User login
Official news magazine of the Society of Hospital Medicine
Copyright by Society of Hospital Medicine or related companies. All rights reserved. ISSN 1553-085X
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
div[contains(@class, 'pane-pub-article-hospitalist')]


Here’s what’s trending at SHM – Nov. 2017
Recognizing American Diabetes Month, COPD Awareness Month, and CDC’s Get Smart Week with QI Solutions
- There’s no better time than during American Diabetes Month to learn more about SHM’s Glycemic Control programs. Find out how your institution can submit point-of-care data to SHM’s Data Center, generate monthly reports and be included in the national glucometrics benchmark report. Hospital systems are also encouraged to subscribe to track and compare their individual as well as overall performance. Be one of the 100-plus hospitals nationwide that are supported by SHM’s respected Glycemic Control Programs. Contact Sara Platt for a free demo at [email protected] or by phone at 267-702-2672. For additional information, visit hospitalmedicine.org/gc.
- November marks Chronic Obstructive Pulmonary Disease (COPD) Month, and it is critical that hospitals begin to direct QI resources to improving care for COPD patients. SHM developed a free guide to help you make changes to COPD care at both the individual patient and the institutional levels. Whether you are a clinician, medical director, VP of quality or chief medical officer, these resources can help you. Visit hospitalmedicine.org/COPD to download the guide.
- And, in conjunction with the Centers for Disease Control & Prevention’s (CDC’s) Get Smart Week, SHM is committed to promoting improved antibiotic prescribing behaviors among U.S. hospitalists. Through the Fight the Resistance campaign, SHM has developed many antimicrobial stewardship resources, including an implementation guide, four educational modules, and posters to hang in your hospital. Learn more at hospitalmedicine.org/abx.
Present your abstract in front of a national audience at HM18
SHM is accepting submissions for the Research, Innovations, and Clinical Vignettes (RIV) Competition at Hospital Medicine 2018 (HM18). Based on past experience, the RIV Competition is likely to be one of the most popular events at HM18, enabling hospitalists from across the country to discuss emerging science and clinical cases, share feedback, and make valuable professional connections.
Many of the cutting-edge abstracts that are first presented at SHM’s RIV sessions go on to be published in highly respected medical journals. The competition also includes a special Trainee Award category for resident and student authors.
SHM is excited to launch the Resident Travel Grant for 10 residents to receive funding to help cover the costs of travel and accommodations to attend SHM’s annual conference. See full details on how to apply and the selection process at shmannualconference.org/riv.
The submission deadline is Sunday, Dec. 3, 2017.
Distinguish yourself as a Class of 2018 Fellow in Hospital Medicine
SHM’s Fellows designation is a prestigious way to differentiate yourself in the rapidly growing profession of hospital medicine. There are currently over 2,000 hospitalists who have earned the Fellow in Hospital Medicine (FHM) or Senior Fellow in Hospital Medicine (SFHM) designation by demonstrating the core values of leadership, teamwork, and quality improvement.
Apply now and learn how you can join this prestigious group of hospitalists at hospitalmedicine.org/fellows. Applications officially close on Nov. 30, 2017.
The hospital observation care problem: Perspectives and solutions from SHM
Hospitalists provide the majority of observation care to Medicare beneficiaries and are often the primary points of contact for patients as they navigate the impact of inpatient and observation care determinations during and after their hospitalizations.
In 2017, SHM re-surveyed members to understand the state of hospital observation care after several legislative and regulatory changes. Through this new survey, hospitalists reported on their experience with the two-midnight rule and the impact of the recent Notification of Observation Treatment and Implication of Care Eligibility (NOTICE) Act, which requires hospitals to inform patients through the Medicare Outpatient Observation Notice (MOON) form that they are hospitalized under observation. Read the white paper to get perspectives and solutions from SHM at hospitalmedicine.org/advocacy.
Introducing ‘Ultrasonography: Essentials in Critical Care’
Brought to you by SHM and CHEST®, the Ultrasonography: Essentials in Critical Care course will be held Dec. 1-3, 2017, at the CHEST Innovation, Simulation, and Training Center in Glenview, Ill.
Enhance your point-of-care ultrasonography skills through hands-on training by experts in the field. Discover key elements of critical care ultrasonography and practice image acquisition with human models using high-quality ultrasound machines in this intensive 3-day course. Participants will earn 20.50 AMA PRA Category 1 Credits™ and MOC points.
Topics include:
- Vascular Access
- Vascular Diagnostic
- Echocardiography: Techniques and Standard Views
- Basic Critical Care and Echocardiography Overview
- Common Clinical Application of Ultrasonography to Guide Management of the Critically Ill
Learn more and register at livelearning.chestnet.org/ultrasonography.
Not a member? Join the movement today
Over 15,000 members have joined SHM to show their commitment to transforming health care and revolutionizing patient care. As an SHM member you will be connected to a wealth of opportunities designed to help you grow professionally, network with colleagues nationwide, and shape the practice of hospital medicine. See a full list of SHM member benefits or become a member today by visiting hospitalmedicine.org/join.
Mr. Radler is marketing communications manager at the Society of Hospital Medicine.
Recognizing American Diabetes Month, COPD Awareness Month, and CDC’s Get Smart Week with QI Solutions
- There’s no better time than during American Diabetes Month to learn more about SHM’s Glycemic Control programs. Find out how your institution can submit point-of-care data to SHM’s Data Center, generate monthly reports and be included in the national glucometrics benchmark report. Hospital systems are also encouraged to subscribe to track and compare their individual as well as overall performance. Be one of the 100-plus hospitals nationwide that are supported by SHM’s respected Glycemic Control Programs. Contact Sara Platt for a free demo at [email protected] or by phone at 267-702-2672. For additional information, visit hospitalmedicine.org/gc.
- November marks Chronic Obstructive Pulmonary Disease (COPD) Month, and it is critical that hospitals begin to direct QI resources to improving care for COPD patients. SHM developed a free guide to help you make changes to COPD care at both the individual patient and the institutional levels. Whether you are a clinician, medical director, VP of quality or chief medical officer, these resources can help you. Visit hospitalmedicine.org/COPD to download the guide.
- And, in conjunction with the Centers for Disease Control & Prevention’s (CDC’s) Get Smart Week, SHM is committed to promoting improved antibiotic prescribing behaviors among U.S. hospitalists. Through the Fight the Resistance campaign, SHM has developed many antimicrobial stewardship resources, including an implementation guide, four educational modules, and posters to hang in your hospital. Learn more at hospitalmedicine.org/abx.
Present your abstract in front of a national audience at HM18
SHM is accepting submissions for the Research, Innovations, and Clinical Vignettes (RIV) Competition at Hospital Medicine 2018 (HM18). Based on past experience, the RIV Competition is likely to be one of the most popular events at HM18, enabling hospitalists from across the country to discuss emerging science and clinical cases, share feedback, and make valuable professional connections.
Many of the cutting-edge abstracts that are first presented at SHM’s RIV sessions go on to be published in highly respected medical journals. The competition also includes a special Trainee Award category for resident and student authors.
SHM is excited to launch the Resident Travel Grant for 10 residents to receive funding to help cover the costs of travel and accommodations to attend SHM’s annual conference. See full details on how to apply and the selection process at shmannualconference.org/riv.
The submission deadline is Sunday, Dec. 3, 2017.
Distinguish yourself as a Class of 2018 Fellow in Hospital Medicine
SHM’s Fellows designation is a prestigious way to differentiate yourself in the rapidly growing profession of hospital medicine. There are currently over 2,000 hospitalists who have earned the Fellow in Hospital Medicine (FHM) or Senior Fellow in Hospital Medicine (SFHM) designation by demonstrating the core values of leadership, teamwork, and quality improvement.
Apply now and learn how you can join this prestigious group of hospitalists at hospitalmedicine.org/fellows. Applications officially close on Nov. 30, 2017.
The hospital observation care problem: Perspectives and solutions from SHM
Hospitalists provide the majority of observation care to Medicare beneficiaries and are often the primary points of contact for patients as they navigate the impact of inpatient and observation care determinations during and after their hospitalizations.
In 2017, SHM re-surveyed members to understand the state of hospital observation care after several legislative and regulatory changes. Through this new survey, hospitalists reported on their experience with the two-midnight rule and the impact of the recent Notification of Observation Treatment and Implication of Care Eligibility (NOTICE) Act, which requires hospitals to inform patients through the Medicare Outpatient Observation Notice (MOON) form that they are hospitalized under observation. Read the white paper to get perspectives and solutions from SHM at hospitalmedicine.org/advocacy.
Introducing ‘Ultrasonography: Essentials in Critical Care’
Brought to you by SHM and CHEST®, the Ultrasonography: Essentials in Critical Care course will be held Dec. 1-3, 2017, at the CHEST Innovation, Simulation, and Training Center in Glenview, Ill.
Enhance your point-of-care ultrasonography skills through hands-on training by experts in the field. Discover key elements of critical care ultrasonography and practice image acquisition with human models using high-quality ultrasound machines in this intensive 3-day course. Participants will earn 20.50 AMA PRA Category 1 Credits™ and MOC points.
Topics include:
- Vascular Access
- Vascular Diagnostic
- Echocardiography: Techniques and Standard Views
- Basic Critical Care and Echocardiography Overview
- Common Clinical Application of Ultrasonography to Guide Management of the Critically Ill
Learn more and register at livelearning.chestnet.org/ultrasonography.
Not a member? Join the movement today
Over 15,000 members have joined SHM to show their commitment to transforming health care and revolutionizing patient care. As an SHM member you will be connected to a wealth of opportunities designed to help you grow professionally, network with colleagues nationwide, and shape the practice of hospital medicine. See a full list of SHM member benefits or become a member today by visiting hospitalmedicine.org/join.
Mr. Radler is marketing communications manager at the Society of Hospital Medicine.
Recognizing American Diabetes Month, COPD Awareness Month, and CDC’s Get Smart Week with QI Solutions
- There’s no better time than during American Diabetes Month to learn more about SHM’s Glycemic Control programs. Find out how your institution can submit point-of-care data to SHM’s Data Center, generate monthly reports and be included in the national glucometrics benchmark report. Hospital systems are also encouraged to subscribe to track and compare their individual as well as overall performance. Be one of the 100-plus hospitals nationwide that are supported by SHM’s respected Glycemic Control Programs. Contact Sara Platt for a free demo at [email protected] or by phone at 267-702-2672. For additional information, visit hospitalmedicine.org/gc.
- November marks Chronic Obstructive Pulmonary Disease (COPD) Month, and it is critical that hospitals begin to direct QI resources to improving care for COPD patients. SHM developed a free guide to help you make changes to COPD care at both the individual patient and the institutional levels. Whether you are a clinician, medical director, VP of quality or chief medical officer, these resources can help you. Visit hospitalmedicine.org/COPD to download the guide.
- And, in conjunction with the Centers for Disease Control & Prevention’s (CDC’s) Get Smart Week, SHM is committed to promoting improved antibiotic prescribing behaviors among U.S. hospitalists. Through the Fight the Resistance campaign, SHM has developed many antimicrobial stewardship resources, including an implementation guide, four educational modules, and posters to hang in your hospital. Learn more at hospitalmedicine.org/abx.
Present your abstract in front of a national audience at HM18
SHM is accepting submissions for the Research, Innovations, and Clinical Vignettes (RIV) Competition at Hospital Medicine 2018 (HM18). Based on past experience, the RIV Competition is likely to be one of the most popular events at HM18, enabling hospitalists from across the country to discuss emerging science and clinical cases, share feedback, and make valuable professional connections.
Many of the cutting-edge abstracts that are first presented at SHM’s RIV sessions go on to be published in highly respected medical journals. The competition also includes a special Trainee Award category for resident and student authors.
SHM is excited to launch the Resident Travel Grant for 10 residents to receive funding to help cover the costs of travel and accommodations to attend SHM’s annual conference. See full details on how to apply and the selection process at shmannualconference.org/riv.
The submission deadline is Sunday, Dec. 3, 2017.
Distinguish yourself as a Class of 2018 Fellow in Hospital Medicine
SHM’s Fellows designation is a prestigious way to differentiate yourself in the rapidly growing profession of hospital medicine. There are currently over 2,000 hospitalists who have earned the Fellow in Hospital Medicine (FHM) or Senior Fellow in Hospital Medicine (SFHM) designation by demonstrating the core values of leadership, teamwork, and quality improvement.
Apply now and learn how you can join this prestigious group of hospitalists at hospitalmedicine.org/fellows. Applications officially close on Nov. 30, 2017.
The hospital observation care problem: Perspectives and solutions from SHM
Hospitalists provide the majority of observation care to Medicare beneficiaries and are often the primary points of contact for patients as they navigate the impact of inpatient and observation care determinations during and after their hospitalizations.
In 2017, SHM re-surveyed members to understand the state of hospital observation care after several legislative and regulatory changes. Through this new survey, hospitalists reported on their experience with the two-midnight rule and the impact of the recent Notification of Observation Treatment and Implication of Care Eligibility (NOTICE) Act, which requires hospitals to inform patients through the Medicare Outpatient Observation Notice (MOON) form that they are hospitalized under observation. Read the white paper to get perspectives and solutions from SHM at hospitalmedicine.org/advocacy.
Introducing ‘Ultrasonography: Essentials in Critical Care’
Brought to you by SHM and CHEST®, the Ultrasonography: Essentials in Critical Care course will be held Dec. 1-3, 2017, at the CHEST Innovation, Simulation, and Training Center in Glenview, Ill.
Enhance your point-of-care ultrasonography skills through hands-on training by experts in the field. Discover key elements of critical care ultrasonography and practice image acquisition with human models using high-quality ultrasound machines in this intensive 3-day course. Participants will earn 20.50 AMA PRA Category 1 Credits™ and MOC points.
Topics include:
- Vascular Access
- Vascular Diagnostic
- Echocardiography: Techniques and Standard Views
- Basic Critical Care and Echocardiography Overview
- Common Clinical Application of Ultrasonography to Guide Management of the Critically Ill
Learn more and register at livelearning.chestnet.org/ultrasonography.
Not a member? Join the movement today
Over 15,000 members have joined SHM to show their commitment to transforming health care and revolutionizing patient care. As an SHM member you will be connected to a wealth of opportunities designed to help you grow professionally, network with colleagues nationwide, and shape the practice of hospital medicine. See a full list of SHM member benefits or become a member today by visiting hospitalmedicine.org/join.
Mr. Radler is marketing communications manager at the Society of Hospital Medicine.
Safety-net hospitals would be hurt by hospital-wide 30-day readmission penalties
Considering all readmissions within 30 days of discharge in the Hospital Readmissions Reduction Program would modestly increase the number of hospitals eligible for penalties and would have a bigger impact on safety-net hospitals, based on a study of two years of Medicare claims data from 3,443 hospitals.
“Transition to a hospital-wide measure would require an adjustment in the penalty formula to keep penalties in the same range for most hospitals and without a change in procedures would have a deleterious effect on safety-net hospitals,” according to Rachael B. Zuckerman, PhD, from the Department of Health and Human Services, Washington, and her co-authors.
Analyzing 6,807,899 admissions for hospital-wide readmission measures and 4,392,658 admissions for condition-specific measures, the researchers found that a condition-specific approach would result in 3,238 hospitals being eligible for penalties for at least one condition. A hospital-wide measure of readmissions would result in 76 additional hospitals being eligible for penalties based on one year of admissions data, and 128 additional hospitals based on 3 years of admissions data (NEJM 2017, 377:1551-58. DOI: 10.1056/NEJMsa1701791).
Moving to a hospital-wide measure of readmissions also would significantly increase mean annual penalty rates across all hospitals by 0.89% of base diagnosis-related group (DRG) payments or $393,000; 43% of hospitals would be penalized under this standard.
“Moving to the hospital-wide readmission measure would also substantially increase the disparity between safety-net and other hospitals: the mean penalty as a percentage of base DRG payments would be 0.41 percentage points ($198,000) higher among safety net hospitals,” the authors wrote.
“Since safety-net hospitals tend to perform slightly worse on the hospital-wide measure, they are more likely to receive a penalty, which would increase the disparity in penalties between the two groups.”
The study was supported by the Department of Health and Human Services. One author declared grants from funding bodies and universities outside the submitted work. One author is an associate editor of the New England Journal of Medicine. One author was an employee of the Department of Health and Human Services at the time of the study. No other conflicts of interest were declared.
Considering all readmissions within 30 days of discharge in the Hospital Readmissions Reduction Program would modestly increase the number of hospitals eligible for penalties and would have a bigger impact on safety-net hospitals, based on a study of two years of Medicare claims data from 3,443 hospitals.
“Transition to a hospital-wide measure would require an adjustment in the penalty formula to keep penalties in the same range for most hospitals and without a change in procedures would have a deleterious effect on safety-net hospitals,” according to Rachael B. Zuckerman, PhD, from the Department of Health and Human Services, Washington, and her co-authors.
Analyzing 6,807,899 admissions for hospital-wide readmission measures and 4,392,658 admissions for condition-specific measures, the researchers found that a condition-specific approach would result in 3,238 hospitals being eligible for penalties for at least one condition. A hospital-wide measure of readmissions would result in 76 additional hospitals being eligible for penalties based on one year of admissions data, and 128 additional hospitals based on 3 years of admissions data (NEJM 2017, 377:1551-58. DOI: 10.1056/NEJMsa1701791).
Moving to a hospital-wide measure of readmissions also would significantly increase mean annual penalty rates across all hospitals by 0.89% of base diagnosis-related group (DRG) payments or $393,000; 43% of hospitals would be penalized under this standard.
“Moving to the hospital-wide readmission measure would also substantially increase the disparity between safety-net and other hospitals: the mean penalty as a percentage of base DRG payments would be 0.41 percentage points ($198,000) higher among safety net hospitals,” the authors wrote.
“Since safety-net hospitals tend to perform slightly worse on the hospital-wide measure, they are more likely to receive a penalty, which would increase the disparity in penalties between the two groups.”
The study was supported by the Department of Health and Human Services. One author declared grants from funding bodies and universities outside the submitted work. One author is an associate editor of the New England Journal of Medicine. One author was an employee of the Department of Health and Human Services at the time of the study. No other conflicts of interest were declared.
Considering all readmissions within 30 days of discharge in the Hospital Readmissions Reduction Program would modestly increase the number of hospitals eligible for penalties and would have a bigger impact on safety-net hospitals, based on a study of two years of Medicare claims data from 3,443 hospitals.
“Transition to a hospital-wide measure would require an adjustment in the penalty formula to keep penalties in the same range for most hospitals and without a change in procedures would have a deleterious effect on safety-net hospitals,” according to Rachael B. Zuckerman, PhD, from the Department of Health and Human Services, Washington, and her co-authors.
Analyzing 6,807,899 admissions for hospital-wide readmission measures and 4,392,658 admissions for condition-specific measures, the researchers found that a condition-specific approach would result in 3,238 hospitals being eligible for penalties for at least one condition. A hospital-wide measure of readmissions would result in 76 additional hospitals being eligible for penalties based on one year of admissions data, and 128 additional hospitals based on 3 years of admissions data (NEJM 2017, 377:1551-58. DOI: 10.1056/NEJMsa1701791).
Moving to a hospital-wide measure of readmissions also would significantly increase mean annual penalty rates across all hospitals by 0.89% of base diagnosis-related group (DRG) payments or $393,000; 43% of hospitals would be penalized under this standard.
“Moving to the hospital-wide readmission measure would also substantially increase the disparity between safety-net and other hospitals: the mean penalty as a percentage of base DRG payments would be 0.41 percentage points ($198,000) higher among safety net hospitals,” the authors wrote.
“Since safety-net hospitals tend to perform slightly worse on the hospital-wide measure, they are more likely to receive a penalty, which would increase the disparity in penalties between the two groups.”
The study was supported by the Department of Health and Human Services. One author declared grants from funding bodies and universities outside the submitted work. One author is an associate editor of the New England Journal of Medicine. One author was an employee of the Department of Health and Human Services at the time of the study. No other conflicts of interest were declared.
FROM NEJM
Key clinical point: Adopting a hospital-wide measure of 30-day readmissions for the Hospital Readmissions Reduction Program would modestly increase the number of hospitals eligible for penalties and would have a bigger impact on safety-net hospitals.
Major finding: With a hospital-wide measure of readmissions in the Hospital Readmissions Reduction Program, the mean penalty as a percentage of base DRG payments would be 0.41 percentage points ($198,000) higher among safety net hospitals.
Data source: Analysis of two years of Medicare claims data from 3,443 hospitals.
Disclosures: The study was supported by the Department of Health and Human Services. One author declared grants from funding bodies and universities outside the submitted work. One author is an associated editor of the New England Journal of Medicine. One author was an employee of the Department of Health and Human Services at the time of the study. No other conflicts of interest were declared.
Heart failure readmission penalties linked with rise in deaths
ANAHEIM, CALIF. – Evidence continues to mount that Medicare’s penalization of hospitals with excess heart failure readmissions has cut readmissions but at the apparent price of more deaths.
During the penalty phase of the Hospital Readmission Reduction Program (HRRP), which started in Oct. 2012, 30-day all-cause mortality following a heart failure hospitalization was 18% higher compared with the adjusted rate during 2006-2010, based on Medicare data from 2006-2014 that underwent “extensive” risk adjustment using prospectively-collected clinical data, Gregg C. Fonarow, MD, and his associates reported in a poster at the American Heart Association scientific sessions. During the same 2012-2014 period with imposed penalties, 30-day all-cause readmissions following an index heart failure hospitalization fell by a risk-adjusted 9% compared to the era just before the HRRP. Both the drop in readmissions and rise in deaths were statistically significant.
A similar pattern existed for the risk-adjusted readmissions and mortality rates during the year following the index hospitalization: readmissions fell by 8% compared with the time before the program but deaths rose by a relative 10%, also statistically significant differences.
“This is urgent and alarming. The Centers for Medicare & Medicaid Services needs to revamp the program to exclude heart failure patients and take steps to mitigate the damage,” Dr. Fonarow said in an interview. He estimated that the uptick in mortality following heart failure hospitalizations is causing 5,000-10,000 excess annual deaths among U.S. heart failure patients that are directly attributable to the HRRP. Similar effects have not been seen for patients with an index hospitalization of pneumonia or acute MI, two other targets of the HRRP, he noted.
The HRRP “currently has penalties for readmissions that are 15-fold higher than for mortality. They need to penalize equally, and they need to get at the gaming that hospitals are doing” to shift outcomes away from readmissions even if it means more patients will die. Heart failure patients “who need hospitalization are being denied admission by hospitals out of fear of the readmissions penalty,” said Dr. Fonarow, professor and co-chief of cardiology at the University of California, Los Angeles. “Seeing increased mortality linked with implementation of the penalty is completely unacceptable.”
Although a prior report used similar Medicare data from 2008-2014 to initially find this inverse association, that analysis relied entirely on administrative data collected in Medicare records to perform risk adjustments (JAMA. 2017 July 17;318[3]:270-8). The new analysis reported by Dr. Fonarow and his associates combined the Medicare data with detailed clinical records for the same patients collected by the Get With the Guidelines--Heart Failure program. The extensive clinical data that the researchers used for risk-adjustment allowed for a more reliable attribution to the HRRP of readmission and mortality differences between the two time periods. Despite the extensive risk adjustment “we see exactly the same result” as initially reported, Dr. Fonarow said.
The findings “remind us that it is very important to look at the unintended consequences” of interventions that might initially seem reasonable, commented Lynne Warner Stevenson, MD, professor and director of cardiomyopathy at Vanderbilt University in Nashville, Tenn.
Concurrent with the presentation at the meeting the results also appeared in an article published online (JAMA Cardiol. 2017 Nov 12;doi:10.1001/jamacardio.2017.4265).
A separate analysis of data collected in the Get With the Guidelines--Heart Failure during 2005-2009 showed that within the past decade the 5-year survival of U.S. hospitalized heart failure patients has remained dismally low, and similar regardless of whether patients had heart failure with reduced ejection fraction (HFrEF, 46% of all heart failure patients in the analysis), heart failure with preserved ejection fraction (HFpEF, also 46% of patients), or the in-between patients who had heart failure with borderline ejection fraction (HFbEF, an ejection fraction of 41%-49%, in 8% of patients).
The results, from 39,982 patients, showed a 75% mortality rate during 5-years of follow-up, with similar mortality rates regardless of the patient’s ejection-fraction level, reported Dr. Fonarow and his associates in a separate poster. In every age group examined, patients with heart failure had dramatically reduced life expectancies compared with the general population. For example, among heart failure patients aged 65-69 years in the study, median survival was less than 4 years compared with a 19-year expected median survival for people in the general U.S. population in the same age range.
These very low survival rates of heart failure patients initially hospitalized for heart failure during the relatively recent era of 2005-2009 “is a call to action to prevent heart failure,” said Dr. Fonarow.
The poor prognosis most heart failure patients face should also spur aggressive treatment of HFrEF patients with all proven treatments, Dr. Fonarow said. It should also spur more effort to find effective treatments for HFpEF, which currently has no clearly-proven effective treatment.
These results also appeared in a report simultaneously published online (J Amer Coll Cardiol. 2017 Nov 12;doi: 10.1016/j.jacc.2017.08.074).
[email protected]
On Twitter @mitchelzoler
ANAHEIM, CALIF. – Evidence continues to mount that Medicare’s penalization of hospitals with excess heart failure readmissions has cut readmissions but at the apparent price of more deaths.
During the penalty phase of the Hospital Readmission Reduction Program (HRRP), which started in Oct. 2012, 30-day all-cause mortality following a heart failure hospitalization was 18% higher compared with the adjusted rate during 2006-2010, based on Medicare data from 2006-2014 that underwent “extensive” risk adjustment using prospectively-collected clinical data, Gregg C. Fonarow, MD, and his associates reported in a poster at the American Heart Association scientific sessions. During the same 2012-2014 period with imposed penalties, 30-day all-cause readmissions following an index heart failure hospitalization fell by a risk-adjusted 9% compared to the era just before the HRRP. Both the drop in readmissions and rise in deaths were statistically significant.
A similar pattern existed for the risk-adjusted readmissions and mortality rates during the year following the index hospitalization: readmissions fell by 8% compared with the time before the program but deaths rose by a relative 10%, also statistically significant differences.
“This is urgent and alarming. The Centers for Medicare & Medicaid Services needs to revamp the program to exclude heart failure patients and take steps to mitigate the damage,” Dr. Fonarow said in an interview. He estimated that the uptick in mortality following heart failure hospitalizations is causing 5,000-10,000 excess annual deaths among U.S. heart failure patients that are directly attributable to the HRRP. Similar effects have not been seen for patients with an index hospitalization of pneumonia or acute MI, two other targets of the HRRP, he noted.
The HRRP “currently has penalties for readmissions that are 15-fold higher than for mortality. They need to penalize equally, and they need to get at the gaming that hospitals are doing” to shift outcomes away from readmissions even if it means more patients will die. Heart failure patients “who need hospitalization are being denied admission by hospitals out of fear of the readmissions penalty,” said Dr. Fonarow, professor and co-chief of cardiology at the University of California, Los Angeles. “Seeing increased mortality linked with implementation of the penalty is completely unacceptable.”
Although a prior report used similar Medicare data from 2008-2014 to initially find this inverse association, that analysis relied entirely on administrative data collected in Medicare records to perform risk adjustments (JAMA. 2017 July 17;318[3]:270-8). The new analysis reported by Dr. Fonarow and his associates combined the Medicare data with detailed clinical records for the same patients collected by the Get With the Guidelines--Heart Failure program. The extensive clinical data that the researchers used for risk-adjustment allowed for a more reliable attribution to the HRRP of readmission and mortality differences between the two time periods. Despite the extensive risk adjustment “we see exactly the same result” as initially reported, Dr. Fonarow said.
The findings “remind us that it is very important to look at the unintended consequences” of interventions that might initially seem reasonable, commented Lynne Warner Stevenson, MD, professor and director of cardiomyopathy at Vanderbilt University in Nashville, Tenn.
Concurrent with the presentation at the meeting the results also appeared in an article published online (JAMA Cardiol. 2017 Nov 12;doi:10.1001/jamacardio.2017.4265).
A separate analysis of data collected in the Get With the Guidelines--Heart Failure during 2005-2009 showed that within the past decade the 5-year survival of U.S. hospitalized heart failure patients has remained dismally low, and similar regardless of whether patients had heart failure with reduced ejection fraction (HFrEF, 46% of all heart failure patients in the analysis), heart failure with preserved ejection fraction (HFpEF, also 46% of patients), or the in-between patients who had heart failure with borderline ejection fraction (HFbEF, an ejection fraction of 41%-49%, in 8% of patients).
The results, from 39,982 patients, showed a 75% mortality rate during 5-years of follow-up, with similar mortality rates regardless of the patient’s ejection-fraction level, reported Dr. Fonarow and his associates in a separate poster. In every age group examined, patients with heart failure had dramatically reduced life expectancies compared with the general population. For example, among heart failure patients aged 65-69 years in the study, median survival was less than 4 years compared with a 19-year expected median survival for people in the general U.S. population in the same age range.
These very low survival rates of heart failure patients initially hospitalized for heart failure during the relatively recent era of 2005-2009 “is a call to action to prevent heart failure,” said Dr. Fonarow.
The poor prognosis most heart failure patients face should also spur aggressive treatment of HFrEF patients with all proven treatments, Dr. Fonarow said. It should also spur more effort to find effective treatments for HFpEF, which currently has no clearly-proven effective treatment.
These results also appeared in a report simultaneously published online (J Amer Coll Cardiol. 2017 Nov 12;doi: 10.1016/j.jacc.2017.08.074).
[email protected]
On Twitter @mitchelzoler
ANAHEIM, CALIF. – Evidence continues to mount that Medicare’s penalization of hospitals with excess heart failure readmissions has cut readmissions but at the apparent price of more deaths.
During the penalty phase of the Hospital Readmission Reduction Program (HRRP), which started in Oct. 2012, 30-day all-cause mortality following a heart failure hospitalization was 18% higher compared with the adjusted rate during 2006-2010, based on Medicare data from 2006-2014 that underwent “extensive” risk adjustment using prospectively-collected clinical data, Gregg C. Fonarow, MD, and his associates reported in a poster at the American Heart Association scientific sessions. During the same 2012-2014 period with imposed penalties, 30-day all-cause readmissions following an index heart failure hospitalization fell by a risk-adjusted 9% compared to the era just before the HRRP. Both the drop in readmissions and rise in deaths were statistically significant.
A similar pattern existed for the risk-adjusted readmissions and mortality rates during the year following the index hospitalization: readmissions fell by 8% compared with the time before the program but deaths rose by a relative 10%, also statistically significant differences.
“This is urgent and alarming. The Centers for Medicare & Medicaid Services needs to revamp the program to exclude heart failure patients and take steps to mitigate the damage,” Dr. Fonarow said in an interview. He estimated that the uptick in mortality following heart failure hospitalizations is causing 5,000-10,000 excess annual deaths among U.S. heart failure patients that are directly attributable to the HRRP. Similar effects have not been seen for patients with an index hospitalization of pneumonia or acute MI, two other targets of the HRRP, he noted.
The HRRP “currently has penalties for readmissions that are 15-fold higher than for mortality. They need to penalize equally, and they need to get at the gaming that hospitals are doing” to shift outcomes away from readmissions even if it means more patients will die. Heart failure patients “who need hospitalization are being denied admission by hospitals out of fear of the readmissions penalty,” said Dr. Fonarow, professor and co-chief of cardiology at the University of California, Los Angeles. “Seeing increased mortality linked with implementation of the penalty is completely unacceptable.”
Although a prior report used similar Medicare data from 2008-2014 to initially find this inverse association, that analysis relied entirely on administrative data collected in Medicare records to perform risk adjustments (JAMA. 2017 July 17;318[3]:270-8). The new analysis reported by Dr. Fonarow and his associates combined the Medicare data with detailed clinical records for the same patients collected by the Get With the Guidelines--Heart Failure program. The extensive clinical data that the researchers used for risk-adjustment allowed for a more reliable attribution to the HRRP of readmission and mortality differences between the two time periods. Despite the extensive risk adjustment “we see exactly the same result” as initially reported, Dr. Fonarow said.
The findings “remind us that it is very important to look at the unintended consequences” of interventions that might initially seem reasonable, commented Lynne Warner Stevenson, MD, professor and director of cardiomyopathy at Vanderbilt University in Nashville, Tenn.
Concurrent with the presentation at the meeting the results also appeared in an article published online (JAMA Cardiol. 2017 Nov 12;doi:10.1001/jamacardio.2017.4265).
A separate analysis of data collected in the Get With the Guidelines--Heart Failure during 2005-2009 showed that within the past decade the 5-year survival of U.S. hospitalized heart failure patients has remained dismally low, and similar regardless of whether patients had heart failure with reduced ejection fraction (HFrEF, 46% of all heart failure patients in the analysis), heart failure with preserved ejection fraction (HFpEF, also 46% of patients), or the in-between patients who had heart failure with borderline ejection fraction (HFbEF, an ejection fraction of 41%-49%, in 8% of patients).
The results, from 39,982 patients, showed a 75% mortality rate during 5-years of follow-up, with similar mortality rates regardless of the patient’s ejection-fraction level, reported Dr. Fonarow and his associates in a separate poster. In every age group examined, patients with heart failure had dramatically reduced life expectancies compared with the general population. For example, among heart failure patients aged 65-69 years in the study, median survival was less than 4 years compared with a 19-year expected median survival for people in the general U.S. population in the same age range.
These very low survival rates of heart failure patients initially hospitalized for heart failure during the relatively recent era of 2005-2009 “is a call to action to prevent heart failure,” said Dr. Fonarow.
The poor prognosis most heart failure patients face should also spur aggressive treatment of HFrEF patients with all proven treatments, Dr. Fonarow said. It should also spur more effort to find effective treatments for HFpEF, which currently has no clearly-proven effective treatment.
These results also appeared in a report simultaneously published online (J Amer Coll Cardiol. 2017 Nov 12;doi: 10.1016/j.jacc.2017.08.074).
[email protected]
On Twitter @mitchelzoler
AT THE AHA SCIENTIFIC SESSIONS
Key clinical point:
Major finding: Risk-adjusted 30-day readmissions fell by a relative 9% during the reduction program but relative mortality rose by 18%.
Data source: Review of 115,245 Medicare beneficiaries with heart failure treated at hospitals in the Get With the Guidelines--Heart Failure program.
Disclosures: Dr. Fonarow has been a consultant to Amgen, Janssen, Medtronic, Novartis, and St. Jude. Dr. Stevenson has been a consultant to Abbott, has received travel support from St. Jude, and has received research funding and food from Novartis.
Price transparency of laboratory testing does not change provider ordering habits
Clinical question: Does price transparency of laboratory tests at the point of order entry affect provider ordering behavior?
Background: Up to 30% of laboratory testing may be unnecessary, and health systems are seeking ways to effectively influence provider ordering of tests to reduce costs and improve value to patients. Price transparency and cost displaying is one strategy that has had mixed results in influencing provider ordering and reducing the amount of unnecessary laboratory testing.
Setting: Three urban academic hospitals in Philadelphia.
Synopsis: Sixty inpatient laboratory tests were randomized to either display Medicare fees at the point of order entry or not. Changes in outcomes were followed for 1 year preintervention and 1 year post intervention. The population included 98,529 patients comprising 142,921 hospital admissions. Tests ordered per patient-day and Medicare-associated fees did not significantly change in the intervention group or the control group in the year after the intervention, compared to the year preintervention.
Bottom line: Displaying laboratory testing fees at the point of order entry did not lead to a significant change in provider ordering behavior or reduction in costs.
Citation: Sedrak MS, Myers JS, Small DS, et al. Effect of a price transparency intervention in the electronic health record on clinician ordering of inpatient laboratory tests: The PRICE randomized clinical trial. JAMA Intern Med. 2017 Jul 1;177(7):939-45.
Dr. Chung is hospitalist and assistant professor of medicine, Icahn School of Medicine of the Mount Sinai Health System.
Clinical question: Does price transparency of laboratory tests at the point of order entry affect provider ordering behavior?
Background: Up to 30% of laboratory testing may be unnecessary, and health systems are seeking ways to effectively influence provider ordering of tests to reduce costs and improve value to patients. Price transparency and cost displaying is one strategy that has had mixed results in influencing provider ordering and reducing the amount of unnecessary laboratory testing.
Setting: Three urban academic hospitals in Philadelphia.
Synopsis: Sixty inpatient laboratory tests were randomized to either display Medicare fees at the point of order entry or not. Changes in outcomes were followed for 1 year preintervention and 1 year post intervention. The population included 98,529 patients comprising 142,921 hospital admissions. Tests ordered per patient-day and Medicare-associated fees did not significantly change in the intervention group or the control group in the year after the intervention, compared to the year preintervention.
Bottom line: Displaying laboratory testing fees at the point of order entry did not lead to a significant change in provider ordering behavior or reduction in costs.
Citation: Sedrak MS, Myers JS, Small DS, et al. Effect of a price transparency intervention in the electronic health record on clinician ordering of inpatient laboratory tests: The PRICE randomized clinical trial. JAMA Intern Med. 2017 Jul 1;177(7):939-45.
Dr. Chung is hospitalist and assistant professor of medicine, Icahn School of Medicine of the Mount Sinai Health System.
Clinical question: Does price transparency of laboratory tests at the point of order entry affect provider ordering behavior?
Background: Up to 30% of laboratory testing may be unnecessary, and health systems are seeking ways to effectively influence provider ordering of tests to reduce costs and improve value to patients. Price transparency and cost displaying is one strategy that has had mixed results in influencing provider ordering and reducing the amount of unnecessary laboratory testing.
Setting: Three urban academic hospitals in Philadelphia.
Synopsis: Sixty inpatient laboratory tests were randomized to either display Medicare fees at the point of order entry or not. Changes in outcomes were followed for 1 year preintervention and 1 year post intervention. The population included 98,529 patients comprising 142,921 hospital admissions. Tests ordered per patient-day and Medicare-associated fees did not significantly change in the intervention group or the control group in the year after the intervention, compared to the year preintervention.
Bottom line: Displaying laboratory testing fees at the point of order entry did not lead to a significant change in provider ordering behavior or reduction in costs.
Citation: Sedrak MS, Myers JS, Small DS, et al. Effect of a price transparency intervention in the electronic health record on clinician ordering of inpatient laboratory tests: The PRICE randomized clinical trial. JAMA Intern Med. 2017 Jul 1;177(7):939-45.
Dr. Chung is hospitalist and assistant professor of medicine, Icahn School of Medicine of the Mount Sinai Health System.
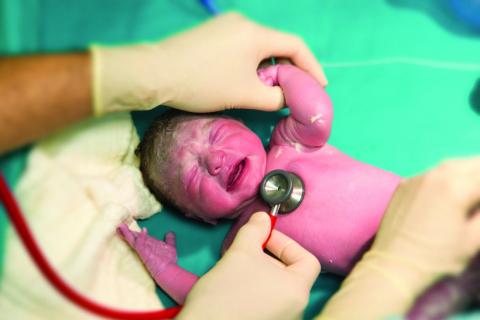
Two changes are made to resuscitation practice in delivery room
CHICAGO – , according to Gary M. Weiner, MD, of the department of pediatrics and neonatal-perinatal medicine at the University of Michigan and C.S. Mott Children’s Hospital in Ann Arbor.
One is recommending an electronic cardiac (EC) monitor to assess heart rate during resuscitation instead of relying on pulse oximetry, and the other is no longer recommending routine tracheal suction in nonvigorous babies with meconium-stained fluid, he told attendees at the American Academy of Pediatrics annual meeting.
About two-thirds of all births have a risk factor for needing resuscitation, and about 10%-20% of babies with a risk factor will need positive pressure ventilation (PPV). But risk factors do not identify all newborns who will need it. The risk is greatest for newborns less than 36 weeks’ or greater than 40 weeks’ gestational age, but 7% of term newborns will need PPV despite having no risk factors.
Situations in which there is the highest risk for advanced resuscitation include the following:
- Fetal bradycardia: 24-fold greater odds.
- Intrauterine growth restriction (IUGR): 20-fold greater odds.
- Clinical chorioamnionitis: 17-fold greater odds.
- Forceps or vacuum: 17-fold greater odds.
- Meconium-stained amniotic fluid (MSAF): 17-fold greater odds.
- Gestational diabetes: 16-fold greater odds.
- Abruption: 12-fold greater odds.
- General anesthesia: 11-fold greater odds.
These risks were determined in a prospective multicenter, case-control study of 61,593 births (Arch Dis Child Fetal Neonatal Ed. 2017 Jan;102[1]:F44-F50).
Assembling a team and using checklists
Teamwork and communication are key in delivery room emergencies, and teams should debrief afterward, ideally having videotaped the resuscitation, if possible, Dr. Weiner said.
He discussed preparation for a very-low-birth-weight birth, a “routine emergency” requiring many tasks in a short period of time: 130 tasks in the first hour and 40 in the first 3 minutes.
“Decisions made during the first hour have long-term implications, so you need multiple caregivers and a high-performance team,” Dr. Weiner said. In addition to a thorough understanding of the clinical situation, a high-performance team should have both effective leadership, and clearly defined roles and responsibilities for each member. Clinicians on the team need highly developed technical skills that they reliably and consistently execute with precision. “Practice, refine, practice, refine,” he emphasized.
It’s also important to make use of preset protocols, scripts, and checklists, Dr. Weiner said. These tools assure consistency, facilitate communication among team members, and improve outcomes. Research has shown that use of protocols, scripts, and checklists leads to improved stroke and trauma care, decreased complications during intubation, fewer central-line complications, and decreased perioperative mortality and complications.
He also recommended implementing a standardized equipment check and team briefing “time-out,” similar to a surgical time-out. This time-out gives teams an opportunity to identify a team leader, define member roles and responsibilities, check all equipment and supplies, discuss risk factors and possible scenarios, talk with the obstetrician and, if possible, introduce the leader or another team member to the parents.
In a study from University of California, San Diego, Medical Center, using checklists as part of resuscitation of potentially high-risk infants reduced the occurrence of communication problems from 24% to 4% of resuscitations (P less than 0.001) over a 3-year period (Resuscitation. 2013 Nov;84[11]:1552-7).
Delayed cord clamping
Dr. Weiner also discussed the benefits of placental transfusion. The fetal-placental unit includes approximately 110 mL/kg of blood, and about one-third of its volume remains in the placenta immediately after birth. Immediate cord clamping means a loss of 10-20 mL/kg of “potential” newborn blood volume, and could contribute to unstable pulmonary blood flow or a carotid artery pressure spike (Matern Health Neonatol Perinatol. 2016. doi: 10.1186/s40748-016-0032-y).
“Umbilical blood flow is complex,” he said. Blood flows toward the baby via the umbilical vein during inhalation, but stops or reverses during crying. The umbilical artery primarily carries blood to the placenta, and flow stops after about 4 minutes in more than half of infants. Gravity’s role in blood flow is controversial (Lancet. 2014 Jul 19;384[9939]:235-40).
The two options for placental transfusion are delayed cord clamping and milking the umbilical cord (also called “stripping”). In vaginal births, delayed clamping allows 20 mL/kg blood to transfer to the baby by 3 minutes after birth, with 90% of that reaching the baby in the first minute (Lancet. 1969 Oct 25;294[7626]:871-3).
Blood transfer is less efficient in cesarean births, so milking may be more efficient than simply delaying clamping, according to a small randomized controlled trial of preterm infants around 28 weeks’ gestational age. No difference between the methods was seen in vaginal births. To milk the cord, pinch it near the placenta and squeeze it toward the newborn for 2 seconds; then release, refill and repeat.
The biggest benefits in delayed cord clamping or milking occur among preterm infants: decreased mortality, higher mean arterial pressure on day 1, and a lower risk of blood transfusion, necrotizing enterocolitis, and a Bayley Motor score below 85 at 18-22 months. Term babies also get benefits, though: increased hemoglobin at birth (approximately 2 g/dL), a 0.5- to 5-point average increase in boys’ Ages & Stages fine motor and social domain scores at age 4 years, and among high-risk infants, a lower risk of iron deficiency anemia at age 1 year (JAMA Pediatr. 2017;171[3]:264-70).
According to current guidelines from the American Academy of Pediatrics, “delayed cord clamping longer than 30 seconds is reasonable for both term and preterm infants who do not require resuscitation at birth,” but “there is insufficient evidence to recommend an approach to cord clamping for infants who require resuscitation.” They also recommend against routine milking for newborns less than 29 weeks’ gestation (Pediatrics. 2015 Nov;136 Suppl 2:S196-218).
Meconium-related complications
Meconium-stained amniotic fluid (MSAF) is common, occurring in about 8% of deliveries and increasing with gestational age, but meconium aspiration syndrome (MAS) is less common, occurring in about 2% of all MSAF cases (Int J Pediatr. 2012. doi: 10.1155/2012/321545).
Risk factors for severe MAS include thick meconium and an abnormal fetal heart rate. But about two-thirds of MAS cases are mild, not requiring ventilation or continuous positive airway pressure (CPAP), Dr. Weiner said. Practice should be driven by evidence from randomized controlled trials (RCTs).
“Nonrandomized observational studies can be misleading, and rational conjecture has led to many mistakes in medicine,” he said. “Be willing to challenge conventional wisdom.”
For example, the standard of care in the 1970s, based on two nonrandomized retrospective reviews of 175 babies, included orapharyngeal and nasopharyngeal suction by the obstetrician and endotracheal tube (ETT) suction by the pediatrician. In the 2000s, however, an RCT of 2,500 infants found no benefit from orapharyngeal and nasopharyngeal suction, even with thick MSAF, (Lancet. 2004 Aug 14-20;364[9434]:597-602) and another RCT with 2,100 infants found no benefit from ETT suction (Pediatrics. 2000 Jan;105[1 Pt 1]:1-7).
More recent, smaller studies have confirmed those conclusions and found similar lack of benefit from ETT in non-vigorous infants, contributing to the new recommendation (Resuscitation. 2016 Aug;105:79-84; Indian J Pediatr. 2016 Oct;83[10]:1125-30).
“Routine tracheal suction is no longer recommended for nonvigorous babies with meconium stained fluid,” Dr. Weiner said. Since MSAF is risk factor for resuscitation, though, at least two clinicians with Neonatal Resuscitation Program (NRP) training should be present, as well as a full team if resuscitation is expected.
Heart rate assessment and tracking
“The baby’s heart rate needs to be monitored during PPV [positive pressure ventilation] because a prompt increase in the baby’s heart rate is the most important indicator of effective PPV,” Dr. Weiner said in an interview. “Half of errors made during NRP [Neonatal Resuscitation Program] simulations are the result of incorrect heart rate assessment.”
Recent evidence comparing pulse oximetry to an EC monitor favored the latter for tracking heart rate, leading to the other new recommendation.
“The baby’s heart rate can be monitored using the pulse oximeter,” Dr. Weiner said. “However, health providers should consider using an electronic cardiac monitor in addition to pulse oximetry because studies show that it achieves a reliable signal faster.” He cited a study of 20 newborns that showed an EC monitor determined the heart rate in a median 34 seconds, compared with 122 seconds with the pulse oximeter (Pediatr Int. 2012 Apr;54[2]:205-7).
Pulse oximetry takes 90-120 seconds to attain a reliable signal and may not work if there’s poor perfusion, but an EC monitor provides continuous heart rate monitoring even with poor perfusion. So an initial heart rate assessment by auscultation is fine, but if PPV begins, EC monitoring may be better and is the preferred method with anticipated resuscitation or chest compressions.
However, pulse oximetry is still recommended “whenever positive pressure ventilation is started or oxygen is administered in order to guide the appropriate amount of oxygen supplementation,” Dr. Weiner noted.
He added that “preliminary studies suggest that handheld Doppler fetal heart monitors correlate well with ECG, provide a rapid audible heart rate and may be a promising alternative in the future” (Pediatr Int. 2017 Oct;59[10]:1069-73).
Correct ventilation techniques
“Ventilation of the lungs is the single most important and most effective step in cardiopulmonary resuscitation of the compromised newborn,” Dr. Weiner said. “If the heart rate is not rapidly increasing, ask if the chest is moving.”
He emphasized that no compressions should occur until after at least 30 seconds of PPV that moves the chest. He provided a “MR. SOPA” acronym: Mask adjustment, Reposition airway, Suction, Open mouth, Pressure increase, Alternative airway.
You also should be aware of possible leaking or obstruction around the mask, which is common, he said, so monitor pressure instead of volume.
“We are not good at identifying leak, obstruction, or adequate tidal volume,” Dr. Weiner said. “A colorimetric CO2 detector attached to the mask is a simple indicator of gas exchange” (Resuscitation. 2014 Nov;85[11]:1568-72).
He also strongly recommended inserting an alternative airway before starting chest compressions with either intubation or a laryngeal mask.
Dr. Weiner concluded with the following list of clinical practice changes you may consider:
- Use a standardized equipment checklist.
- Develop and practice standardized scripts.
- Debrief after all resuscitations; use videotape if you can.
- Delay cord clamping for most term and preterm babies.
- Do not routinely intubate/suction nonvigorous newborns with MSAF. Initiate resuscitation.
- Use an electronic cardiac monitor if resuscitation is required.
- Use a colorimetric CO2 detector with PPV.
- Intubate or place a laryngeal mask before starting compressions.
Dr. Weiner reported having no disclosures, and no external funding was used for the presentation.
CHICAGO – , according to Gary M. Weiner, MD, of the department of pediatrics and neonatal-perinatal medicine at the University of Michigan and C.S. Mott Children’s Hospital in Ann Arbor.
One is recommending an electronic cardiac (EC) monitor to assess heart rate during resuscitation instead of relying on pulse oximetry, and the other is no longer recommending routine tracheal suction in nonvigorous babies with meconium-stained fluid, he told attendees at the American Academy of Pediatrics annual meeting.
About two-thirds of all births have a risk factor for needing resuscitation, and about 10%-20% of babies with a risk factor will need positive pressure ventilation (PPV). But risk factors do not identify all newborns who will need it. The risk is greatest for newborns less than 36 weeks’ or greater than 40 weeks’ gestational age, but 7% of term newborns will need PPV despite having no risk factors.
Situations in which there is the highest risk for advanced resuscitation include the following:
- Fetal bradycardia: 24-fold greater odds.
- Intrauterine growth restriction (IUGR): 20-fold greater odds.
- Clinical chorioamnionitis: 17-fold greater odds.
- Forceps or vacuum: 17-fold greater odds.
- Meconium-stained amniotic fluid (MSAF): 17-fold greater odds.
- Gestational diabetes: 16-fold greater odds.
- Abruption: 12-fold greater odds.
- General anesthesia: 11-fold greater odds.
These risks were determined in a prospective multicenter, case-control study of 61,593 births (Arch Dis Child Fetal Neonatal Ed. 2017 Jan;102[1]:F44-F50).
Assembling a team and using checklists
Teamwork and communication are key in delivery room emergencies, and teams should debrief afterward, ideally having videotaped the resuscitation, if possible, Dr. Weiner said.
He discussed preparation for a very-low-birth-weight birth, a “routine emergency” requiring many tasks in a short period of time: 130 tasks in the first hour and 40 in the first 3 minutes.
“Decisions made during the first hour have long-term implications, so you need multiple caregivers and a high-performance team,” Dr. Weiner said. In addition to a thorough understanding of the clinical situation, a high-performance team should have both effective leadership, and clearly defined roles and responsibilities for each member. Clinicians on the team need highly developed technical skills that they reliably and consistently execute with precision. “Practice, refine, practice, refine,” he emphasized.
It’s also important to make use of preset protocols, scripts, and checklists, Dr. Weiner said. These tools assure consistency, facilitate communication among team members, and improve outcomes. Research has shown that use of protocols, scripts, and checklists leads to improved stroke and trauma care, decreased complications during intubation, fewer central-line complications, and decreased perioperative mortality and complications.
He also recommended implementing a standardized equipment check and team briefing “time-out,” similar to a surgical time-out. This time-out gives teams an opportunity to identify a team leader, define member roles and responsibilities, check all equipment and supplies, discuss risk factors and possible scenarios, talk with the obstetrician and, if possible, introduce the leader or another team member to the parents.
In a study from University of California, San Diego, Medical Center, using checklists as part of resuscitation of potentially high-risk infants reduced the occurrence of communication problems from 24% to 4% of resuscitations (P less than 0.001) over a 3-year period (Resuscitation. 2013 Nov;84[11]:1552-7).
Delayed cord clamping
Dr. Weiner also discussed the benefits of placental transfusion. The fetal-placental unit includes approximately 110 mL/kg of blood, and about one-third of its volume remains in the placenta immediately after birth. Immediate cord clamping means a loss of 10-20 mL/kg of “potential” newborn blood volume, and could contribute to unstable pulmonary blood flow or a carotid artery pressure spike (Matern Health Neonatol Perinatol. 2016. doi: 10.1186/s40748-016-0032-y).
“Umbilical blood flow is complex,” he said. Blood flows toward the baby via the umbilical vein during inhalation, but stops or reverses during crying. The umbilical artery primarily carries blood to the placenta, and flow stops after about 4 minutes in more than half of infants. Gravity’s role in blood flow is controversial (Lancet. 2014 Jul 19;384[9939]:235-40).
The two options for placental transfusion are delayed cord clamping and milking the umbilical cord (also called “stripping”). In vaginal births, delayed clamping allows 20 mL/kg blood to transfer to the baby by 3 minutes after birth, with 90% of that reaching the baby in the first minute (Lancet. 1969 Oct 25;294[7626]:871-3).
Blood transfer is less efficient in cesarean births, so milking may be more efficient than simply delaying clamping, according to a small randomized controlled trial of preterm infants around 28 weeks’ gestational age. No difference between the methods was seen in vaginal births. To milk the cord, pinch it near the placenta and squeeze it toward the newborn for 2 seconds; then release, refill and repeat.
The biggest benefits in delayed cord clamping or milking occur among preterm infants: decreased mortality, higher mean arterial pressure on day 1, and a lower risk of blood transfusion, necrotizing enterocolitis, and a Bayley Motor score below 85 at 18-22 months. Term babies also get benefits, though: increased hemoglobin at birth (approximately 2 g/dL), a 0.5- to 5-point average increase in boys’ Ages & Stages fine motor and social domain scores at age 4 years, and among high-risk infants, a lower risk of iron deficiency anemia at age 1 year (JAMA Pediatr. 2017;171[3]:264-70).
According to current guidelines from the American Academy of Pediatrics, “delayed cord clamping longer than 30 seconds is reasonable for both term and preterm infants who do not require resuscitation at birth,” but “there is insufficient evidence to recommend an approach to cord clamping for infants who require resuscitation.” They also recommend against routine milking for newborns less than 29 weeks’ gestation (Pediatrics. 2015 Nov;136 Suppl 2:S196-218).
Meconium-related complications
Meconium-stained amniotic fluid (MSAF) is common, occurring in about 8% of deliveries and increasing with gestational age, but meconium aspiration syndrome (MAS) is less common, occurring in about 2% of all MSAF cases (Int J Pediatr. 2012. doi: 10.1155/2012/321545).
Risk factors for severe MAS include thick meconium and an abnormal fetal heart rate. But about two-thirds of MAS cases are mild, not requiring ventilation or continuous positive airway pressure (CPAP), Dr. Weiner said. Practice should be driven by evidence from randomized controlled trials (RCTs).
“Nonrandomized observational studies can be misleading, and rational conjecture has led to many mistakes in medicine,” he said. “Be willing to challenge conventional wisdom.”
For example, the standard of care in the 1970s, based on two nonrandomized retrospective reviews of 175 babies, included orapharyngeal and nasopharyngeal suction by the obstetrician and endotracheal tube (ETT) suction by the pediatrician. In the 2000s, however, an RCT of 2,500 infants found no benefit from orapharyngeal and nasopharyngeal suction, even with thick MSAF, (Lancet. 2004 Aug 14-20;364[9434]:597-602) and another RCT with 2,100 infants found no benefit from ETT suction (Pediatrics. 2000 Jan;105[1 Pt 1]:1-7).
More recent, smaller studies have confirmed those conclusions and found similar lack of benefit from ETT in non-vigorous infants, contributing to the new recommendation (Resuscitation. 2016 Aug;105:79-84; Indian J Pediatr. 2016 Oct;83[10]:1125-30).
“Routine tracheal suction is no longer recommended for nonvigorous babies with meconium stained fluid,” Dr. Weiner said. Since MSAF is risk factor for resuscitation, though, at least two clinicians with Neonatal Resuscitation Program (NRP) training should be present, as well as a full team if resuscitation is expected.
Heart rate assessment and tracking
“The baby’s heart rate needs to be monitored during PPV [positive pressure ventilation] because a prompt increase in the baby’s heart rate is the most important indicator of effective PPV,” Dr. Weiner said in an interview. “Half of errors made during NRP [Neonatal Resuscitation Program] simulations are the result of incorrect heart rate assessment.”
Recent evidence comparing pulse oximetry to an EC monitor favored the latter for tracking heart rate, leading to the other new recommendation.
“The baby’s heart rate can be monitored using the pulse oximeter,” Dr. Weiner said. “However, health providers should consider using an electronic cardiac monitor in addition to pulse oximetry because studies show that it achieves a reliable signal faster.” He cited a study of 20 newborns that showed an EC monitor determined the heart rate in a median 34 seconds, compared with 122 seconds with the pulse oximeter (Pediatr Int. 2012 Apr;54[2]:205-7).
Pulse oximetry takes 90-120 seconds to attain a reliable signal and may not work if there’s poor perfusion, but an EC monitor provides continuous heart rate monitoring even with poor perfusion. So an initial heart rate assessment by auscultation is fine, but if PPV begins, EC monitoring may be better and is the preferred method with anticipated resuscitation or chest compressions.
However, pulse oximetry is still recommended “whenever positive pressure ventilation is started or oxygen is administered in order to guide the appropriate amount of oxygen supplementation,” Dr. Weiner noted.
He added that “preliminary studies suggest that handheld Doppler fetal heart monitors correlate well with ECG, provide a rapid audible heart rate and may be a promising alternative in the future” (Pediatr Int. 2017 Oct;59[10]:1069-73).
Correct ventilation techniques
“Ventilation of the lungs is the single most important and most effective step in cardiopulmonary resuscitation of the compromised newborn,” Dr. Weiner said. “If the heart rate is not rapidly increasing, ask if the chest is moving.”
He emphasized that no compressions should occur until after at least 30 seconds of PPV that moves the chest. He provided a “MR. SOPA” acronym: Mask adjustment, Reposition airway, Suction, Open mouth, Pressure increase, Alternative airway.
You also should be aware of possible leaking or obstruction around the mask, which is common, he said, so monitor pressure instead of volume.
“We are not good at identifying leak, obstruction, or adequate tidal volume,” Dr. Weiner said. “A colorimetric CO2 detector attached to the mask is a simple indicator of gas exchange” (Resuscitation. 2014 Nov;85[11]:1568-72).
He also strongly recommended inserting an alternative airway before starting chest compressions with either intubation or a laryngeal mask.
Dr. Weiner concluded with the following list of clinical practice changes you may consider:
- Use a standardized equipment checklist.
- Develop and practice standardized scripts.
- Debrief after all resuscitations; use videotape if you can.
- Delay cord clamping for most term and preterm babies.
- Do not routinely intubate/suction nonvigorous newborns with MSAF. Initiate resuscitation.
- Use an electronic cardiac monitor if resuscitation is required.
- Use a colorimetric CO2 detector with PPV.
- Intubate or place a laryngeal mask before starting compressions.
Dr. Weiner reported having no disclosures, and no external funding was used for the presentation.
CHICAGO – , according to Gary M. Weiner, MD, of the department of pediatrics and neonatal-perinatal medicine at the University of Michigan and C.S. Mott Children’s Hospital in Ann Arbor.
One is recommending an electronic cardiac (EC) monitor to assess heart rate during resuscitation instead of relying on pulse oximetry, and the other is no longer recommending routine tracheal suction in nonvigorous babies with meconium-stained fluid, he told attendees at the American Academy of Pediatrics annual meeting.
About two-thirds of all births have a risk factor for needing resuscitation, and about 10%-20% of babies with a risk factor will need positive pressure ventilation (PPV). But risk factors do not identify all newborns who will need it. The risk is greatest for newborns less than 36 weeks’ or greater than 40 weeks’ gestational age, but 7% of term newborns will need PPV despite having no risk factors.
Situations in which there is the highest risk for advanced resuscitation include the following:
- Fetal bradycardia: 24-fold greater odds.
- Intrauterine growth restriction (IUGR): 20-fold greater odds.
- Clinical chorioamnionitis: 17-fold greater odds.
- Forceps or vacuum: 17-fold greater odds.
- Meconium-stained amniotic fluid (MSAF): 17-fold greater odds.
- Gestational diabetes: 16-fold greater odds.
- Abruption: 12-fold greater odds.
- General anesthesia: 11-fold greater odds.
These risks were determined in a prospective multicenter, case-control study of 61,593 births (Arch Dis Child Fetal Neonatal Ed. 2017 Jan;102[1]:F44-F50).
Assembling a team and using checklists
Teamwork and communication are key in delivery room emergencies, and teams should debrief afterward, ideally having videotaped the resuscitation, if possible, Dr. Weiner said.
He discussed preparation for a very-low-birth-weight birth, a “routine emergency” requiring many tasks in a short period of time: 130 tasks in the first hour and 40 in the first 3 minutes.
“Decisions made during the first hour have long-term implications, so you need multiple caregivers and a high-performance team,” Dr. Weiner said. In addition to a thorough understanding of the clinical situation, a high-performance team should have both effective leadership, and clearly defined roles and responsibilities for each member. Clinicians on the team need highly developed technical skills that they reliably and consistently execute with precision. “Practice, refine, practice, refine,” he emphasized.
It’s also important to make use of preset protocols, scripts, and checklists, Dr. Weiner said. These tools assure consistency, facilitate communication among team members, and improve outcomes. Research has shown that use of protocols, scripts, and checklists leads to improved stroke and trauma care, decreased complications during intubation, fewer central-line complications, and decreased perioperative mortality and complications.
He also recommended implementing a standardized equipment check and team briefing “time-out,” similar to a surgical time-out. This time-out gives teams an opportunity to identify a team leader, define member roles and responsibilities, check all equipment and supplies, discuss risk factors and possible scenarios, talk with the obstetrician and, if possible, introduce the leader or another team member to the parents.
In a study from University of California, San Diego, Medical Center, using checklists as part of resuscitation of potentially high-risk infants reduced the occurrence of communication problems from 24% to 4% of resuscitations (P less than 0.001) over a 3-year period (Resuscitation. 2013 Nov;84[11]:1552-7).
Delayed cord clamping
Dr. Weiner also discussed the benefits of placental transfusion. The fetal-placental unit includes approximately 110 mL/kg of blood, and about one-third of its volume remains in the placenta immediately after birth. Immediate cord clamping means a loss of 10-20 mL/kg of “potential” newborn blood volume, and could contribute to unstable pulmonary blood flow or a carotid artery pressure spike (Matern Health Neonatol Perinatol. 2016. doi: 10.1186/s40748-016-0032-y).
“Umbilical blood flow is complex,” he said. Blood flows toward the baby via the umbilical vein during inhalation, but stops or reverses during crying. The umbilical artery primarily carries blood to the placenta, and flow stops after about 4 minutes in more than half of infants. Gravity’s role in blood flow is controversial (Lancet. 2014 Jul 19;384[9939]:235-40).
The two options for placental transfusion are delayed cord clamping and milking the umbilical cord (also called “stripping”). In vaginal births, delayed clamping allows 20 mL/kg blood to transfer to the baby by 3 minutes after birth, with 90% of that reaching the baby in the first minute (Lancet. 1969 Oct 25;294[7626]:871-3).
Blood transfer is less efficient in cesarean births, so milking may be more efficient than simply delaying clamping, according to a small randomized controlled trial of preterm infants around 28 weeks’ gestational age. No difference between the methods was seen in vaginal births. To milk the cord, pinch it near the placenta and squeeze it toward the newborn for 2 seconds; then release, refill and repeat.
The biggest benefits in delayed cord clamping or milking occur among preterm infants: decreased mortality, higher mean arterial pressure on day 1, and a lower risk of blood transfusion, necrotizing enterocolitis, and a Bayley Motor score below 85 at 18-22 months. Term babies also get benefits, though: increased hemoglobin at birth (approximately 2 g/dL), a 0.5- to 5-point average increase in boys’ Ages & Stages fine motor and social domain scores at age 4 years, and among high-risk infants, a lower risk of iron deficiency anemia at age 1 year (JAMA Pediatr. 2017;171[3]:264-70).
According to current guidelines from the American Academy of Pediatrics, “delayed cord clamping longer than 30 seconds is reasonable for both term and preterm infants who do not require resuscitation at birth,” but “there is insufficient evidence to recommend an approach to cord clamping for infants who require resuscitation.” They also recommend against routine milking for newborns less than 29 weeks’ gestation (Pediatrics. 2015 Nov;136 Suppl 2:S196-218).
Meconium-related complications
Meconium-stained amniotic fluid (MSAF) is common, occurring in about 8% of deliveries and increasing with gestational age, but meconium aspiration syndrome (MAS) is less common, occurring in about 2% of all MSAF cases (Int J Pediatr. 2012. doi: 10.1155/2012/321545).
Risk factors for severe MAS include thick meconium and an abnormal fetal heart rate. But about two-thirds of MAS cases are mild, not requiring ventilation or continuous positive airway pressure (CPAP), Dr. Weiner said. Practice should be driven by evidence from randomized controlled trials (RCTs).
“Nonrandomized observational studies can be misleading, and rational conjecture has led to many mistakes in medicine,” he said. “Be willing to challenge conventional wisdom.”
For example, the standard of care in the 1970s, based on two nonrandomized retrospective reviews of 175 babies, included orapharyngeal and nasopharyngeal suction by the obstetrician and endotracheal tube (ETT) suction by the pediatrician. In the 2000s, however, an RCT of 2,500 infants found no benefit from orapharyngeal and nasopharyngeal suction, even with thick MSAF, (Lancet. 2004 Aug 14-20;364[9434]:597-602) and another RCT with 2,100 infants found no benefit from ETT suction (Pediatrics. 2000 Jan;105[1 Pt 1]:1-7).
More recent, smaller studies have confirmed those conclusions and found similar lack of benefit from ETT in non-vigorous infants, contributing to the new recommendation (Resuscitation. 2016 Aug;105:79-84; Indian J Pediatr. 2016 Oct;83[10]:1125-30).
“Routine tracheal suction is no longer recommended for nonvigorous babies with meconium stained fluid,” Dr. Weiner said. Since MSAF is risk factor for resuscitation, though, at least two clinicians with Neonatal Resuscitation Program (NRP) training should be present, as well as a full team if resuscitation is expected.
Heart rate assessment and tracking
“The baby’s heart rate needs to be monitored during PPV [positive pressure ventilation] because a prompt increase in the baby’s heart rate is the most important indicator of effective PPV,” Dr. Weiner said in an interview. “Half of errors made during NRP [Neonatal Resuscitation Program] simulations are the result of incorrect heart rate assessment.”
Recent evidence comparing pulse oximetry to an EC monitor favored the latter for tracking heart rate, leading to the other new recommendation.
“The baby’s heart rate can be monitored using the pulse oximeter,” Dr. Weiner said. “However, health providers should consider using an electronic cardiac monitor in addition to pulse oximetry because studies show that it achieves a reliable signal faster.” He cited a study of 20 newborns that showed an EC monitor determined the heart rate in a median 34 seconds, compared with 122 seconds with the pulse oximeter (Pediatr Int. 2012 Apr;54[2]:205-7).
Pulse oximetry takes 90-120 seconds to attain a reliable signal and may not work if there’s poor perfusion, but an EC monitor provides continuous heart rate monitoring even with poor perfusion. So an initial heart rate assessment by auscultation is fine, but if PPV begins, EC monitoring may be better and is the preferred method with anticipated resuscitation or chest compressions.
However, pulse oximetry is still recommended “whenever positive pressure ventilation is started or oxygen is administered in order to guide the appropriate amount of oxygen supplementation,” Dr. Weiner noted.
He added that “preliminary studies suggest that handheld Doppler fetal heart monitors correlate well with ECG, provide a rapid audible heart rate and may be a promising alternative in the future” (Pediatr Int. 2017 Oct;59[10]:1069-73).
Correct ventilation techniques
“Ventilation of the lungs is the single most important and most effective step in cardiopulmonary resuscitation of the compromised newborn,” Dr. Weiner said. “If the heart rate is not rapidly increasing, ask if the chest is moving.”
He emphasized that no compressions should occur until after at least 30 seconds of PPV that moves the chest. He provided a “MR. SOPA” acronym: Mask adjustment, Reposition airway, Suction, Open mouth, Pressure increase, Alternative airway.
You also should be aware of possible leaking or obstruction around the mask, which is common, he said, so monitor pressure instead of volume.
“We are not good at identifying leak, obstruction, or adequate tidal volume,” Dr. Weiner said. “A colorimetric CO2 detector attached to the mask is a simple indicator of gas exchange” (Resuscitation. 2014 Nov;85[11]:1568-72).
He also strongly recommended inserting an alternative airway before starting chest compressions with either intubation or a laryngeal mask.
Dr. Weiner concluded with the following list of clinical practice changes you may consider:
- Use a standardized equipment checklist.
- Develop and practice standardized scripts.
- Debrief after all resuscitations; use videotape if you can.
- Delay cord clamping for most term and preterm babies.
- Do not routinely intubate/suction nonvigorous newborns with MSAF. Initiate resuscitation.
- Use an electronic cardiac monitor if resuscitation is required.
- Use a colorimetric CO2 detector with PPV.
- Intubate or place a laryngeal mask before starting compressions.
Dr. Weiner reported having no disclosures, and no external funding was used for the presentation.
EXPERT ANALYSIS FROM AAP 2017
Antibiotics after incision and drainage for simple abscesses improves outcomes
Clinical question: Does antibiotic administration after incision and drainage of simple abscesses improve cure rates?
Background: Abscesses are the most common skin and soft-tissue infection, and most patients seek outpatient treatment. Limited data have shown that antibiotic treatment directed at Staphylococcus aureus after incision and drainage is effective, though the efficacy of adjunctive antibiotic therapy versus incision and drainage alone is unclear.
Setting: Multicenter outpatient facilities across the United States.
Synopsis: After successful incision and drainage of a simple abscess, 786 outpatients (505 adults, 281 children) were randomized to receive either clindamycin, trimethoprim-sulfamethoxazole (TMP-SMX), or placebo for 10 days. The rate of clinical cure was 83.1% in the clindamycin group, 81.7% in the TMP-SMX group, and 68.9% in the placebo group. The cure rate was significantly lower in the placebo group, compared with either of the antibiotic groups. The difference in cure rate was not significant between the clindamycin and TMP-SMX groups. Cure rates in culture-positive S. aureus patients were significantly higher in both antibiotic groups, compared with placebo. Rates of adverse events were higher in the clindamycin group (21.9%) than the TMP-SMX group (11.1%) or the placebo group (12.5%), though all adverse events resolved without sequelae.
Bottom line: Antibiotic therapy after incision and drainage for simple abscesses is associated with improved cure rate and decreased recurrence.
Citation: Daum RS, Miller LG, Immergluck L, et al. A placebo-controlled trial of antibiotics for smaller skin abscesses. N Engl J Med. 2017 Jun 29;376(26):2545-55.
Dr. Chung is hospitalist and assistant professor of medicine, Icahn School of Medicine of the Mount Sinai Health System.
Clinical question: Does antibiotic administration after incision and drainage of simple abscesses improve cure rates?
Background: Abscesses are the most common skin and soft-tissue infection, and most patients seek outpatient treatment. Limited data have shown that antibiotic treatment directed at Staphylococcus aureus after incision and drainage is effective, though the efficacy of adjunctive antibiotic therapy versus incision and drainage alone is unclear.
Setting: Multicenter outpatient facilities across the United States.
Synopsis: After successful incision and drainage of a simple abscess, 786 outpatients (505 adults, 281 children) were randomized to receive either clindamycin, trimethoprim-sulfamethoxazole (TMP-SMX), or placebo for 10 days. The rate of clinical cure was 83.1% in the clindamycin group, 81.7% in the TMP-SMX group, and 68.9% in the placebo group. The cure rate was significantly lower in the placebo group, compared with either of the antibiotic groups. The difference in cure rate was not significant between the clindamycin and TMP-SMX groups. Cure rates in culture-positive S. aureus patients were significantly higher in both antibiotic groups, compared with placebo. Rates of adverse events were higher in the clindamycin group (21.9%) than the TMP-SMX group (11.1%) or the placebo group (12.5%), though all adverse events resolved without sequelae.
Bottom line: Antibiotic therapy after incision and drainage for simple abscesses is associated with improved cure rate and decreased recurrence.
Citation: Daum RS, Miller LG, Immergluck L, et al. A placebo-controlled trial of antibiotics for smaller skin abscesses. N Engl J Med. 2017 Jun 29;376(26):2545-55.
Dr. Chung is hospitalist and assistant professor of medicine, Icahn School of Medicine of the Mount Sinai Health System.
Clinical question: Does antibiotic administration after incision and drainage of simple abscesses improve cure rates?
Background: Abscesses are the most common skin and soft-tissue infection, and most patients seek outpatient treatment. Limited data have shown that antibiotic treatment directed at Staphylococcus aureus after incision and drainage is effective, though the efficacy of adjunctive antibiotic therapy versus incision and drainage alone is unclear.
Setting: Multicenter outpatient facilities across the United States.
Synopsis: After successful incision and drainage of a simple abscess, 786 outpatients (505 adults, 281 children) were randomized to receive either clindamycin, trimethoprim-sulfamethoxazole (TMP-SMX), or placebo for 10 days. The rate of clinical cure was 83.1% in the clindamycin group, 81.7% in the TMP-SMX group, and 68.9% in the placebo group. The cure rate was significantly lower in the placebo group, compared with either of the antibiotic groups. The difference in cure rate was not significant between the clindamycin and TMP-SMX groups. Cure rates in culture-positive S. aureus patients were significantly higher in both antibiotic groups, compared with placebo. Rates of adverse events were higher in the clindamycin group (21.9%) than the TMP-SMX group (11.1%) or the placebo group (12.5%), though all adverse events resolved without sequelae.
Bottom line: Antibiotic therapy after incision and drainage for simple abscesses is associated with improved cure rate and decreased recurrence.
Citation: Daum RS, Miller LG, Immergluck L, et al. A placebo-controlled trial of antibiotics for smaller skin abscesses. N Engl J Med. 2017 Jun 29;376(26):2545-55.
Dr. Chung is hospitalist and assistant professor of medicine, Icahn School of Medicine of the Mount Sinai Health System.
Prescribers mostly ignore clopidogrel pharmacogenomic profiling
ANAHEIM, CALIF. – The boxed warning recommending pharmacogenomic testing of patients receiving clopidogrel to identify reduced metabolizers seems to be playing to a largely deaf audience.
Even when handed information on whether each clopidogrel-treated patient was a poor metabolizer of the drug, treating physicians usually did not switch them to a different antiplatelet drug, ticagrelor, that would be fully effective despite the patient’s reduced-metabolizer status. And clinicians who started patients on ticagrelor did not usually switch those with a good clopidogrel-metabolizing profile to the safer drug, clopidogrel, after learning that clopidogrel would be fully effective.
“Routine reporting of pharmacogenomics test results for acute coronary syndrome patients treated with P2Y12-inhibitor therapy had an uncertain yield and little impact on P2Y12-inhibitor switching,” E. Magnus Ohman, MBBS, said at the American Heart Association scientific sessions.
The study’s design gave each participating clinician free rein on whether to prescribe clopidogrel or ticagrelor (Brilinta) initially, and switching between the drugs was possible at any time after the initial prescription. At the trial’s start, 1,704 patients (56%) were on ticagrelor and 1,333 (44%) were on clopidogrel.
Pharmacogenomic testing showed that 34% of all patients were ultrametabolizers and 38% were extensive metabolizers. Patients in either of these categories metabolize enough clopidogrel into the active form to get full benefit from the drug and derive no additional efficacy benefit from switching to another P2Y12 inhibitor, such as ticagrelor or prasugrel (Effient) – drugs unaffected by metabolizer status. Testing also identified 25% of patients as intermediate metabolizers, who carry one loss-of-function allele for the CYP2C19 gene, and 3% were reduced metabolizers, who are homozygous for loss-of-function alleles. Standard practice is not to treat intermediate or reduced metabolizers with clopidogrel because they would not get an adequate antiplatelet effect; instead, these patients are usually treated with ticagrelor or with prasugrel when it’s an option.
After receiving the results regarding the clopidogrel-metabolizing status for each patient, attending physicians switched the drugs prescribed for only 7% of all patients: 9% of patients initially on ticagrelor and 4% of those initially on clopidogrel, reported Dr. Ohman, professor of medicine at Duke University in Durham, N.C. In addition, Dr. Ohman and his associates asked each participating physician who made a switch about his or her reasons for doing so. Of the patients who switched from clopidogrel to ticagrelor, only 23 were switched because of their pharmacogenomic results; this represents fewer than half of those who switched and only 2% of all patients who took clopidogrel. Only one patient changed from ticagrelor to clopidogrel based on pharmacogenomic results, representing 0.06% of all patients on ticagrelor.
“We believed the findings do not support the utility of mandatory testing in this context, as most did not act on the information,” Dr. Ohman said.
A major reason for the inertia, Dr. Gurbel suggested, may be the absence of any compelling data proving whether there’s any effect on clinical outcomes for switching reduced metabolizers off of clopidogrel or switching good metabolizers onto it.
“We have no large-scale, prospective data supporting pharmacogenomic-based personalization” of clopidogrel treatment leading to improved outcomes, but “we need to get over that,” he said. “It’s a challenge to get funding for this.” But “the answer is not to give ticagrelor or prasugrel to everyone because then the bleeding rate is too high.”
The findings Dr. Ohman reported came from the Study to Compare the Safety of Rivaroxaban Versus Acetylsalicylic Acid in Addition to Either Clopidogrel or Ticagrelor Therapy in Participants With Acute Coronary Syndrome (GEMINI-ACS-1), which had the primary goal of comparing the safety in acute coronary syndrome patients of a reduced dosage of rivaroxaban plus either clopidogrel or ticagrelor with the safety of aspirin plus one of these P2Y12 inhibitors. The primary endpoint was the rate of clinically significant bleeding events during a year of treatment. The study ran at 371 centers in 21 countries and showed similar bleeding rates in both treatment arms (Lancet. 2017 May 6; 389[10081]:1799-808).
The analysis also showed that patients identified as reduced metabolizers were fivefold more likely to be switched than patients identified as ultra metabolizers, and intermediate metabolizes had a 50% higher switching rate than ultra metabolizers. The rates of both ischemic and major bleeding outcomes were roughly similar across the spectrum of metabolizers, but Dr. Ohman cautioned that the trial was not designed to assess this. Dr. Gurbel urged the investigators to report on outcomes analyzed not just by metabolizer status but also by the treatment they received.
The boxed warning that clopidogrel received in 2010 regarding poor metabolizers led to “regulatory guidance” during design of the GEMINI-ACS-1 trial requiring routine pharmacogenomic testing for clopidogrel-metabolizing status, Dr. Ohman explained.
The trial was funded by Janssen and Bayer, the two companies that jointly market rivaroxaban (Xarelto). Dr. Ohman has been a consultant to Bayer and several other companies including AstraZeneca, the company that markets ticagrelor (Brilinta). He has also received research funding from Janssen, as well as Daiichi Sankyo and Gilead Sciences. Dr. Gurbel holds patents on platelet-function testing methods.
[email protected]
On Twitter @mitchelzoler
ANAHEIM, CALIF. – The boxed warning recommending pharmacogenomic testing of patients receiving clopidogrel to identify reduced metabolizers seems to be playing to a largely deaf audience.
Even when handed information on whether each clopidogrel-treated patient was a poor metabolizer of the drug, treating physicians usually did not switch them to a different antiplatelet drug, ticagrelor, that would be fully effective despite the patient’s reduced-metabolizer status. And clinicians who started patients on ticagrelor did not usually switch those with a good clopidogrel-metabolizing profile to the safer drug, clopidogrel, after learning that clopidogrel would be fully effective.
“Routine reporting of pharmacogenomics test results for acute coronary syndrome patients treated with P2Y12-inhibitor therapy had an uncertain yield and little impact on P2Y12-inhibitor switching,” E. Magnus Ohman, MBBS, said at the American Heart Association scientific sessions.
The study’s design gave each participating clinician free rein on whether to prescribe clopidogrel or ticagrelor (Brilinta) initially, and switching between the drugs was possible at any time after the initial prescription. At the trial’s start, 1,704 patients (56%) were on ticagrelor and 1,333 (44%) were on clopidogrel.
Pharmacogenomic testing showed that 34% of all patients were ultrametabolizers and 38% were extensive metabolizers. Patients in either of these categories metabolize enough clopidogrel into the active form to get full benefit from the drug and derive no additional efficacy benefit from switching to another P2Y12 inhibitor, such as ticagrelor or prasugrel (Effient) – drugs unaffected by metabolizer status. Testing also identified 25% of patients as intermediate metabolizers, who carry one loss-of-function allele for the CYP2C19 gene, and 3% were reduced metabolizers, who are homozygous for loss-of-function alleles. Standard practice is not to treat intermediate or reduced metabolizers with clopidogrel because they would not get an adequate antiplatelet effect; instead, these patients are usually treated with ticagrelor or with prasugrel when it’s an option.
After receiving the results regarding the clopidogrel-metabolizing status for each patient, attending physicians switched the drugs prescribed for only 7% of all patients: 9% of patients initially on ticagrelor and 4% of those initially on clopidogrel, reported Dr. Ohman, professor of medicine at Duke University in Durham, N.C. In addition, Dr. Ohman and his associates asked each participating physician who made a switch about his or her reasons for doing so. Of the patients who switched from clopidogrel to ticagrelor, only 23 were switched because of their pharmacogenomic results; this represents fewer than half of those who switched and only 2% of all patients who took clopidogrel. Only one patient changed from ticagrelor to clopidogrel based on pharmacogenomic results, representing 0.06% of all patients on ticagrelor.
“We believed the findings do not support the utility of mandatory testing in this context, as most did not act on the information,” Dr. Ohman said.
A major reason for the inertia, Dr. Gurbel suggested, may be the absence of any compelling data proving whether there’s any effect on clinical outcomes for switching reduced metabolizers off of clopidogrel or switching good metabolizers onto it.
“We have no large-scale, prospective data supporting pharmacogenomic-based personalization” of clopidogrel treatment leading to improved outcomes, but “we need to get over that,” he said. “It’s a challenge to get funding for this.” But “the answer is not to give ticagrelor or prasugrel to everyone because then the bleeding rate is too high.”
The findings Dr. Ohman reported came from the Study to Compare the Safety of Rivaroxaban Versus Acetylsalicylic Acid in Addition to Either Clopidogrel or Ticagrelor Therapy in Participants With Acute Coronary Syndrome (GEMINI-ACS-1), which had the primary goal of comparing the safety in acute coronary syndrome patients of a reduced dosage of rivaroxaban plus either clopidogrel or ticagrelor with the safety of aspirin plus one of these P2Y12 inhibitors. The primary endpoint was the rate of clinically significant bleeding events during a year of treatment. The study ran at 371 centers in 21 countries and showed similar bleeding rates in both treatment arms (Lancet. 2017 May 6; 389[10081]:1799-808).
The analysis also showed that patients identified as reduced metabolizers were fivefold more likely to be switched than patients identified as ultra metabolizers, and intermediate metabolizes had a 50% higher switching rate than ultra metabolizers. The rates of both ischemic and major bleeding outcomes were roughly similar across the spectrum of metabolizers, but Dr. Ohman cautioned that the trial was not designed to assess this. Dr. Gurbel urged the investigators to report on outcomes analyzed not just by metabolizer status but also by the treatment they received.
The boxed warning that clopidogrel received in 2010 regarding poor metabolizers led to “regulatory guidance” during design of the GEMINI-ACS-1 trial requiring routine pharmacogenomic testing for clopidogrel-metabolizing status, Dr. Ohman explained.
The trial was funded by Janssen and Bayer, the two companies that jointly market rivaroxaban (Xarelto). Dr. Ohman has been a consultant to Bayer and several other companies including AstraZeneca, the company that markets ticagrelor (Brilinta). He has also received research funding from Janssen, as well as Daiichi Sankyo and Gilead Sciences. Dr. Gurbel holds patents on platelet-function testing methods.
[email protected]
On Twitter @mitchelzoler
ANAHEIM, CALIF. – The boxed warning recommending pharmacogenomic testing of patients receiving clopidogrel to identify reduced metabolizers seems to be playing to a largely deaf audience.
Even when handed information on whether each clopidogrel-treated patient was a poor metabolizer of the drug, treating physicians usually did not switch them to a different antiplatelet drug, ticagrelor, that would be fully effective despite the patient’s reduced-metabolizer status. And clinicians who started patients on ticagrelor did not usually switch those with a good clopidogrel-metabolizing profile to the safer drug, clopidogrel, after learning that clopidogrel would be fully effective.
“Routine reporting of pharmacogenomics test results for acute coronary syndrome patients treated with P2Y12-inhibitor therapy had an uncertain yield and little impact on P2Y12-inhibitor switching,” E. Magnus Ohman, MBBS, said at the American Heart Association scientific sessions.
The study’s design gave each participating clinician free rein on whether to prescribe clopidogrel or ticagrelor (Brilinta) initially, and switching between the drugs was possible at any time after the initial prescription. At the trial’s start, 1,704 patients (56%) were on ticagrelor and 1,333 (44%) were on clopidogrel.
Pharmacogenomic testing showed that 34% of all patients were ultrametabolizers and 38% were extensive metabolizers. Patients in either of these categories metabolize enough clopidogrel into the active form to get full benefit from the drug and derive no additional efficacy benefit from switching to another P2Y12 inhibitor, such as ticagrelor or prasugrel (Effient) – drugs unaffected by metabolizer status. Testing also identified 25% of patients as intermediate metabolizers, who carry one loss-of-function allele for the CYP2C19 gene, and 3% were reduced metabolizers, who are homozygous for loss-of-function alleles. Standard practice is not to treat intermediate or reduced metabolizers with clopidogrel because they would not get an adequate antiplatelet effect; instead, these patients are usually treated with ticagrelor or with prasugrel when it’s an option.
After receiving the results regarding the clopidogrel-metabolizing status for each patient, attending physicians switched the drugs prescribed for only 7% of all patients: 9% of patients initially on ticagrelor and 4% of those initially on clopidogrel, reported Dr. Ohman, professor of medicine at Duke University in Durham, N.C. In addition, Dr. Ohman and his associates asked each participating physician who made a switch about his or her reasons for doing so. Of the patients who switched from clopidogrel to ticagrelor, only 23 were switched because of their pharmacogenomic results; this represents fewer than half of those who switched and only 2% of all patients who took clopidogrel. Only one patient changed from ticagrelor to clopidogrel based on pharmacogenomic results, representing 0.06% of all patients on ticagrelor.
“We believed the findings do not support the utility of mandatory testing in this context, as most did not act on the information,” Dr. Ohman said.
A major reason for the inertia, Dr. Gurbel suggested, may be the absence of any compelling data proving whether there’s any effect on clinical outcomes for switching reduced metabolizers off of clopidogrel or switching good metabolizers onto it.
“We have no large-scale, prospective data supporting pharmacogenomic-based personalization” of clopidogrel treatment leading to improved outcomes, but “we need to get over that,” he said. “It’s a challenge to get funding for this.” But “the answer is not to give ticagrelor or prasugrel to everyone because then the bleeding rate is too high.”
The findings Dr. Ohman reported came from the Study to Compare the Safety of Rivaroxaban Versus Acetylsalicylic Acid in Addition to Either Clopidogrel or Ticagrelor Therapy in Participants With Acute Coronary Syndrome (GEMINI-ACS-1), which had the primary goal of comparing the safety in acute coronary syndrome patients of a reduced dosage of rivaroxaban plus either clopidogrel or ticagrelor with the safety of aspirin plus one of these P2Y12 inhibitors. The primary endpoint was the rate of clinically significant bleeding events during a year of treatment. The study ran at 371 centers in 21 countries and showed similar bleeding rates in both treatment arms (Lancet. 2017 May 6; 389[10081]:1799-808).
The analysis also showed that patients identified as reduced metabolizers were fivefold more likely to be switched than patients identified as ultra metabolizers, and intermediate metabolizes had a 50% higher switching rate than ultra metabolizers. The rates of both ischemic and major bleeding outcomes were roughly similar across the spectrum of metabolizers, but Dr. Ohman cautioned that the trial was not designed to assess this. Dr. Gurbel urged the investigators to report on outcomes analyzed not just by metabolizer status but also by the treatment they received.
The boxed warning that clopidogrel received in 2010 regarding poor metabolizers led to “regulatory guidance” during design of the GEMINI-ACS-1 trial requiring routine pharmacogenomic testing for clopidogrel-metabolizing status, Dr. Ohman explained.
The trial was funded by Janssen and Bayer, the two companies that jointly market rivaroxaban (Xarelto). Dr. Ohman has been a consultant to Bayer and several other companies including AstraZeneca, the company that markets ticagrelor (Brilinta). He has also received research funding from Janssen, as well as Daiichi Sankyo and Gilead Sciences. Dr. Gurbel holds patents on platelet-function testing methods.
[email protected]
On Twitter @mitchelzoler
AT THE AHA SCIENTIFIC SESSIONS
Key clinical point:
Major finding: Physicians switched P2Y12 inhibitors for only 2% of patients on clopidogrel and only 0.06% on ticagrelor on the basis of their pharmacogenomic results.
Data source: GEMINI-ACS-1, a multicenter, prospective trial with 3,037 patients.
Disclosures: The GEMINI-ACS-1 trial was funded by Janssen and Bayer, the two companies that jointly market rivaroxaban (Xarelto). Dr. Ohman has been a consultant to Bayer and several other companies, including AstraZeneca, the company that markets ticagrelor (Brilinta). He has also received research funding from Janssen, as well as Daiichi Sankyo and Gilead Sciences. Dr. Gurbel holds patents on platelet-function testing methods.
Summary of guidelines for DMARDs for elective surgery
Clinical question: What is the best management for disease-modifying antirheumatic drugs (DMARDs) for patients with RA, ankylosing spondylitis, psoriatic arthritis, juvenile idiopathic arthritis, or systemic lupus erythematosus (SLE) undergoing elective total knee arthroplasty (TKA) or total hip arthroplasty (THA)?
Background: There are limited data in the evaluation of risks of flare with stopping DMARDs versus the risks of infection with continuing them perioperatively for elective TKA or THA, which are procedures frequently required by this patient population.
Study design: Multistep systematic literature review.
Setting: Collaboration between American College of Rheumatology and American Association of Hip and Knee Surgeons.
Synopsis: Through literature review and a requirement of 80% agreement by the panel, seven recommendations were created. Continue methotrexate, leflunomide, hydroxychloroquine, and sulfasalazine. Biologic agents should be held with surgery scheduled at the end of dosing cycle and restarted when the wound is healed, sutures/staples are removed, and there are no signs of infection (~14 days). Tofacitinib should be held for all conditions except SLE for 1 week. For severe SLE, continue mycophenolate mofetil, azathioprine, cyclosporine, or tacrolimus but hold for 1 week for nonsevere SLE. If current dose of glucocorticoids is less than 20 mg/day, the current dose should be administered rather than administering stress-dose steroids.
Limitations include a limited number of studies conducted in the perioperative period, the existing data are based on lower dosages, and it is unknown whether results can be extrapolated to surgical procedures beyond TKA and THA. Additionally there is a need for further studies on glucocorticoid management and biologic agents.
Bottom line: Perioperative management of DMARDs is complex and understudied, but the review provides an evidence-based guide for patients undergoing TKA and THA.
Citation: Goodman SM, Springer B, Gordon G, et. al. 2017 American College of Rheumatology/American Association of Hip and Knee Surgeons Guideline for the Perioperative Management of Antirheumatic Medication in Patients With Rheumatic Diseases Undergoing Elective Total Hip or Total Knee Arthroplasty. Arthritis Care Res. 2017 Aug;69(8):1111-24.
Dr. Kochar is hospitalist and assistant professor of medicine, Icahn School of Medicine of the Mount Sinai Health System.
Clinical question: What is the best management for disease-modifying antirheumatic drugs (DMARDs) for patients with RA, ankylosing spondylitis, psoriatic arthritis, juvenile idiopathic arthritis, or systemic lupus erythematosus (SLE) undergoing elective total knee arthroplasty (TKA) or total hip arthroplasty (THA)?
Background: There are limited data in the evaluation of risks of flare with stopping DMARDs versus the risks of infection with continuing them perioperatively for elective TKA or THA, which are procedures frequently required by this patient population.
Study design: Multistep systematic literature review.
Setting: Collaboration between American College of Rheumatology and American Association of Hip and Knee Surgeons.
Synopsis: Through literature review and a requirement of 80% agreement by the panel, seven recommendations were created. Continue methotrexate, leflunomide, hydroxychloroquine, and sulfasalazine. Biologic agents should be held with surgery scheduled at the end of dosing cycle and restarted when the wound is healed, sutures/staples are removed, and there are no signs of infection (~14 days). Tofacitinib should be held for all conditions except SLE for 1 week. For severe SLE, continue mycophenolate mofetil, azathioprine, cyclosporine, or tacrolimus but hold for 1 week for nonsevere SLE. If current dose of glucocorticoids is less than 20 mg/day, the current dose should be administered rather than administering stress-dose steroids.
Limitations include a limited number of studies conducted in the perioperative period, the existing data are based on lower dosages, and it is unknown whether results can be extrapolated to surgical procedures beyond TKA and THA. Additionally there is a need for further studies on glucocorticoid management and biologic agents.
Bottom line: Perioperative management of DMARDs is complex and understudied, but the review provides an evidence-based guide for patients undergoing TKA and THA.
Citation: Goodman SM, Springer B, Gordon G, et. al. 2017 American College of Rheumatology/American Association of Hip and Knee Surgeons Guideline for the Perioperative Management of Antirheumatic Medication in Patients With Rheumatic Diseases Undergoing Elective Total Hip or Total Knee Arthroplasty. Arthritis Care Res. 2017 Aug;69(8):1111-24.
Dr. Kochar is hospitalist and assistant professor of medicine, Icahn School of Medicine of the Mount Sinai Health System.
Clinical question: What is the best management for disease-modifying antirheumatic drugs (DMARDs) for patients with RA, ankylosing spondylitis, psoriatic arthritis, juvenile idiopathic arthritis, or systemic lupus erythematosus (SLE) undergoing elective total knee arthroplasty (TKA) or total hip arthroplasty (THA)?
Background: There are limited data in the evaluation of risks of flare with stopping DMARDs versus the risks of infection with continuing them perioperatively for elective TKA or THA, which are procedures frequently required by this patient population.
Study design: Multistep systematic literature review.
Setting: Collaboration between American College of Rheumatology and American Association of Hip and Knee Surgeons.
Synopsis: Through literature review and a requirement of 80% agreement by the panel, seven recommendations were created. Continue methotrexate, leflunomide, hydroxychloroquine, and sulfasalazine. Biologic agents should be held with surgery scheduled at the end of dosing cycle and restarted when the wound is healed, sutures/staples are removed, and there are no signs of infection (~14 days). Tofacitinib should be held for all conditions except SLE for 1 week. For severe SLE, continue mycophenolate mofetil, azathioprine, cyclosporine, or tacrolimus but hold for 1 week for nonsevere SLE. If current dose of glucocorticoids is less than 20 mg/day, the current dose should be administered rather than administering stress-dose steroids.
Limitations include a limited number of studies conducted in the perioperative period, the existing data are based on lower dosages, and it is unknown whether results can be extrapolated to surgical procedures beyond TKA and THA. Additionally there is a need for further studies on glucocorticoid management and biologic agents.
Bottom line: Perioperative management of DMARDs is complex and understudied, but the review provides an evidence-based guide for patients undergoing TKA and THA.
Citation: Goodman SM, Springer B, Gordon G, et. al. 2017 American College of Rheumatology/American Association of Hip and Knee Surgeons Guideline for the Perioperative Management of Antirheumatic Medication in Patients With Rheumatic Diseases Undergoing Elective Total Hip or Total Knee Arthroplasty. Arthritis Care Res. 2017 Aug;69(8):1111-24.
Dr. Kochar is hospitalist and assistant professor of medicine, Icahn School of Medicine of the Mount Sinai Health System.
Keep PCI patients on aspirin for noncardiac surgery
ANAHEIM, CALIF. – For every 1,000 patients with a history of percutaneous coronary intervention undergoing noncardiac surgery, perioperative aspirin would prevent 59 myocardial infarctions but cause 8 major/life-threatening bleeds, according to a substudy of the POISE-2 trial presented at the American Heart Association scientific sessions.
For patients with previous PCI undergoing noncardiac surgery, “I think aspirin will be more likely to benefit them than harm them,” so long as they are not having an operation where bleeding would be devastating.” These include “delicate neurosurgery in which, if you bleed into your spine, you end up paralyzed,” said lead investigator Michelle Graham, MD, an interventional cardiologist and professor of cardiology at the University of Alberta, Edmonton.
The original multisite POISE-2 trial (Perioperative Ischemic Evaluation 2) evaluated the effect of perioperative aspirin for noncardiac surgery. Patients were randomized to receive 200 mg aspirin or placebo within 4 hours of surgery and then 100 mg aspirin or placebo in the early postoperative period. There was no significant effect on the composite rate of death or myocardial infarction, but an increased risk of serious bleeding (N Engl J Med. 2014 Apr 17;370[16]:1494-503).
The new substudy focused on the 470 patients with previous PCIs, because such patients are known to have a higher risk for postop complications. More than half received bare-metal stents and a quarter got drug-eluting stents; in most of the rest, the stent type was not known. The median duration from PCI to noncardiac surgery was 64 months, ranging from 34 to 113 months. Patients with bare-metal stents placed within 6 weeks or drug-eluting stents within a year, were excluded.
Overall, 234 patients were randomized to the aspirin group, and 236 to placebo. Among those who came in on chronic, daily aspirin therapy – as almost all of the PCI subjects did – those who were randomized to perioperative aspirin stayed on daily 100 mg aspirin for a week postop, and then flipped back to whatever dose they were on at home. Likewise, placebo patients resumed their home aspirin after 1 week.
The results were very different from the main trial. At 30 days’ follow-up, just 6% of patients in the aspirin arm reached the primary endpoint of death or MI, versus 11.5% in the placebo group, a statistically significant 50% reduction.
This difference was driven almost entirely by a reduction in MIs. Whereas 5.1% of patients in the aspirin arm had MIs, 11% of the placebo group did, a significant 64% reduction. Meanwhile, the risk of major or life-threatening bleeding was not only similar between groups, but also to the overall trial, noted in 5.6% of aspirin and 4.2% of placebo subjects.
Over 75% of the participants were men, almost 60% were undergoing a major surgery, 30% had diabetes, and many had hypertension. Very few were on direct oral anticoagulants. The two arms were well matched, with a median age of about 68 years.
Simultaneously with Dr. Graham’s presentation, the results were published online (Ann Intern Med. 2017 Nov 14; doi: 10.7326/M17-2341)
The work was funded mostly by the Canadian Institutes of Health Research. Bayer supplied the aspirin. Dr. Graham has no industry disclosures.
ANAHEIM, CALIF. – For every 1,000 patients with a history of percutaneous coronary intervention undergoing noncardiac surgery, perioperative aspirin would prevent 59 myocardial infarctions but cause 8 major/life-threatening bleeds, according to a substudy of the POISE-2 trial presented at the American Heart Association scientific sessions.
For patients with previous PCI undergoing noncardiac surgery, “I think aspirin will be more likely to benefit them than harm them,” so long as they are not having an operation where bleeding would be devastating.” These include “delicate neurosurgery in which, if you bleed into your spine, you end up paralyzed,” said lead investigator Michelle Graham, MD, an interventional cardiologist and professor of cardiology at the University of Alberta, Edmonton.
The original multisite POISE-2 trial (Perioperative Ischemic Evaluation 2) evaluated the effect of perioperative aspirin for noncardiac surgery. Patients were randomized to receive 200 mg aspirin or placebo within 4 hours of surgery and then 100 mg aspirin or placebo in the early postoperative period. There was no significant effect on the composite rate of death or myocardial infarction, but an increased risk of serious bleeding (N Engl J Med. 2014 Apr 17;370[16]:1494-503).
The new substudy focused on the 470 patients with previous PCIs, because such patients are known to have a higher risk for postop complications. More than half received bare-metal stents and a quarter got drug-eluting stents; in most of the rest, the stent type was not known. The median duration from PCI to noncardiac surgery was 64 months, ranging from 34 to 113 months. Patients with bare-metal stents placed within 6 weeks or drug-eluting stents within a year, were excluded.
Overall, 234 patients were randomized to the aspirin group, and 236 to placebo. Among those who came in on chronic, daily aspirin therapy – as almost all of the PCI subjects did – those who were randomized to perioperative aspirin stayed on daily 100 mg aspirin for a week postop, and then flipped back to whatever dose they were on at home. Likewise, placebo patients resumed their home aspirin after 1 week.
The results were very different from the main trial. At 30 days’ follow-up, just 6% of patients in the aspirin arm reached the primary endpoint of death or MI, versus 11.5% in the placebo group, a statistically significant 50% reduction.
This difference was driven almost entirely by a reduction in MIs. Whereas 5.1% of patients in the aspirin arm had MIs, 11% of the placebo group did, a significant 64% reduction. Meanwhile, the risk of major or life-threatening bleeding was not only similar between groups, but also to the overall trial, noted in 5.6% of aspirin and 4.2% of placebo subjects.
Over 75% of the participants were men, almost 60% were undergoing a major surgery, 30% had diabetes, and many had hypertension. Very few were on direct oral anticoagulants. The two arms were well matched, with a median age of about 68 years.
Simultaneously with Dr. Graham’s presentation, the results were published online (Ann Intern Med. 2017 Nov 14; doi: 10.7326/M17-2341)
The work was funded mostly by the Canadian Institutes of Health Research. Bayer supplied the aspirin. Dr. Graham has no industry disclosures.
ANAHEIM, CALIF. – For every 1,000 patients with a history of percutaneous coronary intervention undergoing noncardiac surgery, perioperative aspirin would prevent 59 myocardial infarctions but cause 8 major/life-threatening bleeds, according to a substudy of the POISE-2 trial presented at the American Heart Association scientific sessions.
For patients with previous PCI undergoing noncardiac surgery, “I think aspirin will be more likely to benefit them than harm them,” so long as they are not having an operation where bleeding would be devastating.” These include “delicate neurosurgery in which, if you bleed into your spine, you end up paralyzed,” said lead investigator Michelle Graham, MD, an interventional cardiologist and professor of cardiology at the University of Alberta, Edmonton.
The original multisite POISE-2 trial (Perioperative Ischemic Evaluation 2) evaluated the effect of perioperative aspirin for noncardiac surgery. Patients were randomized to receive 200 mg aspirin or placebo within 4 hours of surgery and then 100 mg aspirin or placebo in the early postoperative period. There was no significant effect on the composite rate of death or myocardial infarction, but an increased risk of serious bleeding (N Engl J Med. 2014 Apr 17;370[16]:1494-503).
The new substudy focused on the 470 patients with previous PCIs, because such patients are known to have a higher risk for postop complications. More than half received bare-metal stents and a quarter got drug-eluting stents; in most of the rest, the stent type was not known. The median duration from PCI to noncardiac surgery was 64 months, ranging from 34 to 113 months. Patients with bare-metal stents placed within 6 weeks or drug-eluting stents within a year, were excluded.
Overall, 234 patients were randomized to the aspirin group, and 236 to placebo. Among those who came in on chronic, daily aspirin therapy – as almost all of the PCI subjects did – those who were randomized to perioperative aspirin stayed on daily 100 mg aspirin for a week postop, and then flipped back to whatever dose they were on at home. Likewise, placebo patients resumed their home aspirin after 1 week.
The results were very different from the main trial. At 30 days’ follow-up, just 6% of patients in the aspirin arm reached the primary endpoint of death or MI, versus 11.5% in the placebo group, a statistically significant 50% reduction.
This difference was driven almost entirely by a reduction in MIs. Whereas 5.1% of patients in the aspirin arm had MIs, 11% of the placebo group did, a significant 64% reduction. Meanwhile, the risk of major or life-threatening bleeding was not only similar between groups, but also to the overall trial, noted in 5.6% of aspirin and 4.2% of placebo subjects.
Over 75% of the participants were men, almost 60% were undergoing a major surgery, 30% had diabetes, and many had hypertension. Very few were on direct oral anticoagulants. The two arms were well matched, with a median age of about 68 years.
Simultaneously with Dr. Graham’s presentation, the results were published online (Ann Intern Med. 2017 Nov 14; doi: 10.7326/M17-2341)
The work was funded mostly by the Canadian Institutes of Health Research. Bayer supplied the aspirin. Dr. Graham has no industry disclosures.
AT THE AHA SCIENTIFIC SESSIONS
Key clinical point:
Major finding: For every 1,000 patients with a history of percutaneous coronary intervention undergoing noncardiac surgery, perioperative aspirin would prevent 59 myocardial infarctions but cause 8 major/life-threatening bleeds.
Data source: POISE-2, a randomized trial of 470 PCI patients.
Disclosures: The work was funded mostly by the Canadian Institutes of Health Research. Bayer supplied the aspirin. The lead investigator has no industry disclosures.
VIDEO: Regionalized STEMI care slashes in-hospital mortality
ANAHEIM, CALIF. – An American Heart Association program aimed at streamlining care of patients with ST-elevation MI resulted in a dramatic near-halving of in-hospital mortality, compared with STEMI patients treated in hospitals not participating in the project, James G. Jollis, MD, reported at the American Heart Association scientific sessions.
He presented the results of the STEMI ACCELERATOR 2 study, which involved 12 participating metropolitan regions across the United States, 132 percutaneous coronary intervention–capable hospitals, and 946 emergency medical services agencies. The ACCELERATOR 2 program entailed regional implementation of a structured STEMI care plan in which EMS personnel were trained to obtain prehospital ECGs and to activate cardiac catheterization labs prior to hospital arrival, bypassing the emergency department when appropriate.
Key elements of the project, which was part of the AHA’s Mission: Lifeline program, included having participating hospitals measure their performance of key processes and send that information as well as patient outcome data to the National Cardiovascular Data Registry’s ACTION–Get With The Guidelines registry. The hospitals in turn received quarterly feedback reports containing blinded hospital comparisons.
Dr. Jollis and his coinvestigators worked to obtain buy-in from local stakeholders, organize regional leadership, and help in drafting a central regional STEMI plan featuring prespecified treatment protocols.
The STEMI ACCELERATOR 2 study was carried out in 2015-2017, during which 10,730 patients with STEMI were transported directly to participating hospitals with PCI capability.
The primary study outcome was the change from the first to the final quarter of the study in the proportion of EMS-transported patients with a time from first medical contact to treatment in the cath lab of 90 minutes or less. This improved significantly, from 67% at baseline to 74% in the final quarter. Nine of the 12 participating regions reduced their time from first medical contact to treatment in the cath lab, and eight reached the national of goal of having 75% of STEMI patients treated within 90 minutes.
The other key time-to-care measures improved, too: At baseline, only 38% of patients had a time from first medical contact to cath lab activation of 20 minutes or less; by the final quarter, this figure had climbed to 56%. That’s an important metric, as evidenced by the study finding that in-hospital mortality occurred in 4.5% of patients with a time from first medical contact to cath lab activation of more than 20 minutes, compared with 2.2% in those with a time of 20 minutes or less.
Also, the proportion of patients who spent 20 minutes or less in the emergency department improved from 33% to 43%.
In-hospital mortality improved from 4.4% in the baseline quarter to 2.3% in the final quarter. No similar improvement in in-hospital mortality occurred in a comparison group of 22,651 STEMI patients treated at hospitals not involved in ACCELERATOR 2.
A significant reduction in the rate of in-hospital congestive heart failure occurred in the ACCELERATOR 2 centers, from 7.4% at baseline to 5.0%. In contrast, stroke, cardiogenic shock, and major bleeding rates were unchanged over time.
The ACCELERATOR 2 model of emergency cardiovascular care is designed to be highly generalizable, according to Dr. Jollis.
“This study supports the implementation of regionally coordinated systems across the United States to abort heart attacks, save lives, and enable heart attack victims to return to their families and productive lives,” he said.
The ACCELERATOR 2 operations manual – essentially a blueprint for organizing a regional STEMI system of care – is available gratis.
Dr. Allen, a cardiologist at the University of Colorado, Denver, said the ACCELERATOR 2 model has been successful because it is consistent with a fundamental principle of implementation science as described by Carolyn Clancy, MD, Executive in Charge at the Veterans Health Affairs Administration, who has said it’s a matter of making the right thing to do the easy thing to do.
Gregg C. Fonarow, MD, founder of the Get With the Guidelines program, predicted that the success of this program will lead to a ramping up of efforts to regionalize and coordinate STEMI care across the country. “I hope and anticipate that the AHA will take and run with the ACCELERATOR 2 model and adopt this into Mission: Lifeline, hoping to make this the standard approach to further improving care and outcomes in these patients,” said Dr. Fonarow, professor and cochief of cardiology at the University of California, Los Angeles, in a video interview.
Simultaneous with his presentation at the AHA conference, the results of STEMI ACCELERATOR 2 were published online in Circulation (2017 Nov 14; doi: 0.1161/CIRCULATIONAHA.117.032446).
The trial was sponsored by research and educational grants from AstraZeneca and The Medicines Company. Dr. Jollis reported having no financial conflicts of interest.
ANAHEIM, CALIF. – An American Heart Association program aimed at streamlining care of patients with ST-elevation MI resulted in a dramatic near-halving of in-hospital mortality, compared with STEMI patients treated in hospitals not participating in the project, James G. Jollis, MD, reported at the American Heart Association scientific sessions.
He presented the results of the STEMI ACCELERATOR 2 study, which involved 12 participating metropolitan regions across the United States, 132 percutaneous coronary intervention–capable hospitals, and 946 emergency medical services agencies. The ACCELERATOR 2 program entailed regional implementation of a structured STEMI care plan in which EMS personnel were trained to obtain prehospital ECGs and to activate cardiac catheterization labs prior to hospital arrival, bypassing the emergency department when appropriate.
Key elements of the project, which was part of the AHA’s Mission: Lifeline program, included having participating hospitals measure their performance of key processes and send that information as well as patient outcome data to the National Cardiovascular Data Registry’s ACTION–Get With The Guidelines registry. The hospitals in turn received quarterly feedback reports containing blinded hospital comparisons.
Dr. Jollis and his coinvestigators worked to obtain buy-in from local stakeholders, organize regional leadership, and help in drafting a central regional STEMI plan featuring prespecified treatment protocols.
The STEMI ACCELERATOR 2 study was carried out in 2015-2017, during which 10,730 patients with STEMI were transported directly to participating hospitals with PCI capability.
The primary study outcome was the change from the first to the final quarter of the study in the proportion of EMS-transported patients with a time from first medical contact to treatment in the cath lab of 90 minutes or less. This improved significantly, from 67% at baseline to 74% in the final quarter. Nine of the 12 participating regions reduced their time from first medical contact to treatment in the cath lab, and eight reached the national of goal of having 75% of STEMI patients treated within 90 minutes.
The other key time-to-care measures improved, too: At baseline, only 38% of patients had a time from first medical contact to cath lab activation of 20 minutes or less; by the final quarter, this figure had climbed to 56%. That’s an important metric, as evidenced by the study finding that in-hospital mortality occurred in 4.5% of patients with a time from first medical contact to cath lab activation of more than 20 minutes, compared with 2.2% in those with a time of 20 minutes or less.
Also, the proportion of patients who spent 20 minutes or less in the emergency department improved from 33% to 43%.
In-hospital mortality improved from 4.4% in the baseline quarter to 2.3% in the final quarter. No similar improvement in in-hospital mortality occurred in a comparison group of 22,651 STEMI patients treated at hospitals not involved in ACCELERATOR 2.
A significant reduction in the rate of in-hospital congestive heart failure occurred in the ACCELERATOR 2 centers, from 7.4% at baseline to 5.0%. In contrast, stroke, cardiogenic shock, and major bleeding rates were unchanged over time.
The ACCELERATOR 2 model of emergency cardiovascular care is designed to be highly generalizable, according to Dr. Jollis.
“This study supports the implementation of regionally coordinated systems across the United States to abort heart attacks, save lives, and enable heart attack victims to return to their families and productive lives,” he said.
The ACCELERATOR 2 operations manual – essentially a blueprint for organizing a regional STEMI system of care – is available gratis.
Dr. Allen, a cardiologist at the University of Colorado, Denver, said the ACCELERATOR 2 model has been successful because it is consistent with a fundamental principle of implementation science as described by Carolyn Clancy, MD, Executive in Charge at the Veterans Health Affairs Administration, who has said it’s a matter of making the right thing to do the easy thing to do.
Gregg C. Fonarow, MD, founder of the Get With the Guidelines program, predicted that the success of this program will lead to a ramping up of efforts to regionalize and coordinate STEMI care across the country. “I hope and anticipate that the AHA will take and run with the ACCELERATOR 2 model and adopt this into Mission: Lifeline, hoping to make this the standard approach to further improving care and outcomes in these patients,” said Dr. Fonarow, professor and cochief of cardiology at the University of California, Los Angeles, in a video interview.
Simultaneous with his presentation at the AHA conference, the results of STEMI ACCELERATOR 2 were published online in Circulation (2017 Nov 14; doi: 0.1161/CIRCULATIONAHA.117.032446).
The trial was sponsored by research and educational grants from AstraZeneca and The Medicines Company. Dr. Jollis reported having no financial conflicts of interest.
ANAHEIM, CALIF. – An American Heart Association program aimed at streamlining care of patients with ST-elevation MI resulted in a dramatic near-halving of in-hospital mortality, compared with STEMI patients treated in hospitals not participating in the project, James G. Jollis, MD, reported at the American Heart Association scientific sessions.
He presented the results of the STEMI ACCELERATOR 2 study, which involved 12 participating metropolitan regions across the United States, 132 percutaneous coronary intervention–capable hospitals, and 946 emergency medical services agencies. The ACCELERATOR 2 program entailed regional implementation of a structured STEMI care plan in which EMS personnel were trained to obtain prehospital ECGs and to activate cardiac catheterization labs prior to hospital arrival, bypassing the emergency department when appropriate.
Key elements of the project, which was part of the AHA’s Mission: Lifeline program, included having participating hospitals measure their performance of key processes and send that information as well as patient outcome data to the National Cardiovascular Data Registry’s ACTION–Get With The Guidelines registry. The hospitals in turn received quarterly feedback reports containing blinded hospital comparisons.
Dr. Jollis and his coinvestigators worked to obtain buy-in from local stakeholders, organize regional leadership, and help in drafting a central regional STEMI plan featuring prespecified treatment protocols.
The STEMI ACCELERATOR 2 study was carried out in 2015-2017, during which 10,730 patients with STEMI were transported directly to participating hospitals with PCI capability.
The primary study outcome was the change from the first to the final quarter of the study in the proportion of EMS-transported patients with a time from first medical contact to treatment in the cath lab of 90 minutes or less. This improved significantly, from 67% at baseline to 74% in the final quarter. Nine of the 12 participating regions reduced their time from first medical contact to treatment in the cath lab, and eight reached the national of goal of having 75% of STEMI patients treated within 90 minutes.
The other key time-to-care measures improved, too: At baseline, only 38% of patients had a time from first medical contact to cath lab activation of 20 minutes or less; by the final quarter, this figure had climbed to 56%. That’s an important metric, as evidenced by the study finding that in-hospital mortality occurred in 4.5% of patients with a time from first medical contact to cath lab activation of more than 20 minutes, compared with 2.2% in those with a time of 20 minutes or less.
Also, the proportion of patients who spent 20 minutes or less in the emergency department improved from 33% to 43%.
In-hospital mortality improved from 4.4% in the baseline quarter to 2.3% in the final quarter. No similar improvement in in-hospital mortality occurred in a comparison group of 22,651 STEMI patients treated at hospitals not involved in ACCELERATOR 2.
A significant reduction in the rate of in-hospital congestive heart failure occurred in the ACCELERATOR 2 centers, from 7.4% at baseline to 5.0%. In contrast, stroke, cardiogenic shock, and major bleeding rates were unchanged over time.
The ACCELERATOR 2 model of emergency cardiovascular care is designed to be highly generalizable, according to Dr. Jollis.
“This study supports the implementation of regionally coordinated systems across the United States to abort heart attacks, save lives, and enable heart attack victims to return to their families and productive lives,” he said.
The ACCELERATOR 2 operations manual – essentially a blueprint for organizing a regional STEMI system of care – is available gratis.
Dr. Allen, a cardiologist at the University of Colorado, Denver, said the ACCELERATOR 2 model has been successful because it is consistent with a fundamental principle of implementation science as described by Carolyn Clancy, MD, Executive in Charge at the Veterans Health Affairs Administration, who has said it’s a matter of making the right thing to do the easy thing to do.
Gregg C. Fonarow, MD, founder of the Get With the Guidelines program, predicted that the success of this program will lead to a ramping up of efforts to regionalize and coordinate STEMI care across the country. “I hope and anticipate that the AHA will take and run with the ACCELERATOR 2 model and adopt this into Mission: Lifeline, hoping to make this the standard approach to further improving care and outcomes in these patients,” said Dr. Fonarow, professor and cochief of cardiology at the University of California, Los Angeles, in a video interview.
Simultaneous with his presentation at the AHA conference, the results of STEMI ACCELERATOR 2 were published online in Circulation (2017 Nov 14; doi: 0.1161/CIRCULATIONAHA.117.032446).
The trial was sponsored by research and educational grants from AstraZeneca and The Medicines Company. Dr. Jollis reported having no financial conflicts of interest.
AT THE AHA SCIENTIFIC SESSIONS
Key clinical point:
Major finding: The in-hospital mortality rate of STEMI patients dropped from 4.4% in the baseline quarter to 2.3% in the final quarter of a study that examined the impact of introducing regionalized STEMI care.
Data source: This was a prospective study of an intervention that involved implementation of regionalized STEMI care in a dozen U.S. metropolitan areas.
Disclosures: The study was sponsored by research and educational grants from AstraZeneca and The Medicines Company. The presenter reported having no financial conflicts of interest.