User login
Formerly Skin & Allergy News
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')]
The leading independent newspaper covering dermatology news and commentary.
CDC: Risk in U.S. from 2019-nCoV remains low
A total of 165 persons in the United States are under investigation for infection with the 2019 Novel Coronavirus (2019-nCoV), with 68 testing negative and only 5 confirming positive, according to data presented Jan. 29 during a Centers for Disease Control and Prevention (CDC) briefing.

The remaining samples are in transit or are being processed at the CDC for testing, Nancy Messonnier, MD, director of the National Center for Immunization and Respiratory Diseases, said during the briefing.
“The genetic sequence for all five viruses detected in the United States to date has been uploaded to the CDC website,” she said. “We are working quickly through the process to get the CDC-developed test into the hands of public health partners in the U.S. and internationally.”
Dr. Messonnier reported that the CDC is expanding screening efforts to U.S. ports of entry that house CDC quarantine stations. Also, in collaboration with U.S. Customs and Border Protection, the agency is expanding distribution of travel health education materials to all travelers from China.
“The good news here is that, despite an aggressive public health investigation to find new cases [of 2019-nCoV], we have not,” she said. “The situation in China is concerning, however, we are looking hard here in the U.S. We will continue to be proactive. I still expect that we will find additional cases.”
In another development, the federal government facilitated the return of a plane full of U.S. citizens living in Wuhan, China, to March Air Reserve Force Base in Riverside County, Calif. “We have taken every precaution to ensure their safety while also continuing to protect the health of our nation and the people around them,” Dr. Messonnier said.
All 195 passengers have been screened, monitored, and evaluated by medical personnel “every step of the way,” including before takeoff, during the flight, during a refueling stop in Alaska, and again upon landing at March Air Reserve Force Base on Jan. 28. “All 195 patients are without the symptoms of the novel coronavirus, and all have been assigned living quarters at the Air Force base,” Dr. Messonnier said.
The CDC has launched a second stage of further screening and information gathering from the passengers, who will be offered testing as part of a thorough risk assessment.
“I understand that many people in the U.S. are worried about this virus and whether it will affect them,” Dr. Messonnier said. “Outbreaks like this are always concerning, particularly when a new virus is emerging. But we are well prepared and working closely with federal, state, and local partners to protect our communities and others nationwide from this public health threat. At this time, we continue to believe that the immediate health risk from this new virus to the general American public is low.”
A total of 165 persons in the United States are under investigation for infection with the 2019 Novel Coronavirus (2019-nCoV), with 68 testing negative and only 5 confirming positive, according to data presented Jan. 29 during a Centers for Disease Control and Prevention (CDC) briefing.

The remaining samples are in transit or are being processed at the CDC for testing, Nancy Messonnier, MD, director of the National Center for Immunization and Respiratory Diseases, said during the briefing.
“The genetic sequence for all five viruses detected in the United States to date has been uploaded to the CDC website,” she said. “We are working quickly through the process to get the CDC-developed test into the hands of public health partners in the U.S. and internationally.”
Dr. Messonnier reported that the CDC is expanding screening efforts to U.S. ports of entry that house CDC quarantine stations. Also, in collaboration with U.S. Customs and Border Protection, the agency is expanding distribution of travel health education materials to all travelers from China.
“The good news here is that, despite an aggressive public health investigation to find new cases [of 2019-nCoV], we have not,” she said. “The situation in China is concerning, however, we are looking hard here in the U.S. We will continue to be proactive. I still expect that we will find additional cases.”
In another development, the federal government facilitated the return of a plane full of U.S. citizens living in Wuhan, China, to March Air Reserve Force Base in Riverside County, Calif. “We have taken every precaution to ensure their safety while also continuing to protect the health of our nation and the people around them,” Dr. Messonnier said.
All 195 passengers have been screened, monitored, and evaluated by medical personnel “every step of the way,” including before takeoff, during the flight, during a refueling stop in Alaska, and again upon landing at March Air Reserve Force Base on Jan. 28. “All 195 patients are without the symptoms of the novel coronavirus, and all have been assigned living quarters at the Air Force base,” Dr. Messonnier said.
The CDC has launched a second stage of further screening and information gathering from the passengers, who will be offered testing as part of a thorough risk assessment.
“I understand that many people in the U.S. are worried about this virus and whether it will affect them,” Dr. Messonnier said. “Outbreaks like this are always concerning, particularly when a new virus is emerging. But we are well prepared and working closely with federal, state, and local partners to protect our communities and others nationwide from this public health threat. At this time, we continue to believe that the immediate health risk from this new virus to the general American public is low.”
A total of 165 persons in the United States are under investigation for infection with the 2019 Novel Coronavirus (2019-nCoV), with 68 testing negative and only 5 confirming positive, according to data presented Jan. 29 during a Centers for Disease Control and Prevention (CDC) briefing.

The remaining samples are in transit or are being processed at the CDC for testing, Nancy Messonnier, MD, director of the National Center for Immunization and Respiratory Diseases, said during the briefing.
“The genetic sequence for all five viruses detected in the United States to date has been uploaded to the CDC website,” she said. “We are working quickly through the process to get the CDC-developed test into the hands of public health partners in the U.S. and internationally.”
Dr. Messonnier reported that the CDC is expanding screening efforts to U.S. ports of entry that house CDC quarantine stations. Also, in collaboration with U.S. Customs and Border Protection, the agency is expanding distribution of travel health education materials to all travelers from China.
“The good news here is that, despite an aggressive public health investigation to find new cases [of 2019-nCoV], we have not,” she said. “The situation in China is concerning, however, we are looking hard here in the U.S. We will continue to be proactive. I still expect that we will find additional cases.”
In another development, the federal government facilitated the return of a plane full of U.S. citizens living in Wuhan, China, to March Air Reserve Force Base in Riverside County, Calif. “We have taken every precaution to ensure their safety while also continuing to protect the health of our nation and the people around them,” Dr. Messonnier said.
All 195 passengers have been screened, monitored, and evaluated by medical personnel “every step of the way,” including before takeoff, during the flight, during a refueling stop in Alaska, and again upon landing at March Air Reserve Force Base on Jan. 28. “All 195 patients are without the symptoms of the novel coronavirus, and all have been assigned living quarters at the Air Force base,” Dr. Messonnier said.
The CDC has launched a second stage of further screening and information gathering from the passengers, who will be offered testing as part of a thorough risk assessment.
“I understand that many people in the U.S. are worried about this virus and whether it will affect them,” Dr. Messonnier said. “Outbreaks like this are always concerning, particularly when a new virus is emerging. But we are well prepared and working closely with federal, state, and local partners to protect our communities and others nationwide from this public health threat. At this time, we continue to believe that the immediate health risk from this new virus to the general American public is low.”
Costs are keeping Americans out of the doctor’s office
The cost of health care is keeping more Americans from seeing a doctor, even as the number of individuals with insurance coverage increases, according to a new study.
“Despite short-term gains owing to the [Affordable Care Act], over the past 20 years the portion of adults aged 18-64 years unable to see a physician owing to the cost increased, mostly because of an increase among persons with insurance,” Laura Hawks, MD, of Cambridge (Mass.) Health Alliance and Harvard Medical School in Boston and colleagues wrote in a new research report published in JAMA Internal Medicine.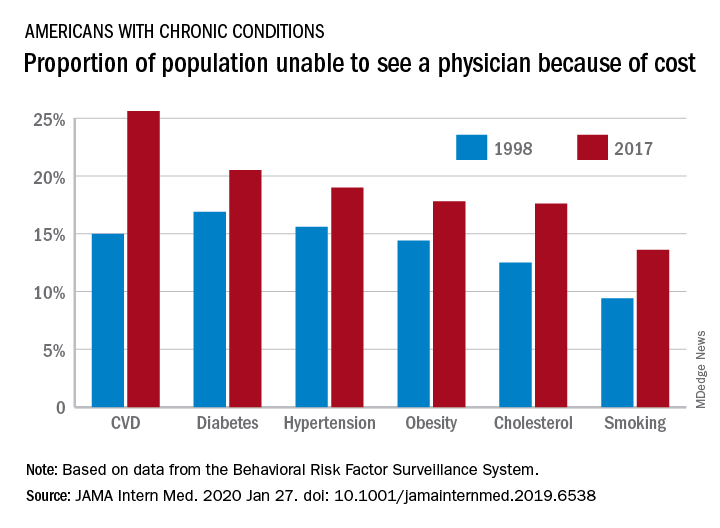
“In 2017, nearly one-fifth of individuals with any chronic condition (diabetes, obesity, or cardiovascular disease) said they were unable to see a physician owing to cost,” they continued.
Researchers examined 20 years of data (January 1998 through December 2017) from the Centers for Disease Control and Prevention’s Behavioral Risk Factor Surveillance System to identify trends in unmet need for physician and preventive services.
Among adults aged 18-64 years who responded to the survey in 1998 and 2017, uninsurance decreased by 2.1 percentage points, falling from 16.9% to 14.8%. But at the same time, the portion of adults who were unable to see a physician because of cost rose by 2.7 percentage points, from 11.4% to 15.7%. Looking specifically at adults who had insurance coverage, the researchers found that cost was a barrier for 11.5% of them in 2017, up from 7.1% in 1998.
These results come against a backdrop of growing medical costs, increasing deductibles and copayments, an increasing use of cost containment measures like prior authorization, and narrow provider networks in the wake of the transition to value-based payment structures, the authors noted.
“Our finding that financial access to physician care worsened is concerning,” Dr. Hawks and her colleagues wrote. “Persons with conditions such as diabetes, hypertension, cardiovascular disease, and poor health status risk substantial harms if they forgo physician care. Financial barriers to care have been associated with increased hospitalizations and worse health outcomes in patients with cardiovascular disease and hypertension and increased morbidity among patients with diabetes.”
One of the trends highlighted by the study authors is the growing number of employers offering plans with a high deductible.
“Enrollment in a high-deductible health plan, which has become increasingly common in the last decade, a trend uninterrupted by the ACA, is associated with forgoing needed care, especially among those of lower socioeconomic status,” the authors wrote. “Other changes in insurance benefit design, such as imposing tiered copayments and coinsurance obligations, eliminating coverage for some services (e.g., eyeglasses) and narrowing provider networks (which can force some patients to go out-of-network for care) may also have undermined the affordability of care.”
There was some positive news among the findings, however.
“The main encouraging finding from our analysis is the increase in the proportion of persons – both insured and uninsured – receiving cholesterol checks and flu shots,” Dr. Hawk and her colleagues wrote, adding that this increase “may be attributable to the increasing implementation of quality metrics, financial incentives, and improved systems for the delivery of these services.”
However, not all preventive services that had cost barriers eliminated under the ACA saw improvement, such as cancer screening. They note that the proportion of women who did not receive mammography increased during the study period and then plateaued, but did not improve following the implementation of the ACA. The authors described the reasons for this as “unclear.”
Dr. Hawks received funding support from an Institutional National Research Service award and from Cambridge Health Alliance, her employer. Other authors reported membership in Physicians for a National Health Program.
SOURCE: Hawks L et al. JAMA Intern Med. 2020 Jan 27. doi: 10.1001/jamainternmed.2019.6538.
The cost of health care is keeping more Americans from seeing a doctor, even as the number of individuals with insurance coverage increases, according to a new study.
“Despite short-term gains owing to the [Affordable Care Act], over the past 20 years the portion of adults aged 18-64 years unable to see a physician owing to the cost increased, mostly because of an increase among persons with insurance,” Laura Hawks, MD, of Cambridge (Mass.) Health Alliance and Harvard Medical School in Boston and colleagues wrote in a new research report published in JAMA Internal Medicine.
“In 2017, nearly one-fifth of individuals with any chronic condition (diabetes, obesity, or cardiovascular disease) said they were unable to see a physician owing to cost,” they continued.
Researchers examined 20 years of data (January 1998 through December 2017) from the Centers for Disease Control and Prevention’s Behavioral Risk Factor Surveillance System to identify trends in unmet need for physician and preventive services.
Among adults aged 18-64 years who responded to the survey in 1998 and 2017, uninsurance decreased by 2.1 percentage points, falling from 16.9% to 14.8%. But at the same time, the portion of adults who were unable to see a physician because of cost rose by 2.7 percentage points, from 11.4% to 15.7%. Looking specifically at adults who had insurance coverage, the researchers found that cost was a barrier for 11.5% of them in 2017, up from 7.1% in 1998.
These results come against a backdrop of growing medical costs, increasing deductibles and copayments, an increasing use of cost containment measures like prior authorization, and narrow provider networks in the wake of the transition to value-based payment structures, the authors noted.
“Our finding that financial access to physician care worsened is concerning,” Dr. Hawks and her colleagues wrote. “Persons with conditions such as diabetes, hypertension, cardiovascular disease, and poor health status risk substantial harms if they forgo physician care. Financial barriers to care have been associated with increased hospitalizations and worse health outcomes in patients with cardiovascular disease and hypertension and increased morbidity among patients with diabetes.”
One of the trends highlighted by the study authors is the growing number of employers offering plans with a high deductible.
“Enrollment in a high-deductible health plan, which has become increasingly common in the last decade, a trend uninterrupted by the ACA, is associated with forgoing needed care, especially among those of lower socioeconomic status,” the authors wrote. “Other changes in insurance benefit design, such as imposing tiered copayments and coinsurance obligations, eliminating coverage for some services (e.g., eyeglasses) and narrowing provider networks (which can force some patients to go out-of-network for care) may also have undermined the affordability of care.”
There was some positive news among the findings, however.
“The main encouraging finding from our analysis is the increase in the proportion of persons – both insured and uninsured – receiving cholesterol checks and flu shots,” Dr. Hawk and her colleagues wrote, adding that this increase “may be attributable to the increasing implementation of quality metrics, financial incentives, and improved systems for the delivery of these services.”
However, not all preventive services that had cost barriers eliminated under the ACA saw improvement, such as cancer screening. They note that the proportion of women who did not receive mammography increased during the study period and then plateaued, but did not improve following the implementation of the ACA. The authors described the reasons for this as “unclear.”
Dr. Hawks received funding support from an Institutional National Research Service award and from Cambridge Health Alliance, her employer. Other authors reported membership in Physicians for a National Health Program.
SOURCE: Hawks L et al. JAMA Intern Med. 2020 Jan 27. doi: 10.1001/jamainternmed.2019.6538.
The cost of health care is keeping more Americans from seeing a doctor, even as the number of individuals with insurance coverage increases, according to a new study.
“Despite short-term gains owing to the [Affordable Care Act], over the past 20 years the portion of adults aged 18-64 years unable to see a physician owing to the cost increased, mostly because of an increase among persons with insurance,” Laura Hawks, MD, of Cambridge (Mass.) Health Alliance and Harvard Medical School in Boston and colleagues wrote in a new research report published in JAMA Internal Medicine.
“In 2017, nearly one-fifth of individuals with any chronic condition (diabetes, obesity, or cardiovascular disease) said they were unable to see a physician owing to cost,” they continued.
Researchers examined 20 years of data (January 1998 through December 2017) from the Centers for Disease Control and Prevention’s Behavioral Risk Factor Surveillance System to identify trends in unmet need for physician and preventive services.
Among adults aged 18-64 years who responded to the survey in 1998 and 2017, uninsurance decreased by 2.1 percentage points, falling from 16.9% to 14.8%. But at the same time, the portion of adults who were unable to see a physician because of cost rose by 2.7 percentage points, from 11.4% to 15.7%. Looking specifically at adults who had insurance coverage, the researchers found that cost was a barrier for 11.5% of them in 2017, up from 7.1% in 1998.
These results come against a backdrop of growing medical costs, increasing deductibles and copayments, an increasing use of cost containment measures like prior authorization, and narrow provider networks in the wake of the transition to value-based payment structures, the authors noted.
“Our finding that financial access to physician care worsened is concerning,” Dr. Hawks and her colleagues wrote. “Persons with conditions such as diabetes, hypertension, cardiovascular disease, and poor health status risk substantial harms if they forgo physician care. Financial barriers to care have been associated with increased hospitalizations and worse health outcomes in patients with cardiovascular disease and hypertension and increased morbidity among patients with diabetes.”
One of the trends highlighted by the study authors is the growing number of employers offering plans with a high deductible.
“Enrollment in a high-deductible health plan, which has become increasingly common in the last decade, a trend uninterrupted by the ACA, is associated with forgoing needed care, especially among those of lower socioeconomic status,” the authors wrote. “Other changes in insurance benefit design, such as imposing tiered copayments and coinsurance obligations, eliminating coverage for some services (e.g., eyeglasses) and narrowing provider networks (which can force some patients to go out-of-network for care) may also have undermined the affordability of care.”
There was some positive news among the findings, however.
“The main encouraging finding from our analysis is the increase in the proportion of persons – both insured and uninsured – receiving cholesterol checks and flu shots,” Dr. Hawk and her colleagues wrote, adding that this increase “may be attributable to the increasing implementation of quality metrics, financial incentives, and improved systems for the delivery of these services.”
However, not all preventive services that had cost barriers eliminated under the ACA saw improvement, such as cancer screening. They note that the proportion of women who did not receive mammography increased during the study period and then plateaued, but did not improve following the implementation of the ACA. The authors described the reasons for this as “unclear.”
Dr. Hawks received funding support from an Institutional National Research Service award and from Cambridge Health Alliance, her employer. Other authors reported membership in Physicians for a National Health Program.
SOURCE: Hawks L et al. JAMA Intern Med. 2020 Jan 27. doi: 10.1001/jamainternmed.2019.6538.
FROM JAMA INTERNAL MEDICINE
Psoriasis: A look back over the past 50 years, and forward to next steps
Imagine a patient suffering with horrible psoriasis for decades having failed “every available treatment.” Imagine him living all that time with “flaking, cracking, painful, itchy skin,” only to develop cirrhosis after exposure to toxic therapies.
Then imagine the experience for that patient when, 2 weeks after initiating treatment with a new interleukin-17 inhibitor, his skin clears completely.
“Two weeks later it’s all gone – it was a moment to behold,” said Joel M. Gelfand, MD, professor of dermatology and epidemiology at the University of Pennsylvania, Philadelphia, who had cared for the man for many years before a psoriasis treatment revolution of sorts took the field of dermatology by storm.
“The progress has been breathtaking – there’s no other way to describe it – and it feels like a miracle every time I see a new patient who has tough disease and I have all these things to offer them,” he continued. “For most patients, I can really help them and make a major difference in their life.”
said Mark Lebwohl, MD, Waldman professor of dermatology and chair of the Kimberly and Eric J. Waldman department of dermatology at the Icahn School of Medicine at Mount Sinai, New York.
Dr. Lebwohl recounted some of his own experiences with psoriasis patients before the advent of treatments – particularly biologics – that have transformed practice.
There was a time when psoriasis patients had little more to turn to than the effective – but “disgusting” – Goeckerman Regimen involving cycles of UVB light exposure and topical crude coal tar application. Initially, the regimen, which was introduced in the 1920s, was used around the clock on an inpatient basis until the skin cleared, Dr. Lebwohl said.
In the 1970s, the immunosuppressive chemotherapy drug methotrexate became the first oral systemic therapy approved for severe psoriasis. For those with disabling disease, it offered some hope for relief, but only about 40% of patients achieved at least a 75% reduction in the Psoriasis Area and Severity Index score (PASI 75), he said, adding that they did so at the expense of the liver and bone marrow. “But it was the only thing we had for severe psoriasis other than light treatments.”
In the 1980s and 1990s, oral retinoids emerged as a treatment for psoriasis, and the immunosuppressive drug cyclosporine used to prevent organ rejection in some transplant patients was found to clear psoriasis in affected transplant recipients. Although they brought relief to some patients with severe, disabling disease, these also came with a high price. “It’s not that effective, and it has lots of side effects ... and causes kidney damage in essentially 100% of patients,” Dr. Lebwohl said of cyclosporine.
“So we had treatments that worked, but because the side effects were sufficiently severe, a lot of patients were not treated,” he said.
Enter the biologics era
The early 2000s brought the first two approvals for psoriasis: alefacept (Amevive), a “modestly effective, but quite safe” immunosuppressive dimeric fusion protein approved in early 2003 for moderate to severe plaque psoriasis, and efalizumab (Raptiva), a recombinant humanized monoclonal antibody approved in October 2003; both were T-cell–targeted therapies. The former was withdrawn from the market voluntarily as newer agents became available, and the latter was withdrawn in 2009 because of a link with development of progressive multifocal leukoencephalopathy.
Tumor necrosis factor (TNF) blockers, which had been used effectively for RA and Crohn’s disease, emerged next, and were highly effective, much safer than the systemic treatments, and gained “very widespread use,” Dr. Lebwohl said.
His colleague Alice B. Gottlieb, MD, PhD, was among the pioneers in the development of TNF blockers for the treatment of psoriasis. Her seminal, investigator-initiated paper on the efficacy and safety of infliximab (Remicade) monotherapy for plaque-type psoriasis published in the Lancet in 2001 helped launch the current era in which many psoriasis patients achieve 100% PASI responses with limited side effects, he said, explaining that subsequent research elucidated the role of IL-12 and -23 – leading to effective treatments like ustekinumab (Stelara), and later IL-17, which is, “in fact, the molecule closest to the pathogenesis of psoriasis.”
“If you block IL-17, you get rid of psoriasis,” he said, noting that there are now several companies with approved antibodies to IL-17. “Taltz [ixekizumab] and Cosentyx [secukinumab] are the leading ones, and Siliq [brodalumab] blocks the receptor for IL-17, so it is very effective.”
Another novel biologic – bimekizumab – is on the horizon. It blocks both IL-17a and IL-17f, and appears highly effective in psoriasis and psoriatic arthritis (PsA). “Biologics were the real start of the [psoriasis treatment] revolution,” he said. “When I started out I would speak at patient meetings and the patients were angry at their physicians; they thought they weren’t aggressive enough, they were very frustrated.”
Dr. Lebwohl described patients he would see at annual National Psoriasis Foundation meetings: “There were patients in wheel chairs, because they couldn’t walk. They would be red and scaly all over ... you could have literally swept up scale like it was snow after one of those meetings.
“You go forward to around 2010 – nobody’s in wheelchairs anymore, everybody has clear skin, and it’s become a party; patients are no longer angry – they are thrilled with the results they are getting from much safer and much more effective drugs,” he said. “So it’s been a pleasure taking care of those patients and going from a very difficult time of treating them, to a time where we’ve done a great job treating them.”
Dr. Lebwohl noted that a “large number of dermatologists have been involved with the development of these drugs and making sure they succeed, and that has also been a pleasure to see.”
Dr. Gottlieb, who Dr. Lebwohl has described as “a superstar” in the fields of dermatology and rheumatology, is one such researcher. In an interview, she looked back on her work and the ways that her work “opened the field,” led to many of her trainees also doing “great work,” and changed the lives of patients.
“It’s nice to feel that I really did change, fundamentally, how psoriasis patients are treated,” said Dr. Gottlieb, who is a clinical professor in the department of dermatology at the Icahn School of Medicine at Mount Sinai. “That obviously feels great.”
She recalled a patient – “a 6-foot-5 biker with bad psoriasis” – who “literally, the minute the door closed, he was crying about how horrible his disease was.”
“And I cleared him ... and then you get big hugs – it just feels extremely good ... giving somebody their life back,” she said.
Dr. Gottlieb has been involved in much of the work in developing biologics for psoriasis, including the ongoing work with bimekizumab for PsA as mentioned by Dr. Lebwohl.
If the phase 2 data with bimekizumab are replicated in the ongoing phase 3 trials now underway at her center, “that can really raise the bar ... so if it’s reproducible, it’s very exciting.”
“It’s exciting to have an IL-23 blocker that, at least in clinical trials, showed inhibition of radiographic progression [in PsA],” she said. “That’s guselkumab those data are already out, and I was involved with that.”
The early work of Dr. Gottlieb and others has also “spread to other diseases,” like hidradenitis suppurativa and atopic dermatitis, she said, noting that numerous studies are underway.
Aside from curing all patients, her ultimate goal is getting to a point where psoriasis has no effect on patients’ quality of life.
“And I see it already,” she said. “It’s happening, and it’s nice to see that it’s happening in children now, too; several of the drugs are approved in kids.”
Alan Menter, MD, chairman of the division of dermatology at Baylor University Medical Center, Dallas, also a prolific researcher – and chair of the guidelines committee that published two new sets of guidelines for psoriasis treatment in 2019 – said that the field of dermatology was “late to the biologic evolution,” as many of the early biologics were first approved for PsA.
“But over the last 10 years, things have changed dramatically,” he said. “After that we suddenly leapt ahead of everybody. ... We now have 11 biologic drugs approved for psoriasis, which is more than any other disease has available.”
It’s been “highly exciting” to see this “evolution and revolution,” he commented, adding that one of the next challenges is to address the comorbidities, such as cardiovascular disease, associated with psoriasis.
“The big question now ... is if you improve skin and you improve joints, can you potentially reduce the risk of coronary artery disease,” he said. “Everybody is looking at that, and to me it’s one of the most exciting things that we’re doing.”
Work is ongoing to look at whether the IL-17s and IL-23s have “other indications outside of the skin and joints,” both within and outside of dermatology.
Like Dr. Gottlieb, Dr. Menter also mentioned the potential for hidradenitis suppurativa, and also for a condition that is rarely discussed or studied: genital psoriasis. Ixekizumab has recently been shown to work in about 75% of patients with genital psoriasis, he noted.
Another important area of research is the identification of biomarkers for predicting response and relapse, he said. For now, biomarker research has disappointed, he added, predicting that it will take at least 3-5 years before biomarkers to help guide treatment are identified.
Indeed, Dr. Gelfand, who also is director of the Psoriasis and Phototherapy Treatment Center, vice chair of clinical research, and medical director of the dermatology clinical studies unit at the University of Pennsylvania, agreed there is a need for research to improve treatment selection.
Advances are being made in genetics – with more than 80 different genes now identified as being related to psoriasis – and in medical informatics – which allow thousands of patients to be followed for years, he said, noting that this could elucidate immunopathological features that can improve treatments, predict and prevent comorbidity, and further improve outcomes.
“We also need care that is more patient centered,” he said, describing the ongoing pragmatic LITE trial of home- or office-based phototherapy for which he is the lead investigator, and other studies that he hopes will expand access to care.
Kenneth Brian Gordon, MD, chair and professor of dermatology at the Medical College of Wisconsin, Milwaukee, whose career started in the basic science immunology arena, added the need for expanding benefit to patients with more-moderate disease. Like Dr. Menter, he identified psoriasis as the area in medicine that has had the greatest degree of advancement, except perhaps for hepatitis C.
He described the process not as a “bench-to-bedside” story, but as a bedside-to-bench, then “back-to-bedside” story.
It was really about taking those early T-cell–targeted biologics and anti-TNF agents from bedside to bench with the realization of the importance of the IL-23 and IL-17 pathways, and that understanding led back to the bedside with the development of the newest agents – and to a “huge difference in patient’s lives.”
“But we’ve gotten so good at treating patients with severe disease ... the question now is how to take care of those with more-moderate disease,” he said, noting that a focus on cost and better delivery systems will be needed for that population.
That research is underway, and the future looks bright – and clear.
“I think with psoriasis therapy and where we’ve come in the last 20 years ... we have a hard time remembering what it was like before we had biologic agents” he said. “Our perspective has changed a lot, and sometimes we forget that.”
In fact, “psoriasis has sort of dragged dermatology into the world of modern clinical trial science, and we can now apply that to all sorts of other diseases,” he said. “The psoriasis trials were the first really well-done large-scale trials in dermatology, and I think that has given dermatology a real leg up in how we do clinical research and how we do evidence-based medicine.”
All of the doctors interviewed for this story have received funds and/or honoraria from, consulted with, are employed with, or served on the advisory boards of manufacturers of biologics. Dr. Gelfand is a copatent holder of resiquimod for treatment of cutaneous T-cell lymphoma and is deputy editor of the Journal of Investigative Dermatology.
Imagine a patient suffering with horrible psoriasis for decades having failed “every available treatment.” Imagine him living all that time with “flaking, cracking, painful, itchy skin,” only to develop cirrhosis after exposure to toxic therapies.
Then imagine the experience for that patient when, 2 weeks after initiating treatment with a new interleukin-17 inhibitor, his skin clears completely.
“Two weeks later it’s all gone – it was a moment to behold,” said Joel M. Gelfand, MD, professor of dermatology and epidemiology at the University of Pennsylvania, Philadelphia, who had cared for the man for many years before a psoriasis treatment revolution of sorts took the field of dermatology by storm.
“The progress has been breathtaking – there’s no other way to describe it – and it feels like a miracle every time I see a new patient who has tough disease and I have all these things to offer them,” he continued. “For most patients, I can really help them and make a major difference in their life.”
said Mark Lebwohl, MD, Waldman professor of dermatology and chair of the Kimberly and Eric J. Waldman department of dermatology at the Icahn School of Medicine at Mount Sinai, New York.
Dr. Lebwohl recounted some of his own experiences with psoriasis patients before the advent of treatments – particularly biologics – that have transformed practice.
There was a time when psoriasis patients had little more to turn to than the effective – but “disgusting” – Goeckerman Regimen involving cycles of UVB light exposure and topical crude coal tar application. Initially, the regimen, which was introduced in the 1920s, was used around the clock on an inpatient basis until the skin cleared, Dr. Lebwohl said.
In the 1970s, the immunosuppressive chemotherapy drug methotrexate became the first oral systemic therapy approved for severe psoriasis. For those with disabling disease, it offered some hope for relief, but only about 40% of patients achieved at least a 75% reduction in the Psoriasis Area and Severity Index score (PASI 75), he said, adding that they did so at the expense of the liver and bone marrow. “But it was the only thing we had for severe psoriasis other than light treatments.”
In the 1980s and 1990s, oral retinoids emerged as a treatment for psoriasis, and the immunosuppressive drug cyclosporine used to prevent organ rejection in some transplant patients was found to clear psoriasis in affected transplant recipients. Although they brought relief to some patients with severe, disabling disease, these also came with a high price. “It’s not that effective, and it has lots of side effects ... and causes kidney damage in essentially 100% of patients,” Dr. Lebwohl said of cyclosporine.
“So we had treatments that worked, but because the side effects were sufficiently severe, a lot of patients were not treated,” he said.
Enter the biologics era
The early 2000s brought the first two approvals for psoriasis: alefacept (Amevive), a “modestly effective, but quite safe” immunosuppressive dimeric fusion protein approved in early 2003 for moderate to severe plaque psoriasis, and efalizumab (Raptiva), a recombinant humanized monoclonal antibody approved in October 2003; both were T-cell–targeted therapies. The former was withdrawn from the market voluntarily as newer agents became available, and the latter was withdrawn in 2009 because of a link with development of progressive multifocal leukoencephalopathy.
Tumor necrosis factor (TNF) blockers, which had been used effectively for RA and Crohn’s disease, emerged next, and were highly effective, much safer than the systemic treatments, and gained “very widespread use,” Dr. Lebwohl said.
His colleague Alice B. Gottlieb, MD, PhD, was among the pioneers in the development of TNF blockers for the treatment of psoriasis. Her seminal, investigator-initiated paper on the efficacy and safety of infliximab (Remicade) monotherapy for plaque-type psoriasis published in the Lancet in 2001 helped launch the current era in which many psoriasis patients achieve 100% PASI responses with limited side effects, he said, explaining that subsequent research elucidated the role of IL-12 and -23 – leading to effective treatments like ustekinumab (Stelara), and later IL-17, which is, “in fact, the molecule closest to the pathogenesis of psoriasis.”
“If you block IL-17, you get rid of psoriasis,” he said, noting that there are now several companies with approved antibodies to IL-17. “Taltz [ixekizumab] and Cosentyx [secukinumab] are the leading ones, and Siliq [brodalumab] blocks the receptor for IL-17, so it is very effective.”
Another novel biologic – bimekizumab – is on the horizon. It blocks both IL-17a and IL-17f, and appears highly effective in psoriasis and psoriatic arthritis (PsA). “Biologics were the real start of the [psoriasis treatment] revolution,” he said. “When I started out I would speak at patient meetings and the patients were angry at their physicians; they thought they weren’t aggressive enough, they were very frustrated.”
Dr. Lebwohl described patients he would see at annual National Psoriasis Foundation meetings: “There were patients in wheel chairs, because they couldn’t walk. They would be red and scaly all over ... you could have literally swept up scale like it was snow after one of those meetings.
“You go forward to around 2010 – nobody’s in wheelchairs anymore, everybody has clear skin, and it’s become a party; patients are no longer angry – they are thrilled with the results they are getting from much safer and much more effective drugs,” he said. “So it’s been a pleasure taking care of those patients and going from a very difficult time of treating them, to a time where we’ve done a great job treating them.”
Dr. Lebwohl noted that a “large number of dermatologists have been involved with the development of these drugs and making sure they succeed, and that has also been a pleasure to see.”
Dr. Gottlieb, who Dr. Lebwohl has described as “a superstar” in the fields of dermatology and rheumatology, is one such researcher. In an interview, she looked back on her work and the ways that her work “opened the field,” led to many of her trainees also doing “great work,” and changed the lives of patients.
“It’s nice to feel that I really did change, fundamentally, how psoriasis patients are treated,” said Dr. Gottlieb, who is a clinical professor in the department of dermatology at the Icahn School of Medicine at Mount Sinai. “That obviously feels great.”
She recalled a patient – “a 6-foot-5 biker with bad psoriasis” – who “literally, the minute the door closed, he was crying about how horrible his disease was.”
“And I cleared him ... and then you get big hugs – it just feels extremely good ... giving somebody their life back,” she said.
Dr. Gottlieb has been involved in much of the work in developing biologics for psoriasis, including the ongoing work with bimekizumab for PsA as mentioned by Dr. Lebwohl.
If the phase 2 data with bimekizumab are replicated in the ongoing phase 3 trials now underway at her center, “that can really raise the bar ... so if it’s reproducible, it’s very exciting.”
“It’s exciting to have an IL-23 blocker that, at least in clinical trials, showed inhibition of radiographic progression [in PsA],” she said. “That’s guselkumab those data are already out, and I was involved with that.”
The early work of Dr. Gottlieb and others has also “spread to other diseases,” like hidradenitis suppurativa and atopic dermatitis, she said, noting that numerous studies are underway.
Aside from curing all patients, her ultimate goal is getting to a point where psoriasis has no effect on patients’ quality of life.
“And I see it already,” she said. “It’s happening, and it’s nice to see that it’s happening in children now, too; several of the drugs are approved in kids.”
Alan Menter, MD, chairman of the division of dermatology at Baylor University Medical Center, Dallas, also a prolific researcher – and chair of the guidelines committee that published two new sets of guidelines for psoriasis treatment in 2019 – said that the field of dermatology was “late to the biologic evolution,” as many of the early biologics were first approved for PsA.
“But over the last 10 years, things have changed dramatically,” he said. “After that we suddenly leapt ahead of everybody. ... We now have 11 biologic drugs approved for psoriasis, which is more than any other disease has available.”
It’s been “highly exciting” to see this “evolution and revolution,” he commented, adding that one of the next challenges is to address the comorbidities, such as cardiovascular disease, associated with psoriasis.
“The big question now ... is if you improve skin and you improve joints, can you potentially reduce the risk of coronary artery disease,” he said. “Everybody is looking at that, and to me it’s one of the most exciting things that we’re doing.”
Work is ongoing to look at whether the IL-17s and IL-23s have “other indications outside of the skin and joints,” both within and outside of dermatology.
Like Dr. Gottlieb, Dr. Menter also mentioned the potential for hidradenitis suppurativa, and also for a condition that is rarely discussed or studied: genital psoriasis. Ixekizumab has recently been shown to work in about 75% of patients with genital psoriasis, he noted.
Another important area of research is the identification of biomarkers for predicting response and relapse, he said. For now, biomarker research has disappointed, he added, predicting that it will take at least 3-5 years before biomarkers to help guide treatment are identified.
Indeed, Dr. Gelfand, who also is director of the Psoriasis and Phototherapy Treatment Center, vice chair of clinical research, and medical director of the dermatology clinical studies unit at the University of Pennsylvania, agreed there is a need for research to improve treatment selection.
Advances are being made in genetics – with more than 80 different genes now identified as being related to psoriasis – and in medical informatics – which allow thousands of patients to be followed for years, he said, noting that this could elucidate immunopathological features that can improve treatments, predict and prevent comorbidity, and further improve outcomes.
“We also need care that is more patient centered,” he said, describing the ongoing pragmatic LITE trial of home- or office-based phototherapy for which he is the lead investigator, and other studies that he hopes will expand access to care.
Kenneth Brian Gordon, MD, chair and professor of dermatology at the Medical College of Wisconsin, Milwaukee, whose career started in the basic science immunology arena, added the need for expanding benefit to patients with more-moderate disease. Like Dr. Menter, he identified psoriasis as the area in medicine that has had the greatest degree of advancement, except perhaps for hepatitis C.
He described the process not as a “bench-to-bedside” story, but as a bedside-to-bench, then “back-to-bedside” story.
It was really about taking those early T-cell–targeted biologics and anti-TNF agents from bedside to bench with the realization of the importance of the IL-23 and IL-17 pathways, and that understanding led back to the bedside with the development of the newest agents – and to a “huge difference in patient’s lives.”
“But we’ve gotten so good at treating patients with severe disease ... the question now is how to take care of those with more-moderate disease,” he said, noting that a focus on cost and better delivery systems will be needed for that population.
That research is underway, and the future looks bright – and clear.
“I think with psoriasis therapy and where we’ve come in the last 20 years ... we have a hard time remembering what it was like before we had biologic agents” he said. “Our perspective has changed a lot, and sometimes we forget that.”
In fact, “psoriasis has sort of dragged dermatology into the world of modern clinical trial science, and we can now apply that to all sorts of other diseases,” he said. “The psoriasis trials were the first really well-done large-scale trials in dermatology, and I think that has given dermatology a real leg up in how we do clinical research and how we do evidence-based medicine.”
All of the doctors interviewed for this story have received funds and/or honoraria from, consulted with, are employed with, or served on the advisory boards of manufacturers of biologics. Dr. Gelfand is a copatent holder of resiquimod for treatment of cutaneous T-cell lymphoma and is deputy editor of the Journal of Investigative Dermatology.
Imagine a patient suffering with horrible psoriasis for decades having failed “every available treatment.” Imagine him living all that time with “flaking, cracking, painful, itchy skin,” only to develop cirrhosis after exposure to toxic therapies.
Then imagine the experience for that patient when, 2 weeks after initiating treatment with a new interleukin-17 inhibitor, his skin clears completely.
“Two weeks later it’s all gone – it was a moment to behold,” said Joel M. Gelfand, MD, professor of dermatology and epidemiology at the University of Pennsylvania, Philadelphia, who had cared for the man for many years before a psoriasis treatment revolution of sorts took the field of dermatology by storm.
“The progress has been breathtaking – there’s no other way to describe it – and it feels like a miracle every time I see a new patient who has tough disease and I have all these things to offer them,” he continued. “For most patients, I can really help them and make a major difference in their life.”
said Mark Lebwohl, MD, Waldman professor of dermatology and chair of the Kimberly and Eric J. Waldman department of dermatology at the Icahn School of Medicine at Mount Sinai, New York.
Dr. Lebwohl recounted some of his own experiences with psoriasis patients before the advent of treatments – particularly biologics – that have transformed practice.
There was a time when psoriasis patients had little more to turn to than the effective – but “disgusting” – Goeckerman Regimen involving cycles of UVB light exposure and topical crude coal tar application. Initially, the regimen, which was introduced in the 1920s, was used around the clock on an inpatient basis until the skin cleared, Dr. Lebwohl said.
In the 1970s, the immunosuppressive chemotherapy drug methotrexate became the first oral systemic therapy approved for severe psoriasis. For those with disabling disease, it offered some hope for relief, but only about 40% of patients achieved at least a 75% reduction in the Psoriasis Area and Severity Index score (PASI 75), he said, adding that they did so at the expense of the liver and bone marrow. “But it was the only thing we had for severe psoriasis other than light treatments.”
In the 1980s and 1990s, oral retinoids emerged as a treatment for psoriasis, and the immunosuppressive drug cyclosporine used to prevent organ rejection in some transplant patients was found to clear psoriasis in affected transplant recipients. Although they brought relief to some patients with severe, disabling disease, these also came with a high price. “It’s not that effective, and it has lots of side effects ... and causes kidney damage in essentially 100% of patients,” Dr. Lebwohl said of cyclosporine.
“So we had treatments that worked, but because the side effects were sufficiently severe, a lot of patients were not treated,” he said.
Enter the biologics era
The early 2000s brought the first two approvals for psoriasis: alefacept (Amevive), a “modestly effective, but quite safe” immunosuppressive dimeric fusion protein approved in early 2003 for moderate to severe plaque psoriasis, and efalizumab (Raptiva), a recombinant humanized monoclonal antibody approved in October 2003; both were T-cell–targeted therapies. The former was withdrawn from the market voluntarily as newer agents became available, and the latter was withdrawn in 2009 because of a link with development of progressive multifocal leukoencephalopathy.
Tumor necrosis factor (TNF) blockers, which had been used effectively for RA and Crohn’s disease, emerged next, and were highly effective, much safer than the systemic treatments, and gained “very widespread use,” Dr. Lebwohl said.
His colleague Alice B. Gottlieb, MD, PhD, was among the pioneers in the development of TNF blockers for the treatment of psoriasis. Her seminal, investigator-initiated paper on the efficacy and safety of infliximab (Remicade) monotherapy for plaque-type psoriasis published in the Lancet in 2001 helped launch the current era in which many psoriasis patients achieve 100% PASI responses with limited side effects, he said, explaining that subsequent research elucidated the role of IL-12 and -23 – leading to effective treatments like ustekinumab (Stelara), and later IL-17, which is, “in fact, the molecule closest to the pathogenesis of psoriasis.”
“If you block IL-17, you get rid of psoriasis,” he said, noting that there are now several companies with approved antibodies to IL-17. “Taltz [ixekizumab] and Cosentyx [secukinumab] are the leading ones, and Siliq [brodalumab] blocks the receptor for IL-17, so it is very effective.”
Another novel biologic – bimekizumab – is on the horizon. It blocks both IL-17a and IL-17f, and appears highly effective in psoriasis and psoriatic arthritis (PsA). “Biologics were the real start of the [psoriasis treatment] revolution,” he said. “When I started out I would speak at patient meetings and the patients were angry at their physicians; they thought they weren’t aggressive enough, they were very frustrated.”
Dr. Lebwohl described patients he would see at annual National Psoriasis Foundation meetings: “There were patients in wheel chairs, because they couldn’t walk. They would be red and scaly all over ... you could have literally swept up scale like it was snow after one of those meetings.
“You go forward to around 2010 – nobody’s in wheelchairs anymore, everybody has clear skin, and it’s become a party; patients are no longer angry – they are thrilled with the results they are getting from much safer and much more effective drugs,” he said. “So it’s been a pleasure taking care of those patients and going from a very difficult time of treating them, to a time where we’ve done a great job treating them.”
Dr. Lebwohl noted that a “large number of dermatologists have been involved with the development of these drugs and making sure they succeed, and that has also been a pleasure to see.”
Dr. Gottlieb, who Dr. Lebwohl has described as “a superstar” in the fields of dermatology and rheumatology, is one such researcher. In an interview, she looked back on her work and the ways that her work “opened the field,” led to many of her trainees also doing “great work,” and changed the lives of patients.
“It’s nice to feel that I really did change, fundamentally, how psoriasis patients are treated,” said Dr. Gottlieb, who is a clinical professor in the department of dermatology at the Icahn School of Medicine at Mount Sinai. “That obviously feels great.”
She recalled a patient – “a 6-foot-5 biker with bad psoriasis” – who “literally, the minute the door closed, he was crying about how horrible his disease was.”
“And I cleared him ... and then you get big hugs – it just feels extremely good ... giving somebody their life back,” she said.
Dr. Gottlieb has been involved in much of the work in developing biologics for psoriasis, including the ongoing work with bimekizumab for PsA as mentioned by Dr. Lebwohl.
If the phase 2 data with bimekizumab are replicated in the ongoing phase 3 trials now underway at her center, “that can really raise the bar ... so if it’s reproducible, it’s very exciting.”
“It’s exciting to have an IL-23 blocker that, at least in clinical trials, showed inhibition of radiographic progression [in PsA],” she said. “That’s guselkumab those data are already out, and I was involved with that.”
The early work of Dr. Gottlieb and others has also “spread to other diseases,” like hidradenitis suppurativa and atopic dermatitis, she said, noting that numerous studies are underway.
Aside from curing all patients, her ultimate goal is getting to a point where psoriasis has no effect on patients’ quality of life.
“And I see it already,” she said. “It’s happening, and it’s nice to see that it’s happening in children now, too; several of the drugs are approved in kids.”
Alan Menter, MD, chairman of the division of dermatology at Baylor University Medical Center, Dallas, also a prolific researcher – and chair of the guidelines committee that published two new sets of guidelines for psoriasis treatment in 2019 – said that the field of dermatology was “late to the biologic evolution,” as many of the early biologics were first approved for PsA.
“But over the last 10 years, things have changed dramatically,” he said. “After that we suddenly leapt ahead of everybody. ... We now have 11 biologic drugs approved for psoriasis, which is more than any other disease has available.”
It’s been “highly exciting” to see this “evolution and revolution,” he commented, adding that one of the next challenges is to address the comorbidities, such as cardiovascular disease, associated with psoriasis.
“The big question now ... is if you improve skin and you improve joints, can you potentially reduce the risk of coronary artery disease,” he said. “Everybody is looking at that, and to me it’s one of the most exciting things that we’re doing.”
Work is ongoing to look at whether the IL-17s and IL-23s have “other indications outside of the skin and joints,” both within and outside of dermatology.
Like Dr. Gottlieb, Dr. Menter also mentioned the potential for hidradenitis suppurativa, and also for a condition that is rarely discussed or studied: genital psoriasis. Ixekizumab has recently been shown to work in about 75% of patients with genital psoriasis, he noted.
Another important area of research is the identification of biomarkers for predicting response and relapse, he said. For now, biomarker research has disappointed, he added, predicting that it will take at least 3-5 years before biomarkers to help guide treatment are identified.
Indeed, Dr. Gelfand, who also is director of the Psoriasis and Phototherapy Treatment Center, vice chair of clinical research, and medical director of the dermatology clinical studies unit at the University of Pennsylvania, agreed there is a need for research to improve treatment selection.
Advances are being made in genetics – with more than 80 different genes now identified as being related to psoriasis – and in medical informatics – which allow thousands of patients to be followed for years, he said, noting that this could elucidate immunopathological features that can improve treatments, predict and prevent comorbidity, and further improve outcomes.
“We also need care that is more patient centered,” he said, describing the ongoing pragmatic LITE trial of home- or office-based phototherapy for which he is the lead investigator, and other studies that he hopes will expand access to care.
Kenneth Brian Gordon, MD, chair and professor of dermatology at the Medical College of Wisconsin, Milwaukee, whose career started in the basic science immunology arena, added the need for expanding benefit to patients with more-moderate disease. Like Dr. Menter, he identified psoriasis as the area in medicine that has had the greatest degree of advancement, except perhaps for hepatitis C.
He described the process not as a “bench-to-bedside” story, but as a bedside-to-bench, then “back-to-bedside” story.
It was really about taking those early T-cell–targeted biologics and anti-TNF agents from bedside to bench with the realization of the importance of the IL-23 and IL-17 pathways, and that understanding led back to the bedside with the development of the newest agents – and to a “huge difference in patient’s lives.”
“But we’ve gotten so good at treating patients with severe disease ... the question now is how to take care of those with more-moderate disease,” he said, noting that a focus on cost and better delivery systems will be needed for that population.
That research is underway, and the future looks bright – and clear.
“I think with psoriasis therapy and where we’ve come in the last 20 years ... we have a hard time remembering what it was like before we had biologic agents” he said. “Our perspective has changed a lot, and sometimes we forget that.”
In fact, “psoriasis has sort of dragged dermatology into the world of modern clinical trial science, and we can now apply that to all sorts of other diseases,” he said. “The psoriasis trials were the first really well-done large-scale trials in dermatology, and I think that has given dermatology a real leg up in how we do clinical research and how we do evidence-based medicine.”
All of the doctors interviewed for this story have received funds and/or honoraria from, consulted with, are employed with, or served on the advisory boards of manufacturers of biologics. Dr. Gelfand is a copatent holder of resiquimod for treatment of cutaneous T-cell lymphoma and is deputy editor of the Journal of Investigative Dermatology.
ID Blog: Wuhan coronavirus – just a stop on the zoonotic highway
Emerging viruses that spread to humans from an animal host are commonplace and represent some of the deadliest diseases known. Given the details of the Wuhan coronavirus (2019-nCoV) outbreak, including the genetic profile of the disease agent, the hypothesis of a snake origin was the first raised in the peer-reviewed literature.
It is a highly controversial origin story, however, given that mammals have been the sources of all other such zoonotic coronaviruses, as well as a host of other zoonotic diseases.
An animal source for emerging infections such as the 2019-nCoV is the default hypothesis, because “around 60% of all infectious diseases in humans are zoonotic, as are 75% of all emerging infectious diseases,” according to a United Nations report. The report goes on to say that, “on average, one new infectious disease emerges in humans every 4 months.”
To appreciate the emergence and nature of 2019-nCoV, it is important to examine the history of zoonotic outbreaks of other such diseases, especially with regard to the “mixing-vessel” phenomenon, which has been noted in closely related coronaviruses, including SARS and MERS, as well as the widely disparate HIV, Ebola, and influenza viruses.
Mutants in the mixing vessel
The mixing-vessel phenomenon is conceptually easy but molecularly complex. A single animal is coinfected with two related viruses; the virus genomes recombine together (virus “sex”) in that animal to form a new variant of virus. Such new mutant viruses can be more or less infective, more or less deadly, and more or less able to jump the species or even genus barrier. An emerging viral zoonosis can occur when a human being is exposed to one of these new viruses (either from the origin species or another species intermediate) that is capable of also infecting a human cell. Such exposure can occur from close proximity to animal waste or body fluids, as in the farm environment, or from wildlife pets or the capturing and slaughtering of wildlife for food, as is proposed in the case of the Wuhan seafood market scenario. In fact, the scientists who postulated a snake intermediary as the potential mixing vessel also stated that 2019‐nCoV appears to be a recombinant virus between a bat coronavirus and an origin‐unknown coronavirus.
Coronaviruses in particular have a history of moving from animal to human hosts (and even back again), and their detailed genetic pattern and taxonomy can reveal the animal origin of these diseases.
Going batty
Bats, in particular, have been shown to be a reservoir species for both alphacoronaviruses and betacoronaviruses. Given their ecology and behavior, they have been found to play a key role in transmitting coronaviruses between species. A highly pertinent example of this is the SARS coronavirus, which was shown to have likely originated in Chinese horseshoe bats. The SARS virus, which is genetically closely related to the new Wuhan coronavirus, first infected humans in the Guangdong province of southern China in 2002.
Scientists speculate that the virus was then either transmitted directly to humans from bats, or passed through an intermediate host species, with SARS-like viruses isolated from Himalayan palm civets found in a live-animal market in Guangdong. The virus infection was also detected in other animals (including a raccoon dog, Nyctereutes procyonoides) and in humans working at the market.
The MERS coronavirus is a betacoronavirus that was first reported in Saudi Arabia in 2012. It turned out to be far more deadly than either SARS or the Wuhan virus (at least as far as current estimates of the new coronavirus’s behavior). The MERS genotype was found to be closely related to MERS-like viruses in bats in Saudi Arabia, Africa, Europe, and Asia. Studies done on the cell receptor for MERS showed an apparently conserved viral receptor in both bats and humans. And an identical strain of MERS was found in bats in a nearby cave and near the workplace of the first known human patient.
However, in many of the other locations of the outbreak in the Middle East, there appeared to be limited contact between bats and humans, so scientists looked for another vector species, perhaps one that was acting as an intermediate. A high seroprevalence of MERS-CoV or a closely related virus was found in camels across the Arabian Peninsula and parts of eastern and northern Africa, while tests for MERS antibodies were negative in the most-likely other species of livestock or pet animals, including chickens, cows, goats, horses, and sheep.
In addition, the MERS-related CoV carried by camels was genetically highly similar to that detected in humans, as demonstrated in one particular outbreak on a farm in Qatar where the genetic sequences of MERS-CoV in the nasal swabs from 3 of 14 seropositive camels were similar to those of 2 human cases on the same farm. Similar genomic results were found in MERS-CoV from nasal swabs from camels in Saudi Arabia.
Other mixing-vessel zoonoses
HIV, the viral cause of AIDS, provides an almost-textbook origin story of the rise of a zoonotic supervillain. The virus was genetically traced to have a chimpanzee-to-human origin, but it was found to be more complicated than that. The virus first emerged in the 1920s in Africa in what is now the Democratic Republic of the Congo, well before its rise to a global pandemic in the 1980s.
Researchers believe the chimpanzee virus is a hybrid of the simian immunodeficiency viruses (SIVs) naturally infecting two different monkey species: the red-capped mangabey (Cercocebus torquatus) and the greater spot-nosed monkey (Cercopithecus nictitans). Chimpanzees kill and eat monkeys, which is likely how they acquired the monkey viruses. The viruses hybridized in a chimpanzee; the hybrid virus then spread through the chimpanzee population and was later transmitted to humans who captured and slaughtered chimps for meat (becoming exposed to their blood). This was the most likely origin of HIV-1.
HIV-1 also shows one of the major risks of zoonotic infections. They can continue to mutate in its human host, increasing the risk of greater virulence, but also interfering with the production of a universally effective vaccine. Since its transmission to humans, for example, many subtypes of the HIV-1 strain have developed, with genetic differences even in the same subtypes found to be up to 20%.
Ebolavirus, first detected in 1976, is another case of bats being the potential culprit. Genetic analysis has shown that African fruit bats are likely involved in the spread of the virus and may be its reservoir host. Further evidence of this was found in the most recent human-infecting Bombali variant of the virus, which was identified in samples from bats collected from Sierra Leone.
It was also found that pigs can also become infected with Zaire ebolavirus, leading to the fear that pigs could serve as a mixing vessel for it and other filoviruses. Pigs have their own forms of Ebola-like disease viruses, which are not currently transmissible to humans, but could provide a potential mixing-vessel reservoir.
Emergent influenzas
The Western world has been most affected by these highly mutable, multispecies zoonotic viruses. The 1957 and 1968 flu pandemics contained a mixture of gene segments from human and avian influenza viruses. “What is clear from genetic analysis of the viruses that caused these past pandemics is that reassortment (gene swapping) occurred to produce novel influenza viruses that caused the pandemics. In both of these cases, the new viruses that emerged showed major differences from the parent viruses,” according to the Centers for Disease Control and Prevention.
Influenza is, however, a good example that all zoonoses are not the result of a mixing-vessel phenomenon, with evidence showing that the origin of the catastrophic 1918 virus pandemic likely resulted from a bird influenza virus directly infecting humans and pigs at about the same time without reassortment, according to the CDC.
Building a protective infrastructure
The first 2 decades of the 21st century saw a huge increase in efforts to develop an infrastructure to monitor and potentially prevent the spread of new zoonoses. As part of a global effort led by the United Nations, the U.S. Agency for International AID developed the PREDICT program in 2009 “to strengthen global capacity for detection and discovery of zoonotic viruses with pandemic potential. Those include coronaviruses, the family to which SARS and MERS belong; paramyxoviruses, like Nipah virus; influenza viruses; and filoviruses, like the ebolavirus.”
PREDICT funding to the EcoHealth Alliance led to discovery of the likely bat origins of the Zaire ebolavirus during the 2013-2016 outbreak. And throughout the existence of PREDICT, more than 145,000 animals and people were surveyed in areas of likely zoonotic outbreaks, leading to the detection of more than “1,100 unique viruses, including zoonotic diseases of public health concern such as Bombali ebolavirus, Zaire ebolavirus, Marburg virus, and MERS- and SARS-like coronaviruses,” according to PREDICT partner, the University of California, Davis.
PREDICT-2 was launched in 2014 with the continuing goals of “identifying and better characterizing pathogens of known epidemic and unknown pandemic potential; recognizing animal reservoirs and amplification hosts of human-infectious viruses; and efficiently targeting intervention action at human behaviors which amplify disease transmission at critical animal-animal and animal-human interfaces in hotspots of viral evolution, spillover, amplification, and spread.”
However, in October 2019, the Trump administration cut all funding to the PREDICT program, leading to its shutdown. In a New York Times interview, Peter Daszak, president of the EcoHealth Alliance, stated: “PREDICT was an approach to heading off pandemics, instead of sitting there waiting for them to emerge and then mobilizing.”
Ultimately, in addition to its human cost, the current Wuhan coronavirus outbreak can be looked at an object lesson – a test of the pandemic surveillance and control systems currently in place, and a practice run for the next and potentially deadlier zoonotic outbreaks to come. Perhaps it is also a reminder that cutting resources to detect zoonoses at their source in their animal hosts – before they enter the human chain– is perhaps not the most prudent of ideas.
Mark Lesney is the managing editor of MDedge.com/IDPractioner. He has a PhD in plant virology and a PhD in the history of science, with a focus on the history of biotechnology and medicine. He has served as an adjunct assistant professor of the department of biochemistry and molecular & celluar biology at Georgetown University, Washington.
Emerging viruses that spread to humans from an animal host are commonplace and represent some of the deadliest diseases known. Given the details of the Wuhan coronavirus (2019-nCoV) outbreak, including the genetic profile of the disease agent, the hypothesis of a snake origin was the first raised in the peer-reviewed literature.
It is a highly controversial origin story, however, given that mammals have been the sources of all other such zoonotic coronaviruses, as well as a host of other zoonotic diseases.
An animal source for emerging infections such as the 2019-nCoV is the default hypothesis, because “around 60% of all infectious diseases in humans are zoonotic, as are 75% of all emerging infectious diseases,” according to a United Nations report. The report goes on to say that, “on average, one new infectious disease emerges in humans every 4 months.”
To appreciate the emergence and nature of 2019-nCoV, it is important to examine the history of zoonotic outbreaks of other such diseases, especially with regard to the “mixing-vessel” phenomenon, which has been noted in closely related coronaviruses, including SARS and MERS, as well as the widely disparate HIV, Ebola, and influenza viruses.
Mutants in the mixing vessel
The mixing-vessel phenomenon is conceptually easy but molecularly complex. A single animal is coinfected with two related viruses; the virus genomes recombine together (virus “sex”) in that animal to form a new variant of virus. Such new mutant viruses can be more or less infective, more or less deadly, and more or less able to jump the species or even genus barrier. An emerging viral zoonosis can occur when a human being is exposed to one of these new viruses (either from the origin species or another species intermediate) that is capable of also infecting a human cell. Such exposure can occur from close proximity to animal waste or body fluids, as in the farm environment, or from wildlife pets or the capturing and slaughtering of wildlife for food, as is proposed in the case of the Wuhan seafood market scenario. In fact, the scientists who postulated a snake intermediary as the potential mixing vessel also stated that 2019‐nCoV appears to be a recombinant virus between a bat coronavirus and an origin‐unknown coronavirus.
Coronaviruses in particular have a history of moving from animal to human hosts (and even back again), and their detailed genetic pattern and taxonomy can reveal the animal origin of these diseases.
Going batty
Bats, in particular, have been shown to be a reservoir species for both alphacoronaviruses and betacoronaviruses. Given their ecology and behavior, they have been found to play a key role in transmitting coronaviruses between species. A highly pertinent example of this is the SARS coronavirus, which was shown to have likely originated in Chinese horseshoe bats. The SARS virus, which is genetically closely related to the new Wuhan coronavirus, first infected humans in the Guangdong province of southern China in 2002.
Scientists speculate that the virus was then either transmitted directly to humans from bats, or passed through an intermediate host species, with SARS-like viruses isolated from Himalayan palm civets found in a live-animal market in Guangdong. The virus infection was also detected in other animals (including a raccoon dog, Nyctereutes procyonoides) and in humans working at the market.
The MERS coronavirus is a betacoronavirus that was first reported in Saudi Arabia in 2012. It turned out to be far more deadly than either SARS or the Wuhan virus (at least as far as current estimates of the new coronavirus’s behavior). The MERS genotype was found to be closely related to MERS-like viruses in bats in Saudi Arabia, Africa, Europe, and Asia. Studies done on the cell receptor for MERS showed an apparently conserved viral receptor in both bats and humans. And an identical strain of MERS was found in bats in a nearby cave and near the workplace of the first known human patient.
However, in many of the other locations of the outbreak in the Middle East, there appeared to be limited contact between bats and humans, so scientists looked for another vector species, perhaps one that was acting as an intermediate. A high seroprevalence of MERS-CoV or a closely related virus was found in camels across the Arabian Peninsula and parts of eastern and northern Africa, while tests for MERS antibodies were negative in the most-likely other species of livestock or pet animals, including chickens, cows, goats, horses, and sheep.
In addition, the MERS-related CoV carried by camels was genetically highly similar to that detected in humans, as demonstrated in one particular outbreak on a farm in Qatar where the genetic sequences of MERS-CoV in the nasal swabs from 3 of 14 seropositive camels were similar to those of 2 human cases on the same farm. Similar genomic results were found in MERS-CoV from nasal swabs from camels in Saudi Arabia.
Other mixing-vessel zoonoses
HIV, the viral cause of AIDS, provides an almost-textbook origin story of the rise of a zoonotic supervillain. The virus was genetically traced to have a chimpanzee-to-human origin, but it was found to be more complicated than that. The virus first emerged in the 1920s in Africa in what is now the Democratic Republic of the Congo, well before its rise to a global pandemic in the 1980s.
Researchers believe the chimpanzee virus is a hybrid of the simian immunodeficiency viruses (SIVs) naturally infecting two different monkey species: the red-capped mangabey (Cercocebus torquatus) and the greater spot-nosed monkey (Cercopithecus nictitans). Chimpanzees kill and eat monkeys, which is likely how they acquired the monkey viruses. The viruses hybridized in a chimpanzee; the hybrid virus then spread through the chimpanzee population and was later transmitted to humans who captured and slaughtered chimps for meat (becoming exposed to their blood). This was the most likely origin of HIV-1.
HIV-1 also shows one of the major risks of zoonotic infections. They can continue to mutate in its human host, increasing the risk of greater virulence, but also interfering with the production of a universally effective vaccine. Since its transmission to humans, for example, many subtypes of the HIV-1 strain have developed, with genetic differences even in the same subtypes found to be up to 20%.
Ebolavirus, first detected in 1976, is another case of bats being the potential culprit. Genetic analysis has shown that African fruit bats are likely involved in the spread of the virus and may be its reservoir host. Further evidence of this was found in the most recent human-infecting Bombali variant of the virus, which was identified in samples from bats collected from Sierra Leone.
It was also found that pigs can also become infected with Zaire ebolavirus, leading to the fear that pigs could serve as a mixing vessel for it and other filoviruses. Pigs have their own forms of Ebola-like disease viruses, which are not currently transmissible to humans, but could provide a potential mixing-vessel reservoir.
Emergent influenzas
The Western world has been most affected by these highly mutable, multispecies zoonotic viruses. The 1957 and 1968 flu pandemics contained a mixture of gene segments from human and avian influenza viruses. “What is clear from genetic analysis of the viruses that caused these past pandemics is that reassortment (gene swapping) occurred to produce novel influenza viruses that caused the pandemics. In both of these cases, the new viruses that emerged showed major differences from the parent viruses,” according to the Centers for Disease Control and Prevention.
Influenza is, however, a good example that all zoonoses are not the result of a mixing-vessel phenomenon, with evidence showing that the origin of the catastrophic 1918 virus pandemic likely resulted from a bird influenza virus directly infecting humans and pigs at about the same time without reassortment, according to the CDC.
Building a protective infrastructure
The first 2 decades of the 21st century saw a huge increase in efforts to develop an infrastructure to monitor and potentially prevent the spread of new zoonoses. As part of a global effort led by the United Nations, the U.S. Agency for International AID developed the PREDICT program in 2009 “to strengthen global capacity for detection and discovery of zoonotic viruses with pandemic potential. Those include coronaviruses, the family to which SARS and MERS belong; paramyxoviruses, like Nipah virus; influenza viruses; and filoviruses, like the ebolavirus.”
PREDICT funding to the EcoHealth Alliance led to discovery of the likely bat origins of the Zaire ebolavirus during the 2013-2016 outbreak. And throughout the existence of PREDICT, more than 145,000 animals and people were surveyed in areas of likely zoonotic outbreaks, leading to the detection of more than “1,100 unique viruses, including zoonotic diseases of public health concern such as Bombali ebolavirus, Zaire ebolavirus, Marburg virus, and MERS- and SARS-like coronaviruses,” according to PREDICT partner, the University of California, Davis.
PREDICT-2 was launched in 2014 with the continuing goals of “identifying and better characterizing pathogens of known epidemic and unknown pandemic potential; recognizing animal reservoirs and amplification hosts of human-infectious viruses; and efficiently targeting intervention action at human behaviors which amplify disease transmission at critical animal-animal and animal-human interfaces in hotspots of viral evolution, spillover, amplification, and spread.”
However, in October 2019, the Trump administration cut all funding to the PREDICT program, leading to its shutdown. In a New York Times interview, Peter Daszak, president of the EcoHealth Alliance, stated: “PREDICT was an approach to heading off pandemics, instead of sitting there waiting for them to emerge and then mobilizing.”
Ultimately, in addition to its human cost, the current Wuhan coronavirus outbreak can be looked at an object lesson – a test of the pandemic surveillance and control systems currently in place, and a practice run for the next and potentially deadlier zoonotic outbreaks to come. Perhaps it is also a reminder that cutting resources to detect zoonoses at their source in their animal hosts – before they enter the human chain– is perhaps not the most prudent of ideas.
Mark Lesney is the managing editor of MDedge.com/IDPractioner. He has a PhD in plant virology and a PhD in the history of science, with a focus on the history of biotechnology and medicine. He has served as an adjunct assistant professor of the department of biochemistry and molecular & celluar biology at Georgetown University, Washington.
Emerging viruses that spread to humans from an animal host are commonplace and represent some of the deadliest diseases known. Given the details of the Wuhan coronavirus (2019-nCoV) outbreak, including the genetic profile of the disease agent, the hypothesis of a snake origin was the first raised in the peer-reviewed literature.
It is a highly controversial origin story, however, given that mammals have been the sources of all other such zoonotic coronaviruses, as well as a host of other zoonotic diseases.
An animal source for emerging infections such as the 2019-nCoV is the default hypothesis, because “around 60% of all infectious diseases in humans are zoonotic, as are 75% of all emerging infectious diseases,” according to a United Nations report. The report goes on to say that, “on average, one new infectious disease emerges in humans every 4 months.”
To appreciate the emergence and nature of 2019-nCoV, it is important to examine the history of zoonotic outbreaks of other such diseases, especially with regard to the “mixing-vessel” phenomenon, which has been noted in closely related coronaviruses, including SARS and MERS, as well as the widely disparate HIV, Ebola, and influenza viruses.
Mutants in the mixing vessel
The mixing-vessel phenomenon is conceptually easy but molecularly complex. A single animal is coinfected with two related viruses; the virus genomes recombine together (virus “sex”) in that animal to form a new variant of virus. Such new mutant viruses can be more or less infective, more or less deadly, and more or less able to jump the species or even genus barrier. An emerging viral zoonosis can occur when a human being is exposed to one of these new viruses (either from the origin species or another species intermediate) that is capable of also infecting a human cell. Such exposure can occur from close proximity to animal waste or body fluids, as in the farm environment, or from wildlife pets or the capturing and slaughtering of wildlife for food, as is proposed in the case of the Wuhan seafood market scenario. In fact, the scientists who postulated a snake intermediary as the potential mixing vessel also stated that 2019‐nCoV appears to be a recombinant virus between a bat coronavirus and an origin‐unknown coronavirus.
Coronaviruses in particular have a history of moving from animal to human hosts (and even back again), and their detailed genetic pattern and taxonomy can reveal the animal origin of these diseases.
Going batty
Bats, in particular, have been shown to be a reservoir species for both alphacoronaviruses and betacoronaviruses. Given their ecology and behavior, they have been found to play a key role in transmitting coronaviruses between species. A highly pertinent example of this is the SARS coronavirus, which was shown to have likely originated in Chinese horseshoe bats. The SARS virus, which is genetically closely related to the new Wuhan coronavirus, first infected humans in the Guangdong province of southern China in 2002.
Scientists speculate that the virus was then either transmitted directly to humans from bats, or passed through an intermediate host species, with SARS-like viruses isolated from Himalayan palm civets found in a live-animal market in Guangdong. The virus infection was also detected in other animals (including a raccoon dog, Nyctereutes procyonoides) and in humans working at the market.
The MERS coronavirus is a betacoronavirus that was first reported in Saudi Arabia in 2012. It turned out to be far more deadly than either SARS or the Wuhan virus (at least as far as current estimates of the new coronavirus’s behavior). The MERS genotype was found to be closely related to MERS-like viruses in bats in Saudi Arabia, Africa, Europe, and Asia. Studies done on the cell receptor for MERS showed an apparently conserved viral receptor in both bats and humans. And an identical strain of MERS was found in bats in a nearby cave and near the workplace of the first known human patient.
However, in many of the other locations of the outbreak in the Middle East, there appeared to be limited contact between bats and humans, so scientists looked for another vector species, perhaps one that was acting as an intermediate. A high seroprevalence of MERS-CoV or a closely related virus was found in camels across the Arabian Peninsula and parts of eastern and northern Africa, while tests for MERS antibodies were negative in the most-likely other species of livestock or pet animals, including chickens, cows, goats, horses, and sheep.
In addition, the MERS-related CoV carried by camels was genetically highly similar to that detected in humans, as demonstrated in one particular outbreak on a farm in Qatar where the genetic sequences of MERS-CoV in the nasal swabs from 3 of 14 seropositive camels were similar to those of 2 human cases on the same farm. Similar genomic results were found in MERS-CoV from nasal swabs from camels in Saudi Arabia.
Other mixing-vessel zoonoses
HIV, the viral cause of AIDS, provides an almost-textbook origin story of the rise of a zoonotic supervillain. The virus was genetically traced to have a chimpanzee-to-human origin, but it was found to be more complicated than that. The virus first emerged in the 1920s in Africa in what is now the Democratic Republic of the Congo, well before its rise to a global pandemic in the 1980s.
Researchers believe the chimpanzee virus is a hybrid of the simian immunodeficiency viruses (SIVs) naturally infecting two different monkey species: the red-capped mangabey (Cercocebus torquatus) and the greater spot-nosed monkey (Cercopithecus nictitans). Chimpanzees kill and eat monkeys, which is likely how they acquired the monkey viruses. The viruses hybridized in a chimpanzee; the hybrid virus then spread through the chimpanzee population and was later transmitted to humans who captured and slaughtered chimps for meat (becoming exposed to their blood). This was the most likely origin of HIV-1.
HIV-1 also shows one of the major risks of zoonotic infections. They can continue to mutate in its human host, increasing the risk of greater virulence, but also interfering with the production of a universally effective vaccine. Since its transmission to humans, for example, many subtypes of the HIV-1 strain have developed, with genetic differences even in the same subtypes found to be up to 20%.
Ebolavirus, first detected in 1976, is another case of bats being the potential culprit. Genetic analysis has shown that African fruit bats are likely involved in the spread of the virus and may be its reservoir host. Further evidence of this was found in the most recent human-infecting Bombali variant of the virus, which was identified in samples from bats collected from Sierra Leone.
It was also found that pigs can also become infected with Zaire ebolavirus, leading to the fear that pigs could serve as a mixing vessel for it and other filoviruses. Pigs have their own forms of Ebola-like disease viruses, which are not currently transmissible to humans, but could provide a potential mixing-vessel reservoir.
Emergent influenzas
The Western world has been most affected by these highly mutable, multispecies zoonotic viruses. The 1957 and 1968 flu pandemics contained a mixture of gene segments from human and avian influenza viruses. “What is clear from genetic analysis of the viruses that caused these past pandemics is that reassortment (gene swapping) occurred to produce novel influenza viruses that caused the pandemics. In both of these cases, the new viruses that emerged showed major differences from the parent viruses,” according to the Centers for Disease Control and Prevention.
Influenza is, however, a good example that all zoonoses are not the result of a mixing-vessel phenomenon, with evidence showing that the origin of the catastrophic 1918 virus pandemic likely resulted from a bird influenza virus directly infecting humans and pigs at about the same time without reassortment, according to the CDC.
Building a protective infrastructure
The first 2 decades of the 21st century saw a huge increase in efforts to develop an infrastructure to monitor and potentially prevent the spread of new zoonoses. As part of a global effort led by the United Nations, the U.S. Agency for International AID developed the PREDICT program in 2009 “to strengthen global capacity for detection and discovery of zoonotic viruses with pandemic potential. Those include coronaviruses, the family to which SARS and MERS belong; paramyxoviruses, like Nipah virus; influenza viruses; and filoviruses, like the ebolavirus.”
PREDICT funding to the EcoHealth Alliance led to discovery of the likely bat origins of the Zaire ebolavirus during the 2013-2016 outbreak. And throughout the existence of PREDICT, more than 145,000 animals and people were surveyed in areas of likely zoonotic outbreaks, leading to the detection of more than “1,100 unique viruses, including zoonotic diseases of public health concern such as Bombali ebolavirus, Zaire ebolavirus, Marburg virus, and MERS- and SARS-like coronaviruses,” according to PREDICT partner, the University of California, Davis.
PREDICT-2 was launched in 2014 with the continuing goals of “identifying and better characterizing pathogens of known epidemic and unknown pandemic potential; recognizing animal reservoirs and amplification hosts of human-infectious viruses; and efficiently targeting intervention action at human behaviors which amplify disease transmission at critical animal-animal and animal-human interfaces in hotspots of viral evolution, spillover, amplification, and spread.”
However, in October 2019, the Trump administration cut all funding to the PREDICT program, leading to its shutdown. In a New York Times interview, Peter Daszak, president of the EcoHealth Alliance, stated: “PREDICT was an approach to heading off pandemics, instead of sitting there waiting for them to emerge and then mobilizing.”
Ultimately, in addition to its human cost, the current Wuhan coronavirus outbreak can be looked at an object lesson – a test of the pandemic surveillance and control systems currently in place, and a practice run for the next and potentially deadlier zoonotic outbreaks to come. Perhaps it is also a reminder that cutting resources to detect zoonoses at their source in their animal hosts – before they enter the human chain– is perhaps not the most prudent of ideas.
Mark Lesney is the managing editor of MDedge.com/IDPractioner. He has a PhD in plant virology and a PhD in the history of science, with a focus on the history of biotechnology and medicine. He has served as an adjunct assistant professor of the department of biochemistry and molecular & celluar biology at Georgetown University, Washington.
Journal editors seek more complete disclosure from authors
A group of leading medical journal editors is seeking to improve the completeness and transparency of financial disclosure reporting with a proposed new disclosure form that puts more onus on readers to decide whether relationships and activities should influence how they view published papers.
The proposed changes are described in an editorial published simultaneously today in the Annals of Internal Medicine, British Medical Journal, Journal of the American Medical Association, The Lancet, New England Journal of Medicine, and several other journals whose editors are members of the International Committee of Medical Journal Editors (ICMJE).
“While no approach to disclosure will be perfect or foolproof, we hope the changes we propose will help promote transparency and trust,” the editorial stated (Ann Intern Med. 2020 Jan 27. doi: 10.7326/M19-3933).
The ICMJE adopted its currently used electronic form – the “ICMJE Form for the Disclosure of Potential Conflicts of Interest” – 10 years ago in an effort to create some uniformity amidst a patchwork of differing disclosure requirements for authors.
It’s not known how many journals outside of the ICMJE’s member journals routinely use the disclosure form, but the organization’s website houses an extensive list of journals whose editors or publishers have requested to be listed as following the ICMJE’s recommendations for editing, reporting, and publishing, including those concerning disclosures. The ICMJE does not “certify” journals. The full set of recommendations was updated in December 2019.
Most authors are committed to transparent reporting, but “opinions differ over which relationships or activities to report,” the editorial stated.
An author might choose to omit an item that others deem important because of a difference in opinion regarding “relevance,” confusion over definitions, or a simple oversight. Some authors may be “concerned that readers will interpret the listing of any item as a ‘potential conflict of interest’ as indicative of problematic influence and wrongdoing,” the editorial stated.
The revised form, like the current one, asks authors to disclose relationships and activities that are directly related to the reported work, as well as those that are topically related (within the broadly defined field addressed in the work). But unlike the current form, the new version provides a checklist of relationships and activities and asks authors to check ‘yes’ or ‘no’ for each one (and to name them when the answer is ‘yes’).
Items in the checklist include grants, payments/honoraria for lectures, patents issued or planned, stock/stock options, and leadership or fiduciary roles in committees, boards, or societies.
The proposed new form makes no mention of “potential conflicts of interest” or “relevancy,” per say. Authors aren’t asked to determine what might be interpreted as a potential conflict of interest, but instead are asked for a “complete listing” of what readers may find “pertinent” to their work.
“We’re trying to move away from calling everything a [potential] ‘conflict,’ ” Darren B. Taichman, MD, PhD, secretary of ICMJE and executive editor of the Annals of Internal Medicine, said in an interview. “We want to remove for authors the concern or stigma, if you will, that anything listed on a form implies that there is something wrong, because that’s just not true. … We want readers to decide what relationships are important as they interpret the work.”
Dr. Taichman said in the interview that the ICMJE’s updating of the form was more a function of “good housekeeping” and continuous appreciation of disclosure as an important issue, rather than any one specific issue, such as concern over a “relevancy” approach to disclosures.
The ICMJE is seeking feedback about its proposed form, which is available with a link for providing comments, at www.icmje.org.
Broader national efforts
Editors and others have been increasingly moving, however, toward asking for more complete disclosures where authors aren’t asked to judge “relevancy” and where readers can make decisions on their own. The American Society of Clinical Oncology, which produces the Journal of Clinical Oncology (JCO) as well as practice guidelines and continuing medical education programs, moved about 5 years ago to a system of general disclosure that asks physicians and others to disclose all financial interests and industry relationships, with no qualifiers.
Earlier in January 2020, the Accreditation Council for Continuing Medical Education issued proposed revisions to its Standards for Integrity and Independence in Accredited Continuing Education. These revisions, which are open for comment, require CME providers to collect disclosure information about all financial relationships of speakers and presenters. It’s up to the CME provider to then determine which relationships are relevant, according to the proposed document.
More change is on the way, as disclosure issues are being deliberated nationally in the wake of a highly publicized disclosure failure at Memorial Sloan Kettering Cancer Center in 2018. Chief medical officer José Baselga, MD, PhD, failed to report millions of dollars of industry payments and ownership interests in journal articles he wrote or cowrote over several years.
In February 2019, leaders from journals, academia, medical societies, and other institutions gathered in Washington for a closed-door meeting to hash out various disclosure related issues.
Hosted by the Association of American Medical Colleges and cosponsored by Memorial Sloan Kettering Cancer Center, ASCO, JAMA, and the Council of Medical Specialty Societies, the meeting led to a series of working groups that are creating additional recommendations “due out soon in 2020,” Heather Pierce, senior director of science policy and regulatory counsel for the AAMC, said in an interview.
Among the questions being discussed: What disclosures should be verified and who should do so? How can disclosures be made more complete and easier for researchers? And, “most importantly,” said Ms. Pierce, how can policy requirements across each of these sectors be aligned so that there’s more coordination and oversight – and with it, public trust?
Some critics of current disclosure policies have called for more reporting of compensation amounts, and Ms. Pierce said that this has been part of cross-sector discussions.
The ICMJE’s proposed form invites, but does not require, authors to indicate what payments were made to them or their institutions. “Part of this is due to the fact that it’s hard to define, let alone agree on, what’s an important amount,” Dr. Taichman said.
A push for registries
The ICMJE is also aiming to make the disclosure process more efficient for authors – and to eliminate inconsistent and incomplete disclosures – by accepting disclosures from web-based repositories, according to the editorial. Repositories allow authors to maintain an inventory of their relationships and activities and then create electronic disclosures that are tailored to the requirements of the ICMJE, medical societies, and other entities.
The AAMC-run repository, called Convey, is consistent with ICMJE reporting requirements and other criteria (e.g., there are no fees for individuals to enter, store, or export their data), but the development of other repositories may be helpful “for meeting regional, linguistic, and regulatory needs” of authors across the world, the editorial stated.
The Annals of Internal Medicine and the New England Journal of Medicine are both currently collecting disclosures through Convey. The platform was born from discussions that followed a 2009 Institute of Medicine report on conflicts of interest.
Signers of the ICMJE editorial include representatives of the National Library of Medicine and the World Association of Medical Editors, in addition to editors in chief and other leaders of the ICMJE member journals.
A group of leading medical journal editors is seeking to improve the completeness and transparency of financial disclosure reporting with a proposed new disclosure form that puts more onus on readers to decide whether relationships and activities should influence how they view published papers.
The proposed changes are described in an editorial published simultaneously today in the Annals of Internal Medicine, British Medical Journal, Journal of the American Medical Association, The Lancet, New England Journal of Medicine, and several other journals whose editors are members of the International Committee of Medical Journal Editors (ICMJE).
“While no approach to disclosure will be perfect or foolproof, we hope the changes we propose will help promote transparency and trust,” the editorial stated (Ann Intern Med. 2020 Jan 27. doi: 10.7326/M19-3933).
The ICMJE adopted its currently used electronic form – the “ICMJE Form for the Disclosure of Potential Conflicts of Interest” – 10 years ago in an effort to create some uniformity amidst a patchwork of differing disclosure requirements for authors.
It’s not known how many journals outside of the ICMJE’s member journals routinely use the disclosure form, but the organization’s website houses an extensive list of journals whose editors or publishers have requested to be listed as following the ICMJE’s recommendations for editing, reporting, and publishing, including those concerning disclosures. The ICMJE does not “certify” journals. The full set of recommendations was updated in December 2019.
Most authors are committed to transparent reporting, but “opinions differ over which relationships or activities to report,” the editorial stated.
An author might choose to omit an item that others deem important because of a difference in opinion regarding “relevance,” confusion over definitions, or a simple oversight. Some authors may be “concerned that readers will interpret the listing of any item as a ‘potential conflict of interest’ as indicative of problematic influence and wrongdoing,” the editorial stated.
The revised form, like the current one, asks authors to disclose relationships and activities that are directly related to the reported work, as well as those that are topically related (within the broadly defined field addressed in the work). But unlike the current form, the new version provides a checklist of relationships and activities and asks authors to check ‘yes’ or ‘no’ for each one (and to name them when the answer is ‘yes’).
Items in the checklist include grants, payments/honoraria for lectures, patents issued or planned, stock/stock options, and leadership or fiduciary roles in committees, boards, or societies.
The proposed new form makes no mention of “potential conflicts of interest” or “relevancy,” per say. Authors aren’t asked to determine what might be interpreted as a potential conflict of interest, but instead are asked for a “complete listing” of what readers may find “pertinent” to their work.
“We’re trying to move away from calling everything a [potential] ‘conflict,’ ” Darren B. Taichman, MD, PhD, secretary of ICMJE and executive editor of the Annals of Internal Medicine, said in an interview. “We want to remove for authors the concern or stigma, if you will, that anything listed on a form implies that there is something wrong, because that’s just not true. … We want readers to decide what relationships are important as they interpret the work.”
Dr. Taichman said in the interview that the ICMJE’s updating of the form was more a function of “good housekeeping” and continuous appreciation of disclosure as an important issue, rather than any one specific issue, such as concern over a “relevancy” approach to disclosures.
The ICMJE is seeking feedback about its proposed form, which is available with a link for providing comments, at www.icmje.org.
Broader national efforts
Editors and others have been increasingly moving, however, toward asking for more complete disclosures where authors aren’t asked to judge “relevancy” and where readers can make decisions on their own. The American Society of Clinical Oncology, which produces the Journal of Clinical Oncology (JCO) as well as practice guidelines and continuing medical education programs, moved about 5 years ago to a system of general disclosure that asks physicians and others to disclose all financial interests and industry relationships, with no qualifiers.
Earlier in January 2020, the Accreditation Council for Continuing Medical Education issued proposed revisions to its Standards for Integrity and Independence in Accredited Continuing Education. These revisions, which are open for comment, require CME providers to collect disclosure information about all financial relationships of speakers and presenters. It’s up to the CME provider to then determine which relationships are relevant, according to the proposed document.
More change is on the way, as disclosure issues are being deliberated nationally in the wake of a highly publicized disclosure failure at Memorial Sloan Kettering Cancer Center in 2018. Chief medical officer José Baselga, MD, PhD, failed to report millions of dollars of industry payments and ownership interests in journal articles he wrote or cowrote over several years.
In February 2019, leaders from journals, academia, medical societies, and other institutions gathered in Washington for a closed-door meeting to hash out various disclosure related issues.
Hosted by the Association of American Medical Colleges and cosponsored by Memorial Sloan Kettering Cancer Center, ASCO, JAMA, and the Council of Medical Specialty Societies, the meeting led to a series of working groups that are creating additional recommendations “due out soon in 2020,” Heather Pierce, senior director of science policy and regulatory counsel for the AAMC, said in an interview.
Among the questions being discussed: What disclosures should be verified and who should do so? How can disclosures be made more complete and easier for researchers? And, “most importantly,” said Ms. Pierce, how can policy requirements across each of these sectors be aligned so that there’s more coordination and oversight – and with it, public trust?
Some critics of current disclosure policies have called for more reporting of compensation amounts, and Ms. Pierce said that this has been part of cross-sector discussions.
The ICMJE’s proposed form invites, but does not require, authors to indicate what payments were made to them or their institutions. “Part of this is due to the fact that it’s hard to define, let alone agree on, what’s an important amount,” Dr. Taichman said.
A push for registries
The ICMJE is also aiming to make the disclosure process more efficient for authors – and to eliminate inconsistent and incomplete disclosures – by accepting disclosures from web-based repositories, according to the editorial. Repositories allow authors to maintain an inventory of their relationships and activities and then create electronic disclosures that are tailored to the requirements of the ICMJE, medical societies, and other entities.
The AAMC-run repository, called Convey, is consistent with ICMJE reporting requirements and other criteria (e.g., there are no fees for individuals to enter, store, or export their data), but the development of other repositories may be helpful “for meeting regional, linguistic, and regulatory needs” of authors across the world, the editorial stated.
The Annals of Internal Medicine and the New England Journal of Medicine are both currently collecting disclosures through Convey. The platform was born from discussions that followed a 2009 Institute of Medicine report on conflicts of interest.
Signers of the ICMJE editorial include representatives of the National Library of Medicine and the World Association of Medical Editors, in addition to editors in chief and other leaders of the ICMJE member journals.
A group of leading medical journal editors is seeking to improve the completeness and transparency of financial disclosure reporting with a proposed new disclosure form that puts more onus on readers to decide whether relationships and activities should influence how they view published papers.
The proposed changes are described in an editorial published simultaneously today in the Annals of Internal Medicine, British Medical Journal, Journal of the American Medical Association, The Lancet, New England Journal of Medicine, and several other journals whose editors are members of the International Committee of Medical Journal Editors (ICMJE).
“While no approach to disclosure will be perfect or foolproof, we hope the changes we propose will help promote transparency and trust,” the editorial stated (Ann Intern Med. 2020 Jan 27. doi: 10.7326/M19-3933).
The ICMJE adopted its currently used electronic form – the “ICMJE Form for the Disclosure of Potential Conflicts of Interest” – 10 years ago in an effort to create some uniformity amidst a patchwork of differing disclosure requirements for authors.
It’s not known how many journals outside of the ICMJE’s member journals routinely use the disclosure form, but the organization’s website houses an extensive list of journals whose editors or publishers have requested to be listed as following the ICMJE’s recommendations for editing, reporting, and publishing, including those concerning disclosures. The ICMJE does not “certify” journals. The full set of recommendations was updated in December 2019.
Most authors are committed to transparent reporting, but “opinions differ over which relationships or activities to report,” the editorial stated.
An author might choose to omit an item that others deem important because of a difference in opinion regarding “relevance,” confusion over definitions, or a simple oversight. Some authors may be “concerned that readers will interpret the listing of any item as a ‘potential conflict of interest’ as indicative of problematic influence and wrongdoing,” the editorial stated.
The revised form, like the current one, asks authors to disclose relationships and activities that are directly related to the reported work, as well as those that are topically related (within the broadly defined field addressed in the work). But unlike the current form, the new version provides a checklist of relationships and activities and asks authors to check ‘yes’ or ‘no’ for each one (and to name them when the answer is ‘yes’).
Items in the checklist include grants, payments/honoraria for lectures, patents issued or planned, stock/stock options, and leadership or fiduciary roles in committees, boards, or societies.
The proposed new form makes no mention of “potential conflicts of interest” or “relevancy,” per say. Authors aren’t asked to determine what might be interpreted as a potential conflict of interest, but instead are asked for a “complete listing” of what readers may find “pertinent” to their work.
“We’re trying to move away from calling everything a [potential] ‘conflict,’ ” Darren B. Taichman, MD, PhD, secretary of ICMJE and executive editor of the Annals of Internal Medicine, said in an interview. “We want to remove for authors the concern or stigma, if you will, that anything listed on a form implies that there is something wrong, because that’s just not true. … We want readers to decide what relationships are important as they interpret the work.”
Dr. Taichman said in the interview that the ICMJE’s updating of the form was more a function of “good housekeeping” and continuous appreciation of disclosure as an important issue, rather than any one specific issue, such as concern over a “relevancy” approach to disclosures.
The ICMJE is seeking feedback about its proposed form, which is available with a link for providing comments, at www.icmje.org.
Broader national efforts
Editors and others have been increasingly moving, however, toward asking for more complete disclosures where authors aren’t asked to judge “relevancy” and where readers can make decisions on their own. The American Society of Clinical Oncology, which produces the Journal of Clinical Oncology (JCO) as well as practice guidelines and continuing medical education programs, moved about 5 years ago to a system of general disclosure that asks physicians and others to disclose all financial interests and industry relationships, with no qualifiers.
Earlier in January 2020, the Accreditation Council for Continuing Medical Education issued proposed revisions to its Standards for Integrity and Independence in Accredited Continuing Education. These revisions, which are open for comment, require CME providers to collect disclosure information about all financial relationships of speakers and presenters. It’s up to the CME provider to then determine which relationships are relevant, according to the proposed document.
More change is on the way, as disclosure issues are being deliberated nationally in the wake of a highly publicized disclosure failure at Memorial Sloan Kettering Cancer Center in 2018. Chief medical officer José Baselga, MD, PhD, failed to report millions of dollars of industry payments and ownership interests in journal articles he wrote or cowrote over several years.
In February 2019, leaders from journals, academia, medical societies, and other institutions gathered in Washington for a closed-door meeting to hash out various disclosure related issues.
Hosted by the Association of American Medical Colleges and cosponsored by Memorial Sloan Kettering Cancer Center, ASCO, JAMA, and the Council of Medical Specialty Societies, the meeting led to a series of working groups that are creating additional recommendations “due out soon in 2020,” Heather Pierce, senior director of science policy and regulatory counsel for the AAMC, said in an interview.
Among the questions being discussed: What disclosures should be verified and who should do so? How can disclosures be made more complete and easier for researchers? And, “most importantly,” said Ms. Pierce, how can policy requirements across each of these sectors be aligned so that there’s more coordination and oversight – and with it, public trust?
Some critics of current disclosure policies have called for more reporting of compensation amounts, and Ms. Pierce said that this has been part of cross-sector discussions.
The ICMJE’s proposed form invites, but does not require, authors to indicate what payments were made to them or their institutions. “Part of this is due to the fact that it’s hard to define, let alone agree on, what’s an important amount,” Dr. Taichman said.
A push for registries
The ICMJE is also aiming to make the disclosure process more efficient for authors – and to eliminate inconsistent and incomplete disclosures – by accepting disclosures from web-based repositories, according to the editorial. Repositories allow authors to maintain an inventory of their relationships and activities and then create electronic disclosures that are tailored to the requirements of the ICMJE, medical societies, and other entities.
The AAMC-run repository, called Convey, is consistent with ICMJE reporting requirements and other criteria (e.g., there are no fees for individuals to enter, store, or export their data), but the development of other repositories may be helpful “for meeting regional, linguistic, and regulatory needs” of authors across the world, the editorial stated.
The Annals of Internal Medicine and the New England Journal of Medicine are both currently collecting disclosures through Convey. The platform was born from discussions that followed a 2009 Institute of Medicine report on conflicts of interest.
Signers of the ICMJE editorial include representatives of the National Library of Medicine and the World Association of Medical Editors, in addition to editors in chief and other leaders of the ICMJE member journals.
FROM ANNALS OF INTERNAL MEDICINE
Wuhan coronavirus cluster suggests human-to-human spread
A Chinese man became ill from a novel coronavirus (2019-nCoV) 4 days after arriving in Vietnam to visit his 27-year-old son. Three days later the healthy young man was also stricken, according to a report published online Jan. 28 in the New England Journal of Medicine.
“This family cluster of 2019-nCoV infection that occurred outside China arouses concern regarding human-to-human transmission,” the authors wrote.
The father, age 65 years and with multiple comorbidities including hypertension, type 2 diabetes, coronary heart disease with stent placement, and lung cancer, flew to Hanoi with his wife on January 13; they traveled from the Wuchang district in Wuhan, China, where outbreaks of 2019-nCoV have been occurring.
On Jan. 17, the older man and his wife met their adult son in Ho Chi Minh City, Vietnam, and shared a hotel room with him for 3 days. The father developed a fever that same day and the son developed a dry cough, fever, diarrhea, and vomiting on Jan. 20. Both men went to a hospital ED on Jan. 22.
The authors say the timing of the son’s symptoms suggests the incubation period may have been 3 days or fewer.
Upon admission to the hospital, the father reported that he had not visited a “wet market” where live and dead animals are sold while he was in Wuhan. Throat swabs were positive for 2019-nCoV on real-time reverse-transcription–polymerase-chain-reaction assays.
The man was placed in isolation and “treated empirically with antiviral agents, broad-spectrum antibiotics, and supportive therapies,” wrote Lan T. Phan, PhD, from the Pasteur Institute Ho Chi Minh City and coauthors.
On admission, chest radiographs revealed an infiltrate in the upper lobe of his left lung; he developed worsening dyspnea with hypoxemia on Jan. 25 and required supplemental oxygen at 5 L/min by nasal cannula. Chest radiographs showed a progressive infiltrate and consolidation. His fever resolved on that day and he has progressively improved.
The man’s son had a fever of 39° C (102.2° F) when the two men arrived at the hospital on Jan. 22; hospital staff isolated the son, and chest radiographs and other laboratory tests were normal with the exception of an increased C-reactive protein level.
The son’s throat swab was positive for 2019-nCoV and he is believed to have been exposed from his father; however, the strains have not been ascertained.
“This family had traveled to four cities across Vietnam using various forms of transportation, including planes, trains, and taxis,” the authors wrote. A total of 28 close contacts were identified, none of whom have developed respiratory symptoms. The older man’s wife has been healthy as well.
The authors have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
A Chinese man became ill from a novel coronavirus (2019-nCoV) 4 days after arriving in Vietnam to visit his 27-year-old son. Three days later the healthy young man was also stricken, according to a report published online Jan. 28 in the New England Journal of Medicine.
“This family cluster of 2019-nCoV infection that occurred outside China arouses concern regarding human-to-human transmission,” the authors wrote.
The father, age 65 years and with multiple comorbidities including hypertension, type 2 diabetes, coronary heart disease with stent placement, and lung cancer, flew to Hanoi with his wife on January 13; they traveled from the Wuchang district in Wuhan, China, where outbreaks of 2019-nCoV have been occurring.
On Jan. 17, the older man and his wife met their adult son in Ho Chi Minh City, Vietnam, and shared a hotel room with him for 3 days. The father developed a fever that same day and the son developed a dry cough, fever, diarrhea, and vomiting on Jan. 20. Both men went to a hospital ED on Jan. 22.
The authors say the timing of the son’s symptoms suggests the incubation period may have been 3 days or fewer.
Upon admission to the hospital, the father reported that he had not visited a “wet market” where live and dead animals are sold while he was in Wuhan. Throat swabs were positive for 2019-nCoV on real-time reverse-transcription–polymerase-chain-reaction assays.
The man was placed in isolation and “treated empirically with antiviral agents, broad-spectrum antibiotics, and supportive therapies,” wrote Lan T. Phan, PhD, from the Pasteur Institute Ho Chi Minh City and coauthors.
On admission, chest radiographs revealed an infiltrate in the upper lobe of his left lung; he developed worsening dyspnea with hypoxemia on Jan. 25 and required supplemental oxygen at 5 L/min by nasal cannula. Chest radiographs showed a progressive infiltrate and consolidation. His fever resolved on that day and he has progressively improved.
The man’s son had a fever of 39° C (102.2° F) when the two men arrived at the hospital on Jan. 22; hospital staff isolated the son, and chest radiographs and other laboratory tests were normal with the exception of an increased C-reactive protein level.
The son’s throat swab was positive for 2019-nCoV and he is believed to have been exposed from his father; however, the strains have not been ascertained.
“This family had traveled to four cities across Vietnam using various forms of transportation, including planes, trains, and taxis,” the authors wrote. A total of 28 close contacts were identified, none of whom have developed respiratory symptoms. The older man’s wife has been healthy as well.
The authors have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
A Chinese man became ill from a novel coronavirus (2019-nCoV) 4 days after arriving in Vietnam to visit his 27-year-old son. Three days later the healthy young man was also stricken, according to a report published online Jan. 28 in the New England Journal of Medicine.
“This family cluster of 2019-nCoV infection that occurred outside China arouses concern regarding human-to-human transmission,” the authors wrote.
The father, age 65 years and with multiple comorbidities including hypertension, type 2 diabetes, coronary heart disease with stent placement, and lung cancer, flew to Hanoi with his wife on January 13; they traveled from the Wuchang district in Wuhan, China, where outbreaks of 2019-nCoV have been occurring.
On Jan. 17, the older man and his wife met their adult son in Ho Chi Minh City, Vietnam, and shared a hotel room with him for 3 days. The father developed a fever that same day and the son developed a dry cough, fever, diarrhea, and vomiting on Jan. 20. Both men went to a hospital ED on Jan. 22.
The authors say the timing of the son’s symptoms suggests the incubation period may have been 3 days or fewer.
Upon admission to the hospital, the father reported that he had not visited a “wet market” where live and dead animals are sold while he was in Wuhan. Throat swabs were positive for 2019-nCoV on real-time reverse-transcription–polymerase-chain-reaction assays.
The man was placed in isolation and “treated empirically with antiviral agents, broad-spectrum antibiotics, and supportive therapies,” wrote Lan T. Phan, PhD, from the Pasteur Institute Ho Chi Minh City and coauthors.
On admission, chest radiographs revealed an infiltrate in the upper lobe of his left lung; he developed worsening dyspnea with hypoxemia on Jan. 25 and required supplemental oxygen at 5 L/min by nasal cannula. Chest radiographs showed a progressive infiltrate and consolidation. His fever resolved on that day and he has progressively improved.
The man’s son had a fever of 39° C (102.2° F) when the two men arrived at the hospital on Jan. 22; hospital staff isolated the son, and chest radiographs and other laboratory tests were normal with the exception of an increased C-reactive protein level.
The son’s throat swab was positive for 2019-nCoV and he is believed to have been exposed from his father; however, the strains have not been ascertained.
“This family had traveled to four cities across Vietnam using various forms of transportation, including planes, trains, and taxis,” the authors wrote. A total of 28 close contacts were identified, none of whom have developed respiratory symptoms. The older man’s wife has been healthy as well.
The authors have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Registry data reveal temporal relationship between psoriasis symptoms and PsA onset
ATLANTA – Psoriasis type and patient age at presentation among patients with psoriatic arthritis predict the timing of arthritis symptom synchronicity, according to findings from the Psoriatic Arthritis Registry of Turkey International Database.
However, in those who develop arthritis symptoms first, age at onset is not predictive of psoriatic arthritis (PsA) symptom synchronicity, Umut Kalyoncu, MD, reported at the annual meeting of the American College of Rheumatology.
Of 1,631 patients from the registry, 1,251 had psoriasis first, 71 had arthritis first, and 309 had synchronous onset, which was defined as the onset of both psoriasis and arthritis symptoms within a 12-month period. The time from skin disease to PsA was 155.6 months, –67.4 months, and 1.8 months, among the groups, respectively, and the mean age at PsA onset was similar, ranging from about 41 to 42 years in those who developed arthritis first, said Dr. Kalyoncu, of the department of rheumatology at Hacettepe University, Ankara, Turkey.
However, the mean age of PsA onset among those who developed psoriasis first was 29.4 years, compared with 46.3 years in those who developed arthritis first.
“So there is a really big difference between psoriasis beginning age,” he said.
PsA types also differed by onset symptoms: Axial involvement was more common with arthritis-first onset at 38.0%, compared with 28.8% for psoriasis first and 27.8% for synchronous onset). Oligoarthritis occurred more often with arthritis-first onset (45.1% vs. 30.7% and 29.4%, respectively), and polyarthritis occurred less often with arthritis-first onset (33.8% vs. 49.4% and 47.6%, respectively), he said.
Psoriasis type also differed among the groups: Pustular skin involvement was more common in arthritis-first patients (18.3% vs. 11.9% and 16.5% of psoriasis-first and synchronous-onset patients), scalp lesions as the initial lesion were more common in psoriasis-first patients (48.3% vs. 35.2% of arthritis-first patients and 39.8% of synchronous-onset patients), and genital involvement was present more often in arthritis-first patients (12.7% vs. 6.2% and 4.9% of psoriasis-first and synchronous-onset patients).
Early-onset (type 1) psoriasis was more common in psoriasis-first patients (74% vs. 28.1% and 51.8% of arthritis-first and synchronous-onset patients), whereas late-onset (type 2) psoriasis was more common in arthritis-first patients (71.9% vs. 26.0% and 48.2% for psoriasis-first and synchronous-onset patients).
A family history of psoriasis or PsA was more common in psoriasis-first patients (35.6% vs. 26.3% and 28.2% of arthritis-first and synchronous-onset patients), Dr. Kalyoncu said.
Treatment types did not differ between the groups.
Multiple linear regression analysis for the time elapsed from psoriasis to PsA symptom synchronicity, with all other independent variables set to baseline values, showed an overall intercept interval of 66 months, but with nail involvement, family history, or plaque psoriasis, the interval was extended by 28, 24, and 20 months, respectively. However, the presence of pustular psoriasis decreased the intercept interval by 28 months.
A temporal relationship between the onset of skin psoriasis and PsA is a well-known feature of psoriatic disease, with prior studies showing that the majority of cases involve psoriasis-first onset, Dr. Kalyoncu said, adding that heterogeneity in musculoskeletal and skin involvement is also a known feature.
However, little is known about the role of genetics, he noted.
Therefore, he and his colleagues used the Psoriatic Arthritis Registry of Turkey International Database, which was established in 2014 and now also includes data from patients in Canada and Italy, to explore the associations between disease characteristics and the temporal relationship of skin and musculoskeletal disease.
Based on the findings, age at the onset of psoriasis was the main factor that determined PsA symptom synchronicity, he said.
“We know that HLA-Cw6 is important in genetic susceptibility of psoriatic arthritis, but it is important only for early-onset arthritis, not late-onset psoriasis,” Dr. Kalyoncu said. “So our results make an indirect contribution [to the understanding of] these genetic and immunochemical differences between early-onset and late-onset psoriasis, and we need further future studies about this topic.”
Dr. Kalyoncu reported having no relevant disclosures.
SOURCE: Kalyoncu U et al. Arthritis Rheumatol. 2019;71(suppl 10), Abstract 2854.
ATLANTA – Psoriasis type and patient age at presentation among patients with psoriatic arthritis predict the timing of arthritis symptom synchronicity, according to findings from the Psoriatic Arthritis Registry of Turkey International Database.
However, in those who develop arthritis symptoms first, age at onset is not predictive of psoriatic arthritis (PsA) symptom synchronicity, Umut Kalyoncu, MD, reported at the annual meeting of the American College of Rheumatology.
Of 1,631 patients from the registry, 1,251 had psoriasis first, 71 had arthritis first, and 309 had synchronous onset, which was defined as the onset of both psoriasis and arthritis symptoms within a 12-month period. The time from skin disease to PsA was 155.6 months, –67.4 months, and 1.8 months, among the groups, respectively, and the mean age at PsA onset was similar, ranging from about 41 to 42 years in those who developed arthritis first, said Dr. Kalyoncu, of the department of rheumatology at Hacettepe University, Ankara, Turkey.
However, the mean age of PsA onset among those who developed psoriasis first was 29.4 years, compared with 46.3 years in those who developed arthritis first.
“So there is a really big difference between psoriasis beginning age,” he said.
PsA types also differed by onset symptoms: Axial involvement was more common with arthritis-first onset at 38.0%, compared with 28.8% for psoriasis first and 27.8% for synchronous onset). Oligoarthritis occurred more often with arthritis-first onset (45.1% vs. 30.7% and 29.4%, respectively), and polyarthritis occurred less often with arthritis-first onset (33.8% vs. 49.4% and 47.6%, respectively), he said.
Psoriasis type also differed among the groups: Pustular skin involvement was more common in arthritis-first patients (18.3% vs. 11.9% and 16.5% of psoriasis-first and synchronous-onset patients), scalp lesions as the initial lesion were more common in psoriasis-first patients (48.3% vs. 35.2% of arthritis-first patients and 39.8% of synchronous-onset patients), and genital involvement was present more often in arthritis-first patients (12.7% vs. 6.2% and 4.9% of psoriasis-first and synchronous-onset patients).
Early-onset (type 1) psoriasis was more common in psoriasis-first patients (74% vs. 28.1% and 51.8% of arthritis-first and synchronous-onset patients), whereas late-onset (type 2) psoriasis was more common in arthritis-first patients (71.9% vs. 26.0% and 48.2% for psoriasis-first and synchronous-onset patients).
A family history of psoriasis or PsA was more common in psoriasis-first patients (35.6% vs. 26.3% and 28.2% of arthritis-first and synchronous-onset patients), Dr. Kalyoncu said.
Treatment types did not differ between the groups.
Multiple linear regression analysis for the time elapsed from psoriasis to PsA symptom synchronicity, with all other independent variables set to baseline values, showed an overall intercept interval of 66 months, but with nail involvement, family history, or plaque psoriasis, the interval was extended by 28, 24, and 20 months, respectively. However, the presence of pustular psoriasis decreased the intercept interval by 28 months.
A temporal relationship between the onset of skin psoriasis and PsA is a well-known feature of psoriatic disease, with prior studies showing that the majority of cases involve psoriasis-first onset, Dr. Kalyoncu said, adding that heterogeneity in musculoskeletal and skin involvement is also a known feature.
However, little is known about the role of genetics, he noted.
Therefore, he and his colleagues used the Psoriatic Arthritis Registry of Turkey International Database, which was established in 2014 and now also includes data from patients in Canada and Italy, to explore the associations between disease characteristics and the temporal relationship of skin and musculoskeletal disease.
Based on the findings, age at the onset of psoriasis was the main factor that determined PsA symptom synchronicity, he said.
“We know that HLA-Cw6 is important in genetic susceptibility of psoriatic arthritis, but it is important only for early-onset arthritis, not late-onset psoriasis,” Dr. Kalyoncu said. “So our results make an indirect contribution [to the understanding of] these genetic and immunochemical differences between early-onset and late-onset psoriasis, and we need further future studies about this topic.”
Dr. Kalyoncu reported having no relevant disclosures.
SOURCE: Kalyoncu U et al. Arthritis Rheumatol. 2019;71(suppl 10), Abstract 2854.
ATLANTA – Psoriasis type and patient age at presentation among patients with psoriatic arthritis predict the timing of arthritis symptom synchronicity, according to findings from the Psoriatic Arthritis Registry of Turkey International Database.
However, in those who develop arthritis symptoms first, age at onset is not predictive of psoriatic arthritis (PsA) symptom synchronicity, Umut Kalyoncu, MD, reported at the annual meeting of the American College of Rheumatology.
Of 1,631 patients from the registry, 1,251 had psoriasis first, 71 had arthritis first, and 309 had synchronous onset, which was defined as the onset of both psoriasis and arthritis symptoms within a 12-month period. The time from skin disease to PsA was 155.6 months, –67.4 months, and 1.8 months, among the groups, respectively, and the mean age at PsA onset was similar, ranging from about 41 to 42 years in those who developed arthritis first, said Dr. Kalyoncu, of the department of rheumatology at Hacettepe University, Ankara, Turkey.
However, the mean age of PsA onset among those who developed psoriasis first was 29.4 years, compared with 46.3 years in those who developed arthritis first.
“So there is a really big difference between psoriasis beginning age,” he said.
PsA types also differed by onset symptoms: Axial involvement was more common with arthritis-first onset at 38.0%, compared with 28.8% for psoriasis first and 27.8% for synchronous onset). Oligoarthritis occurred more often with arthritis-first onset (45.1% vs. 30.7% and 29.4%, respectively), and polyarthritis occurred less often with arthritis-first onset (33.8% vs. 49.4% and 47.6%, respectively), he said.
Psoriasis type also differed among the groups: Pustular skin involvement was more common in arthritis-first patients (18.3% vs. 11.9% and 16.5% of psoriasis-first and synchronous-onset patients), scalp lesions as the initial lesion were more common in psoriasis-first patients (48.3% vs. 35.2% of arthritis-first patients and 39.8% of synchronous-onset patients), and genital involvement was present more often in arthritis-first patients (12.7% vs. 6.2% and 4.9% of psoriasis-first and synchronous-onset patients).
Early-onset (type 1) psoriasis was more common in psoriasis-first patients (74% vs. 28.1% and 51.8% of arthritis-first and synchronous-onset patients), whereas late-onset (type 2) psoriasis was more common in arthritis-first patients (71.9% vs. 26.0% and 48.2% for psoriasis-first and synchronous-onset patients).
A family history of psoriasis or PsA was more common in psoriasis-first patients (35.6% vs. 26.3% and 28.2% of arthritis-first and synchronous-onset patients), Dr. Kalyoncu said.
Treatment types did not differ between the groups.
Multiple linear regression analysis for the time elapsed from psoriasis to PsA symptom synchronicity, with all other independent variables set to baseline values, showed an overall intercept interval of 66 months, but with nail involvement, family history, or plaque psoriasis, the interval was extended by 28, 24, and 20 months, respectively. However, the presence of pustular psoriasis decreased the intercept interval by 28 months.
A temporal relationship between the onset of skin psoriasis and PsA is a well-known feature of psoriatic disease, with prior studies showing that the majority of cases involve psoriasis-first onset, Dr. Kalyoncu said, adding that heterogeneity in musculoskeletal and skin involvement is also a known feature.
However, little is known about the role of genetics, he noted.
Therefore, he and his colleagues used the Psoriatic Arthritis Registry of Turkey International Database, which was established in 2014 and now also includes data from patients in Canada and Italy, to explore the associations between disease characteristics and the temporal relationship of skin and musculoskeletal disease.
Based on the findings, age at the onset of psoriasis was the main factor that determined PsA symptom synchronicity, he said.
“We know that HLA-Cw6 is important in genetic susceptibility of psoriatic arthritis, but it is important only for early-onset arthritis, not late-onset psoriasis,” Dr. Kalyoncu said. “So our results make an indirect contribution [to the understanding of] these genetic and immunochemical differences between early-onset and late-onset psoriasis, and we need further future studies about this topic.”
Dr. Kalyoncu reported having no relevant disclosures.
SOURCE: Kalyoncu U et al. Arthritis Rheumatol. 2019;71(suppl 10), Abstract 2854.
REPORTING FROM ACR 2019
HHS: Coronavirus risk low in U.S., vaccine development underway
U.S. public health officials attempted to stymie concerns about the coronavirus during a press conference on Tuesday,

“Right now, there is no spread of this virus in our communities here at home,” Centers for Disease Control and Prevention director Robert Redfield, MD, said during the Jan. 28 press conference. “This is why our current assessment is that the immediate health risk of this new virus to the general public is low in our nation. The coming days and weeks are likely to bring more confirmed cases here and around the world, including the possibility of some person-to-person spreading, but our goal of the ongoing U.S. public health response is to contain this outbreak and prevent sustained spread of the virus in our country.”
During the press conference, Department Health & Human Services Secretary Alex M. Azar II, reiterated there have been only five confirmed U.S. cases of the coronavirus thus far and all were associated with travel to Wuhan, China, where the virus first appeared. The number of confirmed cases in China, meanwhile, has risen to more than 4,500 with about 100 associated deaths.
U.S. health providers should be on the lookout for any patient who has traveled to China recently, particularly to Hubei province, and they should pay close attention to any relevant symptoms, Secretary Azar said during the press conference.
He defended the decision not to declare a public health emergency at this time, stressing that such a move is based on standards and requirements not yet met by the coronavirus.
“It’s important to remember where we are right now; we have five cases in the United States, each of those individuals with direct contact to Wuhan and no person-to-person transmission in the United States,” Secretary Azar said. “I won’t hesitate at all to invoke any authorities that I need to ensure that we’re taking all the steps to protect the American people, but I’ll do it when it’s appropriate under the standards that we have and the authorities that I need.”
In the meantime, a number of efforts are underway by U.S. agencies to assess the nation’s emergency preparedness stockpile, to assist American families in China with evacuation, and to pursue research into diagnostics and a potential vaccine for the virus, Secretary Azar said.
With regard to countermeasures, the CDC has rapidly developed a diagnostic based on the published sequence of the virus, said Anthony Fauci, MD, director for the National Institute of Allergy and Infectious Diseases (NIAID). The National Institutes of Health and the CDC are now working on the development of next-generation diagnostics to better identify the virus in the United States and throughout the world, Dr. Fauci said during the press conference.
Currently, there are no proven therapeutics for the coronavirus infection, Dr. Fauci said. Based on experiences with SARS and MERS, however, researchers are studying certain antiviral drugs that could potentially treat the virus, he said. This includes the antiviral drug remdesivir, which was developed for the treatment of the Ebola virus, and lopinavir/ritonavir (Kaletra), a combination therapy commonly used to treat HIV. In addition, monoclonal antibodies developed during the SARS outbreak are also being studied.
“Given the somewhat close homology between SARS and the new novel coronavirus, there could be some cross reactivity there that could be utilized,” he said.
Most importantly, he said, vaccine development is underway. Since China isolated the virus and published its sequence, U.S. researchers have already analyzed the components and determined an immunogen to be used in a vaccine, Dr. Fauci said. He anticipates moving to a Phase 1 trial within the next 3 months. The trial would then move to Phase 2 after another few more months for safety data.
“What we do from that point will be determined by what has happened with the outbreak over those months,” he said. “We are proceeding as if we will have to deploy a vaccine. In other words, we’re looking at the worst scenario that this becomes a bigger outbreak.”
Federal health officials, however, stressed that more data about infected patients in China is needed for research. HHS has repeatedly offered to send a CDC team to China to help with public health efforts, research, and response, but China has so far declined the offer, Secretary Azar added.
In addition, the CDC has updated its travel advisory in response to the illness. The latest travel guidance recommends that travelers avoid all nonessential travel to all parts of China.
U.S. public health officials attempted to stymie concerns about the coronavirus during a press conference on Tuesday,

“Right now, there is no spread of this virus in our communities here at home,” Centers for Disease Control and Prevention director Robert Redfield, MD, said during the Jan. 28 press conference. “This is why our current assessment is that the immediate health risk of this new virus to the general public is low in our nation. The coming days and weeks are likely to bring more confirmed cases here and around the world, including the possibility of some person-to-person spreading, but our goal of the ongoing U.S. public health response is to contain this outbreak and prevent sustained spread of the virus in our country.”
During the press conference, Department Health & Human Services Secretary Alex M. Azar II, reiterated there have been only five confirmed U.S. cases of the coronavirus thus far and all were associated with travel to Wuhan, China, where the virus first appeared. The number of confirmed cases in China, meanwhile, has risen to more than 4,500 with about 100 associated deaths.
U.S. health providers should be on the lookout for any patient who has traveled to China recently, particularly to Hubei province, and they should pay close attention to any relevant symptoms, Secretary Azar said during the press conference.
He defended the decision not to declare a public health emergency at this time, stressing that such a move is based on standards and requirements not yet met by the coronavirus.
“It’s important to remember where we are right now; we have five cases in the United States, each of those individuals with direct contact to Wuhan and no person-to-person transmission in the United States,” Secretary Azar said. “I won’t hesitate at all to invoke any authorities that I need to ensure that we’re taking all the steps to protect the American people, but I’ll do it when it’s appropriate under the standards that we have and the authorities that I need.”
In the meantime, a number of efforts are underway by U.S. agencies to assess the nation’s emergency preparedness stockpile, to assist American families in China with evacuation, and to pursue research into diagnostics and a potential vaccine for the virus, Secretary Azar said.
With regard to countermeasures, the CDC has rapidly developed a diagnostic based on the published sequence of the virus, said Anthony Fauci, MD, director for the National Institute of Allergy and Infectious Diseases (NIAID). The National Institutes of Health and the CDC are now working on the development of next-generation diagnostics to better identify the virus in the United States and throughout the world, Dr. Fauci said during the press conference.
Currently, there are no proven therapeutics for the coronavirus infection, Dr. Fauci said. Based on experiences with SARS and MERS, however, researchers are studying certain antiviral drugs that could potentially treat the virus, he said. This includes the antiviral drug remdesivir, which was developed for the treatment of the Ebola virus, and lopinavir/ritonavir (Kaletra), a combination therapy commonly used to treat HIV. In addition, monoclonal antibodies developed during the SARS outbreak are also being studied.
“Given the somewhat close homology between SARS and the new novel coronavirus, there could be some cross reactivity there that could be utilized,” he said.
Most importantly, he said, vaccine development is underway. Since China isolated the virus and published its sequence, U.S. researchers have already analyzed the components and determined an immunogen to be used in a vaccine, Dr. Fauci said. He anticipates moving to a Phase 1 trial within the next 3 months. The trial would then move to Phase 2 after another few more months for safety data.
“What we do from that point will be determined by what has happened with the outbreak over those months,” he said. “We are proceeding as if we will have to deploy a vaccine. In other words, we’re looking at the worst scenario that this becomes a bigger outbreak.”
Federal health officials, however, stressed that more data about infected patients in China is needed for research. HHS has repeatedly offered to send a CDC team to China to help with public health efforts, research, and response, but China has so far declined the offer, Secretary Azar added.
In addition, the CDC has updated its travel advisory in response to the illness. The latest travel guidance recommends that travelers avoid all nonessential travel to all parts of China.
U.S. public health officials attempted to stymie concerns about the coronavirus during a press conference on Tuesday,

“Right now, there is no spread of this virus in our communities here at home,” Centers for Disease Control and Prevention director Robert Redfield, MD, said during the Jan. 28 press conference. “This is why our current assessment is that the immediate health risk of this new virus to the general public is low in our nation. The coming days and weeks are likely to bring more confirmed cases here and around the world, including the possibility of some person-to-person spreading, but our goal of the ongoing U.S. public health response is to contain this outbreak and prevent sustained spread of the virus in our country.”
During the press conference, Department Health & Human Services Secretary Alex M. Azar II, reiterated there have been only five confirmed U.S. cases of the coronavirus thus far and all were associated with travel to Wuhan, China, where the virus first appeared. The number of confirmed cases in China, meanwhile, has risen to more than 4,500 with about 100 associated deaths.
U.S. health providers should be on the lookout for any patient who has traveled to China recently, particularly to Hubei province, and they should pay close attention to any relevant symptoms, Secretary Azar said during the press conference.
He defended the decision not to declare a public health emergency at this time, stressing that such a move is based on standards and requirements not yet met by the coronavirus.
“It’s important to remember where we are right now; we have five cases in the United States, each of those individuals with direct contact to Wuhan and no person-to-person transmission in the United States,” Secretary Azar said. “I won’t hesitate at all to invoke any authorities that I need to ensure that we’re taking all the steps to protect the American people, but I’ll do it when it’s appropriate under the standards that we have and the authorities that I need.”
In the meantime, a number of efforts are underway by U.S. agencies to assess the nation’s emergency preparedness stockpile, to assist American families in China with evacuation, and to pursue research into diagnostics and a potential vaccine for the virus, Secretary Azar said.
With regard to countermeasures, the CDC has rapidly developed a diagnostic based on the published sequence of the virus, said Anthony Fauci, MD, director for the National Institute of Allergy and Infectious Diseases (NIAID). The National Institutes of Health and the CDC are now working on the development of next-generation diagnostics to better identify the virus in the United States and throughout the world, Dr. Fauci said during the press conference.
Currently, there are no proven therapeutics for the coronavirus infection, Dr. Fauci said. Based on experiences with SARS and MERS, however, researchers are studying certain antiviral drugs that could potentially treat the virus, he said. This includes the antiviral drug remdesivir, which was developed for the treatment of the Ebola virus, and lopinavir/ritonavir (Kaletra), a combination therapy commonly used to treat HIV. In addition, monoclonal antibodies developed during the SARS outbreak are also being studied.
“Given the somewhat close homology between SARS and the new novel coronavirus, there could be some cross reactivity there that could be utilized,” he said.
Most importantly, he said, vaccine development is underway. Since China isolated the virus and published its sequence, U.S. researchers have already analyzed the components and determined an immunogen to be used in a vaccine, Dr. Fauci said. He anticipates moving to a Phase 1 trial within the next 3 months. The trial would then move to Phase 2 after another few more months for safety data.
“What we do from that point will be determined by what has happened with the outbreak over those months,” he said. “We are proceeding as if we will have to deploy a vaccine. In other words, we’re looking at the worst scenario that this becomes a bigger outbreak.”
Federal health officials, however, stressed that more data about infected patients in China is needed for research. HHS has repeatedly offered to send a CDC team to China to help with public health efforts, research, and response, but China has so far declined the offer, Secretary Azar added.
In addition, the CDC has updated its travel advisory in response to the illness. The latest travel guidance recommends that travelers avoid all nonessential travel to all parts of China.
Dermatologists are uniquely suited to help sexual-, gender-minority patients
ORLANDO – There is a these patients, Angelo Landriscina, MD, said at the ODAC Dermatology, Aesthetic, & Clinical Conference.
“Our knowledge lagging behind is really to our own detriment,” said Dr. Landriscina, chief resident of dermatology at George Washington University, Washington. “The unique comorbidities and lived experiences of queer patients can impact their dermatologic disease. In addition to that, we are in a really unique position to provide life-changing care for these patients.”
The topic of dermatologic care for SGM patients has been discussed with interest in the dermatology community, but it has not been well studied. In one of two papers recently published in Pediatric Dermatology, Markus D. Boos, MD, PhD, from the University of Washington, Seattle, and Seattle Children’s Hospital, and coauthors noted that there is a particular knowledge gap in how to care for SGM pediatric and adolescent patients (Pediatr Dermatol. 2019 Sep;36[5]:581-6; 587-93).
In his presentation, Dr. Landriscina outlined the differences between sexual orientation – an emotional, romantic, or sexual attraction to others – and gender identity, or how one perceives their own gender. Lesbian, gay, transgender, and queer/questioning are classical definitions, but SGM patients may also self-identify in any number of other ways. Some SGM patients may identify as gender-fluid or nonbinary, while genderqueer is an umbrella term for individuals who don’t identify with typical gender roles. On the topic of pronouns, asking SGM patients how they want to be referred to is ideal, but the singular they is considered a gender-neutral term that should work for most situations.
There are also terms to avoid: “Homosexual” may be acceptable to some SGM patients, but is from an era when same-sex attraction was pathologized; “sexual preference” characterizes sexuality as a choice; the term “lifestyle” perpetuates the idea that all SGM patients are the same; and referring to transgender patients as "pre-op" or "post-op" is problematic as these terms imply that transgender identity is defined by a medical transition. Using transgender or gay as a noun, or the word transsexual, is offensive and shouldn’t be used at all, according to Dr. Landriscina.
As these terms continue to change and be redefined, dermatologists are likely to encounter terms they are unfamiliar with in the clinic, but he emphasized that attendees need not know every term to care for these patients. “The easiest way to be right in all of these situations is to let your patients define themselves,” he said, but noted that “assumptions are your worst enemy. You’re going to have to ask the hard questions.”
Updating understanding of SGM patients
Much of the medical community’s understanding of SGM patients hasn’t been updated in decades, Dr. Landriscina said. For example, medical school students typically learn about SGM risk factors that only apply to men who have sex with men (MSM), but there is new interest in caring for transgender patients within dermatology. The focus on classical notions for treating MSM can be reductive, Dr. Landriscina explained. “It boils a whole community of people down to one disease process. I think that we need to expand the thought that there are other associations here. There are other risks these patients face.”
In contrast, dermatologists who strive to understand their patients can better see them as a whole person, can engage in a better differential diagnosis, are aware of the current comorbidities SGM patients face that affect dermatologic disease, and can even work with the patient toward preventative care.
“It’s been decades since we’ve had a paradigm shift about this, and I think it’s time,” he said.
Overall, SGM patients have a higher likelihood of suffering from mental illness and suicidal ideation, with 10%-20% of lesbian, gay, and bisexual patients attempting suicide. Gender-minority patients have a significantly higher rate of attempting suicide at 40%. SGM patients are also more likely to be homeless and uninsured, and have the highest rates of tobacco, alcohol, and illicit drug use. In the SGM population, there is a higher likelihood of being victimized, and discriminated against, with this risk being much higher in transgender patients.
For MSM, there is a high risk of HIV and other STIs such as herpes simplex virus type 2 (HSV2), human papillomavirus (HPV), gonorrhea, and chlamydia, Dr. Landriscina said. They are also at risk for hepatitis A, B, and C; clusters of meningococcal meningitis; and human herpes virus 8. While MSM are more likely to use sunscreen, they also are more likely to use tanning beds and not wear protective clothing outdoors, and are at a greater risk of skin cancer. The risks of body dysmorphia and eating disorders are also increased for MSM.
While there is not as much research on risk factors for women who have sex with women (WSW), they are still at risk for HIV, HSV, and HPV and are less likely to engage in safe sex practices. For women who have sex with both men and other women, there is an even greater risk of STIs. While WSW are more likely to perceive less need for screening, they should be given the same screening as all other women, and dermatologists can help by ensuring these patients are connected with primary care providers, he said.
Dermatologic sequelae for transgender patients
For patients who transition from male to female, there is little information on their sexual risk from studies, but their care should be managed similarly to MSM, Dr. Landriscina said. When seeing transgender patients, dermatologists should be aware of issues of gender dysphoria, but not offer any intervention without first having a conversation about the patient’s hopes and goals. “Not every patient will have the means or desire to have everything that you can offer,” he said.
For patients who choose to undergo a female-to-male transition, dermatological sequelae may include classical manifestations of androgen excess from hormone therapy such as acne and androgenic alopecia; acne, miliaria, tinea corporis, contact dermatitis from chest binding; and surgical scars and keloids. For acne, isotretinoin is an option if a case is severe, but dermatologists should be aware these patients may still be able to become pregnant. There is no consensus on treatment for androgenic alopecia, but use of finasteride might block wanted secondary sex characteristics, Dr. Landriscina noted.
Patients who undergo a male-to-female transition may develop melasma or asteatotic eczema while receiving estrogen therapy; unwanted facial or body hair; and complications from illicit “filler” injections that may cause foreign-body granulomas, bacterial or atypical mycobacterial infection, lymphedema, and scarring.
Transgender patients may choose to undergo aesthetic treatments that can affirm their gender and decrease their gender dysphoria, augment the effects of hormone therapy and gender-confirmation surgery, and improve their quality of life. “While this has classically fallen under the purview of plastic surgery, I feel like we’re uniquely positioned to provide really life-changing aesthetic services to these patients,” Dr. Landriscina said.
Creating an inclusive environment is key to successfully caring for SGM patients. Any practice policies should have SGM-inclusive language, employees should receive mandatory LGBTQ+ focused training, and a point person should oversee LGBTQ+ matters, according to the Joint Commission’s LGBT Guide. Practices should also begin collecting data on sexual orientation and gender identity, which may help patients who are reluctant to vocally disclose their sexual orientation and gender identity and expand understanding of SGM patients. “Before you even walk into that visit, you know what the patient’s identity is, how they want to be addressed,” said Dr. Landriscina. “It also shows patients that you value what their identities are and that you’re competent in taking care of them.”
Dr. Landriscina encouraged attendees to take the information they learned in the session and “run with it.”
“Go for it. Keep learning,” he said. “There’s more about this topic than I even had the chance to include. Your patients are going to appreciate your dedication to them.”
In an interview, Adam Friedman, MD, professor and interim chair of dermatology at George Washington University and medical director of ODAC, acknowledged the large gaps in care for SGM patients and the unique role dermatologists can play in their care, both in terms of medical and surgical procedures.
“There are specific considerations that we as dermatologists need to think about in terms of just quality of life, potentially mental disease, homelessness, access to care. I think if we consider the whole picture, we can not only provide dermatologic care, but maybe serve as a pivot point to direct them to other specialists, and other physicians, and even nonphysicians who play a role in all facets of life to really get these individuals all the care, in broader senses, that they need,” he said.
Dr. Landriscina reported no relevant conflicts of interest.
ORLANDO – There is a these patients, Angelo Landriscina, MD, said at the ODAC Dermatology, Aesthetic, & Clinical Conference.
“Our knowledge lagging behind is really to our own detriment,” said Dr. Landriscina, chief resident of dermatology at George Washington University, Washington. “The unique comorbidities and lived experiences of queer patients can impact their dermatologic disease. In addition to that, we are in a really unique position to provide life-changing care for these patients.”
The topic of dermatologic care for SGM patients has been discussed with interest in the dermatology community, but it has not been well studied. In one of two papers recently published in Pediatric Dermatology, Markus D. Boos, MD, PhD, from the University of Washington, Seattle, and Seattle Children’s Hospital, and coauthors noted that there is a particular knowledge gap in how to care for SGM pediatric and adolescent patients (Pediatr Dermatol. 2019 Sep;36[5]:581-6; 587-93).
In his presentation, Dr. Landriscina outlined the differences between sexual orientation – an emotional, romantic, or sexual attraction to others – and gender identity, or how one perceives their own gender. Lesbian, gay, transgender, and queer/questioning are classical definitions, but SGM patients may also self-identify in any number of other ways. Some SGM patients may identify as gender-fluid or nonbinary, while genderqueer is an umbrella term for individuals who don’t identify with typical gender roles. On the topic of pronouns, asking SGM patients how they want to be referred to is ideal, but the singular they is considered a gender-neutral term that should work for most situations.
There are also terms to avoid: “Homosexual” may be acceptable to some SGM patients, but is from an era when same-sex attraction was pathologized; “sexual preference” characterizes sexuality as a choice; the term “lifestyle” perpetuates the idea that all SGM patients are the same; and referring to transgender patients as "pre-op" or "post-op" is problematic as these terms imply that transgender identity is defined by a medical transition. Using transgender or gay as a noun, or the word transsexual, is offensive and shouldn’t be used at all, according to Dr. Landriscina.
As these terms continue to change and be redefined, dermatologists are likely to encounter terms they are unfamiliar with in the clinic, but he emphasized that attendees need not know every term to care for these patients. “The easiest way to be right in all of these situations is to let your patients define themselves,” he said, but noted that “assumptions are your worst enemy. You’re going to have to ask the hard questions.”
Updating understanding of SGM patients
Much of the medical community’s understanding of SGM patients hasn’t been updated in decades, Dr. Landriscina said. For example, medical school students typically learn about SGM risk factors that only apply to men who have sex with men (MSM), but there is new interest in caring for transgender patients within dermatology. The focus on classical notions for treating MSM can be reductive, Dr. Landriscina explained. “It boils a whole community of people down to one disease process. I think that we need to expand the thought that there are other associations here. There are other risks these patients face.”
In contrast, dermatologists who strive to understand their patients can better see them as a whole person, can engage in a better differential diagnosis, are aware of the current comorbidities SGM patients face that affect dermatologic disease, and can even work with the patient toward preventative care.
“It’s been decades since we’ve had a paradigm shift about this, and I think it’s time,” he said.
Overall, SGM patients have a higher likelihood of suffering from mental illness and suicidal ideation, with 10%-20% of lesbian, gay, and bisexual patients attempting suicide. Gender-minority patients have a significantly higher rate of attempting suicide at 40%. SGM patients are also more likely to be homeless and uninsured, and have the highest rates of tobacco, alcohol, and illicit drug use. In the SGM population, there is a higher likelihood of being victimized, and discriminated against, with this risk being much higher in transgender patients.
For MSM, there is a high risk of HIV and other STIs such as herpes simplex virus type 2 (HSV2), human papillomavirus (HPV), gonorrhea, and chlamydia, Dr. Landriscina said. They are also at risk for hepatitis A, B, and C; clusters of meningococcal meningitis; and human herpes virus 8. While MSM are more likely to use sunscreen, they also are more likely to use tanning beds and not wear protective clothing outdoors, and are at a greater risk of skin cancer. The risks of body dysmorphia and eating disorders are also increased for MSM.
While there is not as much research on risk factors for women who have sex with women (WSW), they are still at risk for HIV, HSV, and HPV and are less likely to engage in safe sex practices. For women who have sex with both men and other women, there is an even greater risk of STIs. While WSW are more likely to perceive less need for screening, they should be given the same screening as all other women, and dermatologists can help by ensuring these patients are connected with primary care providers, he said.
Dermatologic sequelae for transgender patients
For patients who transition from male to female, there is little information on their sexual risk from studies, but their care should be managed similarly to MSM, Dr. Landriscina said. When seeing transgender patients, dermatologists should be aware of issues of gender dysphoria, but not offer any intervention without first having a conversation about the patient’s hopes and goals. “Not every patient will have the means or desire to have everything that you can offer,” he said.
For patients who choose to undergo a female-to-male transition, dermatological sequelae may include classical manifestations of androgen excess from hormone therapy such as acne and androgenic alopecia; acne, miliaria, tinea corporis, contact dermatitis from chest binding; and surgical scars and keloids. For acne, isotretinoin is an option if a case is severe, but dermatologists should be aware these patients may still be able to become pregnant. There is no consensus on treatment for androgenic alopecia, but use of finasteride might block wanted secondary sex characteristics, Dr. Landriscina noted.
Patients who undergo a male-to-female transition may develop melasma or asteatotic eczema while receiving estrogen therapy; unwanted facial or body hair; and complications from illicit “filler” injections that may cause foreign-body granulomas, bacterial or atypical mycobacterial infection, lymphedema, and scarring.
Transgender patients may choose to undergo aesthetic treatments that can affirm their gender and decrease their gender dysphoria, augment the effects of hormone therapy and gender-confirmation surgery, and improve their quality of life. “While this has classically fallen under the purview of plastic surgery, I feel like we’re uniquely positioned to provide really life-changing aesthetic services to these patients,” Dr. Landriscina said.
Creating an inclusive environment is key to successfully caring for SGM patients. Any practice policies should have SGM-inclusive language, employees should receive mandatory LGBTQ+ focused training, and a point person should oversee LGBTQ+ matters, according to the Joint Commission’s LGBT Guide. Practices should also begin collecting data on sexual orientation and gender identity, which may help patients who are reluctant to vocally disclose their sexual orientation and gender identity and expand understanding of SGM patients. “Before you even walk into that visit, you know what the patient’s identity is, how they want to be addressed,” said Dr. Landriscina. “It also shows patients that you value what their identities are and that you’re competent in taking care of them.”
Dr. Landriscina encouraged attendees to take the information they learned in the session and “run with it.”
“Go for it. Keep learning,” he said. “There’s more about this topic than I even had the chance to include. Your patients are going to appreciate your dedication to them.”
In an interview, Adam Friedman, MD, professor and interim chair of dermatology at George Washington University and medical director of ODAC, acknowledged the large gaps in care for SGM patients and the unique role dermatologists can play in their care, both in terms of medical and surgical procedures.
“There are specific considerations that we as dermatologists need to think about in terms of just quality of life, potentially mental disease, homelessness, access to care. I think if we consider the whole picture, we can not only provide dermatologic care, but maybe serve as a pivot point to direct them to other specialists, and other physicians, and even nonphysicians who play a role in all facets of life to really get these individuals all the care, in broader senses, that they need,” he said.
Dr. Landriscina reported no relevant conflicts of interest.
ORLANDO – There is a these patients, Angelo Landriscina, MD, said at the ODAC Dermatology, Aesthetic, & Clinical Conference.
“Our knowledge lagging behind is really to our own detriment,” said Dr. Landriscina, chief resident of dermatology at George Washington University, Washington. “The unique comorbidities and lived experiences of queer patients can impact their dermatologic disease. In addition to that, we are in a really unique position to provide life-changing care for these patients.”
The topic of dermatologic care for SGM patients has been discussed with interest in the dermatology community, but it has not been well studied. In one of two papers recently published in Pediatric Dermatology, Markus D. Boos, MD, PhD, from the University of Washington, Seattle, and Seattle Children’s Hospital, and coauthors noted that there is a particular knowledge gap in how to care for SGM pediatric and adolescent patients (Pediatr Dermatol. 2019 Sep;36[5]:581-6; 587-93).
In his presentation, Dr. Landriscina outlined the differences between sexual orientation – an emotional, romantic, or sexual attraction to others – and gender identity, or how one perceives their own gender. Lesbian, gay, transgender, and queer/questioning are classical definitions, but SGM patients may also self-identify in any number of other ways. Some SGM patients may identify as gender-fluid or nonbinary, while genderqueer is an umbrella term for individuals who don’t identify with typical gender roles. On the topic of pronouns, asking SGM patients how they want to be referred to is ideal, but the singular they is considered a gender-neutral term that should work for most situations.
There are also terms to avoid: “Homosexual” may be acceptable to some SGM patients, but is from an era when same-sex attraction was pathologized; “sexual preference” characterizes sexuality as a choice; the term “lifestyle” perpetuates the idea that all SGM patients are the same; and referring to transgender patients as "pre-op" or "post-op" is problematic as these terms imply that transgender identity is defined by a medical transition. Using transgender or gay as a noun, or the word transsexual, is offensive and shouldn’t be used at all, according to Dr. Landriscina.
As these terms continue to change and be redefined, dermatologists are likely to encounter terms they are unfamiliar with in the clinic, but he emphasized that attendees need not know every term to care for these patients. “The easiest way to be right in all of these situations is to let your patients define themselves,” he said, but noted that “assumptions are your worst enemy. You’re going to have to ask the hard questions.”
Updating understanding of SGM patients
Much of the medical community’s understanding of SGM patients hasn’t been updated in decades, Dr. Landriscina said. For example, medical school students typically learn about SGM risk factors that only apply to men who have sex with men (MSM), but there is new interest in caring for transgender patients within dermatology. The focus on classical notions for treating MSM can be reductive, Dr. Landriscina explained. “It boils a whole community of people down to one disease process. I think that we need to expand the thought that there are other associations here. There are other risks these patients face.”
In contrast, dermatologists who strive to understand their patients can better see them as a whole person, can engage in a better differential diagnosis, are aware of the current comorbidities SGM patients face that affect dermatologic disease, and can even work with the patient toward preventative care.
“It’s been decades since we’ve had a paradigm shift about this, and I think it’s time,” he said.
Overall, SGM patients have a higher likelihood of suffering from mental illness and suicidal ideation, with 10%-20% of lesbian, gay, and bisexual patients attempting suicide. Gender-minority patients have a significantly higher rate of attempting suicide at 40%. SGM patients are also more likely to be homeless and uninsured, and have the highest rates of tobacco, alcohol, and illicit drug use. In the SGM population, there is a higher likelihood of being victimized, and discriminated against, with this risk being much higher in transgender patients.
For MSM, there is a high risk of HIV and other STIs such as herpes simplex virus type 2 (HSV2), human papillomavirus (HPV), gonorrhea, and chlamydia, Dr. Landriscina said. They are also at risk for hepatitis A, B, and C; clusters of meningococcal meningitis; and human herpes virus 8. While MSM are more likely to use sunscreen, they also are more likely to use tanning beds and not wear protective clothing outdoors, and are at a greater risk of skin cancer. The risks of body dysmorphia and eating disorders are also increased for MSM.
While there is not as much research on risk factors for women who have sex with women (WSW), they are still at risk for HIV, HSV, and HPV and are less likely to engage in safe sex practices. For women who have sex with both men and other women, there is an even greater risk of STIs. While WSW are more likely to perceive less need for screening, they should be given the same screening as all other women, and dermatologists can help by ensuring these patients are connected with primary care providers, he said.
Dermatologic sequelae for transgender patients
For patients who transition from male to female, there is little information on their sexual risk from studies, but their care should be managed similarly to MSM, Dr. Landriscina said. When seeing transgender patients, dermatologists should be aware of issues of gender dysphoria, but not offer any intervention without first having a conversation about the patient’s hopes and goals. “Not every patient will have the means or desire to have everything that you can offer,” he said.
For patients who choose to undergo a female-to-male transition, dermatological sequelae may include classical manifestations of androgen excess from hormone therapy such as acne and androgenic alopecia; acne, miliaria, tinea corporis, contact dermatitis from chest binding; and surgical scars and keloids. For acne, isotretinoin is an option if a case is severe, but dermatologists should be aware these patients may still be able to become pregnant. There is no consensus on treatment for androgenic alopecia, but use of finasteride might block wanted secondary sex characteristics, Dr. Landriscina noted.
Patients who undergo a male-to-female transition may develop melasma or asteatotic eczema while receiving estrogen therapy; unwanted facial or body hair; and complications from illicit “filler” injections that may cause foreign-body granulomas, bacterial or atypical mycobacterial infection, lymphedema, and scarring.
Transgender patients may choose to undergo aesthetic treatments that can affirm their gender and decrease their gender dysphoria, augment the effects of hormone therapy and gender-confirmation surgery, and improve their quality of life. “While this has classically fallen under the purview of plastic surgery, I feel like we’re uniquely positioned to provide really life-changing aesthetic services to these patients,” Dr. Landriscina said.
Creating an inclusive environment is key to successfully caring for SGM patients. Any practice policies should have SGM-inclusive language, employees should receive mandatory LGBTQ+ focused training, and a point person should oversee LGBTQ+ matters, according to the Joint Commission’s LGBT Guide. Practices should also begin collecting data on sexual orientation and gender identity, which may help patients who are reluctant to vocally disclose their sexual orientation and gender identity and expand understanding of SGM patients. “Before you even walk into that visit, you know what the patient’s identity is, how they want to be addressed,” said Dr. Landriscina. “It also shows patients that you value what their identities are and that you’re competent in taking care of them.”
Dr. Landriscina encouraged attendees to take the information they learned in the session and “run with it.”
“Go for it. Keep learning,” he said. “There’s more about this topic than I even had the chance to include. Your patients are going to appreciate your dedication to them.”
In an interview, Adam Friedman, MD, professor and interim chair of dermatology at George Washington University and medical director of ODAC, acknowledged the large gaps in care for SGM patients and the unique role dermatologists can play in their care, both in terms of medical and surgical procedures.
“There are specific considerations that we as dermatologists need to think about in terms of just quality of life, potentially mental disease, homelessness, access to care. I think if we consider the whole picture, we can not only provide dermatologic care, but maybe serve as a pivot point to direct them to other specialists, and other physicians, and even nonphysicians who play a role in all facets of life to really get these individuals all the care, in broader senses, that they need,” he said.
Dr. Landriscina reported no relevant conflicts of interest.
EXPERT ANALYSIS FROM ODAC 2020
Wuhan virus: What clinicians need to know
As the Wuhan coronavirus story unfolds, , according to infectious disease experts.

“We are asking that of everyone with fever and respiratory symptoms who comes to our clinics, hospital, or emergency room. It’s a powerful screening tool,” said William Schaffner, MD, professor of preventive medicine and infectious diseases at Vanderbilt University Medical Center, Nashville, Tenn.
In addition to fever, common signs of infection include cough, shortness of breath, and breathing difficulties. Some patients have had diarrhea, vomiting, and other gastrointestinal symptoms. In more severe cases, infection can cause pneumonia, severe acute respiratory syndrome, kidney failure, and death. The incubation period appears to be up to 2 weeks, according to the World Health Organization (WHO).
If patients exhibit symptoms and either they or a close contact has returned from China recently, take standard airborne precautions and send specimens – a serum sample, oral and nasal pharyngeal swabs, and lower respiratory tract specimens if available – to the local health department, which will forward them to the Centers for Disease Control and Prevention (CDC) for testing. Turnaround time is 24-48 hours.
The 2019 Novel Coronavirus (2019-nCoV), identified as the cause of an outbreak of respiratory illness first detected in December in association with a live animal market in Wuhan, China, has been implicated in almost 2,000 cases and 56 deaths in that country. Cases have been reported in 13 countries besides China. Five cases of 2019-nCoV infection have been confirmed in the United States, all in people recently returned from Wuhan. As the virus spreads in China, however, it’s almost certain more cases will show up in the United States. Travel history is key, Dr. Schaffner and others said.
Plan and rehearse
The first step to prepare is to use the CDC’s Interim Guidance for Healthcare Professionals to make a written plan specific to your practice to respond to a potential case. The plan must include notifying the local health department, the CDC liaison for testing, and tracking down patient contacts.
“It’s not good enough to just download CDC’s guidance; use it to make your own local plan and know what to do 24/7,” said Daniel Lucey, MD, an infectious disease expert at Georgetown University Medical Center, Washington, D.C.
“Know who is on call at the health department on weekends and nights,” he said. Know where the patient is going to be isolated; figure out what to do if there’s more than one, and tests come back positive. Have masks on hand, and rehearse the response. “Make a coronavirus team, and absolutely have the nurses involved,” as well as other providers who may come into contact with a case, he added.
“You want to be able to do as well as your counterparts in Washington state and Chicago,” where the first two U.S. cases emerged. “They were prepared. They knew what to do,” Dr. Lucey said.
Those first two U.S. patients – a man in Everett, Wash., and a Chicago woman – developed symptoms after returning from Wuhan, a city of 11 million just over 400 miles inland from the port city of Shanghai. On Jan. 26 three more cases were confirmed by the CDC, two in California and one in Arizona, and each had recently traveled to Wuhan. All five patients remain hospitalized, and there’s no evidence they spread the infection further. There is also no evidence of human-to-human transmission of other cases exported from China to any other countries, according to the WHO.
WHO declined to declare a global health emergency – a Public Health Emergency of International Concern, in its parlance – on Jan. 23. The step would have triggered travel and trade restrictions in member states, including the United States. For now, at least, the group said it wasn’t warranted at this point.
Fatality rates
The focus right now is China. The outbreak has spread beyond Wuhan to other parts of the country, and there’s evidence of fourth-generation spread.
Transportation into and out of Wuhan and other cities has been curtailed, Lunar New Year festivals have been canceled, and the Shanghai Disneyland has been closed, among other measures taken by Chinese officials.
The government could be taking drastic measures in part to prevent the public criticism it took in the early 2000’s for the delayed response and lack of transparency during the global outbreak of another wildlife market coronavirus epidemic, severe acute respiratory syndrome (SARS). In a press conference Jan. 22, WHO officials commended the government’s containment efforts but did not say they recommended them.
According to WHO, serious cases in China have mostly been in people over 40 years old with significant comorbidities and have skewed towards men. Spread seems to be limited to family members, health care providers, and other close contacts, probably by respiratory droplets. If that pattern holds, WHO officials said, the outbreak is containable.
The fatality rate appears to be around 3%, a good deal lower than the 10% reported for SARS and much lower than the nearly 40% reported for Middle East respiratory syndrome (MERS), another recent coronavirus mutation from the animal trade.
The Wuhan virus fatality rate might drop as milder cases are detected and added to the denominator. “It definitely appears to be less severe than SARS and MERS,” said Amesh Adalja, MD, an infectious disease physician in Pittsburgh and emerging infectious disease researcher at Johns Hopkins University, Baltimore.
SARS: Lessons learned
In general, the world is much better equipped for coronavirus outbreaks than when SARS, in particular, emerged in 2003.
WHO officials in their press conference lauded China for it openness with the current outbreak, and for isolating and sequencing the virus immediately, which gave the world a diagnostic test in the first days of the outbreak, something that wasn’t available for SARS. China and other countries also are cooperating and working closely to contain the Wuhan virus.
“What we know today might change tomorrow, so we have to keep tuned in to new information, but we learned a lot from SARS,” Dr. Shaffner said. Overall, it’s likely “the impact on the United States of this new coronavirus is going to be trivial,” he predicted.
Dr. Lucey, however, recalled that the SARS outbreak in Toronto in 2003 started with one missed case. A woman returned asymptomatic from Hong Kong and spread the infection to her family members before she died. Her cause of death wasn’t immediately recognized, nor was the reason her family members were sick, since they hadn’t been to Hong Kong recently.
The infection ultimately spread to more than 200 people, about half of them health care workers. A few people died.
If a virus is sufficiently contagious, “it just takes one. You don’t want to be the one who misses that first patient,” Dr. Lucey said.
Currently, there are no antivirals or vaccines for coronaviruses; researchers are working on both, but for now, care is supportive.
This article was updated with new case numbers on 1/26/20.
As the Wuhan coronavirus story unfolds, , according to infectious disease experts.

“We are asking that of everyone with fever and respiratory symptoms who comes to our clinics, hospital, or emergency room. It’s a powerful screening tool,” said William Schaffner, MD, professor of preventive medicine and infectious diseases at Vanderbilt University Medical Center, Nashville, Tenn.
In addition to fever, common signs of infection include cough, shortness of breath, and breathing difficulties. Some patients have had diarrhea, vomiting, and other gastrointestinal symptoms. In more severe cases, infection can cause pneumonia, severe acute respiratory syndrome, kidney failure, and death. The incubation period appears to be up to 2 weeks, according to the World Health Organization (WHO).
If patients exhibit symptoms and either they or a close contact has returned from China recently, take standard airborne precautions and send specimens – a serum sample, oral and nasal pharyngeal swabs, and lower respiratory tract specimens if available – to the local health department, which will forward them to the Centers for Disease Control and Prevention (CDC) for testing. Turnaround time is 24-48 hours.
The 2019 Novel Coronavirus (2019-nCoV), identified as the cause of an outbreak of respiratory illness first detected in December in association with a live animal market in Wuhan, China, has been implicated in almost 2,000 cases and 56 deaths in that country. Cases have been reported in 13 countries besides China. Five cases of 2019-nCoV infection have been confirmed in the United States, all in people recently returned from Wuhan. As the virus spreads in China, however, it’s almost certain more cases will show up in the United States. Travel history is key, Dr. Schaffner and others said.
Plan and rehearse
The first step to prepare is to use the CDC’s Interim Guidance for Healthcare Professionals to make a written plan specific to your practice to respond to a potential case. The plan must include notifying the local health department, the CDC liaison for testing, and tracking down patient contacts.
“It’s not good enough to just download CDC’s guidance; use it to make your own local plan and know what to do 24/7,” said Daniel Lucey, MD, an infectious disease expert at Georgetown University Medical Center, Washington, D.C.
“Know who is on call at the health department on weekends and nights,” he said. Know where the patient is going to be isolated; figure out what to do if there’s more than one, and tests come back positive. Have masks on hand, and rehearse the response. “Make a coronavirus team, and absolutely have the nurses involved,” as well as other providers who may come into contact with a case, he added.
“You want to be able to do as well as your counterparts in Washington state and Chicago,” where the first two U.S. cases emerged. “They were prepared. They knew what to do,” Dr. Lucey said.
Those first two U.S. patients – a man in Everett, Wash., and a Chicago woman – developed symptoms after returning from Wuhan, a city of 11 million just over 400 miles inland from the port city of Shanghai. On Jan. 26 three more cases were confirmed by the CDC, two in California and one in Arizona, and each had recently traveled to Wuhan. All five patients remain hospitalized, and there’s no evidence they spread the infection further. There is also no evidence of human-to-human transmission of other cases exported from China to any other countries, according to the WHO.
WHO declined to declare a global health emergency – a Public Health Emergency of International Concern, in its parlance – on Jan. 23. The step would have triggered travel and trade restrictions in member states, including the United States. For now, at least, the group said it wasn’t warranted at this point.
Fatality rates
The focus right now is China. The outbreak has spread beyond Wuhan to other parts of the country, and there’s evidence of fourth-generation spread.
Transportation into and out of Wuhan and other cities has been curtailed, Lunar New Year festivals have been canceled, and the Shanghai Disneyland has been closed, among other measures taken by Chinese officials.
The government could be taking drastic measures in part to prevent the public criticism it took in the early 2000’s for the delayed response and lack of transparency during the global outbreak of another wildlife market coronavirus epidemic, severe acute respiratory syndrome (SARS). In a press conference Jan. 22, WHO officials commended the government’s containment efforts but did not say they recommended them.
According to WHO, serious cases in China have mostly been in people over 40 years old with significant comorbidities and have skewed towards men. Spread seems to be limited to family members, health care providers, and other close contacts, probably by respiratory droplets. If that pattern holds, WHO officials said, the outbreak is containable.
The fatality rate appears to be around 3%, a good deal lower than the 10% reported for SARS and much lower than the nearly 40% reported for Middle East respiratory syndrome (MERS), another recent coronavirus mutation from the animal trade.
The Wuhan virus fatality rate might drop as milder cases are detected and added to the denominator. “It definitely appears to be less severe than SARS and MERS,” said Amesh Adalja, MD, an infectious disease physician in Pittsburgh and emerging infectious disease researcher at Johns Hopkins University, Baltimore.
SARS: Lessons learned
In general, the world is much better equipped for coronavirus outbreaks than when SARS, in particular, emerged in 2003.
WHO officials in their press conference lauded China for it openness with the current outbreak, and for isolating and sequencing the virus immediately, which gave the world a diagnostic test in the first days of the outbreak, something that wasn’t available for SARS. China and other countries also are cooperating and working closely to contain the Wuhan virus.
“What we know today might change tomorrow, so we have to keep tuned in to new information, but we learned a lot from SARS,” Dr. Shaffner said. Overall, it’s likely “the impact on the United States of this new coronavirus is going to be trivial,” he predicted.
Dr. Lucey, however, recalled that the SARS outbreak in Toronto in 2003 started with one missed case. A woman returned asymptomatic from Hong Kong and spread the infection to her family members before she died. Her cause of death wasn’t immediately recognized, nor was the reason her family members were sick, since they hadn’t been to Hong Kong recently.
The infection ultimately spread to more than 200 people, about half of them health care workers. A few people died.
If a virus is sufficiently contagious, “it just takes one. You don’t want to be the one who misses that first patient,” Dr. Lucey said.
Currently, there are no antivirals or vaccines for coronaviruses; researchers are working on both, but for now, care is supportive.
This article was updated with new case numbers on 1/26/20.
As the Wuhan coronavirus story unfolds, , according to infectious disease experts.

“We are asking that of everyone with fever and respiratory symptoms who comes to our clinics, hospital, or emergency room. It’s a powerful screening tool,” said William Schaffner, MD, professor of preventive medicine and infectious diseases at Vanderbilt University Medical Center, Nashville, Tenn.
In addition to fever, common signs of infection include cough, shortness of breath, and breathing difficulties. Some patients have had diarrhea, vomiting, and other gastrointestinal symptoms. In more severe cases, infection can cause pneumonia, severe acute respiratory syndrome, kidney failure, and death. The incubation period appears to be up to 2 weeks, according to the World Health Organization (WHO).
If patients exhibit symptoms and either they or a close contact has returned from China recently, take standard airborne precautions and send specimens – a serum sample, oral and nasal pharyngeal swabs, and lower respiratory tract specimens if available – to the local health department, which will forward them to the Centers for Disease Control and Prevention (CDC) for testing. Turnaround time is 24-48 hours.
The 2019 Novel Coronavirus (2019-nCoV), identified as the cause of an outbreak of respiratory illness first detected in December in association with a live animal market in Wuhan, China, has been implicated in almost 2,000 cases and 56 deaths in that country. Cases have been reported in 13 countries besides China. Five cases of 2019-nCoV infection have been confirmed in the United States, all in people recently returned from Wuhan. As the virus spreads in China, however, it’s almost certain more cases will show up in the United States. Travel history is key, Dr. Schaffner and others said.
Plan and rehearse
The first step to prepare is to use the CDC’s Interim Guidance for Healthcare Professionals to make a written plan specific to your practice to respond to a potential case. The plan must include notifying the local health department, the CDC liaison for testing, and tracking down patient contacts.
“It’s not good enough to just download CDC’s guidance; use it to make your own local plan and know what to do 24/7,” said Daniel Lucey, MD, an infectious disease expert at Georgetown University Medical Center, Washington, D.C.
“Know who is on call at the health department on weekends and nights,” he said. Know where the patient is going to be isolated; figure out what to do if there’s more than one, and tests come back positive. Have masks on hand, and rehearse the response. “Make a coronavirus team, and absolutely have the nurses involved,” as well as other providers who may come into contact with a case, he added.
“You want to be able to do as well as your counterparts in Washington state and Chicago,” where the first two U.S. cases emerged. “They were prepared. They knew what to do,” Dr. Lucey said.
Those first two U.S. patients – a man in Everett, Wash., and a Chicago woman – developed symptoms after returning from Wuhan, a city of 11 million just over 400 miles inland from the port city of Shanghai. On Jan. 26 three more cases were confirmed by the CDC, two in California and one in Arizona, and each had recently traveled to Wuhan. All five patients remain hospitalized, and there’s no evidence they spread the infection further. There is also no evidence of human-to-human transmission of other cases exported from China to any other countries, according to the WHO.
WHO declined to declare a global health emergency – a Public Health Emergency of International Concern, in its parlance – on Jan. 23. The step would have triggered travel and trade restrictions in member states, including the United States. For now, at least, the group said it wasn’t warranted at this point.
Fatality rates
The focus right now is China. The outbreak has spread beyond Wuhan to other parts of the country, and there’s evidence of fourth-generation spread.
Transportation into and out of Wuhan and other cities has been curtailed, Lunar New Year festivals have been canceled, and the Shanghai Disneyland has been closed, among other measures taken by Chinese officials.
The government could be taking drastic measures in part to prevent the public criticism it took in the early 2000’s for the delayed response and lack of transparency during the global outbreak of another wildlife market coronavirus epidemic, severe acute respiratory syndrome (SARS). In a press conference Jan. 22, WHO officials commended the government’s containment efforts but did not say they recommended them.
According to WHO, serious cases in China have mostly been in people over 40 years old with significant comorbidities and have skewed towards men. Spread seems to be limited to family members, health care providers, and other close contacts, probably by respiratory droplets. If that pattern holds, WHO officials said, the outbreak is containable.
The fatality rate appears to be around 3%, a good deal lower than the 10% reported for SARS and much lower than the nearly 40% reported for Middle East respiratory syndrome (MERS), another recent coronavirus mutation from the animal trade.
The Wuhan virus fatality rate might drop as milder cases are detected and added to the denominator. “It definitely appears to be less severe than SARS and MERS,” said Amesh Adalja, MD, an infectious disease physician in Pittsburgh and emerging infectious disease researcher at Johns Hopkins University, Baltimore.
SARS: Lessons learned
In general, the world is much better equipped for coronavirus outbreaks than when SARS, in particular, emerged in 2003.
WHO officials in their press conference lauded China for it openness with the current outbreak, and for isolating and sequencing the virus immediately, which gave the world a diagnostic test in the first days of the outbreak, something that wasn’t available for SARS. China and other countries also are cooperating and working closely to contain the Wuhan virus.
“What we know today might change tomorrow, so we have to keep tuned in to new information, but we learned a lot from SARS,” Dr. Shaffner said. Overall, it’s likely “the impact on the United States of this new coronavirus is going to be trivial,” he predicted.
Dr. Lucey, however, recalled that the SARS outbreak in Toronto in 2003 started with one missed case. A woman returned asymptomatic from Hong Kong and spread the infection to her family members before she died. Her cause of death wasn’t immediately recognized, nor was the reason her family members were sick, since they hadn’t been to Hong Kong recently.
The infection ultimately spread to more than 200 people, about half of them health care workers. A few people died.
If a virus is sufficiently contagious, “it just takes one. You don’t want to be the one who misses that first patient,” Dr. Lucey said.
Currently, there are no antivirals or vaccines for coronaviruses; researchers are working on both, but for now, care is supportive.
This article was updated with new case numbers on 1/26/20.