User login
In Case You Missed It: COVID
COVID bivalent booster better vs. recent Omicron subvariants: Pfizer
the company reported on Nov. 4, supporting calls by public health officials for eligible people to get this booster before a potential COVID-19 surge this winter.
The company’s ongoing phase 2/3 study of their Omicron BA.4 and BA.5 bivalent – which targets both the virus’ original strain and the two subvariants – shows that the vaccine offered the strongest protection in people older than 55 years.
One month after receiving a 30-mcg booster with the bivalent vaccine, those older than 55 had four times more neutralizing antibodies against these Omicron subvariants, compared with people who received the original monovalent vaccine as a booster in the study.
Researchers compared the geometric mean titer (GMT) levels of these antibodies in three groups before and 1 month after boosting. The 36 people older than 55 years in the released study findings had an GMT level of 896 with the bivalent booster, a level 13 times higher than before this immunization.
For the 38 adults ages 18-55 in the study, the GMT level increased to 606 at 1 month after the bivalent booster, an increase of almost 10-fold, compared with baseline. In a comparator group of 40 people receiving the original vaccine as a fourth dose, the GMT level was 236, or threefold higher than before their booster shot.
The newly released data is “very encouraging and consistent now with three studies all showing a substantial 3-4 fold increased level of neutralizing antibodies versus BA.5 as compared with the original booster,” said Eric Topol, MD, director of the Scripps Research Translational Institute in La Jolla, Calif., and editor-in-chief of Medscape Medical News.
Pfizer and BioNTech announced the updated findings in a Nov. 4 press release.
A booster dose of the BA.4/BA.5-adapted bivalent vaccine is authorized for emergency use by the Food and Drug Administration for ages 5 years and older. The safety and tolerability profile of the Pfizer/BioNTech bivalent booster remains favorable and similar to the original COVID-19 vaccine, the company reported.
Until recently, the BA.5 Omicron variant was the dominant strain in the United States, but is now getting elbowed out by the subvariants BQ.1.1, BQ.1, and BA.4.6, which together make up almost 45% of the circulating virus.
Some skepticism
“It is important to note that these data are press-release level, which does not allow a view of the data totality,” Hana El Sahly, MD, professor of molecular virology and microbiology, Baylor College of Medicine, Houston, said in an interview.
“For example, there may be significant differences between the groups, and the release mentions at least one difference that is of importance: the interval since the last vaccination which often affects the response to subsequent boosting,” she said.
Dr. El Sahly added that the findings are not surprising. “In the short term, a variant-specific vaccine produces a higher level of antibody against the variant in the vaccine than the vaccines based on the ancestral strains.”
More researcher results are warranted. “These data do not indicate that these differences between the two vaccines translate into a meaningful clinical benefit at a population level,” Dr. El Sahly said.
An uncertain winter ahead
“As we head into the holiday season, we hope these updated data will encourage people to seek out a COVID-19 bivalent booster as soon as they are eligible in order to maintain high levels of protection against the widely circulating Omicron BA.4 and BA.5 sublineages,” Albert Bourla, Pfizer chairman and CEO, stated in the release.
The updated data from the Pfizer/BioNTech study are “all the more reason to get a booster, with added protection also versus BQ.1.1, which will soon become dominant in the U.S.,” Dr. Topol predicted.
It is unclear when the next surge will happen, as COVID-19 does not always follow a seasonal pattern, at least not yet, Dr. El Sahly said. “Regardless, it is reasonable to recommend additional vaccine doses to immunocompromised and frail or older persons. More importantly, influenza vaccination and being up to date on pneumococcal vaccines are highly recommended as soon as feasible, given the early and intense flu season.”
A version of this article first appeared on Medscape.com.
the company reported on Nov. 4, supporting calls by public health officials for eligible people to get this booster before a potential COVID-19 surge this winter.
The company’s ongoing phase 2/3 study of their Omicron BA.4 and BA.5 bivalent – which targets both the virus’ original strain and the two subvariants – shows that the vaccine offered the strongest protection in people older than 55 years.
One month after receiving a 30-mcg booster with the bivalent vaccine, those older than 55 had four times more neutralizing antibodies against these Omicron subvariants, compared with people who received the original monovalent vaccine as a booster in the study.
Researchers compared the geometric mean titer (GMT) levels of these antibodies in three groups before and 1 month after boosting. The 36 people older than 55 years in the released study findings had an GMT level of 896 with the bivalent booster, a level 13 times higher than before this immunization.
For the 38 adults ages 18-55 in the study, the GMT level increased to 606 at 1 month after the bivalent booster, an increase of almost 10-fold, compared with baseline. In a comparator group of 40 people receiving the original vaccine as a fourth dose, the GMT level was 236, or threefold higher than before their booster shot.
The newly released data is “very encouraging and consistent now with three studies all showing a substantial 3-4 fold increased level of neutralizing antibodies versus BA.5 as compared with the original booster,” said Eric Topol, MD, director of the Scripps Research Translational Institute in La Jolla, Calif., and editor-in-chief of Medscape Medical News.
Pfizer and BioNTech announced the updated findings in a Nov. 4 press release.
A booster dose of the BA.4/BA.5-adapted bivalent vaccine is authorized for emergency use by the Food and Drug Administration for ages 5 years and older. The safety and tolerability profile of the Pfizer/BioNTech bivalent booster remains favorable and similar to the original COVID-19 vaccine, the company reported.
Until recently, the BA.5 Omicron variant was the dominant strain in the United States, but is now getting elbowed out by the subvariants BQ.1.1, BQ.1, and BA.4.6, which together make up almost 45% of the circulating virus.
Some skepticism
“It is important to note that these data are press-release level, which does not allow a view of the data totality,” Hana El Sahly, MD, professor of molecular virology and microbiology, Baylor College of Medicine, Houston, said in an interview.
“For example, there may be significant differences between the groups, and the release mentions at least one difference that is of importance: the interval since the last vaccination which often affects the response to subsequent boosting,” she said.
Dr. El Sahly added that the findings are not surprising. “In the short term, a variant-specific vaccine produces a higher level of antibody against the variant in the vaccine than the vaccines based on the ancestral strains.”
More researcher results are warranted. “These data do not indicate that these differences between the two vaccines translate into a meaningful clinical benefit at a population level,” Dr. El Sahly said.
An uncertain winter ahead
“As we head into the holiday season, we hope these updated data will encourage people to seek out a COVID-19 bivalent booster as soon as they are eligible in order to maintain high levels of protection against the widely circulating Omicron BA.4 and BA.5 sublineages,” Albert Bourla, Pfizer chairman and CEO, stated in the release.
The updated data from the Pfizer/BioNTech study are “all the more reason to get a booster, with added protection also versus BQ.1.1, which will soon become dominant in the U.S.,” Dr. Topol predicted.
It is unclear when the next surge will happen, as COVID-19 does not always follow a seasonal pattern, at least not yet, Dr. El Sahly said. “Regardless, it is reasonable to recommend additional vaccine doses to immunocompromised and frail or older persons. More importantly, influenza vaccination and being up to date on pneumococcal vaccines are highly recommended as soon as feasible, given the early and intense flu season.”
A version of this article first appeared on Medscape.com.
the company reported on Nov. 4, supporting calls by public health officials for eligible people to get this booster before a potential COVID-19 surge this winter.
The company’s ongoing phase 2/3 study of their Omicron BA.4 and BA.5 bivalent – which targets both the virus’ original strain and the two subvariants – shows that the vaccine offered the strongest protection in people older than 55 years.
One month after receiving a 30-mcg booster with the bivalent vaccine, those older than 55 had four times more neutralizing antibodies against these Omicron subvariants, compared with people who received the original monovalent vaccine as a booster in the study.
Researchers compared the geometric mean titer (GMT) levels of these antibodies in three groups before and 1 month after boosting. The 36 people older than 55 years in the released study findings had an GMT level of 896 with the bivalent booster, a level 13 times higher than before this immunization.
For the 38 adults ages 18-55 in the study, the GMT level increased to 606 at 1 month after the bivalent booster, an increase of almost 10-fold, compared with baseline. In a comparator group of 40 people receiving the original vaccine as a fourth dose, the GMT level was 236, or threefold higher than before their booster shot.
The newly released data is “very encouraging and consistent now with three studies all showing a substantial 3-4 fold increased level of neutralizing antibodies versus BA.5 as compared with the original booster,” said Eric Topol, MD, director of the Scripps Research Translational Institute in La Jolla, Calif., and editor-in-chief of Medscape Medical News.
Pfizer and BioNTech announced the updated findings in a Nov. 4 press release.
A booster dose of the BA.4/BA.5-adapted bivalent vaccine is authorized for emergency use by the Food and Drug Administration for ages 5 years and older. The safety and tolerability profile of the Pfizer/BioNTech bivalent booster remains favorable and similar to the original COVID-19 vaccine, the company reported.
Until recently, the BA.5 Omicron variant was the dominant strain in the United States, but is now getting elbowed out by the subvariants BQ.1.1, BQ.1, and BA.4.6, which together make up almost 45% of the circulating virus.
Some skepticism
“It is important to note that these data are press-release level, which does not allow a view of the data totality,” Hana El Sahly, MD, professor of molecular virology and microbiology, Baylor College of Medicine, Houston, said in an interview.
“For example, there may be significant differences between the groups, and the release mentions at least one difference that is of importance: the interval since the last vaccination which often affects the response to subsequent boosting,” she said.
Dr. El Sahly added that the findings are not surprising. “In the short term, a variant-specific vaccine produces a higher level of antibody against the variant in the vaccine than the vaccines based on the ancestral strains.”
More researcher results are warranted. “These data do not indicate that these differences between the two vaccines translate into a meaningful clinical benefit at a population level,” Dr. El Sahly said.
An uncertain winter ahead
“As we head into the holiday season, we hope these updated data will encourage people to seek out a COVID-19 bivalent booster as soon as they are eligible in order to maintain high levels of protection against the widely circulating Omicron BA.4 and BA.5 sublineages,” Albert Bourla, Pfizer chairman and CEO, stated in the release.
The updated data from the Pfizer/BioNTech study are “all the more reason to get a booster, with added protection also versus BQ.1.1, which will soon become dominant in the U.S.,” Dr. Topol predicted.
It is unclear when the next surge will happen, as COVID-19 does not always follow a seasonal pattern, at least not yet, Dr. El Sahly said. “Regardless, it is reasonable to recommend additional vaccine doses to immunocompromised and frail or older persons. More importantly, influenza vaccination and being up to date on pneumococcal vaccines are highly recommended as soon as feasible, given the early and intense flu season.”
A version of this article first appeared on Medscape.com.
Working while sick: Why doctors don’t stay home when ill
The reasons are likely as varied as, “you weren’t feeling bad enough to miss work,” “you couldn’t afford to miss pay,” “you had too many patients to see,” or “too much work to do.”
In Medscape’s Employed Physicians Report: Loving the Focus, Hating the Bureaucracy, 61% of physicians reported that they sometimes or often come to work sick. Only 2% of respondents said they never come to work unwell.
Medscape wanted to know more about how often you call in sick, how often you come to work feeling unwell, what symptoms you have, and the dogma of your workplace culture regarding sick days. Not to mention the brutal ethos that starts in medical school, in which calling in sick shows weakness or is unacceptable.
So, we polled 2,347 physicians in the United States and abroad and asked them about their sniffling, sneezing, cold, flu, and fever symptoms, and, of course, COVID. Results were split about 50-50 among male and female physicians. The poll ran from Sept. 28 through Oct. 11.
Coming to work sick
It’s no surprise that the majority of physicians who were polled (85%) have come to work sick in 2022. In the last prepandemic year (2019), about 70% came to work feeling sick one to five times, and 13% worked while sick six to ten times.
When asked about the symptoms that they’ve previously come to work with, 48% of U.S. physicians said multiple symptoms. They gave high marks for runny nose, cough, congestion, and sore throat. Only 27% have worked with a fever, 22% have worked with other symptoms, and 7% have worked with both strep throat and COVID.
“My workplace, especially in the COVID years, accommodates persons who honestly do not feel well enough to report. Sooner or later, everyone covers for someone else who has to be out,” says Kenneth Abbott, MD, an oncologist in Maryland.
The culture of working while sick
Why doctors come to work when they’re sick is complicated. The overwhelming majority of U.S. respondents cited professional obligations; 73% noted that they feel a professional obligation to their patients, and 72% feel a professional obligation to their co-workers. Half of the polled U.S. physicians said they didn’t feel bad enough to stay home, while 48% said they had too much work to do to stay home.
Some 45% said the expectation at their workplace is to come to work unless seriously ill; 43% had too many patients to see; and 18% didn’t think they were contagious when they headed to work sick. Unfortunately, 15% chose to work while sick because otherwise they would lose pay.
In light of these responses, it’s not surprising that 93% reported they’d seen other medical professionals working when sick.
“My schedule is almost always booked weeks in advance. If someone misses or has to cancel their appointment, they typically have 2-4 weeks to wait to get back in. If I was sick and a full day of patients (or God forbid more than a day) had to be canceled because I called in, it’s so much more work when I return,” says Caitlin Briggs, MD, a psychiatrist in Lexington, Ky.
Doctors’ workplace sick day policy
Most employees’ benefits allow at least a few sick days, but doctors who treat society’s ill patients don’t seem to stay home from work when they’re suffering. So, we asked physicians, official policy aside, whether they thought going to work sick was expected in their workplace. The majority (76%) said yes, while 24% said no.
“Unless I’m dying or extremely contagious, I usually work. At least now, I have the telehealth option. Not saying any of this is right, but it’s the reality we deal with and the choice we must make,” says Dr. Briggs.
Additionally, 58% of polled physicians said their workplace did not have a clearly defined policy against coming to work sick, while 20% said theirs did, and 22% weren’t sure.
“The first thing I heard on the subject as a medical student was that sick people come to the hospital, so if you’re sick, then you come to the hospital too ... to work. If you can’t work, then you will be admitted. Another aphorism was from Churchill, that ‘most of the world’s work is done by people who don’t feel very well,’ ” says Paul Andreason, MD, a psychiatrist in Bethesda, Md.
Working in the time of COVID
Working while ill during ordinary times is one thing, but what about working in the time of COVID? Has the pandemic changed the culture of coming to work sick because medical facilities, such as doctor’s offices and hospitals, don’t want their staff coming in when they have COVID?
Surprisingly, when we asked physicians whether the pandemic has made it more or less acceptable to come to work sick, only 61% thought COVID has made it less acceptable to work while sick, while 16% thought it made it more acceptable, and 23% said there’s no change.
“I draw the line at fevers/chills, feeling like you’ve just been run over, or significant enteritis,” says Dr. Abbott. “Also, if I have to take palliative meds that interfere with alertness, I’m not doing my patients any favors.”
While a minority of physicians may call in sick, most still suffer through their sneezing, coughing, chills, and fever while seeing patients as usual.
A version of this article first appeared on Medscape.com.
The reasons are likely as varied as, “you weren’t feeling bad enough to miss work,” “you couldn’t afford to miss pay,” “you had too many patients to see,” or “too much work to do.”
In Medscape’s Employed Physicians Report: Loving the Focus, Hating the Bureaucracy, 61% of physicians reported that they sometimes or often come to work sick. Only 2% of respondents said they never come to work unwell.
Medscape wanted to know more about how often you call in sick, how often you come to work feeling unwell, what symptoms you have, and the dogma of your workplace culture regarding sick days. Not to mention the brutal ethos that starts in medical school, in which calling in sick shows weakness or is unacceptable.
So, we polled 2,347 physicians in the United States and abroad and asked them about their sniffling, sneezing, cold, flu, and fever symptoms, and, of course, COVID. Results were split about 50-50 among male and female physicians. The poll ran from Sept. 28 through Oct. 11.
Coming to work sick
It’s no surprise that the majority of physicians who were polled (85%) have come to work sick in 2022. In the last prepandemic year (2019), about 70% came to work feeling sick one to five times, and 13% worked while sick six to ten times.
When asked about the symptoms that they’ve previously come to work with, 48% of U.S. physicians said multiple symptoms. They gave high marks for runny nose, cough, congestion, and sore throat. Only 27% have worked with a fever, 22% have worked with other symptoms, and 7% have worked with both strep throat and COVID.
“My workplace, especially in the COVID years, accommodates persons who honestly do not feel well enough to report. Sooner or later, everyone covers for someone else who has to be out,” says Kenneth Abbott, MD, an oncologist in Maryland.
The culture of working while sick
Why doctors come to work when they’re sick is complicated. The overwhelming majority of U.S. respondents cited professional obligations; 73% noted that they feel a professional obligation to their patients, and 72% feel a professional obligation to their co-workers. Half of the polled U.S. physicians said they didn’t feel bad enough to stay home, while 48% said they had too much work to do to stay home.
Some 45% said the expectation at their workplace is to come to work unless seriously ill; 43% had too many patients to see; and 18% didn’t think they were contagious when they headed to work sick. Unfortunately, 15% chose to work while sick because otherwise they would lose pay.
In light of these responses, it’s not surprising that 93% reported they’d seen other medical professionals working when sick.
“My schedule is almost always booked weeks in advance. If someone misses or has to cancel their appointment, they typically have 2-4 weeks to wait to get back in. If I was sick and a full day of patients (or God forbid more than a day) had to be canceled because I called in, it’s so much more work when I return,” says Caitlin Briggs, MD, a psychiatrist in Lexington, Ky.
Doctors’ workplace sick day policy
Most employees’ benefits allow at least a few sick days, but doctors who treat society’s ill patients don’t seem to stay home from work when they’re suffering. So, we asked physicians, official policy aside, whether they thought going to work sick was expected in their workplace. The majority (76%) said yes, while 24% said no.
“Unless I’m dying or extremely contagious, I usually work. At least now, I have the telehealth option. Not saying any of this is right, but it’s the reality we deal with and the choice we must make,” says Dr. Briggs.
Additionally, 58% of polled physicians said their workplace did not have a clearly defined policy against coming to work sick, while 20% said theirs did, and 22% weren’t sure.
“The first thing I heard on the subject as a medical student was that sick people come to the hospital, so if you’re sick, then you come to the hospital too ... to work. If you can’t work, then you will be admitted. Another aphorism was from Churchill, that ‘most of the world’s work is done by people who don’t feel very well,’ ” says Paul Andreason, MD, a psychiatrist in Bethesda, Md.
Working in the time of COVID
Working while ill during ordinary times is one thing, but what about working in the time of COVID? Has the pandemic changed the culture of coming to work sick because medical facilities, such as doctor’s offices and hospitals, don’t want their staff coming in when they have COVID?
Surprisingly, when we asked physicians whether the pandemic has made it more or less acceptable to come to work sick, only 61% thought COVID has made it less acceptable to work while sick, while 16% thought it made it more acceptable, and 23% said there’s no change.
“I draw the line at fevers/chills, feeling like you’ve just been run over, or significant enteritis,” says Dr. Abbott. “Also, if I have to take palliative meds that interfere with alertness, I’m not doing my patients any favors.”
While a minority of physicians may call in sick, most still suffer through their sneezing, coughing, chills, and fever while seeing patients as usual.
A version of this article first appeared on Medscape.com.
The reasons are likely as varied as, “you weren’t feeling bad enough to miss work,” “you couldn’t afford to miss pay,” “you had too many patients to see,” or “too much work to do.”
In Medscape’s Employed Physicians Report: Loving the Focus, Hating the Bureaucracy, 61% of physicians reported that they sometimes or often come to work sick. Only 2% of respondents said they never come to work unwell.
Medscape wanted to know more about how often you call in sick, how often you come to work feeling unwell, what symptoms you have, and the dogma of your workplace culture regarding sick days. Not to mention the brutal ethos that starts in medical school, in which calling in sick shows weakness or is unacceptable.
So, we polled 2,347 physicians in the United States and abroad and asked them about their sniffling, sneezing, cold, flu, and fever symptoms, and, of course, COVID. Results were split about 50-50 among male and female physicians. The poll ran from Sept. 28 through Oct. 11.
Coming to work sick
It’s no surprise that the majority of physicians who were polled (85%) have come to work sick in 2022. In the last prepandemic year (2019), about 70% came to work feeling sick one to five times, and 13% worked while sick six to ten times.
When asked about the symptoms that they’ve previously come to work with, 48% of U.S. physicians said multiple symptoms. They gave high marks for runny nose, cough, congestion, and sore throat. Only 27% have worked with a fever, 22% have worked with other symptoms, and 7% have worked with both strep throat and COVID.
“My workplace, especially in the COVID years, accommodates persons who honestly do not feel well enough to report. Sooner or later, everyone covers for someone else who has to be out,” says Kenneth Abbott, MD, an oncologist in Maryland.
The culture of working while sick
Why doctors come to work when they’re sick is complicated. The overwhelming majority of U.S. respondents cited professional obligations; 73% noted that they feel a professional obligation to their patients, and 72% feel a professional obligation to their co-workers. Half of the polled U.S. physicians said they didn’t feel bad enough to stay home, while 48% said they had too much work to do to stay home.
Some 45% said the expectation at their workplace is to come to work unless seriously ill; 43% had too many patients to see; and 18% didn’t think they were contagious when they headed to work sick. Unfortunately, 15% chose to work while sick because otherwise they would lose pay.
In light of these responses, it’s not surprising that 93% reported they’d seen other medical professionals working when sick.
“My schedule is almost always booked weeks in advance. If someone misses or has to cancel their appointment, they typically have 2-4 weeks to wait to get back in. If I was sick and a full day of patients (or God forbid more than a day) had to be canceled because I called in, it’s so much more work when I return,” says Caitlin Briggs, MD, a psychiatrist in Lexington, Ky.
Doctors’ workplace sick day policy
Most employees’ benefits allow at least a few sick days, but doctors who treat society’s ill patients don’t seem to stay home from work when they’re suffering. So, we asked physicians, official policy aside, whether they thought going to work sick was expected in their workplace. The majority (76%) said yes, while 24% said no.
“Unless I’m dying or extremely contagious, I usually work. At least now, I have the telehealth option. Not saying any of this is right, but it’s the reality we deal with and the choice we must make,” says Dr. Briggs.
Additionally, 58% of polled physicians said their workplace did not have a clearly defined policy against coming to work sick, while 20% said theirs did, and 22% weren’t sure.
“The first thing I heard on the subject as a medical student was that sick people come to the hospital, so if you’re sick, then you come to the hospital too ... to work. If you can’t work, then you will be admitted. Another aphorism was from Churchill, that ‘most of the world’s work is done by people who don’t feel very well,’ ” says Paul Andreason, MD, a psychiatrist in Bethesda, Md.
Working in the time of COVID
Working while ill during ordinary times is one thing, but what about working in the time of COVID? Has the pandemic changed the culture of coming to work sick because medical facilities, such as doctor’s offices and hospitals, don’t want their staff coming in when they have COVID?
Surprisingly, when we asked physicians whether the pandemic has made it more or less acceptable to come to work sick, only 61% thought COVID has made it less acceptable to work while sick, while 16% thought it made it more acceptable, and 23% said there’s no change.
“I draw the line at fevers/chills, feeling like you’ve just been run over, or significant enteritis,” says Dr. Abbott. “Also, if I have to take palliative meds that interfere with alertness, I’m not doing my patients any favors.”
While a minority of physicians may call in sick, most still suffer through their sneezing, coughing, chills, and fever while seeing patients as usual.
A version of this article first appeared on Medscape.com.
New research confirms recommendations on COVID-19 boosters in MS
, as currently recommended.
“We have shown that even MS patients whose B cells were depleted from circulation with ocrelizumab can mount immune responses to COVID-19 vaccines,” said lead study author Ilya Kister, MD, of NYU Langone’s Multiple Sclerosis Comprehensive Care Center in New York.
The findings were presented at the annual meeting of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS).
VIOLA study
The data stem from VIOLA, an ongoing prospective study of humoral and cellular immune responses to COVID-19 vaccines in 60 patients with MS receiving ocrelizumab at MS care centers at NYU Langone and the University of Colorado Denver.
The mean age of participants was 38 years, 73% were women, all had been taking ocrelizumab for a mean of 1.7 years, and 45% had had COVID-19 prior to vaccination.
The researchers examined antibody and cellular responses to the two-dose series of mRNA COVID-19 vaccine (80% received the Pfizer-BioNTech vaccine, 18% the Moderna vaccine, and 2% unknown) over 24 weeks. In addition, 57% of the participants received the third dose/booster.
Results showed that antibody and cellular responses to SARS-CoV-2 spike protein significantly increased after the two-dose mRNA COVID-19 vaccination, though antibody responses tended to peak between 4 and 12 weeks and declined thereafter. There was no significant decline in cellular responses at week 24.
“The third dose ‘booster’ again significantly increased antibody and cellular responses compared with the pre–third dose levels,” Dr. Kister said.
“Importantly, cellular responses remained elevated or even increased from 4 weeks to 12 weeks after third dose/booster. Overall, these data strongly support the need for a third dose in MS patients on ocrelizumab,” Dr. Kister added.
Participants with “hybrid immunity” (those who had been infected with SARS-CoV-2 and who had also been vaccinated for COVID) had markedly higher SARS-CoV-2–specific antibody and cellular responses than those of peers with vaccine-only immunity.
CDC recs
Looking ahead, Dr. Kister said the VIOLA investigators plan to present data on the durability of COVID-19 vaccines in ocrelizumab-treated patients up to 48 weeks after the third dose.
For immunocompromised patients, such as those taking ocrelizumab, the Centers for Disease Control and Prevention considers the third dose of mRNA vaccine not as a “booster” but as part of the regular vaccine series.
“In other words, all these patients should receive three doses as part of their ‘primary’ series,” Dr. Kister noted.
The CDC also recommends receiving the updated booster for COVID-19 that became available in September 2022 (the fourth dose of the vaccine).
“Our study did not evaluate the efficacy of this fourth dose; but based on our results, it is reasonable to suppose that the fourth dose would also lead to a further increase in immune defenses,” Dr. Kister said.
The VIOLA study is an investigator-initiated collaboration supported by F. Hoffmann-La Roche Ltd/Genentech Inc. Dr. Kister has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, as currently recommended.
“We have shown that even MS patients whose B cells were depleted from circulation with ocrelizumab can mount immune responses to COVID-19 vaccines,” said lead study author Ilya Kister, MD, of NYU Langone’s Multiple Sclerosis Comprehensive Care Center in New York.
The findings were presented at the annual meeting of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS).
VIOLA study
The data stem from VIOLA, an ongoing prospective study of humoral and cellular immune responses to COVID-19 vaccines in 60 patients with MS receiving ocrelizumab at MS care centers at NYU Langone and the University of Colorado Denver.
The mean age of participants was 38 years, 73% were women, all had been taking ocrelizumab for a mean of 1.7 years, and 45% had had COVID-19 prior to vaccination.
The researchers examined antibody and cellular responses to the two-dose series of mRNA COVID-19 vaccine (80% received the Pfizer-BioNTech vaccine, 18% the Moderna vaccine, and 2% unknown) over 24 weeks. In addition, 57% of the participants received the third dose/booster.
Results showed that antibody and cellular responses to SARS-CoV-2 spike protein significantly increased after the two-dose mRNA COVID-19 vaccination, though antibody responses tended to peak between 4 and 12 weeks and declined thereafter. There was no significant decline in cellular responses at week 24.
“The third dose ‘booster’ again significantly increased antibody and cellular responses compared with the pre–third dose levels,” Dr. Kister said.
“Importantly, cellular responses remained elevated or even increased from 4 weeks to 12 weeks after third dose/booster. Overall, these data strongly support the need for a third dose in MS patients on ocrelizumab,” Dr. Kister added.
Participants with “hybrid immunity” (those who had been infected with SARS-CoV-2 and who had also been vaccinated for COVID) had markedly higher SARS-CoV-2–specific antibody and cellular responses than those of peers with vaccine-only immunity.
CDC recs
Looking ahead, Dr. Kister said the VIOLA investigators plan to present data on the durability of COVID-19 vaccines in ocrelizumab-treated patients up to 48 weeks after the third dose.
For immunocompromised patients, such as those taking ocrelizumab, the Centers for Disease Control and Prevention considers the third dose of mRNA vaccine not as a “booster” but as part of the regular vaccine series.
“In other words, all these patients should receive three doses as part of their ‘primary’ series,” Dr. Kister noted.
The CDC also recommends receiving the updated booster for COVID-19 that became available in September 2022 (the fourth dose of the vaccine).
“Our study did not evaluate the efficacy of this fourth dose; but based on our results, it is reasonable to suppose that the fourth dose would also lead to a further increase in immune defenses,” Dr. Kister said.
The VIOLA study is an investigator-initiated collaboration supported by F. Hoffmann-La Roche Ltd/Genentech Inc. Dr. Kister has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, as currently recommended.
“We have shown that even MS patients whose B cells were depleted from circulation with ocrelizumab can mount immune responses to COVID-19 vaccines,” said lead study author Ilya Kister, MD, of NYU Langone’s Multiple Sclerosis Comprehensive Care Center in New York.
The findings were presented at the annual meeting of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS).
VIOLA study
The data stem from VIOLA, an ongoing prospective study of humoral and cellular immune responses to COVID-19 vaccines in 60 patients with MS receiving ocrelizumab at MS care centers at NYU Langone and the University of Colorado Denver.
The mean age of participants was 38 years, 73% were women, all had been taking ocrelizumab for a mean of 1.7 years, and 45% had had COVID-19 prior to vaccination.
The researchers examined antibody and cellular responses to the two-dose series of mRNA COVID-19 vaccine (80% received the Pfizer-BioNTech vaccine, 18% the Moderna vaccine, and 2% unknown) over 24 weeks. In addition, 57% of the participants received the third dose/booster.
Results showed that antibody and cellular responses to SARS-CoV-2 spike protein significantly increased after the two-dose mRNA COVID-19 vaccination, though antibody responses tended to peak between 4 and 12 weeks and declined thereafter. There was no significant decline in cellular responses at week 24.
“The third dose ‘booster’ again significantly increased antibody and cellular responses compared with the pre–third dose levels,” Dr. Kister said.
“Importantly, cellular responses remained elevated or even increased from 4 weeks to 12 weeks after third dose/booster. Overall, these data strongly support the need for a third dose in MS patients on ocrelizumab,” Dr. Kister added.
Participants with “hybrid immunity” (those who had been infected with SARS-CoV-2 and who had also been vaccinated for COVID) had markedly higher SARS-CoV-2–specific antibody and cellular responses than those of peers with vaccine-only immunity.
CDC recs
Looking ahead, Dr. Kister said the VIOLA investigators plan to present data on the durability of COVID-19 vaccines in ocrelizumab-treated patients up to 48 weeks after the third dose.
For immunocompromised patients, such as those taking ocrelizumab, the Centers for Disease Control and Prevention considers the third dose of mRNA vaccine not as a “booster” but as part of the regular vaccine series.
“In other words, all these patients should receive three doses as part of their ‘primary’ series,” Dr. Kister noted.
The CDC also recommends receiving the updated booster for COVID-19 that became available in September 2022 (the fourth dose of the vaccine).
“Our study did not evaluate the efficacy of this fourth dose; but based on our results, it is reasonable to suppose that the fourth dose would also lead to a further increase in immune defenses,” Dr. Kister said.
The VIOLA study is an investigator-initiated collaboration supported by F. Hoffmann-La Roche Ltd/Genentech Inc. Dr. Kister has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
From ECTRIMS 2022
Mid-October flulike illness cases higher than past 5 years
Outpatient visits for influenzalike illness (ILI), which includes influenza, SARS-CoV-2, and RSV, were higher after 3 weeks than for any of the previous five flu seasons: 3.3% of visits reported through the CDC’s Outpatient Influenza-like Illness Surveillance Network involved ILI as of Oct. 22. The highest comparable rate in the previous 5 years was the 1.9% recorded in late October of 2021, shortly after the definition of ILI was changed to also include illnesses other than influenza.
This season’s higher flu activity is in contrast to the previous two, which were unusually mild. The change, however, is not unexpected, as William Schaffner, MD, an infectious disease expert and professor of preventive medicine at Vanderbilt University, recently told CNN.
“Here we are in the middle of October – not the middle of November – we’re already seeing scattered influenza cases, even hospitalized influenza cases, around the country,” he said. “So we know that this virus is now spreading out in the community already. It’s gathering speed already. It looks to me to be about a month early.”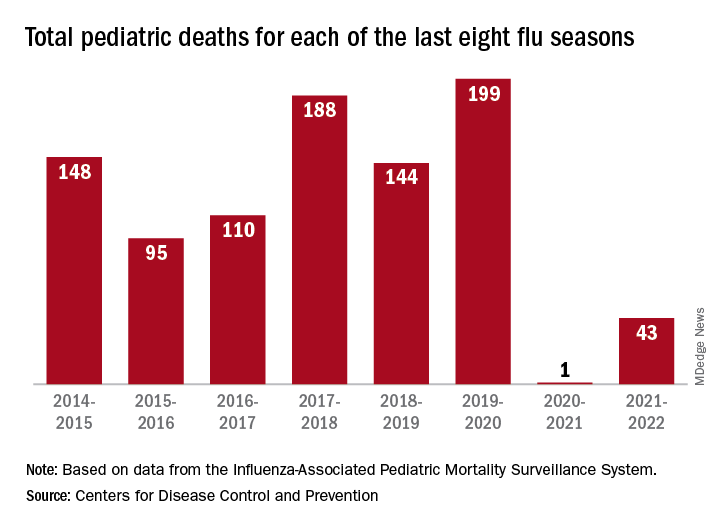
One indication of the mildness of the previous two flu seasons was the number of deaths, both pediatric and overall. Influenza-associated pediatric deaths had averaged about 110 per season over the previous eight seasons, compared with just 1 for 2020-2021 and 43 in 2021-2022. Overall flu deaths never reached 1% of all weekly deaths for either season, well below baseline levels for the flu, which range from 5.5% to 6.8%, CDC data show.
Other indicators of early severity
This season’s early rise in viral activity also can be seen in hospitalizations. The cumulative rate of flu-related admissions was 1.5 per 100,000 population as of Oct. 22, higher than the rate observed in the comparable week of previous seasons going back to 2010-2011, according to the CDC’s Influenza Hospitalization Surveillance Network.
A look at state reports of ILI outpatient visit rates shows that the District of Columbia and South Carolina are already in the very high range of the CDC’s severity scale, while 11 states are in the high range. Again going back to 2010-2011, no jurisdiction has ever been in the very high range this early in the season, based on data from the Outpatient Influenza-like Illness Surveillance Network.
Outpatient visits for influenzalike illness (ILI), which includes influenza, SARS-CoV-2, and RSV, were higher after 3 weeks than for any of the previous five flu seasons: 3.3% of visits reported through the CDC’s Outpatient Influenza-like Illness Surveillance Network involved ILI as of Oct. 22. The highest comparable rate in the previous 5 years was the 1.9% recorded in late October of 2021, shortly after the definition of ILI was changed to also include illnesses other than influenza.
This season’s higher flu activity is in contrast to the previous two, which were unusually mild. The change, however, is not unexpected, as William Schaffner, MD, an infectious disease expert and professor of preventive medicine at Vanderbilt University, recently told CNN.
“Here we are in the middle of October – not the middle of November – we’re already seeing scattered influenza cases, even hospitalized influenza cases, around the country,” he said. “So we know that this virus is now spreading out in the community already. It’s gathering speed already. It looks to me to be about a month early.”
One indication of the mildness of the previous two flu seasons was the number of deaths, both pediatric and overall. Influenza-associated pediatric deaths had averaged about 110 per season over the previous eight seasons, compared with just 1 for 2020-2021 and 43 in 2021-2022. Overall flu deaths never reached 1% of all weekly deaths for either season, well below baseline levels for the flu, which range from 5.5% to 6.8%, CDC data show.
Other indicators of early severity
This season’s early rise in viral activity also can be seen in hospitalizations. The cumulative rate of flu-related admissions was 1.5 per 100,000 population as of Oct. 22, higher than the rate observed in the comparable week of previous seasons going back to 2010-2011, according to the CDC’s Influenza Hospitalization Surveillance Network.
A look at state reports of ILI outpatient visit rates shows that the District of Columbia and South Carolina are already in the very high range of the CDC’s severity scale, while 11 states are in the high range. Again going back to 2010-2011, no jurisdiction has ever been in the very high range this early in the season, based on data from the Outpatient Influenza-like Illness Surveillance Network.
Outpatient visits for influenzalike illness (ILI), which includes influenza, SARS-CoV-2, and RSV, were higher after 3 weeks than for any of the previous five flu seasons: 3.3% of visits reported through the CDC’s Outpatient Influenza-like Illness Surveillance Network involved ILI as of Oct. 22. The highest comparable rate in the previous 5 years was the 1.9% recorded in late October of 2021, shortly after the definition of ILI was changed to also include illnesses other than influenza.
This season’s higher flu activity is in contrast to the previous two, which were unusually mild. The change, however, is not unexpected, as William Schaffner, MD, an infectious disease expert and professor of preventive medicine at Vanderbilt University, recently told CNN.
“Here we are in the middle of October – not the middle of November – we’re already seeing scattered influenza cases, even hospitalized influenza cases, around the country,” he said. “So we know that this virus is now spreading out in the community already. It’s gathering speed already. It looks to me to be about a month early.”
One indication of the mildness of the previous two flu seasons was the number of deaths, both pediatric and overall. Influenza-associated pediatric deaths had averaged about 110 per season over the previous eight seasons, compared with just 1 for 2020-2021 and 43 in 2021-2022. Overall flu deaths never reached 1% of all weekly deaths for either season, well below baseline levels for the flu, which range from 5.5% to 6.8%, CDC data show.
Other indicators of early severity
This season’s early rise in viral activity also can be seen in hospitalizations. The cumulative rate of flu-related admissions was 1.5 per 100,000 population as of Oct. 22, higher than the rate observed in the comparable week of previous seasons going back to 2010-2011, according to the CDC’s Influenza Hospitalization Surveillance Network.
A look at state reports of ILI outpatient visit rates shows that the District of Columbia and South Carolina are already in the very high range of the CDC’s severity scale, while 11 states are in the high range. Again going back to 2010-2011, no jurisdiction has ever been in the very high range this early in the season, based on data from the Outpatient Influenza-like Illness Surveillance Network.
‘Unappreciated’ ties between COVID and gut dysbiosis
(BSIs), new research suggests.
“Collectively, these results reveal an unappreciated link between SARS-CoV-2 infection, gut microbiome dysbiosis, and a severe complication of COVID-19, BSIs,” the study team reported in Nature Communications.
“Our findings suggest that coronavirus infection directly interferes with the healthy balance of microbes in the gut, further endangering patients in the process,” microbiologist and co–senior author Ken Cadwell, PhD, New York University, added in a news release. “Now that we have uncovered the source of this bacterial imbalance, physicians can better identify those coronavirus patients most at risk of a secondary bloodstream infection.”
In a mouse model, the researchers first demonstrated that the SARS-CoV-2 infection alone induces gut microbiome dysbiosis and gut epithelial cell alterations, which correlate with markers of gut barrier permeability.
Next, they analyzed the bacterial composition of stool samples from 96 adults hospitalized with COVID-19 in 2020 in New York and New Haven, Conn.
In line with their observations in mice, they found that the SARS-CoV-2 infection is associated with “severe microbiome injury,” characterized by the loss of gut microbiome diversity.
They also observed an increase in populations of several microbes known to include antibiotic-resistant species. An analysis of stool samples paired with blood cultures found that antibiotic-resistant bacteria in the gut migrated to the bloodstream in 20% of patients.
This migration could be caused by a combination of the immune-compromising effects of the viral infection and the antibiotic-driven depletion of commensal gut microbes, the researchers said.
However, COVID-19 patients are also uniquely exposed to other potential factors predisposing them to bacteremia, including immunosuppressive drugs, long hospital stays, and catheters, the investigators noted. The study is limited in its ability to investigate the individual effects of these factors.
“Our findings support a scenario in which gut-to-blood translocation of microorganisms following microbiome dysbiosis leads to dangerous BSIs during COVID-19, a complication seen in other immunocompromised patients, including patients with cancer, acute respiratory distress syndrome, and in ICU patients receiving probiotics,” the researchers wrote.
Investigating the underlying mechanism behind their observations could help inform “the judicious application of antibiotics and immunosuppressives in patients with respiratory viral infections and increase our resilience to pandemics,” they added.
Funding for the study was provided by the National Institutes of Health, the Yale School of Public Health, and numerous other sources. Dr. Cadwell has received research support from Pfizer, Takeda, Pacific Biosciences, Genentech, and AbbVie; consulted for or received an honoraria from PureTech Health, Genentech, and AbbVie; and is named as an inventor on US patent 10,722,600 and provisional patents 62/935,035 and 63/157,225.
A version of this article first appeared on Medscape.com.
(BSIs), new research suggests.
“Collectively, these results reveal an unappreciated link between SARS-CoV-2 infection, gut microbiome dysbiosis, and a severe complication of COVID-19, BSIs,” the study team reported in Nature Communications.
“Our findings suggest that coronavirus infection directly interferes with the healthy balance of microbes in the gut, further endangering patients in the process,” microbiologist and co–senior author Ken Cadwell, PhD, New York University, added in a news release. “Now that we have uncovered the source of this bacterial imbalance, physicians can better identify those coronavirus patients most at risk of a secondary bloodstream infection.”
In a mouse model, the researchers first demonstrated that the SARS-CoV-2 infection alone induces gut microbiome dysbiosis and gut epithelial cell alterations, which correlate with markers of gut barrier permeability.
Next, they analyzed the bacterial composition of stool samples from 96 adults hospitalized with COVID-19 in 2020 in New York and New Haven, Conn.
In line with their observations in mice, they found that the SARS-CoV-2 infection is associated with “severe microbiome injury,” characterized by the loss of gut microbiome diversity.
They also observed an increase in populations of several microbes known to include antibiotic-resistant species. An analysis of stool samples paired with blood cultures found that antibiotic-resistant bacteria in the gut migrated to the bloodstream in 20% of patients.
This migration could be caused by a combination of the immune-compromising effects of the viral infection and the antibiotic-driven depletion of commensal gut microbes, the researchers said.
However, COVID-19 patients are also uniquely exposed to other potential factors predisposing them to bacteremia, including immunosuppressive drugs, long hospital stays, and catheters, the investigators noted. The study is limited in its ability to investigate the individual effects of these factors.
“Our findings support a scenario in which gut-to-blood translocation of microorganisms following microbiome dysbiosis leads to dangerous BSIs during COVID-19, a complication seen in other immunocompromised patients, including patients with cancer, acute respiratory distress syndrome, and in ICU patients receiving probiotics,” the researchers wrote.
Investigating the underlying mechanism behind their observations could help inform “the judicious application of antibiotics and immunosuppressives in patients with respiratory viral infections and increase our resilience to pandemics,” they added.
Funding for the study was provided by the National Institutes of Health, the Yale School of Public Health, and numerous other sources. Dr. Cadwell has received research support from Pfizer, Takeda, Pacific Biosciences, Genentech, and AbbVie; consulted for or received an honoraria from PureTech Health, Genentech, and AbbVie; and is named as an inventor on US patent 10,722,600 and provisional patents 62/935,035 and 63/157,225.
A version of this article first appeared on Medscape.com.
(BSIs), new research suggests.
“Collectively, these results reveal an unappreciated link between SARS-CoV-2 infection, gut microbiome dysbiosis, and a severe complication of COVID-19, BSIs,” the study team reported in Nature Communications.
“Our findings suggest that coronavirus infection directly interferes with the healthy balance of microbes in the gut, further endangering patients in the process,” microbiologist and co–senior author Ken Cadwell, PhD, New York University, added in a news release. “Now that we have uncovered the source of this bacterial imbalance, physicians can better identify those coronavirus patients most at risk of a secondary bloodstream infection.”
In a mouse model, the researchers first demonstrated that the SARS-CoV-2 infection alone induces gut microbiome dysbiosis and gut epithelial cell alterations, which correlate with markers of gut barrier permeability.
Next, they analyzed the bacterial composition of stool samples from 96 adults hospitalized with COVID-19 in 2020 in New York and New Haven, Conn.
In line with their observations in mice, they found that the SARS-CoV-2 infection is associated with “severe microbiome injury,” characterized by the loss of gut microbiome diversity.
They also observed an increase in populations of several microbes known to include antibiotic-resistant species. An analysis of stool samples paired with blood cultures found that antibiotic-resistant bacteria in the gut migrated to the bloodstream in 20% of patients.
This migration could be caused by a combination of the immune-compromising effects of the viral infection and the antibiotic-driven depletion of commensal gut microbes, the researchers said.
However, COVID-19 patients are also uniquely exposed to other potential factors predisposing them to bacteremia, including immunosuppressive drugs, long hospital stays, and catheters, the investigators noted. The study is limited in its ability to investigate the individual effects of these factors.
“Our findings support a scenario in which gut-to-blood translocation of microorganisms following microbiome dysbiosis leads to dangerous BSIs during COVID-19, a complication seen in other immunocompromised patients, including patients with cancer, acute respiratory distress syndrome, and in ICU patients receiving probiotics,” the researchers wrote.
Investigating the underlying mechanism behind their observations could help inform “the judicious application of antibiotics and immunosuppressives in patients with respiratory viral infections and increase our resilience to pandemics,” they added.
Funding for the study was provided by the National Institutes of Health, the Yale School of Public Health, and numerous other sources. Dr. Cadwell has received research support from Pfizer, Takeda, Pacific Biosciences, Genentech, and AbbVie; consulted for or received an honoraria from PureTech Health, Genentech, and AbbVie; and is named as an inventor on US patent 10,722,600 and provisional patents 62/935,035 and 63/157,225.
A version of this article first appeared on Medscape.com.
FROM NATURE COMMUNICATIONS
Children and COVID: Weekly cases can’t sustain downward trend
New COVID-19 cases in children inched up in late October, just 1 week after dipping to their lowest level in more than a year, and some measures of pediatric emergency visits and hospital admissions rose as well.
There was an 8% increase in the number of cases for the week of Oct. 21-27, compared with the previous week, but this week’s total was still below 25,000, and the overall trend since the beginning of September is still one of decline, based on data from the American Academy of Pediatrics and the Children’s Hospital Association.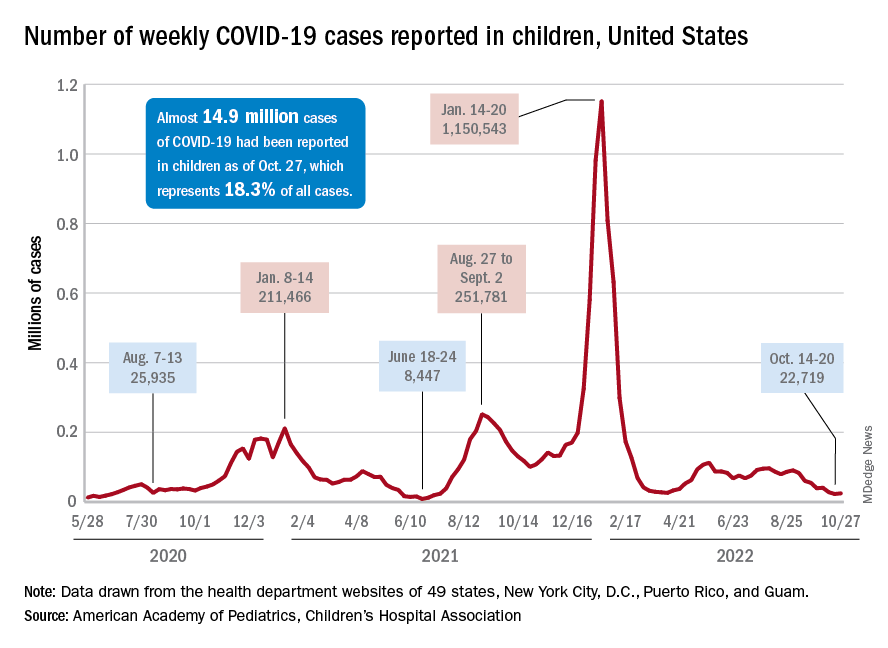
A similar increase can be seen for hospitalizations with confirmed COVID. The rate for children aged 0-17 years fell from 0.44 admissions per 100,000 population at the end of August to 0.16 per 100,000 on Oct. 23. Hospitalizations have since ticked up to 0.17 per 100,000, according to the Centers for Disease Control and Prevention.
Emergency department visits with diagnosed COVID among children aged 16-17 years, as a percentage of all ED visits, rose from 0.6% on Oct. 21 to 0.8% on Oct. 26. ED visits for 12- to 15-year-olds rose from 0.6% to 0.7% at about the same time, with both increases coming after declines that started in late August. No such increase has occurred yet among children aged 0-11 years, the CDC reported on its COVID Data Tracker.
One small milestone reached in the past week involved the proportion of all COVID cases that have occurred in children. The total number of child cases as of Oct. 27 was almost 14.9 million, which represents 18.3% of cases in all Americans, according to the AAP and CHA. That figure had been sitting at 18.4% since mid-August after reaching as high as 19.0% during the spring.
The CDC puts total COVID-related hospital admissions for children aged 0-17 at 163,588 since Aug. 1, 2020, which is 3.0% of all U.S. admissions. Total pediatric deaths number 1,843, or just about 0.2% of all COVID-related fatalities since the start of the pandemic, the CDC data show.
The latest vaccination figures show that 71.3% of children aged 12-17 years have received at least one dose, as have 38.8% of 5- to 11-year-olds, 8.4% of 2- to 4-year-olds, and 5.5% of those under age 2. Full vaccination by age group looks like this: 60.9% (12-17 years), 31.7% (5-11 years), 3.7% (2-4 years), and 2.1% (<2 years), the CDC reported. Almost 30% of children aged 12-17 have gotten a first booster dose, as have 16% of 5- to 11-year-olds.
New COVID-19 cases in children inched up in late October, just 1 week after dipping to their lowest level in more than a year, and some measures of pediatric emergency visits and hospital admissions rose as well.
There was an 8% increase in the number of cases for the week of Oct. 21-27, compared with the previous week, but this week’s total was still below 25,000, and the overall trend since the beginning of September is still one of decline, based on data from the American Academy of Pediatrics and the Children’s Hospital Association.
A similar increase can be seen for hospitalizations with confirmed COVID. The rate for children aged 0-17 years fell from 0.44 admissions per 100,000 population at the end of August to 0.16 per 100,000 on Oct. 23. Hospitalizations have since ticked up to 0.17 per 100,000, according to the Centers for Disease Control and Prevention.
Emergency department visits with diagnosed COVID among children aged 16-17 years, as a percentage of all ED visits, rose from 0.6% on Oct. 21 to 0.8% on Oct. 26. ED visits for 12- to 15-year-olds rose from 0.6% to 0.7% at about the same time, with both increases coming after declines that started in late August. No such increase has occurred yet among children aged 0-11 years, the CDC reported on its COVID Data Tracker.
One small milestone reached in the past week involved the proportion of all COVID cases that have occurred in children. The total number of child cases as of Oct. 27 was almost 14.9 million, which represents 18.3% of cases in all Americans, according to the AAP and CHA. That figure had been sitting at 18.4% since mid-August after reaching as high as 19.0% during the spring.
The CDC puts total COVID-related hospital admissions for children aged 0-17 at 163,588 since Aug. 1, 2020, which is 3.0% of all U.S. admissions. Total pediatric deaths number 1,843, or just about 0.2% of all COVID-related fatalities since the start of the pandemic, the CDC data show.
The latest vaccination figures show that 71.3% of children aged 12-17 years have received at least one dose, as have 38.8% of 5- to 11-year-olds, 8.4% of 2- to 4-year-olds, and 5.5% of those under age 2. Full vaccination by age group looks like this: 60.9% (12-17 years), 31.7% (5-11 years), 3.7% (2-4 years), and 2.1% (<2 years), the CDC reported. Almost 30% of children aged 12-17 have gotten a first booster dose, as have 16% of 5- to 11-year-olds.
New COVID-19 cases in children inched up in late October, just 1 week after dipping to their lowest level in more than a year, and some measures of pediatric emergency visits and hospital admissions rose as well.
There was an 8% increase in the number of cases for the week of Oct. 21-27, compared with the previous week, but this week’s total was still below 25,000, and the overall trend since the beginning of September is still one of decline, based on data from the American Academy of Pediatrics and the Children’s Hospital Association.
A similar increase can be seen for hospitalizations with confirmed COVID. The rate for children aged 0-17 years fell from 0.44 admissions per 100,000 population at the end of August to 0.16 per 100,000 on Oct. 23. Hospitalizations have since ticked up to 0.17 per 100,000, according to the Centers for Disease Control and Prevention.
Emergency department visits with diagnosed COVID among children aged 16-17 years, as a percentage of all ED visits, rose from 0.6% on Oct. 21 to 0.8% on Oct. 26. ED visits for 12- to 15-year-olds rose from 0.6% to 0.7% at about the same time, with both increases coming after declines that started in late August. No such increase has occurred yet among children aged 0-11 years, the CDC reported on its COVID Data Tracker.
One small milestone reached in the past week involved the proportion of all COVID cases that have occurred in children. The total number of child cases as of Oct. 27 was almost 14.9 million, which represents 18.3% of cases in all Americans, according to the AAP and CHA. That figure had been sitting at 18.4% since mid-August after reaching as high as 19.0% during the spring.
The CDC puts total COVID-related hospital admissions for children aged 0-17 at 163,588 since Aug. 1, 2020, which is 3.0% of all U.S. admissions. Total pediatric deaths number 1,843, or just about 0.2% of all COVID-related fatalities since the start of the pandemic, the CDC data show.
The latest vaccination figures show that 71.3% of children aged 12-17 years have received at least one dose, as have 38.8% of 5- to 11-year-olds, 8.4% of 2- to 4-year-olds, and 5.5% of those under age 2. Full vaccination by age group looks like this: 60.9% (12-17 years), 31.7% (5-11 years), 3.7% (2-4 years), and 2.1% (<2 years), the CDC reported. Almost 30% of children aged 12-17 have gotten a first booster dose, as have 16% of 5- to 11-year-olds.
Original COVID-19 vaccines fall short against Omicron subvariants for the immunocompromised
The effectiveness of up to three doses of COVID-19 vaccine was moderate overall and significantly lower among individuals with immunocompromising conditions, compared with the general population during the period of Omicron dominance, according to an analysis of data from more than 34,000 hospitalizations.
Previous studies have suggested lower COVID-19 vaccine effectiveness among immunocompromised individuals, compared with healthy individuals from the general population, but data from the period in which Omicron subvariants have been dominant are limited, wrote Amadea Britton, MD, of the Centers for Disease Control and Prevention’s COVID-19 Emergency Response Team, and colleagues.
The CDC currently recommends an expanded primary vaccine series of three doses of an mRNA vaccine, and the Advisory Committee on Immunization Practices has recommended a fourth dose with the new bivalent booster that contains elements of the Omicron variant, the researchers noted.
In a study published in the CDC’s Morbidity and Mortality Weekly Report, the researchers identified 34,220 adults with immunocompromising conditions who were hospitalized for COVID-19–like illness between Dec. 16, 2021, and Aug. 20, 2022. These conditions included solid malignancy (40.5%), hematologic malignancy (14.6%), rheumatologic or inflammatory disorder (24.4%), other intrinsic immune condition or immunodeficiency (38.5%), or organ or stem cell transplant (8.6%). They used data from the CDC’s VISION Network, a multistate database. The data include spring and summer 2022, when the BA.4 and BA.5 Omicron subvariants dominated other strains, and adults with immunocompromising conditions were eligible for a total of four vaccine doses (two primary doses and two boosters). The median age of the study population was 69 years, and 25.7%, 41.7%, and 7.0% had received two, three, and four doses, respectively, of COVID-19 vaccine.
Overall, vaccine effectiveness (VE) among immunocompromised patients was 34% after two vaccine doses, increasing to 71% during days 7-89 after a third dose, then declining to 41% 90 days or more after that dose.
During the full Omicron period, VE was 36% for 14 or more days after dose two, 69% for 7-89 days after dose three, and 44% for 90 or more days after dose three.
When VE was stratified by sublineage period, VE was higher 7 or more days after dose three during the predominance of BA.1 (67%), compared with VE during the dominant periods of BA.2/BA.2.12.1 (32%) and BA.4/BA.5 (35%).
In the later periods when Omicron BA.2/BA.2.12.1 and BA.4/BA.5 variants dominated, and individuals who had received three doses of vaccine were eligible for a fourth, VE against these variants was 32% 90 or more days after dose three and 43% 7 or more days after dose four.
VE was lowest among individuals with potentially more severe immunocompromising conditions, notably solid organ or stem cell transplants, the researchers wrote in their discussion.
The study findings were limited by several factors including the use of ICD-9 and -10 discharge diagnosis codes for immunocompromising conditions, potential confounding in VE models, lack of data on outpatient treatments such as nirmatelvir/ritonavir (Paxlovid), and lack of COVID-19 genomic sequencing data that may have affected which sublineage was identified, the researchers noted.
However, “this study confirms that even with boosters, immunocompromised adults, because of their weakened immune systems, are still at high risk of moderate to severe COVID,” said coauthor Brian Dixon, PhD, of the Regenstrief Institute and Indiana University Richard M. Fairbanks School of Public Health, Indianapolis, in a press release about the study.
“Given the incomplete protection against hospitalization afforded by monovalent COVID-19 vaccines, persons with immunocompromising conditions might benefit from updated bivalent vaccine booster doses that target recently circulating Omicron sublineages, in line with ACIP [Advisory Committee on Immunization Practices] recommendations,” the researchers concluded in the study.
The study was funded by the CDC. The researchers had no financial conflicts to disclose. The VISION Network is a collaboration between the CDC, the Regenstrief Institute, and seven health care systems across the United States: Columbia University Irving Medical Center (New York), HealthPartners (Wisconsin), Intermountain Healthcare (Utah), Kaiser Permanente Northern California, Kaiser Permanente Northwest (Washington State), the University of Colorado, and Paso Del Norte Health Information Exchange (Texas).
The effectiveness of up to three doses of COVID-19 vaccine was moderate overall and significantly lower among individuals with immunocompromising conditions, compared with the general population during the period of Omicron dominance, according to an analysis of data from more than 34,000 hospitalizations.
Previous studies have suggested lower COVID-19 vaccine effectiveness among immunocompromised individuals, compared with healthy individuals from the general population, but data from the period in which Omicron subvariants have been dominant are limited, wrote Amadea Britton, MD, of the Centers for Disease Control and Prevention’s COVID-19 Emergency Response Team, and colleagues.
The CDC currently recommends an expanded primary vaccine series of three doses of an mRNA vaccine, and the Advisory Committee on Immunization Practices has recommended a fourth dose with the new bivalent booster that contains elements of the Omicron variant, the researchers noted.
In a study published in the CDC’s Morbidity and Mortality Weekly Report, the researchers identified 34,220 adults with immunocompromising conditions who were hospitalized for COVID-19–like illness between Dec. 16, 2021, and Aug. 20, 2022. These conditions included solid malignancy (40.5%), hematologic malignancy (14.6%), rheumatologic or inflammatory disorder (24.4%), other intrinsic immune condition or immunodeficiency (38.5%), or organ or stem cell transplant (8.6%). They used data from the CDC’s VISION Network, a multistate database. The data include spring and summer 2022, when the BA.4 and BA.5 Omicron subvariants dominated other strains, and adults with immunocompromising conditions were eligible for a total of four vaccine doses (two primary doses and two boosters). The median age of the study population was 69 years, and 25.7%, 41.7%, and 7.0% had received two, three, and four doses, respectively, of COVID-19 vaccine.
Overall, vaccine effectiveness (VE) among immunocompromised patients was 34% after two vaccine doses, increasing to 71% during days 7-89 after a third dose, then declining to 41% 90 days or more after that dose.
During the full Omicron period, VE was 36% for 14 or more days after dose two, 69% for 7-89 days after dose three, and 44% for 90 or more days after dose three.
When VE was stratified by sublineage period, VE was higher 7 or more days after dose three during the predominance of BA.1 (67%), compared with VE during the dominant periods of BA.2/BA.2.12.1 (32%) and BA.4/BA.5 (35%).
In the later periods when Omicron BA.2/BA.2.12.1 and BA.4/BA.5 variants dominated, and individuals who had received three doses of vaccine were eligible for a fourth, VE against these variants was 32% 90 or more days after dose three and 43% 7 or more days after dose four.
VE was lowest among individuals with potentially more severe immunocompromising conditions, notably solid organ or stem cell transplants, the researchers wrote in their discussion.
The study findings were limited by several factors including the use of ICD-9 and -10 discharge diagnosis codes for immunocompromising conditions, potential confounding in VE models, lack of data on outpatient treatments such as nirmatelvir/ritonavir (Paxlovid), and lack of COVID-19 genomic sequencing data that may have affected which sublineage was identified, the researchers noted.
However, “this study confirms that even with boosters, immunocompromised adults, because of their weakened immune systems, are still at high risk of moderate to severe COVID,” said coauthor Brian Dixon, PhD, of the Regenstrief Institute and Indiana University Richard M. Fairbanks School of Public Health, Indianapolis, in a press release about the study.
“Given the incomplete protection against hospitalization afforded by monovalent COVID-19 vaccines, persons with immunocompromising conditions might benefit from updated bivalent vaccine booster doses that target recently circulating Omicron sublineages, in line with ACIP [Advisory Committee on Immunization Practices] recommendations,” the researchers concluded in the study.
The study was funded by the CDC. The researchers had no financial conflicts to disclose. The VISION Network is a collaboration between the CDC, the Regenstrief Institute, and seven health care systems across the United States: Columbia University Irving Medical Center (New York), HealthPartners (Wisconsin), Intermountain Healthcare (Utah), Kaiser Permanente Northern California, Kaiser Permanente Northwest (Washington State), the University of Colorado, and Paso Del Norte Health Information Exchange (Texas).
The effectiveness of up to three doses of COVID-19 vaccine was moderate overall and significantly lower among individuals with immunocompromising conditions, compared with the general population during the period of Omicron dominance, according to an analysis of data from more than 34,000 hospitalizations.
Previous studies have suggested lower COVID-19 vaccine effectiveness among immunocompromised individuals, compared with healthy individuals from the general population, but data from the period in which Omicron subvariants have been dominant are limited, wrote Amadea Britton, MD, of the Centers for Disease Control and Prevention’s COVID-19 Emergency Response Team, and colleagues.
The CDC currently recommends an expanded primary vaccine series of three doses of an mRNA vaccine, and the Advisory Committee on Immunization Practices has recommended a fourth dose with the new bivalent booster that contains elements of the Omicron variant, the researchers noted.
In a study published in the CDC’s Morbidity and Mortality Weekly Report, the researchers identified 34,220 adults with immunocompromising conditions who were hospitalized for COVID-19–like illness between Dec. 16, 2021, and Aug. 20, 2022. These conditions included solid malignancy (40.5%), hematologic malignancy (14.6%), rheumatologic or inflammatory disorder (24.4%), other intrinsic immune condition or immunodeficiency (38.5%), or organ or stem cell transplant (8.6%). They used data from the CDC’s VISION Network, a multistate database. The data include spring and summer 2022, when the BA.4 and BA.5 Omicron subvariants dominated other strains, and adults with immunocompromising conditions were eligible for a total of four vaccine doses (two primary doses and two boosters). The median age of the study population was 69 years, and 25.7%, 41.7%, and 7.0% had received two, three, and four doses, respectively, of COVID-19 vaccine.
Overall, vaccine effectiveness (VE) among immunocompromised patients was 34% after two vaccine doses, increasing to 71% during days 7-89 after a third dose, then declining to 41% 90 days or more after that dose.
During the full Omicron period, VE was 36% for 14 or more days after dose two, 69% for 7-89 days after dose three, and 44% for 90 or more days after dose three.
When VE was stratified by sublineage period, VE was higher 7 or more days after dose three during the predominance of BA.1 (67%), compared with VE during the dominant periods of BA.2/BA.2.12.1 (32%) and BA.4/BA.5 (35%).
In the later periods when Omicron BA.2/BA.2.12.1 and BA.4/BA.5 variants dominated, and individuals who had received three doses of vaccine were eligible for a fourth, VE against these variants was 32% 90 or more days after dose three and 43% 7 or more days after dose four.
VE was lowest among individuals with potentially more severe immunocompromising conditions, notably solid organ or stem cell transplants, the researchers wrote in their discussion.
The study findings were limited by several factors including the use of ICD-9 and -10 discharge diagnosis codes for immunocompromising conditions, potential confounding in VE models, lack of data on outpatient treatments such as nirmatelvir/ritonavir (Paxlovid), and lack of COVID-19 genomic sequencing data that may have affected which sublineage was identified, the researchers noted.
However, “this study confirms that even with boosters, immunocompromised adults, because of their weakened immune systems, are still at high risk of moderate to severe COVID,” said coauthor Brian Dixon, PhD, of the Regenstrief Institute and Indiana University Richard M. Fairbanks School of Public Health, Indianapolis, in a press release about the study.
“Given the incomplete protection against hospitalization afforded by monovalent COVID-19 vaccines, persons with immunocompromising conditions might benefit from updated bivalent vaccine booster doses that target recently circulating Omicron sublineages, in line with ACIP [Advisory Committee on Immunization Practices] recommendations,” the researchers concluded in the study.
The study was funded by the CDC. The researchers had no financial conflicts to disclose. The VISION Network is a collaboration between the CDC, the Regenstrief Institute, and seven health care systems across the United States: Columbia University Irving Medical Center (New York), HealthPartners (Wisconsin), Intermountain Healthcare (Utah), Kaiser Permanente Northern California, Kaiser Permanente Northwest (Washington State), the University of Colorado, and Paso Del Norte Health Information Exchange (Texas).
FROM MMWR
I’m a physician battling long COVID. I can assure you it’s real
One in 5. It almost seems unimaginable that this is the real number of people who are struggling with long COVID, especially considering how many people in the United States have had COVID-19 at this point (more than 96 million).
Even more unimaginable at this time is that it’s happening to me. I’ve experienced not only the disabling effects of long COVID, but I’ve also seen, firsthand, the frustration of navigating diagnosis and treatment. It’s given me a taste of what millions of other patients are going through.
Vaxxed, masked, and (too) relaxed
I caught COVID-19 (probably Omicron BA.5) that presented as sniffles, making me think it was probably just allergies. However, my resting heart rate was up on my Garmin watch, so of course I got tested and was positive.
With my symptoms virtually nonexistent, it seemed, at the time, merely an inconvenience, because I was forced to isolate away from family and friends, who all stayed negative.
But 2 weeks later, I began to have urticaria – hives – after physical exertion. Did that mean my mast cells were angry? There’s some evidence these immune cells become overactivated in some patients with COVID. Next, I began to experience lightheadedness and the rapid heartbeat of tachycardia. The tachycardia was especially bad any time I physically exerted myself, including on a walk. Imagine me – a lover of all bargain shopping – cutting short a trip to the outlet mall on a particularly bad day when my heart rate was 140 after taking just a few steps. This was orthostatic intolerance.
Then came the severe worsening of my migraines – which are often vestibular, making me nauseated and dizzy on top of the throbbing.
I was of course familiar with these symptoms, as professor and chair of the department of rehabilitation medicine at the Joe R. and Teresa Lozano Long School of Medicine at University of Texas Health Science Center, San Antonio. I developed a post-COVID recovery clinic to help patients.
So I knew about postexertional malaise (PEM) and postexertional symptom exacerbation (PESE), but I was now experiencing these distressing symptoms firsthand.
Clinicians really need to look for this cardinal sign of long COVID as well as evidence of myalgic encephalomyelitis or chronic fatigue syndrome (ME/CFS). ME/CFS is marked by exacerbation of fatigue or symptoms after an activity that could previously be done without these aftereffects. In my case, as an All-American Masters miler with several marathons under my belt, running 5 miles is a walk in the park. But now, I pay for those 5 miles for the rest of the day on the couch or with palpitations, dizziness, and fatigue the following day. Busy clinic day full of procedures? I would have to be sitting by the end of it. Bed by 9 PM was not always early enough.
Becoming a statistic
Here I am, one of the leading experts in the country on caring for people with long COVID, featured in the national news and having testified in front of Congress, and now I am part of that lived experience. Me – a healthy athlete, with no comorbidities, a normal BMI, vaccinated and boosted, and after an almost asymptomatic bout of COVID-19, a victim to long COVID.
You just never know how your body is going to react. Neuroinflammation occurred in studies with mice with mild respiratory COVID and could be happening to me. I did not want a chronic immune-mediated vasculopathy.
So, I did what any other hyperaware physician-researcher would do. I enrolled in the RECOVER trial – a study my own institution is taking part in and one that I recommend to my own patients.
I also decided that I need to access care and not just ignore my symptoms or try to treat them myself.
That’s when things got difficult. There was a wait of at least a month to see my primary care provider – but I was able to use my privileged position as a physician to get in sooner.
My provider said that she had limited knowledge of long COVID, and she hesitated to order some of the tests and treatments that I recommended because they were not yet considered standard of care. I can understand the hesitation. It is engrained in medical education to follow evidence based on the highest-quality research studies. We are slowly learning more about long COVID, but acknowledging the learning curve offers little to patients who need help now.
This has made me realize that we cannot wait on an evidence-based approach – which can take decades to develop – while people are suffering. And it’s important that everyone on the front line learn about some of the manifestations and disease management of long COVID.
I left this first physician visit feeling more defeated than anything and decided to try to push through. That, I quickly realized, was not the right thing to do.
So again, after a couple of significant crashes and days of severe migraines, I phoned a friend: Ratna Bhavaraju-Sanka, MD, the amazing neurologist who treats patients with long COVID alongside me. She squeezed me in on a non-clinic day. Again, I had the privilege to see a specialist most people wait half a year to see. I was diagnosed with both autonomic dysfunction and intractable migraine.
She ordered some intravenous fluids and IV magnesium that would probably help both. But then another obstacle arose. My institution’s infusion center is focused on patients with cancer, and I was unable to schedule treatments there.
Luckily, I knew about the concierge mobile IV hydration therapy companies that come to your house – mostly offering a hangover treatment service. And I am thankful that I had the health literacy and financial ability to pay for some fluids at home.
On another particularly bad day, I phoned other friends – higher-ups at the hospital – who expedited a slot at the hospital infusion center and approval for the IV magnesium.
Thanks to my access, knowledge, and other privileges, I got fairly quick if imperfect care, enrolled in a research trial, and received medications. I knew to pace myself. The vast majority of others with long COVID lack these advantages.
The patient with long COVID
Things I have learned that others can learn, too:
- Acknowledge and recognize that long COVID is a disease that is affecting 1 in 5 Americans who catch COVID. Many look completely “normal on the outside.” Please listen to your patients.
- Autonomic dysfunction is a common manifestation of long COVID. A 10-minute stand test goes a long way in diagnosing this condition, from the American Academy of Physical Medicine and Rehabilitation. It is not just anxiety.
- “That’s only in research” is dismissive and harmful. Think outside the box. Follow guidelines. Consider encouraging patients to sign up for trials.
- Screen for PEM/PESE and teach your patients to pace themselves, because pushing through it or doing graded exercises will be harmful.
- We need to train more physicians to treat postacute sequelae of SARS-CoV-2 infection () and other postinfectious conditions, such as ME/CFS.
If long COVID is hard for physicians to understand and deal with, imagine how difficult it is for patients with no expertise in this area.
It is exponentially harder for those with fewer resources, time, and health literacy. My lived experience with long COVID has shown me that being a patient is never easy. You put your body and fate into the hands of trusted professionals and expect validation and assistance, not gaslighting or gatekeeping.
Along with millions of others, I am tired of waiting.
Dr. Gutierrez is Professor and Distinguished Chair, department of rehabilitation medicine, University of Texas Health Science Center at San Antonio. She reported receiving honoraria for lecturing on long COVID and receiving a research grant from Co-PI for the NIH RECOVER trial.
A version of this article first appeared on Medscape.com.
One in 5. It almost seems unimaginable that this is the real number of people who are struggling with long COVID, especially considering how many people in the United States have had COVID-19 at this point (more than 96 million).
Even more unimaginable at this time is that it’s happening to me. I’ve experienced not only the disabling effects of long COVID, but I’ve also seen, firsthand, the frustration of navigating diagnosis and treatment. It’s given me a taste of what millions of other patients are going through.
Vaxxed, masked, and (too) relaxed
I caught COVID-19 (probably Omicron BA.5) that presented as sniffles, making me think it was probably just allergies. However, my resting heart rate was up on my Garmin watch, so of course I got tested and was positive.
With my symptoms virtually nonexistent, it seemed, at the time, merely an inconvenience, because I was forced to isolate away from family and friends, who all stayed negative.
But 2 weeks later, I began to have urticaria – hives – after physical exertion. Did that mean my mast cells were angry? There’s some evidence these immune cells become overactivated in some patients with COVID. Next, I began to experience lightheadedness and the rapid heartbeat of tachycardia. The tachycardia was especially bad any time I physically exerted myself, including on a walk. Imagine me – a lover of all bargain shopping – cutting short a trip to the outlet mall on a particularly bad day when my heart rate was 140 after taking just a few steps. This was orthostatic intolerance.
Then came the severe worsening of my migraines – which are often vestibular, making me nauseated and dizzy on top of the throbbing.
I was of course familiar with these symptoms, as professor and chair of the department of rehabilitation medicine at the Joe R. and Teresa Lozano Long School of Medicine at University of Texas Health Science Center, San Antonio. I developed a post-COVID recovery clinic to help patients.
So I knew about postexertional malaise (PEM) and postexertional symptom exacerbation (PESE), but I was now experiencing these distressing symptoms firsthand.
Clinicians really need to look for this cardinal sign of long COVID as well as evidence of myalgic encephalomyelitis or chronic fatigue syndrome (ME/CFS). ME/CFS is marked by exacerbation of fatigue or symptoms after an activity that could previously be done without these aftereffects. In my case, as an All-American Masters miler with several marathons under my belt, running 5 miles is a walk in the park. But now, I pay for those 5 miles for the rest of the day on the couch or with palpitations, dizziness, and fatigue the following day. Busy clinic day full of procedures? I would have to be sitting by the end of it. Bed by 9 PM was not always early enough.
Becoming a statistic
Here I am, one of the leading experts in the country on caring for people with long COVID, featured in the national news and having testified in front of Congress, and now I am part of that lived experience. Me – a healthy athlete, with no comorbidities, a normal BMI, vaccinated and boosted, and after an almost asymptomatic bout of COVID-19, a victim to long COVID.
You just never know how your body is going to react. Neuroinflammation occurred in studies with mice with mild respiratory COVID and could be happening to me. I did not want a chronic immune-mediated vasculopathy.
So, I did what any other hyperaware physician-researcher would do. I enrolled in the RECOVER trial – a study my own institution is taking part in and one that I recommend to my own patients.
I also decided that I need to access care and not just ignore my symptoms or try to treat them myself.
That’s when things got difficult. There was a wait of at least a month to see my primary care provider – but I was able to use my privileged position as a physician to get in sooner.
My provider said that she had limited knowledge of long COVID, and she hesitated to order some of the tests and treatments that I recommended because they were not yet considered standard of care. I can understand the hesitation. It is engrained in medical education to follow evidence based on the highest-quality research studies. We are slowly learning more about long COVID, but acknowledging the learning curve offers little to patients who need help now.
This has made me realize that we cannot wait on an evidence-based approach – which can take decades to develop – while people are suffering. And it’s important that everyone on the front line learn about some of the manifestations and disease management of long COVID.
I left this first physician visit feeling more defeated than anything and decided to try to push through. That, I quickly realized, was not the right thing to do.
So again, after a couple of significant crashes and days of severe migraines, I phoned a friend: Ratna Bhavaraju-Sanka, MD, the amazing neurologist who treats patients with long COVID alongside me. She squeezed me in on a non-clinic day. Again, I had the privilege to see a specialist most people wait half a year to see. I was diagnosed with both autonomic dysfunction and intractable migraine.
She ordered some intravenous fluids and IV magnesium that would probably help both. But then another obstacle arose. My institution’s infusion center is focused on patients with cancer, and I was unable to schedule treatments there.
Luckily, I knew about the concierge mobile IV hydration therapy companies that come to your house – mostly offering a hangover treatment service. And I am thankful that I had the health literacy and financial ability to pay for some fluids at home.
On another particularly bad day, I phoned other friends – higher-ups at the hospital – who expedited a slot at the hospital infusion center and approval for the IV magnesium.
Thanks to my access, knowledge, and other privileges, I got fairly quick if imperfect care, enrolled in a research trial, and received medications. I knew to pace myself. The vast majority of others with long COVID lack these advantages.
The patient with long COVID
Things I have learned that others can learn, too:
- Acknowledge and recognize that long COVID is a disease that is affecting 1 in 5 Americans who catch COVID. Many look completely “normal on the outside.” Please listen to your patients.
- Autonomic dysfunction is a common manifestation of long COVID. A 10-minute stand test goes a long way in diagnosing this condition, from the American Academy of Physical Medicine and Rehabilitation. It is not just anxiety.
- “That’s only in research” is dismissive and harmful. Think outside the box. Follow guidelines. Consider encouraging patients to sign up for trials.
- Screen for PEM/PESE and teach your patients to pace themselves, because pushing through it or doing graded exercises will be harmful.
- We need to train more physicians to treat postacute sequelae of SARS-CoV-2 infection () and other postinfectious conditions, such as ME/CFS.
If long COVID is hard for physicians to understand and deal with, imagine how difficult it is for patients with no expertise in this area.
It is exponentially harder for those with fewer resources, time, and health literacy. My lived experience with long COVID has shown me that being a patient is never easy. You put your body and fate into the hands of trusted professionals and expect validation and assistance, not gaslighting or gatekeeping.
Along with millions of others, I am tired of waiting.
Dr. Gutierrez is Professor and Distinguished Chair, department of rehabilitation medicine, University of Texas Health Science Center at San Antonio. She reported receiving honoraria for lecturing on long COVID and receiving a research grant from Co-PI for the NIH RECOVER trial.
A version of this article first appeared on Medscape.com.
One in 5. It almost seems unimaginable that this is the real number of people who are struggling with long COVID, especially considering how many people in the United States have had COVID-19 at this point (more than 96 million).
Even more unimaginable at this time is that it’s happening to me. I’ve experienced not only the disabling effects of long COVID, but I’ve also seen, firsthand, the frustration of navigating diagnosis and treatment. It’s given me a taste of what millions of other patients are going through.
Vaxxed, masked, and (too) relaxed
I caught COVID-19 (probably Omicron BA.5) that presented as sniffles, making me think it was probably just allergies. However, my resting heart rate was up on my Garmin watch, so of course I got tested and was positive.
With my symptoms virtually nonexistent, it seemed, at the time, merely an inconvenience, because I was forced to isolate away from family and friends, who all stayed negative.
But 2 weeks later, I began to have urticaria – hives – after physical exertion. Did that mean my mast cells were angry? There’s some evidence these immune cells become overactivated in some patients with COVID. Next, I began to experience lightheadedness and the rapid heartbeat of tachycardia. The tachycardia was especially bad any time I physically exerted myself, including on a walk. Imagine me – a lover of all bargain shopping – cutting short a trip to the outlet mall on a particularly bad day when my heart rate was 140 after taking just a few steps. This was orthostatic intolerance.
Then came the severe worsening of my migraines – which are often vestibular, making me nauseated and dizzy on top of the throbbing.
I was of course familiar with these symptoms, as professor and chair of the department of rehabilitation medicine at the Joe R. and Teresa Lozano Long School of Medicine at University of Texas Health Science Center, San Antonio. I developed a post-COVID recovery clinic to help patients.
So I knew about postexertional malaise (PEM) and postexertional symptom exacerbation (PESE), but I was now experiencing these distressing symptoms firsthand.
Clinicians really need to look for this cardinal sign of long COVID as well as evidence of myalgic encephalomyelitis or chronic fatigue syndrome (ME/CFS). ME/CFS is marked by exacerbation of fatigue or symptoms after an activity that could previously be done without these aftereffects. In my case, as an All-American Masters miler with several marathons under my belt, running 5 miles is a walk in the park. But now, I pay for those 5 miles for the rest of the day on the couch or with palpitations, dizziness, and fatigue the following day. Busy clinic day full of procedures? I would have to be sitting by the end of it. Bed by 9 PM was not always early enough.
Becoming a statistic
Here I am, one of the leading experts in the country on caring for people with long COVID, featured in the national news and having testified in front of Congress, and now I am part of that lived experience. Me – a healthy athlete, with no comorbidities, a normal BMI, vaccinated and boosted, and after an almost asymptomatic bout of COVID-19, a victim to long COVID.
You just never know how your body is going to react. Neuroinflammation occurred in studies with mice with mild respiratory COVID and could be happening to me. I did not want a chronic immune-mediated vasculopathy.
So, I did what any other hyperaware physician-researcher would do. I enrolled in the RECOVER trial – a study my own institution is taking part in and one that I recommend to my own patients.
I also decided that I need to access care and not just ignore my symptoms or try to treat them myself.
That’s when things got difficult. There was a wait of at least a month to see my primary care provider – but I was able to use my privileged position as a physician to get in sooner.
My provider said that she had limited knowledge of long COVID, and she hesitated to order some of the tests and treatments that I recommended because they were not yet considered standard of care. I can understand the hesitation. It is engrained in medical education to follow evidence based on the highest-quality research studies. We are slowly learning more about long COVID, but acknowledging the learning curve offers little to patients who need help now.
This has made me realize that we cannot wait on an evidence-based approach – which can take decades to develop – while people are suffering. And it’s important that everyone on the front line learn about some of the manifestations and disease management of long COVID.
I left this first physician visit feeling more defeated than anything and decided to try to push through. That, I quickly realized, was not the right thing to do.
So again, after a couple of significant crashes and days of severe migraines, I phoned a friend: Ratna Bhavaraju-Sanka, MD, the amazing neurologist who treats patients with long COVID alongside me. She squeezed me in on a non-clinic day. Again, I had the privilege to see a specialist most people wait half a year to see. I was diagnosed with both autonomic dysfunction and intractable migraine.
She ordered some intravenous fluids and IV magnesium that would probably help both. But then another obstacle arose. My institution’s infusion center is focused on patients with cancer, and I was unable to schedule treatments there.
Luckily, I knew about the concierge mobile IV hydration therapy companies that come to your house – mostly offering a hangover treatment service. And I am thankful that I had the health literacy and financial ability to pay for some fluids at home.
On another particularly bad day, I phoned other friends – higher-ups at the hospital – who expedited a slot at the hospital infusion center and approval for the IV magnesium.
Thanks to my access, knowledge, and other privileges, I got fairly quick if imperfect care, enrolled in a research trial, and received medications. I knew to pace myself. The vast majority of others with long COVID lack these advantages.
The patient with long COVID
Things I have learned that others can learn, too:
- Acknowledge and recognize that long COVID is a disease that is affecting 1 in 5 Americans who catch COVID. Many look completely “normal on the outside.” Please listen to your patients.
- Autonomic dysfunction is a common manifestation of long COVID. A 10-minute stand test goes a long way in diagnosing this condition, from the American Academy of Physical Medicine and Rehabilitation. It is not just anxiety.
- “That’s only in research” is dismissive and harmful. Think outside the box. Follow guidelines. Consider encouraging patients to sign up for trials.
- Screen for PEM/PESE and teach your patients to pace themselves, because pushing through it or doing graded exercises will be harmful.
- We need to train more physicians to treat postacute sequelae of SARS-CoV-2 infection () and other postinfectious conditions, such as ME/CFS.
If long COVID is hard for physicians to understand and deal with, imagine how difficult it is for patients with no expertise in this area.
It is exponentially harder for those with fewer resources, time, and health literacy. My lived experience with long COVID has shown me that being a patient is never easy. You put your body and fate into the hands of trusted professionals and expect validation and assistance, not gaslighting or gatekeeping.
Along with millions of others, I am tired of waiting.
Dr. Gutierrez is Professor and Distinguished Chair, department of rehabilitation medicine, University of Texas Health Science Center at San Antonio. She reported receiving honoraria for lecturing on long COVID and receiving a research grant from Co-PI for the NIH RECOVER trial.
A version of this article first appeared on Medscape.com.
Has the pandemic affected babies’ brain development?
There’s some good overall news in a large analysis that looked at whether a mother’s COVID-19 infection or birth during the pandemic could affect a baby’s brain development.
Researchers studied 21,419 infants who had neurodevelopmental screening during the pandemic (from January 2020 to January 2021) and compared them with babies born before the pandemic (2015-2019).
They found in an analysis of eight studies that, generally, brain development in infants ages 6-12 months old was not changed by COVID-19.
Communication skill scores lower than prepandemic
However, one area did see a significant difference when they looked at answers to the Ages and Stages Questionnaire, 3rd edition (ASQ-3): Scores were lower in communication skills.
Compared with the prepandemic babies, the pandemic group of babies was more likely to have communication impairment (odds were 1.7 times higher).
Additionally, mothers’ SARS-CoV-2 infection was not associated with significant differences in any neurodevelopment sector in offspring, with one exception: Odds were 3.5 times higher for fine motor impairment in the pandemic baby group.
The babies in this study were either exposed in the womb to the SARS-CoV-2 infection or screened during the pandemic regardless of whether they were exposed to the virus.
The study, led by Kamran Hessami, MD, with the Maternal Fetal Care Center at Boston Children’s Hospital and Harvard Medical School in Boston, was published in JAMA Network Open.
Potential reasons for lower communication skills
The study points to some factors of the pandemic that may be tied to impaired communication skills.
“Higher levels of COVID-19–related stress were reported for both mothers and fathers of infants aged 0-6 months and were associated with insensitive parenting practices, including decreased emotional responsiveness in only mothers, which could lessen the reciprocal exchanges that support language development in early childhood,” they write. “Additionally, opportunities to promote language and social development through new experiences outside the home, including visits with extended family and friends or attendance at a child care center, were lessened for many during the pandemic.”
Viviana M. Fajardo Martinez, MD, with neonatal/perinatal medicine at University of California, Los Angeles, Health, told this publication her team is also studying child development before and after the pandemic over a 3-year period, and delayed communication skills is something she is seeing in clinic there.
She says some parents have been concerned, saying their babies aren’t talking enough or are behind in vocabulary.
Babies can catch up after 12 months
One thing she tells parents is that babies who are a bit delayed at 12 months can catch up.
Up to 18 months, they can catch up, she said, adding that they can be reevaluated then for improvement. If, at that point, the baby is not catching up, “that’s when we refer for early intervention,” she said.
Dr. Martinez also tells parents concerned about their infant’s communication skills that it’s important to talk, read, and sing to their child. She said amid pandemic stress, corners may have been cut in asking children to use language skills.
For instance, if a child points to an apple, a stressed parent may just give the child the apple instead of asking the child to request it by name and repeat the word several times.
She also said a limitation of this study is the use of the ASQ-3 questionnaire, which is filled out by parents. Answers are subjective, she notes, and sometimes differ between one child’s two parents. The questionnaire was commonly used during the pandemic because a more objective, professional evaluation has been more difficult.
However, a measure like the Bayley Scales of Infant and Toddler Development Screening Test adds objectivity and will likely give a better picture as research progresses, Dr. Martinez said.
Some information missing
Andréane Lavallée, PhD, and Dani Dumitriu, MD, PhD, both with the department of pediatrics at Columbia University, New York, write in an invited commentary that the overall positive message of the study “should not make researchers complacent” and results should be viewed with caution.
They point out that the precise effects of this novel virus are still unclear and the age group and variables studied may not tell the whole story.
“It should be noted that this systematic review did not consider timing of exposure during pregnancy, maternal infection severity, or exposure to various SARS-CoV-2 variants – all factors that could eventually be proven to contribute to subtle adverse neurodevelopmental outcomes,” they write.
Additionally, past pandemics “such as the 1918 Spanish flu, 1964 rubella, and 2009 H1N1” have taught researchers to watch for increases in diagnoses such as autism spectrum disorder (ASD) and schizophrenia in subsequent years.
“ASD is generally diagnosed at age 3-5 years (and often not until early teens), while schizophrenia is generally diagnosed in mid-to-late 20s,” the editorialists point out. The authors agree and emphasize the need for long-term studies.
Authors report no relevant financial relationships. Editorialist Dr. Dumitriu reports grants from National Institute of Mental Health, the U.S. Centers for Disease Control and Prevention, and the W. K. Kellogg Foundation; and has received gift funds from Einhorn Collaborative during the conduct of the study to the Nurture Science Program, for which Dr Dumitriu serves as director. Dr. Dumitriu received personal fees from Medela outside the submitted work; and is the corresponding author for one of the studies (Shuffrey et al., 2022) included in the systematic review conducted by Dr. Hessami et al. Dr. Lavallée reports grants from the Canadian Institutes of Health Research. Dr. Martinez reports no relevant financial relationships.
There’s some good overall news in a large analysis that looked at whether a mother’s COVID-19 infection or birth during the pandemic could affect a baby’s brain development.
Researchers studied 21,419 infants who had neurodevelopmental screening during the pandemic (from January 2020 to January 2021) and compared them with babies born before the pandemic (2015-2019).
They found in an analysis of eight studies that, generally, brain development in infants ages 6-12 months old was not changed by COVID-19.
Communication skill scores lower than prepandemic
However, one area did see a significant difference when they looked at answers to the Ages and Stages Questionnaire, 3rd edition (ASQ-3): Scores were lower in communication skills.
Compared with the prepandemic babies, the pandemic group of babies was more likely to have communication impairment (odds were 1.7 times higher).
Additionally, mothers’ SARS-CoV-2 infection was not associated with significant differences in any neurodevelopment sector in offspring, with one exception: Odds were 3.5 times higher for fine motor impairment in the pandemic baby group.
The babies in this study were either exposed in the womb to the SARS-CoV-2 infection or screened during the pandemic regardless of whether they were exposed to the virus.
The study, led by Kamran Hessami, MD, with the Maternal Fetal Care Center at Boston Children’s Hospital and Harvard Medical School in Boston, was published in JAMA Network Open.
Potential reasons for lower communication skills
The study points to some factors of the pandemic that may be tied to impaired communication skills.
“Higher levels of COVID-19–related stress were reported for both mothers and fathers of infants aged 0-6 months and were associated with insensitive parenting practices, including decreased emotional responsiveness in only mothers, which could lessen the reciprocal exchanges that support language development in early childhood,” they write. “Additionally, opportunities to promote language and social development through new experiences outside the home, including visits with extended family and friends or attendance at a child care center, were lessened for many during the pandemic.”
Viviana M. Fajardo Martinez, MD, with neonatal/perinatal medicine at University of California, Los Angeles, Health, told this publication her team is also studying child development before and after the pandemic over a 3-year period, and delayed communication skills is something she is seeing in clinic there.
She says some parents have been concerned, saying their babies aren’t talking enough or are behind in vocabulary.
Babies can catch up after 12 months
One thing she tells parents is that babies who are a bit delayed at 12 months can catch up.
Up to 18 months, they can catch up, she said, adding that they can be reevaluated then for improvement. If, at that point, the baby is not catching up, “that’s when we refer for early intervention,” she said.
Dr. Martinez also tells parents concerned about their infant’s communication skills that it’s important to talk, read, and sing to their child. She said amid pandemic stress, corners may have been cut in asking children to use language skills.
For instance, if a child points to an apple, a stressed parent may just give the child the apple instead of asking the child to request it by name and repeat the word several times.
She also said a limitation of this study is the use of the ASQ-3 questionnaire, which is filled out by parents. Answers are subjective, she notes, and sometimes differ between one child’s two parents. The questionnaire was commonly used during the pandemic because a more objective, professional evaluation has been more difficult.
However, a measure like the Bayley Scales of Infant and Toddler Development Screening Test adds objectivity and will likely give a better picture as research progresses, Dr. Martinez said.
Some information missing
Andréane Lavallée, PhD, and Dani Dumitriu, MD, PhD, both with the department of pediatrics at Columbia University, New York, write in an invited commentary that the overall positive message of the study “should not make researchers complacent” and results should be viewed with caution.
They point out that the precise effects of this novel virus are still unclear and the age group and variables studied may not tell the whole story.
“It should be noted that this systematic review did not consider timing of exposure during pregnancy, maternal infection severity, or exposure to various SARS-CoV-2 variants – all factors that could eventually be proven to contribute to subtle adverse neurodevelopmental outcomes,” they write.
Additionally, past pandemics “such as the 1918 Spanish flu, 1964 rubella, and 2009 H1N1” have taught researchers to watch for increases in diagnoses such as autism spectrum disorder (ASD) and schizophrenia in subsequent years.
“ASD is generally diagnosed at age 3-5 years (and often not until early teens), while schizophrenia is generally diagnosed in mid-to-late 20s,” the editorialists point out. The authors agree and emphasize the need for long-term studies.
Authors report no relevant financial relationships. Editorialist Dr. Dumitriu reports grants from National Institute of Mental Health, the U.S. Centers for Disease Control and Prevention, and the W. K. Kellogg Foundation; and has received gift funds from Einhorn Collaborative during the conduct of the study to the Nurture Science Program, for which Dr Dumitriu serves as director. Dr. Dumitriu received personal fees from Medela outside the submitted work; and is the corresponding author for one of the studies (Shuffrey et al., 2022) included in the systematic review conducted by Dr. Hessami et al. Dr. Lavallée reports grants from the Canadian Institutes of Health Research. Dr. Martinez reports no relevant financial relationships.
There’s some good overall news in a large analysis that looked at whether a mother’s COVID-19 infection or birth during the pandemic could affect a baby’s brain development.
Researchers studied 21,419 infants who had neurodevelopmental screening during the pandemic (from January 2020 to January 2021) and compared them with babies born before the pandemic (2015-2019).
They found in an analysis of eight studies that, generally, brain development in infants ages 6-12 months old was not changed by COVID-19.
Communication skill scores lower than prepandemic
However, one area did see a significant difference when they looked at answers to the Ages and Stages Questionnaire, 3rd edition (ASQ-3): Scores were lower in communication skills.
Compared with the prepandemic babies, the pandemic group of babies was more likely to have communication impairment (odds were 1.7 times higher).
Additionally, mothers’ SARS-CoV-2 infection was not associated with significant differences in any neurodevelopment sector in offspring, with one exception: Odds were 3.5 times higher for fine motor impairment in the pandemic baby group.
The babies in this study were either exposed in the womb to the SARS-CoV-2 infection or screened during the pandemic regardless of whether they were exposed to the virus.
The study, led by Kamran Hessami, MD, with the Maternal Fetal Care Center at Boston Children’s Hospital and Harvard Medical School in Boston, was published in JAMA Network Open.
Potential reasons for lower communication skills
The study points to some factors of the pandemic that may be tied to impaired communication skills.
“Higher levels of COVID-19–related stress were reported for both mothers and fathers of infants aged 0-6 months and were associated with insensitive parenting practices, including decreased emotional responsiveness in only mothers, which could lessen the reciprocal exchanges that support language development in early childhood,” they write. “Additionally, opportunities to promote language and social development through new experiences outside the home, including visits with extended family and friends or attendance at a child care center, were lessened for many during the pandemic.”
Viviana M. Fajardo Martinez, MD, with neonatal/perinatal medicine at University of California, Los Angeles, Health, told this publication her team is also studying child development before and after the pandemic over a 3-year period, and delayed communication skills is something she is seeing in clinic there.
She says some parents have been concerned, saying their babies aren’t talking enough or are behind in vocabulary.
Babies can catch up after 12 months
One thing she tells parents is that babies who are a bit delayed at 12 months can catch up.
Up to 18 months, they can catch up, she said, adding that they can be reevaluated then for improvement. If, at that point, the baby is not catching up, “that’s when we refer for early intervention,” she said.
Dr. Martinez also tells parents concerned about their infant’s communication skills that it’s important to talk, read, and sing to their child. She said amid pandemic stress, corners may have been cut in asking children to use language skills.
For instance, if a child points to an apple, a stressed parent may just give the child the apple instead of asking the child to request it by name and repeat the word several times.
She also said a limitation of this study is the use of the ASQ-3 questionnaire, which is filled out by parents. Answers are subjective, she notes, and sometimes differ between one child’s two parents. The questionnaire was commonly used during the pandemic because a more objective, professional evaluation has been more difficult.
However, a measure like the Bayley Scales of Infant and Toddler Development Screening Test adds objectivity and will likely give a better picture as research progresses, Dr. Martinez said.
Some information missing
Andréane Lavallée, PhD, and Dani Dumitriu, MD, PhD, both with the department of pediatrics at Columbia University, New York, write in an invited commentary that the overall positive message of the study “should not make researchers complacent” and results should be viewed with caution.
They point out that the precise effects of this novel virus are still unclear and the age group and variables studied may not tell the whole story.
“It should be noted that this systematic review did not consider timing of exposure during pregnancy, maternal infection severity, or exposure to various SARS-CoV-2 variants – all factors that could eventually be proven to contribute to subtle adverse neurodevelopmental outcomes,” they write.
Additionally, past pandemics “such as the 1918 Spanish flu, 1964 rubella, and 2009 H1N1” have taught researchers to watch for increases in diagnoses such as autism spectrum disorder (ASD) and schizophrenia in subsequent years.
“ASD is generally diagnosed at age 3-5 years (and often not until early teens), while schizophrenia is generally diagnosed in mid-to-late 20s,” the editorialists point out. The authors agree and emphasize the need for long-term studies.
Authors report no relevant financial relationships. Editorialist Dr. Dumitriu reports grants from National Institute of Mental Health, the U.S. Centers for Disease Control and Prevention, and the W. K. Kellogg Foundation; and has received gift funds from Einhorn Collaborative during the conduct of the study to the Nurture Science Program, for which Dr Dumitriu serves as director. Dr. Dumitriu received personal fees from Medela outside the submitted work; and is the corresponding author for one of the studies (Shuffrey et al., 2022) included in the systematic review conducted by Dr. Hessami et al. Dr. Lavallée reports grants from the Canadian Institutes of Health Research. Dr. Martinez reports no relevant financial relationships.
FROM JAMA NETWORK OPEN
Finerenone: ‘Striking’ cut in pneumonia, COVID-19 risks
The nonsteroidal mineralocorticoid receptor antagonist finerenone (Kerendia) unexpectedly showed that it might protect against incident infective pneumonia and COVID-19. The finding was based on secondary analyses run on more than 13,000 people enrolled in the two pivotal trials for finerenone.
Finerenone was approved by the Food and Drug Administration in 2021 for slowing progressive renal dysfunction and preventing cardiovascular events in adults with type 2 diabetes and chronic kidney disease (CKD).
‘Striking reduction in the risk of pneumonia’
The “striking reduction in risk of pneumonia” in a new analysis suggests that “the propagation of pulmonary infection into lobar or bronchial consolidation may be reduced by finerenone,” write Bertram Pitt, MD, and coauthors in a report published on October 26 in JAMA Network Open.
They also suggest that if further studies confirm that finerenone treatment reduces complications from pneumonia and COVID-19, it would have “significant medical implications,” especially because of the limited treatment options now available for complications from COVID-19.
The new analyses used the FIDELITY dataset, a prespecified merging of results from the FIDELIO-DKD and FIGARO-DKD trials, which together enrolled 13,026 people with type 2 diabetes and CKD, as determined on the basis of the patients’ having a urine albumin-to-creatinine ratio of at least 30 mg/g.
The primary outcomes of these trials showed that treatment with finerenone led to significant slowing of the progression of CKD and a significant reduction in the incidence of cardiovascular events, compared with placebo during median follow-up of 3 years.
The new, secondary analyses focused on the 6.0% of participants in whom there was evidence of pneumonia and the 1.6% in whom there was evidence of having COVID-19. Pneumonia was the most common serious adverse event in the two trials, a finding consistent with the documented risk for pneumonia faced by people with CKD.
Finerenone linked with a 29% relative reduction in pneumonia
When analyzed by treatment, the incidence of pneumonia was 4.7% among those who received finerenone and 6.7% among those who received placebo. This translated into a significant relative risk reduction of 29% associated with finerenone treatment.
Analysis of COVID-19 adverse events showed a 1.3% incidence among those who received finerenone and a 1.8% incidence among those in the placebo group, which translated into a significant 27% relative risk reduction linked with finerenone treatment.
In contrast, the data showed no reduced incidence of several other respiratory infections among the finerenone recipients, including nasopharyngitis, bronchitis, and influenza. The data also showed no signal that pneumonia or COVID-19 was more severe among the people who did not receive finerenone, nor did finerenone treatment appear to affect pneumonia recovery.
Analysis based on adverse events reports
These secondary analyses are far from definitive. The authors relied on pneumonia and COVID-19 being reported as adverse events. Each investigator diagnosed pneumonia at their discretion, and the trials did not specify diagnostic criteria. The authors also acknowledge that testing for COVID-19 was “not widespread” and that one of the two pivotal trials largely ran prior to the onset of the COVID-19 pandemic so that only 6 participants developed COVID-19 symptoms out of more than 5,700 enrolled.
The authors hypothesize that several actions of finerenone might potentially help mediate an effect on pneumonia and COVID-19: improvements in pulmonary inflammation and fibrosis, upregulation of expression of angiotensin converting enzyme 2, and amelioration of right heart pressure and pulmonary congestion. Also, antagonizing the mineralocorticoid receptor on monocytes and macrophages may block macrophage infiltration and accumulation of active macrophages, which can mediate the pulmonary tissue damage caused by COVID-19.
The FIDELIO-DKD and FIGARO-DKD trials and the FIDELITY combined database were sponsored by Bayer, the company that markets finerenone (Kerendia). Dr. Pitt has received personal fees from Bayer and personal fees and stock options from numerous other companies. Several coauthors reported having a financial relationship with Bayer, as well as with other companies.
A version of this article first appeared on Medscape.com.
The nonsteroidal mineralocorticoid receptor antagonist finerenone (Kerendia) unexpectedly showed that it might protect against incident infective pneumonia and COVID-19. The finding was based on secondary analyses run on more than 13,000 people enrolled in the two pivotal trials for finerenone.
Finerenone was approved by the Food and Drug Administration in 2021 for slowing progressive renal dysfunction and preventing cardiovascular events in adults with type 2 diabetes and chronic kidney disease (CKD).
‘Striking reduction in the risk of pneumonia’
The “striking reduction in risk of pneumonia” in a new analysis suggests that “the propagation of pulmonary infection into lobar or bronchial consolidation may be reduced by finerenone,” write Bertram Pitt, MD, and coauthors in a report published on October 26 in JAMA Network Open.
They also suggest that if further studies confirm that finerenone treatment reduces complications from pneumonia and COVID-19, it would have “significant medical implications,” especially because of the limited treatment options now available for complications from COVID-19.
The new analyses used the FIDELITY dataset, a prespecified merging of results from the FIDELIO-DKD and FIGARO-DKD trials, which together enrolled 13,026 people with type 2 diabetes and CKD, as determined on the basis of the patients’ having a urine albumin-to-creatinine ratio of at least 30 mg/g.
The primary outcomes of these trials showed that treatment with finerenone led to significant slowing of the progression of CKD and a significant reduction in the incidence of cardiovascular events, compared with placebo during median follow-up of 3 years.
The new, secondary analyses focused on the 6.0% of participants in whom there was evidence of pneumonia and the 1.6% in whom there was evidence of having COVID-19. Pneumonia was the most common serious adverse event in the two trials, a finding consistent with the documented risk for pneumonia faced by people with CKD.
Finerenone linked with a 29% relative reduction in pneumonia
When analyzed by treatment, the incidence of pneumonia was 4.7% among those who received finerenone and 6.7% among those who received placebo. This translated into a significant relative risk reduction of 29% associated with finerenone treatment.
Analysis of COVID-19 adverse events showed a 1.3% incidence among those who received finerenone and a 1.8% incidence among those in the placebo group, which translated into a significant 27% relative risk reduction linked with finerenone treatment.
In contrast, the data showed no reduced incidence of several other respiratory infections among the finerenone recipients, including nasopharyngitis, bronchitis, and influenza. The data also showed no signal that pneumonia or COVID-19 was more severe among the people who did not receive finerenone, nor did finerenone treatment appear to affect pneumonia recovery.
Analysis based on adverse events reports
These secondary analyses are far from definitive. The authors relied on pneumonia and COVID-19 being reported as adverse events. Each investigator diagnosed pneumonia at their discretion, and the trials did not specify diagnostic criteria. The authors also acknowledge that testing for COVID-19 was “not widespread” and that one of the two pivotal trials largely ran prior to the onset of the COVID-19 pandemic so that only 6 participants developed COVID-19 symptoms out of more than 5,700 enrolled.
The authors hypothesize that several actions of finerenone might potentially help mediate an effect on pneumonia and COVID-19: improvements in pulmonary inflammation and fibrosis, upregulation of expression of angiotensin converting enzyme 2, and amelioration of right heart pressure and pulmonary congestion. Also, antagonizing the mineralocorticoid receptor on monocytes and macrophages may block macrophage infiltration and accumulation of active macrophages, which can mediate the pulmonary tissue damage caused by COVID-19.
The FIDELIO-DKD and FIGARO-DKD trials and the FIDELITY combined database were sponsored by Bayer, the company that markets finerenone (Kerendia). Dr. Pitt has received personal fees from Bayer and personal fees and stock options from numerous other companies. Several coauthors reported having a financial relationship with Bayer, as well as with other companies.
A version of this article first appeared on Medscape.com.
The nonsteroidal mineralocorticoid receptor antagonist finerenone (Kerendia) unexpectedly showed that it might protect against incident infective pneumonia and COVID-19. The finding was based on secondary analyses run on more than 13,000 people enrolled in the two pivotal trials for finerenone.
Finerenone was approved by the Food and Drug Administration in 2021 for slowing progressive renal dysfunction and preventing cardiovascular events in adults with type 2 diabetes and chronic kidney disease (CKD).
‘Striking reduction in the risk of pneumonia’
The “striking reduction in risk of pneumonia” in a new analysis suggests that “the propagation of pulmonary infection into lobar or bronchial consolidation may be reduced by finerenone,” write Bertram Pitt, MD, and coauthors in a report published on October 26 in JAMA Network Open.
They also suggest that if further studies confirm that finerenone treatment reduces complications from pneumonia and COVID-19, it would have “significant medical implications,” especially because of the limited treatment options now available for complications from COVID-19.
The new analyses used the FIDELITY dataset, a prespecified merging of results from the FIDELIO-DKD and FIGARO-DKD trials, which together enrolled 13,026 people with type 2 diabetes and CKD, as determined on the basis of the patients’ having a urine albumin-to-creatinine ratio of at least 30 mg/g.
The primary outcomes of these trials showed that treatment with finerenone led to significant slowing of the progression of CKD and a significant reduction in the incidence of cardiovascular events, compared with placebo during median follow-up of 3 years.
The new, secondary analyses focused on the 6.0% of participants in whom there was evidence of pneumonia and the 1.6% in whom there was evidence of having COVID-19. Pneumonia was the most common serious adverse event in the two trials, a finding consistent with the documented risk for pneumonia faced by people with CKD.
Finerenone linked with a 29% relative reduction in pneumonia
When analyzed by treatment, the incidence of pneumonia was 4.7% among those who received finerenone and 6.7% among those who received placebo. This translated into a significant relative risk reduction of 29% associated with finerenone treatment.
Analysis of COVID-19 adverse events showed a 1.3% incidence among those who received finerenone and a 1.8% incidence among those in the placebo group, which translated into a significant 27% relative risk reduction linked with finerenone treatment.
In contrast, the data showed no reduced incidence of several other respiratory infections among the finerenone recipients, including nasopharyngitis, bronchitis, and influenza. The data also showed no signal that pneumonia or COVID-19 was more severe among the people who did not receive finerenone, nor did finerenone treatment appear to affect pneumonia recovery.
Analysis based on adverse events reports
These secondary analyses are far from definitive. The authors relied on pneumonia and COVID-19 being reported as adverse events. Each investigator diagnosed pneumonia at their discretion, and the trials did not specify diagnostic criteria. The authors also acknowledge that testing for COVID-19 was “not widespread” and that one of the two pivotal trials largely ran prior to the onset of the COVID-19 pandemic so that only 6 participants developed COVID-19 symptoms out of more than 5,700 enrolled.
The authors hypothesize that several actions of finerenone might potentially help mediate an effect on pneumonia and COVID-19: improvements in pulmonary inflammation and fibrosis, upregulation of expression of angiotensin converting enzyme 2, and amelioration of right heart pressure and pulmonary congestion. Also, antagonizing the mineralocorticoid receptor on monocytes and macrophages may block macrophage infiltration and accumulation of active macrophages, which can mediate the pulmonary tissue damage caused by COVID-19.
The FIDELIO-DKD and FIGARO-DKD trials and the FIDELITY combined database were sponsored by Bayer, the company that markets finerenone (Kerendia). Dr. Pitt has received personal fees from Bayer and personal fees and stock options from numerous other companies. Several coauthors reported having a financial relationship with Bayer, as well as with other companies.
A version of this article first appeared on Medscape.com.