User login
ID Practitioner is an independent news source that provides infectious disease specialists with timely and relevant news and commentary about clinical developments and the impact of health care policy on the infectious disease specialist’s practice. Specialty focus topics include antimicrobial resistance, emerging infections, global ID, hepatitis, HIV, hospital-acquired infections, immunizations and vaccines, influenza, mycoses, pediatric infections, and STIs. Infectious Diseases News is owned by Frontline Medical Communications.
sofosbuvir
ritonavir with dasabuvir
discount
support path
program
ritonavir
greedy
ledipasvir
assistance
viekira pak
vpak
advocacy
needy
protest
abbvie
paritaprevir
ombitasvir
direct-acting antivirals
dasabuvir
gilead
fake-ovir
support
v pak
oasis
harvoni
section[contains(@class, 'footer-nav-section-wrapper')]
div[contains(@class, 'pane-pub-article-idp')]
div[contains(@class, 'pane-medstat-latest-articles-articles-section')]
div[contains(@class, 'pane-pub-home-idp')]
div[contains(@class, 'pane-pub-topic-idp')]
Biden’s COVID-19 challenge: 100 million vaccinations in the first 100 days. It won’t be easy.
It’s in the nature of presidential candidates and new presidents to promise big things. Just months after his 1961 inauguration, President John F. Kennedy vowed to send a man to the moon by the end of the decade. That pledge was kept, but many others haven’t been, such as candidate Bill Clinton’s promise to provide universal health care and presidential hopeful George H.W. Bush’s guarantee of no new taxes.
Now, during a once-in-a-century pandemic, incoming President Joe Biden has promised to provide 100 million COVID-19 vaccinations in his first 100 days in office.
“This team will help get … at least 100 million covid vaccine shots into the arms of the American people in the first 100 days,” Biden said during a Dec. 8 news conference introducing key members of his health team.
When first asked about his pledge, the Biden team said the president-elect meant 50 million people would get their two-dose regimen. The incoming administration has since updated this plan, saying it will release vaccine doses as soon as they’re available instead of holding back some of that supply for second doses.
Either way, Biden may run into difficulty meeting that 100 million mark.
“I think it’s an attainable goal. I think it’s going to be extremely challenging,” said Claire Hannan, executive director of the Association of Immunization Managers.
While a pace of 1 million doses a day is “somewhat of an increase over what we’re already doing,” a much higher rate of vaccinations will be necessary to stem the pandemic, said Larry Levitt, executive vice president for health policy at Kaiser Family Foundation. (KHN is an editorially independent program of KFF.) “The Biden administration has plans to rationalize vaccine distribution, but increasing the supply quickly” could be a difficult task.
Under the Trump administration, vaccine deployment has been much slower than Biden’s plan. The rollout began on Dec. 14. Since then, 12 million shots have been given and 31 million doses have been shipped out, according to the Centers for Disease Control and Prevention’s vaccine tracker.
This sluggishness has been attributed to a lack of communication between the federal government and state and local health departments, not enough funding for large-scale vaccination efforts, and confusing federal guidance on distribution of the vaccines.
The same problems could plague the Biden administration, said experts.
States still aren’t sure how much vaccine they’ll get and whether there will be a sufficient supply, said Dr. Marcus Plescia, chief medical officer for the Association of State and Territorial Health Officials, which represents state public health agencies.
“We have been given little information about the amount of vaccine the states will receive in the near future and are of the impression that there may not be 1 million doses available per day in the first 100 days of the Biden administration,” said Dr. Plescia. “Or at least not in the early stages of the 100 days.”
Another challenge has been a lack of funding. Public health departments have had to start vaccination campaigns while also operating testing centers and conducting contact tracing efforts with budgets that have been critically underfunded for years.
“States have to pay for creating the systems, identifying the personnel, training, staffing, tracking people, information campaigns – all the things that go into getting a shot in someone’s arm,” said Jennifer Kates, director of global health & HIV policy at KFF. “They’re having to create an unprecedented mass vaccination program on a shaky foundation.”
The latest covid stimulus bill, signed into law in December, allocates almost $9 billion in funding to the CDC for vaccination efforts. About $4.5 billion is supposed to go to states, territories and tribal organizations, and $3 billion of that is slated to arrive soon.
But it’s not clear that level of funding can sustain mass vaccination campaigns as more groups become eligible for the vaccine.
Biden released a $1.9 trillion plan last week to address covid and the struggling economy. It includes $160 billion to create national vaccination and testing programs, but also earmarks funds for $1,400 stimulus payments to individuals, state and local government aid, extension of unemployment insurance, and financial assistance for schools to reopen safely.
Though it took Congress almost eight months to pass the last covid relief bill after Republican objections to the cost, Biden seems optimistic he’ll get some Republicans on board for his plan. But it’s not yet clear that will work.
There’s also the question of whether outgoing President Donald Trump’s impeachment trial will get in the way of Biden’s legislative priorities.
In addition, states have complained about a lack of guidance and confusing instructions on which groups should be given priority status for vaccination, an issue the Biden administration will need to address.
On Dec. 3, the CDC recommended health care personnel, residents of long-term care facilities, those 75 and older, and front-line essential workers should be immunized first. But on Jan. 12, the CDC shifted course and recommended that everyone over age 65 should be immunized. In a speech Biden gave on Jan. 15 detailing his vaccination plan, he said he would stick to the CDC’s recommendation to prioritize those over 65.
Outgoing Health and Human Services Secretary Alex Azar also said on Jan. 12 that states that moved their vaccine supply fastest would be prioritized in getting more shipments. It’s not known yet whether the Biden administration’s CDC will stick to this guidance. Critics have said it could make vaccine distribution less equitable.
In general, taking over with a strong vision and clear communication will be key to ramping up vaccine distribution, said Ms. Hannan.
“Everyone needs to understand what the goal is and how it’s going to work,” she said.
A challenge for Biden will be tamping expectations that the vaccine is all that is needed to end the pandemic. Across the country, covid cases are higher than ever, and in many locations officials cannot control the spread.
Public health experts said Biden must amp up efforts to increase testing across the country, as he has suggested he will do by promising to establish a national pandemic testing board.
With so much focus on vaccine distribution, it’s important that this part of the equation not be lost. Right now, “it’s completely all over the map,” said KFF’s Ms. Kates, adding that the federal government will need a “good sense” of who is and is not being tested in different areas in order to “fix” public health capacity.
Jan. 20, 2021, marks the launch of The Biden Promise Tracker, which monitors the 100 most important campaign promises of President Joseph R. Biden. Biden listed the coronavirus and a variety of other health-related issues among his top priorities. You can see the entire list – including improving the economy, responding to calls for racial justice and combating climate change – here. As part of KHN’s partnership with PolitiFact, we will follow the health-related issues and then rate them on whether the promise was achieved: Promise Kept, Promise Broken, Compromise, Stalled, In the Works or Not Yet Rated. We rate the promise not on the president’s intentions or effort, but on verifiable outcomes. PolitiFact previously tracked the promises of President Donald Trump and President Barack Obama.
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF, which is not affiliated with Kaiser Permanente.
It’s in the nature of presidential candidates and new presidents to promise big things. Just months after his 1961 inauguration, President John F. Kennedy vowed to send a man to the moon by the end of the decade. That pledge was kept, but many others haven’t been, such as candidate Bill Clinton’s promise to provide universal health care and presidential hopeful George H.W. Bush’s guarantee of no new taxes.
Now, during a once-in-a-century pandemic, incoming President Joe Biden has promised to provide 100 million COVID-19 vaccinations in his first 100 days in office.
“This team will help get … at least 100 million covid vaccine shots into the arms of the American people in the first 100 days,” Biden said during a Dec. 8 news conference introducing key members of his health team.
When first asked about his pledge, the Biden team said the president-elect meant 50 million people would get their two-dose regimen. The incoming administration has since updated this plan, saying it will release vaccine doses as soon as they’re available instead of holding back some of that supply for second doses.
Either way, Biden may run into difficulty meeting that 100 million mark.
“I think it’s an attainable goal. I think it’s going to be extremely challenging,” said Claire Hannan, executive director of the Association of Immunization Managers.
While a pace of 1 million doses a day is “somewhat of an increase over what we’re already doing,” a much higher rate of vaccinations will be necessary to stem the pandemic, said Larry Levitt, executive vice president for health policy at Kaiser Family Foundation. (KHN is an editorially independent program of KFF.) “The Biden administration has plans to rationalize vaccine distribution, but increasing the supply quickly” could be a difficult task.
Under the Trump administration, vaccine deployment has been much slower than Biden’s plan. The rollout began on Dec. 14. Since then, 12 million shots have been given and 31 million doses have been shipped out, according to the Centers for Disease Control and Prevention’s vaccine tracker.
This sluggishness has been attributed to a lack of communication between the federal government and state and local health departments, not enough funding for large-scale vaccination efforts, and confusing federal guidance on distribution of the vaccines.
The same problems could plague the Biden administration, said experts.
States still aren’t sure how much vaccine they’ll get and whether there will be a sufficient supply, said Dr. Marcus Plescia, chief medical officer for the Association of State and Territorial Health Officials, which represents state public health agencies.
“We have been given little information about the amount of vaccine the states will receive in the near future and are of the impression that there may not be 1 million doses available per day in the first 100 days of the Biden administration,” said Dr. Plescia. “Or at least not in the early stages of the 100 days.”
Another challenge has been a lack of funding. Public health departments have had to start vaccination campaigns while also operating testing centers and conducting contact tracing efforts with budgets that have been critically underfunded for years.
“States have to pay for creating the systems, identifying the personnel, training, staffing, tracking people, information campaigns – all the things that go into getting a shot in someone’s arm,” said Jennifer Kates, director of global health & HIV policy at KFF. “They’re having to create an unprecedented mass vaccination program on a shaky foundation.”
The latest covid stimulus bill, signed into law in December, allocates almost $9 billion in funding to the CDC for vaccination efforts. About $4.5 billion is supposed to go to states, territories and tribal organizations, and $3 billion of that is slated to arrive soon.
But it’s not clear that level of funding can sustain mass vaccination campaigns as more groups become eligible for the vaccine.
Biden released a $1.9 trillion plan last week to address covid and the struggling economy. It includes $160 billion to create national vaccination and testing programs, but also earmarks funds for $1,400 stimulus payments to individuals, state and local government aid, extension of unemployment insurance, and financial assistance for schools to reopen safely.
Though it took Congress almost eight months to pass the last covid relief bill after Republican objections to the cost, Biden seems optimistic he’ll get some Republicans on board for his plan. But it’s not yet clear that will work.
There’s also the question of whether outgoing President Donald Trump’s impeachment trial will get in the way of Biden’s legislative priorities.
In addition, states have complained about a lack of guidance and confusing instructions on which groups should be given priority status for vaccination, an issue the Biden administration will need to address.
On Dec. 3, the CDC recommended health care personnel, residents of long-term care facilities, those 75 and older, and front-line essential workers should be immunized first. But on Jan. 12, the CDC shifted course and recommended that everyone over age 65 should be immunized. In a speech Biden gave on Jan. 15 detailing his vaccination plan, he said he would stick to the CDC’s recommendation to prioritize those over 65.
Outgoing Health and Human Services Secretary Alex Azar also said on Jan. 12 that states that moved their vaccine supply fastest would be prioritized in getting more shipments. It’s not known yet whether the Biden administration’s CDC will stick to this guidance. Critics have said it could make vaccine distribution less equitable.
In general, taking over with a strong vision and clear communication will be key to ramping up vaccine distribution, said Ms. Hannan.
“Everyone needs to understand what the goal is and how it’s going to work,” she said.
A challenge for Biden will be tamping expectations that the vaccine is all that is needed to end the pandemic. Across the country, covid cases are higher than ever, and in many locations officials cannot control the spread.
Public health experts said Biden must amp up efforts to increase testing across the country, as he has suggested he will do by promising to establish a national pandemic testing board.
With so much focus on vaccine distribution, it’s important that this part of the equation not be lost. Right now, “it’s completely all over the map,” said KFF’s Ms. Kates, adding that the federal government will need a “good sense” of who is and is not being tested in different areas in order to “fix” public health capacity.
Jan. 20, 2021, marks the launch of The Biden Promise Tracker, which monitors the 100 most important campaign promises of President Joseph R. Biden. Biden listed the coronavirus and a variety of other health-related issues among his top priorities. You can see the entire list – including improving the economy, responding to calls for racial justice and combating climate change – here. As part of KHN’s partnership with PolitiFact, we will follow the health-related issues and then rate them on whether the promise was achieved: Promise Kept, Promise Broken, Compromise, Stalled, In the Works or Not Yet Rated. We rate the promise not on the president’s intentions or effort, but on verifiable outcomes. PolitiFact previously tracked the promises of President Donald Trump and President Barack Obama.
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF, which is not affiliated with Kaiser Permanente.
It’s in the nature of presidential candidates and new presidents to promise big things. Just months after his 1961 inauguration, President John F. Kennedy vowed to send a man to the moon by the end of the decade. That pledge was kept, but many others haven’t been, such as candidate Bill Clinton’s promise to provide universal health care and presidential hopeful George H.W. Bush’s guarantee of no new taxes.
Now, during a once-in-a-century pandemic, incoming President Joe Biden has promised to provide 100 million COVID-19 vaccinations in his first 100 days in office.
“This team will help get … at least 100 million covid vaccine shots into the arms of the American people in the first 100 days,” Biden said during a Dec. 8 news conference introducing key members of his health team.
When first asked about his pledge, the Biden team said the president-elect meant 50 million people would get their two-dose regimen. The incoming administration has since updated this plan, saying it will release vaccine doses as soon as they’re available instead of holding back some of that supply for second doses.
Either way, Biden may run into difficulty meeting that 100 million mark.
“I think it’s an attainable goal. I think it’s going to be extremely challenging,” said Claire Hannan, executive director of the Association of Immunization Managers.
While a pace of 1 million doses a day is “somewhat of an increase over what we’re already doing,” a much higher rate of vaccinations will be necessary to stem the pandemic, said Larry Levitt, executive vice president for health policy at Kaiser Family Foundation. (KHN is an editorially independent program of KFF.) “The Biden administration has plans to rationalize vaccine distribution, but increasing the supply quickly” could be a difficult task.
Under the Trump administration, vaccine deployment has been much slower than Biden’s plan. The rollout began on Dec. 14. Since then, 12 million shots have been given and 31 million doses have been shipped out, according to the Centers for Disease Control and Prevention’s vaccine tracker.
This sluggishness has been attributed to a lack of communication between the federal government and state and local health departments, not enough funding for large-scale vaccination efforts, and confusing federal guidance on distribution of the vaccines.
The same problems could plague the Biden administration, said experts.
States still aren’t sure how much vaccine they’ll get and whether there will be a sufficient supply, said Dr. Marcus Plescia, chief medical officer for the Association of State and Territorial Health Officials, which represents state public health agencies.
“We have been given little information about the amount of vaccine the states will receive in the near future and are of the impression that there may not be 1 million doses available per day in the first 100 days of the Biden administration,” said Dr. Plescia. “Or at least not in the early stages of the 100 days.”
Another challenge has been a lack of funding. Public health departments have had to start vaccination campaigns while also operating testing centers and conducting contact tracing efforts with budgets that have been critically underfunded for years.
“States have to pay for creating the systems, identifying the personnel, training, staffing, tracking people, information campaigns – all the things that go into getting a shot in someone’s arm,” said Jennifer Kates, director of global health & HIV policy at KFF. “They’re having to create an unprecedented mass vaccination program on a shaky foundation.”
The latest covid stimulus bill, signed into law in December, allocates almost $9 billion in funding to the CDC for vaccination efforts. About $4.5 billion is supposed to go to states, territories and tribal organizations, and $3 billion of that is slated to arrive soon.
But it’s not clear that level of funding can sustain mass vaccination campaigns as more groups become eligible for the vaccine.
Biden released a $1.9 trillion plan last week to address covid and the struggling economy. It includes $160 billion to create national vaccination and testing programs, but also earmarks funds for $1,400 stimulus payments to individuals, state and local government aid, extension of unemployment insurance, and financial assistance for schools to reopen safely.
Though it took Congress almost eight months to pass the last covid relief bill after Republican objections to the cost, Biden seems optimistic he’ll get some Republicans on board for his plan. But it’s not yet clear that will work.
There’s also the question of whether outgoing President Donald Trump’s impeachment trial will get in the way of Biden’s legislative priorities.
In addition, states have complained about a lack of guidance and confusing instructions on which groups should be given priority status for vaccination, an issue the Biden administration will need to address.
On Dec. 3, the CDC recommended health care personnel, residents of long-term care facilities, those 75 and older, and front-line essential workers should be immunized first. But on Jan. 12, the CDC shifted course and recommended that everyone over age 65 should be immunized. In a speech Biden gave on Jan. 15 detailing his vaccination plan, he said he would stick to the CDC’s recommendation to prioritize those over 65.
Outgoing Health and Human Services Secretary Alex Azar also said on Jan. 12 that states that moved their vaccine supply fastest would be prioritized in getting more shipments. It’s not known yet whether the Biden administration’s CDC will stick to this guidance. Critics have said it could make vaccine distribution less equitable.
In general, taking over with a strong vision and clear communication will be key to ramping up vaccine distribution, said Ms. Hannan.
“Everyone needs to understand what the goal is and how it’s going to work,” she said.
A challenge for Biden will be tamping expectations that the vaccine is all that is needed to end the pandemic. Across the country, covid cases are higher than ever, and in many locations officials cannot control the spread.
Public health experts said Biden must amp up efforts to increase testing across the country, as he has suggested he will do by promising to establish a national pandemic testing board.
With so much focus on vaccine distribution, it’s important that this part of the equation not be lost. Right now, “it’s completely all over the map,” said KFF’s Ms. Kates, adding that the federal government will need a “good sense” of who is and is not being tested in different areas in order to “fix” public health capacity.
Jan. 20, 2021, marks the launch of The Biden Promise Tracker, which monitors the 100 most important campaign promises of President Joseph R. Biden. Biden listed the coronavirus and a variety of other health-related issues among his top priorities. You can see the entire list – including improving the economy, responding to calls for racial justice and combating climate change – here. As part of KHN’s partnership with PolitiFact, we will follow the health-related issues and then rate them on whether the promise was achieved: Promise Kept, Promise Broken, Compromise, Stalled, In the Works or Not Yet Rated. We rate the promise not on the president’s intentions or effort, but on verifiable outcomes. PolitiFact previously tracked the promises of President Donald Trump and President Barack Obama.
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF, which is not affiliated with Kaiser Permanente.
Many EM docs have treated COVID-19 patients without proper PPE: Survey
Many emergency medicine (EM) physicians who responded to a Medscape survey said they have treated COVID-19 patients without appropriate personal protective equipment (PPE).
In the Medscape Emergency Medicine Physicians’ COVID-19 Experience Report, 21% of respondents said that that was sometimes the case; 7% said that it was often the case; and 1% said they always treat patients without appropriate PPE.
EM physicians were the physicians most likely to treat COVID-19 patients in person.
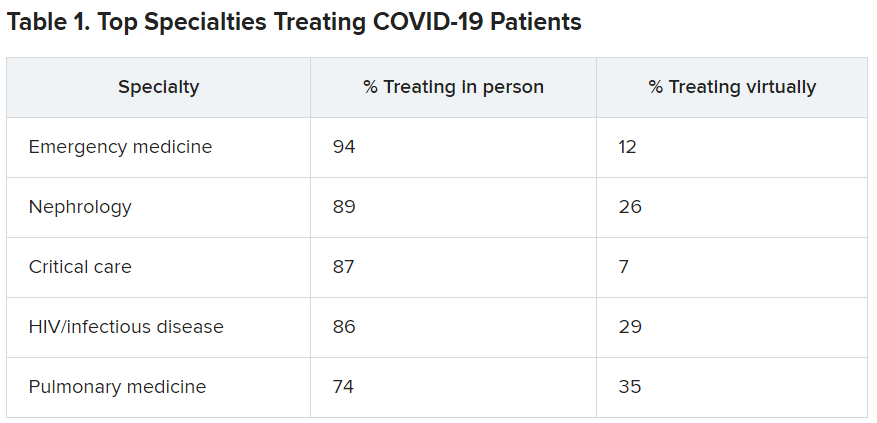
For comparison, among family medicine physicians, 58% said that they have treated COVID-19 patients in person, and 45% said they were treating them via telemedicine.
Data for the report were gathered from June 9 to July 20 as part of Medscape’s COVID-19 experience survey for all physicians. That survey drew more than 5,000 responses.
Nearly all (98%) of EM physicians who have treated COVID-19 patients said that they have done so since the beginning, when the World Health Organization declared a pandemic on March 11, 2020. For all U.S. physicians, the percentage was much higher than that – 73% said they had treated COVID-19 patients from the start.
EM physicians have often found themselves sacrificing their own safety for the sake of patients. More than half of EM physicians (54%) said that they had knowingly taken personal safety risks to treat a COVID-19 emergency, a percentage far higher than the 30% of all physicians who said they had done so.
Four percent of EM physicians have received a positive diagnosis of COVID-19 via testing. An additional 2% have been confirmed as having COVID on the basis of symptoms.
Steep income drops
Survey authors wrote that two-thirds of EM physicians have experienced income loss during the pandemic. Most (71%) saw their income drop by between 11% and 50%; 11% saw a decrease of more than 50%. Among other specialties, the percentages of those who have experienced a drop of more than 50% are far higher. Among ophthalmologists, 51% said they had experienced such a drop; among allergists, 46%; plastic surgeons, 46%; and otolaryngologists, 45%.
Asked whether their burnout levels have increased in the wake of COVID-19, 74% of EM physicians said burnout had intensified; 23% reported no change; and 3% said burnout had lessened.
Reports of loneliness have been widespread during the pandemic, owing to stay-at-home orders and social distancing. More EM physicians than physicians in general said feelings of loneliness had increased for them in the past year.
More than half of EM doctors (55%) said they are experiencing more loneliness in the pandemic, compared with 46% of all physicians who felt that way; 42% said those feelings have not changed; and 3% said they have been less lonely.
Grief and stress relief
Fewer than half (42%) of the respondents reported that their workplace offers clinician activities to help with grief and stress; 39% said their workplace didn’t offer such help; and 19% said they were unsure.
The percentages were nearly identical to the percentages of physicians overall who answered whether their workplace offered help for grief and stress.
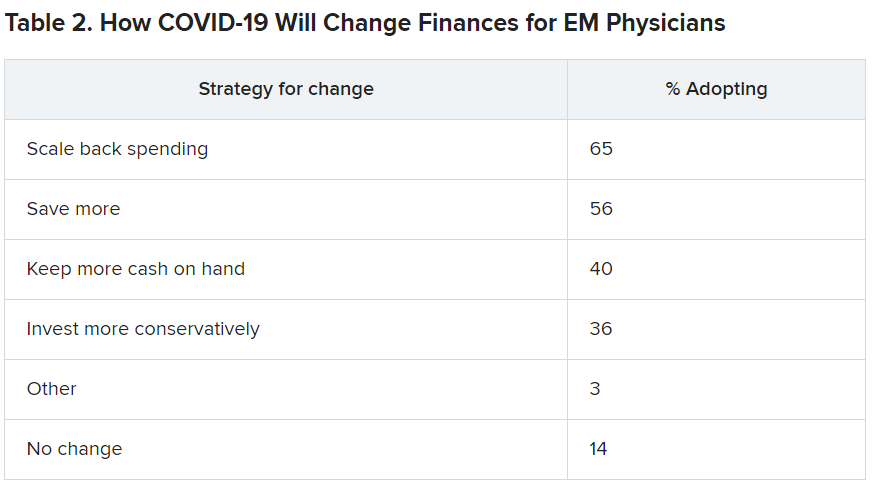
Along with insecurity regarding physical and mental health, COVID-19 has introduced more questions about financial health. Here’s a look at how emergency physicians said they would change the way they save and spend.
Challenges to daily practice
By the time this survey was taken, a large percentage of patients had delayed or avoided urgent or routine medical care for reasons related to COVID-19, so survey authors asked whether EM physicians’ patient population had changed.
Survey authors wrote that “most EM physicians (82%) are seeing patients with non-COVID diseases, such as cardiovascular problems or diabetes, who otherwise probably would have sought treatment earlier.”
COVID-19 has also thrown a major obstacle into most EM physicians’ careers by preventing them from doing the job to the best of their ability. That loss is one of the three primary components of burnout.
More than two-thirds (67%) said COVID-19 has hampered their ability to be as good a doctor as they would like.
A version of this article first appeared on Medscape.com.
Many emergency medicine (EM) physicians who responded to a Medscape survey said they have treated COVID-19 patients without appropriate personal protective equipment (PPE).
In the Medscape Emergency Medicine Physicians’ COVID-19 Experience Report, 21% of respondents said that that was sometimes the case; 7% said that it was often the case; and 1% said they always treat patients without appropriate PPE.
EM physicians were the physicians most likely to treat COVID-19 patients in person.

For comparison, among family medicine physicians, 58% said that they have treated COVID-19 patients in person, and 45% said they were treating them via telemedicine.
Data for the report were gathered from June 9 to July 20 as part of Medscape’s COVID-19 experience survey for all physicians. That survey drew more than 5,000 responses.
Nearly all (98%) of EM physicians who have treated COVID-19 patients said that they have done so since the beginning, when the World Health Organization declared a pandemic on March 11, 2020. For all U.S. physicians, the percentage was much higher than that – 73% said they had treated COVID-19 patients from the start.
EM physicians have often found themselves sacrificing their own safety for the sake of patients. More than half of EM physicians (54%) said that they had knowingly taken personal safety risks to treat a COVID-19 emergency, a percentage far higher than the 30% of all physicians who said they had done so.
Four percent of EM physicians have received a positive diagnosis of COVID-19 via testing. An additional 2% have been confirmed as having COVID on the basis of symptoms.
Steep income drops
Survey authors wrote that two-thirds of EM physicians have experienced income loss during the pandemic. Most (71%) saw their income drop by between 11% and 50%; 11% saw a decrease of more than 50%. Among other specialties, the percentages of those who have experienced a drop of more than 50% are far higher. Among ophthalmologists, 51% said they had experienced such a drop; among allergists, 46%; plastic surgeons, 46%; and otolaryngologists, 45%.
Asked whether their burnout levels have increased in the wake of COVID-19, 74% of EM physicians said burnout had intensified; 23% reported no change; and 3% said burnout had lessened.
Reports of loneliness have been widespread during the pandemic, owing to stay-at-home orders and social distancing. More EM physicians than physicians in general said feelings of loneliness had increased for them in the past year.
More than half of EM doctors (55%) said they are experiencing more loneliness in the pandemic, compared with 46% of all physicians who felt that way; 42% said those feelings have not changed; and 3% said they have been less lonely.
Grief and stress relief
Fewer than half (42%) of the respondents reported that their workplace offers clinician activities to help with grief and stress; 39% said their workplace didn’t offer such help; and 19% said they were unsure.
The percentages were nearly identical to the percentages of physicians overall who answered whether their workplace offered help for grief and stress.
Along with insecurity regarding physical and mental health, COVID-19 has introduced more questions about financial health. Here’s a look at how emergency physicians said they would change the way they save and spend.

Challenges to daily practice
By the time this survey was taken, a large percentage of patients had delayed or avoided urgent or routine medical care for reasons related to COVID-19, so survey authors asked whether EM physicians’ patient population had changed.
Survey authors wrote that “most EM physicians (82%) are seeing patients with non-COVID diseases, such as cardiovascular problems or diabetes, who otherwise probably would have sought treatment earlier.”
COVID-19 has also thrown a major obstacle into most EM physicians’ careers by preventing them from doing the job to the best of their ability. That loss is one of the three primary components of burnout.
More than two-thirds (67%) said COVID-19 has hampered their ability to be as good a doctor as they would like.
A version of this article first appeared on Medscape.com.
Many emergency medicine (EM) physicians who responded to a Medscape survey said they have treated COVID-19 patients without appropriate personal protective equipment (PPE).
In the Medscape Emergency Medicine Physicians’ COVID-19 Experience Report, 21% of respondents said that that was sometimes the case; 7% said that it was often the case; and 1% said they always treat patients without appropriate PPE.
EM physicians were the physicians most likely to treat COVID-19 patients in person.

For comparison, among family medicine physicians, 58% said that they have treated COVID-19 patients in person, and 45% said they were treating them via telemedicine.
Data for the report were gathered from June 9 to July 20 as part of Medscape’s COVID-19 experience survey for all physicians. That survey drew more than 5,000 responses.
Nearly all (98%) of EM physicians who have treated COVID-19 patients said that they have done so since the beginning, when the World Health Organization declared a pandemic on March 11, 2020. For all U.S. physicians, the percentage was much higher than that – 73% said they had treated COVID-19 patients from the start.
EM physicians have often found themselves sacrificing their own safety for the sake of patients. More than half of EM physicians (54%) said that they had knowingly taken personal safety risks to treat a COVID-19 emergency, a percentage far higher than the 30% of all physicians who said they had done so.
Four percent of EM physicians have received a positive diagnosis of COVID-19 via testing. An additional 2% have been confirmed as having COVID on the basis of symptoms.
Steep income drops
Survey authors wrote that two-thirds of EM physicians have experienced income loss during the pandemic. Most (71%) saw their income drop by between 11% and 50%; 11% saw a decrease of more than 50%. Among other specialties, the percentages of those who have experienced a drop of more than 50% are far higher. Among ophthalmologists, 51% said they had experienced such a drop; among allergists, 46%; plastic surgeons, 46%; and otolaryngologists, 45%.
Asked whether their burnout levels have increased in the wake of COVID-19, 74% of EM physicians said burnout had intensified; 23% reported no change; and 3% said burnout had lessened.
Reports of loneliness have been widespread during the pandemic, owing to stay-at-home orders and social distancing. More EM physicians than physicians in general said feelings of loneliness had increased for them in the past year.
More than half of EM doctors (55%) said they are experiencing more loneliness in the pandemic, compared with 46% of all physicians who felt that way; 42% said those feelings have not changed; and 3% said they have been less lonely.
Grief and stress relief
Fewer than half (42%) of the respondents reported that their workplace offers clinician activities to help with grief and stress; 39% said their workplace didn’t offer such help; and 19% said they were unsure.
The percentages were nearly identical to the percentages of physicians overall who answered whether their workplace offered help for grief and stress.
Along with insecurity regarding physical and mental health, COVID-19 has introduced more questions about financial health. Here’s a look at how emergency physicians said they would change the way they save and spend.

Challenges to daily practice
By the time this survey was taken, a large percentage of patients had delayed or avoided urgent or routine medical care for reasons related to COVID-19, so survey authors asked whether EM physicians’ patient population had changed.
Survey authors wrote that “most EM physicians (82%) are seeing patients with non-COVID diseases, such as cardiovascular problems or diabetes, who otherwise probably would have sought treatment earlier.”
COVID-19 has also thrown a major obstacle into most EM physicians’ careers by preventing them from doing the job to the best of their ability. That loss is one of the three primary components of burnout.
More than two-thirds (67%) said COVID-19 has hampered their ability to be as good a doctor as they would like.
A version of this article first appeared on Medscape.com.
Further warning on SGLT2 inhibitor use and DKA risk in COVID-19
a new case series suggests.
Five patients with type 2 diabetes who were taking SGLT2 inhibitors presented in DKA despite having glucose levels below 300 mg/dL. The report was published online last month in AACE Clinical Case Reports by Rebecca J. Vitale, MD, and colleagues at Brigham and Women’s Hospital, Boston.
“A cluster of euglycemic DKA cases at our hospital during the first wave of the pandemic suggests that patients with diabetes taking SGLT2 inhibitors may be at enhanced risk for euDKA when they contract COVID-19,” senior author Naomi D.L. Fisher, MD, said in an interview.
Dr. Fisher, an endocrinologist, added: “This complication is preventable with the simple measure of holding the drug. We are hopeful that widespread patient and physician education will prevent future cases of euDKA as COVID-19 infections continue to surge.”
These cases underscore recommendations published early in the COVID-19 pandemic by an international panel, she noted.
“Patients who are acutely ill with nausea, vomiting, abdominal pain, or diarrhea, or who are experiencing loss of appetite with reduced food and fluid intake, should be advised to hold their SGLT2 inhibitor. This medication should not be resumed until patients are feeling better and eating and drinking normally.”
On the other hand, “If patients with asymptomatic or mild COVID-19 infection are otherwise well, and are eating and drinking normally, there is no evidence that SGLT2 inhibitors need to be stopped. These patients should monitor [themselves] closely for worsening symptoms, especially resulting in poor hydration and nutrition, which would be reason to discontinue their medication.”
Pay special attention to the elderly, those with complications
However, special consideration should be given to elderly patients and those with medical conditions known to increase the likelihood of severe infection, like heart failure and chronic obstructive pulmonary disease, Dr. Fisher added.
The SGLT2 inhibitor class of drugs causes significant urinary glucose excretion, and they are also diuretics. A decrease in available glucose and volume depletion are probably both important contributors to euDKA, she explained.
With COVID-19 infection the euDKA risk is compounded by several mechanisms. Most cases of euDKA are associated with an underlying state of starvation that can be triggered by vomiting, diarrhea, loss of appetite, and poor oral intake.
In addition – although not yet known for certain – SARS-CoV-2 may also be toxic to pancreatic beta cells and thus reduce insulin secretion. The maladaptive inflammatory response seen with COVID-19 may also contribute, she said.
The patients in the current case series were three men and two women seen between March and May 2020. They ranged in age from 52 to 79 years.
None had a prior history of DKA or any known diabetes complications. In all of them, antihyperglycemic medications, including SGLT2 inhibitors, were stopped on hospital admission. The patients were initially treated with intravenous insulin, and then subcutaneous insulin after the DKA diagnosis.
Three of the patients were discharged to rehabilitation facilities on hospital days 28-47 and one (age 53 years) was discharged home on day 11. The other patient also had hypertension and nonalcoholic steatohepatitis.
A version of this article first appeared on Medscape.com.
a new case series suggests.
Five patients with type 2 diabetes who were taking SGLT2 inhibitors presented in DKA despite having glucose levels below 300 mg/dL. The report was published online last month in AACE Clinical Case Reports by Rebecca J. Vitale, MD, and colleagues at Brigham and Women’s Hospital, Boston.
“A cluster of euglycemic DKA cases at our hospital during the first wave of the pandemic suggests that patients with diabetes taking SGLT2 inhibitors may be at enhanced risk for euDKA when they contract COVID-19,” senior author Naomi D.L. Fisher, MD, said in an interview.
Dr. Fisher, an endocrinologist, added: “This complication is preventable with the simple measure of holding the drug. We are hopeful that widespread patient and physician education will prevent future cases of euDKA as COVID-19 infections continue to surge.”
These cases underscore recommendations published early in the COVID-19 pandemic by an international panel, she noted.
“Patients who are acutely ill with nausea, vomiting, abdominal pain, or diarrhea, or who are experiencing loss of appetite with reduced food and fluid intake, should be advised to hold their SGLT2 inhibitor. This medication should not be resumed until patients are feeling better and eating and drinking normally.”
On the other hand, “If patients with asymptomatic or mild COVID-19 infection are otherwise well, and are eating and drinking normally, there is no evidence that SGLT2 inhibitors need to be stopped. These patients should monitor [themselves] closely for worsening symptoms, especially resulting in poor hydration and nutrition, which would be reason to discontinue their medication.”
Pay special attention to the elderly, those with complications
However, special consideration should be given to elderly patients and those with medical conditions known to increase the likelihood of severe infection, like heart failure and chronic obstructive pulmonary disease, Dr. Fisher added.
The SGLT2 inhibitor class of drugs causes significant urinary glucose excretion, and they are also diuretics. A decrease in available glucose and volume depletion are probably both important contributors to euDKA, she explained.
With COVID-19 infection the euDKA risk is compounded by several mechanisms. Most cases of euDKA are associated with an underlying state of starvation that can be triggered by vomiting, diarrhea, loss of appetite, and poor oral intake.
In addition – although not yet known for certain – SARS-CoV-2 may also be toxic to pancreatic beta cells and thus reduce insulin secretion. The maladaptive inflammatory response seen with COVID-19 may also contribute, she said.
The patients in the current case series were three men and two women seen between March and May 2020. They ranged in age from 52 to 79 years.
None had a prior history of DKA or any known diabetes complications. In all of them, antihyperglycemic medications, including SGLT2 inhibitors, were stopped on hospital admission. The patients were initially treated with intravenous insulin, and then subcutaneous insulin after the DKA diagnosis.
Three of the patients were discharged to rehabilitation facilities on hospital days 28-47 and one (age 53 years) was discharged home on day 11. The other patient also had hypertension and nonalcoholic steatohepatitis.
A version of this article first appeared on Medscape.com.
a new case series suggests.
Five patients with type 2 diabetes who were taking SGLT2 inhibitors presented in DKA despite having glucose levels below 300 mg/dL. The report was published online last month in AACE Clinical Case Reports by Rebecca J. Vitale, MD, and colleagues at Brigham and Women’s Hospital, Boston.
“A cluster of euglycemic DKA cases at our hospital during the first wave of the pandemic suggests that patients with diabetes taking SGLT2 inhibitors may be at enhanced risk for euDKA when they contract COVID-19,” senior author Naomi D.L. Fisher, MD, said in an interview.
Dr. Fisher, an endocrinologist, added: “This complication is preventable with the simple measure of holding the drug. We are hopeful that widespread patient and physician education will prevent future cases of euDKA as COVID-19 infections continue to surge.”
These cases underscore recommendations published early in the COVID-19 pandemic by an international panel, she noted.
“Patients who are acutely ill with nausea, vomiting, abdominal pain, or diarrhea, or who are experiencing loss of appetite with reduced food and fluid intake, should be advised to hold their SGLT2 inhibitor. This medication should not be resumed until patients are feeling better and eating and drinking normally.”
On the other hand, “If patients with asymptomatic or mild COVID-19 infection are otherwise well, and are eating and drinking normally, there is no evidence that SGLT2 inhibitors need to be stopped. These patients should monitor [themselves] closely for worsening symptoms, especially resulting in poor hydration and nutrition, which would be reason to discontinue their medication.”
Pay special attention to the elderly, those with complications
However, special consideration should be given to elderly patients and those with medical conditions known to increase the likelihood of severe infection, like heart failure and chronic obstructive pulmonary disease, Dr. Fisher added.
The SGLT2 inhibitor class of drugs causes significant urinary glucose excretion, and they are also diuretics. A decrease in available glucose and volume depletion are probably both important contributors to euDKA, she explained.
With COVID-19 infection the euDKA risk is compounded by several mechanisms. Most cases of euDKA are associated with an underlying state of starvation that can be triggered by vomiting, diarrhea, loss of appetite, and poor oral intake.
In addition – although not yet known for certain – SARS-CoV-2 may also be toxic to pancreatic beta cells and thus reduce insulin secretion. The maladaptive inflammatory response seen with COVID-19 may also contribute, she said.
The patients in the current case series were three men and two women seen between March and May 2020. They ranged in age from 52 to 79 years.
None had a prior history of DKA or any known diabetes complications. In all of them, antihyperglycemic medications, including SGLT2 inhibitors, were stopped on hospital admission. The patients were initially treated with intravenous insulin, and then subcutaneous insulin after the DKA diagnosis.
Three of the patients were discharged to rehabilitation facilities on hospital days 28-47 and one (age 53 years) was discharged home on day 11. The other patient also had hypertension and nonalcoholic steatohepatitis.
A version of this article first appeared on Medscape.com.
COVID-19 in children: Latest weekly increase is largest yet
according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
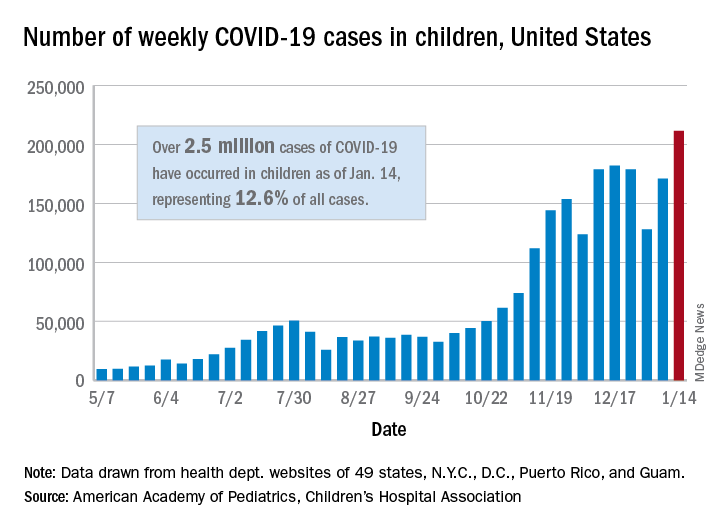
There were 211,466 new cases reported in children during the week of Jan. 8-14, topping the previous high (Dec. 11-17) by almost 30,000. Those new cases bring the total for the pandemic to over 2.5 million children infected with the coronavirus, which represents 12.6% of all reported cases, the AAP and the CHA said Jan. 19 in their weekly COVID-19 report.
The rise in cases also brought an increase in the proportion reported among children. The week before (Jan. 1-7), cases in children were 12.9% of all cases reported, but the most recent week saw that number rise to 14.5% of all cases, the highest it’s been since early October, based on data collected from the health department websites of 49 states (excluding New York), the District of Columbia, New York City, Puerto Rio, and Guam.
The corresponding figures for severe illness continue to be low: Children represent 1.8% of all hospitalizations from COVID-19 in 24 states and New York City and 0.06% of all deaths in 43 states and New York City. Three deaths were reported for the week of Jan. 8-14, making for a total of 191 since the pandemic started, the AAP and CHA said in their report.
Among the states, California has the most overall cases at just over 350,000, Wyoming has the highest proportion of cases in children (20.3%), and North Dakota has the highest rate of infection (over 8,100 per 100,000 children). The infection rate for the nation is now above 3,300 per 100,000 children, and 11 states reported rates over 5,000, according to the AAP and the CHA.
according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

There were 211,466 new cases reported in children during the week of Jan. 8-14, topping the previous high (Dec. 11-17) by almost 30,000. Those new cases bring the total for the pandemic to over 2.5 million children infected with the coronavirus, which represents 12.6% of all reported cases, the AAP and the CHA said Jan. 19 in their weekly COVID-19 report.
The rise in cases also brought an increase in the proportion reported among children. The week before (Jan. 1-7), cases in children were 12.9% of all cases reported, but the most recent week saw that number rise to 14.5% of all cases, the highest it’s been since early October, based on data collected from the health department websites of 49 states (excluding New York), the District of Columbia, New York City, Puerto Rio, and Guam.
The corresponding figures for severe illness continue to be low: Children represent 1.8% of all hospitalizations from COVID-19 in 24 states and New York City and 0.06% of all deaths in 43 states and New York City. Three deaths were reported for the week of Jan. 8-14, making for a total of 191 since the pandemic started, the AAP and CHA said in their report.
Among the states, California has the most overall cases at just over 350,000, Wyoming has the highest proportion of cases in children (20.3%), and North Dakota has the highest rate of infection (over 8,100 per 100,000 children). The infection rate for the nation is now above 3,300 per 100,000 children, and 11 states reported rates over 5,000, according to the AAP and the CHA.
according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

There were 211,466 new cases reported in children during the week of Jan. 8-14, topping the previous high (Dec. 11-17) by almost 30,000. Those new cases bring the total for the pandemic to over 2.5 million children infected with the coronavirus, which represents 12.6% of all reported cases, the AAP and the CHA said Jan. 19 in their weekly COVID-19 report.
The rise in cases also brought an increase in the proportion reported among children. The week before (Jan. 1-7), cases in children were 12.9% of all cases reported, but the most recent week saw that number rise to 14.5% of all cases, the highest it’s been since early October, based on data collected from the health department websites of 49 states (excluding New York), the District of Columbia, New York City, Puerto Rio, and Guam.
The corresponding figures for severe illness continue to be low: Children represent 1.8% of all hospitalizations from COVID-19 in 24 states and New York City and 0.06% of all deaths in 43 states and New York City. Three deaths were reported for the week of Jan. 8-14, making for a total of 191 since the pandemic started, the AAP and CHA said in their report.
Among the states, California has the most overall cases at just over 350,000, Wyoming has the highest proportion of cases in children (20.3%), and North Dakota has the highest rate of infection (over 8,100 per 100,000 children). The infection rate for the nation is now above 3,300 per 100,000 children, and 11 states reported rates over 5,000, according to the AAP and the CHA.
Moderna needs more kids for COVID vaccine trials
according to the company CEO and a federal official.
The Moderna vaccine was authorized for use in December and is now being given to people 18 and over. But children would receive lower doses, so new clinical trials must be done, Moderna CEO Stephane Bancel said at the JPMorgan virtual Health Care Conference on Monday.
Clinical trials on children 11 and younger “will take much longer, because we have to age deescalate and start at a lower dose. So we should not anticipate clinical data in 2021, but more in 2022,” Ms. Bancel said, according to Business Insider.
Moderna’s clinical trials for 12- to 17-year-olds started 4 weeks ago, but the company is having trouble getting enough participants, said Moncef Slaoui, PhD, the scientific head of Operation Warp Speed, the U.S. government’s vaccine effort. That could delay Food and Drug Administration approval, he said.
“It’s really very important for all of us, for all the population in America, to realize that we can’t have that indication unless adolescents aged 12-18 decide to participate,” Dr. Slaoui said, according to USA Today.
He said the adolescent trials are getting only about 800 volunteers a month, but need at least 3,000 volunteers to complete the study, USA Today reported. Parents interested in having their child participate can check eligibility and sign at this website.
The Pfizer/BioNTech vaccine won authorization for use in 16- to 17-year-olds as well as adults.
The coronavirus doesn’t appear to have as serious complications for children as for adults.
“At this time, it appears that severe illness due to COVID-19 is rare among children,” the American Association of Pediatrics says. “However, there is an urgent need to collect more data on longer-term impacts of the pandemic on children, including ways the virus may harm the long-term physical health of infected children, as well as its emotional and mental health effects.”
The association says 179 children had died of COVID-related reasons in 43 states and New York City as of Dec. 31, 2020. That’s about 0.06% of total COVID deaths, it says.
But children do get sick. As of Jan. 7, 2021, nearly 2.3 million children had tested positive for COVID-19 since the start of the pandemic, the association says.
A version of this article first appeared on WebMD.com.
according to the company CEO and a federal official.
The Moderna vaccine was authorized for use in December and is now being given to people 18 and over. But children would receive lower doses, so new clinical trials must be done, Moderna CEO Stephane Bancel said at the JPMorgan virtual Health Care Conference on Monday.
Clinical trials on children 11 and younger “will take much longer, because we have to age deescalate and start at a lower dose. So we should not anticipate clinical data in 2021, but more in 2022,” Ms. Bancel said, according to Business Insider.
Moderna’s clinical trials for 12- to 17-year-olds started 4 weeks ago, but the company is having trouble getting enough participants, said Moncef Slaoui, PhD, the scientific head of Operation Warp Speed, the U.S. government’s vaccine effort. That could delay Food and Drug Administration approval, he said.
“It’s really very important for all of us, for all the population in America, to realize that we can’t have that indication unless adolescents aged 12-18 decide to participate,” Dr. Slaoui said, according to USA Today.
He said the adolescent trials are getting only about 800 volunteers a month, but need at least 3,000 volunteers to complete the study, USA Today reported. Parents interested in having their child participate can check eligibility and sign at this website.
The Pfizer/BioNTech vaccine won authorization for use in 16- to 17-year-olds as well as adults.
The coronavirus doesn’t appear to have as serious complications for children as for adults.
“At this time, it appears that severe illness due to COVID-19 is rare among children,” the American Association of Pediatrics says. “However, there is an urgent need to collect more data on longer-term impacts of the pandemic on children, including ways the virus may harm the long-term physical health of infected children, as well as its emotional and mental health effects.”
The association says 179 children had died of COVID-related reasons in 43 states and New York City as of Dec. 31, 2020. That’s about 0.06% of total COVID deaths, it says.
But children do get sick. As of Jan. 7, 2021, nearly 2.3 million children had tested positive for COVID-19 since the start of the pandemic, the association says.
A version of this article first appeared on WebMD.com.
according to the company CEO and a federal official.
The Moderna vaccine was authorized for use in December and is now being given to people 18 and over. But children would receive lower doses, so new clinical trials must be done, Moderna CEO Stephane Bancel said at the JPMorgan virtual Health Care Conference on Monday.
Clinical trials on children 11 and younger “will take much longer, because we have to age deescalate and start at a lower dose. So we should not anticipate clinical data in 2021, but more in 2022,” Ms. Bancel said, according to Business Insider.
Moderna’s clinical trials for 12- to 17-year-olds started 4 weeks ago, but the company is having trouble getting enough participants, said Moncef Slaoui, PhD, the scientific head of Operation Warp Speed, the U.S. government’s vaccine effort. That could delay Food and Drug Administration approval, he said.
“It’s really very important for all of us, for all the population in America, to realize that we can’t have that indication unless adolescents aged 12-18 decide to participate,” Dr. Slaoui said, according to USA Today.
He said the adolescent trials are getting only about 800 volunteers a month, but need at least 3,000 volunteers to complete the study, USA Today reported. Parents interested in having their child participate can check eligibility and sign at this website.
The Pfizer/BioNTech vaccine won authorization for use in 16- to 17-year-olds as well as adults.
The coronavirus doesn’t appear to have as serious complications for children as for adults.
“At this time, it appears that severe illness due to COVID-19 is rare among children,” the American Association of Pediatrics says. “However, there is an urgent need to collect more data on longer-term impacts of the pandemic on children, including ways the virus may harm the long-term physical health of infected children, as well as its emotional and mental health effects.”
The association says 179 children had died of COVID-related reasons in 43 states and New York City as of Dec. 31, 2020. That’s about 0.06% of total COVID deaths, it says.
But children do get sick. As of Jan. 7, 2021, nearly 2.3 million children had tested positive for COVID-19 since the start of the pandemic, the association says.
A version of this article first appeared on WebMD.com.
Arthritis drugs ‘impressive’ for severe COVID but not ‘magic cure’
New findings suggest that monoclonal antibodies used to treat RA could improve severe COVID-19 outcomes, including risk for death.
Given within 24 hours of critical illness, tocilizumab (Actemra) was associated with a median of 10 days free of respiratory and cardiovascular support up to day 21, the primary outcome. Similarly, sarilumab (Kevzara) was linked to a median of 11 days. In contrast, the usual care control group experienced zero such days in the hospital.
However, the Randomized, Embedded, Multifactorial Adaptive Platform Trial for Community-Acquired Pneumonia (REMAP-CAP) trial comes with a caveat. The preprint findings have not yet been peer reviewed and “should not be used to guide clinical practice,” the authors stated.
The results were published online Jan. 7 in MedRxiv.
Nevertheless, the trial also revealed a mortality benefit associated with the two interleukin-6 antagonists. The hospital mortality rate was 22% with sarilumab, 28% with tocilizumab, and almost 36% with usual care.
“That’s a big change in survival. They are both lifesaving drugs,” lead coinvestigator Anthony Gordon, an Imperial College London professor of anesthesia and critical care, commented in a recent story by Reuters.
Consider the big picture
“What I think is important is ... this is one of many trials,” Paul Auwaerter, MD, MBA, said in an interview. Many other studies looking at monoclonal antibody therapy for people with COVID-19 were halted because they did not show improvement.
One exception is the EMPACTA trial, which suggested that tocilizumab was effective if given before a person becomes ill enough to be placed on a ventilator, said Dr. Auwaerter, clinical director of the division of infectious diseases at Johns Hopkins Medicine and a contributor to this news organization. “It appeared to reduce the need for mechanical ventilation or death.”
“These two trials are the first randomized, prospective trials that show a benefit on a background of others which have not,” Dr. Auwaerter added.
Interim findings
The REMAP-CAP investigators randomly assigned adults within 24 hours of critical care for COVID-19 to 8 mg/kg tocilizumab, 400 mg sarilumab, or usual care at 113 sites in six countries. There were 353 participants in the tocilizumab arm, 48 in the sarilumab group, and 402 in the control group.
Compared with the control group, the 10 days free of organ support in the tocilizumab cohort was associated with an adjusted odds ratio of 1.64 (95% confidence interval, 1.25-2.14). The 11 days free of organ support in the sarilumab cohort was likewise superior to control (adjusted odds ratio, 1.76; 95% CI, 1.17-2.91).
“All secondary outcomes and analyses supported efficacy of these IL-6 receptor antagonists,” the authors note. These endpoints included 90-day survival, time to intensive care unit discharge, and hospital discharge.
Cautious optimism?
“The results were quite impressive – having 10 or 11 fewer days in the ICU, compared to standard of care,” Deepa Gotur, MD, said in an interview. “Choosing the right patient population and providing the anti-IL-6 treatment at the right time would be the key here.”
In addition to not yet receiving peer review, an open-label design, a relatively short follow-up of 21 days, and steroids becoming standard of care about halfway through the trial are potential limitations, said Dr. Gotur, an intensivist at Houston Methodist Hospital and associate professor of clinical medicine at Weill Cornell Medicine, New York.
“This is an interesting study,” Carl J. Fichtenbaum, MD, professor of clinical medicine at the University of Cincinnati, said in a comment.
Additional detail on how many participants in each group received steroids is warranted, Dr. Fichtenbaum said. “The analysis did not carefully adjust for the use of steroids that might have influenced outcomes.”
Dr. Fichtenbaum said it’s important to look at what is distinctive about REMAP-CAP because “there are several other studies showing opposite results.”
Dr. Gotur was an investigator on a previous study evaluating tocilizumab for patients already on mechanical ventilation. “One of the key differences between this and other studies is that they included more of the ICU population,” she said. “They also included patients within 24 hours of requiring organ support, cardiac, as well as respiratory support.” Some other research included less-acute patients, including all comers into the ED who required oxygen and received tocilizumab.
The prior studies also evaluated cytokine or inflammatory markers. In contrast, REMAP-CAP researchers “looked at organ failure itself ... which I think makes sense,” Dr. Gotur said.
Cytokine release syndrome can cause organ damage or organ failure, she added, “but these markers are all over the place. I’ve seen patients who are very, very sick despite having a low [C-reactive protein] or IL-6 level.”
Backing from the British
Citing the combined 24% decrease in the risk for death associated with these agents in the REMAP-CAP trial, the U.K. government announced Jan. 7 it will work to make tocilizumab and sarilumab available to citizens with severe COVID-19.
Experts in the United Kingdom shared their perspectives on the REMAP-CAP interim findings through the U.K. Science Media Centre.
“There are few treatments for severe COVID-19,” said Robin Ferner, MD, honorary professor of clinical pharmacology at the University of Birmingham (England) and honorary consultant physician at City Hospital Birmingham. “If the published data from REMAP-CAP are supported by further studies, this suggests that two IL-6 receptor antagonists can reduce the death rate in the most severely ill patients.”
Dr. Ferner added that the findings are not a “magic cure,” however. He pointed out that of 401 patients given the drugs, 109 died, and with standard treatment, 144 out of 402 died.
Peter Horby, MD, PhD, was more optimistic. “It is great to see a positive result at a time that we really need good news and more tools to fight COVID. This is great achievement for REMAP-CAP,” he said.
“We hope to soon have results from RECOVERY on the effect of tocilizumab in less severely ill patients in the hospital,” said Dr. Horby, cochief investigator of the RECOVERY trial and professor of emerging infectious diseases at the Centre for Tropical Medicine and Global Health at the University of Oxford (England).
Stephen Evans, BA, MSc, FRCP, professor of pharmacoepidemiology at the London School of Hygiene & Tropical Medicine, said, “This is a high-quality trial, and although published as a preprint, is of much higher quality than many non–peer-reviewed papers.”
Dr. Evans also noted the addition of steroid therapy for many participants. “Partway through the trial, the RECOVERY trial findings showed that the corticosteroid drug dexamethasone had notable mortality benefits. Consequently, quite a number of the patients in this trial had also received a corticosteroid.”
“It does look as though these drugs give some additional benefit beyond that given by dexamethasone,” he added.
Awaiting peer review
“We need to wait for the final results and ensure it was adequately powered with enough observations to make us confident in the results,” Dr. Fichtenbaum said.
“We in the United States have to step back and look at the entire set of studies and also, for this particular one, REMAP-CAP, to be in a peer-reviewed publication,” Dr. Auwaerter said. Preprints are often released “in the setting of the pandemic, where there may be important findings, especially if they impact mortality or severity of illness.”
“We need to make sure these findings, as outlined, hold up,” he said.
In the meantime, Dr. Auwaerter added, “Exactly how this will fit in is unclear. But it’s important to me as another potential drug that can help our critically ill patients.”
The REMAP-CAP study is ongoing and updated results will be provided online.
Dr. Auwaerter disclosed that he is a consultant for EMD Serono and a member of the data monitoring safety board for Humanigen. Dr. Gotur, Dr. Fichtenbaum, Dr. Ferner, and Dr. Evans disclosed no relevant financial relationships. Dr. Horby reported that Oxford University receives funding for the RECOVERY trial from U.K. Research and Innovation and the National Institute for Health Research. Roche Products and Sanofi supported REMAP-CAP through provision of tocilizumab and sarilumab in the United Kingdom.
A version of this article first appeared on Medscape.com.
New findings suggest that monoclonal antibodies used to treat RA could improve severe COVID-19 outcomes, including risk for death.
Given within 24 hours of critical illness, tocilizumab (Actemra) was associated with a median of 10 days free of respiratory and cardiovascular support up to day 21, the primary outcome. Similarly, sarilumab (Kevzara) was linked to a median of 11 days. In contrast, the usual care control group experienced zero such days in the hospital.
However, the Randomized, Embedded, Multifactorial Adaptive Platform Trial for Community-Acquired Pneumonia (REMAP-CAP) trial comes with a caveat. The preprint findings have not yet been peer reviewed and “should not be used to guide clinical practice,” the authors stated.
The results were published online Jan. 7 in MedRxiv.
Nevertheless, the trial also revealed a mortality benefit associated with the two interleukin-6 antagonists. The hospital mortality rate was 22% with sarilumab, 28% with tocilizumab, and almost 36% with usual care.
“That’s a big change in survival. They are both lifesaving drugs,” lead coinvestigator Anthony Gordon, an Imperial College London professor of anesthesia and critical care, commented in a recent story by Reuters.
Consider the big picture
“What I think is important is ... this is one of many trials,” Paul Auwaerter, MD, MBA, said in an interview. Many other studies looking at monoclonal antibody therapy for people with COVID-19 were halted because they did not show improvement.
One exception is the EMPACTA trial, which suggested that tocilizumab was effective if given before a person becomes ill enough to be placed on a ventilator, said Dr. Auwaerter, clinical director of the division of infectious diseases at Johns Hopkins Medicine and a contributor to this news organization. “It appeared to reduce the need for mechanical ventilation or death.”
“These two trials are the first randomized, prospective trials that show a benefit on a background of others which have not,” Dr. Auwaerter added.
Interim findings
The REMAP-CAP investigators randomly assigned adults within 24 hours of critical care for COVID-19 to 8 mg/kg tocilizumab, 400 mg sarilumab, or usual care at 113 sites in six countries. There were 353 participants in the tocilizumab arm, 48 in the sarilumab group, and 402 in the control group.
Compared with the control group, the 10 days free of organ support in the tocilizumab cohort was associated with an adjusted odds ratio of 1.64 (95% confidence interval, 1.25-2.14). The 11 days free of organ support in the sarilumab cohort was likewise superior to control (adjusted odds ratio, 1.76; 95% CI, 1.17-2.91).
“All secondary outcomes and analyses supported efficacy of these IL-6 receptor antagonists,” the authors note. These endpoints included 90-day survival, time to intensive care unit discharge, and hospital discharge.
Cautious optimism?
“The results were quite impressive – having 10 or 11 fewer days in the ICU, compared to standard of care,” Deepa Gotur, MD, said in an interview. “Choosing the right patient population and providing the anti-IL-6 treatment at the right time would be the key here.”
In addition to not yet receiving peer review, an open-label design, a relatively short follow-up of 21 days, and steroids becoming standard of care about halfway through the trial are potential limitations, said Dr. Gotur, an intensivist at Houston Methodist Hospital and associate professor of clinical medicine at Weill Cornell Medicine, New York.
“This is an interesting study,” Carl J. Fichtenbaum, MD, professor of clinical medicine at the University of Cincinnati, said in a comment.
Additional detail on how many participants in each group received steroids is warranted, Dr. Fichtenbaum said. “The analysis did not carefully adjust for the use of steroids that might have influenced outcomes.”
Dr. Fichtenbaum said it’s important to look at what is distinctive about REMAP-CAP because “there are several other studies showing opposite results.”
Dr. Gotur was an investigator on a previous study evaluating tocilizumab for patients already on mechanical ventilation. “One of the key differences between this and other studies is that they included more of the ICU population,” she said. “They also included patients within 24 hours of requiring organ support, cardiac, as well as respiratory support.” Some other research included less-acute patients, including all comers into the ED who required oxygen and received tocilizumab.
The prior studies also evaluated cytokine or inflammatory markers. In contrast, REMAP-CAP researchers “looked at organ failure itself ... which I think makes sense,” Dr. Gotur said.
Cytokine release syndrome can cause organ damage or organ failure, she added, “but these markers are all over the place. I’ve seen patients who are very, very sick despite having a low [C-reactive protein] or IL-6 level.”
Backing from the British
Citing the combined 24% decrease in the risk for death associated with these agents in the REMAP-CAP trial, the U.K. government announced Jan. 7 it will work to make tocilizumab and sarilumab available to citizens with severe COVID-19.
Experts in the United Kingdom shared their perspectives on the REMAP-CAP interim findings through the U.K. Science Media Centre.
“There are few treatments for severe COVID-19,” said Robin Ferner, MD, honorary professor of clinical pharmacology at the University of Birmingham (England) and honorary consultant physician at City Hospital Birmingham. “If the published data from REMAP-CAP are supported by further studies, this suggests that two IL-6 receptor antagonists can reduce the death rate in the most severely ill patients.”
Dr. Ferner added that the findings are not a “magic cure,” however. He pointed out that of 401 patients given the drugs, 109 died, and with standard treatment, 144 out of 402 died.
Peter Horby, MD, PhD, was more optimistic. “It is great to see a positive result at a time that we really need good news and more tools to fight COVID. This is great achievement for REMAP-CAP,” he said.
“We hope to soon have results from RECOVERY on the effect of tocilizumab in less severely ill patients in the hospital,” said Dr. Horby, cochief investigator of the RECOVERY trial and professor of emerging infectious diseases at the Centre for Tropical Medicine and Global Health at the University of Oxford (England).
Stephen Evans, BA, MSc, FRCP, professor of pharmacoepidemiology at the London School of Hygiene & Tropical Medicine, said, “This is a high-quality trial, and although published as a preprint, is of much higher quality than many non–peer-reviewed papers.”
Dr. Evans also noted the addition of steroid therapy for many participants. “Partway through the trial, the RECOVERY trial findings showed that the corticosteroid drug dexamethasone had notable mortality benefits. Consequently, quite a number of the patients in this trial had also received a corticosteroid.”
“It does look as though these drugs give some additional benefit beyond that given by dexamethasone,” he added.
Awaiting peer review
“We need to wait for the final results and ensure it was adequately powered with enough observations to make us confident in the results,” Dr. Fichtenbaum said.
“We in the United States have to step back and look at the entire set of studies and also, for this particular one, REMAP-CAP, to be in a peer-reviewed publication,” Dr. Auwaerter said. Preprints are often released “in the setting of the pandemic, where there may be important findings, especially if they impact mortality or severity of illness.”
“We need to make sure these findings, as outlined, hold up,” he said.
In the meantime, Dr. Auwaerter added, “Exactly how this will fit in is unclear. But it’s important to me as another potential drug that can help our critically ill patients.”
The REMAP-CAP study is ongoing and updated results will be provided online.
Dr. Auwaerter disclosed that he is a consultant for EMD Serono and a member of the data monitoring safety board for Humanigen. Dr. Gotur, Dr. Fichtenbaum, Dr. Ferner, and Dr. Evans disclosed no relevant financial relationships. Dr. Horby reported that Oxford University receives funding for the RECOVERY trial from U.K. Research and Innovation and the National Institute for Health Research. Roche Products and Sanofi supported REMAP-CAP through provision of tocilizumab and sarilumab in the United Kingdom.
A version of this article first appeared on Medscape.com.
New findings suggest that monoclonal antibodies used to treat RA could improve severe COVID-19 outcomes, including risk for death.
Given within 24 hours of critical illness, tocilizumab (Actemra) was associated with a median of 10 days free of respiratory and cardiovascular support up to day 21, the primary outcome. Similarly, sarilumab (Kevzara) was linked to a median of 11 days. In contrast, the usual care control group experienced zero such days in the hospital.
However, the Randomized, Embedded, Multifactorial Adaptive Platform Trial for Community-Acquired Pneumonia (REMAP-CAP) trial comes with a caveat. The preprint findings have not yet been peer reviewed and “should not be used to guide clinical practice,” the authors stated.
The results were published online Jan. 7 in MedRxiv.
Nevertheless, the trial also revealed a mortality benefit associated with the two interleukin-6 antagonists. The hospital mortality rate was 22% with sarilumab, 28% with tocilizumab, and almost 36% with usual care.
“That’s a big change in survival. They are both lifesaving drugs,” lead coinvestigator Anthony Gordon, an Imperial College London professor of anesthesia and critical care, commented in a recent story by Reuters.
Consider the big picture
“What I think is important is ... this is one of many trials,” Paul Auwaerter, MD, MBA, said in an interview. Many other studies looking at monoclonal antibody therapy for people with COVID-19 were halted because they did not show improvement.
One exception is the EMPACTA trial, which suggested that tocilizumab was effective if given before a person becomes ill enough to be placed on a ventilator, said Dr. Auwaerter, clinical director of the division of infectious diseases at Johns Hopkins Medicine and a contributor to this news organization. “It appeared to reduce the need for mechanical ventilation or death.”
“These two trials are the first randomized, prospective trials that show a benefit on a background of others which have not,” Dr. Auwaerter added.
Interim findings
The REMAP-CAP investigators randomly assigned adults within 24 hours of critical care for COVID-19 to 8 mg/kg tocilizumab, 400 mg sarilumab, or usual care at 113 sites in six countries. There were 353 participants in the tocilizumab arm, 48 in the sarilumab group, and 402 in the control group.
Compared with the control group, the 10 days free of organ support in the tocilizumab cohort was associated with an adjusted odds ratio of 1.64 (95% confidence interval, 1.25-2.14). The 11 days free of organ support in the sarilumab cohort was likewise superior to control (adjusted odds ratio, 1.76; 95% CI, 1.17-2.91).
“All secondary outcomes and analyses supported efficacy of these IL-6 receptor antagonists,” the authors note. These endpoints included 90-day survival, time to intensive care unit discharge, and hospital discharge.
Cautious optimism?
“The results were quite impressive – having 10 or 11 fewer days in the ICU, compared to standard of care,” Deepa Gotur, MD, said in an interview. “Choosing the right patient population and providing the anti-IL-6 treatment at the right time would be the key here.”
In addition to not yet receiving peer review, an open-label design, a relatively short follow-up of 21 days, and steroids becoming standard of care about halfway through the trial are potential limitations, said Dr. Gotur, an intensivist at Houston Methodist Hospital and associate professor of clinical medicine at Weill Cornell Medicine, New York.
“This is an interesting study,” Carl J. Fichtenbaum, MD, professor of clinical medicine at the University of Cincinnati, said in a comment.
Additional detail on how many participants in each group received steroids is warranted, Dr. Fichtenbaum said. “The analysis did not carefully adjust for the use of steroids that might have influenced outcomes.”
Dr. Fichtenbaum said it’s important to look at what is distinctive about REMAP-CAP because “there are several other studies showing opposite results.”
Dr. Gotur was an investigator on a previous study evaluating tocilizumab for patients already on mechanical ventilation. “One of the key differences between this and other studies is that they included more of the ICU population,” she said. “They also included patients within 24 hours of requiring organ support, cardiac, as well as respiratory support.” Some other research included less-acute patients, including all comers into the ED who required oxygen and received tocilizumab.
The prior studies also evaluated cytokine or inflammatory markers. In contrast, REMAP-CAP researchers “looked at organ failure itself ... which I think makes sense,” Dr. Gotur said.
Cytokine release syndrome can cause organ damage or organ failure, she added, “but these markers are all over the place. I’ve seen patients who are very, very sick despite having a low [C-reactive protein] or IL-6 level.”
Backing from the British
Citing the combined 24% decrease in the risk for death associated with these agents in the REMAP-CAP trial, the U.K. government announced Jan. 7 it will work to make tocilizumab and sarilumab available to citizens with severe COVID-19.
Experts in the United Kingdom shared their perspectives on the REMAP-CAP interim findings through the U.K. Science Media Centre.
“There are few treatments for severe COVID-19,” said Robin Ferner, MD, honorary professor of clinical pharmacology at the University of Birmingham (England) and honorary consultant physician at City Hospital Birmingham. “If the published data from REMAP-CAP are supported by further studies, this suggests that two IL-6 receptor antagonists can reduce the death rate in the most severely ill patients.”
Dr. Ferner added that the findings are not a “magic cure,” however. He pointed out that of 401 patients given the drugs, 109 died, and with standard treatment, 144 out of 402 died.
Peter Horby, MD, PhD, was more optimistic. “It is great to see a positive result at a time that we really need good news and more tools to fight COVID. This is great achievement for REMAP-CAP,” he said.
“We hope to soon have results from RECOVERY on the effect of tocilizumab in less severely ill patients in the hospital,” said Dr. Horby, cochief investigator of the RECOVERY trial and professor of emerging infectious diseases at the Centre for Tropical Medicine and Global Health at the University of Oxford (England).
Stephen Evans, BA, MSc, FRCP, professor of pharmacoepidemiology at the London School of Hygiene & Tropical Medicine, said, “This is a high-quality trial, and although published as a preprint, is of much higher quality than many non–peer-reviewed papers.”
Dr. Evans also noted the addition of steroid therapy for many participants. “Partway through the trial, the RECOVERY trial findings showed that the corticosteroid drug dexamethasone had notable mortality benefits. Consequently, quite a number of the patients in this trial had also received a corticosteroid.”
“It does look as though these drugs give some additional benefit beyond that given by dexamethasone,” he added.
Awaiting peer review
“We need to wait for the final results and ensure it was adequately powered with enough observations to make us confident in the results,” Dr. Fichtenbaum said.
“We in the United States have to step back and look at the entire set of studies and also, for this particular one, REMAP-CAP, to be in a peer-reviewed publication,” Dr. Auwaerter said. Preprints are often released “in the setting of the pandemic, where there may be important findings, especially if they impact mortality or severity of illness.”
“We need to make sure these findings, as outlined, hold up,” he said.
In the meantime, Dr. Auwaerter added, “Exactly how this will fit in is unclear. But it’s important to me as another potential drug that can help our critically ill patients.”
The REMAP-CAP study is ongoing and updated results will be provided online.
Dr. Auwaerter disclosed that he is a consultant for EMD Serono and a member of the data monitoring safety board for Humanigen. Dr. Gotur, Dr. Fichtenbaum, Dr. Ferner, and Dr. Evans disclosed no relevant financial relationships. Dr. Horby reported that Oxford University receives funding for the RECOVERY trial from U.K. Research and Innovation and the National Institute for Health Research. Roche Products and Sanofi supported REMAP-CAP through provision of tocilizumab and sarilumab in the United Kingdom.
A version of this article first appeared on Medscape.com.
Long-haul COVID-19 cases rise as stigma of chronic fatigue taunts
When Margot Gage-Witvliet began feeling run down after her family returned from a trip to the Netherlands in late February 2020, she initially chalked up her symptoms to jet lag. Three days later, however, her situation went from concerning to alarming as she struggled to breathe. “It felt like there was an elephant sitting on my chest,” she said.
Her husband and daughters also became ill with COVID-19, but Ms. Gage-Witvliet was the only one in her family who didn’t get better. After an early improvement, a rare coronavirus-induced tonic-clonic seizure in early April sent her spiraling back down. Ms. Gage-Witvliet spent the next several weeks in bed with the curtains drawn, unable to tolerate light or sound.
Today, Ms. Gage-Witvliet’s life looks nothing like it did 6 months ago when she first got sick. As one of COVID-19’s so called long-haulers, she continues to struggle with crushing fatigue, brain fog, and headaches – symptoms that worsen when she pushes herself to do more. Across the country, as many as 1 in 10 COVID-19 patients are reporting illnesses that continue for weeks and months after their initial diagnosis. Nearly all report neurologic issues like Ms. Gage-Witvliet, as well as shortness of breath and psychiatric concerns.
For Avindra Nath, MD, a neurologist at the National Institutes of Health, the experience of these long-haul COVID-19 patients feels familiar and reminds him of myalgic encephalomyelitis, also known as chronic fatigue syndrome.
Dr. Nath has long been interested in the lingering neurologic issues connected to chronic fatigue. An estimated three-quarters of all patients with chronic fatigue syndrome report that their symptoms started after a viral infection, and they suffer unrelenting exhaustion, difficulties regulating pulse and blood pressure, aches and pains, and brain fog. When Dr. Nath first read about the novel coronavirus, he began to worry that the virus would trigger symptoms in a subset of those infected. Hearing about the experiences of long-haulers like Ms. Gage-Witvliet raised his suspicions even more.
Unlike COVID-19 long-haulers, however, many patients with chronic fatigue syndrome go at least a year with these symptoms before receiving a diagnosis, according to a British survey. That means researchers have had few opportunities to study the early stages of the syndrome. “When we see patients with myalgic encephalomyelitis, whatever infection they might have had occurred in the remote past, so there’s no way for us to know how they got infected with it, what the infection was, or what the effects of it were in that early phase. We’re seeing them 2 years afterward,” Dr. Nath said.
Dr. Nath quickly realized that studying patients like Ms. Gage-Witvliet would give physicians and scientists a unique opportunity to understand not only long-term outcomes of COVID-19 infections, but also other postviral syndromes, including chronic fatigue syndrome at their earliest stages. It’s why Dr. Nath has spent the past several months scrambling to launch two NIH studies to examine the phenomenon.
Although Dr. Nath said that the parallels between COVID-19 long-haulers and those with chronic fatigue syndrome are obvious, he cautions against assuming that they are the same phenomenon. Some long-haulers might simply be taking a much slower path to recovery, or they might have a condition that looks similar on the surface but differs from chronic fatigue syndrome on a molecular level. But even if Dr. Nath fails to see links to chronic fatigue syndrome, with more than 92.5 million documented cases of COVID-19 around the world, the work will be relevant to the substantial number of infected individuals who don’t recover quickly.
“With so many people having exposure to the same virus over a similar time period, we really have the opportunity to look at these manifestations and at the very least to understand postviral syndromes,” said Mady Hornig, MD, a psychiatrist at Columbia University, New York.
The origins of chronic fatigue syndrome date back to 1985, when the Centers for Disease Control and Prevention received a request from two physicians – Paul Cheney, MD, and Daniel Peterson, MD – to investigate a mysterious disease outbreak in Nevada. In November 1984, residents in and around the idyllic vacation spot of Incline Village, a small town tucked into the north shore of Lake Tahoe, had begun reporting flu-like symptoms that persisted for weeks, even months. The doctors had searched high and low for a cause, but they couldn’t figure out what was making their patients sick.
They reported a range of symptoms – including muscle aches and pains, low-grade fevers, sore throats, and headaches – but everyone said that crippling fatigue was the most debilitating issue. This wasn’t the kind of fatigue that could be cured by a nap or even a long holiday. No matter how much their patients slept – and some were almost completely bedbound – their fatigue didn’t abate. What’s more, the fatigue got worse whenever they tried to push themselves to do more. Puzzled, the CDC sent two epidemic intelligence service (EIS) officers to try to get to the bottom of what might be happening.
Muscle aches and pains with crippling fatigue
After their visit to Incline Village, however, the CDC was just as perplexed as Dr. Cheney and Dr. Peterson. Many of the people with the condition reported flu-like symptoms right around the time they first got sick, and the physicians’ leading hypothesis was that the outbreak and its lasting symptoms were caused by chronic Epstein-Barr virus infection. But neither the CDC nor anyone else could identify the infection or any other microbial cause. The two EIS officers duly wrote up a report for the CDC’s flagship publication, Morbidity and Mortality Weekly ReportI, titled “Chronic Fatigue Possibly Related to Epstein-Barr Virus – Nevada”.
That investigators focused on the fatigue aspect made sense, says Leonard A. Jason, PhD, professor of psychology at DePaul University and director of the Center for Community Research, both in Chicago, because it was one of the few symptoms shared by all the individuals studied and it was also the most debilitating. But that focus – and the name “chronic fatigue syndrome” – led to broad public dismissal of the condition’s severity, as did an editorial note in MMWR urging physicians to look for “more definable, and possibly treatable, conditions.” Subsequent research failed to confirm a specific link to the Epstein-Barr virus, which only added to the condition’s phony reputation. Rather than being considered a potentially disabling illness, it was disregarded as a “yuppie flu” or a fancy name for malingering.
“It’s not a surprise that patients are being dismissed because there’s already this sort of grandfathered-in sense that fatigue is not real,” said Jennifer Frankovich, MD, a pediatric rheumatologist at Stanford (Calif.) University’s Lucile Packard Children’s Hospital in Palo Alto. “I’m sure that’s frustrating for them to be tired and then to have the clinician not believe them or dismiss them or think they’re making it up. It would be more helpful to the families to say: ‘You know what, we don’t know, we do not have the answer, and we believe you.’ ”
A syndrome’s shame
As time passed, patient advocacy groups began pushing back against the negative way the condition was being perceived. This criticism came as organizations like the CDC worked to develop a set of diagnostic criteria that researchers and clinicians dealing with chronic fatigue syndrome could use. With such a heterogeneous group of patients and symptoms, the task was no small challenge. The discussions, which took place over nearly 2 decades, played a key role in helping scientists home in on the single factor that was central to chronic fatigue: postexertional malaise.
“This is quite unique for chronic fatigue syndrome. With other diseases, yes, you may have fatigue as one of the components of the disease, but postexertional fatigue is very specific,” said Alain Moreau, PhD, a molecular biologist at the University of Montreal.
Of course, plenty of people have pushed themselves too hard physically and paid the price the next day. But those with chronic fatigue syndrome weren’t running marathons. To them, exertion could be anything from getting the mail to reading a book. Nor could the resulting exhaustion be resolved by an afternoon on the couch or a long vacation.
“If they do these activities, they can crash for weeks, even months,” Dr. Moreau said. It was deep, persistent, and – for 40% of those with chronic fatigue syndrome – disabling. In 2015, a study group from the Institute of Medicine proposed renaming chronic fatigue to “systemic exercise intolerance disease” because of the centrality of this symptom. Although that effort mostly stalled, their report did bring the condition out of its historic place as a scientific backwater. What resulted was an uptick in research on chronic fatigue syndrome, which helped define some of the physiological issues that either contribute to or result from the condition.
Researchers had long known about the link between infection and fatigue, said Dr. Frankovich. Work included mysterious outbreaks like the one in Lake Tahoe and well-documented issues like the wave of encephalitis lethargica (a condition that leaves patients in an almost vegetative state) that followed the 1918 H1N1 influenza pandemic.
“As a clinician, when you see someone who comes in with a chronic infection, they’re tired. I think that’s why, in the chronic-fatigue world, people are desperately looking for the infection so we can treat it, and maybe these poor suffering people will feel better,” Dr. Frankovich added. Then the pandemic struck, giving him yet another opportunity to study postviral syndromes.
Immunologic symptoms
Given the close link between a nonspecific viral illness and the onset of symptoms in chronic fatigue syndrome, scientists like Dr. Hornig opted to focus on immunologic symptoms. In a 2015 analysis published in Science, Dr. Hornig and colleagues showed that immune problems can be found in the earliest stages of chronic fatigue syndrome, and that they change as the illness progresses. Patients who had been sick for less than 3 years showed significant increases in levels of both pro- and anti-inflammatory cytokines, and the factor most strongly correlated to this inability to regulate cytokine levels was the duration of symptoms, not their severity. A series of other studies also revealed problems with regulation of the immune system, although no one could show what might have set these problems in motion.
Other researchers found signs of mitochondrial dysfunction in those with chronic fatigue syndrome. Because mitochondria make energy for cells, it wasn’t an intellectual stretch to believe that glitches in this process could contribute to fatigue. As early as 1991, scientists had discovered signs of mitochondrial degeneration in muscle biopsies from people with chronic fatigue syndrome. Subsequent studies showed that those affected by chronic fatigue were missing segments of mitochondrial DNA and had significantly reduced levels of mitochondrial activity. Although exercise normally improves mitochondrial functioning, the opposite appears to happen in chronic fatigue.
To Dr. Nath, these dual hypotheses aren’t necessarily mutually exclusive. Some studies have hinted that infection with the common human herpesvirus–6 (HHV-6) can lead to an autoimmune condition in which the body makes antibodies against the mitochondria. Mitochondria also play a key role in the ability of the innate immune system to produce interferon and other proinflammatory cytokines. It might also be that the link between immune and mitochondrial problems is more convoluted than originally thought, or that the two systems are affected independent of one another, Dr. Nath said.
Finding answers, especially those that could lead to potential treatments, wouldn’t be easy, however. In 2016, the NIH launched an in-depth study of a small number of individuals with chronic fatigue, hoping to find clues about what the condition was and how it might be treated.
For scientists like Dr. Nath, the NIH study provided a way to get at the underlying biology of chronic fatigue syndrome. Then the pandemic struck, giving him yet another opportunity to study postviral syndromes.
Chronic post-SARS syndrome
In March 2020, retired physician Harvey Moldofsky, MD, began receiving inquiries about a 2011 study he and his colleague, John Patcai, MD, had published in BMC Neurology about something they dubbed “chronic post-SARS syndrome.” The small case-control study, which involved mainly health care workers in Toronto, received little attention when it was first published, but with COVID-19, it was suddenly relevant.
Early clusters of similar cases in Miami made local physicians desperate for Dr. Moldofsky’s expertise. Luckily, he was nearby; he had fled the frigid Canadian winter for the warmth of Sarasota, Fla.
“I had people from various countries around the world writing to me and asking what they should do. And of course I don’t have any answers,” he said. But the study contained one of the world’s only references to the syndrome.
In 2003, a woman arrived in Toronto from Hong Kong. She didn’t know it at the time, but her preairport stay at the Hotel Metropole had infected her with the first SARS (severe acute respiratory syndrome) coronavirus. Her subsequent hospitalization in Toronto sparked a city-wide outbreak of SARS in which 273 people became ill and 44 died. Many of those affected were health care workers, including nurses and respiratory therapists. Although most eventually returned to work, a subset couldn’t. They complained of energy-sapping fatigue, poor sleep, brain fog, and assorted body aches and pains that persisted for more than 18 months. The aches and pains brought them to the attention of Dr. Moldofsky, then director of the Centre for the Study of Pain at the University of Toronto.
His primary interest at the time was fibromyalgia, which caused symptoms similar to those reported by the original SARS long-haulers. Intrigued, Dr. Moldofsky agreed to take a look. Their chest x-rays were clear and the nurses showed no signs of lingering viral infection. Dr. Moldofsky could see that the nurses were ill and suffering, but no lab tests or anything else could identify what was causing their symptoms.
In 2011, Dr. Moldofsky and Dr. Patcai found a strong overlap between chronic SARS, fibromyalgia, and chronic fatigue syndrome when they compared 22 patients with long-term SARS issues with 21 who had fibromyalgia. “Their problems are exactly the same. They have strange symptoms and nobody can figure out what they’re about. And these symptoms are aches and pains, and they have trouble thinking and concentrating,” Dr. Moldofsky said. Reports of COVID-19 long-haulers didn’t surprise Dr. Moldofsky, and he immediately recognized that Nath’s intention to follow these patients could provide insights into both fibromyalgia and chronic fatigue syndrome.
That’s exactly what Dr. Nath is proposing with the two NIH studies. One will focus solely on the neurologic impacts of COVID-19, including stroke, loss of taste and smell, and brain fog. The other will bring patients who have had COVID-19 symptoms for at least 6 months to the NIH Clinical Center for an inpatient stay during which they will undergo detailed physiologic tests.
Scientists around the world are launching their own post–COVID-19 studies. Dr. Moreau’s group in Montreal has laid the groundwork for such an endeavor, and the CoroNerve group in the United Kingdom is monitoring neurologic complications from the coronavirus. Many of them have the same goals as the NIH studies: Leverage the large number of COVID-19 long-haulers to better understand the earliest stages of postviral syndrome.
“At this juncture, after all the reports that we’ve seen so far, I think it’s very unlikely that there will be no relationship whatsoever between COVID-19 and chronic fatigue syndrome,” Dr. Hornig said. “I think there certainly will be some, but again, what’s the scope, what’s the size? And then, of course, even more importantly, if it is happening, what is the mechanism and how is it happening?”
For people like Ms. Gage-Witvliet, the answers can’t come soon enough. For the first time in more than a decade, the full-time professor of epidemiology didn’t prepare to teach this year because she simply can’t. It’s too taxing for her brain to deal with impromptu student questions. Ms. Gage-Witvliet hopes that, by sharing her own experiences with post COVID-19, she can help others.
“In my work, I use data to give a voice to people who don’t have a voice,” she said. “Now, I am one of those people.”
A version of this article first appeared on Medscape.com.
When Margot Gage-Witvliet began feeling run down after her family returned from a trip to the Netherlands in late February 2020, she initially chalked up her symptoms to jet lag. Three days later, however, her situation went from concerning to alarming as she struggled to breathe. “It felt like there was an elephant sitting on my chest,” she said.
Her husband and daughters also became ill with COVID-19, but Ms. Gage-Witvliet was the only one in her family who didn’t get better. After an early improvement, a rare coronavirus-induced tonic-clonic seizure in early April sent her spiraling back down. Ms. Gage-Witvliet spent the next several weeks in bed with the curtains drawn, unable to tolerate light or sound.
Today, Ms. Gage-Witvliet’s life looks nothing like it did 6 months ago when she first got sick. As one of COVID-19’s so called long-haulers, she continues to struggle with crushing fatigue, brain fog, and headaches – symptoms that worsen when she pushes herself to do more. Across the country, as many as 1 in 10 COVID-19 patients are reporting illnesses that continue for weeks and months after their initial diagnosis. Nearly all report neurologic issues like Ms. Gage-Witvliet, as well as shortness of breath and psychiatric concerns.
For Avindra Nath, MD, a neurologist at the National Institutes of Health, the experience of these long-haul COVID-19 patients feels familiar and reminds him of myalgic encephalomyelitis, also known as chronic fatigue syndrome.
Dr. Nath has long been interested in the lingering neurologic issues connected to chronic fatigue. An estimated three-quarters of all patients with chronic fatigue syndrome report that their symptoms started after a viral infection, and they suffer unrelenting exhaustion, difficulties regulating pulse and blood pressure, aches and pains, and brain fog. When Dr. Nath first read about the novel coronavirus, he began to worry that the virus would trigger symptoms in a subset of those infected. Hearing about the experiences of long-haulers like Ms. Gage-Witvliet raised his suspicions even more.
Unlike COVID-19 long-haulers, however, many patients with chronic fatigue syndrome go at least a year with these symptoms before receiving a diagnosis, according to a British survey. That means researchers have had few opportunities to study the early stages of the syndrome. “When we see patients with myalgic encephalomyelitis, whatever infection they might have had occurred in the remote past, so there’s no way for us to know how they got infected with it, what the infection was, or what the effects of it were in that early phase. We’re seeing them 2 years afterward,” Dr. Nath said.
Dr. Nath quickly realized that studying patients like Ms. Gage-Witvliet would give physicians and scientists a unique opportunity to understand not only long-term outcomes of COVID-19 infections, but also other postviral syndromes, including chronic fatigue syndrome at their earliest stages. It’s why Dr. Nath has spent the past several months scrambling to launch two NIH studies to examine the phenomenon.
Although Dr. Nath said that the parallels between COVID-19 long-haulers and those with chronic fatigue syndrome are obvious, he cautions against assuming that they are the same phenomenon. Some long-haulers might simply be taking a much slower path to recovery, or they might have a condition that looks similar on the surface but differs from chronic fatigue syndrome on a molecular level. But even if Dr. Nath fails to see links to chronic fatigue syndrome, with more than 92.5 million documented cases of COVID-19 around the world, the work will be relevant to the substantial number of infected individuals who don’t recover quickly.
“With so many people having exposure to the same virus over a similar time period, we really have the opportunity to look at these manifestations and at the very least to understand postviral syndromes,” said Mady Hornig, MD, a psychiatrist at Columbia University, New York.
The origins of chronic fatigue syndrome date back to 1985, when the Centers for Disease Control and Prevention received a request from two physicians – Paul Cheney, MD, and Daniel Peterson, MD – to investigate a mysterious disease outbreak in Nevada. In November 1984, residents in and around the idyllic vacation spot of Incline Village, a small town tucked into the north shore of Lake Tahoe, had begun reporting flu-like symptoms that persisted for weeks, even months. The doctors had searched high and low for a cause, but they couldn’t figure out what was making their patients sick.
They reported a range of symptoms – including muscle aches and pains, low-grade fevers, sore throats, and headaches – but everyone said that crippling fatigue was the most debilitating issue. This wasn’t the kind of fatigue that could be cured by a nap or even a long holiday. No matter how much their patients slept – and some were almost completely bedbound – their fatigue didn’t abate. What’s more, the fatigue got worse whenever they tried to push themselves to do more. Puzzled, the CDC sent two epidemic intelligence service (EIS) officers to try to get to the bottom of what might be happening.
Muscle aches and pains with crippling fatigue
After their visit to Incline Village, however, the CDC was just as perplexed as Dr. Cheney and Dr. Peterson. Many of the people with the condition reported flu-like symptoms right around the time they first got sick, and the physicians’ leading hypothesis was that the outbreak and its lasting symptoms were caused by chronic Epstein-Barr virus infection. But neither the CDC nor anyone else could identify the infection or any other microbial cause. The two EIS officers duly wrote up a report for the CDC’s flagship publication, Morbidity and Mortality Weekly ReportI, titled “Chronic Fatigue Possibly Related to Epstein-Barr Virus – Nevada”.
That investigators focused on the fatigue aspect made sense, says Leonard A. Jason, PhD, professor of psychology at DePaul University and director of the Center for Community Research, both in Chicago, because it was one of the few symptoms shared by all the individuals studied and it was also the most debilitating. But that focus – and the name “chronic fatigue syndrome” – led to broad public dismissal of the condition’s severity, as did an editorial note in MMWR urging physicians to look for “more definable, and possibly treatable, conditions.” Subsequent research failed to confirm a specific link to the Epstein-Barr virus, which only added to the condition’s phony reputation. Rather than being considered a potentially disabling illness, it was disregarded as a “yuppie flu” or a fancy name for malingering.
“It’s not a surprise that patients are being dismissed because there’s already this sort of grandfathered-in sense that fatigue is not real,” said Jennifer Frankovich, MD, a pediatric rheumatologist at Stanford (Calif.) University’s Lucile Packard Children’s Hospital in Palo Alto. “I’m sure that’s frustrating for them to be tired and then to have the clinician not believe them or dismiss them or think they’re making it up. It would be more helpful to the families to say: ‘You know what, we don’t know, we do not have the answer, and we believe you.’ ”
A syndrome’s shame
As time passed, patient advocacy groups began pushing back against the negative way the condition was being perceived. This criticism came as organizations like the CDC worked to develop a set of diagnostic criteria that researchers and clinicians dealing with chronic fatigue syndrome could use. With such a heterogeneous group of patients and symptoms, the task was no small challenge. The discussions, which took place over nearly 2 decades, played a key role in helping scientists home in on the single factor that was central to chronic fatigue: postexertional malaise.
“This is quite unique for chronic fatigue syndrome. With other diseases, yes, you may have fatigue as one of the components of the disease, but postexertional fatigue is very specific,” said Alain Moreau, PhD, a molecular biologist at the University of Montreal.
Of course, plenty of people have pushed themselves too hard physically and paid the price the next day. But those with chronic fatigue syndrome weren’t running marathons. To them, exertion could be anything from getting the mail to reading a book. Nor could the resulting exhaustion be resolved by an afternoon on the couch or a long vacation.
“If they do these activities, they can crash for weeks, even months,” Dr. Moreau said. It was deep, persistent, and – for 40% of those with chronic fatigue syndrome – disabling. In 2015, a study group from the Institute of Medicine proposed renaming chronic fatigue to “systemic exercise intolerance disease” because of the centrality of this symptom. Although that effort mostly stalled, their report did bring the condition out of its historic place as a scientific backwater. What resulted was an uptick in research on chronic fatigue syndrome, which helped define some of the physiological issues that either contribute to or result from the condition.
Researchers had long known about the link between infection and fatigue, said Dr. Frankovich. Work included mysterious outbreaks like the one in Lake Tahoe and well-documented issues like the wave of encephalitis lethargica (a condition that leaves patients in an almost vegetative state) that followed the 1918 H1N1 influenza pandemic.
“As a clinician, when you see someone who comes in with a chronic infection, they’re tired. I think that’s why, in the chronic-fatigue world, people are desperately looking for the infection so we can treat it, and maybe these poor suffering people will feel better,” Dr. Frankovich added. Then the pandemic struck, giving him yet another opportunity to study postviral syndromes.
Immunologic symptoms
Given the close link between a nonspecific viral illness and the onset of symptoms in chronic fatigue syndrome, scientists like Dr. Hornig opted to focus on immunologic symptoms. In a 2015 analysis published in Science, Dr. Hornig and colleagues showed that immune problems can be found in the earliest stages of chronic fatigue syndrome, and that they change as the illness progresses. Patients who had been sick for less than 3 years showed significant increases in levels of both pro- and anti-inflammatory cytokines, and the factor most strongly correlated to this inability to regulate cytokine levels was the duration of symptoms, not their severity. A series of other studies also revealed problems with regulation of the immune system, although no one could show what might have set these problems in motion.
Other researchers found signs of mitochondrial dysfunction in those with chronic fatigue syndrome. Because mitochondria make energy for cells, it wasn’t an intellectual stretch to believe that glitches in this process could contribute to fatigue. As early as 1991, scientists had discovered signs of mitochondrial degeneration in muscle biopsies from people with chronic fatigue syndrome. Subsequent studies showed that those affected by chronic fatigue were missing segments of mitochondrial DNA and had significantly reduced levels of mitochondrial activity. Although exercise normally improves mitochondrial functioning, the opposite appears to happen in chronic fatigue.
To Dr. Nath, these dual hypotheses aren’t necessarily mutually exclusive. Some studies have hinted that infection with the common human herpesvirus–6 (HHV-6) can lead to an autoimmune condition in which the body makes antibodies against the mitochondria. Mitochondria also play a key role in the ability of the innate immune system to produce interferon and other proinflammatory cytokines. It might also be that the link between immune and mitochondrial problems is more convoluted than originally thought, or that the two systems are affected independent of one another, Dr. Nath said.
Finding answers, especially those that could lead to potential treatments, wouldn’t be easy, however. In 2016, the NIH launched an in-depth study of a small number of individuals with chronic fatigue, hoping to find clues about what the condition was and how it might be treated.
For scientists like Dr. Nath, the NIH study provided a way to get at the underlying biology of chronic fatigue syndrome. Then the pandemic struck, giving him yet another opportunity to study postviral syndromes.
Chronic post-SARS syndrome
In March 2020, retired physician Harvey Moldofsky, MD, began receiving inquiries about a 2011 study he and his colleague, John Patcai, MD, had published in BMC Neurology about something they dubbed “chronic post-SARS syndrome.” The small case-control study, which involved mainly health care workers in Toronto, received little attention when it was first published, but with COVID-19, it was suddenly relevant.
Early clusters of similar cases in Miami made local physicians desperate for Dr. Moldofsky’s expertise. Luckily, he was nearby; he had fled the frigid Canadian winter for the warmth of Sarasota, Fla.
“I had people from various countries around the world writing to me and asking what they should do. And of course I don’t have any answers,” he said. But the study contained one of the world’s only references to the syndrome.
In 2003, a woman arrived in Toronto from Hong Kong. She didn’t know it at the time, but her preairport stay at the Hotel Metropole had infected her with the first SARS (severe acute respiratory syndrome) coronavirus. Her subsequent hospitalization in Toronto sparked a city-wide outbreak of SARS in which 273 people became ill and 44 died. Many of those affected were health care workers, including nurses and respiratory therapists. Although most eventually returned to work, a subset couldn’t. They complained of energy-sapping fatigue, poor sleep, brain fog, and assorted body aches and pains that persisted for more than 18 months. The aches and pains brought them to the attention of Dr. Moldofsky, then director of the Centre for the Study of Pain at the University of Toronto.
His primary interest at the time was fibromyalgia, which caused symptoms similar to those reported by the original SARS long-haulers. Intrigued, Dr. Moldofsky agreed to take a look. Their chest x-rays were clear and the nurses showed no signs of lingering viral infection. Dr. Moldofsky could see that the nurses were ill and suffering, but no lab tests or anything else could identify what was causing their symptoms.
In 2011, Dr. Moldofsky and Dr. Patcai found a strong overlap between chronic SARS, fibromyalgia, and chronic fatigue syndrome when they compared 22 patients with long-term SARS issues with 21 who had fibromyalgia. “Their problems are exactly the same. They have strange symptoms and nobody can figure out what they’re about. And these symptoms are aches and pains, and they have trouble thinking and concentrating,” Dr. Moldofsky said. Reports of COVID-19 long-haulers didn’t surprise Dr. Moldofsky, and he immediately recognized that Nath’s intention to follow these patients could provide insights into both fibromyalgia and chronic fatigue syndrome.
That’s exactly what Dr. Nath is proposing with the two NIH studies. One will focus solely on the neurologic impacts of COVID-19, including stroke, loss of taste and smell, and brain fog. The other will bring patients who have had COVID-19 symptoms for at least 6 months to the NIH Clinical Center for an inpatient stay during which they will undergo detailed physiologic tests.
Scientists around the world are launching their own post–COVID-19 studies. Dr. Moreau’s group in Montreal has laid the groundwork for such an endeavor, and the CoroNerve group in the United Kingdom is monitoring neurologic complications from the coronavirus. Many of them have the same goals as the NIH studies: Leverage the large number of COVID-19 long-haulers to better understand the earliest stages of postviral syndrome.
“At this juncture, after all the reports that we’ve seen so far, I think it’s very unlikely that there will be no relationship whatsoever between COVID-19 and chronic fatigue syndrome,” Dr. Hornig said. “I think there certainly will be some, but again, what’s the scope, what’s the size? And then, of course, even more importantly, if it is happening, what is the mechanism and how is it happening?”
For people like Ms. Gage-Witvliet, the answers can’t come soon enough. For the first time in more than a decade, the full-time professor of epidemiology didn’t prepare to teach this year because she simply can’t. It’s too taxing for her brain to deal with impromptu student questions. Ms. Gage-Witvliet hopes that, by sharing her own experiences with post COVID-19, she can help others.
“In my work, I use data to give a voice to people who don’t have a voice,” she said. “Now, I am one of those people.”
A version of this article first appeared on Medscape.com.
When Margot Gage-Witvliet began feeling run down after her family returned from a trip to the Netherlands in late February 2020, she initially chalked up her symptoms to jet lag. Three days later, however, her situation went from concerning to alarming as she struggled to breathe. “It felt like there was an elephant sitting on my chest,” she said.
Her husband and daughters also became ill with COVID-19, but Ms. Gage-Witvliet was the only one in her family who didn’t get better. After an early improvement, a rare coronavirus-induced tonic-clonic seizure in early April sent her spiraling back down. Ms. Gage-Witvliet spent the next several weeks in bed with the curtains drawn, unable to tolerate light or sound.
Today, Ms. Gage-Witvliet’s life looks nothing like it did 6 months ago when she first got sick. As one of COVID-19’s so called long-haulers, she continues to struggle with crushing fatigue, brain fog, and headaches – symptoms that worsen when she pushes herself to do more. Across the country, as many as 1 in 10 COVID-19 patients are reporting illnesses that continue for weeks and months after their initial diagnosis. Nearly all report neurologic issues like Ms. Gage-Witvliet, as well as shortness of breath and psychiatric concerns.
For Avindra Nath, MD, a neurologist at the National Institutes of Health, the experience of these long-haul COVID-19 patients feels familiar and reminds him of myalgic encephalomyelitis, also known as chronic fatigue syndrome.
Dr. Nath has long been interested in the lingering neurologic issues connected to chronic fatigue. An estimated three-quarters of all patients with chronic fatigue syndrome report that their symptoms started after a viral infection, and they suffer unrelenting exhaustion, difficulties regulating pulse and blood pressure, aches and pains, and brain fog. When Dr. Nath first read about the novel coronavirus, he began to worry that the virus would trigger symptoms in a subset of those infected. Hearing about the experiences of long-haulers like Ms. Gage-Witvliet raised his suspicions even more.
Unlike COVID-19 long-haulers, however, many patients with chronic fatigue syndrome go at least a year with these symptoms before receiving a diagnosis, according to a British survey. That means researchers have had few opportunities to study the early stages of the syndrome. “When we see patients with myalgic encephalomyelitis, whatever infection they might have had occurred in the remote past, so there’s no way for us to know how they got infected with it, what the infection was, or what the effects of it were in that early phase. We’re seeing them 2 years afterward,” Dr. Nath said.
Dr. Nath quickly realized that studying patients like Ms. Gage-Witvliet would give physicians and scientists a unique opportunity to understand not only long-term outcomes of COVID-19 infections, but also other postviral syndromes, including chronic fatigue syndrome at their earliest stages. It’s why Dr. Nath has spent the past several months scrambling to launch two NIH studies to examine the phenomenon.
Although Dr. Nath said that the parallels between COVID-19 long-haulers and those with chronic fatigue syndrome are obvious, he cautions against assuming that they are the same phenomenon. Some long-haulers might simply be taking a much slower path to recovery, or they might have a condition that looks similar on the surface but differs from chronic fatigue syndrome on a molecular level. But even if Dr. Nath fails to see links to chronic fatigue syndrome, with more than 92.5 million documented cases of COVID-19 around the world, the work will be relevant to the substantial number of infected individuals who don’t recover quickly.
“With so many people having exposure to the same virus over a similar time period, we really have the opportunity to look at these manifestations and at the very least to understand postviral syndromes,” said Mady Hornig, MD, a psychiatrist at Columbia University, New York.
The origins of chronic fatigue syndrome date back to 1985, when the Centers for Disease Control and Prevention received a request from two physicians – Paul Cheney, MD, and Daniel Peterson, MD – to investigate a mysterious disease outbreak in Nevada. In November 1984, residents in and around the idyllic vacation spot of Incline Village, a small town tucked into the north shore of Lake Tahoe, had begun reporting flu-like symptoms that persisted for weeks, even months. The doctors had searched high and low for a cause, but they couldn’t figure out what was making their patients sick.
They reported a range of symptoms – including muscle aches and pains, low-grade fevers, sore throats, and headaches – but everyone said that crippling fatigue was the most debilitating issue. This wasn’t the kind of fatigue that could be cured by a nap or even a long holiday. No matter how much their patients slept – and some were almost completely bedbound – their fatigue didn’t abate. What’s more, the fatigue got worse whenever they tried to push themselves to do more. Puzzled, the CDC sent two epidemic intelligence service (EIS) officers to try to get to the bottom of what might be happening.
Muscle aches and pains with crippling fatigue
After their visit to Incline Village, however, the CDC was just as perplexed as Dr. Cheney and Dr. Peterson. Many of the people with the condition reported flu-like symptoms right around the time they first got sick, and the physicians’ leading hypothesis was that the outbreak and its lasting symptoms were caused by chronic Epstein-Barr virus infection. But neither the CDC nor anyone else could identify the infection or any other microbial cause. The two EIS officers duly wrote up a report for the CDC’s flagship publication, Morbidity and Mortality Weekly ReportI, titled “Chronic Fatigue Possibly Related to Epstein-Barr Virus – Nevada”.
That investigators focused on the fatigue aspect made sense, says Leonard A. Jason, PhD, professor of psychology at DePaul University and director of the Center for Community Research, both in Chicago, because it was one of the few symptoms shared by all the individuals studied and it was also the most debilitating. But that focus – and the name “chronic fatigue syndrome” – led to broad public dismissal of the condition’s severity, as did an editorial note in MMWR urging physicians to look for “more definable, and possibly treatable, conditions.” Subsequent research failed to confirm a specific link to the Epstein-Barr virus, which only added to the condition’s phony reputation. Rather than being considered a potentially disabling illness, it was disregarded as a “yuppie flu” or a fancy name for malingering.
“It’s not a surprise that patients are being dismissed because there’s already this sort of grandfathered-in sense that fatigue is not real,” said Jennifer Frankovich, MD, a pediatric rheumatologist at Stanford (Calif.) University’s Lucile Packard Children’s Hospital in Palo Alto. “I’m sure that’s frustrating for them to be tired and then to have the clinician not believe them or dismiss them or think they’re making it up. It would be more helpful to the families to say: ‘You know what, we don’t know, we do not have the answer, and we believe you.’ ”
A syndrome’s shame
As time passed, patient advocacy groups began pushing back against the negative way the condition was being perceived. This criticism came as organizations like the CDC worked to develop a set of diagnostic criteria that researchers and clinicians dealing with chronic fatigue syndrome could use. With such a heterogeneous group of patients and symptoms, the task was no small challenge. The discussions, which took place over nearly 2 decades, played a key role in helping scientists home in on the single factor that was central to chronic fatigue: postexertional malaise.
“This is quite unique for chronic fatigue syndrome. With other diseases, yes, you may have fatigue as one of the components of the disease, but postexertional fatigue is very specific,” said Alain Moreau, PhD, a molecular biologist at the University of Montreal.
Of course, plenty of people have pushed themselves too hard physically and paid the price the next day. But those with chronic fatigue syndrome weren’t running marathons. To them, exertion could be anything from getting the mail to reading a book. Nor could the resulting exhaustion be resolved by an afternoon on the couch or a long vacation.
“If they do these activities, they can crash for weeks, even months,” Dr. Moreau said. It was deep, persistent, and – for 40% of those with chronic fatigue syndrome – disabling. In 2015, a study group from the Institute of Medicine proposed renaming chronic fatigue to “systemic exercise intolerance disease” because of the centrality of this symptom. Although that effort mostly stalled, their report did bring the condition out of its historic place as a scientific backwater. What resulted was an uptick in research on chronic fatigue syndrome, which helped define some of the physiological issues that either contribute to or result from the condition.
Researchers had long known about the link between infection and fatigue, said Dr. Frankovich. Work included mysterious outbreaks like the one in Lake Tahoe and well-documented issues like the wave of encephalitis lethargica (a condition that leaves patients in an almost vegetative state) that followed the 1918 H1N1 influenza pandemic.
“As a clinician, when you see someone who comes in with a chronic infection, they’re tired. I think that’s why, in the chronic-fatigue world, people are desperately looking for the infection so we can treat it, and maybe these poor suffering people will feel better,” Dr. Frankovich added. Then the pandemic struck, giving him yet another opportunity to study postviral syndromes.
Immunologic symptoms
Given the close link between a nonspecific viral illness and the onset of symptoms in chronic fatigue syndrome, scientists like Dr. Hornig opted to focus on immunologic symptoms. In a 2015 analysis published in Science, Dr. Hornig and colleagues showed that immune problems can be found in the earliest stages of chronic fatigue syndrome, and that they change as the illness progresses. Patients who had been sick for less than 3 years showed significant increases in levels of both pro- and anti-inflammatory cytokines, and the factor most strongly correlated to this inability to regulate cytokine levels was the duration of symptoms, not their severity. A series of other studies also revealed problems with regulation of the immune system, although no one could show what might have set these problems in motion.
Other researchers found signs of mitochondrial dysfunction in those with chronic fatigue syndrome. Because mitochondria make energy for cells, it wasn’t an intellectual stretch to believe that glitches in this process could contribute to fatigue. As early as 1991, scientists had discovered signs of mitochondrial degeneration in muscle biopsies from people with chronic fatigue syndrome. Subsequent studies showed that those affected by chronic fatigue were missing segments of mitochondrial DNA and had significantly reduced levels of mitochondrial activity. Although exercise normally improves mitochondrial functioning, the opposite appears to happen in chronic fatigue.
To Dr. Nath, these dual hypotheses aren’t necessarily mutually exclusive. Some studies have hinted that infection with the common human herpesvirus–6 (HHV-6) can lead to an autoimmune condition in which the body makes antibodies against the mitochondria. Mitochondria also play a key role in the ability of the innate immune system to produce interferon and other proinflammatory cytokines. It might also be that the link between immune and mitochondrial problems is more convoluted than originally thought, or that the two systems are affected independent of one another, Dr. Nath said.
Finding answers, especially those that could lead to potential treatments, wouldn’t be easy, however. In 2016, the NIH launched an in-depth study of a small number of individuals with chronic fatigue, hoping to find clues about what the condition was and how it might be treated.
For scientists like Dr. Nath, the NIH study provided a way to get at the underlying biology of chronic fatigue syndrome. Then the pandemic struck, giving him yet another opportunity to study postviral syndromes.
Chronic post-SARS syndrome
In March 2020, retired physician Harvey Moldofsky, MD, began receiving inquiries about a 2011 study he and his colleague, John Patcai, MD, had published in BMC Neurology about something they dubbed “chronic post-SARS syndrome.” The small case-control study, which involved mainly health care workers in Toronto, received little attention when it was first published, but with COVID-19, it was suddenly relevant.
Early clusters of similar cases in Miami made local physicians desperate for Dr. Moldofsky’s expertise. Luckily, he was nearby; he had fled the frigid Canadian winter for the warmth of Sarasota, Fla.
“I had people from various countries around the world writing to me and asking what they should do. And of course I don’t have any answers,” he said. But the study contained one of the world’s only references to the syndrome.
In 2003, a woman arrived in Toronto from Hong Kong. She didn’t know it at the time, but her preairport stay at the Hotel Metropole had infected her with the first SARS (severe acute respiratory syndrome) coronavirus. Her subsequent hospitalization in Toronto sparked a city-wide outbreak of SARS in which 273 people became ill and 44 died. Many of those affected were health care workers, including nurses and respiratory therapists. Although most eventually returned to work, a subset couldn’t. They complained of energy-sapping fatigue, poor sleep, brain fog, and assorted body aches and pains that persisted for more than 18 months. The aches and pains brought them to the attention of Dr. Moldofsky, then director of the Centre for the Study of Pain at the University of Toronto.
His primary interest at the time was fibromyalgia, which caused symptoms similar to those reported by the original SARS long-haulers. Intrigued, Dr. Moldofsky agreed to take a look. Their chest x-rays were clear and the nurses showed no signs of lingering viral infection. Dr. Moldofsky could see that the nurses were ill and suffering, but no lab tests or anything else could identify what was causing their symptoms.
In 2011, Dr. Moldofsky and Dr. Patcai found a strong overlap between chronic SARS, fibromyalgia, and chronic fatigue syndrome when they compared 22 patients with long-term SARS issues with 21 who had fibromyalgia. “Their problems are exactly the same. They have strange symptoms and nobody can figure out what they’re about. And these symptoms are aches and pains, and they have trouble thinking and concentrating,” Dr. Moldofsky said. Reports of COVID-19 long-haulers didn’t surprise Dr. Moldofsky, and he immediately recognized that Nath’s intention to follow these patients could provide insights into both fibromyalgia and chronic fatigue syndrome.
That’s exactly what Dr. Nath is proposing with the two NIH studies. One will focus solely on the neurologic impacts of COVID-19, including stroke, loss of taste and smell, and brain fog. The other will bring patients who have had COVID-19 symptoms for at least 6 months to the NIH Clinical Center for an inpatient stay during which they will undergo detailed physiologic tests.
Scientists around the world are launching their own post–COVID-19 studies. Dr. Moreau’s group in Montreal has laid the groundwork for such an endeavor, and the CoroNerve group in the United Kingdom is monitoring neurologic complications from the coronavirus. Many of them have the same goals as the NIH studies: Leverage the large number of COVID-19 long-haulers to better understand the earliest stages of postviral syndrome.
“At this juncture, after all the reports that we’ve seen so far, I think it’s very unlikely that there will be no relationship whatsoever between COVID-19 and chronic fatigue syndrome,” Dr. Hornig said. “I think there certainly will be some, but again, what’s the scope, what’s the size? And then, of course, even more importantly, if it is happening, what is the mechanism and how is it happening?”
For people like Ms. Gage-Witvliet, the answers can’t come soon enough. For the first time in more than a decade, the full-time professor of epidemiology didn’t prepare to teach this year because she simply can’t. It’s too taxing for her brain to deal with impromptu student questions. Ms. Gage-Witvliet hopes that, by sharing her own experiences with post COVID-19, she can help others.
“In my work, I use data to give a voice to people who don’t have a voice,” she said. “Now, I am one of those people.”
A version of this article first appeared on Medscape.com.
The next likely COVID-19 vaccine has its advantages
Among the multiple vaccine candidates around the globe, next up in the arsenal against COVID-19 is likely the single-dose Ad26.COV2.S vaccine in development from Johnson & Johnson/Janssen, infectious disease experts predict.
And it got closer with promising interim phase 1/2a trial results, published online Jan. 13 in The New England Journal of Medicine.
A single Ad26.COV2.S dose was associated with S-binding and neutralizing antibodies in more than 90% of the participants. The finding was observed in both adults aged 18-55 years and participants 65 and older, as well as for participants given low-dose or high-dose vaccinations.
The results also suggest a durable vaccine response. “The take-home message [includes] a high neutralizing antibody responder rate to a single dose of our Ad26.COV2.S COVID-19 vaccine candidate. In addition, we see that these responses and antibody titers are stable for at least 71 days,” senior study author Hanneke Schuitemaker, PhD, global head of viral vaccine discovery and translational medicine at Johnson & Johnson in Leiden, the Netherlands, said in an interview.
If the single-dose Johnson & Johnson product gains Food and Drug Administration emergency use authorization (EUA), it could significantly boost the number of overall immunizations available. Less stringent storage requirements – only regular refrigeration vs. a need to freeze the Pfizer/BioNTech and Moderna COVID-19 vaccines – is another potential advantage. The Ad26.COV2.S vaccine can be refrigerated for up to 3 months at 36°-46 °F (2°-8 °C).
“Phase 1-2 trial data on the J&J vaccine: If it works as well as the mRNA options, it will have substantial advantages,” Jeremy Faust, MD, an emergency room physician affiliated with Brigham & Women’s Hospital and Harvard Medical School, Boston, tweeted on Jan. 13.
Unlike the Pfizer/BioNTech and Moderna messenger RNA vaccines, the Johnson & Johnson product is a recombinant, replication-incompetent adenovirus serotype 26 (Ad26) vector encoding a full-length and stabilized SARS-CoV-2 spike (S) protein.
Phase 3 efficacy/safety results pending
Under normal circumstances, phase 3 trial results would not be anticipated within weeks of phase 1/2a trial findings. However, the urgency of the COVID-19 pandemic accelerated the vaccine development process, so preclinical trials were conducted simultaneously and not sequentially. For this reason, phase 3 interim results for the Johnson & Johnson vaccine are expected within weeks, and a company executive told Reuters that the rollout is on track for March.
“We hope to report data from our first phase 3 study, ENSEMBLE, in which we are testing the protective efficacy of a single dose of Ad26.COV2.S, by the end of this month or early February,” Dr. Schuitemaker said.
In the meantime, the phase 1/2a ongoing, multicenter, randomized, double-blind, and placebo-controlled trial interim results have drawn positive reactions.
“Data is highly encouraging and supports the single inoculation approach that makes this vaccine unique,” Carlos del Rio, executive associate dean for Emory University at Grady in Atlanta, wrote in a tweet on Jan. 13.
“Encouraging COVID vaccine data from J&J published [Jan. 13]. Solid antibody, CD4 T cell, and CD8 T cell responses – a nice trifecta of vaccine immune responses to see! And safe!” tweeted Shane Crotty, PhD, vaccine scientist and professor at the La Jolla (Calif.) Institute for Immunology.
First results in 800+ participants
At baseline for the phase 1/2a trial, 2% of the younger group and 1% of the 65+ group were seropositive for SARS-CoV-2 S-specific antibodies.
A total of 402 people in the younger age cohort and 403 in the 65 and older group received a first dose of the Johnson & Johnson vaccine. Many participants also received a second dose 56 days later for a separate trial, ENSEMBLE2, designed to compare safety and efficacy between single- and double-dose regimens. Results of that trial are still pending.
Safety profile
A single dose was associated with a higher incidence of solicited systemic adverse events in the higher vaccine dose group. They also found that grade 3 adverse events decreased with increasing age.
Injection site pain on the day of immunization or the next day was the most common local reaction. The pain generally resolved within 24 hours. Fever was reported by 15% of the low-dose vaccine group and 39% of the high-dose cohort. Fatigue, headache, and myalgia were the most common grade 1 or 2 solicited systemic adverse events reported.
Five serious adverse events were reported, including four that investigators deemed unrelated to vaccination: hypotension, bilateral nephrolithiasis, legionella pneumonia, and one case of worsening of multiple sclerosis. The vaccine-related serious adverse event was a fever that resulted in hospitalization because of suspicion of COVID-19. The patient recovered within 12 hours.
“These data confirm our previous experience with vaccine candidates based on our Ad26 viral vector platform in the younger age group. The almost similar performance in older adults is promising,” Dr. Schuitemaker said.
A potential limitation of the phase 1/2a trial is “the lack of representation of minority groups,” the researchers noted. Johnson & Johnson is working on improving the diversity of study participants “with respect to groups that seem to be affected most by the COVID-19 pandemic.”
AstraZeneca/Oxford vaccine status
The AstraZeneca/Oxford AZD1222 vaccine in development received approval for use in the United Kingdom on Dec. 30. The approval came after Public Health England said the country was facing “unprecedented” levels of infections, the BBC reported. AstraZeneca applied for European Medical Agency approval earlier in the week of Jan. 10, which could lead to more widespread use across Europe.
The status of the vaccine remains uncertain in the United States. A phase 3 trial that started in August was paused for about 6 weeks in September and October after an adverse event in a British volunteer halted studies worldwide. On Oct. 23, the FDA permitted researchers to continue the trial with approximately 40,000 participants.
There was some suggestion in the clinical trials that a half dose of the AstraZeneca vaccine was more effective than a full dose, 90% vs. 62%, but some irregularities in the research require further investigation.
Although the AstraZeneca vaccine is delivered to cells by an adenovirus – as with the Johnson & Johnson product – it is designed to be delivered in two doses 28 days apart, like the administration schedule of the Moderna mRNA vaccine.
A need for speed, and more doses
Regardless of which vaccine product is next to gain an EUA in the United States, many experts agree the COVID-19 vaccine rollouts so far have been problematic, at a time when cases are climbing to record-breaking levels, and likely more related to logistics over administration of the vaccine than production of the doses.
“Lots of doses being manufactured. In December 20 million, January 40 million, February 80 million and J&J hopefully soon to add to the count. The shortage is the number arms not getting vaccinated. Freezers do not get COVID. They do not need all those vaccines,” Daniel Griffin, MD, PhD, an infectious disease expert in Port Washington, N.Y., tweeted on Jan. 12.
“Unfortunately, the rollout has not gone smoothly, partly due to a lack of resources for this distribution phase we’re in,” Andrew T. Pavia, MD, chief of the division of pediatric infectious diseases at the University of Utah, Salt Lake City, said during a media briefing Jan. 14 sponsored by the Infectious Diseases Society of America (IDSA).
“We’re concerned about the mismatch between the number of people who are being told they are eligible and the amount of vaccine that is being distributed,” he said.
Complicating the rollout is a directive from U.S. Health and Human Services Secretary Alex Azar that states should start vaccinating everyone 65 and older as well as those with underlying conditions.
Expanding distribution to the 15% of Americans in just this age group is a big challenge, Dr. Pavia said. “We have enough vaccine maybe to vaccinate 40 million by the end of this month. There is a huge disconnect, and that creates a lot of problems.”
“One of the biggest problems is we are trying to do this mass vaccination program in the middle of the biggest surge we’ve ever seen,” Julie Vaishampayan, MD, MPH, chair of the IDSA Public Health Committee, said during the briefing. Without sufficient time for public health officials to plan for vaccinating a larger population, “people will come and stand in extremely long lines.”
Trying to expand immunization access without a proportionate increase in available doses prompted Dr. Vaishampayan to share an analogy from a colleague: “We are trying to fill a lake with a garden hose. Rather than making the lake bigger, what we really need is more water.”
Dr. Pavia emphasized that infectious disease experts “know the measures that work.” Not using masks, physical distancing, and hand hygiene, he said, “is a bit like knowing that really good shark repellents will be available in summer, so I’m going to jump into the ocean covered in blood while the great whites are swimming around.”
An official at the World Health Organization agreed. “Vaccines are coming online and I do believe vaccines will make a huge difference. But they are not here yet in enough quantities and in enough people to make that difference,” Michael Ryan, MB, WHO executive director of health emergencies, said during an online media briefing Jan. 13, held in conjunction with Emory University.
Dr. Ryan predicted that “we’ve got weeks if not months ahead of us in which our weapon is our knowledge ... what we know about this virus, its transmission, and stopping that transmission.
“And as the vaccines roll in, we can hopefully end this horrific pandemic.”
Dr. Schuitemaker reports grants from BARDA during the conduct of the study; personal fees and other from Janssen Vaccines and Prevention, a J&J company, outside the submitted work. Johnson & Johnson and the Biomedical Advanced Research and Development Authority of the Department of Health and Human Services funded the phase 1/2a study.
A version of this article first appeared on Medscape.com.
Among the multiple vaccine candidates around the globe, next up in the arsenal against COVID-19 is likely the single-dose Ad26.COV2.S vaccine in development from Johnson & Johnson/Janssen, infectious disease experts predict.
And it got closer with promising interim phase 1/2a trial results, published online Jan. 13 in The New England Journal of Medicine.
A single Ad26.COV2.S dose was associated with S-binding and neutralizing antibodies in more than 90% of the participants. The finding was observed in both adults aged 18-55 years and participants 65 and older, as well as for participants given low-dose or high-dose vaccinations.
The results also suggest a durable vaccine response. “The take-home message [includes] a high neutralizing antibody responder rate to a single dose of our Ad26.COV2.S COVID-19 vaccine candidate. In addition, we see that these responses and antibody titers are stable for at least 71 days,” senior study author Hanneke Schuitemaker, PhD, global head of viral vaccine discovery and translational medicine at Johnson & Johnson in Leiden, the Netherlands, said in an interview.
If the single-dose Johnson & Johnson product gains Food and Drug Administration emergency use authorization (EUA), it could significantly boost the number of overall immunizations available. Less stringent storage requirements – only regular refrigeration vs. a need to freeze the Pfizer/BioNTech and Moderna COVID-19 vaccines – is another potential advantage. The Ad26.COV2.S vaccine can be refrigerated for up to 3 months at 36°-46 °F (2°-8 °C).
“Phase 1-2 trial data on the J&J vaccine: If it works as well as the mRNA options, it will have substantial advantages,” Jeremy Faust, MD, an emergency room physician affiliated with Brigham & Women’s Hospital and Harvard Medical School, Boston, tweeted on Jan. 13.
Unlike the Pfizer/BioNTech and Moderna messenger RNA vaccines, the Johnson & Johnson product is a recombinant, replication-incompetent adenovirus serotype 26 (Ad26) vector encoding a full-length and stabilized SARS-CoV-2 spike (S) protein.
Phase 3 efficacy/safety results pending
Under normal circumstances, phase 3 trial results would not be anticipated within weeks of phase 1/2a trial findings. However, the urgency of the COVID-19 pandemic accelerated the vaccine development process, so preclinical trials were conducted simultaneously and not sequentially. For this reason, phase 3 interim results for the Johnson & Johnson vaccine are expected within weeks, and a company executive told Reuters that the rollout is on track for March.
“We hope to report data from our first phase 3 study, ENSEMBLE, in which we are testing the protective efficacy of a single dose of Ad26.COV2.S, by the end of this month or early February,” Dr. Schuitemaker said.
In the meantime, the phase 1/2a ongoing, multicenter, randomized, double-blind, and placebo-controlled trial interim results have drawn positive reactions.
“Data is highly encouraging and supports the single inoculation approach that makes this vaccine unique,” Carlos del Rio, executive associate dean for Emory University at Grady in Atlanta, wrote in a tweet on Jan. 13.
“Encouraging COVID vaccine data from J&J published [Jan. 13]. Solid antibody, CD4 T cell, and CD8 T cell responses – a nice trifecta of vaccine immune responses to see! And safe!” tweeted Shane Crotty, PhD, vaccine scientist and professor at the La Jolla (Calif.) Institute for Immunology.
First results in 800+ participants
At baseline for the phase 1/2a trial, 2% of the younger group and 1% of the 65+ group were seropositive for SARS-CoV-2 S-specific antibodies.
A total of 402 people in the younger age cohort and 403 in the 65 and older group received a first dose of the Johnson & Johnson vaccine. Many participants also received a second dose 56 days later for a separate trial, ENSEMBLE2, designed to compare safety and efficacy between single- and double-dose regimens. Results of that trial are still pending.
Safety profile
A single dose was associated with a higher incidence of solicited systemic adverse events in the higher vaccine dose group. They also found that grade 3 adverse events decreased with increasing age.
Injection site pain on the day of immunization or the next day was the most common local reaction. The pain generally resolved within 24 hours. Fever was reported by 15% of the low-dose vaccine group and 39% of the high-dose cohort. Fatigue, headache, and myalgia were the most common grade 1 or 2 solicited systemic adverse events reported.
Five serious adverse events were reported, including four that investigators deemed unrelated to vaccination: hypotension, bilateral nephrolithiasis, legionella pneumonia, and one case of worsening of multiple sclerosis. The vaccine-related serious adverse event was a fever that resulted in hospitalization because of suspicion of COVID-19. The patient recovered within 12 hours.
“These data confirm our previous experience with vaccine candidates based on our Ad26 viral vector platform in the younger age group. The almost similar performance in older adults is promising,” Dr. Schuitemaker said.
A potential limitation of the phase 1/2a trial is “the lack of representation of minority groups,” the researchers noted. Johnson & Johnson is working on improving the diversity of study participants “with respect to groups that seem to be affected most by the COVID-19 pandemic.”
AstraZeneca/Oxford vaccine status
The AstraZeneca/Oxford AZD1222 vaccine in development received approval for use in the United Kingdom on Dec. 30. The approval came after Public Health England said the country was facing “unprecedented” levels of infections, the BBC reported. AstraZeneca applied for European Medical Agency approval earlier in the week of Jan. 10, which could lead to more widespread use across Europe.
The status of the vaccine remains uncertain in the United States. A phase 3 trial that started in August was paused for about 6 weeks in September and October after an adverse event in a British volunteer halted studies worldwide. On Oct. 23, the FDA permitted researchers to continue the trial with approximately 40,000 participants.
There was some suggestion in the clinical trials that a half dose of the AstraZeneca vaccine was more effective than a full dose, 90% vs. 62%, but some irregularities in the research require further investigation.
Although the AstraZeneca vaccine is delivered to cells by an adenovirus – as with the Johnson & Johnson product – it is designed to be delivered in two doses 28 days apart, like the administration schedule of the Moderna mRNA vaccine.
A need for speed, and more doses
Regardless of which vaccine product is next to gain an EUA in the United States, many experts agree the COVID-19 vaccine rollouts so far have been problematic, at a time when cases are climbing to record-breaking levels, and likely more related to logistics over administration of the vaccine than production of the doses.
“Lots of doses being manufactured. In December 20 million, January 40 million, February 80 million and J&J hopefully soon to add to the count. The shortage is the number arms not getting vaccinated. Freezers do not get COVID. They do not need all those vaccines,” Daniel Griffin, MD, PhD, an infectious disease expert in Port Washington, N.Y., tweeted on Jan. 12.
“Unfortunately, the rollout has not gone smoothly, partly due to a lack of resources for this distribution phase we’re in,” Andrew T. Pavia, MD, chief of the division of pediatric infectious diseases at the University of Utah, Salt Lake City, said during a media briefing Jan. 14 sponsored by the Infectious Diseases Society of America (IDSA).
“We’re concerned about the mismatch between the number of people who are being told they are eligible and the amount of vaccine that is being distributed,” he said.
Complicating the rollout is a directive from U.S. Health and Human Services Secretary Alex Azar that states should start vaccinating everyone 65 and older as well as those with underlying conditions.
Expanding distribution to the 15% of Americans in just this age group is a big challenge, Dr. Pavia said. “We have enough vaccine maybe to vaccinate 40 million by the end of this month. There is a huge disconnect, and that creates a lot of problems.”
“One of the biggest problems is we are trying to do this mass vaccination program in the middle of the biggest surge we’ve ever seen,” Julie Vaishampayan, MD, MPH, chair of the IDSA Public Health Committee, said during the briefing. Without sufficient time for public health officials to plan for vaccinating a larger population, “people will come and stand in extremely long lines.”
Trying to expand immunization access without a proportionate increase in available doses prompted Dr. Vaishampayan to share an analogy from a colleague: “We are trying to fill a lake with a garden hose. Rather than making the lake bigger, what we really need is more water.”
Dr. Pavia emphasized that infectious disease experts “know the measures that work.” Not using masks, physical distancing, and hand hygiene, he said, “is a bit like knowing that really good shark repellents will be available in summer, so I’m going to jump into the ocean covered in blood while the great whites are swimming around.”
An official at the World Health Organization agreed. “Vaccines are coming online and I do believe vaccines will make a huge difference. But they are not here yet in enough quantities and in enough people to make that difference,” Michael Ryan, MB, WHO executive director of health emergencies, said during an online media briefing Jan. 13, held in conjunction with Emory University.
Dr. Ryan predicted that “we’ve got weeks if not months ahead of us in which our weapon is our knowledge ... what we know about this virus, its transmission, and stopping that transmission.
“And as the vaccines roll in, we can hopefully end this horrific pandemic.”
Dr. Schuitemaker reports grants from BARDA during the conduct of the study; personal fees and other from Janssen Vaccines and Prevention, a J&J company, outside the submitted work. Johnson & Johnson and the Biomedical Advanced Research and Development Authority of the Department of Health and Human Services funded the phase 1/2a study.
A version of this article first appeared on Medscape.com.
Among the multiple vaccine candidates around the globe, next up in the arsenal against COVID-19 is likely the single-dose Ad26.COV2.S vaccine in development from Johnson & Johnson/Janssen, infectious disease experts predict.
And it got closer with promising interim phase 1/2a trial results, published online Jan. 13 in The New England Journal of Medicine.
A single Ad26.COV2.S dose was associated with S-binding and neutralizing antibodies in more than 90% of the participants. The finding was observed in both adults aged 18-55 years and participants 65 and older, as well as for participants given low-dose or high-dose vaccinations.
The results also suggest a durable vaccine response. “The take-home message [includes] a high neutralizing antibody responder rate to a single dose of our Ad26.COV2.S COVID-19 vaccine candidate. In addition, we see that these responses and antibody titers are stable for at least 71 days,” senior study author Hanneke Schuitemaker, PhD, global head of viral vaccine discovery and translational medicine at Johnson & Johnson in Leiden, the Netherlands, said in an interview.
If the single-dose Johnson & Johnson product gains Food and Drug Administration emergency use authorization (EUA), it could significantly boost the number of overall immunizations available. Less stringent storage requirements – only regular refrigeration vs. a need to freeze the Pfizer/BioNTech and Moderna COVID-19 vaccines – is another potential advantage. The Ad26.COV2.S vaccine can be refrigerated for up to 3 months at 36°-46 °F (2°-8 °C).
“Phase 1-2 trial data on the J&J vaccine: If it works as well as the mRNA options, it will have substantial advantages,” Jeremy Faust, MD, an emergency room physician affiliated with Brigham & Women’s Hospital and Harvard Medical School, Boston, tweeted on Jan. 13.
Unlike the Pfizer/BioNTech and Moderna messenger RNA vaccines, the Johnson & Johnson product is a recombinant, replication-incompetent adenovirus serotype 26 (Ad26) vector encoding a full-length and stabilized SARS-CoV-2 spike (S) protein.
Phase 3 efficacy/safety results pending
Under normal circumstances, phase 3 trial results would not be anticipated within weeks of phase 1/2a trial findings. However, the urgency of the COVID-19 pandemic accelerated the vaccine development process, so preclinical trials were conducted simultaneously and not sequentially. For this reason, phase 3 interim results for the Johnson & Johnson vaccine are expected within weeks, and a company executive told Reuters that the rollout is on track for March.
“We hope to report data from our first phase 3 study, ENSEMBLE, in which we are testing the protective efficacy of a single dose of Ad26.COV2.S, by the end of this month or early February,” Dr. Schuitemaker said.
In the meantime, the phase 1/2a ongoing, multicenter, randomized, double-blind, and placebo-controlled trial interim results have drawn positive reactions.
“Data is highly encouraging and supports the single inoculation approach that makes this vaccine unique,” Carlos del Rio, executive associate dean for Emory University at Grady in Atlanta, wrote in a tweet on Jan. 13.
“Encouraging COVID vaccine data from J&J published [Jan. 13]. Solid antibody, CD4 T cell, and CD8 T cell responses – a nice trifecta of vaccine immune responses to see! And safe!” tweeted Shane Crotty, PhD, vaccine scientist and professor at the La Jolla (Calif.) Institute for Immunology.
First results in 800+ participants
At baseline for the phase 1/2a trial, 2% of the younger group and 1% of the 65+ group were seropositive for SARS-CoV-2 S-specific antibodies.
A total of 402 people in the younger age cohort and 403 in the 65 and older group received a first dose of the Johnson & Johnson vaccine. Many participants also received a second dose 56 days later for a separate trial, ENSEMBLE2, designed to compare safety and efficacy between single- and double-dose regimens. Results of that trial are still pending.
Safety profile
A single dose was associated with a higher incidence of solicited systemic adverse events in the higher vaccine dose group. They also found that grade 3 adverse events decreased with increasing age.
Injection site pain on the day of immunization or the next day was the most common local reaction. The pain generally resolved within 24 hours. Fever was reported by 15% of the low-dose vaccine group and 39% of the high-dose cohort. Fatigue, headache, and myalgia were the most common grade 1 or 2 solicited systemic adverse events reported.
Five serious adverse events were reported, including four that investigators deemed unrelated to vaccination: hypotension, bilateral nephrolithiasis, legionella pneumonia, and one case of worsening of multiple sclerosis. The vaccine-related serious adverse event was a fever that resulted in hospitalization because of suspicion of COVID-19. The patient recovered within 12 hours.
“These data confirm our previous experience with vaccine candidates based on our Ad26 viral vector platform in the younger age group. The almost similar performance in older adults is promising,” Dr. Schuitemaker said.
A potential limitation of the phase 1/2a trial is “the lack of representation of minority groups,” the researchers noted. Johnson & Johnson is working on improving the diversity of study participants “with respect to groups that seem to be affected most by the COVID-19 pandemic.”
AstraZeneca/Oxford vaccine status
The AstraZeneca/Oxford AZD1222 vaccine in development received approval for use in the United Kingdom on Dec. 30. The approval came after Public Health England said the country was facing “unprecedented” levels of infections, the BBC reported. AstraZeneca applied for European Medical Agency approval earlier in the week of Jan. 10, which could lead to more widespread use across Europe.
The status of the vaccine remains uncertain in the United States. A phase 3 trial that started in August was paused for about 6 weeks in September and October after an adverse event in a British volunteer halted studies worldwide. On Oct. 23, the FDA permitted researchers to continue the trial with approximately 40,000 participants.
There was some suggestion in the clinical trials that a half dose of the AstraZeneca vaccine was more effective than a full dose, 90% vs. 62%, but some irregularities in the research require further investigation.
Although the AstraZeneca vaccine is delivered to cells by an adenovirus – as with the Johnson & Johnson product – it is designed to be delivered in two doses 28 days apart, like the administration schedule of the Moderna mRNA vaccine.
A need for speed, and more doses
Regardless of which vaccine product is next to gain an EUA in the United States, many experts agree the COVID-19 vaccine rollouts so far have been problematic, at a time when cases are climbing to record-breaking levels, and likely more related to logistics over administration of the vaccine than production of the doses.
“Lots of doses being manufactured. In December 20 million, January 40 million, February 80 million and J&J hopefully soon to add to the count. The shortage is the number arms not getting vaccinated. Freezers do not get COVID. They do not need all those vaccines,” Daniel Griffin, MD, PhD, an infectious disease expert in Port Washington, N.Y., tweeted on Jan. 12.
“Unfortunately, the rollout has not gone smoothly, partly due to a lack of resources for this distribution phase we’re in,” Andrew T. Pavia, MD, chief of the division of pediatric infectious diseases at the University of Utah, Salt Lake City, said during a media briefing Jan. 14 sponsored by the Infectious Diseases Society of America (IDSA).
“We’re concerned about the mismatch between the number of people who are being told they are eligible and the amount of vaccine that is being distributed,” he said.
Complicating the rollout is a directive from U.S. Health and Human Services Secretary Alex Azar that states should start vaccinating everyone 65 and older as well as those with underlying conditions.
Expanding distribution to the 15% of Americans in just this age group is a big challenge, Dr. Pavia said. “We have enough vaccine maybe to vaccinate 40 million by the end of this month. There is a huge disconnect, and that creates a lot of problems.”
“One of the biggest problems is we are trying to do this mass vaccination program in the middle of the biggest surge we’ve ever seen,” Julie Vaishampayan, MD, MPH, chair of the IDSA Public Health Committee, said during the briefing. Without sufficient time for public health officials to plan for vaccinating a larger population, “people will come and stand in extremely long lines.”
Trying to expand immunization access without a proportionate increase in available doses prompted Dr. Vaishampayan to share an analogy from a colleague: “We are trying to fill a lake with a garden hose. Rather than making the lake bigger, what we really need is more water.”
Dr. Pavia emphasized that infectious disease experts “know the measures that work.” Not using masks, physical distancing, and hand hygiene, he said, “is a bit like knowing that really good shark repellents will be available in summer, so I’m going to jump into the ocean covered in blood while the great whites are swimming around.”
An official at the World Health Organization agreed. “Vaccines are coming online and I do believe vaccines will make a huge difference. But they are not here yet in enough quantities and in enough people to make that difference,” Michael Ryan, MB, WHO executive director of health emergencies, said during an online media briefing Jan. 13, held in conjunction with Emory University.
Dr. Ryan predicted that “we’ve got weeks if not months ahead of us in which our weapon is our knowledge ... what we know about this virus, its transmission, and stopping that transmission.
“And as the vaccines roll in, we can hopefully end this horrific pandemic.”
Dr. Schuitemaker reports grants from BARDA during the conduct of the study; personal fees and other from Janssen Vaccines and Prevention, a J&J company, outside the submitted work. Johnson & Johnson and the Biomedical Advanced Research and Development Authority of the Department of Health and Human Services funded the phase 1/2a study.
A version of this article first appeared on Medscape.com.
Pityriasis rosea carries few risks for pregnant women
according to a review of 33 patients.
“Though generally considered benign, PR may be associated with an increased risk of birth complications if acquired during pregnancy,” and previous studies have shown increased rates of complications including miscarriage and neonatal hypotonia in these patients, wrote Julian Stashower of the University of Virginia, Charlottesville, and colleagues.
In a retrospective study published in the Journal of the American Academy of Dermatology, the researchers assessed pregnancy outcomes in women who developed PR during pregnancy. They were identified from medical records at three institutions between September 2010 and June 2020. Diagnosis of PR, a papulosquamous skin eruption associated with human herpesvirus (HHV)–6/7 reactivation, was based on history and physical examination.
Overall, 8 of the 33 women (24%) had birth complications; the rates of preterm delivery, spontaneous pregnancy loss in clinically detectable pregnancies, and oligohydramnios were 6%, 0%, and 3%, respectively. The average onset of PR during pregnancy was earlier among women with complications, compared with those without complications (10.75 weeks’ gestation vs. 15.21 weeks’ gestation), but the difference was not statistically significant.
The researchers noted that their findings differed from the most recent study of PR in pregnancy, which included 60 patients and found a notably higher incidence of overall birth complications (50%), as well as higher incidence of neonatal hypotonia (25%), and miscarriage (13%).
The previous study also showed an increased risk of birth complications when PR onset occurred prior to 15 weeks’ gestation, but the current study did not reflect that finding, they wrote.
The current study findings were limited by several factors including the small sample size, retrospective design, and lack of confirmation of PR with HHV-6/7 testing, as well as lack of exclusion of atypical PR cases, the researchers noted. However, the results suggest that birth complications associated with PR may be lower than previously reported. “Further research is needed to guide future care and fully elucidate this possible association, which has important implications for both pregnant women with PR and their providers.”
The study received no outside funding. The researchers had no financial conflict to disclose.
according to a review of 33 patients.
“Though generally considered benign, PR may be associated with an increased risk of birth complications if acquired during pregnancy,” and previous studies have shown increased rates of complications including miscarriage and neonatal hypotonia in these patients, wrote Julian Stashower of the University of Virginia, Charlottesville, and colleagues.
In a retrospective study published in the Journal of the American Academy of Dermatology, the researchers assessed pregnancy outcomes in women who developed PR during pregnancy. They were identified from medical records at three institutions between September 2010 and June 2020. Diagnosis of PR, a papulosquamous skin eruption associated with human herpesvirus (HHV)–6/7 reactivation, was based on history and physical examination.
Overall, 8 of the 33 women (24%) had birth complications; the rates of preterm delivery, spontaneous pregnancy loss in clinically detectable pregnancies, and oligohydramnios were 6%, 0%, and 3%, respectively. The average onset of PR during pregnancy was earlier among women with complications, compared with those without complications (10.75 weeks’ gestation vs. 15.21 weeks’ gestation), but the difference was not statistically significant.
The researchers noted that their findings differed from the most recent study of PR in pregnancy, which included 60 patients and found a notably higher incidence of overall birth complications (50%), as well as higher incidence of neonatal hypotonia (25%), and miscarriage (13%).
The previous study also showed an increased risk of birth complications when PR onset occurred prior to 15 weeks’ gestation, but the current study did not reflect that finding, they wrote.
The current study findings were limited by several factors including the small sample size, retrospective design, and lack of confirmation of PR with HHV-6/7 testing, as well as lack of exclusion of atypical PR cases, the researchers noted. However, the results suggest that birth complications associated with PR may be lower than previously reported. “Further research is needed to guide future care and fully elucidate this possible association, which has important implications for both pregnant women with PR and their providers.”
The study received no outside funding. The researchers had no financial conflict to disclose.
according to a review of 33 patients.
“Though generally considered benign, PR may be associated with an increased risk of birth complications if acquired during pregnancy,” and previous studies have shown increased rates of complications including miscarriage and neonatal hypotonia in these patients, wrote Julian Stashower of the University of Virginia, Charlottesville, and colleagues.
In a retrospective study published in the Journal of the American Academy of Dermatology, the researchers assessed pregnancy outcomes in women who developed PR during pregnancy. They were identified from medical records at three institutions between September 2010 and June 2020. Diagnosis of PR, a papulosquamous skin eruption associated with human herpesvirus (HHV)–6/7 reactivation, was based on history and physical examination.
Overall, 8 of the 33 women (24%) had birth complications; the rates of preterm delivery, spontaneous pregnancy loss in clinically detectable pregnancies, and oligohydramnios were 6%, 0%, and 3%, respectively. The average onset of PR during pregnancy was earlier among women with complications, compared with those without complications (10.75 weeks’ gestation vs. 15.21 weeks’ gestation), but the difference was not statistically significant.
The researchers noted that their findings differed from the most recent study of PR in pregnancy, which included 60 patients and found a notably higher incidence of overall birth complications (50%), as well as higher incidence of neonatal hypotonia (25%), and miscarriage (13%).
The previous study also showed an increased risk of birth complications when PR onset occurred prior to 15 weeks’ gestation, but the current study did not reflect that finding, they wrote.
The current study findings were limited by several factors including the small sample size, retrospective design, and lack of confirmation of PR with HHV-6/7 testing, as well as lack of exclusion of atypical PR cases, the researchers noted. However, the results suggest that birth complications associated with PR may be lower than previously reported. “Further research is needed to guide future care and fully elucidate this possible association, which has important implications for both pregnant women with PR and their providers.”
The study received no outside funding. The researchers had no financial conflict to disclose.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Family physicians can help achieve national goals on STIs
Among these are the U.S. Department of Health and Human Services’ first “Sexually Transmitted Infections (STIs) National Strategic Plan for the United States,” which has a strong encompassing vision.
“The United States will be a place where sexually transmitted infections are prevented and where every person has high-quality STI prevention care, and treatment while living free from stigma and discrimination. The vision includes all people, regardless of age, sex, gender identity, sexual orientation, race, ethnicity, religion, disability, geographic location, or socioeconomic circumstance,” the new HHS plan states.1
Family physicians can and should play important roles in helping our country meet this plan’s goals particularly by following two important updated clinical guidelines, one from the U.S. Preventive Services Task Force (USPSTF) and another from the Centers for Disease Control and Prevention (CDC).
This strategic plan includes the following five overarching goals with associated objectives:
- Prevent New STIs.
- Improve the health of people by reducing adverse outcomes of STIs.
- Accelerate progress in STI research, technology, and innovation.
- Reduce STI-related health disparities and health inequities.
- Achieve integrated, coordinated efforts that address the STI epidemic.1
In my opinion, family physicians have important roles to play in order for each of these goals to be achieved.Unfortunately, there are approximately 20 million new cases of STIs each year, and the U.S. has seen increases in the rates of STIs in the past decade.
“Sexually transmitted infections are frequently asymptomatic, which may delay diagnosis and treatment and lead persons to unknowingly transmit STIs to others,” according to a new recommendation statement from the USPSTF.2 STIs may lead to serious health consequences for patients, cause harms to a mother and infant during pregnancy, and lead to cases of cancer among other concerning outcomes. As such, following the HHS new national strategic plan is critical for us to address the needs of our communities.
Preventing new STIs
Family physicians can be vital in achieving the first goal of the plan by helping to prevent new STIs. In August 2020, the USPSTF updated its guideline on behavioral counseling interventions to prevent STIs. In my opinion, the USPSTF offers some practical improvements from the earlier version of this guideline.
The task force provides a grade B recommendation that all sexually active adolescents and adults at increased risk for STIs be provided with behavioral counseling to prevent STIs. The guideline indicates that behavioral counseling interventions reduce the likelihood of those at increased risk for acquiring STIs.2
The 2014 guideline had recommended intensive interventions with a minimum of 30 minutes of counseling. Many family physicians may have found this previous recommendation impractical to implement. These updated recommendations now include a variety of interventions, such as those that take less than 30 minutes.
Although interventions with more than 120 minutes of contact time had the most effect, those with less than 30 minutes still demonstrated statistically significant fewer acquisitions of STIs during follow-up. These options include in-person counseling, and providing written materials, websites, videos, and telephone and text support to patients. These interventions can be delivered directly by the family physician, or patients may be referred to other settings or the media interventions.
The task force’s updated recommendation statement refers to a variety of resources that can be used to identify these interventions. Many of the studies reviewed for this guideline were conducted in STI clinics, and the guideline authors recommended further studies in primary care as opportunities for more generalizability.
In addition to behavioral counseling for STI prevention, family physicians can help prevent STIs in their patients through HPV vaccination and HIV pre-exposure prophylaxis (PrEP provision) within their practices. As the first contact for health care for many patients, we have an opportunity to significantly impact this first goal of prevention.
Treating STIs
Within the second goal of the national strategic plan is treatment of STIs, which family physicians should include in their practices as well as the diagnosis of STIs.
In December 2020, an update to the CDC’s treatment guideline for gonococcal infection was released. Prior to the publishing of this updated recommendation, the CDC recommended combination therapy of 250 mg intramuscular (IM) dose of ceftriaxone and either doxycycline or azithromycin. This recommendation has been changed to a single 500-mg IM dose of ceftriaxone for uncomplicated urogenital, anorectal, and pharyngeal gonorrhea. If chlamydia cannot be excluded, then the addition of oral doxycycline 100 mg twice daily for 7 days is recommended for nonpregnant persons, and 1 g oral azithromycin for pregnant persons. The previous treatment was recommended based on a concern for gonococcal resistance.
This updated guideline reflects increasing concerns for antimicrobial stewardship and emerging azithromycin resistance. It does not recommend a test-of-cure for urogenital or rectal gonorrhea, though did recommend a test-of-cure 7-14 days after treatment of pharyngeal gonorrhea. The guideline also recommends testing for reinfection 3-12 months after treatment as the rate of reinfection ranges from 7% to 12% among those previously treated.3
For some offices, the provision of the IM injection may be challenging, though having this medication in stock with the possibility of provision can greatly improve access and ease of treatment for patients. Family physicians can incorporate these updated recommendations along with those for other STIs such as chlamydia and syphilis with standing orders for treatment and testing within their offices.
Accelerating progress in STI research
Family physicians can also support the national strategic plan by participating in studies looking at the impact of behavioral counseling in the primary care office as opposed to in STI clinics. In addition, by following the STI treatment and screening guidelines, family physicians will contribute to the body of knowledge of prevalence, treatment failure, and reinfection rates of STIs. We can also help advance the research by providing feedback on interventions that have success within our practices.
Reducing STI-related health disparities and inequities
Family physicians are also in important places to support the strategic plan’s fourth goal of reducing health disparities and health inequities.
If we continue to ask the questions to identify those at high risk and ensure that we are offering appropriate STI prevention, care, and treatment services within our clinics, we can expand access to all who need services and improve equity. By offering these services within the primary care office, we may be able to decrease the stigma some may feel going to an STI clinic for services.
By incorporating additional screening and counseling in our practices we may identify some patients who were not aware that they were at risk for an STI and offer them preventive services.
Achieving integrated and coordinated efforts
Finally, as many family physicians have integrated practices, we are uniquely poised to support the fifth goal of the strategic plan of achieving integrated and coordinated efforts addressing the STI epidemic. In our practices we can participate in, lead, and refer to programs for substance use disorders, viral hepatitis, STIs, and HIV as part of full scope primary care.
Family physicians and other primary care providers should work to support the entire strategic plan to ensure that we are fully caring for our patients and communities and stopping the past decade’s increase in STIs. We have an opportunity to use this strategy and make a large impact in our communities.
Dr. Wheat is a family physician at Erie Family Health Center in Chicago. She is program director of Northwestern’s McGaw Family Medicine residency program at Humboldt Park, Chicago. Dr. Wheat serves on the editorial advisory board of Family Practice News. You can contact her at [email protected].
References
1. U.S. Department of Health and Human Services. 2020. Sexually Transmitted Infections National Strategic Plan for the United States: 2021-2025. Washington.
2. U.S. Preventive Services Task Force. Behavioral counseling interventions to prevent sexually transmitted infections: U.S. Preventive Services Task Force Recommendation Statement. JAMA. 2020;324(7):674-81. doi: 10.1001/jama.2020.13095.
3. St. Cyr S et al. Update to CDC’s Treatment Guideline for Gonococcal Infection, 2020. MMWR Morb Mortal Wkly Rep 2020;69:1911-6. doi: 10.15585/mmwr.mm6950a6external_icon.
Among these are the U.S. Department of Health and Human Services’ first “Sexually Transmitted Infections (STIs) National Strategic Plan for the United States,” which has a strong encompassing vision.
“The United States will be a place where sexually transmitted infections are prevented and where every person has high-quality STI prevention care, and treatment while living free from stigma and discrimination. The vision includes all people, regardless of age, sex, gender identity, sexual orientation, race, ethnicity, religion, disability, geographic location, or socioeconomic circumstance,” the new HHS plan states.1
Family physicians can and should play important roles in helping our country meet this plan’s goals particularly by following two important updated clinical guidelines, one from the U.S. Preventive Services Task Force (USPSTF) and another from the Centers for Disease Control and Prevention (CDC).
This strategic plan includes the following five overarching goals with associated objectives:
- Prevent New STIs.
- Improve the health of people by reducing adverse outcomes of STIs.
- Accelerate progress in STI research, technology, and innovation.
- Reduce STI-related health disparities and health inequities.
- Achieve integrated, coordinated efforts that address the STI epidemic.1
In my opinion, family physicians have important roles to play in order for each of these goals to be achieved.Unfortunately, there are approximately 20 million new cases of STIs each year, and the U.S. has seen increases in the rates of STIs in the past decade.
“Sexually transmitted infections are frequently asymptomatic, which may delay diagnosis and treatment and lead persons to unknowingly transmit STIs to others,” according to a new recommendation statement from the USPSTF.2 STIs may lead to serious health consequences for patients, cause harms to a mother and infant during pregnancy, and lead to cases of cancer among other concerning outcomes. As such, following the HHS new national strategic plan is critical for us to address the needs of our communities.
Preventing new STIs
Family physicians can be vital in achieving the first goal of the plan by helping to prevent new STIs. In August 2020, the USPSTF updated its guideline on behavioral counseling interventions to prevent STIs. In my opinion, the USPSTF offers some practical improvements from the earlier version of this guideline.
The task force provides a grade B recommendation that all sexually active adolescents and adults at increased risk for STIs be provided with behavioral counseling to prevent STIs. The guideline indicates that behavioral counseling interventions reduce the likelihood of those at increased risk for acquiring STIs.2
The 2014 guideline had recommended intensive interventions with a minimum of 30 minutes of counseling. Many family physicians may have found this previous recommendation impractical to implement. These updated recommendations now include a variety of interventions, such as those that take less than 30 minutes.
Although interventions with more than 120 minutes of contact time had the most effect, those with less than 30 minutes still demonstrated statistically significant fewer acquisitions of STIs during follow-up. These options include in-person counseling, and providing written materials, websites, videos, and telephone and text support to patients. These interventions can be delivered directly by the family physician, or patients may be referred to other settings or the media interventions.
The task force’s updated recommendation statement refers to a variety of resources that can be used to identify these interventions. Many of the studies reviewed for this guideline were conducted in STI clinics, and the guideline authors recommended further studies in primary care as opportunities for more generalizability.
In addition to behavioral counseling for STI prevention, family physicians can help prevent STIs in their patients through HPV vaccination and HIV pre-exposure prophylaxis (PrEP provision) within their practices. As the first contact for health care for many patients, we have an opportunity to significantly impact this first goal of prevention.
Treating STIs
Within the second goal of the national strategic plan is treatment of STIs, which family physicians should include in their practices as well as the diagnosis of STIs.
In December 2020, an update to the CDC’s treatment guideline for gonococcal infection was released. Prior to the publishing of this updated recommendation, the CDC recommended combination therapy of 250 mg intramuscular (IM) dose of ceftriaxone and either doxycycline or azithromycin. This recommendation has been changed to a single 500-mg IM dose of ceftriaxone for uncomplicated urogenital, anorectal, and pharyngeal gonorrhea. If chlamydia cannot be excluded, then the addition of oral doxycycline 100 mg twice daily for 7 days is recommended for nonpregnant persons, and 1 g oral azithromycin for pregnant persons. The previous treatment was recommended based on a concern for gonococcal resistance.
This updated guideline reflects increasing concerns for antimicrobial stewardship and emerging azithromycin resistance. It does not recommend a test-of-cure for urogenital or rectal gonorrhea, though did recommend a test-of-cure 7-14 days after treatment of pharyngeal gonorrhea. The guideline also recommends testing for reinfection 3-12 months after treatment as the rate of reinfection ranges from 7% to 12% among those previously treated.3
For some offices, the provision of the IM injection may be challenging, though having this medication in stock with the possibility of provision can greatly improve access and ease of treatment for patients. Family physicians can incorporate these updated recommendations along with those for other STIs such as chlamydia and syphilis with standing orders for treatment and testing within their offices.
Accelerating progress in STI research
Family physicians can also support the national strategic plan by participating in studies looking at the impact of behavioral counseling in the primary care office as opposed to in STI clinics. In addition, by following the STI treatment and screening guidelines, family physicians will contribute to the body of knowledge of prevalence, treatment failure, and reinfection rates of STIs. We can also help advance the research by providing feedback on interventions that have success within our practices.
Reducing STI-related health disparities and inequities
Family physicians are also in important places to support the strategic plan’s fourth goal of reducing health disparities and health inequities.
If we continue to ask the questions to identify those at high risk and ensure that we are offering appropriate STI prevention, care, and treatment services within our clinics, we can expand access to all who need services and improve equity. By offering these services within the primary care office, we may be able to decrease the stigma some may feel going to an STI clinic for services.
By incorporating additional screening and counseling in our practices we may identify some patients who were not aware that they were at risk for an STI and offer them preventive services.
Achieving integrated and coordinated efforts
Finally, as many family physicians have integrated practices, we are uniquely poised to support the fifth goal of the strategic plan of achieving integrated and coordinated efforts addressing the STI epidemic. In our practices we can participate in, lead, and refer to programs for substance use disorders, viral hepatitis, STIs, and HIV as part of full scope primary care.
Family physicians and other primary care providers should work to support the entire strategic plan to ensure that we are fully caring for our patients and communities and stopping the past decade’s increase in STIs. We have an opportunity to use this strategy and make a large impact in our communities.
Dr. Wheat is a family physician at Erie Family Health Center in Chicago. She is program director of Northwestern’s McGaw Family Medicine residency program at Humboldt Park, Chicago. Dr. Wheat serves on the editorial advisory board of Family Practice News. You can contact her at [email protected].
References
1. U.S. Department of Health and Human Services. 2020. Sexually Transmitted Infections National Strategic Plan for the United States: 2021-2025. Washington.
2. U.S. Preventive Services Task Force. Behavioral counseling interventions to prevent sexually transmitted infections: U.S. Preventive Services Task Force Recommendation Statement. JAMA. 2020;324(7):674-81. doi: 10.1001/jama.2020.13095.
3. St. Cyr S et al. Update to CDC’s Treatment Guideline for Gonococcal Infection, 2020. MMWR Morb Mortal Wkly Rep 2020;69:1911-6. doi: 10.15585/mmwr.mm6950a6external_icon.
Among these are the U.S. Department of Health and Human Services’ first “Sexually Transmitted Infections (STIs) National Strategic Plan for the United States,” which has a strong encompassing vision.
“The United States will be a place where sexually transmitted infections are prevented and where every person has high-quality STI prevention care, and treatment while living free from stigma and discrimination. The vision includes all people, regardless of age, sex, gender identity, sexual orientation, race, ethnicity, religion, disability, geographic location, or socioeconomic circumstance,” the new HHS plan states.1
Family physicians can and should play important roles in helping our country meet this plan’s goals particularly by following two important updated clinical guidelines, one from the U.S. Preventive Services Task Force (USPSTF) and another from the Centers for Disease Control and Prevention (CDC).
This strategic plan includes the following five overarching goals with associated objectives:
- Prevent New STIs.
- Improve the health of people by reducing adverse outcomes of STIs.
- Accelerate progress in STI research, technology, and innovation.
- Reduce STI-related health disparities and health inequities.
- Achieve integrated, coordinated efforts that address the STI epidemic.1
In my opinion, family physicians have important roles to play in order for each of these goals to be achieved.Unfortunately, there are approximately 20 million new cases of STIs each year, and the U.S. has seen increases in the rates of STIs in the past decade.
“Sexually transmitted infections are frequently asymptomatic, which may delay diagnosis and treatment and lead persons to unknowingly transmit STIs to others,” according to a new recommendation statement from the USPSTF.2 STIs may lead to serious health consequences for patients, cause harms to a mother and infant during pregnancy, and lead to cases of cancer among other concerning outcomes. As such, following the HHS new national strategic plan is critical for us to address the needs of our communities.
Preventing new STIs
Family physicians can be vital in achieving the first goal of the plan by helping to prevent new STIs. In August 2020, the USPSTF updated its guideline on behavioral counseling interventions to prevent STIs. In my opinion, the USPSTF offers some practical improvements from the earlier version of this guideline.
The task force provides a grade B recommendation that all sexually active adolescents and adults at increased risk for STIs be provided with behavioral counseling to prevent STIs. The guideline indicates that behavioral counseling interventions reduce the likelihood of those at increased risk for acquiring STIs.2
The 2014 guideline had recommended intensive interventions with a minimum of 30 minutes of counseling. Many family physicians may have found this previous recommendation impractical to implement. These updated recommendations now include a variety of interventions, such as those that take less than 30 minutes.
Although interventions with more than 120 minutes of contact time had the most effect, those with less than 30 minutes still demonstrated statistically significant fewer acquisitions of STIs during follow-up. These options include in-person counseling, and providing written materials, websites, videos, and telephone and text support to patients. These interventions can be delivered directly by the family physician, or patients may be referred to other settings or the media interventions.
The task force’s updated recommendation statement refers to a variety of resources that can be used to identify these interventions. Many of the studies reviewed for this guideline were conducted in STI clinics, and the guideline authors recommended further studies in primary care as opportunities for more generalizability.
In addition to behavioral counseling for STI prevention, family physicians can help prevent STIs in their patients through HPV vaccination and HIV pre-exposure prophylaxis (PrEP provision) within their practices. As the first contact for health care for many patients, we have an opportunity to significantly impact this first goal of prevention.
Treating STIs
Within the second goal of the national strategic plan is treatment of STIs, which family physicians should include in their practices as well as the diagnosis of STIs.
In December 2020, an update to the CDC’s treatment guideline for gonococcal infection was released. Prior to the publishing of this updated recommendation, the CDC recommended combination therapy of 250 mg intramuscular (IM) dose of ceftriaxone and either doxycycline or azithromycin. This recommendation has been changed to a single 500-mg IM dose of ceftriaxone for uncomplicated urogenital, anorectal, and pharyngeal gonorrhea. If chlamydia cannot be excluded, then the addition of oral doxycycline 100 mg twice daily for 7 days is recommended for nonpregnant persons, and 1 g oral azithromycin for pregnant persons. The previous treatment was recommended based on a concern for gonococcal resistance.
This updated guideline reflects increasing concerns for antimicrobial stewardship and emerging azithromycin resistance. It does not recommend a test-of-cure for urogenital or rectal gonorrhea, though did recommend a test-of-cure 7-14 days after treatment of pharyngeal gonorrhea. The guideline also recommends testing for reinfection 3-12 months after treatment as the rate of reinfection ranges from 7% to 12% among those previously treated.3
For some offices, the provision of the IM injection may be challenging, though having this medication in stock with the possibility of provision can greatly improve access and ease of treatment for patients. Family physicians can incorporate these updated recommendations along with those for other STIs such as chlamydia and syphilis with standing orders for treatment and testing within their offices.
Accelerating progress in STI research
Family physicians can also support the national strategic plan by participating in studies looking at the impact of behavioral counseling in the primary care office as opposed to in STI clinics. In addition, by following the STI treatment and screening guidelines, family physicians will contribute to the body of knowledge of prevalence, treatment failure, and reinfection rates of STIs. We can also help advance the research by providing feedback on interventions that have success within our practices.
Reducing STI-related health disparities and inequities
Family physicians are also in important places to support the strategic plan’s fourth goal of reducing health disparities and health inequities.
If we continue to ask the questions to identify those at high risk and ensure that we are offering appropriate STI prevention, care, and treatment services within our clinics, we can expand access to all who need services and improve equity. By offering these services within the primary care office, we may be able to decrease the stigma some may feel going to an STI clinic for services.
By incorporating additional screening and counseling in our practices we may identify some patients who were not aware that they were at risk for an STI and offer them preventive services.
Achieving integrated and coordinated efforts
Finally, as many family physicians have integrated practices, we are uniquely poised to support the fifth goal of the strategic plan of achieving integrated and coordinated efforts addressing the STI epidemic. In our practices we can participate in, lead, and refer to programs for substance use disorders, viral hepatitis, STIs, and HIV as part of full scope primary care.
Family physicians and other primary care providers should work to support the entire strategic plan to ensure that we are fully caring for our patients and communities and stopping the past decade’s increase in STIs. We have an opportunity to use this strategy and make a large impact in our communities.
Dr. Wheat is a family physician at Erie Family Health Center in Chicago. She is program director of Northwestern’s McGaw Family Medicine residency program at Humboldt Park, Chicago. Dr. Wheat serves on the editorial advisory board of Family Practice News. You can contact her at [email protected].
References
1. U.S. Department of Health and Human Services. 2020. Sexually Transmitted Infections National Strategic Plan for the United States: 2021-2025. Washington.
2. U.S. Preventive Services Task Force. Behavioral counseling interventions to prevent sexually transmitted infections: U.S. Preventive Services Task Force Recommendation Statement. JAMA. 2020;324(7):674-81. doi: 10.1001/jama.2020.13095.
3. St. Cyr S et al. Update to CDC’s Treatment Guideline for Gonococcal Infection, 2020. MMWR Morb Mortal Wkly Rep 2020;69:1911-6. doi: 10.15585/mmwr.mm6950a6external_icon.





