User login
ID Practitioner is an independent news source that provides infectious disease specialists with timely and relevant news and commentary about clinical developments and the impact of health care policy on the infectious disease specialist’s practice. Specialty focus topics include antimicrobial resistance, emerging infections, global ID, hepatitis, HIV, hospital-acquired infections, immunizations and vaccines, influenza, mycoses, pediatric infections, and STIs. Infectious Diseases News is owned by Frontline Medical Communications.
sofosbuvir
ritonavir with dasabuvir
discount
support path
program
ritonavir
greedy
ledipasvir
assistance
viekira pak
vpak
advocacy
needy
protest
abbvie
paritaprevir
ombitasvir
direct-acting antivirals
dasabuvir
gilead
fake-ovir
support
v pak
oasis
harvoni
section[contains(@class, 'footer-nav-section-wrapper')]
div[contains(@class, 'pane-pub-article-idp')]
div[contains(@class, 'pane-medstat-latest-articles-articles-section')]
div[contains(@class, 'pane-pub-home-idp')]
div[contains(@class, 'pane-pub-topic-idp')]
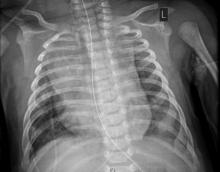
Infant’s COVID-19–related myocardial injury reversed
Reports of signs of heart failure in adults with COVID-19 have been rare – just four such cases have been published since the outbreak started in China – and now a team of pediatric cardiologists in New York have reported a case of acute but reversible myocardial injury in an infant with COVID-19.
and right upper lobe atelectasis.
The 2-month-old infant went home after more than 2 weeks in the hospital with no apparent lingering cardiac effects of the illness and not needing any oral heart failure medications, Madhu Sharma, MD, of the Children’s Hospital and Montefiore in New York and colleagues reported in JACC Case Reports. With close follow-up, the child’s left ventricle size and systolic function have remained normal and mitral regurgitation resolved. The case report didn’t mention the infant’s gender.
But before the straightforward postdischarge course emerged, the infant was in a precarious state, and Dr. Sharma and her team were challenged to diagnose the underlying causes.
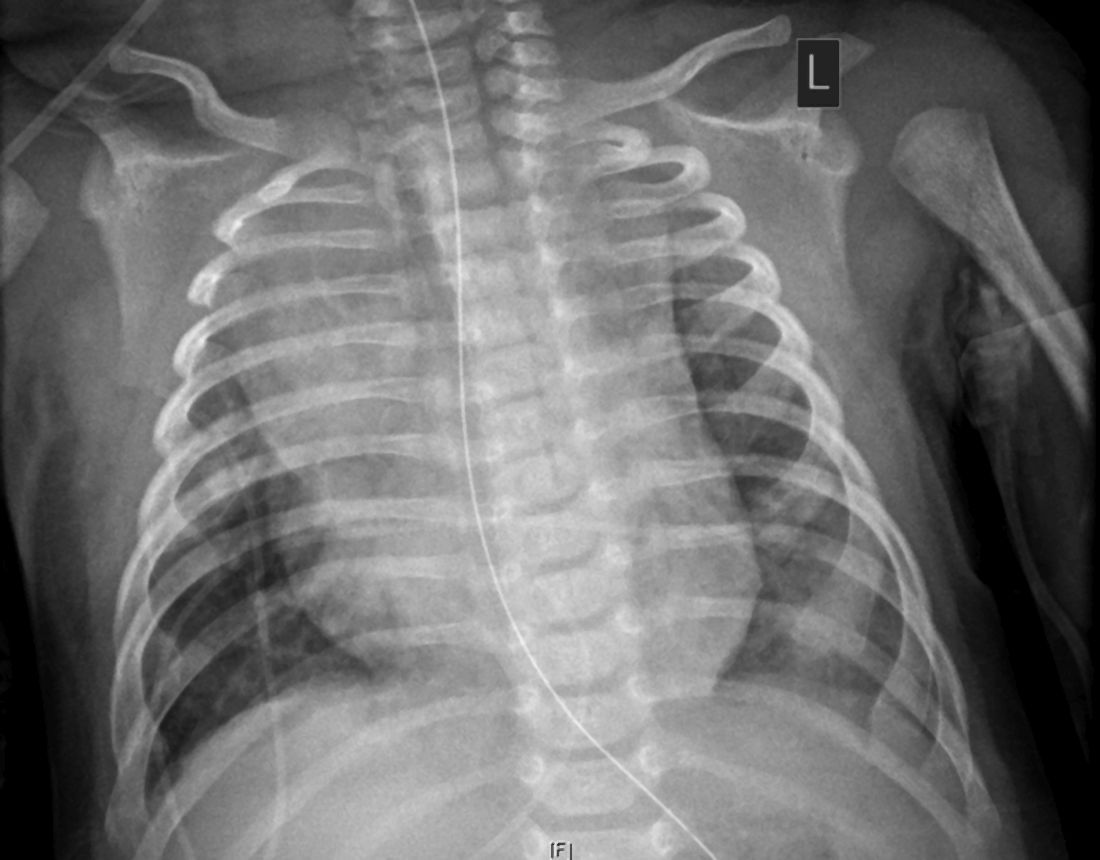
The child, who was born about 7 weeks premature, first came to the hospital having turned blue after choking on food. Nonrebreather mask ventilation was initiated in the ED, and an examination detected a holosystolic murmur. A test for COVID-19 was negative, but a later test was positive, and a chest x-ray exhibited cardiomegaly and signs of fluid and inflammation in the lungs.
An electrocardiogram detected sinus tachycardia, ST-segment depression and other anomalies in cardiac function. Further investigation with a transthoracic ECG showed severely depressed left ventricle systolic function with an ejection fraction of 30%, severe mitral regurgitation, and normal right ventricular systolic function.
Treatment included remdesivir and intravenous antibiotics. Through the hospital course, the patient was extubated to noninvasive ventilation, reintubated, put on intravenous steroid (methylprednisolone) and low-molecular-weight heparin, extubated, and tested throughout for cardiac function.
By day 14, left ventricle size and function normalized, and while the mitral regurgitation remained severe, it improved later without HF therapies. Left ventricle ejection fraction had recovered to 60%, and key cardiac biomarkers had normalized. On day 16, milrinone was discontinued, and the care team determined the patient no longer needed oral heart failure therapies.
“Most children with COVID-19 are either asymptomatic or have mild symptoms, but our case shows the potential for reversible myocardial injury in infants with COVID-19,” said Dr. Sharma. “Testing for COVID-19 in children presenting with signs and symptoms of heart failure is very important as we learn more about the impact of this virus.”
Dr. Sharma and coauthors have no relevant financial relationships to disclose.
SOURCE: Sharma M et al. JACC Case Rep. 2020. doi: 10.1016/j.jaccas.2020.09.031.
Reports of signs of heart failure in adults with COVID-19 have been rare – just four such cases have been published since the outbreak started in China – and now a team of pediatric cardiologists in New York have reported a case of acute but reversible myocardial injury in an infant with COVID-19.
and right upper lobe atelectasis.
The 2-month-old infant went home after more than 2 weeks in the hospital with no apparent lingering cardiac effects of the illness and not needing any oral heart failure medications, Madhu Sharma, MD, of the Children’s Hospital and Montefiore in New York and colleagues reported in JACC Case Reports. With close follow-up, the child’s left ventricle size and systolic function have remained normal and mitral regurgitation resolved. The case report didn’t mention the infant’s gender.
But before the straightforward postdischarge course emerged, the infant was in a precarious state, and Dr. Sharma and her team were challenged to diagnose the underlying causes.
The child, who was born about 7 weeks premature, first came to the hospital having turned blue after choking on food. Nonrebreather mask ventilation was initiated in the ED, and an examination detected a holosystolic murmur. A test for COVID-19 was negative, but a later test was positive, and a chest x-ray exhibited cardiomegaly and signs of fluid and inflammation in the lungs.
An electrocardiogram detected sinus tachycardia, ST-segment depression and other anomalies in cardiac function. Further investigation with a transthoracic ECG showed severely depressed left ventricle systolic function with an ejection fraction of 30%, severe mitral regurgitation, and normal right ventricular systolic function.
Treatment included remdesivir and intravenous antibiotics. Through the hospital course, the patient was extubated to noninvasive ventilation, reintubated, put on intravenous steroid (methylprednisolone) and low-molecular-weight heparin, extubated, and tested throughout for cardiac function.
By day 14, left ventricle size and function normalized, and while the mitral regurgitation remained severe, it improved later without HF therapies. Left ventricle ejection fraction had recovered to 60%, and key cardiac biomarkers had normalized. On day 16, milrinone was discontinued, and the care team determined the patient no longer needed oral heart failure therapies.
“Most children with COVID-19 are either asymptomatic or have mild symptoms, but our case shows the potential for reversible myocardial injury in infants with COVID-19,” said Dr. Sharma. “Testing for COVID-19 in children presenting with signs and symptoms of heart failure is very important as we learn more about the impact of this virus.”
Dr. Sharma and coauthors have no relevant financial relationships to disclose.
SOURCE: Sharma M et al. JACC Case Rep. 2020. doi: 10.1016/j.jaccas.2020.09.031.
Reports of signs of heart failure in adults with COVID-19 have been rare – just four such cases have been published since the outbreak started in China – and now a team of pediatric cardiologists in New York have reported a case of acute but reversible myocardial injury in an infant with COVID-19.
and right upper lobe atelectasis.
The 2-month-old infant went home after more than 2 weeks in the hospital with no apparent lingering cardiac effects of the illness and not needing any oral heart failure medications, Madhu Sharma, MD, of the Children’s Hospital and Montefiore in New York and colleagues reported in JACC Case Reports. With close follow-up, the child’s left ventricle size and systolic function have remained normal and mitral regurgitation resolved. The case report didn’t mention the infant’s gender.
But before the straightforward postdischarge course emerged, the infant was in a precarious state, and Dr. Sharma and her team were challenged to diagnose the underlying causes.
The child, who was born about 7 weeks premature, first came to the hospital having turned blue after choking on food. Nonrebreather mask ventilation was initiated in the ED, and an examination detected a holosystolic murmur. A test for COVID-19 was negative, but a later test was positive, and a chest x-ray exhibited cardiomegaly and signs of fluid and inflammation in the lungs.
An electrocardiogram detected sinus tachycardia, ST-segment depression and other anomalies in cardiac function. Further investigation with a transthoracic ECG showed severely depressed left ventricle systolic function with an ejection fraction of 30%, severe mitral regurgitation, and normal right ventricular systolic function.
Treatment included remdesivir and intravenous antibiotics. Through the hospital course, the patient was extubated to noninvasive ventilation, reintubated, put on intravenous steroid (methylprednisolone) and low-molecular-weight heparin, extubated, and tested throughout for cardiac function.
By day 14, left ventricle size and function normalized, and while the mitral regurgitation remained severe, it improved later without HF therapies. Left ventricle ejection fraction had recovered to 60%, and key cardiac biomarkers had normalized. On day 16, milrinone was discontinued, and the care team determined the patient no longer needed oral heart failure therapies.
“Most children with COVID-19 are either asymptomatic or have mild symptoms, but our case shows the potential for reversible myocardial injury in infants with COVID-19,” said Dr. Sharma. “Testing for COVID-19 in children presenting with signs and symptoms of heart failure is very important as we learn more about the impact of this virus.”
Dr. Sharma and coauthors have no relevant financial relationships to disclose.
SOURCE: Sharma M et al. JACC Case Rep. 2020. doi: 10.1016/j.jaccas.2020.09.031.
FROM JACC CASE REPORTS
Key clinical point: Children presenting with COVID-19 should be tested for heart failure.
Major finding: A 2-month-old infant with COVID-19 had acute but reversible myocardial injury.
Study details: Single case report.
Disclosures: Dr. Sharma, MD, has no relevant financial relationships to disclose.
Source: Sharma M et al. JACC Case Rep. 2020. doi: 10.1016/j.jaccas.2020.09.031.
Obesity, hypoxia predict severity in children with COVID-19
based on data from 281 patients at 8 locations.
Manifestations of COVID-19 in children include respiratory disease similar to that seen in adults, but the full spectrum of disease in children has been studied mainly in single settings or with a focus on one clinical manifestation, wrote Danielle M. Fernandes, MD, of Albert Einstein College of Medicine, New York, and colleagues.
In a study published in the Journal of Pediatrics, the researchers identified 281 children hospitalized with COVID-19 and/or multisystem inflammatory syndrome in children (MIS-C) at 8 sites in Connecticut, New Jersey, and New York. A total of 143 (51%) had respiratory disease, 69 (25%) had MIS-C, and 69 (25%) had other manifestations of illness including 32 patients with gastrointestinal problems, 21 infants with fever, 6 cases of neurologic disease, 6 cases of diabetic ketoacidosis, and 4 patients with other indications. The median age of the patients was 10 years, 60% were male, 51% were Hispanic, and 23% were non-Hispanic Black. The most common comorbidities were obesity (34%) and asthma (14%).
Independent predictors of disease severity in children found
After controlling for multiple variables, obesity and hypoxia at hospital admission were significant independent predictors of severe respiratory disease, with odds ratios of 3.39 and 4.01, respectively. In addition, lower absolute lymphocyte count (OR, 8.33 per unit decrease in 109 cells/L) and higher C-reactive protein (OR, 1.06 per unit increase in mg/dL) were significantly predictive of severe MIS-C (P = .001 and P = .017, respectively).
“The association between weight and severe respiratory COVID-19 is consistent with the adult literature; however, the mechanisms of this association require further study,” Dr. Fernandes and associates noted.
Overall, children with MIS-C were significantly more likely to be non-Hispanic Black, compared with children with respiratory disease, an 18% difference. However, neither race/ethnicity nor socioeconomic status were significant predictors of disease severity, the researchers wrote.
During the study period, 7 patients (2%) died and 114 (41%) were admitted to the ICU.
“We found a wide array of clinical manifestations in children and youth hospitalized with SARS-CoV-2,” Dr. Fernandes and associates wrote. Notably, gastrointestinal symptoms, ocular symptoms, and dermatologic symptoms have rarely been noted in adults with COVID-19, but occurred in more than 30% of the pediatric patients.
“We also found that SARS-CoV-2 can be an incidental finding in a substantial number of hospitalized pediatric patients,” the researchers said.
The findings were limited by several factors including a population of patients only from Connecticut, New Jersey, and New York, and the possibility that decisions on hospital and ICU admission may have varied by location, the researchers said. In addition, approaches may have varied in the absence of data on the optimal treatment of MIS-C.
“This study builds on the growing body of evidence showing that mortality in hospitalized pediatric patients is low, compared with adults,” Dr. Fernandes and associates said. “However, it highlights that the young population is not universally spared from morbidity, and that even previously healthy children and youth can develop severe disease requiring supportive therapy.”
Findings confirm other clinical experience
The study was important to show that, “although most children are spared severe illness from COVID-19, some children are hospitalized both with acute COVID-19 respiratory disease, with MIS-C and with a range of other complications,” Adrienne Randolph, MD, of Boston Children’s Hospital and Harvard Medical School, Boston, said in an interview.
Dr. Randolph said she was not surprised by the study findings, “as we are also seeing these types of complications at Boston Children’s Hospital where I work.”
Additional research is needed on the outcomes of these patients, “especially the longer-term sequelae of having COVID-19 or MIS-C early in life,” she emphasized.
The take-home message to clinicians from the findings at this time is to be aware that children and adolescents can become severely ill from COVID-19–related complications, said Dr. Randolph. “Some of the laboratory values on presentation appear to be associated with disease severity.”
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Randolph disclosed funding from the Centers for Disease Control and Prevention to lead the Overcoming COVID-19 Study in U.S. Children and Adults.
SOURCE: Fernandes DM et al. J Pediatr. 2020 Nov 13. doi: 10.1016/j.jpeds.2020.11.016.
based on data from 281 patients at 8 locations.
Manifestations of COVID-19 in children include respiratory disease similar to that seen in adults, but the full spectrum of disease in children has been studied mainly in single settings or with a focus on one clinical manifestation, wrote Danielle M. Fernandes, MD, of Albert Einstein College of Medicine, New York, and colleagues.
In a study published in the Journal of Pediatrics, the researchers identified 281 children hospitalized with COVID-19 and/or multisystem inflammatory syndrome in children (MIS-C) at 8 sites in Connecticut, New Jersey, and New York. A total of 143 (51%) had respiratory disease, 69 (25%) had MIS-C, and 69 (25%) had other manifestations of illness including 32 patients with gastrointestinal problems, 21 infants with fever, 6 cases of neurologic disease, 6 cases of diabetic ketoacidosis, and 4 patients with other indications. The median age of the patients was 10 years, 60% were male, 51% were Hispanic, and 23% were non-Hispanic Black. The most common comorbidities were obesity (34%) and asthma (14%).
Independent predictors of disease severity in children found
After controlling for multiple variables, obesity and hypoxia at hospital admission were significant independent predictors of severe respiratory disease, with odds ratios of 3.39 and 4.01, respectively. In addition, lower absolute lymphocyte count (OR, 8.33 per unit decrease in 109 cells/L) and higher C-reactive protein (OR, 1.06 per unit increase in mg/dL) were significantly predictive of severe MIS-C (P = .001 and P = .017, respectively).
“The association between weight and severe respiratory COVID-19 is consistent with the adult literature; however, the mechanisms of this association require further study,” Dr. Fernandes and associates noted.
Overall, children with MIS-C were significantly more likely to be non-Hispanic Black, compared with children with respiratory disease, an 18% difference. However, neither race/ethnicity nor socioeconomic status were significant predictors of disease severity, the researchers wrote.
During the study period, 7 patients (2%) died and 114 (41%) were admitted to the ICU.
“We found a wide array of clinical manifestations in children and youth hospitalized with SARS-CoV-2,” Dr. Fernandes and associates wrote. Notably, gastrointestinal symptoms, ocular symptoms, and dermatologic symptoms have rarely been noted in adults with COVID-19, but occurred in more than 30% of the pediatric patients.
“We also found that SARS-CoV-2 can be an incidental finding in a substantial number of hospitalized pediatric patients,” the researchers said.
The findings were limited by several factors including a population of patients only from Connecticut, New Jersey, and New York, and the possibility that decisions on hospital and ICU admission may have varied by location, the researchers said. In addition, approaches may have varied in the absence of data on the optimal treatment of MIS-C.
“This study builds on the growing body of evidence showing that mortality in hospitalized pediatric patients is low, compared with adults,” Dr. Fernandes and associates said. “However, it highlights that the young population is not universally spared from morbidity, and that even previously healthy children and youth can develop severe disease requiring supportive therapy.”
Findings confirm other clinical experience
The study was important to show that, “although most children are spared severe illness from COVID-19, some children are hospitalized both with acute COVID-19 respiratory disease, with MIS-C and with a range of other complications,” Adrienne Randolph, MD, of Boston Children’s Hospital and Harvard Medical School, Boston, said in an interview.
Dr. Randolph said she was not surprised by the study findings, “as we are also seeing these types of complications at Boston Children’s Hospital where I work.”
Additional research is needed on the outcomes of these patients, “especially the longer-term sequelae of having COVID-19 or MIS-C early in life,” she emphasized.
The take-home message to clinicians from the findings at this time is to be aware that children and adolescents can become severely ill from COVID-19–related complications, said Dr. Randolph. “Some of the laboratory values on presentation appear to be associated with disease severity.”
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Randolph disclosed funding from the Centers for Disease Control and Prevention to lead the Overcoming COVID-19 Study in U.S. Children and Adults.
SOURCE: Fernandes DM et al. J Pediatr. 2020 Nov 13. doi: 10.1016/j.jpeds.2020.11.016.
based on data from 281 patients at 8 locations.
Manifestations of COVID-19 in children include respiratory disease similar to that seen in adults, but the full spectrum of disease in children has been studied mainly in single settings or with a focus on one clinical manifestation, wrote Danielle M. Fernandes, MD, of Albert Einstein College of Medicine, New York, and colleagues.
In a study published in the Journal of Pediatrics, the researchers identified 281 children hospitalized with COVID-19 and/or multisystem inflammatory syndrome in children (MIS-C) at 8 sites in Connecticut, New Jersey, and New York. A total of 143 (51%) had respiratory disease, 69 (25%) had MIS-C, and 69 (25%) had other manifestations of illness including 32 patients with gastrointestinal problems, 21 infants with fever, 6 cases of neurologic disease, 6 cases of diabetic ketoacidosis, and 4 patients with other indications. The median age of the patients was 10 years, 60% were male, 51% were Hispanic, and 23% were non-Hispanic Black. The most common comorbidities were obesity (34%) and asthma (14%).
Independent predictors of disease severity in children found
After controlling for multiple variables, obesity and hypoxia at hospital admission were significant independent predictors of severe respiratory disease, with odds ratios of 3.39 and 4.01, respectively. In addition, lower absolute lymphocyte count (OR, 8.33 per unit decrease in 109 cells/L) and higher C-reactive protein (OR, 1.06 per unit increase in mg/dL) were significantly predictive of severe MIS-C (P = .001 and P = .017, respectively).
“The association between weight and severe respiratory COVID-19 is consistent with the adult literature; however, the mechanisms of this association require further study,” Dr. Fernandes and associates noted.
Overall, children with MIS-C were significantly more likely to be non-Hispanic Black, compared with children with respiratory disease, an 18% difference. However, neither race/ethnicity nor socioeconomic status were significant predictors of disease severity, the researchers wrote.
During the study period, 7 patients (2%) died and 114 (41%) were admitted to the ICU.
“We found a wide array of clinical manifestations in children and youth hospitalized with SARS-CoV-2,” Dr. Fernandes and associates wrote. Notably, gastrointestinal symptoms, ocular symptoms, and dermatologic symptoms have rarely been noted in adults with COVID-19, but occurred in more than 30% of the pediatric patients.
“We also found that SARS-CoV-2 can be an incidental finding in a substantial number of hospitalized pediatric patients,” the researchers said.
The findings were limited by several factors including a population of patients only from Connecticut, New Jersey, and New York, and the possibility that decisions on hospital and ICU admission may have varied by location, the researchers said. In addition, approaches may have varied in the absence of data on the optimal treatment of MIS-C.
“This study builds on the growing body of evidence showing that mortality in hospitalized pediatric patients is low, compared with adults,” Dr. Fernandes and associates said. “However, it highlights that the young population is not universally spared from morbidity, and that even previously healthy children and youth can develop severe disease requiring supportive therapy.”
Findings confirm other clinical experience
The study was important to show that, “although most children are spared severe illness from COVID-19, some children are hospitalized both with acute COVID-19 respiratory disease, with MIS-C and with a range of other complications,” Adrienne Randolph, MD, of Boston Children’s Hospital and Harvard Medical School, Boston, said in an interview.
Dr. Randolph said she was not surprised by the study findings, “as we are also seeing these types of complications at Boston Children’s Hospital where I work.”
Additional research is needed on the outcomes of these patients, “especially the longer-term sequelae of having COVID-19 or MIS-C early in life,” she emphasized.
The take-home message to clinicians from the findings at this time is to be aware that children and adolescents can become severely ill from COVID-19–related complications, said Dr. Randolph. “Some of the laboratory values on presentation appear to be associated with disease severity.”
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Randolph disclosed funding from the Centers for Disease Control and Prevention to lead the Overcoming COVID-19 Study in U.S. Children and Adults.
SOURCE: Fernandes DM et al. J Pediatr. 2020 Nov 13. doi: 10.1016/j.jpeds.2020.11.016.
FROM THE JOURNAL OF PEDIATRICS
Two consecutive negative FUBC results clear S. aureus bacteremia
reported Caitlin Cardenas-Comfort, MD, of the section of pediatric infectious diseases at Baylor College of Medicine, Houston, and colleagues.
In a retrospective cohort study of 122 pediatric patients with documented Staphylococcus aureus bacteremia (SAB) that were hospitalized at one of three hospitals in the Texas Children’s Hospital network in Houston, Dr. Cardenas-Comfort and colleagues sought to determine whether specific recommendations can be made on the number of follow-up blood cultures (FUBC) needed to document clearance of SAB. Patients included in the study were under 18 years of age and had confirmed diagnosis of SAB between Jan. 1, and Dec. 31, 2018.
Most cases of bacteremia resolve in under 48 hours
In the majority of cases, patients had bacteremia for less than 48 hours and few to no complications. Only 16% of patients experienced bacteremia lasting 3 or more days, and they had either central line-associated bloodstream infection, endocarditis, or osteomyelitis. In such cases, “patients with endovascular and closed-space infections are at an increased risk of persistent bacteremia,” warranting more conservative monitoring and follow-up, cautioned the researchers.
Although Dr. Cardenas-Comfort and colleagues did note an association between the duration of bacteremia and a diagnosis of infectious disease, increased risk for persistent SAB did not appear to be tied to an underlying medical condition, including immunosuppression.
Fewer than 5% of patients with SAB had intermittent positive cultures and fewer than 1% had repeat positive cultures following two negative FUBC results. For those patients with intermittent positive cultures, the risk of being diagnosed with endocarditis or osteomyelitis is more than double. The authors suggested that “source control could be a critical variable” increasing the risk for intermittent positive cultures, noting that surgical debridement occurred more than 24 hours following initial blood draw for every patient in the osteomyelitis group. In contrast, of those who had consistently negative FUBC results, only 2 of 33 (6%) had debridement in the same period, and only 6 of 33 (18%) required more than one debridement.
Children are less likely to have intermittent positive cultures
Dr. Cardenas-Comfort and colleagues also observed that intermittent positive cultures may appear less frequently in children than adults, consistent with a recent study of adults in which intermittent cultures were found in 13% of 1.071 SAB cases. In just 4% of the cases in that study, more than 2 days of negative blood cultures preceded a repeat positive culture.
The researchers noted several study limitations in their own research. Because more than half (61%) of patients had two or less FUBCs collected, and 21% one or less, they acknowledged that their conclusions are based on the presumption that the 61% of patients would not have any further positive cultures if they had been drawn. Relying on provider documentation also suggested that cases of bacteremia without an identified source also likely were overrepresented. The retrospective nature of the study only allowed for limited collection of standardized follow-up metrics with the limited patient sample available. Patient characteristics also may have affected the quality of study results because a large number of patients had underlying medical conditions or were premature infants.
Look for ongoing hemodynamic instability before third FUBC
Dr. Cardenas-Comfort and colleagues only recommend a third FUBC in cases where patients demonstrate ongoing hemodynamic instability. Applying this to their study population, in retrospect, the authors noted that unnecessary FUBCs could have been prevented in 26% of patients included in the study. They further recommend a thorough clinical evaluation for any patients with SAB lasting 3 or more days with an unidentified infection source. Further research could be beneficial in evaluating cost savings that come from eliminating unnecessary cultures. Additionally, performing a powered analysis would help to determine the probability of an increase in complications based on implementation of these recommendations.
In a separate interview, Tina Q. Tan, MD, infectious disease specialist at Ann & Robert H. Lurie Children’s Hospital of Chicago noted: “This study provides some importance evidence-based guidance on deciding how many blood cultures are needed to demonstrate clearance of S. aureus bacteremia, even in children who have intermittent positive cultures after having negative FUBCs. The recommendation that additional blood cultures to document sterility are not needed after 2 FUBC results are negative in well-appearing children is one that has the potential to decrease cost and unnecessary discomfort in patients. The recommendation currently is for well-appearing children; children who are ill appearing may require further blood cultures to document sterility. Even though this is a single-center study with a relatively small number of patients (n = 122), the information provided is a very useful guide to all clinicians who deal with this issue. Further studies are needed to determine the impact on cost reduction by the elimination of unnecessary blood cultures and whether the rate of complications would increase as a result of not obtaining further cultures in well-appearing children who have two negative follow up blood cultures.”
Dr. Cardenas-Comfort and colleagues as well as Dr. Tan had no conflicts of interest and no relevant financial disclosures. There was no external funding for the study.
SOURCE: Cardenas-Comfort C et al. Pediatrics. 2020. doi: 10.1542/peds.2020-1821.
reported Caitlin Cardenas-Comfort, MD, of the section of pediatric infectious diseases at Baylor College of Medicine, Houston, and colleagues.

In a retrospective cohort study of 122 pediatric patients with documented Staphylococcus aureus bacteremia (SAB) that were hospitalized at one of three hospitals in the Texas Children’s Hospital network in Houston, Dr. Cardenas-Comfort and colleagues sought to determine whether specific recommendations can be made on the number of follow-up blood cultures (FUBC) needed to document clearance of SAB. Patients included in the study were under 18 years of age and had confirmed diagnosis of SAB between Jan. 1, and Dec. 31, 2018.
Most cases of bacteremia resolve in under 48 hours
In the majority of cases, patients had bacteremia for less than 48 hours and few to no complications. Only 16% of patients experienced bacteremia lasting 3 or more days, and they had either central line-associated bloodstream infection, endocarditis, or osteomyelitis. In such cases, “patients with endovascular and closed-space infections are at an increased risk of persistent bacteremia,” warranting more conservative monitoring and follow-up, cautioned the researchers.
Although Dr. Cardenas-Comfort and colleagues did note an association between the duration of bacteremia and a diagnosis of infectious disease, increased risk for persistent SAB did not appear to be tied to an underlying medical condition, including immunosuppression.
Fewer than 5% of patients with SAB had intermittent positive cultures and fewer than 1% had repeat positive cultures following two negative FUBC results. For those patients with intermittent positive cultures, the risk of being diagnosed with endocarditis or osteomyelitis is more than double. The authors suggested that “source control could be a critical variable” increasing the risk for intermittent positive cultures, noting that surgical debridement occurred more than 24 hours following initial blood draw for every patient in the osteomyelitis group. In contrast, of those who had consistently negative FUBC results, only 2 of 33 (6%) had debridement in the same period, and only 6 of 33 (18%) required more than one debridement.
Children are less likely to have intermittent positive cultures
Dr. Cardenas-Comfort and colleagues also observed that intermittent positive cultures may appear less frequently in children than adults, consistent with a recent study of adults in which intermittent cultures were found in 13% of 1.071 SAB cases. In just 4% of the cases in that study, more than 2 days of negative blood cultures preceded a repeat positive culture.
The researchers noted several study limitations in their own research. Because more than half (61%) of patients had two or less FUBCs collected, and 21% one or less, they acknowledged that their conclusions are based on the presumption that the 61% of patients would not have any further positive cultures if they had been drawn. Relying on provider documentation also suggested that cases of bacteremia without an identified source also likely were overrepresented. The retrospective nature of the study only allowed for limited collection of standardized follow-up metrics with the limited patient sample available. Patient characteristics also may have affected the quality of study results because a large number of patients had underlying medical conditions or were premature infants.
Look for ongoing hemodynamic instability before third FUBC
Dr. Cardenas-Comfort and colleagues only recommend a third FUBC in cases where patients demonstrate ongoing hemodynamic instability. Applying this to their study population, in retrospect, the authors noted that unnecessary FUBCs could have been prevented in 26% of patients included in the study. They further recommend a thorough clinical evaluation for any patients with SAB lasting 3 or more days with an unidentified infection source. Further research could be beneficial in evaluating cost savings that come from eliminating unnecessary cultures. Additionally, performing a powered analysis would help to determine the probability of an increase in complications based on implementation of these recommendations.
In a separate interview, Tina Q. Tan, MD, infectious disease specialist at Ann & Robert H. Lurie Children’s Hospital of Chicago noted: “This study provides some importance evidence-based guidance on deciding how many blood cultures are needed to demonstrate clearance of S. aureus bacteremia, even in children who have intermittent positive cultures after having negative FUBCs. The recommendation that additional blood cultures to document sterility are not needed after 2 FUBC results are negative in well-appearing children is one that has the potential to decrease cost and unnecessary discomfort in patients. The recommendation currently is for well-appearing children; children who are ill appearing may require further blood cultures to document sterility. Even though this is a single-center study with a relatively small number of patients (n = 122), the information provided is a very useful guide to all clinicians who deal with this issue. Further studies are needed to determine the impact on cost reduction by the elimination of unnecessary blood cultures and whether the rate of complications would increase as a result of not obtaining further cultures in well-appearing children who have two negative follow up blood cultures.”
Dr. Cardenas-Comfort and colleagues as well as Dr. Tan had no conflicts of interest and no relevant financial disclosures. There was no external funding for the study.
SOURCE: Cardenas-Comfort C et al. Pediatrics. 2020. doi: 10.1542/peds.2020-1821.
reported Caitlin Cardenas-Comfort, MD, of the section of pediatric infectious diseases at Baylor College of Medicine, Houston, and colleagues.

In a retrospective cohort study of 122 pediatric patients with documented Staphylococcus aureus bacteremia (SAB) that were hospitalized at one of three hospitals in the Texas Children’s Hospital network in Houston, Dr. Cardenas-Comfort and colleagues sought to determine whether specific recommendations can be made on the number of follow-up blood cultures (FUBC) needed to document clearance of SAB. Patients included in the study were under 18 years of age and had confirmed diagnosis of SAB between Jan. 1, and Dec. 31, 2018.
Most cases of bacteremia resolve in under 48 hours
In the majority of cases, patients had bacteremia for less than 48 hours and few to no complications. Only 16% of patients experienced bacteremia lasting 3 or more days, and they had either central line-associated bloodstream infection, endocarditis, or osteomyelitis. In such cases, “patients with endovascular and closed-space infections are at an increased risk of persistent bacteremia,” warranting more conservative monitoring and follow-up, cautioned the researchers.
Although Dr. Cardenas-Comfort and colleagues did note an association between the duration of bacteremia and a diagnosis of infectious disease, increased risk for persistent SAB did not appear to be tied to an underlying medical condition, including immunosuppression.
Fewer than 5% of patients with SAB had intermittent positive cultures and fewer than 1% had repeat positive cultures following two negative FUBC results. For those patients with intermittent positive cultures, the risk of being diagnosed with endocarditis or osteomyelitis is more than double. The authors suggested that “source control could be a critical variable” increasing the risk for intermittent positive cultures, noting that surgical debridement occurred more than 24 hours following initial blood draw for every patient in the osteomyelitis group. In contrast, of those who had consistently negative FUBC results, only 2 of 33 (6%) had debridement in the same period, and only 6 of 33 (18%) required more than one debridement.
Children are less likely to have intermittent positive cultures
Dr. Cardenas-Comfort and colleagues also observed that intermittent positive cultures may appear less frequently in children than adults, consistent with a recent study of adults in which intermittent cultures were found in 13% of 1.071 SAB cases. In just 4% of the cases in that study, more than 2 days of negative blood cultures preceded a repeat positive culture.
The researchers noted several study limitations in their own research. Because more than half (61%) of patients had two or less FUBCs collected, and 21% one or less, they acknowledged that their conclusions are based on the presumption that the 61% of patients would not have any further positive cultures if they had been drawn. Relying on provider documentation also suggested that cases of bacteremia without an identified source also likely were overrepresented. The retrospective nature of the study only allowed for limited collection of standardized follow-up metrics with the limited patient sample available. Patient characteristics also may have affected the quality of study results because a large number of patients had underlying medical conditions or were premature infants.
Look for ongoing hemodynamic instability before third FUBC
Dr. Cardenas-Comfort and colleagues only recommend a third FUBC in cases where patients demonstrate ongoing hemodynamic instability. Applying this to their study population, in retrospect, the authors noted that unnecessary FUBCs could have been prevented in 26% of patients included in the study. They further recommend a thorough clinical evaluation for any patients with SAB lasting 3 or more days with an unidentified infection source. Further research could be beneficial in evaluating cost savings that come from eliminating unnecessary cultures. Additionally, performing a powered analysis would help to determine the probability of an increase in complications based on implementation of these recommendations.
In a separate interview, Tina Q. Tan, MD, infectious disease specialist at Ann & Robert H. Lurie Children’s Hospital of Chicago noted: “This study provides some importance evidence-based guidance on deciding how many blood cultures are needed to demonstrate clearance of S. aureus bacteremia, even in children who have intermittent positive cultures after having negative FUBCs. The recommendation that additional blood cultures to document sterility are not needed after 2 FUBC results are negative in well-appearing children is one that has the potential to decrease cost and unnecessary discomfort in patients. The recommendation currently is for well-appearing children; children who are ill appearing may require further blood cultures to document sterility. Even though this is a single-center study with a relatively small number of patients (n = 122), the information provided is a very useful guide to all clinicians who deal with this issue. Further studies are needed to determine the impact on cost reduction by the elimination of unnecessary blood cultures and whether the rate of complications would increase as a result of not obtaining further cultures in well-appearing children who have two negative follow up blood cultures.”
Dr. Cardenas-Comfort and colleagues as well as Dr. Tan had no conflicts of interest and no relevant financial disclosures. There was no external funding for the study.
SOURCE: Cardenas-Comfort C et al. Pediatrics. 2020. doi: 10.1542/peds.2020-1821.
FROM PEDIATRICS
Diabetic retinopathy may predict greater risk of COVID-19 severity
Risk of intubation for COVID-19 in very sick hospitalized patients was increased over fivefold in those with diabetic retinopathy, compared with those without, in a small single-center study from the United Kingdom.
Importantly, the risk of intubation was independent of conventional risk factors for poor COVID-19 outcomes.
“People with preexisting diabetes-related vascular damage, such as retinopathy, might be predisposed to a more severe form of COVID-19 requiring ventilation in the intensive therapy unit,” said lead investigator Janaka Karalliedde, MBBS, PhD.
Dr. Karalliedde and colleagues note that this is “the first description of diabetic retinopathy as a potential risk factor for poor COVID-19 outcomes.”
“For this reason, looking for the presence or history of retinopathy or other vascular complications of diabetes may help health care professionals identify patients at high risk of severe COVID-19,” added Dr. Karalliedde, of Guy’s and St Thomas’ NHS Foundation Trust, London.
The study was published online in Diabetes Research and Clinical Practice.
Preexisting diabetic retinopathy and COVID-19 outcomes
The prevalence of diabetic retinopathy is thought to be around 55% in people with type 1 diabetes and 30% in people with type 2 diabetes, on average.
Dr. Karalliedde is part of a research group at King’s College London that has been focused on how vascular disease may predispose to more severe COVID-19.
“COVID-19 affects the blood vessels all over the body,” he said, so they wondered whether having preexisting retinopathy “would predispose to a severe manifestation of COVID-19.”
The observational study included 187 patients with diabetes (179 patients with type 2 diabetes and 8 patients with type 1 diabetes) hospitalized with COVID-19 at Guy’s and St Thomas’ NHS Foundation Trust between March 12 and April 7 (the peak of the first wave of the pandemic in the United Kingdom).
“It was an ethnically diverse population who were very sick and provides a clinical observation of real life,” Dr. Karalliedde said.
Nearly half of patients were African Caribbean (44%), 39% were White, and 17% were of other ethnicities, including 8% who were Asian. The mean age of the cohort was 68 years (range, 22-97 years), and 60% were men.
Diabetic retinopathy was reported in 67 (36%) patients, of whom 80% had background retinopathy and 20% had more advanced retinopathy.
They then looked at whether the presence of retinopathy was associated with a more severe manifestation of COVID-19 as defined by the need for tracheal intubation.
Of the 187 patients, 26% were intubated and 45% of these patients had diabetic retinopathy.
The analysis showed those with diabetic retinopathy had an over-fivefold increased risk for intubation (odds ratio, 5.81; 95% confidence interval, 1.37-24.66).
Of the entire cohort, 32% of patients died, although no association was observed between retinopathy and mortality.
“A greater number of diabetes patients with COVID-19 ended up on the intensive therapy unit. Upon multivariate analysis, we found retinopathy was independently associated with ending up on the intensive therapy unit,” stressed Dr. Karalliedde.
However, they noted that, “due to the cross-sectional design of our study, we cannot prove causality [between retinopathy and intubation]. Further studies are required to understand the mechanisms that explain the associations between retinopathy and other indices of microangiopathy with severe COVID-19.”
A version of this article originally appeared on Medscape.com.
Risk of intubation for COVID-19 in very sick hospitalized patients was increased over fivefold in those with diabetic retinopathy, compared with those without, in a small single-center study from the United Kingdom.
Importantly, the risk of intubation was independent of conventional risk factors for poor COVID-19 outcomes.
“People with preexisting diabetes-related vascular damage, such as retinopathy, might be predisposed to a more severe form of COVID-19 requiring ventilation in the intensive therapy unit,” said lead investigator Janaka Karalliedde, MBBS, PhD.
Dr. Karalliedde and colleagues note that this is “the first description of diabetic retinopathy as a potential risk factor for poor COVID-19 outcomes.”
“For this reason, looking for the presence or history of retinopathy or other vascular complications of diabetes may help health care professionals identify patients at high risk of severe COVID-19,” added Dr. Karalliedde, of Guy’s and St Thomas’ NHS Foundation Trust, London.
The study was published online in Diabetes Research and Clinical Practice.
Preexisting diabetic retinopathy and COVID-19 outcomes
The prevalence of diabetic retinopathy is thought to be around 55% in people with type 1 diabetes and 30% in people with type 2 diabetes, on average.
Dr. Karalliedde is part of a research group at King’s College London that has been focused on how vascular disease may predispose to more severe COVID-19.
“COVID-19 affects the blood vessels all over the body,” he said, so they wondered whether having preexisting retinopathy “would predispose to a severe manifestation of COVID-19.”
The observational study included 187 patients with diabetes (179 patients with type 2 diabetes and 8 patients with type 1 diabetes) hospitalized with COVID-19 at Guy’s and St Thomas’ NHS Foundation Trust between March 12 and April 7 (the peak of the first wave of the pandemic in the United Kingdom).
“It was an ethnically diverse population who were very sick and provides a clinical observation of real life,” Dr. Karalliedde said.
Nearly half of patients were African Caribbean (44%), 39% were White, and 17% were of other ethnicities, including 8% who were Asian. The mean age of the cohort was 68 years (range, 22-97 years), and 60% were men.
Diabetic retinopathy was reported in 67 (36%) patients, of whom 80% had background retinopathy and 20% had more advanced retinopathy.
They then looked at whether the presence of retinopathy was associated with a more severe manifestation of COVID-19 as defined by the need for tracheal intubation.
Of the 187 patients, 26% were intubated and 45% of these patients had diabetic retinopathy.
The analysis showed those with diabetic retinopathy had an over-fivefold increased risk for intubation (odds ratio, 5.81; 95% confidence interval, 1.37-24.66).
Of the entire cohort, 32% of patients died, although no association was observed between retinopathy and mortality.
“A greater number of diabetes patients with COVID-19 ended up on the intensive therapy unit. Upon multivariate analysis, we found retinopathy was independently associated with ending up on the intensive therapy unit,” stressed Dr. Karalliedde.
However, they noted that, “due to the cross-sectional design of our study, we cannot prove causality [between retinopathy and intubation]. Further studies are required to understand the mechanisms that explain the associations between retinopathy and other indices of microangiopathy with severe COVID-19.”
A version of this article originally appeared on Medscape.com.
Risk of intubation for COVID-19 in very sick hospitalized patients was increased over fivefold in those with diabetic retinopathy, compared with those without, in a small single-center study from the United Kingdom.
Importantly, the risk of intubation was independent of conventional risk factors for poor COVID-19 outcomes.
“People with preexisting diabetes-related vascular damage, such as retinopathy, might be predisposed to a more severe form of COVID-19 requiring ventilation in the intensive therapy unit,” said lead investigator Janaka Karalliedde, MBBS, PhD.
Dr. Karalliedde and colleagues note that this is “the first description of diabetic retinopathy as a potential risk factor for poor COVID-19 outcomes.”
“For this reason, looking for the presence or history of retinopathy or other vascular complications of diabetes may help health care professionals identify patients at high risk of severe COVID-19,” added Dr. Karalliedde, of Guy’s and St Thomas’ NHS Foundation Trust, London.
The study was published online in Diabetes Research and Clinical Practice.
Preexisting diabetic retinopathy and COVID-19 outcomes
The prevalence of diabetic retinopathy is thought to be around 55% in people with type 1 diabetes and 30% in people with type 2 diabetes, on average.
Dr. Karalliedde is part of a research group at King’s College London that has been focused on how vascular disease may predispose to more severe COVID-19.
“COVID-19 affects the blood vessels all over the body,” he said, so they wondered whether having preexisting retinopathy “would predispose to a severe manifestation of COVID-19.”
The observational study included 187 patients with diabetes (179 patients with type 2 diabetes and 8 patients with type 1 diabetes) hospitalized with COVID-19 at Guy’s and St Thomas’ NHS Foundation Trust between March 12 and April 7 (the peak of the first wave of the pandemic in the United Kingdom).
“It was an ethnically diverse population who were very sick and provides a clinical observation of real life,” Dr. Karalliedde said.
Nearly half of patients were African Caribbean (44%), 39% were White, and 17% were of other ethnicities, including 8% who were Asian. The mean age of the cohort was 68 years (range, 22-97 years), and 60% were men.
Diabetic retinopathy was reported in 67 (36%) patients, of whom 80% had background retinopathy and 20% had more advanced retinopathy.
They then looked at whether the presence of retinopathy was associated with a more severe manifestation of COVID-19 as defined by the need for tracheal intubation.
Of the 187 patients, 26% were intubated and 45% of these patients had diabetic retinopathy.
The analysis showed those with diabetic retinopathy had an over-fivefold increased risk for intubation (odds ratio, 5.81; 95% confidence interval, 1.37-24.66).
Of the entire cohort, 32% of patients died, although no association was observed between retinopathy and mortality.
“A greater number of diabetes patients with COVID-19 ended up on the intensive therapy unit. Upon multivariate analysis, we found retinopathy was independently associated with ending up on the intensive therapy unit,” stressed Dr. Karalliedde.
However, they noted that, “due to the cross-sectional design of our study, we cannot prove causality [between retinopathy and intubation]. Further studies are required to understand the mechanisms that explain the associations between retinopathy and other indices of microangiopathy with severe COVID-19.”
A version of this article originally appeared on Medscape.com.
COVID-19 vaccine distribution could start in 2 weeks, Pence says
Initial doses of a coronavirus vaccine could be sent out as early as mid-December, Vice President Mike Pence told governors during a call on Monday.
The distribution process could start during the week of Dec. 14, according to audio of a White House Coronavirus Task Force call obtained by CBS News. The call focused on the timeline of vaccine approval and distribution.
“With this morning’s news that Moderna is joining Pfizer in submitting an emergency-use authorization [to the Food and Drug Administration], we continue to be on pace,” Pence said.
The FDA is scheduled to make a decision about Pfizer’s emergency use authorization after an advisory panel meets on Dec. 10 to review the company’s application. FDA Commissioner Stephen Hahn, MD, didn’t commit to the Dec. 14 date, CBS News reported.
“We do all the number crunching ourselves,” Dr. Hahn said. “We look line by line by line on all the data, on all the patients and manufacturing. We do statistical analyses and we come to our own conclusions to support a decision of either thumbs-up or thumbs-down.”
According to a meeting agenda, Pfizer vaccine deliveries should start on Dec. 15, followed by the Moderna vaccine on Dec. 22, CBS News reported.
Between Dec. 13-19, Pfizer is slated to deliver 6.4 million doses, which is enough to immunize about 3 million people with two shots. An “undetermined number” are reserved for backup doses, the news outlet reported.
During the next week, Pfizer and Moderna are scheduled to produce enough doses to vaccinate an additional 10 million people. By the end of the month, about 30 million people should receive doses.
As vaccines begin to roll out, Mr. Pence said “we have a ways to go” in reassuring the public about immunization. He urged governors to use their “bully pulpit” to educate their states and “develop public confidence” in the vaccines.
During the call, Anthony Fauci, MD, director of the National Institute for Allergy and Infectious Diseases, supported the safety and efficacy of the vaccines. Although the vaccine development and approval process was accelerated this year, he said, it “does not at all compromise safety, nor does it compromise scientific integrity.”
“Any misrepresentation that the vaccines had government interference or company interference is patently untrue,” he said.
This article first appeared on Medscape.com.
Initial doses of a coronavirus vaccine could be sent out as early as mid-December, Vice President Mike Pence told governors during a call on Monday.
The distribution process could start during the week of Dec. 14, according to audio of a White House Coronavirus Task Force call obtained by CBS News. The call focused on the timeline of vaccine approval and distribution.
“With this morning’s news that Moderna is joining Pfizer in submitting an emergency-use authorization [to the Food and Drug Administration], we continue to be on pace,” Pence said.
The FDA is scheduled to make a decision about Pfizer’s emergency use authorization after an advisory panel meets on Dec. 10 to review the company’s application. FDA Commissioner Stephen Hahn, MD, didn’t commit to the Dec. 14 date, CBS News reported.
“We do all the number crunching ourselves,” Dr. Hahn said. “We look line by line by line on all the data, on all the patients and manufacturing. We do statistical analyses and we come to our own conclusions to support a decision of either thumbs-up or thumbs-down.”
According to a meeting agenda, Pfizer vaccine deliveries should start on Dec. 15, followed by the Moderna vaccine on Dec. 22, CBS News reported.
Between Dec. 13-19, Pfizer is slated to deliver 6.4 million doses, which is enough to immunize about 3 million people with two shots. An “undetermined number” are reserved for backup doses, the news outlet reported.
During the next week, Pfizer and Moderna are scheduled to produce enough doses to vaccinate an additional 10 million people. By the end of the month, about 30 million people should receive doses.
As vaccines begin to roll out, Mr. Pence said “we have a ways to go” in reassuring the public about immunization. He urged governors to use their “bully pulpit” to educate their states and “develop public confidence” in the vaccines.
During the call, Anthony Fauci, MD, director of the National Institute for Allergy and Infectious Diseases, supported the safety and efficacy of the vaccines. Although the vaccine development and approval process was accelerated this year, he said, it “does not at all compromise safety, nor does it compromise scientific integrity.”
“Any misrepresentation that the vaccines had government interference or company interference is patently untrue,” he said.
This article first appeared on Medscape.com.
Initial doses of a coronavirus vaccine could be sent out as early as mid-December, Vice President Mike Pence told governors during a call on Monday.
The distribution process could start during the week of Dec. 14, according to audio of a White House Coronavirus Task Force call obtained by CBS News. The call focused on the timeline of vaccine approval and distribution.
“With this morning’s news that Moderna is joining Pfizer in submitting an emergency-use authorization [to the Food and Drug Administration], we continue to be on pace,” Pence said.
The FDA is scheduled to make a decision about Pfizer’s emergency use authorization after an advisory panel meets on Dec. 10 to review the company’s application. FDA Commissioner Stephen Hahn, MD, didn’t commit to the Dec. 14 date, CBS News reported.
“We do all the number crunching ourselves,” Dr. Hahn said. “We look line by line by line on all the data, on all the patients and manufacturing. We do statistical analyses and we come to our own conclusions to support a decision of either thumbs-up or thumbs-down.”
According to a meeting agenda, Pfizer vaccine deliveries should start on Dec. 15, followed by the Moderna vaccine on Dec. 22, CBS News reported.
Between Dec. 13-19, Pfizer is slated to deliver 6.4 million doses, which is enough to immunize about 3 million people with two shots. An “undetermined number” are reserved for backup doses, the news outlet reported.
During the next week, Pfizer and Moderna are scheduled to produce enough doses to vaccinate an additional 10 million people. By the end of the month, about 30 million people should receive doses.
As vaccines begin to roll out, Mr. Pence said “we have a ways to go” in reassuring the public about immunization. He urged governors to use their “bully pulpit” to educate their states and “develop public confidence” in the vaccines.
During the call, Anthony Fauci, MD, director of the National Institute for Allergy and Infectious Diseases, supported the safety and efficacy of the vaccines. Although the vaccine development and approval process was accelerated this year, he said, it “does not at all compromise safety, nor does it compromise scientific integrity.”
“Any misrepresentation that the vaccines had government interference or company interference is patently untrue,” he said.
This article first appeared on Medscape.com.
Medicare finalizes 2021 physician pay rule with E/M changes
Medicare officials stuck with their plan to increase payments for office visits for primary care and several other specialties that focus on helping patients manage complex conditions such as diabetes. In doing so, Medicare also finalized cuts for other fields, triggering a new wave of protests.
The final version of the 2021 Medicare physician fee schedule was unveiled on the night of Dec. 1. The Centers for Medicare & Medicaid Services posted an unofficial copy of the rule, which will later be published in the Federal Register.
CMS said it completed work on this massive annual review of payments for clinicians later than it usually does because of the demands of the federal response to the COVID-19 pandemic. The 2021 physician fee rule will take effect within a 30-day period instead of the usual 60-day time frame.
The most contentious item proposed for 2021 was a reshuffling of payments among specialties as part of an overhaul of Medicare’s approach to valuing evaluation and management (E/M) services. There was broader support for other aspects of the E/M overhaul, which are intended to cut some of the administrative hassle clinicians face.
“This finalized policy marks the most significant updates to E/M codes in 30 years, reducing burden on doctors imposed by the coding system and rewarding time spent evaluating and managing their patients’ care,” CMS Administrator Seema Verma said in a statement. “In the past, the system has rewarded interventions and procedures over time spent with patients – time taken preventing disease and managing chronic illnesses.”
In the final rule, CMS summarized these results of the E/M changes in Table 106. CMS largely stuck with the approach outlined in a draft rule released in August, with minor changes in the amounts of cuts and increases.
Specialties in line for increases under the 2021 final physician fee schedule include allergy/immunology (9%), endocrinology (16%), family practice (13%), general practice (7%), geriatrics (3%), hematology/oncology (14%), internal medicine (4%), nephrology (6%), physician assistants (8%), psychiatry (7%), rheumatology (15%), and urology (8%).
In line for cuts would be anesthesiology (–8%), cardiac surgery (–8%), emergency medicine (–6%), general surgery (–6%), infectious disease (–4%), neurosurgery (–6%), physical/occupational therapy (–9%), plastic surgery (–7%), radiology (–10%), and thoracic surgery (–8%).
CMS had initially set these changes in 2021 pay in motion in the 2020 physician fee schedule. The agency subsequently faced significant opposition to its plans. Many physician groups sought to waive a “budget-neutral” approach to the E/M overhaul, which makes the offsetting of cuts necessary. They argued this would allow increased compensation for clinicians whose practices focus on office visits without requiring offsetting cuts from other fields of medicine.
The American Medical Association is among those urging Congress to prevent or postpone the payment reductions resulting from Medicare’s budget neutrality requirement as applied to the E/M overhaul.
In a Tuesday statement, AMA President Susan R. Bailey, MD, noted that many physicians are facing “substantial economic hardships due to COVID-19.”
By AMA’s calculations, CMS’ planned 2021 E/M overhaul could result in “a shocking reduction of 10.2% to Medicare payment rates,” according to Bailey’s statement. The AMA strongly supports other aspects of the E/M changes CMS finalized, which Bailey said will result in “simpler and more flexible” coding and documentation.
The Surgical Care Coalition, which represents about a dozen medical specialty associations, is asking members of Congress to block the full implementation of the E/M overhaul.
In a Dec. 1 statement, the coalition urged the passage of a bill (HR 8702) that has been introduced in the House by a bipartisan duo of physicians, Rep. Ami Bera, MD (D-Calif.), and Rep. Larry Bucshon, MD (R-Ind.). Their bill would effectively block the cuts from going into effect on January 1, 2021. It would provide an additional Medicare payment for certain services in 2021 and 2022 if the otherwise applicable payment is less than it would have been in 2020.
The Medicare E/M overhaul “was a dangerous policy even before the pandemic, and enacting it during the worst health care crisis in a century is unconscionable. If Congress fails to act, it will further strain a health care system that’s already been pushed to the brink due to the COVID-19 pandemic and undermine patient care,” said John A. Wilson, MD, president of the American Association of Neurological Surgeons, in a statement.
Also backing the Bera-Bucshon bill is the American College of Emergency Physicians. In a statement on Tuesday, ACEP President Mark Rosenberg, DO, MBA, urged Congress to act on this measure.
“Emergency physicians and other health care providers battling on the front lines of the ongoing pandemic are already under unprecedented financial strain as they continue to bear the brunt of COVID-19,” Dr. Rosenberg said. “These cuts would have a devastating impact for the future of emergency medicine and could seriously impede patients’ access to emergency care when they need it most.”
“Long overdue”
But there also are champions for the approach CMS took in the E/M overhaul. The influential Medicare Payment Advisory Commission (MedPAC) has argued strongly for keeping the budget-neutral approach to the E/M overhaul.
In an Oct. 2 comment to CMS about the draft 2021 physician fee schedule, MedPAC Chairman Michael E. Chernew, PhD, said this approach would “help rebalance the fee schedule from services that have become overvalued to services that have become undervalued.”
This budget-neutral approach also “will go further in reducing the large gap in compensation between primary care physicians (who had a median income of $243,000 in 2018) and specialists such as surgeons (whose median income was $426,000 in 2018),” Dr. Chernew wrote.
In a Tuesday tweet, Robert B. Doherty, senior vice president of governmental affairs and public policy for the American College of Physicians, said CMS had “finalized long overdue payment increases for primary and comprehensive care including an add-in for more complex visits.”
The American Academy of Family Physicians joined ACP in a November 30 letter to congressional leaders, urging them to allow Medicare “to increase investment in primary care, benefiting millions of Medicare patients and the program itself, and reject last minute efforts to prevent these essential and long-overdue changes from going fully into effect on January 1, 2021.”
In the letter, AAFP and ACP and their cosigners argued for a need to address “underinvestment” in primary care by finalizing the E/M overhaul.
“Given that six in ten American adults have a chronic disease and four in ten have two or more chronic conditions, why would we, as a country, accept such an inadequate investment in the very care model that stands to provide maximum value to these patients?” they wrote. “Since we know that individuals with a longitudinal relationship with a primary care physician have better health outcomes and use fewer health care resources, why would we continue to direct money to higher-cost, marginal value services?”
A version of this article originally appeared on Medscape.com.
Medicare officials stuck with their plan to increase payments for office visits for primary care and several other specialties that focus on helping patients manage complex conditions such as diabetes. In doing so, Medicare also finalized cuts for other fields, triggering a new wave of protests.
The final version of the 2021 Medicare physician fee schedule was unveiled on the night of Dec. 1. The Centers for Medicare & Medicaid Services posted an unofficial copy of the rule, which will later be published in the Federal Register.
CMS said it completed work on this massive annual review of payments for clinicians later than it usually does because of the demands of the federal response to the COVID-19 pandemic. The 2021 physician fee rule will take effect within a 30-day period instead of the usual 60-day time frame.
The most contentious item proposed for 2021 was a reshuffling of payments among specialties as part of an overhaul of Medicare’s approach to valuing evaluation and management (E/M) services. There was broader support for other aspects of the E/M overhaul, which are intended to cut some of the administrative hassle clinicians face.
“This finalized policy marks the most significant updates to E/M codes in 30 years, reducing burden on doctors imposed by the coding system and rewarding time spent evaluating and managing their patients’ care,” CMS Administrator Seema Verma said in a statement. “In the past, the system has rewarded interventions and procedures over time spent with patients – time taken preventing disease and managing chronic illnesses.”
In the final rule, CMS summarized these results of the E/M changes in Table 106. CMS largely stuck with the approach outlined in a draft rule released in August, with minor changes in the amounts of cuts and increases.
Specialties in line for increases under the 2021 final physician fee schedule include allergy/immunology (9%), endocrinology (16%), family practice (13%), general practice (7%), geriatrics (3%), hematology/oncology (14%), internal medicine (4%), nephrology (6%), physician assistants (8%), psychiatry (7%), rheumatology (15%), and urology (8%).
In line for cuts would be anesthesiology (–8%), cardiac surgery (–8%), emergency medicine (–6%), general surgery (–6%), infectious disease (–4%), neurosurgery (–6%), physical/occupational therapy (–9%), plastic surgery (–7%), radiology (–10%), and thoracic surgery (–8%).
CMS had initially set these changes in 2021 pay in motion in the 2020 physician fee schedule. The agency subsequently faced significant opposition to its plans. Many physician groups sought to waive a “budget-neutral” approach to the E/M overhaul, which makes the offsetting of cuts necessary. They argued this would allow increased compensation for clinicians whose practices focus on office visits without requiring offsetting cuts from other fields of medicine.
The American Medical Association is among those urging Congress to prevent or postpone the payment reductions resulting from Medicare’s budget neutrality requirement as applied to the E/M overhaul.
In a Tuesday statement, AMA President Susan R. Bailey, MD, noted that many physicians are facing “substantial economic hardships due to COVID-19.”
By AMA’s calculations, CMS’ planned 2021 E/M overhaul could result in “a shocking reduction of 10.2% to Medicare payment rates,” according to Bailey’s statement. The AMA strongly supports other aspects of the E/M changes CMS finalized, which Bailey said will result in “simpler and more flexible” coding and documentation.
The Surgical Care Coalition, which represents about a dozen medical specialty associations, is asking members of Congress to block the full implementation of the E/M overhaul.
In a Dec. 1 statement, the coalition urged the passage of a bill (HR 8702) that has been introduced in the House by a bipartisan duo of physicians, Rep. Ami Bera, MD (D-Calif.), and Rep. Larry Bucshon, MD (R-Ind.). Their bill would effectively block the cuts from going into effect on January 1, 2021. It would provide an additional Medicare payment for certain services in 2021 and 2022 if the otherwise applicable payment is less than it would have been in 2020.
The Medicare E/M overhaul “was a dangerous policy even before the pandemic, and enacting it during the worst health care crisis in a century is unconscionable. If Congress fails to act, it will further strain a health care system that’s already been pushed to the brink due to the COVID-19 pandemic and undermine patient care,” said John A. Wilson, MD, president of the American Association of Neurological Surgeons, in a statement.
Also backing the Bera-Bucshon bill is the American College of Emergency Physicians. In a statement on Tuesday, ACEP President Mark Rosenberg, DO, MBA, urged Congress to act on this measure.
“Emergency physicians and other health care providers battling on the front lines of the ongoing pandemic are already under unprecedented financial strain as they continue to bear the brunt of COVID-19,” Dr. Rosenberg said. “These cuts would have a devastating impact for the future of emergency medicine and could seriously impede patients’ access to emergency care when they need it most.”
“Long overdue”
But there also are champions for the approach CMS took in the E/M overhaul. The influential Medicare Payment Advisory Commission (MedPAC) has argued strongly for keeping the budget-neutral approach to the E/M overhaul.
In an Oct. 2 comment to CMS about the draft 2021 physician fee schedule, MedPAC Chairman Michael E. Chernew, PhD, said this approach would “help rebalance the fee schedule from services that have become overvalued to services that have become undervalued.”
This budget-neutral approach also “will go further in reducing the large gap in compensation between primary care physicians (who had a median income of $243,000 in 2018) and specialists such as surgeons (whose median income was $426,000 in 2018),” Dr. Chernew wrote.
In a Tuesday tweet, Robert B. Doherty, senior vice president of governmental affairs and public policy for the American College of Physicians, said CMS had “finalized long overdue payment increases for primary and comprehensive care including an add-in for more complex visits.”
The American Academy of Family Physicians joined ACP in a November 30 letter to congressional leaders, urging them to allow Medicare “to increase investment in primary care, benefiting millions of Medicare patients and the program itself, and reject last minute efforts to prevent these essential and long-overdue changes from going fully into effect on January 1, 2021.”
In the letter, AAFP and ACP and their cosigners argued for a need to address “underinvestment” in primary care by finalizing the E/M overhaul.
“Given that six in ten American adults have a chronic disease and four in ten have two or more chronic conditions, why would we, as a country, accept such an inadequate investment in the very care model that stands to provide maximum value to these patients?” they wrote. “Since we know that individuals with a longitudinal relationship with a primary care physician have better health outcomes and use fewer health care resources, why would we continue to direct money to higher-cost, marginal value services?”
A version of this article originally appeared on Medscape.com.
Medicare officials stuck with their plan to increase payments for office visits for primary care and several other specialties that focus on helping patients manage complex conditions such as diabetes. In doing so, Medicare also finalized cuts for other fields, triggering a new wave of protests.
The final version of the 2021 Medicare physician fee schedule was unveiled on the night of Dec. 1. The Centers for Medicare & Medicaid Services posted an unofficial copy of the rule, which will later be published in the Federal Register.
CMS said it completed work on this massive annual review of payments for clinicians later than it usually does because of the demands of the federal response to the COVID-19 pandemic. The 2021 physician fee rule will take effect within a 30-day period instead of the usual 60-day time frame.
The most contentious item proposed for 2021 was a reshuffling of payments among specialties as part of an overhaul of Medicare’s approach to valuing evaluation and management (E/M) services. There was broader support for other aspects of the E/M overhaul, which are intended to cut some of the administrative hassle clinicians face.
“This finalized policy marks the most significant updates to E/M codes in 30 years, reducing burden on doctors imposed by the coding system and rewarding time spent evaluating and managing their patients’ care,” CMS Administrator Seema Verma said in a statement. “In the past, the system has rewarded interventions and procedures over time spent with patients – time taken preventing disease and managing chronic illnesses.”
In the final rule, CMS summarized these results of the E/M changes in Table 106. CMS largely stuck with the approach outlined in a draft rule released in August, with minor changes in the amounts of cuts and increases.
Specialties in line for increases under the 2021 final physician fee schedule include allergy/immunology (9%), endocrinology (16%), family practice (13%), general practice (7%), geriatrics (3%), hematology/oncology (14%), internal medicine (4%), nephrology (6%), physician assistants (8%), psychiatry (7%), rheumatology (15%), and urology (8%).
In line for cuts would be anesthesiology (–8%), cardiac surgery (–8%), emergency medicine (–6%), general surgery (–6%), infectious disease (–4%), neurosurgery (–6%), physical/occupational therapy (–9%), plastic surgery (–7%), radiology (–10%), and thoracic surgery (–8%).
CMS had initially set these changes in 2021 pay in motion in the 2020 physician fee schedule. The agency subsequently faced significant opposition to its plans. Many physician groups sought to waive a “budget-neutral” approach to the E/M overhaul, which makes the offsetting of cuts necessary. They argued this would allow increased compensation for clinicians whose practices focus on office visits without requiring offsetting cuts from other fields of medicine.
The American Medical Association is among those urging Congress to prevent or postpone the payment reductions resulting from Medicare’s budget neutrality requirement as applied to the E/M overhaul.
In a Tuesday statement, AMA President Susan R. Bailey, MD, noted that many physicians are facing “substantial economic hardships due to COVID-19.”
By AMA’s calculations, CMS’ planned 2021 E/M overhaul could result in “a shocking reduction of 10.2% to Medicare payment rates,” according to Bailey’s statement. The AMA strongly supports other aspects of the E/M changes CMS finalized, which Bailey said will result in “simpler and more flexible” coding and documentation.
The Surgical Care Coalition, which represents about a dozen medical specialty associations, is asking members of Congress to block the full implementation of the E/M overhaul.
In a Dec. 1 statement, the coalition urged the passage of a bill (HR 8702) that has been introduced in the House by a bipartisan duo of physicians, Rep. Ami Bera, MD (D-Calif.), and Rep. Larry Bucshon, MD (R-Ind.). Their bill would effectively block the cuts from going into effect on January 1, 2021. It would provide an additional Medicare payment for certain services in 2021 and 2022 if the otherwise applicable payment is less than it would have been in 2020.
The Medicare E/M overhaul “was a dangerous policy even before the pandemic, and enacting it during the worst health care crisis in a century is unconscionable. If Congress fails to act, it will further strain a health care system that’s already been pushed to the brink due to the COVID-19 pandemic and undermine patient care,” said John A. Wilson, MD, president of the American Association of Neurological Surgeons, in a statement.
Also backing the Bera-Bucshon bill is the American College of Emergency Physicians. In a statement on Tuesday, ACEP President Mark Rosenberg, DO, MBA, urged Congress to act on this measure.
“Emergency physicians and other health care providers battling on the front lines of the ongoing pandemic are already under unprecedented financial strain as they continue to bear the brunt of COVID-19,” Dr. Rosenberg said. “These cuts would have a devastating impact for the future of emergency medicine and could seriously impede patients’ access to emergency care when they need it most.”
“Long overdue”
But there also are champions for the approach CMS took in the E/M overhaul. The influential Medicare Payment Advisory Commission (MedPAC) has argued strongly for keeping the budget-neutral approach to the E/M overhaul.
In an Oct. 2 comment to CMS about the draft 2021 physician fee schedule, MedPAC Chairman Michael E. Chernew, PhD, said this approach would “help rebalance the fee schedule from services that have become overvalued to services that have become undervalued.”
This budget-neutral approach also “will go further in reducing the large gap in compensation between primary care physicians (who had a median income of $243,000 in 2018) and specialists such as surgeons (whose median income was $426,000 in 2018),” Dr. Chernew wrote.
In a Tuesday tweet, Robert B. Doherty, senior vice president of governmental affairs and public policy for the American College of Physicians, said CMS had “finalized long overdue payment increases for primary and comprehensive care including an add-in for more complex visits.”
The American Academy of Family Physicians joined ACP in a November 30 letter to congressional leaders, urging them to allow Medicare “to increase investment in primary care, benefiting millions of Medicare patients and the program itself, and reject last minute efforts to prevent these essential and long-overdue changes from going fully into effect on January 1, 2021.”
In the letter, AAFP and ACP and their cosigners argued for a need to address “underinvestment” in primary care by finalizing the E/M overhaul.
“Given that six in ten American adults have a chronic disease and four in ten have two or more chronic conditions, why would we, as a country, accept such an inadequate investment in the very care model that stands to provide maximum value to these patients?” they wrote. “Since we know that individuals with a longitudinal relationship with a primary care physician have better health outcomes and use fewer health care resources, why would we continue to direct money to higher-cost, marginal value services?”
A version of this article originally appeared on Medscape.com.
Colchicine a case study for what’s wrong with U.S. drug pricing
Public spending on colchicine has grown exponentially over the past decade despite generics suggesting an uphill slog for patients seeking access to long-term therapy for gout or cardiac conditions.
Medicaid spending on single-ingredient colchicine jumped 2,833%, from $1.1 million in 2008 to $32.2 million in 2017, new findings show. Medicaid expansion likely played a role in the increase, but 58% was due to price hikes alone.
The centuries-old drug sold for pennies in the United States before increasing 50-fold to about $5 per pill in 2009 after the first FDA-approved colchicine product, Colcrys, was granted 3 years’ market exclusivity for the treatment of acute gout based on a 1-week trial.
If prices had remained at pre-Colcrys levels, Medicaid spending in 2017 would have totaled just $2.1 million rather than $32.2 million according to the analysis, published online Nov. 30 in JAMA Internal Medicine (doi: 10.1001/jamainternmed.2020.5017).
The study was motivated by difficulties gout patients have in accessing colchicine, but also last year’s COLCOT trial, which reported fewer ischemic cardiovascular events in patients receiving colchicine after MI, observed Natalie McCormick, PhD, of Massachusetts General Hospital and Harvard Medical School, both in Boston.
“They were suggesting it could be a cost-effective way for secondary prevention and it is fairly inexpensive in most countries, but not the U.S.,” she said in an interview. “So there’s really a potential to increase public spending if more and more patients are then taking colchicine for prevention of cardiovascular events and the prices don’t change.”
The current pandemic could potentially further increase demand. Results initially slated for September are expected this month from the COLCORONA trial, which is testing whether the anti-inflammatory agent can prevent hospitalizations, lung complications, and death when given early in the course of COVID-19.
University of Oxford (England) researchers also announced last week that colchicine is being added to the massive RECOVERY trial, which is studying treatments for hospitalized COVID-19 patients.
Notably, the Canadian-based COLCOT trial did not use Colcrys, but rather a colchicine product that costs just $0.26 a pill in Canada, roughly the price of most generics available worldwide.
Authorized generics typically drive down drug prices when competing with independent generics, but this competition is missing in the United States, where Colcrys holds patents until 2029, Dr. McCormick and colleagues noted. More than a half-dozen independent generics have FDA approval to date, but only authorized generics with price points set by the brand-name companies are available to treat acute gout, pericarditis, and potentially millions with MI.
“One of the key takeaways is this difference between the brand names and the authorized generics and the independents,” she said. “The authorized [generics] have really not saved money. The list prices were just slightly lower and patients can also have more difficulty in getting those covered.”
For this analysis, the investigators used Medicaid and Medicare data to examine prices for all available forms of colchicine from 2008 to 2017, including unregulated/unapproved colchicine (2008-2010), generic combination probenecid-colchicine (2008-2017), Colcrys (2009-2017), brand-name single-ingredient colchicine Mitigare (approved in late 2014 but not marketed until 2015), and their authorized generics (2015-2017). Medicare trends from 2012 to 2017 were analyzed separately because pre-Colcrys Medicare data were not available.
Based on the results, combined spending on Medicare and Medicaid claims for single-ingredient colchicine exceeded $340 million in 2017.
Inflation- and rebate-adjusted Medicaid unit prices rose from $0.24 a pill in 2008, when unapproved formulations were still available, to $4.20 a pill in 2011 (Colcrys only), and peaked at $4.66 a pill in 2015 (Colcrys plus authorized generics).
Prescribing of lower-priced probenecid-colchicine ($0.66/pill in 2017) remained stable throughout. Medicaid rebate-adjusted prices in 2017 were $3.99/pill for all single-ingredient colchicine products, $5.13/pill for Colcrys, $4.49/pill for Mitigare, and $3.88/pill for authorized generics.
Medicare rebate-adjusted 2017 per-pill prices were $5.81 for all single-ingredient colchicine products, $6.78 for Colcrys, $5.68 for Mitigare, $5.16 for authorized generics, and $0.70 for probenecid-colchicine.
“Authorized generics have still driven high spending,” Dr. McCormick said. “We really need to encourage more competition in order to improve access.”
In an accompanying commentary, B. Joseph Guglielmo, PharmD, University of California, San Francisco, pointed out that the estimated median research and development cost to bring a drug to market is between $985 million and $1,335 million, which inevitably translates into a high selling price for the drug. Such investment and its resultant cost, however, should be associated with potential worth to society.
“Only a fraction of an investment was required for Colcrys, a product that has provided no increased value and an unnecessary, long-term cost burden to the health care system,” he wrote. “The current study findings illustrate that we can never allow such an egregious case to take place again.”
Dr. McCormick reported grants from Canadian Institutes of Health Research during the conduct of the study. Dr. Guglielmo reported having no relevant conflicts of interest.
This article first appeared on Medscape.com.
Public spending on colchicine has grown exponentially over the past decade despite generics suggesting an uphill slog for patients seeking access to long-term therapy for gout or cardiac conditions.
Medicaid spending on single-ingredient colchicine jumped 2,833%, from $1.1 million in 2008 to $32.2 million in 2017, new findings show. Medicaid expansion likely played a role in the increase, but 58% was due to price hikes alone.
The centuries-old drug sold for pennies in the United States before increasing 50-fold to about $5 per pill in 2009 after the first FDA-approved colchicine product, Colcrys, was granted 3 years’ market exclusivity for the treatment of acute gout based on a 1-week trial.
If prices had remained at pre-Colcrys levels, Medicaid spending in 2017 would have totaled just $2.1 million rather than $32.2 million according to the analysis, published online Nov. 30 in JAMA Internal Medicine (doi: 10.1001/jamainternmed.2020.5017).
The study was motivated by difficulties gout patients have in accessing colchicine, but also last year’s COLCOT trial, which reported fewer ischemic cardiovascular events in patients receiving colchicine after MI, observed Natalie McCormick, PhD, of Massachusetts General Hospital and Harvard Medical School, both in Boston.
“They were suggesting it could be a cost-effective way for secondary prevention and it is fairly inexpensive in most countries, but not the U.S.,” she said in an interview. “So there’s really a potential to increase public spending if more and more patients are then taking colchicine for prevention of cardiovascular events and the prices don’t change.”
The current pandemic could potentially further increase demand. Results initially slated for September are expected this month from the COLCORONA trial, which is testing whether the anti-inflammatory agent can prevent hospitalizations, lung complications, and death when given early in the course of COVID-19.
University of Oxford (England) researchers also announced last week that colchicine is being added to the massive RECOVERY trial, which is studying treatments for hospitalized COVID-19 patients.
Notably, the Canadian-based COLCOT trial did not use Colcrys, but rather a colchicine product that costs just $0.26 a pill in Canada, roughly the price of most generics available worldwide.
Authorized generics typically drive down drug prices when competing with independent generics, but this competition is missing in the United States, where Colcrys holds patents until 2029, Dr. McCormick and colleagues noted. More than a half-dozen independent generics have FDA approval to date, but only authorized generics with price points set by the brand-name companies are available to treat acute gout, pericarditis, and potentially millions with MI.
“One of the key takeaways is this difference between the brand names and the authorized generics and the independents,” she said. “The authorized [generics] have really not saved money. The list prices were just slightly lower and patients can also have more difficulty in getting those covered.”
For this analysis, the investigators used Medicaid and Medicare data to examine prices for all available forms of colchicine from 2008 to 2017, including unregulated/unapproved colchicine (2008-2010), generic combination probenecid-colchicine (2008-2017), Colcrys (2009-2017), brand-name single-ingredient colchicine Mitigare (approved in late 2014 but not marketed until 2015), and their authorized generics (2015-2017). Medicare trends from 2012 to 2017 were analyzed separately because pre-Colcrys Medicare data were not available.
Based on the results, combined spending on Medicare and Medicaid claims for single-ingredient colchicine exceeded $340 million in 2017.
Inflation- and rebate-adjusted Medicaid unit prices rose from $0.24 a pill in 2008, when unapproved formulations were still available, to $4.20 a pill in 2011 (Colcrys only), and peaked at $4.66 a pill in 2015 (Colcrys plus authorized generics).
Prescribing of lower-priced probenecid-colchicine ($0.66/pill in 2017) remained stable throughout. Medicaid rebate-adjusted prices in 2017 were $3.99/pill for all single-ingredient colchicine products, $5.13/pill for Colcrys, $4.49/pill for Mitigare, and $3.88/pill for authorized generics.
Medicare rebate-adjusted 2017 per-pill prices were $5.81 for all single-ingredient colchicine products, $6.78 for Colcrys, $5.68 for Mitigare, $5.16 for authorized generics, and $0.70 for probenecid-colchicine.
“Authorized generics have still driven high spending,” Dr. McCormick said. “We really need to encourage more competition in order to improve access.”
In an accompanying commentary, B. Joseph Guglielmo, PharmD, University of California, San Francisco, pointed out that the estimated median research and development cost to bring a drug to market is between $985 million and $1,335 million, which inevitably translates into a high selling price for the drug. Such investment and its resultant cost, however, should be associated with potential worth to society.
“Only a fraction of an investment was required for Colcrys, a product that has provided no increased value and an unnecessary, long-term cost burden to the health care system,” he wrote. “The current study findings illustrate that we can never allow such an egregious case to take place again.”
Dr. McCormick reported grants from Canadian Institutes of Health Research during the conduct of the study. Dr. Guglielmo reported having no relevant conflicts of interest.
This article first appeared on Medscape.com.
Public spending on colchicine has grown exponentially over the past decade despite generics suggesting an uphill slog for patients seeking access to long-term therapy for gout or cardiac conditions.
Medicaid spending on single-ingredient colchicine jumped 2,833%, from $1.1 million in 2008 to $32.2 million in 2017, new findings show. Medicaid expansion likely played a role in the increase, but 58% was due to price hikes alone.
The centuries-old drug sold for pennies in the United States before increasing 50-fold to about $5 per pill in 2009 after the first FDA-approved colchicine product, Colcrys, was granted 3 years’ market exclusivity for the treatment of acute gout based on a 1-week trial.
If prices had remained at pre-Colcrys levels, Medicaid spending in 2017 would have totaled just $2.1 million rather than $32.2 million according to the analysis, published online Nov. 30 in JAMA Internal Medicine (doi: 10.1001/jamainternmed.2020.5017).
The study was motivated by difficulties gout patients have in accessing colchicine, but also last year’s COLCOT trial, which reported fewer ischemic cardiovascular events in patients receiving colchicine after MI, observed Natalie McCormick, PhD, of Massachusetts General Hospital and Harvard Medical School, both in Boston.
“They were suggesting it could be a cost-effective way for secondary prevention and it is fairly inexpensive in most countries, but not the U.S.,” she said in an interview. “So there’s really a potential to increase public spending if more and more patients are then taking colchicine for prevention of cardiovascular events and the prices don’t change.”
The current pandemic could potentially further increase demand. Results initially slated for September are expected this month from the COLCORONA trial, which is testing whether the anti-inflammatory agent can prevent hospitalizations, lung complications, and death when given early in the course of COVID-19.
University of Oxford (England) researchers also announced last week that colchicine is being added to the massive RECOVERY trial, which is studying treatments for hospitalized COVID-19 patients.
Notably, the Canadian-based COLCOT trial did not use Colcrys, but rather a colchicine product that costs just $0.26 a pill in Canada, roughly the price of most generics available worldwide.
Authorized generics typically drive down drug prices when competing with independent generics, but this competition is missing in the United States, where Colcrys holds patents until 2029, Dr. McCormick and colleagues noted. More than a half-dozen independent generics have FDA approval to date, but only authorized generics with price points set by the brand-name companies are available to treat acute gout, pericarditis, and potentially millions with MI.
“One of the key takeaways is this difference between the brand names and the authorized generics and the independents,” she said. “The authorized [generics] have really not saved money. The list prices were just slightly lower and patients can also have more difficulty in getting those covered.”
For this analysis, the investigators used Medicaid and Medicare data to examine prices for all available forms of colchicine from 2008 to 2017, including unregulated/unapproved colchicine (2008-2010), generic combination probenecid-colchicine (2008-2017), Colcrys (2009-2017), brand-name single-ingredient colchicine Mitigare (approved in late 2014 but not marketed until 2015), and their authorized generics (2015-2017). Medicare trends from 2012 to 2017 were analyzed separately because pre-Colcrys Medicare data were not available.
Based on the results, combined spending on Medicare and Medicaid claims for single-ingredient colchicine exceeded $340 million in 2017.
Inflation- and rebate-adjusted Medicaid unit prices rose from $0.24 a pill in 2008, when unapproved formulations were still available, to $4.20 a pill in 2011 (Colcrys only), and peaked at $4.66 a pill in 2015 (Colcrys plus authorized generics).
Prescribing of lower-priced probenecid-colchicine ($0.66/pill in 2017) remained stable throughout. Medicaid rebate-adjusted prices in 2017 were $3.99/pill for all single-ingredient colchicine products, $5.13/pill for Colcrys, $4.49/pill for Mitigare, and $3.88/pill for authorized generics.
Medicare rebate-adjusted 2017 per-pill prices were $5.81 for all single-ingredient colchicine products, $6.78 for Colcrys, $5.68 for Mitigare, $5.16 for authorized generics, and $0.70 for probenecid-colchicine.
“Authorized generics have still driven high spending,” Dr. McCormick said. “We really need to encourage more competition in order to improve access.”
In an accompanying commentary, B. Joseph Guglielmo, PharmD, University of California, San Francisco, pointed out that the estimated median research and development cost to bring a drug to market is between $985 million and $1,335 million, which inevitably translates into a high selling price for the drug. Such investment and its resultant cost, however, should be associated with potential worth to society.
“Only a fraction of an investment was required for Colcrys, a product that has provided no increased value and an unnecessary, long-term cost burden to the health care system,” he wrote. “The current study findings illustrate that we can never allow such an egregious case to take place again.”
Dr. McCormick reported grants from Canadian Institutes of Health Research during the conduct of the study. Dr. Guglielmo reported having no relevant conflicts of interest.
This article first appeared on Medscape.com.
CDC shortens COVID-19 quarantine time to 10 or 7 days, with conditions
Citing new evidence and an “acceptable risk” of transmission, the agency hopes reducing the 14-day quarantine will increase overall compliance and improve public health and economic constraints.
The agency also suggested people postpone travel during the upcoming winter holidays and stay home because of the pandemic.
These shorter quarantine options do not replace initial CDC guidance. “CDC continues to recommend quarantining for 14 days as the best way to reduce risk for spreading COVID-19,” said Henry Walke, MD, MPH, the CDC’s COVID-19 incident manager, during a media briefing on Wednesday.
However, “after reviewing and analyzing new research and data, CDC has identified two acceptable alternative quarantine periods.”
People can now quarantine for 10 days without a COVID-19 test if they have no symptoms. Alternatively, a quarantine can end after 7 days for someone with a negative test and no symptoms. The agency recommends a polymerase chain reaction test or an antigen assay within 48 hours before the end of a quarantine.
The agency also suggests people still monitor for symptoms for a full 14 days.
Reducing the length of quarantine “may make it easier for people to take this critical public health action, by reducing the economic hardship associated with a longer period, especially if they cannot work during that time,” Dr. Walke said. “In addition, a shorter quarantine period can lessen stress on the public health system and communities, especially when new infections are rapidly rising.”
The federal guidance leaves flexibility for local jurisdictions to make their own quarantine recommendations, as warranted, he added.
An ‘acceptable risk’ calculation
Modeling by the CDC and academic and public health partners led to the new quarantine recommendations, said John Brooks, MD, chief medical officer for the CDC’s COVID-19 response. Multiple studies “point in the same direction, which is that we can safely reduce the length of quarantine but accept there is a small residual risk that a person who is leaving quarantine early could transmit to someone else.”
The residual risk is approximately 1%, with an upper limit of 10%, when people quarantine for 10 days. A 7-day quarantine carries a residual risk of about 5% and an upper limit of 12%.
“Ten days is where the risk got into a sweet spot we like, at about 1%,” Dr. Brooks said. “That is a very acceptable risk, I think, for many people.”
Although it remains unknown what proportion of people spending 14 days in quarantine leave early, “we are hearing anecdotally from our partners in public health that many people are discontinuing quarantine ahead of time because there is pressure to go back to work, to get people back into school – and it imposes a burden on the individual,” Dr. Brooks said.
“One of our hopes is that ... if we reduce the amount of time they have to spend in quarantine, people will be more compliant,” he added.
A reporter asked why the CDC is shortening quarantines when the pandemic numbers are increasing nationwide. The timing has to do with capacity, Dr. Brooks said. “We are in situation where the number of cases is rising, the number of contacts is rising and the number of people who require quarantine is rising. That is a lot of burden, not just on the people who have to quarantine, but on public health.”
Home for the holidays
Similar to its pre-Thanksgiving advisory, the CDC also recommends people avoid travel during the upcoming winter holidays. “The best way to protect yourself and others is to postpone travel and stay home,” Dr. Walke said.
If people do decide to travel, the agency recommends COVID-19 testing 1-3 days prior to travel and again 3-5 days afterward, as well as reducing nonessential activities for a full 7 days after returning home. Furthermore, if someone does not have follow-up testing, the CDC recommends reducing nonessential activities for 10 days.
Testing does not eliminate all risk, Dr. Walke said, “but when combined with reducing nonessential activities, symptom screening and continuing with precautions like wearing masks, social distancing and hand washing, it can make travel safer.”
“We are trying to reduce the number of infections by postponing travel over the winter holiday,” Cindy Friedman, MD, chief of the CDC Travelers’ Health Branch, said during the media briefing.
“Travel volume was high during Thanksgiving,” she said, “and even if only a small percentage of those travelers were asymptomatically infected, this can translate into hundreds of thousands of additional infections moving from one community to another.”
This article first appeared on Medscape.com.
Citing new evidence and an “acceptable risk” of transmission, the agency hopes reducing the 14-day quarantine will increase overall compliance and improve public health and economic constraints.
The agency also suggested people postpone travel during the upcoming winter holidays and stay home because of the pandemic.
These shorter quarantine options do not replace initial CDC guidance. “CDC continues to recommend quarantining for 14 days as the best way to reduce risk for spreading COVID-19,” said Henry Walke, MD, MPH, the CDC’s COVID-19 incident manager, during a media briefing on Wednesday.
However, “after reviewing and analyzing new research and data, CDC has identified two acceptable alternative quarantine periods.”
People can now quarantine for 10 days without a COVID-19 test if they have no symptoms. Alternatively, a quarantine can end after 7 days for someone with a negative test and no symptoms. The agency recommends a polymerase chain reaction test or an antigen assay within 48 hours before the end of a quarantine.
The agency also suggests people still monitor for symptoms for a full 14 days.
Reducing the length of quarantine “may make it easier for people to take this critical public health action, by reducing the economic hardship associated with a longer period, especially if they cannot work during that time,” Dr. Walke said. “In addition, a shorter quarantine period can lessen stress on the public health system and communities, especially when new infections are rapidly rising.”
The federal guidance leaves flexibility for local jurisdictions to make their own quarantine recommendations, as warranted, he added.
An ‘acceptable risk’ calculation
Modeling by the CDC and academic and public health partners led to the new quarantine recommendations, said John Brooks, MD, chief medical officer for the CDC’s COVID-19 response. Multiple studies “point in the same direction, which is that we can safely reduce the length of quarantine but accept there is a small residual risk that a person who is leaving quarantine early could transmit to someone else.”
The residual risk is approximately 1%, with an upper limit of 10%, when people quarantine for 10 days. A 7-day quarantine carries a residual risk of about 5% and an upper limit of 12%.
“Ten days is where the risk got into a sweet spot we like, at about 1%,” Dr. Brooks said. “That is a very acceptable risk, I think, for many people.”
Although it remains unknown what proportion of people spending 14 days in quarantine leave early, “we are hearing anecdotally from our partners in public health that many people are discontinuing quarantine ahead of time because there is pressure to go back to work, to get people back into school – and it imposes a burden on the individual,” Dr. Brooks said.
“One of our hopes is that ... if we reduce the amount of time they have to spend in quarantine, people will be more compliant,” he added.
A reporter asked why the CDC is shortening quarantines when the pandemic numbers are increasing nationwide. The timing has to do with capacity, Dr. Brooks said. “We are in situation where the number of cases is rising, the number of contacts is rising and the number of people who require quarantine is rising. That is a lot of burden, not just on the people who have to quarantine, but on public health.”
Home for the holidays
Similar to its pre-Thanksgiving advisory, the CDC also recommends people avoid travel during the upcoming winter holidays. “The best way to protect yourself and others is to postpone travel and stay home,” Dr. Walke said.
If people do decide to travel, the agency recommends COVID-19 testing 1-3 days prior to travel and again 3-5 days afterward, as well as reducing nonessential activities for a full 7 days after returning home. Furthermore, if someone does not have follow-up testing, the CDC recommends reducing nonessential activities for 10 days.
Testing does not eliminate all risk, Dr. Walke said, “but when combined with reducing nonessential activities, symptom screening and continuing with precautions like wearing masks, social distancing and hand washing, it can make travel safer.”
“We are trying to reduce the number of infections by postponing travel over the winter holiday,” Cindy Friedman, MD, chief of the CDC Travelers’ Health Branch, said during the media briefing.
“Travel volume was high during Thanksgiving,” she said, “and even if only a small percentage of those travelers were asymptomatically infected, this can translate into hundreds of thousands of additional infections moving from one community to another.”
This article first appeared on Medscape.com.
Citing new evidence and an “acceptable risk” of transmission, the agency hopes reducing the 14-day quarantine will increase overall compliance and improve public health and economic constraints.
The agency also suggested people postpone travel during the upcoming winter holidays and stay home because of the pandemic.
These shorter quarantine options do not replace initial CDC guidance. “CDC continues to recommend quarantining for 14 days as the best way to reduce risk for spreading COVID-19,” said Henry Walke, MD, MPH, the CDC’s COVID-19 incident manager, during a media briefing on Wednesday.
However, “after reviewing and analyzing new research and data, CDC has identified two acceptable alternative quarantine periods.”
People can now quarantine for 10 days without a COVID-19 test if they have no symptoms. Alternatively, a quarantine can end after 7 days for someone with a negative test and no symptoms. The agency recommends a polymerase chain reaction test or an antigen assay within 48 hours before the end of a quarantine.
The agency also suggests people still monitor for symptoms for a full 14 days.
Reducing the length of quarantine “may make it easier for people to take this critical public health action, by reducing the economic hardship associated with a longer period, especially if they cannot work during that time,” Dr. Walke said. “In addition, a shorter quarantine period can lessen stress on the public health system and communities, especially when new infections are rapidly rising.”
The federal guidance leaves flexibility for local jurisdictions to make their own quarantine recommendations, as warranted, he added.
An ‘acceptable risk’ calculation
Modeling by the CDC and academic and public health partners led to the new quarantine recommendations, said John Brooks, MD, chief medical officer for the CDC’s COVID-19 response. Multiple studies “point in the same direction, which is that we can safely reduce the length of quarantine but accept there is a small residual risk that a person who is leaving quarantine early could transmit to someone else.”
The residual risk is approximately 1%, with an upper limit of 10%, when people quarantine for 10 days. A 7-day quarantine carries a residual risk of about 5% and an upper limit of 12%.
“Ten days is where the risk got into a sweet spot we like, at about 1%,” Dr. Brooks said. “That is a very acceptable risk, I think, for many people.”
Although it remains unknown what proportion of people spending 14 days in quarantine leave early, “we are hearing anecdotally from our partners in public health that many people are discontinuing quarantine ahead of time because there is pressure to go back to work, to get people back into school – and it imposes a burden on the individual,” Dr. Brooks said.
“One of our hopes is that ... if we reduce the amount of time they have to spend in quarantine, people will be more compliant,” he added.
A reporter asked why the CDC is shortening quarantines when the pandemic numbers are increasing nationwide. The timing has to do with capacity, Dr. Brooks said. “We are in situation where the number of cases is rising, the number of contacts is rising and the number of people who require quarantine is rising. That is a lot of burden, not just on the people who have to quarantine, but on public health.”
Home for the holidays
Similar to its pre-Thanksgiving advisory, the CDC also recommends people avoid travel during the upcoming winter holidays. “The best way to protect yourself and others is to postpone travel and stay home,” Dr. Walke said.
If people do decide to travel, the agency recommends COVID-19 testing 1-3 days prior to travel and again 3-5 days afterward, as well as reducing nonessential activities for a full 7 days after returning home. Furthermore, if someone does not have follow-up testing, the CDC recommends reducing nonessential activities for 10 days.
Testing does not eliminate all risk, Dr. Walke said, “but when combined with reducing nonessential activities, symptom screening and continuing with precautions like wearing masks, social distancing and hand washing, it can make travel safer.”
“We are trying to reduce the number of infections by postponing travel over the winter holiday,” Cindy Friedman, MD, chief of the CDC Travelers’ Health Branch, said during the media briefing.
“Travel volume was high during Thanksgiving,” she said, “and even if only a small percentage of those travelers were asymptomatically infected, this can translate into hundreds of thousands of additional infections moving from one community to another.”
This article first appeared on Medscape.com.
Pandemic increases need for home-based care with remote monitoring of patients
While the concept of home-based care and remote monitoring of patients may not be a new concept, the importance of this option for managing patients has taken on great importance during this COVID-19 pandemic.
We are currently living and working in unprecedented times and the impact of the pandemic is quite evident, and it plays an important part in every health care worker’s daily life. The high volumes of patients presenting to emergency rooms and urgent care/walk-in clinics and seeking posthospitalization visits with their physicians is stressing the health care environment. In such difficult times, the hospital-at-home model of care provides a valuable and viable option to provide appropriate care to those patients who may require close monitoring of their health without being hospitalized and using valuable inpatient resources that could then be used for the higher-acuity patients. As a physician who lives this every day and as a practicing internist and a part-time administrator, I welcome the hospital-at-home approach that complements the care provided in the emergency room, inpatient and ambulatory practice settings. I believe this type of approach to patient care would benefit those patients who, while being acutely ill, may not require the 24/7 intensive care that more critically ill individuals may need. As long as the patients are provided with appropriate telemonitoring devices such as a blood pressure cuff, pulse oximeter, and thermometer, and have access to video telemonitoring, the appropriately selected patients would benefit from this method of care provision for their acute illness.
Mental health benefits
I see several benefits for patients who can be triaged/assigned to this telemonitoring model of care. A patient would probably be happier being at home because they could sleep in their own bed and eat their own food and be able to walk around their house or even venture outdoors to enjoy the fresh air and nature. Being able to do these things will contribute positively to their emotional and psychological well-being.
For some elderly individuals, having access to the familiarity of their surroundings would mean these patients would have fewer incidences of hospital-associated delirium or falls. Additionally, they would be able to enjoy the company of their family members, which, during this COVID pandemic, is not possible in many hospitals. This would reduce emotional tensions for the patients and their families and the risk of transmission of infections to the patients and their visitors in the hospitals.
Freeing up resources
More importantly, this model would help physicians and hospitals provide the much needed care to the appropriate patients in the appropriate settings, thereby leading to decreased use of emergency rooms, health care workers, and personal protective equipment – all of which are currently in high demand.
Having a dedicated team of physicians, nurses, respiratory therapists, and other health care workers available to monitor these home-based patients on a daily or more frequent basis, depending on their health status, would result in these patients receiving equivalent care to what they would have received in a hospital.
Another positive outcome of using this home-based care model in the pandemic is that it would free up hospital beds for non–COVID-19 patients who might need hospitalization for management of their acute illnesses or exacerbation of chronic health conditions.
Possible limitations
This model of care has some limitations, including that it is not geared toward high volumes in my opinion and will not work in every home. Patients need to have Internet capabilities, phone services, and other features in their homes that make it possible for them to access this type of care. Additionally, patients may not be able to get their insurance companies to pay for these services. While the Centers for Medicare & Medicaid Services recently authorized patients to be transferred from EDs or inpatient wards to hospital-level care at home, for how long will reimbursements for this kind of care continue? If insurance will not pay for this monitoring at home, then will physician practices and hospital based practices provide this non reimbursed service?
Also, patients and their families may not be accepting of this model of care because they may feel it is inferior to inpatient hospitalization.
Despite these limitations, as long as Medicare and other health insurance programs provide reimbursement for such hospital-at-home services, I foresee this concept being highly used and benefiting health care entities in the United States.
Dr. Deep is a general internist in a multispecialty group practice with Aspirus Antigo (Wis.) Clinic and the chief medical officer and a staff physician at Aspirus Langlade Hospital in Antigo. He is also assistant clinical professor at the Medical College of Wisconsin, Central Wisconsin Campus, and the governor of the Wisconsin chapter of the American College of Physicians. Dr. Deep serves on the editorial advisory board of Internal Medicine News. Contact him at [email protected].
While the concept of home-based care and remote monitoring of patients may not be a new concept, the importance of this option for managing patients has taken on great importance during this COVID-19 pandemic.
We are currently living and working in unprecedented times and the impact of the pandemic is quite evident, and it plays an important part in every health care worker’s daily life. The high volumes of patients presenting to emergency rooms and urgent care/walk-in clinics and seeking posthospitalization visits with their physicians is stressing the health care environment. In such difficult times, the hospital-at-home model of care provides a valuable and viable option to provide appropriate care to those patients who may require close monitoring of their health without being hospitalized and using valuable inpatient resources that could then be used for the higher-acuity patients. As a physician who lives this every day and as a practicing internist and a part-time administrator, I welcome the hospital-at-home approach that complements the care provided in the emergency room, inpatient and ambulatory practice settings. I believe this type of approach to patient care would benefit those patients who, while being acutely ill, may not require the 24/7 intensive care that more critically ill individuals may need. As long as the patients are provided with appropriate telemonitoring devices such as a blood pressure cuff, pulse oximeter, and thermometer, and have access to video telemonitoring, the appropriately selected patients would benefit from this method of care provision for their acute illness.
Mental health benefits
I see several benefits for patients who can be triaged/assigned to this telemonitoring model of care. A patient would probably be happier being at home because they could sleep in their own bed and eat their own food and be able to walk around their house or even venture outdoors to enjoy the fresh air and nature. Being able to do these things will contribute positively to their emotional and psychological well-being.
For some elderly individuals, having access to the familiarity of their surroundings would mean these patients would have fewer incidences of hospital-associated delirium or falls. Additionally, they would be able to enjoy the company of their family members, which, during this COVID pandemic, is not possible in many hospitals. This would reduce emotional tensions for the patients and their families and the risk of transmission of infections to the patients and their visitors in the hospitals.
Freeing up resources
More importantly, this model would help physicians and hospitals provide the much needed care to the appropriate patients in the appropriate settings, thereby leading to decreased use of emergency rooms, health care workers, and personal protective equipment – all of which are currently in high demand.
Having a dedicated team of physicians, nurses, respiratory therapists, and other health care workers available to monitor these home-based patients on a daily or more frequent basis, depending on their health status, would result in these patients receiving equivalent care to what they would have received in a hospital.
Another positive outcome of using this home-based care model in the pandemic is that it would free up hospital beds for non–COVID-19 patients who might need hospitalization for management of their acute illnesses or exacerbation of chronic health conditions.
Possible limitations
This model of care has some limitations, including that it is not geared toward high volumes in my opinion and will not work in every home. Patients need to have Internet capabilities, phone services, and other features in their homes that make it possible for them to access this type of care. Additionally, patients may not be able to get their insurance companies to pay for these services. While the Centers for Medicare & Medicaid Services recently authorized patients to be transferred from EDs or inpatient wards to hospital-level care at home, for how long will reimbursements for this kind of care continue? If insurance will not pay for this monitoring at home, then will physician practices and hospital based practices provide this non reimbursed service?
Also, patients and their families may not be accepting of this model of care because they may feel it is inferior to inpatient hospitalization.
Despite these limitations, as long as Medicare and other health insurance programs provide reimbursement for such hospital-at-home services, I foresee this concept being highly used and benefiting health care entities in the United States.
Dr. Deep is a general internist in a multispecialty group practice with Aspirus Antigo (Wis.) Clinic and the chief medical officer and a staff physician at Aspirus Langlade Hospital in Antigo. He is also assistant clinical professor at the Medical College of Wisconsin, Central Wisconsin Campus, and the governor of the Wisconsin chapter of the American College of Physicians. Dr. Deep serves on the editorial advisory board of Internal Medicine News. Contact him at [email protected].
While the concept of home-based care and remote monitoring of patients may not be a new concept, the importance of this option for managing patients has taken on great importance during this COVID-19 pandemic.
We are currently living and working in unprecedented times and the impact of the pandemic is quite evident, and it plays an important part in every health care worker’s daily life. The high volumes of patients presenting to emergency rooms and urgent care/walk-in clinics and seeking posthospitalization visits with their physicians is stressing the health care environment. In such difficult times, the hospital-at-home model of care provides a valuable and viable option to provide appropriate care to those patients who may require close monitoring of their health without being hospitalized and using valuable inpatient resources that could then be used for the higher-acuity patients. As a physician who lives this every day and as a practicing internist and a part-time administrator, I welcome the hospital-at-home approach that complements the care provided in the emergency room, inpatient and ambulatory practice settings. I believe this type of approach to patient care would benefit those patients who, while being acutely ill, may not require the 24/7 intensive care that more critically ill individuals may need. As long as the patients are provided with appropriate telemonitoring devices such as a blood pressure cuff, pulse oximeter, and thermometer, and have access to video telemonitoring, the appropriately selected patients would benefit from this method of care provision for their acute illness.
Mental health benefits
I see several benefits for patients who can be triaged/assigned to this telemonitoring model of care. A patient would probably be happier being at home because they could sleep in their own bed and eat their own food and be able to walk around their house or even venture outdoors to enjoy the fresh air and nature. Being able to do these things will contribute positively to their emotional and psychological well-being.
For some elderly individuals, having access to the familiarity of their surroundings would mean these patients would have fewer incidences of hospital-associated delirium or falls. Additionally, they would be able to enjoy the company of their family members, which, during this COVID pandemic, is not possible in many hospitals. This would reduce emotional tensions for the patients and their families and the risk of transmission of infections to the patients and their visitors in the hospitals.
Freeing up resources
More importantly, this model would help physicians and hospitals provide the much needed care to the appropriate patients in the appropriate settings, thereby leading to decreased use of emergency rooms, health care workers, and personal protective equipment – all of which are currently in high demand.
Having a dedicated team of physicians, nurses, respiratory therapists, and other health care workers available to monitor these home-based patients on a daily or more frequent basis, depending on their health status, would result in these patients receiving equivalent care to what they would have received in a hospital.
Another positive outcome of using this home-based care model in the pandemic is that it would free up hospital beds for non–COVID-19 patients who might need hospitalization for management of their acute illnesses or exacerbation of chronic health conditions.
Possible limitations
This model of care has some limitations, including that it is not geared toward high volumes in my opinion and will not work in every home. Patients need to have Internet capabilities, phone services, and other features in their homes that make it possible for them to access this type of care. Additionally, patients may not be able to get their insurance companies to pay for these services. While the Centers for Medicare & Medicaid Services recently authorized patients to be transferred from EDs or inpatient wards to hospital-level care at home, for how long will reimbursements for this kind of care continue? If insurance will not pay for this monitoring at home, then will physician practices and hospital based practices provide this non reimbursed service?
Also, patients and their families may not be accepting of this model of care because they may feel it is inferior to inpatient hospitalization.
Despite these limitations, as long as Medicare and other health insurance programs provide reimbursement for such hospital-at-home services, I foresee this concept being highly used and benefiting health care entities in the United States.
Dr. Deep is a general internist in a multispecialty group practice with Aspirus Antigo (Wis.) Clinic and the chief medical officer and a staff physician at Aspirus Langlade Hospital in Antigo. He is also assistant clinical professor at the Medical College of Wisconsin, Central Wisconsin Campus, and the governor of the Wisconsin chapter of the American College of Physicians. Dr. Deep serves on the editorial advisory board of Internal Medicine News. Contact him at [email protected].
U.S. passes 1.3 million COVID-19 cases in children
The news on children and COVID-19 for Thanksgiving week does not provide a lot of room for thankfulness.
the American Academy of Pediatrics and the Children’s Hospital Association said in their latest weekly report.
For those not counting, the week ending Nov. 26 was the fifth in a row to show “the highest weekly increase since the pandemic began,” based on data the AAP and CHA have been collecting from 49 state health departments (New York does not report ages), as well as the District of Columbia, New York City, Puerto Rico, and Guam.
The 153,608 new cases bring the total number of COVID-19 cases in children to almost 1.34 million in those jurisdictions, which is 12% of the total number of cases (11.2 million) among all ages. For just the week ending Nov. 26, children represented 13.7% of all new cases in the United States, down from 14.1% the previous week, according to the AAP/CHA data.
Among the states reporting child cases, Florida has the lowest cumulative proportion of child cases, 6.4%, but the state is using an age range of 0-14 years (no other state goes lower than 17 years). New Jersey and Texas are next at 6.9%, although Texas “reported age for only 6% of total confirmed cases,” the AAP and CHA noted.
There are 35 states above the national number of 12.0%, the highest being Wyoming at 23.3%, followed by Tennessee at 18.3% and South Carolina at 18.2%. The two southern states are the only ones to use an age range of 0-20 years for child cases, the two groups said in this week’s report, which did not include the usual data on testing, hospitalization, and mortality because of the holiday.
The news on children and COVID-19 for Thanksgiving week does not provide a lot of room for thankfulness.

the American Academy of Pediatrics and the Children’s Hospital Association said in their latest weekly report.
For those not counting, the week ending Nov. 26 was the fifth in a row to show “the highest weekly increase since the pandemic began,” based on data the AAP and CHA have been collecting from 49 state health departments (New York does not report ages), as well as the District of Columbia, New York City, Puerto Rico, and Guam.
The 153,608 new cases bring the total number of COVID-19 cases in children to almost 1.34 million in those jurisdictions, which is 12% of the total number of cases (11.2 million) among all ages. For just the week ending Nov. 26, children represented 13.7% of all new cases in the United States, down from 14.1% the previous week, according to the AAP/CHA data.
Among the states reporting child cases, Florida has the lowest cumulative proportion of child cases, 6.4%, but the state is using an age range of 0-14 years (no other state goes lower than 17 years). New Jersey and Texas are next at 6.9%, although Texas “reported age for only 6% of total confirmed cases,” the AAP and CHA noted.
There are 35 states above the national number of 12.0%, the highest being Wyoming at 23.3%, followed by Tennessee at 18.3% and South Carolina at 18.2%. The two southern states are the only ones to use an age range of 0-20 years for child cases, the two groups said in this week’s report, which did not include the usual data on testing, hospitalization, and mortality because of the holiday.
The news on children and COVID-19 for Thanksgiving week does not provide a lot of room for thankfulness.

the American Academy of Pediatrics and the Children’s Hospital Association said in their latest weekly report.
For those not counting, the week ending Nov. 26 was the fifth in a row to show “the highest weekly increase since the pandemic began,” based on data the AAP and CHA have been collecting from 49 state health departments (New York does not report ages), as well as the District of Columbia, New York City, Puerto Rico, and Guam.
The 153,608 new cases bring the total number of COVID-19 cases in children to almost 1.34 million in those jurisdictions, which is 12% of the total number of cases (11.2 million) among all ages. For just the week ending Nov. 26, children represented 13.7% of all new cases in the United States, down from 14.1% the previous week, according to the AAP/CHA data.
Among the states reporting child cases, Florida has the lowest cumulative proportion of child cases, 6.4%, but the state is using an age range of 0-14 years (no other state goes lower than 17 years). New Jersey and Texas are next at 6.9%, although Texas “reported age for only 6% of total confirmed cases,” the AAP and CHA noted.
There are 35 states above the national number of 12.0%, the highest being Wyoming at 23.3%, followed by Tennessee at 18.3% and South Carolina at 18.2%. The two southern states are the only ones to use an age range of 0-20 years for child cases, the two groups said in this week’s report, which did not include the usual data on testing, hospitalization, and mortality because of the holiday.