User login
Official news magazine of the Society of Hospital Medicine
Copyright by Society of Hospital Medicine or related companies. All rights reserved. ISSN 1553-085X
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
div[contains(@class, 'pane-pub-article-hospitalist')]


BP accuracy is the ghost in the machine
SAN FRANCISCO – Amid all the talk about subgroup blood pressure targets and tiny differences in drug regimens at a recent hypertension meeting, there was an elephant in the room that attendees refused to ignore.
“We do it wrong,” said Dr. Steven Yarows, a primary care physician in Chelsea, Mich., who estimated he’s taken 44,000 blood pressures in his 36 years of practice.
Everyone in medicine is taught that people should rest a bit and not talk while their blood pressure is taken; that the last measurement matters more than the first; and that most Americans need a large-sized cuff. Current guidelines are based on patients sitting for 5-10 minutes alone in a quiet room while an automatic machine averages their last 3-5 blood pressures.
But when Dr. Yarows asked his 300 or so audience members – hypertension physicians who paid to come to the meeting – how many actually followed those rules, four hands went up. It’s not good enough; “if you are going to make a diagnosis that lasts a lifetime, you have to be accurate,” he said at the joint scientific sessions of the American Heart Association Council on Hypertension, AHA Council on Kidney Cardiovascular Disease, and American Society of Hypertension.
There’s resistance. No one has a room set aside for blood pressure; staff don’t want to deal with it; and at a time when primary care doctors are nickel and dimed for everything they do, insurers haven’t stepped up to pay to make accurate blood pressure a priority.
To do it right, you have to ask patients to come in 10 minutes early and have a room set up for them where they can sit alone with a large oscillometric cuff to average a few blood pressures at rest, Dr. Yarows said. They also need at least one 24-hour monitoring.
“Most of the time, the patient walks over from the waiting room, they get on the scale which automatically elevates the blood pressure tremendously, and then they sit down and talk about their family while their blood pressure is being taken.” Even in normotensive patients, that alone could raise systolic pressure 20 mm Hg or more, he said. It makes one-time blood pressure pretty much meaningless.
The biggest problem is that blood pressure is hugely variable, so it’s hard to know what matters. In one of Dr. Yarows’ normotensive patients, BP varied 44 mm Hg systolic and 37 mm Hg diastolic over 24 hours. In a hypertensive patient, systolic pressure varied 62 mm Hg and diastolic 48 mm Hg over 24 hours. Another patient was 114/85 mm Hg at noon, and 159/73 mm Hg an hour later. “That’s a huge spread,” he said.
Twenty-four hour monitoring is the only way to really know if patients are hypertensive and need treatment. “Any person you suspect of having hypertension, before you place them on medicine, you should have 24 hour blood pressure monitoring. This is the most effective way to determine if they do have high blood pressure,” and how much it needs to be lowered, he said.
Dr. Yarows had no disclosures.
SAN FRANCISCO – Amid all the talk about subgroup blood pressure targets and tiny differences in drug regimens at a recent hypertension meeting, there was an elephant in the room that attendees refused to ignore.
“We do it wrong,” said Dr. Steven Yarows, a primary care physician in Chelsea, Mich., who estimated he’s taken 44,000 blood pressures in his 36 years of practice.
Everyone in medicine is taught that people should rest a bit and not talk while their blood pressure is taken; that the last measurement matters more than the first; and that most Americans need a large-sized cuff. Current guidelines are based on patients sitting for 5-10 minutes alone in a quiet room while an automatic machine averages their last 3-5 blood pressures.
But when Dr. Yarows asked his 300 or so audience members – hypertension physicians who paid to come to the meeting – how many actually followed those rules, four hands went up. It’s not good enough; “if you are going to make a diagnosis that lasts a lifetime, you have to be accurate,” he said at the joint scientific sessions of the American Heart Association Council on Hypertension, AHA Council on Kidney Cardiovascular Disease, and American Society of Hypertension.
There’s resistance. No one has a room set aside for blood pressure; staff don’t want to deal with it; and at a time when primary care doctors are nickel and dimed for everything they do, insurers haven’t stepped up to pay to make accurate blood pressure a priority.
To do it right, you have to ask patients to come in 10 minutes early and have a room set up for them where they can sit alone with a large oscillometric cuff to average a few blood pressures at rest, Dr. Yarows said. They also need at least one 24-hour monitoring.
“Most of the time, the patient walks over from the waiting room, they get on the scale which automatically elevates the blood pressure tremendously, and then they sit down and talk about their family while their blood pressure is being taken.” Even in normotensive patients, that alone could raise systolic pressure 20 mm Hg or more, he said. It makes one-time blood pressure pretty much meaningless.
The biggest problem is that blood pressure is hugely variable, so it’s hard to know what matters. In one of Dr. Yarows’ normotensive patients, BP varied 44 mm Hg systolic and 37 mm Hg diastolic over 24 hours. In a hypertensive patient, systolic pressure varied 62 mm Hg and diastolic 48 mm Hg over 24 hours. Another patient was 114/85 mm Hg at noon, and 159/73 mm Hg an hour later. “That’s a huge spread,” he said.
Twenty-four hour monitoring is the only way to really know if patients are hypertensive and need treatment. “Any person you suspect of having hypertension, before you place them on medicine, you should have 24 hour blood pressure monitoring. This is the most effective way to determine if they do have high blood pressure,” and how much it needs to be lowered, he said.
Dr. Yarows had no disclosures.
SAN FRANCISCO – Amid all the talk about subgroup blood pressure targets and tiny differences in drug regimens at a recent hypertension meeting, there was an elephant in the room that attendees refused to ignore.
“We do it wrong,” said Dr. Steven Yarows, a primary care physician in Chelsea, Mich., who estimated he’s taken 44,000 blood pressures in his 36 years of practice.
Everyone in medicine is taught that people should rest a bit and not talk while their blood pressure is taken; that the last measurement matters more than the first; and that most Americans need a large-sized cuff. Current guidelines are based on patients sitting for 5-10 minutes alone in a quiet room while an automatic machine averages their last 3-5 blood pressures.
But when Dr. Yarows asked his 300 or so audience members – hypertension physicians who paid to come to the meeting – how many actually followed those rules, four hands went up. It’s not good enough; “if you are going to make a diagnosis that lasts a lifetime, you have to be accurate,” he said at the joint scientific sessions of the American Heart Association Council on Hypertension, AHA Council on Kidney Cardiovascular Disease, and American Society of Hypertension.
There’s resistance. No one has a room set aside for blood pressure; staff don’t want to deal with it; and at a time when primary care doctors are nickel and dimed for everything they do, insurers haven’t stepped up to pay to make accurate blood pressure a priority.
To do it right, you have to ask patients to come in 10 minutes early and have a room set up for them where they can sit alone with a large oscillometric cuff to average a few blood pressures at rest, Dr. Yarows said. They also need at least one 24-hour monitoring.
“Most of the time, the patient walks over from the waiting room, they get on the scale which automatically elevates the blood pressure tremendously, and then they sit down and talk about their family while their blood pressure is being taken.” Even in normotensive patients, that alone could raise systolic pressure 20 mm Hg or more, he said. It makes one-time blood pressure pretty much meaningless.
The biggest problem is that blood pressure is hugely variable, so it’s hard to know what matters. In one of Dr. Yarows’ normotensive patients, BP varied 44 mm Hg systolic and 37 mm Hg diastolic over 24 hours. In a hypertensive patient, systolic pressure varied 62 mm Hg and diastolic 48 mm Hg over 24 hours. Another patient was 114/85 mm Hg at noon, and 159/73 mm Hg an hour later. “That’s a huge spread,” he said.
Twenty-four hour monitoring is the only way to really know if patients are hypertensive and need treatment. “Any person you suspect of having hypertension, before you place them on medicine, you should have 24 hour blood pressure monitoring. This is the most effective way to determine if they do have high blood pressure,” and how much it needs to be lowered, he said.
Dr. Yarows had no disclosures.
EXPERT ANALYSIS FROM JOINT HYPERTENSION 2017
Behavioral approach to appropriate antimicrobial prescribing in hospitals: The DUMAS study
Clinical question: How effective is an antimicrobial stewardship approach grounded in behavioral theory and focused on preserving prescriber autonomy and participation in improving appropriateness of antimicrobial prescribing in hospitals?
Background: Antimicrobial stewardship programs aim to achieve the goal of improving antimicrobial prescribing. This leads to significant benefits, including decreased antimicrobial resistance, improved clinical outcomes (lower morbidity and mortality), and lower health care costs. Stewardship programs do not often focus on the human behavior element of the prescribing physicians. Changing antimicrobial prescribing is a complex behavioral process, and there is a known persistent resistance between prescribers and the stewardship team. In a simple sense, this resistance is generated by tension created when prescribers do not have autonomy.
Previous studies that used interventions based on behavioral theory found promising results, but none of them were in a hospital setting. Rather, most of these studies had a narrow focus of respiratory tract infections in outpatient clinics.
Setting: Seven clinical departments (two medical, three surgical, and two pediatric) in a tertiary care medical center and a general teaching hospital, both in Amsterdam. The first hospital was a 700-bed tertiary care center with salaried specialists, while the second hospital was a 550-bed general medical center with self-employed specialists.
Synopsis: During a baseline period of 16 months and an intervention period of 12 months, physicians who prescribed systemic antimicrobial drugs for any indication were included in the study. In all, 1,121 patient cases with 700 prescriptions were studied during the baseline period and 882 patient cases with 531 prescriptions were studied during the intervention period. The intervention was as follows: Prescribers were offered a free choice of how to improve their antimicrobial prescribing. They were stimulated to choose interventions with higher potential for success based on a root cause analysis of inappropriate prescribing. The study was inspired by the participatory action research paradigm, which focuses on collaboration and empowerment of the stakeholders in the change process. In this study, prescribers were given reports of root cause analysis of their prior prescribing patterns. Then, they were invited to choose and codevelop one or more interventions that were tailored and individualized to improve their own prescribing. This approach draws on the following three behavioral principles: 1) respect for the prescriber’s autonomy to avoid feelings of resistance; 2) the inclination that people have to value a product higher and feel more ownership of it if they made it themselves (the IKEA effect); 3) the tendency of people to follow up on an active and public commitment.
The primary outcome was antimicrobial appropriateness, measured with a validated appropriateness assessment instrument. One of three infectious disease specialists assessed the adult prescriptions, and one of three infectious disease/immunology pediatricians assessed the pediatric prescriptions for appropriateness. Appropriateness criteria were as follows: indication, choice of antimicrobial, dosage, route, and duration. A secondary outcome was antimicrobial consumption, reported as days of therapy per 100 admissions per month. Other outcomes were changes in specific appropriateness categories, intravenous antimicrobial consumption, consumption of specific antimicrobial subgroups, and length of hospital stay.
The mean antimicrobial appropriateness increased from 64.1% at intervention start to 77.4% at 12-month follow-up (+13.3%; relative risk, 1.17; 95% CI, 1.04-1.27), without a change in slope. Antimicrobial consumption remained the same during both study periods. Length of hospital stay did not change relative to the start of the intervention approach.
This is the first study of its kind, as a hospital antimicrobial stewardship program study grounded in behavioral science, with the key element being the free choice allowed to the prescribers, who made their own autonomous decisions about how to improve their prescribing. The authors hypothesize that the prescribers felt relatively nonthreatened by their approach since the prescribers maintained their free will to change their own behavior if so desired. The prescribers were given a free intervention choice. For example, they could have just chosen “education” as an easy out; however, the root cause analysis seemed to be an impetus for the prescribers to choose interventions that would be more effective. A prior study in a nursing home setting was unsuccessful; aside from other differences, that study used a predetermined set of interventions, thus lacking the autonomy and IKEA effect seen in this study.
Bottom line: Use of a behavioral approach that preserves prescriber autonomy resulted in an increase in antimicrobial appropriateness sustained for at least 12 months. The intervention is effective, inexpensive, and transferable to various health care settings.
Citation: Sikkens JJ, van Agtmael MA, Peters EJG, et al. Behavioral approach to appropriate antimicrobial prescribing in hospitals: The dutch unique method for antimicrobial stewardship (DUMAS) participatoryintervention study. JAMA Intern Med. Published online May 1, 2017. doi: 10.1001/jamainternmed.2017.0946.
Dr. Ramee is a hospitalist at Ochsner Health System, New Orleans.
Clinical question: How effective is an antimicrobial stewardship approach grounded in behavioral theory and focused on preserving prescriber autonomy and participation in improving appropriateness of antimicrobial prescribing in hospitals?
Background: Antimicrobial stewardship programs aim to achieve the goal of improving antimicrobial prescribing. This leads to significant benefits, including decreased antimicrobial resistance, improved clinical outcomes (lower morbidity and mortality), and lower health care costs. Stewardship programs do not often focus on the human behavior element of the prescribing physicians. Changing antimicrobial prescribing is a complex behavioral process, and there is a known persistent resistance between prescribers and the stewardship team. In a simple sense, this resistance is generated by tension created when prescribers do not have autonomy.
Previous studies that used interventions based on behavioral theory found promising results, but none of them were in a hospital setting. Rather, most of these studies had a narrow focus of respiratory tract infections in outpatient clinics.
Setting: Seven clinical departments (two medical, three surgical, and two pediatric) in a tertiary care medical center and a general teaching hospital, both in Amsterdam. The first hospital was a 700-bed tertiary care center with salaried specialists, while the second hospital was a 550-bed general medical center with self-employed specialists.
Synopsis: During a baseline period of 16 months and an intervention period of 12 months, physicians who prescribed systemic antimicrobial drugs for any indication were included in the study. In all, 1,121 patient cases with 700 prescriptions were studied during the baseline period and 882 patient cases with 531 prescriptions were studied during the intervention period. The intervention was as follows: Prescribers were offered a free choice of how to improve their antimicrobial prescribing. They were stimulated to choose interventions with higher potential for success based on a root cause analysis of inappropriate prescribing. The study was inspired by the participatory action research paradigm, which focuses on collaboration and empowerment of the stakeholders in the change process. In this study, prescribers were given reports of root cause analysis of their prior prescribing patterns. Then, they were invited to choose and codevelop one or more interventions that were tailored and individualized to improve their own prescribing. This approach draws on the following three behavioral principles: 1) respect for the prescriber’s autonomy to avoid feelings of resistance; 2) the inclination that people have to value a product higher and feel more ownership of it if they made it themselves (the IKEA effect); 3) the tendency of people to follow up on an active and public commitment.
The primary outcome was antimicrobial appropriateness, measured with a validated appropriateness assessment instrument. One of three infectious disease specialists assessed the adult prescriptions, and one of three infectious disease/immunology pediatricians assessed the pediatric prescriptions for appropriateness. Appropriateness criteria were as follows: indication, choice of antimicrobial, dosage, route, and duration. A secondary outcome was antimicrobial consumption, reported as days of therapy per 100 admissions per month. Other outcomes were changes in specific appropriateness categories, intravenous antimicrobial consumption, consumption of specific antimicrobial subgroups, and length of hospital stay.
The mean antimicrobial appropriateness increased from 64.1% at intervention start to 77.4% at 12-month follow-up (+13.3%; relative risk, 1.17; 95% CI, 1.04-1.27), without a change in slope. Antimicrobial consumption remained the same during both study periods. Length of hospital stay did not change relative to the start of the intervention approach.
This is the first study of its kind, as a hospital antimicrobial stewardship program study grounded in behavioral science, with the key element being the free choice allowed to the prescribers, who made their own autonomous decisions about how to improve their prescribing. The authors hypothesize that the prescribers felt relatively nonthreatened by their approach since the prescribers maintained their free will to change their own behavior if so desired. The prescribers were given a free intervention choice. For example, they could have just chosen “education” as an easy out; however, the root cause analysis seemed to be an impetus for the prescribers to choose interventions that would be more effective. A prior study in a nursing home setting was unsuccessful; aside from other differences, that study used a predetermined set of interventions, thus lacking the autonomy and IKEA effect seen in this study.
Bottom line: Use of a behavioral approach that preserves prescriber autonomy resulted in an increase in antimicrobial appropriateness sustained for at least 12 months. The intervention is effective, inexpensive, and transferable to various health care settings.
Citation: Sikkens JJ, van Agtmael MA, Peters EJG, et al. Behavioral approach to appropriate antimicrobial prescribing in hospitals: The dutch unique method for antimicrobial stewardship (DUMAS) participatoryintervention study. JAMA Intern Med. Published online May 1, 2017. doi: 10.1001/jamainternmed.2017.0946.
Dr. Ramee is a hospitalist at Ochsner Health System, New Orleans.
Clinical question: How effective is an antimicrobial stewardship approach grounded in behavioral theory and focused on preserving prescriber autonomy and participation in improving appropriateness of antimicrobial prescribing in hospitals?
Background: Antimicrobial stewardship programs aim to achieve the goal of improving antimicrobial prescribing. This leads to significant benefits, including decreased antimicrobial resistance, improved clinical outcomes (lower morbidity and mortality), and lower health care costs. Stewardship programs do not often focus on the human behavior element of the prescribing physicians. Changing antimicrobial prescribing is a complex behavioral process, and there is a known persistent resistance between prescribers and the stewardship team. In a simple sense, this resistance is generated by tension created when prescribers do not have autonomy.
Previous studies that used interventions based on behavioral theory found promising results, but none of them were in a hospital setting. Rather, most of these studies had a narrow focus of respiratory tract infections in outpatient clinics.
Setting: Seven clinical departments (two medical, three surgical, and two pediatric) in a tertiary care medical center and a general teaching hospital, both in Amsterdam. The first hospital was a 700-bed tertiary care center with salaried specialists, while the second hospital was a 550-bed general medical center with self-employed specialists.
Synopsis: During a baseline period of 16 months and an intervention period of 12 months, physicians who prescribed systemic antimicrobial drugs for any indication were included in the study. In all, 1,121 patient cases with 700 prescriptions were studied during the baseline period and 882 patient cases with 531 prescriptions were studied during the intervention period. The intervention was as follows: Prescribers were offered a free choice of how to improve their antimicrobial prescribing. They were stimulated to choose interventions with higher potential for success based on a root cause analysis of inappropriate prescribing. The study was inspired by the participatory action research paradigm, which focuses on collaboration and empowerment of the stakeholders in the change process. In this study, prescribers were given reports of root cause analysis of their prior prescribing patterns. Then, they were invited to choose and codevelop one or more interventions that were tailored and individualized to improve their own prescribing. This approach draws on the following three behavioral principles: 1) respect for the prescriber’s autonomy to avoid feelings of resistance; 2) the inclination that people have to value a product higher and feel more ownership of it if they made it themselves (the IKEA effect); 3) the tendency of people to follow up on an active and public commitment.
The primary outcome was antimicrobial appropriateness, measured with a validated appropriateness assessment instrument. One of three infectious disease specialists assessed the adult prescriptions, and one of three infectious disease/immunology pediatricians assessed the pediatric prescriptions for appropriateness. Appropriateness criteria were as follows: indication, choice of antimicrobial, dosage, route, and duration. A secondary outcome was antimicrobial consumption, reported as days of therapy per 100 admissions per month. Other outcomes were changes in specific appropriateness categories, intravenous antimicrobial consumption, consumption of specific antimicrobial subgroups, and length of hospital stay.
The mean antimicrobial appropriateness increased from 64.1% at intervention start to 77.4% at 12-month follow-up (+13.3%; relative risk, 1.17; 95% CI, 1.04-1.27), without a change in slope. Antimicrobial consumption remained the same during both study periods. Length of hospital stay did not change relative to the start of the intervention approach.
This is the first study of its kind, as a hospital antimicrobial stewardship program study grounded in behavioral science, with the key element being the free choice allowed to the prescribers, who made their own autonomous decisions about how to improve their prescribing. The authors hypothesize that the prescribers felt relatively nonthreatened by their approach since the prescribers maintained their free will to change their own behavior if so desired. The prescribers were given a free intervention choice. For example, they could have just chosen “education” as an easy out; however, the root cause analysis seemed to be an impetus for the prescribers to choose interventions that would be more effective. A prior study in a nursing home setting was unsuccessful; aside from other differences, that study used a predetermined set of interventions, thus lacking the autonomy and IKEA effect seen in this study.
Bottom line: Use of a behavioral approach that preserves prescriber autonomy resulted in an increase in antimicrobial appropriateness sustained for at least 12 months. The intervention is effective, inexpensive, and transferable to various health care settings.
Citation: Sikkens JJ, van Agtmael MA, Peters EJG, et al. Behavioral approach to appropriate antimicrobial prescribing in hospitals: The dutch unique method for antimicrobial stewardship (DUMAS) participatoryintervention study. JAMA Intern Med. Published online May 1, 2017. doi: 10.1001/jamainternmed.2017.0946.
Dr. Ramee is a hospitalist at Ochsner Health System, New Orleans.
Optimal empiric treatment for uncomplicated cellulitis
Clinical question: Is empiric MRSA coverage for nonpurulent cellulitis necessary?
Background: Most nonpurulent skin and soft tissue infections are caused by beta-hemolytic streptococci and methicillin-susceptible Staphylococcus aureus. However, there is a growing incidence of community-acquired methicillin-resistant S. aureus infections. The authors of this study attempted to answer whether adding empiric methicillin-resistant S. aureus coverage reduces the risk of treatment failure.
Setting: Five emergency departments in the United States.
Synopsis: The authors of this study randomized 500 patients with cellulitis without purulent drainage or evidence of abscess as confirmed by sonography to receive a 7-day course of either cephalexin with placebo or cephalexin plus trimethoprimsulfamethoxazole. When analyzing those patients who took most of the prescribed pills (greater than 75% of doses) according to treatment protocol, there was no significant difference in clinical cure rate between the two arms of the study, reaffirming current guidelines that advocate against empiric methicillin-resistant S. aureus coverage for uncomplicated cellulitis.
When the authors analyzed the result of their data with the assumption that patients who were lost to follow-up had treatment failure, there was a trend favoring the addition of trimethoprim-sulfamethoxazole with cephalexin over monotherapy with cephalexin (P = .07). Although the authors concluded that this finding may warrant further investigation, this was essentially a negative study.
Bottom line: Empirically adding community-acquired methicillin-resistant S. aureus coverage with trimethoprim-sulfamethoxazole to uncomplicated cellulitis did not statistically improve a clinical cure, compared with empiric treatment with monotherapy with cephalexin.
Citation: Moran GJ, Krishnadasan A, Mower WR, et al. Effect of cephalexin plus trimethoprim-sulfamethoxazole vs. cephalexin alone on clinical cure of uncomplicated cellulitis. JAMA. 2017;317(20):2088-96.
Dr. Ramee is a hospitalist at Ochsner Health System, New Orleans.
Clinical question: Is empiric MRSA coverage for nonpurulent cellulitis necessary?
Background: Most nonpurulent skin and soft tissue infections are caused by beta-hemolytic streptococci and methicillin-susceptible Staphylococcus aureus. However, there is a growing incidence of community-acquired methicillin-resistant S. aureus infections. The authors of this study attempted to answer whether adding empiric methicillin-resistant S. aureus coverage reduces the risk of treatment failure.
Setting: Five emergency departments in the United States.
Synopsis: The authors of this study randomized 500 patients with cellulitis without purulent drainage or evidence of abscess as confirmed by sonography to receive a 7-day course of either cephalexin with placebo or cephalexin plus trimethoprimsulfamethoxazole. When analyzing those patients who took most of the prescribed pills (greater than 75% of doses) according to treatment protocol, there was no significant difference in clinical cure rate between the two arms of the study, reaffirming current guidelines that advocate against empiric methicillin-resistant S. aureus coverage for uncomplicated cellulitis.
When the authors analyzed the result of their data with the assumption that patients who were lost to follow-up had treatment failure, there was a trend favoring the addition of trimethoprim-sulfamethoxazole with cephalexin over monotherapy with cephalexin (P = .07). Although the authors concluded that this finding may warrant further investigation, this was essentially a negative study.
Bottom line: Empirically adding community-acquired methicillin-resistant S. aureus coverage with trimethoprim-sulfamethoxazole to uncomplicated cellulitis did not statistically improve a clinical cure, compared with empiric treatment with monotherapy with cephalexin.
Citation: Moran GJ, Krishnadasan A, Mower WR, et al. Effect of cephalexin plus trimethoprim-sulfamethoxazole vs. cephalexin alone on clinical cure of uncomplicated cellulitis. JAMA. 2017;317(20):2088-96.
Dr. Ramee is a hospitalist at Ochsner Health System, New Orleans.
Clinical question: Is empiric MRSA coverage for nonpurulent cellulitis necessary?
Background: Most nonpurulent skin and soft tissue infections are caused by beta-hemolytic streptococci and methicillin-susceptible Staphylococcus aureus. However, there is a growing incidence of community-acquired methicillin-resistant S. aureus infections. The authors of this study attempted to answer whether adding empiric methicillin-resistant S. aureus coverage reduces the risk of treatment failure.
Setting: Five emergency departments in the United States.
Synopsis: The authors of this study randomized 500 patients with cellulitis without purulent drainage or evidence of abscess as confirmed by sonography to receive a 7-day course of either cephalexin with placebo or cephalexin plus trimethoprimsulfamethoxazole. When analyzing those patients who took most of the prescribed pills (greater than 75% of doses) according to treatment protocol, there was no significant difference in clinical cure rate between the two arms of the study, reaffirming current guidelines that advocate against empiric methicillin-resistant S. aureus coverage for uncomplicated cellulitis.
When the authors analyzed the result of their data with the assumption that patients who were lost to follow-up had treatment failure, there was a trend favoring the addition of trimethoprim-sulfamethoxazole with cephalexin over monotherapy with cephalexin (P = .07). Although the authors concluded that this finding may warrant further investigation, this was essentially a negative study.
Bottom line: Empirically adding community-acquired methicillin-resistant S. aureus coverage with trimethoprim-sulfamethoxazole to uncomplicated cellulitis did not statistically improve a clinical cure, compared with empiric treatment with monotherapy with cephalexin.
Citation: Moran GJ, Krishnadasan A, Mower WR, et al. Effect of cephalexin plus trimethoprim-sulfamethoxazole vs. cephalexin alone on clinical cure of uncomplicated cellulitis. JAMA. 2017;317(20):2088-96.
Dr. Ramee is a hospitalist at Ochsner Health System, New Orleans.
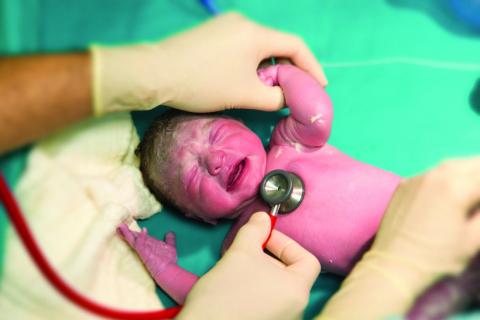
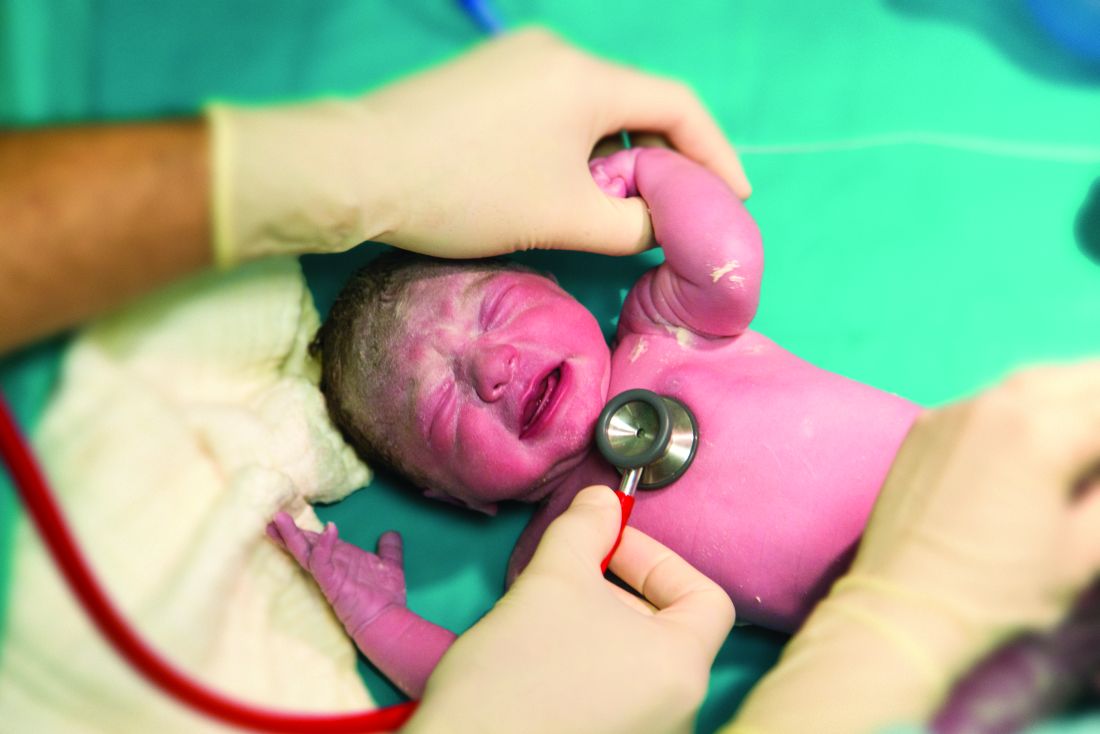
Rectal temps in the nursery
It seems to me that the closer one could get to the center of the child’s body, the more likely you would get a true reading – and the less likely you would fall victim to operator error. However, a study reported on the Pediatric News website suggests that our intuition is wrong again (“Axillary thermometry is the best choice for newborns,” by M. Alexander Otto, Aug. 24, 2017). In the study of 205 newborns at the University of North Carolina at Chapel Hill Medical Center, multiple temperatures were recorded using three methods over a 15-minute period. Rectal temperatures were accurate but less reliable than axillary readings, while temporal artery measurements tended to “overestimate temperatures by an average of about a quarter of a degree.”
However, before we jump on the no-rectal-temps in the nursery bandwagon, let’s look at the rectal probe not just as a way to assess a newborn’s temperature, but as a tool for examining the baby’s rectum. For a variety of reasons, the newborn perineum often seems to escape the careful examination it deserves, particularly if the initial exam is performed with the parents watching.
Of course, parents are interested in their baby’s hair and eye color, and whether it has the requisite number of fingers and toes. They will wait anxiously until you have lifted your stethoscope off the baby’s chest and given them a nod and smile. However, doing a thorough exam of the infant’s genitalia may appear a bit invasive and improper to some parents. Whether it is because we sense some unspoken parental discomfort or because we are trying to save time, the nether regions of little girls are inadequately examined.
But back to rectal temperatures. It seems to me that it would be prudent to adopt a guideline that says that a newborn’s first temperature be taken rectally. Not because it is any more accurate than an axillary temperature – which this study suggests that it is not. But because the process of taking the temperature would make it more likely (I hesitate to say guarantee) that someone will be taking a careful look at the newborn’s rectum. That initial rectal temperature is not going to detect every genital anomaly, but it may help find some in a more timely fashion. If nothing else, it will get that meconium moving.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.”
It seems to me that the closer one could get to the center of the child’s body, the more likely you would get a true reading – and the less likely you would fall victim to operator error. However, a study reported on the Pediatric News website suggests that our intuition is wrong again (“Axillary thermometry is the best choice for newborns,” by M. Alexander Otto, Aug. 24, 2017). In the study of 205 newborns at the University of North Carolina at Chapel Hill Medical Center, multiple temperatures were recorded using three methods over a 15-minute period. Rectal temperatures were accurate but less reliable than axillary readings, while temporal artery measurements tended to “overestimate temperatures by an average of about a quarter of a degree.”
However, before we jump on the no-rectal-temps in the nursery bandwagon, let’s look at the rectal probe not just as a way to assess a newborn’s temperature, but as a tool for examining the baby’s rectum. For a variety of reasons, the newborn perineum often seems to escape the careful examination it deserves, particularly if the initial exam is performed with the parents watching.
Of course, parents are interested in their baby’s hair and eye color, and whether it has the requisite number of fingers and toes. They will wait anxiously until you have lifted your stethoscope off the baby’s chest and given them a nod and smile. However, doing a thorough exam of the infant’s genitalia may appear a bit invasive and improper to some parents. Whether it is because we sense some unspoken parental discomfort or because we are trying to save time, the nether regions of little girls are inadequately examined.
But back to rectal temperatures. It seems to me that it would be prudent to adopt a guideline that says that a newborn’s first temperature be taken rectally. Not because it is any more accurate than an axillary temperature – which this study suggests that it is not. But because the process of taking the temperature would make it more likely (I hesitate to say guarantee) that someone will be taking a careful look at the newborn’s rectum. That initial rectal temperature is not going to detect every genital anomaly, but it may help find some in a more timely fashion. If nothing else, it will get that meconium moving.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.”
It seems to me that the closer one could get to the center of the child’s body, the more likely you would get a true reading – and the less likely you would fall victim to operator error. However, a study reported on the Pediatric News website suggests that our intuition is wrong again (“Axillary thermometry is the best choice for newborns,” by M. Alexander Otto, Aug. 24, 2017). In the study of 205 newborns at the University of North Carolina at Chapel Hill Medical Center, multiple temperatures were recorded using three methods over a 15-minute period. Rectal temperatures were accurate but less reliable than axillary readings, while temporal artery measurements tended to “overestimate temperatures by an average of about a quarter of a degree.”
However, before we jump on the no-rectal-temps in the nursery bandwagon, let’s look at the rectal probe not just as a way to assess a newborn’s temperature, but as a tool for examining the baby’s rectum. For a variety of reasons, the newborn perineum often seems to escape the careful examination it deserves, particularly if the initial exam is performed with the parents watching.
Of course, parents are interested in their baby’s hair and eye color, and whether it has the requisite number of fingers and toes. They will wait anxiously until you have lifted your stethoscope off the baby’s chest and given them a nod and smile. However, doing a thorough exam of the infant’s genitalia may appear a bit invasive and improper to some parents. Whether it is because we sense some unspoken parental discomfort or because we are trying to save time, the nether regions of little girls are inadequately examined.
But back to rectal temperatures. It seems to me that it would be prudent to adopt a guideline that says that a newborn’s first temperature be taken rectally. Not because it is any more accurate than an axillary temperature – which this study suggests that it is not. But because the process of taking the temperature would make it more likely (I hesitate to say guarantee) that someone will be taking a careful look at the newborn’s rectum. That initial rectal temperature is not going to detect every genital anomaly, but it may help find some in a more timely fashion. If nothing else, it will get that meconium moving.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.”
SHM suggests tweaks to CMS QPP proposal
The Society of Hospital Medicine approves of the direction the Centers for Medicare & Medicaid Services is heading when it comes to measuring pay-for-performance for hospitalists in its Quality Payment Program (QPP) but is suggesting some tweaks to make it a better system.
The proposed CMS 2018 update to the QPP, the value-based payment scheme developed by the Medicare Access and CHIP Reauthorization Act (MACRA), included an option that would allow all physicians who primarily practice in a hospital setting to report as a unified group under the hospital umbrella – as an alternative to reporting as an individual in the Merit-Based Incentive Payment System (MIPS) track.
He said there is lot to like in that option, although there are things that need to be changed as well.
One key area SHM would like to see changed is how time spent in a hospital is measured. In the CMS proposal, codes related to site of service capture only those in the emergency room and those admitted for in-patient services. Doctors who are seeing patients on an observation basis before they are admitted are not captured and could not be included in the facility payment.
“Observation services are virtually indistinguishable from inpatient care and frequently occur on the same wards of the hospital,” SHM said in Aug. 21, 2017, comments to CMS on the proposed QPP update, noting that observational care is built around the two-midnight rule.
“We disagree with this interpretation,” the SHM letter continues. “While it is true observation is generally time limited for a given patient, practice structures and provider scheduling have a profound [impact] on the proportion of observation care an individual clinician provides.” The letter noted that hospitalists who are on observation service could have a high proportion of observation (outpatient) billing, which could in turn exclude them from qualifying for a facility-based reporting option “despite the fact they are truly hospital-based inpatient providers.”
Dr. Greeno noted that some hospitals have hospitalists that exclusively provide observational care.
The proposal designates physicians who meet a 75% threshold of providing care in an emergency room or in-patient setting as eligible to opt into facility-based reporting.
SHM suggests that if observation services cannot be included in the 75% threshold, those services should be included and “couple the calculation with a cross-check to ensure most other billing is also hospital-based. As a further check, CMS could look at specialty codes – is the provider also enrolled in Medicare as a hospitalist?” SHM also recommends lowering the threshold “to 70% or, ideally, 60%. Due to the wide variation in hospitalist practice, we are uncomfortable with the use of thresholds in general, but lowering this threshold would at least provide a kind of safety net for hospitalists who are caring for high numbers of patients on observation.”
Another key area that needs to be addressed is the quality metrics that are used for scoring, which Dr. Greeno acknowledged is “surprisingly hard to do.”
For the 2018 reporting year, CMS is proposing that the required number of measures for the MIPS program be six, that same is it currently is for 2017. While SHM agrees with this level, “we remind CMS that even six measures may be a challenge for some providers, including hospitalists, to meet. Concerted efforts should be made to ensure that those providers who have fewer than six measures available for reporting are not disadvantaged in any way.”
Two of the six hospitalist-specific quality metrics relate to heart attacks, Dr. Seymann noted.
“Most hospitalists do take care of these patients, but they can only be reported via registry or via an electronic health record, and I don’t know that all hospitalist groups have access to reporting those ways,” Dr. Seymann said. “Most folks are reporting when they submit their billing claims. That takes two measures away from them. That may significantly decrease your score, even if you are trying your best.”
While Dr. Seymann applauded CMS for the slow rollout of the MIPS program in general, “we haven’t seen great progress as far as the growth of available relevant measures for hospitalists, and I am not confident that 2 years down the line we are going to have 12 measures to choose from.”
He did suggest that hospitalists would like a greater variety of measures and want to be measured on the quality of care they provide.
“We truly believe that the majority of hospitalist groups are really heavily invested in improving the quality of care that is provided at their hospitals – that is a big part of the culture of hospital medicine in general,” Dr. Seymann said. “We want to make our ability to succeed and participate in this program as effective as we can. We want to try to minimize barriers to hospitalists hitting this one out of the park.”
SHM also noted that certain measures rarely meet the volume threshold, which could ultimately put hospitalists at a disadvantage when it comes to receiving bonus payments.
“This is not an acceptable outcome, and we strongly urge CMS to develop a solution for providers with low-volume measures, such as removing low-volume measures from the Quality category score,” SHM wrote.
Ultimately, Dr. Greeno believes the facility reporting opt-in will survive when the rule is finalized.
“We fully expect there to be a facility-based option for hospital-based doctors, including hospitalists,” he said. “So rather than reporting on physician metrics, especially metrics through MIPS, they can get rewarded or penalized based on the hospital value-based purchasing metrics for their hospital.”
The Society of Hospital Medicine approves of the direction the Centers for Medicare & Medicaid Services is heading when it comes to measuring pay-for-performance for hospitalists in its Quality Payment Program (QPP) but is suggesting some tweaks to make it a better system.
The proposed CMS 2018 update to the QPP, the value-based payment scheme developed by the Medicare Access and CHIP Reauthorization Act (MACRA), included an option that would allow all physicians who primarily practice in a hospital setting to report as a unified group under the hospital umbrella – as an alternative to reporting as an individual in the Merit-Based Incentive Payment System (MIPS) track.
He said there is lot to like in that option, although there are things that need to be changed as well.
One key area SHM would like to see changed is how time spent in a hospital is measured. In the CMS proposal, codes related to site of service capture only those in the emergency room and those admitted for in-patient services. Doctors who are seeing patients on an observation basis before they are admitted are not captured and could not be included in the facility payment.
“Observation services are virtually indistinguishable from inpatient care and frequently occur on the same wards of the hospital,” SHM said in Aug. 21, 2017, comments to CMS on the proposed QPP update, noting that observational care is built around the two-midnight rule.
“We disagree with this interpretation,” the SHM letter continues. “While it is true observation is generally time limited for a given patient, practice structures and provider scheduling have a profound [impact] on the proportion of observation care an individual clinician provides.” The letter noted that hospitalists who are on observation service could have a high proportion of observation (outpatient) billing, which could in turn exclude them from qualifying for a facility-based reporting option “despite the fact they are truly hospital-based inpatient providers.”
Dr. Greeno noted that some hospitals have hospitalists that exclusively provide observational care.
The proposal designates physicians who meet a 75% threshold of providing care in an emergency room or in-patient setting as eligible to opt into facility-based reporting.
SHM suggests that if observation services cannot be included in the 75% threshold, those services should be included and “couple the calculation with a cross-check to ensure most other billing is also hospital-based. As a further check, CMS could look at specialty codes – is the provider also enrolled in Medicare as a hospitalist?” SHM also recommends lowering the threshold “to 70% or, ideally, 60%. Due to the wide variation in hospitalist practice, we are uncomfortable with the use of thresholds in general, but lowering this threshold would at least provide a kind of safety net for hospitalists who are caring for high numbers of patients on observation.”
Another key area that needs to be addressed is the quality metrics that are used for scoring, which Dr. Greeno acknowledged is “surprisingly hard to do.”
For the 2018 reporting year, CMS is proposing that the required number of measures for the MIPS program be six, that same is it currently is for 2017. While SHM agrees with this level, “we remind CMS that even six measures may be a challenge for some providers, including hospitalists, to meet. Concerted efforts should be made to ensure that those providers who have fewer than six measures available for reporting are not disadvantaged in any way.”
Two of the six hospitalist-specific quality metrics relate to heart attacks, Dr. Seymann noted.
“Most hospitalists do take care of these patients, but they can only be reported via registry or via an electronic health record, and I don’t know that all hospitalist groups have access to reporting those ways,” Dr. Seymann said. “Most folks are reporting when they submit their billing claims. That takes two measures away from them. That may significantly decrease your score, even if you are trying your best.”
While Dr. Seymann applauded CMS for the slow rollout of the MIPS program in general, “we haven’t seen great progress as far as the growth of available relevant measures for hospitalists, and I am not confident that 2 years down the line we are going to have 12 measures to choose from.”
He did suggest that hospitalists would like a greater variety of measures and want to be measured on the quality of care they provide.
“We truly believe that the majority of hospitalist groups are really heavily invested in improving the quality of care that is provided at their hospitals – that is a big part of the culture of hospital medicine in general,” Dr. Seymann said. “We want to make our ability to succeed and participate in this program as effective as we can. We want to try to minimize barriers to hospitalists hitting this one out of the park.”
SHM also noted that certain measures rarely meet the volume threshold, which could ultimately put hospitalists at a disadvantage when it comes to receiving bonus payments.
“This is not an acceptable outcome, and we strongly urge CMS to develop a solution for providers with low-volume measures, such as removing low-volume measures from the Quality category score,” SHM wrote.
Ultimately, Dr. Greeno believes the facility reporting opt-in will survive when the rule is finalized.
“We fully expect there to be a facility-based option for hospital-based doctors, including hospitalists,” he said. “So rather than reporting on physician metrics, especially metrics through MIPS, they can get rewarded or penalized based on the hospital value-based purchasing metrics for their hospital.”
The Society of Hospital Medicine approves of the direction the Centers for Medicare & Medicaid Services is heading when it comes to measuring pay-for-performance for hospitalists in its Quality Payment Program (QPP) but is suggesting some tweaks to make it a better system.
The proposed CMS 2018 update to the QPP, the value-based payment scheme developed by the Medicare Access and CHIP Reauthorization Act (MACRA), included an option that would allow all physicians who primarily practice in a hospital setting to report as a unified group under the hospital umbrella – as an alternative to reporting as an individual in the Merit-Based Incentive Payment System (MIPS) track.
He said there is lot to like in that option, although there are things that need to be changed as well.
One key area SHM would like to see changed is how time spent in a hospital is measured. In the CMS proposal, codes related to site of service capture only those in the emergency room and those admitted for in-patient services. Doctors who are seeing patients on an observation basis before they are admitted are not captured and could not be included in the facility payment.
“Observation services are virtually indistinguishable from inpatient care and frequently occur on the same wards of the hospital,” SHM said in Aug. 21, 2017, comments to CMS on the proposed QPP update, noting that observational care is built around the two-midnight rule.
“We disagree with this interpretation,” the SHM letter continues. “While it is true observation is generally time limited for a given patient, practice structures and provider scheduling have a profound [impact] on the proportion of observation care an individual clinician provides.” The letter noted that hospitalists who are on observation service could have a high proportion of observation (outpatient) billing, which could in turn exclude them from qualifying for a facility-based reporting option “despite the fact they are truly hospital-based inpatient providers.”
Dr. Greeno noted that some hospitals have hospitalists that exclusively provide observational care.
The proposal designates physicians who meet a 75% threshold of providing care in an emergency room or in-patient setting as eligible to opt into facility-based reporting.
SHM suggests that if observation services cannot be included in the 75% threshold, those services should be included and “couple the calculation with a cross-check to ensure most other billing is also hospital-based. As a further check, CMS could look at specialty codes – is the provider also enrolled in Medicare as a hospitalist?” SHM also recommends lowering the threshold “to 70% or, ideally, 60%. Due to the wide variation in hospitalist practice, we are uncomfortable with the use of thresholds in general, but lowering this threshold would at least provide a kind of safety net for hospitalists who are caring for high numbers of patients on observation.”
Another key area that needs to be addressed is the quality metrics that are used for scoring, which Dr. Greeno acknowledged is “surprisingly hard to do.”
For the 2018 reporting year, CMS is proposing that the required number of measures for the MIPS program be six, that same is it currently is for 2017. While SHM agrees with this level, “we remind CMS that even six measures may be a challenge for some providers, including hospitalists, to meet. Concerted efforts should be made to ensure that those providers who have fewer than six measures available for reporting are not disadvantaged in any way.”
Two of the six hospitalist-specific quality metrics relate to heart attacks, Dr. Seymann noted.
“Most hospitalists do take care of these patients, but they can only be reported via registry or via an electronic health record, and I don’t know that all hospitalist groups have access to reporting those ways,” Dr. Seymann said. “Most folks are reporting when they submit their billing claims. That takes two measures away from them. That may significantly decrease your score, even if you are trying your best.”
While Dr. Seymann applauded CMS for the slow rollout of the MIPS program in general, “we haven’t seen great progress as far as the growth of available relevant measures for hospitalists, and I am not confident that 2 years down the line we are going to have 12 measures to choose from.”
He did suggest that hospitalists would like a greater variety of measures and want to be measured on the quality of care they provide.
“We truly believe that the majority of hospitalist groups are really heavily invested in improving the quality of care that is provided at their hospitals – that is a big part of the culture of hospital medicine in general,” Dr. Seymann said. “We want to make our ability to succeed and participate in this program as effective as we can. We want to try to minimize barriers to hospitalists hitting this one out of the park.”
SHM also noted that certain measures rarely meet the volume threshold, which could ultimately put hospitalists at a disadvantage when it comes to receiving bonus payments.
“This is not an acceptable outcome, and we strongly urge CMS to develop a solution for providers with low-volume measures, such as removing low-volume measures from the Quality category score,” SHM wrote.
Ultimately, Dr. Greeno believes the facility reporting opt-in will survive when the rule is finalized.
“We fully expect there to be a facility-based option for hospital-based doctors, including hospitalists,” he said. “So rather than reporting on physician metrics, especially metrics through MIPS, they can get rewarded or penalized based on the hospital value-based purchasing metrics for their hospital.”
Here’s what’s trending at SHM – Oct. 2017
Don’t miss pre-courses at HM18
Enrich your educational experience and earn additional CME credit and MOC points with pre-courses at Hospital Medicine 2018 (HM18), to be held from April 8-11, 2018, at the Orlando (Fla.) World Center Marriott.
Broaden your skills, fine-tune your practice, and immerse yourself in a day of learning by enrolling in one of the following:
• Bedside procedures for the hospitalist
• Essentials of perioperative medicine and comanagement for the hospitalist
• Hospitalist practice management: How to thrive in a time of intense change
• Sepsis: New insights into detection and management
• Keep your finger on the pulse – cardiology update for the hospitalist
• Maintenance of certification and board prep
• Point-of-care ultrasound for the hospitalist
Pre-course day is Sunday, April 8, 2018. Learn more and register at shmannualconference.org/precourse.
Improve quality at your institution with SHM
The National Association for Healthcare Quality’s (NAHQ) Healthcare Quality Week is Oct. 15-21, 2017, a week dedicated to celebrating the contributions professionals have made in the field and bringing awareness to the profession of health care quality. SHM’s Center for Quality Improvement provides a variety of resources, tools, and programs to address quality and patient safety issues at your institution. Learn more at hospitalmedicine.org/QI.
Distinguish yourself as a Class of 2018 Fellow in Hospital Medicine
SHM’s Fellows designation is a prestigious way to differentiate yourself in the rapidly growing profession of hospital medicine. There are currently over 2,000 hospitalists who have earned the Fellow in Hospital Medicine (FHM) or Senior Fellow in Hospital Medicine (SFHM) designation by demonstrating the core values of leadership, teamwork, and quality improvement.
Apply now and learn how you can join this prestigious group of hospitalists at hospitalmedicine.org/fellows. Applications officially close on Nov. 30, 2017.
Critical care for the hospitalist: Now on the SHM Learning Portal
Many hospitalists provide critical care services without adequate support or training, putting patients at risk and exposing hospitalists to medical liability. Don’t miss the newest SHM Learning Portal series, Critical Care for the Hospitalist. The four courses in this educational series cover common or high-risk clinical scenarios that hospitalists encounter in and out of the intensive care unit, including:
1. Airway management for the hospitalist
2. Noninvasive positive pressure ventilation for the hospitalist
3. Arrhythmias
4. High-risk pulmonary embolism
This series is free for SHM members and $45 per module for nonmembers. Earn 0.75 AMA PRA Category 1 Credit™ and ABIM MOC points per each module. Visit shmlearningportal.org to get started today.
Connect with SHM locally at a chapter meeting near you
Attend a chapter meeting to experience SHM at the local level. Chapter meetings provide focused educational topics through keynote speakers, presentations, and opportunities to network with other hospitalists in your area. Find a chapter meeting close to you at hospitalmedicine.org/chapters.
Stay on top of trending topics in practice management
SHM recently released white papers on trending topics in practice management: Hospitalist Perspectives on EMRs, Telemedicine in Hospital Medicine, and the Evolution of Co-Management in Hospital Medicine. These resources are free to download to members and can be found at hospitalmedicine.org under the Practice Management tab.
Enhance your coding skills and earn CME
SHM’s Clinical Documentation & Coding for Hospitalists (formerly CODE-H) recently launched an updated program with all-new content that offers hospitalists the latest information on best practices in coding, documentation, and compliance from national experts. It provides eight recorded webinar sessions presented by expert faculty, downloadable resources, and an interactive discussion forum on SHM’s online community.
CME credits are offered through an evaluation following the webinars. Each participant is eligible for CME credits for completion of the series.
To learn more, visit hospitalmedicine.org/CODEH. If you have questions on the new program, please contact [email protected].
Mr. Radler is marketing communications manager at the Society of Hospital Medicine.
Don’t miss pre-courses at HM18
Enrich your educational experience and earn additional CME credit and MOC points with pre-courses at Hospital Medicine 2018 (HM18), to be held from April 8-11, 2018, at the Orlando (Fla.) World Center Marriott.
Broaden your skills, fine-tune your practice, and immerse yourself in a day of learning by enrolling in one of the following:
• Bedside procedures for the hospitalist
• Essentials of perioperative medicine and comanagement for the hospitalist
• Hospitalist practice management: How to thrive in a time of intense change
• Sepsis: New insights into detection and management
• Keep your finger on the pulse – cardiology update for the hospitalist
• Maintenance of certification and board prep
• Point-of-care ultrasound for the hospitalist
Pre-course day is Sunday, April 8, 2018. Learn more and register at shmannualconference.org/precourse.
Improve quality at your institution with SHM
The National Association for Healthcare Quality’s (NAHQ) Healthcare Quality Week is Oct. 15-21, 2017, a week dedicated to celebrating the contributions professionals have made in the field and bringing awareness to the profession of health care quality. SHM’s Center for Quality Improvement provides a variety of resources, tools, and programs to address quality and patient safety issues at your institution. Learn more at hospitalmedicine.org/QI.
Distinguish yourself as a Class of 2018 Fellow in Hospital Medicine
SHM’s Fellows designation is a prestigious way to differentiate yourself in the rapidly growing profession of hospital medicine. There are currently over 2,000 hospitalists who have earned the Fellow in Hospital Medicine (FHM) or Senior Fellow in Hospital Medicine (SFHM) designation by demonstrating the core values of leadership, teamwork, and quality improvement.
Apply now and learn how you can join this prestigious group of hospitalists at hospitalmedicine.org/fellows. Applications officially close on Nov. 30, 2017.
Critical care for the hospitalist: Now on the SHM Learning Portal
Many hospitalists provide critical care services without adequate support or training, putting patients at risk and exposing hospitalists to medical liability. Don’t miss the newest SHM Learning Portal series, Critical Care for the Hospitalist. The four courses in this educational series cover common or high-risk clinical scenarios that hospitalists encounter in and out of the intensive care unit, including:
1. Airway management for the hospitalist
2. Noninvasive positive pressure ventilation for the hospitalist
3. Arrhythmias
4. High-risk pulmonary embolism
This series is free for SHM members and $45 per module for nonmembers. Earn 0.75 AMA PRA Category 1 Credit™ and ABIM MOC points per each module. Visit shmlearningportal.org to get started today.
Connect with SHM locally at a chapter meeting near you
Attend a chapter meeting to experience SHM at the local level. Chapter meetings provide focused educational topics through keynote speakers, presentations, and opportunities to network with other hospitalists in your area. Find a chapter meeting close to you at hospitalmedicine.org/chapters.
Stay on top of trending topics in practice management
SHM recently released white papers on trending topics in practice management: Hospitalist Perspectives on EMRs, Telemedicine in Hospital Medicine, and the Evolution of Co-Management in Hospital Medicine. These resources are free to download to members and can be found at hospitalmedicine.org under the Practice Management tab.
Enhance your coding skills and earn CME
SHM’s Clinical Documentation & Coding for Hospitalists (formerly CODE-H) recently launched an updated program with all-new content that offers hospitalists the latest information on best practices in coding, documentation, and compliance from national experts. It provides eight recorded webinar sessions presented by expert faculty, downloadable resources, and an interactive discussion forum on SHM’s online community.
CME credits are offered through an evaluation following the webinars. Each participant is eligible for CME credits for completion of the series.
To learn more, visit hospitalmedicine.org/CODEH. If you have questions on the new program, please contact [email protected].
Mr. Radler is marketing communications manager at the Society of Hospital Medicine.
Don’t miss pre-courses at HM18
Enrich your educational experience and earn additional CME credit and MOC points with pre-courses at Hospital Medicine 2018 (HM18), to be held from April 8-11, 2018, at the Orlando (Fla.) World Center Marriott.
Broaden your skills, fine-tune your practice, and immerse yourself in a day of learning by enrolling in one of the following:
• Bedside procedures for the hospitalist
• Essentials of perioperative medicine and comanagement for the hospitalist
• Hospitalist practice management: How to thrive in a time of intense change
• Sepsis: New insights into detection and management
• Keep your finger on the pulse – cardiology update for the hospitalist
• Maintenance of certification and board prep
• Point-of-care ultrasound for the hospitalist
Pre-course day is Sunday, April 8, 2018. Learn more and register at shmannualconference.org/precourse.
Improve quality at your institution with SHM
The National Association for Healthcare Quality’s (NAHQ) Healthcare Quality Week is Oct. 15-21, 2017, a week dedicated to celebrating the contributions professionals have made in the field and bringing awareness to the profession of health care quality. SHM’s Center for Quality Improvement provides a variety of resources, tools, and programs to address quality and patient safety issues at your institution. Learn more at hospitalmedicine.org/QI.
Distinguish yourself as a Class of 2018 Fellow in Hospital Medicine
SHM’s Fellows designation is a prestigious way to differentiate yourself in the rapidly growing profession of hospital medicine. There are currently over 2,000 hospitalists who have earned the Fellow in Hospital Medicine (FHM) or Senior Fellow in Hospital Medicine (SFHM) designation by demonstrating the core values of leadership, teamwork, and quality improvement.
Apply now and learn how you can join this prestigious group of hospitalists at hospitalmedicine.org/fellows. Applications officially close on Nov. 30, 2017.
Critical care for the hospitalist: Now on the SHM Learning Portal
Many hospitalists provide critical care services without adequate support or training, putting patients at risk and exposing hospitalists to medical liability. Don’t miss the newest SHM Learning Portal series, Critical Care for the Hospitalist. The four courses in this educational series cover common or high-risk clinical scenarios that hospitalists encounter in and out of the intensive care unit, including:
1. Airway management for the hospitalist
2. Noninvasive positive pressure ventilation for the hospitalist
3. Arrhythmias
4. High-risk pulmonary embolism
This series is free for SHM members and $45 per module for nonmembers. Earn 0.75 AMA PRA Category 1 Credit™ and ABIM MOC points per each module. Visit shmlearningportal.org to get started today.
Connect with SHM locally at a chapter meeting near you
Attend a chapter meeting to experience SHM at the local level. Chapter meetings provide focused educational topics through keynote speakers, presentations, and opportunities to network with other hospitalists in your area. Find a chapter meeting close to you at hospitalmedicine.org/chapters.
Stay on top of trending topics in practice management
SHM recently released white papers on trending topics in practice management: Hospitalist Perspectives on EMRs, Telemedicine in Hospital Medicine, and the Evolution of Co-Management in Hospital Medicine. These resources are free to download to members and can be found at hospitalmedicine.org under the Practice Management tab.
Enhance your coding skills and earn CME
SHM’s Clinical Documentation & Coding for Hospitalists (formerly CODE-H) recently launched an updated program with all-new content that offers hospitalists the latest information on best practices in coding, documentation, and compliance from national experts. It provides eight recorded webinar sessions presented by expert faculty, downloadable resources, and an interactive discussion forum on SHM’s online community.
CME credits are offered through an evaluation following the webinars. Each participant is eligible for CME credits for completion of the series.
To learn more, visit hospitalmedicine.org/CODEH. If you have questions on the new program, please contact [email protected].
Mr. Radler is marketing communications manager at the Society of Hospital Medicine.
Oral anticoagulation ‘reasonable’ in advanced kidney disease with A-fib
BARCELONA – Oral anticoagulation had a net overall benefit for patients with atrial fibrillation and advanced chronic kidney disease, based on results of a large observational study reported at the annual congress of the European Society of Cardiology.
The novel direct-acting oral anticoagulants (NOACs) and warfarin were all similarly effective in this study of 39,241 patients who had stage 4 or 5 chronic kidney disease (CKD), atrial fibrillation, and were not on dialysis. Compared with no oral anticoagulation, the drugs cut in half the risk of stroke or systemic embolism, with no increased risk of major bleeding.
“In patients with advanced CKD, it appears that OACs [oral anticoagulants] are reasonable,” concluded Peter A. Noseworthy, MD, of the Mayo Clinic in Rochester, Minn.
This is a potentially practice-changing finding given the “striking underutilization” of OACs in advanced CKD, he noted. Indeed, only one-third of the patients in this study were prescribed an OAC and picked up their prescriptions. And while the study has the limitations inherent to an observational study reliant upon data from a large U.S. administrative database – chiefly, the potential for residual confounding because of factors that couldn’t be adjusted for statistically – these real-world data may be as good as it gets, since patients with advanced CKD were excluded from the pivotal trials of the NOACs.
Apixaban (Eliquis) was the winner in this study: It separated itself from the pack by reducing the major bleeding risk by 57%, compared with warfarin, although it wasn’t significantly more effective than the other drugs in terms of stroke prevention. In contrast, the major bleeding rates for dabigatran (Pradaxa) and rivaroxaban (Xarelto) weren’t significantly different from warfarin in this challenging patient population.
In a related analysis of 10,712 patients with atrial fibrillation and advanced CKD who were on dialysis, use of an OAC was once again a winning strategy: It resulted not only in an impressive 58% reduction in the risk of stroke or systemic embolism, but also a 26% reduction in the risk of major bleeding, compared with no OAC.
Here again, apixaban was arguably the drug of choice. None of the 125 dialysis patients on apixaban experienced a stroke or systemic embolism. In contrast, dabigatran and rivaroxaban were associated with greater than threefold higher stroke rates than in patients on warfarin, although these differences didn’t achieve statistical significance because of small numbers, just 36 patients on dabigatran and 56 on rivaroxaban, the cardiologist continued.
For these analyses of the relationship between OAC exposure and stroke and bleeding outcomes, Dr. Noseworthy and his coinvestigators used propensity scores based upon 59 clinical and sociodemographic characteristics.
Asked why rates of utilization of OACs are so low in patients with advanced CKD, Dr. Noseworthy replied that he didn’t find that particularly surprising.
“Even if you look only at patients without renal dysfunction, there is incredible undertreatment of atrial fibrillation with OACs. And adherence is very poor,” he observed.
Moreover, in talking with nephrologists, he finds many of them have legitimate reservations about prescribing OACs for patients with end-stage renal disease on hemodialysis.
“They’re undergoing a lot of procedures. They’re having a ton of lines placed; they’re having fistulas revised; and they have very high rates of GI bleeding. In some studies the annual risk of bleeding is 20%-40% in this population. And they’re a frail population with frequent falls,” Dr. Noseworthy said.
He reported having no financial conflicts of interest regarding his study, which was conducted free of commercial support.
BARCELONA – Oral anticoagulation had a net overall benefit for patients with atrial fibrillation and advanced chronic kidney disease, based on results of a large observational study reported at the annual congress of the European Society of Cardiology.
The novel direct-acting oral anticoagulants (NOACs) and warfarin were all similarly effective in this study of 39,241 patients who had stage 4 or 5 chronic kidney disease (CKD), atrial fibrillation, and were not on dialysis. Compared with no oral anticoagulation, the drugs cut in half the risk of stroke or systemic embolism, with no increased risk of major bleeding.
“In patients with advanced CKD, it appears that OACs [oral anticoagulants] are reasonable,” concluded Peter A. Noseworthy, MD, of the Mayo Clinic in Rochester, Minn.
This is a potentially practice-changing finding given the “striking underutilization” of OACs in advanced CKD, he noted. Indeed, only one-third of the patients in this study were prescribed an OAC and picked up their prescriptions. And while the study has the limitations inherent to an observational study reliant upon data from a large U.S. administrative database – chiefly, the potential for residual confounding because of factors that couldn’t be adjusted for statistically – these real-world data may be as good as it gets, since patients with advanced CKD were excluded from the pivotal trials of the NOACs.
Apixaban (Eliquis) was the winner in this study: It separated itself from the pack by reducing the major bleeding risk by 57%, compared with warfarin, although it wasn’t significantly more effective than the other drugs in terms of stroke prevention. In contrast, the major bleeding rates for dabigatran (Pradaxa) and rivaroxaban (Xarelto) weren’t significantly different from warfarin in this challenging patient population.
In a related analysis of 10,712 patients with atrial fibrillation and advanced CKD who were on dialysis, use of an OAC was once again a winning strategy: It resulted not only in an impressive 58% reduction in the risk of stroke or systemic embolism, but also a 26% reduction in the risk of major bleeding, compared with no OAC.
Here again, apixaban was arguably the drug of choice. None of the 125 dialysis patients on apixaban experienced a stroke or systemic embolism. In contrast, dabigatran and rivaroxaban were associated with greater than threefold higher stroke rates than in patients on warfarin, although these differences didn’t achieve statistical significance because of small numbers, just 36 patients on dabigatran and 56 on rivaroxaban, the cardiologist continued.
For these analyses of the relationship between OAC exposure and stroke and bleeding outcomes, Dr. Noseworthy and his coinvestigators used propensity scores based upon 59 clinical and sociodemographic characteristics.
Asked why rates of utilization of OACs are so low in patients with advanced CKD, Dr. Noseworthy replied that he didn’t find that particularly surprising.
“Even if you look only at patients without renal dysfunction, there is incredible undertreatment of atrial fibrillation with OACs. And adherence is very poor,” he observed.
Moreover, in talking with nephrologists, he finds many of them have legitimate reservations about prescribing OACs for patients with end-stage renal disease on hemodialysis.
“They’re undergoing a lot of procedures. They’re having a ton of lines placed; they’re having fistulas revised; and they have very high rates of GI bleeding. In some studies the annual risk of bleeding is 20%-40% in this population. And they’re a frail population with frequent falls,” Dr. Noseworthy said.
He reported having no financial conflicts of interest regarding his study, which was conducted free of commercial support.
BARCELONA – Oral anticoagulation had a net overall benefit for patients with atrial fibrillation and advanced chronic kidney disease, based on results of a large observational study reported at the annual congress of the European Society of Cardiology.
The novel direct-acting oral anticoagulants (NOACs) and warfarin were all similarly effective in this study of 39,241 patients who had stage 4 or 5 chronic kidney disease (CKD), atrial fibrillation, and were not on dialysis. Compared with no oral anticoagulation, the drugs cut in half the risk of stroke or systemic embolism, with no increased risk of major bleeding.
“In patients with advanced CKD, it appears that OACs [oral anticoagulants] are reasonable,” concluded Peter A. Noseworthy, MD, of the Mayo Clinic in Rochester, Minn.
This is a potentially practice-changing finding given the “striking underutilization” of OACs in advanced CKD, he noted. Indeed, only one-third of the patients in this study were prescribed an OAC and picked up their prescriptions. And while the study has the limitations inherent to an observational study reliant upon data from a large U.S. administrative database – chiefly, the potential for residual confounding because of factors that couldn’t be adjusted for statistically – these real-world data may be as good as it gets, since patients with advanced CKD were excluded from the pivotal trials of the NOACs.
Apixaban (Eliquis) was the winner in this study: It separated itself from the pack by reducing the major bleeding risk by 57%, compared with warfarin, although it wasn’t significantly more effective than the other drugs in terms of stroke prevention. In contrast, the major bleeding rates for dabigatran (Pradaxa) and rivaroxaban (Xarelto) weren’t significantly different from warfarin in this challenging patient population.
In a related analysis of 10,712 patients with atrial fibrillation and advanced CKD who were on dialysis, use of an OAC was once again a winning strategy: It resulted not only in an impressive 58% reduction in the risk of stroke or systemic embolism, but also a 26% reduction in the risk of major bleeding, compared with no OAC.
Here again, apixaban was arguably the drug of choice. None of the 125 dialysis patients on apixaban experienced a stroke or systemic embolism. In contrast, dabigatran and rivaroxaban were associated with greater than threefold higher stroke rates than in patients on warfarin, although these differences didn’t achieve statistical significance because of small numbers, just 36 patients on dabigatran and 56 on rivaroxaban, the cardiologist continued.
For these analyses of the relationship between OAC exposure and stroke and bleeding outcomes, Dr. Noseworthy and his coinvestigators used propensity scores based upon 59 clinical and sociodemographic characteristics.
Asked why rates of utilization of OACs are so low in patients with advanced CKD, Dr. Noseworthy replied that he didn’t find that particularly surprising.
“Even if you look only at patients without renal dysfunction, there is incredible undertreatment of atrial fibrillation with OACs. And adherence is very poor,” he observed.
Moreover, in talking with nephrologists, he finds many of them have legitimate reservations about prescribing OACs for patients with end-stage renal disease on hemodialysis.
“They’re undergoing a lot of procedures. They’re having a ton of lines placed; they’re having fistulas revised; and they have very high rates of GI bleeding. In some studies the annual risk of bleeding is 20%-40% in this population. And they’re a frail population with frequent falls,” Dr. Noseworthy said.
He reported having no financial conflicts of interest regarding his study, which was conducted free of commercial support.
AT THE ESC CONGRESS 2017
Key clinical point:
Major finding: The risk of stroke/systemic embolism in patients with advanced chronic kidney disease who were on oral anticoagulation was reduced by 49% among those not on hemodialysis and by 58% in those who were, compared with similar patients not on oral anticoagulation.
Data source: This was an observational study of nearly 50,000 patients with atrial fibrillation and stage 4 or 5 chronic kidney disease in a large U.S. administrative database.
Disclosures: The presenter reported having no financial conflicts of interest regarding his study, which was conducted free of commercial support.
Use of BZD and sedative-hypnotics among hospitalized elderly
Clinical question: Which hospitalized older patients are inappropriately prescribed benzodiazepines or sedative hypnotics post discharge, and who is prescribing these medications?
Background: During hospitalization, older patients commonly suffer from agitation and insomnia. Unfortunately, benzodiazepines and sedative hypnotics are commonly used as first-line treatments for these conditions despite significant risk which includes cognitive impairment, postural instability, increased risk of falls and hip fracture as well as lack of effectiveness. The purpose of this study is to determine the magnitude of the issue, discover root causes, and determine the type or types of corrective action needed.
Study Design: Single-center retrospective observational study.
Setting: Urban academic medical center in Toronto.
There was significant increase in these prescriptions if the patient was admitted to a surgical or specialty service compared to the general internal medicine service (odds ratio, 6.61; 95% confidence interval, 2.70-16.17). First-year trainees prescribed these medications more than did attending or fellows (OR, 0.28; 95% CI, 0.08-0.93).
Study limitations include being from a single institution, not being blinded, and inadequate statistical power. Therefore, it may lack generalizability, may be subjected to observer bias, and may not detect significant effects of covariates.
Bottom line: Sleep disruption and poor quality of sleep were the primary reason for the majority of potentially inappropriate newly prescribed benzodiazepines and sedative hypnotics, with first-year trainees being more likely to prescribe these medications compared to attendings and fellows.
Citation: Pek EA, Ramfry A, Pendrith C, et al. High prevalence of inappropriate benzodiazepine and sedative hypnotic prescriptions among hospitalized older adults. J Hosp Med. 2017 May;12(5):310-6.
Dr. Choe is a hospitalist at Ochsner Health System, New Orleans.
Clinical question: Which hospitalized older patients are inappropriately prescribed benzodiazepines or sedative hypnotics post discharge, and who is prescribing these medications?
Background: During hospitalization, older patients commonly suffer from agitation and insomnia. Unfortunately, benzodiazepines and sedative hypnotics are commonly used as first-line treatments for these conditions despite significant risk which includes cognitive impairment, postural instability, increased risk of falls and hip fracture as well as lack of effectiveness. The purpose of this study is to determine the magnitude of the issue, discover root causes, and determine the type or types of corrective action needed.
Study Design: Single-center retrospective observational study.
Setting: Urban academic medical center in Toronto.
There was significant increase in these prescriptions if the patient was admitted to a surgical or specialty service compared to the general internal medicine service (odds ratio, 6.61; 95% confidence interval, 2.70-16.17). First-year trainees prescribed these medications more than did attending or fellows (OR, 0.28; 95% CI, 0.08-0.93).
Study limitations include being from a single institution, not being blinded, and inadequate statistical power. Therefore, it may lack generalizability, may be subjected to observer bias, and may not detect significant effects of covariates.
Bottom line: Sleep disruption and poor quality of sleep were the primary reason for the majority of potentially inappropriate newly prescribed benzodiazepines and sedative hypnotics, with first-year trainees being more likely to prescribe these medications compared to attendings and fellows.
Citation: Pek EA, Ramfry A, Pendrith C, et al. High prevalence of inappropriate benzodiazepine and sedative hypnotic prescriptions among hospitalized older adults. J Hosp Med. 2017 May;12(5):310-6.
Dr. Choe is a hospitalist at Ochsner Health System, New Orleans.
Clinical question: Which hospitalized older patients are inappropriately prescribed benzodiazepines or sedative hypnotics post discharge, and who is prescribing these medications?
Background: During hospitalization, older patients commonly suffer from agitation and insomnia. Unfortunately, benzodiazepines and sedative hypnotics are commonly used as first-line treatments for these conditions despite significant risk which includes cognitive impairment, postural instability, increased risk of falls and hip fracture as well as lack of effectiveness. The purpose of this study is to determine the magnitude of the issue, discover root causes, and determine the type or types of corrective action needed.
Study Design: Single-center retrospective observational study.
Setting: Urban academic medical center in Toronto.
There was significant increase in these prescriptions if the patient was admitted to a surgical or specialty service compared to the general internal medicine service (odds ratio, 6.61; 95% confidence interval, 2.70-16.17). First-year trainees prescribed these medications more than did attending or fellows (OR, 0.28; 95% CI, 0.08-0.93).
Study limitations include being from a single institution, not being blinded, and inadequate statistical power. Therefore, it may lack generalizability, may be subjected to observer bias, and may not detect significant effects of covariates.
Bottom line: Sleep disruption and poor quality of sleep were the primary reason for the majority of potentially inappropriate newly prescribed benzodiazepines and sedative hypnotics, with first-year trainees being more likely to prescribe these medications compared to attendings and fellows.
Citation: Pek EA, Ramfry A, Pendrith C, et al. High prevalence of inappropriate benzodiazepine and sedative hypnotic prescriptions among hospitalized older adults. J Hosp Med. 2017 May;12(5):310-6.
Dr. Choe is a hospitalist at Ochsner Health System, New Orleans.
LVAD use soars in elderly Americans
DALLAS – The percentage of left ventricular assist devices placed in U.S. heart failure patients at least 75 years of age jumped sharply during 2003-2014, and concurrently the short-term survival of these patients improved dramatically, according to data collected by the National Inpatient Sample.
During the 12-year period examined, the percentage of left-ventricular assist devices (LVADs) placed in U.S. heart failure patients aged 75 years and older rose from 3% of all LVADs in 2003 to 11% in 2014, Aniket S. Rali, MD, said at the annual scientific meeting of the Heart Failure Society of America.
The U.S. national numbers also showed that throughout the period studied, elderly U.S. patients who received an LVAD were increasingly sicker, with steadily increasing numbers of patients with a Charlson Comorbidity Index score of four or greater. Despite this, in-hospital mortality rates of elderly patients receiving an LVAD plummeted, dropping from 61% of elderly LVAD recipients in 2003 to 18% in 2014. During the same time, the percentage of elderly patients with a Charlson Comorbidity Index score greater than four doubled from 33% in 2003 to 66% in 2014, said Dr. Rali, a cardiologist at the University of Kansas Medical Center in Kansas City.
“If the Charlson Comorbidity Index score is increasing but in-hospital mortality is decreasing, then increased LVAD use is not a bad trend,” Dr. Rali said in an interview. He hopes that future analysis of longitudinal data from patients could identify clinical factors that link with better patient survival and help target LVAD placement to the patients who stand to gain the most benefit.
“We may be able to give these elderly patients not just longer life but improved quality of life” by a more informed targeting of LVADs, he suggested. “I think these numbers will help convince people that all is not lost,” he noted, for elderly heart failure patients who receive an LVAD as destination therapy. Patients at least 75 years old are not eligible for heart transplantation, so when these patients receive an LVAD it is, by definition, destination therapy.
The data also showed a marked sex disparity in LVAD use, with LVAD placement in men at least 75 years old rising from 1.4/1,000 patients in 2003 to 2.78/1,000 patients in 2014. In contrast, among women these rates rose from 0.8/1,000 patients in 2003 to 1.36/1,000 patients in 2014.
The average age for elderly U.S. LVAD recipients for the entire 12-year period studied was 77.6 years among a total of 2,090 recipients. For all 21,323 U.S. LVAD recipients during 2003-2014 the average age was 51.5 years old.
[email protected]
On Twitter @mitchelzoler
DALLAS – The percentage of left ventricular assist devices placed in U.S. heart failure patients at least 75 years of age jumped sharply during 2003-2014, and concurrently the short-term survival of these patients improved dramatically, according to data collected by the National Inpatient Sample.
During the 12-year period examined, the percentage of left-ventricular assist devices (LVADs) placed in U.S. heart failure patients aged 75 years and older rose from 3% of all LVADs in 2003 to 11% in 2014, Aniket S. Rali, MD, said at the annual scientific meeting of the Heart Failure Society of America.
The U.S. national numbers also showed that throughout the period studied, elderly U.S. patients who received an LVAD were increasingly sicker, with steadily increasing numbers of patients with a Charlson Comorbidity Index score of four or greater. Despite this, in-hospital mortality rates of elderly patients receiving an LVAD plummeted, dropping from 61% of elderly LVAD recipients in 2003 to 18% in 2014. During the same time, the percentage of elderly patients with a Charlson Comorbidity Index score greater than four doubled from 33% in 2003 to 66% in 2014, said Dr. Rali, a cardiologist at the University of Kansas Medical Center in Kansas City.
“If the Charlson Comorbidity Index score is increasing but in-hospital mortality is decreasing, then increased LVAD use is not a bad trend,” Dr. Rali said in an interview. He hopes that future analysis of longitudinal data from patients could identify clinical factors that link with better patient survival and help target LVAD placement to the patients who stand to gain the most benefit.
“We may be able to give these elderly patients not just longer life but improved quality of life” by a more informed targeting of LVADs, he suggested. “I think these numbers will help convince people that all is not lost,” he noted, for elderly heart failure patients who receive an LVAD as destination therapy. Patients at least 75 years old are not eligible for heart transplantation, so when these patients receive an LVAD it is, by definition, destination therapy.
The data also showed a marked sex disparity in LVAD use, with LVAD placement in men at least 75 years old rising from 1.4/1,000 patients in 2003 to 2.78/1,000 patients in 2014. In contrast, among women these rates rose from 0.8/1,000 patients in 2003 to 1.36/1,000 patients in 2014.
The average age for elderly U.S. LVAD recipients for the entire 12-year period studied was 77.6 years among a total of 2,090 recipients. For all 21,323 U.S. LVAD recipients during 2003-2014 the average age was 51.5 years old.
[email protected]
On Twitter @mitchelzoler
DALLAS – The percentage of left ventricular assist devices placed in U.S. heart failure patients at least 75 years of age jumped sharply during 2003-2014, and concurrently the short-term survival of these patients improved dramatically, according to data collected by the National Inpatient Sample.
During the 12-year period examined, the percentage of left-ventricular assist devices (LVADs) placed in U.S. heart failure patients aged 75 years and older rose from 3% of all LVADs in 2003 to 11% in 2014, Aniket S. Rali, MD, said at the annual scientific meeting of the Heart Failure Society of America.
The U.S. national numbers also showed that throughout the period studied, elderly U.S. patients who received an LVAD were increasingly sicker, with steadily increasing numbers of patients with a Charlson Comorbidity Index score of four or greater. Despite this, in-hospital mortality rates of elderly patients receiving an LVAD plummeted, dropping from 61% of elderly LVAD recipients in 2003 to 18% in 2014. During the same time, the percentage of elderly patients with a Charlson Comorbidity Index score greater than four doubled from 33% in 2003 to 66% in 2014, said Dr. Rali, a cardiologist at the University of Kansas Medical Center in Kansas City.
“If the Charlson Comorbidity Index score is increasing but in-hospital mortality is decreasing, then increased LVAD use is not a bad trend,” Dr. Rali said in an interview. He hopes that future analysis of longitudinal data from patients could identify clinical factors that link with better patient survival and help target LVAD placement to the patients who stand to gain the most benefit.
“We may be able to give these elderly patients not just longer life but improved quality of life” by a more informed targeting of LVADs, he suggested. “I think these numbers will help convince people that all is not lost,” he noted, for elderly heart failure patients who receive an LVAD as destination therapy. Patients at least 75 years old are not eligible for heart transplantation, so when these patients receive an LVAD it is, by definition, destination therapy.
The data also showed a marked sex disparity in LVAD use, with LVAD placement in men at least 75 years old rising from 1.4/1,000 patients in 2003 to 2.78/1,000 patients in 2014. In contrast, among women these rates rose from 0.8/1,000 patients in 2003 to 1.36/1,000 patients in 2014.
The average age for elderly U.S. LVAD recipients for the entire 12-year period studied was 77.6 years among a total of 2,090 recipients. For all 21,323 U.S. LVAD recipients during 2003-2014 the average age was 51.5 years old.
[email protected]
On Twitter @mitchelzoler
AT THE HFSA ANNUAL SCIENTIFIC MEETING
Key clinical point:
Major finding: Elderly U.S. patients receiving an LVAD rose from 3% of all LVADs placed in 2003 to 11% in 2014.
Data source: The U.S. National Inpatient Survey during 2003-2014.
Disclosures: Dr. Rali had no disclosures.
Sepsis time to treatment
Clinical question: Does early identification and treatment of sepsis using protocols improve outcomes?
Background: International clinical guidelines recommend early detection and treatment of sepsis with broad spectrum antibiotics and intravenous fluids which are supported by preclinical and observation studies that show a reduction in avoidable deaths. However, controversy remains in the timing of these treatments on how it relates to patient outcomes such as risk-adjusted mortality.
Study design: Retrospective cohort study using data reported to the New York State Department of Health from April 1, 2014, to June 30, 2016.
Setting: New York hospitals.
Synopsis: For patients with sepsis and septic shock, a sepsis protocol was initiated within 6 hours after arrival in the emergency department and had all items in a 3-hour bundle of care (that is, blood cultures, broad-spectrum antibiotic, and lactate measurement) completed within 12 hours. Among 49,331 patients at 149 hospitals, higher risk-adjusted in-hospital mortality was associated with longer time to the completion of the bundle (P less than .001), administration of antibiotics (P less than .001), but not completion of a bolus of intravenous fluids (P = .21).
Study limitations include being nonrandomized, hospitals all from one state possibly introducing epidemiologic distinct features of sepsis inherent to the region, and accuracy of the data collection (that is, start time).
No association was found between time to completion of the initial bolus of fluids and improved outcomes in risk-adjusted mortality; however, the analysis of time of the initial fluid bolus was most vulnerable to confounding; a causal relationship will need further study.
Bottom line: A lower risk-adjusted in-hospital mortality was associated with rapid administration of antibiotics and a faster completion of a 3-hour bundle of sepsis care, but there was no discernable association with the rapid administration of initial bolus of intravenous fluids.
Citation: Seymour CW, Gesten F, Prescott HC, et al. Time to treatment and mortality during mandated emergency care for sepsis. N Engl J Med. 2017; 376:2235-44.
Dr. Choe is a hospitalist at Ochsner Health System, New Orleans.
Clinical question: Does early identification and treatment of sepsis using protocols improve outcomes?
Background: International clinical guidelines recommend early detection and treatment of sepsis with broad spectrum antibiotics and intravenous fluids which are supported by preclinical and observation studies that show a reduction in avoidable deaths. However, controversy remains in the timing of these treatments on how it relates to patient outcomes such as risk-adjusted mortality.
Study design: Retrospective cohort study using data reported to the New York State Department of Health from April 1, 2014, to June 30, 2016.
Setting: New York hospitals.
Synopsis: For patients with sepsis and septic shock, a sepsis protocol was initiated within 6 hours after arrival in the emergency department and had all items in a 3-hour bundle of care (that is, blood cultures, broad-spectrum antibiotic, and lactate measurement) completed within 12 hours. Among 49,331 patients at 149 hospitals, higher risk-adjusted in-hospital mortality was associated with longer time to the completion of the bundle (P less than .001), administration of antibiotics (P less than .001), but not completion of a bolus of intravenous fluids (P = .21).
Study limitations include being nonrandomized, hospitals all from one state possibly introducing epidemiologic distinct features of sepsis inherent to the region, and accuracy of the data collection (that is, start time).
No association was found between time to completion of the initial bolus of fluids and improved outcomes in risk-adjusted mortality; however, the analysis of time of the initial fluid bolus was most vulnerable to confounding; a causal relationship will need further study.
Bottom line: A lower risk-adjusted in-hospital mortality was associated with rapid administration of antibiotics and a faster completion of a 3-hour bundle of sepsis care, but there was no discernable association with the rapid administration of initial bolus of intravenous fluids.
Citation: Seymour CW, Gesten F, Prescott HC, et al. Time to treatment and mortality during mandated emergency care for sepsis. N Engl J Med. 2017; 376:2235-44.
Dr. Choe is a hospitalist at Ochsner Health System, New Orleans.
Clinical question: Does early identification and treatment of sepsis using protocols improve outcomes?
Background: International clinical guidelines recommend early detection and treatment of sepsis with broad spectrum antibiotics and intravenous fluids which are supported by preclinical and observation studies that show a reduction in avoidable deaths. However, controversy remains in the timing of these treatments on how it relates to patient outcomes such as risk-adjusted mortality.
Study design: Retrospective cohort study using data reported to the New York State Department of Health from April 1, 2014, to June 30, 2016.
Setting: New York hospitals.
Synopsis: For patients with sepsis and septic shock, a sepsis protocol was initiated within 6 hours after arrival in the emergency department and had all items in a 3-hour bundle of care (that is, blood cultures, broad-spectrum antibiotic, and lactate measurement) completed within 12 hours. Among 49,331 patients at 149 hospitals, higher risk-adjusted in-hospital mortality was associated with longer time to the completion of the bundle (P less than .001), administration of antibiotics (P less than .001), but not completion of a bolus of intravenous fluids (P = .21).
Study limitations include being nonrandomized, hospitals all from one state possibly introducing epidemiologic distinct features of sepsis inherent to the region, and accuracy of the data collection (that is, start time).
No association was found between time to completion of the initial bolus of fluids and improved outcomes in risk-adjusted mortality; however, the analysis of time of the initial fluid bolus was most vulnerable to confounding; a causal relationship will need further study.
Bottom line: A lower risk-adjusted in-hospital mortality was associated with rapid administration of antibiotics and a faster completion of a 3-hour bundle of sepsis care, but there was no discernable association with the rapid administration of initial bolus of intravenous fluids.
Citation: Seymour CW, Gesten F, Prescott HC, et al. Time to treatment and mortality during mandated emergency care for sepsis. N Engl J Med. 2017; 376:2235-44.
Dr. Choe is a hospitalist at Ochsner Health System, New Orleans.