User login
Official news magazine of the Society of Hospital Medicine
Copyright by Society of Hospital Medicine or related companies. All rights reserved. ISSN 1553-085X
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
div[contains(@class, 'pane-pub-article-hospitalist')]


ABIM to allow do-overs for all subspecialties with Knowledge Check-In
ABIM previously announced that, beginning in 2018, physicians taking the Knowledge Check-In in 2018 would get another chance to take it in 2 years if they were unsuccessful, even if they were due to pass the maintenance of certification (MOC) exam later that year. In 2018, Knowledge Check-Ins will be offered in internal medicine and nephrology.
“Based on feedback ABIM has received from the physician community, we are happy to let you know that we are extending this policy to include all other internal medicine subspecialties in the future,” ABIM said in a Dec. 4 announcement on its website. “This means that if a physician takes the Knowledge Check-In in the first year it is offered in their subspecialty and is unsuccessful, they will get at least one additional opportunity to take and pass it 2 years later.”
The Knowledge Check-In is an alternative to the traditional MOC process, and is administered every 2 years rather than the standard decade between MOC exams. ABIM noted that a single failure on a Knowledge Check-In will not result in a status change to a physician’s certification status.
Separately, ABIM also announced that it will continue to make practice assessment activities (part IV of the MOC program) a part of the portfolio of options that can be used to satisfy MOC requirements.
“Our intent is to support physicians completing MOC activities that are most meaningful to their practice, including those that enhance and improve medical knowledge, as well as many existing quality improvement activities, and those that blend both,” ABIM said in its announcement.
ABIM previously announced that, beginning in 2018, physicians taking the Knowledge Check-In in 2018 would get another chance to take it in 2 years if they were unsuccessful, even if they were due to pass the maintenance of certification (MOC) exam later that year. In 2018, Knowledge Check-Ins will be offered in internal medicine and nephrology.
“Based on feedback ABIM has received from the physician community, we are happy to let you know that we are extending this policy to include all other internal medicine subspecialties in the future,” ABIM said in a Dec. 4 announcement on its website. “This means that if a physician takes the Knowledge Check-In in the first year it is offered in their subspecialty and is unsuccessful, they will get at least one additional opportunity to take and pass it 2 years later.”
The Knowledge Check-In is an alternative to the traditional MOC process, and is administered every 2 years rather than the standard decade between MOC exams. ABIM noted that a single failure on a Knowledge Check-In will not result in a status change to a physician’s certification status.
Separately, ABIM also announced that it will continue to make practice assessment activities (part IV of the MOC program) a part of the portfolio of options that can be used to satisfy MOC requirements.
“Our intent is to support physicians completing MOC activities that are most meaningful to their practice, including those that enhance and improve medical knowledge, as well as many existing quality improvement activities, and those that blend both,” ABIM said in its announcement.
ABIM previously announced that, beginning in 2018, physicians taking the Knowledge Check-In in 2018 would get another chance to take it in 2 years if they were unsuccessful, even if they were due to pass the maintenance of certification (MOC) exam later that year. In 2018, Knowledge Check-Ins will be offered in internal medicine and nephrology.
“Based on feedback ABIM has received from the physician community, we are happy to let you know that we are extending this policy to include all other internal medicine subspecialties in the future,” ABIM said in a Dec. 4 announcement on its website. “This means that if a physician takes the Knowledge Check-In in the first year it is offered in their subspecialty and is unsuccessful, they will get at least one additional opportunity to take and pass it 2 years later.”
The Knowledge Check-In is an alternative to the traditional MOC process, and is administered every 2 years rather than the standard decade between MOC exams. ABIM noted that a single failure on a Knowledge Check-In will not result in a status change to a physician’s certification status.
Separately, ABIM also announced that it will continue to make practice assessment activities (part IV of the MOC program) a part of the portfolio of options that can be used to satisfy MOC requirements.
“Our intent is to support physicians completing MOC activities that are most meaningful to their practice, including those that enhance and improve medical knowledge, as well as many existing quality improvement activities, and those that blend both,” ABIM said in its announcement.
Alarm reductions don’t improve ICU response times
TORONTO – It will take more than a reduction in alarms to address the issue of alarm fatigue in the ICU; a change in the ICU staff culture is needed, suggests new research.
“It may take years to recondition clinicians [to realize] that alarms are actionable and must get a response,” Afua Kunadu, MD, said during her presentation on the study at the CHEST annual meeting. Results from prior studies had suggested that as many as 99% of clinical alarms do not result in clinical intervention, noted Dr. Kunadu, an internal medicine physician at Harlem Hospital Center in New York.
She described the program, which started in the 20-bed adult ICU of Harlem Hospital Center, following a 2014 National Patient Safety Goal issued by The Joint Commission to improve the safety of clinical alarm systems by reducing unneeded alarms and alarm fatigue. The Harlem Hospital task force that ran the program began with an audit of alarms that went off in the ICU and used the results to identify the three most common alarms: bedside cardiac monitors, infusion pumps, and mechanical ventilators. The task force arranged to reset the default settings on these devices to decrease alarm frequency and boost the clinical importance of each alarm that still sounded. Concurrently, they ran educational sessions about the new alarm thresholds, the anticipated drop in alarm number, and the increased urgency to respond to the remaining alarms very quickly for the ICU staff.
The raised thresholds effectively cut the number of alarms. The average number of alarms per patient per hour fell from 4.5 at baseline during September 2016 to about 2 after 1 month, during December 2016. Then the rate further declined to reach a steady nadir that stayed at about 1.3 alarms per patient per hour 4 months into the program.
But timely responses, measured as the percentage of alarm responses occurring within 60 seconds after the alarm went off, fell from 60% at 1 month into the program down to 12% after 4 months, Dr. Kunadu reported.
She had no disclosures.
[email protected]
On Twitter @mitchelzoler
TORONTO – It will take more than a reduction in alarms to address the issue of alarm fatigue in the ICU; a change in the ICU staff culture is needed, suggests new research.
“It may take years to recondition clinicians [to realize] that alarms are actionable and must get a response,” Afua Kunadu, MD, said during her presentation on the study at the CHEST annual meeting. Results from prior studies had suggested that as many as 99% of clinical alarms do not result in clinical intervention, noted Dr. Kunadu, an internal medicine physician at Harlem Hospital Center in New York.
She described the program, which started in the 20-bed adult ICU of Harlem Hospital Center, following a 2014 National Patient Safety Goal issued by The Joint Commission to improve the safety of clinical alarm systems by reducing unneeded alarms and alarm fatigue. The Harlem Hospital task force that ran the program began with an audit of alarms that went off in the ICU and used the results to identify the three most common alarms: bedside cardiac monitors, infusion pumps, and mechanical ventilators. The task force arranged to reset the default settings on these devices to decrease alarm frequency and boost the clinical importance of each alarm that still sounded. Concurrently, they ran educational sessions about the new alarm thresholds, the anticipated drop in alarm number, and the increased urgency to respond to the remaining alarms very quickly for the ICU staff.
The raised thresholds effectively cut the number of alarms. The average number of alarms per patient per hour fell from 4.5 at baseline during September 2016 to about 2 after 1 month, during December 2016. Then the rate further declined to reach a steady nadir that stayed at about 1.3 alarms per patient per hour 4 months into the program.
But timely responses, measured as the percentage of alarm responses occurring within 60 seconds after the alarm went off, fell from 60% at 1 month into the program down to 12% after 4 months, Dr. Kunadu reported.
She had no disclosures.
[email protected]
On Twitter @mitchelzoler
TORONTO – It will take more than a reduction in alarms to address the issue of alarm fatigue in the ICU; a change in the ICU staff culture is needed, suggests new research.
“It may take years to recondition clinicians [to realize] that alarms are actionable and must get a response,” Afua Kunadu, MD, said during her presentation on the study at the CHEST annual meeting. Results from prior studies had suggested that as many as 99% of clinical alarms do not result in clinical intervention, noted Dr. Kunadu, an internal medicine physician at Harlem Hospital Center in New York.
She described the program, which started in the 20-bed adult ICU of Harlem Hospital Center, following a 2014 National Patient Safety Goal issued by The Joint Commission to improve the safety of clinical alarm systems by reducing unneeded alarms and alarm fatigue. The Harlem Hospital task force that ran the program began with an audit of alarms that went off in the ICU and used the results to identify the three most common alarms: bedside cardiac monitors, infusion pumps, and mechanical ventilators. The task force arranged to reset the default settings on these devices to decrease alarm frequency and boost the clinical importance of each alarm that still sounded. Concurrently, they ran educational sessions about the new alarm thresholds, the anticipated drop in alarm number, and the increased urgency to respond to the remaining alarms very quickly for the ICU staff.
The raised thresholds effectively cut the number of alarms. The average number of alarms per patient per hour fell from 4.5 at baseline during September 2016 to about 2 after 1 month, during December 2016. Then the rate further declined to reach a steady nadir that stayed at about 1.3 alarms per patient per hour 4 months into the program.
But timely responses, measured as the percentage of alarm responses occurring within 60 seconds after the alarm went off, fell from 60% at 1 month into the program down to 12% after 4 months, Dr. Kunadu reported.
She had no disclosures.
[email protected]
On Twitter @mitchelzoler
AT CHEST 2017
Key clinical point:
Major finding: Average alarms/patient/hour fell from 4.5 to 1.3, but the percentage of responses in less than 60 seconds fell from 60% to 12%.
Data source: An observational study at a single adult ICU in the United States.
Disclosures: Dr. Kunadu had no disclosures.
Underlying peripheral arterial or venous disease in patients with lower extremity SSTIs
Clinical case
A 56-year-old woman with type 2 diabetes, morbid obesity, and hypertension presents with right lower extremity erythema, weeping, and exquisite tenderness associated with chills. She reports a 2-year history of chronic lower extremity swelling and cramps with a more recent development of scaling and two superficial ulcers on lower third of her leg. For 1 month, she has noted significant pain circumferentially around the ankles with focal tautness and pallor of the skin. She has tried acetaminophen and oxycodone with little relief.
Over the past week, she noted foul smelling discharge from one of the superficial ulcers with redness extending up to the knee prompting presentation to the emergency department. She had a fever to 101.2° F, tachycardia to 105 beats per minute, and leukocytosis to 14.7. She is admitted to the hospitalist service for sepsis secondary to right lower extremity cellulitis.
Introduction
Skin and soft tissue infections (SSTIs) remain among the most common inpatient diagnoses cared for by hospitalists. Most patients admitted to a hospitalist service with an SSTI meet the criteria for either moderate or severe infection as outlined by the Infectious Disease Society of America – systemic signs of infection by SIRS criteria or a high likelihood of an immunocompromised state, methicillin-resistant Staphylococcus aureus infection, trauma, or wounds.1
Often these patients have several comorbid conditions such as diabetes, morbid obesity, or peripheral arterial and venous disease. Though most hospitalists are adept at managing diabetes, blood pressure, and other comorbidities, the ability to recognize and manage peripheral vascular disease can be challenging. This article will discuss ways to help providers better identify and manage underlying peripheral arterial disease (PAD) and/or chronic venous insufficiency (CVI) in patients admitted with lower extremity SSTIs.
1. In addition to an infection, could there also be underlying peripheral arterial or venous disease?
Patients with peripheral edema and vascular disease are predisposed to recurrent lower extremity SSTIs. When assessing for vascular disease, it is important to consider PAD and CVI separately.
CVI refers to the spectrum of syndromes caused by venous valvular incompetency, venous obstruction, or decreased muscle contraction. Veins cannot maximally deliver venous blood back to the heart resulting in venous pooling in the lower extremities. The exact mechanism of the skin changes that accompany venous insufficiency is unknown but may be related to cytokine cascades that result in perivascular inflammation and a weakening of the dermal barrier. Over time, this can develop into spontaneous ulceration of the skin.2,3
PAD refers to atherosclerosis of the noncerebral, noncoronary arteries, which leads to ischemic symptoms and atrophy of the supplied territory. Ulceration usually results from mild trauma due to poor wound healing.4,5 A thorough history, assessment of risk factors, and physical exam are essential to identifying these two potential diagnoses in patients admitted with SSTIs.
First, the provider should assess risk factors for underlying vascular disease. For PAD, these include risk factors similar to those of coronary artery disease (CAD): hypertension, hyperlipidemia, history of smoking, and poorly-controlled diabetes. Chronic kidney disease and family history are also associated with PAD. Since PAD and CAD share similar risk factors, it is often common for patients with CAD (as well as patients with cerebrovascular disease) to have PAD. Risk factors for CVI include obesity, chronic sedentary lifestyle, multiple pregnancies, family history, and prior superficial or deep venous thrombosis.2,4
Next, the provider should ask the patient about symptoms experienced prior to the onset of the current SSTI. Patients with either arterial or venous disease will typically report lower extremity symptoms that have been occurring for months to years, long before the acute SSTI. The classic symptom for PAD is claudication – leg pain or cramping that occurs on exertion and improves with rest. This is due to decreased arterial blood flow to the affected limb, felt most acutely during exercise. Other symptoms include numbness, a cool lower extremity, and lower extremity hair loss. As PAD progresses, a patient may also have rest pain, which may indicate more critical ischemia, as well nonhealing wounds after mild trauma.
In contrast, symptoms of CVI present more variably. CVI can be associated with heaviness, cramping, and pain that are usually worse in the dependent position and relieved with elevation. Patients may also report dry skin, edema, pruritus, scaling, skin tightness, and indolent ulcers at advanced stages.2-6
The physical exam can help the provider distinguish between venous and arterial disease. Patients with PAD often have diminished or nonpalpable distal pulses, bruits in proximal arteries, pallor, hair loss, nail thickening, decreased capillary refill time, and ulceration of the toes. CVI shares some common characteristics but can be distinguished by evidence of varicose veins, telangiectasia, edema (which spares the foot), lipodermatosclerosis, and atrophie blanche (white scarring around the ankle). Patients with venous disease tend to have warm lower extremities and palpable pulses. Often, there is hyperpigmentation, especially around the ankles, and associated eczematous changes with scaling, erythema, and weeping. CVI can also present with ulcers. In addition, if the SSTI is not responding to appropriate antibiotics in the typical time frame, this may be a clue that there is an underlying vascular issue.2-6
Ulcers, whether arterial or venous, comprise a break in the skin’s protective barrier and give bacteria a point of entry. Thus, ulcers often get superinfected, leading to an SSTI rather than SSTIs causing ulcers. The anatomic location can help differentiate between venous and arterial ulcers. Arterial ulcers tend to occur on the toes, heels, and lateral and medial malleoli. Venous ulcers are classically present above the medial malleolus but can occur anywhere on the medial lower third of the leg. Venous ulcers are more superficial and have an irregular shape, while arterial ulcers are deeper, have smoother edges and a “punched-out” shape. Both arterial and venous ulcers can be exudative though venous ulcers are rarely necrotic. Both arterial and venous ulcers can be painful.7-9
2. There are signs and symptoms of underlying vascular disease in a patient with a lower extremity SSTI. Now what?
Neither PAD nor CVI is a clinical diagnosis, thus further work-up is required to confirm the diagnosis and accurately classify disease severity. The timing of this work-up is of unique interest to hospitalists.
Most patients who are hospitalized with cellulitis or a superficial wound infection do not need urgent inpatient work-up of suspected peripheral arterial or venous disease. The one notable exception to this is patients with diabetic foot infections or infected arterial ulcers that need prompt evaluation for possible critical limb ischemia. Barring cases of critical limb ischemia, the main objective of identifying PAD or CVI in patients hospitalized for SSTIs is to appropriately arrange testing and follow-up after discharge.
To address specific management strategies, it is useful to stratify patients by symptom and exam severity as follows: mild/moderate PAD symptoms without ulcer; infected ulcer with PAD features; mild/moderate CVI symptoms without ulcer; and infected ulcer with CVI features. As specific guidelines for the inpatient work-up and management of suspected peripheral arterial and venous disease are sparse, we rely on guidelines and best practices used in the outpatient setting and adapt them to these potential inpatient presentations.
Mild/Moderate PAD symptoms with superimposed cellulitis but no ulceration
In a patient admitted for cellulitis without open wounds, history and review of systems might reveal the presence of claudication or other symptoms suspicious for PAD. While the U.S. Preventative Services Task Force and American College of Cardiology discourages the routine screening of asymptomatic patients for PAD, patients with risk factors who endorse symptoms should undergo initial testing for PAD with an ankle-brachial index (ABI).10
The ABI is the ratio of ankle blood pressure to arm blood pressure, and is measured via sphygmomanometry with a Doppler probe. The ABI remains the simplest, most inexpensive first-line test for PAD. An ABI value of less than 0.9 is considered diagnostic for PAD and has been found to be more than 95% specific for arterial stenoses of greater than 50% on angiography across multiple studies.11
In an inpatient with risk factors for PAD and claudication symptoms, referral for outpatient ABIs with subsequent follow-up by a primary care physician should be arranged. If a diagnosis of PAD is made via ABI, the PCP should reinforce risk factor modification (tobacco cessation, diet, exercise, and aggressive lipid, blood pressure and blood glucose control) and start medical management with a single anti-platelet agent to reduce the risk of MI, stroke, or “vascular death.” The most recent ACC guidelines recommend either aspirin or clopidogrel as an acceptable anti-platelet agent (grade 1A).12 Cilostazol may be considered if claudication symptoms are significantly interfering with lifestyle. If this management fails, the patient may be referred to a vascular specialist for consideration of revascularization.
Infected ulcer with PAD features
Unlike cellulitis, arterial ulcers are a direct sequela of arterial insufficiency and represent the far end of the spectrum of disease severity and in certain cases treatment failure. Patients who present with advanced ischemic and/or diabetic foot ulcers may have never been evaluated for PAD as an outpatient. Prompt work-up and management is required given the high degree of morbidity and mortality associated with arterial ulcers. Whether an urgent inpatient evaluation is indicated depends on the clinical evaluation.
The first step is to determine the depth of the ulceration. Critical limb ischemia may be present if the ulcer is deep, gangrenous, overlies a bony prominence, or is associated with systemic signs of sepsis. A physical exam should include an assessment of the pulses including femoral, popliteal, PT and DP, preferably with bedside Doppler ultrasound. If pulses are absent, urgent vascular surgery evaluation is warranted to prevent loss of limb; the work-up generally involves imaging such as computed tomography angiography or magnetic resonance angiography to identify culprit lesions, or if sufficiently suspicious, immediate invasive angiogram with the potential for endovascular intervention.
While palpable pulses can be reassuring and raise the possibility of a nonarterial etiology of ulceration – such as a microvascular, neuropathic or venous disease – it is important to remember that pulse exams are often unreliable and provider dependent.13 Moreover, the presence of pulses does not effectively exclude severe PAD or critical limb ischemia in patients with a high pretest probability.14 Thus, in cases of deep, complex lower extremity and foot ulcers, it is prudent to obtain urgent evaluation by a surgical wound specialist, which depending on the institution may be podiatry, vascular surgery, or wound care. This may lead to a better clinical assessment of the wound and clearer recommendations regarding the need for additional testing, such as imaging, to rule out osteomyelitis, surgical debridement, or amputation.
Inpatient ABIs in this situation may help diagnose and quantify the severity of PAD. Newer classification schemes such as the Society of Vascular Surgery Wound Ischemia Foot Infection score take into account clinical findings as well as ABI scores to better prognosticate limb loss and select patients for intervention.15 If the clinical picture is deemed sufficiently suspicious for critical limb ischemia, the patient may be taken directly for invasive testing with possible intervention.
If an infected ulcer is superficial, shows no signs of gangrene, and has been present for less than 30 days, further work-up for suspected PAD can generally be deferred to an outpatient setting after resolution of the acute infection. Management of the wound is highly institution dependent. When available, a wound care specialist (physician or nurse) or a plastic surgeon can be consulted as an inpatient to give specific recommendations that can range anywhere from enzymatic debridement to simple dressing. If this service is unavailable, we recommend dressing the wound with moist nonocclusive dressings with frequent changes. Referrals for ABI testing and follow up in podiatry, wound care, or vascular clinic should be arranged. Finally, educating the patient on what to expect can increase compliance with the outpatient treatment plan.
Mild to moderate CVI symptoms with superimposed cellulitis but no ulceration
Chronic venous insufficiency is a syndrome that has variable presentations based on the location and degree of valvular incompetence in the superficial or, less commonly, deep venous systems. For a patient with cellulitis and CVI, the clinical exam findings may be associated with venous hypertension syndrome – in which there is deep axial reflux and possible obstruction – and could also represent complex varicose disease which is usually caused by superficial reflux of the greater saphenous vein.3 The lack of advanced skin changes and ulceration raises the suspicion of mild to moderate CVI.
Guidelines from the American Venous Forum and the Society for Vascular Surgery recommend that all patients with suspected CVI, regardless of severity, undergo venous duplex ultrasound scanning as a first diagnostic test (grade 1A) to accurately classify the disease according to the Clinical Etiological Anatomical Pathophysiology (CEAP) system (Table 1).16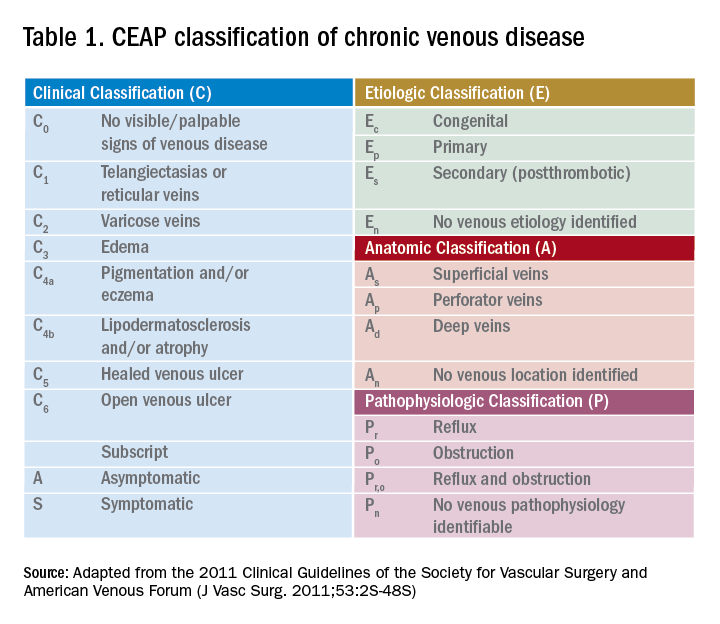
Compression therapy is commonly accepted as a noninvasive treatment option for all levels of CVI, yet most of the evidence comes from secondary prevention studies in patients with advanced CVI with venous ulcers.18 Strong evidence for the role of compression stockings in mild to moderate CVI is lacking. In fact, recent guidelines from the Society of Vascular Surgery, reviewed by the American Heart Association, do not recommend compression therapy as a primary treatment modality in patients with symptomatic varicose veins (without ulcers) if the patient is a candidate for saphenous vein ablation.19 This recommendation is based on clinical trial data that showed greater efficacy and cost-effectiveness of surgery versus conservative management in patients with CEAP2 (low severity) CVI as well as studies noting noncompliance with compression therapy as high as 75%.20-21
However, determining a patient’s candidacy for ablative or surgical therapy requires ultrasound data for accurate CEAP scoring, which is often not achieved as an inpatient. Given the potential benefit and lack of severe adverse effects, hospitalists can consider initiating compression therapy at the time of discharge in a patient with mild to moderate signs of CVI and a low risk profile for severe PAD. The prescription should specify knee-length elastic stockings with graduated compression between 20 to 30 mm Hg.22 The patient should also be encouraged to complete the outpatient duplex ultrasound testing prior to the PCP visit so that he or she can be referred to a vascular specialist appropriately.
Infected ulcer with CVI features
If the patient’s exam is suspicious for advanced venous disease with ulceration, the clinician should evaluate for the presence of scarring. This would indicate that there has been long-standing venous disease with recurrent ulceration. This patient should be asked about a previous diagnosis of CVI, prior compression therapy, and barriers to compliance with compression therapy such as poor fit or difficulty of use due to obesity or immobility. It is important to note that mixed ulcers are present in up to 20% of patients; a careful assessment of risk factors for PAD, pulse exam, and referral for outpatient ABI testing is warranted to rule out arterial insufficiency in this patient with likely venous ulcer.23
The AHA recommends prompt specialist evaluation for CEAP scores greater than or equal to 4; based on physical exam alone, this patient’s active venous ulcer yields the highest possible score of 6.2 If not previously done, this patient with advanced CVI and ulceration should be referred for an outpatient venous duplex ultrasound as well as urgent follow-up with a vascular specialist soon after discharge.
There is significant consensus in the literature that multilayer compression therapy between 30 and 40 mm Hg is the first-line treatment in patients with venous ulcers as it has been shown to promote ulcer healing and prevent recurrence.24-25 In addition, superficial venous surgery, including minimally invasive ablation, can reduce the recurrence of ulcers if used as adjunctive therapy in selected patients.26 However, compressive therapy should generally not be prescribed in patients with venous ulcers until PAD has been ruled out.
If ABI results are available, the clinician can consider compression at 30-40 mm Hg for ABI values greater than 0.8 and reduced compression at 20-30 mm Hg for values of 0.5-0.8; compression is contraindicated if the ABI is less than 0.5. Prompt follow-up with a vascular specialist can help direct compressive and/or surgical therapy. Wound care consultation as an inpatient can assist with dressing recommendations, though the evidence has not shown that dressings of any type worn under compressive garments improve ulcer healing.27
Bottom line
Hospitalists are in a unique position to identify patients with underlying peripheral arterial and venous disease when they are admitted for lower extremity skin and soft tissue infections. A focused history and physical exam can yield significant clinical clues and should prompt either inpatient or outpatient work-up.
In patients with deep ulcers and concern for critical limb ischemia, inpatient consultation should be sought. In patients with superficial venous or arterial ulcers, referral for outpatient ABI, color duplex ultrasound, or both should be made; most of these patients should also be directly referred to a vascular and/or wound specialist. Patients with more benign forms of disease who endorse chronic symptoms suspicious for mild to moderate PAD or CVI can be seen by a PCP for further management. All patients should be educated about the importance of follow-up as it remains their best chance to curb the progression of disease, reduce the risks for recurrent infection, and improve overall quality of life.
Back to the original case
Our patient’s lower extremity erythema, fever, and leukocytosis improved with 3 days of IV vancomycin treatment. Her wound was kept clean with moist dressings and showed no signs of deep infection; with elevation, her bilateral lower extremity edema also improved. Her physical exam findings and clinical history were highly suspicious for long-standing CVI. She was discharged with oral antibiotics and a referral to wound care for ongoing management of her superficial ulcers. An outpatient venous duplex ultrasound and ABI were scheduled prior to her vascular surgery appointment to effectively rule out PAD before consideration of further therapy for severe CVI.
Key Points
- Hospitalists are in a unique position to identify patients with peripheral vascular disease when they are admitted with SSTIs.
- When assessing patients, it is important to consider peripheral arterial disease (PAD) and chronic venous insufficiency (CVI) separately.
- The classic symptom for PAD is claudication. In contrast, symptoms of CVI present more variably.
- Barring cases of critical limb ischemia, the main objective of identifying PAD or CVI is to arrange testing and follow-up after discharge.
References
1. Stevens, DL, et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2014;59(2):147-59.
2. Eberhardt, RT, et al. Chronic venous insufficiency. Circulation. 2014;130:333-46.
3. Raju, S, et al. Chronic venous insufficiency and varicose veins. N Engl J Med. 2009;360:2319-27.
4. Kullo, IJ, et al. Peripheral artery disease. N Engl J Med. 2016;374(9):861-71.
5. Hennion D, et al. Diagnosis and treatment of peripheral arterial disease. Am Fam Physician. 2013 Sep 1;88(5):306-10.
6. Henke P, et al. ACP Observer Extra: Peripheral arterial disease. June 2007.
7. Vivas A. Venous leg ulcers. Ann Intern Med. 2016;165(3):ITC17-32.
8. Sumpio BE. Foot ulcers. N Engl J Med. 2000;343(11):787-93.
9. Bazari H, et al. Case 7–2007. 59-year-old woman with diabetic renal disease and nonhealing skin ulcers. N Engl J Med. 2007 Mar 8; 356(10):1049-57.
10. Moyer VA. Screening for peripheral artery disease and cardiovascular disease risk assessment with the ankle-brachial index in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2013 Sep 3.159(5):342-8.
11. Khan TH, et al. Critical review of the ankle brachial index. Curr Cardiol Rev. 2008 May;4(2):101-6.
12. Gerhard-Herman MD, et al. 2016 AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease. A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. 2016.
13. Brearley S, et al. Peripheral pulse palpation: An unreliable physical sign. Ann R Coll Surg Engl. 1992;74:169-71.
14. Khan NA, et al. Does the clinical examination predict lower extremity peripheral arterial disease? JAMA. 2006;295(5):536-46.
15. Mills JL Sr., et al. The Society for Vascular Surgery Lower Extremity Threatened Limb Classification System: Risk stratification based on wound, ischemia, and foot infection (WIfI). J Vasc Surg. 2014 Jan;59(1):220-34.e1-2.
16. Gloviczki P, et al. The care of patients with varicose veins and associated chronic venous diseases: Clinical practice guidelines of the Society for Vascular Surgery and the American Venous Forum. J Vasc Surg. 2011;53(5, Suppl):2S-48S.
17. Hamper UM,et al. Ultrasound evaluation of the lower extremity veins. Radiol Clin North Am. 2007 May;45(3):525-47.
18. Vivas A. Venous leg ulcers. Ann Intern Med. 2016;165(3):ITC17-32.
19. Gloviczki P, et al. The care of patients with varicose veins and associated chronic venous diseases: Clinical practice guidelines of the Society for Vascular Surgery and the American Venous Forum. J Vasc Surg. 2011;53(5, Suppl):2S-48S.
20. Michaels JA, et al. Randomized clinical trial comparing surgery with conservative treatment for uncomplicated varicose veins. Br J Surg. 2006 Feb;93(2):175-81.
21. Raju S, et al. Use of compression stockings in chronic venous disease: Patient compliance and efficacy. Ann Vasc Surg. 2007 Nov;21(6):790-5.
22. Gloviczki P, et al. The care of patients with varicose veins and associated chronic venous diseases: Clinical practice guidelines of the Society for Vascular Surgery and the American Venous Forum. J Vasc Surg. 2011;53(5, Suppl):2S-48S.
23. Humphreys ML, et al. Management of mixed arterial and venous leg ulcers. Br J Surg. 2007 Sep;94(9):1104-7.
24. O’Meara S, et al. Compression for venous leg ulcers. Cochrane Database Syst Rev. 2012 Nov 14;11:CD000265.
25. Dolibog P, et al. A comparative clinical study on five types of compression therapy in patients with venous leg ulcers. Int J Med Sci. 2013;11(1):34-43.
26. Gohel MS, et al. Long term results of compression therapy alone versus compression plus surgery in chronic venous ulceration (ESCHAR): Randomised controlled trial. BMJ. 2007 Jul 14;335(7610):83.
27. Palfreyman S, et al. Dressings for venous leg ulcers: systematic review and meta-analysis. BMJ. 2007 Aug 4;335(7613):244.
Clinical case
A 56-year-old woman with type 2 diabetes, morbid obesity, and hypertension presents with right lower extremity erythema, weeping, and exquisite tenderness associated with chills. She reports a 2-year history of chronic lower extremity swelling and cramps with a more recent development of scaling and two superficial ulcers on lower third of her leg. For 1 month, she has noted significant pain circumferentially around the ankles with focal tautness and pallor of the skin. She has tried acetaminophen and oxycodone with little relief.
Over the past week, she noted foul smelling discharge from one of the superficial ulcers with redness extending up to the knee prompting presentation to the emergency department. She had a fever to 101.2° F, tachycardia to 105 beats per minute, and leukocytosis to 14.7. She is admitted to the hospitalist service for sepsis secondary to right lower extremity cellulitis.
Introduction
Skin and soft tissue infections (SSTIs) remain among the most common inpatient diagnoses cared for by hospitalists. Most patients admitted to a hospitalist service with an SSTI meet the criteria for either moderate or severe infection as outlined by the Infectious Disease Society of America – systemic signs of infection by SIRS criteria or a high likelihood of an immunocompromised state, methicillin-resistant Staphylococcus aureus infection, trauma, or wounds.1
Often these patients have several comorbid conditions such as diabetes, morbid obesity, or peripheral arterial and venous disease. Though most hospitalists are adept at managing diabetes, blood pressure, and other comorbidities, the ability to recognize and manage peripheral vascular disease can be challenging. This article will discuss ways to help providers better identify and manage underlying peripheral arterial disease (PAD) and/or chronic venous insufficiency (CVI) in patients admitted with lower extremity SSTIs.
1. In addition to an infection, could there also be underlying peripheral arterial or venous disease?
Patients with peripheral edema and vascular disease are predisposed to recurrent lower extremity SSTIs. When assessing for vascular disease, it is important to consider PAD and CVI separately.
CVI refers to the spectrum of syndromes caused by venous valvular incompetency, venous obstruction, or decreased muscle contraction. Veins cannot maximally deliver venous blood back to the heart resulting in venous pooling in the lower extremities. The exact mechanism of the skin changes that accompany venous insufficiency is unknown but may be related to cytokine cascades that result in perivascular inflammation and a weakening of the dermal barrier. Over time, this can develop into spontaneous ulceration of the skin.2,3
PAD refers to atherosclerosis of the noncerebral, noncoronary arteries, which leads to ischemic symptoms and atrophy of the supplied territory. Ulceration usually results from mild trauma due to poor wound healing.4,5 A thorough history, assessment of risk factors, and physical exam are essential to identifying these two potential diagnoses in patients admitted with SSTIs.
First, the provider should assess risk factors for underlying vascular disease. For PAD, these include risk factors similar to those of coronary artery disease (CAD): hypertension, hyperlipidemia, history of smoking, and poorly-controlled diabetes. Chronic kidney disease and family history are also associated with PAD. Since PAD and CAD share similar risk factors, it is often common for patients with CAD (as well as patients with cerebrovascular disease) to have PAD. Risk factors for CVI include obesity, chronic sedentary lifestyle, multiple pregnancies, family history, and prior superficial or deep venous thrombosis.2,4
Next, the provider should ask the patient about symptoms experienced prior to the onset of the current SSTI. Patients with either arterial or venous disease will typically report lower extremity symptoms that have been occurring for months to years, long before the acute SSTI. The classic symptom for PAD is claudication – leg pain or cramping that occurs on exertion and improves with rest. This is due to decreased arterial blood flow to the affected limb, felt most acutely during exercise. Other symptoms include numbness, a cool lower extremity, and lower extremity hair loss. As PAD progresses, a patient may also have rest pain, which may indicate more critical ischemia, as well nonhealing wounds after mild trauma.
In contrast, symptoms of CVI present more variably. CVI can be associated with heaviness, cramping, and pain that are usually worse in the dependent position and relieved with elevation. Patients may also report dry skin, edema, pruritus, scaling, skin tightness, and indolent ulcers at advanced stages.2-6
The physical exam can help the provider distinguish between venous and arterial disease. Patients with PAD often have diminished or nonpalpable distal pulses, bruits in proximal arteries, pallor, hair loss, nail thickening, decreased capillary refill time, and ulceration of the toes. CVI shares some common characteristics but can be distinguished by evidence of varicose veins, telangiectasia, edema (which spares the foot), lipodermatosclerosis, and atrophie blanche (white scarring around the ankle). Patients with venous disease tend to have warm lower extremities and palpable pulses. Often, there is hyperpigmentation, especially around the ankles, and associated eczematous changes with scaling, erythema, and weeping. CVI can also present with ulcers. In addition, if the SSTI is not responding to appropriate antibiotics in the typical time frame, this may be a clue that there is an underlying vascular issue.2-6
Ulcers, whether arterial or venous, comprise a break in the skin’s protective barrier and give bacteria a point of entry. Thus, ulcers often get superinfected, leading to an SSTI rather than SSTIs causing ulcers. The anatomic location can help differentiate between venous and arterial ulcers. Arterial ulcers tend to occur on the toes, heels, and lateral and medial malleoli. Venous ulcers are classically present above the medial malleolus but can occur anywhere on the medial lower third of the leg. Venous ulcers are more superficial and have an irregular shape, while arterial ulcers are deeper, have smoother edges and a “punched-out” shape. Both arterial and venous ulcers can be exudative though venous ulcers are rarely necrotic. Both arterial and venous ulcers can be painful.7-9
2. There are signs and symptoms of underlying vascular disease in a patient with a lower extremity SSTI. Now what?
Neither PAD nor CVI is a clinical diagnosis, thus further work-up is required to confirm the diagnosis and accurately classify disease severity. The timing of this work-up is of unique interest to hospitalists.
Most patients who are hospitalized with cellulitis or a superficial wound infection do not need urgent inpatient work-up of suspected peripheral arterial or venous disease. The one notable exception to this is patients with diabetic foot infections or infected arterial ulcers that need prompt evaluation for possible critical limb ischemia. Barring cases of critical limb ischemia, the main objective of identifying PAD or CVI in patients hospitalized for SSTIs is to appropriately arrange testing and follow-up after discharge.
To address specific management strategies, it is useful to stratify patients by symptom and exam severity as follows: mild/moderate PAD symptoms without ulcer; infected ulcer with PAD features; mild/moderate CVI symptoms without ulcer; and infected ulcer with CVI features. As specific guidelines for the inpatient work-up and management of suspected peripheral arterial and venous disease are sparse, we rely on guidelines and best practices used in the outpatient setting and adapt them to these potential inpatient presentations.
Mild/Moderate PAD symptoms with superimposed cellulitis but no ulceration
In a patient admitted for cellulitis without open wounds, history and review of systems might reveal the presence of claudication or other symptoms suspicious for PAD. While the U.S. Preventative Services Task Force and American College of Cardiology discourages the routine screening of asymptomatic patients for PAD, patients with risk factors who endorse symptoms should undergo initial testing for PAD with an ankle-brachial index (ABI).10
The ABI is the ratio of ankle blood pressure to arm blood pressure, and is measured via sphygmomanometry with a Doppler probe. The ABI remains the simplest, most inexpensive first-line test for PAD. An ABI value of less than 0.9 is considered diagnostic for PAD and has been found to be more than 95% specific for arterial stenoses of greater than 50% on angiography across multiple studies.11
In an inpatient with risk factors for PAD and claudication symptoms, referral for outpatient ABIs with subsequent follow-up by a primary care physician should be arranged. If a diagnosis of PAD is made via ABI, the PCP should reinforce risk factor modification (tobacco cessation, diet, exercise, and aggressive lipid, blood pressure and blood glucose control) and start medical management with a single anti-platelet agent to reduce the risk of MI, stroke, or “vascular death.” The most recent ACC guidelines recommend either aspirin or clopidogrel as an acceptable anti-platelet agent (grade 1A).12 Cilostazol may be considered if claudication symptoms are significantly interfering with lifestyle. If this management fails, the patient may be referred to a vascular specialist for consideration of revascularization.
Infected ulcer with PAD features
Unlike cellulitis, arterial ulcers are a direct sequela of arterial insufficiency and represent the far end of the spectrum of disease severity and in certain cases treatment failure. Patients who present with advanced ischemic and/or diabetic foot ulcers may have never been evaluated for PAD as an outpatient. Prompt work-up and management is required given the high degree of morbidity and mortality associated with arterial ulcers. Whether an urgent inpatient evaluation is indicated depends on the clinical evaluation.
The first step is to determine the depth of the ulceration. Critical limb ischemia may be present if the ulcer is deep, gangrenous, overlies a bony prominence, or is associated with systemic signs of sepsis. A physical exam should include an assessment of the pulses including femoral, popliteal, PT and DP, preferably with bedside Doppler ultrasound. If pulses are absent, urgent vascular surgery evaluation is warranted to prevent loss of limb; the work-up generally involves imaging such as computed tomography angiography or magnetic resonance angiography to identify culprit lesions, or if sufficiently suspicious, immediate invasive angiogram with the potential for endovascular intervention.
While palpable pulses can be reassuring and raise the possibility of a nonarterial etiology of ulceration – such as a microvascular, neuropathic or venous disease – it is important to remember that pulse exams are often unreliable and provider dependent.13 Moreover, the presence of pulses does not effectively exclude severe PAD or critical limb ischemia in patients with a high pretest probability.14 Thus, in cases of deep, complex lower extremity and foot ulcers, it is prudent to obtain urgent evaluation by a surgical wound specialist, which depending on the institution may be podiatry, vascular surgery, or wound care. This may lead to a better clinical assessment of the wound and clearer recommendations regarding the need for additional testing, such as imaging, to rule out osteomyelitis, surgical debridement, or amputation.
Inpatient ABIs in this situation may help diagnose and quantify the severity of PAD. Newer classification schemes such as the Society of Vascular Surgery Wound Ischemia Foot Infection score take into account clinical findings as well as ABI scores to better prognosticate limb loss and select patients for intervention.15 If the clinical picture is deemed sufficiently suspicious for critical limb ischemia, the patient may be taken directly for invasive testing with possible intervention.
If an infected ulcer is superficial, shows no signs of gangrene, and has been present for less than 30 days, further work-up for suspected PAD can generally be deferred to an outpatient setting after resolution of the acute infection. Management of the wound is highly institution dependent. When available, a wound care specialist (physician or nurse) or a plastic surgeon can be consulted as an inpatient to give specific recommendations that can range anywhere from enzymatic debridement to simple dressing. If this service is unavailable, we recommend dressing the wound with moist nonocclusive dressings with frequent changes. Referrals for ABI testing and follow up in podiatry, wound care, or vascular clinic should be arranged. Finally, educating the patient on what to expect can increase compliance with the outpatient treatment plan.
Mild to moderate CVI symptoms with superimposed cellulitis but no ulceration
Chronic venous insufficiency is a syndrome that has variable presentations based on the location and degree of valvular incompetence in the superficial or, less commonly, deep venous systems. For a patient with cellulitis and CVI, the clinical exam findings may be associated with venous hypertension syndrome – in which there is deep axial reflux and possible obstruction – and could also represent complex varicose disease which is usually caused by superficial reflux of the greater saphenous vein.3 The lack of advanced skin changes and ulceration raises the suspicion of mild to moderate CVI.
Guidelines from the American Venous Forum and the Society for Vascular Surgery recommend that all patients with suspected CVI, regardless of severity, undergo venous duplex ultrasound scanning as a first diagnostic test (grade 1A) to accurately classify the disease according to the Clinical Etiological Anatomical Pathophysiology (CEAP) system (Table 1).16
Compression therapy is commonly accepted as a noninvasive treatment option for all levels of CVI, yet most of the evidence comes from secondary prevention studies in patients with advanced CVI with venous ulcers.18 Strong evidence for the role of compression stockings in mild to moderate CVI is lacking. In fact, recent guidelines from the Society of Vascular Surgery, reviewed by the American Heart Association, do not recommend compression therapy as a primary treatment modality in patients with symptomatic varicose veins (without ulcers) if the patient is a candidate for saphenous vein ablation.19 This recommendation is based on clinical trial data that showed greater efficacy and cost-effectiveness of surgery versus conservative management in patients with CEAP2 (low severity) CVI as well as studies noting noncompliance with compression therapy as high as 75%.20-21
However, determining a patient’s candidacy for ablative or surgical therapy requires ultrasound data for accurate CEAP scoring, which is often not achieved as an inpatient. Given the potential benefit and lack of severe adverse effects, hospitalists can consider initiating compression therapy at the time of discharge in a patient with mild to moderate signs of CVI and a low risk profile for severe PAD. The prescription should specify knee-length elastic stockings with graduated compression between 20 to 30 mm Hg.22 The patient should also be encouraged to complete the outpatient duplex ultrasound testing prior to the PCP visit so that he or she can be referred to a vascular specialist appropriately.
Infected ulcer with CVI features
If the patient’s exam is suspicious for advanced venous disease with ulceration, the clinician should evaluate for the presence of scarring. This would indicate that there has been long-standing venous disease with recurrent ulceration. This patient should be asked about a previous diagnosis of CVI, prior compression therapy, and barriers to compliance with compression therapy such as poor fit or difficulty of use due to obesity or immobility. It is important to note that mixed ulcers are present in up to 20% of patients; a careful assessment of risk factors for PAD, pulse exam, and referral for outpatient ABI testing is warranted to rule out arterial insufficiency in this patient with likely venous ulcer.23
The AHA recommends prompt specialist evaluation for CEAP scores greater than or equal to 4; based on physical exam alone, this patient’s active venous ulcer yields the highest possible score of 6.2 If not previously done, this patient with advanced CVI and ulceration should be referred for an outpatient venous duplex ultrasound as well as urgent follow-up with a vascular specialist soon after discharge.
There is significant consensus in the literature that multilayer compression therapy between 30 and 40 mm Hg is the first-line treatment in patients with venous ulcers as it has been shown to promote ulcer healing and prevent recurrence.24-25 In addition, superficial venous surgery, including minimally invasive ablation, can reduce the recurrence of ulcers if used as adjunctive therapy in selected patients.26 However, compressive therapy should generally not be prescribed in patients with venous ulcers until PAD has been ruled out.
If ABI results are available, the clinician can consider compression at 30-40 mm Hg for ABI values greater than 0.8 and reduced compression at 20-30 mm Hg for values of 0.5-0.8; compression is contraindicated if the ABI is less than 0.5. Prompt follow-up with a vascular specialist can help direct compressive and/or surgical therapy. Wound care consultation as an inpatient can assist with dressing recommendations, though the evidence has not shown that dressings of any type worn under compressive garments improve ulcer healing.27
Bottom line
Hospitalists are in a unique position to identify patients with underlying peripheral arterial and venous disease when they are admitted for lower extremity skin and soft tissue infections. A focused history and physical exam can yield significant clinical clues and should prompt either inpatient or outpatient work-up.
In patients with deep ulcers and concern for critical limb ischemia, inpatient consultation should be sought. In patients with superficial venous or arterial ulcers, referral for outpatient ABI, color duplex ultrasound, or both should be made; most of these patients should also be directly referred to a vascular and/or wound specialist. Patients with more benign forms of disease who endorse chronic symptoms suspicious for mild to moderate PAD or CVI can be seen by a PCP for further management. All patients should be educated about the importance of follow-up as it remains their best chance to curb the progression of disease, reduce the risks for recurrent infection, and improve overall quality of life.
Back to the original case
Our patient’s lower extremity erythema, fever, and leukocytosis improved with 3 days of IV vancomycin treatment. Her wound was kept clean with moist dressings and showed no signs of deep infection; with elevation, her bilateral lower extremity edema also improved. Her physical exam findings and clinical history were highly suspicious for long-standing CVI. She was discharged with oral antibiotics and a referral to wound care for ongoing management of her superficial ulcers. An outpatient venous duplex ultrasound and ABI were scheduled prior to her vascular surgery appointment to effectively rule out PAD before consideration of further therapy for severe CVI.
Key Points
- Hospitalists are in a unique position to identify patients with peripheral vascular disease when they are admitted with SSTIs.
- When assessing patients, it is important to consider peripheral arterial disease (PAD) and chronic venous insufficiency (CVI) separately.
- The classic symptom for PAD is claudication. In contrast, symptoms of CVI present more variably.
- Barring cases of critical limb ischemia, the main objective of identifying PAD or CVI is to arrange testing and follow-up after discharge.
References
1. Stevens, DL, et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2014;59(2):147-59.
2. Eberhardt, RT, et al. Chronic venous insufficiency. Circulation. 2014;130:333-46.
3. Raju, S, et al. Chronic venous insufficiency and varicose veins. N Engl J Med. 2009;360:2319-27.
4. Kullo, IJ, et al. Peripheral artery disease. N Engl J Med. 2016;374(9):861-71.
5. Hennion D, et al. Diagnosis and treatment of peripheral arterial disease. Am Fam Physician. 2013 Sep 1;88(5):306-10.
6. Henke P, et al. ACP Observer Extra: Peripheral arterial disease. June 2007.
7. Vivas A. Venous leg ulcers. Ann Intern Med. 2016;165(3):ITC17-32.
8. Sumpio BE. Foot ulcers. N Engl J Med. 2000;343(11):787-93.
9. Bazari H, et al. Case 7–2007. 59-year-old woman with diabetic renal disease and nonhealing skin ulcers. N Engl J Med. 2007 Mar 8; 356(10):1049-57.
10. Moyer VA. Screening for peripheral artery disease and cardiovascular disease risk assessment with the ankle-brachial index in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2013 Sep 3.159(5):342-8.
11. Khan TH, et al. Critical review of the ankle brachial index. Curr Cardiol Rev. 2008 May;4(2):101-6.
12. Gerhard-Herman MD, et al. 2016 AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease. A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. 2016.
13. Brearley S, et al. Peripheral pulse palpation: An unreliable physical sign. Ann R Coll Surg Engl. 1992;74:169-71.
14. Khan NA, et al. Does the clinical examination predict lower extremity peripheral arterial disease? JAMA. 2006;295(5):536-46.
15. Mills JL Sr., et al. The Society for Vascular Surgery Lower Extremity Threatened Limb Classification System: Risk stratification based on wound, ischemia, and foot infection (WIfI). J Vasc Surg. 2014 Jan;59(1):220-34.e1-2.
16. Gloviczki P, et al. The care of patients with varicose veins and associated chronic venous diseases: Clinical practice guidelines of the Society for Vascular Surgery and the American Venous Forum. J Vasc Surg. 2011;53(5, Suppl):2S-48S.
17. Hamper UM,et al. Ultrasound evaluation of the lower extremity veins. Radiol Clin North Am. 2007 May;45(3):525-47.
18. Vivas A. Venous leg ulcers. Ann Intern Med. 2016;165(3):ITC17-32.
19. Gloviczki P, et al. The care of patients with varicose veins and associated chronic venous diseases: Clinical practice guidelines of the Society for Vascular Surgery and the American Venous Forum. J Vasc Surg. 2011;53(5, Suppl):2S-48S.
20. Michaels JA, et al. Randomized clinical trial comparing surgery with conservative treatment for uncomplicated varicose veins. Br J Surg. 2006 Feb;93(2):175-81.
21. Raju S, et al. Use of compression stockings in chronic venous disease: Patient compliance and efficacy. Ann Vasc Surg. 2007 Nov;21(6):790-5.
22. Gloviczki P, et al. The care of patients with varicose veins and associated chronic venous diseases: Clinical practice guidelines of the Society for Vascular Surgery and the American Venous Forum. J Vasc Surg. 2011;53(5, Suppl):2S-48S.
23. Humphreys ML, et al. Management of mixed arterial and venous leg ulcers. Br J Surg. 2007 Sep;94(9):1104-7.
24. O’Meara S, et al. Compression for venous leg ulcers. Cochrane Database Syst Rev. 2012 Nov 14;11:CD000265.
25. Dolibog P, et al. A comparative clinical study on five types of compression therapy in patients with venous leg ulcers. Int J Med Sci. 2013;11(1):34-43.
26. Gohel MS, et al. Long term results of compression therapy alone versus compression plus surgery in chronic venous ulceration (ESCHAR): Randomised controlled trial. BMJ. 2007 Jul 14;335(7610):83.
27. Palfreyman S, et al. Dressings for venous leg ulcers: systematic review and meta-analysis. BMJ. 2007 Aug 4;335(7613):244.
Clinical case
A 56-year-old woman with type 2 diabetes, morbid obesity, and hypertension presents with right lower extremity erythema, weeping, and exquisite tenderness associated with chills. She reports a 2-year history of chronic lower extremity swelling and cramps with a more recent development of scaling and two superficial ulcers on lower third of her leg. For 1 month, she has noted significant pain circumferentially around the ankles with focal tautness and pallor of the skin. She has tried acetaminophen and oxycodone with little relief.
Over the past week, she noted foul smelling discharge from one of the superficial ulcers with redness extending up to the knee prompting presentation to the emergency department. She had a fever to 101.2° F, tachycardia to 105 beats per minute, and leukocytosis to 14.7. She is admitted to the hospitalist service for sepsis secondary to right lower extremity cellulitis.
Introduction
Skin and soft tissue infections (SSTIs) remain among the most common inpatient diagnoses cared for by hospitalists. Most patients admitted to a hospitalist service with an SSTI meet the criteria for either moderate or severe infection as outlined by the Infectious Disease Society of America – systemic signs of infection by SIRS criteria or a high likelihood of an immunocompromised state, methicillin-resistant Staphylococcus aureus infection, trauma, or wounds.1
Often these patients have several comorbid conditions such as diabetes, morbid obesity, or peripheral arterial and venous disease. Though most hospitalists are adept at managing diabetes, blood pressure, and other comorbidities, the ability to recognize and manage peripheral vascular disease can be challenging. This article will discuss ways to help providers better identify and manage underlying peripheral arterial disease (PAD) and/or chronic venous insufficiency (CVI) in patients admitted with lower extremity SSTIs.
1. In addition to an infection, could there also be underlying peripheral arterial or venous disease?
Patients with peripheral edema and vascular disease are predisposed to recurrent lower extremity SSTIs. When assessing for vascular disease, it is important to consider PAD and CVI separately.
CVI refers to the spectrum of syndromes caused by venous valvular incompetency, venous obstruction, or decreased muscle contraction. Veins cannot maximally deliver venous blood back to the heart resulting in venous pooling in the lower extremities. The exact mechanism of the skin changes that accompany venous insufficiency is unknown but may be related to cytokine cascades that result in perivascular inflammation and a weakening of the dermal barrier. Over time, this can develop into spontaneous ulceration of the skin.2,3
PAD refers to atherosclerosis of the noncerebral, noncoronary arteries, which leads to ischemic symptoms and atrophy of the supplied territory. Ulceration usually results from mild trauma due to poor wound healing.4,5 A thorough history, assessment of risk factors, and physical exam are essential to identifying these two potential diagnoses in patients admitted with SSTIs.
First, the provider should assess risk factors for underlying vascular disease. For PAD, these include risk factors similar to those of coronary artery disease (CAD): hypertension, hyperlipidemia, history of smoking, and poorly-controlled diabetes. Chronic kidney disease and family history are also associated with PAD. Since PAD and CAD share similar risk factors, it is often common for patients with CAD (as well as patients with cerebrovascular disease) to have PAD. Risk factors for CVI include obesity, chronic sedentary lifestyle, multiple pregnancies, family history, and prior superficial or deep venous thrombosis.2,4
Next, the provider should ask the patient about symptoms experienced prior to the onset of the current SSTI. Patients with either arterial or venous disease will typically report lower extremity symptoms that have been occurring for months to years, long before the acute SSTI. The classic symptom for PAD is claudication – leg pain or cramping that occurs on exertion and improves with rest. This is due to decreased arterial blood flow to the affected limb, felt most acutely during exercise. Other symptoms include numbness, a cool lower extremity, and lower extremity hair loss. As PAD progresses, a patient may also have rest pain, which may indicate more critical ischemia, as well nonhealing wounds after mild trauma.
In contrast, symptoms of CVI present more variably. CVI can be associated with heaviness, cramping, and pain that are usually worse in the dependent position and relieved with elevation. Patients may also report dry skin, edema, pruritus, scaling, skin tightness, and indolent ulcers at advanced stages.2-6
The physical exam can help the provider distinguish between venous and arterial disease. Patients with PAD often have diminished or nonpalpable distal pulses, bruits in proximal arteries, pallor, hair loss, nail thickening, decreased capillary refill time, and ulceration of the toes. CVI shares some common characteristics but can be distinguished by evidence of varicose veins, telangiectasia, edema (which spares the foot), lipodermatosclerosis, and atrophie blanche (white scarring around the ankle). Patients with venous disease tend to have warm lower extremities and palpable pulses. Often, there is hyperpigmentation, especially around the ankles, and associated eczematous changes with scaling, erythema, and weeping. CVI can also present with ulcers. In addition, if the SSTI is not responding to appropriate antibiotics in the typical time frame, this may be a clue that there is an underlying vascular issue.2-6
Ulcers, whether arterial or venous, comprise a break in the skin’s protective barrier and give bacteria a point of entry. Thus, ulcers often get superinfected, leading to an SSTI rather than SSTIs causing ulcers. The anatomic location can help differentiate between venous and arterial ulcers. Arterial ulcers tend to occur on the toes, heels, and lateral and medial malleoli. Venous ulcers are classically present above the medial malleolus but can occur anywhere on the medial lower third of the leg. Venous ulcers are more superficial and have an irregular shape, while arterial ulcers are deeper, have smoother edges and a “punched-out” shape. Both arterial and venous ulcers can be exudative though venous ulcers are rarely necrotic. Both arterial and venous ulcers can be painful.7-9
2. There are signs and symptoms of underlying vascular disease in a patient with a lower extremity SSTI. Now what?
Neither PAD nor CVI is a clinical diagnosis, thus further work-up is required to confirm the diagnosis and accurately classify disease severity. The timing of this work-up is of unique interest to hospitalists.
Most patients who are hospitalized with cellulitis or a superficial wound infection do not need urgent inpatient work-up of suspected peripheral arterial or venous disease. The one notable exception to this is patients with diabetic foot infections or infected arterial ulcers that need prompt evaluation for possible critical limb ischemia. Barring cases of critical limb ischemia, the main objective of identifying PAD or CVI in patients hospitalized for SSTIs is to appropriately arrange testing and follow-up after discharge.
To address specific management strategies, it is useful to stratify patients by symptom and exam severity as follows: mild/moderate PAD symptoms without ulcer; infected ulcer with PAD features; mild/moderate CVI symptoms without ulcer; and infected ulcer with CVI features. As specific guidelines for the inpatient work-up and management of suspected peripheral arterial and venous disease are sparse, we rely on guidelines and best practices used in the outpatient setting and adapt them to these potential inpatient presentations.
Mild/Moderate PAD symptoms with superimposed cellulitis but no ulceration
In a patient admitted for cellulitis without open wounds, history and review of systems might reveal the presence of claudication or other symptoms suspicious for PAD. While the U.S. Preventative Services Task Force and American College of Cardiology discourages the routine screening of asymptomatic patients for PAD, patients with risk factors who endorse symptoms should undergo initial testing for PAD with an ankle-brachial index (ABI).10
The ABI is the ratio of ankle blood pressure to arm blood pressure, and is measured via sphygmomanometry with a Doppler probe. The ABI remains the simplest, most inexpensive first-line test for PAD. An ABI value of less than 0.9 is considered diagnostic for PAD and has been found to be more than 95% specific for arterial stenoses of greater than 50% on angiography across multiple studies.11
In an inpatient with risk factors for PAD and claudication symptoms, referral for outpatient ABIs with subsequent follow-up by a primary care physician should be arranged. If a diagnosis of PAD is made via ABI, the PCP should reinforce risk factor modification (tobacco cessation, diet, exercise, and aggressive lipid, blood pressure and blood glucose control) and start medical management with a single anti-platelet agent to reduce the risk of MI, stroke, or “vascular death.” The most recent ACC guidelines recommend either aspirin or clopidogrel as an acceptable anti-platelet agent (grade 1A).12 Cilostazol may be considered if claudication symptoms are significantly interfering with lifestyle. If this management fails, the patient may be referred to a vascular specialist for consideration of revascularization.
Infected ulcer with PAD features
Unlike cellulitis, arterial ulcers are a direct sequela of arterial insufficiency and represent the far end of the spectrum of disease severity and in certain cases treatment failure. Patients who present with advanced ischemic and/or diabetic foot ulcers may have never been evaluated for PAD as an outpatient. Prompt work-up and management is required given the high degree of morbidity and mortality associated with arterial ulcers. Whether an urgent inpatient evaluation is indicated depends on the clinical evaluation.
The first step is to determine the depth of the ulceration. Critical limb ischemia may be present if the ulcer is deep, gangrenous, overlies a bony prominence, or is associated with systemic signs of sepsis. A physical exam should include an assessment of the pulses including femoral, popliteal, PT and DP, preferably with bedside Doppler ultrasound. If pulses are absent, urgent vascular surgery evaluation is warranted to prevent loss of limb; the work-up generally involves imaging such as computed tomography angiography or magnetic resonance angiography to identify culprit lesions, or if sufficiently suspicious, immediate invasive angiogram with the potential for endovascular intervention.
While palpable pulses can be reassuring and raise the possibility of a nonarterial etiology of ulceration – such as a microvascular, neuropathic or venous disease – it is important to remember that pulse exams are often unreliable and provider dependent.13 Moreover, the presence of pulses does not effectively exclude severe PAD or critical limb ischemia in patients with a high pretest probability.14 Thus, in cases of deep, complex lower extremity and foot ulcers, it is prudent to obtain urgent evaluation by a surgical wound specialist, which depending on the institution may be podiatry, vascular surgery, or wound care. This may lead to a better clinical assessment of the wound and clearer recommendations regarding the need for additional testing, such as imaging, to rule out osteomyelitis, surgical debridement, or amputation.
Inpatient ABIs in this situation may help diagnose and quantify the severity of PAD. Newer classification schemes such as the Society of Vascular Surgery Wound Ischemia Foot Infection score take into account clinical findings as well as ABI scores to better prognosticate limb loss and select patients for intervention.15 If the clinical picture is deemed sufficiently suspicious for critical limb ischemia, the patient may be taken directly for invasive testing with possible intervention.
If an infected ulcer is superficial, shows no signs of gangrene, and has been present for less than 30 days, further work-up for suspected PAD can generally be deferred to an outpatient setting after resolution of the acute infection. Management of the wound is highly institution dependent. When available, a wound care specialist (physician or nurse) or a plastic surgeon can be consulted as an inpatient to give specific recommendations that can range anywhere from enzymatic debridement to simple dressing. If this service is unavailable, we recommend dressing the wound with moist nonocclusive dressings with frequent changes. Referrals for ABI testing and follow up in podiatry, wound care, or vascular clinic should be arranged. Finally, educating the patient on what to expect can increase compliance with the outpatient treatment plan.
Mild to moderate CVI symptoms with superimposed cellulitis but no ulceration
Chronic venous insufficiency is a syndrome that has variable presentations based on the location and degree of valvular incompetence in the superficial or, less commonly, deep venous systems. For a patient with cellulitis and CVI, the clinical exam findings may be associated with venous hypertension syndrome – in which there is deep axial reflux and possible obstruction – and could also represent complex varicose disease which is usually caused by superficial reflux of the greater saphenous vein.3 The lack of advanced skin changes and ulceration raises the suspicion of mild to moderate CVI.
Guidelines from the American Venous Forum and the Society for Vascular Surgery recommend that all patients with suspected CVI, regardless of severity, undergo venous duplex ultrasound scanning as a first diagnostic test (grade 1A) to accurately classify the disease according to the Clinical Etiological Anatomical Pathophysiology (CEAP) system (Table 1).16
Compression therapy is commonly accepted as a noninvasive treatment option for all levels of CVI, yet most of the evidence comes from secondary prevention studies in patients with advanced CVI with venous ulcers.18 Strong evidence for the role of compression stockings in mild to moderate CVI is lacking. In fact, recent guidelines from the Society of Vascular Surgery, reviewed by the American Heart Association, do not recommend compression therapy as a primary treatment modality in patients with symptomatic varicose veins (without ulcers) if the patient is a candidate for saphenous vein ablation.19 This recommendation is based on clinical trial data that showed greater efficacy and cost-effectiveness of surgery versus conservative management in patients with CEAP2 (low severity) CVI as well as studies noting noncompliance with compression therapy as high as 75%.20-21
However, determining a patient’s candidacy for ablative or surgical therapy requires ultrasound data for accurate CEAP scoring, which is often not achieved as an inpatient. Given the potential benefit and lack of severe adverse effects, hospitalists can consider initiating compression therapy at the time of discharge in a patient with mild to moderate signs of CVI and a low risk profile for severe PAD. The prescription should specify knee-length elastic stockings with graduated compression between 20 to 30 mm Hg.22 The patient should also be encouraged to complete the outpatient duplex ultrasound testing prior to the PCP visit so that he or she can be referred to a vascular specialist appropriately.
Infected ulcer with CVI features
If the patient’s exam is suspicious for advanced venous disease with ulceration, the clinician should evaluate for the presence of scarring. This would indicate that there has been long-standing venous disease with recurrent ulceration. This patient should be asked about a previous diagnosis of CVI, prior compression therapy, and barriers to compliance with compression therapy such as poor fit or difficulty of use due to obesity or immobility. It is important to note that mixed ulcers are present in up to 20% of patients; a careful assessment of risk factors for PAD, pulse exam, and referral for outpatient ABI testing is warranted to rule out arterial insufficiency in this patient with likely venous ulcer.23
The AHA recommends prompt specialist evaluation for CEAP scores greater than or equal to 4; based on physical exam alone, this patient’s active venous ulcer yields the highest possible score of 6.2 If not previously done, this patient with advanced CVI and ulceration should be referred for an outpatient venous duplex ultrasound as well as urgent follow-up with a vascular specialist soon after discharge.
There is significant consensus in the literature that multilayer compression therapy between 30 and 40 mm Hg is the first-line treatment in patients with venous ulcers as it has been shown to promote ulcer healing and prevent recurrence.24-25 In addition, superficial venous surgery, including minimally invasive ablation, can reduce the recurrence of ulcers if used as adjunctive therapy in selected patients.26 However, compressive therapy should generally not be prescribed in patients with venous ulcers until PAD has been ruled out.
If ABI results are available, the clinician can consider compression at 30-40 mm Hg for ABI values greater than 0.8 and reduced compression at 20-30 mm Hg for values of 0.5-0.8; compression is contraindicated if the ABI is less than 0.5. Prompt follow-up with a vascular specialist can help direct compressive and/or surgical therapy. Wound care consultation as an inpatient can assist with dressing recommendations, though the evidence has not shown that dressings of any type worn under compressive garments improve ulcer healing.27
Bottom line
Hospitalists are in a unique position to identify patients with underlying peripheral arterial and venous disease when they are admitted for lower extremity skin and soft tissue infections. A focused history and physical exam can yield significant clinical clues and should prompt either inpatient or outpatient work-up.
In patients with deep ulcers and concern for critical limb ischemia, inpatient consultation should be sought. In patients with superficial venous or arterial ulcers, referral for outpatient ABI, color duplex ultrasound, or both should be made; most of these patients should also be directly referred to a vascular and/or wound specialist. Patients with more benign forms of disease who endorse chronic symptoms suspicious for mild to moderate PAD or CVI can be seen by a PCP for further management. All patients should be educated about the importance of follow-up as it remains their best chance to curb the progression of disease, reduce the risks for recurrent infection, and improve overall quality of life.
Back to the original case
Our patient’s lower extremity erythema, fever, and leukocytosis improved with 3 days of IV vancomycin treatment. Her wound was kept clean with moist dressings and showed no signs of deep infection; with elevation, her bilateral lower extremity edema also improved. Her physical exam findings and clinical history were highly suspicious for long-standing CVI. She was discharged with oral antibiotics and a referral to wound care for ongoing management of her superficial ulcers. An outpatient venous duplex ultrasound and ABI were scheduled prior to her vascular surgery appointment to effectively rule out PAD before consideration of further therapy for severe CVI.
Key Points
- Hospitalists are in a unique position to identify patients with peripheral vascular disease when they are admitted with SSTIs.
- When assessing patients, it is important to consider peripheral arterial disease (PAD) and chronic venous insufficiency (CVI) separately.
- The classic symptom for PAD is claudication. In contrast, symptoms of CVI present more variably.
- Barring cases of critical limb ischemia, the main objective of identifying PAD or CVI is to arrange testing and follow-up after discharge.
References
1. Stevens, DL, et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2014;59(2):147-59.
2. Eberhardt, RT, et al. Chronic venous insufficiency. Circulation. 2014;130:333-46.
3. Raju, S, et al. Chronic venous insufficiency and varicose veins. N Engl J Med. 2009;360:2319-27.
4. Kullo, IJ, et al. Peripheral artery disease. N Engl J Med. 2016;374(9):861-71.
5. Hennion D, et al. Diagnosis and treatment of peripheral arterial disease. Am Fam Physician. 2013 Sep 1;88(5):306-10.
6. Henke P, et al. ACP Observer Extra: Peripheral arterial disease. June 2007.
7. Vivas A. Venous leg ulcers. Ann Intern Med. 2016;165(3):ITC17-32.
8. Sumpio BE. Foot ulcers. N Engl J Med. 2000;343(11):787-93.
9. Bazari H, et al. Case 7–2007. 59-year-old woman with diabetic renal disease and nonhealing skin ulcers. N Engl J Med. 2007 Mar 8; 356(10):1049-57.
10. Moyer VA. Screening for peripheral artery disease and cardiovascular disease risk assessment with the ankle-brachial index in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2013 Sep 3.159(5):342-8.
11. Khan TH, et al. Critical review of the ankle brachial index. Curr Cardiol Rev. 2008 May;4(2):101-6.
12. Gerhard-Herman MD, et al. 2016 AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease. A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. 2016.
13. Brearley S, et al. Peripheral pulse palpation: An unreliable physical sign. Ann R Coll Surg Engl. 1992;74:169-71.
14. Khan NA, et al. Does the clinical examination predict lower extremity peripheral arterial disease? JAMA. 2006;295(5):536-46.
15. Mills JL Sr., et al. The Society for Vascular Surgery Lower Extremity Threatened Limb Classification System: Risk stratification based on wound, ischemia, and foot infection (WIfI). J Vasc Surg. 2014 Jan;59(1):220-34.e1-2.
16. Gloviczki P, et al. The care of patients with varicose veins and associated chronic venous diseases: Clinical practice guidelines of the Society for Vascular Surgery and the American Venous Forum. J Vasc Surg. 2011;53(5, Suppl):2S-48S.
17. Hamper UM,et al. Ultrasound evaluation of the lower extremity veins. Radiol Clin North Am. 2007 May;45(3):525-47.
18. Vivas A. Venous leg ulcers. Ann Intern Med. 2016;165(3):ITC17-32.
19. Gloviczki P, et al. The care of patients with varicose veins and associated chronic venous diseases: Clinical practice guidelines of the Society for Vascular Surgery and the American Venous Forum. J Vasc Surg. 2011;53(5, Suppl):2S-48S.
20. Michaels JA, et al. Randomized clinical trial comparing surgery with conservative treatment for uncomplicated varicose veins. Br J Surg. 2006 Feb;93(2):175-81.
21. Raju S, et al. Use of compression stockings in chronic venous disease: Patient compliance and efficacy. Ann Vasc Surg. 2007 Nov;21(6):790-5.
22. Gloviczki P, et al. The care of patients with varicose veins and associated chronic venous diseases: Clinical practice guidelines of the Society for Vascular Surgery and the American Venous Forum. J Vasc Surg. 2011;53(5, Suppl):2S-48S.
23. Humphreys ML, et al. Management of mixed arterial and venous leg ulcers. Br J Surg. 2007 Sep;94(9):1104-7.
24. O’Meara S, et al. Compression for venous leg ulcers. Cochrane Database Syst Rev. 2012 Nov 14;11:CD000265.
25. Dolibog P, et al. A comparative clinical study on five types of compression therapy in patients with venous leg ulcers. Int J Med Sci. 2013;11(1):34-43.
26. Gohel MS, et al. Long term results of compression therapy alone versus compression plus surgery in chronic venous ulceration (ESCHAR): Randomised controlled trial. BMJ. 2007 Jul 14;335(7610):83.
27. Palfreyman S, et al. Dressings for venous leg ulcers: systematic review and meta-analysis. BMJ. 2007 Aug 4;335(7613):244.
Swarm and suspicion leadership
During your career, you serve as staff member and leader to many different professional groups. Some are collaborative, collegial, and supportive. Others are competitive, antagonistic, or even combative. What are the benefits and downsides of each of these cultures and what can you do, as a hospitalist leader, to influence the character of your workplace?
The field of “game theory” provides insights into the distinction. The first questions to ask are “What is the game you are playing?” and then “Who is the competition?” In a “winner-takes-all” scenario, such as a sporting event, each team seeks strategic advantage over the other team. In baseball terms, the winner gets more points when at bat and denies more points when on the field. However, when competing as a team, winning together requires collaboration to build strategy, execute plays, and reach victory. You compete against the other team and collaborate within your own team.
Scientists who study negotiation strategies and conflict resolution find that collaborative groups spend less time countering one another and, instead, investing that same effort into building constructive outcomes, a force multiplier.
In the winner-takes-all model, the baseball team that gets “outs,” makes plays, and advances team members to home plate, wins. If there is contest within the team, players invest that same effort into seeking their own gain at the expense of others. Benefits derived from shared effort are shunned in favor of benefits accrued to one player over the other. It is a distinction between “I won” versus “We won.”
Hospital medicine is not a win/lose sport, yet over the years, hospitalists have shared with me that their institution or group at times feels like a competitive field with winners and losers. If this distinction is placed on a continuum, what factors encourage a more collaborative environment and what factors do the opposite, toward the adversarial side of the continuum? It makes a substantive difference in the interactions and accomplishments that a group achieves.
My colleagues and I at Harvard study leaders in times of crisis. A crisis makes apparent what is often more subtle during routine times. Our study of leaders in the wake of the Boston Marathon bombings was among our most revealing.
During most crises, an operational leader is designated to oversee the whole of the response. This is an individual with organizational authority and subject-matter expertise appropriate to the situation at hand. In Boston, however, there were so many different jurisdictions – federal, state, and local – and so many different agencies, that no one leader stood above the others. They worked in a remarkably collaborative fashion. While the bombings themselves were tragic, the response itself was a success: All who survived the initial blasts lived, a function of remarkable emergency care, distribution to hospitals, and good medical care. The perpetrators were caught in 102 hours, and “Boston Strong” reflected a genuine city resilience.
These leaders worked together in ways that we had rarely seen before. What we discovered was a phenomenon we call “swarm leadership,” inspired by the ways ants, bees, and termites engage in collective work and decision making. These creatures have clear lines of communication and structures for judgment calls, often about food sources, nesting locations, and threats.
There are five principles of swarm leadership:
- Unity of mission – In Boston, that was to “save lives,” and it motivated and activated the whole of the response.
- Generosity of spirit and action – Across the community, people were eager to assist in the response.
- Everyone stayed in their own lanes of responsibility and helped others succeed in theirs – There were law enforcement, medical, and resilience activities and the theme across the leaders was “how can I help make you a success?”
- No ego and no blame – There was a level of emotional intelligence and maturity among the leaders.
- A foundation of trusting relations – These leaders had known one another for years and, though the decisions were tough, they were confident in the motives and actions of the others.
While the discovery emerged from our crisis research, the findings equally apply to other, more routine work and interactions. Conduct your own assessment. Have you worked in groups in which these principles of swarm leadership characterized the experience? People were focused on a shared mission: They were available to assist one another; accomplished their work in ways that were respectful and supportive of their different responsibilities; did not claim undue credit or swipe at each another; and knew one another well enough to trust the others’ actions and motives.
The flip side of this continuum of collaboration and competition we term “suspicion leadership.” This is characterized by selfish ambitions; narcissistic actions; grabs for authority and resources; credit taking for the good and accusations for the bad; and an environment of mistrust and back stabbing.
Leaders influence the tone and tenor of their own group’s interactions as well as interactions among different working groups. As role models, if they articulate and demonstrate a mission that others can rally around, they forge that critical unity of mission. By contrast, suspicion leaders make it clear that “it is all about me and my priorities.” There is much work to be done, and swarm leaders ensure that people have the resources, autonomy, and support necessary to get the job done. On the other end, the work environment is burdened by the uncertainties about who does what and who is responsible. Swarm leaders are focused on “we” and suspicion leaders are caught up on “me.” There is no trust when people are suspicious of one another. Much can be accomplished when people believe in themselves, their colleagues, and the reasons that bring them together.
As a hospitalist leader, you influence where on this continuum your group will lie. It is your choice to be a role model for the principles of swarm, encouraging the same among others. When those principles become the beacons by which you work and relate, you will find an environment that inspires people to be and to do their best.
In the next column, how to build trust within your teams.
Dr. Marcus is director, Program on Health Care Negotiation and Conflict Resolution, at the Harvard T.H. Chan School of Public Health, in Boston.
During your career, you serve as staff member and leader to many different professional groups. Some are collaborative, collegial, and supportive. Others are competitive, antagonistic, or even combative. What are the benefits and downsides of each of these cultures and what can you do, as a hospitalist leader, to influence the character of your workplace?
The field of “game theory” provides insights into the distinction. The first questions to ask are “What is the game you are playing?” and then “Who is the competition?” In a “winner-takes-all” scenario, such as a sporting event, each team seeks strategic advantage over the other team. In baseball terms, the winner gets more points when at bat and denies more points when on the field. However, when competing as a team, winning together requires collaboration to build strategy, execute plays, and reach victory. You compete against the other team and collaborate within your own team.
Scientists who study negotiation strategies and conflict resolution find that collaborative groups spend less time countering one another and, instead, investing that same effort into building constructive outcomes, a force multiplier.
In the winner-takes-all model, the baseball team that gets “outs,” makes plays, and advances team members to home plate, wins. If there is contest within the team, players invest that same effort into seeking their own gain at the expense of others. Benefits derived from shared effort are shunned in favor of benefits accrued to one player over the other. It is a distinction between “I won” versus “We won.”
Hospital medicine is not a win/lose sport, yet over the years, hospitalists have shared with me that their institution or group at times feels like a competitive field with winners and losers. If this distinction is placed on a continuum, what factors encourage a more collaborative environment and what factors do the opposite, toward the adversarial side of the continuum? It makes a substantive difference in the interactions and accomplishments that a group achieves.
My colleagues and I at Harvard study leaders in times of crisis. A crisis makes apparent what is often more subtle during routine times. Our study of leaders in the wake of the Boston Marathon bombings was among our most revealing.
During most crises, an operational leader is designated to oversee the whole of the response. This is an individual with organizational authority and subject-matter expertise appropriate to the situation at hand. In Boston, however, there were so many different jurisdictions – federal, state, and local – and so many different agencies, that no one leader stood above the others. They worked in a remarkably collaborative fashion. While the bombings themselves were tragic, the response itself was a success: All who survived the initial blasts lived, a function of remarkable emergency care, distribution to hospitals, and good medical care. The perpetrators were caught in 102 hours, and “Boston Strong” reflected a genuine city resilience.
These leaders worked together in ways that we had rarely seen before. What we discovered was a phenomenon we call “swarm leadership,” inspired by the ways ants, bees, and termites engage in collective work and decision making. These creatures have clear lines of communication and structures for judgment calls, often about food sources, nesting locations, and threats.
There are five principles of swarm leadership:
- Unity of mission – In Boston, that was to “save lives,” and it motivated and activated the whole of the response.
- Generosity of spirit and action – Across the community, people were eager to assist in the response.
- Everyone stayed in their own lanes of responsibility and helped others succeed in theirs – There were law enforcement, medical, and resilience activities and the theme across the leaders was “how can I help make you a success?”
- No ego and no blame – There was a level of emotional intelligence and maturity among the leaders.
- A foundation of trusting relations – These leaders had known one another for years and, though the decisions were tough, they were confident in the motives and actions of the others.
While the discovery emerged from our crisis research, the findings equally apply to other, more routine work and interactions. Conduct your own assessment. Have you worked in groups in which these principles of swarm leadership characterized the experience? People were focused on a shared mission: They were available to assist one another; accomplished their work in ways that were respectful and supportive of their different responsibilities; did not claim undue credit or swipe at each another; and knew one another well enough to trust the others’ actions and motives.
The flip side of this continuum of collaboration and competition we term “suspicion leadership.” This is characterized by selfish ambitions; narcissistic actions; grabs for authority and resources; credit taking for the good and accusations for the bad; and an environment of mistrust and back stabbing.
Leaders influence the tone and tenor of their own group’s interactions as well as interactions among different working groups. As role models, if they articulate and demonstrate a mission that others can rally around, they forge that critical unity of mission. By contrast, suspicion leaders make it clear that “it is all about me and my priorities.” There is much work to be done, and swarm leaders ensure that people have the resources, autonomy, and support necessary to get the job done. On the other end, the work environment is burdened by the uncertainties about who does what and who is responsible. Swarm leaders are focused on “we” and suspicion leaders are caught up on “me.” There is no trust when people are suspicious of one another. Much can be accomplished when people believe in themselves, their colleagues, and the reasons that bring them together.
As a hospitalist leader, you influence where on this continuum your group will lie. It is your choice to be a role model for the principles of swarm, encouraging the same among others. When those principles become the beacons by which you work and relate, you will find an environment that inspires people to be and to do their best.
In the next column, how to build trust within your teams.
Dr. Marcus is director, Program on Health Care Negotiation and Conflict Resolution, at the Harvard T.H. Chan School of Public Health, in Boston.
During your career, you serve as staff member and leader to many different professional groups. Some are collaborative, collegial, and supportive. Others are competitive, antagonistic, or even combative. What are the benefits and downsides of each of these cultures and what can you do, as a hospitalist leader, to influence the character of your workplace?
The field of “game theory” provides insights into the distinction. The first questions to ask are “What is the game you are playing?” and then “Who is the competition?” In a “winner-takes-all” scenario, such as a sporting event, each team seeks strategic advantage over the other team. In baseball terms, the winner gets more points when at bat and denies more points when on the field. However, when competing as a team, winning together requires collaboration to build strategy, execute plays, and reach victory. You compete against the other team and collaborate within your own team.
Scientists who study negotiation strategies and conflict resolution find that collaborative groups spend less time countering one another and, instead, investing that same effort into building constructive outcomes, a force multiplier.
In the winner-takes-all model, the baseball team that gets “outs,” makes plays, and advances team members to home plate, wins. If there is contest within the team, players invest that same effort into seeking their own gain at the expense of others. Benefits derived from shared effort are shunned in favor of benefits accrued to one player over the other. It is a distinction between “I won” versus “We won.”
Hospital medicine is not a win/lose sport, yet over the years, hospitalists have shared with me that their institution or group at times feels like a competitive field with winners and losers. If this distinction is placed on a continuum, what factors encourage a more collaborative environment and what factors do the opposite, toward the adversarial side of the continuum? It makes a substantive difference in the interactions and accomplishments that a group achieves.
My colleagues and I at Harvard study leaders in times of crisis. A crisis makes apparent what is often more subtle during routine times. Our study of leaders in the wake of the Boston Marathon bombings was among our most revealing.
During most crises, an operational leader is designated to oversee the whole of the response. This is an individual with organizational authority and subject-matter expertise appropriate to the situation at hand. In Boston, however, there were so many different jurisdictions – federal, state, and local – and so many different agencies, that no one leader stood above the others. They worked in a remarkably collaborative fashion. While the bombings themselves were tragic, the response itself was a success: All who survived the initial blasts lived, a function of remarkable emergency care, distribution to hospitals, and good medical care. The perpetrators were caught in 102 hours, and “Boston Strong” reflected a genuine city resilience.
These leaders worked together in ways that we had rarely seen before. What we discovered was a phenomenon we call “swarm leadership,” inspired by the ways ants, bees, and termites engage in collective work and decision making. These creatures have clear lines of communication and structures for judgment calls, often about food sources, nesting locations, and threats.
There are five principles of swarm leadership:
- Unity of mission – In Boston, that was to “save lives,” and it motivated and activated the whole of the response.
- Generosity of spirit and action – Across the community, people were eager to assist in the response.
- Everyone stayed in their own lanes of responsibility and helped others succeed in theirs – There were law enforcement, medical, and resilience activities and the theme across the leaders was “how can I help make you a success?”
- No ego and no blame – There was a level of emotional intelligence and maturity among the leaders.
- A foundation of trusting relations – These leaders had known one another for years and, though the decisions were tough, they were confident in the motives and actions of the others.
While the discovery emerged from our crisis research, the findings equally apply to other, more routine work and interactions. Conduct your own assessment. Have you worked in groups in which these principles of swarm leadership characterized the experience? People were focused on a shared mission: They were available to assist one another; accomplished their work in ways that were respectful and supportive of their different responsibilities; did not claim undue credit or swipe at each another; and knew one another well enough to trust the others’ actions and motives.
The flip side of this continuum of collaboration and competition we term “suspicion leadership.” This is characterized by selfish ambitions; narcissistic actions; grabs for authority and resources; credit taking for the good and accusations for the bad; and an environment of mistrust and back stabbing.
Leaders influence the tone and tenor of their own group’s interactions as well as interactions among different working groups. As role models, if they articulate and demonstrate a mission that others can rally around, they forge that critical unity of mission. By contrast, suspicion leaders make it clear that “it is all about me and my priorities.” There is much work to be done, and swarm leaders ensure that people have the resources, autonomy, and support necessary to get the job done. On the other end, the work environment is burdened by the uncertainties about who does what and who is responsible. Swarm leaders are focused on “we” and suspicion leaders are caught up on “me.” There is no trust when people are suspicious of one another. Much can be accomplished when people believe in themselves, their colleagues, and the reasons that bring them together.
As a hospitalist leader, you influence where on this continuum your group will lie. It is your choice to be a role model for the principles of swarm, encouraging the same among others. When those principles become the beacons by which you work and relate, you will find an environment that inspires people to be and to do their best.
In the next column, how to build trust within your teams.
Dr. Marcus is director, Program on Health Care Negotiation and Conflict Resolution, at the Harvard T.H. Chan School of Public Health, in Boston.
Benefit of dabigatran over warfarin persists in AF patient subgroups undergoing PCI
ANAHEIM, CALIF. – The benefit of dabigatran dual therapy versus warfarin triple therapy after percutaneous coronary intervention in patients with atrial fibrillation was consistent whether patients had drug-eluting or bare-metal stents, concomitant treatment with ticagrelor or clopidogrel, or acute coronary syndrome or stable disease as the indication for PCI, according to a subgroup analysis of the RE-DUAL PCI trial.
The trial, presented at the American Heart Association scientific sessions, randomized 2,725 patients to triple therapy with warfarin plus a P2Y12 inhibitor (clopidogrel or ticagrelor) and aspirin – the triple therapy group – or dabigatran 110 mg or 150 mg twice daily plus clopidogrel or ticagrelor – the dual therapy groups (N Engl J Med. 2017 Oct 19;377[16]:1513-24).
After a mean follow-up 14 months, the incidence of the major or clinically relevant nonmajor bleeding was 15.4% in the 110-mg dual-therapy group (hazard ratio, 0.52; 95% CI, 0.42-0.63; P less than .001) and 20.2% in the 150-mg dual-therapy group (HR, 0.72; 95% CI, 0.58-0.88; P less than .001), versus about 26% with triple-therapy.
The incidence of the composite efficacy endpoint – death, unplanned revascularization, myocardial infarction, stroke, or systemic embolism – was 13.7% in the two dual-therapy groups versus 13.4% with triple-therapy (HR, 1.04; 95% CI, 0.84-1.29; P = .005).
The investigators found consistent results when they analyzed their prespecified subgroups.
Drug-eluting stents were placed in 83% of patients; the rest had bare metal stents (BMS). The groups were well-balanced, except BMS patients were again more likely to be new to oral anticoagulation. Bleeding, thromboembolic events, and mortality were consistent with the main results regardless of the stent type, Most of the subjects were on clopidogrel, with just 12% on ticagrelor in both the dabigatran and warfarin groups. Ticagrelor patients were more likely to have ACS as their PCI indication and be new to oral anticoagulation. Ticagrelor patients were also more clinically complex, with a higher bleeding risk. Even so, they had relative bleeding risk reduction and efficacy results with dabigatran that were consistent with the overall finding, Dr. Oldgren said.
Patients were eligible for RE-DUAL PCI (Evaluation of Dual Therapy with Dabigatran vs. Triple Therapy with Warfarin in Patients with AF That Undergo a PCI with Stenting) if they had nonvalvular atrial fibrillation and a successful PCI within 120 hours. Those with bioprosthetic or mechanical heart valves, severe renal insufficiency, or other major comorbidities were excluded.
The trial was funded by Boehringer Ingelheim, the maker of dabigatran. Several investigators were employees. Dr. Oldgren is an adviser to Boehringer Ingelheim. Other authors reported financial ties to the company as well.
ANAHEIM, CALIF. – The benefit of dabigatran dual therapy versus warfarin triple therapy after percutaneous coronary intervention in patients with atrial fibrillation was consistent whether patients had drug-eluting or bare-metal stents, concomitant treatment with ticagrelor or clopidogrel, or acute coronary syndrome or stable disease as the indication for PCI, according to a subgroup analysis of the RE-DUAL PCI trial.
The trial, presented at the American Heart Association scientific sessions, randomized 2,725 patients to triple therapy with warfarin plus a P2Y12 inhibitor (clopidogrel or ticagrelor) and aspirin – the triple therapy group – or dabigatran 110 mg or 150 mg twice daily plus clopidogrel or ticagrelor – the dual therapy groups (N Engl J Med. 2017 Oct 19;377[16]:1513-24).
After a mean follow-up 14 months, the incidence of the major or clinically relevant nonmajor bleeding was 15.4% in the 110-mg dual-therapy group (hazard ratio, 0.52; 95% CI, 0.42-0.63; P less than .001) and 20.2% in the 150-mg dual-therapy group (HR, 0.72; 95% CI, 0.58-0.88; P less than .001), versus about 26% with triple-therapy.
The incidence of the composite efficacy endpoint – death, unplanned revascularization, myocardial infarction, stroke, or systemic embolism – was 13.7% in the two dual-therapy groups versus 13.4% with triple-therapy (HR, 1.04; 95% CI, 0.84-1.29; P = .005).
The investigators found consistent results when they analyzed their prespecified subgroups.
Drug-eluting stents were placed in 83% of patients; the rest had bare metal stents (BMS). The groups were well-balanced, except BMS patients were again more likely to be new to oral anticoagulation. Bleeding, thromboembolic events, and mortality were consistent with the main results regardless of the stent type, Most of the subjects were on clopidogrel, with just 12% on ticagrelor in both the dabigatran and warfarin groups. Ticagrelor patients were more likely to have ACS as their PCI indication and be new to oral anticoagulation. Ticagrelor patients were also more clinically complex, with a higher bleeding risk. Even so, they had relative bleeding risk reduction and efficacy results with dabigatran that were consistent with the overall finding, Dr. Oldgren said.
Patients were eligible for RE-DUAL PCI (Evaluation of Dual Therapy with Dabigatran vs. Triple Therapy with Warfarin in Patients with AF That Undergo a PCI with Stenting) if they had nonvalvular atrial fibrillation and a successful PCI within 120 hours. Those with bioprosthetic or mechanical heart valves, severe renal insufficiency, or other major comorbidities were excluded.
The trial was funded by Boehringer Ingelheim, the maker of dabigatran. Several investigators were employees. Dr. Oldgren is an adviser to Boehringer Ingelheim. Other authors reported financial ties to the company as well.
ANAHEIM, CALIF. – The benefit of dabigatran dual therapy versus warfarin triple therapy after percutaneous coronary intervention in patients with atrial fibrillation was consistent whether patients had drug-eluting or bare-metal stents, concomitant treatment with ticagrelor or clopidogrel, or acute coronary syndrome or stable disease as the indication for PCI, according to a subgroup analysis of the RE-DUAL PCI trial.
The trial, presented at the American Heart Association scientific sessions, randomized 2,725 patients to triple therapy with warfarin plus a P2Y12 inhibitor (clopidogrel or ticagrelor) and aspirin – the triple therapy group – or dabigatran 110 mg or 150 mg twice daily plus clopidogrel or ticagrelor – the dual therapy groups (N Engl J Med. 2017 Oct 19;377[16]:1513-24).
After a mean follow-up 14 months, the incidence of the major or clinically relevant nonmajor bleeding was 15.4% in the 110-mg dual-therapy group (hazard ratio, 0.52; 95% CI, 0.42-0.63; P less than .001) and 20.2% in the 150-mg dual-therapy group (HR, 0.72; 95% CI, 0.58-0.88; P less than .001), versus about 26% with triple-therapy.
The incidence of the composite efficacy endpoint – death, unplanned revascularization, myocardial infarction, stroke, or systemic embolism – was 13.7% in the two dual-therapy groups versus 13.4% with triple-therapy (HR, 1.04; 95% CI, 0.84-1.29; P = .005).
The investigators found consistent results when they analyzed their prespecified subgroups.
Drug-eluting stents were placed in 83% of patients; the rest had bare metal stents (BMS). The groups were well-balanced, except BMS patients were again more likely to be new to oral anticoagulation. Bleeding, thromboembolic events, and mortality were consistent with the main results regardless of the stent type, Most of the subjects were on clopidogrel, with just 12% on ticagrelor in both the dabigatran and warfarin groups. Ticagrelor patients were more likely to have ACS as their PCI indication and be new to oral anticoagulation. Ticagrelor patients were also more clinically complex, with a higher bleeding risk. Even so, they had relative bleeding risk reduction and efficacy results with dabigatran that were consistent with the overall finding, Dr. Oldgren said.
Patients were eligible for RE-DUAL PCI (Evaluation of Dual Therapy with Dabigatran vs. Triple Therapy with Warfarin in Patients with AF That Undergo a PCI with Stenting) if they had nonvalvular atrial fibrillation and a successful PCI within 120 hours. Those with bioprosthetic or mechanical heart valves, severe renal insufficiency, or other major comorbidities were excluded.
The trial was funded by Boehringer Ingelheim, the maker of dabigatran. Several investigators were employees. Dr. Oldgren is an adviser to Boehringer Ingelheim. Other authors reported financial ties to the company as well.
AT THE AHA SCIENTIFIC SESSIONS
Key clinical point:
Major finding: After a mean follow-up of 14 months, the incidence of major or clinically relevant nonmajor bleeding was 15.4% in the 110-mg dual-therapy group (HR, 0.52; 95% CI, 0.42-0.63, P less than .001) and 20.2% in the 150-mg dual-therapy group (HR, 0.72; 95% CI, 0.58-0.88; P less than .001), versus about 26% with triple-therapy.
Data source: Subgroup analysis of RE-DUAL PCI trial
Disclosures: The trial was funded by Boehringer Ingelheim, the maker of dabigatran. Several investigators were employees. Authors disclosed various financial ties to the company.
Intense urine output monitoring beneficial in ICU
Intense monitoring of urine output could be a useful tool in detecting acute kidney injury (AKI), according to a study conducted at the University of Pittsburgh.
Kui Jin, MD, of the University of Pittsburgh and his associates found that, after adjustment for baseline characteristics, intensive monitoring of urine output (UO) was associated with higher rates of AKI, with an odds ratio of 1.22. Intensive UO monitoring also was strongly associated with improved 30-day survival among patients developing AKI.
This retrospective cohort study included 15,724 adult patients admitted to the center’s ICUs during 2000-2008. All patients had either their UO or serum creatinine (SC) monitored. These patients were then divided into subcohorts that were monitored at one of two different intensities. UO intensive monitoring was defined by hourly recordings, with gaps no greater than 3 hours for the first 48 hours after ICU admission. The group receiving less intensive UO monitoring comprised patients who did not meet intensive monitoring criteria, regardless of their UO in the 7 days following ICU admission. The patients who had their SC intensively monitored had 3 calendar days of samples taken after their ICU admissions. Those who did not meet SC intensive monitoring criteria were placed into the less intensive SC monitoring group.
To understand the effect of the monitoring strategies on detecting the development of AKI, the researchers determined each patient’s baseline, admission, and reference serum creatinine levels. Baseline creatinine was defined as the lowest value in the year prior to hospital admission. Reference creatinine was the baseline creatinine, if available, or the lowest creatinine level recorded within 24 hours after ICU admission. A third method for determining reference creatinine levels was used for some patients, which involved making an estimation based on the Modification of Diet in Renal Disease equation for serum creatinine.
The crude rates of stage 2-3 AKI 7 days after admission to the ICU were similar between patients from both groups that had their UO monitored; 62.5% of intensive and 63.9% of less intensive patients displayed symptoms. After the researchers adjusted for baseline characteristics, however, intensive monitoring of UO was associated with greater rates of stage 2-3 AKI (OR, 1.22; P less than .001). Crude rates were higher in the patients who received intensive monitoring for SC, compared with patients who received less intensive monitoring for SC. Ultimately, Dr. Jin and his associates found that, when caring for patients with or without AKI, fluid management is one of the most important factors. Patients who underwent intensive UO monitoring received less fluid in their first 24 hours (3.6 L) in the ICU, compared with patients who received less intense UO monitoring (4.2 L). Patients who received intensive monitoring of their UO also were less likely to use vasopressors (29.9% vs. 43.3%; P less than .001), suggesting these patients were more hemodynamically stable. Further, the percentage of patients at or above 10% of fluid overload was lower in the group who received intensive monitoring of their UO (2.49% vs. 5.68%; P less than .001), during the first 72 hours in the ICU.
“Our results should help inform clinical decisions and ICU policy around frequency of monitoring of UO, especially for patients at high risk of AKI,” Dr. Jin and his colleagues wrote.
None of the authors had financial disclosures to report. Partial funding was provided by a research grant from C.R. Bard.
Intense monitoring of urine output could be a useful tool in detecting acute kidney injury (AKI), according to a study conducted at the University of Pittsburgh.
Kui Jin, MD, of the University of Pittsburgh and his associates found that, after adjustment for baseline characteristics, intensive monitoring of urine output (UO) was associated with higher rates of AKI, with an odds ratio of 1.22. Intensive UO monitoring also was strongly associated with improved 30-day survival among patients developing AKI.
This retrospective cohort study included 15,724 adult patients admitted to the center’s ICUs during 2000-2008. All patients had either their UO or serum creatinine (SC) monitored. These patients were then divided into subcohorts that were monitored at one of two different intensities. UO intensive monitoring was defined by hourly recordings, with gaps no greater than 3 hours for the first 48 hours after ICU admission. The group receiving less intensive UO monitoring comprised patients who did not meet intensive monitoring criteria, regardless of their UO in the 7 days following ICU admission. The patients who had their SC intensively monitored had 3 calendar days of samples taken after their ICU admissions. Those who did not meet SC intensive monitoring criteria were placed into the less intensive SC monitoring group.
To understand the effect of the monitoring strategies on detecting the development of AKI, the researchers determined each patient’s baseline, admission, and reference serum creatinine levels. Baseline creatinine was defined as the lowest value in the year prior to hospital admission. Reference creatinine was the baseline creatinine, if available, or the lowest creatinine level recorded within 24 hours after ICU admission. A third method for determining reference creatinine levels was used for some patients, which involved making an estimation based on the Modification of Diet in Renal Disease equation for serum creatinine.
The crude rates of stage 2-3 AKI 7 days after admission to the ICU were similar between patients from both groups that had their UO monitored; 62.5% of intensive and 63.9% of less intensive patients displayed symptoms. After the researchers adjusted for baseline characteristics, however, intensive monitoring of UO was associated with greater rates of stage 2-3 AKI (OR, 1.22; P less than .001). Crude rates were higher in the patients who received intensive monitoring for SC, compared with patients who received less intensive monitoring for SC. Ultimately, Dr. Jin and his associates found that, when caring for patients with or without AKI, fluid management is one of the most important factors. Patients who underwent intensive UO monitoring received less fluid in their first 24 hours (3.6 L) in the ICU, compared with patients who received less intense UO monitoring (4.2 L). Patients who received intensive monitoring of their UO also were less likely to use vasopressors (29.9% vs. 43.3%; P less than .001), suggesting these patients were more hemodynamically stable. Further, the percentage of patients at or above 10% of fluid overload was lower in the group who received intensive monitoring of their UO (2.49% vs. 5.68%; P less than .001), during the first 72 hours in the ICU.
“Our results should help inform clinical decisions and ICU policy around frequency of monitoring of UO, especially for patients at high risk of AKI,” Dr. Jin and his colleagues wrote.
None of the authors had financial disclosures to report. Partial funding was provided by a research grant from C.R. Bard.
Intense monitoring of urine output could be a useful tool in detecting acute kidney injury (AKI), according to a study conducted at the University of Pittsburgh.
Kui Jin, MD, of the University of Pittsburgh and his associates found that, after adjustment for baseline characteristics, intensive monitoring of urine output (UO) was associated with higher rates of AKI, with an odds ratio of 1.22. Intensive UO monitoring also was strongly associated with improved 30-day survival among patients developing AKI.
This retrospective cohort study included 15,724 adult patients admitted to the center’s ICUs during 2000-2008. All patients had either their UO or serum creatinine (SC) monitored. These patients were then divided into subcohorts that were monitored at one of two different intensities. UO intensive monitoring was defined by hourly recordings, with gaps no greater than 3 hours for the first 48 hours after ICU admission. The group receiving less intensive UO monitoring comprised patients who did not meet intensive monitoring criteria, regardless of their UO in the 7 days following ICU admission. The patients who had their SC intensively monitored had 3 calendar days of samples taken after their ICU admissions. Those who did not meet SC intensive monitoring criteria were placed into the less intensive SC monitoring group.
To understand the effect of the monitoring strategies on detecting the development of AKI, the researchers determined each patient’s baseline, admission, and reference serum creatinine levels. Baseline creatinine was defined as the lowest value in the year prior to hospital admission. Reference creatinine was the baseline creatinine, if available, or the lowest creatinine level recorded within 24 hours after ICU admission. A third method for determining reference creatinine levels was used for some patients, which involved making an estimation based on the Modification of Diet in Renal Disease equation for serum creatinine.
The crude rates of stage 2-3 AKI 7 days after admission to the ICU were similar between patients from both groups that had their UO monitored; 62.5% of intensive and 63.9% of less intensive patients displayed symptoms. After the researchers adjusted for baseline characteristics, however, intensive monitoring of UO was associated with greater rates of stage 2-3 AKI (OR, 1.22; P less than .001). Crude rates were higher in the patients who received intensive monitoring for SC, compared with patients who received less intensive monitoring for SC. Ultimately, Dr. Jin and his associates found that, when caring for patients with or without AKI, fluid management is one of the most important factors. Patients who underwent intensive UO monitoring received less fluid in their first 24 hours (3.6 L) in the ICU, compared with patients who received less intense UO monitoring (4.2 L). Patients who received intensive monitoring of their UO also were less likely to use vasopressors (29.9% vs. 43.3%; P less than .001), suggesting these patients were more hemodynamically stable. Further, the percentage of patients at or above 10% of fluid overload was lower in the group who received intensive monitoring of their UO (2.49% vs. 5.68%; P less than .001), during the first 72 hours in the ICU.
“Our results should help inform clinical decisions and ICU policy around frequency of monitoring of UO, especially for patients at high risk of AKI,” Dr. Jin and his colleagues wrote.
None of the authors had financial disclosures to report. Partial funding was provided by a research grant from C.R. Bard.
FROM CHEST
Key clinical point:
Major finding: AKI was more likely to be seen in patients who received intensive monitoring of their urine output (OR,1.22; P less than .001).
Data source: Retrospective cohort study at a single academic medical center of 15,724 adult patients admitted to the center’s ICUs during 2000-2008.
Disclosures: None of the authors had financial disclosures to report. Partial funding was provided by a research grant from C.R. Bard.
New buprenorphine formulation approved for medication-assisted treatment
The Food and Drug Administration has approved an extended-release, subcutaneous injection formulation of buprenorphine for use in treating moderate to severe opioid use disorder (OUD), the manufacturer of the drug announced Nov. 30.
The new product, called Sublocade, is a monthly injection intended for use in patients who have already begun treatment of OUD with transmucosal buprenorphine products, followed by a dose adjustment for a minimum of 7 days. Sublocade contains the partial mu-opioid agonist buprenorphine. By administering a consistent level of buprenorphine into the body, it ensures that levels of buprenorphine are delivered to the mu-opioid receptors, diminishing the effects of opioids, including the euphoric sensations associated with opioid use. During the clinical trial program, buprenorphine plasma concentrations of 2-3 ng/mL were found to bind to greater than 70% of mu-opioid receptors.
According to a statement from the FDA, Sublocade will be distributed only to health care providers as part of a Risk Evaluation and Mitigation Strategy to ensure that the product is not distributed directly to patients. Sublocade should be administered only by a health care professional. Self-injection of Sublocade into the blood stream instead of subcutaneously could lead to occlusion of blood vessels and embolism, according to one of the drug’s boxed warnings. It also should be used as part of a complete treatment program that includes counseling and psychosocial support.
The FDA is also requiring the manufacturer to conduct postmarketing studies to assess which patients would benefit from a higher dosing regimen, to determine whether Sublocade can be safely initiated without a dose stabilization period of sublingual buprenorphine, to assess the feasibility of administering Sublocade at a longer inter-dose interval than once monthly, and to determine a process for transitioning patients with long-term stability on a transmucosal buprenorphine dose to a monthly dose of Sublocade without the use of a higher dose for the first 2 months of treatment.
At recent joint meetings of the FDA’s Psychopharmacologic Drugs and Drug Safety and Risk Management advisory committees, panelists voted on Oct. 31 to recommend approval of Sublocade and on Nov. 1 for another subcutaneous buprenorphine injection formulation. These actions have not gone unnoticed by the American Medical Association.
“The AMA enthusiastically supports Food and Drug Administration Commissioner Scott Gottlieb’s efforts to advance policies and actions to treat those suffering from an opioid use disorder,” Patrice Harris, MD, immediate past chair of the American Medical Association Board of Trustees and a member of the AMA Opioid Task Force, said in a statement. “We also second his bold acknowledgment that criminal justice systems should offer [medication-assisted treatment] to those being detained. As he points out, ‘At the very moment when the criminal justice system could be dramatically lowering the risk of overdose, it is creating the conditions of reduced tolerance to opioids that substantially raises the risk of death upon release.’ With his clear explanation of the problem and solution, this situation can be remedied.”
The Food and Drug Administration has approved an extended-release, subcutaneous injection formulation of buprenorphine for use in treating moderate to severe opioid use disorder (OUD), the manufacturer of the drug announced Nov. 30.
The new product, called Sublocade, is a monthly injection intended for use in patients who have already begun treatment of OUD with transmucosal buprenorphine products, followed by a dose adjustment for a minimum of 7 days. Sublocade contains the partial mu-opioid agonist buprenorphine. By administering a consistent level of buprenorphine into the body, it ensures that levels of buprenorphine are delivered to the mu-opioid receptors, diminishing the effects of opioids, including the euphoric sensations associated with opioid use. During the clinical trial program, buprenorphine plasma concentrations of 2-3 ng/mL were found to bind to greater than 70% of mu-opioid receptors.
According to a statement from the FDA, Sublocade will be distributed only to health care providers as part of a Risk Evaluation and Mitigation Strategy to ensure that the product is not distributed directly to patients. Sublocade should be administered only by a health care professional. Self-injection of Sublocade into the blood stream instead of subcutaneously could lead to occlusion of blood vessels and embolism, according to one of the drug’s boxed warnings. It also should be used as part of a complete treatment program that includes counseling and psychosocial support.
The FDA is also requiring the manufacturer to conduct postmarketing studies to assess which patients would benefit from a higher dosing regimen, to determine whether Sublocade can be safely initiated without a dose stabilization period of sublingual buprenorphine, to assess the feasibility of administering Sublocade at a longer inter-dose interval than once monthly, and to determine a process for transitioning patients with long-term stability on a transmucosal buprenorphine dose to a monthly dose of Sublocade without the use of a higher dose for the first 2 months of treatment.
At recent joint meetings of the FDA’s Psychopharmacologic Drugs and Drug Safety and Risk Management advisory committees, panelists voted on Oct. 31 to recommend approval of Sublocade and on Nov. 1 for another subcutaneous buprenorphine injection formulation. These actions have not gone unnoticed by the American Medical Association.
“The AMA enthusiastically supports Food and Drug Administration Commissioner Scott Gottlieb’s efforts to advance policies and actions to treat those suffering from an opioid use disorder,” Patrice Harris, MD, immediate past chair of the American Medical Association Board of Trustees and a member of the AMA Opioid Task Force, said in a statement. “We also second his bold acknowledgment that criminal justice systems should offer [medication-assisted treatment] to those being detained. As he points out, ‘At the very moment when the criminal justice system could be dramatically lowering the risk of overdose, it is creating the conditions of reduced tolerance to opioids that substantially raises the risk of death upon release.’ With his clear explanation of the problem and solution, this situation can be remedied.”
The Food and Drug Administration has approved an extended-release, subcutaneous injection formulation of buprenorphine for use in treating moderate to severe opioid use disorder (OUD), the manufacturer of the drug announced Nov. 30.
The new product, called Sublocade, is a monthly injection intended for use in patients who have already begun treatment of OUD with transmucosal buprenorphine products, followed by a dose adjustment for a minimum of 7 days. Sublocade contains the partial mu-opioid agonist buprenorphine. By administering a consistent level of buprenorphine into the body, it ensures that levels of buprenorphine are delivered to the mu-opioid receptors, diminishing the effects of opioids, including the euphoric sensations associated with opioid use. During the clinical trial program, buprenorphine plasma concentrations of 2-3 ng/mL were found to bind to greater than 70% of mu-opioid receptors.
According to a statement from the FDA, Sublocade will be distributed only to health care providers as part of a Risk Evaluation and Mitigation Strategy to ensure that the product is not distributed directly to patients. Sublocade should be administered only by a health care professional. Self-injection of Sublocade into the blood stream instead of subcutaneously could lead to occlusion of blood vessels and embolism, according to one of the drug’s boxed warnings. It also should be used as part of a complete treatment program that includes counseling and psychosocial support.
The FDA is also requiring the manufacturer to conduct postmarketing studies to assess which patients would benefit from a higher dosing regimen, to determine whether Sublocade can be safely initiated without a dose stabilization period of sublingual buprenorphine, to assess the feasibility of administering Sublocade at a longer inter-dose interval than once monthly, and to determine a process for transitioning patients with long-term stability on a transmucosal buprenorphine dose to a monthly dose of Sublocade without the use of a higher dose for the first 2 months of treatment.
At recent joint meetings of the FDA’s Psychopharmacologic Drugs and Drug Safety and Risk Management advisory committees, panelists voted on Oct. 31 to recommend approval of Sublocade and on Nov. 1 for another subcutaneous buprenorphine injection formulation. These actions have not gone unnoticed by the American Medical Association.
“The AMA enthusiastically supports Food and Drug Administration Commissioner Scott Gottlieb’s efforts to advance policies and actions to treat those suffering from an opioid use disorder,” Patrice Harris, MD, immediate past chair of the American Medical Association Board of Trustees and a member of the AMA Opioid Task Force, said in a statement. “We also second his bold acknowledgment that criminal justice systems should offer [medication-assisted treatment] to those being detained. As he points out, ‘At the very moment when the criminal justice system could be dramatically lowering the risk of overdose, it is creating the conditions of reduced tolerance to opioids that substantially raises the risk of death upon release.’ With his clear explanation of the problem and solution, this situation can be remedied.”
Ensuring a smooth data collection process
Editor’s note: The Society of Hospital Medicine’s (SHM’s) Physician in Training Committee launched a scholarship program in 2015 for medical students to help transform health care and revolutionize patient care. The program has been expanded for the 2017-18 year, offering two options for students to receive funding and engage in scholarly work during their first, second and third years of medical school. As a part of the longitudinal (18-month) program, recipients are required to write about their experience on a monthly basis.
Piloting of data collection is finally underway! My mentor, Dr. Ian Jenkins, an attending in the Division of Hospital Medicine at the University of California, San Diego, and I are currently collaborating with the Surgical Intensive Care Unit at UC San Diego to conduct a daily review of urinary catheter (UC) necessity for patients on the unit, and subsequently coordinating with nursing staff on the unit to look for opportunities to implement UC alternatives.
As far as timeline, we are past the halfway point. One thing that has surprised me is how long it has taken to get piloting phase underway. To that end, I think that our initial project timeline was ambitious, especially because we were unclear on how well initial project enthusiasm would translate into subsequent project participation. Up until this point, our research approach has largely been to fine tune each process prospectively. For instance, we decided a pilot run of data collection prior to final project data collection would allow us to ensure a smoother data collection process. While this has slowed things initially, we are optimistic that this will allow us to progress more quickly and smoothly in the latter stages of the project. We are not currently planning to change this research approach for the time being, but we are open to the idea depending on how well the data piloting phase progresses.
Outside of data collection, the project has provided an excellent opportunity to learn and improve clinical skills. Specifically, the project has improved my understanding of the indications for urinary catheter use, as well as helped me to develop a more critical mindset regarding medical indications in general. The project has made me more aware of the importance of really asking and thinking about why a patient is on a specific medication or using a specific medical device, which is something that is very helpful for anticipating and avoiding errors in the clinical setting.
Overall, I have enjoyed my participation in the project to date and it has increased my enthusiasm for participating in a quality improvement project.
Victor Ekuta is a third-year medical student at UC San Diego.
Editor’s note: The Society of Hospital Medicine’s (SHM’s) Physician in Training Committee launched a scholarship program in 2015 for medical students to help transform health care and revolutionize patient care. The program has been expanded for the 2017-18 year, offering two options for students to receive funding and engage in scholarly work during their first, second and third years of medical school. As a part of the longitudinal (18-month) program, recipients are required to write about their experience on a monthly basis.
Piloting of data collection is finally underway! My mentor, Dr. Ian Jenkins, an attending in the Division of Hospital Medicine at the University of California, San Diego, and I are currently collaborating with the Surgical Intensive Care Unit at UC San Diego to conduct a daily review of urinary catheter (UC) necessity for patients on the unit, and subsequently coordinating with nursing staff on the unit to look for opportunities to implement UC alternatives.
As far as timeline, we are past the halfway point. One thing that has surprised me is how long it has taken to get piloting phase underway. To that end, I think that our initial project timeline was ambitious, especially because we were unclear on how well initial project enthusiasm would translate into subsequent project participation. Up until this point, our research approach has largely been to fine tune each process prospectively. For instance, we decided a pilot run of data collection prior to final project data collection would allow us to ensure a smoother data collection process. While this has slowed things initially, we are optimistic that this will allow us to progress more quickly and smoothly in the latter stages of the project. We are not currently planning to change this research approach for the time being, but we are open to the idea depending on how well the data piloting phase progresses.
Outside of data collection, the project has provided an excellent opportunity to learn and improve clinical skills. Specifically, the project has improved my understanding of the indications for urinary catheter use, as well as helped me to develop a more critical mindset regarding medical indications in general. The project has made me more aware of the importance of really asking and thinking about why a patient is on a specific medication or using a specific medical device, which is something that is very helpful for anticipating and avoiding errors in the clinical setting.
Overall, I have enjoyed my participation in the project to date and it has increased my enthusiasm for participating in a quality improvement project.
Victor Ekuta is a third-year medical student at UC San Diego.
Editor’s note: The Society of Hospital Medicine’s (SHM’s) Physician in Training Committee launched a scholarship program in 2015 for medical students to help transform health care and revolutionize patient care. The program has been expanded for the 2017-18 year, offering two options for students to receive funding and engage in scholarly work during their first, second and third years of medical school. As a part of the longitudinal (18-month) program, recipients are required to write about their experience on a monthly basis.
Piloting of data collection is finally underway! My mentor, Dr. Ian Jenkins, an attending in the Division of Hospital Medicine at the University of California, San Diego, and I are currently collaborating with the Surgical Intensive Care Unit at UC San Diego to conduct a daily review of urinary catheter (UC) necessity for patients on the unit, and subsequently coordinating with nursing staff on the unit to look for opportunities to implement UC alternatives.
As far as timeline, we are past the halfway point. One thing that has surprised me is how long it has taken to get piloting phase underway. To that end, I think that our initial project timeline was ambitious, especially because we were unclear on how well initial project enthusiasm would translate into subsequent project participation. Up until this point, our research approach has largely been to fine tune each process prospectively. For instance, we decided a pilot run of data collection prior to final project data collection would allow us to ensure a smoother data collection process. While this has slowed things initially, we are optimistic that this will allow us to progress more quickly and smoothly in the latter stages of the project. We are not currently planning to change this research approach for the time being, but we are open to the idea depending on how well the data piloting phase progresses.
Outside of data collection, the project has provided an excellent opportunity to learn and improve clinical skills. Specifically, the project has improved my understanding of the indications for urinary catheter use, as well as helped me to develop a more critical mindset regarding medical indications in general. The project has made me more aware of the importance of really asking and thinking about why a patient is on a specific medication or using a specific medical device, which is something that is very helpful for anticipating and avoiding errors in the clinical setting.
Overall, I have enjoyed my participation in the project to date and it has increased my enthusiasm for participating in a quality improvement project.
Victor Ekuta is a third-year medical student at UC San Diego.
Pediatric acute appendicitis: Is it time for nonoperative treatment (NOT)?
Clinical question
What are the differences in rates of treatment failure, duration of hospitalization, and cost between nonoperative treatment (NOT) for acute uncomplicated appendicitis versus urgent appendectomy?
Background
Acute appendicitis is found in around 5% of children presenting for urgent or emergent evaluation of abdominal pain. It is the most common illness prompting emergency abdominal surgery in children.
Several trials in adults have shown that urgent surgery may not be necessary, and NOT of uncomplicated appendicitis may be both effective and safe. NOT involves a course of IV antibiotics and careful clinical monitoring while hospitalized, then a course of oral antibiotics after discharge. Regimens vary but include coverage for aerobic and anaerobic gut flora, such as piperacillin-tazobactam followed by amoxicillin. Little is known about the safety and efficacy of NOT in children.
Study design
Meta-analysis.
Search strategy
PubMed, MEDLINE, EMBASE, and Cochrane Library were searched for relevant studies. This search identified 527 potential articles, of which the authors examined the full text of 68 and ultimately identified 5 single-center trials for analysis (4 prospective cohort trials and 1 randomized, controlled trial).
Synopsis
A total of 404 patients with uncomplicated appendicitis were seen in all trials: 168 received NOT and 236 received standard surgical care (urgent appendectomy). In the single randomized, controlled trial, patients were assigned NOT or surgical care randomly. In the other trials parental preference directed therapy.
The heterogeneity of the design, populations, definitions of illness, duration of follow-up, and NOT treatment regimens made the meta-analysis challenging. Antibiotic options for NOT varied by center but included a course of IV antibiotics followed by 7-10 days of oral antibiotics. NOT success was defined as no need for surgery within 48 hours and no recurrence of appendicitis within 1 month. Of the 236 patients who received standard surgical care, all had appendicitis and 1 had a complication requiring repeat operation. Of the NOT group, 16 (9.5%) had treatment failures, including 3 with perforated appendicitis, and 45 (27%) went on to have an appendectomy within the following year, yielding a risk ratio of failure versus standard treatment of 8.9 (95% confidence interval, 2.7-29.8). A subgroup analysis of patients with appendicoliths who received NOT found that these patients experienced a substantially increased risk of treatment failures and recurrent appendicitis with the risk ratio versus NOT without appendicolith of 10.4 (95% CI, 1.5-74). Of the 30 patients who experienced treatment failure with NOT, 15 had appendicoliths. NOT lengthened hospital stays by 14.3 hours (95% CI, 7.5-21.1) but led to lower total costs by $1,310 (95% CI, $920-$1,690).
Bottom line
NOT may be a reasonable alternative to standard surgical management for acute uncomplicated appendicitis without appendicolith in children, with a success rate of greater than 90%. Further larger, randomized prospective studies are required to establish its safety and efficacy.
Citation
Huang L et al. Comparison of antibiotic therapy and appendectomy for acute uncomplicated appendicitis in children: A meta-analysis. JAMA Pediatr. 2017;171(5):426-34.
Dr. Stubblefield is a pediatric hospitalist at Nemours/Alfred I. duPont Hospital for Children in Wilmington, Del., and a clinical assistant professor of pediatrics at Jefferson Medical College in Philadelphia.
Clinical question
What are the differences in rates of treatment failure, duration of hospitalization, and cost between nonoperative treatment (NOT) for acute uncomplicated appendicitis versus urgent appendectomy?
Background
Acute appendicitis is found in around 5% of children presenting for urgent or emergent evaluation of abdominal pain. It is the most common illness prompting emergency abdominal surgery in children.
Several trials in adults have shown that urgent surgery may not be necessary, and NOT of uncomplicated appendicitis may be both effective and safe. NOT involves a course of IV antibiotics and careful clinical monitoring while hospitalized, then a course of oral antibiotics after discharge. Regimens vary but include coverage for aerobic and anaerobic gut flora, such as piperacillin-tazobactam followed by amoxicillin. Little is known about the safety and efficacy of NOT in children.
Study design
Meta-analysis.
Search strategy
PubMed, MEDLINE, EMBASE, and Cochrane Library were searched for relevant studies. This search identified 527 potential articles, of which the authors examined the full text of 68 and ultimately identified 5 single-center trials for analysis (4 prospective cohort trials and 1 randomized, controlled trial).
Synopsis
A total of 404 patients with uncomplicated appendicitis were seen in all trials: 168 received NOT and 236 received standard surgical care (urgent appendectomy). In the single randomized, controlled trial, patients were assigned NOT or surgical care randomly. In the other trials parental preference directed therapy.
The heterogeneity of the design, populations, definitions of illness, duration of follow-up, and NOT treatment regimens made the meta-analysis challenging. Antibiotic options for NOT varied by center but included a course of IV antibiotics followed by 7-10 days of oral antibiotics. NOT success was defined as no need for surgery within 48 hours and no recurrence of appendicitis within 1 month. Of the 236 patients who received standard surgical care, all had appendicitis and 1 had a complication requiring repeat operation. Of the NOT group, 16 (9.5%) had treatment failures, including 3 with perforated appendicitis, and 45 (27%) went on to have an appendectomy within the following year, yielding a risk ratio of failure versus standard treatment of 8.9 (95% confidence interval, 2.7-29.8). A subgroup analysis of patients with appendicoliths who received NOT found that these patients experienced a substantially increased risk of treatment failures and recurrent appendicitis with the risk ratio versus NOT without appendicolith of 10.4 (95% CI, 1.5-74). Of the 30 patients who experienced treatment failure with NOT, 15 had appendicoliths. NOT lengthened hospital stays by 14.3 hours (95% CI, 7.5-21.1) but led to lower total costs by $1,310 (95% CI, $920-$1,690).
Bottom line
NOT may be a reasonable alternative to standard surgical management for acute uncomplicated appendicitis without appendicolith in children, with a success rate of greater than 90%. Further larger, randomized prospective studies are required to establish its safety and efficacy.
Citation
Huang L et al. Comparison of antibiotic therapy and appendectomy for acute uncomplicated appendicitis in children: A meta-analysis. JAMA Pediatr. 2017;171(5):426-34.
Dr. Stubblefield is a pediatric hospitalist at Nemours/Alfred I. duPont Hospital for Children in Wilmington, Del., and a clinical assistant professor of pediatrics at Jefferson Medical College in Philadelphia.
Clinical question
What are the differences in rates of treatment failure, duration of hospitalization, and cost between nonoperative treatment (NOT) for acute uncomplicated appendicitis versus urgent appendectomy?
Background
Acute appendicitis is found in around 5% of children presenting for urgent or emergent evaluation of abdominal pain. It is the most common illness prompting emergency abdominal surgery in children.
Several trials in adults have shown that urgent surgery may not be necessary, and NOT of uncomplicated appendicitis may be both effective and safe. NOT involves a course of IV antibiotics and careful clinical monitoring while hospitalized, then a course of oral antibiotics after discharge. Regimens vary but include coverage for aerobic and anaerobic gut flora, such as piperacillin-tazobactam followed by amoxicillin. Little is known about the safety and efficacy of NOT in children.
Study design
Meta-analysis.
Search strategy
PubMed, MEDLINE, EMBASE, and Cochrane Library were searched for relevant studies. This search identified 527 potential articles, of which the authors examined the full text of 68 and ultimately identified 5 single-center trials for analysis (4 prospective cohort trials and 1 randomized, controlled trial).
Synopsis
A total of 404 patients with uncomplicated appendicitis were seen in all trials: 168 received NOT and 236 received standard surgical care (urgent appendectomy). In the single randomized, controlled trial, patients were assigned NOT or surgical care randomly. In the other trials parental preference directed therapy.
The heterogeneity of the design, populations, definitions of illness, duration of follow-up, and NOT treatment regimens made the meta-analysis challenging. Antibiotic options for NOT varied by center but included a course of IV antibiotics followed by 7-10 days of oral antibiotics. NOT success was defined as no need for surgery within 48 hours and no recurrence of appendicitis within 1 month. Of the 236 patients who received standard surgical care, all had appendicitis and 1 had a complication requiring repeat operation. Of the NOT group, 16 (9.5%) had treatment failures, including 3 with perforated appendicitis, and 45 (27%) went on to have an appendectomy within the following year, yielding a risk ratio of failure versus standard treatment of 8.9 (95% confidence interval, 2.7-29.8). A subgroup analysis of patients with appendicoliths who received NOT found that these patients experienced a substantially increased risk of treatment failures and recurrent appendicitis with the risk ratio versus NOT without appendicolith of 10.4 (95% CI, 1.5-74). Of the 30 patients who experienced treatment failure with NOT, 15 had appendicoliths. NOT lengthened hospital stays by 14.3 hours (95% CI, 7.5-21.1) but led to lower total costs by $1,310 (95% CI, $920-$1,690).
Bottom line
NOT may be a reasonable alternative to standard surgical management for acute uncomplicated appendicitis without appendicolith in children, with a success rate of greater than 90%. Further larger, randomized prospective studies are required to establish its safety and efficacy.
Citation
Huang L et al. Comparison of antibiotic therapy and appendectomy for acute uncomplicated appendicitis in children: A meta-analysis. JAMA Pediatr. 2017;171(5):426-34.
Dr. Stubblefield is a pediatric hospitalist at Nemours/Alfred I. duPont Hospital for Children in Wilmington, Del., and a clinical assistant professor of pediatrics at Jefferson Medical College in Philadelphia.
Hospitalist movers and shakers – Nov. 2017
Pediatric hospitalist Patrick Conway, MD, has been named president and chief executive officer of Blue Cross and Blue Shield of North Carolina. Dr. Conway will take over for the retiring Brad Wilson on Oct. 1.
Dr. Conway is currently the deputy administrator for Innovation and Quality, and the director of the Center for Medicare and Medicaid Innovation for the Centers for Medicare and Medicaid Services (CMS). Previously, he was CMO at CMS, having served both the Obama and Trump administrations.
Hossam Hafez, MD, recently claimed the role of chief of Hospitalist Service with Health Quest Medical Practice (LaGrangeville, N.Y.). Dr. Hafez will be based out of Health Quest’s Vassar Brothers Medical Center in Poughkeepsie, N.Y., coordinating care in that hospital and throughout the Health Quest system.
Dr. Hafez has served full-time hospitalist stints with MidMichigan Health’s Physician Hospitalist Group, as well as with RiteMed Urgent Care. A native of Egypt, Dr. Hafez is fluent in both English and Arabic.
Caldwell UNC Healthcare (Lenoir, N.C.) has promoted David Lowry, MD, to chief medical officer as of Aug. 1, 2017.
Dr. Lowry, a longtime hospitalist and veteran in hospital medicine in general, will lead the building’s hospitalist program, support the chief of staff, and provide direct patient care, as well. He will serve as physician advisor for Caldwell’s Clinical Documentation, Utilization Review, Respiratory Care, and Rehabilitation departments.
Dr. Lowry boasts more than 25 years experience in hospital medicine and led in the creation of Caldwell’s hospitalist program. Since joining Caldwell, he has held leadership positions including chief of medicine. He received the hospital’s Donald D. McNeill Jr. Award for Outstanding Physician Leadership in 2014, as voted by his peers.
Joahd Toure, MD, recently was hired by Adirondack Health (Saranac Lake, N.Y.) as its new chief medical officer. He started his new position in late June 2017.
A Massachusetts native, Dr. Toure most recently worked as chief of hospitalist medicine with AdvantageCare Physicians in New York City. There, he helped manage care for patients in that system’s 16 hospitals in the New York metro area. Previously, he was regional medical director for Essex Inpatient Physicians (Boxford, Mass.) and a staff hospitalist at South Shore Hospital (South Weymouth, Mass.).
Longtime employee Emily Chapman, MD, has been promoted to chief medical officer and vice president of medical affairs at Children’s Minnesota Hospital (Minneapolis). The former vice CMO took on her new role on July 5, 2017.
Previously, Dr. Chapman served Children’s as its hospitalist program director, and as director of graduate medical education. She is an American Academy of Pediatrics Fellow.
Mark Sockell, MD, is the new chief medical officer at Meritage Medical Network in Novato, Calif. Meritage is a physician-run network that includes more than 700 board-certified physicians in both primary care and specialist fields.
Dr. Sockell has been a member of Meritage’s Board of Directors since 2014, and he specializes in risk adjustment and quality measures. His career has focused on medical education, headed by a stint as director of medical education at St. Mary’s Medical Center (San Francisco). There, he created and ran the inpatient hospitalist program.
Business Moves
Hammond-Henry Hospital (Geneseo, Ill.) recently announced the creation of a hospitalist program, utilizing the facility’s own emergency room physicians. Hammond-Henry will staff one emergency room doctor available for rounds outside of their ER work throughout the day.
The center’s hospitalist program will be led by medical director Kevin Jeffries, MD, who also will serve as one of the hospital’s ER physicians/hospitalists.
Avera Queen of Peace Hospital (Mitchell, S.D.) started its own hospitalist program on Aug. 1, 2017, launching with the goal of improving patient experience within the building. Avera’s hospitalists will be on-site for 12 hours each day, assisting specialists and working with patients who do not have a local primary care physician.
Queen of Peace is the regional referral center for an 11-county area, part of Avera Health System’s 330 facilities across North and South Dakota, Minnesota, Iowa, and Nebraska.
Pediatric hospitalist Patrick Conway, MD, has been named president and chief executive officer of Blue Cross and Blue Shield of North Carolina. Dr. Conway will take over for the retiring Brad Wilson on Oct. 1.
Dr. Conway is currently the deputy administrator for Innovation and Quality, and the director of the Center for Medicare and Medicaid Innovation for the Centers for Medicare and Medicaid Services (CMS). Previously, he was CMO at CMS, having served both the Obama and Trump administrations.
Hossam Hafez, MD, recently claimed the role of chief of Hospitalist Service with Health Quest Medical Practice (LaGrangeville, N.Y.). Dr. Hafez will be based out of Health Quest’s Vassar Brothers Medical Center in Poughkeepsie, N.Y., coordinating care in that hospital and throughout the Health Quest system.
Dr. Hafez has served full-time hospitalist stints with MidMichigan Health’s Physician Hospitalist Group, as well as with RiteMed Urgent Care. A native of Egypt, Dr. Hafez is fluent in both English and Arabic.
Caldwell UNC Healthcare (Lenoir, N.C.) has promoted David Lowry, MD, to chief medical officer as of Aug. 1, 2017.
Dr. Lowry, a longtime hospitalist and veteran in hospital medicine in general, will lead the building’s hospitalist program, support the chief of staff, and provide direct patient care, as well. He will serve as physician advisor for Caldwell’s Clinical Documentation, Utilization Review, Respiratory Care, and Rehabilitation departments.
Dr. Lowry boasts more than 25 years experience in hospital medicine and led in the creation of Caldwell’s hospitalist program. Since joining Caldwell, he has held leadership positions including chief of medicine. He received the hospital’s Donald D. McNeill Jr. Award for Outstanding Physician Leadership in 2014, as voted by his peers.
Joahd Toure, MD, recently was hired by Adirondack Health (Saranac Lake, N.Y.) as its new chief medical officer. He started his new position in late June 2017.
A Massachusetts native, Dr. Toure most recently worked as chief of hospitalist medicine with AdvantageCare Physicians in New York City. There, he helped manage care for patients in that system’s 16 hospitals in the New York metro area. Previously, he was regional medical director for Essex Inpatient Physicians (Boxford, Mass.) and a staff hospitalist at South Shore Hospital (South Weymouth, Mass.).
Longtime employee Emily Chapman, MD, has been promoted to chief medical officer and vice president of medical affairs at Children’s Minnesota Hospital (Minneapolis). The former vice CMO took on her new role on July 5, 2017.
Previously, Dr. Chapman served Children’s as its hospitalist program director, and as director of graduate medical education. She is an American Academy of Pediatrics Fellow.
Mark Sockell, MD, is the new chief medical officer at Meritage Medical Network in Novato, Calif. Meritage is a physician-run network that includes more than 700 board-certified physicians in both primary care and specialist fields.
Dr. Sockell has been a member of Meritage’s Board of Directors since 2014, and he specializes in risk adjustment and quality measures. His career has focused on medical education, headed by a stint as director of medical education at St. Mary’s Medical Center (San Francisco). There, he created and ran the inpatient hospitalist program.
Business Moves
Hammond-Henry Hospital (Geneseo, Ill.) recently announced the creation of a hospitalist program, utilizing the facility’s own emergency room physicians. Hammond-Henry will staff one emergency room doctor available for rounds outside of their ER work throughout the day.
The center’s hospitalist program will be led by medical director Kevin Jeffries, MD, who also will serve as one of the hospital’s ER physicians/hospitalists.
Avera Queen of Peace Hospital (Mitchell, S.D.) started its own hospitalist program on Aug. 1, 2017, launching with the goal of improving patient experience within the building. Avera’s hospitalists will be on-site for 12 hours each day, assisting specialists and working with patients who do not have a local primary care physician.
Queen of Peace is the regional referral center for an 11-county area, part of Avera Health System’s 330 facilities across North and South Dakota, Minnesota, Iowa, and Nebraska.
Pediatric hospitalist Patrick Conway, MD, has been named president and chief executive officer of Blue Cross and Blue Shield of North Carolina. Dr. Conway will take over for the retiring Brad Wilson on Oct. 1.
Dr. Conway is currently the deputy administrator for Innovation and Quality, and the director of the Center for Medicare and Medicaid Innovation for the Centers for Medicare and Medicaid Services (CMS). Previously, he was CMO at CMS, having served both the Obama and Trump administrations.
Hossam Hafez, MD, recently claimed the role of chief of Hospitalist Service with Health Quest Medical Practice (LaGrangeville, N.Y.). Dr. Hafez will be based out of Health Quest’s Vassar Brothers Medical Center in Poughkeepsie, N.Y., coordinating care in that hospital and throughout the Health Quest system.
Dr. Hafez has served full-time hospitalist stints with MidMichigan Health’s Physician Hospitalist Group, as well as with RiteMed Urgent Care. A native of Egypt, Dr. Hafez is fluent in both English and Arabic.
Caldwell UNC Healthcare (Lenoir, N.C.) has promoted David Lowry, MD, to chief medical officer as of Aug. 1, 2017.
Dr. Lowry, a longtime hospitalist and veteran in hospital medicine in general, will lead the building’s hospitalist program, support the chief of staff, and provide direct patient care, as well. He will serve as physician advisor for Caldwell’s Clinical Documentation, Utilization Review, Respiratory Care, and Rehabilitation departments.
Dr. Lowry boasts more than 25 years experience in hospital medicine and led in the creation of Caldwell’s hospitalist program. Since joining Caldwell, he has held leadership positions including chief of medicine. He received the hospital’s Donald D. McNeill Jr. Award for Outstanding Physician Leadership in 2014, as voted by his peers.
Joahd Toure, MD, recently was hired by Adirondack Health (Saranac Lake, N.Y.) as its new chief medical officer. He started his new position in late June 2017.
A Massachusetts native, Dr. Toure most recently worked as chief of hospitalist medicine with AdvantageCare Physicians in New York City. There, he helped manage care for patients in that system’s 16 hospitals in the New York metro area. Previously, he was regional medical director for Essex Inpatient Physicians (Boxford, Mass.) and a staff hospitalist at South Shore Hospital (South Weymouth, Mass.).
Longtime employee Emily Chapman, MD, has been promoted to chief medical officer and vice president of medical affairs at Children’s Minnesota Hospital (Minneapolis). The former vice CMO took on her new role on July 5, 2017.
Previously, Dr. Chapman served Children’s as its hospitalist program director, and as director of graduate medical education. She is an American Academy of Pediatrics Fellow.
Mark Sockell, MD, is the new chief medical officer at Meritage Medical Network in Novato, Calif. Meritage is a physician-run network that includes more than 700 board-certified physicians in both primary care and specialist fields.
Dr. Sockell has been a member of Meritage’s Board of Directors since 2014, and he specializes in risk adjustment and quality measures. His career has focused on medical education, headed by a stint as director of medical education at St. Mary’s Medical Center (San Francisco). There, he created and ran the inpatient hospitalist program.
Business Moves
Hammond-Henry Hospital (Geneseo, Ill.) recently announced the creation of a hospitalist program, utilizing the facility’s own emergency room physicians. Hammond-Henry will staff one emergency room doctor available for rounds outside of their ER work throughout the day.
The center’s hospitalist program will be led by medical director Kevin Jeffries, MD, who also will serve as one of the hospital’s ER physicians/hospitalists.
Avera Queen of Peace Hospital (Mitchell, S.D.) started its own hospitalist program on Aug. 1, 2017, launching with the goal of improving patient experience within the building. Avera’s hospitalists will be on-site for 12 hours each day, assisting specialists and working with patients who do not have a local primary care physician.
Queen of Peace is the regional referral center for an 11-county area, part of Avera Health System’s 330 facilities across North and South Dakota, Minnesota, Iowa, and Nebraska.