User login
The Journal of Clinical Outcomes Management® is an independent, peer-reviewed journal offering evidence-based, practical information for improving the quality, safety, and value of health care.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Any level of physical activity tied to better later-life memory
new research suggests.
A prospective study of 1,400 participants showed that those who exercised to any extent in adulthood had significantly better cognitive scores later in life, compared with their peers who were physically inactive.
Maintaining an exercise routine throughout adulthood showed the strongest link to subsequent mental acuity.
Although these associations lessened when investigators controlled for childhood cognitive ability, socioeconomic background, and education, they remained statistically significant.
“Our findings support recommendations for greater participation in physical activity across adulthood,” lead investigator Sarah-Naomi James, PhD, research fellow at the Medical Research Council Unit for Lifelong Health and Ageing at the University College London, told this news organization.
“We provide evidence to encourage inactive adults to be active even to a small extent … at any point during adulthood,” which can improve cognition and memory later in life, Dr. James said.
The findings were published online in the Journal of Neurology, Neurosurgery & Psychiatry.
Exercise timing
Previous studies have established a link between fitness training and cognitive benefit later in life, but the researchers wanted to explore whether the timing or type of exercise influenced cognitive outcomes in later life.
The investigators asked more than 1,400 participants in the 1946 British birth cohort how much they had exercised at ages 36, 43, 60, and 69 years.
The questions changed slightly for each assessment period, but in general, participants were asked whether in the past month they had exercised or participated in such activities as badminton, swimming, fitness exercises, yoga, dancing, football, mountain climbing, jogging, or brisk walks for 30 minutes or more; and if so, how many times they participated per month.
Prior research showed that when the participants were aged 60 years, the most commonly reported activities were walking (71%), swimming (33%), floor exercises (24%), and cycling (15%).
When they turned 69, researchers tested participants’ cognitive performance using the Addenbrooke’s Cognitive Examination–III, which measures attention and orientation, verbal fluency, memory, language, and visuospatial function. In this study sample, 53% were women, and all were White.
Physical activity levels were classified as inactive, moderately active (one to four times per month), and most active (five or more times per month). In addition, they were summed across all five assessments to create a total score ranging from 0 (inactive at all ages) to 5 (active at all ages).
Overall, 11% of participants were physically inactive at all five time points; 17% were active at one time point; 20% were active at two and three time points; 17% were active at four time points; and 15% were active at all five time points.
‘Cradle to grave’ study?
Results showed that being physically active at all study time points was significantly associated with higher cognitive performance, verbal memory, and processing speed when participants were aged 69 (P < .01).
Those who exercised to any extent in adulthood – even just once a month during one of the time periods, fared better cognitively in later life, compared with physically inactive participants. (P < .01).
Study limitations cited include a lack of diversity among participants and a disproportionately high attrition rate among those who were socially disadvantaged.
“Our findings show that being active during every decade from their 30s on was associated with better cognition at around 70. Indeed, those who were active for longer had the highest cognitive function,” Dr. James said.
“However, it is also never too late to start. People in our study who only started being active in their 50s or 60s still had higher cognitive scores at age 70, compared to people of the same age who had never been active,” she added.
Dr. James intends to continue following the study sample to determine whether physical activity is linked to preserved cognitive aging “and buffers the effects of cognitive deterioration in the presence of disease markers that cause dementia, ultimately delaying dementia onset.
“We hope the cohort we study will be the first ‘cradle to grave’ study in the world, where we have followed people for their entire lives,” she said.
Encouraging finding
In a comment, Joel Hughes, PhD, professor of psychology and director of clinical training at Kent (Ohio) State University, said the study contributes to the idea that “accumulation of physical activity over one’s lifetime fits the data better than a ‘sensitive period’ – which suggests that it’s never too late to start exercising.”
Dr. Hughes, who was not involved in the research, noted that “exercise can improve cerebral blood flow and hemodynamic function, as well as greater activation of relevant brain regions such as the frontal lobes.”
While observing that the effects of exercise on cognition are likely complex from a mechanistic point of view, the finding that “exercise preserves or improves cognition later in life is encouraging,” he said.
The study received funding from the UK Medical Research Council and Alzheimer’s Research UK. The investigators and Dr. Hughes report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
new research suggests.
A prospective study of 1,400 participants showed that those who exercised to any extent in adulthood had significantly better cognitive scores later in life, compared with their peers who were physically inactive.
Maintaining an exercise routine throughout adulthood showed the strongest link to subsequent mental acuity.
Although these associations lessened when investigators controlled for childhood cognitive ability, socioeconomic background, and education, they remained statistically significant.
“Our findings support recommendations for greater participation in physical activity across adulthood,” lead investigator Sarah-Naomi James, PhD, research fellow at the Medical Research Council Unit for Lifelong Health and Ageing at the University College London, told this news organization.
“We provide evidence to encourage inactive adults to be active even to a small extent … at any point during adulthood,” which can improve cognition and memory later in life, Dr. James said.
The findings were published online in the Journal of Neurology, Neurosurgery & Psychiatry.
Exercise timing
Previous studies have established a link between fitness training and cognitive benefit later in life, but the researchers wanted to explore whether the timing or type of exercise influenced cognitive outcomes in later life.
The investigators asked more than 1,400 participants in the 1946 British birth cohort how much they had exercised at ages 36, 43, 60, and 69 years.
The questions changed slightly for each assessment period, but in general, participants were asked whether in the past month they had exercised or participated in such activities as badminton, swimming, fitness exercises, yoga, dancing, football, mountain climbing, jogging, or brisk walks for 30 minutes or more; and if so, how many times they participated per month.
Prior research showed that when the participants were aged 60 years, the most commonly reported activities were walking (71%), swimming (33%), floor exercises (24%), and cycling (15%).
When they turned 69, researchers tested participants’ cognitive performance using the Addenbrooke’s Cognitive Examination–III, which measures attention and orientation, verbal fluency, memory, language, and visuospatial function. In this study sample, 53% were women, and all were White.
Physical activity levels were classified as inactive, moderately active (one to four times per month), and most active (five or more times per month). In addition, they were summed across all five assessments to create a total score ranging from 0 (inactive at all ages) to 5 (active at all ages).
Overall, 11% of participants were physically inactive at all five time points; 17% were active at one time point; 20% were active at two and three time points; 17% were active at four time points; and 15% were active at all five time points.
‘Cradle to grave’ study?
Results showed that being physically active at all study time points was significantly associated with higher cognitive performance, verbal memory, and processing speed when participants were aged 69 (P < .01).
Those who exercised to any extent in adulthood – even just once a month during one of the time periods, fared better cognitively in later life, compared with physically inactive participants. (P < .01).
Study limitations cited include a lack of diversity among participants and a disproportionately high attrition rate among those who were socially disadvantaged.
“Our findings show that being active during every decade from their 30s on was associated with better cognition at around 70. Indeed, those who were active for longer had the highest cognitive function,” Dr. James said.
“However, it is also never too late to start. People in our study who only started being active in their 50s or 60s still had higher cognitive scores at age 70, compared to people of the same age who had never been active,” she added.
Dr. James intends to continue following the study sample to determine whether physical activity is linked to preserved cognitive aging “and buffers the effects of cognitive deterioration in the presence of disease markers that cause dementia, ultimately delaying dementia onset.
“We hope the cohort we study will be the first ‘cradle to grave’ study in the world, where we have followed people for their entire lives,” she said.
Encouraging finding
In a comment, Joel Hughes, PhD, professor of psychology and director of clinical training at Kent (Ohio) State University, said the study contributes to the idea that “accumulation of physical activity over one’s lifetime fits the data better than a ‘sensitive period’ – which suggests that it’s never too late to start exercising.”
Dr. Hughes, who was not involved in the research, noted that “exercise can improve cerebral blood flow and hemodynamic function, as well as greater activation of relevant brain regions such as the frontal lobes.”
While observing that the effects of exercise on cognition are likely complex from a mechanistic point of view, the finding that “exercise preserves or improves cognition later in life is encouraging,” he said.
The study received funding from the UK Medical Research Council and Alzheimer’s Research UK. The investigators and Dr. Hughes report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
new research suggests.
A prospective study of 1,400 participants showed that those who exercised to any extent in adulthood had significantly better cognitive scores later in life, compared with their peers who were physically inactive.
Maintaining an exercise routine throughout adulthood showed the strongest link to subsequent mental acuity.
Although these associations lessened when investigators controlled for childhood cognitive ability, socioeconomic background, and education, they remained statistically significant.
“Our findings support recommendations for greater participation in physical activity across adulthood,” lead investigator Sarah-Naomi James, PhD, research fellow at the Medical Research Council Unit for Lifelong Health and Ageing at the University College London, told this news organization.
“We provide evidence to encourage inactive adults to be active even to a small extent … at any point during adulthood,” which can improve cognition and memory later in life, Dr. James said.
The findings were published online in the Journal of Neurology, Neurosurgery & Psychiatry.
Exercise timing
Previous studies have established a link between fitness training and cognitive benefit later in life, but the researchers wanted to explore whether the timing or type of exercise influenced cognitive outcomes in later life.
The investigators asked more than 1,400 participants in the 1946 British birth cohort how much they had exercised at ages 36, 43, 60, and 69 years.
The questions changed slightly for each assessment period, but in general, participants were asked whether in the past month they had exercised or participated in such activities as badminton, swimming, fitness exercises, yoga, dancing, football, mountain climbing, jogging, or brisk walks for 30 minutes or more; and if so, how many times they participated per month.
Prior research showed that when the participants were aged 60 years, the most commonly reported activities were walking (71%), swimming (33%), floor exercises (24%), and cycling (15%).
When they turned 69, researchers tested participants’ cognitive performance using the Addenbrooke’s Cognitive Examination–III, which measures attention and orientation, verbal fluency, memory, language, and visuospatial function. In this study sample, 53% were women, and all were White.
Physical activity levels were classified as inactive, moderately active (one to four times per month), and most active (five or more times per month). In addition, they were summed across all five assessments to create a total score ranging from 0 (inactive at all ages) to 5 (active at all ages).
Overall, 11% of participants were physically inactive at all five time points; 17% were active at one time point; 20% were active at two and three time points; 17% were active at four time points; and 15% were active at all five time points.
‘Cradle to grave’ study?
Results showed that being physically active at all study time points was significantly associated with higher cognitive performance, verbal memory, and processing speed when participants were aged 69 (P < .01).
Those who exercised to any extent in adulthood – even just once a month during one of the time periods, fared better cognitively in later life, compared with physically inactive participants. (P < .01).
Study limitations cited include a lack of diversity among participants and a disproportionately high attrition rate among those who were socially disadvantaged.
“Our findings show that being active during every decade from their 30s on was associated with better cognition at around 70. Indeed, those who were active for longer had the highest cognitive function,” Dr. James said.
“However, it is also never too late to start. People in our study who only started being active in their 50s or 60s still had higher cognitive scores at age 70, compared to people of the same age who had never been active,” she added.
Dr. James intends to continue following the study sample to determine whether physical activity is linked to preserved cognitive aging “and buffers the effects of cognitive deterioration in the presence of disease markers that cause dementia, ultimately delaying dementia onset.
“We hope the cohort we study will be the first ‘cradle to grave’ study in the world, where we have followed people for their entire lives,” she said.
Encouraging finding
In a comment, Joel Hughes, PhD, professor of psychology and director of clinical training at Kent (Ohio) State University, said the study contributes to the idea that “accumulation of physical activity over one’s lifetime fits the data better than a ‘sensitive period’ – which suggests that it’s never too late to start exercising.”
Dr. Hughes, who was not involved in the research, noted that “exercise can improve cerebral blood flow and hemodynamic function, as well as greater activation of relevant brain regions such as the frontal lobes.”
While observing that the effects of exercise on cognition are likely complex from a mechanistic point of view, the finding that “exercise preserves or improves cognition later in life is encouraging,” he said.
The study received funding from the UK Medical Research Council and Alzheimer’s Research UK. The investigators and Dr. Hughes report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF NEUROLOGY, NEUROSURGERY & PSYCHIATRY
Colorectal cancer incidence doubled in younger adults
according to a new report from the American Cancer Society.
Diagnoses in people younger than 55 years doubled from 11% (1 in 10) in 1995 to 20% (1 in 5) in 2019.
In addition, more advanced disease is being diagnosed; the proportion of individuals of all ages presenting with advanced-stage CRC increased from 52% in the mid-2000s to 60% in 2019.
“We know rates are increasing in young people, but it’s alarming to see how rapidly the whole patient population is shifting younger, despite shrinking numbers in the overall population,” said Rebecca Siegel, MPH, senior scientific director of surveillance research at the American Cancer Society and lead author of the report.
“The trend toward more advanced disease in people of all ages is also surprising and should motivate everyone 45 and older to get screened,” she added.
The report was published online in CA: A Cancer Journal for Clinicians.
CRC is the third most commonly diagnosed cancer and the third leading cause of cancer death of both men and women in the United States. It is estimated that there will be 153,020 new cases of CRC in the U.S. in 2023, including 106,970 tumors in the colon and 46,050 in the rectum.
Overall, in 2023, an estimated 153,020 people will be diagnosed with CRC in the U.S., and of those, 52,550 people will die from the disease.
The incidence of CRC rapidly decreased during the 2000s among people aged 50 and older, largely because of an increase in cancer screening with colonoscopy. But progress slowed during the past decade, and now the trends toward declining incidence is largely confined to those aged 65 and older.
The authors point out that more than half of all cases and deaths are associated with modifiable risk factors, including smoking, an unhealthy diet, high alcohol consumption, physical inactivity, and excess body weight. A large proportion of CRC incidence and mortality is preventable through recommended screening, surveillance, and high-quality treatment.
But it remains unclear why rates are rising among younger adults and why there is a trend toward the disase being initially diagnosed at more advanced stages.
“We have to address why the rates in young adults continue to trend in the wrong direction,” said Ahmedin Jemal, DVM, PhD, senior vice president of surveillance and health equity science at the American Cancer Society and senior author of the study. “We need to invest more in research to uncover the causes of the rising trends and to discover new treatment for advanced-stage diseases to reduce the morbidity and mortality associated with this disease in this young population, who are raising families and supporting other family members.”
For their report, Ms. Siegel and colleagues used incidence data from 1995 to 2019 from 50 states and the District of Columbia. The data came from the Surveillance, Epidemiology, and End Results (SEER) program of the National Cancer Institute and the National Program of Cancer Registries of the U.S. Centers for Disease Control and Prevention, as provided by the North American Association of Central Cancer Registries. National mortality data through 2020 were provided by the National Center for Health Statistics.
The authors note that while overall, deaths from CRC are continuing to fall, “this progress is tempered by a rapidly changing landscape of disease that foreshadows less favorable trends ahead.”
The incidence rates have increased by 2% per year among people younger than 50 years as well as among those aged 50-54 years while, for the past decade, the rates have declined among those aged 65 and older. Incidence rates have stabilized among persons aged 50-64 years.
Although the majority of diagnoses continue to occur among people aged 65 years and older, 19,550 cases (13%) will occur in those younger than age 50 years, and one-third will be diagnosed in those aged 50-64 years.
Other key findings include the following.
- Declines in incidence and mortality have slowed, from 3% to 4% per year during the 2000s to 1% per year for incidence and 2% per year for mortality during the past decade.
- The incidence rate was 33% higher among men than women from 2015 to 2019, which may reflect differences in risk factors, such as excess body weight, processed meat consumption, and a history of smoking.
- The percentage of patients who present with advanced-stage disease has increased from a low of 52% in the mid-2000s to 60% in 2019 despite an increase in the use of screening.
- Death rates from CRC have risen since around 2005 by 1% annually among those younger than 50 years and by 0.6% in people aged 50-54.
- The report also identified racial/ethnic differences in incidence and mortality: Incidence was highest among Alaska Natives (88.5 per 100,000), American Indians (46.0 per 100,000), and Black persons, compared with White persons (41.7 per 100,000 vs. 35.7 per 100,000). Mortality followed a similar pattern; the highest rates were observed among Alaska Natives (50.5 per 100,000), American Indians (17.5 per 100,000), and Black persons, compared with White persons (17.6 per 100,000 vs. 13.1 per 100,000).
- There was also a shift to left-sided tumors, despite greater efficacy of screening for preventing left-sided lesions. The proportion of CRCs occurring in the rectum has steadily risen from 27% in 1995 to 31% in 2019.
“These highly concerning data illustrate the urgent need to invest in targeted cancer research studies dedicated to understanding and preventing early-onset colorectal cancer,” said Karen E. Knudsen, MBA, PhD, and CEO of the American Cancer Society. “The shift to diagnosis of more advanced disease also underscores the importance of screening and early detection, which saves lives.”
The study was supported by the American Cancer Society.
A version of this article first appeared on Medscape.com.
according to a new report from the American Cancer Society.
Diagnoses in people younger than 55 years doubled from 11% (1 in 10) in 1995 to 20% (1 in 5) in 2019.
In addition, more advanced disease is being diagnosed; the proportion of individuals of all ages presenting with advanced-stage CRC increased from 52% in the mid-2000s to 60% in 2019.
“We know rates are increasing in young people, but it’s alarming to see how rapidly the whole patient population is shifting younger, despite shrinking numbers in the overall population,” said Rebecca Siegel, MPH, senior scientific director of surveillance research at the American Cancer Society and lead author of the report.
“The trend toward more advanced disease in people of all ages is also surprising and should motivate everyone 45 and older to get screened,” she added.
The report was published online in CA: A Cancer Journal for Clinicians.
CRC is the third most commonly diagnosed cancer and the third leading cause of cancer death of both men and women in the United States. It is estimated that there will be 153,020 new cases of CRC in the U.S. in 2023, including 106,970 tumors in the colon and 46,050 in the rectum.
Overall, in 2023, an estimated 153,020 people will be diagnosed with CRC in the U.S., and of those, 52,550 people will die from the disease.
The incidence of CRC rapidly decreased during the 2000s among people aged 50 and older, largely because of an increase in cancer screening with colonoscopy. But progress slowed during the past decade, and now the trends toward declining incidence is largely confined to those aged 65 and older.
The authors point out that more than half of all cases and deaths are associated with modifiable risk factors, including smoking, an unhealthy diet, high alcohol consumption, physical inactivity, and excess body weight. A large proportion of CRC incidence and mortality is preventable through recommended screening, surveillance, and high-quality treatment.
But it remains unclear why rates are rising among younger adults and why there is a trend toward the disase being initially diagnosed at more advanced stages.
“We have to address why the rates in young adults continue to trend in the wrong direction,” said Ahmedin Jemal, DVM, PhD, senior vice president of surveillance and health equity science at the American Cancer Society and senior author of the study. “We need to invest more in research to uncover the causes of the rising trends and to discover new treatment for advanced-stage diseases to reduce the morbidity and mortality associated with this disease in this young population, who are raising families and supporting other family members.”
For their report, Ms. Siegel and colleagues used incidence data from 1995 to 2019 from 50 states and the District of Columbia. The data came from the Surveillance, Epidemiology, and End Results (SEER) program of the National Cancer Institute and the National Program of Cancer Registries of the U.S. Centers for Disease Control and Prevention, as provided by the North American Association of Central Cancer Registries. National mortality data through 2020 were provided by the National Center for Health Statistics.
The authors note that while overall, deaths from CRC are continuing to fall, “this progress is tempered by a rapidly changing landscape of disease that foreshadows less favorable trends ahead.”
The incidence rates have increased by 2% per year among people younger than 50 years as well as among those aged 50-54 years while, for the past decade, the rates have declined among those aged 65 and older. Incidence rates have stabilized among persons aged 50-64 years.
Although the majority of diagnoses continue to occur among people aged 65 years and older, 19,550 cases (13%) will occur in those younger than age 50 years, and one-third will be diagnosed in those aged 50-64 years.
Other key findings include the following.
- Declines in incidence and mortality have slowed, from 3% to 4% per year during the 2000s to 1% per year for incidence and 2% per year for mortality during the past decade.
- The incidence rate was 33% higher among men than women from 2015 to 2019, which may reflect differences in risk factors, such as excess body weight, processed meat consumption, and a history of smoking.
- The percentage of patients who present with advanced-stage disease has increased from a low of 52% in the mid-2000s to 60% in 2019 despite an increase in the use of screening.
- Death rates from CRC have risen since around 2005 by 1% annually among those younger than 50 years and by 0.6% in people aged 50-54.
- The report also identified racial/ethnic differences in incidence and mortality: Incidence was highest among Alaska Natives (88.5 per 100,000), American Indians (46.0 per 100,000), and Black persons, compared with White persons (41.7 per 100,000 vs. 35.7 per 100,000). Mortality followed a similar pattern; the highest rates were observed among Alaska Natives (50.5 per 100,000), American Indians (17.5 per 100,000), and Black persons, compared with White persons (17.6 per 100,000 vs. 13.1 per 100,000).
- There was also a shift to left-sided tumors, despite greater efficacy of screening for preventing left-sided lesions. The proportion of CRCs occurring in the rectum has steadily risen from 27% in 1995 to 31% in 2019.
“These highly concerning data illustrate the urgent need to invest in targeted cancer research studies dedicated to understanding and preventing early-onset colorectal cancer,” said Karen E. Knudsen, MBA, PhD, and CEO of the American Cancer Society. “The shift to diagnosis of more advanced disease also underscores the importance of screening and early detection, which saves lives.”
The study was supported by the American Cancer Society.
A version of this article first appeared on Medscape.com.
according to a new report from the American Cancer Society.
Diagnoses in people younger than 55 years doubled from 11% (1 in 10) in 1995 to 20% (1 in 5) in 2019.
In addition, more advanced disease is being diagnosed; the proportion of individuals of all ages presenting with advanced-stage CRC increased from 52% in the mid-2000s to 60% in 2019.
“We know rates are increasing in young people, but it’s alarming to see how rapidly the whole patient population is shifting younger, despite shrinking numbers in the overall population,” said Rebecca Siegel, MPH, senior scientific director of surveillance research at the American Cancer Society and lead author of the report.
“The trend toward more advanced disease in people of all ages is also surprising and should motivate everyone 45 and older to get screened,” she added.
The report was published online in CA: A Cancer Journal for Clinicians.
CRC is the third most commonly diagnosed cancer and the third leading cause of cancer death of both men and women in the United States. It is estimated that there will be 153,020 new cases of CRC in the U.S. in 2023, including 106,970 tumors in the colon and 46,050 in the rectum.
Overall, in 2023, an estimated 153,020 people will be diagnosed with CRC in the U.S., and of those, 52,550 people will die from the disease.
The incidence of CRC rapidly decreased during the 2000s among people aged 50 and older, largely because of an increase in cancer screening with colonoscopy. But progress slowed during the past decade, and now the trends toward declining incidence is largely confined to those aged 65 and older.
The authors point out that more than half of all cases and deaths are associated with modifiable risk factors, including smoking, an unhealthy diet, high alcohol consumption, physical inactivity, and excess body weight. A large proportion of CRC incidence and mortality is preventable through recommended screening, surveillance, and high-quality treatment.
But it remains unclear why rates are rising among younger adults and why there is a trend toward the disase being initially diagnosed at more advanced stages.
“We have to address why the rates in young adults continue to trend in the wrong direction,” said Ahmedin Jemal, DVM, PhD, senior vice president of surveillance and health equity science at the American Cancer Society and senior author of the study. “We need to invest more in research to uncover the causes of the rising trends and to discover new treatment for advanced-stage diseases to reduce the morbidity and mortality associated with this disease in this young population, who are raising families and supporting other family members.”
For their report, Ms. Siegel and colleagues used incidence data from 1995 to 2019 from 50 states and the District of Columbia. The data came from the Surveillance, Epidemiology, and End Results (SEER) program of the National Cancer Institute and the National Program of Cancer Registries of the U.S. Centers for Disease Control and Prevention, as provided by the North American Association of Central Cancer Registries. National mortality data through 2020 were provided by the National Center for Health Statistics.
The authors note that while overall, deaths from CRC are continuing to fall, “this progress is tempered by a rapidly changing landscape of disease that foreshadows less favorable trends ahead.”
The incidence rates have increased by 2% per year among people younger than 50 years as well as among those aged 50-54 years while, for the past decade, the rates have declined among those aged 65 and older. Incidence rates have stabilized among persons aged 50-64 years.
Although the majority of diagnoses continue to occur among people aged 65 years and older, 19,550 cases (13%) will occur in those younger than age 50 years, and one-third will be diagnosed in those aged 50-64 years.
Other key findings include the following.
- Declines in incidence and mortality have slowed, from 3% to 4% per year during the 2000s to 1% per year for incidence and 2% per year for mortality during the past decade.
- The incidence rate was 33% higher among men than women from 2015 to 2019, which may reflect differences in risk factors, such as excess body weight, processed meat consumption, and a history of smoking.
- The percentage of patients who present with advanced-stage disease has increased from a low of 52% in the mid-2000s to 60% in 2019 despite an increase in the use of screening.
- Death rates from CRC have risen since around 2005 by 1% annually among those younger than 50 years and by 0.6% in people aged 50-54.
- The report also identified racial/ethnic differences in incidence and mortality: Incidence was highest among Alaska Natives (88.5 per 100,000), American Indians (46.0 per 100,000), and Black persons, compared with White persons (41.7 per 100,000 vs. 35.7 per 100,000). Mortality followed a similar pattern; the highest rates were observed among Alaska Natives (50.5 per 100,000), American Indians (17.5 per 100,000), and Black persons, compared with White persons (17.6 per 100,000 vs. 13.1 per 100,000).
- There was also a shift to left-sided tumors, despite greater efficacy of screening for preventing left-sided lesions. The proportion of CRCs occurring in the rectum has steadily risen from 27% in 1995 to 31% in 2019.
“These highly concerning data illustrate the urgent need to invest in targeted cancer research studies dedicated to understanding and preventing early-onset colorectal cancer,” said Karen E. Knudsen, MBA, PhD, and CEO of the American Cancer Society. “The shift to diagnosis of more advanced disease also underscores the importance of screening and early detection, which saves lives.”
The study was supported by the American Cancer Society.
A version of this article first appeared on Medscape.com.
FROM CA: A CANCER JOURNAL FOR CLINICIANS
Lilly cuts insulin price by 70%, caps out-of-pocket cost
Eli Lilly will cut prices for most of its insulins in the United States by 70% and cap out-of-pocket costs for insulin at $35 per month, the company announced on March 1.
“Lilly is taking these actions to make it easier to access Lilly insulin and help Americans who may have difficulty navigating a complex healthcare system that may keep them from getting affordable insulin,” the company said in a statement.
The $35 price cap is effective immediately at participating retail pharmacies for people with commercial insurance. Those without insurance can go to InsulinAffordability.com and download the Lilly Insulin Value Program savings card to receive Lilly insulins for $35 per month.
The company says it will cut the list price of its nonbranded Insulin Lispro Injection 100 units/mL to $25 a vial, effective May 1, 2023. The list price of the branded Humalog (insulin lispro injection) 100 units/mL will be cut by 70%, effective in the fourth quarter of 2023.
Lilly is among the three main companies that manufacture insulin, along with Novo Nordisk and Sanofi, that have come under fire over the cost of insulin in the US. Studies have shown that up to 25% of people with type 1 diabetes ration insulin because of costs, putting their health and often their lives in jeopardy.
Prices in the United States are around 10 times higher than in other countries. California is the latest state to say it plans to sue these big three companies over the high price of insulin and has announced plans to make its own cheaper versions.
Asked at a telephone press briefing if the lawsuit prompted the company’s move, Lilly chair and CEO David A. Ricks said: “Of course there are complaints against the industry and the company. We see those as completely unfounded. However, we can probably all agree that patients should have a consistent and lower-cost experience at the pharmacy counter, and that’s what today’s announcement is about. We’re doing this completely voluntarily because it’s time and it’s the right thing to do.”
On hearing the company announcement, Laura Nally, MD, a pediatric endocrinologist living with type 1 diabetes, @drnallypants, tweeted: “YES. After years of advocacy, the list price of Lispro/Humalog is now similar to what it was in the late 1990s. Cheers to all the #pwd [people with diabetes] who have advocated through #insulin4all! But we still have work to do to improve access to other diabetes medications & supplies.”
#insulin4all is a worldwide campaign to ensure that people with type 1 diabetes have access to affordable insulin and other supplies needed to manage the condition, such as glucose strips. It is supported, among others, by the advocacy group T1International.
Also giving his reaction to the Lilly announcement, Chuck Henderson, CEO of the American Diabetes Association, said: “We applaud Eli Lilly for taking the important step to limit cost-sharing for its insulin, and we encourage other insulin manufacturers to do the same.
“While we have been able to help achieve significant progress on the issue of insulin affordability, including Medicare’s new out-of-pocket cost cap on insulin, state copay caps, and patient assistance developments from insulin manufacturers, we know that our work is not done,” he added.
“ADA will work to ensure that Eli Lilly’s patient assistance program is benefiting patients as intended and continue the fight so that everyone who needs insulin has access.”
And Endocrine Society chief medical officer Robert Lash, MD, said: “Lilly’s move to apply a $35/month cap for people with private insurance will be a significant improvement for adults and children with diabetes who use Lilly’s products.
“We encourage all insulin manufacturers to join in the effort to reduce out-of-pocket costs for people who need insulin.”
Lilly will also launch a new insulin biosimilar, Rezvoglar (insulin glargine-aglr) injection, which is similar to and interchangeable with insulin glargine (Lantus). The cost will by $92 for a five pack of KwikPens, a 78% discount, compared with the cost of Lantus, beginning April 1, 2023.
A version of this article first appeared on Medscape.com.
Eli Lilly will cut prices for most of its insulins in the United States by 70% and cap out-of-pocket costs for insulin at $35 per month, the company announced on March 1.
“Lilly is taking these actions to make it easier to access Lilly insulin and help Americans who may have difficulty navigating a complex healthcare system that may keep them from getting affordable insulin,” the company said in a statement.
The $35 price cap is effective immediately at participating retail pharmacies for people with commercial insurance. Those without insurance can go to InsulinAffordability.com and download the Lilly Insulin Value Program savings card to receive Lilly insulins for $35 per month.
The company says it will cut the list price of its nonbranded Insulin Lispro Injection 100 units/mL to $25 a vial, effective May 1, 2023. The list price of the branded Humalog (insulin lispro injection) 100 units/mL will be cut by 70%, effective in the fourth quarter of 2023.
Lilly is among the three main companies that manufacture insulin, along with Novo Nordisk and Sanofi, that have come under fire over the cost of insulin in the US. Studies have shown that up to 25% of people with type 1 diabetes ration insulin because of costs, putting their health and often their lives in jeopardy.
Prices in the United States are around 10 times higher than in other countries. California is the latest state to say it plans to sue these big three companies over the high price of insulin and has announced plans to make its own cheaper versions.
Asked at a telephone press briefing if the lawsuit prompted the company’s move, Lilly chair and CEO David A. Ricks said: “Of course there are complaints against the industry and the company. We see those as completely unfounded. However, we can probably all agree that patients should have a consistent and lower-cost experience at the pharmacy counter, and that’s what today’s announcement is about. We’re doing this completely voluntarily because it’s time and it’s the right thing to do.”
On hearing the company announcement, Laura Nally, MD, a pediatric endocrinologist living with type 1 diabetes, @drnallypants, tweeted: “YES. After years of advocacy, the list price of Lispro/Humalog is now similar to what it was in the late 1990s. Cheers to all the #pwd [people with diabetes] who have advocated through #insulin4all! But we still have work to do to improve access to other diabetes medications & supplies.”
#insulin4all is a worldwide campaign to ensure that people with type 1 diabetes have access to affordable insulin and other supplies needed to manage the condition, such as glucose strips. It is supported, among others, by the advocacy group T1International.
Also giving his reaction to the Lilly announcement, Chuck Henderson, CEO of the American Diabetes Association, said: “We applaud Eli Lilly for taking the important step to limit cost-sharing for its insulin, and we encourage other insulin manufacturers to do the same.
“While we have been able to help achieve significant progress on the issue of insulin affordability, including Medicare’s new out-of-pocket cost cap on insulin, state copay caps, and patient assistance developments from insulin manufacturers, we know that our work is not done,” he added.
“ADA will work to ensure that Eli Lilly’s patient assistance program is benefiting patients as intended and continue the fight so that everyone who needs insulin has access.”
And Endocrine Society chief medical officer Robert Lash, MD, said: “Lilly’s move to apply a $35/month cap for people with private insurance will be a significant improvement for adults and children with diabetes who use Lilly’s products.
“We encourage all insulin manufacturers to join in the effort to reduce out-of-pocket costs for people who need insulin.”
Lilly will also launch a new insulin biosimilar, Rezvoglar (insulin glargine-aglr) injection, which is similar to and interchangeable with insulin glargine (Lantus). The cost will by $92 for a five pack of KwikPens, a 78% discount, compared with the cost of Lantus, beginning April 1, 2023.
A version of this article first appeared on Medscape.com.
Eli Lilly will cut prices for most of its insulins in the United States by 70% and cap out-of-pocket costs for insulin at $35 per month, the company announced on March 1.
“Lilly is taking these actions to make it easier to access Lilly insulin and help Americans who may have difficulty navigating a complex healthcare system that may keep them from getting affordable insulin,” the company said in a statement.
The $35 price cap is effective immediately at participating retail pharmacies for people with commercial insurance. Those without insurance can go to InsulinAffordability.com and download the Lilly Insulin Value Program savings card to receive Lilly insulins for $35 per month.
The company says it will cut the list price of its nonbranded Insulin Lispro Injection 100 units/mL to $25 a vial, effective May 1, 2023. The list price of the branded Humalog (insulin lispro injection) 100 units/mL will be cut by 70%, effective in the fourth quarter of 2023.
Lilly is among the three main companies that manufacture insulin, along with Novo Nordisk and Sanofi, that have come under fire over the cost of insulin in the US. Studies have shown that up to 25% of people with type 1 diabetes ration insulin because of costs, putting their health and often their lives in jeopardy.
Prices in the United States are around 10 times higher than in other countries. California is the latest state to say it plans to sue these big three companies over the high price of insulin and has announced plans to make its own cheaper versions.
Asked at a telephone press briefing if the lawsuit prompted the company’s move, Lilly chair and CEO David A. Ricks said: “Of course there are complaints against the industry and the company. We see those as completely unfounded. However, we can probably all agree that patients should have a consistent and lower-cost experience at the pharmacy counter, and that’s what today’s announcement is about. We’re doing this completely voluntarily because it’s time and it’s the right thing to do.”
On hearing the company announcement, Laura Nally, MD, a pediatric endocrinologist living with type 1 diabetes, @drnallypants, tweeted: “YES. After years of advocacy, the list price of Lispro/Humalog is now similar to what it was in the late 1990s. Cheers to all the #pwd [people with diabetes] who have advocated through #insulin4all! But we still have work to do to improve access to other diabetes medications & supplies.”
#insulin4all is a worldwide campaign to ensure that people with type 1 diabetes have access to affordable insulin and other supplies needed to manage the condition, such as glucose strips. It is supported, among others, by the advocacy group T1International.
Also giving his reaction to the Lilly announcement, Chuck Henderson, CEO of the American Diabetes Association, said: “We applaud Eli Lilly for taking the important step to limit cost-sharing for its insulin, and we encourage other insulin manufacturers to do the same.
“While we have been able to help achieve significant progress on the issue of insulin affordability, including Medicare’s new out-of-pocket cost cap on insulin, state copay caps, and patient assistance developments from insulin manufacturers, we know that our work is not done,” he added.
“ADA will work to ensure that Eli Lilly’s patient assistance program is benefiting patients as intended and continue the fight so that everyone who needs insulin has access.”
And Endocrine Society chief medical officer Robert Lash, MD, said: “Lilly’s move to apply a $35/month cap for people with private insurance will be a significant improvement for adults and children with diabetes who use Lilly’s products.
“We encourage all insulin manufacturers to join in the effort to reduce out-of-pocket costs for people who need insulin.”
Lilly will also launch a new insulin biosimilar, Rezvoglar (insulin glargine-aglr) injection, which is similar to and interchangeable with insulin glargine (Lantus). The cost will by $92 for a five pack of KwikPens, a 78% discount, compared with the cost of Lantus, beginning April 1, 2023.
A version of this article first appeared on Medscape.com.
Is cellular senescence related to post–COVID-19 syndrome?
Proinflammatory elements mediated through metabolic pathways related to obesity and increased cellular senescence in CD57 expression in CD8+ T cells are associated with postacute sequelae of COVID-19 (PASC), according to a Mexican study. The researchers followed a Mexican cohort of 102 patients 3 months and 6 months after acute SARS-CoV-2 infection.
The study’s principal investigator was Diana Gómez-Martín, MD, PhD, of the department of immunology and rheumatology at the Salvador Zubirán National Institute of Medical Sciences and Nutrition, Mexico City. She told this news organization that follow-up of the patients began with the objective of understanding the determinative clinical, genetic, metabolic, and immunological factors in the progression of the acute disease. However, clinical aspects associated with PASC developed in the selected cohort. As a result, the study was extended, and the clinical, metabolic, and immunologic conditions in this single-center Mexican cohort were evaluated 3 months 6 months after the onset of infection.
Dr. Gómez-Martín explained that the immune senescence in CD57 of CD8+ T cells is one of the best-known findings of the present study. If it is confirmed in future studies, it could have important implications. “Its main implication is the possibility of better understanding the physiopathology of the clinical aspects associated with postacute sequelae of COVID-19, potentially being used for early detection and to provide follow-up aimed at patients, in addition to eventually developing targeted therapeutic strategies, such as immunometabolism regulation, in certain populations.”
Patients with PASC
The study was conducted from August 2020 to August 2021. Investigators recruited 102 patients (median age, 50.5 years; 55% were women) at the Mexico City Temporary Unit with a confirmed diagnosis of SARS-CoV-2. Of the patients, 44% had mild or moderate COVID-19, 30% had severe cases, and 26% of patients had critical cases. The most frequent comorbidities were obesity (44%), hypertension (24%), and type 2 diabetes (24%). The authors used a questionnaire to assess the presence of symptoms during follow-up. They analyzed immunologic variables at the time of recruitment, as well as levels of cytokines, immunoglobulin G against SARS-CoV-2, and neutrophil extracellular traps (NETs) at 1, 3, and 6 months. At 6 months’ follow-up, 12.7% of the cohort had symptoms compatible with PASC, which was defined for the study as the presence and report of three or more symptoms at 6 months’ follow-up.
As in similar studies, the authors found that female gender, remaining in intensive care, and having had more symptoms and greater titers of anti-SARS-CoV-2 antibodies during the acute infection were associated with the development of clinical aspects associated with PASC. Patients who had the disease at 6 months had increased serum levels of interleukin-1 alpha (6.21 pg/mL vs. 2.21 pg/mL), granulocyte colony-stimulating factor (55.08 pg/mL vs. 14.68 pg/mL), and interferon gamma-induced protein 10 (2,309.40 pg/mL vs. 780 pg/mL). Also, there was a trend toward an increase in serum concentration of interleukin-1 beta, interleukin-6, and interferon-gamma.
Patients whose condition met the definition of persistent PASC had increased expression of CD57 in CD8+ T cells (42,714 arbitrary units vs. 28,506) 6 months after the acute infection. The authors reported that there was no association between the persistence of PASC and the baseline amount of NETs, TRIM63, and anticellular antibodies. Nor was there an association between PASC and the titers of anti-SARS-CoV-2 antibodies at baseline and 1 month after COVID-19 diagnosis. Nonetheless, patients with persistent PASC had higher titers of anti-SARS-CoV-2 IgGs 3 months after the onset of COVID-19.
On the basis of previous data, the researchers aimed to construct a preliminary explanatory model to address the clinical and immunologic features associated with persistent PASC 6 months after SARS-CoV-2 infection. In the univariate analysis, the variables associated with the diagnosis of persistent PASC were the serum levels of granulocyte colony-stimulating factor (odds ratio, 1.01), macrophage inflammatory protein-1 alpha (OR, 1.13), interferon gamma-induced protein 10 (OR, 1.00), interleukin-6 (OR, 1.03), the expression of CD57 in CD8+ T cells (OR, 1.00), and the titers of anti-SARS-CoV-2 IgG at 1 month (OR, 1.45).
, such as obesity, greater levels of macrophage inflammatory protein-1 alpha and interferon gamma-induced protein 10 in peripheral blood, greater expression of the senescence CD57 marker in CD8+ T lymphocytes, and persistent symptoms at 3 months.
Using these parameters to construct a predictive model after 3 months, the authors found a sensitivity of 97.7%, specificity of 53.8%, positive predictive value of 93.5%, and a negative predictive value of 77.7% for the diagnosis of clinical aspects associated with PASC at 6 months.
Interpreting CD57
One of the researchers who participated in the study was Luis Martínez-Juárez, MD, MPH, DrPH. He is on the operative solutions team at the Carlos Slim Foundation. Dr. Martínez-Juárez pointed out that one of the contributions of this study was that it specifically examined the Mexican population. He noted that “according to the findings, obesity is not only a comorbidity associated with more severe progressions during acute COVID-19 disease, but also, through inflammation parameters, such as interleukin-6, interferon gamma-induced protein 10, and macrophage inflammatory protein-1 alpha, it’s involved in the development of clinical aspects related to postacute sequelae of COVID-19.”
Dr. Gómez-Martín added that finding proinflammatory and obesity parameters in the patients could potentially support the hypothesis of the persistence of virus fragments in adipose tissue as possibly involved in clinical aspects associated with PASC, as some groups have reported in the medical literature.
Angélica Cuapio, MD, DrMed, an immunologist and senior investigator at the Karolinska Institute, Stockholm, who did not participate in the study, said in an interview that the authors’ findings on the sustained increase of the CD57 marker in CD8+ lymphocytes are of notable interest. They may be associated with senescence states or cellular aging or with a stage of chronic viral infections. Therefore, Dr. Cuapio argued, it would have been valuable to include cellular markers of the innate system, such as natural killer cells, since in various infections, an increase in CD57 in lymphocytes is accompanied by an almost proportional increase of this marker in natural killer cells.
“This information would help to determine more accurately if we are talking about a cellular senescence or more about a chronic infection in persistent COVID-19.” The finding is important, but future research is needed in this developing field.
Dr. Cuapio pointed out that the authors found an interesting elevation in interleukin-1 alpha in patients with clinical aspects associated with PASC in a clinically well-characterized population in Mexico. “It is possible that this is a specific marker either of a specific population or location, or this could be an association with a humoral response. Despite the fact that this finding is new and unclear, it is worth investigating. This study is of great value for the scientific community because it’s one more piece in the complex puzzle of clinical aspects associated with postacute sequelae of COVID-19.”
Dr. Gómez-Martín noted that the main limitations of the study consist of its single-center design and the small patient sample. Dr. Martínez-Juárez added that the study did not consider reinfections. In future studies, it would be ideal to integrate other molecular assessments associated with various hypotheses of the physiopathology of clinical aspects associated with PASC, such as microbiota alteration, coagulation anomalies, endothelial damage, and dysfunctional neurologic signaling.
The study was supported and funded by the Carlos Slim Foundation. Dr. Gómez-Martín, Dr. Martínez-Juárez, and Dr. Cuapio have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Proinflammatory elements mediated through metabolic pathways related to obesity and increased cellular senescence in CD57 expression in CD8+ T cells are associated with postacute sequelae of COVID-19 (PASC), according to a Mexican study. The researchers followed a Mexican cohort of 102 patients 3 months and 6 months after acute SARS-CoV-2 infection.
The study’s principal investigator was Diana Gómez-Martín, MD, PhD, of the department of immunology and rheumatology at the Salvador Zubirán National Institute of Medical Sciences and Nutrition, Mexico City. She told this news organization that follow-up of the patients began with the objective of understanding the determinative clinical, genetic, metabolic, and immunological factors in the progression of the acute disease. However, clinical aspects associated with PASC developed in the selected cohort. As a result, the study was extended, and the clinical, metabolic, and immunologic conditions in this single-center Mexican cohort were evaluated 3 months 6 months after the onset of infection.
Dr. Gómez-Martín explained that the immune senescence in CD57 of CD8+ T cells is one of the best-known findings of the present study. If it is confirmed in future studies, it could have important implications. “Its main implication is the possibility of better understanding the physiopathology of the clinical aspects associated with postacute sequelae of COVID-19, potentially being used for early detection and to provide follow-up aimed at patients, in addition to eventually developing targeted therapeutic strategies, such as immunometabolism regulation, in certain populations.”
Patients with PASC
The study was conducted from August 2020 to August 2021. Investigators recruited 102 patients (median age, 50.5 years; 55% were women) at the Mexico City Temporary Unit with a confirmed diagnosis of SARS-CoV-2. Of the patients, 44% had mild or moderate COVID-19, 30% had severe cases, and 26% of patients had critical cases. The most frequent comorbidities were obesity (44%), hypertension (24%), and type 2 diabetes (24%). The authors used a questionnaire to assess the presence of symptoms during follow-up. They analyzed immunologic variables at the time of recruitment, as well as levels of cytokines, immunoglobulin G against SARS-CoV-2, and neutrophil extracellular traps (NETs) at 1, 3, and 6 months. At 6 months’ follow-up, 12.7% of the cohort had symptoms compatible with PASC, which was defined for the study as the presence and report of three or more symptoms at 6 months’ follow-up.
As in similar studies, the authors found that female gender, remaining in intensive care, and having had more symptoms and greater titers of anti-SARS-CoV-2 antibodies during the acute infection were associated with the development of clinical aspects associated with PASC. Patients who had the disease at 6 months had increased serum levels of interleukin-1 alpha (6.21 pg/mL vs. 2.21 pg/mL), granulocyte colony-stimulating factor (55.08 pg/mL vs. 14.68 pg/mL), and interferon gamma-induced protein 10 (2,309.40 pg/mL vs. 780 pg/mL). Also, there was a trend toward an increase in serum concentration of interleukin-1 beta, interleukin-6, and interferon-gamma.
Patients whose condition met the definition of persistent PASC had increased expression of CD57 in CD8+ T cells (42,714 arbitrary units vs. 28,506) 6 months after the acute infection. The authors reported that there was no association between the persistence of PASC and the baseline amount of NETs, TRIM63, and anticellular antibodies. Nor was there an association between PASC and the titers of anti-SARS-CoV-2 antibodies at baseline and 1 month after COVID-19 diagnosis. Nonetheless, patients with persistent PASC had higher titers of anti-SARS-CoV-2 IgGs 3 months after the onset of COVID-19.
On the basis of previous data, the researchers aimed to construct a preliminary explanatory model to address the clinical and immunologic features associated with persistent PASC 6 months after SARS-CoV-2 infection. In the univariate analysis, the variables associated with the diagnosis of persistent PASC were the serum levels of granulocyte colony-stimulating factor (odds ratio, 1.01), macrophage inflammatory protein-1 alpha (OR, 1.13), interferon gamma-induced protein 10 (OR, 1.00), interleukin-6 (OR, 1.03), the expression of CD57 in CD8+ T cells (OR, 1.00), and the titers of anti-SARS-CoV-2 IgG at 1 month (OR, 1.45).
, such as obesity, greater levels of macrophage inflammatory protein-1 alpha and interferon gamma-induced protein 10 in peripheral blood, greater expression of the senescence CD57 marker in CD8+ T lymphocytes, and persistent symptoms at 3 months.
Using these parameters to construct a predictive model after 3 months, the authors found a sensitivity of 97.7%, specificity of 53.8%, positive predictive value of 93.5%, and a negative predictive value of 77.7% for the diagnosis of clinical aspects associated with PASC at 6 months.
Interpreting CD57
One of the researchers who participated in the study was Luis Martínez-Juárez, MD, MPH, DrPH. He is on the operative solutions team at the Carlos Slim Foundation. Dr. Martínez-Juárez pointed out that one of the contributions of this study was that it specifically examined the Mexican population. He noted that “according to the findings, obesity is not only a comorbidity associated with more severe progressions during acute COVID-19 disease, but also, through inflammation parameters, such as interleukin-6, interferon gamma-induced protein 10, and macrophage inflammatory protein-1 alpha, it’s involved in the development of clinical aspects related to postacute sequelae of COVID-19.”
Dr. Gómez-Martín added that finding proinflammatory and obesity parameters in the patients could potentially support the hypothesis of the persistence of virus fragments in adipose tissue as possibly involved in clinical aspects associated with PASC, as some groups have reported in the medical literature.
Angélica Cuapio, MD, DrMed, an immunologist and senior investigator at the Karolinska Institute, Stockholm, who did not participate in the study, said in an interview that the authors’ findings on the sustained increase of the CD57 marker in CD8+ lymphocytes are of notable interest. They may be associated with senescence states or cellular aging or with a stage of chronic viral infections. Therefore, Dr. Cuapio argued, it would have been valuable to include cellular markers of the innate system, such as natural killer cells, since in various infections, an increase in CD57 in lymphocytes is accompanied by an almost proportional increase of this marker in natural killer cells.
“This information would help to determine more accurately if we are talking about a cellular senescence or more about a chronic infection in persistent COVID-19.” The finding is important, but future research is needed in this developing field.
Dr. Cuapio pointed out that the authors found an interesting elevation in interleukin-1 alpha in patients with clinical aspects associated with PASC in a clinically well-characterized population in Mexico. “It is possible that this is a specific marker either of a specific population or location, or this could be an association with a humoral response. Despite the fact that this finding is new and unclear, it is worth investigating. This study is of great value for the scientific community because it’s one more piece in the complex puzzle of clinical aspects associated with postacute sequelae of COVID-19.”
Dr. Gómez-Martín noted that the main limitations of the study consist of its single-center design and the small patient sample. Dr. Martínez-Juárez added that the study did not consider reinfections. In future studies, it would be ideal to integrate other molecular assessments associated with various hypotheses of the physiopathology of clinical aspects associated with PASC, such as microbiota alteration, coagulation anomalies, endothelial damage, and dysfunctional neurologic signaling.
The study was supported and funded by the Carlos Slim Foundation. Dr. Gómez-Martín, Dr. Martínez-Juárez, and Dr. Cuapio have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Proinflammatory elements mediated through metabolic pathways related to obesity and increased cellular senescence in CD57 expression in CD8+ T cells are associated with postacute sequelae of COVID-19 (PASC), according to a Mexican study. The researchers followed a Mexican cohort of 102 patients 3 months and 6 months after acute SARS-CoV-2 infection.
The study’s principal investigator was Diana Gómez-Martín, MD, PhD, of the department of immunology and rheumatology at the Salvador Zubirán National Institute of Medical Sciences and Nutrition, Mexico City. She told this news organization that follow-up of the patients began with the objective of understanding the determinative clinical, genetic, metabolic, and immunological factors in the progression of the acute disease. However, clinical aspects associated with PASC developed in the selected cohort. As a result, the study was extended, and the clinical, metabolic, and immunologic conditions in this single-center Mexican cohort were evaluated 3 months 6 months after the onset of infection.
Dr. Gómez-Martín explained that the immune senescence in CD57 of CD8+ T cells is one of the best-known findings of the present study. If it is confirmed in future studies, it could have important implications. “Its main implication is the possibility of better understanding the physiopathology of the clinical aspects associated with postacute sequelae of COVID-19, potentially being used for early detection and to provide follow-up aimed at patients, in addition to eventually developing targeted therapeutic strategies, such as immunometabolism regulation, in certain populations.”
Patients with PASC
The study was conducted from August 2020 to August 2021. Investigators recruited 102 patients (median age, 50.5 years; 55% were women) at the Mexico City Temporary Unit with a confirmed diagnosis of SARS-CoV-2. Of the patients, 44% had mild or moderate COVID-19, 30% had severe cases, and 26% of patients had critical cases. The most frequent comorbidities were obesity (44%), hypertension (24%), and type 2 diabetes (24%). The authors used a questionnaire to assess the presence of symptoms during follow-up. They analyzed immunologic variables at the time of recruitment, as well as levels of cytokines, immunoglobulin G against SARS-CoV-2, and neutrophil extracellular traps (NETs) at 1, 3, and 6 months. At 6 months’ follow-up, 12.7% of the cohort had symptoms compatible with PASC, which was defined for the study as the presence and report of three or more symptoms at 6 months’ follow-up.
As in similar studies, the authors found that female gender, remaining in intensive care, and having had more symptoms and greater titers of anti-SARS-CoV-2 antibodies during the acute infection were associated with the development of clinical aspects associated with PASC. Patients who had the disease at 6 months had increased serum levels of interleukin-1 alpha (6.21 pg/mL vs. 2.21 pg/mL), granulocyte colony-stimulating factor (55.08 pg/mL vs. 14.68 pg/mL), and interferon gamma-induced protein 10 (2,309.40 pg/mL vs. 780 pg/mL). Also, there was a trend toward an increase in serum concentration of interleukin-1 beta, interleukin-6, and interferon-gamma.
Patients whose condition met the definition of persistent PASC had increased expression of CD57 in CD8+ T cells (42,714 arbitrary units vs. 28,506) 6 months after the acute infection. The authors reported that there was no association between the persistence of PASC and the baseline amount of NETs, TRIM63, and anticellular antibodies. Nor was there an association between PASC and the titers of anti-SARS-CoV-2 antibodies at baseline and 1 month after COVID-19 diagnosis. Nonetheless, patients with persistent PASC had higher titers of anti-SARS-CoV-2 IgGs 3 months after the onset of COVID-19.
On the basis of previous data, the researchers aimed to construct a preliminary explanatory model to address the clinical and immunologic features associated with persistent PASC 6 months after SARS-CoV-2 infection. In the univariate analysis, the variables associated with the diagnosis of persistent PASC were the serum levels of granulocyte colony-stimulating factor (odds ratio, 1.01), macrophage inflammatory protein-1 alpha (OR, 1.13), interferon gamma-induced protein 10 (OR, 1.00), interleukin-6 (OR, 1.03), the expression of CD57 in CD8+ T cells (OR, 1.00), and the titers of anti-SARS-CoV-2 IgG at 1 month (OR, 1.45).
, such as obesity, greater levels of macrophage inflammatory protein-1 alpha and interferon gamma-induced protein 10 in peripheral blood, greater expression of the senescence CD57 marker in CD8+ T lymphocytes, and persistent symptoms at 3 months.
Using these parameters to construct a predictive model after 3 months, the authors found a sensitivity of 97.7%, specificity of 53.8%, positive predictive value of 93.5%, and a negative predictive value of 77.7% for the diagnosis of clinical aspects associated with PASC at 6 months.
Interpreting CD57
One of the researchers who participated in the study was Luis Martínez-Juárez, MD, MPH, DrPH. He is on the operative solutions team at the Carlos Slim Foundation. Dr. Martínez-Juárez pointed out that one of the contributions of this study was that it specifically examined the Mexican population. He noted that “according to the findings, obesity is not only a comorbidity associated with more severe progressions during acute COVID-19 disease, but also, through inflammation parameters, such as interleukin-6, interferon gamma-induced protein 10, and macrophage inflammatory protein-1 alpha, it’s involved in the development of clinical aspects related to postacute sequelae of COVID-19.”
Dr. Gómez-Martín added that finding proinflammatory and obesity parameters in the patients could potentially support the hypothesis of the persistence of virus fragments in adipose tissue as possibly involved in clinical aspects associated with PASC, as some groups have reported in the medical literature.
Angélica Cuapio, MD, DrMed, an immunologist and senior investigator at the Karolinska Institute, Stockholm, who did not participate in the study, said in an interview that the authors’ findings on the sustained increase of the CD57 marker in CD8+ lymphocytes are of notable interest. They may be associated with senescence states or cellular aging or with a stage of chronic viral infections. Therefore, Dr. Cuapio argued, it would have been valuable to include cellular markers of the innate system, such as natural killer cells, since in various infections, an increase in CD57 in lymphocytes is accompanied by an almost proportional increase of this marker in natural killer cells.
“This information would help to determine more accurately if we are talking about a cellular senescence or more about a chronic infection in persistent COVID-19.” The finding is important, but future research is needed in this developing field.
Dr. Cuapio pointed out that the authors found an interesting elevation in interleukin-1 alpha in patients with clinical aspects associated with PASC in a clinically well-characterized population in Mexico. “It is possible that this is a specific marker either of a specific population or location, or this could be an association with a humoral response. Despite the fact that this finding is new and unclear, it is worth investigating. This study is of great value for the scientific community because it’s one more piece in the complex puzzle of clinical aspects associated with postacute sequelae of COVID-19.”
Dr. Gómez-Martín noted that the main limitations of the study consist of its single-center design and the small patient sample. Dr. Martínez-Juárez added that the study did not consider reinfections. In future studies, it would be ideal to integrate other molecular assessments associated with various hypotheses of the physiopathology of clinical aspects associated with PASC, such as microbiota alteration, coagulation anomalies, endothelial damage, and dysfunctional neurologic signaling.
The study was supported and funded by the Carlos Slim Foundation. Dr. Gómez-Martín, Dr. Martínez-Juárez, and Dr. Cuapio have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Frequent cannabis use tied to coronary artery disease
In the first part, in an observational study, daily cannabis use was associated with 34% higher odds for CAD, compared with never-users, in a large population-based U.S. cohort. Less frequent use was not associated with increased odds for CAD.
In the second part, people with a genetic susceptibility to cannabis use disorder or severe cannabis dependency had an increased risk for CAD, compared with other people.
Ishan Paranjpe, MD, the study’s lead author, reported these results in a press briefing and will present the study at the upcoming joint scientific sessions of the American College of Cardiology and the World Heart Federation 2023.
“A couple of takeaway points are that daily cannabis use, but not less frequent cannabis use, was associated with CAD” in the large population-based cohort, said Dr. Paranjpe, a resident physician at Stanford (Calif.) University, during the press conference.
“This analysis was adjusted for several possible confounders including age, sex at birth, [body mass index (BMI)], race, education, cigarette use, hypertension, high cholesterol, and diabetes,” he noted, and even after accounting for these risk factors, the association with heart disease remained.
“And the next thing, using Mendelian randomization, we sort of implied that there might be a causal relationship between cannabis and heart disease. Importantly this effect is independent of alcohol and cigarette use.
“The notion that cannabis is completely benign is probably wrong, and there might be certain risk of certain cardiovascular effects of cannabis we should be more on the lookout for,” Dr. Paranjpe said in an interview.
“Our main conclusion was that prevalent CAD is associated with cannabis consumption,” he added. “Other mechanistic work published in Cell has also shown that cannabis causes vascular inflammation that may lead to CAD.
“Thus, there is growing evidence from both laboratory and population studies that cannabis consumption may be harmful for cardiovascular health,” he said. “However, we still need more work on whether it affects the risk of incident cardiovascular events (i.e., stroke, heart attack) in patient[s] with existing CAD.”
ASCVD risk
Invited to comment, Robert L. Page II, PharmD, chair of the writing group for the American Heart Association’s scientific statement Medical Marijuana, Recreational Cannabis, and Cardiovascular Health, published in 2020, said, “This adds to our hypothesis that if you are using marijuana over a longer period, greater exposure, you’re going to see an increase in the risk” for atherosclerotic cardiovascular disease (ASCVD).
“We’re seeing this increased risk for ASCVD in young adults between ages 18 to 40 – people who think that they’re invincible,” Dr. Page, a professor at the University of Colorado at Denver, Aurora, who was not involved with this research, told this news organization in an interview.
“The bottom line is that the risk that they are seeing is what has also been documented in other observational studies, and it adds fuel to the fire. We need to be paying close attention to this,” he said.
“Primary care [clinicians], cardiologists, need to address this, particularly in younger adults – because that’s where you’re seeing the highest amount of use.”
‘All of Us’ observational study
In the first part of the study, the researchers analyzed data from the “All of Us” cohort comprising adults age 18 and older from 340 inpatient and outpatient sites across the United States.
They identified 57,958 individuals who replied to a questionnaire asking about cannabis use (medicinal or recreational and whether it was edible or used by smoking or vaping) over the past 3 months.
There were 39,678 never-users, 8,749 who used it once or twice, 2,075 who used it monthly, 2,720 who used it weekly, and 4,736 who used it daily.
Of these, 3,506 individuals had CAD, based on medical records.
Only daily users had a significantly higher risk for CAD, compared with never-users (odds ratio, 1.34; P = .001) after adjusting for age, sex, hypertension, hyperlipidemia, type 2 diabetes, BMI, education, insurance status, and cigarette use.
The median age for daily users was 41, whereas the median age for never-users was 59.
GWAS analyses
The researchers then performed a Mendelian randomization analysis based on genome-wide association studies (GWAS) of cannabis use disorder and of CAD.
“Cannabis use disorder is a psychiatric diagnosis of severe cannabis dependency, equivalent to ‘alcohol use disorder’ for alcohol consumption,” Dr. Paranjpe explained. “The exact definition involves frequent use leading to significant dependence (but does not specify how often it is used).”
The GWAS data for cannabis use disorder came from a recent meta-analysis of three cohorts: the Psychiatric Genomics Consortium Substance Use Disorders working group, iPSYCH, and deCODE.
The GWAS statistics for CAD were obtained from the CARDIoGRAMplusC4D Consortium.
Cannabis use disorder was associated with significantly increased odds for CAD (OR, 1.05; P = .001), which remained after adjusting for both cigarette and alcohol use (OR, 1.04).
A version of this article first appeared on Medscape.com.
In the first part, in an observational study, daily cannabis use was associated with 34% higher odds for CAD, compared with never-users, in a large population-based U.S. cohort. Less frequent use was not associated with increased odds for CAD.
In the second part, people with a genetic susceptibility to cannabis use disorder or severe cannabis dependency had an increased risk for CAD, compared with other people.
Ishan Paranjpe, MD, the study’s lead author, reported these results in a press briefing and will present the study at the upcoming joint scientific sessions of the American College of Cardiology and the World Heart Federation 2023.
“A couple of takeaway points are that daily cannabis use, but not less frequent cannabis use, was associated with CAD” in the large population-based cohort, said Dr. Paranjpe, a resident physician at Stanford (Calif.) University, during the press conference.
“This analysis was adjusted for several possible confounders including age, sex at birth, [body mass index (BMI)], race, education, cigarette use, hypertension, high cholesterol, and diabetes,” he noted, and even after accounting for these risk factors, the association with heart disease remained.
“And the next thing, using Mendelian randomization, we sort of implied that there might be a causal relationship between cannabis and heart disease. Importantly this effect is independent of alcohol and cigarette use.
“The notion that cannabis is completely benign is probably wrong, and there might be certain risk of certain cardiovascular effects of cannabis we should be more on the lookout for,” Dr. Paranjpe said in an interview.
“Our main conclusion was that prevalent CAD is associated with cannabis consumption,” he added. “Other mechanistic work published in Cell has also shown that cannabis causes vascular inflammation that may lead to CAD.
“Thus, there is growing evidence from both laboratory and population studies that cannabis consumption may be harmful for cardiovascular health,” he said. “However, we still need more work on whether it affects the risk of incident cardiovascular events (i.e., stroke, heart attack) in patient[s] with existing CAD.”
ASCVD risk
Invited to comment, Robert L. Page II, PharmD, chair of the writing group for the American Heart Association’s scientific statement Medical Marijuana, Recreational Cannabis, and Cardiovascular Health, published in 2020, said, “This adds to our hypothesis that if you are using marijuana over a longer period, greater exposure, you’re going to see an increase in the risk” for atherosclerotic cardiovascular disease (ASCVD).
“We’re seeing this increased risk for ASCVD in young adults between ages 18 to 40 – people who think that they’re invincible,” Dr. Page, a professor at the University of Colorado at Denver, Aurora, who was not involved with this research, told this news organization in an interview.
“The bottom line is that the risk that they are seeing is what has also been documented in other observational studies, and it adds fuel to the fire. We need to be paying close attention to this,” he said.
“Primary care [clinicians], cardiologists, need to address this, particularly in younger adults – because that’s where you’re seeing the highest amount of use.”
‘All of Us’ observational study
In the first part of the study, the researchers analyzed data from the “All of Us” cohort comprising adults age 18 and older from 340 inpatient and outpatient sites across the United States.
They identified 57,958 individuals who replied to a questionnaire asking about cannabis use (medicinal or recreational and whether it was edible or used by smoking or vaping) over the past 3 months.
There were 39,678 never-users, 8,749 who used it once or twice, 2,075 who used it monthly, 2,720 who used it weekly, and 4,736 who used it daily.
Of these, 3,506 individuals had CAD, based on medical records.
Only daily users had a significantly higher risk for CAD, compared with never-users (odds ratio, 1.34; P = .001) after adjusting for age, sex, hypertension, hyperlipidemia, type 2 diabetes, BMI, education, insurance status, and cigarette use.
The median age for daily users was 41, whereas the median age for never-users was 59.
GWAS analyses
The researchers then performed a Mendelian randomization analysis based on genome-wide association studies (GWAS) of cannabis use disorder and of CAD.
“Cannabis use disorder is a psychiatric diagnosis of severe cannabis dependency, equivalent to ‘alcohol use disorder’ for alcohol consumption,” Dr. Paranjpe explained. “The exact definition involves frequent use leading to significant dependence (but does not specify how often it is used).”
The GWAS data for cannabis use disorder came from a recent meta-analysis of three cohorts: the Psychiatric Genomics Consortium Substance Use Disorders working group, iPSYCH, and deCODE.
The GWAS statistics for CAD were obtained from the CARDIoGRAMplusC4D Consortium.
Cannabis use disorder was associated with significantly increased odds for CAD (OR, 1.05; P = .001), which remained after adjusting for both cigarette and alcohol use (OR, 1.04).
A version of this article first appeared on Medscape.com.
In the first part, in an observational study, daily cannabis use was associated with 34% higher odds for CAD, compared with never-users, in a large population-based U.S. cohort. Less frequent use was not associated with increased odds for CAD.
In the second part, people with a genetic susceptibility to cannabis use disorder or severe cannabis dependency had an increased risk for CAD, compared with other people.
Ishan Paranjpe, MD, the study’s lead author, reported these results in a press briefing and will present the study at the upcoming joint scientific sessions of the American College of Cardiology and the World Heart Federation 2023.
“A couple of takeaway points are that daily cannabis use, but not less frequent cannabis use, was associated with CAD” in the large population-based cohort, said Dr. Paranjpe, a resident physician at Stanford (Calif.) University, during the press conference.
“This analysis was adjusted for several possible confounders including age, sex at birth, [body mass index (BMI)], race, education, cigarette use, hypertension, high cholesterol, and diabetes,” he noted, and even after accounting for these risk factors, the association with heart disease remained.
“And the next thing, using Mendelian randomization, we sort of implied that there might be a causal relationship between cannabis and heart disease. Importantly this effect is independent of alcohol and cigarette use.
“The notion that cannabis is completely benign is probably wrong, and there might be certain risk of certain cardiovascular effects of cannabis we should be more on the lookout for,” Dr. Paranjpe said in an interview.
“Our main conclusion was that prevalent CAD is associated with cannabis consumption,” he added. “Other mechanistic work published in Cell has also shown that cannabis causes vascular inflammation that may lead to CAD.
“Thus, there is growing evidence from both laboratory and population studies that cannabis consumption may be harmful for cardiovascular health,” he said. “However, we still need more work on whether it affects the risk of incident cardiovascular events (i.e., stroke, heart attack) in patient[s] with existing CAD.”
ASCVD risk
Invited to comment, Robert L. Page II, PharmD, chair of the writing group for the American Heart Association’s scientific statement Medical Marijuana, Recreational Cannabis, and Cardiovascular Health, published in 2020, said, “This adds to our hypothesis that if you are using marijuana over a longer period, greater exposure, you’re going to see an increase in the risk” for atherosclerotic cardiovascular disease (ASCVD).
“We’re seeing this increased risk for ASCVD in young adults between ages 18 to 40 – people who think that they’re invincible,” Dr. Page, a professor at the University of Colorado at Denver, Aurora, who was not involved with this research, told this news organization in an interview.
“The bottom line is that the risk that they are seeing is what has also been documented in other observational studies, and it adds fuel to the fire. We need to be paying close attention to this,” he said.
“Primary care [clinicians], cardiologists, need to address this, particularly in younger adults – because that’s where you’re seeing the highest amount of use.”
‘All of Us’ observational study
In the first part of the study, the researchers analyzed data from the “All of Us” cohort comprising adults age 18 and older from 340 inpatient and outpatient sites across the United States.
They identified 57,958 individuals who replied to a questionnaire asking about cannabis use (medicinal or recreational and whether it was edible or used by smoking or vaping) over the past 3 months.
There were 39,678 never-users, 8,749 who used it once or twice, 2,075 who used it monthly, 2,720 who used it weekly, and 4,736 who used it daily.
Of these, 3,506 individuals had CAD, based on medical records.
Only daily users had a significantly higher risk for CAD, compared with never-users (odds ratio, 1.34; P = .001) after adjusting for age, sex, hypertension, hyperlipidemia, type 2 diabetes, BMI, education, insurance status, and cigarette use.
The median age for daily users was 41, whereas the median age for never-users was 59.
GWAS analyses
The researchers then performed a Mendelian randomization analysis based on genome-wide association studies (GWAS) of cannabis use disorder and of CAD.
“Cannabis use disorder is a psychiatric diagnosis of severe cannabis dependency, equivalent to ‘alcohol use disorder’ for alcohol consumption,” Dr. Paranjpe explained. “The exact definition involves frequent use leading to significant dependence (but does not specify how often it is used).”
The GWAS data for cannabis use disorder came from a recent meta-analysis of three cohorts: the Psychiatric Genomics Consortium Substance Use Disorders working group, iPSYCH, and deCODE.
The GWAS statistics for CAD were obtained from the CARDIoGRAMplusC4D Consortium.
Cannabis use disorder was associated with significantly increased odds for CAD (OR, 1.05; P = .001), which remained after adjusting for both cigarette and alcohol use (OR, 1.04).
A version of this article first appeared on Medscape.com.
FROM ACC 2023
Old drug verapamil may have new use in type 1 diabetes
In children and adolescents with new-onset type 1 diabetes, the calcium channel blocker verapamil slowed the destruction of insulin-producing pancreatic beta cells for up to a year, new data show.
Use of daily verapamil within a month of diagnosis resulted in a 30% increase in C-peptide secretion (a measure of preserved beta-cell function), compared with placebo at 52 weeks, without serious adverse events.
To put it another way, verapamil delayed the expected decline in C-peptide production from 3 months after diagnosis of type 1 diabetes to 6 months after diagnosis.
“We think this is a really, really exciting finding that’s hopefully going to impact the care for children with type 1 diabetes in the new-onset period,” lead author Gregory P. Forlenza, MD, said during his presentation of the data on Feb. 24 at the annual Advanced Technologies & Treatments for Diabetes (ATTD) meeting in Berlin.
“In view of the favorable safety profile, particularly compared with immune-suppressive agents, once-a-day oral administration, and low cost, initiation of verapamil should be a consideration for newly diagnosed patients with type 1 diabetes,” added Dr. Forlenza, a pediatric endocrinologist at the Barbara Davis Center for Diabetes, Anschutz Medical Campus, University of Colorado, Aurora.
The data were also simultaneously published in JAMA, as part of the CLVer (Hybrid Closed Loop Therapy and Verapamil for Beta Cell Preservation in New Onset Type 1 Diabetes) trial.
The randomized, double-blind, six-center trial involved 113 participants, aged 7-17 years, with newly diagnosed type 1 diabetes. They were randomized to the most advanced commercially available automated insulin delivery systems available or standard care to test the effects of intensive glucose control on C-peptide levels for 52 weeks during the COVID-19 pandemic (July 2020 to September 2022). Eighty-eight patients who weighed 30 kg (66 lb) or more were further randomized (1:1) to daily extended-release verapamil or placebo for the same duration.
The positive findings for verapamil, published in one paper, contrasted with the negative ones for the automated insulin delivery (AID) system. The latter did not prevent the expected decline in C-peptide, putting to rest a long-held hypothesis that reducing glucotoxicity might preserve beta-cell function in newly diagnosed individuals with type 1 diabetes, noted Dr. Forlenza.
Could combination therapy work?
In recent years, immune-modulating agents have increasingly been shown to preserve beta-cell function in both new-onset and preclinical type 1 diabetes. One such agent, teplizumab (Tzield, Provention Bio), was approved by the U.S. Food and Drug Administration in November 2022 to delay type 1 diabetes onset in those at high risk.
Calcium channel blockers such as verapamil – used for years to treat hypertension and cardiac arrhythmias – may accomplish the same goal as teplizumab but in a different way, by reducing the protein overexpression that induces beta-cell apoptosis and death.
Dr. Forlenza showed a slide comparing the preservation of C-peptide, which was much lower with verapamil, at 30%, than with teplizumab, at 75%.
Asked to comment, session moderator Torben Biester, MD, a pediatric diabetologist at Auf der Bult-Zentrum Diabetes-Center for Children and Adolescents, Hanover, Germany, said: “[Verapamil] is a very cheap [daily] pill. [Teplizumab] is a very high-priced ... immune therapy in the United States ... an infusion twice for 10 days, so it’s a lot more burden for the patients and a lot more risk of side effects.”
“The future might be combination therapy,” added Dr. Biester.
And in an editorial published in JAMA and accompanying the two CLVer papers, Jennifer Couper, MD, of the University of Adelaide, agrees: “A well-tolerated, inexpensive, oral treatment such as verapamil with modest benefits on C-peptide production is relevant to practice.”
The new work “supports investigation of verapamil in combination with other effective agents during the earlier stages of type 1 diabetes before insulin dependence develops,” she noted.
Verapamil results ‘brilliant’ but more work needed
In the verapamil part of the CLVer trial, by 52 weeks, verapamil doses in the youth who received it ranged from 120-360 mg/day based on weight and tolerance.
The primary outcome, C-peptide area under the curve, stayed stable, from 0.66 pmol/mL at baseline to 0.65 pmol/mL at 52 weeks in the verapamil group, compared with a drop from 0.60 pmol/mL down to 0.44 pmol/mL with placebo, a significant difference of 0.14 pmol/mL (P = .04), representing a 30% higher C-peptide level in the verapamil group.
“For us, this is a phenomenally exciting result,” Dr. Forlenza commented during his presentation.
At 52 weeks, A1c was 6.6% in the verapamil group versus 6.9% with placebo, which was not significantly different. Daily insulin dose was 0.65 versus 0.74 units/kg per day, respectively, also not significantly different.
One severe hypoglycemic event occurred in each group, and one diabetic ketoacidosis event occurred in the placebo group. In the verapamil group, three participants experienced “nonserious” electrocardiogram abnormalities and one had hypertension.
Dr. Biester said he isn’t “that concerned” about the small number of mild ECG abnormalities seen in the study with verapamil, as this is a known side effect. But overall, he said, “I would think that for a recommendation for routine use it’s too early after one study, even though the results are brilliant.”
He noted that he is involved in a similar ongoing study of verapamil in adults with new-onset type 1 diabetes, called Ver-A-T1D.
No C-peptide effect of tight glycemic control: ‘A tough pill’
In the AID part of the study, the 113 participants were randomized 2:1 to one of two commercially available AID systems (Tandem t:slim X2 with Control-IQ or Medtronic 670G or 780G) plus frequent contact (a median of 35 times) by study staff, or standard management using a continuous glucose monitor (CGM) with an insulin pump or multiple daily injections.
At 52 weeks, A1c was 6.5% for the intensive group versus 7.1% with standard care, a significant difference. Time in blood glucose range of 70-180 mg/dL was significantly longer with intensive management, at 78%, compared with standard care, at 64%.
Nonetheless, the change in C-peptide area under the curve did not differ between the two groups, decreasing from 0.57 pmol/mL at baseline to 0.45 pmol/mL at 52 weeks with the AID system, compared with a decrease from 0.60 pmol/L down to 0.50 pmol/L with standard care (P = .89).
Dr. Forlenza commented that the hypothesis that tight glycemic control would delay the decline in C-peptide secretion “is something I think a lot of endocrinologists assumed to be true and something I’ve heard lots of colleagues over the years talk about.”
Consequently, he said these findings are “a tough pill for us to swallow ... but it’s important for us in the field to understand.”
“Even with frequent contacts that are well above the level we’d be able to do in standard clinical care, and even with use of the most advanced AID systems we have ... we saw absolutely no difference in stimulated C-peptide levels at any of the timepoints throughout the first year or at 52 weeks.”
“So, in our opinion, this,” combined with a prior study from 2022, “should put this hypothesis to rest,” he said.
“Excellent glycemic control has a benefit in and of itself, but it was not a successful intervention for beta-cell preservation.”
Dr. Forlenza has reported serving as a consultant, speaker, or advisory board member for Medtronic, Dexcom, Abbott, Tandem Diabetes Care, Insulet, Lilly, and Beta Bionics, and his institution has also received funding on his behalf for research grants from these companies. Dr. Biester has reported receiving speaker’s fees from DexCom, Medtronic, Novo Nordisk, F. Hoffmann–La Roche, Sanofi, and Ypsomed Holding; serving on advisory boards for Ascensia Diabetes Care Holdings, AstraZeneca, DexCom, and Medtronic; and receiving personal fees from SYNLAB; and is a member of the European Commission Expert Panel for Medical Devices for Endocrinology and Diabetes. Dr. Couper has reported no relevant financial relationships.
The rationale for the companion CLVer analysis of the effect of reducing glucose toxicity via tight glycemic control on C-peptide progression dates back to an inpatient study published in 1989 involving 26 adolescents using an early artificial pancreas prototype called a Biostator, in which beta-cell preservation was achieved. However, two more recent studies of this approach, including one published in late 2022, did not show a difference. The CLVer analysis involved 113 participants randomized 2:1 to one of two commercially available AID systems (Tandem t:slim X2 with Control-IQ or Medtronic 670G or 780G) plus frequent contact by study staff, or standard management using a CGM with a pump or multiple daily injections.
A version of this article originally appeared on Medscape.com.
In children and adolescents with new-onset type 1 diabetes, the calcium channel blocker verapamil slowed the destruction of insulin-producing pancreatic beta cells for up to a year, new data show.
Use of daily verapamil within a month of diagnosis resulted in a 30% increase in C-peptide secretion (a measure of preserved beta-cell function), compared with placebo at 52 weeks, without serious adverse events.
To put it another way, verapamil delayed the expected decline in C-peptide production from 3 months after diagnosis of type 1 diabetes to 6 months after diagnosis.
“We think this is a really, really exciting finding that’s hopefully going to impact the care for children with type 1 diabetes in the new-onset period,” lead author Gregory P. Forlenza, MD, said during his presentation of the data on Feb. 24 at the annual Advanced Technologies & Treatments for Diabetes (ATTD) meeting in Berlin.
“In view of the favorable safety profile, particularly compared with immune-suppressive agents, once-a-day oral administration, and low cost, initiation of verapamil should be a consideration for newly diagnosed patients with type 1 diabetes,” added Dr. Forlenza, a pediatric endocrinologist at the Barbara Davis Center for Diabetes, Anschutz Medical Campus, University of Colorado, Aurora.
The data were also simultaneously published in JAMA, as part of the CLVer (Hybrid Closed Loop Therapy and Verapamil for Beta Cell Preservation in New Onset Type 1 Diabetes) trial.
The randomized, double-blind, six-center trial involved 113 participants, aged 7-17 years, with newly diagnosed type 1 diabetes. They were randomized to the most advanced commercially available automated insulin delivery systems available or standard care to test the effects of intensive glucose control on C-peptide levels for 52 weeks during the COVID-19 pandemic (July 2020 to September 2022). Eighty-eight patients who weighed 30 kg (66 lb) or more were further randomized (1:1) to daily extended-release verapamil or placebo for the same duration.
The positive findings for verapamil, published in one paper, contrasted with the negative ones for the automated insulin delivery (AID) system. The latter did not prevent the expected decline in C-peptide, putting to rest a long-held hypothesis that reducing glucotoxicity might preserve beta-cell function in newly diagnosed individuals with type 1 diabetes, noted Dr. Forlenza.
Could combination therapy work?
In recent years, immune-modulating agents have increasingly been shown to preserve beta-cell function in both new-onset and preclinical type 1 diabetes. One such agent, teplizumab (Tzield, Provention Bio), was approved by the U.S. Food and Drug Administration in November 2022 to delay type 1 diabetes onset in those at high risk.
Calcium channel blockers such as verapamil – used for years to treat hypertension and cardiac arrhythmias – may accomplish the same goal as teplizumab but in a different way, by reducing the protein overexpression that induces beta-cell apoptosis and death.
Dr. Forlenza showed a slide comparing the preservation of C-peptide, which was much lower with verapamil, at 30%, than with teplizumab, at 75%.
Asked to comment, session moderator Torben Biester, MD, a pediatric diabetologist at Auf der Bult-Zentrum Diabetes-Center for Children and Adolescents, Hanover, Germany, said: “[Verapamil] is a very cheap [daily] pill. [Teplizumab] is a very high-priced ... immune therapy in the United States ... an infusion twice for 10 days, so it’s a lot more burden for the patients and a lot more risk of side effects.”
“The future might be combination therapy,” added Dr. Biester.
And in an editorial published in JAMA and accompanying the two CLVer papers, Jennifer Couper, MD, of the University of Adelaide, agrees: “A well-tolerated, inexpensive, oral treatment such as verapamil with modest benefits on C-peptide production is relevant to practice.”
The new work “supports investigation of verapamil in combination with other effective agents during the earlier stages of type 1 diabetes before insulin dependence develops,” she noted.
Verapamil results ‘brilliant’ but more work needed
In the verapamil part of the CLVer trial, by 52 weeks, verapamil doses in the youth who received it ranged from 120-360 mg/day based on weight and tolerance.
The primary outcome, C-peptide area under the curve, stayed stable, from 0.66 pmol/mL at baseline to 0.65 pmol/mL at 52 weeks in the verapamil group, compared with a drop from 0.60 pmol/mL down to 0.44 pmol/mL with placebo, a significant difference of 0.14 pmol/mL (P = .04), representing a 30% higher C-peptide level in the verapamil group.
“For us, this is a phenomenally exciting result,” Dr. Forlenza commented during his presentation.
At 52 weeks, A1c was 6.6% in the verapamil group versus 6.9% with placebo, which was not significantly different. Daily insulin dose was 0.65 versus 0.74 units/kg per day, respectively, also not significantly different.
One severe hypoglycemic event occurred in each group, and one diabetic ketoacidosis event occurred in the placebo group. In the verapamil group, three participants experienced “nonserious” electrocardiogram abnormalities and one had hypertension.
Dr. Biester said he isn’t “that concerned” about the small number of mild ECG abnormalities seen in the study with verapamil, as this is a known side effect. But overall, he said, “I would think that for a recommendation for routine use it’s too early after one study, even though the results are brilliant.”
He noted that he is involved in a similar ongoing study of verapamil in adults with new-onset type 1 diabetes, called Ver-A-T1D.
No C-peptide effect of tight glycemic control: ‘A tough pill’
In the AID part of the study, the 113 participants were randomized 2:1 to one of two commercially available AID systems (Tandem t:slim X2 with Control-IQ or Medtronic 670G or 780G) plus frequent contact (a median of 35 times) by study staff, or standard management using a continuous glucose monitor (CGM) with an insulin pump or multiple daily injections.
At 52 weeks, A1c was 6.5% for the intensive group versus 7.1% with standard care, a significant difference. Time in blood glucose range of 70-180 mg/dL was significantly longer with intensive management, at 78%, compared with standard care, at 64%.
Nonetheless, the change in C-peptide area under the curve did not differ between the two groups, decreasing from 0.57 pmol/mL at baseline to 0.45 pmol/mL at 52 weeks with the AID system, compared with a decrease from 0.60 pmol/L down to 0.50 pmol/L with standard care (P = .89).
Dr. Forlenza commented that the hypothesis that tight glycemic control would delay the decline in C-peptide secretion “is something I think a lot of endocrinologists assumed to be true and something I’ve heard lots of colleagues over the years talk about.”
Consequently, he said these findings are “a tough pill for us to swallow ... but it’s important for us in the field to understand.”
“Even with frequent contacts that are well above the level we’d be able to do in standard clinical care, and even with use of the most advanced AID systems we have ... we saw absolutely no difference in stimulated C-peptide levels at any of the timepoints throughout the first year or at 52 weeks.”
“So, in our opinion, this,” combined with a prior study from 2022, “should put this hypothesis to rest,” he said.
“Excellent glycemic control has a benefit in and of itself, but it was not a successful intervention for beta-cell preservation.”
Dr. Forlenza has reported serving as a consultant, speaker, or advisory board member for Medtronic, Dexcom, Abbott, Tandem Diabetes Care, Insulet, Lilly, and Beta Bionics, and his institution has also received funding on his behalf for research grants from these companies. Dr. Biester has reported receiving speaker’s fees from DexCom, Medtronic, Novo Nordisk, F. Hoffmann–La Roche, Sanofi, and Ypsomed Holding; serving on advisory boards for Ascensia Diabetes Care Holdings, AstraZeneca, DexCom, and Medtronic; and receiving personal fees from SYNLAB; and is a member of the European Commission Expert Panel for Medical Devices for Endocrinology and Diabetes. Dr. Couper has reported no relevant financial relationships.
The rationale for the companion CLVer analysis of the effect of reducing glucose toxicity via tight glycemic control on C-peptide progression dates back to an inpatient study published in 1989 involving 26 adolescents using an early artificial pancreas prototype called a Biostator, in which beta-cell preservation was achieved. However, two more recent studies of this approach, including one published in late 2022, did not show a difference. The CLVer analysis involved 113 participants randomized 2:1 to one of two commercially available AID systems (Tandem t:slim X2 with Control-IQ or Medtronic 670G or 780G) plus frequent contact by study staff, or standard management using a CGM with a pump or multiple daily injections.
A version of this article originally appeared on Medscape.com.
In children and adolescents with new-onset type 1 diabetes, the calcium channel blocker verapamil slowed the destruction of insulin-producing pancreatic beta cells for up to a year, new data show.
Use of daily verapamil within a month of diagnosis resulted in a 30% increase in C-peptide secretion (a measure of preserved beta-cell function), compared with placebo at 52 weeks, without serious adverse events.
To put it another way, verapamil delayed the expected decline in C-peptide production from 3 months after diagnosis of type 1 diabetes to 6 months after diagnosis.
“We think this is a really, really exciting finding that’s hopefully going to impact the care for children with type 1 diabetes in the new-onset period,” lead author Gregory P. Forlenza, MD, said during his presentation of the data on Feb. 24 at the annual Advanced Technologies & Treatments for Diabetes (ATTD) meeting in Berlin.
“In view of the favorable safety profile, particularly compared with immune-suppressive agents, once-a-day oral administration, and low cost, initiation of verapamil should be a consideration for newly diagnosed patients with type 1 diabetes,” added Dr. Forlenza, a pediatric endocrinologist at the Barbara Davis Center for Diabetes, Anschutz Medical Campus, University of Colorado, Aurora.
The data were also simultaneously published in JAMA, as part of the CLVer (Hybrid Closed Loop Therapy and Verapamil for Beta Cell Preservation in New Onset Type 1 Diabetes) trial.
The randomized, double-blind, six-center trial involved 113 participants, aged 7-17 years, with newly diagnosed type 1 diabetes. They were randomized to the most advanced commercially available automated insulin delivery systems available or standard care to test the effects of intensive glucose control on C-peptide levels for 52 weeks during the COVID-19 pandemic (July 2020 to September 2022). Eighty-eight patients who weighed 30 kg (66 lb) or more were further randomized (1:1) to daily extended-release verapamil or placebo for the same duration.
The positive findings for verapamil, published in one paper, contrasted with the negative ones for the automated insulin delivery (AID) system. The latter did not prevent the expected decline in C-peptide, putting to rest a long-held hypothesis that reducing glucotoxicity might preserve beta-cell function in newly diagnosed individuals with type 1 diabetes, noted Dr. Forlenza.
Could combination therapy work?
In recent years, immune-modulating agents have increasingly been shown to preserve beta-cell function in both new-onset and preclinical type 1 diabetes. One such agent, teplizumab (Tzield, Provention Bio), was approved by the U.S. Food and Drug Administration in November 2022 to delay type 1 diabetes onset in those at high risk.
Calcium channel blockers such as verapamil – used for years to treat hypertension and cardiac arrhythmias – may accomplish the same goal as teplizumab but in a different way, by reducing the protein overexpression that induces beta-cell apoptosis and death.
Dr. Forlenza showed a slide comparing the preservation of C-peptide, which was much lower with verapamil, at 30%, than with teplizumab, at 75%.
Asked to comment, session moderator Torben Biester, MD, a pediatric diabetologist at Auf der Bult-Zentrum Diabetes-Center for Children and Adolescents, Hanover, Germany, said: “[Verapamil] is a very cheap [daily] pill. [Teplizumab] is a very high-priced ... immune therapy in the United States ... an infusion twice for 10 days, so it’s a lot more burden for the patients and a lot more risk of side effects.”
“The future might be combination therapy,” added Dr. Biester.
And in an editorial published in JAMA and accompanying the two CLVer papers, Jennifer Couper, MD, of the University of Adelaide, agrees: “A well-tolerated, inexpensive, oral treatment such as verapamil with modest benefits on C-peptide production is relevant to practice.”
The new work “supports investigation of verapamil in combination with other effective agents during the earlier stages of type 1 diabetes before insulin dependence develops,” she noted.
Verapamil results ‘brilliant’ but more work needed
In the verapamil part of the CLVer trial, by 52 weeks, verapamil doses in the youth who received it ranged from 120-360 mg/day based on weight and tolerance.
The primary outcome, C-peptide area under the curve, stayed stable, from 0.66 pmol/mL at baseline to 0.65 pmol/mL at 52 weeks in the verapamil group, compared with a drop from 0.60 pmol/mL down to 0.44 pmol/mL with placebo, a significant difference of 0.14 pmol/mL (P = .04), representing a 30% higher C-peptide level in the verapamil group.
“For us, this is a phenomenally exciting result,” Dr. Forlenza commented during his presentation.
At 52 weeks, A1c was 6.6% in the verapamil group versus 6.9% with placebo, which was not significantly different. Daily insulin dose was 0.65 versus 0.74 units/kg per day, respectively, also not significantly different.
One severe hypoglycemic event occurred in each group, and one diabetic ketoacidosis event occurred in the placebo group. In the verapamil group, three participants experienced “nonserious” electrocardiogram abnormalities and one had hypertension.
Dr. Biester said he isn’t “that concerned” about the small number of mild ECG abnormalities seen in the study with verapamil, as this is a known side effect. But overall, he said, “I would think that for a recommendation for routine use it’s too early after one study, even though the results are brilliant.”
He noted that he is involved in a similar ongoing study of verapamil in adults with new-onset type 1 diabetes, called Ver-A-T1D.
No C-peptide effect of tight glycemic control: ‘A tough pill’
In the AID part of the study, the 113 participants were randomized 2:1 to one of two commercially available AID systems (Tandem t:slim X2 with Control-IQ or Medtronic 670G or 780G) plus frequent contact (a median of 35 times) by study staff, or standard management using a continuous glucose monitor (CGM) with an insulin pump or multiple daily injections.
At 52 weeks, A1c was 6.5% for the intensive group versus 7.1% with standard care, a significant difference. Time in blood glucose range of 70-180 mg/dL was significantly longer with intensive management, at 78%, compared with standard care, at 64%.
Nonetheless, the change in C-peptide area under the curve did not differ between the two groups, decreasing from 0.57 pmol/mL at baseline to 0.45 pmol/mL at 52 weeks with the AID system, compared with a decrease from 0.60 pmol/L down to 0.50 pmol/L with standard care (P = .89).
Dr. Forlenza commented that the hypothesis that tight glycemic control would delay the decline in C-peptide secretion “is something I think a lot of endocrinologists assumed to be true and something I’ve heard lots of colleagues over the years talk about.”
Consequently, he said these findings are “a tough pill for us to swallow ... but it’s important for us in the field to understand.”
“Even with frequent contacts that are well above the level we’d be able to do in standard clinical care, and even with use of the most advanced AID systems we have ... we saw absolutely no difference in stimulated C-peptide levels at any of the timepoints throughout the first year or at 52 weeks.”
“So, in our opinion, this,” combined with a prior study from 2022, “should put this hypothesis to rest,” he said.
“Excellent glycemic control has a benefit in and of itself, but it was not a successful intervention for beta-cell preservation.”
Dr. Forlenza has reported serving as a consultant, speaker, or advisory board member for Medtronic, Dexcom, Abbott, Tandem Diabetes Care, Insulet, Lilly, and Beta Bionics, and his institution has also received funding on his behalf for research grants from these companies. Dr. Biester has reported receiving speaker’s fees from DexCom, Medtronic, Novo Nordisk, F. Hoffmann–La Roche, Sanofi, and Ypsomed Holding; serving on advisory boards for Ascensia Diabetes Care Holdings, AstraZeneca, DexCom, and Medtronic; and receiving personal fees from SYNLAB; and is a member of the European Commission Expert Panel for Medical Devices for Endocrinology and Diabetes. Dr. Couper has reported no relevant financial relationships.
The rationale for the companion CLVer analysis of the effect of reducing glucose toxicity via tight glycemic control on C-peptide progression dates back to an inpatient study published in 1989 involving 26 adolescents using an early artificial pancreas prototype called a Biostator, in which beta-cell preservation was achieved. However, two more recent studies of this approach, including one published in late 2022, did not show a difference. The CLVer analysis involved 113 participants randomized 2:1 to one of two commercially available AID systems (Tandem t:slim X2 with Control-IQ or Medtronic 670G or 780G) plus frequent contact by study staff, or standard management using a CGM with a pump or multiple daily injections.
A version of this article originally appeared on Medscape.com.
Insomnia, short sleep linked to greater risk for MI
Insomnia – difficulty falling or staying asleep – was associated with a 69% greater risk of having a myocardial infarction than among adults without insomnia, according to new research.
Those who slept 5 or fewer hours per night had the highest risk for MI, and those with both diabetes and insomnia had double the risk for MI, compared with patients without these comorbidities.
The findings are from a meta-analysis of studies in more than 1 million patients, almost all without prior MI who were, on average, in their early 50s and followed for 9 years.
Yomna E. Dean, a medical student at Alexandria (Egypt) University, reported these results in a press briefing, and the study was simultaneously published in Clinical Cardiology. It will be presented at the upcoming at the annual scientific sessions of the American College of Cardiology.
“Insomnia and ]at least] 5 hours of sleep are highly associated with increased incidence of MI, an association comparable to that of other MI risk factors and as such, it should be considered as a risk factor for MI and to be incorporated into MI prevention guidelines,” the researchers concluded.
“We believe that [insomnia] should be screened and patients should be educated about the importance of sleep because nowadays insomnia is no longer a disease – sleep deprivation could also be a life choice,” Ms, Dean told a press conference prior to the meeting.
“Clinicians must educate the patients about the importance of sleep in maintaining a healthy heart and encourage proper sleep hygiene,” Ms. Dean reiterated in an email. “And if a patient still has insomnia, other methods should be considered such as cognitive-behavior[al] therapy for insomnia [CBT-I].”
Adds to growing evidence
This study does not allow any conclusion about whether treating insomnia will reduce heart attack risk, Jennifer L. Martin, PhD, president of the American Academy of Sleep Medicine, noted in a comment. Nor does it report the diversity of study participants, since insomnia is also a health equity issue, she noted, and insomnia symptoms and comorbidities were self-reported.
However, this analysis “adds to the growing evidence that poor quality or insufficient sleep is associated with poor health,” said Dr. Martin, professor of medicine at the University of California, Los Angeles, who was not involved with this research.
The study reinforces the recommendation from the American Heart Association, which includes “Get Healthy Sleep” as one of “Life’s Essential 8” for heart health, Dr. Martin noted.
“Particularly in primary care where disease prevention and health promotion are important, clinicians should be asking all patients about their sleep – just like they ask about diet and exercise – as a key aspect of maintaining heart health,” she said.
Advice about basic sleep hygiene advice is a first step, she noted.
When improved sleep hygiene is not enough to address chronic insomnia, the AASM’s clinical practice guidelines and the guidelines of the Department of Veterans Affairs/Department of Defense, recommend first-line treatment with CBT-I, typically offered by a sleep specialist or mental health clinician.
Similarly, the American College of Physicians suggests that sleeping pills should be reserved for short-term use in patients who may not benefit sufficiently from CBT-I.
Sleeping too little, too much, equally harmful
“Studies have found that insomnia and subsequent sleep deprivation puts the body under stress,” Ms. Dean said. “This triggers cortisol release which could accelerate atherosclerosis,” and increase risk of MI.
For this analysis, the researchers identified nine observational studies, published from 1998 to 2019, with data on incident MI in adults who had insomnia.
The diagnosis of insomnia was based on ICD diagnostic codes or on the DSM‐5, which defines insomnia as the presence of any of the following three symptoms: difficulty initiating sleep, difficulty maintaining sleep, or early morning awakening with inability to return to sleep. Patients with sleep apnea were excluded.
The studies were in populations in China, Germany, Norway, Taiwan, United Kingdom, and United States, in 1.1 million adults aged 18 and older. The patients had a mean age of 52 years and 13% had insomnia.
During follow-up, 2,406 of 153,881 patients with insomnia, and 12,398 of 1,030,375 patients without insomnia had an MI.
In the pooled analysis, patients with insomnia had a significantly increased risk of MI (relative risk, 1.69; P < .00001), after adjusting for age, gender, diabetes, hypertension, high cholesterol, and smoking.
Sleeping 5 hours or less was associated with a greater risk for MI than sleeping 6 hours, or 7-8 hours, but sleeping 9 hours or more was just as harmful.
Patients who had difficulty initiating and maintaining sleep – two symptoms of insomnia – had a 13% increased risk for MI compared with other patients (RR, 1.13; P = .003).
However, patients who had nonrestorative sleep and daytime dysfunction despite adequate sleep – which is common – did not have an increased risk of MI, compared with other patients (RR, 1.06; P = .46).
Women with insomnia had a 2.24-fold greater risk for MI than other women, whereas men with insomnia had a 2.03-fold greater risk for MI than other men.
Patients with insomnia had a greater risk for MI than those without insomnia in subgroups based on patients’ age (< 65 and > 65), follow up duration (≤ 5 years and > 5 years), and comorbidities (diabetes, hypertension, and hyperlipidemia).
The authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Insomnia – difficulty falling or staying asleep – was associated with a 69% greater risk of having a myocardial infarction than among adults without insomnia, according to new research.
Those who slept 5 or fewer hours per night had the highest risk for MI, and those with both diabetes and insomnia had double the risk for MI, compared with patients without these comorbidities.
The findings are from a meta-analysis of studies in more than 1 million patients, almost all without prior MI who were, on average, in their early 50s and followed for 9 years.
Yomna E. Dean, a medical student at Alexandria (Egypt) University, reported these results in a press briefing, and the study was simultaneously published in Clinical Cardiology. It will be presented at the upcoming at the annual scientific sessions of the American College of Cardiology.
“Insomnia and ]at least] 5 hours of sleep are highly associated with increased incidence of MI, an association comparable to that of other MI risk factors and as such, it should be considered as a risk factor for MI and to be incorporated into MI prevention guidelines,” the researchers concluded.
“We believe that [insomnia] should be screened and patients should be educated about the importance of sleep because nowadays insomnia is no longer a disease – sleep deprivation could also be a life choice,” Ms, Dean told a press conference prior to the meeting.
“Clinicians must educate the patients about the importance of sleep in maintaining a healthy heart and encourage proper sleep hygiene,” Ms. Dean reiterated in an email. “And if a patient still has insomnia, other methods should be considered such as cognitive-behavior[al] therapy for insomnia [CBT-I].”
Adds to growing evidence
This study does not allow any conclusion about whether treating insomnia will reduce heart attack risk, Jennifer L. Martin, PhD, president of the American Academy of Sleep Medicine, noted in a comment. Nor does it report the diversity of study participants, since insomnia is also a health equity issue, she noted, and insomnia symptoms and comorbidities were self-reported.
However, this analysis “adds to the growing evidence that poor quality or insufficient sleep is associated with poor health,” said Dr. Martin, professor of medicine at the University of California, Los Angeles, who was not involved with this research.
The study reinforces the recommendation from the American Heart Association, which includes “Get Healthy Sleep” as one of “Life’s Essential 8” for heart health, Dr. Martin noted.
“Particularly in primary care where disease prevention and health promotion are important, clinicians should be asking all patients about their sleep – just like they ask about diet and exercise – as a key aspect of maintaining heart health,” she said.
Advice about basic sleep hygiene advice is a first step, she noted.
When improved sleep hygiene is not enough to address chronic insomnia, the AASM’s clinical practice guidelines and the guidelines of the Department of Veterans Affairs/Department of Defense, recommend first-line treatment with CBT-I, typically offered by a sleep specialist or mental health clinician.
Similarly, the American College of Physicians suggests that sleeping pills should be reserved for short-term use in patients who may not benefit sufficiently from CBT-I.
Sleeping too little, too much, equally harmful
“Studies have found that insomnia and subsequent sleep deprivation puts the body under stress,” Ms. Dean said. “This triggers cortisol release which could accelerate atherosclerosis,” and increase risk of MI.
For this analysis, the researchers identified nine observational studies, published from 1998 to 2019, with data on incident MI in adults who had insomnia.
The diagnosis of insomnia was based on ICD diagnostic codes or on the DSM‐5, which defines insomnia as the presence of any of the following three symptoms: difficulty initiating sleep, difficulty maintaining sleep, or early morning awakening with inability to return to sleep. Patients with sleep apnea were excluded.
The studies were in populations in China, Germany, Norway, Taiwan, United Kingdom, and United States, in 1.1 million adults aged 18 and older. The patients had a mean age of 52 years and 13% had insomnia.
During follow-up, 2,406 of 153,881 patients with insomnia, and 12,398 of 1,030,375 patients without insomnia had an MI.
In the pooled analysis, patients with insomnia had a significantly increased risk of MI (relative risk, 1.69; P < .00001), after adjusting for age, gender, diabetes, hypertension, high cholesterol, and smoking.
Sleeping 5 hours or less was associated with a greater risk for MI than sleeping 6 hours, or 7-8 hours, but sleeping 9 hours or more was just as harmful.
Patients who had difficulty initiating and maintaining sleep – two symptoms of insomnia – had a 13% increased risk for MI compared with other patients (RR, 1.13; P = .003).
However, patients who had nonrestorative sleep and daytime dysfunction despite adequate sleep – which is common – did not have an increased risk of MI, compared with other patients (RR, 1.06; P = .46).
Women with insomnia had a 2.24-fold greater risk for MI than other women, whereas men with insomnia had a 2.03-fold greater risk for MI than other men.
Patients with insomnia had a greater risk for MI than those without insomnia in subgroups based on patients’ age (< 65 and > 65), follow up duration (≤ 5 years and > 5 years), and comorbidities (diabetes, hypertension, and hyperlipidemia).
The authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Insomnia – difficulty falling or staying asleep – was associated with a 69% greater risk of having a myocardial infarction than among adults without insomnia, according to new research.
Those who slept 5 or fewer hours per night had the highest risk for MI, and those with both diabetes and insomnia had double the risk for MI, compared with patients without these comorbidities.
The findings are from a meta-analysis of studies in more than 1 million patients, almost all without prior MI who were, on average, in their early 50s and followed for 9 years.
Yomna E. Dean, a medical student at Alexandria (Egypt) University, reported these results in a press briefing, and the study was simultaneously published in Clinical Cardiology. It will be presented at the upcoming at the annual scientific sessions of the American College of Cardiology.
“Insomnia and ]at least] 5 hours of sleep are highly associated with increased incidence of MI, an association comparable to that of other MI risk factors and as such, it should be considered as a risk factor for MI and to be incorporated into MI prevention guidelines,” the researchers concluded.
“We believe that [insomnia] should be screened and patients should be educated about the importance of sleep because nowadays insomnia is no longer a disease – sleep deprivation could also be a life choice,” Ms, Dean told a press conference prior to the meeting.
“Clinicians must educate the patients about the importance of sleep in maintaining a healthy heart and encourage proper sleep hygiene,” Ms. Dean reiterated in an email. “And if a patient still has insomnia, other methods should be considered such as cognitive-behavior[al] therapy for insomnia [CBT-I].”
Adds to growing evidence
This study does not allow any conclusion about whether treating insomnia will reduce heart attack risk, Jennifer L. Martin, PhD, president of the American Academy of Sleep Medicine, noted in a comment. Nor does it report the diversity of study participants, since insomnia is also a health equity issue, she noted, and insomnia symptoms and comorbidities were self-reported.
However, this analysis “adds to the growing evidence that poor quality or insufficient sleep is associated with poor health,” said Dr. Martin, professor of medicine at the University of California, Los Angeles, who was not involved with this research.
The study reinforces the recommendation from the American Heart Association, which includes “Get Healthy Sleep” as one of “Life’s Essential 8” for heart health, Dr. Martin noted.
“Particularly in primary care where disease prevention and health promotion are important, clinicians should be asking all patients about their sleep – just like they ask about diet and exercise – as a key aspect of maintaining heart health,” she said.
Advice about basic sleep hygiene advice is a first step, she noted.
When improved sleep hygiene is not enough to address chronic insomnia, the AASM’s clinical practice guidelines and the guidelines of the Department of Veterans Affairs/Department of Defense, recommend first-line treatment with CBT-I, typically offered by a sleep specialist or mental health clinician.
Similarly, the American College of Physicians suggests that sleeping pills should be reserved for short-term use in patients who may not benefit sufficiently from CBT-I.
Sleeping too little, too much, equally harmful
“Studies have found that insomnia and subsequent sleep deprivation puts the body under stress,” Ms. Dean said. “This triggers cortisol release which could accelerate atherosclerosis,” and increase risk of MI.
For this analysis, the researchers identified nine observational studies, published from 1998 to 2019, with data on incident MI in adults who had insomnia.
The diagnosis of insomnia was based on ICD diagnostic codes or on the DSM‐5, which defines insomnia as the presence of any of the following three symptoms: difficulty initiating sleep, difficulty maintaining sleep, or early morning awakening with inability to return to sleep. Patients with sleep apnea were excluded.
The studies were in populations in China, Germany, Norway, Taiwan, United Kingdom, and United States, in 1.1 million adults aged 18 and older. The patients had a mean age of 52 years and 13% had insomnia.
During follow-up, 2,406 of 153,881 patients with insomnia, and 12,398 of 1,030,375 patients without insomnia had an MI.
In the pooled analysis, patients with insomnia had a significantly increased risk of MI (relative risk, 1.69; P < .00001), after adjusting for age, gender, diabetes, hypertension, high cholesterol, and smoking.
Sleeping 5 hours or less was associated with a greater risk for MI than sleeping 6 hours, or 7-8 hours, but sleeping 9 hours or more was just as harmful.
Patients who had difficulty initiating and maintaining sleep – two symptoms of insomnia – had a 13% increased risk for MI compared with other patients (RR, 1.13; P = .003).
However, patients who had nonrestorative sleep and daytime dysfunction despite adequate sleep – which is common – did not have an increased risk of MI, compared with other patients (RR, 1.06; P = .46).
Women with insomnia had a 2.24-fold greater risk for MI than other women, whereas men with insomnia had a 2.03-fold greater risk for MI than other men.
Patients with insomnia had a greater risk for MI than those without insomnia in subgroups based on patients’ age (< 65 and > 65), follow up duration (≤ 5 years and > 5 years), and comorbidities (diabetes, hypertension, and hyperlipidemia).
The authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ACC 2023
Chest lesion
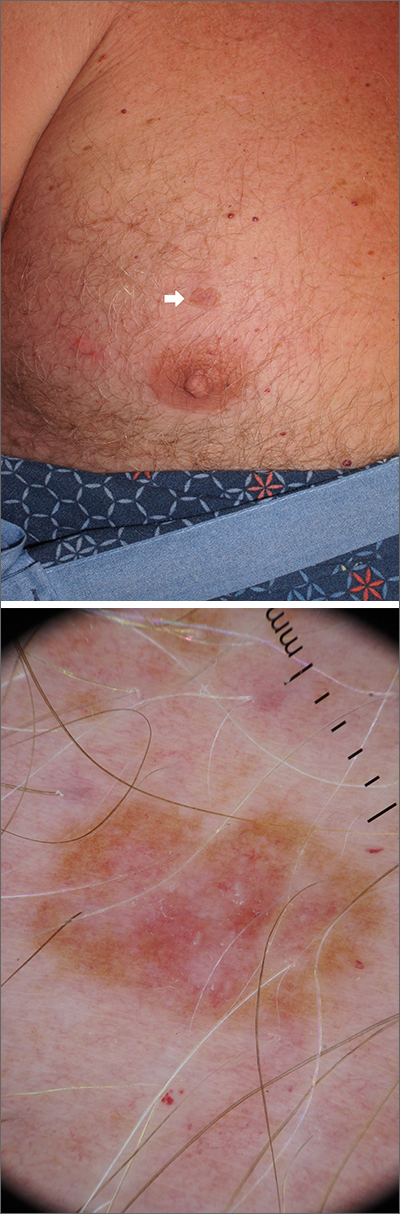
A scoop shave biopsy was performed, including at least a 1-mm margin of normal-looking skin. Pathology was consistent with melanoma in situ.
Melanoma in situ, also called Stage 0 melanoma, is defined by atypical melanocytes that have not begun to invade the dermis and, therefore, have a Breslow thickness of 0 mm. While invasive melanoma is responsible for the largest number of skin cancer deaths in the United States (estimated to be 7990 in 2023), melanoma in situ maintains a very high cure rate when treated appropriately.1
Dermoscopy can help differentiate melanoma from benign nevi or other benign skin lesions. In this case, dermoscopy revealed a fine pigment network at the periphery that indicated this lesion was made up of melanocytes. There were also atypical vascular markings (the milky red color) in the center. Taken together, these findings were strongly indicative of melanoma.
Standard of care for melanoma in situ is a wide local excision with a margin of 5 to 10 mm. Melanoma in situ does not require sentinel lymph node biopsy. However, a lymph node biopsy would have been necessary if the melanoma had been ≥ 1 mm in thickness or if it had been ≥ 0.8 mm in thickness with higher-risk features, such as an increased number of mitoses per high-power field on pathology. Mohs micrographic surgery (MMS) is emerging as an alternative method to wide local excision to treat melanoma in situ. However, it can only be done in specialized centers that can do rapid immunohistochemical staining on frozen sections. MMS is especially useful in cosmetically sensitive areas of the body and in areas where the true size of the melanoma in situ is unclear.
This patient subsequently underwent a wide local excision in the office with a margin of 6 mm. A sentinel lymph node biopsy was not performed. The patient will continue with skin surveillance consisting of full skin exams 3 to 4 times in the first year of diagnosis, then twice annually for Years 2 to 5. He will then come in for annual skin exams after that.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
1. American Cancer Society. Cancer Facts & Figures 2023. Atlanta: American Cancer Society; 2023. Accessed February 20, 2023. www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2023/2023-cancer-facts-and-figures.pdf

A scoop shave biopsy was performed, including at least a 1-mm margin of normal-looking skin. Pathology was consistent with melanoma in situ.
Melanoma in situ, also called Stage 0 melanoma, is defined by atypical melanocytes that have not begun to invade the dermis and, therefore, have a Breslow thickness of 0 mm. While invasive melanoma is responsible for the largest number of skin cancer deaths in the United States (estimated to be 7990 in 2023), melanoma in situ maintains a very high cure rate when treated appropriately.1
Dermoscopy can help differentiate melanoma from benign nevi or other benign skin lesions. In this case, dermoscopy revealed a fine pigment network at the periphery that indicated this lesion was made up of melanocytes. There were also atypical vascular markings (the milky red color) in the center. Taken together, these findings were strongly indicative of melanoma.
Standard of care for melanoma in situ is a wide local excision with a margin of 5 to 10 mm. Melanoma in situ does not require sentinel lymph node biopsy. However, a lymph node biopsy would have been necessary if the melanoma had been ≥ 1 mm in thickness or if it had been ≥ 0.8 mm in thickness with higher-risk features, such as an increased number of mitoses per high-power field on pathology. Mohs micrographic surgery (MMS) is emerging as an alternative method to wide local excision to treat melanoma in situ. However, it can only be done in specialized centers that can do rapid immunohistochemical staining on frozen sections. MMS is especially useful in cosmetically sensitive areas of the body and in areas where the true size of the melanoma in situ is unclear.
This patient subsequently underwent a wide local excision in the office with a margin of 6 mm. A sentinel lymph node biopsy was not performed. The patient will continue with skin surveillance consisting of full skin exams 3 to 4 times in the first year of diagnosis, then twice annually for Years 2 to 5. He will then come in for annual skin exams after that.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.

A scoop shave biopsy was performed, including at least a 1-mm margin of normal-looking skin. Pathology was consistent with melanoma in situ.
Melanoma in situ, also called Stage 0 melanoma, is defined by atypical melanocytes that have not begun to invade the dermis and, therefore, have a Breslow thickness of 0 mm. While invasive melanoma is responsible for the largest number of skin cancer deaths in the United States (estimated to be 7990 in 2023), melanoma in situ maintains a very high cure rate when treated appropriately.1
Dermoscopy can help differentiate melanoma from benign nevi or other benign skin lesions. In this case, dermoscopy revealed a fine pigment network at the periphery that indicated this lesion was made up of melanocytes. There were also atypical vascular markings (the milky red color) in the center. Taken together, these findings were strongly indicative of melanoma.
Standard of care for melanoma in situ is a wide local excision with a margin of 5 to 10 mm. Melanoma in situ does not require sentinel lymph node biopsy. However, a lymph node biopsy would have been necessary if the melanoma had been ≥ 1 mm in thickness or if it had been ≥ 0.8 mm in thickness with higher-risk features, such as an increased number of mitoses per high-power field on pathology. Mohs micrographic surgery (MMS) is emerging as an alternative method to wide local excision to treat melanoma in situ. However, it can only be done in specialized centers that can do rapid immunohistochemical staining on frozen sections. MMS is especially useful in cosmetically sensitive areas of the body and in areas where the true size of the melanoma in situ is unclear.
This patient subsequently underwent a wide local excision in the office with a margin of 6 mm. A sentinel lymph node biopsy was not performed. The patient will continue with skin surveillance consisting of full skin exams 3 to 4 times in the first year of diagnosis, then twice annually for Years 2 to 5. He will then come in for annual skin exams after that.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
1. American Cancer Society. Cancer Facts & Figures 2023. Atlanta: American Cancer Society; 2023. Accessed February 20, 2023. www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2023/2023-cancer-facts-and-figures.pdf
1. American Cancer Society. Cancer Facts & Figures 2023. Atlanta: American Cancer Society; 2023. Accessed February 20, 2023. www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2023/2023-cancer-facts-and-figures.pdf
Artificial sweetener in ‘keto foods’ tied to cardiovascular risk
Erythritol is one of the most widely used artificial sweeteners with rapidly increasing prevalence in processed and “keto-related” foods. Artificial sweeteners are “generally recognized as safe” (GRAS) by the U.S. Food and Drug Administration, so there is no requirement for long-term safety studies, and little is known about the long-term health effects.
The current research, published online in Nature Medicine by Marco Witkowski, MD, of the Lerner Research Institute at Cleveland Clinic and colleagues, had multiple parts.
First, in a group of patients undergoing cardiac risk assessment, the researchers found that high levels of polyols, especially erythritol, were associated with increased 3-year risk of MACE, defined as cardiovascular death or nonfatal myocardial infarction or stroke.
Next, the association of erythritol with this outcome was reproduced in two large U.S. and European groups of stable patients undergoing elective cardiac evaluation.
Next, adding erythritol to whole blood or platelets led to clot activation. And lastly, in eight healthy volunteers, ingesting 30 g of an erythritol-sweetened drink – comparable to a single can of commercially available beverage or a pint of keto ice cream – induced marked and sustained (> 2 day) increases in levels of plasma erythritol.
“Our study shows that when participants consumed an artificially sweetened beverage with an amount of erythritol found in many processed foods, markedly elevated levels in the blood are observed for days – levels well above those observed to enhance clotting risks,” said senior author Stanley L. Hazen, MD, PhD.
“It is important that further safety studies are conducted to examine the long-term effects of artificial sweeteners in general, and erythritol specifically, on risks for heart attack and stroke, particularly in people at higher risk for cardiovascular disease,” Dr. Hazen, co–section head of preventive cardiology at Cleveland Clinic, said in a press release from his institution.
“Sweeteners like erythritol have rapidly increased in popularity in recent years, but there needs to be more in-depth research into their long-term effects. Cardiovascular disease builds over time, and heart disease is the leading cause of death globally. We need to make sure the foods we eat aren’t hidden contributors,” Dr. Hazen urged.
The topic remains controversial.
Duane Mellor, PhD, a registered dietitian and senior teaching fellow at Aston University, Birmingham, England, told the U.K. Science Media Centre: “This paper effectively shows multiple pieces of a jigsaw exploring the effects of erythritol – although it claims to show an associated risk with the use of erythritol as an artificial sweetener and cardiovascular disease, I believe it fails to do so, as ultimately, erythritol can be made inside our bodies and the intake in most people’s diet is much lower than the amount given in this study.”
Dr. Hazen countered that data from the 2013-2014 National Health and Nutrition Examination Survey (NHANES) in the United States show that, in some individuals, daily intake of erythritol is estimated to reach 30 g/day.
“Many try and reduce sugar intake by taking many teaspoons of erythritol in their tea, coffee, etc., instead of sugar,” Dr. Hazen added. “Or they eat keto processed foods that have significant quantities of erythritol within it.”
“These studies are a warning for how our processed food (keto and zero sugar, especially) may inadvertently be causing risk/harm. … in the very subset of subjects who are most vulnerable,” according to Dr. Hazen.
Erythritol marketed as ‘zero calorie’, ‘non-nutritive’, or ‘natural’
Patients with type 2 diabetes and obesity are often advised to replace sugar with artificial sweeteners for better glucose control and weight loss, but growing epidemiologic evidence links artificial sweetener consumption with weight gain, insulin resistance, type 2 diabetes, and cardiovascular disease, the researchers write.
Erythritol is naturally present in low amounts in fruits and vegetables; the artificial sweetener erythritol that is produced from corn is only 70% as sweet as sugar.
Upon ingestion it is poorly metabolized, and most is excreted in the urine, so it is characterized as a “zero-calorie,” “non-nutritive,” or “natural sweetener.” It is predicted to double in marketshare in the sweetener sector in the next 5 years.
Multipart study
In the first part of their study, in a discovery cohort in 1,157 patients undergoing cardiovascular assessment with 3-year outcomes, the researchers identified polyols that were associated with MACE, and erythritol was among the top MACE-associated molecules.
Next, in a U.S. validation cohort of 2,149 patients, over a 3-year follow-up, patients with plasma levels of erythritol in the highest quartile had a 1.8-fold higher risk of MACE than patients in the lowest quartile (P = .007), after adjusting for cardiovascular risk factors.
In a European validation cohort of 833 patients, over a 3-year follow-up, patients with plasma levels of erythritol in the highest quartile had a 2.21-fold higher risk of MACE than patients in the lowest quartile (P = .010, after adjustment).
At physiologic levels, erythritol enhanced platelet reactivity in vitro and thrombosis formation in vivo.
Finally, in a prospective pilot intervention study, erythritol ingestion in healthy volunteers induced marked and sustained increases in plasma erythritol levels well above thresholds associated with heightened platelet reactivity and thrombosis potential in in vitro and in vivo studies.
Others weigh in
“While I think the finding certainly warrants further investigation, don’t throw out your sweeteners just yet,” commented Oliver Jones, PhD, professor of chemistry at the Royal Melbourne Institute of Technology.
“This study only looks at erythritol, and artificial sweeteners are generally considered safe. Any possible (and, as yet unproven) risks of excess erythritol would also need to be balanced against the very real health risks of excess glucose consumption,” he said.
Dr. Hazen responded: “True enough. Erythritol is but one of many artificial sweeteners. That is why it is important to read labels. This study can make patients be informed about how to potentially avoid something that might cause them inadvertent harm.”
“The key findings of this study are that high blood levels of erythritol are strongly associated with cardiovascular outcomes in high-risk patients, which has been replicated in separate validation studies,” said Tom Sanders, DSc, PhD, professor emeritus of nutrition and dietetics, King’s College London.
“Diabetes UK currently advises diabetes patients not to use polyols,” he added.
Dr. Hazen noted that “About three-quarters of the participants had coronary disease, high blood pressure, and about a fifth had diabetes.”
The researchers acknowledge, however, that the observational studies cannot show cause and effect.
The study was supported by the Office of Dietary Supplements at the National Institutes of Health, the Leducq Foundation, and the German Research Foundation (Deutsche Forschungsgemeinschaft). Dr. Mellor, Dr. Jones, and Dr. Sanders have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Erythritol is one of the most widely used artificial sweeteners with rapidly increasing prevalence in processed and “keto-related” foods. Artificial sweeteners are “generally recognized as safe” (GRAS) by the U.S. Food and Drug Administration, so there is no requirement for long-term safety studies, and little is known about the long-term health effects.
The current research, published online in Nature Medicine by Marco Witkowski, MD, of the Lerner Research Institute at Cleveland Clinic and colleagues, had multiple parts.
First, in a group of patients undergoing cardiac risk assessment, the researchers found that high levels of polyols, especially erythritol, were associated with increased 3-year risk of MACE, defined as cardiovascular death or nonfatal myocardial infarction or stroke.
Next, the association of erythritol with this outcome was reproduced in two large U.S. and European groups of stable patients undergoing elective cardiac evaluation.
Next, adding erythritol to whole blood or platelets led to clot activation. And lastly, in eight healthy volunteers, ingesting 30 g of an erythritol-sweetened drink – comparable to a single can of commercially available beverage or a pint of keto ice cream – induced marked and sustained (> 2 day) increases in levels of plasma erythritol.
“Our study shows that when participants consumed an artificially sweetened beverage with an amount of erythritol found in many processed foods, markedly elevated levels in the blood are observed for days – levels well above those observed to enhance clotting risks,” said senior author Stanley L. Hazen, MD, PhD.
“It is important that further safety studies are conducted to examine the long-term effects of artificial sweeteners in general, and erythritol specifically, on risks for heart attack and stroke, particularly in people at higher risk for cardiovascular disease,” Dr. Hazen, co–section head of preventive cardiology at Cleveland Clinic, said in a press release from his institution.
“Sweeteners like erythritol have rapidly increased in popularity in recent years, but there needs to be more in-depth research into their long-term effects. Cardiovascular disease builds over time, and heart disease is the leading cause of death globally. We need to make sure the foods we eat aren’t hidden contributors,” Dr. Hazen urged.
The topic remains controversial.
Duane Mellor, PhD, a registered dietitian and senior teaching fellow at Aston University, Birmingham, England, told the U.K. Science Media Centre: “This paper effectively shows multiple pieces of a jigsaw exploring the effects of erythritol – although it claims to show an associated risk with the use of erythritol as an artificial sweetener and cardiovascular disease, I believe it fails to do so, as ultimately, erythritol can be made inside our bodies and the intake in most people’s diet is much lower than the amount given in this study.”
Dr. Hazen countered that data from the 2013-2014 National Health and Nutrition Examination Survey (NHANES) in the United States show that, in some individuals, daily intake of erythritol is estimated to reach 30 g/day.
“Many try and reduce sugar intake by taking many teaspoons of erythritol in their tea, coffee, etc., instead of sugar,” Dr. Hazen added. “Or they eat keto processed foods that have significant quantities of erythritol within it.”
“These studies are a warning for how our processed food (keto and zero sugar, especially) may inadvertently be causing risk/harm. … in the very subset of subjects who are most vulnerable,” according to Dr. Hazen.
Erythritol marketed as ‘zero calorie’, ‘non-nutritive’, or ‘natural’
Patients with type 2 diabetes and obesity are often advised to replace sugar with artificial sweeteners for better glucose control and weight loss, but growing epidemiologic evidence links artificial sweetener consumption with weight gain, insulin resistance, type 2 diabetes, and cardiovascular disease, the researchers write.
Erythritol is naturally present in low amounts in fruits and vegetables; the artificial sweetener erythritol that is produced from corn is only 70% as sweet as sugar.
Upon ingestion it is poorly metabolized, and most is excreted in the urine, so it is characterized as a “zero-calorie,” “non-nutritive,” or “natural sweetener.” It is predicted to double in marketshare in the sweetener sector in the next 5 years.
Multipart study
In the first part of their study, in a discovery cohort in 1,157 patients undergoing cardiovascular assessment with 3-year outcomes, the researchers identified polyols that were associated with MACE, and erythritol was among the top MACE-associated molecules.
Next, in a U.S. validation cohort of 2,149 patients, over a 3-year follow-up, patients with plasma levels of erythritol in the highest quartile had a 1.8-fold higher risk of MACE than patients in the lowest quartile (P = .007), after adjusting for cardiovascular risk factors.
In a European validation cohort of 833 patients, over a 3-year follow-up, patients with plasma levels of erythritol in the highest quartile had a 2.21-fold higher risk of MACE than patients in the lowest quartile (P = .010, after adjustment).
At physiologic levels, erythritol enhanced platelet reactivity in vitro and thrombosis formation in vivo.
Finally, in a prospective pilot intervention study, erythritol ingestion in healthy volunteers induced marked and sustained increases in plasma erythritol levels well above thresholds associated with heightened platelet reactivity and thrombosis potential in in vitro and in vivo studies.
Others weigh in
“While I think the finding certainly warrants further investigation, don’t throw out your sweeteners just yet,” commented Oliver Jones, PhD, professor of chemistry at the Royal Melbourne Institute of Technology.
“This study only looks at erythritol, and artificial sweeteners are generally considered safe. Any possible (and, as yet unproven) risks of excess erythritol would also need to be balanced against the very real health risks of excess glucose consumption,” he said.
Dr. Hazen responded: “True enough. Erythritol is but one of many artificial sweeteners. That is why it is important to read labels. This study can make patients be informed about how to potentially avoid something that might cause them inadvertent harm.”
“The key findings of this study are that high blood levels of erythritol are strongly associated with cardiovascular outcomes in high-risk patients, which has been replicated in separate validation studies,” said Tom Sanders, DSc, PhD, professor emeritus of nutrition and dietetics, King’s College London.
“Diabetes UK currently advises diabetes patients not to use polyols,” he added.
Dr. Hazen noted that “About three-quarters of the participants had coronary disease, high blood pressure, and about a fifth had diabetes.”
The researchers acknowledge, however, that the observational studies cannot show cause and effect.
The study was supported by the Office of Dietary Supplements at the National Institutes of Health, the Leducq Foundation, and the German Research Foundation (Deutsche Forschungsgemeinschaft). Dr. Mellor, Dr. Jones, and Dr. Sanders have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Erythritol is one of the most widely used artificial sweeteners with rapidly increasing prevalence in processed and “keto-related” foods. Artificial sweeteners are “generally recognized as safe” (GRAS) by the U.S. Food and Drug Administration, so there is no requirement for long-term safety studies, and little is known about the long-term health effects.
The current research, published online in Nature Medicine by Marco Witkowski, MD, of the Lerner Research Institute at Cleveland Clinic and colleagues, had multiple parts.
First, in a group of patients undergoing cardiac risk assessment, the researchers found that high levels of polyols, especially erythritol, were associated with increased 3-year risk of MACE, defined as cardiovascular death or nonfatal myocardial infarction or stroke.
Next, the association of erythritol with this outcome was reproduced in two large U.S. and European groups of stable patients undergoing elective cardiac evaluation.
Next, adding erythritol to whole blood or platelets led to clot activation. And lastly, in eight healthy volunteers, ingesting 30 g of an erythritol-sweetened drink – comparable to a single can of commercially available beverage or a pint of keto ice cream – induced marked and sustained (> 2 day) increases in levels of plasma erythritol.
“Our study shows that when participants consumed an artificially sweetened beverage with an amount of erythritol found in many processed foods, markedly elevated levels in the blood are observed for days – levels well above those observed to enhance clotting risks,” said senior author Stanley L. Hazen, MD, PhD.
“It is important that further safety studies are conducted to examine the long-term effects of artificial sweeteners in general, and erythritol specifically, on risks for heart attack and stroke, particularly in people at higher risk for cardiovascular disease,” Dr. Hazen, co–section head of preventive cardiology at Cleveland Clinic, said in a press release from his institution.
“Sweeteners like erythritol have rapidly increased in popularity in recent years, but there needs to be more in-depth research into their long-term effects. Cardiovascular disease builds over time, and heart disease is the leading cause of death globally. We need to make sure the foods we eat aren’t hidden contributors,” Dr. Hazen urged.
The topic remains controversial.
Duane Mellor, PhD, a registered dietitian and senior teaching fellow at Aston University, Birmingham, England, told the U.K. Science Media Centre: “This paper effectively shows multiple pieces of a jigsaw exploring the effects of erythritol – although it claims to show an associated risk with the use of erythritol as an artificial sweetener and cardiovascular disease, I believe it fails to do so, as ultimately, erythritol can be made inside our bodies and the intake in most people’s diet is much lower than the amount given in this study.”
Dr. Hazen countered that data from the 2013-2014 National Health and Nutrition Examination Survey (NHANES) in the United States show that, in some individuals, daily intake of erythritol is estimated to reach 30 g/day.
“Many try and reduce sugar intake by taking many teaspoons of erythritol in their tea, coffee, etc., instead of sugar,” Dr. Hazen added. “Or they eat keto processed foods that have significant quantities of erythritol within it.”
“These studies are a warning for how our processed food (keto and zero sugar, especially) may inadvertently be causing risk/harm. … in the very subset of subjects who are most vulnerable,” according to Dr. Hazen.
Erythritol marketed as ‘zero calorie’, ‘non-nutritive’, or ‘natural’
Patients with type 2 diabetes and obesity are often advised to replace sugar with artificial sweeteners for better glucose control and weight loss, but growing epidemiologic evidence links artificial sweetener consumption with weight gain, insulin resistance, type 2 diabetes, and cardiovascular disease, the researchers write.
Erythritol is naturally present in low amounts in fruits and vegetables; the artificial sweetener erythritol that is produced from corn is only 70% as sweet as sugar.
Upon ingestion it is poorly metabolized, and most is excreted in the urine, so it is characterized as a “zero-calorie,” “non-nutritive,” or “natural sweetener.” It is predicted to double in marketshare in the sweetener sector in the next 5 years.
Multipart study
In the first part of their study, in a discovery cohort in 1,157 patients undergoing cardiovascular assessment with 3-year outcomes, the researchers identified polyols that were associated with MACE, and erythritol was among the top MACE-associated molecules.
Next, in a U.S. validation cohort of 2,149 patients, over a 3-year follow-up, patients with plasma levels of erythritol in the highest quartile had a 1.8-fold higher risk of MACE than patients in the lowest quartile (P = .007), after adjusting for cardiovascular risk factors.
In a European validation cohort of 833 patients, over a 3-year follow-up, patients with plasma levels of erythritol in the highest quartile had a 2.21-fold higher risk of MACE than patients in the lowest quartile (P = .010, after adjustment).
At physiologic levels, erythritol enhanced platelet reactivity in vitro and thrombosis formation in vivo.
Finally, in a prospective pilot intervention study, erythritol ingestion in healthy volunteers induced marked and sustained increases in plasma erythritol levels well above thresholds associated with heightened platelet reactivity and thrombosis potential in in vitro and in vivo studies.
Others weigh in
“While I think the finding certainly warrants further investigation, don’t throw out your sweeteners just yet,” commented Oliver Jones, PhD, professor of chemistry at the Royal Melbourne Institute of Technology.
“This study only looks at erythritol, and artificial sweeteners are generally considered safe. Any possible (and, as yet unproven) risks of excess erythritol would also need to be balanced against the very real health risks of excess glucose consumption,” he said.
Dr. Hazen responded: “True enough. Erythritol is but one of many artificial sweeteners. That is why it is important to read labels. This study can make patients be informed about how to potentially avoid something that might cause them inadvertent harm.”
“The key findings of this study are that high blood levels of erythritol are strongly associated with cardiovascular outcomes in high-risk patients, which has been replicated in separate validation studies,” said Tom Sanders, DSc, PhD, professor emeritus of nutrition and dietetics, King’s College London.
“Diabetes UK currently advises diabetes patients not to use polyols,” he added.
Dr. Hazen noted that “About three-quarters of the participants had coronary disease, high blood pressure, and about a fifth had diabetes.”
The researchers acknowledge, however, that the observational studies cannot show cause and effect.
The study was supported by the Office of Dietary Supplements at the National Institutes of Health, the Leducq Foundation, and the German Research Foundation (Deutsche Forschungsgemeinschaft). Dr. Mellor, Dr. Jones, and Dr. Sanders have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM NATURE MEDICINE
Drinking beet juice tied to reduced post-PCI restenosis
WASHINGTON – Late lumen loss (LLL) after percutaneous interventions (PCI) can be reduced significantly by a daily glass of beet juice, according to a phase 2 randomized trial.
The protection against LLL, attributed to the nitrate contained in beet juice, was accompanied by a trend for a reduced risk of major adverse cardiovascular events (MACE), according to Krishnaraj Rathod, MBBS, BMedSci, PhD, who presented results at the Cardiovascular Research Technologies conference, sponsored by MedStar Heart & Vascular Institute.
The study grew out of relatively recent evidence that ingestion of nitrate-rich foods, such as beets, can trigger noncanonical pathways for nitric oxide generation, sometimes referred to as the nitrate-nitrite-nitric oxide sequence. Dr. Rathod cited experimental evidence associating this pathway with the traditional benefits of NO generation, such as anti-inflammatory and antithrombotic effects.
In this study, 300 patients scheduled for PCI to treat stable angina were randomized to the experimental arm of nitrate-rich beetroot juice or the control arm of nitrate-depleted beetroot juice. Each had a 70-mL glass of juice once daily. Dr. Rathod, a senior interventional cardiology registrar, Barts Heart Centre, London, described this as the equivalent of about four beets.
The primary endpoint of the study was in-stent LLL assessed by quantitative coronary angiography (QCA) at 6 months.
MACE, defined as death, MI, need for revascularization, and in-stent thrombosis, was assessed at 3, 9, 12, and 24 months. In addition, markers of NO activation, platelet reactivity, and inflammation were monitored.
Lumen loss reduced less than 50%
On OCA, the median stent LLL at 6 months was 0.244 mm in the nitrate-depleted beet juice group and 0.117 mm (P = .0165) in the group that received natural beet juice. The mean segment LLL similarly favored the natural beet juice (0.269 vs. 0.050 mm; P = .0011).
The same effect was reflected in the measurement of mean change in minimum lumen diameter at 6 months. From baseline, this in-stent measure was reduced at 6 months by 0.244 mm in the control group, but by only 0.117 mm in the group receiving the dietary nitrate (P = .0154 for two-way analysis of variance).
Over 24 months of follow-up, there were 18 MACE events in the control arm versus 9 in the arm randomized to dietary nitrate (P = .0718). There were no in-stent thromboses observed in either group, but death (two vs. five), MI (one vs. six), and target-vessel revascularization (six vs. seven) were all numerically lower in the group receiving dietary nitrate.
“Once-a-day oral dietary nitrate for 6 months was well tolerated and safe,” Dr. Rathod reported at the meeting.
Asked specifically about the taste of the daily glass of beet juice, Dr. Rathod acknowledged that some patients were not enamored, but many had no objections or even liked the taste.
The patients were reasonably representative of a PCI population. The mean age in both groups was 61 years. There were no significant differences in body mass index (approximately 29 kg/m2) or proportion with diabetes (22%), hypertension, or hypercholesterolemia (about 70% in both groups) and other comorbidities.
More PCI was performed in the left anterior descending artery (36.7% vs. 44.0%) in the control group, while less PCI was performed in the right coronary (27.3% vs. 30.7%). Neither difference was significant. The vast majority (~90%) of patients received drug-eluting stents with a mean of 1.4 implanted. Procedural success was 100% in both groups.
Discharge medications, including antiplatelet and antithrombotic therapies, were similar in the two groups.
Results characterized as highly positive
Based on the 53% reduction in LLL at 6 months and the trend for a MACE reduction, Dr. Rathod concluded that the results were highly positive.
“These results suggest that dietary nitrate may have a therapeutic role in reducing restenosis following PCI for stable angina,” he said.
In the discussion, several panelists pointed out that nearly one-third of patients were not available for evaluation at 6 months (41 of 150 in the experimental group and 51 of 150 in the control group) with further attrition at 1 and 2 years of follow-up. Of these about half were lost to follow-up and the other half withdrew.
The lack of follow-up on such a high proportion of participants is one weakness of this study,” acknowledged Hector M. Garcia-Garcia, MD, PhD, a cardiovascular researcher at MedStar Washington Hospital Center. However, he remains enthusiastic about the premise.
“It was encouraging to see every signal moving in the right direction,” said Dr. Garcia, who consulted with Dr. Rathod’s group on the design of the study. He called these data “promising,” and said they provide support for larger trial for a treatment with potential benefits at low cost.
George Dangas, MD, PhD, professor of medicine at the Icahn School of Medicine at Mount Sinai, New York, was among panelists who seemed surprised by such positive findings from a simple but novel concept. However, he remains open to further evaluations.
“As with any surprising result, further confirmation in a large and multicenter trial should be anticipated,” he said in an interview. If, as this study suggests, dietary changes are capable of providing therapeutic NO at the vascular level, he suggested studies to demonstrate anti-inflammatory effects or other mechanistic benefits would be helpful.
“Other sources of oral nitrate would also be a worthwhile investigation,” he said.
Dr. Rathod reports no potential conflicts of interest. Dr. Garcia-Garcia reports ties to Abbott, Biotronik, Boston Scientific, CorFlow, Medtronic, Neovasc, Phillips, and Shockwave. Dr. Dangas reports financial relationships with Abbott Vascular, AstraZeneca, Boston Scientific, Daiichi-Sankyo, and Medtronic.
WASHINGTON – Late lumen loss (LLL) after percutaneous interventions (PCI) can be reduced significantly by a daily glass of beet juice, according to a phase 2 randomized trial.
The protection against LLL, attributed to the nitrate contained in beet juice, was accompanied by a trend for a reduced risk of major adverse cardiovascular events (MACE), according to Krishnaraj Rathod, MBBS, BMedSci, PhD, who presented results at the Cardiovascular Research Technologies conference, sponsored by MedStar Heart & Vascular Institute.
The study grew out of relatively recent evidence that ingestion of nitrate-rich foods, such as beets, can trigger noncanonical pathways for nitric oxide generation, sometimes referred to as the nitrate-nitrite-nitric oxide sequence. Dr. Rathod cited experimental evidence associating this pathway with the traditional benefits of NO generation, such as anti-inflammatory and antithrombotic effects.
In this study, 300 patients scheduled for PCI to treat stable angina were randomized to the experimental arm of nitrate-rich beetroot juice or the control arm of nitrate-depleted beetroot juice. Each had a 70-mL glass of juice once daily. Dr. Rathod, a senior interventional cardiology registrar, Barts Heart Centre, London, described this as the equivalent of about four beets.
The primary endpoint of the study was in-stent LLL assessed by quantitative coronary angiography (QCA) at 6 months.
MACE, defined as death, MI, need for revascularization, and in-stent thrombosis, was assessed at 3, 9, 12, and 24 months. In addition, markers of NO activation, platelet reactivity, and inflammation were monitored.
Lumen loss reduced less than 50%
On OCA, the median stent LLL at 6 months was 0.244 mm in the nitrate-depleted beet juice group and 0.117 mm (P = .0165) in the group that received natural beet juice. The mean segment LLL similarly favored the natural beet juice (0.269 vs. 0.050 mm; P = .0011).
The same effect was reflected in the measurement of mean change in minimum lumen diameter at 6 months. From baseline, this in-stent measure was reduced at 6 months by 0.244 mm in the control group, but by only 0.117 mm in the group receiving the dietary nitrate (P = .0154 for two-way analysis of variance).
Over 24 months of follow-up, there were 18 MACE events in the control arm versus 9 in the arm randomized to dietary nitrate (P = .0718). There were no in-stent thromboses observed in either group, but death (two vs. five), MI (one vs. six), and target-vessel revascularization (six vs. seven) were all numerically lower in the group receiving dietary nitrate.
“Once-a-day oral dietary nitrate for 6 months was well tolerated and safe,” Dr. Rathod reported at the meeting.
Asked specifically about the taste of the daily glass of beet juice, Dr. Rathod acknowledged that some patients were not enamored, but many had no objections or even liked the taste.
The patients were reasonably representative of a PCI population. The mean age in both groups was 61 years. There were no significant differences in body mass index (approximately 29 kg/m2) or proportion with diabetes (22%), hypertension, or hypercholesterolemia (about 70% in both groups) and other comorbidities.
More PCI was performed in the left anterior descending artery (36.7% vs. 44.0%) in the control group, while less PCI was performed in the right coronary (27.3% vs. 30.7%). Neither difference was significant. The vast majority (~90%) of patients received drug-eluting stents with a mean of 1.4 implanted. Procedural success was 100% in both groups.
Discharge medications, including antiplatelet and antithrombotic therapies, were similar in the two groups.
Results characterized as highly positive
Based on the 53% reduction in LLL at 6 months and the trend for a MACE reduction, Dr. Rathod concluded that the results were highly positive.
“These results suggest that dietary nitrate may have a therapeutic role in reducing restenosis following PCI for stable angina,” he said.
In the discussion, several panelists pointed out that nearly one-third of patients were not available for evaluation at 6 months (41 of 150 in the experimental group and 51 of 150 in the control group) with further attrition at 1 and 2 years of follow-up. Of these about half were lost to follow-up and the other half withdrew.
The lack of follow-up on such a high proportion of participants is one weakness of this study,” acknowledged Hector M. Garcia-Garcia, MD, PhD, a cardiovascular researcher at MedStar Washington Hospital Center. However, he remains enthusiastic about the premise.
“It was encouraging to see every signal moving in the right direction,” said Dr. Garcia, who consulted with Dr. Rathod’s group on the design of the study. He called these data “promising,” and said they provide support for larger trial for a treatment with potential benefits at low cost.
George Dangas, MD, PhD, professor of medicine at the Icahn School of Medicine at Mount Sinai, New York, was among panelists who seemed surprised by such positive findings from a simple but novel concept. However, he remains open to further evaluations.
“As with any surprising result, further confirmation in a large and multicenter trial should be anticipated,” he said in an interview. If, as this study suggests, dietary changes are capable of providing therapeutic NO at the vascular level, he suggested studies to demonstrate anti-inflammatory effects or other mechanistic benefits would be helpful.
“Other sources of oral nitrate would also be a worthwhile investigation,” he said.
Dr. Rathod reports no potential conflicts of interest. Dr. Garcia-Garcia reports ties to Abbott, Biotronik, Boston Scientific, CorFlow, Medtronic, Neovasc, Phillips, and Shockwave. Dr. Dangas reports financial relationships with Abbott Vascular, AstraZeneca, Boston Scientific, Daiichi-Sankyo, and Medtronic.
WASHINGTON – Late lumen loss (LLL) after percutaneous interventions (PCI) can be reduced significantly by a daily glass of beet juice, according to a phase 2 randomized trial.
The protection against LLL, attributed to the nitrate contained in beet juice, was accompanied by a trend for a reduced risk of major adverse cardiovascular events (MACE), according to Krishnaraj Rathod, MBBS, BMedSci, PhD, who presented results at the Cardiovascular Research Technologies conference, sponsored by MedStar Heart & Vascular Institute.
The study grew out of relatively recent evidence that ingestion of nitrate-rich foods, such as beets, can trigger noncanonical pathways for nitric oxide generation, sometimes referred to as the nitrate-nitrite-nitric oxide sequence. Dr. Rathod cited experimental evidence associating this pathway with the traditional benefits of NO generation, such as anti-inflammatory and antithrombotic effects.
In this study, 300 patients scheduled for PCI to treat stable angina were randomized to the experimental arm of nitrate-rich beetroot juice or the control arm of nitrate-depleted beetroot juice. Each had a 70-mL glass of juice once daily. Dr. Rathod, a senior interventional cardiology registrar, Barts Heart Centre, London, described this as the equivalent of about four beets.
The primary endpoint of the study was in-stent LLL assessed by quantitative coronary angiography (QCA) at 6 months.
MACE, defined as death, MI, need for revascularization, and in-stent thrombosis, was assessed at 3, 9, 12, and 24 months. In addition, markers of NO activation, platelet reactivity, and inflammation were monitored.
Lumen loss reduced less than 50%
On OCA, the median stent LLL at 6 months was 0.244 mm in the nitrate-depleted beet juice group and 0.117 mm (P = .0165) in the group that received natural beet juice. The mean segment LLL similarly favored the natural beet juice (0.269 vs. 0.050 mm; P = .0011).
The same effect was reflected in the measurement of mean change in minimum lumen diameter at 6 months. From baseline, this in-stent measure was reduced at 6 months by 0.244 mm in the control group, but by only 0.117 mm in the group receiving the dietary nitrate (P = .0154 for two-way analysis of variance).
Over 24 months of follow-up, there were 18 MACE events in the control arm versus 9 in the arm randomized to dietary nitrate (P = .0718). There were no in-stent thromboses observed in either group, but death (two vs. five), MI (one vs. six), and target-vessel revascularization (six vs. seven) were all numerically lower in the group receiving dietary nitrate.
“Once-a-day oral dietary nitrate for 6 months was well tolerated and safe,” Dr. Rathod reported at the meeting.
Asked specifically about the taste of the daily glass of beet juice, Dr. Rathod acknowledged that some patients were not enamored, but many had no objections or even liked the taste.
The patients were reasonably representative of a PCI population. The mean age in both groups was 61 years. There were no significant differences in body mass index (approximately 29 kg/m2) or proportion with diabetes (22%), hypertension, or hypercholesterolemia (about 70% in both groups) and other comorbidities.
More PCI was performed in the left anterior descending artery (36.7% vs. 44.0%) in the control group, while less PCI was performed in the right coronary (27.3% vs. 30.7%). Neither difference was significant. The vast majority (~90%) of patients received drug-eluting stents with a mean of 1.4 implanted. Procedural success was 100% in both groups.
Discharge medications, including antiplatelet and antithrombotic therapies, were similar in the two groups.
Results characterized as highly positive
Based on the 53% reduction in LLL at 6 months and the trend for a MACE reduction, Dr. Rathod concluded that the results were highly positive.
“These results suggest that dietary nitrate may have a therapeutic role in reducing restenosis following PCI for stable angina,” he said.
In the discussion, several panelists pointed out that nearly one-third of patients were not available for evaluation at 6 months (41 of 150 in the experimental group and 51 of 150 in the control group) with further attrition at 1 and 2 years of follow-up. Of these about half were lost to follow-up and the other half withdrew.
The lack of follow-up on such a high proportion of participants is one weakness of this study,” acknowledged Hector M. Garcia-Garcia, MD, PhD, a cardiovascular researcher at MedStar Washington Hospital Center. However, he remains enthusiastic about the premise.
“It was encouraging to see every signal moving in the right direction,” said Dr. Garcia, who consulted with Dr. Rathod’s group on the design of the study. He called these data “promising,” and said they provide support for larger trial for a treatment with potential benefits at low cost.
George Dangas, MD, PhD, professor of medicine at the Icahn School of Medicine at Mount Sinai, New York, was among panelists who seemed surprised by such positive findings from a simple but novel concept. However, he remains open to further evaluations.
“As with any surprising result, further confirmation in a large and multicenter trial should be anticipated,” he said in an interview. If, as this study suggests, dietary changes are capable of providing therapeutic NO at the vascular level, he suggested studies to demonstrate anti-inflammatory effects or other mechanistic benefits would be helpful.
“Other sources of oral nitrate would also be a worthwhile investigation,” he said.
Dr. Rathod reports no potential conflicts of interest. Dr. Garcia-Garcia reports ties to Abbott, Biotronik, Boston Scientific, CorFlow, Medtronic, Neovasc, Phillips, and Shockwave. Dr. Dangas reports financial relationships with Abbott Vascular, AstraZeneca, Boston Scientific, Daiichi-Sankyo, and Medtronic.
AT CRT 2023