.”
Based on their findings, he said, “dermatologists can utilize this information to facilitate evaluations of high-risk patients so they can take steps to prevent progression to more severe toxicities and reduce reliance or systemic immunosuppression.”
The 25% real-world incidence of cutaneous irAEs observed in the study, Dr. Semenov said, is “somewhat lower than previous clinical trial estimates of over one-third of patients presenting with cutaneous toxicities” but he added that previous estimates were based primarily on studies of patients with melanoma.
That some patients delayed presentation with these conditions “should revise clinicians’ understanding of when to expect patients to present with these toxicities, and not to rule out a delayed onset of symptoms as being unrelated to immunotherapy,” Dr. Semenov said.
Most cutaneous irAEs are ‘manageable’
In an interview, Naiara Braghiroli, MD, PhD, a dermatologist at Baptist Health’s Miami Cancer Institute, Plantation, Fla., who was not an investigator in the study, noted that over the last decade, ICIs have “revolutionized the treatment of metastatic melanoma” and, more recently, the treatment of nonmelanoma skin cancers, with regard to survival rates and side effects.
She said that the results of the study show that “most of the cutaneous side effects are manageable with very few exceptions, like the cutaneous bullous disorders and rarely, more serious reactions [such as] Stevens-Johnson syndrome.”
The majority of the side effects are treatable “and when well controlled, the patient can have a good quality of life” during treatment, she added.
For future research, Dr. Braghiroli noted, it would be interesting to know more about whether the development of any specific cutaneous reaction associated with ICIs “is associated with a higher chance of good antitumor response,” as seen with other anticancer therapies such as epidermal growth factor receptor inhibitors.
Dr. Semenov and Dr. Braghiroli report having no relevant financial disclosures.
A , according to research presented at the annual meeting of the Society for Investigative Dermatology, held virtually.
What’s more, many of the cutaneous immune-related adverse events (irAEs) from immune checkpoint inhibitors (ICIs) observed in the study may be unreported in clinical trial settings and by providers, according to one of the investigators, Yevgeniy Semenov, MD, MA, a dermatologist at Massachusetts General Hospital, Boston.
“Most cutaneous irAEs are low grade and might go unreported outside of clinical trial settings, as patients might not seek medical care, or when they do, providers might not report them in patient charts. As a result, the diagnoses identified in this study likely represent the most clinically relevant cutaneous events in the ICI population,” said Dr. Semenov, who presented the results at the meeting.
In the study, he said that one of the first issues he and his colleagues encountered was how to classify cutaneous irAEs, as they “can vary widely in morphology and severity.” Immune-related adverse events from ICIs are a “unique constellation of inflammatory toxicities,” affecting nearly every organ system, and may require treatment with immunosuppressive agents that can impact the effectiveness of the ICI. The matter is further complicated by a “lack of definitional standards of what constitutes a cutaneous immune-related adverse event, which greatly limits the research in this area,” Dr. Semenov said. There is also potential for misdiagnosis of irAEs as cutaneous eruptions occurring in patients receiving ICI therapy because of failure to account for the presence of skin disease at baseline, he pointed out.
Dr. Semenov noted that more than 40 cutaneous eruptions have been associated with ICI treatment. “Much of the observational data on cutaneous immune-related adverse events has been riddled with case reports and case series of cutaneous events that happen to be occurring in the setting of ICI therapy. These lack rigorous control groups and often associate events with little to no relationship to the actual ICI, which may have instead occurred in the setting of a competing medication,” he explained.
Real-world data
The researchers thus sought to identify the real-world incidence of cutaneous irAEs with population-level data. Using data from a national claims insurance database from January 2011 through 2019, they compared 8,637 of patients with cancer, treated with an ICI (who had not been treated with other cancer treatments within 6 months of starting an ICI) with 8,637 patients with cancer who were not treated with an ICI, matched for demographics, primary cancer type, and Charlson Comorbidity Index (CCI) score.
In both groups, the mean age of the patients was 67.5 years, 59.2% were men, and 93% had a severe CCI score. The most common cancer types were lung cancer (40%), melanoma (26.6%), and renal cell carcinoma (12.3%). The median follow-up time was 1.9 years, and the median treatment duration was 2.0 years.
Dr. Semenov and colleagues selected 42 dermatoses reported in the literature to evaluate and found an overall incidence of 25% within 2 years of starting ICI therapy. Of those 42 dermatoses, there were 10 with a significantly higher incidence among patients receiving ICIs, compared with controls: drug eruption or other nonspecific eruption (4.2%; incidence rate ratio, 5.00), bullous pemphigoid (0.3%; IRR, 4.91), maculopapular eruption (0.9%; IRR, 4.75), vitiligo (0.7%; IRR, 3.79), Grover’s disease (0.2%; IRR, 3.43), rash and other nonspecific eruption (9.0%; IRR, 2.34), mucositis (1.5%; IRR, 2.33), pruritus (4.8%; IRR, 1.92), lichen planus (0.5%; IRR, 1.75), and erythroderma (1.1%; IRR, 1.70).
After adjusting for a baseline history of squamous cell carcinoma and actinic keratosis, the researchers found that both were significantly less likely in patients receiving ICIs.
A delay in presentation of any cutaneous irAE after starting ICI therapy was also observed (a median of 16.1 weeks), which Dr. Semenov noted was longer than the 5 weeks reported in clinical trials. This delay in presentation increased to a median of 37.5 weeks for the 10 dermatoses with a significantly higher incidence among patients receiving ICIs, with 17.6% of patients presenting in the first month, 63.1% presenting by 6 months, and 84.6% presenting by 1 year.
Use of immunosuppressive treatment
The researchers also examined use of systemic immunosuppression for treating cutaneous toxicities, defined as “a new prescription for systemic glucocorticoids greater than 10 mg per day, prednisone equivalent, or nonsteroidal systemic immunosuppression,” administered within 7 days of the diagnosis of the cutaneous event. They found that 5% of patients overall received systemic immunosuppressive treatment within 7 days of a cutaneous event, which was “at the higher end of what was reported in clinical trials for the treatment of cutaneous toxicities,” Dr. Semenov noted.
“This is likely the result of the delays in diagnosis in nonclinical trial settings ... allowing more time for these events to progress to a higher grade. Also, there may be a greater willingness by providers to initiate systemic immunosuppression due to less stringent treatment protocols in real-world clinical settings,” he said.
Using a multivariable risk prediction model for cutaneous toxicities, the researchers identified use of ipilimumab, a CTLA-4-blocking antibody, as having a protective effect for not developing a cutaneous irAE, compared with the PD-1 blocker pembrolizumab (odds ratio, 0.78; 95% confidence interval, 0.62-0.98; P < .01). But combination ICI therapy (OR, 1.53; 95% CI, 1.25-1.88; P < .001), a melanoma diagnosis (OR, 2.47; 95% CI, 2.11-2.89; P < .001), and a renal cell carcinoma diagnosis (OR, 1.65; 95% CI, 1.36-2.00; P < .001) were found to be risk factors for developing cutaneous irAEs.
“The protective effect of ipilimumab identified in the study is interesting, as historically ipilimumab has been more likely to cause cutaneous toxicities,” Dr. Semenov said. “However, we believe that the majority of this association is mediated by the melanoma, for which ipilimumab was primarily used since its introduction. Independent of this relationship, it seems to be less likely to cause cutaneous toxicity than PD-1 inhibition, according to this data.”
Based on their findings, he said, “dermatologists can utilize this information to facilitate evaluations of high-risk patients so they can take steps to prevent progression to more severe toxicities and reduce reliance or systemic immunosuppression.”
The 25% real-world incidence of cutaneous irAEs observed in the study, Dr. Semenov said, is “somewhat lower than previous clinical trial estimates of over one-third of patients presenting with cutaneous toxicities” but he added that previous estimates were based primarily on studies of patients with melanoma.
That some patients delayed presentation with these conditions “should revise clinicians’ understanding of when to expect patients to present with these toxicities, and not to rule out a delayed onset of symptoms as being unrelated to immunotherapy,” Dr. Semenov said.
Most cutaneous irAEs are ‘manageable’
In an interview, Naiara Braghiroli, MD, PhD, a dermatologist at Baptist Health’s Miami Cancer Institute, Plantation, Fla., who was not an investigator in the study, noted that over the last decade, ICIs have “revolutionized the treatment of metastatic melanoma” and, more recently, the treatment of nonmelanoma skin cancers, with regard to survival rates and side effects.
She said that the results of the study show that “most of the cutaneous side effects are manageable with very few exceptions, like the cutaneous bullous disorders and rarely, more serious reactions [such as] Stevens-Johnson syndrome.”
The majority of the side effects are treatable “and when well controlled, the patient can have a good quality of life” during treatment, she added.
For future research, Dr. Braghiroli noted, it would be interesting to know more about whether the development of any specific cutaneous reaction associated with ICIs “is associated with a higher chance of good antitumor response,” as seen with other anticancer therapies such as epidermal growth factor receptor inhibitors.
Dr. Semenov and Dr. Braghiroli report having no relevant financial disclosures.
FROM SID 2021
Baricitinib found effective for moderate to severe AD out to 52 weeks
“With long-term therapy, the baricitinib 2 mg response remains stable or slightly improved, compared with week 16 for skin inflammation, itch, sleep, and quality of life,” presenting study author Eric L. Simpson, MD, said during the Revolutionizing Atopic Dermatitis symposium.
Baricitinib is an oral selective Janus kinase 1/JAK2 inhibitor being developed for the treatment of moderate to severe AD in adults who are candidates for systemic therapy. The drug is already approved for AD in Europe at the 2-mg and 4-mg doses. A 16-week placebo-controlled study conducted in North America known as BREEZE-AD5 found that 2 mg of baricitinib improved disease in adults with moderate to severe AD.
For the current analysis, Dr. Simpson, professor of dermatology at Oregon Health and Science University, Portland, and colleagues integrated data from BREEZE-AD5 and BREEZE-AD6, an ongoing, open-label study of BREEZE-AD5, to evaluate the long-term efficacy and safety of baricitinib 2 mg in patients with moderate to severe AD.
At week 16, patients from BREEZE-AD5 who were on baricitinib 2 mg could either continue the trial out to week 52, or they could transition to BREEZE-AD6 if they were nonresponders. The use of low-potency corticosteroids was permitted after week 16 in BREEZE-AD5 and throughout BREEZE-AD6. Endpoints of interest at week 52 in both trials were the proportions of patients with 75% or greater improvement from baseline in the Eczema and Severity Index (EASI75), a Validated Investigator Global Assessment for Atopic Dermatitis (vIGA-AD) score of 0 or 1, a Dermatology Life Quality Index (DLQI) score of 5 or less, as well as mean SCORing AD (SCORAD) visual analog scales of itch and sleeplessness scores, and the mean percent change from baseline in EASI score.
Dr. Simpson presented data on 146 patients from both trials who were randomized to baricitinib 2 mg. Their mean age was 40 years, 53% were female, 58% were White, 21% were Black, 15% were Asian, and the remainder were from other backgrounds. Their mean duration of AD was 16 years and their average EASI score was 26.6. At weeks 16, 32, and 52, the proportion of patients who achieved an EASI75 response was 40%, 51%, and 49%, respectively, while the mean percent change from baseline in EASI score was –50%, –59%, and –57%.
At weeks 16, 32, and 52, the vIGA-AD responses of 0 or 1 were observed in 27%, 38%, and 31% of patients. The mean SCORAD pruritus score improved from 7.7 at baseline to 4.8 at week 16 and was maintained at weeks 32 (3.8) and 52 (4.3). The mean SCORAD sleeplessness score also improved from 6.5 at baseline to 3.9 at week 16 and remained stable through weeks 32 (3.4) and 52 (3.7).
Finally, among 129 patients who had a baseline DLQI of greater than 5, 39% had DLQI scores of 5 or lower at week 16, compared with 49% at week 32 and 45% at week 52, indicating a small or no effect of AD on quality of life.
The study was sponsored by Eli Lilly, which is developing baricitinib. Dr. Simpson disclosed that he is a consultant to and/or an investigator for several pharmaceutical companies, including Eli Lilly.
“With long-term therapy, the baricitinib 2 mg response remains stable or slightly improved, compared with week 16 for skin inflammation, itch, sleep, and quality of life,” presenting study author Eric L. Simpson, MD, said during the Revolutionizing Atopic Dermatitis symposium.
Baricitinib is an oral selective Janus kinase 1/JAK2 inhibitor being developed for the treatment of moderate to severe AD in adults who are candidates for systemic therapy. The drug is already approved for AD in Europe at the 2-mg and 4-mg doses. A 16-week placebo-controlled study conducted in North America known as BREEZE-AD5 found that 2 mg of baricitinib improved disease in adults with moderate to severe AD.
For the current analysis, Dr. Simpson, professor of dermatology at Oregon Health and Science University, Portland, and colleagues integrated data from BREEZE-AD5 and BREEZE-AD6, an ongoing, open-label study of BREEZE-AD5, to evaluate the long-term efficacy and safety of baricitinib 2 mg in patients with moderate to severe AD.
At week 16, patients from BREEZE-AD5 who were on baricitinib 2 mg could either continue the trial out to week 52, or they could transition to BREEZE-AD6 if they were nonresponders. The use of low-potency corticosteroids was permitted after week 16 in BREEZE-AD5 and throughout BREEZE-AD6. Endpoints of interest at week 52 in both trials were the proportions of patients with 75% or greater improvement from baseline in the Eczema and Severity Index (EASI75), a Validated Investigator Global Assessment for Atopic Dermatitis (vIGA-AD) score of 0 or 1, a Dermatology Life Quality Index (DLQI) score of 5 or less, as well as mean SCORing AD (SCORAD) visual analog scales of itch and sleeplessness scores, and the mean percent change from baseline in EASI score.
Dr. Simpson presented data on 146 patients from both trials who were randomized to baricitinib 2 mg. Their mean age was 40 years, 53% were female, 58% were White, 21% were Black, 15% were Asian, and the remainder were from other backgrounds. Their mean duration of AD was 16 years and their average EASI score was 26.6. At weeks 16, 32, and 52, the proportion of patients who achieved an EASI75 response was 40%, 51%, and 49%, respectively, while the mean percent change from baseline in EASI score was –50%, –59%, and –57%.
At weeks 16, 32, and 52, the vIGA-AD responses of 0 or 1 were observed in 27%, 38%, and 31% of patients. The mean SCORAD pruritus score improved from 7.7 at baseline to 4.8 at week 16 and was maintained at weeks 32 (3.8) and 52 (4.3). The mean SCORAD sleeplessness score also improved from 6.5 at baseline to 3.9 at week 16 and remained stable through weeks 32 (3.4) and 52 (3.7).
Finally, among 129 patients who had a baseline DLQI of greater than 5, 39% had DLQI scores of 5 or lower at week 16, compared with 49% at week 32 and 45% at week 52, indicating a small or no effect of AD on quality of life.
The study was sponsored by Eli Lilly, which is developing baricitinib. Dr. Simpson disclosed that he is a consultant to and/or an investigator for several pharmaceutical companies, including Eli Lilly.
“With long-term therapy, the baricitinib 2 mg response remains stable or slightly improved, compared with week 16 for skin inflammation, itch, sleep, and quality of life,” presenting study author Eric L. Simpson, MD, said during the Revolutionizing Atopic Dermatitis symposium.
Baricitinib is an oral selective Janus kinase 1/JAK2 inhibitor being developed for the treatment of moderate to severe AD in adults who are candidates for systemic therapy. The drug is already approved for AD in Europe at the 2-mg and 4-mg doses. A 16-week placebo-controlled study conducted in North America known as BREEZE-AD5 found that 2 mg of baricitinib improved disease in adults with moderate to severe AD.
For the current analysis, Dr. Simpson, professor of dermatology at Oregon Health and Science University, Portland, and colleagues integrated data from BREEZE-AD5 and BREEZE-AD6, an ongoing, open-label study of BREEZE-AD5, to evaluate the long-term efficacy and safety of baricitinib 2 mg in patients with moderate to severe AD.
At week 16, patients from BREEZE-AD5 who were on baricitinib 2 mg could either continue the trial out to week 52, or they could transition to BREEZE-AD6 if they were nonresponders. The use of low-potency corticosteroids was permitted after week 16 in BREEZE-AD5 and throughout BREEZE-AD6. Endpoints of interest at week 52 in both trials were the proportions of patients with 75% or greater improvement from baseline in the Eczema and Severity Index (EASI75), a Validated Investigator Global Assessment for Atopic Dermatitis (vIGA-AD) score of 0 or 1, a Dermatology Life Quality Index (DLQI) score of 5 or less, as well as mean SCORing AD (SCORAD) visual analog scales of itch and sleeplessness scores, and the mean percent change from baseline in EASI score.
Dr. Simpson presented data on 146 patients from both trials who were randomized to baricitinib 2 mg. Their mean age was 40 years, 53% were female, 58% were White, 21% were Black, 15% were Asian, and the remainder were from other backgrounds. Their mean duration of AD was 16 years and their average EASI score was 26.6. At weeks 16, 32, and 52, the proportion of patients who achieved an EASI75 response was 40%, 51%, and 49%, respectively, while the mean percent change from baseline in EASI score was –50%, –59%, and –57%.
At weeks 16, 32, and 52, the vIGA-AD responses of 0 or 1 were observed in 27%, 38%, and 31% of patients. The mean SCORAD pruritus score improved from 7.7 at baseline to 4.8 at week 16 and was maintained at weeks 32 (3.8) and 52 (4.3). The mean SCORAD sleeplessness score also improved from 6.5 at baseline to 3.9 at week 16 and remained stable through weeks 32 (3.4) and 52 (3.7).
Finally, among 129 patients who had a baseline DLQI of greater than 5, 39% had DLQI scores of 5 or lower at week 16, compared with 49% at week 32 and 45% at week 52, indicating a small or no effect of AD on quality of life.
The study was sponsored by Eli Lilly, which is developing baricitinib. Dr. Simpson disclosed that he is a consultant to and/or an investigator for several pharmaceutical companies, including Eli Lilly.
FROM REVOLUTIONIZING AD 2021
EC approves cemiplimab for advanced or metastatic BCC after HHI therapy
The
The programmed death-1 (PD-1) inhibitor, which is being jointly developed by Regeneron and Sanofi under a global collaboration agreement, was approved by the Food and Drug Administration for this indication in the United States in February; the FDA granted full approval for its use in patients with locally advanced BCC and accelerated approval for use in patients with metastatic BCC.
The EC’s thumbs-up for cemiplimab as a treatment for BCC marks the third such approval for an advanced cancer in the European Union: The immunotherapy was concurrently approved by the EC for the first-line treatment of adults with advanced non–small cell lung cancer (NSCLC) whose tumor cells have ≥ 50% PD-L1 expression and no EGFR, ALK or ROS1 aberrations, and was approved in 2019 for the treatment of adults with metastatic or locally advanced cutaneous squamous cell carcinoma (CSCC) who are not candidates for curative surgery or curative radiation.
The FDA granted approval of cemiplimab for NSCLC in February, and for CSCC in 2018.
The latest BCC approval is based on data from an ongoing, open-label, prospective phase 2 clinical trial of 119 patients with advanced BCC who were previously treated with an HHI. The objective response rates in cemiplimab-treated patients were 32% (partial responses in 25%; complete responses in 7%) in those with locally advanced BCC, and 29% (partial responses in 26%; complete responses in 3%) in those with metastatic BCC.
About 90% of all patients had a duration of response (DOR) of 6 months or longer. Median DOR was not reached in either group at median follow-up of 16 months for locally advanced BCC and 9 months for metastatic BCC.
The safety profile of cemiplimab has been generally consistent across approved indications. Serious adverse events have been reported in 30% of 816 patients from all four cemiplimab monotherapy pivotal trials, and these led to permanent discontinuation of treatment in 8% of patients.
Immune-related adverse reactions occurred in 22% of patients, and led to permanent discontinuation in 4%. The most common such reactions were hypothyroidism (8%), hyperthyroidism (3%), pneumonitis (3%), hepatitis (2%), colitis (2%) and immune-related skin adverse reactions (2%).
Cemiplimab is administered by intravenous infusion over 30 minutes every 3 weeks until disease progression or unacceptable toxicity. The recommended dose is 350 mg.
A press release from Regeneron notes that research efforts with respect to cemiplimab – both as monotherapy and in combination with other agents – are focused on difficult-to-treat cancers, including advanced NSCLC, cervical cancer, and other solid tumors and blood cancers.
The
The programmed death-1 (PD-1) inhibitor, which is being jointly developed by Regeneron and Sanofi under a global collaboration agreement, was approved by the Food and Drug Administration for this indication in the United States in February; the FDA granted full approval for its use in patients with locally advanced BCC and accelerated approval for use in patients with metastatic BCC.
The EC’s thumbs-up for cemiplimab as a treatment for BCC marks the third such approval for an advanced cancer in the European Union: The immunotherapy was concurrently approved by the EC for the first-line treatment of adults with advanced non–small cell lung cancer (NSCLC) whose tumor cells have ≥ 50% PD-L1 expression and no EGFR, ALK or ROS1 aberrations, and was approved in 2019 for the treatment of adults with metastatic or locally advanced cutaneous squamous cell carcinoma (CSCC) who are not candidates for curative surgery or curative radiation.
The FDA granted approval of cemiplimab for NSCLC in February, and for CSCC in 2018.
The latest BCC approval is based on data from an ongoing, open-label, prospective phase 2 clinical trial of 119 patients with advanced BCC who were previously treated with an HHI. The objective response rates in cemiplimab-treated patients were 32% (partial responses in 25%; complete responses in 7%) in those with locally advanced BCC, and 29% (partial responses in 26%; complete responses in 3%) in those with metastatic BCC.
About 90% of all patients had a duration of response (DOR) of 6 months or longer. Median DOR was not reached in either group at median follow-up of 16 months for locally advanced BCC and 9 months for metastatic BCC.
The safety profile of cemiplimab has been generally consistent across approved indications. Serious adverse events have been reported in 30% of 816 patients from all four cemiplimab monotherapy pivotal trials, and these led to permanent discontinuation of treatment in 8% of patients.
Immune-related adverse reactions occurred in 22% of patients, and led to permanent discontinuation in 4%. The most common such reactions were hypothyroidism (8%), hyperthyroidism (3%), pneumonitis (3%), hepatitis (2%), colitis (2%) and immune-related skin adverse reactions (2%).
Cemiplimab is administered by intravenous infusion over 30 minutes every 3 weeks until disease progression or unacceptable toxicity. The recommended dose is 350 mg.
A press release from Regeneron notes that research efforts with respect to cemiplimab – both as monotherapy and in combination with other agents – are focused on difficult-to-treat cancers, including advanced NSCLC, cervical cancer, and other solid tumors and blood cancers.
The
The programmed death-1 (PD-1) inhibitor, which is being jointly developed by Regeneron and Sanofi under a global collaboration agreement, was approved by the Food and Drug Administration for this indication in the United States in February; the FDA granted full approval for its use in patients with locally advanced BCC and accelerated approval for use in patients with metastatic BCC.
The EC’s thumbs-up for cemiplimab as a treatment for BCC marks the third such approval for an advanced cancer in the European Union: The immunotherapy was concurrently approved by the EC for the first-line treatment of adults with advanced non–small cell lung cancer (NSCLC) whose tumor cells have ≥ 50% PD-L1 expression and no EGFR, ALK or ROS1 aberrations, and was approved in 2019 for the treatment of adults with metastatic or locally advanced cutaneous squamous cell carcinoma (CSCC) who are not candidates for curative surgery or curative radiation.
The FDA granted approval of cemiplimab for NSCLC in February, and for CSCC in 2018.
The latest BCC approval is based on data from an ongoing, open-label, prospective phase 2 clinical trial of 119 patients with advanced BCC who were previously treated with an HHI. The objective response rates in cemiplimab-treated patients were 32% (partial responses in 25%; complete responses in 7%) in those with locally advanced BCC, and 29% (partial responses in 26%; complete responses in 3%) in those with metastatic BCC.
About 90% of all patients had a duration of response (DOR) of 6 months or longer. Median DOR was not reached in either group at median follow-up of 16 months for locally advanced BCC and 9 months for metastatic BCC.
The safety profile of cemiplimab has been generally consistent across approved indications. Serious adverse events have been reported in 30% of 816 patients from all four cemiplimab monotherapy pivotal trials, and these led to permanent discontinuation of treatment in 8% of patients.
Immune-related adverse reactions occurred in 22% of patients, and led to permanent discontinuation in 4%. The most common such reactions were hypothyroidism (8%), hyperthyroidism (3%), pneumonitis (3%), hepatitis (2%), colitis (2%) and immune-related skin adverse reactions (2%).
Cemiplimab is administered by intravenous infusion over 30 minutes every 3 weeks until disease progression or unacceptable toxicity. The recommended dose is 350 mg.
A press release from Regeneron notes that research efforts with respect to cemiplimab – both as monotherapy and in combination with other agents – are focused on difficult-to-treat cancers, including advanced NSCLC, cervical cancer, and other solid tumors and blood cancers.
Type 1 diabetes amputation rates fall in Sweden, rise in U.S.
according to a national registry analysis.
The incidence of any amputation trended downward from 2011 to 2019, Sara Hallström, MD, reported at the annual scientific sessions of the American Diabetes Association.
Levels of hemoglobin A1c have also trended downward over time in Sweden among those with type 1 diabetes, while renal function has remained stable among patients who did not undergo amputations, Dr. Hallström said in a virtual presentation.
“Observing stable renal function and decreasing levels of [hemoglobin] A1c, along with decreasing incidence of amputation, indicates a shift in the prognosis of persons with type 1 diabetes,” she said.
Drilling down on amputation risk in type 1 diabetes
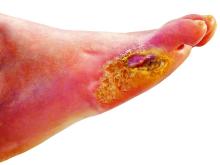
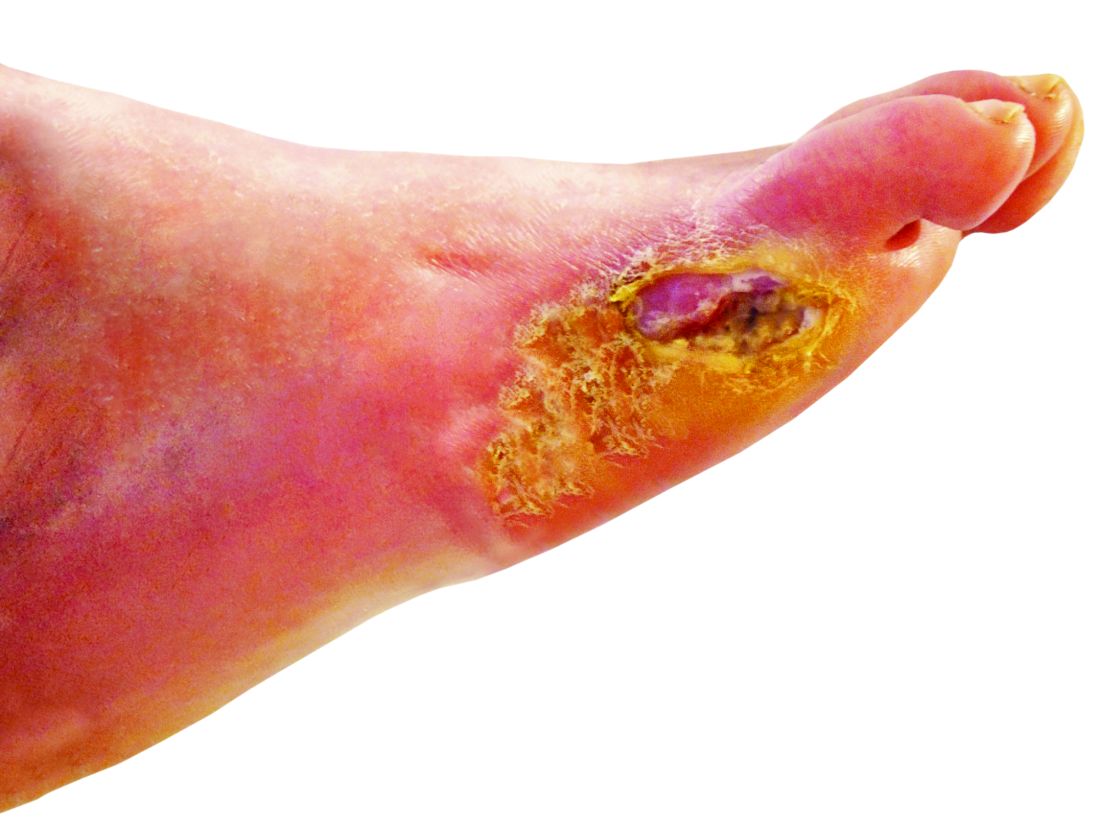
Lower-extremity amputation is a major source of disability and distress in people with diabetes, and also poses a significant financial burden for the health care system, according to Dr. Hallström of Sahlgrenska University Hospital and the University of Gothenburg (Sweden).
“Limb loss due to amputation is not seldom a final outcome of diabetic foot ulcers,” she said in the presentation.
Most studies of amputation incidence and risk factors have grouped patients with different types of diabetes, though a few recent studies have singled out type 1 diabetes.
Among these is a 2019 study indicating a 40-fold higher risk of amputation among individuals with type 1 diabetes, compared with the general population, based on analysis of Swedish National Diabetes Register data from 1998 to 2013.
Trends over time
In the present study, Dr. Hallström and coinvestigators queried that same Swedish registry and identified 46,008 individuals with type 1 diabetes from 1998 to 2019. The mean age was 32.5 years and 55% were male. Overall, 1,519 of these individuals (3.3%) underwent amputation.
The incidence of any amputation fluctuated from 1998 to 2011, followed by a “decreasing trend over time” from 2011 to 2019, Dr. Hallström said.
The incidence of amputation per 1,000 patient-years was 2.84 in the earliest time period of 1998-2001, decreasing to 1.64 in 2017-2019.
Levels of A1c decreased over time, starting at 2012, both in participants with and without amputations, Dr. Hallström said. Renal function over that period remained stable in persons without amputation, and showed a decreasing trend in persons with amputation.
Compared with individuals with no amputations, those undergoing amputation were older (50 years vs. 32 years), had a longer duration of diabetes (34.9 years vs. 16.5 years), and had higher mean A1c, Dr. Hellström said. The amputee group also included a higher proportion of smokers, at 19.4% versus 14.0%, data show.
Risk factors for amputation included renal dysfunction, hyperglycemia, older age, smoking, hypertension, and cardiovascular comorbidities, according to the researcher.
U.S. amputations on the rise overall
While authors say results of this study point to a potentially improved prognosis for individuals with type 1 diabetes in Sweden, Robert A. Gabbay, MD, PhD, chief scientific and medical officer of the ADA, said amputation rates remains “concerning” based on U.S. data focused largely on type 2 diabetes.
“The amputation rate is unfortunately rising,” he said. “Sadly, this continues to be an issue.”
Significant health disparities persist, he added, with Black Americans having two- to threefold higher rates of amputations.
To help reduce amputation rates, clinicians should be asking patient about claudication and using simple screening techniques such as inspecting patient’s feet. “The big deal here is preventing ulcer formation, because once the ulcer forms, it often doesn’t heal, and it’s a downward spiral,” he said.
In addition, recent research suggests seeking a second opinion may help: “Many of those amputations could be avoided, in part because people aren’t aware of some of the treatments that can open up the arteries and reestablish blood flow,” he added.
Dr. Hallström reported no conflicts of interest. One coauthor on the study provided disclosures related to Abbott, AstraZeneca, Boehringer Ingelheim, Lilly Diabetes, and Novo Nordisk.
according to a national registry analysis.
The incidence of any amputation trended downward from 2011 to 2019, Sara Hallström, MD, reported at the annual scientific sessions of the American Diabetes Association.
Levels of hemoglobin A1c have also trended downward over time in Sweden among those with type 1 diabetes, while renal function has remained stable among patients who did not undergo amputations, Dr. Hallström said in a virtual presentation.
“Observing stable renal function and decreasing levels of [hemoglobin] A1c, along with decreasing incidence of amputation, indicates a shift in the prognosis of persons with type 1 diabetes,” she said.
Drilling down on amputation risk in type 1 diabetes
Lower-extremity amputation is a major source of disability and distress in people with diabetes, and also poses a significant financial burden for the health care system, according to Dr. Hallström of Sahlgrenska University Hospital and the University of Gothenburg (Sweden).
“Limb loss due to amputation is not seldom a final outcome of diabetic foot ulcers,” she said in the presentation.
Most studies of amputation incidence and risk factors have grouped patients with different types of diabetes, though a few recent studies have singled out type 1 diabetes.
Among these is a 2019 study indicating a 40-fold higher risk of amputation among individuals with type 1 diabetes, compared with the general population, based on analysis of Swedish National Diabetes Register data from 1998 to 2013.
Trends over time
In the present study, Dr. Hallström and coinvestigators queried that same Swedish registry and identified 46,008 individuals with type 1 diabetes from 1998 to 2019. The mean age was 32.5 years and 55% were male. Overall, 1,519 of these individuals (3.3%) underwent amputation.
The incidence of any amputation fluctuated from 1998 to 2011, followed by a “decreasing trend over time” from 2011 to 2019, Dr. Hallström said.
The incidence of amputation per 1,000 patient-years was 2.84 in the earliest time period of 1998-2001, decreasing to 1.64 in 2017-2019.
Levels of A1c decreased over time, starting at 2012, both in participants with and without amputations, Dr. Hallström said. Renal function over that period remained stable in persons without amputation, and showed a decreasing trend in persons with amputation.
Compared with individuals with no amputations, those undergoing amputation were older (50 years vs. 32 years), had a longer duration of diabetes (34.9 years vs. 16.5 years), and had higher mean A1c, Dr. Hellström said. The amputee group also included a higher proportion of smokers, at 19.4% versus 14.0%, data show.
Risk factors for amputation included renal dysfunction, hyperglycemia, older age, smoking, hypertension, and cardiovascular comorbidities, according to the researcher.
U.S. amputations on the rise overall
While authors say results of this study point to a potentially improved prognosis for individuals with type 1 diabetes in Sweden, Robert A. Gabbay, MD, PhD, chief scientific and medical officer of the ADA, said amputation rates remains “concerning” based on U.S. data focused largely on type 2 diabetes.
“The amputation rate is unfortunately rising,” he said. “Sadly, this continues to be an issue.”
Significant health disparities persist, he added, with Black Americans having two- to threefold higher rates of amputations.
To help reduce amputation rates, clinicians should be asking patient about claudication and using simple screening techniques such as inspecting patient’s feet. “The big deal here is preventing ulcer formation, because once the ulcer forms, it often doesn’t heal, and it’s a downward spiral,” he said.
In addition, recent research suggests seeking a second opinion may help: “Many of those amputations could be avoided, in part because people aren’t aware of some of the treatments that can open up the arteries and reestablish blood flow,” he added.
Dr. Hallström reported no conflicts of interest. One coauthor on the study provided disclosures related to Abbott, AstraZeneca, Boehringer Ingelheim, Lilly Diabetes, and Novo Nordisk.
according to a national registry analysis.
The incidence of any amputation trended downward from 2011 to 2019, Sara Hallström, MD, reported at the annual scientific sessions of the American Diabetes Association.
Levels of hemoglobin A1c have also trended downward over time in Sweden among those with type 1 diabetes, while renal function has remained stable among patients who did not undergo amputations, Dr. Hallström said in a virtual presentation.
“Observing stable renal function and decreasing levels of [hemoglobin] A1c, along with decreasing incidence of amputation, indicates a shift in the prognosis of persons with type 1 diabetes,” she said.
Drilling down on amputation risk in type 1 diabetes
Lower-extremity amputation is a major source of disability and distress in people with diabetes, and also poses a significant financial burden for the health care system, according to Dr. Hallström of Sahlgrenska University Hospital and the University of Gothenburg (Sweden).
“Limb loss due to amputation is not seldom a final outcome of diabetic foot ulcers,” she said in the presentation.
Most studies of amputation incidence and risk factors have grouped patients with different types of diabetes, though a few recent studies have singled out type 1 diabetes.
Among these is a 2019 study indicating a 40-fold higher risk of amputation among individuals with type 1 diabetes, compared with the general population, based on analysis of Swedish National Diabetes Register data from 1998 to 2013.
Trends over time
In the present study, Dr. Hallström and coinvestigators queried that same Swedish registry and identified 46,008 individuals with type 1 diabetes from 1998 to 2019. The mean age was 32.5 years and 55% were male. Overall, 1,519 of these individuals (3.3%) underwent amputation.
The incidence of any amputation fluctuated from 1998 to 2011, followed by a “decreasing trend over time” from 2011 to 2019, Dr. Hallström said.
The incidence of amputation per 1,000 patient-years was 2.84 in the earliest time period of 1998-2001, decreasing to 1.64 in 2017-2019.
Levels of A1c decreased over time, starting at 2012, both in participants with and without amputations, Dr. Hallström said. Renal function over that period remained stable in persons without amputation, and showed a decreasing trend in persons with amputation.
Compared with individuals with no amputations, those undergoing amputation were older (50 years vs. 32 years), had a longer duration of diabetes (34.9 years vs. 16.5 years), and had higher mean A1c, Dr. Hellström said. The amputee group also included a higher proportion of smokers, at 19.4% versus 14.0%, data show.
Risk factors for amputation included renal dysfunction, hyperglycemia, older age, smoking, hypertension, and cardiovascular comorbidities, according to the researcher.
U.S. amputations on the rise overall
While authors say results of this study point to a potentially improved prognosis for individuals with type 1 diabetes in Sweden, Robert A. Gabbay, MD, PhD, chief scientific and medical officer of the ADA, said amputation rates remains “concerning” based on U.S. data focused largely on type 2 diabetes.
“The amputation rate is unfortunately rising,” he said. “Sadly, this continues to be an issue.”
Significant health disparities persist, he added, with Black Americans having two- to threefold higher rates of amputations.
To help reduce amputation rates, clinicians should be asking patient about claudication and using simple screening techniques such as inspecting patient’s feet. “The big deal here is preventing ulcer formation, because once the ulcer forms, it often doesn’t heal, and it’s a downward spiral,” he said.
In addition, recent research suggests seeking a second opinion may help: “Many of those amputations could be avoided, in part because people aren’t aware of some of the treatments that can open up the arteries and reestablish blood flow,” he added.
Dr. Hallström reported no conflicts of interest. One coauthor on the study provided disclosures related to Abbott, AstraZeneca, Boehringer Ingelheim, Lilly Diabetes, and Novo Nordisk.
FROM ADA 2020
In Black patients, acne scarring might not mean what you think
Treating the needs of patients of color requires an understanding of differences that may not be readily apparent, a dermatologist told colleagues. For example, of the term that may be misinterpreted in the doctor’s office.
“Scarring is not usually what they’re talking about, although they may have some of that as well. They’re [typically] talking about what we know as postinflammatory hyperpigmentation, not scarring. So right away, you have to clarify,” Amy McMichael, MD, professor and chair of dermatology at Wake Forest Baptist Medical Center in Winston-Salem, N.C., said in a presentation at the Inaugural Symposium for Inflammatory Skin Disease. “When you’re talking about scarring, do you mean the dark spots? What exactly are you concerned about?”
Dr. McMichael highlighted a 2014 study that reported the results of a survey of 208 women (51% were White; 49% were non-White), which included 51 Black, 23 Hispanic, and 16 Asian women aged 25-45 (mean age, 35) with 25 or more lesions. White women were more troubled by facial acne than were women of color (89% vs. 76%, respectively, P < .05), and they were more likely to say lesion clearance was most important to them (58% vs. 32%, respectively, P < .001).
Meanwhile, non-White women were much more likely than were White women to say that clearance of postinflammatory hyperpigmentation was most important to them (42% vs. 8%, respectively, P < .0001).
“Seventy percent of [non-White women] felt that their race and ethnicity required targeted attention [in treatment], and two-thirds desired acne treatment that was designed to meet the needs of their skin type,” Dr. McMichael said. “If you don’t address the issues, if you don’t talk about the pigmentation with them or explain how you’re going to address it, people don’t feel heard. They don’t feel like they’ve really seen a dermatologist who understands their needs.”
She added that it’s crucial to ask about over-the-counter products. “If you don’t discuss them, they’ll assume that what they’re doing is okay.” She warns her patients against using and exposing their skin and face to cocoa butter and oils such as tea tree oil.
Research has suggested that among people of color, Blacks and Hispanics are most likely to experience dyspigmentation and scarring, Dr. McMichael said. She advised colleagues to be aware of pomade acne in these two groups of patients. Pomade acne appears along the hair line and is caused by the use of hair products. She also cautioned about acne cosmetica, which can be triggered by products such as makeup, used to cover up acne and postinflammatory hyperpigmentation.
As for acne treatments, Dr. McMichael highlighted a long list of familiar topical and oral agents and procedural options. Less familiar strategies include laser and light-based therapies, she said.
As for up-and-coming options, she pointed to topical minocycline, “which allows us to use an anti-inflammatory agent topically rather than orally when we’re trying to get away from using a lot of oral antibiotics.”
Also consider whether female patients have polycystic ovary syndrome, she said. “Then you might consider spironolactone. I certainly use a lot more of that these days to try to avoid long-term oral antibiotics.”
She recommended earlier use of isotretinoin in patients overall, and she urged colleagues to proceed with their standard retinoid approaches. However, she noted that she lets patients know that she’ll focus first on treating the acne itself and then work on the dark spots in later treatments. “If you give people a bleaching agent in the beginning, they’re going to stop using their main products, and they’re going to chase those dark spots. That’s just something that they can’t help doing.”
Dr. McMichael disclosed investigator and consultant relationships with multiple drug makers.
Treating the needs of patients of color requires an understanding of differences that may not be readily apparent, a dermatologist told colleagues. For example, of the term that may be misinterpreted in the doctor’s office.
“Scarring is not usually what they’re talking about, although they may have some of that as well. They’re [typically] talking about what we know as postinflammatory hyperpigmentation, not scarring. So right away, you have to clarify,” Amy McMichael, MD, professor and chair of dermatology at Wake Forest Baptist Medical Center in Winston-Salem, N.C., said in a presentation at the Inaugural Symposium for Inflammatory Skin Disease. “When you’re talking about scarring, do you mean the dark spots? What exactly are you concerned about?”
Dr. McMichael highlighted a 2014 study that reported the results of a survey of 208 women (51% were White; 49% were non-White), which included 51 Black, 23 Hispanic, and 16 Asian women aged 25-45 (mean age, 35) with 25 or more lesions. White women were more troubled by facial acne than were women of color (89% vs. 76%, respectively, P < .05), and they were more likely to say lesion clearance was most important to them (58% vs. 32%, respectively, P < .001).
Meanwhile, non-White women were much more likely than were White women to say that clearance of postinflammatory hyperpigmentation was most important to them (42% vs. 8%, respectively, P < .0001).
“Seventy percent of [non-White women] felt that their race and ethnicity required targeted attention [in treatment], and two-thirds desired acne treatment that was designed to meet the needs of their skin type,” Dr. McMichael said. “If you don’t address the issues, if you don’t talk about the pigmentation with them or explain how you’re going to address it, people don’t feel heard. They don’t feel like they’ve really seen a dermatologist who understands their needs.”
She added that it’s crucial to ask about over-the-counter products. “If you don’t discuss them, they’ll assume that what they’re doing is okay.” She warns her patients against using and exposing their skin and face to cocoa butter and oils such as tea tree oil.
Research has suggested that among people of color, Blacks and Hispanics are most likely to experience dyspigmentation and scarring, Dr. McMichael said. She advised colleagues to be aware of pomade acne in these two groups of patients. Pomade acne appears along the hair line and is caused by the use of hair products. She also cautioned about acne cosmetica, which can be triggered by products such as makeup, used to cover up acne and postinflammatory hyperpigmentation.
As for acne treatments, Dr. McMichael highlighted a long list of familiar topical and oral agents and procedural options. Less familiar strategies include laser and light-based therapies, she said.
As for up-and-coming options, she pointed to topical minocycline, “which allows us to use an anti-inflammatory agent topically rather than orally when we’re trying to get away from using a lot of oral antibiotics.”
Also consider whether female patients have polycystic ovary syndrome, she said. “Then you might consider spironolactone. I certainly use a lot more of that these days to try to avoid long-term oral antibiotics.”
She recommended earlier use of isotretinoin in patients overall, and she urged colleagues to proceed with their standard retinoid approaches. However, she noted that she lets patients know that she’ll focus first on treating the acne itself and then work on the dark spots in later treatments. “If you give people a bleaching agent in the beginning, they’re going to stop using their main products, and they’re going to chase those dark spots. That’s just something that they can’t help doing.”
Dr. McMichael disclosed investigator and consultant relationships with multiple drug makers.
Treating the needs of patients of color requires an understanding of differences that may not be readily apparent, a dermatologist told colleagues. For example, of the term that may be misinterpreted in the doctor’s office.
“Scarring is not usually what they’re talking about, although they may have some of that as well. They’re [typically] talking about what we know as postinflammatory hyperpigmentation, not scarring. So right away, you have to clarify,” Amy McMichael, MD, professor and chair of dermatology at Wake Forest Baptist Medical Center in Winston-Salem, N.C., said in a presentation at the Inaugural Symposium for Inflammatory Skin Disease. “When you’re talking about scarring, do you mean the dark spots? What exactly are you concerned about?”
Dr. McMichael highlighted a 2014 study that reported the results of a survey of 208 women (51% were White; 49% were non-White), which included 51 Black, 23 Hispanic, and 16 Asian women aged 25-45 (mean age, 35) with 25 or more lesions. White women were more troubled by facial acne than were women of color (89% vs. 76%, respectively, P < .05), and they were more likely to say lesion clearance was most important to them (58% vs. 32%, respectively, P < .001).
Meanwhile, non-White women were much more likely than were White women to say that clearance of postinflammatory hyperpigmentation was most important to them (42% vs. 8%, respectively, P < .0001).
“Seventy percent of [non-White women] felt that their race and ethnicity required targeted attention [in treatment], and two-thirds desired acne treatment that was designed to meet the needs of their skin type,” Dr. McMichael said. “If you don’t address the issues, if you don’t talk about the pigmentation with them or explain how you’re going to address it, people don’t feel heard. They don’t feel like they’ve really seen a dermatologist who understands their needs.”
She added that it’s crucial to ask about over-the-counter products. “If you don’t discuss them, they’ll assume that what they’re doing is okay.” She warns her patients against using and exposing their skin and face to cocoa butter and oils such as tea tree oil.
Research has suggested that among people of color, Blacks and Hispanics are most likely to experience dyspigmentation and scarring, Dr. McMichael said. She advised colleagues to be aware of pomade acne in these two groups of patients. Pomade acne appears along the hair line and is caused by the use of hair products. She also cautioned about acne cosmetica, which can be triggered by products such as makeup, used to cover up acne and postinflammatory hyperpigmentation.
As for acne treatments, Dr. McMichael highlighted a long list of familiar topical and oral agents and procedural options. Less familiar strategies include laser and light-based therapies, she said.
As for up-and-coming options, she pointed to topical minocycline, “which allows us to use an anti-inflammatory agent topically rather than orally when we’re trying to get away from using a lot of oral antibiotics.”
Also consider whether female patients have polycystic ovary syndrome, she said. “Then you might consider spironolactone. I certainly use a lot more of that these days to try to avoid long-term oral antibiotics.”
She recommended earlier use of isotretinoin in patients overall, and she urged colleagues to proceed with their standard retinoid approaches. However, she noted that she lets patients know that she’ll focus first on treating the acne itself and then work on the dark spots in later treatments. “If you give people a bleaching agent in the beginning, they’re going to stop using their main products, and they’re going to chase those dark spots. That’s just something that they can’t help doing.”
Dr. McMichael disclosed investigator and consultant relationships with multiple drug makers.
FROM SISD 2021
Ruxolitinib cream for atopic dermatitis found to be effective, safe up to 52 weeks
results from a long-term analysis of clinical trial data showed.
“The incidence of application-site reactions was low, and there were no clinically meaningful changes or trends in hematologic parameters,” Kim Papp, MD, PhD, said during the Revolutionizing Atopic Dermatitis symposium.
Ruxolitinib cream is a selective Janus kinase 1/JAK2 inhibitor being developed by Incyte for the treatment of atopic dermatitis (AD).
According to a press release from the company, the Food and Drug Administration has extended the New Drug Application review period for the agent by 3 months to September 2021. If approved, it would become first topical JAK inhibitor for use in dermatology.
In two phase 3, randomized studies of identical design involving 1,249 patients aged 12 and older with AD – TRuE-AD1 and TRuE-AD2 – ruxolitinib cream demonstrated anti-inflammatory activity, with rapid and sustained antipruritic action, compared with vehicle. To be eligible for the trials patients with an Investigator’s Global Assessment (IGA) score of 2 or 3 and 3%-20% of affected body surface area (BSA) were randomized (2:2:1) to twice-daily 0.75% ruxolitinib cream, 1.5% ruxolitinib cream, or vehicle cream for 8 continuous weeks.
A recently published report found that significantly more patients in TRuE-AD1 and TRuE-AD2 achieved IGA treatment success with 0.75% (50% vs. 39%, respectively) and 1.5% ruxolitinib cream (53.8% vs. 51.3%), compared with vehicle (15.1% vs. 7.6%; P < .0001) at week 8. In addition, significant reductions in itch, compared with vehicle, were reported within 12 hours of first applying 1.5% ruxolitinib cream (P < .05).
Longterm data
During the symposium, Dr. Papp presented long-term safety data of ruxolitinib cream in patients who were followed for an additional 44 weeks. Those initially randomized to vehicle were rerandomized 1:1 (blinded) to either ruxolitinib cream regimen. They were instructed to treat skin areas with active AD only and to stop treatment 3 days after clearance of lesions, and to restart treatment with ruxolitinib cream at the first sign of recurrence. Safety and tolerability were assessed by frequency and severity of adverse events, while disease control was measured by the proportion of patients with an IGA score of 0 or 1 and the affected BSA.
Dr. Papp, a dermatologist and founder of Probity Medical Research, Waterloo, Ont., reported that 543 patients from TRuE-AD1 and 530 from TRuE-AD2 entered the long-term analysis and that about 78% of these patients completed the study. From weeks 12 to 52, the proportion of patients with an IGA score of 0 or 1 with 0.75% and 1.5% ruxolitinib cream ranged from 62%-77% and 67%-77%, respectively, in TRuE-AD1 to 60%-77% and 72%-80% in TRuE-AD2.
The measured mean total affected BSA was less than 3% throughout the follow-up period in the 1.5% ruxolitinib cream arm in TRuE-AD1 and TRuE-AD2 and was less than 3% in the 0.75% ruxolitinib cream arm during most of the study period.
In a pooled safety analysis, treatment-emergent adverse events (TEAEs) were reported in 60% and 54% of patients who applied 0.75% and 1.5% ruxolitinib cream, respectively, over 44 weeks. The frequency of application-site reactions remained low. Specifically, treatment-related adverse events were reported in 5% of patients who applied 0.75% ruxolitinib cream and in 3% of patients who applied 1.5% ruxolitinib cream; none were serious. TEAEs led to discontinuation in 2% of patients in the 0.75% ruxolitinib cream group, and no patients in the 1.5% ruxolitinib cream group.
“The most common treatment adverse events were upper respiratory tract infections and nasopharyngitis,” Dr. Papp said. “When looking at exposure-adjusted adverse events, we see that there is a high degree of similarity between any of the TEAEs across all of the treatment groups in both studies. We also see that it was patients on the vehicle who experienced the greatest number of application-site reactions.”
Dr. Papp disclosed that he has received honoraria or clinical research grants as a consultant, speaker, scientific officer, advisory board member, and/or steering committee member for several pharmaceutical companies, including Incyte.
results from a long-term analysis of clinical trial data showed.
“The incidence of application-site reactions was low, and there were no clinically meaningful changes or trends in hematologic parameters,” Kim Papp, MD, PhD, said during the Revolutionizing Atopic Dermatitis symposium.
Ruxolitinib cream is a selective Janus kinase 1/JAK2 inhibitor being developed by Incyte for the treatment of atopic dermatitis (AD).
According to a press release from the company, the Food and Drug Administration has extended the New Drug Application review period for the agent by 3 months to September 2021. If approved, it would become first topical JAK inhibitor for use in dermatology.
In two phase 3, randomized studies of identical design involving 1,249 patients aged 12 and older with AD – TRuE-AD1 and TRuE-AD2 – ruxolitinib cream demonstrated anti-inflammatory activity, with rapid and sustained antipruritic action, compared with vehicle. To be eligible for the trials patients with an Investigator’s Global Assessment (IGA) score of 2 or 3 and 3%-20% of affected body surface area (BSA) were randomized (2:2:1) to twice-daily 0.75% ruxolitinib cream, 1.5% ruxolitinib cream, or vehicle cream for 8 continuous weeks.
A recently published report found that significantly more patients in TRuE-AD1 and TRuE-AD2 achieved IGA treatment success with 0.75% (50% vs. 39%, respectively) and 1.5% ruxolitinib cream (53.8% vs. 51.3%), compared with vehicle (15.1% vs. 7.6%; P < .0001) at week 8. In addition, significant reductions in itch, compared with vehicle, were reported within 12 hours of first applying 1.5% ruxolitinib cream (P < .05).
Longterm data
During the symposium, Dr. Papp presented long-term safety data of ruxolitinib cream in patients who were followed for an additional 44 weeks. Those initially randomized to vehicle were rerandomized 1:1 (blinded) to either ruxolitinib cream regimen. They were instructed to treat skin areas with active AD only and to stop treatment 3 days after clearance of lesions, and to restart treatment with ruxolitinib cream at the first sign of recurrence. Safety and tolerability were assessed by frequency and severity of adverse events, while disease control was measured by the proportion of patients with an IGA score of 0 or 1 and the affected BSA.
Dr. Papp, a dermatologist and founder of Probity Medical Research, Waterloo, Ont., reported that 543 patients from TRuE-AD1 and 530 from TRuE-AD2 entered the long-term analysis and that about 78% of these patients completed the study. From weeks 12 to 52, the proportion of patients with an IGA score of 0 or 1 with 0.75% and 1.5% ruxolitinib cream ranged from 62%-77% and 67%-77%, respectively, in TRuE-AD1 to 60%-77% and 72%-80% in TRuE-AD2.
The measured mean total affected BSA was less than 3% throughout the follow-up period in the 1.5% ruxolitinib cream arm in TRuE-AD1 and TRuE-AD2 and was less than 3% in the 0.75% ruxolitinib cream arm during most of the study period.
In a pooled safety analysis, treatment-emergent adverse events (TEAEs) were reported in 60% and 54% of patients who applied 0.75% and 1.5% ruxolitinib cream, respectively, over 44 weeks. The frequency of application-site reactions remained low. Specifically, treatment-related adverse events were reported in 5% of patients who applied 0.75% ruxolitinib cream and in 3% of patients who applied 1.5% ruxolitinib cream; none were serious. TEAEs led to discontinuation in 2% of patients in the 0.75% ruxolitinib cream group, and no patients in the 1.5% ruxolitinib cream group.
“The most common treatment adverse events were upper respiratory tract infections and nasopharyngitis,” Dr. Papp said. “When looking at exposure-adjusted adverse events, we see that there is a high degree of similarity between any of the TEAEs across all of the treatment groups in both studies. We also see that it was patients on the vehicle who experienced the greatest number of application-site reactions.”
Dr. Papp disclosed that he has received honoraria or clinical research grants as a consultant, speaker, scientific officer, advisory board member, and/or steering committee member for several pharmaceutical companies, including Incyte.
results from a long-term analysis of clinical trial data showed.
“The incidence of application-site reactions was low, and there were no clinically meaningful changes or trends in hematologic parameters,” Kim Papp, MD, PhD, said during the Revolutionizing Atopic Dermatitis symposium.
Ruxolitinib cream is a selective Janus kinase 1/JAK2 inhibitor being developed by Incyte for the treatment of atopic dermatitis (AD).
According to a press release from the company, the Food and Drug Administration has extended the New Drug Application review period for the agent by 3 months to September 2021. If approved, it would become first topical JAK inhibitor for use in dermatology.
In two phase 3, randomized studies of identical design involving 1,249 patients aged 12 and older with AD – TRuE-AD1 and TRuE-AD2 – ruxolitinib cream demonstrated anti-inflammatory activity, with rapid and sustained antipruritic action, compared with vehicle. To be eligible for the trials patients with an Investigator’s Global Assessment (IGA) score of 2 or 3 and 3%-20% of affected body surface area (BSA) were randomized (2:2:1) to twice-daily 0.75% ruxolitinib cream, 1.5% ruxolitinib cream, or vehicle cream for 8 continuous weeks.
A recently published report found that significantly more patients in TRuE-AD1 and TRuE-AD2 achieved IGA treatment success with 0.75% (50% vs. 39%, respectively) and 1.5% ruxolitinib cream (53.8% vs. 51.3%), compared with vehicle (15.1% vs. 7.6%; P < .0001) at week 8. In addition, significant reductions in itch, compared with vehicle, were reported within 12 hours of first applying 1.5% ruxolitinib cream (P < .05).
Longterm data
During the symposium, Dr. Papp presented long-term safety data of ruxolitinib cream in patients who were followed for an additional 44 weeks. Those initially randomized to vehicle were rerandomized 1:1 (blinded) to either ruxolitinib cream regimen. They were instructed to treat skin areas with active AD only and to stop treatment 3 days after clearance of lesions, and to restart treatment with ruxolitinib cream at the first sign of recurrence. Safety and tolerability were assessed by frequency and severity of adverse events, while disease control was measured by the proportion of patients with an IGA score of 0 or 1 and the affected BSA.
Dr. Papp, a dermatologist and founder of Probity Medical Research, Waterloo, Ont., reported that 543 patients from TRuE-AD1 and 530 from TRuE-AD2 entered the long-term analysis and that about 78% of these patients completed the study. From weeks 12 to 52, the proportion of patients with an IGA score of 0 or 1 with 0.75% and 1.5% ruxolitinib cream ranged from 62%-77% and 67%-77%, respectively, in TRuE-AD1 to 60%-77% and 72%-80% in TRuE-AD2.
The measured mean total affected BSA was less than 3% throughout the follow-up period in the 1.5% ruxolitinib cream arm in TRuE-AD1 and TRuE-AD2 and was less than 3% in the 0.75% ruxolitinib cream arm during most of the study period.
In a pooled safety analysis, treatment-emergent adverse events (TEAEs) were reported in 60% and 54% of patients who applied 0.75% and 1.5% ruxolitinib cream, respectively, over 44 weeks. The frequency of application-site reactions remained low. Specifically, treatment-related adverse events were reported in 5% of patients who applied 0.75% ruxolitinib cream and in 3% of patients who applied 1.5% ruxolitinib cream; none were serious. TEAEs led to discontinuation in 2% of patients in the 0.75% ruxolitinib cream group, and no patients in the 1.5% ruxolitinib cream group.
“The most common treatment adverse events were upper respiratory tract infections and nasopharyngitis,” Dr. Papp said. “When looking at exposure-adjusted adverse events, we see that there is a high degree of similarity between any of the TEAEs across all of the treatment groups in both studies. We also see that it was patients on the vehicle who experienced the greatest number of application-site reactions.”
Dr. Papp disclosed that he has received honoraria or clinical research grants as a consultant, speaker, scientific officer, advisory board member, and/or steering committee member for several pharmaceutical companies, including Incyte.
FROM REVOLUTIONIZING AD 2021
Survey spotlights the out-of-pocket burden on Blacks with atopic dermatitis
They also have significantly poorer disease control and an increased rate of comorbid skin infections.
Those are among the key findings from a 25-question survey administered to members of the National Eczema Association.
“Black individuals with AD have a unique sociodemographic and disease profile,” lead study investigator Raj Chovatiya, MD, PhD, said during the Revolutionizing Atopic Dermatitis symposium. “Out-of-pocket expenses are just one component of the real-world burden faced by this population.”
According to Dr. Chovatiya, of the department of dermatology at Northwestern University, Chicago, the clinical phenotype and burden of AD can vary across racial and ethnic groups. Black race, for example, is associated with a higher prevalence of AD, a higher burden of moderate to severe disease, increased rates of allergic comorbidities, greater AD-related impact on health-related quality of life, and more treatment-resistant AD.
“These features can make long-term AD control very difficult,” he said. “Given the variable long-term efficacy and safety of current treatments, health care providers and patients often have to combine therapies, seek new treatments, and consider adjunctive approaches – all of which can contribute to increased costs.”
AD is also associated with a considerable financial burden, he continued, including direct health care costs, lost work productivity and out-of-pocket health care expenses. “Previous population-based studies suggest that there are multifactorial increases in overall out-of-pocket health expenses in AD,” Dr. Chovatiya said. “Black race in particular is thought to be associated with increased health care utilization in AD, but little is known about the out-of-pocket health care expenses.”
To characterize the categories and impact of out-of-pocket health care expenses associated with AD management among Black individuals, he and his colleagues administered a 25-question voluntary survey to 113,502 members of the NEA between Nov. 14 and Dec. 21, 2019. They included adults with a self-reported diagnosis of AD or children, teens, or young adults who had a caregiver responding for them. In all, 1,118 respondents met inclusion criteria. Questions included those about out-of-pocket expenses for AD over the past 30 days and over the past year, as well as the disease impact on household finances.
The cohort included 75% of individuals with AD; 25% were primary caregivers of children, teens, and young adults with AD. More than three-quarters of respondents (77%) were female, 73% were White, 11% were Black, 6% were Asian, and the remainder were from other ethnic backgrounds. More than half of respondents (58%) had employer-sponsored insurance coverage and the median annual household income was between $50,000 and $75,000.
Nearly three-quarters of respondents (74%) classified their AD severity as moderate or severe, and 63% reported minimally controlled or somewhat-controlled AD. Black respondents were significantly more likely to be younger, have lower household incomes, live in an urban setting, use Medicaid or state assistance, have poor disease control, and frequent skin infections (P ≤ .02). “A numerically higher proportion of Black respondents also had increased AD severity and reported the use of step-up therapy with systemic agents, prescription polypharmacy with three or more prescriptions, and a higher monthly out-of-pocket cost,” Dr. Chovatiya said.
Compared with their non-Black counterparts, Black survey respondents reported more out-of-pocket costs for prescription medications covered by insurance (74.2% vs. 63.6%, P = .04), prescription medications not covered by insurance (65.1% vs. 46.5%, P = .0004), ED visits (22.1% vs. 11.8%, P = .005), and outpatient laboratory testing (33.3% vs. 21.8%, P = .01). Black race was associated with increased household financial impact from out-of-pocket expenses (P = .0009), and predictors of financial impact included minimally controlled AD (adjusted odds ratio, 13.88; P = .02), comorbid anxiety and/or depression (aOR, 4.34; P = .01), systemic therapy (aOR, 4.34; P = .003), out-of-pocket costs that exceeded $200 per month (aOR, 14.28; P = .0003), and Medicaid insurance (aOR, 4.02; P = .03). Blacks with Medicaid had higher odds of harmful financial impact (aOR, 3.32; P = .0002) than respondents who were Black (aOR, 1.81; P = .04) or those with Medicaid alone (aOR, 1.39; P = .04).
“I looked at some of the findings from recent studies that have talked about this burden, including an increased prevalence among Black children, a higher likelihood of moderate to severe disease, higher rates of ED visits and hospitalizations, and increased prescription medications,” Dr. Chovatiya said.“Our findings reflect these racial and socioeconomic disparities and provide another piece of evidence for increased financial burden among Black individuals with AD and support the need for targeted strategies to address these inequities.”
The study received funding support from the NEA. Dr. Chovatiya disclosed that he is a consultant to, a speaker for, and/or a member of the advisory board for AbbVie, Incyte, and Regeneron/Sanofi-Genzyme.
They also have significantly poorer disease control and an increased rate of comorbid skin infections.
Those are among the key findings from a 25-question survey administered to members of the National Eczema Association.
“Black individuals with AD have a unique sociodemographic and disease profile,” lead study investigator Raj Chovatiya, MD, PhD, said during the Revolutionizing Atopic Dermatitis symposium. “Out-of-pocket expenses are just one component of the real-world burden faced by this population.”
According to Dr. Chovatiya, of the department of dermatology at Northwestern University, Chicago, the clinical phenotype and burden of AD can vary across racial and ethnic groups. Black race, for example, is associated with a higher prevalence of AD, a higher burden of moderate to severe disease, increased rates of allergic comorbidities, greater AD-related impact on health-related quality of life, and more treatment-resistant AD.
“These features can make long-term AD control very difficult,” he said. “Given the variable long-term efficacy and safety of current treatments, health care providers and patients often have to combine therapies, seek new treatments, and consider adjunctive approaches – all of which can contribute to increased costs.”
AD is also associated with a considerable financial burden, he continued, including direct health care costs, lost work productivity and out-of-pocket health care expenses. “Previous population-based studies suggest that there are multifactorial increases in overall out-of-pocket health expenses in AD,” Dr. Chovatiya said. “Black race in particular is thought to be associated with increased health care utilization in AD, but little is known about the out-of-pocket health care expenses.”
To characterize the categories and impact of out-of-pocket health care expenses associated with AD management among Black individuals, he and his colleagues administered a 25-question voluntary survey to 113,502 members of the NEA between Nov. 14 and Dec. 21, 2019. They included adults with a self-reported diagnosis of AD or children, teens, or young adults who had a caregiver responding for them. In all, 1,118 respondents met inclusion criteria. Questions included those about out-of-pocket expenses for AD over the past 30 days and over the past year, as well as the disease impact on household finances.
The cohort included 75% of individuals with AD; 25% were primary caregivers of children, teens, and young adults with AD. More than three-quarters of respondents (77%) were female, 73% were White, 11% were Black, 6% were Asian, and the remainder were from other ethnic backgrounds. More than half of respondents (58%) had employer-sponsored insurance coverage and the median annual household income was between $50,000 and $75,000.
Nearly three-quarters of respondents (74%) classified their AD severity as moderate or severe, and 63% reported minimally controlled or somewhat-controlled AD. Black respondents were significantly more likely to be younger, have lower household incomes, live in an urban setting, use Medicaid or state assistance, have poor disease control, and frequent skin infections (P ≤ .02). “A numerically higher proportion of Black respondents also had increased AD severity and reported the use of step-up therapy with systemic agents, prescription polypharmacy with three or more prescriptions, and a higher monthly out-of-pocket cost,” Dr. Chovatiya said.
Compared with their non-Black counterparts, Black survey respondents reported more out-of-pocket costs for prescription medications covered by insurance (74.2% vs. 63.6%, P = .04), prescription medications not covered by insurance (65.1% vs. 46.5%, P = .0004), ED visits (22.1% vs. 11.8%, P = .005), and outpatient laboratory testing (33.3% vs. 21.8%, P = .01). Black race was associated with increased household financial impact from out-of-pocket expenses (P = .0009), and predictors of financial impact included minimally controlled AD (adjusted odds ratio, 13.88; P = .02), comorbid anxiety and/or depression (aOR, 4.34; P = .01), systemic therapy (aOR, 4.34; P = .003), out-of-pocket costs that exceeded $200 per month (aOR, 14.28; P = .0003), and Medicaid insurance (aOR, 4.02; P = .03). Blacks with Medicaid had higher odds of harmful financial impact (aOR, 3.32; P = .0002) than respondents who were Black (aOR, 1.81; P = .04) or those with Medicaid alone (aOR, 1.39; P = .04).
“I looked at some of the findings from recent studies that have talked about this burden, including an increased prevalence among Black children, a higher likelihood of moderate to severe disease, higher rates of ED visits and hospitalizations, and increased prescription medications,” Dr. Chovatiya said.“Our findings reflect these racial and socioeconomic disparities and provide another piece of evidence for increased financial burden among Black individuals with AD and support the need for targeted strategies to address these inequities.”
The study received funding support from the NEA. Dr. Chovatiya disclosed that he is a consultant to, a speaker for, and/or a member of the advisory board for AbbVie, Incyte, and Regeneron/Sanofi-Genzyme.
They also have significantly poorer disease control and an increased rate of comorbid skin infections.
Those are among the key findings from a 25-question survey administered to members of the National Eczema Association.
“Black individuals with AD have a unique sociodemographic and disease profile,” lead study investigator Raj Chovatiya, MD, PhD, said during the Revolutionizing Atopic Dermatitis symposium. “Out-of-pocket expenses are just one component of the real-world burden faced by this population.”
According to Dr. Chovatiya, of the department of dermatology at Northwestern University, Chicago, the clinical phenotype and burden of AD can vary across racial and ethnic groups. Black race, for example, is associated with a higher prevalence of AD, a higher burden of moderate to severe disease, increased rates of allergic comorbidities, greater AD-related impact on health-related quality of life, and more treatment-resistant AD.
“These features can make long-term AD control very difficult,” he said. “Given the variable long-term efficacy and safety of current treatments, health care providers and patients often have to combine therapies, seek new treatments, and consider adjunctive approaches – all of which can contribute to increased costs.”
AD is also associated with a considerable financial burden, he continued, including direct health care costs, lost work productivity and out-of-pocket health care expenses. “Previous population-based studies suggest that there are multifactorial increases in overall out-of-pocket health expenses in AD,” Dr. Chovatiya said. “Black race in particular is thought to be associated with increased health care utilization in AD, but little is known about the out-of-pocket health care expenses.”
To characterize the categories and impact of out-of-pocket health care expenses associated with AD management among Black individuals, he and his colleagues administered a 25-question voluntary survey to 113,502 members of the NEA between Nov. 14 and Dec. 21, 2019. They included adults with a self-reported diagnosis of AD or children, teens, or young adults who had a caregiver responding for them. In all, 1,118 respondents met inclusion criteria. Questions included those about out-of-pocket expenses for AD over the past 30 days and over the past year, as well as the disease impact on household finances.
The cohort included 75% of individuals with AD; 25% were primary caregivers of children, teens, and young adults with AD. More than three-quarters of respondents (77%) were female, 73% were White, 11% were Black, 6% were Asian, and the remainder were from other ethnic backgrounds. More than half of respondents (58%) had employer-sponsored insurance coverage and the median annual household income was between $50,000 and $75,000.
Nearly three-quarters of respondents (74%) classified their AD severity as moderate or severe, and 63% reported minimally controlled or somewhat-controlled AD. Black respondents were significantly more likely to be younger, have lower household incomes, live in an urban setting, use Medicaid or state assistance, have poor disease control, and frequent skin infections (P ≤ .02). “A numerically higher proportion of Black respondents also had increased AD severity and reported the use of step-up therapy with systemic agents, prescription polypharmacy with three or more prescriptions, and a higher monthly out-of-pocket cost,” Dr. Chovatiya said.
Compared with their non-Black counterparts, Black survey respondents reported more out-of-pocket costs for prescription medications covered by insurance (74.2% vs. 63.6%, P = .04), prescription medications not covered by insurance (65.1% vs. 46.5%, P = .0004), ED visits (22.1% vs. 11.8%, P = .005), and outpatient laboratory testing (33.3% vs. 21.8%, P = .01). Black race was associated with increased household financial impact from out-of-pocket expenses (P = .0009), and predictors of financial impact included minimally controlled AD (adjusted odds ratio, 13.88; P = .02), comorbid anxiety and/or depression (aOR, 4.34; P = .01), systemic therapy (aOR, 4.34; P = .003), out-of-pocket costs that exceeded $200 per month (aOR, 14.28; P = .0003), and Medicaid insurance (aOR, 4.02; P = .03). Blacks with Medicaid had higher odds of harmful financial impact (aOR, 3.32; P = .0002) than respondents who were Black (aOR, 1.81; P = .04) or those with Medicaid alone (aOR, 1.39; P = .04).
“I looked at some of the findings from recent studies that have talked about this burden, including an increased prevalence among Black children, a higher likelihood of moderate to severe disease, higher rates of ED visits and hospitalizations, and increased prescription medications,” Dr. Chovatiya said.“Our findings reflect these racial and socioeconomic disparities and provide another piece of evidence for increased financial burden among Black individuals with AD and support the need for targeted strategies to address these inequities.”
The study received funding support from the NEA. Dr. Chovatiya disclosed that he is a consultant to, a speaker for, and/or a member of the advisory board for AbbVie, Incyte, and Regeneron/Sanofi-Genzyme.
FROM REVOLUTIONIZING AD 2021
Could the Surgisphere Lancet and NEJM retractions debacle happen again?
In May 2020, two major scientific journals published and subsequently retracted studies that relied on data provided by the now-disgraced data analytics company Surgisphere.
One of the studies, published in The Lancet, reported an association between the antimalarial drugs hydroxychloroquine and chloroquine and increased in-hospital mortality and cardiac arrhythmias in patients with COVID-19. The second study, which appeared in the New England Journal of Medicine, described an association between underlying cardiovascular disease, but not related drug therapy, with increased mortality in COVID-19 patients.
The retractions in June 2020 followed an open letter to each publication penned by scientists, ethicists, and clinicians who flagged serious methodological and ethical anomalies in the data used in the studies.
On the 1-year anniversary, researchers and journal editors spoke about what was learned to reduce the risk of something like this happening again.
“The Surgisphere incident served as a wake-up call for everyone involved with scientific research to make sure that data have integrity and are robust,” Sunil Rao, MD, professor of medicine, Duke University Health System, Durham, N.C., and editor-in-chief of Circulation: Cardiovascular Interventions, said in an interview.
“I’m sure this isn’t going to be the last incident of this nature, and we have to be vigilant about new datasets or datasets that we haven’t heard of as having a track record of publication,” Dr. Rao said.
Spotlight on authors
The editors of the Lancet Group responded to the “wake-up call” with a statement, Learning From a Retraction, which announced changes to reduce the risks of research and publication misconduct.
The changes affect multiple phases of the publication process. For example, the declaration form that authors must sign “will require that more than one author has directly accessed and verified the data reported in the manuscript.” Additionally, when a research article is the result of an academic and commercial partnership – as was the case in the two retracted studies – “one of the authors named as having accessed and verified data must be from the academic team.”
This was particularly important because it appears that the academic coauthors of the retracted studies did not have access to the data provided by Surgisphere, a private commercial entity.
Mandeep R. Mehra, MD, William Harvey Distinguished Chair in Advanced Cardiovascular Medicine, Brigham and Women’s Hospital, Boston, who was the lead author of both studies, declined to be interviewed for this article. In a letter to the New England Journal of Medicine editors requesting that the article be retracted, he wrote: “Because all the authors were not granted access to the raw data and the raw data could not be made available to a third-party auditor, we are unable to validate the primary data sources underlying our article.”
In a similar communication with The Lancet, Dr. Mehra wrote even more pointedly that, in light of the refusal of Surgisphere to make the data available to the third-party auditor, “we can no longer vouch for the veracity of the primary data sources.”
“It is very disturbing that the authors were willing to put their names on a paper without ever seeing and verifying the data,” Mario Malički, MD, PhD, a postdoctoral researcher at METRICS at Stanford (Calif.) University, said in an interview. “Saying that they could ‘no longer vouch’ suggests that at one point they could vouch for it. Most likely they took its existence and veracity entirely on trust.”
Dr. Malički pointed out that one of the four criteria of the International Committee of Medical Journal Editors for being an author on a study is the “agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.”
The new policies put forth by The Lancet are “encouraging,” but perhaps do not go far enough. “Every author, not only one or two authors, should personally take responsibility for the integrity of data,” he stated.
Many journals “adhere to ICMJE rules in principle and have checkboxes for authors to confirm that they guarantee the veracity of the data.” However, they “do not have the resources to verify the authors’ statements.”
Ideally, “it is the institutions where the researchers work that should guarantee the veracity of the raw data – but I do not know any university or institute that does this,” he said.
No ‘good-housekeeping’ seal
For articles based on large, real-world datasets, the Lancet Group will now require that editors ensure that at least one peer reviewer is “knowledgeable about the details of the dataset being reported and can understand its strengths and limitations in relation to the question being addressed.”
For studies that use “very large datasets,” the editors are now required to ensure that, in addition to a statistical peer review, a review from an “expert in data science” is obtained. Reviewers will also be explicitly asked if they have “concerns about research integrity or publication ethics regarding the manuscript they are reviewing.”
Although these changes are encouraging, Harlan Krumholz, MD, professor of medicine (cardiology), Yale University, New Haven, Conn., is not convinced that they are realistic.
Dr. Krumholz, who is also the founder and director of the Yale New Haven Hospital Center for Outcome Research and Evaluation, said in an interview that “large, real-world datasets” are of two varieties. Datasets drawn from publicly available sources, such as Medicare or Medicaid health records, are utterly transparent.
By contrast, Surgisphere was a privately owned database, and “it is not unusual for privately owned databases to have proprietary data from multiple sources that the company may choose to keep confidential,” Dr. Krumholz said.
He noted that several large datasets are widely used for research purposes, such as IBM, Optum, and Komodo – a data analytics company that recently entered into partnership with a fourth company, PicnicHealth.
These companies receive deidentified electronic health records from health systems and insurers nationwide. Komodo boasts “real-time and longitudinal data on more than 325 million patients, representing more than 65 billion clinical encounters with 15 million new encounters added daily.”
“One has to raise an eyebrow – how were these data acquired? And, given that the U.S. has a population of around 328 million people, is it really plausible that a single company has health records of almost the entire U.S. population?” Dr. Krumholz commented. (A spokesperson for Komodo said in an interview that the company has records on 325 million U.S. patients.)
This is “an issue across the board with ‘real-world evidence,’ which is that it’s like the ‘Wild West’ – the transparencies of private databases are less than optimal and there are no common standards to help us move forward,” Dr. Krumholz said, noting that there is “no external authority overseeing, validating, or auditing these databases. In the end, we are trusting the companies.”
Although the Food and Drug Administration has laid out a framework for how real-world data and real-world evidence can be used to advance scientific research, the FDA does not oversee the databases.
“Thus, there is no ‘good housekeeping seal’ that a peer reviewer or author would be in a position to evaluate,” Dr. Krumholz said. “No journal can do an audit of these types of private databases, so ultimately, it boils down to trust.”
Nevertheless, there were red flags with Surgisphere, Dr. Rao pointed out. Unlike more established and widely used databases, the Surgisphere database had been catapulted from relative obscurity onto center stage, which should have given researchers pause.
AI-assisted peer review
A series of investigative reports by The Guardian raised questions about Sapan Desai, the CEO of Surgisphere, including the fact that hospitals purporting to have contributed data to Surgisphere had never heard of the company.
However, peer reviewers are not expected to be investigative reporters, explained Dr. Malički.
“In an ideal world, editors and peer reviewers would have a chance to look at raw data or would have a certificate from the academic institution the authors are affiliated with that the data have been inspected by the institution, but in the real world, of course, this does not happen,” he said.
Artificial intelligence software is being developed and deployed to assist in the peer review process, Dr. Malički noted. In July 2020, Frontiers Science News debuted its Artificial Intelligence Review Assistant to help editors, reviewers, and authors evaluate the quality of a manuscript. The program can make up to 20 recommendations, including “the assessment of language quality, the detection of plagiarism, and identification of potential conflicts of interest.” The program is now in use in all 103 journals published by Frontiers. Preliminary software is also available to detect statistical errors.
Another system under development is FAIRware, an initiative of the Research on Research Institute in partnership with the Stanford Center for Biomedical Informatics Research. The partnership’s goal is to “develop an automated online tool (or suite of tools) to help researchers ensure that the datasets they produce are ‘FAIR’ at the point of creation,” said Dr. Malički, referring to the findability, accessibility, interoperability, and reusability (FAIR) guiding principles for data management. The principles aim to increase the ability of machines to automatically find and use the data, as well as to support its reuse by individuals.
He added that these advanced tools cannot replace human reviewers, who will “likely always be a necessary quality check in the process.”
Greater transparency needed
Another limitation of peer review is the reviewers themselves, according to Dr. Malički. “It’s a step in the right direction that The Lancet is now requesting a peer reviewer with expertise in big datasets, but it does not go far enough to increase accountability of peer reviewers,” he said.
Dr. Malički is the co–editor-in-chief of the journal Research Integrity and Peer Review , which has “an open and transparent review process – meaning that we reveal the names of the reviewers to the public and we publish the full review report alongside the paper.” The publication also allows the authors to make public the original version they sent.
Dr. Malički cited several advantages to transparent peer review, particularly the increased accountability that results from placing potential conflicts of interest under the microscope.
As for the concern that identifying the reviewers might soften the review process, “there is little evidence to substantiate that concern,” he added.
Dr. Malički emphasized that making reviews public “is not a problem – people voice strong opinions at conferences and elsewhere. The question remains, who gets to decide if the criticism has been adequately addressed, so that the findings of the study still stand?”
He acknowledged that, “as in politics and on many social platforms, rage, hatred, and personal attacks divert the discussion from the topic at hand, which is why a good moderator is needed.”
A journal editor or a moderator at a scientific conference may be tasked with “stopping all talk not directly related to the topic.”
Widening the circle of scrutiny
Dr. Malički added: “A published paper should not be considered the ‘final word,’ even if it has gone through peer review and is published in a reputable journal. The peer-review process means that a limited number of people have seen the study.”
Once the study is published, “the whole world gets to see it and criticize it, and that widens the circle of scrutiny.”
One classic way to raise concerns about a study post publication is to write a letter to the journal editor. But there is no guarantee that the letter will be published or the authors notified of the feedback.
Dr. Malički encourages readers to use PubPeer, an online forum in which members of the public can post comments on scientific studies and articles.
Once a comment is posted, the authors are alerted. “There is no ‘police department’ that forces authors to acknowledge comments or forces journal editors to take action, but at least PubPeer guarantees that readers’ messages will reach the authors and – depending on how many people raise similar issues – the comments can lead to errata or even full retractions,” he said.
PubPeer was key in pointing out errors in a suspect study from France (which did not involve Surgisphere) that supported the use of hydroxychloroquine in COVID-19.
A message to policy makers
High stakes are involved in ensuring the integrity of scientific publications: The French government revoked a decree that allowed hospitals to prescribe hydroxychloroquine for certain COVID-19 patients.
After the Surgisphere Lancet article, the World Health Organization temporarily halted enrollment in the hydroxychloroquine component of the Solidarity international randomized trial of medications to treat COVID-19.
Similarly, the U.K. Medicines and Healthcare Products Regulatory Agency instructed the organizers of COPCOV, an international trial of the use of hydroxychloroquine as prophylaxis against COVID-19, to suspend recruitment of patients. The SOLIDARITY trial briefly resumed, but that arm of the trial was ultimately suspended after a preliminary analysis suggested that hydroxychloroquine provided no benefit for patients with COVID-19.
Dr. Malički emphasized that governments and organizations should not “blindly trust journal articles” and make policy decisions based exclusively on study findings in published journals – even with the current improvements in the peer review process – without having their own experts conduct a thorough review of the data.
“If you are not willing to do your own due diligence, then at least be brave enough and say transparently why you are making this policy, or any other changes, and clearly state if your decision is based primarily or solely on the fact that ‘X’ study was published in ‘Y’ journal,” he stated.
Dr. Rao believes that the most important take-home message of the Surgisphere scandal is “that we should be skeptical and do our own due diligence about the kinds of data published – a responsibility that applies to all of us, whether we are investigators, editors at journals, the press, scientists, and readers.”
Dr. Rao reported being on the steering committee of the National Heart, Lung, and Blood Institute–sponsored MINT trial and the Bayer-sponsored PACIFIC AMI trial. Dr. Malički reports being a postdoc at METRICS Stanford in the past 3 years. Dr. Krumholz received expenses and/or personal fees from UnitedHealth, Element Science, Aetna, Facebook, the Siegfried and Jensen Law Firm, Arnold and Porter Law Firm, Martin/Baughman Law Firm, F-Prime, and the National Center for Cardiovascular Diseases in Beijing. He is an owner of Refactor Health and HugoHealth and had grants and/or contracts from the Centers for Medicare & Medicaid Services, the FDA, Johnson & Johnson, and the Shenzhen Center for Health Information.
A version of this article first appeared on Medscape.com.
In May 2020, two major scientific journals published and subsequently retracted studies that relied on data provided by the now-disgraced data analytics company Surgisphere.
One of the studies, published in The Lancet, reported an association between the antimalarial drugs hydroxychloroquine and chloroquine and increased in-hospital mortality and cardiac arrhythmias in patients with COVID-19. The second study, which appeared in the New England Journal of Medicine, described an association between underlying cardiovascular disease, but not related drug therapy, with increased mortality in COVID-19 patients.
The retractions in June 2020 followed an open letter to each publication penned by scientists, ethicists, and clinicians who flagged serious methodological and ethical anomalies in the data used in the studies.
On the 1-year anniversary, researchers and journal editors spoke about what was learned to reduce the risk of something like this happening again.
“The Surgisphere incident served as a wake-up call for everyone involved with scientific research to make sure that data have integrity and are robust,” Sunil Rao, MD, professor of medicine, Duke University Health System, Durham, N.C., and editor-in-chief of Circulation: Cardiovascular Interventions, said in an interview.
“I’m sure this isn’t going to be the last incident of this nature, and we have to be vigilant about new datasets or datasets that we haven’t heard of as having a track record of publication,” Dr. Rao said.
Spotlight on authors
The editors of the Lancet Group responded to the “wake-up call” with a statement, Learning From a Retraction, which announced changes to reduce the risks of research and publication misconduct.
The changes affect multiple phases of the publication process. For example, the declaration form that authors must sign “will require that more than one author has directly accessed and verified the data reported in the manuscript.” Additionally, when a research article is the result of an academic and commercial partnership – as was the case in the two retracted studies – “one of the authors named as having accessed and verified data must be from the academic team.”
This was particularly important because it appears that the academic coauthors of the retracted studies did not have access to the data provided by Surgisphere, a private commercial entity.
Mandeep R. Mehra, MD, William Harvey Distinguished Chair in Advanced Cardiovascular Medicine, Brigham and Women’s Hospital, Boston, who was the lead author of both studies, declined to be interviewed for this article. In a letter to the New England Journal of Medicine editors requesting that the article be retracted, he wrote: “Because all the authors were not granted access to the raw data and the raw data could not be made available to a third-party auditor, we are unable to validate the primary data sources underlying our article.”
In a similar communication with The Lancet, Dr. Mehra wrote even more pointedly that, in light of the refusal of Surgisphere to make the data available to the third-party auditor, “we can no longer vouch for the veracity of the primary data sources.”
“It is very disturbing that the authors were willing to put their names on a paper without ever seeing and verifying the data,” Mario Malički, MD, PhD, a postdoctoral researcher at METRICS at Stanford (Calif.) University, said in an interview. “Saying that they could ‘no longer vouch’ suggests that at one point they could vouch for it. Most likely they took its existence and veracity entirely on trust.”
Dr. Malički pointed out that one of the four criteria of the International Committee of Medical Journal Editors for being an author on a study is the “agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.”
The new policies put forth by The Lancet are “encouraging,” but perhaps do not go far enough. “Every author, not only one or two authors, should personally take responsibility for the integrity of data,” he stated.
Many journals “adhere to ICMJE rules in principle and have checkboxes for authors to confirm that they guarantee the veracity of the data.” However, they “do not have the resources to verify the authors’ statements.”
Ideally, “it is the institutions where the researchers work that should guarantee the veracity of the raw data – but I do not know any university or institute that does this,” he said.
No ‘good-housekeeping’ seal
For articles based on large, real-world datasets, the Lancet Group will now require that editors ensure that at least one peer reviewer is “knowledgeable about the details of the dataset being reported and can understand its strengths and limitations in relation to the question being addressed.”
For studies that use “very large datasets,” the editors are now required to ensure that, in addition to a statistical peer review, a review from an “expert in data science” is obtained. Reviewers will also be explicitly asked if they have “concerns about research integrity or publication ethics regarding the manuscript they are reviewing.”
Although these changes are encouraging, Harlan Krumholz, MD, professor of medicine (cardiology), Yale University, New Haven, Conn., is not convinced that they are realistic.
Dr. Krumholz, who is also the founder and director of the Yale New Haven Hospital Center for Outcome Research and Evaluation, said in an interview that “large, real-world datasets” are of two varieties. Datasets drawn from publicly available sources, such as Medicare or Medicaid health records, are utterly transparent.
By contrast, Surgisphere was a privately owned database, and “it is not unusual for privately owned databases to have proprietary data from multiple sources that the company may choose to keep confidential,” Dr. Krumholz said.
He noted that several large datasets are widely used for research purposes, such as IBM, Optum, and Komodo – a data analytics company that recently entered into partnership with a fourth company, PicnicHealth.
These companies receive deidentified electronic health records from health systems and insurers nationwide. Komodo boasts “real-time and longitudinal data on more than 325 million patients, representing more than 65 billion clinical encounters with 15 million new encounters added daily.”
“One has to raise an eyebrow – how were these data acquired? And, given that the U.S. has a population of around 328 million people, is it really plausible that a single company has health records of almost the entire U.S. population?” Dr. Krumholz commented. (A spokesperson for Komodo said in an interview that the company has records on 325 million U.S. patients.)
This is “an issue across the board with ‘real-world evidence,’ which is that it’s like the ‘Wild West’ – the transparencies of private databases are less than optimal and there are no common standards to help us move forward,” Dr. Krumholz said, noting that there is “no external authority overseeing, validating, or auditing these databases. In the end, we are trusting the companies.”
Although the Food and Drug Administration has laid out a framework for how real-world data and real-world evidence can be used to advance scientific research, the FDA does not oversee the databases.
“Thus, there is no ‘good housekeeping seal’ that a peer reviewer or author would be in a position to evaluate,” Dr. Krumholz said. “No journal can do an audit of these types of private databases, so ultimately, it boils down to trust.”
Nevertheless, there were red flags with Surgisphere, Dr. Rao pointed out. Unlike more established and widely used databases, the Surgisphere database had been catapulted from relative obscurity onto center stage, which should have given researchers pause.
AI-assisted peer review
A series of investigative reports by The Guardian raised questions about Sapan Desai, the CEO of Surgisphere, including the fact that hospitals purporting to have contributed data to Surgisphere had never heard of the company.
However, peer reviewers are not expected to be investigative reporters, explained Dr. Malički.
“In an ideal world, editors and peer reviewers would have a chance to look at raw data or would have a certificate from the academic institution the authors are affiliated with that the data have been inspected by the institution, but in the real world, of course, this does not happen,” he said.
Artificial intelligence software is being developed and deployed to assist in the peer review process, Dr. Malički noted. In July 2020, Frontiers Science News debuted its Artificial Intelligence Review Assistant to help editors, reviewers, and authors evaluate the quality of a manuscript. The program can make up to 20 recommendations, including “the assessment of language quality, the detection of plagiarism, and identification of potential conflicts of interest.” The program is now in use in all 103 journals published by Frontiers. Preliminary software is also available to detect statistical errors.
Another system under development is FAIRware, an initiative of the Research on Research Institute in partnership with the Stanford Center for Biomedical Informatics Research. The partnership’s goal is to “develop an automated online tool (or suite of tools) to help researchers ensure that the datasets they produce are ‘FAIR’ at the point of creation,” said Dr. Malički, referring to the findability, accessibility, interoperability, and reusability (FAIR) guiding principles for data management. The principles aim to increase the ability of machines to automatically find and use the data, as well as to support its reuse by individuals.
He added that these advanced tools cannot replace human reviewers, who will “likely always be a necessary quality check in the process.”
Greater transparency needed
Another limitation of peer review is the reviewers themselves, according to Dr. Malički. “It’s a step in the right direction that The Lancet is now requesting a peer reviewer with expertise in big datasets, but it does not go far enough to increase accountability of peer reviewers,” he said.
Dr. Malički is the co–editor-in-chief of the journal Research Integrity and Peer Review , which has “an open and transparent review process – meaning that we reveal the names of the reviewers to the public and we publish the full review report alongside the paper.” The publication also allows the authors to make public the original version they sent.
Dr. Malički cited several advantages to transparent peer review, particularly the increased accountability that results from placing potential conflicts of interest under the microscope.
As for the concern that identifying the reviewers might soften the review process, “there is little evidence to substantiate that concern,” he added.
Dr. Malički emphasized that making reviews public “is not a problem – people voice strong opinions at conferences and elsewhere. The question remains, who gets to decide if the criticism has been adequately addressed, so that the findings of the study still stand?”
He acknowledged that, “as in politics and on many social platforms, rage, hatred, and personal attacks divert the discussion from the topic at hand, which is why a good moderator is needed.”
A journal editor or a moderator at a scientific conference may be tasked with “stopping all talk not directly related to the topic.”
Widening the circle of scrutiny
Dr. Malički added: “A published paper should not be considered the ‘final word,’ even if it has gone through peer review and is published in a reputable journal. The peer-review process means that a limited number of people have seen the study.”
Once the study is published, “the whole world gets to see it and criticize it, and that widens the circle of scrutiny.”
One classic way to raise concerns about a study post publication is to write a letter to the journal editor. But there is no guarantee that the letter will be published or the authors notified of the feedback.
Dr. Malički encourages readers to use PubPeer, an online forum in which members of the public can post comments on scientific studies and articles.
Once a comment is posted, the authors are alerted. “There is no ‘police department’ that forces authors to acknowledge comments or forces journal editors to take action, but at least PubPeer guarantees that readers’ messages will reach the authors and – depending on how many people raise similar issues – the comments can lead to errata or even full retractions,” he said.
PubPeer was key in pointing out errors in a suspect study from France (which did not involve Surgisphere) that supported the use of hydroxychloroquine in COVID-19.
A message to policy makers
High stakes are involved in ensuring the integrity of scientific publications: The French government revoked a decree that allowed hospitals to prescribe hydroxychloroquine for certain COVID-19 patients.
After the Surgisphere Lancet article, the World Health Organization temporarily halted enrollment in the hydroxychloroquine component of the Solidarity international randomized trial of medications to treat COVID-19.
Similarly, the U.K. Medicines and Healthcare Products Regulatory Agency instructed the organizers of COPCOV, an international trial of the use of hydroxychloroquine as prophylaxis against COVID-19, to suspend recruitment of patients. The SOLIDARITY trial briefly resumed, but that arm of the trial was ultimately suspended after a preliminary analysis suggested that hydroxychloroquine provided no benefit for patients with COVID-19.
Dr. Malički emphasized that governments and organizations should not “blindly trust journal articles” and make policy decisions based exclusively on study findings in published journals – even with the current improvements in the peer review process – without having their own experts conduct a thorough review of the data.
“If you are not willing to do your own due diligence, then at least be brave enough and say transparently why you are making this policy, or any other changes, and clearly state if your decision is based primarily or solely on the fact that ‘X’ study was published in ‘Y’ journal,” he stated.
Dr. Rao believes that the most important take-home message of the Surgisphere scandal is “that we should be skeptical and do our own due diligence about the kinds of data published – a responsibility that applies to all of us, whether we are investigators, editors at journals, the press, scientists, and readers.”
Dr. Rao reported being on the steering committee of the National Heart, Lung, and Blood Institute–sponsored MINT trial and the Bayer-sponsored PACIFIC AMI trial. Dr. Malički reports being a postdoc at METRICS Stanford in the past 3 years. Dr. Krumholz received expenses and/or personal fees from UnitedHealth, Element Science, Aetna, Facebook, the Siegfried and Jensen Law Firm, Arnold and Porter Law Firm, Martin/Baughman Law Firm, F-Prime, and the National Center for Cardiovascular Diseases in Beijing. He is an owner of Refactor Health and HugoHealth and had grants and/or contracts from the Centers for Medicare & Medicaid Services, the FDA, Johnson & Johnson, and the Shenzhen Center for Health Information.
A version of this article first appeared on Medscape.com.
In May 2020, two major scientific journals published and subsequently retracted studies that relied on data provided by the now-disgraced data analytics company Surgisphere.
One of the studies, published in The Lancet, reported an association between the antimalarial drugs hydroxychloroquine and chloroquine and increased in-hospital mortality and cardiac arrhythmias in patients with COVID-19. The second study, which appeared in the New England Journal of Medicine, described an association between underlying cardiovascular disease, but not related drug therapy, with increased mortality in COVID-19 patients.
The retractions in June 2020 followed an open letter to each publication penned by scientists, ethicists, and clinicians who flagged serious methodological and ethical anomalies in the data used in the studies.
On the 1-year anniversary, researchers and journal editors spoke about what was learned to reduce the risk of something like this happening again.
“The Surgisphere incident served as a wake-up call for everyone involved with scientific research to make sure that data have integrity and are robust,” Sunil Rao, MD, professor of medicine, Duke University Health System, Durham, N.C., and editor-in-chief of Circulation: Cardiovascular Interventions, said in an interview.
“I’m sure this isn’t going to be the last incident of this nature, and we have to be vigilant about new datasets or datasets that we haven’t heard of as having a track record of publication,” Dr. Rao said.
Spotlight on authors
The editors of the Lancet Group responded to the “wake-up call” with a statement, Learning From a Retraction, which announced changes to reduce the risks of research and publication misconduct.
The changes affect multiple phases of the publication process. For example, the declaration form that authors must sign “will require that more than one author has directly accessed and verified the data reported in the manuscript.” Additionally, when a research article is the result of an academic and commercial partnership – as was the case in the two retracted studies – “one of the authors named as having accessed and verified data must be from the academic team.”
This was particularly important because it appears that the academic coauthors of the retracted studies did not have access to the data provided by Surgisphere, a private commercial entity.
Mandeep R. Mehra, MD, William Harvey Distinguished Chair in Advanced Cardiovascular Medicine, Brigham and Women’s Hospital, Boston, who was the lead author of both studies, declined to be interviewed for this article. In a letter to the New England Journal of Medicine editors requesting that the article be retracted, he wrote: “Because all the authors were not granted access to the raw data and the raw data could not be made available to a third-party auditor, we are unable to validate the primary data sources underlying our article.”
In a similar communication with The Lancet, Dr. Mehra wrote even more pointedly that, in light of the refusal of Surgisphere to make the data available to the third-party auditor, “we can no longer vouch for the veracity of the primary data sources.”
“It is very disturbing that the authors were willing to put their names on a paper without ever seeing and verifying the data,” Mario Malički, MD, PhD, a postdoctoral researcher at METRICS at Stanford (Calif.) University, said in an interview. “Saying that they could ‘no longer vouch’ suggests that at one point they could vouch for it. Most likely they took its existence and veracity entirely on trust.”
Dr. Malički pointed out that one of the four criteria of the International Committee of Medical Journal Editors for being an author on a study is the “agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.”
The new policies put forth by The Lancet are “encouraging,” but perhaps do not go far enough. “Every author, not only one or two authors, should personally take responsibility for the integrity of data,” he stated.
Many journals “adhere to ICMJE rules in principle and have checkboxes for authors to confirm that they guarantee the veracity of the data.” However, they “do not have the resources to verify the authors’ statements.”
Ideally, “it is the institutions where the researchers work that should guarantee the veracity of the raw data – but I do not know any university or institute that does this,” he said.
No ‘good-housekeeping’ seal
For articles based on large, real-world datasets, the Lancet Group will now require that editors ensure that at least one peer reviewer is “knowledgeable about the details of the dataset being reported and can understand its strengths and limitations in relation to the question being addressed.”
For studies that use “very large datasets,” the editors are now required to ensure that, in addition to a statistical peer review, a review from an “expert in data science” is obtained. Reviewers will also be explicitly asked if they have “concerns about research integrity or publication ethics regarding the manuscript they are reviewing.”
Although these changes are encouraging, Harlan Krumholz, MD, professor of medicine (cardiology), Yale University, New Haven, Conn., is not convinced that they are realistic.
Dr. Krumholz, who is also the founder and director of the Yale New Haven Hospital Center for Outcome Research and Evaluation, said in an interview that “large, real-world datasets” are of two varieties. Datasets drawn from publicly available sources, such as Medicare or Medicaid health records, are utterly transparent.
By contrast, Surgisphere was a privately owned database, and “it is not unusual for privately owned databases to have proprietary data from multiple sources that the company may choose to keep confidential,” Dr. Krumholz said.
He noted that several large datasets are widely used for research purposes, such as IBM, Optum, and Komodo – a data analytics company that recently entered into partnership with a fourth company, PicnicHealth.
These companies receive deidentified electronic health records from health systems and insurers nationwide. Komodo boasts “real-time and longitudinal data on more than 325 million patients, representing more than 65 billion clinical encounters with 15 million new encounters added daily.”
“One has to raise an eyebrow – how were these data acquired? And, given that the U.S. has a population of around 328 million people, is it really plausible that a single company has health records of almost the entire U.S. population?” Dr. Krumholz commented. (A spokesperson for Komodo said in an interview that the company has records on 325 million U.S. patients.)
This is “an issue across the board with ‘real-world evidence,’ which is that it’s like the ‘Wild West’ – the transparencies of private databases are less than optimal and there are no common standards to help us move forward,” Dr. Krumholz said, noting that there is “no external authority overseeing, validating, or auditing these databases. In the end, we are trusting the companies.”
Although the Food and Drug Administration has laid out a framework for how real-world data and real-world evidence can be used to advance scientific research, the FDA does not oversee the databases.
“Thus, there is no ‘good housekeeping seal’ that a peer reviewer or author would be in a position to evaluate,” Dr. Krumholz said. “No journal can do an audit of these types of private databases, so ultimately, it boils down to trust.”
Nevertheless, there were red flags with Surgisphere, Dr. Rao pointed out. Unlike more established and widely used databases, the Surgisphere database had been catapulted from relative obscurity onto center stage, which should have given researchers pause.
AI-assisted peer review
A series of investigative reports by The Guardian raised questions about Sapan Desai, the CEO of Surgisphere, including the fact that hospitals purporting to have contributed data to Surgisphere had never heard of the company.
However, peer reviewers are not expected to be investigative reporters, explained Dr. Malički.
“In an ideal world, editors and peer reviewers would have a chance to look at raw data or would have a certificate from the academic institution the authors are affiliated with that the data have been inspected by the institution, but in the real world, of course, this does not happen,” he said.
Artificial intelligence software is being developed and deployed to assist in the peer review process, Dr. Malički noted. In July 2020, Frontiers Science News debuted its Artificial Intelligence Review Assistant to help editors, reviewers, and authors evaluate the quality of a manuscript. The program can make up to 20 recommendations, including “the assessment of language quality, the detection of plagiarism, and identification of potential conflicts of interest.” The program is now in use in all 103 journals published by Frontiers. Preliminary software is also available to detect statistical errors.
Another system under development is FAIRware, an initiative of the Research on Research Institute in partnership with the Stanford Center for Biomedical Informatics Research. The partnership’s goal is to “develop an automated online tool (or suite of tools) to help researchers ensure that the datasets they produce are ‘FAIR’ at the point of creation,” said Dr. Malički, referring to the findability, accessibility, interoperability, and reusability (FAIR) guiding principles for data management. The principles aim to increase the ability of machines to automatically find and use the data, as well as to support its reuse by individuals.
He added that these advanced tools cannot replace human reviewers, who will “likely always be a necessary quality check in the process.”
Greater transparency needed
Another limitation of peer review is the reviewers themselves, according to Dr. Malički. “It’s a step in the right direction that The Lancet is now requesting a peer reviewer with expertise in big datasets, but it does not go far enough to increase accountability of peer reviewers,” he said.
Dr. Malički is the co–editor-in-chief of the journal Research Integrity and Peer Review , which has “an open and transparent review process – meaning that we reveal the names of the reviewers to the public and we publish the full review report alongside the paper.” The publication also allows the authors to make public the original version they sent.
Dr. Malički cited several advantages to transparent peer review, particularly the increased accountability that results from placing potential conflicts of interest under the microscope.
As for the concern that identifying the reviewers might soften the review process, “there is little evidence to substantiate that concern,” he added.
Dr. Malički emphasized that making reviews public “is not a problem – people voice strong opinions at conferences and elsewhere. The question remains, who gets to decide if the criticism has been adequately addressed, so that the findings of the study still stand?”
He acknowledged that, “as in politics and on many social platforms, rage, hatred, and personal attacks divert the discussion from the topic at hand, which is why a good moderator is needed.”
A journal editor or a moderator at a scientific conference may be tasked with “stopping all talk not directly related to the topic.”
Widening the circle of scrutiny
Dr. Malički added: “A published paper should not be considered the ‘final word,’ even if it has gone through peer review and is published in a reputable journal. The peer-review process means that a limited number of people have seen the study.”
Once the study is published, “the whole world gets to see it and criticize it, and that widens the circle of scrutiny.”
One classic way to raise concerns about a study post publication is to write a letter to the journal editor. But there is no guarantee that the letter will be published or the authors notified of the feedback.
Dr. Malički encourages readers to use PubPeer, an online forum in which members of the public can post comments on scientific studies and articles.
Once a comment is posted, the authors are alerted. “There is no ‘police department’ that forces authors to acknowledge comments or forces journal editors to take action, but at least PubPeer guarantees that readers’ messages will reach the authors and – depending on how many people raise similar issues – the comments can lead to errata or even full retractions,” he said.
PubPeer was key in pointing out errors in a suspect study from France (which did not involve Surgisphere) that supported the use of hydroxychloroquine in COVID-19.
A message to policy makers
High stakes are involved in ensuring the integrity of scientific publications: The French government revoked a decree that allowed hospitals to prescribe hydroxychloroquine for certain COVID-19 patients.
After the Surgisphere Lancet article, the World Health Organization temporarily halted enrollment in the hydroxychloroquine component of the Solidarity international randomized trial of medications to treat COVID-19.
Similarly, the U.K. Medicines and Healthcare Products Regulatory Agency instructed the organizers of COPCOV, an international trial of the use of hydroxychloroquine as prophylaxis against COVID-19, to suspend recruitment of patients. The SOLIDARITY trial briefly resumed, but that arm of the trial was ultimately suspended after a preliminary analysis suggested that hydroxychloroquine provided no benefit for patients with COVID-19.
Dr. Malički emphasized that governments and organizations should not “blindly trust journal articles” and make policy decisions based exclusively on study findings in published journals – even with the current improvements in the peer review process – without having their own experts conduct a thorough review of the data.
“If you are not willing to do your own due diligence, then at least be brave enough and say transparently why you are making this policy, or any other changes, and clearly state if your decision is based primarily or solely on the fact that ‘X’ study was published in ‘Y’ journal,” he stated.
Dr. Rao believes that the most important take-home message of the Surgisphere scandal is “that we should be skeptical and do our own due diligence about the kinds of data published – a responsibility that applies to all of us, whether we are investigators, editors at journals, the press, scientists, and readers.”
Dr. Rao reported being on the steering committee of the National Heart, Lung, and Blood Institute–sponsored MINT trial and the Bayer-sponsored PACIFIC AMI trial. Dr. Malički reports being a postdoc at METRICS Stanford in the past 3 years. Dr. Krumholz received expenses and/or personal fees from UnitedHealth, Element Science, Aetna, Facebook, the Siegfried and Jensen Law Firm, Arnold and Porter Law Firm, Martin/Baughman Law Firm, F-Prime, and the National Center for Cardiovascular Diseases in Beijing. He is an owner of Refactor Health and HugoHealth and had grants and/or contracts from the Centers for Medicare & Medicaid Services, the FDA, Johnson & Johnson, and the Shenzhen Center for Health Information.
A version of this article first appeared on Medscape.com.
Few clinical guidelines exist for treating post-COVID symptoms
As doctors struggled through several surges of COVID-19 infections, most of what we learned was acquired through real-life experience. While many treatment options were promoted, most flat-out failed to be real therapeutics at all. Now that we have a safe and effective vaccine, we can prevent many infections from this virus. However, we are still left to manage the many post-COVID symptoms our patients continue to suffer with.
Symptoms following infection can last for months and range widely from “brain fog,” fatigue, dyspnea, chest pain, generalized weakness, depression, and a host of others. Patients may experience one or all of these symptoms, and there is currently no good way to predict who will go on to become a COVID “long hauler”.
Following the example of being educated by COVID as it happened, the same is true for managing post-COVID symptoms. The medical community still has a poor understanding of why some people develop it and there are few evidence-based studies to support any treatment modalities.
which they define as “new, recurring, or ongoing symptoms more than 4 weeks after infection, sometimes after initial symptom recovery.” It is important to note that these symptoms can occur in any degree of sickness during the acute infection, including in those who were asymptomatic. Even the actual name of this post-COVID syndrome is still being developed, with several other names being used for it as well.
While the guidelines are quite extensive, the actual clinical recommendations are still vague. For example, it is advised to let the patient know that post-COVID symptoms are still not well understood. While it is important to be transparent with patients, this does little to reassure them. Patients look to doctors, especially their primary care physicians, to guide them on the best treatment paths. Yet, we currently have none for post-COVID syndrome.
It is also advised to treat the patients’ symptoms and help improve functioning. For many diseases, doctors like to get to the root cause of the problem. Treating a symptom often masks an underlying condition. It may make the patient feel better and improve what they are capable of doing, which is important, but it also fails to unmask the real problem. It is also important to note that symptoms can be out of proportion to clinical findings and should not be dismissed: we just don’t have the answers yet.
One helpful recommendation is having a patient keep a diary of their symptoms. This will help both the patient and doctor learn what may be triggering factors. If it is, for example, exertion that induces breathlessness, perhaps the patient can gradually increase their level of activity to minimize symptoms. Additionally, a “comprehensive rehabilitation program” is also advised and this can greatly assist addressing all the issues a patient is experiencing, physically and medically.
It is also advised that management of underlying medical conditions be optimized. While this is very important, it is not something specific to post-COVID syndrome: All patients should have their underlying medical conditions well controlled. It might be that the patient is paying more attention to their overall health, which is a good thing. However, this does not necessarily reduce the current symptoms a patient is experiencing.
The CDC makes a good attempt to offer guidance in the frustrating management of post-COVID syndrome. However, their clinical guidelines fail to offer specific management tools specific to treating post-COVID patients. The recommendations offered are more helpful to health in general. The fact that more specific recommendations are lacking is simply caused by the lack of knowledge of this condition at present. As more research is conducted and more knowledge obtained, new guidelines should become more detailed.
Dr. Girgis practices family medicine in South River, N.J., and is a clinical assistant professor of family medicine at Robert Wood Johnson Medical School, New Brunswick, N.J. You can contact her at [email protected].
As doctors struggled through several surges of COVID-19 infections, most of what we learned was acquired through real-life experience. While many treatment options were promoted, most flat-out failed to be real therapeutics at all. Now that we have a safe and effective vaccine, we can prevent many infections from this virus. However, we are still left to manage the many post-COVID symptoms our patients continue to suffer with.
Symptoms following infection can last for months and range widely from “brain fog,” fatigue, dyspnea, chest pain, generalized weakness, depression, and a host of others. Patients may experience one or all of these symptoms, and there is currently no good way to predict who will go on to become a COVID “long hauler”.
Following the example of being educated by COVID as it happened, the same is true for managing post-COVID symptoms. The medical community still has a poor understanding of why some people develop it and there are few evidence-based studies to support any treatment modalities.
which they define as “new, recurring, or ongoing symptoms more than 4 weeks after infection, sometimes after initial symptom recovery.” It is important to note that these symptoms can occur in any degree of sickness during the acute infection, including in those who were asymptomatic. Even the actual name of this post-COVID syndrome is still being developed, with several other names being used for it as well.
While the guidelines are quite extensive, the actual clinical recommendations are still vague. For example, it is advised to let the patient know that post-COVID symptoms are still not well understood. While it is important to be transparent with patients, this does little to reassure them. Patients look to doctors, especially their primary care physicians, to guide them on the best treatment paths. Yet, we currently have none for post-COVID syndrome.
It is also advised to treat the patients’ symptoms and help improve functioning. For many diseases, doctors like to get to the root cause of the problem. Treating a symptom often masks an underlying condition. It may make the patient feel better and improve what they are capable of doing, which is important, but it also fails to unmask the real problem. It is also important to note that symptoms can be out of proportion to clinical findings and should not be dismissed: we just don’t have the answers yet.
One helpful recommendation is having a patient keep a diary of their symptoms. This will help both the patient and doctor learn what may be triggering factors. If it is, for example, exertion that induces breathlessness, perhaps the patient can gradually increase their level of activity to minimize symptoms. Additionally, a “comprehensive rehabilitation program” is also advised and this can greatly assist addressing all the issues a patient is experiencing, physically and medically.
It is also advised that management of underlying medical conditions be optimized. While this is very important, it is not something specific to post-COVID syndrome: All patients should have their underlying medical conditions well controlled. It might be that the patient is paying more attention to their overall health, which is a good thing. However, this does not necessarily reduce the current symptoms a patient is experiencing.
The CDC makes a good attempt to offer guidance in the frustrating management of post-COVID syndrome. However, their clinical guidelines fail to offer specific management tools specific to treating post-COVID patients. The recommendations offered are more helpful to health in general. The fact that more specific recommendations are lacking is simply caused by the lack of knowledge of this condition at present. As more research is conducted and more knowledge obtained, new guidelines should become more detailed.
Dr. Girgis practices family medicine in South River, N.J., and is a clinical assistant professor of family medicine at Robert Wood Johnson Medical School, New Brunswick, N.J. You can contact her at [email protected].
As doctors struggled through several surges of COVID-19 infections, most of what we learned was acquired through real-life experience. While many treatment options were promoted, most flat-out failed to be real therapeutics at all. Now that we have a safe and effective vaccine, we can prevent many infections from this virus. However, we are still left to manage the many post-COVID symptoms our patients continue to suffer with.
Symptoms following infection can last for months and range widely from “brain fog,” fatigue, dyspnea, chest pain, generalized weakness, depression, and a host of others. Patients may experience one or all of these symptoms, and there is currently no good way to predict who will go on to become a COVID “long hauler”.
Following the example of being educated by COVID as it happened, the same is true for managing post-COVID symptoms. The medical community still has a poor understanding of why some people develop it and there are few evidence-based studies to support any treatment modalities.
which they define as “new, recurring, or ongoing symptoms more than 4 weeks after infection, sometimes after initial symptom recovery.” It is important to note that these symptoms can occur in any degree of sickness during the acute infection, including in those who were asymptomatic. Even the actual name of this post-COVID syndrome is still being developed, with several other names being used for it as well.
While the guidelines are quite extensive, the actual clinical recommendations are still vague. For example, it is advised to let the patient know that post-COVID symptoms are still not well understood. While it is important to be transparent with patients, this does little to reassure them. Patients look to doctors, especially their primary care physicians, to guide them on the best treatment paths. Yet, we currently have none for post-COVID syndrome.
It is also advised to treat the patients’ symptoms and help improve functioning. For many diseases, doctors like to get to the root cause of the problem. Treating a symptom often masks an underlying condition. It may make the patient feel better and improve what they are capable of doing, which is important, but it also fails to unmask the real problem. It is also important to note that symptoms can be out of proportion to clinical findings and should not be dismissed: we just don’t have the answers yet.
One helpful recommendation is having a patient keep a diary of their symptoms. This will help both the patient and doctor learn what may be triggering factors. If it is, for example, exertion that induces breathlessness, perhaps the patient can gradually increase their level of activity to minimize symptoms. Additionally, a “comprehensive rehabilitation program” is also advised and this can greatly assist addressing all the issues a patient is experiencing, physically and medically.
It is also advised that management of underlying medical conditions be optimized. While this is very important, it is not something specific to post-COVID syndrome: All patients should have their underlying medical conditions well controlled. It might be that the patient is paying more attention to their overall health, which is a good thing. However, this does not necessarily reduce the current symptoms a patient is experiencing.
The CDC makes a good attempt to offer guidance in the frustrating management of post-COVID syndrome. However, their clinical guidelines fail to offer specific management tools specific to treating post-COVID patients. The recommendations offered are more helpful to health in general. The fact that more specific recommendations are lacking is simply caused by the lack of knowledge of this condition at present. As more research is conducted and more knowledge obtained, new guidelines should become more detailed.
Dr. Girgis practices family medicine in South River, N.J., and is a clinical assistant professor of family medicine at Robert Wood Johnson Medical School, New Brunswick, N.J. You can contact her at [email protected].