User login
ID Practitioner is an independent news source that provides infectious disease specialists with timely and relevant news and commentary about clinical developments and the impact of health care policy on the infectious disease specialist’s practice. Specialty focus topics include antimicrobial resistance, emerging infections, global ID, hepatitis, HIV, hospital-acquired infections, immunizations and vaccines, influenza, mycoses, pediatric infections, and STIs. Infectious Diseases News is owned by Frontline Medical Communications.
sofosbuvir
ritonavir with dasabuvir
discount
support path
program
ritonavir
greedy
ledipasvir
assistance
viekira pak
vpak
advocacy
needy
protest
abbvie
paritaprevir
ombitasvir
direct-acting antivirals
dasabuvir
gilead
fake-ovir
support
v pak
oasis
harvoni
section[contains(@class, 'footer-nav-section-wrapper')]
div[contains(@class, 'pane-pub-article-idp')]
div[contains(@class, 'pane-medstat-latest-articles-articles-section')]
div[contains(@class, 'pane-pub-home-idp')]
div[contains(@class, 'pane-pub-topic-idp')]
The evidence is not clear: Rheumatic diseases, drugs, and COVID-19
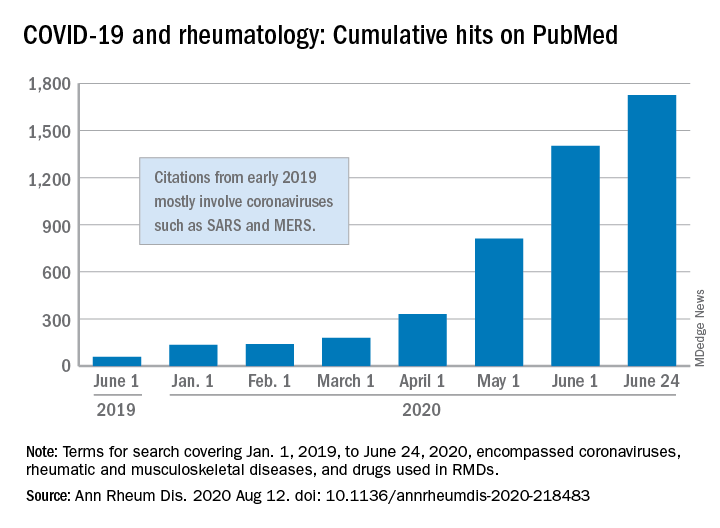
“We are faced by the worldwide spread of a disease that was nonexistent less than a year ago,” Féline P.B. Kroon, MD, and associates said in Annals of the Rheumatic Diseases. “To date, no robust evidence is available to allow strong conclusions on the effects of COVID-19 in patients with RMDs or whether RMDs or [their] treatment impact incidence of infection or outcomes.”
When it comes to quantity of evidence, “the exponential increase in publications over time is evident,” they said. From Jan. 1, 2019 to June 24, 2020, there were 1,725 hits on PubMed for published reports combining COVID-19 with RMDs and drugs used in RMDs. At the beginning of the year, there were only 135 such publications.
The early start of the search, well before identification of the novel coronavirus in China, was meant to ensure that nothing was missed, so “citations that came up in the first months of 2019 mostly encompass papers about other coronaviruses, such as SARS and MERS,” said Dr. Kroon of Zuyderland Medical Center, Heerlen, the Netherlands, when asked for clarification.
The quality of that evidence, however, is another matter. A majority of publications (60%) are “viewpoints or (narrative) literature reviews, and only a small proportion actually presents original data in the form of case reports or case series (15%), observational cohort studies (10%), or clinical trials (<1%),” the investigators explained.
Very few of the published studies, about 10%, specifically involve COVID-19 and RMDs. Even well-regarded sources such as systematic literature reviews or meta-analyses, “which will undoubtedly appear more frequently in the next few months in response to requests by users who feel overwhelmed by a multitude of data, will not eliminate the internal bias present in individual studies,” Dr. Kroon and associates wrote.
The lack of evidence also brings into question one particular form of guidance: recommendations “issued by groups of the so-called experts and (inter)national societies, such as, among others, American College of Rheumatology and European League Against Rheumatism,” the investigators said.
“The rapid increase in research on COVID-19 is encouraging,” but at the same time it “also poses risks of ‘information overload’ or ‘fake news,’ ” they said. “As researchers and clinicians, it is our responsibility to carefully interpret study results that emerge, even more so in this ‘digital era,’ in which published data can quickly have a large societal impact.”
SOURCE: Kroon FPB et al. Ann Rheum Dis. 2020 Aug 12. doi: 10.1136/annrheumdis-2020-218483.

“We are faced by the worldwide spread of a disease that was nonexistent less than a year ago,” Féline P.B. Kroon, MD, and associates said in Annals of the Rheumatic Diseases. “To date, no robust evidence is available to allow strong conclusions on the effects of COVID-19 in patients with RMDs or whether RMDs or [their] treatment impact incidence of infection or outcomes.”
When it comes to quantity of evidence, “the exponential increase in publications over time is evident,” they said. From Jan. 1, 2019 to June 24, 2020, there were 1,725 hits on PubMed for published reports combining COVID-19 with RMDs and drugs used in RMDs. At the beginning of the year, there were only 135 such publications.
The early start of the search, well before identification of the novel coronavirus in China, was meant to ensure that nothing was missed, so “citations that came up in the first months of 2019 mostly encompass papers about other coronaviruses, such as SARS and MERS,” said Dr. Kroon of Zuyderland Medical Center, Heerlen, the Netherlands, when asked for clarification.
The quality of that evidence, however, is another matter. A majority of publications (60%) are “viewpoints or (narrative) literature reviews, and only a small proportion actually presents original data in the form of case reports or case series (15%), observational cohort studies (10%), or clinical trials (<1%),” the investigators explained.
Very few of the published studies, about 10%, specifically involve COVID-19 and RMDs. Even well-regarded sources such as systematic literature reviews or meta-analyses, “which will undoubtedly appear more frequently in the next few months in response to requests by users who feel overwhelmed by a multitude of data, will not eliminate the internal bias present in individual studies,” Dr. Kroon and associates wrote.
The lack of evidence also brings into question one particular form of guidance: recommendations “issued by groups of the so-called experts and (inter)national societies, such as, among others, American College of Rheumatology and European League Against Rheumatism,” the investigators said.
“The rapid increase in research on COVID-19 is encouraging,” but at the same time it “also poses risks of ‘information overload’ or ‘fake news,’ ” they said. “As researchers and clinicians, it is our responsibility to carefully interpret study results that emerge, even more so in this ‘digital era,’ in which published data can quickly have a large societal impact.”
SOURCE: Kroon FPB et al. Ann Rheum Dis. 2020 Aug 12. doi: 10.1136/annrheumdis-2020-218483.

“We are faced by the worldwide spread of a disease that was nonexistent less than a year ago,” Féline P.B. Kroon, MD, and associates said in Annals of the Rheumatic Diseases. “To date, no robust evidence is available to allow strong conclusions on the effects of COVID-19 in patients with RMDs or whether RMDs or [their] treatment impact incidence of infection or outcomes.”
When it comes to quantity of evidence, “the exponential increase in publications over time is evident,” they said. From Jan. 1, 2019 to June 24, 2020, there were 1,725 hits on PubMed for published reports combining COVID-19 with RMDs and drugs used in RMDs. At the beginning of the year, there were only 135 such publications.
The early start of the search, well before identification of the novel coronavirus in China, was meant to ensure that nothing was missed, so “citations that came up in the first months of 2019 mostly encompass papers about other coronaviruses, such as SARS and MERS,” said Dr. Kroon of Zuyderland Medical Center, Heerlen, the Netherlands, when asked for clarification.
The quality of that evidence, however, is another matter. A majority of publications (60%) are “viewpoints or (narrative) literature reviews, and only a small proportion actually presents original data in the form of case reports or case series (15%), observational cohort studies (10%), or clinical trials (<1%),” the investigators explained.
Very few of the published studies, about 10%, specifically involve COVID-19 and RMDs. Even well-regarded sources such as systematic literature reviews or meta-analyses, “which will undoubtedly appear more frequently in the next few months in response to requests by users who feel overwhelmed by a multitude of data, will not eliminate the internal bias present in individual studies,” Dr. Kroon and associates wrote.
The lack of evidence also brings into question one particular form of guidance: recommendations “issued by groups of the so-called experts and (inter)national societies, such as, among others, American College of Rheumatology and European League Against Rheumatism,” the investigators said.
“The rapid increase in research on COVID-19 is encouraging,” but at the same time it “also poses risks of ‘information overload’ or ‘fake news,’ ” they said. “As researchers and clinicians, it is our responsibility to carefully interpret study results that emerge, even more so in this ‘digital era,’ in which published data can quickly have a large societal impact.”
SOURCE: Kroon FPB et al. Ann Rheum Dis. 2020 Aug 12. doi: 10.1136/annrheumdis-2020-218483.
FROM ANNALS OF THE RHEUMATIC DISEASES
Pooled COVID-19 testing feasible, greatly reduces supply use
‘Straightforward, cost effective, and efficient’
Combining specimens from several low-risk inpatients in a single test for SARS-CoV-2 infection allowed hospital staff to stretch testing supplies and provide test results quickly for many more patients than they might have otherwise, researchers found.
“We believe this strategy conserved [personal protective equipment (PPE)], led to a marked reduction in staff and patient anxiety, and improved patient care,” wrote David Mastrianni, MD, and colleagues from Saratoga Hospital in Saratoga Springs, N.Y. “Our impression is that testing all admitted patients has also been reassuring to our community.”
The researchers published their findings July 20 in the Journal of Hospital Medicine.
“What was really important about this study was they were actually able to implement pooled testing after communication with the [Food and Drug Administration],” Samir S. Shah, MD, MSCE, SFHM, the journal’s editor-in-chief, said in an interview.
“Pooled testing combines samples from multiple people within a single test. The benefit is, if the test is negative [you know that] everyone whose sample was combined … is negative. So you’ve effectively tested anywhere from three to five people with the resources required for only one test,” Dr. Shah continued.
The challenge is that, if the test is positive, everyone in that testing group must be retested individually because one or more of them has the infection, said Dr. Shah, director of hospital medicine at Cincinnati Children’s Hospital Medical Center.
Dr. Mastrianni said early in the pandemic they started getting the “New York surge” at their hospital, located approximately 3 hours from New York City. They wanted to test all of the inpatients at their hospital for COVID-19 and they had a rapid in-house test that worked well, “but we just didn’t have enough cartridges, and we couldn’t get deliveries, and we started pooling.” In fact, they ran out of testing supplies at one point during the study but were able to replenish their supply in about a day, he noted.
For the current study, all patients admitted to the hospital, including those admitted for observation, underwent testing for SARS-CoV-2. Staff in the emergency department designated patients as low risk if they had no symptoms or other clinical evidence of COVID-19; those patients underwent pooled testing.
Patients with clinical evidence of COVID-19, such as respiratory symptoms or laboratory or radiographic findings consistent with infection, were considered high risk and were tested on an individual basis and thus excluded from the current analysis.
The pooled testing strategy required some patients to be held in the emergency department until there were three available for pooled testing. On several occasions when this was not practical, specimens from two patients were pooled.
Between April 17 and May 11, clinicians tested 530 patients via pooled testing using 179 cartridges (172 with swabs from three patients and 7 with swabs from two patients). There were four positive pooled tests, which necessitated the use of an additional 11 cartridges. Overall, the testing used 190 cartridges, which is 340 fewer than would have been used if all patients had been tested individually.
Among the low-risk patients, the positive rate was 0.8% (4/530). No patients from pools that were negative tested positive later during their hospitalization or developed evidence of the infection.
Team effort, flexibility needed
Dr. Mastrianni said he expected their study to find that pooled testing saved testing resources, but he “was surprised by the complexity of the logistics in the hospital, and how it really required getting everybody to work together. …There were a lot of details, and it really took a lot of teamwork.”
The nursing supervisor in the emergency department was in charge of the batch and coordinated with the laboratory, he explained. There were many moving parts to manage, including monitoring how many patients were being admitted, what their conditions were, whether they were high or low risk, and where they would house those patients as the emergency department became increasingly busy. “It’s a lot for them, but they’ve adapted really well,” Dr. Mastrianni said.
Pooling tests seems to work best for three to five patients at a time; larger batches increase the chance of having a positive test, and thus identifying the sick individual(s) becomes more challenging and expensive, Dr. Shah said.
“It’s a fine line between having a pool large enough that you save on testing supplies and testing costs but not having the pool so large that you dramatically increase your likelihood of having a positive test,” Dr. Shah said.
Hospitals will likely need to be flexible and adapt as the local positivity rate changes and supply levels vary, according to the authors.
“Pooled testing is mainly dependent on the COVID-19 positive rate in the population of interest in addition to the sensitivity of the [reverse transcriptase-polymerase chain reaction (RT-PCR)] method used for COVID-19 testing,” said Baha Abdalhamid, MD, PhD, of the department of pathology and microbiology at the University of Nebraska Medical Center in Omaha.
“Each laboratory and hospital needs to do their own validation testing because it is dependent on the positive rate of COVID-19,” added Dr. Abdalhamid, who was not involved in the current study.
It’s important for clinicians to “do a good history to find who’s high risk and who’s low risk,” Dr. Mastrianni said. Clinicians also need to remember that, although a patient may test negative initially, they may still have COVID-19, he warned. That test reflects a single point in time, and a patient could be infected and not yet be ill, so clinicians need to be alert to a change in the patient’s status.
Best for settings with low-risk individuals
“Pooled COVID-19 testing is a straightforward, cost-effective, and efficient approach,” Dr. Abdalhamid said. He and his colleagues found pooled testing could increase testing capability by 69% or more when the incidence rate of SARS-CoV-2 infection is 10% or lower.
He said the approach would be helpful in other settings “as long as the positive rate is equal to or less than 10%. Asymptomatic population or surveillance groups such as students, athletes, and military service members are [an] interesting population to test using pooling testing because we expect these populations to have low positive rates, which makes pooled testing ideal.”
Benefit outweighs risk
“There is risk of missing specimens with low concentration of the virus,” Dr. Abdalhamid cautioned. “These specimens might be missed due to the dilution factor of pooling [false-negative specimens]. We did not have a single false-negative specimen in our proof-of-concept study. In addition, there are practical approaches to deal with false-negative pooled specimens.
“The benefit definitely outweighs the risk of false-negative specimens because false-negative results rarely occur, if any. In addition, there is significant saving of time, reagents, and supplies in [a] pooled specimens approach as well as expansion of the test for higher number of patients,” Dr. Abdalhamid continued.
Dr. Mastrianni’s hospital currently has enough testing cartridges, but they are continuing to conduct pooled testing to conserve resources for the benefit of their own hospital and for the nation as a whole, he said.
The authors have disclosed no relevant financial relationships. Dr. Abdalhamid and Dr. Shah have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
‘Straightforward, cost effective, and efficient’
‘Straightforward, cost effective, and efficient’
Combining specimens from several low-risk inpatients in a single test for SARS-CoV-2 infection allowed hospital staff to stretch testing supplies and provide test results quickly for many more patients than they might have otherwise, researchers found.
“We believe this strategy conserved [personal protective equipment (PPE)], led to a marked reduction in staff and patient anxiety, and improved patient care,” wrote David Mastrianni, MD, and colleagues from Saratoga Hospital in Saratoga Springs, N.Y. “Our impression is that testing all admitted patients has also been reassuring to our community.”
The researchers published their findings July 20 in the Journal of Hospital Medicine.
“What was really important about this study was they were actually able to implement pooled testing after communication with the [Food and Drug Administration],” Samir S. Shah, MD, MSCE, SFHM, the journal’s editor-in-chief, said in an interview.
“Pooled testing combines samples from multiple people within a single test. The benefit is, if the test is negative [you know that] everyone whose sample was combined … is negative. So you’ve effectively tested anywhere from three to five people with the resources required for only one test,” Dr. Shah continued.
The challenge is that, if the test is positive, everyone in that testing group must be retested individually because one or more of them has the infection, said Dr. Shah, director of hospital medicine at Cincinnati Children’s Hospital Medical Center.
Dr. Mastrianni said early in the pandemic they started getting the “New York surge” at their hospital, located approximately 3 hours from New York City. They wanted to test all of the inpatients at their hospital for COVID-19 and they had a rapid in-house test that worked well, “but we just didn’t have enough cartridges, and we couldn’t get deliveries, and we started pooling.” In fact, they ran out of testing supplies at one point during the study but were able to replenish their supply in about a day, he noted.
For the current study, all patients admitted to the hospital, including those admitted for observation, underwent testing for SARS-CoV-2. Staff in the emergency department designated patients as low risk if they had no symptoms or other clinical evidence of COVID-19; those patients underwent pooled testing.
Patients with clinical evidence of COVID-19, such as respiratory symptoms or laboratory or radiographic findings consistent with infection, were considered high risk and were tested on an individual basis and thus excluded from the current analysis.
The pooled testing strategy required some patients to be held in the emergency department until there were three available for pooled testing. On several occasions when this was not practical, specimens from two patients were pooled.
Between April 17 and May 11, clinicians tested 530 patients via pooled testing using 179 cartridges (172 with swabs from three patients and 7 with swabs from two patients). There were four positive pooled tests, which necessitated the use of an additional 11 cartridges. Overall, the testing used 190 cartridges, which is 340 fewer than would have been used if all patients had been tested individually.
Among the low-risk patients, the positive rate was 0.8% (4/530). No patients from pools that were negative tested positive later during their hospitalization or developed evidence of the infection.
Team effort, flexibility needed
Dr. Mastrianni said he expected their study to find that pooled testing saved testing resources, but he “was surprised by the complexity of the logistics in the hospital, and how it really required getting everybody to work together. …There were a lot of details, and it really took a lot of teamwork.”
The nursing supervisor in the emergency department was in charge of the batch and coordinated with the laboratory, he explained. There were many moving parts to manage, including monitoring how many patients were being admitted, what their conditions were, whether they were high or low risk, and where they would house those patients as the emergency department became increasingly busy. “It’s a lot for them, but they’ve adapted really well,” Dr. Mastrianni said.
Pooling tests seems to work best for three to five patients at a time; larger batches increase the chance of having a positive test, and thus identifying the sick individual(s) becomes more challenging and expensive, Dr. Shah said.
“It’s a fine line between having a pool large enough that you save on testing supplies and testing costs but not having the pool so large that you dramatically increase your likelihood of having a positive test,” Dr. Shah said.
Hospitals will likely need to be flexible and adapt as the local positivity rate changes and supply levels vary, according to the authors.
“Pooled testing is mainly dependent on the COVID-19 positive rate in the population of interest in addition to the sensitivity of the [reverse transcriptase-polymerase chain reaction (RT-PCR)] method used for COVID-19 testing,” said Baha Abdalhamid, MD, PhD, of the department of pathology and microbiology at the University of Nebraska Medical Center in Omaha.
“Each laboratory and hospital needs to do their own validation testing because it is dependent on the positive rate of COVID-19,” added Dr. Abdalhamid, who was not involved in the current study.
It’s important for clinicians to “do a good history to find who’s high risk and who’s low risk,” Dr. Mastrianni said. Clinicians also need to remember that, although a patient may test negative initially, they may still have COVID-19, he warned. That test reflects a single point in time, and a patient could be infected and not yet be ill, so clinicians need to be alert to a change in the patient’s status.
Best for settings with low-risk individuals
“Pooled COVID-19 testing is a straightforward, cost-effective, and efficient approach,” Dr. Abdalhamid said. He and his colleagues found pooled testing could increase testing capability by 69% or more when the incidence rate of SARS-CoV-2 infection is 10% or lower.
He said the approach would be helpful in other settings “as long as the positive rate is equal to or less than 10%. Asymptomatic population or surveillance groups such as students, athletes, and military service members are [an] interesting population to test using pooling testing because we expect these populations to have low positive rates, which makes pooled testing ideal.”
Benefit outweighs risk
“There is risk of missing specimens with low concentration of the virus,” Dr. Abdalhamid cautioned. “These specimens might be missed due to the dilution factor of pooling [false-negative specimens]. We did not have a single false-negative specimen in our proof-of-concept study. In addition, there are practical approaches to deal with false-negative pooled specimens.
“The benefit definitely outweighs the risk of false-negative specimens because false-negative results rarely occur, if any. In addition, there is significant saving of time, reagents, and supplies in [a] pooled specimens approach as well as expansion of the test for higher number of patients,” Dr. Abdalhamid continued.
Dr. Mastrianni’s hospital currently has enough testing cartridges, but they are continuing to conduct pooled testing to conserve resources for the benefit of their own hospital and for the nation as a whole, he said.
The authors have disclosed no relevant financial relationships. Dr. Abdalhamid and Dr. Shah have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Combining specimens from several low-risk inpatients in a single test for SARS-CoV-2 infection allowed hospital staff to stretch testing supplies and provide test results quickly for many more patients than they might have otherwise, researchers found.
“We believe this strategy conserved [personal protective equipment (PPE)], led to a marked reduction in staff and patient anxiety, and improved patient care,” wrote David Mastrianni, MD, and colleagues from Saratoga Hospital in Saratoga Springs, N.Y. “Our impression is that testing all admitted patients has also been reassuring to our community.”
The researchers published their findings July 20 in the Journal of Hospital Medicine.
“What was really important about this study was they were actually able to implement pooled testing after communication with the [Food and Drug Administration],” Samir S. Shah, MD, MSCE, SFHM, the journal’s editor-in-chief, said in an interview.
“Pooled testing combines samples from multiple people within a single test. The benefit is, if the test is negative [you know that] everyone whose sample was combined … is negative. So you’ve effectively tested anywhere from three to five people with the resources required for only one test,” Dr. Shah continued.
The challenge is that, if the test is positive, everyone in that testing group must be retested individually because one or more of them has the infection, said Dr. Shah, director of hospital medicine at Cincinnati Children’s Hospital Medical Center.
Dr. Mastrianni said early in the pandemic they started getting the “New York surge” at their hospital, located approximately 3 hours from New York City. They wanted to test all of the inpatients at their hospital for COVID-19 and they had a rapid in-house test that worked well, “but we just didn’t have enough cartridges, and we couldn’t get deliveries, and we started pooling.” In fact, they ran out of testing supplies at one point during the study but were able to replenish their supply in about a day, he noted.
For the current study, all patients admitted to the hospital, including those admitted for observation, underwent testing for SARS-CoV-2. Staff in the emergency department designated patients as low risk if they had no symptoms or other clinical evidence of COVID-19; those patients underwent pooled testing.
Patients with clinical evidence of COVID-19, such as respiratory symptoms or laboratory or radiographic findings consistent with infection, were considered high risk and were tested on an individual basis and thus excluded from the current analysis.
The pooled testing strategy required some patients to be held in the emergency department until there were three available for pooled testing. On several occasions when this was not practical, specimens from two patients were pooled.
Between April 17 and May 11, clinicians tested 530 patients via pooled testing using 179 cartridges (172 with swabs from three patients and 7 with swabs from two patients). There were four positive pooled tests, which necessitated the use of an additional 11 cartridges. Overall, the testing used 190 cartridges, which is 340 fewer than would have been used if all patients had been tested individually.
Among the low-risk patients, the positive rate was 0.8% (4/530). No patients from pools that were negative tested positive later during their hospitalization or developed evidence of the infection.
Team effort, flexibility needed
Dr. Mastrianni said he expected their study to find that pooled testing saved testing resources, but he “was surprised by the complexity of the logistics in the hospital, and how it really required getting everybody to work together. …There were a lot of details, and it really took a lot of teamwork.”
The nursing supervisor in the emergency department was in charge of the batch and coordinated with the laboratory, he explained. There were many moving parts to manage, including monitoring how many patients were being admitted, what their conditions were, whether they were high or low risk, and where they would house those patients as the emergency department became increasingly busy. “It’s a lot for them, but they’ve adapted really well,” Dr. Mastrianni said.
Pooling tests seems to work best for three to five patients at a time; larger batches increase the chance of having a positive test, and thus identifying the sick individual(s) becomes more challenging and expensive, Dr. Shah said.
“It’s a fine line between having a pool large enough that you save on testing supplies and testing costs but not having the pool so large that you dramatically increase your likelihood of having a positive test,” Dr. Shah said.
Hospitals will likely need to be flexible and adapt as the local positivity rate changes and supply levels vary, according to the authors.
“Pooled testing is mainly dependent on the COVID-19 positive rate in the population of interest in addition to the sensitivity of the [reverse transcriptase-polymerase chain reaction (RT-PCR)] method used for COVID-19 testing,” said Baha Abdalhamid, MD, PhD, of the department of pathology and microbiology at the University of Nebraska Medical Center in Omaha.
“Each laboratory and hospital needs to do their own validation testing because it is dependent on the positive rate of COVID-19,” added Dr. Abdalhamid, who was not involved in the current study.
It’s important for clinicians to “do a good history to find who’s high risk and who’s low risk,” Dr. Mastrianni said. Clinicians also need to remember that, although a patient may test negative initially, they may still have COVID-19, he warned. That test reflects a single point in time, and a patient could be infected and not yet be ill, so clinicians need to be alert to a change in the patient’s status.
Best for settings with low-risk individuals
“Pooled COVID-19 testing is a straightforward, cost-effective, and efficient approach,” Dr. Abdalhamid said. He and his colleagues found pooled testing could increase testing capability by 69% or more when the incidence rate of SARS-CoV-2 infection is 10% or lower.
He said the approach would be helpful in other settings “as long as the positive rate is equal to or less than 10%. Asymptomatic population or surveillance groups such as students, athletes, and military service members are [an] interesting population to test using pooling testing because we expect these populations to have low positive rates, which makes pooled testing ideal.”
Benefit outweighs risk
“There is risk of missing specimens with low concentration of the virus,” Dr. Abdalhamid cautioned. “These specimens might be missed due to the dilution factor of pooling [false-negative specimens]. We did not have a single false-negative specimen in our proof-of-concept study. In addition, there are practical approaches to deal with false-negative pooled specimens.
“The benefit definitely outweighs the risk of false-negative specimens because false-negative results rarely occur, if any. In addition, there is significant saving of time, reagents, and supplies in [a] pooled specimens approach as well as expansion of the test for higher number of patients,” Dr. Abdalhamid continued.
Dr. Mastrianni’s hospital currently has enough testing cartridges, but they are continuing to conduct pooled testing to conserve resources for the benefit of their own hospital and for the nation as a whole, he said.
The authors have disclosed no relevant financial relationships. Dr. Abdalhamid and Dr. Shah have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
COVID-19 and the myth of the super doctor
Let us begin with a thought exercise. Close your eyes and picture the word, “hero.” What comes to mind? A relative, a teacher, a fictional character wielding a hammer or flying gracefully through the air?
Several months ago, our country was introduced to a foe that brought us to our knees. Before that time, the idea of a hero had fluctuated with circumstance and had been guided by aging and maturity; however, since the moment COVID-19 struck, a new image has emerged. Not all heroes wear capes, but some wield stethoscopes.
Over these past months the phrase, “Health Care Heroes” has spread throughout our collective consciousness, highlighted everywhere from talk shows and news media to billboards and journals. Doctors, nurses, and other health care professionals are lauded for their strength, dedication, resilience, and compassion. Citizens line up to clap, honk horns, and shower praise in recognition of those who have risked their health, sacrificed their personal lives, and committed themselves to the greater good. Yet, what does it mean to be a hero, and what is the cost of hero worship?
The focus of medical training has gradually shifted to include the physical as well as mental well-being of future physicians, but the remnants of traditional doctrine linger. Hours of focused training through study and direct clinical interaction reinforce dedication to patient care. Rewards are given for time spent and compassion lent, and research is lauded, but family time is rarely applauded. We are encouraged to do our greatest, work our hardest, be the best, rise and defeat every test. Failure (or the perception thereof) is not an option.
According to Rikinkumar S. Patel, MD, MPH, and associates, physicians have nearly twice the burnout rate of other professionals (Behav Sci. [Basel]. 2018 Nov;8[11]:98). The dedication to our craft propels excellence as well as sacrifice. When COVID-19 entered our lives, many of my colleagues did not hesitate to heed to the call for action. They immersed themselves in the ICU, led triage units, and extended work hours in the service of the sick and dying. Several were years removed from emergency/intensive care, while others were allocated from their chosen residency programs and voluntarily thrust into an environment they had never before traversed.
These individuals are praised as “brave,” “dedicated,” “selfless.” A few even provided insight into their experiences through various publications highlighting their appreciation and gratitude toward such a treacherous, albeit, tremendous experience. Even though their words are an honest perspective of life through one of the worst health care crises in 100 years, in effect, they perpetuate the noble hero; the myth of the super doctor.
In a profession that has borne witness to multiple suicides over the past few months, why do we not encourage open dialogue of our victories as well as our defeats? Our wins as much as our losses? Why does an esteemed veteran physician feel guilt over declining to provide emergency services to patients whom they have long forgotten how to manage? What drives the guilt and the self-doubt? Are we ashamed of what others will think? Is it that the fear of not living up to our cherished medical oath outweighs our own boundaries and acknowledgment of our limitations?
A hero is an entity, a person encompassing a state of being, yet health care professionals are bestowed this title and this burden on a near-daily basis. We are perfectly imperfect. The more in tune we are to vulnerability, the more honest we can become with ourselves and one another.
Dr. Thomas is a board-certified adult psychiatrist with an interest in chronic illness, women’s behavioral health, and minority mental health. She currently practices in North Kingstown and East Providence, R.I. She has no conflicts of interest.
Let us begin with a thought exercise. Close your eyes and picture the word, “hero.” What comes to mind? A relative, a teacher, a fictional character wielding a hammer or flying gracefully through the air?
Several months ago, our country was introduced to a foe that brought us to our knees. Before that time, the idea of a hero had fluctuated with circumstance and had been guided by aging and maturity; however, since the moment COVID-19 struck, a new image has emerged. Not all heroes wear capes, but some wield stethoscopes.
Over these past months the phrase, “Health Care Heroes” has spread throughout our collective consciousness, highlighted everywhere from talk shows and news media to billboards and journals. Doctors, nurses, and other health care professionals are lauded for their strength, dedication, resilience, and compassion. Citizens line up to clap, honk horns, and shower praise in recognition of those who have risked their health, sacrificed their personal lives, and committed themselves to the greater good. Yet, what does it mean to be a hero, and what is the cost of hero worship?
The focus of medical training has gradually shifted to include the physical as well as mental well-being of future physicians, but the remnants of traditional doctrine linger. Hours of focused training through study and direct clinical interaction reinforce dedication to patient care. Rewards are given for time spent and compassion lent, and research is lauded, but family time is rarely applauded. We are encouraged to do our greatest, work our hardest, be the best, rise and defeat every test. Failure (or the perception thereof) is not an option.
According to Rikinkumar S. Patel, MD, MPH, and associates, physicians have nearly twice the burnout rate of other professionals (Behav Sci. [Basel]. 2018 Nov;8[11]:98). The dedication to our craft propels excellence as well as sacrifice. When COVID-19 entered our lives, many of my colleagues did not hesitate to heed to the call for action. They immersed themselves in the ICU, led triage units, and extended work hours in the service of the sick and dying. Several were years removed from emergency/intensive care, while others were allocated from their chosen residency programs and voluntarily thrust into an environment they had never before traversed.
These individuals are praised as “brave,” “dedicated,” “selfless.” A few even provided insight into their experiences through various publications highlighting their appreciation and gratitude toward such a treacherous, albeit, tremendous experience. Even though their words are an honest perspective of life through one of the worst health care crises in 100 years, in effect, they perpetuate the noble hero; the myth of the super doctor.
In a profession that has borne witness to multiple suicides over the past few months, why do we not encourage open dialogue of our victories as well as our defeats? Our wins as much as our losses? Why does an esteemed veteran physician feel guilt over declining to provide emergency services to patients whom they have long forgotten how to manage? What drives the guilt and the self-doubt? Are we ashamed of what others will think? Is it that the fear of not living up to our cherished medical oath outweighs our own boundaries and acknowledgment of our limitations?
A hero is an entity, a person encompassing a state of being, yet health care professionals are bestowed this title and this burden on a near-daily basis. We are perfectly imperfect. The more in tune we are to vulnerability, the more honest we can become with ourselves and one another.
Dr. Thomas is a board-certified adult psychiatrist with an interest in chronic illness, women’s behavioral health, and minority mental health. She currently practices in North Kingstown and East Providence, R.I. She has no conflicts of interest.
Let us begin with a thought exercise. Close your eyes and picture the word, “hero.” What comes to mind? A relative, a teacher, a fictional character wielding a hammer or flying gracefully through the air?
Several months ago, our country was introduced to a foe that brought us to our knees. Before that time, the idea of a hero had fluctuated with circumstance and had been guided by aging and maturity; however, since the moment COVID-19 struck, a new image has emerged. Not all heroes wear capes, but some wield stethoscopes.
Over these past months the phrase, “Health Care Heroes” has spread throughout our collective consciousness, highlighted everywhere from talk shows and news media to billboards and journals. Doctors, nurses, and other health care professionals are lauded for their strength, dedication, resilience, and compassion. Citizens line up to clap, honk horns, and shower praise in recognition of those who have risked their health, sacrificed their personal lives, and committed themselves to the greater good. Yet, what does it mean to be a hero, and what is the cost of hero worship?
The focus of medical training has gradually shifted to include the physical as well as mental well-being of future physicians, but the remnants of traditional doctrine linger. Hours of focused training through study and direct clinical interaction reinforce dedication to patient care. Rewards are given for time spent and compassion lent, and research is lauded, but family time is rarely applauded. We are encouraged to do our greatest, work our hardest, be the best, rise and defeat every test. Failure (or the perception thereof) is not an option.
According to Rikinkumar S. Patel, MD, MPH, and associates, physicians have nearly twice the burnout rate of other professionals (Behav Sci. [Basel]. 2018 Nov;8[11]:98). The dedication to our craft propels excellence as well as sacrifice. When COVID-19 entered our lives, many of my colleagues did not hesitate to heed to the call for action. They immersed themselves in the ICU, led triage units, and extended work hours in the service of the sick and dying. Several were years removed from emergency/intensive care, while others were allocated from their chosen residency programs and voluntarily thrust into an environment they had never before traversed.
These individuals are praised as “brave,” “dedicated,” “selfless.” A few even provided insight into their experiences through various publications highlighting their appreciation and gratitude toward such a treacherous, albeit, tremendous experience. Even though their words are an honest perspective of life through one of the worst health care crises in 100 years, in effect, they perpetuate the noble hero; the myth of the super doctor.
In a profession that has borne witness to multiple suicides over the past few months, why do we not encourage open dialogue of our victories as well as our defeats? Our wins as much as our losses? Why does an esteemed veteran physician feel guilt over declining to provide emergency services to patients whom they have long forgotten how to manage? What drives the guilt and the self-doubt? Are we ashamed of what others will think? Is it that the fear of not living up to our cherished medical oath outweighs our own boundaries and acknowledgment of our limitations?
A hero is an entity, a person encompassing a state of being, yet health care professionals are bestowed this title and this burden on a near-daily basis. We are perfectly imperfect. The more in tune we are to vulnerability, the more honest we can become with ourselves and one another.
Dr. Thomas is a board-certified adult psychiatrist with an interest in chronic illness, women’s behavioral health, and minority mental health. She currently practices in North Kingstown and East Providence, R.I. She has no conflicts of interest.
Does metformin reduce risk for death in COVID-19?
Accumulating observational data suggest that metformin use in patients with type 2 diabetes might reduce the risk for death from COVID-19, but the randomized trials needed to prove this are unlikely to be carried out, according to experts.
The latest results, which are not yet peer reviewed, were published online July 31. The study was conducted by Andrew B. Crouse, PhD, of the Hugh Kaul Precision Medicine Institute, University of Alabama at Birmingham, and colleagues.
The researchers found that among more than 600 patients with diabetes and COVID-19, use of metformin was associated with a nearly 70% reduction in mortality after adjustment for multiple confounders.
Data from four previous studies that also show a reduction in mortality among metformin users compared to nonusers were summarized in a “mini review” by André J. Scheen, MD, PhD, published Aug. 1 in Diabetes and Metabolism.
Dr. Scheen, of the division of diabetes, nutrition, and metabolic disorders and the division of clinical pharmacology at Liège (Belgium) University, discussed possible mechanisms behind this observation.
“Because metformin exerts various effects beyond its glucose-lowering action, among which are anti-inflammatory effects, it may be speculated that this biguanide might positively influence the prognosis of patients with [type 2 diabetes] hospitalized for COVID-19,” he said.
“However, given the potential confounders inherently found in observational studies, caution is required before drawing any firm conclusions in the absence of randomized controlled trials,” Dr. Scheen wrote.
Indeed, when asked to comment, endocrinologist Kasia Lipska, MD, of Yale University, New Haven, Conn., said in an interview: “Metformin users tend to do better in many different settings with respect to many different outcomes. To me, it is still unclear whether metformin is truly a miracle drug or whether it is simply used more often among people who are healthier and who do not have contraindications to its use.”
She added, “I don’t think we have enough data to suggest metformin use for COVID-19 mitigation at this point.”
Alabama authors say confounding effects ‘unlikely’
In the retrospective analysis of electronic health records from their institution, Dr. Crouse and colleagues reviewed data from 604 patients who were confirmed to have tested positive for COVID-19 between Feb. 25 and June 22, 2020. Of those individuals, 40% had diabetes.
Death occurred in 11% (n = 67); the odds ratio (OR) for death among those with, vs. without, diabetes was 3.62 (P < .0001).
Individuals with diabetes accounted for >60% of all deaths. In multiple logistic regression, age 50-70 vs. <50, male sex, and diabetes emerged as independent predictors of death.
Of the 42 patients with diabetes who died, 8 (19%) had used metformin, and 34 (81%) had not*, a significant difference (OR, 0.38; P = .0221). Insulin use, on the other hand, had no effect on mortality (P = .5728).
“In fact, with 11% [being] the mortality of metformin users, [this] was comparable to that of the general COVID-19-positive population and dramatically lower than the 23% mortality observed in subjects with diabetes and not on metformin,” the authors said.
The survival benefit observed with metformin remained after exclusion of patients with classic metformin contraindications, such as chronic kidney disease and heart failure (OR, 0.17; P = .0231).
“This makes any potential confounding effects from skewing metformin users toward healthier subjects without these additional comorbidities very unlikely,” Dr. Crouse and colleagues contended.
After further analysis that controlled for other covariates (age, sex, obesity status, and hypertension), age, sex, and metformin use remained independent predictors of mortality.
For metformin, the odds ratio was 0.33 (P = .0210).
But, Dr. Lipska pointed out, “Observational studies can take into account confounders that are measured. However, unmeasured confounders may still affect the conclusions of these studies ... Propensity score matching to account for the likelihood of use of metformin could be used to better account for differences between metformin users and nonusers.”
If metformin does reduce COVID-19 deaths, multiple mechanisms likely
In his article, Dr. Scheen noted that several mechanisms have been proposed for the possible beneficial effect of metformin on COVID-19 outcomes, including direct improvements in glucose control, body weight, and insulin resistance; reduction in inflammation; inhibition of virus penetration via phosphorylation of ACE2; inhibition of an immune hyperactivation pathway; and neutrophil reduction. All remain theoretical, he emphasized.
He noted that some authors have raised concerns about possible harms from the use of metformin by patients with type 2 diabetes who are hospitalized for COVID-19, particularly because of the potential risk for lactic acidosis in cases of multiple organ failure.
In totality, four studies suggest 25% death reduction with metformin
Taken together, the four observational studies that Dr. Scheen reviewed showed that metformin had a positive effect, with an overall 25% reduction in death (P < .00001), albeit with relatively high heterogeneity (I² = 61%).
The largest of these, from the United States, included 6,256 patients hospitalized with COVID-19 and involved propensity matching. A significant reduction in mortality with metformin use was seen in women but not men (odds ratio, 0.759).
The French Coronavirus-SARS-CoV-2 and Diabetes Outcomes (CORONADO) study of 1,317 patients with diabetes and confirmed COVID-19 who were admitted to 53 French hospitals also showed a significant survival benefit for metformin, although the study wasn’t designed to address that issue.
In that study, the odds ratio for death on day 7 in prior metformin users compared to nonusers was 0.59. This finding lost significance but remained a trend after full adjustments (0.80).
Two smaller observational studies produced similar trends toward survival benefit with metformin.
Nonetheless, Dr. Scheen cautioned: “Firm conclusions about the impact of metformin therapy can only be drawn from double-blind randomized controlled trials (RCTs), and such trials are almost impossible in the context of COVID-19.”
He added: “Because metformin is out of patent and very inexpensive, no pharmaceutical company is likely to be interested in planning a study to demonstrate the benefits of metformin on COVID-19-related clinical outcomes.”
Dr. Lipska agreed: “RCTs are unlikely to be conducted to settle these issues. In their absence, metformin use should be based on its safety and effectiveness profile.”
Dr. Scheen concluded, however, that “there are at least no negative safety indications, so there is no reason to stop metformin therapy during COVID-19 infection except in cases of severe gastrointestinal symptoms, hypoxia and/or multiple organ failure.”
Dr. Lipska has received grants from the National Institutes of Health and works under contract for the Centers for Medicare & Medicaid Services to develop publicly reported quality measures. Dr. Scheen has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
*A previous version reversed these two outcomes in error.
Accumulating observational data suggest that metformin use in patients with type 2 diabetes might reduce the risk for death from COVID-19, but the randomized trials needed to prove this are unlikely to be carried out, according to experts.
The latest results, which are not yet peer reviewed, were published online July 31. The study was conducted by Andrew B. Crouse, PhD, of the Hugh Kaul Precision Medicine Institute, University of Alabama at Birmingham, and colleagues.
The researchers found that among more than 600 patients with diabetes and COVID-19, use of metformin was associated with a nearly 70% reduction in mortality after adjustment for multiple confounders.
Data from four previous studies that also show a reduction in mortality among metformin users compared to nonusers were summarized in a “mini review” by André J. Scheen, MD, PhD, published Aug. 1 in Diabetes and Metabolism.
Dr. Scheen, of the division of diabetes, nutrition, and metabolic disorders and the division of clinical pharmacology at Liège (Belgium) University, discussed possible mechanisms behind this observation.
“Because metformin exerts various effects beyond its glucose-lowering action, among which are anti-inflammatory effects, it may be speculated that this biguanide might positively influence the prognosis of patients with [type 2 diabetes] hospitalized for COVID-19,” he said.
“However, given the potential confounders inherently found in observational studies, caution is required before drawing any firm conclusions in the absence of randomized controlled trials,” Dr. Scheen wrote.
Indeed, when asked to comment, endocrinologist Kasia Lipska, MD, of Yale University, New Haven, Conn., said in an interview: “Metformin users tend to do better in many different settings with respect to many different outcomes. To me, it is still unclear whether metformin is truly a miracle drug or whether it is simply used more often among people who are healthier and who do not have contraindications to its use.”
She added, “I don’t think we have enough data to suggest metformin use for COVID-19 mitigation at this point.”
Alabama authors say confounding effects ‘unlikely’
In the retrospective analysis of electronic health records from their institution, Dr. Crouse and colleagues reviewed data from 604 patients who were confirmed to have tested positive for COVID-19 between Feb. 25 and June 22, 2020. Of those individuals, 40% had diabetes.
Death occurred in 11% (n = 67); the odds ratio (OR) for death among those with, vs. without, diabetes was 3.62 (P < .0001).
Individuals with diabetes accounted for >60% of all deaths. In multiple logistic regression, age 50-70 vs. <50, male sex, and diabetes emerged as independent predictors of death.
Of the 42 patients with diabetes who died, 8 (19%) had used metformin, and 34 (81%) had not*, a significant difference (OR, 0.38; P = .0221). Insulin use, on the other hand, had no effect on mortality (P = .5728).
“In fact, with 11% [being] the mortality of metformin users, [this] was comparable to that of the general COVID-19-positive population and dramatically lower than the 23% mortality observed in subjects with diabetes and not on metformin,” the authors said.
The survival benefit observed with metformin remained after exclusion of patients with classic metformin contraindications, such as chronic kidney disease and heart failure (OR, 0.17; P = .0231).
“This makes any potential confounding effects from skewing metformin users toward healthier subjects without these additional comorbidities very unlikely,” Dr. Crouse and colleagues contended.
After further analysis that controlled for other covariates (age, sex, obesity status, and hypertension), age, sex, and metformin use remained independent predictors of mortality.
For metformin, the odds ratio was 0.33 (P = .0210).
But, Dr. Lipska pointed out, “Observational studies can take into account confounders that are measured. However, unmeasured confounders may still affect the conclusions of these studies ... Propensity score matching to account for the likelihood of use of metformin could be used to better account for differences between metformin users and nonusers.”
If metformin does reduce COVID-19 deaths, multiple mechanisms likely
In his article, Dr. Scheen noted that several mechanisms have been proposed for the possible beneficial effect of metformin on COVID-19 outcomes, including direct improvements in glucose control, body weight, and insulin resistance; reduction in inflammation; inhibition of virus penetration via phosphorylation of ACE2; inhibition of an immune hyperactivation pathway; and neutrophil reduction. All remain theoretical, he emphasized.
He noted that some authors have raised concerns about possible harms from the use of metformin by patients with type 2 diabetes who are hospitalized for COVID-19, particularly because of the potential risk for lactic acidosis in cases of multiple organ failure.
In totality, four studies suggest 25% death reduction with metformin
Taken together, the four observational studies that Dr. Scheen reviewed showed that metformin had a positive effect, with an overall 25% reduction in death (P < .00001), albeit with relatively high heterogeneity (I² = 61%).
The largest of these, from the United States, included 6,256 patients hospitalized with COVID-19 and involved propensity matching. A significant reduction in mortality with metformin use was seen in women but not men (odds ratio, 0.759).
The French Coronavirus-SARS-CoV-2 and Diabetes Outcomes (CORONADO) study of 1,317 patients with diabetes and confirmed COVID-19 who were admitted to 53 French hospitals also showed a significant survival benefit for metformin, although the study wasn’t designed to address that issue.
In that study, the odds ratio for death on day 7 in prior metformin users compared to nonusers was 0.59. This finding lost significance but remained a trend after full adjustments (0.80).
Two smaller observational studies produced similar trends toward survival benefit with metformin.
Nonetheless, Dr. Scheen cautioned: “Firm conclusions about the impact of metformin therapy can only be drawn from double-blind randomized controlled trials (RCTs), and such trials are almost impossible in the context of COVID-19.”
He added: “Because metformin is out of patent and very inexpensive, no pharmaceutical company is likely to be interested in planning a study to demonstrate the benefits of metformin on COVID-19-related clinical outcomes.”
Dr. Lipska agreed: “RCTs are unlikely to be conducted to settle these issues. In their absence, metformin use should be based on its safety and effectiveness profile.”
Dr. Scheen concluded, however, that “there are at least no negative safety indications, so there is no reason to stop metformin therapy during COVID-19 infection except in cases of severe gastrointestinal symptoms, hypoxia and/or multiple organ failure.”
Dr. Lipska has received grants from the National Institutes of Health and works under contract for the Centers for Medicare & Medicaid Services to develop publicly reported quality measures. Dr. Scheen has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
*A previous version reversed these two outcomes in error.
Accumulating observational data suggest that metformin use in patients with type 2 diabetes might reduce the risk for death from COVID-19, but the randomized trials needed to prove this are unlikely to be carried out, according to experts.
The latest results, which are not yet peer reviewed, were published online July 31. The study was conducted by Andrew B. Crouse, PhD, of the Hugh Kaul Precision Medicine Institute, University of Alabama at Birmingham, and colleagues.
The researchers found that among more than 600 patients with diabetes and COVID-19, use of metformin was associated with a nearly 70% reduction in mortality after adjustment for multiple confounders.
Data from four previous studies that also show a reduction in mortality among metformin users compared to nonusers were summarized in a “mini review” by André J. Scheen, MD, PhD, published Aug. 1 in Diabetes and Metabolism.
Dr. Scheen, of the division of diabetes, nutrition, and metabolic disorders and the division of clinical pharmacology at Liège (Belgium) University, discussed possible mechanisms behind this observation.
“Because metformin exerts various effects beyond its glucose-lowering action, among which are anti-inflammatory effects, it may be speculated that this biguanide might positively influence the prognosis of patients with [type 2 diabetes] hospitalized for COVID-19,” he said.
“However, given the potential confounders inherently found in observational studies, caution is required before drawing any firm conclusions in the absence of randomized controlled trials,” Dr. Scheen wrote.
Indeed, when asked to comment, endocrinologist Kasia Lipska, MD, of Yale University, New Haven, Conn., said in an interview: “Metformin users tend to do better in many different settings with respect to many different outcomes. To me, it is still unclear whether metformin is truly a miracle drug or whether it is simply used more often among people who are healthier and who do not have contraindications to its use.”
She added, “I don’t think we have enough data to suggest metformin use for COVID-19 mitigation at this point.”
Alabama authors say confounding effects ‘unlikely’
In the retrospective analysis of electronic health records from their institution, Dr. Crouse and colleagues reviewed data from 604 patients who were confirmed to have tested positive for COVID-19 between Feb. 25 and June 22, 2020. Of those individuals, 40% had diabetes.
Death occurred in 11% (n = 67); the odds ratio (OR) for death among those with, vs. without, diabetes was 3.62 (P < .0001).
Individuals with diabetes accounted for >60% of all deaths. In multiple logistic regression, age 50-70 vs. <50, male sex, and diabetes emerged as independent predictors of death.
Of the 42 patients with diabetes who died, 8 (19%) had used metformin, and 34 (81%) had not*, a significant difference (OR, 0.38; P = .0221). Insulin use, on the other hand, had no effect on mortality (P = .5728).
“In fact, with 11% [being] the mortality of metformin users, [this] was comparable to that of the general COVID-19-positive population and dramatically lower than the 23% mortality observed in subjects with diabetes and not on metformin,” the authors said.
The survival benefit observed with metformin remained after exclusion of patients with classic metformin contraindications, such as chronic kidney disease and heart failure (OR, 0.17; P = .0231).
“This makes any potential confounding effects from skewing metformin users toward healthier subjects without these additional comorbidities very unlikely,” Dr. Crouse and colleagues contended.
After further analysis that controlled for other covariates (age, sex, obesity status, and hypertension), age, sex, and metformin use remained independent predictors of mortality.
For metformin, the odds ratio was 0.33 (P = .0210).
But, Dr. Lipska pointed out, “Observational studies can take into account confounders that are measured. However, unmeasured confounders may still affect the conclusions of these studies ... Propensity score matching to account for the likelihood of use of metformin could be used to better account for differences between metformin users and nonusers.”
If metformin does reduce COVID-19 deaths, multiple mechanisms likely
In his article, Dr. Scheen noted that several mechanisms have been proposed for the possible beneficial effect of metformin on COVID-19 outcomes, including direct improvements in glucose control, body weight, and insulin resistance; reduction in inflammation; inhibition of virus penetration via phosphorylation of ACE2; inhibition of an immune hyperactivation pathway; and neutrophil reduction. All remain theoretical, he emphasized.
He noted that some authors have raised concerns about possible harms from the use of metformin by patients with type 2 diabetes who are hospitalized for COVID-19, particularly because of the potential risk for lactic acidosis in cases of multiple organ failure.
In totality, four studies suggest 25% death reduction with metformin
Taken together, the four observational studies that Dr. Scheen reviewed showed that metformin had a positive effect, with an overall 25% reduction in death (P < .00001), albeit with relatively high heterogeneity (I² = 61%).
The largest of these, from the United States, included 6,256 patients hospitalized with COVID-19 and involved propensity matching. A significant reduction in mortality with metformin use was seen in women but not men (odds ratio, 0.759).
The French Coronavirus-SARS-CoV-2 and Diabetes Outcomes (CORONADO) study of 1,317 patients with diabetes and confirmed COVID-19 who were admitted to 53 French hospitals also showed a significant survival benefit for metformin, although the study wasn’t designed to address that issue.
In that study, the odds ratio for death on day 7 in prior metformin users compared to nonusers was 0.59. This finding lost significance but remained a trend after full adjustments (0.80).
Two smaller observational studies produced similar trends toward survival benefit with metformin.
Nonetheless, Dr. Scheen cautioned: “Firm conclusions about the impact of metformin therapy can only be drawn from double-blind randomized controlled trials (RCTs), and such trials are almost impossible in the context of COVID-19.”
He added: “Because metformin is out of patent and very inexpensive, no pharmaceutical company is likely to be interested in planning a study to demonstrate the benefits of metformin on COVID-19-related clinical outcomes.”
Dr. Lipska agreed: “RCTs are unlikely to be conducted to settle these issues. In their absence, metformin use should be based on its safety and effectiveness profile.”
Dr. Scheen concluded, however, that “there are at least no negative safety indications, so there is no reason to stop metformin therapy during COVID-19 infection except in cases of severe gastrointestinal symptoms, hypoxia and/or multiple organ failure.”
Dr. Lipska has received grants from the National Institutes of Health and works under contract for the Centers for Medicare & Medicaid Services to develop publicly reported quality measures. Dr. Scheen has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
*A previous version reversed these two outcomes in error.
COVID-19 and masks: Doctor, may I be excused?
In the last 2 months, at least 10 patients have asked Constantine George, MD, for a written medical exemption so they won’t have to wear a mask in public. Dr. George, the chief medical officer of Vedius, an app for a travelers’ concierge medical service in Las Vegas, turned them all down.
Elena Christofides, MD, an endocrinologist in Columbus, Ohio, has also refused patients’ requests for exemptions.
“It’s very rare for someone to need an exemption,” says Albert Rizzo, MD, chief medical officer for the American Lung Association and a lung specialist at ChristianaCare Health System in Newark, Del.
The opposition is sometimes strong. Recently, a video of Lenka Koloma of Laguna Niguel, Calif., who founded the antimask Freedom to Breathe Agency, went viral. She was in a California supermarket, maskless, telling an employee she was breaking the law by requiring patrons to wear masks.
“People need oxygen,” she said. “That alone is a medical condition.” Her webpage has a “Face Mask Exempt Card” that cites the Americans with Disabilities Act and posts a Department of Justice ADA violation reporting number. The DOJ issued a statement calling the cards fraudulent.
Figuring out if a patient’s request to opt out of wearing a mask is legitimate is a ‘’new frontier” for doctors, says Mical Raz, MD, a professor in public policy and health at the University of Rochester (N.Y.), and a hospitalist at the university medical center.
Should some people skip masks?
Experts say there are very few medical reasons for people to skip masks. “If you look at the research, patients with COPD [chronic obstructive pulmonary disorder], those with reactive airway, even those can breathe through a mask,” Dr. George said. Requests for exemptions due to medical reasons are usually without basis. “Obviously, if someone is incapacitated, for example, with mental health issues, that’s case by case.”
Dr. Christofides said one of her patients cited anxiety and the other cited headaches as reasons not to wear a mask. “I told the one who asked for anxiety [reasons] that she could wear ones that were less tight.” The patient with headaches told Dr. Christofides that she had a buildup of carbon dioxide in the mask because of industrial exposure. Baloney, Dr. Christofides told her.
Dr. Rizzo says one rare example of someone who can’t wear a mask might be a patient with an advanced lung condition so severe, they need extra oxygen. “These are the extreme patients where any change in oxygen and carbon dioxide could make a difference,” he said. But “that’s also the population that shouldn’t be going out in the first place.”
Dr. Raz cowrote a commentary about mask exemptions, saying doctors are faced with difficult decisions and must keep a delicate balance between public health and individual disability needs. “Inappropriate medical exemptions may inadvertently hasten viral spread and threaten public health,” she wrote.
In an interview, she says that some people do have a hard time tolerating a mask. “Probably the most common reasons are mental health issues, such as anxiety, panic and PTSD, and children with sensory processing disorders (making them oversensitive to their environment). I think there are very few pulmonary reasons.”
CDC, professional organization guidelines
The CDC says people should wear masks in public and when around people who don’t live in the same household. Beyond that, it simply says masks should not be worn by children under age 2, “or anyone who has trouble breathing, is unconscious, incapacitated, or otherwise unable to remove the mask without assistance.”
In mid-July, four professional organizations released a statement in response to the CDC recommendation for facial coverings. Jointly issued by the American College of Chest Physicians, the American Lung Association, the American Thoracic Society and the COPD Foundation, it states in part that people with normal lungs and “even many individuals with underlying chronic lung disease should be able to wear a non-N95 facial covering without affecting their oxygen or carbon dioxide levels.”
It acknowledges that some people will seek an exemption and doctors must weigh the patient’s concerns against the need to stop the spread of the virus. “In some instances, physician reassurance regarding the safety of the facial coverings may be all that is needed,” it states.
Addressing the excuses
Here are some of the common medical reasons people give for not being able to tolerate a mask:
Claustrophobia or anxiety. Dr. Raz and others suggests a “desensitizing” period, wearing the mask for longer and longer periods of time to get used to it. Parents could suggest kids wear a mask when doing something they like, such as watching television, so they equate it with something pleasant. Switching to a different kind of mask or one that fits better could also help.
Masks cause Legionnaires’ disease. Not true, experts say. Legionnaires’ is a severe form of pneumonia, the result of inhaling tiny water droplets with legionella bacteria.
It’s difficult to read lips. People can buy masks with a clear window that makes their mouth and lips visible.
Trouble breathing. Brief periods of mask use won’t have a bad effect on oxygen levels for most people.
“There is not an inherent right to be out in a pandemic with an unmasked face,” Dr. Raz says. But “you are entitled to an accommodation.” That might be using curbside pickup for food and medication. That requires much less time wearing a mask than entering a store would.
There are no “boilerplate” cards or letters to excuse people provided by the four organizations that addressed the issue, Dr. Rizzo said. If he were to write a letter asking for an exemption, he would personalize it for an individual patient’s medical condition. As to whether a state would honor it, he cannot say. The states have a patchwork of recommendations, making it difficult to say.
Dr. Rizzo tells lung disease patients who are able to go out that wearing a mask for 15-20 minutes to do an errand won’t harm their oxygen levels. And he reminds them that having an exemption, in the form of a doctor’s letter, may bring more problems. “Even with an exemption, someone may confront them” for their lack of a face covering. People with COPD have a higher risk of getting a severe illness from COVID-19, according to the CDC.
This article first appeared on WebMD.com.
In the last 2 months, at least 10 patients have asked Constantine George, MD, for a written medical exemption so they won’t have to wear a mask in public. Dr. George, the chief medical officer of Vedius, an app for a travelers’ concierge medical service in Las Vegas, turned them all down.
Elena Christofides, MD, an endocrinologist in Columbus, Ohio, has also refused patients’ requests for exemptions.
“It’s very rare for someone to need an exemption,” says Albert Rizzo, MD, chief medical officer for the American Lung Association and a lung specialist at ChristianaCare Health System in Newark, Del.
The opposition is sometimes strong. Recently, a video of Lenka Koloma of Laguna Niguel, Calif., who founded the antimask Freedom to Breathe Agency, went viral. She was in a California supermarket, maskless, telling an employee she was breaking the law by requiring patrons to wear masks.
“People need oxygen,” she said. “That alone is a medical condition.” Her webpage has a “Face Mask Exempt Card” that cites the Americans with Disabilities Act and posts a Department of Justice ADA violation reporting number. The DOJ issued a statement calling the cards fraudulent.
Figuring out if a patient’s request to opt out of wearing a mask is legitimate is a ‘’new frontier” for doctors, says Mical Raz, MD, a professor in public policy and health at the University of Rochester (N.Y.), and a hospitalist at the university medical center.
Should some people skip masks?
Experts say there are very few medical reasons for people to skip masks. “If you look at the research, patients with COPD [chronic obstructive pulmonary disorder], those with reactive airway, even those can breathe through a mask,” Dr. George said. Requests for exemptions due to medical reasons are usually without basis. “Obviously, if someone is incapacitated, for example, with mental health issues, that’s case by case.”
Dr. Christofides said one of her patients cited anxiety and the other cited headaches as reasons not to wear a mask. “I told the one who asked for anxiety [reasons] that she could wear ones that were less tight.” The patient with headaches told Dr. Christofides that she had a buildup of carbon dioxide in the mask because of industrial exposure. Baloney, Dr. Christofides told her.
Dr. Rizzo says one rare example of someone who can’t wear a mask might be a patient with an advanced lung condition so severe, they need extra oxygen. “These are the extreme patients where any change in oxygen and carbon dioxide could make a difference,” he said. But “that’s also the population that shouldn’t be going out in the first place.”
Dr. Raz cowrote a commentary about mask exemptions, saying doctors are faced with difficult decisions and must keep a delicate balance between public health and individual disability needs. “Inappropriate medical exemptions may inadvertently hasten viral spread and threaten public health,” she wrote.
In an interview, she says that some people do have a hard time tolerating a mask. “Probably the most common reasons are mental health issues, such as anxiety, panic and PTSD, and children with sensory processing disorders (making them oversensitive to their environment). I think there are very few pulmonary reasons.”
CDC, professional organization guidelines
The CDC says people should wear masks in public and when around people who don’t live in the same household. Beyond that, it simply says masks should not be worn by children under age 2, “or anyone who has trouble breathing, is unconscious, incapacitated, or otherwise unable to remove the mask without assistance.”
In mid-July, four professional organizations released a statement in response to the CDC recommendation for facial coverings. Jointly issued by the American College of Chest Physicians, the American Lung Association, the American Thoracic Society and the COPD Foundation, it states in part that people with normal lungs and “even many individuals with underlying chronic lung disease should be able to wear a non-N95 facial covering without affecting their oxygen or carbon dioxide levels.”
It acknowledges that some people will seek an exemption and doctors must weigh the patient’s concerns against the need to stop the spread of the virus. “In some instances, physician reassurance regarding the safety of the facial coverings may be all that is needed,” it states.
Addressing the excuses
Here are some of the common medical reasons people give for not being able to tolerate a mask:
Claustrophobia or anxiety. Dr. Raz and others suggests a “desensitizing” period, wearing the mask for longer and longer periods of time to get used to it. Parents could suggest kids wear a mask when doing something they like, such as watching television, so they equate it with something pleasant. Switching to a different kind of mask or one that fits better could also help.
Masks cause Legionnaires’ disease. Not true, experts say. Legionnaires’ is a severe form of pneumonia, the result of inhaling tiny water droplets with legionella bacteria.
It’s difficult to read lips. People can buy masks with a clear window that makes their mouth and lips visible.
Trouble breathing. Brief periods of mask use won’t have a bad effect on oxygen levels for most people.
“There is not an inherent right to be out in a pandemic with an unmasked face,” Dr. Raz says. But “you are entitled to an accommodation.” That might be using curbside pickup for food and medication. That requires much less time wearing a mask than entering a store would.
There are no “boilerplate” cards or letters to excuse people provided by the four organizations that addressed the issue, Dr. Rizzo said. If he were to write a letter asking for an exemption, he would personalize it for an individual patient’s medical condition. As to whether a state would honor it, he cannot say. The states have a patchwork of recommendations, making it difficult to say.
Dr. Rizzo tells lung disease patients who are able to go out that wearing a mask for 15-20 minutes to do an errand won’t harm their oxygen levels. And he reminds them that having an exemption, in the form of a doctor’s letter, may bring more problems. “Even with an exemption, someone may confront them” for their lack of a face covering. People with COPD have a higher risk of getting a severe illness from COVID-19, according to the CDC.
This article first appeared on WebMD.com.
In the last 2 months, at least 10 patients have asked Constantine George, MD, for a written medical exemption so they won’t have to wear a mask in public. Dr. George, the chief medical officer of Vedius, an app for a travelers’ concierge medical service in Las Vegas, turned them all down.
Elena Christofides, MD, an endocrinologist in Columbus, Ohio, has also refused patients’ requests for exemptions.
“It’s very rare for someone to need an exemption,” says Albert Rizzo, MD, chief medical officer for the American Lung Association and a lung specialist at ChristianaCare Health System in Newark, Del.
The opposition is sometimes strong. Recently, a video of Lenka Koloma of Laguna Niguel, Calif., who founded the antimask Freedom to Breathe Agency, went viral. She was in a California supermarket, maskless, telling an employee she was breaking the law by requiring patrons to wear masks.
“People need oxygen,” she said. “That alone is a medical condition.” Her webpage has a “Face Mask Exempt Card” that cites the Americans with Disabilities Act and posts a Department of Justice ADA violation reporting number. The DOJ issued a statement calling the cards fraudulent.
Figuring out if a patient’s request to opt out of wearing a mask is legitimate is a ‘’new frontier” for doctors, says Mical Raz, MD, a professor in public policy and health at the University of Rochester (N.Y.), and a hospitalist at the university medical center.
Should some people skip masks?
Experts say there are very few medical reasons for people to skip masks. “If you look at the research, patients with COPD [chronic obstructive pulmonary disorder], those with reactive airway, even those can breathe through a mask,” Dr. George said. Requests for exemptions due to medical reasons are usually without basis. “Obviously, if someone is incapacitated, for example, with mental health issues, that’s case by case.”
Dr. Christofides said one of her patients cited anxiety and the other cited headaches as reasons not to wear a mask. “I told the one who asked for anxiety [reasons] that she could wear ones that were less tight.” The patient with headaches told Dr. Christofides that she had a buildup of carbon dioxide in the mask because of industrial exposure. Baloney, Dr. Christofides told her.
Dr. Rizzo says one rare example of someone who can’t wear a mask might be a patient with an advanced lung condition so severe, they need extra oxygen. “These are the extreme patients where any change in oxygen and carbon dioxide could make a difference,” he said. But “that’s also the population that shouldn’t be going out in the first place.”
Dr. Raz cowrote a commentary about mask exemptions, saying doctors are faced with difficult decisions and must keep a delicate balance between public health and individual disability needs. “Inappropriate medical exemptions may inadvertently hasten viral spread and threaten public health,” she wrote.
In an interview, she says that some people do have a hard time tolerating a mask. “Probably the most common reasons are mental health issues, such as anxiety, panic and PTSD, and children with sensory processing disorders (making them oversensitive to their environment). I think there are very few pulmonary reasons.”
CDC, professional organization guidelines
The CDC says people should wear masks in public and when around people who don’t live in the same household. Beyond that, it simply says masks should not be worn by children under age 2, “or anyone who has trouble breathing, is unconscious, incapacitated, or otherwise unable to remove the mask without assistance.”
In mid-July, four professional organizations released a statement in response to the CDC recommendation for facial coverings. Jointly issued by the American College of Chest Physicians, the American Lung Association, the American Thoracic Society and the COPD Foundation, it states in part that people with normal lungs and “even many individuals with underlying chronic lung disease should be able to wear a non-N95 facial covering without affecting their oxygen or carbon dioxide levels.”
It acknowledges that some people will seek an exemption and doctors must weigh the patient’s concerns against the need to stop the spread of the virus. “In some instances, physician reassurance regarding the safety of the facial coverings may be all that is needed,” it states.
Addressing the excuses
Here are some of the common medical reasons people give for not being able to tolerate a mask:
Claustrophobia or anxiety. Dr. Raz and others suggests a “desensitizing” period, wearing the mask for longer and longer periods of time to get used to it. Parents could suggest kids wear a mask when doing something they like, such as watching television, so they equate it with something pleasant. Switching to a different kind of mask or one that fits better could also help.
Masks cause Legionnaires’ disease. Not true, experts say. Legionnaires’ is a severe form of pneumonia, the result of inhaling tiny water droplets with legionella bacteria.
It’s difficult to read lips. People can buy masks with a clear window that makes their mouth and lips visible.
Trouble breathing. Brief periods of mask use won’t have a bad effect on oxygen levels for most people.
“There is not an inherent right to be out in a pandemic with an unmasked face,” Dr. Raz says. But “you are entitled to an accommodation.” That might be using curbside pickup for food and medication. That requires much less time wearing a mask than entering a store would.
There are no “boilerplate” cards or letters to excuse people provided by the four organizations that addressed the issue, Dr. Rizzo said. If he were to write a letter asking for an exemption, he would personalize it for an individual patient’s medical condition. As to whether a state would honor it, he cannot say. The states have a patchwork of recommendations, making it difficult to say.
Dr. Rizzo tells lung disease patients who are able to go out that wearing a mask for 15-20 minutes to do an errand won’t harm their oxygen levels. And he reminds them that having an exemption, in the form of a doctor’s letter, may bring more problems. “Even with an exemption, someone may confront them” for their lack of a face covering. People with COPD have a higher risk of getting a severe illness from COVID-19, according to the CDC.
This article first appeared on WebMD.com.
Action and awareness are needed to increase immunization rates
August was National Immunization Awareness Month. ... just in time to address the precipitous drop in immunization delivered during the early months of the pandemic.
In May, the Centers for Disease Control and Prevention reported substantial reductions in vaccine doses ordered through the Vaccines for Children program after the declaration of national emergency because of COVID-19 on March 13. Approximately 2.5 million fewer doses of routine, noninfluenza vaccines were administered between Jan. 6 and April 2020, compared with a similar period last year (MMWR Morb Mortal Wkly Rep. 2020 May 15;69[19]:591-3). Declines in immunization rates were echoed by states and municipalities across the United States. Last month, the health system in which I work reported 40,000 children behind on at least one vaccine.
We all know that, when immunization rates drop, outbreaks of vaccine-preventable diseases follow. In order and that is going to take more than a single month.
Identify patients who’ve missed vaccinations
Simply being open and ready to vaccinate is not enough. The Centers for Disease Control and Prevention urges providers to identify patients who have missed vaccines, and call them to schedule in-person visits. Proactively let parents know about strategies implemented in your office to ensure a safe environment.
Pediatricians are accustomed to an influx of patients in the summer, as parents make sure their children have all of the vaccines required for school attendance. As noted in a Washington Post article from Aug. 4, 2020, schools have traditionally served as a backstop for immunization rates. But as many school districts opt to take education online this fall, the implications for vaccine requirements are unclear. District of Columbia public schools continue to require immunization for virtual school attendance, but it is not clear how easily this can be enforced. To read about how other school districts have chosen to address – or not address – immunization requirements for school, visit the the Immunization Action Coalition’s Repository of Resources for Maintaining Immunization during the COVID-19 Pandemic. The repository links to international, national, and state-level policies and guidance and advocacy materials, including talking points, webinars, press releases, media articles from around the United States and social media posts, as well as telehealth resources.
Get some inspiration to talk about vaccination
Need a little inspiration for talking to parents about vaccines? Check out the CDC’s #HowIRecommend video series. These are short videos, most under a minute in length, that explain the importance of vaccination, how to effectively address questions from parents about vaccine safety, and how clinicians routinely recommend same day vaccination to their patients. These videos are part of the CDC’s National Immunization Awareness Month (NIAM) toolkit for communication with health care professionals. A companion toolkit for communicating with parents and patients contains sample social media messages with graphics, along with educational resources to share with parents.
The “Comprehensive Vaccine Education Program – From Training to Practice,” a free online program offered by the Pediatric Infectious Diseases Society, takes a deeper dive into strategies to combat vaccine misinformation and address vaccine hesitancy. Available modules cover vaccine fundamentals, vaccine safety, clinical manifestations of vaccine-preventable diseases, and communication skills that lead to more effective conversations with patients and parents. The curriculum also includes the newest edition of The Vaccine Handbook app, a comprehensive source of practical information for vaccine providers.
Educate young children about vaccines
Don’t leave young children out of the conversation. Vax-Force is a children’s book that explores how vaccination works inside the human body. Dr. Vaxson the pediatrician explains how trusted doctors and scientists made Vicky the Vaccine. Her mission is to tell Willy the White Blood Cell and his Antibuddies how to find and fight bad-guy germs like measles, tetanus, and polio. The book was written by Kelsey Rowe, MD, while she was a medical student at Saint Louis University School of Medicine. Dr. Rowe, now a pediatric resident, notes, “In a world where anti-vaccination rhetoric threatens the health of our global community, this book’s mission is to teach children and adults alike that getting vaccinations is a safe, effective, and even exciting thing to do.” The book is available for purchase at https://www.vax-force.com/, and a small part of every sale is donated to Unicef USA.
Consider vaccination advocacy in your communities
Vaccinate Your Family, a national, nonprofit organization dedicated to protecting people of all ages from vaccine-preventable diseases, suggests that health care providers need to take an active role in raising immunization rates, not just in their own practices, but in their communities. One way to do this is to submit an opinion piece or letter to the editor to a local newspaper describing why it’s important for parents to make sure their child’s immunizations are current. Those who have never written an opinion-editorial should look at the guidance developed by Voices for Vaccines.
How are we doing?
Early data suggest a rebound in immunization rates in May and June, but that is unlikely to close the gap created by disruptions in health care delivery earlier in the year. Collectively, we need to set ambitious goals. Are we just trying to reach prepandemic immunization levels? In Kentucky, where I practice, only 71% of kids aged 19-45 months had received all doses of seven routinely recommended vaccines (≥4 DTaP doses, ≥3 polio doses, ≥1 MMR dose, Hib full series, ≥3 HepB doses, ≥1 varicella dose, and ≥4 PCV doses) based on 2017 National Immunization Survey data. The Healthy People 2020 target goal is 80%. Only 55% of Kentucky girls aged 13-17 years received at least one dose of HPV vaccine, and rates in boys were even lower. Flu vaccine coverage in children 6 months to 17 years also was 55%. The status quo sets the bar too low. To see how your state is doing, check out the interactive map developed by the American Academy of Pediatrics.
Are we attempting to avoid disaster or can we seize the opportunity to protect more children than ever from vaccine-preventable diseases? The latter would really be something to celebrate.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She said she had no relevant financial disclosures. Email her at [email protected].
August was National Immunization Awareness Month. ... just in time to address the precipitous drop in immunization delivered during the early months of the pandemic.
In May, the Centers for Disease Control and Prevention reported substantial reductions in vaccine doses ordered through the Vaccines for Children program after the declaration of national emergency because of COVID-19 on March 13. Approximately 2.5 million fewer doses of routine, noninfluenza vaccines were administered between Jan. 6 and April 2020, compared with a similar period last year (MMWR Morb Mortal Wkly Rep. 2020 May 15;69[19]:591-3). Declines in immunization rates were echoed by states and municipalities across the United States. Last month, the health system in which I work reported 40,000 children behind on at least one vaccine.
We all know that, when immunization rates drop, outbreaks of vaccine-preventable diseases follow. In order and that is going to take more than a single month.
Identify patients who’ve missed vaccinations
Simply being open and ready to vaccinate is not enough. The Centers for Disease Control and Prevention urges providers to identify patients who have missed vaccines, and call them to schedule in-person visits. Proactively let parents know about strategies implemented in your office to ensure a safe environment.
Pediatricians are accustomed to an influx of patients in the summer, as parents make sure their children have all of the vaccines required for school attendance. As noted in a Washington Post article from Aug. 4, 2020, schools have traditionally served as a backstop for immunization rates. But as many school districts opt to take education online this fall, the implications for vaccine requirements are unclear. District of Columbia public schools continue to require immunization for virtual school attendance, but it is not clear how easily this can be enforced. To read about how other school districts have chosen to address – or not address – immunization requirements for school, visit the the Immunization Action Coalition’s Repository of Resources for Maintaining Immunization during the COVID-19 Pandemic. The repository links to international, national, and state-level policies and guidance and advocacy materials, including talking points, webinars, press releases, media articles from around the United States and social media posts, as well as telehealth resources.
Get some inspiration to talk about vaccination
Need a little inspiration for talking to parents about vaccines? Check out the CDC’s #HowIRecommend video series. These are short videos, most under a minute in length, that explain the importance of vaccination, how to effectively address questions from parents about vaccine safety, and how clinicians routinely recommend same day vaccination to their patients. These videos are part of the CDC’s National Immunization Awareness Month (NIAM) toolkit for communication with health care professionals. A companion toolkit for communicating with parents and patients contains sample social media messages with graphics, along with educational resources to share with parents.
The “Comprehensive Vaccine Education Program – From Training to Practice,” a free online program offered by the Pediatric Infectious Diseases Society, takes a deeper dive into strategies to combat vaccine misinformation and address vaccine hesitancy. Available modules cover vaccine fundamentals, vaccine safety, clinical manifestations of vaccine-preventable diseases, and communication skills that lead to more effective conversations with patients and parents. The curriculum also includes the newest edition of The Vaccine Handbook app, a comprehensive source of practical information for vaccine providers.
Educate young children about vaccines
Don’t leave young children out of the conversation. Vax-Force is a children’s book that explores how vaccination works inside the human body. Dr. Vaxson the pediatrician explains how trusted doctors and scientists made Vicky the Vaccine. Her mission is to tell Willy the White Blood Cell and his Antibuddies how to find and fight bad-guy germs like measles, tetanus, and polio. The book was written by Kelsey Rowe, MD, while she was a medical student at Saint Louis University School of Medicine. Dr. Rowe, now a pediatric resident, notes, “In a world where anti-vaccination rhetoric threatens the health of our global community, this book’s mission is to teach children and adults alike that getting vaccinations is a safe, effective, and even exciting thing to do.” The book is available for purchase at https://www.vax-force.com/, and a small part of every sale is donated to Unicef USA.
Consider vaccination advocacy in your communities
Vaccinate Your Family, a national, nonprofit organization dedicated to protecting people of all ages from vaccine-preventable diseases, suggests that health care providers need to take an active role in raising immunization rates, not just in their own practices, but in their communities. One way to do this is to submit an opinion piece or letter to the editor to a local newspaper describing why it’s important for parents to make sure their child’s immunizations are current. Those who have never written an opinion-editorial should look at the guidance developed by Voices for Vaccines.
How are we doing?
Early data suggest a rebound in immunization rates in May and June, but that is unlikely to close the gap created by disruptions in health care delivery earlier in the year. Collectively, we need to set ambitious goals. Are we just trying to reach prepandemic immunization levels? In Kentucky, where I practice, only 71% of kids aged 19-45 months had received all doses of seven routinely recommended vaccines (≥4 DTaP doses, ≥3 polio doses, ≥1 MMR dose, Hib full series, ≥3 HepB doses, ≥1 varicella dose, and ≥4 PCV doses) based on 2017 National Immunization Survey data. The Healthy People 2020 target goal is 80%. Only 55% of Kentucky girls aged 13-17 years received at least one dose of HPV vaccine, and rates in boys were even lower. Flu vaccine coverage in children 6 months to 17 years also was 55%. The status quo sets the bar too low. To see how your state is doing, check out the interactive map developed by the American Academy of Pediatrics.
Are we attempting to avoid disaster or can we seize the opportunity to protect more children than ever from vaccine-preventable diseases? The latter would really be something to celebrate.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She said she had no relevant financial disclosures. Email her at [email protected].
August was National Immunization Awareness Month. ... just in time to address the precipitous drop in immunization delivered during the early months of the pandemic.
In May, the Centers for Disease Control and Prevention reported substantial reductions in vaccine doses ordered through the Vaccines for Children program after the declaration of national emergency because of COVID-19 on March 13. Approximately 2.5 million fewer doses of routine, noninfluenza vaccines were administered between Jan. 6 and April 2020, compared with a similar period last year (MMWR Morb Mortal Wkly Rep. 2020 May 15;69[19]:591-3). Declines in immunization rates were echoed by states and municipalities across the United States. Last month, the health system in which I work reported 40,000 children behind on at least one vaccine.
We all know that, when immunization rates drop, outbreaks of vaccine-preventable diseases follow. In order and that is going to take more than a single month.
Identify patients who’ve missed vaccinations
Simply being open and ready to vaccinate is not enough. The Centers for Disease Control and Prevention urges providers to identify patients who have missed vaccines, and call them to schedule in-person visits. Proactively let parents know about strategies implemented in your office to ensure a safe environment.
Pediatricians are accustomed to an influx of patients in the summer, as parents make sure their children have all of the vaccines required for school attendance. As noted in a Washington Post article from Aug. 4, 2020, schools have traditionally served as a backstop for immunization rates. But as many school districts opt to take education online this fall, the implications for vaccine requirements are unclear. District of Columbia public schools continue to require immunization for virtual school attendance, but it is not clear how easily this can be enforced. To read about how other school districts have chosen to address – or not address – immunization requirements for school, visit the the Immunization Action Coalition’s Repository of Resources for Maintaining Immunization during the COVID-19 Pandemic. The repository links to international, national, and state-level policies and guidance and advocacy materials, including talking points, webinars, press releases, media articles from around the United States and social media posts, as well as telehealth resources.
Get some inspiration to talk about vaccination
Need a little inspiration for talking to parents about vaccines? Check out the CDC’s #HowIRecommend video series. These are short videos, most under a minute in length, that explain the importance of vaccination, how to effectively address questions from parents about vaccine safety, and how clinicians routinely recommend same day vaccination to their patients. These videos are part of the CDC’s National Immunization Awareness Month (NIAM) toolkit for communication with health care professionals. A companion toolkit for communicating with parents and patients contains sample social media messages with graphics, along with educational resources to share with parents.
The “Comprehensive Vaccine Education Program – From Training to Practice,” a free online program offered by the Pediatric Infectious Diseases Society, takes a deeper dive into strategies to combat vaccine misinformation and address vaccine hesitancy. Available modules cover vaccine fundamentals, vaccine safety, clinical manifestations of vaccine-preventable diseases, and communication skills that lead to more effective conversations with patients and parents. The curriculum also includes the newest edition of The Vaccine Handbook app, a comprehensive source of practical information for vaccine providers.
Educate young children about vaccines
Don’t leave young children out of the conversation. Vax-Force is a children’s book that explores how vaccination works inside the human body. Dr. Vaxson the pediatrician explains how trusted doctors and scientists made Vicky the Vaccine. Her mission is to tell Willy the White Blood Cell and his Antibuddies how to find and fight bad-guy germs like measles, tetanus, and polio. The book was written by Kelsey Rowe, MD, while she was a medical student at Saint Louis University School of Medicine. Dr. Rowe, now a pediatric resident, notes, “In a world where anti-vaccination rhetoric threatens the health of our global community, this book’s mission is to teach children and adults alike that getting vaccinations is a safe, effective, and even exciting thing to do.” The book is available for purchase at https://www.vax-force.com/, and a small part of every sale is donated to Unicef USA.
Consider vaccination advocacy in your communities
Vaccinate Your Family, a national, nonprofit organization dedicated to protecting people of all ages from vaccine-preventable diseases, suggests that health care providers need to take an active role in raising immunization rates, not just in their own practices, but in their communities. One way to do this is to submit an opinion piece or letter to the editor to a local newspaper describing why it’s important for parents to make sure their child’s immunizations are current. Those who have never written an opinion-editorial should look at the guidance developed by Voices for Vaccines.
How are we doing?
Early data suggest a rebound in immunization rates in May and June, but that is unlikely to close the gap created by disruptions in health care delivery earlier in the year. Collectively, we need to set ambitious goals. Are we just trying to reach prepandemic immunization levels? In Kentucky, where I practice, only 71% of kids aged 19-45 months had received all doses of seven routinely recommended vaccines (≥4 DTaP doses, ≥3 polio doses, ≥1 MMR dose, Hib full series, ≥3 HepB doses, ≥1 varicella dose, and ≥4 PCV doses) based on 2017 National Immunization Survey data. The Healthy People 2020 target goal is 80%. Only 55% of Kentucky girls aged 13-17 years received at least one dose of HPV vaccine, and rates in boys were even lower. Flu vaccine coverage in children 6 months to 17 years also was 55%. The status quo sets the bar too low. To see how your state is doing, check out the interactive map developed by the American Academy of Pediatrics.
Are we attempting to avoid disaster or can we seize the opportunity to protect more children than ever from vaccine-preventable diseases? The latter would really be something to celebrate.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She said she had no relevant financial disclosures. Email her at [email protected].
Determining cause of skin lesions in COVID-19 patients remains challenging
published in the Journal of the American Academy of Dermatology.
SARS-CoV-2 infection has been associated with a range of skin conditions, wrote Antonio Martinez-Lopez, MD, of Virgen de las Nieves University Hospital, Granada, Spain, and colleagues, who provided an overview of the cutaneous side effects associated with drugs used to treat COVID-19 infection.
“Cutaneous manifestations have recently been described in patients with the new coronavirus infection, similar to cutaneous involvement occurring in common viral infections,” they said. Infected individuals have experienced maculopapular eruption, pseudo-chilblain lesions, urticaria, monomorphic disseminated vesicular lesions, acral vesicular-pustulous lesions, and livedo or necrosis, they noted.
Diagnosing skin manifestations in patients with COVID-19 remains a challenge, because it is unclear whether the skin lesions are related to the virus, the authors said. “Skin diseases not related to coronavirus, other seasonal viral infections, and drug reactions should be considered in the differential diagnosis, especially in those patients suffering from nonspecific manifestations such as urticaria or maculopapular eruptions,” they wrote.
However, “urticarial lesions and maculopapular eruptions in SARS-CoV-2 infections usually appear at the same time as the systemic symptoms, while drug adverse reactions are likely to arise hours to days after the start of the treatment,” they said.
The reviewers noted several cutaneous side effects associated with several of the often-prescribed drugs for COVID-19 infection. The antimalarials hydroxychloroquine and chloroquine had been authorized for COVID-19 treatment by the Food and Drug Administration, but this emergency authorization was rescinded in June. They noted that up to 11.5% of patients on these drugs may experience cutaneous adverse effects, including some that “can be mistaken for skin manifestations of SARS-CoV-2, especially those with maculopapular rash or exanthematous reactions.” Another side effect is exacerbation of psoriasis, which has been described in patients with COVID-19, the authors said.
The oral antiretroviral combination lopinavir/ritonavir, under investigation in clinical trials for COVID-19, has been associated with skin rashes in as many as 5% of adults in HIV studies. Usually appearing after treatment is started, the maculopapular pruritic rash is “usually well tolerated,” they said, although there have been reports of Stevens-Johnson syndrome. Alopecia areata is among the other side effects reported.
Remdesivir also has been authorized for emergency treatment of COVID-19, and the small amount of data available suggest that cutaneous manifestations may be infrequent, the reviewers said. In a recent study of 53 patients treated with remdesivir for 10 days, approximately 8% developed a rash, but the study did not include any information “about rash morphology, distribution, or timeline in relation to remdesivir that may help clinicians differentiate from cutaneous manifestations of COVID-19,” they said.
Other potential treatments for complications of COVID-19 include imatinib, tocilizumab, anakinra, immunoglobulins, corticosteroids, colchicine, and low molecular weight heparins; all have the potential for association with skin reactions, but data on skin manifestations associated with COVID-19 are limited, the authors wrote.
Notably, data on the use of systemic corticosteroids for COVID-19 patients are controversial, although preliminary data showed some reduced mortality in COVID-19 patients who were on respiratory support, they noted. “With regard to differential diagnosis of cutaneous manifestations of COVID-19, the vascular fragility associated with corticosteroid use, especially in elderly patients, may be similar to the thrombotic complications of COVID-19 infection.”
Knowledge about the virology of COVID-19 continues to evolve rapidly, and the number of drugs being studied as treatments continues to expand, the authors pointed out.
“By considering adverse drug reactions in the differential diagnosis, dermatologists can be useful in assisting in the care of these patients,” they wrote. Drugs, rather than the infection, may be the cause of skin reactions in some COVID-19 patients, and “management is often symptomatic, but it is sometimes necessary to modify or discontinue the treatment, and some conditions can even be life-threatening,” they concluded.
The study received no outside funding. The researchers had no financial conflicts to disclose.
SOURCE: Martinez-Lopez A et al. J Am Acad Dermatol. 2020 doi: 10.1016/j.jaad.2020.08.006.
published in the Journal of the American Academy of Dermatology.
SARS-CoV-2 infection has been associated with a range of skin conditions, wrote Antonio Martinez-Lopez, MD, of Virgen de las Nieves University Hospital, Granada, Spain, and colleagues, who provided an overview of the cutaneous side effects associated with drugs used to treat COVID-19 infection.
“Cutaneous manifestations have recently been described in patients with the new coronavirus infection, similar to cutaneous involvement occurring in common viral infections,” they said. Infected individuals have experienced maculopapular eruption, pseudo-chilblain lesions, urticaria, monomorphic disseminated vesicular lesions, acral vesicular-pustulous lesions, and livedo or necrosis, they noted.
Diagnosing skin manifestations in patients with COVID-19 remains a challenge, because it is unclear whether the skin lesions are related to the virus, the authors said. “Skin diseases not related to coronavirus, other seasonal viral infections, and drug reactions should be considered in the differential diagnosis, especially in those patients suffering from nonspecific manifestations such as urticaria or maculopapular eruptions,” they wrote.
However, “urticarial lesions and maculopapular eruptions in SARS-CoV-2 infections usually appear at the same time as the systemic symptoms, while drug adverse reactions are likely to arise hours to days after the start of the treatment,” they said.
The reviewers noted several cutaneous side effects associated with several of the often-prescribed drugs for COVID-19 infection. The antimalarials hydroxychloroquine and chloroquine had been authorized for COVID-19 treatment by the Food and Drug Administration, but this emergency authorization was rescinded in June. They noted that up to 11.5% of patients on these drugs may experience cutaneous adverse effects, including some that “can be mistaken for skin manifestations of SARS-CoV-2, especially those with maculopapular rash or exanthematous reactions.” Another side effect is exacerbation of psoriasis, which has been described in patients with COVID-19, the authors said.
The oral antiretroviral combination lopinavir/ritonavir, under investigation in clinical trials for COVID-19, has been associated with skin rashes in as many as 5% of adults in HIV studies. Usually appearing after treatment is started, the maculopapular pruritic rash is “usually well tolerated,” they said, although there have been reports of Stevens-Johnson syndrome. Alopecia areata is among the other side effects reported.
Remdesivir also has been authorized for emergency treatment of COVID-19, and the small amount of data available suggest that cutaneous manifestations may be infrequent, the reviewers said. In a recent study of 53 patients treated with remdesivir for 10 days, approximately 8% developed a rash, but the study did not include any information “about rash morphology, distribution, or timeline in relation to remdesivir that may help clinicians differentiate from cutaneous manifestations of COVID-19,” they said.
Other potential treatments for complications of COVID-19 include imatinib, tocilizumab, anakinra, immunoglobulins, corticosteroids, colchicine, and low molecular weight heparins; all have the potential for association with skin reactions, but data on skin manifestations associated with COVID-19 are limited, the authors wrote.
Notably, data on the use of systemic corticosteroids for COVID-19 patients are controversial, although preliminary data showed some reduced mortality in COVID-19 patients who were on respiratory support, they noted. “With regard to differential diagnosis of cutaneous manifestations of COVID-19, the vascular fragility associated with corticosteroid use, especially in elderly patients, may be similar to the thrombotic complications of COVID-19 infection.”
Knowledge about the virology of COVID-19 continues to evolve rapidly, and the number of drugs being studied as treatments continues to expand, the authors pointed out.
“By considering adverse drug reactions in the differential diagnosis, dermatologists can be useful in assisting in the care of these patients,” they wrote. Drugs, rather than the infection, may be the cause of skin reactions in some COVID-19 patients, and “management is often symptomatic, but it is sometimes necessary to modify or discontinue the treatment, and some conditions can even be life-threatening,” they concluded.
The study received no outside funding. The researchers had no financial conflicts to disclose.
SOURCE: Martinez-Lopez A et al. J Am Acad Dermatol. 2020 doi: 10.1016/j.jaad.2020.08.006.
published in the Journal of the American Academy of Dermatology.
SARS-CoV-2 infection has been associated with a range of skin conditions, wrote Antonio Martinez-Lopez, MD, of Virgen de las Nieves University Hospital, Granada, Spain, and colleagues, who provided an overview of the cutaneous side effects associated with drugs used to treat COVID-19 infection.
“Cutaneous manifestations have recently been described in patients with the new coronavirus infection, similar to cutaneous involvement occurring in common viral infections,” they said. Infected individuals have experienced maculopapular eruption, pseudo-chilblain lesions, urticaria, monomorphic disseminated vesicular lesions, acral vesicular-pustulous lesions, and livedo or necrosis, they noted.
Diagnosing skin manifestations in patients with COVID-19 remains a challenge, because it is unclear whether the skin lesions are related to the virus, the authors said. “Skin diseases not related to coronavirus, other seasonal viral infections, and drug reactions should be considered in the differential diagnosis, especially in those patients suffering from nonspecific manifestations such as urticaria or maculopapular eruptions,” they wrote.
However, “urticarial lesions and maculopapular eruptions in SARS-CoV-2 infections usually appear at the same time as the systemic symptoms, while drug adverse reactions are likely to arise hours to days after the start of the treatment,” they said.
The reviewers noted several cutaneous side effects associated with several of the often-prescribed drugs for COVID-19 infection. The antimalarials hydroxychloroquine and chloroquine had been authorized for COVID-19 treatment by the Food and Drug Administration, but this emergency authorization was rescinded in June. They noted that up to 11.5% of patients on these drugs may experience cutaneous adverse effects, including some that “can be mistaken for skin manifestations of SARS-CoV-2, especially those with maculopapular rash or exanthematous reactions.” Another side effect is exacerbation of psoriasis, which has been described in patients with COVID-19, the authors said.
The oral antiretroviral combination lopinavir/ritonavir, under investigation in clinical trials for COVID-19, has been associated with skin rashes in as many as 5% of adults in HIV studies. Usually appearing after treatment is started, the maculopapular pruritic rash is “usually well tolerated,” they said, although there have been reports of Stevens-Johnson syndrome. Alopecia areata is among the other side effects reported.
Remdesivir also has been authorized for emergency treatment of COVID-19, and the small amount of data available suggest that cutaneous manifestations may be infrequent, the reviewers said. In a recent study of 53 patients treated with remdesivir for 10 days, approximately 8% developed a rash, but the study did not include any information “about rash morphology, distribution, or timeline in relation to remdesivir that may help clinicians differentiate from cutaneous manifestations of COVID-19,” they said.
Other potential treatments for complications of COVID-19 include imatinib, tocilizumab, anakinra, immunoglobulins, corticosteroids, colchicine, and low molecular weight heparins; all have the potential for association with skin reactions, but data on skin manifestations associated with COVID-19 are limited, the authors wrote.
Notably, data on the use of systemic corticosteroids for COVID-19 patients are controversial, although preliminary data showed some reduced mortality in COVID-19 patients who were on respiratory support, they noted. “With regard to differential diagnosis of cutaneous manifestations of COVID-19, the vascular fragility associated with corticosteroid use, especially in elderly patients, may be similar to the thrombotic complications of COVID-19 infection.”
Knowledge about the virology of COVID-19 continues to evolve rapidly, and the number of drugs being studied as treatments continues to expand, the authors pointed out.
“By considering adverse drug reactions in the differential diagnosis, dermatologists can be useful in assisting in the care of these patients,” they wrote. Drugs, rather than the infection, may be the cause of skin reactions in some COVID-19 patients, and “management is often symptomatic, but it is sometimes necessary to modify or discontinue the treatment, and some conditions can even be life-threatening,” they concluded.
The study received no outside funding. The researchers had no financial conflicts to disclose.
SOURCE: Martinez-Lopez A et al. J Am Acad Dermatol. 2020 doi: 10.1016/j.jaad.2020.08.006.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Since COVID-19 onset, admissions for MI are down, mortality rates are up
A substantial decrease in hospital admissions for acute MI was accompanied by a rise in mortality, particularly for ST-segment elevation MI (STEMI), following the onset of the COVID-19 pandemic, according to a cross-sectional retrospective study.
Although it can’t be confirmed from these results that the observed increase in in-hospital acute MI (AMI) mortality are related to delays in seeking treatment, this is a reasonable working hypothesis until more is known, commented Harlan Krumholz, MD, who was not involved in the study.
The analysis, derived from data collected at 49 centers in a hospital system spread across six states, supports previous reports that patients with AMI were avoiding hospitalization, according to the investigators, who were led by Tyler J. Gluckman, MD, medical director of the Center for Cardiovascular Analytics, Providence Heart Institute, Portland, Ore.
When compared with a nearly 14-month period that preceded the COVID-19 pandemic, the rate of AMI-associated hospitalization fell by 19 cases per week (95% confidence interval, –29.0 to –9.0 cases) in the early COVID-19 period, which was defined by the investigators as spanning from Feb. 23, 2020 to March 28, 2020.
The case rate per week then increased by 10.5 (95% CI, 4.6-16.5 cases) in a subsequent 8-week period spanning between March 29, 2020, and May 16, 2020. Although a substantial increase from the early COVID-19 period, the case rate remained below the baseline established before COVID-19.
The analysis looked at 15,244 AMI hospitalizations among 14,724 patients treated in the Providence St. Joseph Hospital System, which has facilities in Alaska, California, Montana, Oregon, Texas, and Washington. The 1,915 AMI cases captured from Feb. 23, 2020, represented 13% of the total.
Differences in mortality, patients, treatment
In the early period, the ratio of observed-to-expected (O/E) mortality relative to the pre–COVID-19 baseline increased by 27% (odds ratio, 1.27; 95% CI, 1.07-1.48). When STEMI was analyzed separately, the O/E mortality was nearly double that of the baseline period (OR, 1.96; 95% CI, 1.22-2.70). In the latter post–COVID-19 period of observation, the overall increase in AMI-associated mortality on the basis of an O/E ratio was no longer significant relative to the baseline period (OR, 1.23; 95% CI, 0.98-1.47). However, the relative increase in STEMI-associated mortality on an O/E basis was even greater (OR, 2.40; 95% CI, 1.65-3.16) in the second COVID-19 period analyzed. Even after risk adjustment, the OR for STEMI mortality remained significantly elevated relative to baseline (1.52; 95% CI, 1.02-2.26).
The differences in AMI patients treated before the onset of the COVID-19 pandemic and those treated afterwards might be relevant, according to the investigators. Specifically, patients hospitalized after Feb. 23, 2020 were 1-3 years younger (P < .001) depending on type of AMI, and more likely to be Asian (P = .01).
The length of stay was 6 hours shorter in the early COVID-19 period and 7 hours shorter in the latter period relative to baseline, but an analysis of treatment approaches to non-STEMI and STEMI during the COVID-19 pandemic were not found to be significantly different from baseline.
Prior to the COVID-19 pandemic, 79% of STEMI patients and 77% of non-STEMI patients were discharged home, which was significantly lower than in the early COVID-19 period, when 83% (P = .02) of STEMI and 81% (P = .006) of non-STEMI patients were discharged home. In the latter period, discharge to home care was also significantly higher than in the baseline period.
More than fear of COVID-19?
One theory to account for the reduction in AMI hospitalizations and the increase in AMI-related mortality is the possibility that patients were slow to seek care at acute care hospitals because of concern about COVID-19 infection, according to Dr. Gluckman and coinvestigators.
“Given the time-sensitive nature of STEMI, any delay by patients, emergency medical services, the emergency department, or cardiac catheterization laboratory may have played a role,” they suggested.
In an interview, Dr. Gluckman said that further effort to identify the reasons for the increased AMI-related mortality is planned. Pulling data from the electronic medical records of the patients included in this retrospective analysis might be a “challenge,” but Dr. Gluckman reported that he and his coinvestigators plan to look at a different set of registry data that might provide information on sources of delay, particularly in the STEMI population.
“This includes looking at a number of time factors, such as symptom onset to first medical contact, first medical contact to device, and door-in-door-out times,” Dr. Gluckman said. The goal is to “better understand if delays [in treatment] occurred during the pandemic and, if so, how they may have contributed to increases in risk adjusted mortality.”
Dr. Krumholz, director of the Yale Center for Outcomes Research and Evaluation, New Haven, Conn., called this study a “useful” confirmation of changes in AMI-related care with the onset of the COVID-19 pandemic. As reported anecdotally, the study “indicates marked decreases in hospitalizations of patients with AMI even in areas that were not experiencing big outbreaks but did have some restrictions to limit spread,” he noted.
More data gathered by other centers might provide information about what it all means.
“There remain so many questions about what happened and what consequences accrued,” Dr. Krumholz observed. “In the meantime, we need to continue to send the message that people with symptoms that suggest a heart attack need to rapidly seek care.”
The investigators reported having no financial conflicts of interest.
SOURCE: Gluckman TJ et al. JAMA Cardiol. 2020 Aug 7. doi: 10.1001/jamacardio.2020.3629.
A substantial decrease in hospital admissions for acute MI was accompanied by a rise in mortality, particularly for ST-segment elevation MI (STEMI), following the onset of the COVID-19 pandemic, according to a cross-sectional retrospective study.
Although it can’t be confirmed from these results that the observed increase in in-hospital acute MI (AMI) mortality are related to delays in seeking treatment, this is a reasonable working hypothesis until more is known, commented Harlan Krumholz, MD, who was not involved in the study.
The analysis, derived from data collected at 49 centers in a hospital system spread across six states, supports previous reports that patients with AMI were avoiding hospitalization, according to the investigators, who were led by Tyler J. Gluckman, MD, medical director of the Center for Cardiovascular Analytics, Providence Heart Institute, Portland, Ore.
When compared with a nearly 14-month period that preceded the COVID-19 pandemic, the rate of AMI-associated hospitalization fell by 19 cases per week (95% confidence interval, –29.0 to –9.0 cases) in the early COVID-19 period, which was defined by the investigators as spanning from Feb. 23, 2020 to March 28, 2020.
The case rate per week then increased by 10.5 (95% CI, 4.6-16.5 cases) in a subsequent 8-week period spanning between March 29, 2020, and May 16, 2020. Although a substantial increase from the early COVID-19 period, the case rate remained below the baseline established before COVID-19.
The analysis looked at 15,244 AMI hospitalizations among 14,724 patients treated in the Providence St. Joseph Hospital System, which has facilities in Alaska, California, Montana, Oregon, Texas, and Washington. The 1,915 AMI cases captured from Feb. 23, 2020, represented 13% of the total.
Differences in mortality, patients, treatment
In the early period, the ratio of observed-to-expected (O/E) mortality relative to the pre–COVID-19 baseline increased by 27% (odds ratio, 1.27; 95% CI, 1.07-1.48). When STEMI was analyzed separately, the O/E mortality was nearly double that of the baseline period (OR, 1.96; 95% CI, 1.22-2.70). In the latter post–COVID-19 period of observation, the overall increase in AMI-associated mortality on the basis of an O/E ratio was no longer significant relative to the baseline period (OR, 1.23; 95% CI, 0.98-1.47). However, the relative increase in STEMI-associated mortality on an O/E basis was even greater (OR, 2.40; 95% CI, 1.65-3.16) in the second COVID-19 period analyzed. Even after risk adjustment, the OR for STEMI mortality remained significantly elevated relative to baseline (1.52; 95% CI, 1.02-2.26).
The differences in AMI patients treated before the onset of the COVID-19 pandemic and those treated afterwards might be relevant, according to the investigators. Specifically, patients hospitalized after Feb. 23, 2020 were 1-3 years younger (P < .001) depending on type of AMI, and more likely to be Asian (P = .01).
The length of stay was 6 hours shorter in the early COVID-19 period and 7 hours shorter in the latter period relative to baseline, but an analysis of treatment approaches to non-STEMI and STEMI during the COVID-19 pandemic were not found to be significantly different from baseline.
Prior to the COVID-19 pandemic, 79% of STEMI patients and 77% of non-STEMI patients were discharged home, which was significantly lower than in the early COVID-19 period, when 83% (P = .02) of STEMI and 81% (P = .006) of non-STEMI patients were discharged home. In the latter period, discharge to home care was also significantly higher than in the baseline period.
More than fear of COVID-19?
One theory to account for the reduction in AMI hospitalizations and the increase in AMI-related mortality is the possibility that patients were slow to seek care at acute care hospitals because of concern about COVID-19 infection, according to Dr. Gluckman and coinvestigators.
“Given the time-sensitive nature of STEMI, any delay by patients, emergency medical services, the emergency department, or cardiac catheterization laboratory may have played a role,” they suggested.
In an interview, Dr. Gluckman said that further effort to identify the reasons for the increased AMI-related mortality is planned. Pulling data from the electronic medical records of the patients included in this retrospective analysis might be a “challenge,” but Dr. Gluckman reported that he and his coinvestigators plan to look at a different set of registry data that might provide information on sources of delay, particularly in the STEMI population.
“This includes looking at a number of time factors, such as symptom onset to first medical contact, first medical contact to device, and door-in-door-out times,” Dr. Gluckman said. The goal is to “better understand if delays [in treatment] occurred during the pandemic and, if so, how they may have contributed to increases in risk adjusted mortality.”
Dr. Krumholz, director of the Yale Center for Outcomes Research and Evaluation, New Haven, Conn., called this study a “useful” confirmation of changes in AMI-related care with the onset of the COVID-19 pandemic. As reported anecdotally, the study “indicates marked decreases in hospitalizations of patients with AMI even in areas that were not experiencing big outbreaks but did have some restrictions to limit spread,” he noted.
More data gathered by other centers might provide information about what it all means.
“There remain so many questions about what happened and what consequences accrued,” Dr. Krumholz observed. “In the meantime, we need to continue to send the message that people with symptoms that suggest a heart attack need to rapidly seek care.”
The investigators reported having no financial conflicts of interest.
SOURCE: Gluckman TJ et al. JAMA Cardiol. 2020 Aug 7. doi: 10.1001/jamacardio.2020.3629.
A substantial decrease in hospital admissions for acute MI was accompanied by a rise in mortality, particularly for ST-segment elevation MI (STEMI), following the onset of the COVID-19 pandemic, according to a cross-sectional retrospective study.
Although it can’t be confirmed from these results that the observed increase in in-hospital acute MI (AMI) mortality are related to delays in seeking treatment, this is a reasonable working hypothesis until more is known, commented Harlan Krumholz, MD, who was not involved in the study.
The analysis, derived from data collected at 49 centers in a hospital system spread across six states, supports previous reports that patients with AMI were avoiding hospitalization, according to the investigators, who were led by Tyler J. Gluckman, MD, medical director of the Center for Cardiovascular Analytics, Providence Heart Institute, Portland, Ore.
When compared with a nearly 14-month period that preceded the COVID-19 pandemic, the rate of AMI-associated hospitalization fell by 19 cases per week (95% confidence interval, –29.0 to –9.0 cases) in the early COVID-19 period, which was defined by the investigators as spanning from Feb. 23, 2020 to March 28, 2020.
The case rate per week then increased by 10.5 (95% CI, 4.6-16.5 cases) in a subsequent 8-week period spanning between March 29, 2020, and May 16, 2020. Although a substantial increase from the early COVID-19 period, the case rate remained below the baseline established before COVID-19.
The analysis looked at 15,244 AMI hospitalizations among 14,724 patients treated in the Providence St. Joseph Hospital System, which has facilities in Alaska, California, Montana, Oregon, Texas, and Washington. The 1,915 AMI cases captured from Feb. 23, 2020, represented 13% of the total.
Differences in mortality, patients, treatment
In the early period, the ratio of observed-to-expected (O/E) mortality relative to the pre–COVID-19 baseline increased by 27% (odds ratio, 1.27; 95% CI, 1.07-1.48). When STEMI was analyzed separately, the O/E mortality was nearly double that of the baseline period (OR, 1.96; 95% CI, 1.22-2.70). In the latter post–COVID-19 period of observation, the overall increase in AMI-associated mortality on the basis of an O/E ratio was no longer significant relative to the baseline period (OR, 1.23; 95% CI, 0.98-1.47). However, the relative increase in STEMI-associated mortality on an O/E basis was even greater (OR, 2.40; 95% CI, 1.65-3.16) in the second COVID-19 period analyzed. Even after risk adjustment, the OR for STEMI mortality remained significantly elevated relative to baseline (1.52; 95% CI, 1.02-2.26).
The differences in AMI patients treated before the onset of the COVID-19 pandemic and those treated afterwards might be relevant, according to the investigators. Specifically, patients hospitalized after Feb. 23, 2020 were 1-3 years younger (P < .001) depending on type of AMI, and more likely to be Asian (P = .01).
The length of stay was 6 hours shorter in the early COVID-19 period and 7 hours shorter in the latter period relative to baseline, but an analysis of treatment approaches to non-STEMI and STEMI during the COVID-19 pandemic were not found to be significantly different from baseline.
Prior to the COVID-19 pandemic, 79% of STEMI patients and 77% of non-STEMI patients were discharged home, which was significantly lower than in the early COVID-19 period, when 83% (P = .02) of STEMI and 81% (P = .006) of non-STEMI patients were discharged home. In the latter period, discharge to home care was also significantly higher than in the baseline period.
More than fear of COVID-19?
One theory to account for the reduction in AMI hospitalizations and the increase in AMI-related mortality is the possibility that patients were slow to seek care at acute care hospitals because of concern about COVID-19 infection, according to Dr. Gluckman and coinvestigators.
“Given the time-sensitive nature of STEMI, any delay by patients, emergency medical services, the emergency department, or cardiac catheterization laboratory may have played a role,” they suggested.
In an interview, Dr. Gluckman said that further effort to identify the reasons for the increased AMI-related mortality is planned. Pulling data from the electronic medical records of the patients included in this retrospective analysis might be a “challenge,” but Dr. Gluckman reported that he and his coinvestigators plan to look at a different set of registry data that might provide information on sources of delay, particularly in the STEMI population.
“This includes looking at a number of time factors, such as symptom onset to first medical contact, first medical contact to device, and door-in-door-out times,” Dr. Gluckman said. The goal is to “better understand if delays [in treatment] occurred during the pandemic and, if so, how they may have contributed to increases in risk adjusted mortality.”
Dr. Krumholz, director of the Yale Center for Outcomes Research and Evaluation, New Haven, Conn., called this study a “useful” confirmation of changes in AMI-related care with the onset of the COVID-19 pandemic. As reported anecdotally, the study “indicates marked decreases in hospitalizations of patients with AMI even in areas that were not experiencing big outbreaks but did have some restrictions to limit spread,” he noted.
More data gathered by other centers might provide information about what it all means.
“There remain so many questions about what happened and what consequences accrued,” Dr. Krumholz observed. “In the meantime, we need to continue to send the message that people with symptoms that suggest a heart attack need to rapidly seek care.”
The investigators reported having no financial conflicts of interest.
SOURCE: Gluckman TJ et al. JAMA Cardiol. 2020 Aug 7. doi: 10.1001/jamacardio.2020.3629.
FROM JAMA CARDIOLOGY
Pandemic effect: Telemedicine is now a ‘must-have’ service
If people try telemedicine, they’ll like telemedicine. And if they want to avoid a doctor’s office, as most people do these days, they’ll try telemedicine. That is the message coming from 1,000 people surveyed for DocASAP, a provider of online patient access and engagement systems.

Here are a couple of numbers: 92% of those who made a telemedicine visit said they were satisfied with the overall appointment experience, and 91% said that they are more likely to schedule a telemedicine visit instead of an in-person appointment. All of the survey respondents had visited a health care provider in the past year, and 40% already had made a telemedicine visit, DocASAP reported.
Puneet Maheshwari, DocASAP cofounder and CEO, said in a statement. “As providers continue to adopt innovative technology to power a more seamless, end-to-end digital consumer experience, I expect telehealth to become fully integrated into overall care management.”
For now, though, COVID-19 is an overriding concern and health care facilities are suspect. When respondents were asked to identify the types of public facilities where they felt safe, hospitals were named by 32%, doctors’ offices by 26%, and ED/urgent care by just 12%, the DocASAP report said. Even public transportation got 13%.
The safest place to be, according to 42% of the respondents? The grocery store.
Of those surveyed, 43% “indicated they will not feel safe entering any health care setting until at least the fall,” the company said. An even higher share of patients, 68%, canceled or postponed an in-person appointment during the pandemic.
“No longer are remote health services viewed as ‘nice to have’ – they are now a must-have care delivery option,” DocASAP said in their report.
Safety concerns involving COVID-19, named by 47% of the sample, were the leading factor that would influence patients’ decision to schedule a telemedicine visit. Insurance coverage was next at 43%, followed by “ease of accessing quality care” at 40%, the report said.
Among those who had made a telemedicine visit, scheduling the appointment was the most satisfying aspect of the experience, according to 54% of respondents, with day-of-appointment wait time next at 38% and quality of the video/audio technology tied with preappointment communication at almost 33%, the survey data show.
Conversely, scheduling the appointment also was declared the most frustrating aspect of the telemedicine experience, although the total in that category was a much lower 29%.
“The pandemic has thrust profound change on every aspect of life, particularly health care. … Innovations – like digital and telehealth solutions – designed to meet patient needs will likely become embedded into the health care delivery system,” DocASAP said.
The survey was commissioned by DocASAP and conducted by marketing research company OnePoll on June 29-30, 2020.
If people try telemedicine, they’ll like telemedicine. And if they want to avoid a doctor’s office, as most people do these days, they’ll try telemedicine. That is the message coming from 1,000 people surveyed for DocASAP, a provider of online patient access and engagement systems.

Here are a couple of numbers: 92% of those who made a telemedicine visit said they were satisfied with the overall appointment experience, and 91% said that they are more likely to schedule a telemedicine visit instead of an in-person appointment. All of the survey respondents had visited a health care provider in the past year, and 40% already had made a telemedicine visit, DocASAP reported.
Puneet Maheshwari, DocASAP cofounder and CEO, said in a statement. “As providers continue to adopt innovative technology to power a more seamless, end-to-end digital consumer experience, I expect telehealth to become fully integrated into overall care management.”
For now, though, COVID-19 is an overriding concern and health care facilities are suspect. When respondents were asked to identify the types of public facilities where they felt safe, hospitals were named by 32%, doctors’ offices by 26%, and ED/urgent care by just 12%, the DocASAP report said. Even public transportation got 13%.
The safest place to be, according to 42% of the respondents? The grocery store.
Of those surveyed, 43% “indicated they will not feel safe entering any health care setting until at least the fall,” the company said. An even higher share of patients, 68%, canceled or postponed an in-person appointment during the pandemic.
“No longer are remote health services viewed as ‘nice to have’ – they are now a must-have care delivery option,” DocASAP said in their report.
Safety concerns involving COVID-19, named by 47% of the sample, were the leading factor that would influence patients’ decision to schedule a telemedicine visit. Insurance coverage was next at 43%, followed by “ease of accessing quality care” at 40%, the report said.
Among those who had made a telemedicine visit, scheduling the appointment was the most satisfying aspect of the experience, according to 54% of respondents, with day-of-appointment wait time next at 38% and quality of the video/audio technology tied with preappointment communication at almost 33%, the survey data show.
Conversely, scheduling the appointment also was declared the most frustrating aspect of the telemedicine experience, although the total in that category was a much lower 29%.
“The pandemic has thrust profound change on every aspect of life, particularly health care. … Innovations – like digital and telehealth solutions – designed to meet patient needs will likely become embedded into the health care delivery system,” DocASAP said.
The survey was commissioned by DocASAP and conducted by marketing research company OnePoll on June 29-30, 2020.
If people try telemedicine, they’ll like telemedicine. And if they want to avoid a doctor’s office, as most people do these days, they’ll try telemedicine. That is the message coming from 1,000 people surveyed for DocASAP, a provider of online patient access and engagement systems.

Here are a couple of numbers: 92% of those who made a telemedicine visit said they were satisfied with the overall appointment experience, and 91% said that they are more likely to schedule a telemedicine visit instead of an in-person appointment. All of the survey respondents had visited a health care provider in the past year, and 40% already had made a telemedicine visit, DocASAP reported.
Puneet Maheshwari, DocASAP cofounder and CEO, said in a statement. “As providers continue to adopt innovative technology to power a more seamless, end-to-end digital consumer experience, I expect telehealth to become fully integrated into overall care management.”
For now, though, COVID-19 is an overriding concern and health care facilities are suspect. When respondents were asked to identify the types of public facilities where they felt safe, hospitals were named by 32%, doctors’ offices by 26%, and ED/urgent care by just 12%, the DocASAP report said. Even public transportation got 13%.
The safest place to be, according to 42% of the respondents? The grocery store.
Of those surveyed, 43% “indicated they will not feel safe entering any health care setting until at least the fall,” the company said. An even higher share of patients, 68%, canceled or postponed an in-person appointment during the pandemic.
“No longer are remote health services viewed as ‘nice to have’ – they are now a must-have care delivery option,” DocASAP said in their report.
Safety concerns involving COVID-19, named by 47% of the sample, were the leading factor that would influence patients’ decision to schedule a telemedicine visit. Insurance coverage was next at 43%, followed by “ease of accessing quality care” at 40%, the report said.
Among those who had made a telemedicine visit, scheduling the appointment was the most satisfying aspect of the experience, according to 54% of respondents, with day-of-appointment wait time next at 38% and quality of the video/audio technology tied with preappointment communication at almost 33%, the survey data show.
Conversely, scheduling the appointment also was declared the most frustrating aspect of the telemedicine experience, although the total in that category was a much lower 29%.
“The pandemic has thrust profound change on every aspect of life, particularly health care. … Innovations – like digital and telehealth solutions – designed to meet patient needs will likely become embedded into the health care delivery system,” DocASAP said.
The survey was commissioned by DocASAP and conducted by marketing research company OnePoll on June 29-30, 2020.
Long-lasting COVID-19 symptoms: Patients want answers
Q&A with Dr. Sachin Gupta
For some patients, a bout of COVID-19 may not be over after hospital discharge, acute symptoms subside, or a couple of tests for SARS-CoV-2 come back negative. Those who have reached these milestones of conquering the disease may find that their recovery journey has only begun. Debilitating symptoms such as fatigue, headache, and dyspnea may linger for weeks or longer. Patients with persistent symptoms, often referred to as “long haulers” in reference to the duration of their recovery, are looking for answers about their condition and when their COVID-19 illness will finally resolve.
Long-haul patients organize
What started as an accumulation of anecdotal evidence in social media, blogs, and the mainstream press about slow recovery and long-lasting symptoms of COVID-19 is now the focus of clinical trials in the population of recovering patients. Projects such as the COVID Symptom Study, initiated by the Massachusetts General Hospital, Boston; the Harvard School of Public Health, Boston; King’s College London; and Stanford (Calif.) University, are collecting data on symptoms from millions of patients and will eventually contribute to a better understanding of prolonged recovery.
Patients looking for answers have created groups on social media such as Facebook to exchange information about their experiences (e.g., Survivor Corps, COVID-19 Support Group, COVID-19 Recovered Survivors). Recovering patients have created patient-led research organizations (Body Politic COVID-19 Support Group) to explore persistent symptoms and begin to create data for research.
Some data on lingering symptoms
A small study of 143 previously hospitalized, recovering patients in Italy found that 87.4% of the cohort had at least one persistent symptom 2 months or longer after initial onset and at more than a month after discharge. In this sample, only 5% had been intubated. (JAMA 2020 Jul 9. doi: 10.1001/jama.2020.12603).
One study found that even patients who have had relatively mild symptoms and were not hospitalized can have persistent symptoms. The Centers for Disease Control and Prevention conducted a survey of adults who tested positive for the positive reverse transcription–polymerase chain reaction test for SARS-CoV-2 and found that, among the 292 respondents, 35% were still feeling the impact of the disease 2-3 weeks after testing. Fatigue (71%), cough (61%), and headache (61%) were the most commonly reported symptoms. The survey found that delayed recovery was evident in nearly a quarter of 18- to 34-year-olds and in a third of 35- to 49-year-olds who were not sick enough to require hospitalization (MMWR. 2020 Jul 24. doi: 10.15585/mmwr.mm6930e1).
Sachin Gupta, MD, FCCP, ATSF, a pulmonologist and member of the CHEST Physician editorial advisory board, has treated patients with COVID-19 and shared some of his thoughts on the problem of prolonged symptoms of COVID-19.
Q: Should clinicians expect to see COVID-19 patients who have symptoms persisting weeks after they are diagnosed?
Dr. Gupta: I think clinicians, especially in primary care, are already seeing many patients with lingering symptoms, both respiratory and nonrespiratory related, and debility. A few patients here in the San Francisco Bay Area that I have spoken with 4-6 weeks out from their acute illness have complained of persisting, though improving, fatigue and cough. Early studies are confirming this as a topical issue. There may be other long-lasting sequelae of COVID-19 beyond the common mild lingering symptoms. It will also be important to consider (and get more data on) to what degree asymptomatic patients develop some degree of mild inflammatory and subsequent fibrotic changes in organs like the lungs and heart
Q: How does the recovery phase of COVID-19 compare with recovery from severe influenza or ARDS?
Dr. Gupta: Most prior influenza and acute respiratory distress syndrome (ARDS) studies have provided initial follow-up at 3 months and beyond, so technically speaking, it is a little difficult to compare the symptomatology patterns in the JAMA study of 2 months on follow-up. Nevertheless, the key takeaway is that, even though few patients in the study had ARDS requiring intubation (severe disease), many patients with milder disease had significant lingering symptoms (55% with three or more symptoms) at 2 months.
This fits logically with the premise, which we have some limited data on with ARDS (N Engl J Med. 2003;348:683-93. doi: 10.1056/NEJMoa022450) and severe influenza infection survivors (Nature Sci Rep. 2017;7:17275. doi: 10.1038/s41598-017-17497-6) that varying degrees of the inflammation cascade triggered by certain viruses can lead to changes in important patient-reported outcomes, and objective measures such as pulmonary function over the long term.
Q: What can you do for patients with lingering symptoms of COVID-19 or what can you tell them about their symptoms?
Dr. Gupta: For many patients, there is fear, given the novel nature of the virus/pandemic, that their symptoms may persist long term. Acknowledgment of their symptoms is validating and important for us to recognize as we learn more about the virus. As we are finding, many patients are going online to find answers, after sometimes feeling rushed or dismissed initially in the clinical setting.
In my experience, the bar is fairly high for most patients to reach out to their physicians with complaints of lingering symptoms after acute infection. For the ones who do reach out, they tend to have either a greater constellation of symptoms or higher severity of one or two key symptoms. After assessing and, when appropriate, ruling out secondary infections or newly developed conditions, I shift toward symptom management. I encourage such patients to build up slowly. I suggest they work first on their activities of daily living (bathing, grooming), then their instrumental activities of daily living (cooking, cleaning, checking the mail), and then to engage, based on their tolerance of symptoms, to light purposeful exercise. There are many online resources for at-home exercise activities that I recommend to patients who are more debilitated; some larger centers are beginning to offer some forms of telepulmonary rehab.
Based on what we know about other causes of viral pneumonitis and ARDS, I ask such symptomatic patients to expect a slow, gradual, and in most cases a complete recovery, and depending on the individual case, I recommend pulmonary function tests and imaging that may be helpful to track that progress.
I remind myself, and patients, that our understanding may change as we learn more over time. Checking in at set intervals, even if not in person but through a phone call, can go a long way in a setting where we do not have a specific therapy, other than gradual exercise training, to help these patients recover faster. Reassurance and encouragement are vital for patients who are struggling with the lingering burden of disease and who may find it difficult to return to work or function as usual at home. The final point is to be mindful of our patient’s mental health and, where our reassurance is not enough, to consider appropriate mental health referrals.
Q: Can you handle this kind of problem with telemedicine or which patients with lingering symptoms need to come into the office – or failing that, the ED?
Dr. Gupta: Telemedicine in the outpatient setting provides a helpful tool to assess and manage patients, in my experience, with limited and straightforward complaints. Its scope is limited diagnostically (assessing symptoms and signs) as is its reach (ability to connect with elderly, disabled, or patients without/limited telemedicine access). In many instances, telemedicine limits our ability to connect with patients emotionally and build trust. Many patients who have gone through the acute illness that we see in pulmonary clinic on follow-up are older in age, and for many, video visits are not a practical solution. Telemedicine visits can sometimes present challenges for me as well in terms of thoroughly conveying lifestyle and symptom management strategies. Health literacy is typically easier to gauge and address in person.
For patients with any degree of enduring dyspnea, more so in the acute phase, I recommend home pulse oximetry for monitoring their oxygen saturation if it is financially and technically feasible for them to obtain one. Sending a patient to the ED is an option of last resort, but one that is necessary in some cases. I expect patients with lingering symptoms to tell me that symptoms may be persisting, hopefully gradually improving, and not getting worse. If post–COVID-19 symptoms such as fever, dyspnea, fatigue, or lightheadedness are new or worsening, particularly rapidly, the safest and best option I advise patients is to go to the ED for further assessment and testing. Postviral bacterial pneumonia is something we should consider, and there is some potential for aspergillosis as well.
Q: Do you have any concerns about patients with asthma, chronic obstructive pulmonary disease, or other pulmonary issues having lingering symptoms that may mask exacerbations or may cause exacerbation of their disease?
Dr. Gupta: So far, patients with chronic lung conditions do not appear to have not been disproportionately affected by the pandemic in terms of absolute numbers or percentage wise compared to the general public. I think that sheltering in place has been readily followed by many of these patients, and in addition, I assume better adherence to their maintenance therapies has likely helped. The very few cases of patients with underlying chronic obstructive pulmonary disease and interstitial lung disease that I have seen have fared very poorly when they were diagnosed with COVID-19 in the hospital. There are emerging data about short-term outcomes from severe COVID-19 infection in patients with interstitial lung disease in Europe (medRxiv. 2020 Jul 17. doi: 10.1101/2020.07.15.20152967), and from physicians treating pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension (Ann Am Thorac Soc. 2020 Jul 29. doi: 10.1513/AnnalsATS.202005-521OC). But so far, little has been published on the outcomes of mild disease in these patients with chronic lung disease.
Q: It’s still early days to know the significance of lingering symptoms. But at what point do you begin to consider the possibility of some kind of relapse? And what is your next move if the symptoms get worse?
Dr. Gupta: COVID-19 recurrence, whether because of reinfection or relapse, is a potential concern but not one that is very commonly seen so far in my purview. Generally, symptoms of post–COVID-19 infection that are lingering trend toward getting better, even if slowly. If post–COVID-19 infection symptoms are progressing (particularly if rapidly), that would be a strong indication to evaluate that patient in the ED (less likely in clinic), reswab them for SARS-CoV-2, and obtain further testing such as blood work and imaging. A significant challenge from a research perspective will be determining if coinfection with another virus is playing a role as we move closer to the fall season.
Q&A with Dr. Sachin Gupta
Q&A with Dr. Sachin Gupta
For some patients, a bout of COVID-19 may not be over after hospital discharge, acute symptoms subside, or a couple of tests for SARS-CoV-2 come back negative. Those who have reached these milestones of conquering the disease may find that their recovery journey has only begun. Debilitating symptoms such as fatigue, headache, and dyspnea may linger for weeks or longer. Patients with persistent symptoms, often referred to as “long haulers” in reference to the duration of their recovery, are looking for answers about their condition and when their COVID-19 illness will finally resolve.
Long-haul patients organize
What started as an accumulation of anecdotal evidence in social media, blogs, and the mainstream press about slow recovery and long-lasting symptoms of COVID-19 is now the focus of clinical trials in the population of recovering patients. Projects such as the COVID Symptom Study, initiated by the Massachusetts General Hospital, Boston; the Harvard School of Public Health, Boston; King’s College London; and Stanford (Calif.) University, are collecting data on symptoms from millions of patients and will eventually contribute to a better understanding of prolonged recovery.
Patients looking for answers have created groups on social media such as Facebook to exchange information about their experiences (e.g., Survivor Corps, COVID-19 Support Group, COVID-19 Recovered Survivors). Recovering patients have created patient-led research organizations (Body Politic COVID-19 Support Group) to explore persistent symptoms and begin to create data for research.
Some data on lingering symptoms
A small study of 143 previously hospitalized, recovering patients in Italy found that 87.4% of the cohort had at least one persistent symptom 2 months or longer after initial onset and at more than a month after discharge. In this sample, only 5% had been intubated. (JAMA 2020 Jul 9. doi: 10.1001/jama.2020.12603).
One study found that even patients who have had relatively mild symptoms and were not hospitalized can have persistent symptoms. The Centers for Disease Control and Prevention conducted a survey of adults who tested positive for the positive reverse transcription–polymerase chain reaction test for SARS-CoV-2 and found that, among the 292 respondents, 35% were still feeling the impact of the disease 2-3 weeks after testing. Fatigue (71%), cough (61%), and headache (61%) were the most commonly reported symptoms. The survey found that delayed recovery was evident in nearly a quarter of 18- to 34-year-olds and in a third of 35- to 49-year-olds who were not sick enough to require hospitalization (MMWR. 2020 Jul 24. doi: 10.15585/mmwr.mm6930e1).
Sachin Gupta, MD, FCCP, ATSF, a pulmonologist and member of the CHEST Physician editorial advisory board, has treated patients with COVID-19 and shared some of his thoughts on the problem of prolonged symptoms of COVID-19.
Q: Should clinicians expect to see COVID-19 patients who have symptoms persisting weeks after they are diagnosed?
Dr. Gupta: I think clinicians, especially in primary care, are already seeing many patients with lingering symptoms, both respiratory and nonrespiratory related, and debility. A few patients here in the San Francisco Bay Area that I have spoken with 4-6 weeks out from their acute illness have complained of persisting, though improving, fatigue and cough. Early studies are confirming this as a topical issue. There may be other long-lasting sequelae of COVID-19 beyond the common mild lingering symptoms. It will also be important to consider (and get more data on) to what degree asymptomatic patients develop some degree of mild inflammatory and subsequent fibrotic changes in organs like the lungs and heart
Q: How does the recovery phase of COVID-19 compare with recovery from severe influenza or ARDS?
Dr. Gupta: Most prior influenza and acute respiratory distress syndrome (ARDS) studies have provided initial follow-up at 3 months and beyond, so technically speaking, it is a little difficult to compare the symptomatology patterns in the JAMA study of 2 months on follow-up. Nevertheless, the key takeaway is that, even though few patients in the study had ARDS requiring intubation (severe disease), many patients with milder disease had significant lingering symptoms (55% with three or more symptoms) at 2 months.
This fits logically with the premise, which we have some limited data on with ARDS (N Engl J Med. 2003;348:683-93. doi: 10.1056/NEJMoa022450) and severe influenza infection survivors (Nature Sci Rep. 2017;7:17275. doi: 10.1038/s41598-017-17497-6) that varying degrees of the inflammation cascade triggered by certain viruses can lead to changes in important patient-reported outcomes, and objective measures such as pulmonary function over the long term.
Q: What can you do for patients with lingering symptoms of COVID-19 or what can you tell them about their symptoms?
Dr. Gupta: For many patients, there is fear, given the novel nature of the virus/pandemic, that their symptoms may persist long term. Acknowledgment of their symptoms is validating and important for us to recognize as we learn more about the virus. As we are finding, many patients are going online to find answers, after sometimes feeling rushed or dismissed initially in the clinical setting.
In my experience, the bar is fairly high for most patients to reach out to their physicians with complaints of lingering symptoms after acute infection. For the ones who do reach out, they tend to have either a greater constellation of symptoms or higher severity of one or two key symptoms. After assessing and, when appropriate, ruling out secondary infections or newly developed conditions, I shift toward symptom management. I encourage such patients to build up slowly. I suggest they work first on their activities of daily living (bathing, grooming), then their instrumental activities of daily living (cooking, cleaning, checking the mail), and then to engage, based on their tolerance of symptoms, to light purposeful exercise. There are many online resources for at-home exercise activities that I recommend to patients who are more debilitated; some larger centers are beginning to offer some forms of telepulmonary rehab.
Based on what we know about other causes of viral pneumonitis and ARDS, I ask such symptomatic patients to expect a slow, gradual, and in most cases a complete recovery, and depending on the individual case, I recommend pulmonary function tests and imaging that may be helpful to track that progress.
I remind myself, and patients, that our understanding may change as we learn more over time. Checking in at set intervals, even if not in person but through a phone call, can go a long way in a setting where we do not have a specific therapy, other than gradual exercise training, to help these patients recover faster. Reassurance and encouragement are vital for patients who are struggling with the lingering burden of disease and who may find it difficult to return to work or function as usual at home. The final point is to be mindful of our patient’s mental health and, where our reassurance is not enough, to consider appropriate mental health referrals.
Q: Can you handle this kind of problem with telemedicine or which patients with lingering symptoms need to come into the office – or failing that, the ED?
Dr. Gupta: Telemedicine in the outpatient setting provides a helpful tool to assess and manage patients, in my experience, with limited and straightforward complaints. Its scope is limited diagnostically (assessing symptoms and signs) as is its reach (ability to connect with elderly, disabled, or patients without/limited telemedicine access). In many instances, telemedicine limits our ability to connect with patients emotionally and build trust. Many patients who have gone through the acute illness that we see in pulmonary clinic on follow-up are older in age, and for many, video visits are not a practical solution. Telemedicine visits can sometimes present challenges for me as well in terms of thoroughly conveying lifestyle and symptom management strategies. Health literacy is typically easier to gauge and address in person.
For patients with any degree of enduring dyspnea, more so in the acute phase, I recommend home pulse oximetry for monitoring their oxygen saturation if it is financially and technically feasible for them to obtain one. Sending a patient to the ED is an option of last resort, but one that is necessary in some cases. I expect patients with lingering symptoms to tell me that symptoms may be persisting, hopefully gradually improving, and not getting worse. If post–COVID-19 symptoms such as fever, dyspnea, fatigue, or lightheadedness are new or worsening, particularly rapidly, the safest and best option I advise patients is to go to the ED for further assessment and testing. Postviral bacterial pneumonia is something we should consider, and there is some potential for aspergillosis as well.
Q: Do you have any concerns about patients with asthma, chronic obstructive pulmonary disease, or other pulmonary issues having lingering symptoms that may mask exacerbations or may cause exacerbation of their disease?
Dr. Gupta: So far, patients with chronic lung conditions do not appear to have not been disproportionately affected by the pandemic in terms of absolute numbers or percentage wise compared to the general public. I think that sheltering in place has been readily followed by many of these patients, and in addition, I assume better adherence to their maintenance therapies has likely helped. The very few cases of patients with underlying chronic obstructive pulmonary disease and interstitial lung disease that I have seen have fared very poorly when they were diagnosed with COVID-19 in the hospital. There are emerging data about short-term outcomes from severe COVID-19 infection in patients with interstitial lung disease in Europe (medRxiv. 2020 Jul 17. doi: 10.1101/2020.07.15.20152967), and from physicians treating pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension (Ann Am Thorac Soc. 2020 Jul 29. doi: 10.1513/AnnalsATS.202005-521OC). But so far, little has been published on the outcomes of mild disease in these patients with chronic lung disease.
Q: It’s still early days to know the significance of lingering symptoms. But at what point do you begin to consider the possibility of some kind of relapse? And what is your next move if the symptoms get worse?
Dr. Gupta: COVID-19 recurrence, whether because of reinfection or relapse, is a potential concern but not one that is very commonly seen so far in my purview. Generally, symptoms of post–COVID-19 infection that are lingering trend toward getting better, even if slowly. If post–COVID-19 infection symptoms are progressing (particularly if rapidly), that would be a strong indication to evaluate that patient in the ED (less likely in clinic), reswab them for SARS-CoV-2, and obtain further testing such as blood work and imaging. A significant challenge from a research perspective will be determining if coinfection with another virus is playing a role as we move closer to the fall season.
For some patients, a bout of COVID-19 may not be over after hospital discharge, acute symptoms subside, or a couple of tests for SARS-CoV-2 come back negative. Those who have reached these milestones of conquering the disease may find that their recovery journey has only begun. Debilitating symptoms such as fatigue, headache, and dyspnea may linger for weeks or longer. Patients with persistent symptoms, often referred to as “long haulers” in reference to the duration of their recovery, are looking for answers about their condition and when their COVID-19 illness will finally resolve.
Long-haul patients organize
What started as an accumulation of anecdotal evidence in social media, blogs, and the mainstream press about slow recovery and long-lasting symptoms of COVID-19 is now the focus of clinical trials in the population of recovering patients. Projects such as the COVID Symptom Study, initiated by the Massachusetts General Hospital, Boston; the Harvard School of Public Health, Boston; King’s College London; and Stanford (Calif.) University, are collecting data on symptoms from millions of patients and will eventually contribute to a better understanding of prolonged recovery.
Patients looking for answers have created groups on social media such as Facebook to exchange information about their experiences (e.g., Survivor Corps, COVID-19 Support Group, COVID-19 Recovered Survivors). Recovering patients have created patient-led research organizations (Body Politic COVID-19 Support Group) to explore persistent symptoms and begin to create data for research.
Some data on lingering symptoms
A small study of 143 previously hospitalized, recovering patients in Italy found that 87.4% of the cohort had at least one persistent symptom 2 months or longer after initial onset and at more than a month after discharge. In this sample, only 5% had been intubated. (JAMA 2020 Jul 9. doi: 10.1001/jama.2020.12603).
One study found that even patients who have had relatively mild symptoms and were not hospitalized can have persistent symptoms. The Centers for Disease Control and Prevention conducted a survey of adults who tested positive for the positive reverse transcription–polymerase chain reaction test for SARS-CoV-2 and found that, among the 292 respondents, 35% were still feeling the impact of the disease 2-3 weeks after testing. Fatigue (71%), cough (61%), and headache (61%) were the most commonly reported symptoms. The survey found that delayed recovery was evident in nearly a quarter of 18- to 34-year-olds and in a third of 35- to 49-year-olds who were not sick enough to require hospitalization (MMWR. 2020 Jul 24. doi: 10.15585/mmwr.mm6930e1).
Sachin Gupta, MD, FCCP, ATSF, a pulmonologist and member of the CHEST Physician editorial advisory board, has treated patients with COVID-19 and shared some of his thoughts on the problem of prolonged symptoms of COVID-19.
Q: Should clinicians expect to see COVID-19 patients who have symptoms persisting weeks after they are diagnosed?
Dr. Gupta: I think clinicians, especially in primary care, are already seeing many patients with lingering symptoms, both respiratory and nonrespiratory related, and debility. A few patients here in the San Francisco Bay Area that I have spoken with 4-6 weeks out from their acute illness have complained of persisting, though improving, fatigue and cough. Early studies are confirming this as a topical issue. There may be other long-lasting sequelae of COVID-19 beyond the common mild lingering symptoms. It will also be important to consider (and get more data on) to what degree asymptomatic patients develop some degree of mild inflammatory and subsequent fibrotic changes in organs like the lungs and heart
Q: How does the recovery phase of COVID-19 compare with recovery from severe influenza or ARDS?
Dr. Gupta: Most prior influenza and acute respiratory distress syndrome (ARDS) studies have provided initial follow-up at 3 months and beyond, so technically speaking, it is a little difficult to compare the symptomatology patterns in the JAMA study of 2 months on follow-up. Nevertheless, the key takeaway is that, even though few patients in the study had ARDS requiring intubation (severe disease), many patients with milder disease had significant lingering symptoms (55% with three or more symptoms) at 2 months.
This fits logically with the premise, which we have some limited data on with ARDS (N Engl J Med. 2003;348:683-93. doi: 10.1056/NEJMoa022450) and severe influenza infection survivors (Nature Sci Rep. 2017;7:17275. doi: 10.1038/s41598-017-17497-6) that varying degrees of the inflammation cascade triggered by certain viruses can lead to changes in important patient-reported outcomes, and objective measures such as pulmonary function over the long term.
Q: What can you do for patients with lingering symptoms of COVID-19 or what can you tell them about their symptoms?
Dr. Gupta: For many patients, there is fear, given the novel nature of the virus/pandemic, that their symptoms may persist long term. Acknowledgment of their symptoms is validating and important for us to recognize as we learn more about the virus. As we are finding, many patients are going online to find answers, after sometimes feeling rushed or dismissed initially in the clinical setting.
In my experience, the bar is fairly high for most patients to reach out to their physicians with complaints of lingering symptoms after acute infection. For the ones who do reach out, they tend to have either a greater constellation of symptoms or higher severity of one or two key symptoms. After assessing and, when appropriate, ruling out secondary infections or newly developed conditions, I shift toward symptom management. I encourage such patients to build up slowly. I suggest they work first on their activities of daily living (bathing, grooming), then their instrumental activities of daily living (cooking, cleaning, checking the mail), and then to engage, based on their tolerance of symptoms, to light purposeful exercise. There are many online resources for at-home exercise activities that I recommend to patients who are more debilitated; some larger centers are beginning to offer some forms of telepulmonary rehab.
Based on what we know about other causes of viral pneumonitis and ARDS, I ask such symptomatic patients to expect a slow, gradual, and in most cases a complete recovery, and depending on the individual case, I recommend pulmonary function tests and imaging that may be helpful to track that progress.
I remind myself, and patients, that our understanding may change as we learn more over time. Checking in at set intervals, even if not in person but through a phone call, can go a long way in a setting where we do not have a specific therapy, other than gradual exercise training, to help these patients recover faster. Reassurance and encouragement are vital for patients who are struggling with the lingering burden of disease and who may find it difficult to return to work or function as usual at home. The final point is to be mindful of our patient’s mental health and, where our reassurance is not enough, to consider appropriate mental health referrals.
Q: Can you handle this kind of problem with telemedicine or which patients with lingering symptoms need to come into the office – or failing that, the ED?
Dr. Gupta: Telemedicine in the outpatient setting provides a helpful tool to assess and manage patients, in my experience, with limited and straightforward complaints. Its scope is limited diagnostically (assessing symptoms and signs) as is its reach (ability to connect with elderly, disabled, or patients without/limited telemedicine access). In many instances, telemedicine limits our ability to connect with patients emotionally and build trust. Many patients who have gone through the acute illness that we see in pulmonary clinic on follow-up are older in age, and for many, video visits are not a practical solution. Telemedicine visits can sometimes present challenges for me as well in terms of thoroughly conveying lifestyle and symptom management strategies. Health literacy is typically easier to gauge and address in person.
For patients with any degree of enduring dyspnea, more so in the acute phase, I recommend home pulse oximetry for monitoring their oxygen saturation if it is financially and technically feasible for them to obtain one. Sending a patient to the ED is an option of last resort, but one that is necessary in some cases. I expect patients with lingering symptoms to tell me that symptoms may be persisting, hopefully gradually improving, and not getting worse. If post–COVID-19 symptoms such as fever, dyspnea, fatigue, or lightheadedness are new or worsening, particularly rapidly, the safest and best option I advise patients is to go to the ED for further assessment and testing. Postviral bacterial pneumonia is something we should consider, and there is some potential for aspergillosis as well.
Q: Do you have any concerns about patients with asthma, chronic obstructive pulmonary disease, or other pulmonary issues having lingering symptoms that may mask exacerbations or may cause exacerbation of their disease?
Dr. Gupta: So far, patients with chronic lung conditions do not appear to have not been disproportionately affected by the pandemic in terms of absolute numbers or percentage wise compared to the general public. I think that sheltering in place has been readily followed by many of these patients, and in addition, I assume better adherence to their maintenance therapies has likely helped. The very few cases of patients with underlying chronic obstructive pulmonary disease and interstitial lung disease that I have seen have fared very poorly when they were diagnosed with COVID-19 in the hospital. There are emerging data about short-term outcomes from severe COVID-19 infection in patients with interstitial lung disease in Europe (medRxiv. 2020 Jul 17. doi: 10.1101/2020.07.15.20152967), and from physicians treating pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension (Ann Am Thorac Soc. 2020 Jul 29. doi: 10.1513/AnnalsATS.202005-521OC). But so far, little has been published on the outcomes of mild disease in these patients with chronic lung disease.
Q: It’s still early days to know the significance of lingering symptoms. But at what point do you begin to consider the possibility of some kind of relapse? And what is your next move if the symptoms get worse?
Dr. Gupta: COVID-19 recurrence, whether because of reinfection or relapse, is a potential concern but not one that is very commonly seen so far in my purview. Generally, symptoms of post–COVID-19 infection that are lingering trend toward getting better, even if slowly. If post–COVID-19 infection symptoms are progressing (particularly if rapidly), that would be a strong indication to evaluate that patient in the ED (less likely in clinic), reswab them for SARS-CoV-2, and obtain further testing such as blood work and imaging. A significant challenge from a research perspective will be determining if coinfection with another virus is playing a role as we move closer to the fall season.