User login
In Case You Missed It: COVID
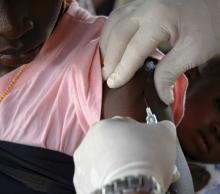
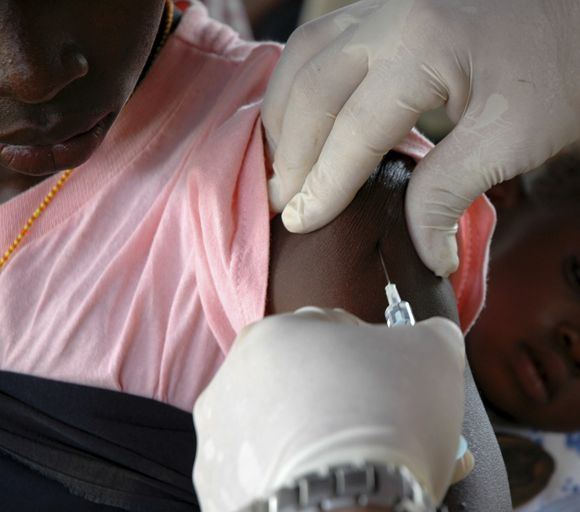
FDA, CDC urge pause of J&J COVID vaccine
The Food and Drug Administration and Centers for Disease Control and Prevention on April 13 recommended that use of the Johnson & Johnson COVID-19 vaccine be paused after reports of blood clots in patients receiving the shot, the agencies have announced.
In a statement, FDA said 6.8 million doses of the J&J vaccine have been administered and the agency is investigating six reported cases of a rare and severe blood clot occurring in patients who received the vaccine.
The pause is intended to give time to alert the public to this "very rare" condition, experts said during a joint CDC-FDA media briefing April 13.
"It was clear to us that we needed to alert the public," Janet Woodcock, MD, acting FDA commissioner, said. The move also will allow "time for the healthcare community to learn what they need to know about how to diagnose, treat and report" any additional cases.
The CDC will convene a meeting of the Advisory Committee on Immunization Practices on April 14 to review the cases.
"I know the information today will be very concerning to Americans who have already received the Johnson & Johnson vaccine," said Anne Schuchat, MD, principal deputy director at the CDC.
"For people who got the vaccine more than one month ago, the risk is very low at this time," she added. "For people who recently got the vaccine, in the last couple of weeks, look for symptoms."
Headache, leg pain, abdominal pain, and shortness of breath were among the reported symptoms. All six cases arose within 6 to 13 days of receipt of the Johnson & Johnson vaccine.
Traditional treatment dangerous
Importantly, treatment for traditional blood clots, such as the drug heparin, should not be used for these clots. "The issue here with these types of blood clots is that if one administers the standard treatment we give for blood clots, one can cause tremendous harm or it can be fatal," said Peter Marks, MD, director of the FDA Center for Biologics Evaluation and Research.
If health care providers see people with these symptoms along with a low platelet count or blood clots, they should ask about any recent vaccinations, Dr. Marks added.
Headache is a common side effect of COVID-19 vaccination, Dr. Marks said, but it typically happens within a day or two. In contrast, the headaches associated with these blood clots come 1 to 2 weeks later and were very severe.
Not all of the six women involved in the events had a pre-existing condition or risk factor, Dr. Schuchat said.
Severe but 'extremely rare'
To put the numbers in context, the six reported events occurred among millions of people who received the Johnson & Johnson vaccine to date.
"There have been six reports of a severe stroke-like illness due to low platelet count and more than six million doses of the Johnson & Johnson vaccine have been administered so far," Dr. Schuchat said.
"I would like to stress these events are extremely rare," Dr. Woodcock said, "but we take all reports of adverse events after vaccination very seriously."
The company response
Johnson & Johnson in a statement said, "We are aware of an extremely rare disorder involving people with blood clots in combination with low platelets in a small number of individuals who have received our COVID-19 vaccine. The United States Centers for Disease Control (CDC) and Food and Drug Administration (FDA) are reviewing data involving six reported U.S. cases out of more than 6.8 million doses administered. Out of an abundance of caution, the CDC and FDA have recommended a pause in the use of our vaccine."
The company said they are also reviewing these cases with European regulators and "we have made the decision to proactively delay the rollout of our vaccine in Europe."
Overall vaccinations continuing apace
"This announcement will not have a significant impact on our vaccination plan. Johnson & Johnson vaccine makes up less than 5% of the recorded shots in arms in the United States to date," Jeff Zients, White House COVID-19 Response Coordinator, said in a statement.
"Based on actions taken by the president earlier this year, the United States has secured enough Pfizer and Moderna doses for 300 million Americans. We are working now with our state and federal partners to get anyone scheduled for a J&J vaccine quickly rescheduled for a Pfizer or Moderna vaccine," he added.
The likely duration of the pause remains unclear.
"I know this has been a long and difficult pandemic, and people are tired of the steps they have to take," Dr. Schuchat said. "Steps taken today make sure the health care system is ready to diagnose, treat and report [any additional cases] and the public has the information necessary to stay safe."
A version of this article first appeared on WebMD.com.
This article was updated 4/13/21.
The Food and Drug Administration and Centers for Disease Control and Prevention on April 13 recommended that use of the Johnson & Johnson COVID-19 vaccine be paused after reports of blood clots in patients receiving the shot, the agencies have announced.
In a statement, FDA said 6.8 million doses of the J&J vaccine have been administered and the agency is investigating six reported cases of a rare and severe blood clot occurring in patients who received the vaccine.
The pause is intended to give time to alert the public to this "very rare" condition, experts said during a joint CDC-FDA media briefing April 13.
"It was clear to us that we needed to alert the public," Janet Woodcock, MD, acting FDA commissioner, said. The move also will allow "time for the healthcare community to learn what they need to know about how to diagnose, treat and report" any additional cases.
The CDC will convene a meeting of the Advisory Committee on Immunization Practices on April 14 to review the cases.
"I know the information today will be very concerning to Americans who have already received the Johnson & Johnson vaccine," said Anne Schuchat, MD, principal deputy director at the CDC.
"For people who got the vaccine more than one month ago, the risk is very low at this time," she added. "For people who recently got the vaccine, in the last couple of weeks, look for symptoms."
Headache, leg pain, abdominal pain, and shortness of breath were among the reported symptoms. All six cases arose within 6 to 13 days of receipt of the Johnson & Johnson vaccine.
Traditional treatment dangerous
Importantly, treatment for traditional blood clots, such as the drug heparin, should not be used for these clots. "The issue here with these types of blood clots is that if one administers the standard treatment we give for blood clots, one can cause tremendous harm or it can be fatal," said Peter Marks, MD, director of the FDA Center for Biologics Evaluation and Research.
If health care providers see people with these symptoms along with a low platelet count or blood clots, they should ask about any recent vaccinations, Dr. Marks added.
Headache is a common side effect of COVID-19 vaccination, Dr. Marks said, but it typically happens within a day or two. In contrast, the headaches associated with these blood clots come 1 to 2 weeks later and were very severe.
Not all of the six women involved in the events had a pre-existing condition or risk factor, Dr. Schuchat said.
Severe but 'extremely rare'
To put the numbers in context, the six reported events occurred among millions of people who received the Johnson & Johnson vaccine to date.
"There have been six reports of a severe stroke-like illness due to low platelet count and more than six million doses of the Johnson & Johnson vaccine have been administered so far," Dr. Schuchat said.
"I would like to stress these events are extremely rare," Dr. Woodcock said, "but we take all reports of adverse events after vaccination very seriously."
The company response
Johnson & Johnson in a statement said, "We are aware of an extremely rare disorder involving people with blood clots in combination with low platelets in a small number of individuals who have received our COVID-19 vaccine. The United States Centers for Disease Control (CDC) and Food and Drug Administration (FDA) are reviewing data involving six reported U.S. cases out of more than 6.8 million doses administered. Out of an abundance of caution, the CDC and FDA have recommended a pause in the use of our vaccine."
The company said they are also reviewing these cases with European regulators and "we have made the decision to proactively delay the rollout of our vaccine in Europe."
Overall vaccinations continuing apace
"This announcement will not have a significant impact on our vaccination plan. Johnson & Johnson vaccine makes up less than 5% of the recorded shots in arms in the United States to date," Jeff Zients, White House COVID-19 Response Coordinator, said in a statement.
"Based on actions taken by the president earlier this year, the United States has secured enough Pfizer and Moderna doses for 300 million Americans. We are working now with our state and federal partners to get anyone scheduled for a J&J vaccine quickly rescheduled for a Pfizer or Moderna vaccine," he added.
The likely duration of the pause remains unclear.
"I know this has been a long and difficult pandemic, and people are tired of the steps they have to take," Dr. Schuchat said. "Steps taken today make sure the health care system is ready to diagnose, treat and report [any additional cases] and the public has the information necessary to stay safe."
A version of this article first appeared on WebMD.com.
This article was updated 4/13/21.
The Food and Drug Administration and Centers for Disease Control and Prevention on April 13 recommended that use of the Johnson & Johnson COVID-19 vaccine be paused after reports of blood clots in patients receiving the shot, the agencies have announced.
In a statement, FDA said 6.8 million doses of the J&J vaccine have been administered and the agency is investigating six reported cases of a rare and severe blood clot occurring in patients who received the vaccine.
The pause is intended to give time to alert the public to this "very rare" condition, experts said during a joint CDC-FDA media briefing April 13.
"It was clear to us that we needed to alert the public," Janet Woodcock, MD, acting FDA commissioner, said. The move also will allow "time for the healthcare community to learn what they need to know about how to diagnose, treat and report" any additional cases.
The CDC will convene a meeting of the Advisory Committee on Immunization Practices on April 14 to review the cases.
"I know the information today will be very concerning to Americans who have already received the Johnson & Johnson vaccine," said Anne Schuchat, MD, principal deputy director at the CDC.
"For people who got the vaccine more than one month ago, the risk is very low at this time," she added. "For people who recently got the vaccine, in the last couple of weeks, look for symptoms."
Headache, leg pain, abdominal pain, and shortness of breath were among the reported symptoms. All six cases arose within 6 to 13 days of receipt of the Johnson & Johnson vaccine.
Traditional treatment dangerous
Importantly, treatment for traditional blood clots, such as the drug heparin, should not be used for these clots. "The issue here with these types of blood clots is that if one administers the standard treatment we give for blood clots, one can cause tremendous harm or it can be fatal," said Peter Marks, MD, director of the FDA Center for Biologics Evaluation and Research.
If health care providers see people with these symptoms along with a low platelet count or blood clots, they should ask about any recent vaccinations, Dr. Marks added.
Headache is a common side effect of COVID-19 vaccination, Dr. Marks said, but it typically happens within a day or two. In contrast, the headaches associated with these blood clots come 1 to 2 weeks later and were very severe.
Not all of the six women involved in the events had a pre-existing condition or risk factor, Dr. Schuchat said.
Severe but 'extremely rare'
To put the numbers in context, the six reported events occurred among millions of people who received the Johnson & Johnson vaccine to date.
"There have been six reports of a severe stroke-like illness due to low platelet count and more than six million doses of the Johnson & Johnson vaccine have been administered so far," Dr. Schuchat said.
"I would like to stress these events are extremely rare," Dr. Woodcock said, "but we take all reports of adverse events after vaccination very seriously."
The company response
Johnson & Johnson in a statement said, "We are aware of an extremely rare disorder involving people with blood clots in combination with low platelets in a small number of individuals who have received our COVID-19 vaccine. The United States Centers for Disease Control (CDC) and Food and Drug Administration (FDA) are reviewing data involving six reported U.S. cases out of more than 6.8 million doses administered. Out of an abundance of caution, the CDC and FDA have recommended a pause in the use of our vaccine."
The company said they are also reviewing these cases with European regulators and "we have made the decision to proactively delay the rollout of our vaccine in Europe."
Overall vaccinations continuing apace
"This announcement will not have a significant impact on our vaccination plan. Johnson & Johnson vaccine makes up less than 5% of the recorded shots in arms in the United States to date," Jeff Zients, White House COVID-19 Response Coordinator, said in a statement.
"Based on actions taken by the president earlier this year, the United States has secured enough Pfizer and Moderna doses for 300 million Americans. We are working now with our state and federal partners to get anyone scheduled for a J&J vaccine quickly rescheduled for a Pfizer or Moderna vaccine," he added.
The likely duration of the pause remains unclear.
"I know this has been a long and difficult pandemic, and people are tired of the steps they have to take," Dr. Schuchat said. "Steps taken today make sure the health care system is ready to diagnose, treat and report [any additional cases] and the public has the information necessary to stay safe."
A version of this article first appeared on WebMD.com.
This article was updated 4/13/21.
Study IDs most common lingering symptoms 8 months after mild COVID
Loss of smell, loss of taste, dyspnea, and fatigue are the four most common symptoms that health care professionals in Sweden report 8 months after mild COVID-19 illness, new evidence reveals.
according to the study.
“We see that a substantial portion of health care workers suffer from long-term symptoms after mild COVID-19,” senior author Charlotte Thålin, MD, PhD, said in an interview. She added that loss of smell and taste “may seem trivial, but have a negative impact on work, social, and home life in the long run.”
The study is noteworthy not only for tracking the COVID-19-related experiences of health care workers over time, but also for what it did not find. There was no increased prevalence of cognitive issues – including memory or concentration – that others have linked to what’s often called long-haul COVID-19.
The research letter was published online April 7, 2021, in JAMA.
“Even if you are young and previously healthy, a mild COVID-19 infection may result in long-term consequences,” said Dr. Thålin, from the department of clinical sciences at Danderyd Hospital, Karolinska Institute, Stockholm.
The researchers did not observe an increased risk for long-term symptoms after asymptomatic COVID-19.
Adding to existing evidence
This research letter “adds to the growing body of literature showing that people recovering from COVID have reported a diverse array of symptoms lasting for months after initial infection,” Lekshmi Santhosh, MD, said in an interview. She is physician faculty lead at the University of California, San Francisco Post-COVID OPTIMAL Clinic.
Previous research revealed severe long-term symptoms, including heart palpitations and neurologic impairments, among people hospitalized with COVID-19. However, “there is limited data on the long-term effects after mild COVID-19, and these studies are often hampered by selection bias and without proper control groups,” Dr. Thålin said.
The absence of these more severe symptoms after mild COVID-19 is “reassuring,” she added.
The current findings are part of the ongoing COMMUNITY (COVID-19 Biomarker and Immunity) study looking at long-term immunity. Health care professionals enrolled in the research between April 15 and May 8, 2020, and have initial blood tests repeated every 4 months.
Dr. Thålin, lead author Sebastian Havervall, MD, and their colleagues compared symptom reporting between 323 hospital employees who had mild COVID-19 at least 8 months earlier with 1,072 employees who did not have COVID-19 throughout the study.
The results show that 26% of those who had COVID-19 previously had at least one moderate to severe symptom that lasted more than 2 months, compared with 9% in the control group.
The group with a history of mild COVID-19 was a median 43 years old and 83% were women. The controls were a median 47 years old and 86% were women.
“These data mirror what we have seen across long-term cohorts of patients with COVID-19 infection. Notably, mild illness among previously healthy individuals may be associated with long-term persistent symptoms,” Sarah Jolley, MD, a pulmonologist specializing in critical care at the University of Colorado Hospital in Aurora and director of the Post-COVID Clinic, said in an interview.
“In this cohort, similar to others, this seems to be more pronounced in women,” Dr. Jolley added.
Key findings on functioning
At 8 months, using a smartphone app, participants reported presence, duration, and severity of 23 predefined symptoms. Researchers used the Sheehan Disability Scale to gauge functional impairment.
A total of 11% participants reported at least one symptom that negatively affected work or social or home life at 8 months versus only 2% of the control group.
Seropositive participants were almost two times more likely to report that their long-term symptoms moderately to markedly disrupted their work life, 8% versus 4% of seronegative healthcare workers (relative risk, 1.8; 95%; confidence interval, 1.2-2.9).
Disruptions to a social life from long-term symptoms were 2.5 times more likely in the seropositive group. A total 15% of this cohort reported moderate to marked effects, compared with 6% of the seronegative group (RR, 2.5; 95% CI, 1.8-3.6).
The researchers also inquired about home life disruptions, which were reported by 12% of the seropositive health care workers and 5% of the seronegative participants (RR, 2.3; 95% CI, 1.6-3.4).
The study’s findings “tracks with a lot of the other work we’re seeing,” David Putrino, PT, PhD, director of rehabilitation innovation at Mount Sinai Health System in New York, said in an interview. He and his colleagues are responsible for managing the rehabilitation of patients with long COVID.
Interestingly, the proportion of people with persistent symptoms might be underestimated in this research, Dr. Putrino said. “Antibodies are not an entirely reliable biomarker. So what the researchers are using here is the most conservative measure of who may have had the virus.”
Potential recall bias and the subjective rating of symptoms were possible limitations of the study.
When asked to speculate why researchers did not find higher levels of cognitive dysfunction, Dr. Putrino said that self-reports are generally less reliable than measures like the Montreal Cognitive Assessment for detecting cognitive impairment.
Furthermore, unlike many of the people with long-haul COVID-19 whom he treats clinically – ones who are “really struggling” – the health care workers studied in Sweden are functioning well enough to perform their duties at the hospital, so the study population may not represent the population at large.
More research required
“More research needs to be conducted to investigate the mechanisms underlying these persistent symptoms, and several centers, including UCSF, are conducting research into why this might be,” Dr. Santhosh said.
Dr. Thålin and colleagues plan to continue following participants. “The primary aim of the COMMUNITY study is to investigate long-term immunity after COVID-19, but we will also look into possible underlying pathophysiological mechanisms behind COVID-19–related long-term symptoms,” she said.
“I hope to see that taste and smell will return,” Dr. Thålin added.
“We’re really just starting to understand the long-term effects of COVID-19,” Putrino said. “This is something we’re going to see a lot of moving forward.”
Dr. Thålin, Dr. Santhosh, Dr. Jolley, and Dr. Putrino disclosed no relevant financial relationships. The research was funded by grants from the Knut and Alice Wallenberg Foundation, Jonas and Christina af Jochnick Foundation, Leif Lundblad Family Foundation, Region Stockholm, and Erling-Persson Family Foundation.
A version of this article first appeared on Medscape.com.
Loss of smell, loss of taste, dyspnea, and fatigue are the four most common symptoms that health care professionals in Sweden report 8 months after mild COVID-19 illness, new evidence reveals.
according to the study.
“We see that a substantial portion of health care workers suffer from long-term symptoms after mild COVID-19,” senior author Charlotte Thålin, MD, PhD, said in an interview. She added that loss of smell and taste “may seem trivial, but have a negative impact on work, social, and home life in the long run.”
The study is noteworthy not only for tracking the COVID-19-related experiences of health care workers over time, but also for what it did not find. There was no increased prevalence of cognitive issues – including memory or concentration – that others have linked to what’s often called long-haul COVID-19.
The research letter was published online April 7, 2021, in JAMA.
“Even if you are young and previously healthy, a mild COVID-19 infection may result in long-term consequences,” said Dr. Thålin, from the department of clinical sciences at Danderyd Hospital, Karolinska Institute, Stockholm.
The researchers did not observe an increased risk for long-term symptoms after asymptomatic COVID-19.
Adding to existing evidence
This research letter “adds to the growing body of literature showing that people recovering from COVID have reported a diverse array of symptoms lasting for months after initial infection,” Lekshmi Santhosh, MD, said in an interview. She is physician faculty lead at the University of California, San Francisco Post-COVID OPTIMAL Clinic.
Previous research revealed severe long-term symptoms, including heart palpitations and neurologic impairments, among people hospitalized with COVID-19. However, “there is limited data on the long-term effects after mild COVID-19, and these studies are often hampered by selection bias and without proper control groups,” Dr. Thålin said.
The absence of these more severe symptoms after mild COVID-19 is “reassuring,” she added.
The current findings are part of the ongoing COMMUNITY (COVID-19 Biomarker and Immunity) study looking at long-term immunity. Health care professionals enrolled in the research between April 15 and May 8, 2020, and have initial blood tests repeated every 4 months.
Dr. Thålin, lead author Sebastian Havervall, MD, and their colleagues compared symptom reporting between 323 hospital employees who had mild COVID-19 at least 8 months earlier with 1,072 employees who did not have COVID-19 throughout the study.
The results show that 26% of those who had COVID-19 previously had at least one moderate to severe symptom that lasted more than 2 months, compared with 9% in the control group.
The group with a history of mild COVID-19 was a median 43 years old and 83% were women. The controls were a median 47 years old and 86% were women.
“These data mirror what we have seen across long-term cohorts of patients with COVID-19 infection. Notably, mild illness among previously healthy individuals may be associated with long-term persistent symptoms,” Sarah Jolley, MD, a pulmonologist specializing in critical care at the University of Colorado Hospital in Aurora and director of the Post-COVID Clinic, said in an interview.
“In this cohort, similar to others, this seems to be more pronounced in women,” Dr. Jolley added.
Key findings on functioning
At 8 months, using a smartphone app, participants reported presence, duration, and severity of 23 predefined symptoms. Researchers used the Sheehan Disability Scale to gauge functional impairment.
A total of 11% participants reported at least one symptom that negatively affected work or social or home life at 8 months versus only 2% of the control group.
Seropositive participants were almost two times more likely to report that their long-term symptoms moderately to markedly disrupted their work life, 8% versus 4% of seronegative healthcare workers (relative risk, 1.8; 95%; confidence interval, 1.2-2.9).
Disruptions to a social life from long-term symptoms were 2.5 times more likely in the seropositive group. A total 15% of this cohort reported moderate to marked effects, compared with 6% of the seronegative group (RR, 2.5; 95% CI, 1.8-3.6).
The researchers also inquired about home life disruptions, which were reported by 12% of the seropositive health care workers and 5% of the seronegative participants (RR, 2.3; 95% CI, 1.6-3.4).
The study’s findings “tracks with a lot of the other work we’re seeing,” David Putrino, PT, PhD, director of rehabilitation innovation at Mount Sinai Health System in New York, said in an interview. He and his colleagues are responsible for managing the rehabilitation of patients with long COVID.
Interestingly, the proportion of people with persistent symptoms might be underestimated in this research, Dr. Putrino said. “Antibodies are not an entirely reliable biomarker. So what the researchers are using here is the most conservative measure of who may have had the virus.”
Potential recall bias and the subjective rating of symptoms were possible limitations of the study.
When asked to speculate why researchers did not find higher levels of cognitive dysfunction, Dr. Putrino said that self-reports are generally less reliable than measures like the Montreal Cognitive Assessment for detecting cognitive impairment.
Furthermore, unlike many of the people with long-haul COVID-19 whom he treats clinically – ones who are “really struggling” – the health care workers studied in Sweden are functioning well enough to perform their duties at the hospital, so the study population may not represent the population at large.
More research required
“More research needs to be conducted to investigate the mechanisms underlying these persistent symptoms, and several centers, including UCSF, are conducting research into why this might be,” Dr. Santhosh said.
Dr. Thålin and colleagues plan to continue following participants. “The primary aim of the COMMUNITY study is to investigate long-term immunity after COVID-19, but we will also look into possible underlying pathophysiological mechanisms behind COVID-19–related long-term symptoms,” she said.
“I hope to see that taste and smell will return,” Dr. Thålin added.
“We’re really just starting to understand the long-term effects of COVID-19,” Putrino said. “This is something we’re going to see a lot of moving forward.”
Dr. Thålin, Dr. Santhosh, Dr. Jolley, and Dr. Putrino disclosed no relevant financial relationships. The research was funded by grants from the Knut and Alice Wallenberg Foundation, Jonas and Christina af Jochnick Foundation, Leif Lundblad Family Foundation, Region Stockholm, and Erling-Persson Family Foundation.
A version of this article first appeared on Medscape.com.
Loss of smell, loss of taste, dyspnea, and fatigue are the four most common symptoms that health care professionals in Sweden report 8 months after mild COVID-19 illness, new evidence reveals.
according to the study.
“We see that a substantial portion of health care workers suffer from long-term symptoms after mild COVID-19,” senior author Charlotte Thålin, MD, PhD, said in an interview. She added that loss of smell and taste “may seem trivial, but have a negative impact on work, social, and home life in the long run.”
The study is noteworthy not only for tracking the COVID-19-related experiences of health care workers over time, but also for what it did not find. There was no increased prevalence of cognitive issues – including memory or concentration – that others have linked to what’s often called long-haul COVID-19.
The research letter was published online April 7, 2021, in JAMA.
“Even if you are young and previously healthy, a mild COVID-19 infection may result in long-term consequences,” said Dr. Thålin, from the department of clinical sciences at Danderyd Hospital, Karolinska Institute, Stockholm.
The researchers did not observe an increased risk for long-term symptoms after asymptomatic COVID-19.
Adding to existing evidence
This research letter “adds to the growing body of literature showing that people recovering from COVID have reported a diverse array of symptoms lasting for months after initial infection,” Lekshmi Santhosh, MD, said in an interview. She is physician faculty lead at the University of California, San Francisco Post-COVID OPTIMAL Clinic.
Previous research revealed severe long-term symptoms, including heart palpitations and neurologic impairments, among people hospitalized with COVID-19. However, “there is limited data on the long-term effects after mild COVID-19, and these studies are often hampered by selection bias and without proper control groups,” Dr. Thålin said.
The absence of these more severe symptoms after mild COVID-19 is “reassuring,” she added.
The current findings are part of the ongoing COMMUNITY (COVID-19 Biomarker and Immunity) study looking at long-term immunity. Health care professionals enrolled in the research between April 15 and May 8, 2020, and have initial blood tests repeated every 4 months.
Dr. Thålin, lead author Sebastian Havervall, MD, and their colleagues compared symptom reporting between 323 hospital employees who had mild COVID-19 at least 8 months earlier with 1,072 employees who did not have COVID-19 throughout the study.
The results show that 26% of those who had COVID-19 previously had at least one moderate to severe symptom that lasted more than 2 months, compared with 9% in the control group.
The group with a history of mild COVID-19 was a median 43 years old and 83% were women. The controls were a median 47 years old and 86% were women.
“These data mirror what we have seen across long-term cohorts of patients with COVID-19 infection. Notably, mild illness among previously healthy individuals may be associated with long-term persistent symptoms,” Sarah Jolley, MD, a pulmonologist specializing in critical care at the University of Colorado Hospital in Aurora and director of the Post-COVID Clinic, said in an interview.
“In this cohort, similar to others, this seems to be more pronounced in women,” Dr. Jolley added.
Key findings on functioning
At 8 months, using a smartphone app, participants reported presence, duration, and severity of 23 predefined symptoms. Researchers used the Sheehan Disability Scale to gauge functional impairment.
A total of 11% participants reported at least one symptom that negatively affected work or social or home life at 8 months versus only 2% of the control group.
Seropositive participants were almost two times more likely to report that their long-term symptoms moderately to markedly disrupted their work life, 8% versus 4% of seronegative healthcare workers (relative risk, 1.8; 95%; confidence interval, 1.2-2.9).
Disruptions to a social life from long-term symptoms were 2.5 times more likely in the seropositive group. A total 15% of this cohort reported moderate to marked effects, compared with 6% of the seronegative group (RR, 2.5; 95% CI, 1.8-3.6).
The researchers also inquired about home life disruptions, which were reported by 12% of the seropositive health care workers and 5% of the seronegative participants (RR, 2.3; 95% CI, 1.6-3.4).
The study’s findings “tracks with a lot of the other work we’re seeing,” David Putrino, PT, PhD, director of rehabilitation innovation at Mount Sinai Health System in New York, said in an interview. He and his colleagues are responsible for managing the rehabilitation of patients with long COVID.
Interestingly, the proportion of people with persistent symptoms might be underestimated in this research, Dr. Putrino said. “Antibodies are not an entirely reliable biomarker. So what the researchers are using here is the most conservative measure of who may have had the virus.”
Potential recall bias and the subjective rating of symptoms were possible limitations of the study.
When asked to speculate why researchers did not find higher levels of cognitive dysfunction, Dr. Putrino said that self-reports are generally less reliable than measures like the Montreal Cognitive Assessment for detecting cognitive impairment.
Furthermore, unlike many of the people with long-haul COVID-19 whom he treats clinically – ones who are “really struggling” – the health care workers studied in Sweden are functioning well enough to perform their duties at the hospital, so the study population may not represent the population at large.
More research required
“More research needs to be conducted to investigate the mechanisms underlying these persistent symptoms, and several centers, including UCSF, are conducting research into why this might be,” Dr. Santhosh said.
Dr. Thålin and colleagues plan to continue following participants. “The primary aim of the COMMUNITY study is to investigate long-term immunity after COVID-19, but we will also look into possible underlying pathophysiological mechanisms behind COVID-19–related long-term symptoms,” she said.
“I hope to see that taste and smell will return,” Dr. Thålin added.
“We’re really just starting to understand the long-term effects of COVID-19,” Putrino said. “This is something we’re going to see a lot of moving forward.”
Dr. Thålin, Dr. Santhosh, Dr. Jolley, and Dr. Putrino disclosed no relevant financial relationships. The research was funded by grants from the Knut and Alice Wallenberg Foundation, Jonas and Christina af Jochnick Foundation, Leif Lundblad Family Foundation, Region Stockholm, and Erling-Persson Family Foundation.
A version of this article first appeared on Medscape.com.
U.S. finally hits its stride with COVID-19 vaccination rollouts
Each afternoon, Cyrus Shahpar, MD, the data guru for the White House COVID-19 Response Team, sends an email to staffers with the daily count of COVID-19 vaccinations delivered in the United States.
The numbers, collected from states ahead of the final figures being posted on the Centers for Disease Control and Prevention website, act as a report card of sorts on the team’s efforts.
On Saturday, April 3, it was a new record: 4.1 million vaccinations delivered in a single day, more than the total population of some states.
While the United States has a long way to go before it is done with COVID-19, there’s finally some good news in the nation’s long and blundering slog through the pandemic.
After a rocky start in December 2020 and January 2021, vaccination is happening faster than nearly anyone thought possible. As more people see their friends and family roll up their sleeves, hesitancy is dropping, too.
In settings where large numbers of people are vaccinated, such as nursing homes, COVID-19 cases and deaths have plunged.
Those gains, however, haven’t been shared equally. According to CDC data, 69% of people who are fully vaccinated are White, while just 8% are Black and about 9% are Hispanic, a group that now represents most new COVID-19 cases.
Officials say that’s partly because the vaccines were rolled out to the elderly first. The average life expectancy for Black people in the United States is now age 72, which means there were fewer people of color represented in the first groups to become eligible. Experts are hopeful that underrepresented groups will start to catch up as more states open up vaccinations to younger people.
Based on overall numbers of daily vaccine doses, the United States ranks third, behind China and India. America ranks fourth – behind Israel, the United Kingdom, and Chile – in the total share of the population that’s been vaccinated, according to the website Our World in Data.
A positive development
It’s a stunning turnaround for a country that failed for months to develop effective tests, and still struggles in some quarters to investigate new cases and quarantine their contacts.
The 7-day rolling average of vaccines administered in the United States is currently more than 3 million a day.
“We knew that we needed to get to 3 million a day at some point, if we were going to get most people vaccinated this year, but I don’t think that most people expected it to happen this early,” said Eric Toner, MD, a senior scholar with the Johns Hopkins Center for Health Security in Baltimore.
Before taking office, President Joe Biden pledged to get 100 million shots in arms within his first 100 days in office. After hitting that goal in late March, he doubled it, to 200 million vaccinations by April 30. After first saying all adults should be eligible to get in line for the vaccine by May 1, on April 6, he bumped up that date to April 19.
Some media reports have seen this repeated moving of the goalposts as calculated – an unstated strategy of underpromising and overdelivering with the aim of rebuilding public trust.
But others pointed out that, even if that’s true, the goals being set aren’t easy, and hitting them has never been a given.
“I think the Biden administration really gets a lot of credit for pushing the companies to get more vaccine out faster than they had planned to,” Dr. Toner said. “And the states have really responded as well as the federal government in terms of getting vaccination sites going. So we’re not only getting the vaccines, we’re getting it into people’s arms faster than expected.”
Others agree.
“We’re doing an amazing job, and I think the U.S. is really beginning to bend the curve,” said Carlos del Rio, MD, an infectious disease specialist and distinguished professor of medicine at Emory University, Atlanta.
“I think overall it’s just that everybody’s putting in a ton of work to get it done,” he said.
On April 3, the day the United States hit its vaccination record, he was volunteering to give vaccinations.
“I mean, of all the bad things we do to people as clinicians, this is one thing that people are very happy about, right?” Dr. del Rio said.
He said he vaccinated a young woman who asked if she could video chat with her mom, who was feeling nervous about getting the shot. He answered her mom’s questions, and later that day, she came down to be vaccinated herself.
‘We view it as a war’
The White House COVID-19 Response Team has worked hard to better coordinate the work of so many people at both the federal and state levels, Andy Slavitt, senior adviser for the team, said in an interview.
“We view it as a war, and in a war, you do everything: You bring experienced personnel; you bring all the resources to bear; you create multiple routes,” Mr. Slavitt said. “You don’t leave anything to chance.”
Among the levers the administration has pulled, using the Defense Production Act has helped vaccine manufacturers get needed supplies, Mr. Slavitt said.
The administration has set up an array of Federal Emergency Management Agency–run community vaccination centers and mobile vaccination sites to complement state-led efforts, and it’s activated a federal health law called the Public Readiness and Emergency Preparedness Act, which provides immunity from liability for retired doctors and nurses, among others, who sign up to help give vaccinations. That’s helped get more people into the field giving shots.
The administration also canceled a plan to allocate vaccines to states based on their pace of administration, which would have punished underperforming states. Instead, doses are allocated based on population.
In a media call on April 7, when asked whether the administration would send additional vaccines to Michigan, a state that’s seeing a surge of COVID-19 cases with more transmissible variants, Mr. Slavitt said they weren’t managing vaccine supply “according to some formula.”
He said they were distributing based on population “because that’s fundamental,” but were also locating vaccines “surgically in places that have had the greatest disease and where people have the greatest exposure.”
He said sites like community health centers and retail pharmacies have the power to order vaccines directly from the federal government, which helps get more supply to harder-hit areas.
Mr. Slavitt said hitting 4.1 million daily vaccinations on April 3 was gratifying.
“I’ve seen photographs ... of people breaking down in tears when they get their vaccine, people who are giving standing ovations to active military for taking care of them,” he said, “and I think about people who have gone for a long time without hope, or who have been very scared.
“It’s incredibly encouraging to think about maybe a few million people taking a step back to normal life again,” he said.
A version of this article first appeared on Medscape.com.
Each afternoon, Cyrus Shahpar, MD, the data guru for the White House COVID-19 Response Team, sends an email to staffers with the daily count of COVID-19 vaccinations delivered in the United States.
The numbers, collected from states ahead of the final figures being posted on the Centers for Disease Control and Prevention website, act as a report card of sorts on the team’s efforts.
On Saturday, April 3, it was a new record: 4.1 million vaccinations delivered in a single day, more than the total population of some states.
While the United States has a long way to go before it is done with COVID-19, there’s finally some good news in the nation’s long and blundering slog through the pandemic.
After a rocky start in December 2020 and January 2021, vaccination is happening faster than nearly anyone thought possible. As more people see their friends and family roll up their sleeves, hesitancy is dropping, too.
In settings where large numbers of people are vaccinated, such as nursing homes, COVID-19 cases and deaths have plunged.
Those gains, however, haven’t been shared equally. According to CDC data, 69% of people who are fully vaccinated are White, while just 8% are Black and about 9% are Hispanic, a group that now represents most new COVID-19 cases.
Officials say that’s partly because the vaccines were rolled out to the elderly first. The average life expectancy for Black people in the United States is now age 72, which means there were fewer people of color represented in the first groups to become eligible. Experts are hopeful that underrepresented groups will start to catch up as more states open up vaccinations to younger people.
Based on overall numbers of daily vaccine doses, the United States ranks third, behind China and India. America ranks fourth – behind Israel, the United Kingdom, and Chile – in the total share of the population that’s been vaccinated, according to the website Our World in Data.
A positive development
It’s a stunning turnaround for a country that failed for months to develop effective tests, and still struggles in some quarters to investigate new cases and quarantine their contacts.
The 7-day rolling average of vaccines administered in the United States is currently more than 3 million a day.
“We knew that we needed to get to 3 million a day at some point, if we were going to get most people vaccinated this year, but I don’t think that most people expected it to happen this early,” said Eric Toner, MD, a senior scholar with the Johns Hopkins Center for Health Security in Baltimore.
Before taking office, President Joe Biden pledged to get 100 million shots in arms within his first 100 days in office. After hitting that goal in late March, he doubled it, to 200 million vaccinations by April 30. After first saying all adults should be eligible to get in line for the vaccine by May 1, on April 6, he bumped up that date to April 19.
Some media reports have seen this repeated moving of the goalposts as calculated – an unstated strategy of underpromising and overdelivering with the aim of rebuilding public trust.
But others pointed out that, even if that’s true, the goals being set aren’t easy, and hitting them has never been a given.
“I think the Biden administration really gets a lot of credit for pushing the companies to get more vaccine out faster than they had planned to,” Dr. Toner said. “And the states have really responded as well as the federal government in terms of getting vaccination sites going. So we’re not only getting the vaccines, we’re getting it into people’s arms faster than expected.”
Others agree.
“We’re doing an amazing job, and I think the U.S. is really beginning to bend the curve,” said Carlos del Rio, MD, an infectious disease specialist and distinguished professor of medicine at Emory University, Atlanta.
“I think overall it’s just that everybody’s putting in a ton of work to get it done,” he said.
On April 3, the day the United States hit its vaccination record, he was volunteering to give vaccinations.
“I mean, of all the bad things we do to people as clinicians, this is one thing that people are very happy about, right?” Dr. del Rio said.
He said he vaccinated a young woman who asked if she could video chat with her mom, who was feeling nervous about getting the shot. He answered her mom’s questions, and later that day, she came down to be vaccinated herself.
‘We view it as a war’
The White House COVID-19 Response Team has worked hard to better coordinate the work of so many people at both the federal and state levels, Andy Slavitt, senior adviser for the team, said in an interview.
“We view it as a war, and in a war, you do everything: You bring experienced personnel; you bring all the resources to bear; you create multiple routes,” Mr. Slavitt said. “You don’t leave anything to chance.”
Among the levers the administration has pulled, using the Defense Production Act has helped vaccine manufacturers get needed supplies, Mr. Slavitt said.
The administration has set up an array of Federal Emergency Management Agency–run community vaccination centers and mobile vaccination sites to complement state-led efforts, and it’s activated a federal health law called the Public Readiness and Emergency Preparedness Act, which provides immunity from liability for retired doctors and nurses, among others, who sign up to help give vaccinations. That’s helped get more people into the field giving shots.
The administration also canceled a plan to allocate vaccines to states based on their pace of administration, which would have punished underperforming states. Instead, doses are allocated based on population.
In a media call on April 7, when asked whether the administration would send additional vaccines to Michigan, a state that’s seeing a surge of COVID-19 cases with more transmissible variants, Mr. Slavitt said they weren’t managing vaccine supply “according to some formula.”
He said they were distributing based on population “because that’s fundamental,” but were also locating vaccines “surgically in places that have had the greatest disease and where people have the greatest exposure.”
He said sites like community health centers and retail pharmacies have the power to order vaccines directly from the federal government, which helps get more supply to harder-hit areas.
Mr. Slavitt said hitting 4.1 million daily vaccinations on April 3 was gratifying.
“I’ve seen photographs ... of people breaking down in tears when they get their vaccine, people who are giving standing ovations to active military for taking care of them,” he said, “and I think about people who have gone for a long time without hope, or who have been very scared.
“It’s incredibly encouraging to think about maybe a few million people taking a step back to normal life again,” he said.
A version of this article first appeared on Medscape.com.
Each afternoon, Cyrus Shahpar, MD, the data guru for the White House COVID-19 Response Team, sends an email to staffers with the daily count of COVID-19 vaccinations delivered in the United States.
The numbers, collected from states ahead of the final figures being posted on the Centers for Disease Control and Prevention website, act as a report card of sorts on the team’s efforts.
On Saturday, April 3, it was a new record: 4.1 million vaccinations delivered in a single day, more than the total population of some states.
While the United States has a long way to go before it is done with COVID-19, there’s finally some good news in the nation’s long and blundering slog through the pandemic.
After a rocky start in December 2020 and January 2021, vaccination is happening faster than nearly anyone thought possible. As more people see their friends and family roll up their sleeves, hesitancy is dropping, too.
In settings where large numbers of people are vaccinated, such as nursing homes, COVID-19 cases and deaths have plunged.
Those gains, however, haven’t been shared equally. According to CDC data, 69% of people who are fully vaccinated are White, while just 8% are Black and about 9% are Hispanic, a group that now represents most new COVID-19 cases.
Officials say that’s partly because the vaccines were rolled out to the elderly first. The average life expectancy for Black people in the United States is now age 72, which means there were fewer people of color represented in the first groups to become eligible. Experts are hopeful that underrepresented groups will start to catch up as more states open up vaccinations to younger people.
Based on overall numbers of daily vaccine doses, the United States ranks third, behind China and India. America ranks fourth – behind Israel, the United Kingdom, and Chile – in the total share of the population that’s been vaccinated, according to the website Our World in Data.
A positive development
It’s a stunning turnaround for a country that failed for months to develop effective tests, and still struggles in some quarters to investigate new cases and quarantine their contacts.
The 7-day rolling average of vaccines administered in the United States is currently more than 3 million a day.
“We knew that we needed to get to 3 million a day at some point, if we were going to get most people vaccinated this year, but I don’t think that most people expected it to happen this early,” said Eric Toner, MD, a senior scholar with the Johns Hopkins Center for Health Security in Baltimore.
Before taking office, President Joe Biden pledged to get 100 million shots in arms within his first 100 days in office. After hitting that goal in late March, he doubled it, to 200 million vaccinations by April 30. After first saying all adults should be eligible to get in line for the vaccine by May 1, on April 6, he bumped up that date to April 19.
Some media reports have seen this repeated moving of the goalposts as calculated – an unstated strategy of underpromising and overdelivering with the aim of rebuilding public trust.
But others pointed out that, even if that’s true, the goals being set aren’t easy, and hitting them has never been a given.
“I think the Biden administration really gets a lot of credit for pushing the companies to get more vaccine out faster than they had planned to,” Dr. Toner said. “And the states have really responded as well as the federal government in terms of getting vaccination sites going. So we’re not only getting the vaccines, we’re getting it into people’s arms faster than expected.”
Others agree.
“We’re doing an amazing job, and I think the U.S. is really beginning to bend the curve,” said Carlos del Rio, MD, an infectious disease specialist and distinguished professor of medicine at Emory University, Atlanta.
“I think overall it’s just that everybody’s putting in a ton of work to get it done,” he said.
On April 3, the day the United States hit its vaccination record, he was volunteering to give vaccinations.
“I mean, of all the bad things we do to people as clinicians, this is one thing that people are very happy about, right?” Dr. del Rio said.
He said he vaccinated a young woman who asked if she could video chat with her mom, who was feeling nervous about getting the shot. He answered her mom’s questions, and later that day, she came down to be vaccinated herself.
‘We view it as a war’
The White House COVID-19 Response Team has worked hard to better coordinate the work of so many people at both the federal and state levels, Andy Slavitt, senior adviser for the team, said in an interview.
“We view it as a war, and in a war, you do everything: You bring experienced personnel; you bring all the resources to bear; you create multiple routes,” Mr. Slavitt said. “You don’t leave anything to chance.”
Among the levers the administration has pulled, using the Defense Production Act has helped vaccine manufacturers get needed supplies, Mr. Slavitt said.
The administration has set up an array of Federal Emergency Management Agency–run community vaccination centers and mobile vaccination sites to complement state-led efforts, and it’s activated a federal health law called the Public Readiness and Emergency Preparedness Act, which provides immunity from liability for retired doctors and nurses, among others, who sign up to help give vaccinations. That’s helped get more people into the field giving shots.
The administration also canceled a plan to allocate vaccines to states based on their pace of administration, which would have punished underperforming states. Instead, doses are allocated based on population.
In a media call on April 7, when asked whether the administration would send additional vaccines to Michigan, a state that’s seeing a surge of COVID-19 cases with more transmissible variants, Mr. Slavitt said they weren’t managing vaccine supply “according to some formula.”
He said they were distributing based on population “because that’s fundamental,” but were also locating vaccines “surgically in places that have had the greatest disease and where people have the greatest exposure.”
He said sites like community health centers and retail pharmacies have the power to order vaccines directly from the federal government, which helps get more supply to harder-hit areas.
Mr. Slavitt said hitting 4.1 million daily vaccinations on April 3 was gratifying.
“I’ve seen photographs ... of people breaking down in tears when they get their vaccine, people who are giving standing ovations to active military for taking care of them,” he said, “and I think about people who have gone for a long time without hope, or who have been very scared.
“It’s incredibly encouraging to think about maybe a few million people taking a step back to normal life again,” he said.
A version of this article first appeared on Medscape.com.
The Plague Year Revisited
In April 2020, I pledged to focus my editorials on the pandemic. In subsequent editorials I renewed that intention. And it is a promise I have kept during the long plague year for all my editorials. When I announced my plan to write solely on COVID-19, my astute editor asked me, “How are you going to know when to stop?” I reminded myself of his question as I sat down to write each month and never arrived at a satisfactory answer. Nor do I have an answer now for why I am asking readers to release me from my vow—except for the somewhat trivial reason that a year seems enough. Is there more to say about the pandemic? Yes, there is so much more that needs to be discovered and unraveled, contemplated and analyzed; no doubt oceans of print and electronic pages will wash over us in the coming decade from thousands of scientists and journalists commenting on the topic of this public health crisis.2
Nevertheless, I have run the gauntlet of salient subjects within my wheelhouse: The plague year of editorials opened with a primer on public health ethics; the May column studied the duty to care for health care professionals in the midst of the first surge of virus; June examined the controversy around remdesivir and hydroxcholoroquine as medicine frantically sought some way to treat the sick; in July, I took a lighter look at the “Dog Days” of COVID-19 staring my Labrador Retriever mix, Reed, snoozing on his couch on the patio; August celebrated the amazing outreach of the US Department of Defense, US Public Health Service, and US Department of Veterans Affairs (VA) in service to the community; September discussed the adverse effects of the prolonged pandemic on the human psyche and some positive ways of handling the stress; October lamented the exponential rise in substance misuse as human beings struggled to manage the emotional toll of the pandemic; in December, COVID-19 was the sole subject of my annual Best and Worst ethics column; the new year saw the emergency use authorizations of the first and second vaccines and the editorial laid out the critical challenges for vaccination; in February my esteemed colleague Anita Tarzian joined me in an article explaining the ethical approach to vaccine allocation developed by the VA.3-12
A reader might aptly ask whether I am laying down the COVID-19 gauntlet because I believe the pandemic is over and done with us. The news is full of pundits opining when things will return to normal (if that ever existed or will again) and soothsayers divining the signs of the plague’s end.13 What I think is that we are more than done with the pandemic and unfortunately that may be the central cause of its perpetuation; which brings me to Daniel Defoe’s A Journal of the Plague Year.1
Defoe is better known to most of us if at all from modern films of his best-seller Robinson Crusoe. Yet A Journal of the Plague Year and other books about epidemics have become popular reading as we seek clues to the mystery of how to affirm life amid a death-dealing infectious disease.14 There is even an emerging lockdown literature genre. (Before anyone asks, I am in no way so pretentious as to suggest my columns should be included in that scholarly body of work).
Defoe’s book chronicles the last episode of the bubonic plague that afflicted London in 1665 and claimed 100,000 lives. Defoe was only 5 years old when the epidemic devastated one of the greatest cities in Europe. In 1772 he published what one recent reviewer called “a fascinating record of trying to cope with the capital’s last plague.”15 Defoe presciently documented the central reason I think the pandemic may not end anytime soon despite the increasing success of vaccination, at least in the United States. “But the Case was this...that the infection was propagated insensibly, and by such Persons, as were not visibly infected, who neither knew who they infected, or who they were infected by.”1
Ignorance and apathy are not confined to the streets of 17th century England: We see state after state lift restrictions prematurely, guaranteeing the scientists prediction that the wave now hitting Europe could again breach our shores. Defoe wrote long before germ theory and the ascendancy of public health, yet he knew that the inability or unwillingness to stick close to home kept the plague circulating. “And here I must observe again, that this Necessity of going out of our Houses to buy Provisions, was in a Great Measure the Ruin of the whole City, for the people catch’d the Distemper, on those Occasions, one of another...”1 While provisions may equate to food for many, for others necessities include going to bars, dining inside restaurants, and working out at gyms—all are natural laboratories for the spread and mutation of COVID-19 into variants against which physicians warn that the vaccine may not offer protection.
Defoe’s insights were at least in part due to his distance from the horror of the plague, which enabled him to study it with both empathy and objectivity, critical thinking, and creative observation. Similarly, it is time to take a brief breathing space from the pandemic as the central preoccupation of our existence: not just for me but for all of us to the extent possible given that unlike Defoe’s epoch it is still very much our reality. Even a few moments imagining a world without COVID-19 or more accurately one where it is under some reasonable control can help us reconceive how we want to live in it.
Can we use that luminal period to reenvision society along the lines Defoe idealistically drew even while we contribute to the collective search for the Holy Grail of herd immunity? During this second plague year, in coming editorials and in my own small circle of concern I will try to take a different less frustrated, embittered view of our lives scarred as they may be. It is only such a reorientation of perspectives in the shadow of so much death and suffering that can give us the energy and empathy to wear masks, go only where we must, follow public health measures and direction, and persuade the hesitant to be vaccinated so this truly is the last plague year at least for a long, quiet while.
1. Defoe D. A Journal of the Plague Year . Revised edition. Oxford World Classics; 2010.
2. Balch BT. One year into COVID, scientists are still learning about how the virus spreads, why disease symptoms and severity vary, and more. Published March 11, 2021. Accessed March 22, 2021. https://www.aamc.org/news-insights/one-year-covid-scientists-are-still-learning-about-how-virus-spreads-why-disease-symptoms-and
3. Geppert CMA. The return of the plague: a primer on pandemic ethics. Fed Pract. 2020;37(4):158-159.
4. Geppert CMA. The duty to care and its exceptions in a pandemic. Fed Pract. 2020;37(5):210-211.
5. Geppert CMA. A tale of 2 medications: a desperate race for hope. Fed Pract. 2020;37(6):256-257.
6. Geppert CMA. The dog days of COVID-19. Fed Pract. 2020;37(7):300-301.
7. Geppert CMA. All hands on deck: the federal health care response to the COVID-19 national emergency. Fed Pract. 2020;37(8):346-347. doi:10.12788/fp.0036
8. Geppert CMA. The brain in COVID-19: no one is okay. Fed Pract. 2020;37(9):396-397. doi:10.12788/fp.0046
9. Geppert CMA. The other pandemic: addiction. Fed Pract. 2020;37(10):440-441. doi:10.12788/fp.0059
10. Geppert CMA. Recalled to life: the best and worst of 2020 is the year 2020. Fed Pract . 2020;37(12):550-551. doi:10.12788/fp.0077
11. Geppert CMA. Trust in a vial. Fed Pract. 2021;38(1):4-5. doi:10.12788/fp.0084
12. Tarzian AJ, Geppert CMA. The Veterans Health Administration approach to COVID-19 vaccine allocation-balancing utility and equity. Fed Pract. 2021;38(2):52-54. doi:10.12788/fp.0093
13. Madrigal AG. A simple rule of thumb for knowing when the pandemic is over. Published February 23, 2021. Accessed March 22, 2021. https://www.theatlantic.com/health/archive/2021/02/how-know-when-pandemic-over/618122
14. Ford-Smith A. A Journal of the Plague Year book review. Med History. 2012;56(1):98-99. doi:10.1017/S0025727300000338
15. Jordison S. A Journal of the Plague Year by Daniel Defoe is our reading group book for May. The Guardian . Published April 28, 2020. Accessed March 22, 2021. https://www.theguardian.com/books/booksblog/2020/apr/28/a-journal-of-the-plague-year-by-daniel-defoe-is-our-reading-group-book-for-may
In April 2020, I pledged to focus my editorials on the pandemic. In subsequent editorials I renewed that intention. And it is a promise I have kept during the long plague year for all my editorials. When I announced my plan to write solely on COVID-19, my astute editor asked me, “How are you going to know when to stop?” I reminded myself of his question as I sat down to write each month and never arrived at a satisfactory answer. Nor do I have an answer now for why I am asking readers to release me from my vow—except for the somewhat trivial reason that a year seems enough. Is there more to say about the pandemic? Yes, there is so much more that needs to be discovered and unraveled, contemplated and analyzed; no doubt oceans of print and electronic pages will wash over us in the coming decade from thousands of scientists and journalists commenting on the topic of this public health crisis.2
Nevertheless, I have run the gauntlet of salient subjects within my wheelhouse: The plague year of editorials opened with a primer on public health ethics; the May column studied the duty to care for health care professionals in the midst of the first surge of virus; June examined the controversy around remdesivir and hydroxcholoroquine as medicine frantically sought some way to treat the sick; in July, I took a lighter look at the “Dog Days” of COVID-19 staring my Labrador Retriever mix, Reed, snoozing on his couch on the patio; August celebrated the amazing outreach of the US Department of Defense, US Public Health Service, and US Department of Veterans Affairs (VA) in service to the community; September discussed the adverse effects of the prolonged pandemic on the human psyche and some positive ways of handling the stress; October lamented the exponential rise in substance misuse as human beings struggled to manage the emotional toll of the pandemic; in December, COVID-19 was the sole subject of my annual Best and Worst ethics column; the new year saw the emergency use authorizations of the first and second vaccines and the editorial laid out the critical challenges for vaccination; in February my esteemed colleague Anita Tarzian joined me in an article explaining the ethical approach to vaccine allocation developed by the VA.3-12
A reader might aptly ask whether I am laying down the COVID-19 gauntlet because I believe the pandemic is over and done with us. The news is full of pundits opining when things will return to normal (if that ever existed or will again) and soothsayers divining the signs of the plague’s end.13 What I think is that we are more than done with the pandemic and unfortunately that may be the central cause of its perpetuation; which brings me to Daniel Defoe’s A Journal of the Plague Year.1
Defoe is better known to most of us if at all from modern films of his best-seller Robinson Crusoe. Yet A Journal of the Plague Year and other books about epidemics have become popular reading as we seek clues to the mystery of how to affirm life amid a death-dealing infectious disease.14 There is even an emerging lockdown literature genre. (Before anyone asks, I am in no way so pretentious as to suggest my columns should be included in that scholarly body of work).
Defoe’s book chronicles the last episode of the bubonic plague that afflicted London in 1665 and claimed 100,000 lives. Defoe was only 5 years old when the epidemic devastated one of the greatest cities in Europe. In 1772 he published what one recent reviewer called “a fascinating record of trying to cope with the capital’s last plague.”15 Defoe presciently documented the central reason I think the pandemic may not end anytime soon despite the increasing success of vaccination, at least in the United States. “But the Case was this...that the infection was propagated insensibly, and by such Persons, as were not visibly infected, who neither knew who they infected, or who they were infected by.”1
Ignorance and apathy are not confined to the streets of 17th century England: We see state after state lift restrictions prematurely, guaranteeing the scientists prediction that the wave now hitting Europe could again breach our shores. Defoe wrote long before germ theory and the ascendancy of public health, yet he knew that the inability or unwillingness to stick close to home kept the plague circulating. “And here I must observe again, that this Necessity of going out of our Houses to buy Provisions, was in a Great Measure the Ruin of the whole City, for the people catch’d the Distemper, on those Occasions, one of another...”1 While provisions may equate to food for many, for others necessities include going to bars, dining inside restaurants, and working out at gyms—all are natural laboratories for the spread and mutation of COVID-19 into variants against which physicians warn that the vaccine may not offer protection.
Defoe’s insights were at least in part due to his distance from the horror of the plague, which enabled him to study it with both empathy and objectivity, critical thinking, and creative observation. Similarly, it is time to take a brief breathing space from the pandemic as the central preoccupation of our existence: not just for me but for all of us to the extent possible given that unlike Defoe’s epoch it is still very much our reality. Even a few moments imagining a world without COVID-19 or more accurately one where it is under some reasonable control can help us reconceive how we want to live in it.
Can we use that luminal period to reenvision society along the lines Defoe idealistically drew even while we contribute to the collective search for the Holy Grail of herd immunity? During this second plague year, in coming editorials and in my own small circle of concern I will try to take a different less frustrated, embittered view of our lives scarred as they may be. It is only such a reorientation of perspectives in the shadow of so much death and suffering that can give us the energy and empathy to wear masks, go only where we must, follow public health measures and direction, and persuade the hesitant to be vaccinated so this truly is the last plague year at least for a long, quiet while.
In April 2020, I pledged to focus my editorials on the pandemic. In subsequent editorials I renewed that intention. And it is a promise I have kept during the long plague year for all my editorials. When I announced my plan to write solely on COVID-19, my astute editor asked me, “How are you going to know when to stop?” I reminded myself of his question as I sat down to write each month and never arrived at a satisfactory answer. Nor do I have an answer now for why I am asking readers to release me from my vow—except for the somewhat trivial reason that a year seems enough. Is there more to say about the pandemic? Yes, there is so much more that needs to be discovered and unraveled, contemplated and analyzed; no doubt oceans of print and electronic pages will wash over us in the coming decade from thousands of scientists and journalists commenting on the topic of this public health crisis.2
Nevertheless, I have run the gauntlet of salient subjects within my wheelhouse: The plague year of editorials opened with a primer on public health ethics; the May column studied the duty to care for health care professionals in the midst of the first surge of virus; June examined the controversy around remdesivir and hydroxcholoroquine as medicine frantically sought some way to treat the sick; in July, I took a lighter look at the “Dog Days” of COVID-19 staring my Labrador Retriever mix, Reed, snoozing on his couch on the patio; August celebrated the amazing outreach of the US Department of Defense, US Public Health Service, and US Department of Veterans Affairs (VA) in service to the community; September discussed the adverse effects of the prolonged pandemic on the human psyche and some positive ways of handling the stress; October lamented the exponential rise in substance misuse as human beings struggled to manage the emotional toll of the pandemic; in December, COVID-19 was the sole subject of my annual Best and Worst ethics column; the new year saw the emergency use authorizations of the first and second vaccines and the editorial laid out the critical challenges for vaccination; in February my esteemed colleague Anita Tarzian joined me in an article explaining the ethical approach to vaccine allocation developed by the VA.3-12
A reader might aptly ask whether I am laying down the COVID-19 gauntlet because I believe the pandemic is over and done with us. The news is full of pundits opining when things will return to normal (if that ever existed or will again) and soothsayers divining the signs of the plague’s end.13 What I think is that we are more than done with the pandemic and unfortunately that may be the central cause of its perpetuation; which brings me to Daniel Defoe’s A Journal of the Plague Year.1
Defoe is better known to most of us if at all from modern films of his best-seller Robinson Crusoe. Yet A Journal of the Plague Year and other books about epidemics have become popular reading as we seek clues to the mystery of how to affirm life amid a death-dealing infectious disease.14 There is even an emerging lockdown literature genre. (Before anyone asks, I am in no way so pretentious as to suggest my columns should be included in that scholarly body of work).
Defoe’s book chronicles the last episode of the bubonic plague that afflicted London in 1665 and claimed 100,000 lives. Defoe was only 5 years old when the epidemic devastated one of the greatest cities in Europe. In 1772 he published what one recent reviewer called “a fascinating record of trying to cope with the capital’s last plague.”15 Defoe presciently documented the central reason I think the pandemic may not end anytime soon despite the increasing success of vaccination, at least in the United States. “But the Case was this...that the infection was propagated insensibly, and by such Persons, as were not visibly infected, who neither knew who they infected, or who they were infected by.”1
Ignorance and apathy are not confined to the streets of 17th century England: We see state after state lift restrictions prematurely, guaranteeing the scientists prediction that the wave now hitting Europe could again breach our shores. Defoe wrote long before germ theory and the ascendancy of public health, yet he knew that the inability or unwillingness to stick close to home kept the plague circulating. “And here I must observe again, that this Necessity of going out of our Houses to buy Provisions, was in a Great Measure the Ruin of the whole City, for the people catch’d the Distemper, on those Occasions, one of another...”1 While provisions may equate to food for many, for others necessities include going to bars, dining inside restaurants, and working out at gyms—all are natural laboratories for the spread and mutation of COVID-19 into variants against which physicians warn that the vaccine may not offer protection.
Defoe’s insights were at least in part due to his distance from the horror of the plague, which enabled him to study it with both empathy and objectivity, critical thinking, and creative observation. Similarly, it is time to take a brief breathing space from the pandemic as the central preoccupation of our existence: not just for me but for all of us to the extent possible given that unlike Defoe’s epoch it is still very much our reality. Even a few moments imagining a world without COVID-19 or more accurately one where it is under some reasonable control can help us reconceive how we want to live in it.
Can we use that luminal period to reenvision society along the lines Defoe idealistically drew even while we contribute to the collective search for the Holy Grail of herd immunity? During this second plague year, in coming editorials and in my own small circle of concern I will try to take a different less frustrated, embittered view of our lives scarred as they may be. It is only such a reorientation of perspectives in the shadow of so much death and suffering that can give us the energy and empathy to wear masks, go only where we must, follow public health measures and direction, and persuade the hesitant to be vaccinated so this truly is the last plague year at least for a long, quiet while.
1. Defoe D. A Journal of the Plague Year . Revised edition. Oxford World Classics; 2010.
2. Balch BT. One year into COVID, scientists are still learning about how the virus spreads, why disease symptoms and severity vary, and more. Published March 11, 2021. Accessed March 22, 2021. https://www.aamc.org/news-insights/one-year-covid-scientists-are-still-learning-about-how-virus-spreads-why-disease-symptoms-and
3. Geppert CMA. The return of the plague: a primer on pandemic ethics. Fed Pract. 2020;37(4):158-159.
4. Geppert CMA. The duty to care and its exceptions in a pandemic. Fed Pract. 2020;37(5):210-211.
5. Geppert CMA. A tale of 2 medications: a desperate race for hope. Fed Pract. 2020;37(6):256-257.
6. Geppert CMA. The dog days of COVID-19. Fed Pract. 2020;37(7):300-301.
7. Geppert CMA. All hands on deck: the federal health care response to the COVID-19 national emergency. Fed Pract. 2020;37(8):346-347. doi:10.12788/fp.0036
8. Geppert CMA. The brain in COVID-19: no one is okay. Fed Pract. 2020;37(9):396-397. doi:10.12788/fp.0046
9. Geppert CMA. The other pandemic: addiction. Fed Pract. 2020;37(10):440-441. doi:10.12788/fp.0059
10. Geppert CMA. Recalled to life: the best and worst of 2020 is the year 2020. Fed Pract . 2020;37(12):550-551. doi:10.12788/fp.0077
11. Geppert CMA. Trust in a vial. Fed Pract. 2021;38(1):4-5. doi:10.12788/fp.0084
12. Tarzian AJ, Geppert CMA. The Veterans Health Administration approach to COVID-19 vaccine allocation-balancing utility and equity. Fed Pract. 2021;38(2):52-54. doi:10.12788/fp.0093
13. Madrigal AG. A simple rule of thumb for knowing when the pandemic is over. Published February 23, 2021. Accessed March 22, 2021. https://www.theatlantic.com/health/archive/2021/02/how-know-when-pandemic-over/618122
14. Ford-Smith A. A Journal of the Plague Year book review. Med History. 2012;56(1):98-99. doi:10.1017/S0025727300000338
15. Jordison S. A Journal of the Plague Year by Daniel Defoe is our reading group book for May. The Guardian . Published April 28, 2020. Accessed March 22, 2021. https://www.theguardian.com/books/booksblog/2020/apr/28/a-journal-of-the-plague-year-by-daniel-defoe-is-our-reading-group-book-for-may
1. Defoe D. A Journal of the Plague Year . Revised edition. Oxford World Classics; 2010.
2. Balch BT. One year into COVID, scientists are still learning about how the virus spreads, why disease symptoms and severity vary, and more. Published March 11, 2021. Accessed March 22, 2021. https://www.aamc.org/news-insights/one-year-covid-scientists-are-still-learning-about-how-virus-spreads-why-disease-symptoms-and
3. Geppert CMA. The return of the plague: a primer on pandemic ethics. Fed Pract. 2020;37(4):158-159.
4. Geppert CMA. The duty to care and its exceptions in a pandemic. Fed Pract. 2020;37(5):210-211.
5. Geppert CMA. A tale of 2 medications: a desperate race for hope. Fed Pract. 2020;37(6):256-257.
6. Geppert CMA. The dog days of COVID-19. Fed Pract. 2020;37(7):300-301.
7. Geppert CMA. All hands on deck: the federal health care response to the COVID-19 national emergency. Fed Pract. 2020;37(8):346-347. doi:10.12788/fp.0036
8. Geppert CMA. The brain in COVID-19: no one is okay. Fed Pract. 2020;37(9):396-397. doi:10.12788/fp.0046
9. Geppert CMA. The other pandemic: addiction. Fed Pract. 2020;37(10):440-441. doi:10.12788/fp.0059
10. Geppert CMA. Recalled to life: the best and worst of 2020 is the year 2020. Fed Pract . 2020;37(12):550-551. doi:10.12788/fp.0077
11. Geppert CMA. Trust in a vial. Fed Pract. 2021;38(1):4-5. doi:10.12788/fp.0084
12. Tarzian AJ, Geppert CMA. The Veterans Health Administration approach to COVID-19 vaccine allocation-balancing utility and equity. Fed Pract. 2021;38(2):52-54. doi:10.12788/fp.0093
13. Madrigal AG. A simple rule of thumb for knowing when the pandemic is over. Published February 23, 2021. Accessed March 22, 2021. https://www.theatlantic.com/health/archive/2021/02/how-know-when-pandemic-over/618122
14. Ford-Smith A. A Journal of the Plague Year book review. Med History. 2012;56(1):98-99. doi:10.1017/S0025727300000338
15. Jordison S. A Journal of the Plague Year by Daniel Defoe is our reading group book for May. The Guardian . Published April 28, 2020. Accessed March 22, 2021. https://www.theguardian.com/books/booksblog/2020/apr/28/a-journal-of-the-plague-year-by-daniel-defoe-is-our-reading-group-book-for-may
Cancer screening stopped by pandemic: Repercussions to come?
Last year, cancer screening programs around the world ground to a halt as SARS-CoV-2 infection rates surged globally. The effect of this slowdown is now becoming clear.
Thousands of cancer diagnoses are “missing,” and oncologists worry that this will lead to more advanced cancers and higher mortality for years to come.
“I feel like this is an earthquake that’s rocked our health care system. My guess is that you’ll probably still see repercussions of this over the next couple of years at least,” said Sharon Chang, MD, an attending surgical oncologist in the Permanente Medical Group, Fremont, Calif.
She was senior author of a study that analyzed the effects of the slowdown in mammography screening as a result of California’s “shelter-in-place” order on March 17, 2020. In the 2 months that followed, there were 64% fewer breast cancer diagnoses at 21 Kaiser Permanente medical centers, compared with the same period in 2019 (250 vs. 703).
In effect, approximately 450 breast cancer patients had “disappeared,” said coauthor Annie Tang, MD, a research fellow at the University of California, San Francisco, East Bay surgery program.
“What surprised me most from our data was the sheer number of breast cancer patients that were missing,” Dr. Tang said in an interview.
A similar picture has emerged elsewhere.
In Boston, an estimated 1,438 cancerous and precancerous lesions “went missing” during the first 3 months of pandemic shutdown, according to a study from the Massachusetts General Brigham health care system.
In this study, the investigators assessed screening rates for five cancers – breast cancer (mammography), prostate cancer (prostate-specific antigen testing), colorectal cancer (colonoscopy), cervical cancer (Papanicolaou tests), and lung cancer (low-dose CT).
Screening rates during the first peak of the pandemic (March 2 to June 2, 2020) were compared with those during the preceding and following 3 months and during the same 3 months in 2019.
The results showed a pronounced drop in screening rates during the peak pandemic period, compared with the three control periods. Decreases occurred for all screening tests and ranged from –60% to –82%.
There were also significant decreases in cancer diagnoses resulting from the decreases in screening tests, ranging from –19% to –78%.
“Quantifying the actual problem made us realize how much work needs to be done to get us back to prepandemic numbers,” said senior author Quoc-Dien Trinh, MD, FACS, codirector of the Dana Farber/Brigham and Women’s prostate cancer program.
In the Canadian province of Alberta, a similar decrease in cancer diagnoses occurred during the early days of the pandemic.
By the end of 2020, Alberta was “missing” approximately 2,000 cases of invasive cancers and 1,000 cases of noninvasive cancers, Doug Stewart, MD, senior medical director at the Cancer Strategic Clinical Network (SCN) of Alberta Health Services, told this news organization.
Dr. Stewart is able to track cancer diagnoses in Alberta almost in real time through a mandatory cancer registry. Within a month of shutdown, there was a 30% decrease in diagnoses of invasive cancers and a 50% decrease “in the kind of preinvasive cancers that, for the most part, are picked up by screening programs,” said Dr. Stewart.
After the health care system opened up again in the summer, Stewart said, noninvasive cancer diagnoses continued to be 20% lower than expected. There was a 10% shortfall in invasive cancer diagnoses.
The number of diagnoses had returned to normal by December 2020. However, Dr. Stewart is worried that this fact conceals a terrible truth.
The worry is over the backlog. Although the number of diagnoses is now similar to what it was before the pandemic, “people are presenting later, and maybe the cancer is more advanced,” he speculated.
His team at Alberta Health Services is assessing whether the cancers that are being diagnosed now are more advanced. Initial results are anticipated by late April 2021.
In the United Kingdom, there was a similar halt in cancer screening as a result of the country’s lockdown. Researchers now predict an uptick in cancer diagnoses.
Ajay Aggarwal, MD, PhD, consultant clinical oncologist and associate professor at the London School of Hygiene and Tropical Medicine, and colleagues have estimated that at least 3,500 deaths from breast, colorectal, esophageal, and lung cancer will occur during the next 5 years in England that could have been avoided had it not been for the lockdown measures necessitated by the pandemic.
Speaking to this news organization, Dr. Aggarwal warned that these numbers, which are from a modeling study published in August 2020, are “extremely conservative,” because the investigators considered diagnostic delays over only a 3-month period, the analysis involved only four cancers, and it did not reflect deferral of cancer treatment.
“It felt like it was the tip of the iceberg,” Dr. Aggarwal said. He warns that more recent data suggest that “diagnostic delays are probably worse than we predicted.”
He suspects that there is more at play than screening cancellations.
In another study conducted in the United Kingdom, data show “a falling edge of referrals” from primary care to cancer centers early in the pandemic. In that study, investigators analyzed real-time weekly hospital data from eight large British hospitals and found that urgent cancer referrals fell 70% at their lowest point.
“It really surprised me that the urgent referrals dropped so drastically,” said lead author Alvina Lai, PhD, a lecturer in health data analytics at University College London.
She attributed this in part to patients’ adherence to lockdown rules. “Patients are trying to follow government guidelines to stay home and not go to [general practitioners] unless necessary,” Dr. Lai explained in an interview.
Canada, like the United Kingdom, has a publicly funded health care system. Dr. Stewart came to a similar conclusion. “Some patients who have been diagnosed with cancer ... have told me it took them an extra couple of months to even contact the family doc, because they ... didn’t want to bother the family doctor with something that wasn’t COVID, this kind of guilt. They want to do something good for society. You know, most people are just really nice people, and they don’t want to bother the health care system if they don’t have COVID,” Dr. Stewart said.
Shelley Fuld Nasso, CEO of the National Coalition for Cancer Survivorship, a nonprofit organization based in Silver Spring, Md., agreed that screening shutdowns are not the only danger. “While we agree that screening is really important, we also want to make sure patients are following up with their physicians about symptoms that they have,” she said.
“Some of the speculation or concern about increased mortality for cancer is related to screening, but some of it is related to delayed diagnosis because of not following up on symptoms. ... What concerns me is not everyone has that ability or willingness to advocate for themselves,” she said.
Speaking at a press briefing held by the American Society for Radiation Oncology on March 30, Dr. Nasso related a case involving a patient who experienced severe arm pain. In a teleconsultation with her primary care physician, her condition was diagnosed as arthritis. She was subsequently diagnosed in the ED as having multiple myeloma.
Patients who “feel fine” may postpone their checkups to avoid going to the hospital and risking exposure to COVID-19.
“Some patients are still hesitant about returning for their mammograms or coming in if they feel a breast lump,” Dr. Tang said. “That fear of COVID-19 is still out there, and we don’t know how long patients are going to delay.”
In London, Dr. Aggarwal saw a similar response to the pandemic. “People were overestimating quite significantly what their risk of death was from acquiring COVID-19, and I think that balance was never [redressed] explicitly,” he said.
Public health initiatives to rebalance the messaging are now underway.
Public Health England and National Health Service England launched their Help Us Help You campaign in October 2020. The public information campaign urges people to speak to their doctors if they were “worried about a symptom that could be cancer.”
In Canada, the provincial government in Alberta has launched a public awareness campaign that conveys the message, “cancer has not gone away.”
“Cancer is still the No. 1 cause of potential life-years lost, despite COVID,” Dr. Stewart said. “We need to do what we can to make sure there’s no slippage in survival rates.”
Dr. Tang, Dr. Chang, Dr. Lai, Dr. Stewart, and Dr. Aggarwal have disclosed no relevant financial relationship. Dr. Trinh has received personal fees from Astellas, Bayer, and Janssen and grants from Intuitive Surgical.
A version of this article first appeared on Medscape.com.
Last year, cancer screening programs around the world ground to a halt as SARS-CoV-2 infection rates surged globally. The effect of this slowdown is now becoming clear.
Thousands of cancer diagnoses are “missing,” and oncologists worry that this will lead to more advanced cancers and higher mortality for years to come.
“I feel like this is an earthquake that’s rocked our health care system. My guess is that you’ll probably still see repercussions of this over the next couple of years at least,” said Sharon Chang, MD, an attending surgical oncologist in the Permanente Medical Group, Fremont, Calif.
She was senior author of a study that analyzed the effects of the slowdown in mammography screening as a result of California’s “shelter-in-place” order on March 17, 2020. In the 2 months that followed, there were 64% fewer breast cancer diagnoses at 21 Kaiser Permanente medical centers, compared with the same period in 2019 (250 vs. 703).
In effect, approximately 450 breast cancer patients had “disappeared,” said coauthor Annie Tang, MD, a research fellow at the University of California, San Francisco, East Bay surgery program.
“What surprised me most from our data was the sheer number of breast cancer patients that were missing,” Dr. Tang said in an interview.
A similar picture has emerged elsewhere.
In Boston, an estimated 1,438 cancerous and precancerous lesions “went missing” during the first 3 months of pandemic shutdown, according to a study from the Massachusetts General Brigham health care system.
In this study, the investigators assessed screening rates for five cancers – breast cancer (mammography), prostate cancer (prostate-specific antigen testing), colorectal cancer (colonoscopy), cervical cancer (Papanicolaou tests), and lung cancer (low-dose CT).
Screening rates during the first peak of the pandemic (March 2 to June 2, 2020) were compared with those during the preceding and following 3 months and during the same 3 months in 2019.
The results showed a pronounced drop in screening rates during the peak pandemic period, compared with the three control periods. Decreases occurred for all screening tests and ranged from –60% to –82%.
There were also significant decreases in cancer diagnoses resulting from the decreases in screening tests, ranging from –19% to –78%.
“Quantifying the actual problem made us realize how much work needs to be done to get us back to prepandemic numbers,” said senior author Quoc-Dien Trinh, MD, FACS, codirector of the Dana Farber/Brigham and Women’s prostate cancer program.
In the Canadian province of Alberta, a similar decrease in cancer diagnoses occurred during the early days of the pandemic.
By the end of 2020, Alberta was “missing” approximately 2,000 cases of invasive cancers and 1,000 cases of noninvasive cancers, Doug Stewart, MD, senior medical director at the Cancer Strategic Clinical Network (SCN) of Alberta Health Services, told this news organization.
Dr. Stewart is able to track cancer diagnoses in Alberta almost in real time through a mandatory cancer registry. Within a month of shutdown, there was a 30% decrease in diagnoses of invasive cancers and a 50% decrease “in the kind of preinvasive cancers that, for the most part, are picked up by screening programs,” said Dr. Stewart.
After the health care system opened up again in the summer, Stewart said, noninvasive cancer diagnoses continued to be 20% lower than expected. There was a 10% shortfall in invasive cancer diagnoses.
The number of diagnoses had returned to normal by December 2020. However, Dr. Stewart is worried that this fact conceals a terrible truth.
The worry is over the backlog. Although the number of diagnoses is now similar to what it was before the pandemic, “people are presenting later, and maybe the cancer is more advanced,” he speculated.
His team at Alberta Health Services is assessing whether the cancers that are being diagnosed now are more advanced. Initial results are anticipated by late April 2021.
In the United Kingdom, there was a similar halt in cancer screening as a result of the country’s lockdown. Researchers now predict an uptick in cancer diagnoses.
Ajay Aggarwal, MD, PhD, consultant clinical oncologist and associate professor at the London School of Hygiene and Tropical Medicine, and colleagues have estimated that at least 3,500 deaths from breast, colorectal, esophageal, and lung cancer will occur during the next 5 years in England that could have been avoided had it not been for the lockdown measures necessitated by the pandemic.
Speaking to this news organization, Dr. Aggarwal warned that these numbers, which are from a modeling study published in August 2020, are “extremely conservative,” because the investigators considered diagnostic delays over only a 3-month period, the analysis involved only four cancers, and it did not reflect deferral of cancer treatment.
“It felt like it was the tip of the iceberg,” Dr. Aggarwal said. He warns that more recent data suggest that “diagnostic delays are probably worse than we predicted.”
He suspects that there is more at play than screening cancellations.
In another study conducted in the United Kingdom, data show “a falling edge of referrals” from primary care to cancer centers early in the pandemic. In that study, investigators analyzed real-time weekly hospital data from eight large British hospitals and found that urgent cancer referrals fell 70% at their lowest point.
“It really surprised me that the urgent referrals dropped so drastically,” said lead author Alvina Lai, PhD, a lecturer in health data analytics at University College London.
She attributed this in part to patients’ adherence to lockdown rules. “Patients are trying to follow government guidelines to stay home and not go to [general practitioners] unless necessary,” Dr. Lai explained in an interview.
Canada, like the United Kingdom, has a publicly funded health care system. Dr. Stewart came to a similar conclusion. “Some patients who have been diagnosed with cancer ... have told me it took them an extra couple of months to even contact the family doc, because they ... didn’t want to bother the family doctor with something that wasn’t COVID, this kind of guilt. They want to do something good for society. You know, most people are just really nice people, and they don’t want to bother the health care system if they don’t have COVID,” Dr. Stewart said.
Shelley Fuld Nasso, CEO of the National Coalition for Cancer Survivorship, a nonprofit organization based in Silver Spring, Md., agreed that screening shutdowns are not the only danger. “While we agree that screening is really important, we also want to make sure patients are following up with their physicians about symptoms that they have,” she said.
“Some of the speculation or concern about increased mortality for cancer is related to screening, but some of it is related to delayed diagnosis because of not following up on symptoms. ... What concerns me is not everyone has that ability or willingness to advocate for themselves,” she said.
Speaking at a press briefing held by the American Society for Radiation Oncology on March 30, Dr. Nasso related a case involving a patient who experienced severe arm pain. In a teleconsultation with her primary care physician, her condition was diagnosed as arthritis. She was subsequently diagnosed in the ED as having multiple myeloma.
Patients who “feel fine” may postpone their checkups to avoid going to the hospital and risking exposure to COVID-19.
“Some patients are still hesitant about returning for their mammograms or coming in if they feel a breast lump,” Dr. Tang said. “That fear of COVID-19 is still out there, and we don’t know how long patients are going to delay.”
In London, Dr. Aggarwal saw a similar response to the pandemic. “People were overestimating quite significantly what their risk of death was from acquiring COVID-19, and I think that balance was never [redressed] explicitly,” he said.
Public health initiatives to rebalance the messaging are now underway.
Public Health England and National Health Service England launched their Help Us Help You campaign in October 2020. The public information campaign urges people to speak to their doctors if they were “worried about a symptom that could be cancer.”
In Canada, the provincial government in Alberta has launched a public awareness campaign that conveys the message, “cancer has not gone away.”
“Cancer is still the No. 1 cause of potential life-years lost, despite COVID,” Dr. Stewart said. “We need to do what we can to make sure there’s no slippage in survival rates.”
Dr. Tang, Dr. Chang, Dr. Lai, Dr. Stewart, and Dr. Aggarwal have disclosed no relevant financial relationship. Dr. Trinh has received personal fees from Astellas, Bayer, and Janssen and grants from Intuitive Surgical.
A version of this article first appeared on Medscape.com.
Last year, cancer screening programs around the world ground to a halt as SARS-CoV-2 infection rates surged globally. The effect of this slowdown is now becoming clear.
Thousands of cancer diagnoses are “missing,” and oncologists worry that this will lead to more advanced cancers and higher mortality for years to come.
“I feel like this is an earthquake that’s rocked our health care system. My guess is that you’ll probably still see repercussions of this over the next couple of years at least,” said Sharon Chang, MD, an attending surgical oncologist in the Permanente Medical Group, Fremont, Calif.
She was senior author of a study that analyzed the effects of the slowdown in mammography screening as a result of California’s “shelter-in-place” order on March 17, 2020. In the 2 months that followed, there were 64% fewer breast cancer diagnoses at 21 Kaiser Permanente medical centers, compared with the same period in 2019 (250 vs. 703).
In effect, approximately 450 breast cancer patients had “disappeared,” said coauthor Annie Tang, MD, a research fellow at the University of California, San Francisco, East Bay surgery program.
“What surprised me most from our data was the sheer number of breast cancer patients that were missing,” Dr. Tang said in an interview.
A similar picture has emerged elsewhere.
In Boston, an estimated 1,438 cancerous and precancerous lesions “went missing” during the first 3 months of pandemic shutdown, according to a study from the Massachusetts General Brigham health care system.
In this study, the investigators assessed screening rates for five cancers – breast cancer (mammography), prostate cancer (prostate-specific antigen testing), colorectal cancer (colonoscopy), cervical cancer (Papanicolaou tests), and lung cancer (low-dose CT).
Screening rates during the first peak of the pandemic (March 2 to June 2, 2020) were compared with those during the preceding and following 3 months and during the same 3 months in 2019.
The results showed a pronounced drop in screening rates during the peak pandemic period, compared with the three control periods. Decreases occurred for all screening tests and ranged from –60% to –82%.
There were also significant decreases in cancer diagnoses resulting from the decreases in screening tests, ranging from –19% to –78%.
“Quantifying the actual problem made us realize how much work needs to be done to get us back to prepandemic numbers,” said senior author Quoc-Dien Trinh, MD, FACS, codirector of the Dana Farber/Brigham and Women’s prostate cancer program.
In the Canadian province of Alberta, a similar decrease in cancer diagnoses occurred during the early days of the pandemic.
By the end of 2020, Alberta was “missing” approximately 2,000 cases of invasive cancers and 1,000 cases of noninvasive cancers, Doug Stewart, MD, senior medical director at the Cancer Strategic Clinical Network (SCN) of Alberta Health Services, told this news organization.
Dr. Stewart is able to track cancer diagnoses in Alberta almost in real time through a mandatory cancer registry. Within a month of shutdown, there was a 30% decrease in diagnoses of invasive cancers and a 50% decrease “in the kind of preinvasive cancers that, for the most part, are picked up by screening programs,” said Dr. Stewart.
After the health care system opened up again in the summer, Stewart said, noninvasive cancer diagnoses continued to be 20% lower than expected. There was a 10% shortfall in invasive cancer diagnoses.
The number of diagnoses had returned to normal by December 2020. However, Dr. Stewart is worried that this fact conceals a terrible truth.
The worry is over the backlog. Although the number of diagnoses is now similar to what it was before the pandemic, “people are presenting later, and maybe the cancer is more advanced,” he speculated.
His team at Alberta Health Services is assessing whether the cancers that are being diagnosed now are more advanced. Initial results are anticipated by late April 2021.
In the United Kingdom, there was a similar halt in cancer screening as a result of the country’s lockdown. Researchers now predict an uptick in cancer diagnoses.
Ajay Aggarwal, MD, PhD, consultant clinical oncologist and associate professor at the London School of Hygiene and Tropical Medicine, and colleagues have estimated that at least 3,500 deaths from breast, colorectal, esophageal, and lung cancer will occur during the next 5 years in England that could have been avoided had it not been for the lockdown measures necessitated by the pandemic.
Speaking to this news organization, Dr. Aggarwal warned that these numbers, which are from a modeling study published in August 2020, are “extremely conservative,” because the investigators considered diagnostic delays over only a 3-month period, the analysis involved only four cancers, and it did not reflect deferral of cancer treatment.
“It felt like it was the tip of the iceberg,” Dr. Aggarwal said. He warns that more recent data suggest that “diagnostic delays are probably worse than we predicted.”
He suspects that there is more at play than screening cancellations.
In another study conducted in the United Kingdom, data show “a falling edge of referrals” from primary care to cancer centers early in the pandemic. In that study, investigators analyzed real-time weekly hospital data from eight large British hospitals and found that urgent cancer referrals fell 70% at their lowest point.
“It really surprised me that the urgent referrals dropped so drastically,” said lead author Alvina Lai, PhD, a lecturer in health data analytics at University College London.
She attributed this in part to patients’ adherence to lockdown rules. “Patients are trying to follow government guidelines to stay home and not go to [general practitioners] unless necessary,” Dr. Lai explained in an interview.
Canada, like the United Kingdom, has a publicly funded health care system. Dr. Stewart came to a similar conclusion. “Some patients who have been diagnosed with cancer ... have told me it took them an extra couple of months to even contact the family doc, because they ... didn’t want to bother the family doctor with something that wasn’t COVID, this kind of guilt. They want to do something good for society. You know, most people are just really nice people, and they don’t want to bother the health care system if they don’t have COVID,” Dr. Stewart said.
Shelley Fuld Nasso, CEO of the National Coalition for Cancer Survivorship, a nonprofit organization based in Silver Spring, Md., agreed that screening shutdowns are not the only danger. “While we agree that screening is really important, we also want to make sure patients are following up with their physicians about symptoms that they have,” she said.
“Some of the speculation or concern about increased mortality for cancer is related to screening, but some of it is related to delayed diagnosis because of not following up on symptoms. ... What concerns me is not everyone has that ability or willingness to advocate for themselves,” she said.
Speaking at a press briefing held by the American Society for Radiation Oncology on March 30, Dr. Nasso related a case involving a patient who experienced severe arm pain. In a teleconsultation with her primary care physician, her condition was diagnosed as arthritis. She was subsequently diagnosed in the ED as having multiple myeloma.
Patients who “feel fine” may postpone their checkups to avoid going to the hospital and risking exposure to COVID-19.
“Some patients are still hesitant about returning for their mammograms or coming in if they feel a breast lump,” Dr. Tang said. “That fear of COVID-19 is still out there, and we don’t know how long patients are going to delay.”
In London, Dr. Aggarwal saw a similar response to the pandemic. “People were overestimating quite significantly what their risk of death was from acquiring COVID-19, and I think that balance was never [redressed] explicitly,” he said.
Public health initiatives to rebalance the messaging are now underway.
Public Health England and National Health Service England launched their Help Us Help You campaign in October 2020. The public information campaign urges people to speak to their doctors if they were “worried about a symptom that could be cancer.”
In Canada, the provincial government in Alberta has launched a public awareness campaign that conveys the message, “cancer has not gone away.”
“Cancer is still the No. 1 cause of potential life-years lost, despite COVID,” Dr. Stewart said. “We need to do what we can to make sure there’s no slippage in survival rates.”
Dr. Tang, Dr. Chang, Dr. Lai, Dr. Stewart, and Dr. Aggarwal have disclosed no relevant financial relationship. Dr. Trinh has received personal fees from Astellas, Bayer, and Janssen and grants from Intuitive Surgical.
A version of this article first appeared on Medscape.com.
University taking aim at racial disparities in COVID vaccine trials
Although recent months have seen the arrival of several promising vaccines to combat COVID-19, many researchers have been concerned about the shortage of Black and Latinx volunteers in their pivotal trials.
Minority groups have long been underrepresented in clinical research. The pandemic’s inequitable fallout has heightened the need for more inclusive COVID-19 trials. By one estimate, Black Americans are three times more likely to become infected with SARS-Cov-2 and twice as likely to die from it, compared with their White counterparts.
It was therefore welcome news this past November when the Maryland-based biotech company Novavax unveiled their plans to boost participation among specific minority groups during the phase 3 trial of their COVID-19 vaccine candidate NVX-CoV2373. To help them in their efforts, the company tapped Howard University, in Washington, D.C., to be a clinical test site. The goal was to enroll 300 Black and Latinx volunteers through a recruitment registry at the Coronavirus Prevention Network.
“We have seen quite a good number of participants in the registry, and many are African American, who are the ones we are trying to reach in the trial,” explained Siham Mahgoub, MD, medical director of the Center of Infectious Diseases Management and Research and principal investigator for the Novavax trial at Howard University, Washington. “It’s very important for people of color to participate in the trial because we want to make sure these vaccines work in people of color,” Dr. Mahgoub said.
Over the years, Howard University has hosted several important clinical trials and studies, and its participation in the multi-institutional Georgetown–Howard Universities Center for Clinical and Translational Science consortium brings crucial infrastructural value. By bringing this vaccine trial to one of the most esteemed historically Black colleges or universities (HBCUs), researchers hoped to address a sense of hesitancy among possible participants that is prompted in part by the tragic history of medical testing in the Black community.
“The community trusts Howard,” said Dr. Mahgoub. “I think it’s great having Howard and an HBCU host this trial, because these are people who look like them.”
Lisa M. Dunkle, MD, vice president and global medical lead for coronavirus vaccine at Novavax, explained that, in addition to Howard being located close to the company’s headquarters, the university seemed like a great fit for the overall mission.
“As part of our goal to achieve a representative trial population that includes communities who are disproportionately impacted by the pandemic, we sought out some of the HBCUs to include in our trial sites. We hoped that this might encourage people of color to enroll and to increase their comfort level with vaccines in general,” Dr. Dunkle said.
Building more representative clinical trials
For decades, research on some of the most groundbreaking vaccines and treatments have been based on the results of studies conducted with predominately White participants, despite the fact that a much more demographically varied general population would ultimately receive them. This has led to calls to include people of different races and ethnic backgrounds in trials.
Homogeneity in clinical trials is discouraged, but trials are not heavily regulated in this regard. In 1993, Congress passed the Revitalization Act, which requires that trials that are conducted by the National Institutes of Health include women and members of minority groups among their cohorts. However, the number or proportion of such participants is not specified.
Underrepresentation in clinical trials also reflects a general unwillingness by members of ethnic minorities to volunteer because of the deeply unsettling history of such trials in minority communities. Among some Black persons, it is not uncommon for names like Tuskegee, Henrietta Lacks, and J. Marion Simms to be mentioned when giving reasons for not participating.
“There is certainly some dark history in how minorities have been treated by our health care system, and it’s not surprising that there is some fear and distrust,” said Dr. Dunkle. “By recruiting people of color into clinical trials that are governed with strict standards, we can begin to change perceptions and attitudes.”
Vaccine hesitancy is not only rooted in the past. The current state of medical care also has some potential trial participants worried. Misinformation, inequity in health care access, and low health literacy contribute to the current fears of scientific development.
A trial designed to engender trust
Having information about the vaccine come from trusted voices in the community is a key means of overcoming hesitancy. Howard University President Wayne Frederick, MD, reached out to a pastor of a local Black church to have more participants enroll in the trial. One who answered the call to action was Stephanie Williams, an elementary school teacher in Montgomery County, Maryland. When she saw that her pastor was participating in the Novavax trial and when she considered the devastation she had seen from COVID-19, she was on board.
“We had about three sessions where he shared his experiences. He also shared some links to read about it more,” Ms. Williams said. “When I saw that he took it, that gave me a lot of confidence. Since I’m going be going into the classroom, I wanted to be sure that I was well protected.”
Transparency is key to gaining more participation, explained Dr. Maghoub. Webinar-based information sessions have proven particularly important in achieving this.
“We do a lot of explaining in very simple language to make sure everyone understands about the vaccine. The participants have time to ask questions during the webinar, and at any time [during the trial], if a participant feels that it is not right for them, they can stop. They have time to learn about the trial and give consent. People often think they are like guinea pigs in trials, but they are not. They must give consent.”
There are signs that the approach has been successful. Over a period of 4-5 weeks, the Howard site enrolled 150 participants, of whom 30% were Black and 20% were Latinx.
Novavax has been in business for more than 3 decades but hasn’t seen the booming success that their competitors have. The company has noted progress in developing vaccines against Middle East respiratory syndrome and severe acute respiratory syndrome. However, they missed the mark in clinical trials, failing twice in 3 years to develop a respiratory syncytial virus vaccine administered through maternal immunizations.
From being on the verge of closing, Novavax has since made a dramatic turnaround after former President Trump awarded the company $1.6 billion dollars in July 2020 as part of Operation Warp Speed. If trial results are promising, the Novavax vaccine could enter the market in a few months, representing not only a new therapeutic option but perhaps a new model for building inclusivity in clinical trials.
A version of this article first appeared on Medscape.com.
Although recent months have seen the arrival of several promising vaccines to combat COVID-19, many researchers have been concerned about the shortage of Black and Latinx volunteers in their pivotal trials.
Minority groups have long been underrepresented in clinical research. The pandemic’s inequitable fallout has heightened the need for more inclusive COVID-19 trials. By one estimate, Black Americans are three times more likely to become infected with SARS-Cov-2 and twice as likely to die from it, compared with their White counterparts.
It was therefore welcome news this past November when the Maryland-based biotech company Novavax unveiled their plans to boost participation among specific minority groups during the phase 3 trial of their COVID-19 vaccine candidate NVX-CoV2373. To help them in their efforts, the company tapped Howard University, in Washington, D.C., to be a clinical test site. The goal was to enroll 300 Black and Latinx volunteers through a recruitment registry at the Coronavirus Prevention Network.
“We have seen quite a good number of participants in the registry, and many are African American, who are the ones we are trying to reach in the trial,” explained Siham Mahgoub, MD, medical director of the Center of Infectious Diseases Management and Research and principal investigator for the Novavax trial at Howard University, Washington. “It’s very important for people of color to participate in the trial because we want to make sure these vaccines work in people of color,” Dr. Mahgoub said.
Over the years, Howard University has hosted several important clinical trials and studies, and its participation in the multi-institutional Georgetown–Howard Universities Center for Clinical and Translational Science consortium brings crucial infrastructural value. By bringing this vaccine trial to one of the most esteemed historically Black colleges or universities (HBCUs), researchers hoped to address a sense of hesitancy among possible participants that is prompted in part by the tragic history of medical testing in the Black community.
“The community trusts Howard,” said Dr. Mahgoub. “I think it’s great having Howard and an HBCU host this trial, because these are people who look like them.”
Lisa M. Dunkle, MD, vice president and global medical lead for coronavirus vaccine at Novavax, explained that, in addition to Howard being located close to the company’s headquarters, the university seemed like a great fit for the overall mission.
“As part of our goal to achieve a representative trial population that includes communities who are disproportionately impacted by the pandemic, we sought out some of the HBCUs to include in our trial sites. We hoped that this might encourage people of color to enroll and to increase their comfort level with vaccines in general,” Dr. Dunkle said.
Building more representative clinical trials
For decades, research on some of the most groundbreaking vaccines and treatments have been based on the results of studies conducted with predominately White participants, despite the fact that a much more demographically varied general population would ultimately receive them. This has led to calls to include people of different races and ethnic backgrounds in trials.
Homogeneity in clinical trials is discouraged, but trials are not heavily regulated in this regard. In 1993, Congress passed the Revitalization Act, which requires that trials that are conducted by the National Institutes of Health include women and members of minority groups among their cohorts. However, the number or proportion of such participants is not specified.
Underrepresentation in clinical trials also reflects a general unwillingness by members of ethnic minorities to volunteer because of the deeply unsettling history of such trials in minority communities. Among some Black persons, it is not uncommon for names like Tuskegee, Henrietta Lacks, and J. Marion Simms to be mentioned when giving reasons for not participating.
“There is certainly some dark history in how minorities have been treated by our health care system, and it’s not surprising that there is some fear and distrust,” said Dr. Dunkle. “By recruiting people of color into clinical trials that are governed with strict standards, we can begin to change perceptions and attitudes.”
Vaccine hesitancy is not only rooted in the past. The current state of medical care also has some potential trial participants worried. Misinformation, inequity in health care access, and low health literacy contribute to the current fears of scientific development.
A trial designed to engender trust
Having information about the vaccine come from trusted voices in the community is a key means of overcoming hesitancy. Howard University President Wayne Frederick, MD, reached out to a pastor of a local Black church to have more participants enroll in the trial. One who answered the call to action was Stephanie Williams, an elementary school teacher in Montgomery County, Maryland. When she saw that her pastor was participating in the Novavax trial and when she considered the devastation she had seen from COVID-19, she was on board.
“We had about three sessions where he shared his experiences. He also shared some links to read about it more,” Ms. Williams said. “When I saw that he took it, that gave me a lot of confidence. Since I’m going be going into the classroom, I wanted to be sure that I was well protected.”
Transparency is key to gaining more participation, explained Dr. Maghoub. Webinar-based information sessions have proven particularly important in achieving this.
“We do a lot of explaining in very simple language to make sure everyone understands about the vaccine. The participants have time to ask questions during the webinar, and at any time [during the trial], if a participant feels that it is not right for them, they can stop. They have time to learn about the trial and give consent. People often think they are like guinea pigs in trials, but they are not. They must give consent.”
There are signs that the approach has been successful. Over a period of 4-5 weeks, the Howard site enrolled 150 participants, of whom 30% were Black and 20% were Latinx.
Novavax has been in business for more than 3 decades but hasn’t seen the booming success that their competitors have. The company has noted progress in developing vaccines against Middle East respiratory syndrome and severe acute respiratory syndrome. However, they missed the mark in clinical trials, failing twice in 3 years to develop a respiratory syncytial virus vaccine administered through maternal immunizations.
From being on the verge of closing, Novavax has since made a dramatic turnaround after former President Trump awarded the company $1.6 billion dollars in July 2020 as part of Operation Warp Speed. If trial results are promising, the Novavax vaccine could enter the market in a few months, representing not only a new therapeutic option but perhaps a new model for building inclusivity in clinical trials.
A version of this article first appeared on Medscape.com.
Although recent months have seen the arrival of several promising vaccines to combat COVID-19, many researchers have been concerned about the shortage of Black and Latinx volunteers in their pivotal trials.
Minority groups have long been underrepresented in clinical research. The pandemic’s inequitable fallout has heightened the need for more inclusive COVID-19 trials. By one estimate, Black Americans are three times more likely to become infected with SARS-Cov-2 and twice as likely to die from it, compared with their White counterparts.
It was therefore welcome news this past November when the Maryland-based biotech company Novavax unveiled their plans to boost participation among specific minority groups during the phase 3 trial of their COVID-19 vaccine candidate NVX-CoV2373. To help them in their efforts, the company tapped Howard University, in Washington, D.C., to be a clinical test site. The goal was to enroll 300 Black and Latinx volunteers through a recruitment registry at the Coronavirus Prevention Network.
“We have seen quite a good number of participants in the registry, and many are African American, who are the ones we are trying to reach in the trial,” explained Siham Mahgoub, MD, medical director of the Center of Infectious Diseases Management and Research and principal investigator for the Novavax trial at Howard University, Washington. “It’s very important for people of color to participate in the trial because we want to make sure these vaccines work in people of color,” Dr. Mahgoub said.
Over the years, Howard University has hosted several important clinical trials and studies, and its participation in the multi-institutional Georgetown–Howard Universities Center for Clinical and Translational Science consortium brings crucial infrastructural value. By bringing this vaccine trial to one of the most esteemed historically Black colleges or universities (HBCUs), researchers hoped to address a sense of hesitancy among possible participants that is prompted in part by the tragic history of medical testing in the Black community.
“The community trusts Howard,” said Dr. Mahgoub. “I think it’s great having Howard and an HBCU host this trial, because these are people who look like them.”
Lisa M. Dunkle, MD, vice president and global medical lead for coronavirus vaccine at Novavax, explained that, in addition to Howard being located close to the company’s headquarters, the university seemed like a great fit for the overall mission.
“As part of our goal to achieve a representative trial population that includes communities who are disproportionately impacted by the pandemic, we sought out some of the HBCUs to include in our trial sites. We hoped that this might encourage people of color to enroll and to increase their comfort level with vaccines in general,” Dr. Dunkle said.
Building more representative clinical trials
For decades, research on some of the most groundbreaking vaccines and treatments have been based on the results of studies conducted with predominately White participants, despite the fact that a much more demographically varied general population would ultimately receive them. This has led to calls to include people of different races and ethnic backgrounds in trials.
Homogeneity in clinical trials is discouraged, but trials are not heavily regulated in this regard. In 1993, Congress passed the Revitalization Act, which requires that trials that are conducted by the National Institutes of Health include women and members of minority groups among their cohorts. However, the number or proportion of such participants is not specified.
Underrepresentation in clinical trials also reflects a general unwillingness by members of ethnic minorities to volunteer because of the deeply unsettling history of such trials in minority communities. Among some Black persons, it is not uncommon for names like Tuskegee, Henrietta Lacks, and J. Marion Simms to be mentioned when giving reasons for not participating.
“There is certainly some dark history in how minorities have been treated by our health care system, and it’s not surprising that there is some fear and distrust,” said Dr. Dunkle. “By recruiting people of color into clinical trials that are governed with strict standards, we can begin to change perceptions and attitudes.”
Vaccine hesitancy is not only rooted in the past. The current state of medical care also has some potential trial participants worried. Misinformation, inequity in health care access, and low health literacy contribute to the current fears of scientific development.
A trial designed to engender trust
Having information about the vaccine come from trusted voices in the community is a key means of overcoming hesitancy. Howard University President Wayne Frederick, MD, reached out to a pastor of a local Black church to have more participants enroll in the trial. One who answered the call to action was Stephanie Williams, an elementary school teacher in Montgomery County, Maryland. When she saw that her pastor was participating in the Novavax trial and when she considered the devastation she had seen from COVID-19, she was on board.
“We had about three sessions where he shared his experiences. He also shared some links to read about it more,” Ms. Williams said. “When I saw that he took it, that gave me a lot of confidence. Since I’m going be going into the classroom, I wanted to be sure that I was well protected.”
Transparency is key to gaining more participation, explained Dr. Maghoub. Webinar-based information sessions have proven particularly important in achieving this.
“We do a lot of explaining in very simple language to make sure everyone understands about the vaccine. The participants have time to ask questions during the webinar, and at any time [during the trial], if a participant feels that it is not right for them, they can stop. They have time to learn about the trial and give consent. People often think they are like guinea pigs in trials, but they are not. They must give consent.”
There are signs that the approach has been successful. Over a period of 4-5 weeks, the Howard site enrolled 150 participants, of whom 30% were Black and 20% were Latinx.
Novavax has been in business for more than 3 decades but hasn’t seen the booming success that their competitors have. The company has noted progress in developing vaccines against Middle East respiratory syndrome and severe acute respiratory syndrome. However, they missed the mark in clinical trials, failing twice in 3 years to develop a respiratory syncytial virus vaccine administered through maternal immunizations.
From being on the verge of closing, Novavax has since made a dramatic turnaround after former President Trump awarded the company $1.6 billion dollars in July 2020 as part of Operation Warp Speed. If trial results are promising, the Novavax vaccine could enter the market in a few months, representing not only a new therapeutic option but perhaps a new model for building inclusivity in clinical trials.
A version of this article first appeared on Medscape.com.
COVID-19 leaves thousands of U.S. children without a parent
Approximately 40,000 children in the United States have lost a parent to COVID-19, based on data from a combination of death counts and simulation models.
The scale of mortality from COVID-19 among adults in the United States merits efforts to monitor how many children have lost a parent as a result of the pandemic, wrote Rachel Kidman, PhD, of Stony Brook (N.Y.) University and colleagues.
In a study published in JAMA Pediatrics, the researchers used kinship networks of White and Black individuals in the United States to estimate parental bereavement. They combined deaths from COVID-19 as of February 2021 and combined them with excess deaths, and estimated future bereavement based on a herd immunity scenario.
Overall, the model suggested that each death from COVID-19 results in potential parental bereavement for 0.78 children aged 0-17 years, representing an increase of 17.5%-20.2% in parental bereavement. The model indicated that, as of February 2021, 37,337 children aged 0-17 years had lost a parent to COVID-19, including 11,366 children age 0-9 years and 31,661 children and teens aged 10-17 years. A total of 20,600 of these children were non-Hispanic White and 7,600 were Black. Black children accounted for 20% of the bereaved children, although they account for approximately 14% of children aged 0-17 years in the United States, the researchers noted.
Including the excess death estimate, which refers to the difference between observed and expected deaths for the remainder of the pandemic, raised the total bereaved children to 43,000. A future mortality scenario using a total of 1,500,000 deaths from COVID-19 based on a natural herd immunity strategy increased the total estimate of bereaved children to 116,922.
The study findings were limited by several factors including the lack of data on nonparental primary caregivers, and the use of demographic models rather than survey or administrative data, the researchers noted.
However, the huge number of children who have experienced the death of a parent because of COVID-19 emphasizes the need for reforms to address health, educational, and economic impacts of this mass bereavement on children and teens, they said.
“Parentally bereaved children will also need targeted support to help with grief, particularly during this period of heightened social isolation,” they emphasized.
Establishment of a national child bereavement cohort could identify children early in the bereavement process to help ensure that they are connected to local supportive care and monitored for health and behavior problems, the researchers said. In addition, such a cohort could be used as a basis for a longitudinal study of the impact of mass parental bereavement during a unique period of social isolation and economic uncertainty, they concluded.
Study spotlights gaps in mental health care
The study is an important reminder of how COVID-19 has disrupted children’s lives, said Herschel Lessin, MD, of Children’s Medical Group in Poughkeepsie, N.Y., in an interview. Losing a parent because of COVID-19 is one more tragedy on the list of social and emotional disasters the pandemic has wrought on children, he said.
“There has to be some sort of national response to help children through all of this, not just one item at a time,” Dr. Lessin said. However, the management of children’s mental health in the United States has been subpar for decades, he noted, with few clinicians trained to specialize in treating behavioral and mental health issues in children. Consequently, more general pediatricians will continue to be faced with the mental health issues of bereaved children who desperately need support, he said.
Money remains a key barrier, as it keeps qualified clinicians from entering the field of pediatric mental and behavioral health, and even where there are mental health providers, most do not take insurance and have long waiting lists, Dr. Lessin noted.
General pediatricians were seeing more patients with ADHD, anxiety, and depression before the advent of COVID-19, though most are not trained in managing these conditions, said Dr. Lessin. “Approximately 25%-30% of my visits now are mental health related, and the pandemic will make it geometrically worse,” he said.
The current study, with its dramatic estimates of the number of children who have lost a parent because of COVID-19, may bring attention to the fact that more training and money are needed to support mental health programs for children, he said.
Lead author Dr. Kidman had no financial conflicts to disclose. The study was supported by grants to corresponding author Ashton M. Verdery, PhD, from the National Institute on Aging and the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Dr. Lessin had no financial conflicts but serves on the Pediatric News editorial advisory board.
SOURCE: Kidman R et al. JAMA Pediatr. .
Approximately 40,000 children in the United States have lost a parent to COVID-19, based on data from a combination of death counts and simulation models.
The scale of mortality from COVID-19 among adults in the United States merits efforts to monitor how many children have lost a parent as a result of the pandemic, wrote Rachel Kidman, PhD, of Stony Brook (N.Y.) University and colleagues.
In a study published in JAMA Pediatrics, the researchers used kinship networks of White and Black individuals in the United States to estimate parental bereavement. They combined deaths from COVID-19 as of February 2021 and combined them with excess deaths, and estimated future bereavement based on a herd immunity scenario.
Overall, the model suggested that each death from COVID-19 results in potential parental bereavement for 0.78 children aged 0-17 years, representing an increase of 17.5%-20.2% in parental bereavement. The model indicated that, as of February 2021, 37,337 children aged 0-17 years had lost a parent to COVID-19, including 11,366 children age 0-9 years and 31,661 children and teens aged 10-17 years. A total of 20,600 of these children were non-Hispanic White and 7,600 were Black. Black children accounted for 20% of the bereaved children, although they account for approximately 14% of children aged 0-17 years in the United States, the researchers noted.
Including the excess death estimate, which refers to the difference between observed and expected deaths for the remainder of the pandemic, raised the total bereaved children to 43,000. A future mortality scenario using a total of 1,500,000 deaths from COVID-19 based on a natural herd immunity strategy increased the total estimate of bereaved children to 116,922.
The study findings were limited by several factors including the lack of data on nonparental primary caregivers, and the use of demographic models rather than survey or administrative data, the researchers noted.
However, the huge number of children who have experienced the death of a parent because of COVID-19 emphasizes the need for reforms to address health, educational, and economic impacts of this mass bereavement on children and teens, they said.
“Parentally bereaved children will also need targeted support to help with grief, particularly during this period of heightened social isolation,” they emphasized.
Establishment of a national child bereavement cohort could identify children early in the bereavement process to help ensure that they are connected to local supportive care and monitored for health and behavior problems, the researchers said. In addition, such a cohort could be used as a basis for a longitudinal study of the impact of mass parental bereavement during a unique period of social isolation and economic uncertainty, they concluded.
Study spotlights gaps in mental health care
The study is an important reminder of how COVID-19 has disrupted children’s lives, said Herschel Lessin, MD, of Children’s Medical Group in Poughkeepsie, N.Y., in an interview. Losing a parent because of COVID-19 is one more tragedy on the list of social and emotional disasters the pandemic has wrought on children, he said.
“There has to be some sort of national response to help children through all of this, not just one item at a time,” Dr. Lessin said. However, the management of children’s mental health in the United States has been subpar for decades, he noted, with few clinicians trained to specialize in treating behavioral and mental health issues in children. Consequently, more general pediatricians will continue to be faced with the mental health issues of bereaved children who desperately need support, he said.
Money remains a key barrier, as it keeps qualified clinicians from entering the field of pediatric mental and behavioral health, and even where there are mental health providers, most do not take insurance and have long waiting lists, Dr. Lessin noted.
General pediatricians were seeing more patients with ADHD, anxiety, and depression before the advent of COVID-19, though most are not trained in managing these conditions, said Dr. Lessin. “Approximately 25%-30% of my visits now are mental health related, and the pandemic will make it geometrically worse,” he said.
The current study, with its dramatic estimates of the number of children who have lost a parent because of COVID-19, may bring attention to the fact that more training and money are needed to support mental health programs for children, he said.
Lead author Dr. Kidman had no financial conflicts to disclose. The study was supported by grants to corresponding author Ashton M. Verdery, PhD, from the National Institute on Aging and the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Dr. Lessin had no financial conflicts but serves on the Pediatric News editorial advisory board.
SOURCE: Kidman R et al. JAMA Pediatr. .
Approximately 40,000 children in the United States have lost a parent to COVID-19, based on data from a combination of death counts and simulation models.
The scale of mortality from COVID-19 among adults in the United States merits efforts to monitor how many children have lost a parent as a result of the pandemic, wrote Rachel Kidman, PhD, of Stony Brook (N.Y.) University and colleagues.
In a study published in JAMA Pediatrics, the researchers used kinship networks of White and Black individuals in the United States to estimate parental bereavement. They combined deaths from COVID-19 as of February 2021 and combined them with excess deaths, and estimated future bereavement based on a herd immunity scenario.
Overall, the model suggested that each death from COVID-19 results in potential parental bereavement for 0.78 children aged 0-17 years, representing an increase of 17.5%-20.2% in parental bereavement. The model indicated that, as of February 2021, 37,337 children aged 0-17 years had lost a parent to COVID-19, including 11,366 children age 0-9 years and 31,661 children and teens aged 10-17 years. A total of 20,600 of these children were non-Hispanic White and 7,600 were Black. Black children accounted for 20% of the bereaved children, although they account for approximately 14% of children aged 0-17 years in the United States, the researchers noted.
Including the excess death estimate, which refers to the difference between observed and expected deaths for the remainder of the pandemic, raised the total bereaved children to 43,000. A future mortality scenario using a total of 1,500,000 deaths from COVID-19 based on a natural herd immunity strategy increased the total estimate of bereaved children to 116,922.
The study findings were limited by several factors including the lack of data on nonparental primary caregivers, and the use of demographic models rather than survey or administrative data, the researchers noted.
However, the huge number of children who have experienced the death of a parent because of COVID-19 emphasizes the need for reforms to address health, educational, and economic impacts of this mass bereavement on children and teens, they said.
“Parentally bereaved children will also need targeted support to help with grief, particularly during this period of heightened social isolation,” they emphasized.
Establishment of a national child bereavement cohort could identify children early in the bereavement process to help ensure that they are connected to local supportive care and monitored for health and behavior problems, the researchers said. In addition, such a cohort could be used as a basis for a longitudinal study of the impact of mass parental bereavement during a unique period of social isolation and economic uncertainty, they concluded.
Study spotlights gaps in mental health care
The study is an important reminder of how COVID-19 has disrupted children’s lives, said Herschel Lessin, MD, of Children’s Medical Group in Poughkeepsie, N.Y., in an interview. Losing a parent because of COVID-19 is one more tragedy on the list of social and emotional disasters the pandemic has wrought on children, he said.
“There has to be some sort of national response to help children through all of this, not just one item at a time,” Dr. Lessin said. However, the management of children’s mental health in the United States has been subpar for decades, he noted, with few clinicians trained to specialize in treating behavioral and mental health issues in children. Consequently, more general pediatricians will continue to be faced with the mental health issues of bereaved children who desperately need support, he said.
Money remains a key barrier, as it keeps qualified clinicians from entering the field of pediatric mental and behavioral health, and even where there are mental health providers, most do not take insurance and have long waiting lists, Dr. Lessin noted.
General pediatricians were seeing more patients with ADHD, anxiety, and depression before the advent of COVID-19, though most are not trained in managing these conditions, said Dr. Lessin. “Approximately 25%-30% of my visits now are mental health related, and the pandemic will make it geometrically worse,” he said.
The current study, with its dramatic estimates of the number of children who have lost a parent because of COVID-19, may bring attention to the fact that more training and money are needed to support mental health programs for children, he said.
Lead author Dr. Kidman had no financial conflicts to disclose. The study was supported by grants to corresponding author Ashton M. Verdery, PhD, from the National Institute on Aging and the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Dr. Lessin had no financial conflicts but serves on the Pediatric News editorial advisory board.
SOURCE: Kidman R et al. JAMA Pediatr. .
FROM JAMA PEDIATRICS
‘Beyond a reasonable doubt’: COVID-19 brain health fallout is real, severe
COVID-19 survivors face a sharply elevated risk of developing psychiatric or neurologic disorders in the 6 months after they contract the virus – a danger that mounts with symptom severity, new research shows.
In what is purported to be the largest study of its kind to date, results showed that among 236,379 COVID-19 patients, one-third were diagnosed with at least 1 of 14 psychiatric or neurologic disorders within a 6-month span.
The rate of illnesses, which ranged from depression to stroke, rose sharply among those with COVID-19 symptoms acute enough to require hospitalization.
“If we look at patients who were hospitalized, that rate increased to 39%, and then increased to about just under 1 in 2 patients who needed ICU admission at the time of the COVID-19 diagnosis,” Maxime Taquet, PhD, University of Oxford (England) department of psychiatry, said at a media briefing.
Incidence jumps to almost two-thirds in patients with encephalopathy at the time of COVID-19 diagnosis, he added.
The study, which examined the brain health of 236,379 survivors of COVID-19 via a U.S. database of 81 million electronic health records, was published online April 6 in The Lancet Psychiatry.
High rate of neurologic, psychiatric disorders
The research team looked at the first-time diagnosis or recurrence of 14 neurologic and psychiatric outcomes in patients with confirmed SARS-CoV-2 infections. They also compared the brain health of this cohort with a control group of those with influenza or with non–COVID-19 respiratory infections over the same period.
All study participants were older than 10 years, diagnosed with COVID-19 on or after Jan. 20, 2020, and still alive as of Dec. 13, 2020.
The psychiatric and neurologic conditions examined included intracranial hemorrhage; ischemic stroke; parkinsonism; Guillain-Barré syndrome; nerve, nerve root and plexus disorders; myoneural junction and muscle disease; encephalitis; dementia; psychotic, mood, and anxiety disorders; substance use disorder; and insomnia.
The investigators used hospitalization, intensive care admissions, and encephalopathy as an indication of the severity of COVID-19 symptoms.
The study benchmarked the primary cohort with four populations of patients diagnosed in the same period with nonrespiratory illnesses, including skin infection, urolithiasis, bone fractures, and pulmonary embolisms.
Results showed that substantially more COVID-19 patients were diagnosed with a neurologic or psychiatric disorder compared with those with other respiratory illnesses.
“On average, in terms of the relative numbers, there was a 44% increased risk of having a neurological or psychiatric diagnosis after COVID-19 than after the flu and a 16% increased risk compared to other respiratory tract infections,” Dr. Taquet told reporters.
Health services should be prepared for an increase in psychiatric and neurologic issues in the months to come, he said, adding that further investigations are needed into why, and how, the coronavirus affects brain health.
Largest study to date
Although previous research suggests a link between the two, this is the largest study of its kind, examines a wider range of neurologic outcomes, and spans the longest time frame to date, said study coinvestigator Paul Harrison, BM BCh, associate head of the University of Oxford department of psychiatry.
There was a lower incidence of mood and anxiety disorders vs. neurologic disorders in patients with severe COVID-19 symptoms, a finding that Dr. Harrison said may indicate pandemic-related psychological stress is driving these disorders vs. biological factors.
“This paper follows up on an earlier study we did where we found much the same association, and our view is that a lot of the mental health consequences of COVID are … to do with the stress of knowing that one has had COVID and all the implications that go with that, rather than its being a direct effect, for example, of the virus on the brain, or of the immune response to the virus on the brain,” he added.
In contrast, neurologic diagnoses were more likely to be “mediated by some direct consequence of the COVID infection,” he added.
Psychosis and dementia, for instance, were less frequent in the overall COVID-19 population but became much more frequent among those with severe symptoms. The research team said these findings, along with those related to the incidence of ischemic stroke, were “concerning.”
“We found that 1 in 50 patients with COVID-19 go on to have an ischemic stroke in the 6 months after the COVID-19 illness,” Dr. Taquet told reporters. “And that rate increased to 1 in 11 patients if we look at patients with encephalopathy at the time of the COVID-19 diagnosis.”
Rates of brain hemorrhages also rose sharply among those with acute symptoms. Just over 1 in 200 total COVID-19 patients were diagnosed with this neurological condition, but that jumped to 1 in 25 of those who experienced encephalopathy at the time of their COVID-19 diagnosis.
Need for replication
Study coauthor Masud Husain, PhD, of University of Oxford’s cognitive neurology department, told reporters that while there is evidence from other neurologic studies that the virus can access the brain, there has been little sign the neurons themselves are affected.
“There isn’t much evidence that the virus itself attacks neurons in the brain, but it can cause inflammation, and it can activate inflammatory cells in the brain,” he said.
“And those effects are probably very important in some of the biological effects on the brain. In addition, of course, we know that the virus can change clotting and the likelihood of thrombosis in the blood, and those effects can also impact upon the brain,” he added.
Dr. Harrison said it would be helpful to replicate the results garnered from the U.S. database in other populations.
“It goes without saying that replication of these results with other electronic health records and in other countries is a priority,” he said, adding that investigations are essential into how and why the virus affects brain health.
Dr. Harrison cited a U.K. Research and Innovation–funded study called COVID CNS that will follow patients with neurologic and/or psychiatric issues during acute COVID-19 in hopes of exploring possible causes.
Beyond a reasonable doubt
Commenting on the findings, Sir Simon Wessely, MD, Regius chair of psychiatry, King’s College London, said in a release: “This is a very important paper. It confirms beyond any reasonable doubt that COVID-19 affects both brain and mind in equal measure.”
Some of these effects, including stroke and anxiety disorders, were already known, but others such as dementia and psychosis were less well known, he added.
“What is very new is the comparisons with all respiratory viruses or influenza, which suggests that these increases are specifically related to COVID-19, and not a general impact of viral infection,” Dr. Wessely said. “In general, the worse the illness, the greater the neurological or psychiatric outcomes, which is perhaps not surprising.
“The worst outcomes were in those with encephalopathy – inflammation of the brain – again, not surprising. The association with dementia was, however, small and might reflect diagnostic issues, whilst so far there doesn’t seem early evidence of a link with parkinsonism, which was a major factor after the great Spanish Flu pandemic, although the authors caution that it is too early to rule this out.”
A version of this article first appeared on Medscape.com.
COVID-19 survivors face a sharply elevated risk of developing psychiatric or neurologic disorders in the 6 months after they contract the virus – a danger that mounts with symptom severity, new research shows.
In what is purported to be the largest study of its kind to date, results showed that among 236,379 COVID-19 patients, one-third were diagnosed with at least 1 of 14 psychiatric or neurologic disorders within a 6-month span.
The rate of illnesses, which ranged from depression to stroke, rose sharply among those with COVID-19 symptoms acute enough to require hospitalization.
“If we look at patients who were hospitalized, that rate increased to 39%, and then increased to about just under 1 in 2 patients who needed ICU admission at the time of the COVID-19 diagnosis,” Maxime Taquet, PhD, University of Oxford (England) department of psychiatry, said at a media briefing.
Incidence jumps to almost two-thirds in patients with encephalopathy at the time of COVID-19 diagnosis, he added.
The study, which examined the brain health of 236,379 survivors of COVID-19 via a U.S. database of 81 million electronic health records, was published online April 6 in The Lancet Psychiatry.
High rate of neurologic, psychiatric disorders
The research team looked at the first-time diagnosis or recurrence of 14 neurologic and psychiatric outcomes in patients with confirmed SARS-CoV-2 infections. They also compared the brain health of this cohort with a control group of those with influenza or with non–COVID-19 respiratory infections over the same period.
All study participants were older than 10 years, diagnosed with COVID-19 on or after Jan. 20, 2020, and still alive as of Dec. 13, 2020.
The psychiatric and neurologic conditions examined included intracranial hemorrhage; ischemic stroke; parkinsonism; Guillain-Barré syndrome; nerve, nerve root and plexus disorders; myoneural junction and muscle disease; encephalitis; dementia; psychotic, mood, and anxiety disorders; substance use disorder; and insomnia.
The investigators used hospitalization, intensive care admissions, and encephalopathy as an indication of the severity of COVID-19 symptoms.
The study benchmarked the primary cohort with four populations of patients diagnosed in the same period with nonrespiratory illnesses, including skin infection, urolithiasis, bone fractures, and pulmonary embolisms.
Results showed that substantially more COVID-19 patients were diagnosed with a neurologic or psychiatric disorder compared with those with other respiratory illnesses.
“On average, in terms of the relative numbers, there was a 44% increased risk of having a neurological or psychiatric diagnosis after COVID-19 than after the flu and a 16% increased risk compared to other respiratory tract infections,” Dr. Taquet told reporters.
Health services should be prepared for an increase in psychiatric and neurologic issues in the months to come, he said, adding that further investigations are needed into why, and how, the coronavirus affects brain health.
Largest study to date
Although previous research suggests a link between the two, this is the largest study of its kind, examines a wider range of neurologic outcomes, and spans the longest time frame to date, said study coinvestigator Paul Harrison, BM BCh, associate head of the University of Oxford department of psychiatry.
There was a lower incidence of mood and anxiety disorders vs. neurologic disorders in patients with severe COVID-19 symptoms, a finding that Dr. Harrison said may indicate pandemic-related psychological stress is driving these disorders vs. biological factors.
“This paper follows up on an earlier study we did where we found much the same association, and our view is that a lot of the mental health consequences of COVID are … to do with the stress of knowing that one has had COVID and all the implications that go with that, rather than its being a direct effect, for example, of the virus on the brain, or of the immune response to the virus on the brain,” he added.
In contrast, neurologic diagnoses were more likely to be “mediated by some direct consequence of the COVID infection,” he added.
Psychosis and dementia, for instance, were less frequent in the overall COVID-19 population but became much more frequent among those with severe symptoms. The research team said these findings, along with those related to the incidence of ischemic stroke, were “concerning.”
“We found that 1 in 50 patients with COVID-19 go on to have an ischemic stroke in the 6 months after the COVID-19 illness,” Dr. Taquet told reporters. “And that rate increased to 1 in 11 patients if we look at patients with encephalopathy at the time of the COVID-19 diagnosis.”
Rates of brain hemorrhages also rose sharply among those with acute symptoms. Just over 1 in 200 total COVID-19 patients were diagnosed with this neurological condition, but that jumped to 1 in 25 of those who experienced encephalopathy at the time of their COVID-19 diagnosis.
Need for replication
Study coauthor Masud Husain, PhD, of University of Oxford’s cognitive neurology department, told reporters that while there is evidence from other neurologic studies that the virus can access the brain, there has been little sign the neurons themselves are affected.
“There isn’t much evidence that the virus itself attacks neurons in the brain, but it can cause inflammation, and it can activate inflammatory cells in the brain,” he said.
“And those effects are probably very important in some of the biological effects on the brain. In addition, of course, we know that the virus can change clotting and the likelihood of thrombosis in the blood, and those effects can also impact upon the brain,” he added.
Dr. Harrison said it would be helpful to replicate the results garnered from the U.S. database in other populations.
“It goes without saying that replication of these results with other electronic health records and in other countries is a priority,” he said, adding that investigations are essential into how and why the virus affects brain health.
Dr. Harrison cited a U.K. Research and Innovation–funded study called COVID CNS that will follow patients with neurologic and/or psychiatric issues during acute COVID-19 in hopes of exploring possible causes.
Beyond a reasonable doubt
Commenting on the findings, Sir Simon Wessely, MD, Regius chair of psychiatry, King’s College London, said in a release: “This is a very important paper. It confirms beyond any reasonable doubt that COVID-19 affects both brain and mind in equal measure.”
Some of these effects, including stroke and anxiety disorders, were already known, but others such as dementia and psychosis were less well known, he added.
“What is very new is the comparisons with all respiratory viruses or influenza, which suggests that these increases are specifically related to COVID-19, and not a general impact of viral infection,” Dr. Wessely said. “In general, the worse the illness, the greater the neurological or psychiatric outcomes, which is perhaps not surprising.
“The worst outcomes were in those with encephalopathy – inflammation of the brain – again, not surprising. The association with dementia was, however, small and might reflect diagnostic issues, whilst so far there doesn’t seem early evidence of a link with parkinsonism, which was a major factor after the great Spanish Flu pandemic, although the authors caution that it is too early to rule this out.”
A version of this article first appeared on Medscape.com.
COVID-19 survivors face a sharply elevated risk of developing psychiatric or neurologic disorders in the 6 months after they contract the virus – a danger that mounts with symptom severity, new research shows.
In what is purported to be the largest study of its kind to date, results showed that among 236,379 COVID-19 patients, one-third were diagnosed with at least 1 of 14 psychiatric or neurologic disorders within a 6-month span.
The rate of illnesses, which ranged from depression to stroke, rose sharply among those with COVID-19 symptoms acute enough to require hospitalization.
“If we look at patients who were hospitalized, that rate increased to 39%, and then increased to about just under 1 in 2 patients who needed ICU admission at the time of the COVID-19 diagnosis,” Maxime Taquet, PhD, University of Oxford (England) department of psychiatry, said at a media briefing.
Incidence jumps to almost two-thirds in patients with encephalopathy at the time of COVID-19 diagnosis, he added.
The study, which examined the brain health of 236,379 survivors of COVID-19 via a U.S. database of 81 million electronic health records, was published online April 6 in The Lancet Psychiatry.
High rate of neurologic, psychiatric disorders
The research team looked at the first-time diagnosis or recurrence of 14 neurologic and psychiatric outcomes in patients with confirmed SARS-CoV-2 infections. They also compared the brain health of this cohort with a control group of those with influenza or with non–COVID-19 respiratory infections over the same period.
All study participants were older than 10 years, diagnosed with COVID-19 on or after Jan. 20, 2020, and still alive as of Dec. 13, 2020.
The psychiatric and neurologic conditions examined included intracranial hemorrhage; ischemic stroke; parkinsonism; Guillain-Barré syndrome; nerve, nerve root and plexus disorders; myoneural junction and muscle disease; encephalitis; dementia; psychotic, mood, and anxiety disorders; substance use disorder; and insomnia.
The investigators used hospitalization, intensive care admissions, and encephalopathy as an indication of the severity of COVID-19 symptoms.
The study benchmarked the primary cohort with four populations of patients diagnosed in the same period with nonrespiratory illnesses, including skin infection, urolithiasis, bone fractures, and pulmonary embolisms.
Results showed that substantially more COVID-19 patients were diagnosed with a neurologic or psychiatric disorder compared with those with other respiratory illnesses.
“On average, in terms of the relative numbers, there was a 44% increased risk of having a neurological or psychiatric diagnosis after COVID-19 than after the flu and a 16% increased risk compared to other respiratory tract infections,” Dr. Taquet told reporters.
Health services should be prepared for an increase in psychiatric and neurologic issues in the months to come, he said, adding that further investigations are needed into why, and how, the coronavirus affects brain health.
Largest study to date
Although previous research suggests a link between the two, this is the largest study of its kind, examines a wider range of neurologic outcomes, and spans the longest time frame to date, said study coinvestigator Paul Harrison, BM BCh, associate head of the University of Oxford department of psychiatry.
There was a lower incidence of mood and anxiety disorders vs. neurologic disorders in patients with severe COVID-19 symptoms, a finding that Dr. Harrison said may indicate pandemic-related psychological stress is driving these disorders vs. biological factors.
“This paper follows up on an earlier study we did where we found much the same association, and our view is that a lot of the mental health consequences of COVID are … to do with the stress of knowing that one has had COVID and all the implications that go with that, rather than its being a direct effect, for example, of the virus on the brain, or of the immune response to the virus on the brain,” he added.
In contrast, neurologic diagnoses were more likely to be “mediated by some direct consequence of the COVID infection,” he added.
Psychosis and dementia, for instance, were less frequent in the overall COVID-19 population but became much more frequent among those with severe symptoms. The research team said these findings, along with those related to the incidence of ischemic stroke, were “concerning.”
“We found that 1 in 50 patients with COVID-19 go on to have an ischemic stroke in the 6 months after the COVID-19 illness,” Dr. Taquet told reporters. “And that rate increased to 1 in 11 patients if we look at patients with encephalopathy at the time of the COVID-19 diagnosis.”
Rates of brain hemorrhages also rose sharply among those with acute symptoms. Just over 1 in 200 total COVID-19 patients were diagnosed with this neurological condition, but that jumped to 1 in 25 of those who experienced encephalopathy at the time of their COVID-19 diagnosis.
Need for replication
Study coauthor Masud Husain, PhD, of University of Oxford’s cognitive neurology department, told reporters that while there is evidence from other neurologic studies that the virus can access the brain, there has been little sign the neurons themselves are affected.
“There isn’t much evidence that the virus itself attacks neurons in the brain, but it can cause inflammation, and it can activate inflammatory cells in the brain,” he said.
“And those effects are probably very important in some of the biological effects on the brain. In addition, of course, we know that the virus can change clotting and the likelihood of thrombosis in the blood, and those effects can also impact upon the brain,” he added.
Dr. Harrison said it would be helpful to replicate the results garnered from the U.S. database in other populations.
“It goes without saying that replication of these results with other electronic health records and in other countries is a priority,” he said, adding that investigations are essential into how and why the virus affects brain health.
Dr. Harrison cited a U.K. Research and Innovation–funded study called COVID CNS that will follow patients with neurologic and/or psychiatric issues during acute COVID-19 in hopes of exploring possible causes.
Beyond a reasonable doubt
Commenting on the findings, Sir Simon Wessely, MD, Regius chair of psychiatry, King’s College London, said in a release: “This is a very important paper. It confirms beyond any reasonable doubt that COVID-19 affects both brain and mind in equal measure.”
Some of these effects, including stroke and anxiety disorders, were already known, but others such as dementia and psychosis were less well known, he added.
“What is very new is the comparisons with all respiratory viruses or influenza, which suggests that these increases are specifically related to COVID-19, and not a general impact of viral infection,” Dr. Wessely said. “In general, the worse the illness, the greater the neurological or psychiatric outcomes, which is perhaps not surprising.
“The worst outcomes were in those with encephalopathy – inflammation of the brain – again, not surprising. The association with dementia was, however, small and might reflect diagnostic issues, whilst so far there doesn’t seem early evidence of a link with parkinsonism, which was a major factor after the great Spanish Flu pandemic, although the authors caution that it is too early to rule this out.”
A version of this article first appeared on Medscape.com.
About one in five clinicians considers quitting because of pandemic
a new survey of more than 5,000 clinicians at an academic medical center illustrates.
About one in five people reported considering leaving the workforce because of the challenges of working during the COVID-19 pandemic. In addition, 30% reported they are considering cutting back work hours.
“There are a substantial number of employees and trainees who are experiencing major stress and work disruptions because of the pandemic,” lead author Rebecca K. Delaney, PhD, said in an interview. “It is particularly alarming that people who have spent 5 or more years in training for their specialty are struggling with their work, so much so that they have even considered leaving the workforce or reducing their hours.”
“Being a caregiver adds another layer of difficulty for faculty, staff, and trainees who are trying to manage work and child care,” added Dr. Delaney, a researcher in the department of population health sciences, University of Utah, Salt Lake City.
The study was published online April 2 in JAMA Network Open.
“This looks like an excellent survey,” Carol A Bernstein, MD, said in an interview when asked to comment. “I do not think it provides particularly new information as these challenges in the workplace, especially for women during COVID, have been well documented in the media and the medical literature to date.”
“That said, to the extent that data helps drive solutions, I would hope that information such as this would be considered as strong further evidence that health care systems must pay close attention to the wellbeing of the workforce,” added Dr. Bernstein, professor and vice chair of faculty development and well-being, departments of psychiatry and behavioral sciences and obstetrics and gynecology and women’s health, Montefiore Medical Center/Albert Einstein College of Medicine, New York.
When the pandemic hits home
A total of 42% of the American workforce rapidly transitioned to working from home at the onset of the COVID-19 pandemic. At the same time, many employees had to provide child care and assistance with schoolwork. This placed a burden on many individuals at academic medical centers, and women in particular.
“Women comprise 74.9% of hospital employees, many of whom are essential clinical workers,” the researchers noted. “The extent of the needs and difficulties for these workers during the pandemic remain largely unknown.”
To learn more, Dr. Delaney, senior author Angie Fagerlin, PhD, and their colleagues emailed a Qualtrics survey to 27,700 faculty, staff, and trainees at University of Utah Health. The survey was conducted Aug. 5-20, 2020 as part of a quality improvement initiative. All responses were anonymous.
Survey questions included if, because of the pandemic, people had considered leaving the workforce, considered reducing their hours, or experienced reduced productivity. The researchers also asked about career impacts and potential solutions in terms of “work culture adaptations.”
Respondents with children aged under 18 years also were asked about child care options. Dr. Delaney and colleagues also inquired about race and ethnicity because they hypothesized that employees from underrepresented groups would likely experience the pandemic differently.
The mean age of the 5,951 (21%) faculty, staff, and trainees who completed the survey was 40 years. A majority of respondents were women, reflecting the higher proportion of women within the health system.
A majority (86%) identified as White or European American. About two-thirds of respondents (66%) were staff, 16% were faculty, and 13% were trainees.
COVID-19 career concerns
Overall, 1,061 respondents (21%) “moderately or very seriously” considered leaving the workforce and 1,505 (30%) considered reducing hours. Respondents who were younger, married, a member of an underrepresented racial/ethnic group, and worked in a clinical setting were more likely to consider leaving the workforce.
The survey showed 27% felt their productivity increased whereas 39% believed their productivity decreased.
Of the 2,412 survey participants with children aged 18 years or younger, 66% reported that they did not have child care fully available.
“Failure to address and provide for child care has long been one of the many significant deficits in U.S. health care systems,” said Dr. Bernstein, lead author of a March 2021 report evaluating staff emotional support at Montefiore Medical Center during the pandemic in The Joint Commission Journal on Quality and Patient Safety.
Furthermore, 47% were “moderately or very seriously worried” about COVID-19 impacting their career development.
Women trainees were significantly more likely than male counterparts to consider leaving the workforce and reducing their work hours. Women in a faculty or trainee role were also more likely to worry about COVID-19’s impact on their career, compared with men, and compared with women in staff positions.
“It was disheartening to have our data support the gender and racial/ethnic disparity that has been highlighted in the media during the pandemic,” Dr. Delaney said. “Women and in some cases racial/ethnic groups that are underrepresented in medicine were most likely to consider leaving the workforce, reducing hours, and were worried about their career development.
“It is critical that we strategically address these important disparities,” she said.
Women also are disproportionately affected by burnout, particularly during the pandemic, according to an analysis of Medscape’s Physician Burnout and Suicide Report.
Furthermore, the COVID-19 pandemic has shifted the medical specialties now considered highest risk for burnout: critical care physicians ranked first in the report, followed by rheumatologists and infectious disease specialists.
Potential solutions
“Given the disproportionate impact COVID-19 has on employees of health systems, institutions must find ways to support their employees, both in terms of workplace cultural adaptations and assistance with familial responsibilities,” the researchers noted.
Telecommuting policies, scheduling flexibility, and expanding employee support programs are potential solutions. Institutional policies also could address the educational and direct care needs of employee children.
Limitations of the study include its generalizability beyond employees of University of Utah Health. Also, respondents included a lower proportion of racial and ethnic groups, compared with national figures, “although this is mostly accounted for by the overall low population of such groups in the state of Utah,” the researchers added.
“Our results suggest that respondents were struggling during the COVID-19 pandemic,” the researchers noted. “As a result, even after investing substantial amounts of time in years of training, many were considering leaving the workforce because of stress and caregiving responsibilities related to the pandemic.”
The Jon M. Huntsman Presidential Endowed Chair supported the work with a financial award to Dr. Fagerlin. Dr. Delaney and Dr. Bernstein disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
a new survey of more than 5,000 clinicians at an academic medical center illustrates.
About one in five people reported considering leaving the workforce because of the challenges of working during the COVID-19 pandemic. In addition, 30% reported they are considering cutting back work hours.
“There are a substantial number of employees and trainees who are experiencing major stress and work disruptions because of the pandemic,” lead author Rebecca K. Delaney, PhD, said in an interview. “It is particularly alarming that people who have spent 5 or more years in training for their specialty are struggling with their work, so much so that they have even considered leaving the workforce or reducing their hours.”
“Being a caregiver adds another layer of difficulty for faculty, staff, and trainees who are trying to manage work and child care,” added Dr. Delaney, a researcher in the department of population health sciences, University of Utah, Salt Lake City.
The study was published online April 2 in JAMA Network Open.
“This looks like an excellent survey,” Carol A Bernstein, MD, said in an interview when asked to comment. “I do not think it provides particularly new information as these challenges in the workplace, especially for women during COVID, have been well documented in the media and the medical literature to date.”
“That said, to the extent that data helps drive solutions, I would hope that information such as this would be considered as strong further evidence that health care systems must pay close attention to the wellbeing of the workforce,” added Dr. Bernstein, professor and vice chair of faculty development and well-being, departments of psychiatry and behavioral sciences and obstetrics and gynecology and women’s health, Montefiore Medical Center/Albert Einstein College of Medicine, New York.
When the pandemic hits home
A total of 42% of the American workforce rapidly transitioned to working from home at the onset of the COVID-19 pandemic. At the same time, many employees had to provide child care and assistance with schoolwork. This placed a burden on many individuals at academic medical centers, and women in particular.
“Women comprise 74.9% of hospital employees, many of whom are essential clinical workers,” the researchers noted. “The extent of the needs and difficulties for these workers during the pandemic remain largely unknown.”
To learn more, Dr. Delaney, senior author Angie Fagerlin, PhD, and their colleagues emailed a Qualtrics survey to 27,700 faculty, staff, and trainees at University of Utah Health. The survey was conducted Aug. 5-20, 2020 as part of a quality improvement initiative. All responses were anonymous.
Survey questions included if, because of the pandemic, people had considered leaving the workforce, considered reducing their hours, or experienced reduced productivity. The researchers also asked about career impacts and potential solutions in terms of “work culture adaptations.”
Respondents with children aged under 18 years also were asked about child care options. Dr. Delaney and colleagues also inquired about race and ethnicity because they hypothesized that employees from underrepresented groups would likely experience the pandemic differently.
The mean age of the 5,951 (21%) faculty, staff, and trainees who completed the survey was 40 years. A majority of respondents were women, reflecting the higher proportion of women within the health system.
A majority (86%) identified as White or European American. About two-thirds of respondents (66%) were staff, 16% were faculty, and 13% were trainees.
COVID-19 career concerns
Overall, 1,061 respondents (21%) “moderately or very seriously” considered leaving the workforce and 1,505 (30%) considered reducing hours. Respondents who were younger, married, a member of an underrepresented racial/ethnic group, and worked in a clinical setting were more likely to consider leaving the workforce.
The survey showed 27% felt their productivity increased whereas 39% believed their productivity decreased.
Of the 2,412 survey participants with children aged 18 years or younger, 66% reported that they did not have child care fully available.
“Failure to address and provide for child care has long been one of the many significant deficits in U.S. health care systems,” said Dr. Bernstein, lead author of a March 2021 report evaluating staff emotional support at Montefiore Medical Center during the pandemic in The Joint Commission Journal on Quality and Patient Safety.
Furthermore, 47% were “moderately or very seriously worried” about COVID-19 impacting their career development.
Women trainees were significantly more likely than male counterparts to consider leaving the workforce and reducing their work hours. Women in a faculty or trainee role were also more likely to worry about COVID-19’s impact on their career, compared with men, and compared with women in staff positions.
“It was disheartening to have our data support the gender and racial/ethnic disparity that has been highlighted in the media during the pandemic,” Dr. Delaney said. “Women and in some cases racial/ethnic groups that are underrepresented in medicine were most likely to consider leaving the workforce, reducing hours, and were worried about their career development.
“It is critical that we strategically address these important disparities,” she said.
Women also are disproportionately affected by burnout, particularly during the pandemic, according to an analysis of Medscape’s Physician Burnout and Suicide Report.
Furthermore, the COVID-19 pandemic has shifted the medical specialties now considered highest risk for burnout: critical care physicians ranked first in the report, followed by rheumatologists and infectious disease specialists.
Potential solutions
“Given the disproportionate impact COVID-19 has on employees of health systems, institutions must find ways to support their employees, both in terms of workplace cultural adaptations and assistance with familial responsibilities,” the researchers noted.
Telecommuting policies, scheduling flexibility, and expanding employee support programs are potential solutions. Institutional policies also could address the educational and direct care needs of employee children.
Limitations of the study include its generalizability beyond employees of University of Utah Health. Also, respondents included a lower proportion of racial and ethnic groups, compared with national figures, “although this is mostly accounted for by the overall low population of such groups in the state of Utah,” the researchers added.
“Our results suggest that respondents were struggling during the COVID-19 pandemic,” the researchers noted. “As a result, even after investing substantial amounts of time in years of training, many were considering leaving the workforce because of stress and caregiving responsibilities related to the pandemic.”
The Jon M. Huntsman Presidential Endowed Chair supported the work with a financial award to Dr. Fagerlin. Dr. Delaney and Dr. Bernstein disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
a new survey of more than 5,000 clinicians at an academic medical center illustrates.
About one in five people reported considering leaving the workforce because of the challenges of working during the COVID-19 pandemic. In addition, 30% reported they are considering cutting back work hours.
“There are a substantial number of employees and trainees who are experiencing major stress and work disruptions because of the pandemic,” lead author Rebecca K. Delaney, PhD, said in an interview. “It is particularly alarming that people who have spent 5 or more years in training for their specialty are struggling with their work, so much so that they have even considered leaving the workforce or reducing their hours.”
“Being a caregiver adds another layer of difficulty for faculty, staff, and trainees who are trying to manage work and child care,” added Dr. Delaney, a researcher in the department of population health sciences, University of Utah, Salt Lake City.
The study was published online April 2 in JAMA Network Open.
“This looks like an excellent survey,” Carol A Bernstein, MD, said in an interview when asked to comment. “I do not think it provides particularly new information as these challenges in the workplace, especially for women during COVID, have been well documented in the media and the medical literature to date.”
“That said, to the extent that data helps drive solutions, I would hope that information such as this would be considered as strong further evidence that health care systems must pay close attention to the wellbeing of the workforce,” added Dr. Bernstein, professor and vice chair of faculty development and well-being, departments of psychiatry and behavioral sciences and obstetrics and gynecology and women’s health, Montefiore Medical Center/Albert Einstein College of Medicine, New York.
When the pandemic hits home
A total of 42% of the American workforce rapidly transitioned to working from home at the onset of the COVID-19 pandemic. At the same time, many employees had to provide child care and assistance with schoolwork. This placed a burden on many individuals at academic medical centers, and women in particular.
“Women comprise 74.9% of hospital employees, many of whom are essential clinical workers,” the researchers noted. “The extent of the needs and difficulties for these workers during the pandemic remain largely unknown.”
To learn more, Dr. Delaney, senior author Angie Fagerlin, PhD, and their colleagues emailed a Qualtrics survey to 27,700 faculty, staff, and trainees at University of Utah Health. The survey was conducted Aug. 5-20, 2020 as part of a quality improvement initiative. All responses were anonymous.
Survey questions included if, because of the pandemic, people had considered leaving the workforce, considered reducing their hours, or experienced reduced productivity. The researchers also asked about career impacts and potential solutions in terms of “work culture adaptations.”
Respondents with children aged under 18 years also were asked about child care options. Dr. Delaney and colleagues also inquired about race and ethnicity because they hypothesized that employees from underrepresented groups would likely experience the pandemic differently.
The mean age of the 5,951 (21%) faculty, staff, and trainees who completed the survey was 40 years. A majority of respondents were women, reflecting the higher proportion of women within the health system.
A majority (86%) identified as White or European American. About two-thirds of respondents (66%) were staff, 16% were faculty, and 13% were trainees.
COVID-19 career concerns
Overall, 1,061 respondents (21%) “moderately or very seriously” considered leaving the workforce and 1,505 (30%) considered reducing hours. Respondents who were younger, married, a member of an underrepresented racial/ethnic group, and worked in a clinical setting were more likely to consider leaving the workforce.
The survey showed 27% felt their productivity increased whereas 39% believed their productivity decreased.
Of the 2,412 survey participants with children aged 18 years or younger, 66% reported that they did not have child care fully available.
“Failure to address and provide for child care has long been one of the many significant deficits in U.S. health care systems,” said Dr. Bernstein, lead author of a March 2021 report evaluating staff emotional support at Montefiore Medical Center during the pandemic in The Joint Commission Journal on Quality and Patient Safety.
Furthermore, 47% were “moderately or very seriously worried” about COVID-19 impacting their career development.
Women trainees were significantly more likely than male counterparts to consider leaving the workforce and reducing their work hours. Women in a faculty or trainee role were also more likely to worry about COVID-19’s impact on their career, compared with men, and compared with women in staff positions.
“It was disheartening to have our data support the gender and racial/ethnic disparity that has been highlighted in the media during the pandemic,” Dr. Delaney said. “Women and in some cases racial/ethnic groups that are underrepresented in medicine were most likely to consider leaving the workforce, reducing hours, and were worried about their career development.
“It is critical that we strategically address these important disparities,” she said.
Women also are disproportionately affected by burnout, particularly during the pandemic, according to an analysis of Medscape’s Physician Burnout and Suicide Report.
Furthermore, the COVID-19 pandemic has shifted the medical specialties now considered highest risk for burnout: critical care physicians ranked first in the report, followed by rheumatologists and infectious disease specialists.
Potential solutions
“Given the disproportionate impact COVID-19 has on employees of health systems, institutions must find ways to support their employees, both in terms of workplace cultural adaptations and assistance with familial responsibilities,” the researchers noted.
Telecommuting policies, scheduling flexibility, and expanding employee support programs are potential solutions. Institutional policies also could address the educational and direct care needs of employee children.
Limitations of the study include its generalizability beyond employees of University of Utah Health. Also, respondents included a lower proportion of racial and ethnic groups, compared with national figures, “although this is mostly accounted for by the overall low population of such groups in the state of Utah,” the researchers added.
“Our results suggest that respondents were struggling during the COVID-19 pandemic,” the researchers noted. “As a result, even after investing substantial amounts of time in years of training, many were considering leaving the workforce because of stress and caregiving responsibilities related to the pandemic.”
The Jon M. Huntsman Presidential Endowed Chair supported the work with a financial award to Dr. Fagerlin. Dr. Delaney and Dr. Bernstein disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Hypofractionated radiotherapy: New normal for lung cancer?
The U.K.-based study showed that patients with stage I-III lung cancer who were set to undergo radiotherapy with curative intent were more likely to receive fewer fractions at higher doses when treated between April and October 2020. During that period, 19% of patients had their radiotherapy dose or fractionation schedule changed to deviate from standard care.
In addition, 8% of patients who were set to undergo surgery ultimately received radiotherapy instead, presumably to ease pressures on already struggling intensive care services, said Kathryn Banfill, MBChB, of Christie NHS Foundation Trust in Manchester, England.
Dr. Banfill presented results from the COVID-RT Lung study at the European Lung Cancer Virtual Congress 2021 (Abstract 203MO).
New guidelines prompt study
When the COVID-19 pandemic began, European and joint European and North American guidelines were issued to try to ensure that lung cancer patients would continue to receive the best possible treatment under the circumstances. This included guidance on how and when to use treatments such as radiotherapy.
One U.K. guideline included recommendations on the use of hypofractionation in the COVID-19 era. The recommendations focused on altering the dosage or length of radiotherapy treatments to try to reduce the number of hospital visits, thereby reducing the risk of exposing patients to SARS-CoV-2.
“The aim of these guidelines is very much to reduce the risk to patients,” Dr. Banfill said. “These patients are often at higher risk of serious COVID-19, both as a result of their cancer and also as a result of many of the coexisting medical conditions that they have, such as COPD [chronic obstructive pulmonary disease],” she explained.
The COVID-RT Lung study was essentially born out of these guidelines. The goals of the study were to see what changes to radiotherapy practice occurred as a result of the guidelines and to assess how the changes have affected patient outcomes.
Changes to diagnosis and treatment
COVID-RT Lung is an ongoing, prospective study of patients with biopsy- or imaging-proven stage I–III lung cancer who were referred for, or treated with, radical radiotherapy at one of 26 oncology centers in the United Kingdom between April and October 2020.
Records on 1,117 patients were available for the initial analysis. The patients’ median age was 72 years (range, 38-93 years), and half were women.
The records showed changes to diagnostic investigations in 14% of patients (n = 160). Changes included not obtaining histology (4.6%, n = 51), not conducting nodal sampling (3.1%, n = 35), not performing pulmonary function tests (1.8%, n = 20), not conducting brain imaging (2.9%, n = 32), not performing PET/CT scans or having out-of-date scans (4.2%, n = 47), and delays in diagnosis (0.6%, n = 7).
Changes to treatment – deviations from standard care – occurred in 37% of patients (n = 415). This included 19% of patients (n = 210) having changes to radiotherapy dose or fractionation schedule, 8% (n = 86) undergoing radiotherapy instead of surgery, and 13% (n = 143) having their chemotherapy omitted or reduced.
The median number of radiotherapy fractions was 15 for patients who had their radiotherapy adjusted and 20 for those who had no treatment amendments.
“Those who had their treatment changed were more likely to have hypofractionated or ultra-hypofractionated radiotherapy,” Dr. Banfill said.
This was particularly true for patients with early-stage disease, she noted, where there was an increase in the percentage of patients getting more than 15 Gy per fraction. Even in stage III disease, there was an increased use of 3–5 Gy per fraction, although “virtually nobody” who had a change in treatment received less than 2 Gy per fraction, Dr. Banfill said.
“The changes are in line with what was reported in international recommendations,” observed Yolande Lievens, MD, PhD, of Ghent University Hospital in Belgium, who discussed the findings at the meeting.
Few patients had COVID-19
“It was striking to me to see that so few patients developed COVID-19 prior to radiotherapy or during radiotherapy,” Dr. Lievens noted. “This is actually something that we’ve also experienced in our setting.”
Indeed, just 15 patients (1%) were diagnosed with COVID-19, 10 of whom were diagnosed before receiving radiotherapy.
Dr. Banfill observed that the COVID-19 diagnosis had been “a reasonable time” before the patients started radiotherapy, and some had been diagnosed with lung cancer as a result of having a chest x-ray for suspected COVID-19.
Of the four patients who were diagnosed during treatment, two had their radiotherapy interrupted as a result.
The low COVID-19 rate is perhaps a result of the protective measures recommended in the United Kingdom, such as advising patients to shield from others, Dr. Banfill said.
Are changes to practice likely to hold?
“Part of the reason we actually stopped the data collection in October was that people were starting to go, ‘Well, is this actually a change?’ because they’d been doing it for 6 months,” Dr. Banfill observed during the discussion session.
“It was becoming almost normal for some of these hypofractionated changes. I think there is potential for these to become more embedded going forward,” she said. Data on how these changes might affect patients in the long term is going to be the focus of a future analysis.
“There is ongoing data collection on recurrence and survival and toxicity, which will hopefully provide more information on the outcomes of this patient group,” Dr. Banfill said.
The COVID-RT Lung project is supported by the NIHR Manchester Biomedical Research Centre. Dr. Banfill and Dr. Lievens reported no relevant conflicts of interest.
The U.K.-based study showed that patients with stage I-III lung cancer who were set to undergo radiotherapy with curative intent were more likely to receive fewer fractions at higher doses when treated between April and October 2020. During that period, 19% of patients had their radiotherapy dose or fractionation schedule changed to deviate from standard care.
In addition, 8% of patients who were set to undergo surgery ultimately received radiotherapy instead, presumably to ease pressures on already struggling intensive care services, said Kathryn Banfill, MBChB, of Christie NHS Foundation Trust in Manchester, England.
Dr. Banfill presented results from the COVID-RT Lung study at the European Lung Cancer Virtual Congress 2021 (Abstract 203MO).
New guidelines prompt study
When the COVID-19 pandemic began, European and joint European and North American guidelines were issued to try to ensure that lung cancer patients would continue to receive the best possible treatment under the circumstances. This included guidance on how and when to use treatments such as radiotherapy.
One U.K. guideline included recommendations on the use of hypofractionation in the COVID-19 era. The recommendations focused on altering the dosage or length of radiotherapy treatments to try to reduce the number of hospital visits, thereby reducing the risk of exposing patients to SARS-CoV-2.
“The aim of these guidelines is very much to reduce the risk to patients,” Dr. Banfill said. “These patients are often at higher risk of serious COVID-19, both as a result of their cancer and also as a result of many of the coexisting medical conditions that they have, such as COPD [chronic obstructive pulmonary disease],” she explained.
The COVID-RT Lung study was essentially born out of these guidelines. The goals of the study were to see what changes to radiotherapy practice occurred as a result of the guidelines and to assess how the changes have affected patient outcomes.
Changes to diagnosis and treatment
COVID-RT Lung is an ongoing, prospective study of patients with biopsy- or imaging-proven stage I–III lung cancer who were referred for, or treated with, radical radiotherapy at one of 26 oncology centers in the United Kingdom between April and October 2020.
Records on 1,117 patients were available for the initial analysis. The patients’ median age was 72 years (range, 38-93 years), and half were women.
The records showed changes to diagnostic investigations in 14% of patients (n = 160). Changes included not obtaining histology (4.6%, n = 51), not conducting nodal sampling (3.1%, n = 35), not performing pulmonary function tests (1.8%, n = 20), not conducting brain imaging (2.9%, n = 32), not performing PET/CT scans or having out-of-date scans (4.2%, n = 47), and delays in diagnosis (0.6%, n = 7).
Changes to treatment – deviations from standard care – occurred in 37% of patients (n = 415). This included 19% of patients (n = 210) having changes to radiotherapy dose or fractionation schedule, 8% (n = 86) undergoing radiotherapy instead of surgery, and 13% (n = 143) having their chemotherapy omitted or reduced.
The median number of radiotherapy fractions was 15 for patients who had their radiotherapy adjusted and 20 for those who had no treatment amendments.
“Those who had their treatment changed were more likely to have hypofractionated or ultra-hypofractionated radiotherapy,” Dr. Banfill said.
This was particularly true for patients with early-stage disease, she noted, where there was an increase in the percentage of patients getting more than 15 Gy per fraction. Even in stage III disease, there was an increased use of 3–5 Gy per fraction, although “virtually nobody” who had a change in treatment received less than 2 Gy per fraction, Dr. Banfill said.
“The changes are in line with what was reported in international recommendations,” observed Yolande Lievens, MD, PhD, of Ghent University Hospital in Belgium, who discussed the findings at the meeting.
Few patients had COVID-19
“It was striking to me to see that so few patients developed COVID-19 prior to radiotherapy or during radiotherapy,” Dr. Lievens noted. “This is actually something that we’ve also experienced in our setting.”
Indeed, just 15 patients (1%) were diagnosed with COVID-19, 10 of whom were diagnosed before receiving radiotherapy.
Dr. Banfill observed that the COVID-19 diagnosis had been “a reasonable time” before the patients started radiotherapy, and some had been diagnosed with lung cancer as a result of having a chest x-ray for suspected COVID-19.
Of the four patients who were diagnosed during treatment, two had their radiotherapy interrupted as a result.
The low COVID-19 rate is perhaps a result of the protective measures recommended in the United Kingdom, such as advising patients to shield from others, Dr. Banfill said.
Are changes to practice likely to hold?
“Part of the reason we actually stopped the data collection in October was that people were starting to go, ‘Well, is this actually a change?’ because they’d been doing it for 6 months,” Dr. Banfill observed during the discussion session.
“It was becoming almost normal for some of these hypofractionated changes. I think there is potential for these to become more embedded going forward,” she said. Data on how these changes might affect patients in the long term is going to be the focus of a future analysis.
“There is ongoing data collection on recurrence and survival and toxicity, which will hopefully provide more information on the outcomes of this patient group,” Dr. Banfill said.
The COVID-RT Lung project is supported by the NIHR Manchester Biomedical Research Centre. Dr. Banfill and Dr. Lievens reported no relevant conflicts of interest.
The U.K.-based study showed that patients with stage I-III lung cancer who were set to undergo radiotherapy with curative intent were more likely to receive fewer fractions at higher doses when treated between April and October 2020. During that period, 19% of patients had their radiotherapy dose or fractionation schedule changed to deviate from standard care.
In addition, 8% of patients who were set to undergo surgery ultimately received radiotherapy instead, presumably to ease pressures on already struggling intensive care services, said Kathryn Banfill, MBChB, of Christie NHS Foundation Trust in Manchester, England.
Dr. Banfill presented results from the COVID-RT Lung study at the European Lung Cancer Virtual Congress 2021 (Abstract 203MO).
New guidelines prompt study
When the COVID-19 pandemic began, European and joint European and North American guidelines were issued to try to ensure that lung cancer patients would continue to receive the best possible treatment under the circumstances. This included guidance on how and when to use treatments such as radiotherapy.
One U.K. guideline included recommendations on the use of hypofractionation in the COVID-19 era. The recommendations focused on altering the dosage or length of radiotherapy treatments to try to reduce the number of hospital visits, thereby reducing the risk of exposing patients to SARS-CoV-2.
“The aim of these guidelines is very much to reduce the risk to patients,” Dr. Banfill said. “These patients are often at higher risk of serious COVID-19, both as a result of their cancer and also as a result of many of the coexisting medical conditions that they have, such as COPD [chronic obstructive pulmonary disease],” she explained.
The COVID-RT Lung study was essentially born out of these guidelines. The goals of the study were to see what changes to radiotherapy practice occurred as a result of the guidelines and to assess how the changes have affected patient outcomes.
Changes to diagnosis and treatment
COVID-RT Lung is an ongoing, prospective study of patients with biopsy- or imaging-proven stage I–III lung cancer who were referred for, or treated with, radical radiotherapy at one of 26 oncology centers in the United Kingdom between April and October 2020.
Records on 1,117 patients were available for the initial analysis. The patients’ median age was 72 years (range, 38-93 years), and half were women.
The records showed changes to diagnostic investigations in 14% of patients (n = 160). Changes included not obtaining histology (4.6%, n = 51), not conducting nodal sampling (3.1%, n = 35), not performing pulmonary function tests (1.8%, n = 20), not conducting brain imaging (2.9%, n = 32), not performing PET/CT scans or having out-of-date scans (4.2%, n = 47), and delays in diagnosis (0.6%, n = 7).
Changes to treatment – deviations from standard care – occurred in 37% of patients (n = 415). This included 19% of patients (n = 210) having changes to radiotherapy dose or fractionation schedule, 8% (n = 86) undergoing radiotherapy instead of surgery, and 13% (n = 143) having their chemotherapy omitted or reduced.
The median number of radiotherapy fractions was 15 for patients who had their radiotherapy adjusted and 20 for those who had no treatment amendments.
“Those who had their treatment changed were more likely to have hypofractionated or ultra-hypofractionated radiotherapy,” Dr. Banfill said.
This was particularly true for patients with early-stage disease, she noted, where there was an increase in the percentage of patients getting more than 15 Gy per fraction. Even in stage III disease, there was an increased use of 3–5 Gy per fraction, although “virtually nobody” who had a change in treatment received less than 2 Gy per fraction, Dr. Banfill said.
“The changes are in line with what was reported in international recommendations,” observed Yolande Lievens, MD, PhD, of Ghent University Hospital in Belgium, who discussed the findings at the meeting.
Few patients had COVID-19
“It was striking to me to see that so few patients developed COVID-19 prior to radiotherapy or during radiotherapy,” Dr. Lievens noted. “This is actually something that we’ve also experienced in our setting.”
Indeed, just 15 patients (1%) were diagnosed with COVID-19, 10 of whom were diagnosed before receiving radiotherapy.
Dr. Banfill observed that the COVID-19 diagnosis had been “a reasonable time” before the patients started radiotherapy, and some had been diagnosed with lung cancer as a result of having a chest x-ray for suspected COVID-19.
Of the four patients who were diagnosed during treatment, two had their radiotherapy interrupted as a result.
The low COVID-19 rate is perhaps a result of the protective measures recommended in the United Kingdom, such as advising patients to shield from others, Dr. Banfill said.
Are changes to practice likely to hold?
“Part of the reason we actually stopped the data collection in October was that people were starting to go, ‘Well, is this actually a change?’ because they’d been doing it for 6 months,” Dr. Banfill observed during the discussion session.
“It was becoming almost normal for some of these hypofractionated changes. I think there is potential for these to become more embedded going forward,” she said. Data on how these changes might affect patients in the long term is going to be the focus of a future analysis.
“There is ongoing data collection on recurrence and survival and toxicity, which will hopefully provide more information on the outcomes of this patient group,” Dr. Banfill said.
The COVID-RT Lung project is supported by the NIHR Manchester Biomedical Research Centre. Dr. Banfill and Dr. Lievens reported no relevant conflicts of interest.
FROM ELCC 2021