User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
No benefit of anti-inflammatory strategy in acute myocarditis
AMSTERDAM – A short course of the interleukin-1 receptor antagonist, anakinra, appeared safe but did not reduce complications of acute myocarditis in the ARAMIS trial.
The trial was presented at the annual congress of the European Society of Cardiology.
Lead investigator, Mathieu Kerneis, MD, Pitie Salpetriere APHP University Hospital, Paris, said this was the largest randomized controlled trial of patients with acute myocarditis and probably the first ever study in the acute setting of myocarditis patients diagnosed with cardiac magnetic resonance (CMR) imaging, not on biopsy, who are mostly at low risk for events.
He suggested that one of the reasons for the neutral result could have been the low-risk population involved and the low complication rate. “We enrolled an all-comer acute myocarditis population diagnosed with CMR, who were mostly at a low risk of complications,” he noted.
“I don’t think the story of anti-inflammatory drugs in acute myocarditis is over. This is just the beginning. This was the first trial, and it was just a phase 2 trial. We need further randomized trials to explore the potential benefit of an anti-inflammatory strategy in acute myocarditis patients at higher risk of complications. In addition, larger studies are needed to evaluate prolonged anti-inflammatory strategies in acute myocarditis patients at low-to-moderate risk of complications,” Dr. Kerneis concluded.
“It is very challenging to do a trial in high-risk patients with myocarditis as these patients are quite rare,” he added.
Inflammation of the myocardium
Dr. Kerneis explained that acute myocarditis is an inflammation of the myocardium that can cause permanent damage to the heart muscle and lead to myocardial infarction, stroke, heart failure, arrhythmias, and death. The condition can occur in individuals of all ages but is most frequent in young people. There is no specific treatment, but patients are generally treated with beta-blockers, angiotensin-converting enzyme (ACE) inhibitors, and sometimes steroids.
Anakinra is an interleukin-1 receptor antagonist that works by targeting the interleukin-1β innate immune pathway. Anakinra is used for the treatment of rheumatoid arthritis and has shown efficacy in pericarditis. Dr. Kerneis noted that there have been several case reports of successful treatment with anakinra in acute myocarditis.
The ARAMIS trial – conducted at six academic centers in France – was the first randomized study to evaluate inhibition of the interleukin-1β innate immune pathway in myocarditis patients. The trial enrolled 120 hospitalized, symptomatic patients with chest pain, increased cardiac troponin, and acute myocarditis diagnosed using CMR. More than half had had a recent bacterial or viral infection.
Patients were randomized within 72 hours of hospital admission to a daily subcutaneous dose of anakinra 100 mg or placebo until hospital discharge. Patients in both groups received standard-of-care treatments, including an ACE inhibitor, for at least 1 month. Consistent with prior data, the median age of participants was 28 years and 90% were men.
The primary endpoint was the number of days free of myocarditis complications (heart failure requiring hospitalization, chest pain requiring medication, left ventricular ejection fraction less than 50%, and ventricular arrhythmias) within 28 days postdischarge.
There was no significant difference in this endpoint between the two arms, with a median of 30 days for anakinra versus 31 days for placebo.
Overall, the rate of the composite endpoint of myocarditis complications occurred in 13.7% of patients, and there was a numerical reduction in the number of patients with these myocarditis complications with anakinra – 6 patients (10.5%) in the anakinra group versus 10 patients (16.5%) in the placebo group (odds ratio, 0.59; 95% confidence interval, 0.19-1.78). This was driven by fewer patients with chest pain requiring new medication (two patients versus six patients).
The safety endpoint was the number of serious adverse events within 28 days postdischarge. This endpoint occurred in seven patients (12.1%) in the anakinra arm and six patients (10.2%) in the placebo arm, with no significant difference between groups. Cases of severe infection within 28 days postdischarge were reported in both arms.
Low-risk population
Designated discussant of the study at the ESC Hotline session, Enrico Ammirati, MD, PhD, University of Milano-Bicocca, Monza, Italy, said that patients involved in ARAMIS fit the profile of acute myocarditis and that the CMR diagnosis was positive in all the patients enrolled.
Dr. Ammirati agreed with Dr. Kerneis that the neutral results of the study were probably caused by the low-risk population. “If we look at retrospective registries, at 30 days there are zero cardiac deaths or heart transplants at 30 days in patients with a low-risk presentation.
“The ARAMIS trial has shown the feasibility of conducting studies in the setting of acute myocarditis, and even if the primary endpoint was neutral, some important data are still missing, such as change in ejection fraction and troponin levels,” he noted.
“In terms of future perspective, we are moving to assessing efficacy of anakinra or other immunosuppressive drugs from acute low risk patients to higher risk patients with heart failure and severe dysfunction,” he said.
Dr. Ammirati is the lead investigator of another ongoing study in such a higher-risk population; the MYTHS trial is investigating the use of intravenous steroids in patients with suspected acute myocarditis complicated by acute heart failure or cardiogenic shock, and an ejection fraction below 41%.
“So, we will have more results on the best treatment in this higher risk group of patients,” he concluded.
The ARAMIS trial was an academic study funded by the French Health Ministry and coordinated by the ACTION Group. Dr. Kerneis reports having received consulting fees from Kiniksa, Sanofi, and Bayer, and holds a patent for use of abatacept in immune checkpoint inhibitor (ICI)–induced myocarditis.
A version of this article first appeared on Medscape.com.
AMSTERDAM – A short course of the interleukin-1 receptor antagonist, anakinra, appeared safe but did not reduce complications of acute myocarditis in the ARAMIS trial.
The trial was presented at the annual congress of the European Society of Cardiology.
Lead investigator, Mathieu Kerneis, MD, Pitie Salpetriere APHP University Hospital, Paris, said this was the largest randomized controlled trial of patients with acute myocarditis and probably the first ever study in the acute setting of myocarditis patients diagnosed with cardiac magnetic resonance (CMR) imaging, not on biopsy, who are mostly at low risk for events.
He suggested that one of the reasons for the neutral result could have been the low-risk population involved and the low complication rate. “We enrolled an all-comer acute myocarditis population diagnosed with CMR, who were mostly at a low risk of complications,” he noted.
“I don’t think the story of anti-inflammatory drugs in acute myocarditis is over. This is just the beginning. This was the first trial, and it was just a phase 2 trial. We need further randomized trials to explore the potential benefit of an anti-inflammatory strategy in acute myocarditis patients at higher risk of complications. In addition, larger studies are needed to evaluate prolonged anti-inflammatory strategies in acute myocarditis patients at low-to-moderate risk of complications,” Dr. Kerneis concluded.
“It is very challenging to do a trial in high-risk patients with myocarditis as these patients are quite rare,” he added.
Inflammation of the myocardium
Dr. Kerneis explained that acute myocarditis is an inflammation of the myocardium that can cause permanent damage to the heart muscle and lead to myocardial infarction, stroke, heart failure, arrhythmias, and death. The condition can occur in individuals of all ages but is most frequent in young people. There is no specific treatment, but patients are generally treated with beta-blockers, angiotensin-converting enzyme (ACE) inhibitors, and sometimes steroids.
Anakinra is an interleukin-1 receptor antagonist that works by targeting the interleukin-1β innate immune pathway. Anakinra is used for the treatment of rheumatoid arthritis and has shown efficacy in pericarditis. Dr. Kerneis noted that there have been several case reports of successful treatment with anakinra in acute myocarditis.
The ARAMIS trial – conducted at six academic centers in France – was the first randomized study to evaluate inhibition of the interleukin-1β innate immune pathway in myocarditis patients. The trial enrolled 120 hospitalized, symptomatic patients with chest pain, increased cardiac troponin, and acute myocarditis diagnosed using CMR. More than half had had a recent bacterial or viral infection.
Patients were randomized within 72 hours of hospital admission to a daily subcutaneous dose of anakinra 100 mg or placebo until hospital discharge. Patients in both groups received standard-of-care treatments, including an ACE inhibitor, for at least 1 month. Consistent with prior data, the median age of participants was 28 years and 90% were men.
The primary endpoint was the number of days free of myocarditis complications (heart failure requiring hospitalization, chest pain requiring medication, left ventricular ejection fraction less than 50%, and ventricular arrhythmias) within 28 days postdischarge.
There was no significant difference in this endpoint between the two arms, with a median of 30 days for anakinra versus 31 days for placebo.
Overall, the rate of the composite endpoint of myocarditis complications occurred in 13.7% of patients, and there was a numerical reduction in the number of patients with these myocarditis complications with anakinra – 6 patients (10.5%) in the anakinra group versus 10 patients (16.5%) in the placebo group (odds ratio, 0.59; 95% confidence interval, 0.19-1.78). This was driven by fewer patients with chest pain requiring new medication (two patients versus six patients).
The safety endpoint was the number of serious adverse events within 28 days postdischarge. This endpoint occurred in seven patients (12.1%) in the anakinra arm and six patients (10.2%) in the placebo arm, with no significant difference between groups. Cases of severe infection within 28 days postdischarge were reported in both arms.
Low-risk population
Designated discussant of the study at the ESC Hotline session, Enrico Ammirati, MD, PhD, University of Milano-Bicocca, Monza, Italy, said that patients involved in ARAMIS fit the profile of acute myocarditis and that the CMR diagnosis was positive in all the patients enrolled.
Dr. Ammirati agreed with Dr. Kerneis that the neutral results of the study were probably caused by the low-risk population. “If we look at retrospective registries, at 30 days there are zero cardiac deaths or heart transplants at 30 days in patients with a low-risk presentation.
“The ARAMIS trial has shown the feasibility of conducting studies in the setting of acute myocarditis, and even if the primary endpoint was neutral, some important data are still missing, such as change in ejection fraction and troponin levels,” he noted.
“In terms of future perspective, we are moving to assessing efficacy of anakinra or other immunosuppressive drugs from acute low risk patients to higher risk patients with heart failure and severe dysfunction,” he said.
Dr. Ammirati is the lead investigator of another ongoing study in such a higher-risk population; the MYTHS trial is investigating the use of intravenous steroids in patients with suspected acute myocarditis complicated by acute heart failure or cardiogenic shock, and an ejection fraction below 41%.
“So, we will have more results on the best treatment in this higher risk group of patients,” he concluded.
The ARAMIS trial was an academic study funded by the French Health Ministry and coordinated by the ACTION Group. Dr. Kerneis reports having received consulting fees from Kiniksa, Sanofi, and Bayer, and holds a patent for use of abatacept in immune checkpoint inhibitor (ICI)–induced myocarditis.
A version of this article first appeared on Medscape.com.
AMSTERDAM – A short course of the interleukin-1 receptor antagonist, anakinra, appeared safe but did not reduce complications of acute myocarditis in the ARAMIS trial.
The trial was presented at the annual congress of the European Society of Cardiology.
Lead investigator, Mathieu Kerneis, MD, Pitie Salpetriere APHP University Hospital, Paris, said this was the largest randomized controlled trial of patients with acute myocarditis and probably the first ever study in the acute setting of myocarditis patients diagnosed with cardiac magnetic resonance (CMR) imaging, not on biopsy, who are mostly at low risk for events.
He suggested that one of the reasons for the neutral result could have been the low-risk population involved and the low complication rate. “We enrolled an all-comer acute myocarditis population diagnosed with CMR, who were mostly at a low risk of complications,” he noted.
“I don’t think the story of anti-inflammatory drugs in acute myocarditis is over. This is just the beginning. This was the first trial, and it was just a phase 2 trial. We need further randomized trials to explore the potential benefit of an anti-inflammatory strategy in acute myocarditis patients at higher risk of complications. In addition, larger studies are needed to evaluate prolonged anti-inflammatory strategies in acute myocarditis patients at low-to-moderate risk of complications,” Dr. Kerneis concluded.
“It is very challenging to do a trial in high-risk patients with myocarditis as these patients are quite rare,” he added.
Inflammation of the myocardium
Dr. Kerneis explained that acute myocarditis is an inflammation of the myocardium that can cause permanent damage to the heart muscle and lead to myocardial infarction, stroke, heart failure, arrhythmias, and death. The condition can occur in individuals of all ages but is most frequent in young people. There is no specific treatment, but patients are generally treated with beta-blockers, angiotensin-converting enzyme (ACE) inhibitors, and sometimes steroids.
Anakinra is an interleukin-1 receptor antagonist that works by targeting the interleukin-1β innate immune pathway. Anakinra is used for the treatment of rheumatoid arthritis and has shown efficacy in pericarditis. Dr. Kerneis noted that there have been several case reports of successful treatment with anakinra in acute myocarditis.
The ARAMIS trial – conducted at six academic centers in France – was the first randomized study to evaluate inhibition of the interleukin-1β innate immune pathway in myocarditis patients. The trial enrolled 120 hospitalized, symptomatic patients with chest pain, increased cardiac troponin, and acute myocarditis diagnosed using CMR. More than half had had a recent bacterial or viral infection.
Patients were randomized within 72 hours of hospital admission to a daily subcutaneous dose of anakinra 100 mg or placebo until hospital discharge. Patients in both groups received standard-of-care treatments, including an ACE inhibitor, for at least 1 month. Consistent with prior data, the median age of participants was 28 years and 90% were men.
The primary endpoint was the number of days free of myocarditis complications (heart failure requiring hospitalization, chest pain requiring medication, left ventricular ejection fraction less than 50%, and ventricular arrhythmias) within 28 days postdischarge.
There was no significant difference in this endpoint between the two arms, with a median of 30 days for anakinra versus 31 days for placebo.
Overall, the rate of the composite endpoint of myocarditis complications occurred in 13.7% of patients, and there was a numerical reduction in the number of patients with these myocarditis complications with anakinra – 6 patients (10.5%) in the anakinra group versus 10 patients (16.5%) in the placebo group (odds ratio, 0.59; 95% confidence interval, 0.19-1.78). This was driven by fewer patients with chest pain requiring new medication (two patients versus six patients).
The safety endpoint was the number of serious adverse events within 28 days postdischarge. This endpoint occurred in seven patients (12.1%) in the anakinra arm and six patients (10.2%) in the placebo arm, with no significant difference between groups. Cases of severe infection within 28 days postdischarge were reported in both arms.
Low-risk population
Designated discussant of the study at the ESC Hotline session, Enrico Ammirati, MD, PhD, University of Milano-Bicocca, Monza, Italy, said that patients involved in ARAMIS fit the profile of acute myocarditis and that the CMR diagnosis was positive in all the patients enrolled.
Dr. Ammirati agreed with Dr. Kerneis that the neutral results of the study were probably caused by the low-risk population. “If we look at retrospective registries, at 30 days there are zero cardiac deaths or heart transplants at 30 days in patients with a low-risk presentation.
“The ARAMIS trial has shown the feasibility of conducting studies in the setting of acute myocarditis, and even if the primary endpoint was neutral, some important data are still missing, such as change in ejection fraction and troponin levels,” he noted.
“In terms of future perspective, we are moving to assessing efficacy of anakinra or other immunosuppressive drugs from acute low risk patients to higher risk patients with heart failure and severe dysfunction,” he said.
Dr. Ammirati is the lead investigator of another ongoing study in such a higher-risk population; the MYTHS trial is investigating the use of intravenous steroids in patients with suspected acute myocarditis complicated by acute heart failure or cardiogenic shock, and an ejection fraction below 41%.
“So, we will have more results on the best treatment in this higher risk group of patients,” he concluded.
The ARAMIS trial was an academic study funded by the French Health Ministry and coordinated by the ACTION Group. Dr. Kerneis reports having received consulting fees from Kiniksa, Sanofi, and Bayer, and holds a patent for use of abatacept in immune checkpoint inhibitor (ICI)–induced myocarditis.
A version of this article first appeared on Medscape.com.
AT ESC CONGRESS 2023
The new normal in body temperature
This transcript has been edited for clarity.
Every branch of science has its constants. Physics has the speed of light, the gravitational constant, the Planck constant. Chemistry gives us Avogadro’s number, Faraday’s constant, the charge of an electron. Medicine isn’t quite as reliable as physics when it comes to these things, but insofar as there are any constants in medicine, might I suggest normal body temperature: 37° Celsius, 98.6° Fahrenheit.
Sure, serum sodium may be less variable and lactate concentration more clinically relevant, but even my 7-year-old knows that normal body temperature is 98.6°.
Except, as it turns out, 98.6° isn’t normal at all.
How did we arrive at 37.0° C for normal body temperature? We got it from this guy – German physician Carl Reinhold August Wunderlich, who, in addition to looking eerily like Luciano Pavarotti, was the first to realize that fever was not itself a disease but a symptom of one.
In 1851, Dr. Wunderlich released his measurements of more than 1 million body temperatures taken from 25,000 Germans – a painstaking process at the time, which employed a foot-long thermometer and took 20 minutes to obtain a measurement.
The average temperature measured, of course, was 37° C.
We’re more than 150 years post-Wunderlich right now, and the average person in the United States might be quite a bit different from the average German in 1850. Moreover, we can do a lot better than just measuring a ton of people and taking the average, because we have statistics. The problem with measuring a bunch of people and taking the average temperature as normal is that you can’t be sure that the people you are measuring are normal. There are obvious causes of elevated temperature that you could exclude. Let’s not take people with a respiratory infection or who are taking Tylenol, for example. But as highlighted in this paper in JAMA Internal Medicine, we can do a lot better than that.
The study leverages the fact that body temperature is typically measured during all medical office visits and recorded in the ever-present electronic medical record.
Researchers from Stanford identified 724,199 patient encounters with outpatient temperature data. They excluded extreme temperatures – less than 34° C or greater than 40° C – excluded patients under 20 or above 80 years, and excluded those with extremes of height, weight, or body mass index.
You end up with a distribution like this. Note that the peak is clearly lower than 37° C.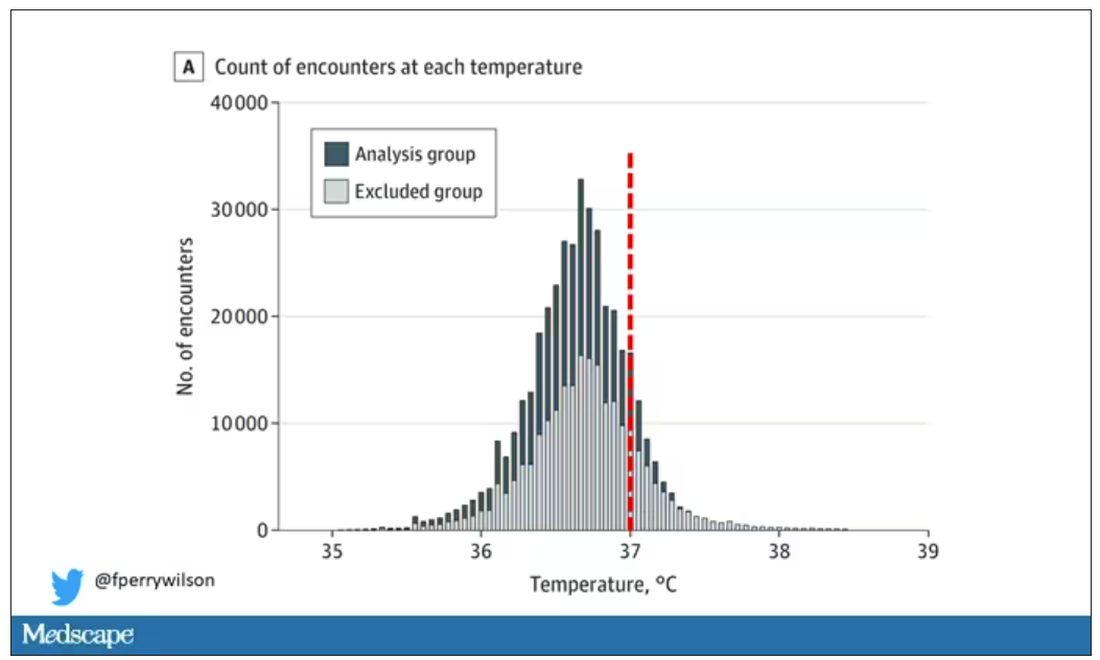
But we’re still not at “normal.” Some people would be seeing their doctor for conditions that affect body temperature, such as infection. You could use diagnosis codes to flag these individuals and drop them, but that feels a bit arbitrary.
I really love how the researchers used data to fix this problem. They used a technique called LIMIT (Laboratory Information Mining for Individualized Thresholds). It works like this:
Take all the temperature measurements and then identify the outliers – the very tails of the distribution.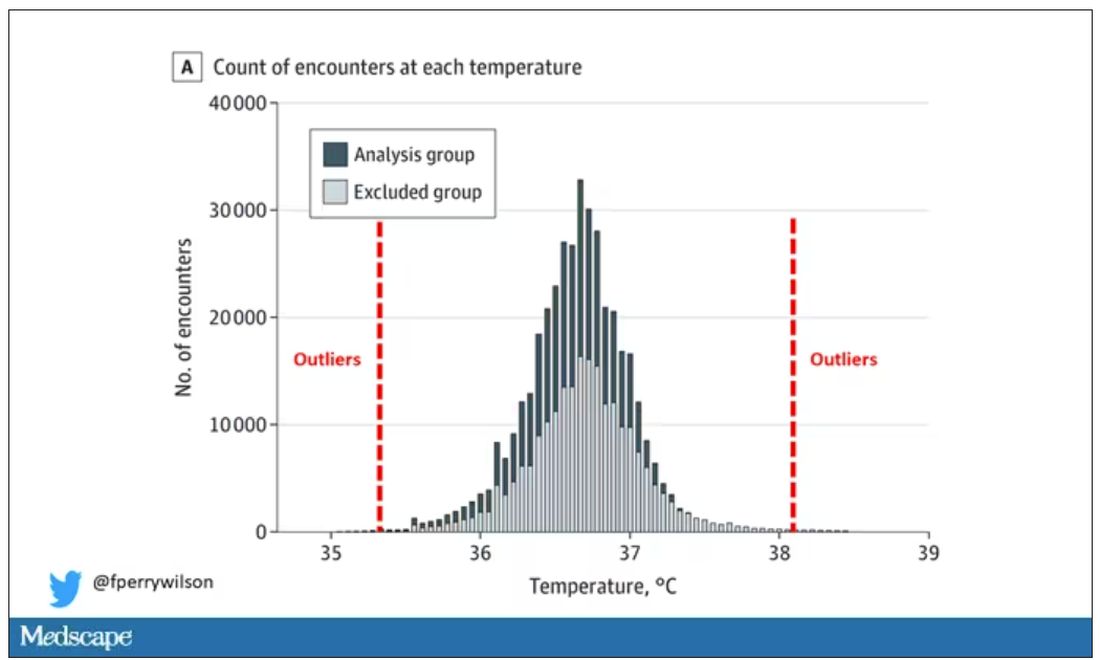
Look at all the diagnosis codes in those distributions. Determine which diagnosis codes are overrepresented in those distributions. Now you have a data-driven way to say that yes, these diagnoses are associated with weird temperatures. Next, eliminate everyone with those diagnoses from the dataset. What you are left with is a normal population, or at least a population that doesn’t have a condition that seems to meaningfully affect temperature.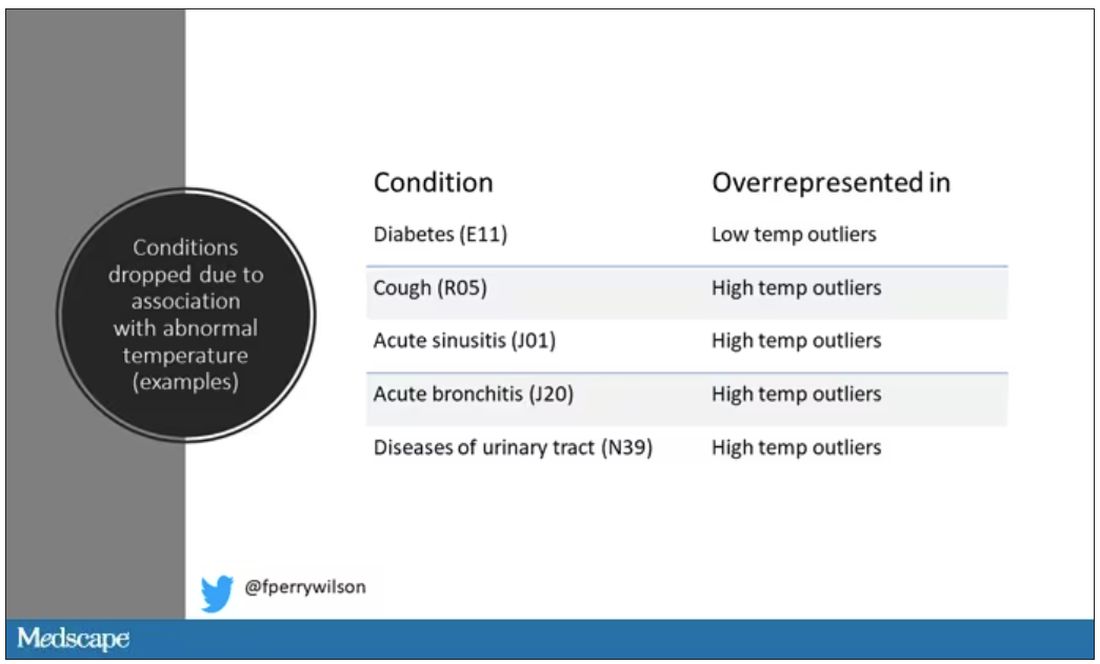
So, who was dropped? Well, a lot of people, actually. It turned out that diabetes was way overrepresented in the outlier group. Although 9.2% of the population had diabetes, 26% of people with very low temperatures did, so everyone with diabetes is removed from the dataset. While 5% of the population had a cough at their encounter, 7% of the people with very high temperature and 7% of the people with very low temperature had a cough, so everyone with cough gets thrown out.
The algorithm excluded people on antibiotics or who had sinusitis, urinary tract infections, pneumonia, and, yes, a diagnosis of “fever.” The list makes sense, which is always nice when you have a purely algorithmic classification system.
What do we have left? What is the real normal temperature? Ready?
It’s 36.64° C, or about 98.0° F.
Of course, normal temperature varied depending on the time of day it was measured – higher in the afternoon.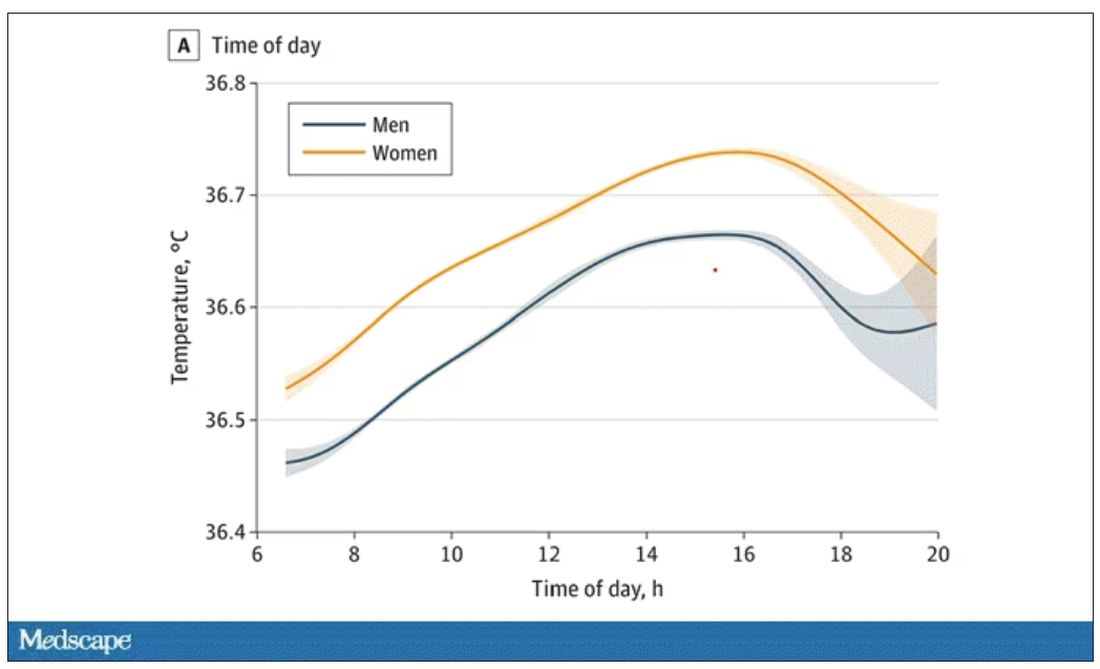
The normal temperature in women tended to be higher than in men. The normal temperature declined with age as well.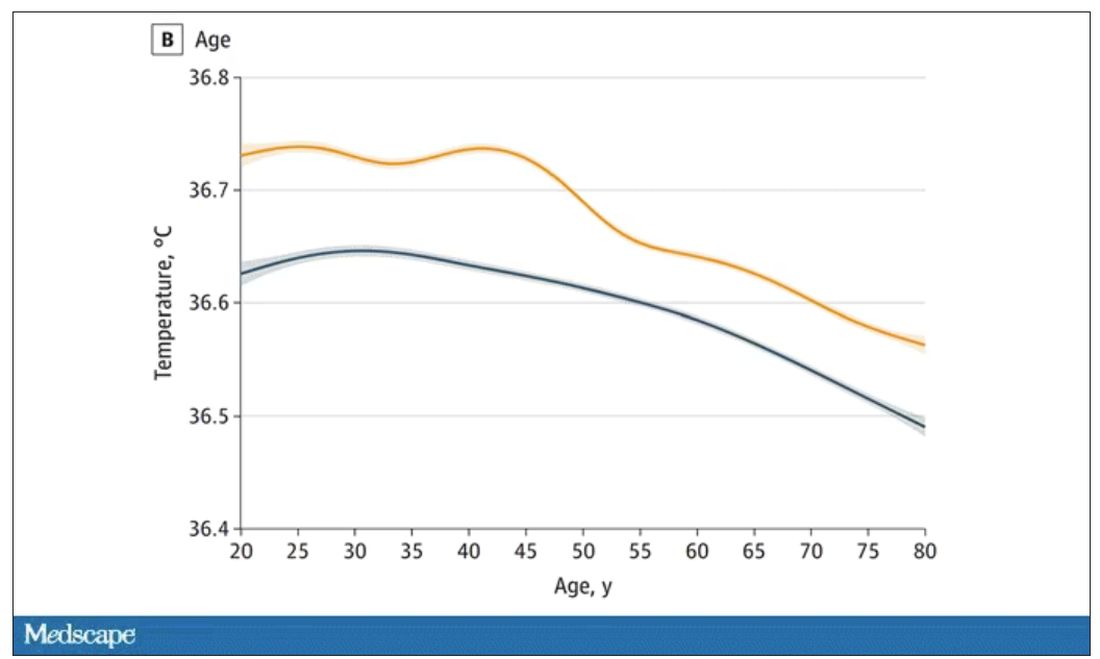
In fact, the researchers built a nice online calculator where you can enter your own, or your patient’s, parameters and calculate a normal body temperature for them. Here’s mine. My normal temperature at around 2 p.m. should be 36.7° C.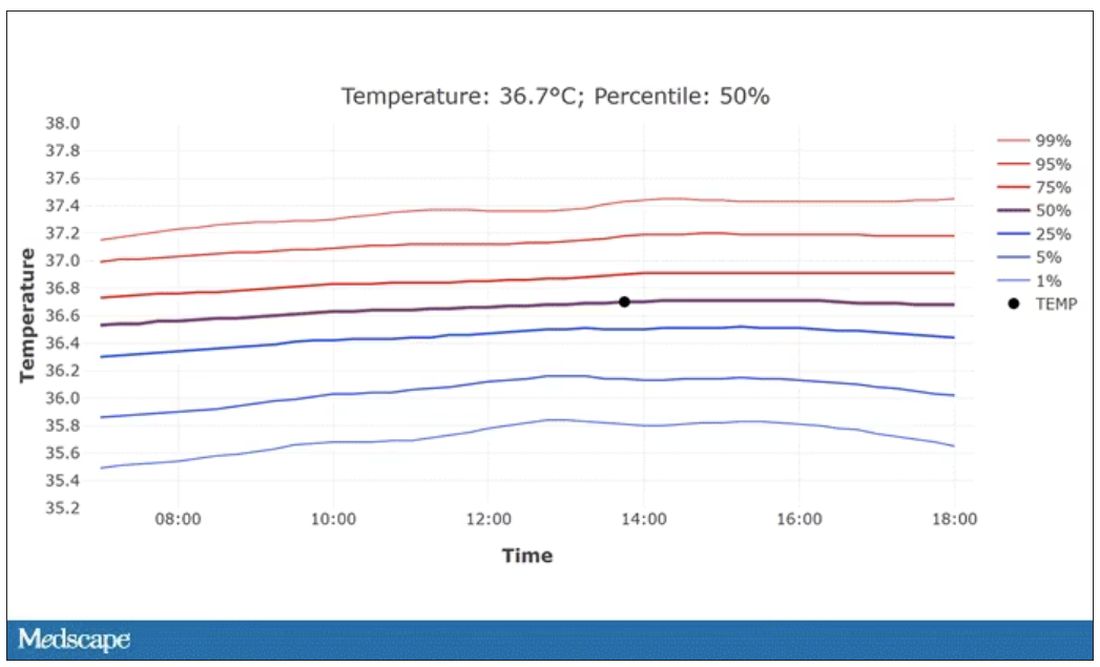
So, we’re all more cold-blooded than we thought. Is this just because of better methods? Maybe. But studies have actually shown that body temperature may be decreasing over time in humans, possibly because of the lower levels of inflammation we face in modern life (thanks to improvements in hygiene and antibiotics).
Of course, I’m sure some of you are asking yourselves whether any of this really matters. Is 37° C close enough?
Sure, this may be sort of puttering around the edges of physical diagnosis, but I think the methodology is really interesting and can obviously be applied to other broadly collected data points. But these data show us that thin, older individuals really do run cooler, and that we may need to pay more attention to a low-grade fever in that population than we otherwise would.
In any case, it’s time for a little re-education. If someone asks you what normal body temperature is, just say 36.6° C, 98.0° F. For his work in this area, I suggest we call it Wunderlich’s constant.
Dr. Wilson is associate professor of medicine and public health at Yale University, New Haven, Conn., and director of Yale’s Clinical and Translational Research Accelerator. He has no disclosures.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Every branch of science has its constants. Physics has the speed of light, the gravitational constant, the Planck constant. Chemistry gives us Avogadro’s number, Faraday’s constant, the charge of an electron. Medicine isn’t quite as reliable as physics when it comes to these things, but insofar as there are any constants in medicine, might I suggest normal body temperature: 37° Celsius, 98.6° Fahrenheit.
Sure, serum sodium may be less variable and lactate concentration more clinically relevant, but even my 7-year-old knows that normal body temperature is 98.6°.
Except, as it turns out, 98.6° isn’t normal at all.
How did we arrive at 37.0° C for normal body temperature? We got it from this guy – German physician Carl Reinhold August Wunderlich, who, in addition to looking eerily like Luciano Pavarotti, was the first to realize that fever was not itself a disease but a symptom of one.
In 1851, Dr. Wunderlich released his measurements of more than 1 million body temperatures taken from 25,000 Germans – a painstaking process at the time, which employed a foot-long thermometer and took 20 minutes to obtain a measurement.
The average temperature measured, of course, was 37° C.
We’re more than 150 years post-Wunderlich right now, and the average person in the United States might be quite a bit different from the average German in 1850. Moreover, we can do a lot better than just measuring a ton of people and taking the average, because we have statistics. The problem with measuring a bunch of people and taking the average temperature as normal is that you can’t be sure that the people you are measuring are normal. There are obvious causes of elevated temperature that you could exclude. Let’s not take people with a respiratory infection or who are taking Tylenol, for example. But as highlighted in this paper in JAMA Internal Medicine, we can do a lot better than that.
The study leverages the fact that body temperature is typically measured during all medical office visits and recorded in the ever-present electronic medical record.
Researchers from Stanford identified 724,199 patient encounters with outpatient temperature data. They excluded extreme temperatures – less than 34° C or greater than 40° C – excluded patients under 20 or above 80 years, and excluded those with extremes of height, weight, or body mass index.
You end up with a distribution like this. Note that the peak is clearly lower than 37° C.
But we’re still not at “normal.” Some people would be seeing their doctor for conditions that affect body temperature, such as infection. You could use diagnosis codes to flag these individuals and drop them, but that feels a bit arbitrary.
I really love how the researchers used data to fix this problem. They used a technique called LIMIT (Laboratory Information Mining for Individualized Thresholds). It works like this:
Take all the temperature measurements and then identify the outliers – the very tails of the distribution.
Look at all the diagnosis codes in those distributions. Determine which diagnosis codes are overrepresented in those distributions. Now you have a data-driven way to say that yes, these diagnoses are associated with weird temperatures. Next, eliminate everyone with those diagnoses from the dataset. What you are left with is a normal population, or at least a population that doesn’t have a condition that seems to meaningfully affect temperature.
So, who was dropped? Well, a lot of people, actually. It turned out that diabetes was way overrepresented in the outlier group. Although 9.2% of the population had diabetes, 26% of people with very low temperatures did, so everyone with diabetes is removed from the dataset. While 5% of the population had a cough at their encounter, 7% of the people with very high temperature and 7% of the people with very low temperature had a cough, so everyone with cough gets thrown out.
The algorithm excluded people on antibiotics or who had sinusitis, urinary tract infections, pneumonia, and, yes, a diagnosis of “fever.” The list makes sense, which is always nice when you have a purely algorithmic classification system.
What do we have left? What is the real normal temperature? Ready?
It’s 36.64° C, or about 98.0° F.
Of course, normal temperature varied depending on the time of day it was measured – higher in the afternoon.
The normal temperature in women tended to be higher than in men. The normal temperature declined with age as well.
In fact, the researchers built a nice online calculator where you can enter your own, or your patient’s, parameters and calculate a normal body temperature for them. Here’s mine. My normal temperature at around 2 p.m. should be 36.7° C.
So, we’re all more cold-blooded than we thought. Is this just because of better methods? Maybe. But studies have actually shown that body temperature may be decreasing over time in humans, possibly because of the lower levels of inflammation we face in modern life (thanks to improvements in hygiene and antibiotics).
Of course, I’m sure some of you are asking yourselves whether any of this really matters. Is 37° C close enough?
Sure, this may be sort of puttering around the edges of physical diagnosis, but I think the methodology is really interesting and can obviously be applied to other broadly collected data points. But these data show us that thin, older individuals really do run cooler, and that we may need to pay more attention to a low-grade fever in that population than we otherwise would.
In any case, it’s time for a little re-education. If someone asks you what normal body temperature is, just say 36.6° C, 98.0° F. For his work in this area, I suggest we call it Wunderlich’s constant.
Dr. Wilson is associate professor of medicine and public health at Yale University, New Haven, Conn., and director of Yale’s Clinical and Translational Research Accelerator. He has no disclosures.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Every branch of science has its constants. Physics has the speed of light, the gravitational constant, the Planck constant. Chemistry gives us Avogadro’s number, Faraday’s constant, the charge of an electron. Medicine isn’t quite as reliable as physics when it comes to these things, but insofar as there are any constants in medicine, might I suggest normal body temperature: 37° Celsius, 98.6° Fahrenheit.
Sure, serum sodium may be less variable and lactate concentration more clinically relevant, but even my 7-year-old knows that normal body temperature is 98.6°.
Except, as it turns out, 98.6° isn’t normal at all.
How did we arrive at 37.0° C for normal body temperature? We got it from this guy – German physician Carl Reinhold August Wunderlich, who, in addition to looking eerily like Luciano Pavarotti, was the first to realize that fever was not itself a disease but a symptom of one.
In 1851, Dr. Wunderlich released his measurements of more than 1 million body temperatures taken from 25,000 Germans – a painstaking process at the time, which employed a foot-long thermometer and took 20 minutes to obtain a measurement.
The average temperature measured, of course, was 37° C.
We’re more than 150 years post-Wunderlich right now, and the average person in the United States might be quite a bit different from the average German in 1850. Moreover, we can do a lot better than just measuring a ton of people and taking the average, because we have statistics. The problem with measuring a bunch of people and taking the average temperature as normal is that you can’t be sure that the people you are measuring are normal. There are obvious causes of elevated temperature that you could exclude. Let’s not take people with a respiratory infection or who are taking Tylenol, for example. But as highlighted in this paper in JAMA Internal Medicine, we can do a lot better than that.
The study leverages the fact that body temperature is typically measured during all medical office visits and recorded in the ever-present electronic medical record.
Researchers from Stanford identified 724,199 patient encounters with outpatient temperature data. They excluded extreme temperatures – less than 34° C or greater than 40° C – excluded patients under 20 or above 80 years, and excluded those with extremes of height, weight, or body mass index.
You end up with a distribution like this. Note that the peak is clearly lower than 37° C.
But we’re still not at “normal.” Some people would be seeing their doctor for conditions that affect body temperature, such as infection. You could use diagnosis codes to flag these individuals and drop them, but that feels a bit arbitrary.
I really love how the researchers used data to fix this problem. They used a technique called LIMIT (Laboratory Information Mining for Individualized Thresholds). It works like this:
Take all the temperature measurements and then identify the outliers – the very tails of the distribution.
Look at all the diagnosis codes in those distributions. Determine which diagnosis codes are overrepresented in those distributions. Now you have a data-driven way to say that yes, these diagnoses are associated with weird temperatures. Next, eliminate everyone with those diagnoses from the dataset. What you are left with is a normal population, or at least a population that doesn’t have a condition that seems to meaningfully affect temperature.
So, who was dropped? Well, a lot of people, actually. It turned out that diabetes was way overrepresented in the outlier group. Although 9.2% of the population had diabetes, 26% of people with very low temperatures did, so everyone with diabetes is removed from the dataset. While 5% of the population had a cough at their encounter, 7% of the people with very high temperature and 7% of the people with very low temperature had a cough, so everyone with cough gets thrown out.
The algorithm excluded people on antibiotics or who had sinusitis, urinary tract infections, pneumonia, and, yes, a diagnosis of “fever.” The list makes sense, which is always nice when you have a purely algorithmic classification system.
What do we have left? What is the real normal temperature? Ready?
It’s 36.64° C, or about 98.0° F.
Of course, normal temperature varied depending on the time of day it was measured – higher in the afternoon.
The normal temperature in women tended to be higher than in men. The normal temperature declined with age as well.
In fact, the researchers built a nice online calculator where you can enter your own, or your patient’s, parameters and calculate a normal body temperature for them. Here’s mine. My normal temperature at around 2 p.m. should be 36.7° C.
So, we’re all more cold-blooded than we thought. Is this just because of better methods? Maybe. But studies have actually shown that body temperature may be decreasing over time in humans, possibly because of the lower levels of inflammation we face in modern life (thanks to improvements in hygiene and antibiotics).
Of course, I’m sure some of you are asking yourselves whether any of this really matters. Is 37° C close enough?
Sure, this may be sort of puttering around the edges of physical diagnosis, but I think the methodology is really interesting and can obviously be applied to other broadly collected data points. But these data show us that thin, older individuals really do run cooler, and that we may need to pay more attention to a low-grade fever in that population than we otherwise would.
In any case, it’s time for a little re-education. If someone asks you what normal body temperature is, just say 36.6° C, 98.0° F. For his work in this area, I suggest we call it Wunderlich’s constant.
Dr. Wilson is associate professor of medicine and public health at Yale University, New Haven, Conn., and director of Yale’s Clinical and Translational Research Accelerator. He has no disclosures.
A version of this article appeared on Medscape.com.
New AI-enhanced bandages poised to transform wound treatment
You cut yourself. You put on a bandage. In a week or so, your wound heals.
Most people take this routine for granted. But for the more than 8.2 million Americans who have chronic wounds, it’s not so simple.
Traumatic injuries, post-surgical complications, advanced age, and chronic illnesses like diabetes and vascular disease can all disrupt the delicate healing process, leading to wounds that last months or years.
Left untreated, about 30% led to amputation. And recent studies show the risk of dying from a chronic wound complication within 5 years rivals that of most cancers.
Yet until recently, medical technology had not kept up with what experts say is a snowballing threat to public health.
“Wound care – even with all of the billions of products that are sold – still exists on kind of a medieval level,” said Geoffrey Gurtner, MD, chair of the department of surgery and professor of biomedical engineering at the University of Arizona College of Medicine. “We’re still putting on poultices and salves ... and when it comes to diagnosing infection, it’s really an art. I think we can do better.”
Old-school bandage meets AI
Dr. Gurtner is among dozens of clinicians and researchers reimagining the humble bandage, combining cutting-edge materials science with artificial intelligence and patient data to develop “smart bandages” that do far more than shield a wound.
Someday soon, these paper-thin bandages embedded with miniaturized electronics could monitor the healing process in real time, alerting the patient – or a doctor – when things go wrong. With the press of a smartphone button, that bandage could deliver medicine to fight an infection or an electrical pulse to stimulate healing.
Some “closed-loop” designs need no prompting, instead monitoring the wound and automatically giving it what it needs.
Others in development could halt a battlefield wound from hemorrhaging or kick-start healing in a blast wound, preventing longer-term disability.
The same technologies could – if the price is right – speed up healing and reduce scarring in minor cuts and scrapes, too, said Dr. Gurtner.
And unlike many cutting-edge medical innovations, these next-generation bandages could be made relatively cheaply and benefit some of the most vulnerable populations, including older adults, people with low incomes, and those in developing countries.
They could also save the health care system money, as the U.S. spends more than $28 billion annually treating chronic wounds.
“This is a condition that many patients find shameful and embarrassing, so there hasn’t been a lot of advocacy,” said Dr. Gurtner, outgoing board president of the Wound Healing Society. “It’s a relatively ignored problem afflicting an underserved population that has a huge cost. It’s a perfect storm.”
How wounds heal, or don’t
Wound healing is one of the most complex processes of the human body.
First platelets rush to the injury, prompting blood to clot. Then immune cells emit compounds called inflammatory cytokines, helping to fight off pathogens and keep infection at bay. Other compounds, including nitric oxide, spark the growth of new blood vessels and collagen to rebuild skin and connective tissue. As inflammation slows and stops, the flesh continues to reform.
But some conditions can stall the process, often in the inflammatory stage.
In people with diabetes, high glucose levels and poor circulation tend to sabotage the process. And people with nerve damage from spinal cord injuries, diabetes, or other ailments may not be able to feel it when a wound is getting worse or reinjured.
“We end up with patients going months with open wounds that are festering and infected,” said Roslyn Rivkah Isseroff, MD, professor of dermatology at the University of California Davis and head of the VA Northern California Health Care System’s wound healing clinic. “The patients are upset with the smell. These open ulcers put the patient at risk for systemic infection, like sepsis.” It can impact mental health, draining the patient’s ability to care for their wound.
“We see them once a week and send them home and say change your dressing every day, and they say, ‘I can barely move. I can’t do this,’ ” said Dr. Isseroff.
Checking for infection means removing bandages and culturing the wound. That can be painful, and results take time.
A lot can happen to a wound in a week.
“Sometimes, they come back and it’s a disaster, and they have to be admitted to the ER or even get an amputation,” Dr. Gurtner said.
People who are housing insecure or lack access to health care are even more vulnerable to complications.
“If you had the ability to say ‘there is something bad happening,’ you could do a lot to prevent this cascade and downward spiral.”
Bandages 2.0
In 2019, the Defense Advanced Research Projects Agency, the research arm of the Department of Defense, launched the Bioelectronics for Tissue Regeneration program to encourage scientists to develop a “closed-loop” bandage capable of both monitoring and hastening healing.
Tens of millions in funding has kick-started a flood of innovation since.
“It’s kind of a race to the finish,” said Marco Rolandi, PhD, associate professor of electrical and computer engineering at the University of California Santa Cruz and the principal investigator for a team including engineers, medical doctors, and computer scientists from UC Santa Cruz, UC Davis, and Tufts. “I’ve been amazed and impressed at all the work coming out.”
His team’s goal is to cut healing time in half by using (a) real-time monitoring of how a wound is healing – using indicators like temperature, pH level, oxygen, moisture, glucose, electrical activity, and certain proteins, and (b) appropriate stimulation.
“Every wound is different, so there is no one solution,” said Dr. Isseroff, the team’s clinical lead. “The idea is that it will be able to sense different parameters unique to the wound, use AI to figure out what stage it is in, and provide the right stimulus to kick it out of that stalled stage.”
The team has developed a proof-of-concept prototype: a bandage embedded with a tiny camera that takes pictures and transmits them to a computer algorithm to assess the wound’s progress. Miniaturized battery-powered actuators, or motors, automatically deliver medication.
Phase I trials in rodents went well, Dr. Rolandi said. The team is now testing the bandage on pigs.
Across the globe, other promising developments are underway.
In a scientific paper published in May, researchers at the University of Glasgow described a new “low-cost, environmentally friendly” bandage embedded with light-emitting diodes that use ultraviolet light to kill bacteria – no antibiotics needed. The fabric is stitched with a slim, flexible coil that powers the lights without a battery using wireless power transfer. In lab studies, it eradicated gram-negative bacteria (some of the nastiest bugs) in 6 hours.
Also in May, in the journal Bioactive Materials, a Penn State team detailed a bandage with medicine-injecting microneedles that can halt bleeding immediately after injury. In lab and animal tests, it reduced clotting time from 11.5 minutes to 1.3 minutes and bleeding by 90%.
“With hemorrhaging injuries, it is often the loss of blood – not the injury itself – that causes death,” said study author Amir Sheikhi, PhD, assistant professor of chemical and biomedical engineering at Penn State. “Those 10 minutes could be the difference between life and death.”
Another smart bandage, developed at Northwestern University, Chicago, harmlessly dissolves – electrodes and all – into the body after it is no longer needed, eliminating what can be a painful removal.
Guillermo Ameer, DSc, a study author reporting on the technology in Science Advances, hopes it could be made cheaply and used in developing countries.
“We’d like to create something that you could use in your home, even in a very remote village,” said Dr. Ameer, professor of biomedical engineering at Northwestern.
Timeline for clinical use
These are early days for the smart bandage, scientists say. Most studies have been in rodents and more work is needed to develop human-scale bandages, reduce cost, solve long-term data storage, and ensure material adheres well without irritating the skin.
But Dr. Gurtner is hopeful that some iteration could be used in clinical practice within a few years.
In May, he and colleagues at Stanford (Calif.) University published a paper in Nature Biotechnology describing their smart bandage. It includes a microcontroller unit, a radio antenna, biosensors, and an electrical stimulator all affixed to a rubbery, skin-like polymer (or hydrogel) about the thickness of a single coat of latex paint.
The bandage senses changes in temperature and electrical conductivity as the wound heals, and it gives electrical stimulation to accelerate that healing.
Animals treated with the bandage healed 25% faster, with 50% less scarring.
Electrical currents are already used for wound healing in clinical practice, Dr. Gurtner said. Because the stimulus is already approved and the cost to make the bandage could be low (as little as $10 to $50), he believes it could be ushered through the approval processes relatively quickly.
“Is this the ultimate embodiment of all the bells and whistles that are possible in a smart bandage? No. Not yet,” he said. “But we think it will help people. And right now, that’s good enough.”
A version of this article appeared on WebMD.com.
You cut yourself. You put on a bandage. In a week or so, your wound heals.
Most people take this routine for granted. But for the more than 8.2 million Americans who have chronic wounds, it’s not so simple.
Traumatic injuries, post-surgical complications, advanced age, and chronic illnesses like diabetes and vascular disease can all disrupt the delicate healing process, leading to wounds that last months or years.
Left untreated, about 30% led to amputation. And recent studies show the risk of dying from a chronic wound complication within 5 years rivals that of most cancers.
Yet until recently, medical technology had not kept up with what experts say is a snowballing threat to public health.
“Wound care – even with all of the billions of products that are sold – still exists on kind of a medieval level,” said Geoffrey Gurtner, MD, chair of the department of surgery and professor of biomedical engineering at the University of Arizona College of Medicine. “We’re still putting on poultices and salves ... and when it comes to diagnosing infection, it’s really an art. I think we can do better.”
Old-school bandage meets AI
Dr. Gurtner is among dozens of clinicians and researchers reimagining the humble bandage, combining cutting-edge materials science with artificial intelligence and patient data to develop “smart bandages” that do far more than shield a wound.
Someday soon, these paper-thin bandages embedded with miniaturized electronics could monitor the healing process in real time, alerting the patient – or a doctor – when things go wrong. With the press of a smartphone button, that bandage could deliver medicine to fight an infection or an electrical pulse to stimulate healing.
Some “closed-loop” designs need no prompting, instead monitoring the wound and automatically giving it what it needs.
Others in development could halt a battlefield wound from hemorrhaging or kick-start healing in a blast wound, preventing longer-term disability.
The same technologies could – if the price is right – speed up healing and reduce scarring in minor cuts and scrapes, too, said Dr. Gurtner.
And unlike many cutting-edge medical innovations, these next-generation bandages could be made relatively cheaply and benefit some of the most vulnerable populations, including older adults, people with low incomes, and those in developing countries.
They could also save the health care system money, as the U.S. spends more than $28 billion annually treating chronic wounds.
“This is a condition that many patients find shameful and embarrassing, so there hasn’t been a lot of advocacy,” said Dr. Gurtner, outgoing board president of the Wound Healing Society. “It’s a relatively ignored problem afflicting an underserved population that has a huge cost. It’s a perfect storm.”
How wounds heal, or don’t
Wound healing is one of the most complex processes of the human body.
First platelets rush to the injury, prompting blood to clot. Then immune cells emit compounds called inflammatory cytokines, helping to fight off pathogens and keep infection at bay. Other compounds, including nitric oxide, spark the growth of new blood vessels and collagen to rebuild skin and connective tissue. As inflammation slows and stops, the flesh continues to reform.
But some conditions can stall the process, often in the inflammatory stage.
In people with diabetes, high glucose levels and poor circulation tend to sabotage the process. And people with nerve damage from spinal cord injuries, diabetes, or other ailments may not be able to feel it when a wound is getting worse or reinjured.
“We end up with patients going months with open wounds that are festering and infected,” said Roslyn Rivkah Isseroff, MD, professor of dermatology at the University of California Davis and head of the VA Northern California Health Care System’s wound healing clinic. “The patients are upset with the smell. These open ulcers put the patient at risk for systemic infection, like sepsis.” It can impact mental health, draining the patient’s ability to care for their wound.
“We see them once a week and send them home and say change your dressing every day, and they say, ‘I can barely move. I can’t do this,’ ” said Dr. Isseroff.
Checking for infection means removing bandages and culturing the wound. That can be painful, and results take time.
A lot can happen to a wound in a week.
“Sometimes, they come back and it’s a disaster, and they have to be admitted to the ER or even get an amputation,” Dr. Gurtner said.
People who are housing insecure or lack access to health care are even more vulnerable to complications.
“If you had the ability to say ‘there is something bad happening,’ you could do a lot to prevent this cascade and downward spiral.”
Bandages 2.0
In 2019, the Defense Advanced Research Projects Agency, the research arm of the Department of Defense, launched the Bioelectronics for Tissue Regeneration program to encourage scientists to develop a “closed-loop” bandage capable of both monitoring and hastening healing.
Tens of millions in funding has kick-started a flood of innovation since.
“It’s kind of a race to the finish,” said Marco Rolandi, PhD, associate professor of electrical and computer engineering at the University of California Santa Cruz and the principal investigator for a team including engineers, medical doctors, and computer scientists from UC Santa Cruz, UC Davis, and Tufts. “I’ve been amazed and impressed at all the work coming out.”
His team’s goal is to cut healing time in half by using (a) real-time monitoring of how a wound is healing – using indicators like temperature, pH level, oxygen, moisture, glucose, electrical activity, and certain proteins, and (b) appropriate stimulation.
“Every wound is different, so there is no one solution,” said Dr. Isseroff, the team’s clinical lead. “The idea is that it will be able to sense different parameters unique to the wound, use AI to figure out what stage it is in, and provide the right stimulus to kick it out of that stalled stage.”
The team has developed a proof-of-concept prototype: a bandage embedded with a tiny camera that takes pictures and transmits them to a computer algorithm to assess the wound’s progress. Miniaturized battery-powered actuators, or motors, automatically deliver medication.
Phase I trials in rodents went well, Dr. Rolandi said. The team is now testing the bandage on pigs.
Across the globe, other promising developments are underway.
In a scientific paper published in May, researchers at the University of Glasgow described a new “low-cost, environmentally friendly” bandage embedded with light-emitting diodes that use ultraviolet light to kill bacteria – no antibiotics needed. The fabric is stitched with a slim, flexible coil that powers the lights without a battery using wireless power transfer. In lab studies, it eradicated gram-negative bacteria (some of the nastiest bugs) in 6 hours.
Also in May, in the journal Bioactive Materials, a Penn State team detailed a bandage with medicine-injecting microneedles that can halt bleeding immediately after injury. In lab and animal tests, it reduced clotting time from 11.5 minutes to 1.3 minutes and bleeding by 90%.
“With hemorrhaging injuries, it is often the loss of blood – not the injury itself – that causes death,” said study author Amir Sheikhi, PhD, assistant professor of chemical and biomedical engineering at Penn State. “Those 10 minutes could be the difference between life and death.”
Another smart bandage, developed at Northwestern University, Chicago, harmlessly dissolves – electrodes and all – into the body after it is no longer needed, eliminating what can be a painful removal.
Guillermo Ameer, DSc, a study author reporting on the technology in Science Advances, hopes it could be made cheaply and used in developing countries.
“We’d like to create something that you could use in your home, even in a very remote village,” said Dr. Ameer, professor of biomedical engineering at Northwestern.
Timeline for clinical use
These are early days for the smart bandage, scientists say. Most studies have been in rodents and more work is needed to develop human-scale bandages, reduce cost, solve long-term data storage, and ensure material adheres well without irritating the skin.
But Dr. Gurtner is hopeful that some iteration could be used in clinical practice within a few years.
In May, he and colleagues at Stanford (Calif.) University published a paper in Nature Biotechnology describing their smart bandage. It includes a microcontroller unit, a radio antenna, biosensors, and an electrical stimulator all affixed to a rubbery, skin-like polymer (or hydrogel) about the thickness of a single coat of latex paint.
The bandage senses changes in temperature and electrical conductivity as the wound heals, and it gives electrical stimulation to accelerate that healing.
Animals treated with the bandage healed 25% faster, with 50% less scarring.
Electrical currents are already used for wound healing in clinical practice, Dr. Gurtner said. Because the stimulus is already approved and the cost to make the bandage could be low (as little as $10 to $50), he believes it could be ushered through the approval processes relatively quickly.
“Is this the ultimate embodiment of all the bells and whistles that are possible in a smart bandage? No. Not yet,” he said. “But we think it will help people. And right now, that’s good enough.”
A version of this article appeared on WebMD.com.
You cut yourself. You put on a bandage. In a week or so, your wound heals.
Most people take this routine for granted. But for the more than 8.2 million Americans who have chronic wounds, it’s not so simple.
Traumatic injuries, post-surgical complications, advanced age, and chronic illnesses like diabetes and vascular disease can all disrupt the delicate healing process, leading to wounds that last months or years.
Left untreated, about 30% led to amputation. And recent studies show the risk of dying from a chronic wound complication within 5 years rivals that of most cancers.
Yet until recently, medical technology had not kept up with what experts say is a snowballing threat to public health.
“Wound care – even with all of the billions of products that are sold – still exists on kind of a medieval level,” said Geoffrey Gurtner, MD, chair of the department of surgery and professor of biomedical engineering at the University of Arizona College of Medicine. “We’re still putting on poultices and salves ... and when it comes to diagnosing infection, it’s really an art. I think we can do better.”
Old-school bandage meets AI
Dr. Gurtner is among dozens of clinicians and researchers reimagining the humble bandage, combining cutting-edge materials science with artificial intelligence and patient data to develop “smart bandages” that do far more than shield a wound.
Someday soon, these paper-thin bandages embedded with miniaturized electronics could monitor the healing process in real time, alerting the patient – or a doctor – when things go wrong. With the press of a smartphone button, that bandage could deliver medicine to fight an infection or an electrical pulse to stimulate healing.
Some “closed-loop” designs need no prompting, instead monitoring the wound and automatically giving it what it needs.
Others in development could halt a battlefield wound from hemorrhaging or kick-start healing in a blast wound, preventing longer-term disability.
The same technologies could – if the price is right – speed up healing and reduce scarring in minor cuts and scrapes, too, said Dr. Gurtner.
And unlike many cutting-edge medical innovations, these next-generation bandages could be made relatively cheaply and benefit some of the most vulnerable populations, including older adults, people with low incomes, and those in developing countries.
They could also save the health care system money, as the U.S. spends more than $28 billion annually treating chronic wounds.
“This is a condition that many patients find shameful and embarrassing, so there hasn’t been a lot of advocacy,” said Dr. Gurtner, outgoing board president of the Wound Healing Society. “It’s a relatively ignored problem afflicting an underserved population that has a huge cost. It’s a perfect storm.”
How wounds heal, or don’t
Wound healing is one of the most complex processes of the human body.
First platelets rush to the injury, prompting blood to clot. Then immune cells emit compounds called inflammatory cytokines, helping to fight off pathogens and keep infection at bay. Other compounds, including nitric oxide, spark the growth of new blood vessels and collagen to rebuild skin and connective tissue. As inflammation slows and stops, the flesh continues to reform.
But some conditions can stall the process, often in the inflammatory stage.
In people with diabetes, high glucose levels and poor circulation tend to sabotage the process. And people with nerve damage from spinal cord injuries, diabetes, or other ailments may not be able to feel it when a wound is getting worse or reinjured.
“We end up with patients going months with open wounds that are festering and infected,” said Roslyn Rivkah Isseroff, MD, professor of dermatology at the University of California Davis and head of the VA Northern California Health Care System’s wound healing clinic. “The patients are upset with the smell. These open ulcers put the patient at risk for systemic infection, like sepsis.” It can impact mental health, draining the patient’s ability to care for their wound.
“We see them once a week and send them home and say change your dressing every day, and they say, ‘I can barely move. I can’t do this,’ ” said Dr. Isseroff.
Checking for infection means removing bandages and culturing the wound. That can be painful, and results take time.
A lot can happen to a wound in a week.
“Sometimes, they come back and it’s a disaster, and they have to be admitted to the ER or even get an amputation,” Dr. Gurtner said.
People who are housing insecure or lack access to health care are even more vulnerable to complications.
“If you had the ability to say ‘there is something bad happening,’ you could do a lot to prevent this cascade and downward spiral.”
Bandages 2.0
In 2019, the Defense Advanced Research Projects Agency, the research arm of the Department of Defense, launched the Bioelectronics for Tissue Regeneration program to encourage scientists to develop a “closed-loop” bandage capable of both monitoring and hastening healing.
Tens of millions in funding has kick-started a flood of innovation since.
“It’s kind of a race to the finish,” said Marco Rolandi, PhD, associate professor of electrical and computer engineering at the University of California Santa Cruz and the principal investigator for a team including engineers, medical doctors, and computer scientists from UC Santa Cruz, UC Davis, and Tufts. “I’ve been amazed and impressed at all the work coming out.”
His team’s goal is to cut healing time in half by using (a) real-time monitoring of how a wound is healing – using indicators like temperature, pH level, oxygen, moisture, glucose, electrical activity, and certain proteins, and (b) appropriate stimulation.
“Every wound is different, so there is no one solution,” said Dr. Isseroff, the team’s clinical lead. “The idea is that it will be able to sense different parameters unique to the wound, use AI to figure out what stage it is in, and provide the right stimulus to kick it out of that stalled stage.”
The team has developed a proof-of-concept prototype: a bandage embedded with a tiny camera that takes pictures and transmits them to a computer algorithm to assess the wound’s progress. Miniaturized battery-powered actuators, or motors, automatically deliver medication.
Phase I trials in rodents went well, Dr. Rolandi said. The team is now testing the bandage on pigs.
Across the globe, other promising developments are underway.
In a scientific paper published in May, researchers at the University of Glasgow described a new “low-cost, environmentally friendly” bandage embedded with light-emitting diodes that use ultraviolet light to kill bacteria – no antibiotics needed. The fabric is stitched with a slim, flexible coil that powers the lights without a battery using wireless power transfer. In lab studies, it eradicated gram-negative bacteria (some of the nastiest bugs) in 6 hours.
Also in May, in the journal Bioactive Materials, a Penn State team detailed a bandage with medicine-injecting microneedles that can halt bleeding immediately after injury. In lab and animal tests, it reduced clotting time from 11.5 minutes to 1.3 minutes and bleeding by 90%.
“With hemorrhaging injuries, it is often the loss of blood – not the injury itself – that causes death,” said study author Amir Sheikhi, PhD, assistant professor of chemical and biomedical engineering at Penn State. “Those 10 minutes could be the difference between life and death.”
Another smart bandage, developed at Northwestern University, Chicago, harmlessly dissolves – electrodes and all – into the body after it is no longer needed, eliminating what can be a painful removal.
Guillermo Ameer, DSc, a study author reporting on the technology in Science Advances, hopes it could be made cheaply and used in developing countries.
“We’d like to create something that you could use in your home, even in a very remote village,” said Dr. Ameer, professor of biomedical engineering at Northwestern.
Timeline for clinical use
These are early days for the smart bandage, scientists say. Most studies have been in rodents and more work is needed to develop human-scale bandages, reduce cost, solve long-term data storage, and ensure material adheres well without irritating the skin.
But Dr. Gurtner is hopeful that some iteration could be used in clinical practice within a few years.
In May, he and colleagues at Stanford (Calif.) University published a paper in Nature Biotechnology describing their smart bandage. It includes a microcontroller unit, a radio antenna, biosensors, and an electrical stimulator all affixed to a rubbery, skin-like polymer (or hydrogel) about the thickness of a single coat of latex paint.
The bandage senses changes in temperature and electrical conductivity as the wound heals, and it gives electrical stimulation to accelerate that healing.
Animals treated with the bandage healed 25% faster, with 50% less scarring.
Electrical currents are already used for wound healing in clinical practice, Dr. Gurtner said. Because the stimulus is already approved and the cost to make the bandage could be low (as little as $10 to $50), he believes it could be ushered through the approval processes relatively quickly.
“Is this the ultimate embodiment of all the bells and whistles that are possible in a smart bandage? No. Not yet,” he said. “But we think it will help people. And right now, that’s good enough.”
A version of this article appeared on WebMD.com.
‘Missed opportunities’ for accurate diagnosing of women with vaginitis
Although the standard of care of diagnosing vaginitis is clinical evaluation, many practices do not perform accurate and comprehensive clinical examinations for a variety for reasons, and the Centers for Disease Control and Prevention currently recommends molecular testing, wrote Casey N. Pinto, PhD, of Penn State University, Hershey, and colleagues. The CDC also recommends testing women with vaginitis for Chlamydia trachomatis (CT) and Neissaria gonorrhoeae (NG) given the high rate of coinfections between vaginitis and these sexually transmitted infections, but data on cotesting in clinical practice are limited, they said.
In a study published in Sexually Transmitted Diseases, the researchers reviewed data from a commercial administrative claims database for 1,359,289 women aged 18-50 years who were diagnosed with vaginitis between 2012 and 2017.
The women were categorized into groups based on type of vaginitis diagnosis: nucleic amplification test (NAAT), DNA probe test, traditional lab test, and those diagnosed clinically at an index visit but with no CPT code for further testing.
Overall, nearly half of the women (49.2%) had no CPT code for further vaginitis testing beyond clinical diagnosis. Of those with CPT codes for testing, 50.9% underwent traditional point-of-care testing, wet mount, or culture, 23.5% had a DNA probe, and 20.6% had NAAT testing.
Approximately one-third (34%) of women were cotested for CT/NG. Testing rates varied widely across the type of vaginitis test, from 70.8% of women who received NAAT to 22.8% of women with no CPT code. In multivariate analysis including age, region, and the Charlson Comorbidity Index (CCI), those tested with NAAT were eight times more likely to be cotested for CT/NG than those with no CPT code (odds ratio, 8.77; P < .0001).
Women who received a traditional test or DNA probe test for vaginitis also were more likely to have CT/NG testing than women with no CPT code, but only 1.8-2.5 times as likely.
“Our data suggest that most clinicians are not engaging the standard of care for testing and diagnosing vaginitis, or not engaging in comprehensive care by cotesting for vaginitis and CT/NG when patients may be at risk, resulting in missed opportunities for accurate diagnosis and potential associated coinfections,” the researchers wrote in their discussion. The higher rates for CT/NG testing among women receiving either NAAT or DNA probe vaginitis testing could be attributed to bundled testing, they noted, and the lower rate of CT/NG testing for patients with no CPT code could stem from limited access to microscopy or clinician preference for clinical diagnosis only, they said.
The findings were limited by several factors, including the lack of data on testing and diagnoses prior to the study period and not billed to insurance, and by the inability to account for variables including race, ethnicity, and socioeconomic status, the researchers noted.
However, the results highlight the need for more comprehensive care in vaginitis testing to take advantage of opportunities to identify CT or NG in women diagnosed with vaginitis, they concluded.
The study was supported by Becton, Dickinson and Company. Lead author Dr. Pinto disclosed consulting for Becton, Dickinson and Company, and receiving an honorarium from Roche.
Although the standard of care of diagnosing vaginitis is clinical evaluation, many practices do not perform accurate and comprehensive clinical examinations for a variety for reasons, and the Centers for Disease Control and Prevention currently recommends molecular testing, wrote Casey N. Pinto, PhD, of Penn State University, Hershey, and colleagues. The CDC also recommends testing women with vaginitis for Chlamydia trachomatis (CT) and Neissaria gonorrhoeae (NG) given the high rate of coinfections between vaginitis and these sexually transmitted infections, but data on cotesting in clinical practice are limited, they said.
In a study published in Sexually Transmitted Diseases, the researchers reviewed data from a commercial administrative claims database for 1,359,289 women aged 18-50 years who were diagnosed with vaginitis between 2012 and 2017.
The women were categorized into groups based on type of vaginitis diagnosis: nucleic amplification test (NAAT), DNA probe test, traditional lab test, and those diagnosed clinically at an index visit but with no CPT code for further testing.
Overall, nearly half of the women (49.2%) had no CPT code for further vaginitis testing beyond clinical diagnosis. Of those with CPT codes for testing, 50.9% underwent traditional point-of-care testing, wet mount, or culture, 23.5% had a DNA probe, and 20.6% had NAAT testing.
Approximately one-third (34%) of women were cotested for CT/NG. Testing rates varied widely across the type of vaginitis test, from 70.8% of women who received NAAT to 22.8% of women with no CPT code. In multivariate analysis including age, region, and the Charlson Comorbidity Index (CCI), those tested with NAAT were eight times more likely to be cotested for CT/NG than those with no CPT code (odds ratio, 8.77; P < .0001).
Women who received a traditional test or DNA probe test for vaginitis also were more likely to have CT/NG testing than women with no CPT code, but only 1.8-2.5 times as likely.
“Our data suggest that most clinicians are not engaging the standard of care for testing and diagnosing vaginitis, or not engaging in comprehensive care by cotesting for vaginitis and CT/NG when patients may be at risk, resulting in missed opportunities for accurate diagnosis and potential associated coinfections,” the researchers wrote in their discussion. The higher rates for CT/NG testing among women receiving either NAAT or DNA probe vaginitis testing could be attributed to bundled testing, they noted, and the lower rate of CT/NG testing for patients with no CPT code could stem from limited access to microscopy or clinician preference for clinical diagnosis only, they said.
The findings were limited by several factors, including the lack of data on testing and diagnoses prior to the study period and not billed to insurance, and by the inability to account for variables including race, ethnicity, and socioeconomic status, the researchers noted.
However, the results highlight the need for more comprehensive care in vaginitis testing to take advantage of opportunities to identify CT or NG in women diagnosed with vaginitis, they concluded.
The study was supported by Becton, Dickinson and Company. Lead author Dr. Pinto disclosed consulting for Becton, Dickinson and Company, and receiving an honorarium from Roche.
Although the standard of care of diagnosing vaginitis is clinical evaluation, many practices do not perform accurate and comprehensive clinical examinations for a variety for reasons, and the Centers for Disease Control and Prevention currently recommends molecular testing, wrote Casey N. Pinto, PhD, of Penn State University, Hershey, and colleagues. The CDC also recommends testing women with vaginitis for Chlamydia trachomatis (CT) and Neissaria gonorrhoeae (NG) given the high rate of coinfections between vaginitis and these sexually transmitted infections, but data on cotesting in clinical practice are limited, they said.
In a study published in Sexually Transmitted Diseases, the researchers reviewed data from a commercial administrative claims database for 1,359,289 women aged 18-50 years who were diagnosed with vaginitis between 2012 and 2017.
The women were categorized into groups based on type of vaginitis diagnosis: nucleic amplification test (NAAT), DNA probe test, traditional lab test, and those diagnosed clinically at an index visit but with no CPT code for further testing.
Overall, nearly half of the women (49.2%) had no CPT code for further vaginitis testing beyond clinical diagnosis. Of those with CPT codes for testing, 50.9% underwent traditional point-of-care testing, wet mount, or culture, 23.5% had a DNA probe, and 20.6% had NAAT testing.
Approximately one-third (34%) of women were cotested for CT/NG. Testing rates varied widely across the type of vaginitis test, from 70.8% of women who received NAAT to 22.8% of women with no CPT code. In multivariate analysis including age, region, and the Charlson Comorbidity Index (CCI), those tested with NAAT were eight times more likely to be cotested for CT/NG than those with no CPT code (odds ratio, 8.77; P < .0001).
Women who received a traditional test or DNA probe test for vaginitis also were more likely to have CT/NG testing than women with no CPT code, but only 1.8-2.5 times as likely.
“Our data suggest that most clinicians are not engaging the standard of care for testing and diagnosing vaginitis, or not engaging in comprehensive care by cotesting for vaginitis and CT/NG when patients may be at risk, resulting in missed opportunities for accurate diagnosis and potential associated coinfections,” the researchers wrote in their discussion. The higher rates for CT/NG testing among women receiving either NAAT or DNA probe vaginitis testing could be attributed to bundled testing, they noted, and the lower rate of CT/NG testing for patients with no CPT code could stem from limited access to microscopy or clinician preference for clinical diagnosis only, they said.
The findings were limited by several factors, including the lack of data on testing and diagnoses prior to the study period and not billed to insurance, and by the inability to account for variables including race, ethnicity, and socioeconomic status, the researchers noted.
However, the results highlight the need for more comprehensive care in vaginitis testing to take advantage of opportunities to identify CT or NG in women diagnosed with vaginitis, they concluded.
The study was supported by Becton, Dickinson and Company. Lead author Dr. Pinto disclosed consulting for Becton, Dickinson and Company, and receiving an honorarium from Roche.
FROM SEXUALLY TRANSMITTED DISEASES
Sepsis too often neglected in hospitals
according to a recent survey by the Centers for Disease Control and Prevention.
For the hospitals that do have sepsis teams, only 55% of them report that their team leaders get dedicated time to manage their sepsis programs.
“One in three people who dies in a hospital has sepsis during that hospitalization,” CDC Director Mandy Cohen, MD, MPH, noted in a statement. “That’s why CDC is calling on all U.S. hospitals to have a sepsis program and raise the bar on sepsis care by incorporating seven core elements.”
The sepsis seven
- Leadership: Dedicating the necessary human, financial, and information technology resources.
- Accountability: Appointing a leader responsible for program outcomes and setting concrete goals.
- Multiprofessional: Engaging key partners throughout the organization.
- Action: Implementing structures and processes to improve the identification, management, and recovery from sepsis.
- Tracking: Measuring sepsis epidemiology, outcomes, and progress toward program goals and the impact of sepsis initiatives.
- Reporting: Providing usable information on sepsis treatment and outcomes to relevant partners.
- Education: Providing sepsis education to health care professionals during onboarding and annually.
Craig Weinert, MD, MPH, a pulmonologist and critical care physician and professor of medicine at the University of Minnesota, Minneapolis, says the point the CDC is making with the announcement is that when these sepsis programs have been implemented at hospitals, they have been successful at reducing mortality. And now, the agency is urging all hospitals to implement them and support them properly.
“It’s not asking hospitals to develop new, innovative kinds of sepsis programs. This is not about new drugs or new antibiotics or new devices,” Dr. Weinert says. “This is about having hospitals dedicate organizational resources to implementing sepsis programs.”
The CDC’s announcement is aimed toward hospital administrators, Dr. Weinert adds. The agency is making the case that sepsis needs more funding in hospitals that either don’t have the programs or aren’t supporting them with dedicated resources.
There’s another message as well, Dr. Weinert says.
“COVID basically obliterated sepsis programs for two and a half years,” he says. Now the CDC is saying it’s time to divert staff back to sepsis care.
Stepping up sepsis care
Raymund Dantes, MD, assistant professor of medicine at Emory University, Atlanta, one of the developers of the core elements, says this is like a recipe for sepsis care.
Dr. Dantes compares the instructions for hospitals with getting a good restaurant up and running. And in the restaurant business, “you need more than the recipes. You need a leader or manager to ensure you have the right people working together, with the right supplies, getting the right feedback on their work to continuously improve,” he explains.
Dr. Dantes, who is also the physician lead for the Emory Healthcare Sepsis Program, says the approach is meant to be flexible to the size of the hospital, population served, and available resources.
He points out that a well-run sepsis program at a 25-bed rural hospital will look very different from the program at a 1,000-bed tertiary care hospital.
Some hospitals, Dr. Dantes says, will be starting from scratch when getting a sepsis program, and for those hospitals, the developers included a “Getting Started” section as part of the detailed, 29-page full report.
In September, Sepsis Awareness Month, the CDC will provide educational information to health care professionals, patients, families, and caregivers about preventing infections that can lead to sepsis through its ongoing Get Ahead of Sepsis campaign.
A version of this article first appeared on Medscape.com.
according to a recent survey by the Centers for Disease Control and Prevention.
For the hospitals that do have sepsis teams, only 55% of them report that their team leaders get dedicated time to manage their sepsis programs.
“One in three people who dies in a hospital has sepsis during that hospitalization,” CDC Director Mandy Cohen, MD, MPH, noted in a statement. “That’s why CDC is calling on all U.S. hospitals to have a sepsis program and raise the bar on sepsis care by incorporating seven core elements.”
The sepsis seven
- Leadership: Dedicating the necessary human, financial, and information technology resources.
- Accountability: Appointing a leader responsible for program outcomes and setting concrete goals.
- Multiprofessional: Engaging key partners throughout the organization.
- Action: Implementing structures and processes to improve the identification, management, and recovery from sepsis.
- Tracking: Measuring sepsis epidemiology, outcomes, and progress toward program goals and the impact of sepsis initiatives.
- Reporting: Providing usable information on sepsis treatment and outcomes to relevant partners.
- Education: Providing sepsis education to health care professionals during onboarding and annually.
Craig Weinert, MD, MPH, a pulmonologist and critical care physician and professor of medicine at the University of Minnesota, Minneapolis, says the point the CDC is making with the announcement is that when these sepsis programs have been implemented at hospitals, they have been successful at reducing mortality. And now, the agency is urging all hospitals to implement them and support them properly.
“It’s not asking hospitals to develop new, innovative kinds of sepsis programs. This is not about new drugs or new antibiotics or new devices,” Dr. Weinert says. “This is about having hospitals dedicate organizational resources to implementing sepsis programs.”
The CDC’s announcement is aimed toward hospital administrators, Dr. Weinert adds. The agency is making the case that sepsis needs more funding in hospitals that either don’t have the programs or aren’t supporting them with dedicated resources.
There’s another message as well, Dr. Weinert says.
“COVID basically obliterated sepsis programs for two and a half years,” he says. Now the CDC is saying it’s time to divert staff back to sepsis care.
Stepping up sepsis care
Raymund Dantes, MD, assistant professor of medicine at Emory University, Atlanta, one of the developers of the core elements, says this is like a recipe for sepsis care.
Dr. Dantes compares the instructions for hospitals with getting a good restaurant up and running. And in the restaurant business, “you need more than the recipes. You need a leader or manager to ensure you have the right people working together, with the right supplies, getting the right feedback on their work to continuously improve,” he explains.
Dr. Dantes, who is also the physician lead for the Emory Healthcare Sepsis Program, says the approach is meant to be flexible to the size of the hospital, population served, and available resources.
He points out that a well-run sepsis program at a 25-bed rural hospital will look very different from the program at a 1,000-bed tertiary care hospital.
Some hospitals, Dr. Dantes says, will be starting from scratch when getting a sepsis program, and for those hospitals, the developers included a “Getting Started” section as part of the detailed, 29-page full report.
In September, Sepsis Awareness Month, the CDC will provide educational information to health care professionals, patients, families, and caregivers about preventing infections that can lead to sepsis through its ongoing Get Ahead of Sepsis campaign.
A version of this article first appeared on Medscape.com.
according to a recent survey by the Centers for Disease Control and Prevention.
For the hospitals that do have sepsis teams, only 55% of them report that their team leaders get dedicated time to manage their sepsis programs.
“One in three people who dies in a hospital has sepsis during that hospitalization,” CDC Director Mandy Cohen, MD, MPH, noted in a statement. “That’s why CDC is calling on all U.S. hospitals to have a sepsis program and raise the bar on sepsis care by incorporating seven core elements.”
The sepsis seven
- Leadership: Dedicating the necessary human, financial, and information technology resources.
- Accountability: Appointing a leader responsible for program outcomes and setting concrete goals.
- Multiprofessional: Engaging key partners throughout the organization.
- Action: Implementing structures and processes to improve the identification, management, and recovery from sepsis.
- Tracking: Measuring sepsis epidemiology, outcomes, and progress toward program goals and the impact of sepsis initiatives.
- Reporting: Providing usable information on sepsis treatment and outcomes to relevant partners.
- Education: Providing sepsis education to health care professionals during onboarding and annually.
Craig Weinert, MD, MPH, a pulmonologist and critical care physician and professor of medicine at the University of Minnesota, Minneapolis, says the point the CDC is making with the announcement is that when these sepsis programs have been implemented at hospitals, they have been successful at reducing mortality. And now, the agency is urging all hospitals to implement them and support them properly.
“It’s not asking hospitals to develop new, innovative kinds of sepsis programs. This is not about new drugs or new antibiotics or new devices,” Dr. Weinert says. “This is about having hospitals dedicate organizational resources to implementing sepsis programs.”
The CDC’s announcement is aimed toward hospital administrators, Dr. Weinert adds. The agency is making the case that sepsis needs more funding in hospitals that either don’t have the programs or aren’t supporting them with dedicated resources.
There’s another message as well, Dr. Weinert says.
“COVID basically obliterated sepsis programs for two and a half years,” he says. Now the CDC is saying it’s time to divert staff back to sepsis care.
Stepping up sepsis care
Raymund Dantes, MD, assistant professor of medicine at Emory University, Atlanta, one of the developers of the core elements, says this is like a recipe for sepsis care.
Dr. Dantes compares the instructions for hospitals with getting a good restaurant up and running. And in the restaurant business, “you need more than the recipes. You need a leader or manager to ensure you have the right people working together, with the right supplies, getting the right feedback on their work to continuously improve,” he explains.
Dr. Dantes, who is also the physician lead for the Emory Healthcare Sepsis Program, says the approach is meant to be flexible to the size of the hospital, population served, and available resources.
He points out that a well-run sepsis program at a 25-bed rural hospital will look very different from the program at a 1,000-bed tertiary care hospital.
Some hospitals, Dr. Dantes says, will be starting from scratch when getting a sepsis program, and for those hospitals, the developers included a “Getting Started” section as part of the detailed, 29-page full report.
In September, Sepsis Awareness Month, the CDC will provide educational information to health care professionals, patients, families, and caregivers about preventing infections that can lead to sepsis through its ongoing Get Ahead of Sepsis campaign.
A version of this article first appeared on Medscape.com.
Five ways to avert a malpractice lawsuit with better EHR techniques
Although most physicians have gotten used to working with EHRs, despite their irritations, the use of EHRs has contributed to a growing number of malpractice lawsuits. Defense attorneys say that
According to a study in the Journal of Patient Safety, more than 30% of all EHR-related malpractice cases are associated with medication errors; 28% with diagnosis; and 31% with a complication of treatment, such as entering wrong information, entering information in the wrong place, and overlooking EHR flags and warnings for interactions or contraindications.
The study gave these examples of EHR-related errors that led to patient harm and ultimately to malpractice lawsuits:
- A discharge order omitted a patient’s medication that prevented strokes; the patient had a stroke days later.
- An electronic order for morphine failed to state the upper dose limit; the patient died.
- A physician meant to click on “discontinue” for an anticoagulant but mistakenly clicked on “continue” for home use.
Catching potential issues such as drug interactions or critical medical history that should inform treatment is more important than ever. “We know from safety engineering principles that just relying on vigilance is not a long-term safety strategy,” says Aaron Zach Hettinger, MD, chief research information officer at MedStar Health Research Institute, Washington, D.C. “So, it’s critical that we design these safe systems and leverage the data that’s in them.”
Here are five smart EHR practices to help protect your patients’ health and your own liability.
1. Double-check dropdown boxes
When it comes to user error, it’s easy to click the wrong choice from a drop-down menu. Better to take the time to explain your answer in a box, even if it takes a few more minutes. Or if you are choosing from a menu, proofread any information it auto-fills in the chart.
Dr. Hettinger says you can strike a balance between these templated approaches to diagnosis and long-term care by working with third-party systems and your organization or vendor IT department to help with follow-up questions to keep populated data in check.
“Make sure you have a back-end system that can help monitor that structured data,” says Dr. Hettinger. Structured data are the patient’s demographic information, like name, address, age, height, weight, vital signs, and data elements like diagnosis, medications, and lab results. “Wherever you can leverage the underlying tools that are part of the electronic health record to make sure that we’re constantly checking the right results, that helps reduce the workload so that clinicians can focus on taking care of the patients and doing the right thing and not be as focused on entering data into the system.”
2. Supplement EHR notes with direct communication
The failure to diagnose cancer because one physician doesn’t know what another physician saw in an imaging report is one of the most common claims in the cases he tries, says Aaron Boeder, a plaintiff’s medical negligence lawyer in Chicago.
Physicians often assume that if they put a note in the electronic chart, others will look for it, but Mr. Boeder says it’s far more prudent to communicate directly.
“Let’s say a radiologist interprets a scan and sees what might be cancer,” he says. “If the ordering doctor is an orthopedist who’s ordered a CT scan for DVT, there’s going to be a report for that scan. It’s going to get auto-populated back into that physician’s note,” says Mr. Boeder.
The physician may or may not look at it, but it will be in their note, and they’re supposed to follow up on it because they ordered the scan. “But they may not follow up on it, and they may not get a call from the radiologist,” he says.
“Next thing you know, 2 or 3 years later, that patient is diagnosed with very advanced cancer.”
3. Tailor auto-fill information to your common practices
Suppose, as a physician, you find that you need to change a default setting time and time again. Dr. Hettinger says it’s worth your time to take an extra couple of minutes to work with your vendor or your health system to try and make changes to auto-population settings that align with your practices.
“Let’s say a default dose of 20 milligrams of a medication is what automatically pops up, but in reality, your practice is to use a smaller dose because it’s safer, even though they’re all within the acceptable realm of what you would order,” he says. “Rather than have the default to the higher dose, see if you can change the default to a lower dose. And that way, you don’t have to catch yourself every time.”
If your auto-fills are amounts that constantly need changing, an interruption could easily knock you off course before you make that correction.
“If there are ways to have the system defaults be safer or more in line with your clinical practice, and especially across a group, then you’re designing a safer system and not relying on vigilance or memory prone to interruptions,” says Dr. Hettinger.
4. Curb the copy and paste
It’s tempting to copy a note from a previous patient visit and make only minimal changes as needed, but you risk including outdated information if you do. Even if you’re repeating questions asked by the intake nurse, it is safer to not to rely on that information, says Beth Kanik, a defense medical malpractice attorney in Atlanta.
“If it later goes into litigation, the argument then becomes that it looks like you didn’t do your job,” says Ms. Kanik. “Instead, try to ask questions in a way that would elicit responses that may be a little different than what the nurse got, so that it’s clear you asked the questions and didn’t just simply rely upon someone else’s information.”
5. Separate typing from listening
While EHR may be an excellent tool for data collection and safety checking, it’s not a stand-in for doctor-patient interaction. As technology practices push medicine toward more and more efficiency, Mr. Boeder says it’s most often listening over all else that makes the difference in the quality of care. And good listening requires full attention.
“A real concern for physicians is the number of visits they’re expected to accomplish in a set amount of time,” says Mr. Boeder. “Often this translates into a doctor talking to a patient while typing notes or while reading a note from the last time the patient was in.”
Taking the time to pause after entering data and briefly reviewing your understanding of what your patient has told you can be invaluable and may save you – and your patient – problems later.
“In so many cases, it comes down to people not being heard,” says Mr. Boeder. “So listen to what your patients are saying.”
A version of this article first appeared on Medscape.com.
Although most physicians have gotten used to working with EHRs, despite their irritations, the use of EHRs has contributed to a growing number of malpractice lawsuits. Defense attorneys say that
According to a study in the Journal of Patient Safety, more than 30% of all EHR-related malpractice cases are associated with medication errors; 28% with diagnosis; and 31% with a complication of treatment, such as entering wrong information, entering information in the wrong place, and overlooking EHR flags and warnings for interactions or contraindications.
The study gave these examples of EHR-related errors that led to patient harm and ultimately to malpractice lawsuits:
- A discharge order omitted a patient’s medication that prevented strokes; the patient had a stroke days later.
- An electronic order for morphine failed to state the upper dose limit; the patient died.
- A physician meant to click on “discontinue” for an anticoagulant but mistakenly clicked on “continue” for home use.
Catching potential issues such as drug interactions or critical medical history that should inform treatment is more important than ever. “We know from safety engineering principles that just relying on vigilance is not a long-term safety strategy,” says Aaron Zach Hettinger, MD, chief research information officer at MedStar Health Research Institute, Washington, D.C. “So, it’s critical that we design these safe systems and leverage the data that’s in them.”
Here are five smart EHR practices to help protect your patients’ health and your own liability.
1. Double-check dropdown boxes
When it comes to user error, it’s easy to click the wrong choice from a drop-down menu. Better to take the time to explain your answer in a box, even if it takes a few more minutes. Or if you are choosing from a menu, proofread any information it auto-fills in the chart.
Dr. Hettinger says you can strike a balance between these templated approaches to diagnosis and long-term care by working with third-party systems and your organization or vendor IT department to help with follow-up questions to keep populated data in check.
“Make sure you have a back-end system that can help monitor that structured data,” says Dr. Hettinger. Structured data are the patient’s demographic information, like name, address, age, height, weight, vital signs, and data elements like diagnosis, medications, and lab results. “Wherever you can leverage the underlying tools that are part of the electronic health record to make sure that we’re constantly checking the right results, that helps reduce the workload so that clinicians can focus on taking care of the patients and doing the right thing and not be as focused on entering data into the system.”
2. Supplement EHR notes with direct communication
The failure to diagnose cancer because one physician doesn’t know what another physician saw in an imaging report is one of the most common claims in the cases he tries, says Aaron Boeder, a plaintiff’s medical negligence lawyer in Chicago.
Physicians often assume that if they put a note in the electronic chart, others will look for it, but Mr. Boeder says it’s far more prudent to communicate directly.
“Let’s say a radiologist interprets a scan and sees what might be cancer,” he says. “If the ordering doctor is an orthopedist who’s ordered a CT scan for DVT, there’s going to be a report for that scan. It’s going to get auto-populated back into that physician’s note,” says Mr. Boeder.
The physician may or may not look at it, but it will be in their note, and they’re supposed to follow up on it because they ordered the scan. “But they may not follow up on it, and they may not get a call from the radiologist,” he says.
“Next thing you know, 2 or 3 years later, that patient is diagnosed with very advanced cancer.”
3. Tailor auto-fill information to your common practices
Suppose, as a physician, you find that you need to change a default setting time and time again. Dr. Hettinger says it’s worth your time to take an extra couple of minutes to work with your vendor or your health system to try and make changes to auto-population settings that align with your practices.
“Let’s say a default dose of 20 milligrams of a medication is what automatically pops up, but in reality, your practice is to use a smaller dose because it’s safer, even though they’re all within the acceptable realm of what you would order,” he says. “Rather than have the default to the higher dose, see if you can change the default to a lower dose. And that way, you don’t have to catch yourself every time.”
If your auto-fills are amounts that constantly need changing, an interruption could easily knock you off course before you make that correction.
“If there are ways to have the system defaults be safer or more in line with your clinical practice, and especially across a group, then you’re designing a safer system and not relying on vigilance or memory prone to interruptions,” says Dr. Hettinger.
4. Curb the copy and paste
It’s tempting to copy a note from a previous patient visit and make only minimal changes as needed, but you risk including outdated information if you do. Even if you’re repeating questions asked by the intake nurse, it is safer to not to rely on that information, says Beth Kanik, a defense medical malpractice attorney in Atlanta.
“If it later goes into litigation, the argument then becomes that it looks like you didn’t do your job,” says Ms. Kanik. “Instead, try to ask questions in a way that would elicit responses that may be a little different than what the nurse got, so that it’s clear you asked the questions and didn’t just simply rely upon someone else’s information.”
5. Separate typing from listening
While EHR may be an excellent tool for data collection and safety checking, it’s not a stand-in for doctor-patient interaction. As technology practices push medicine toward more and more efficiency, Mr. Boeder says it’s most often listening over all else that makes the difference in the quality of care. And good listening requires full attention.
“A real concern for physicians is the number of visits they’re expected to accomplish in a set amount of time,” says Mr. Boeder. “Often this translates into a doctor talking to a patient while typing notes or while reading a note from the last time the patient was in.”
Taking the time to pause after entering data and briefly reviewing your understanding of what your patient has told you can be invaluable and may save you – and your patient – problems later.
“In so many cases, it comes down to people not being heard,” says Mr. Boeder. “So listen to what your patients are saying.”
A version of this article first appeared on Medscape.com.
Although most physicians have gotten used to working with EHRs, despite their irritations, the use of EHRs has contributed to a growing number of malpractice lawsuits. Defense attorneys say that
According to a study in the Journal of Patient Safety, more than 30% of all EHR-related malpractice cases are associated with medication errors; 28% with diagnosis; and 31% with a complication of treatment, such as entering wrong information, entering information in the wrong place, and overlooking EHR flags and warnings for interactions or contraindications.
The study gave these examples of EHR-related errors that led to patient harm and ultimately to malpractice lawsuits:
- A discharge order omitted a patient’s medication that prevented strokes; the patient had a stroke days later.
- An electronic order for morphine failed to state the upper dose limit; the patient died.
- A physician meant to click on “discontinue” for an anticoagulant but mistakenly clicked on “continue” for home use.
Catching potential issues such as drug interactions or critical medical history that should inform treatment is more important than ever. “We know from safety engineering principles that just relying on vigilance is not a long-term safety strategy,” says Aaron Zach Hettinger, MD, chief research information officer at MedStar Health Research Institute, Washington, D.C. “So, it’s critical that we design these safe systems and leverage the data that’s in them.”
Here are five smart EHR practices to help protect your patients’ health and your own liability.
1. Double-check dropdown boxes
When it comes to user error, it’s easy to click the wrong choice from a drop-down menu. Better to take the time to explain your answer in a box, even if it takes a few more minutes. Or if you are choosing from a menu, proofread any information it auto-fills in the chart.
Dr. Hettinger says you can strike a balance between these templated approaches to diagnosis and long-term care by working with third-party systems and your organization or vendor IT department to help with follow-up questions to keep populated data in check.
“Make sure you have a back-end system that can help monitor that structured data,” says Dr. Hettinger. Structured data are the patient’s demographic information, like name, address, age, height, weight, vital signs, and data elements like diagnosis, medications, and lab results. “Wherever you can leverage the underlying tools that are part of the electronic health record to make sure that we’re constantly checking the right results, that helps reduce the workload so that clinicians can focus on taking care of the patients and doing the right thing and not be as focused on entering data into the system.”
2. Supplement EHR notes with direct communication
The failure to diagnose cancer because one physician doesn’t know what another physician saw in an imaging report is one of the most common claims in the cases he tries, says Aaron Boeder, a plaintiff’s medical negligence lawyer in Chicago.
Physicians often assume that if they put a note in the electronic chart, others will look for it, but Mr. Boeder says it’s far more prudent to communicate directly.
“Let’s say a radiologist interprets a scan and sees what might be cancer,” he says. “If the ordering doctor is an orthopedist who’s ordered a CT scan for DVT, there’s going to be a report for that scan. It’s going to get auto-populated back into that physician’s note,” says Mr. Boeder.
The physician may or may not look at it, but it will be in their note, and they’re supposed to follow up on it because they ordered the scan. “But they may not follow up on it, and they may not get a call from the radiologist,” he says.
“Next thing you know, 2 or 3 years later, that patient is diagnosed with very advanced cancer.”
3. Tailor auto-fill information to your common practices
Suppose, as a physician, you find that you need to change a default setting time and time again. Dr. Hettinger says it’s worth your time to take an extra couple of minutes to work with your vendor or your health system to try and make changes to auto-population settings that align with your practices.
“Let’s say a default dose of 20 milligrams of a medication is what automatically pops up, but in reality, your practice is to use a smaller dose because it’s safer, even though they’re all within the acceptable realm of what you would order,” he says. “Rather than have the default to the higher dose, see if you can change the default to a lower dose. And that way, you don’t have to catch yourself every time.”
If your auto-fills are amounts that constantly need changing, an interruption could easily knock you off course before you make that correction.
“If there are ways to have the system defaults be safer or more in line with your clinical practice, and especially across a group, then you’re designing a safer system and not relying on vigilance or memory prone to interruptions,” says Dr. Hettinger.
4. Curb the copy and paste
It’s tempting to copy a note from a previous patient visit and make only minimal changes as needed, but you risk including outdated information if you do. Even if you’re repeating questions asked by the intake nurse, it is safer to not to rely on that information, says Beth Kanik, a defense medical malpractice attorney in Atlanta.
“If it later goes into litigation, the argument then becomes that it looks like you didn’t do your job,” says Ms. Kanik. “Instead, try to ask questions in a way that would elicit responses that may be a little different than what the nurse got, so that it’s clear you asked the questions and didn’t just simply rely upon someone else’s information.”
5. Separate typing from listening
While EHR may be an excellent tool for data collection and safety checking, it’s not a stand-in for doctor-patient interaction. As technology practices push medicine toward more and more efficiency, Mr. Boeder says it’s most often listening over all else that makes the difference in the quality of care. And good listening requires full attention.
“A real concern for physicians is the number of visits they’re expected to accomplish in a set amount of time,” says Mr. Boeder. “Often this translates into a doctor talking to a patient while typing notes or while reading a note from the last time the patient was in.”
Taking the time to pause after entering data and briefly reviewing your understanding of what your patient has told you can be invaluable and may save you – and your patient – problems later.
“In so many cases, it comes down to people not being heard,” says Mr. Boeder. “So listen to what your patients are saying.”
A version of this article first appeared on Medscape.com.
Domestic violence in health care is real and underreported
To protect survivors’ identities, some names have been changed or shortened.
Natasha Abadilla, MD, met the man who would become her abuser while working abroad for a public health nonprofit. When he began emotionally and physically abusing her, she did everything she could to hide it.
“My coworkers knew nothing of the abuse. I became an expert in applying makeup to hide the bruises,” recalls Dr. Abadilla, now a second-year resident and pediatric neurologist at Lucile Packard Children’s Hospital at Stanford.
Dr. Abadilla says she strongly identifies as a hard worker and – to this day – hopes her work did not falter despite her partner’s constant drain on her. But the impact of the abuse continued to affect her for years. Like many survivors of domestic violence, she struggled with PTSD and depression.
Health care workers are often the first point of contact for survivors of domestic violence. Experts and advocates continue to push for more training for clinicians to identify and respond to signs among their patients. Often missing from this conversation is the reality that those tasked with screening can also be victims of intimate partner violence themselves.
What’s more: The very strengths that medical professionals often pride themselves on – perfectionism, empathy, grit – can make it harder for them to identify abuse in their own relationships and push through humiliation and shame to seek help.
Dr. Abadilla is exceptional among survivors in the medical field. Rather than keep her experience quiet, she has shared it publicly.
Awareness, she believes, can save lives.
An understudied problem in an underserved group
The majority of research on health care workers in this area has focused on workplace violence, which 62% experience worldwide. But intimate partner violence remains understudied and underdiscussed. Some medical professionals are even saddled with a “double burden,” facing trauma at work and at home, note the authors of a 2022 meta-analysis published in the journal Trauma, Violence, & Abuse.
The problem has had dire consequences. In recent years, many health care workers have been killed by their abusers:
- In 2016, Casey M. Drawert, MD, a Texas-based critical care anesthesiologist, was fatally shot by her husband in a murder-suicide.
- In 2018, Tamara O’Neal, MD, an ER physician, and Dayna Less, a first-year pharmacy resident, were killed by Dr. O’Neal’s ex-fiancé at Mercy Hospital in Chicago.
- In 2019, Sarah Hawley, MD, a first-year University of Utah resident, was fatally shot by her boyfriend in a murder-suicide.
- In 2021, Moria Kinsey, a nurse practitioner in Tahlequah, Okla., was murdered by a physician.
- In July of 2023, Gwendolyn Lavonne Riddick, DO, an ob.gyn. in North Carolina, was fatally shot by the father of her 3-year-old son.
There are others.
In the wake of these tragedies, calls for health care workers to screen each other as well as patients have grown. But for an untold number of survivors, breaking the silence is still not possible due to concerns about their reputation, professional consequences, the threat of harassment from abusers who are often in the same field, a medical culture of selfless endurance, and a lack of appropriate resources.
While the vast majority have stayed silent, those who have spoken out say there’s a need for targeted interventions to educate medical professionals as well as more supportive policies throughout the health care system.
Are health care workers more at risk?
Although more studies are needed, research indicates health care workers experience domestic violence at rates comparable to those of other populations, whereas some data suggest rates may be higher.
In the United States, more than one in three women and one in four men experience some form of intimate partner violence in their lifetime. Similarly, a 2020 study found that 24% of 400 physicians responding to a survey reported a history of domestic violence, with 15% reporting verbal abuse, 8% reporting physical violence, 4% reporting sexual abuse, and 4% reporting stalking.
Meanwhile, in an anonymous survey completed by 882 practicing surgeons and trainees in the United States from late 2018 to early 2019, more than 60% reported experiencing some type of intimate partner violence, most commonly emotional abuse.
Recent studies in the United Kingdom, Australia, and elsewhere show that significant numbers of medical professionals are fighting this battle. A 2019 study of more than 2,000 nurses, midwives, and health care assistants in the United Kingdom found that nurses were three times more likely to experience domestic violence than the average person.
What would help solve this problem: More study of health care worker-survivors as a unique group with unique risk factors. In general, domestic violence is most prevalent among women and people in marginalized groups. But young adults, such as medical students and trainees, can face an increased risk due to economic strain. Major life changes, such as relocating for residency, can also drive up stress and fray social connections, further isolating victims.
Why it’s so much harder for medical professionals to reveal abuse
For medical professionals accustomed to being strong and forging on, identifying as a victim of abuse can seem like a personal contradiction. It can feel easier to separate their personal and professional lives rather than face a complex reality.
In a personal essay on KevinMD.com, medical student Chloe N. L. Lee describes this emotional turmoil. “As an aspiring psychiatrist, I questioned my character judgment (how did I end up with a misogynistic abuser?) and wondered if I ought to have known better. I worried that my colleagues would deem me unfit to care for patients. And I thought that this was not supposed to happen to women like me,” Ms. Lee writes.
Kimberly, a licensed therapist, experienced a similar pattern of self-blame when her partner began exhibiting violent behavior. “For a long time, I felt guilty because I said to myself, You’re a therapist. You’re supposed to know this,” she recalls. At the same time, she felt driven to help him and sought couples therapy as his violence escalated.
Whitney, a pharmacist, recognized the “hallmarks” of abuse in her relationship, but she coped by compartmentalizing. Whitney says she was vulnerable to her abuser as a young college student who struggled financially. As he showered her with gifts, she found herself waving away red flags like aggressiveness or overprotectiveness.
After Whitney graduated, her partner’s emotional manipulation escalated into frequent physical assaults. When he gave her a black eye, she could not bring herself to go into work. She quit her job without notice. Despite a spotless record, none of her coworkers ever reached out to investigate her sudden departure.
It would take 8 years for Whitney to acknowledge the abuse and seize a moment to escape. She fled with just her purse and started over in a new city, rebuilding her life in the midst of harassment and threats from her ex. She says she’s grateful to be alive.
An imperfect system doesn’t help
Health care workers rarely ask for support or disclose abuse at work. Some have cited stigma, a lack of confidentiality (especially when the abuser is also in health care), fears about colleagues’ judgment, and a culture that doesn’t prioritize self-care.
Sometimes policies get in the way: In a 2021 qualitative study of interviews with 21 female physician-survivors in the United Kingdom, many said that despite the intense stress of abuse and recovery, they were unable to take any time off.
Of 180 UK-based midwife-survivors interviewed in a 2018 study, only 60 sought support at work and 30 received it. Many said their supervisors pressured them to report the abuse and get back to work, called social services behind their back, or reported them to their professional regulator. “I was treated like the perpetrator,” one said. Barbara Hernandez, PhD, a researcher who studies physician-survivors and director of physician vitality at Loma Linda University in southern California, says workplace violence and mistreatment from patients or colleagues – and a poor institutional response – can make those in health care feel like they have to “shut up and put up,” priming them to also tolerate abuse at home.
When survivors do reach out, there can be a disconnect between the resources they need and those they’re offered, Dr. Hernandez adds. In a recent survey of 400 physicians she conducted, respondents typically said they would advise a physician-survivor to “get to a shelter quickly.” But when roles were reversed, they admitted going to a shelter was the least feasible option. Support groups can also be problematic in smaller communities where physicians might be recognized or see their own patients.
Complicating matters further, the violence often comes from within the medical community. This can lead to particularly malicious abuse tactics like sending false accusations to a victim’s regulatory college or board; prolonged court and custody battles to drain them of all resources and their ability to hold a job; or even sabotage, harassment, or violence at work. The sheen of the abuser’s public persona, on the other hand, can guard them from any accountability.
For example, one physician-survivor said her ex-partner, a psychiatrist, coerced her into believing she was mentally ill, claimed she was “psychotic” in order to take back their children after she left, and had numerous colleagues serve as character witnesses in court for him, “saying he couldn’t have done any of these things, how great he is, and what a wonderful father he is.”
Slow progress is still progress
After Sherilyn M. Gordon-Burroughs, MD, a Texas-based transplant surgeon, mother, and educator, was killed by her husband in a murder-suicide in 2017, her friends Barbara Lee Bass, MD, president of the American College of Surgeons, and Patricia L. Turner, MD, were spurred into action. Together, they founded the ACS Intimate Partner Violence Task Force. Their mission is to educate surgeons to identify the signs of intimate partner violence (IPV) in themselves and their colleagues and connect them with resources.
“There is a concerted effort to close that gap,” says D’Andrea K. Joseph, MD, cochair of the task force and chief of trauma and acute care surgery at NYU Langone in New York. In the future, Dr. Joseph predicts, “making this a part of the curriculum, that it’s standardized for residents and trainees, that there is a safe place for victims ... and that we can band together and really recognize and assist our colleagues who are in trouble.”
Resources created by the ACS IPV task force, such as the toolkit and curriculum, provide a model for other health care leaders. But there have been few similar initiatives aimed at increasing IPV intervention within the medical system.
What you can do in your workplace
In her essay, Ms. Lee explains that a major turning point came when a physician friend explicitly asked if she was experiencing abuse. He then gently confirmed she was, and asked without judgment how he could support her, an approach that mirrors advice from the National Domestic Violence Hotline.
“Having a physician validate that this was, indeed, an abusive situation helped enormously ... I believe it may have saved my life,” she writes.
That validation can be crucial, and Dr. Abadilla urges other physicians to regularly check in with colleagues, especially those who seem particularly positive with a go-getter attitude and yet may not seem themselves. That was how she presented when she was struggling the most.
Supporting systemic changes within your organization and beyond is also important. The authors of the 2022 meta-analysis stress the need for domestic violence training, legislative changes, paid leave, and union support.
Finding strength in recovery
Over a decade after escaping her abuser, Whitney says she’s only just begun to share her experience, but what she’s learned has made her a better pharmacist. She says she’s more attuned to subtle signs something could be off with patients and coworkers. When someone makes comments about feeling anxious or that they can’t do anything right, it’s important to ask why, she says.
Recently, Kimberly has opened up to her mentor and other therapists, many of whom have shared that they’re also survivors.
“The last thing I said to [my abuser] is you think you’ve won and you’re hurting me, but what you’ve done to me – I’m going to utilize this and I’m going to help other people,” Kimberly says. “This pain that I have will go away, and I’m going to save the lives of others.”
A version of this article first appeared on Medscape.com.
To protect survivors’ identities, some names have been changed or shortened.
Natasha Abadilla, MD, met the man who would become her abuser while working abroad for a public health nonprofit. When he began emotionally and physically abusing her, she did everything she could to hide it.
“My coworkers knew nothing of the abuse. I became an expert in applying makeup to hide the bruises,” recalls Dr. Abadilla, now a second-year resident and pediatric neurologist at Lucile Packard Children’s Hospital at Stanford.
Dr. Abadilla says she strongly identifies as a hard worker and – to this day – hopes her work did not falter despite her partner’s constant drain on her. But the impact of the abuse continued to affect her for years. Like many survivors of domestic violence, she struggled with PTSD and depression.
Health care workers are often the first point of contact for survivors of domestic violence. Experts and advocates continue to push for more training for clinicians to identify and respond to signs among their patients. Often missing from this conversation is the reality that those tasked with screening can also be victims of intimate partner violence themselves.
What’s more: The very strengths that medical professionals often pride themselves on – perfectionism, empathy, grit – can make it harder for them to identify abuse in their own relationships and push through humiliation and shame to seek help.
Dr. Abadilla is exceptional among survivors in the medical field. Rather than keep her experience quiet, she has shared it publicly.
Awareness, she believes, can save lives.
An understudied problem in an underserved group
The majority of research on health care workers in this area has focused on workplace violence, which 62% experience worldwide. But intimate partner violence remains understudied and underdiscussed. Some medical professionals are even saddled with a “double burden,” facing trauma at work and at home, note the authors of a 2022 meta-analysis published in the journal Trauma, Violence, & Abuse.
The problem has had dire consequences. In recent years, many health care workers have been killed by their abusers:
- In 2016, Casey M. Drawert, MD, a Texas-based critical care anesthesiologist, was fatally shot by her husband in a murder-suicide.
- In 2018, Tamara O’Neal, MD, an ER physician, and Dayna Less, a first-year pharmacy resident, were killed by Dr. O’Neal’s ex-fiancé at Mercy Hospital in Chicago.
- In 2019, Sarah Hawley, MD, a first-year University of Utah resident, was fatally shot by her boyfriend in a murder-suicide.
- In 2021, Moria Kinsey, a nurse practitioner in Tahlequah, Okla., was murdered by a physician.
- In July of 2023, Gwendolyn Lavonne Riddick, DO, an ob.gyn. in North Carolina, was fatally shot by the father of her 3-year-old son.
There are others.
In the wake of these tragedies, calls for health care workers to screen each other as well as patients have grown. But for an untold number of survivors, breaking the silence is still not possible due to concerns about their reputation, professional consequences, the threat of harassment from abusers who are often in the same field, a medical culture of selfless endurance, and a lack of appropriate resources.
While the vast majority have stayed silent, those who have spoken out say there’s a need for targeted interventions to educate medical professionals as well as more supportive policies throughout the health care system.
Are health care workers more at risk?
Although more studies are needed, research indicates health care workers experience domestic violence at rates comparable to those of other populations, whereas some data suggest rates may be higher.
In the United States, more than one in three women and one in four men experience some form of intimate partner violence in their lifetime. Similarly, a 2020 study found that 24% of 400 physicians responding to a survey reported a history of domestic violence, with 15% reporting verbal abuse, 8% reporting physical violence, 4% reporting sexual abuse, and 4% reporting stalking.
Meanwhile, in an anonymous survey completed by 882 practicing surgeons and trainees in the United States from late 2018 to early 2019, more than 60% reported experiencing some type of intimate partner violence, most commonly emotional abuse.
Recent studies in the United Kingdom, Australia, and elsewhere show that significant numbers of medical professionals are fighting this battle. A 2019 study of more than 2,000 nurses, midwives, and health care assistants in the United Kingdom found that nurses were three times more likely to experience domestic violence than the average person.
What would help solve this problem: More study of health care worker-survivors as a unique group with unique risk factors. In general, domestic violence is most prevalent among women and people in marginalized groups. But young adults, such as medical students and trainees, can face an increased risk due to economic strain. Major life changes, such as relocating for residency, can also drive up stress and fray social connections, further isolating victims.
Why it’s so much harder for medical professionals to reveal abuse
For medical professionals accustomed to being strong and forging on, identifying as a victim of abuse can seem like a personal contradiction. It can feel easier to separate their personal and professional lives rather than face a complex reality.
In a personal essay on KevinMD.com, medical student Chloe N. L. Lee describes this emotional turmoil. “As an aspiring psychiatrist, I questioned my character judgment (how did I end up with a misogynistic abuser?) and wondered if I ought to have known better. I worried that my colleagues would deem me unfit to care for patients. And I thought that this was not supposed to happen to women like me,” Ms. Lee writes.
Kimberly, a licensed therapist, experienced a similar pattern of self-blame when her partner began exhibiting violent behavior. “For a long time, I felt guilty because I said to myself, You’re a therapist. You’re supposed to know this,” she recalls. At the same time, she felt driven to help him and sought couples therapy as his violence escalated.
Whitney, a pharmacist, recognized the “hallmarks” of abuse in her relationship, but she coped by compartmentalizing. Whitney says she was vulnerable to her abuser as a young college student who struggled financially. As he showered her with gifts, she found herself waving away red flags like aggressiveness or overprotectiveness.
After Whitney graduated, her partner’s emotional manipulation escalated into frequent physical assaults. When he gave her a black eye, she could not bring herself to go into work. She quit her job without notice. Despite a spotless record, none of her coworkers ever reached out to investigate her sudden departure.
It would take 8 years for Whitney to acknowledge the abuse and seize a moment to escape. She fled with just her purse and started over in a new city, rebuilding her life in the midst of harassment and threats from her ex. She says she’s grateful to be alive.
An imperfect system doesn’t help
Health care workers rarely ask for support or disclose abuse at work. Some have cited stigma, a lack of confidentiality (especially when the abuser is also in health care), fears about colleagues’ judgment, and a culture that doesn’t prioritize self-care.
Sometimes policies get in the way: In a 2021 qualitative study of interviews with 21 female physician-survivors in the United Kingdom, many said that despite the intense stress of abuse and recovery, they were unable to take any time off.
Of 180 UK-based midwife-survivors interviewed in a 2018 study, only 60 sought support at work and 30 received it. Many said their supervisors pressured them to report the abuse and get back to work, called social services behind their back, or reported them to their professional regulator. “I was treated like the perpetrator,” one said. Barbara Hernandez, PhD, a researcher who studies physician-survivors and director of physician vitality at Loma Linda University in southern California, says workplace violence and mistreatment from patients or colleagues – and a poor institutional response – can make those in health care feel like they have to “shut up and put up,” priming them to also tolerate abuse at home.
When survivors do reach out, there can be a disconnect between the resources they need and those they’re offered, Dr. Hernandez adds. In a recent survey of 400 physicians she conducted, respondents typically said they would advise a physician-survivor to “get to a shelter quickly.” But when roles were reversed, they admitted going to a shelter was the least feasible option. Support groups can also be problematic in smaller communities where physicians might be recognized or see their own patients.
Complicating matters further, the violence often comes from within the medical community. This can lead to particularly malicious abuse tactics like sending false accusations to a victim’s regulatory college or board; prolonged court and custody battles to drain them of all resources and their ability to hold a job; or even sabotage, harassment, or violence at work. The sheen of the abuser’s public persona, on the other hand, can guard them from any accountability.
For example, one physician-survivor said her ex-partner, a psychiatrist, coerced her into believing she was mentally ill, claimed she was “psychotic” in order to take back their children after she left, and had numerous colleagues serve as character witnesses in court for him, “saying he couldn’t have done any of these things, how great he is, and what a wonderful father he is.”
Slow progress is still progress
After Sherilyn M. Gordon-Burroughs, MD, a Texas-based transplant surgeon, mother, and educator, was killed by her husband in a murder-suicide in 2017, her friends Barbara Lee Bass, MD, president of the American College of Surgeons, and Patricia L. Turner, MD, were spurred into action. Together, they founded the ACS Intimate Partner Violence Task Force. Their mission is to educate surgeons to identify the signs of intimate partner violence (IPV) in themselves and their colleagues and connect them with resources.
“There is a concerted effort to close that gap,” says D’Andrea K. Joseph, MD, cochair of the task force and chief of trauma and acute care surgery at NYU Langone in New York. In the future, Dr. Joseph predicts, “making this a part of the curriculum, that it’s standardized for residents and trainees, that there is a safe place for victims ... and that we can band together and really recognize and assist our colleagues who are in trouble.”
Resources created by the ACS IPV task force, such as the toolkit and curriculum, provide a model for other health care leaders. But there have been few similar initiatives aimed at increasing IPV intervention within the medical system.
What you can do in your workplace
In her essay, Ms. Lee explains that a major turning point came when a physician friend explicitly asked if she was experiencing abuse. He then gently confirmed she was, and asked without judgment how he could support her, an approach that mirrors advice from the National Domestic Violence Hotline.
“Having a physician validate that this was, indeed, an abusive situation helped enormously ... I believe it may have saved my life,” she writes.
That validation can be crucial, and Dr. Abadilla urges other physicians to regularly check in with colleagues, especially those who seem particularly positive with a go-getter attitude and yet may not seem themselves. That was how she presented when she was struggling the most.
Supporting systemic changes within your organization and beyond is also important. The authors of the 2022 meta-analysis stress the need for domestic violence training, legislative changes, paid leave, and union support.
Finding strength in recovery
Over a decade after escaping her abuser, Whitney says she’s only just begun to share her experience, but what she’s learned has made her a better pharmacist. She says she’s more attuned to subtle signs something could be off with patients and coworkers. When someone makes comments about feeling anxious or that they can’t do anything right, it’s important to ask why, she says.
Recently, Kimberly has opened up to her mentor and other therapists, many of whom have shared that they’re also survivors.
“The last thing I said to [my abuser] is you think you’ve won and you’re hurting me, but what you’ve done to me – I’m going to utilize this and I’m going to help other people,” Kimberly says. “This pain that I have will go away, and I’m going to save the lives of others.”
A version of this article first appeared on Medscape.com.
To protect survivors’ identities, some names have been changed or shortened.
Natasha Abadilla, MD, met the man who would become her abuser while working abroad for a public health nonprofit. When he began emotionally and physically abusing her, she did everything she could to hide it.
“My coworkers knew nothing of the abuse. I became an expert in applying makeup to hide the bruises,” recalls Dr. Abadilla, now a second-year resident and pediatric neurologist at Lucile Packard Children’s Hospital at Stanford.
Dr. Abadilla says she strongly identifies as a hard worker and – to this day – hopes her work did not falter despite her partner’s constant drain on her. But the impact of the abuse continued to affect her for years. Like many survivors of domestic violence, she struggled with PTSD and depression.
Health care workers are often the first point of contact for survivors of domestic violence. Experts and advocates continue to push for more training for clinicians to identify and respond to signs among their patients. Often missing from this conversation is the reality that those tasked with screening can also be victims of intimate partner violence themselves.
What’s more: The very strengths that medical professionals often pride themselves on – perfectionism, empathy, grit – can make it harder for them to identify abuse in their own relationships and push through humiliation and shame to seek help.
Dr. Abadilla is exceptional among survivors in the medical field. Rather than keep her experience quiet, she has shared it publicly.
Awareness, she believes, can save lives.
An understudied problem in an underserved group
The majority of research on health care workers in this area has focused on workplace violence, which 62% experience worldwide. But intimate partner violence remains understudied and underdiscussed. Some medical professionals are even saddled with a “double burden,” facing trauma at work and at home, note the authors of a 2022 meta-analysis published in the journal Trauma, Violence, & Abuse.
The problem has had dire consequences. In recent years, many health care workers have been killed by their abusers:
- In 2016, Casey M. Drawert, MD, a Texas-based critical care anesthesiologist, was fatally shot by her husband in a murder-suicide.
- In 2018, Tamara O’Neal, MD, an ER physician, and Dayna Less, a first-year pharmacy resident, were killed by Dr. O’Neal’s ex-fiancé at Mercy Hospital in Chicago.
- In 2019, Sarah Hawley, MD, a first-year University of Utah resident, was fatally shot by her boyfriend in a murder-suicide.
- In 2021, Moria Kinsey, a nurse practitioner in Tahlequah, Okla., was murdered by a physician.
- In July of 2023, Gwendolyn Lavonne Riddick, DO, an ob.gyn. in North Carolina, was fatally shot by the father of her 3-year-old son.
There are others.
In the wake of these tragedies, calls for health care workers to screen each other as well as patients have grown. But for an untold number of survivors, breaking the silence is still not possible due to concerns about their reputation, professional consequences, the threat of harassment from abusers who are often in the same field, a medical culture of selfless endurance, and a lack of appropriate resources.
While the vast majority have stayed silent, those who have spoken out say there’s a need for targeted interventions to educate medical professionals as well as more supportive policies throughout the health care system.
Are health care workers more at risk?
Although more studies are needed, research indicates health care workers experience domestic violence at rates comparable to those of other populations, whereas some data suggest rates may be higher.
In the United States, more than one in three women and one in four men experience some form of intimate partner violence in their lifetime. Similarly, a 2020 study found that 24% of 400 physicians responding to a survey reported a history of domestic violence, with 15% reporting verbal abuse, 8% reporting physical violence, 4% reporting sexual abuse, and 4% reporting stalking.
Meanwhile, in an anonymous survey completed by 882 practicing surgeons and trainees in the United States from late 2018 to early 2019, more than 60% reported experiencing some type of intimate partner violence, most commonly emotional abuse.
Recent studies in the United Kingdom, Australia, and elsewhere show that significant numbers of medical professionals are fighting this battle. A 2019 study of more than 2,000 nurses, midwives, and health care assistants in the United Kingdom found that nurses were three times more likely to experience domestic violence than the average person.
What would help solve this problem: More study of health care worker-survivors as a unique group with unique risk factors. In general, domestic violence is most prevalent among women and people in marginalized groups. But young adults, such as medical students and trainees, can face an increased risk due to economic strain. Major life changes, such as relocating for residency, can also drive up stress and fray social connections, further isolating victims.
Why it’s so much harder for medical professionals to reveal abuse
For medical professionals accustomed to being strong and forging on, identifying as a victim of abuse can seem like a personal contradiction. It can feel easier to separate their personal and professional lives rather than face a complex reality.
In a personal essay on KevinMD.com, medical student Chloe N. L. Lee describes this emotional turmoil. “As an aspiring psychiatrist, I questioned my character judgment (how did I end up with a misogynistic abuser?) and wondered if I ought to have known better. I worried that my colleagues would deem me unfit to care for patients. And I thought that this was not supposed to happen to women like me,” Ms. Lee writes.
Kimberly, a licensed therapist, experienced a similar pattern of self-blame when her partner began exhibiting violent behavior. “For a long time, I felt guilty because I said to myself, You’re a therapist. You’re supposed to know this,” she recalls. At the same time, she felt driven to help him and sought couples therapy as his violence escalated.
Whitney, a pharmacist, recognized the “hallmarks” of abuse in her relationship, but she coped by compartmentalizing. Whitney says she was vulnerable to her abuser as a young college student who struggled financially. As he showered her with gifts, she found herself waving away red flags like aggressiveness or overprotectiveness.
After Whitney graduated, her partner’s emotional manipulation escalated into frequent physical assaults. When he gave her a black eye, she could not bring herself to go into work. She quit her job without notice. Despite a spotless record, none of her coworkers ever reached out to investigate her sudden departure.
It would take 8 years for Whitney to acknowledge the abuse and seize a moment to escape. She fled with just her purse and started over in a new city, rebuilding her life in the midst of harassment and threats from her ex. She says she’s grateful to be alive.
An imperfect system doesn’t help
Health care workers rarely ask for support or disclose abuse at work. Some have cited stigma, a lack of confidentiality (especially when the abuser is also in health care), fears about colleagues’ judgment, and a culture that doesn’t prioritize self-care.
Sometimes policies get in the way: In a 2021 qualitative study of interviews with 21 female physician-survivors in the United Kingdom, many said that despite the intense stress of abuse and recovery, they were unable to take any time off.
Of 180 UK-based midwife-survivors interviewed in a 2018 study, only 60 sought support at work and 30 received it. Many said their supervisors pressured them to report the abuse and get back to work, called social services behind their back, or reported them to their professional regulator. “I was treated like the perpetrator,” one said. Barbara Hernandez, PhD, a researcher who studies physician-survivors and director of physician vitality at Loma Linda University in southern California, says workplace violence and mistreatment from patients or colleagues – and a poor institutional response – can make those in health care feel like they have to “shut up and put up,” priming them to also tolerate abuse at home.
When survivors do reach out, there can be a disconnect between the resources they need and those they’re offered, Dr. Hernandez adds. In a recent survey of 400 physicians she conducted, respondents typically said they would advise a physician-survivor to “get to a shelter quickly.” But when roles were reversed, they admitted going to a shelter was the least feasible option. Support groups can also be problematic in smaller communities where physicians might be recognized or see their own patients.
Complicating matters further, the violence often comes from within the medical community. This can lead to particularly malicious abuse tactics like sending false accusations to a victim’s regulatory college or board; prolonged court and custody battles to drain them of all resources and their ability to hold a job; or even sabotage, harassment, or violence at work. The sheen of the abuser’s public persona, on the other hand, can guard them from any accountability.
For example, one physician-survivor said her ex-partner, a psychiatrist, coerced her into believing she was mentally ill, claimed she was “psychotic” in order to take back their children after she left, and had numerous colleagues serve as character witnesses in court for him, “saying he couldn’t have done any of these things, how great he is, and what a wonderful father he is.”
Slow progress is still progress
After Sherilyn M. Gordon-Burroughs, MD, a Texas-based transplant surgeon, mother, and educator, was killed by her husband in a murder-suicide in 2017, her friends Barbara Lee Bass, MD, president of the American College of Surgeons, and Patricia L. Turner, MD, were spurred into action. Together, they founded the ACS Intimate Partner Violence Task Force. Their mission is to educate surgeons to identify the signs of intimate partner violence (IPV) in themselves and their colleagues and connect them with resources.
“There is a concerted effort to close that gap,” says D’Andrea K. Joseph, MD, cochair of the task force and chief of trauma and acute care surgery at NYU Langone in New York. In the future, Dr. Joseph predicts, “making this a part of the curriculum, that it’s standardized for residents and trainees, that there is a safe place for victims ... and that we can band together and really recognize and assist our colleagues who are in trouble.”
Resources created by the ACS IPV task force, such as the toolkit and curriculum, provide a model for other health care leaders. But there have been few similar initiatives aimed at increasing IPV intervention within the medical system.
What you can do in your workplace
In her essay, Ms. Lee explains that a major turning point came when a physician friend explicitly asked if she was experiencing abuse. He then gently confirmed she was, and asked without judgment how he could support her, an approach that mirrors advice from the National Domestic Violence Hotline.
“Having a physician validate that this was, indeed, an abusive situation helped enormously ... I believe it may have saved my life,” she writes.
That validation can be crucial, and Dr. Abadilla urges other physicians to regularly check in with colleagues, especially those who seem particularly positive with a go-getter attitude and yet may not seem themselves. That was how she presented when she was struggling the most.
Supporting systemic changes within your organization and beyond is also important. The authors of the 2022 meta-analysis stress the need for domestic violence training, legislative changes, paid leave, and union support.
Finding strength in recovery
Over a decade after escaping her abuser, Whitney says she’s only just begun to share her experience, but what she’s learned has made her a better pharmacist. She says she’s more attuned to subtle signs something could be off with patients and coworkers. When someone makes comments about feeling anxious or that they can’t do anything right, it’s important to ask why, she says.
Recently, Kimberly has opened up to her mentor and other therapists, many of whom have shared that they’re also survivors.
“The last thing I said to [my abuser] is you think you’ve won and you’re hurting me, but what you’ve done to me – I’m going to utilize this and I’m going to help other people,” Kimberly says. “This pain that I have will go away, and I’m going to save the lives of others.”
A version of this article first appeared on Medscape.com.
One in five doctors with long COVID can no longer work: Survey
Crippling symptoms, lost careers, and eroded incomes: This is the harsh reality for doctors suffering with long COVID, according to the first major survey of physicians with the condition.
The survey, conducted by the British Medical Association and the Long COVID Doctors for Action support group, sheds light on the lingering effects of long COVID on more than 600 chronically ill and disabled doctors with the condition. It also spotlights what they describe as a lack of medical and financial support from their government and employers at the National Health Service.
“We feel betrayed and abandoned,” said Kelly Fearnley, MBChB, chair and cofounder of Long COVID Doctors for Action. “At a time of national crisis, when health care workers were asked to step up, we did. When the nation needed us, we stepped up. We put our lives on the line. We put our families’ lives on the line. And now that we are injured after knowingly being unprotected and deliberately and repeatedly exposed to a level 3 biohazard, we now find ourselves in this position.”
Dr. Fearnley fell ill while working in a hospital’s COVID ward in November 2020. She is one of an estimated 2 million people in the United Kingdom – including thousands of NHS employees – with long COVID. She hasn’t been able to return to work in nearly 3 years.
Long COVID affects more than 65 million people worldwide. It is estimated that 1 in 10 people infected with the virus develop long-term symptoms. In the United Kingdom, health care and social care workers are seven times more likely to have had severe COVID-19 than other types of employees.
Doctors responding to the BMA survey reported a wide range of long COVID symptoms, including fatigue, headaches, muscular pain, nerve damage, joint pain, and respiratory problems.
Among the survey’s key findings, 60% of doctors said long COVID has affected their ability to carry out day-to-day tasks on a regular basis. Almost one in five (18%) said they were no longer able to work, while fewer than one in three (31%) were working full time. This compares with more than half (57%) of respondents working full time before the onset of their COVID illness – a decline of 46%.
Nearly half (48%) of respondents said they have experienced some form of loss of earnings as a result of long COVID, and almost half of the doctors were never referred to an NHS long COVID clinic. The survey included the following first-person accounts from doctors living with the condition.
- One doctor said: “I nearly lost my life, my home, my partner and my career. I have received little support to help keep these. The impact on my mental health nearly cost [me] my life again.”
- A senior consulting physician commented: “Life is absolutely miserable. Every day is a struggle. I wake up exhausted, the insomnia and night terrors are horrendous as I live through my worst fears every night. Any activity such as eating meals, washing, etc., will mean I have to go to bed for a few hours. I am unable to look after myself or my child, exercise or maintain social relationships. I have no financial security. Long COVID has totally destroyed my life.”
- A salaried general practitioner said: “I can no longer work, finances are ruined. I didn’t have employment protection so am now unemployed and penniless.”
Calls for action from the BMA include the following:
- Financial support for doctors and health care staff with long COVID.
- The recognition of long COVID as an occupational disease among health care workers, along with a definition of the condition that covers all of the debilitating disease’s symptoms.
- Improved access to physical and mental health services to help comprehensive assessment, investigations, and treatment.
- Greater workplace protection for health care staff who risk their lives for others.
- Better support for long COVID sufferers to return to work safely if they can, including a flexible approach to the use of workplace adjustments.
“One would think, given the circumstances under which we fell ill and current workforce shortages, NHS employers would be eager to do everything to facilitate the return to work of people with long COVID,” said Dr. Fearnley. “However, NHS employers are legally required to implement only ‘reasonable adjustments,’ and so things such as extended phased return or adjustments to shift patterns are not always being facilitated. Instead, an increasing number of employers are choosing to terminate contracts.”
Raymond Agius, the BMA’s occupational medicine committee cochair, also put the blame on inadequate safety measures for doctors. Those inadequate measures persist to this day, inasmuch as U.K. hospitals have dropped masking requirements.
“During the COVID-19 pandemic, doctors were left exposed and unprotected at work,” he said in a BMA press release. “They often did not have access to the right PPE. ... Too many risk assessments of workplaces and especially of vulnerable doctors were not undertaken.”
A small minority of doctors who were surveyed said they had access to respiratory protective equipment about the time they contracted COVID-19. Only 11% had access to an FFP2 respirator (the equivalent of an N95 mask); 16% had an FFP3 respirator (the equivalent of an N99 mask).
To date, the British government hasn’t issued much of a response to the survey, saying only that it has invested more than ₤50 million to better understand long COVID.
A version of this article first appeared on Medscape.com.
Crippling symptoms, lost careers, and eroded incomes: This is the harsh reality for doctors suffering with long COVID, according to the first major survey of physicians with the condition.
The survey, conducted by the British Medical Association and the Long COVID Doctors for Action support group, sheds light on the lingering effects of long COVID on more than 600 chronically ill and disabled doctors with the condition. It also spotlights what they describe as a lack of medical and financial support from their government and employers at the National Health Service.
“We feel betrayed and abandoned,” said Kelly Fearnley, MBChB, chair and cofounder of Long COVID Doctors for Action. “At a time of national crisis, when health care workers were asked to step up, we did. When the nation needed us, we stepped up. We put our lives on the line. We put our families’ lives on the line. And now that we are injured after knowingly being unprotected and deliberately and repeatedly exposed to a level 3 biohazard, we now find ourselves in this position.”
Dr. Fearnley fell ill while working in a hospital’s COVID ward in November 2020. She is one of an estimated 2 million people in the United Kingdom – including thousands of NHS employees – with long COVID. She hasn’t been able to return to work in nearly 3 years.
Long COVID affects more than 65 million people worldwide. It is estimated that 1 in 10 people infected with the virus develop long-term symptoms. In the United Kingdom, health care and social care workers are seven times more likely to have had severe COVID-19 than other types of employees.
Doctors responding to the BMA survey reported a wide range of long COVID symptoms, including fatigue, headaches, muscular pain, nerve damage, joint pain, and respiratory problems.
Among the survey’s key findings, 60% of doctors said long COVID has affected their ability to carry out day-to-day tasks on a regular basis. Almost one in five (18%) said they were no longer able to work, while fewer than one in three (31%) were working full time. This compares with more than half (57%) of respondents working full time before the onset of their COVID illness – a decline of 46%.
Nearly half (48%) of respondents said they have experienced some form of loss of earnings as a result of long COVID, and almost half of the doctors were never referred to an NHS long COVID clinic. The survey included the following first-person accounts from doctors living with the condition.
- One doctor said: “I nearly lost my life, my home, my partner and my career. I have received little support to help keep these. The impact on my mental health nearly cost [me] my life again.”
- A senior consulting physician commented: “Life is absolutely miserable. Every day is a struggle. I wake up exhausted, the insomnia and night terrors are horrendous as I live through my worst fears every night. Any activity such as eating meals, washing, etc., will mean I have to go to bed for a few hours. I am unable to look after myself or my child, exercise or maintain social relationships. I have no financial security. Long COVID has totally destroyed my life.”
- A salaried general practitioner said: “I can no longer work, finances are ruined. I didn’t have employment protection so am now unemployed and penniless.”
Calls for action from the BMA include the following:
- Financial support for doctors and health care staff with long COVID.
- The recognition of long COVID as an occupational disease among health care workers, along with a definition of the condition that covers all of the debilitating disease’s symptoms.
- Improved access to physical and mental health services to help comprehensive assessment, investigations, and treatment.
- Greater workplace protection for health care staff who risk their lives for others.
- Better support for long COVID sufferers to return to work safely if they can, including a flexible approach to the use of workplace adjustments.
“One would think, given the circumstances under which we fell ill and current workforce shortages, NHS employers would be eager to do everything to facilitate the return to work of people with long COVID,” said Dr. Fearnley. “However, NHS employers are legally required to implement only ‘reasonable adjustments,’ and so things such as extended phased return or adjustments to shift patterns are not always being facilitated. Instead, an increasing number of employers are choosing to terminate contracts.”
Raymond Agius, the BMA’s occupational medicine committee cochair, also put the blame on inadequate safety measures for doctors. Those inadequate measures persist to this day, inasmuch as U.K. hospitals have dropped masking requirements.
“During the COVID-19 pandemic, doctors were left exposed and unprotected at work,” he said in a BMA press release. “They often did not have access to the right PPE. ... Too many risk assessments of workplaces and especially of vulnerable doctors were not undertaken.”
A small minority of doctors who were surveyed said they had access to respiratory protective equipment about the time they contracted COVID-19. Only 11% had access to an FFP2 respirator (the equivalent of an N95 mask); 16% had an FFP3 respirator (the equivalent of an N99 mask).
To date, the British government hasn’t issued much of a response to the survey, saying only that it has invested more than ₤50 million to better understand long COVID.
A version of this article first appeared on Medscape.com.
Crippling symptoms, lost careers, and eroded incomes: This is the harsh reality for doctors suffering with long COVID, according to the first major survey of physicians with the condition.
The survey, conducted by the British Medical Association and the Long COVID Doctors for Action support group, sheds light on the lingering effects of long COVID on more than 600 chronically ill and disabled doctors with the condition. It also spotlights what they describe as a lack of medical and financial support from their government and employers at the National Health Service.
“We feel betrayed and abandoned,” said Kelly Fearnley, MBChB, chair and cofounder of Long COVID Doctors for Action. “At a time of national crisis, when health care workers were asked to step up, we did. When the nation needed us, we stepped up. We put our lives on the line. We put our families’ lives on the line. And now that we are injured after knowingly being unprotected and deliberately and repeatedly exposed to a level 3 biohazard, we now find ourselves in this position.”
Dr. Fearnley fell ill while working in a hospital’s COVID ward in November 2020. She is one of an estimated 2 million people in the United Kingdom – including thousands of NHS employees – with long COVID. She hasn’t been able to return to work in nearly 3 years.
Long COVID affects more than 65 million people worldwide. It is estimated that 1 in 10 people infected with the virus develop long-term symptoms. In the United Kingdom, health care and social care workers are seven times more likely to have had severe COVID-19 than other types of employees.
Doctors responding to the BMA survey reported a wide range of long COVID symptoms, including fatigue, headaches, muscular pain, nerve damage, joint pain, and respiratory problems.
Among the survey’s key findings, 60% of doctors said long COVID has affected their ability to carry out day-to-day tasks on a regular basis. Almost one in five (18%) said they were no longer able to work, while fewer than one in three (31%) were working full time. This compares with more than half (57%) of respondents working full time before the onset of their COVID illness – a decline of 46%.
Nearly half (48%) of respondents said they have experienced some form of loss of earnings as a result of long COVID, and almost half of the doctors were never referred to an NHS long COVID clinic. The survey included the following first-person accounts from doctors living with the condition.
- One doctor said: “I nearly lost my life, my home, my partner and my career. I have received little support to help keep these. The impact on my mental health nearly cost [me] my life again.”
- A senior consulting physician commented: “Life is absolutely miserable. Every day is a struggle. I wake up exhausted, the insomnia and night terrors are horrendous as I live through my worst fears every night. Any activity such as eating meals, washing, etc., will mean I have to go to bed for a few hours. I am unable to look after myself or my child, exercise or maintain social relationships. I have no financial security. Long COVID has totally destroyed my life.”
- A salaried general practitioner said: “I can no longer work, finances are ruined. I didn’t have employment protection so am now unemployed and penniless.”
Calls for action from the BMA include the following:
- Financial support for doctors and health care staff with long COVID.
- The recognition of long COVID as an occupational disease among health care workers, along with a definition of the condition that covers all of the debilitating disease’s symptoms.
- Improved access to physical and mental health services to help comprehensive assessment, investigations, and treatment.
- Greater workplace protection for health care staff who risk their lives for others.
- Better support for long COVID sufferers to return to work safely if they can, including a flexible approach to the use of workplace adjustments.
“One would think, given the circumstances under which we fell ill and current workforce shortages, NHS employers would be eager to do everything to facilitate the return to work of people with long COVID,” said Dr. Fearnley. “However, NHS employers are legally required to implement only ‘reasonable adjustments,’ and so things such as extended phased return or adjustments to shift patterns are not always being facilitated. Instead, an increasing number of employers are choosing to terminate contracts.”
Raymond Agius, the BMA’s occupational medicine committee cochair, also put the blame on inadequate safety measures for doctors. Those inadequate measures persist to this day, inasmuch as U.K. hospitals have dropped masking requirements.
“During the COVID-19 pandemic, doctors were left exposed and unprotected at work,” he said in a BMA press release. “They often did not have access to the right PPE. ... Too many risk assessments of workplaces and especially of vulnerable doctors were not undertaken.”
A small minority of doctors who were surveyed said they had access to respiratory protective equipment about the time they contracted COVID-19. Only 11% had access to an FFP2 respirator (the equivalent of an N95 mask); 16% had an FFP3 respirator (the equivalent of an N99 mask).
To date, the British government hasn’t issued much of a response to the survey, saying only that it has invested more than ₤50 million to better understand long COVID.
A version of this article first appeared on Medscape.com.
Resident creates AI alternative to U.S. News med school ranking
For decades, pre-med students depended on the annual medical school rankings by U.S. News and World Report to decide where to apply for physician education. But after several prominent med schools pulled out of the rankings, one resident began experimenting with artificial intelligence (AI) to create an alternative.
Brandon Turner MD, MSc, a radiation oncology resident at Massachusetts General Hospital in Boston, developed a free do-it-yourself tool using AI that allows prospective students to rank medical schools based on considerations that are most important to them. His research was published online in JAMA Network Open.
“One of the flaws with conventional ranking systems is that the metrics used in these tools are weighted based on the preferences and views of the people who developed these rankings, but those may not work for everyone,” Dr. Turner told this news organization.
He explained that there are different types of metrics used in the U.S. News ranking: one for research and the other for primary care. “The research rankings carry the most prestige and are the ones that most people know about,” he explained. These metrics take into account factors such as how many grant dollars the medical school receives and the average size of those grants per faculty member, Dr. Turner said.
Admission metrics are also included – for example, the median grade point average or MCAT scores of students who have been accepted. “These don’t tell you anything about the research output of the school, only about how selective the school is,” he said.
Primary care metrics might focus on how many graduates of a given school go into primary care, or how other schools rate the quality of primary care training at a given school – a process called peer assessment, Dr. Turner said.
But even though these might be helpful, students may be more interested in the cost of attendance, average debt, representation of minorities, and how many graduates pass their boards, he said. “U.S. News metrics don’t capture these things, but I included them in my algorithm.”
A U.S. News spokesperson said that the publication continues to help students and their families make decisions about their future education. The spokesperson cited U.S. News’ explanation of how it calculates its rankings. “A school’s overall Best Medical Schools rank should be one consideration and not the lone determinant in where a student applies and accepts,” the article states.
Dr. Turner agreed ranking systems are a good starting point when researching med schools, “but the values reflected in the ranking may not reflect an individual’s goals.”
Tyra-Lee Brett, a premed student at the University of South Florida, Tampa, believes an additional tool for students to evaluate medical schools is needed – and she could potentially see herself using Dr. Turner’s creation.
Still, Ms. Brett, a premed trustee of the American Medical Student Association, doesn’t regard any ranking tool as the “be all and end all.” Rather, she feels that the most effective tool would be based on students’ lived experiences. The AMSA is developing a scorecard in which students grade schools based on their opinions about such issues as housing, family planning, and environmental health, she said.
No prior judgments
To develop his algorithm, Dr. Turner used a branch of AI called “unsupervised learning.” It doesn’t make a prior judgment about what the data should look like, Dr. Turner explained.
“You’re just analyzing natural trends within the data.”
The algorithm tries to find and discover clusters or patterns within the data. “It’s like saying to the algorithm: ‘I want you to tell me what schools you think should be grouped together based on the data I feed you,’ which is the data that the user selects based on his or her personal preferences.”
U.S. News has been transparent about the metrics it uses, Dr. Turner notes. “When I started looking into how rankings are developed, I saw that there was transparency, and the reasoning for choosing the metrics used to develop the ranking was pretty sound,” he said.
“But I didn’t see any justification as to why they chose the particular metrics and weighted them in the way that they did.”
Dr. Turner extracted data from the 2023 U.S. News report, which ranked 109 allopathic medical schools, and applied several scenarios to the results to create his alternative ranking system.
In one scenario, he used the same research metrics used by U.S. News, such as a peer research assessment, median federal research activity per full-time faculty member, median GPA, median MCAT, acceptance rate, and faculty-student ratio.
In another scenario, he included four additional metrics: debt, in-state cost of attendance, USMLE Step 1 passing rate, and percentage of underrepresented students with minority race or ethnicity at the school.
For example, a user can rank the importance of the diversity of the class, amount of debt students expect to incur, and amount of research funding the medical school receives. After selecting those factors, the tool generates tiered results displayed in a circle, a shape chosen to avoid the appearance of the hierarchy associated with traditional rankings, Dr. Turner said.
“A prospective student might not care about acceptance rates and MCAT scores, and instead cares about diversity and debt,” Dr. Turner said. He looks forward to extending this approach to the ranking of colleges as well.
‘Imperfect measures’
“The model and interesting online tool that Dr. Turner created allows a premed [student] to generate custom rankings that are in line with their own priorities,” said Christopher Worsham, MD, MPH, a critical care physician in Mass General’s division of pulmonary and critical care medicine.
But Dr. Worsham, also a teaching associate at Harvard Medical School’s department of health care policy, expressed concern that factors figuring into the rankings by U.S. News and Dr. Turner’s alternative “are imperfect measures of medical school quality.”
For example, a student interested in research might favor federal research funding in their customized rankings with Dr. Turner’s model. “But higher research funding doesn’t necessarily translate into a better education for students, particularly when differentiating between two major research systems,” Dr. Worsham noted.
Dr. Worsham added that neither ranking system accurately predicts the quality of doctors graduating from the schools. Instead, he’d like to see ranking systems based on which schools’ graduates deliver the best patient outcomes, whether that’s through direct patient care, impactful research, or leadership within the health care system.
Michael Sauder, PhD, professor of sociology at the University of Iowa, Iowa City, said the model could offer a valuable alternative to the U.S. News ranking system. It might help users develop their own criteria for determining the ranking of medical schools, which is a big improvement over a “one-size-fits-all” approach, Dr. Sauder said.
And Hanna Stotland, an admission consultant based in Chicago, noted that most students rely on rankings because they “don’t have the luxury of advisers who know the ins and outs of different medical schools.” Given the role that rankings play, Ms. Stotland expects that every new ranking tool will have some influence on students.
This tool in particular “has the potential to be useful for students who have identified values they want their medical school to share.” For example, students who care about racial diversity “could use it to easily identify schools that are successful on that metric,” Ms. Stotland said.
Sujay Ratna, a 2nd-year med student at Icahn School of Medicine at Mount Sinai in New York, said he considered the U.S. News ranking his “go-to tool” when he was applying to med school.
But after reading Dr. Turner’s article, the AMSA membership vice president tried the algorithm. “I definitely would have used it had it existed when I was thinking of what schools to apply to and what [schools] to attend.”
The study had no specific funding. Dr. Turner, Dr. Worsham, Dr. Sauder, Ms. Stotland, Ms. Brett, and Mr. Ratna report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
For decades, pre-med students depended on the annual medical school rankings by U.S. News and World Report to decide where to apply for physician education. But after several prominent med schools pulled out of the rankings, one resident began experimenting with artificial intelligence (AI) to create an alternative.
Brandon Turner MD, MSc, a radiation oncology resident at Massachusetts General Hospital in Boston, developed a free do-it-yourself tool using AI that allows prospective students to rank medical schools based on considerations that are most important to them. His research was published online in JAMA Network Open.
“One of the flaws with conventional ranking systems is that the metrics used in these tools are weighted based on the preferences and views of the people who developed these rankings, but those may not work for everyone,” Dr. Turner told this news organization.
He explained that there are different types of metrics used in the U.S. News ranking: one for research and the other for primary care. “The research rankings carry the most prestige and are the ones that most people know about,” he explained. These metrics take into account factors such as how many grant dollars the medical school receives and the average size of those grants per faculty member, Dr. Turner said.
Admission metrics are also included – for example, the median grade point average or MCAT scores of students who have been accepted. “These don’t tell you anything about the research output of the school, only about how selective the school is,” he said.
Primary care metrics might focus on how many graduates of a given school go into primary care, or how other schools rate the quality of primary care training at a given school – a process called peer assessment, Dr. Turner said.
But even though these might be helpful, students may be more interested in the cost of attendance, average debt, representation of minorities, and how many graduates pass their boards, he said. “U.S. News metrics don’t capture these things, but I included them in my algorithm.”
A U.S. News spokesperson said that the publication continues to help students and their families make decisions about their future education. The spokesperson cited U.S. News’ explanation of how it calculates its rankings. “A school’s overall Best Medical Schools rank should be one consideration and not the lone determinant in where a student applies and accepts,” the article states.
Dr. Turner agreed ranking systems are a good starting point when researching med schools, “but the values reflected in the ranking may not reflect an individual’s goals.”
Tyra-Lee Brett, a premed student at the University of South Florida, Tampa, believes an additional tool for students to evaluate medical schools is needed – and she could potentially see herself using Dr. Turner’s creation.
Still, Ms. Brett, a premed trustee of the American Medical Student Association, doesn’t regard any ranking tool as the “be all and end all.” Rather, she feels that the most effective tool would be based on students’ lived experiences. The AMSA is developing a scorecard in which students grade schools based on their opinions about such issues as housing, family planning, and environmental health, she said.
No prior judgments
To develop his algorithm, Dr. Turner used a branch of AI called “unsupervised learning.” It doesn’t make a prior judgment about what the data should look like, Dr. Turner explained.
“You’re just analyzing natural trends within the data.”
The algorithm tries to find and discover clusters or patterns within the data. “It’s like saying to the algorithm: ‘I want you to tell me what schools you think should be grouped together based on the data I feed you,’ which is the data that the user selects based on his or her personal preferences.”
U.S. News has been transparent about the metrics it uses, Dr. Turner notes. “When I started looking into how rankings are developed, I saw that there was transparency, and the reasoning for choosing the metrics used to develop the ranking was pretty sound,” he said.
“But I didn’t see any justification as to why they chose the particular metrics and weighted them in the way that they did.”
Dr. Turner extracted data from the 2023 U.S. News report, which ranked 109 allopathic medical schools, and applied several scenarios to the results to create his alternative ranking system.
In one scenario, he used the same research metrics used by U.S. News, such as a peer research assessment, median federal research activity per full-time faculty member, median GPA, median MCAT, acceptance rate, and faculty-student ratio.
In another scenario, he included four additional metrics: debt, in-state cost of attendance, USMLE Step 1 passing rate, and percentage of underrepresented students with minority race or ethnicity at the school.
For example, a user can rank the importance of the diversity of the class, amount of debt students expect to incur, and amount of research funding the medical school receives. After selecting those factors, the tool generates tiered results displayed in a circle, a shape chosen to avoid the appearance of the hierarchy associated with traditional rankings, Dr. Turner said.
“A prospective student might not care about acceptance rates and MCAT scores, and instead cares about diversity and debt,” Dr. Turner said. He looks forward to extending this approach to the ranking of colleges as well.
‘Imperfect measures’
“The model and interesting online tool that Dr. Turner created allows a premed [student] to generate custom rankings that are in line with their own priorities,” said Christopher Worsham, MD, MPH, a critical care physician in Mass General’s division of pulmonary and critical care medicine.
But Dr. Worsham, also a teaching associate at Harvard Medical School’s department of health care policy, expressed concern that factors figuring into the rankings by U.S. News and Dr. Turner’s alternative “are imperfect measures of medical school quality.”
For example, a student interested in research might favor federal research funding in their customized rankings with Dr. Turner’s model. “But higher research funding doesn’t necessarily translate into a better education for students, particularly when differentiating between two major research systems,” Dr. Worsham noted.
Dr. Worsham added that neither ranking system accurately predicts the quality of doctors graduating from the schools. Instead, he’d like to see ranking systems based on which schools’ graduates deliver the best patient outcomes, whether that’s through direct patient care, impactful research, or leadership within the health care system.
Michael Sauder, PhD, professor of sociology at the University of Iowa, Iowa City, said the model could offer a valuable alternative to the U.S. News ranking system. It might help users develop their own criteria for determining the ranking of medical schools, which is a big improvement over a “one-size-fits-all” approach, Dr. Sauder said.
And Hanna Stotland, an admission consultant based in Chicago, noted that most students rely on rankings because they “don’t have the luxury of advisers who know the ins and outs of different medical schools.” Given the role that rankings play, Ms. Stotland expects that every new ranking tool will have some influence on students.
This tool in particular “has the potential to be useful for students who have identified values they want their medical school to share.” For example, students who care about racial diversity “could use it to easily identify schools that are successful on that metric,” Ms. Stotland said.
Sujay Ratna, a 2nd-year med student at Icahn School of Medicine at Mount Sinai in New York, said he considered the U.S. News ranking his “go-to tool” when he was applying to med school.
But after reading Dr. Turner’s article, the AMSA membership vice president tried the algorithm. “I definitely would have used it had it existed when I was thinking of what schools to apply to and what [schools] to attend.”
The study had no specific funding. Dr. Turner, Dr. Worsham, Dr. Sauder, Ms. Stotland, Ms. Brett, and Mr. Ratna report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
For decades, pre-med students depended on the annual medical school rankings by U.S. News and World Report to decide where to apply for physician education. But after several prominent med schools pulled out of the rankings, one resident began experimenting with artificial intelligence (AI) to create an alternative.
Brandon Turner MD, MSc, a radiation oncology resident at Massachusetts General Hospital in Boston, developed a free do-it-yourself tool using AI that allows prospective students to rank medical schools based on considerations that are most important to them. His research was published online in JAMA Network Open.
“One of the flaws with conventional ranking systems is that the metrics used in these tools are weighted based on the preferences and views of the people who developed these rankings, but those may not work for everyone,” Dr. Turner told this news organization.
He explained that there are different types of metrics used in the U.S. News ranking: one for research and the other for primary care. “The research rankings carry the most prestige and are the ones that most people know about,” he explained. These metrics take into account factors such as how many grant dollars the medical school receives and the average size of those grants per faculty member, Dr. Turner said.
Admission metrics are also included – for example, the median grade point average or MCAT scores of students who have been accepted. “These don’t tell you anything about the research output of the school, only about how selective the school is,” he said.
Primary care metrics might focus on how many graduates of a given school go into primary care, or how other schools rate the quality of primary care training at a given school – a process called peer assessment, Dr. Turner said.
But even though these might be helpful, students may be more interested in the cost of attendance, average debt, representation of minorities, and how many graduates pass their boards, he said. “U.S. News metrics don’t capture these things, but I included them in my algorithm.”
A U.S. News spokesperson said that the publication continues to help students and their families make decisions about their future education. The spokesperson cited U.S. News’ explanation of how it calculates its rankings. “A school’s overall Best Medical Schools rank should be one consideration and not the lone determinant in where a student applies and accepts,” the article states.
Dr. Turner agreed ranking systems are a good starting point when researching med schools, “but the values reflected in the ranking may not reflect an individual’s goals.”
Tyra-Lee Brett, a premed student at the University of South Florida, Tampa, believes an additional tool for students to evaluate medical schools is needed – and she could potentially see herself using Dr. Turner’s creation.
Still, Ms. Brett, a premed trustee of the American Medical Student Association, doesn’t regard any ranking tool as the “be all and end all.” Rather, she feels that the most effective tool would be based on students’ lived experiences. The AMSA is developing a scorecard in which students grade schools based on their opinions about such issues as housing, family planning, and environmental health, she said.
No prior judgments
To develop his algorithm, Dr. Turner used a branch of AI called “unsupervised learning.” It doesn’t make a prior judgment about what the data should look like, Dr. Turner explained.
“You’re just analyzing natural trends within the data.”
The algorithm tries to find and discover clusters or patterns within the data. “It’s like saying to the algorithm: ‘I want you to tell me what schools you think should be grouped together based on the data I feed you,’ which is the data that the user selects based on his or her personal preferences.”
U.S. News has been transparent about the metrics it uses, Dr. Turner notes. “When I started looking into how rankings are developed, I saw that there was transparency, and the reasoning for choosing the metrics used to develop the ranking was pretty sound,” he said.
“But I didn’t see any justification as to why they chose the particular metrics and weighted them in the way that they did.”
Dr. Turner extracted data from the 2023 U.S. News report, which ranked 109 allopathic medical schools, and applied several scenarios to the results to create his alternative ranking system.
In one scenario, he used the same research metrics used by U.S. News, such as a peer research assessment, median federal research activity per full-time faculty member, median GPA, median MCAT, acceptance rate, and faculty-student ratio.
In another scenario, he included four additional metrics: debt, in-state cost of attendance, USMLE Step 1 passing rate, and percentage of underrepresented students with minority race or ethnicity at the school.
For example, a user can rank the importance of the diversity of the class, amount of debt students expect to incur, and amount of research funding the medical school receives. After selecting those factors, the tool generates tiered results displayed in a circle, a shape chosen to avoid the appearance of the hierarchy associated with traditional rankings, Dr. Turner said.
“A prospective student might not care about acceptance rates and MCAT scores, and instead cares about diversity and debt,” Dr. Turner said. He looks forward to extending this approach to the ranking of colleges as well.
‘Imperfect measures’
“The model and interesting online tool that Dr. Turner created allows a premed [student] to generate custom rankings that are in line with their own priorities,” said Christopher Worsham, MD, MPH, a critical care physician in Mass General’s division of pulmonary and critical care medicine.
But Dr. Worsham, also a teaching associate at Harvard Medical School’s department of health care policy, expressed concern that factors figuring into the rankings by U.S. News and Dr. Turner’s alternative “are imperfect measures of medical school quality.”
For example, a student interested in research might favor federal research funding in their customized rankings with Dr. Turner’s model. “But higher research funding doesn’t necessarily translate into a better education for students, particularly when differentiating between two major research systems,” Dr. Worsham noted.
Dr. Worsham added that neither ranking system accurately predicts the quality of doctors graduating from the schools. Instead, he’d like to see ranking systems based on which schools’ graduates deliver the best patient outcomes, whether that’s through direct patient care, impactful research, or leadership within the health care system.
Michael Sauder, PhD, professor of sociology at the University of Iowa, Iowa City, said the model could offer a valuable alternative to the U.S. News ranking system. It might help users develop their own criteria for determining the ranking of medical schools, which is a big improvement over a “one-size-fits-all” approach, Dr. Sauder said.
And Hanna Stotland, an admission consultant based in Chicago, noted that most students rely on rankings because they “don’t have the luxury of advisers who know the ins and outs of different medical schools.” Given the role that rankings play, Ms. Stotland expects that every new ranking tool will have some influence on students.
This tool in particular “has the potential to be useful for students who have identified values they want their medical school to share.” For example, students who care about racial diversity “could use it to easily identify schools that are successful on that metric,” Ms. Stotland said.
Sujay Ratna, a 2nd-year med student at Icahn School of Medicine at Mount Sinai in New York, said he considered the U.S. News ranking his “go-to tool” when he was applying to med school.
But after reading Dr. Turner’s article, the AMSA membership vice president tried the algorithm. “I definitely would have used it had it existed when I was thinking of what schools to apply to and what [schools] to attend.”
The study had no specific funding. Dr. Turner, Dr. Worsham, Dr. Sauder, Ms. Stotland, Ms. Brett, and Mr. Ratna report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Q&A: What to know about the new BA 2.86 COVID variant
The Centers for Disease Control and Prevention and the World Health Organization have dubbed the BA 2.86 variant of COVID-19 as a variant to watch.
So far, only 26 cases of “Pirola,” as the new variant is being called, have been identified: 10 in Denmark, four each in Sweden and the United States, three in South Africa, two in Portugal, and one each the United Kingdom, Israel, and Canada. BA 2.86 is a subvariant of Omicron, but according to reports from the CDC, the strain has many more mutations than the ones that came before it.
With so many facts still unknown about this new variant, this news organization asked experts what people need to be aware of as it continues to spread.
What is unique about the BA 2.86 variant?
“It is unique in that it has more than three mutations on the spike protein,” said Purvi S. Parikh, MD, an infectious disease expert at New York University’s Langone Health. The virus uses the spike proteins to enter our cells.
This “may mean it will be more transmissible, cause more severe disease, and/or our vaccines and treatments may not work as well, as compared to other variants,” she said.
What do we need to watch with BA 2.86 going forward?
“We don’t know if this variant will be associated with a change in the disease severity. We currently see increased numbers of cases in general, even though we don’t yet see the BA.2.86 in our system,” said Heba Mostafa, PhD, director of the molecular virology laboratory at Johns Hopkins Hospital in Baltimore.
“It is important to monitor BA.2.86 (and other variants) and understand how its evolution impacts the number of cases and disease outcomes,” she said. “We should all be aware of the current increase in cases, though, and try to get tested and be treated as soon as possible, as antivirals should be effective against the circulating variants.”
What should doctors know?
Dr. Parikh said doctors should generally expect more COVID cases in their clinics and make sure to screen patients even if their symptoms are mild.
“We have tools that can be used – antivirals like Paxlovid are still efficacious with current dominant strains such as EG.5,” she said. “And encourage your patients to get their boosters, mask, wash hands, and social distance.”
How well can our vaccines fight BA 2.86?
“Vaccine coverage for the BA.2.86 is an area of uncertainty right now,” said Dr. Mostafa.
In its report, the CDC said scientists are still figuring out how well the updated COVID vaccine works. It’s expected to be available in the fall, and for now, they believe the new shot will still make infections less severe, new variants and all.
Prior vaccinations and infections have created antibodies in many people, and that will likely provide some protection, Dr. Mostafa said. “When we experienced the Omicron wave in December 2021, even though the variant was distant from what circulated before its emergence and was associated with a very large increase in the number of cases, vaccinations were still protective against severe disease.”
What is the most important thing to keep track of when it comes to this variant?
According to Dr. Parikh, “it’s most important to monitor how transmissible [BA 2.86] is, how severe it is, and if our current treatments and vaccines work.”
Dr. Mostafa said how well the new variants escape existing antibody protection should also be studied and watched closely.
What does this stage of the virus mutation tell us about where we are in the pandemic?
The history of the coronavirus over the past few years shows that variants with many changes evolve and can spread very quickly, Dr. Mostafa said. “Now that the virus is endemic, it is essential to monitor, update vaccinations if necessary, diagnose, treat, and implement infection control measures when necessary.”
With the limited data we have so far, experts seem to agree that while the variant’s makeup raises some red flags, it is too soon to jump to any conclusions about how easy it is to catch it and the ways it may change how the virus impacts those who contract it.
A version of this article first appeared on WebMD.com.
The Centers for Disease Control and Prevention and the World Health Organization have dubbed the BA 2.86 variant of COVID-19 as a variant to watch.
So far, only 26 cases of “Pirola,” as the new variant is being called, have been identified: 10 in Denmark, four each in Sweden and the United States, three in South Africa, two in Portugal, and one each the United Kingdom, Israel, and Canada. BA 2.86 is a subvariant of Omicron, but according to reports from the CDC, the strain has many more mutations than the ones that came before it.
With so many facts still unknown about this new variant, this news organization asked experts what people need to be aware of as it continues to spread.
What is unique about the BA 2.86 variant?
“It is unique in that it has more than three mutations on the spike protein,” said Purvi S. Parikh, MD, an infectious disease expert at New York University’s Langone Health. The virus uses the spike proteins to enter our cells.
This “may mean it will be more transmissible, cause more severe disease, and/or our vaccines and treatments may not work as well, as compared to other variants,” she said.
What do we need to watch with BA 2.86 going forward?
“We don’t know if this variant will be associated with a change in the disease severity. We currently see increased numbers of cases in general, even though we don’t yet see the BA.2.86 in our system,” said Heba Mostafa, PhD, director of the molecular virology laboratory at Johns Hopkins Hospital in Baltimore.
“It is important to monitor BA.2.86 (and other variants) and understand how its evolution impacts the number of cases and disease outcomes,” she said. “We should all be aware of the current increase in cases, though, and try to get tested and be treated as soon as possible, as antivirals should be effective against the circulating variants.”
What should doctors know?
Dr. Parikh said doctors should generally expect more COVID cases in their clinics and make sure to screen patients even if their symptoms are mild.
“We have tools that can be used – antivirals like Paxlovid are still efficacious with current dominant strains such as EG.5,” she said. “And encourage your patients to get their boosters, mask, wash hands, and social distance.”
How well can our vaccines fight BA 2.86?
“Vaccine coverage for the BA.2.86 is an area of uncertainty right now,” said Dr. Mostafa.
In its report, the CDC said scientists are still figuring out how well the updated COVID vaccine works. It’s expected to be available in the fall, and for now, they believe the new shot will still make infections less severe, new variants and all.
Prior vaccinations and infections have created antibodies in many people, and that will likely provide some protection, Dr. Mostafa said. “When we experienced the Omicron wave in December 2021, even though the variant was distant from what circulated before its emergence and was associated with a very large increase in the number of cases, vaccinations were still protective against severe disease.”
What is the most important thing to keep track of when it comes to this variant?
According to Dr. Parikh, “it’s most important to monitor how transmissible [BA 2.86] is, how severe it is, and if our current treatments and vaccines work.”
Dr. Mostafa said how well the new variants escape existing antibody protection should also be studied and watched closely.
What does this stage of the virus mutation tell us about where we are in the pandemic?
The history of the coronavirus over the past few years shows that variants with many changes evolve and can spread very quickly, Dr. Mostafa said. “Now that the virus is endemic, it is essential to monitor, update vaccinations if necessary, diagnose, treat, and implement infection control measures when necessary.”
With the limited data we have so far, experts seem to agree that while the variant’s makeup raises some red flags, it is too soon to jump to any conclusions about how easy it is to catch it and the ways it may change how the virus impacts those who contract it.
A version of this article first appeared on WebMD.com.
The Centers for Disease Control and Prevention and the World Health Organization have dubbed the BA 2.86 variant of COVID-19 as a variant to watch.
So far, only 26 cases of “Pirola,” as the new variant is being called, have been identified: 10 in Denmark, four each in Sweden and the United States, three in South Africa, two in Portugal, and one each the United Kingdom, Israel, and Canada. BA 2.86 is a subvariant of Omicron, but according to reports from the CDC, the strain has many more mutations than the ones that came before it.
With so many facts still unknown about this new variant, this news organization asked experts what people need to be aware of as it continues to spread.
What is unique about the BA 2.86 variant?
“It is unique in that it has more than three mutations on the spike protein,” said Purvi S. Parikh, MD, an infectious disease expert at New York University’s Langone Health. The virus uses the spike proteins to enter our cells.
This “may mean it will be more transmissible, cause more severe disease, and/or our vaccines and treatments may not work as well, as compared to other variants,” she said.
What do we need to watch with BA 2.86 going forward?
“We don’t know if this variant will be associated with a change in the disease severity. We currently see increased numbers of cases in general, even though we don’t yet see the BA.2.86 in our system,” said Heba Mostafa, PhD, director of the molecular virology laboratory at Johns Hopkins Hospital in Baltimore.
“It is important to monitor BA.2.86 (and other variants) and understand how its evolution impacts the number of cases and disease outcomes,” she said. “We should all be aware of the current increase in cases, though, and try to get tested and be treated as soon as possible, as antivirals should be effective against the circulating variants.”
What should doctors know?
Dr. Parikh said doctors should generally expect more COVID cases in their clinics and make sure to screen patients even if their symptoms are mild.
“We have tools that can be used – antivirals like Paxlovid are still efficacious with current dominant strains such as EG.5,” she said. “And encourage your patients to get their boosters, mask, wash hands, and social distance.”
How well can our vaccines fight BA 2.86?
“Vaccine coverage for the BA.2.86 is an area of uncertainty right now,” said Dr. Mostafa.
In its report, the CDC said scientists are still figuring out how well the updated COVID vaccine works. It’s expected to be available in the fall, and for now, they believe the new shot will still make infections less severe, new variants and all.
Prior vaccinations and infections have created antibodies in many people, and that will likely provide some protection, Dr. Mostafa said. “When we experienced the Omicron wave in December 2021, even though the variant was distant from what circulated before its emergence and was associated with a very large increase in the number of cases, vaccinations were still protective against severe disease.”
What is the most important thing to keep track of when it comes to this variant?
According to Dr. Parikh, “it’s most important to monitor how transmissible [BA 2.86] is, how severe it is, and if our current treatments and vaccines work.”
Dr. Mostafa said how well the new variants escape existing antibody protection should also be studied and watched closely.
What does this stage of the virus mutation tell us about where we are in the pandemic?
The history of the coronavirus over the past few years shows that variants with many changes evolve and can spread very quickly, Dr. Mostafa said. “Now that the virus is endemic, it is essential to monitor, update vaccinations if necessary, diagnose, treat, and implement infection control measures when necessary.”
With the limited data we have so far, experts seem to agree that while the variant’s makeup raises some red flags, it is too soon to jump to any conclusions about how easy it is to catch it and the ways it may change how the virus impacts those who contract it.
A version of this article first appeared on WebMD.com.

