User login
ID Practitioner is an independent news source that provides infectious disease specialists with timely and relevant news and commentary about clinical developments and the impact of health care policy on the infectious disease specialist’s practice. Specialty focus topics include antimicrobial resistance, emerging infections, global ID, hepatitis, HIV, hospital-acquired infections, immunizations and vaccines, influenza, mycoses, pediatric infections, and STIs. Infectious Diseases News is owned by Frontline Medical Communications.
sofosbuvir
ritonavir with dasabuvir
discount
support path
program
ritonavir
greedy
ledipasvir
assistance
viekira pak
vpak
advocacy
needy
protest
abbvie
paritaprevir
ombitasvir
direct-acting antivirals
dasabuvir
gilead
fake-ovir
support
v pak
oasis
harvoni
section[contains(@class, 'footer-nav-section-wrapper')]
div[contains(@class, 'pane-pub-article-idp')]
div[contains(@class, 'pane-medstat-latest-articles-articles-section')]
div[contains(@class, 'pane-pub-home-idp')]
div[contains(@class, 'pane-pub-topic-idp')]
Post-COVID clinics get jump-start from patients with lingering illness
Clarence Troutman survived a 2-month hospital stay with COVID-19, then went home in early June. But he’s far from over the disease, still suffering from limited endurance, shortness of breath and hands that can be stiff and swollen.
“Before COVID, I was a 59-year-old, relatively healthy man,” said the broadband technician from Denver. “If I had to say where I’m at now, I’d say about 50% of where I was, but when I first went home, I was at 20%.”
He credits much of his progress to the “motivation and education” gleaned from a new program for post-COVID patients at the University of Colorado at Denver, Aurora, one of a small but growing number of clinics aimed at treating and studying those who have had the unpredictable coronavirus.
As the election nears, much attention is focused on daily infection numbers or the climbing death toll, but another measure matters: Patients who survive but continue to wrestle with a range of physical or mental effects, including lung damage, heart or neurologic concerns, anxiety, and depression.
“We need to think about how we’re going to provide care for patients who may be recovering for years after the virus,” said Sarah Jolley, MD, a pulmonologist with UCHealth University of Colorado Hospital and director of UCHealth’s Post-Covid Clinic, where Mr. Troutman is seen.
That need has jump-started post-COVID clinics, which bring together a range of specialists into a one-stop shop.
One of the first and largest such clinics is at Mount Sinai in New York City, but programs have also launched at the University of California,San Francisco; Stanford (Calif.) University Medical Center; and the University of Pennsylvania, Philadelphia. The Cleveland Clinic plans to open one early next year. And it’s not just academic medical centers: St. John’s Well Child and Family Center, part of a network of community clinics in south central Los Angeles, said this month it aims to test thousands of its patients who were diagnosed with COVID-19 since March for long-term effects.
Mental health specialists are also involved, along with social workers and pharmacists. Many of the centers also do research studies, aiming to better understand why the virus hits certain patients so hard.
“Some of our patients, even those on a ventilator on death’s door, will come out remarkably unscathed,” said Lekshmi Santhosh, MD, an assistant professor of pulmonary critical care and a leader of the post-COVID program at UCSF, called the OPTIMAL clinic. “Others, even those who were never hospitalized, have disabling fatigue, ongoing chest pain, and shortness of breath, and there’s a whole spectrum in between.”
‘Staggering’ medical need
It’s too early to know how long the persistent medical effects and symptoms will linger, or to make accurate estimates on the percentage of patients affected.
Some early studies are sobering. An Austrian report released this month found that 76 of the first 86 patients studied had evidence of lung damage 6 weeks after hospital discharge, but that dropped to 48 patients at 12 weeks.
Some researchers and clinics say about 10% of U.S. COVID patients they see may have longer-running effects, said Zijian Chen, MD, medical director of the Center for Post-COVID Care at Mount Sinai, which has enrolled 400 patients so far.
If that estimate is correct – and Dr. Chen emphasized that more research is needed to make sure – it translates to patients entering the medical system in droves, often with multiple issues.
How health systems and insurers respond will be key, he said. More than 6.5 million U.S. residents have tested positive for the disease. If fewer than 10% – say 500,000 – already have long-lasting symptoms, “that number is staggering,” Dr. Chen said. “How much medical care will be needed for that?”
Though start-up costs could be a hurdle, the clinics themselves may eventually draw much-needed revenue to medical centers by attracting patients, many of whom have insurance to cover some or all of the cost of repeated visits.
Dr. Chen said the specialized centers can help lower health spending by providing more cost effective, coordinated care that avoids duplicative testing a patient might otherwise undergo.
“We’ve seen patients that when they come in, they’ve already had four MRI or CT scans and a stack of bloodwork,” he said.
The program consolidates those earlier results and determines if any additional testing is needed. Sometimes the answer to what’s causing patients’ long-lasting symptoms remains elusive. One problem for patients seeking help outside of dedicated clinics is that when there is no clear cause for their condition, they may be told the symptoms are imagined.
“I believe in the patients,” said Dr. Chen.
About half the clinic’s patients have received test results showing damage, said Dr. Chen, an endocrinologist and internal medicine physician. For those patients, the clinic can develop a treatment plan. But, frustratingly, the other half have inconclusive test results yet exhibit a range of symptoms.
“That makes it more difficult to treat,” said Dr. Chen.
Experts see parallels to a push in the past decade to establish special clinics to treat patients released from ICU wards, who may have problems related to long-term bed rest or the delirium many experience while hospitalized. Some of the current post-COVID clinics are modeled after the post-ICU clinics or are expanded versions of them.
The ICU Recovery Center at Vanderbilt University Medical Center, Nashville, Tenn., for instance, which opened in 2012, is accepting post-COVID patients.
There are about a dozen post-ICU clinics nationally, some of which are also now working with COVID patients, said James Jackson, director of long-term outcomes at the Vanderbilt center. In addition, he’s heard of at least another dozen post-COVID centers in development.
The centers generally do an initial assessment a few weeks after a patient is diagnosed or discharged from the hospital, often by video call. Check-in and repeat visits are scheduled every month or so after that.
“In an ideal world, with these post-COVID clinics, you can identify the patients and get them into rehab,” he said. “Even if the primary thing these clinics did was to say to patients: ‘This is real, it is not all in your head,’ that impact would be important.”
A question of feasibility
Financing is the largest obstacle, program proponents said. Many hospitals lost substantial revenue to canceled elective procedures during stay-at-home periods.
“So, it’s not a great time to be pitching a new activity that requires a start-up subsidy,” said Glenn Melnick, PhD, a professor of health economics at the University of Southern California.
At UCSF, a select group of faculty members staff the post-COVID clinics and some mental health professionals volunteer their time, said Dr. Santhosh.
Dr. Chen said he was able to recruit team members and support staff from the ranks of those whose elective patient caseload had dropped.
Dr. Jackson said unfortunately there’s not been enough research into the cost-and-clinical effectiveness of post-ICU centers.
“In the early days, there may have been questions about how much value does this add,” he noted. “Now, the question is not so much is it a good idea, but is it feasible?”
Right now, the post-COVID centers are foremost a research effort, said Len Nichols, an economist and nonresident fellow at the Urban Institute. “If these guys get good at treating long-term symptoms, that’s good for all of us. There’s not enough patients to make it a business model yet, but if they become the place to go when you get it, it could become a business model for some of the elite institutions.”
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
Clarence Troutman survived a 2-month hospital stay with COVID-19, then went home in early June. But he’s far from over the disease, still suffering from limited endurance, shortness of breath and hands that can be stiff and swollen.
“Before COVID, I was a 59-year-old, relatively healthy man,” said the broadband technician from Denver. “If I had to say where I’m at now, I’d say about 50% of where I was, but when I first went home, I was at 20%.”
He credits much of his progress to the “motivation and education” gleaned from a new program for post-COVID patients at the University of Colorado at Denver, Aurora, one of a small but growing number of clinics aimed at treating and studying those who have had the unpredictable coronavirus.
As the election nears, much attention is focused on daily infection numbers or the climbing death toll, but another measure matters: Patients who survive but continue to wrestle with a range of physical or mental effects, including lung damage, heart or neurologic concerns, anxiety, and depression.
“We need to think about how we’re going to provide care for patients who may be recovering for years after the virus,” said Sarah Jolley, MD, a pulmonologist with UCHealth University of Colorado Hospital and director of UCHealth’s Post-Covid Clinic, where Mr. Troutman is seen.
That need has jump-started post-COVID clinics, which bring together a range of specialists into a one-stop shop.
One of the first and largest such clinics is at Mount Sinai in New York City, but programs have also launched at the University of California,San Francisco; Stanford (Calif.) University Medical Center; and the University of Pennsylvania, Philadelphia. The Cleveland Clinic plans to open one early next year. And it’s not just academic medical centers: St. John’s Well Child and Family Center, part of a network of community clinics in south central Los Angeles, said this month it aims to test thousands of its patients who were diagnosed with COVID-19 since March for long-term effects.
Mental health specialists are also involved, along with social workers and pharmacists. Many of the centers also do research studies, aiming to better understand why the virus hits certain patients so hard.
“Some of our patients, even those on a ventilator on death’s door, will come out remarkably unscathed,” said Lekshmi Santhosh, MD, an assistant professor of pulmonary critical care and a leader of the post-COVID program at UCSF, called the OPTIMAL clinic. “Others, even those who were never hospitalized, have disabling fatigue, ongoing chest pain, and shortness of breath, and there’s a whole spectrum in between.”
‘Staggering’ medical need
It’s too early to know how long the persistent medical effects and symptoms will linger, or to make accurate estimates on the percentage of patients affected.
Some early studies are sobering. An Austrian report released this month found that 76 of the first 86 patients studied had evidence of lung damage 6 weeks after hospital discharge, but that dropped to 48 patients at 12 weeks.
Some researchers and clinics say about 10% of U.S. COVID patients they see may have longer-running effects, said Zijian Chen, MD, medical director of the Center for Post-COVID Care at Mount Sinai, which has enrolled 400 patients so far.
If that estimate is correct – and Dr. Chen emphasized that more research is needed to make sure – it translates to patients entering the medical system in droves, often with multiple issues.
How health systems and insurers respond will be key, he said. More than 6.5 million U.S. residents have tested positive for the disease. If fewer than 10% – say 500,000 – already have long-lasting symptoms, “that number is staggering,” Dr. Chen said. “How much medical care will be needed for that?”
Though start-up costs could be a hurdle, the clinics themselves may eventually draw much-needed revenue to medical centers by attracting patients, many of whom have insurance to cover some or all of the cost of repeated visits.
Dr. Chen said the specialized centers can help lower health spending by providing more cost effective, coordinated care that avoids duplicative testing a patient might otherwise undergo.
“We’ve seen patients that when they come in, they’ve already had four MRI or CT scans and a stack of bloodwork,” he said.
The program consolidates those earlier results and determines if any additional testing is needed. Sometimes the answer to what’s causing patients’ long-lasting symptoms remains elusive. One problem for patients seeking help outside of dedicated clinics is that when there is no clear cause for their condition, they may be told the symptoms are imagined.
“I believe in the patients,” said Dr. Chen.
About half the clinic’s patients have received test results showing damage, said Dr. Chen, an endocrinologist and internal medicine physician. For those patients, the clinic can develop a treatment plan. But, frustratingly, the other half have inconclusive test results yet exhibit a range of symptoms.
“That makes it more difficult to treat,” said Dr. Chen.
Experts see parallels to a push in the past decade to establish special clinics to treat patients released from ICU wards, who may have problems related to long-term bed rest or the delirium many experience while hospitalized. Some of the current post-COVID clinics are modeled after the post-ICU clinics or are expanded versions of them.
The ICU Recovery Center at Vanderbilt University Medical Center, Nashville, Tenn., for instance, which opened in 2012, is accepting post-COVID patients.
There are about a dozen post-ICU clinics nationally, some of which are also now working with COVID patients, said James Jackson, director of long-term outcomes at the Vanderbilt center. In addition, he’s heard of at least another dozen post-COVID centers in development.
The centers generally do an initial assessment a few weeks after a patient is diagnosed or discharged from the hospital, often by video call. Check-in and repeat visits are scheduled every month or so after that.
“In an ideal world, with these post-COVID clinics, you can identify the patients and get them into rehab,” he said. “Even if the primary thing these clinics did was to say to patients: ‘This is real, it is not all in your head,’ that impact would be important.”
A question of feasibility
Financing is the largest obstacle, program proponents said. Many hospitals lost substantial revenue to canceled elective procedures during stay-at-home periods.
“So, it’s not a great time to be pitching a new activity that requires a start-up subsidy,” said Glenn Melnick, PhD, a professor of health economics at the University of Southern California.
At UCSF, a select group of faculty members staff the post-COVID clinics and some mental health professionals volunteer their time, said Dr. Santhosh.
Dr. Chen said he was able to recruit team members and support staff from the ranks of those whose elective patient caseload had dropped.
Dr. Jackson said unfortunately there’s not been enough research into the cost-and-clinical effectiveness of post-ICU centers.
“In the early days, there may have been questions about how much value does this add,” he noted. “Now, the question is not so much is it a good idea, but is it feasible?”
Right now, the post-COVID centers are foremost a research effort, said Len Nichols, an economist and nonresident fellow at the Urban Institute. “If these guys get good at treating long-term symptoms, that’s good for all of us. There’s not enough patients to make it a business model yet, but if they become the place to go when you get it, it could become a business model for some of the elite institutions.”
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
Clarence Troutman survived a 2-month hospital stay with COVID-19, then went home in early June. But he’s far from over the disease, still suffering from limited endurance, shortness of breath and hands that can be stiff and swollen.
“Before COVID, I was a 59-year-old, relatively healthy man,” said the broadband technician from Denver. “If I had to say where I’m at now, I’d say about 50% of where I was, but when I first went home, I was at 20%.”
He credits much of his progress to the “motivation and education” gleaned from a new program for post-COVID patients at the University of Colorado at Denver, Aurora, one of a small but growing number of clinics aimed at treating and studying those who have had the unpredictable coronavirus.
As the election nears, much attention is focused on daily infection numbers or the climbing death toll, but another measure matters: Patients who survive but continue to wrestle with a range of physical or mental effects, including lung damage, heart or neurologic concerns, anxiety, and depression.
“We need to think about how we’re going to provide care for patients who may be recovering for years after the virus,” said Sarah Jolley, MD, a pulmonologist with UCHealth University of Colorado Hospital and director of UCHealth’s Post-Covid Clinic, where Mr. Troutman is seen.
That need has jump-started post-COVID clinics, which bring together a range of specialists into a one-stop shop.
One of the first and largest such clinics is at Mount Sinai in New York City, but programs have also launched at the University of California,San Francisco; Stanford (Calif.) University Medical Center; and the University of Pennsylvania, Philadelphia. The Cleveland Clinic plans to open one early next year. And it’s not just academic medical centers: St. John’s Well Child and Family Center, part of a network of community clinics in south central Los Angeles, said this month it aims to test thousands of its patients who were diagnosed with COVID-19 since March for long-term effects.
Mental health specialists are also involved, along with social workers and pharmacists. Many of the centers also do research studies, aiming to better understand why the virus hits certain patients so hard.
“Some of our patients, even those on a ventilator on death’s door, will come out remarkably unscathed,” said Lekshmi Santhosh, MD, an assistant professor of pulmonary critical care and a leader of the post-COVID program at UCSF, called the OPTIMAL clinic. “Others, even those who were never hospitalized, have disabling fatigue, ongoing chest pain, and shortness of breath, and there’s a whole spectrum in between.”
‘Staggering’ medical need
It’s too early to know how long the persistent medical effects and symptoms will linger, or to make accurate estimates on the percentage of patients affected.
Some early studies are sobering. An Austrian report released this month found that 76 of the first 86 patients studied had evidence of lung damage 6 weeks after hospital discharge, but that dropped to 48 patients at 12 weeks.
Some researchers and clinics say about 10% of U.S. COVID patients they see may have longer-running effects, said Zijian Chen, MD, medical director of the Center for Post-COVID Care at Mount Sinai, which has enrolled 400 patients so far.
If that estimate is correct – and Dr. Chen emphasized that more research is needed to make sure – it translates to patients entering the medical system in droves, often with multiple issues.
How health systems and insurers respond will be key, he said. More than 6.5 million U.S. residents have tested positive for the disease. If fewer than 10% – say 500,000 – already have long-lasting symptoms, “that number is staggering,” Dr. Chen said. “How much medical care will be needed for that?”
Though start-up costs could be a hurdle, the clinics themselves may eventually draw much-needed revenue to medical centers by attracting patients, many of whom have insurance to cover some or all of the cost of repeated visits.
Dr. Chen said the specialized centers can help lower health spending by providing more cost effective, coordinated care that avoids duplicative testing a patient might otherwise undergo.
“We’ve seen patients that when they come in, they’ve already had four MRI or CT scans and a stack of bloodwork,” he said.
The program consolidates those earlier results and determines if any additional testing is needed. Sometimes the answer to what’s causing patients’ long-lasting symptoms remains elusive. One problem for patients seeking help outside of dedicated clinics is that when there is no clear cause for their condition, they may be told the symptoms are imagined.
“I believe in the patients,” said Dr. Chen.
About half the clinic’s patients have received test results showing damage, said Dr. Chen, an endocrinologist and internal medicine physician. For those patients, the clinic can develop a treatment plan. But, frustratingly, the other half have inconclusive test results yet exhibit a range of symptoms.
“That makes it more difficult to treat,” said Dr. Chen.
Experts see parallels to a push in the past decade to establish special clinics to treat patients released from ICU wards, who may have problems related to long-term bed rest or the delirium many experience while hospitalized. Some of the current post-COVID clinics are modeled after the post-ICU clinics or are expanded versions of them.
The ICU Recovery Center at Vanderbilt University Medical Center, Nashville, Tenn., for instance, which opened in 2012, is accepting post-COVID patients.
There are about a dozen post-ICU clinics nationally, some of which are also now working with COVID patients, said James Jackson, director of long-term outcomes at the Vanderbilt center. In addition, he’s heard of at least another dozen post-COVID centers in development.
The centers generally do an initial assessment a few weeks after a patient is diagnosed or discharged from the hospital, often by video call. Check-in and repeat visits are scheduled every month or so after that.
“In an ideal world, with these post-COVID clinics, you can identify the patients and get them into rehab,” he said. “Even if the primary thing these clinics did was to say to patients: ‘This is real, it is not all in your head,’ that impact would be important.”
A question of feasibility
Financing is the largest obstacle, program proponents said. Many hospitals lost substantial revenue to canceled elective procedures during stay-at-home periods.
“So, it’s not a great time to be pitching a new activity that requires a start-up subsidy,” said Glenn Melnick, PhD, a professor of health economics at the University of Southern California.
At UCSF, a select group of faculty members staff the post-COVID clinics and some mental health professionals volunteer their time, said Dr. Santhosh.
Dr. Chen said he was able to recruit team members and support staff from the ranks of those whose elective patient caseload had dropped.
Dr. Jackson said unfortunately there’s not been enough research into the cost-and-clinical effectiveness of post-ICU centers.
“In the early days, there may have been questions about how much value does this add,” he noted. “Now, the question is not so much is it a good idea, but is it feasible?”
Right now, the post-COVID centers are foremost a research effort, said Len Nichols, an economist and nonresident fellow at the Urban Institute. “If these guys get good at treating long-term symptoms, that’s good for all of us. There’s not enough patients to make it a business model yet, but if they become the place to go when you get it, it could become a business model for some of the elite institutions.”
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
Nerve damage linked to prone positioning in COVID-19
A new case series describes peripheral nerve injuries associated with this type of positioning and suggests ways to minimize the potential damage.
“Physicians should remain aware of increased susceptibility to peripheral nerve damage in patients with severe COVID-19 after prone positioning, since it is surprisingly common among these patients, and should refine standard protocols accordingly to reduce that risk,” said senior author Colin Franz, MD, PhD, director of the Electrodiagnostic Laboratory, Shirley Ryan AbilityLab, Chicago.
The article was published online Sept. 4 in the British Journal of Anaesthesiology.
Unique type of nerve injury
Many patients who are admitted to the intensive care unit with COVID-19 undergo invasive mechanical ventilation because of acute respiratory distress syndrome (ARDS). Clinical guidelines recommend that such patients lie in the prone position 12-16 hours per day.
“Prone positioning for up to 16 hours is a therapy we use for patients with more severe forms of ARDS, and high-level evidence points to mortality benefit in patients with moderate to severe ARDS if [mechanical] ventilation occurs,” said study coauthor James McCauley Walter, MD, of the pulmonary division at Northwestern University, Chicago.
With a “significant number of COVID-19 patients flooding the ICU, we quickly started to prone a lot of them, but if you are in a specific position for multiple hours a day, coupled with the neurotoxic effects of the SARS-CoV-2 virus itself, you may be exposed to a unique type of nerve injury,” he said.
Dr. Walter said that the “incidence of asymmetric neuropathies seems out of proportion to what has been reported in non–COVID-19 settings, which is what caught our attention.”
Many of these patients are discharged to rehabilitation hospitals, and “what we noticed, which was unique about COVID-19 patients coming to our rehab hospital, was that, compared with other patients who had been critically ill with a long hospital stay, there was a significantly higher percentage of COVID-19 patients who had peripheral nerve damage,” Dr. Franz said.
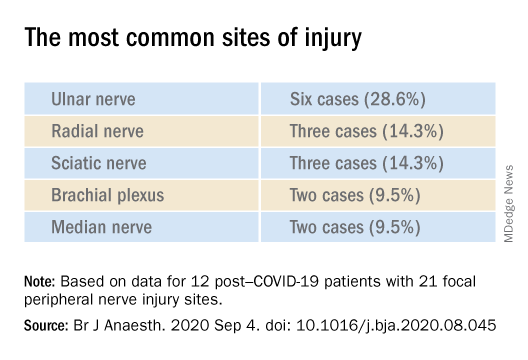
The authors described 12 of these patients who were admitted between April 24 and June 30, 2020 (mean age, 60.3 years; range, 23-80 years). The sample included White, Black, and Hispanic individuals. Eleven of the 12 post–COVID-19 patients with peripheral nerve damage had experienced prone positioning during acute management.
The average number of days patients received mechanical ventilation was 33.6 (range, 12-62 days). The average number of proning sessions was 4.5 (range, 1-16) with an average of 81.2 hours (range, 16-252 hours) spent prone.
A major contributor
Dr. Franz suggested that prone positioning is likely not the only cause of peripheral nerve damage but “may play a big role in these patients who are vulnerable because of viral infection and the critical illness that causes damage and nerve injuries.”
“The first component of lifesaving care for the critically ill in the ICU is intravenous fluids, mechanical ventilation, steroids, and antibiotics for infection,” said Dr. Walter.
“We are trying to come up with ways to place patients in prone position in safer ways, to pay attention to pressure points and areas of injury that we have seen and try to offload them, to see if we can decrease the rate of these injuries,” he added.
The researchers’ article includes a heat map diagram as a “template for where to focus the most efforts, in terms of decreasing pressure,” Dr. Walter said.
“The nerves are accepting too much force for gravely ill COVID-19 patients to handle, so we suggest using the template to determine where extra padding might be needed, or a protocol that might include changes in positioning,” he added.
Dr. Franz described the interventions used for COVID-19 patients with prone positioning–related peripheral nerve damage. “The first step is trying to address the problems one by one, either trying to solve them through exercise or teaching new skills, new ways to compensate, beginning with basic activities, such as getting out of bed and self-care,” he said.
Long-term recovery of nerve injuries depends on how severe the injuries are. Some nerves can slowly regenerate – possibly at the rate of 1 inch per month – which can be a long process, taking between a year and 18 months.
Dr. Franz said that therapies for this condition are “extrapolated from clinical trial work” on promoting nerve regeneration after surgery using electrical stimulation to enable nerves to regrow at a faster rate.
“Regeneration is not only slow, but it may not happen completely, leaving the patient with permanent nerve damage – in fact, based on our experience and what has been reported, the percentage of patients with full recovery is only 10%,” he said.
The most common symptomatic complaint other than lack of movement or feeling is neuropathic pain, “which may require medication to take the edge off the pain,” Dr. Franz added.
Irreversible damage?
Commenting on the study, Tae Chung, MD, of the departments of physical medicine, rehabilitation, and neurology, Johns Hopkins University, Baltimore, said the study “provides one of the first and the largest description of peripheral nerve injury associated with prone positioning for management of ARDS from COVID-19.”
Dr. Chung, who was not involved in the research, noted that “various neurological complications from COVID-19 have been reported, and some of them may result in irreversible neurological damage or delay the recovery from COVID-19 infection,” so “accurate and timely diagnosis of such neurological complications is critical for rehabilitation of the COVID-19 survivors.”
The study received no funding. Dr. Franz, Dr. Walter, study coauthors, and Dr. Chung report no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
A new case series describes peripheral nerve injuries associated with this type of positioning and suggests ways to minimize the potential damage.

“Physicians should remain aware of increased susceptibility to peripheral nerve damage in patients with severe COVID-19 after prone positioning, since it is surprisingly common among these patients, and should refine standard protocols accordingly to reduce that risk,” said senior author Colin Franz, MD, PhD, director of the Electrodiagnostic Laboratory, Shirley Ryan AbilityLab, Chicago.
The article was published online Sept. 4 in the British Journal of Anaesthesiology.
Unique type of nerve injury
Many patients who are admitted to the intensive care unit with COVID-19 undergo invasive mechanical ventilation because of acute respiratory distress syndrome (ARDS). Clinical guidelines recommend that such patients lie in the prone position 12-16 hours per day.
“Prone positioning for up to 16 hours is a therapy we use for patients with more severe forms of ARDS, and high-level evidence points to mortality benefit in patients with moderate to severe ARDS if [mechanical] ventilation occurs,” said study coauthor James McCauley Walter, MD, of the pulmonary division at Northwestern University, Chicago.
With a “significant number of COVID-19 patients flooding the ICU, we quickly started to prone a lot of them, but if you are in a specific position for multiple hours a day, coupled with the neurotoxic effects of the SARS-CoV-2 virus itself, you may be exposed to a unique type of nerve injury,” he said.
Dr. Walter said that the “incidence of asymmetric neuropathies seems out of proportion to what has been reported in non–COVID-19 settings, which is what caught our attention.”
Many of these patients are discharged to rehabilitation hospitals, and “what we noticed, which was unique about COVID-19 patients coming to our rehab hospital, was that, compared with other patients who had been critically ill with a long hospital stay, there was a significantly higher percentage of COVID-19 patients who had peripheral nerve damage,” Dr. Franz said.
The authors described 12 of these patients who were admitted between April 24 and June 30, 2020 (mean age, 60.3 years; range, 23-80 years). The sample included White, Black, and Hispanic individuals. Eleven of the 12 post–COVID-19 patients with peripheral nerve damage had experienced prone positioning during acute management.
The average number of days patients received mechanical ventilation was 33.6 (range, 12-62 days). The average number of proning sessions was 4.5 (range, 1-16) with an average of 81.2 hours (range, 16-252 hours) spent prone.
A major contributor
Dr. Franz suggested that prone positioning is likely not the only cause of peripheral nerve damage but “may play a big role in these patients who are vulnerable because of viral infection and the critical illness that causes damage and nerve injuries.”
“The first component of lifesaving care for the critically ill in the ICU is intravenous fluids, mechanical ventilation, steroids, and antibiotics for infection,” said Dr. Walter.
“We are trying to come up with ways to place patients in prone position in safer ways, to pay attention to pressure points and areas of injury that we have seen and try to offload them, to see if we can decrease the rate of these injuries,” he added.
The researchers’ article includes a heat map diagram as a “template for where to focus the most efforts, in terms of decreasing pressure,” Dr. Walter said.
“The nerves are accepting too much force for gravely ill COVID-19 patients to handle, so we suggest using the template to determine where extra padding might be needed, or a protocol that might include changes in positioning,” he added.
Dr. Franz described the interventions used for COVID-19 patients with prone positioning–related peripheral nerve damage. “The first step is trying to address the problems one by one, either trying to solve them through exercise or teaching new skills, new ways to compensate, beginning with basic activities, such as getting out of bed and self-care,” he said.
Long-term recovery of nerve injuries depends on how severe the injuries are. Some nerves can slowly regenerate – possibly at the rate of 1 inch per month – which can be a long process, taking between a year and 18 months.
Dr. Franz said that therapies for this condition are “extrapolated from clinical trial work” on promoting nerve regeneration after surgery using electrical stimulation to enable nerves to regrow at a faster rate.
“Regeneration is not only slow, but it may not happen completely, leaving the patient with permanent nerve damage – in fact, based on our experience and what has been reported, the percentage of patients with full recovery is only 10%,” he said.
The most common symptomatic complaint other than lack of movement or feeling is neuropathic pain, “which may require medication to take the edge off the pain,” Dr. Franz added.
Irreversible damage?
Commenting on the study, Tae Chung, MD, of the departments of physical medicine, rehabilitation, and neurology, Johns Hopkins University, Baltimore, said the study “provides one of the first and the largest description of peripheral nerve injury associated with prone positioning for management of ARDS from COVID-19.”
Dr. Chung, who was not involved in the research, noted that “various neurological complications from COVID-19 have been reported, and some of them may result in irreversible neurological damage or delay the recovery from COVID-19 infection,” so “accurate and timely diagnosis of such neurological complications is critical for rehabilitation of the COVID-19 survivors.”
The study received no funding. Dr. Franz, Dr. Walter, study coauthors, and Dr. Chung report no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
A new case series describes peripheral nerve injuries associated with this type of positioning and suggests ways to minimize the potential damage.

“Physicians should remain aware of increased susceptibility to peripheral nerve damage in patients with severe COVID-19 after prone positioning, since it is surprisingly common among these patients, and should refine standard protocols accordingly to reduce that risk,” said senior author Colin Franz, MD, PhD, director of the Electrodiagnostic Laboratory, Shirley Ryan AbilityLab, Chicago.
The article was published online Sept. 4 in the British Journal of Anaesthesiology.
Unique type of nerve injury
Many patients who are admitted to the intensive care unit with COVID-19 undergo invasive mechanical ventilation because of acute respiratory distress syndrome (ARDS). Clinical guidelines recommend that such patients lie in the prone position 12-16 hours per day.
“Prone positioning for up to 16 hours is a therapy we use for patients with more severe forms of ARDS, and high-level evidence points to mortality benefit in patients with moderate to severe ARDS if [mechanical] ventilation occurs,” said study coauthor James McCauley Walter, MD, of the pulmonary division at Northwestern University, Chicago.
With a “significant number of COVID-19 patients flooding the ICU, we quickly started to prone a lot of them, but if you are in a specific position for multiple hours a day, coupled with the neurotoxic effects of the SARS-CoV-2 virus itself, you may be exposed to a unique type of nerve injury,” he said.
Dr. Walter said that the “incidence of asymmetric neuropathies seems out of proportion to what has been reported in non–COVID-19 settings, which is what caught our attention.”
Many of these patients are discharged to rehabilitation hospitals, and “what we noticed, which was unique about COVID-19 patients coming to our rehab hospital, was that, compared with other patients who had been critically ill with a long hospital stay, there was a significantly higher percentage of COVID-19 patients who had peripheral nerve damage,” Dr. Franz said.
The authors described 12 of these patients who were admitted between April 24 and June 30, 2020 (mean age, 60.3 years; range, 23-80 years). The sample included White, Black, and Hispanic individuals. Eleven of the 12 post–COVID-19 patients with peripheral nerve damage had experienced prone positioning during acute management.
The average number of days patients received mechanical ventilation was 33.6 (range, 12-62 days). The average number of proning sessions was 4.5 (range, 1-16) with an average of 81.2 hours (range, 16-252 hours) spent prone.
A major contributor
Dr. Franz suggested that prone positioning is likely not the only cause of peripheral nerve damage but “may play a big role in these patients who are vulnerable because of viral infection and the critical illness that causes damage and nerve injuries.”
“The first component of lifesaving care for the critically ill in the ICU is intravenous fluids, mechanical ventilation, steroids, and antibiotics for infection,” said Dr. Walter.
“We are trying to come up with ways to place patients in prone position in safer ways, to pay attention to pressure points and areas of injury that we have seen and try to offload them, to see if we can decrease the rate of these injuries,” he added.
The researchers’ article includes a heat map diagram as a “template for where to focus the most efforts, in terms of decreasing pressure,” Dr. Walter said.
“The nerves are accepting too much force for gravely ill COVID-19 patients to handle, so we suggest using the template to determine where extra padding might be needed, or a protocol that might include changes in positioning,” he added.
Dr. Franz described the interventions used for COVID-19 patients with prone positioning–related peripheral nerve damage. “The first step is trying to address the problems one by one, either trying to solve them through exercise or teaching new skills, new ways to compensate, beginning with basic activities, such as getting out of bed and self-care,” he said.
Long-term recovery of nerve injuries depends on how severe the injuries are. Some nerves can slowly regenerate – possibly at the rate of 1 inch per month – which can be a long process, taking between a year and 18 months.
Dr. Franz said that therapies for this condition are “extrapolated from clinical trial work” on promoting nerve regeneration after surgery using electrical stimulation to enable nerves to regrow at a faster rate.
“Regeneration is not only slow, but it may not happen completely, leaving the patient with permanent nerve damage – in fact, based on our experience and what has been reported, the percentage of patients with full recovery is only 10%,” he said.
The most common symptomatic complaint other than lack of movement or feeling is neuropathic pain, “which may require medication to take the edge off the pain,” Dr. Franz added.
Irreversible damage?
Commenting on the study, Tae Chung, MD, of the departments of physical medicine, rehabilitation, and neurology, Johns Hopkins University, Baltimore, said the study “provides one of the first and the largest description of peripheral nerve injury associated with prone positioning for management of ARDS from COVID-19.”
Dr. Chung, who was not involved in the research, noted that “various neurological complications from COVID-19 have been reported, and some of them may result in irreversible neurological damage or delay the recovery from COVID-19 infection,” so “accurate and timely diagnosis of such neurological complications is critical for rehabilitation of the COVID-19 survivors.”
The study received no funding. Dr. Franz, Dr. Walter, study coauthors, and Dr. Chung report no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM THE BRITISH JOURNAL OF ANAESTHESIOLOGY
Trump signs Medicare loan relief bill delaying repayments
President Trump on Oct. 1 signed a bill to keep the federal government running through December 11. This “continuing resolution” (CR), which was approved by the Senate Wednesday on an 84-10 vote, according to The New York Times, includes provisions to delay repayment by physicians of pandemic-related Medicare loans and to reduce the loans’ interest rate.
In an earlier news release, the American Medical Association reported that Congress and the White House had agreed to include the provisions on Medicare loans in the CR.
Under the Medicare Accelerated and Advance Payments (AAP) program, the Centers for Medicare & Medicaid Services advanced money to physicians who were financially impacted by the pandemic. The program, created in March, was suspended in late April.
Physicians who received the Medicare loans were supposed to start paying them back 120 days after they were made. CMS planned to recoup the advances by offsetting them against Medicare claims payments due to physicians. Practices had up to 210 days (7 months) to repay the loans through this process before being asked to repay them directly with interest of 10.25%.
For the practices that received these advances, that meant their Medicare cash flow was scheduled to dry up, starting in August. However, CMS quietly abstained from collecting these payments when they came due, according to Modern Healthcare.
New terms
The amount to be recouped from each claim is reduced from 100% to 25% of the claim for the first 11 months and to 50% of claims withheld for an additional 6 months. If the loan is not repaid in full by then, the provider must pay the balance with interest of 4%.
More than 80% of the $100 billion that CMS loaned to healthcare providers through May 2 went to hospitals, Modern Healthcare calculated. Of the remainder, specialty or multispecialty practices received $3.5 billion, internal medicine specialists got $24 million, family physicians were loaned $15 million, and federally qualified health centers received $20 million.
In the AMA’s news release, AMA President Susan Bailey, MD, who assumed the post in June, called the original loan repayment plan an “economic sword hanging over physician practices.”
This article first appeared on Medscape.com.
President Trump on Oct. 1 signed a bill to keep the federal government running through December 11. This “continuing resolution” (CR), which was approved by the Senate Wednesday on an 84-10 vote, according to The New York Times, includes provisions to delay repayment by physicians of pandemic-related Medicare loans and to reduce the loans’ interest rate.
In an earlier news release, the American Medical Association reported that Congress and the White House had agreed to include the provisions on Medicare loans in the CR.
Under the Medicare Accelerated and Advance Payments (AAP) program, the Centers for Medicare & Medicaid Services advanced money to physicians who were financially impacted by the pandemic. The program, created in March, was suspended in late April.
Physicians who received the Medicare loans were supposed to start paying them back 120 days after they were made. CMS planned to recoup the advances by offsetting them against Medicare claims payments due to physicians. Practices had up to 210 days (7 months) to repay the loans through this process before being asked to repay them directly with interest of 10.25%.
For the practices that received these advances, that meant their Medicare cash flow was scheduled to dry up, starting in August. However, CMS quietly abstained from collecting these payments when they came due, according to Modern Healthcare.
New terms
The amount to be recouped from each claim is reduced from 100% to 25% of the claim for the first 11 months and to 50% of claims withheld for an additional 6 months. If the loan is not repaid in full by then, the provider must pay the balance with interest of 4%.
More than 80% of the $100 billion that CMS loaned to healthcare providers through May 2 went to hospitals, Modern Healthcare calculated. Of the remainder, specialty or multispecialty practices received $3.5 billion, internal medicine specialists got $24 million, family physicians were loaned $15 million, and federally qualified health centers received $20 million.
In the AMA’s news release, AMA President Susan Bailey, MD, who assumed the post in June, called the original loan repayment plan an “economic sword hanging over physician practices.”
This article first appeared on Medscape.com.
President Trump on Oct. 1 signed a bill to keep the federal government running through December 11. This “continuing resolution” (CR), which was approved by the Senate Wednesday on an 84-10 vote, according to The New York Times, includes provisions to delay repayment by physicians of pandemic-related Medicare loans and to reduce the loans’ interest rate.
In an earlier news release, the American Medical Association reported that Congress and the White House had agreed to include the provisions on Medicare loans in the CR.
Under the Medicare Accelerated and Advance Payments (AAP) program, the Centers for Medicare & Medicaid Services advanced money to physicians who were financially impacted by the pandemic. The program, created in March, was suspended in late April.
Physicians who received the Medicare loans were supposed to start paying them back 120 days after they were made. CMS planned to recoup the advances by offsetting them against Medicare claims payments due to physicians. Practices had up to 210 days (7 months) to repay the loans through this process before being asked to repay them directly with interest of 10.25%.
For the practices that received these advances, that meant their Medicare cash flow was scheduled to dry up, starting in August. However, CMS quietly abstained from collecting these payments when they came due, according to Modern Healthcare.
New terms
The amount to be recouped from each claim is reduced from 100% to 25% of the claim for the first 11 months and to 50% of claims withheld for an additional 6 months. If the loan is not repaid in full by then, the provider must pay the balance with interest of 4%.
More than 80% of the $100 billion that CMS loaned to healthcare providers through May 2 went to hospitals, Modern Healthcare calculated. Of the remainder, specialty or multispecialty practices received $3.5 billion, internal medicine specialists got $24 million, family physicians were loaned $15 million, and federally qualified health centers received $20 million.
In the AMA’s news release, AMA President Susan Bailey, MD, who assumed the post in June, called the original loan repayment plan an “economic sword hanging over physician practices.”
This article first appeared on Medscape.com.
Children’s share of new COVID-19 cases is on the rise
The cumulative percentage of COVID-19 cases reported in children continues to climb, but “the history behind that cumulative number shows substantial change,” according to a new analysis of state health department data.
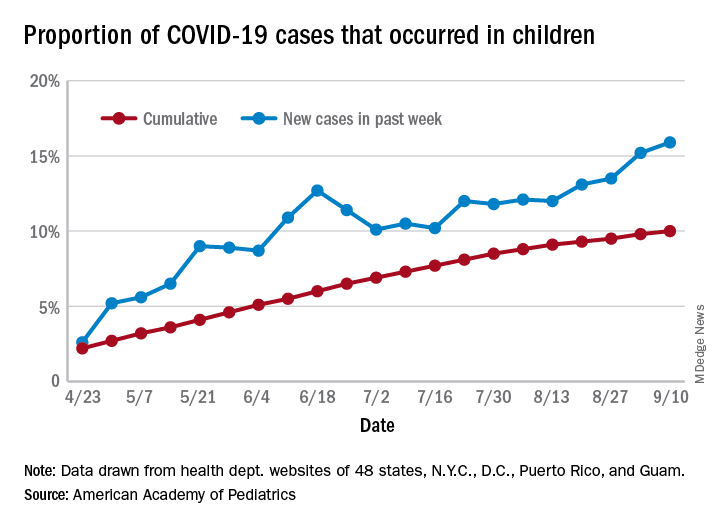
As of Sept. 10, the 549,432 cases in children represented 10.0% of all reported COVID-19 cases in the United States following a substantial rise over the course of the pandemic – the figure was 7.7% on July 16 and 3.2% on May 7, Blake Sisk, PhD, of the American Academy of Pediatrics and associates reported Sept. 29 in Pediatrics.
Unlike the cumulative number, the weekly proportion of cases in children fell early in the summer but then started climbing again in late July. Dr. Sisk and associates wrote.
Despite the increase, however, the proportion of pediatric COVID-19 cases is still well below children’s share of the overall population (22.6%). Also, “it is unclear how much of the increase in child cases is due to increased testing capacity, although CDC data from public and commercial laboratories show the share of all tests administered to children ages 0-17 has remained stable at 5%-7% since late April,” they said.
Data for the current report were drawn from 49 state health department websites (New York state does not report ages for COVID-19 cases), along with New York City, the District of Columbia, Puerto Rico, and Guam. Alabama changed its definition of a child case in August and was not included in the trend analysis (see graph), the investigators explained.
Those data show “substantial variation in case growth by region: in April, a preponderance of cases was in the Northeast. In June, cases surged in the South and West, followed by mid-July increases in the Midwest,” Dr. Sisk and associates said.
The increase among children in Midwest states is ongoing with the number of new cases reaching its highest level yet during the week ending Sept. 10, they reported.
SOURCE: Sisk B et al. Pediatrics. 2020 Sep 29. doi: 10.1542/peds.2020-027425.
The cumulative percentage of COVID-19 cases reported in children continues to climb, but “the history behind that cumulative number shows substantial change,” according to a new analysis of state health department data.

As of Sept. 10, the 549,432 cases in children represented 10.0% of all reported COVID-19 cases in the United States following a substantial rise over the course of the pandemic – the figure was 7.7% on July 16 and 3.2% on May 7, Blake Sisk, PhD, of the American Academy of Pediatrics and associates reported Sept. 29 in Pediatrics.
Unlike the cumulative number, the weekly proportion of cases in children fell early in the summer but then started climbing again in late July. Dr. Sisk and associates wrote.
Despite the increase, however, the proportion of pediatric COVID-19 cases is still well below children’s share of the overall population (22.6%). Also, “it is unclear how much of the increase in child cases is due to increased testing capacity, although CDC data from public and commercial laboratories show the share of all tests administered to children ages 0-17 has remained stable at 5%-7% since late April,” they said.
Data for the current report were drawn from 49 state health department websites (New York state does not report ages for COVID-19 cases), along with New York City, the District of Columbia, Puerto Rico, and Guam. Alabama changed its definition of a child case in August and was not included in the trend analysis (see graph), the investigators explained.
Those data show “substantial variation in case growth by region: in April, a preponderance of cases was in the Northeast. In June, cases surged in the South and West, followed by mid-July increases in the Midwest,” Dr. Sisk and associates said.
The increase among children in Midwest states is ongoing with the number of new cases reaching its highest level yet during the week ending Sept. 10, they reported.
SOURCE: Sisk B et al. Pediatrics. 2020 Sep 29. doi: 10.1542/peds.2020-027425.
The cumulative percentage of COVID-19 cases reported in children continues to climb, but “the history behind that cumulative number shows substantial change,” according to a new analysis of state health department data.

As of Sept. 10, the 549,432 cases in children represented 10.0% of all reported COVID-19 cases in the United States following a substantial rise over the course of the pandemic – the figure was 7.7% on July 16 and 3.2% on May 7, Blake Sisk, PhD, of the American Academy of Pediatrics and associates reported Sept. 29 in Pediatrics.
Unlike the cumulative number, the weekly proportion of cases in children fell early in the summer but then started climbing again in late July. Dr. Sisk and associates wrote.
Despite the increase, however, the proportion of pediatric COVID-19 cases is still well below children’s share of the overall population (22.6%). Also, “it is unclear how much of the increase in child cases is due to increased testing capacity, although CDC data from public and commercial laboratories show the share of all tests administered to children ages 0-17 has remained stable at 5%-7% since late April,” they said.
Data for the current report were drawn from 49 state health department websites (New York state does not report ages for COVID-19 cases), along with New York City, the District of Columbia, Puerto Rico, and Guam. Alabama changed its definition of a child case in August and was not included in the trend analysis (see graph), the investigators explained.
Those data show “substantial variation in case growth by region: in April, a preponderance of cases was in the Northeast. In June, cases surged in the South and West, followed by mid-July increases in the Midwest,” Dr. Sisk and associates said.
The increase among children in Midwest states is ongoing with the number of new cases reaching its highest level yet during the week ending Sept. 10, they reported.
SOURCE: Sisk B et al. Pediatrics. 2020 Sep 29. doi: 10.1542/peds.2020-027425.
FROM PEDIATRICS
J&J’s one-shot COVID-19 vaccine advances to phase 3 testing
The National Institute of Allergy and Infectious Diseases, which is aiding Johnson & Johnson with development, described this in a news release as the fourth phase 3 clinical trial of evaluating an investigational vaccine for coronavirus disease.
This NIAID tally tracks products likely to be presented soon for Food and Drug Administration approval. (The World Health Organization’s COVID vaccine tracker lists nine candidates as having reached this stage, including products developed in Russia and China.)
As many as 60,000 volunteers will be enrolled in the trial, with about 215 clinical research sites expected to participate, NIAID said. The vaccine will be tested in the United States and abroad.
The start of this test, known as the ENSEMBLE trial, follows positive results from a Phase 1/2a clinical study, which involved a single vaccination. The results of this study have been submitted to medRxiv and are set to be published online imminently.
New Brunswick, N.J–based J&J said it intends to offer the vaccine on “a not-for-profit basis for emergency pandemic use.” If testing proceeds well, J&J might seek an emergency use clearance for the vaccine, which could possibly allow the first batches to be made available in early 2021.
J&J’s vaccine is unusual in that it will be tested based on a single dose, while other advanced candidates have been tested in two-dose regimens.
J&J on Wednesday also released the study protocol for its phase 3 test. The developers of the other late-stage COVID vaccine candidates also have done this, as reported by Medscape Medical News. Because of the great interest in the COVID vaccine, the American Medical Association had last month asked the FDA to keep physicians informed of their COVID-19 vaccine review process.
Trials and tribulations
One of these experimental COVID vaccines already has had a setback in phase 3 testing, which is a fairly routine occurrence in drug development. But with a pandemic still causing deaths and disrupting lives around the world, there has been intense interest in each step of the effort to develop a COVID vaccine.
AstraZeneca PLC earlier this month announced a temporary cessation of all their coronavirus vaccine trials to investigate an “unexplained illness” that arose in a participant, as reported by Medscape Medical News.
On September 12, AstraZeneca announced that clinical trials for the AZD1222, which it developed with Oxford University, had resumed in the United Kingdom. On Wednesday, CNBC said Health and Human Services Secretary Alex Azar told the news station that AstraZeneca’s late-stage coronavirus vaccine trial in the United States remains on hold until safety concerns are resolved, a critical issue with all the fast-track COVID vaccines now being tested.
“Look at the AstraZeneca program, phase 3 clinical trial, a lot of hope. [A] single serious adverse event report in the United Kingdom, global shutdown, and [a] hold of the clinical trials,” Mr. Azar told CNBC.
The New York Times has reported on concerns stemming from serious neurologic illnesses in two participants, both women, who received AstraZeneca’s experimental vaccine in Britain.
The Senate Health, Education, Labor and Pensions Committee on Wednesday separately held a hearing with the leaders of the FDA and the Centers of Disease Control and Prevention, allowing an airing of lawmakers’ concerns about a potential rush to approve a COVID vaccine.
Details of J&J trial
The J&J trial is designed primarily to determine if the investigational vaccine can prevent moderate to severe COVID-19 after a single dose. It also is designed to examine whether the vaccine can prevent COVID-19 requiring medical intervention and if the vaccine can prevent milder cases of COVID-19 and asymptomatic SARS-CoV-2 infection, NIAID said.
Principal investigators for the phase 3 trial of the J & J vaccine are Paul A. Goepfert, MD, director of the Alabama Vaccine Research Clinic at the University of Alabama in Birmingham; Beatriz Grinsztejn, MD, PhD, director of the Laboratory of Clinical Research on HIV/AIDS at the Evandro Chagas National Institute of Infectious Diseases-Oswaldo Cruz Foundation in Rio de Janeiro, Brazil; and Glenda E. Gray, MBBCh, president and chief executive officer of the South African Medical Research Council and coprincipal investigator of the HIV Vaccine Trials Network.
This article first appeared on Medscape.com.
The National Institute of Allergy and Infectious Diseases, which is aiding Johnson & Johnson with development, described this in a news release as the fourth phase 3 clinical trial of evaluating an investigational vaccine for coronavirus disease.
This NIAID tally tracks products likely to be presented soon for Food and Drug Administration approval. (The World Health Organization’s COVID vaccine tracker lists nine candidates as having reached this stage, including products developed in Russia and China.)
As many as 60,000 volunteers will be enrolled in the trial, with about 215 clinical research sites expected to participate, NIAID said. The vaccine will be tested in the United States and abroad.
The start of this test, known as the ENSEMBLE trial, follows positive results from a Phase 1/2a clinical study, which involved a single vaccination. The results of this study have been submitted to medRxiv and are set to be published online imminently.
New Brunswick, N.J–based J&J said it intends to offer the vaccine on “a not-for-profit basis for emergency pandemic use.” If testing proceeds well, J&J might seek an emergency use clearance for the vaccine, which could possibly allow the first batches to be made available in early 2021.
J&J’s vaccine is unusual in that it will be tested based on a single dose, while other advanced candidates have been tested in two-dose regimens.
J&J on Wednesday also released the study protocol for its phase 3 test. The developers of the other late-stage COVID vaccine candidates also have done this, as reported by Medscape Medical News. Because of the great interest in the COVID vaccine, the American Medical Association had last month asked the FDA to keep physicians informed of their COVID-19 vaccine review process.
Trials and tribulations
One of these experimental COVID vaccines already has had a setback in phase 3 testing, which is a fairly routine occurrence in drug development. But with a pandemic still causing deaths and disrupting lives around the world, there has been intense interest in each step of the effort to develop a COVID vaccine.
AstraZeneca PLC earlier this month announced a temporary cessation of all their coronavirus vaccine trials to investigate an “unexplained illness” that arose in a participant, as reported by Medscape Medical News.
On September 12, AstraZeneca announced that clinical trials for the AZD1222, which it developed with Oxford University, had resumed in the United Kingdom. On Wednesday, CNBC said Health and Human Services Secretary Alex Azar told the news station that AstraZeneca’s late-stage coronavirus vaccine trial in the United States remains on hold until safety concerns are resolved, a critical issue with all the fast-track COVID vaccines now being tested.
“Look at the AstraZeneca program, phase 3 clinical trial, a lot of hope. [A] single serious adverse event report in the United Kingdom, global shutdown, and [a] hold of the clinical trials,” Mr. Azar told CNBC.
The New York Times has reported on concerns stemming from serious neurologic illnesses in two participants, both women, who received AstraZeneca’s experimental vaccine in Britain.
The Senate Health, Education, Labor and Pensions Committee on Wednesday separately held a hearing with the leaders of the FDA and the Centers of Disease Control and Prevention, allowing an airing of lawmakers’ concerns about a potential rush to approve a COVID vaccine.
Details of J&J trial
The J&J trial is designed primarily to determine if the investigational vaccine can prevent moderate to severe COVID-19 after a single dose. It also is designed to examine whether the vaccine can prevent COVID-19 requiring medical intervention and if the vaccine can prevent milder cases of COVID-19 and asymptomatic SARS-CoV-2 infection, NIAID said.
Principal investigators for the phase 3 trial of the J & J vaccine are Paul A. Goepfert, MD, director of the Alabama Vaccine Research Clinic at the University of Alabama in Birmingham; Beatriz Grinsztejn, MD, PhD, director of the Laboratory of Clinical Research on HIV/AIDS at the Evandro Chagas National Institute of Infectious Diseases-Oswaldo Cruz Foundation in Rio de Janeiro, Brazil; and Glenda E. Gray, MBBCh, president and chief executive officer of the South African Medical Research Council and coprincipal investigator of the HIV Vaccine Trials Network.
This article first appeared on Medscape.com.
The National Institute of Allergy and Infectious Diseases, which is aiding Johnson & Johnson with development, described this in a news release as the fourth phase 3 clinical trial of evaluating an investigational vaccine for coronavirus disease.
This NIAID tally tracks products likely to be presented soon for Food and Drug Administration approval. (The World Health Organization’s COVID vaccine tracker lists nine candidates as having reached this stage, including products developed in Russia and China.)
As many as 60,000 volunteers will be enrolled in the trial, with about 215 clinical research sites expected to participate, NIAID said. The vaccine will be tested in the United States and abroad.
The start of this test, known as the ENSEMBLE trial, follows positive results from a Phase 1/2a clinical study, which involved a single vaccination. The results of this study have been submitted to medRxiv and are set to be published online imminently.
New Brunswick, N.J–based J&J said it intends to offer the vaccine on “a not-for-profit basis for emergency pandemic use.” If testing proceeds well, J&J might seek an emergency use clearance for the vaccine, which could possibly allow the first batches to be made available in early 2021.
J&J’s vaccine is unusual in that it will be tested based on a single dose, while other advanced candidates have been tested in two-dose regimens.
J&J on Wednesday also released the study protocol for its phase 3 test. The developers of the other late-stage COVID vaccine candidates also have done this, as reported by Medscape Medical News. Because of the great interest in the COVID vaccine, the American Medical Association had last month asked the FDA to keep physicians informed of their COVID-19 vaccine review process.
Trials and tribulations
One of these experimental COVID vaccines already has had a setback in phase 3 testing, which is a fairly routine occurrence in drug development. But with a pandemic still causing deaths and disrupting lives around the world, there has been intense interest in each step of the effort to develop a COVID vaccine.
AstraZeneca PLC earlier this month announced a temporary cessation of all their coronavirus vaccine trials to investigate an “unexplained illness” that arose in a participant, as reported by Medscape Medical News.
On September 12, AstraZeneca announced that clinical trials for the AZD1222, which it developed with Oxford University, had resumed in the United Kingdom. On Wednesday, CNBC said Health and Human Services Secretary Alex Azar told the news station that AstraZeneca’s late-stage coronavirus vaccine trial in the United States remains on hold until safety concerns are resolved, a critical issue with all the fast-track COVID vaccines now being tested.
“Look at the AstraZeneca program, phase 3 clinical trial, a lot of hope. [A] single serious adverse event report in the United Kingdom, global shutdown, and [a] hold of the clinical trials,” Mr. Azar told CNBC.
The New York Times has reported on concerns stemming from serious neurologic illnesses in two participants, both women, who received AstraZeneca’s experimental vaccine in Britain.
The Senate Health, Education, Labor and Pensions Committee on Wednesday separately held a hearing with the leaders of the FDA and the Centers of Disease Control and Prevention, allowing an airing of lawmakers’ concerns about a potential rush to approve a COVID vaccine.
Details of J&J trial
The J&J trial is designed primarily to determine if the investigational vaccine can prevent moderate to severe COVID-19 after a single dose. It also is designed to examine whether the vaccine can prevent COVID-19 requiring medical intervention and if the vaccine can prevent milder cases of COVID-19 and asymptomatic SARS-CoV-2 infection, NIAID said.
Principal investigators for the phase 3 trial of the J & J vaccine are Paul A. Goepfert, MD, director of the Alabama Vaccine Research Clinic at the University of Alabama in Birmingham; Beatriz Grinsztejn, MD, PhD, director of the Laboratory of Clinical Research on HIV/AIDS at the Evandro Chagas National Institute of Infectious Diseases-Oswaldo Cruz Foundation in Rio de Janeiro, Brazil; and Glenda E. Gray, MBBCh, president and chief executive officer of the South African Medical Research Council and coprincipal investigator of the HIV Vaccine Trials Network.
This article first appeared on Medscape.com.
Hepatocellular carcinoma shows risk factor shift
Rates of hepatocellular carcinoma (HCC) continue to rise in the United States, but unevenly so given how the incidence has become highest in the Hispanic population, which is reflected in increased rates in the southern and western states, Hashem B. El-Serag, MD, of Baylor College of Medicine, Houston, said in a virtual presentation at the annual Digestive Diseases: New Advances, which is jointly provided by Rutgers and Global Academy for Medical Education.
In addition to this demographic shift, the risk factors for HCC are shifting, he said. Hepatitis C virus (HCV) has been the dominant risk factor for HCC; for patients with active HCV, the factors historically associated with increased HCC risk have included alcohol consumption, obesity, diabetes, coinfection, and genetics, he said.
This pattern is starting to change. In fact, for patients with active HCV, antiviral treatment with a sustained virologic response has surfaced as the most significant risk factor in the development of HCC, said Dr. El-Serag: Among these patients, sustained virologic response from direct-acting antivirals is associated with a significant reduction in HCC risk. However, it is important to recognize that a residual risk of HCC remains that doesn’t go away for several years, he noted.
“Who are those people who got treated, got cured, and still developed HCC? Those with cirrhosis at the time of treatment,” he said. Those with cirrhosis have cumulative incidence of 1.8% per year, but those without cirrhosis had very low risk, he said.
Some good news in HCC is that rates appear to be declining among young men, and this is thought to be one of the groups who are achieving a cure of HCV, he said.
“One would hope, if goals for HCV elimination are met, that will translate into massive reduction of HCC,” he said.
“The issue now for hepatitis is finding infected patients and curing them,” he noted.
Dr. El-Serag touched on hepatitis B (HBV), which continues to be the driving force of hepatitis infections globally. However, in patients who receive and respond to antiviral treatment “there is a significant and considerable reduction in HCC in the context of hepatitis B” similar to that seen with hepatitis C. Vaccination programs for HBV have started to make the desired impact of reducing HCC in HBV-endemic areas, he noted.
However, current risk factors for HCC are related less to HCV and HBV and more to metabolic syndrome because more people are treated for HCV and HBV, Dr. El-Serag said. He went on to address the new dominant global risk factor for HCC: obesity. Based on data from multiple studies, those who are obese, defined as a body mass index greater than 30 kg/m2, carry a twofold increased risk of developing HCC, he said.
To reduce this risk, treatment targets might address intermediate factors such as abdominal obesity, said Dr. El-Serag. He cited a study published in Hepatology in which individuals in the highest tertile for waist-hip ratio had a threefold higher risk of HCC, compared with those in the lowest tertile.
In addition, consideration of obesity must include type 2 diabetes, which is often linked to obesity and occurs in approximately one-third of adults in the United States, Dr. El-Serag said.
Treatment of type 2 diabetes may make a difference in HCC risk reduction, Dr. El-Serag noted. “The impact of treatment of diabetes on HCC risk is an area of intense interest,” he said. Based on the latest research, “the bottom line is that those treated with metformin experience a 50% reduction in the risk of HCC,” he said
Dr. El-Serag also acknowledged the impact of other risk factors for HCC: the use of statins and the presence of nonalcoholic fatty liver disease (NAFLD).
Dr. El-Serag noted that, among NAFLD patients, subgroups at even greater risk for HCC include those with diabetes, those older than 65 years, Hispanic race, and those with cirrhosis. These patients should be candidates for surveillance. Metabolic dysfunction traits such as obesity and diabetes are very common conditions, so it’s important to look at other, more specific factors, he added. “I hope that there will be tools to help clinicians classify or risk-stratify patients into different buckets,” he said.
Areas for further research on HCC continue to include risk stratification, mechanisms of action, and HCC prevention related to treatment of metabolic syndrome, he emphasized.
Dr. El-Serag had no financial conflicts to disclose. Global Academy for Medical Education and this news organization are owned by the same parent company.
*This story was updated on Oct. 28, 2020.
SOURCE: El-Serag HB. Digestive Diseases: New Advances 2020.
Rates of hepatocellular carcinoma (HCC) continue to rise in the United States, but unevenly so given how the incidence has become highest in the Hispanic population, which is reflected in increased rates in the southern and western states, Hashem B. El-Serag, MD, of Baylor College of Medicine, Houston, said in a virtual presentation at the annual Digestive Diseases: New Advances, which is jointly provided by Rutgers and Global Academy for Medical Education.
In addition to this demographic shift, the risk factors for HCC are shifting, he said. Hepatitis C virus (HCV) has been the dominant risk factor for HCC; for patients with active HCV, the factors historically associated with increased HCC risk have included alcohol consumption, obesity, diabetes, coinfection, and genetics, he said.
This pattern is starting to change. In fact, for patients with active HCV, antiviral treatment with a sustained virologic response has surfaced as the most significant risk factor in the development of HCC, said Dr. El-Serag: Among these patients, sustained virologic response from direct-acting antivirals is associated with a significant reduction in HCC risk. However, it is important to recognize that a residual risk of HCC remains that doesn’t go away for several years, he noted.
“Who are those people who got treated, got cured, and still developed HCC? Those with cirrhosis at the time of treatment,” he said. Those with cirrhosis have cumulative incidence of 1.8% per year, but those without cirrhosis had very low risk, he said.
Some good news in HCC is that rates appear to be declining among young men, and this is thought to be one of the groups who are achieving a cure of HCV, he said.
“One would hope, if goals for HCV elimination are met, that will translate into massive reduction of HCC,” he said.
“The issue now for hepatitis is finding infected patients and curing them,” he noted.
Dr. El-Serag touched on hepatitis B (HBV), which continues to be the driving force of hepatitis infections globally. However, in patients who receive and respond to antiviral treatment “there is a significant and considerable reduction in HCC in the context of hepatitis B” similar to that seen with hepatitis C. Vaccination programs for HBV have started to make the desired impact of reducing HCC in HBV-endemic areas, he noted.
However, current risk factors for HCC are related less to HCV and HBV and more to metabolic syndrome because more people are treated for HCV and HBV, Dr. El-Serag said. He went on to address the new dominant global risk factor for HCC: obesity. Based on data from multiple studies, those who are obese, defined as a body mass index greater than 30 kg/m2, carry a twofold increased risk of developing HCC, he said.
To reduce this risk, treatment targets might address intermediate factors such as abdominal obesity, said Dr. El-Serag. He cited a study published in Hepatology in which individuals in the highest tertile for waist-hip ratio had a threefold higher risk of HCC, compared with those in the lowest tertile.
In addition, consideration of obesity must include type 2 diabetes, which is often linked to obesity and occurs in approximately one-third of adults in the United States, Dr. El-Serag said.
Treatment of type 2 diabetes may make a difference in HCC risk reduction, Dr. El-Serag noted. “The impact of treatment of diabetes on HCC risk is an area of intense interest,” he said. Based on the latest research, “the bottom line is that those treated with metformin experience a 50% reduction in the risk of HCC,” he said
Dr. El-Serag also acknowledged the impact of other risk factors for HCC: the use of statins and the presence of nonalcoholic fatty liver disease (NAFLD).
Dr. El-Serag noted that, among NAFLD patients, subgroups at even greater risk for HCC include those with diabetes, those older than 65 years, Hispanic race, and those with cirrhosis. These patients should be candidates for surveillance. Metabolic dysfunction traits such as obesity and diabetes are very common conditions, so it’s important to look at other, more specific factors, he added. “I hope that there will be tools to help clinicians classify or risk-stratify patients into different buckets,” he said.
Areas for further research on HCC continue to include risk stratification, mechanisms of action, and HCC prevention related to treatment of metabolic syndrome, he emphasized.
Dr. El-Serag had no financial conflicts to disclose. Global Academy for Medical Education and this news organization are owned by the same parent company.
*This story was updated on Oct. 28, 2020.
SOURCE: El-Serag HB. Digestive Diseases: New Advances 2020.
Rates of hepatocellular carcinoma (HCC) continue to rise in the United States, but unevenly so given how the incidence has become highest in the Hispanic population, which is reflected in increased rates in the southern and western states, Hashem B. El-Serag, MD, of Baylor College of Medicine, Houston, said in a virtual presentation at the annual Digestive Diseases: New Advances, which is jointly provided by Rutgers and Global Academy for Medical Education.
In addition to this demographic shift, the risk factors for HCC are shifting, he said. Hepatitis C virus (HCV) has been the dominant risk factor for HCC; for patients with active HCV, the factors historically associated with increased HCC risk have included alcohol consumption, obesity, diabetes, coinfection, and genetics, he said.
This pattern is starting to change. In fact, for patients with active HCV, antiviral treatment with a sustained virologic response has surfaced as the most significant risk factor in the development of HCC, said Dr. El-Serag: Among these patients, sustained virologic response from direct-acting antivirals is associated with a significant reduction in HCC risk. However, it is important to recognize that a residual risk of HCC remains that doesn’t go away for several years, he noted.
“Who are those people who got treated, got cured, and still developed HCC? Those with cirrhosis at the time of treatment,” he said. Those with cirrhosis have cumulative incidence of 1.8% per year, but those without cirrhosis had very low risk, he said.
Some good news in HCC is that rates appear to be declining among young men, and this is thought to be one of the groups who are achieving a cure of HCV, he said.
“One would hope, if goals for HCV elimination are met, that will translate into massive reduction of HCC,” he said.
“The issue now for hepatitis is finding infected patients and curing them,” he noted.
Dr. El-Serag touched on hepatitis B (HBV), which continues to be the driving force of hepatitis infections globally. However, in patients who receive and respond to antiviral treatment “there is a significant and considerable reduction in HCC in the context of hepatitis B” similar to that seen with hepatitis C. Vaccination programs for HBV have started to make the desired impact of reducing HCC in HBV-endemic areas, he noted.
However, current risk factors for HCC are related less to HCV and HBV and more to metabolic syndrome because more people are treated for HCV and HBV, Dr. El-Serag said. He went on to address the new dominant global risk factor for HCC: obesity. Based on data from multiple studies, those who are obese, defined as a body mass index greater than 30 kg/m2, carry a twofold increased risk of developing HCC, he said.
To reduce this risk, treatment targets might address intermediate factors such as abdominal obesity, said Dr. El-Serag. He cited a study published in Hepatology in which individuals in the highest tertile for waist-hip ratio had a threefold higher risk of HCC, compared with those in the lowest tertile.
In addition, consideration of obesity must include type 2 diabetes, which is often linked to obesity and occurs in approximately one-third of adults in the United States, Dr. El-Serag said.
Treatment of type 2 diabetes may make a difference in HCC risk reduction, Dr. El-Serag noted. “The impact of treatment of diabetes on HCC risk is an area of intense interest,” he said. Based on the latest research, “the bottom line is that those treated with metformin experience a 50% reduction in the risk of HCC,” he said
Dr. El-Serag also acknowledged the impact of other risk factors for HCC: the use of statins and the presence of nonalcoholic fatty liver disease (NAFLD).
Dr. El-Serag noted that, among NAFLD patients, subgroups at even greater risk for HCC include those with diabetes, those older than 65 years, Hispanic race, and those with cirrhosis. These patients should be candidates for surveillance. Metabolic dysfunction traits such as obesity and diabetes are very common conditions, so it’s important to look at other, more specific factors, he added. “I hope that there will be tools to help clinicians classify or risk-stratify patients into different buckets,” he said.
Areas for further research on HCC continue to include risk stratification, mechanisms of action, and HCC prevention related to treatment of metabolic syndrome, he emphasized.
Dr. El-Serag had no financial conflicts to disclose. Global Academy for Medical Education and this news organization are owned by the same parent company.
*This story was updated on Oct. 28, 2020.
SOURCE: El-Serag HB. Digestive Diseases: New Advances 2020.
FROM DIGESTIVE DISEASES: NEW ADVANCES
CDC playbook prepares states for rollout of COVID-19 vaccine if one is approved
States have begun preparing to distribute a COVID-19 vaccine if one is approved, a CDC official said today.
The CDC released guidance for states on Sept. 16 titled COVID-19 Vaccination Program Interim Playbook for Jurisdiction Operations. The document discusses vaccine ordering, storage, and handling and says that states should submit their plans for vaccine distribution to the agency by Oct. 16.
“Every jurisdiction is heavily involved right now in their plan development,” CDC official Janell Routh, MD, told the Advisory Committee on Immunization Practices during its Sept. 22 meeting. “It was really impressive to me that, even though the playbook only went out last week, states and jurisdictions have been thinking about this for quite some time.”
However, one committee member suggested that setting a deadline before more safety, efficacy, and storage information is known may be premature.
“I cannot imagine that we will actually know the final storage requirements for this vaccine by Oct. 16, which makes me a little concerned about finalizing state plans,” said Helen “Keipp” Talbot, MD, MPH, associate professor of medicine at Vanderbilt University Medical Center in Nashville, Tenn. “We also don’t know the best populations yet when it comes to efficacy and safety.”
Dr. Routh said the CDC is asking states to plan on the basis of assumptions. “We know those plans will constantly be improving, changing, as we learn more information,” Dr. Routh said. States agreed to return a plan 30 days after the playbook was released, which is how the Oct. 16 deadline was established, she said.
States are encouraged to think broadly. Plans may include contingencies for a product that requires ultracold storage or for distributing more than one vaccine product, Dr. Routh said.
“One goal is to be ready on the first day that we can actually distribute vaccine,” Nancy Messonnier, MD, director of the National Center for Immunization and Respiratory Diseases, said during the meeting. “Our colleagues in Operation Warp Speed say that they expect there will be vaccine as early as November, and therefore we need to be ready so there is no delay in distributing that vaccine. And that phase, that early phase, is really close upon us.”
Many states have already developed plans, and the CDC is providing technical assistance as needed to monitor the plans regularly, Dr. Routh said.
Key issues identified
From holding pilot meetings with five jurisdictions, officials learned that public confidence in the vaccine is among states’ greatest concerns, Dr. Routh said. In addition, distribution is resource intensive, and social distancing adds logistical complexity.
Specific guidance on whom to vaccinate in the early stages will smooth the process, officials suggested during the pilot meetings. For the first several weeks, vaccine doses may be limited to priority populations, such as health care workers.
“This interim playbook is a living document,” Dr. Routh emphasized. “We definitely plan to update the content regularly as we learn more information about what vaccines and when they will be released.”
During the early stages of COVID-19 vaccination, officials plan to implement an enhanced monitoring program in which vaccine recipients would complete surveys about adverse events, in addition to the traditional vaccine safety monitoring programs that already exist, officials said.
A version of this article originally appeared on Medscape.com.
States have begun preparing to distribute a COVID-19 vaccine if one is approved, a CDC official said today.
The CDC released guidance for states on Sept. 16 titled COVID-19 Vaccination Program Interim Playbook for Jurisdiction Operations. The document discusses vaccine ordering, storage, and handling and says that states should submit their plans for vaccine distribution to the agency by Oct. 16.
“Every jurisdiction is heavily involved right now in their plan development,” CDC official Janell Routh, MD, told the Advisory Committee on Immunization Practices during its Sept. 22 meeting. “It was really impressive to me that, even though the playbook only went out last week, states and jurisdictions have been thinking about this for quite some time.”
However, one committee member suggested that setting a deadline before more safety, efficacy, and storage information is known may be premature.
“I cannot imagine that we will actually know the final storage requirements for this vaccine by Oct. 16, which makes me a little concerned about finalizing state plans,” said Helen “Keipp” Talbot, MD, MPH, associate professor of medicine at Vanderbilt University Medical Center in Nashville, Tenn. “We also don’t know the best populations yet when it comes to efficacy and safety.”
Dr. Routh said the CDC is asking states to plan on the basis of assumptions. “We know those plans will constantly be improving, changing, as we learn more information,” Dr. Routh said. States agreed to return a plan 30 days after the playbook was released, which is how the Oct. 16 deadline was established, she said.
States are encouraged to think broadly. Plans may include contingencies for a product that requires ultracold storage or for distributing more than one vaccine product, Dr. Routh said.
“One goal is to be ready on the first day that we can actually distribute vaccine,” Nancy Messonnier, MD, director of the National Center for Immunization and Respiratory Diseases, said during the meeting. “Our colleagues in Operation Warp Speed say that they expect there will be vaccine as early as November, and therefore we need to be ready so there is no delay in distributing that vaccine. And that phase, that early phase, is really close upon us.”
Many states have already developed plans, and the CDC is providing technical assistance as needed to monitor the plans regularly, Dr. Routh said.
Key issues identified
From holding pilot meetings with five jurisdictions, officials learned that public confidence in the vaccine is among states’ greatest concerns, Dr. Routh said. In addition, distribution is resource intensive, and social distancing adds logistical complexity.
Specific guidance on whom to vaccinate in the early stages will smooth the process, officials suggested during the pilot meetings. For the first several weeks, vaccine doses may be limited to priority populations, such as health care workers.
“This interim playbook is a living document,” Dr. Routh emphasized. “We definitely plan to update the content regularly as we learn more information about what vaccines and when they will be released.”
During the early stages of COVID-19 vaccination, officials plan to implement an enhanced monitoring program in which vaccine recipients would complete surveys about adverse events, in addition to the traditional vaccine safety monitoring programs that already exist, officials said.
A version of this article originally appeared on Medscape.com.
States have begun preparing to distribute a COVID-19 vaccine if one is approved, a CDC official said today.
The CDC released guidance for states on Sept. 16 titled COVID-19 Vaccination Program Interim Playbook for Jurisdiction Operations. The document discusses vaccine ordering, storage, and handling and says that states should submit their plans for vaccine distribution to the agency by Oct. 16.
“Every jurisdiction is heavily involved right now in their plan development,” CDC official Janell Routh, MD, told the Advisory Committee on Immunization Practices during its Sept. 22 meeting. “It was really impressive to me that, even though the playbook only went out last week, states and jurisdictions have been thinking about this for quite some time.”
However, one committee member suggested that setting a deadline before more safety, efficacy, and storage information is known may be premature.
“I cannot imagine that we will actually know the final storage requirements for this vaccine by Oct. 16, which makes me a little concerned about finalizing state plans,” said Helen “Keipp” Talbot, MD, MPH, associate professor of medicine at Vanderbilt University Medical Center in Nashville, Tenn. “We also don’t know the best populations yet when it comes to efficacy and safety.”
Dr. Routh said the CDC is asking states to plan on the basis of assumptions. “We know those plans will constantly be improving, changing, as we learn more information,” Dr. Routh said. States agreed to return a plan 30 days after the playbook was released, which is how the Oct. 16 deadline was established, she said.
States are encouraged to think broadly. Plans may include contingencies for a product that requires ultracold storage or for distributing more than one vaccine product, Dr. Routh said.
“One goal is to be ready on the first day that we can actually distribute vaccine,” Nancy Messonnier, MD, director of the National Center for Immunization and Respiratory Diseases, said during the meeting. “Our colleagues in Operation Warp Speed say that they expect there will be vaccine as early as November, and therefore we need to be ready so there is no delay in distributing that vaccine. And that phase, that early phase, is really close upon us.”
Many states have already developed plans, and the CDC is providing technical assistance as needed to monitor the plans regularly, Dr. Routh said.
Key issues identified
From holding pilot meetings with five jurisdictions, officials learned that public confidence in the vaccine is among states’ greatest concerns, Dr. Routh said. In addition, distribution is resource intensive, and social distancing adds logistical complexity.
Specific guidance on whom to vaccinate in the early stages will smooth the process, officials suggested during the pilot meetings. For the first several weeks, vaccine doses may be limited to priority populations, such as health care workers.
“This interim playbook is a living document,” Dr. Routh emphasized. “We definitely plan to update the content regularly as we learn more information about what vaccines and when they will be released.”
During the early stages of COVID-19 vaccination, officials plan to implement an enhanced monitoring program in which vaccine recipients would complete surveys about adverse events, in addition to the traditional vaccine safety monitoring programs that already exist, officials said.
A version of this article originally appeared on Medscape.com.
Three major COVID vaccine developers release detailed trial protocols
Typically, manufacturers guard the specifics of preclinical vaccine trials. This rare move follows calls for greater transparency. For example, the American Medical Association wrote a letter in late August asking the Food and Drug Administration to keep physicians informed of their COVID-19 vaccine review process.
On September 17, ModernaTx released the phase 3 trial protocol for its mRNA-1273 SARS-CoV-2 vaccine. In short order, on September 19, Pfizer/BioNTech shared their phase 1/2/3 trial vaccine protocol. AstraZeneca, which is developing a vaccine along with Oxford University, also released its protocol.
The AstraZeneca vaccine trial made headlines recently for having to be temporarily halted because of unexpected illnesses that arose in two participants, according to the New York Times and other sources.
“I applaud the release of the clinical trial protocols by the companies. The public trust in any COVID-19 vaccine is paramount, especially given the fast timeline and perceived political pressures of these candidates,” Robert Kruse, MD, PhD, told Medscape Medical News when asked to comment.
AstraZeneca takes a shot at transparency
The three primary objectives of the AstraZeneca AZD1222 trial outlined in the 110-page protocol include estimating the efficacy, safety, tolerability, and reactogenicity associated with two intramuscular doses of the vaccine in comparison with placebo in adults.
The projected enrollment is 30,000 participants, and the estimated primary completion date is Dec. 2, 2020, according to information on clinicaltrials.gov.
“Given the unprecedented global impact of the coronavirus pandemic and the need for public information, AstraZeneca has published the detailed protocol and design of our AZD1222 clinical trial,” the company said in a statement. “As with most clinical development, protocols are not typically shared publicly due to the importance of maintaining confidentiality and integrity of trials.
“AstraZeneca continues to work with industry peers to ensure a consistent approach to sharing timely clinical trial information,” the company added.
Moderna methodology
The ModernaTX 135-page protocol outlines the primary trial objectives of evaluating efficacy, safety, and reactogenicity of two injections of the vaccine administered 28 days apart. Researchers also plan to randomly assign 30,000 adults to receive either vaccine or placebo. The estimated primary completion date is Oct. 27, 2022.
A statement that was requested from ModernaTX was not received by press time.
Pfizer protocol
In the Pfizer/BioNTech vaccine trial, researchers plan to evaluate different doses in different age groups in a multistep protocol. The trial features 20 primary safety objectives, which include reporting adverse events and serious adverse events, including any local or systemic events.
Efficacy endpoints are secondary objectives. The estimated enrollment is 29,481 adults; the estimated primary completion date is April 19, 2021.
“Pfizer and BioNTech recognize that the COVID-19 pandemic is a unique circumstance, and the need for transparency is clear,” Pfizer spokesperson Sharon Castillo told Medscape Medical News. By making the full protocol available, “we believe this will reinforce our long-standing commitment to scientific and regulatory rigor that benefits patients,” she said.
“Based on current infection rates, Pfizer and BioNTech continue to expect that a conclusive read-out on efficacy is likely by the end of October. Neither Pfizer nor the FDA can move faster than the data we are generating through our clinical trial,” Castillo said.
If clinical work and regulatory approval or authorization proceed as planned, Pfizer and BioNTech expect to supply up to 100 million doses worldwide by the end of 2020 and approximately 1.3 billion doses worldwide by the end of 2021.
Pfizer is not willing to sacrifice safety and efficacy in the name of expediency, Castillo said. “We will not cut corners in this pursuit. Patient safety is our highest priority, and Pfizer will not bring a vaccine to market without adequate evidence of safety and efficacy.”
A positive move
“COVID-19 vaccines will only be useful if many people are willing to receive them,” said Kruse, a postgraduate year 3 resident in the Department of Pathology at Johns Hopkins Medicine in Baltimore, Maryland.
“By giving the general public along with other scientists and physicians the opportunity to critique the protocols, everyone can understand what the metrics would be for an early look at efficacy,” Kruse said. He noted that information could help inform a potential FDA emergency use authorization.
Kruse has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Typically, manufacturers guard the specifics of preclinical vaccine trials. This rare move follows calls for greater transparency. For example, the American Medical Association wrote a letter in late August asking the Food and Drug Administration to keep physicians informed of their COVID-19 vaccine review process.
On September 17, ModernaTx released the phase 3 trial protocol for its mRNA-1273 SARS-CoV-2 vaccine. In short order, on September 19, Pfizer/BioNTech shared their phase 1/2/3 trial vaccine protocol. AstraZeneca, which is developing a vaccine along with Oxford University, also released its protocol.
The AstraZeneca vaccine trial made headlines recently for having to be temporarily halted because of unexpected illnesses that arose in two participants, according to the New York Times and other sources.
“I applaud the release of the clinical trial protocols by the companies. The public trust in any COVID-19 vaccine is paramount, especially given the fast timeline and perceived political pressures of these candidates,” Robert Kruse, MD, PhD, told Medscape Medical News when asked to comment.
AstraZeneca takes a shot at transparency
The three primary objectives of the AstraZeneca AZD1222 trial outlined in the 110-page protocol include estimating the efficacy, safety, tolerability, and reactogenicity associated with two intramuscular doses of the vaccine in comparison with placebo in adults.
The projected enrollment is 30,000 participants, and the estimated primary completion date is Dec. 2, 2020, according to information on clinicaltrials.gov.
“Given the unprecedented global impact of the coronavirus pandemic and the need for public information, AstraZeneca has published the detailed protocol and design of our AZD1222 clinical trial,” the company said in a statement. “As with most clinical development, protocols are not typically shared publicly due to the importance of maintaining confidentiality and integrity of trials.
“AstraZeneca continues to work with industry peers to ensure a consistent approach to sharing timely clinical trial information,” the company added.
Moderna methodology
The ModernaTX 135-page protocol outlines the primary trial objectives of evaluating efficacy, safety, and reactogenicity of two injections of the vaccine administered 28 days apart. Researchers also plan to randomly assign 30,000 adults to receive either vaccine or placebo. The estimated primary completion date is Oct. 27, 2022.
A statement that was requested from ModernaTX was not received by press time.
Pfizer protocol
In the Pfizer/BioNTech vaccine trial, researchers plan to evaluate different doses in different age groups in a multistep protocol. The trial features 20 primary safety objectives, which include reporting adverse events and serious adverse events, including any local or systemic events.
Efficacy endpoints are secondary objectives. The estimated enrollment is 29,481 adults; the estimated primary completion date is April 19, 2021.
“Pfizer and BioNTech recognize that the COVID-19 pandemic is a unique circumstance, and the need for transparency is clear,” Pfizer spokesperson Sharon Castillo told Medscape Medical News. By making the full protocol available, “we believe this will reinforce our long-standing commitment to scientific and regulatory rigor that benefits patients,” she said.
“Based on current infection rates, Pfizer and BioNTech continue to expect that a conclusive read-out on efficacy is likely by the end of October. Neither Pfizer nor the FDA can move faster than the data we are generating through our clinical trial,” Castillo said.
If clinical work and regulatory approval or authorization proceed as planned, Pfizer and BioNTech expect to supply up to 100 million doses worldwide by the end of 2020 and approximately 1.3 billion doses worldwide by the end of 2021.
Pfizer is not willing to sacrifice safety and efficacy in the name of expediency, Castillo said. “We will not cut corners in this pursuit. Patient safety is our highest priority, and Pfizer will not bring a vaccine to market without adequate evidence of safety and efficacy.”
A positive move
“COVID-19 vaccines will only be useful if many people are willing to receive them,” said Kruse, a postgraduate year 3 resident in the Department of Pathology at Johns Hopkins Medicine in Baltimore, Maryland.
“By giving the general public along with other scientists and physicians the opportunity to critique the protocols, everyone can understand what the metrics would be for an early look at efficacy,” Kruse said. He noted that information could help inform a potential FDA emergency use authorization.
Kruse has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Typically, manufacturers guard the specifics of preclinical vaccine trials. This rare move follows calls for greater transparency. For example, the American Medical Association wrote a letter in late August asking the Food and Drug Administration to keep physicians informed of their COVID-19 vaccine review process.
On September 17, ModernaTx released the phase 3 trial protocol for its mRNA-1273 SARS-CoV-2 vaccine. In short order, on September 19, Pfizer/BioNTech shared their phase 1/2/3 trial vaccine protocol. AstraZeneca, which is developing a vaccine along with Oxford University, also released its protocol.
The AstraZeneca vaccine trial made headlines recently for having to be temporarily halted because of unexpected illnesses that arose in two participants, according to the New York Times and other sources.
“I applaud the release of the clinical trial protocols by the companies. The public trust in any COVID-19 vaccine is paramount, especially given the fast timeline and perceived political pressures of these candidates,” Robert Kruse, MD, PhD, told Medscape Medical News when asked to comment.
AstraZeneca takes a shot at transparency
The three primary objectives of the AstraZeneca AZD1222 trial outlined in the 110-page protocol include estimating the efficacy, safety, tolerability, and reactogenicity associated with two intramuscular doses of the vaccine in comparison with placebo in adults.
The projected enrollment is 30,000 participants, and the estimated primary completion date is Dec. 2, 2020, according to information on clinicaltrials.gov.
“Given the unprecedented global impact of the coronavirus pandemic and the need for public information, AstraZeneca has published the detailed protocol and design of our AZD1222 clinical trial,” the company said in a statement. “As with most clinical development, protocols are not typically shared publicly due to the importance of maintaining confidentiality and integrity of trials.
“AstraZeneca continues to work with industry peers to ensure a consistent approach to sharing timely clinical trial information,” the company added.
Moderna methodology
The ModernaTX 135-page protocol outlines the primary trial objectives of evaluating efficacy, safety, and reactogenicity of two injections of the vaccine administered 28 days apart. Researchers also plan to randomly assign 30,000 adults to receive either vaccine or placebo. The estimated primary completion date is Oct. 27, 2022.
A statement that was requested from ModernaTX was not received by press time.
Pfizer protocol
In the Pfizer/BioNTech vaccine trial, researchers plan to evaluate different doses in different age groups in a multistep protocol. The trial features 20 primary safety objectives, which include reporting adverse events and serious adverse events, including any local or systemic events.
Efficacy endpoints are secondary objectives. The estimated enrollment is 29,481 adults; the estimated primary completion date is April 19, 2021.
“Pfizer and BioNTech recognize that the COVID-19 pandemic is a unique circumstance, and the need for transparency is clear,” Pfizer spokesperson Sharon Castillo told Medscape Medical News. By making the full protocol available, “we believe this will reinforce our long-standing commitment to scientific and regulatory rigor that benefits patients,” she said.
“Based on current infection rates, Pfizer and BioNTech continue to expect that a conclusive read-out on efficacy is likely by the end of October. Neither Pfizer nor the FDA can move faster than the data we are generating through our clinical trial,” Castillo said.
If clinical work and regulatory approval or authorization proceed as planned, Pfizer and BioNTech expect to supply up to 100 million doses worldwide by the end of 2020 and approximately 1.3 billion doses worldwide by the end of 2021.
Pfizer is not willing to sacrifice safety and efficacy in the name of expediency, Castillo said. “We will not cut corners in this pursuit. Patient safety is our highest priority, and Pfizer will not bring a vaccine to market without adequate evidence of safety and efficacy.”
A positive move
“COVID-19 vaccines will only be useful if many people are willing to receive them,” said Kruse, a postgraduate year 3 resident in the Department of Pathology at Johns Hopkins Medicine in Baltimore, Maryland.
“By giving the general public along with other scientists and physicians the opportunity to critique the protocols, everyone can understand what the metrics would be for an early look at efficacy,” Kruse said. He noted that information could help inform a potential FDA emergency use authorization.
Kruse has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Smart health devices – promises and pitfalls
What needs to be done before the data deluge hits the office
Hurricane Sally recently crossed the Gulf of Mexico and landed with torrential rainfalls along the Alabama coast. A little rainfall is important for crops; too much leads to devastation. As physicians, we need data in order to help manage patients’ illnesses and to help to keep them healthy. Our fear though is that too much data provided too quickly may have the opposite effect.
Personal monitoring devices
When I bought my first Fitbit 7 years ago, I was enamored with the technology. The Fitbit was little more than a step tracker, yet I proudly wore its black rubber strap on my wrist. It was my first foray into wearable technology, and it felt quite empowering to have an objective way to track my fitness beyond just using my bathroom scale. Now less than a decade later, that Fitbit looks archaic in comparison with the wrist-top technology currently available.
As I write this, the world’s largest technology company is in the process of releasing its sixth-generation Apple Watch. In addition to acting as a smartphone, this new device, which is barely larger than a postage stamp, offers GPS-based movement tracking, the ability to detect falls, continuous heart rate monitoring, a built-in EKG capable of diagnosing atrial fibrillation, and an oxygen saturation sensor. These features weren’t added thoughtlessly. Apple is marketing this as a health-focused device, with their primary advertising campaign claiming that “the future of health is on your wrist,” and they aren’t the only company making this play.
Along with Apple, Samsung, Withings, Fitbit, and other companies continue to bring products to market that monitor our activity and provide new insights into our health. Typically linked to smartphone-based apps, these devices record all of their measurements for later review, while software helps interpret the findings to make them actionable. From heart rate tracking to sleep analysis, these options now provide access to volumes of data that promise to improve our wellness and change our lives. Of course, those promises will only be fulfilled if our behavior is altered as a consequence of having more detailed information. Whether that will happen remains to be seen.
Health system–linked devices
Major advancements in medical monitoring technology are now enabling physicians to get much deeper insight into their patients’ health status. Internet-connected scales, blood pressure cuffs, and exercise equipment offer the ability to upload information into patient portals and integrate that information into EHRs. New devices provide access to information that previously was impossible to obtain. For example, wearable continuous blood glucose monitors, such as the FreeStyle Libre or DexCom’s G6, allow patients and physicians to follow blood sugar readings 24 hours a day. This provides unprecedented awareness of diabetes control and relieves the pain and inconvenience of finger sticks and blood draws. It also aids with compliance because patients don’t need to remember to check their sugar levels on a schedule.
Other compliance-boosting breakthroughs, such as Bluetooth-enabled asthma inhalers and cellular-connected continuous positive airway pressure machines, assist patients with managing chronic respiratory conditions. Many companies are developing technologies to manage acute conditions as well. One such company, an on-demand telemedicine provider called TytoCare, has developed a $299 suite of instruments that includes a digital stethoscope, thermometer, and camera-based otoscope. In concert with a virtual visit, their providers can remotely use these tools to examine and assess sick individuals. This virtual “laying on of hands” may have sounded like science fiction and likely would have been rejected by patients just a few years ago. Now it is becoming commonplace and will soon be an expectation of many seeking care.
But if we are to be successful, everyone must acknowledge that this revolution in health care brings many challenges along with it. One of those is the deluge of data that connected devices provide.
Information overload
There is such a thing as “too much of a good thing.” Described by journalist David Shenk as “data smog” in his 1997 book of the same name, the idea is clear: There is only so much information we can assimilate.
Even after years of using EHRs and with government-implemented incentives that promote “meaningful use,” physicians are still struggling with EHRs. Additionally, many have expressed frustration with the connectedness that EHRs provide and lament their inability to ever really “leave the office.” As more and more data become available to physicians, the challenge of how to assimilate and act on those data will continue to grow. The addition of patient-provided health statistics will only make information overload worse, with clinicians will feeling an ever-growing burden to know, understand, and act on this information.
Unless we develop systems to sort, filter, and prioritize the flow of information, there is potential for liability from not acting on the amount of virtual information doctors receive. This new risk for already fatigued and overburdened physicians combined with an increase in the amount of virtual information at doctors’ fingertips may lead to the value of patient data being lost.
Dr. Notte is a family physician and chief medical officer of Abington (Pa.) Hospital–Jefferson Health. Follow him on Twitter (@doctornotte). Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Philadelphia, and associate director of the family medicine residency program at Abington Hospital–Jefferson Health. They have no conflicts related to the content of this piece.
What needs to be done before the data deluge hits the office
What needs to be done before the data deluge hits the office
Hurricane Sally recently crossed the Gulf of Mexico and landed with torrential rainfalls along the Alabama coast. A little rainfall is important for crops; too much leads to devastation. As physicians, we need data in order to help manage patients’ illnesses and to help to keep them healthy. Our fear though is that too much data provided too quickly may have the opposite effect.
Personal monitoring devices
When I bought my first Fitbit 7 years ago, I was enamored with the technology. The Fitbit was little more than a step tracker, yet I proudly wore its black rubber strap on my wrist. It was my first foray into wearable technology, and it felt quite empowering to have an objective way to track my fitness beyond just using my bathroom scale. Now less than a decade later, that Fitbit looks archaic in comparison with the wrist-top technology currently available.
As I write this, the world’s largest technology company is in the process of releasing its sixth-generation Apple Watch. In addition to acting as a smartphone, this new device, which is barely larger than a postage stamp, offers GPS-based movement tracking, the ability to detect falls, continuous heart rate monitoring, a built-in EKG capable of diagnosing atrial fibrillation, and an oxygen saturation sensor. These features weren’t added thoughtlessly. Apple is marketing this as a health-focused device, with their primary advertising campaign claiming that “the future of health is on your wrist,” and they aren’t the only company making this play.
Along with Apple, Samsung, Withings, Fitbit, and other companies continue to bring products to market that monitor our activity and provide new insights into our health. Typically linked to smartphone-based apps, these devices record all of their measurements for later review, while software helps interpret the findings to make them actionable. From heart rate tracking to sleep analysis, these options now provide access to volumes of data that promise to improve our wellness and change our lives. Of course, those promises will only be fulfilled if our behavior is altered as a consequence of having more detailed information. Whether that will happen remains to be seen.
Health system–linked devices
Major advancements in medical monitoring technology are now enabling physicians to get much deeper insight into their patients’ health status. Internet-connected scales, blood pressure cuffs, and exercise equipment offer the ability to upload information into patient portals and integrate that information into EHRs. New devices provide access to information that previously was impossible to obtain. For example, wearable continuous blood glucose monitors, such as the FreeStyle Libre or DexCom’s G6, allow patients and physicians to follow blood sugar readings 24 hours a day. This provides unprecedented awareness of diabetes control and relieves the pain and inconvenience of finger sticks and blood draws. It also aids with compliance because patients don’t need to remember to check their sugar levels on a schedule.
Other compliance-boosting breakthroughs, such as Bluetooth-enabled asthma inhalers and cellular-connected continuous positive airway pressure machines, assist patients with managing chronic respiratory conditions. Many companies are developing technologies to manage acute conditions as well. One such company, an on-demand telemedicine provider called TytoCare, has developed a $299 suite of instruments that includes a digital stethoscope, thermometer, and camera-based otoscope. In concert with a virtual visit, their providers can remotely use these tools to examine and assess sick individuals. This virtual “laying on of hands” may have sounded like science fiction and likely would have been rejected by patients just a few years ago. Now it is becoming commonplace and will soon be an expectation of many seeking care.
But if we are to be successful, everyone must acknowledge that this revolution in health care brings many challenges along with it. One of those is the deluge of data that connected devices provide.
Information overload
There is such a thing as “too much of a good thing.” Described by journalist David Shenk as “data smog” in his 1997 book of the same name, the idea is clear: There is only so much information we can assimilate.
Even after years of using EHRs and with government-implemented incentives that promote “meaningful use,” physicians are still struggling with EHRs. Additionally, many have expressed frustration with the connectedness that EHRs provide and lament their inability to ever really “leave the office.” As more and more data become available to physicians, the challenge of how to assimilate and act on those data will continue to grow. The addition of patient-provided health statistics will only make information overload worse, with clinicians will feeling an ever-growing burden to know, understand, and act on this information.
Unless we develop systems to sort, filter, and prioritize the flow of information, there is potential for liability from not acting on the amount of virtual information doctors receive. This new risk for already fatigued and overburdened physicians combined with an increase in the amount of virtual information at doctors’ fingertips may lead to the value of patient data being lost.
Dr. Notte is a family physician and chief medical officer of Abington (Pa.) Hospital–Jefferson Health. Follow him on Twitter (@doctornotte). Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Philadelphia, and associate director of the family medicine residency program at Abington Hospital–Jefferson Health. They have no conflicts related to the content of this piece.
Hurricane Sally recently crossed the Gulf of Mexico and landed with torrential rainfalls along the Alabama coast. A little rainfall is important for crops; too much leads to devastation. As physicians, we need data in order to help manage patients’ illnesses and to help to keep them healthy. Our fear though is that too much data provided too quickly may have the opposite effect.
Personal monitoring devices
When I bought my first Fitbit 7 years ago, I was enamored with the technology. The Fitbit was little more than a step tracker, yet I proudly wore its black rubber strap on my wrist. It was my first foray into wearable technology, and it felt quite empowering to have an objective way to track my fitness beyond just using my bathroom scale. Now less than a decade later, that Fitbit looks archaic in comparison with the wrist-top technology currently available.
As I write this, the world’s largest technology company is in the process of releasing its sixth-generation Apple Watch. In addition to acting as a smartphone, this new device, which is barely larger than a postage stamp, offers GPS-based movement tracking, the ability to detect falls, continuous heart rate monitoring, a built-in EKG capable of diagnosing atrial fibrillation, and an oxygen saturation sensor. These features weren’t added thoughtlessly. Apple is marketing this as a health-focused device, with their primary advertising campaign claiming that “the future of health is on your wrist,” and they aren’t the only company making this play.
Along with Apple, Samsung, Withings, Fitbit, and other companies continue to bring products to market that monitor our activity and provide new insights into our health. Typically linked to smartphone-based apps, these devices record all of their measurements for later review, while software helps interpret the findings to make them actionable. From heart rate tracking to sleep analysis, these options now provide access to volumes of data that promise to improve our wellness and change our lives. Of course, those promises will only be fulfilled if our behavior is altered as a consequence of having more detailed information. Whether that will happen remains to be seen.
Health system–linked devices
Major advancements in medical monitoring technology are now enabling physicians to get much deeper insight into their patients’ health status. Internet-connected scales, blood pressure cuffs, and exercise equipment offer the ability to upload information into patient portals and integrate that information into EHRs. New devices provide access to information that previously was impossible to obtain. For example, wearable continuous blood glucose monitors, such as the FreeStyle Libre or DexCom’s G6, allow patients and physicians to follow blood sugar readings 24 hours a day. This provides unprecedented awareness of diabetes control and relieves the pain and inconvenience of finger sticks and blood draws. It also aids with compliance because patients don’t need to remember to check their sugar levels on a schedule.
Other compliance-boosting breakthroughs, such as Bluetooth-enabled asthma inhalers and cellular-connected continuous positive airway pressure machines, assist patients with managing chronic respiratory conditions. Many companies are developing technologies to manage acute conditions as well. One such company, an on-demand telemedicine provider called TytoCare, has developed a $299 suite of instruments that includes a digital stethoscope, thermometer, and camera-based otoscope. In concert with a virtual visit, their providers can remotely use these tools to examine and assess sick individuals. This virtual “laying on of hands” may have sounded like science fiction and likely would have been rejected by patients just a few years ago. Now it is becoming commonplace and will soon be an expectation of many seeking care.
But if we are to be successful, everyone must acknowledge that this revolution in health care brings many challenges along with it. One of those is the deluge of data that connected devices provide.
Information overload
There is such a thing as “too much of a good thing.” Described by journalist David Shenk as “data smog” in his 1997 book of the same name, the idea is clear: There is only so much information we can assimilate.
Even after years of using EHRs and with government-implemented incentives that promote “meaningful use,” physicians are still struggling with EHRs. Additionally, many have expressed frustration with the connectedness that EHRs provide and lament their inability to ever really “leave the office.” As more and more data become available to physicians, the challenge of how to assimilate and act on those data will continue to grow. The addition of patient-provided health statistics will only make information overload worse, with clinicians will feeling an ever-growing burden to know, understand, and act on this information.
Unless we develop systems to sort, filter, and prioritize the flow of information, there is potential for liability from not acting on the amount of virtual information doctors receive. This new risk for already fatigued and overburdened physicians combined with an increase in the amount of virtual information at doctors’ fingertips may lead to the value of patient data being lost.
Dr. Notte is a family physician and chief medical officer of Abington (Pa.) Hospital–Jefferson Health. Follow him on Twitter (@doctornotte). Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Philadelphia, and associate director of the family medicine residency program at Abington Hospital–Jefferson Health. They have no conflicts related to the content of this piece.
Children’s share of COVID-19 burden continues to increase
Children continue to represent an increasing proportion of reported COVID-19 cases in the United States, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
The previous week, children represented 10.0% of all cases, and that proportion has continued to rise throughout the pandemic, the AAP and CHA report shows.
Looking at just new cases for the latest week, the 38,000+ pediatric cases made up almost 17% of the 228,396 cases reported for all ages, compared with 16% and 15% the two previous weeks. For the weeks ending Aug. 13 and Aug. 6, the corresponding figures were 8% and 13%, based on the data in the AAP/CHA report, which cover 49 states (New York City but not New York state), the District of Columbia, Puerto Rico, and Guam.
The state with the highest proportion of child COVID-19 cases as of Sept. 17 was Wyoming, with 20.6%, followed by North Dakota at 18.3% and Tennessee at 17.9%. New York City has a cumulative rate of just 3.4%, but New Jersey is the state with the lowest rate at 3.6%. Florida comes in at 5.9% but is using an age range of 0-14 years for children, and Texas has a rate of 6.0% but has reported ages for only 8% of confirmed cases, the AAP and CHA noted.
Severe illness, however, continues to be rare in children. The overall hospitalization rate for children was down to 1.7% among the 26 jurisdictions providing ages as Sept. 17 – down from 1.8% the week before and 2.3% on Aug. 20. The death rate is just 0.02% among 43 jurisdictions, the report said.
Children continue to represent an increasing proportion of reported COVID-19 cases in the United States, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
The previous week, children represented 10.0% of all cases, and that proportion has continued to rise throughout the pandemic, the AAP and CHA report shows.
Looking at just new cases for the latest week, the 38,000+ pediatric cases made up almost 17% of the 228,396 cases reported for all ages, compared with 16% and 15% the two previous weeks. For the weeks ending Aug. 13 and Aug. 6, the corresponding figures were 8% and 13%, based on the data in the AAP/CHA report, which cover 49 states (New York City but not New York state), the District of Columbia, Puerto Rico, and Guam.
The state with the highest proportion of child COVID-19 cases as of Sept. 17 was Wyoming, with 20.6%, followed by North Dakota at 18.3% and Tennessee at 17.9%. New York City has a cumulative rate of just 3.4%, but New Jersey is the state with the lowest rate at 3.6%. Florida comes in at 5.9% but is using an age range of 0-14 years for children, and Texas has a rate of 6.0% but has reported ages for only 8% of confirmed cases, the AAP and CHA noted.
Severe illness, however, continues to be rare in children. The overall hospitalization rate for children was down to 1.7% among the 26 jurisdictions providing ages as Sept. 17 – down from 1.8% the week before and 2.3% on Aug. 20. The death rate is just 0.02% among 43 jurisdictions, the report said.
Children continue to represent an increasing proportion of reported COVID-19 cases in the United States, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
The previous week, children represented 10.0% of all cases, and that proportion has continued to rise throughout the pandemic, the AAP and CHA report shows.
Looking at just new cases for the latest week, the 38,000+ pediatric cases made up almost 17% of the 228,396 cases reported for all ages, compared with 16% and 15% the two previous weeks. For the weeks ending Aug. 13 and Aug. 6, the corresponding figures were 8% and 13%, based on the data in the AAP/CHA report, which cover 49 states (New York City but not New York state), the District of Columbia, Puerto Rico, and Guam.
The state with the highest proportion of child COVID-19 cases as of Sept. 17 was Wyoming, with 20.6%, followed by North Dakota at 18.3% and Tennessee at 17.9%. New York City has a cumulative rate of just 3.4%, but New Jersey is the state with the lowest rate at 3.6%. Florida comes in at 5.9% but is using an age range of 0-14 years for children, and Texas has a rate of 6.0% but has reported ages for only 8% of confirmed cases, the AAP and CHA noted.
Severe illness, however, continues to be rare in children. The overall hospitalization rate for children was down to 1.7% among the 26 jurisdictions providing ages as Sept. 17 – down from 1.8% the week before and 2.3% on Aug. 20. The death rate is just 0.02% among 43 jurisdictions, the report said.



