User login
The Journal of Clinical Outcomes Management® is an independent, peer-reviewed journal offering evidence-based, practical information for improving the quality, safety, and value of health care.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Even mild COVID is hard on the brain
early research suggests.
“Our results suggest a severe pattern of changes in how the brain communicates as well as its structure, mainly in people with anxiety and depression with long-COVID syndrome, which affects so many people,” study investigator Clarissa Yasuda, MD, PhD, from University of Campinas, São Paulo, said in a news release.
“The magnitude of these changes suggests that they could lead to problems with memory and thinking skills, so we need to be exploring holistic treatments even for people mildly affected by COVID-19,” Dr. Yasuda added.
The findings were released March 6 ahead of the study’s scheduled presentation at the annual meeting of the American Academy of Neurology.
Brain shrinkage
Some studies have shown a high prevalence of symptoms of anxiety and depression in COVID-19 survivors, but few have investigated the associated cerebral changes, Dr. Yasuda told this news organization.
The study included 254 adults (177 women, 77 men, median age 41 years) who had mild COVID-19 a median of 82 days earlier. A total of 102 had symptoms of both anxiety and depression, and 152 had no such symptoms.
On brain imaging, those with COVID-19 and anxiety and depression had atrophy in the limbic area of the brain, which plays a role in memory and emotional processing.
No shrinkage in this area was evident in people who had COVID-19 without anxiety and depression or in a healthy control group of individuals without COVID-19.
The researchers also observed a “severe” pattern of abnormal cerebral functional connectivity in those with COVID-19 and anxiety and depression.
In this functional connectivity analysis, individuals with COVID-19 and anxiety and depression had widespread functional changes in each of the 12 networks assessed, while those with COVID-19 but without symptoms of anxiety and depression showed changes in only 5 networks.
Mechanisms unclear
“Unfortunately, the underpinning mechanisms associated with brain changes and neuropsychiatric dysfunction after COVID-19 infection are unclear,” Dr. Yasuda told this news organization.
“Some studies have demonstrated an association between symptoms of anxiety and depression with inflammation. However, we hypothesize that these cerebral alterations may result from a more complex interaction of social, psychological, and systemic stressors, including inflammation. It is indeed intriguing that such alterations are present in individuals who presented mild acute infection,” Dr. Yasuda added.
“Symptoms of anxiety and depression are frequently observed after COVID-19 and are part of long-COVID syndrome for some individuals. These symptoms require adequate treatment to improve the quality of life, cognition, and work capacity,” she said.
Treating these symptoms may induce “brain plasticity, which may result in some degree of gray matter increase and eventually prevent further structural and functional damage,” Dr. Yasuda said.
A limitation of the study was that symptoms of anxiety and depression were self-reported, meaning people may have misjudged or misreported symptoms.
Commenting on the findings for this news organization, Cyrus Raji, MD, PhD, with the Mallinckrodt Institute of Radiology, Washington University, St. Louis, said the idea that COVID-19 is bad for the brain isn’t new. Dr. Raji was not involved with the study.
Early in the pandemic, Dr. Raji and colleagues published a paper detailing COVID-19’s effects on the brain, and Dr. Raji followed it up with a TED talk on the subject.
“Within the growing framework of what we already know about COVID-19 infection and its adverse effects on the brain, this work incrementally adds to this knowledge by identifying functional and structural neuroimaging abnormalities related to anxiety and depression in persons suffering from COVID-19 infection,” Dr. Raji said.
The study was supported by the São Paulo Research Foundation. The authors have no relevant disclosures. Raji is a consultant for Brainreader, Apollo Health, Pacific Neuroscience Foundation, and Neurevolution LLC.
early research suggests.
“Our results suggest a severe pattern of changes in how the brain communicates as well as its structure, mainly in people with anxiety and depression with long-COVID syndrome, which affects so many people,” study investigator Clarissa Yasuda, MD, PhD, from University of Campinas, São Paulo, said in a news release.
“The magnitude of these changes suggests that they could lead to problems with memory and thinking skills, so we need to be exploring holistic treatments even for people mildly affected by COVID-19,” Dr. Yasuda added.
The findings were released March 6 ahead of the study’s scheduled presentation at the annual meeting of the American Academy of Neurology.
Brain shrinkage
Some studies have shown a high prevalence of symptoms of anxiety and depression in COVID-19 survivors, but few have investigated the associated cerebral changes, Dr. Yasuda told this news organization.
The study included 254 adults (177 women, 77 men, median age 41 years) who had mild COVID-19 a median of 82 days earlier. A total of 102 had symptoms of both anxiety and depression, and 152 had no such symptoms.
On brain imaging, those with COVID-19 and anxiety and depression had atrophy in the limbic area of the brain, which plays a role in memory and emotional processing.
No shrinkage in this area was evident in people who had COVID-19 without anxiety and depression or in a healthy control group of individuals without COVID-19.
The researchers also observed a “severe” pattern of abnormal cerebral functional connectivity in those with COVID-19 and anxiety and depression.
In this functional connectivity analysis, individuals with COVID-19 and anxiety and depression had widespread functional changes in each of the 12 networks assessed, while those with COVID-19 but without symptoms of anxiety and depression showed changes in only 5 networks.
Mechanisms unclear
“Unfortunately, the underpinning mechanisms associated with brain changes and neuropsychiatric dysfunction after COVID-19 infection are unclear,” Dr. Yasuda told this news organization.
“Some studies have demonstrated an association between symptoms of anxiety and depression with inflammation. However, we hypothesize that these cerebral alterations may result from a more complex interaction of social, psychological, and systemic stressors, including inflammation. It is indeed intriguing that such alterations are present in individuals who presented mild acute infection,” Dr. Yasuda added.
“Symptoms of anxiety and depression are frequently observed after COVID-19 and are part of long-COVID syndrome for some individuals. These symptoms require adequate treatment to improve the quality of life, cognition, and work capacity,” she said.
Treating these symptoms may induce “brain plasticity, which may result in some degree of gray matter increase and eventually prevent further structural and functional damage,” Dr. Yasuda said.
A limitation of the study was that symptoms of anxiety and depression were self-reported, meaning people may have misjudged or misreported symptoms.
Commenting on the findings for this news organization, Cyrus Raji, MD, PhD, with the Mallinckrodt Institute of Radiology, Washington University, St. Louis, said the idea that COVID-19 is bad for the brain isn’t new. Dr. Raji was not involved with the study.
Early in the pandemic, Dr. Raji and colleagues published a paper detailing COVID-19’s effects on the brain, and Dr. Raji followed it up with a TED talk on the subject.
“Within the growing framework of what we already know about COVID-19 infection and its adverse effects on the brain, this work incrementally adds to this knowledge by identifying functional and structural neuroimaging abnormalities related to anxiety and depression in persons suffering from COVID-19 infection,” Dr. Raji said.
The study was supported by the São Paulo Research Foundation. The authors have no relevant disclosures. Raji is a consultant for Brainreader, Apollo Health, Pacific Neuroscience Foundation, and Neurevolution LLC.
early research suggests.
“Our results suggest a severe pattern of changes in how the brain communicates as well as its structure, mainly in people with anxiety and depression with long-COVID syndrome, which affects so many people,” study investigator Clarissa Yasuda, MD, PhD, from University of Campinas, São Paulo, said in a news release.
“The magnitude of these changes suggests that they could lead to problems with memory and thinking skills, so we need to be exploring holistic treatments even for people mildly affected by COVID-19,” Dr. Yasuda added.
The findings were released March 6 ahead of the study’s scheduled presentation at the annual meeting of the American Academy of Neurology.
Brain shrinkage
Some studies have shown a high prevalence of symptoms of anxiety and depression in COVID-19 survivors, but few have investigated the associated cerebral changes, Dr. Yasuda told this news organization.
The study included 254 adults (177 women, 77 men, median age 41 years) who had mild COVID-19 a median of 82 days earlier. A total of 102 had symptoms of both anxiety and depression, and 152 had no such symptoms.
On brain imaging, those with COVID-19 and anxiety and depression had atrophy in the limbic area of the brain, which plays a role in memory and emotional processing.
No shrinkage in this area was evident in people who had COVID-19 without anxiety and depression or in a healthy control group of individuals without COVID-19.
The researchers also observed a “severe” pattern of abnormal cerebral functional connectivity in those with COVID-19 and anxiety and depression.
In this functional connectivity analysis, individuals with COVID-19 and anxiety and depression had widespread functional changes in each of the 12 networks assessed, while those with COVID-19 but without symptoms of anxiety and depression showed changes in only 5 networks.
Mechanisms unclear
“Unfortunately, the underpinning mechanisms associated with brain changes and neuropsychiatric dysfunction after COVID-19 infection are unclear,” Dr. Yasuda told this news organization.
“Some studies have demonstrated an association between symptoms of anxiety and depression with inflammation. However, we hypothesize that these cerebral alterations may result from a more complex interaction of social, psychological, and systemic stressors, including inflammation. It is indeed intriguing that such alterations are present in individuals who presented mild acute infection,” Dr. Yasuda added.
“Symptoms of anxiety and depression are frequently observed after COVID-19 and are part of long-COVID syndrome for some individuals. These symptoms require adequate treatment to improve the quality of life, cognition, and work capacity,” she said.
Treating these symptoms may induce “brain plasticity, which may result in some degree of gray matter increase and eventually prevent further structural and functional damage,” Dr. Yasuda said.
A limitation of the study was that symptoms of anxiety and depression were self-reported, meaning people may have misjudged or misreported symptoms.
Commenting on the findings for this news organization, Cyrus Raji, MD, PhD, with the Mallinckrodt Institute of Radiology, Washington University, St. Louis, said the idea that COVID-19 is bad for the brain isn’t new. Dr. Raji was not involved with the study.
Early in the pandemic, Dr. Raji and colleagues published a paper detailing COVID-19’s effects on the brain, and Dr. Raji followed it up with a TED talk on the subject.
“Within the growing framework of what we already know about COVID-19 infection and its adverse effects on the brain, this work incrementally adds to this knowledge by identifying functional and structural neuroimaging abnormalities related to anxiety and depression in persons suffering from COVID-19 infection,” Dr. Raji said.
The study was supported by the São Paulo Research Foundation. The authors have no relevant disclosures. Raji is a consultant for Brainreader, Apollo Health, Pacific Neuroscience Foundation, and Neurevolution LLC.
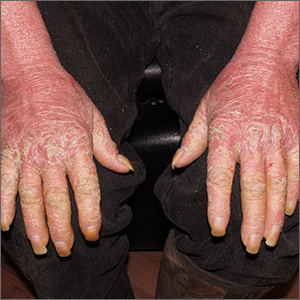
Widespread flaky red skin
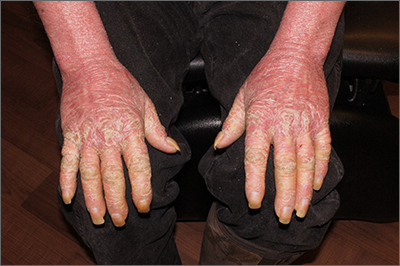
This patient had erythroderma, which involves widespread erythema and scaling of the majority of the skin. Erythroderma can be caused by severe variants of several skin disorders, including atopic dermatitis, contact dermatitis, and psoriasis. In this case, a punch biopsy from the forearm was most consistent with erythrodermic psoriasis.
Erythrodermic psoriasis is a rare subtype of psoriasis and most often develops as an exacerbation of preexisting plaque psoriasis and is defined by erythema, scale, and desquamation covering 75% to 90% of the body surface.1 The alteration in the skin negatively affects heat exchange and hemodynamics and can be life threatening. Many cases develop as a rebound reaction in patients with preexisting psoriasis treated with systemic steroids that are discontinued. Patients with dehydration, poor urinary output, hypotension, or significant weakness may benefit from supportive inpatient care while treatment is initiated.1
Initial treatment options for patients with erythrodermic psoriasis include biologics and steroid-sparing immunosuppressants, such as cyclosporine and acitretin. While a patient awaits the initiation of a definitive therapy, topical triamcinolone 0.1% may be applied over the entire skin surface twice daily and covered with 2 layers of scrubs or pajamas. The pair closest to the skin should be slightly damp and the outer pair should be dry to help retain heat. These are referred to as wet wraps or wet pajama wraps.
The patient described here was hemodynamically stable and was allowed to initiate wet pajama wrap therapy at home while awaiting initiation of adalimumab as an outpatient. He has improved dramatically with adalimumab given subcutaneously every 2 weeks.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
1. Lo Y, Tsai TF. Updates on the treatment of erythrodermic psoriasis. Psoriasis (Auckl). 2021;11:59-73. doi: 10.2147/PTT.S288345

This patient had erythroderma, which involves widespread erythema and scaling of the majority of the skin. Erythroderma can be caused by severe variants of several skin disorders, including atopic dermatitis, contact dermatitis, and psoriasis. In this case, a punch biopsy from the forearm was most consistent with erythrodermic psoriasis.
Erythrodermic psoriasis is a rare subtype of psoriasis and most often develops as an exacerbation of preexisting plaque psoriasis and is defined by erythema, scale, and desquamation covering 75% to 90% of the body surface.1 The alteration in the skin negatively affects heat exchange and hemodynamics and can be life threatening. Many cases develop as a rebound reaction in patients with preexisting psoriasis treated with systemic steroids that are discontinued. Patients with dehydration, poor urinary output, hypotension, or significant weakness may benefit from supportive inpatient care while treatment is initiated.1
Initial treatment options for patients with erythrodermic psoriasis include biologics and steroid-sparing immunosuppressants, such as cyclosporine and acitretin. While a patient awaits the initiation of a definitive therapy, topical triamcinolone 0.1% may be applied over the entire skin surface twice daily and covered with 2 layers of scrubs or pajamas. The pair closest to the skin should be slightly damp and the outer pair should be dry to help retain heat. These are referred to as wet wraps or wet pajama wraps.
The patient described here was hemodynamically stable and was allowed to initiate wet pajama wrap therapy at home while awaiting initiation of adalimumab as an outpatient. He has improved dramatically with adalimumab given subcutaneously every 2 weeks.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.

This patient had erythroderma, which involves widespread erythema and scaling of the majority of the skin. Erythroderma can be caused by severe variants of several skin disorders, including atopic dermatitis, contact dermatitis, and psoriasis. In this case, a punch biopsy from the forearm was most consistent with erythrodermic psoriasis.
Erythrodermic psoriasis is a rare subtype of psoriasis and most often develops as an exacerbation of preexisting plaque psoriasis and is defined by erythema, scale, and desquamation covering 75% to 90% of the body surface.1 The alteration in the skin negatively affects heat exchange and hemodynamics and can be life threatening. Many cases develop as a rebound reaction in patients with preexisting psoriasis treated with systemic steroids that are discontinued. Patients with dehydration, poor urinary output, hypotension, or significant weakness may benefit from supportive inpatient care while treatment is initiated.1
Initial treatment options for patients with erythrodermic psoriasis include biologics and steroid-sparing immunosuppressants, such as cyclosporine and acitretin. While a patient awaits the initiation of a definitive therapy, topical triamcinolone 0.1% may be applied over the entire skin surface twice daily and covered with 2 layers of scrubs or pajamas. The pair closest to the skin should be slightly damp and the outer pair should be dry to help retain heat. These are referred to as wet wraps or wet pajama wraps.
The patient described here was hemodynamically stable and was allowed to initiate wet pajama wrap therapy at home while awaiting initiation of adalimumab as an outpatient. He has improved dramatically with adalimumab given subcutaneously every 2 weeks.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
1. Lo Y, Tsai TF. Updates on the treatment of erythrodermic psoriasis. Psoriasis (Auckl). 2021;11:59-73. doi: 10.2147/PTT.S288345
1. Lo Y, Tsai TF. Updates on the treatment of erythrodermic psoriasis. Psoriasis (Auckl). 2021;11:59-73. doi: 10.2147/PTT.S288345
COORDINATEd effort boosts optimal therapy in patients with T2D and ASCVD
NEW ORLEANS – Twenty cardiology clinics successfully intensified the medical care they gave patients with type 2 diabetes (T2D) and atherosclerotic cardiovascular disease (ASCVD) after receiving a simple and scalable investigational intervention that gave the clinics’ staffs guidance on best prescribing practices and implementation and also provided quality-improvement feedback.
Within a year, these clinics quadrupled optimal medical management of these patients, compared with control clinics, in a randomized trial involving a total of 43 clinics and 1,049 patients.
“This multifaceted intervention is effective in increasing the prescription of evidence-based therapies in adults with T2D and ASCVD,” Neha J. Pagidipati, MD, said at the joint scientific sessions of the American College of Cardiology and the World Heart Federation.
“The next step is to scale this intervention across cardiology practices” interested in improving the quality of care they deliver to these patients, added Dr. Pagidipati, a cardiologist specializing in cardiometabolic disease prevention at Duke University in Durham, N.C.
The goal is getting patients on triple therapy
The primary outcome of the COORDINATE-Diabetes trial was the change in the number of patients with T2D and ASCVD who received prescriptions for agents from three recommended medication classes and at recommended dosages: a high-intensity statin, a renin-angiotensin system inhibitor (RASi), and at least one agent from either of two classes that have both cardiovascular-protective and antihyperglycemic effects: the sodium-glucose cotransporter 2 (SGLT2) inhibitors, or the glucagonlike peptide 1 (GLP-1)–receptor agonists.
Among the 457 patients treated at the 20 cardiology clinics who received the quality-improvement intervention, 37.9% were on the promoted triple therapy after 12 months, compared with 14.5% of the 588 patients treated at the 23 clinics that continued with their usual care approach. This 23.4–percentage point increase in triple-class prescribing at recommended dosages represented a significant 4.4-fold increase in the goal prescribing endpoint after adjustment for possible confounders, Dr. Pagidipati reported.
Simultaneously with her report, the findings also appeared online in JAMA.
At baseline, 41%-50% of the patients were on both a high-intensity statin and a RASi, with a total of about 58%-67% on a high-intensity statin and about 70%-75% on a RASi. Fewer than 1% of patients were on SGLT2 inhibitors or GLP-1–receptor agonists at baseline. By design, no patient could be on all three categories of medication at baseline.
At their last follow-up visit (after 12 months for 97% of patients, or after 6 months for the remainder) 71% of the patients at practices that received the intervention were on a high-intensity statin, 81% were taking a RASi, and 60% were on an SGLT2 inhibitor or GLP-1–receptor agonist. Among the control patients, 58% were on a high-intensity statin, 68% on a RASi, and 36% were on one of the antihyperglycemic agents.
Effective interventions and the need for a champion
The clinics randomized to the active arm received instruction from a three-member team, either from an in-person or virtual one-time visit, on an intervention comprising several initiatives:
- Analysis of the barriers to evidence-based care at each clinic.
- Development of local interdisciplinary care pathways to address the identified barriers.
- Facilitation of care coordination among clinicians – particularly among cardiology, endocrinology, and primary care clinicians.
- Education of the clinic staff, including provision of educational materials.
- Auditing of clinic performance using specified metrics and feedback on the findings.
Clinics in the usual care group were given current clinical practice guidelines.
The investigational intervention was, by design, “low-tech and designed to be scalable,” explained Dr. Pagidipati, and once the COVID pandemic started the intervention team shifted to a virtual consultation with participating practices that was mostly front-loaded, followed by monthly phone calls to give clinics feedback on their progress.
Among the most helpful aspects of the intervention was involving the entire clinic staff, including pharmacists, nurses, and advanced care practitioners; boosting familiarity with the relevant medications and their appropriate use; and advice on navigating insurance-coverage barriers such as prior authorizations.
“What was most critical was having a local champion who took on making this effort an important part” of what the clinic was trying to do, she explained. “All it takes is passion, and the tenacity of a bulldog,” Dr. Pagidipati said.
Research advances often don’t translate into management changes
“We don’t do a great job of translating findings from trials to patient care, so any method we can use to improve that will improve practice,” commented Kristen B. Campbell, PharmD, a clinical pharmacist at Duke who was not involved in the study.
“Although the trial was not powered to look at patient outcomes, we think that patients will benefit” because all the recommended medication uses have been proven to help patients in prior trials, Dr. Campbell noted.
“A particular strength of this study was its simple design. All the interventions are low-tech and scalable.”
The low level of use of guideline-directed medical therapy in American adults with type 2 diabetes and atherosclerotic cardiovascular disease is “incredible,” said Christopher B. Granger, MD, a senior investigator on the study and a cardiologist and professor at Duke.
The researchers who ran the study are now focused on evaluating which cardiology clinics and patients had the most success from the intervention and are using that information to further refine implementation. They are also planning to encourage cardiology practices as well as other relevant medical groups to incorporate the intervention and implementation model used in the trial. The intervention program is detailed and available at no charge on the COORDINATE-Diabetes website.
COORDINATE-Diabetes received funding from Boehringer Ingelheim and Eli Lilly. Dr. Pagidipati has received personal fees from Boehringer Ingelheim, Lilly, AstraZeneca, Novartis, Novo Nordisk, Merck, and CRISPR Therapeutics, and she has received research grants from Amgen, Novartis, Novo Nordisk, and Eggland’s Best. Dr. Campbell had no disclosures. Dr. Granger has received personal fees and research funding from numerous companies.
NEW ORLEANS – Twenty cardiology clinics successfully intensified the medical care they gave patients with type 2 diabetes (T2D) and atherosclerotic cardiovascular disease (ASCVD) after receiving a simple and scalable investigational intervention that gave the clinics’ staffs guidance on best prescribing practices and implementation and also provided quality-improvement feedback.
Within a year, these clinics quadrupled optimal medical management of these patients, compared with control clinics, in a randomized trial involving a total of 43 clinics and 1,049 patients.
“This multifaceted intervention is effective in increasing the prescription of evidence-based therapies in adults with T2D and ASCVD,” Neha J. Pagidipati, MD, said at the joint scientific sessions of the American College of Cardiology and the World Heart Federation.
“The next step is to scale this intervention across cardiology practices” interested in improving the quality of care they deliver to these patients, added Dr. Pagidipati, a cardiologist specializing in cardiometabolic disease prevention at Duke University in Durham, N.C.
The goal is getting patients on triple therapy
The primary outcome of the COORDINATE-Diabetes trial was the change in the number of patients with T2D and ASCVD who received prescriptions for agents from three recommended medication classes and at recommended dosages: a high-intensity statin, a renin-angiotensin system inhibitor (RASi), and at least one agent from either of two classes that have both cardiovascular-protective and antihyperglycemic effects: the sodium-glucose cotransporter 2 (SGLT2) inhibitors, or the glucagonlike peptide 1 (GLP-1)–receptor agonists.
Among the 457 patients treated at the 20 cardiology clinics who received the quality-improvement intervention, 37.9% were on the promoted triple therapy after 12 months, compared with 14.5% of the 588 patients treated at the 23 clinics that continued with their usual care approach. This 23.4–percentage point increase in triple-class prescribing at recommended dosages represented a significant 4.4-fold increase in the goal prescribing endpoint after adjustment for possible confounders, Dr. Pagidipati reported.
Simultaneously with her report, the findings also appeared online in JAMA.
At baseline, 41%-50% of the patients were on both a high-intensity statin and a RASi, with a total of about 58%-67% on a high-intensity statin and about 70%-75% on a RASi. Fewer than 1% of patients were on SGLT2 inhibitors or GLP-1–receptor agonists at baseline. By design, no patient could be on all three categories of medication at baseline.
At their last follow-up visit (after 12 months for 97% of patients, or after 6 months for the remainder) 71% of the patients at practices that received the intervention were on a high-intensity statin, 81% were taking a RASi, and 60% were on an SGLT2 inhibitor or GLP-1–receptor agonist. Among the control patients, 58% were on a high-intensity statin, 68% on a RASi, and 36% were on one of the antihyperglycemic agents.
Effective interventions and the need for a champion
The clinics randomized to the active arm received instruction from a three-member team, either from an in-person or virtual one-time visit, on an intervention comprising several initiatives:
- Analysis of the barriers to evidence-based care at each clinic.
- Development of local interdisciplinary care pathways to address the identified barriers.
- Facilitation of care coordination among clinicians – particularly among cardiology, endocrinology, and primary care clinicians.
- Education of the clinic staff, including provision of educational materials.
- Auditing of clinic performance using specified metrics and feedback on the findings.
Clinics in the usual care group were given current clinical practice guidelines.
The investigational intervention was, by design, “low-tech and designed to be scalable,” explained Dr. Pagidipati, and once the COVID pandemic started the intervention team shifted to a virtual consultation with participating practices that was mostly front-loaded, followed by monthly phone calls to give clinics feedback on their progress.
Among the most helpful aspects of the intervention was involving the entire clinic staff, including pharmacists, nurses, and advanced care practitioners; boosting familiarity with the relevant medications and their appropriate use; and advice on navigating insurance-coverage barriers such as prior authorizations.
“What was most critical was having a local champion who took on making this effort an important part” of what the clinic was trying to do, she explained. “All it takes is passion, and the tenacity of a bulldog,” Dr. Pagidipati said.
Research advances often don’t translate into management changes
“We don’t do a great job of translating findings from trials to patient care, so any method we can use to improve that will improve practice,” commented Kristen B. Campbell, PharmD, a clinical pharmacist at Duke who was not involved in the study.
“Although the trial was not powered to look at patient outcomes, we think that patients will benefit” because all the recommended medication uses have been proven to help patients in prior trials, Dr. Campbell noted.
“A particular strength of this study was its simple design. All the interventions are low-tech and scalable.”
The low level of use of guideline-directed medical therapy in American adults with type 2 diabetes and atherosclerotic cardiovascular disease is “incredible,” said Christopher B. Granger, MD, a senior investigator on the study and a cardiologist and professor at Duke.
The researchers who ran the study are now focused on evaluating which cardiology clinics and patients had the most success from the intervention and are using that information to further refine implementation. They are also planning to encourage cardiology practices as well as other relevant medical groups to incorporate the intervention and implementation model used in the trial. The intervention program is detailed and available at no charge on the COORDINATE-Diabetes website.
COORDINATE-Diabetes received funding from Boehringer Ingelheim and Eli Lilly. Dr. Pagidipati has received personal fees from Boehringer Ingelheim, Lilly, AstraZeneca, Novartis, Novo Nordisk, Merck, and CRISPR Therapeutics, and she has received research grants from Amgen, Novartis, Novo Nordisk, and Eggland’s Best. Dr. Campbell had no disclosures. Dr. Granger has received personal fees and research funding from numerous companies.
NEW ORLEANS – Twenty cardiology clinics successfully intensified the medical care they gave patients with type 2 diabetes (T2D) and atherosclerotic cardiovascular disease (ASCVD) after receiving a simple and scalable investigational intervention that gave the clinics’ staffs guidance on best prescribing practices and implementation and also provided quality-improvement feedback.
Within a year, these clinics quadrupled optimal medical management of these patients, compared with control clinics, in a randomized trial involving a total of 43 clinics and 1,049 patients.
“This multifaceted intervention is effective in increasing the prescription of evidence-based therapies in adults with T2D and ASCVD,” Neha J. Pagidipati, MD, said at the joint scientific sessions of the American College of Cardiology and the World Heart Federation.
“The next step is to scale this intervention across cardiology practices” interested in improving the quality of care they deliver to these patients, added Dr. Pagidipati, a cardiologist specializing in cardiometabolic disease prevention at Duke University in Durham, N.C.
The goal is getting patients on triple therapy
The primary outcome of the COORDINATE-Diabetes trial was the change in the number of patients with T2D and ASCVD who received prescriptions for agents from three recommended medication classes and at recommended dosages: a high-intensity statin, a renin-angiotensin system inhibitor (RASi), and at least one agent from either of two classes that have both cardiovascular-protective and antihyperglycemic effects: the sodium-glucose cotransporter 2 (SGLT2) inhibitors, or the glucagonlike peptide 1 (GLP-1)–receptor agonists.
Among the 457 patients treated at the 20 cardiology clinics who received the quality-improvement intervention, 37.9% were on the promoted triple therapy after 12 months, compared with 14.5% of the 588 patients treated at the 23 clinics that continued with their usual care approach. This 23.4–percentage point increase in triple-class prescribing at recommended dosages represented a significant 4.4-fold increase in the goal prescribing endpoint after adjustment for possible confounders, Dr. Pagidipati reported.
Simultaneously with her report, the findings also appeared online in JAMA.
At baseline, 41%-50% of the patients were on both a high-intensity statin and a RASi, with a total of about 58%-67% on a high-intensity statin and about 70%-75% on a RASi. Fewer than 1% of patients were on SGLT2 inhibitors or GLP-1–receptor agonists at baseline. By design, no patient could be on all three categories of medication at baseline.
At their last follow-up visit (after 12 months for 97% of patients, or after 6 months for the remainder) 71% of the patients at practices that received the intervention were on a high-intensity statin, 81% were taking a RASi, and 60% were on an SGLT2 inhibitor or GLP-1–receptor agonist. Among the control patients, 58% were on a high-intensity statin, 68% on a RASi, and 36% were on one of the antihyperglycemic agents.
Effective interventions and the need for a champion
The clinics randomized to the active arm received instruction from a three-member team, either from an in-person or virtual one-time visit, on an intervention comprising several initiatives:
- Analysis of the barriers to evidence-based care at each clinic.
- Development of local interdisciplinary care pathways to address the identified barriers.
- Facilitation of care coordination among clinicians – particularly among cardiology, endocrinology, and primary care clinicians.
- Education of the clinic staff, including provision of educational materials.
- Auditing of clinic performance using specified metrics and feedback on the findings.
Clinics in the usual care group were given current clinical practice guidelines.
The investigational intervention was, by design, “low-tech and designed to be scalable,” explained Dr. Pagidipati, and once the COVID pandemic started the intervention team shifted to a virtual consultation with participating practices that was mostly front-loaded, followed by monthly phone calls to give clinics feedback on their progress.
Among the most helpful aspects of the intervention was involving the entire clinic staff, including pharmacists, nurses, and advanced care practitioners; boosting familiarity with the relevant medications and their appropriate use; and advice on navigating insurance-coverage barriers such as prior authorizations.
“What was most critical was having a local champion who took on making this effort an important part” of what the clinic was trying to do, she explained. “All it takes is passion, and the tenacity of a bulldog,” Dr. Pagidipati said.
Research advances often don’t translate into management changes
“We don’t do a great job of translating findings from trials to patient care, so any method we can use to improve that will improve practice,” commented Kristen B. Campbell, PharmD, a clinical pharmacist at Duke who was not involved in the study.
“Although the trial was not powered to look at patient outcomes, we think that patients will benefit” because all the recommended medication uses have been proven to help patients in prior trials, Dr. Campbell noted.
“A particular strength of this study was its simple design. All the interventions are low-tech and scalable.”
The low level of use of guideline-directed medical therapy in American adults with type 2 diabetes and atherosclerotic cardiovascular disease is “incredible,” said Christopher B. Granger, MD, a senior investigator on the study and a cardiologist and professor at Duke.
The researchers who ran the study are now focused on evaluating which cardiology clinics and patients had the most success from the intervention and are using that information to further refine implementation. They are also planning to encourage cardiology practices as well as other relevant medical groups to incorporate the intervention and implementation model used in the trial. The intervention program is detailed and available at no charge on the COORDINATE-Diabetes website.
COORDINATE-Diabetes received funding from Boehringer Ingelheim and Eli Lilly. Dr. Pagidipati has received personal fees from Boehringer Ingelheim, Lilly, AstraZeneca, Novartis, Novo Nordisk, Merck, and CRISPR Therapeutics, and she has received research grants from Amgen, Novartis, Novo Nordisk, and Eggland’s Best. Dr. Campbell had no disclosures. Dr. Granger has received personal fees and research funding from numerous companies.
AT ACC 2023
Med center and top cardio surgeon must pay $8.5 million for fraud, concurrent surgeries
the Department of Justice (DOJ) announced.
The lawsuit alleges that James L. Luketich, MD, the longtime chair of the school’s cardiothoracic surgery department, regularly performed up to three complex surgical procedures simultaneously, moving among multiple operating rooms and attending to matters other than patient care. The investigation began after Jonathan D’Cunha, MD, a former UPMC surgeon, raised concerns about his colleague’s surgical scheduling and billing practices.
Dr. Luketich’s overbooking of procedures led to patients enduring hours of medically unnecessary anesthesia time and risking surgical complications, according to court documents.
In addition, the complaint states that these practices violated the False Claims Act, which prohibits “teaching physicians” like Dr. Luketich from billing Medicare and other government health plans for “concurrent surgeries” – regulations federal authorities say UPMC leadership were aware of and the University of Pittsburgh Physicians (UPP), also named in the suit, permitted Dr. Luketich to skirt.
The whistleblower provision of the False Claims Act allows private parties to file an action on behalf of the United States and receive a portion of the recovery to help deter health care fraud, says the DOJ.
The defendants previously asked the court to dismiss the case, but a judge denied the request in June 2022.
Paul Wood, vice president and chief communications officer for UPMC, told this news organization that the lawsuit pertained to Dr. Luketich’s “most complicated, team-based surgical procedures.”
“At issue was compliance with the Centers for Medicare & Medicaid Services’ (CMS’s) Teaching Physician Regulation and related billing guidance as well as with UPMC’s internal surgical policies,” he said.
“While UPMC continues to believe Dr. Luketich’s surgical practice complies with CMS requirements, it has agreed to [the settlement] to avoid the distraction and expense of further litigation,” said Mr. Wood, adding that all parties agree that UPMC can seek clarity from CMS regarding future billing of these surgeries.
Efrem Grail, JD, Dr. Luketich’s attorney, said in an interview that he and Dr. Luketich are pleased that the settlement puts an end to the case and that he hopes the United States will issue “authoritative guidance” on billing regulations for teaching physicians, something medical schools and hospitals have sought for years.
Dr. Luketich, UPMC, and UPP face more legal challenges from a separate medical malpractice lawsuit. In March 2018, Bernadette Fedorka underwent a lung transplant at UPMC. Although Dr. Luketich did not perform the surgery, Ms. Fedorka alleges that his poor leadership caused understaffing of the lung transplant program and contributed to surgical complications, including a 4-inch piece of wire left in her neck.
Ms. Fedorka claims that suboxone impaired Dr. Luketich’s decision-making. He began taking the drug in 2008 to manage the pain from a slipped disc injury after a history of prescription drug abuse. Both UPMC and Dr. Luketich have denied the validity of Ms. Fedorka’s claims.
The malpractice suit centers on a recording of a conversation between Dr. Luketich and David Wilson, MD, who prescribed the suboxone and treated the surgeon’s opioid use disorder for several years. Dr. Luketich has accused former colleagues, Dr. D’Cunha and Lara Schaheen, MD, of illegally recording the private conversation that discussed Dr. Luketich’s suboxone prescription – something both physicians deny.
For the billing fraud case, Dr. Luketich has agreed to complete a corrective action plan and submit to a third-party audit of his Medicare billings for 1 year.
“This is an important settlement and a just conclusion to the United States’ investigation into Dr. Luketich’s surgical and billing practices and UPMC and UPP’s acceptance of those practices,” Acting U.S. Attorney Troy Rivetti said in a statement that, “no medical provider – however renowned – is excepted from scrutiny or above the law.”
A version of this article first appeared on Medscape.com.
the Department of Justice (DOJ) announced.
The lawsuit alleges that James L. Luketich, MD, the longtime chair of the school’s cardiothoracic surgery department, regularly performed up to three complex surgical procedures simultaneously, moving among multiple operating rooms and attending to matters other than patient care. The investigation began after Jonathan D’Cunha, MD, a former UPMC surgeon, raised concerns about his colleague’s surgical scheduling and billing practices.
Dr. Luketich’s overbooking of procedures led to patients enduring hours of medically unnecessary anesthesia time and risking surgical complications, according to court documents.
In addition, the complaint states that these practices violated the False Claims Act, which prohibits “teaching physicians” like Dr. Luketich from billing Medicare and other government health plans for “concurrent surgeries” – regulations federal authorities say UPMC leadership were aware of and the University of Pittsburgh Physicians (UPP), also named in the suit, permitted Dr. Luketich to skirt.
The whistleblower provision of the False Claims Act allows private parties to file an action on behalf of the United States and receive a portion of the recovery to help deter health care fraud, says the DOJ.
The defendants previously asked the court to dismiss the case, but a judge denied the request in June 2022.
Paul Wood, vice president and chief communications officer for UPMC, told this news organization that the lawsuit pertained to Dr. Luketich’s “most complicated, team-based surgical procedures.”
“At issue was compliance with the Centers for Medicare & Medicaid Services’ (CMS’s) Teaching Physician Regulation and related billing guidance as well as with UPMC’s internal surgical policies,” he said.
“While UPMC continues to believe Dr. Luketich’s surgical practice complies with CMS requirements, it has agreed to [the settlement] to avoid the distraction and expense of further litigation,” said Mr. Wood, adding that all parties agree that UPMC can seek clarity from CMS regarding future billing of these surgeries.
Efrem Grail, JD, Dr. Luketich’s attorney, said in an interview that he and Dr. Luketich are pleased that the settlement puts an end to the case and that he hopes the United States will issue “authoritative guidance” on billing regulations for teaching physicians, something medical schools and hospitals have sought for years.
Dr. Luketich, UPMC, and UPP face more legal challenges from a separate medical malpractice lawsuit. In March 2018, Bernadette Fedorka underwent a lung transplant at UPMC. Although Dr. Luketich did not perform the surgery, Ms. Fedorka alleges that his poor leadership caused understaffing of the lung transplant program and contributed to surgical complications, including a 4-inch piece of wire left in her neck.
Ms. Fedorka claims that suboxone impaired Dr. Luketich’s decision-making. He began taking the drug in 2008 to manage the pain from a slipped disc injury after a history of prescription drug abuse. Both UPMC and Dr. Luketich have denied the validity of Ms. Fedorka’s claims.
The malpractice suit centers on a recording of a conversation between Dr. Luketich and David Wilson, MD, who prescribed the suboxone and treated the surgeon’s opioid use disorder for several years. Dr. Luketich has accused former colleagues, Dr. D’Cunha and Lara Schaheen, MD, of illegally recording the private conversation that discussed Dr. Luketich’s suboxone prescription – something both physicians deny.
For the billing fraud case, Dr. Luketich has agreed to complete a corrective action plan and submit to a third-party audit of his Medicare billings for 1 year.
“This is an important settlement and a just conclusion to the United States’ investigation into Dr. Luketich’s surgical and billing practices and UPMC and UPP’s acceptance of those practices,” Acting U.S. Attorney Troy Rivetti said in a statement that, “no medical provider – however renowned – is excepted from scrutiny or above the law.”
A version of this article first appeared on Medscape.com.
the Department of Justice (DOJ) announced.
The lawsuit alleges that James L. Luketich, MD, the longtime chair of the school’s cardiothoracic surgery department, regularly performed up to three complex surgical procedures simultaneously, moving among multiple operating rooms and attending to matters other than patient care. The investigation began after Jonathan D’Cunha, MD, a former UPMC surgeon, raised concerns about his colleague’s surgical scheduling and billing practices.
Dr. Luketich’s overbooking of procedures led to patients enduring hours of medically unnecessary anesthesia time and risking surgical complications, according to court documents.
In addition, the complaint states that these practices violated the False Claims Act, which prohibits “teaching physicians” like Dr. Luketich from billing Medicare and other government health plans for “concurrent surgeries” – regulations federal authorities say UPMC leadership were aware of and the University of Pittsburgh Physicians (UPP), also named in the suit, permitted Dr. Luketich to skirt.
The whistleblower provision of the False Claims Act allows private parties to file an action on behalf of the United States and receive a portion of the recovery to help deter health care fraud, says the DOJ.
The defendants previously asked the court to dismiss the case, but a judge denied the request in June 2022.
Paul Wood, vice president and chief communications officer for UPMC, told this news organization that the lawsuit pertained to Dr. Luketich’s “most complicated, team-based surgical procedures.”
“At issue was compliance with the Centers for Medicare & Medicaid Services’ (CMS’s) Teaching Physician Regulation and related billing guidance as well as with UPMC’s internal surgical policies,” he said.
“While UPMC continues to believe Dr. Luketich’s surgical practice complies with CMS requirements, it has agreed to [the settlement] to avoid the distraction and expense of further litigation,” said Mr. Wood, adding that all parties agree that UPMC can seek clarity from CMS regarding future billing of these surgeries.
Efrem Grail, JD, Dr. Luketich’s attorney, said in an interview that he and Dr. Luketich are pleased that the settlement puts an end to the case and that he hopes the United States will issue “authoritative guidance” on billing regulations for teaching physicians, something medical schools and hospitals have sought for years.
Dr. Luketich, UPMC, and UPP face more legal challenges from a separate medical malpractice lawsuit. In March 2018, Bernadette Fedorka underwent a lung transplant at UPMC. Although Dr. Luketich did not perform the surgery, Ms. Fedorka alleges that his poor leadership caused understaffing of the lung transplant program and contributed to surgical complications, including a 4-inch piece of wire left in her neck.
Ms. Fedorka claims that suboxone impaired Dr. Luketich’s decision-making. He began taking the drug in 2008 to manage the pain from a slipped disc injury after a history of prescription drug abuse. Both UPMC and Dr. Luketich have denied the validity of Ms. Fedorka’s claims.
The malpractice suit centers on a recording of a conversation between Dr. Luketich and David Wilson, MD, who prescribed the suboxone and treated the surgeon’s opioid use disorder for several years. Dr. Luketich has accused former colleagues, Dr. D’Cunha and Lara Schaheen, MD, of illegally recording the private conversation that discussed Dr. Luketich’s suboxone prescription – something both physicians deny.
For the billing fraud case, Dr. Luketich has agreed to complete a corrective action plan and submit to a third-party audit of his Medicare billings for 1 year.
“This is an important settlement and a just conclusion to the United States’ investigation into Dr. Luketich’s surgical and billing practices and UPMC and UPP’s acceptance of those practices,” Acting U.S. Attorney Troy Rivetti said in a statement that, “no medical provider – however renowned – is excepted from scrutiny or above the law.”
A version of this article first appeared on Medscape.com.
What happens if we sit for more than 8 hours per day?
according to a recent Latin American study published in BMC Public Health.
These data come from almost 8,000 people aged 20-65 years (half of whom are women) who participated in the Latin American Study on Nutrition and Health (ELANS). The cross-sectional survey included representative samples from urban populations in Argentina, Brazil, Chile, Colombia, Costa Rica, Ecuador, Peru, and Venezuela. The average time spent sitting was 420 min/d. Ecuador had the lowest time (300 min/day), and Argentina and Peru had the highest (480 min/day).
No amount of sitting time has been associated with a greater health risk, but the World Health Organization recommends that sitting time be minimal.
“We used to believe that any intense physical exercise could compensate for a sedentary life. But now we know that a sedentary lifestyle in general and sitting time in particular have a direct effect and are an independent risk factor for chronic diseases,” said study author Irina Kovalskys, PhD, a pediatric specialist in nutrition and a professor of nutrition at the Catholic University of Argentina, Buenos Aires, and a principal investigator of ELANS.
Dr. Kovalskys stated that the 420-min average sitting time is worrying in a population such as the one studied, in which 60% of adults are obese and there are high rates of cardiometabolic risk factors. She affirmed that it is important to raise awareness among the population and focus on adolescents.
Felipe Lobelo, PhD, is a Colombian physician, an associate professor of global health at Emory University and director of epidemiology at Kaiser Permanente Georgia, both in Atlanta. He did not participate in this study but promotes the concept of exercise in medicine. The activity of the patient must be included in a clinical setting, and improving the level of physical activity can have a positive impact on health prognosis, he said.
“To make public health recommendations or even advise patients, a cutoff point is needed. Guidelines recommend 150 minutes per week of moderate to vigorous physical activity, and some countries have started to indicate that we should be concerned about people’s sitting time. There is still no equivalent to the 150 minutes, therefore, these studies are important, especially in the Latin American population,” said Dr. Lobelo.
He explained that the concept of an increased risk of death or chronic disease because of a lack of physical activity arose in the past 50 years, but only in the past 2 decades have we started thinking about sitting time.
“Spending more than 8 hours sitting per day clearly causes a much higher risk of chronic diseases, including obesity and diabetes. It may be a continuous and progressive association, and the point at which this increase becomes exponential is clearly between 6 and 8 hours of sitting time,” Dr. Lobelo added.
The authors expected to find a linear association with risk for being overweight or obese after 4 hours, but they did not find one. “This study has limitations. Among them was that other indicators were not considered, such as health indicators. Collaborations are starting with other research groups, and other studies are being designed,” said study author Gerson Ferrari, PhD, an associate professor at Santiago de Chile University.
Comparing indicators
The Latin American study tried to establish a sitting cutoff time after which the risk of becoming overweight or obese increases. It used three indicators of excess weight: body mass index (BMI), waist circumference, and neck circumference.
Sitting for more than 8 hours increased the chances of excess weight by 10% when measured by BMI and by 13% when neck circumference was used.
Dr. Ferrari stated that the result obtained measuring BMI is the one that should be considered, because it is used in public policy. Neck circumference is a more recent measurement of detection and it is less studied, but it is a valid indicator, with good sensitivity and advantages over others, such as ease of measurement and lack of variation over time.
According to the results of this study, measuring neck circumference may be the most sensitive method of the three. Neck circumference was proportionally greater in people who sat for at least 4, at least 6, and at least 8 hours/day than in those who sat for less than 4, less than 6, and less than 8 hours/day. This relationship was not observed with the other indicators.
Broaching the topic
“What is important is uninterrupted sitting time. The recommendation is to break up those sitting times with active periods. Health professionals have already incorporated the concept of moderate to vigorous physical exercise, but nonintense activities are sufficient to reduce sitting time. Yoga may not be vigorous, but it is valuable at reducing sitting time,” said Dr. Kovalskys.
Dr. Ferrari recommended giving patients concrete messages so that they spend as little time possible sitting. “It is better to stand on the bus or the subway even when there is a place to sit. Are you going to talk on the phone? It is better to do it while walking or at least standing instead of sitting.”
A recent literature review conducted by investigators of the University of Birmingham (England) studied the possible molecular and physiologic mechanisms of inactivity time, health consequences, and protection strategies. It offers an evaluation of interventions that can compensate for the immediate negative consequences of inactivity.
Physical activity
Some studies suggest that more than 60 min/day of moderate-intensity exercise or more than 150 min/week of moderate to vigorous exercise may be effective at mitigating the increased risk for mortality associated with sitting time, but reduced intensity may not be enough.
Active pauses
Interrupting sitting every 30-60 min to walk or cycle (2-10 min), performing 3 minutes of simple resistance activities every 30 minutes, such as calf or knee lifts, performing intermittent leg movements (1 minute of activity for every 4 minutes of inactivity during a 3-hour protocol session), or pausing to climb stairs (5 minutes every hour) may be beneficial for vascular health. However, not all studies have demonstrated these positive effects, therefore, some populations may need exercise of greater intensity or duration to counteract the negative vascular effects of acute inactivity periods.
Standing workstations
Standing workstations are effective at reducing sitting time in offices but may be ineffective at reducing vascular alterations related to sitting time. Although some experimental studies indicate vascular benefits, epidemiologic studies suggest that long periods of standing can be harmful to vascular health, especially for venous diseases. Recommendations for use should be accompanied by specific regimens on the frequency and duration of the position to attain the maximum benefits and minimize other vascular complications.
One problem that Dr. Lobelo noted is that some doctors ask their patients how active they are, but they do so in a nonstandardized manner. This observation led him to publish, together with the American Heart Association, an article on the importance for health systems of considering physical activity as a vital sign and including it in records in a standardized manner.
He said that “one advantage of having physical activity as a vital sign in patient medical records is that it allows us to identify individuals who are at greater risk.”
Kaiser Permanente asks the following questions: how many minutes of physical activity do you perform regularly per week, and what is the average intensity of that activity? Patients can be classified into the following three groups: those who follow the recommendations, those with almost no activity, and those who perform some physical activity but do not meet the recommended 150 min/week of moderate to vigorous activity.
Recording sitting time is more difficult. Dr. Lobelo indicated that “it is easier for a person to remember how much time they spent running than how long they were sitting.” Regarding the use of technology, he commented that most watches provide a good estimate. Without technology, it can be estimated by asking how much time is spent in the car, on the bus, or in front of the computer or television and then adding up these times.
Dr. Lobelo emphasized that the two behaviors, lack of physical activity and excessive sitting time, have independent associations with health outcomes. But if both are combined, the risk of obesity, diabetes, and cardiovascular diseases is not just added but rather is multiplied. These behaviors contribute to the epidemic of obesity and diabetes, since most people do not follow either of the two recommendations.
“Studies show that of the two behaviors, the more negative for health would be not following the physical activity recommendations,” said Dr. Lobelo. “If the recommendation of 150 min/week of moderate to vigorous physical activity is followed, the associated risk of sitting too much declines by 80%-90%. Additionally, we can prevent, help to manage, and decrease the risk of complications in more than 100 diseases, including infections. During the pandemic, it was observed that more active people had a lower risk of dying or of being hospitalized due to COVID-19 than less active people, independently of other factors, such as hypertension, diabetes, and obesity.”
Moreover, Dr. Lobelo believes in “practicing what you preach” and advocates that doctors become healthy models.
Dr. Lobelo, Dr. Ferrari, and Dr. Kovalskys disclosed no relevant financial relationships.
This article was translated from the Medscape Spanish edition. A version appeared on Medscape.com.
according to a recent Latin American study published in BMC Public Health.
These data come from almost 8,000 people aged 20-65 years (half of whom are women) who participated in the Latin American Study on Nutrition and Health (ELANS). The cross-sectional survey included representative samples from urban populations in Argentina, Brazil, Chile, Colombia, Costa Rica, Ecuador, Peru, and Venezuela. The average time spent sitting was 420 min/d. Ecuador had the lowest time (300 min/day), and Argentina and Peru had the highest (480 min/day).
No amount of sitting time has been associated with a greater health risk, but the World Health Organization recommends that sitting time be minimal.
“We used to believe that any intense physical exercise could compensate for a sedentary life. But now we know that a sedentary lifestyle in general and sitting time in particular have a direct effect and are an independent risk factor for chronic diseases,” said study author Irina Kovalskys, PhD, a pediatric specialist in nutrition and a professor of nutrition at the Catholic University of Argentina, Buenos Aires, and a principal investigator of ELANS.
Dr. Kovalskys stated that the 420-min average sitting time is worrying in a population such as the one studied, in which 60% of adults are obese and there are high rates of cardiometabolic risk factors. She affirmed that it is important to raise awareness among the population and focus on adolescents.
Felipe Lobelo, PhD, is a Colombian physician, an associate professor of global health at Emory University and director of epidemiology at Kaiser Permanente Georgia, both in Atlanta. He did not participate in this study but promotes the concept of exercise in medicine. The activity of the patient must be included in a clinical setting, and improving the level of physical activity can have a positive impact on health prognosis, he said.
“To make public health recommendations or even advise patients, a cutoff point is needed. Guidelines recommend 150 minutes per week of moderate to vigorous physical activity, and some countries have started to indicate that we should be concerned about people’s sitting time. There is still no equivalent to the 150 minutes, therefore, these studies are important, especially in the Latin American population,” said Dr. Lobelo.
He explained that the concept of an increased risk of death or chronic disease because of a lack of physical activity arose in the past 50 years, but only in the past 2 decades have we started thinking about sitting time.
“Spending more than 8 hours sitting per day clearly causes a much higher risk of chronic diseases, including obesity and diabetes. It may be a continuous and progressive association, and the point at which this increase becomes exponential is clearly between 6 and 8 hours of sitting time,” Dr. Lobelo added.
The authors expected to find a linear association with risk for being overweight or obese after 4 hours, but they did not find one. “This study has limitations. Among them was that other indicators were not considered, such as health indicators. Collaborations are starting with other research groups, and other studies are being designed,” said study author Gerson Ferrari, PhD, an associate professor at Santiago de Chile University.
Comparing indicators
The Latin American study tried to establish a sitting cutoff time after which the risk of becoming overweight or obese increases. It used three indicators of excess weight: body mass index (BMI), waist circumference, and neck circumference.
Sitting for more than 8 hours increased the chances of excess weight by 10% when measured by BMI and by 13% when neck circumference was used.
Dr. Ferrari stated that the result obtained measuring BMI is the one that should be considered, because it is used in public policy. Neck circumference is a more recent measurement of detection and it is less studied, but it is a valid indicator, with good sensitivity and advantages over others, such as ease of measurement and lack of variation over time.
According to the results of this study, measuring neck circumference may be the most sensitive method of the three. Neck circumference was proportionally greater in people who sat for at least 4, at least 6, and at least 8 hours/day than in those who sat for less than 4, less than 6, and less than 8 hours/day. This relationship was not observed with the other indicators.
Broaching the topic
“What is important is uninterrupted sitting time. The recommendation is to break up those sitting times with active periods. Health professionals have already incorporated the concept of moderate to vigorous physical exercise, but nonintense activities are sufficient to reduce sitting time. Yoga may not be vigorous, but it is valuable at reducing sitting time,” said Dr. Kovalskys.
Dr. Ferrari recommended giving patients concrete messages so that they spend as little time possible sitting. “It is better to stand on the bus or the subway even when there is a place to sit. Are you going to talk on the phone? It is better to do it while walking or at least standing instead of sitting.”
A recent literature review conducted by investigators of the University of Birmingham (England) studied the possible molecular and physiologic mechanisms of inactivity time, health consequences, and protection strategies. It offers an evaluation of interventions that can compensate for the immediate negative consequences of inactivity.
Physical activity
Some studies suggest that more than 60 min/day of moderate-intensity exercise or more than 150 min/week of moderate to vigorous exercise may be effective at mitigating the increased risk for mortality associated with sitting time, but reduced intensity may not be enough.
Active pauses
Interrupting sitting every 30-60 min to walk or cycle (2-10 min), performing 3 minutes of simple resistance activities every 30 minutes, such as calf or knee lifts, performing intermittent leg movements (1 minute of activity for every 4 minutes of inactivity during a 3-hour protocol session), or pausing to climb stairs (5 minutes every hour) may be beneficial for vascular health. However, not all studies have demonstrated these positive effects, therefore, some populations may need exercise of greater intensity or duration to counteract the negative vascular effects of acute inactivity periods.
Standing workstations
Standing workstations are effective at reducing sitting time in offices but may be ineffective at reducing vascular alterations related to sitting time. Although some experimental studies indicate vascular benefits, epidemiologic studies suggest that long periods of standing can be harmful to vascular health, especially for venous diseases. Recommendations for use should be accompanied by specific regimens on the frequency and duration of the position to attain the maximum benefits and minimize other vascular complications.
One problem that Dr. Lobelo noted is that some doctors ask their patients how active they are, but they do so in a nonstandardized manner. This observation led him to publish, together with the American Heart Association, an article on the importance for health systems of considering physical activity as a vital sign and including it in records in a standardized manner.
He said that “one advantage of having physical activity as a vital sign in patient medical records is that it allows us to identify individuals who are at greater risk.”
Kaiser Permanente asks the following questions: how many minutes of physical activity do you perform regularly per week, and what is the average intensity of that activity? Patients can be classified into the following three groups: those who follow the recommendations, those with almost no activity, and those who perform some physical activity but do not meet the recommended 150 min/week of moderate to vigorous activity.
Recording sitting time is more difficult. Dr. Lobelo indicated that “it is easier for a person to remember how much time they spent running than how long they were sitting.” Regarding the use of technology, he commented that most watches provide a good estimate. Without technology, it can be estimated by asking how much time is spent in the car, on the bus, or in front of the computer or television and then adding up these times.
Dr. Lobelo emphasized that the two behaviors, lack of physical activity and excessive sitting time, have independent associations with health outcomes. But if both are combined, the risk of obesity, diabetes, and cardiovascular diseases is not just added but rather is multiplied. These behaviors contribute to the epidemic of obesity and diabetes, since most people do not follow either of the two recommendations.
“Studies show that of the two behaviors, the more negative for health would be not following the physical activity recommendations,” said Dr. Lobelo. “If the recommendation of 150 min/week of moderate to vigorous physical activity is followed, the associated risk of sitting too much declines by 80%-90%. Additionally, we can prevent, help to manage, and decrease the risk of complications in more than 100 diseases, including infections. During the pandemic, it was observed that more active people had a lower risk of dying or of being hospitalized due to COVID-19 than less active people, independently of other factors, such as hypertension, diabetes, and obesity.”
Moreover, Dr. Lobelo believes in “practicing what you preach” and advocates that doctors become healthy models.
Dr. Lobelo, Dr. Ferrari, and Dr. Kovalskys disclosed no relevant financial relationships.
This article was translated from the Medscape Spanish edition. A version appeared on Medscape.com.
according to a recent Latin American study published in BMC Public Health.
These data come from almost 8,000 people aged 20-65 years (half of whom are women) who participated in the Latin American Study on Nutrition and Health (ELANS). The cross-sectional survey included representative samples from urban populations in Argentina, Brazil, Chile, Colombia, Costa Rica, Ecuador, Peru, and Venezuela. The average time spent sitting was 420 min/d. Ecuador had the lowest time (300 min/day), and Argentina and Peru had the highest (480 min/day).
No amount of sitting time has been associated with a greater health risk, but the World Health Organization recommends that sitting time be minimal.
“We used to believe that any intense physical exercise could compensate for a sedentary life. But now we know that a sedentary lifestyle in general and sitting time in particular have a direct effect and are an independent risk factor for chronic diseases,” said study author Irina Kovalskys, PhD, a pediatric specialist in nutrition and a professor of nutrition at the Catholic University of Argentina, Buenos Aires, and a principal investigator of ELANS.
Dr. Kovalskys stated that the 420-min average sitting time is worrying in a population such as the one studied, in which 60% of adults are obese and there are high rates of cardiometabolic risk factors. She affirmed that it is important to raise awareness among the population and focus on adolescents.
Felipe Lobelo, PhD, is a Colombian physician, an associate professor of global health at Emory University and director of epidemiology at Kaiser Permanente Georgia, both in Atlanta. He did not participate in this study but promotes the concept of exercise in medicine. The activity of the patient must be included in a clinical setting, and improving the level of physical activity can have a positive impact on health prognosis, he said.
“To make public health recommendations or even advise patients, a cutoff point is needed. Guidelines recommend 150 minutes per week of moderate to vigorous physical activity, and some countries have started to indicate that we should be concerned about people’s sitting time. There is still no equivalent to the 150 minutes, therefore, these studies are important, especially in the Latin American population,” said Dr. Lobelo.
He explained that the concept of an increased risk of death or chronic disease because of a lack of physical activity arose in the past 50 years, but only in the past 2 decades have we started thinking about sitting time.
“Spending more than 8 hours sitting per day clearly causes a much higher risk of chronic diseases, including obesity and diabetes. It may be a continuous and progressive association, and the point at which this increase becomes exponential is clearly between 6 and 8 hours of sitting time,” Dr. Lobelo added.
The authors expected to find a linear association with risk for being overweight or obese after 4 hours, but they did not find one. “This study has limitations. Among them was that other indicators were not considered, such as health indicators. Collaborations are starting with other research groups, and other studies are being designed,” said study author Gerson Ferrari, PhD, an associate professor at Santiago de Chile University.
Comparing indicators
The Latin American study tried to establish a sitting cutoff time after which the risk of becoming overweight or obese increases. It used three indicators of excess weight: body mass index (BMI), waist circumference, and neck circumference.
Sitting for more than 8 hours increased the chances of excess weight by 10% when measured by BMI and by 13% when neck circumference was used.
Dr. Ferrari stated that the result obtained measuring BMI is the one that should be considered, because it is used in public policy. Neck circumference is a more recent measurement of detection and it is less studied, but it is a valid indicator, with good sensitivity and advantages over others, such as ease of measurement and lack of variation over time.
According to the results of this study, measuring neck circumference may be the most sensitive method of the three. Neck circumference was proportionally greater in people who sat for at least 4, at least 6, and at least 8 hours/day than in those who sat for less than 4, less than 6, and less than 8 hours/day. This relationship was not observed with the other indicators.
Broaching the topic
“What is important is uninterrupted sitting time. The recommendation is to break up those sitting times with active periods. Health professionals have already incorporated the concept of moderate to vigorous physical exercise, but nonintense activities are sufficient to reduce sitting time. Yoga may not be vigorous, but it is valuable at reducing sitting time,” said Dr. Kovalskys.
Dr. Ferrari recommended giving patients concrete messages so that they spend as little time possible sitting. “It is better to stand on the bus or the subway even when there is a place to sit. Are you going to talk on the phone? It is better to do it while walking or at least standing instead of sitting.”
A recent literature review conducted by investigators of the University of Birmingham (England) studied the possible molecular and physiologic mechanisms of inactivity time, health consequences, and protection strategies. It offers an evaluation of interventions that can compensate for the immediate negative consequences of inactivity.
Physical activity
Some studies suggest that more than 60 min/day of moderate-intensity exercise or more than 150 min/week of moderate to vigorous exercise may be effective at mitigating the increased risk for mortality associated with sitting time, but reduced intensity may not be enough.
Active pauses
Interrupting sitting every 30-60 min to walk or cycle (2-10 min), performing 3 minutes of simple resistance activities every 30 minutes, such as calf or knee lifts, performing intermittent leg movements (1 minute of activity for every 4 minutes of inactivity during a 3-hour protocol session), or pausing to climb stairs (5 minutes every hour) may be beneficial for vascular health. However, not all studies have demonstrated these positive effects, therefore, some populations may need exercise of greater intensity or duration to counteract the negative vascular effects of acute inactivity periods.
Standing workstations
Standing workstations are effective at reducing sitting time in offices but may be ineffective at reducing vascular alterations related to sitting time. Although some experimental studies indicate vascular benefits, epidemiologic studies suggest that long periods of standing can be harmful to vascular health, especially for venous diseases. Recommendations for use should be accompanied by specific regimens on the frequency and duration of the position to attain the maximum benefits and minimize other vascular complications.
One problem that Dr. Lobelo noted is that some doctors ask their patients how active they are, but they do so in a nonstandardized manner. This observation led him to publish, together with the American Heart Association, an article on the importance for health systems of considering physical activity as a vital sign and including it in records in a standardized manner.
He said that “one advantage of having physical activity as a vital sign in patient medical records is that it allows us to identify individuals who are at greater risk.”
Kaiser Permanente asks the following questions: how many minutes of physical activity do you perform regularly per week, and what is the average intensity of that activity? Patients can be classified into the following three groups: those who follow the recommendations, those with almost no activity, and those who perform some physical activity but do not meet the recommended 150 min/week of moderate to vigorous activity.
Recording sitting time is more difficult. Dr. Lobelo indicated that “it is easier for a person to remember how much time they spent running than how long they were sitting.” Regarding the use of technology, he commented that most watches provide a good estimate. Without technology, it can be estimated by asking how much time is spent in the car, on the bus, or in front of the computer or television and then adding up these times.
Dr. Lobelo emphasized that the two behaviors, lack of physical activity and excessive sitting time, have independent associations with health outcomes. But if both are combined, the risk of obesity, diabetes, and cardiovascular diseases is not just added but rather is multiplied. These behaviors contribute to the epidemic of obesity and diabetes, since most people do not follow either of the two recommendations.
“Studies show that of the two behaviors, the more negative for health would be not following the physical activity recommendations,” said Dr. Lobelo. “If the recommendation of 150 min/week of moderate to vigorous physical activity is followed, the associated risk of sitting too much declines by 80%-90%. Additionally, we can prevent, help to manage, and decrease the risk of complications in more than 100 diseases, including infections. During the pandemic, it was observed that more active people had a lower risk of dying or of being hospitalized due to COVID-19 than less active people, independently of other factors, such as hypertension, diabetes, and obesity.”
Moreover, Dr. Lobelo believes in “practicing what you preach” and advocates that doctors become healthy models.
Dr. Lobelo, Dr. Ferrari, and Dr. Kovalskys disclosed no relevant financial relationships.
This article was translated from the Medscape Spanish edition. A version appeared on Medscape.com.
FROM BMC PUBLIC HEALTH
NP-PA turf fights: Where the relationship can improve
– The U.S. Bureau of Labor Statistics forecasts a 40% increase in the NP workforce by 2031, coupled with a 28% rise in PAs.
In recent reports on the quality of the relationships involving these health care professions, survey respondents mostly gave positive accounts of collaboration, using words such as like “comradery,” “teamwork,” “congenial,” and “cohesion.” But all was not perfect. Where and how could these important health care provider relationships improve?
PAs: “Competition and collaboration’ with RNs
In a Medscape survey of more than 770 PAs about their working relationships with other health care professionals; 83% of them supported the idea of PAs and NPs practicing more independently from physicians, but sometimes it’s not easy to stay in their individual lanes.
One PA respondent complained that NPs get “more opportunities and preference,” another pointed to PA-NP “turf issues,” and a third griped about NPs’ “strong unions,” which have stoked more fighting about practice abilities and available settings.
Robert Blumm, MA, PA-C, a retired surgical and emergency medicine PA who regards himself as an advocate for both PAs and NPs, describes their interaction as a “mixture of competition and collaboration.”
On one hand, the two groups typically “cooperate and do an excellent job, incurring patient errors similar to or less than physician colleagues or senior residents.” On the other hand, Mr. Blumm conceded, there is some jealousy among PAs over NPs’ advantage in staffing and hiring decisions, “since they don’t need [direct physician] supervision ... and there are limits on how many PAs can be supervised by one physician.”
Most PA-NP interactions are collaborative, although many people emphasize the relatively few conflicts, said Jennifer Orozco, DMSc, PA-C, president and chair of the American Academy of PAs.
“We see that a lot in this country,” she said. “People try to drive a wedge, but it’s often a misnomer that there’s a lot of arguing and infighting.”
NPs: Different backgrounds, same goal
The Medscape survey also included information from 750 NPs on working relationships; 93% of them favored nurses and PAs working more independently from doctors.
April Kapu, DNP, ARPN, has worked closely with PAs for more than 20 years. “In my experience ... they complement one another as health team members, although the education and training are somewhat different,” said Ms. Kapu, , president of the American Association of Nurse Practitioners.
Some respondents noted the different educational trajectories for NPs and PAs. “Doctors and PAs are taught using the same model, but NPs are taught under the nursing model,” wrote a family medicine PA.
In emergency departments where Mr. Blumm has worked, ICU NPs have an edge over PAs in terms of preparation, organization, and the tabulation of formulas. On the other hand, some of Mr. Blumm’s fellow PAs were also emergency medicine technicians or respiratory therapists, who had “2 years of classroom training, on par with that of medical students.”
Must these differences in training and education foment conflict between NPs and PAs? “We all bring something different to the table,” said Ms. Kapu, who also is associate dean for clinical and community partnerships at Vanderbilt University, Nashville, Tenn. “It is important to respect each person’s entry point, education, and training.”
Differing personalities and environments
Numerous PA respondents said that individual personalities and work environments are more likely to trigger issues with NPs than are differences in training.
“It depends on the team and situation and who the people are, not the letters behind their names,” an emergency medicine PA wrote. A surgical PA noted that “group dynamics and work culture differ from place to place,” while a third PA agreed that “it’s personality dependent, not title dependent.”
No single formula will resolve areas of NP-PA conflict, Ms. Orozco said. “What works in Chicago might not work in rural Colorado or Texas or California, but we do have to come together. The overall focus should be on greater flexibility for PAs and NPs. Patients will fare better.”
Joint research, publishing could help
About a decade ago, Mr. Blumm joined with another PA and an NP to form the American College of Clinicians, the first joint PA-NP national professional organization. Although it disbanded after 6 years, owing to low membership, he hopes a similar collaboration will take off in the future.
“I also recommend that PAs and NPs publish articles together, with research as an excellent place to start,” he added. “PAs and NPs should stand together and be a source of healing for all our patients. Regardless of our titles, our responsibility is to bring healing together.”
A version of this article first appeared on Medscape.com.
– The U.S. Bureau of Labor Statistics forecasts a 40% increase in the NP workforce by 2031, coupled with a 28% rise in PAs.
In recent reports on the quality of the relationships involving these health care professions, survey respondents mostly gave positive accounts of collaboration, using words such as like “comradery,” “teamwork,” “congenial,” and “cohesion.” But all was not perfect. Where and how could these important health care provider relationships improve?
PAs: “Competition and collaboration’ with RNs
In a Medscape survey of more than 770 PAs about their working relationships with other health care professionals; 83% of them supported the idea of PAs and NPs practicing more independently from physicians, but sometimes it’s not easy to stay in their individual lanes.
One PA respondent complained that NPs get “more opportunities and preference,” another pointed to PA-NP “turf issues,” and a third griped about NPs’ “strong unions,” which have stoked more fighting about practice abilities and available settings.
Robert Blumm, MA, PA-C, a retired surgical and emergency medicine PA who regards himself as an advocate for both PAs and NPs, describes their interaction as a “mixture of competition and collaboration.”
On one hand, the two groups typically “cooperate and do an excellent job, incurring patient errors similar to or less than physician colleagues or senior residents.” On the other hand, Mr. Blumm conceded, there is some jealousy among PAs over NPs’ advantage in staffing and hiring decisions, “since they don’t need [direct physician] supervision ... and there are limits on how many PAs can be supervised by one physician.”
Most PA-NP interactions are collaborative, although many people emphasize the relatively few conflicts, said Jennifer Orozco, DMSc, PA-C, president and chair of the American Academy of PAs.
“We see that a lot in this country,” she said. “People try to drive a wedge, but it’s often a misnomer that there’s a lot of arguing and infighting.”
NPs: Different backgrounds, same goal
The Medscape survey also included information from 750 NPs on working relationships; 93% of them favored nurses and PAs working more independently from doctors.
April Kapu, DNP, ARPN, has worked closely with PAs for more than 20 years. “In my experience ... they complement one another as health team members, although the education and training are somewhat different,” said Ms. Kapu, , president of the American Association of Nurse Practitioners.
Some respondents noted the different educational trajectories for NPs and PAs. “Doctors and PAs are taught using the same model, but NPs are taught under the nursing model,” wrote a family medicine PA.
In emergency departments where Mr. Blumm has worked, ICU NPs have an edge over PAs in terms of preparation, organization, and the tabulation of formulas. On the other hand, some of Mr. Blumm’s fellow PAs were also emergency medicine technicians or respiratory therapists, who had “2 years of classroom training, on par with that of medical students.”
Must these differences in training and education foment conflict between NPs and PAs? “We all bring something different to the table,” said Ms. Kapu, who also is associate dean for clinical and community partnerships at Vanderbilt University, Nashville, Tenn. “It is important to respect each person’s entry point, education, and training.”
Differing personalities and environments
Numerous PA respondents said that individual personalities and work environments are more likely to trigger issues with NPs than are differences in training.
“It depends on the team and situation and who the people are, not the letters behind their names,” an emergency medicine PA wrote. A surgical PA noted that “group dynamics and work culture differ from place to place,” while a third PA agreed that “it’s personality dependent, not title dependent.”
No single formula will resolve areas of NP-PA conflict, Ms. Orozco said. “What works in Chicago might not work in rural Colorado or Texas or California, but we do have to come together. The overall focus should be on greater flexibility for PAs and NPs. Patients will fare better.”
Joint research, publishing could help
About a decade ago, Mr. Blumm joined with another PA and an NP to form the American College of Clinicians, the first joint PA-NP national professional organization. Although it disbanded after 6 years, owing to low membership, he hopes a similar collaboration will take off in the future.
“I also recommend that PAs and NPs publish articles together, with research as an excellent place to start,” he added. “PAs and NPs should stand together and be a source of healing for all our patients. Regardless of our titles, our responsibility is to bring healing together.”
A version of this article first appeared on Medscape.com.
– The U.S. Bureau of Labor Statistics forecasts a 40% increase in the NP workforce by 2031, coupled with a 28% rise in PAs.
In recent reports on the quality of the relationships involving these health care professions, survey respondents mostly gave positive accounts of collaboration, using words such as like “comradery,” “teamwork,” “congenial,” and “cohesion.” But all was not perfect. Where and how could these important health care provider relationships improve?
PAs: “Competition and collaboration’ with RNs
In a Medscape survey of more than 770 PAs about their working relationships with other health care professionals; 83% of them supported the idea of PAs and NPs practicing more independently from physicians, but sometimes it’s not easy to stay in their individual lanes.
One PA respondent complained that NPs get “more opportunities and preference,” another pointed to PA-NP “turf issues,” and a third griped about NPs’ “strong unions,” which have stoked more fighting about practice abilities and available settings.
Robert Blumm, MA, PA-C, a retired surgical and emergency medicine PA who regards himself as an advocate for both PAs and NPs, describes their interaction as a “mixture of competition and collaboration.”
On one hand, the two groups typically “cooperate and do an excellent job, incurring patient errors similar to or less than physician colleagues or senior residents.” On the other hand, Mr. Blumm conceded, there is some jealousy among PAs over NPs’ advantage in staffing and hiring decisions, “since they don’t need [direct physician] supervision ... and there are limits on how many PAs can be supervised by one physician.”
Most PA-NP interactions are collaborative, although many people emphasize the relatively few conflicts, said Jennifer Orozco, DMSc, PA-C, president and chair of the American Academy of PAs.
“We see that a lot in this country,” she said. “People try to drive a wedge, but it’s often a misnomer that there’s a lot of arguing and infighting.”
NPs: Different backgrounds, same goal
The Medscape survey also included information from 750 NPs on working relationships; 93% of them favored nurses and PAs working more independently from doctors.
April Kapu, DNP, ARPN, has worked closely with PAs for more than 20 years. “In my experience ... they complement one another as health team members, although the education and training are somewhat different,” said Ms. Kapu, , president of the American Association of Nurse Practitioners.
Some respondents noted the different educational trajectories for NPs and PAs. “Doctors and PAs are taught using the same model, but NPs are taught under the nursing model,” wrote a family medicine PA.
In emergency departments where Mr. Blumm has worked, ICU NPs have an edge over PAs in terms of preparation, organization, and the tabulation of formulas. On the other hand, some of Mr. Blumm’s fellow PAs were also emergency medicine technicians or respiratory therapists, who had “2 years of classroom training, on par with that of medical students.”
Must these differences in training and education foment conflict between NPs and PAs? “We all bring something different to the table,” said Ms. Kapu, who also is associate dean for clinical and community partnerships at Vanderbilt University, Nashville, Tenn. “It is important to respect each person’s entry point, education, and training.”
Differing personalities and environments
Numerous PA respondents said that individual personalities and work environments are more likely to trigger issues with NPs than are differences in training.
“It depends on the team and situation and who the people are, not the letters behind their names,” an emergency medicine PA wrote. A surgical PA noted that “group dynamics and work culture differ from place to place,” while a third PA agreed that “it’s personality dependent, not title dependent.”
No single formula will resolve areas of NP-PA conflict, Ms. Orozco said. “What works in Chicago might not work in rural Colorado or Texas or California, but we do have to come together. The overall focus should be on greater flexibility for PAs and NPs. Patients will fare better.”
Joint research, publishing could help
About a decade ago, Mr. Blumm joined with another PA and an NP to form the American College of Clinicians, the first joint PA-NP national professional organization. Although it disbanded after 6 years, owing to low membership, he hopes a similar collaboration will take off in the future.
“I also recommend that PAs and NPs publish articles together, with research as an excellent place to start,” he added. “PAs and NPs should stand together and be a source of healing for all our patients. Regardless of our titles, our responsibility is to bring healing together.”
A version of this article first appeared on Medscape.com.
Celiac disease appears to double COVID-19 hospitalization risk
, a single-center U.S. study shows.
Vaccination against COVID-19 reduced the risk for hospitalization by almost half for both groups, however, the study finds.
“To our knowledge this is the first study that demonstrated a vaccination effect on mitigation of the risk of hospitalization in celiac disease patients with COVID-19 infection,” write Alberto Rubio-Tapia, MD, director, Celiac Disease Program, Digestive Disease and Surgery Institute, Cleveland Clinic Foundation, and colleagues.
Despite the increased risk for hospitalization among patients with celiac disease, there were no significant differences between those with and without the condition with respect to intensive care unit requirement, mortality, or thrombosis, the researchers found.
The findings suggest that celiac disease patients with COVID-19 are “not inherently at greater risk for more severe outcomes,” they wrote.
The study was published online in Clinical Gastroenterology and Hepatology.
Comparing outcomes
Although it has been shown that patients with celiac disease have increased susceptibility to viral illnesses, research to date has found similar COVID-19 incidence and outcomes, including hospitalization, between patients with celiac disease and the general population, the researchers wrote.
However, the impact of COVID-19 vaccination is less clear, so the researchers set out to compare the frequency of COVID-19–related outcomes between patients with and without celiac disease before and after vaccination.
Through an analysis of patient medical records, researchers found 171,763 patients diagnosed and treated for COVID-19 at their institution between March 1, 2020, and Jan 1, 2022. Of them, 110 adults had biopsy-proven celiac disease.
The median time from biopsy diagnosis of celiac disease to COVID-19 was 217 months, 66.3% of patients were documented to be following a gluten-free diet, and tissue transglutaminase IgA was positive in 46.2% at the time of COVID-19.
The celiac group was matched by age, ethnicity, sex, and date of COVID-19 diagnosis with a control group of 220 adults without a clinical diagnosis of celiac disease. The two cohorts had similar rates of comorbid obesity, type 2 diabetes, preexisting lung disease, and tobacco use.
Patients with celiac disease were significantly more likely to be hospitalized for COVID-19 than were the control participants, at 24% vs. 11% (hazard ratio, 2.1; P = .009), the researchers wrote.
However, hospitalized patients with celiac disease were less likely to require supplementary oxygen than were the control participants, at 63% vs. 84%.
Vaccination rates for COVID-19 were similar between the two groups, at 64.5% among patients with celiac disease and 70% in the control group. Vaccination was associated with a lower risk for hospitalization on multivariate analysis (HR, 0.53; P = .026).
There was no significant difference in hospitalization rates between vaccinated patients with celiac disease and vaccinated patients in the control group (odds ratio, 1.12; P = .79), the team reported.
The secondary outcomes of ICU requirement, mortality, and thrombosis were minimal in both groups, the researchers wrote.
Vaccination’s importance
The different findings regarding hospitalization risk among patients with celiac disease between this study and previous research are likely due to earlier studies not accounting for vaccination status, the researchers wrote.
“This study shows significantly different rates of hospitalization among patients with [celiac disease] depending on their vaccination status, with strong evidence for mitigation of hospitalization risk through vaccination,” they added.
“Vaccination against COVID-19 should be strongly recommended in patients with celiac disease,” the researchers concluded.
No funding was declared. Dr. Rubio-Tapia reported a relationship with Takeda. No other financial relationships were declared.
A version of this article first appeared on Medscape.com.
, a single-center U.S. study shows.
Vaccination against COVID-19 reduced the risk for hospitalization by almost half for both groups, however, the study finds.
“To our knowledge this is the first study that demonstrated a vaccination effect on mitigation of the risk of hospitalization in celiac disease patients with COVID-19 infection,” write Alberto Rubio-Tapia, MD, director, Celiac Disease Program, Digestive Disease and Surgery Institute, Cleveland Clinic Foundation, and colleagues.
Despite the increased risk for hospitalization among patients with celiac disease, there were no significant differences between those with and without the condition with respect to intensive care unit requirement, mortality, or thrombosis, the researchers found.
The findings suggest that celiac disease patients with COVID-19 are “not inherently at greater risk for more severe outcomes,” they wrote.
The study was published online in Clinical Gastroenterology and Hepatology.
Comparing outcomes
Although it has been shown that patients with celiac disease have increased susceptibility to viral illnesses, research to date has found similar COVID-19 incidence and outcomes, including hospitalization, between patients with celiac disease and the general population, the researchers wrote.
However, the impact of COVID-19 vaccination is less clear, so the researchers set out to compare the frequency of COVID-19–related outcomes between patients with and without celiac disease before and after vaccination.
Through an analysis of patient medical records, researchers found 171,763 patients diagnosed and treated for COVID-19 at their institution between March 1, 2020, and Jan 1, 2022. Of them, 110 adults had biopsy-proven celiac disease.
The median time from biopsy diagnosis of celiac disease to COVID-19 was 217 months, 66.3% of patients were documented to be following a gluten-free diet, and tissue transglutaminase IgA was positive in 46.2% at the time of COVID-19.
The celiac group was matched by age, ethnicity, sex, and date of COVID-19 diagnosis with a control group of 220 adults without a clinical diagnosis of celiac disease. The two cohorts had similar rates of comorbid obesity, type 2 diabetes, preexisting lung disease, and tobacco use.
Patients with celiac disease were significantly more likely to be hospitalized for COVID-19 than were the control participants, at 24% vs. 11% (hazard ratio, 2.1; P = .009), the researchers wrote.
However, hospitalized patients with celiac disease were less likely to require supplementary oxygen than were the control participants, at 63% vs. 84%.
Vaccination rates for COVID-19 were similar between the two groups, at 64.5% among patients with celiac disease and 70% in the control group. Vaccination was associated with a lower risk for hospitalization on multivariate analysis (HR, 0.53; P = .026).
There was no significant difference in hospitalization rates between vaccinated patients with celiac disease and vaccinated patients in the control group (odds ratio, 1.12; P = .79), the team reported.
The secondary outcomes of ICU requirement, mortality, and thrombosis were minimal in both groups, the researchers wrote.
Vaccination’s importance
The different findings regarding hospitalization risk among patients with celiac disease between this study and previous research are likely due to earlier studies not accounting for vaccination status, the researchers wrote.
“This study shows significantly different rates of hospitalization among patients with [celiac disease] depending on their vaccination status, with strong evidence for mitigation of hospitalization risk through vaccination,” they added.
“Vaccination against COVID-19 should be strongly recommended in patients with celiac disease,” the researchers concluded.
No funding was declared. Dr. Rubio-Tapia reported a relationship with Takeda. No other financial relationships were declared.
A version of this article first appeared on Medscape.com.
, a single-center U.S. study shows.
Vaccination against COVID-19 reduced the risk for hospitalization by almost half for both groups, however, the study finds.
“To our knowledge this is the first study that demonstrated a vaccination effect on mitigation of the risk of hospitalization in celiac disease patients with COVID-19 infection,” write Alberto Rubio-Tapia, MD, director, Celiac Disease Program, Digestive Disease and Surgery Institute, Cleveland Clinic Foundation, and colleagues.
Despite the increased risk for hospitalization among patients with celiac disease, there were no significant differences between those with and without the condition with respect to intensive care unit requirement, mortality, or thrombosis, the researchers found.
The findings suggest that celiac disease patients with COVID-19 are “not inherently at greater risk for more severe outcomes,” they wrote.
The study was published online in Clinical Gastroenterology and Hepatology.
Comparing outcomes
Although it has been shown that patients with celiac disease have increased susceptibility to viral illnesses, research to date has found similar COVID-19 incidence and outcomes, including hospitalization, between patients with celiac disease and the general population, the researchers wrote.
However, the impact of COVID-19 vaccination is less clear, so the researchers set out to compare the frequency of COVID-19–related outcomes between patients with and without celiac disease before and after vaccination.
Through an analysis of patient medical records, researchers found 171,763 patients diagnosed and treated for COVID-19 at their institution between March 1, 2020, and Jan 1, 2022. Of them, 110 adults had biopsy-proven celiac disease.
The median time from biopsy diagnosis of celiac disease to COVID-19 was 217 months, 66.3% of patients were documented to be following a gluten-free diet, and tissue transglutaminase IgA was positive in 46.2% at the time of COVID-19.
The celiac group was matched by age, ethnicity, sex, and date of COVID-19 diagnosis with a control group of 220 adults without a clinical diagnosis of celiac disease. The two cohorts had similar rates of comorbid obesity, type 2 diabetes, preexisting lung disease, and tobacco use.
Patients with celiac disease were significantly more likely to be hospitalized for COVID-19 than were the control participants, at 24% vs. 11% (hazard ratio, 2.1; P = .009), the researchers wrote.
However, hospitalized patients with celiac disease were less likely to require supplementary oxygen than were the control participants, at 63% vs. 84%.
Vaccination rates for COVID-19 were similar between the two groups, at 64.5% among patients with celiac disease and 70% in the control group. Vaccination was associated with a lower risk for hospitalization on multivariate analysis (HR, 0.53; P = .026).
There was no significant difference in hospitalization rates between vaccinated patients with celiac disease and vaccinated patients in the control group (odds ratio, 1.12; P = .79), the team reported.
The secondary outcomes of ICU requirement, mortality, and thrombosis were minimal in both groups, the researchers wrote.
Vaccination’s importance
The different findings regarding hospitalization risk among patients with celiac disease between this study and previous research are likely due to earlier studies not accounting for vaccination status, the researchers wrote.
“This study shows significantly different rates of hospitalization among patients with [celiac disease] depending on their vaccination status, with strong evidence for mitigation of hospitalization risk through vaccination,” they added.
“Vaccination against COVID-19 should be strongly recommended in patients with celiac disease,” the researchers concluded.
No funding was declared. Dr. Rubio-Tapia reported a relationship with Takeda. No other financial relationships were declared.
A version of this article first appeared on Medscape.com.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Risk of stent infection low, but may be underreported
Infections of coronary stents appear to be uncommon, but it is not clear if they are often missed, underreported, or truly rare, according to a new analysis.
In a search of multiple databases, 79 cases of coronary stent infections (CSI) were found in 65 published reports, according to Venkatakrishnan Ramakumar, MBBS, MD, department of cardiology, All India Institute of Medical Sciences, New Delhi.
Over the period of evaluation, which had no defined starting point but stretched to November 2021, the 79 infections reported worldwide occurred when millions of percutaneous coronary intervention (PCI) procedures were performed. In the United States alone, the current estimated annual number of PCIs is 600,000, according to an article published in the Journal of the American Heart Association.
If the number of reported CSI cases represented even a modest fraction of those that occurred, the risk would still be almost negligible. Yet, Dr. Ramakumar insisted that there has been little attention paid to the potential for CSI, creating a situation in which many or almost all cases are simply being missed.
“We do not know how many infections have gone unrecognized,” Dr. Ramakumar said in presenting his results at the Cardiovascular Research Technologies conference, sponsored by MedStar Heart & Vascular Institute. And even if they are identified and promptly treated, there “is the potential for a publication bias,” he added, referring to the reluctance of investigators to submit and publishers to accept manuscripts with negative results.
Regardless of the frequency with which they occur, CSI is associated with bad outcomes, according to the data evaluated by Dr. Ramakumar. On the basis of in-hospital mortality, the primary endpoint of this analysis, the rate of death in patients developing CSI was 30.3%.
Successful treatment varied by hospital type
This risk was not uniform. Rather, rates of in-hospital mortality and proportion of patients treated successfully varied substantially by type of hospital. At private teaching hospitals for example, successful treatment – whether medical alone or followed by bailout surgery – was 80%. The rates fell to 40% at public teaching hospitals and then to 25% at private nonteaching hospitals.
The full-text articles included in this analysis were evaluated and selected by two reviewers working independently. A CSI diagnosis made clinically or with imaging and treatment outcomes were among criteria for the case studies to be included. Dr. Ramakumar said the study, which he claimed is the largest systematic review of CSI ever conducted, has been registered with PROSPERO, an international prospective registry of systematic reviews.
The presenting symptom was fever in 72% of cases and chest pain in the others, although there was one asymptomatic CSI reported. On angiography, 62% had a concomitant mycotic aneurysm. Intramyocardial abscess (13.9%), rupture (11.3%), and coronary fistula (7.5%) were also common findings, but no angiographic abnormalities could be identified in 53% of patients.
Following PCI, most CSI developed within 8 days (43%) or the first month (23%), but CSI was reported more than 6 months after the procedure in 19%. Complex PCI accounted for 51% of cases. Of stent types, 56% were drug eluting and 13% were bare metal.
When comparing characteristics of those who survived CSI with those who did not, most (89%) of those with a non–ST-segment elevated acute coronary syndrome ultimately survived, while survival from CSI in those with structural heart disease was only 17%.
Microbiological findings were not a criterion for study inclusion, but Staphylococcus species accounted for 65% of the infections for which positive cultures were reported. Pseudomonas accounted for 13%. Less than 4% (3.8%) tested positive for multiple pathogens. A small proportion of patients had unusual infectious organisms.
As part of this analysis, the investigators developed an artificial intelligence model to predict CSI based on patient characteristics and other variables. However, the specificity of only around 70% led Dr. Ramakumar to conclude that it does not yet have practical value.
However, he believes that better methodology to detect CSI is needed, and he proposed a diagnostic algorithm that he believes would both improve detection rates and accelerate the time to diagnosis.
Algorithm proposed for detection of CSI
In this algorithm, the first step in symptomatic patients with a positive blood culture suspected of CSI is imaging, such as transthoracic echocardiography, to identify features of infective endocarditis or endarteritis. If the imaging is positive, further imaging, such as PET, that supports the diagnosis, should be adequate to support a diagnosis and treatment.
If initial imaging is negative, alternative diagnoses should be considered, but Dr. Ramakumar advised repeat imaging after 48 hours if symptoms persist and no other causes are found.
Dr. Ramakumar acknowledged the many limitations of this analysis, including the small sample size and the challenges of assembling coherent data from case reports with variable types of information submitted during different eras of PCI evolution. However, reiterating that CSI might be frequently missed, he emphasized that this problem might be bigger than currently understood.
It is difficult to rule out any possibility that CSI is frequently missed, but Andrew Sharp, MD, PhD, a consultant interventional cardiologist at the University Hospital of Wales, Cardiff, is skeptical.
“One might think this is a potential problem, but I cannot think of one patient in whom this has occurred,” Dr. Sharp said in an interview. He is fairly confident that they are extremely rare.
“When there is infection associated with a foreign body, such as a pacemaker, they do not typically resolve by themselves,” he explained. “Often the device has to be removed. If this was true for CSI, then I think we would be aware of these complications.”
However, he praised the investigators for taking a look at CSI in a systematic approach. An invited panelist during the CRT featured research, which is where these data were presented, Dr. Sharp was more interested in understanding why they do not occur now that data are available to suggest they are rare.
“Is there something in the coronary environment, such as the consistent blood flow, that protects against infection?” he asked. CSI is a valid area of further research, according to Dr. Sharp, but he does not consider infected stents to be a common threat based on his own sizable case series.
Dr. Ramakumar and Dr. Sharp reported no potential conflicts of interest.
Infections of coronary stents appear to be uncommon, but it is not clear if they are often missed, underreported, or truly rare, according to a new analysis.
In a search of multiple databases, 79 cases of coronary stent infections (CSI) were found in 65 published reports, according to Venkatakrishnan Ramakumar, MBBS, MD, department of cardiology, All India Institute of Medical Sciences, New Delhi.
Over the period of evaluation, which had no defined starting point but stretched to November 2021, the 79 infections reported worldwide occurred when millions of percutaneous coronary intervention (PCI) procedures were performed. In the United States alone, the current estimated annual number of PCIs is 600,000, according to an article published in the Journal of the American Heart Association.
If the number of reported CSI cases represented even a modest fraction of those that occurred, the risk would still be almost negligible. Yet, Dr. Ramakumar insisted that there has been little attention paid to the potential for CSI, creating a situation in which many or almost all cases are simply being missed.
“We do not know how many infections have gone unrecognized,” Dr. Ramakumar said in presenting his results at the Cardiovascular Research Technologies conference, sponsored by MedStar Heart & Vascular Institute. And even if they are identified and promptly treated, there “is the potential for a publication bias,” he added, referring to the reluctance of investigators to submit and publishers to accept manuscripts with negative results.
Regardless of the frequency with which they occur, CSI is associated with bad outcomes, according to the data evaluated by Dr. Ramakumar. On the basis of in-hospital mortality, the primary endpoint of this analysis, the rate of death in patients developing CSI was 30.3%.
Successful treatment varied by hospital type
This risk was not uniform. Rather, rates of in-hospital mortality and proportion of patients treated successfully varied substantially by type of hospital. At private teaching hospitals for example, successful treatment – whether medical alone or followed by bailout surgery – was 80%. The rates fell to 40% at public teaching hospitals and then to 25% at private nonteaching hospitals.
The full-text articles included in this analysis were evaluated and selected by two reviewers working independently. A CSI diagnosis made clinically or with imaging and treatment outcomes were among criteria for the case studies to be included. Dr. Ramakumar said the study, which he claimed is the largest systematic review of CSI ever conducted, has been registered with PROSPERO, an international prospective registry of systematic reviews.
The presenting symptom was fever in 72% of cases and chest pain in the others, although there was one asymptomatic CSI reported. On angiography, 62% had a concomitant mycotic aneurysm. Intramyocardial abscess (13.9%), rupture (11.3%), and coronary fistula (7.5%) were also common findings, but no angiographic abnormalities could be identified in 53% of patients.
Following PCI, most CSI developed within 8 days (43%) or the first month (23%), but CSI was reported more than 6 months after the procedure in 19%. Complex PCI accounted for 51% of cases. Of stent types, 56% were drug eluting and 13% were bare metal.
When comparing characteristics of those who survived CSI with those who did not, most (89%) of those with a non–ST-segment elevated acute coronary syndrome ultimately survived, while survival from CSI in those with structural heart disease was only 17%.
Microbiological findings were not a criterion for study inclusion, but Staphylococcus species accounted for 65% of the infections for which positive cultures were reported. Pseudomonas accounted for 13%. Less than 4% (3.8%) tested positive for multiple pathogens. A small proportion of patients had unusual infectious organisms.
As part of this analysis, the investigators developed an artificial intelligence model to predict CSI based on patient characteristics and other variables. However, the specificity of only around 70% led Dr. Ramakumar to conclude that it does not yet have practical value.
However, he believes that better methodology to detect CSI is needed, and he proposed a diagnostic algorithm that he believes would both improve detection rates and accelerate the time to diagnosis.
Algorithm proposed for detection of CSI
In this algorithm, the first step in symptomatic patients with a positive blood culture suspected of CSI is imaging, such as transthoracic echocardiography, to identify features of infective endocarditis or endarteritis. If the imaging is positive, further imaging, such as PET, that supports the diagnosis, should be adequate to support a diagnosis and treatment.
If initial imaging is negative, alternative diagnoses should be considered, but Dr. Ramakumar advised repeat imaging after 48 hours if symptoms persist and no other causes are found.
Dr. Ramakumar acknowledged the many limitations of this analysis, including the small sample size and the challenges of assembling coherent data from case reports with variable types of information submitted during different eras of PCI evolution. However, reiterating that CSI might be frequently missed, he emphasized that this problem might be bigger than currently understood.
It is difficult to rule out any possibility that CSI is frequently missed, but Andrew Sharp, MD, PhD, a consultant interventional cardiologist at the University Hospital of Wales, Cardiff, is skeptical.
“One might think this is a potential problem, but I cannot think of one patient in whom this has occurred,” Dr. Sharp said in an interview. He is fairly confident that they are extremely rare.
“When there is infection associated with a foreign body, such as a pacemaker, they do not typically resolve by themselves,” he explained. “Often the device has to be removed. If this was true for CSI, then I think we would be aware of these complications.”
However, he praised the investigators for taking a look at CSI in a systematic approach. An invited panelist during the CRT featured research, which is where these data were presented, Dr. Sharp was more interested in understanding why they do not occur now that data are available to suggest they are rare.
“Is there something in the coronary environment, such as the consistent blood flow, that protects against infection?” he asked. CSI is a valid area of further research, according to Dr. Sharp, but he does not consider infected stents to be a common threat based on his own sizable case series.
Dr. Ramakumar and Dr. Sharp reported no potential conflicts of interest.
Infections of coronary stents appear to be uncommon, but it is not clear if they are often missed, underreported, or truly rare, according to a new analysis.
In a search of multiple databases, 79 cases of coronary stent infections (CSI) were found in 65 published reports, according to Venkatakrishnan Ramakumar, MBBS, MD, department of cardiology, All India Institute of Medical Sciences, New Delhi.
Over the period of evaluation, which had no defined starting point but stretched to November 2021, the 79 infections reported worldwide occurred when millions of percutaneous coronary intervention (PCI) procedures were performed. In the United States alone, the current estimated annual number of PCIs is 600,000, according to an article published in the Journal of the American Heart Association.
If the number of reported CSI cases represented even a modest fraction of those that occurred, the risk would still be almost negligible. Yet, Dr. Ramakumar insisted that there has been little attention paid to the potential for CSI, creating a situation in which many or almost all cases are simply being missed.
“We do not know how many infections have gone unrecognized,” Dr. Ramakumar said in presenting his results at the Cardiovascular Research Technologies conference, sponsored by MedStar Heart & Vascular Institute. And even if they are identified and promptly treated, there “is the potential for a publication bias,” he added, referring to the reluctance of investigators to submit and publishers to accept manuscripts with negative results.
Regardless of the frequency with which they occur, CSI is associated with bad outcomes, according to the data evaluated by Dr. Ramakumar. On the basis of in-hospital mortality, the primary endpoint of this analysis, the rate of death in patients developing CSI was 30.3%.
Successful treatment varied by hospital type
This risk was not uniform. Rather, rates of in-hospital mortality and proportion of patients treated successfully varied substantially by type of hospital. At private teaching hospitals for example, successful treatment – whether medical alone or followed by bailout surgery – was 80%. The rates fell to 40% at public teaching hospitals and then to 25% at private nonteaching hospitals.
The full-text articles included in this analysis were evaluated and selected by two reviewers working independently. A CSI diagnosis made clinically or with imaging and treatment outcomes were among criteria for the case studies to be included. Dr. Ramakumar said the study, which he claimed is the largest systematic review of CSI ever conducted, has been registered with PROSPERO, an international prospective registry of systematic reviews.
The presenting symptom was fever in 72% of cases and chest pain in the others, although there was one asymptomatic CSI reported. On angiography, 62% had a concomitant mycotic aneurysm. Intramyocardial abscess (13.9%), rupture (11.3%), and coronary fistula (7.5%) were also common findings, but no angiographic abnormalities could be identified in 53% of patients.
Following PCI, most CSI developed within 8 days (43%) or the first month (23%), but CSI was reported more than 6 months after the procedure in 19%. Complex PCI accounted for 51% of cases. Of stent types, 56% were drug eluting and 13% were bare metal.
When comparing characteristics of those who survived CSI with those who did not, most (89%) of those with a non–ST-segment elevated acute coronary syndrome ultimately survived, while survival from CSI in those with structural heart disease was only 17%.
Microbiological findings were not a criterion for study inclusion, but Staphylococcus species accounted for 65% of the infections for which positive cultures were reported. Pseudomonas accounted for 13%. Less than 4% (3.8%) tested positive for multiple pathogens. A small proportion of patients had unusual infectious organisms.
As part of this analysis, the investigators developed an artificial intelligence model to predict CSI based on patient characteristics and other variables. However, the specificity of only around 70% led Dr. Ramakumar to conclude that it does not yet have practical value.
However, he believes that better methodology to detect CSI is needed, and he proposed a diagnostic algorithm that he believes would both improve detection rates and accelerate the time to diagnosis.
Algorithm proposed for detection of CSI
In this algorithm, the first step in symptomatic patients with a positive blood culture suspected of CSI is imaging, such as transthoracic echocardiography, to identify features of infective endocarditis or endarteritis. If the imaging is positive, further imaging, such as PET, that supports the diagnosis, should be adequate to support a diagnosis and treatment.
If initial imaging is negative, alternative diagnoses should be considered, but Dr. Ramakumar advised repeat imaging after 48 hours if symptoms persist and no other causes are found.
Dr. Ramakumar acknowledged the many limitations of this analysis, including the small sample size and the challenges of assembling coherent data from case reports with variable types of information submitted during different eras of PCI evolution. However, reiterating that CSI might be frequently missed, he emphasized that this problem might be bigger than currently understood.
It is difficult to rule out any possibility that CSI is frequently missed, but Andrew Sharp, MD, PhD, a consultant interventional cardiologist at the University Hospital of Wales, Cardiff, is skeptical.
“One might think this is a potential problem, but I cannot think of one patient in whom this has occurred,” Dr. Sharp said in an interview. He is fairly confident that they are extremely rare.
“When there is infection associated with a foreign body, such as a pacemaker, they do not typically resolve by themselves,” he explained. “Often the device has to be removed. If this was true for CSI, then I think we would be aware of these complications.”
However, he praised the investigators for taking a look at CSI in a systematic approach. An invited panelist during the CRT featured research, which is where these data were presented, Dr. Sharp was more interested in understanding why they do not occur now that data are available to suggest they are rare.
“Is there something in the coronary environment, such as the consistent blood flow, that protects against infection?” he asked. CSI is a valid area of further research, according to Dr. Sharp, but he does not consider infected stents to be a common threat based on his own sizable case series.
Dr. Ramakumar and Dr. Sharp reported no potential conflicts of interest.
FROM CRT 2023
Empathy meltdown? Why burnout busts your empathy levels
Compassion is borne out of a sense of empathy – the ability to understand and share the feelings of others. Studies on empathy show it to be crucial to quality health care and not just for patients.
In one study on empathy ratings among doctors, 87% of the public believe that compassion, or a clear and obvious desire to relieve suffering, is the most critical factor when choosing a doctor. In fact, it eclipses travel time, wait time, and cost on the list of sought-after physician features.
Wendie Trubow, MD, an ob.gyn. in Newton, Mass., with over 25 years of experience in the medical field, says empathy has absolutely helped her be a better physician.
“Patients consistently mention how grateful they are that someone has listened to them and validated them,” she says. “When patients feel heard and validated, they are more likely to communicate openly, and this raises the potential of being able to create treatment plans that they will actually participate in. Ultimately, it enriches patient care.”
Mohammadreza Hojat, PhD, research professor of psychiatry and human behavior at the Asano-Gonnella Center for Research in Medical Education and Health Care at Thomas Jefferson University in Philadelphia, says that empirical research he and colleagues have done on empathy in health profession education and patient care over the past 20 years shows that empathic engagement in patient care is reciprocally beneficial for both clinicians and patients.
For example, Dr. Hojat notes that in one study, diabetic patients treated by empathic physicians (measured by the Jefferson Scale of Empathy) had more control over their disease when measured with laboratory test results such as hemoglobin A1c and LDL-C. In another, patients with diabetes treated by more empathic physicians had significantly lower rates of acute metabolic complications that required hospitalization.
For physicians, empathic relationships with your patients lead to fewer disputes, higher reimbursements, greater patient satisfaction, fewer malpractice lawsuits, and a more rewarding experience treating patients.
Different types of empathy
The importance of empathy in doctoring is evident, but Dr. Hojat says it’s crucial to differentiate between clinical empathy and emotional empathy. One can enhance care, while the other, when overused, may lead to physician burnout.
In fact, he says, clinical empathy and emotional empathy have different consequences in a medical setting.
“The relationship between clinical empathy and clinical outcomes is linear, meaning that more empathic engagement leads to more positive clinical outcomes,” says Dr. Hojat. “However, the relationship between emotional empathy and clinical outcomes is curvilinear, or an inverted U shape, similar to the association between anxiety and performance, meaning that limited emotional empathy or limited sympathetic engagement could be helpful, but its overabundance can hamper clinical relationships and objective clinical decision-making.”
The takeaway is that when physicians don’t regulate their emotional empathy, it becomes an obstacle to clinical empathy, ultimately detrimental to health care outcomes.
When burnout hinders empathy
Of course, the reverse is also true – burnout can make it harder for physicians to muster up empathy of any kind toward their patients. At least 53% of physicians show one or more symptoms of burnout, such as exhaustion, questioning the point of the work, cynicism, sarcasm, and the need to “vent” about patients or the job, according to Medscape’s ‘I Cry but No One Cares’: Physician Burnout & Depression Report 2023.
Venting about patients can also be called “compassion fatigue,” which is a sign that your ability to empathize with patients is compromised. You can still practice medicine, but you’re not operating anywhere close to your optimum abilities.
“Generally, physicians who are burned out struggle with empathy since it’s exactly what they’re missing for themselves, and [they] often find it difficult to generate,” says Dr. Trubow.
How to manage burnout and boost your empathy
Burnout can happen for various reasons – pressure to cycle through scores of patients, too many bureaucratic tasks, less autonomy, frustration with electronic health record requirements, and too many work hours, according to the Medscape report.
A report in Family Practice Management finds there are two main goals for physicians to tackle when trying to reduce burnout symptoms: Lower your stress levels and improve your ability to recharge your energy accounts.
“For physicians experiencing burnout [and thus, a lack of empathy], the best approach to this situation is to first take a break and evaluate whether there are any structures to put in place to improve the situation; this can often improve a provider’s empathy,” says Dr. Trubow.
For example, physicians can look at ways to alleviate burnout by investing in leadership development, finding flexible work arrangements, reducing technological burdens, and limiting nonclinical activities.
Other strategies that can build up your reserves include connecting with colleagues, gaining a greater sense of control over your work, and having opportunities to grow and excel in your field. This requires not only a personal approach by physicians, but a buy-in at an institutional level as well.
In Medscape’s report, where 65% of physicians say burnout affects their relationships, physicians’ coping methods include exercise, time with family and friends, time alone, sleep, music, and meditation.
“Clinical empathy must be placed in the realm of ‘evidence-based’ medicine,” says Dr. Hojat. “Given our research findings that clinical empathy tends to erode as students progress through medical school, it is important that assessment and enhancement of clinical empathy be integrated into formal educational curriculum of medical schools and postgraduate training programs for professional development of physicians–in-training and –in-practice.”
“Burnout also leads to a large swath of physicians who aren’t as empathetic toward their patients as they could be.”
–Danielle Ofri, “What Doctors Feel: How Emotions Affect the Practice of Medicine”.
A version of this article first appeared on Medscape.com.
Compassion is borne out of a sense of empathy – the ability to understand and share the feelings of others. Studies on empathy show it to be crucial to quality health care and not just for patients.
In one study on empathy ratings among doctors, 87% of the public believe that compassion, or a clear and obvious desire to relieve suffering, is the most critical factor when choosing a doctor. In fact, it eclipses travel time, wait time, and cost on the list of sought-after physician features.
Wendie Trubow, MD, an ob.gyn. in Newton, Mass., with over 25 years of experience in the medical field, says empathy has absolutely helped her be a better physician.
“Patients consistently mention how grateful they are that someone has listened to them and validated them,” she says. “When patients feel heard and validated, they are more likely to communicate openly, and this raises the potential of being able to create treatment plans that they will actually participate in. Ultimately, it enriches patient care.”
Mohammadreza Hojat, PhD, research professor of psychiatry and human behavior at the Asano-Gonnella Center for Research in Medical Education and Health Care at Thomas Jefferson University in Philadelphia, says that empirical research he and colleagues have done on empathy in health profession education and patient care over the past 20 years shows that empathic engagement in patient care is reciprocally beneficial for both clinicians and patients.
For example, Dr. Hojat notes that in one study, diabetic patients treated by empathic physicians (measured by the Jefferson Scale of Empathy) had more control over their disease when measured with laboratory test results such as hemoglobin A1c and LDL-C. In another, patients with diabetes treated by more empathic physicians had significantly lower rates of acute metabolic complications that required hospitalization.
For physicians, empathic relationships with your patients lead to fewer disputes, higher reimbursements, greater patient satisfaction, fewer malpractice lawsuits, and a more rewarding experience treating patients.
Different types of empathy
The importance of empathy in doctoring is evident, but Dr. Hojat says it’s crucial to differentiate between clinical empathy and emotional empathy. One can enhance care, while the other, when overused, may lead to physician burnout.
In fact, he says, clinical empathy and emotional empathy have different consequences in a medical setting.
“The relationship between clinical empathy and clinical outcomes is linear, meaning that more empathic engagement leads to more positive clinical outcomes,” says Dr. Hojat. “However, the relationship between emotional empathy and clinical outcomes is curvilinear, or an inverted U shape, similar to the association between anxiety and performance, meaning that limited emotional empathy or limited sympathetic engagement could be helpful, but its overabundance can hamper clinical relationships and objective clinical decision-making.”
The takeaway is that when physicians don’t regulate their emotional empathy, it becomes an obstacle to clinical empathy, ultimately detrimental to health care outcomes.
When burnout hinders empathy
Of course, the reverse is also true – burnout can make it harder for physicians to muster up empathy of any kind toward their patients. At least 53% of physicians show one or more symptoms of burnout, such as exhaustion, questioning the point of the work, cynicism, sarcasm, and the need to “vent” about patients or the job, according to Medscape’s ‘I Cry but No One Cares’: Physician Burnout & Depression Report 2023.
Venting about patients can also be called “compassion fatigue,” which is a sign that your ability to empathize with patients is compromised. You can still practice medicine, but you’re not operating anywhere close to your optimum abilities.
“Generally, physicians who are burned out struggle with empathy since it’s exactly what they’re missing for themselves, and [they] often find it difficult to generate,” says Dr. Trubow.
How to manage burnout and boost your empathy
Burnout can happen for various reasons – pressure to cycle through scores of patients, too many bureaucratic tasks, less autonomy, frustration with electronic health record requirements, and too many work hours, according to the Medscape report.
A report in Family Practice Management finds there are two main goals for physicians to tackle when trying to reduce burnout symptoms: Lower your stress levels and improve your ability to recharge your energy accounts.
“For physicians experiencing burnout [and thus, a lack of empathy], the best approach to this situation is to first take a break and evaluate whether there are any structures to put in place to improve the situation; this can often improve a provider’s empathy,” says Dr. Trubow.
For example, physicians can look at ways to alleviate burnout by investing in leadership development, finding flexible work arrangements, reducing technological burdens, and limiting nonclinical activities.
Other strategies that can build up your reserves include connecting with colleagues, gaining a greater sense of control over your work, and having opportunities to grow and excel in your field. This requires not only a personal approach by physicians, but a buy-in at an institutional level as well.
In Medscape’s report, where 65% of physicians say burnout affects their relationships, physicians’ coping methods include exercise, time with family and friends, time alone, sleep, music, and meditation.
“Clinical empathy must be placed in the realm of ‘evidence-based’ medicine,” says Dr. Hojat. “Given our research findings that clinical empathy tends to erode as students progress through medical school, it is important that assessment and enhancement of clinical empathy be integrated into formal educational curriculum of medical schools and postgraduate training programs for professional development of physicians–in-training and –in-practice.”
“Burnout also leads to a large swath of physicians who aren’t as empathetic toward their patients as they could be.”
–Danielle Ofri, “What Doctors Feel: How Emotions Affect the Practice of Medicine”.
A version of this article first appeared on Medscape.com.
Compassion is borne out of a sense of empathy – the ability to understand and share the feelings of others. Studies on empathy show it to be crucial to quality health care and not just for patients.
In one study on empathy ratings among doctors, 87% of the public believe that compassion, or a clear and obvious desire to relieve suffering, is the most critical factor when choosing a doctor. In fact, it eclipses travel time, wait time, and cost on the list of sought-after physician features.
Wendie Trubow, MD, an ob.gyn. in Newton, Mass., with over 25 years of experience in the medical field, says empathy has absolutely helped her be a better physician.
“Patients consistently mention how grateful they are that someone has listened to them and validated them,” she says. “When patients feel heard and validated, they are more likely to communicate openly, and this raises the potential of being able to create treatment plans that they will actually participate in. Ultimately, it enriches patient care.”
Mohammadreza Hojat, PhD, research professor of psychiatry and human behavior at the Asano-Gonnella Center for Research in Medical Education and Health Care at Thomas Jefferson University in Philadelphia, says that empirical research he and colleagues have done on empathy in health profession education and patient care over the past 20 years shows that empathic engagement in patient care is reciprocally beneficial for both clinicians and patients.
For example, Dr. Hojat notes that in one study, diabetic patients treated by empathic physicians (measured by the Jefferson Scale of Empathy) had more control over their disease when measured with laboratory test results such as hemoglobin A1c and LDL-C. In another, patients with diabetes treated by more empathic physicians had significantly lower rates of acute metabolic complications that required hospitalization.
For physicians, empathic relationships with your patients lead to fewer disputes, higher reimbursements, greater patient satisfaction, fewer malpractice lawsuits, and a more rewarding experience treating patients.
Different types of empathy
The importance of empathy in doctoring is evident, but Dr. Hojat says it’s crucial to differentiate between clinical empathy and emotional empathy. One can enhance care, while the other, when overused, may lead to physician burnout.
In fact, he says, clinical empathy and emotional empathy have different consequences in a medical setting.
“The relationship between clinical empathy and clinical outcomes is linear, meaning that more empathic engagement leads to more positive clinical outcomes,” says Dr. Hojat. “However, the relationship between emotional empathy and clinical outcomes is curvilinear, or an inverted U shape, similar to the association between anxiety and performance, meaning that limited emotional empathy or limited sympathetic engagement could be helpful, but its overabundance can hamper clinical relationships and objective clinical decision-making.”
The takeaway is that when physicians don’t regulate their emotional empathy, it becomes an obstacle to clinical empathy, ultimately detrimental to health care outcomes.
When burnout hinders empathy
Of course, the reverse is also true – burnout can make it harder for physicians to muster up empathy of any kind toward their patients. At least 53% of physicians show one or more symptoms of burnout, such as exhaustion, questioning the point of the work, cynicism, sarcasm, and the need to “vent” about patients or the job, according to Medscape’s ‘I Cry but No One Cares’: Physician Burnout & Depression Report 2023.
Venting about patients can also be called “compassion fatigue,” which is a sign that your ability to empathize with patients is compromised. You can still practice medicine, but you’re not operating anywhere close to your optimum abilities.
“Generally, physicians who are burned out struggle with empathy since it’s exactly what they’re missing for themselves, and [they] often find it difficult to generate,” says Dr. Trubow.
How to manage burnout and boost your empathy
Burnout can happen for various reasons – pressure to cycle through scores of patients, too many bureaucratic tasks, less autonomy, frustration with electronic health record requirements, and too many work hours, according to the Medscape report.
A report in Family Practice Management finds there are two main goals for physicians to tackle when trying to reduce burnout symptoms: Lower your stress levels and improve your ability to recharge your energy accounts.
“For physicians experiencing burnout [and thus, a lack of empathy], the best approach to this situation is to first take a break and evaluate whether there are any structures to put in place to improve the situation; this can often improve a provider’s empathy,” says Dr. Trubow.
For example, physicians can look at ways to alleviate burnout by investing in leadership development, finding flexible work arrangements, reducing technological burdens, and limiting nonclinical activities.
Other strategies that can build up your reserves include connecting with colleagues, gaining a greater sense of control over your work, and having opportunities to grow and excel in your field. This requires not only a personal approach by physicians, but a buy-in at an institutional level as well.
In Medscape’s report, where 65% of physicians say burnout affects their relationships, physicians’ coping methods include exercise, time with family and friends, time alone, sleep, music, and meditation.
“Clinical empathy must be placed in the realm of ‘evidence-based’ medicine,” says Dr. Hojat. “Given our research findings that clinical empathy tends to erode as students progress through medical school, it is important that assessment and enhancement of clinical empathy be integrated into formal educational curriculum of medical schools and postgraduate training programs for professional development of physicians–in-training and –in-practice.”
“Burnout also leads to a large swath of physicians who aren’t as empathetic toward their patients as they could be.”
–Danielle Ofri, “What Doctors Feel: How Emotions Affect the Practice of Medicine”.
A version of this article first appeared on Medscape.com.
Which nonopioid meds are best for easing acute low back pain?
based on data from more than 3,000 individuals.
Acute low back pain (LBP) remains a common cause of disability worldwide, with a high socioeconomic burden, write Alice Baroncini, MD, of RWTH University Hospital, Aachen, Germany, and colleagues.
In an analysis published in the Journal of Orthopaedic Research, a team of investigators from Germany examined which nonopioid drugs are best for treating LBP.
The researchers identified 18 studies totaling 3,478 patients with acute low back pain of less than 12 weeks’ duration. They selected studies that only investigated the lumbar spine, and studies involving opioids were excluded. The mean age of the patients across all the studies was 42.5 years, and 54% were women. The mean duration of symptoms before treatment was 15.1 days.
Overall, muscle relaxants and NSAIDs demonstrated effectiveness in reducing pain and disability for acute LBP patients after about 1 week of use.
In addition, studies of a combination of NSAIDs and paracetamol (also known as acetaminophen) showed a greater improvement than NSAIDs alone, but paracetamol/acetaminophen alone had no significant impact on LBP.
Most patients with acute LBP experience spontaneous recovery and reduction of symptoms, thus the real impact of most medications is uncertain, the researchers write in their discussion. The lack of a placebo effect in the selected studies reinforces the hypothesis that nonopioid medications improve LBP symptoms, they say.
However, “while this work only focuses on the pharmacological management of acute LBP, it is fundamental to highlight that the use of drugs should always be a second-line strategy once other nonpharmacological, noninvasive therapies have proved to be insufficient,” the researchers write.
The study findings were limited by several factors, including the inability to distinguish among different NSAID classes, the inability to conduct a subanalysis of the best drug or treatment protocol for a given drug class, and the short follow-up period for the included studies, the researchers note.
More research is needed to address the effects of different drugs on LBP recurrence, they add.
However, the results support the current opinion that NSAIDs can be effectively used for LBP, strengthened by the large number of studies and relatively low risk of bias, the researchers conclude.
The current study addresses a common cause of morbidity among patients and highlights alternatives to opioid analgesics for its management, Suman Pal, MBBS, a specialist in hospital medicine at the University of New Mexico, Albuquerque, said in an interview.
Dr. Pal said he was not surprised by the results. “The findings of the study mirror prior studies,” he said. “However, the lack of benefit of paracetamol alone needs to be highlighted as important to clinical practice.”
A key message for clinicians is the role of NSAIDs in LBP, Dr. Pal said. “NSAIDs, either alone or in combination with paracetamol or myorelaxants, can be effective therapy for select patients with acute LBP.” However, “further research is needed to better identify which patients would derive most benefit from this approach,” he said.
Other research needs include more evidence to better understand the appropriate duration of therapy, given the potential for adverse effects with chronic NSAID use, Dr. Pal said.
The study received no outside funding. The researchers and Dr. Pal have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
based on data from more than 3,000 individuals.
Acute low back pain (LBP) remains a common cause of disability worldwide, with a high socioeconomic burden, write Alice Baroncini, MD, of RWTH University Hospital, Aachen, Germany, and colleagues.
In an analysis published in the Journal of Orthopaedic Research, a team of investigators from Germany examined which nonopioid drugs are best for treating LBP.
The researchers identified 18 studies totaling 3,478 patients with acute low back pain of less than 12 weeks’ duration. They selected studies that only investigated the lumbar spine, and studies involving opioids were excluded. The mean age of the patients across all the studies was 42.5 years, and 54% were women. The mean duration of symptoms before treatment was 15.1 days.
Overall, muscle relaxants and NSAIDs demonstrated effectiveness in reducing pain and disability for acute LBP patients after about 1 week of use.
In addition, studies of a combination of NSAIDs and paracetamol (also known as acetaminophen) showed a greater improvement than NSAIDs alone, but paracetamol/acetaminophen alone had no significant impact on LBP.
Most patients with acute LBP experience spontaneous recovery and reduction of symptoms, thus the real impact of most medications is uncertain, the researchers write in their discussion. The lack of a placebo effect in the selected studies reinforces the hypothesis that nonopioid medications improve LBP symptoms, they say.
However, “while this work only focuses on the pharmacological management of acute LBP, it is fundamental to highlight that the use of drugs should always be a second-line strategy once other nonpharmacological, noninvasive therapies have proved to be insufficient,” the researchers write.
The study findings were limited by several factors, including the inability to distinguish among different NSAID classes, the inability to conduct a subanalysis of the best drug or treatment protocol for a given drug class, and the short follow-up period for the included studies, the researchers note.
More research is needed to address the effects of different drugs on LBP recurrence, they add.
However, the results support the current opinion that NSAIDs can be effectively used for LBP, strengthened by the large number of studies and relatively low risk of bias, the researchers conclude.
The current study addresses a common cause of morbidity among patients and highlights alternatives to opioid analgesics for its management, Suman Pal, MBBS, a specialist in hospital medicine at the University of New Mexico, Albuquerque, said in an interview.
Dr. Pal said he was not surprised by the results. “The findings of the study mirror prior studies,” he said. “However, the lack of benefit of paracetamol alone needs to be highlighted as important to clinical practice.”
A key message for clinicians is the role of NSAIDs in LBP, Dr. Pal said. “NSAIDs, either alone or in combination with paracetamol or myorelaxants, can be effective therapy for select patients with acute LBP.” However, “further research is needed to better identify which patients would derive most benefit from this approach,” he said.
Other research needs include more evidence to better understand the appropriate duration of therapy, given the potential for adverse effects with chronic NSAID use, Dr. Pal said.
The study received no outside funding. The researchers and Dr. Pal have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
based on data from more than 3,000 individuals.
Acute low back pain (LBP) remains a common cause of disability worldwide, with a high socioeconomic burden, write Alice Baroncini, MD, of RWTH University Hospital, Aachen, Germany, and colleagues.
In an analysis published in the Journal of Orthopaedic Research, a team of investigators from Germany examined which nonopioid drugs are best for treating LBP.
The researchers identified 18 studies totaling 3,478 patients with acute low back pain of less than 12 weeks’ duration. They selected studies that only investigated the lumbar spine, and studies involving opioids were excluded. The mean age of the patients across all the studies was 42.5 years, and 54% were women. The mean duration of symptoms before treatment was 15.1 days.
Overall, muscle relaxants and NSAIDs demonstrated effectiveness in reducing pain and disability for acute LBP patients after about 1 week of use.
In addition, studies of a combination of NSAIDs and paracetamol (also known as acetaminophen) showed a greater improvement than NSAIDs alone, but paracetamol/acetaminophen alone had no significant impact on LBP.
Most patients with acute LBP experience spontaneous recovery and reduction of symptoms, thus the real impact of most medications is uncertain, the researchers write in their discussion. The lack of a placebo effect in the selected studies reinforces the hypothesis that nonopioid medications improve LBP symptoms, they say.
However, “while this work only focuses on the pharmacological management of acute LBP, it is fundamental to highlight that the use of drugs should always be a second-line strategy once other nonpharmacological, noninvasive therapies have proved to be insufficient,” the researchers write.
The study findings were limited by several factors, including the inability to distinguish among different NSAID classes, the inability to conduct a subanalysis of the best drug or treatment protocol for a given drug class, and the short follow-up period for the included studies, the researchers note.
More research is needed to address the effects of different drugs on LBP recurrence, they add.
However, the results support the current opinion that NSAIDs can be effectively used for LBP, strengthened by the large number of studies and relatively low risk of bias, the researchers conclude.
The current study addresses a common cause of morbidity among patients and highlights alternatives to opioid analgesics for its management, Suman Pal, MBBS, a specialist in hospital medicine at the University of New Mexico, Albuquerque, said in an interview.
Dr. Pal said he was not surprised by the results. “The findings of the study mirror prior studies,” he said. “However, the lack of benefit of paracetamol alone needs to be highlighted as important to clinical practice.”
A key message for clinicians is the role of NSAIDs in LBP, Dr. Pal said. “NSAIDs, either alone or in combination with paracetamol or myorelaxants, can be effective therapy for select patients with acute LBP.” However, “further research is needed to better identify which patients would derive most benefit from this approach,” he said.
Other research needs include more evidence to better understand the appropriate duration of therapy, given the potential for adverse effects with chronic NSAID use, Dr. Pal said.
The study received no outside funding. The researchers and Dr. Pal have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF ORTHOPAEDIC RESEARCH