User login
Formerly Skin & Allergy News
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')]
The leading independent newspaper covering dermatology news and commentary.
List of COVID-19 high-risk comorbidities expanded
The list of medical according to the Centers for Disease Control and Prevention.
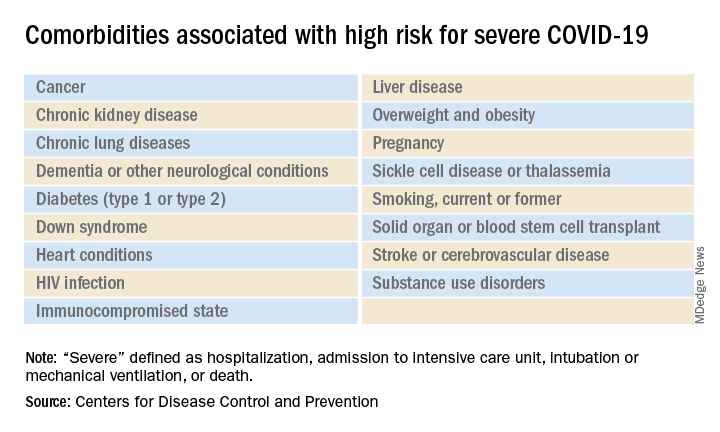
The CDC’s latest list consists of 17 conditions or groups of related conditions that may increase patients’ risk of developing severe outcomes of COVID-19, the CDC said on a web page intended for the general public.
On a separate page, the CDC defines severe outcomes “as hospitalization, admission to the intensive care unit, intubation or mechanical ventilation, or death.”
Asthma is included in the newly expanded list with other chronic lung diseases such as chronic obstructive pulmonary disease and cystic fibrosis; the list’s heart disease entry covers coronary artery disease, heart failure, cardiomyopathies, and hypertension, the CDC said.
The list of medical according to the Centers for Disease Control and Prevention.

The CDC’s latest list consists of 17 conditions or groups of related conditions that may increase patients’ risk of developing severe outcomes of COVID-19, the CDC said on a web page intended for the general public.
On a separate page, the CDC defines severe outcomes “as hospitalization, admission to the intensive care unit, intubation or mechanical ventilation, or death.”
Asthma is included in the newly expanded list with other chronic lung diseases such as chronic obstructive pulmonary disease and cystic fibrosis; the list’s heart disease entry covers coronary artery disease, heart failure, cardiomyopathies, and hypertension, the CDC said.
The list of medical according to the Centers for Disease Control and Prevention.

The CDC’s latest list consists of 17 conditions or groups of related conditions that may increase patients’ risk of developing severe outcomes of COVID-19, the CDC said on a web page intended for the general public.
On a separate page, the CDC defines severe outcomes “as hospitalization, admission to the intensive care unit, intubation or mechanical ventilation, or death.”
Asthma is included in the newly expanded list with other chronic lung diseases such as chronic obstructive pulmonary disease and cystic fibrosis; the list’s heart disease entry covers coronary artery disease, heart failure, cardiomyopathies, and hypertension, the CDC said.
Researchers stress importance of second COVID-19 vaccine dose for infliximab users
Patients being treated with infliximab had weakened immune responses to the first dose of the ChAdOx1 nCoV-19 (Oxford/AstraZeneca) and BNT162b2 (Pfizer/BioNTech) vaccines, compared with patients on vedolizumab (Entyvio), although a very significant number of patients from both groups seroconverted after their second dose, according to a new U.K. study of patients with inflammatory bowel disease (IBD).
“Antibody testing and adapted vaccine schedules should be considered to protect these at-risk patients,” Nicholas A. Kennedy, PhD, MBBS, of the University of Exeter (England) and colleagues wrote in a preprint published March 29 on MedRxiv.
Infliximab is an anti–tumor necrosis factor (anti-TNF) monoclonal antibody that’s approved to treat adult and pediatric Crohn’s disease and ulcerative colitis, as well as rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis, and plaque psoriasis, whereas vedolizumab, a gut selective anti-integrin alpha4beta7 monoclonal antibody that is not associated with impaired systemic immune responses, is approved to treat Crohn’s disease and ulcerative colitis in adults.
A previous study from Kennedy and colleagues revealed that IBD patients on infliximab showed a weakened COVID-19 antibody response compared with patients on vedolizumab. To determine if treatment with anti-TNF drugs impacted the efficacy of the first shot of these two-dose COVID-19 vaccines, the researchers used data from the CLARITY IBD study to assess 865 infliximab- and 428 vedolizumab-treated participants without evidence of prior SARS-CoV-2 infection who had received uninterrupted biologic therapy since being recruited between Sept. 22 and Dec. 23, 2020.
In the 3-10 weeks after initial vaccination, geometric mean concentrations for SARS-CoV-2 anti-spike protein receptor-binding protein antibodies were lower in patients on infliximab, compared with patients on vedolizumab for both the Pfizer (6.0 U/mL [5.9] versus 28.8 U/mL [5.4], P < .0001) and AstraZeneca (4.7 U/mL [4.9] versus 13.8 U/mL [5.9]; P < .0001) vaccines. The researchers’ multivariable models reinforced those findings, with antibody concentrations lower in infliximab-treated patients for both the Pfizer (fold change, 0.29; 95% confidence interval, 0.21-0.40; P < .0001) and AstraZeneca (FC, 0.39; 95% CI, 0.30-0.51; P < .0001) vaccines.
After second doses of the two-dose Pfizer vaccine, 85% of patients on infliximab and 86% of patients on vedolizumab seroconverted (P = .68); similarly high seroconversion rates were seen in patients who had been infected with SARS-CoV-2 prior to receiving either vaccine. Several patient characteristics were associated with lower antibody concentrations regardless of vaccine type: being 60 years or older, use of immunomodulators, having Crohn’s disease, and being a smoker. Alternatively, non-White ethnicity was associated with higher antibody concentrations.
Evidence has ‘unclear clinical significance’
“These data, which require peer review, do not change my opinion on the safety and efficacy of COVID-19 vaccines in patients taking TNF inhibitors such as infliximab as monotherapy for the treatment of psoriatic disease,” Joel M. Gelfand MD, director of the psoriasis and phototherapy treatment center at the University of Pennsylvania, Philadelphia, said in an interview.
“First, two peer-reviewed studies found good antibody response in patients on TNF inhibitors receiving COVID-19 vaccines (doi: 10.1136/annrheumdis-2021-220289; 10.1136/annrheumdis-2021-220272). Second, antibody responses were robust in the small cohort that received the second dose of a COVID-19 vaccine. We already know that, for the two messenger RNA-based vaccines available under emergency use authorization in the U.S., a second dose is required for optimal efficacy. Thus, evidence of a reduced antibody response after just one dose is of unclear clinical significance. Third, antibody responses are only a surrogate marker, and a low antibody response doesn’t necessarily mean the patient will not be protected by the vaccine.”
Focus on the second dose of a two-dose regimen
“Tell me about the response in people who got both doses of a vaccine that you’re supposed to get both doses of,” Jeffrey Curtis, MD, professor of medicine in the division of clinical immunology and rheumatology at the University of Alabama at Birmingham, said in an interview. “The number of patients in that subset was small [n = 27] but in my opinion that’s the most clinically relevant analysis and the one that patients and clinicians want answered.”
He also emphasized the uncertainty around what ‘protection’ means in these early days of studying COVID-19 vaccine responses. “You can define seroprotection or seroconversion as some absolute level of an antibody response, but if you want to say ‘Mrs. Smith, your antibody level was X,’ on whatever arbitrary scale with whoever’s arbitrary lab test, nobody actually knows that Mrs. Smith is now protected from SARS-CoV-2, or how protected,” he said.
“What is not terribly controversial is: If you can’t detect antibodies, the vaccine didn’t ‘take,’ if you will. But if I tell you that the mean antibody level was X with one drug and then 2X with another drug, does that mean that you’re twice as protected? We don’t know that. I’m fearful that people are looking at these studies and thinking that more is better. It might be, but we don’t know that to be true.”
Debating the cause of weakened immune responses
“The biological plausibility of being on an anti-TNF affecting your immune reaction to a messenger RNA or even a replication-deficient viral vector vaccine doesn’t make sense,” David T. Rubin, MD, professor of medicine at the University of Chicago and chair of the National Scientific Advisory Committee of the Crohn’s and Colitis Foundation, said in an interview.
“I’m sure immunologists may differ with me on this, but given what we have come to appreciate about these vaccine mechanisms, this finding doesn’t make intuitive sense. So we need to make sure that, when this happens, we look to the next studies and try to understand, was there any other confounder that may have resulted in these findings that was not adequately adjusted for or addressed in some other way?
“When you have a study of this size, you argue, ‘Because it’s so large, any effect that was seen must be real,’ ” he added. “Alternatively, to have a study of this size, by its very nature you are limited in being able to control for certain other factors or differences between the groups.”
That said, he commended the authors for their study and acknowledged the potential questions it raises about the single-shot Johnson & Johnson vaccine. “If you only get one and you’re on infliximab, this study implies that maybe that’s not enough,” he said. “Despite the fact that Johnson & Johnson was approved as a single dose, it may be necessary to think about it as the first of two, or maybe it’s not the preferred vaccine in this group of patients.”
The study was supported by the Royal Devon and Exeter and Hull University Hospital Foundation NHS Trusts and unrestricted educational grants from Biogen (Switzerland), Celltrion Healthcare (South Korea), Galapagos NV (Belgium), and F. Hoffmann-La Roche (Switzerland). The authors acknowledged numerous potential conflicts of interest, including receiving grants, personal fees, and nonfinancial support from various pharmaceutical companies.
Patients being treated with infliximab had weakened immune responses to the first dose of the ChAdOx1 nCoV-19 (Oxford/AstraZeneca) and BNT162b2 (Pfizer/BioNTech) vaccines, compared with patients on vedolizumab (Entyvio), although a very significant number of patients from both groups seroconverted after their second dose, according to a new U.K. study of patients with inflammatory bowel disease (IBD).
“Antibody testing and adapted vaccine schedules should be considered to protect these at-risk patients,” Nicholas A. Kennedy, PhD, MBBS, of the University of Exeter (England) and colleagues wrote in a preprint published March 29 on MedRxiv.
Infliximab is an anti–tumor necrosis factor (anti-TNF) monoclonal antibody that’s approved to treat adult and pediatric Crohn’s disease and ulcerative colitis, as well as rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis, and plaque psoriasis, whereas vedolizumab, a gut selective anti-integrin alpha4beta7 monoclonal antibody that is not associated with impaired systemic immune responses, is approved to treat Crohn’s disease and ulcerative colitis in adults.
A previous study from Kennedy and colleagues revealed that IBD patients on infliximab showed a weakened COVID-19 antibody response compared with patients on vedolizumab. To determine if treatment with anti-TNF drugs impacted the efficacy of the first shot of these two-dose COVID-19 vaccines, the researchers used data from the CLARITY IBD study to assess 865 infliximab- and 428 vedolizumab-treated participants without evidence of prior SARS-CoV-2 infection who had received uninterrupted biologic therapy since being recruited between Sept. 22 and Dec. 23, 2020.
In the 3-10 weeks after initial vaccination, geometric mean concentrations for SARS-CoV-2 anti-spike protein receptor-binding protein antibodies were lower in patients on infliximab, compared with patients on vedolizumab for both the Pfizer (6.0 U/mL [5.9] versus 28.8 U/mL [5.4], P < .0001) and AstraZeneca (4.7 U/mL [4.9] versus 13.8 U/mL [5.9]; P < .0001) vaccines. The researchers’ multivariable models reinforced those findings, with antibody concentrations lower in infliximab-treated patients for both the Pfizer (fold change, 0.29; 95% confidence interval, 0.21-0.40; P < .0001) and AstraZeneca (FC, 0.39; 95% CI, 0.30-0.51; P < .0001) vaccines.
After second doses of the two-dose Pfizer vaccine, 85% of patients on infliximab and 86% of patients on vedolizumab seroconverted (P = .68); similarly high seroconversion rates were seen in patients who had been infected with SARS-CoV-2 prior to receiving either vaccine. Several patient characteristics were associated with lower antibody concentrations regardless of vaccine type: being 60 years or older, use of immunomodulators, having Crohn’s disease, and being a smoker. Alternatively, non-White ethnicity was associated with higher antibody concentrations.
Evidence has ‘unclear clinical significance’
“These data, which require peer review, do not change my opinion on the safety and efficacy of COVID-19 vaccines in patients taking TNF inhibitors such as infliximab as monotherapy for the treatment of psoriatic disease,” Joel M. Gelfand MD, director of the psoriasis and phototherapy treatment center at the University of Pennsylvania, Philadelphia, said in an interview.
“First, two peer-reviewed studies found good antibody response in patients on TNF inhibitors receiving COVID-19 vaccines (doi: 10.1136/annrheumdis-2021-220289; 10.1136/annrheumdis-2021-220272). Second, antibody responses were robust in the small cohort that received the second dose of a COVID-19 vaccine. We already know that, for the two messenger RNA-based vaccines available under emergency use authorization in the U.S., a second dose is required for optimal efficacy. Thus, evidence of a reduced antibody response after just one dose is of unclear clinical significance. Third, antibody responses are only a surrogate marker, and a low antibody response doesn’t necessarily mean the patient will not be protected by the vaccine.”
Focus on the second dose of a two-dose regimen
“Tell me about the response in people who got both doses of a vaccine that you’re supposed to get both doses of,” Jeffrey Curtis, MD, professor of medicine in the division of clinical immunology and rheumatology at the University of Alabama at Birmingham, said in an interview. “The number of patients in that subset was small [n = 27] but in my opinion that’s the most clinically relevant analysis and the one that patients and clinicians want answered.”
He also emphasized the uncertainty around what ‘protection’ means in these early days of studying COVID-19 vaccine responses. “You can define seroprotection or seroconversion as some absolute level of an antibody response, but if you want to say ‘Mrs. Smith, your antibody level was X,’ on whatever arbitrary scale with whoever’s arbitrary lab test, nobody actually knows that Mrs. Smith is now protected from SARS-CoV-2, or how protected,” he said.
“What is not terribly controversial is: If you can’t detect antibodies, the vaccine didn’t ‘take,’ if you will. But if I tell you that the mean antibody level was X with one drug and then 2X with another drug, does that mean that you’re twice as protected? We don’t know that. I’m fearful that people are looking at these studies and thinking that more is better. It might be, but we don’t know that to be true.”
Debating the cause of weakened immune responses
“The biological plausibility of being on an anti-TNF affecting your immune reaction to a messenger RNA or even a replication-deficient viral vector vaccine doesn’t make sense,” David T. Rubin, MD, professor of medicine at the University of Chicago and chair of the National Scientific Advisory Committee of the Crohn’s and Colitis Foundation, said in an interview.
“I’m sure immunologists may differ with me on this, but given what we have come to appreciate about these vaccine mechanisms, this finding doesn’t make intuitive sense. So we need to make sure that, when this happens, we look to the next studies and try to understand, was there any other confounder that may have resulted in these findings that was not adequately adjusted for or addressed in some other way?
“When you have a study of this size, you argue, ‘Because it’s so large, any effect that was seen must be real,’ ” he added. “Alternatively, to have a study of this size, by its very nature you are limited in being able to control for certain other factors or differences between the groups.”
That said, he commended the authors for their study and acknowledged the potential questions it raises about the single-shot Johnson & Johnson vaccine. “If you only get one and you’re on infliximab, this study implies that maybe that’s not enough,” he said. “Despite the fact that Johnson & Johnson was approved as a single dose, it may be necessary to think about it as the first of two, or maybe it’s not the preferred vaccine in this group of patients.”
The study was supported by the Royal Devon and Exeter and Hull University Hospital Foundation NHS Trusts and unrestricted educational grants from Biogen (Switzerland), Celltrion Healthcare (South Korea), Galapagos NV (Belgium), and F. Hoffmann-La Roche (Switzerland). The authors acknowledged numerous potential conflicts of interest, including receiving grants, personal fees, and nonfinancial support from various pharmaceutical companies.
Patients being treated with infliximab had weakened immune responses to the first dose of the ChAdOx1 nCoV-19 (Oxford/AstraZeneca) and BNT162b2 (Pfizer/BioNTech) vaccines, compared with patients on vedolizumab (Entyvio), although a very significant number of patients from both groups seroconverted after their second dose, according to a new U.K. study of patients with inflammatory bowel disease (IBD).
“Antibody testing and adapted vaccine schedules should be considered to protect these at-risk patients,” Nicholas A. Kennedy, PhD, MBBS, of the University of Exeter (England) and colleagues wrote in a preprint published March 29 on MedRxiv.
Infliximab is an anti–tumor necrosis factor (anti-TNF) monoclonal antibody that’s approved to treat adult and pediatric Crohn’s disease and ulcerative colitis, as well as rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis, and plaque psoriasis, whereas vedolizumab, a gut selective anti-integrin alpha4beta7 monoclonal antibody that is not associated with impaired systemic immune responses, is approved to treat Crohn’s disease and ulcerative colitis in adults.
A previous study from Kennedy and colleagues revealed that IBD patients on infliximab showed a weakened COVID-19 antibody response compared with patients on vedolizumab. To determine if treatment with anti-TNF drugs impacted the efficacy of the first shot of these two-dose COVID-19 vaccines, the researchers used data from the CLARITY IBD study to assess 865 infliximab- and 428 vedolizumab-treated participants without evidence of prior SARS-CoV-2 infection who had received uninterrupted biologic therapy since being recruited between Sept. 22 and Dec. 23, 2020.
In the 3-10 weeks after initial vaccination, geometric mean concentrations for SARS-CoV-2 anti-spike protein receptor-binding protein antibodies were lower in patients on infliximab, compared with patients on vedolizumab for both the Pfizer (6.0 U/mL [5.9] versus 28.8 U/mL [5.4], P < .0001) and AstraZeneca (4.7 U/mL [4.9] versus 13.8 U/mL [5.9]; P < .0001) vaccines. The researchers’ multivariable models reinforced those findings, with antibody concentrations lower in infliximab-treated patients for both the Pfizer (fold change, 0.29; 95% confidence interval, 0.21-0.40; P < .0001) and AstraZeneca (FC, 0.39; 95% CI, 0.30-0.51; P < .0001) vaccines.
After second doses of the two-dose Pfizer vaccine, 85% of patients on infliximab and 86% of patients on vedolizumab seroconverted (P = .68); similarly high seroconversion rates were seen in patients who had been infected with SARS-CoV-2 prior to receiving either vaccine. Several patient characteristics were associated with lower antibody concentrations regardless of vaccine type: being 60 years or older, use of immunomodulators, having Crohn’s disease, and being a smoker. Alternatively, non-White ethnicity was associated with higher antibody concentrations.
Evidence has ‘unclear clinical significance’
“These data, which require peer review, do not change my opinion on the safety and efficacy of COVID-19 vaccines in patients taking TNF inhibitors such as infliximab as monotherapy for the treatment of psoriatic disease,” Joel M. Gelfand MD, director of the psoriasis and phototherapy treatment center at the University of Pennsylvania, Philadelphia, said in an interview.
“First, two peer-reviewed studies found good antibody response in patients on TNF inhibitors receiving COVID-19 vaccines (doi: 10.1136/annrheumdis-2021-220289; 10.1136/annrheumdis-2021-220272). Second, antibody responses were robust in the small cohort that received the second dose of a COVID-19 vaccine. We already know that, for the two messenger RNA-based vaccines available under emergency use authorization in the U.S., a second dose is required for optimal efficacy. Thus, evidence of a reduced antibody response after just one dose is of unclear clinical significance. Third, antibody responses are only a surrogate marker, and a low antibody response doesn’t necessarily mean the patient will not be protected by the vaccine.”
Focus on the second dose of a two-dose regimen
“Tell me about the response in people who got both doses of a vaccine that you’re supposed to get both doses of,” Jeffrey Curtis, MD, professor of medicine in the division of clinical immunology and rheumatology at the University of Alabama at Birmingham, said in an interview. “The number of patients in that subset was small [n = 27] but in my opinion that’s the most clinically relevant analysis and the one that patients and clinicians want answered.”
He also emphasized the uncertainty around what ‘protection’ means in these early days of studying COVID-19 vaccine responses. “You can define seroprotection or seroconversion as some absolute level of an antibody response, but if you want to say ‘Mrs. Smith, your antibody level was X,’ on whatever arbitrary scale with whoever’s arbitrary lab test, nobody actually knows that Mrs. Smith is now protected from SARS-CoV-2, or how protected,” he said.
“What is not terribly controversial is: If you can’t detect antibodies, the vaccine didn’t ‘take,’ if you will. But if I tell you that the mean antibody level was X with one drug and then 2X with another drug, does that mean that you’re twice as protected? We don’t know that. I’m fearful that people are looking at these studies and thinking that more is better. It might be, but we don’t know that to be true.”
Debating the cause of weakened immune responses
“The biological plausibility of being on an anti-TNF affecting your immune reaction to a messenger RNA or even a replication-deficient viral vector vaccine doesn’t make sense,” David T. Rubin, MD, professor of medicine at the University of Chicago and chair of the National Scientific Advisory Committee of the Crohn’s and Colitis Foundation, said in an interview.
“I’m sure immunologists may differ with me on this, but given what we have come to appreciate about these vaccine mechanisms, this finding doesn’t make intuitive sense. So we need to make sure that, when this happens, we look to the next studies and try to understand, was there any other confounder that may have resulted in these findings that was not adequately adjusted for or addressed in some other way?
“When you have a study of this size, you argue, ‘Because it’s so large, any effect that was seen must be real,’ ” he added. “Alternatively, to have a study of this size, by its very nature you are limited in being able to control for certain other factors or differences between the groups.”
That said, he commended the authors for their study and acknowledged the potential questions it raises about the single-shot Johnson & Johnson vaccine. “If you only get one and you’re on infliximab, this study implies that maybe that’s not enough,” he said. “Despite the fact that Johnson & Johnson was approved as a single dose, it may be necessary to think about it as the first of two, or maybe it’s not the preferred vaccine in this group of patients.”
The study was supported by the Royal Devon and Exeter and Hull University Hospital Foundation NHS Trusts and unrestricted educational grants from Biogen (Switzerland), Celltrion Healthcare (South Korea), Galapagos NV (Belgium), and F. Hoffmann-La Roche (Switzerland). The authors acknowledged numerous potential conflicts of interest, including receiving grants, personal fees, and nonfinancial support from various pharmaceutical companies.
FROM MEDRXIV
Bimekizumab superior to adalimumab in head-to-head psoriasis study
for treatment of moderate to severe plaque psoriasis in the head-to-head, phase 3 BE SURE trial, Jerry Bagel, MD, said at Innovations in Dermatology: Virtual Spring Conference 2021.
“Results demonstrated that bimekizumab was superior to adalimumab over 16 weeks of treatment in terms of the speed, depth, and durability of skin clearance,” reported Dr. Bagel, a dermatologist at the Psoriasis Center of Central New Jersey, East Windsor.
The Food and Drug Administration is now reviewing UCB’s application for marketing approval of bimekizumab for treatment of moderate to severe psoriasis in adults.
BE SURE was a 478-patient, double-blind, phase 3 trial in which patients were randomized to one of three regimens: 320 mg of bimekizumab every 4 weeks; the tumor necrosis factor blocker adalimumab (Humira) at 40 mg every 2 weeks for 24 weeks, followed by a switch to bimekizumab at 320 mg every 4 weeks; or 320 mg of bimekizumab every 4 weeks for 16 weeks, then ratcheting back to dosing every 8 weeks. The trial concluded at week 56, Dr. Bagel explained at the conference sponsored by MedscapeLIVE! and the producers of the Hawaii Dermatology Seminar and Caribbean Dermatology Symposium.
The two coprimary endpoints were the 16-week rates of a 90% improvement from baseline in Psoriasis Area and Severity Index score, or PASI 90 response, and an Investigator’s Global Assessment (IGA) score of 0 or 1, meaning clear or almost clear. Bimekizumab every 4 weeks bested adalimumab on both endpoints, with a PASI 90 rate of 86.2%, compared with 47.2%, and a IGA 0/1 rate of 85.3% versus 57.2%. The 16-week PASI 100 response rate was 60.8% with bimekizumab and 23.9% with adalimumab.
The response to bimekizumab was notably fast: already by week 4, the PASI 75 rate was 76.4%, compared with 31.4% with adalimumab. And once patients switched from adalimumab to bimekizumab at week 24, their response rates shot up rapidly. Bimekizumab was equally effective whether dosed at 320 mg every 4 weeks or at maintenance dosing every 8 weeks, such that at week 56 patients in all three study arms had PASI 90 rates of 82%-84%.
The most frequent treatment-emergent adverse events associated with bimekizumab were oral candidiasis, nasopharyngitis, and upper respiratory tract infection. The oral candidiasis, which occurred in 13.2% of patients on bimekizumab every 4 weeks, was mainly mild to moderate, localized, and in no instance led to discontinuation of therapy, according to Dr. Bagel.
“Very impressive data,” commented session comoderator Linda Stein Gold, MD. “This study shows some data that’s potentially unprecedented. Bimekizumab was superior to one of the drugs that we know, we’ve used, and know is very, very effective.”
“Note the speed of this drug,” added comoderator Bruce E. Strober, MD, PhD, of Yale University, New Haven, Conn., and Central Connecticut Dermatology, Cromwell, Conn. “It achieved at week 4 the efficacy that it took adalimumab until week 16 to reach. So it is a very fast drug. Bimekizumab will be the fastest drug you’ve ever, ever worked with.”
“You’ll see in the bimekizumab studies about a fivefold increased frequency of oral candidiasis relative to our more legacy IL-17 inhibitors, such as ixekizumab, secukinumab, and brodalumab. I think that means approximately one in five or one in six patients will have some form of candidiasis when you treat them with bimekizumab,” he said. Therefore, he added, “in some patients you’ll have to manage oral candidiasis. Most affected patients don’t leave the studies, so it’s manageable, but you’ll have to become something of an authority on how to treat with, for example, oral antifungal swish-and-swallow, swish-and-spit, or oral fluconazole. And some of these patients will have recurrent infections.”
It’s a prospect that doesn’t concern Dr. Stein Gold. “This is a side effect that we can treat. We can see it, we’re comfortable with it, and it’s certainly something we can get a handle on,” said Dr. Stein Gold, director of dermatology clinical research at the Henry Ford Health System in Detroit.
BE SURE was funded by UCB. Dr. Bagel reported serving as a speaker for, consultant to, and paid investigator for AbbVie, Celgene, Eli Lilly, Leo Pharma, Novartis, and Ortho Pharmaceuticals. Dr. Stein Gold and Dr. Strober reported having financial relationships with numerous pharmaceutical companies.
MedscapeLIVE! and this news organization are owned by the same parent company.
for treatment of moderate to severe plaque psoriasis in the head-to-head, phase 3 BE SURE trial, Jerry Bagel, MD, said at Innovations in Dermatology: Virtual Spring Conference 2021.
“Results demonstrated that bimekizumab was superior to adalimumab over 16 weeks of treatment in terms of the speed, depth, and durability of skin clearance,” reported Dr. Bagel, a dermatologist at the Psoriasis Center of Central New Jersey, East Windsor.
The Food and Drug Administration is now reviewing UCB’s application for marketing approval of bimekizumab for treatment of moderate to severe psoriasis in adults.
BE SURE was a 478-patient, double-blind, phase 3 trial in which patients were randomized to one of three regimens: 320 mg of bimekizumab every 4 weeks; the tumor necrosis factor blocker adalimumab (Humira) at 40 mg every 2 weeks for 24 weeks, followed by a switch to bimekizumab at 320 mg every 4 weeks; or 320 mg of bimekizumab every 4 weeks for 16 weeks, then ratcheting back to dosing every 8 weeks. The trial concluded at week 56, Dr. Bagel explained at the conference sponsored by MedscapeLIVE! and the producers of the Hawaii Dermatology Seminar and Caribbean Dermatology Symposium.
The two coprimary endpoints were the 16-week rates of a 90% improvement from baseline in Psoriasis Area and Severity Index score, or PASI 90 response, and an Investigator’s Global Assessment (IGA) score of 0 or 1, meaning clear or almost clear. Bimekizumab every 4 weeks bested adalimumab on both endpoints, with a PASI 90 rate of 86.2%, compared with 47.2%, and a IGA 0/1 rate of 85.3% versus 57.2%. The 16-week PASI 100 response rate was 60.8% with bimekizumab and 23.9% with adalimumab.
The response to bimekizumab was notably fast: already by week 4, the PASI 75 rate was 76.4%, compared with 31.4% with adalimumab. And once patients switched from adalimumab to bimekizumab at week 24, their response rates shot up rapidly. Bimekizumab was equally effective whether dosed at 320 mg every 4 weeks or at maintenance dosing every 8 weeks, such that at week 56 patients in all three study arms had PASI 90 rates of 82%-84%.
The most frequent treatment-emergent adverse events associated with bimekizumab were oral candidiasis, nasopharyngitis, and upper respiratory tract infection. The oral candidiasis, which occurred in 13.2% of patients on bimekizumab every 4 weeks, was mainly mild to moderate, localized, and in no instance led to discontinuation of therapy, according to Dr. Bagel.
“Very impressive data,” commented session comoderator Linda Stein Gold, MD. “This study shows some data that’s potentially unprecedented. Bimekizumab was superior to one of the drugs that we know, we’ve used, and know is very, very effective.”
“Note the speed of this drug,” added comoderator Bruce E. Strober, MD, PhD, of Yale University, New Haven, Conn., and Central Connecticut Dermatology, Cromwell, Conn. “It achieved at week 4 the efficacy that it took adalimumab until week 16 to reach. So it is a very fast drug. Bimekizumab will be the fastest drug you’ve ever, ever worked with.”
“You’ll see in the bimekizumab studies about a fivefold increased frequency of oral candidiasis relative to our more legacy IL-17 inhibitors, such as ixekizumab, secukinumab, and brodalumab. I think that means approximately one in five or one in six patients will have some form of candidiasis when you treat them with bimekizumab,” he said. Therefore, he added, “in some patients you’ll have to manage oral candidiasis. Most affected patients don’t leave the studies, so it’s manageable, but you’ll have to become something of an authority on how to treat with, for example, oral antifungal swish-and-swallow, swish-and-spit, or oral fluconazole. And some of these patients will have recurrent infections.”
It’s a prospect that doesn’t concern Dr. Stein Gold. “This is a side effect that we can treat. We can see it, we’re comfortable with it, and it’s certainly something we can get a handle on,” said Dr. Stein Gold, director of dermatology clinical research at the Henry Ford Health System in Detroit.
BE SURE was funded by UCB. Dr. Bagel reported serving as a speaker for, consultant to, and paid investigator for AbbVie, Celgene, Eli Lilly, Leo Pharma, Novartis, and Ortho Pharmaceuticals. Dr. Stein Gold and Dr. Strober reported having financial relationships with numerous pharmaceutical companies.
MedscapeLIVE! and this news organization are owned by the same parent company.
for treatment of moderate to severe plaque psoriasis in the head-to-head, phase 3 BE SURE trial, Jerry Bagel, MD, said at Innovations in Dermatology: Virtual Spring Conference 2021.
“Results demonstrated that bimekizumab was superior to adalimumab over 16 weeks of treatment in terms of the speed, depth, and durability of skin clearance,” reported Dr. Bagel, a dermatologist at the Psoriasis Center of Central New Jersey, East Windsor.
The Food and Drug Administration is now reviewing UCB’s application for marketing approval of bimekizumab for treatment of moderate to severe psoriasis in adults.
BE SURE was a 478-patient, double-blind, phase 3 trial in which patients were randomized to one of three regimens: 320 mg of bimekizumab every 4 weeks; the tumor necrosis factor blocker adalimumab (Humira) at 40 mg every 2 weeks for 24 weeks, followed by a switch to bimekizumab at 320 mg every 4 weeks; or 320 mg of bimekizumab every 4 weeks for 16 weeks, then ratcheting back to dosing every 8 weeks. The trial concluded at week 56, Dr. Bagel explained at the conference sponsored by MedscapeLIVE! and the producers of the Hawaii Dermatology Seminar and Caribbean Dermatology Symposium.
The two coprimary endpoints were the 16-week rates of a 90% improvement from baseline in Psoriasis Area and Severity Index score, or PASI 90 response, and an Investigator’s Global Assessment (IGA) score of 0 or 1, meaning clear or almost clear. Bimekizumab every 4 weeks bested adalimumab on both endpoints, with a PASI 90 rate of 86.2%, compared with 47.2%, and a IGA 0/1 rate of 85.3% versus 57.2%. The 16-week PASI 100 response rate was 60.8% with bimekizumab and 23.9% with adalimumab.
The response to bimekizumab was notably fast: already by week 4, the PASI 75 rate was 76.4%, compared with 31.4% with adalimumab. And once patients switched from adalimumab to bimekizumab at week 24, their response rates shot up rapidly. Bimekizumab was equally effective whether dosed at 320 mg every 4 weeks or at maintenance dosing every 8 weeks, such that at week 56 patients in all three study arms had PASI 90 rates of 82%-84%.
The most frequent treatment-emergent adverse events associated with bimekizumab were oral candidiasis, nasopharyngitis, and upper respiratory tract infection. The oral candidiasis, which occurred in 13.2% of patients on bimekizumab every 4 weeks, was mainly mild to moderate, localized, and in no instance led to discontinuation of therapy, according to Dr. Bagel.
“Very impressive data,” commented session comoderator Linda Stein Gold, MD. “This study shows some data that’s potentially unprecedented. Bimekizumab was superior to one of the drugs that we know, we’ve used, and know is very, very effective.”
“Note the speed of this drug,” added comoderator Bruce E. Strober, MD, PhD, of Yale University, New Haven, Conn., and Central Connecticut Dermatology, Cromwell, Conn. “It achieved at week 4 the efficacy that it took adalimumab until week 16 to reach. So it is a very fast drug. Bimekizumab will be the fastest drug you’ve ever, ever worked with.”
“You’ll see in the bimekizumab studies about a fivefold increased frequency of oral candidiasis relative to our more legacy IL-17 inhibitors, such as ixekizumab, secukinumab, and brodalumab. I think that means approximately one in five or one in six patients will have some form of candidiasis when you treat them with bimekizumab,” he said. Therefore, he added, “in some patients you’ll have to manage oral candidiasis. Most affected patients don’t leave the studies, so it’s manageable, but you’ll have to become something of an authority on how to treat with, for example, oral antifungal swish-and-swallow, swish-and-spit, or oral fluconazole. And some of these patients will have recurrent infections.”
It’s a prospect that doesn’t concern Dr. Stein Gold. “This is a side effect that we can treat. We can see it, we’re comfortable with it, and it’s certainly something we can get a handle on,” said Dr. Stein Gold, director of dermatology clinical research at the Henry Ford Health System in Detroit.
BE SURE was funded by UCB. Dr. Bagel reported serving as a speaker for, consultant to, and paid investigator for AbbVie, Celgene, Eli Lilly, Leo Pharma, Novartis, and Ortho Pharmaceuticals. Dr. Stein Gold and Dr. Strober reported having financial relationships with numerous pharmaceutical companies.
MedscapeLIVE! and this news organization are owned by the same parent company.
FROM INNOVATIONS IN DERMATOLOGY
Hedgehog inhibitor alternative dosing advantageous for BCC
in a successful effort to maintain efficacy while reducing treatment discontinuation caused by unacceptable side effects, Vishal Patel, MD, said at Innovations in Dermatology: Virtual Spring Conference 2021.
“It’s the tolerability issues that make these drugs very difficult to prescribe and use regularly. What we’ve seen in the last few years is that a lot of alternative dosing regimens have been published that have been both effective at treating the tumor and keeping the tumor clear and at bay while lowering the side-effect profile,” explained Dr. Patel, a Mohs surgeon and director of the cutaneous oncology program at the George Washington University Cancer Center in Washington, D.C.
Product labeling for the two available hedgehog pathway inhibitors, vismodegib (Erivedge) and sonidegib (Odomzo), calls for once-daily therapy until disease progression or unacceptable toxicity. Studies show that, when used in this way, these agents achieve objective response rates in the 40% range for patients with locally advanced BCC and 15%-33% for those with metastatic BCC.
“The critical thing in these patients is not that the drugs work – although they can work in quite remarkable ways – but rather it’s that nearly all patients experience at least one side effect. And grade 3 or 4 adverse effects that can lead to cessation of drug occur in about 25% of patients,” he said at the conference sponsored by MedscapeLIVE! and the producers of the Hawaii Dermatology Seminar and Caribbean Dermatology Symposium.
The classic side effects of the hedgehog pathway inhibitors are muscle spasms, hair loss, fatigue, loss of taste, diarrhea, and weight loss.
Among the alternative dosing regimens that have been published with good results, mostly in single-center retrospective case series, are a weekdays-on/weekends-off strategy at the Cleveland Clinic and an Italian approach entailing an initial 3-4 months of daily therapy followed by a switch to alternate-day therapy.
But Dr. Patel favors a different off-label regimen in lieu of Food and Drug Administration–recommended daily dosing indefinitely. It takes advantage of the fact that most patients don’t begin to get the classic side effects until about the 3-month mark.
“What we’ve begun to recommend as a much better option for patients who need to be on the drug potentially forever is that the drug is dosed daily for 3 months to shrink the tumor and get the optimal effect, and then at that point we taper the dose down to every other day, then every third day, or even up to a week as long as the tumor continues to stay at bay. If there’s any sign of recurrence or a scouting biopsy shows tumor, we reinstitute the daily medicine,” the dermatologist said.
This strategy requires careful monitoring for emergence of the typical side effects. Also, an important caveat regarding sonidegib is that it shouldn’t be given concomitantly with medications that are moderate or strong inhibitors of CYP3A, so it’s essential to get a complete medical history when giving this drug, Dr. Patel noted.
He reported having no financial conflicts regarding his presentation.
MedscapeLIVE! and this news organization are owned by the same parent company.
in a successful effort to maintain efficacy while reducing treatment discontinuation caused by unacceptable side effects, Vishal Patel, MD, said at Innovations in Dermatology: Virtual Spring Conference 2021.
“It’s the tolerability issues that make these drugs very difficult to prescribe and use regularly. What we’ve seen in the last few years is that a lot of alternative dosing regimens have been published that have been both effective at treating the tumor and keeping the tumor clear and at bay while lowering the side-effect profile,” explained Dr. Patel, a Mohs surgeon and director of the cutaneous oncology program at the George Washington University Cancer Center in Washington, D.C.
Product labeling for the two available hedgehog pathway inhibitors, vismodegib (Erivedge) and sonidegib (Odomzo), calls for once-daily therapy until disease progression or unacceptable toxicity. Studies show that, when used in this way, these agents achieve objective response rates in the 40% range for patients with locally advanced BCC and 15%-33% for those with metastatic BCC.
“The critical thing in these patients is not that the drugs work – although they can work in quite remarkable ways – but rather it’s that nearly all patients experience at least one side effect. And grade 3 or 4 adverse effects that can lead to cessation of drug occur in about 25% of patients,” he said at the conference sponsored by MedscapeLIVE! and the producers of the Hawaii Dermatology Seminar and Caribbean Dermatology Symposium.
The classic side effects of the hedgehog pathway inhibitors are muscle spasms, hair loss, fatigue, loss of taste, diarrhea, and weight loss.
Among the alternative dosing regimens that have been published with good results, mostly in single-center retrospective case series, are a weekdays-on/weekends-off strategy at the Cleveland Clinic and an Italian approach entailing an initial 3-4 months of daily therapy followed by a switch to alternate-day therapy.
But Dr. Patel favors a different off-label regimen in lieu of Food and Drug Administration–recommended daily dosing indefinitely. It takes advantage of the fact that most patients don’t begin to get the classic side effects until about the 3-month mark.
“What we’ve begun to recommend as a much better option for patients who need to be on the drug potentially forever is that the drug is dosed daily for 3 months to shrink the tumor and get the optimal effect, and then at that point we taper the dose down to every other day, then every third day, or even up to a week as long as the tumor continues to stay at bay. If there’s any sign of recurrence or a scouting biopsy shows tumor, we reinstitute the daily medicine,” the dermatologist said.
This strategy requires careful monitoring for emergence of the typical side effects. Also, an important caveat regarding sonidegib is that it shouldn’t be given concomitantly with medications that are moderate or strong inhibitors of CYP3A, so it’s essential to get a complete medical history when giving this drug, Dr. Patel noted.
He reported having no financial conflicts regarding his presentation.
MedscapeLIVE! and this news organization are owned by the same parent company.
in a successful effort to maintain efficacy while reducing treatment discontinuation caused by unacceptable side effects, Vishal Patel, MD, said at Innovations in Dermatology: Virtual Spring Conference 2021.
“It’s the tolerability issues that make these drugs very difficult to prescribe and use regularly. What we’ve seen in the last few years is that a lot of alternative dosing regimens have been published that have been both effective at treating the tumor and keeping the tumor clear and at bay while lowering the side-effect profile,” explained Dr. Patel, a Mohs surgeon and director of the cutaneous oncology program at the George Washington University Cancer Center in Washington, D.C.
Product labeling for the two available hedgehog pathway inhibitors, vismodegib (Erivedge) and sonidegib (Odomzo), calls for once-daily therapy until disease progression or unacceptable toxicity. Studies show that, when used in this way, these agents achieve objective response rates in the 40% range for patients with locally advanced BCC and 15%-33% for those with metastatic BCC.
“The critical thing in these patients is not that the drugs work – although they can work in quite remarkable ways – but rather it’s that nearly all patients experience at least one side effect. And grade 3 or 4 adverse effects that can lead to cessation of drug occur in about 25% of patients,” he said at the conference sponsored by MedscapeLIVE! and the producers of the Hawaii Dermatology Seminar and Caribbean Dermatology Symposium.
The classic side effects of the hedgehog pathway inhibitors are muscle spasms, hair loss, fatigue, loss of taste, diarrhea, and weight loss.
Among the alternative dosing regimens that have been published with good results, mostly in single-center retrospective case series, are a weekdays-on/weekends-off strategy at the Cleveland Clinic and an Italian approach entailing an initial 3-4 months of daily therapy followed by a switch to alternate-day therapy.
But Dr. Patel favors a different off-label regimen in lieu of Food and Drug Administration–recommended daily dosing indefinitely. It takes advantage of the fact that most patients don’t begin to get the classic side effects until about the 3-month mark.
“What we’ve begun to recommend as a much better option for patients who need to be on the drug potentially forever is that the drug is dosed daily for 3 months to shrink the tumor and get the optimal effect, and then at that point we taper the dose down to every other day, then every third day, or even up to a week as long as the tumor continues to stay at bay. If there’s any sign of recurrence or a scouting biopsy shows tumor, we reinstitute the daily medicine,” the dermatologist said.
This strategy requires careful monitoring for emergence of the typical side effects. Also, an important caveat regarding sonidegib is that it shouldn’t be given concomitantly with medications that are moderate or strong inhibitors of CYP3A, so it’s essential to get a complete medical history when giving this drug, Dr. Patel noted.
He reported having no financial conflicts regarding his presentation.
MedscapeLIVE! and this news organization are owned by the same parent company.
FROM INNOVATIONS IN DERMATOLOGY
Excess deaths jump 23% in U.S. in 2020, mostly because of COVID-19
The United States saw nearly 23% more deaths than expected during the first 9 months of the pandemic, and almost three-quarters of those deaths involved COVID-19.
For comparison, the death rate increased by 2.5% or less annually in recent years.
At the same time, rates of deaths from heart disease, Alzheimer’s disease or dementia, and diabetes also increased from March 1, 2020, to Jan. 2, 2021, especially during COVID-19 surges.
“Excess deaths surged in the east in April, followed by extended summer and early winter surges concentrated in Southern and Western states, respectively. Many of these states weakly embraced, or discouraged, pandemic control measures and lifted restrictions earlier than other states,” lead author Steven H. Woolf, MD, MPH, from the Virginia Commonwealth University, Richmond, and colleagues wrote in a research letter published online April 2, 2021, in JAMA.
COVID-19 mortality included all deaths for which it was cited as an underlying or contributing cause in records from the District of Columbia and 49 states. North Carolina was excluded for insufficient data.
More than half a million excess deaths
Between March 1, 2020, and Jan. 2, 2021, the United States experienced 2,801,439 deaths, or 522,368 excess deaths. A total 72.4% of these events were attributed to COVID-19.
Not all racial and ethnic groups were equally represented. For example, the rate of excess deaths was higher among non-Hispanic Black populations, at 208.4 deaths per 100,000. Non-Hispanic White populations experienced 157 deaths per 100,000, and Hispanic populations experienced 139.8 deaths per 100,000.
Further, non-Hispanic Black individuals accounted for 16.9% of the excess deaths but only 12.5% of the U.S. population, which reflects “racial disparities in COVID-19 mortality,” the authors noted.
Not adjusting for population aging is a potential limitation, as was reliance on provisional data and the likelihood that some death certificates were inaccurate.
In February, Anthony S. Fauci, MD, chief medical adviser to President Joe Biden, stated that political divisions likely played a role in the 500,000-plus COVID-19–related deaths in the United States.
Then a report came out on March 26 indicating that a different U.S. response to the pandemic could have avoided almost 400,000 COVID-19 deaths. In addition, an April 1 study in the CDC’s Morbidity and Mortality Weekly Report revealed that COVID-19 is now the third leading cause of death in the United States, after heart disease and cancer.
‘Massive’ excessive mortality
“There is no more visible or alarming manifestation of the toll of the COVID-19 pandemic than the deaths it has caused. In this issue of JAMA, Dr. Woolf and colleagues provide updated analyses that demonstrate that the excess mortality in the U.S. between March 1, 2020, and Jan. 2, 2021, has been massive,” Alan Garber, MD, PhD, wrote in an accompanying editorial.
“It seems likely that COVID-19 will have contributed to nearly as many deaths in the U.S. as the great influenza pandemic of 1918, and more than in any influenza outbreak in the U.S. since then,” added Dr. Garber, provost of Harvard University in Cambridge, Mass.
This study of excess mortality illustrates what is at stake, he added. “Despite the scientific, medical and public health progress of recent decades, the loss of life attributable to the COVID-19 pandemic exceeds the mortality of major wars. No nation should squander this opportunity to do what it takes to prepare for the next one.”
Dr. Woolf and Dr. Garber disclosed no relevant financial relationships. The National Institutes of Health supported the research through its National Center for Advancing Translational Sciences and the National Institute on Aging.
A version of this article first appeared on Medscape.com.
The United States saw nearly 23% more deaths than expected during the first 9 months of the pandemic, and almost three-quarters of those deaths involved COVID-19.
For comparison, the death rate increased by 2.5% or less annually in recent years.
At the same time, rates of deaths from heart disease, Alzheimer’s disease or dementia, and diabetes also increased from March 1, 2020, to Jan. 2, 2021, especially during COVID-19 surges.
“Excess deaths surged in the east in April, followed by extended summer and early winter surges concentrated in Southern and Western states, respectively. Many of these states weakly embraced, or discouraged, pandemic control measures and lifted restrictions earlier than other states,” lead author Steven H. Woolf, MD, MPH, from the Virginia Commonwealth University, Richmond, and colleagues wrote in a research letter published online April 2, 2021, in JAMA.
COVID-19 mortality included all deaths for which it was cited as an underlying or contributing cause in records from the District of Columbia and 49 states. North Carolina was excluded for insufficient data.
More than half a million excess deaths
Between March 1, 2020, and Jan. 2, 2021, the United States experienced 2,801,439 deaths, or 522,368 excess deaths. A total 72.4% of these events were attributed to COVID-19.
Not all racial and ethnic groups were equally represented. For example, the rate of excess deaths was higher among non-Hispanic Black populations, at 208.4 deaths per 100,000. Non-Hispanic White populations experienced 157 deaths per 100,000, and Hispanic populations experienced 139.8 deaths per 100,000.
Further, non-Hispanic Black individuals accounted for 16.9% of the excess deaths but only 12.5% of the U.S. population, which reflects “racial disparities in COVID-19 mortality,” the authors noted.
Not adjusting for population aging is a potential limitation, as was reliance on provisional data and the likelihood that some death certificates were inaccurate.
In February, Anthony S. Fauci, MD, chief medical adviser to President Joe Biden, stated that political divisions likely played a role in the 500,000-plus COVID-19–related deaths in the United States.
Then a report came out on March 26 indicating that a different U.S. response to the pandemic could have avoided almost 400,000 COVID-19 deaths. In addition, an April 1 study in the CDC’s Morbidity and Mortality Weekly Report revealed that COVID-19 is now the third leading cause of death in the United States, after heart disease and cancer.
‘Massive’ excessive mortality
“There is no more visible or alarming manifestation of the toll of the COVID-19 pandemic than the deaths it has caused. In this issue of JAMA, Dr. Woolf and colleagues provide updated analyses that demonstrate that the excess mortality in the U.S. between March 1, 2020, and Jan. 2, 2021, has been massive,” Alan Garber, MD, PhD, wrote in an accompanying editorial.
“It seems likely that COVID-19 will have contributed to nearly as many deaths in the U.S. as the great influenza pandemic of 1918, and more than in any influenza outbreak in the U.S. since then,” added Dr. Garber, provost of Harvard University in Cambridge, Mass.
This study of excess mortality illustrates what is at stake, he added. “Despite the scientific, medical and public health progress of recent decades, the loss of life attributable to the COVID-19 pandemic exceeds the mortality of major wars. No nation should squander this opportunity to do what it takes to prepare for the next one.”
Dr. Woolf and Dr. Garber disclosed no relevant financial relationships. The National Institutes of Health supported the research through its National Center for Advancing Translational Sciences and the National Institute on Aging.
A version of this article first appeared on Medscape.com.
The United States saw nearly 23% more deaths than expected during the first 9 months of the pandemic, and almost three-quarters of those deaths involved COVID-19.
For comparison, the death rate increased by 2.5% or less annually in recent years.
At the same time, rates of deaths from heart disease, Alzheimer’s disease or dementia, and diabetes also increased from March 1, 2020, to Jan. 2, 2021, especially during COVID-19 surges.
“Excess deaths surged in the east in April, followed by extended summer and early winter surges concentrated in Southern and Western states, respectively. Many of these states weakly embraced, or discouraged, pandemic control measures and lifted restrictions earlier than other states,” lead author Steven H. Woolf, MD, MPH, from the Virginia Commonwealth University, Richmond, and colleagues wrote in a research letter published online April 2, 2021, in JAMA.
COVID-19 mortality included all deaths for which it was cited as an underlying or contributing cause in records from the District of Columbia and 49 states. North Carolina was excluded for insufficient data.
More than half a million excess deaths
Between March 1, 2020, and Jan. 2, 2021, the United States experienced 2,801,439 deaths, or 522,368 excess deaths. A total 72.4% of these events were attributed to COVID-19.
Not all racial and ethnic groups were equally represented. For example, the rate of excess deaths was higher among non-Hispanic Black populations, at 208.4 deaths per 100,000. Non-Hispanic White populations experienced 157 deaths per 100,000, and Hispanic populations experienced 139.8 deaths per 100,000.
Further, non-Hispanic Black individuals accounted for 16.9% of the excess deaths but only 12.5% of the U.S. population, which reflects “racial disparities in COVID-19 mortality,” the authors noted.
Not adjusting for population aging is a potential limitation, as was reliance on provisional data and the likelihood that some death certificates were inaccurate.
In February, Anthony S. Fauci, MD, chief medical adviser to President Joe Biden, stated that political divisions likely played a role in the 500,000-plus COVID-19–related deaths in the United States.
Then a report came out on March 26 indicating that a different U.S. response to the pandemic could have avoided almost 400,000 COVID-19 deaths. In addition, an April 1 study in the CDC’s Morbidity and Mortality Weekly Report revealed that COVID-19 is now the third leading cause of death in the United States, after heart disease and cancer.
‘Massive’ excessive mortality
“There is no more visible or alarming manifestation of the toll of the COVID-19 pandemic than the deaths it has caused. In this issue of JAMA, Dr. Woolf and colleagues provide updated analyses that demonstrate that the excess mortality in the U.S. between March 1, 2020, and Jan. 2, 2021, has been massive,” Alan Garber, MD, PhD, wrote in an accompanying editorial.
“It seems likely that COVID-19 will have contributed to nearly as many deaths in the U.S. as the great influenza pandemic of 1918, and more than in any influenza outbreak in the U.S. since then,” added Dr. Garber, provost of Harvard University in Cambridge, Mass.
This study of excess mortality illustrates what is at stake, he added. “Despite the scientific, medical and public health progress of recent decades, the loss of life attributable to the COVID-19 pandemic exceeds the mortality of major wars. No nation should squander this opportunity to do what it takes to prepare for the next one.”
Dr. Woolf and Dr. Garber disclosed no relevant financial relationships. The National Institutes of Health supported the research through its National Center for Advancing Translational Sciences and the National Institute on Aging.
A version of this article first appeared on Medscape.com.
Novel analysis quantifies the benefit of melanoma screening
Patients at very high risk for melanoma, including those with a family history or with inherited pathogenic variants of genes that increase the risk, likely benefit from routine whole-body screening for melanoma and education about UV protection.
Those are key findings from the first prospective cohort study to quantify the benefit of screening in melanoma-prone families, which was published online April 2 in Cancer Epidemiology, Biomarkers & Prevention.
“Whole-body screening for melanoma is currently routine for individuals at high risk for melanoma, which includes people from melanoma-prone families (at least two relatives who have had melanoma) and those with inherited pathogenic gene variants of the CDKN2A or CDK4 genes, which increase risk for melanoma,” lead author Michael R. Sargen, MD, said in an interview. “In our study, we investigated whether screening and educational interventions, including education about the appearance of melanoma and strategies for protecting skin from ultraviolet damage, contributed to early diagnosis of melanoma in individuals from melanoma-prone families.”
Of the 293 individuals who enrolled in the study between 1976 and 2014, 246 were diagnosed with melanoma before enrollment (the prestudy cohort) and 47 were diagnosed after enrollment (the prospective cohort). The researchers compared differences in melanoma thickness and tumor stage between participants in the prestudy and prospective cohorts, and compared tumor-thickness trends between participants in their study and cases in the general population using data from Surveillance, Epidemiology, and End Results (SEER) registries between 1973 and 2016. Because information on melanoma thickness was missing for 24% of melanoma cases in the NCI Familial Melanoma Study and 8.7% of melanoma cases found in the SEER registry, the researchers imputed the missing data.
After adjusting for gender and age, Dr. Sargen and his colleagues found that participants in the prospective cohort had significantly thinner melanomas, compared with those in the prestudy cohort (0.6 mm vs. 1.1 mm, respectively; P < .001). In addition, 83% of those in the prospective cohort were significantly more likely to be diagnosed at the early T1 stage, compared with 40% of those in the prestudy cohort (P < .001).
In their analysis, they also determined that after adjusting for gender and age, “all NCI family cases had systematically lower thickness than SEER cases during the study period.” The reductions in melanoma thickness and tumor stage, they concluded, “were not fully explained by calendar period effects of decreasing thickness in the general population and point to the potential benefit of skin cancer screening for patients with a family history of melanoma and those with pathogenic germline variants of melanoma-susceptibility genes.”
“Our data provide reassuring evidence that screening, alongside education about proper UV protection and the appearance of melanoma, is likely benefiting patients with a significantly elevated risk for melanoma,” Dr. Sargen said in the interview “Further studies are needed to determine whether individuals without a family history of melanoma may benefit from whole-body screening, and whether the benefits vary by ethnicity.”
He acknowledged certain limitations of the study, including the relatively small sample size of melanoma cases in the NCI Familial Melanoma Study and the imputation of missing melanoma-thickness data. “Additionally, since this was a prospective cohort study, we were not able to distinguish the independent effect of each intervention,” he said. “Randomized controlled studies are needed to understand the impact of each aspect of the intervention, such as whole-body screening, melanoma education, or strategies for skin protection.”
In an interview, Maryam M. Asgari, MD, professor of dermatology at Harvard University, Boston, called the analysis “well done,” but commented on the potential role of selection bias impacting the findings. “People who have a strong family history of melanoma and who are opting to engage in an NCI study and come in for full-body skin checks and go through that education process may have very different health-seeking behaviors than individuals in the general population that would be reported to SEER,” she said.
She also raised the question of whether the results were driven by the early detection through the NCI’s program of provider screening or through the educational component that enables earlier self-detection. “If you’re an individual involved in a study and that brings attention to your moles and you have a strong family history of melanoma to begin with, it is not surprising that you are going to have heightened awareness of any changing mole and therefore are more likely to have melanoma detected at an earlier stage,” Dr. Asgari said.
The study was supported by the Intramural Research Program of the Division of Cancer Epidemiology and Genetics at the National Cancer Institute. Dr. Sargen reported having no financial disclosures.
Dr. Asgari disclosed that she has received research support from the Melanoma Research Alliance.
Patients at very high risk for melanoma, including those with a family history or with inherited pathogenic variants of genes that increase the risk, likely benefit from routine whole-body screening for melanoma and education about UV protection.
Those are key findings from the first prospective cohort study to quantify the benefit of screening in melanoma-prone families, which was published online April 2 in Cancer Epidemiology, Biomarkers & Prevention.
“Whole-body screening for melanoma is currently routine for individuals at high risk for melanoma, which includes people from melanoma-prone families (at least two relatives who have had melanoma) and those with inherited pathogenic gene variants of the CDKN2A or CDK4 genes, which increase risk for melanoma,” lead author Michael R. Sargen, MD, said in an interview. “In our study, we investigated whether screening and educational interventions, including education about the appearance of melanoma and strategies for protecting skin from ultraviolet damage, contributed to early diagnosis of melanoma in individuals from melanoma-prone families.”
Of the 293 individuals who enrolled in the study between 1976 and 2014, 246 were diagnosed with melanoma before enrollment (the prestudy cohort) and 47 were diagnosed after enrollment (the prospective cohort). The researchers compared differences in melanoma thickness and tumor stage between participants in the prestudy and prospective cohorts, and compared tumor-thickness trends between participants in their study and cases in the general population using data from Surveillance, Epidemiology, and End Results (SEER) registries between 1973 and 2016. Because information on melanoma thickness was missing for 24% of melanoma cases in the NCI Familial Melanoma Study and 8.7% of melanoma cases found in the SEER registry, the researchers imputed the missing data.
After adjusting for gender and age, Dr. Sargen and his colleagues found that participants in the prospective cohort had significantly thinner melanomas, compared with those in the prestudy cohort (0.6 mm vs. 1.1 mm, respectively; P < .001). In addition, 83% of those in the prospective cohort were significantly more likely to be diagnosed at the early T1 stage, compared with 40% of those in the prestudy cohort (P < .001).
In their analysis, they also determined that after adjusting for gender and age, “all NCI family cases had systematically lower thickness than SEER cases during the study period.” The reductions in melanoma thickness and tumor stage, they concluded, “were not fully explained by calendar period effects of decreasing thickness in the general population and point to the potential benefit of skin cancer screening for patients with a family history of melanoma and those with pathogenic germline variants of melanoma-susceptibility genes.”
“Our data provide reassuring evidence that screening, alongside education about proper UV protection and the appearance of melanoma, is likely benefiting patients with a significantly elevated risk for melanoma,” Dr. Sargen said in the interview “Further studies are needed to determine whether individuals without a family history of melanoma may benefit from whole-body screening, and whether the benefits vary by ethnicity.”
He acknowledged certain limitations of the study, including the relatively small sample size of melanoma cases in the NCI Familial Melanoma Study and the imputation of missing melanoma-thickness data. “Additionally, since this was a prospective cohort study, we were not able to distinguish the independent effect of each intervention,” he said. “Randomized controlled studies are needed to understand the impact of each aspect of the intervention, such as whole-body screening, melanoma education, or strategies for skin protection.”
In an interview, Maryam M. Asgari, MD, professor of dermatology at Harvard University, Boston, called the analysis “well done,” but commented on the potential role of selection bias impacting the findings. “People who have a strong family history of melanoma and who are opting to engage in an NCI study and come in for full-body skin checks and go through that education process may have very different health-seeking behaviors than individuals in the general population that would be reported to SEER,” she said.
She also raised the question of whether the results were driven by the early detection through the NCI’s program of provider screening or through the educational component that enables earlier self-detection. “If you’re an individual involved in a study and that brings attention to your moles and you have a strong family history of melanoma to begin with, it is not surprising that you are going to have heightened awareness of any changing mole and therefore are more likely to have melanoma detected at an earlier stage,” Dr. Asgari said.
The study was supported by the Intramural Research Program of the Division of Cancer Epidemiology and Genetics at the National Cancer Institute. Dr. Sargen reported having no financial disclosures.
Dr. Asgari disclosed that she has received research support from the Melanoma Research Alliance.
Patients at very high risk for melanoma, including those with a family history or with inherited pathogenic variants of genes that increase the risk, likely benefit from routine whole-body screening for melanoma and education about UV protection.
Those are key findings from the first prospective cohort study to quantify the benefit of screening in melanoma-prone families, which was published online April 2 in Cancer Epidemiology, Biomarkers & Prevention.
“Whole-body screening for melanoma is currently routine for individuals at high risk for melanoma, which includes people from melanoma-prone families (at least two relatives who have had melanoma) and those with inherited pathogenic gene variants of the CDKN2A or CDK4 genes, which increase risk for melanoma,” lead author Michael R. Sargen, MD, said in an interview. “In our study, we investigated whether screening and educational interventions, including education about the appearance of melanoma and strategies for protecting skin from ultraviolet damage, contributed to early diagnosis of melanoma in individuals from melanoma-prone families.”
Of the 293 individuals who enrolled in the study between 1976 and 2014, 246 were diagnosed with melanoma before enrollment (the prestudy cohort) and 47 were diagnosed after enrollment (the prospective cohort). The researchers compared differences in melanoma thickness and tumor stage between participants in the prestudy and prospective cohorts, and compared tumor-thickness trends between participants in their study and cases in the general population using data from Surveillance, Epidemiology, and End Results (SEER) registries between 1973 and 2016. Because information on melanoma thickness was missing for 24% of melanoma cases in the NCI Familial Melanoma Study and 8.7% of melanoma cases found in the SEER registry, the researchers imputed the missing data.
After adjusting for gender and age, Dr. Sargen and his colleagues found that participants in the prospective cohort had significantly thinner melanomas, compared with those in the prestudy cohort (0.6 mm vs. 1.1 mm, respectively; P < .001). In addition, 83% of those in the prospective cohort were significantly more likely to be diagnosed at the early T1 stage, compared with 40% of those in the prestudy cohort (P < .001).
In their analysis, they also determined that after adjusting for gender and age, “all NCI family cases had systematically lower thickness than SEER cases during the study period.” The reductions in melanoma thickness and tumor stage, they concluded, “were not fully explained by calendar period effects of decreasing thickness in the general population and point to the potential benefit of skin cancer screening for patients with a family history of melanoma and those with pathogenic germline variants of melanoma-susceptibility genes.”
“Our data provide reassuring evidence that screening, alongside education about proper UV protection and the appearance of melanoma, is likely benefiting patients with a significantly elevated risk for melanoma,” Dr. Sargen said in the interview “Further studies are needed to determine whether individuals without a family history of melanoma may benefit from whole-body screening, and whether the benefits vary by ethnicity.”
He acknowledged certain limitations of the study, including the relatively small sample size of melanoma cases in the NCI Familial Melanoma Study and the imputation of missing melanoma-thickness data. “Additionally, since this was a prospective cohort study, we were not able to distinguish the independent effect of each intervention,” he said. “Randomized controlled studies are needed to understand the impact of each aspect of the intervention, such as whole-body screening, melanoma education, or strategies for skin protection.”
In an interview, Maryam M. Asgari, MD, professor of dermatology at Harvard University, Boston, called the analysis “well done,” but commented on the potential role of selection bias impacting the findings. “People who have a strong family history of melanoma and who are opting to engage in an NCI study and come in for full-body skin checks and go through that education process may have very different health-seeking behaviors than individuals in the general population that would be reported to SEER,” she said.
She also raised the question of whether the results were driven by the early detection through the NCI’s program of provider screening or through the educational component that enables earlier self-detection. “If you’re an individual involved in a study and that brings attention to your moles and you have a strong family history of melanoma to begin with, it is not surprising that you are going to have heightened awareness of any changing mole and therefore are more likely to have melanoma detected at an earlier stage,” Dr. Asgari said.
The study was supported by the Intramural Research Program of the Division of Cancer Epidemiology and Genetics at the National Cancer Institute. Dr. Sargen reported having no financial disclosures.
Dr. Asgari disclosed that she has received research support from the Melanoma Research Alliance.
FROM CANCER EPIDEMIOLOGY, BIOMARKERS, AND PREVENTION
Starting April 5, patients can read your notes: 5 things to consider
Change in writing style is not mandated
The mandate, called “open notes” by many, is part of the 21st Century Cures Act, a wide-ranging piece of federal health care legislation. The previous deadline of Nov. 2, 2020, for enacting open notes was extended last year because of the exigencies of the COVID-19 pandemic.
Organizations must provide access via patient portals to the following types of notes: consultations, discharge summaries, histories, physical examination findings, imaging narratives, laboratory and pathology report narratives, and procedure and progress notes. Noncompliant organizations will eventually be subject to fines from the Department of Health & Human Services for “information blocking.”
This news organization reported on the mandate in 2020, and some readers said it was an unwelcome intrusion into practice. Since then, this news organization has run additional open notes stories about physician concerns, a perspective essay addressing those fears, and a reader poll about the phenomenon.
Now, as the legislation turns into a practical clinical matter, there are five key points clinicians should consider.
Clinicians don’t have to change writing style.
The new law mandates timely patient access to notes and test results, but it doesn’t require that clinicians alter their writing, said Scott MacDonald, MD, an internist and electronic health record medical director at University of California Davis Health in Sacramento.
“You don’t have to change your notes,” he said. However, patients are now part of the note audience and some health care systems are directing clinicians to make patient-friendly style changes.
Everyday experience should guide clinicians when writing notes, said one expert.
“When you’re not sure [of how to write a note], just mirror the way you would speak in the office – that’s going to get you right, including for mental health issues,” advised Leonor Fernandez, MD, an internist at Beth Deaconess Israel Medical Center, Boston, in her “take-away” comments in the online video, How to Write an Open Note.
According to a 2020 Medscape poll of 1,050 physicians, a majority (56%) anticipate that they will write notes differently, knowing that patients can read them via open notes. Nearly two-thirds (64%) believe that this new wrinkle in medical records will increase their workload. However, actual practice suggests that this is true for a minority of practitioners, according to the results from a recent study of more than 1,000 physicians in Boston, Seattle, and rural Pennsylvania, who already work in open notes settings. Only about one-third (37%) reported “spending more time on documentation.”
Note writing is going to change because of the addition of the patient reader, and something will be lost, argued Steven Reidbord, MD, a psychiatrist in private practice in San Francisco. By watering down the language for patients, “you are trading away the technical precision and other advantages of having a professional language,” commented Dr. Reidbord, who blogs for Psychology Today and has criticized the open notes movement in the past.
However, years of investigation from OpenNotes, the Boston-based advocacy and research organization, indicates that there are many gains with patient-accessible notes, including improved medical record accuracy, greater medication adherence, and potentially improved health care disparities among a range of patient types. In a 2019 study, researchers said that worry and confusion among note-reading patients are uncommon (5% and 3%, respectively), which addresses two criticisms voiced by multiple people last year.
Some clinical notes can be withheld.
The new rules from the federal government permit information blocking if there is clear evidence that doing so “will substantially reduce the risk of harm” to patients or to other third parties, Tom Delbanco, MD, and Charlotte Blease, PhD, of OpenNotes in Boston wrote in a commentary in February 2021.
There are also state-level laws that can supersede the new U.S. law and block access to notes, points out MacDonald. For example, California law dictates that providers cannot post cancer test results without talking with the patient first.
The OpenNotes organization also points out that, with regard to sensitive psychotherapy notes that are separated from the rest of a medical record, those notes “can be kept from patients without their permission, and such rules vary state by state.”
Some patients are more likely readers.
Some patients are more likely to peer into their files than others, said Liz Salmi, senior strategist at OpenNotes, who is also a brain cancer patient.
“Those patients who have more serious or chronic conditions ... are more likely to read their notes,” she said in an interview.
A new study of nearly 6,000 medical oncology patients at the University of Wisconsin confirmed that opinion. Patients with incurable metastatic disease were much more likely than those with early-stage, curable disease to read notes. Notably, younger patients were more likely than older ones to access notes, likely the result of generational tech savvy.
Despite the unpredictability of serious disease such as cancer, oncology patients find satisfaction in reading their notes, say experts. “We’ve overwhelmingly heard that patients like it,” Thomas LeBlanc, MD, medical oncologist at Duke University, Durham, N.C., where all patients already have access to clinicians’ notes, told this news organization in 2018.
You are part of the avant garde.
The United States and Scandinavian countries are the world leaders in implementing open notes in clinical practice, Dr. Blease said in an interview.
“It’s a phenomenal achievement” to have enacted open notes nationally, she said. For example, there are no open notes in Northern Ireland, Dr. Blease’s home country, or most of Europe.
In the United States, there are more than 200 medical organizations, including at least one in every state, that were voluntarily providing open notes before April 5, including interstate giants such as Banner Health and big-name medical centers such as Cleveland Clinic.
It may be hard for the United States to top Sweden’s embrace of the practice. The national open notes program now has 7.2 million patient accounts in a country of 10 million people, noted Maria Häggland, PhD, of Uppsala (Sweden) MedTech Science Innovation Center during a webinar last year.
The start day will come, and you may not notice.
“When April 5 happens, something brand new is going to happen symbolically,” Ms. Salmi said. Its importance is hard to measure.
“Patients say they trust their doctor more because they understand their thinking with open notes. How do you value that? We don’t have metrics for that,” she said.
Dr. MacDonald suggested that open notes are both new and not new. In the fall of 2020, he predicted that the launch day would come, and few clinicians would notice, in part because many patients already access truncated information via patient portals.
However, there are “sensitive issues,” such as with adolescents and reproductive health, where “we know that some parents have sign-in information for their teen’s portal,” he commented. With clinical notes now on full display, potential problems “may be out of our control.”
Still, the Sacramento-based physician and IT officer acknowledged that concerns about open notes may be a bit inflated. “I’ve been more worried about reassuring physicians that everything will be okay than what’s actually going to happen [as the law takes effect],” Dr. MacDonald said.
The OpenNotes organization is grant funded, and staff disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Change in writing style is not mandated
Change in writing style is not mandated
The mandate, called “open notes” by many, is part of the 21st Century Cures Act, a wide-ranging piece of federal health care legislation. The previous deadline of Nov. 2, 2020, for enacting open notes was extended last year because of the exigencies of the COVID-19 pandemic.
Organizations must provide access via patient portals to the following types of notes: consultations, discharge summaries, histories, physical examination findings, imaging narratives, laboratory and pathology report narratives, and procedure and progress notes. Noncompliant organizations will eventually be subject to fines from the Department of Health & Human Services for “information blocking.”
This news organization reported on the mandate in 2020, and some readers said it was an unwelcome intrusion into practice. Since then, this news organization has run additional open notes stories about physician concerns, a perspective essay addressing those fears, and a reader poll about the phenomenon.
Now, as the legislation turns into a practical clinical matter, there are five key points clinicians should consider.
Clinicians don’t have to change writing style.
The new law mandates timely patient access to notes and test results, but it doesn’t require that clinicians alter their writing, said Scott MacDonald, MD, an internist and electronic health record medical director at University of California Davis Health in Sacramento.
“You don’t have to change your notes,” he said. However, patients are now part of the note audience and some health care systems are directing clinicians to make patient-friendly style changes.
Everyday experience should guide clinicians when writing notes, said one expert.
“When you’re not sure [of how to write a note], just mirror the way you would speak in the office – that’s going to get you right, including for mental health issues,” advised Leonor Fernandez, MD, an internist at Beth Deaconess Israel Medical Center, Boston, in her “take-away” comments in the online video, How to Write an Open Note.
According to a 2020 Medscape poll of 1,050 physicians, a majority (56%) anticipate that they will write notes differently, knowing that patients can read them via open notes. Nearly two-thirds (64%) believe that this new wrinkle in medical records will increase their workload. However, actual practice suggests that this is true for a minority of practitioners, according to the results from a recent study of more than 1,000 physicians in Boston, Seattle, and rural Pennsylvania, who already work in open notes settings. Only about one-third (37%) reported “spending more time on documentation.”
Note writing is going to change because of the addition of the patient reader, and something will be lost, argued Steven Reidbord, MD, a psychiatrist in private practice in San Francisco. By watering down the language for patients, “you are trading away the technical precision and other advantages of having a professional language,” commented Dr. Reidbord, who blogs for Psychology Today and has criticized the open notes movement in the past.
However, years of investigation from OpenNotes, the Boston-based advocacy and research organization, indicates that there are many gains with patient-accessible notes, including improved medical record accuracy, greater medication adherence, and potentially improved health care disparities among a range of patient types. In a 2019 study, researchers said that worry and confusion among note-reading patients are uncommon (5% and 3%, respectively), which addresses two criticisms voiced by multiple people last year.
Some clinical notes can be withheld.
The new rules from the federal government permit information blocking if there is clear evidence that doing so “will substantially reduce the risk of harm” to patients or to other third parties, Tom Delbanco, MD, and Charlotte Blease, PhD, of OpenNotes in Boston wrote in a commentary in February 2021.
There are also state-level laws that can supersede the new U.S. law and block access to notes, points out MacDonald. For example, California law dictates that providers cannot post cancer test results without talking with the patient first.
The OpenNotes organization also points out that, with regard to sensitive psychotherapy notes that are separated from the rest of a medical record, those notes “can be kept from patients without their permission, and such rules vary state by state.”
Some patients are more likely readers.
Some patients are more likely to peer into their files than others, said Liz Salmi, senior strategist at OpenNotes, who is also a brain cancer patient.
“Those patients who have more serious or chronic conditions ... are more likely to read their notes,” she said in an interview.
A new study of nearly 6,000 medical oncology patients at the University of Wisconsin confirmed that opinion. Patients with incurable metastatic disease were much more likely than those with early-stage, curable disease to read notes. Notably, younger patients were more likely than older ones to access notes, likely the result of generational tech savvy.
Despite the unpredictability of serious disease such as cancer, oncology patients find satisfaction in reading their notes, say experts. “We’ve overwhelmingly heard that patients like it,” Thomas LeBlanc, MD, medical oncologist at Duke University, Durham, N.C., where all patients already have access to clinicians’ notes, told this news organization in 2018.
You are part of the avant garde.
The United States and Scandinavian countries are the world leaders in implementing open notes in clinical practice, Dr. Blease said in an interview.
“It’s a phenomenal achievement” to have enacted open notes nationally, she said. For example, there are no open notes in Northern Ireland, Dr. Blease’s home country, or most of Europe.
In the United States, there are more than 200 medical organizations, including at least one in every state, that were voluntarily providing open notes before April 5, including interstate giants such as Banner Health and big-name medical centers such as Cleveland Clinic.
It may be hard for the United States to top Sweden’s embrace of the practice. The national open notes program now has 7.2 million patient accounts in a country of 10 million people, noted Maria Häggland, PhD, of Uppsala (Sweden) MedTech Science Innovation Center during a webinar last year.
The start day will come, and you may not notice.
“When April 5 happens, something brand new is going to happen symbolically,” Ms. Salmi said. Its importance is hard to measure.
“Patients say they trust their doctor more because they understand their thinking with open notes. How do you value that? We don’t have metrics for that,” she said.
Dr. MacDonald suggested that open notes are both new and not new. In the fall of 2020, he predicted that the launch day would come, and few clinicians would notice, in part because many patients already access truncated information via patient portals.
However, there are “sensitive issues,” such as with adolescents and reproductive health, where “we know that some parents have sign-in information for their teen’s portal,” he commented. With clinical notes now on full display, potential problems “may be out of our control.”
Still, the Sacramento-based physician and IT officer acknowledged that concerns about open notes may be a bit inflated. “I’ve been more worried about reassuring physicians that everything will be okay than what’s actually going to happen [as the law takes effect],” Dr. MacDonald said.
The OpenNotes organization is grant funded, and staff disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The mandate, called “open notes” by many, is part of the 21st Century Cures Act, a wide-ranging piece of federal health care legislation. The previous deadline of Nov. 2, 2020, for enacting open notes was extended last year because of the exigencies of the COVID-19 pandemic.
Organizations must provide access via patient portals to the following types of notes: consultations, discharge summaries, histories, physical examination findings, imaging narratives, laboratory and pathology report narratives, and procedure and progress notes. Noncompliant organizations will eventually be subject to fines from the Department of Health & Human Services for “information blocking.”
This news organization reported on the mandate in 2020, and some readers said it was an unwelcome intrusion into practice. Since then, this news organization has run additional open notes stories about physician concerns, a perspective essay addressing those fears, and a reader poll about the phenomenon.
Now, as the legislation turns into a practical clinical matter, there are five key points clinicians should consider.
Clinicians don’t have to change writing style.
The new law mandates timely patient access to notes and test results, but it doesn’t require that clinicians alter their writing, said Scott MacDonald, MD, an internist and electronic health record medical director at University of California Davis Health in Sacramento.
“You don’t have to change your notes,” he said. However, patients are now part of the note audience and some health care systems are directing clinicians to make patient-friendly style changes.
Everyday experience should guide clinicians when writing notes, said one expert.
“When you’re not sure [of how to write a note], just mirror the way you would speak in the office – that’s going to get you right, including for mental health issues,” advised Leonor Fernandez, MD, an internist at Beth Deaconess Israel Medical Center, Boston, in her “take-away” comments in the online video, How to Write an Open Note.
According to a 2020 Medscape poll of 1,050 physicians, a majority (56%) anticipate that they will write notes differently, knowing that patients can read them via open notes. Nearly two-thirds (64%) believe that this new wrinkle in medical records will increase their workload. However, actual practice suggests that this is true for a minority of practitioners, according to the results from a recent study of more than 1,000 physicians in Boston, Seattle, and rural Pennsylvania, who already work in open notes settings. Only about one-third (37%) reported “spending more time on documentation.”
Note writing is going to change because of the addition of the patient reader, and something will be lost, argued Steven Reidbord, MD, a psychiatrist in private practice in San Francisco. By watering down the language for patients, “you are trading away the technical precision and other advantages of having a professional language,” commented Dr. Reidbord, who blogs for Psychology Today and has criticized the open notes movement in the past.
However, years of investigation from OpenNotes, the Boston-based advocacy and research organization, indicates that there are many gains with patient-accessible notes, including improved medical record accuracy, greater medication adherence, and potentially improved health care disparities among a range of patient types. In a 2019 study, researchers said that worry and confusion among note-reading patients are uncommon (5% and 3%, respectively), which addresses two criticisms voiced by multiple people last year.
Some clinical notes can be withheld.
The new rules from the federal government permit information blocking if there is clear evidence that doing so “will substantially reduce the risk of harm” to patients or to other third parties, Tom Delbanco, MD, and Charlotte Blease, PhD, of OpenNotes in Boston wrote in a commentary in February 2021.
There are also state-level laws that can supersede the new U.S. law and block access to notes, points out MacDonald. For example, California law dictates that providers cannot post cancer test results without talking with the patient first.
The OpenNotes organization also points out that, with regard to sensitive psychotherapy notes that are separated from the rest of a medical record, those notes “can be kept from patients without their permission, and such rules vary state by state.”
Some patients are more likely readers.
Some patients are more likely to peer into their files than others, said Liz Salmi, senior strategist at OpenNotes, who is also a brain cancer patient.
“Those patients who have more serious or chronic conditions ... are more likely to read their notes,” she said in an interview.
A new study of nearly 6,000 medical oncology patients at the University of Wisconsin confirmed that opinion. Patients with incurable metastatic disease were much more likely than those with early-stage, curable disease to read notes. Notably, younger patients were more likely than older ones to access notes, likely the result of generational tech savvy.
Despite the unpredictability of serious disease such as cancer, oncology patients find satisfaction in reading their notes, say experts. “We’ve overwhelmingly heard that patients like it,” Thomas LeBlanc, MD, medical oncologist at Duke University, Durham, N.C., where all patients already have access to clinicians’ notes, told this news organization in 2018.
You are part of the avant garde.
The United States and Scandinavian countries are the world leaders in implementing open notes in clinical practice, Dr. Blease said in an interview.
“It’s a phenomenal achievement” to have enacted open notes nationally, she said. For example, there are no open notes in Northern Ireland, Dr. Blease’s home country, or most of Europe.
In the United States, there are more than 200 medical organizations, including at least one in every state, that were voluntarily providing open notes before April 5, including interstate giants such as Banner Health and big-name medical centers such as Cleveland Clinic.
It may be hard for the United States to top Sweden’s embrace of the practice. The national open notes program now has 7.2 million patient accounts in a country of 10 million people, noted Maria Häggland, PhD, of Uppsala (Sweden) MedTech Science Innovation Center during a webinar last year.
The start day will come, and you may not notice.
“When April 5 happens, something brand new is going to happen symbolically,” Ms. Salmi said. Its importance is hard to measure.
“Patients say they trust their doctor more because they understand their thinking with open notes. How do you value that? We don’t have metrics for that,” she said.
Dr. MacDonald suggested that open notes are both new and not new. In the fall of 2020, he predicted that the launch day would come, and few clinicians would notice, in part because many patients already access truncated information via patient portals.
However, there are “sensitive issues,” such as with adolescents and reproductive health, where “we know that some parents have sign-in information for their teen’s portal,” he commented. With clinical notes now on full display, potential problems “may be out of our control.”
Still, the Sacramento-based physician and IT officer acknowledged that concerns about open notes may be a bit inflated. “I’ve been more worried about reassuring physicians that everything will be okay than what’s actually going to happen [as the law takes effect],” Dr. MacDonald said.
The OpenNotes organization is grant funded, and staff disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
COVID-19 in 2020: Deaths and disparities
COVID-19 was the third-leading cause of death in the United States in 2020, but that mortality burden did not fall evenly along racial/ethnic lines, according to a provisional report from the Centers for Disease Control and Prevention.

Only heart disease and cancer caused more deaths than SARS-CoV-2, which took the lives of almost 378,000 Americans last year, Farida B. Ahmad, MPH, and associates at the National Center for Health Statistics noted March 31 in the Morbidity and Mortality Weekly Report.
That represents 11.2% of the almost 3.36 million total deaths recorded in 2020. The racial/ethnics demographics, however, show that 22.4% of all deaths among Hispanic Americans were COVID-19–related, as were 18.6% of deaths in American Indians/Alaska Natives. Deaths among Asian persons, at 14.7%, and African Americans, at 13.5%, were closer but still above the national figure, while Whites (9.3%) were the only major subgroup below it, based on data from the National Vital Statistics System.
Age-adjusted death rates tell a somewhat different story: American Indian/Alaska native persons were highest with a rate of 187.8 COVID-19–associated deaths per 100,000 standard population, with Hispanic persons second at 164.3 per 100,000. Blacks were next at 151.1 deaths per 100,000, but Whites had a higher rate (72.5) than did Asian Americans (66.7), the CDC investigators reported.
“During January-December 2020, the estimated 2020 age-adjusted death rate increased for the first time since 2017, with an increase of 15.9% compared with 2019, from 715.2 to 828.7 deaths per 100,000 population,” they wrote, noting that “certain categories of race (i.e., AI/AN and Asian) and Hispanic ethnicity reported on death certificates might have been misclassified, possibly resulting in underestimates of death rates for some groups.”
COVID-19 was the third-leading cause of death in the United States in 2020, but that mortality burden did not fall evenly along racial/ethnic lines, according to a provisional report from the Centers for Disease Control and Prevention.

Only heart disease and cancer caused more deaths than SARS-CoV-2, which took the lives of almost 378,000 Americans last year, Farida B. Ahmad, MPH, and associates at the National Center for Health Statistics noted March 31 in the Morbidity and Mortality Weekly Report.
That represents 11.2% of the almost 3.36 million total deaths recorded in 2020. The racial/ethnics demographics, however, show that 22.4% of all deaths among Hispanic Americans were COVID-19–related, as were 18.6% of deaths in American Indians/Alaska Natives. Deaths among Asian persons, at 14.7%, and African Americans, at 13.5%, were closer but still above the national figure, while Whites (9.3%) were the only major subgroup below it, based on data from the National Vital Statistics System.
Age-adjusted death rates tell a somewhat different story: American Indian/Alaska native persons were highest with a rate of 187.8 COVID-19–associated deaths per 100,000 standard population, with Hispanic persons second at 164.3 per 100,000. Blacks were next at 151.1 deaths per 100,000, but Whites had a higher rate (72.5) than did Asian Americans (66.7), the CDC investigators reported.
“During January-December 2020, the estimated 2020 age-adjusted death rate increased for the first time since 2017, with an increase of 15.9% compared with 2019, from 715.2 to 828.7 deaths per 100,000 population,” they wrote, noting that “certain categories of race (i.e., AI/AN and Asian) and Hispanic ethnicity reported on death certificates might have been misclassified, possibly resulting in underestimates of death rates for some groups.”
COVID-19 was the third-leading cause of death in the United States in 2020, but that mortality burden did not fall evenly along racial/ethnic lines, according to a provisional report from the Centers for Disease Control and Prevention.

Only heart disease and cancer caused more deaths than SARS-CoV-2, which took the lives of almost 378,000 Americans last year, Farida B. Ahmad, MPH, and associates at the National Center for Health Statistics noted March 31 in the Morbidity and Mortality Weekly Report.
That represents 11.2% of the almost 3.36 million total deaths recorded in 2020. The racial/ethnics demographics, however, show that 22.4% of all deaths among Hispanic Americans were COVID-19–related, as were 18.6% of deaths in American Indians/Alaska Natives. Deaths among Asian persons, at 14.7%, and African Americans, at 13.5%, were closer but still above the national figure, while Whites (9.3%) were the only major subgroup below it, based on data from the National Vital Statistics System.
Age-adjusted death rates tell a somewhat different story: American Indian/Alaska native persons were highest with a rate of 187.8 COVID-19–associated deaths per 100,000 standard population, with Hispanic persons second at 164.3 per 100,000. Blacks were next at 151.1 deaths per 100,000, but Whites had a higher rate (72.5) than did Asian Americans (66.7), the CDC investigators reported.
“During January-December 2020, the estimated 2020 age-adjusted death rate increased for the first time since 2017, with an increase of 15.9% compared with 2019, from 715.2 to 828.7 deaths per 100,000 population,” they wrote, noting that “certain categories of race (i.e., AI/AN and Asian) and Hispanic ethnicity reported on death certificates might have been misclassified, possibly resulting in underestimates of death rates for some groups.”
FROM MMWR
Children could become eligible for a COVID-19 vaccine by fall, expert predicts
If everything goes as planned,
According to Yvonne Maldonado, MD, Pfizer has fully enrolled adolescent trials and Moderna is currently enrolling 3,000 adolescents in a safety and reactogenicity trial known as TeenCOVE, in which participants will receive an intramuscular injection of 100 mcg mRNA-1273 on day 1 and on day 29. Meanwhile, Johnson & Johnson and AstraZeneca will be starting to enroll older children and adolescents into studies within the next several weeks.
The companies are also planning to enroll younger children, Dr. Maldonado, the Taube professor of global health and infectious diseases at Stanford (Calif.) University, said during the Society for Pediatric Dermatology pre-AAD meeting. “At least two of the vaccine companies have indicated that they would like to start enrolling children as young as 2-5 years of age and eventually getting down to infants and toddlers if the vaccines prove to be safe and effective in the older children. Eventually, we hope to get to the level where we can have several vaccine candidates for all children 6 months of age and older.”
In the future, she said, infectious disease experts hope to see antiviral, immunomodulatory, anti-inflammatory, and monoclonal therapies for all populations including children, although trials in this population have not begun. “Clinical trials must be flexible and adaptive to deal with children and adolescents,” added Dr. Maldonado, who is also senior associate dean for faculty development and diversity at Stanford.
“We would ideally like to have new correlates of protection, as well as biomarkers to follow for evidence of effectiveness. We also would love to see vaccines in the pediatric population as soon as possible, because herd immunity is the ultimate goal for protection against this disease and prevention of additional transmission over time.” However, she said, the degree and durability of immunity has yet to be determined, and vaccine-associated immune effects are unknown. In the meantime, infectious disease researchers expect nonpharmacologic interventions, such as wearing face masks and social distancing to continue for an undefined period.
(Less than 2 weeks after Dr. Maldonado spoke at the SPD meeting, Pfizer announced in a press release that, in phase 3 clinical trials, the company’s coronavirus vaccine was 100% effective in protecting children aged 12-15 years from infection, with a “robust” antibody responses and side effects similar to those experienced by those aged 16-25 years. The company also announced that it plans to seek Food and Drug Administration EUA for this age group. Asked to comment on this update, Dr. Maldonado said the results released by Pfizer “suggest that their COVID-19 vaccine is very safe and highly effective in preventing COVID-19 among children 12-15 years of age.” She added that additional data from the Pfizer trials as well as from Moderna and Johnson & Johnson vaccine trials “will hopefully lead to FDA EUA review in the coming weeks,” and that COVID-19 vaccinations for children “may be possible by this summer.”)
Children with underlying diseases or on immune suppressants
At the SPD meeting, an attendee asked if there were any pediatric patients for whom she would not recommend receiving a COVID-19 vaccine because of an underlying disease or concurrent therapy with immune suppressants. “We don’t have those data yet,” Dr. Maldonado said. “Based on what we’re seeing with adults, it does appear that those with underlying conditions are at somewhat higher risk of developing severe infection and may therefore most likely to need vaccination. Most of those risks are cardiovascular, obesity, and other factors, but not necessarily immunocompromising conditions. More likely what we’re seeing is that people with underlying immunocompromising conditions may not mount a good response to the vaccines at this time. It doesn’t mean we shouldn’t give the vaccines, but we need to learn more about that.”
Dr. Maldonado went on to note that, as vaccine manufacturers commence pediatric trials, healthy children will be tested first, followed in due time with children who have immunocompromised conditions. “The question will be whether or not we should give monoclonal antibodies to those particular children to help boost their immunity to SARS-CoV-2, because they might not have a good response to the vaccines,” she said. “Those things need to be sorted out, but there’s no safety signal or concerns at this point for vaccine to be given to immunocompromised individuals.”
Another meeting attendee asked Dr. Maldonado if she thinks there is a practical role for assessing markers of T-cell immunity when evaluating suspected COVID-19 patients who may test negative on serology, Dr. Maldonado said that she and her colleagues are seeking pediatric patients who were treated for COVID-19 at Stanford, in an effort to sort this out.
They are checking peripheral blood mononuclear cells in these patients “to try and tease out what the immune response is in kids who have serious disease, versus those who came in with acute disease, versus those who are asymptomatic,” and comparing them with children who don’t have infection, she explained. “The question is, what is the role of T cells and how much do they contribute? One of the biggest questions we have is, do we have an immune correlate? Can we detect a particular level of neutralizing antibody that seems to be protective? If so, how long is it protective, and can we look for T- and B-cell memory cells and effector vector cells and see how long those effector vector cells can be active in protection? Those are studies that are ongoing now.”
Dr. Maldonado disclosed that she is a member of the data safety monitoring board for a non–COVID-19 vaccine being developed by Pfizer.
If everything goes as planned,
According to Yvonne Maldonado, MD, Pfizer has fully enrolled adolescent trials and Moderna is currently enrolling 3,000 adolescents in a safety and reactogenicity trial known as TeenCOVE, in which participants will receive an intramuscular injection of 100 mcg mRNA-1273 on day 1 and on day 29. Meanwhile, Johnson & Johnson and AstraZeneca will be starting to enroll older children and adolescents into studies within the next several weeks.
The companies are also planning to enroll younger children, Dr. Maldonado, the Taube professor of global health and infectious diseases at Stanford (Calif.) University, said during the Society for Pediatric Dermatology pre-AAD meeting. “At least two of the vaccine companies have indicated that they would like to start enrolling children as young as 2-5 years of age and eventually getting down to infants and toddlers if the vaccines prove to be safe and effective in the older children. Eventually, we hope to get to the level where we can have several vaccine candidates for all children 6 months of age and older.”
In the future, she said, infectious disease experts hope to see antiviral, immunomodulatory, anti-inflammatory, and monoclonal therapies for all populations including children, although trials in this population have not begun. “Clinical trials must be flexible and adaptive to deal with children and adolescents,” added Dr. Maldonado, who is also senior associate dean for faculty development and diversity at Stanford.
“We would ideally like to have new correlates of protection, as well as biomarkers to follow for evidence of effectiveness. We also would love to see vaccines in the pediatric population as soon as possible, because herd immunity is the ultimate goal for protection against this disease and prevention of additional transmission over time.” However, she said, the degree and durability of immunity has yet to be determined, and vaccine-associated immune effects are unknown. In the meantime, infectious disease researchers expect nonpharmacologic interventions, such as wearing face masks and social distancing to continue for an undefined period.
(Less than 2 weeks after Dr. Maldonado spoke at the SPD meeting, Pfizer announced in a press release that, in phase 3 clinical trials, the company’s coronavirus vaccine was 100% effective in protecting children aged 12-15 years from infection, with a “robust” antibody responses and side effects similar to those experienced by those aged 16-25 years. The company also announced that it plans to seek Food and Drug Administration EUA for this age group. Asked to comment on this update, Dr. Maldonado said the results released by Pfizer “suggest that their COVID-19 vaccine is very safe and highly effective in preventing COVID-19 among children 12-15 years of age.” She added that additional data from the Pfizer trials as well as from Moderna and Johnson & Johnson vaccine trials “will hopefully lead to FDA EUA review in the coming weeks,” and that COVID-19 vaccinations for children “may be possible by this summer.”)
Children with underlying diseases or on immune suppressants
At the SPD meeting, an attendee asked if there were any pediatric patients for whom she would not recommend receiving a COVID-19 vaccine because of an underlying disease or concurrent therapy with immune suppressants. “We don’t have those data yet,” Dr. Maldonado said. “Based on what we’re seeing with adults, it does appear that those with underlying conditions are at somewhat higher risk of developing severe infection and may therefore most likely to need vaccination. Most of those risks are cardiovascular, obesity, and other factors, but not necessarily immunocompromising conditions. More likely what we’re seeing is that people with underlying immunocompromising conditions may not mount a good response to the vaccines at this time. It doesn’t mean we shouldn’t give the vaccines, but we need to learn more about that.”
Dr. Maldonado went on to note that, as vaccine manufacturers commence pediatric trials, healthy children will be tested first, followed in due time with children who have immunocompromised conditions. “The question will be whether or not we should give monoclonal antibodies to those particular children to help boost their immunity to SARS-CoV-2, because they might not have a good response to the vaccines,” she said. “Those things need to be sorted out, but there’s no safety signal or concerns at this point for vaccine to be given to immunocompromised individuals.”
Another meeting attendee asked Dr. Maldonado if she thinks there is a practical role for assessing markers of T-cell immunity when evaluating suspected COVID-19 patients who may test negative on serology, Dr. Maldonado said that she and her colleagues are seeking pediatric patients who were treated for COVID-19 at Stanford, in an effort to sort this out.
They are checking peripheral blood mononuclear cells in these patients “to try and tease out what the immune response is in kids who have serious disease, versus those who came in with acute disease, versus those who are asymptomatic,” and comparing them with children who don’t have infection, she explained. “The question is, what is the role of T cells and how much do they contribute? One of the biggest questions we have is, do we have an immune correlate? Can we detect a particular level of neutralizing antibody that seems to be protective? If so, how long is it protective, and can we look for T- and B-cell memory cells and effector vector cells and see how long those effector vector cells can be active in protection? Those are studies that are ongoing now.”
Dr. Maldonado disclosed that she is a member of the data safety monitoring board for a non–COVID-19 vaccine being developed by Pfizer.
If everything goes as planned,
According to Yvonne Maldonado, MD, Pfizer has fully enrolled adolescent trials and Moderna is currently enrolling 3,000 adolescents in a safety and reactogenicity trial known as TeenCOVE, in which participants will receive an intramuscular injection of 100 mcg mRNA-1273 on day 1 and on day 29. Meanwhile, Johnson & Johnson and AstraZeneca will be starting to enroll older children and adolescents into studies within the next several weeks.
The companies are also planning to enroll younger children, Dr. Maldonado, the Taube professor of global health and infectious diseases at Stanford (Calif.) University, said during the Society for Pediatric Dermatology pre-AAD meeting. “At least two of the vaccine companies have indicated that they would like to start enrolling children as young as 2-5 years of age and eventually getting down to infants and toddlers if the vaccines prove to be safe and effective in the older children. Eventually, we hope to get to the level where we can have several vaccine candidates for all children 6 months of age and older.”
In the future, she said, infectious disease experts hope to see antiviral, immunomodulatory, anti-inflammatory, and monoclonal therapies for all populations including children, although trials in this population have not begun. “Clinical trials must be flexible and adaptive to deal with children and adolescents,” added Dr. Maldonado, who is also senior associate dean for faculty development and diversity at Stanford.
“We would ideally like to have new correlates of protection, as well as biomarkers to follow for evidence of effectiveness. We also would love to see vaccines in the pediatric population as soon as possible, because herd immunity is the ultimate goal for protection against this disease and prevention of additional transmission over time.” However, she said, the degree and durability of immunity has yet to be determined, and vaccine-associated immune effects are unknown. In the meantime, infectious disease researchers expect nonpharmacologic interventions, such as wearing face masks and social distancing to continue for an undefined period.
(Less than 2 weeks after Dr. Maldonado spoke at the SPD meeting, Pfizer announced in a press release that, in phase 3 clinical trials, the company’s coronavirus vaccine was 100% effective in protecting children aged 12-15 years from infection, with a “robust” antibody responses and side effects similar to those experienced by those aged 16-25 years. The company also announced that it plans to seek Food and Drug Administration EUA for this age group. Asked to comment on this update, Dr. Maldonado said the results released by Pfizer “suggest that their COVID-19 vaccine is very safe and highly effective in preventing COVID-19 among children 12-15 years of age.” She added that additional data from the Pfizer trials as well as from Moderna and Johnson & Johnson vaccine trials “will hopefully lead to FDA EUA review in the coming weeks,” and that COVID-19 vaccinations for children “may be possible by this summer.”)
Children with underlying diseases or on immune suppressants
At the SPD meeting, an attendee asked if there were any pediatric patients for whom she would not recommend receiving a COVID-19 vaccine because of an underlying disease or concurrent therapy with immune suppressants. “We don’t have those data yet,” Dr. Maldonado said. “Based on what we’re seeing with adults, it does appear that those with underlying conditions are at somewhat higher risk of developing severe infection and may therefore most likely to need vaccination. Most of those risks are cardiovascular, obesity, and other factors, but not necessarily immunocompromising conditions. More likely what we’re seeing is that people with underlying immunocompromising conditions may not mount a good response to the vaccines at this time. It doesn’t mean we shouldn’t give the vaccines, but we need to learn more about that.”
Dr. Maldonado went on to note that, as vaccine manufacturers commence pediatric trials, healthy children will be tested first, followed in due time with children who have immunocompromised conditions. “The question will be whether or not we should give monoclonal antibodies to those particular children to help boost their immunity to SARS-CoV-2, because they might not have a good response to the vaccines,” she said. “Those things need to be sorted out, but there’s no safety signal or concerns at this point for vaccine to be given to immunocompromised individuals.”
Another meeting attendee asked Dr. Maldonado if she thinks there is a practical role for assessing markers of T-cell immunity when evaluating suspected COVID-19 patients who may test negative on serology, Dr. Maldonado said that she and her colleagues are seeking pediatric patients who were treated for COVID-19 at Stanford, in an effort to sort this out.
They are checking peripheral blood mononuclear cells in these patients “to try and tease out what the immune response is in kids who have serious disease, versus those who came in with acute disease, versus those who are asymptomatic,” and comparing them with children who don’t have infection, she explained. “The question is, what is the role of T cells and how much do they contribute? One of the biggest questions we have is, do we have an immune correlate? Can we detect a particular level of neutralizing antibody that seems to be protective? If so, how long is it protective, and can we look for T- and B-cell memory cells and effector vector cells and see how long those effector vector cells can be active in protection? Those are studies that are ongoing now.”
Dr. Maldonado disclosed that she is a member of the data safety monitoring board for a non–COVID-19 vaccine being developed by Pfizer.
FROM THE SPD PRE-AAD MEETING
Vitiligo patients share their experiences, frustrations with treatment options with FDA
Patients with vitiligo have faced significant impacts psychosocially and in many cases, profound losses of identity – and they’ve had only minimal success with treatment, according to participants who spoke at and provided input at a public meeting on patient-focused drug development for the disease.
The virtual meeting, held in March, was part of the Food and Drug Administration’s Patient-Focused Drug Development (PFDD) initiative, which began in 2012 and aims to provide a systematic way for patients’ experiences, needs and priorities to be “captured and meaningfully incorporated” into drug development and evaluation.
Seemal Desai, MD, of the department of dermatology at the University of Texas, Dallas, who attended the meeting as an observer, said in a later interview that while “all skin diseases have a psychosocial component … vitiligo is a really unique one, because it really relates to the patient’s own identity.
“What I heard loud and clear from the FDA [leaders who ran and attended the meeting] is recognition that patients are suffering. They needed to hear about the emotional devastation of the disease and how it is a medical condition,” Dr. Desai said.
The meeting was the “first-ever vitiligo meeting at the FDA” and was a “historic moment for the vitiligo community,” he added.
The pigmentation disorder affects 1% of the world’s population. Nearly 50% have an onset before age 20, and onset before age 12 is common, Brenda Carr, MD, medical officer with the FDA’s Division of Dermatology and Dentistry in the Center for Drug Evaluation and Research, said in an introductory overview.
The only FDA-approved treatment for vitiligo is monobenzone cream, but this is indicated for final depigmentation in extensive vitiligo and is no longer marketed. Treatment options include corticosteroids, calcineurin inhibitors, vitamin D analogues, phototherapy, surgical treatments (tissue grafts and cellular grafts), and camouflage (make-up, tattoos, self-tanning products), Dr. Carr said.
Patients participated in one of two panels – one about the health effects and daily impacts of vitiligo and the other about treatments – or submitted input electronically. All patients were invited to answer poll questions and open-ended queries, including questions about how they would assess new treatments.
Several panel members who are Black shared series of photos that showed the evolution of defined white patches into widespread, generalized depigmentation. One man with skin of color who lives in the Netherlands said he has had vitiligo since the age of 12, but that when he became older, over a 4-year period, he was “transformed from a man of Indonesian roots to a totally white man.”
Experiencing only minimal benefit from treatment and the short-term effectiveness of treatments were the top two answers to a poll question asking participants about the most burdensome impacts of the medical products and interventions they have used. Difficulty in accessing treatment, concern about serious risks of treatment, and uncertainty about long-term effects of treatment were other frequently chosen answers.
Patients described the onerous nature of phototherapy (treatments repeated several times a week over long periods) and other treatments, and several described feeling that some physicians did not take the condition seriously or fully know of treatment options.
In her closing remarks, Kendall Marcus, MD, director of the Division of Dermatology and Dentistry at the FDA, acknowledged the input. “Some of you have had difficulty having your disease taken seriously by physicians who view it as a cosmetic condition and are reluctant to treat because they believe your expectations will not be met, that it will be an exercise in frustration,” she said.
Regarding the impacts of treatments that have been utilized, “some of the treatments make it impossible to do other activities such as work or care for yourself in other ways,” Dr. Marcus said. “Certainly that’s not the kind of treatment … that anybody wants to have.”
Dr. Desai, who utilizes an array of oral and topical treatments and phototherapies in his practice, said he was surprised and disheartened to hear the level of concern about side effects of treatment. Most of those who expressed concerns alluded to phototherapy. “I think light treatments are very safe and effective,” he said in the interview. “I might equate [such concerns] to the older PUVA [psoralen plus UVA ultraviolet light] therapy, but not so much the newer therapies.”
The FDA participants probed patients for their perspective on a meaningful level of repigmentation and an acceptable level of risk for any new hypothetical treatment. Specifically, they asked whether patients would use a new topical cream approved for vitiligo if the cream needed to be applied once a day, would have up to 50% efficacy in some people, and would have common side effects of redness and irritation at the application site, mild acne, and burning, as well as several rarer but more serious side effects.
Only 36% answered yes; 24% said no, and 40% answered maybe. Some patients said during the meeting that they had accepted their condition and were not pursuing any treatment. Others said they were very interested in treatment but only if the level of repigmentation were significantly higher than 50%. Some described their fear that positive treatment effects would be short term only.
Meri Izrail Kohen, who lives in France and has lost half of her skin’s pigmentation, said that treatment efficacy is “not only about how much recovery of pigment it allows, but how long the recovery will last.” Some treatments will work for some patients, she said, “but even in these cases when we stop the treatment, it will come back somehow.”
Lee Thomas, a TV anchor in Detroit, and a reporter and author of the book “Turning White,” described how he tried “every treatment he could afford” but stopped trying 10 years ago. A treatment in Germany “gave me 80% of my pigment back, but it has gone again,” he said. “I would love to have my face back again. I was born a Black child, and I’d like to die a Black man.”
Patients also spoke of their skin burning easily outdoors; skin sensitivity, itchiness, and burning with the spread of disease; treatment expenses and not being able to afford treatment; and worsening of their vitiligo with the stress of the pandemic. Parents expressed having fear that their children would develop vitiligo and experience bullying, isolation, or other emotional or psychosocial impacts that they had experienced; one described having an almost-paralyzing anxiety when he saw patchy white spots on his 20-month-old daughter (it was not diagnosed as vitiligo).
Calls for further advancement with home phototherapy – which Dr. Desai said is a growing market but not yet adequately covered by insurance plans – were also made, as were pleas for research on the root causes of the disease.
Patients clearly indicated “that they need more efficacious treatments, and more comprehensive treatments,” said Dr. Desai, who chairs the advisory committee of the Global Vitiligo Foundation. “It’s disappointing to me that patients come in with a not fully optimistic viewpoint, with a lot of anxiety and angst that treatments are not going to work. … But the Agency needs to hear that. This means that there haven’t been good treatments and we need more.”
The FDA will accept public comments until May 10, 2021, at which time comments will be compiled into a summary report. FDA officials assured patients that the report would be visible and circulated not only within the FDA but among drug companies, researchers, and other product developers.
Patients with vitiligo have faced significant impacts psychosocially and in many cases, profound losses of identity – and they’ve had only minimal success with treatment, according to participants who spoke at and provided input at a public meeting on patient-focused drug development for the disease.
The virtual meeting, held in March, was part of the Food and Drug Administration’s Patient-Focused Drug Development (PFDD) initiative, which began in 2012 and aims to provide a systematic way for patients’ experiences, needs and priorities to be “captured and meaningfully incorporated” into drug development and evaluation.
Seemal Desai, MD, of the department of dermatology at the University of Texas, Dallas, who attended the meeting as an observer, said in a later interview that while “all skin diseases have a psychosocial component … vitiligo is a really unique one, because it really relates to the patient’s own identity.
“What I heard loud and clear from the FDA [leaders who ran and attended the meeting] is recognition that patients are suffering. They needed to hear about the emotional devastation of the disease and how it is a medical condition,” Dr. Desai said.
The meeting was the “first-ever vitiligo meeting at the FDA” and was a “historic moment for the vitiligo community,” he added.
The pigmentation disorder affects 1% of the world’s population. Nearly 50% have an onset before age 20, and onset before age 12 is common, Brenda Carr, MD, medical officer with the FDA’s Division of Dermatology and Dentistry in the Center for Drug Evaluation and Research, said in an introductory overview.
The only FDA-approved treatment for vitiligo is monobenzone cream, but this is indicated for final depigmentation in extensive vitiligo and is no longer marketed. Treatment options include corticosteroids, calcineurin inhibitors, vitamin D analogues, phototherapy, surgical treatments (tissue grafts and cellular grafts), and camouflage (make-up, tattoos, self-tanning products), Dr. Carr said.
Patients participated in one of two panels – one about the health effects and daily impacts of vitiligo and the other about treatments – or submitted input electronically. All patients were invited to answer poll questions and open-ended queries, including questions about how they would assess new treatments.
Several panel members who are Black shared series of photos that showed the evolution of defined white patches into widespread, generalized depigmentation. One man with skin of color who lives in the Netherlands said he has had vitiligo since the age of 12, but that when he became older, over a 4-year period, he was “transformed from a man of Indonesian roots to a totally white man.”
Experiencing only minimal benefit from treatment and the short-term effectiveness of treatments were the top two answers to a poll question asking participants about the most burdensome impacts of the medical products and interventions they have used. Difficulty in accessing treatment, concern about serious risks of treatment, and uncertainty about long-term effects of treatment were other frequently chosen answers.
Patients described the onerous nature of phototherapy (treatments repeated several times a week over long periods) and other treatments, and several described feeling that some physicians did not take the condition seriously or fully know of treatment options.
In her closing remarks, Kendall Marcus, MD, director of the Division of Dermatology and Dentistry at the FDA, acknowledged the input. “Some of you have had difficulty having your disease taken seriously by physicians who view it as a cosmetic condition and are reluctant to treat because they believe your expectations will not be met, that it will be an exercise in frustration,” she said.
Regarding the impacts of treatments that have been utilized, “some of the treatments make it impossible to do other activities such as work or care for yourself in other ways,” Dr. Marcus said. “Certainly that’s not the kind of treatment … that anybody wants to have.”
Dr. Desai, who utilizes an array of oral and topical treatments and phototherapies in his practice, said he was surprised and disheartened to hear the level of concern about side effects of treatment. Most of those who expressed concerns alluded to phototherapy. “I think light treatments are very safe and effective,” he said in the interview. “I might equate [such concerns] to the older PUVA [psoralen plus UVA ultraviolet light] therapy, but not so much the newer therapies.”
The FDA participants probed patients for their perspective on a meaningful level of repigmentation and an acceptable level of risk for any new hypothetical treatment. Specifically, they asked whether patients would use a new topical cream approved for vitiligo if the cream needed to be applied once a day, would have up to 50% efficacy in some people, and would have common side effects of redness and irritation at the application site, mild acne, and burning, as well as several rarer but more serious side effects.
Only 36% answered yes; 24% said no, and 40% answered maybe. Some patients said during the meeting that they had accepted their condition and were not pursuing any treatment. Others said they were very interested in treatment but only if the level of repigmentation were significantly higher than 50%. Some described their fear that positive treatment effects would be short term only.
Meri Izrail Kohen, who lives in France and has lost half of her skin’s pigmentation, said that treatment efficacy is “not only about how much recovery of pigment it allows, but how long the recovery will last.” Some treatments will work for some patients, she said, “but even in these cases when we stop the treatment, it will come back somehow.”
Lee Thomas, a TV anchor in Detroit, and a reporter and author of the book “Turning White,” described how he tried “every treatment he could afford” but stopped trying 10 years ago. A treatment in Germany “gave me 80% of my pigment back, but it has gone again,” he said. “I would love to have my face back again. I was born a Black child, and I’d like to die a Black man.”
Patients also spoke of their skin burning easily outdoors; skin sensitivity, itchiness, and burning with the spread of disease; treatment expenses and not being able to afford treatment; and worsening of their vitiligo with the stress of the pandemic. Parents expressed having fear that their children would develop vitiligo and experience bullying, isolation, or other emotional or psychosocial impacts that they had experienced; one described having an almost-paralyzing anxiety when he saw patchy white spots on his 20-month-old daughter (it was not diagnosed as vitiligo).
Calls for further advancement with home phototherapy – which Dr. Desai said is a growing market but not yet adequately covered by insurance plans – were also made, as were pleas for research on the root causes of the disease.
Patients clearly indicated “that they need more efficacious treatments, and more comprehensive treatments,” said Dr. Desai, who chairs the advisory committee of the Global Vitiligo Foundation. “It’s disappointing to me that patients come in with a not fully optimistic viewpoint, with a lot of anxiety and angst that treatments are not going to work. … But the Agency needs to hear that. This means that there haven’t been good treatments and we need more.”
The FDA will accept public comments until May 10, 2021, at which time comments will be compiled into a summary report. FDA officials assured patients that the report would be visible and circulated not only within the FDA but among drug companies, researchers, and other product developers.
Patients with vitiligo have faced significant impacts psychosocially and in many cases, profound losses of identity – and they’ve had only minimal success with treatment, according to participants who spoke at and provided input at a public meeting on patient-focused drug development for the disease.
The virtual meeting, held in March, was part of the Food and Drug Administration’s Patient-Focused Drug Development (PFDD) initiative, which began in 2012 and aims to provide a systematic way for patients’ experiences, needs and priorities to be “captured and meaningfully incorporated” into drug development and evaluation.
Seemal Desai, MD, of the department of dermatology at the University of Texas, Dallas, who attended the meeting as an observer, said in a later interview that while “all skin diseases have a psychosocial component … vitiligo is a really unique one, because it really relates to the patient’s own identity.
“What I heard loud and clear from the FDA [leaders who ran and attended the meeting] is recognition that patients are suffering. They needed to hear about the emotional devastation of the disease and how it is a medical condition,” Dr. Desai said.
The meeting was the “first-ever vitiligo meeting at the FDA” and was a “historic moment for the vitiligo community,” he added.
The pigmentation disorder affects 1% of the world’s population. Nearly 50% have an onset before age 20, and onset before age 12 is common, Brenda Carr, MD, medical officer with the FDA’s Division of Dermatology and Dentistry in the Center for Drug Evaluation and Research, said in an introductory overview.
The only FDA-approved treatment for vitiligo is monobenzone cream, but this is indicated for final depigmentation in extensive vitiligo and is no longer marketed. Treatment options include corticosteroids, calcineurin inhibitors, vitamin D analogues, phototherapy, surgical treatments (tissue grafts and cellular grafts), and camouflage (make-up, tattoos, self-tanning products), Dr. Carr said.
Patients participated in one of two panels – one about the health effects and daily impacts of vitiligo and the other about treatments – or submitted input electronically. All patients were invited to answer poll questions and open-ended queries, including questions about how they would assess new treatments.
Several panel members who are Black shared series of photos that showed the evolution of defined white patches into widespread, generalized depigmentation. One man with skin of color who lives in the Netherlands said he has had vitiligo since the age of 12, but that when he became older, over a 4-year period, he was “transformed from a man of Indonesian roots to a totally white man.”
Experiencing only minimal benefit from treatment and the short-term effectiveness of treatments were the top two answers to a poll question asking participants about the most burdensome impacts of the medical products and interventions they have used. Difficulty in accessing treatment, concern about serious risks of treatment, and uncertainty about long-term effects of treatment were other frequently chosen answers.
Patients described the onerous nature of phototherapy (treatments repeated several times a week over long periods) and other treatments, and several described feeling that some physicians did not take the condition seriously or fully know of treatment options.
In her closing remarks, Kendall Marcus, MD, director of the Division of Dermatology and Dentistry at the FDA, acknowledged the input. “Some of you have had difficulty having your disease taken seriously by physicians who view it as a cosmetic condition and are reluctant to treat because they believe your expectations will not be met, that it will be an exercise in frustration,” she said.
Regarding the impacts of treatments that have been utilized, “some of the treatments make it impossible to do other activities such as work or care for yourself in other ways,” Dr. Marcus said. “Certainly that’s not the kind of treatment … that anybody wants to have.”
Dr. Desai, who utilizes an array of oral and topical treatments and phototherapies in his practice, said he was surprised and disheartened to hear the level of concern about side effects of treatment. Most of those who expressed concerns alluded to phototherapy. “I think light treatments are very safe and effective,” he said in the interview. “I might equate [such concerns] to the older PUVA [psoralen plus UVA ultraviolet light] therapy, but not so much the newer therapies.”
The FDA participants probed patients for their perspective on a meaningful level of repigmentation and an acceptable level of risk for any new hypothetical treatment. Specifically, they asked whether patients would use a new topical cream approved for vitiligo if the cream needed to be applied once a day, would have up to 50% efficacy in some people, and would have common side effects of redness and irritation at the application site, mild acne, and burning, as well as several rarer but more serious side effects.
Only 36% answered yes; 24% said no, and 40% answered maybe. Some patients said during the meeting that they had accepted their condition and were not pursuing any treatment. Others said they were very interested in treatment but only if the level of repigmentation were significantly higher than 50%. Some described their fear that positive treatment effects would be short term only.
Meri Izrail Kohen, who lives in France and has lost half of her skin’s pigmentation, said that treatment efficacy is “not only about how much recovery of pigment it allows, but how long the recovery will last.” Some treatments will work for some patients, she said, “but even in these cases when we stop the treatment, it will come back somehow.”
Lee Thomas, a TV anchor in Detroit, and a reporter and author of the book “Turning White,” described how he tried “every treatment he could afford” but stopped trying 10 years ago. A treatment in Germany “gave me 80% of my pigment back, but it has gone again,” he said. “I would love to have my face back again. I was born a Black child, and I’d like to die a Black man.”
Patients also spoke of their skin burning easily outdoors; skin sensitivity, itchiness, and burning with the spread of disease; treatment expenses and not being able to afford treatment; and worsening of their vitiligo with the stress of the pandemic. Parents expressed having fear that their children would develop vitiligo and experience bullying, isolation, or other emotional or psychosocial impacts that they had experienced; one described having an almost-paralyzing anxiety when he saw patchy white spots on his 20-month-old daughter (it was not diagnosed as vitiligo).
Calls for further advancement with home phototherapy – which Dr. Desai said is a growing market but not yet adequately covered by insurance plans – were also made, as were pleas for research on the root causes of the disease.
Patients clearly indicated “that they need more efficacious treatments, and more comprehensive treatments,” said Dr. Desai, who chairs the advisory committee of the Global Vitiligo Foundation. “It’s disappointing to me that patients come in with a not fully optimistic viewpoint, with a lot of anxiety and angst that treatments are not going to work. … But the Agency needs to hear that. This means that there haven’t been good treatments and we need more.”
The FDA will accept public comments until May 10, 2021, at which time comments will be compiled into a summary report. FDA officials assured patients that the report would be visible and circulated not only within the FDA but among drug companies, researchers, and other product developers.
FROM AN FDA PATIENT-FOCUSED DRUG DEVELOPMENT MEETING