User login
News and Views that Matter to Physicians
Genes that drive glucose levels also drive heart disease
MUNICH – A group of 12 genes that influence blood sugar appears to help drive the risk of heart disease, independent of type 2 diabetes.
The genome-wide association study determined that every 1 mmol/L increase in fasting glucose associated with these genes increased the risk of coronary heart disease by 43%, Jordi Merino, PhD, said at the annual meeting of the European Association for the Study of Diabetes.
“Our results quantify the causal relationship between isolated, genetically increased fasting glucose and heart disease risk beyond the genetic effect of type 2 diabetes,” said Dr. Merino of Massachusetts General Hospital, Boston. “They suggest that modulating glycemia may provide cardiovascular benefit.”
It’s known that patients with type 2 diabetes have a higher incidence of coronary heart disease, even after accounting for traditional cardiovascular risk factors, he said. But five large prospective randomized studies – including the much-vaunted ACCORD – failed to find convincing evidence that managing blood glucose in patients with diabetes exerts any benefit on cardiovascular outcomes. In fact, patients assigned to intensive management (blood glucose targeted to below 6%) had a relative increase in all-cause mortality of 22% and an absolute increase of 1%, without any differences in cardiovascular mortality (5% vs. 4%; hazard ratio, 1.22) (N Engl J Med. 2008;358:2545-59).
However, a 2014 subanalysis of ACCORD found that outcomes for ischemic heart disease were significantly better in the intensively managed group. There was a 20% reduction in the risk of heart attack; a 19% reduction in a combined endpoint of heart attack; and similar reductions in the risk of coronary revascularization and unstable angina (Lancet. 2014;384:1936-41).
“We believed genetics might help to answer the question about this discrepancies in findings,” Dr. Merino said.
To investigate this, he and his colleagues plumbed the largest meta-analyses of genome-wide association studies of glucose and insulin regulation. MAGIC (the Meta-Analyses of Glucose and Insulin-related traits Consortium) is a collaborative effort that has combined genetic data from 55 studies.
MAGIC investigators have identified dozens of loci that influence levels of fasting glucose, fasting insulin, and hemoglobin A1c. The project includes data on 133,000 subjects without type 2 diabetes.
They used these data to conduct a Mendelian randomization analysis – a way of establishing causality between a specific gene and a specific clinical trait. Such an analysis is valid only when there are no other functional pathways between the genetic variant and the outcome and when confounding factors that could also affect the outcome can be controlled for.
MAGIC found 234 genetic variants that influence fasting glucose. Some of these also increase the risk of type 2 diabetes; after excluding those, Dr. Merino was left with 107 candidate genes. A disequilibrium analysis further pruned the group, leaving 12 genes that are independently associated with fasting glucose regulation.
He and his colleagues then applied data from the CARDIoGRAMplusC4D Consortium, which is searching for multiple risk loci for coronary artery disease and myocardial infarction in several large genetic studies. They created a five-level risk score for the glycemia-modulating genes and used to it determine how much genetically driven glucose variability affected the risk of heart disease in 5,000 subjects included in the Framingham Heart Study. The analysis controlled for lipids, blood pressure, and body mass index, he noted.
In a model that included all 12 of the variants, the investigators found that every 1 mmol/L increase in fasting glucose was associated with a significant 43% increase in the risk of heart disease.
A second analysis excluded one of the genes, but the significant association with increased risk of heart disease was preserved, at 34% per 1 mmol/L increase in fasting glucose. Individually, 10 of the genes raised the risk of coronary heart disease from a low of 6% (OR 1.06) to a high of almost 400% (OR 3.8).
The final pleiotropic analysis excluded all genes that could have more than one effect on heart disease; five genes survived to this level. Overall, they raised the risk of heart disease by 33%. Individually, the relative increased risks ranged from a low of 12% (odds ratio, 1.12) to a high of 87% (OR, 1.87). One gene was associated with a 25% risk reduction.
Dr. Merino had no financial disclosures.
MUNICH – A group of 12 genes that influence blood sugar appears to help drive the risk of heart disease, independent of type 2 diabetes.
The genome-wide association study determined that every 1 mmol/L increase in fasting glucose associated with these genes increased the risk of coronary heart disease by 43%, Jordi Merino, PhD, said at the annual meeting of the European Association for the Study of Diabetes.
“Our results quantify the causal relationship between isolated, genetically increased fasting glucose and heart disease risk beyond the genetic effect of type 2 diabetes,” said Dr. Merino of Massachusetts General Hospital, Boston. “They suggest that modulating glycemia may provide cardiovascular benefit.”
It’s known that patients with type 2 diabetes have a higher incidence of coronary heart disease, even after accounting for traditional cardiovascular risk factors, he said. But five large prospective randomized studies – including the much-vaunted ACCORD – failed to find convincing evidence that managing blood glucose in patients with diabetes exerts any benefit on cardiovascular outcomes. In fact, patients assigned to intensive management (blood glucose targeted to below 6%) had a relative increase in all-cause mortality of 22% and an absolute increase of 1%, without any differences in cardiovascular mortality (5% vs. 4%; hazard ratio, 1.22) (N Engl J Med. 2008;358:2545-59).
However, a 2014 subanalysis of ACCORD found that outcomes for ischemic heart disease were significantly better in the intensively managed group. There was a 20% reduction in the risk of heart attack; a 19% reduction in a combined endpoint of heart attack; and similar reductions in the risk of coronary revascularization and unstable angina (Lancet. 2014;384:1936-41).
“We believed genetics might help to answer the question about this discrepancies in findings,” Dr. Merino said.
To investigate this, he and his colleagues plumbed the largest meta-analyses of genome-wide association studies of glucose and insulin regulation. MAGIC (the Meta-Analyses of Glucose and Insulin-related traits Consortium) is a collaborative effort that has combined genetic data from 55 studies.
MAGIC investigators have identified dozens of loci that influence levels of fasting glucose, fasting insulin, and hemoglobin A1c. The project includes data on 133,000 subjects without type 2 diabetes.
They used these data to conduct a Mendelian randomization analysis – a way of establishing causality between a specific gene and a specific clinical trait. Such an analysis is valid only when there are no other functional pathways between the genetic variant and the outcome and when confounding factors that could also affect the outcome can be controlled for.
MAGIC found 234 genetic variants that influence fasting glucose. Some of these also increase the risk of type 2 diabetes; after excluding those, Dr. Merino was left with 107 candidate genes. A disequilibrium analysis further pruned the group, leaving 12 genes that are independently associated with fasting glucose regulation.
He and his colleagues then applied data from the CARDIoGRAMplusC4D Consortium, which is searching for multiple risk loci for coronary artery disease and myocardial infarction in several large genetic studies. They created a five-level risk score for the glycemia-modulating genes and used to it determine how much genetically driven glucose variability affected the risk of heart disease in 5,000 subjects included in the Framingham Heart Study. The analysis controlled for lipids, blood pressure, and body mass index, he noted.
In a model that included all 12 of the variants, the investigators found that every 1 mmol/L increase in fasting glucose was associated with a significant 43% increase in the risk of heart disease.
A second analysis excluded one of the genes, but the significant association with increased risk of heart disease was preserved, at 34% per 1 mmol/L increase in fasting glucose. Individually, 10 of the genes raised the risk of coronary heart disease from a low of 6% (OR 1.06) to a high of almost 400% (OR 3.8).
The final pleiotropic analysis excluded all genes that could have more than one effect on heart disease; five genes survived to this level. Overall, they raised the risk of heart disease by 33%. Individually, the relative increased risks ranged from a low of 12% (odds ratio, 1.12) to a high of 87% (OR, 1.87). One gene was associated with a 25% risk reduction.
Dr. Merino had no financial disclosures.
MUNICH – A group of 12 genes that influence blood sugar appears to help drive the risk of heart disease, independent of type 2 diabetes.
The genome-wide association study determined that every 1 mmol/L increase in fasting glucose associated with these genes increased the risk of coronary heart disease by 43%, Jordi Merino, PhD, said at the annual meeting of the European Association for the Study of Diabetes.
“Our results quantify the causal relationship between isolated, genetically increased fasting glucose and heart disease risk beyond the genetic effect of type 2 diabetes,” said Dr. Merino of Massachusetts General Hospital, Boston. “They suggest that modulating glycemia may provide cardiovascular benefit.”
It’s known that patients with type 2 diabetes have a higher incidence of coronary heart disease, even after accounting for traditional cardiovascular risk factors, he said. But five large prospective randomized studies – including the much-vaunted ACCORD – failed to find convincing evidence that managing blood glucose in patients with diabetes exerts any benefit on cardiovascular outcomes. In fact, patients assigned to intensive management (blood glucose targeted to below 6%) had a relative increase in all-cause mortality of 22% and an absolute increase of 1%, without any differences in cardiovascular mortality (5% vs. 4%; hazard ratio, 1.22) (N Engl J Med. 2008;358:2545-59).
However, a 2014 subanalysis of ACCORD found that outcomes for ischemic heart disease were significantly better in the intensively managed group. There was a 20% reduction in the risk of heart attack; a 19% reduction in a combined endpoint of heart attack; and similar reductions in the risk of coronary revascularization and unstable angina (Lancet. 2014;384:1936-41).
“We believed genetics might help to answer the question about this discrepancies in findings,” Dr. Merino said.
To investigate this, he and his colleagues plumbed the largest meta-analyses of genome-wide association studies of glucose and insulin regulation. MAGIC (the Meta-Analyses of Glucose and Insulin-related traits Consortium) is a collaborative effort that has combined genetic data from 55 studies.
MAGIC investigators have identified dozens of loci that influence levels of fasting glucose, fasting insulin, and hemoglobin A1c. The project includes data on 133,000 subjects without type 2 diabetes.
They used these data to conduct a Mendelian randomization analysis – a way of establishing causality between a specific gene and a specific clinical trait. Such an analysis is valid only when there are no other functional pathways between the genetic variant and the outcome and when confounding factors that could also affect the outcome can be controlled for.
MAGIC found 234 genetic variants that influence fasting glucose. Some of these also increase the risk of type 2 diabetes; after excluding those, Dr. Merino was left with 107 candidate genes. A disequilibrium analysis further pruned the group, leaving 12 genes that are independently associated with fasting glucose regulation.
He and his colleagues then applied data from the CARDIoGRAMplusC4D Consortium, which is searching for multiple risk loci for coronary artery disease and myocardial infarction in several large genetic studies. They created a five-level risk score for the glycemia-modulating genes and used to it determine how much genetically driven glucose variability affected the risk of heart disease in 5,000 subjects included in the Framingham Heart Study. The analysis controlled for lipids, blood pressure, and body mass index, he noted.
In a model that included all 12 of the variants, the investigators found that every 1 mmol/L increase in fasting glucose was associated with a significant 43% increase in the risk of heart disease.
A second analysis excluded one of the genes, but the significant association with increased risk of heart disease was preserved, at 34% per 1 mmol/L increase in fasting glucose. Individually, 10 of the genes raised the risk of coronary heart disease from a low of 6% (OR 1.06) to a high of almost 400% (OR 3.8).
The final pleiotropic analysis excluded all genes that could have more than one effect on heart disease; five genes survived to this level. Overall, they raised the risk of heart disease by 33%. Individually, the relative increased risks ranged from a low of 12% (odds ratio, 1.12) to a high of 87% (OR, 1.87). One gene was associated with a 25% risk reduction.
Dr. Merino had no financial disclosures.
AT EASD 2016
Key clinical point: Twelve newly identified genes associated with glucose levels appear to be independent drivers of coronary heart disease.
Major finding: Altogether, the constellation of genes raises the risk of heart disease by 43% for every 1 mmol/L increase in blood glucose.
Data source: Analysis of 133,000 subjects without diabetes.
Disclosures: Dr. Merino had no financial disclosures.
Medical quality beats patient comfort
A good way to survive an acute myocardial infarction is to go to the best hospital.
Some patients seem to have found out where those are. For those of us who want to know where to go and what to look for, a recent analysis of 800,000 Medicare patients admitted with acute myocardial infarction (AMI) and heart failure in 10,000 hospitals between 2008 and 2009 provides some reassuring news (National Bureau of Economic Research Working Paper 21603). Its findings indicated that in an era when health care choice is seemingly influenced by testimonial TV ads and the creation of hospitals that look like hotels, technical medical quality outranks all the glitz and bricks. Quality was measured by hospital mortality, 30-day readmissions, adherence to well-established guidelines, and patient satisfaction questionnaires. The investigators measured the effect that medical quality and the “comfort quotient” had on the growth of hospital patient volume through the emergency departments and interhospital referrals.
Hospital admissions increased in hospitals with the highest-quality performance. Over the 2-year period, the hospitals with the highest-quality performance had increases in hospital volume. Hospitals with a 1% improvement in the adjusted AMI mortality had a 17% increase in market share and a 1.5 % growth rate.
The authors estimated that patients with an AMI (or their family) were willing to travel an additional 1.8 miles for an ED admission to a hospital with a higher survival rate, and 34 miles further for a transfer to a hospital with a higher survival rate. When patients had the option to choose a hospital to be transferred to for further care, quality of care measures had an even greater impact on choice. Postdischarge evaluation of patient satisfaction had little or no effect on growth.
Patients admitted through the ED have the least chance for hospital choice, but even in these patients knowledge about quality influenced the choice of the hospital and the long-term hospital growth rate. Considering the fact that there is scant information available either to patients or even doctors about quality measures, there appears to be a choice process either by patient family, doctor, or ambulance driver to direct patients to the hospital with the best survival rate.
How they made those decisions is not clear. Comparative hospital survival data are rarely transmitted to staff physicians and are not widely available to the public. I have never seen any data like these in the multitude of hospital TV ads, yet somehow those numbers, real or perceived, affected admission and transfer. Maybe it’s just reputation; we all know about that. If you are really interested, you can find hospital medical quality and patient experience data at Medicare’s Hospital Compare site.
All this is good. Medical quality wins. Other studies, however, suggest that usually the “comfort quotient” and measures of medical quality are more closely linked. It has also been suggested that volume is the driving force for the improvement in both quality measures by providing the resources and logistics for better care. Whatever the mechanism, it seems that high-quality medical care is not a bad way to choose which neighborhood hospital to go to in order to survive an AMI.
Dr. Goldstein, medical editor of Cardiology News, is professor of medicine at Wayne State University and division head emeritus of cardiovascular medicine at Henry Ford Hospital, both in Detroit. He is on data safety monitoring committees for the National Institutes of Health and several pharmaceutical companies.
A good way to survive an acute myocardial infarction is to go to the best hospital.
Some patients seem to have found out where those are. For those of us who want to know where to go and what to look for, a recent analysis of 800,000 Medicare patients admitted with acute myocardial infarction (AMI) and heart failure in 10,000 hospitals between 2008 and 2009 provides some reassuring news (National Bureau of Economic Research Working Paper 21603). Its findings indicated that in an era when health care choice is seemingly influenced by testimonial TV ads and the creation of hospitals that look like hotels, technical medical quality outranks all the glitz and bricks. Quality was measured by hospital mortality, 30-day readmissions, adherence to well-established guidelines, and patient satisfaction questionnaires. The investigators measured the effect that medical quality and the “comfort quotient” had on the growth of hospital patient volume through the emergency departments and interhospital referrals.
Hospital admissions increased in hospitals with the highest-quality performance. Over the 2-year period, the hospitals with the highest-quality performance had increases in hospital volume. Hospitals with a 1% improvement in the adjusted AMI mortality had a 17% increase in market share and a 1.5 % growth rate.
The authors estimated that patients with an AMI (or their family) were willing to travel an additional 1.8 miles for an ED admission to a hospital with a higher survival rate, and 34 miles further for a transfer to a hospital with a higher survival rate. When patients had the option to choose a hospital to be transferred to for further care, quality of care measures had an even greater impact on choice. Postdischarge evaluation of patient satisfaction had little or no effect on growth.
Patients admitted through the ED have the least chance for hospital choice, but even in these patients knowledge about quality influenced the choice of the hospital and the long-term hospital growth rate. Considering the fact that there is scant information available either to patients or even doctors about quality measures, there appears to be a choice process either by patient family, doctor, or ambulance driver to direct patients to the hospital with the best survival rate.
How they made those decisions is not clear. Comparative hospital survival data are rarely transmitted to staff physicians and are not widely available to the public. I have never seen any data like these in the multitude of hospital TV ads, yet somehow those numbers, real or perceived, affected admission and transfer. Maybe it’s just reputation; we all know about that. If you are really interested, you can find hospital medical quality and patient experience data at Medicare’s Hospital Compare site.
All this is good. Medical quality wins. Other studies, however, suggest that usually the “comfort quotient” and measures of medical quality are more closely linked. It has also been suggested that volume is the driving force for the improvement in both quality measures by providing the resources and logistics for better care. Whatever the mechanism, it seems that high-quality medical care is not a bad way to choose which neighborhood hospital to go to in order to survive an AMI.
Dr. Goldstein, medical editor of Cardiology News, is professor of medicine at Wayne State University and division head emeritus of cardiovascular medicine at Henry Ford Hospital, both in Detroit. He is on data safety monitoring committees for the National Institutes of Health and several pharmaceutical companies.
A good way to survive an acute myocardial infarction is to go to the best hospital.
Some patients seem to have found out where those are. For those of us who want to know where to go and what to look for, a recent analysis of 800,000 Medicare patients admitted with acute myocardial infarction (AMI) and heart failure in 10,000 hospitals between 2008 and 2009 provides some reassuring news (National Bureau of Economic Research Working Paper 21603). Its findings indicated that in an era when health care choice is seemingly influenced by testimonial TV ads and the creation of hospitals that look like hotels, technical medical quality outranks all the glitz and bricks. Quality was measured by hospital mortality, 30-day readmissions, adherence to well-established guidelines, and patient satisfaction questionnaires. The investigators measured the effect that medical quality and the “comfort quotient” had on the growth of hospital patient volume through the emergency departments and interhospital referrals.
Hospital admissions increased in hospitals with the highest-quality performance. Over the 2-year period, the hospitals with the highest-quality performance had increases in hospital volume. Hospitals with a 1% improvement in the adjusted AMI mortality had a 17% increase in market share and a 1.5 % growth rate.
The authors estimated that patients with an AMI (or their family) were willing to travel an additional 1.8 miles for an ED admission to a hospital with a higher survival rate, and 34 miles further for a transfer to a hospital with a higher survival rate. When patients had the option to choose a hospital to be transferred to for further care, quality of care measures had an even greater impact on choice. Postdischarge evaluation of patient satisfaction had little or no effect on growth.
Patients admitted through the ED have the least chance for hospital choice, but even in these patients knowledge about quality influenced the choice of the hospital and the long-term hospital growth rate. Considering the fact that there is scant information available either to patients or even doctors about quality measures, there appears to be a choice process either by patient family, doctor, or ambulance driver to direct patients to the hospital with the best survival rate.
How they made those decisions is not clear. Comparative hospital survival data are rarely transmitted to staff physicians and are not widely available to the public. I have never seen any data like these in the multitude of hospital TV ads, yet somehow those numbers, real or perceived, affected admission and transfer. Maybe it’s just reputation; we all know about that. If you are really interested, you can find hospital medical quality and patient experience data at Medicare’s Hospital Compare site.
All this is good. Medical quality wins. Other studies, however, suggest that usually the “comfort quotient” and measures of medical quality are more closely linked. It has also been suggested that volume is the driving force for the improvement in both quality measures by providing the resources and logistics for better care. Whatever the mechanism, it seems that high-quality medical care is not a bad way to choose which neighborhood hospital to go to in order to survive an AMI.
Dr. Goldstein, medical editor of Cardiology News, is professor of medicine at Wayne State University and division head emeritus of cardiovascular medicine at Henry Ford Hospital, both in Detroit. He is on data safety monitoring committees for the National Institutes of Health and several pharmaceutical companies.
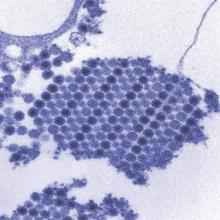
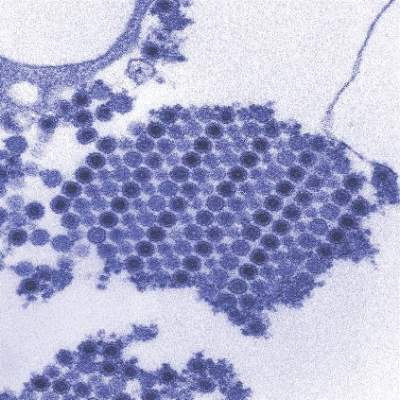
Postnatally acquired Zika infection in children usually mild
The clinical course of postnatally acquired Zika virus disease in children younger than 18 years is mild and rarely results in severe illness or death, reported Alyson Goodman, MD, and her associates at the Centers for Disease Control and Prevention (CDC), Atlanta.
There were a total of 158 confirmed or probable postnatally acquired Zika virus disease cases among children younger than 18 years reported to the CDC in the United States between January 2015 and July 2016, wrote researchers in a case series that described the epidemiology, clinical findings, and outcomes of the cases (MMWR. 2016 Sep 30;65:1-4).
The cases were reported in 30 d ifferent states, and the states with the highest numbers of reported cases were Florida (23%), New York (11%), and California (9%). All patients acquired Zika virus infections during travel to a location where mosquito-borne transmission had been documented, according to researchers.
The median patient age was 14 years, the majority of the patients were female (56%), and five patients were pregnant.
Of Zika’s four primary clinical signs and symptoms, 82% of the pediatric population had a rash, 55% had a fever, 29% had conjunctivitis, and 28% had arthralgia, with 70% of the children presenting with two or more of these symptoms.
Only two children were hospitalized because of their infections. No children were reported to have meningitis, encephalitis, or Guillain-Barré syndrome, and no patients with Zika virus infection died, researchers reported.
This data “corroborates previously published reports suggesting that the clinical course of Zika virus disease is typically mild in children, as it is in adults,” Dr. Goodman and her associates wrote.
Severe disease and death in children with postnatally acquired Zika virus infection are rare, the researchers pointed out, but they encouraged physicians to consider a Zika virus disease diagnosis for children with the four common symptoms and who reside in or traveled to an area with active Zika virus transmission. All Zika virus disease cases should be reported to state health departments.
On Twitter @jessnicolecraig
The clinical course of postnatally acquired Zika virus disease in children younger than 18 years is mild and rarely results in severe illness or death, reported Alyson Goodman, MD, and her associates at the Centers for Disease Control and Prevention (CDC), Atlanta.
There were a total of 158 confirmed or probable postnatally acquired Zika virus disease cases among children younger than 18 years reported to the CDC in the United States between January 2015 and July 2016, wrote researchers in a case series that described the epidemiology, clinical findings, and outcomes of the cases (MMWR. 2016 Sep 30;65:1-4).
The cases were reported in 30 d ifferent states, and the states with the highest numbers of reported cases were Florida (23%), New York (11%), and California (9%). All patients acquired Zika virus infections during travel to a location where mosquito-borne transmission had been documented, according to researchers.
The median patient age was 14 years, the majority of the patients were female (56%), and five patients were pregnant.
Of Zika’s four primary clinical signs and symptoms, 82% of the pediatric population had a rash, 55% had a fever, 29% had conjunctivitis, and 28% had arthralgia, with 70% of the children presenting with two or more of these symptoms.
Only two children were hospitalized because of their infections. No children were reported to have meningitis, encephalitis, or Guillain-Barré syndrome, and no patients with Zika virus infection died, researchers reported.
This data “corroborates previously published reports suggesting that the clinical course of Zika virus disease is typically mild in children, as it is in adults,” Dr. Goodman and her associates wrote.
Severe disease and death in children with postnatally acquired Zika virus infection are rare, the researchers pointed out, but they encouraged physicians to consider a Zika virus disease diagnosis for children with the four common symptoms and who reside in or traveled to an area with active Zika virus transmission. All Zika virus disease cases should be reported to state health departments.
On Twitter @jessnicolecraig
The clinical course of postnatally acquired Zika virus disease in children younger than 18 years is mild and rarely results in severe illness or death, reported Alyson Goodman, MD, and her associates at the Centers for Disease Control and Prevention (CDC), Atlanta.
There were a total of 158 confirmed or probable postnatally acquired Zika virus disease cases among children younger than 18 years reported to the CDC in the United States between January 2015 and July 2016, wrote researchers in a case series that described the epidemiology, clinical findings, and outcomes of the cases (MMWR. 2016 Sep 30;65:1-4).
The cases were reported in 30 d ifferent states, and the states with the highest numbers of reported cases were Florida (23%), New York (11%), and California (9%). All patients acquired Zika virus infections during travel to a location where mosquito-borne transmission had been documented, according to researchers.
The median patient age was 14 years, the majority of the patients were female (56%), and five patients were pregnant.
Of Zika’s four primary clinical signs and symptoms, 82% of the pediatric population had a rash, 55% had a fever, 29% had conjunctivitis, and 28% had arthralgia, with 70% of the children presenting with two or more of these symptoms.
Only two children were hospitalized because of their infections. No children were reported to have meningitis, encephalitis, or Guillain-Barré syndrome, and no patients with Zika virus infection died, researchers reported.
This data “corroborates previously published reports suggesting that the clinical course of Zika virus disease is typically mild in children, as it is in adults,” Dr. Goodman and her associates wrote.
Severe disease and death in children with postnatally acquired Zika virus infection are rare, the researchers pointed out, but they encouraged physicians to consider a Zika virus disease diagnosis for children with the four common symptoms and who reside in or traveled to an area with active Zika virus transmission. All Zika virus disease cases should be reported to state health departments.
On Twitter @jessnicolecraig
FROM MMWR
Key clinical point: Postnatally acquired Zika virus disease in children younger than 18 years is mild and rarely results in severe illness or death.
Major finding: Only two children were hospitalized because of their infections, and no children died.
Data source: Case series of 158 confirmed or probable postnatally acquired Zika virus disease cases among children.
Disclosures: The study was funded by the CDC. Author disclosures were not reported.
GAO report calls out HHS for misdirecting ACA reinsurance funds
The Health and Human Services department is failing to properly administer a reinsurance program under the Affordable Care Act by unlawfully diverting funds intended for the U.S. Treasury, according to a nonpartisan watchdog report.
The Government Accountability Office (GAO) report, released Sept. 29, finds that HHS is breaking ACA regulations that require a portion of funds collected under its Transitional Reinsurance Program to go to the Treasury. Instead, the agency has redirected $5 billion so far to pay health insurers that enroll high-cost patients under the program.
“In light of the foregoing analysis, we conclude that HHS lacks authority to ignore the statute’s directive to deposit amounts from collections under the Transitional Reinsurance Program to the Treasury and instead make deposits in the Treasury only if its collections reach the amounts for reinsurance payments specified in section 1341,” Susan A. Poling, GAO general counsel, wrote in a legal opinion. “The agency is not authorized to prioritize collections in this manner.”
Republican lawmakers, including Sen. John Barrasso III (Wyo.), chair of the Senate Republican Policy Committee, praised the legal opinion, saying it shows the Obama administration is bending the rules when it comes to rolling out the ACA.
“This is a major victory for the American people who are suffering with higher premiums and fewer choices because of this failed law,” said Sen. Barrasso, who with several fellow Republican legislators requested the GAO investigation. “The administration should end this illegal scheme immediately, and focus on providing relief from the burdens of this law.”
At press time, neither the White House nor HHS had responded to request for comment on the report.
The Transitional Reinsurance Program is a 3-year initiative under the ACA that collects fees from employers and other private health insurance plans and directs the funds to health plans that face large claims for patients with high-cost medical conditions. The ACA specifies that between 2014 and 2016, HHS would collect $25 billion in fees, of which $5 billion would go into the Treasury.
But when HHS was unable to collect the full amount over the 3 years, it did not distribute funds into the Treasury, but instead used it pay the health plans. To justify that decision, HHS officials announced that the department would allocate all collections first for reinsurance payments until collections totaled the target amount set forth for reinsurance payments under the law, and that any remaining collections would go toward to administrative expenses and the Treasury, according to the GAO report.
But the GAO argues that HHS falling short of the projected collections does not alter the meaning of the statute.
“Specifically, where actual funding has fallen short of an agency’s original expectations, courts have directed the agency to distribute available funds to approximate the allocation plan Congress designed in anticipation of full funding,” Ms. Poling wrote. The HHS “assertion that the statute is silent with respect to allocation of collections overlooks the fact that section 1341 expressly directs HHS to collect amounts for the Treasury and prohibits the use of these amounts for any purpose other than deposit in the Treasury. HHS’s analysis focuses on words and phrases in the statute in isolation rather than in their appropriate context.”
While the GAO cannot force HHS to act with the opinion, lawmakers could use the report to craft legislation forcing HHS to repay the Treasury.
“This issue has been brewing for a while, and is another example of a series of attempts to challenge or limit payments being made to carriers under the ACA,” Katherine Hempstead, senior adviser to vice president at the Robert Wood Johnson Foundation, said in an interview. “It’s no mystery why making these payments is a pretty high priority for the [Centers for Medicare & Medicaid Services] right now, given the losses sustained by many market participants. My guess is that the can will probably be kicked down the road on this and a number of other issues for the next administration and Congress to resolve.”
The reinsurance program was one of three programs intended to protect against the negative impacts of adverse selection and risk selection, while working to stabilize premiums during the initial years of the health law’s implementation. The program aims to protect against premium increases in the individual market by offsetting expenses of high-cost patients.
On Twitter @legal_med
The Health and Human Services department is failing to properly administer a reinsurance program under the Affordable Care Act by unlawfully diverting funds intended for the U.S. Treasury, according to a nonpartisan watchdog report.
The Government Accountability Office (GAO) report, released Sept. 29, finds that HHS is breaking ACA regulations that require a portion of funds collected under its Transitional Reinsurance Program to go to the Treasury. Instead, the agency has redirected $5 billion so far to pay health insurers that enroll high-cost patients under the program.
“In light of the foregoing analysis, we conclude that HHS lacks authority to ignore the statute’s directive to deposit amounts from collections under the Transitional Reinsurance Program to the Treasury and instead make deposits in the Treasury only if its collections reach the amounts for reinsurance payments specified in section 1341,” Susan A. Poling, GAO general counsel, wrote in a legal opinion. “The agency is not authorized to prioritize collections in this manner.”
Republican lawmakers, including Sen. John Barrasso III (Wyo.), chair of the Senate Republican Policy Committee, praised the legal opinion, saying it shows the Obama administration is bending the rules when it comes to rolling out the ACA.
“This is a major victory for the American people who are suffering with higher premiums and fewer choices because of this failed law,” said Sen. Barrasso, who with several fellow Republican legislators requested the GAO investigation. “The administration should end this illegal scheme immediately, and focus on providing relief from the burdens of this law.”
At press time, neither the White House nor HHS had responded to request for comment on the report.
The Transitional Reinsurance Program is a 3-year initiative under the ACA that collects fees from employers and other private health insurance plans and directs the funds to health plans that face large claims for patients with high-cost medical conditions. The ACA specifies that between 2014 and 2016, HHS would collect $25 billion in fees, of which $5 billion would go into the Treasury.
But when HHS was unable to collect the full amount over the 3 years, it did not distribute funds into the Treasury, but instead used it pay the health plans. To justify that decision, HHS officials announced that the department would allocate all collections first for reinsurance payments until collections totaled the target amount set forth for reinsurance payments under the law, and that any remaining collections would go toward to administrative expenses and the Treasury, according to the GAO report.
But the GAO argues that HHS falling short of the projected collections does not alter the meaning of the statute.
“Specifically, where actual funding has fallen short of an agency’s original expectations, courts have directed the agency to distribute available funds to approximate the allocation plan Congress designed in anticipation of full funding,” Ms. Poling wrote. The HHS “assertion that the statute is silent with respect to allocation of collections overlooks the fact that section 1341 expressly directs HHS to collect amounts for the Treasury and prohibits the use of these amounts for any purpose other than deposit in the Treasury. HHS’s analysis focuses on words and phrases in the statute in isolation rather than in their appropriate context.”
While the GAO cannot force HHS to act with the opinion, lawmakers could use the report to craft legislation forcing HHS to repay the Treasury.
“This issue has been brewing for a while, and is another example of a series of attempts to challenge or limit payments being made to carriers under the ACA,” Katherine Hempstead, senior adviser to vice president at the Robert Wood Johnson Foundation, said in an interview. “It’s no mystery why making these payments is a pretty high priority for the [Centers for Medicare & Medicaid Services] right now, given the losses sustained by many market participants. My guess is that the can will probably be kicked down the road on this and a number of other issues for the next administration and Congress to resolve.”
The reinsurance program was one of three programs intended to protect against the negative impacts of adverse selection and risk selection, while working to stabilize premiums during the initial years of the health law’s implementation. The program aims to protect against premium increases in the individual market by offsetting expenses of high-cost patients.
On Twitter @legal_med
The Health and Human Services department is failing to properly administer a reinsurance program under the Affordable Care Act by unlawfully diverting funds intended for the U.S. Treasury, according to a nonpartisan watchdog report.
The Government Accountability Office (GAO) report, released Sept. 29, finds that HHS is breaking ACA regulations that require a portion of funds collected under its Transitional Reinsurance Program to go to the Treasury. Instead, the agency has redirected $5 billion so far to pay health insurers that enroll high-cost patients under the program.
“In light of the foregoing analysis, we conclude that HHS lacks authority to ignore the statute’s directive to deposit amounts from collections under the Transitional Reinsurance Program to the Treasury and instead make deposits in the Treasury only if its collections reach the amounts for reinsurance payments specified in section 1341,” Susan A. Poling, GAO general counsel, wrote in a legal opinion. “The agency is not authorized to prioritize collections in this manner.”
Republican lawmakers, including Sen. John Barrasso III (Wyo.), chair of the Senate Republican Policy Committee, praised the legal opinion, saying it shows the Obama administration is bending the rules when it comes to rolling out the ACA.
“This is a major victory for the American people who are suffering with higher premiums and fewer choices because of this failed law,” said Sen. Barrasso, who with several fellow Republican legislators requested the GAO investigation. “The administration should end this illegal scheme immediately, and focus on providing relief from the burdens of this law.”
At press time, neither the White House nor HHS had responded to request for comment on the report.
The Transitional Reinsurance Program is a 3-year initiative under the ACA that collects fees from employers and other private health insurance plans and directs the funds to health plans that face large claims for patients with high-cost medical conditions. The ACA specifies that between 2014 and 2016, HHS would collect $25 billion in fees, of which $5 billion would go into the Treasury.
But when HHS was unable to collect the full amount over the 3 years, it did not distribute funds into the Treasury, but instead used it pay the health plans. To justify that decision, HHS officials announced that the department would allocate all collections first for reinsurance payments until collections totaled the target amount set forth for reinsurance payments under the law, and that any remaining collections would go toward to administrative expenses and the Treasury, according to the GAO report.
But the GAO argues that HHS falling short of the projected collections does not alter the meaning of the statute.
“Specifically, where actual funding has fallen short of an agency’s original expectations, courts have directed the agency to distribute available funds to approximate the allocation plan Congress designed in anticipation of full funding,” Ms. Poling wrote. The HHS “assertion that the statute is silent with respect to allocation of collections overlooks the fact that section 1341 expressly directs HHS to collect amounts for the Treasury and prohibits the use of these amounts for any purpose other than deposit in the Treasury. HHS’s analysis focuses on words and phrases in the statute in isolation rather than in their appropriate context.”
While the GAO cannot force HHS to act with the opinion, lawmakers could use the report to craft legislation forcing HHS to repay the Treasury.
“This issue has been brewing for a while, and is another example of a series of attempts to challenge or limit payments being made to carriers under the ACA,” Katherine Hempstead, senior adviser to vice president at the Robert Wood Johnson Foundation, said in an interview. “It’s no mystery why making these payments is a pretty high priority for the [Centers for Medicare & Medicaid Services] right now, given the losses sustained by many market participants. My guess is that the can will probably be kicked down the road on this and a number of other issues for the next administration and Congress to resolve.”
The reinsurance program was one of three programs intended to protect against the negative impacts of adverse selection and risk selection, while working to stabilize premiums during the initial years of the health law’s implementation. The program aims to protect against premium increases in the individual market by offsetting expenses of high-cost patients.
On Twitter @legal_med
New chikungunya diagnostic assay proves quick, effective
A reverse transcription recombinase polymerase amplification (RT-RPA) assay was able to quickly and effectively identify chikungunya virus (CHIKV), according to a study published in PLOS Neglected Tropical Diseases.
Using chikungunya virus RNA samples, the RT-RPA assay detected down to 80 genome copies per reaction within 15 minutes, a time period four to six times faster than other molecular diagnostic techniques, such as reverse transcription-polymerase chain reaction (RT-PCR). In a sensitivity test involving all chikungunya serotypes and various alphaviruses, flaviviruses, and one phlebovirus, the RT-RPA assay identified all virus genotypes, with the only cross-reaction occurring with O’nyong’nyong virus.
In a test involving 58 plasma samples of suspected chikungunya fever from a trial in Thailand, two real-time RT-PCR tests identified 36 out of 58 samples (62%) as positive for chikungunya. The RT-RPA test successfully detected the virus in all 36 positive samples and did not detect the virus in any of the negative samples, giving a sensitivity and specificity of 100%.
“The CHIKV RPA assay presented here is a promising tool for CHIKV diagnostics at the point of need,” the investigators wrote. “Integration into a multimer or multiplex assay for simultaneous and differential detection of CHIKV, Dengue virus, and Zika virus, as well as an internal positive control would improve outbreak investigations, since the three viruses induce the same clinical picture upon infection and increasingly cocirculate in many parts of the world.”
Find the full study in PLOS Neglected Tropical Diseases (doi: 10.1371/journal.pntd.0004953).
A reverse transcription recombinase polymerase amplification (RT-RPA) assay was able to quickly and effectively identify chikungunya virus (CHIKV), according to a study published in PLOS Neglected Tropical Diseases.
Using chikungunya virus RNA samples, the RT-RPA assay detected down to 80 genome copies per reaction within 15 minutes, a time period four to six times faster than other molecular diagnostic techniques, such as reverse transcription-polymerase chain reaction (RT-PCR). In a sensitivity test involving all chikungunya serotypes and various alphaviruses, flaviviruses, and one phlebovirus, the RT-RPA assay identified all virus genotypes, with the only cross-reaction occurring with O’nyong’nyong virus.
In a test involving 58 plasma samples of suspected chikungunya fever from a trial in Thailand, two real-time RT-PCR tests identified 36 out of 58 samples (62%) as positive for chikungunya. The RT-RPA test successfully detected the virus in all 36 positive samples and did not detect the virus in any of the negative samples, giving a sensitivity and specificity of 100%.
“The CHIKV RPA assay presented here is a promising tool for CHIKV diagnostics at the point of need,” the investigators wrote. “Integration into a multimer or multiplex assay for simultaneous and differential detection of CHIKV, Dengue virus, and Zika virus, as well as an internal positive control would improve outbreak investigations, since the three viruses induce the same clinical picture upon infection and increasingly cocirculate in many parts of the world.”
Find the full study in PLOS Neglected Tropical Diseases (doi: 10.1371/journal.pntd.0004953).
A reverse transcription recombinase polymerase amplification (RT-RPA) assay was able to quickly and effectively identify chikungunya virus (CHIKV), according to a study published in PLOS Neglected Tropical Diseases.
Using chikungunya virus RNA samples, the RT-RPA assay detected down to 80 genome copies per reaction within 15 minutes, a time period four to six times faster than other molecular diagnostic techniques, such as reverse transcription-polymerase chain reaction (RT-PCR). In a sensitivity test involving all chikungunya serotypes and various alphaviruses, flaviviruses, and one phlebovirus, the RT-RPA assay identified all virus genotypes, with the only cross-reaction occurring with O’nyong’nyong virus.
In a test involving 58 plasma samples of suspected chikungunya fever from a trial in Thailand, two real-time RT-PCR tests identified 36 out of 58 samples (62%) as positive for chikungunya. The RT-RPA test successfully detected the virus in all 36 positive samples and did not detect the virus in any of the negative samples, giving a sensitivity and specificity of 100%.
“The CHIKV RPA assay presented here is a promising tool for CHIKV diagnostics at the point of need,” the investigators wrote. “Integration into a multimer or multiplex assay for simultaneous and differential detection of CHIKV, Dengue virus, and Zika virus, as well as an internal positive control would improve outbreak investigations, since the three viruses induce the same clinical picture upon infection and increasingly cocirculate in many parts of the world.”
Find the full study in PLOS Neglected Tropical Diseases (doi: 10.1371/journal.pntd.0004953).
FROM PLOS NEGLECTED TROPICAL DISEASES
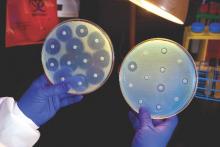
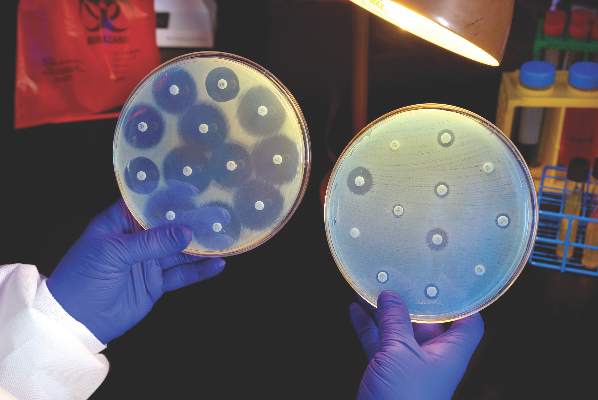
Ceftazidime-avibactam stands up to CRE, but resistance a problem
Intravenous ceftazidime-avibactam successfully treated 59% of carbapenem-resistant Enterobacteriaceae (CRE) infections, and 76% of patients remained alive at 30 days, according to a retrospective cohort study published in Clinical Infectious Diseases.
Those rates resemble previous reports of treatment with in vitro active agents, while the rate of acute kidney injury was about a third lower, said Ryan K. Shields, PharmD, of the University of Pittsburgh, and his associates. But 8% of CRE infections developed ceftazidime-avibactam resistance, which accounted for about a third of microbiological failures, the researchers said. “It is incumbent upon health care providers to share their clinical experiences with ceftazidime-avibactam and other new beta-lactamase inhibitors, so these agents can be used most effectively for the longest period of time,” they added.
Ceftazidime-avibactam (Avycaz) is a novel beta-lactam/beta-lactamase inhibitor combination approved by the Food and Drug Administration in 2015 for complicated intra-abdominal and urinary tract infections. It was hoped that the newly approved combination would prove safer and more effective than previously developed agents that showed in vitro activity against CRE, such as colistin, gentamicin, and tigecycline, the researchers noted.
They described CRE-infected patients treated with ceftazidime-avibactam (median, 14 days; range, 4-71 days) between April 2015 and February 2016. The average age of the patients was 64 years (range 26-78 years), and 57% were men. Infections included ventilator or health care–associated pneumonia, primary bacteremia, intra-abdominal infection, skin and soft tissue infections, pyelonephritis, mediastinitis, subdural empyema/ventriculitis, and purulent tracheobronchitis. All the CRE isolates were susceptible to ceftazidime-avibactam at baseline. In all, 70% of patients received ceftazidime-avibactam as monotherapy, while 30% received it in combination with intravenous or inhaled gentamicin, intravenous or intrathecal colistin, or tigecycline (Clin Infect Dis. 2016 Sep 13. doi: 10.1093/cid/ciw636).
A total of 28 (76%) patients were alive at 30 days and 62% were alive at 90 days, the investigators said. They calculated a 59% rate of clinical success, defined as absence of recurrence within 30 days of onset, resolution of signs and symptoms, and sterilization of site-specific cultures within 7 days of treatment. Combination therapy did not improve the chances of clinical success, they noted. Among the 15 clinical failures, 9 patients died, 4 developed recurrent CRE infections, and 2 did not clinically improve. Clinical success was less likely when patients needed continuous renal replacement therapy (17% vs. 68% for other patients; P = .03) or had higher Sequential Organ Failure Assessment (SOFA) scores (average, 5.2 in clinical successes vs. 8.8 in clinical failures; P = .047). In addition, 10% of patients developed acute kidney injury within 7 days of starting treatment, which was “considerably lower than the approximately 30% rate we previously reported with carbapenem-colistin or aminoglycoside-based combinations,” the investigators said.
The sample size was too small to definitively answer questions about whether combination regimens can overcome resistance, improve outcomes, or effectively treat specific types of CRE infection, the researchers noted. “Nevertheless, we can conclude that ceftazidime-avibactam offers an important advance in the treatment of CRE infections,” they wrote. “The development of resistance after as few as 10 days of therapy is troubling, and treatment failures and deaths in a significant minority of patients highlight the need for more agents with activity against CRE.”
The University of Pittsburgh Medical Center and the National Institutes of Health provided funding. One coauthor disclosed ties to Meiji, Shionogi, Tetraphase, Achaogen, Merck, and The Medicines Company. The other authors had no disclosures.
In the movie “Jaws,” after confidently setting out with an experienced shark hunter, upon catching his first glimpse of the predator, Chief Brody famously uttered, “We’re gonna need a bigger boat.” Similarly, we rejoiced at our triumph when ceftazidime-avibactam became available to treat our patients infected with [Klebsiella pneumoniae carbapenemase]-producing bacteria, and confidently set out to combat this killer. But like Chief Brody, we appear to have underestimated our foe; we too need a “bigger boat.”
We must not let the past repeat itself; hubris about the sudden availability of effective antibiotics has led to overconfidence and complacency among the medical and microbiological communities on several prior occasions in the last 80 years, with serious societal consequences. Shields and colleagues have provided us with a sobering reminder that there is no endpoint in our struggle against microbes. They will never stop adapting to what we conceive of to combat them, and in turn we must never stop conceiving of new ways to stay one step ahead.
Brad Spellberg, MD, is at Los Angeles County–USC Medical Center in Los Angeles. He disclosed ties to Cempra, The Medicines Company, MedImmune/AstraZeneca, PTC Therapeutics, Entasis, Tetraphase, Merck, Genentech, Dipexium, Motif, BioAIM, and Synthetic Biologics. He has received grants from AstraZeneca, Merck, Melinta, Steris, NIH, and Veterans Affairs Merit Review. These comments are from an editorial (Clin Infect Dis. 2016 Sept 13. doi: 10.1093/cid/ciw639).
In the movie “Jaws,” after confidently setting out with an experienced shark hunter, upon catching his first glimpse of the predator, Chief Brody famously uttered, “We’re gonna need a bigger boat.” Similarly, we rejoiced at our triumph when ceftazidime-avibactam became available to treat our patients infected with [Klebsiella pneumoniae carbapenemase]-producing bacteria, and confidently set out to combat this killer. But like Chief Brody, we appear to have underestimated our foe; we too need a “bigger boat.”
We must not let the past repeat itself; hubris about the sudden availability of effective antibiotics has led to overconfidence and complacency among the medical and microbiological communities on several prior occasions in the last 80 years, with serious societal consequences. Shields and colleagues have provided us with a sobering reminder that there is no endpoint in our struggle against microbes. They will never stop adapting to what we conceive of to combat them, and in turn we must never stop conceiving of new ways to stay one step ahead.
Brad Spellberg, MD, is at Los Angeles County–USC Medical Center in Los Angeles. He disclosed ties to Cempra, The Medicines Company, MedImmune/AstraZeneca, PTC Therapeutics, Entasis, Tetraphase, Merck, Genentech, Dipexium, Motif, BioAIM, and Synthetic Biologics. He has received grants from AstraZeneca, Merck, Melinta, Steris, NIH, and Veterans Affairs Merit Review. These comments are from an editorial (Clin Infect Dis. 2016 Sept 13. doi: 10.1093/cid/ciw639).
In the movie “Jaws,” after confidently setting out with an experienced shark hunter, upon catching his first glimpse of the predator, Chief Brody famously uttered, “We’re gonna need a bigger boat.” Similarly, we rejoiced at our triumph when ceftazidime-avibactam became available to treat our patients infected with [Klebsiella pneumoniae carbapenemase]-producing bacteria, and confidently set out to combat this killer. But like Chief Brody, we appear to have underestimated our foe; we too need a “bigger boat.”
We must not let the past repeat itself; hubris about the sudden availability of effective antibiotics has led to overconfidence and complacency among the medical and microbiological communities on several prior occasions in the last 80 years, with serious societal consequences. Shields and colleagues have provided us with a sobering reminder that there is no endpoint in our struggle against microbes. They will never stop adapting to what we conceive of to combat them, and in turn we must never stop conceiving of new ways to stay one step ahead.
Brad Spellberg, MD, is at Los Angeles County–USC Medical Center in Los Angeles. He disclosed ties to Cempra, The Medicines Company, MedImmune/AstraZeneca, PTC Therapeutics, Entasis, Tetraphase, Merck, Genentech, Dipexium, Motif, BioAIM, and Synthetic Biologics. He has received grants from AstraZeneca, Merck, Melinta, Steris, NIH, and Veterans Affairs Merit Review. These comments are from an editorial (Clin Infect Dis. 2016 Sept 13. doi: 10.1093/cid/ciw639).
Intravenous ceftazidime-avibactam successfully treated 59% of carbapenem-resistant Enterobacteriaceae (CRE) infections, and 76% of patients remained alive at 30 days, according to a retrospective cohort study published in Clinical Infectious Diseases.
Those rates resemble previous reports of treatment with in vitro active agents, while the rate of acute kidney injury was about a third lower, said Ryan K. Shields, PharmD, of the University of Pittsburgh, and his associates. But 8% of CRE infections developed ceftazidime-avibactam resistance, which accounted for about a third of microbiological failures, the researchers said. “It is incumbent upon health care providers to share their clinical experiences with ceftazidime-avibactam and other new beta-lactamase inhibitors, so these agents can be used most effectively for the longest period of time,” they added.
Ceftazidime-avibactam (Avycaz) is a novel beta-lactam/beta-lactamase inhibitor combination approved by the Food and Drug Administration in 2015 for complicated intra-abdominal and urinary tract infections. It was hoped that the newly approved combination would prove safer and more effective than previously developed agents that showed in vitro activity against CRE, such as colistin, gentamicin, and tigecycline, the researchers noted.
They described CRE-infected patients treated with ceftazidime-avibactam (median, 14 days; range, 4-71 days) between April 2015 and February 2016. The average age of the patients was 64 years (range 26-78 years), and 57% were men. Infections included ventilator or health care–associated pneumonia, primary bacteremia, intra-abdominal infection, skin and soft tissue infections, pyelonephritis, mediastinitis, subdural empyema/ventriculitis, and purulent tracheobronchitis. All the CRE isolates were susceptible to ceftazidime-avibactam at baseline. In all, 70% of patients received ceftazidime-avibactam as monotherapy, while 30% received it in combination with intravenous or inhaled gentamicin, intravenous or intrathecal colistin, or tigecycline (Clin Infect Dis. 2016 Sep 13. doi: 10.1093/cid/ciw636).
A total of 28 (76%) patients were alive at 30 days and 62% were alive at 90 days, the investigators said. They calculated a 59% rate of clinical success, defined as absence of recurrence within 30 days of onset, resolution of signs and symptoms, and sterilization of site-specific cultures within 7 days of treatment. Combination therapy did not improve the chances of clinical success, they noted. Among the 15 clinical failures, 9 patients died, 4 developed recurrent CRE infections, and 2 did not clinically improve. Clinical success was less likely when patients needed continuous renal replacement therapy (17% vs. 68% for other patients; P = .03) or had higher Sequential Organ Failure Assessment (SOFA) scores (average, 5.2 in clinical successes vs. 8.8 in clinical failures; P = .047). In addition, 10% of patients developed acute kidney injury within 7 days of starting treatment, which was “considerably lower than the approximately 30% rate we previously reported with carbapenem-colistin or aminoglycoside-based combinations,” the investigators said.
The sample size was too small to definitively answer questions about whether combination regimens can overcome resistance, improve outcomes, or effectively treat specific types of CRE infection, the researchers noted. “Nevertheless, we can conclude that ceftazidime-avibactam offers an important advance in the treatment of CRE infections,” they wrote. “The development of resistance after as few as 10 days of therapy is troubling, and treatment failures and deaths in a significant minority of patients highlight the need for more agents with activity against CRE.”
The University of Pittsburgh Medical Center and the National Institutes of Health provided funding. One coauthor disclosed ties to Meiji, Shionogi, Tetraphase, Achaogen, Merck, and The Medicines Company. The other authors had no disclosures.
Intravenous ceftazidime-avibactam successfully treated 59% of carbapenem-resistant Enterobacteriaceae (CRE) infections, and 76% of patients remained alive at 30 days, according to a retrospective cohort study published in Clinical Infectious Diseases.
Those rates resemble previous reports of treatment with in vitro active agents, while the rate of acute kidney injury was about a third lower, said Ryan K. Shields, PharmD, of the University of Pittsburgh, and his associates. But 8% of CRE infections developed ceftazidime-avibactam resistance, which accounted for about a third of microbiological failures, the researchers said. “It is incumbent upon health care providers to share their clinical experiences with ceftazidime-avibactam and other new beta-lactamase inhibitors, so these agents can be used most effectively for the longest period of time,” they added.
Ceftazidime-avibactam (Avycaz) is a novel beta-lactam/beta-lactamase inhibitor combination approved by the Food and Drug Administration in 2015 for complicated intra-abdominal and urinary tract infections. It was hoped that the newly approved combination would prove safer and more effective than previously developed agents that showed in vitro activity against CRE, such as colistin, gentamicin, and tigecycline, the researchers noted.
They described CRE-infected patients treated with ceftazidime-avibactam (median, 14 days; range, 4-71 days) between April 2015 and February 2016. The average age of the patients was 64 years (range 26-78 years), and 57% were men. Infections included ventilator or health care–associated pneumonia, primary bacteremia, intra-abdominal infection, skin and soft tissue infections, pyelonephritis, mediastinitis, subdural empyema/ventriculitis, and purulent tracheobronchitis. All the CRE isolates were susceptible to ceftazidime-avibactam at baseline. In all, 70% of patients received ceftazidime-avibactam as monotherapy, while 30% received it in combination with intravenous or inhaled gentamicin, intravenous or intrathecal colistin, or tigecycline (Clin Infect Dis. 2016 Sep 13. doi: 10.1093/cid/ciw636).
A total of 28 (76%) patients were alive at 30 days and 62% were alive at 90 days, the investigators said. They calculated a 59% rate of clinical success, defined as absence of recurrence within 30 days of onset, resolution of signs and symptoms, and sterilization of site-specific cultures within 7 days of treatment. Combination therapy did not improve the chances of clinical success, they noted. Among the 15 clinical failures, 9 patients died, 4 developed recurrent CRE infections, and 2 did not clinically improve. Clinical success was less likely when patients needed continuous renal replacement therapy (17% vs. 68% for other patients; P = .03) or had higher Sequential Organ Failure Assessment (SOFA) scores (average, 5.2 in clinical successes vs. 8.8 in clinical failures; P = .047). In addition, 10% of patients developed acute kidney injury within 7 days of starting treatment, which was “considerably lower than the approximately 30% rate we previously reported with carbapenem-colistin or aminoglycoside-based combinations,” the investigators said.
The sample size was too small to definitively answer questions about whether combination regimens can overcome resistance, improve outcomes, or effectively treat specific types of CRE infection, the researchers noted. “Nevertheless, we can conclude that ceftazidime-avibactam offers an important advance in the treatment of CRE infections,” they wrote. “The development of resistance after as few as 10 days of therapy is troubling, and treatment failures and deaths in a significant minority of patients highlight the need for more agents with activity against CRE.”
The University of Pittsburgh Medical Center and the National Institutes of Health provided funding. One coauthor disclosed ties to Meiji, Shionogi, Tetraphase, Achaogen, Merck, and The Medicines Company. The other authors had no disclosures.
FROM CLINICAL INFECTIOUS DISEASES
Key clinical point: Ceftazidime-avibactam effectively treated carbapenem-resistant Enterobacteriaceae (CRE) infections, but resistance emerged rapidly and in some cases led to microbiological failure.
Major finding: The rate of clinical success was 59%; 10% of patients developed acute kidney injury within 7 days of starting treatment; 8% developed resistance.
Data source: A single-center retrospective study of 37 patients with carbapenem-resistant Enterobacteriaceae infections treated with ceftazidime-avibactam.
Disclosures: The University of Pittsburgh Medical Center and the National Institutes of Health provided funding. A coauthor disclosed ties to Meiji, Shionogi, Tetraphase, Achaogen, Merck, and The Medicines Company. The other authors had no disclosures.
The choice in November could not be more important
Editor’s note: For the last five presidential elections, this news organization has offered the Republican and Democrat presidential candidate the opportunity to present their ideas directly to U.S. physicians via side-by-side Guest Editorials. The candidates – or their proxies – have used these pages to reach out to you, our readers, with their views on medicine, health care, and other issues. We have taken pride in the ability to offer you a balanced view in the weeks leading up to the general election. This year, we cannot provide you with that balanced view. Despite repeated efforts via every medium at our disposal – telephone calls, emails, Twitter, LinkedIn, and more – the Donald J. Trump for President organization has not responded to our request for a contribution. Here we present the contribution from Secretary Hillary Clinton’s proxy.
Guest Editorial
As physicians, we have the unique privilege of serving patients, often at their most vulnerable moments. We also bear witness to how our health care system works – and too often, where it falls short – through our patients’ eyes.
That view could change dramatically depending on the outcome of this year’s presidential election. Hillary Clinton has a long track record of expanding affordable health care, and wants to accelerate the march toward universal access to high-quality care. Her opponent, Donald Trump, wants to roll back the progress we’ve made, with a plan that takes health insurance away from more than 20 million Americans.
Secretary Clinton’s career demonstrates her commitment to the ideal of health care as a human right. For example, she was instrumental in the bipartisan effort to pass the Children’s Health Insurance Program. Despite recent gains in health coverage, too many Americans still struggle to access the care they need – where and when they need it. Secretary Clinton’s plan for health care would expand access to care by building on the Affordable Care Act – with more relief for high premiums and out-of-pocket costs, particularly for prescription drugs; by working with states to expand Medicaid and give people the choice of a “public option” health plan. She also worked with Sen. Bernie Sanders (I-Vt.) on a plan to double funding for community health centers and substantially expand our commitment to the National Health Service Corps – so that millions more Americans have access to primary care, especially in rural and medically underserved urban areas, and so that we can offer more loan repayment and scholarships to early-career physicians.
Anyone taking care of patients today knows that improving access to care is only the first step. We must improve the way we deliver care, refocusing around the patient-doctor relationship. Too often there are barriers – regulations, paperwork, or insurance restrictions – to taking care of patients in the way that they deserve. Secretary Clinton wants to ensure an advanced and coordinated health care system that supports patient-doctor relationships instead of getting in the way. She wants to spur delivery system reform to reward value and quality. She was one of the first elected officials to call for modernizing health information technology, reaching across the aisle to work with physician and then-Sen. Bill Frist (R-Tenn.). And she’s offered plans to address major contemporary challenges, such as preventing and better treating Alzheimer’s disease, destigmatizing mental illness, and improving care for substance use disorders.
In addition to improving our health care system, Secretary Clinton believes we must take a number of proactive steps so that all Americans – regardless of location, income, or history – have the opportunity to live full, healthy lives. She believes we must invest in our public health infrastructure to ensure preparedness for emerging threats like Zika at home and abroad; to prevent illness and injury in communities; and to promote health equity. Of course, some of the most important determinants of well-being lie outside the walls of our clinics and hospitals. Secretary Clinton also will move us forward on these fundamental issues, such as women’s rights, criminal justice reform, and climate change.
Meanwhile, Donald Trump offers a very different vision for health care in the United States. His proposals would repeal the Affordable Care Act, instantly stripping millions of people of lifesaving health insurance. He would cut Medicaid through block grants, leaving millions of the poorest Americans without a safety net. And he would once again allow insurers to discriminate, based on preexisting conditions.
The choice in November could not be more important, for our patients and for the practice of medicine. Secretary Clinton’s long track record of fighting for universal, high-quality, affordable health care speaks for itself. With so much more left to do to improve health in our country, she brings the thoughtful leadership and steely determination needed to get the job done.
Dr. Chokshi practices internal medicine at Bellevue Hospital in New York and is a health policy adviser to Hillary for America.
Editor’s note: For the last five presidential elections, this news organization has offered the Republican and Democrat presidential candidate the opportunity to present their ideas directly to U.S. physicians via side-by-side Guest Editorials. The candidates – or their proxies – have used these pages to reach out to you, our readers, with their views on medicine, health care, and other issues. We have taken pride in the ability to offer you a balanced view in the weeks leading up to the general election. This year, we cannot provide you with that balanced view. Despite repeated efforts via every medium at our disposal – telephone calls, emails, Twitter, LinkedIn, and more – the Donald J. Trump for President organization has not responded to our request for a contribution. Here we present the contribution from Secretary Hillary Clinton’s proxy.
Guest Editorial
As physicians, we have the unique privilege of serving patients, often at their most vulnerable moments. We also bear witness to how our health care system works – and too often, where it falls short – through our patients’ eyes.
That view could change dramatically depending on the outcome of this year’s presidential election. Hillary Clinton has a long track record of expanding affordable health care, and wants to accelerate the march toward universal access to high-quality care. Her opponent, Donald Trump, wants to roll back the progress we’ve made, with a plan that takes health insurance away from more than 20 million Americans.
Secretary Clinton’s career demonstrates her commitment to the ideal of health care as a human right. For example, she was instrumental in the bipartisan effort to pass the Children’s Health Insurance Program. Despite recent gains in health coverage, too many Americans still struggle to access the care they need – where and when they need it. Secretary Clinton’s plan for health care would expand access to care by building on the Affordable Care Act – with more relief for high premiums and out-of-pocket costs, particularly for prescription drugs; by working with states to expand Medicaid and give people the choice of a “public option” health plan. She also worked with Sen. Bernie Sanders (I-Vt.) on a plan to double funding for community health centers and substantially expand our commitment to the National Health Service Corps – so that millions more Americans have access to primary care, especially in rural and medically underserved urban areas, and so that we can offer more loan repayment and scholarships to early-career physicians.
Anyone taking care of patients today knows that improving access to care is only the first step. We must improve the way we deliver care, refocusing around the patient-doctor relationship. Too often there are barriers – regulations, paperwork, or insurance restrictions – to taking care of patients in the way that they deserve. Secretary Clinton wants to ensure an advanced and coordinated health care system that supports patient-doctor relationships instead of getting in the way. She wants to spur delivery system reform to reward value and quality. She was one of the first elected officials to call for modernizing health information technology, reaching across the aisle to work with physician and then-Sen. Bill Frist (R-Tenn.). And she’s offered plans to address major contemporary challenges, such as preventing and better treating Alzheimer’s disease, destigmatizing mental illness, and improving care for substance use disorders.
In addition to improving our health care system, Secretary Clinton believes we must take a number of proactive steps so that all Americans – regardless of location, income, or history – have the opportunity to live full, healthy lives. She believes we must invest in our public health infrastructure to ensure preparedness for emerging threats like Zika at home and abroad; to prevent illness and injury in communities; and to promote health equity. Of course, some of the most important determinants of well-being lie outside the walls of our clinics and hospitals. Secretary Clinton also will move us forward on these fundamental issues, such as women’s rights, criminal justice reform, and climate change.
Meanwhile, Donald Trump offers a very different vision for health care in the United States. His proposals would repeal the Affordable Care Act, instantly stripping millions of people of lifesaving health insurance. He would cut Medicaid through block grants, leaving millions of the poorest Americans without a safety net. And he would once again allow insurers to discriminate, based on preexisting conditions.
The choice in November could not be more important, for our patients and for the practice of medicine. Secretary Clinton’s long track record of fighting for universal, high-quality, affordable health care speaks for itself. With so much more left to do to improve health in our country, she brings the thoughtful leadership and steely determination needed to get the job done.
Dr. Chokshi practices internal medicine at Bellevue Hospital in New York and is a health policy adviser to Hillary for America.
Editor’s note: For the last five presidential elections, this news organization has offered the Republican and Democrat presidential candidate the opportunity to present their ideas directly to U.S. physicians via side-by-side Guest Editorials. The candidates – or their proxies – have used these pages to reach out to you, our readers, with their views on medicine, health care, and other issues. We have taken pride in the ability to offer you a balanced view in the weeks leading up to the general election. This year, we cannot provide you with that balanced view. Despite repeated efforts via every medium at our disposal – telephone calls, emails, Twitter, LinkedIn, and more – the Donald J. Trump for President organization has not responded to our request for a contribution. Here we present the contribution from Secretary Hillary Clinton’s proxy.
Guest Editorial
As physicians, we have the unique privilege of serving patients, often at their most vulnerable moments. We also bear witness to how our health care system works – and too often, where it falls short – through our patients’ eyes.
That view could change dramatically depending on the outcome of this year’s presidential election. Hillary Clinton has a long track record of expanding affordable health care, and wants to accelerate the march toward universal access to high-quality care. Her opponent, Donald Trump, wants to roll back the progress we’ve made, with a plan that takes health insurance away from more than 20 million Americans.
Secretary Clinton’s career demonstrates her commitment to the ideal of health care as a human right. For example, she was instrumental in the bipartisan effort to pass the Children’s Health Insurance Program. Despite recent gains in health coverage, too many Americans still struggle to access the care they need – where and when they need it. Secretary Clinton’s plan for health care would expand access to care by building on the Affordable Care Act – with more relief for high premiums and out-of-pocket costs, particularly for prescription drugs; by working with states to expand Medicaid and give people the choice of a “public option” health plan. She also worked with Sen. Bernie Sanders (I-Vt.) on a plan to double funding for community health centers and substantially expand our commitment to the National Health Service Corps – so that millions more Americans have access to primary care, especially in rural and medically underserved urban areas, and so that we can offer more loan repayment and scholarships to early-career physicians.
Anyone taking care of patients today knows that improving access to care is only the first step. We must improve the way we deliver care, refocusing around the patient-doctor relationship. Too often there are barriers – regulations, paperwork, or insurance restrictions – to taking care of patients in the way that they deserve. Secretary Clinton wants to ensure an advanced and coordinated health care system that supports patient-doctor relationships instead of getting in the way. She wants to spur delivery system reform to reward value and quality. She was one of the first elected officials to call for modernizing health information technology, reaching across the aisle to work with physician and then-Sen. Bill Frist (R-Tenn.). And she’s offered plans to address major contemporary challenges, such as preventing and better treating Alzheimer’s disease, destigmatizing mental illness, and improving care for substance use disorders.
In addition to improving our health care system, Secretary Clinton believes we must take a number of proactive steps so that all Americans – regardless of location, income, or history – have the opportunity to live full, healthy lives. She believes we must invest in our public health infrastructure to ensure preparedness for emerging threats like Zika at home and abroad; to prevent illness and injury in communities; and to promote health equity. Of course, some of the most important determinants of well-being lie outside the walls of our clinics and hospitals. Secretary Clinton also will move us forward on these fundamental issues, such as women’s rights, criminal justice reform, and climate change.
Meanwhile, Donald Trump offers a very different vision for health care in the United States. His proposals would repeal the Affordable Care Act, instantly stripping millions of people of lifesaving health insurance. He would cut Medicaid through block grants, leaving millions of the poorest Americans without a safety net. And he would once again allow insurers to discriminate, based on preexisting conditions.
The choice in November could not be more important, for our patients and for the practice of medicine. Secretary Clinton’s long track record of fighting for universal, high-quality, affordable health care speaks for itself. With so much more left to do to improve health in our country, she brings the thoughtful leadership and steely determination needed to get the job done.
Dr. Chokshi practices internal medicine at Bellevue Hospital in New York and is a health policy adviser to Hillary for America.
Nonsexual secondary Zika virus case confirmed in Utah
The first case of nonsexual secondary Zika virus transmission has occurred in the United States, according to a research letter published by the New England Journal of Medicine.
“We report a rapidly progressive, fatal [Zika virus] infection acquired outside the United States and secondary local transmission in the absence of known risk factors,” wrote the authors of the report, led by Sankar Swaminathan, MD, of the University of Utah in Salt Lake City.
The individual infected via secondary transmission, dubbed Patient Two in the report, is suspected to have contracted the disease from Patient One, a 73-year-old man who visited the southwestern coast of Mexico – a known hotbed of Zika virus – for a 3-week trip before returning to the United States. Eight days after returning, Patient One was admitted to a Salt Lake City hospital with symptoms consistent with a flavivirus infection and told doctors that he had been bitten by mosquitoes during his trip (N Engl J Med. 2016 Sep 28. doi: 10.1056/NEJMc1610613).
After undergoing tourniquet and laboratory testing, a diagnosis of dengue shock syndrome was made, but Patient One’s condition continued to deteriorate rapidly. Patient One died 4 days after initial hospitalization; real-time PCR assay confirmed Zika virus infection shortly thereafter.
Patient Two came into contact with Patient One during the latter’s hospitalization, reporting that he “assisted a nurse in repositioning Patient One in bed without using gloves,” according to the report. Patient Two began experiencing conjunctivitis, fever, myalgia, and a maculopapular rash on his face 5 days after Patient One died. The rash resolved itself after 7 days, and while PCR analysis of Patient Two’s serum was negative for Zika, his urinalysis was positive.
Because Patient Two had not traveled to a Zika-endemic area within 9 months of experiencing Zika-like symptoms and had not engaged in sexual intercourse with anyone who traveled to a Zika-endemic area, the authors conclude that he contracted the disease from contact with Patient One. The authors posit that, given the high levels of viremia in Patient One, the Zika virus could have been transmitted to Patient Two via sweat or tears, which Patient Two came into contact with while not wearing gloves. Local transmission via Aedis aegypti mosquito bite was highly unlikely to be the cause of transmission because of the lack of such mosquitoes in the Salt Lake City area.
“These two cases illustrate several important points,” the authors concluded. “The spectrum of those at risk for fulminant [Zika virus] infection may be broader than previously recognized, and those who are not severely immunocompromised or chronically ill may nevertheless be at risk for fatal infection.”
The first case of nonsexual secondary Zika virus transmission has occurred in the United States, according to a research letter published by the New England Journal of Medicine.
“We report a rapidly progressive, fatal [Zika virus] infection acquired outside the United States and secondary local transmission in the absence of known risk factors,” wrote the authors of the report, led by Sankar Swaminathan, MD, of the University of Utah in Salt Lake City.
The individual infected via secondary transmission, dubbed Patient Two in the report, is suspected to have contracted the disease from Patient One, a 73-year-old man who visited the southwestern coast of Mexico – a known hotbed of Zika virus – for a 3-week trip before returning to the United States. Eight days after returning, Patient One was admitted to a Salt Lake City hospital with symptoms consistent with a flavivirus infection and told doctors that he had been bitten by mosquitoes during his trip (N Engl J Med. 2016 Sep 28. doi: 10.1056/NEJMc1610613).
After undergoing tourniquet and laboratory testing, a diagnosis of dengue shock syndrome was made, but Patient One’s condition continued to deteriorate rapidly. Patient One died 4 days after initial hospitalization; real-time PCR assay confirmed Zika virus infection shortly thereafter.
Patient Two came into contact with Patient One during the latter’s hospitalization, reporting that he “assisted a nurse in repositioning Patient One in bed without using gloves,” according to the report. Patient Two began experiencing conjunctivitis, fever, myalgia, and a maculopapular rash on his face 5 days after Patient One died. The rash resolved itself after 7 days, and while PCR analysis of Patient Two’s serum was negative for Zika, his urinalysis was positive.
Because Patient Two had not traveled to a Zika-endemic area within 9 months of experiencing Zika-like symptoms and had not engaged in sexual intercourse with anyone who traveled to a Zika-endemic area, the authors conclude that he contracted the disease from contact with Patient One. The authors posit that, given the high levels of viremia in Patient One, the Zika virus could have been transmitted to Patient Two via sweat or tears, which Patient Two came into contact with while not wearing gloves. Local transmission via Aedis aegypti mosquito bite was highly unlikely to be the cause of transmission because of the lack of such mosquitoes in the Salt Lake City area.
“These two cases illustrate several important points,” the authors concluded. “The spectrum of those at risk for fulminant [Zika virus] infection may be broader than previously recognized, and those who are not severely immunocompromised or chronically ill may nevertheless be at risk for fatal infection.”
The first case of nonsexual secondary Zika virus transmission has occurred in the United States, according to a research letter published by the New England Journal of Medicine.
“We report a rapidly progressive, fatal [Zika virus] infection acquired outside the United States and secondary local transmission in the absence of known risk factors,” wrote the authors of the report, led by Sankar Swaminathan, MD, of the University of Utah in Salt Lake City.
The individual infected via secondary transmission, dubbed Patient Two in the report, is suspected to have contracted the disease from Patient One, a 73-year-old man who visited the southwestern coast of Mexico – a known hotbed of Zika virus – for a 3-week trip before returning to the United States. Eight days after returning, Patient One was admitted to a Salt Lake City hospital with symptoms consistent with a flavivirus infection and told doctors that he had been bitten by mosquitoes during his trip (N Engl J Med. 2016 Sep 28. doi: 10.1056/NEJMc1610613).
After undergoing tourniquet and laboratory testing, a diagnosis of dengue shock syndrome was made, but Patient One’s condition continued to deteriorate rapidly. Patient One died 4 days after initial hospitalization; real-time PCR assay confirmed Zika virus infection shortly thereafter.
Patient Two came into contact with Patient One during the latter’s hospitalization, reporting that he “assisted a nurse in repositioning Patient One in bed without using gloves,” according to the report. Patient Two began experiencing conjunctivitis, fever, myalgia, and a maculopapular rash on his face 5 days after Patient One died. The rash resolved itself after 7 days, and while PCR analysis of Patient Two’s serum was negative for Zika, his urinalysis was positive.
Because Patient Two had not traveled to a Zika-endemic area within 9 months of experiencing Zika-like symptoms and had not engaged in sexual intercourse with anyone who traveled to a Zika-endemic area, the authors conclude that he contracted the disease from contact with Patient One. The authors posit that, given the high levels of viremia in Patient One, the Zika virus could have been transmitted to Patient Two via sweat or tears, which Patient Two came into contact with while not wearing gloves. Local transmission via Aedis aegypti mosquito bite was highly unlikely to be the cause of transmission because of the lack of such mosquitoes in the Salt Lake City area.
“These two cases illustrate several important points,” the authors concluded. “The spectrum of those at risk for fulminant [Zika virus] infection may be broader than previously recognized, and those who are not severely immunocompromised or chronically ill may nevertheless be at risk for fatal infection.”
FROM NEW ENGLAND JOURNAL OF MEDICINE
Heart failure risk with individual NSAIDs examined in study
Nine popular painkillers – including traditional NSAIDs and cyclo-oxygenase-2 (COX-2) inhibitors – are associated with an increased risk of hospitalization for heart failure in adults, based on data from a case-control study of approximately 92,000 hospital admissions. The findings were published online Sept. 28 in BMJ.
Although data from previous large studies suggest that high doses of NSAIDs as well as COX-2 inhibitors increase the risk of hospital admission for heart failure, “there is still limited information on the risk of heart failure associated with the use of individual NSAIDs in clinical practice,” wrote Andrea Arfè of the University of Milano-Bicocca, Milan, and his colleagues (BMJ. 2016 Sep;354:i4857 doi: 10.1136/bmj.i4857).
The researchers reviewed data from five electronic health databases in the Netherlands, Italy, Germany, and the United Kingdom as part of the SOS (Safety of Non-Steroidal Anti-Inflammatory Drugs) project and conducted a nested, case-control study including 92,163 hospital admissions for heart failure and 8,246,403 controls matched for age, sex, and year of study entry. The study included 23 traditional NSAIDs and four selective COX-2 inhibitors.
Overall, individual use of any of nine different NSAIDs within 14 days was associated with a nearly 20% higher likelihood of hospital admission for heart failure, compared with NSAID use more than 183 days in the past (odds ratio, 1.19). For seven traditional NSAIDS (diclofenac, ibuprofen, indomethacin, ketorolac, naproxen, nimesulide, and piroxicam) and the COX-2 inhibitors etoricoxib and rofecoxib, the odds ratios for heart failure with current use ranged from 1.16 for naproxen to 1.83 for ketorolac, compared with past use.
In addition, the odds of hospitalization for heart failure doubled for diclofenac, etoricoxib, indomethacin, piroxicam, and rofecoxib when dosed at two or more daily dose equivalents, the researchers noted. There was no increase in the odds of hospitalization for heart failure with celecoxib when dosed at standard levels, but “indomethacin and etoricoxib seemed to increase the risk of hospital admission for heart failure, even if used at medium doses,” they said. Other lesser-used NSAIDs were associated with an increased risk, but it was not statistically significant.
“The effect of individual NSAIDs could depend on a complex interaction of pharmacological properties, including duration and extent of platelet inhibition, extent of blood pressure increase, and properties possibly unique to the molecule,” the researchers said.
The findings were limited by several factors including misclassification of outcomes and the observational nature of the study, which prevents conclusions about cause and effect, the researchers noted. However, “Because any potential increased risk could have a considerable impact on public health, the risk effect estimates provided by this study may help inform both clinical practice and regulatory activities,” they said.
The study was funded in part by the European Community’s seventh Framework Programme. Mr. Arfè had no financial conflicts to disclose. Several study coauthors disclosed relationships with multiple companies including AstraZeneca, Bayer, Celgene, GlaxoSmithKline, Schwabe, and Novartis.
This report provides additional weight backing the association between increased risk of heart failure with NSAID use and gives better insight on the dose-response relationship between individual NSAIDs and heart failure. However, beyond that, its clinical impact is hurt by the lack of data on the magnitude of excess absolute risk of heart failure with NSAID use, which varies according to baseline cardiovascular risk.
Even though the risk of heart failure associated with NSAID use in the study occurred independent of a history of heart failure, it still is prudent to restrict NSAID use in patients with heart failure because of the high risk noted in this group in other studies.
The widespread use and ease of access to NSAIDs fuels the common misconception that NSAIDs are harmless drugs that are safe for everyone, and this warrants a more restrictive policy by regulatory authorities on the availability of NSAIDs and requirements for health care professionals providing advice on their use.
Gunnar H. Gislason, MD, PhD, is a professor of cardiology at Copenhagen University Hospital Herlev and Gentofte, Denmark, and Christian Torp-Pedersen, MD, is a professor of cardiology at Aalborg (Denmark) University. Dr. Gislason had no disclosures and Dr. Torp-Pedersen advises Bayer on anticoagulation for atrial fibrillation. Their remarks are taken from an editorial accompanying the study by Mr. Arfè and his colleagues (BMJ. 2016 Sep;354:i5163 doi: 10.1136/bmj.i5163).
This report provides additional weight backing the association between increased risk of heart failure with NSAID use and gives better insight on the dose-response relationship between individual NSAIDs and heart failure. However, beyond that, its clinical impact is hurt by the lack of data on the magnitude of excess absolute risk of heart failure with NSAID use, which varies according to baseline cardiovascular risk.
Even though the risk of heart failure associated with NSAID use in the study occurred independent of a history of heart failure, it still is prudent to restrict NSAID use in patients with heart failure because of the high risk noted in this group in other studies.
The widespread use and ease of access to NSAIDs fuels the common misconception that NSAIDs are harmless drugs that are safe for everyone, and this warrants a more restrictive policy by regulatory authorities on the availability of NSAIDs and requirements for health care professionals providing advice on their use.
Gunnar H. Gislason, MD, PhD, is a professor of cardiology at Copenhagen University Hospital Herlev and Gentofte, Denmark, and Christian Torp-Pedersen, MD, is a professor of cardiology at Aalborg (Denmark) University. Dr. Gislason had no disclosures and Dr. Torp-Pedersen advises Bayer on anticoagulation for atrial fibrillation. Their remarks are taken from an editorial accompanying the study by Mr. Arfè and his colleagues (BMJ. 2016 Sep;354:i5163 doi: 10.1136/bmj.i5163).
This report provides additional weight backing the association between increased risk of heart failure with NSAID use and gives better insight on the dose-response relationship between individual NSAIDs and heart failure. However, beyond that, its clinical impact is hurt by the lack of data on the magnitude of excess absolute risk of heart failure with NSAID use, which varies according to baseline cardiovascular risk.
Even though the risk of heart failure associated with NSAID use in the study occurred independent of a history of heart failure, it still is prudent to restrict NSAID use in patients with heart failure because of the high risk noted in this group in other studies.
The widespread use and ease of access to NSAIDs fuels the common misconception that NSAIDs are harmless drugs that are safe for everyone, and this warrants a more restrictive policy by regulatory authorities on the availability of NSAIDs and requirements for health care professionals providing advice on their use.
Gunnar H. Gislason, MD, PhD, is a professor of cardiology at Copenhagen University Hospital Herlev and Gentofte, Denmark, and Christian Torp-Pedersen, MD, is a professor of cardiology at Aalborg (Denmark) University. Dr. Gislason had no disclosures and Dr. Torp-Pedersen advises Bayer on anticoagulation for atrial fibrillation. Their remarks are taken from an editorial accompanying the study by Mr. Arfè and his colleagues (BMJ. 2016 Sep;354:i5163 doi: 10.1136/bmj.i5163).
Nine popular painkillers – including traditional NSAIDs and cyclo-oxygenase-2 (COX-2) inhibitors – are associated with an increased risk of hospitalization for heart failure in adults, based on data from a case-control study of approximately 92,000 hospital admissions. The findings were published online Sept. 28 in BMJ.
Although data from previous large studies suggest that high doses of NSAIDs as well as COX-2 inhibitors increase the risk of hospital admission for heart failure, “there is still limited information on the risk of heart failure associated with the use of individual NSAIDs in clinical practice,” wrote Andrea Arfè of the University of Milano-Bicocca, Milan, and his colleagues (BMJ. 2016 Sep;354:i4857 doi: 10.1136/bmj.i4857).
The researchers reviewed data from five electronic health databases in the Netherlands, Italy, Germany, and the United Kingdom as part of the SOS (Safety of Non-Steroidal Anti-Inflammatory Drugs) project and conducted a nested, case-control study including 92,163 hospital admissions for heart failure and 8,246,403 controls matched for age, sex, and year of study entry. The study included 23 traditional NSAIDs and four selective COX-2 inhibitors.
Overall, individual use of any of nine different NSAIDs within 14 days was associated with a nearly 20% higher likelihood of hospital admission for heart failure, compared with NSAID use more than 183 days in the past (odds ratio, 1.19). For seven traditional NSAIDS (diclofenac, ibuprofen, indomethacin, ketorolac, naproxen, nimesulide, and piroxicam) and the COX-2 inhibitors etoricoxib and rofecoxib, the odds ratios for heart failure with current use ranged from 1.16 for naproxen to 1.83 for ketorolac, compared with past use.
In addition, the odds of hospitalization for heart failure doubled for diclofenac, etoricoxib, indomethacin, piroxicam, and rofecoxib when dosed at two or more daily dose equivalents, the researchers noted. There was no increase in the odds of hospitalization for heart failure with celecoxib when dosed at standard levels, but “indomethacin and etoricoxib seemed to increase the risk of hospital admission for heart failure, even if used at medium doses,” they said. Other lesser-used NSAIDs were associated with an increased risk, but it was not statistically significant.
“The effect of individual NSAIDs could depend on a complex interaction of pharmacological properties, including duration and extent of platelet inhibition, extent of blood pressure increase, and properties possibly unique to the molecule,” the researchers said.
The findings were limited by several factors including misclassification of outcomes and the observational nature of the study, which prevents conclusions about cause and effect, the researchers noted. However, “Because any potential increased risk could have a considerable impact on public health, the risk effect estimates provided by this study may help inform both clinical practice and regulatory activities,” they said.
The study was funded in part by the European Community’s seventh Framework Programme. Mr. Arfè had no financial conflicts to disclose. Several study coauthors disclosed relationships with multiple companies including AstraZeneca, Bayer, Celgene, GlaxoSmithKline, Schwabe, and Novartis.
Nine popular painkillers – including traditional NSAIDs and cyclo-oxygenase-2 (COX-2) inhibitors – are associated with an increased risk of hospitalization for heart failure in adults, based on data from a case-control study of approximately 92,000 hospital admissions. The findings were published online Sept. 28 in BMJ.
Although data from previous large studies suggest that high doses of NSAIDs as well as COX-2 inhibitors increase the risk of hospital admission for heart failure, “there is still limited information on the risk of heart failure associated with the use of individual NSAIDs in clinical practice,” wrote Andrea Arfè of the University of Milano-Bicocca, Milan, and his colleagues (BMJ. 2016 Sep;354:i4857 doi: 10.1136/bmj.i4857).
The researchers reviewed data from five electronic health databases in the Netherlands, Italy, Germany, and the United Kingdom as part of the SOS (Safety of Non-Steroidal Anti-Inflammatory Drugs) project and conducted a nested, case-control study including 92,163 hospital admissions for heart failure and 8,246,403 controls matched for age, sex, and year of study entry. The study included 23 traditional NSAIDs and four selective COX-2 inhibitors.
Overall, individual use of any of nine different NSAIDs within 14 days was associated with a nearly 20% higher likelihood of hospital admission for heart failure, compared with NSAID use more than 183 days in the past (odds ratio, 1.19). For seven traditional NSAIDS (diclofenac, ibuprofen, indomethacin, ketorolac, naproxen, nimesulide, and piroxicam) and the COX-2 inhibitors etoricoxib and rofecoxib, the odds ratios for heart failure with current use ranged from 1.16 for naproxen to 1.83 for ketorolac, compared with past use.
In addition, the odds of hospitalization for heart failure doubled for diclofenac, etoricoxib, indomethacin, piroxicam, and rofecoxib when dosed at two or more daily dose equivalents, the researchers noted. There was no increase in the odds of hospitalization for heart failure with celecoxib when dosed at standard levels, but “indomethacin and etoricoxib seemed to increase the risk of hospital admission for heart failure, even if used at medium doses,” they said. Other lesser-used NSAIDs were associated with an increased risk, but it was not statistically significant.
“The effect of individual NSAIDs could depend on a complex interaction of pharmacological properties, including duration and extent of platelet inhibition, extent of blood pressure increase, and properties possibly unique to the molecule,” the researchers said.
The findings were limited by several factors including misclassification of outcomes and the observational nature of the study, which prevents conclusions about cause and effect, the researchers noted. However, “Because any potential increased risk could have a considerable impact on public health, the risk effect estimates provided by this study may help inform both clinical practice and regulatory activities,” they said.
The study was funded in part by the European Community’s seventh Framework Programme. Mr. Arfè had no financial conflicts to disclose. Several study coauthors disclosed relationships with multiple companies including AstraZeneca, Bayer, Celgene, GlaxoSmithKline, Schwabe, and Novartis.
FROM BMJ
Key clinical point: Patients taking high doses of certain NSAIDS had significantly higher odds of hospital admission for heart failure, compared with controls not currently taking the medications.
Major finding: The odds of hospitalization for heart failure increased by 19% overall for adults currently using certain NSAIDS and doubled for users of certain NSAIDs at high doses.
Data source: The data come from approximately 10 million hospital admissions taken from databases in the Netherlands, Italy, Germany, and the United Kingdom.
Disclosures: The study was funded in part by the European Community’s seventh Framework Programme. Mr. Arfè had no financial conflicts to disclose. Several study coauthors disclosed relationships with multiple companies including AstraZeneca, Bayer, Celgene, GlaxoSmithKline, Schwabe, and Novartis.
Adjunctive azithromycin cuts postcesarean infection
Adding a single intravenous dose of azithromycin to standard antibiotic prophylaxis further reduces maternal infections without increasing neonatal adverse outcomes after nonelective cesarean delivery, according to a report published in the New England Journal of Medicine.
The adjunctive azithromycin also significantly decreased rates of postpartum fever and of readmission or unscheduled office visits, wrote Alan T.N. Tita, MD, PhD, of the University of Alabama at Birmingham, and his colleagues.
Recent studies have suggested that extended-spectrum prophylaxis using azithromycin, when added to standard cephalosporin prophylaxis, would further reduce the incidence of post-cesarean infection, chiefly because of azithromycin’s coverage of ureaplasma species that are frequently associated with these infections. The C/SOAP (Cesarean Section Optimal Antibiotic Prophylaxis) trial tested this hypothesis in 2,013 women who underwent nonelective cesarean delivery of singleton neonates at 14 U.S. hospitals during a 3.5-year period.
All the women received standard antibiotic prophylaxis (usually with cefazolin) and were randomly assigned to receive either a 500-mg dose of azithromycin (1,019 participants) or a matching placebo (994 participants) before surgical incision.
The primary outcome measure – a composite of endometritis; wound infection; or other infections such as abdominopelvic abscess, maternal sepsis, pelvic septic thrombophlebitis, pyelonephritis, pneumonia, or meningitis occurring up to 6 weeks after surgery – developed in half as many women in the azithromycin group (6.1%) as in the placebo group (12.0%). The relative risk (RR) was 0.51 (P less than .001).
Azithromycin, in particular, was associated with significantly lower rates of endometritis (3.8% vs. 6.1%; RR, 0.62; P = .02) and wound infection (2.4% vs. 6.6%; RR, 0.35; P less than .001). This benefit extended across all subgroups of patients regardless of study site, maternal obesity status, the presence or absence of membrane rupture at randomization, preterm or term delivery, or maternal diabetes status.
The number of patients who would need to be treated to prevent one study outcome was 17 for the primary outcome, 43 for endometritis, and 24 for wound infections, the researchers reported (N Engl J Med. 2016 Sep 29;375:1231-41).
Serious maternal adverse events also were less common with azithromycin (1.5%) than with placebo (2.9%). Neonatal outcomes did not differ between the study groups. The rate of combined neonatal death or complications was 14.3% with azithromycin and 13.6% with placebo, a nonsignificant difference.
The study was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Pfizer donated the azithromycin used in the trial. Dr. Tita reported having no relevant financial disclosures; his colleagues reported ties to numerous industry sources.
This well-designed, pragmatic, multicenter trial shows that a single adjunctive dose of azithromycin likely would reduce the number of infectious complications for women undergoing nonelective cesarean section.
The addition of azithromycin may have been particularly effective for the 73% of this study population who had a body mass index of 30 kg/m2 or more. Obesity is known to double the risk of infectious complications, and previous studies have suggested that cefazolin may be underdosed in women with increased BMI values. It appears that azithromycin improved outcomes on the basis of the additive effects of the two drugs against common surgical pathogens, such as staphylococcus species. Information provided in the Supplementary Appendix accompanying the article indicates that routine bacterial cultures, when done, were much-less-frequently positive in the azithromycin group.
Robert A. Weinstein, MD, and Kenneth M. Boyer, MD, are at Rush University Medical Center and Cook County Health and Hospital System, both in Chicago. Dr. Weinstein reported receiving support from Merck outside of this work, and Dr. Boyer reported having no relevant financial disclosures. These remarks are adapted from an accompanying editorial (N Engl J Med. 2016 Sept 29. doi: 10.1056/NEJMe1610010).
This well-designed, pragmatic, multicenter trial shows that a single adjunctive dose of azithromycin likely would reduce the number of infectious complications for women undergoing nonelective cesarean section.
The addition of azithromycin may have been particularly effective for the 73% of this study population who had a body mass index of 30 kg/m2 or more. Obesity is known to double the risk of infectious complications, and previous studies have suggested that cefazolin may be underdosed in women with increased BMI values. It appears that azithromycin improved outcomes on the basis of the additive effects of the two drugs against common surgical pathogens, such as staphylococcus species. Information provided in the Supplementary Appendix accompanying the article indicates that routine bacterial cultures, when done, were much-less-frequently positive in the azithromycin group.
Robert A. Weinstein, MD, and Kenneth M. Boyer, MD, are at Rush University Medical Center and Cook County Health and Hospital System, both in Chicago. Dr. Weinstein reported receiving support from Merck outside of this work, and Dr. Boyer reported having no relevant financial disclosures. These remarks are adapted from an accompanying editorial (N Engl J Med. 2016 Sept 29. doi: 10.1056/NEJMe1610010).
This well-designed, pragmatic, multicenter trial shows that a single adjunctive dose of azithromycin likely would reduce the number of infectious complications for women undergoing nonelective cesarean section.
The addition of azithromycin may have been particularly effective for the 73% of this study population who had a body mass index of 30 kg/m2 or more. Obesity is known to double the risk of infectious complications, and previous studies have suggested that cefazolin may be underdosed in women with increased BMI values. It appears that azithromycin improved outcomes on the basis of the additive effects of the two drugs against common surgical pathogens, such as staphylococcus species. Information provided in the Supplementary Appendix accompanying the article indicates that routine bacterial cultures, when done, were much-less-frequently positive in the azithromycin group.
Robert A. Weinstein, MD, and Kenneth M. Boyer, MD, are at Rush University Medical Center and Cook County Health and Hospital System, both in Chicago. Dr. Weinstein reported receiving support from Merck outside of this work, and Dr. Boyer reported having no relevant financial disclosures. These remarks are adapted from an accompanying editorial (N Engl J Med. 2016 Sept 29. doi: 10.1056/NEJMe1610010).
Adding a single intravenous dose of azithromycin to standard antibiotic prophylaxis further reduces maternal infections without increasing neonatal adverse outcomes after nonelective cesarean delivery, according to a report published in the New England Journal of Medicine.
The adjunctive azithromycin also significantly decreased rates of postpartum fever and of readmission or unscheduled office visits, wrote Alan T.N. Tita, MD, PhD, of the University of Alabama at Birmingham, and his colleagues.
Recent studies have suggested that extended-spectrum prophylaxis using azithromycin, when added to standard cephalosporin prophylaxis, would further reduce the incidence of post-cesarean infection, chiefly because of azithromycin’s coverage of ureaplasma species that are frequently associated with these infections. The C/SOAP (Cesarean Section Optimal Antibiotic Prophylaxis) trial tested this hypothesis in 2,013 women who underwent nonelective cesarean delivery of singleton neonates at 14 U.S. hospitals during a 3.5-year period.
All the women received standard antibiotic prophylaxis (usually with cefazolin) and were randomly assigned to receive either a 500-mg dose of azithromycin (1,019 participants) or a matching placebo (994 participants) before surgical incision.
The primary outcome measure – a composite of endometritis; wound infection; or other infections such as abdominopelvic abscess, maternal sepsis, pelvic septic thrombophlebitis, pyelonephritis, pneumonia, or meningitis occurring up to 6 weeks after surgery – developed in half as many women in the azithromycin group (6.1%) as in the placebo group (12.0%). The relative risk (RR) was 0.51 (P less than .001).
Azithromycin, in particular, was associated with significantly lower rates of endometritis (3.8% vs. 6.1%; RR, 0.62; P = .02) and wound infection (2.4% vs. 6.6%; RR, 0.35; P less than .001). This benefit extended across all subgroups of patients regardless of study site, maternal obesity status, the presence or absence of membrane rupture at randomization, preterm or term delivery, or maternal diabetes status.
The number of patients who would need to be treated to prevent one study outcome was 17 for the primary outcome, 43 for endometritis, and 24 for wound infections, the researchers reported (N Engl J Med. 2016 Sep 29;375:1231-41).
Serious maternal adverse events also were less common with azithromycin (1.5%) than with placebo (2.9%). Neonatal outcomes did not differ between the study groups. The rate of combined neonatal death or complications was 14.3% with azithromycin and 13.6% with placebo, a nonsignificant difference.
The study was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Pfizer donated the azithromycin used in the trial. Dr. Tita reported having no relevant financial disclosures; his colleagues reported ties to numerous industry sources.
Adding a single intravenous dose of azithromycin to standard antibiotic prophylaxis further reduces maternal infections without increasing neonatal adverse outcomes after nonelective cesarean delivery, according to a report published in the New England Journal of Medicine.
The adjunctive azithromycin also significantly decreased rates of postpartum fever and of readmission or unscheduled office visits, wrote Alan T.N. Tita, MD, PhD, of the University of Alabama at Birmingham, and his colleagues.
Recent studies have suggested that extended-spectrum prophylaxis using azithromycin, when added to standard cephalosporin prophylaxis, would further reduce the incidence of post-cesarean infection, chiefly because of azithromycin’s coverage of ureaplasma species that are frequently associated with these infections. The C/SOAP (Cesarean Section Optimal Antibiotic Prophylaxis) trial tested this hypothesis in 2,013 women who underwent nonelective cesarean delivery of singleton neonates at 14 U.S. hospitals during a 3.5-year period.
All the women received standard antibiotic prophylaxis (usually with cefazolin) and were randomly assigned to receive either a 500-mg dose of azithromycin (1,019 participants) or a matching placebo (994 participants) before surgical incision.
The primary outcome measure – a composite of endometritis; wound infection; or other infections such as abdominopelvic abscess, maternal sepsis, pelvic septic thrombophlebitis, pyelonephritis, pneumonia, or meningitis occurring up to 6 weeks after surgery – developed in half as many women in the azithromycin group (6.1%) as in the placebo group (12.0%). The relative risk (RR) was 0.51 (P less than .001).
Azithromycin, in particular, was associated with significantly lower rates of endometritis (3.8% vs. 6.1%; RR, 0.62; P = .02) and wound infection (2.4% vs. 6.6%; RR, 0.35; P less than .001). This benefit extended across all subgroups of patients regardless of study site, maternal obesity status, the presence or absence of membrane rupture at randomization, preterm or term delivery, or maternal diabetes status.
The number of patients who would need to be treated to prevent one study outcome was 17 for the primary outcome, 43 for endometritis, and 24 for wound infections, the researchers reported (N Engl J Med. 2016 Sep 29;375:1231-41).
Serious maternal adverse events also were less common with azithromycin (1.5%) than with placebo (2.9%). Neonatal outcomes did not differ between the study groups. The rate of combined neonatal death or complications was 14.3% with azithromycin and 13.6% with placebo, a nonsignificant difference.
The study was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Pfizer donated the azithromycin used in the trial. Dr. Tita reported having no relevant financial disclosures; his colleagues reported ties to numerous industry sources.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE