User login
Official news magazine of the Society of Hospital Medicine
Copyright by Society of Hospital Medicine or related companies. All rights reserved. ISSN 1553-085X
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
div[contains(@class, 'pane-pub-article-hospitalist')]


In California, opioids most often prescribed in low-income, mostly white areas
There is a higher prevalence of opioid prescribing and opioid-related overdose deaths concentrated in regions with mostly low-income, white residents, compared with regions with high income and the lowest proportion of white residents, according to a new analysis of data on people living in California.
The findings of this study provide further evidence that the opioid epidemic affects a large proportion of low-income white communities (JAMA Intern Med. 2019 Feb 11. doi: 10.1001/jamainternmed.2018.6721).
“Whereas most epidemics predominate within social minority groups and previous US drug epidemics have typically been concentrated in nonwhite communities, Joseph Friedman, MPH, from the University of California, Los Angeles, and his colleagues wrote in their study. “Our analysis suggests that, at least in California, an important determinant of this phenomenon may be that white individuals have a higher level of exposure than nonwhite individuals to opioid prescriptions on a per capita basis through the health care system.”
Mr. Friedman and his colleagues analyzed 29.7 million prescription drug records from California’s Controlled Substance Utilization Review and Evaluation System in and examined the prevalence of opioids, benzodiazepines, and stimulants by race, ethnicity, and income level in 1,760 zip codes during 2011-2015. The researchers estimated the prevalence of opioid prescriptions in each zip code by calculating the number of people per zip code receiving an opioid prescription divided by the population of the zip code during each year.
Overall, 23.6% of California residents received at least one opioid prescription each year of the study. The researchers found 44.2% of individuals in zip codes with the lowest income but highest proportion of white residents and 16.1% of individuals in areas with the highest income and lowest proportion of white residents had received a minimum of one opioid prescription each year. The prevalence of stimulant prescriptions was 3.8% in zip codes with high income, and a high proportion of white population, compared with a prevalence of 0.6% in areas with low income and a low proportion of white residents. The researchers noted there was no association between income and benzodiazepine prescription, but the prevalence of benzodiazepine prescriptions was 15.7% in zip codes with the highest proportion of white residents, compared with 7.0% in zip codes with a low proportion of white residents.
During the same time period, there were 9,534 opioid overdose deaths in California from causes such as fentanyl, synthetic opioids, and prescription opioids. “Overdose deaths were highly concentrated in lower-income and mostly white areas,” Mr. Friedman and his colleagues wrote. “We observed an approximate 10-fold difference in overdose rates across the race/ethnicity–income gradient in California.”
Although the number of opioids prescribed each year has decreased since 2012, in a research letter published in the same issue noted that the rate of prescribing is still higher than it was in 1999 (JAMA Intern Med. 2019 Feb 11. doi: 10.1001/jamainternmed.2018.6989). The authors also pointed out increases in the duration of opioid prescriptions and wide regional variations in opioid prescribing rates.
In their study, Gery P. Guy Jr., PhD, and his colleagues used data from the IQVIA Xponent database from approximately 50,400 retail pharmacies and discovered the average morphine milligram equivalent (MME) per capita had decreased from 641.4 MME per capita in 2015 to 512.6 MME per capita in 2017 (20.1%). The number of opioid prescriptions also decreased from 6.7 per 100 persons in 2015 to 5.0 per 100 persons in 2017 (25.3%). However, during 2015-2017, the average duration of opioid prescriptions increased from 17.7 days to 18.3 days (3.4%), while the median duration increased during the same time from 15.0 days to 20.0 days (33.3%).
While 74.7% of counties reduced the number of opioids prescribed during 2015-2017 and there also were reductions in the rate of high-dose prescribing (76.6%) and overall prescribing rates (74.7%), Dr. Guy of the Centers for Disease Control and Prevention and his colleagues found “substantial variation” in 2017 prescription rates at the county level, with opioids prescribed at 1,061.0 MME per capita at the highest quartile, compared with 182.8 MME per capita at the lowest quartile.
“Recent reductions could be related to policies and strategies aimed at reducing inappropriate prescribing, increased awareness of the risks associated with opioids, and release of the CDC Guideline for Prescribing Opioids for Chronic Pain–United States, 2016,” Dr. Guy and his colleagues noted.
In an additional article published in the same JAMA Internal Medicine issue, Bennett Allen, a research associate at the New York City Department of Health and Mental Hygiene and his colleagues examined the rate of opioid overdose deaths for non-Hispanic white, non-Hispanic black, Hispanic, and undefined other races in New York (JAMA Intern Med. 2019 Feb 11. doi: 10.1001/jamainternmed.2018.7700). They identified 1,487 deaths in 2017, which included 556 white (37.0%), 421 black (28.0%), 455 Hispanic (31.0%), and 55 undefined (4.0%) opioid overdose deaths. There was a higher rate of fentanyl and/or heroin overdose deaths from younger (aged 15-34 years) white New Yorkers (22.2/100,000 persons; 95% confidence interval, 19.0-25.5), compared with younger black New Yorkers (5.8/100,000; 95% CI, 4.0-8.2) and Hispanic (9.7/100,000; 95% CI, 7.6-12.1).
Among older residents (aged 55-84 years), Mr. Allen and his colleagues found higher rates of fentanyl and/or heroin overdose for black New Yorkers (25.4/100,000 persons; 95% CI, 20.9-30.0), compared with older white New Yorkers (9.4/100,000 persons; 95% CI, 7.3-11.8), as well as significantly higher rates of cocaine overdose (25.4/100,000 persons; 95% CI, 20.9-30.0), compared with white (5.1/100,000 persons; 95% CI, 3.6-7.0) and Hispanic residents (11.8/100,000 persons; 95% CI, 8.9-15.4).
“The distinct age distribution and drug involvement of overdose deaths among New York City blacks, Latinos, and whites, along with complementary evidence about drug use trajectories, highlight the need for heterogeneous approaches to treatment and the equitable allocation of treatment and health care resources to reach diverse populations at risk of overdose,” Mr. Allen and his colleagues wrote.
Dr. Schriger reported support from Korein Foundation for his time working on the study by Friedman et al. The other authors reported no conflicts of interest.
The results published by Friedman et al. are a reminder that we can use regional prescribing trends to identify communities most susceptible to the opioid epidemic and give them the resources they need to combat opioid addiction, Vice Adm. Jerome M. Adams, MD, MPH, and Adm. Brett P. Giroir, MD, wrote in a related editorial.
“Discussion of overdose risks and coprescribing of naloxone must become routine if we are to make opioid prescribing safer,” the authors wrote.
Physicians also can help respond to the opioid epidemic outside of prescribing by promoting evidence-based nonopioid and nonpharmaceutical pain treatments, screening their patients for OUD and OUD risks, and acknowledging “that the problem cannot be solved by medical interventions alone.” Individual, environmental, and societal factors also contribute to the opioid epidemic, and physicians are uniquely suited to spearhead efforts aimed at addressing comprehensive opioid misuse.
“Physicians stand out as natural leaders to help solve the crises because of the depth of their knowledge, immediacy of their contact with patients, and relatively high level of respect their profession enjoys,” Dr. Adams and Dr. Giroir wrote. “We thereby call on our nation’s doctors to embrace their roles in the clinic and beyond to help educate communities, bring together stakeholders, and be part of the cultural change to support people living free from addiction.”
Dr. Adams is the 20th surgeon general of the United States at the U.S. Public Health Service and HHS; Dr. Giroir is the 16th U.S. assistant secretary for health at the U.S. Public Health Service and HHS. They reported no relevant conflicts of interest. Their invited commentary accompanied the three related articles in the publication (JAMA Intern Med. 2019 Feb 11. doi: 10.1001/jamainternmed.2018.7934 ).
The results published by Friedman et al. are a reminder that we can use regional prescribing trends to identify communities most susceptible to the opioid epidemic and give them the resources they need to combat opioid addiction, Vice Adm. Jerome M. Adams, MD, MPH, and Adm. Brett P. Giroir, MD, wrote in a related editorial.
“Discussion of overdose risks and coprescribing of naloxone must become routine if we are to make opioid prescribing safer,” the authors wrote.
Physicians also can help respond to the opioid epidemic outside of prescribing by promoting evidence-based nonopioid and nonpharmaceutical pain treatments, screening their patients for OUD and OUD risks, and acknowledging “that the problem cannot be solved by medical interventions alone.” Individual, environmental, and societal factors also contribute to the opioid epidemic, and physicians are uniquely suited to spearhead efforts aimed at addressing comprehensive opioid misuse.
“Physicians stand out as natural leaders to help solve the crises because of the depth of their knowledge, immediacy of their contact with patients, and relatively high level of respect their profession enjoys,” Dr. Adams and Dr. Giroir wrote. “We thereby call on our nation’s doctors to embrace their roles in the clinic and beyond to help educate communities, bring together stakeholders, and be part of the cultural change to support people living free from addiction.”
Dr. Adams is the 20th surgeon general of the United States at the U.S. Public Health Service and HHS; Dr. Giroir is the 16th U.S. assistant secretary for health at the U.S. Public Health Service and HHS. They reported no relevant conflicts of interest. Their invited commentary accompanied the three related articles in the publication (JAMA Intern Med. 2019 Feb 11. doi: 10.1001/jamainternmed.2018.7934 ).
The results published by Friedman et al. are a reminder that we can use regional prescribing trends to identify communities most susceptible to the opioid epidemic and give them the resources they need to combat opioid addiction, Vice Adm. Jerome M. Adams, MD, MPH, and Adm. Brett P. Giroir, MD, wrote in a related editorial.
“Discussion of overdose risks and coprescribing of naloxone must become routine if we are to make opioid prescribing safer,” the authors wrote.
Physicians also can help respond to the opioid epidemic outside of prescribing by promoting evidence-based nonopioid and nonpharmaceutical pain treatments, screening their patients for OUD and OUD risks, and acknowledging “that the problem cannot be solved by medical interventions alone.” Individual, environmental, and societal factors also contribute to the opioid epidemic, and physicians are uniquely suited to spearhead efforts aimed at addressing comprehensive opioid misuse.
“Physicians stand out as natural leaders to help solve the crises because of the depth of their knowledge, immediacy of their contact with patients, and relatively high level of respect their profession enjoys,” Dr. Adams and Dr. Giroir wrote. “We thereby call on our nation’s doctors to embrace their roles in the clinic and beyond to help educate communities, bring together stakeholders, and be part of the cultural change to support people living free from addiction.”
Dr. Adams is the 20th surgeon general of the United States at the U.S. Public Health Service and HHS; Dr. Giroir is the 16th U.S. assistant secretary for health at the U.S. Public Health Service and HHS. They reported no relevant conflicts of interest. Their invited commentary accompanied the three related articles in the publication (JAMA Intern Med. 2019 Feb 11. doi: 10.1001/jamainternmed.2018.7934 ).
There is a higher prevalence of opioid prescribing and opioid-related overdose deaths concentrated in regions with mostly low-income, white residents, compared with regions with high income and the lowest proportion of white residents, according to a new analysis of data on people living in California.
The findings of this study provide further evidence that the opioid epidemic affects a large proportion of low-income white communities (JAMA Intern Med. 2019 Feb 11. doi: 10.1001/jamainternmed.2018.6721).
“Whereas most epidemics predominate within social minority groups and previous US drug epidemics have typically been concentrated in nonwhite communities, Joseph Friedman, MPH, from the University of California, Los Angeles, and his colleagues wrote in their study. “Our analysis suggests that, at least in California, an important determinant of this phenomenon may be that white individuals have a higher level of exposure than nonwhite individuals to opioid prescriptions on a per capita basis through the health care system.”
Mr. Friedman and his colleagues analyzed 29.7 million prescription drug records from California’s Controlled Substance Utilization Review and Evaluation System in and examined the prevalence of opioids, benzodiazepines, and stimulants by race, ethnicity, and income level in 1,760 zip codes during 2011-2015. The researchers estimated the prevalence of opioid prescriptions in each zip code by calculating the number of people per zip code receiving an opioid prescription divided by the population of the zip code during each year.
Overall, 23.6% of California residents received at least one opioid prescription each year of the study. The researchers found 44.2% of individuals in zip codes with the lowest income but highest proportion of white residents and 16.1% of individuals in areas with the highest income and lowest proportion of white residents had received a minimum of one opioid prescription each year. The prevalence of stimulant prescriptions was 3.8% in zip codes with high income, and a high proportion of white population, compared with a prevalence of 0.6% in areas with low income and a low proportion of white residents. The researchers noted there was no association between income and benzodiazepine prescription, but the prevalence of benzodiazepine prescriptions was 15.7% in zip codes with the highest proportion of white residents, compared with 7.0% in zip codes with a low proportion of white residents.
During the same time period, there were 9,534 opioid overdose deaths in California from causes such as fentanyl, synthetic opioids, and prescription opioids. “Overdose deaths were highly concentrated in lower-income and mostly white areas,” Mr. Friedman and his colleagues wrote. “We observed an approximate 10-fold difference in overdose rates across the race/ethnicity–income gradient in California.”
Although the number of opioids prescribed each year has decreased since 2012, in a research letter published in the same issue noted that the rate of prescribing is still higher than it was in 1999 (JAMA Intern Med. 2019 Feb 11. doi: 10.1001/jamainternmed.2018.6989). The authors also pointed out increases in the duration of opioid prescriptions and wide regional variations in opioid prescribing rates.
In their study, Gery P. Guy Jr., PhD, and his colleagues used data from the IQVIA Xponent database from approximately 50,400 retail pharmacies and discovered the average morphine milligram equivalent (MME) per capita had decreased from 641.4 MME per capita in 2015 to 512.6 MME per capita in 2017 (20.1%). The number of opioid prescriptions also decreased from 6.7 per 100 persons in 2015 to 5.0 per 100 persons in 2017 (25.3%). However, during 2015-2017, the average duration of opioid prescriptions increased from 17.7 days to 18.3 days (3.4%), while the median duration increased during the same time from 15.0 days to 20.0 days (33.3%).
While 74.7% of counties reduced the number of opioids prescribed during 2015-2017 and there also were reductions in the rate of high-dose prescribing (76.6%) and overall prescribing rates (74.7%), Dr. Guy of the Centers for Disease Control and Prevention and his colleagues found “substantial variation” in 2017 prescription rates at the county level, with opioids prescribed at 1,061.0 MME per capita at the highest quartile, compared with 182.8 MME per capita at the lowest quartile.
“Recent reductions could be related to policies and strategies aimed at reducing inappropriate prescribing, increased awareness of the risks associated with opioids, and release of the CDC Guideline for Prescribing Opioids for Chronic Pain–United States, 2016,” Dr. Guy and his colleagues noted.
In an additional article published in the same JAMA Internal Medicine issue, Bennett Allen, a research associate at the New York City Department of Health and Mental Hygiene and his colleagues examined the rate of opioid overdose deaths for non-Hispanic white, non-Hispanic black, Hispanic, and undefined other races in New York (JAMA Intern Med. 2019 Feb 11. doi: 10.1001/jamainternmed.2018.7700). They identified 1,487 deaths in 2017, which included 556 white (37.0%), 421 black (28.0%), 455 Hispanic (31.0%), and 55 undefined (4.0%) opioid overdose deaths. There was a higher rate of fentanyl and/or heroin overdose deaths from younger (aged 15-34 years) white New Yorkers (22.2/100,000 persons; 95% confidence interval, 19.0-25.5), compared with younger black New Yorkers (5.8/100,000; 95% CI, 4.0-8.2) and Hispanic (9.7/100,000; 95% CI, 7.6-12.1).
Among older residents (aged 55-84 years), Mr. Allen and his colleagues found higher rates of fentanyl and/or heroin overdose for black New Yorkers (25.4/100,000 persons; 95% CI, 20.9-30.0), compared with older white New Yorkers (9.4/100,000 persons; 95% CI, 7.3-11.8), as well as significantly higher rates of cocaine overdose (25.4/100,000 persons; 95% CI, 20.9-30.0), compared with white (5.1/100,000 persons; 95% CI, 3.6-7.0) and Hispanic residents (11.8/100,000 persons; 95% CI, 8.9-15.4).
“The distinct age distribution and drug involvement of overdose deaths among New York City blacks, Latinos, and whites, along with complementary evidence about drug use trajectories, highlight the need for heterogeneous approaches to treatment and the equitable allocation of treatment and health care resources to reach diverse populations at risk of overdose,” Mr. Allen and his colleagues wrote.
Dr. Schriger reported support from Korein Foundation for his time working on the study by Friedman et al. The other authors reported no conflicts of interest.
There is a higher prevalence of opioid prescribing and opioid-related overdose deaths concentrated in regions with mostly low-income, white residents, compared with regions with high income and the lowest proportion of white residents, according to a new analysis of data on people living in California.
The findings of this study provide further evidence that the opioid epidemic affects a large proportion of low-income white communities (JAMA Intern Med. 2019 Feb 11. doi: 10.1001/jamainternmed.2018.6721).
“Whereas most epidemics predominate within social minority groups and previous US drug epidemics have typically been concentrated in nonwhite communities, Joseph Friedman, MPH, from the University of California, Los Angeles, and his colleagues wrote in their study. “Our analysis suggests that, at least in California, an important determinant of this phenomenon may be that white individuals have a higher level of exposure than nonwhite individuals to opioid prescriptions on a per capita basis through the health care system.”
Mr. Friedman and his colleagues analyzed 29.7 million prescription drug records from California’s Controlled Substance Utilization Review and Evaluation System in and examined the prevalence of opioids, benzodiazepines, and stimulants by race, ethnicity, and income level in 1,760 zip codes during 2011-2015. The researchers estimated the prevalence of opioid prescriptions in each zip code by calculating the number of people per zip code receiving an opioid prescription divided by the population of the zip code during each year.
Overall, 23.6% of California residents received at least one opioid prescription each year of the study. The researchers found 44.2% of individuals in zip codes with the lowest income but highest proportion of white residents and 16.1% of individuals in areas with the highest income and lowest proportion of white residents had received a minimum of one opioid prescription each year. The prevalence of stimulant prescriptions was 3.8% in zip codes with high income, and a high proportion of white population, compared with a prevalence of 0.6% in areas with low income and a low proportion of white residents. The researchers noted there was no association between income and benzodiazepine prescription, but the prevalence of benzodiazepine prescriptions was 15.7% in zip codes with the highest proportion of white residents, compared with 7.0% in zip codes with a low proportion of white residents.
During the same time period, there were 9,534 opioid overdose deaths in California from causes such as fentanyl, synthetic opioids, and prescription opioids. “Overdose deaths were highly concentrated in lower-income and mostly white areas,” Mr. Friedman and his colleagues wrote. “We observed an approximate 10-fold difference in overdose rates across the race/ethnicity–income gradient in California.”
Although the number of opioids prescribed each year has decreased since 2012, in a research letter published in the same issue noted that the rate of prescribing is still higher than it was in 1999 (JAMA Intern Med. 2019 Feb 11. doi: 10.1001/jamainternmed.2018.6989). The authors also pointed out increases in the duration of opioid prescriptions and wide regional variations in opioid prescribing rates.
In their study, Gery P. Guy Jr., PhD, and his colleagues used data from the IQVIA Xponent database from approximately 50,400 retail pharmacies and discovered the average morphine milligram equivalent (MME) per capita had decreased from 641.4 MME per capita in 2015 to 512.6 MME per capita in 2017 (20.1%). The number of opioid prescriptions also decreased from 6.7 per 100 persons in 2015 to 5.0 per 100 persons in 2017 (25.3%). However, during 2015-2017, the average duration of opioid prescriptions increased from 17.7 days to 18.3 days (3.4%), while the median duration increased during the same time from 15.0 days to 20.0 days (33.3%).
While 74.7% of counties reduced the number of opioids prescribed during 2015-2017 and there also were reductions in the rate of high-dose prescribing (76.6%) and overall prescribing rates (74.7%), Dr. Guy of the Centers for Disease Control and Prevention and his colleagues found “substantial variation” in 2017 prescription rates at the county level, with opioids prescribed at 1,061.0 MME per capita at the highest quartile, compared with 182.8 MME per capita at the lowest quartile.
“Recent reductions could be related to policies and strategies aimed at reducing inappropriate prescribing, increased awareness of the risks associated with opioids, and release of the CDC Guideline for Prescribing Opioids for Chronic Pain–United States, 2016,” Dr. Guy and his colleagues noted.
In an additional article published in the same JAMA Internal Medicine issue, Bennett Allen, a research associate at the New York City Department of Health and Mental Hygiene and his colleagues examined the rate of opioid overdose deaths for non-Hispanic white, non-Hispanic black, Hispanic, and undefined other races in New York (JAMA Intern Med. 2019 Feb 11. doi: 10.1001/jamainternmed.2018.7700). They identified 1,487 deaths in 2017, which included 556 white (37.0%), 421 black (28.0%), 455 Hispanic (31.0%), and 55 undefined (4.0%) opioid overdose deaths. There was a higher rate of fentanyl and/or heroin overdose deaths from younger (aged 15-34 years) white New Yorkers (22.2/100,000 persons; 95% confidence interval, 19.0-25.5), compared with younger black New Yorkers (5.8/100,000; 95% CI, 4.0-8.2) and Hispanic (9.7/100,000; 95% CI, 7.6-12.1).
Among older residents (aged 55-84 years), Mr. Allen and his colleagues found higher rates of fentanyl and/or heroin overdose for black New Yorkers (25.4/100,000 persons; 95% CI, 20.9-30.0), compared with older white New Yorkers (9.4/100,000 persons; 95% CI, 7.3-11.8), as well as significantly higher rates of cocaine overdose (25.4/100,000 persons; 95% CI, 20.9-30.0), compared with white (5.1/100,000 persons; 95% CI, 3.6-7.0) and Hispanic residents (11.8/100,000 persons; 95% CI, 8.9-15.4).
“The distinct age distribution and drug involvement of overdose deaths among New York City blacks, Latinos, and whites, along with complementary evidence about drug use trajectories, highlight the need for heterogeneous approaches to treatment and the equitable allocation of treatment and health care resources to reach diverse populations at risk of overdose,” Mr. Allen and his colleagues wrote.
Dr. Schriger reported support from Korein Foundation for his time working on the study by Friedman et al. The other authors reported no conflicts of interest.
FROM JAMA INTERNAL MEDICINE
Key clinical point: The most common users of opioids according to prescription drug records are residents of mostly low-income, white neighborhoods.
Major finding: Compared with 23.6% of all Californians, 44.2% of individuals in zip codes containing mostly low-income, white residents had at least one opioid prescription each year, compared with 16.1% of individuals in high-income zip codes with the lowest population of white residents.
Study details: An analysis of 29.7 million opioid prescription drug records by race and income in California during 2011-2015.
Disclosures: Dr. Schriger reported support from the Korein Foundation for his time working on the study by Friedman et al. The other authors from Friedman et al. reported no conflicts of interest.
Improving interview skills for hospitalists
Standardized prep courses are helpful
Are residents generally prepared for fellowship interviews? Applications to the Fellowship Match through the National Residency Matching Program (NRMP) Specialties Matching Service (SMS) are at an all-time high, but there is limited data regarding the preparedness of residents who go through the fellowship interview process, said Kelvin Wong, MD, a coauthor of research describing a new approach to fellowship interview preparation, which may be generalizable to hospitalists in applying for other positions.
“Applicants receive little to no feedback after their interviews and are thus likely to repeat the same mistakes throughout the process. Verbal feedback from our own fellowship directors indicated that residents as a whole are unprepared to interview,” according to an abstract written by Dr. Wong and his colleagues.
Dr. Wong wanted to investigate the effects of a standardized fellowship interview preparation course on resident preparedness. He and his coauthors developed a formal preparation course for the applicants in the summer of 2017.
Precourse surveys showed that only 17.65% of residents felt prepared to go on interviews; postcourse surveys showed a rise in this number to 82.35%. Immediately after their mock interview, only 27.78% of residents rated their overall interview skills as “very good” or “excellent,” whereas 87.5% of interviewers and 70.59% of observers rated their skills to be “very good” or “excellent.”
A final survey will be given to the applicants and the fellowship program directors once the applicants have completed all of their actual interviews.
“This demonstrates the potential positive impact that mock interviews and standardized interview preparation courses can have, which may be generalizable to hospitalists applying for other positions,” Dr. Wong said. “Specifically for teaching hospitalists in teaching hospitals, the institution of such fellowship interview preparation courses may improve resident preparedness for the fellowship application process.”
Reference
1. Wong K et al. A novel approach to improve fellowship interview skills [abstract]. https://www.shmabstracts.com/abstract/a-novel-approach-to-improve-fellowship-interview-skills/.
Standardized prep courses are helpful
Standardized prep courses are helpful
Are residents generally prepared for fellowship interviews? Applications to the Fellowship Match through the National Residency Matching Program (NRMP) Specialties Matching Service (SMS) are at an all-time high, but there is limited data regarding the preparedness of residents who go through the fellowship interview process, said Kelvin Wong, MD, a coauthor of research describing a new approach to fellowship interview preparation, which may be generalizable to hospitalists in applying for other positions.
“Applicants receive little to no feedback after their interviews and are thus likely to repeat the same mistakes throughout the process. Verbal feedback from our own fellowship directors indicated that residents as a whole are unprepared to interview,” according to an abstract written by Dr. Wong and his colleagues.
Dr. Wong wanted to investigate the effects of a standardized fellowship interview preparation course on resident preparedness. He and his coauthors developed a formal preparation course for the applicants in the summer of 2017.
Precourse surveys showed that only 17.65% of residents felt prepared to go on interviews; postcourse surveys showed a rise in this number to 82.35%. Immediately after their mock interview, only 27.78% of residents rated their overall interview skills as “very good” or “excellent,” whereas 87.5% of interviewers and 70.59% of observers rated their skills to be “very good” or “excellent.”
A final survey will be given to the applicants and the fellowship program directors once the applicants have completed all of their actual interviews.
“This demonstrates the potential positive impact that mock interviews and standardized interview preparation courses can have, which may be generalizable to hospitalists applying for other positions,” Dr. Wong said. “Specifically for teaching hospitalists in teaching hospitals, the institution of such fellowship interview preparation courses may improve resident preparedness for the fellowship application process.”
Reference
1. Wong K et al. A novel approach to improve fellowship interview skills [abstract]. https://www.shmabstracts.com/abstract/a-novel-approach-to-improve-fellowship-interview-skills/.
Are residents generally prepared for fellowship interviews? Applications to the Fellowship Match through the National Residency Matching Program (NRMP) Specialties Matching Service (SMS) are at an all-time high, but there is limited data regarding the preparedness of residents who go through the fellowship interview process, said Kelvin Wong, MD, a coauthor of research describing a new approach to fellowship interview preparation, which may be generalizable to hospitalists in applying for other positions.
“Applicants receive little to no feedback after their interviews and are thus likely to repeat the same mistakes throughout the process. Verbal feedback from our own fellowship directors indicated that residents as a whole are unprepared to interview,” according to an abstract written by Dr. Wong and his colleagues.
Dr. Wong wanted to investigate the effects of a standardized fellowship interview preparation course on resident preparedness. He and his coauthors developed a formal preparation course for the applicants in the summer of 2017.
Precourse surveys showed that only 17.65% of residents felt prepared to go on interviews; postcourse surveys showed a rise in this number to 82.35%. Immediately after their mock interview, only 27.78% of residents rated their overall interview skills as “very good” or “excellent,” whereas 87.5% of interviewers and 70.59% of observers rated their skills to be “very good” or “excellent.”
A final survey will be given to the applicants and the fellowship program directors once the applicants have completed all of their actual interviews.
“This demonstrates the potential positive impact that mock interviews and standardized interview preparation courses can have, which may be generalizable to hospitalists applying for other positions,” Dr. Wong said. “Specifically for teaching hospitalists in teaching hospitals, the institution of such fellowship interview preparation courses may improve resident preparedness for the fellowship application process.”
Reference
1. Wong K et al. A novel approach to improve fellowship interview skills [abstract]. https://www.shmabstracts.com/abstract/a-novel-approach-to-improve-fellowship-interview-skills/.
Quick Byte: Needle-free injections
A start-up operating out of MIT in Cambridge, Mass., called Portal Instruments has developed a needleless injection system.
The device, called PRIME, delivers medication into the bloodstream in a high-pressure stream that travels at Mach 0.7 – the speed of a jet. The makers signed a commercial deal in December 2017, and the device is expected to be available soon.
Reference
1. Kerrigan S. The 16 Most Remarkable Healthcare Innovations, Events, and Discoveries of 2018 For World Health Day. https://interestingengineering.com/the-16-most-remarkable-healthcare-innovations-events-and-discoveries-of-2018-for-world-health-day. April 7, 2018. Accessed June 4, 2018.
A start-up operating out of MIT in Cambridge, Mass., called Portal Instruments has developed a needleless injection system.
The device, called PRIME, delivers medication into the bloodstream in a high-pressure stream that travels at Mach 0.7 – the speed of a jet. The makers signed a commercial deal in December 2017, and the device is expected to be available soon.
Reference
1. Kerrigan S. The 16 Most Remarkable Healthcare Innovations, Events, and Discoveries of 2018 For World Health Day. https://interestingengineering.com/the-16-most-remarkable-healthcare-innovations-events-and-discoveries-of-2018-for-world-health-day. April 7, 2018. Accessed June 4, 2018.
A start-up operating out of MIT in Cambridge, Mass., called Portal Instruments has developed a needleless injection system.
The device, called PRIME, delivers medication into the bloodstream in a high-pressure stream that travels at Mach 0.7 – the speed of a jet. The makers signed a commercial deal in December 2017, and the device is expected to be available soon.
Reference
1. Kerrigan S. The 16 Most Remarkable Healthcare Innovations, Events, and Discoveries of 2018 For World Health Day. https://interestingengineering.com/the-16-most-remarkable-healthcare-innovations-events-and-discoveries-of-2018-for-world-health-day. April 7, 2018. Accessed June 4, 2018.
United States now over 100 measles cases for the year
according to the Centers for Disease Control and Prevention.
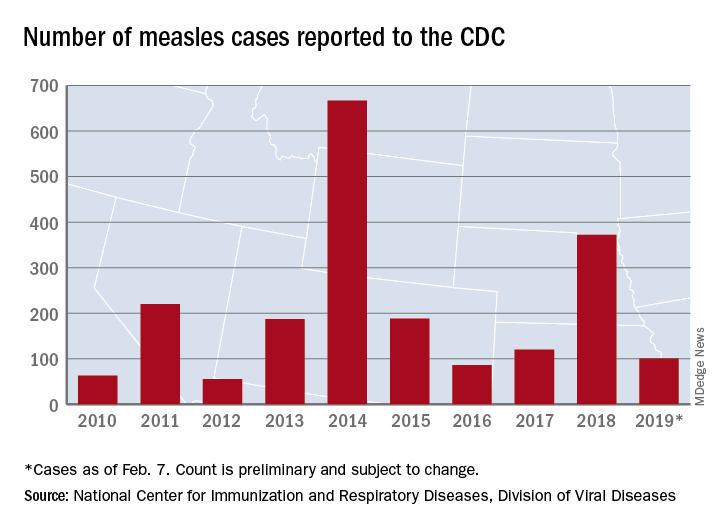
Just over half of the cases in 2019 have occurred in Clark County, Wash., which has reported 53 cases. That outbreak led Gov. Jay Inslee to declare a public health emergency for the entire state on Jan. 25.
The cases in Washington represent one of the five outbreaks – the CDC defines an outbreak as three or more cases – that have occurred so far this year, with three reported in New York State (Rockland County, Monroe County, and New York City) and one in Texas, which has been spread out over five counties, the CDC reported Feb. 11.
“These outbreaks are linked to travelers who brought measles back from other countries such as Israel and Ukraine, where large measles outbreaks are occurring,” the CDC noted. The other states with confirmed cases are California, Colorado, Connecticut, Georgia, Illinois, New Jersey, and Oregon.
In a video released Feb. 1, Surgeon General Jerome Adams stressed the importance of getting vaccinated and noted that an infected person can transmit the measles virus up to 4 days before he or she develops symptoms.
according to the Centers for Disease Control and Prevention.

Just over half of the cases in 2019 have occurred in Clark County, Wash., which has reported 53 cases. That outbreak led Gov. Jay Inslee to declare a public health emergency for the entire state on Jan. 25.
The cases in Washington represent one of the five outbreaks – the CDC defines an outbreak as three or more cases – that have occurred so far this year, with three reported in New York State (Rockland County, Monroe County, and New York City) and one in Texas, which has been spread out over five counties, the CDC reported Feb. 11.
“These outbreaks are linked to travelers who brought measles back from other countries such as Israel and Ukraine, where large measles outbreaks are occurring,” the CDC noted. The other states with confirmed cases are California, Colorado, Connecticut, Georgia, Illinois, New Jersey, and Oregon.
In a video released Feb. 1, Surgeon General Jerome Adams stressed the importance of getting vaccinated and noted that an infected person can transmit the measles virus up to 4 days before he or she develops symptoms.
according to the Centers for Disease Control and Prevention.

Just over half of the cases in 2019 have occurred in Clark County, Wash., which has reported 53 cases. That outbreak led Gov. Jay Inslee to declare a public health emergency for the entire state on Jan. 25.
The cases in Washington represent one of the five outbreaks – the CDC defines an outbreak as three or more cases – that have occurred so far this year, with three reported in New York State (Rockland County, Monroe County, and New York City) and one in Texas, which has been spread out over five counties, the CDC reported Feb. 11.
“These outbreaks are linked to travelers who brought measles back from other countries such as Israel and Ukraine, where large measles outbreaks are occurring,” the CDC noted. The other states with confirmed cases are California, Colorado, Connecticut, Georgia, Illinois, New Jersey, and Oregon.
In a video released Feb. 1, Surgeon General Jerome Adams stressed the importance of getting vaccinated and noted that an infected person can transmit the measles virus up to 4 days before he or she develops symptoms.
Flu activity hits seasonal high
Influenza activity increased for the third consecutive week and has now reached its highest point for the 2018-2019 flu season, according to the Centers for Disease Control and Prevention.
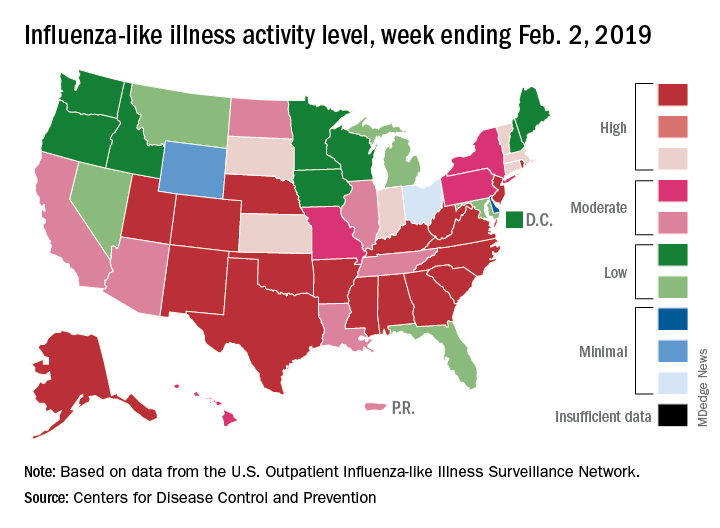
The proportion of outpatient visits for influenza-like illness (ILI) hit 4.3% for the week ending Feb. 2, which topped the previous high of 4.0% that was reached in late December (the national baseline rate is 2.2%). Outpatient ILI visits then dipped down to 3.1% after 2 weeks of decreases before rising again in mid-January, the CDC’s influenza division reported Feb. 8.
Season-high activity also was seen at the state level for the week ending Feb. 2. There were 18 states at level 10 on the CDC’s 1-10 scale of ILI activity, which was up from 16 the week before, and a total of 24 states were in the high range from 8-10, compared with 23 for the previous week. The geographic spread of influenza was reported as widespread in 47 states and Puerto Rico, the CDC said.
Four flu-related pediatric deaths were reported during the week ending Feb. 2, two of which occurred the previous week, which brings the total for the 2018-2019 season to 28, the CDC said.
There were 158 flu-related deaths among all ages during the week ending Jan. 26 – the latest for which such data are available – with reporting almost 75% complete. The previous week saw 177 overall flu deaths, with reporting for that week over 90% complete. During the corresponding weeks of the very severe 2017-2018 flu season, the overall death totals were 1,448 and 1,626, CDC data show.
Influenza activity increased for the third consecutive week and has now reached its highest point for the 2018-2019 flu season, according to the Centers for Disease Control and Prevention.

The proportion of outpatient visits for influenza-like illness (ILI) hit 4.3% for the week ending Feb. 2, which topped the previous high of 4.0% that was reached in late December (the national baseline rate is 2.2%). Outpatient ILI visits then dipped down to 3.1% after 2 weeks of decreases before rising again in mid-January, the CDC’s influenza division reported Feb. 8.
Season-high activity also was seen at the state level for the week ending Feb. 2. There were 18 states at level 10 on the CDC’s 1-10 scale of ILI activity, which was up from 16 the week before, and a total of 24 states were in the high range from 8-10, compared with 23 for the previous week. The geographic spread of influenza was reported as widespread in 47 states and Puerto Rico, the CDC said.
Four flu-related pediatric deaths were reported during the week ending Feb. 2, two of which occurred the previous week, which brings the total for the 2018-2019 season to 28, the CDC said.
There were 158 flu-related deaths among all ages during the week ending Jan. 26 – the latest for which such data are available – with reporting almost 75% complete. The previous week saw 177 overall flu deaths, with reporting for that week over 90% complete. During the corresponding weeks of the very severe 2017-2018 flu season, the overall death totals were 1,448 and 1,626, CDC data show.
Influenza activity increased for the third consecutive week and has now reached its highest point for the 2018-2019 flu season, according to the Centers for Disease Control and Prevention.

The proportion of outpatient visits for influenza-like illness (ILI) hit 4.3% for the week ending Feb. 2, which topped the previous high of 4.0% that was reached in late December (the national baseline rate is 2.2%). Outpatient ILI visits then dipped down to 3.1% after 2 weeks of decreases before rising again in mid-January, the CDC’s influenza division reported Feb. 8.
Season-high activity also was seen at the state level for the week ending Feb. 2. There were 18 states at level 10 on the CDC’s 1-10 scale of ILI activity, which was up from 16 the week before, and a total of 24 states were in the high range from 8-10, compared with 23 for the previous week. The geographic spread of influenza was reported as widespread in 47 states and Puerto Rico, the CDC said.
Four flu-related pediatric deaths were reported during the week ending Feb. 2, two of which occurred the previous week, which brings the total for the 2018-2019 season to 28, the CDC said.
There were 158 flu-related deaths among all ages during the week ending Jan. 26 – the latest for which such data are available – with reporting almost 75% complete. The previous week saw 177 overall flu deaths, with reporting for that week over 90% complete. During the corresponding weeks of the very severe 2017-2018 flu season, the overall death totals were 1,448 and 1,626, CDC data show.
SGLT2 inhibitors morph into HF drugs
SNOWMASS, COLO. – The oral sodium-glucose cotransporter-2 (SGLT2) inhibitors are the focus of a slew of ongoing phase 3 clinical trials in patients with symptomatic heart failure but no diabetes.
“We have a wide array of exciting opportunities to modify cardiovascular risk with agents that were initially developed for the therapy of diabetes. I think we’re increasingly moving to an age where these agents are actually cardiovascular drugs that happen to lower blood glucose, rather than the other way around, which is how they were initially conceived,” Akshay S. Desai, MD, observed at the Annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
These are each multi-thousand-patient trials, variously due to be completed in 2019-2021. Of note, several of them are restricted to nondiabetic patients with heart failure with preserved ejection fraction (HFpEF), a common, serious, understudied, extremely high-cost disease sorely in need of effective pharmacotherapies, added Dr. Desai, director of the cardiomyopathy and heart failure program at Brigham and Women’s Hospital and a cardiologist at Harvard Medical School, Boston.
All of these placebo-controlled trials have as their composite primary endpoint cardiovascular death and heart failure hospitalization.
EMPEROR-Preserved has randomized 4,126 patients with HFpEF to empagliflozin (Jardiance) or placebo, while EMPEROR-Reduced involves 2,850 patients with heart failure with reduced ejection fraction (HFrEF). Both are due to be completed in 2020.
In addition, the DELIVER trial is focused on 4,700 HFpEF patients randomized to dapagliflozin (Farxiga) or placebo, while Dapa-HF employs the SGLT2 inhibitor in a study of 4,500 patients with HFrEF. Dapa-HF will be completed by late 2019. DELIVER wraps up in mid-2021.
Again, remarkably, none of the participants in these trials has diabetes. All have symptomatic heart failure with elevated N-terminal pro b-type natriuretic peptide levels. The impetus for this ongoing round of studies was the impressive reduction in the risk of hospitalization for heart failure seen in the pivotal trials that earned the SGLT2 inhibitors empagliflozin, canagliflozin (Invokana), and dapagliflozin marketing approval for treatment of type 2 diabetes from the Food and Drug Administration.
Dr. Desai called attention to a new systematic review and meta-analysis of cardiovascular outcomes in randomized, placebo-controlled trials of SGLT2 inhibitors in more than 34,000 patients with type 2 diabetes. The conclusion: These drugs impressively reduced the risk of heart failure hospitalization by 32% in patients with a baseline history of heart failure and similarly by 29% in those with no such history. Also notable was the 45% reduction in the risk of progression of renal disease regardless of whether patients had atherosclerotic cardiovascular disease (Lancet. 2019 Jan 5;393[10166]:31-9).
Only one of the ongoing round of phase 3 trials of SGLT2 inhibitors in heart failure is being conducted in patients with comorbid type 2 diabetes: the 4,000-subject SOLOIST-WHF trial. This study features the investigational dual inhibitor of SGLT1 and 2, sotagliflozin, with a primary outcome of cardiovascular death or heart failure hospitalization. Results are expected in early 2021.
What the latest guidelines say
The 2018 American Diabetes Association/European Association for the Study of Diabetes joint consensus statement on management of hyperglycemia in type 2 diabetes reflects an appreciation of the cardiovascular benefits of the SGLT2 inhibitors as well as the injectable glucagon-like peptide-1 receptor (GLP-1) agonists, which have shown significant reductions in major adverse cardiovascular events in pivotal trials including LEADER, HARMONY, and REWIND, albeit without the impressive reduction in heart failure hospitalizations documented with the SGLT2 inhibitors.
The consensus statement emphasizes that aggressive lifestyle modification advice is step No. 1, with the first-line medication being metformin titrated to a target of 1,000 mg twice daily. For patients with clinical heart failure or chronic kidney disease and atherosclerotic cardiovascular heart disease, the next drug recommended is an SGLT2 inhibitor with proven cardiovascular benefit. A GLP-1 agonist is recommended as the first injectable medication, ahead of insulin.
Who will take the lead in this new treatment strategy?
Dr. Desai presented data showing that overall utilization of SGLT2 inhibitors and GLP-1 agonists is going up, but not as steeply as it should.
“Cardiologists need to take a more active role,” he declared.
“It’s increasingly clear that, if we’re interested in modifying cardiovascular outcomes, we need to take ownership of this problem, much as we’ve done for lipids and hypertension, because modulating cardiovascular risk is our job,” Dr. Desai asserted. “These drugs may have modest influence on glycemic control, but the primary goal with these agents is to influence cardiovascular outcomes – and if we leave that job to our colleagues, then it often is just a can that gets kicked down the road.”
As a practical matter in prescribing SGLT2 inhibitors and GLP-1 agonists, he emphasized the value of partnering with a primary care physician, endocrinologist, and/or pharmacist by creating pathways for accelerated referral for pharmacologic teaching and, in the case of GLP-1 agonists, injection-related instruction. Pharmacists are often particularly helpful in obtaining prior authorization and financial approval for these medications, and they are familiar with drug discounts and vouchers.
“A great way to jump start collaboration is to provide the patient with a prescription before leaving your office. I think often what we do is just suggest it to the patient, and then a year later they come back and nothing has changed,” the cardiologist said.
Dr. Desai reported serving as a paid consultant to more than half a dozen pharmaceutical or medical device companies.
SNOWMASS, COLO. – The oral sodium-glucose cotransporter-2 (SGLT2) inhibitors are the focus of a slew of ongoing phase 3 clinical trials in patients with symptomatic heart failure but no diabetes.
“We have a wide array of exciting opportunities to modify cardiovascular risk with agents that were initially developed for the therapy of diabetes. I think we’re increasingly moving to an age where these agents are actually cardiovascular drugs that happen to lower blood glucose, rather than the other way around, which is how they were initially conceived,” Akshay S. Desai, MD, observed at the Annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
These are each multi-thousand-patient trials, variously due to be completed in 2019-2021. Of note, several of them are restricted to nondiabetic patients with heart failure with preserved ejection fraction (HFpEF), a common, serious, understudied, extremely high-cost disease sorely in need of effective pharmacotherapies, added Dr. Desai, director of the cardiomyopathy and heart failure program at Brigham and Women’s Hospital and a cardiologist at Harvard Medical School, Boston.
All of these placebo-controlled trials have as their composite primary endpoint cardiovascular death and heart failure hospitalization.
EMPEROR-Preserved has randomized 4,126 patients with HFpEF to empagliflozin (Jardiance) or placebo, while EMPEROR-Reduced involves 2,850 patients with heart failure with reduced ejection fraction (HFrEF). Both are due to be completed in 2020.
In addition, the DELIVER trial is focused on 4,700 HFpEF patients randomized to dapagliflozin (Farxiga) or placebo, while Dapa-HF employs the SGLT2 inhibitor in a study of 4,500 patients with HFrEF. Dapa-HF will be completed by late 2019. DELIVER wraps up in mid-2021.
Again, remarkably, none of the participants in these trials has diabetes. All have symptomatic heart failure with elevated N-terminal pro b-type natriuretic peptide levels. The impetus for this ongoing round of studies was the impressive reduction in the risk of hospitalization for heart failure seen in the pivotal trials that earned the SGLT2 inhibitors empagliflozin, canagliflozin (Invokana), and dapagliflozin marketing approval for treatment of type 2 diabetes from the Food and Drug Administration.
Dr. Desai called attention to a new systematic review and meta-analysis of cardiovascular outcomes in randomized, placebo-controlled trials of SGLT2 inhibitors in more than 34,000 patients with type 2 diabetes. The conclusion: These drugs impressively reduced the risk of heart failure hospitalization by 32% in patients with a baseline history of heart failure and similarly by 29% in those with no such history. Also notable was the 45% reduction in the risk of progression of renal disease regardless of whether patients had atherosclerotic cardiovascular disease (Lancet. 2019 Jan 5;393[10166]:31-9).
Only one of the ongoing round of phase 3 trials of SGLT2 inhibitors in heart failure is being conducted in patients with comorbid type 2 diabetes: the 4,000-subject SOLOIST-WHF trial. This study features the investigational dual inhibitor of SGLT1 and 2, sotagliflozin, with a primary outcome of cardiovascular death or heart failure hospitalization. Results are expected in early 2021.
What the latest guidelines say
The 2018 American Diabetes Association/European Association for the Study of Diabetes joint consensus statement on management of hyperglycemia in type 2 diabetes reflects an appreciation of the cardiovascular benefits of the SGLT2 inhibitors as well as the injectable glucagon-like peptide-1 receptor (GLP-1) agonists, which have shown significant reductions in major adverse cardiovascular events in pivotal trials including LEADER, HARMONY, and REWIND, albeit without the impressive reduction in heart failure hospitalizations documented with the SGLT2 inhibitors.
The consensus statement emphasizes that aggressive lifestyle modification advice is step No. 1, with the first-line medication being metformin titrated to a target of 1,000 mg twice daily. For patients with clinical heart failure or chronic kidney disease and atherosclerotic cardiovascular heart disease, the next drug recommended is an SGLT2 inhibitor with proven cardiovascular benefit. A GLP-1 agonist is recommended as the first injectable medication, ahead of insulin.
Who will take the lead in this new treatment strategy?
Dr. Desai presented data showing that overall utilization of SGLT2 inhibitors and GLP-1 agonists is going up, but not as steeply as it should.
“Cardiologists need to take a more active role,” he declared.
“It’s increasingly clear that, if we’re interested in modifying cardiovascular outcomes, we need to take ownership of this problem, much as we’ve done for lipids and hypertension, because modulating cardiovascular risk is our job,” Dr. Desai asserted. “These drugs may have modest influence on glycemic control, but the primary goal with these agents is to influence cardiovascular outcomes – and if we leave that job to our colleagues, then it often is just a can that gets kicked down the road.”
As a practical matter in prescribing SGLT2 inhibitors and GLP-1 agonists, he emphasized the value of partnering with a primary care physician, endocrinologist, and/or pharmacist by creating pathways for accelerated referral for pharmacologic teaching and, in the case of GLP-1 agonists, injection-related instruction. Pharmacists are often particularly helpful in obtaining prior authorization and financial approval for these medications, and they are familiar with drug discounts and vouchers.
“A great way to jump start collaboration is to provide the patient with a prescription before leaving your office. I think often what we do is just suggest it to the patient, and then a year later they come back and nothing has changed,” the cardiologist said.
Dr. Desai reported serving as a paid consultant to more than half a dozen pharmaceutical or medical device companies.
SNOWMASS, COLO. – The oral sodium-glucose cotransporter-2 (SGLT2) inhibitors are the focus of a slew of ongoing phase 3 clinical trials in patients with symptomatic heart failure but no diabetes.
“We have a wide array of exciting opportunities to modify cardiovascular risk with agents that were initially developed for the therapy of diabetes. I think we’re increasingly moving to an age where these agents are actually cardiovascular drugs that happen to lower blood glucose, rather than the other way around, which is how they were initially conceived,” Akshay S. Desai, MD, observed at the Annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
These are each multi-thousand-patient trials, variously due to be completed in 2019-2021. Of note, several of them are restricted to nondiabetic patients with heart failure with preserved ejection fraction (HFpEF), a common, serious, understudied, extremely high-cost disease sorely in need of effective pharmacotherapies, added Dr. Desai, director of the cardiomyopathy and heart failure program at Brigham and Women’s Hospital and a cardiologist at Harvard Medical School, Boston.
All of these placebo-controlled trials have as their composite primary endpoint cardiovascular death and heart failure hospitalization.
EMPEROR-Preserved has randomized 4,126 patients with HFpEF to empagliflozin (Jardiance) or placebo, while EMPEROR-Reduced involves 2,850 patients with heart failure with reduced ejection fraction (HFrEF). Both are due to be completed in 2020.
In addition, the DELIVER trial is focused on 4,700 HFpEF patients randomized to dapagliflozin (Farxiga) or placebo, while Dapa-HF employs the SGLT2 inhibitor in a study of 4,500 patients with HFrEF. Dapa-HF will be completed by late 2019. DELIVER wraps up in mid-2021.
Again, remarkably, none of the participants in these trials has diabetes. All have symptomatic heart failure with elevated N-terminal pro b-type natriuretic peptide levels. The impetus for this ongoing round of studies was the impressive reduction in the risk of hospitalization for heart failure seen in the pivotal trials that earned the SGLT2 inhibitors empagliflozin, canagliflozin (Invokana), and dapagliflozin marketing approval for treatment of type 2 diabetes from the Food and Drug Administration.
Dr. Desai called attention to a new systematic review and meta-analysis of cardiovascular outcomes in randomized, placebo-controlled trials of SGLT2 inhibitors in more than 34,000 patients with type 2 diabetes. The conclusion: These drugs impressively reduced the risk of heart failure hospitalization by 32% in patients with a baseline history of heart failure and similarly by 29% in those with no such history. Also notable was the 45% reduction in the risk of progression of renal disease regardless of whether patients had atherosclerotic cardiovascular disease (Lancet. 2019 Jan 5;393[10166]:31-9).
Only one of the ongoing round of phase 3 trials of SGLT2 inhibitors in heart failure is being conducted in patients with comorbid type 2 diabetes: the 4,000-subject SOLOIST-WHF trial. This study features the investigational dual inhibitor of SGLT1 and 2, sotagliflozin, with a primary outcome of cardiovascular death or heart failure hospitalization. Results are expected in early 2021.
What the latest guidelines say
The 2018 American Diabetes Association/European Association for the Study of Diabetes joint consensus statement on management of hyperglycemia in type 2 diabetes reflects an appreciation of the cardiovascular benefits of the SGLT2 inhibitors as well as the injectable glucagon-like peptide-1 receptor (GLP-1) agonists, which have shown significant reductions in major adverse cardiovascular events in pivotal trials including LEADER, HARMONY, and REWIND, albeit without the impressive reduction in heart failure hospitalizations documented with the SGLT2 inhibitors.
The consensus statement emphasizes that aggressive lifestyle modification advice is step No. 1, with the first-line medication being metformin titrated to a target of 1,000 mg twice daily. For patients with clinical heart failure or chronic kidney disease and atherosclerotic cardiovascular heart disease, the next drug recommended is an SGLT2 inhibitor with proven cardiovascular benefit. A GLP-1 agonist is recommended as the first injectable medication, ahead of insulin.
Who will take the lead in this new treatment strategy?
Dr. Desai presented data showing that overall utilization of SGLT2 inhibitors and GLP-1 agonists is going up, but not as steeply as it should.
“Cardiologists need to take a more active role,” he declared.
“It’s increasingly clear that, if we’re interested in modifying cardiovascular outcomes, we need to take ownership of this problem, much as we’ve done for lipids and hypertension, because modulating cardiovascular risk is our job,” Dr. Desai asserted. “These drugs may have modest influence on glycemic control, but the primary goal with these agents is to influence cardiovascular outcomes – and if we leave that job to our colleagues, then it often is just a can that gets kicked down the road.”
As a practical matter in prescribing SGLT2 inhibitors and GLP-1 agonists, he emphasized the value of partnering with a primary care physician, endocrinologist, and/or pharmacist by creating pathways for accelerated referral for pharmacologic teaching and, in the case of GLP-1 agonists, injection-related instruction. Pharmacists are often particularly helpful in obtaining prior authorization and financial approval for these medications, and they are familiar with drug discounts and vouchers.
“A great way to jump start collaboration is to provide the patient with a prescription before leaving your office. I think often what we do is just suggest it to the patient, and then a year later they come back and nothing has changed,” the cardiologist said.
Dr. Desai reported serving as a paid consultant to more than half a dozen pharmaceutical or medical device companies.
EXPERT ANALYSIS FROM ACC SNOWMASS 2019
Cilostazol plus aspirin or clopidogrel reduces the risk of recurrent stroke
HONOLULU – The combination also entails a similar risk of major bleeding, compared with aspirin and clopidogrel alone, according to results from the Cilostazol Stroke Prevention Study for Antiplatelet Combination (CSPS.com).
Dual-antiplatelet therapy with aspirin and clopidogrel reduced the rate of recurrent stroke in previous studies. The benefit of this drug combination is relatively short-lived, however, and long-term concomitant use of aspirin and clopidogrel entails a risk of major bleeding. Other data have indicated that cilostazol, which is approved by the Food and Drug Administration to alleviate intermittent claudication in patients with peripheral vascular disease, prevents stroke recurrence without increasing the incidence of serious bleeding, compared with aspirin, said Kazunori Toyoda, MD, PhD, who presented the results of the CSPS.com trial at the International Stroke Conference sponsored by the American Heart Association.
Dr. Toyoda of the National Cerebral and Cardiovascular Center in Osaka, Japan, and his colleagues randomized 1,879 high-risk patients at 8-180 days after the onset of noncardioembolic ischemic stroke identified on MRI to receive 81 or 100 mg aspirin or 50 or 75 mg clopidogrel alone, or a combination of cilostazol 100 mg twice daily with aspirin or clopidogrel. They conducted their open-label, parallel-group trial at 292 sites in Japan from December 2013 through March 2017.
To be considered at high risk, participants had to meet one or more of the following criteria: 50% or greater stenosis of a major intracranial artery, 50% or greater stenosis of an extracranial artery, and two or more vascular risk factors. The trial’s primary efficacy outcome was the first recurrence of ischemic stroke. Safety outcomes included severe or life-threatening bleeding.
The investigators ended the trial early because of a delay in recruiting patients. They enrolled 1,884 and randomized 1,879 of an anticipated 4,000 patients. At randomization, 41% in the dual-therapy group received aspirin and 59% clopidogrel, and in the monotherapy group, 40% received aspirin and 60% clopidogrel. Baseline characteristics were similar between the treatment groups. The population’s mean age was 70. Approximately 30% of patients were women.
During a median follow-up period of 17 months, ischemic stroke recurred in 29 of 932 patients receiving dual therapy including cilostazol for an annual rate of 2.2% and in 64 of 947 patients receiving monotherapy for an annual rate of 4.5% (hazard ratio, 0.49; 95% confidence interval, 0.31-0.76; P = .001). Severe or life-threatening bleeding occurred in 8 patients (0.6% per year) receiving dual therapy and 13 patients (0.9% per year) receiving monotherapy (HR, 0.66; 95% CI, 0.27-1.60; P = .354).
The study was funded by Otsuka Pharmaceutical, which manufactures cilostazol. Dr. Toyoda reported receiving support from Bayer Yakuhin, Daiichi Sankyo, Bristol-Myers Squibb, and Nippon Boehringer Ingelheim.
SOURCE: Toyoda K et al. ISC 2019, Abstract LB3.
HONOLULU – The combination also entails a similar risk of major bleeding, compared with aspirin and clopidogrel alone, according to results from the Cilostazol Stroke Prevention Study for Antiplatelet Combination (CSPS.com).
Dual-antiplatelet therapy with aspirin and clopidogrel reduced the rate of recurrent stroke in previous studies. The benefit of this drug combination is relatively short-lived, however, and long-term concomitant use of aspirin and clopidogrel entails a risk of major bleeding. Other data have indicated that cilostazol, which is approved by the Food and Drug Administration to alleviate intermittent claudication in patients with peripheral vascular disease, prevents stroke recurrence without increasing the incidence of serious bleeding, compared with aspirin, said Kazunori Toyoda, MD, PhD, who presented the results of the CSPS.com trial at the International Stroke Conference sponsored by the American Heart Association.
Dr. Toyoda of the National Cerebral and Cardiovascular Center in Osaka, Japan, and his colleagues randomized 1,879 high-risk patients at 8-180 days after the onset of noncardioembolic ischemic stroke identified on MRI to receive 81 or 100 mg aspirin or 50 or 75 mg clopidogrel alone, or a combination of cilostazol 100 mg twice daily with aspirin or clopidogrel. They conducted their open-label, parallel-group trial at 292 sites in Japan from December 2013 through March 2017.
To be considered at high risk, participants had to meet one or more of the following criteria: 50% or greater stenosis of a major intracranial artery, 50% or greater stenosis of an extracranial artery, and two or more vascular risk factors. The trial’s primary efficacy outcome was the first recurrence of ischemic stroke. Safety outcomes included severe or life-threatening bleeding.
The investigators ended the trial early because of a delay in recruiting patients. They enrolled 1,884 and randomized 1,879 of an anticipated 4,000 patients. At randomization, 41% in the dual-therapy group received aspirin and 59% clopidogrel, and in the monotherapy group, 40% received aspirin and 60% clopidogrel. Baseline characteristics were similar between the treatment groups. The population’s mean age was 70. Approximately 30% of patients were women.
During a median follow-up period of 17 months, ischemic stroke recurred in 29 of 932 patients receiving dual therapy including cilostazol for an annual rate of 2.2% and in 64 of 947 patients receiving monotherapy for an annual rate of 4.5% (hazard ratio, 0.49; 95% confidence interval, 0.31-0.76; P = .001). Severe or life-threatening bleeding occurred in 8 patients (0.6% per year) receiving dual therapy and 13 patients (0.9% per year) receiving monotherapy (HR, 0.66; 95% CI, 0.27-1.60; P = .354).
The study was funded by Otsuka Pharmaceutical, which manufactures cilostazol. Dr. Toyoda reported receiving support from Bayer Yakuhin, Daiichi Sankyo, Bristol-Myers Squibb, and Nippon Boehringer Ingelheim.
SOURCE: Toyoda K et al. ISC 2019, Abstract LB3.
HONOLULU – The combination also entails a similar risk of major bleeding, compared with aspirin and clopidogrel alone, according to results from the Cilostazol Stroke Prevention Study for Antiplatelet Combination (CSPS.com).
Dual-antiplatelet therapy with aspirin and clopidogrel reduced the rate of recurrent stroke in previous studies. The benefit of this drug combination is relatively short-lived, however, and long-term concomitant use of aspirin and clopidogrel entails a risk of major bleeding. Other data have indicated that cilostazol, which is approved by the Food and Drug Administration to alleviate intermittent claudication in patients with peripheral vascular disease, prevents stroke recurrence without increasing the incidence of serious bleeding, compared with aspirin, said Kazunori Toyoda, MD, PhD, who presented the results of the CSPS.com trial at the International Stroke Conference sponsored by the American Heart Association.
Dr. Toyoda of the National Cerebral and Cardiovascular Center in Osaka, Japan, and his colleagues randomized 1,879 high-risk patients at 8-180 days after the onset of noncardioembolic ischemic stroke identified on MRI to receive 81 or 100 mg aspirin or 50 or 75 mg clopidogrel alone, or a combination of cilostazol 100 mg twice daily with aspirin or clopidogrel. They conducted their open-label, parallel-group trial at 292 sites in Japan from December 2013 through March 2017.
To be considered at high risk, participants had to meet one or more of the following criteria: 50% or greater stenosis of a major intracranial artery, 50% or greater stenosis of an extracranial artery, and two or more vascular risk factors. The trial’s primary efficacy outcome was the first recurrence of ischemic stroke. Safety outcomes included severe or life-threatening bleeding.
The investigators ended the trial early because of a delay in recruiting patients. They enrolled 1,884 and randomized 1,879 of an anticipated 4,000 patients. At randomization, 41% in the dual-therapy group received aspirin and 59% clopidogrel, and in the monotherapy group, 40% received aspirin and 60% clopidogrel. Baseline characteristics were similar between the treatment groups. The population’s mean age was 70. Approximately 30% of patients were women.
During a median follow-up period of 17 months, ischemic stroke recurred in 29 of 932 patients receiving dual therapy including cilostazol for an annual rate of 2.2% and in 64 of 947 patients receiving monotherapy for an annual rate of 4.5% (hazard ratio, 0.49; 95% confidence interval, 0.31-0.76; P = .001). Severe or life-threatening bleeding occurred in 8 patients (0.6% per year) receiving dual therapy and 13 patients (0.9% per year) receiving monotherapy (HR, 0.66; 95% CI, 0.27-1.60; P = .354).
The study was funded by Otsuka Pharmaceutical, which manufactures cilostazol. Dr. Toyoda reported receiving support from Bayer Yakuhin, Daiichi Sankyo, Bristol-Myers Squibb, and Nippon Boehringer Ingelheim.
SOURCE: Toyoda K et al. ISC 2019, Abstract LB3.
REPORTING FROM ISC
Key clinical point: Treating patients at high risk of recurrent stroke with cilostazol and aspirin or clopidogrel reduced the risk of recurrent stroke more than either aspirin or clopidogrel alone and was just as safe.
Major finding: Dual therapy with cilostazol and aspirin or clopidogrel reduced the risk of recurrent stroke by approximately half, compared with aspirin or clopidogrel alone.
Study details: A multicenter, randomized, open-label, parallel-group trial including 1,879 patients at high risk of recurrent stroke.
Disclosures: Otsuka Pharmaceutical funded the study. The presenter reported receiving support from Bayer Yakuhin, Daiichi Sankyo, Bristol-Myers Squibb, and Nippon Boehringer Ingelheim.
Source: Toyoda K et al. ISC 2019, Abstract LB3.
Intensive insulin added no benefit for hyperglycemia after ischemic stroke
HONOLULU – In patients who were hyperglycemic following an acute ischemic stroke, intensive insulin control using a continuous insulin drip and an aggressive blood glucose target of 80-130 mg/dL provided no incremental benefit in clinical outcome, compared with a more standard approach of serial, subcutaneous insulin injections and a moderate blood glucose target in a multicenter, U.S. trial with more than 1,100 patients.
The results also highlighted the potential downside to aggressive insulin treatment, with an associated 2.6% incidence of severe hypoglycemia, defined as blood glucose falling below 40 mg/dL, Karen C. Johnston, MD, said at the International Stroke Conference sponsored by the American Heart Association.
“Our data suggest that subcutaneously administered insulin with a target blood glucose level of less than 180 mg/dL is the preferred treatment” because it produces similar efficacy without causing any episodes of severe hypoglycemia, concluded Dr. Johnston, professor and chair of neurology at the University of Virginia in Charlottesville. “There should be no further debate” over the potential superiority of a glucose target substantially below 180 mg/dL, she added in an interview.
Continuing to use a glucose target of less than 180 mg/dL and treating patients with subcutaneous insulin injections every 6 hours to achieve this will mean substantially less resource use and precludes the need for keeping patients in intensive care beds as is needed with an insulin drip, Dr. Johnston noted. A treatment target of less than 180 mg/dL is also consistent with the most recent American Heart Association stroke treatment guidelines, which listed a blood glucose target of 140-180 mg/dL as a class IIa recommendation (Stroke. 2018 March;49[3]:e66-99).
The SHINE (Stroke Hyperglycemia Insulin Network Effort) trial enrolled 1,151 adults diagnosed with an acute ischemic stroke at 63 U.S. centers during 2012-2018, excluding patients with type 1 diabetes. Patients had to enter the study within 12 hours of their last known well time, and with an elevated blood glucose level, above 110 mg/dL in patients with type 2 diabetes or at or above 150 mg/dL in other patients. The median glucose level of enrolled patients was about 188 mg/dL. Enrolled patients averaged 66 years old, and about 80% had type 2 diabetes. The median time from last known well to randomization was just over 7 hours. Almost two-thirds of the patients received thrombolytic treatment, and about 13% underwent thrombectomy.
During up to 72 hours of treatment following enrollment the patients in the standard-treatment arm showed a fairly steady average blood glucose level of 179 mg/dL; patients in the intensive arm showed a steady average of 118 mg/dL.
The study’s primary end point was the percentage of patients with a favorable outcome 90 days after enrollment based on their modified Rankin scale score at that time, with the scores that qualified for this end point varying depending on stroke severity at baseline. The percentage of patients achieving this was 20.5% among the intensive patients and 21.6% among those who received standard insulin treatment, a difference that was not statistically significant.
The findings left open the question of how to better manage acute ischemic stroke patients who present with hyperglycemia.
“Hyperglycemic stroke patients have worse outcomes than stroke patients without hyperglycemia. More aggressively treating the hyperglycemia did not help these patients, We need to figure out what will help them,” Dr. Johnson said.
SOURCE: Johnston KC et al. ISC 2019, Abstract LB1.
SHINE was a well-designed trial that was run with a high degree of rigor, and its results advance the field. The results left no doubt that the result was neutral, that , while resulting in an excess of severe hypoglycemia episodes.
Using a less intensive insulin regimen that does not require a continuous drip is easier. The question of how aggressive treatment needs to be when managing glucose in acute ischemic stroke patients is something that U.S. clinicians who care for stroke patients argue about virtually daily. At my center, Cedars-Sinai in Los Angeles, we have recently used an approach that blended standard insulin treatment with more aggressive treatment. The SHINE results may not be practice changing, but they will be argument changing. The new results will make a difference. We will now stop arguing. We now know what we need to do.
Patrick D. Lyden, MD , is professor and chair of neurology at Cedars-Sinai Medical Center in Los Angeles. He had no relevant disclosures. He made these comments in an interview.
SHINE was a well-designed trial that was run with a high degree of rigor, and its results advance the field. The results left no doubt that the result was neutral, that , while resulting in an excess of severe hypoglycemia episodes.
Using a less intensive insulin regimen that does not require a continuous drip is easier. The question of how aggressive treatment needs to be when managing glucose in acute ischemic stroke patients is something that U.S. clinicians who care for stroke patients argue about virtually daily. At my center, Cedars-Sinai in Los Angeles, we have recently used an approach that blended standard insulin treatment with more aggressive treatment. The SHINE results may not be practice changing, but they will be argument changing. The new results will make a difference. We will now stop arguing. We now know what we need to do.
Patrick D. Lyden, MD , is professor and chair of neurology at Cedars-Sinai Medical Center in Los Angeles. He had no relevant disclosures. He made these comments in an interview.
SHINE was a well-designed trial that was run with a high degree of rigor, and its results advance the field. The results left no doubt that the result was neutral, that , while resulting in an excess of severe hypoglycemia episodes.
Using a less intensive insulin regimen that does not require a continuous drip is easier. The question of how aggressive treatment needs to be when managing glucose in acute ischemic stroke patients is something that U.S. clinicians who care for stroke patients argue about virtually daily. At my center, Cedars-Sinai in Los Angeles, we have recently used an approach that blended standard insulin treatment with more aggressive treatment. The SHINE results may not be practice changing, but they will be argument changing. The new results will make a difference. We will now stop arguing. We now know what we need to do.
Patrick D. Lyden, MD , is professor and chair of neurology at Cedars-Sinai Medical Center in Los Angeles. He had no relevant disclosures. He made these comments in an interview.
HONOLULU – In patients who were hyperglycemic following an acute ischemic stroke, intensive insulin control using a continuous insulin drip and an aggressive blood glucose target of 80-130 mg/dL provided no incremental benefit in clinical outcome, compared with a more standard approach of serial, subcutaneous insulin injections and a moderate blood glucose target in a multicenter, U.S. trial with more than 1,100 patients.
The results also highlighted the potential downside to aggressive insulin treatment, with an associated 2.6% incidence of severe hypoglycemia, defined as blood glucose falling below 40 mg/dL, Karen C. Johnston, MD, said at the International Stroke Conference sponsored by the American Heart Association.
“Our data suggest that subcutaneously administered insulin with a target blood glucose level of less than 180 mg/dL is the preferred treatment” because it produces similar efficacy without causing any episodes of severe hypoglycemia, concluded Dr. Johnston, professor and chair of neurology at the University of Virginia in Charlottesville. “There should be no further debate” over the potential superiority of a glucose target substantially below 180 mg/dL, she added in an interview.
Continuing to use a glucose target of less than 180 mg/dL and treating patients with subcutaneous insulin injections every 6 hours to achieve this will mean substantially less resource use and precludes the need for keeping patients in intensive care beds as is needed with an insulin drip, Dr. Johnston noted. A treatment target of less than 180 mg/dL is also consistent with the most recent American Heart Association stroke treatment guidelines, which listed a blood glucose target of 140-180 mg/dL as a class IIa recommendation (Stroke. 2018 March;49[3]:e66-99).
The SHINE (Stroke Hyperglycemia Insulin Network Effort) trial enrolled 1,151 adults diagnosed with an acute ischemic stroke at 63 U.S. centers during 2012-2018, excluding patients with type 1 diabetes. Patients had to enter the study within 12 hours of their last known well time, and with an elevated blood glucose level, above 110 mg/dL in patients with type 2 diabetes or at or above 150 mg/dL in other patients. The median glucose level of enrolled patients was about 188 mg/dL. Enrolled patients averaged 66 years old, and about 80% had type 2 diabetes. The median time from last known well to randomization was just over 7 hours. Almost two-thirds of the patients received thrombolytic treatment, and about 13% underwent thrombectomy.
During up to 72 hours of treatment following enrollment the patients in the standard-treatment arm showed a fairly steady average blood glucose level of 179 mg/dL; patients in the intensive arm showed a steady average of 118 mg/dL.
The study’s primary end point was the percentage of patients with a favorable outcome 90 days after enrollment based on their modified Rankin scale score at that time, with the scores that qualified for this end point varying depending on stroke severity at baseline. The percentage of patients achieving this was 20.5% among the intensive patients and 21.6% among those who received standard insulin treatment, a difference that was not statistically significant.
The findings left open the question of how to better manage acute ischemic stroke patients who present with hyperglycemia.
“Hyperglycemic stroke patients have worse outcomes than stroke patients without hyperglycemia. More aggressively treating the hyperglycemia did not help these patients, We need to figure out what will help them,” Dr. Johnson said.
SOURCE: Johnston KC et al. ISC 2019, Abstract LB1.
HONOLULU – In patients who were hyperglycemic following an acute ischemic stroke, intensive insulin control using a continuous insulin drip and an aggressive blood glucose target of 80-130 mg/dL provided no incremental benefit in clinical outcome, compared with a more standard approach of serial, subcutaneous insulin injections and a moderate blood glucose target in a multicenter, U.S. trial with more than 1,100 patients.
The results also highlighted the potential downside to aggressive insulin treatment, with an associated 2.6% incidence of severe hypoglycemia, defined as blood glucose falling below 40 mg/dL, Karen C. Johnston, MD, said at the International Stroke Conference sponsored by the American Heart Association.
“Our data suggest that subcutaneously administered insulin with a target blood glucose level of less than 180 mg/dL is the preferred treatment” because it produces similar efficacy without causing any episodes of severe hypoglycemia, concluded Dr. Johnston, professor and chair of neurology at the University of Virginia in Charlottesville. “There should be no further debate” over the potential superiority of a glucose target substantially below 180 mg/dL, she added in an interview.
Continuing to use a glucose target of less than 180 mg/dL and treating patients with subcutaneous insulin injections every 6 hours to achieve this will mean substantially less resource use and precludes the need for keeping patients in intensive care beds as is needed with an insulin drip, Dr. Johnston noted. A treatment target of less than 180 mg/dL is also consistent with the most recent American Heart Association stroke treatment guidelines, which listed a blood glucose target of 140-180 mg/dL as a class IIa recommendation (Stroke. 2018 March;49[3]:e66-99).
The SHINE (Stroke Hyperglycemia Insulin Network Effort) trial enrolled 1,151 adults diagnosed with an acute ischemic stroke at 63 U.S. centers during 2012-2018, excluding patients with type 1 diabetes. Patients had to enter the study within 12 hours of their last known well time, and with an elevated blood glucose level, above 110 mg/dL in patients with type 2 diabetes or at or above 150 mg/dL in other patients. The median glucose level of enrolled patients was about 188 mg/dL. Enrolled patients averaged 66 years old, and about 80% had type 2 diabetes. The median time from last known well to randomization was just over 7 hours. Almost two-thirds of the patients received thrombolytic treatment, and about 13% underwent thrombectomy.
During up to 72 hours of treatment following enrollment the patients in the standard-treatment arm showed a fairly steady average blood glucose level of 179 mg/dL; patients in the intensive arm showed a steady average of 118 mg/dL.
The study’s primary end point was the percentage of patients with a favorable outcome 90 days after enrollment based on their modified Rankin scale score at that time, with the scores that qualified for this end point varying depending on stroke severity at baseline. The percentage of patients achieving this was 20.5% among the intensive patients and 21.6% among those who received standard insulin treatment, a difference that was not statistically significant.
The findings left open the question of how to better manage acute ischemic stroke patients who present with hyperglycemia.
“Hyperglycemic stroke patients have worse outcomes than stroke patients without hyperglycemia. More aggressively treating the hyperglycemia did not help these patients, We need to figure out what will help them,” Dr. Johnson said.
SOURCE: Johnston KC et al. ISC 2019, Abstract LB1.
REPORTING FROM ISC 2019
Key clinical point: Aggressive insulin management of hyperglycemia following an ischemic stroke gave no clinical benefit, compared with a standard approach.
Major finding: After 90 days, favorable outcomes occurred in 21% of patients on aggressive insulin treatment and 22% on standard treatment.
Study details: SHINE, a multicenter, randomized trial with 1,151 acute ischemic stroke patients.
Disclosures: SHINE received no commercial funding. Dr. Johnston had no disclosures.
Source: Johnston KC et al. ISC 2019, Abstract LB1.
Benefiting from an egalitarian hospital culture
Cultural change linked to improved outcomes
Health care experts have long known of a link between patient outcomes and a hospital’s organizational culture, according to an article in the New York Times by Pauline W. Chen, MD.
“Heart attack patients who are treated at hospitals where nurses feel powerless and senior management is only sporadically involved in patient care tend to fare more poorly than patients hospitalized at institutions where nurses are asked regularly for their input and chief executives hold regular meetings with clinicians to review patient results,” she wrote.
But there is hope for change, Dr. Chen noted, and it’s demonstrable, citing a group of researchers that has written about strategies targeting hospital organizational culture called “Leadership Saves Lives.” The researchers showed hospitals could create significant cultural changes, which could impact patient outcomes, in just 2 years.
“Leadership Saves Lives requires that each hospital create a ‘Guiding Coalition,’ a group of staff members from across the entire institution. The coalition members participate in regular workshops, discussions, and national forums on ways hospitals might improve, then help their respective hospital translate newfound ideas and information into clinical practice,” she wrote.
The researchers monitored heart attack patients to assess the effect of Leadership Saves Lives in 10 hospitals that had below average patient outcomes. Over 2 years, all 10 hospitals changed significantly, but 6 hospitals experienced particularly profound cultural transformations.
“The staff of these hospitals spoke of an institutional shift from ‘because I said so’ to ‘focusing on the why’s,’ ” Dr. Chen wrote. “Instead of accepting that every heart attack patient had to undergo certain testing or take specific drugs because the chief of the department or administrator had previously established such clinical protocols, for example, it became more important to provide the data that proved such rituals were actually helpful. Staff members in these hospitals also said they received, and appreciated, increased support from senior management and a newfound freedom to voice opinions in ‘more of an equal role, no matter what position you are.’ ”
The degree of an institution’s cultural change was directly linked to patient outcomes, the researchers found. Indeed, hospitals that made more substantial changes in their work culture realized larger and more sustained drops in heart attack mortality rates.
References
1. Chen PW. A More Egalitarian Hospital Culture Is Better for Everyone. New York Times. https://www.nytimes.com/2018/05/31/well/live/doctors-patients-hospital-culture-better-health.html. Published May 31, 2018. Accessed June 1, 2018.
2. Curry LA et al. Organizational culture change in U.S. hospitals: A mixed methods longitudinal intervention study. Implementation Science. 2015 Mar 7. doi: 10.1186/s13012-015-0218-0. Accessed June 18, 2018.
Cultural change linked to improved outcomes
Cultural change linked to improved outcomes
Health care experts have long known of a link between patient outcomes and a hospital’s organizational culture, according to an article in the New York Times by Pauline W. Chen, MD.
“Heart attack patients who are treated at hospitals where nurses feel powerless and senior management is only sporadically involved in patient care tend to fare more poorly than patients hospitalized at institutions where nurses are asked regularly for their input and chief executives hold regular meetings with clinicians to review patient results,” she wrote.
But there is hope for change, Dr. Chen noted, and it’s demonstrable, citing a group of researchers that has written about strategies targeting hospital organizational culture called “Leadership Saves Lives.” The researchers showed hospitals could create significant cultural changes, which could impact patient outcomes, in just 2 years.
“Leadership Saves Lives requires that each hospital create a ‘Guiding Coalition,’ a group of staff members from across the entire institution. The coalition members participate in regular workshops, discussions, and national forums on ways hospitals might improve, then help their respective hospital translate newfound ideas and information into clinical practice,” she wrote.
The researchers monitored heart attack patients to assess the effect of Leadership Saves Lives in 10 hospitals that had below average patient outcomes. Over 2 years, all 10 hospitals changed significantly, but 6 hospitals experienced particularly profound cultural transformations.
“The staff of these hospitals spoke of an institutional shift from ‘because I said so’ to ‘focusing on the why’s,’ ” Dr. Chen wrote. “Instead of accepting that every heart attack patient had to undergo certain testing or take specific drugs because the chief of the department or administrator had previously established such clinical protocols, for example, it became more important to provide the data that proved such rituals were actually helpful. Staff members in these hospitals also said they received, and appreciated, increased support from senior management and a newfound freedom to voice opinions in ‘more of an equal role, no matter what position you are.’ ”
The degree of an institution’s cultural change was directly linked to patient outcomes, the researchers found. Indeed, hospitals that made more substantial changes in their work culture realized larger and more sustained drops in heart attack mortality rates.
References
1. Chen PW. A More Egalitarian Hospital Culture Is Better for Everyone. New York Times. https://www.nytimes.com/2018/05/31/well/live/doctors-patients-hospital-culture-better-health.html. Published May 31, 2018. Accessed June 1, 2018.
2. Curry LA et al. Organizational culture change in U.S. hospitals: A mixed methods longitudinal intervention study. Implementation Science. 2015 Mar 7. doi: 10.1186/s13012-015-0218-0. Accessed June 18, 2018.
Health care experts have long known of a link between patient outcomes and a hospital’s organizational culture, according to an article in the New York Times by Pauline W. Chen, MD.
“Heart attack patients who are treated at hospitals where nurses feel powerless and senior management is only sporadically involved in patient care tend to fare more poorly than patients hospitalized at institutions where nurses are asked regularly for their input and chief executives hold regular meetings with clinicians to review patient results,” she wrote.
But there is hope for change, Dr. Chen noted, and it’s demonstrable, citing a group of researchers that has written about strategies targeting hospital organizational culture called “Leadership Saves Lives.” The researchers showed hospitals could create significant cultural changes, which could impact patient outcomes, in just 2 years.
“Leadership Saves Lives requires that each hospital create a ‘Guiding Coalition,’ a group of staff members from across the entire institution. The coalition members participate in regular workshops, discussions, and national forums on ways hospitals might improve, then help their respective hospital translate newfound ideas and information into clinical practice,” she wrote.
The researchers monitored heart attack patients to assess the effect of Leadership Saves Lives in 10 hospitals that had below average patient outcomes. Over 2 years, all 10 hospitals changed significantly, but 6 hospitals experienced particularly profound cultural transformations.
“The staff of these hospitals spoke of an institutional shift from ‘because I said so’ to ‘focusing on the why’s,’ ” Dr. Chen wrote. “Instead of accepting that every heart attack patient had to undergo certain testing or take specific drugs because the chief of the department or administrator had previously established such clinical protocols, for example, it became more important to provide the data that proved such rituals were actually helpful. Staff members in these hospitals also said they received, and appreciated, increased support from senior management and a newfound freedom to voice opinions in ‘more of an equal role, no matter what position you are.’ ”
The degree of an institution’s cultural change was directly linked to patient outcomes, the researchers found. Indeed, hospitals that made more substantial changes in their work culture realized larger and more sustained drops in heart attack mortality rates.
References
1. Chen PW. A More Egalitarian Hospital Culture Is Better for Everyone. New York Times. https://www.nytimes.com/2018/05/31/well/live/doctors-patients-hospital-culture-better-health.html. Published May 31, 2018. Accessed June 1, 2018.
2. Curry LA et al. Organizational culture change in U.S. hospitals: A mixed methods longitudinal intervention study. Implementation Science. 2015 Mar 7. doi: 10.1186/s13012-015-0218-0. Accessed June 18, 2018.
Public insurance income limits and hospitalizations for low-income children
Vulnerable populations at greater risk
Background: Medicaid and the Children’s Health Insurance Program (CHIP) provide health care to over 30 million children in the United States.1,2 As a result, low-income children have had increased access to health care, of all forms, which has increased the utilization of primary care and decreased unnecessary ED visits and hospitalizations. However, this comes at a high cost, both at the state and national level. Medicaid currently subsidizes more than 50% of every state’s public insurance program, spending about $100 billion/year in health care payments for children.3 Given this hefty price tag, there have been myriad strategies proposed to help decrease these costs. One such strategy, includes decreasing enrollment in public insurance through decreasing income eligibility thresholds. As a result, many children from low-income families would lose their public insurance and be eligible for commercial insurance only. Consequently, this would place an undue financial burden on these families and the health care systems that care for them. Furthermore, it is anticipated that poor health care outcomes would increase in these vulnerable populations.
Study design: Retrospective cohort study using 2014 State Inpatient Databases.
Setting: Pediatric hospitalizations (aged less than 18 years) from 14 states during 2014 with public insurance listed as the primary payer. This encompassed about 30% of family households in the United States in 2014.
Synopsis: Simulations were done at three different thresholds of the federal poverty level (FPL), including less than 100%, less than 200% and less than 300%. Of the families included, 43% lived below 300%, 27% below 200% and 11% below 100% of the FPL. Of note, public insurance FPL eligibility limits tended to be lower in states with a greater percentage of the population being below 300% of the FPL. The results, of these reductions, were as follows:
- If reduced to less than 300% of the FPL, about 155,000 hospitalizations became ineligible for reimbursement. The median per-hospitalization estimated costs ranged from approximately $6,000 to approximately $10,000, accumulating $1.2 billion in estimated costs.
- If reduced to less than 200% of the FPL, about 440,000 hospitalizations became ineligible for reimbursement. The median per-hospitalization estimated costs ranged from approximately $2,000 to approximately $10,000, accumulating $3.1 billion in estimated costs.
- If reduced to less than 100% of the FPL, about 650,000 hospitalizations became ineligible for reimbursement. The median per-hospitalization estimated costs ranged from approximately $2,000 to approximately $10,000, accumulating $4.4 billion in estimated costs.
If these reductions occurred, healthy newborns would be disproportionately affected by them, which is important to note because newborn hospitalization is one of the fastest-rising costs in pediatric care. In fact, it can range from approximately $700 to approximately $2,000 per hospitalization, which may represent a huge financial strain for families that are unable to secure commercial insurance. Furthermore, with the average hospitalization of non-newborns ranging from $3,000 to $10,000, it is likely that this cost would constitute a fairly large percentage of a low income family’s annual income, which may represent an untenable financial burden.
Thus, if these families are unable to obtain commercial insurance and/or pay these debts, the financial burden will shift to the institutions that care for these vulnerable populations.
Bottom line: If public insurance eligibility thresholds were decreased, a large number of pediatric hospitalizations would become ineligible for coverage, which would shift the costs to families and institutions that are already financially strained and likely result in poor health care outcomes for some of our most vulnerable pediatric patients.
Citation: Bettenhausen JL et al. The effect of lowering public insurance income limits on hospitalizations for low-income children. Pediatrics. 2018 Aug. doi: 10.1542/peds.2017-3486.
Dr. Darden is a pediatric hospitalist at Phoenix Children’s Hospital and clinical assistant professor, University of Arizona, Phoenix.
References
1. The Henry J. Kaiser Family Foundation. Total Medicaid Spending. 2016. Available at: http://kff.org/medicaid/state-indicator/total-medicaid-spending/.
2. Medicaid and CHIP Payment and Access Commission. Trends in Medicaid Spending. 2016. Available at https://www.macpac.gov/wp-content/uploads/2016/06/Trends-in-Medicaid-Spending.pdf.
3. Medicaid and CHIP Payment and Access Commission. Medicaid’s share of state budgets. 2017. Available at: https://www.macpac.gov/subtopic/medicaids-share-of-state-budgets/.
Vulnerable populations at greater risk
Vulnerable populations at greater risk
Background: Medicaid and the Children’s Health Insurance Program (CHIP) provide health care to over 30 million children in the United States.1,2 As a result, low-income children have had increased access to health care, of all forms, which has increased the utilization of primary care and decreased unnecessary ED visits and hospitalizations. However, this comes at a high cost, both at the state and national level. Medicaid currently subsidizes more than 50% of every state’s public insurance program, spending about $100 billion/year in health care payments for children.3 Given this hefty price tag, there have been myriad strategies proposed to help decrease these costs. One such strategy, includes decreasing enrollment in public insurance through decreasing income eligibility thresholds. As a result, many children from low-income families would lose their public insurance and be eligible for commercial insurance only. Consequently, this would place an undue financial burden on these families and the health care systems that care for them. Furthermore, it is anticipated that poor health care outcomes would increase in these vulnerable populations.
Study design: Retrospective cohort study using 2014 State Inpatient Databases.
Setting: Pediatric hospitalizations (aged less than 18 years) from 14 states during 2014 with public insurance listed as the primary payer. This encompassed about 30% of family households in the United States in 2014.
Synopsis: Simulations were done at three different thresholds of the federal poverty level (FPL), including less than 100%, less than 200% and less than 300%. Of the families included, 43% lived below 300%, 27% below 200% and 11% below 100% of the FPL. Of note, public insurance FPL eligibility limits tended to be lower in states with a greater percentage of the population being below 300% of the FPL. The results, of these reductions, were as follows:
- If reduced to less than 300% of the FPL, about 155,000 hospitalizations became ineligible for reimbursement. The median per-hospitalization estimated costs ranged from approximately $6,000 to approximately $10,000, accumulating $1.2 billion in estimated costs.
- If reduced to less than 200% of the FPL, about 440,000 hospitalizations became ineligible for reimbursement. The median per-hospitalization estimated costs ranged from approximately $2,000 to approximately $10,000, accumulating $3.1 billion in estimated costs.
- If reduced to less than 100% of the FPL, about 650,000 hospitalizations became ineligible for reimbursement. The median per-hospitalization estimated costs ranged from approximately $2,000 to approximately $10,000, accumulating $4.4 billion in estimated costs.
If these reductions occurred, healthy newborns would be disproportionately affected by them, which is important to note because newborn hospitalization is one of the fastest-rising costs in pediatric care. In fact, it can range from approximately $700 to approximately $2,000 per hospitalization, which may represent a huge financial strain for families that are unable to secure commercial insurance. Furthermore, with the average hospitalization of non-newborns ranging from $3,000 to $10,000, it is likely that this cost would constitute a fairly large percentage of a low income family’s annual income, which may represent an untenable financial burden.
Thus, if these families are unable to obtain commercial insurance and/or pay these debts, the financial burden will shift to the institutions that care for these vulnerable populations.
Bottom line: If public insurance eligibility thresholds were decreased, a large number of pediatric hospitalizations would become ineligible for coverage, which would shift the costs to families and institutions that are already financially strained and likely result in poor health care outcomes for some of our most vulnerable pediatric patients.
Citation: Bettenhausen JL et al. The effect of lowering public insurance income limits on hospitalizations for low-income children. Pediatrics. 2018 Aug. doi: 10.1542/peds.2017-3486.
Dr. Darden is a pediatric hospitalist at Phoenix Children’s Hospital and clinical assistant professor, University of Arizona, Phoenix.
References
1. The Henry J. Kaiser Family Foundation. Total Medicaid Spending. 2016. Available at: http://kff.org/medicaid/state-indicator/total-medicaid-spending/.
2. Medicaid and CHIP Payment and Access Commission. Trends in Medicaid Spending. 2016. Available at https://www.macpac.gov/wp-content/uploads/2016/06/Trends-in-Medicaid-Spending.pdf.
3. Medicaid and CHIP Payment and Access Commission. Medicaid’s share of state budgets. 2017. Available at: https://www.macpac.gov/subtopic/medicaids-share-of-state-budgets/.
Background: Medicaid and the Children’s Health Insurance Program (CHIP) provide health care to over 30 million children in the United States.1,2 As a result, low-income children have had increased access to health care, of all forms, which has increased the utilization of primary care and decreased unnecessary ED visits and hospitalizations. However, this comes at a high cost, both at the state and national level. Medicaid currently subsidizes more than 50% of every state’s public insurance program, spending about $100 billion/year in health care payments for children.3 Given this hefty price tag, there have been myriad strategies proposed to help decrease these costs. One such strategy, includes decreasing enrollment in public insurance through decreasing income eligibility thresholds. As a result, many children from low-income families would lose their public insurance and be eligible for commercial insurance only. Consequently, this would place an undue financial burden on these families and the health care systems that care for them. Furthermore, it is anticipated that poor health care outcomes would increase in these vulnerable populations.
Study design: Retrospective cohort study using 2014 State Inpatient Databases.
Setting: Pediatric hospitalizations (aged less than 18 years) from 14 states during 2014 with public insurance listed as the primary payer. This encompassed about 30% of family households in the United States in 2014.
Synopsis: Simulations were done at three different thresholds of the federal poverty level (FPL), including less than 100%, less than 200% and less than 300%. Of the families included, 43% lived below 300%, 27% below 200% and 11% below 100% of the FPL. Of note, public insurance FPL eligibility limits tended to be lower in states with a greater percentage of the population being below 300% of the FPL. The results, of these reductions, were as follows:
- If reduced to less than 300% of the FPL, about 155,000 hospitalizations became ineligible for reimbursement. The median per-hospitalization estimated costs ranged from approximately $6,000 to approximately $10,000, accumulating $1.2 billion in estimated costs.
- If reduced to less than 200% of the FPL, about 440,000 hospitalizations became ineligible for reimbursement. The median per-hospitalization estimated costs ranged from approximately $2,000 to approximately $10,000, accumulating $3.1 billion in estimated costs.
- If reduced to less than 100% of the FPL, about 650,000 hospitalizations became ineligible for reimbursement. The median per-hospitalization estimated costs ranged from approximately $2,000 to approximately $10,000, accumulating $4.4 billion in estimated costs.
If these reductions occurred, healthy newborns would be disproportionately affected by them, which is important to note because newborn hospitalization is one of the fastest-rising costs in pediatric care. In fact, it can range from approximately $700 to approximately $2,000 per hospitalization, which may represent a huge financial strain for families that are unable to secure commercial insurance. Furthermore, with the average hospitalization of non-newborns ranging from $3,000 to $10,000, it is likely that this cost would constitute a fairly large percentage of a low income family’s annual income, which may represent an untenable financial burden.
Thus, if these families are unable to obtain commercial insurance and/or pay these debts, the financial burden will shift to the institutions that care for these vulnerable populations.
Bottom line: If public insurance eligibility thresholds were decreased, a large number of pediatric hospitalizations would become ineligible for coverage, which would shift the costs to families and institutions that are already financially strained and likely result in poor health care outcomes for some of our most vulnerable pediatric patients.
Citation: Bettenhausen JL et al. The effect of lowering public insurance income limits on hospitalizations for low-income children. Pediatrics. 2018 Aug. doi: 10.1542/peds.2017-3486.
Dr. Darden is a pediatric hospitalist at Phoenix Children’s Hospital and clinical assistant professor, University of Arizona, Phoenix.
References
1. The Henry J. Kaiser Family Foundation. Total Medicaid Spending. 2016. Available at: http://kff.org/medicaid/state-indicator/total-medicaid-spending/.
2. Medicaid and CHIP Payment and Access Commission. Trends in Medicaid Spending. 2016. Available at https://www.macpac.gov/wp-content/uploads/2016/06/Trends-in-Medicaid-Spending.pdf.
3. Medicaid and CHIP Payment and Access Commission. Medicaid’s share of state budgets. 2017. Available at: https://www.macpac.gov/subtopic/medicaids-share-of-state-budgets/.