User login
ID Practitioner is an independent news source that provides infectious disease specialists with timely and relevant news and commentary about clinical developments and the impact of health care policy on the infectious disease specialist’s practice. Specialty focus topics include antimicrobial resistance, emerging infections, global ID, hepatitis, HIV, hospital-acquired infections, immunizations and vaccines, influenza, mycoses, pediatric infections, and STIs. Infectious Diseases News is owned by Frontline Medical Communications.
sofosbuvir
ritonavir with dasabuvir
discount
support path
program
ritonavir
greedy
ledipasvir
assistance
viekira pak
vpak
advocacy
needy
protest
abbvie
paritaprevir
ombitasvir
direct-acting antivirals
dasabuvir
gilead
fake-ovir
support
v pak
oasis
harvoni
section[contains(@class, 'footer-nav-section-wrapper')]
div[contains(@class, 'pane-pub-article-idp')]
div[contains(@class, 'pane-medstat-latest-articles-articles-section')]
div[contains(@class, 'pane-pub-home-idp')]
div[contains(@class, 'pane-pub-topic-idp')]
Returning to competition
As we continue to stumble around trying to find our way out of the COVID-19 pandemic, it has become clear that the journey has been a never-ending continuum of exercises in risk/benefit assessment. The population always has sorted itself into a bell-shaped curve from those who are risk averse to those who revel in risk taking. And, of course, with a paucity of facts on which we can base our assessment of risk, the discussion often shifts to our gut feelings about the benefits.
When faced with the question of when it is time for children to return to in-person schooling, there seems to be reasonably good agreement about the benefits of face-to-face learning. The level of risk is still to be determined.
When it comes to the issue of when to return to competitive school sports, the risks are equally indeterminate but there is less agreement on the benefits. This lack of uniformity reflects a long-standing dichotomy between those parents and students with a passion for competitive sports and those who see them as nonessential. This existential tug-of-war has gone on in almost every school system I am aware of when the school budget comes up for a vote.
The debate about a return to competitive sports on a collegiate and professional level unfortunately is colored by enormous revenues from media contracts, which means that high school and middle schools can’t look to what are essentially businesses for guidance. The delay created confusion, fluctuating angst and disappointment, but the end product made some sense. Volleyball (indoor) and football were indefinitely delayed. Heavy breathing between competitors separated by a couple of feet and protected only by a flimsy net or helmet cage seems like a risk not worth taking – at least until we have more information.
Other sports were allowed to start with restrictions based on existing social distancing mandates which include no locker rooms and no fans. Some rules such as no throw-ins for soccer didn’t make sense given what we are learning about the virus. But, for the most part, the compromises should result in a chance to reap the benefits of competition for the students whose families are willing to expose them to the yet to be fully determined risks.
There has been some grumbling from parents who see the no-fans mandate as a step too far. Until we know more about the risk of group gatherings outdoors, having no fans, including parents and grandparents, makes sense. In fact, to me it is a step long overdue and a rare sliver of silver lining to the pandemic. Competitive youth sports are for the kids. They are not meant to be entertainment events. Too often children are exposed to parental pressure (voiced and unvoiced) about their “performance” on the field. Neither my younger sister nor I can remember our parents going to any of my away football games in high school or any of my lacrosse games in college. I never felt the loss.
Will I miss watching my grandchildren compete? Of course I will miss it badly. However, giving kids some space to learn and enjoy the competition for itself in an atmosphere free of parental over-involvement will be a breath of fresh air. Something we need badly during this pandemic.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
As we continue to stumble around trying to find our way out of the COVID-19 pandemic, it has become clear that the journey has been a never-ending continuum of exercises in risk/benefit assessment. The population always has sorted itself into a bell-shaped curve from those who are risk averse to those who revel in risk taking. And, of course, with a paucity of facts on which we can base our assessment of risk, the discussion often shifts to our gut feelings about the benefits.
When faced with the question of when it is time for children to return to in-person schooling, there seems to be reasonably good agreement about the benefits of face-to-face learning. The level of risk is still to be determined.
When it comes to the issue of when to return to competitive school sports, the risks are equally indeterminate but there is less agreement on the benefits. This lack of uniformity reflects a long-standing dichotomy between those parents and students with a passion for competitive sports and those who see them as nonessential. This existential tug-of-war has gone on in almost every school system I am aware of when the school budget comes up for a vote.
The debate about a return to competitive sports on a collegiate and professional level unfortunately is colored by enormous revenues from media contracts, which means that high school and middle schools can’t look to what are essentially businesses for guidance. The delay created confusion, fluctuating angst and disappointment, but the end product made some sense. Volleyball (indoor) and football were indefinitely delayed. Heavy breathing between competitors separated by a couple of feet and protected only by a flimsy net or helmet cage seems like a risk not worth taking – at least until we have more information.
Other sports were allowed to start with restrictions based on existing social distancing mandates which include no locker rooms and no fans. Some rules such as no throw-ins for soccer didn’t make sense given what we are learning about the virus. But, for the most part, the compromises should result in a chance to reap the benefits of competition for the students whose families are willing to expose them to the yet to be fully determined risks.
There has been some grumbling from parents who see the no-fans mandate as a step too far. Until we know more about the risk of group gatherings outdoors, having no fans, including parents and grandparents, makes sense. In fact, to me it is a step long overdue and a rare sliver of silver lining to the pandemic. Competitive youth sports are for the kids. They are not meant to be entertainment events. Too often children are exposed to parental pressure (voiced and unvoiced) about their “performance” on the field. Neither my younger sister nor I can remember our parents going to any of my away football games in high school or any of my lacrosse games in college. I never felt the loss.
Will I miss watching my grandchildren compete? Of course I will miss it badly. However, giving kids some space to learn and enjoy the competition for itself in an atmosphere free of parental over-involvement will be a breath of fresh air. Something we need badly during this pandemic.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
As we continue to stumble around trying to find our way out of the COVID-19 pandemic, it has become clear that the journey has been a never-ending continuum of exercises in risk/benefit assessment. The population always has sorted itself into a bell-shaped curve from those who are risk averse to those who revel in risk taking. And, of course, with a paucity of facts on which we can base our assessment of risk, the discussion often shifts to our gut feelings about the benefits.
When faced with the question of when it is time for children to return to in-person schooling, there seems to be reasonably good agreement about the benefits of face-to-face learning. The level of risk is still to be determined.
When it comes to the issue of when to return to competitive school sports, the risks are equally indeterminate but there is less agreement on the benefits. This lack of uniformity reflects a long-standing dichotomy between those parents and students with a passion for competitive sports and those who see them as nonessential. This existential tug-of-war has gone on in almost every school system I am aware of when the school budget comes up for a vote.
The debate about a return to competitive sports on a collegiate and professional level unfortunately is colored by enormous revenues from media contracts, which means that high school and middle schools can’t look to what are essentially businesses for guidance. The delay created confusion, fluctuating angst and disappointment, but the end product made some sense. Volleyball (indoor) and football were indefinitely delayed. Heavy breathing between competitors separated by a couple of feet and protected only by a flimsy net or helmet cage seems like a risk not worth taking – at least until we have more information.
Other sports were allowed to start with restrictions based on existing social distancing mandates which include no locker rooms and no fans. Some rules such as no throw-ins for soccer didn’t make sense given what we are learning about the virus. But, for the most part, the compromises should result in a chance to reap the benefits of competition for the students whose families are willing to expose them to the yet to be fully determined risks.
There has been some grumbling from parents who see the no-fans mandate as a step too far. Until we know more about the risk of group gatherings outdoors, having no fans, including parents and grandparents, makes sense. In fact, to me it is a step long overdue and a rare sliver of silver lining to the pandemic. Competitive youth sports are for the kids. They are not meant to be entertainment events. Too often children are exposed to parental pressure (voiced and unvoiced) about their “performance” on the field. Neither my younger sister nor I can remember our parents going to any of my away football games in high school or any of my lacrosse games in college. I never felt the loss.
Will I miss watching my grandchildren compete? Of course I will miss it badly. However, giving kids some space to learn and enjoy the competition for itself in an atmosphere free of parental over-involvement will be a breath of fresh air. Something we need badly during this pandemic.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
Remdesivir effective, well-tolerated in final trial report
Drug beats placebo across multiple endpoints in COVID-19 patients
In May 2020, remdesivir received Food and Drug Administration approval for emergency treatment of severe COVID-19 on the basis of a preliminary report on this trial. In August 2020, the FDA expanded the indication to include all hospitalized adult and pediatric patients with suspected or laboratory-confirmed COVID-19 infection irrespective of severity.
“Our findings were consistent with the findings of the preliminary report: a 10-day course of remdesivir was superior to placebo in the treatment of hospitalized patients with COVID-19,” reported a team of investigators led by John H. Beigel, MD, of the Division of Microbiology and Infectious Diseases at the National Institute of Allergy and Infectious Diseases, in the New England Journal of Medicine.
The drug’s broadened indication was not based on the ACTT-1 trial, according to Dr. Beigel. “Other data have demonstrated that remdesivir shortens recovery in patients with lower acuity. In our study, evidence of pneumonia was an enrollment requirement,” he explained in an interview.
In the newly published final ACTT-1 data, the median time to recovery was 10 days for those on active therapy versus 15 days for those randomized to placebo. With a rate ratio of 1.29 (P less than .001), this translated to a recovery that was about one third faster.
In this final report, remdesivir’s significant advantage over placebo regarding the trial’s primary endpoint was reinforced by efficacy on multiple secondary endpoints.
This benefits on multiple secondary endpoints included a 50% greater odds ratio (OR, 1.5; 95% CI, 1.2-1.9) of significant clinical improvement by day 15 after adjustment for baseline severity, a shorter initial length of hospital stay (12 vs. 17 days) and fewer days on oxygen supplementation (13 vs. 21 days) for the subgroup of patients on oxygen at enrollment.
Although the numerically lower mortality in the remdesivir arm (6.75 vs. 11.9%) did not reach statistical significance, Dr. Beigel said, “mortality was moving in the same direction as the other key endpoints.”
According to the study investigators, the types of rates of adverse events on remdesivir, which inhibits viral replication, “were generally similar in the remdesivir and placebo groups.”
In ACTT-1, 1,062 patients were randomized to remdesivir (200 mg loading dose followed by 100 mg daily for up to 9 days) or placebo. Patients were enrolled at study sites in North America, Europe, and Asia.
The data of ACTT-1 confirm a benefit from remdesivir in hospitalized COVID-19 patients with severe disease, but Dr. Beigel said he agrees with the current FDA indication that supports treatment in any hospitalized COVID-19 patient.
“We saw bigger benefits in patients with more severe infections. The benefits are not as large in patients with mild disease, but I think remdesivir should be considered in any hospitalized patient,” Dr. Beigel said.
This point of view is shared.
“I would give this drug to anyone in the hospital infected with COVID-19 assuming there was an ample supply and no need for rationing,” said Donna E. Sweet, MD, professor of internal medicine, University of Kansas, Wichita. She noted that this study has implications for hospital and hospital staff, as well as for patients.
“This type of reduction in recovery time means a reduction in potential exposures to hospital staff, a reduced need for PPE [personal protective equipment], and it will free up beds in the ICU [intensive care unit],” said Dr. Sweet, who also serves as an editorial advisory board member for Internal Medicine News.
An infectious disease specialist at the University of Minnesota also considers remdesivir to have an important role for conserving resources that deserves emphasis.
The reduction in time to recovery “is of benefit to the health system by maintaining hospital bed capacity,” said David R. Boulware, MD, professor of medicine at the University of Minnesota, Minneapolis.
According to his reading of the available data, including those from ACTT-1, the benefit appears to be greatest in those with a moderate degree of illness, which he defined as “sick enough to be hospitalized and require oxygen, yet not severely sick [and] requiring a ventilator or [extracorporeal membrane oxygenation].”
This does not preclude a benefit in those with more severe or milder disease, but patients with mild disease “are likely to recover regardless – or despite – whatever therapy they receive,” he said.
Dr. Beigel, the principal investigator of this trial, reports no potential conflicts of interest.
SOURCE: Beigel JH et al. N Engl J Med. 2020 Oct 8. doi: 10.1056/NEJMoa2007764.
Drug beats placebo across multiple endpoints in COVID-19 patients
Drug beats placebo across multiple endpoints in COVID-19 patients
In May 2020, remdesivir received Food and Drug Administration approval for emergency treatment of severe COVID-19 on the basis of a preliminary report on this trial. In August 2020, the FDA expanded the indication to include all hospitalized adult and pediatric patients with suspected or laboratory-confirmed COVID-19 infection irrespective of severity.
“Our findings were consistent with the findings of the preliminary report: a 10-day course of remdesivir was superior to placebo in the treatment of hospitalized patients with COVID-19,” reported a team of investigators led by John H. Beigel, MD, of the Division of Microbiology and Infectious Diseases at the National Institute of Allergy and Infectious Diseases, in the New England Journal of Medicine.
The drug’s broadened indication was not based on the ACTT-1 trial, according to Dr. Beigel. “Other data have demonstrated that remdesivir shortens recovery in patients with lower acuity. In our study, evidence of pneumonia was an enrollment requirement,” he explained in an interview.
In the newly published final ACTT-1 data, the median time to recovery was 10 days for those on active therapy versus 15 days for those randomized to placebo. With a rate ratio of 1.29 (P less than .001), this translated to a recovery that was about one third faster.
In this final report, remdesivir’s significant advantage over placebo regarding the trial’s primary endpoint was reinforced by efficacy on multiple secondary endpoints.
This benefits on multiple secondary endpoints included a 50% greater odds ratio (OR, 1.5; 95% CI, 1.2-1.9) of significant clinical improvement by day 15 after adjustment for baseline severity, a shorter initial length of hospital stay (12 vs. 17 days) and fewer days on oxygen supplementation (13 vs. 21 days) for the subgroup of patients on oxygen at enrollment.
Although the numerically lower mortality in the remdesivir arm (6.75 vs. 11.9%) did not reach statistical significance, Dr. Beigel said, “mortality was moving in the same direction as the other key endpoints.”
According to the study investigators, the types of rates of adverse events on remdesivir, which inhibits viral replication, “were generally similar in the remdesivir and placebo groups.”
In ACTT-1, 1,062 patients were randomized to remdesivir (200 mg loading dose followed by 100 mg daily for up to 9 days) or placebo. Patients were enrolled at study sites in North America, Europe, and Asia.
The data of ACTT-1 confirm a benefit from remdesivir in hospitalized COVID-19 patients with severe disease, but Dr. Beigel said he agrees with the current FDA indication that supports treatment in any hospitalized COVID-19 patient.
“We saw bigger benefits in patients with more severe infections. The benefits are not as large in patients with mild disease, but I think remdesivir should be considered in any hospitalized patient,” Dr. Beigel said.
This point of view is shared.
“I would give this drug to anyone in the hospital infected with COVID-19 assuming there was an ample supply and no need for rationing,” said Donna E. Sweet, MD, professor of internal medicine, University of Kansas, Wichita. She noted that this study has implications for hospital and hospital staff, as well as for patients.
“This type of reduction in recovery time means a reduction in potential exposures to hospital staff, a reduced need for PPE [personal protective equipment], and it will free up beds in the ICU [intensive care unit],” said Dr. Sweet, who also serves as an editorial advisory board member for Internal Medicine News.
An infectious disease specialist at the University of Minnesota also considers remdesivir to have an important role for conserving resources that deserves emphasis.
The reduction in time to recovery “is of benefit to the health system by maintaining hospital bed capacity,” said David R. Boulware, MD, professor of medicine at the University of Minnesota, Minneapolis.
According to his reading of the available data, including those from ACTT-1, the benefit appears to be greatest in those with a moderate degree of illness, which he defined as “sick enough to be hospitalized and require oxygen, yet not severely sick [and] requiring a ventilator or [extracorporeal membrane oxygenation].”
This does not preclude a benefit in those with more severe or milder disease, but patients with mild disease “are likely to recover regardless – or despite – whatever therapy they receive,” he said.
Dr. Beigel, the principal investigator of this trial, reports no potential conflicts of interest.
SOURCE: Beigel JH et al. N Engl J Med. 2020 Oct 8. doi: 10.1056/NEJMoa2007764.
In May 2020, remdesivir received Food and Drug Administration approval for emergency treatment of severe COVID-19 on the basis of a preliminary report on this trial. In August 2020, the FDA expanded the indication to include all hospitalized adult and pediatric patients with suspected or laboratory-confirmed COVID-19 infection irrespective of severity.
“Our findings were consistent with the findings of the preliminary report: a 10-day course of remdesivir was superior to placebo in the treatment of hospitalized patients with COVID-19,” reported a team of investigators led by John H. Beigel, MD, of the Division of Microbiology and Infectious Diseases at the National Institute of Allergy and Infectious Diseases, in the New England Journal of Medicine.
The drug’s broadened indication was not based on the ACTT-1 trial, according to Dr. Beigel. “Other data have demonstrated that remdesivir shortens recovery in patients with lower acuity. In our study, evidence of pneumonia was an enrollment requirement,” he explained in an interview.
In the newly published final ACTT-1 data, the median time to recovery was 10 days for those on active therapy versus 15 days for those randomized to placebo. With a rate ratio of 1.29 (P less than .001), this translated to a recovery that was about one third faster.
In this final report, remdesivir’s significant advantage over placebo regarding the trial’s primary endpoint was reinforced by efficacy on multiple secondary endpoints.
This benefits on multiple secondary endpoints included a 50% greater odds ratio (OR, 1.5; 95% CI, 1.2-1.9) of significant clinical improvement by day 15 after adjustment for baseline severity, a shorter initial length of hospital stay (12 vs. 17 days) and fewer days on oxygen supplementation (13 vs. 21 days) for the subgroup of patients on oxygen at enrollment.
Although the numerically lower mortality in the remdesivir arm (6.75 vs. 11.9%) did not reach statistical significance, Dr. Beigel said, “mortality was moving in the same direction as the other key endpoints.”
According to the study investigators, the types of rates of adverse events on remdesivir, which inhibits viral replication, “were generally similar in the remdesivir and placebo groups.”
In ACTT-1, 1,062 patients were randomized to remdesivir (200 mg loading dose followed by 100 mg daily for up to 9 days) or placebo. Patients were enrolled at study sites in North America, Europe, and Asia.
The data of ACTT-1 confirm a benefit from remdesivir in hospitalized COVID-19 patients with severe disease, but Dr. Beigel said he agrees with the current FDA indication that supports treatment in any hospitalized COVID-19 patient.
“We saw bigger benefits in patients with more severe infections. The benefits are not as large in patients with mild disease, but I think remdesivir should be considered in any hospitalized patient,” Dr. Beigel said.
This point of view is shared.
“I would give this drug to anyone in the hospital infected with COVID-19 assuming there was an ample supply and no need for rationing,” said Donna E. Sweet, MD, professor of internal medicine, University of Kansas, Wichita. She noted that this study has implications for hospital and hospital staff, as well as for patients.
“This type of reduction in recovery time means a reduction in potential exposures to hospital staff, a reduced need for PPE [personal protective equipment], and it will free up beds in the ICU [intensive care unit],” said Dr. Sweet, who also serves as an editorial advisory board member for Internal Medicine News.
An infectious disease specialist at the University of Minnesota also considers remdesivir to have an important role for conserving resources that deserves emphasis.
The reduction in time to recovery “is of benefit to the health system by maintaining hospital bed capacity,” said David R. Boulware, MD, professor of medicine at the University of Minnesota, Minneapolis.
According to his reading of the available data, including those from ACTT-1, the benefit appears to be greatest in those with a moderate degree of illness, which he defined as “sick enough to be hospitalized and require oxygen, yet not severely sick [and] requiring a ventilator or [extracorporeal membrane oxygenation].”
This does not preclude a benefit in those with more severe or milder disease, but patients with mild disease “are likely to recover regardless – or despite – whatever therapy they receive,” he said.
Dr. Beigel, the principal investigator of this trial, reports no potential conflicts of interest.
SOURCE: Beigel JH et al. N Engl J Med. 2020 Oct 8. doi: 10.1056/NEJMoa2007764.
More data on impact of corticosteroids on COVID-19 mortality in patients with COPD
, a study of almost 1 million individuals in the United Kingdom has shown.
Patients with chronic obstructive pulmonary disease or asthma who used ICS on a regular basis were more likely to die from COVID-19 than COPD or asthma patients who were prescribed non-ICS therapies, reported co-lead author Anna Schultze, PhD, of London School of Hygiene & Tropical Medicine and colleagues.
Of note, the increased risk of death among ICS users likely stemmed from greater severity of preexisting chronic respiratory conditions, instead of directly from ICS usage, which has little apparent impact on COVID-19 mortality, the investigators wrote in Lancet Respiratory Medicine.
These findings conflict with a hypothesis proposed early in the pandemic: that ICS may protect individuals from SARS-CoV-2 infection and poor outcomes with COVID-19.
According to Megan Conroy, MD, of the department of internal medicine at the Ohio State University Wexner Medical Center, Columbus, this hypothesis was based on some unexpected epidemiological findings.
“In general, we tend to think people with underlying lung disease – like COPD or asthma – to be at higher risk for severe forms of lower respiratory tract infections,” Dr. Conroy said. “Somewhat surprisingly, early data in the pandemic showed patients with COPD and asthma [were] underrepresented [among patients with COVID] when compared to the prevalence of these diseases in the population.”
This raised the possibility of an incidental protective effect from regular ICS therapy, which “had some strong theoretic pathophysiologic basis,” Dr. Conroy said, referring to research that demonstrated ICS-mediated downregulation of SARS-CoV-2 entry receptors ACE2 and TMPRSS2.
Dr. Schultze and colleagues noted that investigators for two ongoing randomized controlled trials (NCT04331054, NCT04330586) are studying ICS as an intervention for COVID-19; but neither trial includes individuals already taking ICS for chronic respiratory disease.
The present observational study therefore aimed to assess mortality risk within this population. Data were drawn from electronic health records and a U.K. national mortality database, with follow-up ranging from March 1 to May 6, 2020. Eligibility required a relevant prescription within 4 months of first follow-up. In the COPD group, patients were prescribed a long-acting beta agonist plus a long-acting muscarinic antagonist (LABA–LAMA), LABA alone, LABA plus ICS, LABA–LAMA plus ICS, or ICS alone (if prescribed LABA within 4 months).
In the asthma group, patients received low/medium-dose ICS, high-dose ICS, or a short-acting beta agonist (SABA) alone. Patients with COPD were at least 35 years of age, while those with asthma were 18 years or older. Hazard ratios were adjusted for a variety of covariates, including respiratory disease–exacerbation history, age, sex, body mass index, hypertension, diabetes, and others.
These eligibility criteria returned 148,557 patients with COPD and 818,490 with asthma.
Patients with COPD who were prescribed ICS plus LABA-LAMA or ICS plus LABA had an increased risk of COVID-19-related death, compared with those who did not receive ICS (adjusted hazard ratio, 1.39; 95% confidence interval, 1.10-1.76). Separate analyses of patients who received a triple combination (LABA–LAMA plus ICS) versus those who took a dual combination (LABA plus ICS) showed that triple-combination therapy was significantly associated with increased COVID-19-related mortality (aHR, 1.43; 95% CI, 1.12-1.83), while dual-combination therapy was less so (aHR, 1.29; 95% CI, 0.96-1.74). Non–COVID-19–related mortality was significantly increased for all COPD patients who were prescribed ICS, with or without adjustment for covariates.
Asthma patients prescribed high-dose ICS instead of SABA alone had a slightly greater risk of COVID-19–related death, based on an adjusted hazard ratio of 1.55 (95% CI, 1.10-2.18). Those with asthma who received low/medium–dose ICS demonstrated a slight trend toward increased mortality risk, but this was not significant (aHR, 1.14; 95% CI, 0.85-1.54). ICS usage in the asthma group was not linked with a significant increase in non–COVID-19–related death.
“In summary, we found no evidence of a beneficial effect of regular ICS use among people with COPD and asthma on COVID-19–related mortality,” the investigators concluded.
In agreement with the investigators, Dr. Conroy said that the increased mortality rate among ICS users should not be misconstrued as a medication-related risk.
“While the study found that those with COPD or asthma taking ICS and high-dose ICS were at an increased risk of death, this could easily be explained by the likelihood that those are the patients who are more likely to have more severe underlying lung disease,” Dr. Conroy said. “While this observational study did attempt to control for exacerbation history, the ability to do so by electronic health records data is certainly imperfect.”
With this in mind, patients with chronic respiratory disease should be encouraged to adhere to their usual treatment regimen, Dr. Conroy added.
“There isn’t evidence to increase or decrease medications just because of the pandemic,” she said. “A patient with asthma or COPD should continue to take the medications that are needed to achieve good control of their lung disease.”
The study was funded by the U.K. Medical Research Council. The investigators reported additional relationships with the Wellcome Trust, the Good Thinking Foundation, the Laura and John Arnold Foundation, and others. Dr. Conroy reported no conflicts of interest.
SOURCE: Schultze A et al. Lancet Respir Med. 2020 Sep 24. doi: 10.1016/ S2213-2600(20)30415-X.
, a study of almost 1 million individuals in the United Kingdom has shown.
Patients with chronic obstructive pulmonary disease or asthma who used ICS on a regular basis were more likely to die from COVID-19 than COPD or asthma patients who were prescribed non-ICS therapies, reported co-lead author Anna Schultze, PhD, of London School of Hygiene & Tropical Medicine and colleagues.
Of note, the increased risk of death among ICS users likely stemmed from greater severity of preexisting chronic respiratory conditions, instead of directly from ICS usage, which has little apparent impact on COVID-19 mortality, the investigators wrote in Lancet Respiratory Medicine.
These findings conflict with a hypothesis proposed early in the pandemic: that ICS may protect individuals from SARS-CoV-2 infection and poor outcomes with COVID-19.
According to Megan Conroy, MD, of the department of internal medicine at the Ohio State University Wexner Medical Center, Columbus, this hypothesis was based on some unexpected epidemiological findings.
“In general, we tend to think people with underlying lung disease – like COPD or asthma – to be at higher risk for severe forms of lower respiratory tract infections,” Dr. Conroy said. “Somewhat surprisingly, early data in the pandemic showed patients with COPD and asthma [were] underrepresented [among patients with COVID] when compared to the prevalence of these diseases in the population.”
This raised the possibility of an incidental protective effect from regular ICS therapy, which “had some strong theoretic pathophysiologic basis,” Dr. Conroy said, referring to research that demonstrated ICS-mediated downregulation of SARS-CoV-2 entry receptors ACE2 and TMPRSS2.
Dr. Schultze and colleagues noted that investigators for two ongoing randomized controlled trials (NCT04331054, NCT04330586) are studying ICS as an intervention for COVID-19; but neither trial includes individuals already taking ICS for chronic respiratory disease.
The present observational study therefore aimed to assess mortality risk within this population. Data were drawn from electronic health records and a U.K. national mortality database, with follow-up ranging from March 1 to May 6, 2020. Eligibility required a relevant prescription within 4 months of first follow-up. In the COPD group, patients were prescribed a long-acting beta agonist plus a long-acting muscarinic antagonist (LABA–LAMA), LABA alone, LABA plus ICS, LABA–LAMA plus ICS, or ICS alone (if prescribed LABA within 4 months).
In the asthma group, patients received low/medium-dose ICS, high-dose ICS, or a short-acting beta agonist (SABA) alone. Patients with COPD were at least 35 years of age, while those with asthma were 18 years or older. Hazard ratios were adjusted for a variety of covariates, including respiratory disease–exacerbation history, age, sex, body mass index, hypertension, diabetes, and others.
These eligibility criteria returned 148,557 patients with COPD and 818,490 with asthma.
Patients with COPD who were prescribed ICS plus LABA-LAMA or ICS plus LABA had an increased risk of COVID-19-related death, compared with those who did not receive ICS (adjusted hazard ratio, 1.39; 95% confidence interval, 1.10-1.76). Separate analyses of patients who received a triple combination (LABA–LAMA plus ICS) versus those who took a dual combination (LABA plus ICS) showed that triple-combination therapy was significantly associated with increased COVID-19-related mortality (aHR, 1.43; 95% CI, 1.12-1.83), while dual-combination therapy was less so (aHR, 1.29; 95% CI, 0.96-1.74). Non–COVID-19–related mortality was significantly increased for all COPD patients who were prescribed ICS, with or without adjustment for covariates.
Asthma patients prescribed high-dose ICS instead of SABA alone had a slightly greater risk of COVID-19–related death, based on an adjusted hazard ratio of 1.55 (95% CI, 1.10-2.18). Those with asthma who received low/medium–dose ICS demonstrated a slight trend toward increased mortality risk, but this was not significant (aHR, 1.14; 95% CI, 0.85-1.54). ICS usage in the asthma group was not linked with a significant increase in non–COVID-19–related death.
“In summary, we found no evidence of a beneficial effect of regular ICS use among people with COPD and asthma on COVID-19–related mortality,” the investigators concluded.
In agreement with the investigators, Dr. Conroy said that the increased mortality rate among ICS users should not be misconstrued as a medication-related risk.
“While the study found that those with COPD or asthma taking ICS and high-dose ICS were at an increased risk of death, this could easily be explained by the likelihood that those are the patients who are more likely to have more severe underlying lung disease,” Dr. Conroy said. “While this observational study did attempt to control for exacerbation history, the ability to do so by electronic health records data is certainly imperfect.”
With this in mind, patients with chronic respiratory disease should be encouraged to adhere to their usual treatment regimen, Dr. Conroy added.
“There isn’t evidence to increase or decrease medications just because of the pandemic,” she said. “A patient with asthma or COPD should continue to take the medications that are needed to achieve good control of their lung disease.”
The study was funded by the U.K. Medical Research Council. The investigators reported additional relationships with the Wellcome Trust, the Good Thinking Foundation, the Laura and John Arnold Foundation, and others. Dr. Conroy reported no conflicts of interest.
SOURCE: Schultze A et al. Lancet Respir Med. 2020 Sep 24. doi: 10.1016/ S2213-2600(20)30415-X.
, a study of almost 1 million individuals in the United Kingdom has shown.
Patients with chronic obstructive pulmonary disease or asthma who used ICS on a regular basis were more likely to die from COVID-19 than COPD or asthma patients who were prescribed non-ICS therapies, reported co-lead author Anna Schultze, PhD, of London School of Hygiene & Tropical Medicine and colleagues.
Of note, the increased risk of death among ICS users likely stemmed from greater severity of preexisting chronic respiratory conditions, instead of directly from ICS usage, which has little apparent impact on COVID-19 mortality, the investigators wrote in Lancet Respiratory Medicine.
These findings conflict with a hypothesis proposed early in the pandemic: that ICS may protect individuals from SARS-CoV-2 infection and poor outcomes with COVID-19.
According to Megan Conroy, MD, of the department of internal medicine at the Ohio State University Wexner Medical Center, Columbus, this hypothesis was based on some unexpected epidemiological findings.
“In general, we tend to think people with underlying lung disease – like COPD or asthma – to be at higher risk for severe forms of lower respiratory tract infections,” Dr. Conroy said. “Somewhat surprisingly, early data in the pandemic showed patients with COPD and asthma [were] underrepresented [among patients with COVID] when compared to the prevalence of these diseases in the population.”
This raised the possibility of an incidental protective effect from regular ICS therapy, which “had some strong theoretic pathophysiologic basis,” Dr. Conroy said, referring to research that demonstrated ICS-mediated downregulation of SARS-CoV-2 entry receptors ACE2 and TMPRSS2.
Dr. Schultze and colleagues noted that investigators for two ongoing randomized controlled trials (NCT04331054, NCT04330586) are studying ICS as an intervention for COVID-19; but neither trial includes individuals already taking ICS for chronic respiratory disease.
The present observational study therefore aimed to assess mortality risk within this population. Data were drawn from electronic health records and a U.K. national mortality database, with follow-up ranging from March 1 to May 6, 2020. Eligibility required a relevant prescription within 4 months of first follow-up. In the COPD group, patients were prescribed a long-acting beta agonist plus a long-acting muscarinic antagonist (LABA–LAMA), LABA alone, LABA plus ICS, LABA–LAMA plus ICS, or ICS alone (if prescribed LABA within 4 months).
In the asthma group, patients received low/medium-dose ICS, high-dose ICS, or a short-acting beta agonist (SABA) alone. Patients with COPD were at least 35 years of age, while those with asthma were 18 years or older. Hazard ratios were adjusted for a variety of covariates, including respiratory disease–exacerbation history, age, sex, body mass index, hypertension, diabetes, and others.
These eligibility criteria returned 148,557 patients with COPD and 818,490 with asthma.
Patients with COPD who were prescribed ICS plus LABA-LAMA or ICS plus LABA had an increased risk of COVID-19-related death, compared with those who did not receive ICS (adjusted hazard ratio, 1.39; 95% confidence interval, 1.10-1.76). Separate analyses of patients who received a triple combination (LABA–LAMA plus ICS) versus those who took a dual combination (LABA plus ICS) showed that triple-combination therapy was significantly associated with increased COVID-19-related mortality (aHR, 1.43; 95% CI, 1.12-1.83), while dual-combination therapy was less so (aHR, 1.29; 95% CI, 0.96-1.74). Non–COVID-19–related mortality was significantly increased for all COPD patients who were prescribed ICS, with or without adjustment for covariates.
Asthma patients prescribed high-dose ICS instead of SABA alone had a slightly greater risk of COVID-19–related death, based on an adjusted hazard ratio of 1.55 (95% CI, 1.10-2.18). Those with asthma who received low/medium–dose ICS demonstrated a slight trend toward increased mortality risk, but this was not significant (aHR, 1.14; 95% CI, 0.85-1.54). ICS usage in the asthma group was not linked with a significant increase in non–COVID-19–related death.
“In summary, we found no evidence of a beneficial effect of regular ICS use among people with COPD and asthma on COVID-19–related mortality,” the investigators concluded.
In agreement with the investigators, Dr. Conroy said that the increased mortality rate among ICS users should not be misconstrued as a medication-related risk.
“While the study found that those with COPD or asthma taking ICS and high-dose ICS were at an increased risk of death, this could easily be explained by the likelihood that those are the patients who are more likely to have more severe underlying lung disease,” Dr. Conroy said. “While this observational study did attempt to control for exacerbation history, the ability to do so by electronic health records data is certainly imperfect.”
With this in mind, patients with chronic respiratory disease should be encouraged to adhere to their usual treatment regimen, Dr. Conroy added.
“There isn’t evidence to increase or decrease medications just because of the pandemic,” she said. “A patient with asthma or COPD should continue to take the medications that are needed to achieve good control of their lung disease.”
The study was funded by the U.K. Medical Research Council. The investigators reported additional relationships with the Wellcome Trust, the Good Thinking Foundation, the Laura and John Arnold Foundation, and others. Dr. Conroy reported no conflicts of interest.
SOURCE: Schultze A et al. Lancet Respir Med. 2020 Sep 24. doi: 10.1016/ S2213-2600(20)30415-X.
FROM LANCET RESPIRATORY MEDICINE
COVID-19 vaccine hesitancy ‘somewhat understandable,’ expert says
“I worry that vaccines are going to be sold like magic powder that we sprinkle across the land and make the virus go away,” Paul Offit, MD, said at the virtual American Academy of Pediatrics (AAP) 2020 National Conference. “That’s not true.”
according to Dr. Offit, director of the Vaccine Education Center and an attending physician in the Division of Infectious Diseases at Children’s Hospital of Philadelphia.
“I think we can get a vaccine that’s 75%-80% effective at preventing mild to moderate disease, but that means one of every four people can still get moderate to severe disease,” Dr. Offit continued.
And that’s if there is high uptake of the vaccine, which may not be the case. Recent polls have suggested there is considerable concern about the pending vaccines.
“It’s somewhat understandable,” Dr. Offitt acknowledged, especially given the “frightening” language used to describe vaccine development. Terms such as “warp speed” may suggest that haste might trump safety considerations. Before COVID-19, the fastest vaccine ever developed was for mumps, he said, with the virus isolated in 1963 and a commercial product available in 1967.
Addressing hesitancy in clinics
In a wide-ranging livestream plenary presentation, Dr. Offit, coinventor of a rotavirus vaccine, shed light on SARS-CoV-2 vaccine development and his impressions of vaccine hesitancy among patients and families. He also offered advice for how to reassure those skeptical of the safety and efficacy of any SARS-COV-2 vaccine, given the accelerated development process.
With more than 180 different vaccines in various stages of investigation, Dr. Offit called the effort to develop COVID-19 vaccines “unprecedented.” Part of that is a result of governments relieving pharmaceutical companies of much of the typical financial risk – which often climbs to hundreds of millions of dollars – by underwriting the costs of vaccine development to battle the pandemic-inducing virus, he said.
But this very swiftness is also stoking antivaccine sentiment. Dr. Offit, part of vaccine advisory groups for the National Institutes of Health and U.S. Food and Drug Administration, cited recent research reporting nearly half of American adults definitely or probably would not get a COVID-19 vaccine if it were available today.
“One way you convince skeptics is with data presented in a clear, compassionate, and compelling way,” he said.
“The other group is vaccine cynics, who are basically conspiracy theorists who believe pharmaceutical companies control the world, the government, the medical establishment. I think there’s no talking them down from this.”
Numerous strategies are being used in COVID-19 vaccine development, he noted, including messenger RNA, DNA, viral vectors, purified protein, and whole killed virus. Dr. Offit believes any candidates approved for distribution will likely be in the range of 75% effective at preventing mild to moderate symptoms.
But clinicians should be ready to face immediate questions of safety. “Even if this vaccination is given to 20,000 [trial participants] safely, that’s not 20 million,” Dr. Offit said. “Anyone could reasonably ask questions about if it causes rare, serious side effects.
“The good news is, there are systems in place,” such as adverse event reporting systems, to identify rare events, even those that occur in one in a million vaccine recipients. Reminding patients of that continued surveillance can be reassuring.
Another reassuring point is that COVID-19 vaccine trial participants have included people from many diverse populations, he said. But children, notably absent so far, should be added to trials immediately, Dr. Offit contends.
“This is going to be important when you consider strategies to get children universally back into school,” he said, which is a “critical issue” from both learning and wellness standpoints. “It breaks my heart that we’ve been unable to do this when other countries have.”
Transparency will be paramount
While presenting data transparently to patients is key in helping them accept COVID-19 vaccination, Dr. Offit said, he also believes “telling stories” can be just as effective, if not more so. When the varicella vaccine was approved in 1995, he said, the “uptake the first few years was pretty miserable” until public service messaging emphasized that some children die from chickenpox.
“Fear works,” he said. “You always worry about pushback of something being oversold, but hopefully we’re scared enough about this virus” to convince people that vaccination is wise. “I do think personal stories carry weight on both sides,” Dr. Offit said.
Mark Sawyer, MD, of University of California San Diego School of Medicine and Rady Children’s Hospital in San Diego, California, said Offit’s presentation offered important takeaways for clinicians about how to broach the topic of COVID-19 vaccination with patients and families.
“We need to communicate clearly and transparently to patients about what we do and don’t know” about the vaccines, Dr. Sawyer said in an interview. “We will know if they have common side effects, but we will not know about very rare side effects until we have used the vaccines for a while.
“We will know how well the vaccine works over the short-term, but we won’t know over the long term,” added Dr. Sawyer, a member of the AAP Committee on Infectious Diseases.
“We can reassure the community that SARS-CoV-2 vaccines are being evaluated in trials in the same way and with the same thoroughness as other vaccines have been,” he said. “That should give people confidence that shortcuts are not being taken with regard to safety and effectiveness evaluations.”
Dr. Offit and Dr. Sawyer have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
“I worry that vaccines are going to be sold like magic powder that we sprinkle across the land and make the virus go away,” Paul Offit, MD, said at the virtual American Academy of Pediatrics (AAP) 2020 National Conference. “That’s not true.”
according to Dr. Offit, director of the Vaccine Education Center and an attending physician in the Division of Infectious Diseases at Children’s Hospital of Philadelphia.
“I think we can get a vaccine that’s 75%-80% effective at preventing mild to moderate disease, but that means one of every four people can still get moderate to severe disease,” Dr. Offit continued.
And that’s if there is high uptake of the vaccine, which may not be the case. Recent polls have suggested there is considerable concern about the pending vaccines.
“It’s somewhat understandable,” Dr. Offitt acknowledged, especially given the “frightening” language used to describe vaccine development. Terms such as “warp speed” may suggest that haste might trump safety considerations. Before COVID-19, the fastest vaccine ever developed was for mumps, he said, with the virus isolated in 1963 and a commercial product available in 1967.
Addressing hesitancy in clinics
In a wide-ranging livestream plenary presentation, Dr. Offit, coinventor of a rotavirus vaccine, shed light on SARS-CoV-2 vaccine development and his impressions of vaccine hesitancy among patients and families. He also offered advice for how to reassure those skeptical of the safety and efficacy of any SARS-COV-2 vaccine, given the accelerated development process.
With more than 180 different vaccines in various stages of investigation, Dr. Offit called the effort to develop COVID-19 vaccines “unprecedented.” Part of that is a result of governments relieving pharmaceutical companies of much of the typical financial risk – which often climbs to hundreds of millions of dollars – by underwriting the costs of vaccine development to battle the pandemic-inducing virus, he said.
But this very swiftness is also stoking antivaccine sentiment. Dr. Offit, part of vaccine advisory groups for the National Institutes of Health and U.S. Food and Drug Administration, cited recent research reporting nearly half of American adults definitely or probably would not get a COVID-19 vaccine if it were available today.
“One way you convince skeptics is with data presented in a clear, compassionate, and compelling way,” he said.
“The other group is vaccine cynics, who are basically conspiracy theorists who believe pharmaceutical companies control the world, the government, the medical establishment. I think there’s no talking them down from this.”
Numerous strategies are being used in COVID-19 vaccine development, he noted, including messenger RNA, DNA, viral vectors, purified protein, and whole killed virus. Dr. Offit believes any candidates approved for distribution will likely be in the range of 75% effective at preventing mild to moderate symptoms.
But clinicians should be ready to face immediate questions of safety. “Even if this vaccination is given to 20,000 [trial participants] safely, that’s not 20 million,” Dr. Offit said. “Anyone could reasonably ask questions about if it causes rare, serious side effects.
“The good news is, there are systems in place,” such as adverse event reporting systems, to identify rare events, even those that occur in one in a million vaccine recipients. Reminding patients of that continued surveillance can be reassuring.
Another reassuring point is that COVID-19 vaccine trial participants have included people from many diverse populations, he said. But children, notably absent so far, should be added to trials immediately, Dr. Offit contends.
“This is going to be important when you consider strategies to get children universally back into school,” he said, which is a “critical issue” from both learning and wellness standpoints. “It breaks my heart that we’ve been unable to do this when other countries have.”
Transparency will be paramount
While presenting data transparently to patients is key in helping them accept COVID-19 vaccination, Dr. Offit said, he also believes “telling stories” can be just as effective, if not more so. When the varicella vaccine was approved in 1995, he said, the “uptake the first few years was pretty miserable” until public service messaging emphasized that some children die from chickenpox.
“Fear works,” he said. “You always worry about pushback of something being oversold, but hopefully we’re scared enough about this virus” to convince people that vaccination is wise. “I do think personal stories carry weight on both sides,” Dr. Offit said.
Mark Sawyer, MD, of University of California San Diego School of Medicine and Rady Children’s Hospital in San Diego, California, said Offit’s presentation offered important takeaways for clinicians about how to broach the topic of COVID-19 vaccination with patients and families.
“We need to communicate clearly and transparently to patients about what we do and don’t know” about the vaccines, Dr. Sawyer said in an interview. “We will know if they have common side effects, but we will not know about very rare side effects until we have used the vaccines for a while.
“We will know how well the vaccine works over the short-term, but we won’t know over the long term,” added Dr. Sawyer, a member of the AAP Committee on Infectious Diseases.
“We can reassure the community that SARS-CoV-2 vaccines are being evaluated in trials in the same way and with the same thoroughness as other vaccines have been,” he said. “That should give people confidence that shortcuts are not being taken with regard to safety and effectiveness evaluations.”
Dr. Offit and Dr. Sawyer have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
“I worry that vaccines are going to be sold like magic powder that we sprinkle across the land and make the virus go away,” Paul Offit, MD, said at the virtual American Academy of Pediatrics (AAP) 2020 National Conference. “That’s not true.”
according to Dr. Offit, director of the Vaccine Education Center and an attending physician in the Division of Infectious Diseases at Children’s Hospital of Philadelphia.
“I think we can get a vaccine that’s 75%-80% effective at preventing mild to moderate disease, but that means one of every four people can still get moderate to severe disease,” Dr. Offit continued.
And that’s if there is high uptake of the vaccine, which may not be the case. Recent polls have suggested there is considerable concern about the pending vaccines.
“It’s somewhat understandable,” Dr. Offitt acknowledged, especially given the “frightening” language used to describe vaccine development. Terms such as “warp speed” may suggest that haste might trump safety considerations. Before COVID-19, the fastest vaccine ever developed was for mumps, he said, with the virus isolated in 1963 and a commercial product available in 1967.
Addressing hesitancy in clinics
In a wide-ranging livestream plenary presentation, Dr. Offit, coinventor of a rotavirus vaccine, shed light on SARS-CoV-2 vaccine development and his impressions of vaccine hesitancy among patients and families. He also offered advice for how to reassure those skeptical of the safety and efficacy of any SARS-COV-2 vaccine, given the accelerated development process.
With more than 180 different vaccines in various stages of investigation, Dr. Offit called the effort to develop COVID-19 vaccines “unprecedented.” Part of that is a result of governments relieving pharmaceutical companies of much of the typical financial risk – which often climbs to hundreds of millions of dollars – by underwriting the costs of vaccine development to battle the pandemic-inducing virus, he said.
But this very swiftness is also stoking antivaccine sentiment. Dr. Offit, part of vaccine advisory groups for the National Institutes of Health and U.S. Food and Drug Administration, cited recent research reporting nearly half of American adults definitely or probably would not get a COVID-19 vaccine if it were available today.
“One way you convince skeptics is with data presented in a clear, compassionate, and compelling way,” he said.
“The other group is vaccine cynics, who are basically conspiracy theorists who believe pharmaceutical companies control the world, the government, the medical establishment. I think there’s no talking them down from this.”
Numerous strategies are being used in COVID-19 vaccine development, he noted, including messenger RNA, DNA, viral vectors, purified protein, and whole killed virus. Dr. Offit believes any candidates approved for distribution will likely be in the range of 75% effective at preventing mild to moderate symptoms.
But clinicians should be ready to face immediate questions of safety. “Even if this vaccination is given to 20,000 [trial participants] safely, that’s not 20 million,” Dr. Offit said. “Anyone could reasonably ask questions about if it causes rare, serious side effects.
“The good news is, there are systems in place,” such as adverse event reporting systems, to identify rare events, even those that occur in one in a million vaccine recipients. Reminding patients of that continued surveillance can be reassuring.
Another reassuring point is that COVID-19 vaccine trial participants have included people from many diverse populations, he said. But children, notably absent so far, should be added to trials immediately, Dr. Offit contends.
“This is going to be important when you consider strategies to get children universally back into school,” he said, which is a “critical issue” from both learning and wellness standpoints. “It breaks my heart that we’ve been unable to do this when other countries have.”
Transparency will be paramount
While presenting data transparently to patients is key in helping them accept COVID-19 vaccination, Dr. Offit said, he also believes “telling stories” can be just as effective, if not more so. When the varicella vaccine was approved in 1995, he said, the “uptake the first few years was pretty miserable” until public service messaging emphasized that some children die from chickenpox.
“Fear works,” he said. “You always worry about pushback of something being oversold, but hopefully we’re scared enough about this virus” to convince people that vaccination is wise. “I do think personal stories carry weight on both sides,” Dr. Offit said.
Mark Sawyer, MD, of University of California San Diego School of Medicine and Rady Children’s Hospital in San Diego, California, said Offit’s presentation offered important takeaways for clinicians about how to broach the topic of COVID-19 vaccination with patients and families.
“We need to communicate clearly and transparently to patients about what we do and don’t know” about the vaccines, Dr. Sawyer said in an interview. “We will know if they have common side effects, but we will not know about very rare side effects until we have used the vaccines for a while.
“We will know how well the vaccine works over the short-term, but we won’t know over the long term,” added Dr. Sawyer, a member of the AAP Committee on Infectious Diseases.
“We can reassure the community that SARS-CoV-2 vaccines are being evaluated in trials in the same way and with the same thoroughness as other vaccines have been,” he said. “That should give people confidence that shortcuts are not being taken with regard to safety and effectiveness evaluations.”
Dr. Offit and Dr. Sawyer have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
COVID-19 and the superspreaders: Teens
Although cases of COVID-19 in children is reported to be low, we are seeing a surge in Wisconsin with a 27.6% positivity rate reported on Sept. 27. Numerous other states across the country are reporting similar jumps of 10% or more.
According to the Wisconsin Department of Health Services as of Sept. 20, 2020, there were 10,644 cumulative cases in persons aged less than 18 years. This rise in cases is consistent with a return to school and sports. This cumulative case load amounts to 836.7/100, 000 cases. This population may not experience the level of illness seen in the older populations with hospitalization rates of only 3% under the age of 9 years and 13% of those age 10- 19-years, yet exposing older family and members of the community is driving the death rates. The combined influenza and COVID-19 season may greatly impact hospitalization rates of young and old. Additionally, we may see a surge in pediatric cancer rates and autoimmune diseases secondary to these trends.
I believe the overall number of adolescents with COVID-19 is underreported. Teens admit to a lack of understanding of symptoms. Many do not realize they have COVID-19 until someone points out the symptoms they describe such as a loss of taste or smell are COVID-19 symptoms. Others report they do not report symptoms to prevent quarantine. Additionally, others endorse ridicule from peers if they have tested positive and contract tracing identifies others potentially exposed and forced to sit out of sports because of quarantine. They have been bullied into amnesia when contract tracers call to prevent identifying others at school or in the community. All these behaviors proliferate the spread of disease within the community and will continue to drive both exposures and death rates.
Teens in high schools require increased education of the symptoms of COVID-19, promotion of the flu vaccine, and knowledge of the impact they can have on preventing the spread of viruses.
Ms. Thew is the medical director of the department of adolescent medicine at Children’s Wisconsin in Milwaukee. She is a member of the Pediatric News editorial advisory board. She said she had no relevant financial disclosures. Email her at [email protected].
Reference
COVID-19: Wisconsin Cases, Wisconsin Department of Health Services. Accessed 2020 Sep 27.
Although cases of COVID-19 in children is reported to be low, we are seeing a surge in Wisconsin with a 27.6% positivity rate reported on Sept. 27. Numerous other states across the country are reporting similar jumps of 10% or more.
According to the Wisconsin Department of Health Services as of Sept. 20, 2020, there were 10,644 cumulative cases in persons aged less than 18 years. This rise in cases is consistent with a return to school and sports. This cumulative case load amounts to 836.7/100, 000 cases. This population may not experience the level of illness seen in the older populations with hospitalization rates of only 3% under the age of 9 years and 13% of those age 10- 19-years, yet exposing older family and members of the community is driving the death rates. The combined influenza and COVID-19 season may greatly impact hospitalization rates of young and old. Additionally, we may see a surge in pediatric cancer rates and autoimmune diseases secondary to these trends.
I believe the overall number of adolescents with COVID-19 is underreported. Teens admit to a lack of understanding of symptoms. Many do not realize they have COVID-19 until someone points out the symptoms they describe such as a loss of taste or smell are COVID-19 symptoms. Others report they do not report symptoms to prevent quarantine. Additionally, others endorse ridicule from peers if they have tested positive and contract tracing identifies others potentially exposed and forced to sit out of sports because of quarantine. They have been bullied into amnesia when contract tracers call to prevent identifying others at school or in the community. All these behaviors proliferate the spread of disease within the community and will continue to drive both exposures and death rates.
Teens in high schools require increased education of the symptoms of COVID-19, promotion of the flu vaccine, and knowledge of the impact they can have on preventing the spread of viruses.
Ms. Thew is the medical director of the department of adolescent medicine at Children’s Wisconsin in Milwaukee. She is a member of the Pediatric News editorial advisory board. She said she had no relevant financial disclosures. Email her at [email protected].
Reference
COVID-19: Wisconsin Cases, Wisconsin Department of Health Services. Accessed 2020 Sep 27.
Although cases of COVID-19 in children is reported to be low, we are seeing a surge in Wisconsin with a 27.6% positivity rate reported on Sept. 27. Numerous other states across the country are reporting similar jumps of 10% or more.
According to the Wisconsin Department of Health Services as of Sept. 20, 2020, there were 10,644 cumulative cases in persons aged less than 18 years. This rise in cases is consistent with a return to school and sports. This cumulative case load amounts to 836.7/100, 000 cases. This population may not experience the level of illness seen in the older populations with hospitalization rates of only 3% under the age of 9 years and 13% of those age 10- 19-years, yet exposing older family and members of the community is driving the death rates. The combined influenza and COVID-19 season may greatly impact hospitalization rates of young and old. Additionally, we may see a surge in pediatric cancer rates and autoimmune diseases secondary to these trends.
I believe the overall number of adolescents with COVID-19 is underreported. Teens admit to a lack of understanding of symptoms. Many do not realize they have COVID-19 until someone points out the symptoms they describe such as a loss of taste or smell are COVID-19 symptoms. Others report they do not report symptoms to prevent quarantine. Additionally, others endorse ridicule from peers if they have tested positive and contract tracing identifies others potentially exposed and forced to sit out of sports because of quarantine. They have been bullied into amnesia when contract tracers call to prevent identifying others at school or in the community. All these behaviors proliferate the spread of disease within the community and will continue to drive both exposures and death rates.
Teens in high schools require increased education of the symptoms of COVID-19, promotion of the flu vaccine, and knowledge of the impact they can have on preventing the spread of viruses.
Ms. Thew is the medical director of the department of adolescent medicine at Children’s Wisconsin in Milwaukee. She is a member of the Pediatric News editorial advisory board. She said she had no relevant financial disclosures. Email her at [email protected].
Reference
COVID-19: Wisconsin Cases, Wisconsin Department of Health Services. Accessed 2020 Sep 27.
Pediatric fractures shift during pandemic
Pediatric fractures dropped by 2.5-fold during the early months of the COVID-19 pandemic, but more breaks happened at home and on bicycles, and younger kids were more affected, new research indicates.
The study of 1,745 patients also found that those with distal radius torus fractures were more likely to receive a Velcro splint during the pandemic. Experts said this key trend points toward widespread shifts to streamline treatment, which should persist after the pandemic.
“We expected to see a drop in fracture volume, but what was a bit unexpected was the proportional rise in at-home injuries, which we weren’t immediately aware of,” said senior author Apurva Shah, MD, MBA, of Children’s Hospital of Philadelphia (CHOP) and the University of Pennsylvania in Philadelphia.
“As time went on, it became more apparent that trampoline and bicycle injuries were on the rise, but at the beginning of the pandemic, we didn’t intuitively expect that,” he added.
“Whenever there’s a major shift in how the world is working, we want to understand how that impacts child safety,” Dr. Shah said in an interview. “The message to get out to parents is that it’s obviously difficult to supervise kids while working from home” during the pandemic “and that supervision obviously is not always working as well as intended.”
Joshua T. Bram, a medical student, presented the study at the virtual American Academy of Pediatrics (AAP) 2020 National Conference.
Dr. Bram, Dr. Shah, and colleagues compared patients with acute fractures who presented at CHOP between March and April 2020 with those who presented during the same months in 2018 and 2019.
Overall, the number of patients with pediatric fractures who presented to CHOP fell to an average of just under 10 per day, compared with more than 22 per day in prior years (P < .001). In addition, the age of the patients fell from an average of 9.4 years to 7.5 years (P < .001), with fewer adolescents affected in 2020.
“I think when you cancel a 14-year-old’s baseball season” because of the pandemic, “unfortunately, that lost outdoor time might be substituted with time on a screen,” he explained. “But canceling a 6-year-old’s soccer season might mean substituting that with more time outside on bikes or on a trampoline.”
As noted, because of the pandemic, a higher proportion of pediatric fractures occurred at home (57.8% vs. 32.5%; P < .001) or on bicycles (18.3% vs. 8.2%; P < .001), but there were fewer organized sports–related (7.2% vs. 26.0%; P < .001) or playground-related injuries (5.2% vs. 9.0%; P < .001).
In the study period this year, the researchers saw no increase in the amount of time between injury and presentation. However, data suggest that, in more recent months, “kids are presenting with fractures late, with sometimes great consequences,” Dr. Shah said.
“What has changed is that a lot of adults have lost their jobs, and as a consequence, a lot of children have lost their access to private insurance,” he said. “But fracture is really a major injury, and this is a reminder for pediatricians and primary care physicians to recognize that families are going through these changes and that delays in care can really be detrimental to children.”
Velcro splints more common
A potential upside to shifts seen during the pandemic, Dr. Shah said, is the finding that distal radius torus fractures were more likely to be treated with a Velcro splint than in previous years (44.2% vs. 25.9%; P = .010).
“This is hitting on something important – that sometimes it’s crisis that forces us as physicians to evolve,” he said. “This is something I think is here to stay.
“Although research had already been there suggesting a close equivalent between splints and casting, culturally, a lot of surgeons hadn’t made that shift when historically the gold standard had been casting,” Dr. Shah added. “But with the pandemic, the shift to minimize contact with the health care system to keep families safe in their COVID bubble helped [usage of] splints take off.
“I suspect – and we’ll only know when we’re on the other side of this – when physicians see good results in splints in their own patients, they’re going to adopt those strategies more permanently,” he said.
Benjamin Shore, MD, MPH, of Boston Children’s Hospital, agreed with Dr. Shah’s prediction that fracture care will be more streamlined after the pandemic. Dr. Shore, who wasn’t involved in the study, said not only are more orthopedic providers treating patients with Velcro splints and bivalve casts, but they are also monitoring patients via telehealth.
“All of these are great examples of innovation, and one of the unique parts of the pandemic is it created a lot of rapid change across healthcare because it caused us to scrutinize the ways we practice and make a change,” Dr. Shore said in an interview.
“It wasn’t a very fancy study, but it’s very important in terms of demonstrating a change in practice,” Dr. Shore said. “The research here basically validated what many of us are seeing and hopefully will help us in future pandemics – which hopefully won’t happen – to tell families what to be proactive about.”
Dr. Shah and Dr. Shore agreed that, because fewer fractures are occurring in kids during the pandemic, there is an opportunity to redeploy orthopedic providers to other clinical areas on the basis of volume and need.
Dr. Shah and Dr. Shore have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Pediatric fractures dropped by 2.5-fold during the early months of the COVID-19 pandemic, but more breaks happened at home and on bicycles, and younger kids were more affected, new research indicates.
The study of 1,745 patients also found that those with distal radius torus fractures were more likely to receive a Velcro splint during the pandemic. Experts said this key trend points toward widespread shifts to streamline treatment, which should persist after the pandemic.
“We expected to see a drop in fracture volume, but what was a bit unexpected was the proportional rise in at-home injuries, which we weren’t immediately aware of,” said senior author Apurva Shah, MD, MBA, of Children’s Hospital of Philadelphia (CHOP) and the University of Pennsylvania in Philadelphia.
“As time went on, it became more apparent that trampoline and bicycle injuries were on the rise, but at the beginning of the pandemic, we didn’t intuitively expect that,” he added.
“Whenever there’s a major shift in how the world is working, we want to understand how that impacts child safety,” Dr. Shah said in an interview. “The message to get out to parents is that it’s obviously difficult to supervise kids while working from home” during the pandemic “and that supervision obviously is not always working as well as intended.”
Joshua T. Bram, a medical student, presented the study at the virtual American Academy of Pediatrics (AAP) 2020 National Conference.
Dr. Bram, Dr. Shah, and colleagues compared patients with acute fractures who presented at CHOP between March and April 2020 with those who presented during the same months in 2018 and 2019.
Overall, the number of patients with pediatric fractures who presented to CHOP fell to an average of just under 10 per day, compared with more than 22 per day in prior years (P < .001). In addition, the age of the patients fell from an average of 9.4 years to 7.5 years (P < .001), with fewer adolescents affected in 2020.
“I think when you cancel a 14-year-old’s baseball season” because of the pandemic, “unfortunately, that lost outdoor time might be substituted with time on a screen,” he explained. “But canceling a 6-year-old’s soccer season might mean substituting that with more time outside on bikes or on a trampoline.”
As noted, because of the pandemic, a higher proportion of pediatric fractures occurred at home (57.8% vs. 32.5%; P < .001) or on bicycles (18.3% vs. 8.2%; P < .001), but there were fewer organized sports–related (7.2% vs. 26.0%; P < .001) or playground-related injuries (5.2% vs. 9.0%; P < .001).
In the study period this year, the researchers saw no increase in the amount of time between injury and presentation. However, data suggest that, in more recent months, “kids are presenting with fractures late, with sometimes great consequences,” Dr. Shah said.
“What has changed is that a lot of adults have lost their jobs, and as a consequence, a lot of children have lost their access to private insurance,” he said. “But fracture is really a major injury, and this is a reminder for pediatricians and primary care physicians to recognize that families are going through these changes and that delays in care can really be detrimental to children.”
Velcro splints more common
A potential upside to shifts seen during the pandemic, Dr. Shah said, is the finding that distal radius torus fractures were more likely to be treated with a Velcro splint than in previous years (44.2% vs. 25.9%; P = .010).
“This is hitting on something important – that sometimes it’s crisis that forces us as physicians to evolve,” he said. “This is something I think is here to stay.
“Although research had already been there suggesting a close equivalent between splints and casting, culturally, a lot of surgeons hadn’t made that shift when historically the gold standard had been casting,” Dr. Shah added. “But with the pandemic, the shift to minimize contact with the health care system to keep families safe in their COVID bubble helped [usage of] splints take off.
“I suspect – and we’ll only know when we’re on the other side of this – when physicians see good results in splints in their own patients, they’re going to adopt those strategies more permanently,” he said.
Benjamin Shore, MD, MPH, of Boston Children’s Hospital, agreed with Dr. Shah’s prediction that fracture care will be more streamlined after the pandemic. Dr. Shore, who wasn’t involved in the study, said not only are more orthopedic providers treating patients with Velcro splints and bivalve casts, but they are also monitoring patients via telehealth.
“All of these are great examples of innovation, and one of the unique parts of the pandemic is it created a lot of rapid change across healthcare because it caused us to scrutinize the ways we practice and make a change,” Dr. Shore said in an interview.
“It wasn’t a very fancy study, but it’s very important in terms of demonstrating a change in practice,” Dr. Shore said. “The research here basically validated what many of us are seeing and hopefully will help us in future pandemics – which hopefully won’t happen – to tell families what to be proactive about.”
Dr. Shah and Dr. Shore agreed that, because fewer fractures are occurring in kids during the pandemic, there is an opportunity to redeploy orthopedic providers to other clinical areas on the basis of volume and need.
Dr. Shah and Dr. Shore have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Pediatric fractures dropped by 2.5-fold during the early months of the COVID-19 pandemic, but more breaks happened at home and on bicycles, and younger kids were more affected, new research indicates.
The study of 1,745 patients also found that those with distal radius torus fractures were more likely to receive a Velcro splint during the pandemic. Experts said this key trend points toward widespread shifts to streamline treatment, which should persist after the pandemic.
“We expected to see a drop in fracture volume, but what was a bit unexpected was the proportional rise in at-home injuries, which we weren’t immediately aware of,” said senior author Apurva Shah, MD, MBA, of Children’s Hospital of Philadelphia (CHOP) and the University of Pennsylvania in Philadelphia.
“As time went on, it became more apparent that trampoline and bicycle injuries were on the rise, but at the beginning of the pandemic, we didn’t intuitively expect that,” he added.
“Whenever there’s a major shift in how the world is working, we want to understand how that impacts child safety,” Dr. Shah said in an interview. “The message to get out to parents is that it’s obviously difficult to supervise kids while working from home” during the pandemic “and that supervision obviously is not always working as well as intended.”
Joshua T. Bram, a medical student, presented the study at the virtual American Academy of Pediatrics (AAP) 2020 National Conference.
Dr. Bram, Dr. Shah, and colleagues compared patients with acute fractures who presented at CHOP between March and April 2020 with those who presented during the same months in 2018 and 2019.
Overall, the number of patients with pediatric fractures who presented to CHOP fell to an average of just under 10 per day, compared with more than 22 per day in prior years (P < .001). In addition, the age of the patients fell from an average of 9.4 years to 7.5 years (P < .001), with fewer adolescents affected in 2020.
“I think when you cancel a 14-year-old’s baseball season” because of the pandemic, “unfortunately, that lost outdoor time might be substituted with time on a screen,” he explained. “But canceling a 6-year-old’s soccer season might mean substituting that with more time outside on bikes or on a trampoline.”
As noted, because of the pandemic, a higher proportion of pediatric fractures occurred at home (57.8% vs. 32.5%; P < .001) or on bicycles (18.3% vs. 8.2%; P < .001), but there were fewer organized sports–related (7.2% vs. 26.0%; P < .001) or playground-related injuries (5.2% vs. 9.0%; P < .001).
In the study period this year, the researchers saw no increase in the amount of time between injury and presentation. However, data suggest that, in more recent months, “kids are presenting with fractures late, with sometimes great consequences,” Dr. Shah said.
“What has changed is that a lot of adults have lost their jobs, and as a consequence, a lot of children have lost their access to private insurance,” he said. “But fracture is really a major injury, and this is a reminder for pediatricians and primary care physicians to recognize that families are going through these changes and that delays in care can really be detrimental to children.”
Velcro splints more common
A potential upside to shifts seen during the pandemic, Dr. Shah said, is the finding that distal radius torus fractures were more likely to be treated with a Velcro splint than in previous years (44.2% vs. 25.9%; P = .010).
“This is hitting on something important – that sometimes it’s crisis that forces us as physicians to evolve,” he said. “This is something I think is here to stay.
“Although research had already been there suggesting a close equivalent between splints and casting, culturally, a lot of surgeons hadn’t made that shift when historically the gold standard had been casting,” Dr. Shah added. “But with the pandemic, the shift to minimize contact with the health care system to keep families safe in their COVID bubble helped [usage of] splints take off.
“I suspect – and we’ll only know when we’re on the other side of this – when physicians see good results in splints in their own patients, they’re going to adopt those strategies more permanently,” he said.
Benjamin Shore, MD, MPH, of Boston Children’s Hospital, agreed with Dr. Shah’s prediction that fracture care will be more streamlined after the pandemic. Dr. Shore, who wasn’t involved in the study, said not only are more orthopedic providers treating patients with Velcro splints and bivalve casts, but they are also monitoring patients via telehealth.
“All of these are great examples of innovation, and one of the unique parts of the pandemic is it created a lot of rapid change across healthcare because it caused us to scrutinize the ways we practice and make a change,” Dr. Shore said in an interview.
“It wasn’t a very fancy study, but it’s very important in terms of demonstrating a change in practice,” Dr. Shore said. “The research here basically validated what many of us are seeing and hopefully will help us in future pandemics – which hopefully won’t happen – to tell families what to be proactive about.”
Dr. Shah and Dr. Shore agreed that, because fewer fractures are occurring in kids during the pandemic, there is an opportunity to redeploy orthopedic providers to other clinical areas on the basis of volume and need.
Dr. Shah and Dr. Shore have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
One measure of child COVID-19 may be trending downward
After increasing for several weeks, the proportion of new COVID-19 cases occurring in children has dropped for the second week in a row, according to data in a new report from the American Academy of Pediatrics and the Children’s Hospital Association.
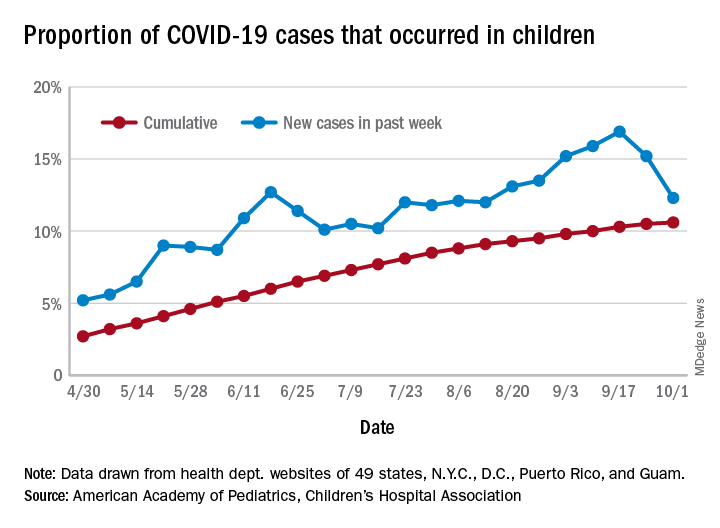
COVID-19 cases in children accounted for 12.3% of all new cases in the United States for the week ending Oct. 1, down from 15.2% the previous week. That measure had reached its highest point, 16.9%, just one week earlier (Sept. 17), the AAP and the CHA said in their weekly COVID-19 report.
based on data from the health departments of 49 states (New York does not provide ages on its website), as well as the District of Columbia, New York City, Puerto Rico, and Guam.
The child COVID-19 rate for the United States was 874 per 100,000 children as of Oct. 1, and that figure has doubled since the end of July. At the state level, the highest rates can be found in Tennessee (2,031.4 per 100,000), North Dakota (2,029.6), and South Carolina (2,002.6), with the lowest rates in Vermont (168.9), Maine (229.1), and New Hampshire (268.3), the AAP/CHA report shows.
The children of Wyoming make up the largest share, 22.4%, of any state’s COVID-19 cases, followed by North Dakota and Tennessee, both at 18.3%. New Jersey is lower than any other state at 3.9%, although New York City is a slightly lower 3.6%, the AAP and CHA said.
“The data are limited because the states differ in how they report the data, and it is unknown how many children have been infected but not tested. It is unclear how much of the increase in child cases is due to increased testing capacity,” the AAP said in an earlier statement.
After increasing for several weeks, the proportion of new COVID-19 cases occurring in children has dropped for the second week in a row, according to data in a new report from the American Academy of Pediatrics and the Children’s Hospital Association.

COVID-19 cases in children accounted for 12.3% of all new cases in the United States for the week ending Oct. 1, down from 15.2% the previous week. That measure had reached its highest point, 16.9%, just one week earlier (Sept. 17), the AAP and the CHA said in their weekly COVID-19 report.
based on data from the health departments of 49 states (New York does not provide ages on its website), as well as the District of Columbia, New York City, Puerto Rico, and Guam.
The child COVID-19 rate for the United States was 874 per 100,000 children as of Oct. 1, and that figure has doubled since the end of July. At the state level, the highest rates can be found in Tennessee (2,031.4 per 100,000), North Dakota (2,029.6), and South Carolina (2,002.6), with the lowest rates in Vermont (168.9), Maine (229.1), and New Hampshire (268.3), the AAP/CHA report shows.
The children of Wyoming make up the largest share, 22.4%, of any state’s COVID-19 cases, followed by North Dakota and Tennessee, both at 18.3%. New Jersey is lower than any other state at 3.9%, although New York City is a slightly lower 3.6%, the AAP and CHA said.
“The data are limited because the states differ in how they report the data, and it is unknown how many children have been infected but not tested. It is unclear how much of the increase in child cases is due to increased testing capacity,” the AAP said in an earlier statement.
After increasing for several weeks, the proportion of new COVID-19 cases occurring in children has dropped for the second week in a row, according to data in a new report from the American Academy of Pediatrics and the Children’s Hospital Association.

COVID-19 cases in children accounted for 12.3% of all new cases in the United States for the week ending Oct. 1, down from 15.2% the previous week. That measure had reached its highest point, 16.9%, just one week earlier (Sept. 17), the AAP and the CHA said in their weekly COVID-19 report.
based on data from the health departments of 49 states (New York does not provide ages on its website), as well as the District of Columbia, New York City, Puerto Rico, and Guam.
The child COVID-19 rate for the United States was 874 per 100,000 children as of Oct. 1, and that figure has doubled since the end of July. At the state level, the highest rates can be found in Tennessee (2,031.4 per 100,000), North Dakota (2,029.6), and South Carolina (2,002.6), with the lowest rates in Vermont (168.9), Maine (229.1), and New Hampshire (268.3), the AAP/CHA report shows.
The children of Wyoming make up the largest share, 22.4%, of any state’s COVID-19 cases, followed by North Dakota and Tennessee, both at 18.3%. New Jersey is lower than any other state at 3.9%, although New York City is a slightly lower 3.6%, the AAP and CHA said.
“The data are limited because the states differ in how they report the data, and it is unknown how many children have been infected but not tested. It is unclear how much of the increase in child cases is due to increased testing capacity,” the AAP said in an earlier statement.
CMS gives hospitals 14 weeks to start daily COVID, flu reports
The federal government is giving hospitals 14 weeks to comply with daily reporting requirements for COVID-19.
The Centers for Medicare & Medicaid Services will send letters on October 7 to all 6,200 hospitals that receive reimbursement from the two federal health programs informing them of how well they are doing now, said CMS Administrator Seema Verma on a press call.
Verma would not give an estimate on how many hospitals are currently not compliant. But Deborah Birx, MD, a member of the White House Coronavirus Task Force, said on the call that 86% of hospitals are currently reporting daily.
Federal officials on the call also announced that hospitals would have the option to begin reporting certain data on influenza starting October 19, but that it would become mandatory a few weeks later.
The reporting is important “to really ensure that we’re triangulating all data to understand where this epidemic is, how it’s moving through different populations, and ensuring that we’re meeting the needs of specific hospitals and communities,” Birx said.
The federal government began a new hospital reporting system in April but did not require hospitals to participate until it quietly issued guidance in mid-July informing facilities that they should no longer report to the Centers for Disease Control and Prevention (CDC).
The move perplexed many public health experts and epidemiologists, who expressed concern that asking hospitals to use a new data system during a pandemic could result in delays and lost information. The new HHS data collection site, HHS Protect, is being managed by a private contractor, not the CDC, which also raised alarms.
The final CMS rule issued in August went into effect immediately, without any chance for comment or revision. CMS said at the time that the pandemic was reason enough to skip over the normal bureaucratic process.
Hospitals were not pleased. But Verma claimed that since then CMS had been working with hospital organizations on enforcement.
“We’re going to do everything we can to facilitate reporting, including an enforcement timeline that will provide hospitals ample opportunity to come into compliance,” she said.
Hospitals that do not comply will get a notice every 3 weeks. Three weeks after the second notice, they’ll get weekly notices for a month, and a final termination notice at 14 weeks.
The Federation of American Hospitals (FAH), however, said their members were still not happy. “It is both inappropriate and frankly overkill for CMS to tie compliance with reporting to Medicare conditions of participation,” said FAH President and CEO Chip Kahn in a statement. He called the CMS proposal “sledgehammer enforcement,” and said that the continuing data request might weaken hospitals’ response to the pandemic because it would divert time and money away from patient care.
Rick Pollack, president and CEO of the American Hospital Association called the CMS rule an “overly heavy-handed approach that could jeopardize access to hospital care for all Americans.” He noted in a statement that barring hospitals from Medicare and Medicaid could harm beneficiaries and the effort to provide COVID care.
Pollack also noted that AHA has “observed errors in data processing and confusion about exactly what was being requested at the hospital, state, contractor, and federal level, and has worked diligently with the federal agencies to identify and correct those problems.”
The document that lays out U.S. Department of Health and Human Services (HHS) Protect reporting requirements were updated again on October 6 to add influenza data. The hospitals must report on total patients with laboratory-confirmed flu; previous day’s flu admissions; total ICU patients with lab-confirmed flu; total inpatients with either flu or COVID-19; and the previous day’s deaths for flu and COVID.
CDC Director Robert Redfield, MD, said on the press call that the new data will give the agency crucial hospital-level information and perhaps better estimates of the flu burden. Flu trends have been tracked using the CDC’s Influenza Hospitalization Surveillance Network (FluSurv-NET), which will not be replaced, Redfield said. But that network only tracks hospitalizations in 14 states and does not provide information in “nearly real-time,” he said.
Having the new data “will give us a true situational awareness of severe respiratory illness, provide local hospitalization trends, and help direct resources such as antiretrovirals to address potential increased impact of flu and COVID cocirculation,” Redfield said.
This article first appeared on Medscape.com.
The federal government is giving hospitals 14 weeks to comply with daily reporting requirements for COVID-19.
The Centers for Medicare & Medicaid Services will send letters on October 7 to all 6,200 hospitals that receive reimbursement from the two federal health programs informing them of how well they are doing now, said CMS Administrator Seema Verma on a press call.
Verma would not give an estimate on how many hospitals are currently not compliant. But Deborah Birx, MD, a member of the White House Coronavirus Task Force, said on the call that 86% of hospitals are currently reporting daily.
Federal officials on the call also announced that hospitals would have the option to begin reporting certain data on influenza starting October 19, but that it would become mandatory a few weeks later.
The reporting is important “to really ensure that we’re triangulating all data to understand where this epidemic is, how it’s moving through different populations, and ensuring that we’re meeting the needs of specific hospitals and communities,” Birx said.
The federal government began a new hospital reporting system in April but did not require hospitals to participate until it quietly issued guidance in mid-July informing facilities that they should no longer report to the Centers for Disease Control and Prevention (CDC).
The move perplexed many public health experts and epidemiologists, who expressed concern that asking hospitals to use a new data system during a pandemic could result in delays and lost information. The new HHS data collection site, HHS Protect, is being managed by a private contractor, not the CDC, which also raised alarms.
The final CMS rule issued in August went into effect immediately, without any chance for comment or revision. CMS said at the time that the pandemic was reason enough to skip over the normal bureaucratic process.
Hospitals were not pleased. But Verma claimed that since then CMS had been working with hospital organizations on enforcement.
“We’re going to do everything we can to facilitate reporting, including an enforcement timeline that will provide hospitals ample opportunity to come into compliance,” she said.
Hospitals that do not comply will get a notice every 3 weeks. Three weeks after the second notice, they’ll get weekly notices for a month, and a final termination notice at 14 weeks.
The Federation of American Hospitals (FAH), however, said their members were still not happy. “It is both inappropriate and frankly overkill for CMS to tie compliance with reporting to Medicare conditions of participation,” said FAH President and CEO Chip Kahn in a statement. He called the CMS proposal “sledgehammer enforcement,” and said that the continuing data request might weaken hospitals’ response to the pandemic because it would divert time and money away from patient care.
Rick Pollack, president and CEO of the American Hospital Association called the CMS rule an “overly heavy-handed approach that could jeopardize access to hospital care for all Americans.” He noted in a statement that barring hospitals from Medicare and Medicaid could harm beneficiaries and the effort to provide COVID care.
Pollack also noted that AHA has “observed errors in data processing and confusion about exactly what was being requested at the hospital, state, contractor, and federal level, and has worked diligently with the federal agencies to identify and correct those problems.”
The document that lays out U.S. Department of Health and Human Services (HHS) Protect reporting requirements were updated again on October 6 to add influenza data. The hospitals must report on total patients with laboratory-confirmed flu; previous day’s flu admissions; total ICU patients with lab-confirmed flu; total inpatients with either flu or COVID-19; and the previous day’s deaths for flu and COVID.
CDC Director Robert Redfield, MD, said on the press call that the new data will give the agency crucial hospital-level information and perhaps better estimates of the flu burden. Flu trends have been tracked using the CDC’s Influenza Hospitalization Surveillance Network (FluSurv-NET), which will not be replaced, Redfield said. But that network only tracks hospitalizations in 14 states and does not provide information in “nearly real-time,” he said.
Having the new data “will give us a true situational awareness of severe respiratory illness, provide local hospitalization trends, and help direct resources such as antiretrovirals to address potential increased impact of flu and COVID cocirculation,” Redfield said.
This article first appeared on Medscape.com.
The federal government is giving hospitals 14 weeks to comply with daily reporting requirements for COVID-19.
The Centers for Medicare & Medicaid Services will send letters on October 7 to all 6,200 hospitals that receive reimbursement from the two federal health programs informing them of how well they are doing now, said CMS Administrator Seema Verma on a press call.
Verma would not give an estimate on how many hospitals are currently not compliant. But Deborah Birx, MD, a member of the White House Coronavirus Task Force, said on the call that 86% of hospitals are currently reporting daily.
Federal officials on the call also announced that hospitals would have the option to begin reporting certain data on influenza starting October 19, but that it would become mandatory a few weeks later.
The reporting is important “to really ensure that we’re triangulating all data to understand where this epidemic is, how it’s moving through different populations, and ensuring that we’re meeting the needs of specific hospitals and communities,” Birx said.
The federal government began a new hospital reporting system in April but did not require hospitals to participate until it quietly issued guidance in mid-July informing facilities that they should no longer report to the Centers for Disease Control and Prevention (CDC).
The move perplexed many public health experts and epidemiologists, who expressed concern that asking hospitals to use a new data system during a pandemic could result in delays and lost information. The new HHS data collection site, HHS Protect, is being managed by a private contractor, not the CDC, which also raised alarms.
The final CMS rule issued in August went into effect immediately, without any chance for comment or revision. CMS said at the time that the pandemic was reason enough to skip over the normal bureaucratic process.
Hospitals were not pleased. But Verma claimed that since then CMS had been working with hospital organizations on enforcement.
“We’re going to do everything we can to facilitate reporting, including an enforcement timeline that will provide hospitals ample opportunity to come into compliance,” she said.
Hospitals that do not comply will get a notice every 3 weeks. Three weeks after the second notice, they’ll get weekly notices for a month, and a final termination notice at 14 weeks.
The Federation of American Hospitals (FAH), however, said their members were still not happy. “It is both inappropriate and frankly overkill for CMS to tie compliance with reporting to Medicare conditions of participation,” said FAH President and CEO Chip Kahn in a statement. He called the CMS proposal “sledgehammer enforcement,” and said that the continuing data request might weaken hospitals’ response to the pandemic because it would divert time and money away from patient care.
Rick Pollack, president and CEO of the American Hospital Association called the CMS rule an “overly heavy-handed approach that could jeopardize access to hospital care for all Americans.” He noted in a statement that barring hospitals from Medicare and Medicaid could harm beneficiaries and the effort to provide COVID care.
Pollack also noted that AHA has “observed errors in data processing and confusion about exactly what was being requested at the hospital, state, contractor, and federal level, and has worked diligently with the federal agencies to identify and correct those problems.”
The document that lays out U.S. Department of Health and Human Services (HHS) Protect reporting requirements were updated again on October 6 to add influenza data. The hospitals must report on total patients with laboratory-confirmed flu; previous day’s flu admissions; total ICU patients with lab-confirmed flu; total inpatients with either flu or COVID-19; and the previous day’s deaths for flu and COVID.
CDC Director Robert Redfield, MD, said on the press call that the new data will give the agency crucial hospital-level information and perhaps better estimates of the flu burden. Flu trends have been tracked using the CDC’s Influenza Hospitalization Surveillance Network (FluSurv-NET), which will not be replaced, Redfield said. But that network only tracks hospitalizations in 14 states and does not provide information in “nearly real-time,” he said.
Having the new data “will give us a true situational awareness of severe respiratory illness, provide local hospitalization trends, and help direct resources such as antiretrovirals to address potential increased impact of flu and COVID cocirculation,” Redfield said.
This article first appeared on Medscape.com.
FDA posts COVID vaccine guidance amid White House pushback
while medical and trade associations called for a thorough review of any such product before approval.
The FDA took the unusual step of posting background materials much earlier than usual for its planned Oct. 22 advisory committee meeting on potential vaccines for COVID-19. The FDA also on Tuesday afternoon released a new guidance document, expanding on a previous set of recommendations the agency released in June.
In the new guidance document, FDA officials outline what will be required for even a limited clearance, known as an emergency use authorization (EUA), for a COVID-19 vaccine.
“Data from phase 3 studies should include a median follow-up duration of at least 2 months after completion of the full vaccination regimen to help provide adequate information to assess a vaccine’s benefit-risk profile,” the FDA said in the document.
FDA staff have emphasized the higher bar that drugmakers and regulators face in considering approval of a COVID-19 vaccine.
“Vaccines are complex biological products, and an EUA for a COVID-19 vaccine may allow for rapid and widespread deployment for administration of the vaccine to millions of individuals, including healthy people,” the agency staff said in the briefing documents.
The FDA’s briefing document for the Oct. 22 meeting appears to be markedly at odds with the claim Trump made in a video Monday night, in which he told the American public that “vaccines are coming momentarily.”
Trump, who is in a tightly contested presidential race against Democratic candidate Joe Biden, has repeatedly made claims of the potential arrival of COVID vaccines that are at odds with timelines offered with guarded optimism by experts in infectious diseases.
But based on these new guidelines from the FDA, it appears that the White House may now endorse the FDA’s stance, according to a Wall Street Journal report based on “people familiar with the matter.”
The publication reports that the White House, which has yet to officially comment, “endorsed the U.S. Food and Drug Administration’s plans for assessing whether a Covid-19 vaccine should be given widely, casting aside objections to requirements that would likely mean a shot won’t be cleared until after Election Day, people familiar with the matter said.”
Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, on Monday night said during a virtual appearance at the twenty-first annual New Yorker Festival that there could be evidence as early as November or December about whether one of the vaccines now in testing will work out. He declared himself to have “cautious optimism” about potential rollout of vaccines as early as late 2020 or early 2021.
Peter Lurie, MD, MPH, who earlier served as the FDA’s associate commissioner for public health strategy and analysis, described the agency’s release of the briefing document as being a positive development.
News organizations, including the New York Times, have reported that the White House had sought to block the FDA from releasing further instructions for companies developing COVID-19 vaccines. The Associated Press on Tuesday said that a senior Trump administration official confirmed that the White House had blocked earlier FDA plans to formally publish the safety guidelines based on the 2-month data requirement, arguing that there was “no clinical or medical reason” for it.
“It is an encouraging sign that, despite opposition from the White House, the Food and Drug Administration has effectively published guidelines for emergency release of a vaccine for COVID-19 by disclosing the advice it has been providing to individual sponsors,” said Dr. Lurie, who is now executive director and president of the Center for Science in the Public Interest.
In a news release, he said the White House had sought to keep the FDA guidance under wraps “so it could maintain the public fiction that a safe and effective vaccine could be available before Election Day or even so that it could force emergency authorization of a vaccine with more limited follow-up.”
“Even the pharmaceutical industry has been clamoring for the release of these guidelines. We all want a safe and effective vaccine to end the pandemic, and we want it sooner rather than later,” Dr. Lurie said. “But we can’t afford for the Trump administration to bungle vaccine review the way they’ve bungled nearly every other aspect of its pandemic response.”
Tuesday also saw a flood of statements in support of FDA officials, including tweets from the chief executive of Pfizer, which is among the leaders in the race to develop a COVID-19 vaccine. Pfizer’s Albert Bourla, DVM, PhD, said that the FDA’s “public servants are known for their high integrity and scientific expertise and we have full faith in their ability to set appropriate standards for the approval of a COVID vaccine or treatment.”
The American Medical Association on Tuesday announced a public webinar on Wednesday where its president, Susan R. Bailey, MD, will discuss the COVID-19 vaccine review process with Peter Marks, MD, PhD, director of the Center for Biologics Evaluation and Research at the FDA. The AMA described this webinar as part of work “to restore trust in science and science-based decision-making among policymakers and the public.”
“To ensure media and the physician community are continuously informed about the federal review process for COVID-19 vaccine candidates, the AMA will host a webinar series to gain fact-based insights from the nation’s highest-ranking subject matter experts working to protect the health of the public,” the organization said in announcing the webinar.
In a statement, leaders of the Association of American Medical Colleges said that the FDA’s Vaccines and Related Biological Products Advisory Committee should evaluate any COVID-19 candidate vaccines prior to the FDA issuing an EUA.
“Full approval of a new vaccine or biologic requires demonstration of safety and effectiveness through a process that includes evaluation by the VRBPAC. Their recommendations are considered by FDA staff who ultimately have the authority to approve the new product,” said AAMC chief scientific officer Ross McKinney Jr, MD, and AAMC CEO David J. Skorton, MD, in the statement.
Thomas M. File Jr., MD, president of the Infectious Diseases Society of America, said in a statement that his association again asked the White House to “follow medical and scientific expertise in efforts to combat COVID-19.”
“It is imperative that a vaccine be approved on the basis of FDA’s quality standards and that its safety and efficacy are established before it is authorized,” Dr. File said. “A vaccine that has been approved with speed, rather than safety and efficacy, at the forefront will compound the challenges posed by this pandemic. FDA guidelines for approval that set standards the American people can trust are essential to the success of a vaccine.”
Stephen J. Ubl, chief executive of the Pharmaceutical Research and Manufacturers of America, said in a statement that his association “supports any efforts by FDA to provide clarifying guidance and we have engaged with the agency to support bringing greater transparency to the review process for COVID-19 vaccines.”
“To help address this public health crisis, our companies have also taken unprecedented steps to share vaccine clinical trial protocols and data in real time,” Mr. Ubl said. “We welcome the agency’s efforts to instill confidence in the rigorous safety of these potential vaccines.”
On Oct. 1, Michelle McMurry-Heath, MD, PhD, president and chief executive of the Biotechnology Innovation Organization, released publicly her letter urging Department of Health & Human Services Secretary Alex Azar to “publicly release all new guidance” related to a COVID-19 vaccine. Such a move would bolster public confidence in the vaccine, she said.
“We cannot allow a lack of transparency to undermine confidence in the vaccine development process. The public must have full faith in the scientific process and the rigor of FDA’s regulatory oversight if we are to end the pandemic,” she wrote in the Oct. 1 letter to Azar. “Releasing any additional guidance on granting emergency use authorization for a vaccine will go a long way in accomplishing this critical goal.”
This article first appeared on Medscape.com.
while medical and trade associations called for a thorough review of any such product before approval.
The FDA took the unusual step of posting background materials much earlier than usual for its planned Oct. 22 advisory committee meeting on potential vaccines for COVID-19. The FDA also on Tuesday afternoon released a new guidance document, expanding on a previous set of recommendations the agency released in June.
In the new guidance document, FDA officials outline what will be required for even a limited clearance, known as an emergency use authorization (EUA), for a COVID-19 vaccine.
“Data from phase 3 studies should include a median follow-up duration of at least 2 months after completion of the full vaccination regimen to help provide adequate information to assess a vaccine’s benefit-risk profile,” the FDA said in the document.
FDA staff have emphasized the higher bar that drugmakers and regulators face in considering approval of a COVID-19 vaccine.
“Vaccines are complex biological products, and an EUA for a COVID-19 vaccine may allow for rapid and widespread deployment for administration of the vaccine to millions of individuals, including healthy people,” the agency staff said in the briefing documents.
The FDA’s briefing document for the Oct. 22 meeting appears to be markedly at odds with the claim Trump made in a video Monday night, in which he told the American public that “vaccines are coming momentarily.”
Trump, who is in a tightly contested presidential race against Democratic candidate Joe Biden, has repeatedly made claims of the potential arrival of COVID vaccines that are at odds with timelines offered with guarded optimism by experts in infectious diseases.
But based on these new guidelines from the FDA, it appears that the White House may now endorse the FDA’s stance, according to a Wall Street Journal report based on “people familiar with the matter.”
The publication reports that the White House, which has yet to officially comment, “endorsed the U.S. Food and Drug Administration’s plans for assessing whether a Covid-19 vaccine should be given widely, casting aside objections to requirements that would likely mean a shot won’t be cleared until after Election Day, people familiar with the matter said.”
Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, on Monday night said during a virtual appearance at the twenty-first annual New Yorker Festival that there could be evidence as early as November or December about whether one of the vaccines now in testing will work out. He declared himself to have “cautious optimism” about potential rollout of vaccines as early as late 2020 or early 2021.
Peter Lurie, MD, MPH, who earlier served as the FDA’s associate commissioner for public health strategy and analysis, described the agency’s release of the briefing document as being a positive development.
News organizations, including the New York Times, have reported that the White House had sought to block the FDA from releasing further instructions for companies developing COVID-19 vaccines. The Associated Press on Tuesday said that a senior Trump administration official confirmed that the White House had blocked earlier FDA plans to formally publish the safety guidelines based on the 2-month data requirement, arguing that there was “no clinical or medical reason” for it.
“It is an encouraging sign that, despite opposition from the White House, the Food and Drug Administration has effectively published guidelines for emergency release of a vaccine for COVID-19 by disclosing the advice it has been providing to individual sponsors,” said Dr. Lurie, who is now executive director and president of the Center for Science in the Public Interest.
In a news release, he said the White House had sought to keep the FDA guidance under wraps “so it could maintain the public fiction that a safe and effective vaccine could be available before Election Day or even so that it could force emergency authorization of a vaccine with more limited follow-up.”
“Even the pharmaceutical industry has been clamoring for the release of these guidelines. We all want a safe and effective vaccine to end the pandemic, and we want it sooner rather than later,” Dr. Lurie said. “But we can’t afford for the Trump administration to bungle vaccine review the way they’ve bungled nearly every other aspect of its pandemic response.”
Tuesday also saw a flood of statements in support of FDA officials, including tweets from the chief executive of Pfizer, which is among the leaders in the race to develop a COVID-19 vaccine. Pfizer’s Albert Bourla, DVM, PhD, said that the FDA’s “public servants are known for their high integrity and scientific expertise and we have full faith in their ability to set appropriate standards for the approval of a COVID vaccine or treatment.”
The American Medical Association on Tuesday announced a public webinar on Wednesday where its president, Susan R. Bailey, MD, will discuss the COVID-19 vaccine review process with Peter Marks, MD, PhD, director of the Center for Biologics Evaluation and Research at the FDA. The AMA described this webinar as part of work “to restore trust in science and science-based decision-making among policymakers and the public.”
“To ensure media and the physician community are continuously informed about the federal review process for COVID-19 vaccine candidates, the AMA will host a webinar series to gain fact-based insights from the nation’s highest-ranking subject matter experts working to protect the health of the public,” the organization said in announcing the webinar.
In a statement, leaders of the Association of American Medical Colleges said that the FDA’s Vaccines and Related Biological Products Advisory Committee should evaluate any COVID-19 candidate vaccines prior to the FDA issuing an EUA.
“Full approval of a new vaccine or biologic requires demonstration of safety and effectiveness through a process that includes evaluation by the VRBPAC. Their recommendations are considered by FDA staff who ultimately have the authority to approve the new product,” said AAMC chief scientific officer Ross McKinney Jr, MD, and AAMC CEO David J. Skorton, MD, in the statement.
Thomas M. File Jr., MD, president of the Infectious Diseases Society of America, said in a statement that his association again asked the White House to “follow medical and scientific expertise in efforts to combat COVID-19.”
“It is imperative that a vaccine be approved on the basis of FDA’s quality standards and that its safety and efficacy are established before it is authorized,” Dr. File said. “A vaccine that has been approved with speed, rather than safety and efficacy, at the forefront will compound the challenges posed by this pandemic. FDA guidelines for approval that set standards the American people can trust are essential to the success of a vaccine.”
Stephen J. Ubl, chief executive of the Pharmaceutical Research and Manufacturers of America, said in a statement that his association “supports any efforts by FDA to provide clarifying guidance and we have engaged with the agency to support bringing greater transparency to the review process for COVID-19 vaccines.”
“To help address this public health crisis, our companies have also taken unprecedented steps to share vaccine clinical trial protocols and data in real time,” Mr. Ubl said. “We welcome the agency’s efforts to instill confidence in the rigorous safety of these potential vaccines.”
On Oct. 1, Michelle McMurry-Heath, MD, PhD, president and chief executive of the Biotechnology Innovation Organization, released publicly her letter urging Department of Health & Human Services Secretary Alex Azar to “publicly release all new guidance” related to a COVID-19 vaccine. Such a move would bolster public confidence in the vaccine, she said.
“We cannot allow a lack of transparency to undermine confidence in the vaccine development process. The public must have full faith in the scientific process and the rigor of FDA’s regulatory oversight if we are to end the pandemic,” she wrote in the Oct. 1 letter to Azar. “Releasing any additional guidance on granting emergency use authorization for a vaccine will go a long way in accomplishing this critical goal.”
This article first appeared on Medscape.com.
while medical and trade associations called for a thorough review of any such product before approval.
The FDA took the unusual step of posting background materials much earlier than usual for its planned Oct. 22 advisory committee meeting on potential vaccines for COVID-19. The FDA also on Tuesday afternoon released a new guidance document, expanding on a previous set of recommendations the agency released in June.
In the new guidance document, FDA officials outline what will be required for even a limited clearance, known as an emergency use authorization (EUA), for a COVID-19 vaccine.
“Data from phase 3 studies should include a median follow-up duration of at least 2 months after completion of the full vaccination regimen to help provide adequate information to assess a vaccine’s benefit-risk profile,” the FDA said in the document.
FDA staff have emphasized the higher bar that drugmakers and regulators face in considering approval of a COVID-19 vaccine.
“Vaccines are complex biological products, and an EUA for a COVID-19 vaccine may allow for rapid and widespread deployment for administration of the vaccine to millions of individuals, including healthy people,” the agency staff said in the briefing documents.
The FDA’s briefing document for the Oct. 22 meeting appears to be markedly at odds with the claim Trump made in a video Monday night, in which he told the American public that “vaccines are coming momentarily.”
Trump, who is in a tightly contested presidential race against Democratic candidate Joe Biden, has repeatedly made claims of the potential arrival of COVID vaccines that are at odds with timelines offered with guarded optimism by experts in infectious diseases.
But based on these new guidelines from the FDA, it appears that the White House may now endorse the FDA’s stance, according to a Wall Street Journal report based on “people familiar with the matter.”
The publication reports that the White House, which has yet to officially comment, “endorsed the U.S. Food and Drug Administration’s plans for assessing whether a Covid-19 vaccine should be given widely, casting aside objections to requirements that would likely mean a shot won’t be cleared until after Election Day, people familiar with the matter said.”
Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, on Monday night said during a virtual appearance at the twenty-first annual New Yorker Festival that there could be evidence as early as November or December about whether one of the vaccines now in testing will work out. He declared himself to have “cautious optimism” about potential rollout of vaccines as early as late 2020 or early 2021.
Peter Lurie, MD, MPH, who earlier served as the FDA’s associate commissioner for public health strategy and analysis, described the agency’s release of the briefing document as being a positive development.
News organizations, including the New York Times, have reported that the White House had sought to block the FDA from releasing further instructions for companies developing COVID-19 vaccines. The Associated Press on Tuesday said that a senior Trump administration official confirmed that the White House had blocked earlier FDA plans to formally publish the safety guidelines based on the 2-month data requirement, arguing that there was “no clinical or medical reason” for it.
“It is an encouraging sign that, despite opposition from the White House, the Food and Drug Administration has effectively published guidelines for emergency release of a vaccine for COVID-19 by disclosing the advice it has been providing to individual sponsors,” said Dr. Lurie, who is now executive director and president of the Center for Science in the Public Interest.
In a news release, he said the White House had sought to keep the FDA guidance under wraps “so it could maintain the public fiction that a safe and effective vaccine could be available before Election Day or even so that it could force emergency authorization of a vaccine with more limited follow-up.”
“Even the pharmaceutical industry has been clamoring for the release of these guidelines. We all want a safe and effective vaccine to end the pandemic, and we want it sooner rather than later,” Dr. Lurie said. “But we can’t afford for the Trump administration to bungle vaccine review the way they’ve bungled nearly every other aspect of its pandemic response.”
Tuesday also saw a flood of statements in support of FDA officials, including tweets from the chief executive of Pfizer, which is among the leaders in the race to develop a COVID-19 vaccine. Pfizer’s Albert Bourla, DVM, PhD, said that the FDA’s “public servants are known for their high integrity and scientific expertise and we have full faith in their ability to set appropriate standards for the approval of a COVID vaccine or treatment.”
The American Medical Association on Tuesday announced a public webinar on Wednesday where its president, Susan R. Bailey, MD, will discuss the COVID-19 vaccine review process with Peter Marks, MD, PhD, director of the Center for Biologics Evaluation and Research at the FDA. The AMA described this webinar as part of work “to restore trust in science and science-based decision-making among policymakers and the public.”
“To ensure media and the physician community are continuously informed about the federal review process for COVID-19 vaccine candidates, the AMA will host a webinar series to gain fact-based insights from the nation’s highest-ranking subject matter experts working to protect the health of the public,” the organization said in announcing the webinar.
In a statement, leaders of the Association of American Medical Colleges said that the FDA’s Vaccines and Related Biological Products Advisory Committee should evaluate any COVID-19 candidate vaccines prior to the FDA issuing an EUA.
“Full approval of a new vaccine or biologic requires demonstration of safety and effectiveness through a process that includes evaluation by the VRBPAC. Their recommendations are considered by FDA staff who ultimately have the authority to approve the new product,” said AAMC chief scientific officer Ross McKinney Jr, MD, and AAMC CEO David J. Skorton, MD, in the statement.
Thomas M. File Jr., MD, president of the Infectious Diseases Society of America, said in a statement that his association again asked the White House to “follow medical and scientific expertise in efforts to combat COVID-19.”
“It is imperative that a vaccine be approved on the basis of FDA’s quality standards and that its safety and efficacy are established before it is authorized,” Dr. File said. “A vaccine that has been approved with speed, rather than safety and efficacy, at the forefront will compound the challenges posed by this pandemic. FDA guidelines for approval that set standards the American people can trust are essential to the success of a vaccine.”
Stephen J. Ubl, chief executive of the Pharmaceutical Research and Manufacturers of America, said in a statement that his association “supports any efforts by FDA to provide clarifying guidance and we have engaged with the agency to support bringing greater transparency to the review process for COVID-19 vaccines.”
“To help address this public health crisis, our companies have also taken unprecedented steps to share vaccine clinical trial protocols and data in real time,” Mr. Ubl said. “We welcome the agency’s efforts to instill confidence in the rigorous safety of these potential vaccines.”
On Oct. 1, Michelle McMurry-Heath, MD, PhD, president and chief executive of the Biotechnology Innovation Organization, released publicly her letter urging Department of Health & Human Services Secretary Alex Azar to “publicly release all new guidance” related to a COVID-19 vaccine. Such a move would bolster public confidence in the vaccine, she said.
“We cannot allow a lack of transparency to undermine confidence in the vaccine development process. The public must have full faith in the scientific process and the rigor of FDA’s regulatory oversight if we are to end the pandemic,” she wrote in the Oct. 1 letter to Azar. “Releasing any additional guidance on granting emergency use authorization for a vaccine will go a long way in accomplishing this critical goal.”
This article first appeared on Medscape.com.
COVID-19 risks are no higher in patients with multiple sclerosis
new U.S. data suggest. A separate study from the United Kingdom also found similar trends of rates of COVID-19 infection in patients with MS and the general population.
Both studies were presented Sept. 26 at a special session on multiple sclerosis and COVID-19 at a final “Encore” event as part of the Joint European Committee for Treatment and Research in Multiple Sclerosis–Americas Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS-ACTRIMS) 2020, this year known as MSVirtual2020.
The U.S. data appear consistent with studies from several other countries, in that worse COVID-19 outcomes increase with age and higher disability levels, both of which would be expected from findings in the general population.
The U.S. data also show a clear effect of race in MS, with higher rates of adverse COVID-19 outcomes in Black patients, again in line with what is seen in the general population.
“I would say the results from our study and in general do not suggest that MS itself is associated with higher risks of severe COVID-19 outcomes, compared with the general population,” said Amber Salter, PhD.
Dr. Salter, who is assistant professor of biostatistics at Washington University, St. Louis, presented data from the COViMS North American registry, set up for health care providers to report persons with MS who are infected with COVID-19.
The COViMS registry so far has information on 858 patients with MS who have COVID-19 (80% verified by a positive test), as reported from 150 different health care providers in the United States and Canada. The average age was 48 years, with average disease duration of 13.6 years. MS clinical course was reported as relapsing remitting in 78%, secondary progressive in 15%, and primary progressive in 5%. Most patients (72%) were fully ambulatory, 16% could walk with assistance, and 12% were nonambulatory.
Severe COVID-19 outcomes were classified as mortality (which occurred in 5.7% of the cohort), mortality/ICU admission (13.6%) and mortality/ICU admission/hospitalization (30.2%).
Results were adjusted for many different covariates, including sex, age, smoking, MS clinical course (relapsing, progressive), disease duration, ambulation, individual comorbidities (cardiovascular disease, cerebrovascular disease, chronic kidney disease, chronic lung disease, diabetes, hypertension, morbid obesity), and disease-modifying therapy use.
In multivariable logistic regression analyses, older age, having chronic renal disease, and being nonambulatory were consistently associated with increased odds of poorer outcomes. Chronic kidney disease had the strongest association with mortality (odds ratio, 28.6; P < .001). Other factors associated with mortality included cardiovascular disease (OR, 4.35; P = .009); age (OR per 10 years, 1.91; P = .012), and male sex (OR, 2.60; P = .041).
Patients who were nonambulatory had a higher risk of mortality/ICU admission/hospitalization (OR, 3.32; P = .003). This endpoint was also increased in patients on anti-CD20 drugs, compared with other disease-modifying treatment (OR, 2.31; P = .002), consistent with results from at least two other studies.
Disease-modifying therapy in general was not associated with an increased risk of worse outcomes. “There was some concern at the outset about the effect of disease-modifying therapies on COVID-19 outcomes, but most studies have not found an increased risk of worse outcomes in patients on such drug treatments, with the possible exception of anti-CD20 drugs,” Dr. Salter said.
“Some disease-modifying therapies may actually be protective (particularly interferon) and studies are investigating whether they may have a role in the treatment of COVID-19,” she added.
“The factors in MS patients that we and others have found to be associated with worse COVID-19 outcomes may not be specific to MS. Older age is known to be a primary risk factor for worse COVID-19 outcomes in the general population, and increasing disability presumably tracks with worse general heath,” Dr. Salter commented.
“I would say the overall data are fairly reassuring for MS patents,” she concluded.
Black patients have higher risk
One worrying finding in the North American data, however, was the effect of race. “We found an independent effect of race for worse COVID-19 outcomes in MS patients,” Dr. Slater said.
Of the 858 patients in the COViMS registry, 65.7% were White and 26.1% were Black. Black individuals were more likely to be younger, never smokers, have shorter MS duration, a relapsing MS course, and have comorbidities, compared with White patients. A higher proportion of Black patients had hypertension (40.2% vs 19.5%) and morbid obesity (17% vs. 9.5%).
Results showed that mortality rates were not statistically different between White and Black patients, but Black race was associated with increased risk of mortality and/or ICU admission, compared with White patients (16.9% vs. 12.8%), and multivariate logistic regression analysis showed Black race was independently associated with mortality/ICU admission after adjustments for covariates (OR, 3.7; P = .002).
Black race was also associated with increased risk of mortality/ICU admission/hospital admission (35.8% vs. 30.2%), and after adjustment for covariates this was found to be an independent predictor (OR, 1.7; P = .04).
“This higher COVID-19 risk in Black individuals is also seen in the general population, so these results are not that surprising and it doesn’t appear to be an effect specific to MS patients,” Dr. Salter commented.
U.K. data on risk of contracting COVID-19
A U.K. study also suggested race to be an independent predictor in the risk of contracting COVID-19 in patients with MS.
The study of more than 5,000 patients with MS showed that those from a Black, Asian, and Minority Ethnic group were twice as likely to report having COVID-19 than those who were White.
The study, which was conducted during the U.K. lockdown, also found that the trend of COVID-19 infection in patients with MS is comparable with that of the U.K. general population.
Presenting the data, Afagh Garjani, MD, concluded: “During a period with strict physical distancing measures, patients with MS are not at an increased risk of contracting COVID-19.”
Dr. Garjani, a neurology clinical research fellow at the University of Nottingham, (England), explained that the COVID-19 pandemic has introduced uncertainties into the MS community, and the focus so far has been the severity of infection among people with MS who have COVID-19.
“This approach has left questions about the risk of contracting disease in people with MS unanswered, which has implications as society gradually returns to normal,” she said.
Dr. Garjani presented data from the United Kingdom MS Register (UKMSR), which has been collecting demographic and MS-related data since 2011 from patients with MS throughout the United Kingdom.
On March 17 – just before the lockdown in United Kingdom – existing participants of the UKMSR were asked to join the COVID-19 study. The study was also advertised through social media. In this ongoing study, people with MS answered a COVID-19–related survey at participation and a different follow-up survey every 2 weeks depending on whether they contracted COVID-19.
The COVID-19 study included 5,309 patients with MS. The mean age of the study population was 52.4 years, 76.1% were female, and 95.7% were White. Of the 5,309 patients, 535 (10%) reported a self-diagnosis of COVID-19. Because of limited availability of tests in the United Kingdom at the time, only 75 patents had a positive polymerase chain reaction result.
“To our knowledge, this is the largest community-based study of COVID-19 in patients with MS worldwide,” Dr. Garjani said. She presented results from the period March 23 to June 24, when the United Kingdom was in a period of lockdown with vulnerable groups encouraged to self-isolate completely.
In this MS cohort, 47% reported self-isolating at some point. Those at older age and higher Expanded Disability Status Scale (EDSS) score were more likely to have self-isolated.
The researchers did not find that patients with progressive MS or those on disease-modifying therapies in general isolated more, but patients on monoclonal antibody drugs and fingolimod were more likely to self-isolate versus those on other therapies. “This may be because there are concerns about infection with these drugs and patients on these therapies may be more concerned about contracting COVID-19,” Dr. Garjani suggested.
In terms of contracting COVID, the researchers found a reduced risk of COVID-19 (self-diagnosed) in patients with older age and higher EDSS. “This is not really surprising that these patients were more likely to self-isolate,” Dr. Garjani commented.
No association was seen between type of MS, disease duration, disease-modifying therapy in general, and risk of COVID-19. No individual drug treatment increased risk versus no therapy or versus self-injectables. But there was an increased risk of contracting the virus in patients whose race was Black, Asian, or Minority Ethnic (OR, 2.2), which is in line with findings from the general population.
“This study is unique – the denominator is all people with MS. We are looking primarily at the risk of contracting COVID-19. Other studies are focusing more on people with MS who have COVID and assessing risk of a severe COVID outcome. Our results are not contradicting the findings from those studies,” Dr. Garjani said.
The results were similar only when patients with a confirmed COVID-19 test were considered.
In terms of outcomes in those who reported COVID-19 infection, preliminary results have not shown any MS factors – such as EDSS, age, type of MS, drug therapy in general – to be associated with outcome.
“Since the COVID-19 outbreak started there has been concern among MS patients, especially among those on disease-modifying therapies, about whether they are at increased risk of infection and severe disease,” Dr. Garjani said.
“We found similar trends of rates of infection in MS patients and the general population, and no signal of increased risks in those with higher EDSS or progressive MS. The caveat is that this study was conducted in a period of lockdown, but we adjusted for self-isolating behavior in the multivariable regression analysis,” she noted.
Dr. Salter is a statistical editor for the American Heart Association journal Circulation: Cardiovascular Imaging. Dr. Garjani has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
new U.S. data suggest. A separate study from the United Kingdom also found similar trends of rates of COVID-19 infection in patients with MS and the general population.
Both studies were presented Sept. 26 at a special session on multiple sclerosis and COVID-19 at a final “Encore” event as part of the Joint European Committee for Treatment and Research in Multiple Sclerosis–Americas Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS-ACTRIMS) 2020, this year known as MSVirtual2020.
The U.S. data appear consistent with studies from several other countries, in that worse COVID-19 outcomes increase with age and higher disability levels, both of which would be expected from findings in the general population.
The U.S. data also show a clear effect of race in MS, with higher rates of adverse COVID-19 outcomes in Black patients, again in line with what is seen in the general population.
“I would say the results from our study and in general do not suggest that MS itself is associated with higher risks of severe COVID-19 outcomes, compared with the general population,” said Amber Salter, PhD.
Dr. Salter, who is assistant professor of biostatistics at Washington University, St. Louis, presented data from the COViMS North American registry, set up for health care providers to report persons with MS who are infected with COVID-19.
The COViMS registry so far has information on 858 patients with MS who have COVID-19 (80% verified by a positive test), as reported from 150 different health care providers in the United States and Canada. The average age was 48 years, with average disease duration of 13.6 years. MS clinical course was reported as relapsing remitting in 78%, secondary progressive in 15%, and primary progressive in 5%. Most patients (72%) were fully ambulatory, 16% could walk with assistance, and 12% were nonambulatory.
Severe COVID-19 outcomes were classified as mortality (which occurred in 5.7% of the cohort), mortality/ICU admission (13.6%) and mortality/ICU admission/hospitalization (30.2%).
Results were adjusted for many different covariates, including sex, age, smoking, MS clinical course (relapsing, progressive), disease duration, ambulation, individual comorbidities (cardiovascular disease, cerebrovascular disease, chronic kidney disease, chronic lung disease, diabetes, hypertension, morbid obesity), and disease-modifying therapy use.
In multivariable logistic regression analyses, older age, having chronic renal disease, and being nonambulatory were consistently associated with increased odds of poorer outcomes. Chronic kidney disease had the strongest association with mortality (odds ratio, 28.6; P < .001). Other factors associated with mortality included cardiovascular disease (OR, 4.35; P = .009); age (OR per 10 years, 1.91; P = .012), and male sex (OR, 2.60; P = .041).
Patients who were nonambulatory had a higher risk of mortality/ICU admission/hospitalization (OR, 3.32; P = .003). This endpoint was also increased in patients on anti-CD20 drugs, compared with other disease-modifying treatment (OR, 2.31; P = .002), consistent with results from at least two other studies.
Disease-modifying therapy in general was not associated with an increased risk of worse outcomes. “There was some concern at the outset about the effect of disease-modifying therapies on COVID-19 outcomes, but most studies have not found an increased risk of worse outcomes in patients on such drug treatments, with the possible exception of anti-CD20 drugs,” Dr. Salter said.
“Some disease-modifying therapies may actually be protective (particularly interferon) and studies are investigating whether they may have a role in the treatment of COVID-19,” she added.
“The factors in MS patients that we and others have found to be associated with worse COVID-19 outcomes may not be specific to MS. Older age is known to be a primary risk factor for worse COVID-19 outcomes in the general population, and increasing disability presumably tracks with worse general heath,” Dr. Salter commented.
“I would say the overall data are fairly reassuring for MS patents,” she concluded.
Black patients have higher risk
One worrying finding in the North American data, however, was the effect of race. “We found an independent effect of race for worse COVID-19 outcomes in MS patients,” Dr. Slater said.
Of the 858 patients in the COViMS registry, 65.7% were White and 26.1% were Black. Black individuals were more likely to be younger, never smokers, have shorter MS duration, a relapsing MS course, and have comorbidities, compared with White patients. A higher proportion of Black patients had hypertension (40.2% vs 19.5%) and morbid obesity (17% vs. 9.5%).
Results showed that mortality rates were not statistically different between White and Black patients, but Black race was associated with increased risk of mortality and/or ICU admission, compared with White patients (16.9% vs. 12.8%), and multivariate logistic regression analysis showed Black race was independently associated with mortality/ICU admission after adjustments for covariates (OR, 3.7; P = .002).
Black race was also associated with increased risk of mortality/ICU admission/hospital admission (35.8% vs. 30.2%), and after adjustment for covariates this was found to be an independent predictor (OR, 1.7; P = .04).
“This higher COVID-19 risk in Black individuals is also seen in the general population, so these results are not that surprising and it doesn’t appear to be an effect specific to MS patients,” Dr. Salter commented.
U.K. data on risk of contracting COVID-19
A U.K. study also suggested race to be an independent predictor in the risk of contracting COVID-19 in patients with MS.
The study of more than 5,000 patients with MS showed that those from a Black, Asian, and Minority Ethnic group were twice as likely to report having COVID-19 than those who were White.
The study, which was conducted during the U.K. lockdown, also found that the trend of COVID-19 infection in patients with MS is comparable with that of the U.K. general population.
Presenting the data, Afagh Garjani, MD, concluded: “During a period with strict physical distancing measures, patients with MS are not at an increased risk of contracting COVID-19.”
Dr. Garjani, a neurology clinical research fellow at the University of Nottingham, (England), explained that the COVID-19 pandemic has introduced uncertainties into the MS community, and the focus so far has been the severity of infection among people with MS who have COVID-19.
“This approach has left questions about the risk of contracting disease in people with MS unanswered, which has implications as society gradually returns to normal,” she said.
Dr. Garjani presented data from the United Kingdom MS Register (UKMSR), which has been collecting demographic and MS-related data since 2011 from patients with MS throughout the United Kingdom.
On March 17 – just before the lockdown in United Kingdom – existing participants of the UKMSR were asked to join the COVID-19 study. The study was also advertised through social media. In this ongoing study, people with MS answered a COVID-19–related survey at participation and a different follow-up survey every 2 weeks depending on whether they contracted COVID-19.
The COVID-19 study included 5,309 patients with MS. The mean age of the study population was 52.4 years, 76.1% were female, and 95.7% were White. Of the 5,309 patients, 535 (10%) reported a self-diagnosis of COVID-19. Because of limited availability of tests in the United Kingdom at the time, only 75 patents had a positive polymerase chain reaction result.
“To our knowledge, this is the largest community-based study of COVID-19 in patients with MS worldwide,” Dr. Garjani said. She presented results from the period March 23 to June 24, when the United Kingdom was in a period of lockdown with vulnerable groups encouraged to self-isolate completely.
In this MS cohort, 47% reported self-isolating at some point. Those at older age and higher Expanded Disability Status Scale (EDSS) score were more likely to have self-isolated.
The researchers did not find that patients with progressive MS or those on disease-modifying therapies in general isolated more, but patients on monoclonal antibody drugs and fingolimod were more likely to self-isolate versus those on other therapies. “This may be because there are concerns about infection with these drugs and patients on these therapies may be more concerned about contracting COVID-19,” Dr. Garjani suggested.
In terms of contracting COVID, the researchers found a reduced risk of COVID-19 (self-diagnosed) in patients with older age and higher EDSS. “This is not really surprising that these patients were more likely to self-isolate,” Dr. Garjani commented.
No association was seen between type of MS, disease duration, disease-modifying therapy in general, and risk of COVID-19. No individual drug treatment increased risk versus no therapy or versus self-injectables. But there was an increased risk of contracting the virus in patients whose race was Black, Asian, or Minority Ethnic (OR, 2.2), which is in line with findings from the general population.
“This study is unique – the denominator is all people with MS. We are looking primarily at the risk of contracting COVID-19. Other studies are focusing more on people with MS who have COVID and assessing risk of a severe COVID outcome. Our results are not contradicting the findings from those studies,” Dr. Garjani said.
The results were similar only when patients with a confirmed COVID-19 test were considered.
In terms of outcomes in those who reported COVID-19 infection, preliminary results have not shown any MS factors – such as EDSS, age, type of MS, drug therapy in general – to be associated with outcome.
“Since the COVID-19 outbreak started there has been concern among MS patients, especially among those on disease-modifying therapies, about whether they are at increased risk of infection and severe disease,” Dr. Garjani said.
“We found similar trends of rates of infection in MS patients and the general population, and no signal of increased risks in those with higher EDSS or progressive MS. The caveat is that this study was conducted in a period of lockdown, but we adjusted for self-isolating behavior in the multivariable regression analysis,” she noted.
Dr. Salter is a statistical editor for the American Heart Association journal Circulation: Cardiovascular Imaging. Dr. Garjani has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
new U.S. data suggest. A separate study from the United Kingdom also found similar trends of rates of COVID-19 infection in patients with MS and the general population.
Both studies were presented Sept. 26 at a special session on multiple sclerosis and COVID-19 at a final “Encore” event as part of the Joint European Committee for Treatment and Research in Multiple Sclerosis–Americas Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS-ACTRIMS) 2020, this year known as MSVirtual2020.
The U.S. data appear consistent with studies from several other countries, in that worse COVID-19 outcomes increase with age and higher disability levels, both of which would be expected from findings in the general population.
The U.S. data also show a clear effect of race in MS, with higher rates of adverse COVID-19 outcomes in Black patients, again in line with what is seen in the general population.
“I would say the results from our study and in general do not suggest that MS itself is associated with higher risks of severe COVID-19 outcomes, compared with the general population,” said Amber Salter, PhD.
Dr. Salter, who is assistant professor of biostatistics at Washington University, St. Louis, presented data from the COViMS North American registry, set up for health care providers to report persons with MS who are infected with COVID-19.
The COViMS registry so far has information on 858 patients with MS who have COVID-19 (80% verified by a positive test), as reported from 150 different health care providers in the United States and Canada. The average age was 48 years, with average disease duration of 13.6 years. MS clinical course was reported as relapsing remitting in 78%, secondary progressive in 15%, and primary progressive in 5%. Most patients (72%) were fully ambulatory, 16% could walk with assistance, and 12% were nonambulatory.
Severe COVID-19 outcomes were classified as mortality (which occurred in 5.7% of the cohort), mortality/ICU admission (13.6%) and mortality/ICU admission/hospitalization (30.2%).
Results were adjusted for many different covariates, including sex, age, smoking, MS clinical course (relapsing, progressive), disease duration, ambulation, individual comorbidities (cardiovascular disease, cerebrovascular disease, chronic kidney disease, chronic lung disease, diabetes, hypertension, morbid obesity), and disease-modifying therapy use.
In multivariable logistic regression analyses, older age, having chronic renal disease, and being nonambulatory were consistently associated with increased odds of poorer outcomes. Chronic kidney disease had the strongest association with mortality (odds ratio, 28.6; P < .001). Other factors associated with mortality included cardiovascular disease (OR, 4.35; P = .009); age (OR per 10 years, 1.91; P = .012), and male sex (OR, 2.60; P = .041).
Patients who were nonambulatory had a higher risk of mortality/ICU admission/hospitalization (OR, 3.32; P = .003). This endpoint was also increased in patients on anti-CD20 drugs, compared with other disease-modifying treatment (OR, 2.31; P = .002), consistent with results from at least two other studies.
Disease-modifying therapy in general was not associated with an increased risk of worse outcomes. “There was some concern at the outset about the effect of disease-modifying therapies on COVID-19 outcomes, but most studies have not found an increased risk of worse outcomes in patients on such drug treatments, with the possible exception of anti-CD20 drugs,” Dr. Salter said.
“Some disease-modifying therapies may actually be protective (particularly interferon) and studies are investigating whether they may have a role in the treatment of COVID-19,” she added.
“The factors in MS patients that we and others have found to be associated with worse COVID-19 outcomes may not be specific to MS. Older age is known to be a primary risk factor for worse COVID-19 outcomes in the general population, and increasing disability presumably tracks with worse general heath,” Dr. Salter commented.
“I would say the overall data are fairly reassuring for MS patents,” she concluded.
Black patients have higher risk
One worrying finding in the North American data, however, was the effect of race. “We found an independent effect of race for worse COVID-19 outcomes in MS patients,” Dr. Slater said.
Of the 858 patients in the COViMS registry, 65.7% were White and 26.1% were Black. Black individuals were more likely to be younger, never smokers, have shorter MS duration, a relapsing MS course, and have comorbidities, compared with White patients. A higher proportion of Black patients had hypertension (40.2% vs 19.5%) and morbid obesity (17% vs. 9.5%).
Results showed that mortality rates were not statistically different between White and Black patients, but Black race was associated with increased risk of mortality and/or ICU admission, compared with White patients (16.9% vs. 12.8%), and multivariate logistic regression analysis showed Black race was independently associated with mortality/ICU admission after adjustments for covariates (OR, 3.7; P = .002).
Black race was also associated with increased risk of mortality/ICU admission/hospital admission (35.8% vs. 30.2%), and after adjustment for covariates this was found to be an independent predictor (OR, 1.7; P = .04).
“This higher COVID-19 risk in Black individuals is also seen in the general population, so these results are not that surprising and it doesn’t appear to be an effect specific to MS patients,” Dr. Salter commented.
U.K. data on risk of contracting COVID-19
A U.K. study also suggested race to be an independent predictor in the risk of contracting COVID-19 in patients with MS.
The study of more than 5,000 patients with MS showed that those from a Black, Asian, and Minority Ethnic group were twice as likely to report having COVID-19 than those who were White.
The study, which was conducted during the U.K. lockdown, also found that the trend of COVID-19 infection in patients with MS is comparable with that of the U.K. general population.
Presenting the data, Afagh Garjani, MD, concluded: “During a period with strict physical distancing measures, patients with MS are not at an increased risk of contracting COVID-19.”
Dr. Garjani, a neurology clinical research fellow at the University of Nottingham, (England), explained that the COVID-19 pandemic has introduced uncertainties into the MS community, and the focus so far has been the severity of infection among people with MS who have COVID-19.
“This approach has left questions about the risk of contracting disease in people with MS unanswered, which has implications as society gradually returns to normal,” she said.
Dr. Garjani presented data from the United Kingdom MS Register (UKMSR), which has been collecting demographic and MS-related data since 2011 from patients with MS throughout the United Kingdom.
On March 17 – just before the lockdown in United Kingdom – existing participants of the UKMSR were asked to join the COVID-19 study. The study was also advertised through social media. In this ongoing study, people with MS answered a COVID-19–related survey at participation and a different follow-up survey every 2 weeks depending on whether they contracted COVID-19.
The COVID-19 study included 5,309 patients with MS. The mean age of the study population was 52.4 years, 76.1% were female, and 95.7% were White. Of the 5,309 patients, 535 (10%) reported a self-diagnosis of COVID-19. Because of limited availability of tests in the United Kingdom at the time, only 75 patents had a positive polymerase chain reaction result.
“To our knowledge, this is the largest community-based study of COVID-19 in patients with MS worldwide,” Dr. Garjani said. She presented results from the period March 23 to June 24, when the United Kingdom was in a period of lockdown with vulnerable groups encouraged to self-isolate completely.
In this MS cohort, 47% reported self-isolating at some point. Those at older age and higher Expanded Disability Status Scale (EDSS) score were more likely to have self-isolated.
The researchers did not find that patients with progressive MS or those on disease-modifying therapies in general isolated more, but patients on monoclonal antibody drugs and fingolimod were more likely to self-isolate versus those on other therapies. “This may be because there are concerns about infection with these drugs and patients on these therapies may be more concerned about contracting COVID-19,” Dr. Garjani suggested.
In terms of contracting COVID, the researchers found a reduced risk of COVID-19 (self-diagnosed) in patients with older age and higher EDSS. “This is not really surprising that these patients were more likely to self-isolate,” Dr. Garjani commented.
No association was seen between type of MS, disease duration, disease-modifying therapy in general, and risk of COVID-19. No individual drug treatment increased risk versus no therapy or versus self-injectables. But there was an increased risk of contracting the virus in patients whose race was Black, Asian, or Minority Ethnic (OR, 2.2), which is in line with findings from the general population.
“This study is unique – the denominator is all people with MS. We are looking primarily at the risk of contracting COVID-19. Other studies are focusing more on people with MS who have COVID and assessing risk of a severe COVID outcome. Our results are not contradicting the findings from those studies,” Dr. Garjani said.
The results were similar only when patients with a confirmed COVID-19 test were considered.
In terms of outcomes in those who reported COVID-19 infection, preliminary results have not shown any MS factors – such as EDSS, age, type of MS, drug therapy in general – to be associated with outcome.
“Since the COVID-19 outbreak started there has been concern among MS patients, especially among those on disease-modifying therapies, about whether they are at increased risk of infection and severe disease,” Dr. Garjani said.
“We found similar trends of rates of infection in MS patients and the general population, and no signal of increased risks in those with higher EDSS or progressive MS. The caveat is that this study was conducted in a period of lockdown, but we adjusted for self-isolating behavior in the multivariable regression analysis,” she noted.
Dr. Salter is a statistical editor for the American Heart Association journal Circulation: Cardiovascular Imaging. Dr. Garjani has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM MSVIRTUAL2020






