User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
New antibiotic could combat multidrug-resistant superbugs
Antibiotic resistance is a major public health problem. Few new molecules are in development, but a new antibiotic called clovibactin brings hope.
The drug was discovered and has been studied by scientists from Utrecht University in the Netherlands, the University of Bonn in Germany, the German Center for Infection Research, Northeastern University in Boston, and NovoBiotic Pharmaceuticals in Cambridge, Mass.
Their research was published in Cell.
“Since clovibactin was isolated from bacteria that could not be grown before, pathogenic bacteria have not seen such an antibiotic before and had no time to develop resistance,” Markus Weingarth, MD, PhD, a researcher in Utrecht University’s chemistry department, said in a press release.
Microbial “dark matter”
Researchers isolated clovibactin from sandy soil from North Carolina and studied it using the iChip device, which was developed in 2015. This technique allowed them to grow “bacterial dark matter,” so-called unculturable bacteria, which compose a group to which 99% of bacteria belong.
This device also paved the way for the discovery of the antibiotic teixobactin in 2020. Teixobactin is effective against gram-positive bacteria and is one of the first truly new antibiotics in decades. Its mechanism of action is like that of clovibactin.
Combats resistant bacteria
In the Cell article, the researchers showed that clovibactin acts via several mechanisms and that it successfully treated mice infected with the superbug Staphylococcus aureus.
Clovibactin exhibited antibacterial activity against a broad range of gram-positive pathogens, including methicillin-resistant S. aureus, daptomycin-resistant and vancomycin-resistant S. aureus strains, and difficult-to-treat vancomycin-resistant Enterococcus faecalis and E faecium (vancomycin-resistant enterococci). Escherichia coli was only marginally affected “compared with an outer membrane deficient E. coli WO153 strain, probably reflecting insufficient penetration of the compound,” the authors wrote.
Original mechanism of action
Clovibactin acts not on one but three molecules, all of which are essential to the construction of bacterial walls: C55PP, lipid II, and lipid IIIWTA, which are from different cell wall biosynthetic pathways. Clovibactin binds to the pyrophosphate portion of these precursors.
“Clovibactin wraps around the pyrophosphate like [a] tight glove, like a cage that encloses its target,” said Dr. Weingarth. This is what gives clovibactin its name, which is derived from Greek word klouvi, meaning cage.
The remarkable aspect of clovibactin’s mechanism is that it only binds to the immutable pyrophosphate that is common to cell wall precursors, but it also ignores the variable sugar-peptide part of the targets. The bacteria therefore have a much harder time developing resistance against it. “In fact, we did not observe any resistance to clovibactin in our studies,” Dr. Weingarth confirmed.
Upon binding the target molecules, it self-assembles into large fibrils on the surface of bacterial membranes. These fibrils are stable for a long time and thereby ensure that the target molecules remain sequestered for as long as necessary to kill bacteria.
Few side effects
Because of the mechanism of action of the antibiotic, few side effects are predicted. Indeed, clovibactin targets bacteria cells but not human cells.
“Since these fibrils only form on bacterial membranes and not on human membranes, they are presumably also the reason why clovibactin selectively damages bacterial cells but is not toxic to human cells,” said Dr. Weingarth.
Other studies – in particular, studies in humans – are needed before the antibiotic can be considered a potential treatment. In the meantime, regulations regarding the proper use of antibiotics must continue to be applied to limit antibiotic resistance.
In 2019, 4.95 million deaths worldwide were associated with bacterial antimicrobial resistance, including 1.27 million deaths directly attributable to bacterial antimicrobial resistance. If this trend continues without new medicines becoming available to treat bacterial infections, it is estimated that by 2050, 10 million people will die every year from antimicrobial drug resistance.
This article was translated from the Medscape French Edition. A version appeared on Medscape.com.
Antibiotic resistance is a major public health problem. Few new molecules are in development, but a new antibiotic called clovibactin brings hope.
The drug was discovered and has been studied by scientists from Utrecht University in the Netherlands, the University of Bonn in Germany, the German Center for Infection Research, Northeastern University in Boston, and NovoBiotic Pharmaceuticals in Cambridge, Mass.
Their research was published in Cell.
“Since clovibactin was isolated from bacteria that could not be grown before, pathogenic bacteria have not seen such an antibiotic before and had no time to develop resistance,” Markus Weingarth, MD, PhD, a researcher in Utrecht University’s chemistry department, said in a press release.
Microbial “dark matter”
Researchers isolated clovibactin from sandy soil from North Carolina and studied it using the iChip device, which was developed in 2015. This technique allowed them to grow “bacterial dark matter,” so-called unculturable bacteria, which compose a group to which 99% of bacteria belong.
This device also paved the way for the discovery of the antibiotic teixobactin in 2020. Teixobactin is effective against gram-positive bacteria and is one of the first truly new antibiotics in decades. Its mechanism of action is like that of clovibactin.
Combats resistant bacteria
In the Cell article, the researchers showed that clovibactin acts via several mechanisms and that it successfully treated mice infected with the superbug Staphylococcus aureus.
Clovibactin exhibited antibacterial activity against a broad range of gram-positive pathogens, including methicillin-resistant S. aureus, daptomycin-resistant and vancomycin-resistant S. aureus strains, and difficult-to-treat vancomycin-resistant Enterococcus faecalis and E faecium (vancomycin-resistant enterococci). Escherichia coli was only marginally affected “compared with an outer membrane deficient E. coli WO153 strain, probably reflecting insufficient penetration of the compound,” the authors wrote.
Original mechanism of action
Clovibactin acts not on one but three molecules, all of which are essential to the construction of bacterial walls: C55PP, lipid II, and lipid IIIWTA, which are from different cell wall biosynthetic pathways. Clovibactin binds to the pyrophosphate portion of these precursors.
“Clovibactin wraps around the pyrophosphate like [a] tight glove, like a cage that encloses its target,” said Dr. Weingarth. This is what gives clovibactin its name, which is derived from Greek word klouvi, meaning cage.
The remarkable aspect of clovibactin’s mechanism is that it only binds to the immutable pyrophosphate that is common to cell wall precursors, but it also ignores the variable sugar-peptide part of the targets. The bacteria therefore have a much harder time developing resistance against it. “In fact, we did not observe any resistance to clovibactin in our studies,” Dr. Weingarth confirmed.
Upon binding the target molecules, it self-assembles into large fibrils on the surface of bacterial membranes. These fibrils are stable for a long time and thereby ensure that the target molecules remain sequestered for as long as necessary to kill bacteria.
Few side effects
Because of the mechanism of action of the antibiotic, few side effects are predicted. Indeed, clovibactin targets bacteria cells but not human cells.
“Since these fibrils only form on bacterial membranes and not on human membranes, they are presumably also the reason why clovibactin selectively damages bacterial cells but is not toxic to human cells,” said Dr. Weingarth.
Other studies – in particular, studies in humans – are needed before the antibiotic can be considered a potential treatment. In the meantime, regulations regarding the proper use of antibiotics must continue to be applied to limit antibiotic resistance.
In 2019, 4.95 million deaths worldwide were associated with bacterial antimicrobial resistance, including 1.27 million deaths directly attributable to bacterial antimicrobial resistance. If this trend continues without new medicines becoming available to treat bacterial infections, it is estimated that by 2050, 10 million people will die every year from antimicrobial drug resistance.
This article was translated from the Medscape French Edition. A version appeared on Medscape.com.
Antibiotic resistance is a major public health problem. Few new molecules are in development, but a new antibiotic called clovibactin brings hope.
The drug was discovered and has been studied by scientists from Utrecht University in the Netherlands, the University of Bonn in Germany, the German Center for Infection Research, Northeastern University in Boston, and NovoBiotic Pharmaceuticals in Cambridge, Mass.
Their research was published in Cell.
“Since clovibactin was isolated from bacteria that could not be grown before, pathogenic bacteria have not seen such an antibiotic before and had no time to develop resistance,” Markus Weingarth, MD, PhD, a researcher in Utrecht University’s chemistry department, said in a press release.
Microbial “dark matter”
Researchers isolated clovibactin from sandy soil from North Carolina and studied it using the iChip device, which was developed in 2015. This technique allowed them to grow “bacterial dark matter,” so-called unculturable bacteria, which compose a group to which 99% of bacteria belong.
This device also paved the way for the discovery of the antibiotic teixobactin in 2020. Teixobactin is effective against gram-positive bacteria and is one of the first truly new antibiotics in decades. Its mechanism of action is like that of clovibactin.
Combats resistant bacteria
In the Cell article, the researchers showed that clovibactin acts via several mechanisms and that it successfully treated mice infected with the superbug Staphylococcus aureus.
Clovibactin exhibited antibacterial activity against a broad range of gram-positive pathogens, including methicillin-resistant S. aureus, daptomycin-resistant and vancomycin-resistant S. aureus strains, and difficult-to-treat vancomycin-resistant Enterococcus faecalis and E faecium (vancomycin-resistant enterococci). Escherichia coli was only marginally affected “compared with an outer membrane deficient E. coli WO153 strain, probably reflecting insufficient penetration of the compound,” the authors wrote.
Original mechanism of action
Clovibactin acts not on one but three molecules, all of which are essential to the construction of bacterial walls: C55PP, lipid II, and lipid IIIWTA, which are from different cell wall biosynthetic pathways. Clovibactin binds to the pyrophosphate portion of these precursors.
“Clovibactin wraps around the pyrophosphate like [a] tight glove, like a cage that encloses its target,” said Dr. Weingarth. This is what gives clovibactin its name, which is derived from Greek word klouvi, meaning cage.
The remarkable aspect of clovibactin’s mechanism is that it only binds to the immutable pyrophosphate that is common to cell wall precursors, but it also ignores the variable sugar-peptide part of the targets. The bacteria therefore have a much harder time developing resistance against it. “In fact, we did not observe any resistance to clovibactin in our studies,” Dr. Weingarth confirmed.
Upon binding the target molecules, it self-assembles into large fibrils on the surface of bacterial membranes. These fibrils are stable for a long time and thereby ensure that the target molecules remain sequestered for as long as necessary to kill bacteria.
Few side effects
Because of the mechanism of action of the antibiotic, few side effects are predicted. Indeed, clovibactin targets bacteria cells but not human cells.
“Since these fibrils only form on bacterial membranes and not on human membranes, they are presumably also the reason why clovibactin selectively damages bacterial cells but is not toxic to human cells,” said Dr. Weingarth.
Other studies – in particular, studies in humans – are needed before the antibiotic can be considered a potential treatment. In the meantime, regulations regarding the proper use of antibiotics must continue to be applied to limit antibiotic resistance.
In 2019, 4.95 million deaths worldwide were associated with bacterial antimicrobial resistance, including 1.27 million deaths directly attributable to bacterial antimicrobial resistance. If this trend continues without new medicines becoming available to treat bacterial infections, it is estimated that by 2050, 10 million people will die every year from antimicrobial drug resistance.
This article was translated from the Medscape French Edition. A version appeared on Medscape.com.
FROM CELL
Antigen tests: After pandemic success, time for bigger role?
Before the pandemic, most of the public probably had a fleeting and limited familiarity with lateral flow tests (LFTs), also known as rapid antigen tests. Perhaps they used, or awaited the results of, a lateral flow home pregnancy test, which detects human chorionic gonadotropin in urine.
Then came COVID-19, and the need for large-scale testing. By late 2022, more than 3 billion tests for SARS-CoV-2 had been done worldwide. Although testing with reverse-transcription polymerase chain reaction (PCR) is the gold standard for diagnosing COVID, LFTs made possible large-scale testing at low cost with rapid results.
As of Sept. 12, the Food and Drug Administration lists 32 rapid antigen tests with emergency use authorizations (EUAs) for home use.
Now, many experts conclude, it’s time to expand the role of LFTs so the technology can help detect a host of other diseases. In a Nature Reviews bioengineering report, global experts from the United States, the United Kingdom, and other countries pointed out that commercial LFTs are currently not available for four of the eight known priority diseases of epidemic potential: Crimean-Congo hemorrhagic fever, Middle East respiratory syndrome coronavirus, Nipah and other henipaviruses, and Rift Valley fever.
Expansion should not only include more tests for more diseases, some experts say, but also make use of existing technology to provide “full-circle” care. After a rapid test, for instance, users could download a mobile phone app, transmit the results to their health care provider, and then set up an appointment if needed or get a prescribed medication at the pharmacy.
Medical community on board
Clinicians support increased availability of LFTs, said Eric J. Topol, MD, professor and executive vice president of Scripps Research, La Jolla, Calif.“Rapid antigen tests are critical, made a big difference in the pandemic, and will be used increasingly for many other applications in the years ahead,” Dr. Topol said in an email.
Physicians welcome their potential, agreed William Schaffner, MD, professor of preventive medicine and infectious disease specialist at Vanderbilt University Medical Center, Nashville, Tenn. At the start of the pandemic, when he was briefed about a lateral flow device in development, he said, “I was blown away by the technology, ease of use, rapidity of getting a result, its reasonable accuracy and its anticipated relatively low price.”
Clinicians would probably see many advantages to having more LFTs for more diseases, Dr. Schaffner said, because they are of use not only at home but also in doctors’ offices and in emergency departments. Their increased use “would help [people] make quick decisions about treatment, especially for flu and COVID.”
How LFTs work
LFTs are capable of targeting antigens, such as for the COVID tests, and antibodies such as IgG or IgM. The tests are also capable of detecting nucleic acids, although the availability of these tests is currently rare.
First, a sample from blood, urine, saliva or other bodily sources is placed onto a sample pad. It travels to a conjugate pad containing antibodies. If the target being looked for is present, the target and antibodies bind and, as the sample moves along to the test line, produces a positive result line along with the control line (to show that the test worked).
Global market outlook
By 2030, the lateral flow assays market is predicted to rise to $14.1 billion, according to a report issued in September by the firm Research and Markets. In 2022, the market was estimated at $9.4 billion, with $3.6 billion of that in the United States.
The report details the performances of 55 major competitors, such as Abbott Laboratories, Siemens, and QuidelOrtho, but smaller companies and start-ups are also involved in LFT development.
LFTs: Pros and cons
Although LFTs give rapid results, their accuracy is lower than that of PCR, especially the sensitivity. For COVID antigen LFTs, the sensitivity ranges from 34.1% to 88.1%, with an overall specificity of 99.6%, according to a Cochrane Review report. The analytical sensitivity performance of PCR testing for COVID is near 100%.
Everyone acknowledges the accuracy challenge of LFTs. The technologies “are generally thought to have limitations of detection that for some applications may present a challenge,” said Douglas C. Bryant, president and CEO of QuidelOrtho, San Diego, which counts the QuickVue rapid test for COVID detection among its products.
However, Mr. Bryant added, “as we saw during the pandemic, there was a place for more sensitive PCR-based technologies that are often run in a lab and there was a place for the use of rapid tests: The key is knowing the strengths and best use cases when applying the different technologies.”
One strength, he said, was that the tests “were shown to be highly effective at detecting active, infectious cases of SARS-CoV-2 and the rapid turnaround time allowed patients to isolate themselves from others quickly to help curb the spread of infection to others.” Another advantage was the ability to screen high-risk populations such as nursing homes to detect positive cases and help prevent outbreaks.
The pandemic familiarized people with the tests, said Jeremy Stackawitz, CEO of Senzo, a start-up in vitro diagnostics company developing an amplified LFT platform for rapid tests for flu, tuberculosis, COVID, and Clostridioides difficile. People liked using them. Physicians generally accepted them. It works great with tele-doc. It works great with personalized medicine.
Now, he said, people used to the COVID self-tests are asking: “Where is my strep test? Where is my sexual health test?”
FDA’s perspective on LFTs
The FDA has no one-size-fits-all standard for evaluating LFTs.
“LFTs are evaluated with respect to their individual indications and the pathway under which they are being reviewed,” said James McKinney, an FDA spokesperson. “A performance recommendation for one type of lateral flow test may not be appropriate for another.”
EUAs, such as those given for the COVID at-home tests, require different levels of evidence than traditional premarket review, he said, whether de novo marketing authorization, 510(k) premarket notification, or premarket approval. The EUAs are evaluated with a risk-benefit analysis to speed up the time it takes to make the devices available.
And, Mr. McKinney said, for some devices, the FDA provides recommendations on the expected performance through guidance documents. For instance, for rapid devices developed to detect influenza A virus antigen, the FDA recommends including enough sample to generate sensitivity of greater than 60% and testing at least 50 samples.
LFTs: The potential, the challenges
Mr. Stackawitz predicted that, as more LFT self-tests become available, more people will seek care, just as they did with the COVID rapid tests. A 22-year-old who thinks he has chlamydia may balk at going to a doctor right away. However, “if he can go buy a soda and a test at CVS, it’s different, it really is. With a little anonymity, people will seek care.”
He has a vision shared by other experts: That testing technology will evolve so that after getting the results at home, people would follow through by sending those results to their health care provider and obtaining needed care or medication. In his opinion, this is superior to the traditional way, which often involves visiting a doctor with symptoms, going for tests, waiting for results, and then beginning treatment.
“It would make more sense if you came in knowing your results,” Mr. Stackawitz said. “It’s a much smarter pathway, gives better outcomes for the patient, is much quicker and at much less cost. And it frees up time for doctors. I think most physicians would embrace that.”
Although rapid testing is gaining well-deserved recognition, funding is an issue, according to the Nature Reviews report. Those experts warned that “a reduction in funding for LFT research post COVID-19 may hamper efforts to capitalize on gains in decentralized testing, especially self-testing, which may be critical to address future pandemic threats.”
A version of this article first appeared on Medscape.com.
Before the pandemic, most of the public probably had a fleeting and limited familiarity with lateral flow tests (LFTs), also known as rapid antigen tests. Perhaps they used, or awaited the results of, a lateral flow home pregnancy test, which detects human chorionic gonadotropin in urine.
Then came COVID-19, and the need for large-scale testing. By late 2022, more than 3 billion tests for SARS-CoV-2 had been done worldwide. Although testing with reverse-transcription polymerase chain reaction (PCR) is the gold standard for diagnosing COVID, LFTs made possible large-scale testing at low cost with rapid results.
As of Sept. 12, the Food and Drug Administration lists 32 rapid antigen tests with emergency use authorizations (EUAs) for home use.
Now, many experts conclude, it’s time to expand the role of LFTs so the technology can help detect a host of other diseases. In a Nature Reviews bioengineering report, global experts from the United States, the United Kingdom, and other countries pointed out that commercial LFTs are currently not available for four of the eight known priority diseases of epidemic potential: Crimean-Congo hemorrhagic fever, Middle East respiratory syndrome coronavirus, Nipah and other henipaviruses, and Rift Valley fever.
Expansion should not only include more tests for more diseases, some experts say, but also make use of existing technology to provide “full-circle” care. After a rapid test, for instance, users could download a mobile phone app, transmit the results to their health care provider, and then set up an appointment if needed or get a prescribed medication at the pharmacy.
Medical community on board
Clinicians support increased availability of LFTs, said Eric J. Topol, MD, professor and executive vice president of Scripps Research, La Jolla, Calif.“Rapid antigen tests are critical, made a big difference in the pandemic, and will be used increasingly for many other applications in the years ahead,” Dr. Topol said in an email.
Physicians welcome their potential, agreed William Schaffner, MD, professor of preventive medicine and infectious disease specialist at Vanderbilt University Medical Center, Nashville, Tenn. At the start of the pandemic, when he was briefed about a lateral flow device in development, he said, “I was blown away by the technology, ease of use, rapidity of getting a result, its reasonable accuracy and its anticipated relatively low price.”
Clinicians would probably see many advantages to having more LFTs for more diseases, Dr. Schaffner said, because they are of use not only at home but also in doctors’ offices and in emergency departments. Their increased use “would help [people] make quick decisions about treatment, especially for flu and COVID.”
How LFTs work
LFTs are capable of targeting antigens, such as for the COVID tests, and antibodies such as IgG or IgM. The tests are also capable of detecting nucleic acids, although the availability of these tests is currently rare.
First, a sample from blood, urine, saliva or other bodily sources is placed onto a sample pad. It travels to a conjugate pad containing antibodies. If the target being looked for is present, the target and antibodies bind and, as the sample moves along to the test line, produces a positive result line along with the control line (to show that the test worked).
Global market outlook
By 2030, the lateral flow assays market is predicted to rise to $14.1 billion, according to a report issued in September by the firm Research and Markets. In 2022, the market was estimated at $9.4 billion, with $3.6 billion of that in the United States.
The report details the performances of 55 major competitors, such as Abbott Laboratories, Siemens, and QuidelOrtho, but smaller companies and start-ups are also involved in LFT development.
LFTs: Pros and cons
Although LFTs give rapid results, their accuracy is lower than that of PCR, especially the sensitivity. For COVID antigen LFTs, the sensitivity ranges from 34.1% to 88.1%, with an overall specificity of 99.6%, according to a Cochrane Review report. The analytical sensitivity performance of PCR testing for COVID is near 100%.
Everyone acknowledges the accuracy challenge of LFTs. The technologies “are generally thought to have limitations of detection that for some applications may present a challenge,” said Douglas C. Bryant, president and CEO of QuidelOrtho, San Diego, which counts the QuickVue rapid test for COVID detection among its products.
However, Mr. Bryant added, “as we saw during the pandemic, there was a place for more sensitive PCR-based technologies that are often run in a lab and there was a place for the use of rapid tests: The key is knowing the strengths and best use cases when applying the different technologies.”
One strength, he said, was that the tests “were shown to be highly effective at detecting active, infectious cases of SARS-CoV-2 and the rapid turnaround time allowed patients to isolate themselves from others quickly to help curb the spread of infection to others.” Another advantage was the ability to screen high-risk populations such as nursing homes to detect positive cases and help prevent outbreaks.
The pandemic familiarized people with the tests, said Jeremy Stackawitz, CEO of Senzo, a start-up in vitro diagnostics company developing an amplified LFT platform for rapid tests for flu, tuberculosis, COVID, and Clostridioides difficile. People liked using them. Physicians generally accepted them. It works great with tele-doc. It works great with personalized medicine.
Now, he said, people used to the COVID self-tests are asking: “Where is my strep test? Where is my sexual health test?”
FDA’s perspective on LFTs
The FDA has no one-size-fits-all standard for evaluating LFTs.
“LFTs are evaluated with respect to their individual indications and the pathway under which they are being reviewed,” said James McKinney, an FDA spokesperson. “A performance recommendation for one type of lateral flow test may not be appropriate for another.”
EUAs, such as those given for the COVID at-home tests, require different levels of evidence than traditional premarket review, he said, whether de novo marketing authorization, 510(k) premarket notification, or premarket approval. The EUAs are evaluated with a risk-benefit analysis to speed up the time it takes to make the devices available.
And, Mr. McKinney said, for some devices, the FDA provides recommendations on the expected performance through guidance documents. For instance, for rapid devices developed to detect influenza A virus antigen, the FDA recommends including enough sample to generate sensitivity of greater than 60% and testing at least 50 samples.
LFTs: The potential, the challenges
Mr. Stackawitz predicted that, as more LFT self-tests become available, more people will seek care, just as they did with the COVID rapid tests. A 22-year-old who thinks he has chlamydia may balk at going to a doctor right away. However, “if he can go buy a soda and a test at CVS, it’s different, it really is. With a little anonymity, people will seek care.”
He has a vision shared by other experts: That testing technology will evolve so that after getting the results at home, people would follow through by sending those results to their health care provider and obtaining needed care or medication. In his opinion, this is superior to the traditional way, which often involves visiting a doctor with symptoms, going for tests, waiting for results, and then beginning treatment.
“It would make more sense if you came in knowing your results,” Mr. Stackawitz said. “It’s a much smarter pathway, gives better outcomes for the patient, is much quicker and at much less cost. And it frees up time for doctors. I think most physicians would embrace that.”
Although rapid testing is gaining well-deserved recognition, funding is an issue, according to the Nature Reviews report. Those experts warned that “a reduction in funding for LFT research post COVID-19 may hamper efforts to capitalize on gains in decentralized testing, especially self-testing, which may be critical to address future pandemic threats.”
A version of this article first appeared on Medscape.com.
Before the pandemic, most of the public probably had a fleeting and limited familiarity with lateral flow tests (LFTs), also known as rapid antigen tests. Perhaps they used, or awaited the results of, a lateral flow home pregnancy test, which detects human chorionic gonadotropin in urine.
Then came COVID-19, and the need for large-scale testing. By late 2022, more than 3 billion tests for SARS-CoV-2 had been done worldwide. Although testing with reverse-transcription polymerase chain reaction (PCR) is the gold standard for diagnosing COVID, LFTs made possible large-scale testing at low cost with rapid results.
As of Sept. 12, the Food and Drug Administration lists 32 rapid antigen tests with emergency use authorizations (EUAs) for home use.
Now, many experts conclude, it’s time to expand the role of LFTs so the technology can help detect a host of other diseases. In a Nature Reviews bioengineering report, global experts from the United States, the United Kingdom, and other countries pointed out that commercial LFTs are currently not available for four of the eight known priority diseases of epidemic potential: Crimean-Congo hemorrhagic fever, Middle East respiratory syndrome coronavirus, Nipah and other henipaviruses, and Rift Valley fever.
Expansion should not only include more tests for more diseases, some experts say, but also make use of existing technology to provide “full-circle” care. After a rapid test, for instance, users could download a mobile phone app, transmit the results to their health care provider, and then set up an appointment if needed or get a prescribed medication at the pharmacy.
Medical community on board
Clinicians support increased availability of LFTs, said Eric J. Topol, MD, professor and executive vice president of Scripps Research, La Jolla, Calif.“Rapid antigen tests are critical, made a big difference in the pandemic, and will be used increasingly for many other applications in the years ahead,” Dr. Topol said in an email.
Physicians welcome their potential, agreed William Schaffner, MD, professor of preventive medicine and infectious disease specialist at Vanderbilt University Medical Center, Nashville, Tenn. At the start of the pandemic, when he was briefed about a lateral flow device in development, he said, “I was blown away by the technology, ease of use, rapidity of getting a result, its reasonable accuracy and its anticipated relatively low price.”
Clinicians would probably see many advantages to having more LFTs for more diseases, Dr. Schaffner said, because they are of use not only at home but also in doctors’ offices and in emergency departments. Their increased use “would help [people] make quick decisions about treatment, especially for flu and COVID.”
How LFTs work
LFTs are capable of targeting antigens, such as for the COVID tests, and antibodies such as IgG or IgM. The tests are also capable of detecting nucleic acids, although the availability of these tests is currently rare.
First, a sample from blood, urine, saliva or other bodily sources is placed onto a sample pad. It travels to a conjugate pad containing antibodies. If the target being looked for is present, the target and antibodies bind and, as the sample moves along to the test line, produces a positive result line along with the control line (to show that the test worked).
Global market outlook
By 2030, the lateral flow assays market is predicted to rise to $14.1 billion, according to a report issued in September by the firm Research and Markets. In 2022, the market was estimated at $9.4 billion, with $3.6 billion of that in the United States.
The report details the performances of 55 major competitors, such as Abbott Laboratories, Siemens, and QuidelOrtho, but smaller companies and start-ups are also involved in LFT development.
LFTs: Pros and cons
Although LFTs give rapid results, their accuracy is lower than that of PCR, especially the sensitivity. For COVID antigen LFTs, the sensitivity ranges from 34.1% to 88.1%, with an overall specificity of 99.6%, according to a Cochrane Review report. The analytical sensitivity performance of PCR testing for COVID is near 100%.
Everyone acknowledges the accuracy challenge of LFTs. The technologies “are generally thought to have limitations of detection that for some applications may present a challenge,” said Douglas C. Bryant, president and CEO of QuidelOrtho, San Diego, which counts the QuickVue rapid test for COVID detection among its products.
However, Mr. Bryant added, “as we saw during the pandemic, there was a place for more sensitive PCR-based technologies that are often run in a lab and there was a place for the use of rapid tests: The key is knowing the strengths and best use cases when applying the different technologies.”
One strength, he said, was that the tests “were shown to be highly effective at detecting active, infectious cases of SARS-CoV-2 and the rapid turnaround time allowed patients to isolate themselves from others quickly to help curb the spread of infection to others.” Another advantage was the ability to screen high-risk populations such as nursing homes to detect positive cases and help prevent outbreaks.
The pandemic familiarized people with the tests, said Jeremy Stackawitz, CEO of Senzo, a start-up in vitro diagnostics company developing an amplified LFT platform for rapid tests for flu, tuberculosis, COVID, and Clostridioides difficile. People liked using them. Physicians generally accepted them. It works great with tele-doc. It works great with personalized medicine.
Now, he said, people used to the COVID self-tests are asking: “Where is my strep test? Where is my sexual health test?”
FDA’s perspective on LFTs
The FDA has no one-size-fits-all standard for evaluating LFTs.
“LFTs are evaluated with respect to their individual indications and the pathway under which they are being reviewed,” said James McKinney, an FDA spokesperson. “A performance recommendation for one type of lateral flow test may not be appropriate for another.”
EUAs, such as those given for the COVID at-home tests, require different levels of evidence than traditional premarket review, he said, whether de novo marketing authorization, 510(k) premarket notification, or premarket approval. The EUAs are evaluated with a risk-benefit analysis to speed up the time it takes to make the devices available.
And, Mr. McKinney said, for some devices, the FDA provides recommendations on the expected performance through guidance documents. For instance, for rapid devices developed to detect influenza A virus antigen, the FDA recommends including enough sample to generate sensitivity of greater than 60% and testing at least 50 samples.
LFTs: The potential, the challenges
Mr. Stackawitz predicted that, as more LFT self-tests become available, more people will seek care, just as they did with the COVID rapid tests. A 22-year-old who thinks he has chlamydia may balk at going to a doctor right away. However, “if he can go buy a soda and a test at CVS, it’s different, it really is. With a little anonymity, people will seek care.”
He has a vision shared by other experts: That testing technology will evolve so that after getting the results at home, people would follow through by sending those results to their health care provider and obtaining needed care or medication. In his opinion, this is superior to the traditional way, which often involves visiting a doctor with symptoms, going for tests, waiting for results, and then beginning treatment.
“It would make more sense if you came in knowing your results,” Mr. Stackawitz said. “It’s a much smarter pathway, gives better outcomes for the patient, is much quicker and at much less cost. And it frees up time for doctors. I think most physicians would embrace that.”
Although rapid testing is gaining well-deserved recognition, funding is an issue, according to the Nature Reviews report. Those experts warned that “a reduction in funding for LFT research post COVID-19 may hamper efforts to capitalize on gains in decentralized testing, especially self-testing, which may be critical to address future pandemic threats.”
A version of this article first appeared on Medscape.com.
Artificial intelligence in your office
It is difficult to go through any publication or website these days without finding an article about artificial intelligence (AI). Many discuss its current status, while others speculate on potential future applications. Often, AI is described as an “existential threat to human health” by commentators who aren’t even aware of the definition of that term as Kierkegaard conceived it, the role of the individual to breathe meaning into life. Others characterize such cataclysmic predictions as “overblown and misdirected”.
The long-term potential for abuse of AI requires discussion, and should be addressed by policy makers, but that is beyond the scope of this column.
Meanwhile, with no “existential” threat to anybody.
The most popular current AI-based medical applications are automated scribes. They transcribe live consultations between physician and patient automatically and create a searchable report, plus notes for charts and billing.
I’ve written about AI scribes before, but the quality and user-friendliness of these products have improved dramatically in recent years. Language processing capabilities now permit you to speak naturally, without having to memorize specific commands. Some scribes can mimic your writing style based on sample notes that you enter into the system. Others allow you to integrate your own knowledge base, or a bibliography of research studies. With some systems, you can dictate notes directly into most EHR software, ask questions regarding medication dosages, or access a patient’s medical history from hospitals or other offices.
Current popular medical scribe products include DeepCura, DeepScribe, Nuance, Suki, Augmedix, Tali AI, Iodine Software, and ScribeLink. Amazon Web Services recently launched its own product, HealthScribe, as well. (As always, I have no financial interest in any product or service mentioned in this column.)
AI scribes aren’t entirely autonomous, of course; you need to read the output and check for potential inaccuracies. Still, users claim that they substantially reduce documentation and charting time, permitting more patient visits and less after-hours work.
AI can also be used to provide useful content for your patients. If you are not particularly good at writing, or don’t have the time for it, generative algorithms like the much-vaunted ChatGPT can generate posts, FAQs, and other informational content for your website, blog, or social media pages. You can ask for ideas about timely health topics and write general information articles, or create content specific to your location or specialty. You can use it to write emails informing your patients about upcoming office events or educate them on a range of topics, from getting their annual flu shots to scheduling regular screening skin exams.
With some of the same techniques and additional software, you can create entire videos for your website at a fraction of the cost of hiring a video production team. After using ChatGPT to write the content – for example, a 5-minute script on the importance of sunscreen in preventing skin cancer – you can employ a text-to-speech algorithm such as Revoicer to transform the script into audio content, and then a preproduction algorithm like Yepic or Synthesia to generate a video with a synthetic human.
If you are unhappy with your current online presence, you can use AI to create an entire website. Through a series of questions, AI website builders such as Wix ADI, Jimdo, Hostinger, and 10Web gather all the information needed to set up a website draft that is already personalized with medical-specific content. Most offer the option to connect to Instagram, Facebook, Google My Business, and similar sites, to which they can import your office’s logo, images, and descriptive texts.
Some of them are capable of pulling up responsive site pages that automatically adjust to the device – mobile or computer – that the visitor is using. This is important, as I’ve written before, because more than half of all searches for doctors are now made on smartphones, so the more “mobile friendly” your site is, the higher it will be ranked. You can test how easily a visitor can use your website on a mobile device with Google’s free Mobile-Friendly Test.
If you give talks at medical meetings, you know how cumbersome and time-consuming it can be to create Powerpoint presentations. Once again, AI can save you time and trouble. Presentation designers such as Presentations.AI, Deck Robot, iA Presenter, and Beautiful.AI can assemble very acceptable presentations from your primary inputs. You typically choose a template, input your basic data, and AI will format the slides and offer you visuals, animations, voice-overs, and other fancy features. You will also have flexibility in changing segments or images or sizes you don’t like.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
It is difficult to go through any publication or website these days without finding an article about artificial intelligence (AI). Many discuss its current status, while others speculate on potential future applications. Often, AI is described as an “existential threat to human health” by commentators who aren’t even aware of the definition of that term as Kierkegaard conceived it, the role of the individual to breathe meaning into life. Others characterize such cataclysmic predictions as “overblown and misdirected”.
The long-term potential for abuse of AI requires discussion, and should be addressed by policy makers, but that is beyond the scope of this column.
Meanwhile, with no “existential” threat to anybody.
The most popular current AI-based medical applications are automated scribes. They transcribe live consultations between physician and patient automatically and create a searchable report, plus notes for charts and billing.
I’ve written about AI scribes before, but the quality and user-friendliness of these products have improved dramatically in recent years. Language processing capabilities now permit you to speak naturally, without having to memorize specific commands. Some scribes can mimic your writing style based on sample notes that you enter into the system. Others allow you to integrate your own knowledge base, or a bibliography of research studies. With some systems, you can dictate notes directly into most EHR software, ask questions regarding medication dosages, or access a patient’s medical history from hospitals or other offices.
Current popular medical scribe products include DeepCura, DeepScribe, Nuance, Suki, Augmedix, Tali AI, Iodine Software, and ScribeLink. Amazon Web Services recently launched its own product, HealthScribe, as well. (As always, I have no financial interest in any product or service mentioned in this column.)
AI scribes aren’t entirely autonomous, of course; you need to read the output and check for potential inaccuracies. Still, users claim that they substantially reduce documentation and charting time, permitting more patient visits and less after-hours work.
AI can also be used to provide useful content for your patients. If you are not particularly good at writing, or don’t have the time for it, generative algorithms like the much-vaunted ChatGPT can generate posts, FAQs, and other informational content for your website, blog, or social media pages. You can ask for ideas about timely health topics and write general information articles, or create content specific to your location or specialty. You can use it to write emails informing your patients about upcoming office events or educate them on a range of topics, from getting their annual flu shots to scheduling regular screening skin exams.
With some of the same techniques and additional software, you can create entire videos for your website at a fraction of the cost of hiring a video production team. After using ChatGPT to write the content – for example, a 5-minute script on the importance of sunscreen in preventing skin cancer – you can employ a text-to-speech algorithm such as Revoicer to transform the script into audio content, and then a preproduction algorithm like Yepic or Synthesia to generate a video with a synthetic human.
If you are unhappy with your current online presence, you can use AI to create an entire website. Through a series of questions, AI website builders such as Wix ADI, Jimdo, Hostinger, and 10Web gather all the information needed to set up a website draft that is already personalized with medical-specific content. Most offer the option to connect to Instagram, Facebook, Google My Business, and similar sites, to which they can import your office’s logo, images, and descriptive texts.
Some of them are capable of pulling up responsive site pages that automatically adjust to the device – mobile or computer – that the visitor is using. This is important, as I’ve written before, because more than half of all searches for doctors are now made on smartphones, so the more “mobile friendly” your site is, the higher it will be ranked. You can test how easily a visitor can use your website on a mobile device with Google’s free Mobile-Friendly Test.
If you give talks at medical meetings, you know how cumbersome and time-consuming it can be to create Powerpoint presentations. Once again, AI can save you time and trouble. Presentation designers such as Presentations.AI, Deck Robot, iA Presenter, and Beautiful.AI can assemble very acceptable presentations from your primary inputs. You typically choose a template, input your basic data, and AI will format the slides and offer you visuals, animations, voice-overs, and other fancy features. You will also have flexibility in changing segments or images or sizes you don’t like.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
It is difficult to go through any publication or website these days without finding an article about artificial intelligence (AI). Many discuss its current status, while others speculate on potential future applications. Often, AI is described as an “existential threat to human health” by commentators who aren’t even aware of the definition of that term as Kierkegaard conceived it, the role of the individual to breathe meaning into life. Others characterize such cataclysmic predictions as “overblown and misdirected”.
The long-term potential for abuse of AI requires discussion, and should be addressed by policy makers, but that is beyond the scope of this column.
Meanwhile, with no “existential” threat to anybody.
The most popular current AI-based medical applications are automated scribes. They transcribe live consultations between physician and patient automatically and create a searchable report, plus notes for charts and billing.
I’ve written about AI scribes before, but the quality and user-friendliness of these products have improved dramatically in recent years. Language processing capabilities now permit you to speak naturally, without having to memorize specific commands. Some scribes can mimic your writing style based on sample notes that you enter into the system. Others allow you to integrate your own knowledge base, or a bibliography of research studies. With some systems, you can dictate notes directly into most EHR software, ask questions regarding medication dosages, or access a patient’s medical history from hospitals or other offices.
Current popular medical scribe products include DeepCura, DeepScribe, Nuance, Suki, Augmedix, Tali AI, Iodine Software, and ScribeLink. Amazon Web Services recently launched its own product, HealthScribe, as well. (As always, I have no financial interest in any product or service mentioned in this column.)
AI scribes aren’t entirely autonomous, of course; you need to read the output and check for potential inaccuracies. Still, users claim that they substantially reduce documentation and charting time, permitting more patient visits and less after-hours work.
AI can also be used to provide useful content for your patients. If you are not particularly good at writing, or don’t have the time for it, generative algorithms like the much-vaunted ChatGPT can generate posts, FAQs, and other informational content for your website, blog, or social media pages. You can ask for ideas about timely health topics and write general information articles, or create content specific to your location or specialty. You can use it to write emails informing your patients about upcoming office events or educate them on a range of topics, from getting their annual flu shots to scheduling regular screening skin exams.
With some of the same techniques and additional software, you can create entire videos for your website at a fraction of the cost of hiring a video production team. After using ChatGPT to write the content – for example, a 5-minute script on the importance of sunscreen in preventing skin cancer – you can employ a text-to-speech algorithm such as Revoicer to transform the script into audio content, and then a preproduction algorithm like Yepic or Synthesia to generate a video with a synthetic human.
If you are unhappy with your current online presence, you can use AI to create an entire website. Through a series of questions, AI website builders such as Wix ADI, Jimdo, Hostinger, and 10Web gather all the information needed to set up a website draft that is already personalized with medical-specific content. Most offer the option to connect to Instagram, Facebook, Google My Business, and similar sites, to which they can import your office’s logo, images, and descriptive texts.
Some of them are capable of pulling up responsive site pages that automatically adjust to the device – mobile or computer – that the visitor is using. This is important, as I’ve written before, because more than half of all searches for doctors are now made on smartphones, so the more “mobile friendly” your site is, the higher it will be ranked. You can test how easily a visitor can use your website on a mobile device with Google’s free Mobile-Friendly Test.
If you give talks at medical meetings, you know how cumbersome and time-consuming it can be to create Powerpoint presentations. Once again, AI can save you time and trouble. Presentation designers such as Presentations.AI, Deck Robot, iA Presenter, and Beautiful.AI can assemble very acceptable presentations from your primary inputs. You typically choose a template, input your basic data, and AI will format the slides and offer you visuals, animations, voice-overs, and other fancy features. You will also have flexibility in changing segments or images or sizes you don’t like.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
COVID booster may transiently raise glucose levels in T1D
TOPLINE:
METHODOLOGY:
- In a single-center prospective cohort study of 21 adults with type 1 diabetes, patients were given a blinded Dexcom G6 Pro continuous glucose monitor (CGM) at the first research clinic visit.
- After 3-4 days, participants received a COVID-19 booster vaccine.
- They returned to the clinic 10 days after the initial visit (5-6 days after booster vaccination) to have the CGM removed and glycemia assessed.
TAKEAWAY:
- Compared with baseline, the mean daily glucose level was significantly increased at day 2 (162.9 mg/dL vs. 172.8 mg/dL; P = .04) and day 3 (173.1 mg/dL; P = .02) post vaccination.
- Glucose excursions at day 0 (173.2 mg/dL; P = .058) and day 1 (173.1 mg/dL; P = .078) didn’t quite reach statistical significance.
- One participant experienced increases in glucose of 36%, 69%, 35%, 26%, 22%, and 19% on days 0-5, respectively, compared with baseline.
- Glucose excursions of at least 25% above baseline occurred in four participants on day 0 and day 1 and in three participants on days 2 and 5.
- Insulin resistance, as measured by Total Daily Insulin Resistance (a metric that integrates daily mean glucose concentration with total daily insulin dose), was also significantly increased from baseline to day 2 post vaccination (7,171 mg/dL vs. 8,070 mg/dL units; P = .03).
- No other measures of glycemia differed significantly, compared with baseline.
- Outcomes didn’t differ significantly by sex, age, or vaccine manufacturer.
IN PRACTICE:
- “To our knowledge this is the first study investigating the effect of the COVID-19 booster vaccine on glycemia specifically in people with type 1 diabetes,” say the authors.
- “Clinicians, pharmacists, and other health care providers may need to counsel people with T1D to be more vigilant with glucose testing and insulin dosing for the first 5 days after vaccination. Most importantly, insulin, required to control glycemia, may need to be transiently increased.”
- “Further studies are warranted to investigate whether other vaccines have similar glycemic effects, and which individuals are at highest risk for profound glucose perturbations post vaccination.”
SOURCE:
The study was conducted by Mihail Zilbermint, MD, of the division of hospital medicine, Johns Hopkins Medicine, Bethesda, Md., and colleagues. It was published in Diabetes Research and Clinical Practice.
LIMITATIONS:
- The sample size was small.
- There were no measurements of inflammatory markers, dietary intake, physical activity, or survey patient symptomatology to adjust for variables that may have influenced glycemic control.
- In the study cohort, glycemia was moderately well controlled at baseline.
DISCLOSURES:
The study was supported by an investigator-initiated study grant from DexCom Inc. Dr. Zilbermint has consulted for EMD Serono.
A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- In a single-center prospective cohort study of 21 adults with type 1 diabetes, patients were given a blinded Dexcom G6 Pro continuous glucose monitor (CGM) at the first research clinic visit.
- After 3-4 days, participants received a COVID-19 booster vaccine.
- They returned to the clinic 10 days after the initial visit (5-6 days after booster vaccination) to have the CGM removed and glycemia assessed.
TAKEAWAY:
- Compared with baseline, the mean daily glucose level was significantly increased at day 2 (162.9 mg/dL vs. 172.8 mg/dL; P = .04) and day 3 (173.1 mg/dL; P = .02) post vaccination.
- Glucose excursions at day 0 (173.2 mg/dL; P = .058) and day 1 (173.1 mg/dL; P = .078) didn’t quite reach statistical significance.
- One participant experienced increases in glucose of 36%, 69%, 35%, 26%, 22%, and 19% on days 0-5, respectively, compared with baseline.
- Glucose excursions of at least 25% above baseline occurred in four participants on day 0 and day 1 and in three participants on days 2 and 5.
- Insulin resistance, as measured by Total Daily Insulin Resistance (a metric that integrates daily mean glucose concentration with total daily insulin dose), was also significantly increased from baseline to day 2 post vaccination (7,171 mg/dL vs. 8,070 mg/dL units; P = .03).
- No other measures of glycemia differed significantly, compared with baseline.
- Outcomes didn’t differ significantly by sex, age, or vaccine manufacturer.
IN PRACTICE:
- “To our knowledge this is the first study investigating the effect of the COVID-19 booster vaccine on glycemia specifically in people with type 1 diabetes,” say the authors.
- “Clinicians, pharmacists, and other health care providers may need to counsel people with T1D to be more vigilant with glucose testing and insulin dosing for the first 5 days after vaccination. Most importantly, insulin, required to control glycemia, may need to be transiently increased.”
- “Further studies are warranted to investigate whether other vaccines have similar glycemic effects, and which individuals are at highest risk for profound glucose perturbations post vaccination.”
SOURCE:
The study was conducted by Mihail Zilbermint, MD, of the division of hospital medicine, Johns Hopkins Medicine, Bethesda, Md., and colleagues. It was published in Diabetes Research and Clinical Practice.
LIMITATIONS:
- The sample size was small.
- There were no measurements of inflammatory markers, dietary intake, physical activity, or survey patient symptomatology to adjust for variables that may have influenced glycemic control.
- In the study cohort, glycemia was moderately well controlled at baseline.
DISCLOSURES:
The study was supported by an investigator-initiated study grant from DexCom Inc. Dr. Zilbermint has consulted for EMD Serono.
A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- In a single-center prospective cohort study of 21 adults with type 1 diabetes, patients were given a blinded Dexcom G6 Pro continuous glucose monitor (CGM) at the first research clinic visit.
- After 3-4 days, participants received a COVID-19 booster vaccine.
- They returned to the clinic 10 days after the initial visit (5-6 days after booster vaccination) to have the CGM removed and glycemia assessed.
TAKEAWAY:
- Compared with baseline, the mean daily glucose level was significantly increased at day 2 (162.9 mg/dL vs. 172.8 mg/dL; P = .04) and day 3 (173.1 mg/dL; P = .02) post vaccination.
- Glucose excursions at day 0 (173.2 mg/dL; P = .058) and day 1 (173.1 mg/dL; P = .078) didn’t quite reach statistical significance.
- One participant experienced increases in glucose of 36%, 69%, 35%, 26%, 22%, and 19% on days 0-5, respectively, compared with baseline.
- Glucose excursions of at least 25% above baseline occurred in four participants on day 0 and day 1 and in three participants on days 2 and 5.
- Insulin resistance, as measured by Total Daily Insulin Resistance (a metric that integrates daily mean glucose concentration with total daily insulin dose), was also significantly increased from baseline to day 2 post vaccination (7,171 mg/dL vs. 8,070 mg/dL units; P = .03).
- No other measures of glycemia differed significantly, compared with baseline.
- Outcomes didn’t differ significantly by sex, age, or vaccine manufacturer.
IN PRACTICE:
- “To our knowledge this is the first study investigating the effect of the COVID-19 booster vaccine on glycemia specifically in people with type 1 diabetes,” say the authors.
- “Clinicians, pharmacists, and other health care providers may need to counsel people with T1D to be more vigilant with glucose testing and insulin dosing for the first 5 days after vaccination. Most importantly, insulin, required to control glycemia, may need to be transiently increased.”
- “Further studies are warranted to investigate whether other vaccines have similar glycemic effects, and which individuals are at highest risk for profound glucose perturbations post vaccination.”
SOURCE:
The study was conducted by Mihail Zilbermint, MD, of the division of hospital medicine, Johns Hopkins Medicine, Bethesda, Md., and colleagues. It was published in Diabetes Research and Clinical Practice.
LIMITATIONS:
- The sample size was small.
- There were no measurements of inflammatory markers, dietary intake, physical activity, or survey patient symptomatology to adjust for variables that may have influenced glycemic control.
- In the study cohort, glycemia was moderately well controlled at baseline.
DISCLOSURES:
The study was supported by an investigator-initiated study grant from DexCom Inc. Dr. Zilbermint has consulted for EMD Serono.
A version of this article first appeared on Medscape.com.
FROM DIABETES RESEARCH AND CLINICAL PRACTICE
SGLT2 inhibitors: No benefit or harm in hospitalized COVID-19
A new meta-analysis has shown that SGLT2 inhibitors do not lead to lower 28-day all-cause mortality, compared with usual care or placebo, in patients hospitalized with COVID-19.
However, no major safety issues were identified with the use of SGLT2 inhibitors in these acutely ill patients, the researchers report.
“While these findings do not support the use of SGLT2-inhibitors as standard of care for patients hospitalized with COVID-19, I think the most important take home message here is that the use of these medications appears to be safe even in really acutely ill hospitalized patients,” lead investigator of the meta-analysis, Mikhail Kosiborod, MD, Saint Luke’s Mid America Heart Institute, Kansas City, Mo., concluded.
He said this was important because the list of indications for SGLT2 inhibitors is rapidly growing.
“These medications are being used in more and more patients. And we know that when we discontinue medications in the hospital they frequently don’t get restarted, which can lead to real risks if SGLT2 inhibitors are stopped in patients with heart failure, chronic kidney disease, or diabetes. So, ,” he added.
The new meta-analysis was presented at the recent annual congress of the European Society of Cardiology, held in Amsterdam.
Discussant of the presentation at the ESC Hotline session, Muthiah Vaduganathan, MD, MPH, Brigham and Women’s Hospital, Boston, agreed with Dr. Kosiborod’s interpretation.
“Until today we have had very limited information on the safety of SGLT2-inhibitors in acute illness, as the pivotal trials which established the use of these drugs in diabetes and chronic kidney disease largely excluded patients who were hospitalized,” Dr. Vaduganathan said.
“While the overall results of this meta-analysis are neutral and SGLT2 inhibitors will not be added as drugs to be used in the primary care of patients with COVID-19, it certainly sends a strong message of safety in acutely ill patients,” he added.
Dr. Vaduganathan explained that from the beginning of the COVID-19 pandemic, there was great interest in repurposing established therapies for alternative indications for their use in the management of COVID-19.
“Conditions that strongly predispose to adverse COVID outcomes strongly overlap with established indications for SGLT2-inhibitors. So many wondered whether these drugs may be an ideal treatment candidate for the management of COVID-19. However, there have been many safety concerns about the use of SGLT2-inhibitors in this acute setting, with worries that they may induce hemodynamic changes such an excessive lowering of blood pressure, or metabolic changes such as ketoacidosis in acutely ill patients,” he noted.
The initial DARE-19 study investigating SGLT2-inhibitors in COVID-19, with 1,250 participants, found a 20% reduction in the primary outcome of organ dysfunction or death, but this did not reach statistical significance, and no safety issues were seen. This “intriguing” result led to two further larger trials – the ACTIV-4a and RECOVERY trials, Dr. Vaduganathan reported.
“Those early signals of benefit seen in DARE-19 were largely not substantiated in the ACTIV-4A and RECOVERY trials, or in this new meta-analysis, and now we have this much larger body of evidence and more stable estimates about the efficacy of these drugs in acutely ill COVID-19 patients,” he said.
“But the story that we will all take forward is one of safety. This set of trials was arguably conducted in some of the sickest patients we’ve seen who have been exposed to SGLT2-inhibitors, and they strongly affirm that these agents can be safely continued in the setting of acute illness, with very low rates of ketoacidosis and kidney injury, and there was no prolongation of hospital stay,” he commented.
In his presentation, Dr. Kosiborod explained that treatments targeting COVID-19 pathobiology such as dysregulated immune responses, endothelial damage, microvascular thrombosis, and inflammation have been shown to improve the key outcomes in this patient group.
SGLT2 inhibitors, which modulate similar pathobiology, provide cardiovascular protection and prevent the progression of kidney disease in patients at risk for these events, including those with type 2 diabetes, heart failure, and kidney disease, and may also lead to organ protection in a setting of acute illness such as COVID-19, he noted. However, the role of SGLT2 inhibitors in patients hospitalized with COVID-19 remains uncertain.
To address the need for more definitive efficacy data, the World Health Organization Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group conducted a prospective meta-analysis using data from the three randomized controlled trials, DARE-19, RECOVERY, and ACTIV-4a, evaluating SGLT2 inhibitors in patients hospitalized with COVID-19.
Overall, these trials randomized 6,096 participants: 3,025 to SGLT2 inhibitors and 3,071 to usual care or placebo. The average age of participants ranged between 62 and 73 years across the trials, 39% were women, and 25% had type 2 diabetes.
By 28 days after randomization, all-cause mortality, the primary endpoint, had occurred in 11.6% of the SGLT2-inhibitor patients, compared with 12.4% of those randomized to usual care or placebo, giving an odds ratio of 0.93 (95% confidence interval, 0.79-1.08; P = .33) for SGLT2 inhibitors, with consistency across trials.
Data on in-hospital and 90-day all-cause mortality were only available for two out of three trials (DARE-19 and ACTIV-4a), but the results were similar to the primary endpoint showing nonsignificant trends toward a possible benefit in the SGLT2-inhibitor group.
The results were also similar for the secondary outcomes of progression to acute kidney injury or requirement for dialysis or death, and progression to invasive mechanical ventilation, extracorporeal membrane oxygenation, or death, both assessed at 28 days.
The primary safety outcome of ketoacidosis by 28 days was observed in seven and two patients allocated to SGLT2 inhibitors and usual care or placebo, respectively, and overall, the incidence of reported serious adverse events was balanced between treatment groups.
The RECOVERY trial was supported by grants to the University of Oxford from UK Research and Innovation, the National Institute for Health and Care Research, and Wellcome. The ACTIV-4a platform was sponsored by the National Heart, Lung, and Blood Institute. DARE-19 was an investigator-initiated collaborative trial supported by AstraZeneca. Dr. Kosiborod reported numerous conflicts of interest.
A version of this article first appeared on Medscape.com.
A new meta-analysis has shown that SGLT2 inhibitors do not lead to lower 28-day all-cause mortality, compared with usual care or placebo, in patients hospitalized with COVID-19.
However, no major safety issues were identified with the use of SGLT2 inhibitors in these acutely ill patients, the researchers report.
“While these findings do not support the use of SGLT2-inhibitors as standard of care for patients hospitalized with COVID-19, I think the most important take home message here is that the use of these medications appears to be safe even in really acutely ill hospitalized patients,” lead investigator of the meta-analysis, Mikhail Kosiborod, MD, Saint Luke’s Mid America Heart Institute, Kansas City, Mo., concluded.
He said this was important because the list of indications for SGLT2 inhibitors is rapidly growing.
“These medications are being used in more and more patients. And we know that when we discontinue medications in the hospital they frequently don’t get restarted, which can lead to real risks if SGLT2 inhibitors are stopped in patients with heart failure, chronic kidney disease, or diabetes. So, ,” he added.
The new meta-analysis was presented at the recent annual congress of the European Society of Cardiology, held in Amsterdam.
Discussant of the presentation at the ESC Hotline session, Muthiah Vaduganathan, MD, MPH, Brigham and Women’s Hospital, Boston, agreed with Dr. Kosiborod’s interpretation.
“Until today we have had very limited information on the safety of SGLT2-inhibitors in acute illness, as the pivotal trials which established the use of these drugs in diabetes and chronic kidney disease largely excluded patients who were hospitalized,” Dr. Vaduganathan said.
“While the overall results of this meta-analysis are neutral and SGLT2 inhibitors will not be added as drugs to be used in the primary care of patients with COVID-19, it certainly sends a strong message of safety in acutely ill patients,” he added.
Dr. Vaduganathan explained that from the beginning of the COVID-19 pandemic, there was great interest in repurposing established therapies for alternative indications for their use in the management of COVID-19.
“Conditions that strongly predispose to adverse COVID outcomes strongly overlap with established indications for SGLT2-inhibitors. So many wondered whether these drugs may be an ideal treatment candidate for the management of COVID-19. However, there have been many safety concerns about the use of SGLT2-inhibitors in this acute setting, with worries that they may induce hemodynamic changes such an excessive lowering of blood pressure, or metabolic changes such as ketoacidosis in acutely ill patients,” he noted.
The initial DARE-19 study investigating SGLT2-inhibitors in COVID-19, with 1,250 participants, found a 20% reduction in the primary outcome of organ dysfunction or death, but this did not reach statistical significance, and no safety issues were seen. This “intriguing” result led to two further larger trials – the ACTIV-4a and RECOVERY trials, Dr. Vaduganathan reported.
“Those early signals of benefit seen in DARE-19 were largely not substantiated in the ACTIV-4A and RECOVERY trials, or in this new meta-analysis, and now we have this much larger body of evidence and more stable estimates about the efficacy of these drugs in acutely ill COVID-19 patients,” he said.
“But the story that we will all take forward is one of safety. This set of trials was arguably conducted in some of the sickest patients we’ve seen who have been exposed to SGLT2-inhibitors, and they strongly affirm that these agents can be safely continued in the setting of acute illness, with very low rates of ketoacidosis and kidney injury, and there was no prolongation of hospital stay,” he commented.
In his presentation, Dr. Kosiborod explained that treatments targeting COVID-19 pathobiology such as dysregulated immune responses, endothelial damage, microvascular thrombosis, and inflammation have been shown to improve the key outcomes in this patient group.
SGLT2 inhibitors, which modulate similar pathobiology, provide cardiovascular protection and prevent the progression of kidney disease in patients at risk for these events, including those with type 2 diabetes, heart failure, and kidney disease, and may also lead to organ protection in a setting of acute illness such as COVID-19, he noted. However, the role of SGLT2 inhibitors in patients hospitalized with COVID-19 remains uncertain.
To address the need for more definitive efficacy data, the World Health Organization Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group conducted a prospective meta-analysis using data from the three randomized controlled trials, DARE-19, RECOVERY, and ACTIV-4a, evaluating SGLT2 inhibitors in patients hospitalized with COVID-19.
Overall, these trials randomized 6,096 participants: 3,025 to SGLT2 inhibitors and 3,071 to usual care or placebo. The average age of participants ranged between 62 and 73 years across the trials, 39% were women, and 25% had type 2 diabetes.
By 28 days after randomization, all-cause mortality, the primary endpoint, had occurred in 11.6% of the SGLT2-inhibitor patients, compared with 12.4% of those randomized to usual care or placebo, giving an odds ratio of 0.93 (95% confidence interval, 0.79-1.08; P = .33) for SGLT2 inhibitors, with consistency across trials.
Data on in-hospital and 90-day all-cause mortality were only available for two out of three trials (DARE-19 and ACTIV-4a), but the results were similar to the primary endpoint showing nonsignificant trends toward a possible benefit in the SGLT2-inhibitor group.
The results were also similar for the secondary outcomes of progression to acute kidney injury or requirement for dialysis or death, and progression to invasive mechanical ventilation, extracorporeal membrane oxygenation, or death, both assessed at 28 days.
The primary safety outcome of ketoacidosis by 28 days was observed in seven and two patients allocated to SGLT2 inhibitors and usual care or placebo, respectively, and overall, the incidence of reported serious adverse events was balanced between treatment groups.
The RECOVERY trial was supported by grants to the University of Oxford from UK Research and Innovation, the National Institute for Health and Care Research, and Wellcome. The ACTIV-4a platform was sponsored by the National Heart, Lung, and Blood Institute. DARE-19 was an investigator-initiated collaborative trial supported by AstraZeneca. Dr. Kosiborod reported numerous conflicts of interest.
A version of this article first appeared on Medscape.com.
A new meta-analysis has shown that SGLT2 inhibitors do not lead to lower 28-day all-cause mortality, compared with usual care or placebo, in patients hospitalized with COVID-19.
However, no major safety issues were identified with the use of SGLT2 inhibitors in these acutely ill patients, the researchers report.
“While these findings do not support the use of SGLT2-inhibitors as standard of care for patients hospitalized with COVID-19, I think the most important take home message here is that the use of these medications appears to be safe even in really acutely ill hospitalized patients,” lead investigator of the meta-analysis, Mikhail Kosiborod, MD, Saint Luke’s Mid America Heart Institute, Kansas City, Mo., concluded.
He said this was important because the list of indications for SGLT2 inhibitors is rapidly growing.
“These medications are being used in more and more patients. And we know that when we discontinue medications in the hospital they frequently don’t get restarted, which can lead to real risks if SGLT2 inhibitors are stopped in patients with heart failure, chronic kidney disease, or diabetes. So, ,” he added.
The new meta-analysis was presented at the recent annual congress of the European Society of Cardiology, held in Amsterdam.
Discussant of the presentation at the ESC Hotline session, Muthiah Vaduganathan, MD, MPH, Brigham and Women’s Hospital, Boston, agreed with Dr. Kosiborod’s interpretation.
“Until today we have had very limited information on the safety of SGLT2-inhibitors in acute illness, as the pivotal trials which established the use of these drugs in diabetes and chronic kidney disease largely excluded patients who were hospitalized,” Dr. Vaduganathan said.
“While the overall results of this meta-analysis are neutral and SGLT2 inhibitors will not be added as drugs to be used in the primary care of patients with COVID-19, it certainly sends a strong message of safety in acutely ill patients,” he added.
Dr. Vaduganathan explained that from the beginning of the COVID-19 pandemic, there was great interest in repurposing established therapies for alternative indications for their use in the management of COVID-19.
“Conditions that strongly predispose to adverse COVID outcomes strongly overlap with established indications for SGLT2-inhibitors. So many wondered whether these drugs may be an ideal treatment candidate for the management of COVID-19. However, there have been many safety concerns about the use of SGLT2-inhibitors in this acute setting, with worries that they may induce hemodynamic changes such an excessive lowering of blood pressure, or metabolic changes such as ketoacidosis in acutely ill patients,” he noted.
The initial DARE-19 study investigating SGLT2-inhibitors in COVID-19, with 1,250 participants, found a 20% reduction in the primary outcome of organ dysfunction or death, but this did not reach statistical significance, and no safety issues were seen. This “intriguing” result led to two further larger trials – the ACTIV-4a and RECOVERY trials, Dr. Vaduganathan reported.
“Those early signals of benefit seen in DARE-19 were largely not substantiated in the ACTIV-4A and RECOVERY trials, or in this new meta-analysis, and now we have this much larger body of evidence and more stable estimates about the efficacy of these drugs in acutely ill COVID-19 patients,” he said.
“But the story that we will all take forward is one of safety. This set of trials was arguably conducted in some of the sickest patients we’ve seen who have been exposed to SGLT2-inhibitors, and they strongly affirm that these agents can be safely continued in the setting of acute illness, with very low rates of ketoacidosis and kidney injury, and there was no prolongation of hospital stay,” he commented.
In his presentation, Dr. Kosiborod explained that treatments targeting COVID-19 pathobiology such as dysregulated immune responses, endothelial damage, microvascular thrombosis, and inflammation have been shown to improve the key outcomes in this patient group.
SGLT2 inhibitors, which modulate similar pathobiology, provide cardiovascular protection and prevent the progression of kidney disease in patients at risk for these events, including those with type 2 diabetes, heart failure, and kidney disease, and may also lead to organ protection in a setting of acute illness such as COVID-19, he noted. However, the role of SGLT2 inhibitors in patients hospitalized with COVID-19 remains uncertain.
To address the need for more definitive efficacy data, the World Health Organization Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group conducted a prospective meta-analysis using data from the three randomized controlled trials, DARE-19, RECOVERY, and ACTIV-4a, evaluating SGLT2 inhibitors in patients hospitalized with COVID-19.
Overall, these trials randomized 6,096 participants: 3,025 to SGLT2 inhibitors and 3,071 to usual care or placebo. The average age of participants ranged between 62 and 73 years across the trials, 39% were women, and 25% had type 2 diabetes.
By 28 days after randomization, all-cause mortality, the primary endpoint, had occurred in 11.6% of the SGLT2-inhibitor patients, compared with 12.4% of those randomized to usual care or placebo, giving an odds ratio of 0.93 (95% confidence interval, 0.79-1.08; P = .33) for SGLT2 inhibitors, with consistency across trials.
Data on in-hospital and 90-day all-cause mortality were only available for two out of three trials (DARE-19 and ACTIV-4a), but the results were similar to the primary endpoint showing nonsignificant trends toward a possible benefit in the SGLT2-inhibitor group.
The results were also similar for the secondary outcomes of progression to acute kidney injury or requirement for dialysis or death, and progression to invasive mechanical ventilation, extracorporeal membrane oxygenation, or death, both assessed at 28 days.
The primary safety outcome of ketoacidosis by 28 days was observed in seven and two patients allocated to SGLT2 inhibitors and usual care or placebo, respectively, and overall, the incidence of reported serious adverse events was balanced between treatment groups.
The RECOVERY trial was supported by grants to the University of Oxford from UK Research and Innovation, the National Institute for Health and Care Research, and Wellcome. The ACTIV-4a platform was sponsored by the National Heart, Lung, and Blood Institute. DARE-19 was an investigator-initiated collaborative trial supported by AstraZeneca. Dr. Kosiborod reported numerous conflicts of interest.
A version of this article first appeared on Medscape.com.
FROM ESC CONGRESS 2023
Your workplace is toxic: Can you make it better?
A physician in your office is hot-tempered, critical, and upsets both the physicians and staff. Two of your partners are arguing over a software vendor and refuse to compromise. One doctor’s spouse is the office manager and snipes at everyone; the lead partner micromanages and second-guesses other doctors’ treatment plans, and no one will stand up to her.
If your practice has similar scenarios, you’re likely dealing with your own anger, irritation, and dread at work. You’re struggling with a toxic practice atmosphere, and you must make changes – fast.
However, this isn’t easy, given that what goes on in a doctor’s office is “high consequence,” says Leonard J. Marcus, PhD, founding director of the program for health care negotiation and conflict resolution at the Harvard School of Public Health in Boston.
The two things that tend to plague medical practices most: A culture of fear and someone who is letting ego run the day-to-day, he says.
“Fear overwhelms any chance for good morale among colleagues,” says Dr. Marcus, who is also the coauthor of “Renegotiating Health Care: Resolving Conflict to Build Collaboration.” “In a work environment where the fear is overwhelming, the ego can take over, and someone at the practice becomes overly concerned about getting credit, taking control, ordering other people around, and deciding who is on top and who is on the bottom.”
Tension, stress, back-biting, and rudeness are also symptoms of a more significant problem, says Jes Montgomery, MD, a psychiatrist and medical director of APN Dallas, a mental health–focused practice.
“If you don’t get toxicity under control, it will blow the office apart,” Dr. Montgomery says.
1. Recognize the signs
Part of the problem with a toxic medical practice is that, culturally, we don’t treat mental health and burnout as real illnesses. “A physician who is depressed is not going to be melancholy or bursting into tears with patients,” Dr. Montgomery says. “They’ll get behind on paperwork, skip meals, or find that it’s difficult to sleep at night. Next, they’ll yell at the partners and staff, always be in a foul mood, and gripe about inconsequential things. Their behavior affects everyone.”
Dr. Montgomery says that physicians aren’t taught to ask for help, making it difficult to see what’s really going on when someone displays toxic behavior in the practice. If it’s a partner, take time to ask what’s going on. If it’s yourself, step back and see if you can ask someone for the help you need.
2. Have difficult conversations
This is tough for most of us, says Jeremy Pollack, PhD, CEO and founder of Pollack Peacebuilding Systems, a conflict resolution consulting firm. If a team member is hot-tempered, disrespectful, or talking to patients in an unproductive manner, see if you can have an effective conversation with that person. The tricky part is critiquing in a way that doesn’t make them feel defensive – and wanting to push back.
For a micromanaging office manager, for example, you could say something like,”You’re doing a great job with the inventory, but I need you to let the staff have some autonomy and not hover over every supply they use in the break room, so that people won’t feel resentful toward us.” Make it clear you’re a team, and this is a team challenge. “However, if a doctor feels like they’ve tried to communicate to that colleague and are still walking on eggshells, it’s time to try to get help from someone – perhaps a practice management organization,” says Dr. Pollack.
3. Open lines of communication
It’s critical to create a comfortable space to speak with your colleagues, says Marisa Garshick, MD, a dermatologist in private practice in New York. “Creating an environment where there is an open line of communication, whether it’s directly to somebody in charge or having a system where you can give feedback more privately or anonymously, is important so that tension doesn’t build.”
“Being a doctor is a social enterprise,” Dr. Marcus says. “The science of medicine is critically important, but patients and the other health care workers on your team are also critically important. In the long run, the most successful physicians pay attention to both. It’s a full package.”
4. Emphasize the positive
Instead of discussing things only when they go wrong, try optimism, Dr. Garshick said. When positive things happen, whether it’s an excellent patient encounter or the office did something really well together, highlight it so everyone has a sense of accomplishment. If a patient compliments a medical assistant or raves about a nurse, share those compliments with the employees so that not every encounter you have calls out problems and staff missteps.
Suppose partners have a conflict with one another or are arguing over something. In that case, you may need to mediate or invest in a meaningful intervention so people can reflect on the narrative they’re contributing to the culture.
5. Practice self-care
Finally, the work of a physician is exhausting, so it’s crucial to practice personal TLC. That may mean taking micro breaks, getting adequate sleep, maintaining a healthy diet, and exercising well and managing stress to maintain energy levels and patience.
“Sometimes, when I’m fed up with the office, I need to get away,” Dr. Montgomery says. “I’ll take a day to go fishing, golfing, and not think about the office.” Just a small break can shift the lens that you see through when you return to the office and put problems in perspective.
A version of this article first appeared on Medscape.com.
A physician in your office is hot-tempered, critical, and upsets both the physicians and staff. Two of your partners are arguing over a software vendor and refuse to compromise. One doctor’s spouse is the office manager and snipes at everyone; the lead partner micromanages and second-guesses other doctors’ treatment plans, and no one will stand up to her.
If your practice has similar scenarios, you’re likely dealing with your own anger, irritation, and dread at work. You’re struggling with a toxic practice atmosphere, and you must make changes – fast.
However, this isn’t easy, given that what goes on in a doctor’s office is “high consequence,” says Leonard J. Marcus, PhD, founding director of the program for health care negotiation and conflict resolution at the Harvard School of Public Health in Boston.
The two things that tend to plague medical practices most: A culture of fear and someone who is letting ego run the day-to-day, he says.
“Fear overwhelms any chance for good morale among colleagues,” says Dr. Marcus, who is also the coauthor of “Renegotiating Health Care: Resolving Conflict to Build Collaboration.” “In a work environment where the fear is overwhelming, the ego can take over, and someone at the practice becomes overly concerned about getting credit, taking control, ordering other people around, and deciding who is on top and who is on the bottom.”
Tension, stress, back-biting, and rudeness are also symptoms of a more significant problem, says Jes Montgomery, MD, a psychiatrist and medical director of APN Dallas, a mental health–focused practice.
“If you don’t get toxicity under control, it will blow the office apart,” Dr. Montgomery says.
1. Recognize the signs
Part of the problem with a toxic medical practice is that, culturally, we don’t treat mental health and burnout as real illnesses. “A physician who is depressed is not going to be melancholy or bursting into tears with patients,” Dr. Montgomery says. “They’ll get behind on paperwork, skip meals, or find that it’s difficult to sleep at night. Next, they’ll yell at the partners and staff, always be in a foul mood, and gripe about inconsequential things. Their behavior affects everyone.”
Dr. Montgomery says that physicians aren’t taught to ask for help, making it difficult to see what’s really going on when someone displays toxic behavior in the practice. If it’s a partner, take time to ask what’s going on. If it’s yourself, step back and see if you can ask someone for the help you need.
2. Have difficult conversations
This is tough for most of us, says Jeremy Pollack, PhD, CEO and founder of Pollack Peacebuilding Systems, a conflict resolution consulting firm. If a team member is hot-tempered, disrespectful, or talking to patients in an unproductive manner, see if you can have an effective conversation with that person. The tricky part is critiquing in a way that doesn’t make them feel defensive – and wanting to push back.
For a micromanaging office manager, for example, you could say something like,”You’re doing a great job with the inventory, but I need you to let the staff have some autonomy and not hover over every supply they use in the break room, so that people won’t feel resentful toward us.” Make it clear you’re a team, and this is a team challenge. “However, if a doctor feels like they’ve tried to communicate to that colleague and are still walking on eggshells, it’s time to try to get help from someone – perhaps a practice management organization,” says Dr. Pollack.
3. Open lines of communication
It’s critical to create a comfortable space to speak with your colleagues, says Marisa Garshick, MD, a dermatologist in private practice in New York. “Creating an environment where there is an open line of communication, whether it’s directly to somebody in charge or having a system where you can give feedback more privately or anonymously, is important so that tension doesn’t build.”
“Being a doctor is a social enterprise,” Dr. Marcus says. “The science of medicine is critically important, but patients and the other health care workers on your team are also critically important. In the long run, the most successful physicians pay attention to both. It’s a full package.”
4. Emphasize the positive
Instead of discussing things only when they go wrong, try optimism, Dr. Garshick said. When positive things happen, whether it’s an excellent patient encounter or the office did something really well together, highlight it so everyone has a sense of accomplishment. If a patient compliments a medical assistant or raves about a nurse, share those compliments with the employees so that not every encounter you have calls out problems and staff missteps.
Suppose partners have a conflict with one another or are arguing over something. In that case, you may need to mediate or invest in a meaningful intervention so people can reflect on the narrative they’re contributing to the culture.
5. Practice self-care
Finally, the work of a physician is exhausting, so it’s crucial to practice personal TLC. That may mean taking micro breaks, getting adequate sleep, maintaining a healthy diet, and exercising well and managing stress to maintain energy levels and patience.
“Sometimes, when I’m fed up with the office, I need to get away,” Dr. Montgomery says. “I’ll take a day to go fishing, golfing, and not think about the office.” Just a small break can shift the lens that you see through when you return to the office and put problems in perspective.
A version of this article first appeared on Medscape.com.
A physician in your office is hot-tempered, critical, and upsets both the physicians and staff. Two of your partners are arguing over a software vendor and refuse to compromise. One doctor’s spouse is the office manager and snipes at everyone; the lead partner micromanages and second-guesses other doctors’ treatment plans, and no one will stand up to her.
If your practice has similar scenarios, you’re likely dealing with your own anger, irritation, and dread at work. You’re struggling with a toxic practice atmosphere, and you must make changes – fast.
However, this isn’t easy, given that what goes on in a doctor’s office is “high consequence,” says Leonard J. Marcus, PhD, founding director of the program for health care negotiation and conflict resolution at the Harvard School of Public Health in Boston.
The two things that tend to plague medical practices most: A culture of fear and someone who is letting ego run the day-to-day, he says.
“Fear overwhelms any chance for good morale among colleagues,” says Dr. Marcus, who is also the coauthor of “Renegotiating Health Care: Resolving Conflict to Build Collaboration.” “In a work environment where the fear is overwhelming, the ego can take over, and someone at the practice becomes overly concerned about getting credit, taking control, ordering other people around, and deciding who is on top and who is on the bottom.”
Tension, stress, back-biting, and rudeness are also symptoms of a more significant problem, says Jes Montgomery, MD, a psychiatrist and medical director of APN Dallas, a mental health–focused practice.
“If you don’t get toxicity under control, it will blow the office apart,” Dr. Montgomery says.
1. Recognize the signs
Part of the problem with a toxic medical practice is that, culturally, we don’t treat mental health and burnout as real illnesses. “A physician who is depressed is not going to be melancholy or bursting into tears with patients,” Dr. Montgomery says. “They’ll get behind on paperwork, skip meals, or find that it’s difficult to sleep at night. Next, they’ll yell at the partners and staff, always be in a foul mood, and gripe about inconsequential things. Their behavior affects everyone.”
Dr. Montgomery says that physicians aren’t taught to ask for help, making it difficult to see what’s really going on when someone displays toxic behavior in the practice. If it’s a partner, take time to ask what’s going on. If it’s yourself, step back and see if you can ask someone for the help you need.
2. Have difficult conversations
This is tough for most of us, says Jeremy Pollack, PhD, CEO and founder of Pollack Peacebuilding Systems, a conflict resolution consulting firm. If a team member is hot-tempered, disrespectful, or talking to patients in an unproductive manner, see if you can have an effective conversation with that person. The tricky part is critiquing in a way that doesn’t make them feel defensive – and wanting to push back.
For a micromanaging office manager, for example, you could say something like,”You’re doing a great job with the inventory, but I need you to let the staff have some autonomy and not hover over every supply they use in the break room, so that people won’t feel resentful toward us.” Make it clear you’re a team, and this is a team challenge. “However, if a doctor feels like they’ve tried to communicate to that colleague and are still walking on eggshells, it’s time to try to get help from someone – perhaps a practice management organization,” says Dr. Pollack.
3. Open lines of communication
It’s critical to create a comfortable space to speak with your colleagues, says Marisa Garshick, MD, a dermatologist in private practice in New York. “Creating an environment where there is an open line of communication, whether it’s directly to somebody in charge or having a system where you can give feedback more privately or anonymously, is important so that tension doesn’t build.”
“Being a doctor is a social enterprise,” Dr. Marcus says. “The science of medicine is critically important, but patients and the other health care workers on your team are also critically important. In the long run, the most successful physicians pay attention to both. It’s a full package.”
4. Emphasize the positive
Instead of discussing things only when they go wrong, try optimism, Dr. Garshick said. When positive things happen, whether it’s an excellent patient encounter or the office did something really well together, highlight it so everyone has a sense of accomplishment. If a patient compliments a medical assistant or raves about a nurse, share those compliments with the employees so that not every encounter you have calls out problems and staff missteps.
Suppose partners have a conflict with one another or are arguing over something. In that case, you may need to mediate or invest in a meaningful intervention so people can reflect on the narrative they’re contributing to the culture.
5. Practice self-care
Finally, the work of a physician is exhausting, so it’s crucial to practice personal TLC. That may mean taking micro breaks, getting adequate sleep, maintaining a healthy diet, and exercising well and managing stress to maintain energy levels and patience.
“Sometimes, when I’m fed up with the office, I need to get away,” Dr. Montgomery says. “I’ll take a day to go fishing, golfing, and not think about the office.” Just a small break can shift the lens that you see through when you return to the office and put problems in perspective.
A version of this article first appeared on Medscape.com.
Bad blood: Could brain bleeds be contagious?
This transcript has been edited for clarity.
How do you tell if a condition is caused by an infection?
It seems like an obvious question, right? In the post–van Leeuwenhoek era we can look at whatever part of the body is diseased under a microscope and see microbes – you know, the usual suspects.
Except when we can’t. And there are plenty of cases where we can’t: where the microbe is too small to be seen without more advanced imaging techniques, like with viruses; or when the pathogen is sparsely populated or hard to culture, like Mycobacterium.
Finding out that a condition is the result of an infection is not only an exercise for 19th century physicians. After all, it was 2008 when Barry Marshall and Robin Warren won their Nobel Prize for proving that stomach ulcers, long thought to be due to “stress,” were actually caused by a tiny microbe called Helicobacter pylori.
And this week, we are looking at a study which, once again, begins to suggest that a condition thought to be more or less random – cerebral amyloid angiopathy – may actually be the result of an infectious disease.
We’re talking about this paper, appearing in JAMA, which is just a great example of old-fashioned shoe-leather epidemiology. But let’s get up to speed on cerebral amyloid angiopathy (CAA) first.
CAA is characterized by the deposition of amyloid protein in the brain. While there are some genetic causes, they are quite rare, and most cases are thought to be idiopathic. Recent analyses suggest that somewhere between 5% and 7% of cognitively normal older adults have CAA, but the rate is much higher among those with intracerebral hemorrhage – brain bleeds. In fact, CAA is the second-most common cause of bleeding in the brain, second only to severe hypertension.
An article in Nature highlights cases that seemed to develop after the administration of cadaveric pituitary hormone.
Other studies have shown potential transmission via dura mater grafts and neurosurgical instruments. But despite those clues, no infectious organism has been identified. Some have suggested that the long latent period and difficulty of finding a responsible microbe points to a prion-like disease not yet known. But these studies are more or less case series. The new JAMA paper gives us, if not a smoking gun, a pretty decent set of fingerprints.
Here’s the idea: If CAA is caused by some infectious agent, it may be transmitted in the blood. We know that a decent percentage of people who have spontaneous brain bleeds have CAA. If those people donated blood in the past, maybe the people who received that blood would be at risk for brain bleeds too.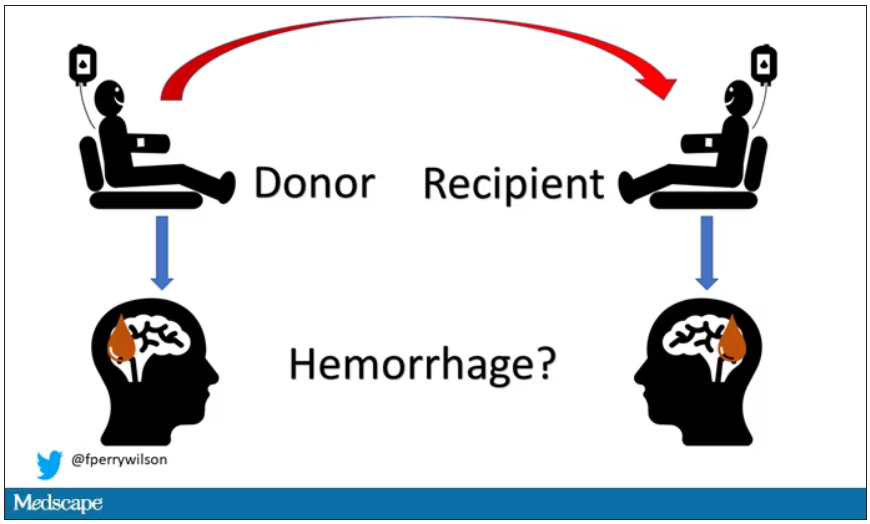
Of course, to really test that hypothesis, you’d need to know who every blood donor in a country was and every person who received that blood and all their subsequent diagnoses for basically their entire lives. No one has that kind of data, right?
Well, if you’ve been watching this space, you’ll know that a few countries do. Enter Sweden and Denmark, with their national electronic health record that captures all of this information, and much more, on every single person who lives or has lived in those countries since before 1970. Unbelievable.
So that’s exactly what the researchers, led by Jingchen Zhao at Karolinska (Sweden) University, did. They identified roughly 760,000 individuals in Sweden and 330,000 people in Denmark who had received a blood transfusion between 1970 and 2017.
Of course, most of those blood donors – 99% of them, actually – never went on to have any bleeding in the brain. It is a rare thing, fortunately.
But some of the donors did, on average within about 5 years of the time they donated blood. The researchers characterized each donor as either never having a brain bleed, having a single bleed, or having multiple bleeds. The latter is most strongly associated with CAA.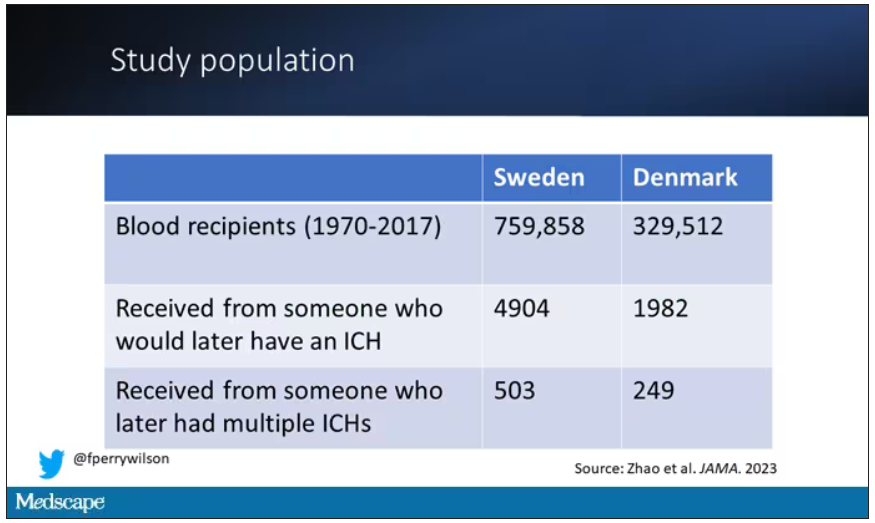
The big question: Would recipients who got blood from individuals who later on had brain bleeds, have brain bleeds themselves?
The answer is yes, though with an asterisk. You can see the results here. The risk of recipients having a brain bleed was lowest if the blood they received was from people who never had a brain bleed, higher if the individual had a single brain bleed, and highest if they got blood from a donor who would go on to have multiple brain bleeds.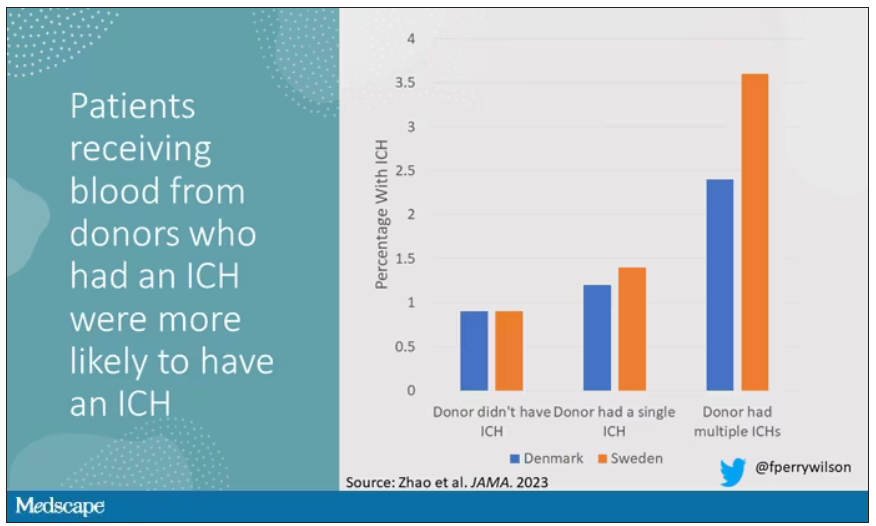
All in all, individuals who received blood from someone who would later have multiple hemorrhages were three times more likely to themselves develop bleeds themselves. It’s fairly compelling evidence of a transmissible agent.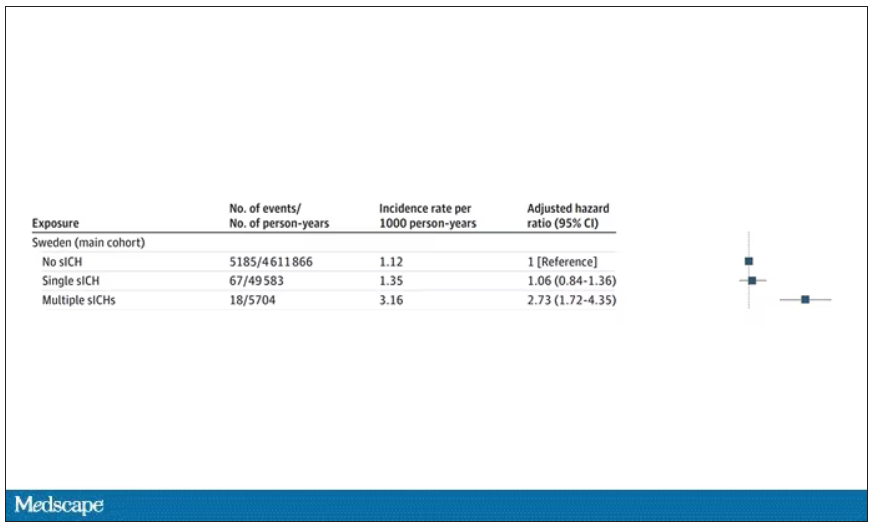
Of course, there are some potential confounders to consider here. Whose blood you get is not totally random. If, for example, people with type O blood are just more likely to have brain bleeds, then you could get results like this, as type O tends to donate to type O and both groups would have higher risk after donation. But the authors adjusted for blood type. They also adjusted for number of transfusions, calendar year, age, sex, and indication for transfusion.
Perhaps most compelling, and most clever, is that they used ischemic stroke as a negative control. Would people who received blood from someone who later had an ischemic stroke themselves be more likely to go on to have an ischemic stroke? No signal at all. It does not appear that there is a transmissible agent associated with ischemic stroke – only the brain bleeds.
I know what you’re thinking. What’s the agent? What’s the microbe, or virus, or prion, or toxin? The study gives us no insight there. These nationwide databases are awesome but they can only do so much. Because of the vagaries of medical coding and the difficulty of making the CAA diagnosis, the authors are using brain bleeds as a proxy here; we don’t even know for sure whether these were CAA-associated brain bleeds.
It’s also worth noting that there’s little we can do about this. None of the blood donors in this study had a brain bleed prior to donation; it’s not like we could screen people out of donating in the future. We have no test for whatever this agent is, if it even exists, nor do we have a potential treatment. Fortunately, whatever it is, it is extremely rare.
Still, this paper feels like a shot across the bow. At this point, the probability has shifted strongly away from CAA being a purely random disease and toward it being an infectious one. It may be time to round up some of the unusual suspects.
Dr. F. Perry Wilson is an associate professor of medicine and public health and director of Yale University’s Clinical and Translational Research Accelerator in New Haven, Conn. He reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
How do you tell if a condition is caused by an infection?
It seems like an obvious question, right? In the post–van Leeuwenhoek era we can look at whatever part of the body is diseased under a microscope and see microbes – you know, the usual suspects.
Except when we can’t. And there are plenty of cases where we can’t: where the microbe is too small to be seen without more advanced imaging techniques, like with viruses; or when the pathogen is sparsely populated or hard to culture, like Mycobacterium.
Finding out that a condition is the result of an infection is not only an exercise for 19th century physicians. After all, it was 2008 when Barry Marshall and Robin Warren won their Nobel Prize for proving that stomach ulcers, long thought to be due to “stress,” were actually caused by a tiny microbe called Helicobacter pylori.
And this week, we are looking at a study which, once again, begins to suggest that a condition thought to be more or less random – cerebral amyloid angiopathy – may actually be the result of an infectious disease.
We’re talking about this paper, appearing in JAMA, which is just a great example of old-fashioned shoe-leather epidemiology. But let’s get up to speed on cerebral amyloid angiopathy (CAA) first.
CAA is characterized by the deposition of amyloid protein in the brain. While there are some genetic causes, they are quite rare, and most cases are thought to be idiopathic. Recent analyses suggest that somewhere between 5% and 7% of cognitively normal older adults have CAA, but the rate is much higher among those with intracerebral hemorrhage – brain bleeds. In fact, CAA is the second-most common cause of bleeding in the brain, second only to severe hypertension.
An article in Nature highlights cases that seemed to develop after the administration of cadaveric pituitary hormone.
Other studies have shown potential transmission via dura mater grafts and neurosurgical instruments. But despite those clues, no infectious organism has been identified. Some have suggested that the long latent period and difficulty of finding a responsible microbe points to a prion-like disease not yet known. But these studies are more or less case series. The new JAMA paper gives us, if not a smoking gun, a pretty decent set of fingerprints.
Here’s the idea: If CAA is caused by some infectious agent, it may be transmitted in the blood. We know that a decent percentage of people who have spontaneous brain bleeds have CAA. If those people donated blood in the past, maybe the people who received that blood would be at risk for brain bleeds too.
Of course, to really test that hypothesis, you’d need to know who every blood donor in a country was and every person who received that blood and all their subsequent diagnoses for basically their entire lives. No one has that kind of data, right?
Well, if you’ve been watching this space, you’ll know that a few countries do. Enter Sweden and Denmark, with their national electronic health record that captures all of this information, and much more, on every single person who lives or has lived in those countries since before 1970. Unbelievable.
So that’s exactly what the researchers, led by Jingchen Zhao at Karolinska (Sweden) University, did. They identified roughly 760,000 individuals in Sweden and 330,000 people in Denmark who had received a blood transfusion between 1970 and 2017.
Of course, most of those blood donors – 99% of them, actually – never went on to have any bleeding in the brain. It is a rare thing, fortunately.
But some of the donors did, on average within about 5 years of the time they donated blood. The researchers characterized each donor as either never having a brain bleed, having a single bleed, or having multiple bleeds. The latter is most strongly associated with CAA.
The big question: Would recipients who got blood from individuals who later on had brain bleeds, have brain bleeds themselves?
The answer is yes, though with an asterisk. You can see the results here. The risk of recipients having a brain bleed was lowest if the blood they received was from people who never had a brain bleed, higher if the individual had a single brain bleed, and highest if they got blood from a donor who would go on to have multiple brain bleeds.
All in all, individuals who received blood from someone who would later have multiple hemorrhages were three times more likely to themselves develop bleeds themselves. It’s fairly compelling evidence of a transmissible agent.
Of course, there are some potential confounders to consider here. Whose blood you get is not totally random. If, for example, people with type O blood are just more likely to have brain bleeds, then you could get results like this, as type O tends to donate to type O and both groups would have higher risk after donation. But the authors adjusted for blood type. They also adjusted for number of transfusions, calendar year, age, sex, and indication for transfusion.
Perhaps most compelling, and most clever, is that they used ischemic stroke as a negative control. Would people who received blood from someone who later had an ischemic stroke themselves be more likely to go on to have an ischemic stroke? No signal at all. It does not appear that there is a transmissible agent associated with ischemic stroke – only the brain bleeds.
I know what you’re thinking. What’s the agent? What’s the microbe, or virus, or prion, or toxin? The study gives us no insight there. These nationwide databases are awesome but they can only do so much. Because of the vagaries of medical coding and the difficulty of making the CAA diagnosis, the authors are using brain bleeds as a proxy here; we don’t even know for sure whether these were CAA-associated brain bleeds.
It’s also worth noting that there’s little we can do about this. None of the blood donors in this study had a brain bleed prior to donation; it’s not like we could screen people out of donating in the future. We have no test for whatever this agent is, if it even exists, nor do we have a potential treatment. Fortunately, whatever it is, it is extremely rare.
Still, this paper feels like a shot across the bow. At this point, the probability has shifted strongly away from CAA being a purely random disease and toward it being an infectious one. It may be time to round up some of the unusual suspects.
Dr. F. Perry Wilson is an associate professor of medicine and public health and director of Yale University’s Clinical and Translational Research Accelerator in New Haven, Conn. He reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
How do you tell if a condition is caused by an infection?
It seems like an obvious question, right? In the post–van Leeuwenhoek era we can look at whatever part of the body is diseased under a microscope and see microbes – you know, the usual suspects.
Except when we can’t. And there are plenty of cases where we can’t: where the microbe is too small to be seen without more advanced imaging techniques, like with viruses; or when the pathogen is sparsely populated or hard to culture, like Mycobacterium.
Finding out that a condition is the result of an infection is not only an exercise for 19th century physicians. After all, it was 2008 when Barry Marshall and Robin Warren won their Nobel Prize for proving that stomach ulcers, long thought to be due to “stress,” were actually caused by a tiny microbe called Helicobacter pylori.
And this week, we are looking at a study which, once again, begins to suggest that a condition thought to be more or less random – cerebral amyloid angiopathy – may actually be the result of an infectious disease.
We’re talking about this paper, appearing in JAMA, which is just a great example of old-fashioned shoe-leather epidemiology. But let’s get up to speed on cerebral amyloid angiopathy (CAA) first.
CAA is characterized by the deposition of amyloid protein in the brain. While there are some genetic causes, they are quite rare, and most cases are thought to be idiopathic. Recent analyses suggest that somewhere between 5% and 7% of cognitively normal older adults have CAA, but the rate is much higher among those with intracerebral hemorrhage – brain bleeds. In fact, CAA is the second-most common cause of bleeding in the brain, second only to severe hypertension.
An article in Nature highlights cases that seemed to develop after the administration of cadaveric pituitary hormone.
Other studies have shown potential transmission via dura mater grafts and neurosurgical instruments. But despite those clues, no infectious organism has been identified. Some have suggested that the long latent period and difficulty of finding a responsible microbe points to a prion-like disease not yet known. But these studies are more or less case series. The new JAMA paper gives us, if not a smoking gun, a pretty decent set of fingerprints.
Here’s the idea: If CAA is caused by some infectious agent, it may be transmitted in the blood. We know that a decent percentage of people who have spontaneous brain bleeds have CAA. If those people donated blood in the past, maybe the people who received that blood would be at risk for brain bleeds too.
Of course, to really test that hypothesis, you’d need to know who every blood donor in a country was and every person who received that blood and all their subsequent diagnoses for basically their entire lives. No one has that kind of data, right?
Well, if you’ve been watching this space, you’ll know that a few countries do. Enter Sweden and Denmark, with their national electronic health record that captures all of this information, and much more, on every single person who lives or has lived in those countries since before 1970. Unbelievable.
So that’s exactly what the researchers, led by Jingchen Zhao at Karolinska (Sweden) University, did. They identified roughly 760,000 individuals in Sweden and 330,000 people in Denmark who had received a blood transfusion between 1970 and 2017.
Of course, most of those blood donors – 99% of them, actually – never went on to have any bleeding in the brain. It is a rare thing, fortunately.
But some of the donors did, on average within about 5 years of the time they donated blood. The researchers characterized each donor as either never having a brain bleed, having a single bleed, or having multiple bleeds. The latter is most strongly associated with CAA.
The big question: Would recipients who got blood from individuals who later on had brain bleeds, have brain bleeds themselves?
The answer is yes, though with an asterisk. You can see the results here. The risk of recipients having a brain bleed was lowest if the blood they received was from people who never had a brain bleed, higher if the individual had a single brain bleed, and highest if they got blood from a donor who would go on to have multiple brain bleeds.
All in all, individuals who received blood from someone who would later have multiple hemorrhages were three times more likely to themselves develop bleeds themselves. It’s fairly compelling evidence of a transmissible agent.
Of course, there are some potential confounders to consider here. Whose blood you get is not totally random. If, for example, people with type O blood are just more likely to have brain bleeds, then you could get results like this, as type O tends to donate to type O and both groups would have higher risk after donation. But the authors adjusted for blood type. They also adjusted for number of transfusions, calendar year, age, sex, and indication for transfusion.
Perhaps most compelling, and most clever, is that they used ischemic stroke as a negative control. Would people who received blood from someone who later had an ischemic stroke themselves be more likely to go on to have an ischemic stroke? No signal at all. It does not appear that there is a transmissible agent associated with ischemic stroke – only the brain bleeds.
I know what you’re thinking. What’s the agent? What’s the microbe, or virus, or prion, or toxin? The study gives us no insight there. These nationwide databases are awesome but they can only do so much. Because of the vagaries of medical coding and the difficulty of making the CAA diagnosis, the authors are using brain bleeds as a proxy here; we don’t even know for sure whether these were CAA-associated brain bleeds.
It’s also worth noting that there’s little we can do about this. None of the blood donors in this study had a brain bleed prior to donation; it’s not like we could screen people out of donating in the future. We have no test for whatever this agent is, if it even exists, nor do we have a potential treatment. Fortunately, whatever it is, it is extremely rare.
Still, this paper feels like a shot across the bow. At this point, the probability has shifted strongly away from CAA being a purely random disease and toward it being an infectious one. It may be time to round up some of the unusual suspects.
Dr. F. Perry Wilson is an associate professor of medicine and public health and director of Yale University’s Clinical and Translational Research Accelerator in New Haven, Conn. He reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
New COVID vaccines force bivalents out
COVID vaccines will have a new formulation in 2023, according to a decision announced by the U.S. Food and Drug Administration, that will focus efforts on circulating variants. The move pushes last year’s bivalent vaccines out of circulation because they will no longer be authorized for use in the United States.
The updated mRNA vaccines for 2023-2024 are being revised to include a single component that corresponds to the Omicron variant XBB.1.5. Like the bivalents offered before, the new monovalents are being manufactured by Moderna and Pfizer.
The new vaccines are authorized for use in individuals age 6 months and older. And the new options are being developed using a similar process as previous formulations, according to the FDA.
Targeting circulating variants
In recent studies, regulators point out the extent of neutralization observed by the updated vaccines against currently circulating viral variants causing COVID-19, including EG.5, BA.2.86, appears to be of a similar magnitude to the extent of neutralization observed with previous versions of the vaccines against corresponding prior variants.
“This suggests that the vaccines are a good match for protecting against the currently circulating COVID-19 variants,” according to the report.
Hundreds of millions of people in the United States have already received previously approved mRNA COVID vaccines, according to regulators who say the benefit-to-risk profile is well understood as they move forward with new formulations.
“Vaccination remains critical to public health and continued protection against serious consequences of COVID-19, including hospitalization and death,” Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, said in a statement. “The public can be assured that these updated vaccines have met the agency’s rigorous scientific standards for safety, effectiveness, and manufacturing quality. We very much encourage those who are eligible to consider getting vaccinated.”
Timing the effort
On Sept. 12 the U.S. Centers for Disease Control and Prevention recommended that everyone 6 months and older get an updated COVID-19 vaccine. Updated vaccines from Pfizer-BioNTech and Moderna will be available later this week, according to the agency.
This article was updated 9/14/23.
A version of this article appeared on Medscape.com.
COVID vaccines will have a new formulation in 2023, according to a decision announced by the U.S. Food and Drug Administration, that will focus efforts on circulating variants. The move pushes last year’s bivalent vaccines out of circulation because they will no longer be authorized for use in the United States.
The updated mRNA vaccines for 2023-2024 are being revised to include a single component that corresponds to the Omicron variant XBB.1.5. Like the bivalents offered before, the new monovalents are being manufactured by Moderna and Pfizer.
The new vaccines are authorized for use in individuals age 6 months and older. And the new options are being developed using a similar process as previous formulations, according to the FDA.
Targeting circulating variants
In recent studies, regulators point out the extent of neutralization observed by the updated vaccines against currently circulating viral variants causing COVID-19, including EG.5, BA.2.86, appears to be of a similar magnitude to the extent of neutralization observed with previous versions of the vaccines against corresponding prior variants.
“This suggests that the vaccines are a good match for protecting against the currently circulating COVID-19 variants,” according to the report.
Hundreds of millions of people in the United States have already received previously approved mRNA COVID vaccines, according to regulators who say the benefit-to-risk profile is well understood as they move forward with new formulations.
“Vaccination remains critical to public health and continued protection against serious consequences of COVID-19, including hospitalization and death,” Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, said in a statement. “The public can be assured that these updated vaccines have met the agency’s rigorous scientific standards for safety, effectiveness, and manufacturing quality. We very much encourage those who are eligible to consider getting vaccinated.”
Timing the effort
On Sept. 12 the U.S. Centers for Disease Control and Prevention recommended that everyone 6 months and older get an updated COVID-19 vaccine. Updated vaccines from Pfizer-BioNTech and Moderna will be available later this week, according to the agency.
This article was updated 9/14/23.
A version of this article appeared on Medscape.com.
COVID vaccines will have a new formulation in 2023, according to a decision announced by the U.S. Food and Drug Administration, that will focus efforts on circulating variants. The move pushes last year’s bivalent vaccines out of circulation because they will no longer be authorized for use in the United States.
The updated mRNA vaccines for 2023-2024 are being revised to include a single component that corresponds to the Omicron variant XBB.1.5. Like the bivalents offered before, the new monovalents are being manufactured by Moderna and Pfizer.
The new vaccines are authorized for use in individuals age 6 months and older. And the new options are being developed using a similar process as previous formulations, according to the FDA.
Targeting circulating variants
In recent studies, regulators point out the extent of neutralization observed by the updated vaccines against currently circulating viral variants causing COVID-19, including EG.5, BA.2.86, appears to be of a similar magnitude to the extent of neutralization observed with previous versions of the vaccines against corresponding prior variants.
“This suggests that the vaccines are a good match for protecting against the currently circulating COVID-19 variants,” according to the report.
Hundreds of millions of people in the United States have already received previously approved mRNA COVID vaccines, according to regulators who say the benefit-to-risk profile is well understood as they move forward with new formulations.
“Vaccination remains critical to public health and continued protection against serious consequences of COVID-19, including hospitalization and death,” Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, said in a statement. “The public can be assured that these updated vaccines have met the agency’s rigorous scientific standards for safety, effectiveness, and manufacturing quality. We very much encourage those who are eligible to consider getting vaccinated.”
Timing the effort
On Sept. 12 the U.S. Centers for Disease Control and Prevention recommended that everyone 6 months and older get an updated COVID-19 vaccine. Updated vaccines from Pfizer-BioNTech and Moderna will be available later this week, according to the agency.
This article was updated 9/14/23.
A version of this article appeared on Medscape.com.
RSV season has started, and this year could be different
The Centers for Disease Control and Prevention issued a national alert to health officials Sept. 5, urging them to offer new medicines that can prevent severe cases of the respiratory virus in very young children and in older people. Those two groups are at the highest risk of potentially deadly complications from RSV.
Typically, the CDC considers the start of RSV season to occur when the rate of positive tests for the virus goes above 3% for 2 consecutive weeks. In Florida, the rate has been around 5% in recent weeks, and in Georgia, there has been an increase in RSV-related hospitalizations. Most of the hospitalizations in Georgia have been among infants less than a year old.
“Historically, such regional increases have predicted the beginning of RSV season nationally, with increased RSV activity spreading north and west over the following 2-3 months,” the CDC said.
Most children have been infected with RSV by the time they are 2 years old. Historically, up to 80,000 children under 5 years old are hospitalized annually because of the virus, and between 100 and 300 die from complications each year.
Those figures could be drastically different this year because new preventive treatments are available.
The CDC recommends that all children under 8 months old receive the newly approved monoclonal antibody treatment nirsevimab (Beyfortus). Children up to 19 months old at high risk of severe complications from RSV are also eligible for the single-dose shot. In clinical trials, the treatment was 80% effective at preventing RSV infections from becoming so severe that children had to be hospitalized. The protection lasted about 5 months.
Older people are also at a heightened risk of severe illness from RSV, and two new vaccines are available this season. The vaccines are called Arexvy and Abrysvo, and the single-dose shots are approved for people ages 60 years and older. They are more than 80% effective at making severe lower respiratory complications less likely.
Last year’s RSV season started during the summer and peaked in October and November, which was earlier than usual. There’s no indication yet of when RSV season may peak this year. Last year and throughout the pandemic, RSV held its historical pattern of starting in Florida.
A version of this article appeared on WebMD.com.
The Centers for Disease Control and Prevention issued a national alert to health officials Sept. 5, urging them to offer new medicines that can prevent severe cases of the respiratory virus in very young children and in older people. Those two groups are at the highest risk of potentially deadly complications from RSV.
Typically, the CDC considers the start of RSV season to occur when the rate of positive tests for the virus goes above 3% for 2 consecutive weeks. In Florida, the rate has been around 5% in recent weeks, and in Georgia, there has been an increase in RSV-related hospitalizations. Most of the hospitalizations in Georgia have been among infants less than a year old.
“Historically, such regional increases have predicted the beginning of RSV season nationally, with increased RSV activity spreading north and west over the following 2-3 months,” the CDC said.
Most children have been infected with RSV by the time they are 2 years old. Historically, up to 80,000 children under 5 years old are hospitalized annually because of the virus, and between 100 and 300 die from complications each year.
Those figures could be drastically different this year because new preventive treatments are available.
The CDC recommends that all children under 8 months old receive the newly approved monoclonal antibody treatment nirsevimab (Beyfortus). Children up to 19 months old at high risk of severe complications from RSV are also eligible for the single-dose shot. In clinical trials, the treatment was 80% effective at preventing RSV infections from becoming so severe that children had to be hospitalized. The protection lasted about 5 months.
Older people are also at a heightened risk of severe illness from RSV, and two new vaccines are available this season. The vaccines are called Arexvy and Abrysvo, and the single-dose shots are approved for people ages 60 years and older. They are more than 80% effective at making severe lower respiratory complications less likely.
Last year’s RSV season started during the summer and peaked in October and November, which was earlier than usual. There’s no indication yet of when RSV season may peak this year. Last year and throughout the pandemic, RSV held its historical pattern of starting in Florida.
A version of this article appeared on WebMD.com.
The Centers for Disease Control and Prevention issued a national alert to health officials Sept. 5, urging them to offer new medicines that can prevent severe cases of the respiratory virus in very young children and in older people. Those two groups are at the highest risk of potentially deadly complications from RSV.
Typically, the CDC considers the start of RSV season to occur when the rate of positive tests for the virus goes above 3% for 2 consecutive weeks. In Florida, the rate has been around 5% in recent weeks, and in Georgia, there has been an increase in RSV-related hospitalizations. Most of the hospitalizations in Georgia have been among infants less than a year old.
“Historically, such regional increases have predicted the beginning of RSV season nationally, with increased RSV activity spreading north and west over the following 2-3 months,” the CDC said.
Most children have been infected with RSV by the time they are 2 years old. Historically, up to 80,000 children under 5 years old are hospitalized annually because of the virus, and between 100 and 300 die from complications each year.
Those figures could be drastically different this year because new preventive treatments are available.
The CDC recommends that all children under 8 months old receive the newly approved monoclonal antibody treatment nirsevimab (Beyfortus). Children up to 19 months old at high risk of severe complications from RSV are also eligible for the single-dose shot. In clinical trials, the treatment was 80% effective at preventing RSV infections from becoming so severe that children had to be hospitalized. The protection lasted about 5 months.
Older people are also at a heightened risk of severe illness from RSV, and two new vaccines are available this season. The vaccines are called Arexvy and Abrysvo, and the single-dose shots are approved for people ages 60 years and older. They are more than 80% effective at making severe lower respiratory complications less likely.
Last year’s RSV season started during the summer and peaked in October and November, which was earlier than usual. There’s no indication yet of when RSV season may peak this year. Last year and throughout the pandemic, RSV held its historical pattern of starting in Florida.
A version of this article appeared on WebMD.com.
New Moderna vaccine to work against recent COVID variant
“The company said its shot generated an 8.7-fold increase in neutralizing antibodies in humans against BA.2.86, which is being tracked by the World Health Organization and the U.S. Centers for Disease Control and Prevention,” Reuters reported.
“We think this is news people will want to hear as they prepare to go out and get their fall boosters,” Jacqueline Miller, Moderna head of infectious diseases, told the news agency.
The CDC said that the BA.2.86 variant might be more likely to infect people who have already had COVID or previous vaccinations. BA.2.86 is an Omicron variant. It has undergone more mutations than XBB.1.5, which has dominated most of this year and was the intended target of the updated shots.
BA.2.86 does not have a strong presence in the United States yet. However, officials are concerned about its high number of mutations, NBC News reported.
The FDA is expected to approve the new Moderna shot by early October.
Pfizer told NBC that its updated booster also generated a strong antibody response against Omicron variants, including BA.2.86.
COVID-19 cases and hospitalizations have been increasing in the U.S. because of the rise of several variants.
Experts told Reuters that BA.2.86 probably won’t cause a wave of severe disease and death because immunity has been built up around the world through previous infections and mass vaccinations.
A version of this article appeared on WebMD.com.
“The company said its shot generated an 8.7-fold increase in neutralizing antibodies in humans against BA.2.86, which is being tracked by the World Health Organization and the U.S. Centers for Disease Control and Prevention,” Reuters reported.
“We think this is news people will want to hear as they prepare to go out and get their fall boosters,” Jacqueline Miller, Moderna head of infectious diseases, told the news agency.
The CDC said that the BA.2.86 variant might be more likely to infect people who have already had COVID or previous vaccinations. BA.2.86 is an Omicron variant. It has undergone more mutations than XBB.1.5, which has dominated most of this year and was the intended target of the updated shots.
BA.2.86 does not have a strong presence in the United States yet. However, officials are concerned about its high number of mutations, NBC News reported.
The FDA is expected to approve the new Moderna shot by early October.
Pfizer told NBC that its updated booster also generated a strong antibody response against Omicron variants, including BA.2.86.
COVID-19 cases and hospitalizations have been increasing in the U.S. because of the rise of several variants.
Experts told Reuters that BA.2.86 probably won’t cause a wave of severe disease and death because immunity has been built up around the world through previous infections and mass vaccinations.
A version of this article appeared on WebMD.com.
“The company said its shot generated an 8.7-fold increase in neutralizing antibodies in humans against BA.2.86, which is being tracked by the World Health Organization and the U.S. Centers for Disease Control and Prevention,” Reuters reported.
“We think this is news people will want to hear as they prepare to go out and get their fall boosters,” Jacqueline Miller, Moderna head of infectious diseases, told the news agency.
The CDC said that the BA.2.86 variant might be more likely to infect people who have already had COVID or previous vaccinations. BA.2.86 is an Omicron variant. It has undergone more mutations than XBB.1.5, which has dominated most of this year and was the intended target of the updated shots.
BA.2.86 does not have a strong presence in the United States yet. However, officials are concerned about its high number of mutations, NBC News reported.
The FDA is expected to approve the new Moderna shot by early October.
Pfizer told NBC that its updated booster also generated a strong antibody response against Omicron variants, including BA.2.86.
COVID-19 cases and hospitalizations have been increasing in the U.S. because of the rise of several variants.
Experts told Reuters that BA.2.86 probably won’t cause a wave of severe disease and death because immunity has been built up around the world through previous infections and mass vaccinations.
A version of this article appeared on WebMD.com.



