User login
Formerly Skin & Allergy News
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')]
The leading independent newspaper covering dermatology news and commentary.
Vitamin D deficiency in COVID-19 quadrupled death rate
Vitamin D deficiency on admission to hospital was associated with a 3.7-fold increase in the odds of dying from COVID-19, according to an observational study looking back at data from the first wave of the pandemic.
Nearly 60% of patients with COVID-19 were vitamin D deficient upon hospitalization, with men in the advanced stages of COVID-19 pneumonia showing the greatest deficit.
Importantly, the results were independent of comorbidities known to be affected by vitamin D deficiency, wrote the authors, led by Dieter De Smet, MD, from AZ Delta General Hospital, Roeselare, Belgium.
“[The findings] highlight the need for randomized, controlled trials specifically targeting vitamin D–deficient patients at intake, and make a call for general avoidance of vitamin D deficiency as a safe and inexpensive possible mitigation of the SARS-CoV-2 pandemic,” Dr. De Smet and colleagues wrote in their article, published online Nov. 25 in the American Journal of Clinical Pathology.
A search of ClinicalTrials.gov reveals there are currently close to 40 ongoing intervention trials with vitamin D in COVID-19 around the world for varying purposes, including prevention, and varying forms of treatment.
Consider vitamin D to prevent COVID-19 infection
With regard to the potential role in prevention, “Numerous observational studies have shown that low vitamin D levels are a major predictor for poor COVID outcomes,” noted Jacob Teitelbaum, MD, an internist who specializes in treating chronic fatigue syndrome and fibromyalgia who also has an interest in COVID-19.
“This study shows how severe a problem this is,” Dr. Teitelbaum said in an interview. “A 3.7-fold increase in death rate if someone’s vitamin D level was below 20 [ng/mL] is staggering. It is arguably one of the most important risk factors to consider.”
“What is not clear is whether vitamin D levels are acting as an acute-phase reactant, dropping because of the infection, with larger drops indicating more severe disease, or whether vitamin D deficiency is causing worse outcomes,” added Dr. Teitelbaum, who is director of the Center for Effective CFIDS/Fibromyalgia Therapies, Kailua-Kona, Hawaii.
Also asked to comment, Andrea Giustina, MD, president of the European Society of Endocrinology, said: “The paper by De Smet et al confirms what we already hypothesized in BMJ last March: that patients with low vitamin D levels are at high risk of hospitalization for COVID-19 and developing severe and lethal disease. This is likely due to the loss in the protective action of vitamin D on the immune system and against the SARS-CoV-2–induced cytokine storm.”
He said it is particularly interesting that the authors of the new study had reported more prevalent vitamin D deficiency among men than women, most likely because women are more often treated with vitamin D for osteoporosis.
The new study should prompt all clinicians and health authorities to seriously consider vitamin D supplementation as an additional tool in the fight against COVID-19, particularly for the prevention of infection in those at high risk of both COVID-19 and hypovitaminosis D, such the elderly, urged Dr. Giustina, of San Raffaele Vita-Salute University, Milan.
Results adjusted for multiple confounders
Dr. De Smet and colleagues looked at serum 25-hydroxyvitamin D (25[OH]D) levels in 186 patients hospitalized for severe COVID-19 infection as a function of radiologic stage of COVID-19 pneumonia as well as the association between vitamin D status on admission and COVID-19 mortality.
Cognizant of the potential for confounding by multiple factors, they adjusted for age, sex, and known vitamin D–affected comorbidities such as diabetes, chronic lung disease, and coronary artery disease.
Patients were hospitalized from March 1 to April 7, 2020 (the peak of the first wave of the pandemic) at their institution, AZ Delta General Hospital, a tertiary network hospital.
The mean age of patients was 69 years, 41% were women, and 59% had coronary artery disease. Upon admission to hospital, median vitamin D level was 18 ng/mL (women, 20.7 ng/mL; men, 17.6 ng/mL).
A remarkably high percentage (59%, 109/186) of patients with COVID-19 were vitamin D deficient (25[OH]D <20 ng/mL) when admitted (47% of women and 67% of men), wrote the authors.
“What surprises me,” said Dr. Teitelbaum, is that almost 60% “of these patients had 25(OH)D under 20 ng/mL but most clinicians consider under 50 to be low.”
All patients had a chest CT scan to determine the radiologic stage of COVID-19 pneumonia and serum vitamin D measurement on admission. Radiologic stage of pneumonia was used as a proxy for immunologic phase of COVID-19.
Vitamin D deficiency correlated with worsening pneumonia
Among men, rates of vitamin D deficiency increased with advancing disease, with rates of 55% in stage 1, 67% in stage 2, and up to 74% in stage 3 pneumonia.
There is therefore “a clear correlation between 25(OH)D level and temporal stages of viral pneumonia, particularly in male patients,” the authors wrote.
“Vitamin D dampens excessive inflammation,” said Dr. Teitelbaum. “In these patients with acute respiratory distress syndrome, the immune system has gone wild.”
“The study was carried out in Belgium, so there’s less sunlight there than some other places, but even here in Hawaii, with plenty of sunshine, we have vitamin D deficiency,” he added.
“More studies are needed, but I think there are enough data to suggest a multivitamin should be used to aid prophylaxis, and this is reflected in [some] infectious disease recommendations,” he noted.
A version of this article originally appeared on Medscape.com.
Vitamin D deficiency on admission to hospital was associated with a 3.7-fold increase in the odds of dying from COVID-19, according to an observational study looking back at data from the first wave of the pandemic.
Nearly 60% of patients with COVID-19 were vitamin D deficient upon hospitalization, with men in the advanced stages of COVID-19 pneumonia showing the greatest deficit.
Importantly, the results were independent of comorbidities known to be affected by vitamin D deficiency, wrote the authors, led by Dieter De Smet, MD, from AZ Delta General Hospital, Roeselare, Belgium.
“[The findings] highlight the need for randomized, controlled trials specifically targeting vitamin D–deficient patients at intake, and make a call for general avoidance of vitamin D deficiency as a safe and inexpensive possible mitigation of the SARS-CoV-2 pandemic,” Dr. De Smet and colleagues wrote in their article, published online Nov. 25 in the American Journal of Clinical Pathology.
A search of ClinicalTrials.gov reveals there are currently close to 40 ongoing intervention trials with vitamin D in COVID-19 around the world for varying purposes, including prevention, and varying forms of treatment.
Consider vitamin D to prevent COVID-19 infection
With regard to the potential role in prevention, “Numerous observational studies have shown that low vitamin D levels are a major predictor for poor COVID outcomes,” noted Jacob Teitelbaum, MD, an internist who specializes in treating chronic fatigue syndrome and fibromyalgia who also has an interest in COVID-19.
“This study shows how severe a problem this is,” Dr. Teitelbaum said in an interview. “A 3.7-fold increase in death rate if someone’s vitamin D level was below 20 [ng/mL] is staggering. It is arguably one of the most important risk factors to consider.”
“What is not clear is whether vitamin D levels are acting as an acute-phase reactant, dropping because of the infection, with larger drops indicating more severe disease, or whether vitamin D deficiency is causing worse outcomes,” added Dr. Teitelbaum, who is director of the Center for Effective CFIDS/Fibromyalgia Therapies, Kailua-Kona, Hawaii.
Also asked to comment, Andrea Giustina, MD, president of the European Society of Endocrinology, said: “The paper by De Smet et al confirms what we already hypothesized in BMJ last March: that patients with low vitamin D levels are at high risk of hospitalization for COVID-19 and developing severe and lethal disease. This is likely due to the loss in the protective action of vitamin D on the immune system and against the SARS-CoV-2–induced cytokine storm.”
He said it is particularly interesting that the authors of the new study had reported more prevalent vitamin D deficiency among men than women, most likely because women are more often treated with vitamin D for osteoporosis.
The new study should prompt all clinicians and health authorities to seriously consider vitamin D supplementation as an additional tool in the fight against COVID-19, particularly for the prevention of infection in those at high risk of both COVID-19 and hypovitaminosis D, such the elderly, urged Dr. Giustina, of San Raffaele Vita-Salute University, Milan.
Results adjusted for multiple confounders
Dr. De Smet and colleagues looked at serum 25-hydroxyvitamin D (25[OH]D) levels in 186 patients hospitalized for severe COVID-19 infection as a function of radiologic stage of COVID-19 pneumonia as well as the association between vitamin D status on admission and COVID-19 mortality.
Cognizant of the potential for confounding by multiple factors, they adjusted for age, sex, and known vitamin D–affected comorbidities such as diabetes, chronic lung disease, and coronary artery disease.
Patients were hospitalized from March 1 to April 7, 2020 (the peak of the first wave of the pandemic) at their institution, AZ Delta General Hospital, a tertiary network hospital.
The mean age of patients was 69 years, 41% were women, and 59% had coronary artery disease. Upon admission to hospital, median vitamin D level was 18 ng/mL (women, 20.7 ng/mL; men, 17.6 ng/mL).
A remarkably high percentage (59%, 109/186) of patients with COVID-19 were vitamin D deficient (25[OH]D <20 ng/mL) when admitted (47% of women and 67% of men), wrote the authors.
“What surprises me,” said Dr. Teitelbaum, is that almost 60% “of these patients had 25(OH)D under 20 ng/mL but most clinicians consider under 50 to be low.”
All patients had a chest CT scan to determine the radiologic stage of COVID-19 pneumonia and serum vitamin D measurement on admission. Radiologic stage of pneumonia was used as a proxy for immunologic phase of COVID-19.
Vitamin D deficiency correlated with worsening pneumonia
Among men, rates of vitamin D deficiency increased with advancing disease, with rates of 55% in stage 1, 67% in stage 2, and up to 74% in stage 3 pneumonia.
There is therefore “a clear correlation between 25(OH)D level and temporal stages of viral pneumonia, particularly in male patients,” the authors wrote.
“Vitamin D dampens excessive inflammation,” said Dr. Teitelbaum. “In these patients with acute respiratory distress syndrome, the immune system has gone wild.”
“The study was carried out in Belgium, so there’s less sunlight there than some other places, but even here in Hawaii, with plenty of sunshine, we have vitamin D deficiency,” he added.
“More studies are needed, but I think there are enough data to suggest a multivitamin should be used to aid prophylaxis, and this is reflected in [some] infectious disease recommendations,” he noted.
A version of this article originally appeared on Medscape.com.
Vitamin D deficiency on admission to hospital was associated with a 3.7-fold increase in the odds of dying from COVID-19, according to an observational study looking back at data from the first wave of the pandemic.
Nearly 60% of patients with COVID-19 were vitamin D deficient upon hospitalization, with men in the advanced stages of COVID-19 pneumonia showing the greatest deficit.
Importantly, the results were independent of comorbidities known to be affected by vitamin D deficiency, wrote the authors, led by Dieter De Smet, MD, from AZ Delta General Hospital, Roeselare, Belgium.
“[The findings] highlight the need for randomized, controlled trials specifically targeting vitamin D–deficient patients at intake, and make a call for general avoidance of vitamin D deficiency as a safe and inexpensive possible mitigation of the SARS-CoV-2 pandemic,” Dr. De Smet and colleagues wrote in their article, published online Nov. 25 in the American Journal of Clinical Pathology.
A search of ClinicalTrials.gov reveals there are currently close to 40 ongoing intervention trials with vitamin D in COVID-19 around the world for varying purposes, including prevention, and varying forms of treatment.
Consider vitamin D to prevent COVID-19 infection
With regard to the potential role in prevention, “Numerous observational studies have shown that low vitamin D levels are a major predictor for poor COVID outcomes,” noted Jacob Teitelbaum, MD, an internist who specializes in treating chronic fatigue syndrome and fibromyalgia who also has an interest in COVID-19.
“This study shows how severe a problem this is,” Dr. Teitelbaum said in an interview. “A 3.7-fold increase in death rate if someone’s vitamin D level was below 20 [ng/mL] is staggering. It is arguably one of the most important risk factors to consider.”
“What is not clear is whether vitamin D levels are acting as an acute-phase reactant, dropping because of the infection, with larger drops indicating more severe disease, or whether vitamin D deficiency is causing worse outcomes,” added Dr. Teitelbaum, who is director of the Center for Effective CFIDS/Fibromyalgia Therapies, Kailua-Kona, Hawaii.
Also asked to comment, Andrea Giustina, MD, president of the European Society of Endocrinology, said: “The paper by De Smet et al confirms what we already hypothesized in BMJ last March: that patients with low vitamin D levels are at high risk of hospitalization for COVID-19 and developing severe and lethal disease. This is likely due to the loss in the protective action of vitamin D on the immune system and against the SARS-CoV-2–induced cytokine storm.”
He said it is particularly interesting that the authors of the new study had reported more prevalent vitamin D deficiency among men than women, most likely because women are more often treated with vitamin D for osteoporosis.
The new study should prompt all clinicians and health authorities to seriously consider vitamin D supplementation as an additional tool in the fight against COVID-19, particularly for the prevention of infection in those at high risk of both COVID-19 and hypovitaminosis D, such the elderly, urged Dr. Giustina, of San Raffaele Vita-Salute University, Milan.
Results adjusted for multiple confounders
Dr. De Smet and colleagues looked at serum 25-hydroxyvitamin D (25[OH]D) levels in 186 patients hospitalized for severe COVID-19 infection as a function of radiologic stage of COVID-19 pneumonia as well as the association between vitamin D status on admission and COVID-19 mortality.
Cognizant of the potential for confounding by multiple factors, they adjusted for age, sex, and known vitamin D–affected comorbidities such as diabetes, chronic lung disease, and coronary artery disease.
Patients were hospitalized from March 1 to April 7, 2020 (the peak of the first wave of the pandemic) at their institution, AZ Delta General Hospital, a tertiary network hospital.
The mean age of patients was 69 years, 41% were women, and 59% had coronary artery disease. Upon admission to hospital, median vitamin D level was 18 ng/mL (women, 20.7 ng/mL; men, 17.6 ng/mL).
A remarkably high percentage (59%, 109/186) of patients with COVID-19 were vitamin D deficient (25[OH]D <20 ng/mL) when admitted (47% of women and 67% of men), wrote the authors.
“What surprises me,” said Dr. Teitelbaum, is that almost 60% “of these patients had 25(OH)D under 20 ng/mL but most clinicians consider under 50 to be low.”
All patients had a chest CT scan to determine the radiologic stage of COVID-19 pneumonia and serum vitamin D measurement on admission. Radiologic stage of pneumonia was used as a proxy for immunologic phase of COVID-19.
Vitamin D deficiency correlated with worsening pneumonia
Among men, rates of vitamin D deficiency increased with advancing disease, with rates of 55% in stage 1, 67% in stage 2, and up to 74% in stage 3 pneumonia.
There is therefore “a clear correlation between 25(OH)D level and temporal stages of viral pneumonia, particularly in male patients,” the authors wrote.
“Vitamin D dampens excessive inflammation,” said Dr. Teitelbaum. “In these patients with acute respiratory distress syndrome, the immune system has gone wild.”
“The study was carried out in Belgium, so there’s less sunlight there than some other places, but even here in Hawaii, with plenty of sunshine, we have vitamin D deficiency,” he added.
“More studies are needed, but I think there are enough data to suggest a multivitamin should be used to aid prophylaxis, and this is reflected in [some] infectious disease recommendations,” he noted.
A version of this article originally appeared on Medscape.com.
Just under three million will get COVID-19 vaccine in first week
The federal government says it will distribute only enough doses of Pfizer’s COVID-19 vaccine to immunize 2.9 million Americans in the first week after the US Food and Drug Administration (FDA) authorizes it, far less than the initially discussed 6.4 million doses.
Theoretically, states have already formulated plans for distribution based on the revised lower amount. But in a briefing with reporters on December 9, officials from Operation Warp Speed and the Department of Health and Human Services (HHS) didn’t make clear exactly what the states were expecting.
Vaccine will be shipped to and allocated by 64 jurisdictions and five federal agencies — the Bureau of Prisons, the Department of Defense, the Department of State, the Indian Health Service, and the Veterans Health Administration — according to the Centers for Disease Control and Prevention’s COVID-19 Vaccination Program Interim Playbook.
It will be up to states — which will receive a supply prorated to population — and these agencies to determine how to prioritize distribution of the 2.9 million doses. Each state and agency has its own plan. Gen. Gustave Perna, the chief operating officer for Operation Warp Speed, said in the briefing that 30 states have told the federal government they will prioritize initial doses for residents and staff of long-term care facilities.
The distribution is contingent on FDA authorization, which could happen soon. The FDA’s Vaccines and Related Biologics Advisory Committee weighed the effectiveness data for the Pfizer vaccine on December 10 and recommended that the agency grant emergency authorization. The FDA could issue a decision at any time.
Fewer doses out of the gate
Perna said the federal government will begin shipping the Pfizer vaccine within 24 hours of an FDA authorization.
He said those shipments will include a total of 2.9 million doses — not the 6.4 million that will be available. The government is holding 500,000 doses in reserve and another 2.9 million to guarantee that the first few million people who are vaccinated will be able to receive a second dose 21 days later, said Perna.
In part, that is because the FDA labeling will require that a first dose be followed by a second exactly 21 days later, said HHS Secretary Alex Azar in the briefing.
Federal officials have calculated how much to hold back on the basis of Pfizer’s production, said Azar. At least initially, “we will not distribute a vaccine knowing that the booster will not be available either from reserve supply by us or ongoing expected predicted production,” he said.
Even with Pfizer having reduced its estimates of how much vaccine it can deliver in December, Azar said, “There will be enough vaccine available for 20 million first vaccinations in the month of December.”
That estimate is predicated, however, on the idea that a vaccine under development by Moderna will receive clearance shortly after the FDA assesses that vaccine’s safety and effectiveness on December 17.
This article first appeared on Medscape.com.
The federal government says it will distribute only enough doses of Pfizer’s COVID-19 vaccine to immunize 2.9 million Americans in the first week after the US Food and Drug Administration (FDA) authorizes it, far less than the initially discussed 6.4 million doses.
Theoretically, states have already formulated plans for distribution based on the revised lower amount. But in a briefing with reporters on December 9, officials from Operation Warp Speed and the Department of Health and Human Services (HHS) didn’t make clear exactly what the states were expecting.
Vaccine will be shipped to and allocated by 64 jurisdictions and five federal agencies — the Bureau of Prisons, the Department of Defense, the Department of State, the Indian Health Service, and the Veterans Health Administration — according to the Centers for Disease Control and Prevention’s COVID-19 Vaccination Program Interim Playbook.
It will be up to states — which will receive a supply prorated to population — and these agencies to determine how to prioritize distribution of the 2.9 million doses. Each state and agency has its own plan. Gen. Gustave Perna, the chief operating officer for Operation Warp Speed, said in the briefing that 30 states have told the federal government they will prioritize initial doses for residents and staff of long-term care facilities.
The distribution is contingent on FDA authorization, which could happen soon. The FDA’s Vaccines and Related Biologics Advisory Committee weighed the effectiveness data for the Pfizer vaccine on December 10 and recommended that the agency grant emergency authorization. The FDA could issue a decision at any time.
Fewer doses out of the gate
Perna said the federal government will begin shipping the Pfizer vaccine within 24 hours of an FDA authorization.
He said those shipments will include a total of 2.9 million doses — not the 6.4 million that will be available. The government is holding 500,000 doses in reserve and another 2.9 million to guarantee that the first few million people who are vaccinated will be able to receive a second dose 21 days later, said Perna.
In part, that is because the FDA labeling will require that a first dose be followed by a second exactly 21 days later, said HHS Secretary Alex Azar in the briefing.
Federal officials have calculated how much to hold back on the basis of Pfizer’s production, said Azar. At least initially, “we will not distribute a vaccine knowing that the booster will not be available either from reserve supply by us or ongoing expected predicted production,” he said.
Even with Pfizer having reduced its estimates of how much vaccine it can deliver in December, Azar said, “There will be enough vaccine available for 20 million first vaccinations in the month of December.”
That estimate is predicated, however, on the idea that a vaccine under development by Moderna will receive clearance shortly after the FDA assesses that vaccine’s safety and effectiveness on December 17.
This article first appeared on Medscape.com.
The federal government says it will distribute only enough doses of Pfizer’s COVID-19 vaccine to immunize 2.9 million Americans in the first week after the US Food and Drug Administration (FDA) authorizes it, far less than the initially discussed 6.4 million doses.
Theoretically, states have already formulated plans for distribution based on the revised lower amount. But in a briefing with reporters on December 9, officials from Operation Warp Speed and the Department of Health and Human Services (HHS) didn’t make clear exactly what the states were expecting.
Vaccine will be shipped to and allocated by 64 jurisdictions and five federal agencies — the Bureau of Prisons, the Department of Defense, the Department of State, the Indian Health Service, and the Veterans Health Administration — according to the Centers for Disease Control and Prevention’s COVID-19 Vaccination Program Interim Playbook.
It will be up to states — which will receive a supply prorated to population — and these agencies to determine how to prioritize distribution of the 2.9 million doses. Each state and agency has its own plan. Gen. Gustave Perna, the chief operating officer for Operation Warp Speed, said in the briefing that 30 states have told the federal government they will prioritize initial doses for residents and staff of long-term care facilities.
The distribution is contingent on FDA authorization, which could happen soon. The FDA’s Vaccines and Related Biologics Advisory Committee weighed the effectiveness data for the Pfizer vaccine on December 10 and recommended that the agency grant emergency authorization. The FDA could issue a decision at any time.
Fewer doses out of the gate
Perna said the federal government will begin shipping the Pfizer vaccine within 24 hours of an FDA authorization.
He said those shipments will include a total of 2.9 million doses — not the 6.4 million that will be available. The government is holding 500,000 doses in reserve and another 2.9 million to guarantee that the first few million people who are vaccinated will be able to receive a second dose 21 days later, said Perna.
In part, that is because the FDA labeling will require that a first dose be followed by a second exactly 21 days later, said HHS Secretary Alex Azar in the briefing.
Federal officials have calculated how much to hold back on the basis of Pfizer’s production, said Azar. At least initially, “we will not distribute a vaccine knowing that the booster will not be available either from reserve supply by us or ongoing expected predicted production,” he said.
Even with Pfizer having reduced its estimates of how much vaccine it can deliver in December, Azar said, “There will be enough vaccine available for 20 million first vaccinations in the month of December.”
That estimate is predicated, however, on the idea that a vaccine under development by Moderna will receive clearance shortly after the FDA assesses that vaccine’s safety and effectiveness on December 17.
This article first appeared on Medscape.com.
Antihistamine prescribing for AD varies by specialty
(AD), according to an analysis of a national database.
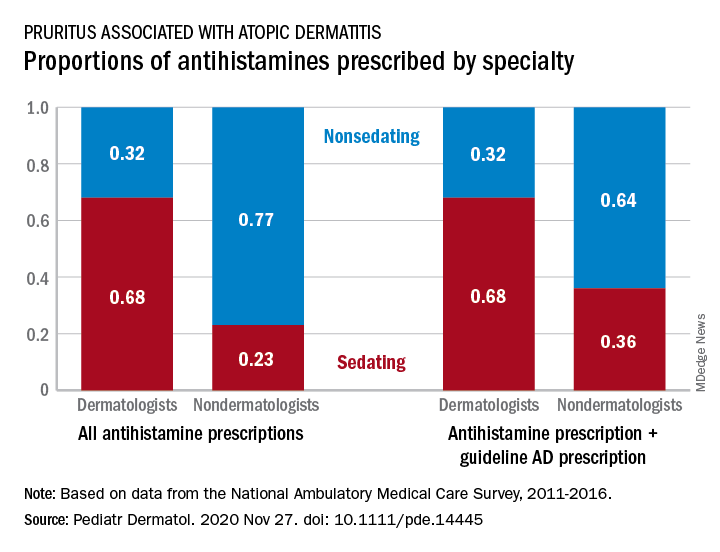
Dermatologists were more likely to prescribe sedating than nonsedating antihistamines (0.68 vs. 0.32) for patients with AD, but the reverse applied to nondermatologists, whose antihistamine distribution was 0.23 sedating and 0.77 nonsedating, based on 2011-2016 data from the National Ambulatory Medical Care Survey.
The numbers were similar for new antihistamine prescriptions, with sedating/nonsedating proportions of 0.60/0.40 for dermatologists and 0.24/0.76 for nondermatologists. Addition of guideline-recommended drugs such as topical corticosteroids and calcineurin inhibitors to the AD equation did not change the result, as dermatologists again showed a preference for sedating antihistamines, compared with nondermatologists, the investigators said.
The data also showed that Black patients with AD were more likely than were White patients to receive prescriptions for first-generation antihistamines and for therapies recommended by the AAD guidelines, and that patients under 21 years received more sedating antihistamines than did patients over age 21, they reported.
The age disparity “may be due to patient preference, as sedation effects may be less desirable to adult patients,” the investigators noted.
SOURCE: Garg S et al. Pediatr Dermatol. 2020 Nov 27. doi: 10.1111/pde.14445.
(AD), according to an analysis of a national database.

Dermatologists were more likely to prescribe sedating than nonsedating antihistamines (0.68 vs. 0.32) for patients with AD, but the reverse applied to nondermatologists, whose antihistamine distribution was 0.23 sedating and 0.77 nonsedating, based on 2011-2016 data from the National Ambulatory Medical Care Survey.
The numbers were similar for new antihistamine prescriptions, with sedating/nonsedating proportions of 0.60/0.40 for dermatologists and 0.24/0.76 for nondermatologists. Addition of guideline-recommended drugs such as topical corticosteroids and calcineurin inhibitors to the AD equation did not change the result, as dermatologists again showed a preference for sedating antihistamines, compared with nondermatologists, the investigators said.
The data also showed that Black patients with AD were more likely than were White patients to receive prescriptions for first-generation antihistamines and for therapies recommended by the AAD guidelines, and that patients under 21 years received more sedating antihistamines than did patients over age 21, they reported.
The age disparity “may be due to patient preference, as sedation effects may be less desirable to adult patients,” the investigators noted.
SOURCE: Garg S et al. Pediatr Dermatol. 2020 Nov 27. doi: 10.1111/pde.14445.
(AD), according to an analysis of a national database.

Dermatologists were more likely to prescribe sedating than nonsedating antihistamines (0.68 vs. 0.32) for patients with AD, but the reverse applied to nondermatologists, whose antihistamine distribution was 0.23 sedating and 0.77 nonsedating, based on 2011-2016 data from the National Ambulatory Medical Care Survey.
The numbers were similar for new antihistamine prescriptions, with sedating/nonsedating proportions of 0.60/0.40 for dermatologists and 0.24/0.76 for nondermatologists. Addition of guideline-recommended drugs such as topical corticosteroids and calcineurin inhibitors to the AD equation did not change the result, as dermatologists again showed a preference for sedating antihistamines, compared with nondermatologists, the investigators said.
The data also showed that Black patients with AD were more likely than were White patients to receive prescriptions for first-generation antihistamines and for therapies recommended by the AAD guidelines, and that patients under 21 years received more sedating antihistamines than did patients over age 21, they reported.
The age disparity “may be due to patient preference, as sedation effects may be less desirable to adult patients,” the investigators noted.
SOURCE: Garg S et al. Pediatr Dermatol. 2020 Nov 27. doi: 10.1111/pde.14445.
FROM PEDIATRIC DERMATOLOGY
FDA panel overwhelmingly backs emergency authorization for Pfizer COVID vaccine
Federal advisers on Thursday told US regulators that the benefits of Pfizer's COVID vaccine outweigh its risks for people aged 16 years and older, moving this product closer to a special emergency clearance.
The US Food and Drug Administration (FDA) put Pfizer's application before its Vaccines and Related Biological Products Advisory Committee (VRBPAC), seeking expert feedback on what is likely to be the first COVID-19 vaccine cleared for use in the United States.
New York-based Pfizer is seeking an emergency use authorization (EUA) for its vaccine, known as BNT162b2, which it developed with Germany's BioNTech. The FDA asked its advisers to vote on a single question regarding this product: "Based on the totality of scientific evidence available, do the benefits of the Pfizer-BioNTech COVID-19 Vaccine outweigh its risks for use in individuals 16 years of age and older?"
The members of VRBPAC voted 17-4 in favor of the Pfizer vaccine, with one panelist abstaining. The FDA considers the recommendations of its panels, but is not bound by them. The agency is expected to quickly grant the special clearance to Pfizer's vaccine, with the company then expected to complete work needed for a more complete biologics license application (BLA).
The FDA often allows members of its advisory committees to explain the reasons for their decisions to vote for or against an application after the tallies are publicly counted.
But the FDA did not give VRBPAC members this opportunity on Thursday, leaving the public without detailed insight into their support or objections.
Before the vote, several panelists had asked if the FDA could rephrase the voting question, raising the age for the approved group to perhaps 18 years of age. During the day, panelists also had questioned whether Pfizer's studies give enough information to judge whether the vaccine works against severe cases of COVID. And there was a discussion about how Pfizer could address concerns about the potential for allergic reactions to the vaccine, given the news of two healthcare workers who experienced allergic reactions after having the vaccine but who have since recovered.
In closing the meeting, VRBPAC chairman, Arnold Monto, MD, noted that the panel will on Dec. 17 meet again to offer recommendations on Moderna Inc.'s COVID vaccine.
"I believe most of us are going to be revisiting some of these issues in about a week," he said.
The panelist who abstained was H. Cody Meissner, MD, an expert in pediatric infectious disease from Tufts University. He earlier was among the several panelists who raised questions about the limited data available about the benefit to those ages 16 and 17. Those voting against the application were Michael Kurilla, MD, PhD; Archana Chatterjee, MD, PhD; A. Oveta Fuller, PhD, and David Kim, MD, MA, according to a tally read by the FDA staff after the vote.
Meanwhile, Sheldon Toubman, JD, voted in favor of the application according to the FDA staff's tally. Toubman had been a chief critic among VRBPAC members in reviewing Pfizer's application at the meeting. He'd suggested limiting the EUA to healthcare workers and residents of nursing homes. Members of these two groups are expected to be the first in the US to get Pfizer's vaccine, for which there will be only a limited initial supply. That idea gained no traction.
Toubman also pressed for more evidence that Pfizer's vaccine will work against severe cases of COVID.
The FDA staff on December 8 released a largely positive agency review of Pfizer vaccine. The efficacy of a two-dose administration of the vaccine has been pegged at 95.0%, with eight COVID-19 cases in the vaccine group and 162 COVID-19 cases in the placebo group. The FDA staff said that the 95% credible interval for the vaccine efficacy was 90.3% to 97.6%.
In that review, the FDA staff said there may be a hint from the results observed to date that the Pfizer vaccine may help ward off severe cases of COVID-19. There were 10 study participants that had severe COVID-19 disease after the first dose: one who received the vaccine and nine who received placebo.
"The total number of severe cases is small, which limits the overall conclusions that can be drawn; however, the case split does suggest protection from severe COVID-19 disease," the FDA staff said.
At the meeting today, Doron Fink, MD, PhD, a lead FDA official on the COVID vaccine review, responded directly to Toubman's concerns. There are many examples of vaccines that protect as well if not better against severe disease as they do against mild to moderate disease, Fink said.
"Protecting against disease of any severity is actually a pretty good predictor of protection against severe disease," Fink said, adding that there's already been a "strong result" shown in terms of the efficacy of Pfizer's vaccine.
Rolling out
Canadian health regulators on December 9 announced their nation's conditional approval of Pfizer's vaccine for people ages 16 and older. In the United Kingdom, a widely publicized rollout of Pfizer's vaccine began on Dec. 8. News quickly spread about two workers in the National Health Service having allergic reactions following vaccination. Both of these workers carry adrenaline autoinjectors, suggesting they have suffered reactions in the past, the Guardian reported. These kinds of autoinjectors are well known in the United States under the brand name EpiPen.
A noted vaccine expert serving on VRBPAC, Paul Offit, MD, of Children's Hospital of Philadelphia, Philadelphia, Pennsylvania, urged the FDA and Pfizer to investigate any connection between reaction to the vaccine and known allergies. If not fully addressed, reports of the reactions seen in initial vaccinations in the UK could prove to unnecessarily frighten people who have allergies away from getting the COVID shot, he said.
Offit suggested running tests where people with egg and peanut allergies would get the Pfizer vaccine under close medical observation "to prove that this is not going to be a problem."
"This is a practical solution because this issue is not going to die until we have better data," Offit said.
More than a dozen COVID-19 vaccines have reached advanced stages of testing, including ones developed in Russia and China, according to the World Health Organization (WHO). The two leading candidates for the US market are the Pfizer/BioNTech vaccine and a similar vaccine developed by Moderna and the National Institute of Allergy and Infectious Diseases. Johnson & Johnson and AstraZeneca are among the other companies with COVID-19 vaccines in testing.
The rapid development of COVID vaccines will create challenges in testing these products. A key issue will be how and whether to continue with placebo-controlled trials, even though such research would be helpful, FDA advisers said.
The FDA tasked Steven Goodman, MD, MHS, PhD, of Stanford University with presenting an overview of considerations for continuing a placebo-controlled trial as COVID vaccines become available. Once a COVID-19 vaccine becomes available to the public, people who have received placebo in the Pfizer trial should not be allowed to immediately receive the vaccine, Goodman said.
There isn't a strong medically-based argument against placebo-controlled research in COVID-19, as many people can take steps to reduce their risk for the infection, Goodman said.
"So as long as there are still important things to learn about the vaccine, placebo-controlled trials should not be regarded as unethical," Goodman said. " I think, however, they might be infeasible. And that is a big issue, because people may not be willing to either remain in the study or to enroll."
During the public comment session, a former FDA official spoke of a need for careful consideration of study volunteers' needs in designing trials of COVID-19 vaccines.
"Reasonable people can disagree over whether study subjects should have priority access to a product whose efficacy they helped demonstrate," said Peter Lurie, MD, president of the nonprofit Center for Science in the Public Interest. "But we ought to be able to agree on this: No subject who has put their body on the line in a vaccine study should be at a disadvantage in terms of vaccine access as a result of their participation."
Lurie argued against extended periods of blinded follow-up after authorization of a COVID-19 vaccine. Such a requirement would be "hard to justify ethically, if it is inconsistent with public health recommendations, particularly with rapidly rising case rates and the reported levels of effectiveness" of the Pfizer vaccine, said Lurie, who served as an associate commissioner at FDA from 2014 to 2017.
Lurie also noted the FDA staff's identification of what he called "disproportionate numbers of Bell's Palsy cases (4 in the vaccine groups vs. 0 in the placebo group)" as a matter that should continue to be monitored, including in the postmarketing phase. He raised no objections to the EUA.
Sidney Wolfe, MD, founder and senior adviser to Public Citizen's Health Research Group, also spoke at the public comment session, citing no objection to an EUA for the Pfizer vaccine. Like Lurie, he urged special consideration of people who have or will receive placebo in COVID-19 vaccine trials.
The Thursday advisory committee on the Pfizer vaccine differed from those held for many other products. The discussion focused more on how to monitor and evaluate the vaccine once approved, while advisory committees sometimes include a detailed look at whether a company has proven that its product works. One of the special advisers serving temporarily on VRBPAC, Eric J. Rubin, MD, PhD, also today published an editorial in The New England Journal of Medicine, titled "SARS-CoV-2 Vaccination — An Ounce (Actually, Much Less) of Prevention."
In the editorial, Rubin and coauthor, Dan L. Longo, MD, called the Pfizer vaccine results seen so far "impressive."
"In the primary analysis, only 8 cases of Covid-19 were seen in the vaccine group, as compared with 162 in the placebo group, for an overall efficacy of 95% (with a 95% credible interval of 90.3 to 97.6%)," they write. "Although the trial does not have the statistical power to assess subgroups, efficacy appeared to be similar in low-risk and high-risk persons, including some from communities that have been disproportionately affected by disease, and in participants older than 55 years of age and those younger than 55."
Intense Scrutiny
The FDA has come under intense scrutiny this year in part because of the aggressive — and ultimately unrealistic — timelines for COVID-19 treatments promoted by the Trump administration. President Donald Trump several times suggested a COVID-19 vaccine could be approved before the November election. Many concerned physicians and scientists including Medscape Editor-in-Chief Eric Topol, MD, called on FDA staff to fight back against any bid to inappropriately speed the approval process for political reasons.
"Any shortcuts will not only jeopardize the vaccine programs but betray the public trust, which is already fragile about vaccines, and has been made more so by your lack of autonomy from the Trump administration and its overt politicization of the FDA," Topol wrote in an August open letter to FDA Commissioner Stephen Hahn, MD.
In an October interview with Topol, Hahn noted that there has been some pushback against the idea of an EUA for a COVID-19 vaccine, with some people preferring to wait for a more complete biological license application.
"When you're talking about a pandemic where people are dying, you want to expedite it as much as possible," Hahn told Topol in the interview.
On Thursday, Hahn issued a public statement about the VRBPAC meeting. Hahn said the FDA's "career staff — made up of physicians, biologists, chemists, epidemiologists, statisticians, and other professionals — have been working around the clock to thoroughly evaluate the data and information in the EUA request."
"I can assure you that no vaccine will be authorized for use in the United States until FDA career officials feel confident in allowing their own families to receive it," Hahn said.
Many clinicians offered their views on the FDA meeting during the day on Twitter.
Robert Wachter, MD, chair of the Department of Medicine at the University of California, San Francisco, who has been a vocal opponent of some of Trump's public statements on COVID-19, urged state officials to stick with the FDA's call on the Pfizer vaccine. In a tweet, he noted that officials in California and several other states have called for independent reviews of COVID-19 vaccines.
If such reviews were to delay distribution of vaccines, this would "lead to more harm than good," Wachter tweeted. "Once FDA says 'go', we should go."
This article was updated 12/10/20.
This article originally appeared on Medscape.com.
Federal advisers on Thursday told US regulators that the benefits of Pfizer's COVID vaccine outweigh its risks for people aged 16 years and older, moving this product closer to a special emergency clearance.
The US Food and Drug Administration (FDA) put Pfizer's application before its Vaccines and Related Biological Products Advisory Committee (VRBPAC), seeking expert feedback on what is likely to be the first COVID-19 vaccine cleared for use in the United States.
New York-based Pfizer is seeking an emergency use authorization (EUA) for its vaccine, known as BNT162b2, which it developed with Germany's BioNTech. The FDA asked its advisers to vote on a single question regarding this product: "Based on the totality of scientific evidence available, do the benefits of the Pfizer-BioNTech COVID-19 Vaccine outweigh its risks for use in individuals 16 years of age and older?"
The members of VRBPAC voted 17-4 in favor of the Pfizer vaccine, with one panelist abstaining. The FDA considers the recommendations of its panels, but is not bound by them. The agency is expected to quickly grant the special clearance to Pfizer's vaccine, with the company then expected to complete work needed for a more complete biologics license application (BLA).
The FDA often allows members of its advisory committees to explain the reasons for their decisions to vote for or against an application after the tallies are publicly counted.
But the FDA did not give VRBPAC members this opportunity on Thursday, leaving the public without detailed insight into their support or objections.
Before the vote, several panelists had asked if the FDA could rephrase the voting question, raising the age for the approved group to perhaps 18 years of age. During the day, panelists also had questioned whether Pfizer's studies give enough information to judge whether the vaccine works against severe cases of COVID. And there was a discussion about how Pfizer could address concerns about the potential for allergic reactions to the vaccine, given the news of two healthcare workers who experienced allergic reactions after having the vaccine but who have since recovered.
In closing the meeting, VRBPAC chairman, Arnold Monto, MD, noted that the panel will on Dec. 17 meet again to offer recommendations on Moderna Inc.'s COVID vaccine.
"I believe most of us are going to be revisiting some of these issues in about a week," he said.
The panelist who abstained was H. Cody Meissner, MD, an expert in pediatric infectious disease from Tufts University. He earlier was among the several panelists who raised questions about the limited data available about the benefit to those ages 16 and 17. Those voting against the application were Michael Kurilla, MD, PhD; Archana Chatterjee, MD, PhD; A. Oveta Fuller, PhD, and David Kim, MD, MA, according to a tally read by the FDA staff after the vote.
Meanwhile, Sheldon Toubman, JD, voted in favor of the application according to the FDA staff's tally. Toubman had been a chief critic among VRBPAC members in reviewing Pfizer's application at the meeting. He'd suggested limiting the EUA to healthcare workers and residents of nursing homes. Members of these two groups are expected to be the first in the US to get Pfizer's vaccine, for which there will be only a limited initial supply. That idea gained no traction.
Toubman also pressed for more evidence that Pfizer's vaccine will work against severe cases of COVID.
The FDA staff on December 8 released a largely positive agency review of Pfizer vaccine. The efficacy of a two-dose administration of the vaccine has been pegged at 95.0%, with eight COVID-19 cases in the vaccine group and 162 COVID-19 cases in the placebo group. The FDA staff said that the 95% credible interval for the vaccine efficacy was 90.3% to 97.6%.
In that review, the FDA staff said there may be a hint from the results observed to date that the Pfizer vaccine may help ward off severe cases of COVID-19. There were 10 study participants that had severe COVID-19 disease after the first dose: one who received the vaccine and nine who received placebo.
"The total number of severe cases is small, which limits the overall conclusions that can be drawn; however, the case split does suggest protection from severe COVID-19 disease," the FDA staff said.
At the meeting today, Doron Fink, MD, PhD, a lead FDA official on the COVID vaccine review, responded directly to Toubman's concerns. There are many examples of vaccines that protect as well if not better against severe disease as they do against mild to moderate disease, Fink said.
"Protecting against disease of any severity is actually a pretty good predictor of protection against severe disease," Fink said, adding that there's already been a "strong result" shown in terms of the efficacy of Pfizer's vaccine.
Rolling out
Canadian health regulators on December 9 announced their nation's conditional approval of Pfizer's vaccine for people ages 16 and older. In the United Kingdom, a widely publicized rollout of Pfizer's vaccine began on Dec. 8. News quickly spread about two workers in the National Health Service having allergic reactions following vaccination. Both of these workers carry adrenaline autoinjectors, suggesting they have suffered reactions in the past, the Guardian reported. These kinds of autoinjectors are well known in the United States under the brand name EpiPen.
A noted vaccine expert serving on VRBPAC, Paul Offit, MD, of Children's Hospital of Philadelphia, Philadelphia, Pennsylvania, urged the FDA and Pfizer to investigate any connection between reaction to the vaccine and known allergies. If not fully addressed, reports of the reactions seen in initial vaccinations in the UK could prove to unnecessarily frighten people who have allergies away from getting the COVID shot, he said.
Offit suggested running tests where people with egg and peanut allergies would get the Pfizer vaccine under close medical observation "to prove that this is not going to be a problem."
"This is a practical solution because this issue is not going to die until we have better data," Offit said.
More than a dozen COVID-19 vaccines have reached advanced stages of testing, including ones developed in Russia and China, according to the World Health Organization (WHO). The two leading candidates for the US market are the Pfizer/BioNTech vaccine and a similar vaccine developed by Moderna and the National Institute of Allergy and Infectious Diseases. Johnson & Johnson and AstraZeneca are among the other companies with COVID-19 vaccines in testing.
The rapid development of COVID vaccines will create challenges in testing these products. A key issue will be how and whether to continue with placebo-controlled trials, even though such research would be helpful, FDA advisers said.
The FDA tasked Steven Goodman, MD, MHS, PhD, of Stanford University with presenting an overview of considerations for continuing a placebo-controlled trial as COVID vaccines become available. Once a COVID-19 vaccine becomes available to the public, people who have received placebo in the Pfizer trial should not be allowed to immediately receive the vaccine, Goodman said.
There isn't a strong medically-based argument against placebo-controlled research in COVID-19, as many people can take steps to reduce their risk for the infection, Goodman said.
"So as long as there are still important things to learn about the vaccine, placebo-controlled trials should not be regarded as unethical," Goodman said. " I think, however, they might be infeasible. And that is a big issue, because people may not be willing to either remain in the study or to enroll."
During the public comment session, a former FDA official spoke of a need for careful consideration of study volunteers' needs in designing trials of COVID-19 vaccines.
"Reasonable people can disagree over whether study subjects should have priority access to a product whose efficacy they helped demonstrate," said Peter Lurie, MD, president of the nonprofit Center for Science in the Public Interest. "But we ought to be able to agree on this: No subject who has put their body on the line in a vaccine study should be at a disadvantage in terms of vaccine access as a result of their participation."
Lurie argued against extended periods of blinded follow-up after authorization of a COVID-19 vaccine. Such a requirement would be "hard to justify ethically, if it is inconsistent with public health recommendations, particularly with rapidly rising case rates and the reported levels of effectiveness" of the Pfizer vaccine, said Lurie, who served as an associate commissioner at FDA from 2014 to 2017.
Lurie also noted the FDA staff's identification of what he called "disproportionate numbers of Bell's Palsy cases (4 in the vaccine groups vs. 0 in the placebo group)" as a matter that should continue to be monitored, including in the postmarketing phase. He raised no objections to the EUA.
Sidney Wolfe, MD, founder and senior adviser to Public Citizen's Health Research Group, also spoke at the public comment session, citing no objection to an EUA for the Pfizer vaccine. Like Lurie, he urged special consideration of people who have or will receive placebo in COVID-19 vaccine trials.
The Thursday advisory committee on the Pfizer vaccine differed from those held for many other products. The discussion focused more on how to monitor and evaluate the vaccine once approved, while advisory committees sometimes include a detailed look at whether a company has proven that its product works. One of the special advisers serving temporarily on VRBPAC, Eric J. Rubin, MD, PhD, also today published an editorial in The New England Journal of Medicine, titled "SARS-CoV-2 Vaccination — An Ounce (Actually, Much Less) of Prevention."
In the editorial, Rubin and coauthor, Dan L. Longo, MD, called the Pfizer vaccine results seen so far "impressive."
"In the primary analysis, only 8 cases of Covid-19 were seen in the vaccine group, as compared with 162 in the placebo group, for an overall efficacy of 95% (with a 95% credible interval of 90.3 to 97.6%)," they write. "Although the trial does not have the statistical power to assess subgroups, efficacy appeared to be similar in low-risk and high-risk persons, including some from communities that have been disproportionately affected by disease, and in participants older than 55 years of age and those younger than 55."
Intense Scrutiny
The FDA has come under intense scrutiny this year in part because of the aggressive — and ultimately unrealistic — timelines for COVID-19 treatments promoted by the Trump administration. President Donald Trump several times suggested a COVID-19 vaccine could be approved before the November election. Many concerned physicians and scientists including Medscape Editor-in-Chief Eric Topol, MD, called on FDA staff to fight back against any bid to inappropriately speed the approval process for political reasons.
"Any shortcuts will not only jeopardize the vaccine programs but betray the public trust, which is already fragile about vaccines, and has been made more so by your lack of autonomy from the Trump administration and its overt politicization of the FDA," Topol wrote in an August open letter to FDA Commissioner Stephen Hahn, MD.
In an October interview with Topol, Hahn noted that there has been some pushback against the idea of an EUA for a COVID-19 vaccine, with some people preferring to wait for a more complete biological license application.
"When you're talking about a pandemic where people are dying, you want to expedite it as much as possible," Hahn told Topol in the interview.
On Thursday, Hahn issued a public statement about the VRBPAC meeting. Hahn said the FDA's "career staff — made up of physicians, biologists, chemists, epidemiologists, statisticians, and other professionals — have been working around the clock to thoroughly evaluate the data and information in the EUA request."
"I can assure you that no vaccine will be authorized for use in the United States until FDA career officials feel confident in allowing their own families to receive it," Hahn said.
Many clinicians offered their views on the FDA meeting during the day on Twitter.
Robert Wachter, MD, chair of the Department of Medicine at the University of California, San Francisco, who has been a vocal opponent of some of Trump's public statements on COVID-19, urged state officials to stick with the FDA's call on the Pfizer vaccine. In a tweet, he noted that officials in California and several other states have called for independent reviews of COVID-19 vaccines.
If such reviews were to delay distribution of vaccines, this would "lead to more harm than good," Wachter tweeted. "Once FDA says 'go', we should go."
This article was updated 12/10/20.
This article originally appeared on Medscape.com.
Federal advisers on Thursday told US regulators that the benefits of Pfizer's COVID vaccine outweigh its risks for people aged 16 years and older, moving this product closer to a special emergency clearance.
The US Food and Drug Administration (FDA) put Pfizer's application before its Vaccines and Related Biological Products Advisory Committee (VRBPAC), seeking expert feedback on what is likely to be the first COVID-19 vaccine cleared for use in the United States.
New York-based Pfizer is seeking an emergency use authorization (EUA) for its vaccine, known as BNT162b2, which it developed with Germany's BioNTech. The FDA asked its advisers to vote on a single question regarding this product: "Based on the totality of scientific evidence available, do the benefits of the Pfizer-BioNTech COVID-19 Vaccine outweigh its risks for use in individuals 16 years of age and older?"
The members of VRBPAC voted 17-4 in favor of the Pfizer vaccine, with one panelist abstaining. The FDA considers the recommendations of its panels, but is not bound by them. The agency is expected to quickly grant the special clearance to Pfizer's vaccine, with the company then expected to complete work needed for a more complete biologics license application (BLA).
The FDA often allows members of its advisory committees to explain the reasons for their decisions to vote for or against an application after the tallies are publicly counted.
But the FDA did not give VRBPAC members this opportunity on Thursday, leaving the public without detailed insight into their support or objections.
Before the vote, several panelists had asked if the FDA could rephrase the voting question, raising the age for the approved group to perhaps 18 years of age. During the day, panelists also had questioned whether Pfizer's studies give enough information to judge whether the vaccine works against severe cases of COVID. And there was a discussion about how Pfizer could address concerns about the potential for allergic reactions to the vaccine, given the news of two healthcare workers who experienced allergic reactions after having the vaccine but who have since recovered.
In closing the meeting, VRBPAC chairman, Arnold Monto, MD, noted that the panel will on Dec. 17 meet again to offer recommendations on Moderna Inc.'s COVID vaccine.
"I believe most of us are going to be revisiting some of these issues in about a week," he said.
The panelist who abstained was H. Cody Meissner, MD, an expert in pediatric infectious disease from Tufts University. He earlier was among the several panelists who raised questions about the limited data available about the benefit to those ages 16 and 17. Those voting against the application were Michael Kurilla, MD, PhD; Archana Chatterjee, MD, PhD; A. Oveta Fuller, PhD, and David Kim, MD, MA, according to a tally read by the FDA staff after the vote.
Meanwhile, Sheldon Toubman, JD, voted in favor of the application according to the FDA staff's tally. Toubman had been a chief critic among VRBPAC members in reviewing Pfizer's application at the meeting. He'd suggested limiting the EUA to healthcare workers and residents of nursing homes. Members of these two groups are expected to be the first in the US to get Pfizer's vaccine, for which there will be only a limited initial supply. That idea gained no traction.
Toubman also pressed for more evidence that Pfizer's vaccine will work against severe cases of COVID.
The FDA staff on December 8 released a largely positive agency review of Pfizer vaccine. The efficacy of a two-dose administration of the vaccine has been pegged at 95.0%, with eight COVID-19 cases in the vaccine group and 162 COVID-19 cases in the placebo group. The FDA staff said that the 95% credible interval for the vaccine efficacy was 90.3% to 97.6%.
In that review, the FDA staff said there may be a hint from the results observed to date that the Pfizer vaccine may help ward off severe cases of COVID-19. There were 10 study participants that had severe COVID-19 disease after the first dose: one who received the vaccine and nine who received placebo.
"The total number of severe cases is small, which limits the overall conclusions that can be drawn; however, the case split does suggest protection from severe COVID-19 disease," the FDA staff said.
At the meeting today, Doron Fink, MD, PhD, a lead FDA official on the COVID vaccine review, responded directly to Toubman's concerns. There are many examples of vaccines that protect as well if not better against severe disease as they do against mild to moderate disease, Fink said.
"Protecting against disease of any severity is actually a pretty good predictor of protection against severe disease," Fink said, adding that there's already been a "strong result" shown in terms of the efficacy of Pfizer's vaccine.
Rolling out
Canadian health regulators on December 9 announced their nation's conditional approval of Pfizer's vaccine for people ages 16 and older. In the United Kingdom, a widely publicized rollout of Pfizer's vaccine began on Dec. 8. News quickly spread about two workers in the National Health Service having allergic reactions following vaccination. Both of these workers carry adrenaline autoinjectors, suggesting they have suffered reactions in the past, the Guardian reported. These kinds of autoinjectors are well known in the United States under the brand name EpiPen.
A noted vaccine expert serving on VRBPAC, Paul Offit, MD, of Children's Hospital of Philadelphia, Philadelphia, Pennsylvania, urged the FDA and Pfizer to investigate any connection between reaction to the vaccine and known allergies. If not fully addressed, reports of the reactions seen in initial vaccinations in the UK could prove to unnecessarily frighten people who have allergies away from getting the COVID shot, he said.
Offit suggested running tests where people with egg and peanut allergies would get the Pfizer vaccine under close medical observation "to prove that this is not going to be a problem."
"This is a practical solution because this issue is not going to die until we have better data," Offit said.
More than a dozen COVID-19 vaccines have reached advanced stages of testing, including ones developed in Russia and China, according to the World Health Organization (WHO). The two leading candidates for the US market are the Pfizer/BioNTech vaccine and a similar vaccine developed by Moderna and the National Institute of Allergy and Infectious Diseases. Johnson & Johnson and AstraZeneca are among the other companies with COVID-19 vaccines in testing.
The rapid development of COVID vaccines will create challenges in testing these products. A key issue will be how and whether to continue with placebo-controlled trials, even though such research would be helpful, FDA advisers said.
The FDA tasked Steven Goodman, MD, MHS, PhD, of Stanford University with presenting an overview of considerations for continuing a placebo-controlled trial as COVID vaccines become available. Once a COVID-19 vaccine becomes available to the public, people who have received placebo in the Pfizer trial should not be allowed to immediately receive the vaccine, Goodman said.
There isn't a strong medically-based argument against placebo-controlled research in COVID-19, as many people can take steps to reduce their risk for the infection, Goodman said.
"So as long as there are still important things to learn about the vaccine, placebo-controlled trials should not be regarded as unethical," Goodman said. " I think, however, they might be infeasible. And that is a big issue, because people may not be willing to either remain in the study or to enroll."
During the public comment session, a former FDA official spoke of a need for careful consideration of study volunteers' needs in designing trials of COVID-19 vaccines.
"Reasonable people can disagree over whether study subjects should have priority access to a product whose efficacy they helped demonstrate," said Peter Lurie, MD, president of the nonprofit Center for Science in the Public Interest. "But we ought to be able to agree on this: No subject who has put their body on the line in a vaccine study should be at a disadvantage in terms of vaccine access as a result of their participation."
Lurie argued against extended periods of blinded follow-up after authorization of a COVID-19 vaccine. Such a requirement would be "hard to justify ethically, if it is inconsistent with public health recommendations, particularly with rapidly rising case rates and the reported levels of effectiveness" of the Pfizer vaccine, said Lurie, who served as an associate commissioner at FDA from 2014 to 2017.
Lurie also noted the FDA staff's identification of what he called "disproportionate numbers of Bell's Palsy cases (4 in the vaccine groups vs. 0 in the placebo group)" as a matter that should continue to be monitored, including in the postmarketing phase. He raised no objections to the EUA.
Sidney Wolfe, MD, founder and senior adviser to Public Citizen's Health Research Group, also spoke at the public comment session, citing no objection to an EUA for the Pfizer vaccine. Like Lurie, he urged special consideration of people who have or will receive placebo in COVID-19 vaccine trials.
The Thursday advisory committee on the Pfizer vaccine differed from those held for many other products. The discussion focused more on how to monitor and evaluate the vaccine once approved, while advisory committees sometimes include a detailed look at whether a company has proven that its product works. One of the special advisers serving temporarily on VRBPAC, Eric J. Rubin, MD, PhD, also today published an editorial in The New England Journal of Medicine, titled "SARS-CoV-2 Vaccination — An Ounce (Actually, Much Less) of Prevention."
In the editorial, Rubin and coauthor, Dan L. Longo, MD, called the Pfizer vaccine results seen so far "impressive."
"In the primary analysis, only 8 cases of Covid-19 were seen in the vaccine group, as compared with 162 in the placebo group, for an overall efficacy of 95% (with a 95% credible interval of 90.3 to 97.6%)," they write. "Although the trial does not have the statistical power to assess subgroups, efficacy appeared to be similar in low-risk and high-risk persons, including some from communities that have been disproportionately affected by disease, and in participants older than 55 years of age and those younger than 55."
Intense Scrutiny
The FDA has come under intense scrutiny this year in part because of the aggressive — and ultimately unrealistic — timelines for COVID-19 treatments promoted by the Trump administration. President Donald Trump several times suggested a COVID-19 vaccine could be approved before the November election. Many concerned physicians and scientists including Medscape Editor-in-Chief Eric Topol, MD, called on FDA staff to fight back against any bid to inappropriately speed the approval process for political reasons.
"Any shortcuts will not only jeopardize the vaccine programs but betray the public trust, which is already fragile about vaccines, and has been made more so by your lack of autonomy from the Trump administration and its overt politicization of the FDA," Topol wrote in an August open letter to FDA Commissioner Stephen Hahn, MD.
In an October interview with Topol, Hahn noted that there has been some pushback against the idea of an EUA for a COVID-19 vaccine, with some people preferring to wait for a more complete biological license application.
"When you're talking about a pandemic where people are dying, you want to expedite it as much as possible," Hahn told Topol in the interview.
On Thursday, Hahn issued a public statement about the VRBPAC meeting. Hahn said the FDA's "career staff — made up of physicians, biologists, chemists, epidemiologists, statisticians, and other professionals — have been working around the clock to thoroughly evaluate the data and information in the EUA request."
"I can assure you that no vaccine will be authorized for use in the United States until FDA career officials feel confident in allowing their own families to receive it," Hahn said.
Many clinicians offered their views on the FDA meeting during the day on Twitter.
Robert Wachter, MD, chair of the Department of Medicine at the University of California, San Francisco, who has been a vocal opponent of some of Trump's public statements on COVID-19, urged state officials to stick with the FDA's call on the Pfizer vaccine. In a tweet, he noted that officials in California and several other states have called for independent reviews of COVID-19 vaccines.
If such reviews were to delay distribution of vaccines, this would "lead to more harm than good," Wachter tweeted. "Once FDA says 'go', we should go."
This article was updated 12/10/20.
This article originally appeared on Medscape.com.
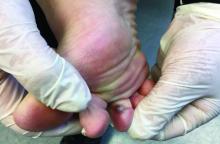
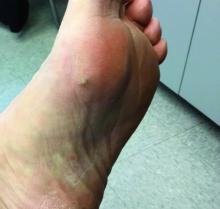
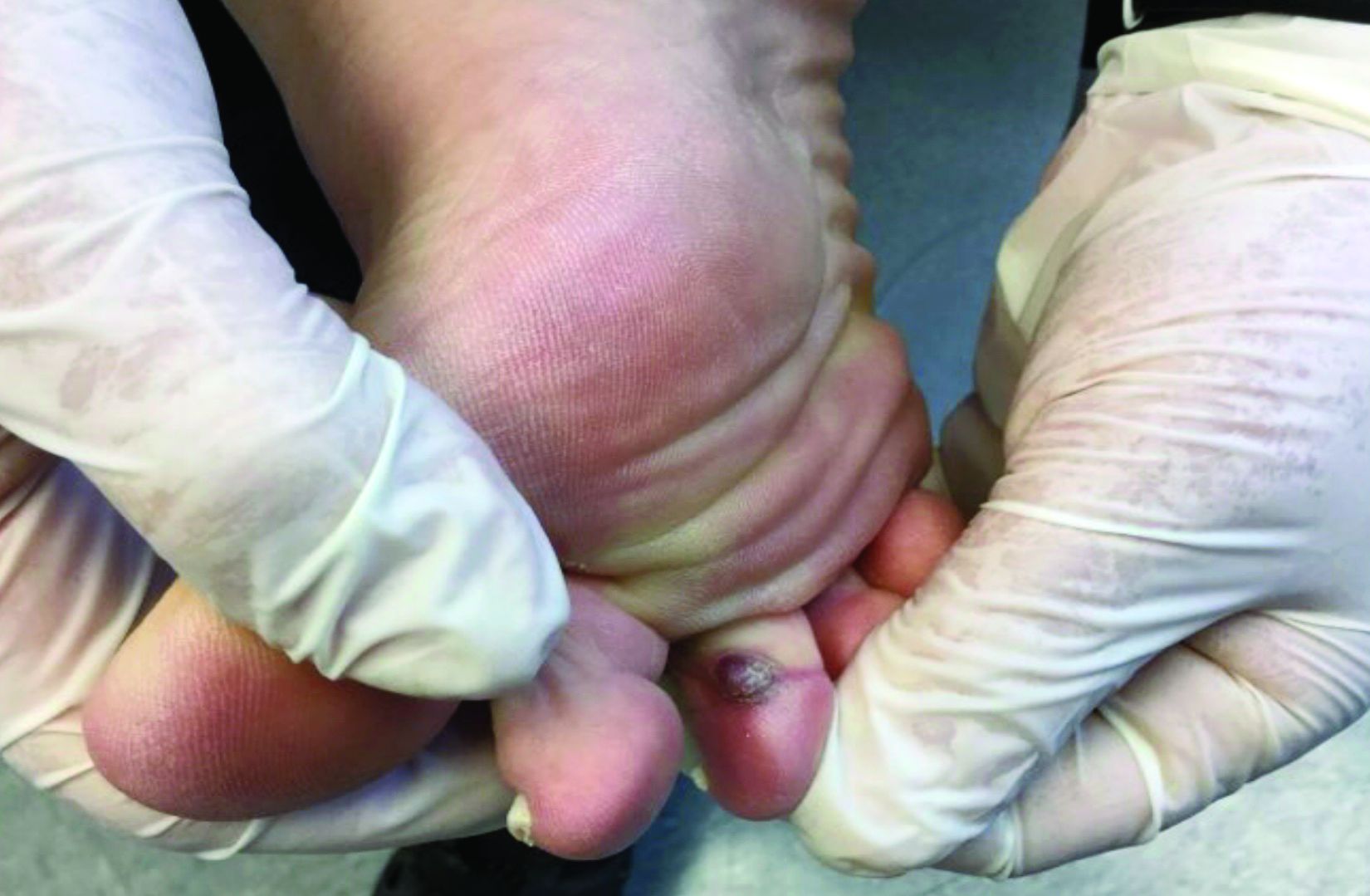
Palpation key when evaluating the skin for suspected MCC
“The lack of a pathognomonic appearance is often what precludes an early diagnosis of this cancer,” Dr. Thakuria, a dermatologist at Brigham and Women’s Hospital, Boston, said during a virtual forum on cutaneous malignancies jointly presented by Postgraduate Institute for Medicine and the Global Academy for Medical Education. “MCCs can vary in appearance in their color, from pink to red to purple, or sometimes they have no color at all. They can be exophytic and obvious, or subtle, deeper tumors. These tumors are generally firm and nontender and are characterized by rapid growth, which is usually but not exclusively the feature that prompts biopsy.”
The typical patient with MCC is elderly, with an average age of 75 years. It affects males more than females by an approximately 2:1 ratio and tends to occur in fair-skinned individuals, although MCC does develop in skin of color. “While the majority of patients with this disease are immunocompetent, immunosuppressed patients are overrepresented in this disease, compared with the general population,” she said.
The clinical differential diagnosis is broad and includes both malignant and benign tumors, which requires a high index of suspicion. Most primary lesions are located on the head and neck, lower limb, and upper limb, but they may appear in non–sun exposed areas, such as the buttocks, as well.
One prospective study of 195 MCC patients found that 56% of clinicians presumed that these tumors were benign at the time of biopsy, and 32% were thought to have a cyst or acneiform lesion. The study authors summarized key clinical features of MCC with the acronym AEIOU: A stands for asymptomatic or nontender; E stands for expanding rapidly, usually over a duration less than 3 months; I stands for immunosuppression; O stands for patient older than age 50 years; and U stands for UV exposed skin location. The researchers found that 89% of the patients studied met three or more of the AEIOU criteria.
Dr. Thakuria, codirector of the Merkel Cell Carcinoma Center of Excellence at the Dana-Farber/Brigham and Women’s Cancer Center and assistant professor of dermatology at Harvard University, both in Boston, shared the following tips for dermatologic evaluation when MCC is suspected:
- Measure and record the clinical diameter of the lesions. “This helps you determine the T staging later, and from there can help you decide on proper treatment,” she said.
- Inspect and palpate the surrounding skin to look for in-transit metastases. “This may actually upstage the patient.”
- For a subcutaneous nodule, hub your punch biopsy. “These tumors can be centered in the deep dermis or fat,” Dr. Thakuria said. “If you really suspect MCC and you don’t get a result on your first biopsy, you may want to consider doing a second deeper biopsy, perhaps even a telescoping biopsy. This is especially true if your first biopsy was via shave technique and showed normal skin.”
- Refer to surgical oncology and radiation oncology ASAP. “You want to call them to ensure speedy consultation, within 1 week if possible,” she said. “Remember that all clinically node-negative MCCs warrant consideration of sentinel lymph node biopsy, regardless of tumor size. Upstaging will occur in 25%-32% of patients.”
Staging workup includes a full skin and lymph node exam to identify in-transit metastases and regional lymphadenopathy. “Palpation is key,” Dr. Thakuria said. “Next, you want to do some form of radiographic examination, so either a scalp to toes PET/CT or CT scan of the chest, abdomen, and pelvis. Finally, sentinel lymph node biopsy is going to be important if you have a clinically node-negative patient but you want to pathologically stage the person appropriately.” Although not formally part of the staging workup, she recommends ordering an AMERK test at diagnosis. AMERK detects antibodies to a Merkel cell polyomavirus oncoprotein, which is a marker of disease status present in about half of MCC patients. It falls with the treatment of cancer and rises with recurrence.
Discussing prognosis with MCC patients “can be challenging and uncomfortable, but even more so if you’re unfamiliar with some of the nuances of the terminology that is used,” Dr. Thakuria said. “Patients who go to Google are often going to encounter overall survival numbers, which are going to be worse than disease-specific numbers in any disease because they take into account death from any cause. This effect is heightened in MCC because this is cancer of predominately older adults, so there are other competing causes of death in this population, which drags down the overall survival estimates.”
Another point to remember when discussing survival with patients is that advances in immunotherapy are not necessarily reflected in national databases. “This is important, because usually in any cancer there’s a 5- to 10-year lag in survival information,” she said. “The last 5 years have brought an incredible change to MCC because of the advent of immunotherapy. Now we’re seeing incredible responses [in the clinic], but we’re not yet seeing those reflected in our survival tables.”
According to an analysis of prognostic factors from 9,387 MCC cases, nodal status is one of most important predictors of lower survival at 5 years, compared with having local disease: 35% versus 51%, respectively. Among patients with macroscopic lymph nodes, having known primary disease is associated with a lower survival at 5 years, compared with having unknown primary disease (27% vs. 42% at five years).
Dr. Thakuria concluded her presentation by recommending a three-step plan for surveillance, starting with a full skin and lymph node exam every 3-6 months for the first 3 years and every 6-12 months thereafter. Second, she advised routine imaging for high risk patients (American Joint Committee on Cancer stage 2 and above) and symptom-directed imaging for low-risk patients. Finally, she recommended the AMERK test every 3 months for the first 2-3 years in patients who were seropositive at diagnosis. A rising titer may be an early indicator of recurrence.
Global Academy for Medical Education and this news organization are owned by the same parent company.
Dr. Thakuria reported having no financial disclosures.
“The lack of a pathognomonic appearance is often what precludes an early diagnosis of this cancer,” Dr. Thakuria, a dermatologist at Brigham and Women’s Hospital, Boston, said during a virtual forum on cutaneous malignancies jointly presented by Postgraduate Institute for Medicine and the Global Academy for Medical Education. “MCCs can vary in appearance in their color, from pink to red to purple, or sometimes they have no color at all. They can be exophytic and obvious, or subtle, deeper tumors. These tumors are generally firm and nontender and are characterized by rapid growth, which is usually but not exclusively the feature that prompts biopsy.”
The typical patient with MCC is elderly, with an average age of 75 years. It affects males more than females by an approximately 2:1 ratio and tends to occur in fair-skinned individuals, although MCC does develop in skin of color. “While the majority of patients with this disease are immunocompetent, immunosuppressed patients are overrepresented in this disease, compared with the general population,” she said.
The clinical differential diagnosis is broad and includes both malignant and benign tumors, which requires a high index of suspicion. Most primary lesions are located on the head and neck, lower limb, and upper limb, but they may appear in non–sun exposed areas, such as the buttocks, as well.
One prospective study of 195 MCC patients found that 56% of clinicians presumed that these tumors were benign at the time of biopsy, and 32% were thought to have a cyst or acneiform lesion. The study authors summarized key clinical features of MCC with the acronym AEIOU: A stands for asymptomatic or nontender; E stands for expanding rapidly, usually over a duration less than 3 months; I stands for immunosuppression; O stands for patient older than age 50 years; and U stands for UV exposed skin location. The researchers found that 89% of the patients studied met three or more of the AEIOU criteria.
Dr. Thakuria, codirector of the Merkel Cell Carcinoma Center of Excellence at the Dana-Farber/Brigham and Women’s Cancer Center and assistant professor of dermatology at Harvard University, both in Boston, shared the following tips for dermatologic evaluation when MCC is suspected:
- Measure and record the clinical diameter of the lesions. “This helps you determine the T staging later, and from there can help you decide on proper treatment,” she said.
- Inspect and palpate the surrounding skin to look for in-transit metastases. “This may actually upstage the patient.”
- For a subcutaneous nodule, hub your punch biopsy. “These tumors can be centered in the deep dermis or fat,” Dr. Thakuria said. “If you really suspect MCC and you don’t get a result on your first biopsy, you may want to consider doing a second deeper biopsy, perhaps even a telescoping biopsy. This is especially true if your first biopsy was via shave technique and showed normal skin.”
- Refer to surgical oncology and radiation oncology ASAP. “You want to call them to ensure speedy consultation, within 1 week if possible,” she said. “Remember that all clinically node-negative MCCs warrant consideration of sentinel lymph node biopsy, regardless of tumor size. Upstaging will occur in 25%-32% of patients.”
Staging workup includes a full skin and lymph node exam to identify in-transit metastases and regional lymphadenopathy. “Palpation is key,” Dr. Thakuria said. “Next, you want to do some form of radiographic examination, so either a scalp to toes PET/CT or CT scan of the chest, abdomen, and pelvis. Finally, sentinel lymph node biopsy is going to be important if you have a clinically node-negative patient but you want to pathologically stage the person appropriately.” Although not formally part of the staging workup, she recommends ordering an AMERK test at diagnosis. AMERK detects antibodies to a Merkel cell polyomavirus oncoprotein, which is a marker of disease status present in about half of MCC patients. It falls with the treatment of cancer and rises with recurrence.
Discussing prognosis with MCC patients “can be challenging and uncomfortable, but even more so if you’re unfamiliar with some of the nuances of the terminology that is used,” Dr. Thakuria said. “Patients who go to Google are often going to encounter overall survival numbers, which are going to be worse than disease-specific numbers in any disease because they take into account death from any cause. This effect is heightened in MCC because this is cancer of predominately older adults, so there are other competing causes of death in this population, which drags down the overall survival estimates.”
Another point to remember when discussing survival with patients is that advances in immunotherapy are not necessarily reflected in national databases. “This is important, because usually in any cancer there’s a 5- to 10-year lag in survival information,” she said. “The last 5 years have brought an incredible change to MCC because of the advent of immunotherapy. Now we’re seeing incredible responses [in the clinic], but we’re not yet seeing those reflected in our survival tables.”
According to an analysis of prognostic factors from 9,387 MCC cases, nodal status is one of most important predictors of lower survival at 5 years, compared with having local disease: 35% versus 51%, respectively. Among patients with macroscopic lymph nodes, having known primary disease is associated with a lower survival at 5 years, compared with having unknown primary disease (27% vs. 42% at five years).
Dr. Thakuria concluded her presentation by recommending a three-step plan for surveillance, starting with a full skin and lymph node exam every 3-6 months for the first 3 years and every 6-12 months thereafter. Second, she advised routine imaging for high risk patients (American Joint Committee on Cancer stage 2 and above) and symptom-directed imaging for low-risk patients. Finally, she recommended the AMERK test every 3 months for the first 2-3 years in patients who were seropositive at diagnosis. A rising titer may be an early indicator of recurrence.
Global Academy for Medical Education and this news organization are owned by the same parent company.
Dr. Thakuria reported having no financial disclosures.
“The lack of a pathognomonic appearance is often what precludes an early diagnosis of this cancer,” Dr. Thakuria, a dermatologist at Brigham and Women’s Hospital, Boston, said during a virtual forum on cutaneous malignancies jointly presented by Postgraduate Institute for Medicine and the Global Academy for Medical Education. “MCCs can vary in appearance in their color, from pink to red to purple, or sometimes they have no color at all. They can be exophytic and obvious, or subtle, deeper tumors. These tumors are generally firm and nontender and are characterized by rapid growth, which is usually but not exclusively the feature that prompts biopsy.”
The typical patient with MCC is elderly, with an average age of 75 years. It affects males more than females by an approximately 2:1 ratio and tends to occur in fair-skinned individuals, although MCC does develop in skin of color. “While the majority of patients with this disease are immunocompetent, immunosuppressed patients are overrepresented in this disease, compared with the general population,” she said.
The clinical differential diagnosis is broad and includes both malignant and benign tumors, which requires a high index of suspicion. Most primary lesions are located on the head and neck, lower limb, and upper limb, but they may appear in non–sun exposed areas, such as the buttocks, as well.
One prospective study of 195 MCC patients found that 56% of clinicians presumed that these tumors were benign at the time of biopsy, and 32% were thought to have a cyst or acneiform lesion. The study authors summarized key clinical features of MCC with the acronym AEIOU: A stands for asymptomatic or nontender; E stands for expanding rapidly, usually over a duration less than 3 months; I stands for immunosuppression; O stands for patient older than age 50 years; and U stands for UV exposed skin location. The researchers found that 89% of the patients studied met three or more of the AEIOU criteria.
Dr. Thakuria, codirector of the Merkel Cell Carcinoma Center of Excellence at the Dana-Farber/Brigham and Women’s Cancer Center and assistant professor of dermatology at Harvard University, both in Boston, shared the following tips for dermatologic evaluation when MCC is suspected:
- Measure and record the clinical diameter of the lesions. “This helps you determine the T staging later, and from there can help you decide on proper treatment,” she said.
- Inspect and palpate the surrounding skin to look for in-transit metastases. “This may actually upstage the patient.”
- For a subcutaneous nodule, hub your punch biopsy. “These tumors can be centered in the deep dermis or fat,” Dr. Thakuria said. “If you really suspect MCC and you don’t get a result on your first biopsy, you may want to consider doing a second deeper biopsy, perhaps even a telescoping biopsy. This is especially true if your first biopsy was via shave technique and showed normal skin.”
- Refer to surgical oncology and radiation oncology ASAP. “You want to call them to ensure speedy consultation, within 1 week if possible,” she said. “Remember that all clinically node-negative MCCs warrant consideration of sentinel lymph node biopsy, regardless of tumor size. Upstaging will occur in 25%-32% of patients.”
Staging workup includes a full skin and lymph node exam to identify in-transit metastases and regional lymphadenopathy. “Palpation is key,” Dr. Thakuria said. “Next, you want to do some form of radiographic examination, so either a scalp to toes PET/CT or CT scan of the chest, abdomen, and pelvis. Finally, sentinel lymph node biopsy is going to be important if you have a clinically node-negative patient but you want to pathologically stage the person appropriately.” Although not formally part of the staging workup, she recommends ordering an AMERK test at diagnosis. AMERK detects antibodies to a Merkel cell polyomavirus oncoprotein, which is a marker of disease status present in about half of MCC patients. It falls with the treatment of cancer and rises with recurrence.
Discussing prognosis with MCC patients “can be challenging and uncomfortable, but even more so if you’re unfamiliar with some of the nuances of the terminology that is used,” Dr. Thakuria said. “Patients who go to Google are often going to encounter overall survival numbers, which are going to be worse than disease-specific numbers in any disease because they take into account death from any cause. This effect is heightened in MCC because this is cancer of predominately older adults, so there are other competing causes of death in this population, which drags down the overall survival estimates.”
Another point to remember when discussing survival with patients is that advances in immunotherapy are not necessarily reflected in national databases. “This is important, because usually in any cancer there’s a 5- to 10-year lag in survival information,” she said. “The last 5 years have brought an incredible change to MCC because of the advent of immunotherapy. Now we’re seeing incredible responses [in the clinic], but we’re not yet seeing those reflected in our survival tables.”
According to an analysis of prognostic factors from 9,387 MCC cases, nodal status is one of most important predictors of lower survival at 5 years, compared with having local disease: 35% versus 51%, respectively. Among patients with macroscopic lymph nodes, having known primary disease is associated with a lower survival at 5 years, compared with having unknown primary disease (27% vs. 42% at five years).
Dr. Thakuria concluded her presentation by recommending a three-step plan for surveillance, starting with a full skin and lymph node exam every 3-6 months for the first 3 years and every 6-12 months thereafter. Second, she advised routine imaging for high risk patients (American Joint Committee on Cancer stage 2 and above) and symptom-directed imaging for low-risk patients. Finally, she recommended the AMERK test every 3 months for the first 2-3 years in patients who were seropositive at diagnosis. A rising titer may be an early indicator of recurrence.
Global Academy for Medical Education and this news organization are owned by the same parent company.
Dr. Thakuria reported having no financial disclosures.
FROM THE CUTANEOUS MALIGNANCIES FORUM
Pfizer can’t supply additional vaccines to U.S. until June
Pfizer won’t be able to provide more COVID-19 vaccine doses to the United States until late June or July because other countries have bought up the available supply, according to The Washington Post.
The U.S. government signed a deal with the giant pharmaceutical company earlier this year to provide 100 million doses for $1.95 billion – enough for 50 million Americans to receive the two-dose vaccine. At that time, Pfizer officials encouraged Operation Warp Speed officials to purchase an additional 100 million doses, The New York Times first reported Dec. 7, but the federal officials declined.
Since then, other countries have signed vaccine deals with Pfizer, so the U.S. may not be able to receive a second major allotment until the summer of 2021, The Washington Post reported. Without a substantial number of additional doses, the U.S. may not be able to follow its schedule of vaccinating the majority of Americans against COVID-19 by April or May.
However, Trump administration officials told the newspaper that there won’t be issues, citing other vaccine companies such as Moderna.
“I’m not concerned about our ability to buy vaccines to offer to all of the American public,” Gen. Paul Ostrowski, who oversees logistics for Operation Warp Speed, told The Washington Post.
“It’s clear that Pfizer made plans with other countries. Many have been announced. We understand those pieces,” he said.
With Pfizer’s COVID-19 vaccine on the verge of FDA approval, federal officials contacted the company last weekend to buy another 100 million doses, but the company said its current supply is already committed, the newspaper reported.
The vaccine from Pfizer and BioNTech is expected to win emergency approval within days and has been shown to be effective against COVID-19.
Pfizer added that it may be able to provide 50 million doses at the end of the second quarter and another 50 million doses during the third quarter. However, the company can’t offer anything “substantial” until next summer.
Beyond the initial 100 million doses that the U.S. has already secured, Pfizer and federal officials would need to negotiate a new, “separate and mutually acceptable agreement,” Amy Rose, a spokeswoman for Pfizer, told the newspaper.
On Dec. 8, President Donald Trump was expected to sign an executive order prioritizing vaccination for Americans first before providing doses to other countries, according to Fox News.
The order will provide guidelines to the Department of Health and Human Services, the U.S. Agency for International Development and the U.S. International Development Finance Corporation for foreign assistance with vaccines, the news outlet reported.
It’s unclear whether the executive order is related to the Pfizer issue, whether the president can prevent a private company from fulfilling contracts with other countries, and whether President-elect Joe Biden will create his own policy, according to CNBC. The order may prove to be mostly symbolic.
The FDA could issue an emergency use authorization for Pfizer’s coronavirus vaccine this week and will likely approve Moderna’s vaccine next week. The U.S. has signed a contract with Moderna for 100 million doses.
During a call with reporters on Dec. 7, a spokeswoman for the Department of Health and Human Services said, “We are confident that we will have 100 million doses of Pfizer’s vaccine as agreed to in our contract, and beyond that, we have five other vaccine candidates, including 100 million doses on the way from Moderna.”
Federal officials are counting on vaccine candidates from AstraZeneca and Johnson & Johnson to seek FDA approval in January and be ready for shipment in February.
“We could have all of them,” Moncef Slaoui, the chief science adviser for Operation Warp Speed, told The Washington Post on Dec. 7.
“And for this reason, we feel confident we could cover the needs without a specific cliff,” he said. “We have planned things in such a way as we would indeed avoid a cliff.”
This article first appeared on WebMD.com.
Pfizer won’t be able to provide more COVID-19 vaccine doses to the United States until late June or July because other countries have bought up the available supply, according to The Washington Post.
The U.S. government signed a deal with the giant pharmaceutical company earlier this year to provide 100 million doses for $1.95 billion – enough for 50 million Americans to receive the two-dose vaccine. At that time, Pfizer officials encouraged Operation Warp Speed officials to purchase an additional 100 million doses, The New York Times first reported Dec. 7, but the federal officials declined.
Since then, other countries have signed vaccine deals with Pfizer, so the U.S. may not be able to receive a second major allotment until the summer of 2021, The Washington Post reported. Without a substantial number of additional doses, the U.S. may not be able to follow its schedule of vaccinating the majority of Americans against COVID-19 by April or May.
However, Trump administration officials told the newspaper that there won’t be issues, citing other vaccine companies such as Moderna.
“I’m not concerned about our ability to buy vaccines to offer to all of the American public,” Gen. Paul Ostrowski, who oversees logistics for Operation Warp Speed, told The Washington Post.
“It’s clear that Pfizer made plans with other countries. Many have been announced. We understand those pieces,” he said.
With Pfizer’s COVID-19 vaccine on the verge of FDA approval, federal officials contacted the company last weekend to buy another 100 million doses, but the company said its current supply is already committed, the newspaper reported.
The vaccine from Pfizer and BioNTech is expected to win emergency approval within days and has been shown to be effective against COVID-19.
Pfizer added that it may be able to provide 50 million doses at the end of the second quarter and another 50 million doses during the third quarter. However, the company can’t offer anything “substantial” until next summer.
Beyond the initial 100 million doses that the U.S. has already secured, Pfizer and federal officials would need to negotiate a new, “separate and mutually acceptable agreement,” Amy Rose, a spokeswoman for Pfizer, told the newspaper.
On Dec. 8, President Donald Trump was expected to sign an executive order prioritizing vaccination for Americans first before providing doses to other countries, according to Fox News.
The order will provide guidelines to the Department of Health and Human Services, the U.S. Agency for International Development and the U.S. International Development Finance Corporation for foreign assistance with vaccines, the news outlet reported.
It’s unclear whether the executive order is related to the Pfizer issue, whether the president can prevent a private company from fulfilling contracts with other countries, and whether President-elect Joe Biden will create his own policy, according to CNBC. The order may prove to be mostly symbolic.
The FDA could issue an emergency use authorization for Pfizer’s coronavirus vaccine this week and will likely approve Moderna’s vaccine next week. The U.S. has signed a contract with Moderna for 100 million doses.
During a call with reporters on Dec. 7, a spokeswoman for the Department of Health and Human Services said, “We are confident that we will have 100 million doses of Pfizer’s vaccine as agreed to in our contract, and beyond that, we have five other vaccine candidates, including 100 million doses on the way from Moderna.”
Federal officials are counting on vaccine candidates from AstraZeneca and Johnson & Johnson to seek FDA approval in January and be ready for shipment in February.
“We could have all of them,” Moncef Slaoui, the chief science adviser for Operation Warp Speed, told The Washington Post on Dec. 7.
“And for this reason, we feel confident we could cover the needs without a specific cliff,” he said. “We have planned things in such a way as we would indeed avoid a cliff.”
This article first appeared on WebMD.com.
Pfizer won’t be able to provide more COVID-19 vaccine doses to the United States until late June or July because other countries have bought up the available supply, according to The Washington Post.
The U.S. government signed a deal with the giant pharmaceutical company earlier this year to provide 100 million doses for $1.95 billion – enough for 50 million Americans to receive the two-dose vaccine. At that time, Pfizer officials encouraged Operation Warp Speed officials to purchase an additional 100 million doses, The New York Times first reported Dec. 7, but the federal officials declined.
Since then, other countries have signed vaccine deals with Pfizer, so the U.S. may not be able to receive a second major allotment until the summer of 2021, The Washington Post reported. Without a substantial number of additional doses, the U.S. may not be able to follow its schedule of vaccinating the majority of Americans against COVID-19 by April or May.
However, Trump administration officials told the newspaper that there won’t be issues, citing other vaccine companies such as Moderna.
“I’m not concerned about our ability to buy vaccines to offer to all of the American public,” Gen. Paul Ostrowski, who oversees logistics for Operation Warp Speed, told The Washington Post.
“It’s clear that Pfizer made plans with other countries. Many have been announced. We understand those pieces,” he said.
With Pfizer’s COVID-19 vaccine on the verge of FDA approval, federal officials contacted the company last weekend to buy another 100 million doses, but the company said its current supply is already committed, the newspaper reported.
The vaccine from Pfizer and BioNTech is expected to win emergency approval within days and has been shown to be effective against COVID-19.
Pfizer added that it may be able to provide 50 million doses at the end of the second quarter and another 50 million doses during the third quarter. However, the company can’t offer anything “substantial” until next summer.
Beyond the initial 100 million doses that the U.S. has already secured, Pfizer and federal officials would need to negotiate a new, “separate and mutually acceptable agreement,” Amy Rose, a spokeswoman for Pfizer, told the newspaper.
On Dec. 8, President Donald Trump was expected to sign an executive order prioritizing vaccination for Americans first before providing doses to other countries, according to Fox News.
The order will provide guidelines to the Department of Health and Human Services, the U.S. Agency for International Development and the U.S. International Development Finance Corporation for foreign assistance with vaccines, the news outlet reported.
It’s unclear whether the executive order is related to the Pfizer issue, whether the president can prevent a private company from fulfilling contracts with other countries, and whether President-elect Joe Biden will create his own policy, according to CNBC. The order may prove to be mostly symbolic.
The FDA could issue an emergency use authorization for Pfizer’s coronavirus vaccine this week and will likely approve Moderna’s vaccine next week. The U.S. has signed a contract with Moderna for 100 million doses.
During a call with reporters on Dec. 7, a spokeswoman for the Department of Health and Human Services said, “We are confident that we will have 100 million doses of Pfizer’s vaccine as agreed to in our contract, and beyond that, we have five other vaccine candidates, including 100 million doses on the way from Moderna.”
Federal officials are counting on vaccine candidates from AstraZeneca and Johnson & Johnson to seek FDA approval in January and be ready for shipment in February.
“We could have all of them,” Moncef Slaoui, the chief science adviser for Operation Warp Speed, told The Washington Post on Dec. 7.
“And for this reason, we feel confident we could cover the needs without a specific cliff,” he said. “We have planned things in such a way as we would indeed avoid a cliff.”
This article first appeared on WebMD.com.
Tattoo removal techniques continue to be refined
According to a 2016 Harris Poll, 29% of Americans have at least one tattoo, up from 21% in 2012. At the same time, 23% of Americans polled in 2016 regret having their tattoo, which means big business for dermatologists who practice laser tattoo removal.
Prior to the theory of selective photothermolysis, tattoo removal mostly consisted of chemical or mechanical abrasion, surgical removal, or using some sort of caustic chemical or thermal destruction of the tattoo, Omar A. Ibrahimi, MD, PhD, said during a virtual course on laser and aesthetic skin therapy. “The earliest lasers prior to refinement by the theory of selective photothermolysis also fell into these categories: just basically crudely removing the skin and trying to get under to where the tattoo is,” said Dr. Ibrahimi, a dermatologist with the Connecticut Skin Institute in Stamford. “These would often heal with horrible scarring.”
Today, clinicians use Q-switched nanosecond and picosecond lasers for tattoo removal, though appropriate wavelengths need to be selected based on the tattoo ink color. Tattoo ink particles average about 0.1 mcm in size, and the thermal relaxation size works out to be about 10 nanoseconds. Black is the most common color dermatologists will treat. “For that, you can typically use a 1064, which has the highest absorption, but you can also use many of the other wavelengths,” he said. “The other colors are less common, followed by red, for which you would use a 532-nm wavelength.”
The clinical endpoint to strive for during tattoo removal is a whitening of the ink. That typically fades after about 20 minutes. “This whitening corresponds to cavitation [the production of gas vacuoles in the cells that were holding the ink],” Dr. Ibrahimi explained during the meeting, which was sponsored by Harvard Medical School, Massachusetts General Hospital, and the Wellman Center for Photomedicine. “These vacuoles are what lead to the whitening when using a high-gigawatt laser in a very short pulse. This causes highly localized heating, cavitation, and cell rupture. We don’t fully understand how tattoos are removed today, but the working models include some of the residual ink coming out through transepidermal elimination, some of it being removed via lymphatics, and some of it being removed by rephagocytosis.”
For optimal results, determine if the tattoo is professional, amateur, traumatic, or cosmetic. “That’s going to give you some insight as to what kind of expectations to set for the patient,” he said. “Black ink is often the easiest to remove, while certain colors like white are more challenging. Certain colors are more prone to paradoxical ink darkening, like red or orange, or pink. These can undergo a chemical reaction where they darken. This is something important to discuss with patients in advance.”
Older tattoos “tend to be less hearty” and usually respond better to laser, he continued. Location of the tattoo also plays a role. “I find that tattoos below the knee are very slow to respond. Smaller tattoos will respond faster.”
During the focused medical exam, ask patients about any history of keloid scarring, vitiligo or any dermatologic conditions with a Koebner phenomenon, and rule out a history of parental gold salt administration for arthritis. “During your informed consent you want to make sure you address the expected healing time and the risks such as hyper- and hypopigmentation, blistering, and scarring,” Dr. Ibrahimi said. “You also want to set the expectation that this is not going to be a one and done procedure. Laser tattoo removal takes a series of treatments, often more than what we think – sometimes in the range of 15-20. And you may not get complete clearance. I liken it to breaking it up enough so that if somebody sees it, they won’t be able to recognize what the tattoo is. But you won’t be able to erase it 100%.”
Black, dark blue, and red tattoo colors respond best to laser light. Light blue, green, and purple colors are slower to respond, while yellow and orange colors respond poorly. “Now that we have picosecond lasers, we’re a little better at treating these tougher colors, but I think we still have a lot of room for improvement,” Dr. Ibrahimi said.
Melanin is a competing chromophore, which complicates treatment of tanned individuals and those with darker skin types. “The Q-switched 1064-nm laser is the safest device to use for these patients but it’s not effective for many ink colors,” he said.
Options to keep patients comfortable during the procedure include application of ice or forced chilled air. “You can also use topical anesthetics such as EMLA or liposomal lidocaine cream under occlusion,” he said. “You can also use injectable lidocaine. If you go that route, I recommend a ring block. If you inject right into the tattoo sometimes the ink can get leeched out after treatment. As for spot size, a larger spot size will penetrate deeper, so I try to treat tattoos with the biggest spot size. It also results in less bleeding, less splatter, less side effects, and you get better results.”
Common adverse events from tattoo removal include prolonged erythema, blistering, hyperpigmentation, hypopigmentation, and scarring. Less frequent complications include ink darkening, chrysiasis, and transient immunoreactivity. “We don’t really know what’s in a lot of these ink residues,” Dr. Ibrahimi said. “We know they’re getting mobilized and some of it’s going into the lymphatics. What’s happening with these ink particles? We don’t fully know.”
He also warned against using hair-removal devices to treat a tattoo. “It is the wrong pulse duration,” he said. “You need a picosecond or nanosecond device. You cannot use any other pulse durations, or you will horribly scar your patient.”
In 2012, R. Rox Anderson, MD, director of the Wellman Center for Photomedicine at Massachusetts General Hospital, and colleagues published results of a study that compared a single Q-switched laser treatment pass with four treatment passes separated by 20 minutes. After treating 18 tattoos in 12 adults, they found that the technique, known as the R20 method, was more effective than a single-pass treatment (P < .01). “Subsequent papers have shown that this result isn’t as impressive as initially reported, but I think it’s a method that persists,” Dr. Ibrahimi said.
Another recent advance is use of a topical square silicone patch infused with perfluorodecalin patch during tattoo removal, which has been shown to reduce epidermal whitening. “So, instead of waiting 20 minutes you wait 0 minutes,” he said. “This is called the R0 method,” he added, noting that there are also some secondary benefits to using this patch, including possibly helping as an optical clearing agent for deeper penetration of the laser. “Often after treatment you can see ink on the underside of the patch, which speaks to the transdermal elimination mechanism of action for removal of tattoos.”
As for future directions, Dr. Ibrahimi predicted that there will be better picosecond lasers coming down the pike. He also anticipates that Soliton’s Rapid Acoustic Pulse (RAP) device will make a significant impact in the field. The device was cleared for tattoo removal in 2019 and is being investigated as an option to improve the appearance of cellulite. The manufacturer anticipates that an upgraded RAP device will be cleared for use by the end of the first quarter of 2021.
Dr. Ibrahimi disclosed that he has received research funding and speaker honorarium from Cutera, Lumenis, Lutronic, and Syneron-Candela. He also holds stock in Soliton.
According to a 2016 Harris Poll, 29% of Americans have at least one tattoo, up from 21% in 2012. At the same time, 23% of Americans polled in 2016 regret having their tattoo, which means big business for dermatologists who practice laser tattoo removal.
Prior to the theory of selective photothermolysis, tattoo removal mostly consisted of chemical or mechanical abrasion, surgical removal, or using some sort of caustic chemical or thermal destruction of the tattoo, Omar A. Ibrahimi, MD, PhD, said during a virtual course on laser and aesthetic skin therapy. “The earliest lasers prior to refinement by the theory of selective photothermolysis also fell into these categories: just basically crudely removing the skin and trying to get under to where the tattoo is,” said Dr. Ibrahimi, a dermatologist with the Connecticut Skin Institute in Stamford. “These would often heal with horrible scarring.”
Today, clinicians use Q-switched nanosecond and picosecond lasers for tattoo removal, though appropriate wavelengths need to be selected based on the tattoo ink color. Tattoo ink particles average about 0.1 mcm in size, and the thermal relaxation size works out to be about 10 nanoseconds. Black is the most common color dermatologists will treat. “For that, you can typically use a 1064, which has the highest absorption, but you can also use many of the other wavelengths,” he said. “The other colors are less common, followed by red, for which you would use a 532-nm wavelength.”
The clinical endpoint to strive for during tattoo removal is a whitening of the ink. That typically fades after about 20 minutes. “This whitening corresponds to cavitation [the production of gas vacuoles in the cells that were holding the ink],” Dr. Ibrahimi explained during the meeting, which was sponsored by Harvard Medical School, Massachusetts General Hospital, and the Wellman Center for Photomedicine. “These vacuoles are what lead to the whitening when using a high-gigawatt laser in a very short pulse. This causes highly localized heating, cavitation, and cell rupture. We don’t fully understand how tattoos are removed today, but the working models include some of the residual ink coming out through transepidermal elimination, some of it being removed via lymphatics, and some of it being removed by rephagocytosis.”
For optimal results, determine if the tattoo is professional, amateur, traumatic, or cosmetic. “That’s going to give you some insight as to what kind of expectations to set for the patient,” he said. “Black ink is often the easiest to remove, while certain colors like white are more challenging. Certain colors are more prone to paradoxical ink darkening, like red or orange, or pink. These can undergo a chemical reaction where they darken. This is something important to discuss with patients in advance.”
Older tattoos “tend to be less hearty” and usually respond better to laser, he continued. Location of the tattoo also plays a role. “I find that tattoos below the knee are very slow to respond. Smaller tattoos will respond faster.”
During the focused medical exam, ask patients about any history of keloid scarring, vitiligo or any dermatologic conditions with a Koebner phenomenon, and rule out a history of parental gold salt administration for arthritis. “During your informed consent you want to make sure you address the expected healing time and the risks such as hyper- and hypopigmentation, blistering, and scarring,” Dr. Ibrahimi said. “You also want to set the expectation that this is not going to be a one and done procedure. Laser tattoo removal takes a series of treatments, often more than what we think – sometimes in the range of 15-20. And you may not get complete clearance. I liken it to breaking it up enough so that if somebody sees it, they won’t be able to recognize what the tattoo is. But you won’t be able to erase it 100%.”
Black, dark blue, and red tattoo colors respond best to laser light. Light blue, green, and purple colors are slower to respond, while yellow and orange colors respond poorly. “Now that we have picosecond lasers, we’re a little better at treating these tougher colors, but I think we still have a lot of room for improvement,” Dr. Ibrahimi said.
Melanin is a competing chromophore, which complicates treatment of tanned individuals and those with darker skin types. “The Q-switched 1064-nm laser is the safest device to use for these patients but it’s not effective for many ink colors,” he said.
Options to keep patients comfortable during the procedure include application of ice or forced chilled air. “You can also use topical anesthetics such as EMLA or liposomal lidocaine cream under occlusion,” he said. “You can also use injectable lidocaine. If you go that route, I recommend a ring block. If you inject right into the tattoo sometimes the ink can get leeched out after treatment. As for spot size, a larger spot size will penetrate deeper, so I try to treat tattoos with the biggest spot size. It also results in less bleeding, less splatter, less side effects, and you get better results.”
Common adverse events from tattoo removal include prolonged erythema, blistering, hyperpigmentation, hypopigmentation, and scarring. Less frequent complications include ink darkening, chrysiasis, and transient immunoreactivity. “We don’t really know what’s in a lot of these ink residues,” Dr. Ibrahimi said. “We know they’re getting mobilized and some of it’s going into the lymphatics. What’s happening with these ink particles? We don’t fully know.”
He also warned against using hair-removal devices to treat a tattoo. “It is the wrong pulse duration,” he said. “You need a picosecond or nanosecond device. You cannot use any other pulse durations, or you will horribly scar your patient.”
In 2012, R. Rox Anderson, MD, director of the Wellman Center for Photomedicine at Massachusetts General Hospital, and colleagues published results of a study that compared a single Q-switched laser treatment pass with four treatment passes separated by 20 minutes. After treating 18 tattoos in 12 adults, they found that the technique, known as the R20 method, was more effective than a single-pass treatment (P < .01). “Subsequent papers have shown that this result isn’t as impressive as initially reported, but I think it’s a method that persists,” Dr. Ibrahimi said.
Another recent advance is use of a topical square silicone patch infused with perfluorodecalin patch during tattoo removal, which has been shown to reduce epidermal whitening. “So, instead of waiting 20 minutes you wait 0 minutes,” he said. “This is called the R0 method,” he added, noting that there are also some secondary benefits to using this patch, including possibly helping as an optical clearing agent for deeper penetration of the laser. “Often after treatment you can see ink on the underside of the patch, which speaks to the transdermal elimination mechanism of action for removal of tattoos.”
As for future directions, Dr. Ibrahimi predicted that there will be better picosecond lasers coming down the pike. He also anticipates that Soliton’s Rapid Acoustic Pulse (RAP) device will make a significant impact in the field. The device was cleared for tattoo removal in 2019 and is being investigated as an option to improve the appearance of cellulite. The manufacturer anticipates that an upgraded RAP device will be cleared for use by the end of the first quarter of 2021.
Dr. Ibrahimi disclosed that he has received research funding and speaker honorarium from Cutera, Lumenis, Lutronic, and Syneron-Candela. He also holds stock in Soliton.
According to a 2016 Harris Poll, 29% of Americans have at least one tattoo, up from 21% in 2012. At the same time, 23% of Americans polled in 2016 regret having their tattoo, which means big business for dermatologists who practice laser tattoo removal.
Prior to the theory of selective photothermolysis, tattoo removal mostly consisted of chemical or mechanical abrasion, surgical removal, or using some sort of caustic chemical or thermal destruction of the tattoo, Omar A. Ibrahimi, MD, PhD, said during a virtual course on laser and aesthetic skin therapy. “The earliest lasers prior to refinement by the theory of selective photothermolysis also fell into these categories: just basically crudely removing the skin and trying to get under to where the tattoo is,” said Dr. Ibrahimi, a dermatologist with the Connecticut Skin Institute in Stamford. “These would often heal with horrible scarring.”
Today, clinicians use Q-switched nanosecond and picosecond lasers for tattoo removal, though appropriate wavelengths need to be selected based on the tattoo ink color. Tattoo ink particles average about 0.1 mcm in size, and the thermal relaxation size works out to be about 10 nanoseconds. Black is the most common color dermatologists will treat. “For that, you can typically use a 1064, which has the highest absorption, but you can also use many of the other wavelengths,” he said. “The other colors are less common, followed by red, for which you would use a 532-nm wavelength.”
The clinical endpoint to strive for during tattoo removal is a whitening of the ink. That typically fades after about 20 minutes. “This whitening corresponds to cavitation [the production of gas vacuoles in the cells that were holding the ink],” Dr. Ibrahimi explained during the meeting, which was sponsored by Harvard Medical School, Massachusetts General Hospital, and the Wellman Center for Photomedicine. “These vacuoles are what lead to the whitening when using a high-gigawatt laser in a very short pulse. This causes highly localized heating, cavitation, and cell rupture. We don’t fully understand how tattoos are removed today, but the working models include some of the residual ink coming out through transepidermal elimination, some of it being removed via lymphatics, and some of it being removed by rephagocytosis.”
For optimal results, determine if the tattoo is professional, amateur, traumatic, or cosmetic. “That’s going to give you some insight as to what kind of expectations to set for the patient,” he said. “Black ink is often the easiest to remove, while certain colors like white are more challenging. Certain colors are more prone to paradoxical ink darkening, like red or orange, or pink. These can undergo a chemical reaction where they darken. This is something important to discuss with patients in advance.”
Older tattoos “tend to be less hearty” and usually respond better to laser, he continued. Location of the tattoo also plays a role. “I find that tattoos below the knee are very slow to respond. Smaller tattoos will respond faster.”
During the focused medical exam, ask patients about any history of keloid scarring, vitiligo or any dermatologic conditions with a Koebner phenomenon, and rule out a history of parental gold salt administration for arthritis. “During your informed consent you want to make sure you address the expected healing time and the risks such as hyper- and hypopigmentation, blistering, and scarring,” Dr. Ibrahimi said. “You also want to set the expectation that this is not going to be a one and done procedure. Laser tattoo removal takes a series of treatments, often more than what we think – sometimes in the range of 15-20. And you may not get complete clearance. I liken it to breaking it up enough so that if somebody sees it, they won’t be able to recognize what the tattoo is. But you won’t be able to erase it 100%.”
Black, dark blue, and red tattoo colors respond best to laser light. Light blue, green, and purple colors are slower to respond, while yellow and orange colors respond poorly. “Now that we have picosecond lasers, we’re a little better at treating these tougher colors, but I think we still have a lot of room for improvement,” Dr. Ibrahimi said.
Melanin is a competing chromophore, which complicates treatment of tanned individuals and those with darker skin types. “The Q-switched 1064-nm laser is the safest device to use for these patients but it’s not effective for many ink colors,” he said.
Options to keep patients comfortable during the procedure include application of ice or forced chilled air. “You can also use topical anesthetics such as EMLA or liposomal lidocaine cream under occlusion,” he said. “You can also use injectable lidocaine. If you go that route, I recommend a ring block. If you inject right into the tattoo sometimes the ink can get leeched out after treatment. As for spot size, a larger spot size will penetrate deeper, so I try to treat tattoos with the biggest spot size. It also results in less bleeding, less splatter, less side effects, and you get better results.”
Common adverse events from tattoo removal include prolonged erythema, blistering, hyperpigmentation, hypopigmentation, and scarring. Less frequent complications include ink darkening, chrysiasis, and transient immunoreactivity. “We don’t really know what’s in a lot of these ink residues,” Dr. Ibrahimi said. “We know they’re getting mobilized and some of it’s going into the lymphatics. What’s happening with these ink particles? We don’t fully know.”
He also warned against using hair-removal devices to treat a tattoo. “It is the wrong pulse duration,” he said. “You need a picosecond or nanosecond device. You cannot use any other pulse durations, or you will horribly scar your patient.”
In 2012, R. Rox Anderson, MD, director of the Wellman Center for Photomedicine at Massachusetts General Hospital, and colleagues published results of a study that compared a single Q-switched laser treatment pass with four treatment passes separated by 20 minutes. After treating 18 tattoos in 12 adults, they found that the technique, known as the R20 method, was more effective than a single-pass treatment (P < .01). “Subsequent papers have shown that this result isn’t as impressive as initially reported, but I think it’s a method that persists,” Dr. Ibrahimi said.
Another recent advance is use of a topical square silicone patch infused with perfluorodecalin patch during tattoo removal, which has been shown to reduce epidermal whitening. “So, instead of waiting 20 minutes you wait 0 minutes,” he said. “This is called the R0 method,” he added, noting that there are also some secondary benefits to using this patch, including possibly helping as an optical clearing agent for deeper penetration of the laser. “Often after treatment you can see ink on the underside of the patch, which speaks to the transdermal elimination mechanism of action for removal of tattoos.”
As for future directions, Dr. Ibrahimi predicted that there will be better picosecond lasers coming down the pike. He also anticipates that Soliton’s Rapid Acoustic Pulse (RAP) device will make a significant impact in the field. The device was cleared for tattoo removal in 2019 and is being investigated as an option to improve the appearance of cellulite. The manufacturer anticipates that an upgraded RAP device will be cleared for use by the end of the first quarter of 2021.
Dr. Ibrahimi disclosed that he has received research funding and speaker honorarium from Cutera, Lumenis, Lutronic, and Syneron-Candela. He also holds stock in Soliton.
FROM A LASER & AESTHETIC SKIN THERAPY COURSE
Can a health care worker refuse the COVID-19 vaccine?
As hospitals across the country develop their plans to vaccinate their health care employees against COVID-19, a key question has come to the fore: What if an employee – whether nurse, physician, or other health care worker – refuses to receive the vaccine? Can hospitals require their employees to be vaccinated against COVID-19? And what consequences could an employee face for refusing the vaccine?
My answer needs to be based, in part, on the law related to previous vaccines – influenza, for example – because at the time of this writing (early December 2020), no vaccine for COVID-19 has been approved, although approval of at least one vaccine is expected within a week. So there have been no offers of vaccine and refusals yet, nor are there any cases to date involving an employee who refused a COVID-19 vaccine. As of December 2020, there are no state or federal laws that either require an employee to be vaccinated against COVID-19 or that protect an employee who refuses vaccination against COVID-19. It will take a while after the vaccine is approved and distributed before refusals, reactions, policies, cases, and laws begin to emerge.
If we look at the law related to health care workers refusing to be vaccinated against the closest relative to COVID-19 – influenza – then the answer would be yes, employers can require employees to be vaccinated.
An employer can fire an employee who refuses influenza vaccination. If an employee who refused and was fired sues the employer for wrongful termination, the employee has more or less chance of success depending on the reason for refusal. Some courts and the Equal Employment Opportunity Commission have held that a refusal on religious grounds is protected by the U.S. Constitution, as in this recent case. The Constitution protects freedom to practice one’s religion. Specific religions may have a range of tenets that support refusal to be vaccinated.
A refusal on medical grounds has been successful if the medical grounds fall under the protections of the Americans with Disabilities Act but may fail when the medical grounds for the claim are not covered by the ADA.
Refusal for secular, nonmedical reasons, such as a health care worker’s policy of treating their body as their temple, has not gone over well with employers or courts. However, in at least one case, a nurse who refused vaccination on secular, nonmedical grounds won her case against her employer, on appeal. The appeals court found that the hospital violated her First Amendment rights.
Employees who refuse vaccination for religious or medical reasons still will need to take measures to protect patients and other employees from infection. An employer such as a hospital can, rather than fire the employee, offer the employee an accommodation, such as requiring that the employee wear a mask or quarantine. There are no cases that have upheld an employee’s right to refuse to wear a mask or quarantine.
The situation with the COVID-19 vaccine is different from the situation surrounding influenza vaccines. There are plenty of data on effectiveness and side effects of influenza vaccines, but there is very little evidence of short- or long-term effects of the COVID-19 vaccines currently being tested and/or considered for approval. One could argue that the process of vaccine development is the same for all virus vaccines. However, public confidence in the vaccine vetting process is not what it once was. It has been widely publicized that the COVID-19 vaccine trials have been rushed. As of December 2020, only 60% of the general population say they would take the vaccine, although researchers say confidence is increasing.
The Centers for Disease Control and Prevention has designated health care workers as first in line to get the vaccine, but some health care workers may not want to be the first to try it. A CDC survey found that 63% of health care workers polled in recent months said they would get a COVID-19 vaccine.
Unions have entered the conversation. A coalition of unions that represent health care workers said, “we need a transparent, evidence-based federal vaccine strategy based on principles of equity, safety, and priority, as well as robust efforts to address a high degree of skepticism about safety of an authorized vaccine.” The organization declined to promote a vaccine until more is known.
As of publication date, the EEOC guidance for employers responding to COVID-19 does not address vaccines.
The CDC’s Interim Guidance for Businesses and Employers Responding to Coronavirus Disease 2019, May 2020, updated Dec. 4, 2020, does not address vaccines. The CDC’s page on COVID-19 vaccination for health care workers does not address a health care worker’s refusal. The site does assure health care workers that the vaccine development process is sound: “The current vaccine safety system is strong and robust, with the capacity to effectively monitor COVID-19 vaccine safety. Existing data systems have validated analytic methods that can rapidly detect statistical signals for possible vaccine safety problems. These systems are being scaled up to fully meet the needs of the nation. Additional systems and data sources are also being developed to further enhance safety monitoring capabilities. CDC is committed to ensuring that COVID-19 vaccines are safe.”
In the coming months, government officials and vaccine manufacturers will be working to reassure the public of the safety of the vaccine and the rigor of the vaccine development process. In November 2020, National Institute of Allergy and Infectious Diseases Director Anthony Fauci, MD, told Kaiser Health News: “The company looks at the data. I look at the data. Then the company puts the data to the FDA. The FDA will make the decision to do an emergency-use authorization or a license application approval. And they have career scientists who are really independent. They’re not beholden to anybody. Then there’s another independent group, the Vaccines and Related Biological Products Advisory Committee. The FDA commissioner has vowed publicly that he will go according to the opinion of the career scientists and the advisory board.” President-elect Joe Biden said he would get a vaccine when Dr. Fauci thinks it is safe.
An employee who, after researching the vaccine and the process, still wants to refuse when offered the vaccine is not likely to be fired for that reason right away, as long as the employee takes other precautions, such as wearing a mask. If the employer does fire the employee and the employee sues the employer, it is impossible to predict how a court would decide the case.
Related legal questions may arise in the coming months. For example:
- Is an employer exempt from paying workers’ compensation to an employee who refuses to be vaccinated and then contracts the virus while on the job?
- Can a prospective employer require COVID-19 vaccination as a precondition of employment?
- Is it within a patient’s rights to receive an answer to the question: Has my health care worker been vaccinated against COVID-19?
- If a hospital allows employees to refuse vaccination and keep working, and an outbreak occurs, and it is suggested through contact tracing that unvaccinated workers infected patients, will a court hold the hospital liable for patients’ damages?
Answers to these questions are yet to be determined.
Carolyn Buppert (www.buppert.com) is an attorney and former nurse practitioner who focuses on the legal issues affecting nurse practitioners.
A version of this article originally appeared on Medscape.com.
As hospitals across the country develop their plans to vaccinate their health care employees against COVID-19, a key question has come to the fore: What if an employee – whether nurse, physician, or other health care worker – refuses to receive the vaccine? Can hospitals require their employees to be vaccinated against COVID-19? And what consequences could an employee face for refusing the vaccine?
My answer needs to be based, in part, on the law related to previous vaccines – influenza, for example – because at the time of this writing (early December 2020), no vaccine for COVID-19 has been approved, although approval of at least one vaccine is expected within a week. So there have been no offers of vaccine and refusals yet, nor are there any cases to date involving an employee who refused a COVID-19 vaccine. As of December 2020, there are no state or federal laws that either require an employee to be vaccinated against COVID-19 or that protect an employee who refuses vaccination against COVID-19. It will take a while after the vaccine is approved and distributed before refusals, reactions, policies, cases, and laws begin to emerge.
If we look at the law related to health care workers refusing to be vaccinated against the closest relative to COVID-19 – influenza – then the answer would be yes, employers can require employees to be vaccinated.
An employer can fire an employee who refuses influenza vaccination. If an employee who refused and was fired sues the employer for wrongful termination, the employee has more or less chance of success depending on the reason for refusal. Some courts and the Equal Employment Opportunity Commission have held that a refusal on religious grounds is protected by the U.S. Constitution, as in this recent case. The Constitution protects freedom to practice one’s religion. Specific religions may have a range of tenets that support refusal to be vaccinated.
A refusal on medical grounds has been successful if the medical grounds fall under the protections of the Americans with Disabilities Act but may fail when the medical grounds for the claim are not covered by the ADA.
Refusal for secular, nonmedical reasons, such as a health care worker’s policy of treating their body as their temple, has not gone over well with employers or courts. However, in at least one case, a nurse who refused vaccination on secular, nonmedical grounds won her case against her employer, on appeal. The appeals court found that the hospital violated her First Amendment rights.
Employees who refuse vaccination for religious or medical reasons still will need to take measures to protect patients and other employees from infection. An employer such as a hospital can, rather than fire the employee, offer the employee an accommodation, such as requiring that the employee wear a mask or quarantine. There are no cases that have upheld an employee’s right to refuse to wear a mask or quarantine.
The situation with the COVID-19 vaccine is different from the situation surrounding influenza vaccines. There are plenty of data on effectiveness and side effects of influenza vaccines, but there is very little evidence of short- or long-term effects of the COVID-19 vaccines currently being tested and/or considered for approval. One could argue that the process of vaccine development is the same for all virus vaccines. However, public confidence in the vaccine vetting process is not what it once was. It has been widely publicized that the COVID-19 vaccine trials have been rushed. As of December 2020, only 60% of the general population say they would take the vaccine, although researchers say confidence is increasing.
The Centers for Disease Control and Prevention has designated health care workers as first in line to get the vaccine, but some health care workers may not want to be the first to try it. A CDC survey found that 63% of health care workers polled in recent months said they would get a COVID-19 vaccine.
Unions have entered the conversation. A coalition of unions that represent health care workers said, “we need a transparent, evidence-based federal vaccine strategy based on principles of equity, safety, and priority, as well as robust efforts to address a high degree of skepticism about safety of an authorized vaccine.” The organization declined to promote a vaccine until more is known.
As of publication date, the EEOC guidance for employers responding to COVID-19 does not address vaccines.
The CDC’s Interim Guidance for Businesses and Employers Responding to Coronavirus Disease 2019, May 2020, updated Dec. 4, 2020, does not address vaccines. The CDC’s page on COVID-19 vaccination for health care workers does not address a health care worker’s refusal. The site does assure health care workers that the vaccine development process is sound: “The current vaccine safety system is strong and robust, with the capacity to effectively monitor COVID-19 vaccine safety. Existing data systems have validated analytic methods that can rapidly detect statistical signals for possible vaccine safety problems. These systems are being scaled up to fully meet the needs of the nation. Additional systems and data sources are also being developed to further enhance safety monitoring capabilities. CDC is committed to ensuring that COVID-19 vaccines are safe.”
In the coming months, government officials and vaccine manufacturers will be working to reassure the public of the safety of the vaccine and the rigor of the vaccine development process. In November 2020, National Institute of Allergy and Infectious Diseases Director Anthony Fauci, MD, told Kaiser Health News: “The company looks at the data. I look at the data. Then the company puts the data to the FDA. The FDA will make the decision to do an emergency-use authorization or a license application approval. And they have career scientists who are really independent. They’re not beholden to anybody. Then there’s another independent group, the Vaccines and Related Biological Products Advisory Committee. The FDA commissioner has vowed publicly that he will go according to the opinion of the career scientists and the advisory board.” President-elect Joe Biden said he would get a vaccine when Dr. Fauci thinks it is safe.
An employee who, after researching the vaccine and the process, still wants to refuse when offered the vaccine is not likely to be fired for that reason right away, as long as the employee takes other precautions, such as wearing a mask. If the employer does fire the employee and the employee sues the employer, it is impossible to predict how a court would decide the case.
Related legal questions may arise in the coming months. For example:
- Is an employer exempt from paying workers’ compensation to an employee who refuses to be vaccinated and then contracts the virus while on the job?
- Can a prospective employer require COVID-19 vaccination as a precondition of employment?
- Is it within a patient’s rights to receive an answer to the question: Has my health care worker been vaccinated against COVID-19?
- If a hospital allows employees to refuse vaccination and keep working, and an outbreak occurs, and it is suggested through contact tracing that unvaccinated workers infected patients, will a court hold the hospital liable for patients’ damages?
Answers to these questions are yet to be determined.
Carolyn Buppert (www.buppert.com) is an attorney and former nurse practitioner who focuses on the legal issues affecting nurse practitioners.
A version of this article originally appeared on Medscape.com.
As hospitals across the country develop their plans to vaccinate their health care employees against COVID-19, a key question has come to the fore: What if an employee – whether nurse, physician, or other health care worker – refuses to receive the vaccine? Can hospitals require their employees to be vaccinated against COVID-19? And what consequences could an employee face for refusing the vaccine?
My answer needs to be based, in part, on the law related to previous vaccines – influenza, for example – because at the time of this writing (early December 2020), no vaccine for COVID-19 has been approved, although approval of at least one vaccine is expected within a week. So there have been no offers of vaccine and refusals yet, nor are there any cases to date involving an employee who refused a COVID-19 vaccine. As of December 2020, there are no state or federal laws that either require an employee to be vaccinated against COVID-19 or that protect an employee who refuses vaccination against COVID-19. It will take a while after the vaccine is approved and distributed before refusals, reactions, policies, cases, and laws begin to emerge.
If we look at the law related to health care workers refusing to be vaccinated against the closest relative to COVID-19 – influenza – then the answer would be yes, employers can require employees to be vaccinated.
An employer can fire an employee who refuses influenza vaccination. If an employee who refused and was fired sues the employer for wrongful termination, the employee has more or less chance of success depending on the reason for refusal. Some courts and the Equal Employment Opportunity Commission have held that a refusal on religious grounds is protected by the U.S. Constitution, as in this recent case. The Constitution protects freedom to practice one’s religion. Specific religions may have a range of tenets that support refusal to be vaccinated.
A refusal on medical grounds has been successful if the medical grounds fall under the protections of the Americans with Disabilities Act but may fail when the medical grounds for the claim are not covered by the ADA.
Refusal for secular, nonmedical reasons, such as a health care worker’s policy of treating their body as their temple, has not gone over well with employers or courts. However, in at least one case, a nurse who refused vaccination on secular, nonmedical grounds won her case against her employer, on appeal. The appeals court found that the hospital violated her First Amendment rights.
Employees who refuse vaccination for religious or medical reasons still will need to take measures to protect patients and other employees from infection. An employer such as a hospital can, rather than fire the employee, offer the employee an accommodation, such as requiring that the employee wear a mask or quarantine. There are no cases that have upheld an employee’s right to refuse to wear a mask or quarantine.
The situation with the COVID-19 vaccine is different from the situation surrounding influenza vaccines. There are plenty of data on effectiveness and side effects of influenza vaccines, but there is very little evidence of short- or long-term effects of the COVID-19 vaccines currently being tested and/or considered for approval. One could argue that the process of vaccine development is the same for all virus vaccines. However, public confidence in the vaccine vetting process is not what it once was. It has been widely publicized that the COVID-19 vaccine trials have been rushed. As of December 2020, only 60% of the general population say they would take the vaccine, although researchers say confidence is increasing.
The Centers for Disease Control and Prevention has designated health care workers as first in line to get the vaccine, but some health care workers may not want to be the first to try it. A CDC survey found that 63% of health care workers polled in recent months said they would get a COVID-19 vaccine.
Unions have entered the conversation. A coalition of unions that represent health care workers said, “we need a transparent, evidence-based federal vaccine strategy based on principles of equity, safety, and priority, as well as robust efforts to address a high degree of skepticism about safety of an authorized vaccine.” The organization declined to promote a vaccine until more is known.
As of publication date, the EEOC guidance for employers responding to COVID-19 does not address vaccines.
The CDC’s Interim Guidance for Businesses and Employers Responding to Coronavirus Disease 2019, May 2020, updated Dec. 4, 2020, does not address vaccines. The CDC’s page on COVID-19 vaccination for health care workers does not address a health care worker’s refusal. The site does assure health care workers that the vaccine development process is sound: “The current vaccine safety system is strong and robust, with the capacity to effectively monitor COVID-19 vaccine safety. Existing data systems have validated analytic methods that can rapidly detect statistical signals for possible vaccine safety problems. These systems are being scaled up to fully meet the needs of the nation. Additional systems and data sources are also being developed to further enhance safety monitoring capabilities. CDC is committed to ensuring that COVID-19 vaccines are safe.”
In the coming months, government officials and vaccine manufacturers will be working to reassure the public of the safety of the vaccine and the rigor of the vaccine development process. In November 2020, National Institute of Allergy and Infectious Diseases Director Anthony Fauci, MD, told Kaiser Health News: “The company looks at the data. I look at the data. Then the company puts the data to the FDA. The FDA will make the decision to do an emergency-use authorization or a license application approval. And they have career scientists who are really independent. They’re not beholden to anybody. Then there’s another independent group, the Vaccines and Related Biological Products Advisory Committee. The FDA commissioner has vowed publicly that he will go according to the opinion of the career scientists and the advisory board.” President-elect Joe Biden said he would get a vaccine when Dr. Fauci thinks it is safe.
An employee who, after researching the vaccine and the process, still wants to refuse when offered the vaccine is not likely to be fired for that reason right away, as long as the employee takes other precautions, such as wearing a mask. If the employer does fire the employee and the employee sues the employer, it is impossible to predict how a court would decide the case.
Related legal questions may arise in the coming months. For example:
- Is an employer exempt from paying workers’ compensation to an employee who refuses to be vaccinated and then contracts the virus while on the job?
- Can a prospective employer require COVID-19 vaccination as a precondition of employment?
- Is it within a patient’s rights to receive an answer to the question: Has my health care worker been vaccinated against COVID-19?
- If a hospital allows employees to refuse vaccination and keep working, and an outbreak occurs, and it is suggested through contact tracing that unvaccinated workers infected patients, will a court hold the hospital liable for patients’ damages?
Answers to these questions are yet to be determined.
Carolyn Buppert (www.buppert.com) is an attorney and former nurse practitioner who focuses on the legal issues affecting nurse practitioners.
A version of this article originally appeared on Medscape.com.
A 70-year-old presented with a 3-week history of asymptomatic violaceous papules on his feet
and named the condition multiple benign pigmented hemorrhagic sarcoma. The disease emerged again at the onset of the AIDS epidemic among homosexual men. There are five variants: HIV/AIDS–related KS, classic KS, African cutaneous KS, African lymphadenopathic KS, and immunosuppression-associated KS (from immunosuppressive therapy or malignancies such as lymphoma).
KS is caused by human herpes virus type 8 (HHV-8). Patients with KS have an increased risk of developing other malignancies such as lymphomas, leukemia, and myeloma. This patient exhibited classic KS.
The various forms of KS may appear different clinically. The lesions may appear as erythematous macules, small violaceous papules, large plaques, or ulcerated nodules. In classic KS, violaceous to bluish-black macules evolve to papules or plaques. Lesions are generally asymptomatic. The most common locations are the toes and soles, although other areas may be affected. Any mucocutaneous surface can be involved. The most common areas of internal involvement are the gastrointestinal system and lymphatics.
Histology reveals angular vessels lined by atypical cells. An associated inflammatory infiltrate containing plasma cells may be present in the upper dermis and perivascular areas. Nodules and plaques reveal a spindle cell neoplasm pattern. Lesions will stain positive for HHV-8.
In patients with HIV/AIDS–related KS, highly active antiretroviral therapy is the most important and beneficial treatment. Since the introduction of HAART, the incidence of KS has greatly decreased. However, there are a proportion of HIV/AIDS–associated Kaposi’s sarcoma patients with well-controlled HIV and undetectable viral loads who require further treatment.
Lesions may spontaneously resolve on their own. Other treatment methods include: cryotherapy, topical alitretinoin (9-cis-retinoic acid), intralesional interferon-alpha or vinblastine, superficial radiotherapy, liposomal doxorubicin, daunorubicin or paclitaxel. Small lesions that are asymptomatic may be monitored.
This patient had no internal involvement and responded well to cryotherapy.
This case and photo were provided by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
and named the condition multiple benign pigmented hemorrhagic sarcoma. The disease emerged again at the onset of the AIDS epidemic among homosexual men. There are five variants: HIV/AIDS–related KS, classic KS, African cutaneous KS, African lymphadenopathic KS, and immunosuppression-associated KS (from immunosuppressive therapy or malignancies such as lymphoma).
KS is caused by human herpes virus type 8 (HHV-8). Patients with KS have an increased risk of developing other malignancies such as lymphomas, leukemia, and myeloma. This patient exhibited classic KS.
The various forms of KS may appear different clinically. The lesions may appear as erythematous macules, small violaceous papules, large plaques, or ulcerated nodules. In classic KS, violaceous to bluish-black macules evolve to papules or plaques. Lesions are generally asymptomatic. The most common locations are the toes and soles, although other areas may be affected. Any mucocutaneous surface can be involved. The most common areas of internal involvement are the gastrointestinal system and lymphatics.
Histology reveals angular vessels lined by atypical cells. An associated inflammatory infiltrate containing plasma cells may be present in the upper dermis and perivascular areas. Nodules and plaques reveal a spindle cell neoplasm pattern. Lesions will stain positive for HHV-8.
In patients with HIV/AIDS–related KS, highly active antiretroviral therapy is the most important and beneficial treatment. Since the introduction of HAART, the incidence of KS has greatly decreased. However, there are a proportion of HIV/AIDS–associated Kaposi’s sarcoma patients with well-controlled HIV and undetectable viral loads who require further treatment.
Lesions may spontaneously resolve on their own. Other treatment methods include: cryotherapy, topical alitretinoin (9-cis-retinoic acid), intralesional interferon-alpha or vinblastine, superficial radiotherapy, liposomal doxorubicin, daunorubicin or paclitaxel. Small lesions that are asymptomatic may be monitored.
This patient had no internal involvement and responded well to cryotherapy.
This case and photo were provided by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
and named the condition multiple benign pigmented hemorrhagic sarcoma. The disease emerged again at the onset of the AIDS epidemic among homosexual men. There are five variants: HIV/AIDS–related KS, classic KS, African cutaneous KS, African lymphadenopathic KS, and immunosuppression-associated KS (from immunosuppressive therapy or malignancies such as lymphoma).
KS is caused by human herpes virus type 8 (HHV-8). Patients with KS have an increased risk of developing other malignancies such as lymphomas, leukemia, and myeloma. This patient exhibited classic KS.
The various forms of KS may appear different clinically. The lesions may appear as erythematous macules, small violaceous papules, large plaques, or ulcerated nodules. In classic KS, violaceous to bluish-black macules evolve to papules or plaques. Lesions are generally asymptomatic. The most common locations are the toes and soles, although other areas may be affected. Any mucocutaneous surface can be involved. The most common areas of internal involvement are the gastrointestinal system and lymphatics.
Histology reveals angular vessels lined by atypical cells. An associated inflammatory infiltrate containing plasma cells may be present in the upper dermis and perivascular areas. Nodules and plaques reveal a spindle cell neoplasm pattern. Lesions will stain positive for HHV-8.
In patients with HIV/AIDS–related KS, highly active antiretroviral therapy is the most important and beneficial treatment. Since the introduction of HAART, the incidence of KS has greatly decreased. However, there are a proportion of HIV/AIDS–associated Kaposi’s sarcoma patients with well-controlled HIV and undetectable viral loads who require further treatment.
Lesions may spontaneously resolve on their own. Other treatment methods include: cryotherapy, topical alitretinoin (9-cis-retinoic acid), intralesional interferon-alpha or vinblastine, superficial radiotherapy, liposomal doxorubicin, daunorubicin or paclitaxel. Small lesions that are asymptomatic may be monitored.
This patient had no internal involvement and responded well to cryotherapy.
This case and photo were provided by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
Study results support screening rosacea patients for cardiometabolic disease
according to the results of a meta-analysis of more than 50,000 patients.
To date, “mounting comorbidities of rosacea have been identified, suggesting that rosacea is not simply a skin disease but has links to multiple systemic illnesses,” wrote Qi Chen, MD, of Central South University, Changsha, China, and colleagues. The association with rosacea and cardiometabolic disease has been controversial, they added.
In a study published in the Journal of the American Academy of Dermatology, they identified 13 studies including 50,442 rosacea patients and 1,525,864 controls. Approximately 71% of the rosacea patients were women.
Overall, patients with rosacea showed a statistically significant association for hypertension (risk ratio, 1.20; 95% confidence interval, 1.08-1.34; P = .001) and dyslipidemia (RR, 1.32; 95% CI, 1.10-1.58; P = .002). Specifically, rosacea patients averaged higher standard mean differences of systolic and diastolic blood pressure, total cholesterol, HDL cholesterol and LDL cholesterol, and triglycerides, compared with controls.
Rosacea was not significantly associated with an increased risk for ischemic heart disease, stroke, or diabetes, although the rosacea patients showed significantly increased risk of higher fasting blood glucose, compared with controls.
Findings don’t show causality
The study findings were limited by several factors, including the observational nature of some of the studies and the inability to perform subgroup analyses based on subtype and disease severity, the researchers noted. In addition, most of the rosacea patients were outpatients. “Further investigations are warranted to identify the relationship between rosacea and [cardiometabolic disease] in general populations to further validate the significance of our findings.”
However, the results support the value of screening for cardiometabolic disease in rosacea patients to facilitate diagnosis and treatment of disease at an early stage, they concluded.
“Rosacea has been linked statistically to many comorbidities including depression, anxiety, hypertension, and diabetes mellitus,” Julie Harper, MD, of the Dermatology and Skin Care Center of Birmingham (Alabama), said in an interview.
“This study looked more specifically at cardiometabolic disease and found a statistically significant correlation between rosacea and hypertension, higher total cholesterol, higher triglycerides and higher fasting blood glucose,” she said. However, “while there is an association present in this meta-analysis, we cannot assume a cause-and-effect relationship.”
Although the analysis does not prove causality, the key message for clinicians is that cardiometabolic disease is quite common in rosacea patients, and risk factors should be identified and treated early, said Dr. Harper. “Our patients with and without rosacea will benefit from age-appropriate screening, physical examination, and laboratory evaluation with a primary care physician. For rosacea patients in particular, we can advise them that early research suggests that individuals with rosacea might have an increased risk of hypertension and/or high cholesterol and triglycerides. It never hurts to make an appointment with primary care and to be checked.”
“We need more confirmatory studies that minimize the influence of confounding,” Dr. Harper added. Rosacea also has also been linked to obesity, which is another risk factor for cardiometabolic disease.
The study was supported by multiple grants from the National Natural Science Foundation of China. The researchers had no financial conflicts to disclose. Dr. Harper had no relevant financial conflicts to disclose.
SOURCE: Chen Q et al. J Am Acad Dermatol. 2020 Nov;83(5):1331-40.
according to the results of a meta-analysis of more than 50,000 patients.
To date, “mounting comorbidities of rosacea have been identified, suggesting that rosacea is not simply a skin disease but has links to multiple systemic illnesses,” wrote Qi Chen, MD, of Central South University, Changsha, China, and colleagues. The association with rosacea and cardiometabolic disease has been controversial, they added.
In a study published in the Journal of the American Academy of Dermatology, they identified 13 studies including 50,442 rosacea patients and 1,525,864 controls. Approximately 71% of the rosacea patients were women.
Overall, patients with rosacea showed a statistically significant association for hypertension (risk ratio, 1.20; 95% confidence interval, 1.08-1.34; P = .001) and dyslipidemia (RR, 1.32; 95% CI, 1.10-1.58; P = .002). Specifically, rosacea patients averaged higher standard mean differences of systolic and diastolic blood pressure, total cholesterol, HDL cholesterol and LDL cholesterol, and triglycerides, compared with controls.
Rosacea was not significantly associated with an increased risk for ischemic heart disease, stroke, or diabetes, although the rosacea patients showed significantly increased risk of higher fasting blood glucose, compared with controls.
Findings don’t show causality
The study findings were limited by several factors, including the observational nature of some of the studies and the inability to perform subgroup analyses based on subtype and disease severity, the researchers noted. In addition, most of the rosacea patients were outpatients. “Further investigations are warranted to identify the relationship between rosacea and [cardiometabolic disease] in general populations to further validate the significance of our findings.”
However, the results support the value of screening for cardiometabolic disease in rosacea patients to facilitate diagnosis and treatment of disease at an early stage, they concluded.
“Rosacea has been linked statistically to many comorbidities including depression, anxiety, hypertension, and diabetes mellitus,” Julie Harper, MD, of the Dermatology and Skin Care Center of Birmingham (Alabama), said in an interview.
“This study looked more specifically at cardiometabolic disease and found a statistically significant correlation between rosacea and hypertension, higher total cholesterol, higher triglycerides and higher fasting blood glucose,” she said. However, “while there is an association present in this meta-analysis, we cannot assume a cause-and-effect relationship.”
Although the analysis does not prove causality, the key message for clinicians is that cardiometabolic disease is quite common in rosacea patients, and risk factors should be identified and treated early, said Dr. Harper. “Our patients with and without rosacea will benefit from age-appropriate screening, physical examination, and laboratory evaluation with a primary care physician. For rosacea patients in particular, we can advise them that early research suggests that individuals with rosacea might have an increased risk of hypertension and/or high cholesterol and triglycerides. It never hurts to make an appointment with primary care and to be checked.”
“We need more confirmatory studies that minimize the influence of confounding,” Dr. Harper added. Rosacea also has also been linked to obesity, which is another risk factor for cardiometabolic disease.
The study was supported by multiple grants from the National Natural Science Foundation of China. The researchers had no financial conflicts to disclose. Dr. Harper had no relevant financial conflicts to disclose.
SOURCE: Chen Q et al. J Am Acad Dermatol. 2020 Nov;83(5):1331-40.
according to the results of a meta-analysis of more than 50,000 patients.
To date, “mounting comorbidities of rosacea have been identified, suggesting that rosacea is not simply a skin disease but has links to multiple systemic illnesses,” wrote Qi Chen, MD, of Central South University, Changsha, China, and colleagues. The association with rosacea and cardiometabolic disease has been controversial, they added.
In a study published in the Journal of the American Academy of Dermatology, they identified 13 studies including 50,442 rosacea patients and 1,525,864 controls. Approximately 71% of the rosacea patients were women.
Overall, patients with rosacea showed a statistically significant association for hypertension (risk ratio, 1.20; 95% confidence interval, 1.08-1.34; P = .001) and dyslipidemia (RR, 1.32; 95% CI, 1.10-1.58; P = .002). Specifically, rosacea patients averaged higher standard mean differences of systolic and diastolic blood pressure, total cholesterol, HDL cholesterol and LDL cholesterol, and triglycerides, compared with controls.
Rosacea was not significantly associated with an increased risk for ischemic heart disease, stroke, or diabetes, although the rosacea patients showed significantly increased risk of higher fasting blood glucose, compared with controls.
Findings don’t show causality
The study findings were limited by several factors, including the observational nature of some of the studies and the inability to perform subgroup analyses based on subtype and disease severity, the researchers noted. In addition, most of the rosacea patients were outpatients. “Further investigations are warranted to identify the relationship between rosacea and [cardiometabolic disease] in general populations to further validate the significance of our findings.”
However, the results support the value of screening for cardiometabolic disease in rosacea patients to facilitate diagnosis and treatment of disease at an early stage, they concluded.
“Rosacea has been linked statistically to many comorbidities including depression, anxiety, hypertension, and diabetes mellitus,” Julie Harper, MD, of the Dermatology and Skin Care Center of Birmingham (Alabama), said in an interview.
“This study looked more specifically at cardiometabolic disease and found a statistically significant correlation between rosacea and hypertension, higher total cholesterol, higher triglycerides and higher fasting blood glucose,” she said. However, “while there is an association present in this meta-analysis, we cannot assume a cause-and-effect relationship.”
Although the analysis does not prove causality, the key message for clinicians is that cardiometabolic disease is quite common in rosacea patients, and risk factors should be identified and treated early, said Dr. Harper. “Our patients with and without rosacea will benefit from age-appropriate screening, physical examination, and laboratory evaluation with a primary care physician. For rosacea patients in particular, we can advise them that early research suggests that individuals with rosacea might have an increased risk of hypertension and/or high cholesterol and triglycerides. It never hurts to make an appointment with primary care and to be checked.”
“We need more confirmatory studies that minimize the influence of confounding,” Dr. Harper added. Rosacea also has also been linked to obesity, which is another risk factor for cardiometabolic disease.
The study was supported by multiple grants from the National Natural Science Foundation of China. The researchers had no financial conflicts to disclose. Dr. Harper had no relevant financial conflicts to disclose.
SOURCE: Chen Q et al. J Am Acad Dermatol. 2020 Nov;83(5):1331-40.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY