User login
Formerly Skin & Allergy News
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')]
The leading independent newspaper covering dermatology news and commentary.
FDA approves first treatment for advanced epithelioid sarcoma
The Food and Drug Administration has granted accelerated approval to tazemetostat (Tazverik) for the treatment of adults and pediatric patients aged 16 years and older with metastatic or locally advanced epithelioid sarcoma not eligible for complete resection.
Approval was based on overall response rate in a trial enrolling 62 patients with metastatic or locally advanced epithelioid sarcoma. The overall response rate was 15%, with 1.6% of patients having a complete response and 13% having a partial response. Of the nine patients that had a response, six (67%) had a response lasting 6 months or longer, the FDA said in a press statement.
The most common side effects for patients taking tazemetostat were pain, fatigue, nausea, decreased appetite, vomiting, and constipation. Patients treated with tazemetostat are at increased risk of developing secondary malignancies, including T-cell lymphoblastic lymphoma, myelodysplastic syndrome, and acute myeloid leukemia.
“Epithelioid sarcoma accounts for less than 1% of all soft-tissue sarcomas,” said Richard Pazdur, MD, director of the FDA’s Oncology Center of Excellence and acting director of the Office of Oncologic Diseases in the Center for Drug Evaluation and Research. “Until today, there were no treatment options specifically for patients with epithelioid sarcoma. The approval of Tazverik provides a treatment option that specifically targets this disease.”
Tazemetostat must be dispensed with a patient medication guide that describes important information about the drug’s uses and risks, the FDA said.
The Food and Drug Administration has granted accelerated approval to tazemetostat (Tazverik) for the treatment of adults and pediatric patients aged 16 years and older with metastatic or locally advanced epithelioid sarcoma not eligible for complete resection.
Approval was based on overall response rate in a trial enrolling 62 patients with metastatic or locally advanced epithelioid sarcoma. The overall response rate was 15%, with 1.6% of patients having a complete response and 13% having a partial response. Of the nine patients that had a response, six (67%) had a response lasting 6 months or longer, the FDA said in a press statement.
The most common side effects for patients taking tazemetostat were pain, fatigue, nausea, decreased appetite, vomiting, and constipation. Patients treated with tazemetostat are at increased risk of developing secondary malignancies, including T-cell lymphoblastic lymphoma, myelodysplastic syndrome, and acute myeloid leukemia.
“Epithelioid sarcoma accounts for less than 1% of all soft-tissue sarcomas,” said Richard Pazdur, MD, director of the FDA’s Oncology Center of Excellence and acting director of the Office of Oncologic Diseases in the Center for Drug Evaluation and Research. “Until today, there were no treatment options specifically for patients with epithelioid sarcoma. The approval of Tazverik provides a treatment option that specifically targets this disease.”
Tazemetostat must be dispensed with a patient medication guide that describes important information about the drug’s uses and risks, the FDA said.
The Food and Drug Administration has granted accelerated approval to tazemetostat (Tazverik) for the treatment of adults and pediatric patients aged 16 years and older with metastatic or locally advanced epithelioid sarcoma not eligible for complete resection.
Approval was based on overall response rate in a trial enrolling 62 patients with metastatic or locally advanced epithelioid sarcoma. The overall response rate was 15%, with 1.6% of patients having a complete response and 13% having a partial response. Of the nine patients that had a response, six (67%) had a response lasting 6 months or longer, the FDA said in a press statement.
The most common side effects for patients taking tazemetostat were pain, fatigue, nausea, decreased appetite, vomiting, and constipation. Patients treated with tazemetostat are at increased risk of developing secondary malignancies, including T-cell lymphoblastic lymphoma, myelodysplastic syndrome, and acute myeloid leukemia.
“Epithelioid sarcoma accounts for less than 1% of all soft-tissue sarcomas,” said Richard Pazdur, MD, director of the FDA’s Oncology Center of Excellence and acting director of the Office of Oncologic Diseases in the Center for Drug Evaluation and Research. “Until today, there were no treatment options specifically for patients with epithelioid sarcoma. The approval of Tazverik provides a treatment option that specifically targets this disease.”
Tazemetostat must be dispensed with a patient medication guide that describes important information about the drug’s uses and risks, the FDA said.
Actor Alan Alda discusses using empathy as an antidote to burnout
LA JOLLA, CALIF. – Physicians and other medical professionals who routinely foster empathic connections with patients may be helping themselves steer clear of burnout.
That’s what iconic actor Alan Alda suggested during a media briefing at Scripps Research on Jan. 16, 2020.

“There’s a tremendous pressure on doctors now to have shorter and shorter visits with their patients,” said the 83-year-old Mr. Alda, who received the Public Welfare Medal from the National Academy of Sciences in 2016 for his work as a champion of science. “A lot of that time is taken up with recording on a computer, which can only put pressure on the doctor.”
Practicing empathy, he continued, “kind of opens people up to one another, which inspirits them.”
Mr. Alda appeared on the research campus to announce that Scripps Research will serve as the new West Coast home of Alda Communication Training, which will work in tandem with the Alan Alda Center for Communicating Science at Stony Brook (N.Y.) University, a nonprofit organization that Mr. Alda helped found in 2009.
“This will be a center where people can come to get training in effective communication,” Mr. Alda, who is the winner of six Emmy Awards and six Golden Globe awards, told an audience of scientists and medical professionals prior to the media briefing.
“It’s an experiential kind of training,” he explained. “We don’t give tips. We don’t give lectures. We put you through exercises that are fun and actually make you laugh, but turn you into a better communicator, so you’re better able to connect to the people you’re talking to.”
During a question-and-answer session, Mr. Alda opened up about his Parkinson’s disease, which he said was diagnosed about 5 years ago. In 2018, he decided to speak publicly about his diagnosis for the first time.
“The reason was that I wanted to communicate to people who had recently been diagnosed not to believe or give into the stereotype that, when you get a diagnosis, your life is over,” said Mr. Alda, who played army surgeon “Hawkeye” Pierce on the TV series “M*A*S*H.”
“Under the burden of that belief, some people won’t tell their family or workplace colleagues,” he said. “There are exercises you can do and medications you can take to prolong the time it takes before Parkinson’s gets much more serious. It’s not to diminish the fact that it can get really bad; but to think that your life is over as soon as you get a diagnosis is wrong.”
The first 2-day training session at Scripps Research will be held in June 2020. Additional sessions are scheduled to take place in October and December. Registration is available at aldacommunicationtraining.com/workshops.
LA JOLLA, CALIF. – Physicians and other medical professionals who routinely foster empathic connections with patients may be helping themselves steer clear of burnout.
That’s what iconic actor Alan Alda suggested during a media briefing at Scripps Research on Jan. 16, 2020.

“There’s a tremendous pressure on doctors now to have shorter and shorter visits with their patients,” said the 83-year-old Mr. Alda, who received the Public Welfare Medal from the National Academy of Sciences in 2016 for his work as a champion of science. “A lot of that time is taken up with recording on a computer, which can only put pressure on the doctor.”
Practicing empathy, he continued, “kind of opens people up to one another, which inspirits them.”
Mr. Alda appeared on the research campus to announce that Scripps Research will serve as the new West Coast home of Alda Communication Training, which will work in tandem with the Alan Alda Center for Communicating Science at Stony Brook (N.Y.) University, a nonprofit organization that Mr. Alda helped found in 2009.
“This will be a center where people can come to get training in effective communication,” Mr. Alda, who is the winner of six Emmy Awards and six Golden Globe awards, told an audience of scientists and medical professionals prior to the media briefing.
“It’s an experiential kind of training,” he explained. “We don’t give tips. We don’t give lectures. We put you through exercises that are fun and actually make you laugh, but turn you into a better communicator, so you’re better able to connect to the people you’re talking to.”
During a question-and-answer session, Mr. Alda opened up about his Parkinson’s disease, which he said was diagnosed about 5 years ago. In 2018, he decided to speak publicly about his diagnosis for the first time.
“The reason was that I wanted to communicate to people who had recently been diagnosed not to believe or give into the stereotype that, when you get a diagnosis, your life is over,” said Mr. Alda, who played army surgeon “Hawkeye” Pierce on the TV series “M*A*S*H.”
“Under the burden of that belief, some people won’t tell their family or workplace colleagues,” he said. “There are exercises you can do and medications you can take to prolong the time it takes before Parkinson’s gets much more serious. It’s not to diminish the fact that it can get really bad; but to think that your life is over as soon as you get a diagnosis is wrong.”
The first 2-day training session at Scripps Research will be held in June 2020. Additional sessions are scheduled to take place in October and December. Registration is available at aldacommunicationtraining.com/workshops.
LA JOLLA, CALIF. – Physicians and other medical professionals who routinely foster empathic connections with patients may be helping themselves steer clear of burnout.
That’s what iconic actor Alan Alda suggested during a media briefing at Scripps Research on Jan. 16, 2020.

“There’s a tremendous pressure on doctors now to have shorter and shorter visits with their patients,” said the 83-year-old Mr. Alda, who received the Public Welfare Medal from the National Academy of Sciences in 2016 for his work as a champion of science. “A lot of that time is taken up with recording on a computer, which can only put pressure on the doctor.”
Practicing empathy, he continued, “kind of opens people up to one another, which inspirits them.”
Mr. Alda appeared on the research campus to announce that Scripps Research will serve as the new West Coast home of Alda Communication Training, which will work in tandem with the Alan Alda Center for Communicating Science at Stony Brook (N.Y.) University, a nonprofit organization that Mr. Alda helped found in 2009.
“This will be a center where people can come to get training in effective communication,” Mr. Alda, who is the winner of six Emmy Awards and six Golden Globe awards, told an audience of scientists and medical professionals prior to the media briefing.
“It’s an experiential kind of training,” he explained. “We don’t give tips. We don’t give lectures. We put you through exercises that are fun and actually make you laugh, but turn you into a better communicator, so you’re better able to connect to the people you’re talking to.”
During a question-and-answer session, Mr. Alda opened up about his Parkinson’s disease, which he said was diagnosed about 5 years ago. In 2018, he decided to speak publicly about his diagnosis for the first time.
“The reason was that I wanted to communicate to people who had recently been diagnosed not to believe or give into the stereotype that, when you get a diagnosis, your life is over,” said Mr. Alda, who played army surgeon “Hawkeye” Pierce on the TV series “M*A*S*H.”
“Under the burden of that belief, some people won’t tell their family or workplace colleagues,” he said. “There are exercises you can do and medications you can take to prolong the time it takes before Parkinson’s gets much more serious. It’s not to diminish the fact that it can get really bad; but to think that your life is over as soon as you get a diagnosis is wrong.”
The first 2-day training session at Scripps Research will be held in June 2020. Additional sessions are scheduled to take place in October and December. Registration is available at aldacommunicationtraining.com/workshops.
Exogenous boosting against shingles not as robust as thought
Exposure to children with chickenpox reduces the incidence of shingles in adults 33% over 2 years, and 27% out to 20 years, according to British investigators.
Being exposed to children with illness due to varicella infection acts as an “exogenous booster” in adults who had chickenpox themselves as children, making shingles less likely, they explained in a BMJ article.
Although that’s good news, it’s been reported previously that exposure to children with chickenpox confers complete protection against shingles in adults for years afterward.
The finding matters in the United Kingdom because varicella vaccine is not part of the pediatric immunization schedule. The United States is the only country that mandates two shots as a requirement for children to attend school.
The United Kingdom, however, is reconsidering its policy. In the past, the exogenous booster idea has been one of the arguments used against mandating the vaccine for children; the concern is that preventing chickenpox in children – and subsequent reexposure to herpes zoster in adults – would kick off a costly wave of shingles in adults.
The study results “are themselves unable to justify for or against specific vaccination schedules, but they do suggest that revised mathematical models are required to estimate the impact of varicella vaccination, with the updated assumption that exogenous boosting is incomplete and only reduces the risk of zoster by about 30%,” noted the investigators, led by Harriet Forbes of the London School of Hygiene and Tropical Medicine.
The researchers identified 9,604 adults with a shingles diagnosis during 1997-2018 who at some point lived with a child who had chickenpox. Data came from the U.K. Clinical Practice Research Datalink, a general practice database.
They then looked at the incidence of shingles within 20 years of exposure to the sick child and compared it with the incidence before exposure and after 20 years, by which time the exogenous booster is thought to wear off. It was a self-controlled case series analysis, “a relatively novel epidemiological study design where individuals act as their own controls. Comparisons are made within individuals rather than between individuals as in a cohort or case control study,” Ms. Forbes and colleagues explained.
After adjustment for age, calendar time, and season, they found that in the 2 years after household exposure to a child with varicella, adults were 33% less likely to develop zoster (incidence ratio 0.67, 95% confidence interval 0.62-0.73), and 27% less likely from 10 to 20 years (IR 0.73, CI 0.62-0.87). The boosting effect appeared to be stronger in men.
“Exogenous boosting provides some protection from the risk of herpes zoster, but not complete immunity, as assumed by previous cost effectiveness estimates of varicella immunization,” the researchers said.
More than two-thirds of the adults with shingles were women, which fits with previous reports. Median age of exposure to a child with varicella was 38 years.
Ms. Forbes and colleagues noted that “the study design required patients with zoster to be living with a child with varicella, therefore the study cohort is younger than a general population with zoster. ... However, when we restricted our analysis to adults aged 50 and older at exposure to varicella, a similar pattern of association was observed, with no evidence of effect modification by age. This suggests that although the median age of our study cohort ... was low, the findings can be generalized to older people.”
There was no external funding for the work, and the lead investigator had no relevant financial disclosures. One investigator reported research grants from GSK and Merck, both makers of chickenpox and shingles vaccines.
SOURCE: Forbes H et al. BMJ. 2020 Jan 22;368:l6987.
Exposure to children with chickenpox reduces the incidence of shingles in adults 33% over 2 years, and 27% out to 20 years, according to British investigators.
Being exposed to children with illness due to varicella infection acts as an “exogenous booster” in adults who had chickenpox themselves as children, making shingles less likely, they explained in a BMJ article.
Although that’s good news, it’s been reported previously that exposure to children with chickenpox confers complete protection against shingles in adults for years afterward.
The finding matters in the United Kingdom because varicella vaccine is not part of the pediatric immunization schedule. The United States is the only country that mandates two shots as a requirement for children to attend school.
The United Kingdom, however, is reconsidering its policy. In the past, the exogenous booster idea has been one of the arguments used against mandating the vaccine for children; the concern is that preventing chickenpox in children – and subsequent reexposure to herpes zoster in adults – would kick off a costly wave of shingles in adults.
The study results “are themselves unable to justify for or against specific vaccination schedules, but they do suggest that revised mathematical models are required to estimate the impact of varicella vaccination, with the updated assumption that exogenous boosting is incomplete and only reduces the risk of zoster by about 30%,” noted the investigators, led by Harriet Forbes of the London School of Hygiene and Tropical Medicine.
The researchers identified 9,604 adults with a shingles diagnosis during 1997-2018 who at some point lived with a child who had chickenpox. Data came from the U.K. Clinical Practice Research Datalink, a general practice database.
They then looked at the incidence of shingles within 20 years of exposure to the sick child and compared it with the incidence before exposure and after 20 years, by which time the exogenous booster is thought to wear off. It was a self-controlled case series analysis, “a relatively novel epidemiological study design where individuals act as their own controls. Comparisons are made within individuals rather than between individuals as in a cohort or case control study,” Ms. Forbes and colleagues explained.
After adjustment for age, calendar time, and season, they found that in the 2 years after household exposure to a child with varicella, adults were 33% less likely to develop zoster (incidence ratio 0.67, 95% confidence interval 0.62-0.73), and 27% less likely from 10 to 20 years (IR 0.73, CI 0.62-0.87). The boosting effect appeared to be stronger in men.
“Exogenous boosting provides some protection from the risk of herpes zoster, but not complete immunity, as assumed by previous cost effectiveness estimates of varicella immunization,” the researchers said.
More than two-thirds of the adults with shingles were women, which fits with previous reports. Median age of exposure to a child with varicella was 38 years.
Ms. Forbes and colleagues noted that “the study design required patients with zoster to be living with a child with varicella, therefore the study cohort is younger than a general population with zoster. ... However, when we restricted our analysis to adults aged 50 and older at exposure to varicella, a similar pattern of association was observed, with no evidence of effect modification by age. This suggests that although the median age of our study cohort ... was low, the findings can be generalized to older people.”
There was no external funding for the work, and the lead investigator had no relevant financial disclosures. One investigator reported research grants from GSK and Merck, both makers of chickenpox and shingles vaccines.
SOURCE: Forbes H et al. BMJ. 2020 Jan 22;368:l6987.
Exposure to children with chickenpox reduces the incidence of shingles in adults 33% over 2 years, and 27% out to 20 years, according to British investigators.
Being exposed to children with illness due to varicella infection acts as an “exogenous booster” in adults who had chickenpox themselves as children, making shingles less likely, they explained in a BMJ article.
Although that’s good news, it’s been reported previously that exposure to children with chickenpox confers complete protection against shingles in adults for years afterward.
The finding matters in the United Kingdom because varicella vaccine is not part of the pediatric immunization schedule. The United States is the only country that mandates two shots as a requirement for children to attend school.
The United Kingdom, however, is reconsidering its policy. In the past, the exogenous booster idea has been one of the arguments used against mandating the vaccine for children; the concern is that preventing chickenpox in children – and subsequent reexposure to herpes zoster in adults – would kick off a costly wave of shingles in adults.
The study results “are themselves unable to justify for or against specific vaccination schedules, but they do suggest that revised mathematical models are required to estimate the impact of varicella vaccination, with the updated assumption that exogenous boosting is incomplete and only reduces the risk of zoster by about 30%,” noted the investigators, led by Harriet Forbes of the London School of Hygiene and Tropical Medicine.
The researchers identified 9,604 adults with a shingles diagnosis during 1997-2018 who at some point lived with a child who had chickenpox. Data came from the U.K. Clinical Practice Research Datalink, a general practice database.
They then looked at the incidence of shingles within 20 years of exposure to the sick child and compared it with the incidence before exposure and after 20 years, by which time the exogenous booster is thought to wear off. It was a self-controlled case series analysis, “a relatively novel epidemiological study design where individuals act as their own controls. Comparisons are made within individuals rather than between individuals as in a cohort or case control study,” Ms. Forbes and colleagues explained.
After adjustment for age, calendar time, and season, they found that in the 2 years after household exposure to a child with varicella, adults were 33% less likely to develop zoster (incidence ratio 0.67, 95% confidence interval 0.62-0.73), and 27% less likely from 10 to 20 years (IR 0.73, CI 0.62-0.87). The boosting effect appeared to be stronger in men.
“Exogenous boosting provides some protection from the risk of herpes zoster, but not complete immunity, as assumed by previous cost effectiveness estimates of varicella immunization,” the researchers said.
More than two-thirds of the adults with shingles were women, which fits with previous reports. Median age of exposure to a child with varicella was 38 years.
Ms. Forbes and colleagues noted that “the study design required patients with zoster to be living with a child with varicella, therefore the study cohort is younger than a general population with zoster. ... However, when we restricted our analysis to adults aged 50 and older at exposure to varicella, a similar pattern of association was observed, with no evidence of effect modification by age. This suggests that although the median age of our study cohort ... was low, the findings can be generalized to older people.”
There was no external funding for the work, and the lead investigator had no relevant financial disclosures. One investigator reported research grants from GSK and Merck, both makers of chickenpox and shingles vaccines.
SOURCE: Forbes H et al. BMJ. 2020 Jan 22;368:l6987.
FROM BMJ
Apremilast more likely to succeed with moderate psoriatic arthritis activity
new research suggests.
A paper published in Arthritis Care & Research presents a pooled analysis of the PALACE 1-3 studies that included a total of 1,493 patients with active psoriatic arthritis whose disease had resisted treatment with tumor necrosis factor inhibitors or conventional disease-modifying antirheumatic drugs. Participants were randomized either to the oral phosphodiesterase 4 inhibitor apremilast (Otezla) 30 mg twice daily, 20 mg twice daily, or placebo for 24 weeks, after which all patients on placebo were rerandomized to one of the two apremilast doses until 52 weeks.
The analysis focused on 494 patients who were randomized to apremilast 30 mg twice daily at baseline.
At week 16, 40% patients with low disease activity at baseline had achieved remission on their clinical Disease Activity Index for Psoriatic Arthritis (cDAPSA) score, compared with 7% of patients with moderate disease activity and 2.1% of patients with high disease activity. The cDAPSA score is calculated as a composite score including swollen and tender joint counts, patient’s assessment of pain, and patient’s global assessment of disease activity, with possible scores from 0 to 154. Based on patients’ cDAPSA score, the researchers defined remission as a score of 4 or less, low disease activity as more than 4 and up to 13, moderate disease activity as more than 13 and up to 27, and high disease activity as greater than 27.
Among patients with moderate disease activity, 29.8% achieved low disease activity by week 16; among patients with high disease activity at baseline, 11.5% achieved low disease activity, and 38.1% achieved moderate disease activity.
The study found that patients who had moderate disease activity at baseline and achieved either low disease activity or remission by week 16 had a 58.9%-88.5% probability of remaining at those treatment targets by week 52. Patients with high disease activity at baseline who achieved low disease activity or remission by week 16 had a 64.3%-77.4% probability of achieving treatment targets by week 52.
Overall, nearly twice as many patients who had moderate disease activity at baseline achieved their treatment targets when compared with those who began with high disease activity (46.9% vs. 24.9%).
Any patient who achieved at least a 30% improvement in cDAPSA score by week 16 had a 63% probability of achieving treatment targets by week 52.
First author Philip J. Mease, MD, from the Swedish Medical Center and the University of Washington, Seattle, and coauthors noted that the absence of treatment response by week 16 should point to the need for a treatment adjustment. “Taken together, these findings provide a framework of reference for the selection and monitoring of patients with the highest likelihood of achieving optimal treatment responses with apremilast in clinical practice,” they wrote.
The authors also commented that their study provided support for the use of clinical Disease Activity Index for Psoriatic Arthritis score to monitor patients treated with apremilast.
The study was sponsored by Celgene. Three authors were employees of Celgene at the time of the study, and nine authors declared a range of consultancies, grants, research, and other support from the pharmaceutical sector, including from Celgene.
SOURCE: Mease P et al. Arthritis Care Res. 2019 Jan 7. doi: 10.1002/acr.24134
new research suggests.
A paper published in Arthritis Care & Research presents a pooled analysis of the PALACE 1-3 studies that included a total of 1,493 patients with active psoriatic arthritis whose disease had resisted treatment with tumor necrosis factor inhibitors or conventional disease-modifying antirheumatic drugs. Participants were randomized either to the oral phosphodiesterase 4 inhibitor apremilast (Otezla) 30 mg twice daily, 20 mg twice daily, or placebo for 24 weeks, after which all patients on placebo were rerandomized to one of the two apremilast doses until 52 weeks.
The analysis focused on 494 patients who were randomized to apremilast 30 mg twice daily at baseline.
At week 16, 40% patients with low disease activity at baseline had achieved remission on their clinical Disease Activity Index for Psoriatic Arthritis (cDAPSA) score, compared with 7% of patients with moderate disease activity and 2.1% of patients with high disease activity. The cDAPSA score is calculated as a composite score including swollen and tender joint counts, patient’s assessment of pain, and patient’s global assessment of disease activity, with possible scores from 0 to 154. Based on patients’ cDAPSA score, the researchers defined remission as a score of 4 or less, low disease activity as more than 4 and up to 13, moderate disease activity as more than 13 and up to 27, and high disease activity as greater than 27.
Among patients with moderate disease activity, 29.8% achieved low disease activity by week 16; among patients with high disease activity at baseline, 11.5% achieved low disease activity, and 38.1% achieved moderate disease activity.
The study found that patients who had moderate disease activity at baseline and achieved either low disease activity or remission by week 16 had a 58.9%-88.5% probability of remaining at those treatment targets by week 52. Patients with high disease activity at baseline who achieved low disease activity or remission by week 16 had a 64.3%-77.4% probability of achieving treatment targets by week 52.
Overall, nearly twice as many patients who had moderate disease activity at baseline achieved their treatment targets when compared with those who began with high disease activity (46.9% vs. 24.9%).
Any patient who achieved at least a 30% improvement in cDAPSA score by week 16 had a 63% probability of achieving treatment targets by week 52.
First author Philip J. Mease, MD, from the Swedish Medical Center and the University of Washington, Seattle, and coauthors noted that the absence of treatment response by week 16 should point to the need for a treatment adjustment. “Taken together, these findings provide a framework of reference for the selection and monitoring of patients with the highest likelihood of achieving optimal treatment responses with apremilast in clinical practice,” they wrote.
The authors also commented that their study provided support for the use of clinical Disease Activity Index for Psoriatic Arthritis score to monitor patients treated with apremilast.
The study was sponsored by Celgene. Three authors were employees of Celgene at the time of the study, and nine authors declared a range of consultancies, grants, research, and other support from the pharmaceutical sector, including from Celgene.
SOURCE: Mease P et al. Arthritis Care Res. 2019 Jan 7. doi: 10.1002/acr.24134
new research suggests.
A paper published in Arthritis Care & Research presents a pooled analysis of the PALACE 1-3 studies that included a total of 1,493 patients with active psoriatic arthritis whose disease had resisted treatment with tumor necrosis factor inhibitors or conventional disease-modifying antirheumatic drugs. Participants were randomized either to the oral phosphodiesterase 4 inhibitor apremilast (Otezla) 30 mg twice daily, 20 mg twice daily, or placebo for 24 weeks, after which all patients on placebo were rerandomized to one of the two apremilast doses until 52 weeks.
The analysis focused on 494 patients who were randomized to apremilast 30 mg twice daily at baseline.
At week 16, 40% patients with low disease activity at baseline had achieved remission on their clinical Disease Activity Index for Psoriatic Arthritis (cDAPSA) score, compared with 7% of patients with moderate disease activity and 2.1% of patients with high disease activity. The cDAPSA score is calculated as a composite score including swollen and tender joint counts, patient’s assessment of pain, and patient’s global assessment of disease activity, with possible scores from 0 to 154. Based on patients’ cDAPSA score, the researchers defined remission as a score of 4 or less, low disease activity as more than 4 and up to 13, moderate disease activity as more than 13 and up to 27, and high disease activity as greater than 27.
Among patients with moderate disease activity, 29.8% achieved low disease activity by week 16; among patients with high disease activity at baseline, 11.5% achieved low disease activity, and 38.1% achieved moderate disease activity.
The study found that patients who had moderate disease activity at baseline and achieved either low disease activity or remission by week 16 had a 58.9%-88.5% probability of remaining at those treatment targets by week 52. Patients with high disease activity at baseline who achieved low disease activity or remission by week 16 had a 64.3%-77.4% probability of achieving treatment targets by week 52.
Overall, nearly twice as many patients who had moderate disease activity at baseline achieved their treatment targets when compared with those who began with high disease activity (46.9% vs. 24.9%).
Any patient who achieved at least a 30% improvement in cDAPSA score by week 16 had a 63% probability of achieving treatment targets by week 52.
First author Philip J. Mease, MD, from the Swedish Medical Center and the University of Washington, Seattle, and coauthors noted that the absence of treatment response by week 16 should point to the need for a treatment adjustment. “Taken together, these findings provide a framework of reference for the selection and monitoring of patients with the highest likelihood of achieving optimal treatment responses with apremilast in clinical practice,” they wrote.
The authors also commented that their study provided support for the use of clinical Disease Activity Index for Psoriatic Arthritis score to monitor patients treated with apremilast.
The study was sponsored by Celgene. Three authors were employees of Celgene at the time of the study, and nine authors declared a range of consultancies, grants, research, and other support from the pharmaceutical sector, including from Celgene.
SOURCE: Mease P et al. Arthritis Care Res. 2019 Jan 7. doi: 10.1002/acr.24134
FROM ARTHRITIS CARE & RESEARCH
February 2020
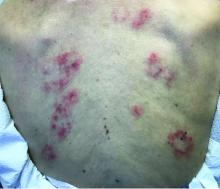
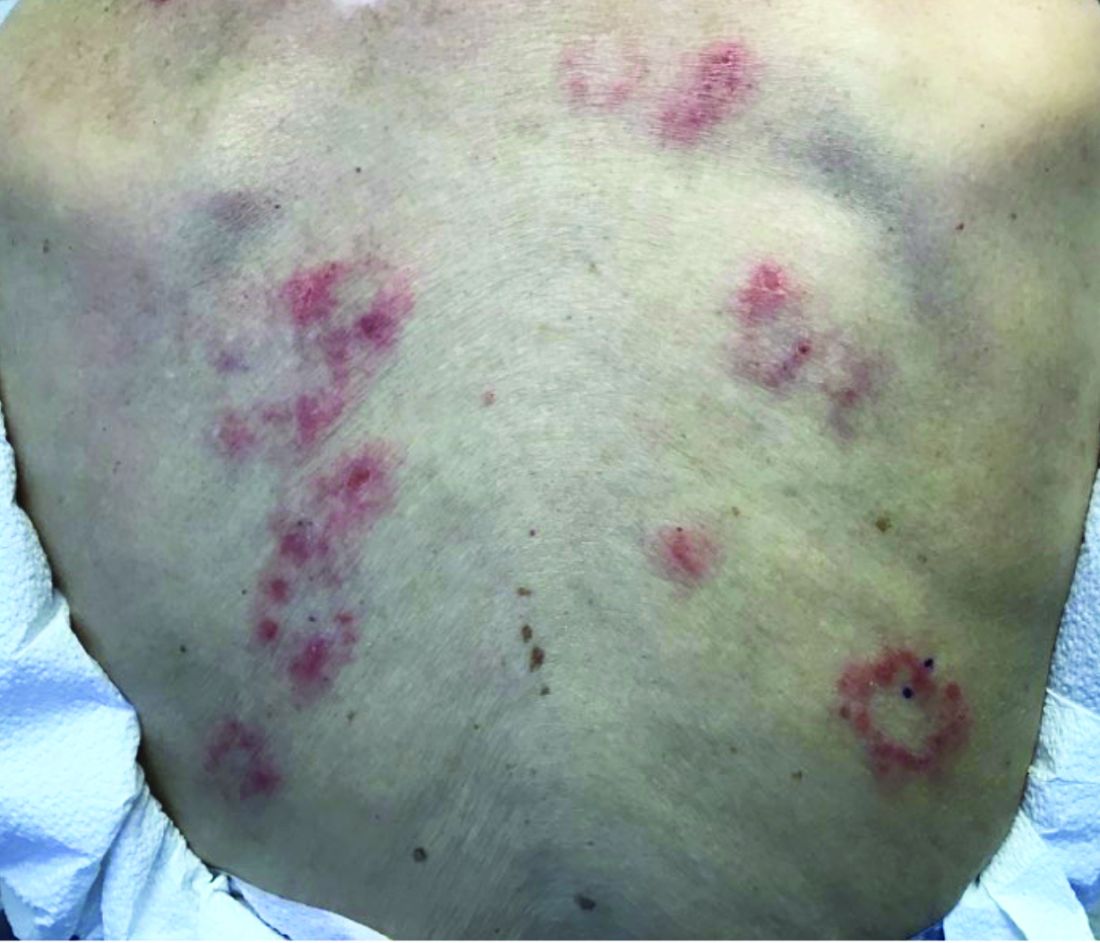
Subacute cutaneous lupus erythematosus
Subacute cutaneous lupus erythematosus (SCLE) is a type of cutaneous lupus erythematosus that may occur independently of or in combination with systemic lupus erythematosus. About 10%-15% of patients with SCLE will develop systemic lupus erythematosus. White females are more typically affected.
SCLE lesions often present as scaly, annular, or polycyclic scaly patches and plaques with central clearing. They may appear psoriasiform. They heal without atrophy or scarring but may leave dyspigmentation. Follicular plugging is absent. Lesions generally occur on sun exposed areas such as the neck, V of the chest, and upper extremities. Up to 75% of patients may exhibit associated symptoms such as photosensitivity, oral ulcers, and arthritis. Less than 20% of patients will develop internal disease, including nephritis and pulmonary disease. Symptoms of Sjögren’s syndrome and SCLE may overlap in some patients, and will portend higher risk for internal disease.
The differential diagnosis includes eczema, psoriasis, dermatophytosis, granuloma annulare, and erythema annulare centrifugum. Histology reveals epidermal atrophy and keratinocyte apoptosis, with a superficial and perivascular lymphohistiocytic infiltrate in the upper dermis. Interface changes at the dermal-epidermal junction can be seen. Direct immunofluorescence of lesional skin is positive in one-third of cases, often revealing granular deposits of IgG and IgM at the dermal-epidermal junction and around hair follicles (called the lupus-band test). Serology in SCLE may reveal a positive antinuclear antigen test, as well as positive Ro/SSA antigen. Other lupus serologies such as La/SSB, dsDNA, antihistone, and Sm antibodies may be positive, but are less commonly seen.
Several drugs may cause SCLE, such as hydrochlorothiazide, terbinafine, ACE inhibitors, NSAIDs, calcium-channel blockers, interferons, anticonvulsants, griseofulvin, penicillamine, spironolactone, tumor necrosis factor–alpha inhibitors, and statins. Discontinuing the offending medications may clear the lesions, but not always.
Treatment includes sunscreen and avoidance of sun exposure. Potent topical corticosteroids are helpful. If systemic treatment is indicated, antimalarials are first line.
This case and photo were submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
Subacute cutaneous lupus erythematosus
Subacute cutaneous lupus erythematosus (SCLE) is a type of cutaneous lupus erythematosus that may occur independently of or in combination with systemic lupus erythematosus. About 10%-15% of patients with SCLE will develop systemic lupus erythematosus. White females are more typically affected.
SCLE lesions often present as scaly, annular, or polycyclic scaly patches and plaques with central clearing. They may appear psoriasiform. They heal without atrophy or scarring but may leave dyspigmentation. Follicular plugging is absent. Lesions generally occur on sun exposed areas such as the neck, V of the chest, and upper extremities. Up to 75% of patients may exhibit associated symptoms such as photosensitivity, oral ulcers, and arthritis. Less than 20% of patients will develop internal disease, including nephritis and pulmonary disease. Symptoms of Sjögren’s syndrome and SCLE may overlap in some patients, and will portend higher risk for internal disease.
The differential diagnosis includes eczema, psoriasis, dermatophytosis, granuloma annulare, and erythema annulare centrifugum. Histology reveals epidermal atrophy and keratinocyte apoptosis, with a superficial and perivascular lymphohistiocytic infiltrate in the upper dermis. Interface changes at the dermal-epidermal junction can be seen. Direct immunofluorescence of lesional skin is positive in one-third of cases, often revealing granular deposits of IgG and IgM at the dermal-epidermal junction and around hair follicles (called the lupus-band test). Serology in SCLE may reveal a positive antinuclear antigen test, as well as positive Ro/SSA antigen. Other lupus serologies such as La/SSB, dsDNA, antihistone, and Sm antibodies may be positive, but are less commonly seen.
Several drugs may cause SCLE, such as hydrochlorothiazide, terbinafine, ACE inhibitors, NSAIDs, calcium-channel blockers, interferons, anticonvulsants, griseofulvin, penicillamine, spironolactone, tumor necrosis factor–alpha inhibitors, and statins. Discontinuing the offending medications may clear the lesions, but not always.
Treatment includes sunscreen and avoidance of sun exposure. Potent topical corticosteroids are helpful. If systemic treatment is indicated, antimalarials are first line.
This case and photo were submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
Subacute cutaneous lupus erythematosus
Subacute cutaneous lupus erythematosus (SCLE) is a type of cutaneous lupus erythematosus that may occur independently of or in combination with systemic lupus erythematosus. About 10%-15% of patients with SCLE will develop systemic lupus erythematosus. White females are more typically affected.
SCLE lesions often present as scaly, annular, or polycyclic scaly patches and plaques with central clearing. They may appear psoriasiform. They heal without atrophy or scarring but may leave dyspigmentation. Follicular plugging is absent. Lesions generally occur on sun exposed areas such as the neck, V of the chest, and upper extremities. Up to 75% of patients may exhibit associated symptoms such as photosensitivity, oral ulcers, and arthritis. Less than 20% of patients will develop internal disease, including nephritis and pulmonary disease. Symptoms of Sjögren’s syndrome and SCLE may overlap in some patients, and will portend higher risk for internal disease.
The differential diagnosis includes eczema, psoriasis, dermatophytosis, granuloma annulare, and erythema annulare centrifugum. Histology reveals epidermal atrophy and keratinocyte apoptosis, with a superficial and perivascular lymphohistiocytic infiltrate in the upper dermis. Interface changes at the dermal-epidermal junction can be seen. Direct immunofluorescence of lesional skin is positive in one-third of cases, often revealing granular deposits of IgG and IgM at the dermal-epidermal junction and around hair follicles (called the lupus-band test). Serology in SCLE may reveal a positive antinuclear antigen test, as well as positive Ro/SSA antigen. Other lupus serologies such as La/SSB, dsDNA, antihistone, and Sm antibodies may be positive, but are less commonly seen.
Several drugs may cause SCLE, such as hydrochlorothiazide, terbinafine, ACE inhibitors, NSAIDs, calcium-channel blockers, interferons, anticonvulsants, griseofulvin, penicillamine, spironolactone, tumor necrosis factor–alpha inhibitors, and statins. Discontinuing the offending medications may clear the lesions, but not always.
Treatment includes sunscreen and avoidance of sun exposure. Potent topical corticosteroids are helpful. If systemic treatment is indicated, antimalarials are first line.
This case and photo were submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
New year, old you
This column should arrive just in time. By this time in February, eighty percent of us will abort what we resolved to do this year. If this was you, it could be considered a catastrophic failure because not only is it a new year, it is a new decade. That’s right, the opportunity to fix the 10-year-imperfect you won’t come again until 2030!
I’m among you. I intended to read fiction daily (starting with “The Great Gatsby,” not “Moby Dick” – I thought I would give myself a fighting chance, but alas ...), to workout at least 5 days every week (I tore my left triangular fibrocartilage complex, so there’s that), to write at least 500 words daily (I’m typing this one-handed: I’m lucky to get 500 letters a day). So I’m out.
If you resolved to do something this year, chances are it was to make a better you: a self-improvement goal such as losing weight, saving more money, or exercising more. According to a Marist Poll, these were the most popular resolutions for 2020. At the bottom of the most-likely-resolutions list were things like “worry less” or “be kinder to others.” These are important goals we’d agree, but we don’t deem them resolution-worthy. Why?
And why do we have New Year’s resolutions in the first place? When I looked into this further, I was surprised by some of the history I discovered.
As far back as the Babylonians, once a year, we’ve tried our best to get better. At the feast of Akitu, the Babylonian new year festival (about March on our modern calendar), people resolved to do a better job of paying debts and returning favors – spin had not been invented, and yoga hadn’t caught on in the Middle East yet. This fundamental desire to be a better human seems hardwired, and long before Bullet Journals we seem to have loved “fresh start” days on the calendar. Yet, we’re doomed to fail, over and over, at least for the last 5,000 or so attempts.
We know so much more now. Put your Nike Renue Fusion shoes next to your bed so you get up and run first thing. Set SMART goals. Sign up for automatic retirement contribution and for automatic, plant-based meal delivery from Blue Apron. (I’ve no conflict of interest in these products).
Good ideas all, but I’m suggesting a different approach: Resolve to do something else this year.
Rather than try the same things we’ve attempted, how about selecting something from the bottom of the Marist Poll list – such as resolving to be more humble. Admit when you don’t know something or don’t understand what’s being discussed. Recognize and acknowledge when you’ve screwed up. Or resolve to be more selfless. Add on someone else’s patient, an extra call without expecting a favor in return, or do what you can to help a curbside consult, even if there is no reward or even a small risk to you. Repay the debt you owe your friends, family, colleagues, staff, and patients.
These things are a little trickier to track, but you can find a way to keep yourself accountable. Add a box to your weekly planner that says “Be humble and kind” and check it off for the next 42 weeks. Good news, March 1 falls on a Sunday this year – let’s call it the feast of Akitu.
Happy New Year! And good luck!
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
This column should arrive just in time. By this time in February, eighty percent of us will abort what we resolved to do this year. If this was you, it could be considered a catastrophic failure because not only is it a new year, it is a new decade. That’s right, the opportunity to fix the 10-year-imperfect you won’t come again until 2030!
I’m among you. I intended to read fiction daily (starting with “The Great Gatsby,” not “Moby Dick” – I thought I would give myself a fighting chance, but alas ...), to workout at least 5 days every week (I tore my left triangular fibrocartilage complex, so there’s that), to write at least 500 words daily (I’m typing this one-handed: I’m lucky to get 500 letters a day). So I’m out.
If you resolved to do something this year, chances are it was to make a better you: a self-improvement goal such as losing weight, saving more money, or exercising more. According to a Marist Poll, these were the most popular resolutions for 2020. At the bottom of the most-likely-resolutions list were things like “worry less” or “be kinder to others.” These are important goals we’d agree, but we don’t deem them resolution-worthy. Why?
And why do we have New Year’s resolutions in the first place? When I looked into this further, I was surprised by some of the history I discovered.
As far back as the Babylonians, once a year, we’ve tried our best to get better. At the feast of Akitu, the Babylonian new year festival (about March on our modern calendar), people resolved to do a better job of paying debts and returning favors – spin had not been invented, and yoga hadn’t caught on in the Middle East yet. This fundamental desire to be a better human seems hardwired, and long before Bullet Journals we seem to have loved “fresh start” days on the calendar. Yet, we’re doomed to fail, over and over, at least for the last 5,000 or so attempts.
We know so much more now. Put your Nike Renue Fusion shoes next to your bed so you get up and run first thing. Set SMART goals. Sign up for automatic retirement contribution and for automatic, plant-based meal delivery from Blue Apron. (I’ve no conflict of interest in these products).
Good ideas all, but I’m suggesting a different approach: Resolve to do something else this year.
Rather than try the same things we’ve attempted, how about selecting something from the bottom of the Marist Poll list – such as resolving to be more humble. Admit when you don’t know something or don’t understand what’s being discussed. Recognize and acknowledge when you’ve screwed up. Or resolve to be more selfless. Add on someone else’s patient, an extra call without expecting a favor in return, or do what you can to help a curbside consult, even if there is no reward or even a small risk to you. Repay the debt you owe your friends, family, colleagues, staff, and patients.
These things are a little trickier to track, but you can find a way to keep yourself accountable. Add a box to your weekly planner that says “Be humble and kind” and check it off for the next 42 weeks. Good news, March 1 falls on a Sunday this year – let’s call it the feast of Akitu.
Happy New Year! And good luck!
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
This column should arrive just in time. By this time in February, eighty percent of us will abort what we resolved to do this year. If this was you, it could be considered a catastrophic failure because not only is it a new year, it is a new decade. That’s right, the opportunity to fix the 10-year-imperfect you won’t come again until 2030!
I’m among you. I intended to read fiction daily (starting with “The Great Gatsby,” not “Moby Dick” – I thought I would give myself a fighting chance, but alas ...), to workout at least 5 days every week (I tore my left triangular fibrocartilage complex, so there’s that), to write at least 500 words daily (I’m typing this one-handed: I’m lucky to get 500 letters a day). So I’m out.
If you resolved to do something this year, chances are it was to make a better you: a self-improvement goal such as losing weight, saving more money, or exercising more. According to a Marist Poll, these were the most popular resolutions for 2020. At the bottom of the most-likely-resolutions list were things like “worry less” or “be kinder to others.” These are important goals we’d agree, but we don’t deem them resolution-worthy. Why?
And why do we have New Year’s resolutions in the first place? When I looked into this further, I was surprised by some of the history I discovered.
As far back as the Babylonians, once a year, we’ve tried our best to get better. At the feast of Akitu, the Babylonian new year festival (about March on our modern calendar), people resolved to do a better job of paying debts and returning favors – spin had not been invented, and yoga hadn’t caught on in the Middle East yet. This fundamental desire to be a better human seems hardwired, and long before Bullet Journals we seem to have loved “fresh start” days on the calendar. Yet, we’re doomed to fail, over and over, at least for the last 5,000 or so attempts.
We know so much more now. Put your Nike Renue Fusion shoes next to your bed so you get up and run first thing. Set SMART goals. Sign up for automatic retirement contribution and for automatic, plant-based meal delivery from Blue Apron. (I’ve no conflict of interest in these products).
Good ideas all, but I’m suggesting a different approach: Resolve to do something else this year.
Rather than try the same things we’ve attempted, how about selecting something from the bottom of the Marist Poll list – such as resolving to be more humble. Admit when you don’t know something or don’t understand what’s being discussed. Recognize and acknowledge when you’ve screwed up. Or resolve to be more selfless. Add on someone else’s patient, an extra call without expecting a favor in return, or do what you can to help a curbside consult, even if there is no reward or even a small risk to you. Repay the debt you owe your friends, family, colleagues, staff, and patients.
These things are a little trickier to track, but you can find a way to keep yourself accountable. Add a box to your weekly planner that says “Be humble and kind” and check it off for the next 42 weeks. Good news, March 1 falls on a Sunday this year – let’s call it the feast of Akitu.
Happy New Year! And good luck!
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
FDA supports sunscreen safety studies
Six active in a randomized trial including four product types. The results were published in JAMA.
The testing was done as part of a proposed rule on sunscreen, published in February 2019, which requested additional information on sunscreen ingredients. Murali K. Matta, PhD, of the Food and Drug Administration and coauthors wrote that these plasma concentrations “surpassed the FDA threshold for potentially waiving additional safety studies for sunscreens.” But, they added, the findings “do not indicate that individuals should refrain from the use of sunscreen.”
This was a follow-up study to a smaller study of 24 health volunteers published last year that determined that the sunscreen active ingredients tested were absorbed systemically (JAMA. 2019;321[21]:2082-91). “This follow-up study expanded the sample size, tested additional sunscreen active ingredients and formulations, and confirmed the finding that sunscreen active ingredients are systemically absorbed,” the authors wrote.
To gather information on the absorption of active ingredients in sunscreens, the investigators randomized 48 adults to one of four sunscreen products (lotion, aerosol spray, nonaerosol spray, or pump spray) with one of six active ingredients (avobenzone, oxybenzone, octocrylene, homosalate, octisalate, and octinoxate). Not all products contained each of the ingredients.
The participants applied the products in amounts of 2 mg/cm2 to 75% of body surface area at baseline, no use on day 1 and four times a day at 2-hour intervals on days 2 through 4. The researchers collected blood samples over 21 days and measured the maximum plasma concentrations. The average age of the participants was 37 years, and half were women. The study was conducted in a clinical pharmacology unit.
The geometric mean maximum plasma concentrations for the primary endpoint of avobenzone in lotion, aerosol spray, nonaerosol spray, and pump spray were 7.1 ng/mL, 3.5 ng/mL, 3.5 ng/mL, and 3.3 ng/mL, respectively.
For oxybenzone, the concentrations were 258.1 ng/mL and 180.1 ng/mL, respectively, for lotion and aerosol spray. The concentrations for octocrylene were 7.8 ng/mL, 6.6 ng/mL, and 6.6 ng/mL, respectively, for lotion, aerosol spray, and nonaerosol spray.
For homosalate, the geometric mean plasma concentrations were 23.1 ng/mL for aerosol spray, 17.9 for nonaerosol spray, and 13.9 for pump spray. For octisalate, the concentrations were 5.1 ng/mL, 5.8 ng/mL, and 4.6 ng/mL, respectively, for aerosol spray, nonaerosol spray, and pump spray. For octinoxate, the concentrations were 7.9 ng/mL for nonaerosol spray and 5.2 ng/mL for pump spray.
“The systemic exposures, as measured by geometric mean maximum plasma concentrations, of all the tested active ingredients were higher than 0.5 ng/mL after a single application,” the researchers noted.
Overall, the most common event was rash, which was reported in 14 participants.
The study findings were limited by several factors including the use of an indoor clinical setting, rather than outdoor exposure; the inability to assess absorption differences by formulation and Fitzpatrick skin type; and the variation in amounts of ingredients among products, the researchers noted. However, the results can be used to design additional studies needed to research the effects of systemic exposure to sunscreen ingredients, they said.
In an accompanying editorial (JAMA. 2020;323:223-4), Adewole S. Adamson, MD, of the University of Texas at Austin, and Kanade Shinkai, MD, of the University of California, San Francisco, wrote that “the study did not address key questions about sunscreen safety,” including the length of time it takes “for plasma concentrations of sunscreen ingredients to fall below the FDA threshold for safety testing.” Dr. Shinkai is also editor in chief of JAMA Dermatology.
“In making an informed decision, clinicians must determine whether the magnitude of the benefit exceeds the risk of potential harm for a specific individual,” they said. “Importantly, this balance may be different, depending on characteristics of the sunscreen user (e.g., for individuals with darker skin types and for children) and may depend on the frequency and duration of application (e.g., daily vs. intermittent use; starting in infancy or later in life),” they noted.
“In the absence of clear data demonstrating harm, the use of chemical sunscreen may still be considered appropriate; the use of mineral-based sunscreen is a well-established safe alternative,” although the potential harms remain uncertain until the sunscreen industry conducts the safety studies recommended by the FDA, Dr. Adamson and Dr. Shinkai concluded.
In a statement released by the FDA on Jan 21, the day the study was published, Janet Woodcock, MD, director of the FDA’s Center for Drug Evaluation and Research, said that, considering the “recognized public health benefits” of using sunscreen, the FDA “urges Americans to use sunscreens in conjunction with other sun protective measures (such as protective clothing).”
Commenting on the study, she said, “results from our study released today show there is evidence that some sunscreen active ingredients may be absorbed. However, the fact that an ingredient is absorbed through the skin and into the body does not mean that the ingredient is unsafe, nor does the FDA seeking further information indicate such. Rather, this finding calls for further industry testing to determine the safety and effect of systemic exposure of sunscreen ingredients, especially with chronic use.”
The study was supported by the FDA. The researchers and editorial authors had no financial conflicts to disclose.
SOURCES: Matta MK et al. JAMA. 2020;323:256-267.
Six active in a randomized trial including four product types. The results were published in JAMA.
The testing was done as part of a proposed rule on sunscreen, published in February 2019, which requested additional information on sunscreen ingredients. Murali K. Matta, PhD, of the Food and Drug Administration and coauthors wrote that these plasma concentrations “surpassed the FDA threshold for potentially waiving additional safety studies for sunscreens.” But, they added, the findings “do not indicate that individuals should refrain from the use of sunscreen.”
This was a follow-up study to a smaller study of 24 health volunteers published last year that determined that the sunscreen active ingredients tested were absorbed systemically (JAMA. 2019;321[21]:2082-91). “This follow-up study expanded the sample size, tested additional sunscreen active ingredients and formulations, and confirmed the finding that sunscreen active ingredients are systemically absorbed,” the authors wrote.
To gather information on the absorption of active ingredients in sunscreens, the investigators randomized 48 adults to one of four sunscreen products (lotion, aerosol spray, nonaerosol spray, or pump spray) with one of six active ingredients (avobenzone, oxybenzone, octocrylene, homosalate, octisalate, and octinoxate). Not all products contained each of the ingredients.
The participants applied the products in amounts of 2 mg/cm2 to 75% of body surface area at baseline, no use on day 1 and four times a day at 2-hour intervals on days 2 through 4. The researchers collected blood samples over 21 days and measured the maximum plasma concentrations. The average age of the participants was 37 years, and half were women. The study was conducted in a clinical pharmacology unit.
The geometric mean maximum plasma concentrations for the primary endpoint of avobenzone in lotion, aerosol spray, nonaerosol spray, and pump spray were 7.1 ng/mL, 3.5 ng/mL, 3.5 ng/mL, and 3.3 ng/mL, respectively.
For oxybenzone, the concentrations were 258.1 ng/mL and 180.1 ng/mL, respectively, for lotion and aerosol spray. The concentrations for octocrylene were 7.8 ng/mL, 6.6 ng/mL, and 6.6 ng/mL, respectively, for lotion, aerosol spray, and nonaerosol spray.
For homosalate, the geometric mean plasma concentrations were 23.1 ng/mL for aerosol spray, 17.9 for nonaerosol spray, and 13.9 for pump spray. For octisalate, the concentrations were 5.1 ng/mL, 5.8 ng/mL, and 4.6 ng/mL, respectively, for aerosol spray, nonaerosol spray, and pump spray. For octinoxate, the concentrations were 7.9 ng/mL for nonaerosol spray and 5.2 ng/mL for pump spray.
“The systemic exposures, as measured by geometric mean maximum plasma concentrations, of all the tested active ingredients were higher than 0.5 ng/mL after a single application,” the researchers noted.
Overall, the most common event was rash, which was reported in 14 participants.
The study findings were limited by several factors including the use of an indoor clinical setting, rather than outdoor exposure; the inability to assess absorption differences by formulation and Fitzpatrick skin type; and the variation in amounts of ingredients among products, the researchers noted. However, the results can be used to design additional studies needed to research the effects of systemic exposure to sunscreen ingredients, they said.
In an accompanying editorial (JAMA. 2020;323:223-4), Adewole S. Adamson, MD, of the University of Texas at Austin, and Kanade Shinkai, MD, of the University of California, San Francisco, wrote that “the study did not address key questions about sunscreen safety,” including the length of time it takes “for plasma concentrations of sunscreen ingredients to fall below the FDA threshold for safety testing.” Dr. Shinkai is also editor in chief of JAMA Dermatology.
“In making an informed decision, clinicians must determine whether the magnitude of the benefit exceeds the risk of potential harm for a specific individual,” they said. “Importantly, this balance may be different, depending on characteristics of the sunscreen user (e.g., for individuals with darker skin types and for children) and may depend on the frequency and duration of application (e.g., daily vs. intermittent use; starting in infancy or later in life),” they noted.
“In the absence of clear data demonstrating harm, the use of chemical sunscreen may still be considered appropriate; the use of mineral-based sunscreen is a well-established safe alternative,” although the potential harms remain uncertain until the sunscreen industry conducts the safety studies recommended by the FDA, Dr. Adamson and Dr. Shinkai concluded.
In a statement released by the FDA on Jan 21, the day the study was published, Janet Woodcock, MD, director of the FDA’s Center for Drug Evaluation and Research, said that, considering the “recognized public health benefits” of using sunscreen, the FDA “urges Americans to use sunscreens in conjunction with other sun protective measures (such as protective clothing).”
Commenting on the study, she said, “results from our study released today show there is evidence that some sunscreen active ingredients may be absorbed. However, the fact that an ingredient is absorbed through the skin and into the body does not mean that the ingredient is unsafe, nor does the FDA seeking further information indicate such. Rather, this finding calls for further industry testing to determine the safety and effect of systemic exposure of sunscreen ingredients, especially with chronic use.”
The study was supported by the FDA. The researchers and editorial authors had no financial conflicts to disclose.
SOURCES: Matta MK et al. JAMA. 2020;323:256-267.
Six active in a randomized trial including four product types. The results were published in JAMA.
The testing was done as part of a proposed rule on sunscreen, published in February 2019, which requested additional information on sunscreen ingredients. Murali K. Matta, PhD, of the Food and Drug Administration and coauthors wrote that these plasma concentrations “surpassed the FDA threshold for potentially waiving additional safety studies for sunscreens.” But, they added, the findings “do not indicate that individuals should refrain from the use of sunscreen.”
This was a follow-up study to a smaller study of 24 health volunteers published last year that determined that the sunscreen active ingredients tested were absorbed systemically (JAMA. 2019;321[21]:2082-91). “This follow-up study expanded the sample size, tested additional sunscreen active ingredients and formulations, and confirmed the finding that sunscreen active ingredients are systemically absorbed,” the authors wrote.
To gather information on the absorption of active ingredients in sunscreens, the investigators randomized 48 adults to one of four sunscreen products (lotion, aerosol spray, nonaerosol spray, or pump spray) with one of six active ingredients (avobenzone, oxybenzone, octocrylene, homosalate, octisalate, and octinoxate). Not all products contained each of the ingredients.
The participants applied the products in amounts of 2 mg/cm2 to 75% of body surface area at baseline, no use on day 1 and four times a day at 2-hour intervals on days 2 through 4. The researchers collected blood samples over 21 days and measured the maximum plasma concentrations. The average age of the participants was 37 years, and half were women. The study was conducted in a clinical pharmacology unit.
The geometric mean maximum plasma concentrations for the primary endpoint of avobenzone in lotion, aerosol spray, nonaerosol spray, and pump spray were 7.1 ng/mL, 3.5 ng/mL, 3.5 ng/mL, and 3.3 ng/mL, respectively.
For oxybenzone, the concentrations were 258.1 ng/mL and 180.1 ng/mL, respectively, for lotion and aerosol spray. The concentrations for octocrylene were 7.8 ng/mL, 6.6 ng/mL, and 6.6 ng/mL, respectively, for lotion, aerosol spray, and nonaerosol spray.
For homosalate, the geometric mean plasma concentrations were 23.1 ng/mL for aerosol spray, 17.9 for nonaerosol spray, and 13.9 for pump spray. For octisalate, the concentrations were 5.1 ng/mL, 5.8 ng/mL, and 4.6 ng/mL, respectively, for aerosol spray, nonaerosol spray, and pump spray. For octinoxate, the concentrations were 7.9 ng/mL for nonaerosol spray and 5.2 ng/mL for pump spray.
“The systemic exposures, as measured by geometric mean maximum plasma concentrations, of all the tested active ingredients were higher than 0.5 ng/mL after a single application,” the researchers noted.
Overall, the most common event was rash, which was reported in 14 participants.
The study findings were limited by several factors including the use of an indoor clinical setting, rather than outdoor exposure; the inability to assess absorption differences by formulation and Fitzpatrick skin type; and the variation in amounts of ingredients among products, the researchers noted. However, the results can be used to design additional studies needed to research the effects of systemic exposure to sunscreen ingredients, they said.
In an accompanying editorial (JAMA. 2020;323:223-4), Adewole S. Adamson, MD, of the University of Texas at Austin, and Kanade Shinkai, MD, of the University of California, San Francisco, wrote that “the study did not address key questions about sunscreen safety,” including the length of time it takes “for plasma concentrations of sunscreen ingredients to fall below the FDA threshold for safety testing.” Dr. Shinkai is also editor in chief of JAMA Dermatology.
“In making an informed decision, clinicians must determine whether the magnitude of the benefit exceeds the risk of potential harm for a specific individual,” they said. “Importantly, this balance may be different, depending on characteristics of the sunscreen user (e.g., for individuals with darker skin types and for children) and may depend on the frequency and duration of application (e.g., daily vs. intermittent use; starting in infancy or later in life),” they noted.
“In the absence of clear data demonstrating harm, the use of chemical sunscreen may still be considered appropriate; the use of mineral-based sunscreen is a well-established safe alternative,” although the potential harms remain uncertain until the sunscreen industry conducts the safety studies recommended by the FDA, Dr. Adamson and Dr. Shinkai concluded.
In a statement released by the FDA on Jan 21, the day the study was published, Janet Woodcock, MD, director of the FDA’s Center for Drug Evaluation and Research, said that, considering the “recognized public health benefits” of using sunscreen, the FDA “urges Americans to use sunscreens in conjunction with other sun protective measures (such as protective clothing).”
Commenting on the study, she said, “results from our study released today show there is evidence that some sunscreen active ingredients may be absorbed. However, the fact that an ingredient is absorbed through the skin and into the body does not mean that the ingredient is unsafe, nor does the FDA seeking further information indicate such. Rather, this finding calls for further industry testing to determine the safety and effect of systemic exposure of sunscreen ingredients, especially with chronic use.”
The study was supported by the FDA. The researchers and editorial authors had no financial conflicts to disclose.
SOURCES: Matta MK et al. JAMA. 2020;323:256-267.
FROM JAMA
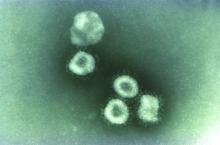
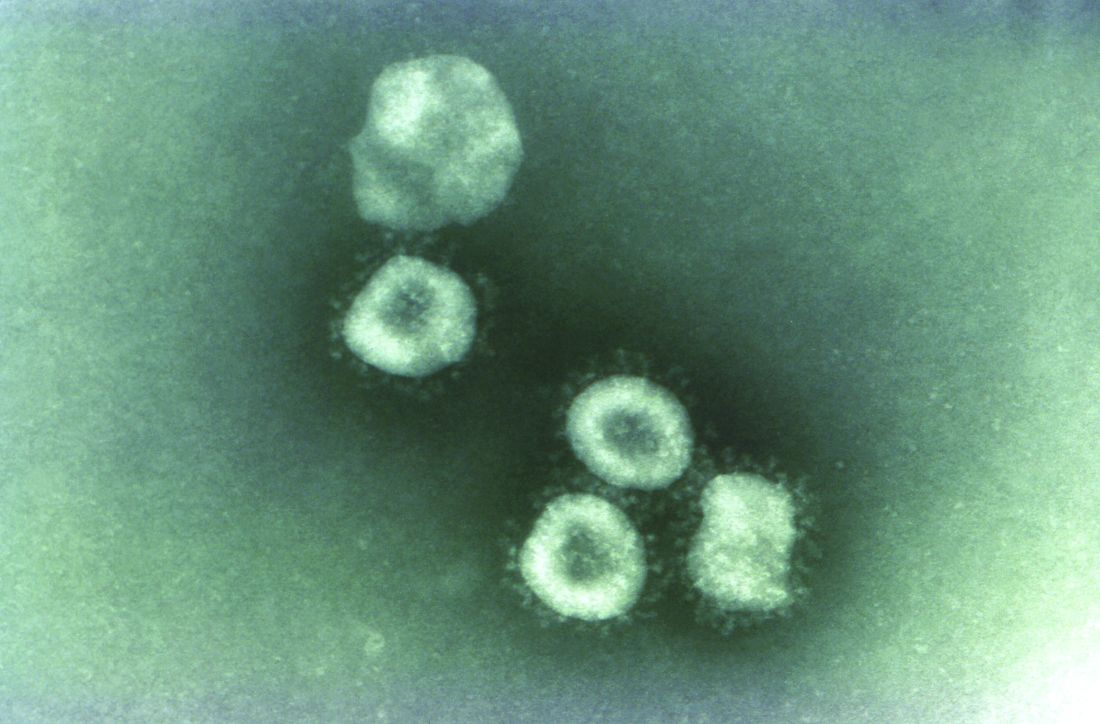
Washington state patient is first U.S. case of novel coronavirus
The first case of the novel coronavirus, named 2019-nCoV, in the United States has been diagnosed in a traveler from China who came through Seattle-Tacoma International Airport on Jan 15, the Centers for Disease Control and Prevention announced today at a press briefing.
The outbreak began at a animal and meat market in China and now has spread to at least three other countries, including Thailand, Japan and South Korea. While originally thought to be spreading from animal to person, it appears that limited person-to-person transmission is occurring, although it is currently unknown how easily this virus spreads between people.
More than 300 cases have been reported and six deaths have occurred. Fourteen health care workers have been infected.
Scott Lindquist, MD, MPH, Washington state epidemiologist, said at the briefing that the patient, a man who had been in Wuhan, arrived at Sea-Tac on Jan. 15, 2 days before airport screening had been initiated. He was symptom free at the time of his arrival and probably would not have been identified as infected with 2019-nCoV. The patient had been aware of the public health and news media coverage of 2019-nCoV and, after developing symptoms, contacted his health care provider on Jan. 19. The patient did not fly directly from Wuhan, but Dr. Lindquist said that he has been fully cooperative and has been helpful to authorities in tracing his route and contacts. The man is being treated at Providence Regional Medical Center, Everett, Wash.
The CDC obtained a specimen from the patient immediately and identified the 2019-nCoV within 24 hours.
Screening at airports is part of a multipart strategy to address this type of infection that includes public health information dissemination, patient education, as well as hospital preparation and training exercises. Currently, a strategy referred to as “funneling” is being implemented wherein travelers from China are rerouted and reticketed to one of the five airports conducting screening. At present, JFK in New York, San Francisco International, Los Angeles International, Hartsfield-Jackson Atlanta International Airport, and Chicago O’Hare International Airport are conducting inbound traveler screening.
The CDC is working in close cooperation with the Department of Homeland Security and the Federal Aviation Administration to coordinate travel screenings and reroutings. In addition, the CDC is working with the World Health Organization and the international global health community to share information about this outbreak. The CDC also has staff on site in Wuhan and is communicating with local health authorities. The CDC has activated its Emergency Operations Center to better provide ongoing support to the 2019-nCoV response. Currently, the focus is on tracing contacts and the means of transmission of this virus.
Updates on the outbreak will be posted on the CDC coronavirus website.
CORRECTION: 1/21/2020: The name of the medical center where the 2019-nCoV patient is being treated was corrected.
The first case of the novel coronavirus, named 2019-nCoV, in the United States has been diagnosed in a traveler from China who came through Seattle-Tacoma International Airport on Jan 15, the Centers for Disease Control and Prevention announced today at a press briefing.
The outbreak began at a animal and meat market in China and now has spread to at least three other countries, including Thailand, Japan and South Korea. While originally thought to be spreading from animal to person, it appears that limited person-to-person transmission is occurring, although it is currently unknown how easily this virus spreads between people.
More than 300 cases have been reported and six deaths have occurred. Fourteen health care workers have been infected.
Scott Lindquist, MD, MPH, Washington state epidemiologist, said at the briefing that the patient, a man who had been in Wuhan, arrived at Sea-Tac on Jan. 15, 2 days before airport screening had been initiated. He was symptom free at the time of his arrival and probably would not have been identified as infected with 2019-nCoV. The patient had been aware of the public health and news media coverage of 2019-nCoV and, after developing symptoms, contacted his health care provider on Jan. 19. The patient did not fly directly from Wuhan, but Dr. Lindquist said that he has been fully cooperative and has been helpful to authorities in tracing his route and contacts. The man is being treated at Providence Regional Medical Center, Everett, Wash.
The CDC obtained a specimen from the patient immediately and identified the 2019-nCoV within 24 hours.
Screening at airports is part of a multipart strategy to address this type of infection that includes public health information dissemination, patient education, as well as hospital preparation and training exercises. Currently, a strategy referred to as “funneling” is being implemented wherein travelers from China are rerouted and reticketed to one of the five airports conducting screening. At present, JFK in New York, San Francisco International, Los Angeles International, Hartsfield-Jackson Atlanta International Airport, and Chicago O’Hare International Airport are conducting inbound traveler screening.
The CDC is working in close cooperation with the Department of Homeland Security and the Federal Aviation Administration to coordinate travel screenings and reroutings. In addition, the CDC is working with the World Health Organization and the international global health community to share information about this outbreak. The CDC also has staff on site in Wuhan and is communicating with local health authorities. The CDC has activated its Emergency Operations Center to better provide ongoing support to the 2019-nCoV response. Currently, the focus is on tracing contacts and the means of transmission of this virus.
Updates on the outbreak will be posted on the CDC coronavirus website.
CORRECTION: 1/21/2020: The name of the medical center where the 2019-nCoV patient is being treated was corrected.
The first case of the novel coronavirus, named 2019-nCoV, in the United States has been diagnosed in a traveler from China who came through Seattle-Tacoma International Airport on Jan 15, the Centers for Disease Control and Prevention announced today at a press briefing.
The outbreak began at a animal and meat market in China and now has spread to at least three other countries, including Thailand, Japan and South Korea. While originally thought to be spreading from animal to person, it appears that limited person-to-person transmission is occurring, although it is currently unknown how easily this virus spreads between people.
More than 300 cases have been reported and six deaths have occurred. Fourteen health care workers have been infected.
Scott Lindquist, MD, MPH, Washington state epidemiologist, said at the briefing that the patient, a man who had been in Wuhan, arrived at Sea-Tac on Jan. 15, 2 days before airport screening had been initiated. He was symptom free at the time of his arrival and probably would not have been identified as infected with 2019-nCoV. The patient had been aware of the public health and news media coverage of 2019-nCoV and, after developing symptoms, contacted his health care provider on Jan. 19. The patient did not fly directly from Wuhan, but Dr. Lindquist said that he has been fully cooperative and has been helpful to authorities in tracing his route and contacts. The man is being treated at Providence Regional Medical Center, Everett, Wash.
The CDC obtained a specimen from the patient immediately and identified the 2019-nCoV within 24 hours.
Screening at airports is part of a multipart strategy to address this type of infection that includes public health information dissemination, patient education, as well as hospital preparation and training exercises. Currently, a strategy referred to as “funneling” is being implemented wherein travelers from China are rerouted and reticketed to one of the five airports conducting screening. At present, JFK in New York, San Francisco International, Los Angeles International, Hartsfield-Jackson Atlanta International Airport, and Chicago O’Hare International Airport are conducting inbound traveler screening.
The CDC is working in close cooperation with the Department of Homeland Security and the Federal Aviation Administration to coordinate travel screenings and reroutings. In addition, the CDC is working with the World Health Organization and the international global health community to share information about this outbreak. The CDC also has staff on site in Wuhan and is communicating with local health authorities. The CDC has activated its Emergency Operations Center to better provide ongoing support to the 2019-nCoV response. Currently, the focus is on tracing contacts and the means of transmission of this virus.
Updates on the outbreak will be posted on the CDC coronavirus website.
CORRECTION: 1/21/2020: The name of the medical center where the 2019-nCoV patient is being treated was corrected.
REPORTING FROM CDC
Dermatology News welcomes new advisory board member Dr. Jonathan I. Silverberg
Dermatology News welcomes Jonathan I. Silverberg, MD, PhD, MPH, to its editorial advisory board. Dr. Silverberg is an associate professor of dermatology at George Washington University in Washington, where he is also director of clinical research and contact dermatitis.
Dr. Silverberg’s clinical subspecialty is inflammatory skin disease, particularly atopic dermatitis and contact dermatitis. His research interests include comorbidities and burden of itch and inflammatory skin disease, evidence-based dermatology, patient-reported outcomes, and dermatoepidemiology, as well as health services research, drug development, clinical trial design, and biomarkers. He has published more than 600 peer-reviewed articles, abstracts, and book chapters, and has appeared frequently on the pages of Dermatology News.
‑Elizabeth Mechcatie, editor
Dermatology News welcomes Jonathan I. Silverberg, MD, PhD, MPH, to its editorial advisory board. Dr. Silverberg is an associate professor of dermatology at George Washington University in Washington, where he is also director of clinical research and contact dermatitis.
Dr. Silverberg’s clinical subspecialty is inflammatory skin disease, particularly atopic dermatitis and contact dermatitis. His research interests include comorbidities and burden of itch and inflammatory skin disease, evidence-based dermatology, patient-reported outcomes, and dermatoepidemiology, as well as health services research, drug development, clinical trial design, and biomarkers. He has published more than 600 peer-reviewed articles, abstracts, and book chapters, and has appeared frequently on the pages of Dermatology News.
‑Elizabeth Mechcatie, editor
Dermatology News welcomes Jonathan I. Silverberg, MD, PhD, MPH, to its editorial advisory board. Dr. Silverberg is an associate professor of dermatology at George Washington University in Washington, where he is also director of clinical research and contact dermatitis.
Dr. Silverberg’s clinical subspecialty is inflammatory skin disease, particularly atopic dermatitis and contact dermatitis. His research interests include comorbidities and burden of itch and inflammatory skin disease, evidence-based dermatology, patient-reported outcomes, and dermatoepidemiology, as well as health services research, drug development, clinical trial design, and biomarkers. He has published more than 600 peer-reviewed articles, abstracts, and book chapters, and has appeared frequently on the pages of Dermatology News.
‑Elizabeth Mechcatie, editor
The rise of U.S. dermatology: A brief history from the 1800s to 1970
As Dermatology News (formerly Skin and Allergy News) reaches its 50th-year milestone, a reflection on the history of the discipline, especially in the United States, up to the time of the launch of this publication is in order. Such an overview must, of course, be cursory in this context. Yet, for those who want to learn more, a large body of historical references and research has been created to fill in the gaps, as modern dermatology has always been cognizant of the importance of its history, with many individuals and groups drawn to the subject.
Two excellent sources for the history of the field can be found in work by William Allen Pusey, MD (1865-1940), and Herbert Rattner, MD (1900-1962), “The History of Dermatology” published in 1933 and research by members of the History of Dermatology Society, founded in 1973 in New York.
Modern dermatology
The development of the field of modern dermatology can be traced back to the early to mid-19th century. During the first half of the 19th century, England and France dominated the study of dermatology, but by the middle of the century, the German revolution in microparasitology shifted that focus “with remarkable German discoveries,” according to Bernard S. Potter, MD, in his review of bibliographic landmarks of the history of dermatology (J Am Acad Dermatol 2003;48:919-32). For example, Johann Lucas Schoenlein (1793-1864) in 1839 discovered the fungal origin of favus, and in 1841 Jacob Henle (1809-1885) discovered Demodex folliculorum. Karl Ferdinand Eichstedt (1816-1892) in 1846 followed with the discovery of the causative agent of pityriasis versicolor, and Friedrich Wilhelm Felix von Barensprung (1822-1864) in 1862 coined the term erythrasma and named the organism responsible for this condition Microsporum minutissimum.
Dr. Potter described how American dermatology originated in New York City in 1836 when Henry Daggett Bulkley, MD, (1803-1872) opened the first dispensary for skin diseases, the Broome Street Infirmary for Diseases of the Skin, thus creating the first institution in the United States for the treatment of cutaneous disease. As the first American dermatologist, he was also the first in the United States to lecture on and to exclusively practice dermatology.
The rise of interest in the importance of dermatology led to the organization of the early American Dermatological Association in 1886.
However, the state of dermatology as a science in the 19th century was not always looked upon favorably, even by its practitioners, especially in the United States. In 1871, in a “Review on Modern Dermatology,” given as a series of lectures on skin disease at Harvard University, James C. White, MD (1833-1916) of Massachusetts General Hospital, stated that: “Were the literature of skin diseases previous to that of the last half-century absolutely annihilated, and with it, the influence it has exercised upon that of the present day, it would be an immense gain to dermatology, although much of real value would perish.” He lamented that America had contributed little so far to the study of dermatology, and that the discipline was only taught in some of its largest schools, and he urged that this be changed. He also lamented that The American Journal of Syphilography and Dermatology, established the year before, had so far proved itself heavy on syphilis, but light on dermatology, a situation he also hoped would change dramatically.
By the late-19th century, the conviction that diseases of the skin needed to be connected to the overall metabolism and physiology of the patient as a whole was becoming more mainstream.
“It has been, and still is, too much the custom to study diseases of the skin in the light of pathological pictures, to name the local manifestation and to so label it as disease. It is much easier to give the disease name and to label it than it is to comprehend the process at work. The former is comparatively unimportant for the patient, the latter point upon which recovery may depend. The nature and meaning of the process in connection with the cutaneous symptoms has not received enough attention, and I believe this to be one reason why the treatment of many of these diseases in the past has been so notoriously unsatisfactory,” Louis A. Duhring, MD (1845-1913) chided his colleagues in the Section of Dermatology and Syphilography, at the Forty-fourth Annual Meeting of the American Medical Association in 1894. (collections.nlm.nih.gov/ext/dw/101489447/PDF/101489447.pdf)
In the early-20th century, German dermatology influenced American dermatology more than any other, according to Karl Holubar, MD, of the Institute for the History of Medicine, University of Vienna, in his lecture on the history of European dermatopathology.
He stated that, with regard to dermatopathology, it was Oscar Gans, MD (1888-1983) who brought the latest knowledge into the United States by delivering a series of lectures at Mayo Clinic in the late 1920s upon the invitation of Paul A. O’Leary, MD, (1891-1955) who then headed the Mayo section of dermatology.
By the 1930s, a flurry of organizational activity overtook American dermatology. In 1932, the American Board of Dermatology was established, with its first exams given in 1933 (20 students passed, 7 failed). The Society for Investigative Dermatology was founded in 1937, and the American Academy of Dermatology and Syphilology (now the American Academy of Dermatology), founded in 1938.
The 1930s also saw a major influx of German and other European Jews fleeing Nazi oppression who would forever change the face of American dermatology. “Between 1933 and 1938, a series of repressive measures eliminated them from the practice of medicine in Germany and other countries. Although some died in concentration camps and others committed suicide, many were able to emigrate from Europe. Dermatology in the United States particularly benefited from the influx of several stellar Jewish dermatologists who were major contributors to the subsequent flowering of academic dermatology in the United States” (JAMA Derm. 2013;149[9]:1090-4).
“The overtures of the holocaust and the rising power of Hitler in Europe finally brought over to the United States the flower of dermatologists and investigators of the German School, e.g., Alexander and Walter Lever, Felix and Hermann Pinkus, the Epsteins, Erich Auerbach, Stephen Rothman, to name just a few. With this exodus and transfer of brain power, Europe lost its leading role to never again regain it,” according to Dr. Holubar. Walter F. Lever, MD (1909-1992) was especially well-known for his landmark textbook on dermatology, “Histopathology of the Skin,” published in the United States in 1949.
The therapeutic era
Throughout the 19th century, a variety of soaps and patent medicines were touted as cure-alls for a host of skin diseases. Other than their benefits to surface cleanliness and their antiseptic properties, however, they were of little effect.
It wasn’t until the 20th century that truly effective therapeutics entered the dermatologic pharmacopoeia. In their 1989 review, Diane Quintal, MD, and Robert Jackson, MD, discussed the origins of the most important of these drugs and pointed out that, “Until this century, the essence of dermatology resided in the realm of morphology. Early contributors largely confined their activities to the classification of skin diseases and to the elaboration of clinical dermatologic entities based on morphologic features. ... but “in the last 50 years, there have been significant scientific discoveries in the field of therapeutics that have revolutionized the practice of dermatology.“ (Clin Dermatol. 1989;7[3]38-47).
These key drugs comprised:
- Quinacrine was introduced in 1932 by Walter Kikuth, MD, as an antimalarial drug. But it was not until 1940, that A.J. Prokoptchouksi, MD, reported on its effectiveness 35 patients with lupus erythematosus.
- Para-aminobenzoic acid (PABA) came into prominence in 1942, when Stephen Rothman, MD, and Jack Rubin, MD, at the University of Chicago, published the results of their experiment, showing that when PABA was incorporated in an ointment base and applied to the skin, it could protect against sunburn.
- Dapsone. The effectiveness of sulfapyridine was demonstrated in 1947 by M.J. Costello, MD, who reported its usefulness in a patient with dermatitis herpetiformis, which he believed to be caused by a bacterial allergy. Sulfapyridine controlled the disease, but gastrointestinal intolerance and sulfonamide sensitivity were side effects. Ultimately, in 1951, Theodore Cornbleet, MD, introduced the use of sulfoxones in his article entitled “Sulfoxone (diasones) sodium for dermatitis herpetiformis,” considered more effective than sulfapyridine. Dapsone is the active principal ingredient.
- Hydrocortisone. In August 1952, Marion Sulzberger, MD, and Victor H. Witten, MD (both members of the first Skin & Allergy News editorial advisory board), described use of Compound F (17-hydroxycorticosterone-21-acetate, hydrocortisone) in seven cases of atopic dermatitis and one case of discoid or subacute lupus erythematosus, reporting improvement in all of these cases.
- Benzoyl peroxide. Canadian dermatologist William E. Pace, MD, reported on the beneficial effects of benzoyl peroxide on acne in 1953. The product had originally been used for chronic Staphylococcus aureus folliculitis of the beard.
- Griseofulvin, a metabolic byproduct of a number of species of Penicillium, was first isolated in 1939. But in 1958, Harvey Blank, MD, at the University of Miami (also on the first Skin & Allergy News editorial advisory board), and Stanley I. Cullen, MD, administered the drug to a patient with Trichophyton rubrum granuloma, in the first human trial. In 1959, they reported the drug’s benefits on 31 patients with various fungal infections.
- Methotrexate. In 1951, R. Gubner, MD, and colleagues noticed the rapid clearing of skin lesions in a patient with rheumatoid arthritis who had been treated with the folic acid antagonist, aminopterin. And in 1958, W.F. Edmundson, MD, and W.B. Guy, MD, reported on the oral use of the folic acid antagonist, methotrexate. This was followed by multiple reports on the successful use of methotrexate in psoriasis.
- 5-fluorouracil (5-FU). In 1957, 5-FU, an antimetabolite of uracil, was first synthesized. In 1962, G. Falkson, MD, and E.J. Schulz, MD, reported on skin changes observed in 85 patients being treated with systemic 5-FU for advanced carcinomatosis. They found that 31 of the 85 patients developed sensitivity to sunlight and subsequent disappearance of actinic keratoses in these same patients.
Technology in skin care also was developing in the era just before the launch of Skin & Allergy News. For example, Leon Goldman, MD, then chairman of the department of dermatology at the University of Cincinnati, was the first physician to use a laser for tattoo removal. His publication in 1965 helped to solidify its use, leading him to be “regarded by many in the dermatologic community as the ‘godfather of lasers in medicine and surgery’ ” (Clin Dermatol. 2007;25:434-42).
So, by 1970, dermatology as a field had established itself fully with a strong societal infrastructure, a vibrant base of journals and books, and an evolving set of scientific and technical tools. The launch of Skin & Allergy News (now Dermatology News) that year would chronicle dermatology’s commitment to the development of new therapeutics and technologies in service of patient needs – the stories of which would grace the newspaper’s pages for 5 decades and counting.
As Dermatology News (formerly Skin and Allergy News) reaches its 50th-year milestone, a reflection on the history of the discipline, especially in the United States, up to the time of the launch of this publication is in order. Such an overview must, of course, be cursory in this context. Yet, for those who want to learn more, a large body of historical references and research has been created to fill in the gaps, as modern dermatology has always been cognizant of the importance of its history, with many individuals and groups drawn to the subject.
Two excellent sources for the history of the field can be found in work by William Allen Pusey, MD (1865-1940), and Herbert Rattner, MD (1900-1962), “The History of Dermatology” published in 1933 and research by members of the History of Dermatology Society, founded in 1973 in New York.
Modern dermatology
The development of the field of modern dermatology can be traced back to the early to mid-19th century. During the first half of the 19th century, England and France dominated the study of dermatology, but by the middle of the century, the German revolution in microparasitology shifted that focus “with remarkable German discoveries,” according to Bernard S. Potter, MD, in his review of bibliographic landmarks of the history of dermatology (J Am Acad Dermatol 2003;48:919-32). For example, Johann Lucas Schoenlein (1793-1864) in 1839 discovered the fungal origin of favus, and in 1841 Jacob Henle (1809-1885) discovered Demodex folliculorum. Karl Ferdinand Eichstedt (1816-1892) in 1846 followed with the discovery of the causative agent of pityriasis versicolor, and Friedrich Wilhelm Felix von Barensprung (1822-1864) in 1862 coined the term erythrasma and named the organism responsible for this condition Microsporum minutissimum.
Dr. Potter described how American dermatology originated in New York City in 1836 when Henry Daggett Bulkley, MD, (1803-1872) opened the first dispensary for skin diseases, the Broome Street Infirmary for Diseases of the Skin, thus creating the first institution in the United States for the treatment of cutaneous disease. As the first American dermatologist, he was also the first in the United States to lecture on and to exclusively practice dermatology.
The rise of interest in the importance of dermatology led to the organization of the early American Dermatological Association in 1886.
However, the state of dermatology as a science in the 19th century was not always looked upon favorably, even by its practitioners, especially in the United States. In 1871, in a “Review on Modern Dermatology,” given as a series of lectures on skin disease at Harvard University, James C. White, MD (1833-1916) of Massachusetts General Hospital, stated that: “Were the literature of skin diseases previous to that of the last half-century absolutely annihilated, and with it, the influence it has exercised upon that of the present day, it would be an immense gain to dermatology, although much of real value would perish.” He lamented that America had contributed little so far to the study of dermatology, and that the discipline was only taught in some of its largest schools, and he urged that this be changed. He also lamented that The American Journal of Syphilography and Dermatology, established the year before, had so far proved itself heavy on syphilis, but light on dermatology, a situation he also hoped would change dramatically.
By the late-19th century, the conviction that diseases of the skin needed to be connected to the overall metabolism and physiology of the patient as a whole was becoming more mainstream.
“It has been, and still is, too much the custom to study diseases of the skin in the light of pathological pictures, to name the local manifestation and to so label it as disease. It is much easier to give the disease name and to label it than it is to comprehend the process at work. The former is comparatively unimportant for the patient, the latter point upon which recovery may depend. The nature and meaning of the process in connection with the cutaneous symptoms has not received enough attention, and I believe this to be one reason why the treatment of many of these diseases in the past has been so notoriously unsatisfactory,” Louis A. Duhring, MD (1845-1913) chided his colleagues in the Section of Dermatology and Syphilography, at the Forty-fourth Annual Meeting of the American Medical Association in 1894. (collections.nlm.nih.gov/ext/dw/101489447/PDF/101489447.pdf)
In the early-20th century, German dermatology influenced American dermatology more than any other, according to Karl Holubar, MD, of the Institute for the History of Medicine, University of Vienna, in his lecture on the history of European dermatopathology.
He stated that, with regard to dermatopathology, it was Oscar Gans, MD (1888-1983) who brought the latest knowledge into the United States by delivering a series of lectures at Mayo Clinic in the late 1920s upon the invitation of Paul A. O’Leary, MD, (1891-1955) who then headed the Mayo section of dermatology.
By the 1930s, a flurry of organizational activity overtook American dermatology. In 1932, the American Board of Dermatology was established, with its first exams given in 1933 (20 students passed, 7 failed). The Society for Investigative Dermatology was founded in 1937, and the American Academy of Dermatology and Syphilology (now the American Academy of Dermatology), founded in 1938.
The 1930s also saw a major influx of German and other European Jews fleeing Nazi oppression who would forever change the face of American dermatology. “Between 1933 and 1938, a series of repressive measures eliminated them from the practice of medicine in Germany and other countries. Although some died in concentration camps and others committed suicide, many were able to emigrate from Europe. Dermatology in the United States particularly benefited from the influx of several stellar Jewish dermatologists who were major contributors to the subsequent flowering of academic dermatology in the United States” (JAMA Derm. 2013;149[9]:1090-4).
“The overtures of the holocaust and the rising power of Hitler in Europe finally brought over to the United States the flower of dermatologists and investigators of the German School, e.g., Alexander and Walter Lever, Felix and Hermann Pinkus, the Epsteins, Erich Auerbach, Stephen Rothman, to name just a few. With this exodus and transfer of brain power, Europe lost its leading role to never again regain it,” according to Dr. Holubar. Walter F. Lever, MD (1909-1992) was especially well-known for his landmark textbook on dermatology, “Histopathology of the Skin,” published in the United States in 1949.
The therapeutic era
Throughout the 19th century, a variety of soaps and patent medicines were touted as cure-alls for a host of skin diseases. Other than their benefits to surface cleanliness and their antiseptic properties, however, they were of little effect.
It wasn’t until the 20th century that truly effective therapeutics entered the dermatologic pharmacopoeia. In their 1989 review, Diane Quintal, MD, and Robert Jackson, MD, discussed the origins of the most important of these drugs and pointed out that, “Until this century, the essence of dermatology resided in the realm of morphology. Early contributors largely confined their activities to the classification of skin diseases and to the elaboration of clinical dermatologic entities based on morphologic features. ... but “in the last 50 years, there have been significant scientific discoveries in the field of therapeutics that have revolutionized the practice of dermatology.“ (Clin Dermatol. 1989;7[3]38-47).
These key drugs comprised:
- Quinacrine was introduced in 1932 by Walter Kikuth, MD, as an antimalarial drug. But it was not until 1940, that A.J. Prokoptchouksi, MD, reported on its effectiveness 35 patients with lupus erythematosus.
- Para-aminobenzoic acid (PABA) came into prominence in 1942, when Stephen Rothman, MD, and Jack Rubin, MD, at the University of Chicago, published the results of their experiment, showing that when PABA was incorporated in an ointment base and applied to the skin, it could protect against sunburn.
- Dapsone. The effectiveness of sulfapyridine was demonstrated in 1947 by M.J. Costello, MD, who reported its usefulness in a patient with dermatitis herpetiformis, which he believed to be caused by a bacterial allergy. Sulfapyridine controlled the disease, but gastrointestinal intolerance and sulfonamide sensitivity were side effects. Ultimately, in 1951, Theodore Cornbleet, MD, introduced the use of sulfoxones in his article entitled “Sulfoxone (diasones) sodium for dermatitis herpetiformis,” considered more effective than sulfapyridine. Dapsone is the active principal ingredient.
- Hydrocortisone. In August 1952, Marion Sulzberger, MD, and Victor H. Witten, MD (both members of the first Skin & Allergy News editorial advisory board), described use of Compound F (17-hydroxycorticosterone-21-acetate, hydrocortisone) in seven cases of atopic dermatitis and one case of discoid or subacute lupus erythematosus, reporting improvement in all of these cases.
- Benzoyl peroxide. Canadian dermatologist William E. Pace, MD, reported on the beneficial effects of benzoyl peroxide on acne in 1953. The product had originally been used for chronic Staphylococcus aureus folliculitis of the beard.
- Griseofulvin, a metabolic byproduct of a number of species of Penicillium, was first isolated in 1939. But in 1958, Harvey Blank, MD, at the University of Miami (also on the first Skin & Allergy News editorial advisory board), and Stanley I. Cullen, MD, administered the drug to a patient with Trichophyton rubrum granuloma, in the first human trial. In 1959, they reported the drug’s benefits on 31 patients with various fungal infections.
- Methotrexate. In 1951, R. Gubner, MD, and colleagues noticed the rapid clearing of skin lesions in a patient with rheumatoid arthritis who had been treated with the folic acid antagonist, aminopterin. And in 1958, W.F. Edmundson, MD, and W.B. Guy, MD, reported on the oral use of the folic acid antagonist, methotrexate. This was followed by multiple reports on the successful use of methotrexate in psoriasis.
- 5-fluorouracil (5-FU). In 1957, 5-FU, an antimetabolite of uracil, was first synthesized. In 1962, G. Falkson, MD, and E.J. Schulz, MD, reported on skin changes observed in 85 patients being treated with systemic 5-FU for advanced carcinomatosis. They found that 31 of the 85 patients developed sensitivity to sunlight and subsequent disappearance of actinic keratoses in these same patients.
Technology in skin care also was developing in the era just before the launch of Skin & Allergy News. For example, Leon Goldman, MD, then chairman of the department of dermatology at the University of Cincinnati, was the first physician to use a laser for tattoo removal. His publication in 1965 helped to solidify its use, leading him to be “regarded by many in the dermatologic community as the ‘godfather of lasers in medicine and surgery’ ” (Clin Dermatol. 2007;25:434-42).
So, by 1970, dermatology as a field had established itself fully with a strong societal infrastructure, a vibrant base of journals and books, and an evolving set of scientific and technical tools. The launch of Skin & Allergy News (now Dermatology News) that year would chronicle dermatology’s commitment to the development of new therapeutics and technologies in service of patient needs – the stories of which would grace the newspaper’s pages for 5 decades and counting.
As Dermatology News (formerly Skin and Allergy News) reaches its 50th-year milestone, a reflection on the history of the discipline, especially in the United States, up to the time of the launch of this publication is in order. Such an overview must, of course, be cursory in this context. Yet, for those who want to learn more, a large body of historical references and research has been created to fill in the gaps, as modern dermatology has always been cognizant of the importance of its history, with many individuals and groups drawn to the subject.
Two excellent sources for the history of the field can be found in work by William Allen Pusey, MD (1865-1940), and Herbert Rattner, MD (1900-1962), “The History of Dermatology” published in 1933 and research by members of the History of Dermatology Society, founded in 1973 in New York.
Modern dermatology
The development of the field of modern dermatology can be traced back to the early to mid-19th century. During the first half of the 19th century, England and France dominated the study of dermatology, but by the middle of the century, the German revolution in microparasitology shifted that focus “with remarkable German discoveries,” according to Bernard S. Potter, MD, in his review of bibliographic landmarks of the history of dermatology (J Am Acad Dermatol 2003;48:919-32). For example, Johann Lucas Schoenlein (1793-1864) in 1839 discovered the fungal origin of favus, and in 1841 Jacob Henle (1809-1885) discovered Demodex folliculorum. Karl Ferdinand Eichstedt (1816-1892) in 1846 followed with the discovery of the causative agent of pityriasis versicolor, and Friedrich Wilhelm Felix von Barensprung (1822-1864) in 1862 coined the term erythrasma and named the organism responsible for this condition Microsporum minutissimum.
Dr. Potter described how American dermatology originated in New York City in 1836 when Henry Daggett Bulkley, MD, (1803-1872) opened the first dispensary for skin diseases, the Broome Street Infirmary for Diseases of the Skin, thus creating the first institution in the United States for the treatment of cutaneous disease. As the first American dermatologist, he was also the first in the United States to lecture on and to exclusively practice dermatology.
The rise of interest in the importance of dermatology led to the organization of the early American Dermatological Association in 1886.
However, the state of dermatology as a science in the 19th century was not always looked upon favorably, even by its practitioners, especially in the United States. In 1871, in a “Review on Modern Dermatology,” given as a series of lectures on skin disease at Harvard University, James C. White, MD (1833-1916) of Massachusetts General Hospital, stated that: “Were the literature of skin diseases previous to that of the last half-century absolutely annihilated, and with it, the influence it has exercised upon that of the present day, it would be an immense gain to dermatology, although much of real value would perish.” He lamented that America had contributed little so far to the study of dermatology, and that the discipline was only taught in some of its largest schools, and he urged that this be changed. He also lamented that The American Journal of Syphilography and Dermatology, established the year before, had so far proved itself heavy on syphilis, but light on dermatology, a situation he also hoped would change dramatically.
By the late-19th century, the conviction that diseases of the skin needed to be connected to the overall metabolism and physiology of the patient as a whole was becoming more mainstream.
“It has been, and still is, too much the custom to study diseases of the skin in the light of pathological pictures, to name the local manifestation and to so label it as disease. It is much easier to give the disease name and to label it than it is to comprehend the process at work. The former is comparatively unimportant for the patient, the latter point upon which recovery may depend. The nature and meaning of the process in connection with the cutaneous symptoms has not received enough attention, and I believe this to be one reason why the treatment of many of these diseases in the past has been so notoriously unsatisfactory,” Louis A. Duhring, MD (1845-1913) chided his colleagues in the Section of Dermatology and Syphilography, at the Forty-fourth Annual Meeting of the American Medical Association in 1894. (collections.nlm.nih.gov/ext/dw/101489447/PDF/101489447.pdf)
In the early-20th century, German dermatology influenced American dermatology more than any other, according to Karl Holubar, MD, of the Institute for the History of Medicine, University of Vienna, in his lecture on the history of European dermatopathology.
He stated that, with regard to dermatopathology, it was Oscar Gans, MD (1888-1983) who brought the latest knowledge into the United States by delivering a series of lectures at Mayo Clinic in the late 1920s upon the invitation of Paul A. O’Leary, MD, (1891-1955) who then headed the Mayo section of dermatology.
By the 1930s, a flurry of organizational activity overtook American dermatology. In 1932, the American Board of Dermatology was established, with its first exams given in 1933 (20 students passed, 7 failed). The Society for Investigative Dermatology was founded in 1937, and the American Academy of Dermatology and Syphilology (now the American Academy of Dermatology), founded in 1938.
The 1930s also saw a major influx of German and other European Jews fleeing Nazi oppression who would forever change the face of American dermatology. “Between 1933 and 1938, a series of repressive measures eliminated them from the practice of medicine in Germany and other countries. Although some died in concentration camps and others committed suicide, many were able to emigrate from Europe. Dermatology in the United States particularly benefited from the influx of several stellar Jewish dermatologists who were major contributors to the subsequent flowering of academic dermatology in the United States” (JAMA Derm. 2013;149[9]:1090-4).
“The overtures of the holocaust and the rising power of Hitler in Europe finally brought over to the United States the flower of dermatologists and investigators of the German School, e.g., Alexander and Walter Lever, Felix and Hermann Pinkus, the Epsteins, Erich Auerbach, Stephen Rothman, to name just a few. With this exodus and transfer of brain power, Europe lost its leading role to never again regain it,” according to Dr. Holubar. Walter F. Lever, MD (1909-1992) was especially well-known for his landmark textbook on dermatology, “Histopathology of the Skin,” published in the United States in 1949.
The therapeutic era
Throughout the 19th century, a variety of soaps and patent medicines were touted as cure-alls for a host of skin diseases. Other than their benefits to surface cleanliness and their antiseptic properties, however, they were of little effect.
It wasn’t until the 20th century that truly effective therapeutics entered the dermatologic pharmacopoeia. In their 1989 review, Diane Quintal, MD, and Robert Jackson, MD, discussed the origins of the most important of these drugs and pointed out that, “Until this century, the essence of dermatology resided in the realm of morphology. Early contributors largely confined their activities to the classification of skin diseases and to the elaboration of clinical dermatologic entities based on morphologic features. ... but “in the last 50 years, there have been significant scientific discoveries in the field of therapeutics that have revolutionized the practice of dermatology.“ (Clin Dermatol. 1989;7[3]38-47).
These key drugs comprised:
- Quinacrine was introduced in 1932 by Walter Kikuth, MD, as an antimalarial drug. But it was not until 1940, that A.J. Prokoptchouksi, MD, reported on its effectiveness 35 patients with lupus erythematosus.
- Para-aminobenzoic acid (PABA) came into prominence in 1942, when Stephen Rothman, MD, and Jack Rubin, MD, at the University of Chicago, published the results of their experiment, showing that when PABA was incorporated in an ointment base and applied to the skin, it could protect against sunburn.
- Dapsone. The effectiveness of sulfapyridine was demonstrated in 1947 by M.J. Costello, MD, who reported its usefulness in a patient with dermatitis herpetiformis, which he believed to be caused by a bacterial allergy. Sulfapyridine controlled the disease, but gastrointestinal intolerance and sulfonamide sensitivity were side effects. Ultimately, in 1951, Theodore Cornbleet, MD, introduced the use of sulfoxones in his article entitled “Sulfoxone (diasones) sodium for dermatitis herpetiformis,” considered more effective than sulfapyridine. Dapsone is the active principal ingredient.
- Hydrocortisone. In August 1952, Marion Sulzberger, MD, and Victor H. Witten, MD (both members of the first Skin & Allergy News editorial advisory board), described use of Compound F (17-hydroxycorticosterone-21-acetate, hydrocortisone) in seven cases of atopic dermatitis and one case of discoid or subacute lupus erythematosus, reporting improvement in all of these cases.
- Benzoyl peroxide. Canadian dermatologist William E. Pace, MD, reported on the beneficial effects of benzoyl peroxide on acne in 1953. The product had originally been used for chronic Staphylococcus aureus folliculitis of the beard.
- Griseofulvin, a metabolic byproduct of a number of species of Penicillium, was first isolated in 1939. But in 1958, Harvey Blank, MD, at the University of Miami (also on the first Skin & Allergy News editorial advisory board), and Stanley I. Cullen, MD, administered the drug to a patient with Trichophyton rubrum granuloma, in the first human trial. In 1959, they reported the drug’s benefits on 31 patients with various fungal infections.
- Methotrexate. In 1951, R. Gubner, MD, and colleagues noticed the rapid clearing of skin lesions in a patient with rheumatoid arthritis who had been treated with the folic acid antagonist, aminopterin. And in 1958, W.F. Edmundson, MD, and W.B. Guy, MD, reported on the oral use of the folic acid antagonist, methotrexate. This was followed by multiple reports on the successful use of methotrexate in psoriasis.
- 5-fluorouracil (5-FU). In 1957, 5-FU, an antimetabolite of uracil, was first synthesized. In 1962, G. Falkson, MD, and E.J. Schulz, MD, reported on skin changes observed in 85 patients being treated with systemic 5-FU for advanced carcinomatosis. They found that 31 of the 85 patients developed sensitivity to sunlight and subsequent disappearance of actinic keratoses in these same patients.
Technology in skin care also was developing in the era just before the launch of Skin & Allergy News. For example, Leon Goldman, MD, then chairman of the department of dermatology at the University of Cincinnati, was the first physician to use a laser for tattoo removal. His publication in 1965 helped to solidify its use, leading him to be “regarded by many in the dermatologic community as the ‘godfather of lasers in medicine and surgery’ ” (Clin Dermatol. 2007;25:434-42).
So, by 1970, dermatology as a field had established itself fully with a strong societal infrastructure, a vibrant base of journals and books, and an evolving set of scientific and technical tools. The launch of Skin & Allergy News (now Dermatology News) that year would chronicle dermatology’s commitment to the development of new therapeutics and technologies in service of patient needs – the stories of which would grace the newspaper’s pages for 5 decades and counting.