User login
In Case You Missed It: COVID
President Biden signs 10 new orders to help fight COVID-19

“For the past year, we couldn’t rely on the federal government to act with the urgency and focus and coordination we needed, and we have seen the tragic cost of that failure,” Mr. Biden said in remarks from the White House, unveiling his 198-page National Strategy for the COVID-19 Response and Pandemic Preparedness.
He said as many as 500,000 Americans will have died by February. “It’s going to take months for us to turn things around,” he said.
“Our national strategy is comprehensive – it’s based on science, not politics; it’s based on truth, not denial,” Mr. Biden said. He also promised to restore public trust, in part by having scientists and public health experts speak to the public. “That’s why you’ll be hearing a lot more from Dr. Fauci again, not from the president,” he said, adding that the experts will be “free from political interference.”
While the president’s executive orders can help accomplish some of the plan’s proposals, the majority will require new funding from Congress and will be included in the $1.9 trillion American Rescue package that Mr. Biden hopes legislators will approve.
Ten new orders
The 10 new pandemic-related orders Biden signed on Jan. 21 follow two he signed on his first day in office.
One establishes a COVID-19 Response Office responsible for coordinating the pandemic response across all federal departments and agencies and also reestablishes the White House Directorate on Global Health Security and Biodefense, which was disabled by the Trump administration.
The other order requires masks and physical distancing in all federal buildings, on all federal lands, and by federal employees and contractors.
Among the new orders will be directives that:
- Require individuals to also wear masks in airports and planes, and when using other modes of public transportation including trains, boats, and intercity buses, and also require international travelers to produce proof of a recent negative COVID-19 test prior to entry and to quarantine after entry.
- Federal agencies use all powers, including the Defense Production Act, to accelerate manufacturing and delivery of supplies such as N95 masks, gowns, gloves, swabs, reagents, pipette tips, rapid test kits, and nitrocellulose material for rapid antigen tests, and all equipment and material needed to accelerate manufacture, delivery, and administration of COVID-19 vaccine.
- Create a Pandemic Testing Board to expand supply and access, to promote more surge capacity, and to ensure equitable access to tests.
- Facilitate discovery, development, and trials of potential COVID-19 treatments, as well as expand access to programs that can meet the long-term health needs of those recovering from the disease.
- Facilitate more and better data sharing that will allow businesses, schools, hospitals, and individuals to make real-time decisions based on spread in their community.
- Direct the Education and Health & Human Services departments to provide schools and child-care operations guidance on how to reopen and operate safely.
- Direct the Occupational Safety and Health Administration (OSHA) to immediately release clear guidance for employers to help keep workers safe and to enforce health and safety requirements.
The plan also sets goals for vaccination – including 100 million shots in the administration’s first 100 days. President Biden had already previewed his goals for vaccination, including setting up mass vaccination sites and mobile vaccination sites. During his remarks, Mr. Biden said that he had already directed the Federal Emergency Management Agency (FEMA) to begin setting up the vaccination centers.
The administration is also going to look into improving reimbursement for giving vaccines. As a start, the HHS will ask the Centers for Medicare & Medicaid Services to consider if a higher rate “may more accurately compensate providers,” according to the Biden plan.
“But the brutal truth is it will take months before we can get the majority of Americans vaccinated,” said Mr. Biden.
As part of the goal of ensuring an equitable pandemic response, the president will sign an order that establishes a COVID-19 Health Equity Task Force. The task force is charged with providing recommendations for allocating resources and funding in communities with inequities in COVID-19 outcomes by race, ethnicity, geography, disability, and other considerations.
Finally, the administration has committed to being more transparent and sharing more information. The national plan calls for the federal government to conduct regular, expert-led, science-based public briefings and to release regular reports on the pandemic. The administration said it will launch massive science-based public information campaigns – in multiple languages – to educate Americans on masks, testing, and vaccines, and also work to counter misinformation and disinformation.
The American Academy of Family Physicians (AAFP) applauded Mr. Biden’s initiative. “If enacted, this bold legislative agenda will provide much-needed support to American families struggling during the pandemic – especially communities of color and those hardest hit by the virus,” Ada D. Stewart, MD, AAFP president, said in a statement.
Dr. Stewart also noted that family physicians “are uniquely positioned in their communities to educate patients, prioritize access, and coordinate administration of the COVID-19 vaccines,” and urged the administration to ensure that family physicians and staff be vaccinated as soon as possible, to help them “more safely provide care to their communities.”
A version of this article first appeared on Medscape.com.

“For the past year, we couldn’t rely on the federal government to act with the urgency and focus and coordination we needed, and we have seen the tragic cost of that failure,” Mr. Biden said in remarks from the White House, unveiling his 198-page National Strategy for the COVID-19 Response and Pandemic Preparedness.
He said as many as 500,000 Americans will have died by February. “It’s going to take months for us to turn things around,” he said.
“Our national strategy is comprehensive – it’s based on science, not politics; it’s based on truth, not denial,” Mr. Biden said. He also promised to restore public trust, in part by having scientists and public health experts speak to the public. “That’s why you’ll be hearing a lot more from Dr. Fauci again, not from the president,” he said, adding that the experts will be “free from political interference.”
While the president’s executive orders can help accomplish some of the plan’s proposals, the majority will require new funding from Congress and will be included in the $1.9 trillion American Rescue package that Mr. Biden hopes legislators will approve.
Ten new orders
The 10 new pandemic-related orders Biden signed on Jan. 21 follow two he signed on his first day in office.
One establishes a COVID-19 Response Office responsible for coordinating the pandemic response across all federal departments and agencies and also reestablishes the White House Directorate on Global Health Security and Biodefense, which was disabled by the Trump administration.
The other order requires masks and physical distancing in all federal buildings, on all federal lands, and by federal employees and contractors.
Among the new orders will be directives that:
- Require individuals to also wear masks in airports and planes, and when using other modes of public transportation including trains, boats, and intercity buses, and also require international travelers to produce proof of a recent negative COVID-19 test prior to entry and to quarantine after entry.
- Federal agencies use all powers, including the Defense Production Act, to accelerate manufacturing and delivery of supplies such as N95 masks, gowns, gloves, swabs, reagents, pipette tips, rapid test kits, and nitrocellulose material for rapid antigen tests, and all equipment and material needed to accelerate manufacture, delivery, and administration of COVID-19 vaccine.
- Create a Pandemic Testing Board to expand supply and access, to promote more surge capacity, and to ensure equitable access to tests.
- Facilitate discovery, development, and trials of potential COVID-19 treatments, as well as expand access to programs that can meet the long-term health needs of those recovering from the disease.
- Facilitate more and better data sharing that will allow businesses, schools, hospitals, and individuals to make real-time decisions based on spread in their community.
- Direct the Education and Health & Human Services departments to provide schools and child-care operations guidance on how to reopen and operate safely.
- Direct the Occupational Safety and Health Administration (OSHA) to immediately release clear guidance for employers to help keep workers safe and to enforce health and safety requirements.
The plan also sets goals for vaccination – including 100 million shots in the administration’s first 100 days. President Biden had already previewed his goals for vaccination, including setting up mass vaccination sites and mobile vaccination sites. During his remarks, Mr. Biden said that he had already directed the Federal Emergency Management Agency (FEMA) to begin setting up the vaccination centers.
The administration is also going to look into improving reimbursement for giving vaccines. As a start, the HHS will ask the Centers for Medicare & Medicaid Services to consider if a higher rate “may more accurately compensate providers,” according to the Biden plan.
“But the brutal truth is it will take months before we can get the majority of Americans vaccinated,” said Mr. Biden.
As part of the goal of ensuring an equitable pandemic response, the president will sign an order that establishes a COVID-19 Health Equity Task Force. The task force is charged with providing recommendations for allocating resources and funding in communities with inequities in COVID-19 outcomes by race, ethnicity, geography, disability, and other considerations.
Finally, the administration has committed to being more transparent and sharing more information. The national plan calls for the federal government to conduct regular, expert-led, science-based public briefings and to release regular reports on the pandemic. The administration said it will launch massive science-based public information campaigns – in multiple languages – to educate Americans on masks, testing, and vaccines, and also work to counter misinformation and disinformation.
The American Academy of Family Physicians (AAFP) applauded Mr. Biden’s initiative. “If enacted, this bold legislative agenda will provide much-needed support to American families struggling during the pandemic – especially communities of color and those hardest hit by the virus,” Ada D. Stewart, MD, AAFP president, said in a statement.
Dr. Stewart also noted that family physicians “are uniquely positioned in their communities to educate patients, prioritize access, and coordinate administration of the COVID-19 vaccines,” and urged the administration to ensure that family physicians and staff be vaccinated as soon as possible, to help them “more safely provide care to their communities.”
A version of this article first appeared on Medscape.com.

“For the past year, we couldn’t rely on the federal government to act with the urgency and focus and coordination we needed, and we have seen the tragic cost of that failure,” Mr. Biden said in remarks from the White House, unveiling his 198-page National Strategy for the COVID-19 Response and Pandemic Preparedness.
He said as many as 500,000 Americans will have died by February. “It’s going to take months for us to turn things around,” he said.
“Our national strategy is comprehensive – it’s based on science, not politics; it’s based on truth, not denial,” Mr. Biden said. He also promised to restore public trust, in part by having scientists and public health experts speak to the public. “That’s why you’ll be hearing a lot more from Dr. Fauci again, not from the president,” he said, adding that the experts will be “free from political interference.”
While the president’s executive orders can help accomplish some of the plan’s proposals, the majority will require new funding from Congress and will be included in the $1.9 trillion American Rescue package that Mr. Biden hopes legislators will approve.
Ten new orders
The 10 new pandemic-related orders Biden signed on Jan. 21 follow two he signed on his first day in office.
One establishes a COVID-19 Response Office responsible for coordinating the pandemic response across all federal departments and agencies and also reestablishes the White House Directorate on Global Health Security and Biodefense, which was disabled by the Trump administration.
The other order requires masks and physical distancing in all federal buildings, on all federal lands, and by federal employees and contractors.
Among the new orders will be directives that:
- Require individuals to also wear masks in airports and planes, and when using other modes of public transportation including trains, boats, and intercity buses, and also require international travelers to produce proof of a recent negative COVID-19 test prior to entry and to quarantine after entry.
- Federal agencies use all powers, including the Defense Production Act, to accelerate manufacturing and delivery of supplies such as N95 masks, gowns, gloves, swabs, reagents, pipette tips, rapid test kits, and nitrocellulose material for rapid antigen tests, and all equipment and material needed to accelerate manufacture, delivery, and administration of COVID-19 vaccine.
- Create a Pandemic Testing Board to expand supply and access, to promote more surge capacity, and to ensure equitable access to tests.
- Facilitate discovery, development, and trials of potential COVID-19 treatments, as well as expand access to programs that can meet the long-term health needs of those recovering from the disease.
- Facilitate more and better data sharing that will allow businesses, schools, hospitals, and individuals to make real-time decisions based on spread in their community.
- Direct the Education and Health & Human Services departments to provide schools and child-care operations guidance on how to reopen and operate safely.
- Direct the Occupational Safety and Health Administration (OSHA) to immediately release clear guidance for employers to help keep workers safe and to enforce health and safety requirements.
The plan also sets goals for vaccination – including 100 million shots in the administration’s first 100 days. President Biden had already previewed his goals for vaccination, including setting up mass vaccination sites and mobile vaccination sites. During his remarks, Mr. Biden said that he had already directed the Federal Emergency Management Agency (FEMA) to begin setting up the vaccination centers.
The administration is also going to look into improving reimbursement for giving vaccines. As a start, the HHS will ask the Centers for Medicare & Medicaid Services to consider if a higher rate “may more accurately compensate providers,” according to the Biden plan.
“But the brutal truth is it will take months before we can get the majority of Americans vaccinated,” said Mr. Biden.
As part of the goal of ensuring an equitable pandemic response, the president will sign an order that establishes a COVID-19 Health Equity Task Force. The task force is charged with providing recommendations for allocating resources and funding in communities with inequities in COVID-19 outcomes by race, ethnicity, geography, disability, and other considerations.
Finally, the administration has committed to being more transparent and sharing more information. The national plan calls for the federal government to conduct regular, expert-led, science-based public briefings and to release regular reports on the pandemic. The administration said it will launch massive science-based public information campaigns – in multiple languages – to educate Americans on masks, testing, and vaccines, and also work to counter misinformation and disinformation.
The American Academy of Family Physicians (AAFP) applauded Mr. Biden’s initiative. “If enacted, this bold legislative agenda will provide much-needed support to American families struggling during the pandemic – especially communities of color and those hardest hit by the virus,” Ada D. Stewart, MD, AAFP president, said in a statement.
Dr. Stewart also noted that family physicians “are uniquely positioned in their communities to educate patients, prioritize access, and coordinate administration of the COVID-19 vaccines,” and urged the administration to ensure that family physicians and staff be vaccinated as soon as possible, to help them “more safely provide care to their communities.”
A version of this article first appeared on Medscape.com.
Metformin treatment again linked to fewer deaths from COVID-19
People with type 2 diabetes who develop COVID-19 show a substantially reduced risk of dying if they are taking metformin, shows a study that adds to prior research indicating the drug might somehow play a role in reducing the severity of infection.
“Unlike several previous analyses, this was a study in a racially diverse population with a high proportion of Blacks/African Americans and [it] revealed that metformin treatment of diabetes prior to diagnosis with COVID-19 was associated with a dramatic threefold reduced mortality in subjects with type 2 diabetes, even after correcting for multiple covariates,” first author Anath Shalev, MD, of the Comprehensive Diabetes Center at the University of Alabama at Birmingham, said in an interview.
But Anne Peters, MD, a professor of clinical medicine at the University of Southern California, Los Angeles, said caution is needed when interpreting these findings.
“It’s hard to tease out the true effects because, for instance, those treated with insulin may be a sicker subset of patients with diabetes than those on metformin, or those with comorbidities such as renal insufficiency may not be treated with metformin” she said in an interview.
“In general, though, treatment obviously matters and people who are better treated tend to do better, so while I think this study raises the question of what role metformin plays in the risk of mortality and COVID-19, I don’t think it necessarily proves the association,” Dr. Peters asserted.
Diverse population
The new study, published this month in Frontiers of Endocrinology, included 25,326 individuals who were tested for COVID-19 at the University of Alabama at Birmingham Hospital between February and June 2020.
Overall, 2.4% tested positive for COVID-19 (n = 604), which the authors note is likely a low figure because screening included asymptomatic hospital staff and patients having elective procedures.
Black/African American patients had a significantly higher risk of COVID-19 positivity, compared with White patients (odds ratio, 2.6; P < .0001). Rates were also higher among those with hypertension (OR, 2.46), diabetes (OR, 2.11), and obesity (OR, 1.93), compared with those without each condition (all P < .0001).
The overall mortality rate in COVID-19-positive patients was 11%. Diabetes was associated with a dramatically increased risk of death (OR, 3.62; P < .0001), and remained an independent risk factor even after adjusting for age, race, sex, obesity, and hypertension.
Notably, the reduction in mortality among those with diabetes taking metformin prior to COVID-19 diagnosis was significant: 11% of those patients died, compared with 23% of those with diabetes not taking metformin (OR, 0.33; P = .021).
Similar findings reported across varied populations
The study adds to mounting research suggesting metformin could have a protective effect on COVID-19 mortality, including an early report from Wuhan, China, findings from the French CORONADO study, and a U.S. study linking treatment with decreased mortality among women with COVID-19.
Of note, the effects of metformin on mortality in the current study were observed in men and women alike, as well as in high-risk subgroups including African Americans.
“The fact that such similar results were obtained in different populations from around the world suggests that the observed reduction in mortality risk, associated with metformin use in subjects with type 2 diabetes and COVID-19, might be generalizable,” the authors wrote.
“Furthermore, these findings underline the importance of following general diabetes treatment and prevention guidelines and not delaying or discontinuing any metformin treatment,” they add.
Speculation of mechanisms includes anti-inflammatory effects
While the mechanisms behind metformin’s potential role in reducing mortality risk in COVID-19 are unknown, the authors note that the most obvious assumption – that improved glycemic control may be a key factor – is disputed by the study’s finding that blood glucose levels and hemoglobin A1c were not significantly different among COVID-19 survivors taking versus not taking metformin.
They point instead to metformin’s known anti-inflammatory and antithrombotic properties.
“We therefore hypothesize that, by exerting some of these effects, metformin might improve outcomes and we are now in the process of investigating this possibility further,” Dr. Shalev said.
Dr. Peters noted that anti-inflammatory properties, themselves, are not necessarily unique to metformin in the treatment of diabetes.
“Many other agents, such as sodium-glucose cotransporter 2 (SGLT2) inhibitors can reduce inflammation, so I don’t know if that would explain it, but it certainly could help,” she said. “[Reducing inflammation] is a hypothesis you see commonly with diabetes drugs, but I think there are also a lot of metabolic benefits from metformin.”
“It was fascinating that they had the A1c data and that survival with metformin didn’t appear to be as related to A1c levels as one might think,” she added.
Notably, a key advantage, should the effects and mechanisms be validated, is metformin’s high accessibility, Dr. Peters added.
“This doesn’t necessarily tell us what we can do to reduce the health care disparities surrounding COVID-19, but the fact that metformin is low cost and easily available is very important, so maybe it will help as we try to grapple with other risk factors.”
The authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
People with type 2 diabetes who develop COVID-19 show a substantially reduced risk of dying if they are taking metformin, shows a study that adds to prior research indicating the drug might somehow play a role in reducing the severity of infection.
“Unlike several previous analyses, this was a study in a racially diverse population with a high proportion of Blacks/African Americans and [it] revealed that metformin treatment of diabetes prior to diagnosis with COVID-19 was associated with a dramatic threefold reduced mortality in subjects with type 2 diabetes, even after correcting for multiple covariates,” first author Anath Shalev, MD, of the Comprehensive Diabetes Center at the University of Alabama at Birmingham, said in an interview.
But Anne Peters, MD, a professor of clinical medicine at the University of Southern California, Los Angeles, said caution is needed when interpreting these findings.
“It’s hard to tease out the true effects because, for instance, those treated with insulin may be a sicker subset of patients with diabetes than those on metformin, or those with comorbidities such as renal insufficiency may not be treated with metformin” she said in an interview.
“In general, though, treatment obviously matters and people who are better treated tend to do better, so while I think this study raises the question of what role metformin plays in the risk of mortality and COVID-19, I don’t think it necessarily proves the association,” Dr. Peters asserted.
Diverse population
The new study, published this month in Frontiers of Endocrinology, included 25,326 individuals who were tested for COVID-19 at the University of Alabama at Birmingham Hospital between February and June 2020.
Overall, 2.4% tested positive for COVID-19 (n = 604), which the authors note is likely a low figure because screening included asymptomatic hospital staff and patients having elective procedures.
Black/African American patients had a significantly higher risk of COVID-19 positivity, compared with White patients (odds ratio, 2.6; P < .0001). Rates were also higher among those with hypertension (OR, 2.46), diabetes (OR, 2.11), and obesity (OR, 1.93), compared with those without each condition (all P < .0001).
The overall mortality rate in COVID-19-positive patients was 11%. Diabetes was associated with a dramatically increased risk of death (OR, 3.62; P < .0001), and remained an independent risk factor even after adjusting for age, race, sex, obesity, and hypertension.
Notably, the reduction in mortality among those with diabetes taking metformin prior to COVID-19 diagnosis was significant: 11% of those patients died, compared with 23% of those with diabetes not taking metformin (OR, 0.33; P = .021).
Similar findings reported across varied populations
The study adds to mounting research suggesting metformin could have a protective effect on COVID-19 mortality, including an early report from Wuhan, China, findings from the French CORONADO study, and a U.S. study linking treatment with decreased mortality among women with COVID-19.
Of note, the effects of metformin on mortality in the current study were observed in men and women alike, as well as in high-risk subgroups including African Americans.
“The fact that such similar results were obtained in different populations from around the world suggests that the observed reduction in mortality risk, associated with metformin use in subjects with type 2 diabetes and COVID-19, might be generalizable,” the authors wrote.
“Furthermore, these findings underline the importance of following general diabetes treatment and prevention guidelines and not delaying or discontinuing any metformin treatment,” they add.
Speculation of mechanisms includes anti-inflammatory effects
While the mechanisms behind metformin’s potential role in reducing mortality risk in COVID-19 are unknown, the authors note that the most obvious assumption – that improved glycemic control may be a key factor – is disputed by the study’s finding that blood glucose levels and hemoglobin A1c were not significantly different among COVID-19 survivors taking versus not taking metformin.
They point instead to metformin’s known anti-inflammatory and antithrombotic properties.
“We therefore hypothesize that, by exerting some of these effects, metformin might improve outcomes and we are now in the process of investigating this possibility further,” Dr. Shalev said.
Dr. Peters noted that anti-inflammatory properties, themselves, are not necessarily unique to metformin in the treatment of diabetes.
“Many other agents, such as sodium-glucose cotransporter 2 (SGLT2) inhibitors can reduce inflammation, so I don’t know if that would explain it, but it certainly could help,” she said. “[Reducing inflammation] is a hypothesis you see commonly with diabetes drugs, but I think there are also a lot of metabolic benefits from metformin.”
“It was fascinating that they had the A1c data and that survival with metformin didn’t appear to be as related to A1c levels as one might think,” she added.
Notably, a key advantage, should the effects and mechanisms be validated, is metformin’s high accessibility, Dr. Peters added.
“This doesn’t necessarily tell us what we can do to reduce the health care disparities surrounding COVID-19, but the fact that metformin is low cost and easily available is very important, so maybe it will help as we try to grapple with other risk factors.”
The authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
People with type 2 diabetes who develop COVID-19 show a substantially reduced risk of dying if they are taking metformin, shows a study that adds to prior research indicating the drug might somehow play a role in reducing the severity of infection.
“Unlike several previous analyses, this was a study in a racially diverse population with a high proportion of Blacks/African Americans and [it] revealed that metformin treatment of diabetes prior to diagnosis with COVID-19 was associated with a dramatic threefold reduced mortality in subjects with type 2 diabetes, even after correcting for multiple covariates,” first author Anath Shalev, MD, of the Comprehensive Diabetes Center at the University of Alabama at Birmingham, said in an interview.
But Anne Peters, MD, a professor of clinical medicine at the University of Southern California, Los Angeles, said caution is needed when interpreting these findings.
“It’s hard to tease out the true effects because, for instance, those treated with insulin may be a sicker subset of patients with diabetes than those on metformin, or those with comorbidities such as renal insufficiency may not be treated with metformin” she said in an interview.
“In general, though, treatment obviously matters and people who are better treated tend to do better, so while I think this study raises the question of what role metformin plays in the risk of mortality and COVID-19, I don’t think it necessarily proves the association,” Dr. Peters asserted.
Diverse population
The new study, published this month in Frontiers of Endocrinology, included 25,326 individuals who were tested for COVID-19 at the University of Alabama at Birmingham Hospital between February and June 2020.
Overall, 2.4% tested positive for COVID-19 (n = 604), which the authors note is likely a low figure because screening included asymptomatic hospital staff and patients having elective procedures.
Black/African American patients had a significantly higher risk of COVID-19 positivity, compared with White patients (odds ratio, 2.6; P < .0001). Rates were also higher among those with hypertension (OR, 2.46), diabetes (OR, 2.11), and obesity (OR, 1.93), compared with those without each condition (all P < .0001).
The overall mortality rate in COVID-19-positive patients was 11%. Diabetes was associated with a dramatically increased risk of death (OR, 3.62; P < .0001), and remained an independent risk factor even after adjusting for age, race, sex, obesity, and hypertension.
Notably, the reduction in mortality among those with diabetes taking metformin prior to COVID-19 diagnosis was significant: 11% of those patients died, compared with 23% of those with diabetes not taking metformin (OR, 0.33; P = .021).
Similar findings reported across varied populations
The study adds to mounting research suggesting metformin could have a protective effect on COVID-19 mortality, including an early report from Wuhan, China, findings from the French CORONADO study, and a U.S. study linking treatment with decreased mortality among women with COVID-19.
Of note, the effects of metformin on mortality in the current study were observed in men and women alike, as well as in high-risk subgroups including African Americans.
“The fact that such similar results were obtained in different populations from around the world suggests that the observed reduction in mortality risk, associated with metformin use in subjects with type 2 diabetes and COVID-19, might be generalizable,” the authors wrote.
“Furthermore, these findings underline the importance of following general diabetes treatment and prevention guidelines and not delaying or discontinuing any metformin treatment,” they add.
Speculation of mechanisms includes anti-inflammatory effects
While the mechanisms behind metformin’s potential role in reducing mortality risk in COVID-19 are unknown, the authors note that the most obvious assumption – that improved glycemic control may be a key factor – is disputed by the study’s finding that blood glucose levels and hemoglobin A1c were not significantly different among COVID-19 survivors taking versus not taking metformin.
They point instead to metformin’s known anti-inflammatory and antithrombotic properties.
“We therefore hypothesize that, by exerting some of these effects, metformin might improve outcomes and we are now in the process of investigating this possibility further,” Dr. Shalev said.
Dr. Peters noted that anti-inflammatory properties, themselves, are not necessarily unique to metformin in the treatment of diabetes.
“Many other agents, such as sodium-glucose cotransporter 2 (SGLT2) inhibitors can reduce inflammation, so I don’t know if that would explain it, but it certainly could help,” she said. “[Reducing inflammation] is a hypothesis you see commonly with diabetes drugs, but I think there are also a lot of metabolic benefits from metformin.”
“It was fascinating that they had the A1c data and that survival with metformin didn’t appear to be as related to A1c levels as one might think,” she added.
Notably, a key advantage, should the effects and mechanisms be validated, is metformin’s high accessibility, Dr. Peters added.
“This doesn’t necessarily tell us what we can do to reduce the health care disparities surrounding COVID-19, but the fact that metformin is low cost and easily available is very important, so maybe it will help as we try to grapple with other risk factors.”
The authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Seven ways President Biden could now change health care
President Joe Biden has come into office after an unexpected shift in Congress. On Jan. 5, Democrats scored an upset by winning two U.S. Senate seats in runoff elections in Georgia, giving them control of the Senate.
Now the Democrats have control of all three levers of power – the Senate, the House, and the presidency – for the first time since the early years of the Obama administration.
How will President Biden use this new concentration of power to shape health care policy?
Democrats’ small majorities in both houses of Congress suggest that moderation and bipartisanship will be necessary to get things done. Moreover, Mr. Biden himself is calling for bipartisanship. “On this January day,” he said in his inauguration speech, “my whole soul is in this: Bringing America together, uniting our people, uniting our nation.”
Key health care actions that Mr. Biden could pursue include the following.
1. Passing a new COVID-19 relief bill
Above all, Mr. Biden is focused on overcoming the COVID-19 pandemic, which has been registering record deaths recently, and getting newly released vaccines to Americans.
“Dealing with the coronavirus pandemic is one of the most important battles our administration will face, and I will be informed by science and by experts,” the president said.
“There is no question that the pandemic is the highest priority for the Biden administration,” said Larry Levitt, executive vice president for health policy at the Henry J. Kaiser Family Foundation. “COVID will dominate the early weeks and months of this administration. His success rests, in particular, on improving the rollout of vaccines.”
Five days before his inauguration, the president-elect unveiled the American Rescue Plan, a massive, $1.9 trillion legislative package intended to hasten rollout of COVID-19 vaccines, improve COVID-19 testing, and provide financial help to businesses and individuals, among many other things.
The bill would add $1,400 to the recently passed $600 government relief payments for each American, amounting to a $2,000 check. It would also enact many non-COVID-19 measures, such as a $15-an-hour minimum wage and measures to bolster the Affordable Care Act (ACA).
If Democrats cannot reach a deal with the Republicans, they might turn the proposal into a reconciliation bill, which could then be passed with a simple majority. However, drafting a reconciliation bill is a long, complicated process that would require removing provisions that don’t meet the requirements of reconciliation, said Hazen Marshall, a Washington lobbyist and former staffer for Sen. Mitch McConnell.
Most importantly, Mr. Marshall said, reconciliation bills bring out diehard partisanship. “They involve a sledgehammer mentality,” he says. “You’re telling the other side that their views aren’t going to matter.” The final version of the ACA, for example, was passed as a reconciliation bill, with not one Republican vote.
In the Trump years, “the last four reconciliation bills did not get any votes from the minority,” added Rodney Whitlock, PhD, a political consultant at McDermott+Consulting, who worked 21 years for Republicans in the House. “When the majority chooses to use reconciliation, it is an admission that it has no interest in working with the minority.”
Hammering out a compromise will be tough, but Robert Pearl MD, former CEO of the Permanente Medical Group and a professor at Stanford (Calif.) University, said that if anyone can do it, it would be President Biden. Having served in the Senate for 36 years, “Biden knows Congress better than any president since Lyndon Johnson,” he said. “He can reach across the aisle and get legislation passed as much as anyone could these days.”
2. Restoring Obamacare
Mr. Biden has vowed to undo a gradual dismantling of the ACA that went on during the Trump administration through executive orders, rule-making, and new laws. “Reinvigorating the ACA was a central part of Biden’s platform as a candidate,” Mr. Levitt said.
Each Trump action against the ACA must be undone in the same way. Presidential orders must be met with presidential orders, regulations with regulations, and legislation with legislation.
The ACA is also being challenged in the Supreme Court. Republicans under Trump passed a law that reduced the penalty for not buying health insurance under the ACA to zero. Then a group of 20 states, led by Texas, filed a lawsuit asserting that this change makes the ACA unconstitutional.
The lawsuit was heard by the Supreme Court in November. From remarks made by the justices then, it appears that the court might well uphold the law when a verdict comes down in June.
But just in case, Mr. Biden wants Congress to enact a small penalty for not buying health insurance, which would remove the basis of the lawsuit.
Mr. Biden’s choice for secretary of Health and Human Services shows his level of commitment to protecting the ACA. His HHS nominee is California Attorney General Xavier Becerra, who led a group of 17 states defending the ACA in the current lawsuit.
In addition to undoing Trump’s changes, Mr. Biden plans to expand the ACA beyond the original legislation. The new COVID-19 bill contains provisions that would expand subsidies to buy insurance on the exchanges and would lower the maximum percentage of income that anyone has to pay for health insurance to 8.5%.
Dealing with Medicaid is also related to the ACA. In 2012, the Supreme Court struck down a mandate that states expand their Medicaid programs, with substantial funding from the federal government.
To date, 12 states still do not participate in the Medicaid expansion. To lure them into the expansion, the Democrat-controlled House last session passed a bill that would offer to pay the entire bill for the first 3 years of Medicaid expansion if they chose to enact an expansion.
3. Undoing other Trump actions in health care
In addition to changes in the ACA, Trump also enacted a number of other changes in health care that President Biden could undo. For example, Mr. Biden says he will reenter the World Health Organization (WHO) so that the United States could better coordinate a COVID-19 response with other nations. Trump exited the WHO with the stroke of a pen, and Mr. Biden can do the same in reverse.
Under Trump, the Centers for Medicare & Medicaid Services used waivers to weaken the ACA and allow states to alter their Medicaid programs. One waiver allows Georgia to leave the ACA exchanges and put brokers in charge of buying coverage. Other waivers allow states to transform federal Medicaid payments into block grants, which several states are planning to do.
The Trump CMS has allowed several states to use Medicaid waivers to add work requirements for Medicaid recipients. The courts have blocked the work rules so far, and the Biden CMS may decide to reverse these waivers or modify them.
“Undoing waivers is normally a fairly simple thing,” Mr. Levitt said. In January, however, the Trump CMS asked some waiver states to sign new contracts in which the CMS pledges not to end a waiver without 9 months’ notice. It’s unclear how many states signed such contracts and what obligation the Biden CMS has to enforce them.
The Trump CMS also stopped reimbursing insurers for waiving deductibles and copayments for low-income customers, as directed by the ACA. Without federal reimbursement, some insurers raised premiums by as much as 20% to cover the costs. It is unclear how the Biden CMS would tackle this change.
4. Negotiating lower drug prices
Allowing Medicare to negotiate drug prices, a major plank in Mr. Biden’s campaign, would seem like a slam dunk for the Democrats. This approach is backed by 89% of Americans, including 84% of Republicans, according to a Kaiser Family Foundation survey in December.
“With that level of support, it’s hard to go wrong politically on this issue,” Mr. Levitt said.
Many Republicans, however, do not favor negotiating drug prices, and the two parties continue to be far apart on how to control drug prices. Trump signed an action that allows Americans to buy cheaper drugs abroad, an approach that Mr. Biden also supports, but it is now tied up in the courts.
“A drug pricing bill has always been difficult to pass,” Dr. Whitlock said. “The issue is popular with the public, but change does not come easily. The drug lobby is one the strongest in Washington, and now it may be even stronger, since it was the drug companies that gave us the COVID vaccines.”
Dr. Whitlock said Republicans will want Democrats to compromise on drug pricing, but he doubts they will do so. The House passed a bill to negotiate drug prices last year, which never was voted on in the Senate. “It is difficult to imagine that the Democrats will be able to move rightward from that House bill,” Dr. Whitlock said. “Democrats are likely to stand pat on drug pricing.”
5. Introducing a public option
President Biden’s campaign proposal for a public option – health insurance offered by the federal government – and to lower the age for Medicare eligibility from 65 years to 60 years, resulted from a compromise between two factions of the Democratic party on how to expand coverage.
Although Mr. Biden and other moderates wanted to focus on fixing the ACA, Democrats led by Sen. Bernie Sanders of Vermont called for a single-payer system, dubbed “Medicare for all.” A public option was seen as the middle ground between the two camps.
“A public option would be a very controversial,” Dr. Whitlock said. Critics say it would pay at Medicare rates, which would reduce doctors’ reimbursements, and save very little money compared with a single-payer system.
Dr. Pearl sees similar problems with lowering the Medicare age. “This would be an expensive change that the federal government could not afford, particularly with all the spending on the pandemic,” he said. “And it would be tough on doctors and hospitals, because Medicare pays less than the private insurance payment they are now getting.”
“The public option is likely to get serious discussion within the Democratic caucus and get onto the Senate floor,” Mr. Levitt said. “The party won’t ignore it.” He notes that in the new Senate, Sen. Sanders chairs the budget committee, and from that position he is likely to push for expanding access to care.
Mr. Levitt says the Biden CMS might allow states to experiment with a statewide public option or even a single-payer model, but he concedes that states, with their budgets ravaged by COVID-19, do not currently have the money to launch such programs.
6. Reviving the CMS
Under President Obama, the CMS was the engine that implemented the ACA and shepherded wider use of value-based reimbursements, which reward providers for quality and outcomes rather than volume.
Under the Trump administration, CMS leadership continued to uphold value-based reimbursement, Dr. Pearl observed. “CMS leadership championed value-based payments, but they encountered a lot of pushback from doctors and hospitals and had to scale back their goals,” he said.
On the other hand, the Trump CMS took a 180-degree turn on the ACA and worked to take it apart. This took a toll on staff morale, according to Donald M. Berwick, MD, who ran the CMS under President Obama. “Many people in CMS did not feel supported during the Trump administration, and some of them left,” Dr. Berwick said.
The CMS needs experienced staff on board to write comprehensible rules and regulations that can overcome court challenges.
Having a fully functioning CMS also requires consistent leadership, which was a problem for Obama. When Mr. Obama nominated Dr. Berwick, 60 Senate votes were needed to confirm him, and Republicans would not vote for him. Mr. Obama eventually brought Dr. Berwick in as a recess appointment, but it meant he could serve for only 17 months.
Since then, Senate confirmation rules have changed so that only a simple majority is needed to confirm appointments. This is important for Biden’s nominees, Dr. Berwick said. “For a president, having your team in place means you are able to execute the policies you want,” he said. “You need to have consistent leadership.”
7. Potentially changing health care without Congress
Even with their newly won control of the Senate, the Democrats’ thin majorities in both houses of Congress may not be enough to pass much legislation if Republicans are solidly opposed.
Democrats in the House also have a narrow path this session in which to pass legislation. The Democratic leadership has an 11-vote majority, but it must contend with 15 moderate representatives in purple districts (where Democrats and Republicans have about equal support).
A bigger problem looms before the Democrats. In 2022, the party may well lose its majorities in both houses. Mr. Whitlock notes that the party of an incoming president normally loses seats in the first midterm election. “The last incoming president to keep both houses of Congress in his first midterm was Jimmy Carter,” he said.
If this happens, President Biden would have to govern without the support of Congress, which is what Barack Obama had to do through most of his presidency. As Mr. Obama’s vice president, Mr. Biden is well aware how that goes. Governing without Congress means relying on presidential orders and decrees.
In health care, Mr. Biden has a powerful policy-making tool, the Center for Medicare & Medicaid Innovation (CMMI). The CMMI was empowered by the ACA to initiate pilot programs for new payment models.
So far, the CMMI’s work has been mainly limited to accountable care organizations, bundled payments, and patient-centered medical homes, but it could also be used to enact new federal policies that would normally require Congressional action, Mr. Levitt said.
Conclusion
Expectations have been very high for what President Joe Biden can do in health care. He needs to unite a very divided political system to defeat a deadly pandemic, restore Obamacare, and sign landmark legislation, such as a drug-pricing bill.
But shepherding bills through Congress will be a challenge. “You need to have accountability, unity, and civility, which is a Herculean task,” Mr. Whitlock said. “You have to keep policies off the table that could blow up the bipartisanship.”
A version of this article first appeared on Medscape.com.
President Joe Biden has come into office after an unexpected shift in Congress. On Jan. 5, Democrats scored an upset by winning two U.S. Senate seats in runoff elections in Georgia, giving them control of the Senate.
Now the Democrats have control of all three levers of power – the Senate, the House, and the presidency – for the first time since the early years of the Obama administration.
How will President Biden use this new concentration of power to shape health care policy?
Democrats’ small majorities in both houses of Congress suggest that moderation and bipartisanship will be necessary to get things done. Moreover, Mr. Biden himself is calling for bipartisanship. “On this January day,” he said in his inauguration speech, “my whole soul is in this: Bringing America together, uniting our people, uniting our nation.”
Key health care actions that Mr. Biden could pursue include the following.
1. Passing a new COVID-19 relief bill
Above all, Mr. Biden is focused on overcoming the COVID-19 pandemic, which has been registering record deaths recently, and getting newly released vaccines to Americans.
“Dealing with the coronavirus pandemic is one of the most important battles our administration will face, and I will be informed by science and by experts,” the president said.
“There is no question that the pandemic is the highest priority for the Biden administration,” said Larry Levitt, executive vice president for health policy at the Henry J. Kaiser Family Foundation. “COVID will dominate the early weeks and months of this administration. His success rests, in particular, on improving the rollout of vaccines.”
Five days before his inauguration, the president-elect unveiled the American Rescue Plan, a massive, $1.9 trillion legislative package intended to hasten rollout of COVID-19 vaccines, improve COVID-19 testing, and provide financial help to businesses and individuals, among many other things.
The bill would add $1,400 to the recently passed $600 government relief payments for each American, amounting to a $2,000 check. It would also enact many non-COVID-19 measures, such as a $15-an-hour minimum wage and measures to bolster the Affordable Care Act (ACA).
If Democrats cannot reach a deal with the Republicans, they might turn the proposal into a reconciliation bill, which could then be passed with a simple majority. However, drafting a reconciliation bill is a long, complicated process that would require removing provisions that don’t meet the requirements of reconciliation, said Hazen Marshall, a Washington lobbyist and former staffer for Sen. Mitch McConnell.
Most importantly, Mr. Marshall said, reconciliation bills bring out diehard partisanship. “They involve a sledgehammer mentality,” he says. “You’re telling the other side that their views aren’t going to matter.” The final version of the ACA, for example, was passed as a reconciliation bill, with not one Republican vote.
In the Trump years, “the last four reconciliation bills did not get any votes from the minority,” added Rodney Whitlock, PhD, a political consultant at McDermott+Consulting, who worked 21 years for Republicans in the House. “When the majority chooses to use reconciliation, it is an admission that it has no interest in working with the minority.”
Hammering out a compromise will be tough, but Robert Pearl MD, former CEO of the Permanente Medical Group and a professor at Stanford (Calif.) University, said that if anyone can do it, it would be President Biden. Having served in the Senate for 36 years, “Biden knows Congress better than any president since Lyndon Johnson,” he said. “He can reach across the aisle and get legislation passed as much as anyone could these days.”
2. Restoring Obamacare
Mr. Biden has vowed to undo a gradual dismantling of the ACA that went on during the Trump administration through executive orders, rule-making, and new laws. “Reinvigorating the ACA was a central part of Biden’s platform as a candidate,” Mr. Levitt said.
Each Trump action against the ACA must be undone in the same way. Presidential orders must be met with presidential orders, regulations with regulations, and legislation with legislation.
The ACA is also being challenged in the Supreme Court. Republicans under Trump passed a law that reduced the penalty for not buying health insurance under the ACA to zero. Then a group of 20 states, led by Texas, filed a lawsuit asserting that this change makes the ACA unconstitutional.
The lawsuit was heard by the Supreme Court in November. From remarks made by the justices then, it appears that the court might well uphold the law when a verdict comes down in June.
But just in case, Mr. Biden wants Congress to enact a small penalty for not buying health insurance, which would remove the basis of the lawsuit.
Mr. Biden’s choice for secretary of Health and Human Services shows his level of commitment to protecting the ACA. His HHS nominee is California Attorney General Xavier Becerra, who led a group of 17 states defending the ACA in the current lawsuit.
In addition to undoing Trump’s changes, Mr. Biden plans to expand the ACA beyond the original legislation. The new COVID-19 bill contains provisions that would expand subsidies to buy insurance on the exchanges and would lower the maximum percentage of income that anyone has to pay for health insurance to 8.5%.
Dealing with Medicaid is also related to the ACA. In 2012, the Supreme Court struck down a mandate that states expand their Medicaid programs, with substantial funding from the federal government.
To date, 12 states still do not participate in the Medicaid expansion. To lure them into the expansion, the Democrat-controlled House last session passed a bill that would offer to pay the entire bill for the first 3 years of Medicaid expansion if they chose to enact an expansion.
3. Undoing other Trump actions in health care
In addition to changes in the ACA, Trump also enacted a number of other changes in health care that President Biden could undo. For example, Mr. Biden says he will reenter the World Health Organization (WHO) so that the United States could better coordinate a COVID-19 response with other nations. Trump exited the WHO with the stroke of a pen, and Mr. Biden can do the same in reverse.
Under Trump, the Centers for Medicare & Medicaid Services used waivers to weaken the ACA and allow states to alter their Medicaid programs. One waiver allows Georgia to leave the ACA exchanges and put brokers in charge of buying coverage. Other waivers allow states to transform federal Medicaid payments into block grants, which several states are planning to do.
The Trump CMS has allowed several states to use Medicaid waivers to add work requirements for Medicaid recipients. The courts have blocked the work rules so far, and the Biden CMS may decide to reverse these waivers or modify them.
“Undoing waivers is normally a fairly simple thing,” Mr. Levitt said. In January, however, the Trump CMS asked some waiver states to sign new contracts in which the CMS pledges not to end a waiver without 9 months’ notice. It’s unclear how many states signed such contracts and what obligation the Biden CMS has to enforce them.
The Trump CMS also stopped reimbursing insurers for waiving deductibles and copayments for low-income customers, as directed by the ACA. Without federal reimbursement, some insurers raised premiums by as much as 20% to cover the costs. It is unclear how the Biden CMS would tackle this change.
4. Negotiating lower drug prices
Allowing Medicare to negotiate drug prices, a major plank in Mr. Biden’s campaign, would seem like a slam dunk for the Democrats. This approach is backed by 89% of Americans, including 84% of Republicans, according to a Kaiser Family Foundation survey in December.
“With that level of support, it’s hard to go wrong politically on this issue,” Mr. Levitt said.
Many Republicans, however, do not favor negotiating drug prices, and the two parties continue to be far apart on how to control drug prices. Trump signed an action that allows Americans to buy cheaper drugs abroad, an approach that Mr. Biden also supports, but it is now tied up in the courts.
“A drug pricing bill has always been difficult to pass,” Dr. Whitlock said. “The issue is popular with the public, but change does not come easily. The drug lobby is one the strongest in Washington, and now it may be even stronger, since it was the drug companies that gave us the COVID vaccines.”
Dr. Whitlock said Republicans will want Democrats to compromise on drug pricing, but he doubts they will do so. The House passed a bill to negotiate drug prices last year, which never was voted on in the Senate. “It is difficult to imagine that the Democrats will be able to move rightward from that House bill,” Dr. Whitlock said. “Democrats are likely to stand pat on drug pricing.”
5. Introducing a public option
President Biden’s campaign proposal for a public option – health insurance offered by the federal government – and to lower the age for Medicare eligibility from 65 years to 60 years, resulted from a compromise between two factions of the Democratic party on how to expand coverage.
Although Mr. Biden and other moderates wanted to focus on fixing the ACA, Democrats led by Sen. Bernie Sanders of Vermont called for a single-payer system, dubbed “Medicare for all.” A public option was seen as the middle ground between the two camps.
“A public option would be a very controversial,” Dr. Whitlock said. Critics say it would pay at Medicare rates, which would reduce doctors’ reimbursements, and save very little money compared with a single-payer system.
Dr. Pearl sees similar problems with lowering the Medicare age. “This would be an expensive change that the federal government could not afford, particularly with all the spending on the pandemic,” he said. “And it would be tough on doctors and hospitals, because Medicare pays less than the private insurance payment they are now getting.”
“The public option is likely to get serious discussion within the Democratic caucus and get onto the Senate floor,” Mr. Levitt said. “The party won’t ignore it.” He notes that in the new Senate, Sen. Sanders chairs the budget committee, and from that position he is likely to push for expanding access to care.
Mr. Levitt says the Biden CMS might allow states to experiment with a statewide public option or even a single-payer model, but he concedes that states, with their budgets ravaged by COVID-19, do not currently have the money to launch such programs.
6. Reviving the CMS
Under President Obama, the CMS was the engine that implemented the ACA and shepherded wider use of value-based reimbursements, which reward providers for quality and outcomes rather than volume.
Under the Trump administration, CMS leadership continued to uphold value-based reimbursement, Dr. Pearl observed. “CMS leadership championed value-based payments, but they encountered a lot of pushback from doctors and hospitals and had to scale back their goals,” he said.
On the other hand, the Trump CMS took a 180-degree turn on the ACA and worked to take it apart. This took a toll on staff morale, according to Donald M. Berwick, MD, who ran the CMS under President Obama. “Many people in CMS did not feel supported during the Trump administration, and some of them left,” Dr. Berwick said.
The CMS needs experienced staff on board to write comprehensible rules and regulations that can overcome court challenges.
Having a fully functioning CMS also requires consistent leadership, which was a problem for Obama. When Mr. Obama nominated Dr. Berwick, 60 Senate votes were needed to confirm him, and Republicans would not vote for him. Mr. Obama eventually brought Dr. Berwick in as a recess appointment, but it meant he could serve for only 17 months.
Since then, Senate confirmation rules have changed so that only a simple majority is needed to confirm appointments. This is important for Biden’s nominees, Dr. Berwick said. “For a president, having your team in place means you are able to execute the policies you want,” he said. “You need to have consistent leadership.”
7. Potentially changing health care without Congress
Even with their newly won control of the Senate, the Democrats’ thin majorities in both houses of Congress may not be enough to pass much legislation if Republicans are solidly opposed.
Democrats in the House also have a narrow path this session in which to pass legislation. The Democratic leadership has an 11-vote majority, but it must contend with 15 moderate representatives in purple districts (where Democrats and Republicans have about equal support).
A bigger problem looms before the Democrats. In 2022, the party may well lose its majorities in both houses. Mr. Whitlock notes that the party of an incoming president normally loses seats in the first midterm election. “The last incoming president to keep both houses of Congress in his first midterm was Jimmy Carter,” he said.
If this happens, President Biden would have to govern without the support of Congress, which is what Barack Obama had to do through most of his presidency. As Mr. Obama’s vice president, Mr. Biden is well aware how that goes. Governing without Congress means relying on presidential orders and decrees.
In health care, Mr. Biden has a powerful policy-making tool, the Center for Medicare & Medicaid Innovation (CMMI). The CMMI was empowered by the ACA to initiate pilot programs for new payment models.
So far, the CMMI’s work has been mainly limited to accountable care organizations, bundled payments, and patient-centered medical homes, but it could also be used to enact new federal policies that would normally require Congressional action, Mr. Levitt said.
Conclusion
Expectations have been very high for what President Joe Biden can do in health care. He needs to unite a very divided political system to defeat a deadly pandemic, restore Obamacare, and sign landmark legislation, such as a drug-pricing bill.
But shepherding bills through Congress will be a challenge. “You need to have accountability, unity, and civility, which is a Herculean task,” Mr. Whitlock said. “You have to keep policies off the table that could blow up the bipartisanship.”
A version of this article first appeared on Medscape.com.
President Joe Biden has come into office after an unexpected shift in Congress. On Jan. 5, Democrats scored an upset by winning two U.S. Senate seats in runoff elections in Georgia, giving them control of the Senate.
Now the Democrats have control of all three levers of power – the Senate, the House, and the presidency – for the first time since the early years of the Obama administration.
How will President Biden use this new concentration of power to shape health care policy?
Democrats’ small majorities in both houses of Congress suggest that moderation and bipartisanship will be necessary to get things done. Moreover, Mr. Biden himself is calling for bipartisanship. “On this January day,” he said in his inauguration speech, “my whole soul is in this: Bringing America together, uniting our people, uniting our nation.”
Key health care actions that Mr. Biden could pursue include the following.
1. Passing a new COVID-19 relief bill
Above all, Mr. Biden is focused on overcoming the COVID-19 pandemic, which has been registering record deaths recently, and getting newly released vaccines to Americans.
“Dealing with the coronavirus pandemic is one of the most important battles our administration will face, and I will be informed by science and by experts,” the president said.
“There is no question that the pandemic is the highest priority for the Biden administration,” said Larry Levitt, executive vice president for health policy at the Henry J. Kaiser Family Foundation. “COVID will dominate the early weeks and months of this administration. His success rests, in particular, on improving the rollout of vaccines.”
Five days before his inauguration, the president-elect unveiled the American Rescue Plan, a massive, $1.9 trillion legislative package intended to hasten rollout of COVID-19 vaccines, improve COVID-19 testing, and provide financial help to businesses and individuals, among many other things.
The bill would add $1,400 to the recently passed $600 government relief payments for each American, amounting to a $2,000 check. It would also enact many non-COVID-19 measures, such as a $15-an-hour minimum wage and measures to bolster the Affordable Care Act (ACA).
If Democrats cannot reach a deal with the Republicans, they might turn the proposal into a reconciliation bill, which could then be passed with a simple majority. However, drafting a reconciliation bill is a long, complicated process that would require removing provisions that don’t meet the requirements of reconciliation, said Hazen Marshall, a Washington lobbyist and former staffer for Sen. Mitch McConnell.
Most importantly, Mr. Marshall said, reconciliation bills bring out diehard partisanship. “They involve a sledgehammer mentality,” he says. “You’re telling the other side that their views aren’t going to matter.” The final version of the ACA, for example, was passed as a reconciliation bill, with not one Republican vote.
In the Trump years, “the last four reconciliation bills did not get any votes from the minority,” added Rodney Whitlock, PhD, a political consultant at McDermott+Consulting, who worked 21 years for Republicans in the House. “When the majority chooses to use reconciliation, it is an admission that it has no interest in working with the minority.”
Hammering out a compromise will be tough, but Robert Pearl MD, former CEO of the Permanente Medical Group and a professor at Stanford (Calif.) University, said that if anyone can do it, it would be President Biden. Having served in the Senate for 36 years, “Biden knows Congress better than any president since Lyndon Johnson,” he said. “He can reach across the aisle and get legislation passed as much as anyone could these days.”
2. Restoring Obamacare
Mr. Biden has vowed to undo a gradual dismantling of the ACA that went on during the Trump administration through executive orders, rule-making, and new laws. “Reinvigorating the ACA was a central part of Biden’s platform as a candidate,” Mr. Levitt said.
Each Trump action against the ACA must be undone in the same way. Presidential orders must be met with presidential orders, regulations with regulations, and legislation with legislation.
The ACA is also being challenged in the Supreme Court. Republicans under Trump passed a law that reduced the penalty for not buying health insurance under the ACA to zero. Then a group of 20 states, led by Texas, filed a lawsuit asserting that this change makes the ACA unconstitutional.
The lawsuit was heard by the Supreme Court in November. From remarks made by the justices then, it appears that the court might well uphold the law when a verdict comes down in June.
But just in case, Mr. Biden wants Congress to enact a small penalty for not buying health insurance, which would remove the basis of the lawsuit.
Mr. Biden’s choice for secretary of Health and Human Services shows his level of commitment to protecting the ACA. His HHS nominee is California Attorney General Xavier Becerra, who led a group of 17 states defending the ACA in the current lawsuit.
In addition to undoing Trump’s changes, Mr. Biden plans to expand the ACA beyond the original legislation. The new COVID-19 bill contains provisions that would expand subsidies to buy insurance on the exchanges and would lower the maximum percentage of income that anyone has to pay for health insurance to 8.5%.
Dealing with Medicaid is also related to the ACA. In 2012, the Supreme Court struck down a mandate that states expand their Medicaid programs, with substantial funding from the federal government.
To date, 12 states still do not participate in the Medicaid expansion. To lure them into the expansion, the Democrat-controlled House last session passed a bill that would offer to pay the entire bill for the first 3 years of Medicaid expansion if they chose to enact an expansion.
3. Undoing other Trump actions in health care
In addition to changes in the ACA, Trump also enacted a number of other changes in health care that President Biden could undo. For example, Mr. Biden says he will reenter the World Health Organization (WHO) so that the United States could better coordinate a COVID-19 response with other nations. Trump exited the WHO with the stroke of a pen, and Mr. Biden can do the same in reverse.
Under Trump, the Centers for Medicare & Medicaid Services used waivers to weaken the ACA and allow states to alter their Medicaid programs. One waiver allows Georgia to leave the ACA exchanges and put brokers in charge of buying coverage. Other waivers allow states to transform federal Medicaid payments into block grants, which several states are planning to do.
The Trump CMS has allowed several states to use Medicaid waivers to add work requirements for Medicaid recipients. The courts have blocked the work rules so far, and the Biden CMS may decide to reverse these waivers or modify them.
“Undoing waivers is normally a fairly simple thing,” Mr. Levitt said. In January, however, the Trump CMS asked some waiver states to sign new contracts in which the CMS pledges not to end a waiver without 9 months’ notice. It’s unclear how many states signed such contracts and what obligation the Biden CMS has to enforce them.
The Trump CMS also stopped reimbursing insurers for waiving deductibles and copayments for low-income customers, as directed by the ACA. Without federal reimbursement, some insurers raised premiums by as much as 20% to cover the costs. It is unclear how the Biden CMS would tackle this change.
4. Negotiating lower drug prices
Allowing Medicare to negotiate drug prices, a major plank in Mr. Biden’s campaign, would seem like a slam dunk for the Democrats. This approach is backed by 89% of Americans, including 84% of Republicans, according to a Kaiser Family Foundation survey in December.
“With that level of support, it’s hard to go wrong politically on this issue,” Mr. Levitt said.
Many Republicans, however, do not favor negotiating drug prices, and the two parties continue to be far apart on how to control drug prices. Trump signed an action that allows Americans to buy cheaper drugs abroad, an approach that Mr. Biden also supports, but it is now tied up in the courts.
“A drug pricing bill has always been difficult to pass,” Dr. Whitlock said. “The issue is popular with the public, but change does not come easily. The drug lobby is one the strongest in Washington, and now it may be even stronger, since it was the drug companies that gave us the COVID vaccines.”
Dr. Whitlock said Republicans will want Democrats to compromise on drug pricing, but he doubts they will do so. The House passed a bill to negotiate drug prices last year, which never was voted on in the Senate. “It is difficult to imagine that the Democrats will be able to move rightward from that House bill,” Dr. Whitlock said. “Democrats are likely to stand pat on drug pricing.”
5. Introducing a public option
President Biden’s campaign proposal for a public option – health insurance offered by the federal government – and to lower the age for Medicare eligibility from 65 years to 60 years, resulted from a compromise between two factions of the Democratic party on how to expand coverage.
Although Mr. Biden and other moderates wanted to focus on fixing the ACA, Democrats led by Sen. Bernie Sanders of Vermont called for a single-payer system, dubbed “Medicare for all.” A public option was seen as the middle ground between the two camps.
“A public option would be a very controversial,” Dr. Whitlock said. Critics say it would pay at Medicare rates, which would reduce doctors’ reimbursements, and save very little money compared with a single-payer system.
Dr. Pearl sees similar problems with lowering the Medicare age. “This would be an expensive change that the federal government could not afford, particularly with all the spending on the pandemic,” he said. “And it would be tough on doctors and hospitals, because Medicare pays less than the private insurance payment they are now getting.”
“The public option is likely to get serious discussion within the Democratic caucus and get onto the Senate floor,” Mr. Levitt said. “The party won’t ignore it.” He notes that in the new Senate, Sen. Sanders chairs the budget committee, and from that position he is likely to push for expanding access to care.
Mr. Levitt says the Biden CMS might allow states to experiment with a statewide public option or even a single-payer model, but he concedes that states, with their budgets ravaged by COVID-19, do not currently have the money to launch such programs.
6. Reviving the CMS
Under President Obama, the CMS was the engine that implemented the ACA and shepherded wider use of value-based reimbursements, which reward providers for quality and outcomes rather than volume.
Under the Trump administration, CMS leadership continued to uphold value-based reimbursement, Dr. Pearl observed. “CMS leadership championed value-based payments, but they encountered a lot of pushback from doctors and hospitals and had to scale back their goals,” he said.
On the other hand, the Trump CMS took a 180-degree turn on the ACA and worked to take it apart. This took a toll on staff morale, according to Donald M. Berwick, MD, who ran the CMS under President Obama. “Many people in CMS did not feel supported during the Trump administration, and some of them left,” Dr. Berwick said.
The CMS needs experienced staff on board to write comprehensible rules and regulations that can overcome court challenges.
Having a fully functioning CMS also requires consistent leadership, which was a problem for Obama. When Mr. Obama nominated Dr. Berwick, 60 Senate votes were needed to confirm him, and Republicans would not vote for him. Mr. Obama eventually brought Dr. Berwick in as a recess appointment, but it meant he could serve for only 17 months.
Since then, Senate confirmation rules have changed so that only a simple majority is needed to confirm appointments. This is important for Biden’s nominees, Dr. Berwick said. “For a president, having your team in place means you are able to execute the policies you want,” he said. “You need to have consistent leadership.”
7. Potentially changing health care without Congress
Even with their newly won control of the Senate, the Democrats’ thin majorities in both houses of Congress may not be enough to pass much legislation if Republicans are solidly opposed.
Democrats in the House also have a narrow path this session in which to pass legislation. The Democratic leadership has an 11-vote majority, but it must contend with 15 moderate representatives in purple districts (where Democrats and Republicans have about equal support).
A bigger problem looms before the Democrats. In 2022, the party may well lose its majorities in both houses. Mr. Whitlock notes that the party of an incoming president normally loses seats in the first midterm election. “The last incoming president to keep both houses of Congress in his first midterm was Jimmy Carter,” he said.
If this happens, President Biden would have to govern without the support of Congress, which is what Barack Obama had to do through most of his presidency. As Mr. Obama’s vice president, Mr. Biden is well aware how that goes. Governing without Congress means relying on presidential orders and decrees.
In health care, Mr. Biden has a powerful policy-making tool, the Center for Medicare & Medicaid Innovation (CMMI). The CMMI was empowered by the ACA to initiate pilot programs for new payment models.
So far, the CMMI’s work has been mainly limited to accountable care organizations, bundled payments, and patient-centered medical homes, but it could also be used to enact new federal policies that would normally require Congressional action, Mr. Levitt said.
Conclusion
Expectations have been very high for what President Joe Biden can do in health care. He needs to unite a very divided political system to defeat a deadly pandemic, restore Obamacare, and sign landmark legislation, such as a drug-pricing bill.
But shepherding bills through Congress will be a challenge. “You need to have accountability, unity, and civility, which is a Herculean task,” Mr. Whitlock said. “You have to keep policies off the table that could blow up the bipartisanship.”
A version of this article first appeared on Medscape.com.
Reproductive psychiatry in 2021: Old questions and new challenges
Across this period of the pandemic, we’ve spent considerable attention focusing on adaptations to clinical care for pregnant and postpartum women across the board. From the start, this has included a shift to telemedicine for the majority of our patients who come to see us with psychiatric disorders either before, during, or after pregnancy.
Specific issues for perinatal patients since the early days of COVID-19 have included the shifts in women’s plans with respect to delivery as well as the limitation on women’s ability to configure the types of support that they had originally planned on with family, friends, and others during delivery. Telemedicine again helped, at least in part, to fill that void by having online digital support by individuals or groups for both pregnant and postpartum women. These supports were always available, but quickly scaled up during the first 6-9 months of the pandemic and have likely seen their greatest increase in participation in the history of support groups for pregnant and postpartum women.
Similarly, at our own center, we have seen a dramatic increase across the last 10 months in requests for consultation by women with psychiatric disorders who have hopes and plans to conceive, to those who are pregnant or post partum and who are trying to sustain emotional well-being despite the added burden of the pandemic. As we heard similar stories regarding interactions with perinatal patients from reproductive psychiatrists across the country, my colleagues and I had to set up an additional resource, Virtual Rounds at the Center for Women’s Mental Health at Massachusetts General Hospital, Boston, which has been mentioned in previous columns, which has only grown during the last 6 months of the pandemic. We have colleagues across the country joining us from 2 p.m. to 3 p.m. on Wednesdays after our own faculty rounds, where we perform case reviews of our own patients, and invite our colleagues to share cases that are then reviewed with expert panelists together with our own faculty in a collaborative environment. Feedback from the community of clinicians has indicated that these virtual rounds have been invaluable to their efforts in taking care of women with perinatal psychiatric issues, particularly during the pandemic.
Of particular note during consultations on our service is the number of women coming to see us for questions about the reproductive safety of the medications on which they are maintained. Hundreds of women present to the center each year for the most up-to-date information regarding the reproductive safety of the most commonly used psychiatric medications in reproductive-age women, including antidepressants (SSRIs, serotonin norepinephrine reuptake inhibitor), mood stabilizers, lithium, lamotrigine, and atypical antipsychotics, as well as other medicines used to treat symptoms that have been a particular issue during the pandemic, such as insomnia and anxiety (benzodiazepines, nonbenzodiazepines, sedative hypnotics, and medicines such as gabapentin).
While consultation regarding risk of fetal exposure to psychotropics has been the cornerstone of our clinical work for 25 years, it has taken on a particularly critical dimension during the pandemic given the wish that women stay euthymic during the pandemic to limit the possibility of patients needing to present in a clinical space that would increase their risk for COVID-19, and to also minimize their risk for postpartum depression. (Psychiatric disorder during pregnancy remains the strongest predictor of emergence or worsening of underlying illness during the postpartum period.)
It is also noteworthy that, during a pandemic year, publications in reproductive psychiatry have been numerous, and we continue to make an effort at our own center to keep up with this and to share with our colleagues our impression of that literature using the weekly blog at womensmentalhealth.org. Last year brought the largest audience to the blog and visits to womensmentalhealth.org in the history of our center.
A case recently at our center presents a unique opportunity to review a confusing question in reproductive psychiatry over the last 15 years. A woman with a longstanding history of mixed anxiety and depression recently came to see us on a regimen of escitalopram and low-dose benzodiazepine. She was doing well, and she and her husband of 4 years were hopeful about starting to try to conceive despite the pandemic. We reviewed the reproductive safety data of the medicines on which she was maintained and made plans to follow-up as her plans galvanized. She notified me several months later that she had become pregnant but had experienced an early miscarriage. The patient was obviously upset and, as she reflected on her decision to maintain treatment with SSRI during her attempts to conceive and across a very early pregnancy, she queried about the extent to which her SSRI use might have contributed to her miscarriage.
The question about the possible association of antidepressant use during pregnancy and increased risk for miscarriage goes back at least 15 years when there were reports of an increased risk of miscarriage in women taking SSRIs during pregnancy. In that early work, there was an apparent increase in miscarriage in women taking SSRIs relative to a control group, but the rate did not exceed the prevalence of miscarriage in the general population. Since those early reports, we are lucky to have had multiple investigators look very closely at this issue, including one meta-analysis of 11 studies done approximately 8 years ago that failed to show an increased risk of miscarriage in the setting of first trimester exposure to SSRIs.
What has been most problematic methodologically, however, has been the failure to account for the potential role of depression in models that predict risk. A subsequent large epidemiologic study from Denmark evaluating over a million women has looked at this question further. The authors found a slightly increased risk of spontaneous abortion associated with the use of antidepressants (12.0% in women with antidepressant exposure vs. 11.1% in women with no exposure). However, looking only at women with a diagnosis of depression, the adjusted risk ratio for spontaneous abortion after any antidepressant exposure was 1.00 (95% confidence interval, 0.80-1.24). Thus, the researchers concluded that exposure to depression – but not exposure to antidepressant – is associated with a slightly higher risk of miscarriage.
Even more recently, a follow-up study examining this question supports the large epidemiologic study by Kjaersgaard and colleagues. For most readers, this effectively answers this very important question for women about rates of miscarriage associated with fetal exposure to SSRIs.
For the patient who presented at the center, reassuring her with this information felt particularly good, especially within the context of the pandemic. After several months of trying to conceive, she again became pregnant and delivered without difficulty. What was palpable in that clinical scenario, as it relates to the practice of reproductive psychiatry during the pandemic, is the even-greater emotional valence that questions about using psychiatric medications during pregnancy has taken on across these past months. While attention and thoughtful consideration about the relative risks of using psychiatric medications during pregnancy should be standard clinical practice, the level of anxiety associated with decisions to sustain or to discontinue treatment during pregnancy seems to have increased for some patients during the pandemic.
Even as the COVID-19 vaccine initiative across the United States is rolled out, 2021 will continue to be a complicated time for women and families. We still need to be vigilant. In addition to screening for perinatal depression during pregnancy and the postpartum period, we should be equally mindful of screening and treating perinatal anxiety, particularly during this challenging time. The challenge to keep pregnant and postpartum women well is perhaps even greater now, 10 months into the pandemic, than it was when we were in crisis mode in March 2020. As clinicians, we need to mobilize the spectrum of both pharmacologic and nonpharmacologic treatment options to sustain emotional well-being among women planning to conceive as well as those who are pregnant or postpartum as we navigate our way to safer times.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications. Email Dr. Cohen at [email protected].
Across this period of the pandemic, we’ve spent considerable attention focusing on adaptations to clinical care for pregnant and postpartum women across the board. From the start, this has included a shift to telemedicine for the majority of our patients who come to see us with psychiatric disorders either before, during, or after pregnancy.
Specific issues for perinatal patients since the early days of COVID-19 have included the shifts in women’s plans with respect to delivery as well as the limitation on women’s ability to configure the types of support that they had originally planned on with family, friends, and others during delivery. Telemedicine again helped, at least in part, to fill that void by having online digital support by individuals or groups for both pregnant and postpartum women. These supports were always available, but quickly scaled up during the first 6-9 months of the pandemic and have likely seen their greatest increase in participation in the history of support groups for pregnant and postpartum women.
Similarly, at our own center, we have seen a dramatic increase across the last 10 months in requests for consultation by women with psychiatric disorders who have hopes and plans to conceive, to those who are pregnant or post partum and who are trying to sustain emotional well-being despite the added burden of the pandemic. As we heard similar stories regarding interactions with perinatal patients from reproductive psychiatrists across the country, my colleagues and I had to set up an additional resource, Virtual Rounds at the Center for Women’s Mental Health at Massachusetts General Hospital, Boston, which has been mentioned in previous columns, which has only grown during the last 6 months of the pandemic. We have colleagues across the country joining us from 2 p.m. to 3 p.m. on Wednesdays after our own faculty rounds, where we perform case reviews of our own patients, and invite our colleagues to share cases that are then reviewed with expert panelists together with our own faculty in a collaborative environment. Feedback from the community of clinicians has indicated that these virtual rounds have been invaluable to their efforts in taking care of women with perinatal psychiatric issues, particularly during the pandemic.
Of particular note during consultations on our service is the number of women coming to see us for questions about the reproductive safety of the medications on which they are maintained. Hundreds of women present to the center each year for the most up-to-date information regarding the reproductive safety of the most commonly used psychiatric medications in reproductive-age women, including antidepressants (SSRIs, serotonin norepinephrine reuptake inhibitor), mood stabilizers, lithium, lamotrigine, and atypical antipsychotics, as well as other medicines used to treat symptoms that have been a particular issue during the pandemic, such as insomnia and anxiety (benzodiazepines, nonbenzodiazepines, sedative hypnotics, and medicines such as gabapentin).
While consultation regarding risk of fetal exposure to psychotropics has been the cornerstone of our clinical work for 25 years, it has taken on a particularly critical dimension during the pandemic given the wish that women stay euthymic during the pandemic to limit the possibility of patients needing to present in a clinical space that would increase their risk for COVID-19, and to also minimize their risk for postpartum depression. (Psychiatric disorder during pregnancy remains the strongest predictor of emergence or worsening of underlying illness during the postpartum period.)
It is also noteworthy that, during a pandemic year, publications in reproductive psychiatry have been numerous, and we continue to make an effort at our own center to keep up with this and to share with our colleagues our impression of that literature using the weekly blog at womensmentalhealth.org. Last year brought the largest audience to the blog and visits to womensmentalhealth.org in the history of our center.
A case recently at our center presents a unique opportunity to review a confusing question in reproductive psychiatry over the last 15 years. A woman with a longstanding history of mixed anxiety and depression recently came to see us on a regimen of escitalopram and low-dose benzodiazepine. She was doing well, and she and her husband of 4 years were hopeful about starting to try to conceive despite the pandemic. We reviewed the reproductive safety data of the medicines on which she was maintained and made plans to follow-up as her plans galvanized. She notified me several months later that she had become pregnant but had experienced an early miscarriage. The patient was obviously upset and, as she reflected on her decision to maintain treatment with SSRI during her attempts to conceive and across a very early pregnancy, she queried about the extent to which her SSRI use might have contributed to her miscarriage.
The question about the possible association of antidepressant use during pregnancy and increased risk for miscarriage goes back at least 15 years when there were reports of an increased risk of miscarriage in women taking SSRIs during pregnancy. In that early work, there was an apparent increase in miscarriage in women taking SSRIs relative to a control group, but the rate did not exceed the prevalence of miscarriage in the general population. Since those early reports, we are lucky to have had multiple investigators look very closely at this issue, including one meta-analysis of 11 studies done approximately 8 years ago that failed to show an increased risk of miscarriage in the setting of first trimester exposure to SSRIs.
What has been most problematic methodologically, however, has been the failure to account for the potential role of depression in models that predict risk. A subsequent large epidemiologic study from Denmark evaluating over a million women has looked at this question further. The authors found a slightly increased risk of spontaneous abortion associated with the use of antidepressants (12.0% in women with antidepressant exposure vs. 11.1% in women with no exposure). However, looking only at women with a diagnosis of depression, the adjusted risk ratio for spontaneous abortion after any antidepressant exposure was 1.00 (95% confidence interval, 0.80-1.24). Thus, the researchers concluded that exposure to depression – but not exposure to antidepressant – is associated with a slightly higher risk of miscarriage.
Even more recently, a follow-up study examining this question supports the large epidemiologic study by Kjaersgaard and colleagues. For most readers, this effectively answers this very important question for women about rates of miscarriage associated with fetal exposure to SSRIs.
For the patient who presented at the center, reassuring her with this information felt particularly good, especially within the context of the pandemic. After several months of trying to conceive, she again became pregnant and delivered without difficulty. What was palpable in that clinical scenario, as it relates to the practice of reproductive psychiatry during the pandemic, is the even-greater emotional valence that questions about using psychiatric medications during pregnancy has taken on across these past months. While attention and thoughtful consideration about the relative risks of using psychiatric medications during pregnancy should be standard clinical practice, the level of anxiety associated with decisions to sustain or to discontinue treatment during pregnancy seems to have increased for some patients during the pandemic.
Even as the COVID-19 vaccine initiative across the United States is rolled out, 2021 will continue to be a complicated time for women and families. We still need to be vigilant. In addition to screening for perinatal depression during pregnancy and the postpartum period, we should be equally mindful of screening and treating perinatal anxiety, particularly during this challenging time. The challenge to keep pregnant and postpartum women well is perhaps even greater now, 10 months into the pandemic, than it was when we were in crisis mode in March 2020. As clinicians, we need to mobilize the spectrum of both pharmacologic and nonpharmacologic treatment options to sustain emotional well-being among women planning to conceive as well as those who are pregnant or postpartum as we navigate our way to safer times.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications. Email Dr. Cohen at [email protected].
Across this period of the pandemic, we’ve spent considerable attention focusing on adaptations to clinical care for pregnant and postpartum women across the board. From the start, this has included a shift to telemedicine for the majority of our patients who come to see us with psychiatric disorders either before, during, or after pregnancy.
Specific issues for perinatal patients since the early days of COVID-19 have included the shifts in women’s plans with respect to delivery as well as the limitation on women’s ability to configure the types of support that they had originally planned on with family, friends, and others during delivery. Telemedicine again helped, at least in part, to fill that void by having online digital support by individuals or groups for both pregnant and postpartum women. These supports were always available, but quickly scaled up during the first 6-9 months of the pandemic and have likely seen their greatest increase in participation in the history of support groups for pregnant and postpartum women.
Similarly, at our own center, we have seen a dramatic increase across the last 10 months in requests for consultation by women with psychiatric disorders who have hopes and plans to conceive, to those who are pregnant or post partum and who are trying to sustain emotional well-being despite the added burden of the pandemic. As we heard similar stories regarding interactions with perinatal patients from reproductive psychiatrists across the country, my colleagues and I had to set up an additional resource, Virtual Rounds at the Center for Women’s Mental Health at Massachusetts General Hospital, Boston, which has been mentioned in previous columns, which has only grown during the last 6 months of the pandemic. We have colleagues across the country joining us from 2 p.m. to 3 p.m. on Wednesdays after our own faculty rounds, where we perform case reviews of our own patients, and invite our colleagues to share cases that are then reviewed with expert panelists together with our own faculty in a collaborative environment. Feedback from the community of clinicians has indicated that these virtual rounds have been invaluable to their efforts in taking care of women with perinatal psychiatric issues, particularly during the pandemic.
Of particular note during consultations on our service is the number of women coming to see us for questions about the reproductive safety of the medications on which they are maintained. Hundreds of women present to the center each year for the most up-to-date information regarding the reproductive safety of the most commonly used psychiatric medications in reproductive-age women, including antidepressants (SSRIs, serotonin norepinephrine reuptake inhibitor), mood stabilizers, lithium, lamotrigine, and atypical antipsychotics, as well as other medicines used to treat symptoms that have been a particular issue during the pandemic, such as insomnia and anxiety (benzodiazepines, nonbenzodiazepines, sedative hypnotics, and medicines such as gabapentin).
While consultation regarding risk of fetal exposure to psychotropics has been the cornerstone of our clinical work for 25 years, it has taken on a particularly critical dimension during the pandemic given the wish that women stay euthymic during the pandemic to limit the possibility of patients needing to present in a clinical space that would increase their risk for COVID-19, and to also minimize their risk for postpartum depression. (Psychiatric disorder during pregnancy remains the strongest predictor of emergence or worsening of underlying illness during the postpartum period.)
It is also noteworthy that, during a pandemic year, publications in reproductive psychiatry have been numerous, and we continue to make an effort at our own center to keep up with this and to share with our colleagues our impression of that literature using the weekly blog at womensmentalhealth.org. Last year brought the largest audience to the blog and visits to womensmentalhealth.org in the history of our center.
A case recently at our center presents a unique opportunity to review a confusing question in reproductive psychiatry over the last 15 years. A woman with a longstanding history of mixed anxiety and depression recently came to see us on a regimen of escitalopram and low-dose benzodiazepine. She was doing well, and she and her husband of 4 years were hopeful about starting to try to conceive despite the pandemic. We reviewed the reproductive safety data of the medicines on which she was maintained and made plans to follow-up as her plans galvanized. She notified me several months later that she had become pregnant but had experienced an early miscarriage. The patient was obviously upset and, as she reflected on her decision to maintain treatment with SSRI during her attempts to conceive and across a very early pregnancy, she queried about the extent to which her SSRI use might have contributed to her miscarriage.
The question about the possible association of antidepressant use during pregnancy and increased risk for miscarriage goes back at least 15 years when there were reports of an increased risk of miscarriage in women taking SSRIs during pregnancy. In that early work, there was an apparent increase in miscarriage in women taking SSRIs relative to a control group, but the rate did not exceed the prevalence of miscarriage in the general population. Since those early reports, we are lucky to have had multiple investigators look very closely at this issue, including one meta-analysis of 11 studies done approximately 8 years ago that failed to show an increased risk of miscarriage in the setting of first trimester exposure to SSRIs.
What has been most problematic methodologically, however, has been the failure to account for the potential role of depression in models that predict risk. A subsequent large epidemiologic study from Denmark evaluating over a million women has looked at this question further. The authors found a slightly increased risk of spontaneous abortion associated with the use of antidepressants (12.0% in women with antidepressant exposure vs. 11.1% in women with no exposure). However, looking only at women with a diagnosis of depression, the adjusted risk ratio for spontaneous abortion after any antidepressant exposure was 1.00 (95% confidence interval, 0.80-1.24). Thus, the researchers concluded that exposure to depression – but not exposure to antidepressant – is associated with a slightly higher risk of miscarriage.
Even more recently, a follow-up study examining this question supports the large epidemiologic study by Kjaersgaard and colleagues. For most readers, this effectively answers this very important question for women about rates of miscarriage associated with fetal exposure to SSRIs.
For the patient who presented at the center, reassuring her with this information felt particularly good, especially within the context of the pandemic. After several months of trying to conceive, she again became pregnant and delivered without difficulty. What was palpable in that clinical scenario, as it relates to the practice of reproductive psychiatry during the pandemic, is the even-greater emotional valence that questions about using psychiatric medications during pregnancy has taken on across these past months. While attention and thoughtful consideration about the relative risks of using psychiatric medications during pregnancy should be standard clinical practice, the level of anxiety associated with decisions to sustain or to discontinue treatment during pregnancy seems to have increased for some patients during the pandemic.
Even as the COVID-19 vaccine initiative across the United States is rolled out, 2021 will continue to be a complicated time for women and families. We still need to be vigilant. In addition to screening for perinatal depression during pregnancy and the postpartum period, we should be equally mindful of screening and treating perinatal anxiety, particularly during this challenging time. The challenge to keep pregnant and postpartum women well is perhaps even greater now, 10 months into the pandemic, than it was when we were in crisis mode in March 2020. As clinicians, we need to mobilize the spectrum of both pharmacologic and nonpharmacologic treatment options to sustain emotional well-being among women planning to conceive as well as those who are pregnant or postpartum as we navigate our way to safer times.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications. Email Dr. Cohen at [email protected].
On receiving the COVID-19 vaccine
This moment, for which I am so grateful and fortunate, represents a link in a remarkable chain of events that spans decades and represents the acme of human achievement.
My gratitude starts with scientists who years before this pandemic, perfected the ability to extract DNA from viruses, sequence it, and transcribe it to RNA. From there my gratitude goes to scientists who years ago developed an ingenious animal model for mRNA vaccines. The next link of gratitude is for scientists who at the start of this year quickly identified a deadly novel coronavirus and to scientists who rapidly sequenced its villainous DNA.
Next, I give thanks to scientists who promptly identified the segment of that DNA that codes for the spike proteins that the virus uses to invade our cells. And then I am grateful to the scientists who made the mRNA that corresponds to that specific DNA sequence, and to the scientists who figured out how create a lipid womb to protect that precious mRNA payload during its perilous journey from factory floor to the depths of our deltoid musculature.
I am no less grateful to the brave people who volunteered for the Pfizer trial, taking the risk of being the first humans ever to participate in an mRNA trial with stakes so high, and to the investigators who ran that trial and the scientists at Pfizer, the Food and Drug Administration, and the Western Coalition who reviewed the data and approved the vaccine without bowing to political pressure.
My gratitude extends to the factory workers who manufactured the vaccine in mass quantities, and the workers who manufactured the equipment that those factories rely on, and the pilots of planes and drivers of trucks who transported the vaccine to my hospital in Seattle, and to the workers who made those planes and trucks that carried that precious cargo. And the workers who devised super-cold storage systems and the workers who built those systems, and the people who fed them and clothed them and housed them so that they could do this life-saving work.
And to the leaders at my hospital who devised our immunization plan, and the ethicists who figured out who should go first (thanks Nancy), and the workers who made the glass vials to hold the vaccine, the plastic syringes to deliver it precisely, and surgically sharp needles so that there would be no pain whatsoever when those beautiful little mRNA filled lipid particles got injected into my left deltoid muscle by a highly skilled and compassionate nurse.
From there, the miracle of nature takes hold causing my cells to transcribe that RNA into spike proteins which will trigger my magical B-cells and T-cells to recognize that nasty spike protein as foreign in case it ever shows its ugly head to my respiratory mucosa, where these cells and the antibodies and chemicals they produce would stomp that wretched virus down without me ever knowing it or missing a beat, and keep me safe not only to live and thrive another day but also hopefully prevent me from spreading the virus to those I love and others I don’t even know but pass within just feet of.
For these miracles of nature and the chain of human toil and genius involving innumerable individuals over many years, many whom will never be thanked or recognized, I am truly and forever grateful.
Dr. Aaronson is a hospitalist and chief medical informatics officer at Virginia Mason Medical Center in Seattle.
This moment, for which I am so grateful and fortunate, represents a link in a remarkable chain of events that spans decades and represents the acme of human achievement.
My gratitude starts with scientists who years before this pandemic, perfected the ability to extract DNA from viruses, sequence it, and transcribe it to RNA. From there my gratitude goes to scientists who years ago developed an ingenious animal model for mRNA vaccines. The next link of gratitude is for scientists who at the start of this year quickly identified a deadly novel coronavirus and to scientists who rapidly sequenced its villainous DNA.
Next, I give thanks to scientists who promptly identified the segment of that DNA that codes for the spike proteins that the virus uses to invade our cells. And then I am grateful to the scientists who made the mRNA that corresponds to that specific DNA sequence, and to the scientists who figured out how create a lipid womb to protect that precious mRNA payload during its perilous journey from factory floor to the depths of our deltoid musculature.
I am no less grateful to the brave people who volunteered for the Pfizer trial, taking the risk of being the first humans ever to participate in an mRNA trial with stakes so high, and to the investigators who ran that trial and the scientists at Pfizer, the Food and Drug Administration, and the Western Coalition who reviewed the data and approved the vaccine without bowing to political pressure.
My gratitude extends to the factory workers who manufactured the vaccine in mass quantities, and the workers who manufactured the equipment that those factories rely on, and the pilots of planes and drivers of trucks who transported the vaccine to my hospital in Seattle, and to the workers who made those planes and trucks that carried that precious cargo. And the workers who devised super-cold storage systems and the workers who built those systems, and the people who fed them and clothed them and housed them so that they could do this life-saving work.
And to the leaders at my hospital who devised our immunization plan, and the ethicists who figured out who should go first (thanks Nancy), and the workers who made the glass vials to hold the vaccine, the plastic syringes to deliver it precisely, and surgically sharp needles so that there would be no pain whatsoever when those beautiful little mRNA filled lipid particles got injected into my left deltoid muscle by a highly skilled and compassionate nurse.
From there, the miracle of nature takes hold causing my cells to transcribe that RNA into spike proteins which will trigger my magical B-cells and T-cells to recognize that nasty spike protein as foreign in case it ever shows its ugly head to my respiratory mucosa, where these cells and the antibodies and chemicals they produce would stomp that wretched virus down without me ever knowing it or missing a beat, and keep me safe not only to live and thrive another day but also hopefully prevent me from spreading the virus to those I love and others I don’t even know but pass within just feet of.
For these miracles of nature and the chain of human toil and genius involving innumerable individuals over many years, many whom will never be thanked or recognized, I am truly and forever grateful.
Dr. Aaronson is a hospitalist and chief medical informatics officer at Virginia Mason Medical Center in Seattle.
This moment, for which I am so grateful and fortunate, represents a link in a remarkable chain of events that spans decades and represents the acme of human achievement.
My gratitude starts with scientists who years before this pandemic, perfected the ability to extract DNA from viruses, sequence it, and transcribe it to RNA. From there my gratitude goes to scientists who years ago developed an ingenious animal model for mRNA vaccines. The next link of gratitude is for scientists who at the start of this year quickly identified a deadly novel coronavirus and to scientists who rapidly sequenced its villainous DNA.
Next, I give thanks to scientists who promptly identified the segment of that DNA that codes for the spike proteins that the virus uses to invade our cells. And then I am grateful to the scientists who made the mRNA that corresponds to that specific DNA sequence, and to the scientists who figured out how create a lipid womb to protect that precious mRNA payload during its perilous journey from factory floor to the depths of our deltoid musculature.
I am no less grateful to the brave people who volunteered for the Pfizer trial, taking the risk of being the first humans ever to participate in an mRNA trial with stakes so high, and to the investigators who ran that trial and the scientists at Pfizer, the Food and Drug Administration, and the Western Coalition who reviewed the data and approved the vaccine without bowing to political pressure.
My gratitude extends to the factory workers who manufactured the vaccine in mass quantities, and the workers who manufactured the equipment that those factories rely on, and the pilots of planes and drivers of trucks who transported the vaccine to my hospital in Seattle, and to the workers who made those planes and trucks that carried that precious cargo. And the workers who devised super-cold storage systems and the workers who built those systems, and the people who fed them and clothed them and housed them so that they could do this life-saving work.
And to the leaders at my hospital who devised our immunization plan, and the ethicists who figured out who should go first (thanks Nancy), and the workers who made the glass vials to hold the vaccine, the plastic syringes to deliver it precisely, and surgically sharp needles so that there would be no pain whatsoever when those beautiful little mRNA filled lipid particles got injected into my left deltoid muscle by a highly skilled and compassionate nurse.
From there, the miracle of nature takes hold causing my cells to transcribe that RNA into spike proteins which will trigger my magical B-cells and T-cells to recognize that nasty spike protein as foreign in case it ever shows its ugly head to my respiratory mucosa, where these cells and the antibodies and chemicals they produce would stomp that wretched virus down without me ever knowing it or missing a beat, and keep me safe not only to live and thrive another day but also hopefully prevent me from spreading the virus to those I love and others I don’t even know but pass within just feet of.
For these miracles of nature and the chain of human toil and genius involving innumerable individuals over many years, many whom will never be thanked or recognized, I am truly and forever grateful.
Dr. Aaronson is a hospitalist and chief medical informatics officer at Virginia Mason Medical Center in Seattle.
Patient Handout: Safe practices during the COVID-19 pandemic
In addition to sharing this handout (see PDF link) with your patients, Dr. Gupta also recommends advising them to watch the video Hand-washing Steps Using the WHO Technique, which is available at https://youtu.be/IisgnbMfKvI
In addition to sharing this handout (see PDF link) with your patients, Dr. Gupta also recommends advising them to watch the video Hand-washing Steps Using the WHO Technique, which is available at https://youtu.be/IisgnbMfKvI
In addition to sharing this handout (see PDF link) with your patients, Dr. Gupta also recommends advising them to watch the video Hand-washing Steps Using the WHO Technique, which is available at https://youtu.be/IisgnbMfKvI
The state of inpatient COVID-19 care
A brief evidence-based review of everything we have learned
Evidence on emerging treatments for COVID-19 has been incomplete, often disappointing, and rapidly changing. The concept of a practice-changing press release is as novel as the coronavirus. The pandemic has created an interdependent set of inpatient challenges: keeping up with evolving science and operationalizing clinical workflows, technology, and therapeutics to adapt what we are learning.
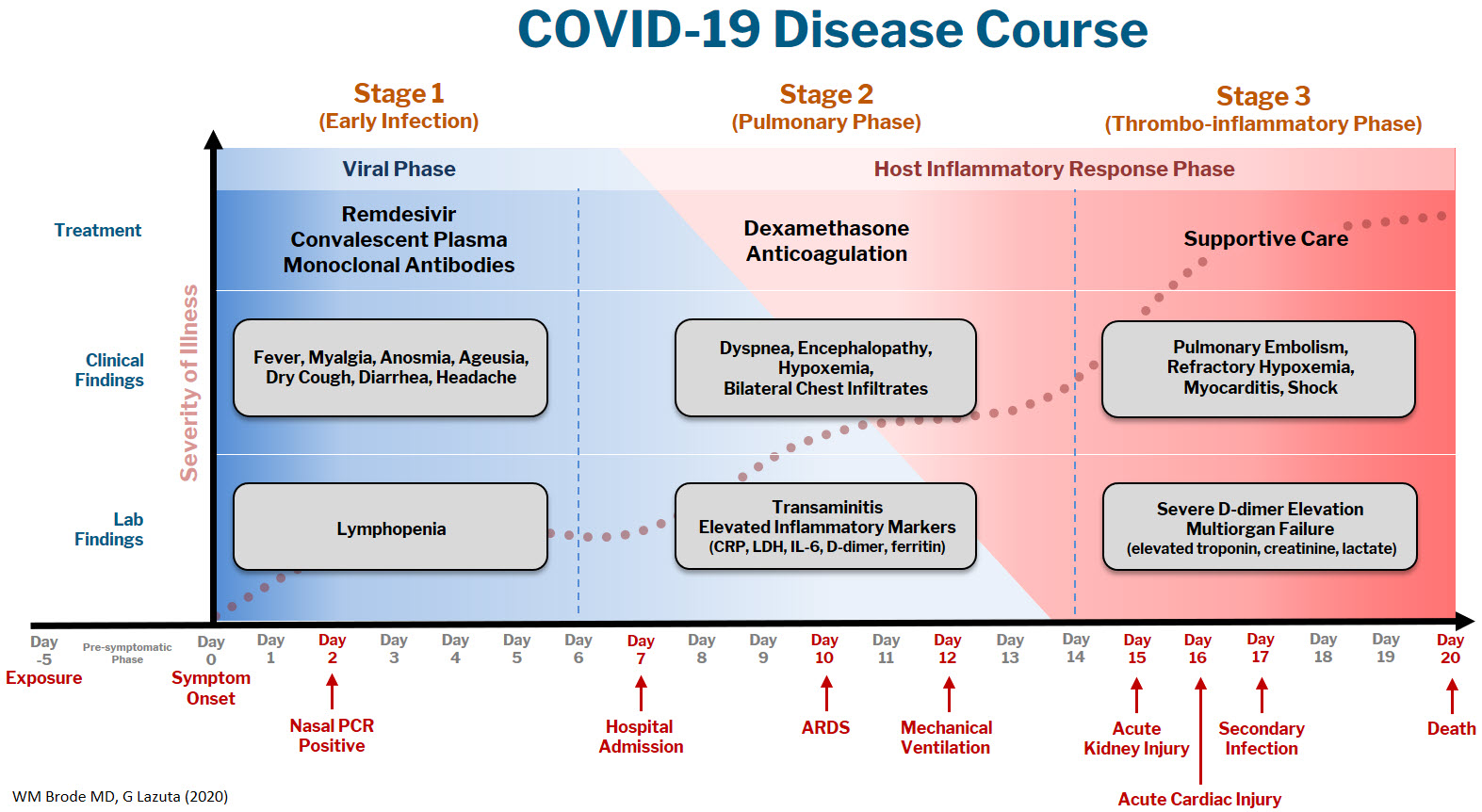
At Dell Medical School, we have created a Therapeutics and Informatics Committee to put evidence into practice in real-time, and below is a brief framework of what we have learned to date:
The COVID-19 disease course can be broken down into 3 stages, and workup and interventions should be targeted to those stages.1–3
Stage 1 is the viral phase following a median 5-day pre-symptomatic phase from exposure; this is indistinguishable from an influenza-like illness with the typical fever, cough, GI symptoms, and the more specific anosmia, ageusia, and orthostasis.
Stage 2 is the pulmonary phase where patients develop COVID-19 pneumonia and will have diffuse chest infiltrates on imaging. This stage usually represents the tail end of the viral phase prior to recovery, but for the ~15% of patients who present to the hospital needing admission because of hypoxemia (the definition of severe COVID-19, typically 5-7 days from symptom onset) this phase is characterized by elevated inflammatory markers and an exuberant host-immune response.
Stage 3 is the dreaded thrombo-inflammatory phase, which is a late manifestation usually >10 days from symptom onset and appears to be independent of viral replication. The morbidity and mortality associated with COVID-19 is likely a result of diffuse microthrombosis, and critical disease should no longer be thought of as a “cytokine storm,” but as life-threatening organ dysfunction caused by a dysregulated host response to infection. Unlike sepsis, the predominant pathology is not vasodilation and shock, but a hypercoagulable state with diffuse endothelial damage.4,5
Workup on presentation to the hospital should focus on identifying which phase of illness the patient is in, based on timing of symptom onset, inflammatory markers, and end-organ damage. CBC, CMP, D-dimer, troponin, and CRP are likely sufficient baseline labs in addition to a chest X-ray. There are many risk stratification tools, but to date, the 4C Mortality 4C Deterioration Scores are recommended due to their large derivation cohort and reliance on only 8 practical variables.6
Remdesivir and convalescent plasma (CVP) disrupt viral replication in stages 1 and 2 of the illness. Remdesivir has shown efficacy reducing hospital length of stay and a small trend towards decreasing mortality, especially if given within 10 days of symptom onset, although its effectiveness in general use is very small, if it exists at all.7,8 CVP efficacy has been disappointing and should not be the standard of care: multiple RCTs do not show any clinical benefit, although the Mayo Clinic registry data suggests that high-titer CVP given within 3 days from diagnosis decreases mortality compared to low-titer plasma.9-11 Monoclonal antibodies are theoretically “supercharged” high-titer CVP, but are approved for outpatient use only. Trials for hospitalized patients requiring oxygen were stopped due to futility. By the time the patient is hospitalized, it is probably too late in the disease course for CVP or monoclonal antibodies to be effective.
Dexamethasone is the only treatment with a proven mortality benefit. The RECOVERY trial showed the greatest mortality benefit (number needed to treat [NNT] of 8) in mechanically ventilated patients > 7 days from symptom onset. While there is a benefit to patients requiring any oxygen (NNT of 35), early administration to patients in the viral phase is associated with higher mortality as corticosteroids can reduce viral clearance.12 Corticosteroids should therefore be targeted to a therapeutic window to reduce the dysregulated host immune response and treat ARDS in phases 2 and 3; earlier is not necessarily better.
Incidence of venous thromboembolism (VTE) increases linearly with disease severity (one metanalysis showing a rate of 24% in the ICU13) and autopsy studies demonstrate diffuse microthrombosis even when VTE was not suspected5. Observational studies have shown VTE pharmacoprophylaxis reduces mortality, but the optimal agent, timing, and intensity of regimens is not yet clear.14-15 A recent press release from the NIH reported that full dose prophylactic anticoagulation in moderately ill patients reduced disease progression and trended toward lower mortality. Interestingly, for critically ill patients requiring high-flow nasal cannula (HFNC) or mechanical ventilation, intensified anticoagulation regiments had potential harm, and enrollment was stopped in this cohort.16 This announcement is a hopeful sign that intensified anticoagulation regimens can prevent thrombo-inflammation, but until the data of multiple ongoing trials is published it remains expert opinion only.
The most important treatment remains delivering oxygen with fidelity, correcting the much-observed “silent” or “happy hypoxemic.”17 Given the high mortality associated with mechanical ventilation and that hypoxemia can be out of proportion to respiratory distress, arbitrary thresholds should not be used to decide when to intubate and instead should evaluate work of breathing, hypercapnia, mentation, or progression of end-organ damage rather than a single cutoff.18 High-flow nasal cannula (HFNC) can correct severe hypoxemia in addition to self-proning, and while there is scant outcomes data for this strategy, it has been adopted widely as ICU capacity is strained nationally. A ventilator can add PEEP for alveolar recruitment or perform the work of breathing for a patient, but a patient will receive 100% FiO2 whether it is delivered through the nares on HFNC or 10 inches lower by an endotracheal tube.
In the absence of a single therapeutic cure or breakthrough, caring for a COVID-19 patient requires the hospital system to instead do a thousand things conscientiously and consistently. This is supportive care: most patients will get better with time and attentive evaluation for end-organ complications like myocarditis, encephalopathy, or pressure ulcers. It requires nursing to patient ratios that allows for this type of vigilance, with shared protocols, order sets, and close communication among team members that provides this support. The treatment of COVID-19 continues to evolve, but as we confront rising hospital volumes nationally, it is important to standardize care for patients throughout each of the 3 stages of illness until we find that single breakthrough.
Dr. Brode is a practicing internal medicine physician at Dell Seton Medical Center and assistant professor in the Department of Internal Medicine at Dell Medical School, both in Austin, Texas. He is a clinician educator who emphasizes knowing the patient as a person first, evidence-based diagnosis, and comprehensive care for the patients who are most vulnerable. This article is part of a series originally published in The Hospital Leader, the official blog of SHM.
References
1. Cummings MJ, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. The Lancet. 2020 June 6;395(10239):1763-1770. doi:10.1016/S0140-6736(20)31189-2.
2. Oudkerk M, et al. Diagnosis, prevention, and treatment of thromboembolic complications in COVID-19: Report of the National Institute for Public Health of the Netherlands. Radiology. 2020;297(1):E216-E222. doi:10.1148/radiol.2020201629.
3. Siddiqi HK, and Mehra MR. COVID-19 illness in native and immunosuppressed states: A clinical–therapeutic staging proposal. J Heart Lung Transplant. 2020;39:405-407.
4. Connors JM, and Levy JH. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;135:2033-2040.
5. Ackermann M, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020 July 9;383:120-128. doi:10.1056/NEJMoa2015432.
6. Knight SR, et al. Risk stratification of patients admitted to hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: Development and validation of the 4C Mortality Score. BMJ. 2020;370:m3339. doi:10.1136/bmj.m3339.
7. Beigel JH, et al. Remdesivir for the treatment of Covid-19 – Final report. N Engl J Med. 2020;383:1813-1826. doi:10.1056/NEJMoa2007764.
8. Repurposed antiviral drugs for COVID-19: Interim WHO SOLIDARITY trial results. medRxiv. 2020;10.15.20209817. doi:10.1101/2020.10.15.20209817.
9. Agarwal A, et al. Convalescent plasma in the management of moderate covid-19 in adults in India: open label phase II multicentre randomised controlled trial (PLACID Trial). BMJ. 2020;371:m3939.
10. Simonovich VA, et al. A randomized trial of convalescent plasma in Covid-19 severe pneumonia. N Engl J Med. 2020 Nov 24. doi:10.1056/NEJMoa2031304.
11. Joyner MJ, et al. Convalescent Plasma Antibody Levels and the Risk of Death from Covid-19. N Engl J Med 2021; 384:1015-1027. doi:10.1056/NEJMoa2031893.
12. The RECOVERY Collaborative Group: Dexamethasone in hospitalized patients with Covid-19 – Preliminary report. N Engl J Med. 2020 July 17. doi:10.1056/NEJMoa2021436.
13. Porfidia A, et al. Venous thromboembolism in patients with COVID-19: Systematic review and meta-analysis. Thromb Res. 2020 Dec;196:67-74.
14. Nadkarni GN, et al. Anticoagulation, mortality, bleeding and pathology among patients hospitalized with COVID-19: A single health system study. J Am Coll Cardiol. 2020 Oct 20;76(16):1815-1826. doi:10.1016/j.jacc.2020.08.041.
15. Paranjpe I, et al. Association of treatment dose anticoagulation with in-hospital survival among hospitalized patients with COVID-19. J Am Coll Cardiol. 2020 Jul 7;76(1):122-124. doi:10.1016/j.jacc.2020.05.001.
16. Full-dose blood thinners decreased need for life support and improved outcome in hospitalized COVID-19 patients. National Institutes of Health. Available at https://www.nih.gov/news-events/news-releases/full-dose-blood-thinners-decreased-need-life-support-improved-outcome-hospitalized-covid-19-patients.
17. Tobin MJ, et al. Why COVID-19 silent hypoxemia is baffling to physicians. Am J Respir Crit Care Med. 2020 Aug 1;202(3):356-360. doi:10.1164/rccm.202006-2157CP.
18. Berlin DA, et al. Severe Covid-19. N Engl J Med. 2020;383:2451-2460. doi:10.1056/NEJMcp2009575.
A brief evidence-based review of everything we have learned
A brief evidence-based review of everything we have learned
Evidence on emerging treatments for COVID-19 has been incomplete, often disappointing, and rapidly changing. The concept of a practice-changing press release is as novel as the coronavirus. The pandemic has created an interdependent set of inpatient challenges: keeping up with evolving science and operationalizing clinical workflows, technology, and therapeutics to adapt what we are learning.

At Dell Medical School, we have created a Therapeutics and Informatics Committee to put evidence into practice in real-time, and below is a brief framework of what we have learned to date:
The COVID-19 disease course can be broken down into 3 stages, and workup and interventions should be targeted to those stages.1–3
Stage 1 is the viral phase following a median 5-day pre-symptomatic phase from exposure; this is indistinguishable from an influenza-like illness with the typical fever, cough, GI symptoms, and the more specific anosmia, ageusia, and orthostasis.
Stage 2 is the pulmonary phase where patients develop COVID-19 pneumonia and will have diffuse chest infiltrates on imaging. This stage usually represents the tail end of the viral phase prior to recovery, but for the ~15% of patients who present to the hospital needing admission because of hypoxemia (the definition of severe COVID-19, typically 5-7 days from symptom onset) this phase is characterized by elevated inflammatory markers and an exuberant host-immune response.
Stage 3 is the dreaded thrombo-inflammatory phase, which is a late manifestation usually >10 days from symptom onset and appears to be independent of viral replication. The morbidity and mortality associated with COVID-19 is likely a result of diffuse microthrombosis, and critical disease should no longer be thought of as a “cytokine storm,” but as life-threatening organ dysfunction caused by a dysregulated host response to infection. Unlike sepsis, the predominant pathology is not vasodilation and shock, but a hypercoagulable state with diffuse endothelial damage.4,5
Workup on presentation to the hospital should focus on identifying which phase of illness the patient is in, based on timing of symptom onset, inflammatory markers, and end-organ damage. CBC, CMP, D-dimer, troponin, and CRP are likely sufficient baseline labs in addition to a chest X-ray. There are many risk stratification tools, but to date, the 4C Mortality 4C Deterioration Scores are recommended due to their large derivation cohort and reliance on only 8 practical variables.6
Remdesivir and convalescent plasma (CVP) disrupt viral replication in stages 1 and 2 of the illness. Remdesivir has shown efficacy reducing hospital length of stay and a small trend towards decreasing mortality, especially if given within 10 days of symptom onset, although its effectiveness in general use is very small, if it exists at all.7,8 CVP efficacy has been disappointing and should not be the standard of care: multiple RCTs do not show any clinical benefit, although the Mayo Clinic registry data suggests that high-titer CVP given within 3 days from diagnosis decreases mortality compared to low-titer plasma.9-11 Monoclonal antibodies are theoretically “supercharged” high-titer CVP, but are approved for outpatient use only. Trials for hospitalized patients requiring oxygen were stopped due to futility. By the time the patient is hospitalized, it is probably too late in the disease course for CVP or monoclonal antibodies to be effective.
Dexamethasone is the only treatment with a proven mortality benefit. The RECOVERY trial showed the greatest mortality benefit (number needed to treat [NNT] of 8) in mechanically ventilated patients > 7 days from symptom onset. While there is a benefit to patients requiring any oxygen (NNT of 35), early administration to patients in the viral phase is associated with higher mortality as corticosteroids can reduce viral clearance.12 Corticosteroids should therefore be targeted to a therapeutic window to reduce the dysregulated host immune response and treat ARDS in phases 2 and 3; earlier is not necessarily better.
Incidence of venous thromboembolism (VTE) increases linearly with disease severity (one metanalysis showing a rate of 24% in the ICU13) and autopsy studies demonstrate diffuse microthrombosis even when VTE was not suspected5. Observational studies have shown VTE pharmacoprophylaxis reduces mortality, but the optimal agent, timing, and intensity of regimens is not yet clear.14-15 A recent press release from the NIH reported that full dose prophylactic anticoagulation in moderately ill patients reduced disease progression and trended toward lower mortality. Interestingly, for critically ill patients requiring high-flow nasal cannula (HFNC) or mechanical ventilation, intensified anticoagulation regiments had potential harm, and enrollment was stopped in this cohort.16 This announcement is a hopeful sign that intensified anticoagulation regimens can prevent thrombo-inflammation, but until the data of multiple ongoing trials is published it remains expert opinion only.
The most important treatment remains delivering oxygen with fidelity, correcting the much-observed “silent” or “happy hypoxemic.”17 Given the high mortality associated with mechanical ventilation and that hypoxemia can be out of proportion to respiratory distress, arbitrary thresholds should not be used to decide when to intubate and instead should evaluate work of breathing, hypercapnia, mentation, or progression of end-organ damage rather than a single cutoff.18 High-flow nasal cannula (HFNC) can correct severe hypoxemia in addition to self-proning, and while there is scant outcomes data for this strategy, it has been adopted widely as ICU capacity is strained nationally. A ventilator can add PEEP for alveolar recruitment or perform the work of breathing for a patient, but a patient will receive 100% FiO2 whether it is delivered through the nares on HFNC or 10 inches lower by an endotracheal tube.
In the absence of a single therapeutic cure or breakthrough, caring for a COVID-19 patient requires the hospital system to instead do a thousand things conscientiously and consistently. This is supportive care: most patients will get better with time and attentive evaluation for end-organ complications like myocarditis, encephalopathy, or pressure ulcers. It requires nursing to patient ratios that allows for this type of vigilance, with shared protocols, order sets, and close communication among team members that provides this support. The treatment of COVID-19 continues to evolve, but as we confront rising hospital volumes nationally, it is important to standardize care for patients throughout each of the 3 stages of illness until we find that single breakthrough.
Dr. Brode is a practicing internal medicine physician at Dell Seton Medical Center and assistant professor in the Department of Internal Medicine at Dell Medical School, both in Austin, Texas. He is a clinician educator who emphasizes knowing the patient as a person first, evidence-based diagnosis, and comprehensive care for the patients who are most vulnerable. This article is part of a series originally published in The Hospital Leader, the official blog of SHM.
References
1. Cummings MJ, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. The Lancet. 2020 June 6;395(10239):1763-1770. doi:10.1016/S0140-6736(20)31189-2.
2. Oudkerk M, et al. Diagnosis, prevention, and treatment of thromboembolic complications in COVID-19: Report of the National Institute for Public Health of the Netherlands. Radiology. 2020;297(1):E216-E222. doi:10.1148/radiol.2020201629.
3. Siddiqi HK, and Mehra MR. COVID-19 illness in native and immunosuppressed states: A clinical–therapeutic staging proposal. J Heart Lung Transplant. 2020;39:405-407.
4. Connors JM, and Levy JH. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;135:2033-2040.
5. Ackermann M, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020 July 9;383:120-128. doi:10.1056/NEJMoa2015432.
6. Knight SR, et al. Risk stratification of patients admitted to hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: Development and validation of the 4C Mortality Score. BMJ. 2020;370:m3339. doi:10.1136/bmj.m3339.
7. Beigel JH, et al. Remdesivir for the treatment of Covid-19 – Final report. N Engl J Med. 2020;383:1813-1826. doi:10.1056/NEJMoa2007764.
8. Repurposed antiviral drugs for COVID-19: Interim WHO SOLIDARITY trial results. medRxiv. 2020;10.15.20209817. doi:10.1101/2020.10.15.20209817.
9. Agarwal A, et al. Convalescent plasma in the management of moderate covid-19 in adults in India: open label phase II multicentre randomised controlled trial (PLACID Trial). BMJ. 2020;371:m3939.
10. Simonovich VA, et al. A randomized trial of convalescent plasma in Covid-19 severe pneumonia. N Engl J Med. 2020 Nov 24. doi:10.1056/NEJMoa2031304.
11. Joyner MJ, et al. Convalescent Plasma Antibody Levels and the Risk of Death from Covid-19. N Engl J Med 2021; 384:1015-1027. doi:10.1056/NEJMoa2031893.
12. The RECOVERY Collaborative Group: Dexamethasone in hospitalized patients with Covid-19 – Preliminary report. N Engl J Med. 2020 July 17. doi:10.1056/NEJMoa2021436.
13. Porfidia A, et al. Venous thromboembolism in patients with COVID-19: Systematic review and meta-analysis. Thromb Res. 2020 Dec;196:67-74.
14. Nadkarni GN, et al. Anticoagulation, mortality, bleeding and pathology among patients hospitalized with COVID-19: A single health system study. J Am Coll Cardiol. 2020 Oct 20;76(16):1815-1826. doi:10.1016/j.jacc.2020.08.041.
15. Paranjpe I, et al. Association of treatment dose anticoagulation with in-hospital survival among hospitalized patients with COVID-19. J Am Coll Cardiol. 2020 Jul 7;76(1):122-124. doi:10.1016/j.jacc.2020.05.001.
16. Full-dose blood thinners decreased need for life support and improved outcome in hospitalized COVID-19 patients. National Institutes of Health. Available at https://www.nih.gov/news-events/news-releases/full-dose-blood-thinners-decreased-need-life-support-improved-outcome-hospitalized-covid-19-patients.
17. Tobin MJ, et al. Why COVID-19 silent hypoxemia is baffling to physicians. Am J Respir Crit Care Med. 2020 Aug 1;202(3):356-360. doi:10.1164/rccm.202006-2157CP.
18. Berlin DA, et al. Severe Covid-19. N Engl J Med. 2020;383:2451-2460. doi:10.1056/NEJMcp2009575.
Evidence on emerging treatments for COVID-19 has been incomplete, often disappointing, and rapidly changing. The concept of a practice-changing press release is as novel as the coronavirus. The pandemic has created an interdependent set of inpatient challenges: keeping up with evolving science and operationalizing clinical workflows, technology, and therapeutics to adapt what we are learning.

At Dell Medical School, we have created a Therapeutics and Informatics Committee to put evidence into practice in real-time, and below is a brief framework of what we have learned to date:
The COVID-19 disease course can be broken down into 3 stages, and workup and interventions should be targeted to those stages.1–3
Stage 1 is the viral phase following a median 5-day pre-symptomatic phase from exposure; this is indistinguishable from an influenza-like illness with the typical fever, cough, GI symptoms, and the more specific anosmia, ageusia, and orthostasis.
Stage 2 is the pulmonary phase where patients develop COVID-19 pneumonia and will have diffuse chest infiltrates on imaging. This stage usually represents the tail end of the viral phase prior to recovery, but for the ~15% of patients who present to the hospital needing admission because of hypoxemia (the definition of severe COVID-19, typically 5-7 days from symptom onset) this phase is characterized by elevated inflammatory markers and an exuberant host-immune response.
Stage 3 is the dreaded thrombo-inflammatory phase, which is a late manifestation usually >10 days from symptom onset and appears to be independent of viral replication. The morbidity and mortality associated with COVID-19 is likely a result of diffuse microthrombosis, and critical disease should no longer be thought of as a “cytokine storm,” but as life-threatening organ dysfunction caused by a dysregulated host response to infection. Unlike sepsis, the predominant pathology is not vasodilation and shock, but a hypercoagulable state with diffuse endothelial damage.4,5
Workup on presentation to the hospital should focus on identifying which phase of illness the patient is in, based on timing of symptom onset, inflammatory markers, and end-organ damage. CBC, CMP, D-dimer, troponin, and CRP are likely sufficient baseline labs in addition to a chest X-ray. There are many risk stratification tools, but to date, the 4C Mortality 4C Deterioration Scores are recommended due to their large derivation cohort and reliance on only 8 practical variables.6
Remdesivir and convalescent plasma (CVP) disrupt viral replication in stages 1 and 2 of the illness. Remdesivir has shown efficacy reducing hospital length of stay and a small trend towards decreasing mortality, especially if given within 10 days of symptom onset, although its effectiveness in general use is very small, if it exists at all.7,8 CVP efficacy has been disappointing and should not be the standard of care: multiple RCTs do not show any clinical benefit, although the Mayo Clinic registry data suggests that high-titer CVP given within 3 days from diagnosis decreases mortality compared to low-titer plasma.9-11 Monoclonal antibodies are theoretically “supercharged” high-titer CVP, but are approved for outpatient use only. Trials for hospitalized patients requiring oxygen were stopped due to futility. By the time the patient is hospitalized, it is probably too late in the disease course for CVP or monoclonal antibodies to be effective.
Dexamethasone is the only treatment with a proven mortality benefit. The RECOVERY trial showed the greatest mortality benefit (number needed to treat [NNT] of 8) in mechanically ventilated patients > 7 days from symptom onset. While there is a benefit to patients requiring any oxygen (NNT of 35), early administration to patients in the viral phase is associated with higher mortality as corticosteroids can reduce viral clearance.12 Corticosteroids should therefore be targeted to a therapeutic window to reduce the dysregulated host immune response and treat ARDS in phases 2 and 3; earlier is not necessarily better.
Incidence of venous thromboembolism (VTE) increases linearly with disease severity (one metanalysis showing a rate of 24% in the ICU13) and autopsy studies demonstrate diffuse microthrombosis even when VTE was not suspected5. Observational studies have shown VTE pharmacoprophylaxis reduces mortality, but the optimal agent, timing, and intensity of regimens is not yet clear.14-15 A recent press release from the NIH reported that full dose prophylactic anticoagulation in moderately ill patients reduced disease progression and trended toward lower mortality. Interestingly, for critically ill patients requiring high-flow nasal cannula (HFNC) or mechanical ventilation, intensified anticoagulation regiments had potential harm, and enrollment was stopped in this cohort.16 This announcement is a hopeful sign that intensified anticoagulation regimens can prevent thrombo-inflammation, but until the data of multiple ongoing trials is published it remains expert opinion only.
The most important treatment remains delivering oxygen with fidelity, correcting the much-observed “silent” or “happy hypoxemic.”17 Given the high mortality associated with mechanical ventilation and that hypoxemia can be out of proportion to respiratory distress, arbitrary thresholds should not be used to decide when to intubate and instead should evaluate work of breathing, hypercapnia, mentation, or progression of end-organ damage rather than a single cutoff.18 High-flow nasal cannula (HFNC) can correct severe hypoxemia in addition to self-proning, and while there is scant outcomes data for this strategy, it has been adopted widely as ICU capacity is strained nationally. A ventilator can add PEEP for alveolar recruitment or perform the work of breathing for a patient, but a patient will receive 100% FiO2 whether it is delivered through the nares on HFNC or 10 inches lower by an endotracheal tube.
In the absence of a single therapeutic cure or breakthrough, caring for a COVID-19 patient requires the hospital system to instead do a thousand things conscientiously and consistently. This is supportive care: most patients will get better with time and attentive evaluation for end-organ complications like myocarditis, encephalopathy, or pressure ulcers. It requires nursing to patient ratios that allows for this type of vigilance, with shared protocols, order sets, and close communication among team members that provides this support. The treatment of COVID-19 continues to evolve, but as we confront rising hospital volumes nationally, it is important to standardize care for patients throughout each of the 3 stages of illness until we find that single breakthrough.
Dr. Brode is a practicing internal medicine physician at Dell Seton Medical Center and assistant professor in the Department of Internal Medicine at Dell Medical School, both in Austin, Texas. He is a clinician educator who emphasizes knowing the patient as a person first, evidence-based diagnosis, and comprehensive care for the patients who are most vulnerable. This article is part of a series originally published in The Hospital Leader, the official blog of SHM.
References
1. Cummings MJ, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. The Lancet. 2020 June 6;395(10239):1763-1770. doi:10.1016/S0140-6736(20)31189-2.
2. Oudkerk M, et al. Diagnosis, prevention, and treatment of thromboembolic complications in COVID-19: Report of the National Institute for Public Health of the Netherlands. Radiology. 2020;297(1):E216-E222. doi:10.1148/radiol.2020201629.
3. Siddiqi HK, and Mehra MR. COVID-19 illness in native and immunosuppressed states: A clinical–therapeutic staging proposal. J Heart Lung Transplant. 2020;39:405-407.
4. Connors JM, and Levy JH. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;135:2033-2040.
5. Ackermann M, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020 July 9;383:120-128. doi:10.1056/NEJMoa2015432.
6. Knight SR, et al. Risk stratification of patients admitted to hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: Development and validation of the 4C Mortality Score. BMJ. 2020;370:m3339. doi:10.1136/bmj.m3339.
7. Beigel JH, et al. Remdesivir for the treatment of Covid-19 – Final report. N Engl J Med. 2020;383:1813-1826. doi:10.1056/NEJMoa2007764.
8. Repurposed antiviral drugs for COVID-19: Interim WHO SOLIDARITY trial results. medRxiv. 2020;10.15.20209817. doi:10.1101/2020.10.15.20209817.
9. Agarwal A, et al. Convalescent plasma in the management of moderate covid-19 in adults in India: open label phase II multicentre randomised controlled trial (PLACID Trial). BMJ. 2020;371:m3939.
10. Simonovich VA, et al. A randomized trial of convalescent plasma in Covid-19 severe pneumonia. N Engl J Med. 2020 Nov 24. doi:10.1056/NEJMoa2031304.
11. Joyner MJ, et al. Convalescent Plasma Antibody Levels and the Risk of Death from Covid-19. N Engl J Med 2021; 384:1015-1027. doi:10.1056/NEJMoa2031893.
12. The RECOVERY Collaborative Group: Dexamethasone in hospitalized patients with Covid-19 – Preliminary report. N Engl J Med. 2020 July 17. doi:10.1056/NEJMoa2021436.
13. Porfidia A, et al. Venous thromboembolism in patients with COVID-19: Systematic review and meta-analysis. Thromb Res. 2020 Dec;196:67-74.
14. Nadkarni GN, et al. Anticoagulation, mortality, bleeding and pathology among patients hospitalized with COVID-19: A single health system study. J Am Coll Cardiol. 2020 Oct 20;76(16):1815-1826. doi:10.1016/j.jacc.2020.08.041.
15. Paranjpe I, et al. Association of treatment dose anticoagulation with in-hospital survival among hospitalized patients with COVID-19. J Am Coll Cardiol. 2020 Jul 7;76(1):122-124. doi:10.1016/j.jacc.2020.05.001.
16. Full-dose blood thinners decreased need for life support and improved outcome in hospitalized COVID-19 patients. National Institutes of Health. Available at https://www.nih.gov/news-events/news-releases/full-dose-blood-thinners-decreased-need-life-support-improved-outcome-hospitalized-covid-19-patients.
17. Tobin MJ, et al. Why COVID-19 silent hypoxemia is baffling to physicians. Am J Respir Crit Care Med. 2020 Aug 1;202(3):356-360. doi:10.1164/rccm.202006-2157CP.
18. Berlin DA, et al. Severe Covid-19. N Engl J Med. 2020;383:2451-2460. doi:10.1056/NEJMcp2009575.
President Biden kicks off health agenda with COVID actions, WHO outreach
President Joe Biden kicked off his new administration Jan. 20 with an immediate focus on attempts to stop the spread of COVID-19, including closer coordination with other nations.
Mr. Biden signed 17 executive orders, memoranda, and directives addressing not only the pandemic but also economic concerns, climate change, and racial inequity.
At the top of the list of actions was what his transition team called a “100 Days Masking Challenge.” Mr. Biden issued an executive order requiring masks and physical distancing in all federal buildings, on all federal lands, and by federal employees and contractors.
The president also halted the Trump administration’s process of withdrawing from the World Health Organization. Instead, Mr. Biden named Anthony Fauci, MD, the director of the National Institute for Allergy and Infectious Diseases, as the head of a delegation to participate in the WHO executive board meeting that is being held this week.
Mr. Biden also signed an executive order creating the position of COVID-19 response coordinator, which will report directly to the president and be responsible for coordinating all elements of the COVID-19 response across government, including the production and distribution of vaccines and medical supplies.
The newly inaugurated president also intends to restore the National Security Council’s Directorate for Global Health Security and Biodefense, which will aid in the response to the pandemic, his transition team said.
The American Medical Association was among the first to commend the first-day actions.
“Defeating COVID-19 requires bold, coordinated federal leadership and strong adherence to the public health steps we know stop the spread of this virus – wearing masks, practicing physical distancing, and washing hands,” said AMA President Susan R. Bailey, MD in a news release. “We are pleased by the Biden administration’s steps today, including universal mask wearing within federal jurisdictions, providing federal leadership for COVID-19 response, and reengaging with the World Health Organization. Taking these actions on day 1 of the administration sends the right message – that our nation is laser focused on stopping the ravages of COVID-19.”
A version of this article first appeared on Medscape.com.
President Joe Biden kicked off his new administration Jan. 20 with an immediate focus on attempts to stop the spread of COVID-19, including closer coordination with other nations.
Mr. Biden signed 17 executive orders, memoranda, and directives addressing not only the pandemic but also economic concerns, climate change, and racial inequity.
At the top of the list of actions was what his transition team called a “100 Days Masking Challenge.” Mr. Biden issued an executive order requiring masks and physical distancing in all federal buildings, on all federal lands, and by federal employees and contractors.
The president also halted the Trump administration’s process of withdrawing from the World Health Organization. Instead, Mr. Biden named Anthony Fauci, MD, the director of the National Institute for Allergy and Infectious Diseases, as the head of a delegation to participate in the WHO executive board meeting that is being held this week.
Mr. Biden also signed an executive order creating the position of COVID-19 response coordinator, which will report directly to the president and be responsible for coordinating all elements of the COVID-19 response across government, including the production and distribution of vaccines and medical supplies.
The newly inaugurated president also intends to restore the National Security Council’s Directorate for Global Health Security and Biodefense, which will aid in the response to the pandemic, his transition team said.
The American Medical Association was among the first to commend the first-day actions.
“Defeating COVID-19 requires bold, coordinated federal leadership and strong adherence to the public health steps we know stop the spread of this virus – wearing masks, practicing physical distancing, and washing hands,” said AMA President Susan R. Bailey, MD in a news release. “We are pleased by the Biden administration’s steps today, including universal mask wearing within federal jurisdictions, providing federal leadership for COVID-19 response, and reengaging with the World Health Organization. Taking these actions on day 1 of the administration sends the right message – that our nation is laser focused on stopping the ravages of COVID-19.”
A version of this article first appeared on Medscape.com.
President Joe Biden kicked off his new administration Jan. 20 with an immediate focus on attempts to stop the spread of COVID-19, including closer coordination with other nations.
Mr. Biden signed 17 executive orders, memoranda, and directives addressing not only the pandemic but also economic concerns, climate change, and racial inequity.
At the top of the list of actions was what his transition team called a “100 Days Masking Challenge.” Mr. Biden issued an executive order requiring masks and physical distancing in all federal buildings, on all federal lands, and by federal employees and contractors.
The president also halted the Trump administration’s process of withdrawing from the World Health Organization. Instead, Mr. Biden named Anthony Fauci, MD, the director of the National Institute for Allergy and Infectious Diseases, as the head of a delegation to participate in the WHO executive board meeting that is being held this week.
Mr. Biden also signed an executive order creating the position of COVID-19 response coordinator, which will report directly to the president and be responsible for coordinating all elements of the COVID-19 response across government, including the production and distribution of vaccines and medical supplies.
The newly inaugurated president also intends to restore the National Security Council’s Directorate for Global Health Security and Biodefense, which will aid in the response to the pandemic, his transition team said.
The American Medical Association was among the first to commend the first-day actions.
“Defeating COVID-19 requires bold, coordinated federal leadership and strong adherence to the public health steps we know stop the spread of this virus – wearing masks, practicing physical distancing, and washing hands,” said AMA President Susan R. Bailey, MD in a news release. “We are pleased by the Biden administration’s steps today, including universal mask wearing within federal jurisdictions, providing federal leadership for COVID-19 response, and reengaging with the World Health Organization. Taking these actions on day 1 of the administration sends the right message – that our nation is laser focused on stopping the ravages of COVID-19.”
A version of this article first appeared on Medscape.com.
COVID-19 may damage blood vessels in the brain
Until now, the neurological manifestations of COVID-19 have been believed to be a result of direct damage to nerve cells. However, a new study suggests that the virus might actually damage the brain’s small blood vessels rather than nerve cells themselves.
The findings add further weight to previous research into neurological complications from COVID-19, according to Anna Cervantes, MD. Dr. Cervantes is assistant professor of neurology at the Boston University and has been studying the neurological effects of COVID-19, though she was not involved in this study. “I can tell from my personal experience, and things we’ve published on and the literature that’s out there – there are patients that are having complications like stroke that aren’t even critically ill from COVID. We’re seeing that not in just the acute setting, but also in a delayed fashion. Even though most of the coagulopathy is largely venous and probably microvascular, this does affect the brain through a myriad of ways,” Dr. Cervantes said.
The research was published online Jan. 12 in the New England Journal of Medicine. Myoung‑Hwa Lee, PhD, was the lead author.
The study included high resolution magnetic resonance imaging and histopathological examination of 13 individuals with a median age of 50 years. Among 10 patients with brain alterations, the researchers conducted further studies in 5 individuals using multiplex fluorescence imaging and chromogenic immunostaining in all 10.
The team conducted conventional histopathology on the brains of 18 individuals. Fourteen had a history of chronic illness, including diabetes, and hypertension, and 11 had died unexpectedly or been found dead. Magnetic resonance microscopy revealed punctuate hypo-intensities in nine subjects, indicating microvascular injury and fibrinogen leakage. Histopathology using fluorescence imaging showed the same features. Collagen IV immunostaining showed thinning of the basal lamina of the endothelial cells in five patients. Ten patients had congested blood vessels and surrounding fibrinogen leakage, but comparatively intact vasculature. The researchers interpreted linear hypo-intensities as micro-hemorrhages.
The researchers found little perivascular inflammation, and no vascular occlusion. Thirteen subjects had perivascular-activated microglia, macrophage infiltrates, and hypertrophic astrocytes. Eight had CD3+ and CD8+ T cells in the perivascular spaces and in lumens next to endothelial cells, which could help explain vascular injury.
The researchers found no evidence of the SARS-CoV-2 virus itself, despite efforts using polymerase chain reaction with multiple primer sets, RNA sequencing within the brain, or RNA in situ hybridization and immunostaining. Subjects may have cleared the virus by the time they died, or viral copy numbers could have been below the detection limit of the assays.
The researchers also obtained a convenience sample of subjects who had died from COVID-19. Magnetic resonance microscopy, histopathology, and immunohistochemical analysis of sections revealed microvascular injury in the brain and olfactory bulb, despite no evidence of viral infection. The authors stressed that they could not draw conclusions about the neurological features of COVID-19 because of a lack of clinical information.
Dr. Cervantes noted that limitation: “We’re seeing a lot of patients with encephalopathy or alterations in their mental status. A lot of things can cause that, and some are common in patients who are critically ill, like medications and metabolic derangement.”
Still, the findings could help to inform future medical management. “There’s going to be a large number of patients who don’t have really bad pulmonary disease but still may have encephalopathy. So if there is small vessel involvement because of inflammation that we might not necessarily catch in a lumbar puncture or routine imaging, there’s still somebody we can make better (using) steroids. Having more information on what’s happening on a pathophysiologic level and on pathology is really helpful.”
The study was supported by internal funds from the National Institute of Neurological Disorders and Stroke. Dr. Cervantes has no relevant financial disclosures.
Until now, the neurological manifestations of COVID-19 have been believed to be a result of direct damage to nerve cells. However, a new study suggests that the virus might actually damage the brain’s small blood vessels rather than nerve cells themselves.
The findings add further weight to previous research into neurological complications from COVID-19, according to Anna Cervantes, MD. Dr. Cervantes is assistant professor of neurology at the Boston University and has been studying the neurological effects of COVID-19, though she was not involved in this study. “I can tell from my personal experience, and things we’ve published on and the literature that’s out there – there are patients that are having complications like stroke that aren’t even critically ill from COVID. We’re seeing that not in just the acute setting, but also in a delayed fashion. Even though most of the coagulopathy is largely venous and probably microvascular, this does affect the brain through a myriad of ways,” Dr. Cervantes said.
The research was published online Jan. 12 in the New England Journal of Medicine. Myoung‑Hwa Lee, PhD, was the lead author.
The study included high resolution magnetic resonance imaging and histopathological examination of 13 individuals with a median age of 50 years. Among 10 patients with brain alterations, the researchers conducted further studies in 5 individuals using multiplex fluorescence imaging and chromogenic immunostaining in all 10.
The team conducted conventional histopathology on the brains of 18 individuals. Fourteen had a history of chronic illness, including diabetes, and hypertension, and 11 had died unexpectedly or been found dead. Magnetic resonance microscopy revealed punctuate hypo-intensities in nine subjects, indicating microvascular injury and fibrinogen leakage. Histopathology using fluorescence imaging showed the same features. Collagen IV immunostaining showed thinning of the basal lamina of the endothelial cells in five patients. Ten patients had congested blood vessels and surrounding fibrinogen leakage, but comparatively intact vasculature. The researchers interpreted linear hypo-intensities as micro-hemorrhages.
The researchers found little perivascular inflammation, and no vascular occlusion. Thirteen subjects had perivascular-activated microglia, macrophage infiltrates, and hypertrophic astrocytes. Eight had CD3+ and CD8+ T cells in the perivascular spaces and in lumens next to endothelial cells, which could help explain vascular injury.
The researchers found no evidence of the SARS-CoV-2 virus itself, despite efforts using polymerase chain reaction with multiple primer sets, RNA sequencing within the brain, or RNA in situ hybridization and immunostaining. Subjects may have cleared the virus by the time they died, or viral copy numbers could have been below the detection limit of the assays.
The researchers also obtained a convenience sample of subjects who had died from COVID-19. Magnetic resonance microscopy, histopathology, and immunohistochemical analysis of sections revealed microvascular injury in the brain and olfactory bulb, despite no evidence of viral infection. The authors stressed that they could not draw conclusions about the neurological features of COVID-19 because of a lack of clinical information.
Dr. Cervantes noted that limitation: “We’re seeing a lot of patients with encephalopathy or alterations in their mental status. A lot of things can cause that, and some are common in patients who are critically ill, like medications and metabolic derangement.”
Still, the findings could help to inform future medical management. “There’s going to be a large number of patients who don’t have really bad pulmonary disease but still may have encephalopathy. So if there is small vessel involvement because of inflammation that we might not necessarily catch in a lumbar puncture or routine imaging, there’s still somebody we can make better (using) steroids. Having more information on what’s happening on a pathophysiologic level and on pathology is really helpful.”
The study was supported by internal funds from the National Institute of Neurological Disorders and Stroke. Dr. Cervantes has no relevant financial disclosures.
Until now, the neurological manifestations of COVID-19 have been believed to be a result of direct damage to nerve cells. However, a new study suggests that the virus might actually damage the brain’s small blood vessels rather than nerve cells themselves.
The findings add further weight to previous research into neurological complications from COVID-19, according to Anna Cervantes, MD. Dr. Cervantes is assistant professor of neurology at the Boston University and has been studying the neurological effects of COVID-19, though she was not involved in this study. “I can tell from my personal experience, and things we’ve published on and the literature that’s out there – there are patients that are having complications like stroke that aren’t even critically ill from COVID. We’re seeing that not in just the acute setting, but also in a delayed fashion. Even though most of the coagulopathy is largely venous and probably microvascular, this does affect the brain through a myriad of ways,” Dr. Cervantes said.
The research was published online Jan. 12 in the New England Journal of Medicine. Myoung‑Hwa Lee, PhD, was the lead author.
The study included high resolution magnetic resonance imaging and histopathological examination of 13 individuals with a median age of 50 years. Among 10 patients with brain alterations, the researchers conducted further studies in 5 individuals using multiplex fluorescence imaging and chromogenic immunostaining in all 10.
The team conducted conventional histopathology on the brains of 18 individuals. Fourteen had a history of chronic illness, including diabetes, and hypertension, and 11 had died unexpectedly or been found dead. Magnetic resonance microscopy revealed punctuate hypo-intensities in nine subjects, indicating microvascular injury and fibrinogen leakage. Histopathology using fluorescence imaging showed the same features. Collagen IV immunostaining showed thinning of the basal lamina of the endothelial cells in five patients. Ten patients had congested blood vessels and surrounding fibrinogen leakage, but comparatively intact vasculature. The researchers interpreted linear hypo-intensities as micro-hemorrhages.
The researchers found little perivascular inflammation, and no vascular occlusion. Thirteen subjects had perivascular-activated microglia, macrophage infiltrates, and hypertrophic astrocytes. Eight had CD3+ and CD8+ T cells in the perivascular spaces and in lumens next to endothelial cells, which could help explain vascular injury.
The researchers found no evidence of the SARS-CoV-2 virus itself, despite efforts using polymerase chain reaction with multiple primer sets, RNA sequencing within the brain, or RNA in situ hybridization and immunostaining. Subjects may have cleared the virus by the time they died, or viral copy numbers could have been below the detection limit of the assays.
The researchers also obtained a convenience sample of subjects who had died from COVID-19. Magnetic resonance microscopy, histopathology, and immunohistochemical analysis of sections revealed microvascular injury in the brain and olfactory bulb, despite no evidence of viral infection. The authors stressed that they could not draw conclusions about the neurological features of COVID-19 because of a lack of clinical information.
Dr. Cervantes noted that limitation: “We’re seeing a lot of patients with encephalopathy or alterations in their mental status. A lot of things can cause that, and some are common in patients who are critically ill, like medications and metabolic derangement.”
Still, the findings could help to inform future medical management. “There’s going to be a large number of patients who don’t have really bad pulmonary disease but still may have encephalopathy. So if there is small vessel involvement because of inflammation that we might not necessarily catch in a lumbar puncture or routine imaging, there’s still somebody we can make better (using) steroids. Having more information on what’s happening on a pathophysiologic level and on pathology is really helpful.”
The study was supported by internal funds from the National Institute of Neurological Disorders and Stroke. Dr. Cervantes has no relevant financial disclosures.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Patients fend for themselves to access highly touted COVID antibody treatments
By the time he tested positive for COVID-19 on Jan. 12, Gary Herritz was feeling pretty sick. He suspects he was infected a week earlier, during a medical appointment in which he saw health workers who were wearing masks beneath their noses or who had removed them entirely.
His scratchy throat had turned to a dry cough, headache, joint pain, and fever – all warning signs to Mr. Herritz, who underwent liver transplant surgery in 2012, followed by a rejection scare in 2018. He knew his compromised immune system left him especially vulnerable to a potentially deadly case of COVID.
“The thing with transplant patients is we can crash in a heartbeat,” said Mr. Herritz, 39. “The outcome for transplant patients [with COVID] is not good.”
On Twitter, Mr. Herritz had read about monoclonal antibody therapy, the treatment famously given to President Donald Trump and other high-profile politicians and authorized by the Food and Drug Administration for emergency use in high-risk COVID patients. But as his symptoms worsened, Mr. Herritz found himself very much on his own as he scrambled for access.
His primary care doctor wasn’t sure he qualified for treatment. His transplant team in Wisconsin, where he’d had the liver surgery, wasn’t calling back. No one was sure exactly where he should go to get it. From bed in Pascagoula, Miss., he spent 2 days punching in phone numbers, reaching out to health officials in four states, before he finally landed an appointment to receive a treatment aimed at keeping patients like him out of the hospital – and, perhaps, the morgue.
“I am not rich, I am not special, I am not a political figure,” Mr. Herritz, a former community service officer, wrote on Twitter. “I just called until someone would listen.”
Months after Mr. Trump emphatically credited an experimental antibody therapy for his quick recovery from covid and even as drugmakers ramp up supplies, only a trickle of the product has found its way into regular people. While hundreds of thousands of vials sit unused, sick patients who, research indicates, could benefit from early treatment – available for free – have largely been fending for themselves.
Federal officials have allocated more than 785,000 doses of two antibody treatments authorized for emergency use during the pandemic, and more than 550,000 doses have been delivered to sites across the nation. The federal government has contracted for nearly 2.5 million doses of the products from drugmakers Eli Lilly and Regeneron Pharmaceuticals at a cost of more than $4.4 billion.
So far, however, only about 30% of the available doses have been administered to patients, U.S. Department of Health & Human Services officials said.
Scores of high-risk COVID patients who are eligible remain unaware or have not been offered the option. Research has shown the therapy is most effective if given early in the illness, within 10 days of a positive COVID test. But many would-be recipients have missed this crucial window because of a patchwork system in the United States that can delay testing and diagnosis.
“The bottleneck here in the funnel is administration, not availability of the product,” said Dr. Janet Woodcock, a veteran FDA official in charge of therapeutics for the federal Operation Warp Speed effort.
Among the daunting hurdles: Until this week, there has been no nationwide system to tell people where they could obtain the drugs, which are delivered through IV infusions that require hours to administer and monitor. Finding space to keep COVID-infected patients separate from others has been difficult in some health centers slammed by the pandemic.
“The health care system is crashing,” Dr. Woodcock told reporters. “What we’ve heard around the country is the No. 1 barrier is staffing.”
At the same time, many hospitals have refused to offer the therapy because doctors were unimpressed with the research federal officials used to justify its use.
Monoclonal antibodies are lab-produced molecules that act as substitutes for the body’s own antibodies that fight infection. The COVID treatments are designed to block the SARS-CoV-2 virus that causes infection from attaching to and entering human cells. Such treatments are usually prohibitively expensive, but for the time being the federal government is footing the bulk of the bill, though patients likely will be charged administrative fees.
Nationwide, nearly 4,000 sites offer the infusion therapies. But for patients and families of people most at risk – those 65 and older or with underlying health conditions – finding the sites and gaining access has been almost impossible, said Brian Nyquist, chief executive officer of the National Infusion Center Association, which is tracking supplies of the antibody products. Like Mr. Herritz, many seeking information about monoclonals find themselves on a lone crusade.
“If they’re not hammering the phones and advocating for access for their loved ones, others often won’t,” he said. “Tenacity is critical.”
Regeneron officials said they’re fielding calls about COVID treatments daily to the company’s medical information line. More than 3,500 people have flooded Eli Lilly’s COVID hotline with questions about access.
As of this week, all states are required to list on a federal locator map sites that have received the monoclonal antibody products, HHS officials said. The updated map shows wide distribution, but a listing doesn’t guarantee availability or access; patients still need to check. It’s best to confer with a primary care provider before reaching out to the centers. For best results, treatment should occur as soon as possible after a positive COVID test.
Some health systems have refused to offer the monoclonal antibody therapies because of doubts about the data used to authorize them. Early studies suggested that Lilly’s therapy, bamlanivimab, reduced the need for hospitalization or emergency treatment in outpatient COVID cases by about 70%, while Regeneron’s antibody cocktail of casirivimab plus imdevimab reduced the need by about 50%.
But those studies were small, just a few hundred subjects, and the results were limited. “A lot of doctors, actually, they’re not impressed with the data,” said Dr. Daniel Griffin, an infectious disease expert at Columbia University who cohosts the podcast “This Week in Virology.” “There really is still that question of, ‘Does this stuff really work?’ ”
As more patients are treated, however, there’s growing evidence that the therapies can keep high-risk patients out of the hospital, not only easing their recovery but also decreasing the burden on health systems struggling with record numbers of patients.
Dr. Raymund Razonable, an infectious disease expert at the Mayo Clinic in Minnesota, said he has treated more than 2,500 COVID patients with monoclonal antibody therapy with promising results. “It’s looking good,” he said, declining to provide details because they’re embargoed for publication. “We are seeing reductions in hospitalizations; we’re seeing reductions in ICU care; we’re also seeing reductions in mortality.”
Banking on observations from Mayo experts and others, federal officials have been pushing for wider use of antibody therapies. HHS officials have partnered with hospitals in three hard-hit states – California, Arizona, and Nevada – to set up infusion centers that are treating dozens of COVID patients each day.
One of those sites went up in late December at El Centro Regional Medical Center in California’s Imperial County, an impoverished farming region on the state’s southern border that has recorded among the highest COVID infection rates in the state. For months, the medical center strained to absorb the overwhelming influx of patients, but chief executive Dr. Adolphe Edward said a new walk-up infusion site has already put a dent in the COVID load.
More than 130 people have been treated, all patients who were able to get the 2-hour infusions and then recuperate at home. “If those folks would not have had the treatment, they would have come through the emergency department and we would have had to admit the lion’s share of them,” he said.
It’s important to make sure people in high-risk groups know to seek out the therapy and to get it early, Dr. Edward said. He and his staff have been working with area doctors’ offices and nonprofit groups and relying on word of mouth.
“On multiple levels, we’re saying, ‘If you’ve tested positive for the virus, come and let us see if you are eligible,’ ” Dr. Edward said.
Greater awareness is a goal of the HHS effort, said Dr. John Redd, chief medical officer for the assistant secretary for preparedness and response. “These antibodies are meant for everyone,” he said. “Everyone across the country should have equal access to these products.”
For now, patients like Mr. Herritz, the Mississippi liver transplant recipient, say reality is falling well short of that goal. If he hadn’t continued to call in search of a referral, he wouldn’t have been treated. And without the therapy, Mr. Herritz believes, he was just days away from hospitalization.
“I think it’s horrible that if I didn’t have Twitter, I wouldn’t know anything about this,” he said. “I think about all the people who have died not knowing this was an option for high-risk individuals.”
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
By the time he tested positive for COVID-19 on Jan. 12, Gary Herritz was feeling pretty sick. He suspects he was infected a week earlier, during a medical appointment in which he saw health workers who were wearing masks beneath their noses or who had removed them entirely.
His scratchy throat had turned to a dry cough, headache, joint pain, and fever – all warning signs to Mr. Herritz, who underwent liver transplant surgery in 2012, followed by a rejection scare in 2018. He knew his compromised immune system left him especially vulnerable to a potentially deadly case of COVID.
“The thing with transplant patients is we can crash in a heartbeat,” said Mr. Herritz, 39. “The outcome for transplant patients [with COVID] is not good.”
On Twitter, Mr. Herritz had read about monoclonal antibody therapy, the treatment famously given to President Donald Trump and other high-profile politicians and authorized by the Food and Drug Administration for emergency use in high-risk COVID patients. But as his symptoms worsened, Mr. Herritz found himself very much on his own as he scrambled for access.
His primary care doctor wasn’t sure he qualified for treatment. His transplant team in Wisconsin, where he’d had the liver surgery, wasn’t calling back. No one was sure exactly where he should go to get it. From bed in Pascagoula, Miss., he spent 2 days punching in phone numbers, reaching out to health officials in four states, before he finally landed an appointment to receive a treatment aimed at keeping patients like him out of the hospital – and, perhaps, the morgue.
“I am not rich, I am not special, I am not a political figure,” Mr. Herritz, a former community service officer, wrote on Twitter. “I just called until someone would listen.”
Months after Mr. Trump emphatically credited an experimental antibody therapy for his quick recovery from covid and even as drugmakers ramp up supplies, only a trickle of the product has found its way into regular people. While hundreds of thousands of vials sit unused, sick patients who, research indicates, could benefit from early treatment – available for free – have largely been fending for themselves.
Federal officials have allocated more than 785,000 doses of two antibody treatments authorized for emergency use during the pandemic, and more than 550,000 doses have been delivered to sites across the nation. The federal government has contracted for nearly 2.5 million doses of the products from drugmakers Eli Lilly and Regeneron Pharmaceuticals at a cost of more than $4.4 billion.
So far, however, only about 30% of the available doses have been administered to patients, U.S. Department of Health & Human Services officials said.
Scores of high-risk COVID patients who are eligible remain unaware or have not been offered the option. Research has shown the therapy is most effective if given early in the illness, within 10 days of a positive COVID test. But many would-be recipients have missed this crucial window because of a patchwork system in the United States that can delay testing and diagnosis.
“The bottleneck here in the funnel is administration, not availability of the product,” said Dr. Janet Woodcock, a veteran FDA official in charge of therapeutics for the federal Operation Warp Speed effort.
Among the daunting hurdles: Until this week, there has been no nationwide system to tell people where they could obtain the drugs, which are delivered through IV infusions that require hours to administer and monitor. Finding space to keep COVID-infected patients separate from others has been difficult in some health centers slammed by the pandemic.
“The health care system is crashing,” Dr. Woodcock told reporters. “What we’ve heard around the country is the No. 1 barrier is staffing.”
At the same time, many hospitals have refused to offer the therapy because doctors were unimpressed with the research federal officials used to justify its use.
Monoclonal antibodies are lab-produced molecules that act as substitutes for the body’s own antibodies that fight infection. The COVID treatments are designed to block the SARS-CoV-2 virus that causes infection from attaching to and entering human cells. Such treatments are usually prohibitively expensive, but for the time being the federal government is footing the bulk of the bill, though patients likely will be charged administrative fees.
Nationwide, nearly 4,000 sites offer the infusion therapies. But for patients and families of people most at risk – those 65 and older or with underlying health conditions – finding the sites and gaining access has been almost impossible, said Brian Nyquist, chief executive officer of the National Infusion Center Association, which is tracking supplies of the antibody products. Like Mr. Herritz, many seeking information about monoclonals find themselves on a lone crusade.
“If they’re not hammering the phones and advocating for access for their loved ones, others often won’t,” he said. “Tenacity is critical.”
Regeneron officials said they’re fielding calls about COVID treatments daily to the company’s medical information line. More than 3,500 people have flooded Eli Lilly’s COVID hotline with questions about access.
As of this week, all states are required to list on a federal locator map sites that have received the monoclonal antibody products, HHS officials said. The updated map shows wide distribution, but a listing doesn’t guarantee availability or access; patients still need to check. It’s best to confer with a primary care provider before reaching out to the centers. For best results, treatment should occur as soon as possible after a positive COVID test.
Some health systems have refused to offer the monoclonal antibody therapies because of doubts about the data used to authorize them. Early studies suggested that Lilly’s therapy, bamlanivimab, reduced the need for hospitalization or emergency treatment in outpatient COVID cases by about 70%, while Regeneron’s antibody cocktail of casirivimab plus imdevimab reduced the need by about 50%.
But those studies were small, just a few hundred subjects, and the results were limited. “A lot of doctors, actually, they’re not impressed with the data,” said Dr. Daniel Griffin, an infectious disease expert at Columbia University who cohosts the podcast “This Week in Virology.” “There really is still that question of, ‘Does this stuff really work?’ ”
As more patients are treated, however, there’s growing evidence that the therapies can keep high-risk patients out of the hospital, not only easing their recovery but also decreasing the burden on health systems struggling with record numbers of patients.
Dr. Raymund Razonable, an infectious disease expert at the Mayo Clinic in Minnesota, said he has treated more than 2,500 COVID patients with monoclonal antibody therapy with promising results. “It’s looking good,” he said, declining to provide details because they’re embargoed for publication. “We are seeing reductions in hospitalizations; we’re seeing reductions in ICU care; we’re also seeing reductions in mortality.”
Banking on observations from Mayo experts and others, federal officials have been pushing for wider use of antibody therapies. HHS officials have partnered with hospitals in three hard-hit states – California, Arizona, and Nevada – to set up infusion centers that are treating dozens of COVID patients each day.
One of those sites went up in late December at El Centro Regional Medical Center in California’s Imperial County, an impoverished farming region on the state’s southern border that has recorded among the highest COVID infection rates in the state. For months, the medical center strained to absorb the overwhelming influx of patients, but chief executive Dr. Adolphe Edward said a new walk-up infusion site has already put a dent in the COVID load.
More than 130 people have been treated, all patients who were able to get the 2-hour infusions and then recuperate at home. “If those folks would not have had the treatment, they would have come through the emergency department and we would have had to admit the lion’s share of them,” he said.
It’s important to make sure people in high-risk groups know to seek out the therapy and to get it early, Dr. Edward said. He and his staff have been working with area doctors’ offices and nonprofit groups and relying on word of mouth.
“On multiple levels, we’re saying, ‘If you’ve tested positive for the virus, come and let us see if you are eligible,’ ” Dr. Edward said.
Greater awareness is a goal of the HHS effort, said Dr. John Redd, chief medical officer for the assistant secretary for preparedness and response. “These antibodies are meant for everyone,” he said. “Everyone across the country should have equal access to these products.”
For now, patients like Mr. Herritz, the Mississippi liver transplant recipient, say reality is falling well short of that goal. If he hadn’t continued to call in search of a referral, he wouldn’t have been treated. And without the therapy, Mr. Herritz believes, he was just days away from hospitalization.
“I think it’s horrible that if I didn’t have Twitter, I wouldn’t know anything about this,” he said. “I think about all the people who have died not knowing this was an option for high-risk individuals.”
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
By the time he tested positive for COVID-19 on Jan. 12, Gary Herritz was feeling pretty sick. He suspects he was infected a week earlier, during a medical appointment in which he saw health workers who were wearing masks beneath their noses or who had removed them entirely.
His scratchy throat had turned to a dry cough, headache, joint pain, and fever – all warning signs to Mr. Herritz, who underwent liver transplant surgery in 2012, followed by a rejection scare in 2018. He knew his compromised immune system left him especially vulnerable to a potentially deadly case of COVID.
“The thing with transplant patients is we can crash in a heartbeat,” said Mr. Herritz, 39. “The outcome for transplant patients [with COVID] is not good.”
On Twitter, Mr. Herritz had read about monoclonal antibody therapy, the treatment famously given to President Donald Trump and other high-profile politicians and authorized by the Food and Drug Administration for emergency use in high-risk COVID patients. But as his symptoms worsened, Mr. Herritz found himself very much on his own as he scrambled for access.
His primary care doctor wasn’t sure he qualified for treatment. His transplant team in Wisconsin, where he’d had the liver surgery, wasn’t calling back. No one was sure exactly where he should go to get it. From bed in Pascagoula, Miss., he spent 2 days punching in phone numbers, reaching out to health officials in four states, before he finally landed an appointment to receive a treatment aimed at keeping patients like him out of the hospital – and, perhaps, the morgue.
“I am not rich, I am not special, I am not a political figure,” Mr. Herritz, a former community service officer, wrote on Twitter. “I just called until someone would listen.”
Months after Mr. Trump emphatically credited an experimental antibody therapy for his quick recovery from covid and even as drugmakers ramp up supplies, only a trickle of the product has found its way into regular people. While hundreds of thousands of vials sit unused, sick patients who, research indicates, could benefit from early treatment – available for free – have largely been fending for themselves.
Federal officials have allocated more than 785,000 doses of two antibody treatments authorized for emergency use during the pandemic, and more than 550,000 doses have been delivered to sites across the nation. The federal government has contracted for nearly 2.5 million doses of the products from drugmakers Eli Lilly and Regeneron Pharmaceuticals at a cost of more than $4.4 billion.
So far, however, only about 30% of the available doses have been administered to patients, U.S. Department of Health & Human Services officials said.
Scores of high-risk COVID patients who are eligible remain unaware or have not been offered the option. Research has shown the therapy is most effective if given early in the illness, within 10 days of a positive COVID test. But many would-be recipients have missed this crucial window because of a patchwork system in the United States that can delay testing and diagnosis.
“The bottleneck here in the funnel is administration, not availability of the product,” said Dr. Janet Woodcock, a veteran FDA official in charge of therapeutics for the federal Operation Warp Speed effort.
Among the daunting hurdles: Until this week, there has been no nationwide system to tell people where they could obtain the drugs, which are delivered through IV infusions that require hours to administer and monitor. Finding space to keep COVID-infected patients separate from others has been difficult in some health centers slammed by the pandemic.
“The health care system is crashing,” Dr. Woodcock told reporters. “What we’ve heard around the country is the No. 1 barrier is staffing.”
At the same time, many hospitals have refused to offer the therapy because doctors were unimpressed with the research federal officials used to justify its use.
Monoclonal antibodies are lab-produced molecules that act as substitutes for the body’s own antibodies that fight infection. The COVID treatments are designed to block the SARS-CoV-2 virus that causes infection from attaching to and entering human cells. Such treatments are usually prohibitively expensive, but for the time being the federal government is footing the bulk of the bill, though patients likely will be charged administrative fees.
Nationwide, nearly 4,000 sites offer the infusion therapies. But for patients and families of people most at risk – those 65 and older or with underlying health conditions – finding the sites and gaining access has been almost impossible, said Brian Nyquist, chief executive officer of the National Infusion Center Association, which is tracking supplies of the antibody products. Like Mr. Herritz, many seeking information about monoclonals find themselves on a lone crusade.
“If they’re not hammering the phones and advocating for access for their loved ones, others often won’t,” he said. “Tenacity is critical.”
Regeneron officials said they’re fielding calls about COVID treatments daily to the company’s medical information line. More than 3,500 people have flooded Eli Lilly’s COVID hotline with questions about access.
As of this week, all states are required to list on a federal locator map sites that have received the monoclonal antibody products, HHS officials said. The updated map shows wide distribution, but a listing doesn’t guarantee availability or access; patients still need to check. It’s best to confer with a primary care provider before reaching out to the centers. For best results, treatment should occur as soon as possible after a positive COVID test.
Some health systems have refused to offer the monoclonal antibody therapies because of doubts about the data used to authorize them. Early studies suggested that Lilly’s therapy, bamlanivimab, reduced the need for hospitalization or emergency treatment in outpatient COVID cases by about 70%, while Regeneron’s antibody cocktail of casirivimab plus imdevimab reduced the need by about 50%.
But those studies were small, just a few hundred subjects, and the results were limited. “A lot of doctors, actually, they’re not impressed with the data,” said Dr. Daniel Griffin, an infectious disease expert at Columbia University who cohosts the podcast “This Week in Virology.” “There really is still that question of, ‘Does this stuff really work?’ ”
As more patients are treated, however, there’s growing evidence that the therapies can keep high-risk patients out of the hospital, not only easing their recovery but also decreasing the burden on health systems struggling with record numbers of patients.
Dr. Raymund Razonable, an infectious disease expert at the Mayo Clinic in Minnesota, said he has treated more than 2,500 COVID patients with monoclonal antibody therapy with promising results. “It’s looking good,” he said, declining to provide details because they’re embargoed for publication. “We are seeing reductions in hospitalizations; we’re seeing reductions in ICU care; we’re also seeing reductions in mortality.”
Banking on observations from Mayo experts and others, federal officials have been pushing for wider use of antibody therapies. HHS officials have partnered with hospitals in three hard-hit states – California, Arizona, and Nevada – to set up infusion centers that are treating dozens of COVID patients each day.
One of those sites went up in late December at El Centro Regional Medical Center in California’s Imperial County, an impoverished farming region on the state’s southern border that has recorded among the highest COVID infection rates in the state. For months, the medical center strained to absorb the overwhelming influx of patients, but chief executive Dr. Adolphe Edward said a new walk-up infusion site has already put a dent in the COVID load.
More than 130 people have been treated, all patients who were able to get the 2-hour infusions and then recuperate at home. “If those folks would not have had the treatment, they would have come through the emergency department and we would have had to admit the lion’s share of them,” he said.
It’s important to make sure people in high-risk groups know to seek out the therapy and to get it early, Dr. Edward said. He and his staff have been working with area doctors’ offices and nonprofit groups and relying on word of mouth.
“On multiple levels, we’re saying, ‘If you’ve tested positive for the virus, come and let us see if you are eligible,’ ” Dr. Edward said.
Greater awareness is a goal of the HHS effort, said Dr. John Redd, chief medical officer for the assistant secretary for preparedness and response. “These antibodies are meant for everyone,” he said. “Everyone across the country should have equal access to these products.”
For now, patients like Mr. Herritz, the Mississippi liver transplant recipient, say reality is falling well short of that goal. If he hadn’t continued to call in search of a referral, he wouldn’t have been treated. And without the therapy, Mr. Herritz believes, he was just days away from hospitalization.
“I think it’s horrible that if I didn’t have Twitter, I wouldn’t know anything about this,” he said. “I think about all the people who have died not knowing this was an option for high-risk individuals.”
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.