User login
News and Views that Matter to Physicians
VIDEO: When is it time to jump into MACRA with both feet?
LAS VEGAS – Change in federal reimbursement for physicians is coming. Though the change is inevitable, physicians still have to weigh choices about when they might want to jump in with both feet, since entry into the full incentive payment system will be optional – for a time.
The Medicare Access and CHIP Reauthorization Act of 2015 (MACRA) is “basically a reorganization of all of these disparate reward and penalty systems” that have existed within the federal health care reimbursement landscape, said Joseph S. Eastern, MD. “The idea was to collect them all within one system.”
The new system is called the Medicare Incentive Payment System, or MIPS. Physicians are already familiar with many MIPS components, including meaningful use of the electronic health record, “which everybody thought was going away, but it isn’t,” said Dr. Eastern, a dermatologist in private practice in Belleville, N.J., who’s affiliated with Seton Hall University, South Orange, N.J. Also included are the Physician Quality Reimbursement System (PQRS) and the value-based modifier system.
MIPS is designed so that “you’ll either get a reward or a penalty depending on how well you do, compared with other physicians,” said Dr. Eastern, speaking at the annual Perspectives in Rheumatic Diseases held by Global Academy for Medical Education.
The alternative, he said, is to opt for one of the Alternative Payment Models, or APMs. However, details about APMs are “really up in the air, because a lot of them have either not been doing very well, or have not been very well defined,” so that physicians often don’t currently have enough data to make an informed choice. He expects the APM landscape to sort out over the next year or two.
Opting not to comply and take the 1%-3% cut in Medicare reimbursement associated with noncompliance might make sense for just a few physicians, though it might seem tempting, Dr. Eastern said in a video interview. Since the penalties will escalate significantly over the next few years, he feels that only physicians who are considering retiring soon or selling their practices should consider opting out.
Global Academy for Medical Education and this news organization are owned by the same parent company.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @karioakes
LAS VEGAS – Change in federal reimbursement for physicians is coming. Though the change is inevitable, physicians still have to weigh choices about when they might want to jump in with both feet, since entry into the full incentive payment system will be optional – for a time.
The Medicare Access and CHIP Reauthorization Act of 2015 (MACRA) is “basically a reorganization of all of these disparate reward and penalty systems” that have existed within the federal health care reimbursement landscape, said Joseph S. Eastern, MD. “The idea was to collect them all within one system.”
The new system is called the Medicare Incentive Payment System, or MIPS. Physicians are already familiar with many MIPS components, including meaningful use of the electronic health record, “which everybody thought was going away, but it isn’t,” said Dr. Eastern, a dermatologist in private practice in Belleville, N.J., who’s affiliated with Seton Hall University, South Orange, N.J. Also included are the Physician Quality Reimbursement System (PQRS) and the value-based modifier system.
MIPS is designed so that “you’ll either get a reward or a penalty depending on how well you do, compared with other physicians,” said Dr. Eastern, speaking at the annual Perspectives in Rheumatic Diseases held by Global Academy for Medical Education.
The alternative, he said, is to opt for one of the Alternative Payment Models, or APMs. However, details about APMs are “really up in the air, because a lot of them have either not been doing very well, or have not been very well defined,” so that physicians often don’t currently have enough data to make an informed choice. He expects the APM landscape to sort out over the next year or two.
Opting not to comply and take the 1%-3% cut in Medicare reimbursement associated with noncompliance might make sense for just a few physicians, though it might seem tempting, Dr. Eastern said in a video interview. Since the penalties will escalate significantly over the next few years, he feels that only physicians who are considering retiring soon or selling their practices should consider opting out.
Global Academy for Medical Education and this news organization are owned by the same parent company.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @karioakes
LAS VEGAS – Change in federal reimbursement for physicians is coming. Though the change is inevitable, physicians still have to weigh choices about when they might want to jump in with both feet, since entry into the full incentive payment system will be optional – for a time.
The Medicare Access and CHIP Reauthorization Act of 2015 (MACRA) is “basically a reorganization of all of these disparate reward and penalty systems” that have existed within the federal health care reimbursement landscape, said Joseph S. Eastern, MD. “The idea was to collect them all within one system.”
The new system is called the Medicare Incentive Payment System, or MIPS. Physicians are already familiar with many MIPS components, including meaningful use of the electronic health record, “which everybody thought was going away, but it isn’t,” said Dr. Eastern, a dermatologist in private practice in Belleville, N.J., who’s affiliated with Seton Hall University, South Orange, N.J. Also included are the Physician Quality Reimbursement System (PQRS) and the value-based modifier system.
MIPS is designed so that “you’ll either get a reward or a penalty depending on how well you do, compared with other physicians,” said Dr. Eastern, speaking at the annual Perspectives in Rheumatic Diseases held by Global Academy for Medical Education.
The alternative, he said, is to opt for one of the Alternative Payment Models, or APMs. However, details about APMs are “really up in the air, because a lot of them have either not been doing very well, or have not been very well defined,” so that physicians often don’t currently have enough data to make an informed choice. He expects the APM landscape to sort out over the next year or two.
Opting not to comply and take the 1%-3% cut in Medicare reimbursement associated with noncompliance might make sense for just a few physicians, though it might seem tempting, Dr. Eastern said in a video interview. Since the penalties will escalate significantly over the next few years, he feels that only physicians who are considering retiring soon or selling their practices should consider opting out.
Global Academy for Medical Education and this news organization are owned by the same parent company.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @karioakes
EXPERT ANALYSIS FROM THE ANNUAL PERSPECTIVES IN RHEUMATIC DISEASES
Empiric warfarin adjustment cut drug-drug interactions with antimicrobials
BOSTON – A medication management strategy to minimize the effect of drug-drug interactions (DDIs) between warfarin and common antimicrobials resulted in significantly greater time within therapeutic range for anticoagulation, as well as a numerically smaller, but nonsignificant, number of bleeding events.
After implementation of a comprehensive inpatient and postdischarge guideline to manage DDIs between warfarin and 16 antibiotics, antivirals, or antifungal medications, patients’ in-hospital time within therapeutic range (TWTR) increased to 72% from 50% preimplementation (P = .043). Warfarin TWTR also improved across care transitions after the guidelines were implemented, rising to 70% from 46% (P = .012). No bleeding events occurred in the group studied after the guidelines were instituted, compared with four events in the comparator preguidelines group (P = .11).
Nghi Ha, PharmD, MPH, and his collaborators sought to determine whether formalizing a process to manage potentially dangerous antimicrobial-warfarin DDIs made a difference in achieving more TWTR for patients, as determined by international normalized ratio (INR) values. Dr. Ha, a clinical pharmacist at University of Michigan Health System, Ann Arbor, presented the results during a poster session at the annual meeting of the American Society for Microbiology.
Secondary outcome measures studied by Dr. Ha and his associates included the incidence of thrombosis or major bleeding events, as well as tracking documentation of medications and the anticoagulation plan in progress and discharge notes.
Patients were included if they were at least 18 years old, and if they were on 3 days or more of an antimicrobial with potential for DDI with warfarin. Patients who were also newly on other medications with the potential for significant DDI with warfarin were excluded to minimize the potential for confounding.
Dr. Ha and his collaborators characterized the study as a retrospective, single-center, quasi-experimental design of a pharmacist-run anticoagulation service. The study examined endpoints before and after implementation of comprehensive guidelines, and included 78 preguideline and 31 postguideline patients.
The guidelines drafted by the investigators and tested in their study included empiric adjustment of warfarin dosing for patients who were placed on an antibiotic with high potential to increase INR. These included many azoles and sulfamethoxazole/trimethoprim, for which initial warfarin doses were empirically reduced 20%-30% for patients whose INRs were therapeutic at the start of antimicrobial therapy. For ciprofloxacin, erythromycin, clarithromycin, and isoniazid, the guidelines recommended initial empiric warfarin dose reductions of 10%-15%.
Patients whose INRs were subtherapeutic at the beginning of therapy and who received these antimicrobials were continued on their maintenance warfarin dosing, but were monitored for rising INRs over the first 48 hours, for consideration of dosing adjustment as needed. Individuals with supratherapeutic INRs at the beginning of antimicrobial dosing had their warfarin doses reduced or held by a more aggressive algorithm based on their initial INR, and based on the potential of the antimicrobial to increase INR.
On discharge, patients were either reverted to their previous warfarin regimen if they had been stable on that regimen, or had their inpatient warfarin dosing increased by 10%-20%.
Drugs that were deemed to have moderate potential to increase INR included doxycycline, levofloxacin, moxifloxacin, quinupristin/dalfopristin, telaprevir, boceprevir, and simeprevir. For these medications, the protocol recommended no initial dose adjustment, but recommended monitoring of INR to consider a dose reduction if needed. On hospital discharge, patients who had been on these medications were to resume their previous warfarin dosing.
Antimicrobials with potential to decrease INR included nafcillin, for which the protocol recommended empiric warfarin dose increases of 25%-50%, starting 3-5 days after nafcillin was begun. Patients on rifampin or rifabutin were to increase their warfarin by 20%-30%, also 3-5 days after beginning the antibiotics. Patients on ritonavir alone, or any protease inhibitor given for HIV along with ritonavir, were closely monitored, but no empiric dosing adjustments were made.
Patients with initial subtherapeutic INRs had dosing increased by 30%-50% for nafcillin and 20%-30% for rifampin and rifabutin. A stepped algorithm for dose adjustment or withholding was also developed for these medications to treat patients with initial supratherapeutic INRs. Patients on these medications were to resume their previous warfarin dosing, with monitoring and adjustment if they had not been previously stable.
Documentation of antimicrobial-warfarin DDI in the anticoagulation service discharge summary improved significantly once the guidelines were implemented (40% compared with 14%, P = .02). There was not a significant improvement in DDI documentation in daily progress notes.
The comprehensive intervention included the formulation of guidelines and requirements to document the medication interaction in the medical chart. Other interventions included training for clinical pharmacists and the development of pocket cards and flyers to educate team members about the new guidelines. The electronic health record had triggers built and implemented to prompt consideration of warfarin/antimicrobial DDIs as well.
Dr. Ha and his coauthors noted that the uncontrolled nature of the pre-post study design was one limitation of the study. Also, the real-world design of the study meant that investigators could not control for diet, comorbidities, and other factors that have the potential to affect INR. “Implementing a process to identify high-risk antimicrobial-warfarin DDIs and provide guidelines for empiric warfarin dose adjustment … can improve INR time within therapeutic range,” noted Dr. Ha and his coauthors.
The study authors reported no external sources of funding and no conflicts of interest.
On Twitter @karioakes
BOSTON – A medication management strategy to minimize the effect of drug-drug interactions (DDIs) between warfarin and common antimicrobials resulted in significantly greater time within therapeutic range for anticoagulation, as well as a numerically smaller, but nonsignificant, number of bleeding events.
After implementation of a comprehensive inpatient and postdischarge guideline to manage DDIs between warfarin and 16 antibiotics, antivirals, or antifungal medications, patients’ in-hospital time within therapeutic range (TWTR) increased to 72% from 50% preimplementation (P = .043). Warfarin TWTR also improved across care transitions after the guidelines were implemented, rising to 70% from 46% (P = .012). No bleeding events occurred in the group studied after the guidelines were instituted, compared with four events in the comparator preguidelines group (P = .11).
Nghi Ha, PharmD, MPH, and his collaborators sought to determine whether formalizing a process to manage potentially dangerous antimicrobial-warfarin DDIs made a difference in achieving more TWTR for patients, as determined by international normalized ratio (INR) values. Dr. Ha, a clinical pharmacist at University of Michigan Health System, Ann Arbor, presented the results during a poster session at the annual meeting of the American Society for Microbiology.
Secondary outcome measures studied by Dr. Ha and his associates included the incidence of thrombosis or major bleeding events, as well as tracking documentation of medications and the anticoagulation plan in progress and discharge notes.
Patients were included if they were at least 18 years old, and if they were on 3 days or more of an antimicrobial with potential for DDI with warfarin. Patients who were also newly on other medications with the potential for significant DDI with warfarin were excluded to minimize the potential for confounding.
Dr. Ha and his collaborators characterized the study as a retrospective, single-center, quasi-experimental design of a pharmacist-run anticoagulation service. The study examined endpoints before and after implementation of comprehensive guidelines, and included 78 preguideline and 31 postguideline patients.
The guidelines drafted by the investigators and tested in their study included empiric adjustment of warfarin dosing for patients who were placed on an antibiotic with high potential to increase INR. These included many azoles and sulfamethoxazole/trimethoprim, for which initial warfarin doses were empirically reduced 20%-30% for patients whose INRs were therapeutic at the start of antimicrobial therapy. For ciprofloxacin, erythromycin, clarithromycin, and isoniazid, the guidelines recommended initial empiric warfarin dose reductions of 10%-15%.
Patients whose INRs were subtherapeutic at the beginning of therapy and who received these antimicrobials were continued on their maintenance warfarin dosing, but were monitored for rising INRs over the first 48 hours, for consideration of dosing adjustment as needed. Individuals with supratherapeutic INRs at the beginning of antimicrobial dosing had their warfarin doses reduced or held by a more aggressive algorithm based on their initial INR, and based on the potential of the antimicrobial to increase INR.
On discharge, patients were either reverted to their previous warfarin regimen if they had been stable on that regimen, or had their inpatient warfarin dosing increased by 10%-20%.
Drugs that were deemed to have moderate potential to increase INR included doxycycline, levofloxacin, moxifloxacin, quinupristin/dalfopristin, telaprevir, boceprevir, and simeprevir. For these medications, the protocol recommended no initial dose adjustment, but recommended monitoring of INR to consider a dose reduction if needed. On hospital discharge, patients who had been on these medications were to resume their previous warfarin dosing.
Antimicrobials with potential to decrease INR included nafcillin, for which the protocol recommended empiric warfarin dose increases of 25%-50%, starting 3-5 days after nafcillin was begun. Patients on rifampin or rifabutin were to increase their warfarin by 20%-30%, also 3-5 days after beginning the antibiotics. Patients on ritonavir alone, or any protease inhibitor given for HIV along with ritonavir, were closely monitored, but no empiric dosing adjustments were made.
Patients with initial subtherapeutic INRs had dosing increased by 30%-50% for nafcillin and 20%-30% for rifampin and rifabutin. A stepped algorithm for dose adjustment or withholding was also developed for these medications to treat patients with initial supratherapeutic INRs. Patients on these medications were to resume their previous warfarin dosing, with monitoring and adjustment if they had not been previously stable.
Documentation of antimicrobial-warfarin DDI in the anticoagulation service discharge summary improved significantly once the guidelines were implemented (40% compared with 14%, P = .02). There was not a significant improvement in DDI documentation in daily progress notes.
The comprehensive intervention included the formulation of guidelines and requirements to document the medication interaction in the medical chart. Other interventions included training for clinical pharmacists and the development of pocket cards and flyers to educate team members about the new guidelines. The electronic health record had triggers built and implemented to prompt consideration of warfarin/antimicrobial DDIs as well.
Dr. Ha and his coauthors noted that the uncontrolled nature of the pre-post study design was one limitation of the study. Also, the real-world design of the study meant that investigators could not control for diet, comorbidities, and other factors that have the potential to affect INR. “Implementing a process to identify high-risk antimicrobial-warfarin DDIs and provide guidelines for empiric warfarin dose adjustment … can improve INR time within therapeutic range,” noted Dr. Ha and his coauthors.
The study authors reported no external sources of funding and no conflicts of interest.
On Twitter @karioakes
BOSTON – A medication management strategy to minimize the effect of drug-drug interactions (DDIs) between warfarin and common antimicrobials resulted in significantly greater time within therapeutic range for anticoagulation, as well as a numerically smaller, but nonsignificant, number of bleeding events.
After implementation of a comprehensive inpatient and postdischarge guideline to manage DDIs between warfarin and 16 antibiotics, antivirals, or antifungal medications, patients’ in-hospital time within therapeutic range (TWTR) increased to 72% from 50% preimplementation (P = .043). Warfarin TWTR also improved across care transitions after the guidelines were implemented, rising to 70% from 46% (P = .012). No bleeding events occurred in the group studied after the guidelines were instituted, compared with four events in the comparator preguidelines group (P = .11).
Nghi Ha, PharmD, MPH, and his collaborators sought to determine whether formalizing a process to manage potentially dangerous antimicrobial-warfarin DDIs made a difference in achieving more TWTR for patients, as determined by international normalized ratio (INR) values. Dr. Ha, a clinical pharmacist at University of Michigan Health System, Ann Arbor, presented the results during a poster session at the annual meeting of the American Society for Microbiology.
Secondary outcome measures studied by Dr. Ha and his associates included the incidence of thrombosis or major bleeding events, as well as tracking documentation of medications and the anticoagulation plan in progress and discharge notes.
Patients were included if they were at least 18 years old, and if they were on 3 days or more of an antimicrobial with potential for DDI with warfarin. Patients who were also newly on other medications with the potential for significant DDI with warfarin were excluded to minimize the potential for confounding.
Dr. Ha and his collaborators characterized the study as a retrospective, single-center, quasi-experimental design of a pharmacist-run anticoagulation service. The study examined endpoints before and after implementation of comprehensive guidelines, and included 78 preguideline and 31 postguideline patients.
The guidelines drafted by the investigators and tested in their study included empiric adjustment of warfarin dosing for patients who were placed on an antibiotic with high potential to increase INR. These included many azoles and sulfamethoxazole/trimethoprim, for which initial warfarin doses were empirically reduced 20%-30% for patients whose INRs were therapeutic at the start of antimicrobial therapy. For ciprofloxacin, erythromycin, clarithromycin, and isoniazid, the guidelines recommended initial empiric warfarin dose reductions of 10%-15%.
Patients whose INRs were subtherapeutic at the beginning of therapy and who received these antimicrobials were continued on their maintenance warfarin dosing, but were monitored for rising INRs over the first 48 hours, for consideration of dosing adjustment as needed. Individuals with supratherapeutic INRs at the beginning of antimicrobial dosing had their warfarin doses reduced or held by a more aggressive algorithm based on their initial INR, and based on the potential of the antimicrobial to increase INR.
On discharge, patients were either reverted to their previous warfarin regimen if they had been stable on that regimen, or had their inpatient warfarin dosing increased by 10%-20%.
Drugs that were deemed to have moderate potential to increase INR included doxycycline, levofloxacin, moxifloxacin, quinupristin/dalfopristin, telaprevir, boceprevir, and simeprevir. For these medications, the protocol recommended no initial dose adjustment, but recommended monitoring of INR to consider a dose reduction if needed. On hospital discharge, patients who had been on these medications were to resume their previous warfarin dosing.
Antimicrobials with potential to decrease INR included nafcillin, for which the protocol recommended empiric warfarin dose increases of 25%-50%, starting 3-5 days after nafcillin was begun. Patients on rifampin or rifabutin were to increase their warfarin by 20%-30%, also 3-5 days after beginning the antibiotics. Patients on ritonavir alone, or any protease inhibitor given for HIV along with ritonavir, were closely monitored, but no empiric dosing adjustments were made.
Patients with initial subtherapeutic INRs had dosing increased by 30%-50% for nafcillin and 20%-30% for rifampin and rifabutin. A stepped algorithm for dose adjustment or withholding was also developed for these medications to treat patients with initial supratherapeutic INRs. Patients on these medications were to resume their previous warfarin dosing, with monitoring and adjustment if they had not been previously stable.
Documentation of antimicrobial-warfarin DDI in the anticoagulation service discharge summary improved significantly once the guidelines were implemented (40% compared with 14%, P = .02). There was not a significant improvement in DDI documentation in daily progress notes.
The comprehensive intervention included the formulation of guidelines and requirements to document the medication interaction in the medical chart. Other interventions included training for clinical pharmacists and the development of pocket cards and flyers to educate team members about the new guidelines. The electronic health record had triggers built and implemented to prompt consideration of warfarin/antimicrobial DDIs as well.
Dr. Ha and his coauthors noted that the uncontrolled nature of the pre-post study design was one limitation of the study. Also, the real-world design of the study meant that investigators could not control for diet, comorbidities, and other factors that have the potential to affect INR. “Implementing a process to identify high-risk antimicrobial-warfarin DDIs and provide guidelines for empiric warfarin dose adjustment … can improve INR time within therapeutic range,” noted Dr. Ha and his coauthors.
The study authors reported no external sources of funding and no conflicts of interest.
On Twitter @karioakes
AT ASM MICROBE 2016
Key clinical point: Clinical guidelines with empiric warfarin adjustments improved time within therapeutic range (TWTR) for inpatients on antimicrobials.
Major finding: In-hospital TWTR increased to 72% from 50% before implementation of clinical guidelines (P = .043).
Data source: Retrospective, single-center study of inpatients on warfarin and antimicrobial with potential for DDI before (n = 78) and after (n = 31) implementation of a comprehensive clinical guideline.
Disclosures: The study investigators reported no outside sources of funding and no disclosures.
Less pain, quicker discharge with post-TORS dexamethasone
SEATTLE – A longer course of dexamethasone was a bit better than the usual single intraoperative dose for controlling pain and dysphagia after transoral robotic surgery in a randomized trial from the Oregon Health and Science University, Portland.
Thirty-five subjects were randomized to the standard 10-mg intraoperative dexamethasone dose plus 8 mg every 8 hours for up to 4 days; 33 others were randomized to the intraoperative dose plus placebo. All the subjects had transoral robotic surgery (TORS) resection for T1 or T2 oropharyngeal squamous cell carcinoma, either partial pharyngectomy/radical tonsillectomy, base of tongue resection, or both.
The dexamethasone group had significantly less pain on postop day 3 (about 1.5 points less on the 10-point visual analogue scale) and were discharged, on average, a day earlier. They also advanced more quickly toward solid food at 1- and 3-weeks’ follow-up. “They were much more likely to be on a full-soft diet, while the placebo group was mostly still on purees, and just starting into soft foods,” said lead investigator Daniel Clayburgh, MD, a head and neck cancer specialist at the university.
Otherwise, however, the extra dexamethasone wasn’t much help; pain scores were the same in both groups for the first couple days after surgery and at follow-up, and both groups used the same amount of post-op opioids. Other than food tolerance, dysphagia metrics were pretty much the same.
“I was actually anticipating a little bit more of a benefit, but there are potentially some benefits to extended corticosteroid courses after TORS. It’s safe, and well tolerated so long as you screen out diabetes and other problems with hyperglycemia,” as was done in the study, he said. “It does decrease post-op length of stay and may provide a modest decrease in post-op pain, and may slightly accelerate advancement of dietary consistency,” Dr. Clayburgh said at the International Conference on Head and Neck Cancer, held by the American Head and Neck Society.
Although he and his colleagues are mulling over what to do with the findings in light of other initiatives to reduce post-TORS pain, they are now likely to extend dexamethasone courses when significant post-op pain seems likely, and doing so is not otherwise contraindicated, he said.
Intraoperative corticosteroids are now routine for TORS, based on the strength of benefit in the tonsillectomy literature. The team decided to try an extended course because “being rather simple minded surgeons, we thought that if one dose is good, more should be better,” Dr. Clayburgh said.
The dexamethasone group was slightly younger than the placebo group (56 vs. 61 years) but otherwise similar; most were men. In addition to patients with hyperglycemia issues, those with confounders for post-op speech and swallowing recovery were among those excluded from the trial. Subjects required nasogastric feeding tubes for a median of 6.5 days postoperatively, lost a mean of 10 pounds in the first 2 post-op weeks, and were hospitalized for a mean of about 5 days. Dexamethasone was delivered orally or by nasogastric tube.
There was no external funding for the study, and Dr. Clayburgh had no relevant financial disclosures.
SEATTLE – A longer course of dexamethasone was a bit better than the usual single intraoperative dose for controlling pain and dysphagia after transoral robotic surgery in a randomized trial from the Oregon Health and Science University, Portland.
Thirty-five subjects were randomized to the standard 10-mg intraoperative dexamethasone dose plus 8 mg every 8 hours for up to 4 days; 33 others were randomized to the intraoperative dose plus placebo. All the subjects had transoral robotic surgery (TORS) resection for T1 or T2 oropharyngeal squamous cell carcinoma, either partial pharyngectomy/radical tonsillectomy, base of tongue resection, or both.
The dexamethasone group had significantly less pain on postop day 3 (about 1.5 points less on the 10-point visual analogue scale) and were discharged, on average, a day earlier. They also advanced more quickly toward solid food at 1- and 3-weeks’ follow-up. “They were much more likely to be on a full-soft diet, while the placebo group was mostly still on purees, and just starting into soft foods,” said lead investigator Daniel Clayburgh, MD, a head and neck cancer specialist at the university.
Otherwise, however, the extra dexamethasone wasn’t much help; pain scores were the same in both groups for the first couple days after surgery and at follow-up, and both groups used the same amount of post-op opioids. Other than food tolerance, dysphagia metrics were pretty much the same.
“I was actually anticipating a little bit more of a benefit, but there are potentially some benefits to extended corticosteroid courses after TORS. It’s safe, and well tolerated so long as you screen out diabetes and other problems with hyperglycemia,” as was done in the study, he said. “It does decrease post-op length of stay and may provide a modest decrease in post-op pain, and may slightly accelerate advancement of dietary consistency,” Dr. Clayburgh said at the International Conference on Head and Neck Cancer, held by the American Head and Neck Society.
Although he and his colleagues are mulling over what to do with the findings in light of other initiatives to reduce post-TORS pain, they are now likely to extend dexamethasone courses when significant post-op pain seems likely, and doing so is not otherwise contraindicated, he said.
Intraoperative corticosteroids are now routine for TORS, based on the strength of benefit in the tonsillectomy literature. The team decided to try an extended course because “being rather simple minded surgeons, we thought that if one dose is good, more should be better,” Dr. Clayburgh said.
The dexamethasone group was slightly younger than the placebo group (56 vs. 61 years) but otherwise similar; most were men. In addition to patients with hyperglycemia issues, those with confounders for post-op speech and swallowing recovery were among those excluded from the trial. Subjects required nasogastric feeding tubes for a median of 6.5 days postoperatively, lost a mean of 10 pounds in the first 2 post-op weeks, and were hospitalized for a mean of about 5 days. Dexamethasone was delivered orally or by nasogastric tube.
There was no external funding for the study, and Dr. Clayburgh had no relevant financial disclosures.
SEATTLE – A longer course of dexamethasone was a bit better than the usual single intraoperative dose for controlling pain and dysphagia after transoral robotic surgery in a randomized trial from the Oregon Health and Science University, Portland.
Thirty-five subjects were randomized to the standard 10-mg intraoperative dexamethasone dose plus 8 mg every 8 hours for up to 4 days; 33 others were randomized to the intraoperative dose plus placebo. All the subjects had transoral robotic surgery (TORS) resection for T1 or T2 oropharyngeal squamous cell carcinoma, either partial pharyngectomy/radical tonsillectomy, base of tongue resection, or both.
The dexamethasone group had significantly less pain on postop day 3 (about 1.5 points less on the 10-point visual analogue scale) and were discharged, on average, a day earlier. They also advanced more quickly toward solid food at 1- and 3-weeks’ follow-up. “They were much more likely to be on a full-soft diet, while the placebo group was mostly still on purees, and just starting into soft foods,” said lead investigator Daniel Clayburgh, MD, a head and neck cancer specialist at the university.
Otherwise, however, the extra dexamethasone wasn’t much help; pain scores were the same in both groups for the first couple days after surgery and at follow-up, and both groups used the same amount of post-op opioids. Other than food tolerance, dysphagia metrics were pretty much the same.
“I was actually anticipating a little bit more of a benefit, but there are potentially some benefits to extended corticosteroid courses after TORS. It’s safe, and well tolerated so long as you screen out diabetes and other problems with hyperglycemia,” as was done in the study, he said. “It does decrease post-op length of stay and may provide a modest decrease in post-op pain, and may slightly accelerate advancement of dietary consistency,” Dr. Clayburgh said at the International Conference on Head and Neck Cancer, held by the American Head and Neck Society.
Although he and his colleagues are mulling over what to do with the findings in light of other initiatives to reduce post-TORS pain, they are now likely to extend dexamethasone courses when significant post-op pain seems likely, and doing so is not otherwise contraindicated, he said.
Intraoperative corticosteroids are now routine for TORS, based on the strength of benefit in the tonsillectomy literature. The team decided to try an extended course because “being rather simple minded surgeons, we thought that if one dose is good, more should be better,” Dr. Clayburgh said.
The dexamethasone group was slightly younger than the placebo group (56 vs. 61 years) but otherwise similar; most were men. In addition to patients with hyperglycemia issues, those with confounders for post-op speech and swallowing recovery were among those excluded from the trial. Subjects required nasogastric feeding tubes for a median of 6.5 days postoperatively, lost a mean of 10 pounds in the first 2 post-op weeks, and were hospitalized for a mean of about 5 days. Dexamethasone was delivered orally or by nasogastric tube.
There was no external funding for the study, and Dr. Clayburgh had no relevant financial disclosures.
AT AHNS 2016
Key clinical point: A longer course of dexamethasone is a bit better than the usual single intraoperative dose for controlling pain and dysphagia after transoral robotic surgery.
Major finding: The dexamethasone group had significantly less pain on post-op day 3 (about 1.5 points less on the 10-point visual analogue scale) and were discharged, on average, a day earlier. They also advanced more quickly toward solid food at 1- and 3-weeks’ follow-up.
Data source: A randomized trial of 68 TORS patients.
Disclosures: There was no external funding for the study, and the lead investigator had no relevant financial disclosures.
Gonorrhea cluster shows increased antibiotic resistance
ATLANTA – A cluster of gonorrhea cases from the state of Hawaii has been identified as the first in the United States to show decreased susceptibility to ceftriaxone and azithromycin, the two most commonly prescribed drugs used to treat the infection.
“We’re seeing new, troubling signs that our current gonorrhea treatment is losing its effectiveness [but] we’ve not seen a treatment failure in the U.S.,” explained Jonathan Mermin, MD, director of the National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention, during a conference on STD prevention sponsored by the Centers for Disease Control and Prevention.
Isolates were collected from seven individuals in Hawaii during April and May of this year, all of which showed “dramatically higher levels” of resistance to azithromycin in laboratory testing than has normally been seen in the U.S. While large-scale resistance to azithromycin is something the CDC has watched for several months, four of these seven isolates also demonstrated less vulnerability to ceftriaxone, the first time that has occurred. Since 2010, the recommended treatment for gonorrhea has consisted of a single ceftriaxone shot and an oral dose of azithromycin; having a cluster with increased resistance to both is problematic on several levels, infectious disease experts say.
According to Alan Katz, MD, of the University of Hawaii in Honolulu, “the state of Hawaii has been a seminal site for monitoring Neisseria gonorrhoeae resistance, and the Hawaii state Department of Health has been one of the original CDC gonococcal isolate surveillance program surveillance sites since its inception in 1986.” Hawaii typically sees more gonorrhea cases than the rest of the country, partially due its location between the U.S. and Asia, the latter of which Dr. Katz explained is “where we believe many [drug]-resistant strains originate.”
Currently, trials are underway to identify a new treatment that can replace the current regimen, with promising early results. The drug in question, known as ETX0914, is a single-dose oral therapy that would substitute for ceftriaxone in the currently recommended treatment protocol. Stephanie N. Taylor, MD, of Louisiana State University in New Orleans, shared results of a randomized controlled trial, in which 179 subjects – 167 males and 12 females – received either 2g or 3g doses of only ETX0914, or only ceftriaxone.
A total of 47 subjects received the 3g ETX0914 dose, while 49 subjects received the 2g dose, and the rest received ceftriaxone. All patients (47/47) receiving the 3g dose were cured, and 98% (48/49) in the 2g dose were cured, with only 21 subjects (12%) in the entire study population reporting mild side effects.
“We are very pleased with these results and look forward to seeing ETX0914 advance through additional clinical trials,” Dr. Taylor said in a statement.
For now, health care providers are encouraged to continue with the currently recommended drug therapy, which is still effective. In a statement, Gail Bolan, MD, director of the Division of STD Prevention at the CDC, reminded providers that infections should be treated immediately in order for the drugs to have their full impact.
“All health care providers should also promptly report any suspected treatment failure to local health officials and CDC to ensure rapid response to cases or clusters of concern,” she added.
ATLANTA – A cluster of gonorrhea cases from the state of Hawaii has been identified as the first in the United States to show decreased susceptibility to ceftriaxone and azithromycin, the two most commonly prescribed drugs used to treat the infection.
“We’re seeing new, troubling signs that our current gonorrhea treatment is losing its effectiveness [but] we’ve not seen a treatment failure in the U.S.,” explained Jonathan Mermin, MD, director of the National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention, during a conference on STD prevention sponsored by the Centers for Disease Control and Prevention.
Isolates were collected from seven individuals in Hawaii during April and May of this year, all of which showed “dramatically higher levels” of resistance to azithromycin in laboratory testing than has normally been seen in the U.S. While large-scale resistance to azithromycin is something the CDC has watched for several months, four of these seven isolates also demonstrated less vulnerability to ceftriaxone, the first time that has occurred. Since 2010, the recommended treatment for gonorrhea has consisted of a single ceftriaxone shot and an oral dose of azithromycin; having a cluster with increased resistance to both is problematic on several levels, infectious disease experts say.
According to Alan Katz, MD, of the University of Hawaii in Honolulu, “the state of Hawaii has been a seminal site for monitoring Neisseria gonorrhoeae resistance, and the Hawaii state Department of Health has been one of the original CDC gonococcal isolate surveillance program surveillance sites since its inception in 1986.” Hawaii typically sees more gonorrhea cases than the rest of the country, partially due its location between the U.S. and Asia, the latter of which Dr. Katz explained is “where we believe many [drug]-resistant strains originate.”
Currently, trials are underway to identify a new treatment that can replace the current regimen, with promising early results. The drug in question, known as ETX0914, is a single-dose oral therapy that would substitute for ceftriaxone in the currently recommended treatment protocol. Stephanie N. Taylor, MD, of Louisiana State University in New Orleans, shared results of a randomized controlled trial, in which 179 subjects – 167 males and 12 females – received either 2g or 3g doses of only ETX0914, or only ceftriaxone.
A total of 47 subjects received the 3g ETX0914 dose, while 49 subjects received the 2g dose, and the rest received ceftriaxone. All patients (47/47) receiving the 3g dose were cured, and 98% (48/49) in the 2g dose were cured, with only 21 subjects (12%) in the entire study population reporting mild side effects.
“We are very pleased with these results and look forward to seeing ETX0914 advance through additional clinical trials,” Dr. Taylor said in a statement.
For now, health care providers are encouraged to continue with the currently recommended drug therapy, which is still effective. In a statement, Gail Bolan, MD, director of the Division of STD Prevention at the CDC, reminded providers that infections should be treated immediately in order for the drugs to have their full impact.
“All health care providers should also promptly report any suspected treatment failure to local health officials and CDC to ensure rapid response to cases or clusters of concern,” she added.
ATLANTA – A cluster of gonorrhea cases from the state of Hawaii has been identified as the first in the United States to show decreased susceptibility to ceftriaxone and azithromycin, the two most commonly prescribed drugs used to treat the infection.
“We’re seeing new, troubling signs that our current gonorrhea treatment is losing its effectiveness [but] we’ve not seen a treatment failure in the U.S.,” explained Jonathan Mermin, MD, director of the National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention, during a conference on STD prevention sponsored by the Centers for Disease Control and Prevention.
Isolates were collected from seven individuals in Hawaii during April and May of this year, all of which showed “dramatically higher levels” of resistance to azithromycin in laboratory testing than has normally been seen in the U.S. While large-scale resistance to azithromycin is something the CDC has watched for several months, four of these seven isolates also demonstrated less vulnerability to ceftriaxone, the first time that has occurred. Since 2010, the recommended treatment for gonorrhea has consisted of a single ceftriaxone shot and an oral dose of azithromycin; having a cluster with increased resistance to both is problematic on several levels, infectious disease experts say.
According to Alan Katz, MD, of the University of Hawaii in Honolulu, “the state of Hawaii has been a seminal site for monitoring Neisseria gonorrhoeae resistance, and the Hawaii state Department of Health has been one of the original CDC gonococcal isolate surveillance program surveillance sites since its inception in 1986.” Hawaii typically sees more gonorrhea cases than the rest of the country, partially due its location between the U.S. and Asia, the latter of which Dr. Katz explained is “where we believe many [drug]-resistant strains originate.”
Currently, trials are underway to identify a new treatment that can replace the current regimen, with promising early results. The drug in question, known as ETX0914, is a single-dose oral therapy that would substitute for ceftriaxone in the currently recommended treatment protocol. Stephanie N. Taylor, MD, of Louisiana State University in New Orleans, shared results of a randomized controlled trial, in which 179 subjects – 167 males and 12 females – received either 2g or 3g doses of only ETX0914, or only ceftriaxone.
A total of 47 subjects received the 3g ETX0914 dose, while 49 subjects received the 2g dose, and the rest received ceftriaxone. All patients (47/47) receiving the 3g dose were cured, and 98% (48/49) in the 2g dose were cured, with only 21 subjects (12%) in the entire study population reporting mild side effects.
“We are very pleased with these results and look forward to seeing ETX0914 advance through additional clinical trials,” Dr. Taylor said in a statement.
For now, health care providers are encouraged to continue with the currently recommended drug therapy, which is still effective. In a statement, Gail Bolan, MD, director of the Division of STD Prevention at the CDC, reminded providers that infections should be treated immediately in order for the drugs to have their full impact.
“All health care providers should also promptly report any suspected treatment failure to local health officials and CDC to ensure rapid response to cases or clusters of concern,” she added.
AT THE 2016 STD PREVENTION CONFERENCE
Artificial intelligence beats standard algorithms for diagnosing lung disease
LONDON – In a proof-of-principle study, artificial intelligence (AI) led more frequently to the correct diagnosis of underlying lung disease than did physicians’ use of standard algorithms, such as those recommended by the American Thoracic Society and the European Respiratory Society, according to late-breaker data presented at the annual congress of the European Respiratory Society.
“The beauty of this approach is that artificial intelligence can simulate the complex reasoning that clinicians use to reach their diagnosis but in a more standardized and objective fashion, so it removes any bias,” reported Wim Janssens, MD, PhD, of the division of medicine and respiratory rehabilitation at University of Leuven (Belgium).
The AI employed in this study was based on a subfield of computer science that relies on patterns within statistics to build decision trees. Often called machine learning, this type of AI grows smarter as it learns from the patterns it finds in the data provided.
In this case, the AI was designed to provide diagnoses for lung diseases based on patterns drawn from clinical and lung function data. The computer-based choices were compared to diagnoses reached by clinicians. The final diagnoses were validated by a consensus of expert clinicians.
“The computer-based choices were in almost all cases better than the choices made by standard diagnostic algorithms,” reported Marko Topalovic, PhD, a researcher in AI who is affiliated with the University of Leuven. Dr. Topalovic presented the data at the ERS.
The study involved 968 patients presenting with lung symptoms to a pulmonary clinic for the first time. Standard clinical data, such as smoking history, body mass index, and age, were collected. Lung function studies conducted in all patients included spirometry, body plethysmography, and airway diffusion, although participating clinicians were permitted to order additional tests at their own discretion. Clinical diagnoses were separated into 10 predefined disease groups.
The average accuracy of clinicians’ diagnoses was 38%. The clinicians were best at identifying chronic obstructive pulmonary disease (COPD), having accurately diagnosed 74% of the cases of this disease. For other disease groups, the clinician’s accuracy rarely exceeded 50%.
The diagnoses made by AI, on the other hand, on average, were 68% accurate. For diagnosing COPD, the AI achieved a positive predictive value of 83% and a sensitivity of 78%. The positive predictive value and sensitivity of AI for asthma (66% and 82%, respectively) and interstitial lung disease (52% and 59%) were both significantly greater than those achieved by the clinicians.
When findings are ambiguous or there are anomalies in the clinical picture, a final clinical diagnosis can be challenging, according to Dr. Janssens. He suggested that automation eliminates the potential for bias, which often occurs when clinicians inadvertently give more weight to one clinical variable relative to another.
The decision-making system tested in this study was characterized as “a first step to automated interpretation of lung function,” Dr. Topalovic said. He added that he expects the AI to improve as it receives more data.
“Not only do we think this system can help nonexperienced clinicians, but it will help experts reach a diagnosis more quickly,” Dr. Topalovic said. Noting that this same approach is being pursued in other fields of medicine, he said he thinks adding AI to respiratory medicine will reduce the number of redundant tests and, in other ways, introduce opportunities for efficiencies and reduced costs.
Dr. Topalovic and Dr. Janssens reported no relevant financial relationships.
LONDON – In a proof-of-principle study, artificial intelligence (AI) led more frequently to the correct diagnosis of underlying lung disease than did physicians’ use of standard algorithms, such as those recommended by the American Thoracic Society and the European Respiratory Society, according to late-breaker data presented at the annual congress of the European Respiratory Society.
“The beauty of this approach is that artificial intelligence can simulate the complex reasoning that clinicians use to reach their diagnosis but in a more standardized and objective fashion, so it removes any bias,” reported Wim Janssens, MD, PhD, of the division of medicine and respiratory rehabilitation at University of Leuven (Belgium).
The AI employed in this study was based on a subfield of computer science that relies on patterns within statistics to build decision trees. Often called machine learning, this type of AI grows smarter as it learns from the patterns it finds in the data provided.
In this case, the AI was designed to provide diagnoses for lung diseases based on patterns drawn from clinical and lung function data. The computer-based choices were compared to diagnoses reached by clinicians. The final diagnoses were validated by a consensus of expert clinicians.
“The computer-based choices were in almost all cases better than the choices made by standard diagnostic algorithms,” reported Marko Topalovic, PhD, a researcher in AI who is affiliated with the University of Leuven. Dr. Topalovic presented the data at the ERS.
The study involved 968 patients presenting with lung symptoms to a pulmonary clinic for the first time. Standard clinical data, such as smoking history, body mass index, and age, were collected. Lung function studies conducted in all patients included spirometry, body plethysmography, and airway diffusion, although participating clinicians were permitted to order additional tests at their own discretion. Clinical diagnoses were separated into 10 predefined disease groups.
The average accuracy of clinicians’ diagnoses was 38%. The clinicians were best at identifying chronic obstructive pulmonary disease (COPD), having accurately diagnosed 74% of the cases of this disease. For other disease groups, the clinician’s accuracy rarely exceeded 50%.
The diagnoses made by AI, on the other hand, on average, were 68% accurate. For diagnosing COPD, the AI achieved a positive predictive value of 83% and a sensitivity of 78%. The positive predictive value and sensitivity of AI for asthma (66% and 82%, respectively) and interstitial lung disease (52% and 59%) were both significantly greater than those achieved by the clinicians.
When findings are ambiguous or there are anomalies in the clinical picture, a final clinical diagnosis can be challenging, according to Dr. Janssens. He suggested that automation eliminates the potential for bias, which often occurs when clinicians inadvertently give more weight to one clinical variable relative to another.
The decision-making system tested in this study was characterized as “a first step to automated interpretation of lung function,” Dr. Topalovic said. He added that he expects the AI to improve as it receives more data.
“Not only do we think this system can help nonexperienced clinicians, but it will help experts reach a diagnosis more quickly,” Dr. Topalovic said. Noting that this same approach is being pursued in other fields of medicine, he said he thinks adding AI to respiratory medicine will reduce the number of redundant tests and, in other ways, introduce opportunities for efficiencies and reduced costs.
Dr. Topalovic and Dr. Janssens reported no relevant financial relationships.
LONDON – In a proof-of-principle study, artificial intelligence (AI) led more frequently to the correct diagnosis of underlying lung disease than did physicians’ use of standard algorithms, such as those recommended by the American Thoracic Society and the European Respiratory Society, according to late-breaker data presented at the annual congress of the European Respiratory Society.
“The beauty of this approach is that artificial intelligence can simulate the complex reasoning that clinicians use to reach their diagnosis but in a more standardized and objective fashion, so it removes any bias,” reported Wim Janssens, MD, PhD, of the division of medicine and respiratory rehabilitation at University of Leuven (Belgium).
The AI employed in this study was based on a subfield of computer science that relies on patterns within statistics to build decision trees. Often called machine learning, this type of AI grows smarter as it learns from the patterns it finds in the data provided.
In this case, the AI was designed to provide diagnoses for lung diseases based on patterns drawn from clinical and lung function data. The computer-based choices were compared to diagnoses reached by clinicians. The final diagnoses were validated by a consensus of expert clinicians.
“The computer-based choices were in almost all cases better than the choices made by standard diagnostic algorithms,” reported Marko Topalovic, PhD, a researcher in AI who is affiliated with the University of Leuven. Dr. Topalovic presented the data at the ERS.
The study involved 968 patients presenting with lung symptoms to a pulmonary clinic for the first time. Standard clinical data, such as smoking history, body mass index, and age, were collected. Lung function studies conducted in all patients included spirometry, body plethysmography, and airway diffusion, although participating clinicians were permitted to order additional tests at their own discretion. Clinical diagnoses were separated into 10 predefined disease groups.
The average accuracy of clinicians’ diagnoses was 38%. The clinicians were best at identifying chronic obstructive pulmonary disease (COPD), having accurately diagnosed 74% of the cases of this disease. For other disease groups, the clinician’s accuracy rarely exceeded 50%.
The diagnoses made by AI, on the other hand, on average, were 68% accurate. For diagnosing COPD, the AI achieved a positive predictive value of 83% and a sensitivity of 78%. The positive predictive value and sensitivity of AI for asthma (66% and 82%, respectively) and interstitial lung disease (52% and 59%) were both significantly greater than those achieved by the clinicians.
When findings are ambiguous or there are anomalies in the clinical picture, a final clinical diagnosis can be challenging, according to Dr. Janssens. He suggested that automation eliminates the potential for bias, which often occurs when clinicians inadvertently give more weight to one clinical variable relative to another.
The decision-making system tested in this study was characterized as “a first step to automated interpretation of lung function,” Dr. Topalovic said. He added that he expects the AI to improve as it receives more data.
“Not only do we think this system can help nonexperienced clinicians, but it will help experts reach a diagnosis more quickly,” Dr. Topalovic said. Noting that this same approach is being pursued in other fields of medicine, he said he thinks adding AI to respiratory medicine will reduce the number of redundant tests and, in other ways, introduce opportunities for efficiencies and reduced costs.
Dr. Topalovic and Dr. Janssens reported no relevant financial relationships.
AT THE ERS CONGRESS 2016
Key clinical point: When given the same clinical information, artificial intelligence is more likely than are clinicians to diagnose lung diseases correctly.
Major finding: The diagnostic label was correct in 38% of cases with standard algorithms, versus 68% with artificial intelligence.
Data source: Prospective study.
Disclosures: Dr. Marko Topalovic and Dr. Wim Janssens reported no relevant financial relationships.
Legislators: Investigate Medicare fraud before paying doctors
Republican leaders in Congress are calling on CMS to impose stricter safeguards against fraudulent Medicare billing by physicians.
The chairmen of the Senate Finance Committee, the House Ways and Means and Energy and Commerce Committees, and the chairmen of key House subcommittees said that the Centers for Medicare & Medicaid Services relies too heavily on the “outdated” pay and chase method and should focus more energy on preventing payment for potential fraudulent claims.
“Improper payments remain an enormous problem for the Medicare program,” the chairmen wrote in a Sept. 12 letter to Acting CMS Administrator Andy Slavitt. “In 2015, the Medicare program had an error rate of 12.1% or $43.3 billion dollars. The billions of dollars lost to Medicare fraud each year underscore the importance of stopping potentially fraudulent payments before they’re made,”
Some health law experts, however, argue that CMS already has a process for in place for pre-identifying inaccurate claims via prepayment audits and reviews. Such efforts can be devastating for physicians who come under scrutiny for unintentional mistakes, said Daniel F. Shay, a Philadelphia health law attorney.
“I can understand why, particularly in an election year, elected officials might send a letter reiterating the need to curb ‘waste, fraud, and abuse,’” Mr. Shay said in an interview. “It’s true that it’s more efficient for the government to investigate a physician’s claim for reimbursement first, and then pay. However, I think we have to take into account the physicians’ perspective, especially physicians in smaller, independent practices.”
The legislators’ letter acknowledges that CMS has taken some proactive steps to prevent health fraud, including creation of the Fraud Prevention System (FPS), which highlights questionable billing patterns and identifies providers who pose high risk to the program. FPS runs analytics on 4.5 million claims daily and has led to more than $820 million in savings, according to CMS. However, legislators said they are still concerned that CMS too often pays claims before investigating whether they’re false. The letter requests that CMS clarify its implementation of the FPS program, including details on fraud investigations and how the agency monitors FPS’s effectiveness.
Houston, Tex.–based health law attorney Michael E. Clark disagrees that CMS is overusing the pay-and-chase method. Quite the contrary, he said.
“The federal government cannot seem to find the right balance on how to address program fraud,” Mr. Clark said in an interview. “While ‘pay and chase’ once was a problem, now the government can effectively destroy health care service providers under a very low threshold without the businesses having a meaningful right to appeal that determination.”
Specifically, CMS can withhold Medicare reimbursement from health providers under an amended 2011 law that permits payments to be suppressed when “credible” allegations of fraud have been made, but are disputed. The term “credible” is a new, lower standard for the administrative action, which was meant to address the pay-and-chase problem, Mr. Clark said. The law defines a “credible allegation of fraud” as an allegation from any source, including but not limited to fraud hotline complaints, data mining of claims, patterns identified through provider audits, civil false claims cases, and law enforcement investigations.
“That standard is easy to meet and agencies have every incentive to claim they’ve got so-called credible allegations of fraud in order to avoid being criticized later on for not preventing the monies from being dissipated,” he said. Because the law precludes health providers from appealing the fraud allegation to a federal court until all administrative remedies have been exhausted, “a health care services provider can quickly be put out of business, even if it turns out that the investigation proves not to be actionable.”
Prepayment reviews of claims can drag on for months, severely impacting a physician’s income, Mr. Shay added. In his experience, the majority of physicians under investigation are not trying to game the system, but rather don’t understand all of the administrative requirements related to filing claims. In some cases, the physicians’ notes are not complete, their bills are too high for services provided, or not enough documentation exists to support medical necessity.
“In the midst of that, you have doctors who are likely well-meaning, who have provided a service to a patient in need, and who are facing real economic hardship without an effective mechanism to challenge or end the prepayment review process,” he said.
Rather than more prepayment investigations, Mr. Shay would like to see CMS focus on physician education.
There needs to be “more emphasis on provider education in terms of compliance with program requirements,” he said. “It shouldn’t require a lawyer getting involved to find out what specifically [CMS] wants them to do. That should be part of the process as a standard.”
On Twitter @legal_med
Republican leaders in Congress are calling on CMS to impose stricter safeguards against fraudulent Medicare billing by physicians.
The chairmen of the Senate Finance Committee, the House Ways and Means and Energy and Commerce Committees, and the chairmen of key House subcommittees said that the Centers for Medicare & Medicaid Services relies too heavily on the “outdated” pay and chase method and should focus more energy on preventing payment for potential fraudulent claims.
“Improper payments remain an enormous problem for the Medicare program,” the chairmen wrote in a Sept. 12 letter to Acting CMS Administrator Andy Slavitt. “In 2015, the Medicare program had an error rate of 12.1% or $43.3 billion dollars. The billions of dollars lost to Medicare fraud each year underscore the importance of stopping potentially fraudulent payments before they’re made,”
Some health law experts, however, argue that CMS already has a process for in place for pre-identifying inaccurate claims via prepayment audits and reviews. Such efforts can be devastating for physicians who come under scrutiny for unintentional mistakes, said Daniel F. Shay, a Philadelphia health law attorney.
“I can understand why, particularly in an election year, elected officials might send a letter reiterating the need to curb ‘waste, fraud, and abuse,’” Mr. Shay said in an interview. “It’s true that it’s more efficient for the government to investigate a physician’s claim for reimbursement first, and then pay. However, I think we have to take into account the physicians’ perspective, especially physicians in smaller, independent practices.”
The legislators’ letter acknowledges that CMS has taken some proactive steps to prevent health fraud, including creation of the Fraud Prevention System (FPS), which highlights questionable billing patterns and identifies providers who pose high risk to the program. FPS runs analytics on 4.5 million claims daily and has led to more than $820 million in savings, according to CMS. However, legislators said they are still concerned that CMS too often pays claims before investigating whether they’re false. The letter requests that CMS clarify its implementation of the FPS program, including details on fraud investigations and how the agency monitors FPS’s effectiveness.
Houston, Tex.–based health law attorney Michael E. Clark disagrees that CMS is overusing the pay-and-chase method. Quite the contrary, he said.
“The federal government cannot seem to find the right balance on how to address program fraud,” Mr. Clark said in an interview. “While ‘pay and chase’ once was a problem, now the government can effectively destroy health care service providers under a very low threshold without the businesses having a meaningful right to appeal that determination.”
Specifically, CMS can withhold Medicare reimbursement from health providers under an amended 2011 law that permits payments to be suppressed when “credible” allegations of fraud have been made, but are disputed. The term “credible” is a new, lower standard for the administrative action, which was meant to address the pay-and-chase problem, Mr. Clark said. The law defines a “credible allegation of fraud” as an allegation from any source, including but not limited to fraud hotline complaints, data mining of claims, patterns identified through provider audits, civil false claims cases, and law enforcement investigations.
“That standard is easy to meet and agencies have every incentive to claim they’ve got so-called credible allegations of fraud in order to avoid being criticized later on for not preventing the monies from being dissipated,” he said. Because the law precludes health providers from appealing the fraud allegation to a federal court until all administrative remedies have been exhausted, “a health care services provider can quickly be put out of business, even if it turns out that the investigation proves not to be actionable.”
Prepayment reviews of claims can drag on for months, severely impacting a physician’s income, Mr. Shay added. In his experience, the majority of physicians under investigation are not trying to game the system, but rather don’t understand all of the administrative requirements related to filing claims. In some cases, the physicians’ notes are not complete, their bills are too high for services provided, or not enough documentation exists to support medical necessity.
“In the midst of that, you have doctors who are likely well-meaning, who have provided a service to a patient in need, and who are facing real economic hardship without an effective mechanism to challenge or end the prepayment review process,” he said.
Rather than more prepayment investigations, Mr. Shay would like to see CMS focus on physician education.
There needs to be “more emphasis on provider education in terms of compliance with program requirements,” he said. “It shouldn’t require a lawyer getting involved to find out what specifically [CMS] wants them to do. That should be part of the process as a standard.”
On Twitter @legal_med
Republican leaders in Congress are calling on CMS to impose stricter safeguards against fraudulent Medicare billing by physicians.
The chairmen of the Senate Finance Committee, the House Ways and Means and Energy and Commerce Committees, and the chairmen of key House subcommittees said that the Centers for Medicare & Medicaid Services relies too heavily on the “outdated” pay and chase method and should focus more energy on preventing payment for potential fraudulent claims.
“Improper payments remain an enormous problem for the Medicare program,” the chairmen wrote in a Sept. 12 letter to Acting CMS Administrator Andy Slavitt. “In 2015, the Medicare program had an error rate of 12.1% or $43.3 billion dollars. The billions of dollars lost to Medicare fraud each year underscore the importance of stopping potentially fraudulent payments before they’re made,”
Some health law experts, however, argue that CMS already has a process for in place for pre-identifying inaccurate claims via prepayment audits and reviews. Such efforts can be devastating for physicians who come under scrutiny for unintentional mistakes, said Daniel F. Shay, a Philadelphia health law attorney.
“I can understand why, particularly in an election year, elected officials might send a letter reiterating the need to curb ‘waste, fraud, and abuse,’” Mr. Shay said in an interview. “It’s true that it’s more efficient for the government to investigate a physician’s claim for reimbursement first, and then pay. However, I think we have to take into account the physicians’ perspective, especially physicians in smaller, independent practices.”
The legislators’ letter acknowledges that CMS has taken some proactive steps to prevent health fraud, including creation of the Fraud Prevention System (FPS), which highlights questionable billing patterns and identifies providers who pose high risk to the program. FPS runs analytics on 4.5 million claims daily and has led to more than $820 million in savings, according to CMS. However, legislators said they are still concerned that CMS too often pays claims before investigating whether they’re false. The letter requests that CMS clarify its implementation of the FPS program, including details on fraud investigations and how the agency monitors FPS’s effectiveness.
Houston, Tex.–based health law attorney Michael E. Clark disagrees that CMS is overusing the pay-and-chase method. Quite the contrary, he said.
“The federal government cannot seem to find the right balance on how to address program fraud,” Mr. Clark said in an interview. “While ‘pay and chase’ once was a problem, now the government can effectively destroy health care service providers under a very low threshold without the businesses having a meaningful right to appeal that determination.”
Specifically, CMS can withhold Medicare reimbursement from health providers under an amended 2011 law that permits payments to be suppressed when “credible” allegations of fraud have been made, but are disputed. The term “credible” is a new, lower standard for the administrative action, which was meant to address the pay-and-chase problem, Mr. Clark said. The law defines a “credible allegation of fraud” as an allegation from any source, including but not limited to fraud hotline complaints, data mining of claims, patterns identified through provider audits, civil false claims cases, and law enforcement investigations.
“That standard is easy to meet and agencies have every incentive to claim they’ve got so-called credible allegations of fraud in order to avoid being criticized later on for not preventing the monies from being dissipated,” he said. Because the law precludes health providers from appealing the fraud allegation to a federal court until all administrative remedies have been exhausted, “a health care services provider can quickly be put out of business, even if it turns out that the investigation proves not to be actionable.”
Prepayment reviews of claims can drag on for months, severely impacting a physician’s income, Mr. Shay added. In his experience, the majority of physicians under investigation are not trying to game the system, but rather don’t understand all of the administrative requirements related to filing claims. In some cases, the physicians’ notes are not complete, their bills are too high for services provided, or not enough documentation exists to support medical necessity.
“In the midst of that, you have doctors who are likely well-meaning, who have provided a service to a patient in need, and who are facing real economic hardship without an effective mechanism to challenge or end the prepayment review process,” he said.
Rather than more prepayment investigations, Mr. Shay would like to see CMS focus on physician education.
There needs to be “more emphasis on provider education in terms of compliance with program requirements,” he said. “It shouldn’t require a lawyer getting involved to find out what specifically [CMS] wants them to do. That should be part of the process as a standard.”
On Twitter @legal_med
ICS/LABA exacerbation benefit outweighs pneumonia risk
LONDON – The benefit of a fixed-dose inhaled corticosteroid (ICS) and long-acting beta-agonist (LABA) combination in reducing exacerbations of chronic obstructive pulmonary disease (COPD) far outweighed any risk for pneumonia in a post hoc analysis of the 48-week FORWARD study.
Although there were 13 extra pneumonia events when a fixed-dose combination of beclometasone diproprionate and formoterol fumarate (Foster, Chiesi Farmaceutici SpA) was used as compared to formoterol fumarate alone, there were 123 fewer moderate to severe COPD exacerbations over a 342-day analysis period.
“Analysis of pneumonia and exacerbation cumulative number of events shows that the number of incident pneumonia remains very small relative to that of moderate to severe exacerbations,” Massimo Corradi, MD, of the University of Parma (Italy), reported at the annual congress of the European Respiratory Society.
Dr. Corradi added that the new analysis confirms that the ICS/LABA combination has a “positive risk-benefit balance over LABA monotherapy, supporting [the argument that] the benefits of adding an ICS to a bronchodilator significantly outweigh potential risks.”
The FORWARD study was a two-arm trial designed to compare the efficacy and safety of fixed-dose treatment with beclometasone diproprionate and formoterol fumarate versus formoterol fumarate alone in 1,199 patients with severe COPD.
For inclusion in the study, patients had to have a post-bronchodilator forced expiratory volume in 1 second below 50% of predicted and a forced vital capacity ratio of less than 0.7. They also had to have a smoking history of 10 pack-years or more, and a history of at least one COPD exacerbation in the previous 12 months that had required treatment or hospitalization (Eur Respir J. 2013;41[1]:12-7).
After a 2-week run-in period, where all patients received a 24-mcg dose of formoterol fumarate, patients were randomized to continue treatment with formoterol fumarate or to receive the fixed-dose combination of beclometasone diproprionate 400 mcg and beclometasone diproprionate 24 mcg for 48 weeks.
A total of 1,186 patients, most of whom were male (69%) with a mean age of 64 years, formed the intention-to-treat population.
Published results (Respir Med. 2014;108[8]:1153-62) showed that the combination of the ICS beclometasone diproprionate and the LABA formoterol fumarate (Chiesi Farmaceutici SpA) was associated with a 28% reduction in the annual rate of moderate to severe exacerbations versus the LABA alone.
The adjusted rate of exacerbations per patient per year was 0.80 in patients treated with the ICS/LABA combination versus 1.12 for those treated with just the LABA, with an adjusted rate ratio of 0.72 (P less than .001).
The published data also showed that pneumonia was reported by 23 patients (3.8%) treated with the ICS/LABA and by 11 (1.8%) treated with the LABA only.
For the new analysis, Dr. Corradi and his coinvestigators looked at the cases of pneumonia and COPD exacerbations in more detail, plotting out the cumulative number of events over time and also characterizing the types of pneumonia in more detail.
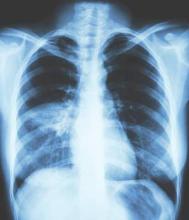
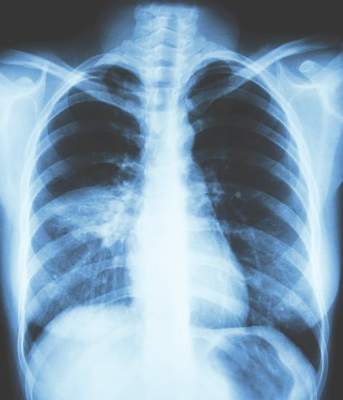
All patients had a chest x-ray to confirm the presence of pneumonia, he said, noting that overall there were 35 cases of pneumonia, 24 occurring in patients treated with the fixed-dose beclometasone diproprionate and formoterol fumarate combination and 11 in patients treated only with formoterol fumarate.
Of these cases, 25 required in-hospital treatment – 16 patients in the ICS/LABA arm and 9 in the LABA-only arm. There were three instances of patients acquiring pneumonia in hospital – two in the ICS/LABA and one in the LABA-only arm.
There were also two fatal cases of pneumonia – one in each treatment group. Neither were thought to be related to either of the treatments.
These findings are in line with a recent review of the use of ICS for COPD by the European Medicines Agency (EMA/488280/2016), which noted that “overall the benefits of inhaled corticosteroid medicines in treating COPD continue to outweigh their risks and there should be no change to the way in which these medicines are used.”
The European Medicines Agency advised that patients and clinicians need to “be alert for signs and symptoms of pneumonia, bearing in mind that the clinical features of pneumonia overlap with those of a worsening (exacerbation) of the underlying disease.”
Dr. Corradi has received speaker fees from Chiesi Farmaceutici SpA, which funded the FORWARD study, and his coauthors are employees of the company.
LONDON – The benefit of a fixed-dose inhaled corticosteroid (ICS) and long-acting beta-agonist (LABA) combination in reducing exacerbations of chronic obstructive pulmonary disease (COPD) far outweighed any risk for pneumonia in a post hoc analysis of the 48-week FORWARD study.
Although there were 13 extra pneumonia events when a fixed-dose combination of beclometasone diproprionate and formoterol fumarate (Foster, Chiesi Farmaceutici SpA) was used as compared to formoterol fumarate alone, there were 123 fewer moderate to severe COPD exacerbations over a 342-day analysis period.
“Analysis of pneumonia and exacerbation cumulative number of events shows that the number of incident pneumonia remains very small relative to that of moderate to severe exacerbations,” Massimo Corradi, MD, of the University of Parma (Italy), reported at the annual congress of the European Respiratory Society.
Dr. Corradi added that the new analysis confirms that the ICS/LABA combination has a “positive risk-benefit balance over LABA monotherapy, supporting [the argument that] the benefits of adding an ICS to a bronchodilator significantly outweigh potential risks.”
The FORWARD study was a two-arm trial designed to compare the efficacy and safety of fixed-dose treatment with beclometasone diproprionate and formoterol fumarate versus formoterol fumarate alone in 1,199 patients with severe COPD.
For inclusion in the study, patients had to have a post-bronchodilator forced expiratory volume in 1 second below 50% of predicted and a forced vital capacity ratio of less than 0.7. They also had to have a smoking history of 10 pack-years or more, and a history of at least one COPD exacerbation in the previous 12 months that had required treatment or hospitalization (Eur Respir J. 2013;41[1]:12-7).
After a 2-week run-in period, where all patients received a 24-mcg dose of formoterol fumarate, patients were randomized to continue treatment with formoterol fumarate or to receive the fixed-dose combination of beclometasone diproprionate 400 mcg and beclometasone diproprionate 24 mcg for 48 weeks.
A total of 1,186 patients, most of whom were male (69%) with a mean age of 64 years, formed the intention-to-treat population.
Published results (Respir Med. 2014;108[8]:1153-62) showed that the combination of the ICS beclometasone diproprionate and the LABA formoterol fumarate (Chiesi Farmaceutici SpA) was associated with a 28% reduction in the annual rate of moderate to severe exacerbations versus the LABA alone.
The adjusted rate of exacerbations per patient per year was 0.80 in patients treated with the ICS/LABA combination versus 1.12 for those treated with just the LABA, with an adjusted rate ratio of 0.72 (P less than .001).
The published data also showed that pneumonia was reported by 23 patients (3.8%) treated with the ICS/LABA and by 11 (1.8%) treated with the LABA only.
For the new analysis, Dr. Corradi and his coinvestigators looked at the cases of pneumonia and COPD exacerbations in more detail, plotting out the cumulative number of events over time and also characterizing the types of pneumonia in more detail.
All patients had a chest x-ray to confirm the presence of pneumonia, he said, noting that overall there were 35 cases of pneumonia, 24 occurring in patients treated with the fixed-dose beclometasone diproprionate and formoterol fumarate combination and 11 in patients treated only with formoterol fumarate.
Of these cases, 25 required in-hospital treatment – 16 patients in the ICS/LABA arm and 9 in the LABA-only arm. There were three instances of patients acquiring pneumonia in hospital – two in the ICS/LABA and one in the LABA-only arm.
There were also two fatal cases of pneumonia – one in each treatment group. Neither were thought to be related to either of the treatments.
These findings are in line with a recent review of the use of ICS for COPD by the European Medicines Agency (EMA/488280/2016), which noted that “overall the benefits of inhaled corticosteroid medicines in treating COPD continue to outweigh their risks and there should be no change to the way in which these medicines are used.”
The European Medicines Agency advised that patients and clinicians need to “be alert for signs and symptoms of pneumonia, bearing in mind that the clinical features of pneumonia overlap with those of a worsening (exacerbation) of the underlying disease.”
Dr. Corradi has received speaker fees from Chiesi Farmaceutici SpA, which funded the FORWARD study, and his coauthors are employees of the company.
LONDON – The benefit of a fixed-dose inhaled corticosteroid (ICS) and long-acting beta-agonist (LABA) combination in reducing exacerbations of chronic obstructive pulmonary disease (COPD) far outweighed any risk for pneumonia in a post hoc analysis of the 48-week FORWARD study.
Although there were 13 extra pneumonia events when a fixed-dose combination of beclometasone diproprionate and formoterol fumarate (Foster, Chiesi Farmaceutici SpA) was used as compared to formoterol fumarate alone, there were 123 fewer moderate to severe COPD exacerbations over a 342-day analysis period.
“Analysis of pneumonia and exacerbation cumulative number of events shows that the number of incident pneumonia remains very small relative to that of moderate to severe exacerbations,” Massimo Corradi, MD, of the University of Parma (Italy), reported at the annual congress of the European Respiratory Society.
Dr. Corradi added that the new analysis confirms that the ICS/LABA combination has a “positive risk-benefit balance over LABA monotherapy, supporting [the argument that] the benefits of adding an ICS to a bronchodilator significantly outweigh potential risks.”
The FORWARD study was a two-arm trial designed to compare the efficacy and safety of fixed-dose treatment with beclometasone diproprionate and formoterol fumarate versus formoterol fumarate alone in 1,199 patients with severe COPD.
For inclusion in the study, patients had to have a post-bronchodilator forced expiratory volume in 1 second below 50% of predicted and a forced vital capacity ratio of less than 0.7. They also had to have a smoking history of 10 pack-years or more, and a history of at least one COPD exacerbation in the previous 12 months that had required treatment or hospitalization (Eur Respir J. 2013;41[1]:12-7).
After a 2-week run-in period, where all patients received a 24-mcg dose of formoterol fumarate, patients were randomized to continue treatment with formoterol fumarate or to receive the fixed-dose combination of beclometasone diproprionate 400 mcg and beclometasone diproprionate 24 mcg for 48 weeks.
A total of 1,186 patients, most of whom were male (69%) with a mean age of 64 years, formed the intention-to-treat population.
Published results (Respir Med. 2014;108[8]:1153-62) showed that the combination of the ICS beclometasone diproprionate and the LABA formoterol fumarate (Chiesi Farmaceutici SpA) was associated with a 28% reduction in the annual rate of moderate to severe exacerbations versus the LABA alone.
The adjusted rate of exacerbations per patient per year was 0.80 in patients treated with the ICS/LABA combination versus 1.12 for those treated with just the LABA, with an adjusted rate ratio of 0.72 (P less than .001).
The published data also showed that pneumonia was reported by 23 patients (3.8%) treated with the ICS/LABA and by 11 (1.8%) treated with the LABA only.
For the new analysis, Dr. Corradi and his coinvestigators looked at the cases of pneumonia and COPD exacerbations in more detail, plotting out the cumulative number of events over time and also characterizing the types of pneumonia in more detail.
All patients had a chest x-ray to confirm the presence of pneumonia, he said, noting that overall there were 35 cases of pneumonia, 24 occurring in patients treated with the fixed-dose beclometasone diproprionate and formoterol fumarate combination and 11 in patients treated only with formoterol fumarate.
Of these cases, 25 required in-hospital treatment – 16 patients in the ICS/LABA arm and 9 in the LABA-only arm. There were three instances of patients acquiring pneumonia in hospital – two in the ICS/LABA and one in the LABA-only arm.
There were also two fatal cases of pneumonia – one in each treatment group. Neither were thought to be related to either of the treatments.
These findings are in line with a recent review of the use of ICS for COPD by the European Medicines Agency (EMA/488280/2016), which noted that “overall the benefits of inhaled corticosteroid medicines in treating COPD continue to outweigh their risks and there should be no change to the way in which these medicines are used.”
The European Medicines Agency advised that patients and clinicians need to “be alert for signs and symptoms of pneumonia, bearing in mind that the clinical features of pneumonia overlap with those of a worsening (exacerbation) of the underlying disease.”
Dr. Corradi has received speaker fees from Chiesi Farmaceutici SpA, which funded the FORWARD study, and his coauthors are employees of the company.
AT THE ERS CONGRESS 2016
Key clinical point: The risk for pneumonia associated with drugs containing inhaled corticosteroids (ICS) is outweighed by the reduction in chronic obstructive pulmonary disease (COPD) exacerbations that can be achieved.
Major finding: There were 13 extra pneumonia events but 123 fewer COPD exacerbations when a fixed dose ICS/long-acting beta-agonist (LABA) combination was used versus a LABA alone.
Data source: Post hoc analysis of the FORWARD study, a multicenter, randomized, double-blind, active-controlled 48-week trial of a fixed-dose ICS/LABA combination versus LABA for reducing exacerbations in 1,186 patients with COPD.
Disclosures: Dr. Corradi has received speaker fees from Chiesi Farmaceutici SpA, which funded the FORWARD study, and his coauthors are employees of the company.
CardioMEMS shows real-world heart failure benefit
ORLANDO – Pulmonary artery pressure monitoring using an implanted device was even more effective for controlling pulmonary artery pressures in 2,000 real-world U.S. heart failure patients than it was in the pivotal trial that led to the device’s regulatory approval.
Data from the first 2,000 U.S. heart failure patients to receive the CardioMEMS pulmonary artery (PA) pressure monitoring device and have at least 6 months of follow-up data since the device received Food and Drug Administration approval in 2014 showed that cumulative PA pressure reductions in these patients during the first 6 months of use averaged 434 mm Hg per patient when compared with their baseline PA pressure when they first received the device. This was nearly threefold better than the average 150–mm Hg cumulative reduction in PA pressure per patient during 6 months of use seen in the CHAMPION trial, Dr. William T. Abraham reported at the annual scientific meeting of the Heart Failure Society of America.
Although this analysis of data from the registry maintained for U.S. patients who receive the CardioMEMS device does not yet include information on how these patients fared clinically, and specifically how often they required rehospitalization for heart failure, the strikingly high level of PA pressure control seen in the first 2,000 U.S. patients bodes well for what the clinical findings will show once they are available.
“In our experience with PA pressure monitoring, there is almost a linear relationship between reduced PA pressures and reduced numbers of events” in the form of rehospitalizations for heart failure, said Dr. Abraham, professor of medicine and director of cardiovascular medicine at Ohio State University in Columbus. Once data on outcomes are analyzed for the registry patients, “I think they will be even better than they were in the trial,” he said in an interview.
The PA pressure data in these initial patients “are very important because they tell us that in general use, clinicians – many of whom are at community hospitals – are very capable of using the CardioMEMS data to control patient pressures, and in CHAMPION we showed that there is a relationship between controlled pressures and improved outcomes,” he said. The findings also help allay a key concern about the potential benefit from implanting a device to monitor PA pressure, which is that clinicians must respond to the information and tweak a patient’s diuretic and vasodilator treatments in order for pressure monitoring to have an effect on heart failure outcomes.
“These data clearly refute that concern,” Dr. Abraham said.
He expressed some surprise that PA pressure control with monitoring was so much more effective in real-world use than in the CHAMPION pivotal trial. “In the trial, it was a paradigm shift to manage heart failure patients based on their PA pressures and not according to their symptoms,” he said. With CardioMEMS pressure monitoring, clinicians are supposed to treat high PA pressure with dose adjustments even if the patient feels okay. The new data suggest that clinicians now using the device “have gotten the message that if you don’t do something with the data the patients won’t improve.”
The registry patients came from 47 states and 427 unique physicians who worked in a range of settings including large and small centers, and academic and nonacademic community centers. The patients averaged 70 years, 40% were women, a third had a left ventricular ejection fraction at or above 40%, and their average PA pressure at the time they had their device implanted was 34.9 mm Hg. This pressure was notably higher than the average 31.6 mm Hg pressure among patients enrolled in CHAMPION, a fact that also helps explain why the registry patients received a larger pressure-reduction benefit: They started from a higher level than the trial patients, and during follow-up, their achieved pressures were always compared back to their high baseline pressures.
The registry patients were also substantially older than the trial patients, who had averaged 62 years, and the registry included substantially more women and more patients with higher ejection fractions. Dr. Abraham did not report data on their New York Heart Association class at entry, but labeling for CardioMEMS specifies that patients should have class III heart failure as well as a recent heart failure hospitalization.
Dr. Abraham’s analysis also showed that the greatest degree of PA pressure control occurred in the patients who began device-based treatment with the highest PA pressures. Nearly half the 2,000 registry patients had an entry PA pressure at or above 35 mm Hg, and over a period of 6 months, they averaged a cumulative 876–mm Hg reduction in their PA pressure relative to their baseline level. The third of patients who began with a PA pressure of 25-34 mm Hg had an average 169–mm Hg cumulative pressure reduction over the 6 month period, and the 18% of patients who began with a PA pressure of less than 25 mm Hg actually had an average cumulative increase in the PA pressure of 163 mm Hg. Target PA pressures are usually in the normal range of 18-25 mm Hg.
The analyses also showed that the impact of PA pressure monitoring on pressure was roughly similar regardless of the left ventricular ejection fraction patients had at baseline, and regardless of their sex.
The registry data were collected by St. Jude, the company that markets the CardioMEMS device. Dr. Abraham is a consultant to St. Jude and was lead investigator for the CHAMPION pivotal trial.
On Twitter @mitchelzoler
ORLANDO – Pulmonary artery pressure monitoring using an implanted device was even more effective for controlling pulmonary artery pressures in 2,000 real-world U.S. heart failure patients than it was in the pivotal trial that led to the device’s regulatory approval.
Data from the first 2,000 U.S. heart failure patients to receive the CardioMEMS pulmonary artery (PA) pressure monitoring device and have at least 6 months of follow-up data since the device received Food and Drug Administration approval in 2014 showed that cumulative PA pressure reductions in these patients during the first 6 months of use averaged 434 mm Hg per patient when compared with their baseline PA pressure when they first received the device. This was nearly threefold better than the average 150–mm Hg cumulative reduction in PA pressure per patient during 6 months of use seen in the CHAMPION trial, Dr. William T. Abraham reported at the annual scientific meeting of the Heart Failure Society of America.
Although this analysis of data from the registry maintained for U.S. patients who receive the CardioMEMS device does not yet include information on how these patients fared clinically, and specifically how often they required rehospitalization for heart failure, the strikingly high level of PA pressure control seen in the first 2,000 U.S. patients bodes well for what the clinical findings will show once they are available.
“In our experience with PA pressure monitoring, there is almost a linear relationship between reduced PA pressures and reduced numbers of events” in the form of rehospitalizations for heart failure, said Dr. Abraham, professor of medicine and director of cardiovascular medicine at Ohio State University in Columbus. Once data on outcomes are analyzed for the registry patients, “I think they will be even better than they were in the trial,” he said in an interview.
The PA pressure data in these initial patients “are very important because they tell us that in general use, clinicians – many of whom are at community hospitals – are very capable of using the CardioMEMS data to control patient pressures, and in CHAMPION we showed that there is a relationship between controlled pressures and improved outcomes,” he said. The findings also help allay a key concern about the potential benefit from implanting a device to monitor PA pressure, which is that clinicians must respond to the information and tweak a patient’s diuretic and vasodilator treatments in order for pressure monitoring to have an effect on heart failure outcomes.
“These data clearly refute that concern,” Dr. Abraham said.
He expressed some surprise that PA pressure control with monitoring was so much more effective in real-world use than in the CHAMPION pivotal trial. “In the trial, it was a paradigm shift to manage heart failure patients based on their PA pressures and not according to their symptoms,” he said. With CardioMEMS pressure monitoring, clinicians are supposed to treat high PA pressure with dose adjustments even if the patient feels okay. The new data suggest that clinicians now using the device “have gotten the message that if you don’t do something with the data the patients won’t improve.”
The registry patients came from 47 states and 427 unique physicians who worked in a range of settings including large and small centers, and academic and nonacademic community centers. The patients averaged 70 years, 40% were women, a third had a left ventricular ejection fraction at or above 40%, and their average PA pressure at the time they had their device implanted was 34.9 mm Hg. This pressure was notably higher than the average 31.6 mm Hg pressure among patients enrolled in CHAMPION, a fact that also helps explain why the registry patients received a larger pressure-reduction benefit: They started from a higher level than the trial patients, and during follow-up, their achieved pressures were always compared back to their high baseline pressures.
The registry patients were also substantially older than the trial patients, who had averaged 62 years, and the registry included substantially more women and more patients with higher ejection fractions. Dr. Abraham did not report data on their New York Heart Association class at entry, but labeling for CardioMEMS specifies that patients should have class III heart failure as well as a recent heart failure hospitalization.
Dr. Abraham’s analysis also showed that the greatest degree of PA pressure control occurred in the patients who began device-based treatment with the highest PA pressures. Nearly half the 2,000 registry patients had an entry PA pressure at or above 35 mm Hg, and over a period of 6 months, they averaged a cumulative 876–mm Hg reduction in their PA pressure relative to their baseline level. The third of patients who began with a PA pressure of 25-34 mm Hg had an average 169–mm Hg cumulative pressure reduction over the 6 month period, and the 18% of patients who began with a PA pressure of less than 25 mm Hg actually had an average cumulative increase in the PA pressure of 163 mm Hg. Target PA pressures are usually in the normal range of 18-25 mm Hg.
The analyses also showed that the impact of PA pressure monitoring on pressure was roughly similar regardless of the left ventricular ejection fraction patients had at baseline, and regardless of their sex.
The registry data were collected by St. Jude, the company that markets the CardioMEMS device. Dr. Abraham is a consultant to St. Jude and was lead investigator for the CHAMPION pivotal trial.
On Twitter @mitchelzoler
ORLANDO – Pulmonary artery pressure monitoring using an implanted device was even more effective for controlling pulmonary artery pressures in 2,000 real-world U.S. heart failure patients than it was in the pivotal trial that led to the device’s regulatory approval.
Data from the first 2,000 U.S. heart failure patients to receive the CardioMEMS pulmonary artery (PA) pressure monitoring device and have at least 6 months of follow-up data since the device received Food and Drug Administration approval in 2014 showed that cumulative PA pressure reductions in these patients during the first 6 months of use averaged 434 mm Hg per patient when compared with their baseline PA pressure when they first received the device. This was nearly threefold better than the average 150–mm Hg cumulative reduction in PA pressure per patient during 6 months of use seen in the CHAMPION trial, Dr. William T. Abraham reported at the annual scientific meeting of the Heart Failure Society of America.
Although this analysis of data from the registry maintained for U.S. patients who receive the CardioMEMS device does not yet include information on how these patients fared clinically, and specifically how often they required rehospitalization for heart failure, the strikingly high level of PA pressure control seen in the first 2,000 U.S. patients bodes well for what the clinical findings will show once they are available.
“In our experience with PA pressure monitoring, there is almost a linear relationship between reduced PA pressures and reduced numbers of events” in the form of rehospitalizations for heart failure, said Dr. Abraham, professor of medicine and director of cardiovascular medicine at Ohio State University in Columbus. Once data on outcomes are analyzed for the registry patients, “I think they will be even better than they were in the trial,” he said in an interview.
The PA pressure data in these initial patients “are very important because they tell us that in general use, clinicians – many of whom are at community hospitals – are very capable of using the CardioMEMS data to control patient pressures, and in CHAMPION we showed that there is a relationship between controlled pressures and improved outcomes,” he said. The findings also help allay a key concern about the potential benefit from implanting a device to monitor PA pressure, which is that clinicians must respond to the information and tweak a patient’s diuretic and vasodilator treatments in order for pressure monitoring to have an effect on heart failure outcomes.
“These data clearly refute that concern,” Dr. Abraham said.
He expressed some surprise that PA pressure control with monitoring was so much more effective in real-world use than in the CHAMPION pivotal trial. “In the trial, it was a paradigm shift to manage heart failure patients based on their PA pressures and not according to their symptoms,” he said. With CardioMEMS pressure monitoring, clinicians are supposed to treat high PA pressure with dose adjustments even if the patient feels okay. The new data suggest that clinicians now using the device “have gotten the message that if you don’t do something with the data the patients won’t improve.”
The registry patients came from 47 states and 427 unique physicians who worked in a range of settings including large and small centers, and academic and nonacademic community centers. The patients averaged 70 years, 40% were women, a third had a left ventricular ejection fraction at or above 40%, and their average PA pressure at the time they had their device implanted was 34.9 mm Hg. This pressure was notably higher than the average 31.6 mm Hg pressure among patients enrolled in CHAMPION, a fact that also helps explain why the registry patients received a larger pressure-reduction benefit: They started from a higher level than the trial patients, and during follow-up, their achieved pressures were always compared back to their high baseline pressures.
The registry patients were also substantially older than the trial patients, who had averaged 62 years, and the registry included substantially more women and more patients with higher ejection fractions. Dr. Abraham did not report data on their New York Heart Association class at entry, but labeling for CardioMEMS specifies that patients should have class III heart failure as well as a recent heart failure hospitalization.
Dr. Abraham’s analysis also showed that the greatest degree of PA pressure control occurred in the patients who began device-based treatment with the highest PA pressures. Nearly half the 2,000 registry patients had an entry PA pressure at or above 35 mm Hg, and over a period of 6 months, they averaged a cumulative 876–mm Hg reduction in their PA pressure relative to their baseline level. The third of patients who began with a PA pressure of 25-34 mm Hg had an average 169–mm Hg cumulative pressure reduction over the 6 month period, and the 18% of patients who began with a PA pressure of less than 25 mm Hg actually had an average cumulative increase in the PA pressure of 163 mm Hg. Target PA pressures are usually in the normal range of 18-25 mm Hg.
The analyses also showed that the impact of PA pressure monitoring on pressure was roughly similar regardless of the left ventricular ejection fraction patients had at baseline, and regardless of their sex.
The registry data were collected by St. Jude, the company that markets the CardioMEMS device. Dr. Abraham is a consultant to St. Jude and was lead investigator for the CHAMPION pivotal trial.
On Twitter @mitchelzoler
AT THE HFSA ANNUAL SCIENTIFIC MEETING
Key clinical point: Registry data from the first 2,000 U.S. heart failure patients who received an implanted pulmonary artery pressure monitor showed a level of pressure control over a period of 6 months nearly triple that seen in the pivotal trial.
Major finding: Cumulative pulmonary artery pressure reductions averaged 434 mm Hg over a period of 6 months, compared with an average reduction of 150 mm Hg in the pivotal trial.
Data source: The first 2,000 U.S. patients who received a CardioMEMS device and were followed for at least 6 months.
Disclosures: The registry data were collected by St. Jude, the company that markets the CardioMEMS device. Dr. Abraham is a consultant to St. Jude and was lead investigator for the CHAMPION pivotal trial.
ACOS definitions under fire
LONDON – A study comparing patient data with six definitions of the Asthma-COPD Overlap Syndrome (ACOS) found only one of the patients analyzed met all definitions. This provoked an animated discussion at the annual congress of the European Respiratory Society about the utility of ACOS as a clinical entity.
Of 864 patients diagnosed with chronic obstructive pulmonary disease (COPD) or asthma drawn from the Netherlands Epidemiology of Obesity cohort (a population-based study with 5,784 patients), 39.1% (338 patients) met at least one of the definitions of ACOS, while 0.1% (one patient) met the criteria for all six definitions.
When this finding was presented, the ERS audience first laughed and then applauded. At the end of the presentation, long lines formed at the microphones. Every comment made was hostile to the concept of ACOS.
“Let us bring ACOS to an honorable death,” said one audience member. His point, reiterated by all who commented subsequently, was that ACOS confuses efforts to treat the underlying respiratory symptoms. Even in those who have both asthma and COPD, the speaker, like other members of the audience, said he considered the diagnosis of ACOS unhelpful.
The six definitions in the study included the latest and just published consensus definition from the ERS (Eur Respir J. 2016;48[3]:664-73). According to the ERS definition, the key features of ACOS are age greater than 40 years, long-term history of asthma (since childhood or early adulthood), and significant exposure to cigarette or biomass smoke.
The other definitions analyzed included a medical history of both asthma and COPD, a self-reported history of both asthma and COPD, and a record of the proportion of a person’s vital capacity that he/she is able to expire in 1 second of forced expiration of less than 0.7 plus a record of fractionated nitric oxide concentration in exhaled breath of greater than or equal to 45 parts per billion.
Although attempted, a Venn diagram that would show overlapping subsets of patients that fell into these definitions “was not possible,” according to Tobias Bonten, MD, University of Leiden, the Netherlands.
Asthma duration was just over 10 years in those identified as having ACOS by medical history alone (registry-based definitions), just over 20 years in those with a medical history and objective evidence of impaired lung function, but about 40 years in those with a self-report of both asthma and COPD.
One area that all groups created by the ACOS definitions did have in common was demographic variables, such as median age, proportion of patients defined as overweight or obese by body mass index, and proportion who were current smokers.
Members of the audience acknowledged the importance of considering the coexistence of asthma and COPD, but expressed skepticism about the value of ACOS as a separate entity in the clinic.
“ACOS is something like the emperor’s new clothes,” one audience member said during the discussion. “It is important to identify asthma patients with obstruction because they have reduced lung function that should be treated more actively, but I find the definition [of ACOS] unnecessary,” he said.
A similar conclusion was drawn in a review article devoted to ACOS published last year (N Engl J Med. 2015;373[13]:1241-9). “It is premature to recommend the designation of ACOS as a disease entity,” the authors wrote.
This is a position widely shared by clinicians, judging from audience comments provoked by this demonstration.
For the sake of time, the moderators were forced to end the discussion with significant lines of clinicians at the microphone.
“It is quite clear that ACOS should die,” said one of the last speakers given a chance to voice an opinion. He suggested that the coexistence of asthma and COPD is something that “quite clearly can happen,” but he objected to definitions he said are unhelpful for clinical care.
Dr. Bonten reported no relevant financial relationships.
LONDON – A study comparing patient data with six definitions of the Asthma-COPD Overlap Syndrome (ACOS) found only one of the patients analyzed met all definitions. This provoked an animated discussion at the annual congress of the European Respiratory Society about the utility of ACOS as a clinical entity.
Of 864 patients diagnosed with chronic obstructive pulmonary disease (COPD) or asthma drawn from the Netherlands Epidemiology of Obesity cohort (a population-based study with 5,784 patients), 39.1% (338 patients) met at least one of the definitions of ACOS, while 0.1% (one patient) met the criteria for all six definitions.
When this finding was presented, the ERS audience first laughed and then applauded. At the end of the presentation, long lines formed at the microphones. Every comment made was hostile to the concept of ACOS.
“Let us bring ACOS to an honorable death,” said one audience member. His point, reiterated by all who commented subsequently, was that ACOS confuses efforts to treat the underlying respiratory symptoms. Even in those who have both asthma and COPD, the speaker, like other members of the audience, said he considered the diagnosis of ACOS unhelpful.
The six definitions in the study included the latest and just published consensus definition from the ERS (Eur Respir J. 2016;48[3]:664-73). According to the ERS definition, the key features of ACOS are age greater than 40 years, long-term history of asthma (since childhood or early adulthood), and significant exposure to cigarette or biomass smoke.
The other definitions analyzed included a medical history of both asthma and COPD, a self-reported history of both asthma and COPD, and a record of the proportion of a person’s vital capacity that he/she is able to expire in 1 second of forced expiration of less than 0.7 plus a record of fractionated nitric oxide concentration in exhaled breath of greater than or equal to 45 parts per billion.
Although attempted, a Venn diagram that would show overlapping subsets of patients that fell into these definitions “was not possible,” according to Tobias Bonten, MD, University of Leiden, the Netherlands.
Asthma duration was just over 10 years in those identified as having ACOS by medical history alone (registry-based definitions), just over 20 years in those with a medical history and objective evidence of impaired lung function, but about 40 years in those with a self-report of both asthma and COPD.
One area that all groups created by the ACOS definitions did have in common was demographic variables, such as median age, proportion of patients defined as overweight or obese by body mass index, and proportion who were current smokers.
Members of the audience acknowledged the importance of considering the coexistence of asthma and COPD, but expressed skepticism about the value of ACOS as a separate entity in the clinic.
“ACOS is something like the emperor’s new clothes,” one audience member said during the discussion. “It is important to identify asthma patients with obstruction because they have reduced lung function that should be treated more actively, but I find the definition [of ACOS] unnecessary,” he said.
A similar conclusion was drawn in a review article devoted to ACOS published last year (N Engl J Med. 2015;373[13]:1241-9). “It is premature to recommend the designation of ACOS as a disease entity,” the authors wrote.
This is a position widely shared by clinicians, judging from audience comments provoked by this demonstration.
For the sake of time, the moderators were forced to end the discussion with significant lines of clinicians at the microphone.
“It is quite clear that ACOS should die,” said one of the last speakers given a chance to voice an opinion. He suggested that the coexistence of asthma and COPD is something that “quite clearly can happen,” but he objected to definitions he said are unhelpful for clinical care.
Dr. Bonten reported no relevant financial relationships.
LONDON – A study comparing patient data with six definitions of the Asthma-COPD Overlap Syndrome (ACOS) found only one of the patients analyzed met all definitions. This provoked an animated discussion at the annual congress of the European Respiratory Society about the utility of ACOS as a clinical entity.
Of 864 patients diagnosed with chronic obstructive pulmonary disease (COPD) or asthma drawn from the Netherlands Epidemiology of Obesity cohort (a population-based study with 5,784 patients), 39.1% (338 patients) met at least one of the definitions of ACOS, while 0.1% (one patient) met the criteria for all six definitions.
When this finding was presented, the ERS audience first laughed and then applauded. At the end of the presentation, long lines formed at the microphones. Every comment made was hostile to the concept of ACOS.
“Let us bring ACOS to an honorable death,” said one audience member. His point, reiterated by all who commented subsequently, was that ACOS confuses efforts to treat the underlying respiratory symptoms. Even in those who have both asthma and COPD, the speaker, like other members of the audience, said he considered the diagnosis of ACOS unhelpful.
The six definitions in the study included the latest and just published consensus definition from the ERS (Eur Respir J. 2016;48[3]:664-73). According to the ERS definition, the key features of ACOS are age greater than 40 years, long-term history of asthma (since childhood or early adulthood), and significant exposure to cigarette or biomass smoke.
The other definitions analyzed included a medical history of both asthma and COPD, a self-reported history of both asthma and COPD, and a record of the proportion of a person’s vital capacity that he/she is able to expire in 1 second of forced expiration of less than 0.7 plus a record of fractionated nitric oxide concentration in exhaled breath of greater than or equal to 45 parts per billion.
Although attempted, a Venn diagram that would show overlapping subsets of patients that fell into these definitions “was not possible,” according to Tobias Bonten, MD, University of Leiden, the Netherlands.
Asthma duration was just over 10 years in those identified as having ACOS by medical history alone (registry-based definitions), just over 20 years in those with a medical history and objective evidence of impaired lung function, but about 40 years in those with a self-report of both asthma and COPD.
One area that all groups created by the ACOS definitions did have in common was demographic variables, such as median age, proportion of patients defined as overweight or obese by body mass index, and proportion who were current smokers.
Members of the audience acknowledged the importance of considering the coexistence of asthma and COPD, but expressed skepticism about the value of ACOS as a separate entity in the clinic.
“ACOS is something like the emperor’s new clothes,” one audience member said during the discussion. “It is important to identify asthma patients with obstruction because they have reduced lung function that should be treated more actively, but I find the definition [of ACOS] unnecessary,” he said.
A similar conclusion was drawn in a review article devoted to ACOS published last year (N Engl J Med. 2015;373[13]:1241-9). “It is premature to recommend the designation of ACOS as a disease entity,” the authors wrote.
This is a position widely shared by clinicians, judging from audience comments provoked by this demonstration.
For the sake of time, the moderators were forced to end the discussion with significant lines of clinicians at the microphone.
“It is quite clear that ACOS should die,” said one of the last speakers given a chance to voice an opinion. He suggested that the coexistence of asthma and COPD is something that “quite clearly can happen,” but he objected to definitions he said are unhelpful for clinical care.
Dr. Bonten reported no relevant financial relationships.
AT THE ERS CONGRESS 2016
Key clinical point: A study comparing current definitions of Asthma-COPD Overlap Syndrome (ACOS) provoked criticism of the very concept.
Major finding: When six definitions of ACOS were compared in a population-based study, only 1 (0.1%) of 864 possible candidates met all criteria of all definitions.
Data source: Population-based cohort study.
Disclosures: Dr. Bonten reported no relevant financial relationships.
CABG reduces cardiovascular mortality in ischemic heart failure regardless of age
ROME – Coronary artery bypass surgery reduces cardiovascular mortality in heart failure patients with ischemic cardiomyopathy to a consistent extent regardless of age at the time of surgery, according to a secondary analysis from the landmark STICH trial, Eric J. Velazquez, MD, reported at the annual congress of the European Society of Cardiology.
“Cardiologists and cardiac surgeons can confidently offer patients CABG in addition to optimal medical therapy with the knowledge that cardiovascular mortality is reduced by CABG to a similar extent across all age groups in this trial through 10 years of follow-up,” said Dr. Velazquez, professor of medicine at Duke University in Durham, N.C.
However, that’s only part of the story. Cardiovascular mortality was a secondary endpoint in STICH (Surgical Treatment for Ischemic Heart Failure). The primary endpoint was all-cause mortality. And CABG’s impact on all-cause mortality was diminished in older STICH participants because of their greater comorbidity burden and the competing risk of noncardiovascular death, he added.
The take-home message is that cardiologists and heart surgeons need to carefully assess competing mortality risks before pursuing CABG in older patients, according to Dr. Velazquez.
Session cochair Kim A. Williams, MD, professor and chief of cardiology at Rush University Medical Center in Chicago, posed a direct question: “Is there an age cutoff for your group for bypass surgery?”
No, Dr. Velazquez replied. He pointed out that cardiovascular mortality remained the No. 1 cause of mortality across all age groups.
“If the expectation is that the major cause of fatal events is going to be cardiovascular, and CABG plus medical therapy reduces that risk consistently regardless of age, we think that there really is no particular age cutoff. There is a point at which the noncardiovascular risk predominates, but in the population we studied we did not see that point,” Dr. Velazquez added.
STICH was a 22-nation trial in which 1,212 patients with a left ventricular ejection fraction of 35% or less and coronary artery disease amenable to CABG were randomized to CABG plus optimal medical therapy or optimal medical therapy alone and followed for a median of 9.8 years (JACC Heart Fail. 2013;1[5]:400-8). For purposes of this secondary analysis, participants were divided into quartiles according to baseline age: Quartile 1 patients were up to 54 years old; quartile 2 were ages 55-60; quartile 3 were ages 61-67; and quartile 4 were ages 68 and up.
Older subjects had more comorbidities. All-cause mortality was significantly higher in older than younger patients: for CABG, 68% vs. 48% in quartiles 4 and 1, respectively; for medical therapy, 79% vs. 60% in the same two quartiles. In contrast, cardiovascular mortality did not differ significantly by age: It was 39% in quartile 4 and 35% in quartile 1 in the CABG group, and 53%, compared with 49%, in medically managed patients in quartiles 4 and 1.
For the secondary composite endpoint of all-cause mortality or cardiovascular hospitalization, the benefit of CABG plus medical management over medical management alone was significantly greater in younger than in older patients.
The rate of noncardiovascular mortality was 5.8% in quartiles 1 and 2, then leapt to 14.7% in quartile 3 and 21.1% in quartile 4.
Although the main focus of Dr. Velazquez’s presentation was the impact of CABG with advancing age, he said he found an important lesson in the younger population as well.
“We saw roughly a 40% relative risk reduction in all-cause mortality with CABG in the youngest quartile, compared with the three older groups. My interpretation of that data is that it’s probably not appropriate to avoid CABG in favor of another strategy in a younger patient when you see this kind of mortality benefit,” the cardiologist said.
One limitation of the STICH analysis, said session cochair Stephan Achenbach, MD, is that the study population was relatively young overall. The oldest patients in STICH were roughly the same age as the average patients undergoing CABG for left ventricular systolic dysfunction today at most centers, according to Dr. Achenbach, professor of cardiology at the University of Erlangen-Nuremberg (Germany).
Dr. Velazquez agreed. “I can’t speak as to whether these trial results would apply to the very elderly, patients age 90 and above,” he said.
Simultaneously with the presentation , the new STICH analysis was published online (Circulation. 2016 Aug 29. doi: 10.1161/CIRCULATIONAHA.116.024800).
STICH was funded by the National Institutes of Health. Dr. Velazquez reported having no relevant financial conflicts.
ROME – Coronary artery bypass surgery reduces cardiovascular mortality in heart failure patients with ischemic cardiomyopathy to a consistent extent regardless of age at the time of surgery, according to a secondary analysis from the landmark STICH trial, Eric J. Velazquez, MD, reported at the annual congress of the European Society of Cardiology.
“Cardiologists and cardiac surgeons can confidently offer patients CABG in addition to optimal medical therapy with the knowledge that cardiovascular mortality is reduced by CABG to a similar extent across all age groups in this trial through 10 years of follow-up,” said Dr. Velazquez, professor of medicine at Duke University in Durham, N.C.
However, that’s only part of the story. Cardiovascular mortality was a secondary endpoint in STICH (Surgical Treatment for Ischemic Heart Failure). The primary endpoint was all-cause mortality. And CABG’s impact on all-cause mortality was diminished in older STICH participants because of their greater comorbidity burden and the competing risk of noncardiovascular death, he added.
The take-home message is that cardiologists and heart surgeons need to carefully assess competing mortality risks before pursuing CABG in older patients, according to Dr. Velazquez.
Session cochair Kim A. Williams, MD, professor and chief of cardiology at Rush University Medical Center in Chicago, posed a direct question: “Is there an age cutoff for your group for bypass surgery?”
No, Dr. Velazquez replied. He pointed out that cardiovascular mortality remained the No. 1 cause of mortality across all age groups.
“If the expectation is that the major cause of fatal events is going to be cardiovascular, and CABG plus medical therapy reduces that risk consistently regardless of age, we think that there really is no particular age cutoff. There is a point at which the noncardiovascular risk predominates, but in the population we studied we did not see that point,” Dr. Velazquez added.
STICH was a 22-nation trial in which 1,212 patients with a left ventricular ejection fraction of 35% or less and coronary artery disease amenable to CABG were randomized to CABG plus optimal medical therapy or optimal medical therapy alone and followed for a median of 9.8 years (JACC Heart Fail. 2013;1[5]:400-8). For purposes of this secondary analysis, participants were divided into quartiles according to baseline age: Quartile 1 patients were up to 54 years old; quartile 2 were ages 55-60; quartile 3 were ages 61-67; and quartile 4 were ages 68 and up.
Older subjects had more comorbidities. All-cause mortality was significantly higher in older than younger patients: for CABG, 68% vs. 48% in quartiles 4 and 1, respectively; for medical therapy, 79% vs. 60% in the same two quartiles. In contrast, cardiovascular mortality did not differ significantly by age: It was 39% in quartile 4 and 35% in quartile 1 in the CABG group, and 53%, compared with 49%, in medically managed patients in quartiles 4 and 1.
For the secondary composite endpoint of all-cause mortality or cardiovascular hospitalization, the benefit of CABG plus medical management over medical management alone was significantly greater in younger than in older patients.
The rate of noncardiovascular mortality was 5.8% in quartiles 1 and 2, then leapt to 14.7% in quartile 3 and 21.1% in quartile 4.
Although the main focus of Dr. Velazquez’s presentation was the impact of CABG with advancing age, he said he found an important lesson in the younger population as well.
“We saw roughly a 40% relative risk reduction in all-cause mortality with CABG in the youngest quartile, compared with the three older groups. My interpretation of that data is that it’s probably not appropriate to avoid CABG in favor of another strategy in a younger patient when you see this kind of mortality benefit,” the cardiologist said.
One limitation of the STICH analysis, said session cochair Stephan Achenbach, MD, is that the study population was relatively young overall. The oldest patients in STICH were roughly the same age as the average patients undergoing CABG for left ventricular systolic dysfunction today at most centers, according to Dr. Achenbach, professor of cardiology at the University of Erlangen-Nuremberg (Germany).
Dr. Velazquez agreed. “I can’t speak as to whether these trial results would apply to the very elderly, patients age 90 and above,” he said.
Simultaneously with the presentation , the new STICH analysis was published online (Circulation. 2016 Aug 29. doi: 10.1161/CIRCULATIONAHA.116.024800).
STICH was funded by the National Institutes of Health. Dr. Velazquez reported having no relevant financial conflicts.
ROME – Coronary artery bypass surgery reduces cardiovascular mortality in heart failure patients with ischemic cardiomyopathy to a consistent extent regardless of age at the time of surgery, according to a secondary analysis from the landmark STICH trial, Eric J. Velazquez, MD, reported at the annual congress of the European Society of Cardiology.
“Cardiologists and cardiac surgeons can confidently offer patients CABG in addition to optimal medical therapy with the knowledge that cardiovascular mortality is reduced by CABG to a similar extent across all age groups in this trial through 10 years of follow-up,” said Dr. Velazquez, professor of medicine at Duke University in Durham, N.C.
However, that’s only part of the story. Cardiovascular mortality was a secondary endpoint in STICH (Surgical Treatment for Ischemic Heart Failure). The primary endpoint was all-cause mortality. And CABG’s impact on all-cause mortality was diminished in older STICH participants because of their greater comorbidity burden and the competing risk of noncardiovascular death, he added.
The take-home message is that cardiologists and heart surgeons need to carefully assess competing mortality risks before pursuing CABG in older patients, according to Dr. Velazquez.
Session cochair Kim A. Williams, MD, professor and chief of cardiology at Rush University Medical Center in Chicago, posed a direct question: “Is there an age cutoff for your group for bypass surgery?”
No, Dr. Velazquez replied. He pointed out that cardiovascular mortality remained the No. 1 cause of mortality across all age groups.
“If the expectation is that the major cause of fatal events is going to be cardiovascular, and CABG plus medical therapy reduces that risk consistently regardless of age, we think that there really is no particular age cutoff. There is a point at which the noncardiovascular risk predominates, but in the population we studied we did not see that point,” Dr. Velazquez added.
STICH was a 22-nation trial in which 1,212 patients with a left ventricular ejection fraction of 35% or less and coronary artery disease amenable to CABG were randomized to CABG plus optimal medical therapy or optimal medical therapy alone and followed for a median of 9.8 years (JACC Heart Fail. 2013;1[5]:400-8). For purposes of this secondary analysis, participants were divided into quartiles according to baseline age: Quartile 1 patients were up to 54 years old; quartile 2 were ages 55-60; quartile 3 were ages 61-67; and quartile 4 were ages 68 and up.
Older subjects had more comorbidities. All-cause mortality was significantly higher in older than younger patients: for CABG, 68% vs. 48% in quartiles 4 and 1, respectively; for medical therapy, 79% vs. 60% in the same two quartiles. In contrast, cardiovascular mortality did not differ significantly by age: It was 39% in quartile 4 and 35% in quartile 1 in the CABG group, and 53%, compared with 49%, in medically managed patients in quartiles 4 and 1.
For the secondary composite endpoint of all-cause mortality or cardiovascular hospitalization, the benefit of CABG plus medical management over medical management alone was significantly greater in younger than in older patients.
The rate of noncardiovascular mortality was 5.8% in quartiles 1 and 2, then leapt to 14.7% in quartile 3 and 21.1% in quartile 4.
Although the main focus of Dr. Velazquez’s presentation was the impact of CABG with advancing age, he said he found an important lesson in the younger population as well.
“We saw roughly a 40% relative risk reduction in all-cause mortality with CABG in the youngest quartile, compared with the three older groups. My interpretation of that data is that it’s probably not appropriate to avoid CABG in favor of another strategy in a younger patient when you see this kind of mortality benefit,” the cardiologist said.
One limitation of the STICH analysis, said session cochair Stephan Achenbach, MD, is that the study population was relatively young overall. The oldest patients in STICH were roughly the same age as the average patients undergoing CABG for left ventricular systolic dysfunction today at most centers, according to Dr. Achenbach, professor of cardiology at the University of Erlangen-Nuremberg (Germany).
Dr. Velazquez agreed. “I can’t speak as to whether these trial results would apply to the very elderly, patients age 90 and above,” he said.
Simultaneously with the presentation , the new STICH analysis was published online (Circulation. 2016 Aug 29. doi: 10.1161/CIRCULATIONAHA.116.024800).
STICH was funded by the National Institutes of Health. Dr. Velazquez reported having no relevant financial conflicts.
AT THE ESC CONGRESS 2016
Key clinical point: There should be no age cutoff in offering CABG to older patients with ischemic heart failure.
Major finding: CABG provided an absolute 14.4% reduction in cardiovascular mortality, compared with medical management, in both the youngest and oldest quartiles of patients with heart failure due to ischemic cardiomyopathy.
Data source: A secondary analysis of the STICH trial, in which 1,212 heart failure patients with ischemic cardiomyopathy were randomized to CABG plus medical therapy or medical therapy alone and followed for nearly 10 years.
Disclosures: The study was funded by the National Institutes of Health. The presenter reported having no relevant financial conflicts.