User login
Official news magazine of the Society of Hospital Medicine
Copyright by Society of Hospital Medicine or related companies. All rights reserved. ISSN 1553-085X
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
div[contains(@class, 'pane-pub-article-hospitalist')]


Naloxone: Difficult conversations about a potential lifesaver
New tools to help minimize the risk of opioid-related adverse events are becoming more widely available, although providers are still struggling over how best to implement them.
A recent study by Shane Mueller, MSW, and Ingrid Binswanger, MD, at Kaiser Permanente Colorado Institute for Health Research, Denver, for instance, found that doctors are frequently uncomfortable prescribing the opioid antagonist naloxone to counteract a potential overdose.1
Although much of the research was conducted in outpatient settings, the researchers say several lessons may be translated to the hospital readily. “Patients were really willing to embrace the idea of naloxone when it was framed to be used in a worst-case scenario,” Mr. Mueller said; some providers, for example, compared it to having a fire extinguisher in the house. “I think one patient said, ‘You know, I don’t plan on starting a fire in my kitchen, but it’s good to have there just in case something goes wrong.’ ”
Another important lesson, Mr. Mueller said, is to consider multiple factors that might heighten the overdose risk, such as a change in the prescription or a medical condition like renal failure. Including those considerations in a conversation might help destigmatize the topic and help patients who are concerned that they might be perceived as misusing the medication.
Ideally, opioid-safety interventions should be more patient centered, emphasizing safer home storage to prevent secondary exposures and educating patients fully about the medication’s downsides, she said. “They may have been on them a long time but never been fully informed of the risks,” she said. Among her group’s future research goals, Dr. Binswanger hopes to investigate how best to communicate such risks to patients.
References
1. Mueller SR, Koester S, Glanz JM, et al. Attitudes toward naloxone prescribing in clinical settings: A qualitative study of patients prescribed high dose opioids for chronic non-cancer pain. J Gen Intern Med. 2017 March;32(3):277-83.
New tools to help minimize the risk of opioid-related adverse events are becoming more widely available, although providers are still struggling over how best to implement them.
A recent study by Shane Mueller, MSW, and Ingrid Binswanger, MD, at Kaiser Permanente Colorado Institute for Health Research, Denver, for instance, found that doctors are frequently uncomfortable prescribing the opioid antagonist naloxone to counteract a potential overdose.1
Although much of the research was conducted in outpatient settings, the researchers say several lessons may be translated to the hospital readily. “Patients were really willing to embrace the idea of naloxone when it was framed to be used in a worst-case scenario,” Mr. Mueller said; some providers, for example, compared it to having a fire extinguisher in the house. “I think one patient said, ‘You know, I don’t plan on starting a fire in my kitchen, but it’s good to have there just in case something goes wrong.’ ”
Another important lesson, Mr. Mueller said, is to consider multiple factors that might heighten the overdose risk, such as a change in the prescription or a medical condition like renal failure. Including those considerations in a conversation might help destigmatize the topic and help patients who are concerned that they might be perceived as misusing the medication.
Ideally, opioid-safety interventions should be more patient centered, emphasizing safer home storage to prevent secondary exposures and educating patients fully about the medication’s downsides, she said. “They may have been on them a long time but never been fully informed of the risks,” she said. Among her group’s future research goals, Dr. Binswanger hopes to investigate how best to communicate such risks to patients.
References
1. Mueller SR, Koester S, Glanz JM, et al. Attitudes toward naloxone prescribing in clinical settings: A qualitative study of patients prescribed high dose opioids for chronic non-cancer pain. J Gen Intern Med. 2017 March;32(3):277-83.
New tools to help minimize the risk of opioid-related adverse events are becoming more widely available, although providers are still struggling over how best to implement them.
A recent study by Shane Mueller, MSW, and Ingrid Binswanger, MD, at Kaiser Permanente Colorado Institute for Health Research, Denver, for instance, found that doctors are frequently uncomfortable prescribing the opioid antagonist naloxone to counteract a potential overdose.1
Although much of the research was conducted in outpatient settings, the researchers say several lessons may be translated to the hospital readily. “Patients were really willing to embrace the idea of naloxone when it was framed to be used in a worst-case scenario,” Mr. Mueller said; some providers, for example, compared it to having a fire extinguisher in the house. “I think one patient said, ‘You know, I don’t plan on starting a fire in my kitchen, but it’s good to have there just in case something goes wrong.’ ”
Another important lesson, Mr. Mueller said, is to consider multiple factors that might heighten the overdose risk, such as a change in the prescription or a medical condition like renal failure. Including those considerations in a conversation might help destigmatize the topic and help patients who are concerned that they might be perceived as misusing the medication.
Ideally, opioid-safety interventions should be more patient centered, emphasizing safer home storage to prevent secondary exposures and educating patients fully about the medication’s downsides, she said. “They may have been on them a long time but never been fully informed of the risks,” she said. Among her group’s future research goals, Dr. Binswanger hopes to investigate how best to communicate such risks to patients.
References
1. Mueller SR, Koester S, Glanz JM, et al. Attitudes toward naloxone prescribing in clinical settings: A qualitative study of patients prescribed high dose opioids for chronic non-cancer pain. J Gen Intern Med. 2017 March;32(3):277-83.
Carvedilol fails to reduce variceal bleeds in acute-on-chronic liver failure
WASHINGTON – Treatment with carvedilol reduced the incidence of sepsis and acute kidney injury and improved survival at 28 days but did not significantly reduce the progression of esophageal varices in patients with acute-on-chronic liver failure.
A total of 136 patients with acute-on-chronic liver failure with small or no esophageal varices and a hepatic venous pressure gradient (HVPG) of 12 mm Hg or greater were enrolled in a single center, prospective, open-label, randomized controlled trial: 66 were randomized to carvedilol and 70 to placebo, according to Sumeet Kainth, MD, of the Institute of Liver and Biliary Sciences in New Delhi.
More than 90% of patients were men with a mean age of 44 years, and composition of the treatment and placebo groups was similar. About 70% in each group had alcoholic hepatitis (the reason for acute liver failure in most). Mean Model for End-Stage Liver Disease (MELD) scores were about 25. Hemodynamic parameters also were comparable, with a mean HVPG of about 19, Dr. Kainth said at the annual meeting of the American Association for the Study of Liver Diseases.
Patients in the treatment group received a median maximum tolerated dose of carvedilol of 12.5 mg, with a range of 3.13 mg to 25 mg.
Morbidity and mortality were high, as is expected with acute-on-chronic liver failure, he noted. A total of 36 patients died before the end of the 90-day study period. Another 23 experienced adverse events and 2 progressed to liver transplant.
HVPG at 90 days decreased significantly in both groups. In the carvedilol group, 90-day HVPG was 16 mm Hg, compared with 19.7 mm Hg at baseline (P less than .01). For placebo patients, 90-day HVPG spontaneously improved to 14.8 mm Hg, compared with a baseline of 17.2 mm Hg (P less than .01).
Carvedilol did not significantly slow the development or growth of varices, however, Dr. Kainth said. At 90 days, varices had progressed in 9 of 40 patients (22.5%) of patients on carvedilol and 8 of 31 (25.8%) of placebo patients.
Significantly fewer patients in the carvedilol group developed acute kidney injury at 28 days (14% vs. 38% on placebo) and sepsis (5% vs. 20%). Mortality also was reduced significantly at 28 days (11% vs. 24%), he reported.
Treatment with carvedilol did not achieve significant reductions in variceal bleeding, “possibly due to the low number of bleeds seen in the study [because of] the exclusion of patients with large varices,” Dr. Kainth said.
The study was sponsored by Institute of Liver and Biliary Sciences. Dr. Kainth reported no relevant conflicts of interest.
[email protected]
On Twitter @denisefulton
WASHINGTON – Treatment with carvedilol reduced the incidence of sepsis and acute kidney injury and improved survival at 28 days but did not significantly reduce the progression of esophageal varices in patients with acute-on-chronic liver failure.
A total of 136 patients with acute-on-chronic liver failure with small or no esophageal varices and a hepatic venous pressure gradient (HVPG) of 12 mm Hg or greater were enrolled in a single center, prospective, open-label, randomized controlled trial: 66 were randomized to carvedilol and 70 to placebo, according to Sumeet Kainth, MD, of the Institute of Liver and Biliary Sciences in New Delhi.
More than 90% of patients were men with a mean age of 44 years, and composition of the treatment and placebo groups was similar. About 70% in each group had alcoholic hepatitis (the reason for acute liver failure in most). Mean Model for End-Stage Liver Disease (MELD) scores were about 25. Hemodynamic parameters also were comparable, with a mean HVPG of about 19, Dr. Kainth said at the annual meeting of the American Association for the Study of Liver Diseases.
Patients in the treatment group received a median maximum tolerated dose of carvedilol of 12.5 mg, with a range of 3.13 mg to 25 mg.
Morbidity and mortality were high, as is expected with acute-on-chronic liver failure, he noted. A total of 36 patients died before the end of the 90-day study period. Another 23 experienced adverse events and 2 progressed to liver transplant.
HVPG at 90 days decreased significantly in both groups. In the carvedilol group, 90-day HVPG was 16 mm Hg, compared with 19.7 mm Hg at baseline (P less than .01). For placebo patients, 90-day HVPG spontaneously improved to 14.8 mm Hg, compared with a baseline of 17.2 mm Hg (P less than .01).
Carvedilol did not significantly slow the development or growth of varices, however, Dr. Kainth said. At 90 days, varices had progressed in 9 of 40 patients (22.5%) of patients on carvedilol and 8 of 31 (25.8%) of placebo patients.
Significantly fewer patients in the carvedilol group developed acute kidney injury at 28 days (14% vs. 38% on placebo) and sepsis (5% vs. 20%). Mortality also was reduced significantly at 28 days (11% vs. 24%), he reported.
Treatment with carvedilol did not achieve significant reductions in variceal bleeding, “possibly due to the low number of bleeds seen in the study [because of] the exclusion of patients with large varices,” Dr. Kainth said.
The study was sponsored by Institute of Liver and Biliary Sciences. Dr. Kainth reported no relevant conflicts of interest.
[email protected]
On Twitter @denisefulton
WASHINGTON – Treatment with carvedilol reduced the incidence of sepsis and acute kidney injury and improved survival at 28 days but did not significantly reduce the progression of esophageal varices in patients with acute-on-chronic liver failure.
A total of 136 patients with acute-on-chronic liver failure with small or no esophageal varices and a hepatic venous pressure gradient (HVPG) of 12 mm Hg or greater were enrolled in a single center, prospective, open-label, randomized controlled trial: 66 were randomized to carvedilol and 70 to placebo, according to Sumeet Kainth, MD, of the Institute of Liver and Biliary Sciences in New Delhi.
More than 90% of patients were men with a mean age of 44 years, and composition of the treatment and placebo groups was similar. About 70% in each group had alcoholic hepatitis (the reason for acute liver failure in most). Mean Model for End-Stage Liver Disease (MELD) scores were about 25. Hemodynamic parameters also were comparable, with a mean HVPG of about 19, Dr. Kainth said at the annual meeting of the American Association for the Study of Liver Diseases.
Patients in the treatment group received a median maximum tolerated dose of carvedilol of 12.5 mg, with a range of 3.13 mg to 25 mg.
Morbidity and mortality were high, as is expected with acute-on-chronic liver failure, he noted. A total of 36 patients died before the end of the 90-day study period. Another 23 experienced adverse events and 2 progressed to liver transplant.
HVPG at 90 days decreased significantly in both groups. In the carvedilol group, 90-day HVPG was 16 mm Hg, compared with 19.7 mm Hg at baseline (P less than .01). For placebo patients, 90-day HVPG spontaneously improved to 14.8 mm Hg, compared with a baseline of 17.2 mm Hg (P less than .01).
Carvedilol did not significantly slow the development or growth of varices, however, Dr. Kainth said. At 90 days, varices had progressed in 9 of 40 patients (22.5%) of patients on carvedilol and 8 of 31 (25.8%) of placebo patients.
Significantly fewer patients in the carvedilol group developed acute kidney injury at 28 days (14% vs. 38% on placebo) and sepsis (5% vs. 20%). Mortality also was reduced significantly at 28 days (11% vs. 24%), he reported.
Treatment with carvedilol did not achieve significant reductions in variceal bleeding, “possibly due to the low number of bleeds seen in the study [because of] the exclusion of patients with large varices,” Dr. Kainth said.
The study was sponsored by Institute of Liver and Biliary Sciences. Dr. Kainth reported no relevant conflicts of interest.
[email protected]
On Twitter @denisefulton
AT THE LIVER MEETING 2017
Key clinical point:
Major finding: At 90 days, varices had progressed in 9 of 40 (22.5%) patients on carvedilol vs. 8 of 31 (25.8%) of placebo patients.
Data source: A single-center, prospective, open-label, randomized controlled trial of 136 patients with acute-on-chronic liver failure.
Disclosures: The study was sponsored by the Institute of Liver and Biliary Sciences. Dr. Kainth reported no relevant conflicts of interest.
Firearms’ injury toll of $3 billion just ‘a drop in the bucket’
SAN DIEGO – The true impact of firearms injuries may be greatly underestimated, according to a study presented at the American College of Surgeons Clinical Congress.
An analysis released earlier this month estimated that firearms injuries cost nearly $3 billion a year in emergency department and inpatient treatment costs. The real cost is likely to be 10-20 times higher, said the lead author of the study, Faiz Gani, MD, a research fellow with the Johns Hopkins Surgery Center for Outcomes Research, Baltimore.
“This is just a drop in the bucket,” Dr. Gani said in an interview at the annual clinical congress of the American College of Surgeons.
Dr. Gani and his colleagues launched their study (Health Affairs 2017;36[10]:1729-38) to better understand the cost of firearms injuries, including nonfatal and accidental injuries.
Most estimates of the cost of firearm injuries are outdated or focused on states or single trauma centers, he said. “Contemporary [costs] for emergency rooms are unknown,” he said. “Also, the numbers come down and shoot up. It’s important to continually study this.”
The statistics are especially important to surgeons, who handle these injuries. “A lot of times the surgeon is the primary health care provider if the patient is injured severely. It’s important that we as surgeons know what’s going on.”
The researchers retrospectively analyzed data from the Nationwide Emergency Department Sample of the Healthcare Cost and Utilization Project for the years 2006-2014. They identified 150,930 patients who appeared alive in emergency departments over that period with firearms injuries, and they estimated the total weighted number at 704,916.
They found that the incidence of firearms injury admissions actually fell during 2006-2013 (from 27.9 visits per 100,000 people to 21.5, P < .001) but bumped up by 23.7% to 26.6 during 2013-2014 (P < .001).
Not surprisingly, more men were injured than women: 45.8 firearms-injured men per 100,000 patients presenting at emergency departments, compared with 5.5 firearms-injured women. Assaults (49.5%) and accidents (35.3%) accounted for most cases, followed by attempted suicides (5.3%) and legal intervention (2.4%).
Those who were assaulted had a higher likelihood of being poor, while those who tried to kill themselves were more likely to have the highest incomes among firearms-injured patients.
The average costs of emergency and inpatient care for patients injured by firearms were $5,254 and $95,887, respectively, collectively amounting to about $2.8 billion each year.
Dr. Gani mentioned that the estimation of the cost and impact of firearms injuries don’t account for people who died of firearms injuries before reaching the emergency department, he says, including patients who committed suicide and died at home.
The cost estimates also don’t take follow-up care, rehabilitation, and lifelong disability into account. The surgical portion of the cost is likely to be much higher because the study doesn’t take future surgical procedures into account, he said.
Based on estimates by the Centers for Disease Control and Prevention of the impact of the injuries, Dr. Gani argued that the true annual cost could be 10 or 20 times the nearly $3 billion estimated by the study.
Discussant Elliott R. Haut, MD, FACS, a trauma surgeon at Johns Hopkins Medicine in Baltimore, agreed that the study estimates of cost and impact estimated in the study represent a small part of a larger toll. Some families and individuals can pay those costs more than once. He recalls hearing from family members of firearm victims who recognize him because they’ve been at the hospital for other shooting incidents. “We’ve all heard someone say, ‘You were here the last time when my brother/cousin/uncle was shot,’ ” he said.
Future research should focus on better understanding the long-term cost of firearm injuries and the influence of socioeconomics and demographics, Dr. Gani said.
Dr. Gani and Dr. Haut reported no relevant disclosures.
SAN DIEGO – The true impact of firearms injuries may be greatly underestimated, according to a study presented at the American College of Surgeons Clinical Congress.
An analysis released earlier this month estimated that firearms injuries cost nearly $3 billion a year in emergency department and inpatient treatment costs. The real cost is likely to be 10-20 times higher, said the lead author of the study, Faiz Gani, MD, a research fellow with the Johns Hopkins Surgery Center for Outcomes Research, Baltimore.
“This is just a drop in the bucket,” Dr. Gani said in an interview at the annual clinical congress of the American College of Surgeons.
Dr. Gani and his colleagues launched their study (Health Affairs 2017;36[10]:1729-38) to better understand the cost of firearms injuries, including nonfatal and accidental injuries.
Most estimates of the cost of firearm injuries are outdated or focused on states or single trauma centers, he said. “Contemporary [costs] for emergency rooms are unknown,” he said. “Also, the numbers come down and shoot up. It’s important to continually study this.”
The statistics are especially important to surgeons, who handle these injuries. “A lot of times the surgeon is the primary health care provider if the patient is injured severely. It’s important that we as surgeons know what’s going on.”
The researchers retrospectively analyzed data from the Nationwide Emergency Department Sample of the Healthcare Cost and Utilization Project for the years 2006-2014. They identified 150,930 patients who appeared alive in emergency departments over that period with firearms injuries, and they estimated the total weighted number at 704,916.
They found that the incidence of firearms injury admissions actually fell during 2006-2013 (from 27.9 visits per 100,000 people to 21.5, P < .001) but bumped up by 23.7% to 26.6 during 2013-2014 (P < .001).
Not surprisingly, more men were injured than women: 45.8 firearms-injured men per 100,000 patients presenting at emergency departments, compared with 5.5 firearms-injured women. Assaults (49.5%) and accidents (35.3%) accounted for most cases, followed by attempted suicides (5.3%) and legal intervention (2.4%).
Those who were assaulted had a higher likelihood of being poor, while those who tried to kill themselves were more likely to have the highest incomes among firearms-injured patients.
The average costs of emergency and inpatient care for patients injured by firearms were $5,254 and $95,887, respectively, collectively amounting to about $2.8 billion each year.
Dr. Gani mentioned that the estimation of the cost and impact of firearms injuries don’t account for people who died of firearms injuries before reaching the emergency department, he says, including patients who committed suicide and died at home.
The cost estimates also don’t take follow-up care, rehabilitation, and lifelong disability into account. The surgical portion of the cost is likely to be much higher because the study doesn’t take future surgical procedures into account, he said.
Based on estimates by the Centers for Disease Control and Prevention of the impact of the injuries, Dr. Gani argued that the true annual cost could be 10 or 20 times the nearly $3 billion estimated by the study.
Discussant Elliott R. Haut, MD, FACS, a trauma surgeon at Johns Hopkins Medicine in Baltimore, agreed that the study estimates of cost and impact estimated in the study represent a small part of a larger toll. Some families and individuals can pay those costs more than once. He recalls hearing from family members of firearm victims who recognize him because they’ve been at the hospital for other shooting incidents. “We’ve all heard someone say, ‘You were here the last time when my brother/cousin/uncle was shot,’ ” he said.
Future research should focus on better understanding the long-term cost of firearm injuries and the influence of socioeconomics and demographics, Dr. Gani said.
Dr. Gani and Dr. Haut reported no relevant disclosures.
SAN DIEGO – The true impact of firearms injuries may be greatly underestimated, according to a study presented at the American College of Surgeons Clinical Congress.
An analysis released earlier this month estimated that firearms injuries cost nearly $3 billion a year in emergency department and inpatient treatment costs. The real cost is likely to be 10-20 times higher, said the lead author of the study, Faiz Gani, MD, a research fellow with the Johns Hopkins Surgery Center for Outcomes Research, Baltimore.
“This is just a drop in the bucket,” Dr. Gani said in an interview at the annual clinical congress of the American College of Surgeons.
Dr. Gani and his colleagues launched their study (Health Affairs 2017;36[10]:1729-38) to better understand the cost of firearms injuries, including nonfatal and accidental injuries.
Most estimates of the cost of firearm injuries are outdated or focused on states or single trauma centers, he said. “Contemporary [costs] for emergency rooms are unknown,” he said. “Also, the numbers come down and shoot up. It’s important to continually study this.”
The statistics are especially important to surgeons, who handle these injuries. “A lot of times the surgeon is the primary health care provider if the patient is injured severely. It’s important that we as surgeons know what’s going on.”
The researchers retrospectively analyzed data from the Nationwide Emergency Department Sample of the Healthcare Cost and Utilization Project for the years 2006-2014. They identified 150,930 patients who appeared alive in emergency departments over that period with firearms injuries, and they estimated the total weighted number at 704,916.
They found that the incidence of firearms injury admissions actually fell during 2006-2013 (from 27.9 visits per 100,000 people to 21.5, P < .001) but bumped up by 23.7% to 26.6 during 2013-2014 (P < .001).
Not surprisingly, more men were injured than women: 45.8 firearms-injured men per 100,000 patients presenting at emergency departments, compared with 5.5 firearms-injured women. Assaults (49.5%) and accidents (35.3%) accounted for most cases, followed by attempted suicides (5.3%) and legal intervention (2.4%).
Those who were assaulted had a higher likelihood of being poor, while those who tried to kill themselves were more likely to have the highest incomes among firearms-injured patients.
The average costs of emergency and inpatient care for patients injured by firearms were $5,254 and $95,887, respectively, collectively amounting to about $2.8 billion each year.
Dr. Gani mentioned that the estimation of the cost and impact of firearms injuries don’t account for people who died of firearms injuries before reaching the emergency department, he says, including patients who committed suicide and died at home.
The cost estimates also don’t take follow-up care, rehabilitation, and lifelong disability into account. The surgical portion of the cost is likely to be much higher because the study doesn’t take future surgical procedures into account, he said.
Based on estimates by the Centers for Disease Control and Prevention of the impact of the injuries, Dr. Gani argued that the true annual cost could be 10 or 20 times the nearly $3 billion estimated by the study.
Discussant Elliott R. Haut, MD, FACS, a trauma surgeon at Johns Hopkins Medicine in Baltimore, agreed that the study estimates of cost and impact estimated in the study represent a small part of a larger toll. Some families and individuals can pay those costs more than once. He recalls hearing from family members of firearm victims who recognize him because they’ve been at the hospital for other shooting incidents. “We’ve all heard someone say, ‘You were here the last time when my brother/cousin/uncle was shot,’ ” he said.
Future research should focus on better understanding the long-term cost of firearm injuries and the influence of socioeconomics and demographics, Dr. Gani said.
Dr. Gani and Dr. Haut reported no relevant disclosures.
AT THE ACS CLINICAL CONGRESS
The opioid epidemic and its impact on the health care system
The United States is in the midst of a public health crisis. Every day, 91 Americans die from opioid overdoses.1 Opioid addiction has a tremendous negative effect on parents and children by destroying lives and breaking up families. When used appropriately, these drugs, such as morphine, hydrocodone, oxycodone, and fentanyl, provide much needed pain relief to patients, especially after a surgical procedure or during treatment for cancer. Unfortunately, opioids also have qualities that make them addictive and prone to overuse and abuse.
Heroin and other opioid drugs are affecting social, health, and economic welfare in communities throughout the United States. Opioid addiction does not discriminate among occupations, socioeconomic statuses, or ethnicities. Once addicted, users find it difficult to fight and overcome the habit. In this context, hospitalists who develop and use opioid policies and tactics are better equipped to deal with the increasing prevalence of opioid addiction and overdoses seen in the medical center environment.
Overview of opioid addiction
Opioids are more prevalent and easily available today in part because of the intense marketing campaigns by pharmaceutical companies. In fact, according to the 2014 National Survey on Drug Use and Health, almost 2 million Americans were dependent on or abused prescription opioids.2 Opioids create artificial endorphins in the brain, amplifying positive feelings and euphoria. Once they become dependent, patients feel sick and become depressed when they aren’t using narcotics. They experience uncontrollable cravings, relieved only by increasing opioid use. Personal relationships and finances are severely affected because patients will do whatever they can to acquire more opioids. Some resort to doctor shopping to obtain more opioids, while others will engage in drug trafficking to purchase and sell narcotics. Most new heroin users report that they started with nonmedical use of opioid pain relievers.3
Unfortunately, in 2015, there were more than 20,000 prescription opioid overdose deaths and almost 13,000 overdose deaths related to heroin.4 In addition to addiction and death, overuse of narcotics can cause many medical issues, including dizziness, constipation, depression, and immune dysfunction.
As with any addiction, there are many problems related to opiate withdrawal. Some of these include anxiety, rhinorrhea, lacrimation, piloerection, mydriasis, nausea, vomiting, abdominal pain, diarrhea, tachycardia, and hypertension. Methadone, buprenorphine, and extended-release naltrexone typically are used to help alleviate cravings and the symptoms of withdrawal. With more than 1,000 patients treated daily by emergency departments for misuse of prescription opioids, it’s imperative for the medical community to address this health care crisis.5
Impact on first responders and hospitalists
The impact of this epidemic on the medical community is dramatic. Emergency system resources, already on overload, are further taxed and drained by the increased 911 calls for overdose incidents. This means that instead of responding to heart attacks, strokes, or other emergencies, first responders are spending time stabilizing overdose patients and taking them to hospitals. This resource drain spreads to emergency rooms and hospitals as they treat these patients. Eventually, the epidemic results in higher insurance costs to cover the impact on medical resources.
Strategies for hospitalists
A multifaceted approach is required to combat this crisis, and hospitalists are one component of that solution. Here are strategies hospitalists can employ to combat the growing use and abuse of narcotics:
- Screen for high-risk conditions. Before prescribing opioids, screen patients for conditions that may be exacerbated by opioid use, including sleep apnea, obesity COPD, and heart failure, as well as whether they are using medications that cause sedation and respiratory depression.
- Develop and follow pain management clinical pathway protocols. For example, start with nonopioid analgesics, such as acetaminophen, ibuprofen, and naproxen, for patients with mild pain. If the patient is in moderate pain, or if the mild pain was unrelieved by the first type of medicine, continue with opioid analgesics including codeine, oxycodone, hydrocodone, or morphine. Finally, if the patient is experiencing severe pain, or if the mild to moderate pain was unrelieved by previous medications, prescribe higher doses of morphine, fentanyl, or hydromorphone or use a patient-controlled analgesia delivery of an intravenous opioid.
- Discuss other treatment options. Instead of prescribing narcotics, assess whether other treatment options are viable. These include physical and occupational therapy, steroid shots, massage, local nerve blocks, and muscle relaxers.
- Evaluate the reason(s) for current use of prescribed narcotics. It’s crucial to determine why patients are using opioids. The medical condition for which they were prescribed these medications may have resolved. If patients are using opioids without a medically indicated reason, offer alternative medications, such as methadone or naltrexone, and provide education to help them slowly taper off the drugs. Do not cease opioid use altogether unless the use is contraindicated because of other medical conditions or unstable vital signs (for example, low blood pressure).
- Train nurses on opioid use and addiction. Educate nurses about opioid addiction risk factors and symptoms of addiction. Instruct them on managing the Ramsay Sedation Scale and using an opioid sedation scale with patients receiving IV narcotics.
- Educate families on opioid use and addiction. Counsel patients and their families on the long-term effects of opioid use and about the warning signs of addiction. Teach them about alternatives to narcotics.
- Offer referrals to specialized clinics. Work with social workers, case managers, and local mental health professionals to refer patients to behavioral counseling, patient and family support groups, and monitored detoxification clinics qualified to treat opioid addiction.
Socioeconomic impact
The effects of the opioid epidemic are felt in all areas of the United States, especially in the health care industry. Emergency department visits are mounting while billions of dollars are spent on medical care for those addicted to opioids. Additionally, the socioeconomic effects of this crisis contribute to increasing depression, anxiety, missed days of work or school, unemployment, drop-out rates, and loss of productivity among those addicted to opioids. Also, the epidemic is adversely affecting families, leading to increased divorce rates, single parent families, and child abuse and neglect. By creating strategies and protocols for medical staff, patients, and families affected by opioid use, addiction, or overdose, hospitalists can have a positive influence on patients’ lives and ultimately the opioid epidemic.
Dr. Kasarla is a hospitalist with Apogee Physicians at Parkway Surgical & Cardiovascular Hospital in Fort Worth, Tex. He also works for Texas Health Physicians Group at Texas Health Harris Methodist Hospital and Texas Health Harris Methodist Hospital Southwest in Fort Worth, as well as at Texas Health Harris Methodist Hospital in Azle, Tex.
You can contact him at [email protected] or [email protected].
References
1. Understanding the Epidemic: Drug overdose deaths in the United States continue to increase in 2015. Accessed Sept. 11, 2017.
2. Behavioral health trends in the United States: Results from the 2014 national survey on drug use and health. Accessed Sept. 11, 2017.
3. Jones CM. Heroin use and heroin use risk behaviors among nonmedical users of prescription opioid pain relievers – United States, 2002-2004 and 2008-2010. Drug Alcohol Depend. 2013;132(1-2):95-100.
4. Rudd RA et al. Increases in drug and opioid-involved overdose deaths – United States, 2010-2015. MMWR Morb Mortal Wkly Rep. 2016;65(50-51):1445-52.
5. Highlights of the 2011 Drug Abuse Warning Network (DAWN) findings on drug-related emergency department visits. Accessed Sept. 11, 2017.
The United States is in the midst of a public health crisis. Every day, 91 Americans die from opioid overdoses.1 Opioid addiction has a tremendous negative effect on parents and children by destroying lives and breaking up families. When used appropriately, these drugs, such as morphine, hydrocodone, oxycodone, and fentanyl, provide much needed pain relief to patients, especially after a surgical procedure or during treatment for cancer. Unfortunately, opioids also have qualities that make them addictive and prone to overuse and abuse.
Heroin and other opioid drugs are affecting social, health, and economic welfare in communities throughout the United States. Opioid addiction does not discriminate among occupations, socioeconomic statuses, or ethnicities. Once addicted, users find it difficult to fight and overcome the habit. In this context, hospitalists who develop and use opioid policies and tactics are better equipped to deal with the increasing prevalence of opioid addiction and overdoses seen in the medical center environment.
Overview of opioid addiction
Opioids are more prevalent and easily available today in part because of the intense marketing campaigns by pharmaceutical companies. In fact, according to the 2014 National Survey on Drug Use and Health, almost 2 million Americans were dependent on or abused prescription opioids.2 Opioids create artificial endorphins in the brain, amplifying positive feelings and euphoria. Once they become dependent, patients feel sick and become depressed when they aren’t using narcotics. They experience uncontrollable cravings, relieved only by increasing opioid use. Personal relationships and finances are severely affected because patients will do whatever they can to acquire more opioids. Some resort to doctor shopping to obtain more opioids, while others will engage in drug trafficking to purchase and sell narcotics. Most new heroin users report that they started with nonmedical use of opioid pain relievers.3
Unfortunately, in 2015, there were more than 20,000 prescription opioid overdose deaths and almost 13,000 overdose deaths related to heroin.4 In addition to addiction and death, overuse of narcotics can cause many medical issues, including dizziness, constipation, depression, and immune dysfunction.
As with any addiction, there are many problems related to opiate withdrawal. Some of these include anxiety, rhinorrhea, lacrimation, piloerection, mydriasis, nausea, vomiting, abdominal pain, diarrhea, tachycardia, and hypertension. Methadone, buprenorphine, and extended-release naltrexone typically are used to help alleviate cravings and the symptoms of withdrawal. With more than 1,000 patients treated daily by emergency departments for misuse of prescription opioids, it’s imperative for the medical community to address this health care crisis.5
Impact on first responders and hospitalists
The impact of this epidemic on the medical community is dramatic. Emergency system resources, already on overload, are further taxed and drained by the increased 911 calls for overdose incidents. This means that instead of responding to heart attacks, strokes, or other emergencies, first responders are spending time stabilizing overdose patients and taking them to hospitals. This resource drain spreads to emergency rooms and hospitals as they treat these patients. Eventually, the epidemic results in higher insurance costs to cover the impact on medical resources.
Strategies for hospitalists
A multifaceted approach is required to combat this crisis, and hospitalists are one component of that solution. Here are strategies hospitalists can employ to combat the growing use and abuse of narcotics:
- Screen for high-risk conditions. Before prescribing opioids, screen patients for conditions that may be exacerbated by opioid use, including sleep apnea, obesity COPD, and heart failure, as well as whether they are using medications that cause sedation and respiratory depression.
- Develop and follow pain management clinical pathway protocols. For example, start with nonopioid analgesics, such as acetaminophen, ibuprofen, and naproxen, for patients with mild pain. If the patient is in moderate pain, or if the mild pain was unrelieved by the first type of medicine, continue with opioid analgesics including codeine, oxycodone, hydrocodone, or morphine. Finally, if the patient is experiencing severe pain, or if the mild to moderate pain was unrelieved by previous medications, prescribe higher doses of morphine, fentanyl, or hydromorphone or use a patient-controlled analgesia delivery of an intravenous opioid.
- Discuss other treatment options. Instead of prescribing narcotics, assess whether other treatment options are viable. These include physical and occupational therapy, steroid shots, massage, local nerve blocks, and muscle relaxers.
- Evaluate the reason(s) for current use of prescribed narcotics. It’s crucial to determine why patients are using opioids. The medical condition for which they were prescribed these medications may have resolved. If patients are using opioids without a medically indicated reason, offer alternative medications, such as methadone or naltrexone, and provide education to help them slowly taper off the drugs. Do not cease opioid use altogether unless the use is contraindicated because of other medical conditions or unstable vital signs (for example, low blood pressure).
- Train nurses on opioid use and addiction. Educate nurses about opioid addiction risk factors and symptoms of addiction. Instruct them on managing the Ramsay Sedation Scale and using an opioid sedation scale with patients receiving IV narcotics.
- Educate families on opioid use and addiction. Counsel patients and their families on the long-term effects of opioid use and about the warning signs of addiction. Teach them about alternatives to narcotics.
- Offer referrals to specialized clinics. Work with social workers, case managers, and local mental health professionals to refer patients to behavioral counseling, patient and family support groups, and monitored detoxification clinics qualified to treat opioid addiction.
Socioeconomic impact
The effects of the opioid epidemic are felt in all areas of the United States, especially in the health care industry. Emergency department visits are mounting while billions of dollars are spent on medical care for those addicted to opioids. Additionally, the socioeconomic effects of this crisis contribute to increasing depression, anxiety, missed days of work or school, unemployment, drop-out rates, and loss of productivity among those addicted to opioids. Also, the epidemic is adversely affecting families, leading to increased divorce rates, single parent families, and child abuse and neglect. By creating strategies and protocols for medical staff, patients, and families affected by opioid use, addiction, or overdose, hospitalists can have a positive influence on patients’ lives and ultimately the opioid epidemic.
Dr. Kasarla is a hospitalist with Apogee Physicians at Parkway Surgical & Cardiovascular Hospital in Fort Worth, Tex. He also works for Texas Health Physicians Group at Texas Health Harris Methodist Hospital and Texas Health Harris Methodist Hospital Southwest in Fort Worth, as well as at Texas Health Harris Methodist Hospital in Azle, Tex.
You can contact him at [email protected] or [email protected].
References
1. Understanding the Epidemic: Drug overdose deaths in the United States continue to increase in 2015. Accessed Sept. 11, 2017.
2. Behavioral health trends in the United States: Results from the 2014 national survey on drug use and health. Accessed Sept. 11, 2017.
3. Jones CM. Heroin use and heroin use risk behaviors among nonmedical users of prescription opioid pain relievers – United States, 2002-2004 and 2008-2010. Drug Alcohol Depend. 2013;132(1-2):95-100.
4. Rudd RA et al. Increases in drug and opioid-involved overdose deaths – United States, 2010-2015. MMWR Morb Mortal Wkly Rep. 2016;65(50-51):1445-52.
5. Highlights of the 2011 Drug Abuse Warning Network (DAWN) findings on drug-related emergency department visits. Accessed Sept. 11, 2017.
The United States is in the midst of a public health crisis. Every day, 91 Americans die from opioid overdoses.1 Opioid addiction has a tremendous negative effect on parents and children by destroying lives and breaking up families. When used appropriately, these drugs, such as morphine, hydrocodone, oxycodone, and fentanyl, provide much needed pain relief to patients, especially after a surgical procedure or during treatment for cancer. Unfortunately, opioids also have qualities that make them addictive and prone to overuse and abuse.
Heroin and other opioid drugs are affecting social, health, and economic welfare in communities throughout the United States. Opioid addiction does not discriminate among occupations, socioeconomic statuses, or ethnicities. Once addicted, users find it difficult to fight and overcome the habit. In this context, hospitalists who develop and use opioid policies and tactics are better equipped to deal with the increasing prevalence of opioid addiction and overdoses seen in the medical center environment.
Overview of opioid addiction
Opioids are more prevalent and easily available today in part because of the intense marketing campaigns by pharmaceutical companies. In fact, according to the 2014 National Survey on Drug Use and Health, almost 2 million Americans were dependent on or abused prescription opioids.2 Opioids create artificial endorphins in the brain, amplifying positive feelings and euphoria. Once they become dependent, patients feel sick and become depressed when they aren’t using narcotics. They experience uncontrollable cravings, relieved only by increasing opioid use. Personal relationships and finances are severely affected because patients will do whatever they can to acquire more opioids. Some resort to doctor shopping to obtain more opioids, while others will engage in drug trafficking to purchase and sell narcotics. Most new heroin users report that they started with nonmedical use of opioid pain relievers.3
Unfortunately, in 2015, there were more than 20,000 prescription opioid overdose deaths and almost 13,000 overdose deaths related to heroin.4 In addition to addiction and death, overuse of narcotics can cause many medical issues, including dizziness, constipation, depression, and immune dysfunction.
As with any addiction, there are many problems related to opiate withdrawal. Some of these include anxiety, rhinorrhea, lacrimation, piloerection, mydriasis, nausea, vomiting, abdominal pain, diarrhea, tachycardia, and hypertension. Methadone, buprenorphine, and extended-release naltrexone typically are used to help alleviate cravings and the symptoms of withdrawal. With more than 1,000 patients treated daily by emergency departments for misuse of prescription opioids, it’s imperative for the medical community to address this health care crisis.5
Impact on first responders and hospitalists
The impact of this epidemic on the medical community is dramatic. Emergency system resources, already on overload, are further taxed and drained by the increased 911 calls for overdose incidents. This means that instead of responding to heart attacks, strokes, or other emergencies, first responders are spending time stabilizing overdose patients and taking them to hospitals. This resource drain spreads to emergency rooms and hospitals as they treat these patients. Eventually, the epidemic results in higher insurance costs to cover the impact on medical resources.
Strategies for hospitalists
A multifaceted approach is required to combat this crisis, and hospitalists are one component of that solution. Here are strategies hospitalists can employ to combat the growing use and abuse of narcotics:
- Screen for high-risk conditions. Before prescribing opioids, screen patients for conditions that may be exacerbated by opioid use, including sleep apnea, obesity COPD, and heart failure, as well as whether they are using medications that cause sedation and respiratory depression.
- Develop and follow pain management clinical pathway protocols. For example, start with nonopioid analgesics, such as acetaminophen, ibuprofen, and naproxen, for patients with mild pain. If the patient is in moderate pain, or if the mild pain was unrelieved by the first type of medicine, continue with opioid analgesics including codeine, oxycodone, hydrocodone, or morphine. Finally, if the patient is experiencing severe pain, or if the mild to moderate pain was unrelieved by previous medications, prescribe higher doses of morphine, fentanyl, or hydromorphone or use a patient-controlled analgesia delivery of an intravenous opioid.
- Discuss other treatment options. Instead of prescribing narcotics, assess whether other treatment options are viable. These include physical and occupational therapy, steroid shots, massage, local nerve blocks, and muscle relaxers.
- Evaluate the reason(s) for current use of prescribed narcotics. It’s crucial to determine why patients are using opioids. The medical condition for which they were prescribed these medications may have resolved. If patients are using opioids without a medically indicated reason, offer alternative medications, such as methadone or naltrexone, and provide education to help them slowly taper off the drugs. Do not cease opioid use altogether unless the use is contraindicated because of other medical conditions or unstable vital signs (for example, low blood pressure).
- Train nurses on opioid use and addiction. Educate nurses about opioid addiction risk factors and symptoms of addiction. Instruct them on managing the Ramsay Sedation Scale and using an opioid sedation scale with patients receiving IV narcotics.
- Educate families on opioid use and addiction. Counsel patients and their families on the long-term effects of opioid use and about the warning signs of addiction. Teach them about alternatives to narcotics.
- Offer referrals to specialized clinics. Work with social workers, case managers, and local mental health professionals to refer patients to behavioral counseling, patient and family support groups, and monitored detoxification clinics qualified to treat opioid addiction.
Socioeconomic impact
The effects of the opioid epidemic are felt in all areas of the United States, especially in the health care industry. Emergency department visits are mounting while billions of dollars are spent on medical care for those addicted to opioids. Additionally, the socioeconomic effects of this crisis contribute to increasing depression, anxiety, missed days of work or school, unemployment, drop-out rates, and loss of productivity among those addicted to opioids. Also, the epidemic is adversely affecting families, leading to increased divorce rates, single parent families, and child abuse and neglect. By creating strategies and protocols for medical staff, patients, and families affected by opioid use, addiction, or overdose, hospitalists can have a positive influence on patients’ lives and ultimately the opioid epidemic.
Dr. Kasarla is a hospitalist with Apogee Physicians at Parkway Surgical & Cardiovascular Hospital in Fort Worth, Tex. He also works for Texas Health Physicians Group at Texas Health Harris Methodist Hospital and Texas Health Harris Methodist Hospital Southwest in Fort Worth, as well as at Texas Health Harris Methodist Hospital in Azle, Tex.
You can contact him at [email protected] or [email protected].
References
1. Understanding the Epidemic: Drug overdose deaths in the United States continue to increase in 2015. Accessed Sept. 11, 2017.
2. Behavioral health trends in the United States: Results from the 2014 national survey on drug use and health. Accessed Sept. 11, 2017.
3. Jones CM. Heroin use and heroin use risk behaviors among nonmedical users of prescription opioid pain relievers – United States, 2002-2004 and 2008-2010. Drug Alcohol Depend. 2013;132(1-2):95-100.
4. Rudd RA et al. Increases in drug and opioid-involved overdose deaths – United States, 2010-2015. MMWR Morb Mortal Wkly Rep. 2016;65(50-51):1445-52.
5. Highlights of the 2011 Drug Abuse Warning Network (DAWN) findings on drug-related emergency department visits. Accessed Sept. 11, 2017.
When do patients with SSTIs require hospital admission and IV antibiotics?
Case
A 54-year old gentleman with a history of type 2 diabetes mellitus presents with several days of progressive left lower extremity redness, pain, swelling, and subjective fevers.
On physical examination the patient is afebrile and hemodynamically stable. The left lower extremity is swollen, warm, and tender to light palpation with an irregular area of erythema extending anteriorly from the ankle to just below the knee. There are no areas of purulence or fluctuance. Labs are notable for a mild leukocytosis of 11,500 cells/mcL. An ultrasound shows no evidence of deep vein thrombosis and the patient is started on vancomycin and ceftazidime and admitted for intravenous antibiotics.
Does the patient require hospital admission and continuation of intravenous antibiotics?
Introduction
The clinical presentation of SSTIs can vary greatly. Consequently, the management of SSTIs can be as simple as a short course of outpatient oral antibiotics or escalate to as complicated as surgical intervention and/or prolonged courses of IV antibiotics. Given the frequency with which these infections result in hospital admission, it is essential for the practicing hospitalist to be able to appropriately triage and treat SSTIs in order to assure adequate therapy, while simultaneously reducing unnecessary hospital days and avoiding indiscriminate exposure to broad spectrum antibiotics.
Pathophysiology and clinical presentation
SSTIs represent a diverse range of presentations and severities from superficial impetigo to life-threatening necrotizing infections, with abscesses and cellulitis being most commonly diagnosed.1
All SSTIs emerge from microbial invasion of the layers of the skin and underlying soft tissues. The accepted minimal criteria for diagnosis of an SSTI are erythema, edema, and warmth and tenderness of the affected area. Comorbid conditions that impair skin integrity, such as lymphedema, chronic inflammation (for example, eczema), intertrigo, or venous insufficiency therefore increase the risk of infection. However, the strongest risk factor for development of an SSTI is disruption of the skin barrier via trauma (foreign body, bite wound), ulceration, laceration, fissures, or surgical wound.2,3
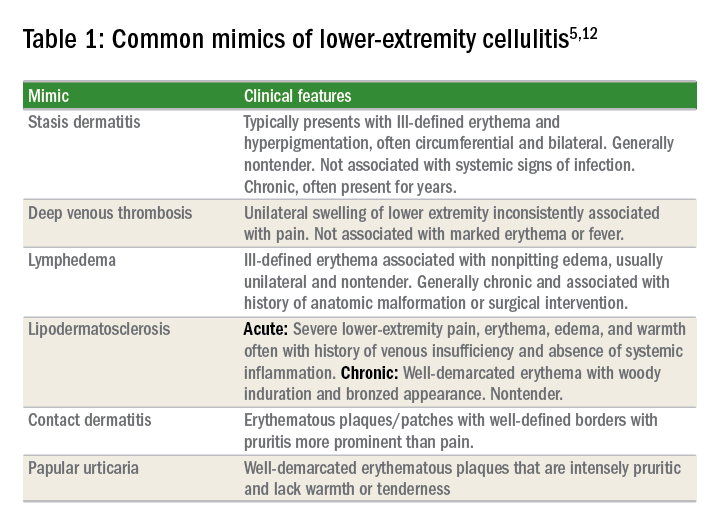
The hallmark features of SSTI are present in other noninfectious skin disorders, thus often yielding misdiagnosis. In a study of 259 patients hospitalized for lower extremity cellulitis, 79 patients (30.5%) were misdiagnosed.4 The most common mimic of SSTI is stasis dermatitis due to chronic venous insufficiency. Other conditions that are often misdiagnosed as SSTI include lymphedema, lipodermatosclerosis, contact dermatitis, papular urticaria and deep venous thrombosis. Differentiating between true SSTI and these “pseudo-cellulitic” conditions is essential to reducing unnecessary hospitalization and exposure to antibiotics, which contribute to nosocomial infection, iatrogenic injury (that is, Clostridium difficile infection, anaphylaxis) and avoidable health care costs.
Microbiology
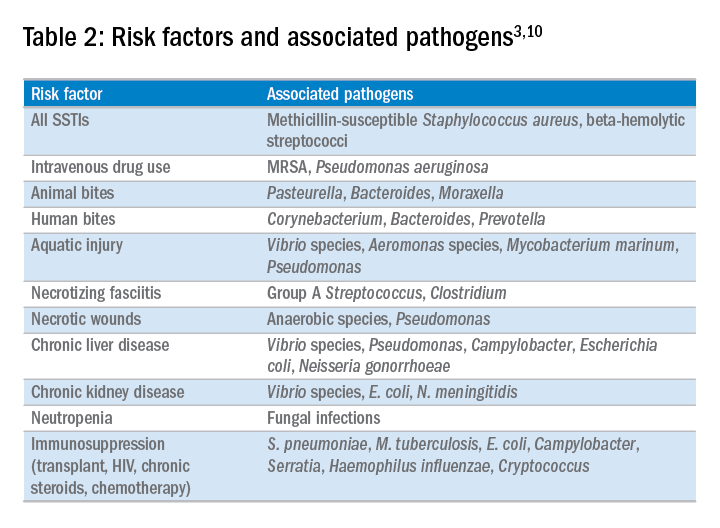
In the majority of cases, the causative pathogen is not identified; superficial culture data is often confounded and positive results do not guarantee pathogenicity of the identified organism. However, the mechanism of bacterial entry, location of infection, and presence of underlying medical conditions also influence the infectious organism(s). For example, infections of the lower extremities may involve enteric organisms such as E. coli and Enterococcus due to fecal runoff. SSTIs due to cat and dog bites commonly involve Pasteurella multocida, while hot tub exposure and intravenous drug use increase the risk of infection with Pseudomonas aeruginosa. Patients with neutropenia are at increased risk for fungal and yeast infections. Consequently, an assessment for potential risk factors is essential in determining appropriate management. Common pathogens associated with various clinical presentations and risk factors are outlined in Table 2.
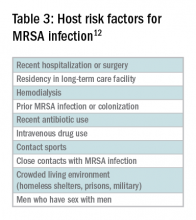
In addition to host risk factors, the type of SSTI may hint at the most likely organisms. Among purulent (“culturable”) SSTIs, up to 76% of infections are due to S. aureus, whereas in diffuse (“nonculturable”) cellulitis, the majority of cases are attributable to B-hemolytic streptococcus.7 The role of S. aureus in SSTIs is further complicated by the rise of methicillin-resistant S. aureus (MRSA), both nosocomial and community-acquired. It is estimated that between 25%-50% of all S. aureus isolates in the United States show methicillin resistance.6,8 Despite the rising prevalence of MRSA, reflexive treatment for MRSA should be avoided in the absence of high-risk presentations (for example, purulent SSTI) or patient risk factors for MRSA (Table 3).
Severity of infection
Given the variety of clinical presentations of SSTIs, an evaluation of the severity of disease is essential to determining appropriate initial management, including the need for hospitalization and intravenous antibiotics. Several grading systems have been proposed to assist in determining severity. High-risk features that are common to these systems include:
• Evidence of systemic infection (fever, tachycardia, altered mental status, tachypnea, hypotension);
• Location of infection with increased risk of local complication (face, brain, hand, perineum);
• Indication of deep tissue infection (for example, crepitus, bullae, or hemorrhage);
• Comorbid conditions predisposing to more severe infection (liver or renal disease, immunocompromised state including neutropenia or active chemotherapy, vascular insufficiency).
In assessing for necrotic infection, the Laboratory Risk Indicator for Necrotizing Fasciitis (LRINEC) score can help to distinguish severe cellulitis from necrotizing infections that require immediate surgical evaluation. The LRINEC score uses readily available laboratory markers to stratify patients into tertiles of risk for necrotizing fasciitis. While this objective score can identify patients who may require immediate surgical intervention, any patient with a clinical history or exam concerning for necrotizing infection should be urgently evaluated for possible surgical debridement.11,12
Management
Nonpurulent disease
Nonpurulent SSTIs include cellulitis and necrotizing infections such as necrotizing fasciitis. In the absence of risk factors for particular infectious agents (see above), mild infections can be managed with a trial of oral antibiotics with coverage for streptococcal species such as cephalexin, clindamycin, or amoxicillin-clavulanate.
Empiric coverage for MRSA is not recommended and has been shown to have little benefit. In a trial of 146 patients with mild nonpurulent cellulitis, there was no significant difference in cure rate at 2 weeks between cephalexin monotherapy and dual therapy with cephalexin and trimethoprim-sulfamethoxazole.13
Moderate infections warrant admission for intravenous antibiotics, also with coverage for streptococcal species and MSSA such as penicillin or cefazolin. In most cases, coverage for MRSA is not required but may be considered in patients with risk factors for MRSA. Generally, blood or cutaneous cultures are not recommended given an expected yield of positive culture to be less than 5%,14 but may be considered in patients with immunosuppression or neutropenia or evidence of systemic inflammatory response.
Severe infections should be evaluated for the need for surgical debridement. Empiric antibiotic coverage for Streptococcus pyogenes, MRSA, and gram-negative and anaerobic species is warranted. If necrotizing infection is suspected or diagnosed, immediate surgical debridement is indicated. Culture data from surgical debridement should be obtained and can be useful for tailoring therapy. While blood cultures remain unlikely to yield useful data, it is reasonable to obtain them in severe disease. Empiric antibiotic coverage should be broad and narrowed based on surgical specimens. The general recommendation regarding duration of antibiotics is 5 days; however, longer courses of up to 10-14 days may be required if there is minimal improvement after initial therapy.9
Purulent disease
Purulent SSTIs by definition involve collections of pus and include abscesses, furuncles, and carbuncles. In all purulent SSTIs, incision and drainage is indicated.
For mild disease, incision and drainage is considered definitive management and deep wound cultures and antibiotics are not required. In moderate purulent SSTI, culture of the drained fluid should be obtained, and antibiotics administered with empiric therapy to include coverage for MRSA. Patients at risk for community-acquired MRSA can be given oral agents such as trimethoprim-sulfamethoxazole, clindamycin, or doxycycline (depending upon local antibiogram data) with antibiotics narrowed based on culture data.
In order to minimize treatment failure with oral agents, dosing should be weight based with a minimum of 5 mg/kg per day of bactrim or 10 mg/kg per day of clindamycin.15 Those with risk factors for nosocomial MRSA may warrant intravenous antibiotics, even in moderate disease. Patients with severe purulent disease require intravenous antibiotics with coverage for MRSA with vancomycin, daptomycin or linezolid and subsequently narrowed based on culture data.9
Back to the case
Our patient presented with a case of mild, nonpurulent cellulitis. While he does have a mildly elevated white blood cell count, he has no other signs of systemic infection or underlying conditions predisposing to more severe disease. Hospital admission is not required and de-escalation of antibiotics to an oral agent is appropriate.
If the patient exhibited other signs of systemic infection (that is, fever or tachycardia) hospital admission or admission to observation status for IV antibiotics would be appropriate; however, de-escalation would still be recommended as MRSA coverage is not warranted. We suggest discharge from the emergency department with oral cephalexin, provided no prohibitive allergy is known, with outpatient follow-up to ensure resolution of infection.
Bottom line
SSTIs encompass a wide variety of clinical presentations and severity, and can be mimicked by a number of noninfectious medical conditions. If an infectious process is considered most likely, the need for hospitalization and broad-spectrum antibiotics should be individually determined based on specific criteria, and not empirically initiated for all presentations.
Dr. Perry is an instructor in the Yale Academic Hospitalist Program at Yale University, New Haven, Conn. Dr. Fogerty is associate professor of medicine in the Yale Academic Hospitalist Program. Dr. Sankey is assistant professor of medicine and interim inpatient medicine clerkship director in the Yale Academic Hospitalist Program.
Key Points
• SSTIs encompass a wide variety of clinical presentations and severity, and can be mimicked by a number of noninfectious medical conditions
• The majority of SSTIs are caused by gram-positive organisms, most notably Staphylococcus aureus and B-hemolytic streptococci
• Evaluation of the severity of disease, using a grading system, is essential to determining appropriate initial management
• Hospitalization and broad-spectrum antibiotics should be individually determined based on specific criteria, not empirically initiated for all presentations
References
1. Miller L, Eisenberg D, Liu H, et al. Incidence of skin and soft tissue infections in ambulatory and inpatient settings, 2005-2010. BMC Infect Dis. 2015 Aug 21;15:362.
2. Dupuy A, Benchikhi H, Roujeau JC, et al. Risk factors for erysipelas of the leg (cellulitis): case control study. BMJ. 1999;318(7198):1591-4.
3. Ki V, Rotstein C. Bacterial skin and soft tissue infections in adults: A review of their epidemiology, pathogenesis, diagnosis, treatment and site of care. Can J Infect Dis Med Microbiol. 2008 Mar;19(2):173-84.
4. Weng QY, Raff AB, Cohen JM, et al. Costs and Consequences Associated with Misdiagnosed Lower Extremity Cellulitis. JAMA Dermatol. 2016 Nov 2.
5. Keller E, Tomecki K, Alraies M. Distinguishing cellulitis from its mimics. Cleve Clin J Med. 2012 Aug;79(8)547-52.
6. Bassetti, M, Carmelutti A, Righi E. The role of methicillin-resistant Staphylococcus aureus in skin and soft tissue infections. Curr Opin Infect Dis. 2017 Apr.
7. Jeng A, Beheshti M, Li J, Ramesh N. The Role of B-hemolytic Streptococci in Causing Diffuse, Nonculturable Cellulitis. Medicine (Baltimore). 2010 Jul;89(4):217-26.
8. Moran G, Krishnadasan A, Gorwitz R. Methicillin-Resistant S. aureus Infections among Patients in the Emergency Department. N Engl J Med. 2006; 355:666-74.
9. Stevens D, Bisno A, Chambers H, et al. Practice Guidelines for the Diagnosis and Management of Skin and Soft tissue Infections: 2014 Update by the Infectious Disease Society of America. Clin Infect Dis. 2014 Jul 15;59(2):e10-52.
10. Raff, AB and Kroshinsky D. Cellulitis: A Review. JAMA. 2016 Jul;316(3):325-37.
11. Wong, CH, Khin LW, Heng KS, Tan KC, Low CO. The LRINEC (Laboratory Risk Indicator for Necrotizing Fasciitis) score: a tool for distinguishing necrotizing fasciitis from other soft tissue infections. Crit Care Med. 2004 Jul;32(7):1535-41.
12. Gunderson CG. Cellulitis: Definition, etiology and clinical features. Am J Med 2011 Dec;124(12):1113-22.
13. Pallin D, et al. Comparative Effectiveness of Cephalexin Plus Trimethoprim-Sulfamethoxazole versus Cephalexin Alone for Treatment of Uncomplicated Cellulitis: A Randomized Controlled Trial. Clin Infect Dis. 2013 Jun;56(12):1754-62.
14. Gunderson CG, Martinello RA. A systematic review of bacteremias in cellulitis and erysipelas. J Infect. 2012;64(2):148-55.
15. Halilovic J, Heintz BH, Brown J.. Risk factors for clinical failure in patients hospitalized with cellulitis and cutaneous abscess. J Infect. 2012;65(2):128-34.
Case
A 54-year old gentleman with a history of type 2 diabetes mellitus presents with several days of progressive left lower extremity redness, pain, swelling, and subjective fevers.
On physical examination the patient is afebrile and hemodynamically stable. The left lower extremity is swollen, warm, and tender to light palpation with an irregular area of erythema extending anteriorly from the ankle to just below the knee. There are no areas of purulence or fluctuance. Labs are notable for a mild leukocytosis of 11,500 cells/mcL. An ultrasound shows no evidence of deep vein thrombosis and the patient is started on vancomycin and ceftazidime and admitted for intravenous antibiotics.
Does the patient require hospital admission and continuation of intravenous antibiotics?
Introduction
The clinical presentation of SSTIs can vary greatly. Consequently, the management of SSTIs can be as simple as a short course of outpatient oral antibiotics or escalate to as complicated as surgical intervention and/or prolonged courses of IV antibiotics. Given the frequency with which these infections result in hospital admission, it is essential for the practicing hospitalist to be able to appropriately triage and treat SSTIs in order to assure adequate therapy, while simultaneously reducing unnecessary hospital days and avoiding indiscriminate exposure to broad spectrum antibiotics.
Pathophysiology and clinical presentation
SSTIs represent a diverse range of presentations and severities from superficial impetigo to life-threatening necrotizing infections, with abscesses and cellulitis being most commonly diagnosed.1
All SSTIs emerge from microbial invasion of the layers of the skin and underlying soft tissues. The accepted minimal criteria for diagnosis of an SSTI are erythema, edema, and warmth and tenderness of the affected area. Comorbid conditions that impair skin integrity, such as lymphedema, chronic inflammation (for example, eczema), intertrigo, or venous insufficiency therefore increase the risk of infection. However, the strongest risk factor for development of an SSTI is disruption of the skin barrier via trauma (foreign body, bite wound), ulceration, laceration, fissures, or surgical wound.2,3
The hallmark features of SSTI are present in other noninfectious skin disorders, thus often yielding misdiagnosis. In a study of 259 patients hospitalized for lower extremity cellulitis, 79 patients (30.5%) were misdiagnosed.4 The most common mimic of SSTI is stasis dermatitis due to chronic venous insufficiency. Other conditions that are often misdiagnosed as SSTI include lymphedema, lipodermatosclerosis, contact dermatitis, papular urticaria and deep venous thrombosis. Differentiating between true SSTI and these “pseudo-cellulitic” conditions is essential to reducing unnecessary hospitalization and exposure to antibiotics, which contribute to nosocomial infection, iatrogenic injury (that is, Clostridium difficile infection, anaphylaxis) and avoidable health care costs.
Microbiology
In the majority of cases, the causative pathogen is not identified; superficial culture data is often confounded and positive results do not guarantee pathogenicity of the identified organism. However, the mechanism of bacterial entry, location of infection, and presence of underlying medical conditions also influence the infectious organism(s). For example, infections of the lower extremities may involve enteric organisms such as E. coli and Enterococcus due to fecal runoff. SSTIs due to cat and dog bites commonly involve Pasteurella multocida, while hot tub exposure and intravenous drug use increase the risk of infection with Pseudomonas aeruginosa. Patients with neutropenia are at increased risk for fungal and yeast infections. Consequently, an assessment for potential risk factors is essential in determining appropriate management. Common pathogens associated with various clinical presentations and risk factors are outlined in Table 2.
In addition to host risk factors, the type of SSTI may hint at the most likely organisms. Among purulent (“culturable”) SSTIs, up to 76% of infections are due to S. aureus, whereas in diffuse (“nonculturable”) cellulitis, the majority of cases are attributable to B-hemolytic streptococcus.7 The role of S. aureus in SSTIs is further complicated by the rise of methicillin-resistant S. aureus (MRSA), both nosocomial and community-acquired. It is estimated that between 25%-50% of all S. aureus isolates in the United States show methicillin resistance.6,8 Despite the rising prevalence of MRSA, reflexive treatment for MRSA should be avoided in the absence of high-risk presentations (for example, purulent SSTI) or patient risk factors for MRSA (Table 3).
Severity of infection
Given the variety of clinical presentations of SSTIs, an evaluation of the severity of disease is essential to determining appropriate initial management, including the need for hospitalization and intravenous antibiotics. Several grading systems have been proposed to assist in determining severity. High-risk features that are common to these systems include:
• Evidence of systemic infection (fever, tachycardia, altered mental status, tachypnea, hypotension);
• Location of infection with increased risk of local complication (face, brain, hand, perineum);
• Indication of deep tissue infection (for example, crepitus, bullae, or hemorrhage);
• Comorbid conditions predisposing to more severe infection (liver or renal disease, immunocompromised state including neutropenia or active chemotherapy, vascular insufficiency).
In assessing for necrotic infection, the Laboratory Risk Indicator for Necrotizing Fasciitis (LRINEC) score can help to distinguish severe cellulitis from necrotizing infections that require immediate surgical evaluation. The LRINEC score uses readily available laboratory markers to stratify patients into tertiles of risk for necrotizing fasciitis. While this objective score can identify patients who may require immediate surgical intervention, any patient with a clinical history or exam concerning for necrotizing infection should be urgently evaluated for possible surgical debridement.11,12
Management
Nonpurulent disease
Nonpurulent SSTIs include cellulitis and necrotizing infections such as necrotizing fasciitis. In the absence of risk factors for particular infectious agents (see above), mild infections can be managed with a trial of oral antibiotics with coverage for streptococcal species such as cephalexin, clindamycin, or amoxicillin-clavulanate.
Empiric coverage for MRSA is not recommended and has been shown to have little benefit. In a trial of 146 patients with mild nonpurulent cellulitis, there was no significant difference in cure rate at 2 weeks between cephalexin monotherapy and dual therapy with cephalexin and trimethoprim-sulfamethoxazole.13
Moderate infections warrant admission for intravenous antibiotics, also with coverage for streptococcal species and MSSA such as penicillin or cefazolin. In most cases, coverage for MRSA is not required but may be considered in patients with risk factors for MRSA. Generally, blood or cutaneous cultures are not recommended given an expected yield of positive culture to be less than 5%,14 but may be considered in patients with immunosuppression or neutropenia or evidence of systemic inflammatory response.
Severe infections should be evaluated for the need for surgical debridement. Empiric antibiotic coverage for Streptococcus pyogenes, MRSA, and gram-negative and anaerobic species is warranted. If necrotizing infection is suspected or diagnosed, immediate surgical debridement is indicated. Culture data from surgical debridement should be obtained and can be useful for tailoring therapy. While blood cultures remain unlikely to yield useful data, it is reasonable to obtain them in severe disease. Empiric antibiotic coverage should be broad and narrowed based on surgical specimens. The general recommendation regarding duration of antibiotics is 5 days; however, longer courses of up to 10-14 days may be required if there is minimal improvement after initial therapy.9
Purulent disease
Purulent SSTIs by definition involve collections of pus and include abscesses, furuncles, and carbuncles. In all purulent SSTIs, incision and drainage is indicated.
For mild disease, incision and drainage is considered definitive management and deep wound cultures and antibiotics are not required. In moderate purulent SSTI, culture of the drained fluid should be obtained, and antibiotics administered with empiric therapy to include coverage for MRSA. Patients at risk for community-acquired MRSA can be given oral agents such as trimethoprim-sulfamethoxazole, clindamycin, or doxycycline (depending upon local antibiogram data) with antibiotics narrowed based on culture data.
In order to minimize treatment failure with oral agents, dosing should be weight based with a minimum of 5 mg/kg per day of bactrim or 10 mg/kg per day of clindamycin.15 Those with risk factors for nosocomial MRSA may warrant intravenous antibiotics, even in moderate disease. Patients with severe purulent disease require intravenous antibiotics with coverage for MRSA with vancomycin, daptomycin or linezolid and subsequently narrowed based on culture data.9
Back to the case
Our patient presented with a case of mild, nonpurulent cellulitis. While he does have a mildly elevated white blood cell count, he has no other signs of systemic infection or underlying conditions predisposing to more severe disease. Hospital admission is not required and de-escalation of antibiotics to an oral agent is appropriate.
If the patient exhibited other signs of systemic infection (that is, fever or tachycardia) hospital admission or admission to observation status for IV antibiotics would be appropriate; however, de-escalation would still be recommended as MRSA coverage is not warranted. We suggest discharge from the emergency department with oral cephalexin, provided no prohibitive allergy is known, with outpatient follow-up to ensure resolution of infection.
Bottom line
SSTIs encompass a wide variety of clinical presentations and severity, and can be mimicked by a number of noninfectious medical conditions. If an infectious process is considered most likely, the need for hospitalization and broad-spectrum antibiotics should be individually determined based on specific criteria, and not empirically initiated for all presentations.
Dr. Perry is an instructor in the Yale Academic Hospitalist Program at Yale University, New Haven, Conn. Dr. Fogerty is associate professor of medicine in the Yale Academic Hospitalist Program. Dr. Sankey is assistant professor of medicine and interim inpatient medicine clerkship director in the Yale Academic Hospitalist Program.
Key Points
• SSTIs encompass a wide variety of clinical presentations and severity, and can be mimicked by a number of noninfectious medical conditions
• The majority of SSTIs are caused by gram-positive organisms, most notably Staphylococcus aureus and B-hemolytic streptococci
• Evaluation of the severity of disease, using a grading system, is essential to determining appropriate initial management
• Hospitalization and broad-spectrum antibiotics should be individually determined based on specific criteria, not empirically initiated for all presentations


References
1. Miller L, Eisenberg D, Liu H, et al. Incidence of skin and soft tissue infections in ambulatory and inpatient settings, 2005-2010. BMC Infect Dis. 2015 Aug 21;15:362.
2. Dupuy A, Benchikhi H, Roujeau JC, et al. Risk factors for erysipelas of the leg (cellulitis): case control study. BMJ. 1999;318(7198):1591-4.
3. Ki V, Rotstein C. Bacterial skin and soft tissue infections in adults: A review of their epidemiology, pathogenesis, diagnosis, treatment and site of care. Can J Infect Dis Med Microbiol. 2008 Mar;19(2):173-84.
4. Weng QY, Raff AB, Cohen JM, et al. Costs and Consequences Associated with Misdiagnosed Lower Extremity Cellulitis. JAMA Dermatol. 2016 Nov 2.
5. Keller E, Tomecki K, Alraies M. Distinguishing cellulitis from its mimics. Cleve Clin J Med. 2012 Aug;79(8)547-52.
6. Bassetti, M, Carmelutti A, Righi E. The role of methicillin-resistant Staphylococcus aureus in skin and soft tissue infections. Curr Opin Infect Dis. 2017 Apr.
7. Jeng A, Beheshti M, Li J, Ramesh N. The Role of B-hemolytic Streptococci in Causing Diffuse, Nonculturable Cellulitis. Medicine (Baltimore). 2010 Jul;89(4):217-26.
8. Moran G, Krishnadasan A, Gorwitz R. Methicillin-Resistant S. aureus Infections among Patients in the Emergency Department. N Engl J Med. 2006; 355:666-74.
9. Stevens D, Bisno A, Chambers H, et al. Practice Guidelines for the Diagnosis and Management of Skin and Soft tissue Infections: 2014 Update by the Infectious Disease Society of America. Clin Infect Dis. 2014 Jul 15;59(2):e10-52.
10. Raff, AB and Kroshinsky D. Cellulitis: A Review. JAMA. 2016 Jul;316(3):325-37.
11. Wong, CH, Khin LW, Heng KS, Tan KC, Low CO. The LRINEC (Laboratory Risk Indicator for Necrotizing Fasciitis) score: a tool for distinguishing necrotizing fasciitis from other soft tissue infections. Crit Care Med. 2004 Jul;32(7):1535-41.
12. Gunderson CG. Cellulitis: Definition, etiology and clinical features. Am J Med 2011 Dec;124(12):1113-22.
13. Pallin D, et al. Comparative Effectiveness of Cephalexin Plus Trimethoprim-Sulfamethoxazole versus Cephalexin Alone for Treatment of Uncomplicated Cellulitis: A Randomized Controlled Trial. Clin Infect Dis. 2013 Jun;56(12):1754-62.
14. Gunderson CG, Martinello RA. A systematic review of bacteremias in cellulitis and erysipelas. J Infect. 2012;64(2):148-55.
15. Halilovic J, Heintz BH, Brown J.. Risk factors for clinical failure in patients hospitalized with cellulitis and cutaneous abscess. J Infect. 2012;65(2):128-34.
Case
A 54-year old gentleman with a history of type 2 diabetes mellitus presents with several days of progressive left lower extremity redness, pain, swelling, and subjective fevers.
On physical examination the patient is afebrile and hemodynamically stable. The left lower extremity is swollen, warm, and tender to light palpation with an irregular area of erythema extending anteriorly from the ankle to just below the knee. There are no areas of purulence or fluctuance. Labs are notable for a mild leukocytosis of 11,500 cells/mcL. An ultrasound shows no evidence of deep vein thrombosis and the patient is started on vancomycin and ceftazidime and admitted for intravenous antibiotics.
Does the patient require hospital admission and continuation of intravenous antibiotics?
Introduction
The clinical presentation of SSTIs can vary greatly. Consequently, the management of SSTIs can be as simple as a short course of outpatient oral antibiotics or escalate to as complicated as surgical intervention and/or prolonged courses of IV antibiotics. Given the frequency with which these infections result in hospital admission, it is essential for the practicing hospitalist to be able to appropriately triage and treat SSTIs in order to assure adequate therapy, while simultaneously reducing unnecessary hospital days and avoiding indiscriminate exposure to broad spectrum antibiotics.
Pathophysiology and clinical presentation
SSTIs represent a diverse range of presentations and severities from superficial impetigo to life-threatening necrotizing infections, with abscesses and cellulitis being most commonly diagnosed.1
All SSTIs emerge from microbial invasion of the layers of the skin and underlying soft tissues. The accepted minimal criteria for diagnosis of an SSTI are erythema, edema, and warmth and tenderness of the affected area. Comorbid conditions that impair skin integrity, such as lymphedema, chronic inflammation (for example, eczema), intertrigo, or venous insufficiency therefore increase the risk of infection. However, the strongest risk factor for development of an SSTI is disruption of the skin barrier via trauma (foreign body, bite wound), ulceration, laceration, fissures, or surgical wound.2,3
The hallmark features of SSTI are present in other noninfectious skin disorders, thus often yielding misdiagnosis. In a study of 259 patients hospitalized for lower extremity cellulitis, 79 patients (30.5%) were misdiagnosed.4 The most common mimic of SSTI is stasis dermatitis due to chronic venous insufficiency. Other conditions that are often misdiagnosed as SSTI include lymphedema, lipodermatosclerosis, contact dermatitis, papular urticaria and deep venous thrombosis. Differentiating between true SSTI and these “pseudo-cellulitic” conditions is essential to reducing unnecessary hospitalization and exposure to antibiotics, which contribute to nosocomial infection, iatrogenic injury (that is, Clostridium difficile infection, anaphylaxis) and avoidable health care costs.
Microbiology
In the majority of cases, the causative pathogen is not identified; superficial culture data is often confounded and positive results do not guarantee pathogenicity of the identified organism. However, the mechanism of bacterial entry, location of infection, and presence of underlying medical conditions also influence the infectious organism(s). For example, infections of the lower extremities may involve enteric organisms such as E. coli and Enterococcus due to fecal runoff. SSTIs due to cat and dog bites commonly involve Pasteurella multocida, while hot tub exposure and intravenous drug use increase the risk of infection with Pseudomonas aeruginosa. Patients with neutropenia are at increased risk for fungal and yeast infections. Consequently, an assessment for potential risk factors is essential in determining appropriate management. Common pathogens associated with various clinical presentations and risk factors are outlined in Table 2.
In addition to host risk factors, the type of SSTI may hint at the most likely organisms. Among purulent (“culturable”) SSTIs, up to 76% of infections are due to S. aureus, whereas in diffuse (“nonculturable”) cellulitis, the majority of cases are attributable to B-hemolytic streptococcus.7 The role of S. aureus in SSTIs is further complicated by the rise of methicillin-resistant S. aureus (MRSA), both nosocomial and community-acquired. It is estimated that between 25%-50% of all S. aureus isolates in the United States show methicillin resistance.6,8 Despite the rising prevalence of MRSA, reflexive treatment for MRSA should be avoided in the absence of high-risk presentations (for example, purulent SSTI) or patient risk factors for MRSA (Table 3).
Severity of infection
Given the variety of clinical presentations of SSTIs, an evaluation of the severity of disease is essential to determining appropriate initial management, including the need for hospitalization and intravenous antibiotics. Several grading systems have been proposed to assist in determining severity. High-risk features that are common to these systems include:
• Evidence of systemic infection (fever, tachycardia, altered mental status, tachypnea, hypotension);
• Location of infection with increased risk of local complication (face, brain, hand, perineum);
• Indication of deep tissue infection (for example, crepitus, bullae, or hemorrhage);
• Comorbid conditions predisposing to more severe infection (liver or renal disease, immunocompromised state including neutropenia or active chemotherapy, vascular insufficiency).
In assessing for necrotic infection, the Laboratory Risk Indicator for Necrotizing Fasciitis (LRINEC) score can help to distinguish severe cellulitis from necrotizing infections that require immediate surgical evaluation. The LRINEC score uses readily available laboratory markers to stratify patients into tertiles of risk for necrotizing fasciitis. While this objective score can identify patients who may require immediate surgical intervention, any patient with a clinical history or exam concerning for necrotizing infection should be urgently evaluated for possible surgical debridement.11,12
Management
Nonpurulent disease
Nonpurulent SSTIs include cellulitis and necrotizing infections such as necrotizing fasciitis. In the absence of risk factors for particular infectious agents (see above), mild infections can be managed with a trial of oral antibiotics with coverage for streptococcal species such as cephalexin, clindamycin, or amoxicillin-clavulanate.
Empiric coverage for MRSA is not recommended and has been shown to have little benefit. In a trial of 146 patients with mild nonpurulent cellulitis, there was no significant difference in cure rate at 2 weeks between cephalexin monotherapy and dual therapy with cephalexin and trimethoprim-sulfamethoxazole.13
Moderate infections warrant admission for intravenous antibiotics, also with coverage for streptococcal species and MSSA such as penicillin or cefazolin. In most cases, coverage for MRSA is not required but may be considered in patients with risk factors for MRSA. Generally, blood or cutaneous cultures are not recommended given an expected yield of positive culture to be less than 5%,14 but may be considered in patients with immunosuppression or neutropenia or evidence of systemic inflammatory response.
Severe infections should be evaluated for the need for surgical debridement. Empiric antibiotic coverage for Streptococcus pyogenes, MRSA, and gram-negative and anaerobic species is warranted. If necrotizing infection is suspected or diagnosed, immediate surgical debridement is indicated. Culture data from surgical debridement should be obtained and can be useful for tailoring therapy. While blood cultures remain unlikely to yield useful data, it is reasonable to obtain them in severe disease. Empiric antibiotic coverage should be broad and narrowed based on surgical specimens. The general recommendation regarding duration of antibiotics is 5 days; however, longer courses of up to 10-14 days may be required if there is minimal improvement after initial therapy.9
Purulent disease
Purulent SSTIs by definition involve collections of pus and include abscesses, furuncles, and carbuncles. In all purulent SSTIs, incision and drainage is indicated.
For mild disease, incision and drainage is considered definitive management and deep wound cultures and antibiotics are not required. In moderate purulent SSTI, culture of the drained fluid should be obtained, and antibiotics administered with empiric therapy to include coverage for MRSA. Patients at risk for community-acquired MRSA can be given oral agents such as trimethoprim-sulfamethoxazole, clindamycin, or doxycycline (depending upon local antibiogram data) with antibiotics narrowed based on culture data.
In order to minimize treatment failure with oral agents, dosing should be weight based with a minimum of 5 mg/kg per day of bactrim or 10 mg/kg per day of clindamycin.15 Those with risk factors for nosocomial MRSA may warrant intravenous antibiotics, even in moderate disease. Patients with severe purulent disease require intravenous antibiotics with coverage for MRSA with vancomycin, daptomycin or linezolid and subsequently narrowed based on culture data.9
Back to the case
Our patient presented with a case of mild, nonpurulent cellulitis. While he does have a mildly elevated white blood cell count, he has no other signs of systemic infection or underlying conditions predisposing to more severe disease. Hospital admission is not required and de-escalation of antibiotics to an oral agent is appropriate.
If the patient exhibited other signs of systemic infection (that is, fever or tachycardia) hospital admission or admission to observation status for IV antibiotics would be appropriate; however, de-escalation would still be recommended as MRSA coverage is not warranted. We suggest discharge from the emergency department with oral cephalexin, provided no prohibitive allergy is known, with outpatient follow-up to ensure resolution of infection.
Bottom line
SSTIs encompass a wide variety of clinical presentations and severity, and can be mimicked by a number of noninfectious medical conditions. If an infectious process is considered most likely, the need for hospitalization and broad-spectrum antibiotics should be individually determined based on specific criteria, and not empirically initiated for all presentations.
Dr. Perry is an instructor in the Yale Academic Hospitalist Program at Yale University, New Haven, Conn. Dr. Fogerty is associate professor of medicine in the Yale Academic Hospitalist Program. Dr. Sankey is assistant professor of medicine and interim inpatient medicine clerkship director in the Yale Academic Hospitalist Program.
Key Points
• SSTIs encompass a wide variety of clinical presentations and severity, and can be mimicked by a number of noninfectious medical conditions
• The majority of SSTIs are caused by gram-positive organisms, most notably Staphylococcus aureus and B-hemolytic streptococci
• Evaluation of the severity of disease, using a grading system, is essential to determining appropriate initial management
• Hospitalization and broad-spectrum antibiotics should be individually determined based on specific criteria, not empirically initiated for all presentations


References
1. Miller L, Eisenberg D, Liu H, et al. Incidence of skin and soft tissue infections in ambulatory and inpatient settings, 2005-2010. BMC Infect Dis. 2015 Aug 21;15:362.
2. Dupuy A, Benchikhi H, Roujeau JC, et al. Risk factors for erysipelas of the leg (cellulitis): case control study. BMJ. 1999;318(7198):1591-4.
3. Ki V, Rotstein C. Bacterial skin and soft tissue infections in adults: A review of their epidemiology, pathogenesis, diagnosis, treatment and site of care. Can J Infect Dis Med Microbiol. 2008 Mar;19(2):173-84.
4. Weng QY, Raff AB, Cohen JM, et al. Costs and Consequences Associated with Misdiagnosed Lower Extremity Cellulitis. JAMA Dermatol. 2016 Nov 2.
5. Keller E, Tomecki K, Alraies M. Distinguishing cellulitis from its mimics. Cleve Clin J Med. 2012 Aug;79(8)547-52.
6. Bassetti, M, Carmelutti A, Righi E. The role of methicillin-resistant Staphylococcus aureus in skin and soft tissue infections. Curr Opin Infect Dis. 2017 Apr.
7. Jeng A, Beheshti M, Li J, Ramesh N. The Role of B-hemolytic Streptococci in Causing Diffuse, Nonculturable Cellulitis. Medicine (Baltimore). 2010 Jul;89(4):217-26.
8. Moran G, Krishnadasan A, Gorwitz R. Methicillin-Resistant S. aureus Infections among Patients in the Emergency Department. N Engl J Med. 2006; 355:666-74.
9. Stevens D, Bisno A, Chambers H, et al. Practice Guidelines for the Diagnosis and Management of Skin and Soft tissue Infections: 2014 Update by the Infectious Disease Society of America. Clin Infect Dis. 2014 Jul 15;59(2):e10-52.
10. Raff, AB and Kroshinsky D. Cellulitis: A Review. JAMA. 2016 Jul;316(3):325-37.
11. Wong, CH, Khin LW, Heng KS, Tan KC, Low CO. The LRINEC (Laboratory Risk Indicator for Necrotizing Fasciitis) score: a tool for distinguishing necrotizing fasciitis from other soft tissue infections. Crit Care Med. 2004 Jul;32(7):1535-41.
12. Gunderson CG. Cellulitis: Definition, etiology and clinical features. Am J Med 2011 Dec;124(12):1113-22.
13. Pallin D, et al. Comparative Effectiveness of Cephalexin Plus Trimethoprim-Sulfamethoxazole versus Cephalexin Alone for Treatment of Uncomplicated Cellulitis: A Randomized Controlled Trial. Clin Infect Dis. 2013 Jun;56(12):1754-62.
14. Gunderson CG, Martinello RA. A systematic review of bacteremias in cellulitis and erysipelas. J Infect. 2012;64(2):148-55.
15. Halilovic J, Heintz BH, Brown J.. Risk factors for clinical failure in patients hospitalized with cellulitis and cutaneous abscess. J Infect. 2012;65(2):128-34.
Sepsis response team does not improve mortality/organ dysfunction
A sepsis response team did not have a positive effect on mortality or organ dysfunction in septic patients, compared with standard treatment by a primary care team, according to a study abstract scheduled to be presented at CHEST 2017.
Compared with the primary care team, the sepsis team was more likely to intervene on patients with a quick Sepsis-Related Organ Failure Assessment score greater than 1 (33.8% vs. 22.8%), change or initiate antibiotics within 3 hours (64.6% vs. 37.2%), and obtain blood cultures on time (66.4% vs 45.2%). An additional difference between the two groups was that the sepsis team had better compliance with the 3-hour bundle (15.2% vs 8.4%).
Despite the sepsis team’s higher level of compliance with certain protocols, the combined outcome measure of mortality and organ dysfunction within 28 days was not significantly higher for patients treated by the sepsis team (11.3% vs. 9.8%; P = .6). In fact, there was at least one downside to being treated by the sepsis team, which was having a 14% longer hospital stay.
Chhaya Patel, MD, is scheduled to present the abstract on Sun., Oct. 29th, at 2:30-2:45 p.m. in Convention Center – 602B. This research is part of the Sepsis & Septic Shock session at the CHEST annual meeting, which will run from 1:30 to 3:00 p.m.
A sepsis response team did not have a positive effect on mortality or organ dysfunction in septic patients, compared with standard treatment by a primary care team, according to a study abstract scheduled to be presented at CHEST 2017.
Compared with the primary care team, the sepsis team was more likely to intervene on patients with a quick Sepsis-Related Organ Failure Assessment score greater than 1 (33.8% vs. 22.8%), change or initiate antibiotics within 3 hours (64.6% vs. 37.2%), and obtain blood cultures on time (66.4% vs 45.2%). An additional difference between the two groups was that the sepsis team had better compliance with the 3-hour bundle (15.2% vs 8.4%).
Despite the sepsis team’s higher level of compliance with certain protocols, the combined outcome measure of mortality and organ dysfunction within 28 days was not significantly higher for patients treated by the sepsis team (11.3% vs. 9.8%; P = .6). In fact, there was at least one downside to being treated by the sepsis team, which was having a 14% longer hospital stay.
Chhaya Patel, MD, is scheduled to present the abstract on Sun., Oct. 29th, at 2:30-2:45 p.m. in Convention Center – 602B. This research is part of the Sepsis & Septic Shock session at the CHEST annual meeting, which will run from 1:30 to 3:00 p.m.
A sepsis response team did not have a positive effect on mortality or organ dysfunction in septic patients, compared with standard treatment by a primary care team, according to a study abstract scheduled to be presented at CHEST 2017.
Compared with the primary care team, the sepsis team was more likely to intervene on patients with a quick Sepsis-Related Organ Failure Assessment score greater than 1 (33.8% vs. 22.8%), change or initiate antibiotics within 3 hours (64.6% vs. 37.2%), and obtain blood cultures on time (66.4% vs 45.2%). An additional difference between the two groups was that the sepsis team had better compliance with the 3-hour bundle (15.2% vs 8.4%).
Despite the sepsis team’s higher level of compliance with certain protocols, the combined outcome measure of mortality and organ dysfunction within 28 days was not significantly higher for patients treated by the sepsis team (11.3% vs. 9.8%; P = .6). In fact, there was at least one downside to being treated by the sepsis team, which was having a 14% longer hospital stay.
Chhaya Patel, MD, is scheduled to present the abstract on Sun., Oct. 29th, at 2:30-2:45 p.m. in Convention Center – 602B. This research is part of the Sepsis & Septic Shock session at the CHEST annual meeting, which will run from 1:30 to 3:00 p.m.
FROM CHEST 2017
Abnormal potassium plus suspected ACS spell trouble
BARCELONA – A serum potassium level of at least 5.0 mmol/L or 3.5 mmol/L or less at admission for suspected acute coronary syndrome is a red flag for increased risk of in-hospital mortality and cardiac arrest, according to a Swedish study of nearly 33,000 consecutive patients.
That’s true even if, as so often ultimately proves to be the case, the patient turns out not to have ACS, Jonas Faxén, MD, of the Karolinska Institute, Stockholm, reported at the annual congress of the European Society of Cardiology.
“This study highlights that, if you have a patient in the emergency department with a possible ACS and potassium imbalance, you should really be cautious,” Dr. Faxén said.
He reported on 32,955 consecutive patients admitted to Stockholm County hospitals for suspected ACS during 2006-2011 and thereby enrolled in the SWEDEHEART (Swedish Web System for Enhancement and Development of Evidence-Based Care in Heart Disease Evaluated According to Recommended Therapies) registry.
Overall in-hospital mortality was 2.7%. In-hospital cardiac arrest occurred in 1.5% of patients. New-onset atrial fibrillation occurred in 2.4% of patients. These key outcomes were compared between the reference group – defined as patients with an admission serum potassium of 3.5 to less than 4.0 mmol/L – and patients with an admission serum potassium above or below those cutoffs.
In a multivariate logistic regression analysis adjusted for 24 potential confounders, including demographics, presentation characteristics, main diagnosis, comorbid conditions, medications on admission, and estimated glomerular filtration rate, patients with a serum potassium of 5.0 to less than 5.5 mmol/L were at 1.8-fold increased risk of in-hospital mortality. Those with a potassium of 5.5 mmol/L or greater were at 2.3-fold increased risk.
In contrast, a low rather than a high serum potassium was an independent risk factor cardiac arrest. An admission potassium of 3.0 to less than 3.5 mmol/L carried a 1.8-fold increased risk of in-hospital cardiac arrest, while a potassium of less than 3.0 was associated with a 2.7-fold increased risk.
A serum potassium below 3.0 mmol/L at admission also was associated with a 1.7-fold increased risk of new-onset atrial fibrillation.
These elevated risks of bad outcomes didn’t differ significantly between patients with ST-elevation MI, non-STEMI ACS, and those whose final diagnosis was not ACS, Dr. Faxén noted.
Session cochair David W. Walker, MD, medical director of the East Sussex (England) Healthcare NHS Trust, observed, “When I was a junior doctor I was always taught that when patients came onto coronary care we had to get their potassium to 4.5-5.0 mmol/L. I think you might want to change that advice now.”
“The implication would be that, if you intervene quickly in a patient with an abnormal potassium level, you might make a difference. Clearly, a potassium that’s too high is much worse than too low, since patients with in-hospital cardiac arrest can often be resuscitated,” Dr. Walker commented.
Dr. Faxén reported having no financial conflicts regarding his study, which was funded by the Swedish Heart and Lung Foundation and the Stockholm County Council.
BARCELONA – A serum potassium level of at least 5.0 mmol/L or 3.5 mmol/L or less at admission for suspected acute coronary syndrome is a red flag for increased risk of in-hospital mortality and cardiac arrest, according to a Swedish study of nearly 33,000 consecutive patients.
That’s true even if, as so often ultimately proves to be the case, the patient turns out not to have ACS, Jonas Faxén, MD, of the Karolinska Institute, Stockholm, reported at the annual congress of the European Society of Cardiology.
“This study highlights that, if you have a patient in the emergency department with a possible ACS and potassium imbalance, you should really be cautious,” Dr. Faxén said.
He reported on 32,955 consecutive patients admitted to Stockholm County hospitals for suspected ACS during 2006-2011 and thereby enrolled in the SWEDEHEART (Swedish Web System for Enhancement and Development of Evidence-Based Care in Heart Disease Evaluated According to Recommended Therapies) registry.
Overall in-hospital mortality was 2.7%. In-hospital cardiac arrest occurred in 1.5% of patients. New-onset atrial fibrillation occurred in 2.4% of patients. These key outcomes were compared between the reference group – defined as patients with an admission serum potassium of 3.5 to less than 4.0 mmol/L – and patients with an admission serum potassium above or below those cutoffs.
In a multivariate logistic regression analysis adjusted for 24 potential confounders, including demographics, presentation characteristics, main diagnosis, comorbid conditions, medications on admission, and estimated glomerular filtration rate, patients with a serum potassium of 5.0 to less than 5.5 mmol/L were at 1.8-fold increased risk of in-hospital mortality. Those with a potassium of 5.5 mmol/L or greater were at 2.3-fold increased risk.
In contrast, a low rather than a high serum potassium was an independent risk factor cardiac arrest. An admission potassium of 3.0 to less than 3.5 mmol/L carried a 1.8-fold increased risk of in-hospital cardiac arrest, while a potassium of less than 3.0 was associated with a 2.7-fold increased risk.
A serum potassium below 3.0 mmol/L at admission also was associated with a 1.7-fold increased risk of new-onset atrial fibrillation.
These elevated risks of bad outcomes didn’t differ significantly between patients with ST-elevation MI, non-STEMI ACS, and those whose final diagnosis was not ACS, Dr. Faxén noted.
Session cochair David W. Walker, MD, medical director of the East Sussex (England) Healthcare NHS Trust, observed, “When I was a junior doctor I was always taught that when patients came onto coronary care we had to get their potassium to 4.5-5.0 mmol/L. I think you might want to change that advice now.”
“The implication would be that, if you intervene quickly in a patient with an abnormal potassium level, you might make a difference. Clearly, a potassium that’s too high is much worse than too low, since patients with in-hospital cardiac arrest can often be resuscitated,” Dr. Walker commented.
Dr. Faxén reported having no financial conflicts regarding his study, which was funded by the Swedish Heart and Lung Foundation and the Stockholm County Council.
BARCELONA – A serum potassium level of at least 5.0 mmol/L or 3.5 mmol/L or less at admission for suspected acute coronary syndrome is a red flag for increased risk of in-hospital mortality and cardiac arrest, according to a Swedish study of nearly 33,000 consecutive patients.
That’s true even if, as so often ultimately proves to be the case, the patient turns out not to have ACS, Jonas Faxén, MD, of the Karolinska Institute, Stockholm, reported at the annual congress of the European Society of Cardiology.
“This study highlights that, if you have a patient in the emergency department with a possible ACS and potassium imbalance, you should really be cautious,” Dr. Faxén said.
He reported on 32,955 consecutive patients admitted to Stockholm County hospitals for suspected ACS during 2006-2011 and thereby enrolled in the SWEDEHEART (Swedish Web System for Enhancement and Development of Evidence-Based Care in Heart Disease Evaluated According to Recommended Therapies) registry.
Overall in-hospital mortality was 2.7%. In-hospital cardiac arrest occurred in 1.5% of patients. New-onset atrial fibrillation occurred in 2.4% of patients. These key outcomes were compared between the reference group – defined as patients with an admission serum potassium of 3.5 to less than 4.0 mmol/L – and patients with an admission serum potassium above or below those cutoffs.
In a multivariate logistic regression analysis adjusted for 24 potential confounders, including demographics, presentation characteristics, main diagnosis, comorbid conditions, medications on admission, and estimated glomerular filtration rate, patients with a serum potassium of 5.0 to less than 5.5 mmol/L were at 1.8-fold increased risk of in-hospital mortality. Those with a potassium of 5.5 mmol/L or greater were at 2.3-fold increased risk.
In contrast, a low rather than a high serum potassium was an independent risk factor cardiac arrest. An admission potassium of 3.0 to less than 3.5 mmol/L carried a 1.8-fold increased risk of in-hospital cardiac arrest, while a potassium of less than 3.0 was associated with a 2.7-fold increased risk.
A serum potassium below 3.0 mmol/L at admission also was associated with a 1.7-fold increased risk of new-onset atrial fibrillation.
These elevated risks of bad outcomes didn’t differ significantly between patients with ST-elevation MI, non-STEMI ACS, and those whose final diagnosis was not ACS, Dr. Faxén noted.
Session cochair David W. Walker, MD, medical director of the East Sussex (England) Healthcare NHS Trust, observed, “When I was a junior doctor I was always taught that when patients came onto coronary care we had to get their potassium to 4.5-5.0 mmol/L. I think you might want to change that advice now.”
“The implication would be that, if you intervene quickly in a patient with an abnormal potassium level, you might make a difference. Clearly, a potassium that’s too high is much worse than too low, since patients with in-hospital cardiac arrest can often be resuscitated,” Dr. Walker commented.
Dr. Faxén reported having no financial conflicts regarding his study, which was funded by the Swedish Heart and Lung Foundation and the Stockholm County Council.
AT THE ESC CONGRESS 2017
Key clinical point:
Major finding: Hyperkalemia of 5.0 to less than 5.5 mmol/L at admission for suspected ACS was associated with close to a twofold increased risk of in-hospital mortality.
Data source: The SWEDEHEART study is an ongoing prospective registry of patients with cardiovascular disease admitted to Stockholm County hospitals.
Disclosures: The presenter reported having no financial conflicts regarding his study, which was funded by the Swedish Heart and Lung Foundation and the Stockholm County Council.
Study: EHR malpractice claims rising
Malpractice claims involving the use of electronic health records (EHRs) are on the rise, according to data from The Doctors Company.
Cases in which EHRs were a factor grew from 2 claims during 2007-2010 to 161 claims from 2011 to December 2016, according an analysis published Oct. 16 by The Doctors Company, a national medical malpractice insurer.
The majority of EHR-related claims during 2014-2016 stemmed from incidents in a doctor’s office or a hospital clinic (35%), while the second most common location was a patient’s room. Malpractice claims involving EHRs were most commonly alleged against ob.gyns, followed by family physicians, and orthopedists. Diagnosis errors and improper medication management were the top most frequent allegations associated with EHR claims.
The analysis shows that while digitization of medicine has improved patient safety, it also has a dark side – as evidenced by the emergence of new kinds of errors, said Robert M. Wachter, MD, a professor at the University of California, San Francisco, and a member of the board of governors for The Doctors Company.
“This study makes an important contribution by chronicling actual errors, such as wrong medications selected from an autopick list, and helps point the way to changes ranging from physician education to EHR software design,” Dr. Wachter said in a statement.
[email protected]
On Twitter @legal_med
Malpractice claims involving the use of electronic health records (EHRs) are on the rise, according to data from The Doctors Company.
Cases in which EHRs were a factor grew from 2 claims during 2007-2010 to 161 claims from 2011 to December 2016, according an analysis published Oct. 16 by The Doctors Company, a national medical malpractice insurer.
The majority of EHR-related claims during 2014-2016 stemmed from incidents in a doctor’s office or a hospital clinic (35%), while the second most common location was a patient’s room. Malpractice claims involving EHRs were most commonly alleged against ob.gyns, followed by family physicians, and orthopedists. Diagnosis errors and improper medication management were the top most frequent allegations associated with EHR claims.
The analysis shows that while digitization of medicine has improved patient safety, it also has a dark side – as evidenced by the emergence of new kinds of errors, said Robert M. Wachter, MD, a professor at the University of California, San Francisco, and a member of the board of governors for The Doctors Company.
“This study makes an important contribution by chronicling actual errors, such as wrong medications selected from an autopick list, and helps point the way to changes ranging from physician education to EHR software design,” Dr. Wachter said in a statement.
[email protected]
On Twitter @legal_med
Malpractice claims involving the use of electronic health records (EHRs) are on the rise, according to data from The Doctors Company.
Cases in which EHRs were a factor grew from 2 claims during 2007-2010 to 161 claims from 2011 to December 2016, according an analysis published Oct. 16 by The Doctors Company, a national medical malpractice insurer.
The majority of EHR-related claims during 2014-2016 stemmed from incidents in a doctor’s office or a hospital clinic (35%), while the second most common location was a patient’s room. Malpractice claims involving EHRs were most commonly alleged against ob.gyns, followed by family physicians, and orthopedists. Diagnosis errors and improper medication management were the top most frequent allegations associated with EHR claims.
The analysis shows that while digitization of medicine has improved patient safety, it also has a dark side – as evidenced by the emergence of new kinds of errors, said Robert M. Wachter, MD, a professor at the University of California, San Francisco, and a member of the board of governors for The Doctors Company.
“This study makes an important contribution by chronicling actual errors, such as wrong medications selected from an autopick list, and helps point the way to changes ranging from physician education to EHR software design,” Dr. Wachter said in a statement.
[email protected]
On Twitter @legal_med
Key clinical point:
Major finding: Cases in which EHRs were a factor grew from 2 claims during 2007-2010 to 161 claims from 2011 to December 2016
Data source: Review of 163 claims from 2007 to 2016 in The Doctors Company database.
Disclosures: The study was funded by The Doctors Company.
Thinking about productivity: Survey data 2017
The 2017 MGMA survey data on compensation and productivity were released last June. While the numbers aren’t surprising, reviewing them always gets me thinking about factors that influence reasonable expectations for compensation and productivity in any individual hospitalist group.
The data were collected in early 2017, reflecting work done in 2016, and show a national median hospitalist compensation for internal medicine physicians of $284,000, up from $278,500 the year before. Since MGMA added a hospitalist category to the survey, compensation has been growing significantly faster than inflation, even though productivity has been essentially flat. I’ve always thought that the high demand for hospitalists, which isn’t letting up much, in the face of a limited supply is probably the most significant force causing hospitalist compensation to rise faster than in most other specialties.
The survey shows a median of 2,114 billed encounters and 4,159 wRVUs (work relative value units) generated per internal medicine hospitalist annually (family medicine hospitalists are reported separately). These numbers have been pretty stable for many years.
Whether it is reasonable to expect hospitalists in your group to produce at this level is a question that can unspool into a lengthy conversation. Below are several assertions I regularly hear others make about productivity, and following each is my commentary.
“Surveys show only what is most typical, not what is optimal. Our field suffers from concerning levels of burnout, essentially proving that median levels of productivity shown in surveys is too high.”
I share this concern, but this is a complicated issue. You’ll have to make up your own mind regarding how significantly workload influences hospitalist burnout. But the modest amount of published research on this topic suggests that workload itself isn’t as strongly associated with burnout as you might think. I’m certain workload does play a role, but other factors such as “occupational solidarity” seem to matter more. Lowering workload in some settings might be appropriate, but without other interventions may not influence work-related stress and burnout as much as might be hoped.
“Surveys don’t capture unbillable activities (‘unbillable wRVUs’), so are a poor frame of reference when thinking about productivity expectations in our own group.”
It’s true that hospitalists do a lot of work that isn’t captured in wRVUs. My work with many groups around the country suggests the amount and difficulty of this unbillable work is reasonably similar across most groups. We all spend time with handoffs, managing paperwork such as charge capture and completing forms, responding to a rapid response call that doesn’t lead to a billable charge, etc. The average amount of this sort of work is built into the survey. Clearly some groups are outliers with meaningfully more unbillable work than elsewhere, but that can be a difficult or impossible thing to prove.
“My hospital has unique barriers to efficiency/productivity, so it’s more difficult to achieve levels of productivity shown in surveys.”
This is another way of expressing the previous issue. To support this assertion hospitalists will mention that it is tougher to be productive at their hospital because they’re a referral center with unusually sick and complicated patients; they teach trainees in addition to clinical care; and/or their patients and families are unusually demanding, so they take much more time than at other places.
Yet for each of these issues I also hear the reverse argument regularly. Hospitalists point out that because they’re a small hospital (not a referral center) they lack the support of other specialties so must manage all aspects of care themselves; they don’t have residents to help do some of the work; and their patients are unsophisticated and lack social support. For these reasons, the argument goes, they shouldn’t be expected to achieve levels of productivity shown in surveys.
I have worked with hospitalist groups that I am convinced do face unusual barriers to efficiency that are meaningful enough that unless the barriers can be addressed, I think productivity expectations should be lower than survey benchmarks. For example, in most academic medical centers and a very small number of nonacademic hospitals, only the attending physician writes orders; consulting doctors don’t. This means that the attending hospitalist must check a patient’s chart repeatedly through the day just to see if the consultant proposed even small things like ordering a routine lab test, advancing the diet, etc., that the hospitalist must order.
A separate daytime admitter shift is a modest barrier to efficiency that is so common it is clearly factored into survey results. Most hospitalist groups with more than about five doctors working daily have one doctor (or more than one in large groups) manage admissions while the rest round and are protected from admissions. While this may have a number of benefits, overall hospitalist efficiency isn’t one of them. It means that all patients, not just those admitted at night, will have a handoff from the admitting provider to a new attending for the first rounding visit. This new attending will spend additional time becoming familiar with the patient – time that wouldn’t be necessary had that doctor performed the admission visit herself.
“Our hospitalist group is always being asked to take on more duties, such as managing med reconciliation, taking referrals from an additional PCP group, or serving as admitting and attending physician for patients previously admitted by a different specialty (which now serves in the consultant role). For this reason, it’s necessary to steadily lower hospitalist productivity expectations over time.”
A hospitalist today probably spends a quarter of the day doing things I didn’t have to do at the outset of my career in the 1980s. So my impulse is to agree that as the breadth of our responsibilities expands, expected wRVU productivity should fall. But surveys over the last 15-20 years don’t show this happening, and the pressure to maintain productivity levels isn’t likely to let up. Rather than generating fewer wRVUs (seeing fewer patients), hospital medicine, like health care as a whole, faces the challenge of continually improving our efficiency.
“Surveys are only one frame of reference for determining expectations at my particular hospitalist group. There are other factors to consider as well.”
This is absolutely true. There may be many reasons for your group to set expectations that are meaningfully different from survey figures. Just make sure your rationale for doing so is well considered and effectively communicated to other stakeholders, such as those in finance and organizational leadership at your organization.
Dr. Nelson has had a career in clinical practice as a hospitalist starting in 1988. He is cofounder and past president of SHM, and principal in Nelson Flores Hospital Medicine Consultants. He is codirector for SHM’s practice management courses. [email protected]
The 2017 MGMA survey data on compensation and productivity were released last June. While the numbers aren’t surprising, reviewing them always gets me thinking about factors that influence reasonable expectations for compensation and productivity in any individual hospitalist group.
The data were collected in early 2017, reflecting work done in 2016, and show a national median hospitalist compensation for internal medicine physicians of $284,000, up from $278,500 the year before. Since MGMA added a hospitalist category to the survey, compensation has been growing significantly faster than inflation, even though productivity has been essentially flat. I’ve always thought that the high demand for hospitalists, which isn’t letting up much, in the face of a limited supply is probably the most significant force causing hospitalist compensation to rise faster than in most other specialties.
The survey shows a median of 2,114 billed encounters and 4,159 wRVUs (work relative value units) generated per internal medicine hospitalist annually (family medicine hospitalists are reported separately). These numbers have been pretty stable for many years.
Whether it is reasonable to expect hospitalists in your group to produce at this level is a question that can unspool into a lengthy conversation. Below are several assertions I regularly hear others make about productivity, and following each is my commentary.
“Surveys show only what is most typical, not what is optimal. Our field suffers from concerning levels of burnout, essentially proving that median levels of productivity shown in surveys is too high.”
I share this concern, but this is a complicated issue. You’ll have to make up your own mind regarding how significantly workload influences hospitalist burnout. But the modest amount of published research on this topic suggests that workload itself isn’t as strongly associated with burnout as you might think. I’m certain workload does play a role, but other factors such as “occupational solidarity” seem to matter more. Lowering workload in some settings might be appropriate, but without other interventions may not influence work-related stress and burnout as much as might be hoped.
“Surveys don’t capture unbillable activities (‘unbillable wRVUs’), so are a poor frame of reference when thinking about productivity expectations in our own group.”
It’s true that hospitalists do a lot of work that isn’t captured in wRVUs. My work with many groups around the country suggests the amount and difficulty of this unbillable work is reasonably similar across most groups. We all spend time with handoffs, managing paperwork such as charge capture and completing forms, responding to a rapid response call that doesn’t lead to a billable charge, etc. The average amount of this sort of work is built into the survey. Clearly some groups are outliers with meaningfully more unbillable work than elsewhere, but that can be a difficult or impossible thing to prove.
“My hospital has unique barriers to efficiency/productivity, so it’s more difficult to achieve levels of productivity shown in surveys.”
This is another way of expressing the previous issue. To support this assertion hospitalists will mention that it is tougher to be productive at their hospital because they’re a referral center with unusually sick and complicated patients; they teach trainees in addition to clinical care; and/or their patients and families are unusually demanding, so they take much more time than at other places.
Yet for each of these issues I also hear the reverse argument regularly. Hospitalists point out that because they’re a small hospital (not a referral center) they lack the support of other specialties so must manage all aspects of care themselves; they don’t have residents to help do some of the work; and their patients are unsophisticated and lack social support. For these reasons, the argument goes, they shouldn’t be expected to achieve levels of productivity shown in surveys.
I have worked with hospitalist groups that I am convinced do face unusual barriers to efficiency that are meaningful enough that unless the barriers can be addressed, I think productivity expectations should be lower than survey benchmarks. For example, in most academic medical centers and a very small number of nonacademic hospitals, only the attending physician writes orders; consulting doctors don’t. This means that the attending hospitalist must check a patient’s chart repeatedly through the day just to see if the consultant proposed even small things like ordering a routine lab test, advancing the diet, etc., that the hospitalist must order.
A separate daytime admitter shift is a modest barrier to efficiency that is so common it is clearly factored into survey results. Most hospitalist groups with more than about five doctors working daily have one doctor (or more than one in large groups) manage admissions while the rest round and are protected from admissions. While this may have a number of benefits, overall hospitalist efficiency isn’t one of them. It means that all patients, not just those admitted at night, will have a handoff from the admitting provider to a new attending for the first rounding visit. This new attending will spend additional time becoming familiar with the patient – time that wouldn’t be necessary had that doctor performed the admission visit herself.
“Our hospitalist group is always being asked to take on more duties, such as managing med reconciliation, taking referrals from an additional PCP group, or serving as admitting and attending physician for patients previously admitted by a different specialty (which now serves in the consultant role). For this reason, it’s necessary to steadily lower hospitalist productivity expectations over time.”
A hospitalist today probably spends a quarter of the day doing things I didn’t have to do at the outset of my career in the 1980s. So my impulse is to agree that as the breadth of our responsibilities expands, expected wRVU productivity should fall. But surveys over the last 15-20 years don’t show this happening, and the pressure to maintain productivity levels isn’t likely to let up. Rather than generating fewer wRVUs (seeing fewer patients), hospital medicine, like health care as a whole, faces the challenge of continually improving our efficiency.
“Surveys are only one frame of reference for determining expectations at my particular hospitalist group. There are other factors to consider as well.”
This is absolutely true. There may be many reasons for your group to set expectations that are meaningfully different from survey figures. Just make sure your rationale for doing so is well considered and effectively communicated to other stakeholders, such as those in finance and organizational leadership at your organization.
Dr. Nelson has had a career in clinical practice as a hospitalist starting in 1988. He is cofounder and past president of SHM, and principal in Nelson Flores Hospital Medicine Consultants. He is codirector for SHM’s practice management courses. [email protected]
The 2017 MGMA survey data on compensation and productivity were released last June. While the numbers aren’t surprising, reviewing them always gets me thinking about factors that influence reasonable expectations for compensation and productivity in any individual hospitalist group.
The data were collected in early 2017, reflecting work done in 2016, and show a national median hospitalist compensation for internal medicine physicians of $284,000, up from $278,500 the year before. Since MGMA added a hospitalist category to the survey, compensation has been growing significantly faster than inflation, even though productivity has been essentially flat. I’ve always thought that the high demand for hospitalists, which isn’t letting up much, in the face of a limited supply is probably the most significant force causing hospitalist compensation to rise faster than in most other specialties.
The survey shows a median of 2,114 billed encounters and 4,159 wRVUs (work relative value units) generated per internal medicine hospitalist annually (family medicine hospitalists are reported separately). These numbers have been pretty stable for many years.
Whether it is reasonable to expect hospitalists in your group to produce at this level is a question that can unspool into a lengthy conversation. Below are several assertions I regularly hear others make about productivity, and following each is my commentary.
“Surveys show only what is most typical, not what is optimal. Our field suffers from concerning levels of burnout, essentially proving that median levels of productivity shown in surveys is too high.”
I share this concern, but this is a complicated issue. You’ll have to make up your own mind regarding how significantly workload influences hospitalist burnout. But the modest amount of published research on this topic suggests that workload itself isn’t as strongly associated with burnout as you might think. I’m certain workload does play a role, but other factors such as “occupational solidarity” seem to matter more. Lowering workload in some settings might be appropriate, but without other interventions may not influence work-related stress and burnout as much as might be hoped.
“Surveys don’t capture unbillable activities (‘unbillable wRVUs’), so are a poor frame of reference when thinking about productivity expectations in our own group.”
It’s true that hospitalists do a lot of work that isn’t captured in wRVUs. My work with many groups around the country suggests the amount and difficulty of this unbillable work is reasonably similar across most groups. We all spend time with handoffs, managing paperwork such as charge capture and completing forms, responding to a rapid response call that doesn’t lead to a billable charge, etc. The average amount of this sort of work is built into the survey. Clearly some groups are outliers with meaningfully more unbillable work than elsewhere, but that can be a difficult or impossible thing to prove.
“My hospital has unique barriers to efficiency/productivity, so it’s more difficult to achieve levels of productivity shown in surveys.”
This is another way of expressing the previous issue. To support this assertion hospitalists will mention that it is tougher to be productive at their hospital because they’re a referral center with unusually sick and complicated patients; they teach trainees in addition to clinical care; and/or their patients and families are unusually demanding, so they take much more time than at other places.
Yet for each of these issues I also hear the reverse argument regularly. Hospitalists point out that because they’re a small hospital (not a referral center) they lack the support of other specialties so must manage all aspects of care themselves; they don’t have residents to help do some of the work; and their patients are unsophisticated and lack social support. For these reasons, the argument goes, they shouldn’t be expected to achieve levels of productivity shown in surveys.
I have worked with hospitalist groups that I am convinced do face unusual barriers to efficiency that are meaningful enough that unless the barriers can be addressed, I think productivity expectations should be lower than survey benchmarks. For example, in most academic medical centers and a very small number of nonacademic hospitals, only the attending physician writes orders; consulting doctors don’t. This means that the attending hospitalist must check a patient’s chart repeatedly through the day just to see if the consultant proposed even small things like ordering a routine lab test, advancing the diet, etc., that the hospitalist must order.
A separate daytime admitter shift is a modest barrier to efficiency that is so common it is clearly factored into survey results. Most hospitalist groups with more than about five doctors working daily have one doctor (or more than one in large groups) manage admissions while the rest round and are protected from admissions. While this may have a number of benefits, overall hospitalist efficiency isn’t one of them. It means that all patients, not just those admitted at night, will have a handoff from the admitting provider to a new attending for the first rounding visit. This new attending will spend additional time becoming familiar with the patient – time that wouldn’t be necessary had that doctor performed the admission visit herself.
“Our hospitalist group is always being asked to take on more duties, such as managing med reconciliation, taking referrals from an additional PCP group, or serving as admitting and attending physician for patients previously admitted by a different specialty (which now serves in the consultant role). For this reason, it’s necessary to steadily lower hospitalist productivity expectations over time.”
A hospitalist today probably spends a quarter of the day doing things I didn’t have to do at the outset of my career in the 1980s. So my impulse is to agree that as the breadth of our responsibilities expands, expected wRVU productivity should fall. But surveys over the last 15-20 years don’t show this happening, and the pressure to maintain productivity levels isn’t likely to let up. Rather than generating fewer wRVUs (seeing fewer patients), hospital medicine, like health care as a whole, faces the challenge of continually improving our efficiency.
“Surveys are only one frame of reference for determining expectations at my particular hospitalist group. There are other factors to consider as well.”
This is absolutely true. There may be many reasons for your group to set expectations that are meaningfully different from survey figures. Just make sure your rationale for doing so is well considered and effectively communicated to other stakeholders, such as those in finance and organizational leadership at your organization.
Dr. Nelson has had a career in clinical practice as a hospitalist starting in 1988. He is cofounder and past president of SHM, and principal in Nelson Flores Hospital Medicine Consultants. He is codirector for SHM’s practice management courses. [email protected]
Pediatric hospitalists take on the challenge of antibiotic stewardship
When Carol Glaser, MD, was in training, the philosophy around antibiotic prescribing often went something like this: “Ten days of antibiotics is good, but let’s do a few more days just to be sure,” she said.
Today, however, the new mantra is “less is more.” Dr. Glaser is an experienced pediatric infectious disease physician and the lead physician for pediatric antimicrobial stewardship at The Permanente Medical Group, Kaiser Permanente, at the Oakland (Calif.) Medical Center. While antibiotic stewardship is an issue relevant to nearly all hospitalists, for pediatric patients, the considerations can be unique and particularly serious.
Dr. Shah, a pediatric infectious disease physician at Cincinnati Children’s Hospital, spoke last spring at HM17, the Society of Hospital Medicine’s annual meeting. His talk drew from issues raised on pediatric hospital medicine electronic mailing lists and from audience questions. These centered on decisions regarding the use of intravenous versus oral antibiotics for pediatric patients – or what he refers to as intravenous-to-oral conversion – as well as antibiotic treatment duration.
“For many conditions in pediatrics, we used to treat with intravenous antibiotics initially – and sometimes for the entire course – and now we’re using oral antibiotics for the entire course,” Dr. Shah said. He noted that urinary tract infections were once treated with IV antibiotics in the hospital but are now routinely treated orally in an outpatient setting.
Dr. Shah cited two studies, both of which he coauthored as part of the Pediatric Research in Inpatient Settings Network, which compared intravenous versus oral antibiotics treatments given after discharge: The first, published in JAMA Pediatrics in 2014, examined treatment for osteomyelitis, while the second, which focused on complicated pneumonia, was published in Pediatrics in 2016.1,2
Both were observational, retrospective studies involving more than 2,000 children across more than 30 hospitals. The JAMA Pediatrics study found that roughly half of the patients were discharged with a peripherally inserted central catheter (PICC) line, and half were prescribed oral antibiotics. In some hospitals, 100% of patients were sent home with a PICC line, and in others, all children were sent home on oral antibiotics. Although treatment failure rates were the same for both groups, 15% of the patients sent home with a PICC line had to return to the emergency department because of PICC-related complications. Some were hospitalized.1
The Pediatrics study found less variation in PICC versus oral antibiotic use across hospitals for patients with complicated pneumonia, but the treatment failure rate was slightly higher for PICC patients at 3.2%, compared with 2.6% for those on oral antibiotics. This difference, however, was not statistically significant. PICC-related complications were observed in 7.1% of patients with PICC lines also were more likely to experience adverse drug reactions, compared with patients on oral antibiotics.2
“PICC lines have some advantages, particularly when children are unable or unwilling to take oral antibiotics, but they also have risks” said Dr. Shah. “If outcomes are equivalent, why would you subject patients to the risks of a catheter? And, every time they get a fever at home with a PICC line, they need urgent evaluation for the possibility of a catheter-associated bacterial infection. There is an emotional cost, as well, to taking care of catheters in the home setting.”
Additionally, economic pressures are compelling hospitals to reduce costs and resource utilization while maintaining or improving the quality of care, Dr. Shah pointed out. “Hospitalists do many things well, and quality improvement is one of those areas. That approach really aligns with antimicrobial stewardship, and there is greater incentive with episode-based payment models and financial penalties for excess readmissions. Reducing post-discharge IV antibiotic use aligns with stewardship goals and reduces the likelihood of hospital readmissions.”
The hospital medicine division at Dr. Shah’s hospital helped assemble a multidisciplinary team involving emergency physicians, pharmacists, nursing staff, hospitalists, and infectious disease physicians to encourage the use of appropriate, narrow-spectrum antibiotics and reduce the duration of antibiotic therapies. For example, skin and soft-tissue infections that were once treated for 10-14 days are now sufficiently treated in 5-7days. These efforts to improve outcomes through better adherence to evidence-based practices, including better stewardship, earned the team the SHM Teamwork in Quality Improvement Award in 2014.
“Quality improvement is really about changing the system, and hospitalists, who excel in QI, are poised to help drive antimicrobial stewardship efforts,” Dr. Shah said.
At Oakland Medical Center, Dr. Glaser helped implement handshake rounds, an idea they adopted from a group in Colorado. Every day, with every patient, the antimicrobial stewardship team meets with representatives of the teams – pediatric intensive care, the wards, the NICU, and others – to review antibiotic treatment plans for the choice of antimicrobial drug, for the duration of treatment, and for specific conditions. “We work really closely with hospitalists and our strong pediatric pharmacy team every day to ask: ‘Do we have the right dose? Do we really need to use this antibiotic?’ ” Dr. Glaser said.
Last year, she also worked to incorporate antimicrobial stewardship principles into the hospital’s residency program. “I think the most important thing we’re doing is changing the culture,” she said. “For these young physicians, we’re giving them the knowledge to empower them rather than telling them what to do and giving them a better, fundamental understanding of infectious disease.”
For instance, most pediatric respiratory illnesses are caused by a virus, yet physicians will still prescribe antibiotics for a host of reasons – including the expectations of parents, the guesswork that can go into diagnosing a young patient who cannot describe what is wrong, and the fear that children will get sicker if an antibiotic is not started early.
“A lot of it is figuring out the best approach with the least amount of side effects but covering what we need to cover for a given patient,” she said.
A number of physicians from Dr. Glaser’s team presented stewardship data from their hospital at the July 2017 Pediatric Hospital Medicine meeting in Nashville, demonstrating that, overall, they are using fewer antibiotics and that fewer of those used are broad spectrum. This satisfies the “pillars of stewardship,” Dr. Glaser said. Use antibiotics only when you need them, use them only as long as you need, and then make sure you use the most narrow-spectrum antibiotic you possibly can, she said.
Oakland Medical Center has benefited from a strong commitment to antimicrobial stewardship efforts, Dr. Glaser said, noting that many programs may lack such support, a problem that can be one of the biggest hurdles antimicrobial stewardship efforts face. The support at her hospital “has been an immense help in getting our program to where it is today.”
References
1. Keren R, Shah SS, Srivastava R, et al. Comparative effectiveness of intravenous vs oral antibiotics for postdischarge treatment of acute osteomyelitis in children. JAMA Pediatr. 2015 Feb:169(2):120-8.
2. Shah SS, Srivastava R, Wu S, et al. Intravenous versus oral antibiotics for postdischarge treatment of complicated pneumonia. Pediatrics. 2016 Dec;138(6). pii: e20161692.
When Carol Glaser, MD, was in training, the philosophy around antibiotic prescribing often went something like this: “Ten days of antibiotics is good, but let’s do a few more days just to be sure,” she said.
Today, however, the new mantra is “less is more.” Dr. Glaser is an experienced pediatric infectious disease physician and the lead physician for pediatric antimicrobial stewardship at The Permanente Medical Group, Kaiser Permanente, at the Oakland (Calif.) Medical Center. While antibiotic stewardship is an issue relevant to nearly all hospitalists, for pediatric patients, the considerations can be unique and particularly serious.
Dr. Shah, a pediatric infectious disease physician at Cincinnati Children’s Hospital, spoke last spring at HM17, the Society of Hospital Medicine’s annual meeting. His talk drew from issues raised on pediatric hospital medicine electronic mailing lists and from audience questions. These centered on decisions regarding the use of intravenous versus oral antibiotics for pediatric patients – or what he refers to as intravenous-to-oral conversion – as well as antibiotic treatment duration.
“For many conditions in pediatrics, we used to treat with intravenous antibiotics initially – and sometimes for the entire course – and now we’re using oral antibiotics for the entire course,” Dr. Shah said. He noted that urinary tract infections were once treated with IV antibiotics in the hospital but are now routinely treated orally in an outpatient setting.
Dr. Shah cited two studies, both of which he coauthored as part of the Pediatric Research in Inpatient Settings Network, which compared intravenous versus oral antibiotics treatments given after discharge: The first, published in JAMA Pediatrics in 2014, examined treatment for osteomyelitis, while the second, which focused on complicated pneumonia, was published in Pediatrics in 2016.1,2
Both were observational, retrospective studies involving more than 2,000 children across more than 30 hospitals. The JAMA Pediatrics study found that roughly half of the patients were discharged with a peripherally inserted central catheter (PICC) line, and half were prescribed oral antibiotics. In some hospitals, 100% of patients were sent home with a PICC line, and in others, all children were sent home on oral antibiotics. Although treatment failure rates were the same for both groups, 15% of the patients sent home with a PICC line had to return to the emergency department because of PICC-related complications. Some were hospitalized.1
The Pediatrics study found less variation in PICC versus oral antibiotic use across hospitals for patients with complicated pneumonia, but the treatment failure rate was slightly higher for PICC patients at 3.2%, compared with 2.6% for those on oral antibiotics. This difference, however, was not statistically significant. PICC-related complications were observed in 7.1% of patients with PICC lines also were more likely to experience adverse drug reactions, compared with patients on oral antibiotics.2
“PICC lines have some advantages, particularly when children are unable or unwilling to take oral antibiotics, but they also have risks” said Dr. Shah. “If outcomes are equivalent, why would you subject patients to the risks of a catheter? And, every time they get a fever at home with a PICC line, they need urgent evaluation for the possibility of a catheter-associated bacterial infection. There is an emotional cost, as well, to taking care of catheters in the home setting.”
Additionally, economic pressures are compelling hospitals to reduce costs and resource utilization while maintaining or improving the quality of care, Dr. Shah pointed out. “Hospitalists do many things well, and quality improvement is one of those areas. That approach really aligns with antimicrobial stewardship, and there is greater incentive with episode-based payment models and financial penalties for excess readmissions. Reducing post-discharge IV antibiotic use aligns with stewardship goals and reduces the likelihood of hospital readmissions.”
The hospital medicine division at Dr. Shah’s hospital helped assemble a multidisciplinary team involving emergency physicians, pharmacists, nursing staff, hospitalists, and infectious disease physicians to encourage the use of appropriate, narrow-spectrum antibiotics and reduce the duration of antibiotic therapies. For example, skin and soft-tissue infections that were once treated for 10-14 days are now sufficiently treated in 5-7days. These efforts to improve outcomes through better adherence to evidence-based practices, including better stewardship, earned the team the SHM Teamwork in Quality Improvement Award in 2014.
“Quality improvement is really about changing the system, and hospitalists, who excel in QI, are poised to help drive antimicrobial stewardship efforts,” Dr. Shah said.
At Oakland Medical Center, Dr. Glaser helped implement handshake rounds, an idea they adopted from a group in Colorado. Every day, with every patient, the antimicrobial stewardship team meets with representatives of the teams – pediatric intensive care, the wards, the NICU, and others – to review antibiotic treatment plans for the choice of antimicrobial drug, for the duration of treatment, and for specific conditions. “We work really closely with hospitalists and our strong pediatric pharmacy team every day to ask: ‘Do we have the right dose? Do we really need to use this antibiotic?’ ” Dr. Glaser said.
Last year, she also worked to incorporate antimicrobial stewardship principles into the hospital’s residency program. “I think the most important thing we’re doing is changing the culture,” she said. “For these young physicians, we’re giving them the knowledge to empower them rather than telling them what to do and giving them a better, fundamental understanding of infectious disease.”
For instance, most pediatric respiratory illnesses are caused by a virus, yet physicians will still prescribe antibiotics for a host of reasons – including the expectations of parents, the guesswork that can go into diagnosing a young patient who cannot describe what is wrong, and the fear that children will get sicker if an antibiotic is not started early.
“A lot of it is figuring out the best approach with the least amount of side effects but covering what we need to cover for a given patient,” she said.
A number of physicians from Dr. Glaser’s team presented stewardship data from their hospital at the July 2017 Pediatric Hospital Medicine meeting in Nashville, demonstrating that, overall, they are using fewer antibiotics and that fewer of those used are broad spectrum. This satisfies the “pillars of stewardship,” Dr. Glaser said. Use antibiotics only when you need them, use them only as long as you need, and then make sure you use the most narrow-spectrum antibiotic you possibly can, she said.
Oakland Medical Center has benefited from a strong commitment to antimicrobial stewardship efforts, Dr. Glaser said, noting that many programs may lack such support, a problem that can be one of the biggest hurdles antimicrobial stewardship efforts face. The support at her hospital “has been an immense help in getting our program to where it is today.”
References
1. Keren R, Shah SS, Srivastava R, et al. Comparative effectiveness of intravenous vs oral antibiotics for postdischarge treatment of acute osteomyelitis in children. JAMA Pediatr. 2015 Feb:169(2):120-8.
2. Shah SS, Srivastava R, Wu S, et al. Intravenous versus oral antibiotics for postdischarge treatment of complicated pneumonia. Pediatrics. 2016 Dec;138(6). pii: e20161692.
When Carol Glaser, MD, was in training, the philosophy around antibiotic prescribing often went something like this: “Ten days of antibiotics is good, but let’s do a few more days just to be sure,” she said.
Today, however, the new mantra is “less is more.” Dr. Glaser is an experienced pediatric infectious disease physician and the lead physician for pediatric antimicrobial stewardship at The Permanente Medical Group, Kaiser Permanente, at the Oakland (Calif.) Medical Center. While antibiotic stewardship is an issue relevant to nearly all hospitalists, for pediatric patients, the considerations can be unique and particularly serious.
Dr. Shah, a pediatric infectious disease physician at Cincinnati Children’s Hospital, spoke last spring at HM17, the Society of Hospital Medicine’s annual meeting. His talk drew from issues raised on pediatric hospital medicine electronic mailing lists and from audience questions. These centered on decisions regarding the use of intravenous versus oral antibiotics for pediatric patients – or what he refers to as intravenous-to-oral conversion – as well as antibiotic treatment duration.
“For many conditions in pediatrics, we used to treat with intravenous antibiotics initially – and sometimes for the entire course – and now we’re using oral antibiotics for the entire course,” Dr. Shah said. He noted that urinary tract infections were once treated with IV antibiotics in the hospital but are now routinely treated orally in an outpatient setting.
Dr. Shah cited two studies, both of which he coauthored as part of the Pediatric Research in Inpatient Settings Network, which compared intravenous versus oral antibiotics treatments given after discharge: The first, published in JAMA Pediatrics in 2014, examined treatment for osteomyelitis, while the second, which focused on complicated pneumonia, was published in Pediatrics in 2016.1,2
Both were observational, retrospective studies involving more than 2,000 children across more than 30 hospitals. The JAMA Pediatrics study found that roughly half of the patients were discharged with a peripherally inserted central catheter (PICC) line, and half were prescribed oral antibiotics. In some hospitals, 100% of patients were sent home with a PICC line, and in others, all children were sent home on oral antibiotics. Although treatment failure rates were the same for both groups, 15% of the patients sent home with a PICC line had to return to the emergency department because of PICC-related complications. Some were hospitalized.1
The Pediatrics study found less variation in PICC versus oral antibiotic use across hospitals for patients with complicated pneumonia, but the treatment failure rate was slightly higher for PICC patients at 3.2%, compared with 2.6% for those on oral antibiotics. This difference, however, was not statistically significant. PICC-related complications were observed in 7.1% of patients with PICC lines also were more likely to experience adverse drug reactions, compared with patients on oral antibiotics.2
“PICC lines have some advantages, particularly when children are unable or unwilling to take oral antibiotics, but they also have risks” said Dr. Shah. “If outcomes are equivalent, why would you subject patients to the risks of a catheter? And, every time they get a fever at home with a PICC line, they need urgent evaluation for the possibility of a catheter-associated bacterial infection. There is an emotional cost, as well, to taking care of catheters in the home setting.”
Additionally, economic pressures are compelling hospitals to reduce costs and resource utilization while maintaining or improving the quality of care, Dr. Shah pointed out. “Hospitalists do many things well, and quality improvement is one of those areas. That approach really aligns with antimicrobial stewardship, and there is greater incentive with episode-based payment models and financial penalties for excess readmissions. Reducing post-discharge IV antibiotic use aligns with stewardship goals and reduces the likelihood of hospital readmissions.”
The hospital medicine division at Dr. Shah’s hospital helped assemble a multidisciplinary team involving emergency physicians, pharmacists, nursing staff, hospitalists, and infectious disease physicians to encourage the use of appropriate, narrow-spectrum antibiotics and reduce the duration of antibiotic therapies. For example, skin and soft-tissue infections that were once treated for 10-14 days are now sufficiently treated in 5-7days. These efforts to improve outcomes through better adherence to evidence-based practices, including better stewardship, earned the team the SHM Teamwork in Quality Improvement Award in 2014.
“Quality improvement is really about changing the system, and hospitalists, who excel in QI, are poised to help drive antimicrobial stewardship efforts,” Dr. Shah said.
At Oakland Medical Center, Dr. Glaser helped implement handshake rounds, an idea they adopted from a group in Colorado. Every day, with every patient, the antimicrobial stewardship team meets with representatives of the teams – pediatric intensive care, the wards, the NICU, and others – to review antibiotic treatment plans for the choice of antimicrobial drug, for the duration of treatment, and for specific conditions. “We work really closely with hospitalists and our strong pediatric pharmacy team every day to ask: ‘Do we have the right dose? Do we really need to use this antibiotic?’ ” Dr. Glaser said.
Last year, she also worked to incorporate antimicrobial stewardship principles into the hospital’s residency program. “I think the most important thing we’re doing is changing the culture,” she said. “For these young physicians, we’re giving them the knowledge to empower them rather than telling them what to do and giving them a better, fundamental understanding of infectious disease.”
For instance, most pediatric respiratory illnesses are caused by a virus, yet physicians will still prescribe antibiotics for a host of reasons – including the expectations of parents, the guesswork that can go into diagnosing a young patient who cannot describe what is wrong, and the fear that children will get sicker if an antibiotic is not started early.
“A lot of it is figuring out the best approach with the least amount of side effects but covering what we need to cover for a given patient,” she said.
A number of physicians from Dr. Glaser’s team presented stewardship data from their hospital at the July 2017 Pediatric Hospital Medicine meeting in Nashville, demonstrating that, overall, they are using fewer antibiotics and that fewer of those used are broad spectrum. This satisfies the “pillars of stewardship,” Dr. Glaser said. Use antibiotics only when you need them, use them only as long as you need, and then make sure you use the most narrow-spectrum antibiotic you possibly can, she said.
Oakland Medical Center has benefited from a strong commitment to antimicrobial stewardship efforts, Dr. Glaser said, noting that many programs may lack such support, a problem that can be one of the biggest hurdles antimicrobial stewardship efforts face. The support at her hospital “has been an immense help in getting our program to where it is today.”
References
1. Keren R, Shah SS, Srivastava R, et al. Comparative effectiveness of intravenous vs oral antibiotics for postdischarge treatment of acute osteomyelitis in children. JAMA Pediatr. 2015 Feb:169(2):120-8.
2. Shah SS, Srivastava R, Wu S, et al. Intravenous versus oral antibiotics for postdischarge treatment of complicated pneumonia. Pediatrics. 2016 Dec;138(6). pii: e20161692.