User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Patient Navigators for Serious Illnesses Can Now Bill Under New Medicare Codes
In a move that acknowledges the gauntlet the US health system poses for people facing serious and fatal illnesses, Medicare will pay for a new class of workers to help patients manage treatments for conditions like cancer and heart failure.
The 2024 Medicare physician fee schedule includes new billing codes, including G0023, to pay for 60 minutes a month of care coordination by certified or trained auxiliary personnel working under the direction of a clinician.
A diagnosis of cancer or another serious illness takes a toll beyond the physical effects of the disease. Patients often scramble to make adjustments in family and work schedules to manage treatment, said Samyukta Mullangi, MD, MBA, medical director of oncology at Thyme Care, a Nashville, Tennessee–based firm that provides navigation and coordination services to oncology practices and insurers.
“It just really does create a bit of a pressure cooker for patients,” Dr. Mullangi told this news organization.
Medicare has for many years paid for medical professionals to help patients cope with the complexities of disease, such as chronic care management (CCM) provided by physicians, nurses, and physician assistants.
The new principal illness navigation (PIN) payments are intended to pay for work that to date typically has been done by people without medical degrees, including those involved in peer support networks and community health programs. The US Centers for Medicare and Medicaid Services(CMS) expects these navigators will undergo training and work under the supervision of clinicians.
The new navigators may coordinate care transitions between medical settings, follow up with patients after emergency department (ED) visits, or communicate with skilled nursing facilities regarding the psychosocial needs and functional deficits of a patient, among other functions.
CMS expects the new navigators may:
- Conduct assessments to understand a patient’s life story, strengths, needs, goals, preferences, and desired outcomes, including understanding cultural and linguistic factors.
- Provide support to accomplish the clinician’s treatment plan.
- Coordinate the receipt of needed services from healthcare facilities, home- and community-based service providers, and caregivers.
Peers as Navigators
The new navigators can be former patients who have undergone similar treatments for serious diseases, CMS said. This approach sets the new program apart from other care management services Medicare already covers, program officials wrote in the 2024 physician fee schedule.
“For some conditions, patients are best able to engage with the healthcare system and access care if they have assistance from a single, dedicated individual who has ‘lived experience,’ ” according to the rule.
The agency has taken a broad initial approach in defining what kinds of illnesses a patient may have to qualify for services. Patients must have a serious condition that is expected to last at least 3 months, such as cancer, heart failure, or substance use disorder.
But those without a definitive diagnosis may also qualify to receive navigator services.
In the rule, CMS cited a case in which a CT scan identified a suspicious mass in a patient’s colon. A clinician might decide this person would benefit from navigation services due to the potential risks for an undiagnosed illness.
“Regardless of the definitive diagnosis of the mass, presence of a colonic mass for that patient may be a serious high-risk condition that could, for example, cause obstruction and lead the patient to present to the emergency department, as well as be potentially indicative of an underlying life-threatening illness such as colon cancer,” CMS wrote in the rule.
Navigators often start their work when cancer patients are screened and guide them through initial diagnosis, potential surgery, radiation, or chemotherapy, said Sharon Gentry, MSN, RN, a former nurse navigator who is now the editor in chief of the Journal of the Academy of Oncology Nurse & Patient Navigators.
The navigators are meant to be a trusted and continual presence for patients, who otherwise might be left to start anew in finding help at each phase of care.
The navigators “see the whole picture. They see the whole journey the patient takes, from pre-diagnosis all the way through diagnosis care out through survival,” Ms. Gentry said.
Gaining a special Medicare payment for these kinds of services will elevate this work, she said.
Many newer drugs can target specific mechanisms and proteins of cancer. Often, oncology treatment involves testing to find out if mutations are allowing the cancer cells to evade a patient’s immune system.
Checking these biomarkers takes time, however. Patients sometimes become frustrated because they are anxious to begin treatment. Patients may receive inaccurate information from friends or family who went through treatment previously. Navigators can provide knowledge on the current state of care for a patient’s disease, helping them better manage anxieties.
“You have to explain to them that things have changed since the guy you drink coffee with was diagnosed with cancer, and there may be a drug that could target that,” Ms. Gentry said.
Potential Challenges
Initial uptake of the new PIN codes may be slow going, however, as clinicians and health systems may already use well-established codes. These include CCM and principal care management services, which may pay higher rates, Mullangi said.
“There might be sensitivity around not wanting to cannibalize existing programs with a new program,” Dr. Mullangi said.
In addition, many patients will have a copay for the services of principal illness navigators, Dr. Mullangi said.
While many patients have additional insurance that would cover the service, not all do. People with traditional Medicare coverage can sometimes pay 20% of the cost of some medical services.
“I think that may give patients pause, particularly if they’re already feeling the financial burden of a cancer treatment journey,” Dr. Mullangi said.
Pay rates for PIN services involve calculations of regional price differences, which are posted publicly by CMS, and potential added fees for services provided by hospital-affiliated organizations.
Consider payments for code G0023, covering 60 minutes of principal navigation services provided in a single month.
A set reimbursement for patients cared for in independent medical practices exists, with variation for local costs. Medicare’s non-facility price for G0023 would be $102.41 in some parts of Silicon Valley in California, including San Jose. In Arkansas, where costs are lower, reimbursement would be $73.14 for this same service.
Patients who get services covered by code G0023 in independent medical practices would have monthly copays of about $15-$20, depending on where they live.
The tab for patients tends to be higher for these same services if delivered through a medical practice owned by a hospital, as this would trigger the addition of facility fees to the payments made to cover the services. Facility fees are difficult for the public to ascertain before getting a treatment or service.
Dr. Mullangi and Ms. Gentry reported no relevant financial disclosures outside of their employers.
A version of this article first appeared on Medscape.com.
In a move that acknowledges the gauntlet the US health system poses for people facing serious and fatal illnesses, Medicare will pay for a new class of workers to help patients manage treatments for conditions like cancer and heart failure.
The 2024 Medicare physician fee schedule includes new billing codes, including G0023, to pay for 60 minutes a month of care coordination by certified or trained auxiliary personnel working under the direction of a clinician.
A diagnosis of cancer or another serious illness takes a toll beyond the physical effects of the disease. Patients often scramble to make adjustments in family and work schedules to manage treatment, said Samyukta Mullangi, MD, MBA, medical director of oncology at Thyme Care, a Nashville, Tennessee–based firm that provides navigation and coordination services to oncology practices and insurers.
“It just really does create a bit of a pressure cooker for patients,” Dr. Mullangi told this news organization.
Medicare has for many years paid for medical professionals to help patients cope with the complexities of disease, such as chronic care management (CCM) provided by physicians, nurses, and physician assistants.
The new principal illness navigation (PIN) payments are intended to pay for work that to date typically has been done by people without medical degrees, including those involved in peer support networks and community health programs. The US Centers for Medicare and Medicaid Services(CMS) expects these navigators will undergo training and work under the supervision of clinicians.
The new navigators may coordinate care transitions between medical settings, follow up with patients after emergency department (ED) visits, or communicate with skilled nursing facilities regarding the psychosocial needs and functional deficits of a patient, among other functions.
CMS expects the new navigators may:
- Conduct assessments to understand a patient’s life story, strengths, needs, goals, preferences, and desired outcomes, including understanding cultural and linguistic factors.
- Provide support to accomplish the clinician’s treatment plan.
- Coordinate the receipt of needed services from healthcare facilities, home- and community-based service providers, and caregivers.
Peers as Navigators
The new navigators can be former patients who have undergone similar treatments for serious diseases, CMS said. This approach sets the new program apart from other care management services Medicare already covers, program officials wrote in the 2024 physician fee schedule.
“For some conditions, patients are best able to engage with the healthcare system and access care if they have assistance from a single, dedicated individual who has ‘lived experience,’ ” according to the rule.
The agency has taken a broad initial approach in defining what kinds of illnesses a patient may have to qualify for services. Patients must have a serious condition that is expected to last at least 3 months, such as cancer, heart failure, or substance use disorder.
But those without a definitive diagnosis may also qualify to receive navigator services.
In the rule, CMS cited a case in which a CT scan identified a suspicious mass in a patient’s colon. A clinician might decide this person would benefit from navigation services due to the potential risks for an undiagnosed illness.
“Regardless of the definitive diagnosis of the mass, presence of a colonic mass for that patient may be a serious high-risk condition that could, for example, cause obstruction and lead the patient to present to the emergency department, as well as be potentially indicative of an underlying life-threatening illness such as colon cancer,” CMS wrote in the rule.
Navigators often start their work when cancer patients are screened and guide them through initial diagnosis, potential surgery, radiation, or chemotherapy, said Sharon Gentry, MSN, RN, a former nurse navigator who is now the editor in chief of the Journal of the Academy of Oncology Nurse & Patient Navigators.
The navigators are meant to be a trusted and continual presence for patients, who otherwise might be left to start anew in finding help at each phase of care.
The navigators “see the whole picture. They see the whole journey the patient takes, from pre-diagnosis all the way through diagnosis care out through survival,” Ms. Gentry said.
Gaining a special Medicare payment for these kinds of services will elevate this work, she said.
Many newer drugs can target specific mechanisms and proteins of cancer. Often, oncology treatment involves testing to find out if mutations are allowing the cancer cells to evade a patient’s immune system.
Checking these biomarkers takes time, however. Patients sometimes become frustrated because they are anxious to begin treatment. Patients may receive inaccurate information from friends or family who went through treatment previously. Navigators can provide knowledge on the current state of care for a patient’s disease, helping them better manage anxieties.
“You have to explain to them that things have changed since the guy you drink coffee with was diagnosed with cancer, and there may be a drug that could target that,” Ms. Gentry said.
Potential Challenges
Initial uptake of the new PIN codes may be slow going, however, as clinicians and health systems may already use well-established codes. These include CCM and principal care management services, which may pay higher rates, Mullangi said.
“There might be sensitivity around not wanting to cannibalize existing programs with a new program,” Dr. Mullangi said.
In addition, many patients will have a copay for the services of principal illness navigators, Dr. Mullangi said.
While many patients have additional insurance that would cover the service, not all do. People with traditional Medicare coverage can sometimes pay 20% of the cost of some medical services.
“I think that may give patients pause, particularly if they’re already feeling the financial burden of a cancer treatment journey,” Dr. Mullangi said.
Pay rates for PIN services involve calculations of regional price differences, which are posted publicly by CMS, and potential added fees for services provided by hospital-affiliated organizations.
Consider payments for code G0023, covering 60 minutes of principal navigation services provided in a single month.
A set reimbursement for patients cared for in independent medical practices exists, with variation for local costs. Medicare’s non-facility price for G0023 would be $102.41 in some parts of Silicon Valley in California, including San Jose. In Arkansas, where costs are lower, reimbursement would be $73.14 for this same service.
Patients who get services covered by code G0023 in independent medical practices would have monthly copays of about $15-$20, depending on where they live.
The tab for patients tends to be higher for these same services if delivered through a medical practice owned by a hospital, as this would trigger the addition of facility fees to the payments made to cover the services. Facility fees are difficult for the public to ascertain before getting a treatment or service.
Dr. Mullangi and Ms. Gentry reported no relevant financial disclosures outside of their employers.
A version of this article first appeared on Medscape.com.
In a move that acknowledges the gauntlet the US health system poses for people facing serious and fatal illnesses, Medicare will pay for a new class of workers to help patients manage treatments for conditions like cancer and heart failure.
The 2024 Medicare physician fee schedule includes new billing codes, including G0023, to pay for 60 minutes a month of care coordination by certified or trained auxiliary personnel working under the direction of a clinician.
A diagnosis of cancer or another serious illness takes a toll beyond the physical effects of the disease. Patients often scramble to make adjustments in family and work schedules to manage treatment, said Samyukta Mullangi, MD, MBA, medical director of oncology at Thyme Care, a Nashville, Tennessee–based firm that provides navigation and coordination services to oncology practices and insurers.
“It just really does create a bit of a pressure cooker for patients,” Dr. Mullangi told this news organization.
Medicare has for many years paid for medical professionals to help patients cope with the complexities of disease, such as chronic care management (CCM) provided by physicians, nurses, and physician assistants.
The new principal illness navigation (PIN) payments are intended to pay for work that to date typically has been done by people without medical degrees, including those involved in peer support networks and community health programs. The US Centers for Medicare and Medicaid Services(CMS) expects these navigators will undergo training and work under the supervision of clinicians.
The new navigators may coordinate care transitions between medical settings, follow up with patients after emergency department (ED) visits, or communicate with skilled nursing facilities regarding the psychosocial needs and functional deficits of a patient, among other functions.
CMS expects the new navigators may:
- Conduct assessments to understand a patient’s life story, strengths, needs, goals, preferences, and desired outcomes, including understanding cultural and linguistic factors.
- Provide support to accomplish the clinician’s treatment plan.
- Coordinate the receipt of needed services from healthcare facilities, home- and community-based service providers, and caregivers.
Peers as Navigators
The new navigators can be former patients who have undergone similar treatments for serious diseases, CMS said. This approach sets the new program apart from other care management services Medicare already covers, program officials wrote in the 2024 physician fee schedule.
“For some conditions, patients are best able to engage with the healthcare system and access care if they have assistance from a single, dedicated individual who has ‘lived experience,’ ” according to the rule.
The agency has taken a broad initial approach in defining what kinds of illnesses a patient may have to qualify for services. Patients must have a serious condition that is expected to last at least 3 months, such as cancer, heart failure, or substance use disorder.
But those without a definitive diagnosis may also qualify to receive navigator services.
In the rule, CMS cited a case in which a CT scan identified a suspicious mass in a patient’s colon. A clinician might decide this person would benefit from navigation services due to the potential risks for an undiagnosed illness.
“Regardless of the definitive diagnosis of the mass, presence of a colonic mass for that patient may be a serious high-risk condition that could, for example, cause obstruction and lead the patient to present to the emergency department, as well as be potentially indicative of an underlying life-threatening illness such as colon cancer,” CMS wrote in the rule.
Navigators often start their work when cancer patients are screened and guide them through initial diagnosis, potential surgery, radiation, or chemotherapy, said Sharon Gentry, MSN, RN, a former nurse navigator who is now the editor in chief of the Journal of the Academy of Oncology Nurse & Patient Navigators.
The navigators are meant to be a trusted and continual presence for patients, who otherwise might be left to start anew in finding help at each phase of care.
The navigators “see the whole picture. They see the whole journey the patient takes, from pre-diagnosis all the way through diagnosis care out through survival,” Ms. Gentry said.
Gaining a special Medicare payment for these kinds of services will elevate this work, she said.
Many newer drugs can target specific mechanisms and proteins of cancer. Often, oncology treatment involves testing to find out if mutations are allowing the cancer cells to evade a patient’s immune system.
Checking these biomarkers takes time, however. Patients sometimes become frustrated because they are anxious to begin treatment. Patients may receive inaccurate information from friends or family who went through treatment previously. Navigators can provide knowledge on the current state of care for a patient’s disease, helping them better manage anxieties.
“You have to explain to them that things have changed since the guy you drink coffee with was diagnosed with cancer, and there may be a drug that could target that,” Ms. Gentry said.
Potential Challenges
Initial uptake of the new PIN codes may be slow going, however, as clinicians and health systems may already use well-established codes. These include CCM and principal care management services, which may pay higher rates, Mullangi said.
“There might be sensitivity around not wanting to cannibalize existing programs with a new program,” Dr. Mullangi said.
In addition, many patients will have a copay for the services of principal illness navigators, Dr. Mullangi said.
While many patients have additional insurance that would cover the service, not all do. People with traditional Medicare coverage can sometimes pay 20% of the cost of some medical services.
“I think that may give patients pause, particularly if they’re already feeling the financial burden of a cancer treatment journey,” Dr. Mullangi said.
Pay rates for PIN services involve calculations of regional price differences, which are posted publicly by CMS, and potential added fees for services provided by hospital-affiliated organizations.
Consider payments for code G0023, covering 60 minutes of principal navigation services provided in a single month.
A set reimbursement for patients cared for in independent medical practices exists, with variation for local costs. Medicare’s non-facility price for G0023 would be $102.41 in some parts of Silicon Valley in California, including San Jose. In Arkansas, where costs are lower, reimbursement would be $73.14 for this same service.
Patients who get services covered by code G0023 in independent medical practices would have monthly copays of about $15-$20, depending on where they live.
The tab for patients tends to be higher for these same services if delivered through a medical practice owned by a hospital, as this would trigger the addition of facility fees to the payments made to cover the services. Facility fees are difficult for the public to ascertain before getting a treatment or service.
Dr. Mullangi and Ms. Gentry reported no relevant financial disclosures outside of their employers.
A version of this article first appeared on Medscape.com.

As GLP-1 Use Surges, Clinicians Weigh Benefits and Risks
Rates of overweight and obesity have more than doubled in the United States during the last three decades, according to a new analysis. By 2050, it’s anticipated that 213 million adults (age, > 25 years) and 43 million children and adolescents will have overweight or obesity. The results led authors of a study to describe obesity as having reached a “crisis point” requiring urgent action and interventions.
Are glucagon-like peptide 1 receptor agonists (GLP-1 RAs), originally developed and prescribed for diabetes and now approved for weight loss, the answer?
Their popularity is certainly surging. Between the last 6 months of 2022 vs the last 6 months of 2024, the number of patients prescribed GLP-1 RAs increased by 132.6%. This is also reflected in a shift in public awareness, with a recent survey of US adults finding that 32% of respondents had heard “a lot” about these drugs, up from 19% in 2023.
GLP-1 RAs (including tirzepatide, which targets not only the GLP-1 receptor but also the glucose-dependent insulinotropic polypeptide receptor) have shown efficacy in weight loss. A 2022 review and meta-analysis of 22 trials (17,183 patients) found that 50.2% and 17.5% of those treated with GLP-1 RAs had a ≥ 5% and ≥ 10% weight loss, respectively, compared with placebo. A 2023 review of 41 trials (15,135 patients) found that compared with controls, GLP-1 RAs significantly reduced body weight, body mass index, waist circumference, and waist-to-hip ratio.
“GLP-1 RAs are great medications,” Andres Acosta, MD, PhD, director of the Precision Medicine for Obesity Laboratory, Mayo Clinic, Rochester, Minnesota, told Medscape Medical News. “We’ve been using them for almost two decades. But now there’s excitement about their utility in treating obesity.”
Treating the Four Categories of Obesity
Daniel Drucker, MD, senior investigator at the Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada, is a pioneer in diabetes treatment and particularly in the development of GLP-1 RAs. Drucker told Medscape Medical News that despite the efficacy and enormous potential of GLP-1 RAs, “we know some people don’t lose much weight when taking these medicines and others don’t feel well and can’t take them.”
The number of individuals who don’t respond to or aren’t able to tolerate GLP-1 RAs “might be small — less than 10% of people who try to take them — but we don’t fully understand the differences in response across different individuals,” Drucker said.
Acosta agreed, adding that it’s “essential for us to identify who will be the best responders, as we do with medications for other conditions, such as cancer and cardiovascular disease.”
Acosta’s group has spent more than a decade engaged in efforts to identify unique characteristics among patients with obesity and has succeeded in identifying four obesity phenotypes.
“What matters in the space of GLP-1 is that using this classification, we can identify the best responders and those who don’t respond.”
The first phenotype, described as “Hungry Gut” (HG), includes patients with abnormal postprandial satiety. “Although they may be satiated at the end of a meal, they have accelerated gastric emptying and therefore feel hungry between meals and want to keep eating,” he said.
There are also patients who experience abnormal satiety during meals. According to Acosta, these are the patients who will return to the table for second and third helpings. “They don’t feel full and continue to eat more and more in a single sitting” — a phenomenon referred to as “Hungry Brain.”
The third phenotype — “Emotional Hunger” — consists of people who are “hedonic” about food or engage in emotional eating behavior, whereas in the fourth group, people have “an abnormal metabolism in which they don’t burn enough calories. They have an inefficient metabolic rate.” This latter phenomenon is called “Slow Burn.”
Acosta and colleagues randomized 312 patients attending a weight management center to phenotype-guided or non–phenotype-guided treatment with anti-obesity medications (phentermine, phentermine/topiramate, bupropion/naltrexone, lorcaserin, and liraglutide). The phenotype-guided approach was associated with a 1.75-fold greater weight loss after 1 year than the non–phenotype-guided approach (mean weight loss, 15.9% vs 9.0%, respectively).
GLP-1 RAs: Not One-Size-Fits-All
Acosta’s group has developed a genetic test that uses patients’ saliva to identify their obesity phenotype, with the aim of predicting the best responders to GLP-1 RAs. The test, MyPhenome genetic obesity test, is licensed by Acosta’s lab and available through Phenomix Sciences.
Acosta and colleagues presented their findings at the American Gastroenterological Association’s 2024 annual meeting regarding a machine-learning gene risk score (ML-GRS) they developed to predict HG, based on saliva and blood samples. Their genetic studies generated a ML-GRS that classified participants with obesity along a continuum from “HG Positive” (HG+) to “HG Negative” (HG−). Compared with the HG− participants, those who were HG+ had superior total body weight loss with semaglutide at 9 and 12 months. When used to predict response, the ML-GRS had an area under the curve of 0.76 (P = .04) and a positive predictive value of 0.95.
According to Acosta, HG+ patients are “the best responders to the GLP-1 RAs, although we don’t yet understand the mechanism of why they have the phenomenon of abnormal postprandial satiety. It may be an abnormal genetic pathway or abnormal secretion of GLP-1. More studies are needed.”
He noted that GLP-1 RAs “might also be helpful with the second [Hungry Brain] category, but these patients do better with phentermine-topiramate,” as demonstrated in a 2023 study conducted by Acosta and colleagues.
His group has also studied which lifestyle interventions are most effective for each phenotype. “When a unique lifestyle intervention targeting each phenotype was applied, patients lost more weight and had greater metabolic improvement,” he reported.
“Treating obesity no longer needs to be trial-and-error, but should be done using precision medicine because one size doesn’t fit all,” Acosta said.
Concerning Side Effects
The popular media has featured stories about individuals who took GLP-1 RAs for weight loss and experienced serious side effects, including a recent account of a British nurse who died after taking tirzepatide. As reported by the BBC, the nurse’s death certificate listed multiple organ failure, septic shock, and pancreatitis as the immediate causes of death, with the “use of prescribed tirzepatide” recorded as a contributing factor. The report went on to note that there were 23 suspected deaths in the United Kingdom tied to semaglutide since 2019.
Beyond brand-name products, there are also risks associated with GLP-1 RAs manufactured by compounding pharmacies. In early November, CNN reported that compounded semaglutide has been linked to at least 10 deaths. Because of a prior shortage of tirzepatide, the US Food and Drug Administration (FDA) had allowed compounding pharmacies to manufacture the drug. In October, the FDA clarified that it won’t take legal action against compounders, even now that the shortage has been resolved.
A pharmacovigilance study using the FDA Adverse Event Reporting System identified “potential safety signals of increased mortality and serious adverse event reporting” associated with certain GLP-1 RAs — especially in younger patients and women (P < .0001 for both groups).
The most common side effects reported with GLP-1 RAs are gastrointestinal events, such as nausea, diarrhea, constipation, and vomiting. Most occur during dose initiation and escalation and wane over the following weeks. However, studies have also reported severe side effects, including a higher risk for pancreatitis, bowel obstruction, and gastroparesis, as well as a significantly higher risk for gallbladder and biliary diseases. In fact, according to one study, patients with diabetes taking GLP-1 RAs reported gastrointestinal-related issues as a “prominent factor” in their decision to discontinue taking these medications.
Several types of cancer are potentially associated with GLP-1 RAs, but findings regarding this potential link have been inconsistent. In a recent review article, Drucker noted there were only inconsistent data linking GLP-1 RAs with thyroid cancer and medullary thyroid cancer and that their potential association with pancreatic has “not been supported by results from randomized controlled trials or real-world data.”
Concerns have been raised about loss of lean mass and muscle strength and function, especially in older individuals with obesity and advanced liver, cardiovascular, or kidney disease. However, as Drucker pointed out in his review article, muscle function may not correlate with the loss of lean mass. In fact, there are “consistent reductions” in lean mass after bariatric surgery, but “little evidence to date for impairment of muscle function.” He added that newer GLP-1 agents under development for obesity treatment are focusing on “developing complementary therapies that preferentially reduce adipose tissue, while sparing lean mass.”
As covered by Medscape Medical News, there have been reports of potential suicidal ideation associated with GLP-1 RAs. This triggered a 2023 review from the European Medicines Agency. However, recent results from a cohort study and a post hoc analysis of randomized controlled trials concluded that there is no evidence that these drugs increase suicidal ideation or behavior.
In early November, the FDA updated the labels for the GLP-1 RAs to include a warning regarding pulmonary aspiration during general anesthesia or deep sedation. Guidance from a group of societies, led by the American Society of Anesthesiologists, contains recommendations regarding nuances of addressing this concern in surgical patients taking these agents.
Not a Standalone Treatment
Marc-Andre Cornier, MD, professor of medicine, James A. Keating Endowed Chair in Diabetes, and director of the Division of Endocrinology, Diabetes and Metabolic Diseases, Department of Medicine, Medical University of South Carolina, Charleston, South Carolina, told Medscape Medical News that GLP-1 RAs should not be viewed as cosmetic interventions but rather as medical treatments, “not only for weight loss but to reverse obesity-associated complications.”
Moreover, they should be used “as an adjunct to lifestyle changes,” emphasized Cornier. “We want our patients to have a high-quality diet with high protein content, fluid, vitamins, and minerals, and we want them to exercise.” Especially with the concern of potential loss of muscle mass with these agents, “resistance exercise might help mitigate that concern.”
Recently published recommendations can assist clinicians in guiding patients taking GLP-1 RAs to optimize nutrition. The recommendations note that patients should be referred to a registered dietitian to “complement and support” treatment with anti-obesity medications.
What Do Patients Want?
Despite the ever-rising popularity of GLP-1 RAs, a new national survey of over 2200 US adults conducted by the Physicians Committee for Responsible Medicine suggests that most Americans don’t want to use them. Among those who wanted to lose weight, almost three-quarters “disagreed” or “strongly disagreed” with the idea of taking a weight-loss injectable, and 68% of those who wanted to lose weight “agreed” or “strongly agreed” that they would be willing to try a plant-based diet, if it could lead to significant weight loss.
Moreover, many individuals treated with GLP-1 RAs discontinue their use, despite the probability of regaining the weight, according to a report that found only 46.3% of GLP-1 users were still taking the medications at 6 months and only 32.3% at 1 year. The authors commented that their real-world findings show a “substantially lower” 1-year persistence rate, compared with the rate reported in clinical trials. They suggest that the financial burden (> $12,000/year) may contribute to discontinuation.
Discontinuation of GLP-1 RAs can lead to worsening cardiometabolic parameters, with a potential increased risk for adverse outcomes; moreover, weight cycling (“yo-yo dieting”) carries its own risks. In light of these concerns, it’s particularly important to select appropriate patients and to determine whether potential short-term therapy has any enduring benefit.
Acosta agreed. “It’s exciting when looking at the data on how to find the best responders and who should make the effort to take these medications — not only in terms of side effects but also in terms of cost and which patients will receive maximum benefits and should be covered by insurance.”
Drucker has served as a consultant or speaker for Altimmune, Amgen, AstraZeneca, Arrowhead, Boehringer Ingelheim, Kallyope, Merck Research Laboratories, Novo Nordisk, Pfizer, and Zealand Pharma. He holds nonexercised options in Kallyope. Mount Sinai Hospital receives research support for investigator-initiated studies in the Drucker laboratory from Amgen, Novo Nordisk, Pfizer, and Zealand Pharma. Gila Therapeutics and Phenomix Sciences have licensed Acosta’s research technologies from University of Florida and Mayo Clinic. Acosta received consultant fees in the last 5 years from Rhythm Pharmaceuticals, Gila Therapeutics, Amgen, General Mills, Regeneron Pharmaceuticals, Boehringer Ingelheim, Novo Nordisk, Currax, Nestlé, Phenomix Sciences, Bausch Health, and Rare Disease. He received funding support from the National Institutes of Health, Vivus Pharmaceuticals, Novo Nordisk, Apollo Endosurgery, Satiogen Pharmaceuticals, Spatz Medical, Rhythm Pharmaceuticals, Regeneron Pharmaceuticals, Boehringer Ingelheim, and Novo Nordisk. In the past, Cornier has served as a consultant for Novo Nordisk.
A version of this article first appeared on Medscape.com.
Rates of overweight and obesity have more than doubled in the United States during the last three decades, according to a new analysis. By 2050, it’s anticipated that 213 million adults (age, > 25 years) and 43 million children and adolescents will have overweight or obesity. The results led authors of a study to describe obesity as having reached a “crisis point” requiring urgent action and interventions.
Are glucagon-like peptide 1 receptor agonists (GLP-1 RAs), originally developed and prescribed for diabetes and now approved for weight loss, the answer?
Their popularity is certainly surging. Between the last 6 months of 2022 vs the last 6 months of 2024, the number of patients prescribed GLP-1 RAs increased by 132.6%. This is also reflected in a shift in public awareness, with a recent survey of US adults finding that 32% of respondents had heard “a lot” about these drugs, up from 19% in 2023.
GLP-1 RAs (including tirzepatide, which targets not only the GLP-1 receptor but also the glucose-dependent insulinotropic polypeptide receptor) have shown efficacy in weight loss. A 2022 review and meta-analysis of 22 trials (17,183 patients) found that 50.2% and 17.5% of those treated with GLP-1 RAs had a ≥ 5% and ≥ 10% weight loss, respectively, compared with placebo. A 2023 review of 41 trials (15,135 patients) found that compared with controls, GLP-1 RAs significantly reduced body weight, body mass index, waist circumference, and waist-to-hip ratio.
“GLP-1 RAs are great medications,” Andres Acosta, MD, PhD, director of the Precision Medicine for Obesity Laboratory, Mayo Clinic, Rochester, Minnesota, told Medscape Medical News. “We’ve been using them for almost two decades. But now there’s excitement about their utility in treating obesity.”
Treating the Four Categories of Obesity
Daniel Drucker, MD, senior investigator at the Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada, is a pioneer in diabetes treatment and particularly in the development of GLP-1 RAs. Drucker told Medscape Medical News that despite the efficacy and enormous potential of GLP-1 RAs, “we know some people don’t lose much weight when taking these medicines and others don’t feel well and can’t take them.”
The number of individuals who don’t respond to or aren’t able to tolerate GLP-1 RAs “might be small — less than 10% of people who try to take them — but we don’t fully understand the differences in response across different individuals,” Drucker said.
Acosta agreed, adding that it’s “essential for us to identify who will be the best responders, as we do with medications for other conditions, such as cancer and cardiovascular disease.”
Acosta’s group has spent more than a decade engaged in efforts to identify unique characteristics among patients with obesity and has succeeded in identifying four obesity phenotypes.
“What matters in the space of GLP-1 is that using this classification, we can identify the best responders and those who don’t respond.”
The first phenotype, described as “Hungry Gut” (HG), includes patients with abnormal postprandial satiety. “Although they may be satiated at the end of a meal, they have accelerated gastric emptying and therefore feel hungry between meals and want to keep eating,” he said.
There are also patients who experience abnormal satiety during meals. According to Acosta, these are the patients who will return to the table for second and third helpings. “They don’t feel full and continue to eat more and more in a single sitting” — a phenomenon referred to as “Hungry Brain.”
The third phenotype — “Emotional Hunger” — consists of people who are “hedonic” about food or engage in emotional eating behavior, whereas in the fourth group, people have “an abnormal metabolism in which they don’t burn enough calories. They have an inefficient metabolic rate.” This latter phenomenon is called “Slow Burn.”
Acosta and colleagues randomized 312 patients attending a weight management center to phenotype-guided or non–phenotype-guided treatment with anti-obesity medications (phentermine, phentermine/topiramate, bupropion/naltrexone, lorcaserin, and liraglutide). The phenotype-guided approach was associated with a 1.75-fold greater weight loss after 1 year than the non–phenotype-guided approach (mean weight loss, 15.9% vs 9.0%, respectively).
GLP-1 RAs: Not One-Size-Fits-All
Acosta’s group has developed a genetic test that uses patients’ saliva to identify their obesity phenotype, with the aim of predicting the best responders to GLP-1 RAs. The test, MyPhenome genetic obesity test, is licensed by Acosta’s lab and available through Phenomix Sciences.
Acosta and colleagues presented their findings at the American Gastroenterological Association’s 2024 annual meeting regarding a machine-learning gene risk score (ML-GRS) they developed to predict HG, based on saliva and blood samples. Their genetic studies generated a ML-GRS that classified participants with obesity along a continuum from “HG Positive” (HG+) to “HG Negative” (HG−). Compared with the HG− participants, those who were HG+ had superior total body weight loss with semaglutide at 9 and 12 months. When used to predict response, the ML-GRS had an area under the curve of 0.76 (P = .04) and a positive predictive value of 0.95.
According to Acosta, HG+ patients are “the best responders to the GLP-1 RAs, although we don’t yet understand the mechanism of why they have the phenomenon of abnormal postprandial satiety. It may be an abnormal genetic pathway or abnormal secretion of GLP-1. More studies are needed.”
He noted that GLP-1 RAs “might also be helpful with the second [Hungry Brain] category, but these patients do better with phentermine-topiramate,” as demonstrated in a 2023 study conducted by Acosta and colleagues.
His group has also studied which lifestyle interventions are most effective for each phenotype. “When a unique lifestyle intervention targeting each phenotype was applied, patients lost more weight and had greater metabolic improvement,” he reported.
“Treating obesity no longer needs to be trial-and-error, but should be done using precision medicine because one size doesn’t fit all,” Acosta said.
Concerning Side Effects
The popular media has featured stories about individuals who took GLP-1 RAs for weight loss and experienced serious side effects, including a recent account of a British nurse who died after taking tirzepatide. As reported by the BBC, the nurse’s death certificate listed multiple organ failure, septic shock, and pancreatitis as the immediate causes of death, with the “use of prescribed tirzepatide” recorded as a contributing factor. The report went on to note that there were 23 suspected deaths in the United Kingdom tied to semaglutide since 2019.
Beyond brand-name products, there are also risks associated with GLP-1 RAs manufactured by compounding pharmacies. In early November, CNN reported that compounded semaglutide has been linked to at least 10 deaths. Because of a prior shortage of tirzepatide, the US Food and Drug Administration (FDA) had allowed compounding pharmacies to manufacture the drug. In October, the FDA clarified that it won’t take legal action against compounders, even now that the shortage has been resolved.
A pharmacovigilance study using the FDA Adverse Event Reporting System identified “potential safety signals of increased mortality and serious adverse event reporting” associated with certain GLP-1 RAs — especially in younger patients and women (P < .0001 for both groups).
The most common side effects reported with GLP-1 RAs are gastrointestinal events, such as nausea, diarrhea, constipation, and vomiting. Most occur during dose initiation and escalation and wane over the following weeks. However, studies have also reported severe side effects, including a higher risk for pancreatitis, bowel obstruction, and gastroparesis, as well as a significantly higher risk for gallbladder and biliary diseases. In fact, according to one study, patients with diabetes taking GLP-1 RAs reported gastrointestinal-related issues as a “prominent factor” in their decision to discontinue taking these medications.
Several types of cancer are potentially associated with GLP-1 RAs, but findings regarding this potential link have been inconsistent. In a recent review article, Drucker noted there were only inconsistent data linking GLP-1 RAs with thyroid cancer and medullary thyroid cancer and that their potential association with pancreatic has “not been supported by results from randomized controlled trials or real-world data.”
Concerns have been raised about loss of lean mass and muscle strength and function, especially in older individuals with obesity and advanced liver, cardiovascular, or kidney disease. However, as Drucker pointed out in his review article, muscle function may not correlate with the loss of lean mass. In fact, there are “consistent reductions” in lean mass after bariatric surgery, but “little evidence to date for impairment of muscle function.” He added that newer GLP-1 agents under development for obesity treatment are focusing on “developing complementary therapies that preferentially reduce adipose tissue, while sparing lean mass.”
As covered by Medscape Medical News, there have been reports of potential suicidal ideation associated with GLP-1 RAs. This triggered a 2023 review from the European Medicines Agency. However, recent results from a cohort study and a post hoc analysis of randomized controlled trials concluded that there is no evidence that these drugs increase suicidal ideation or behavior.
In early November, the FDA updated the labels for the GLP-1 RAs to include a warning regarding pulmonary aspiration during general anesthesia or deep sedation. Guidance from a group of societies, led by the American Society of Anesthesiologists, contains recommendations regarding nuances of addressing this concern in surgical patients taking these agents.
Not a Standalone Treatment
Marc-Andre Cornier, MD, professor of medicine, James A. Keating Endowed Chair in Diabetes, and director of the Division of Endocrinology, Diabetes and Metabolic Diseases, Department of Medicine, Medical University of South Carolina, Charleston, South Carolina, told Medscape Medical News that GLP-1 RAs should not be viewed as cosmetic interventions but rather as medical treatments, “not only for weight loss but to reverse obesity-associated complications.”
Moreover, they should be used “as an adjunct to lifestyle changes,” emphasized Cornier. “We want our patients to have a high-quality diet with high protein content, fluid, vitamins, and minerals, and we want them to exercise.” Especially with the concern of potential loss of muscle mass with these agents, “resistance exercise might help mitigate that concern.”
Recently published recommendations can assist clinicians in guiding patients taking GLP-1 RAs to optimize nutrition. The recommendations note that patients should be referred to a registered dietitian to “complement and support” treatment with anti-obesity medications.
What Do Patients Want?
Despite the ever-rising popularity of GLP-1 RAs, a new national survey of over 2200 US adults conducted by the Physicians Committee for Responsible Medicine suggests that most Americans don’t want to use them. Among those who wanted to lose weight, almost three-quarters “disagreed” or “strongly disagreed” with the idea of taking a weight-loss injectable, and 68% of those who wanted to lose weight “agreed” or “strongly agreed” that they would be willing to try a plant-based diet, if it could lead to significant weight loss.
Moreover, many individuals treated with GLP-1 RAs discontinue their use, despite the probability of regaining the weight, according to a report that found only 46.3% of GLP-1 users were still taking the medications at 6 months and only 32.3% at 1 year. The authors commented that their real-world findings show a “substantially lower” 1-year persistence rate, compared with the rate reported in clinical trials. They suggest that the financial burden (> $12,000/year) may contribute to discontinuation.
Discontinuation of GLP-1 RAs can lead to worsening cardiometabolic parameters, with a potential increased risk for adverse outcomes; moreover, weight cycling (“yo-yo dieting”) carries its own risks. In light of these concerns, it’s particularly important to select appropriate patients and to determine whether potential short-term therapy has any enduring benefit.
Acosta agreed. “It’s exciting when looking at the data on how to find the best responders and who should make the effort to take these medications — not only in terms of side effects but also in terms of cost and which patients will receive maximum benefits and should be covered by insurance.”
Drucker has served as a consultant or speaker for Altimmune, Amgen, AstraZeneca, Arrowhead, Boehringer Ingelheim, Kallyope, Merck Research Laboratories, Novo Nordisk, Pfizer, and Zealand Pharma. He holds nonexercised options in Kallyope. Mount Sinai Hospital receives research support for investigator-initiated studies in the Drucker laboratory from Amgen, Novo Nordisk, Pfizer, and Zealand Pharma. Gila Therapeutics and Phenomix Sciences have licensed Acosta’s research technologies from University of Florida and Mayo Clinic. Acosta received consultant fees in the last 5 years from Rhythm Pharmaceuticals, Gila Therapeutics, Amgen, General Mills, Regeneron Pharmaceuticals, Boehringer Ingelheim, Novo Nordisk, Currax, Nestlé, Phenomix Sciences, Bausch Health, and Rare Disease. He received funding support from the National Institutes of Health, Vivus Pharmaceuticals, Novo Nordisk, Apollo Endosurgery, Satiogen Pharmaceuticals, Spatz Medical, Rhythm Pharmaceuticals, Regeneron Pharmaceuticals, Boehringer Ingelheim, and Novo Nordisk. In the past, Cornier has served as a consultant for Novo Nordisk.
A version of this article first appeared on Medscape.com.
Rates of overweight and obesity have more than doubled in the United States during the last three decades, according to a new analysis. By 2050, it’s anticipated that 213 million adults (age, > 25 years) and 43 million children and adolescents will have overweight or obesity. The results led authors of a study to describe obesity as having reached a “crisis point” requiring urgent action and interventions.
Are glucagon-like peptide 1 receptor agonists (GLP-1 RAs), originally developed and prescribed for diabetes and now approved for weight loss, the answer?
Their popularity is certainly surging. Between the last 6 months of 2022 vs the last 6 months of 2024, the number of patients prescribed GLP-1 RAs increased by 132.6%. This is also reflected in a shift in public awareness, with a recent survey of US adults finding that 32% of respondents had heard “a lot” about these drugs, up from 19% in 2023.
GLP-1 RAs (including tirzepatide, which targets not only the GLP-1 receptor but also the glucose-dependent insulinotropic polypeptide receptor) have shown efficacy in weight loss. A 2022 review and meta-analysis of 22 trials (17,183 patients) found that 50.2% and 17.5% of those treated with GLP-1 RAs had a ≥ 5% and ≥ 10% weight loss, respectively, compared with placebo. A 2023 review of 41 trials (15,135 patients) found that compared with controls, GLP-1 RAs significantly reduced body weight, body mass index, waist circumference, and waist-to-hip ratio.
“GLP-1 RAs are great medications,” Andres Acosta, MD, PhD, director of the Precision Medicine for Obesity Laboratory, Mayo Clinic, Rochester, Minnesota, told Medscape Medical News. “We’ve been using them for almost two decades. But now there’s excitement about their utility in treating obesity.”
Treating the Four Categories of Obesity
Daniel Drucker, MD, senior investigator at the Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada, is a pioneer in diabetes treatment and particularly in the development of GLP-1 RAs. Drucker told Medscape Medical News that despite the efficacy and enormous potential of GLP-1 RAs, “we know some people don’t lose much weight when taking these medicines and others don’t feel well and can’t take them.”
The number of individuals who don’t respond to or aren’t able to tolerate GLP-1 RAs “might be small — less than 10% of people who try to take them — but we don’t fully understand the differences in response across different individuals,” Drucker said.
Acosta agreed, adding that it’s “essential for us to identify who will be the best responders, as we do with medications for other conditions, such as cancer and cardiovascular disease.”
Acosta’s group has spent more than a decade engaged in efforts to identify unique characteristics among patients with obesity and has succeeded in identifying four obesity phenotypes.
“What matters in the space of GLP-1 is that using this classification, we can identify the best responders and those who don’t respond.”
The first phenotype, described as “Hungry Gut” (HG), includes patients with abnormal postprandial satiety. “Although they may be satiated at the end of a meal, they have accelerated gastric emptying and therefore feel hungry between meals and want to keep eating,” he said.
There are also patients who experience abnormal satiety during meals. According to Acosta, these are the patients who will return to the table for second and third helpings. “They don’t feel full and continue to eat more and more in a single sitting” — a phenomenon referred to as “Hungry Brain.”
The third phenotype — “Emotional Hunger” — consists of people who are “hedonic” about food or engage in emotional eating behavior, whereas in the fourth group, people have “an abnormal metabolism in which they don’t burn enough calories. They have an inefficient metabolic rate.” This latter phenomenon is called “Slow Burn.”
Acosta and colleagues randomized 312 patients attending a weight management center to phenotype-guided or non–phenotype-guided treatment with anti-obesity medications (phentermine, phentermine/topiramate, bupropion/naltrexone, lorcaserin, and liraglutide). The phenotype-guided approach was associated with a 1.75-fold greater weight loss after 1 year than the non–phenotype-guided approach (mean weight loss, 15.9% vs 9.0%, respectively).
GLP-1 RAs: Not One-Size-Fits-All
Acosta’s group has developed a genetic test that uses patients’ saliva to identify their obesity phenotype, with the aim of predicting the best responders to GLP-1 RAs. The test, MyPhenome genetic obesity test, is licensed by Acosta’s lab and available through Phenomix Sciences.
Acosta and colleagues presented their findings at the American Gastroenterological Association’s 2024 annual meeting regarding a machine-learning gene risk score (ML-GRS) they developed to predict HG, based on saliva and blood samples. Their genetic studies generated a ML-GRS that classified participants with obesity along a continuum from “HG Positive” (HG+) to “HG Negative” (HG−). Compared with the HG− participants, those who were HG+ had superior total body weight loss with semaglutide at 9 and 12 months. When used to predict response, the ML-GRS had an area under the curve of 0.76 (P = .04) and a positive predictive value of 0.95.
According to Acosta, HG+ patients are “the best responders to the GLP-1 RAs, although we don’t yet understand the mechanism of why they have the phenomenon of abnormal postprandial satiety. It may be an abnormal genetic pathway or abnormal secretion of GLP-1. More studies are needed.”
He noted that GLP-1 RAs “might also be helpful with the second [Hungry Brain] category, but these patients do better with phentermine-topiramate,” as demonstrated in a 2023 study conducted by Acosta and colleagues.
His group has also studied which lifestyle interventions are most effective for each phenotype. “When a unique lifestyle intervention targeting each phenotype was applied, patients lost more weight and had greater metabolic improvement,” he reported.
“Treating obesity no longer needs to be trial-and-error, but should be done using precision medicine because one size doesn’t fit all,” Acosta said.
Concerning Side Effects
The popular media has featured stories about individuals who took GLP-1 RAs for weight loss and experienced serious side effects, including a recent account of a British nurse who died after taking tirzepatide. As reported by the BBC, the nurse’s death certificate listed multiple organ failure, septic shock, and pancreatitis as the immediate causes of death, with the “use of prescribed tirzepatide” recorded as a contributing factor. The report went on to note that there were 23 suspected deaths in the United Kingdom tied to semaglutide since 2019.
Beyond brand-name products, there are also risks associated with GLP-1 RAs manufactured by compounding pharmacies. In early November, CNN reported that compounded semaglutide has been linked to at least 10 deaths. Because of a prior shortage of tirzepatide, the US Food and Drug Administration (FDA) had allowed compounding pharmacies to manufacture the drug. In October, the FDA clarified that it won’t take legal action against compounders, even now that the shortage has been resolved.
A pharmacovigilance study using the FDA Adverse Event Reporting System identified “potential safety signals of increased mortality and serious adverse event reporting” associated with certain GLP-1 RAs — especially in younger patients and women (P < .0001 for both groups).
The most common side effects reported with GLP-1 RAs are gastrointestinal events, such as nausea, diarrhea, constipation, and vomiting. Most occur during dose initiation and escalation and wane over the following weeks. However, studies have also reported severe side effects, including a higher risk for pancreatitis, bowel obstruction, and gastroparesis, as well as a significantly higher risk for gallbladder and biliary diseases. In fact, according to one study, patients with diabetes taking GLP-1 RAs reported gastrointestinal-related issues as a “prominent factor” in their decision to discontinue taking these medications.
Several types of cancer are potentially associated with GLP-1 RAs, but findings regarding this potential link have been inconsistent. In a recent review article, Drucker noted there were only inconsistent data linking GLP-1 RAs with thyroid cancer and medullary thyroid cancer and that their potential association with pancreatic has “not been supported by results from randomized controlled trials or real-world data.”
Concerns have been raised about loss of lean mass and muscle strength and function, especially in older individuals with obesity and advanced liver, cardiovascular, or kidney disease. However, as Drucker pointed out in his review article, muscle function may not correlate with the loss of lean mass. In fact, there are “consistent reductions” in lean mass after bariatric surgery, but “little evidence to date for impairment of muscle function.” He added that newer GLP-1 agents under development for obesity treatment are focusing on “developing complementary therapies that preferentially reduce adipose tissue, while sparing lean mass.”
As covered by Medscape Medical News, there have been reports of potential suicidal ideation associated with GLP-1 RAs. This triggered a 2023 review from the European Medicines Agency. However, recent results from a cohort study and a post hoc analysis of randomized controlled trials concluded that there is no evidence that these drugs increase suicidal ideation or behavior.
In early November, the FDA updated the labels for the GLP-1 RAs to include a warning regarding pulmonary aspiration during general anesthesia or deep sedation. Guidance from a group of societies, led by the American Society of Anesthesiologists, contains recommendations regarding nuances of addressing this concern in surgical patients taking these agents.
Not a Standalone Treatment
Marc-Andre Cornier, MD, professor of medicine, James A. Keating Endowed Chair in Diabetes, and director of the Division of Endocrinology, Diabetes and Metabolic Diseases, Department of Medicine, Medical University of South Carolina, Charleston, South Carolina, told Medscape Medical News that GLP-1 RAs should not be viewed as cosmetic interventions but rather as medical treatments, “not only for weight loss but to reverse obesity-associated complications.”
Moreover, they should be used “as an adjunct to lifestyle changes,” emphasized Cornier. “We want our patients to have a high-quality diet with high protein content, fluid, vitamins, and minerals, and we want them to exercise.” Especially with the concern of potential loss of muscle mass with these agents, “resistance exercise might help mitigate that concern.”
Recently published recommendations can assist clinicians in guiding patients taking GLP-1 RAs to optimize nutrition. The recommendations note that patients should be referred to a registered dietitian to “complement and support” treatment with anti-obesity medications.
What Do Patients Want?
Despite the ever-rising popularity of GLP-1 RAs, a new national survey of over 2200 US adults conducted by the Physicians Committee for Responsible Medicine suggests that most Americans don’t want to use them. Among those who wanted to lose weight, almost three-quarters “disagreed” or “strongly disagreed” with the idea of taking a weight-loss injectable, and 68% of those who wanted to lose weight “agreed” or “strongly agreed” that they would be willing to try a plant-based diet, if it could lead to significant weight loss.
Moreover, many individuals treated with GLP-1 RAs discontinue their use, despite the probability of regaining the weight, according to a report that found only 46.3% of GLP-1 users were still taking the medications at 6 months and only 32.3% at 1 year. The authors commented that their real-world findings show a “substantially lower” 1-year persistence rate, compared with the rate reported in clinical trials. They suggest that the financial burden (> $12,000/year) may contribute to discontinuation.
Discontinuation of GLP-1 RAs can lead to worsening cardiometabolic parameters, with a potential increased risk for adverse outcomes; moreover, weight cycling (“yo-yo dieting”) carries its own risks. In light of these concerns, it’s particularly important to select appropriate patients and to determine whether potential short-term therapy has any enduring benefit.
Acosta agreed. “It’s exciting when looking at the data on how to find the best responders and who should make the effort to take these medications — not only in terms of side effects but also in terms of cost and which patients will receive maximum benefits and should be covered by insurance.”
Drucker has served as a consultant or speaker for Altimmune, Amgen, AstraZeneca, Arrowhead, Boehringer Ingelheim, Kallyope, Merck Research Laboratories, Novo Nordisk, Pfizer, and Zealand Pharma. He holds nonexercised options in Kallyope. Mount Sinai Hospital receives research support for investigator-initiated studies in the Drucker laboratory from Amgen, Novo Nordisk, Pfizer, and Zealand Pharma. Gila Therapeutics and Phenomix Sciences have licensed Acosta’s research technologies from University of Florida and Mayo Clinic. Acosta received consultant fees in the last 5 years from Rhythm Pharmaceuticals, Gila Therapeutics, Amgen, General Mills, Regeneron Pharmaceuticals, Boehringer Ingelheim, Novo Nordisk, Currax, Nestlé, Phenomix Sciences, Bausch Health, and Rare Disease. He received funding support from the National Institutes of Health, Vivus Pharmaceuticals, Novo Nordisk, Apollo Endosurgery, Satiogen Pharmaceuticals, Spatz Medical, Rhythm Pharmaceuticals, Regeneron Pharmaceuticals, Boehringer Ingelheim, and Novo Nordisk. In the past, Cornier has served as a consultant for Novo Nordisk.
A version of this article first appeared on Medscape.com.
How to Manage Patients on GLP-1s Before Surgery
, as does the US Food and Drug Administration’s (FDA’s) labeling for the drugs. The changes can be challenging to keep up with, and endocrinologists seem to be making their own decisions based on clinical experience and their interpretations of the potential impact and value of the emerging information.
The latest FDA label change warns about the risk for pulmonary aspiration but notes “insufficient” data to inform recommendations to mitigate the risk in vulnerable patients. Yet, the latest multi-society guidance, led by the American Society of Anesthesiologists (ASA) and based on consensus, not evidence, has nuanced advice for managing patients at risk.
Does the FDA’s label change make a difference regarding the multi-society guidance, which was published earlier? “The answer is no,” Girish Joshi, MD, vice chair, ASA Committee on Practice Parameters, told this news organization. “The concern of increased pulmonary aspiration in patients who are on GLP-1 receptor agonists has been known, and that concern still exists. So, we started with not an assumption but the premise that patients on GLP-1 receptor agonists are at a higher risk of aspiration during sedation, analgesia, and/or general anesthesia. The FDA basically confirms what we say in the guidance.”
Joshi, professor in the Anesthesiology and Pain Management Department at UT Southwestern Medical Center, Dallas, aimed to make the guidance, which was published simultaneously in several society journals, more implementable with a letter to the editor of Anesthesiology. The key, he said, is to identify patients at higher risk for aspiration; all others would follow treatment as usual.
The letter highlights three overarching recommendations and then expands upon them: Standardized preoperative assessment for risk for delayed gastric emptying (yes/no); selective preoperative care plan based on delayed gastric emptying assessment and shared decision-making; and on the day of the procedure, reassess for delayed gastric emptying and mitigate risk if there is clinical concern.
But it seems as though, for now, endocrinologists are managing these patients as they see fit, within the parameters of any institutional guidance requirements. Here is what they said about their practice:
Amy E. Rothberg, MD, DABOM, director of the Weight Management Program & Rewind at the University of Michigan, Ann Arbor, Michigan, said, “I think it makes sense to inform our patients of the labeling and rare but potential adverse effects if they intend to undergo anesthesia for a scheduled procedure/surgery. There is never no risk of aspiration during anesthesia.”
“I find it a bit curious that ASA implies that those who experience GI side effects are more likely than those who do not to have this potential risk. I doubt there is evidence that those without GI side effects are necessarily ‘safer’ and a study to determine that is unlikely to take be conducted.”
“My institution does require a 1-week pause on GLP-1s for those undergoing anesthesia for surgery,” she added. “That’s not evidence-based either, but probably reduces the risk of aspiration during anesthesia — but I don’t know what the actual denominator is for aspiration in those who continued vs those who took a pause from GLP-1s. Pausing does certainly (anecdotally) increase the traffic of communications between physicians and their patients about what to do in the interval.”
Anne Peters, MD, a professor of clinical medicine and a clinical scholar at the Keck School of Medicine of the University of Southern California, Los Angeles, said, “The FDA label change is a warning that really doesn’t say exactly who on GLP-1 RAs is at highest risk or what to do, and if any intervention has been shown to help. The ASA recommendations seem much more nuanced and practical, including point-of-care gastric ultrasound to see if there is retained food/fluid prior to surgery.”
“In my practice, I individualize what I say, depending on the person and the circumstance,” she said. “Mostly, I have people hold one dose before planned surgery, so they have been 10 days at least without a dose. But if worried about gastrointestinal symptoms or gastroparesis, I have them do a clear liquid diet for 24 hours presurgery. Or at least avoid heavy fat meals the day before.”
“There is a risk of aspiration with anything that slows gastric emptying — maybe even in patients with gastroparesis at baseline due to physiologic, not pharmacological, reasons — and anesthesiologists should be aware of the need to assess patients individually.”
Michael A. Weintraub, MD, of NYU Langone Health Diabetes & Endocrine Associates in New York City, observed, “The risk of a pulmonary aspiration event with GLP-1 medication is quite rare, but not zero. On the other hand, stopping the GLP-1 can cause hyperglycemia or rebound weight gain. Furthermore, it can become complicated to restart GLP1 dosing, particularly given the existing medication shortages.”
“In most cases, stopping a weekly GLP-1 medication 1 week prior to the procedure minimizes the risks of pulmonary aspiration and prevents any worsening hyperglycemia or weight gain,” he said. However, taking the drug 7 days prior to the procedure is optimal. “That way, they would be due for the next dose on the day of the procedure, and taking it the day following procedure minimizes disruption in their once-weekly regimen.”
Malini Gupta, MD, director of G2Endo Endocrinology & Metabolism, Memphis, Tennessee, advised that physicians weigh the risk of stopping the medication (which can cause a glycemic spike) vs risk for aspiration.
“In my opinion, all patients should follow a strict liquid diet or NPO status prior to a surgery to further decrease the risk of aspiration,” she said. “I generally hold the GLP-1 RA for a week before a surgery. If additional glycemic control is necessary, I will add to or adjust one of the patient’s other diabetes medications.”
Jaime Almandoz, MD, associate professor of medicine and medical director of the Weight Wellness Program in Dallas, said, “As endocrinologists, we typically rely on our anesthesia colleagues for guidance on perioperative management. In light of emerging guidelines for holding GLP-1 medications, we also recommend patients adopt a liquid diet 24 hours prior to surgery, along with the fasting protocol.”
“For those managing diabetes with GLP-1 therapies, it is crucial to establish a blood sugar management plan while off these medications, especially during fasting or postoperative periods, which can be further influenced by many factors, including nausea, pain medications, and antibiotics after the procedure.”
Joshi added that at Parkland Hospital in Dallas, “we do a huge number of cases using the same information. We identify patients who are at risk, and then we tell our proceduralists and our surgeons if they’re in the escalating phase of the dosing or if they have GI symptoms; don’t even schedule them as an elective case; wait till the escalation phase is over and then schedule them.”
“That way,” he said, “it becomes logistically easy to manage because the recommendation from the group is that patients who are at higher risk should receive a 24-hour liquid diet — the same as colonoscopy. But sometimes it can be challenging to do so.”
Joshi has received honoraria for consultation from Merck Sharp & Dohme, Vertex Pharmaceuticals, and Haisco-USA Pharmaceuticals. Gupta is on the speakers bureau for Amgen (Tepezza) and IBSA (Tirosint) and is a creative consultant for AbbVie. Almandoz serves on advisory boards for Novo Nordisk, Eli Lilly, and Boehringer Ingelheim. The other experts declared no relevant relationships.
A version of this article first appeared on Medscape.com.
, as does the US Food and Drug Administration’s (FDA’s) labeling for the drugs. The changes can be challenging to keep up with, and endocrinologists seem to be making their own decisions based on clinical experience and their interpretations of the potential impact and value of the emerging information.
The latest FDA label change warns about the risk for pulmonary aspiration but notes “insufficient” data to inform recommendations to mitigate the risk in vulnerable patients. Yet, the latest multi-society guidance, led by the American Society of Anesthesiologists (ASA) and based on consensus, not evidence, has nuanced advice for managing patients at risk.
Does the FDA’s label change make a difference regarding the multi-society guidance, which was published earlier? “The answer is no,” Girish Joshi, MD, vice chair, ASA Committee on Practice Parameters, told this news organization. “The concern of increased pulmonary aspiration in patients who are on GLP-1 receptor agonists has been known, and that concern still exists. So, we started with not an assumption but the premise that patients on GLP-1 receptor agonists are at a higher risk of aspiration during sedation, analgesia, and/or general anesthesia. The FDA basically confirms what we say in the guidance.”
Joshi, professor in the Anesthesiology and Pain Management Department at UT Southwestern Medical Center, Dallas, aimed to make the guidance, which was published simultaneously in several society journals, more implementable with a letter to the editor of Anesthesiology. The key, he said, is to identify patients at higher risk for aspiration; all others would follow treatment as usual.
The letter highlights three overarching recommendations and then expands upon them: Standardized preoperative assessment for risk for delayed gastric emptying (yes/no); selective preoperative care plan based on delayed gastric emptying assessment and shared decision-making; and on the day of the procedure, reassess for delayed gastric emptying and mitigate risk if there is clinical concern.
But it seems as though, for now, endocrinologists are managing these patients as they see fit, within the parameters of any institutional guidance requirements. Here is what they said about their practice:
Amy E. Rothberg, MD, DABOM, director of the Weight Management Program & Rewind at the University of Michigan, Ann Arbor, Michigan, said, “I think it makes sense to inform our patients of the labeling and rare but potential adverse effects if they intend to undergo anesthesia for a scheduled procedure/surgery. There is never no risk of aspiration during anesthesia.”
“I find it a bit curious that ASA implies that those who experience GI side effects are more likely than those who do not to have this potential risk. I doubt there is evidence that those without GI side effects are necessarily ‘safer’ and a study to determine that is unlikely to take be conducted.”
“My institution does require a 1-week pause on GLP-1s for those undergoing anesthesia for surgery,” she added. “That’s not evidence-based either, but probably reduces the risk of aspiration during anesthesia — but I don’t know what the actual denominator is for aspiration in those who continued vs those who took a pause from GLP-1s. Pausing does certainly (anecdotally) increase the traffic of communications between physicians and their patients about what to do in the interval.”
Anne Peters, MD, a professor of clinical medicine and a clinical scholar at the Keck School of Medicine of the University of Southern California, Los Angeles, said, “The FDA label change is a warning that really doesn’t say exactly who on GLP-1 RAs is at highest risk or what to do, and if any intervention has been shown to help. The ASA recommendations seem much more nuanced and practical, including point-of-care gastric ultrasound to see if there is retained food/fluid prior to surgery.”
“In my practice, I individualize what I say, depending on the person and the circumstance,” she said. “Mostly, I have people hold one dose before planned surgery, so they have been 10 days at least without a dose. But if worried about gastrointestinal symptoms or gastroparesis, I have them do a clear liquid diet for 24 hours presurgery. Or at least avoid heavy fat meals the day before.”
“There is a risk of aspiration with anything that slows gastric emptying — maybe even in patients with gastroparesis at baseline due to physiologic, not pharmacological, reasons — and anesthesiologists should be aware of the need to assess patients individually.”
Michael A. Weintraub, MD, of NYU Langone Health Diabetes & Endocrine Associates in New York City, observed, “The risk of a pulmonary aspiration event with GLP-1 medication is quite rare, but not zero. On the other hand, stopping the GLP-1 can cause hyperglycemia or rebound weight gain. Furthermore, it can become complicated to restart GLP1 dosing, particularly given the existing medication shortages.”
“In most cases, stopping a weekly GLP-1 medication 1 week prior to the procedure minimizes the risks of pulmonary aspiration and prevents any worsening hyperglycemia or weight gain,” he said. However, taking the drug 7 days prior to the procedure is optimal. “That way, they would be due for the next dose on the day of the procedure, and taking it the day following procedure minimizes disruption in their once-weekly regimen.”
Malini Gupta, MD, director of G2Endo Endocrinology & Metabolism, Memphis, Tennessee, advised that physicians weigh the risk of stopping the medication (which can cause a glycemic spike) vs risk for aspiration.
“In my opinion, all patients should follow a strict liquid diet or NPO status prior to a surgery to further decrease the risk of aspiration,” she said. “I generally hold the GLP-1 RA for a week before a surgery. If additional glycemic control is necessary, I will add to or adjust one of the patient’s other diabetes medications.”
Jaime Almandoz, MD, associate professor of medicine and medical director of the Weight Wellness Program in Dallas, said, “As endocrinologists, we typically rely on our anesthesia colleagues for guidance on perioperative management. In light of emerging guidelines for holding GLP-1 medications, we also recommend patients adopt a liquid diet 24 hours prior to surgery, along with the fasting protocol.”
“For those managing diabetes with GLP-1 therapies, it is crucial to establish a blood sugar management plan while off these medications, especially during fasting or postoperative periods, which can be further influenced by many factors, including nausea, pain medications, and antibiotics after the procedure.”
Joshi added that at Parkland Hospital in Dallas, “we do a huge number of cases using the same information. We identify patients who are at risk, and then we tell our proceduralists and our surgeons if they’re in the escalating phase of the dosing or if they have GI symptoms; don’t even schedule them as an elective case; wait till the escalation phase is over and then schedule them.”
“That way,” he said, “it becomes logistically easy to manage because the recommendation from the group is that patients who are at higher risk should receive a 24-hour liquid diet — the same as colonoscopy. But sometimes it can be challenging to do so.”
Joshi has received honoraria for consultation from Merck Sharp & Dohme, Vertex Pharmaceuticals, and Haisco-USA Pharmaceuticals. Gupta is on the speakers bureau for Amgen (Tepezza) and IBSA (Tirosint) and is a creative consultant for AbbVie. Almandoz serves on advisory boards for Novo Nordisk, Eli Lilly, and Boehringer Ingelheim. The other experts declared no relevant relationships.
A version of this article first appeared on Medscape.com.
, as does the US Food and Drug Administration’s (FDA’s) labeling for the drugs. The changes can be challenging to keep up with, and endocrinologists seem to be making their own decisions based on clinical experience and their interpretations of the potential impact and value of the emerging information.
The latest FDA label change warns about the risk for pulmonary aspiration but notes “insufficient” data to inform recommendations to mitigate the risk in vulnerable patients. Yet, the latest multi-society guidance, led by the American Society of Anesthesiologists (ASA) and based on consensus, not evidence, has nuanced advice for managing patients at risk.
Does the FDA’s label change make a difference regarding the multi-society guidance, which was published earlier? “The answer is no,” Girish Joshi, MD, vice chair, ASA Committee on Practice Parameters, told this news organization. “The concern of increased pulmonary aspiration in patients who are on GLP-1 receptor agonists has been known, and that concern still exists. So, we started with not an assumption but the premise that patients on GLP-1 receptor agonists are at a higher risk of aspiration during sedation, analgesia, and/or general anesthesia. The FDA basically confirms what we say in the guidance.”
Joshi, professor in the Anesthesiology and Pain Management Department at UT Southwestern Medical Center, Dallas, aimed to make the guidance, which was published simultaneously in several society journals, more implementable with a letter to the editor of Anesthesiology. The key, he said, is to identify patients at higher risk for aspiration; all others would follow treatment as usual.
The letter highlights three overarching recommendations and then expands upon them: Standardized preoperative assessment for risk for delayed gastric emptying (yes/no); selective preoperative care plan based on delayed gastric emptying assessment and shared decision-making; and on the day of the procedure, reassess for delayed gastric emptying and mitigate risk if there is clinical concern.
But it seems as though, for now, endocrinologists are managing these patients as they see fit, within the parameters of any institutional guidance requirements. Here is what they said about their practice:
Amy E. Rothberg, MD, DABOM, director of the Weight Management Program & Rewind at the University of Michigan, Ann Arbor, Michigan, said, “I think it makes sense to inform our patients of the labeling and rare but potential adverse effects if they intend to undergo anesthesia for a scheduled procedure/surgery. There is never no risk of aspiration during anesthesia.”
“I find it a bit curious that ASA implies that those who experience GI side effects are more likely than those who do not to have this potential risk. I doubt there is evidence that those without GI side effects are necessarily ‘safer’ and a study to determine that is unlikely to take be conducted.”
“My institution does require a 1-week pause on GLP-1s for those undergoing anesthesia for surgery,” she added. “That’s not evidence-based either, but probably reduces the risk of aspiration during anesthesia — but I don’t know what the actual denominator is for aspiration in those who continued vs those who took a pause from GLP-1s. Pausing does certainly (anecdotally) increase the traffic of communications between physicians and their patients about what to do in the interval.”
Anne Peters, MD, a professor of clinical medicine and a clinical scholar at the Keck School of Medicine of the University of Southern California, Los Angeles, said, “The FDA label change is a warning that really doesn’t say exactly who on GLP-1 RAs is at highest risk or what to do, and if any intervention has been shown to help. The ASA recommendations seem much more nuanced and practical, including point-of-care gastric ultrasound to see if there is retained food/fluid prior to surgery.”
“In my practice, I individualize what I say, depending on the person and the circumstance,” she said. “Mostly, I have people hold one dose before planned surgery, so they have been 10 days at least without a dose. But if worried about gastrointestinal symptoms or gastroparesis, I have them do a clear liquid diet for 24 hours presurgery. Or at least avoid heavy fat meals the day before.”
“There is a risk of aspiration with anything that slows gastric emptying — maybe even in patients with gastroparesis at baseline due to physiologic, not pharmacological, reasons — and anesthesiologists should be aware of the need to assess patients individually.”
Michael A. Weintraub, MD, of NYU Langone Health Diabetes & Endocrine Associates in New York City, observed, “The risk of a pulmonary aspiration event with GLP-1 medication is quite rare, but not zero. On the other hand, stopping the GLP-1 can cause hyperglycemia or rebound weight gain. Furthermore, it can become complicated to restart GLP1 dosing, particularly given the existing medication shortages.”
“In most cases, stopping a weekly GLP-1 medication 1 week prior to the procedure minimizes the risks of pulmonary aspiration and prevents any worsening hyperglycemia or weight gain,” he said. However, taking the drug 7 days prior to the procedure is optimal. “That way, they would be due for the next dose on the day of the procedure, and taking it the day following procedure minimizes disruption in their once-weekly regimen.”
Malini Gupta, MD, director of G2Endo Endocrinology & Metabolism, Memphis, Tennessee, advised that physicians weigh the risk of stopping the medication (which can cause a glycemic spike) vs risk for aspiration.
“In my opinion, all patients should follow a strict liquid diet or NPO status prior to a surgery to further decrease the risk of aspiration,” she said. “I generally hold the GLP-1 RA for a week before a surgery. If additional glycemic control is necessary, I will add to or adjust one of the patient’s other diabetes medications.”
Jaime Almandoz, MD, associate professor of medicine and medical director of the Weight Wellness Program in Dallas, said, “As endocrinologists, we typically rely on our anesthesia colleagues for guidance on perioperative management. In light of emerging guidelines for holding GLP-1 medications, we also recommend patients adopt a liquid diet 24 hours prior to surgery, along with the fasting protocol.”
“For those managing diabetes with GLP-1 therapies, it is crucial to establish a blood sugar management plan while off these medications, especially during fasting or postoperative periods, which can be further influenced by many factors, including nausea, pain medications, and antibiotics after the procedure.”
Joshi added that at Parkland Hospital in Dallas, “we do a huge number of cases using the same information. We identify patients who are at risk, and then we tell our proceduralists and our surgeons if they’re in the escalating phase of the dosing or if they have GI symptoms; don’t even schedule them as an elective case; wait till the escalation phase is over and then schedule them.”
“That way,” he said, “it becomes logistically easy to manage because the recommendation from the group is that patients who are at higher risk should receive a 24-hour liquid diet — the same as colonoscopy. But sometimes it can be challenging to do so.”
Joshi has received honoraria for consultation from Merck Sharp & Dohme, Vertex Pharmaceuticals, and Haisco-USA Pharmaceuticals. Gupta is on the speakers bureau for Amgen (Tepezza) and IBSA (Tirosint) and is a creative consultant for AbbVie. Almandoz serves on advisory boards for Novo Nordisk, Eli Lilly, and Boehringer Ingelheim. The other experts declared no relevant relationships.
A version of this article first appeared on Medscape.com.
Continuous Glucose Monitors for All? Opinions Remain Mixed
The recent US Food and Drug Administration (FDA) clearance of two over-the-counter (OTC) continuous glucose monitors (CGMs) — Dexcom’s Stelo and Abbott’s Lingo — has sparked interest in potentially expanding their use to those without diabetes or prediabetes.
There are several valid questions about how the general population might benefit from CGMs. Can they motivate those struggling with overweight to shed pounds? Would they prompt users to follow more healthful eating patterns? Can they act as a canary in the coal mine, alerting users to prediabetes?
The short answer to these questions is, we don’t know.
“Glucose levels fluctuate in everyone in response to meals, exercise, stress, etc, but there has been no credible research to support CGM use by most people who do not have diabetes,” Jill Crandall, MD, chief of endocrinology at Albert Einstein College of Medicine and Montefiore Health System in New York City, said in an interview.
“The utility of CGM for people without diabetes hasn’t been established and the drive to market CGM as an OTC device seems largely driven by financial considerations,” Crandall said. She advocates instead for a strategy directed at more meaningful objectives.
“For now, efforts should be focused on making CGMs available to patients who will clearly benefit — ie, people with diabetes, especially those who are using insulin and those who are struggling to achieve desired levels of glucose control.”
Nicole Spartano, PhD, assistant professor of medicine in endocrinology, diabetes, nutrition and weight management at Boston University’s Chobanian & Avedisian School of Medicine in Massachusetts, agreed with this assessment.
“It is definitely too early to make recommendations for patients without diabetes based on their CGM data,” said Spartano, who also serves as the director of the Glucose Monitoring Station at the Framingham Heart Study in Framingham, Massachusetts. “We simply do not have enough follow-up data to tell us which CGM metrics are associated with higher risk for disease.”
Spartano served as the lead author of a recent study showing time spent in various CGM ranges in a large cohort of individuals without diabetes using the Dexcom G6 Pro model. In the future, she said the data may be used to establish reference ranges for clinicians and individuals.
“We are working on another paper surveying diabetologists and CGM experts about how they interpret CGM reports from individuals without diabetes,” she said in an interview. Although the data are not yet published, Spartano said, “we are finding that clinicians are currently very discordant in how they interpret these reports.”
Potential Benefits Right Now
Satish Garg, MD, director of the Adult Clinic at the Barbara Davis Center for Diabetes at the University of Colorado Anschutz Medical Campus, Aurora, and editor-in-chief of Diabetes Technology & Therapeutics, is convinced that glucose should be considered another vital sign, like blood pressure, pulse rate, respiration rate, and body temperature. Therefore, he sees the use of a CGM in people without diabetes as a way to build awareness and perhaps prompt behavior modification.
“Someone with an A1c of 4.9 on a normal day may notice that they’ve gained a little bit of weight, and if they use an OTC CGM and start seeing changes, it might help them to modulate their diet themselves, whether they see a dietitian or not,” Garg said.
He gave the example of “a natural behavioral change” occurring when someone using a CGM declines to eat a post-meal dessert after seeing their blood glucose had already risen to 170.
Wearing a CGM also has the potential to alert the user to high blood glucose, leading them to an earlier diagnosis of prediabetes or diabetes, Shichun Bao, MD, PhD, Diabetes Technology Program Leader at the Vanderbilt Eskind Diabetes Clinic of Vanderbilt University in Nashville, Tennessee, said in an interview. She has had cases where a family member of someone with diabetes used the patient’s fingerstick meter, found that their glucose was 280, and self-diagnosed with diabetes.
“It’s the same thing with the CGM,” she said. “If they somehow did not know they have diabetes and they wear a CGM and it shows their sugar is high, that will help them to know to see their provider to get a diagnosis, get treated, and track progression.”
Given the shortage of endocrinologists and long waits for appointments in the United States and elsewhere, it is very likely that primary care physicians will be the ones fielding questions from individuals without diabetes interested in purchasing an OTC CGM. Internist Douglas Paauw, MD, a professor at the University of Washington School of Medicine, Seattle, said in an interview that, for his practice, “the benefits outweigh some of the limitations.”
“I don’t really think somebody who doesn’t have diabetes needs to be using a CGM all the time or long term,” he said. “But I have used it in a few people without diabetes, and I think if someone can afford to use it for 2-4 weeks, especially if they’ve been gaining weight, then they can really recognize what happens to their bodies when they eat certain foods.”
Paauw added that CGMs are a more effective means of teaching his patients than them receiving a lecture from him on healthy eating. “There’s nothing like immediate feedback on what happens to your body to change behavior.”
Similarly, William Golden, medical director at Arkansas Medicaid and professor of medicine and public health at the University of Arkansas for Medical Sciences, Little Rock, said in an interview that “it is difficult to justify coverage for CGMs on demand — but if people want to invest in their own devices and the technology motivates them to eat better and/or lose weight, then there are benefits to be had.”
Potential Downsides
Although it may seem simple to use an OTC CGM to measure blood glucose on the fly, in the real world it can take patients time to understand these devices, “especially the first day or so, when users are going to get false lows,” Bao said. “Clinicians need to tell them if you don’t feel like your sugar is low and the device says it’s low, whether they do or don’t have diabetes, they should do a fingerstick glucose test to confirm the low before rushing to take in sugar. On the other hand, if they drink a lot of juice, their sugar will go high. So, it can create problems and false results either way.”
Many factors affect glucose, she said. “When you’re sick, glucose can go high, and when you’re very sick, in the ICU, sometimes it can be low. It depends on the situation.” Bao noted that certain vitamins and drugs can also interfere with readings.
Bao doesn’t see value in having people without diabetes monitor their glucose continuously. “If they want to see what foods or exercise do to their body, they will probably benefit from a short trial to gain some insight; otherwise, they’re wasting money,” she said.
Another potential downside is that there’s no head-to-head comparison data with the approved devices, Garg said. “But it’s clear to us that Stelo’s range is very narrow, 70 to 200, whereas the Lingo ranges are pretty much full, from 40 to 400 or 55 to 400. So, we don’t know the accuracy of these sensors.”
Golden observed that for certain patients, CGMs may lead to psychological distress rather than providing a sense of control over their blood glucose levels.
“I have had a nondiabetic patient or two that obsessed about their blood sugars and a device would only magnify their anxiety/neurosis,” he said. “The bottom line is that it’s a tool for a balanced approach to health management, but the daily results must be kept in perspective!”
Educate Patients, Primary Care Physicians
To maximize potential benefits for patients without diabetes, clinicians need to be well trained in the use and interpretation of results from the devices, Bao said. They can then better educate their patients, including discussing with them possible pitfalls surrounding their use.
“For example, a patient may see that their blood glucose, as measured by a fingerstick, is 95, whereas the CGM says 140, and ask, ‘Which one do I trust?’ ”
This is where the patient can be educated about the difference between interstitial glucose, as measured by the CGM, and blood glucose, as measured by the fingerstick. Because it takes about 15 minutes for blood glucose to get to the interstitial tissue, there’s lag time, and the two measurements will differ.
“A discrepancy of 20% is totally acceptable for that reason,” Bao said.
She has also seen several examples where patients were misled by their CGM when its censor became dislodged.
“Sometimes when a sensor has moved, the patient may push it back in because they don’t want to throw it away. But it doesn’t work that way, and they end up with inaccurate readings.”
At a minimum, Bao added, clinicians and patients should read the package insert but also be aware that it doesn’t list everything that might go wrong or interfere with the device’s accuracy.
Manufacturers of OTC devices should be training primary care and family practice doctors in their use, given the expected “huge” influx of patients wanting to use them, according to Garg.
“If you are expecting endos or diabetes specialists to see these people, that’s never going to happen,” he said. “We have a big shortage of these specialists, so industry has to train these doctors. Patients will bring their doctor’s data, and the clinicians need to learn the basics of how to interpret the glucose values they see. Then they can treat these patients rather than shipping all of them to endos who likely are not available.”
Paauw agreed that CGM training should be directed largely toward primary care professionals, who can help their under-resourced endocrinologist colleagues from seeing an uptick in “the worried well.”
“The bottom line is that primary care professionals do need to understand the CGM,” he said. “They do need to get comfortable with it. They do need to come up with opinions on how to use it. The public’s going to be using it, and we need to be competent in it and use our subspecialists appropriately.”
Spartano received funding for an investigator-initiated research grant from Novo Nordisk unrelated to the cited CGM studies. Garg , Bao, Paauw, Golden, and Crandall declared no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
The recent US Food and Drug Administration (FDA) clearance of two over-the-counter (OTC) continuous glucose monitors (CGMs) — Dexcom’s Stelo and Abbott’s Lingo — has sparked interest in potentially expanding their use to those without diabetes or prediabetes.
There are several valid questions about how the general population might benefit from CGMs. Can they motivate those struggling with overweight to shed pounds? Would they prompt users to follow more healthful eating patterns? Can they act as a canary in the coal mine, alerting users to prediabetes?
The short answer to these questions is, we don’t know.
“Glucose levels fluctuate in everyone in response to meals, exercise, stress, etc, but there has been no credible research to support CGM use by most people who do not have diabetes,” Jill Crandall, MD, chief of endocrinology at Albert Einstein College of Medicine and Montefiore Health System in New York City, said in an interview.
“The utility of CGM for people without diabetes hasn’t been established and the drive to market CGM as an OTC device seems largely driven by financial considerations,” Crandall said. She advocates instead for a strategy directed at more meaningful objectives.
“For now, efforts should be focused on making CGMs available to patients who will clearly benefit — ie, people with diabetes, especially those who are using insulin and those who are struggling to achieve desired levels of glucose control.”
Nicole Spartano, PhD, assistant professor of medicine in endocrinology, diabetes, nutrition and weight management at Boston University’s Chobanian & Avedisian School of Medicine in Massachusetts, agreed with this assessment.
“It is definitely too early to make recommendations for patients without diabetes based on their CGM data,” said Spartano, who also serves as the director of the Glucose Monitoring Station at the Framingham Heart Study in Framingham, Massachusetts. “We simply do not have enough follow-up data to tell us which CGM metrics are associated with higher risk for disease.”
Spartano served as the lead author of a recent study showing time spent in various CGM ranges in a large cohort of individuals without diabetes using the Dexcom G6 Pro model. In the future, she said the data may be used to establish reference ranges for clinicians and individuals.
“We are working on another paper surveying diabetologists and CGM experts about how they interpret CGM reports from individuals without diabetes,” she said in an interview. Although the data are not yet published, Spartano said, “we are finding that clinicians are currently very discordant in how they interpret these reports.”
Potential Benefits Right Now
Satish Garg, MD, director of the Adult Clinic at the Barbara Davis Center for Diabetes at the University of Colorado Anschutz Medical Campus, Aurora, and editor-in-chief of Diabetes Technology & Therapeutics, is convinced that glucose should be considered another vital sign, like blood pressure, pulse rate, respiration rate, and body temperature. Therefore, he sees the use of a CGM in people without diabetes as a way to build awareness and perhaps prompt behavior modification.
“Someone with an A1c of 4.9 on a normal day may notice that they’ve gained a little bit of weight, and if they use an OTC CGM and start seeing changes, it might help them to modulate their diet themselves, whether they see a dietitian or not,” Garg said.
He gave the example of “a natural behavioral change” occurring when someone using a CGM declines to eat a post-meal dessert after seeing their blood glucose had already risen to 170.
Wearing a CGM also has the potential to alert the user to high blood glucose, leading them to an earlier diagnosis of prediabetes or diabetes, Shichun Bao, MD, PhD, Diabetes Technology Program Leader at the Vanderbilt Eskind Diabetes Clinic of Vanderbilt University in Nashville, Tennessee, said in an interview. She has had cases where a family member of someone with diabetes used the patient’s fingerstick meter, found that their glucose was 280, and self-diagnosed with diabetes.
“It’s the same thing with the CGM,” she said. “If they somehow did not know they have diabetes and they wear a CGM and it shows their sugar is high, that will help them to know to see their provider to get a diagnosis, get treated, and track progression.”
Given the shortage of endocrinologists and long waits for appointments in the United States and elsewhere, it is very likely that primary care physicians will be the ones fielding questions from individuals without diabetes interested in purchasing an OTC CGM. Internist Douglas Paauw, MD, a professor at the University of Washington School of Medicine, Seattle, said in an interview that, for his practice, “the benefits outweigh some of the limitations.”
“I don’t really think somebody who doesn’t have diabetes needs to be using a CGM all the time or long term,” he said. “But I have used it in a few people without diabetes, and I think if someone can afford to use it for 2-4 weeks, especially if they’ve been gaining weight, then they can really recognize what happens to their bodies when they eat certain foods.”
Paauw added that CGMs are a more effective means of teaching his patients than them receiving a lecture from him on healthy eating. “There’s nothing like immediate feedback on what happens to your body to change behavior.”
Similarly, William Golden, medical director at Arkansas Medicaid and professor of medicine and public health at the University of Arkansas for Medical Sciences, Little Rock, said in an interview that “it is difficult to justify coverage for CGMs on demand — but if people want to invest in their own devices and the technology motivates them to eat better and/or lose weight, then there are benefits to be had.”
Potential Downsides
Although it may seem simple to use an OTC CGM to measure blood glucose on the fly, in the real world it can take patients time to understand these devices, “especially the first day or so, when users are going to get false lows,” Bao said. “Clinicians need to tell them if you don’t feel like your sugar is low and the device says it’s low, whether they do or don’t have diabetes, they should do a fingerstick glucose test to confirm the low before rushing to take in sugar. On the other hand, if they drink a lot of juice, their sugar will go high. So, it can create problems and false results either way.”
Many factors affect glucose, she said. “When you’re sick, glucose can go high, and when you’re very sick, in the ICU, sometimes it can be low. It depends on the situation.” Bao noted that certain vitamins and drugs can also interfere with readings.
Bao doesn’t see value in having people without diabetes monitor their glucose continuously. “If they want to see what foods or exercise do to their body, they will probably benefit from a short trial to gain some insight; otherwise, they’re wasting money,” she said.
Another potential downside is that there’s no head-to-head comparison data with the approved devices, Garg said. “But it’s clear to us that Stelo’s range is very narrow, 70 to 200, whereas the Lingo ranges are pretty much full, from 40 to 400 or 55 to 400. So, we don’t know the accuracy of these sensors.”
Golden observed that for certain patients, CGMs may lead to psychological distress rather than providing a sense of control over their blood glucose levels.
“I have had a nondiabetic patient or two that obsessed about their blood sugars and a device would only magnify their anxiety/neurosis,” he said. “The bottom line is that it’s a tool for a balanced approach to health management, but the daily results must be kept in perspective!”
Educate Patients, Primary Care Physicians
To maximize potential benefits for patients without diabetes, clinicians need to be well trained in the use and interpretation of results from the devices, Bao said. They can then better educate their patients, including discussing with them possible pitfalls surrounding their use.
“For example, a patient may see that their blood glucose, as measured by a fingerstick, is 95, whereas the CGM says 140, and ask, ‘Which one do I trust?’ ”
This is where the patient can be educated about the difference between interstitial glucose, as measured by the CGM, and blood glucose, as measured by the fingerstick. Because it takes about 15 minutes for blood glucose to get to the interstitial tissue, there’s lag time, and the two measurements will differ.
“A discrepancy of 20% is totally acceptable for that reason,” Bao said.
She has also seen several examples where patients were misled by their CGM when its censor became dislodged.
“Sometimes when a sensor has moved, the patient may push it back in because they don’t want to throw it away. But it doesn’t work that way, and they end up with inaccurate readings.”
At a minimum, Bao added, clinicians and patients should read the package insert but also be aware that it doesn’t list everything that might go wrong or interfere with the device’s accuracy.
Manufacturers of OTC devices should be training primary care and family practice doctors in their use, given the expected “huge” influx of patients wanting to use them, according to Garg.
“If you are expecting endos or diabetes specialists to see these people, that’s never going to happen,” he said. “We have a big shortage of these specialists, so industry has to train these doctors. Patients will bring their doctor’s data, and the clinicians need to learn the basics of how to interpret the glucose values they see. Then they can treat these patients rather than shipping all of them to endos who likely are not available.”
Paauw agreed that CGM training should be directed largely toward primary care professionals, who can help their under-resourced endocrinologist colleagues from seeing an uptick in “the worried well.”
“The bottom line is that primary care professionals do need to understand the CGM,” he said. “They do need to get comfortable with it. They do need to come up with opinions on how to use it. The public’s going to be using it, and we need to be competent in it and use our subspecialists appropriately.”
Spartano received funding for an investigator-initiated research grant from Novo Nordisk unrelated to the cited CGM studies. Garg , Bao, Paauw, Golden, and Crandall declared no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
The recent US Food and Drug Administration (FDA) clearance of two over-the-counter (OTC) continuous glucose monitors (CGMs) — Dexcom’s Stelo and Abbott’s Lingo — has sparked interest in potentially expanding their use to those without diabetes or prediabetes.
There are several valid questions about how the general population might benefit from CGMs. Can they motivate those struggling with overweight to shed pounds? Would they prompt users to follow more healthful eating patterns? Can they act as a canary in the coal mine, alerting users to prediabetes?
The short answer to these questions is, we don’t know.
“Glucose levels fluctuate in everyone in response to meals, exercise, stress, etc, but there has been no credible research to support CGM use by most people who do not have diabetes,” Jill Crandall, MD, chief of endocrinology at Albert Einstein College of Medicine and Montefiore Health System in New York City, said in an interview.
“The utility of CGM for people without diabetes hasn’t been established and the drive to market CGM as an OTC device seems largely driven by financial considerations,” Crandall said. She advocates instead for a strategy directed at more meaningful objectives.
“For now, efforts should be focused on making CGMs available to patients who will clearly benefit — ie, people with diabetes, especially those who are using insulin and those who are struggling to achieve desired levels of glucose control.”
Nicole Spartano, PhD, assistant professor of medicine in endocrinology, diabetes, nutrition and weight management at Boston University’s Chobanian & Avedisian School of Medicine in Massachusetts, agreed with this assessment.
“It is definitely too early to make recommendations for patients without diabetes based on their CGM data,” said Spartano, who also serves as the director of the Glucose Monitoring Station at the Framingham Heart Study in Framingham, Massachusetts. “We simply do not have enough follow-up data to tell us which CGM metrics are associated with higher risk for disease.”
Spartano served as the lead author of a recent study showing time spent in various CGM ranges in a large cohort of individuals without diabetes using the Dexcom G6 Pro model. In the future, she said the data may be used to establish reference ranges for clinicians and individuals.
“We are working on another paper surveying diabetologists and CGM experts about how they interpret CGM reports from individuals without diabetes,” she said in an interview. Although the data are not yet published, Spartano said, “we are finding that clinicians are currently very discordant in how they interpret these reports.”
Potential Benefits Right Now
Satish Garg, MD, director of the Adult Clinic at the Barbara Davis Center for Diabetes at the University of Colorado Anschutz Medical Campus, Aurora, and editor-in-chief of Diabetes Technology & Therapeutics, is convinced that glucose should be considered another vital sign, like blood pressure, pulse rate, respiration rate, and body temperature. Therefore, he sees the use of a CGM in people without diabetes as a way to build awareness and perhaps prompt behavior modification.
“Someone with an A1c of 4.9 on a normal day may notice that they’ve gained a little bit of weight, and if they use an OTC CGM and start seeing changes, it might help them to modulate their diet themselves, whether they see a dietitian or not,” Garg said.
He gave the example of “a natural behavioral change” occurring when someone using a CGM declines to eat a post-meal dessert after seeing their blood glucose had already risen to 170.
Wearing a CGM also has the potential to alert the user to high blood glucose, leading them to an earlier diagnosis of prediabetes or diabetes, Shichun Bao, MD, PhD, Diabetes Technology Program Leader at the Vanderbilt Eskind Diabetes Clinic of Vanderbilt University in Nashville, Tennessee, said in an interview. She has had cases where a family member of someone with diabetes used the patient’s fingerstick meter, found that their glucose was 280, and self-diagnosed with diabetes.
“It’s the same thing with the CGM,” she said. “If they somehow did not know they have diabetes and they wear a CGM and it shows their sugar is high, that will help them to know to see their provider to get a diagnosis, get treated, and track progression.”
Given the shortage of endocrinologists and long waits for appointments in the United States and elsewhere, it is very likely that primary care physicians will be the ones fielding questions from individuals without diabetes interested in purchasing an OTC CGM. Internist Douglas Paauw, MD, a professor at the University of Washington School of Medicine, Seattle, said in an interview that, for his practice, “the benefits outweigh some of the limitations.”
“I don’t really think somebody who doesn’t have diabetes needs to be using a CGM all the time or long term,” he said. “But I have used it in a few people without diabetes, and I think if someone can afford to use it for 2-4 weeks, especially if they’ve been gaining weight, then they can really recognize what happens to their bodies when they eat certain foods.”
Paauw added that CGMs are a more effective means of teaching his patients than them receiving a lecture from him on healthy eating. “There’s nothing like immediate feedback on what happens to your body to change behavior.”
Similarly, William Golden, medical director at Arkansas Medicaid and professor of medicine and public health at the University of Arkansas for Medical Sciences, Little Rock, said in an interview that “it is difficult to justify coverage for CGMs on demand — but if people want to invest in their own devices and the technology motivates them to eat better and/or lose weight, then there are benefits to be had.”
Potential Downsides
Although it may seem simple to use an OTC CGM to measure blood glucose on the fly, in the real world it can take patients time to understand these devices, “especially the first day or so, when users are going to get false lows,” Bao said. “Clinicians need to tell them if you don’t feel like your sugar is low and the device says it’s low, whether they do or don’t have diabetes, they should do a fingerstick glucose test to confirm the low before rushing to take in sugar. On the other hand, if they drink a lot of juice, their sugar will go high. So, it can create problems and false results either way.”
Many factors affect glucose, she said. “When you’re sick, glucose can go high, and when you’re very sick, in the ICU, sometimes it can be low. It depends on the situation.” Bao noted that certain vitamins and drugs can also interfere with readings.
Bao doesn’t see value in having people without diabetes monitor their glucose continuously. “If they want to see what foods or exercise do to their body, they will probably benefit from a short trial to gain some insight; otherwise, they’re wasting money,” she said.
Another potential downside is that there’s no head-to-head comparison data with the approved devices, Garg said. “But it’s clear to us that Stelo’s range is very narrow, 70 to 200, whereas the Lingo ranges are pretty much full, from 40 to 400 or 55 to 400. So, we don’t know the accuracy of these sensors.”
Golden observed that for certain patients, CGMs may lead to psychological distress rather than providing a sense of control over their blood glucose levels.
“I have had a nondiabetic patient or two that obsessed about their blood sugars and a device would only magnify their anxiety/neurosis,” he said. “The bottom line is that it’s a tool for a balanced approach to health management, but the daily results must be kept in perspective!”
Educate Patients, Primary Care Physicians
To maximize potential benefits for patients without diabetes, clinicians need to be well trained in the use and interpretation of results from the devices, Bao said. They can then better educate their patients, including discussing with them possible pitfalls surrounding their use.
“For example, a patient may see that their blood glucose, as measured by a fingerstick, is 95, whereas the CGM says 140, and ask, ‘Which one do I trust?’ ”
This is where the patient can be educated about the difference between interstitial glucose, as measured by the CGM, and blood glucose, as measured by the fingerstick. Because it takes about 15 minutes for blood glucose to get to the interstitial tissue, there’s lag time, and the two measurements will differ.
“A discrepancy of 20% is totally acceptable for that reason,” Bao said.
She has also seen several examples where patients were misled by their CGM when its censor became dislodged.
“Sometimes when a sensor has moved, the patient may push it back in because they don’t want to throw it away. But it doesn’t work that way, and they end up with inaccurate readings.”
At a minimum, Bao added, clinicians and patients should read the package insert but also be aware that it doesn’t list everything that might go wrong or interfere with the device’s accuracy.
Manufacturers of OTC devices should be training primary care and family practice doctors in their use, given the expected “huge” influx of patients wanting to use them, according to Garg.
“If you are expecting endos or diabetes specialists to see these people, that’s never going to happen,” he said. “We have a big shortage of these specialists, so industry has to train these doctors. Patients will bring their doctor’s data, and the clinicians need to learn the basics of how to interpret the glucose values they see. Then they can treat these patients rather than shipping all of them to endos who likely are not available.”
Paauw agreed that CGM training should be directed largely toward primary care professionals, who can help their under-resourced endocrinologist colleagues from seeing an uptick in “the worried well.”
“The bottom line is that primary care professionals do need to understand the CGM,” he said. “They do need to get comfortable with it. They do need to come up with opinions on how to use it. The public’s going to be using it, and we need to be competent in it and use our subspecialists appropriately.”
Spartano received funding for an investigator-initiated research grant from Novo Nordisk unrelated to the cited CGM studies. Garg , Bao, Paauw, Golden, and Crandall declared no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Doctors Caution Over Weight Loss Drug Link to Nurse’s Death
Doctors have urged caution in linking the weight loss drug tirzepatide to the death of a 58-year-old nurse from Scotland.
Susan McGowan, from North Lanarkshire, took two low-dose injections of tirzepatide (Mounjaro) over the course of about 2 weeks before her death in September.
BBC News reported that multiple organ failure, septic shock, and pancreatitis were listed on her death certificate as the immediate cause of death, with “the use of prescribed tirzepatide” recorded as a contributing factor.
McGowan worked as a nurse at University Hospital Monklands in Airdrie. A family member said that, apart from carrying a “bit of extra weight,” she had been otherwise healthy and was not taking any other medication.
It is understood that McGowan had sought medical advice before purchasing a prescription for tirzepatide through a registered UK pharmacy. However, days after administering a second injection, she went to A&E at Monklands with severe stomach pain and sickness. She died on September 4.
Expert Insights
Commenting to the Science Media Centre (SMC), Amanda Adler, MD, PhD, professor of diabetic medicine and health policy at the University of Oxford, described the nurse’s death as “sad” but said that “whether or not it was related to tirzepatide may be difficult to prove.” While tirzepatide can be associated with uncommon problems such as acute pancreatitis, “one can develop acute pancreatitis for many other reasons as well,” she said.
Naveed Sattar, MD, PhD, professor of metabolic medicine at the University of Glasgow, noted that data from multiple trials of tirzepatide, involving around 10,000 people living with diabetes or obesity, “do not suggest a higher risk of pancreatitis.” Furthermore, “the data seem to show acceptable safety thus far and a range of benefits including sizable average weight loss (near 20%), strong diabetes prevention, and considerable benefits in people living with sleep apnea,” he told the SMC.
Approved Based on Extensive Assessment
Tirzepatide, a GLP-1 receptor agonist, was approved for use as a weight loss aid in the United Kingdom in November last year by the Medicines and Healthcare products Regulatory Agency (MHRA). It lists nausea, diarrhea, and vomiting as the most common side effects, as well as hypoglycemia for patients with diabetes.
Available figures under the Yellow Card scheme up to 19 May 2024 show that there were 208 adverse drug reactions reported about tirzepatide this year, including 31 serious reactions and one suspected death of a man in his 60s.
In a statement, a spokesperson for the drug’s manufacturer, Eli Lilly, said, “Patient safety is Lilly’s top priority. We are committed to continually monitoring, evaluating, and reporting safety information for all Lilly medicines.
“Mounjaro (tirzepatide) was approved based on extensive assessment of the benefits and risks of the medicine, and we provide information about the benefits and risks of all our medicines to regulators around the world to ensure the latest information is available for prescribers. If anyone is experiencing side effects when taking any Lilly medicine, they should talk to their doctor or other healthcare professional.”
In October, the NHS submitted plans to the National Institute for Health and Care Excellence (NICE) for a phased rollout of tirzepatide in England that would initially prioritize patients with the greatest clinical need. The first phase would see the drug available to people with a body mass index of more than 40 kg/m2 who also suffer from at least three of the main weight-related health problems: hypertension, dyslipidemia, obstructive sleep apnea, and cardiovascular disease.
“Our sincere sympathies are with the family of individual concerned,” said Alison Cave, MHRA Chief Safety Officer.
“Patient safety is our top priority and no medicine would be approved unless it met our expected standards of safety, quality, and effectiveness. Our role is to continually monitor the safety of medicines during their use, such as GLP-1 RAs. We have robust, safety monitoring and surveillance systems in place for all healthcare products.
“New medicines, such as tirzepatide, are more intensively monitored to ensure that any new safety issues are identified promptly. We strongly encourage the reporting of all suspected reactions to newer medicines, which are denoted by an inverted Black Triangle symbol.
“On the basis of the current evidence the benefits of GLP-1 RAs outweigh the potential risks when used for the licensed indications. The decision to start, continue, or stop treatments should be made jointly by patients and their doctor, based on full consideration of the benefits and risks.”
She encouraged patients and healthcare professionals to continue reporting suspected side effects to GLP-1 RAs, such as tirzepatide, through the Yellow Card Scheme. “When a safety issue is confirmed, we always act promptly to inform patients and healthcare professionals and take appropriate steps to mitigate any identified risk.”
The Department of Health and Social Care declined to comment.
Adler disclosed being involved as an unpaid investigator on an Eli Lilly–funded trial for a different drug. Sattar has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Doctors have urged caution in linking the weight loss drug tirzepatide to the death of a 58-year-old nurse from Scotland.
Susan McGowan, from North Lanarkshire, took two low-dose injections of tirzepatide (Mounjaro) over the course of about 2 weeks before her death in September.
BBC News reported that multiple organ failure, septic shock, and pancreatitis were listed on her death certificate as the immediate cause of death, with “the use of prescribed tirzepatide” recorded as a contributing factor.
McGowan worked as a nurse at University Hospital Monklands in Airdrie. A family member said that, apart from carrying a “bit of extra weight,” she had been otherwise healthy and was not taking any other medication.
It is understood that McGowan had sought medical advice before purchasing a prescription for tirzepatide through a registered UK pharmacy. However, days after administering a second injection, she went to A&E at Monklands with severe stomach pain and sickness. She died on September 4.
Expert Insights
Commenting to the Science Media Centre (SMC), Amanda Adler, MD, PhD, professor of diabetic medicine and health policy at the University of Oxford, described the nurse’s death as “sad” but said that “whether or not it was related to tirzepatide may be difficult to prove.” While tirzepatide can be associated with uncommon problems such as acute pancreatitis, “one can develop acute pancreatitis for many other reasons as well,” she said.
Naveed Sattar, MD, PhD, professor of metabolic medicine at the University of Glasgow, noted that data from multiple trials of tirzepatide, involving around 10,000 people living with diabetes or obesity, “do not suggest a higher risk of pancreatitis.” Furthermore, “the data seem to show acceptable safety thus far and a range of benefits including sizable average weight loss (near 20%), strong diabetes prevention, and considerable benefits in people living with sleep apnea,” he told the SMC.
Approved Based on Extensive Assessment
Tirzepatide, a GLP-1 receptor agonist, was approved for use as a weight loss aid in the United Kingdom in November last year by the Medicines and Healthcare products Regulatory Agency (MHRA). It lists nausea, diarrhea, and vomiting as the most common side effects, as well as hypoglycemia for patients with diabetes.
Available figures under the Yellow Card scheme up to 19 May 2024 show that there were 208 adverse drug reactions reported about tirzepatide this year, including 31 serious reactions and one suspected death of a man in his 60s.
In a statement, a spokesperson for the drug’s manufacturer, Eli Lilly, said, “Patient safety is Lilly’s top priority. We are committed to continually monitoring, evaluating, and reporting safety information for all Lilly medicines.
“Mounjaro (tirzepatide) was approved based on extensive assessment of the benefits and risks of the medicine, and we provide information about the benefits and risks of all our medicines to regulators around the world to ensure the latest information is available for prescribers. If anyone is experiencing side effects when taking any Lilly medicine, they should talk to their doctor or other healthcare professional.”
In October, the NHS submitted plans to the National Institute for Health and Care Excellence (NICE) for a phased rollout of tirzepatide in England that would initially prioritize patients with the greatest clinical need. The first phase would see the drug available to people with a body mass index of more than 40 kg/m2 who also suffer from at least three of the main weight-related health problems: hypertension, dyslipidemia, obstructive sleep apnea, and cardiovascular disease.
“Our sincere sympathies are with the family of individual concerned,” said Alison Cave, MHRA Chief Safety Officer.
“Patient safety is our top priority and no medicine would be approved unless it met our expected standards of safety, quality, and effectiveness. Our role is to continually monitor the safety of medicines during their use, such as GLP-1 RAs. We have robust, safety monitoring and surveillance systems in place for all healthcare products.
“New medicines, such as tirzepatide, are more intensively monitored to ensure that any new safety issues are identified promptly. We strongly encourage the reporting of all suspected reactions to newer medicines, which are denoted by an inverted Black Triangle symbol.
“On the basis of the current evidence the benefits of GLP-1 RAs outweigh the potential risks when used for the licensed indications. The decision to start, continue, or stop treatments should be made jointly by patients and their doctor, based on full consideration of the benefits and risks.”
She encouraged patients and healthcare professionals to continue reporting suspected side effects to GLP-1 RAs, such as tirzepatide, through the Yellow Card Scheme. “When a safety issue is confirmed, we always act promptly to inform patients and healthcare professionals and take appropriate steps to mitigate any identified risk.”
The Department of Health and Social Care declined to comment.
Adler disclosed being involved as an unpaid investigator on an Eli Lilly–funded trial for a different drug. Sattar has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Doctors have urged caution in linking the weight loss drug tirzepatide to the death of a 58-year-old nurse from Scotland.
Susan McGowan, from North Lanarkshire, took two low-dose injections of tirzepatide (Mounjaro) over the course of about 2 weeks before her death in September.
BBC News reported that multiple organ failure, septic shock, and pancreatitis were listed on her death certificate as the immediate cause of death, with “the use of prescribed tirzepatide” recorded as a contributing factor.
McGowan worked as a nurse at University Hospital Monklands in Airdrie. A family member said that, apart from carrying a “bit of extra weight,” she had been otherwise healthy and was not taking any other medication.
It is understood that McGowan had sought medical advice before purchasing a prescription for tirzepatide through a registered UK pharmacy. However, days after administering a second injection, she went to A&E at Monklands with severe stomach pain and sickness. She died on September 4.
Expert Insights
Commenting to the Science Media Centre (SMC), Amanda Adler, MD, PhD, professor of diabetic medicine and health policy at the University of Oxford, described the nurse’s death as “sad” but said that “whether or not it was related to tirzepatide may be difficult to prove.” While tirzepatide can be associated with uncommon problems such as acute pancreatitis, “one can develop acute pancreatitis for many other reasons as well,” she said.
Naveed Sattar, MD, PhD, professor of metabolic medicine at the University of Glasgow, noted that data from multiple trials of tirzepatide, involving around 10,000 people living with diabetes or obesity, “do not suggest a higher risk of pancreatitis.” Furthermore, “the data seem to show acceptable safety thus far and a range of benefits including sizable average weight loss (near 20%), strong diabetes prevention, and considerable benefits in people living with sleep apnea,” he told the SMC.
Approved Based on Extensive Assessment
Tirzepatide, a GLP-1 receptor agonist, was approved for use as a weight loss aid in the United Kingdom in November last year by the Medicines and Healthcare products Regulatory Agency (MHRA). It lists nausea, diarrhea, and vomiting as the most common side effects, as well as hypoglycemia for patients with diabetes.
Available figures under the Yellow Card scheme up to 19 May 2024 show that there were 208 adverse drug reactions reported about tirzepatide this year, including 31 serious reactions and one suspected death of a man in his 60s.
In a statement, a spokesperson for the drug’s manufacturer, Eli Lilly, said, “Patient safety is Lilly’s top priority. We are committed to continually monitoring, evaluating, and reporting safety information for all Lilly medicines.
“Mounjaro (tirzepatide) was approved based on extensive assessment of the benefits and risks of the medicine, and we provide information about the benefits and risks of all our medicines to regulators around the world to ensure the latest information is available for prescribers. If anyone is experiencing side effects when taking any Lilly medicine, they should talk to their doctor or other healthcare professional.”
In October, the NHS submitted plans to the National Institute for Health and Care Excellence (NICE) for a phased rollout of tirzepatide in England that would initially prioritize patients with the greatest clinical need. The first phase would see the drug available to people with a body mass index of more than 40 kg/m2 who also suffer from at least three of the main weight-related health problems: hypertension, dyslipidemia, obstructive sleep apnea, and cardiovascular disease.
“Our sincere sympathies are with the family of individual concerned,” said Alison Cave, MHRA Chief Safety Officer.
“Patient safety is our top priority and no medicine would be approved unless it met our expected standards of safety, quality, and effectiveness. Our role is to continually monitor the safety of medicines during their use, such as GLP-1 RAs. We have robust, safety monitoring and surveillance systems in place for all healthcare products.
“New medicines, such as tirzepatide, are more intensively monitored to ensure that any new safety issues are identified promptly. We strongly encourage the reporting of all suspected reactions to newer medicines, which are denoted by an inverted Black Triangle symbol.
“On the basis of the current evidence the benefits of GLP-1 RAs outweigh the potential risks when used for the licensed indications. The decision to start, continue, or stop treatments should be made jointly by patients and their doctor, based on full consideration of the benefits and risks.”
She encouraged patients and healthcare professionals to continue reporting suspected side effects to GLP-1 RAs, such as tirzepatide, through the Yellow Card Scheme. “When a safety issue is confirmed, we always act promptly to inform patients and healthcare professionals and take appropriate steps to mitigate any identified risk.”
The Department of Health and Social Care declined to comment.
Adler disclosed being involved as an unpaid investigator on an Eli Lilly–funded trial for a different drug. Sattar has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Is Acute Kidney Injury Really a Single Disease?
The search for a better biomarker than creatine for acute kidney injury (AKI) has been “long and elusive.” However, could researchers be on the right path now?
“The thinking is moving away from trying to find one biomarker that can be used for different types of kidney injury to a recognition that AKI is not just a single disease that a patient has or doesn’t have,” Rob D. Nerenz, PhD, an associate professor in the Department of Pathology and Laboratory Medicine at the Medical College of Wisconsin, Milwaukee, told this news organization. “It’s lots of different diseases that all affect the kidney in different ways.”
AKI is actually a “loose collection” of hepatorenal, cardiorenal, nephrotoxic, and sepsis-associated syndromes, as well as acute interstitial nephritis (AIN), he said. “So the question is not: ‘Is AKI present — yes or no?’ It’s: ‘What kind of AKI is present, and how do I treat it?’ ”
‘Mediocre Markers’
AKI affects about 10%-30% of hospitalized patients, according to Nerenz. It’s associated with an increased risk for adverse outcomes, including post-AKI chronic kidney disease and a mortality rate of approximately 24%.
Currently, AKI is defined by a rapid increase in serum creatinine, a decrease in urine output, or both.
“Those are mediocre markers,” Nerenz said, as serum creatinine is not very sensitive to acute change, and the increase is often detected after the therapeutic window of intervention has passed. In addition, “it only tells us that the kidneys are unhappy; it doesn’t say anything about the cause.”
Urine output is limited as a marker because many conditions affect it. “If you’re dehydrated, urine output is going to decrease,” he said. “And in some forms of AKI, urine output actually goes up.”
What’s needed, he said, is a more sensitive biomarker that’s detectable within a shorter timeframe of 2-6 hours following injury.
“Right now, we’re looking at 48 hours before a change becomes apparent, and that’s just too long. Plus, it should be kidney specific. One of the major limitations of the biomarkers that have been evaluated to this point is that, yes, they’re released by the kidney, but they’re also released by other tissue types within the body, and that hinders their effectiveness as a marker.”
Neutrophil Gelatinase-Associated Lipocalin (NGAL)
Although research on better biomarkers is ongoing, “there’s also a recognition that some of the protein markers that have been around for a while, if used appropriately, can provide value,” Nerenz said. These include, among others, NGAL.
NGAL works well in pediatric patients without other comorbidities, but it has been less useful in adult patients because it is also released by other cell types. However, recent research suggests it shows promise in patients with both cirrhosis and AKI.
There are three main causes of AKI in cirrhosis, Nerenz explained. The first is prerenal and can be primarily addressed through rehydration.
“When these patients come in, clinicians won’t do anything right away other than provide fluids. If creatinine improves over the 48-hour period of fluid replenishment, then the patient is sent home because there really isn’t extensive damage to the kidneys.”
If improvement isn’t seen after those 48 hours, then it could be one of two things: Hepatorenal syndrome or acute tubular necrosis. Patients with hepatorenal syndrome are candidates for terlipressin, which the Food and Drug Administration (FDA) approved for this indication in 2022 after it displayed notable efficacy in a double-blind study.
“You don’t want to give terlipressin to just anybody because if the issue is not a diminished blood supply to the kidney, it’s not going to help, and comes with some serious side effects, such as respiratory failure,” Nerenz explained. “Having a biomarker that can distinguish between hepatorenal syndrome and acute tubular necrosis really helps clinicians confidently identify which patients are good candidates for this drug. Right now, we’re flying blind to a certain extent, basically using clinical intuition.”
Currently, the determination of NGAL is FDA cleared only for pediatric use. One way hospitals have dealt with that is by making the test in their own labs, using appropriate reagents, validation, and so forth. These tests are then safe for use in adults but haven’t gone through the FDA approval process.
However, the FDA’s recent announcement stating that the agency should oversee lab-developed tests has made this situation unclear, Nerenz said.
“At this point, we don’t know if there’s still an opportunity to take the NGAL test (or any other cleared biomarker) and validate it for use in a different patient population. Many hospital labs simply don’t have the resources to take these tests through the whole FDA approval process.”
A New Biomarker for AIN?
Meanwhile, research is also moving forward on a better biomarker for AIN, which is also under the AKI umbrella.
“It’s important to diagnose AIN because it has a very specific treatment,” Dennis G. Moledina, MD, PhD, Yale School of Medicine in New Haven, Connecticut, told this news organization.
“AIN is caused by a bunch of different medications, such as proton pump inhibitors, cancer drugs, nonsteroidal anti-inflammatory drugs, and antibiotics, so when someone has this condition, you have to stop potentially life-saving medications and give unnecessary and potentially toxic immunosuppressive drugs, like prednisone,” he said. “If you get the diagnosis wrong, you’re stopping vital drugs and giving immunosuppression for no reason. And if you miss the diagnosis, AIN can lead to permanent chronic kidney disease.”
“Right now, the only way to diagnose AIN is to do a kidney biopsy, which is risky because it can often lead to significant bleeding,” he said. “Some people can’t undergo a biopsy because they’re on medications that increase the risk of bleeding, and they can’t be stopped.”
Furthermore, he noted, “the longer a patient takes a drug that’s causing AIN without getting a diagnosis, the less the chances of recovery because the longer you let this kidney inflammation go on, the more fibrosis and permanent damage develops. So it is important to diagnose it as early as possible, and that’s again why we have a real need for a noninvasive biomarker that can be tested rapidly.”
Moledina and colleagues have been working on identifying a suitable biomarker for close to 10 years, the latest example of which is their 2023 study validating urinary CXCL9 as just such a marker.
“We’re most excited about CXCL9 because it’s already used to diagnose some other diseases in plasma,” Moledina said. “We think that we can convince labs to test it in urine.”
In an accompanying editorial, Mark Canney, PhD, and colleagues at the University of Ottawa and The Ottawa Hospital in Ontario, Canada, wrote that the CXCL9 study findings “are exciting because they provide a road map of where diagnostics can get to for this common, yet poorly identified and treated, cause of kidney damage. The need for a different approach can be readily identified from the fact that clinicians’ gestalt for diagnosing AIN was almost tantamount to tossing a coin (AUC, 0.57). CXCL9 alone outperformed not only the clinician’s prebiopsy suspicion but also an existing diagnostic model and other candidate biomarkers both in the discovery and external validation cohorts.”
Like NGAL, CXCL9 will have to go through the FDA approval process before it can be used for AIN. Therefore, it may be a few years before it can become routinely available, Moledina said.
Nevertheless, Nerenz added, “I think the next steps for AKI are probably continuing on this path of context-dependent, selective biomarker use. I anticipate that we’ll see ongoing development in this space, just expanding to a wider variety of clinical scenarios.”
Nerenz declared receiving research funding from Abbott Labs for evaluation of an AKI biomarker. Moledina is a co-inventor on a pending patent, “Methods and Systems for Diagnosis of Acute Interstitial Nephritis”; a cofounder of the diagnostics company Predict AIN; and a consultant for Biohaven.
A version of this article first appeared on Medscape.com.
The search for a better biomarker than creatine for acute kidney injury (AKI) has been “long and elusive.” However, could researchers be on the right path now?
“The thinking is moving away from trying to find one biomarker that can be used for different types of kidney injury to a recognition that AKI is not just a single disease that a patient has or doesn’t have,” Rob D. Nerenz, PhD, an associate professor in the Department of Pathology and Laboratory Medicine at the Medical College of Wisconsin, Milwaukee, told this news organization. “It’s lots of different diseases that all affect the kidney in different ways.”
AKI is actually a “loose collection” of hepatorenal, cardiorenal, nephrotoxic, and sepsis-associated syndromes, as well as acute interstitial nephritis (AIN), he said. “So the question is not: ‘Is AKI present — yes or no?’ It’s: ‘What kind of AKI is present, and how do I treat it?’ ”
‘Mediocre Markers’
AKI affects about 10%-30% of hospitalized patients, according to Nerenz. It’s associated with an increased risk for adverse outcomes, including post-AKI chronic kidney disease and a mortality rate of approximately 24%.
Currently, AKI is defined by a rapid increase in serum creatinine, a decrease in urine output, or both.
“Those are mediocre markers,” Nerenz said, as serum creatinine is not very sensitive to acute change, and the increase is often detected after the therapeutic window of intervention has passed. In addition, “it only tells us that the kidneys are unhappy; it doesn’t say anything about the cause.”
Urine output is limited as a marker because many conditions affect it. “If you’re dehydrated, urine output is going to decrease,” he said. “And in some forms of AKI, urine output actually goes up.”
What’s needed, he said, is a more sensitive biomarker that’s detectable within a shorter timeframe of 2-6 hours following injury.
“Right now, we’re looking at 48 hours before a change becomes apparent, and that’s just too long. Plus, it should be kidney specific. One of the major limitations of the biomarkers that have been evaluated to this point is that, yes, they’re released by the kidney, but they’re also released by other tissue types within the body, and that hinders their effectiveness as a marker.”
Neutrophil Gelatinase-Associated Lipocalin (NGAL)
Although research on better biomarkers is ongoing, “there’s also a recognition that some of the protein markers that have been around for a while, if used appropriately, can provide value,” Nerenz said. These include, among others, NGAL.
NGAL works well in pediatric patients without other comorbidities, but it has been less useful in adult patients because it is also released by other cell types. However, recent research suggests it shows promise in patients with both cirrhosis and AKI.
There are three main causes of AKI in cirrhosis, Nerenz explained. The first is prerenal and can be primarily addressed through rehydration.
“When these patients come in, clinicians won’t do anything right away other than provide fluids. If creatinine improves over the 48-hour period of fluid replenishment, then the patient is sent home because there really isn’t extensive damage to the kidneys.”
If improvement isn’t seen after those 48 hours, then it could be one of two things: Hepatorenal syndrome or acute tubular necrosis. Patients with hepatorenal syndrome are candidates for terlipressin, which the Food and Drug Administration (FDA) approved for this indication in 2022 after it displayed notable efficacy in a double-blind study.
“You don’t want to give terlipressin to just anybody because if the issue is not a diminished blood supply to the kidney, it’s not going to help, and comes with some serious side effects, such as respiratory failure,” Nerenz explained. “Having a biomarker that can distinguish between hepatorenal syndrome and acute tubular necrosis really helps clinicians confidently identify which patients are good candidates for this drug. Right now, we’re flying blind to a certain extent, basically using clinical intuition.”
Currently, the determination of NGAL is FDA cleared only for pediatric use. One way hospitals have dealt with that is by making the test in their own labs, using appropriate reagents, validation, and so forth. These tests are then safe for use in adults but haven’t gone through the FDA approval process.
However, the FDA’s recent announcement stating that the agency should oversee lab-developed tests has made this situation unclear, Nerenz said.
“At this point, we don’t know if there’s still an opportunity to take the NGAL test (or any other cleared biomarker) and validate it for use in a different patient population. Many hospital labs simply don’t have the resources to take these tests through the whole FDA approval process.”
A New Biomarker for AIN?
Meanwhile, research is also moving forward on a better biomarker for AIN, which is also under the AKI umbrella.
“It’s important to diagnose AIN because it has a very specific treatment,” Dennis G. Moledina, MD, PhD, Yale School of Medicine in New Haven, Connecticut, told this news organization.
“AIN is caused by a bunch of different medications, such as proton pump inhibitors, cancer drugs, nonsteroidal anti-inflammatory drugs, and antibiotics, so when someone has this condition, you have to stop potentially life-saving medications and give unnecessary and potentially toxic immunosuppressive drugs, like prednisone,” he said. “If you get the diagnosis wrong, you’re stopping vital drugs and giving immunosuppression for no reason. And if you miss the diagnosis, AIN can lead to permanent chronic kidney disease.”
“Right now, the only way to diagnose AIN is to do a kidney biopsy, which is risky because it can often lead to significant bleeding,” he said. “Some people can’t undergo a biopsy because they’re on medications that increase the risk of bleeding, and they can’t be stopped.”
Furthermore, he noted, “the longer a patient takes a drug that’s causing AIN without getting a diagnosis, the less the chances of recovery because the longer you let this kidney inflammation go on, the more fibrosis and permanent damage develops. So it is important to diagnose it as early as possible, and that’s again why we have a real need for a noninvasive biomarker that can be tested rapidly.”
Moledina and colleagues have been working on identifying a suitable biomarker for close to 10 years, the latest example of which is their 2023 study validating urinary CXCL9 as just such a marker.
“We’re most excited about CXCL9 because it’s already used to diagnose some other diseases in plasma,” Moledina said. “We think that we can convince labs to test it in urine.”
In an accompanying editorial, Mark Canney, PhD, and colleagues at the University of Ottawa and The Ottawa Hospital in Ontario, Canada, wrote that the CXCL9 study findings “are exciting because they provide a road map of where diagnostics can get to for this common, yet poorly identified and treated, cause of kidney damage. The need for a different approach can be readily identified from the fact that clinicians’ gestalt for diagnosing AIN was almost tantamount to tossing a coin (AUC, 0.57). CXCL9 alone outperformed not only the clinician’s prebiopsy suspicion but also an existing diagnostic model and other candidate biomarkers both in the discovery and external validation cohorts.”
Like NGAL, CXCL9 will have to go through the FDA approval process before it can be used for AIN. Therefore, it may be a few years before it can become routinely available, Moledina said.
Nevertheless, Nerenz added, “I think the next steps for AKI are probably continuing on this path of context-dependent, selective biomarker use. I anticipate that we’ll see ongoing development in this space, just expanding to a wider variety of clinical scenarios.”
Nerenz declared receiving research funding from Abbott Labs for evaluation of an AKI biomarker. Moledina is a co-inventor on a pending patent, “Methods and Systems for Diagnosis of Acute Interstitial Nephritis”; a cofounder of the diagnostics company Predict AIN; and a consultant for Biohaven.
A version of this article first appeared on Medscape.com.
The search for a better biomarker than creatine for acute kidney injury (AKI) has been “long and elusive.” However, could researchers be on the right path now?
“The thinking is moving away from trying to find one biomarker that can be used for different types of kidney injury to a recognition that AKI is not just a single disease that a patient has or doesn’t have,” Rob D. Nerenz, PhD, an associate professor in the Department of Pathology and Laboratory Medicine at the Medical College of Wisconsin, Milwaukee, told this news organization. “It’s lots of different diseases that all affect the kidney in different ways.”
AKI is actually a “loose collection” of hepatorenal, cardiorenal, nephrotoxic, and sepsis-associated syndromes, as well as acute interstitial nephritis (AIN), he said. “So the question is not: ‘Is AKI present — yes or no?’ It’s: ‘What kind of AKI is present, and how do I treat it?’ ”
‘Mediocre Markers’
AKI affects about 10%-30% of hospitalized patients, according to Nerenz. It’s associated with an increased risk for adverse outcomes, including post-AKI chronic kidney disease and a mortality rate of approximately 24%.
Currently, AKI is defined by a rapid increase in serum creatinine, a decrease in urine output, or both.
“Those are mediocre markers,” Nerenz said, as serum creatinine is not very sensitive to acute change, and the increase is often detected after the therapeutic window of intervention has passed. In addition, “it only tells us that the kidneys are unhappy; it doesn’t say anything about the cause.”
Urine output is limited as a marker because many conditions affect it. “If you’re dehydrated, urine output is going to decrease,” he said. “And in some forms of AKI, urine output actually goes up.”
What’s needed, he said, is a more sensitive biomarker that’s detectable within a shorter timeframe of 2-6 hours following injury.
“Right now, we’re looking at 48 hours before a change becomes apparent, and that’s just too long. Plus, it should be kidney specific. One of the major limitations of the biomarkers that have been evaluated to this point is that, yes, they’re released by the kidney, but they’re also released by other tissue types within the body, and that hinders their effectiveness as a marker.”
Neutrophil Gelatinase-Associated Lipocalin (NGAL)
Although research on better biomarkers is ongoing, “there’s also a recognition that some of the protein markers that have been around for a while, if used appropriately, can provide value,” Nerenz said. These include, among others, NGAL.
NGAL works well in pediatric patients without other comorbidities, but it has been less useful in adult patients because it is also released by other cell types. However, recent research suggests it shows promise in patients with both cirrhosis and AKI.
There are three main causes of AKI in cirrhosis, Nerenz explained. The first is prerenal and can be primarily addressed through rehydration.
“When these patients come in, clinicians won’t do anything right away other than provide fluids. If creatinine improves over the 48-hour period of fluid replenishment, then the patient is sent home because there really isn’t extensive damage to the kidneys.”
If improvement isn’t seen after those 48 hours, then it could be one of two things: Hepatorenal syndrome or acute tubular necrosis. Patients with hepatorenal syndrome are candidates for terlipressin, which the Food and Drug Administration (FDA) approved for this indication in 2022 after it displayed notable efficacy in a double-blind study.
“You don’t want to give terlipressin to just anybody because if the issue is not a diminished blood supply to the kidney, it’s not going to help, and comes with some serious side effects, such as respiratory failure,” Nerenz explained. “Having a biomarker that can distinguish between hepatorenal syndrome and acute tubular necrosis really helps clinicians confidently identify which patients are good candidates for this drug. Right now, we’re flying blind to a certain extent, basically using clinical intuition.”
Currently, the determination of NGAL is FDA cleared only for pediatric use. One way hospitals have dealt with that is by making the test in their own labs, using appropriate reagents, validation, and so forth. These tests are then safe for use in adults but haven’t gone through the FDA approval process.
However, the FDA’s recent announcement stating that the agency should oversee lab-developed tests has made this situation unclear, Nerenz said.
“At this point, we don’t know if there’s still an opportunity to take the NGAL test (or any other cleared biomarker) and validate it for use in a different patient population. Many hospital labs simply don’t have the resources to take these tests through the whole FDA approval process.”
A New Biomarker for AIN?
Meanwhile, research is also moving forward on a better biomarker for AIN, which is also under the AKI umbrella.
“It’s important to diagnose AIN because it has a very specific treatment,” Dennis G. Moledina, MD, PhD, Yale School of Medicine in New Haven, Connecticut, told this news organization.
“AIN is caused by a bunch of different medications, such as proton pump inhibitors, cancer drugs, nonsteroidal anti-inflammatory drugs, and antibiotics, so when someone has this condition, you have to stop potentially life-saving medications and give unnecessary and potentially toxic immunosuppressive drugs, like prednisone,” he said. “If you get the diagnosis wrong, you’re stopping vital drugs and giving immunosuppression for no reason. And if you miss the diagnosis, AIN can lead to permanent chronic kidney disease.”
“Right now, the only way to diagnose AIN is to do a kidney biopsy, which is risky because it can often lead to significant bleeding,” he said. “Some people can’t undergo a biopsy because they’re on medications that increase the risk of bleeding, and they can’t be stopped.”
Furthermore, he noted, “the longer a patient takes a drug that’s causing AIN without getting a diagnosis, the less the chances of recovery because the longer you let this kidney inflammation go on, the more fibrosis and permanent damage develops. So it is important to diagnose it as early as possible, and that’s again why we have a real need for a noninvasive biomarker that can be tested rapidly.”
Moledina and colleagues have been working on identifying a suitable biomarker for close to 10 years, the latest example of which is their 2023 study validating urinary CXCL9 as just such a marker.
“We’re most excited about CXCL9 because it’s already used to diagnose some other diseases in plasma,” Moledina said. “We think that we can convince labs to test it in urine.”
In an accompanying editorial, Mark Canney, PhD, and colleagues at the University of Ottawa and The Ottawa Hospital in Ontario, Canada, wrote that the CXCL9 study findings “are exciting because they provide a road map of where diagnostics can get to for this common, yet poorly identified and treated, cause of kidney damage. The need for a different approach can be readily identified from the fact that clinicians’ gestalt for diagnosing AIN was almost tantamount to tossing a coin (AUC, 0.57). CXCL9 alone outperformed not only the clinician’s prebiopsy suspicion but also an existing diagnostic model and other candidate biomarkers both in the discovery and external validation cohorts.”
Like NGAL, CXCL9 will have to go through the FDA approval process before it can be used for AIN. Therefore, it may be a few years before it can become routinely available, Moledina said.
Nevertheless, Nerenz added, “I think the next steps for AKI are probably continuing on this path of context-dependent, selective biomarker use. I anticipate that we’ll see ongoing development in this space, just expanding to a wider variety of clinical scenarios.”
Nerenz declared receiving research funding from Abbott Labs for evaluation of an AKI biomarker. Moledina is a co-inventor on a pending patent, “Methods and Systems for Diagnosis of Acute Interstitial Nephritis”; a cofounder of the diagnostics company Predict AIN; and a consultant for Biohaven.
A version of this article first appeared on Medscape.com.
‘Round Face’: A Viral Term’s Real Diagnostic Implications
“Cortisol” has become a household word, popularized by social media and tagged in videos that garnered nearly 800 million views in 2023. This is linked to the also-trending term “moon face,” which TikTok influencers and others have suggested is caused by high cortisol levels and, conversely, can be reduced through stress reduction.
“When we hear the term ‘moon face,’ we’re typically referring to Cushing syndrome [CS] or treatment with prolonged high-dose glucocorticoids,” said Anat Ben-Shlomo, MD, co-director of the Multidisciplinary Adrenal Program, Pituitary Center, Division of Endocrinology, Diabetes and Metabolism at Cedars-Sinai Medical Center, Los Angeles. Medscape Medical News previously discussed moon face in an article detailing how to diagnose CS.
Ben-Shlomo noted that the labels “moon face” and “moon facies” should be avoided for their potentially derogatory, unprofessional-sounding connotations, and that the preferred terms are “rounded face” or “round plethoric face.”
There are several disorders that can be associated with facial roundness, not all of which relate to elevated cortisol.
“It’s important for clinicians to be able distinguish between presentations due to other pathophysiologies, identify the unique constellation of Cushing-associated signs and symptoms, engage in a differential diagnosis, and treat whatever the condition is appropriately,” Katherine Sherif, MD, professor and vice chair of academic affairs, Department of Medicine, Thomas Jefferson University, Philadelphia, said in an interview.
The Unique Presentation of CS
CS results from “prolonged elevation” in plasma cortisol levels caused by either exogenous steroid use or excess endogenous steroid production.
“The shape of the face isn’t the only feature associated with CS,” Ben-Shlomo said. “There’s central obesity, particularly in the neck, supraclavicular area, chest, and abdomen. You sometimes see a posterior cervical thoracic fat pad, colloquially — but unprofessionally — called a ‘cervical hump.’ Simultaneously, the arms and legs are getting thinner.” The development of a round, plethoric face is common in long-standing significant CS, and a reddening of the skin can appear.
Additional symptoms include hirsutism and acne. “These can also be seen in other conditions, such as PCOS [polycystic ovary syndrome] but, combined with the other facial features, are more suggestive of CS,” Ben-Shlomo said.
Deep, wide purple striae appear in the trunk, breast, upper arms, and thighs, but not in the face, Ben-Shlomo advised. These appear as the fragile, thinning under-skin breaks when the patient gains weight.
Additional metabolic issues that can occur comorbidly include insulin resistance and diabetes, hypertension, osteoporosis, dyslipidemia, ecchymoses, increased susceptibility to infections, mood changes, cognitive dysfunction, low libido, infertility, weakness of muscles in the shoulders and thighs, episodes of bleeding and/or clotting, and an increased risk for heart attacks and strokes, Ben-Shlomo said.
“Not everyone presents with full-blown disease, but if you see any of these symptoms, be suspicious of CS and conduct a biochemical evaluation.” Three screening tests to use as a starting point are recommended by the Pituitary Society’s updated Consensus on Diagnosis and Management of Cushing’s Disease. The tests should be repeated to account for intra-patient variability. If two or all three tests are positive, clinicians should be suspicious of CS and move to additional testing to identify the underlying cause, Ben-Shlomo said.
‘Subclinical’ CS
Ben-Shlomo highlighted a condition called minimal autonomous cortisol secretion (formerly “subclinical CS”). “This condition is found when a person has an adrenal nodule that produces cortisol in excess, however not to levels observed in CS. An abnormal finding on the overnight 1-mg low-dose dexamethasone suppression test (LDDST) will identify this disorder, showing mildly unsuppressed morning cortisol level, while all other tests will be within normal range.”
She described minimal autonomous cortisol secretion as a form of “smoldering CS,” which has become more commonly diagnosed. “The condition needs to be treated because the patient can develop insulin resistance, metabolic syndrome, and osteoporosis over time.”
Once a cause has been determined, the optimal course of action is to take a multidisciplinary approach because CS affects multiple systems.
‘Pseudo-Cushing Syndrome’
A variety of abnormalities of the hypothalamus-pituitary adrenal (HPA) axis can be associated with hypercortisolemia and a rounder facial appearance but aren’t actually CS, Ben-Shlomo said.
Often called “pseudo-Cushing syndrome,” these conditions have recently been renamed “non-neoplastic hypercortisolism” or “physiologic non-neoplastic endogenous hypercortisolism.” They share some clinical and biochemical features of CS, but the hypercortisolemia is usually secondary to other factors. They increase the secretion of hypothalamic corticotropin-releasing hormone, which stimulates adrenocorticotropic hormone (ACTH) and adrenal cortisol secretion.
Identifying PCOS
PCOS is often associated with central obesity, Sherif noted, but not all women with PCOS have overweight or a central distribution of fat.
“Ask about menstrual periods and whether they come monthly,” Sherif advised. “If women using hormonal contraception say they have a regular cycle, ask if their cycle was regular prior to starting contraception. So many women with PCOS are undiagnosed because they started contraception in their teens to ‘regulate their periods’ and never realized they had PCOS.”
Additional symptoms of PCOS and its impact are found in the figure below.
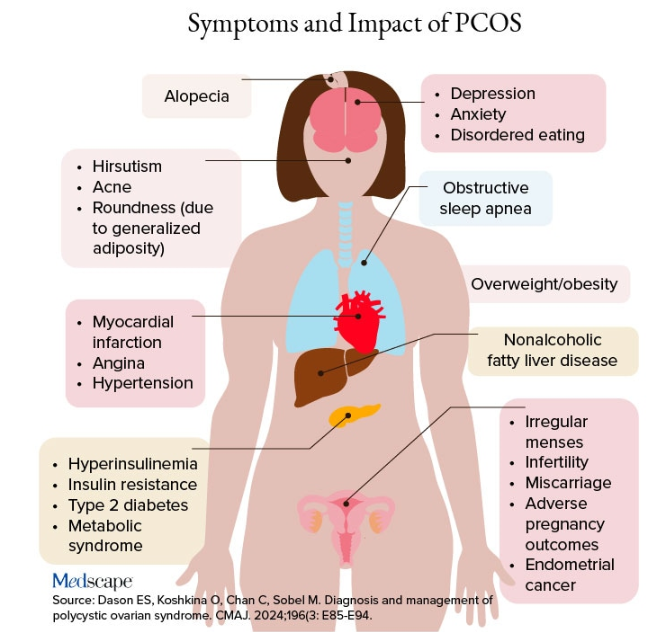
PCOS is diagnosed when two of the following three Rotterdam criteria are met, and other diagnoses are excluded:
- Irregular menstrual cycles
- Clinical hyperandrogenism or biochemical hyperandrogenism
- Polycystic ovarian morphology on transvaginal ultrasonography or high anti-mullerian hormone (applicable only if patient is ≥ 8 years from menarche)
If PCOS is suspected, further tests can be conducted to confirm or rule out the diagnosis.
Alcohol Abuse: Alcohol abuse stimulates hypothalamic corticotropin-releasing hormone, leading to increased ACTH levels. It’s associated with a higher fasting cortisol level, particularly at 8:30 AM or so, and attributable to impaired cortisol clearance due to alcohol-related hepatic dysfunction. The LDDST will show abnormal cortisol suppression.
Sherif advised asking patients about alcohol use, recommending treatment for alcohol use disorder, and repeating clinical and biochemical workup after patients have discontinued alcohol consumption for ≥ 1 month.
Eating Disorders Mimicking CS: Eating disorders, particularly anorexia nervosa, are associated with endocrine abnormalities, amenorrhea, impaired body temperature regulation, and hypercortisolism, likely due to chronic fasting-related stress. Dysregulation of the HPA axis may linger, even after weight recovery.
It’s unlikely that patients with anorexia will display the “rounded face” associated with hypercortisolism, but some research suggests that anorexia can result in a disproportionate accumulation of central adiposity after recovery from the illness.
Neuropsychiatric Disorders: Major depressive disorder (MDD) is associated with HPA axis hyperactivity, with 20%-30% of patients with MDD showing hypercortisolemia. The post-awakening cortisol surge is more pronounced in those with MDD, and about half of patients with MDD also have high evening cortisol levels, suggesting disrupted diurnal cortisol rhythms.
Some patients with MDD have greater resistance to the feedback action of glucocorticoids on HPA axis activity, with weaker sensitivity often restored by effective pharmacotherapy of the depressive condition. Neuropsychiatric disorders are also associated with reduced activity of cortisol-deactivating enzymes. Posttraumatic stress disorder and anxiety are similarly associated with hypercortisolemia.
Addressing neuropsychiatric conditions with appropriate pharmacotherapy and psychotherapy can restore cortisol levels to normal proportions.
Diabetes, Obesity, and Metabolic Syndrome: Diabetes, obesity, and metabolic syndrome can occur comorbidly with CS, and many patients with these conditions may display both a rounder face, some central adiposity, and hypercortisolemia. For example, obesity is often related to a hyperresponsive HPA axis, with elevated cortisol secretion but normal-to-low circulatory concentrations.
Obesity is associated with increased cortisol reactivity after acute physical and/or psychosocial stressors but preserved pituitary sensitivity to feedback inhibition by the LDDST. When these conditions are appropriately managed with pharmacotherapy and lifestyle changes, cortisol levels should normalize, according to the experts.
Hypothyroidism: Hypothyroidism— Hashimoto disease as well as the subclinical variety — can be associated with weight gain, which may take the form of central obesity. Some research suggests a bidirectional relationship between hypothyroidism and obesity.
“Years ago, we didn’t conduct thyroid tests very often but now they’re easy to do, so we usually catch people with hypothyroidism at the beginning of the condition,” Sherif said. “If the patient’s thyroid hasn’t been checked in a year or so, thyroid hormone testing should be conducted.”
Thyroid disease can easily be managed with the administration of thyroid hormones.
Obstructive Sleep Apnea (OSA): OSA has an impact on HPA axis activation, especially when accompanied by obesity and hypertension. A meta-analysis of 22 studies, encompassing over 600 participants, found that continuous positive airway pressure treatment in patients with OSA reduced cortisol levels as well as blood pressure.
Treatment With Exogenous Corticosteroids: Oral corticosteroid treatment is a cornerstone of therapy in transplant, rheumatic, and autoimmune diseases. The impact of chronic exposure to exogenous glucocorticoids is similar to that with endogenous glucocorticoids.
Sherif said corticosteroid treatment can cause facial roundness in as little as 2 weeks and is characteristic in people taking these agents for longer periods. Although the effects are most pronounced with oral agents, systemic effects can be associated with inhaled corticosteroids as well.
Finding alternative anti-inflammatory treatments is advisable, if possible. The co-administration of metformin might lead to improvements in both the metabolic profile and the clinical outcomes of patients receiving glucocorticoids for inflammatory conditions.
Educating Patients: “There’s much we still don’t know about hypercortisolemia and CS, including the reasons for its impact on metabolic derangement and for the accumulation of fat in particular adipose patterns,” Ben-Shlomo said. “But experienced endocrinologists do know relatively well how to diagnose the condition, distinguish it from other conditions presenting with central obesity or a rounder face, and treat it.”
Given the casual use of the terms “moon face” and “extra cortisol” on social media, it’s important for physicians to educate patients about what elevated cortisol does and doesn’t do, and design treatment strategies accordingly.
Neither Ben-Shlomo nor Sherif reported having any disclosures.
A version of this article appeared on Medscape.com.
“Cortisol” has become a household word, popularized by social media and tagged in videos that garnered nearly 800 million views in 2023. This is linked to the also-trending term “moon face,” which TikTok influencers and others have suggested is caused by high cortisol levels and, conversely, can be reduced through stress reduction.
“When we hear the term ‘moon face,’ we’re typically referring to Cushing syndrome [CS] or treatment with prolonged high-dose glucocorticoids,” said Anat Ben-Shlomo, MD, co-director of the Multidisciplinary Adrenal Program, Pituitary Center, Division of Endocrinology, Diabetes and Metabolism at Cedars-Sinai Medical Center, Los Angeles. Medscape Medical News previously discussed moon face in an article detailing how to diagnose CS.
Ben-Shlomo noted that the labels “moon face” and “moon facies” should be avoided for their potentially derogatory, unprofessional-sounding connotations, and that the preferred terms are “rounded face” or “round plethoric face.”
There are several disorders that can be associated with facial roundness, not all of which relate to elevated cortisol.
“It’s important for clinicians to be able distinguish between presentations due to other pathophysiologies, identify the unique constellation of Cushing-associated signs and symptoms, engage in a differential diagnosis, and treat whatever the condition is appropriately,” Katherine Sherif, MD, professor and vice chair of academic affairs, Department of Medicine, Thomas Jefferson University, Philadelphia, said in an interview.
The Unique Presentation of CS
CS results from “prolonged elevation” in plasma cortisol levels caused by either exogenous steroid use or excess endogenous steroid production.
“The shape of the face isn’t the only feature associated with CS,” Ben-Shlomo said. “There’s central obesity, particularly in the neck, supraclavicular area, chest, and abdomen. You sometimes see a posterior cervical thoracic fat pad, colloquially — but unprofessionally — called a ‘cervical hump.’ Simultaneously, the arms and legs are getting thinner.” The development of a round, plethoric face is common in long-standing significant CS, and a reddening of the skin can appear.
Additional symptoms include hirsutism and acne. “These can also be seen in other conditions, such as PCOS [polycystic ovary syndrome] but, combined with the other facial features, are more suggestive of CS,” Ben-Shlomo said.
Deep, wide purple striae appear in the trunk, breast, upper arms, and thighs, but not in the face, Ben-Shlomo advised. These appear as the fragile, thinning under-skin breaks when the patient gains weight.
Additional metabolic issues that can occur comorbidly include insulin resistance and diabetes, hypertension, osteoporosis, dyslipidemia, ecchymoses, increased susceptibility to infections, mood changes, cognitive dysfunction, low libido, infertility, weakness of muscles in the shoulders and thighs, episodes of bleeding and/or clotting, and an increased risk for heart attacks and strokes, Ben-Shlomo said.
“Not everyone presents with full-blown disease, but if you see any of these symptoms, be suspicious of CS and conduct a biochemical evaluation.” Three screening tests to use as a starting point are recommended by the Pituitary Society’s updated Consensus on Diagnosis and Management of Cushing’s Disease. The tests should be repeated to account for intra-patient variability. If two or all three tests are positive, clinicians should be suspicious of CS and move to additional testing to identify the underlying cause, Ben-Shlomo said.
‘Subclinical’ CS
Ben-Shlomo highlighted a condition called minimal autonomous cortisol secretion (formerly “subclinical CS”). “This condition is found when a person has an adrenal nodule that produces cortisol in excess, however not to levels observed in CS. An abnormal finding on the overnight 1-mg low-dose dexamethasone suppression test (LDDST) will identify this disorder, showing mildly unsuppressed morning cortisol level, while all other tests will be within normal range.”
She described minimal autonomous cortisol secretion as a form of “smoldering CS,” which has become more commonly diagnosed. “The condition needs to be treated because the patient can develop insulin resistance, metabolic syndrome, and osteoporosis over time.”
Once a cause has been determined, the optimal course of action is to take a multidisciplinary approach because CS affects multiple systems.
‘Pseudo-Cushing Syndrome’
A variety of abnormalities of the hypothalamus-pituitary adrenal (HPA) axis can be associated with hypercortisolemia and a rounder facial appearance but aren’t actually CS, Ben-Shlomo said.
Often called “pseudo-Cushing syndrome,” these conditions have recently been renamed “non-neoplastic hypercortisolism” or “physiologic non-neoplastic endogenous hypercortisolism.” They share some clinical and biochemical features of CS, but the hypercortisolemia is usually secondary to other factors. They increase the secretion of hypothalamic corticotropin-releasing hormone, which stimulates adrenocorticotropic hormone (ACTH) and adrenal cortisol secretion.
Identifying PCOS
PCOS is often associated with central obesity, Sherif noted, but not all women with PCOS have overweight or a central distribution of fat.
“Ask about menstrual periods and whether they come monthly,” Sherif advised. “If women using hormonal contraception say they have a regular cycle, ask if their cycle was regular prior to starting contraception. So many women with PCOS are undiagnosed because they started contraception in their teens to ‘regulate their periods’ and never realized they had PCOS.”
Additional symptoms of PCOS and its impact are found in the figure below.

PCOS is diagnosed when two of the following three Rotterdam criteria are met, and other diagnoses are excluded:
- Irregular menstrual cycles
- Clinical hyperandrogenism or biochemical hyperandrogenism
- Polycystic ovarian morphology on transvaginal ultrasonography or high anti-mullerian hormone (applicable only if patient is ≥ 8 years from menarche)
If PCOS is suspected, further tests can be conducted to confirm or rule out the diagnosis.
Alcohol Abuse: Alcohol abuse stimulates hypothalamic corticotropin-releasing hormone, leading to increased ACTH levels. It’s associated with a higher fasting cortisol level, particularly at 8:30 AM or so, and attributable to impaired cortisol clearance due to alcohol-related hepatic dysfunction. The LDDST will show abnormal cortisol suppression.
Sherif advised asking patients about alcohol use, recommending treatment for alcohol use disorder, and repeating clinical and biochemical workup after patients have discontinued alcohol consumption for ≥ 1 month.
Eating Disorders Mimicking CS: Eating disorders, particularly anorexia nervosa, are associated with endocrine abnormalities, amenorrhea, impaired body temperature regulation, and hypercortisolism, likely due to chronic fasting-related stress. Dysregulation of the HPA axis may linger, even after weight recovery.
It’s unlikely that patients with anorexia will display the “rounded face” associated with hypercortisolism, but some research suggests that anorexia can result in a disproportionate accumulation of central adiposity after recovery from the illness.
Neuropsychiatric Disorders: Major depressive disorder (MDD) is associated with HPA axis hyperactivity, with 20%-30% of patients with MDD showing hypercortisolemia. The post-awakening cortisol surge is more pronounced in those with MDD, and about half of patients with MDD also have high evening cortisol levels, suggesting disrupted diurnal cortisol rhythms.
Some patients with MDD have greater resistance to the feedback action of glucocorticoids on HPA axis activity, with weaker sensitivity often restored by effective pharmacotherapy of the depressive condition. Neuropsychiatric disorders are also associated with reduced activity of cortisol-deactivating enzymes. Posttraumatic stress disorder and anxiety are similarly associated with hypercortisolemia.
Addressing neuropsychiatric conditions with appropriate pharmacotherapy and psychotherapy can restore cortisol levels to normal proportions.
Diabetes, Obesity, and Metabolic Syndrome: Diabetes, obesity, and metabolic syndrome can occur comorbidly with CS, and many patients with these conditions may display both a rounder face, some central adiposity, and hypercortisolemia. For example, obesity is often related to a hyperresponsive HPA axis, with elevated cortisol secretion but normal-to-low circulatory concentrations.
Obesity is associated with increased cortisol reactivity after acute physical and/or psychosocial stressors but preserved pituitary sensitivity to feedback inhibition by the LDDST. When these conditions are appropriately managed with pharmacotherapy and lifestyle changes, cortisol levels should normalize, according to the experts.
Hypothyroidism: Hypothyroidism— Hashimoto disease as well as the subclinical variety — can be associated with weight gain, which may take the form of central obesity. Some research suggests a bidirectional relationship between hypothyroidism and obesity.
“Years ago, we didn’t conduct thyroid tests very often but now they’re easy to do, so we usually catch people with hypothyroidism at the beginning of the condition,” Sherif said. “If the patient’s thyroid hasn’t been checked in a year or so, thyroid hormone testing should be conducted.”
Thyroid disease can easily be managed with the administration of thyroid hormones.
Obstructive Sleep Apnea (OSA): OSA has an impact on HPA axis activation, especially when accompanied by obesity and hypertension. A meta-analysis of 22 studies, encompassing over 600 participants, found that continuous positive airway pressure treatment in patients with OSA reduced cortisol levels as well as blood pressure.
Treatment With Exogenous Corticosteroids: Oral corticosteroid treatment is a cornerstone of therapy in transplant, rheumatic, and autoimmune diseases. The impact of chronic exposure to exogenous glucocorticoids is similar to that with endogenous glucocorticoids.
Sherif said corticosteroid treatment can cause facial roundness in as little as 2 weeks and is characteristic in people taking these agents for longer periods. Although the effects are most pronounced with oral agents, systemic effects can be associated with inhaled corticosteroids as well.
Finding alternative anti-inflammatory treatments is advisable, if possible. The co-administration of metformin might lead to improvements in both the metabolic profile and the clinical outcomes of patients receiving glucocorticoids for inflammatory conditions.
Educating Patients: “There’s much we still don’t know about hypercortisolemia and CS, including the reasons for its impact on metabolic derangement and for the accumulation of fat in particular adipose patterns,” Ben-Shlomo said. “But experienced endocrinologists do know relatively well how to diagnose the condition, distinguish it from other conditions presenting with central obesity or a rounder face, and treat it.”
Given the casual use of the terms “moon face” and “extra cortisol” on social media, it’s important for physicians to educate patients about what elevated cortisol does and doesn’t do, and design treatment strategies accordingly.
Neither Ben-Shlomo nor Sherif reported having any disclosures.
A version of this article appeared on Medscape.com.
“Cortisol” has become a household word, popularized by social media and tagged in videos that garnered nearly 800 million views in 2023. This is linked to the also-trending term “moon face,” which TikTok influencers and others have suggested is caused by high cortisol levels and, conversely, can be reduced through stress reduction.
“When we hear the term ‘moon face,’ we’re typically referring to Cushing syndrome [CS] or treatment with prolonged high-dose glucocorticoids,” said Anat Ben-Shlomo, MD, co-director of the Multidisciplinary Adrenal Program, Pituitary Center, Division of Endocrinology, Diabetes and Metabolism at Cedars-Sinai Medical Center, Los Angeles. Medscape Medical News previously discussed moon face in an article detailing how to diagnose CS.
Ben-Shlomo noted that the labels “moon face” and “moon facies” should be avoided for their potentially derogatory, unprofessional-sounding connotations, and that the preferred terms are “rounded face” or “round plethoric face.”
There are several disorders that can be associated with facial roundness, not all of which relate to elevated cortisol.
“It’s important for clinicians to be able distinguish between presentations due to other pathophysiologies, identify the unique constellation of Cushing-associated signs and symptoms, engage in a differential diagnosis, and treat whatever the condition is appropriately,” Katherine Sherif, MD, professor and vice chair of academic affairs, Department of Medicine, Thomas Jefferson University, Philadelphia, said in an interview.
The Unique Presentation of CS
CS results from “prolonged elevation” in plasma cortisol levels caused by either exogenous steroid use or excess endogenous steroid production.
“The shape of the face isn’t the only feature associated with CS,” Ben-Shlomo said. “There’s central obesity, particularly in the neck, supraclavicular area, chest, and abdomen. You sometimes see a posterior cervical thoracic fat pad, colloquially — but unprofessionally — called a ‘cervical hump.’ Simultaneously, the arms and legs are getting thinner.” The development of a round, plethoric face is common in long-standing significant CS, and a reddening of the skin can appear.
Additional symptoms include hirsutism and acne. “These can also be seen in other conditions, such as PCOS [polycystic ovary syndrome] but, combined with the other facial features, are more suggestive of CS,” Ben-Shlomo said.
Deep, wide purple striae appear in the trunk, breast, upper arms, and thighs, but not in the face, Ben-Shlomo advised. These appear as the fragile, thinning under-skin breaks when the patient gains weight.
Additional metabolic issues that can occur comorbidly include insulin resistance and diabetes, hypertension, osteoporosis, dyslipidemia, ecchymoses, increased susceptibility to infections, mood changes, cognitive dysfunction, low libido, infertility, weakness of muscles in the shoulders and thighs, episodes of bleeding and/or clotting, and an increased risk for heart attacks and strokes, Ben-Shlomo said.
“Not everyone presents with full-blown disease, but if you see any of these symptoms, be suspicious of CS and conduct a biochemical evaluation.” Three screening tests to use as a starting point are recommended by the Pituitary Society’s updated Consensus on Diagnosis and Management of Cushing’s Disease. The tests should be repeated to account for intra-patient variability. If two or all three tests are positive, clinicians should be suspicious of CS and move to additional testing to identify the underlying cause, Ben-Shlomo said.
‘Subclinical’ CS
Ben-Shlomo highlighted a condition called minimal autonomous cortisol secretion (formerly “subclinical CS”). “This condition is found when a person has an adrenal nodule that produces cortisol in excess, however not to levels observed in CS. An abnormal finding on the overnight 1-mg low-dose dexamethasone suppression test (LDDST) will identify this disorder, showing mildly unsuppressed morning cortisol level, while all other tests will be within normal range.”
She described minimal autonomous cortisol secretion as a form of “smoldering CS,” which has become more commonly diagnosed. “The condition needs to be treated because the patient can develop insulin resistance, metabolic syndrome, and osteoporosis over time.”
Once a cause has been determined, the optimal course of action is to take a multidisciplinary approach because CS affects multiple systems.
‘Pseudo-Cushing Syndrome’
A variety of abnormalities of the hypothalamus-pituitary adrenal (HPA) axis can be associated with hypercortisolemia and a rounder facial appearance but aren’t actually CS, Ben-Shlomo said.
Often called “pseudo-Cushing syndrome,” these conditions have recently been renamed “non-neoplastic hypercortisolism” or “physiologic non-neoplastic endogenous hypercortisolism.” They share some clinical and biochemical features of CS, but the hypercortisolemia is usually secondary to other factors. They increase the secretion of hypothalamic corticotropin-releasing hormone, which stimulates adrenocorticotropic hormone (ACTH) and adrenal cortisol secretion.
Identifying PCOS
PCOS is often associated with central obesity, Sherif noted, but not all women with PCOS have overweight or a central distribution of fat.
“Ask about menstrual periods and whether they come monthly,” Sherif advised. “If women using hormonal contraception say they have a regular cycle, ask if their cycle was regular prior to starting contraception. So many women with PCOS are undiagnosed because they started contraception in their teens to ‘regulate their periods’ and never realized they had PCOS.”
Additional symptoms of PCOS and its impact are found in the figure below.

PCOS is diagnosed when two of the following three Rotterdam criteria are met, and other diagnoses are excluded:
- Irregular menstrual cycles
- Clinical hyperandrogenism or biochemical hyperandrogenism
- Polycystic ovarian morphology on transvaginal ultrasonography or high anti-mullerian hormone (applicable only if patient is ≥ 8 years from menarche)
If PCOS is suspected, further tests can be conducted to confirm or rule out the diagnosis.
Alcohol Abuse: Alcohol abuse stimulates hypothalamic corticotropin-releasing hormone, leading to increased ACTH levels. It’s associated with a higher fasting cortisol level, particularly at 8:30 AM or so, and attributable to impaired cortisol clearance due to alcohol-related hepatic dysfunction. The LDDST will show abnormal cortisol suppression.
Sherif advised asking patients about alcohol use, recommending treatment for alcohol use disorder, and repeating clinical and biochemical workup after patients have discontinued alcohol consumption for ≥ 1 month.
Eating Disorders Mimicking CS: Eating disorders, particularly anorexia nervosa, are associated with endocrine abnormalities, amenorrhea, impaired body temperature regulation, and hypercortisolism, likely due to chronic fasting-related stress. Dysregulation of the HPA axis may linger, even after weight recovery.
It’s unlikely that patients with anorexia will display the “rounded face” associated with hypercortisolism, but some research suggests that anorexia can result in a disproportionate accumulation of central adiposity after recovery from the illness.
Neuropsychiatric Disorders: Major depressive disorder (MDD) is associated with HPA axis hyperactivity, with 20%-30% of patients with MDD showing hypercortisolemia. The post-awakening cortisol surge is more pronounced in those with MDD, and about half of patients with MDD also have high evening cortisol levels, suggesting disrupted diurnal cortisol rhythms.
Some patients with MDD have greater resistance to the feedback action of glucocorticoids on HPA axis activity, with weaker sensitivity often restored by effective pharmacotherapy of the depressive condition. Neuropsychiatric disorders are also associated with reduced activity of cortisol-deactivating enzymes. Posttraumatic stress disorder and anxiety are similarly associated with hypercortisolemia.
Addressing neuropsychiatric conditions with appropriate pharmacotherapy and psychotherapy can restore cortisol levels to normal proportions.
Diabetes, Obesity, and Metabolic Syndrome: Diabetes, obesity, and metabolic syndrome can occur comorbidly with CS, and many patients with these conditions may display both a rounder face, some central adiposity, and hypercortisolemia. For example, obesity is often related to a hyperresponsive HPA axis, with elevated cortisol secretion but normal-to-low circulatory concentrations.
Obesity is associated with increased cortisol reactivity after acute physical and/or psychosocial stressors but preserved pituitary sensitivity to feedback inhibition by the LDDST. When these conditions are appropriately managed with pharmacotherapy and lifestyle changes, cortisol levels should normalize, according to the experts.
Hypothyroidism: Hypothyroidism— Hashimoto disease as well as the subclinical variety — can be associated with weight gain, which may take the form of central obesity. Some research suggests a bidirectional relationship between hypothyroidism and obesity.
“Years ago, we didn’t conduct thyroid tests very often but now they’re easy to do, so we usually catch people with hypothyroidism at the beginning of the condition,” Sherif said. “If the patient’s thyroid hasn’t been checked in a year or so, thyroid hormone testing should be conducted.”
Thyroid disease can easily be managed with the administration of thyroid hormones.
Obstructive Sleep Apnea (OSA): OSA has an impact on HPA axis activation, especially when accompanied by obesity and hypertension. A meta-analysis of 22 studies, encompassing over 600 participants, found that continuous positive airway pressure treatment in patients with OSA reduced cortisol levels as well as blood pressure.
Treatment With Exogenous Corticosteroids: Oral corticosteroid treatment is a cornerstone of therapy in transplant, rheumatic, and autoimmune diseases. The impact of chronic exposure to exogenous glucocorticoids is similar to that with endogenous glucocorticoids.
Sherif said corticosteroid treatment can cause facial roundness in as little as 2 weeks and is characteristic in people taking these agents for longer periods. Although the effects are most pronounced with oral agents, systemic effects can be associated with inhaled corticosteroids as well.
Finding alternative anti-inflammatory treatments is advisable, if possible. The co-administration of metformin might lead to improvements in both the metabolic profile and the clinical outcomes of patients receiving glucocorticoids for inflammatory conditions.
Educating Patients: “There’s much we still don’t know about hypercortisolemia and CS, including the reasons for its impact on metabolic derangement and for the accumulation of fat in particular adipose patterns,” Ben-Shlomo said. “But experienced endocrinologists do know relatively well how to diagnose the condition, distinguish it from other conditions presenting with central obesity or a rounder face, and treat it.”
Given the casual use of the terms “moon face” and “extra cortisol” on social media, it’s important for physicians to educate patients about what elevated cortisol does and doesn’t do, and design treatment strategies accordingly.
Neither Ben-Shlomo nor Sherif reported having any disclosures.
A version of this article appeared on Medscape.com.
Update Coming for Thyroid Disease in Pregnancy Guidelines
CHICAGO — A preview of much-anticipated updates to guidelines on managing thyroid disease in pregnancy shows key changes to recommendations in the evolving field, ranging from consideration of the chance of spontaneous normalization of thyroid levels during pregnancy to a heightened emphasis on shared decision-making and the nuances can factor into personalized treatment.
The guidelines, expected to be published in early 2025, have not been updated since 2017, and with substantial advances and evidence from countless studies since then, the new guidelines were developed with a goal to start afresh, said ATA Thyroid and Pregnancy Guidelines Task Force cochair Tim IM Korevaar, MD, PhD, in presenting the final draft guidelines at the American Thyroid Association (ATA) 2024 Meeting.
“Obviously, we’re not going to ignore the 2017 guidelines, which have been a very good resource for us so far, but we really wanted to start from scratch and follow a ‘blank canvas’ approach in optimizing the evidence,” said Korevaar, an endocrinologist and obstetric internist with the Division of Pharmacology and Vascular Medicine & Academic Center for Thyroid Diseases, Department of Internal Medicine, Erasmus University Medical Center, Rotterdam, The Netherlands.
The guidelines, developed through a collaborative effort involving a wide variety of related medical societies, involved 14 systematic literature reviews. While the pregnancy issues covered by the guidelines is extensive, key highlights include:
Management in Preconception
Beginning with preconception, a key change in the guidelines will be that patients with euthyroid thyroid peroxidase (TPO) antibodies, which can be indicative of thyroid dysfunction, routine treatment with levothyroxine is not recommended, based on new evidence from randomized trials of high-risk patients showing no clear benefit from the treatment.
“In these trials, and across analyses, there was absolutely no beneficial effect of levothyroxine in these patients [with euthyroid TPO antibody positivity],” he said.
With evidence showing, however, that TPO antibody positivity can lead to subclinical or overt hypothyroidism within 1 or 2 years, the guidelines will recommend that TPO antibody–positive patients do have thyroid stimulating hormone (TSH) levels tested every 3-6 months until pregnancy, and existing recommendations to test during pregnancy among those patients remain in place, Korevaar reported.
In terms of preconception subclinical hypothyroidism, the guidelines will emphasize the existing recommendation “to always strive to reassess” thyroid levels, and if subclinical hypothyroidism does persist, to treat with low-dose levothyroxine.
During Pregnancy
During pregnancy, the new proposed recommendations will reflect the important change that three key risk factors, including age over 30 years, having at least two prior pregnancies, and morbid obesity (body mass index [BMI] at least 40 kg/m2), previously considered a risk for thyroid dysfunction in pregnancy, should not, on their own, suggest the need for thyroid testing, based on low evidence of an increased risk in pregnancy.
Research on the issue includes a recent study from Korevaar’s team showing these factors to in fact have low predictability of thyroid dysfunction.
“We deemed that these risk differences weren’t really clinically meaningful (in predicting risk), and so we have removed to maternal age, BMI, and parity as risk factors for thyroid testing indications in pregnancy,” Korevaar said.
Factors considered a risk, resulting in recommended testing at presentation include a history of subclinical or clinical hypo- or hyperthyroidism, postpartum thyroiditis, known thyroid antibody positivity, symptoms of thyroid dysfunction or goiter, and other factors.
Treatment for Subclinical Hypothyroidism in Pregnancy
Whereas current guidelines recommend TPO antibody status in determining when to consider treatment for subclinical hypothyroidism, the new proposed guideline will instead recommend treatment based on the timing of the diagnosis of the subclinical hypothyroidism, with consideration of treatment during the first trimester, but not in the second or third trimester, based on newer evidence of the absolute risk for pregnancy complications and randomized trial data.
“The recommendations are now to no longer based on TPO antibody status, but instead according to the timing of the diagnosis of subclinical hypothyroidism,” Korevaar said.
Based on the collective data, “due to the low risk, we do not recommend for routine levothyroxine treatment in the second or third trimester groups with TSH levels under 10 mU/L now.”
“However, for subclinical hypothyroidism diagnosed in the first trimester, the recommendation would be that you can consider levothyroxine treatment,” he said.
While a clear indication for treatment in any trimester is the presence of overt hypothyroidism, or TSH levels over 10 mU/L, Korevaar underscored the importance of considering nuances of the recommendations that may warrant flexibility, for instance among patients with borderline TSH levels.
Spontaneous Normalization of Thyroid Levels in Pregnancy
Another new recommendation addresses the issue of spontaneous normalization of abnormal thyroid function during pregnancy, with several large studies showing a large proportion of subclinical hypothyroidism cases spontaneously revert to euthyroidism by the third trimester — despite no treatment having been provided.
Under the important proposed recommendation, retesting of subclinical hypothyroidism is suggested within 3 weeks.
“The data shows that a large proportion of patients spontaneously revert to euthyroidism,” Korevaar said.
“Upon identifying subclinical hypothyroidism in the first trimester, there will be essentially two options that clinicians can discuss with their patient — one would be to consider confirmatory tests in 3 weeks or to discuss the starting the lower dose levothyroxine in the first trimester,” he said.
In terms of overt hypothyroidism, likewise, if patients have a TSH levels below 6 mU/L in pregnancy, “you can either consider doing confirmatory testing within 3 weeks, or discussing with the patient starting levothyroxine treatment,” Korevaar added.
Overt Hyperthyroidism
For overt hyperthyroidism, no significant changes from current guidelines are being proposed, with the key exception of a heightened emphasis on the need for shared decision-making with patients, Korevaar said.
“We want to emphasize shared decision-making especially for women who have Graves’ disease prior to pregnancy, because the antithyroid treatment modalities, primarily methimazole (MMI) and propylthiouracil (PTU), have different advantages and disadvantages for an upcoming pregnancy,” he said.
“If you help a patient become involved in the decision-making process, that can also be very helpful in managing the disease and following-up on the pregnancy.”
Under the recommendations, PTU remains the preferred drug in overt hyperthyroidism, due to a more favorable profile in terms of potential birth defects vs MMI, with research showing a higher absolute risk of 3% vs 5%.
The guidelines further suggest the option of stopping the antithyroid medications upon a positive pregnancy test, with the exception of high-risk patients.
Korevaar noted that, if the treatment is stopped early in pregnancy, relapse is not likely to occur until after approximately 3 months, or 12 weeks, at which time, the high-risk teratogenic period, which is between week 5 and week 15, will have passed.
Current guidelines regarding whether to stop treatment in higher-risk hyperthyroid patients are recommended to remain unchanged.
Thyroid Nodules and Cancer
Recommendations regarding thyroid nodules and cancer during pregnancy are also expected to remain largely similar to those in the 2017 guidelines, with the exception of an emphasis on simply considering how the patient would normally be managed outside of pregnancy.
For instance, regarding the question of whether treatment can be withheld for 9 months during pregnancy. “A lot of times, the answer is yes,” Korevaar said.
Other topics that will be largely unchanged include issues of universal screening, definitions of normal and abnormal TSH and free T4 reference ranges and isolated hypothyroxinemia.
Steps Forward in Improving Updates, Readability
In addition to recommendation updates, the new guidelines are being revised to better reflect more recent evidence-based developments and user-friendliness.
“We have now made the step to a more systematic and replicable methodology to ensure for easier updates with a shorter interval,” Korevaar told this news organization.
“Furthermore, since 2006, the ATA guideline documents have followed a question-and-answer format, lacked recommendation tables and had none or only a few graphic illustrations,” he added.
“We are now further developing the typical outline of the guidelines to improve the readability and dissemination of the guideline document.”
Korevaar’s disclosures include lectureship fees from IBSA, Merck, and Berlin Chemie.
A version of this article first appeared on Medscape.com.
CHICAGO — A preview of much-anticipated updates to guidelines on managing thyroid disease in pregnancy shows key changes to recommendations in the evolving field, ranging from consideration of the chance of spontaneous normalization of thyroid levels during pregnancy to a heightened emphasis on shared decision-making and the nuances can factor into personalized treatment.
The guidelines, expected to be published in early 2025, have not been updated since 2017, and with substantial advances and evidence from countless studies since then, the new guidelines were developed with a goal to start afresh, said ATA Thyroid and Pregnancy Guidelines Task Force cochair Tim IM Korevaar, MD, PhD, in presenting the final draft guidelines at the American Thyroid Association (ATA) 2024 Meeting.
“Obviously, we’re not going to ignore the 2017 guidelines, which have been a very good resource for us so far, but we really wanted to start from scratch and follow a ‘blank canvas’ approach in optimizing the evidence,” said Korevaar, an endocrinologist and obstetric internist with the Division of Pharmacology and Vascular Medicine & Academic Center for Thyroid Diseases, Department of Internal Medicine, Erasmus University Medical Center, Rotterdam, The Netherlands.
The guidelines, developed through a collaborative effort involving a wide variety of related medical societies, involved 14 systematic literature reviews. While the pregnancy issues covered by the guidelines is extensive, key highlights include:
Management in Preconception
Beginning with preconception, a key change in the guidelines will be that patients with euthyroid thyroid peroxidase (TPO) antibodies, which can be indicative of thyroid dysfunction, routine treatment with levothyroxine is not recommended, based on new evidence from randomized trials of high-risk patients showing no clear benefit from the treatment.
“In these trials, and across analyses, there was absolutely no beneficial effect of levothyroxine in these patients [with euthyroid TPO antibody positivity],” he said.
With evidence showing, however, that TPO antibody positivity can lead to subclinical or overt hypothyroidism within 1 or 2 years, the guidelines will recommend that TPO antibody–positive patients do have thyroid stimulating hormone (TSH) levels tested every 3-6 months until pregnancy, and existing recommendations to test during pregnancy among those patients remain in place, Korevaar reported.
In terms of preconception subclinical hypothyroidism, the guidelines will emphasize the existing recommendation “to always strive to reassess” thyroid levels, and if subclinical hypothyroidism does persist, to treat with low-dose levothyroxine.
During Pregnancy
During pregnancy, the new proposed recommendations will reflect the important change that three key risk factors, including age over 30 years, having at least two prior pregnancies, and morbid obesity (body mass index [BMI] at least 40 kg/m2), previously considered a risk for thyroid dysfunction in pregnancy, should not, on their own, suggest the need for thyroid testing, based on low evidence of an increased risk in pregnancy.
Research on the issue includes a recent study from Korevaar’s team showing these factors to in fact have low predictability of thyroid dysfunction.
“We deemed that these risk differences weren’t really clinically meaningful (in predicting risk), and so we have removed to maternal age, BMI, and parity as risk factors for thyroid testing indications in pregnancy,” Korevaar said.
Factors considered a risk, resulting in recommended testing at presentation include a history of subclinical or clinical hypo- or hyperthyroidism, postpartum thyroiditis, known thyroid antibody positivity, symptoms of thyroid dysfunction or goiter, and other factors.
Treatment for Subclinical Hypothyroidism in Pregnancy
Whereas current guidelines recommend TPO antibody status in determining when to consider treatment for subclinical hypothyroidism, the new proposed guideline will instead recommend treatment based on the timing of the diagnosis of the subclinical hypothyroidism, with consideration of treatment during the first trimester, but not in the second or third trimester, based on newer evidence of the absolute risk for pregnancy complications and randomized trial data.
“The recommendations are now to no longer based on TPO antibody status, but instead according to the timing of the diagnosis of subclinical hypothyroidism,” Korevaar said.
Based on the collective data, “due to the low risk, we do not recommend for routine levothyroxine treatment in the second or third trimester groups with TSH levels under 10 mU/L now.”
“However, for subclinical hypothyroidism diagnosed in the first trimester, the recommendation would be that you can consider levothyroxine treatment,” he said.
While a clear indication for treatment in any trimester is the presence of overt hypothyroidism, or TSH levels over 10 mU/L, Korevaar underscored the importance of considering nuances of the recommendations that may warrant flexibility, for instance among patients with borderline TSH levels.
Spontaneous Normalization of Thyroid Levels in Pregnancy
Another new recommendation addresses the issue of spontaneous normalization of abnormal thyroid function during pregnancy, with several large studies showing a large proportion of subclinical hypothyroidism cases spontaneously revert to euthyroidism by the third trimester — despite no treatment having been provided.
Under the important proposed recommendation, retesting of subclinical hypothyroidism is suggested within 3 weeks.
“The data shows that a large proportion of patients spontaneously revert to euthyroidism,” Korevaar said.
“Upon identifying subclinical hypothyroidism in the first trimester, there will be essentially two options that clinicians can discuss with their patient — one would be to consider confirmatory tests in 3 weeks or to discuss the starting the lower dose levothyroxine in the first trimester,” he said.
In terms of overt hypothyroidism, likewise, if patients have a TSH levels below 6 mU/L in pregnancy, “you can either consider doing confirmatory testing within 3 weeks, or discussing with the patient starting levothyroxine treatment,” Korevaar added.
Overt Hyperthyroidism
For overt hyperthyroidism, no significant changes from current guidelines are being proposed, with the key exception of a heightened emphasis on the need for shared decision-making with patients, Korevaar said.
“We want to emphasize shared decision-making especially for women who have Graves’ disease prior to pregnancy, because the antithyroid treatment modalities, primarily methimazole (MMI) and propylthiouracil (PTU), have different advantages and disadvantages for an upcoming pregnancy,” he said.
“If you help a patient become involved in the decision-making process, that can also be very helpful in managing the disease and following-up on the pregnancy.”
Under the recommendations, PTU remains the preferred drug in overt hyperthyroidism, due to a more favorable profile in terms of potential birth defects vs MMI, with research showing a higher absolute risk of 3% vs 5%.
The guidelines further suggest the option of stopping the antithyroid medications upon a positive pregnancy test, with the exception of high-risk patients.
Korevaar noted that, if the treatment is stopped early in pregnancy, relapse is not likely to occur until after approximately 3 months, or 12 weeks, at which time, the high-risk teratogenic period, which is between week 5 and week 15, will have passed.
Current guidelines regarding whether to stop treatment in higher-risk hyperthyroid patients are recommended to remain unchanged.
Thyroid Nodules and Cancer
Recommendations regarding thyroid nodules and cancer during pregnancy are also expected to remain largely similar to those in the 2017 guidelines, with the exception of an emphasis on simply considering how the patient would normally be managed outside of pregnancy.
For instance, regarding the question of whether treatment can be withheld for 9 months during pregnancy. “A lot of times, the answer is yes,” Korevaar said.
Other topics that will be largely unchanged include issues of universal screening, definitions of normal and abnormal TSH and free T4 reference ranges and isolated hypothyroxinemia.
Steps Forward in Improving Updates, Readability
In addition to recommendation updates, the new guidelines are being revised to better reflect more recent evidence-based developments and user-friendliness.
“We have now made the step to a more systematic and replicable methodology to ensure for easier updates with a shorter interval,” Korevaar told this news organization.
“Furthermore, since 2006, the ATA guideline documents have followed a question-and-answer format, lacked recommendation tables and had none or only a few graphic illustrations,” he added.
“We are now further developing the typical outline of the guidelines to improve the readability and dissemination of the guideline document.”
Korevaar’s disclosures include lectureship fees from IBSA, Merck, and Berlin Chemie.
A version of this article first appeared on Medscape.com.
CHICAGO — A preview of much-anticipated updates to guidelines on managing thyroid disease in pregnancy shows key changes to recommendations in the evolving field, ranging from consideration of the chance of spontaneous normalization of thyroid levels during pregnancy to a heightened emphasis on shared decision-making and the nuances can factor into personalized treatment.
The guidelines, expected to be published in early 2025, have not been updated since 2017, and with substantial advances and evidence from countless studies since then, the new guidelines were developed with a goal to start afresh, said ATA Thyroid and Pregnancy Guidelines Task Force cochair Tim IM Korevaar, MD, PhD, in presenting the final draft guidelines at the American Thyroid Association (ATA) 2024 Meeting.
“Obviously, we’re not going to ignore the 2017 guidelines, which have been a very good resource for us so far, but we really wanted to start from scratch and follow a ‘blank canvas’ approach in optimizing the evidence,” said Korevaar, an endocrinologist and obstetric internist with the Division of Pharmacology and Vascular Medicine & Academic Center for Thyroid Diseases, Department of Internal Medicine, Erasmus University Medical Center, Rotterdam, The Netherlands.
The guidelines, developed through a collaborative effort involving a wide variety of related medical societies, involved 14 systematic literature reviews. While the pregnancy issues covered by the guidelines is extensive, key highlights include:
Management in Preconception
Beginning with preconception, a key change in the guidelines will be that patients with euthyroid thyroid peroxidase (TPO) antibodies, which can be indicative of thyroid dysfunction, routine treatment with levothyroxine is not recommended, based on new evidence from randomized trials of high-risk patients showing no clear benefit from the treatment.
“In these trials, and across analyses, there was absolutely no beneficial effect of levothyroxine in these patients [with euthyroid TPO antibody positivity],” he said.
With evidence showing, however, that TPO antibody positivity can lead to subclinical or overt hypothyroidism within 1 or 2 years, the guidelines will recommend that TPO antibody–positive patients do have thyroid stimulating hormone (TSH) levels tested every 3-6 months until pregnancy, and existing recommendations to test during pregnancy among those patients remain in place, Korevaar reported.
In terms of preconception subclinical hypothyroidism, the guidelines will emphasize the existing recommendation “to always strive to reassess” thyroid levels, and if subclinical hypothyroidism does persist, to treat with low-dose levothyroxine.
During Pregnancy
During pregnancy, the new proposed recommendations will reflect the important change that three key risk factors, including age over 30 years, having at least two prior pregnancies, and morbid obesity (body mass index [BMI] at least 40 kg/m2), previously considered a risk for thyroid dysfunction in pregnancy, should not, on their own, suggest the need for thyroid testing, based on low evidence of an increased risk in pregnancy.
Research on the issue includes a recent study from Korevaar’s team showing these factors to in fact have low predictability of thyroid dysfunction.
“We deemed that these risk differences weren’t really clinically meaningful (in predicting risk), and so we have removed to maternal age, BMI, and parity as risk factors for thyroid testing indications in pregnancy,” Korevaar said.
Factors considered a risk, resulting in recommended testing at presentation include a history of subclinical or clinical hypo- or hyperthyroidism, postpartum thyroiditis, known thyroid antibody positivity, symptoms of thyroid dysfunction or goiter, and other factors.
Treatment for Subclinical Hypothyroidism in Pregnancy
Whereas current guidelines recommend TPO antibody status in determining when to consider treatment for subclinical hypothyroidism, the new proposed guideline will instead recommend treatment based on the timing of the diagnosis of the subclinical hypothyroidism, with consideration of treatment during the first trimester, but not in the second or third trimester, based on newer evidence of the absolute risk for pregnancy complications and randomized trial data.
“The recommendations are now to no longer based on TPO antibody status, but instead according to the timing of the diagnosis of subclinical hypothyroidism,” Korevaar said.
Based on the collective data, “due to the low risk, we do not recommend for routine levothyroxine treatment in the second or third trimester groups with TSH levels under 10 mU/L now.”
“However, for subclinical hypothyroidism diagnosed in the first trimester, the recommendation would be that you can consider levothyroxine treatment,” he said.
While a clear indication for treatment in any trimester is the presence of overt hypothyroidism, or TSH levels over 10 mU/L, Korevaar underscored the importance of considering nuances of the recommendations that may warrant flexibility, for instance among patients with borderline TSH levels.
Spontaneous Normalization of Thyroid Levels in Pregnancy
Another new recommendation addresses the issue of spontaneous normalization of abnormal thyroid function during pregnancy, with several large studies showing a large proportion of subclinical hypothyroidism cases spontaneously revert to euthyroidism by the third trimester — despite no treatment having been provided.
Under the important proposed recommendation, retesting of subclinical hypothyroidism is suggested within 3 weeks.
“The data shows that a large proportion of patients spontaneously revert to euthyroidism,” Korevaar said.
“Upon identifying subclinical hypothyroidism in the first trimester, there will be essentially two options that clinicians can discuss with their patient — one would be to consider confirmatory tests in 3 weeks or to discuss the starting the lower dose levothyroxine in the first trimester,” he said.
In terms of overt hypothyroidism, likewise, if patients have a TSH levels below 6 mU/L in pregnancy, “you can either consider doing confirmatory testing within 3 weeks, or discussing with the patient starting levothyroxine treatment,” Korevaar added.
Overt Hyperthyroidism
For overt hyperthyroidism, no significant changes from current guidelines are being proposed, with the key exception of a heightened emphasis on the need for shared decision-making with patients, Korevaar said.
“We want to emphasize shared decision-making especially for women who have Graves’ disease prior to pregnancy, because the antithyroid treatment modalities, primarily methimazole (MMI) and propylthiouracil (PTU), have different advantages and disadvantages for an upcoming pregnancy,” he said.
“If you help a patient become involved in the decision-making process, that can also be very helpful in managing the disease and following-up on the pregnancy.”
Under the recommendations, PTU remains the preferred drug in overt hyperthyroidism, due to a more favorable profile in terms of potential birth defects vs MMI, with research showing a higher absolute risk of 3% vs 5%.
The guidelines further suggest the option of stopping the antithyroid medications upon a positive pregnancy test, with the exception of high-risk patients.
Korevaar noted that, if the treatment is stopped early in pregnancy, relapse is not likely to occur until after approximately 3 months, or 12 weeks, at which time, the high-risk teratogenic period, which is between week 5 and week 15, will have passed.
Current guidelines regarding whether to stop treatment in higher-risk hyperthyroid patients are recommended to remain unchanged.
Thyroid Nodules and Cancer
Recommendations regarding thyroid nodules and cancer during pregnancy are also expected to remain largely similar to those in the 2017 guidelines, with the exception of an emphasis on simply considering how the patient would normally be managed outside of pregnancy.
For instance, regarding the question of whether treatment can be withheld for 9 months during pregnancy. “A lot of times, the answer is yes,” Korevaar said.
Other topics that will be largely unchanged include issues of universal screening, definitions of normal and abnormal TSH and free T4 reference ranges and isolated hypothyroxinemia.
Steps Forward in Improving Updates, Readability
In addition to recommendation updates, the new guidelines are being revised to better reflect more recent evidence-based developments and user-friendliness.
“We have now made the step to a more systematic and replicable methodology to ensure for easier updates with a shorter interval,” Korevaar told this news organization.
“Furthermore, since 2006, the ATA guideline documents have followed a question-and-answer format, lacked recommendation tables and had none or only a few graphic illustrations,” he added.
“We are now further developing the typical outline of the guidelines to improve the readability and dissemination of the guideline document.”
Korevaar’s disclosures include lectureship fees from IBSA, Merck, and Berlin Chemie.
A version of this article first appeared on Medscape.com.
FROM ATA 2024
Many Patients With Cancer Visit EDs Before Diagnosis
Researchers examined Institute for Clinical Evaluative Sciences (ICES) data that had been gathered from January 1, 2014, to December 31, 2021. The study focused on patients aged 18 years or older with confirmed primary cancer diagnoses.
Factors associated with an increased likelihood of an ED visit ahead of diagnosis included having certain cancers, living in rural areas, and having less access to primary care, according to study author Keerat Grewal, MD, an emergency physician and clinician scientist at the Schwartz/Reisman Emergency Medicine Institute at Sinai Health in Toronto, Ontario, Canada, and coauthors.
“The ED is a distressing environment for patients to receive a possible cancer diagnosis,” the authors wrote. “Moreover, it is frequently ill equipped to provide ongoing continuity of care, which can lead patients down a poorly defined diagnostic pathway before receiving a confirmed diagnosis based on tissue and a subsequent treatment plan.”
The findings were published online on November 4 in CMAJ).
Neurologic Cancers Prominent
In an interview, Grewal said in an interview that the study reflects her desire as an emergency room physician to understand why so many patients with cancer get the initial reports about their disease from clinicians whom they often have just met for the first time.
Among patients with an ED visit before cancer diagnosis, 51.4% were admitted to hospital from the most recent visit.
Compared with patients with a family physician on whom they could rely for routine care, those who had no outpatient visits (odds ratio [OR], 2.09) or fewer than three outpatient visits (OR, 1.41) in the 6-30 months before cancer diagnosis were more likely to have an ED visit before their cancer diagnosis.
Other factors associated with increased odds of ED use before cancer diagnosis included rurality (OR, 1.15), residence in northern Ontario (northeast region: OR, 1.14 and northwest region: OR, 1.27 vs Toronto region), and living in the most marginalized areas (material resource deprivation: OR, 1.37 and housing stability: OR, 1.09 vs least marginalized area).
The researchers also found that patients with certain cancers were more likely to have sought care in the ED. They compared these cancers with breast cancer, which is often detected through screening.
“Patients with neurologic cancers had extremely high odds of ED use before cancer diagnosis,” the authors wrote. “This is likely because of the emergent nature of presentation, with acute neurologic symptoms such as weakness, confusion, or seizures, which require urgent assessment.” On the other hand, pancreatic, liver, or thoracic cancer can trigger nonspecific symptoms that may be ignored until they reach a crisis level that prompts an ED visit.
The limitations of the study included its inability to identify cancer-related ED visits and its narrow focus on patients in Ontario, according to the researchers. But the use of the ICES databases also allowed researchers access to a broader pool of data than are available in many other cases.
The findings in the new paper echo those of previous research, the authors noted. Research in the United Kingdom found that 24%-31% of cancer diagnoses involved the ED. In addition, a study of people enrolled in the US Medicare program, which serves patients aged 65 years or older, found that 23% were seen in the ED in the 30 days before diagnosis.
‘Unpacking the Data’
The current findings also are consistent with those of an International Cancer Benchmarking Partnership study that was published in 2022 in The Lancet Oncology, said Erika Nicholson, MHS, vice president of cancer systems and innovation at the Canadian Partnership Against Cancer. The latter study analyzed cancer registration and linked hospital admissions data from 14 jurisdictions in Australia, Canada, Denmark, New Zealand, Norway, and the United Kingdom.
“We see similar trends in terms of people visiting EDs and being diagnosed through EDs internationally,” Nicholson said. “We’re working with partners to put in place different strategies to address the challenges” that this phenomenon presents in terms of improving screening and follow-up care.
“Cancer is not one disease, but many diseases,” she said. “They present differently. We’re focused on really unpacking the data and understanding them.”
All this research highlights the need for more services and personnel to address cancer, including people who are trained to help patients cope after getting concerning news through emergency care, she said.
“That means having a system that fully supports you and helps you navigate through that diagnostic process,” Nicholson said. Addressing the added challenges for patients who don’t have secure housing is a special need, she added.
This study was supported by the Canadian Institutes of Health Research (CIHR). Grewal reported receiving grants from CIHR and the Canadian Association of Emergency Physicians. Nicholson reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
Researchers examined Institute for Clinical Evaluative Sciences (ICES) data that had been gathered from January 1, 2014, to December 31, 2021. The study focused on patients aged 18 years or older with confirmed primary cancer diagnoses.
Factors associated with an increased likelihood of an ED visit ahead of diagnosis included having certain cancers, living in rural areas, and having less access to primary care, according to study author Keerat Grewal, MD, an emergency physician and clinician scientist at the Schwartz/Reisman Emergency Medicine Institute at Sinai Health in Toronto, Ontario, Canada, and coauthors.
“The ED is a distressing environment for patients to receive a possible cancer diagnosis,” the authors wrote. “Moreover, it is frequently ill equipped to provide ongoing continuity of care, which can lead patients down a poorly defined diagnostic pathway before receiving a confirmed diagnosis based on tissue and a subsequent treatment plan.”
The findings were published online on November 4 in CMAJ).
Neurologic Cancers Prominent
In an interview, Grewal said in an interview that the study reflects her desire as an emergency room physician to understand why so many patients with cancer get the initial reports about their disease from clinicians whom they often have just met for the first time.
Among patients with an ED visit before cancer diagnosis, 51.4% were admitted to hospital from the most recent visit.
Compared with patients with a family physician on whom they could rely for routine care, those who had no outpatient visits (odds ratio [OR], 2.09) or fewer than three outpatient visits (OR, 1.41) in the 6-30 months before cancer diagnosis were more likely to have an ED visit before their cancer diagnosis.
Other factors associated with increased odds of ED use before cancer diagnosis included rurality (OR, 1.15), residence in northern Ontario (northeast region: OR, 1.14 and northwest region: OR, 1.27 vs Toronto region), and living in the most marginalized areas (material resource deprivation: OR, 1.37 and housing stability: OR, 1.09 vs least marginalized area).
The researchers also found that patients with certain cancers were more likely to have sought care in the ED. They compared these cancers with breast cancer, which is often detected through screening.
“Patients with neurologic cancers had extremely high odds of ED use before cancer diagnosis,” the authors wrote. “This is likely because of the emergent nature of presentation, with acute neurologic symptoms such as weakness, confusion, or seizures, which require urgent assessment.” On the other hand, pancreatic, liver, or thoracic cancer can trigger nonspecific symptoms that may be ignored until they reach a crisis level that prompts an ED visit.
The limitations of the study included its inability to identify cancer-related ED visits and its narrow focus on patients in Ontario, according to the researchers. But the use of the ICES databases also allowed researchers access to a broader pool of data than are available in many other cases.
The findings in the new paper echo those of previous research, the authors noted. Research in the United Kingdom found that 24%-31% of cancer diagnoses involved the ED. In addition, a study of people enrolled in the US Medicare program, which serves patients aged 65 years or older, found that 23% were seen in the ED in the 30 days before diagnosis.
‘Unpacking the Data’
The current findings also are consistent with those of an International Cancer Benchmarking Partnership study that was published in 2022 in The Lancet Oncology, said Erika Nicholson, MHS, vice president of cancer systems and innovation at the Canadian Partnership Against Cancer. The latter study analyzed cancer registration and linked hospital admissions data from 14 jurisdictions in Australia, Canada, Denmark, New Zealand, Norway, and the United Kingdom.
“We see similar trends in terms of people visiting EDs and being diagnosed through EDs internationally,” Nicholson said. “We’re working with partners to put in place different strategies to address the challenges” that this phenomenon presents in terms of improving screening and follow-up care.
“Cancer is not one disease, but many diseases,” she said. “They present differently. We’re focused on really unpacking the data and understanding them.”
All this research highlights the need for more services and personnel to address cancer, including people who are trained to help patients cope after getting concerning news through emergency care, she said.
“That means having a system that fully supports you and helps you navigate through that diagnostic process,” Nicholson said. Addressing the added challenges for patients who don’t have secure housing is a special need, she added.
This study was supported by the Canadian Institutes of Health Research (CIHR). Grewal reported receiving grants from CIHR and the Canadian Association of Emergency Physicians. Nicholson reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
Researchers examined Institute for Clinical Evaluative Sciences (ICES) data that had been gathered from January 1, 2014, to December 31, 2021. The study focused on patients aged 18 years or older with confirmed primary cancer diagnoses.
Factors associated with an increased likelihood of an ED visit ahead of diagnosis included having certain cancers, living in rural areas, and having less access to primary care, according to study author Keerat Grewal, MD, an emergency physician and clinician scientist at the Schwartz/Reisman Emergency Medicine Institute at Sinai Health in Toronto, Ontario, Canada, and coauthors.
“The ED is a distressing environment for patients to receive a possible cancer diagnosis,” the authors wrote. “Moreover, it is frequently ill equipped to provide ongoing continuity of care, which can lead patients down a poorly defined diagnostic pathway before receiving a confirmed diagnosis based on tissue and a subsequent treatment plan.”
The findings were published online on November 4 in CMAJ).
Neurologic Cancers Prominent
In an interview, Grewal said in an interview that the study reflects her desire as an emergency room physician to understand why so many patients with cancer get the initial reports about their disease from clinicians whom they often have just met for the first time.
Among patients with an ED visit before cancer diagnosis, 51.4% were admitted to hospital from the most recent visit.
Compared with patients with a family physician on whom they could rely for routine care, those who had no outpatient visits (odds ratio [OR], 2.09) or fewer than three outpatient visits (OR, 1.41) in the 6-30 months before cancer diagnosis were more likely to have an ED visit before their cancer diagnosis.
Other factors associated with increased odds of ED use before cancer diagnosis included rurality (OR, 1.15), residence in northern Ontario (northeast region: OR, 1.14 and northwest region: OR, 1.27 vs Toronto region), and living in the most marginalized areas (material resource deprivation: OR, 1.37 and housing stability: OR, 1.09 vs least marginalized area).
The researchers also found that patients with certain cancers were more likely to have sought care in the ED. They compared these cancers with breast cancer, which is often detected through screening.
“Patients with neurologic cancers had extremely high odds of ED use before cancer diagnosis,” the authors wrote. “This is likely because of the emergent nature of presentation, with acute neurologic symptoms such as weakness, confusion, or seizures, which require urgent assessment.” On the other hand, pancreatic, liver, or thoracic cancer can trigger nonspecific symptoms that may be ignored until they reach a crisis level that prompts an ED visit.
The limitations of the study included its inability to identify cancer-related ED visits and its narrow focus on patients in Ontario, according to the researchers. But the use of the ICES databases also allowed researchers access to a broader pool of data than are available in many other cases.
The findings in the new paper echo those of previous research, the authors noted. Research in the United Kingdom found that 24%-31% of cancer diagnoses involved the ED. In addition, a study of people enrolled in the US Medicare program, which serves patients aged 65 years or older, found that 23% were seen in the ED in the 30 days before diagnosis.
‘Unpacking the Data’
The current findings also are consistent with those of an International Cancer Benchmarking Partnership study that was published in 2022 in The Lancet Oncology, said Erika Nicholson, MHS, vice president of cancer systems and innovation at the Canadian Partnership Against Cancer. The latter study analyzed cancer registration and linked hospital admissions data from 14 jurisdictions in Australia, Canada, Denmark, New Zealand, Norway, and the United Kingdom.
“We see similar trends in terms of people visiting EDs and being diagnosed through EDs internationally,” Nicholson said. “We’re working with partners to put in place different strategies to address the challenges” that this phenomenon presents in terms of improving screening and follow-up care.
“Cancer is not one disease, but many diseases,” she said. “They present differently. We’re focused on really unpacking the data and understanding them.”
All this research highlights the need for more services and personnel to address cancer, including people who are trained to help patients cope after getting concerning news through emergency care, she said.
“That means having a system that fully supports you and helps you navigate through that diagnostic process,” Nicholson said. Addressing the added challenges for patients who don’t have secure housing is a special need, she added.
This study was supported by the Canadian Institutes of Health Research (CIHR). Grewal reported receiving grants from CIHR and the Canadian Association of Emergency Physicians. Nicholson reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
FROM CMAJ
Are GLP-1s the Newest Fertility Treatment?
First, there were “Ozempic babies.” Now, there is also Ozempic-before-baby.
Unplanned pregnancies are still regularly being reported among people using glucagon-like peptide 1 receptor agonist (GLP-1 RA) drugs, and now fertility specialists are increasingly incorporating the medicines into preconception care plans.
The specialists say their colleagues in other areas of medicine may have an opportunity, too, to talk about weight loss using these new drugs in terms of reproductive health. Motivation and compliance can transform when the goal isn’t simply weight loss but having children.
“We have this really special moment to help patients be healthier, in order to be healthier for their kids,” said Christina Boots, MD, MSci, an associate professor of reproductive endocrinology and infertility at Northwestern University’s Feinberg School of Medicine, Chicago. “And I think that’s also a very motivating moment. It may be hard to get up and go for a run to make my jeans fit better, but when I think about it in terms of, ‘this might someday help my future daughter,’ that is a whole different level of motivation.”
Here’s why, what to know about the current lengthy list of unknowns and risks, and some options for approaching the topic with patients.
What Fertility Docs Are Doing
While overweight and obesity are consistently linked to fertility and pregnancy outcomes, Boots predicts the biggest impact of GLP-1 weight loss for fertility among women will be a specific subset: Those who are not cycling regularly, such as those with polycystic ovary syndrome (PCOS).
“The women who are cycling regularly who have very unexplained infertility and no other comorbidities like high blood pressure or something else going on, I don’t think it’s going to help their fertility very much at all,” she said “It might, but I think there’s probably something else going on in her tubes or with her eggs or his sperm, but it has nothing to do with her metabolic health.
Women who aren’t cycling regularly will benefit, but those with truly unexplained fertility probably won’t, she said.
In their recent narrative review on treating obesity and fertility with GLP-1 RAs that appeared in Fertility and Sterility, Boots and co-author Alyse S. Goldberg, MD, an endocrinologist with the University of Toronto, Ontario, Canada, advocate for the use of GLP-1s as a go-to treatment for obesity as part of preconception care by reproductive endocrinologists, calling the drugs “the most effective, least invasive means of weight loss.”
The paper is timely and necessary because use of GLP-1s is only going to increase, Patricia Jimenez, MD, an associate professor of obstetrics and gynecology at Washington University School of Medicine in St. Louis, Missouri, said in an email to this news organization.
“GLP-1 RAs are becoming a larger part of my practice. More patients are either using them already or interested in using them,” said Jimenez, who is board certified in reproductive endocrinology, obstetrics and gynecology, and obesity medicine. “I specifically see patients to discuss this and do prescribe antiobesity medications, not only GLP-1 RAs. Often this is with people with PCOS who are not planning to conceive soon or patients willing to delay fertility treatment [by] 3-6 months.”
Treating obesity is also important for women who are seeking in vitro fertilization, Boots said, because many IVF clinics have a body mass index cutoff of 40 kg/m2.
Like Jimenez’s approach, Boots and Goldberg call for comprehensive obesity care beyond the use of medication, including nutritional counseling and mental health support. Those supports are important during the transition off of GLP-1 medications, which poses a risk for rapid weight regain. That’s even with the potential support of taking metformin, which Boots often prescribes as a bridge.
Semaglutide should be stopped at least 2 months prior to conception, and tirzepatide should be stopped 1 month prior to conception, according to the manufacturers. (Boots and Goldberg listed the Canadian label recommendation for stopping tirzepatide, noting there is no suggested timeline for stopping prior to conception on the US label.)
Numerous studies have shown rapid weight regain is common when stopping GLP-1s, which presents a unique set of risks for pregnant women including early pregnancy loss, gestational diabetes, preeclampsia, and nonelective cesarean delivery.
Weighing Risks, Benefits, and Unknowns
Early looks at small human data sets, mostly involving semaglutide and earlier short-acting GLP-1s, and their impact on the risk for birth defects are “reassuring,” Boots said.
“But birth defects are just one small aspect. There’s also metabolic health and things like that long-term. Understanding what it does to the growing baby and the proximity of that medication to that growing baby is really important to see, and can’t be answered with animal studies, not perfectly anyway,” Boots said.
There are no published reports, from clinical trials nor case collections, examining the use of tirzepatide among pregnant people.
“One of the most important questions we need to answer is the preconception safety of these medications, and that includes safety for men,” Joshua Halpern, MD, MS, an adjunct assistant professor of urology at Northwestern University’s Feinberg School of Medicine, and chief scientific officer for Posterity Health, said in an email to this news organization.
“For example, a recent study found that men who were taking metformin, another popular medication for diabetes, were more likely to have children with birth defects, compared with those who were not taking the medication,” Halpern said. “Further studies are needed to determine whether a similar effect might hold true for the GLP-1 agonists.”
Small early studies on sperm are encouraging, Halpern said, suggesting that GLP-1 use may be beneficial, but a better understanding of direct effects is needed.
Among women, there may be cases where continuing use of a GLP-1 during pregnancy may offer benefits that outweigh risks, Boots suggested. Manufacturers have also created pregnancy exposure registries to measure the safety of their therapies during pregnancy.
“I have a group of patients whose sugars are so well controlled on these medications, but as soon as they come off, they get weight regain and their glucose is just so poorly controlled,” she said. “There may be a group of women where the benefits of glucose control outweigh the risks of being on the medication the whole pregnancy.”
The list of important unknowns also includes a need to examine how rapid weight loss may impact ovulation rates and spontaneous conception, as well as miscarriage rates, birth weight, and metabolic health of the child.
More detailed rebound weight gain data is coming next year, with additional analysis expected as well on birth weight and pregnancy outcomes, said Jacqueline Maya, MD, first author of the research abstract presented at this year’s American Diabetes Association conference that examined gestational weight gain among people with preexisting type 2 diabetes who were exposed to GLP-1s during pregnancy. The study included 47 exposed pregnancies (based on prescription records and electronic chart information) and compared gestational weight gain to 141 unexposed matched pregnancies. Among the exposed group, 62% exceeded recommended weight gain, compared with 41% in the unexposed group. On average, gestational weight gain in exposed pregnancies exceeded that among matched unexposed pregnancies by about 6 pounds.
The team is now working with an additional data set to examine exposed pregnancies among people with obesity, said Maya, an instructor of pediatrics at Mass General Hospital and Harvard School of Medicine. She is particularly interested in examining weight trajectories during pregnancy to see how they may affect fetal outcomes. Her team’s current project also will likely include analysis to examine other variables like postpartum weight gain and adiposity characteristics of the baby.
Maya said the team hopes to have more to report at the American Diabetes Association conference in June next year.
Offer the Conversation
Using a GLP-1 for weight loss takes time, usually around 1 year to reach a plateau. Boots encouraged nonfertility providers to ask patients of reproductive age about their family plans as an opening.
“I hope for all primary care doctors and gynecologists, that with any patient of reproductive age, you should be bringing this up, asking, ‘Have you thought about having kids? Are you thinking about it soon?’ And if they say they are sometime in the near future, then you can say, ‘Is it OK if I bring up your weight?’ And you should ask permission.”
If the patient declines, it’s OK to bring it up again at a future visit.
“People with obesity have often experienced negative weight bias that impacts their care,” Jimenez said. “Treat obesity as a disease, not a personal failing. Ask permission to discuss weight with the patient beforehand. If they say no, respect that answer. This goes a long way in developing a positive relationship, so they return for care and may be willing to discuss later.”
When patients are open to the conversation, Boots suggests not focusing on the potential for poor outcomes, and instead perhaps saying, “If you’re thinking about having a baby in 5 years, optimizing your health now will not only make your pregnancy healthier, but your child healthier long-term.”
Discussing contraception plans remains important. People starting semaglutide or tirzepatide should use contraception other than oral birth control for 4 weeks while starting the medicine and for 4 weeks after each dose increase.
Boots said that the contraception conversation is particularly important because many people have come to deeply believe that they are infertile and, thus, may perhaps think contraception advice doesn’t apply to them. Maya hypothesized that behavioral changes following weight loss may also be a pathway toward pregnancy.
“Pregnancy while on GLP-1 RAs does happen. I always have a discussion about this possibility and contraception. This can sometimes be challenging for people with infertility to consider,” Jimenez said. “Explaining the risks, benefits, and unknowns can help. As the [Fertility and Sterility] paper describes, the limited data available has not shown increased fetal or maternal complications. We need more high quality data to better understand the impact of exposure or use around the time of conception and during pregnancy.”
It’s also important to introduce the idea to patients that they may someday need to come off the medications, such as when they are ready to have children, and how important lifestyle and behavioral changes will be at that time, Maya said.
“We do know what the alternative is, and we do know what the risks of obesity are,” she said. “So, it’s a tug and pull. We’re not starting off with healthy. We’re starting off with a disease that is physically and emotionally very difficult for the patient, especially when it starts in childhood.”
A version of this article appeared on Medscape.com.
First, there were “Ozempic babies.” Now, there is also Ozempic-before-baby.
Unplanned pregnancies are still regularly being reported among people using glucagon-like peptide 1 receptor agonist (GLP-1 RA) drugs, and now fertility specialists are increasingly incorporating the medicines into preconception care plans.
The specialists say their colleagues in other areas of medicine may have an opportunity, too, to talk about weight loss using these new drugs in terms of reproductive health. Motivation and compliance can transform when the goal isn’t simply weight loss but having children.
“We have this really special moment to help patients be healthier, in order to be healthier for their kids,” said Christina Boots, MD, MSci, an associate professor of reproductive endocrinology and infertility at Northwestern University’s Feinberg School of Medicine, Chicago. “And I think that’s also a very motivating moment. It may be hard to get up and go for a run to make my jeans fit better, but when I think about it in terms of, ‘this might someday help my future daughter,’ that is a whole different level of motivation.”
Here’s why, what to know about the current lengthy list of unknowns and risks, and some options for approaching the topic with patients.
What Fertility Docs Are Doing
While overweight and obesity are consistently linked to fertility and pregnancy outcomes, Boots predicts the biggest impact of GLP-1 weight loss for fertility among women will be a specific subset: Those who are not cycling regularly, such as those with polycystic ovary syndrome (PCOS).
“The women who are cycling regularly who have very unexplained infertility and no other comorbidities like high blood pressure or something else going on, I don’t think it’s going to help their fertility very much at all,” she said “It might, but I think there’s probably something else going on in her tubes or with her eggs or his sperm, but it has nothing to do with her metabolic health.
Women who aren’t cycling regularly will benefit, but those with truly unexplained fertility probably won’t, she said.
In their recent narrative review on treating obesity and fertility with GLP-1 RAs that appeared in Fertility and Sterility, Boots and co-author Alyse S. Goldberg, MD, an endocrinologist with the University of Toronto, Ontario, Canada, advocate for the use of GLP-1s as a go-to treatment for obesity as part of preconception care by reproductive endocrinologists, calling the drugs “the most effective, least invasive means of weight loss.”
The paper is timely and necessary because use of GLP-1s is only going to increase, Patricia Jimenez, MD, an associate professor of obstetrics and gynecology at Washington University School of Medicine in St. Louis, Missouri, said in an email to this news organization.
“GLP-1 RAs are becoming a larger part of my practice. More patients are either using them already or interested in using them,” said Jimenez, who is board certified in reproductive endocrinology, obstetrics and gynecology, and obesity medicine. “I specifically see patients to discuss this and do prescribe antiobesity medications, not only GLP-1 RAs. Often this is with people with PCOS who are not planning to conceive soon or patients willing to delay fertility treatment [by] 3-6 months.”
Treating obesity is also important for women who are seeking in vitro fertilization, Boots said, because many IVF clinics have a body mass index cutoff of 40 kg/m2.
Like Jimenez’s approach, Boots and Goldberg call for comprehensive obesity care beyond the use of medication, including nutritional counseling and mental health support. Those supports are important during the transition off of GLP-1 medications, which poses a risk for rapid weight regain. That’s even with the potential support of taking metformin, which Boots often prescribes as a bridge.
Semaglutide should be stopped at least 2 months prior to conception, and tirzepatide should be stopped 1 month prior to conception, according to the manufacturers. (Boots and Goldberg listed the Canadian label recommendation for stopping tirzepatide, noting there is no suggested timeline for stopping prior to conception on the US label.)
Numerous studies have shown rapid weight regain is common when stopping GLP-1s, which presents a unique set of risks for pregnant women including early pregnancy loss, gestational diabetes, preeclampsia, and nonelective cesarean delivery.
Weighing Risks, Benefits, and Unknowns
Early looks at small human data sets, mostly involving semaglutide and earlier short-acting GLP-1s, and their impact on the risk for birth defects are “reassuring,” Boots said.
“But birth defects are just one small aspect. There’s also metabolic health and things like that long-term. Understanding what it does to the growing baby and the proximity of that medication to that growing baby is really important to see, and can’t be answered with animal studies, not perfectly anyway,” Boots said.
There are no published reports, from clinical trials nor case collections, examining the use of tirzepatide among pregnant people.
“One of the most important questions we need to answer is the preconception safety of these medications, and that includes safety for men,” Joshua Halpern, MD, MS, an adjunct assistant professor of urology at Northwestern University’s Feinberg School of Medicine, and chief scientific officer for Posterity Health, said in an email to this news organization.
“For example, a recent study found that men who were taking metformin, another popular medication for diabetes, were more likely to have children with birth defects, compared with those who were not taking the medication,” Halpern said. “Further studies are needed to determine whether a similar effect might hold true for the GLP-1 agonists.”
Small early studies on sperm are encouraging, Halpern said, suggesting that GLP-1 use may be beneficial, but a better understanding of direct effects is needed.
Among women, there may be cases where continuing use of a GLP-1 during pregnancy may offer benefits that outweigh risks, Boots suggested. Manufacturers have also created pregnancy exposure registries to measure the safety of their therapies during pregnancy.
“I have a group of patients whose sugars are so well controlled on these medications, but as soon as they come off, they get weight regain and their glucose is just so poorly controlled,” she said. “There may be a group of women where the benefits of glucose control outweigh the risks of being on the medication the whole pregnancy.”
The list of important unknowns also includes a need to examine how rapid weight loss may impact ovulation rates and spontaneous conception, as well as miscarriage rates, birth weight, and metabolic health of the child.
More detailed rebound weight gain data is coming next year, with additional analysis expected as well on birth weight and pregnancy outcomes, said Jacqueline Maya, MD, first author of the research abstract presented at this year’s American Diabetes Association conference that examined gestational weight gain among people with preexisting type 2 diabetes who were exposed to GLP-1s during pregnancy. The study included 47 exposed pregnancies (based on prescription records and electronic chart information) and compared gestational weight gain to 141 unexposed matched pregnancies. Among the exposed group, 62% exceeded recommended weight gain, compared with 41% in the unexposed group. On average, gestational weight gain in exposed pregnancies exceeded that among matched unexposed pregnancies by about 6 pounds.
The team is now working with an additional data set to examine exposed pregnancies among people with obesity, said Maya, an instructor of pediatrics at Mass General Hospital and Harvard School of Medicine. She is particularly interested in examining weight trajectories during pregnancy to see how they may affect fetal outcomes. Her team’s current project also will likely include analysis to examine other variables like postpartum weight gain and adiposity characteristics of the baby.
Maya said the team hopes to have more to report at the American Diabetes Association conference in June next year.
Offer the Conversation
Using a GLP-1 for weight loss takes time, usually around 1 year to reach a plateau. Boots encouraged nonfertility providers to ask patients of reproductive age about their family plans as an opening.
“I hope for all primary care doctors and gynecologists, that with any patient of reproductive age, you should be bringing this up, asking, ‘Have you thought about having kids? Are you thinking about it soon?’ And if they say they are sometime in the near future, then you can say, ‘Is it OK if I bring up your weight?’ And you should ask permission.”
If the patient declines, it’s OK to bring it up again at a future visit.
“People with obesity have often experienced negative weight bias that impacts their care,” Jimenez said. “Treat obesity as a disease, not a personal failing. Ask permission to discuss weight with the patient beforehand. If they say no, respect that answer. This goes a long way in developing a positive relationship, so they return for care and may be willing to discuss later.”
When patients are open to the conversation, Boots suggests not focusing on the potential for poor outcomes, and instead perhaps saying, “If you’re thinking about having a baby in 5 years, optimizing your health now will not only make your pregnancy healthier, but your child healthier long-term.”
Discussing contraception plans remains important. People starting semaglutide or tirzepatide should use contraception other than oral birth control for 4 weeks while starting the medicine and for 4 weeks after each dose increase.
Boots said that the contraception conversation is particularly important because many people have come to deeply believe that they are infertile and, thus, may perhaps think contraception advice doesn’t apply to them. Maya hypothesized that behavioral changes following weight loss may also be a pathway toward pregnancy.
“Pregnancy while on GLP-1 RAs does happen. I always have a discussion about this possibility and contraception. This can sometimes be challenging for people with infertility to consider,” Jimenez said. “Explaining the risks, benefits, and unknowns can help. As the [Fertility and Sterility] paper describes, the limited data available has not shown increased fetal or maternal complications. We need more high quality data to better understand the impact of exposure or use around the time of conception and during pregnancy.”
It’s also important to introduce the idea to patients that they may someday need to come off the medications, such as when they are ready to have children, and how important lifestyle and behavioral changes will be at that time, Maya said.
“We do know what the alternative is, and we do know what the risks of obesity are,” she said. “So, it’s a tug and pull. We’re not starting off with healthy. We’re starting off with a disease that is physically and emotionally very difficult for the patient, especially when it starts in childhood.”
A version of this article appeared on Medscape.com.
First, there were “Ozempic babies.” Now, there is also Ozempic-before-baby.
Unplanned pregnancies are still regularly being reported among people using glucagon-like peptide 1 receptor agonist (GLP-1 RA) drugs, and now fertility specialists are increasingly incorporating the medicines into preconception care plans.
The specialists say their colleagues in other areas of medicine may have an opportunity, too, to talk about weight loss using these new drugs in terms of reproductive health. Motivation and compliance can transform when the goal isn’t simply weight loss but having children.
“We have this really special moment to help patients be healthier, in order to be healthier for their kids,” said Christina Boots, MD, MSci, an associate professor of reproductive endocrinology and infertility at Northwestern University’s Feinberg School of Medicine, Chicago. “And I think that’s also a very motivating moment. It may be hard to get up and go for a run to make my jeans fit better, but when I think about it in terms of, ‘this might someday help my future daughter,’ that is a whole different level of motivation.”
Here’s why, what to know about the current lengthy list of unknowns and risks, and some options for approaching the topic with patients.
What Fertility Docs Are Doing
While overweight and obesity are consistently linked to fertility and pregnancy outcomes, Boots predicts the biggest impact of GLP-1 weight loss for fertility among women will be a specific subset: Those who are not cycling regularly, such as those with polycystic ovary syndrome (PCOS).
“The women who are cycling regularly who have very unexplained infertility and no other comorbidities like high blood pressure or something else going on, I don’t think it’s going to help their fertility very much at all,” she said “It might, but I think there’s probably something else going on in her tubes or with her eggs or his sperm, but it has nothing to do with her metabolic health.
Women who aren’t cycling regularly will benefit, but those with truly unexplained fertility probably won’t, she said.
In their recent narrative review on treating obesity and fertility with GLP-1 RAs that appeared in Fertility and Sterility, Boots and co-author Alyse S. Goldberg, MD, an endocrinologist with the University of Toronto, Ontario, Canada, advocate for the use of GLP-1s as a go-to treatment for obesity as part of preconception care by reproductive endocrinologists, calling the drugs “the most effective, least invasive means of weight loss.”
The paper is timely and necessary because use of GLP-1s is only going to increase, Patricia Jimenez, MD, an associate professor of obstetrics and gynecology at Washington University School of Medicine in St. Louis, Missouri, said in an email to this news organization.
“GLP-1 RAs are becoming a larger part of my practice. More patients are either using them already or interested in using them,” said Jimenez, who is board certified in reproductive endocrinology, obstetrics and gynecology, and obesity medicine. “I specifically see patients to discuss this and do prescribe antiobesity medications, not only GLP-1 RAs. Often this is with people with PCOS who are not planning to conceive soon or patients willing to delay fertility treatment [by] 3-6 months.”
Treating obesity is also important for women who are seeking in vitro fertilization, Boots said, because many IVF clinics have a body mass index cutoff of 40 kg/m2.
Like Jimenez’s approach, Boots and Goldberg call for comprehensive obesity care beyond the use of medication, including nutritional counseling and mental health support. Those supports are important during the transition off of GLP-1 medications, which poses a risk for rapid weight regain. That’s even with the potential support of taking metformin, which Boots often prescribes as a bridge.
Semaglutide should be stopped at least 2 months prior to conception, and tirzepatide should be stopped 1 month prior to conception, according to the manufacturers. (Boots and Goldberg listed the Canadian label recommendation for stopping tirzepatide, noting there is no suggested timeline for stopping prior to conception on the US label.)
Numerous studies have shown rapid weight regain is common when stopping GLP-1s, which presents a unique set of risks for pregnant women including early pregnancy loss, gestational diabetes, preeclampsia, and nonelective cesarean delivery.
Weighing Risks, Benefits, and Unknowns
Early looks at small human data sets, mostly involving semaglutide and earlier short-acting GLP-1s, and their impact on the risk for birth defects are “reassuring,” Boots said.
“But birth defects are just one small aspect. There’s also metabolic health and things like that long-term. Understanding what it does to the growing baby and the proximity of that medication to that growing baby is really important to see, and can’t be answered with animal studies, not perfectly anyway,” Boots said.
There are no published reports, from clinical trials nor case collections, examining the use of tirzepatide among pregnant people.
“One of the most important questions we need to answer is the preconception safety of these medications, and that includes safety for men,” Joshua Halpern, MD, MS, an adjunct assistant professor of urology at Northwestern University’s Feinberg School of Medicine, and chief scientific officer for Posterity Health, said in an email to this news organization.
“For example, a recent study found that men who were taking metformin, another popular medication for diabetes, were more likely to have children with birth defects, compared with those who were not taking the medication,” Halpern said. “Further studies are needed to determine whether a similar effect might hold true for the GLP-1 agonists.”
Small early studies on sperm are encouraging, Halpern said, suggesting that GLP-1 use may be beneficial, but a better understanding of direct effects is needed.
Among women, there may be cases where continuing use of a GLP-1 during pregnancy may offer benefits that outweigh risks, Boots suggested. Manufacturers have also created pregnancy exposure registries to measure the safety of their therapies during pregnancy.
“I have a group of patients whose sugars are so well controlled on these medications, but as soon as they come off, they get weight regain and their glucose is just so poorly controlled,” she said. “There may be a group of women where the benefits of glucose control outweigh the risks of being on the medication the whole pregnancy.”
The list of important unknowns also includes a need to examine how rapid weight loss may impact ovulation rates and spontaneous conception, as well as miscarriage rates, birth weight, and metabolic health of the child.
More detailed rebound weight gain data is coming next year, with additional analysis expected as well on birth weight and pregnancy outcomes, said Jacqueline Maya, MD, first author of the research abstract presented at this year’s American Diabetes Association conference that examined gestational weight gain among people with preexisting type 2 diabetes who were exposed to GLP-1s during pregnancy. The study included 47 exposed pregnancies (based on prescription records and electronic chart information) and compared gestational weight gain to 141 unexposed matched pregnancies. Among the exposed group, 62% exceeded recommended weight gain, compared with 41% in the unexposed group. On average, gestational weight gain in exposed pregnancies exceeded that among matched unexposed pregnancies by about 6 pounds.
The team is now working with an additional data set to examine exposed pregnancies among people with obesity, said Maya, an instructor of pediatrics at Mass General Hospital and Harvard School of Medicine. She is particularly interested in examining weight trajectories during pregnancy to see how they may affect fetal outcomes. Her team’s current project also will likely include analysis to examine other variables like postpartum weight gain and adiposity characteristics of the baby.
Maya said the team hopes to have more to report at the American Diabetes Association conference in June next year.
Offer the Conversation
Using a GLP-1 for weight loss takes time, usually around 1 year to reach a plateau. Boots encouraged nonfertility providers to ask patients of reproductive age about their family plans as an opening.
“I hope for all primary care doctors and gynecologists, that with any patient of reproductive age, you should be bringing this up, asking, ‘Have you thought about having kids? Are you thinking about it soon?’ And if they say they are sometime in the near future, then you can say, ‘Is it OK if I bring up your weight?’ And you should ask permission.”
If the patient declines, it’s OK to bring it up again at a future visit.
“People with obesity have often experienced negative weight bias that impacts their care,” Jimenez said. “Treat obesity as a disease, not a personal failing. Ask permission to discuss weight with the patient beforehand. If they say no, respect that answer. This goes a long way in developing a positive relationship, so they return for care and may be willing to discuss later.”
When patients are open to the conversation, Boots suggests not focusing on the potential for poor outcomes, and instead perhaps saying, “If you’re thinking about having a baby in 5 years, optimizing your health now will not only make your pregnancy healthier, but your child healthier long-term.”
Discussing contraception plans remains important. People starting semaglutide or tirzepatide should use contraception other than oral birth control for 4 weeks while starting the medicine and for 4 weeks after each dose increase.
Boots said that the contraception conversation is particularly important because many people have come to deeply believe that they are infertile and, thus, may perhaps think contraception advice doesn’t apply to them. Maya hypothesized that behavioral changes following weight loss may also be a pathway toward pregnancy.
“Pregnancy while on GLP-1 RAs does happen. I always have a discussion about this possibility and contraception. This can sometimes be challenging for people with infertility to consider,” Jimenez said. “Explaining the risks, benefits, and unknowns can help. As the [Fertility and Sterility] paper describes, the limited data available has not shown increased fetal or maternal complications. We need more high quality data to better understand the impact of exposure or use around the time of conception and during pregnancy.”
It’s also important to introduce the idea to patients that they may someday need to come off the medications, such as when they are ready to have children, and how important lifestyle and behavioral changes will be at that time, Maya said.
“We do know what the alternative is, and we do know what the risks of obesity are,” she said. “So, it’s a tug and pull. We’re not starting off with healthy. We’re starting off with a disease that is physically and emotionally very difficult for the patient, especially when it starts in childhood.”
A version of this article appeared on Medscape.com.


