User login
Official news magazine of the Society of Hospital Medicine
Copyright by Society of Hospital Medicine or related companies. All rights reserved. ISSN 1553-085X
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
div[contains(@class, 'pane-pub-article-hospitalist')]


Statins cut vascular events in elderly patients
Statin therapy appears to reduce the risk of major vascular events for patients of all age groups, but there is less evidence that older patients with evidence of occlusive vascular disease benefit from the treatment, according to a recent meta-analysis of 28 trials from the Cholesterol Treatment Trialists’ Collaboration published in The Lancet.
Statins are “useful and affordable drug[s] that reduce heart attacks and strokes in older patients. Until now there has been an evidence gap and we wanted to look at their efficacy and safety in older people,” Jordan Fulcher, BSc (Med), MBBS, from the Cholesterol Treatment Trialists’ (CTT) Collaboration and the University of Sydney stated in a press release. “Our analysis indicates that major cardiovascular events were reduced by about a fifth, per mmol/L lower LDL cholesterol, by statin therapy across all age groups. Despite previous concerns, we found no adverse effect on cancer or nonvascular mortality in any age group.”
The researchers examined 186,854 participants from 28 CTT trials undergoing statin therapy, of whom 14,483 (8%) were older than 75 years. Patients were divided into six groups based on age and examined the risk of major cardiovascular events such as stroke, coronary revascularization and major coronary events, as well as the incidence of cancer and vascular mortality.
Among all age groups, there was a significant reduction in major vascular events, with a 21% proportional per 1.0-mmol/L reduction in LDL cholesterol (risk ratio, 0.79; 95% confidence interval, 0.77-0.81) among patients receiving statin therapy or a more intensive statin regimen, and there was a 24% proportional reduction (RR, 0.76; 95% CI, 0.73-0.79) of major coronary events per 1.0-mmol/L reduction in LDL cholesterol, with older age resulting in a lower proportional reduction of major coronary events (P = .009). The researchers also found a proportional reduction of coronary revascularization procedures by 25% (RR, 0.75; 95% CI, 0.73-0.78) and stroke by 16% (RR, 0.84; 95% CI, 0.80-0.89) among patients of any age group receiving statin therapy or more intensive statin regimen, with no significant differences between age groups.
There was a 12% proportional reduction in vascular mortality per 1.0-mmol/L reduction in LDL cholesterol (RR, 0.88; 95% CI, 0.85-0.91), but this statistic did not remain significant after the researchers excluded four trials that included patients with heart failure or who were receiving renal dialysis. After excluding these trials from the overall analysis, the researchers found the smaller proportional reductions persisted for older patients for major coronary events (P = .01) but was no longer significant for major vascular events.
The researchers noted their study was limited by the highly selected patient population, low percentage of patients older than 75 years, including trials with efficacy endpoints where some nonserious adverse events may not have been recorded, and not including some trials in the meta-analysis if they were not part of the CTT.
This study was funded by Australian National Health and Medical Research Council, National Institute for Health Research Oxford Biomedical Research Centre, UK Medical Research Council, and British Heart Foundation. The authors have reported personal fees, grants, and consulting fees from Abbott, Aegerion, Amgen, Arisaph, AstraZeneca, Bayer, Beckmann, Berlin-Chemie, Boehringer Ingelheim, Daiichi Sankyo, Dalcor, DuPont, Esperion, GlaxoSmithKline, ISIS Pharmaceuticals, Kowa, Mylan, Pfizer, Roche, Sanofi, Singulex, The Medicines Company, and Vatera Capital, as well as the British Heart Foundation, Cancer Research UK, National Institute for Health Research Oxford Biomedical Research Centre, Medical Research Council, Nuffield Department of Population Health, Weill Cornell Medicine, and UK Biobank.
SOURCE: Fulcher J et al. Lancet. 2019;393:407-15.
Statin therapy is often discontinued for older patients who have concomitant disease or other considerations, but it should still be considered in older patients when the benefits outweigh the risks, Bernard M.Y. Cheung, PhD, and Karen S.L. Lam, MD, wrote in a related editorial.
“Even if the relative risk reduction in people older than 75 years is less than expected, statin therapy might still be justified by a high baseline cardiovascular risk, which is usually present in older people,” they said.
One explanation for the decreased relative risk reduction among older patients from the results by Fulcher et al. in the Cholesterol Treatment Trialists’ (CTT) Collaboration trial could have been the inclusion of older patients with cardiac and renal failure, and treating patients with lower cardiac risk or lowering LDL cholesterol in patients at risk of cardiovascular events can help prevent major vascular events later.
Ultimately, no drug is harmless and the risk and benefits must be weighed before making a decision to use statins with older patients just as they would in any other patient population. “The challenge for the health-care profession and the media is to convey risks and benefits in ways that patients can understand, enabling them to make an informed choice,” the authors wrote.
Dr. Cheung and Dr. Lam are from the department of medicine at Queen Mary Hospital, University of Hong Kong in Hong Kong Special Administrative Region, China. They had no relevant disclosures.
Statin therapy is often discontinued for older patients who have concomitant disease or other considerations, but it should still be considered in older patients when the benefits outweigh the risks, Bernard M.Y. Cheung, PhD, and Karen S.L. Lam, MD, wrote in a related editorial.
“Even if the relative risk reduction in people older than 75 years is less than expected, statin therapy might still be justified by a high baseline cardiovascular risk, which is usually present in older people,” they said.
One explanation for the decreased relative risk reduction among older patients from the results by Fulcher et al. in the Cholesterol Treatment Trialists’ (CTT) Collaboration trial could have been the inclusion of older patients with cardiac and renal failure, and treating patients with lower cardiac risk or lowering LDL cholesterol in patients at risk of cardiovascular events can help prevent major vascular events later.
Ultimately, no drug is harmless and the risk and benefits must be weighed before making a decision to use statins with older patients just as they would in any other patient population. “The challenge for the health-care profession and the media is to convey risks and benefits in ways that patients can understand, enabling them to make an informed choice,” the authors wrote.
Dr. Cheung and Dr. Lam are from the department of medicine at Queen Mary Hospital, University of Hong Kong in Hong Kong Special Administrative Region, China. They had no relevant disclosures.
Statin therapy is often discontinued for older patients who have concomitant disease or other considerations, but it should still be considered in older patients when the benefits outweigh the risks, Bernard M.Y. Cheung, PhD, and Karen S.L. Lam, MD, wrote in a related editorial.
“Even if the relative risk reduction in people older than 75 years is less than expected, statin therapy might still be justified by a high baseline cardiovascular risk, which is usually present in older people,” they said.
One explanation for the decreased relative risk reduction among older patients from the results by Fulcher et al. in the Cholesterol Treatment Trialists’ (CTT) Collaboration trial could have been the inclusion of older patients with cardiac and renal failure, and treating patients with lower cardiac risk or lowering LDL cholesterol in patients at risk of cardiovascular events can help prevent major vascular events later.
Ultimately, no drug is harmless and the risk and benefits must be weighed before making a decision to use statins with older patients just as they would in any other patient population. “The challenge for the health-care profession and the media is to convey risks and benefits in ways that patients can understand, enabling them to make an informed choice,” the authors wrote.
Dr. Cheung and Dr. Lam are from the department of medicine at Queen Mary Hospital, University of Hong Kong in Hong Kong Special Administrative Region, China. They had no relevant disclosures.
Statin therapy appears to reduce the risk of major vascular events for patients of all age groups, but there is less evidence that older patients with evidence of occlusive vascular disease benefit from the treatment, according to a recent meta-analysis of 28 trials from the Cholesterol Treatment Trialists’ Collaboration published in The Lancet.
Statins are “useful and affordable drug[s] that reduce heart attacks and strokes in older patients. Until now there has been an evidence gap and we wanted to look at their efficacy and safety in older people,” Jordan Fulcher, BSc (Med), MBBS, from the Cholesterol Treatment Trialists’ (CTT) Collaboration and the University of Sydney stated in a press release. “Our analysis indicates that major cardiovascular events were reduced by about a fifth, per mmol/L lower LDL cholesterol, by statin therapy across all age groups. Despite previous concerns, we found no adverse effect on cancer or nonvascular mortality in any age group.”
The researchers examined 186,854 participants from 28 CTT trials undergoing statin therapy, of whom 14,483 (8%) were older than 75 years. Patients were divided into six groups based on age and examined the risk of major cardiovascular events such as stroke, coronary revascularization and major coronary events, as well as the incidence of cancer and vascular mortality.
Among all age groups, there was a significant reduction in major vascular events, with a 21% proportional per 1.0-mmol/L reduction in LDL cholesterol (risk ratio, 0.79; 95% confidence interval, 0.77-0.81) among patients receiving statin therapy or a more intensive statin regimen, and there was a 24% proportional reduction (RR, 0.76; 95% CI, 0.73-0.79) of major coronary events per 1.0-mmol/L reduction in LDL cholesterol, with older age resulting in a lower proportional reduction of major coronary events (P = .009). The researchers also found a proportional reduction of coronary revascularization procedures by 25% (RR, 0.75; 95% CI, 0.73-0.78) and stroke by 16% (RR, 0.84; 95% CI, 0.80-0.89) among patients of any age group receiving statin therapy or more intensive statin regimen, with no significant differences between age groups.
There was a 12% proportional reduction in vascular mortality per 1.0-mmol/L reduction in LDL cholesterol (RR, 0.88; 95% CI, 0.85-0.91), but this statistic did not remain significant after the researchers excluded four trials that included patients with heart failure or who were receiving renal dialysis. After excluding these trials from the overall analysis, the researchers found the smaller proportional reductions persisted for older patients for major coronary events (P = .01) but was no longer significant for major vascular events.
The researchers noted their study was limited by the highly selected patient population, low percentage of patients older than 75 years, including trials with efficacy endpoints where some nonserious adverse events may not have been recorded, and not including some trials in the meta-analysis if they were not part of the CTT.
This study was funded by Australian National Health and Medical Research Council, National Institute for Health Research Oxford Biomedical Research Centre, UK Medical Research Council, and British Heart Foundation. The authors have reported personal fees, grants, and consulting fees from Abbott, Aegerion, Amgen, Arisaph, AstraZeneca, Bayer, Beckmann, Berlin-Chemie, Boehringer Ingelheim, Daiichi Sankyo, Dalcor, DuPont, Esperion, GlaxoSmithKline, ISIS Pharmaceuticals, Kowa, Mylan, Pfizer, Roche, Sanofi, Singulex, The Medicines Company, and Vatera Capital, as well as the British Heart Foundation, Cancer Research UK, National Institute for Health Research Oxford Biomedical Research Centre, Medical Research Council, Nuffield Department of Population Health, Weill Cornell Medicine, and UK Biobank.
SOURCE: Fulcher J et al. Lancet. 2019;393:407-15.
Statin therapy appears to reduce the risk of major vascular events for patients of all age groups, but there is less evidence that older patients with evidence of occlusive vascular disease benefit from the treatment, according to a recent meta-analysis of 28 trials from the Cholesterol Treatment Trialists’ Collaboration published in The Lancet.
Statins are “useful and affordable drug[s] that reduce heart attacks and strokes in older patients. Until now there has been an evidence gap and we wanted to look at their efficacy and safety in older people,” Jordan Fulcher, BSc (Med), MBBS, from the Cholesterol Treatment Trialists’ (CTT) Collaboration and the University of Sydney stated in a press release. “Our analysis indicates that major cardiovascular events were reduced by about a fifth, per mmol/L lower LDL cholesterol, by statin therapy across all age groups. Despite previous concerns, we found no adverse effect on cancer or nonvascular mortality in any age group.”
The researchers examined 186,854 participants from 28 CTT trials undergoing statin therapy, of whom 14,483 (8%) were older than 75 years. Patients were divided into six groups based on age and examined the risk of major cardiovascular events such as stroke, coronary revascularization and major coronary events, as well as the incidence of cancer and vascular mortality.
Among all age groups, there was a significant reduction in major vascular events, with a 21% proportional per 1.0-mmol/L reduction in LDL cholesterol (risk ratio, 0.79; 95% confidence interval, 0.77-0.81) among patients receiving statin therapy or a more intensive statin regimen, and there was a 24% proportional reduction (RR, 0.76; 95% CI, 0.73-0.79) of major coronary events per 1.0-mmol/L reduction in LDL cholesterol, with older age resulting in a lower proportional reduction of major coronary events (P = .009). The researchers also found a proportional reduction of coronary revascularization procedures by 25% (RR, 0.75; 95% CI, 0.73-0.78) and stroke by 16% (RR, 0.84; 95% CI, 0.80-0.89) among patients of any age group receiving statin therapy or more intensive statin regimen, with no significant differences between age groups.
There was a 12% proportional reduction in vascular mortality per 1.0-mmol/L reduction in LDL cholesterol (RR, 0.88; 95% CI, 0.85-0.91), but this statistic did not remain significant after the researchers excluded four trials that included patients with heart failure or who were receiving renal dialysis. After excluding these trials from the overall analysis, the researchers found the smaller proportional reductions persisted for older patients for major coronary events (P = .01) but was no longer significant for major vascular events.
The researchers noted their study was limited by the highly selected patient population, low percentage of patients older than 75 years, including trials with efficacy endpoints where some nonserious adverse events may not have been recorded, and not including some trials in the meta-analysis if they were not part of the CTT.
This study was funded by Australian National Health and Medical Research Council, National Institute for Health Research Oxford Biomedical Research Centre, UK Medical Research Council, and British Heart Foundation. The authors have reported personal fees, grants, and consulting fees from Abbott, Aegerion, Amgen, Arisaph, AstraZeneca, Bayer, Beckmann, Berlin-Chemie, Boehringer Ingelheim, Daiichi Sankyo, Dalcor, DuPont, Esperion, GlaxoSmithKline, ISIS Pharmaceuticals, Kowa, Mylan, Pfizer, Roche, Sanofi, Singulex, The Medicines Company, and Vatera Capital, as well as the British Heart Foundation, Cancer Research UK, National Institute for Health Research Oxford Biomedical Research Centre, Medical Research Council, Nuffield Department of Population Health, Weill Cornell Medicine, and UK Biobank.
SOURCE: Fulcher J et al. Lancet. 2019;393:407-15.
FROM THE LANCET
Key clinical point: but patients older than 75 years with occlusive vascular disease have a smaller reduction in major coronary events.
Major finding: Major vascular coronary events were reduced by 24% (risk ratio, 0.76; 95% confidence interval, 0.73-0.79) with a decrease in the reduction of coronary events among patients older than 75 years. Study details: A meta-analysis of 28 trials with 186,854 individuals undergoing statin therapy from the Cholesterol Treatment Trialists’ Collaboration.
Disclosures: This study was funded by Australian National Health and Medical Research Council, National Institute for Health Research Oxford Biomedical Research Centre, UK Medical Research Council, and British Heart Foundation. The authors have reported personal fees, grants, and consulting fees from Abbott, Aegerion, Amgen, Arisaph, AstraZeneca, Bayer, Beckmann, Berlin-Chemie, Boehringer Ingelheim, Daiichi Sankyo, Dalcor, DuPont, Esperion, GlaxoSmithKline, ISIS Pharmaceuticals, Kowa, Mylan, Pfizer, Roche, Sanofi, Singulex, The Medicines Company, and Vatera Capital, as well as the British Heart Foundation, Cancer Research UK, National Institute for Health Research Oxford Biomedical Research Centre, Medical Research Council, Nuffield Department of Population Health, Weill Cornell Medicine, and UK Biobank.
Source: Fulcher J et al. Lancet. 2019;393:407-15.
When is it safe to resume anticoagulation in my patient with hemorrhagic stroke?
Balancing risk is critical to decision making
Department of Medicine, Massachusetts General Hospital, Boston
Case
A 75 year-old woman with a history of hypertension, diabetes mellitus, heart failure and nonvalvular atrial fibrillation (CHA2DS2-VASc score, 8) on anticoagulation is admitted with weakness and dysarthria. Exam is notable for hypertension and right-sided hemiparesis. CT of the head shows an intraparenchymal hemorrhage in the left putamen. Her anticoagulation is reversed and blood pressure well controlled. She is discharged 12 days later.
Brief overview of the issue
Intracranial hemorrhage (ICH) is the second most common cause of stroke and is associated with high morbidity and mortality.1 It is estimated that 10%-15% of spontaneous ICH cases occur in patients on therapeutic anticoagulation for atrial fibrillation.2 As our population ages and more people develop atrial fibrillation, anticoagulation for primary or secondary prevention of embolic stroke also will likely increase, placing more people at risk for ICH. Even stringently controlled therapeutic international normalized ratios (INRs) between 2 and 3 may double the risk of ICH.3
Patients with ICH require close monitoring and treatment, including blood pressure control, reversal of anticoagulation, reduction of intracranial pressure and, at times, neurosurgery.4 Although anticoagulation is discontinued and reversed at the onset of ICH, no clear consensus exists as to when it is safe to resume it. Although anticoagulation decreases the risk of stroke/thromboembolism, it may also increase the amount of bleeding associated with the initial ICH or lead to its recurrence.
Factors that may contribute to rebleeding include uncontrolled hypertension, advanced age, time to resumption of anticoagulation, and lobar location of ICH (i.e., in cerebral cortex and/or underlying white matter).5 Traditionally, lobar ICH has high incidence of cerebral amyloid angiopathy and has been associated with higher bleeding rates than has deep ICH (i.e., involving the thalami, basal ganglia, cerebellum, or brainstem) where cerebral amyloid angiopathy is rare and ICH is usually from hypertensive vessel disease. However, in patients with active thromboembolic disease, high-risk atrial fibrillation, and mechanical valves, withholding anticoagulation could place them at high risk of stroke.
Two questions should be addressed in the case presented: Is it safe to restart therapeutic anticoagulation; and if so, what is the optimal time interval between ICH and reinitiation of anticoagulation?
Overview of the data
There is limited guidance from major professional societies regarding the reinitiation of anticoagulation and the optimal timing of safely resuming anticoagulation in patients with prior ICH.
Current European Stroke Organization guidelines provide no specific recommendations for anticoagulation resumption after ICH.7 The American Heart Association/American Stroke Association guideline has a class IIA (weak) recommendation to avoid anticoagulation in spontaneous lobar ICH and a class IIB (very weak) recommendation to consider resuming anticoagulation in nonlobar ICH on a case-by-case basis.4
Two recent meta-analyses have examined outcomes of resuming anticoagulation after ICH. In a meta-analysis of 5,300 patients with nonlobar ICH involving eight retrospective studies, Murthy et al. evaluated the risk of thromboembolic events (described as a composite outcome of MI and stroke) and the risk of recurrent ICH.8 They reported that resumption of therapeutic anticoagulation was associated with a decrease in the rate of thromboembolic events (6.7% vs. 17.6%; risk ratio, 0.35; 95% confidence interval, 0.25-0.45) with no significant change in the rate of repeat ICH (8.7% vs. 7.8%).
A second meta-analysis of three retrospective trials conducted by Biffi et al. examined anticoagulation resumption in 1,012 patients with ICH solely in the setting of thromboprophylaxis for nonvalvular atrial fibrillation.9 Reinitiation of anticoagulation after ICH was associated with decreased mortality (hazard ratio, 0.27; 95% CI, 0.19-0.40; P less than .0001), improved functional outcome (HR, 4.15; 95% CI, 2.92-5.90; P less than .0001), and reduction in all-cause stroke recurrence (HR 0.47; 95% CI, 0.36-0.64; P less than .0001). There was no significant difference in the rate of recurrent ICH when anticoagulation was resumed. Despite the notion that patients with cerebral amyloid angiopathy are at high risk of rebleeding, this positive association still held irrespective of lobar vs. nonlobar location of ICH.
Collectively, these studies suggest that resumption of anticoagulation may be effective in decreasing the rates of thromboembolism, as well as provide a functional and mortality benefit without increasing the risk of rebleeding, irrespective of the location of the bleed.
Less is known about the optimal timing of resumption of therapeutic anticoagulation, with data ranging from 72 hours to 30 weeks.10 The American Heart Association/American Stroke Association has a class IIB (very weak) recommendation to avoid anticoagulation for at least 4 weeks in patients without mechanical heart valves.4 The median time to resumption of therapeutic anticoagulation in aforementioned meta-analyses ranged from 10 to 44 days.8,9
A recent observational study of 2,619 ICH survivors explored the relationship between the timing of reinitiation of anticoagulation and the incidence of thrombotic events (defined as ischemic stroke or death because of MI or systemic arterial thromboembolism) and hemorrhagic events (defined as recurrent ICH or bleeding event leading to death) occurring at least 28 days after initial ICH in patients with atrial fibrillation.11
A decrease in thrombotic events was demonstrated if anticoagulation was started 4-16 weeks after ICH. However, when anticoagulation was started more than 16 weeks after ICH, no benefit was seen. Additionally, there was no significant difference in hemorrhagic events between men and women who resumed anticoagulation. In patients with high venous thromboembolism risk based on CHA2DS2-VASc score, resumption of anticoagulation was associated with a decreased predicted incidence of vascular death and nonfatal stroke, with the greatest benefit observed when anticoagulation was started at 7-8 weeks after ICH.
Unfortunately, published literature to date on anticoagulation after ICH is based entirely on retrospective studies – not randomized, controlled studies – making it more likely that anticoagulation would have been resumed in healthier patients, not those left debilitated by the ICH.
Furthermore, information on the location and size of the hemorrhages – which may serve as another confounding factor – often has not been reported. This is important since patients with smaller hemorrhages in less precarious areas also may be more likely to have resumption of anticoagulation. Another limitation of the current literature is that warfarin is the most common anticoagulant studied, with few studies involving the increasingly prescribed newer direct oral anticoagulants. It is also important to stress that a causal relationship between use of anticoagulants and certain outcomes or adverse effects following ICH may be more difficult to invoke in the absence of randomized controlled study designs.
Application of the data to our patient
Resumption of anticoagulation in our patient with ICH requires balancing the risk of hemorrhage expansion and recurrent ICH with the risk of thromboembolic disease.
Our patient is at higher risk of bleeding because of her advanced age, but adequate control of her blood pressure and nonlobar location of her ICH in the basal ganglia also may decrease her risk of recurrent ICH. Her high CHA2DS2-VASc score places her at high risk of thromboembolic event and stroke, making it more likely for reinitiation of anticoagulation to confer a mortality benefit.
Based on AHA guidelines,4 we should wait at least 4 weeks, or possibly wait until weeks 7-8 after ICH when the greatest benefit may be expected based on prediction models.11
Bottom line
It would likely be safe to resume anticoagulation 4-8 weeks after ICH in our patient.
Dr. Gibson, Dr. Restrepo, Dr. Sasidhara, and Dr. Manian are hospitalists at Massachusetts General Hospital, Boston.
References
1. An SJ et al. Epidemiology, risk factors, and clinical features of intracerebral hemorrhage: An update. J Stroke. 2017 Jan;19:3-10.
2. Horstmann S et al. Intracerebral hemorrhage during anticoagulation with vitamin K antagonists: a consecutive observational study. J Neurol. 2013 Aug;260:2046-51.
3. Rosand J et al. The effect of warfarin and intensity of anticoagulation on outcome of intracerebral hemorrhage. Arch Intern Med. 2004 Apr 26;164:880-4.
4. Hemphill JC et al. Guidelines for the management of spontaneous intracerebral hemorrhage. Stroke. 2015 Jul;46:2032-60.
5. Aguillar MI et al. Update in intracerebral hemorrhage. Neurohospitalist. 2011;1:148-59.
6. Hill MD et al. Rate of stroke recurrence in patients with primary intracerebral hemorrhage. Stroke. 2000;31:123-7.
7. Steiner T et al. European Stroke Organization (ESO) guidelines for the management of spontaneous cerebral hemorrhage. Int J Stroke. 2014;9:840-55.
8. Murthy SB et al. Restarting anticoagulation therapy after intracranial hemorrhage: A systematic review and meta-analysis. Stroke. 2017 Jun;48:1594-600.
9. Biffi A et al. Oral anticoagulation and functional outcome after intracerebral hemorrhage. Ann Neurol. 2017 Nov;82:755-65.
10. Witt DM. What to do after the bleed: Resuming anticoagulation after major bleeding. Hematology Am Soc Hematol Educ Program. 2016 Dec 2;206:620-4.
11. Pennlert J et al. Optimal timing of anticoagulant treatment after intracerebral hemorrhage in patients with atrial fibrillation. Stroke. 2017 Feb;48:314-20.
Key Points
- Robust scientific data on when to resume anticoagulation after ICH does not exist.
- Retrospective studies have shown that anticoagulation resumption after 4-8 weeks decreases the risk of thromboembolic events, decreases mortality, and improves functional status following ICH with no significant change in the risk of its recurrence.
- Prospective, randomized controlled trials are needed to explore risks/benefits of anticoagulation resumption and better define its optimal timing in relation to ICH.
Quiz
Which of the following is false regarding ICH?
A. Lobar ICHs are usually associated with cerebral amyloid angiopathy which are prone to bleeding.
B. Randomized, controlled studies have helped guide the decision as to when to resume anticoagulation in patients with ICH.
C. Current guidelines suggest deferring therapeutic anticoagulation for at least 4 weeks following ICH.
D. Resumption of anticoagulation after 4-8 weeks does not lead to increased risk of rebleeding in patients with prior ICH.
The false answer is B: Current recommendations regarding resumption of anticoagulation in patients with ICH are based solely on retrospective observational studies; there are no randomized, control trials to date.
A is true: In contrast to hypertensive vessel disease associated with deep ICH, lobar hemorrhages are usually associated with cerebral amyloid angiopathy, which are more prone to bleeding.
C is true: The AHA/ASA has a class IIB recommendation to avoid anticoagulation for at least 4 weeks after ICH in patients without mechanical heart valves.
D is true: Several studies have shown that resumption of anticoagulation 4-8 weeks after ICH does not increase the risk of rebleeding.
Balancing risk is critical to decision making
Balancing risk is critical to decision making
Department of Medicine, Massachusetts General Hospital, Boston
Case
A 75 year-old woman with a history of hypertension, diabetes mellitus, heart failure and nonvalvular atrial fibrillation (CHA2DS2-VASc score, 8) on anticoagulation is admitted with weakness and dysarthria. Exam is notable for hypertension and right-sided hemiparesis. CT of the head shows an intraparenchymal hemorrhage in the left putamen. Her anticoagulation is reversed and blood pressure well controlled. She is discharged 12 days later.
Brief overview of the issue
Intracranial hemorrhage (ICH) is the second most common cause of stroke and is associated with high morbidity and mortality.1 It is estimated that 10%-15% of spontaneous ICH cases occur in patients on therapeutic anticoagulation for atrial fibrillation.2 As our population ages and more people develop atrial fibrillation, anticoagulation for primary or secondary prevention of embolic stroke also will likely increase, placing more people at risk for ICH. Even stringently controlled therapeutic international normalized ratios (INRs) between 2 and 3 may double the risk of ICH.3
Patients with ICH require close monitoring and treatment, including blood pressure control, reversal of anticoagulation, reduction of intracranial pressure and, at times, neurosurgery.4 Although anticoagulation is discontinued and reversed at the onset of ICH, no clear consensus exists as to when it is safe to resume it. Although anticoagulation decreases the risk of stroke/thromboembolism, it may also increase the amount of bleeding associated with the initial ICH or lead to its recurrence.
Factors that may contribute to rebleeding include uncontrolled hypertension, advanced age, time to resumption of anticoagulation, and lobar location of ICH (i.e., in cerebral cortex and/or underlying white matter).5 Traditionally, lobar ICH has high incidence of cerebral amyloid angiopathy and has been associated with higher bleeding rates than has deep ICH (i.e., involving the thalami, basal ganglia, cerebellum, or brainstem) where cerebral amyloid angiopathy is rare and ICH is usually from hypertensive vessel disease. However, in patients with active thromboembolic disease, high-risk atrial fibrillation, and mechanical valves, withholding anticoagulation could place them at high risk of stroke.
Two questions should be addressed in the case presented: Is it safe to restart therapeutic anticoagulation; and if so, what is the optimal time interval between ICH and reinitiation of anticoagulation?
Overview of the data
There is limited guidance from major professional societies regarding the reinitiation of anticoagulation and the optimal timing of safely resuming anticoagulation in patients with prior ICH.
Current European Stroke Organization guidelines provide no specific recommendations for anticoagulation resumption after ICH.7 The American Heart Association/American Stroke Association guideline has a class IIA (weak) recommendation to avoid anticoagulation in spontaneous lobar ICH and a class IIB (very weak) recommendation to consider resuming anticoagulation in nonlobar ICH on a case-by-case basis.4
Two recent meta-analyses have examined outcomes of resuming anticoagulation after ICH. In a meta-analysis of 5,300 patients with nonlobar ICH involving eight retrospective studies, Murthy et al. evaluated the risk of thromboembolic events (described as a composite outcome of MI and stroke) and the risk of recurrent ICH.8 They reported that resumption of therapeutic anticoagulation was associated with a decrease in the rate of thromboembolic events (6.7% vs. 17.6%; risk ratio, 0.35; 95% confidence interval, 0.25-0.45) with no significant change in the rate of repeat ICH (8.7% vs. 7.8%).
A second meta-analysis of three retrospective trials conducted by Biffi et al. examined anticoagulation resumption in 1,012 patients with ICH solely in the setting of thromboprophylaxis for nonvalvular atrial fibrillation.9 Reinitiation of anticoagulation after ICH was associated with decreased mortality (hazard ratio, 0.27; 95% CI, 0.19-0.40; P less than .0001), improved functional outcome (HR, 4.15; 95% CI, 2.92-5.90; P less than .0001), and reduction in all-cause stroke recurrence (HR 0.47; 95% CI, 0.36-0.64; P less than .0001). There was no significant difference in the rate of recurrent ICH when anticoagulation was resumed. Despite the notion that patients with cerebral amyloid angiopathy are at high risk of rebleeding, this positive association still held irrespective of lobar vs. nonlobar location of ICH.
Collectively, these studies suggest that resumption of anticoagulation may be effective in decreasing the rates of thromboembolism, as well as provide a functional and mortality benefit without increasing the risk of rebleeding, irrespective of the location of the bleed.
Less is known about the optimal timing of resumption of therapeutic anticoagulation, with data ranging from 72 hours to 30 weeks.10 The American Heart Association/American Stroke Association has a class IIB (very weak) recommendation to avoid anticoagulation for at least 4 weeks in patients without mechanical heart valves.4 The median time to resumption of therapeutic anticoagulation in aforementioned meta-analyses ranged from 10 to 44 days.8,9
A recent observational study of 2,619 ICH survivors explored the relationship between the timing of reinitiation of anticoagulation and the incidence of thrombotic events (defined as ischemic stroke or death because of MI or systemic arterial thromboembolism) and hemorrhagic events (defined as recurrent ICH or bleeding event leading to death) occurring at least 28 days after initial ICH in patients with atrial fibrillation.11
A decrease in thrombotic events was demonstrated if anticoagulation was started 4-16 weeks after ICH. However, when anticoagulation was started more than 16 weeks after ICH, no benefit was seen. Additionally, there was no significant difference in hemorrhagic events between men and women who resumed anticoagulation. In patients with high venous thromboembolism risk based on CHA2DS2-VASc score, resumption of anticoagulation was associated with a decreased predicted incidence of vascular death and nonfatal stroke, with the greatest benefit observed when anticoagulation was started at 7-8 weeks after ICH.
Unfortunately, published literature to date on anticoagulation after ICH is based entirely on retrospective studies – not randomized, controlled studies – making it more likely that anticoagulation would have been resumed in healthier patients, not those left debilitated by the ICH.
Furthermore, information on the location and size of the hemorrhages – which may serve as another confounding factor – often has not been reported. This is important since patients with smaller hemorrhages in less precarious areas also may be more likely to have resumption of anticoagulation. Another limitation of the current literature is that warfarin is the most common anticoagulant studied, with few studies involving the increasingly prescribed newer direct oral anticoagulants. It is also important to stress that a causal relationship between use of anticoagulants and certain outcomes or adverse effects following ICH may be more difficult to invoke in the absence of randomized controlled study designs.
Application of the data to our patient
Resumption of anticoagulation in our patient with ICH requires balancing the risk of hemorrhage expansion and recurrent ICH with the risk of thromboembolic disease.
Our patient is at higher risk of bleeding because of her advanced age, but adequate control of her blood pressure and nonlobar location of her ICH in the basal ganglia also may decrease her risk of recurrent ICH. Her high CHA2DS2-VASc score places her at high risk of thromboembolic event and stroke, making it more likely for reinitiation of anticoagulation to confer a mortality benefit.
Based on AHA guidelines,4 we should wait at least 4 weeks, or possibly wait until weeks 7-8 after ICH when the greatest benefit may be expected based on prediction models.11
Bottom line
It would likely be safe to resume anticoagulation 4-8 weeks after ICH in our patient.
Dr. Gibson, Dr. Restrepo, Dr. Sasidhara, and Dr. Manian are hospitalists at Massachusetts General Hospital, Boston.
References
1. An SJ et al. Epidemiology, risk factors, and clinical features of intracerebral hemorrhage: An update. J Stroke. 2017 Jan;19:3-10.
2. Horstmann S et al. Intracerebral hemorrhage during anticoagulation with vitamin K antagonists: a consecutive observational study. J Neurol. 2013 Aug;260:2046-51.
3. Rosand J et al. The effect of warfarin and intensity of anticoagulation on outcome of intracerebral hemorrhage. Arch Intern Med. 2004 Apr 26;164:880-4.
4. Hemphill JC et al. Guidelines for the management of spontaneous intracerebral hemorrhage. Stroke. 2015 Jul;46:2032-60.
5. Aguillar MI et al. Update in intracerebral hemorrhage. Neurohospitalist. 2011;1:148-59.
6. Hill MD et al. Rate of stroke recurrence in patients with primary intracerebral hemorrhage. Stroke. 2000;31:123-7.
7. Steiner T et al. European Stroke Organization (ESO) guidelines for the management of spontaneous cerebral hemorrhage. Int J Stroke. 2014;9:840-55.
8. Murthy SB et al. Restarting anticoagulation therapy after intracranial hemorrhage: A systematic review and meta-analysis. Stroke. 2017 Jun;48:1594-600.
9. Biffi A et al. Oral anticoagulation and functional outcome after intracerebral hemorrhage. Ann Neurol. 2017 Nov;82:755-65.
10. Witt DM. What to do after the bleed: Resuming anticoagulation after major bleeding. Hematology Am Soc Hematol Educ Program. 2016 Dec 2;206:620-4.
11. Pennlert J et al. Optimal timing of anticoagulant treatment after intracerebral hemorrhage in patients with atrial fibrillation. Stroke. 2017 Feb;48:314-20.
Key Points
- Robust scientific data on when to resume anticoagulation after ICH does not exist.
- Retrospective studies have shown that anticoagulation resumption after 4-8 weeks decreases the risk of thromboembolic events, decreases mortality, and improves functional status following ICH with no significant change in the risk of its recurrence.
- Prospective, randomized controlled trials are needed to explore risks/benefits of anticoagulation resumption and better define its optimal timing in relation to ICH.
Quiz
Which of the following is false regarding ICH?
A. Lobar ICHs are usually associated with cerebral amyloid angiopathy which are prone to bleeding.
B. Randomized, controlled studies have helped guide the decision as to when to resume anticoagulation in patients with ICH.
C. Current guidelines suggest deferring therapeutic anticoagulation for at least 4 weeks following ICH.
D. Resumption of anticoagulation after 4-8 weeks does not lead to increased risk of rebleeding in patients with prior ICH.
The false answer is B: Current recommendations regarding resumption of anticoagulation in patients with ICH are based solely on retrospective observational studies; there are no randomized, control trials to date.
A is true: In contrast to hypertensive vessel disease associated with deep ICH, lobar hemorrhages are usually associated with cerebral amyloid angiopathy, which are more prone to bleeding.
C is true: The AHA/ASA has a class IIB recommendation to avoid anticoagulation for at least 4 weeks after ICH in patients without mechanical heart valves.
D is true: Several studies have shown that resumption of anticoagulation 4-8 weeks after ICH does not increase the risk of rebleeding.
Department of Medicine, Massachusetts General Hospital, Boston
Case
A 75 year-old woman with a history of hypertension, diabetes mellitus, heart failure and nonvalvular atrial fibrillation (CHA2DS2-VASc score, 8) on anticoagulation is admitted with weakness and dysarthria. Exam is notable for hypertension and right-sided hemiparesis. CT of the head shows an intraparenchymal hemorrhage in the left putamen. Her anticoagulation is reversed and blood pressure well controlled. She is discharged 12 days later.
Brief overview of the issue
Intracranial hemorrhage (ICH) is the second most common cause of stroke and is associated with high morbidity and mortality.1 It is estimated that 10%-15% of spontaneous ICH cases occur in patients on therapeutic anticoagulation for atrial fibrillation.2 As our population ages and more people develop atrial fibrillation, anticoagulation for primary or secondary prevention of embolic stroke also will likely increase, placing more people at risk for ICH. Even stringently controlled therapeutic international normalized ratios (INRs) between 2 and 3 may double the risk of ICH.3
Patients with ICH require close monitoring and treatment, including blood pressure control, reversal of anticoagulation, reduction of intracranial pressure and, at times, neurosurgery.4 Although anticoagulation is discontinued and reversed at the onset of ICH, no clear consensus exists as to when it is safe to resume it. Although anticoagulation decreases the risk of stroke/thromboembolism, it may also increase the amount of bleeding associated with the initial ICH or lead to its recurrence.
Factors that may contribute to rebleeding include uncontrolled hypertension, advanced age, time to resumption of anticoagulation, and lobar location of ICH (i.e., in cerebral cortex and/or underlying white matter).5 Traditionally, lobar ICH has high incidence of cerebral amyloid angiopathy and has been associated with higher bleeding rates than has deep ICH (i.e., involving the thalami, basal ganglia, cerebellum, or brainstem) where cerebral amyloid angiopathy is rare and ICH is usually from hypertensive vessel disease. However, in patients with active thromboembolic disease, high-risk atrial fibrillation, and mechanical valves, withholding anticoagulation could place them at high risk of stroke.
Two questions should be addressed in the case presented: Is it safe to restart therapeutic anticoagulation; and if so, what is the optimal time interval between ICH and reinitiation of anticoagulation?
Overview of the data
There is limited guidance from major professional societies regarding the reinitiation of anticoagulation and the optimal timing of safely resuming anticoagulation in patients with prior ICH.
Current European Stroke Organization guidelines provide no specific recommendations for anticoagulation resumption after ICH.7 The American Heart Association/American Stroke Association guideline has a class IIA (weak) recommendation to avoid anticoagulation in spontaneous lobar ICH and a class IIB (very weak) recommendation to consider resuming anticoagulation in nonlobar ICH on a case-by-case basis.4
Two recent meta-analyses have examined outcomes of resuming anticoagulation after ICH. In a meta-analysis of 5,300 patients with nonlobar ICH involving eight retrospective studies, Murthy et al. evaluated the risk of thromboembolic events (described as a composite outcome of MI and stroke) and the risk of recurrent ICH.8 They reported that resumption of therapeutic anticoagulation was associated with a decrease in the rate of thromboembolic events (6.7% vs. 17.6%; risk ratio, 0.35; 95% confidence interval, 0.25-0.45) with no significant change in the rate of repeat ICH (8.7% vs. 7.8%).
A second meta-analysis of three retrospective trials conducted by Biffi et al. examined anticoagulation resumption in 1,012 patients with ICH solely in the setting of thromboprophylaxis for nonvalvular atrial fibrillation.9 Reinitiation of anticoagulation after ICH was associated with decreased mortality (hazard ratio, 0.27; 95% CI, 0.19-0.40; P less than .0001), improved functional outcome (HR, 4.15; 95% CI, 2.92-5.90; P less than .0001), and reduction in all-cause stroke recurrence (HR 0.47; 95% CI, 0.36-0.64; P less than .0001). There was no significant difference in the rate of recurrent ICH when anticoagulation was resumed. Despite the notion that patients with cerebral amyloid angiopathy are at high risk of rebleeding, this positive association still held irrespective of lobar vs. nonlobar location of ICH.
Collectively, these studies suggest that resumption of anticoagulation may be effective in decreasing the rates of thromboembolism, as well as provide a functional and mortality benefit without increasing the risk of rebleeding, irrespective of the location of the bleed.
Less is known about the optimal timing of resumption of therapeutic anticoagulation, with data ranging from 72 hours to 30 weeks.10 The American Heart Association/American Stroke Association has a class IIB (very weak) recommendation to avoid anticoagulation for at least 4 weeks in patients without mechanical heart valves.4 The median time to resumption of therapeutic anticoagulation in aforementioned meta-analyses ranged from 10 to 44 days.8,9
A recent observational study of 2,619 ICH survivors explored the relationship between the timing of reinitiation of anticoagulation and the incidence of thrombotic events (defined as ischemic stroke or death because of MI or systemic arterial thromboembolism) and hemorrhagic events (defined as recurrent ICH or bleeding event leading to death) occurring at least 28 days after initial ICH in patients with atrial fibrillation.11
A decrease in thrombotic events was demonstrated if anticoagulation was started 4-16 weeks after ICH. However, when anticoagulation was started more than 16 weeks after ICH, no benefit was seen. Additionally, there was no significant difference in hemorrhagic events between men and women who resumed anticoagulation. In patients with high venous thromboembolism risk based on CHA2DS2-VASc score, resumption of anticoagulation was associated with a decreased predicted incidence of vascular death and nonfatal stroke, with the greatest benefit observed when anticoagulation was started at 7-8 weeks after ICH.
Unfortunately, published literature to date on anticoagulation after ICH is based entirely on retrospective studies – not randomized, controlled studies – making it more likely that anticoagulation would have been resumed in healthier patients, not those left debilitated by the ICH.
Furthermore, information on the location and size of the hemorrhages – which may serve as another confounding factor – often has not been reported. This is important since patients with smaller hemorrhages in less precarious areas also may be more likely to have resumption of anticoagulation. Another limitation of the current literature is that warfarin is the most common anticoagulant studied, with few studies involving the increasingly prescribed newer direct oral anticoagulants. It is also important to stress that a causal relationship between use of anticoagulants and certain outcomes or adverse effects following ICH may be more difficult to invoke in the absence of randomized controlled study designs.
Application of the data to our patient
Resumption of anticoagulation in our patient with ICH requires balancing the risk of hemorrhage expansion and recurrent ICH with the risk of thromboembolic disease.
Our patient is at higher risk of bleeding because of her advanced age, but adequate control of her blood pressure and nonlobar location of her ICH in the basal ganglia also may decrease her risk of recurrent ICH. Her high CHA2DS2-VASc score places her at high risk of thromboembolic event and stroke, making it more likely for reinitiation of anticoagulation to confer a mortality benefit.
Based on AHA guidelines,4 we should wait at least 4 weeks, or possibly wait until weeks 7-8 after ICH when the greatest benefit may be expected based on prediction models.11
Bottom line
It would likely be safe to resume anticoagulation 4-8 weeks after ICH in our patient.
Dr. Gibson, Dr. Restrepo, Dr. Sasidhara, and Dr. Manian are hospitalists at Massachusetts General Hospital, Boston.
References
1. An SJ et al. Epidemiology, risk factors, and clinical features of intracerebral hemorrhage: An update. J Stroke. 2017 Jan;19:3-10.
2. Horstmann S et al. Intracerebral hemorrhage during anticoagulation with vitamin K antagonists: a consecutive observational study. J Neurol. 2013 Aug;260:2046-51.
3. Rosand J et al. The effect of warfarin and intensity of anticoagulation on outcome of intracerebral hemorrhage. Arch Intern Med. 2004 Apr 26;164:880-4.
4. Hemphill JC et al. Guidelines for the management of spontaneous intracerebral hemorrhage. Stroke. 2015 Jul;46:2032-60.
5. Aguillar MI et al. Update in intracerebral hemorrhage. Neurohospitalist. 2011;1:148-59.
6. Hill MD et al. Rate of stroke recurrence in patients with primary intracerebral hemorrhage. Stroke. 2000;31:123-7.
7. Steiner T et al. European Stroke Organization (ESO) guidelines for the management of spontaneous cerebral hemorrhage. Int J Stroke. 2014;9:840-55.
8. Murthy SB et al. Restarting anticoagulation therapy after intracranial hemorrhage: A systematic review and meta-analysis. Stroke. 2017 Jun;48:1594-600.
9. Biffi A et al. Oral anticoagulation and functional outcome after intracerebral hemorrhage. Ann Neurol. 2017 Nov;82:755-65.
10. Witt DM. What to do after the bleed: Resuming anticoagulation after major bleeding. Hematology Am Soc Hematol Educ Program. 2016 Dec 2;206:620-4.
11. Pennlert J et al. Optimal timing of anticoagulant treatment after intracerebral hemorrhage in patients with atrial fibrillation. Stroke. 2017 Feb;48:314-20.
Key Points
- Robust scientific data on when to resume anticoagulation after ICH does not exist.
- Retrospective studies have shown that anticoagulation resumption after 4-8 weeks decreases the risk of thromboembolic events, decreases mortality, and improves functional status following ICH with no significant change in the risk of its recurrence.
- Prospective, randomized controlled trials are needed to explore risks/benefits of anticoagulation resumption and better define its optimal timing in relation to ICH.
Quiz
Which of the following is false regarding ICH?
A. Lobar ICHs are usually associated with cerebral amyloid angiopathy which are prone to bleeding.
B. Randomized, controlled studies have helped guide the decision as to when to resume anticoagulation in patients with ICH.
C. Current guidelines suggest deferring therapeutic anticoagulation for at least 4 weeks following ICH.
D. Resumption of anticoagulation after 4-8 weeks does not lead to increased risk of rebleeding in patients with prior ICH.
The false answer is B: Current recommendations regarding resumption of anticoagulation in patients with ICH are based solely on retrospective observational studies; there are no randomized, control trials to date.
A is true: In contrast to hypertensive vessel disease associated with deep ICH, lobar hemorrhages are usually associated with cerebral amyloid angiopathy, which are more prone to bleeding.
C is true: The AHA/ASA has a class IIB recommendation to avoid anticoagulation for at least 4 weeks after ICH in patients without mechanical heart valves.
D is true: Several studies have shown that resumption of anticoagulation 4-8 weeks after ICH does not increase the risk of rebleeding.
Positive FIT test should prompt colonoscopy
Patients who test positive on a fecal immunochemical test (FIT), even after a recent colonoscopy, should be offered a repeat colonoscopy. That is the conclusion following a review of 2,228 subjects who were FIT positive, which revealed a greater risk of colorectal cancer (CRC) and advanced colorectal neoplasia (ACRN) the longer the gap since the last colonoscopy. The findings support the recommendations of the U.S. Multi-Society Task Force on CRC Screening to offer repeat colonoscopies to FIT-positive patients, even if they recently underwent a colonoscopy.
That recommendation was based on low-quality supporting evidence, and there is currently little agreement about whether annual FIT should be performed along with colonoscopy.
The researchers set out to detect the frequency of CRC and ACRN among patients with a positive FIT test. They analyzed data from the National Cancer Screening Program in Korea, which offers an annual FIT for adults aged 50 years and older as an initial screening, followed by a colonoscopy in case of a positive result.
The researchers analyzed data from 52,376 individuals who underwent FIT at a single center in Korea during January 2013–July 2017. They excluded patients with a history of CRC or colorectal surgery, inflammatory bowel disease, or poor bowel preparation.
FIT-positive and FIT-negative patients were divided into three groups based on the length of time since their last colonoscopy: less than 3 years, 3-10 years, or more than 10 years or no colonoscopy.
Compared with FIT-negative subjects, FIT-positive individuals were more likely to be diagnosed with any colorectal neoplasia (61.3% vs. 51.8%; P less than .001), ACRN (20.0% vs. 10.3%; P less than .001), and CRC (5.0% vs. 1.9%; P less than .001).
A total of 6% of subjects had a positive FIT result, and data from 2,228 were analyzed after exclusions. They were compared with 6,135 participants who had negative FIT results but underwent a colonoscopy.
Of patients with a positive FIT result, 23.1% had a colonoscopy less than 3 years before, 19.2% had one 3-10 years prior, and 57.8% had a colonoscopy more than 10 years earlier or had never had one.
The more-than-10-year group had a higher frequency of colorectal neoplasia, ACRN, or CRC (26.0%) than did the 3-10-year group (12.6%), and the less-than-3-year group (10.9%; P less than .001 for all). A similar trend was seen for CRC: 7.2%, 1.6%, and 2.1%, respectively (P less than .001).
Of the 6,135 FIT-negative participants, 22.2% were in the less-than-3-years group, 28.9% 3-10 years, and 48.8% more-than-10 years-or-never group. The more-than-10-years group had a higher frequency of ACRN (14.7%) than did the 3-10-year group (0.4%) and the 0-3-year group (0.7%, P less than .001).
Among FIT-positive patients, the more-than-10-year group was at higher risk of ACRN diagnosis during follow-up colonoscopy than was the less-than-3-year group (adjusted OR, 3.63; 95% confidence interval, 2.48-5.31), but not compared with the 3-10-year group (aOR, 1.17; 95% CI, 0.71-1.93). The more-than-10-year group also was at greater risk of a CRC diagnosis than was the less-than-3-year group (aOR, 3.66; 95% CI, 1.74-7.73). There was no significant difference in CRC risk between the less-than-3-year group and the 3-10-year group (aOR, 0.58; 95% CI, 0.17-1.93).
The authors suggest that CRC and ACRN found in patients who had a colonoscopy in the past 3 years are likely to be lesions that were missed in the previous exam, rather than new, fast-growing lesions. That suggests that FIT may help catch lesions that were missed during earlier screenings, though just 2.1% of the less-than-3-year group and 1.6% of the 3-10-year group were diagnosed with CRC, and 10.9% and 12.6% with ACRN, respectively.
The authors conclude that it may not be appropriate to offer interval FIT to all patients, since it can lead to unnecessary colonoscopies. They call for more research to determine which categories of patients are most likely to benefit from interval FIT.
SOURCE: Kim NH et al. Gastrointest Endosc. 2019 Jan 23. doi: 10.1016/j.gie.2019.01.012.
Patients who test positive on a fecal immunochemical test (FIT), even after a recent colonoscopy, should be offered a repeat colonoscopy. That is the conclusion following a review of 2,228 subjects who were FIT positive, which revealed a greater risk of colorectal cancer (CRC) and advanced colorectal neoplasia (ACRN) the longer the gap since the last colonoscopy. The findings support the recommendations of the U.S. Multi-Society Task Force on CRC Screening to offer repeat colonoscopies to FIT-positive patients, even if they recently underwent a colonoscopy.
That recommendation was based on low-quality supporting evidence, and there is currently little agreement about whether annual FIT should be performed along with colonoscopy.
The researchers set out to detect the frequency of CRC and ACRN among patients with a positive FIT test. They analyzed data from the National Cancer Screening Program in Korea, which offers an annual FIT for adults aged 50 years and older as an initial screening, followed by a colonoscopy in case of a positive result.
The researchers analyzed data from 52,376 individuals who underwent FIT at a single center in Korea during January 2013–July 2017. They excluded patients with a history of CRC or colorectal surgery, inflammatory bowel disease, or poor bowel preparation.
FIT-positive and FIT-negative patients were divided into three groups based on the length of time since their last colonoscopy: less than 3 years, 3-10 years, or more than 10 years or no colonoscopy.
Compared with FIT-negative subjects, FIT-positive individuals were more likely to be diagnosed with any colorectal neoplasia (61.3% vs. 51.8%; P less than .001), ACRN (20.0% vs. 10.3%; P less than .001), and CRC (5.0% vs. 1.9%; P less than .001).
A total of 6% of subjects had a positive FIT result, and data from 2,228 were analyzed after exclusions. They were compared with 6,135 participants who had negative FIT results but underwent a colonoscopy.
Of patients with a positive FIT result, 23.1% had a colonoscopy less than 3 years before, 19.2% had one 3-10 years prior, and 57.8% had a colonoscopy more than 10 years earlier or had never had one.
The more-than-10-year group had a higher frequency of colorectal neoplasia, ACRN, or CRC (26.0%) than did the 3-10-year group (12.6%), and the less-than-3-year group (10.9%; P less than .001 for all). A similar trend was seen for CRC: 7.2%, 1.6%, and 2.1%, respectively (P less than .001).
Of the 6,135 FIT-negative participants, 22.2% were in the less-than-3-years group, 28.9% 3-10 years, and 48.8% more-than-10 years-or-never group. The more-than-10-years group had a higher frequency of ACRN (14.7%) than did the 3-10-year group (0.4%) and the 0-3-year group (0.7%, P less than .001).
Among FIT-positive patients, the more-than-10-year group was at higher risk of ACRN diagnosis during follow-up colonoscopy than was the less-than-3-year group (adjusted OR, 3.63; 95% confidence interval, 2.48-5.31), but not compared with the 3-10-year group (aOR, 1.17; 95% CI, 0.71-1.93). The more-than-10-year group also was at greater risk of a CRC diagnosis than was the less-than-3-year group (aOR, 3.66; 95% CI, 1.74-7.73). There was no significant difference in CRC risk between the less-than-3-year group and the 3-10-year group (aOR, 0.58; 95% CI, 0.17-1.93).
The authors suggest that CRC and ACRN found in patients who had a colonoscopy in the past 3 years are likely to be lesions that were missed in the previous exam, rather than new, fast-growing lesions. That suggests that FIT may help catch lesions that were missed during earlier screenings, though just 2.1% of the less-than-3-year group and 1.6% of the 3-10-year group were diagnosed with CRC, and 10.9% and 12.6% with ACRN, respectively.
The authors conclude that it may not be appropriate to offer interval FIT to all patients, since it can lead to unnecessary colonoscopies. They call for more research to determine which categories of patients are most likely to benefit from interval FIT.
SOURCE: Kim NH et al. Gastrointest Endosc. 2019 Jan 23. doi: 10.1016/j.gie.2019.01.012.
Patients who test positive on a fecal immunochemical test (FIT), even after a recent colonoscopy, should be offered a repeat colonoscopy. That is the conclusion following a review of 2,228 subjects who were FIT positive, which revealed a greater risk of colorectal cancer (CRC) and advanced colorectal neoplasia (ACRN) the longer the gap since the last colonoscopy. The findings support the recommendations of the U.S. Multi-Society Task Force on CRC Screening to offer repeat colonoscopies to FIT-positive patients, even if they recently underwent a colonoscopy.
That recommendation was based on low-quality supporting evidence, and there is currently little agreement about whether annual FIT should be performed along with colonoscopy.
The researchers set out to detect the frequency of CRC and ACRN among patients with a positive FIT test. They analyzed data from the National Cancer Screening Program in Korea, which offers an annual FIT for adults aged 50 years and older as an initial screening, followed by a colonoscopy in case of a positive result.
The researchers analyzed data from 52,376 individuals who underwent FIT at a single center in Korea during January 2013–July 2017. They excluded patients with a history of CRC or colorectal surgery, inflammatory bowel disease, or poor bowel preparation.
FIT-positive and FIT-negative patients were divided into three groups based on the length of time since their last colonoscopy: less than 3 years, 3-10 years, or more than 10 years or no colonoscopy.
Compared with FIT-negative subjects, FIT-positive individuals were more likely to be diagnosed with any colorectal neoplasia (61.3% vs. 51.8%; P less than .001), ACRN (20.0% vs. 10.3%; P less than .001), and CRC (5.0% vs. 1.9%; P less than .001).
A total of 6% of subjects had a positive FIT result, and data from 2,228 were analyzed after exclusions. They were compared with 6,135 participants who had negative FIT results but underwent a colonoscopy.
Of patients with a positive FIT result, 23.1% had a colonoscopy less than 3 years before, 19.2% had one 3-10 years prior, and 57.8% had a colonoscopy more than 10 years earlier or had never had one.
The more-than-10-year group had a higher frequency of colorectal neoplasia, ACRN, or CRC (26.0%) than did the 3-10-year group (12.6%), and the less-than-3-year group (10.9%; P less than .001 for all). A similar trend was seen for CRC: 7.2%, 1.6%, and 2.1%, respectively (P less than .001).
Of the 6,135 FIT-negative participants, 22.2% were in the less-than-3-years group, 28.9% 3-10 years, and 48.8% more-than-10 years-or-never group. The more-than-10-years group had a higher frequency of ACRN (14.7%) than did the 3-10-year group (0.4%) and the 0-3-year group (0.7%, P less than .001).
Among FIT-positive patients, the more-than-10-year group was at higher risk of ACRN diagnosis during follow-up colonoscopy than was the less-than-3-year group (adjusted OR, 3.63; 95% confidence interval, 2.48-5.31), but not compared with the 3-10-year group (aOR, 1.17; 95% CI, 0.71-1.93). The more-than-10-year group also was at greater risk of a CRC diagnosis than was the less-than-3-year group (aOR, 3.66; 95% CI, 1.74-7.73). There was no significant difference in CRC risk between the less-than-3-year group and the 3-10-year group (aOR, 0.58; 95% CI, 0.17-1.93).
The authors suggest that CRC and ACRN found in patients who had a colonoscopy in the past 3 years are likely to be lesions that were missed in the previous exam, rather than new, fast-growing lesions. That suggests that FIT may help catch lesions that were missed during earlier screenings, though just 2.1% of the less-than-3-year group and 1.6% of the 3-10-year group were diagnosed with CRC, and 10.9% and 12.6% with ACRN, respectively.
The authors conclude that it may not be appropriate to offer interval FIT to all patients, since it can lead to unnecessary colonoscopies. They call for more research to determine which categories of patients are most likely to benefit from interval FIT.
SOURCE: Kim NH et al. Gastrointest Endosc. 2019 Jan 23. doi: 10.1016/j.gie.2019.01.012.
FROM GASTROINTESTINAL ENDOSCOPY
Key clinical point: Positive findings are linked to greater CRC and advanced colorectal neoplasia risk.
Major finding: Regardless of time since last colonoscopy, CRC and ACRN frequencies were higher in FIT-positive subjects.
Study details: Retrospective analysis of 2,228 FIT-positive and 6,135 FIT-negative subjects
Disclosures: The study received no funding. The authors reported no conflicts of interest.
Source: Kim NH et al. Gastrointest Endosc. 2019 Jan 23. doi: 10.1016/j.gie.2019.01.012.
AHA report highlights CVD burden, declines in smoking, sleep importance
Almost half of U.S. adults now have some form of cardiovascular disease, according to the latest annual statistical update from the American Heart Association.
The prevalence is driven in part by the recently changed definition of hypertension, from 140/90 to 130/80 mm Hg, said authors of the American Heart Association Heart Disease and Stroke Statistics–2019 Update.
Cardiovascular disease (CVD) deaths are up, though smoking rates continue to decline, and adults are getting more exercise (Circulation. 2019;139. doi: 10.1161/CIR.0000000000000659).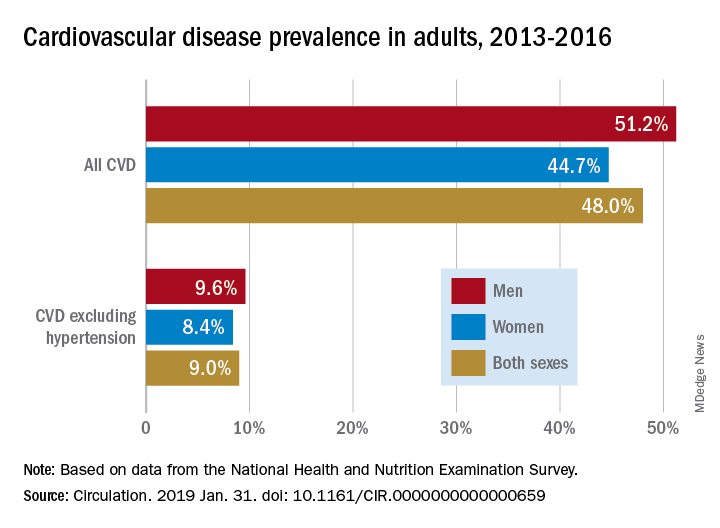
The update includes a new section on sleep and cardiovascular health, an enhanced focus on social determinants of health, and further evidence-based approaches to behavior change, according to the update’s authors, led by chair Emelia J. Benjamin, MD, professor of medicine and epidemiology at Boston University, and vice chair Paul Muntner, PhD, professor of epidemiology at the University of Alabama, Birmingham.
High blood pressure is an “overwhelming presence” that drives heart disease and stroke and can’t be dismissed in the fight against cardiovascular disease, AHA President Ivor J. Benjamin, MD, said in a statement. “Eliminating high blood pressure could have a larger impact on CVD deaths than the elimination of all other risk factors among women, and all except smoking among men.”
Using data from 2013 to 2016, 46% of adults in the United States had hypertension, and in 2016 there were 82,735 deaths attributable primarily to high blood pressure, according to the update.
Total direct costs of hypertension could approach $221 billion by 2035, according to projections in the report.
After decades of decline, U.S. cardiovascular disease deaths increased to 840,678 in 2016, up from 836,546 in 2015, the report says.
Smoking rate declines represent some of the most significant improvements outlined in the report, according to an AHA news release.
Ninety-four percent of adolescents were nonsmokers in the 2015-2016 period, which is up from 76% in 1999-2000, according to the report. The proportion of adult nonsmokers increased to 79% in 2015-2016, up from 73% in 1999-2000.
The new chapter on the importance of sleep cites data from the Centers for Disease Control and Prevention that only 65.2% of Americans have a healthy sleep duration (at least 7 hours), with even lower rates among non-Hispanic blacks, native Hawaiians and Pacific Islanders, and multiracial non-Hispanic individuals.
Short sleep duration is associated with a higher risk of all-cause mortality, total CVD, and coronary heart disease, according to a meta-analysis cited in the report. Long sleep duration, defined as greater than 8 hours, also was associated with higher risk of all-cause mortality, total CVD, coronary heart disease, and stroke.
Members of the statistical update writing group reported disclosures related to the American Heart Association, National Institutes of Health, Amgen, Sanofi, Roche, Abbott, Biogen, Medtronic, and others.
SOURCE: Benjamin EJ et al. Circulation. 2019 Jan 31.
The latest statistics on heart disease and stroke include some metrics that indicate progress, and others that suggest opportunities for improvement.
Tobacco use continues to decline; however, among high school students, e-cigarette use is up to 11.3%, which is concerning.
One bright spot is that the proportion of inactive adults has dropped to 30% in 2016, down from 40% in 2007. Despite that improvement, however, the prevalence of obesity increased significantly over the decade, to the point where nearly 40% of adults are obese and 7.7% are severely obese.
Although 48% of U.S. adults now have cardiovascular disease, according to this latest update, the number drops to just 9% when hypertension is excluded. Even so, 9% represents more than 24.3 million Americans who have coronary artery disease, stroke, or heart failure.
The cost of cardiovascular disease is astronomical, exceeding $351 billion in 2014-1205, with costs projected to increase sharply for older adults over the next few decades.
Starting in 2020, the AHA will begin charting progress in CVD using a metric called health-adjusted life expectancy (HALE), which relies on morbidity and mortality patterns to reflect the number of years a person can expect to live. Patients and the general public may find this metric more understandable than statistics about death rates and cardiovascular risk factors.
Mariell Jessup, MD, is chief science and medical officer for the American Heart Association. Her view on the latest statistical update was derived from a commentary that accompanied the update.
The latest statistics on heart disease and stroke include some metrics that indicate progress, and others that suggest opportunities for improvement.
Tobacco use continues to decline; however, among high school students, e-cigarette use is up to 11.3%, which is concerning.
One bright spot is that the proportion of inactive adults has dropped to 30% in 2016, down from 40% in 2007. Despite that improvement, however, the prevalence of obesity increased significantly over the decade, to the point where nearly 40% of adults are obese and 7.7% are severely obese.
Although 48% of U.S. adults now have cardiovascular disease, according to this latest update, the number drops to just 9% when hypertension is excluded. Even so, 9% represents more than 24.3 million Americans who have coronary artery disease, stroke, or heart failure.
The cost of cardiovascular disease is astronomical, exceeding $351 billion in 2014-1205, with costs projected to increase sharply for older adults over the next few decades.
Starting in 2020, the AHA will begin charting progress in CVD using a metric called health-adjusted life expectancy (HALE), which relies on morbidity and mortality patterns to reflect the number of years a person can expect to live. Patients and the general public may find this metric more understandable than statistics about death rates and cardiovascular risk factors.
Mariell Jessup, MD, is chief science and medical officer for the American Heart Association. Her view on the latest statistical update was derived from a commentary that accompanied the update.
The latest statistics on heart disease and stroke include some metrics that indicate progress, and others that suggest opportunities for improvement.
Tobacco use continues to decline; however, among high school students, e-cigarette use is up to 11.3%, which is concerning.
One bright spot is that the proportion of inactive adults has dropped to 30% in 2016, down from 40% in 2007. Despite that improvement, however, the prevalence of obesity increased significantly over the decade, to the point where nearly 40% of adults are obese and 7.7% are severely obese.
Although 48% of U.S. adults now have cardiovascular disease, according to this latest update, the number drops to just 9% when hypertension is excluded. Even so, 9% represents more than 24.3 million Americans who have coronary artery disease, stroke, or heart failure.
The cost of cardiovascular disease is astronomical, exceeding $351 billion in 2014-1205, with costs projected to increase sharply for older adults over the next few decades.
Starting in 2020, the AHA will begin charting progress in CVD using a metric called health-adjusted life expectancy (HALE), which relies on morbidity and mortality patterns to reflect the number of years a person can expect to live. Patients and the general public may find this metric more understandable than statistics about death rates and cardiovascular risk factors.
Mariell Jessup, MD, is chief science and medical officer for the American Heart Association. Her view on the latest statistical update was derived from a commentary that accompanied the update.
Almost half of U.S. adults now have some form of cardiovascular disease, according to the latest annual statistical update from the American Heart Association.
The prevalence is driven in part by the recently changed definition of hypertension, from 140/90 to 130/80 mm Hg, said authors of the American Heart Association Heart Disease and Stroke Statistics–2019 Update.
Cardiovascular disease (CVD) deaths are up, though smoking rates continue to decline, and adults are getting more exercise (Circulation. 2019;139. doi: 10.1161/CIR.0000000000000659).
The update includes a new section on sleep and cardiovascular health, an enhanced focus on social determinants of health, and further evidence-based approaches to behavior change, according to the update’s authors, led by chair Emelia J. Benjamin, MD, professor of medicine and epidemiology at Boston University, and vice chair Paul Muntner, PhD, professor of epidemiology at the University of Alabama, Birmingham.
High blood pressure is an “overwhelming presence” that drives heart disease and stroke and can’t be dismissed in the fight against cardiovascular disease, AHA President Ivor J. Benjamin, MD, said in a statement. “Eliminating high blood pressure could have a larger impact on CVD deaths than the elimination of all other risk factors among women, and all except smoking among men.”
Using data from 2013 to 2016, 46% of adults in the United States had hypertension, and in 2016 there were 82,735 deaths attributable primarily to high blood pressure, according to the update.
Total direct costs of hypertension could approach $221 billion by 2035, according to projections in the report.
After decades of decline, U.S. cardiovascular disease deaths increased to 840,678 in 2016, up from 836,546 in 2015, the report says.
Smoking rate declines represent some of the most significant improvements outlined in the report, according to an AHA news release.
Ninety-four percent of adolescents were nonsmokers in the 2015-2016 period, which is up from 76% in 1999-2000, according to the report. The proportion of adult nonsmokers increased to 79% in 2015-2016, up from 73% in 1999-2000.
The new chapter on the importance of sleep cites data from the Centers for Disease Control and Prevention that only 65.2% of Americans have a healthy sleep duration (at least 7 hours), with even lower rates among non-Hispanic blacks, native Hawaiians and Pacific Islanders, and multiracial non-Hispanic individuals.
Short sleep duration is associated with a higher risk of all-cause mortality, total CVD, and coronary heart disease, according to a meta-analysis cited in the report. Long sleep duration, defined as greater than 8 hours, also was associated with higher risk of all-cause mortality, total CVD, coronary heart disease, and stroke.
Members of the statistical update writing group reported disclosures related to the American Heart Association, National Institutes of Health, Amgen, Sanofi, Roche, Abbott, Biogen, Medtronic, and others.
SOURCE: Benjamin EJ et al. Circulation. 2019 Jan 31.
Almost half of U.S. adults now have some form of cardiovascular disease, according to the latest annual statistical update from the American Heart Association.
The prevalence is driven in part by the recently changed definition of hypertension, from 140/90 to 130/80 mm Hg, said authors of the American Heart Association Heart Disease and Stroke Statistics–2019 Update.
Cardiovascular disease (CVD) deaths are up, though smoking rates continue to decline, and adults are getting more exercise (Circulation. 2019;139. doi: 10.1161/CIR.0000000000000659).
The update includes a new section on sleep and cardiovascular health, an enhanced focus on social determinants of health, and further evidence-based approaches to behavior change, according to the update’s authors, led by chair Emelia J. Benjamin, MD, professor of medicine and epidemiology at Boston University, and vice chair Paul Muntner, PhD, professor of epidemiology at the University of Alabama, Birmingham.
High blood pressure is an “overwhelming presence” that drives heart disease and stroke and can’t be dismissed in the fight against cardiovascular disease, AHA President Ivor J. Benjamin, MD, said in a statement. “Eliminating high blood pressure could have a larger impact on CVD deaths than the elimination of all other risk factors among women, and all except smoking among men.”
Using data from 2013 to 2016, 46% of adults in the United States had hypertension, and in 2016 there were 82,735 deaths attributable primarily to high blood pressure, according to the update.
Total direct costs of hypertension could approach $221 billion by 2035, according to projections in the report.
After decades of decline, U.S. cardiovascular disease deaths increased to 840,678 in 2016, up from 836,546 in 2015, the report says.
Smoking rate declines represent some of the most significant improvements outlined in the report, according to an AHA news release.
Ninety-four percent of adolescents were nonsmokers in the 2015-2016 period, which is up from 76% in 1999-2000, according to the report. The proportion of adult nonsmokers increased to 79% in 2015-2016, up from 73% in 1999-2000.
The new chapter on the importance of sleep cites data from the Centers for Disease Control and Prevention that only 65.2% of Americans have a healthy sleep duration (at least 7 hours), with even lower rates among non-Hispanic blacks, native Hawaiians and Pacific Islanders, and multiracial non-Hispanic individuals.
Short sleep duration is associated with a higher risk of all-cause mortality, total CVD, and coronary heart disease, according to a meta-analysis cited in the report. Long sleep duration, defined as greater than 8 hours, also was associated with higher risk of all-cause mortality, total CVD, coronary heart disease, and stroke.
Members of the statistical update writing group reported disclosures related to the American Heart Association, National Institutes of Health, Amgen, Sanofi, Roche, Abbott, Biogen, Medtronic, and others.
SOURCE: Benjamin EJ et al. Circulation. 2019 Jan 31.
FROM CIRCULATION
Pulmonary hypertension linked to complications after head and neck procedures
SAN DIEGO – Patients with , compared with their counterparts who do not have the condition. They also face an increase in total charges and length of stay.
The findings come from what is believed to be the first study of its kind to investigate the impact of pulmonary hypertension (PHTN) on major head and neck procedures. “PHTN is a common condition which affects the caliber of lung vasculature, with varied symptom presentation from shortness of breath to syncope, with an estimated prevalence of 2.5 to 7.1 million new cases each year worldwide,” one of the study authors, Nirali M. Patel, said at the Triological Society’s Combined Sections Meeting. “Due to improved therapeutic options, there is an enhanced survival of PHTN patients and higher prevalence of this disease. PHTN has some significant systemic implications. Therefore, cardiopulmonary clearance and a clear understanding of postoperative complications are very important.”
Previous studies have shown PHTN to be an independent predictor of morbidity and mortality in noncardiac procedures, said Ms. Patel, a fourth-year medical student at New Jersey Medical School, Newark. Furthermore, the rate of pulmonary complications is reported to be between 11% and 44.8% following radical head and neck cancer resection. “Despite these findings, there is currently a lack of information regarding perioperative morbidity and mortality of patients with PHTN undergoing major head and neck procedures,” she said.
The researchers queried the National Inpatient Survey from 2002 to 2013 for all cases of major head and neck surgery based on the ICD-9 codes for esophagectomy, glossectomy, laryngectomy, mandibulectomy, and pharyngectomy. They divided patients into two groups: those who had PHTN and those who did not, and performed demographic analyses as well as univariate and multivariate regression analyses.
Ms. Patel reported findings from a cohort of 46,311 patients. Of these, 46,073 had PHTN and 238 did not. The two groups were similar in age (a mean of 69 vs. 60 years in those with and without PHTN, respectively) and race (80% white vs. 79% white, respectively), but there were significantly fewer male patients in the PHTN group (57% vs. 70%; P less than .0001).
Several patient comorbidities were increased in the PHTN group, compared with the non-PHTN group, including coagulopathy (8.4% vs. 3.1%; P less than .0001), chronic heart failure (22.4% vs. 4.1%; P less than .0001), complicated diabetes (4.6% vs. 1.2%; P less than .0001), fluid and electrolyte disorders (30% vs. 18.3%; P less than .0001), and hypertension (63.3% vs. 43.7%; P less than .0001).
Postoperatively, patients with PHTN had a longer length of stay (a mean of 15.80 vs. 11.50 days, respectively; P less than .0001) as well as significantly higher total charges (a mean of $162,021.06 vs. $107,309.46, respectively; P less than .0001). When the researchers evaluated postoperative outcomes between the two cohorts, patients with PHTN had significantly higher rates of cardiac complications (9.2% vs. 3.5%; P less than .0001), iatrogenic pulmonary embolism (2.1% vs. 0.3%; P = .001), pulmonary edema (2.1% vs. 0.4%; P = .003), venous thrombotic events (4.6% vs. 1%; P less than. 0001), pulmonary insufficiency (17.2% vs. 9.7%; P less than .0001), and pneumonia (9.7% vs. 6%; P = .027), as well as higher rates of postoperative tracheostomy (8.4% vs. 4.5%; P = .007).
Multivariate analysis revealed that the following factors predicted in-hospital mortality: coagulopathy, chronic heart failure, fluid and electrolyte disorders, hyperlipidemia, hypertension, hypothyroidism, liver disease, obesity, paralysis, and renal failure. Despite these findings, there was no significant change in overall hospital mortality for those with PHTN, with an odds ratio of 1.055.
“Our study is not without its limitations, many of which are inherent to the use of a database, which is subject to errors in coding and sampling,” Ms. Patel noted at the meeting, which was jointly sponsored by the Triological Society and the American College of Surgeons. “Despite these limitations, the study provides valuable information on the impact of PHTN on major head and neck procedures.
She reported having no financial disclosures.
SOURCE: Patel N et al. Triological CSM, Abstracts.
SAN DIEGO – Patients with , compared with their counterparts who do not have the condition. They also face an increase in total charges and length of stay.
The findings come from what is believed to be the first study of its kind to investigate the impact of pulmonary hypertension (PHTN) on major head and neck procedures. “PHTN is a common condition which affects the caliber of lung vasculature, with varied symptom presentation from shortness of breath to syncope, with an estimated prevalence of 2.5 to 7.1 million new cases each year worldwide,” one of the study authors, Nirali M. Patel, said at the Triological Society’s Combined Sections Meeting. “Due to improved therapeutic options, there is an enhanced survival of PHTN patients and higher prevalence of this disease. PHTN has some significant systemic implications. Therefore, cardiopulmonary clearance and a clear understanding of postoperative complications are very important.”
Previous studies have shown PHTN to be an independent predictor of morbidity and mortality in noncardiac procedures, said Ms. Patel, a fourth-year medical student at New Jersey Medical School, Newark. Furthermore, the rate of pulmonary complications is reported to be between 11% and 44.8% following radical head and neck cancer resection. “Despite these findings, there is currently a lack of information regarding perioperative morbidity and mortality of patients with PHTN undergoing major head and neck procedures,” she said.
The researchers queried the National Inpatient Survey from 2002 to 2013 for all cases of major head and neck surgery based on the ICD-9 codes for esophagectomy, glossectomy, laryngectomy, mandibulectomy, and pharyngectomy. They divided patients into two groups: those who had PHTN and those who did not, and performed demographic analyses as well as univariate and multivariate regression analyses.
Ms. Patel reported findings from a cohort of 46,311 patients. Of these, 46,073 had PHTN and 238 did not. The two groups were similar in age (a mean of 69 vs. 60 years in those with and without PHTN, respectively) and race (80% white vs. 79% white, respectively), but there were significantly fewer male patients in the PHTN group (57% vs. 70%; P less than .0001).
Several patient comorbidities were increased in the PHTN group, compared with the non-PHTN group, including coagulopathy (8.4% vs. 3.1%; P less than .0001), chronic heart failure (22.4% vs. 4.1%; P less than .0001), complicated diabetes (4.6% vs. 1.2%; P less than .0001), fluid and electrolyte disorders (30% vs. 18.3%; P less than .0001), and hypertension (63.3% vs. 43.7%; P less than .0001).
Postoperatively, patients with PHTN had a longer length of stay (a mean of 15.80 vs. 11.50 days, respectively; P less than .0001) as well as significantly higher total charges (a mean of $162,021.06 vs. $107,309.46, respectively; P less than .0001). When the researchers evaluated postoperative outcomes between the two cohorts, patients with PHTN had significantly higher rates of cardiac complications (9.2% vs. 3.5%; P less than .0001), iatrogenic pulmonary embolism (2.1% vs. 0.3%; P = .001), pulmonary edema (2.1% vs. 0.4%; P = .003), venous thrombotic events (4.6% vs. 1%; P less than. 0001), pulmonary insufficiency (17.2% vs. 9.7%; P less than .0001), and pneumonia (9.7% vs. 6%; P = .027), as well as higher rates of postoperative tracheostomy (8.4% vs. 4.5%; P = .007).
Multivariate analysis revealed that the following factors predicted in-hospital mortality: coagulopathy, chronic heart failure, fluid and electrolyte disorders, hyperlipidemia, hypertension, hypothyroidism, liver disease, obesity, paralysis, and renal failure. Despite these findings, there was no significant change in overall hospital mortality for those with PHTN, with an odds ratio of 1.055.
“Our study is not without its limitations, many of which are inherent to the use of a database, which is subject to errors in coding and sampling,” Ms. Patel noted at the meeting, which was jointly sponsored by the Triological Society and the American College of Surgeons. “Despite these limitations, the study provides valuable information on the impact of PHTN on major head and neck procedures.
She reported having no financial disclosures.
SOURCE: Patel N et al. Triological CSM, Abstracts.
SAN DIEGO – Patients with , compared with their counterparts who do not have the condition. They also face an increase in total charges and length of stay.
The findings come from what is believed to be the first study of its kind to investigate the impact of pulmonary hypertension (PHTN) on major head and neck procedures. “PHTN is a common condition which affects the caliber of lung vasculature, with varied symptom presentation from shortness of breath to syncope, with an estimated prevalence of 2.5 to 7.1 million new cases each year worldwide,” one of the study authors, Nirali M. Patel, said at the Triological Society’s Combined Sections Meeting. “Due to improved therapeutic options, there is an enhanced survival of PHTN patients and higher prevalence of this disease. PHTN has some significant systemic implications. Therefore, cardiopulmonary clearance and a clear understanding of postoperative complications are very important.”
Previous studies have shown PHTN to be an independent predictor of morbidity and mortality in noncardiac procedures, said Ms. Patel, a fourth-year medical student at New Jersey Medical School, Newark. Furthermore, the rate of pulmonary complications is reported to be between 11% and 44.8% following radical head and neck cancer resection. “Despite these findings, there is currently a lack of information regarding perioperative morbidity and mortality of patients with PHTN undergoing major head and neck procedures,” she said.
The researchers queried the National Inpatient Survey from 2002 to 2013 for all cases of major head and neck surgery based on the ICD-9 codes for esophagectomy, glossectomy, laryngectomy, mandibulectomy, and pharyngectomy. They divided patients into two groups: those who had PHTN and those who did not, and performed demographic analyses as well as univariate and multivariate regression analyses.
Ms. Patel reported findings from a cohort of 46,311 patients. Of these, 46,073 had PHTN and 238 did not. The two groups were similar in age (a mean of 69 vs. 60 years in those with and without PHTN, respectively) and race (80% white vs. 79% white, respectively), but there were significantly fewer male patients in the PHTN group (57% vs. 70%; P less than .0001).
Several patient comorbidities were increased in the PHTN group, compared with the non-PHTN group, including coagulopathy (8.4% vs. 3.1%; P less than .0001), chronic heart failure (22.4% vs. 4.1%; P less than .0001), complicated diabetes (4.6% vs. 1.2%; P less than .0001), fluid and electrolyte disorders (30% vs. 18.3%; P less than .0001), and hypertension (63.3% vs. 43.7%; P less than .0001).
Postoperatively, patients with PHTN had a longer length of stay (a mean of 15.80 vs. 11.50 days, respectively; P less than .0001) as well as significantly higher total charges (a mean of $162,021.06 vs. $107,309.46, respectively; P less than .0001). When the researchers evaluated postoperative outcomes between the two cohorts, patients with PHTN had significantly higher rates of cardiac complications (9.2% vs. 3.5%; P less than .0001), iatrogenic pulmonary embolism (2.1% vs. 0.3%; P = .001), pulmonary edema (2.1% vs. 0.4%; P = .003), venous thrombotic events (4.6% vs. 1%; P less than. 0001), pulmonary insufficiency (17.2% vs. 9.7%; P less than .0001), and pneumonia (9.7% vs. 6%; P = .027), as well as higher rates of postoperative tracheostomy (8.4% vs. 4.5%; P = .007).
Multivariate analysis revealed that the following factors predicted in-hospital mortality: coagulopathy, chronic heart failure, fluid and electrolyte disorders, hyperlipidemia, hypertension, hypothyroidism, liver disease, obesity, paralysis, and renal failure. Despite these findings, there was no significant change in overall hospital mortality for those with PHTN, with an odds ratio of 1.055.
“Our study is not without its limitations, many of which are inherent to the use of a database, which is subject to errors in coding and sampling,” Ms. Patel noted at the meeting, which was jointly sponsored by the Triological Society and the American College of Surgeons. “Despite these limitations, the study provides valuable information on the impact of PHTN on major head and neck procedures.
She reported having no financial disclosures.
SOURCE: Patel N et al. Triological CSM, Abstracts.
REPORTING FROM THE TRIOLOGICAL CSM
Key clinical point: Pulmonary hypertension (PHTN) is linked with certain head and neck surgical complications.
Major finding: For patients undergoing head and neck surgery, PHTN is associated with an increased risk of cardiac complications (9.2% vs. 3.5%) and iatrogenic pulmonary embolism (2.1% vs. 0.3%).
Study details: A retrospective analysis of 46,311 patient records from the National Inpatient Survey.
Disclosures: The researchers reported having no financial disclosures.
Source: Patel N et al. Triological CSM, Abstracts.
Risk models fail to predict lower-GI bleeding outcomes
In cases of lower gastrointestinal bleeding (LGIB), albumin and hemoglobin levels are the best independent predictors of severe bleeding, according to investigators.
These findings came from a sobering look at LGIB risk-prediction models. While some models could predict specific outcomes with reasonable accuracy, none of the models demonstrated broad predictive power, reported Natalie Tapaskar, MD, of the department of medicine at the University of Chicago, and her colleagues.
LGIB requires intensive resource utilization and proves fatal in 5%-15% of patients, which means timely and appropriate interventions are essential, especially for those with severe bleeding.
“There are limited data on accurately predicting the risk of adverse outcomes for hospitalized patients with LGIB,” the investigators wrote in Gastrointestinal Endoscopy, “especially in comparison to patients with upper gastrointestinal bleeding (UGIB), where tools such as the Glasgow-Blatchford Bleeding Score have been validated to accurately predict important clinical outcomes.”
To assess existing risk models for LGIB, the investigators performed a prospective observational study involving 170 patients with LGIB who underwent colonoscopy during April 2016–September 2017 at the University of Chicago Medical Center. Data were collected through comprehensive medical record review.
The primary outcome was severe bleeding. This was defined by acute bleeding during the first 24 hours of admission that required a transfusion of 2 or more units of packed red blood cells, and/or caused a 20% or greater decrease in hematocrit; and/or recurrent bleeding 24 hours after clinical stability, involving rectal bleeding with an additional drop in hematocrit of 20% or more, and/or readmission for LGIB within 1 week of discharge. Secondary outcomes included blood transfusion requirements, in-hospital recurrent bleeding, length of stay, ICU admission, intervention (surgery, interventional radiology, endoscopy), and the comparative predictive ability of seven clinical risk stratification models: AIMS65, Charlson Comorbidity Index, Glasgow-Blatchford, NOBLADS, Oakland, Sengupta, and Strate. Area under the receiver operating characteristic curve (AUC) was used to compare model predictive power. Risk of adverse outcomes was calculated by univariable and multivariable logistic regression.
Results showed that median patient age was 70 years. Most of the patients (80%) were African American and slightly more than half were female (58%). These demographic factors were not predictive of severe bleeding, which occurred in about half of the cases (52%). Upon admission, patients with severe bleeding were more likely to have chronic renal failure (30% vs. 17%; P = .05), lower albumin (3.6 g/dL vs. 3.95 g/dL; P less than .0001), lower hemoglobin (8.6 g/dL vs. 11.1 g/dL; P = .0001), lower systolic blood pressure (118 mm Hg vs. 132 mm Hg; P = .01), and higher creatinine (1.3 mg/dL vs. 1 mg/dL; P = .04). After adjustment for confounding variables, the strongest independent predictors of severe bleeding were low albumin (odds ratio, 2.56 per 1-g/dL decrease; P = .02) and low hemoglobin (OR, 1.28 per 1-g/dL decrease; P = .0015).
On average, time between admission and colonoscopy was between 2 and 3 days (median, 62.2 hours). In 3 out of 4 patients (77%), etiology of LGIB was confirmed; diverticular bleeding was most common (39%), followed distantly by hemorrhoidal bleeding (15%).
Compared with milder cases, patients with severe bleeding were more likely to stay in the ICU (49% vs. 19%; P less than .0001), have a blood transfusion (85% vs 36%; P less than .0001), and need to remain in the hospital for a longer period of time (6 days vs. 4 days; P = .0009). These findings exemplify the high level of resource utilization required for LGIB and show how severe bleeding dramatically compounds intensity of care.
Further analysis showed that none of the seven risk models were predictive across all outcomes; however, some predicted specific outcomes better than others. Leaders were the Glasgow-Blatchford score for blood transfusion (AUC 0.87; P less than .0001), the Oakland score for severe bleeding (AUC 0.74; P less than .0001), the Sengupta score for ICU stay (AUC 0.74; P less than .0001), and the Strate score for both recurrent bleeding during hospital stay (AUC 0.66; P = .0008) and endoscopic intervention (AUC 0.62; P = .01).
The investigators noted that the Glasgow-Blatchford score, which also is used in cases of UGIB, has previously demonstrated accuracy in predicting blood transfusion, as it did in the present study, suggesting that, “[i]n instances where there may be uncertainty of the origin of the bleeding, the Blatchford score may be a preferential choice of risk score.”
“Overall, we found that no singular score performed best across all the outcomes studied nor did any score have an extremely strong discriminatory power for any individual variable,” the investigators wrote, concluding that “... simpler and more powerful prediction tools are required for better risk stratification in LGIB.”
The investigators reported no financial support or conflicts of interest.
*This story was updated on Jan. 31, 2019.
SOURCE: Tapaskar N et al. Gastrointest Endosc. 2018 Dec 18. doi: 10.1016/j.gie.2018.12.011.
In cases of lower gastrointestinal bleeding (LGIB), albumin and hemoglobin levels are the best independent predictors of severe bleeding, according to investigators.
These findings came from a sobering look at LGIB risk-prediction models. While some models could predict specific outcomes with reasonable accuracy, none of the models demonstrated broad predictive power, reported Natalie Tapaskar, MD, of the department of medicine at the University of Chicago, and her colleagues.
LGIB requires intensive resource utilization and proves fatal in 5%-15% of patients, which means timely and appropriate interventions are essential, especially for those with severe bleeding.
“There are limited data on accurately predicting the risk of adverse outcomes for hospitalized patients with LGIB,” the investigators wrote in Gastrointestinal Endoscopy, “especially in comparison to patients with upper gastrointestinal bleeding (UGIB), where tools such as the Glasgow-Blatchford Bleeding Score have been validated to accurately predict important clinical outcomes.”
To assess existing risk models for LGIB, the investigators performed a prospective observational study involving 170 patients with LGIB who underwent colonoscopy during April 2016–September 2017 at the University of Chicago Medical Center. Data were collected through comprehensive medical record review.
The primary outcome was severe bleeding. This was defined by acute bleeding during the first 24 hours of admission that required a transfusion of 2 or more units of packed red blood cells, and/or caused a 20% or greater decrease in hematocrit; and/or recurrent bleeding 24 hours after clinical stability, involving rectal bleeding with an additional drop in hematocrit of 20% or more, and/or readmission for LGIB within 1 week of discharge. Secondary outcomes included blood transfusion requirements, in-hospital recurrent bleeding, length of stay, ICU admission, intervention (surgery, interventional radiology, endoscopy), and the comparative predictive ability of seven clinical risk stratification models: AIMS65, Charlson Comorbidity Index, Glasgow-Blatchford, NOBLADS, Oakland, Sengupta, and Strate. Area under the receiver operating characteristic curve (AUC) was used to compare model predictive power. Risk of adverse outcomes was calculated by univariable and multivariable logistic regression.
Results showed that median patient age was 70 years. Most of the patients (80%) were African American and slightly more than half were female (58%). These demographic factors were not predictive of severe bleeding, which occurred in about half of the cases (52%). Upon admission, patients with severe bleeding were more likely to have chronic renal failure (30% vs. 17%; P = .05), lower albumin (3.6 g/dL vs. 3.95 g/dL; P less than .0001), lower hemoglobin (8.6 g/dL vs. 11.1 g/dL; P = .0001), lower systolic blood pressure (118 mm Hg vs. 132 mm Hg; P = .01), and higher creatinine (1.3 mg/dL vs. 1 mg/dL; P = .04). After adjustment for confounding variables, the strongest independent predictors of severe bleeding were low albumin (odds ratio, 2.56 per 1-g/dL decrease; P = .02) and low hemoglobin (OR, 1.28 per 1-g/dL decrease; P = .0015).
On average, time between admission and colonoscopy was between 2 and 3 days (median, 62.2 hours). In 3 out of 4 patients (77%), etiology of LGIB was confirmed; diverticular bleeding was most common (39%), followed distantly by hemorrhoidal bleeding (15%).
Compared with milder cases, patients with severe bleeding were more likely to stay in the ICU (49% vs. 19%; P less than .0001), have a blood transfusion (85% vs 36%; P less than .0001), and need to remain in the hospital for a longer period of time (6 days vs. 4 days; P = .0009). These findings exemplify the high level of resource utilization required for LGIB and show how severe bleeding dramatically compounds intensity of care.
Further analysis showed that none of the seven risk models were predictive across all outcomes; however, some predicted specific outcomes better than others. Leaders were the Glasgow-Blatchford score for blood transfusion (AUC 0.87; P less than .0001), the Oakland score for severe bleeding (AUC 0.74; P less than .0001), the Sengupta score for ICU stay (AUC 0.74; P less than .0001), and the Strate score for both recurrent bleeding during hospital stay (AUC 0.66; P = .0008) and endoscopic intervention (AUC 0.62; P = .01).
The investigators noted that the Glasgow-Blatchford score, which also is used in cases of UGIB, has previously demonstrated accuracy in predicting blood transfusion, as it did in the present study, suggesting that, “[i]n instances where there may be uncertainty of the origin of the bleeding, the Blatchford score may be a preferential choice of risk score.”
“Overall, we found that no singular score performed best across all the outcomes studied nor did any score have an extremely strong discriminatory power for any individual variable,” the investigators wrote, concluding that “... simpler and more powerful prediction tools are required for better risk stratification in LGIB.”
The investigators reported no financial support or conflicts of interest.
*This story was updated on Jan. 31, 2019.
SOURCE: Tapaskar N et al. Gastrointest Endosc. 2018 Dec 18. doi: 10.1016/j.gie.2018.12.011.
In cases of lower gastrointestinal bleeding (LGIB), albumin and hemoglobin levels are the best independent predictors of severe bleeding, according to investigators.
These findings came from a sobering look at LGIB risk-prediction models. While some models could predict specific outcomes with reasonable accuracy, none of the models demonstrated broad predictive power, reported Natalie Tapaskar, MD, of the department of medicine at the University of Chicago, and her colleagues.
LGIB requires intensive resource utilization and proves fatal in 5%-15% of patients, which means timely and appropriate interventions are essential, especially for those with severe bleeding.
“There are limited data on accurately predicting the risk of adverse outcomes for hospitalized patients with LGIB,” the investigators wrote in Gastrointestinal Endoscopy, “especially in comparison to patients with upper gastrointestinal bleeding (UGIB), where tools such as the Glasgow-Blatchford Bleeding Score have been validated to accurately predict important clinical outcomes.”
To assess existing risk models for LGIB, the investigators performed a prospective observational study involving 170 patients with LGIB who underwent colonoscopy during April 2016–September 2017 at the University of Chicago Medical Center. Data were collected through comprehensive medical record review.
The primary outcome was severe bleeding. This was defined by acute bleeding during the first 24 hours of admission that required a transfusion of 2 or more units of packed red blood cells, and/or caused a 20% or greater decrease in hematocrit; and/or recurrent bleeding 24 hours after clinical stability, involving rectal bleeding with an additional drop in hematocrit of 20% or more, and/or readmission for LGIB within 1 week of discharge. Secondary outcomes included blood transfusion requirements, in-hospital recurrent bleeding, length of stay, ICU admission, intervention (surgery, interventional radiology, endoscopy), and the comparative predictive ability of seven clinical risk stratification models: AIMS65, Charlson Comorbidity Index, Glasgow-Blatchford, NOBLADS, Oakland, Sengupta, and Strate. Area under the receiver operating characteristic curve (AUC) was used to compare model predictive power. Risk of adverse outcomes was calculated by univariable and multivariable logistic regression.
Results showed that median patient age was 70 years. Most of the patients (80%) were African American and slightly more than half were female (58%). These demographic factors were not predictive of severe bleeding, which occurred in about half of the cases (52%). Upon admission, patients with severe bleeding were more likely to have chronic renal failure (30% vs. 17%; P = .05), lower albumin (3.6 g/dL vs. 3.95 g/dL; P less than .0001), lower hemoglobin (8.6 g/dL vs. 11.1 g/dL; P = .0001), lower systolic blood pressure (118 mm Hg vs. 132 mm Hg; P = .01), and higher creatinine (1.3 mg/dL vs. 1 mg/dL; P = .04). After adjustment for confounding variables, the strongest independent predictors of severe bleeding were low albumin (odds ratio, 2.56 per 1-g/dL decrease; P = .02) and low hemoglobin (OR, 1.28 per 1-g/dL decrease; P = .0015).
On average, time between admission and colonoscopy was between 2 and 3 days (median, 62.2 hours). In 3 out of 4 patients (77%), etiology of LGIB was confirmed; diverticular bleeding was most common (39%), followed distantly by hemorrhoidal bleeding (15%).
Compared with milder cases, patients with severe bleeding were more likely to stay in the ICU (49% vs. 19%; P less than .0001), have a blood transfusion (85% vs 36%; P less than .0001), and need to remain in the hospital for a longer period of time (6 days vs. 4 days; P = .0009). These findings exemplify the high level of resource utilization required for LGIB and show how severe bleeding dramatically compounds intensity of care.
Further analysis showed that none of the seven risk models were predictive across all outcomes; however, some predicted specific outcomes better than others. Leaders were the Glasgow-Blatchford score for blood transfusion (AUC 0.87; P less than .0001), the Oakland score for severe bleeding (AUC 0.74; P less than .0001), the Sengupta score for ICU stay (AUC 0.74; P less than .0001), and the Strate score for both recurrent bleeding during hospital stay (AUC 0.66; P = .0008) and endoscopic intervention (AUC 0.62; P = .01).
The investigators noted that the Glasgow-Blatchford score, which also is used in cases of UGIB, has previously demonstrated accuracy in predicting blood transfusion, as it did in the present study, suggesting that, “[i]n instances where there may be uncertainty of the origin of the bleeding, the Blatchford score may be a preferential choice of risk score.”
“Overall, we found that no singular score performed best across all the outcomes studied nor did any score have an extremely strong discriminatory power for any individual variable,” the investigators wrote, concluding that “... simpler and more powerful prediction tools are required for better risk stratification in LGIB.”
The investigators reported no financial support or conflicts of interest.
*This story was updated on Jan. 31, 2019.
SOURCE: Tapaskar N et al. Gastrointest Endosc. 2018 Dec 18. doi: 10.1016/j.gie.2018.12.011.
FROM GASTROINTESTINAL ENDOSCOPY
Key clinical point: In cases of lower gastrointestinal bleeding (LGIB), albumin and hemoglobin levels are the best independent predictors of severe bleeding.
Major finding: After adjustment for confounding variables, low albumin upon admission was the strongest independent predictor of severe bleeding (OR, 2.56 per 1 g/dL decrease; P = .02).
Study details: A prospective, observational study of 170 patients with LGIB who underwent colonoscopy during April 2016–September 2017 at the University of Chicago Medical Center.
Disclosures: The investigators reported no financial support or conflicts of interest.
Source: Tapaskar N et al. Gastrointest Endosc. 2018 Dec 18. doi: 10.1016/j.gie.2018.12.011.
Defeating the opioid epidemic
The U.S. Surgeon General weighs in.
Vice Admiral Jerome M. Adams, MD, MPH, is the 20th Surgeon General of the United States, a post created in 1871.
Dr. Adams holds degrees in biochemistry and psychology from the University of Maryland, Baltimore County; a master’s degree in public health from the University of California, Berkeley; and a medical degree from the Indiana University, Indianapolis. He is a board-certified anesthesiologist and associate clinical professor of anesthesia at Indiana University.
At the 2018 Executive Advisory Board meeting of the Doctors Company, Richard E. Anderson, MD, FACP, chairman and chief executive officer of the Doctors Company, spoke with Dr. Adams about the opioid epidemic’s enormous impact on communities and health services in the United States.
Dr. Anderson: Dr. Adams, you’ve been busy since taking over as Surgeon General of the United States. What are some of the key challenges that you’re facing in this office?
Dr. Adams: You know, there are many challenges facing our country, but it boils down to a lack of wellness. We know that only 10% of health is due to health care, 20% of health is genetics, and the rest is a combination of behavior and environment.
My motto is “better health through better partnerships,” because I firmly believe that if we break out of our silos and reach across the traditional barriers that have been put up by funding, by reimbursement, and by infrastructure, then we can ultimately achieve wellness in our communities.
You asked what I’ve been focused on as Surgeon General. Well, I’m focused on three main areas right now.
No. 1 is the opioid epidemic. It is a scourge across our country. A person dies every 12½ minutes from an opioid overdose and that’s far too many. Especially when we know that many of those deaths can be prevented.
Another area I’m focused on is demonstrating the link between community health and economic prosperity. We want folks to invest in health because we know that not only will it achieve better health for individuals and communities but it will create a more prosperous nation, also.
And finally, I’m raising awareness about the links between our nation’s health and our safety and security – particularly our national security. Unfortunately, 7 out of 10 young people between the ages of 18 and 24 years old in our country are ineligible for military service. That’s because they can’t pass the physical, they can’t meet the educational requirements, or they have a criminal record.
So, our nation’s poor health is not just a matter of diabetes or heart disease 20 or 30 years down the road. We are literally a less-safe country right now because we’re an unhealthy country.
Dr. Anderson: Regarding the opioid epidemic, what are some of the programs that are available today that you find effective? What would you like to see us do as a nation to respond to the epidemic?
Dr. Adams: Recently, I was at a hospital in Alaska where they have implemented a neonatal abstinence syndrome protocol and program that is being looked at around the country – and others are attempting to replicate it.
We know that if you keep mom and baby together, baby does better, mom does better, hospital stays are shorter, costs go down, and you’re keeping that family unit intact. This prevents future problems for both the baby and the mother. That’s just one small example.
I’m also very happy to see that the prescribing of opioids is going down 20%-25% across the country. And there are even larger decreases in the military and veteran communities. That’s really a testament to doctors and the medical profession finally waking up. And I say this as a physician myself, as an anesthesiologist, as someone who is involved in acute and chronic pain management.
Four out of five people with substance use disorder say they started with a prescription opioid. Many physicians will say, “Those aren’t my patients,” but unfortunately when we look at the PDMP [prescription drug monitoring program] data across the country we do a poor job of predicting who is and who isn’t going to divert. It may not be your patient, but it could be their son or the babysitter who is diverting those overprescribed opioids.
One thing that I really think we need to lean into as health care practitioners is providing medication-assisted treatment, or MAT. We know that the gold standard for treatment and recovery is medication-assisted treatment of some form. But we also know it’s not nearly available enough and that there are barriers on the federal and state levels.
We need you to continue to talk to your congressional representatives and let them know which barriers you perceive because the data waiver comes directly from Congress.
Still, any ER can prescribe up to 3 days of MAT to someone. I’d much rather have our ER doctors putting patients on MAT and then connecting them to treatment, than sending them back out into the arms of a drug dealer after they put them into acute withdrawal with naloxone.
We also have too many pregnant women who want help but can’t find any treatment because no one out there will take care of pregnant moms. We need folks to step up to the plate and get that data waiver in our ob/gyn and primary care sectors.
Ultimately, we need hospitals and health care leaders to create an environment that makes providers feel comfortable providing that service by giving them the training and the support to be able to do it.
We also need to make sure we’re co-prescribing naloxone for those who are at risk for opioid overdose.
Dr. Anderson: Just so we are clear, are you in favor of regular prescribing of naloxone, along with prescriptions for opioids? Is that correct?
Dr. Adams: I issued the first Surgeon General’s advisory from more than 10 years earlier this year to help folks understand that over half of our opioid overdoses occur in a home setting. We all know that an anoxic brain injury occurs in 4-5 minutes. We also know that most ambulances and first responders aren’t going to show up in 4-5 minutes.
If we want to make a dent in this overdose epidemic, we need everyone to consider themselves a first responder. We need to look at it the same as we look at CPR; we need everyone carrying naloxone. That was one of the big pushes from my Surgeon General’s advisory.
How can providers help? Well, they can coprescribe naloxone to folks on high morphine milligram equivalents (MME) who are at risk. If grandma has naloxone at home and her grandson overdoses in the garage, then at least it’s in the same house. Naloxone is not the treatment for the opioid epidemic. But we can’t get someone who is dead into treatment.
I have no illusions that simply making naloxone available is going to turn the tide, but it certainly is an important part of it.
Dr. Anderson: From your unique viewpoint, how much progress do you see in relation to the opioid epidemic? Do you think we’re approaching an inflection point, or do you think there’s a long way to go before this starts to turn around?
Dr. Adams: When I talk about the opioid epidemic, I have two angles. No.1, I want to raise awareness about the opioid epidemic – the severity of it, and how everyone can lean into it in their own way. Whether it’s community citizens, providers, law enforcement, the business community, whomever.
But in addition to raising awareness, I want to instill hope.
I was in Huntington, West Virginia, just a few weeks ago at the epicenter of the opioid epidemic. They’ve been able to turn their opioid overdose rates around by providing peer recovery coaches to individuals and making sure naloxone is available throughout the community. You save the life and then you connect them to care.
We know that the folks who are at highest risk for overdose deaths are the ones who just overdosed. They come out of the ER where we’ve watched them for a few hours and then we send them right back out into the arms of the drug dealer to do exactly what we know they will do medically because we’ve thrown them into withdrawal and they try to get their next fix.
If we can partner with law enforcement, then we can turn our opioid overdose rates around.
A story of recovery that I want to share with you is about a guy named Jonathan, who I met when I was in Rhode Island.
Jonathan overdosed, but his roommate had access to naloxone, which he administered. Jonathan was taken to the ER and then connected with a peer recovery coach. He is now in recovery and has actually become a peer recovery coach himself. Saving this one life will now enable us to save many more.
Yet we still prescribe more than 80% of the world’s opioids to less than 5% of the world’s population. So, we still have an overprescribing epidemic, but we’ve surpassed the inflection point there. Prescribing is coming down.
But another part of this epidemic was that we squeezed the balloon in one place and, as prescribing opioids went down, lots of people switched over to heroin. That’s when we really first started to see overdose rates go up.
Well, it’s important for folks to know that, through law enforcement, through partnerships with the public health community, through an increase in syringe service programs, and through other touch points, heroin use is now going down in most places.
Unfortunately, now we’re seeing the third wave of the epidemic, and that’s fentanyl and carfentanil.
The U.S. Surgeon General weighs in.
The U.S. Surgeon General weighs in.
Vice Admiral Jerome M. Adams, MD, MPH, is the 20th Surgeon General of the United States, a post created in 1871.
Dr. Adams holds degrees in biochemistry and psychology from the University of Maryland, Baltimore County; a master’s degree in public health from the University of California, Berkeley; and a medical degree from the Indiana University, Indianapolis. He is a board-certified anesthesiologist and associate clinical professor of anesthesia at Indiana University.
At the 2018 Executive Advisory Board meeting of the Doctors Company, Richard E. Anderson, MD, FACP, chairman and chief executive officer of the Doctors Company, spoke with Dr. Adams about the opioid epidemic’s enormous impact on communities and health services in the United States.
Dr. Anderson: Dr. Adams, you’ve been busy since taking over as Surgeon General of the United States. What are some of the key challenges that you’re facing in this office?
Dr. Adams: You know, there are many challenges facing our country, but it boils down to a lack of wellness. We know that only 10% of health is due to health care, 20% of health is genetics, and the rest is a combination of behavior and environment.
My motto is “better health through better partnerships,” because I firmly believe that if we break out of our silos and reach across the traditional barriers that have been put up by funding, by reimbursement, and by infrastructure, then we can ultimately achieve wellness in our communities.
You asked what I’ve been focused on as Surgeon General. Well, I’m focused on three main areas right now.
No. 1 is the opioid epidemic. It is a scourge across our country. A person dies every 12½ minutes from an opioid overdose and that’s far too many. Especially when we know that many of those deaths can be prevented.
Another area I’m focused on is demonstrating the link between community health and economic prosperity. We want folks to invest in health because we know that not only will it achieve better health for individuals and communities but it will create a more prosperous nation, also.
And finally, I’m raising awareness about the links between our nation’s health and our safety and security – particularly our national security. Unfortunately, 7 out of 10 young people between the ages of 18 and 24 years old in our country are ineligible for military service. That’s because they can’t pass the physical, they can’t meet the educational requirements, or they have a criminal record.
So, our nation’s poor health is not just a matter of diabetes or heart disease 20 or 30 years down the road. We are literally a less-safe country right now because we’re an unhealthy country.
Dr. Anderson: Regarding the opioid epidemic, what are some of the programs that are available today that you find effective? What would you like to see us do as a nation to respond to the epidemic?
Dr. Adams: Recently, I was at a hospital in Alaska where they have implemented a neonatal abstinence syndrome protocol and program that is being looked at around the country – and others are attempting to replicate it.
We know that if you keep mom and baby together, baby does better, mom does better, hospital stays are shorter, costs go down, and you’re keeping that family unit intact. This prevents future problems for both the baby and the mother. That’s just one small example.
I’m also very happy to see that the prescribing of opioids is going down 20%-25% across the country. And there are even larger decreases in the military and veteran communities. That’s really a testament to doctors and the medical profession finally waking up. And I say this as a physician myself, as an anesthesiologist, as someone who is involved in acute and chronic pain management.
Four out of five people with substance use disorder say they started with a prescription opioid. Many physicians will say, “Those aren’t my patients,” but unfortunately when we look at the PDMP [prescription drug monitoring program] data across the country we do a poor job of predicting who is and who isn’t going to divert. It may not be your patient, but it could be their son or the babysitter who is diverting those overprescribed opioids.
One thing that I really think we need to lean into as health care practitioners is providing medication-assisted treatment, or MAT. We know that the gold standard for treatment and recovery is medication-assisted treatment of some form. But we also know it’s not nearly available enough and that there are barriers on the federal and state levels.
We need you to continue to talk to your congressional representatives and let them know which barriers you perceive because the data waiver comes directly from Congress.
Still, any ER can prescribe up to 3 days of MAT to someone. I’d much rather have our ER doctors putting patients on MAT and then connecting them to treatment, than sending them back out into the arms of a drug dealer after they put them into acute withdrawal with naloxone.
We also have too many pregnant women who want help but can’t find any treatment because no one out there will take care of pregnant moms. We need folks to step up to the plate and get that data waiver in our ob/gyn and primary care sectors.
Ultimately, we need hospitals and health care leaders to create an environment that makes providers feel comfortable providing that service by giving them the training and the support to be able to do it.
We also need to make sure we’re co-prescribing naloxone for those who are at risk for opioid overdose.
Dr. Anderson: Just so we are clear, are you in favor of regular prescribing of naloxone, along with prescriptions for opioids? Is that correct?
Dr. Adams: I issued the first Surgeon General’s advisory from more than 10 years earlier this year to help folks understand that over half of our opioid overdoses occur in a home setting. We all know that an anoxic brain injury occurs in 4-5 minutes. We also know that most ambulances and first responders aren’t going to show up in 4-5 minutes.
If we want to make a dent in this overdose epidemic, we need everyone to consider themselves a first responder. We need to look at it the same as we look at CPR; we need everyone carrying naloxone. That was one of the big pushes from my Surgeon General’s advisory.
How can providers help? Well, they can coprescribe naloxone to folks on high morphine milligram equivalents (MME) who are at risk. If grandma has naloxone at home and her grandson overdoses in the garage, then at least it’s in the same house. Naloxone is not the treatment for the opioid epidemic. But we can’t get someone who is dead into treatment.
I have no illusions that simply making naloxone available is going to turn the tide, but it certainly is an important part of it.
Dr. Anderson: From your unique viewpoint, how much progress do you see in relation to the opioid epidemic? Do you think we’re approaching an inflection point, or do you think there’s a long way to go before this starts to turn around?
Dr. Adams: When I talk about the opioid epidemic, I have two angles. No.1, I want to raise awareness about the opioid epidemic – the severity of it, and how everyone can lean into it in their own way. Whether it’s community citizens, providers, law enforcement, the business community, whomever.
But in addition to raising awareness, I want to instill hope.
I was in Huntington, West Virginia, just a few weeks ago at the epicenter of the opioid epidemic. They’ve been able to turn their opioid overdose rates around by providing peer recovery coaches to individuals and making sure naloxone is available throughout the community. You save the life and then you connect them to care.
We know that the folks who are at highest risk for overdose deaths are the ones who just overdosed. They come out of the ER where we’ve watched them for a few hours and then we send them right back out into the arms of the drug dealer to do exactly what we know they will do medically because we’ve thrown them into withdrawal and they try to get their next fix.
If we can partner with law enforcement, then we can turn our opioid overdose rates around.
A story of recovery that I want to share with you is about a guy named Jonathan, who I met when I was in Rhode Island.
Jonathan overdosed, but his roommate had access to naloxone, which he administered. Jonathan was taken to the ER and then connected with a peer recovery coach. He is now in recovery and has actually become a peer recovery coach himself. Saving this one life will now enable us to save many more.
Yet we still prescribe more than 80% of the world’s opioids to less than 5% of the world’s population. So, we still have an overprescribing epidemic, but we’ve surpassed the inflection point there. Prescribing is coming down.
But another part of this epidemic was that we squeezed the balloon in one place and, as prescribing opioids went down, lots of people switched over to heroin. That’s when we really first started to see overdose rates go up.
Well, it’s important for folks to know that, through law enforcement, through partnerships with the public health community, through an increase in syringe service programs, and through other touch points, heroin use is now going down in most places.
Unfortunately, now we’re seeing the third wave of the epidemic, and that’s fentanyl and carfentanil.
Vice Admiral Jerome M. Adams, MD, MPH, is the 20th Surgeon General of the United States, a post created in 1871.
Dr. Adams holds degrees in biochemistry and psychology from the University of Maryland, Baltimore County; a master’s degree in public health from the University of California, Berkeley; and a medical degree from the Indiana University, Indianapolis. He is a board-certified anesthesiologist and associate clinical professor of anesthesia at Indiana University.
At the 2018 Executive Advisory Board meeting of the Doctors Company, Richard E. Anderson, MD, FACP, chairman and chief executive officer of the Doctors Company, spoke with Dr. Adams about the opioid epidemic’s enormous impact on communities and health services in the United States.
Dr. Anderson: Dr. Adams, you’ve been busy since taking over as Surgeon General of the United States. What are some of the key challenges that you’re facing in this office?
Dr. Adams: You know, there are many challenges facing our country, but it boils down to a lack of wellness. We know that only 10% of health is due to health care, 20% of health is genetics, and the rest is a combination of behavior and environment.
My motto is “better health through better partnerships,” because I firmly believe that if we break out of our silos and reach across the traditional barriers that have been put up by funding, by reimbursement, and by infrastructure, then we can ultimately achieve wellness in our communities.
You asked what I’ve been focused on as Surgeon General. Well, I’m focused on three main areas right now.
No. 1 is the opioid epidemic. It is a scourge across our country. A person dies every 12½ minutes from an opioid overdose and that’s far too many. Especially when we know that many of those deaths can be prevented.
Another area I’m focused on is demonstrating the link between community health and economic prosperity. We want folks to invest in health because we know that not only will it achieve better health for individuals and communities but it will create a more prosperous nation, also.
And finally, I’m raising awareness about the links between our nation’s health and our safety and security – particularly our national security. Unfortunately, 7 out of 10 young people between the ages of 18 and 24 years old in our country are ineligible for military service. That’s because they can’t pass the physical, they can’t meet the educational requirements, or they have a criminal record.
So, our nation’s poor health is not just a matter of diabetes or heart disease 20 or 30 years down the road. We are literally a less-safe country right now because we’re an unhealthy country.
Dr. Anderson: Regarding the opioid epidemic, what are some of the programs that are available today that you find effective? What would you like to see us do as a nation to respond to the epidemic?
Dr. Adams: Recently, I was at a hospital in Alaska where they have implemented a neonatal abstinence syndrome protocol and program that is being looked at around the country – and others are attempting to replicate it.
We know that if you keep mom and baby together, baby does better, mom does better, hospital stays are shorter, costs go down, and you’re keeping that family unit intact. This prevents future problems for both the baby and the mother. That’s just one small example.
I’m also very happy to see that the prescribing of opioids is going down 20%-25% across the country. And there are even larger decreases in the military and veteran communities. That’s really a testament to doctors and the medical profession finally waking up. And I say this as a physician myself, as an anesthesiologist, as someone who is involved in acute and chronic pain management.
Four out of five people with substance use disorder say they started with a prescription opioid. Many physicians will say, “Those aren’t my patients,” but unfortunately when we look at the PDMP [prescription drug monitoring program] data across the country we do a poor job of predicting who is and who isn’t going to divert. It may not be your patient, but it could be their son or the babysitter who is diverting those overprescribed opioids.
One thing that I really think we need to lean into as health care practitioners is providing medication-assisted treatment, or MAT. We know that the gold standard for treatment and recovery is medication-assisted treatment of some form. But we also know it’s not nearly available enough and that there are barriers on the federal and state levels.
We need you to continue to talk to your congressional representatives and let them know which barriers you perceive because the data waiver comes directly from Congress.
Still, any ER can prescribe up to 3 days of MAT to someone. I’d much rather have our ER doctors putting patients on MAT and then connecting them to treatment, than sending them back out into the arms of a drug dealer after they put them into acute withdrawal with naloxone.
We also have too many pregnant women who want help but can’t find any treatment because no one out there will take care of pregnant moms. We need folks to step up to the plate and get that data waiver in our ob/gyn and primary care sectors.
Ultimately, we need hospitals and health care leaders to create an environment that makes providers feel comfortable providing that service by giving them the training and the support to be able to do it.
We also need to make sure we’re co-prescribing naloxone for those who are at risk for opioid overdose.
Dr. Anderson: Just so we are clear, are you in favor of regular prescribing of naloxone, along with prescriptions for opioids? Is that correct?
Dr. Adams: I issued the first Surgeon General’s advisory from more than 10 years earlier this year to help folks understand that over half of our opioid overdoses occur in a home setting. We all know that an anoxic brain injury occurs in 4-5 minutes. We also know that most ambulances and first responders aren’t going to show up in 4-5 minutes.
If we want to make a dent in this overdose epidemic, we need everyone to consider themselves a first responder. We need to look at it the same as we look at CPR; we need everyone carrying naloxone. That was one of the big pushes from my Surgeon General’s advisory.
How can providers help? Well, they can coprescribe naloxone to folks on high morphine milligram equivalents (MME) who are at risk. If grandma has naloxone at home and her grandson overdoses in the garage, then at least it’s in the same house. Naloxone is not the treatment for the opioid epidemic. But we can’t get someone who is dead into treatment.
I have no illusions that simply making naloxone available is going to turn the tide, but it certainly is an important part of it.
Dr. Anderson: From your unique viewpoint, how much progress do you see in relation to the opioid epidemic? Do you think we’re approaching an inflection point, or do you think there’s a long way to go before this starts to turn around?
Dr. Adams: When I talk about the opioid epidemic, I have two angles. No.1, I want to raise awareness about the opioid epidemic – the severity of it, and how everyone can lean into it in their own way. Whether it’s community citizens, providers, law enforcement, the business community, whomever.
But in addition to raising awareness, I want to instill hope.
I was in Huntington, West Virginia, just a few weeks ago at the epicenter of the opioid epidemic. They’ve been able to turn their opioid overdose rates around by providing peer recovery coaches to individuals and making sure naloxone is available throughout the community. You save the life and then you connect them to care.
We know that the folks who are at highest risk for overdose deaths are the ones who just overdosed. They come out of the ER where we’ve watched them for a few hours and then we send them right back out into the arms of the drug dealer to do exactly what we know they will do medically because we’ve thrown them into withdrawal and they try to get their next fix.
If we can partner with law enforcement, then we can turn our opioid overdose rates around.
A story of recovery that I want to share with you is about a guy named Jonathan, who I met when I was in Rhode Island.
Jonathan overdosed, but his roommate had access to naloxone, which he administered. Jonathan was taken to the ER and then connected with a peer recovery coach. He is now in recovery and has actually become a peer recovery coach himself. Saving this one life will now enable us to save many more.
Yet we still prescribe more than 80% of the world’s opioids to less than 5% of the world’s population. So, we still have an overprescribing epidemic, but we’ve surpassed the inflection point there. Prescribing is coming down.
But another part of this epidemic was that we squeezed the balloon in one place and, as prescribing opioids went down, lots of people switched over to heroin. That’s when we really first started to see overdose rates go up.
Well, it’s important for folks to know that, through law enforcement, through partnerships with the public health community, through an increase in syringe service programs, and through other touch points, heroin use is now going down in most places.
Unfortunately, now we’re seeing the third wave of the epidemic, and that’s fentanyl and carfentanil.
Aspirin for primary cardiovascular prevention, RIP
SNOWMASS, COLO. – during the space of a few short weeks in autumn 2018.
“Is aspirin safe and effective for primary prevention? The short answer here is no,” Patrick T. O’Gara, MD, declared at the annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
“Think of all those decades of aspirin therapy in the hopes of making ourselves healthier,” added Dr. O’Gara, professor of medicine at Harvard Medical School, Boston, and a past president of the American College of Cardiology.
He cited the results of three placebo-controlled randomized trials totaling more than 47,000 patients without known cardiovascular disease: ARRIVE, published in late September 2018, followed in October by ASPREE and ASCEND.
• ARRIVE. This double-blind study conducted in seven countries included 12,546 patients deemed at moderate cardiovascular risk, with an estimated 10-year cardiovascular event risk of 17%. Eligibility was restricted to men aged 55 and up and women aged 60 or older. After a median follow-up of 5 years, there was no difference between patients assigned to enteric-coated aspirin at 100 mg/day versus placebo in the incidence of major adverse cardiovascular events, with a hazard ratio of 0.96. However, GI bleeding events were 2.1-fold more common in the aspirin group (Lancet. 2018 Sep 22;392[10152]:1036-46).
• ASPREE. This double-blind trial, conducted in Australia and the United States, included 19,114 community-dwelling participants aged 70 years or older, or 65 years or older for Hispanics and blacks in the United States. After a median 4.7 years of follow-up, there was no difference in major adverse cardiovascular events between subjects randomized to 100 mg/day of enteric-coated aspirin and those on placebo. So, as in ARRIVE, no benefit. However, the rate of major hemorrhage was 38% greater in the aspirin group (N Engl J Med. 2018 Oct 18;379[16]:1509-18).
Moreover, the rate of all-cause mortality was 14% greater in the aspirin group, a statistically significant difference, compared with controls. Drilling down, the investigators showed that the major contributor to this excess mortality in the aspirin group was their 31% greater rate of cancer-related death (N Engl J Med. 2018 Oct 18;379[16]:1519-28).
“Remember, we used to think that taking aspirin reduced the incidence of GI cancer, and, in particular, colon adenocarcinoma? Well, here’s a very startling observation in 19,114 healthy elderly patients showing an increase in cancer-associated death with the use of aspirin,” commented Dr. O’Gara.
• ASCEND. This study randomized 15,480 subjects with diabetes but no known cardiovascular disease to 100 mg/day of aspirin or placebo and followed them for a mean of 7.4 years. There was a significant 12% relative risk reduction in the composite endpoint of serious vascular events in the aspirin group; however, the aspirin-treated patients also had a 29% greater rate of major bleeding events (N Engl J Med. 2018 Oct 18;379[16]:1529-39).
“So in dealing with our diabetic patients, we could perhaps say there is a small reduction in the risk of cardiovascular outcomes that is overwhelmed by more than a factor of two with regard to an increase in the risk of bleeding,” the cardiologist observed.
How did physicians get the aspirin story for primary prevention so wrong for so long? Dr. O’Gara pointed to the Physicians’ Health Study, conducted mainly back in the 1970s, as one of the benchmark studies that led to the widespread use of aspirin in this way.
“I think the aspirin story has now been put into sharp focus just within the course of the last 6 months and should force all of us to reassess what it is that we advise patients,” he concluded.
Dr. O’Gara’s presentation was the talk of the meeting, as many attendees hadn’t yet caught up with the latest aspirin data.
During an Q&A session, Robert A. Vogel, MD, a preventive cardiology authority at the University of Colorado, Denver, was asked, given the new emphasis placed upon coronary artery calcium as a supplemental risk assessment tool in the latest guidelines, at what magnitude of coronary artery calcium score in a patient with no history of coronary disease he would give aspirin for secondary prevention.
“I know I don’t know the answer to that question,” Dr. Vogel replied. “I no longer reflexively give aspirin to, say, a 60-year-old with a calcium score of 200. I will give a statin. Statins in my book are so effective and safe that my threshold for giving a statin in a 60-year-old is virtually nothing. But with a calcium score of 2,000 or 5,000, I worry just like you worry.”
He noted that the primary prevention patients in the three recent major trials were mostly 60-70 years of age or older. It’s safe to assume that by that point in life many of them had silent atherosclerosis and would have had a non-zero coronary artery calcium score, had they been tested. And yet, aspirin didn’t provide any net benefit in those groups, unlike the drug’s rock-solid proven value in patients who have actually experienced a cardiovascular event.
Dr. O’Gara reported receiving funding from the National Heart, Lung and Blood Institute, the National Institute of Dental and Craniofacial Research, from Medtronic in conjunction with the ongoing pivotal APOLLO transcatheter mitral valve replacement trial, and from Edwards Lifesciences for the ongoing EARLY TAVR trial.
This article was updated 1/31/19.
SNOWMASS, COLO. – during the space of a few short weeks in autumn 2018.
“Is aspirin safe and effective for primary prevention? The short answer here is no,” Patrick T. O’Gara, MD, declared at the annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
“Think of all those decades of aspirin therapy in the hopes of making ourselves healthier,” added Dr. O’Gara, professor of medicine at Harvard Medical School, Boston, and a past president of the American College of Cardiology.
He cited the results of three placebo-controlled randomized trials totaling more than 47,000 patients without known cardiovascular disease: ARRIVE, published in late September 2018, followed in October by ASPREE and ASCEND.
• ARRIVE. This double-blind study conducted in seven countries included 12,546 patients deemed at moderate cardiovascular risk, with an estimated 10-year cardiovascular event risk of 17%. Eligibility was restricted to men aged 55 and up and women aged 60 or older. After a median follow-up of 5 years, there was no difference between patients assigned to enteric-coated aspirin at 100 mg/day versus placebo in the incidence of major adverse cardiovascular events, with a hazard ratio of 0.96. However, GI bleeding events were 2.1-fold more common in the aspirin group (Lancet. 2018 Sep 22;392[10152]:1036-46).
• ASPREE. This double-blind trial, conducted in Australia and the United States, included 19,114 community-dwelling participants aged 70 years or older, or 65 years or older for Hispanics and blacks in the United States. After a median 4.7 years of follow-up, there was no difference in major adverse cardiovascular events between subjects randomized to 100 mg/day of enteric-coated aspirin and those on placebo. So, as in ARRIVE, no benefit. However, the rate of major hemorrhage was 38% greater in the aspirin group (N Engl J Med. 2018 Oct 18;379[16]:1509-18).
Moreover, the rate of all-cause mortality was 14% greater in the aspirin group, a statistically significant difference, compared with controls. Drilling down, the investigators showed that the major contributor to this excess mortality in the aspirin group was their 31% greater rate of cancer-related death (N Engl J Med. 2018 Oct 18;379[16]:1519-28).
“Remember, we used to think that taking aspirin reduced the incidence of GI cancer, and, in particular, colon adenocarcinoma? Well, here’s a very startling observation in 19,114 healthy elderly patients showing an increase in cancer-associated death with the use of aspirin,” commented Dr. O’Gara.
• ASCEND. This study randomized 15,480 subjects with diabetes but no known cardiovascular disease to 100 mg/day of aspirin or placebo and followed them for a mean of 7.4 years. There was a significant 12% relative risk reduction in the composite endpoint of serious vascular events in the aspirin group; however, the aspirin-treated patients also had a 29% greater rate of major bleeding events (N Engl J Med. 2018 Oct 18;379[16]:1529-39).
“So in dealing with our diabetic patients, we could perhaps say there is a small reduction in the risk of cardiovascular outcomes that is overwhelmed by more than a factor of two with regard to an increase in the risk of bleeding,” the cardiologist observed.
How did physicians get the aspirin story for primary prevention so wrong for so long? Dr. O’Gara pointed to the Physicians’ Health Study, conducted mainly back in the 1970s, as one of the benchmark studies that led to the widespread use of aspirin in this way.
“I think the aspirin story has now been put into sharp focus just within the course of the last 6 months and should force all of us to reassess what it is that we advise patients,” he concluded.
Dr. O’Gara’s presentation was the talk of the meeting, as many attendees hadn’t yet caught up with the latest aspirin data.
During an Q&A session, Robert A. Vogel, MD, a preventive cardiology authority at the University of Colorado, Denver, was asked, given the new emphasis placed upon coronary artery calcium as a supplemental risk assessment tool in the latest guidelines, at what magnitude of coronary artery calcium score in a patient with no history of coronary disease he would give aspirin for secondary prevention.
“I know I don’t know the answer to that question,” Dr. Vogel replied. “I no longer reflexively give aspirin to, say, a 60-year-old with a calcium score of 200. I will give a statin. Statins in my book are so effective and safe that my threshold for giving a statin in a 60-year-old is virtually nothing. But with a calcium score of 2,000 or 5,000, I worry just like you worry.”
He noted that the primary prevention patients in the three recent major trials were mostly 60-70 years of age or older. It’s safe to assume that by that point in life many of them had silent atherosclerosis and would have had a non-zero coronary artery calcium score, had they been tested. And yet, aspirin didn’t provide any net benefit in those groups, unlike the drug’s rock-solid proven value in patients who have actually experienced a cardiovascular event.
Dr. O’Gara reported receiving funding from the National Heart, Lung and Blood Institute, the National Institute of Dental and Craniofacial Research, from Medtronic in conjunction with the ongoing pivotal APOLLO transcatheter mitral valve replacement trial, and from Edwards Lifesciences for the ongoing EARLY TAVR trial.
This article was updated 1/31/19.
SNOWMASS, COLO. – during the space of a few short weeks in autumn 2018.
“Is aspirin safe and effective for primary prevention? The short answer here is no,” Patrick T. O’Gara, MD, declared at the annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
“Think of all those decades of aspirin therapy in the hopes of making ourselves healthier,” added Dr. O’Gara, professor of medicine at Harvard Medical School, Boston, and a past president of the American College of Cardiology.
He cited the results of three placebo-controlled randomized trials totaling more than 47,000 patients without known cardiovascular disease: ARRIVE, published in late September 2018, followed in October by ASPREE and ASCEND.
• ARRIVE. This double-blind study conducted in seven countries included 12,546 patients deemed at moderate cardiovascular risk, with an estimated 10-year cardiovascular event risk of 17%. Eligibility was restricted to men aged 55 and up and women aged 60 or older. After a median follow-up of 5 years, there was no difference between patients assigned to enteric-coated aspirin at 100 mg/day versus placebo in the incidence of major adverse cardiovascular events, with a hazard ratio of 0.96. However, GI bleeding events were 2.1-fold more common in the aspirin group (Lancet. 2018 Sep 22;392[10152]:1036-46).
• ASPREE. This double-blind trial, conducted in Australia and the United States, included 19,114 community-dwelling participants aged 70 years or older, or 65 years or older for Hispanics and blacks in the United States. After a median 4.7 years of follow-up, there was no difference in major adverse cardiovascular events between subjects randomized to 100 mg/day of enteric-coated aspirin and those on placebo. So, as in ARRIVE, no benefit. However, the rate of major hemorrhage was 38% greater in the aspirin group (N Engl J Med. 2018 Oct 18;379[16]:1509-18).
Moreover, the rate of all-cause mortality was 14% greater in the aspirin group, a statistically significant difference, compared with controls. Drilling down, the investigators showed that the major contributor to this excess mortality in the aspirin group was their 31% greater rate of cancer-related death (N Engl J Med. 2018 Oct 18;379[16]:1519-28).
“Remember, we used to think that taking aspirin reduced the incidence of GI cancer, and, in particular, colon adenocarcinoma? Well, here’s a very startling observation in 19,114 healthy elderly patients showing an increase in cancer-associated death with the use of aspirin,” commented Dr. O’Gara.
• ASCEND. This study randomized 15,480 subjects with diabetes but no known cardiovascular disease to 100 mg/day of aspirin or placebo and followed them for a mean of 7.4 years. There was a significant 12% relative risk reduction in the composite endpoint of serious vascular events in the aspirin group; however, the aspirin-treated patients also had a 29% greater rate of major bleeding events (N Engl J Med. 2018 Oct 18;379[16]:1529-39).
“So in dealing with our diabetic patients, we could perhaps say there is a small reduction in the risk of cardiovascular outcomes that is overwhelmed by more than a factor of two with regard to an increase in the risk of bleeding,” the cardiologist observed.
How did physicians get the aspirin story for primary prevention so wrong for so long? Dr. O’Gara pointed to the Physicians’ Health Study, conducted mainly back in the 1970s, as one of the benchmark studies that led to the widespread use of aspirin in this way.
“I think the aspirin story has now been put into sharp focus just within the course of the last 6 months and should force all of us to reassess what it is that we advise patients,” he concluded.
Dr. O’Gara’s presentation was the talk of the meeting, as many attendees hadn’t yet caught up with the latest aspirin data.
During an Q&A session, Robert A. Vogel, MD, a preventive cardiology authority at the University of Colorado, Denver, was asked, given the new emphasis placed upon coronary artery calcium as a supplemental risk assessment tool in the latest guidelines, at what magnitude of coronary artery calcium score in a patient with no history of coronary disease he would give aspirin for secondary prevention.
“I know I don’t know the answer to that question,” Dr. Vogel replied. “I no longer reflexively give aspirin to, say, a 60-year-old with a calcium score of 200. I will give a statin. Statins in my book are so effective and safe that my threshold for giving a statin in a 60-year-old is virtually nothing. But with a calcium score of 2,000 or 5,000, I worry just like you worry.”
He noted that the primary prevention patients in the three recent major trials were mostly 60-70 years of age or older. It’s safe to assume that by that point in life many of them had silent atherosclerosis and would have had a non-zero coronary artery calcium score, had they been tested. And yet, aspirin didn’t provide any net benefit in those groups, unlike the drug’s rock-solid proven value in patients who have actually experienced a cardiovascular event.
Dr. O’Gara reported receiving funding from the National Heart, Lung and Blood Institute, the National Institute of Dental and Craniofacial Research, from Medtronic in conjunction with the ongoing pivotal APOLLO transcatheter mitral valve replacement trial, and from Edwards Lifesciences for the ongoing EARLY TAVR trial.
This article was updated 1/31/19.
REPORTING FROM ACC SNOWMASS 2019
Hospitalist PA and health system leader: Emilie Thornhill Davis
Building a collaborative practice
Emilie Thornhill Davis, PA-C, is the assistant vice president for advanced practice providers at Ochsner Health System in New Orleans. She is the former chair of SHM’s Nurse Practitioner and Physician Assistant (NP/PA) Committee, and has spoken multiple times at the SHM Annual Conference.
In honor of the inaugural National Hospitalist Day, to be held on Thursday, March 7, 2019, The Hospitalist spoke with Ms. Davis about the unique contributions of NP and PA hospitalists to the specialty of hospital medicine.
Where did you get your education?
I got my undergraduate degree from Mercer University and then went on to get my prerequisites for PA school, and worked clinically for a year prior to starting graduate school in Savannah, Georgia, at South University.
Was your intention always to be a PA?
During my sophomore year of college, Mercer was starting a PA program. Having been taken care of by PAs for most of my life, I realized that this was a profession I was very interested in. I shadowed a lot of PAs and found that they had extremely high levels of satisfaction. I saw the versatility to do so many types of medicine as a PA.
How did you become interested in hospital medicine?
When I was in PA school, we had small groups that were led by a PA who practiced clinically. The PA who was my small group leader was a hospitalist and was a fantastic role model. I did a clinical rotation with her team, and then went on to do my elective with her team in hospital medicine. When I graduated, I got my first job with the hospital medicine group that I had done those clinical rotations with in Savannah. And then in 2013, after about a year and a half, life brought me to New Orleans and I started working at Ochsner in the department of hospital medicine. I was one of the first two PAs that this group had employed.
What is your current role and title at Ochsner?
From 2013 to 2018, I worked in the department of hospital medicine, and for the last 2 years I functioned as the system lead for advanced practice providers in the department of hospital medicine. In September of 2018, I accepted the role of assistant vice president of advanced practice providers for Ochsner Health System.
What are your areas of interest or research?
I’ve had the opportunity to speak at the annual Society of Hospital Medicine Conference for 3 years in a row on innovative models of care, and nurse practitioner and PA utilization in hospital medicine. I was the chair for the NP/PA committee, and during that time we developed a toolkit aimed at providing a resource to hospital medicine groups around nurse practitioner and PA integration to practice in full utilization.
What has your experience taught you about how NPs and PAs can best fit into hospital medicine groups?
Nurse practitioners and PAs are perfectly set up to integrate into practice in hospital medicine. Training for PAs specifically is based on the medicine model, where you have a year of didactic and a year of clinical work in all the major disciplines of medicine. And so in a clinical year as a PA, I would rotate through primary care, internal medicine, general surgery, ob.gyn., psychiatry, emergency medicine, pediatrics. When I come out of school, I’m generalist trained, and depending on where your emphasis was during clinical rotations, that could include a lot of inpatient experience.
I transitioned very smoothly into my first role in hospital medicine as a PA, because I had gained that experience while I was a student on clinical rotations. PAs and nurse practitioners are – when they’re utilized appropriately and at the top of their experience and training – able to provide services to patients that can improve quality outcomes, enhance throughput, decrease length of stay, and improve all the different areas that we focus on as hospitalists.
What roles can a PA occupy in relation to physicians and nurse practitioners in hospital medicine?
When you’re looking at a PA versus a nurse practitioner in hospital medicine, you’ll notice that there are differences in the way that PAs and nurse practitioners are trained. All PAs are trained on a medical model and have a very similar kind of generalist background, whereas a nurse practitioner is typically schooled with nursing training that includes bedside experience that you can’t always guarantee with PAs. But once we enter into practice, our scope and the way that we take care of patients over time becomes very similar. So a PA and a nurse practitioner for the most part can function in very similar capacities in hospital medicine.
The only thing that creates a difference for PAs and NPs are federal and state rules and regulations, as well as hospital policies that might create “scope of practice” barriers. For instance, when I first moved to Louisiana, PAs were not able to prescribe Schedule II medications. That created a barrier whenever I was discharging patients who needed prescriptions for Schedule II. That has since changed in the state of Louisiana; now both PAs and NPs have full prescriptive authority in the state.
I would compare the work of PAs and NPs to that of physicians like this: Once you have NPs or PAs who are trained and have experience in the specialty that they are working in, they are able to provide services that would otherwise be provided by physicians.
How does a hospitalist PA work differently from a PA in other care settings?
The scope of practice for a PA is defined by the physician they’re working with. So my day-to-day work as a PA in hospital medicine looked very similar to a physician’s day-to-day work in hospital medicine. In cardiology, for example, the same would likely hold true, but with tasks unique to that specialty.
How does SHM support hospitalist PAs?
SHM is the home where you have physicians, nurse practitioners, and PAs all represented by one society, which I think is really important whenever we’re talking about a membership organization that reflects what things truly look like in practice. When I am a member of SHM, and the physician I work with is a member of SHM, we are getting the same journals and are both familiar with the changes that occur nationally in our specialty; this really helps us to align ourselves clinically, and to understand what’s going on across the country.
What kind of resources do hospitalist PAs need to succeed, either from SHM or from their own institutions?
I think the first thing we have to do is make sure that we’re getting the nomenclature right, that we’re referring to nurse practitioners and physician assistants by their appropriate names and recognizing their role in hospital medicine. Every year that I spoke at the SHM Annual Conference, I had many hospital medicine leaders come up to me and say they needed help with incorporating NPs and PAs, not only clinically, but also making sure they were represented within their hospital system. That’s why we developed the toolkit, which provides resources for integrating NPs and PAs into practice.
There is an investment early on when you bring PAs into your group to train them. We often use the SHM core competencies when we are referring to a training guide for PAs or NPs as a way to categorize the different materials that they would need to know to practice efficiently, but I do think those could be expanded upon.
What’s on the horizon for NPs and PAs in hospital medicine?
One national trend I see is an increase in the number of NPs and PAs entering hospital medicine. The other big trend is the formal development of postgraduate fellowships for PAs in hospital medicine. As the complexity of our health systems continues to grow, the feeling is that to get a nurse practitioner and PA the training they need, there are benefits to having a protected postgrad year to learn.
One unique thing about nurse practitioners and PAs is their versatility and their ability to move among the various medical specialties. As a PA or a nurse practitioner, if I’m working in hospital medicine and I have a really strong foundation, there’s nothing to say that I couldn’t then accept a job in CV surgery or cardiology and bring those skills with me from hospital medicine.
But this is kind of a double-edged sword, because it also means that you may have a PA or NP leave your HM group after 1-2 years. That kind of turnover is a difficult thing to address, because it means dealing with issues such as workplace culture and compensation. But that shows why training and engagement is important early on in that first year – to make sure that NPs and PAs feel fully supported to meet the demands that hospital medicine requires. All of those things really factor into whether an NP or PA will choose to continue in the field.
Building a collaborative practice
Building a collaborative practice
Emilie Thornhill Davis, PA-C, is the assistant vice president for advanced practice providers at Ochsner Health System in New Orleans. She is the former chair of SHM’s Nurse Practitioner and Physician Assistant (NP/PA) Committee, and has spoken multiple times at the SHM Annual Conference.
In honor of the inaugural National Hospitalist Day, to be held on Thursday, March 7, 2019, The Hospitalist spoke with Ms. Davis about the unique contributions of NP and PA hospitalists to the specialty of hospital medicine.
Where did you get your education?
I got my undergraduate degree from Mercer University and then went on to get my prerequisites for PA school, and worked clinically for a year prior to starting graduate school in Savannah, Georgia, at South University.
Was your intention always to be a PA?
During my sophomore year of college, Mercer was starting a PA program. Having been taken care of by PAs for most of my life, I realized that this was a profession I was very interested in. I shadowed a lot of PAs and found that they had extremely high levels of satisfaction. I saw the versatility to do so many types of medicine as a PA.
How did you become interested in hospital medicine?
When I was in PA school, we had small groups that were led by a PA who practiced clinically. The PA who was my small group leader was a hospitalist and was a fantastic role model. I did a clinical rotation with her team, and then went on to do my elective with her team in hospital medicine. When I graduated, I got my first job with the hospital medicine group that I had done those clinical rotations with in Savannah. And then in 2013, after about a year and a half, life brought me to New Orleans and I started working at Ochsner in the department of hospital medicine. I was one of the first two PAs that this group had employed.
What is your current role and title at Ochsner?
From 2013 to 2018, I worked in the department of hospital medicine, and for the last 2 years I functioned as the system lead for advanced practice providers in the department of hospital medicine. In September of 2018, I accepted the role of assistant vice president of advanced practice providers for Ochsner Health System.
What are your areas of interest or research?
I’ve had the opportunity to speak at the annual Society of Hospital Medicine Conference for 3 years in a row on innovative models of care, and nurse practitioner and PA utilization in hospital medicine. I was the chair for the NP/PA committee, and during that time we developed a toolkit aimed at providing a resource to hospital medicine groups around nurse practitioner and PA integration to practice in full utilization.
What has your experience taught you about how NPs and PAs can best fit into hospital medicine groups?
Nurse practitioners and PAs are perfectly set up to integrate into practice in hospital medicine. Training for PAs specifically is based on the medicine model, where you have a year of didactic and a year of clinical work in all the major disciplines of medicine. And so in a clinical year as a PA, I would rotate through primary care, internal medicine, general surgery, ob.gyn., psychiatry, emergency medicine, pediatrics. When I come out of school, I’m generalist trained, and depending on where your emphasis was during clinical rotations, that could include a lot of inpatient experience.
I transitioned very smoothly into my first role in hospital medicine as a PA, because I had gained that experience while I was a student on clinical rotations. PAs and nurse practitioners are – when they’re utilized appropriately and at the top of their experience and training – able to provide services to patients that can improve quality outcomes, enhance throughput, decrease length of stay, and improve all the different areas that we focus on as hospitalists.
What roles can a PA occupy in relation to physicians and nurse practitioners in hospital medicine?
When you’re looking at a PA versus a nurse practitioner in hospital medicine, you’ll notice that there are differences in the way that PAs and nurse practitioners are trained. All PAs are trained on a medical model and have a very similar kind of generalist background, whereas a nurse practitioner is typically schooled with nursing training that includes bedside experience that you can’t always guarantee with PAs. But once we enter into practice, our scope and the way that we take care of patients over time becomes very similar. So a PA and a nurse practitioner for the most part can function in very similar capacities in hospital medicine.
The only thing that creates a difference for PAs and NPs are federal and state rules and regulations, as well as hospital policies that might create “scope of practice” barriers. For instance, when I first moved to Louisiana, PAs were not able to prescribe Schedule II medications. That created a barrier whenever I was discharging patients who needed prescriptions for Schedule II. That has since changed in the state of Louisiana; now both PAs and NPs have full prescriptive authority in the state.
I would compare the work of PAs and NPs to that of physicians like this: Once you have NPs or PAs who are trained and have experience in the specialty that they are working in, they are able to provide services that would otherwise be provided by physicians.
How does a hospitalist PA work differently from a PA in other care settings?
The scope of practice for a PA is defined by the physician they’re working with. So my day-to-day work as a PA in hospital medicine looked very similar to a physician’s day-to-day work in hospital medicine. In cardiology, for example, the same would likely hold true, but with tasks unique to that specialty.
How does SHM support hospitalist PAs?
SHM is the home where you have physicians, nurse practitioners, and PAs all represented by one society, which I think is really important whenever we’re talking about a membership organization that reflects what things truly look like in practice. When I am a member of SHM, and the physician I work with is a member of SHM, we are getting the same journals and are both familiar with the changes that occur nationally in our specialty; this really helps us to align ourselves clinically, and to understand what’s going on across the country.
What kind of resources do hospitalist PAs need to succeed, either from SHM or from their own institutions?
I think the first thing we have to do is make sure that we’re getting the nomenclature right, that we’re referring to nurse practitioners and physician assistants by their appropriate names and recognizing their role in hospital medicine. Every year that I spoke at the SHM Annual Conference, I had many hospital medicine leaders come up to me and say they needed help with incorporating NPs and PAs, not only clinically, but also making sure they were represented within their hospital system. That’s why we developed the toolkit, which provides resources for integrating NPs and PAs into practice.
There is an investment early on when you bring PAs into your group to train them. We often use the SHM core competencies when we are referring to a training guide for PAs or NPs as a way to categorize the different materials that they would need to know to practice efficiently, but I do think those could be expanded upon.
What’s on the horizon for NPs and PAs in hospital medicine?
One national trend I see is an increase in the number of NPs and PAs entering hospital medicine. The other big trend is the formal development of postgraduate fellowships for PAs in hospital medicine. As the complexity of our health systems continues to grow, the feeling is that to get a nurse practitioner and PA the training they need, there are benefits to having a protected postgrad year to learn.
One unique thing about nurse practitioners and PAs is their versatility and their ability to move among the various medical specialties. As a PA or a nurse practitioner, if I’m working in hospital medicine and I have a really strong foundation, there’s nothing to say that I couldn’t then accept a job in CV surgery or cardiology and bring those skills with me from hospital medicine.
But this is kind of a double-edged sword, because it also means that you may have a PA or NP leave your HM group after 1-2 years. That kind of turnover is a difficult thing to address, because it means dealing with issues such as workplace culture and compensation. But that shows why training and engagement is important early on in that first year – to make sure that NPs and PAs feel fully supported to meet the demands that hospital medicine requires. All of those things really factor into whether an NP or PA will choose to continue in the field.
Emilie Thornhill Davis, PA-C, is the assistant vice president for advanced practice providers at Ochsner Health System in New Orleans. She is the former chair of SHM’s Nurse Practitioner and Physician Assistant (NP/PA) Committee, and has spoken multiple times at the SHM Annual Conference.
In honor of the inaugural National Hospitalist Day, to be held on Thursday, March 7, 2019, The Hospitalist spoke with Ms. Davis about the unique contributions of NP and PA hospitalists to the specialty of hospital medicine.
Where did you get your education?
I got my undergraduate degree from Mercer University and then went on to get my prerequisites for PA school, and worked clinically for a year prior to starting graduate school in Savannah, Georgia, at South University.
Was your intention always to be a PA?
During my sophomore year of college, Mercer was starting a PA program. Having been taken care of by PAs for most of my life, I realized that this was a profession I was very interested in. I shadowed a lot of PAs and found that they had extremely high levels of satisfaction. I saw the versatility to do so many types of medicine as a PA.
How did you become interested in hospital medicine?
When I was in PA school, we had small groups that were led by a PA who practiced clinically. The PA who was my small group leader was a hospitalist and was a fantastic role model. I did a clinical rotation with her team, and then went on to do my elective with her team in hospital medicine. When I graduated, I got my first job with the hospital medicine group that I had done those clinical rotations with in Savannah. And then in 2013, after about a year and a half, life brought me to New Orleans and I started working at Ochsner in the department of hospital medicine. I was one of the first two PAs that this group had employed.
What is your current role and title at Ochsner?
From 2013 to 2018, I worked in the department of hospital medicine, and for the last 2 years I functioned as the system lead for advanced practice providers in the department of hospital medicine. In September of 2018, I accepted the role of assistant vice president of advanced practice providers for Ochsner Health System.
What are your areas of interest or research?
I’ve had the opportunity to speak at the annual Society of Hospital Medicine Conference for 3 years in a row on innovative models of care, and nurse practitioner and PA utilization in hospital medicine. I was the chair for the NP/PA committee, and during that time we developed a toolkit aimed at providing a resource to hospital medicine groups around nurse practitioner and PA integration to practice in full utilization.
What has your experience taught you about how NPs and PAs can best fit into hospital medicine groups?
Nurse practitioners and PAs are perfectly set up to integrate into practice in hospital medicine. Training for PAs specifically is based on the medicine model, where you have a year of didactic and a year of clinical work in all the major disciplines of medicine. And so in a clinical year as a PA, I would rotate through primary care, internal medicine, general surgery, ob.gyn., psychiatry, emergency medicine, pediatrics. When I come out of school, I’m generalist trained, and depending on where your emphasis was during clinical rotations, that could include a lot of inpatient experience.
I transitioned very smoothly into my first role in hospital medicine as a PA, because I had gained that experience while I was a student on clinical rotations. PAs and nurse practitioners are – when they’re utilized appropriately and at the top of their experience and training – able to provide services to patients that can improve quality outcomes, enhance throughput, decrease length of stay, and improve all the different areas that we focus on as hospitalists.
What roles can a PA occupy in relation to physicians and nurse practitioners in hospital medicine?
When you’re looking at a PA versus a nurse practitioner in hospital medicine, you’ll notice that there are differences in the way that PAs and nurse practitioners are trained. All PAs are trained on a medical model and have a very similar kind of generalist background, whereas a nurse practitioner is typically schooled with nursing training that includes bedside experience that you can’t always guarantee with PAs. But once we enter into practice, our scope and the way that we take care of patients over time becomes very similar. So a PA and a nurse practitioner for the most part can function in very similar capacities in hospital medicine.
The only thing that creates a difference for PAs and NPs are federal and state rules and regulations, as well as hospital policies that might create “scope of practice” barriers. For instance, when I first moved to Louisiana, PAs were not able to prescribe Schedule II medications. That created a barrier whenever I was discharging patients who needed prescriptions for Schedule II. That has since changed in the state of Louisiana; now both PAs and NPs have full prescriptive authority in the state.
I would compare the work of PAs and NPs to that of physicians like this: Once you have NPs or PAs who are trained and have experience in the specialty that they are working in, they are able to provide services that would otherwise be provided by physicians.
How does a hospitalist PA work differently from a PA in other care settings?
The scope of practice for a PA is defined by the physician they’re working with. So my day-to-day work as a PA in hospital medicine looked very similar to a physician’s day-to-day work in hospital medicine. In cardiology, for example, the same would likely hold true, but with tasks unique to that specialty.
How does SHM support hospitalist PAs?
SHM is the home where you have physicians, nurse practitioners, and PAs all represented by one society, which I think is really important whenever we’re talking about a membership organization that reflects what things truly look like in practice. When I am a member of SHM, and the physician I work with is a member of SHM, we are getting the same journals and are both familiar with the changes that occur nationally in our specialty; this really helps us to align ourselves clinically, and to understand what’s going on across the country.
What kind of resources do hospitalist PAs need to succeed, either from SHM or from their own institutions?
I think the first thing we have to do is make sure that we’re getting the nomenclature right, that we’re referring to nurse practitioners and physician assistants by their appropriate names and recognizing their role in hospital medicine. Every year that I spoke at the SHM Annual Conference, I had many hospital medicine leaders come up to me and say they needed help with incorporating NPs and PAs, not only clinically, but also making sure they were represented within their hospital system. That’s why we developed the toolkit, which provides resources for integrating NPs and PAs into practice.
There is an investment early on when you bring PAs into your group to train them. We often use the SHM core competencies when we are referring to a training guide for PAs or NPs as a way to categorize the different materials that they would need to know to practice efficiently, but I do think those could be expanded upon.
What’s on the horizon for NPs and PAs in hospital medicine?
One national trend I see is an increase in the number of NPs and PAs entering hospital medicine. The other big trend is the formal development of postgraduate fellowships for PAs in hospital medicine. As the complexity of our health systems continues to grow, the feeling is that to get a nurse practitioner and PA the training they need, there are benefits to having a protected postgrad year to learn.
One unique thing about nurse practitioners and PAs is their versatility and their ability to move among the various medical specialties. As a PA or a nurse practitioner, if I’m working in hospital medicine and I have a really strong foundation, there’s nothing to say that I couldn’t then accept a job in CV surgery or cardiology and bring those skills with me from hospital medicine.
But this is kind of a double-edged sword, because it also means that you may have a PA or NP leave your HM group after 1-2 years. That kind of turnover is a difficult thing to address, because it means dealing with issues such as workplace culture and compensation. But that shows why training and engagement is important early on in that first year – to make sure that NPs and PAs feel fully supported to meet the demands that hospital medicine requires. All of those things really factor into whether an NP or PA will choose to continue in the field.
Revised U.S. A fib guidelines revamp anticoagulation
The first update to U.S. medical-society guidelines for managing atrial fibrillation since 2014 raised the threshold for starting anticoagulant therapy in women, pegged the direct-acting oral anticoagulants (DOACs) as preferred over warfarin, and introduced for the first time weight loss as an important intervention tool for treating patients with an atrial arrhythmia.

On January 28, the American College of Cardiology, American Heart Association, and Heart Rhythm Society posted online a 2019 focused update (Circulation. 2019 Jan 28. doi: 10.1161/CIR.0000000000000665) to the 2014 atrial fibrillation (AF) management guidelines that the groups had previously published (J Am Coll Cardiol. 2014 Dec 2;64[21]:2246-80).
Perhaps the two most important changes, as well as the two that lead off the new document, were a pair of class I recommendations on using oral anticoagulation in AF patients.
This brought U.S. guidelines in line with European guidelines, set by the European Society of Cardiology in 2016 (Eur Heart J. 2016 Oct 7;37[38]:2893-962). It will now also mean that, because of the way the CHA2DS2-VASc score is calculated, women with AF who are at least 65 years old will no longer automatically get flagged as needing oral anticoagulant therapy.
“This is a really important shift. It’s recognition that female sex is not as important a risk factor [for AF-associated stroke] as once was thought,” commented Hugh Calkins, MD, professor of medicine at Johns Hopkins Medicine in Baltimore and a member of the panel that wrote the update. “This will change the number of women with AF who go on anticoagulation,” predicted Dr. Calkins, who directs the cardiac arrhythmia service at his center. “We have been struggling with the notion that all women 65 or older with AF had to be on an anticoagulant. Now a clinician has more leeway. In general, patients with AF remain underanticoagulated, but this clarifies practice and brings us in line with the European guidelines.”
The second important change to the anticoagulation recommendations was to specify the DOACs as recommended over warfarin in AF patients eligible for oral anticoagulation and without moderate to severe mitral stenosis or a mechanical heart valve, which also matches the 2016 European guidelines and updates the prior, 2014, U.S. guidelines, which didn’t even mention DOACs.
Prescribing a DOAC preferentially to AF patients has already become routine among electrophysiologists, but possibly not as routine among primary care physicians, so this change has the potential to shift practice, said Dr. Calkins. But the higher price for DOACs, compared with warfarin, can pose problems. “The cost of DOACs remains an issue that can be a serious limitation to some patients,” said Craig T. January, MD, professor of medicine at the University of Wisconsin in Madison and chair of the guideline-writing panel. He also bemoaned the absence of head-to-head comparisons of individual DOACs that could inform selecting among apixaban, dabigatran, edoxaban, and rivaroxaban.
Another notable change in the 2019 update was inclusion for the first time of weight loss as a recommended intervention, along with other risk factor modification, an addition that Dr. Calkins called “long overdue.”

“This is a new recommendation, and it will potentially be important,” said Dr. January, although the guidelines do not spell out how aggressive clinicians should be about having patients achieve weight loss, how much loss patients should achieve, or how they should do it. “There are a lot of observational data and basic science data suggesting the importance of weight loss. Most electrophysiologists already address weight loss. The problem is how to get patients to do it,” commented Vivek Reddy, MD, professor of medicine and director of cardiac arrhythmia services at Mount Sinai Hospital in New York.
Dr. Reddy expressed surprise over two other features of the updated guidelines. For the first time, the guidelines now address percutaneous left atrial appendage (LAA) occlusion and say: “Percutaneous LAA occlusion may be considered in patients with AF at increased risk of stroke who have contraindications to long-term anticoagulation.” The guidelines’ text acknowledges that this runs counter to the Food and Drug Administration labeling for the Watchman LAA occlusion device, which restricts the device to patients “deemed suitable for long-term warfarin (mirroring the inclusion criteria for enrollment in the clinical trials) but had an appropriate rationale to seek a nonpharmacological alternative to warfarin.”
“We do not take a position on the FDA’s” actions, Dr. January said in an interview.
“The ACC, AHA, and HRS guidelines should reflect what the FDA decided,” Dr. Reddy said in an interview. “I’m a little surprised the guidelines said that anticoagulation had to be contraindicated.
The 2019 update also added a class IIb, “may be reasonable” recommendation for catheter ablation of AF in patients with heart failure with reduced ejection fraction.
“I think a IIb recommendation is unfair; I think it should be a IIa recommendation because there have been positive results from two large, randomized, multicenter trials – CASTLE-AF [Catheter Ablation vs. Standard Conventional Treatment in Patients With LV Dysfunction and AF; N Engl J Med. 2018 Feb 1;378(5):417-27] and AATAC [Ablation vs Amiodarone for Treatment of AFib in Patients With CHF and an ICD; Circulation. 2016 Apr 26;133(7):1637-44], as well as positive results from several smaller randomized studies,” Dr. Reddy said. “I’m really surprised” that the recommendation was not stronger.
Dr. Calkins has been a consultant to Abbott, Altathera, AtriCare, Boehringer-Ingelheim, King, Medtronic, and St. Jude and has received research funding from Boehringer-Ingelheim, Boston Scientific, and St. Jude. Dr. January had no disclosures. Dr. Reddy has been a consultant to, received research funding from, or has an equity interest in more than three dozen companies.
The first update to U.S. medical-society guidelines for managing atrial fibrillation since 2014 raised the threshold for starting anticoagulant therapy in women, pegged the direct-acting oral anticoagulants (DOACs) as preferred over warfarin, and introduced for the first time weight loss as an important intervention tool for treating patients with an atrial arrhythmia.

On January 28, the American College of Cardiology, American Heart Association, and Heart Rhythm Society posted online a 2019 focused update (Circulation. 2019 Jan 28. doi: 10.1161/CIR.0000000000000665) to the 2014 atrial fibrillation (AF) management guidelines that the groups had previously published (J Am Coll Cardiol. 2014 Dec 2;64[21]:2246-80).
Perhaps the two most important changes, as well as the two that lead off the new document, were a pair of class I recommendations on using oral anticoagulation in AF patients.
This brought U.S. guidelines in line with European guidelines, set by the European Society of Cardiology in 2016 (Eur Heart J. 2016 Oct 7;37[38]:2893-962). It will now also mean that, because of the way the CHA2DS2-VASc score is calculated, women with AF who are at least 65 years old will no longer automatically get flagged as needing oral anticoagulant therapy.
“This is a really important shift. It’s recognition that female sex is not as important a risk factor [for AF-associated stroke] as once was thought,” commented Hugh Calkins, MD, professor of medicine at Johns Hopkins Medicine in Baltimore and a member of the panel that wrote the update. “This will change the number of women with AF who go on anticoagulation,” predicted Dr. Calkins, who directs the cardiac arrhythmia service at his center. “We have been struggling with the notion that all women 65 or older with AF had to be on an anticoagulant. Now a clinician has more leeway. In general, patients with AF remain underanticoagulated, but this clarifies practice and brings us in line with the European guidelines.”
The second important change to the anticoagulation recommendations was to specify the DOACs as recommended over warfarin in AF patients eligible for oral anticoagulation and without moderate to severe mitral stenosis or a mechanical heart valve, which also matches the 2016 European guidelines and updates the prior, 2014, U.S. guidelines, which didn’t even mention DOACs.
Prescribing a DOAC preferentially to AF patients has already become routine among electrophysiologists, but possibly not as routine among primary care physicians, so this change has the potential to shift practice, said Dr. Calkins. But the higher price for DOACs, compared with warfarin, can pose problems. “The cost of DOACs remains an issue that can be a serious limitation to some patients,” said Craig T. January, MD, professor of medicine at the University of Wisconsin in Madison and chair of the guideline-writing panel. He also bemoaned the absence of head-to-head comparisons of individual DOACs that could inform selecting among apixaban, dabigatran, edoxaban, and rivaroxaban.
Another notable change in the 2019 update was inclusion for the first time of weight loss as a recommended intervention, along with other risk factor modification, an addition that Dr. Calkins called “long overdue.”

“This is a new recommendation, and it will potentially be important,” said Dr. January, although the guidelines do not spell out how aggressive clinicians should be about having patients achieve weight loss, how much loss patients should achieve, or how they should do it. “There are a lot of observational data and basic science data suggesting the importance of weight loss. Most electrophysiologists already address weight loss. The problem is how to get patients to do it,” commented Vivek Reddy, MD, professor of medicine and director of cardiac arrhythmia services at Mount Sinai Hospital in New York.
Dr. Reddy expressed surprise over two other features of the updated guidelines. For the first time, the guidelines now address percutaneous left atrial appendage (LAA) occlusion and say: “Percutaneous LAA occlusion may be considered in patients with AF at increased risk of stroke who have contraindications to long-term anticoagulation.” The guidelines’ text acknowledges that this runs counter to the Food and Drug Administration labeling for the Watchman LAA occlusion device, which restricts the device to patients “deemed suitable for long-term warfarin (mirroring the inclusion criteria for enrollment in the clinical trials) but had an appropriate rationale to seek a nonpharmacological alternative to warfarin.”
“We do not take a position on the FDA’s” actions, Dr. January said in an interview.
“The ACC, AHA, and HRS guidelines should reflect what the FDA decided,” Dr. Reddy said in an interview. “I’m a little surprised the guidelines said that anticoagulation had to be contraindicated.
The 2019 update also added a class IIb, “may be reasonable” recommendation for catheter ablation of AF in patients with heart failure with reduced ejection fraction.
“I think a IIb recommendation is unfair; I think it should be a IIa recommendation because there have been positive results from two large, randomized, multicenter trials – CASTLE-AF [Catheter Ablation vs. Standard Conventional Treatment in Patients With LV Dysfunction and AF; N Engl J Med. 2018 Feb 1;378(5):417-27] and AATAC [Ablation vs Amiodarone for Treatment of AFib in Patients With CHF and an ICD; Circulation. 2016 Apr 26;133(7):1637-44], as well as positive results from several smaller randomized studies,” Dr. Reddy said. “I’m really surprised” that the recommendation was not stronger.
Dr. Calkins has been a consultant to Abbott, Altathera, AtriCare, Boehringer-Ingelheim, King, Medtronic, and St. Jude and has received research funding from Boehringer-Ingelheim, Boston Scientific, and St. Jude. Dr. January had no disclosures. Dr. Reddy has been a consultant to, received research funding from, or has an equity interest in more than three dozen companies.
The first update to U.S. medical-society guidelines for managing atrial fibrillation since 2014 raised the threshold for starting anticoagulant therapy in women, pegged the direct-acting oral anticoagulants (DOACs) as preferred over warfarin, and introduced for the first time weight loss as an important intervention tool for treating patients with an atrial arrhythmia.

On January 28, the American College of Cardiology, American Heart Association, and Heart Rhythm Society posted online a 2019 focused update (Circulation. 2019 Jan 28. doi: 10.1161/CIR.0000000000000665) to the 2014 atrial fibrillation (AF) management guidelines that the groups had previously published (J Am Coll Cardiol. 2014 Dec 2;64[21]:2246-80).
Perhaps the two most important changes, as well as the two that lead off the new document, were a pair of class I recommendations on using oral anticoagulation in AF patients.
This brought U.S. guidelines in line with European guidelines, set by the European Society of Cardiology in 2016 (Eur Heart J. 2016 Oct 7;37[38]:2893-962). It will now also mean that, because of the way the CHA2DS2-VASc score is calculated, women with AF who are at least 65 years old will no longer automatically get flagged as needing oral anticoagulant therapy.
“This is a really important shift. It’s recognition that female sex is not as important a risk factor [for AF-associated stroke] as once was thought,” commented Hugh Calkins, MD, professor of medicine at Johns Hopkins Medicine in Baltimore and a member of the panel that wrote the update. “This will change the number of women with AF who go on anticoagulation,” predicted Dr. Calkins, who directs the cardiac arrhythmia service at his center. “We have been struggling with the notion that all women 65 or older with AF had to be on an anticoagulant. Now a clinician has more leeway. In general, patients with AF remain underanticoagulated, but this clarifies practice and brings us in line with the European guidelines.”
The second important change to the anticoagulation recommendations was to specify the DOACs as recommended over warfarin in AF patients eligible for oral anticoagulation and without moderate to severe mitral stenosis or a mechanical heart valve, which also matches the 2016 European guidelines and updates the prior, 2014, U.S. guidelines, which didn’t even mention DOACs.
Prescribing a DOAC preferentially to AF patients has already become routine among electrophysiologists, but possibly not as routine among primary care physicians, so this change has the potential to shift practice, said Dr. Calkins. But the higher price for DOACs, compared with warfarin, can pose problems. “The cost of DOACs remains an issue that can be a serious limitation to some patients,” said Craig T. January, MD, professor of medicine at the University of Wisconsin in Madison and chair of the guideline-writing panel. He also bemoaned the absence of head-to-head comparisons of individual DOACs that could inform selecting among apixaban, dabigatran, edoxaban, and rivaroxaban.
Another notable change in the 2019 update was inclusion for the first time of weight loss as a recommended intervention, along with other risk factor modification, an addition that Dr. Calkins called “long overdue.”

“This is a new recommendation, and it will potentially be important,” said Dr. January, although the guidelines do not spell out how aggressive clinicians should be about having patients achieve weight loss, how much loss patients should achieve, or how they should do it. “There are a lot of observational data and basic science data suggesting the importance of weight loss. Most electrophysiologists already address weight loss. The problem is how to get patients to do it,” commented Vivek Reddy, MD, professor of medicine and director of cardiac arrhythmia services at Mount Sinai Hospital in New York.
Dr. Reddy expressed surprise over two other features of the updated guidelines. For the first time, the guidelines now address percutaneous left atrial appendage (LAA) occlusion and say: “Percutaneous LAA occlusion may be considered in patients with AF at increased risk of stroke who have contraindications to long-term anticoagulation.” The guidelines’ text acknowledges that this runs counter to the Food and Drug Administration labeling for the Watchman LAA occlusion device, which restricts the device to patients “deemed suitable for long-term warfarin (mirroring the inclusion criteria for enrollment in the clinical trials) but had an appropriate rationale to seek a nonpharmacological alternative to warfarin.”
“We do not take a position on the FDA’s” actions, Dr. January said in an interview.
“The ACC, AHA, and HRS guidelines should reflect what the FDA decided,” Dr. Reddy said in an interview. “I’m a little surprised the guidelines said that anticoagulation had to be contraindicated.
The 2019 update also added a class IIb, “may be reasonable” recommendation for catheter ablation of AF in patients with heart failure with reduced ejection fraction.
“I think a IIb recommendation is unfair; I think it should be a IIa recommendation because there have been positive results from two large, randomized, multicenter trials – CASTLE-AF [Catheter Ablation vs. Standard Conventional Treatment in Patients With LV Dysfunction and AF; N Engl J Med. 2018 Feb 1;378(5):417-27] and AATAC [Ablation vs Amiodarone for Treatment of AFib in Patients With CHF and an ICD; Circulation. 2016 Apr 26;133(7):1637-44], as well as positive results from several smaller randomized studies,” Dr. Reddy said. “I’m really surprised” that the recommendation was not stronger.
Dr. Calkins has been a consultant to Abbott, Altathera, AtriCare, Boehringer-Ingelheim, King, Medtronic, and St. Jude and has received research funding from Boehringer-Ingelheim, Boston Scientific, and St. Jude. Dr. January had no disclosures. Dr. Reddy has been a consultant to, received research funding from, or has an equity interest in more than three dozen companies.