User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
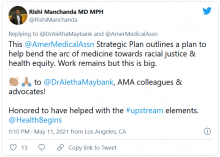
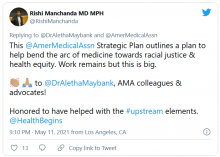
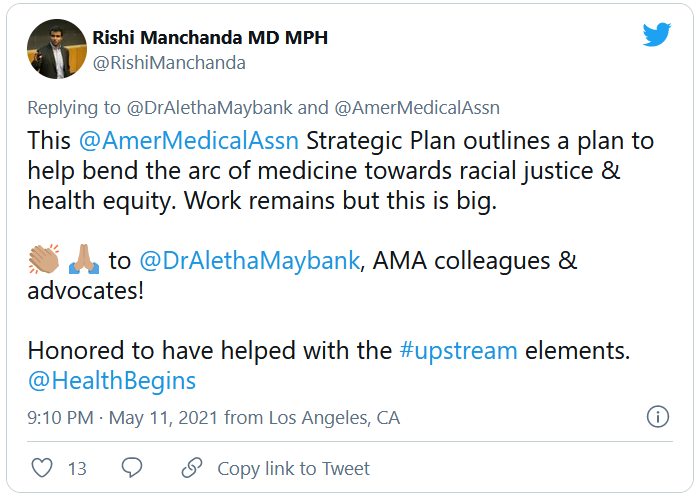
AMA announces major commitment to health equity
The 82-page report, which was created by the association’s Center for Health Equity, argues for both internal changes at the AMA and changes in how the association addresses race-based inequities in general.
The report was released just 2 months after this news organization reported that a podcast hosted by AMA’s top journal was lambasted as racist and out of touch. In the podcast – entitled “Stuctural Racism for Doctors – What Is It?” – one JAMA editor argued that structural racism doesn’t exist. He eventually resigned and the journal’s top editor was placed on administration leave.
The new AMA report’s strategic framework “is driven by the immense need for equity-centered solutions to confront harms produced by systemic racism and other forms of oppression for Black, Latinx, Indigenous, Asian, and other people of color, as well as people who identify as LGBTQ+ and people with disabilities,” the AMA said in a news release. “Its urgency is underscored by ongoing circumstances including inequities exacerbated by the COVID-19 pandemic, ongoing police brutality, and hate crimes targeting Asian, Black, and Brown communities.”
The plan includes five main approaches to addressing inequities in health care and the AMA:
- Implement antiracist equity strategies through AMA practices, programming, policies, and culture.
- Build alliances with marginalized doctors and other stakeholders to elevate the experiences and ideas of historically marginalized and minority health care leaders.
- Strengthen, empower, and equip doctors with the knowledge and tools to dismantle structural and social health inequities.
- Ensure equitable opportunities in innovation.
- Foster truth, racial healing, reconciliation, and transformation for AMA’s past by accounting for how policies and processes excluded, discriminated, and harmed communities.
As the report acknowledges, the AMA has a long history of exclusion of and discrimination against Black physicians, for which the association publicly apologized in 2008. Within the past year, the AMA has reaffirmed its commitment to addressing this legacy and to be proactive on health equity.
Among other things, the association has described racism as a public health crisis, stated that race has nothing to do with biology, said police brutality is a product of structural racism, and called on the federal government to collect and release COVID-19 race/ethnicity data. It also removed the name of AMA founder Nathan Davis, MD, from an annual award and display because of his contribution to explicit racist practices.
Equity-centered solutions
The AMA launched its Center for Health Equity in 2019 with a mandate “to embed health equity across the organization.” Aletha Maybank, MD, was named the AMA’s chief health equity officer to lead the center.
In the report that Dr. Maybank helped write, the AMA discusses the consequences of individual and systemic injustice toward minorities. Among these consequences, the report said, is “segregated and inequitable health care systems.”
The “equity-centered solutions” listed in the report include:
- End segregated health care.
- Establish national health care equity and racial justice standards.
- End the use of race-based clinical decision models.
- Eliminate all forms of discrimination, exclusion and oppression in medical and physician education, training, hiring, and promotion.
- Prevent exclusion of and ensure equal representation of Black, Indigenous and Latinx people in medical school admissions as well as medical school and hospital leadership ranks.
- Ensure equity in innovation, including design, development, implementation along with support for equitable innovation opportunities and entrepreneurship.
- Solidify connections and coordination between health care and public health.
- Acknowledge and repair past harms committed by institutions.
Changing medical education
In an exclusive interview, Gerald E. Harmon, MD, president-elect of the AMA, singled out medical education as an area that is ripe for change. “One of the most threatened phenotypes on the planet is the Black male physician,” he said. “Their numbers among medical school applicants continue to drop. We have increasing numbers of women in medical schools – over 50% of trainees are women – and more Black women are entering medical school, but Black men in medical school are an endangered species.
“We’re trying to get the physician workforce to look like the patient workforce.”
Dr. Harmon cited the “pipeline program” at the Morehouse School of Medicine in Atlanta and the AMA’s “doctors back to school” program as examples of efforts to attract minority high school students to health care careers. Much more needs to be done, he added. “We have to put equity and representation into our medical workforce so we can provide better high quality, more reliable care for underrepresented patients.”
Putting the AMA’s house in order
In its report, the AMA also makes recommendations about how it can improve equity within its own organization. Over the next 3 years, among other things, the association plans to improve the diversity of leadership at the AMA and its journal, JAMA; train all staff on equity requirements; and develop a plan to recruit more racial and ethnic minorities, LGBTQ+ people, and disabled people.
Dr. Maybank, the AMA’s chief health equity officer, said in an interview that she wouldn’t describe these efforts as affirmative action. “This is beyond affirmative action. It’s about intentional activity and action to ensure equity and justice within the AMA.”
The AMA has to thoroughly examine its own processes and determine “how inequity shows up on a day-to-day basis,” she said. “Whether it’s through hiring, innovation, publishing or communications, everybody needs to know how inequity shows up and how their own mental models can exacerbate inequities. People need tools to challenge themselves and ask themselves critical questions about racism in their processes and what they can do to mitigate those.”
A version of this article first appeared on WebMD.com.
The 82-page report, which was created by the association’s Center for Health Equity, argues for both internal changes at the AMA and changes in how the association addresses race-based inequities in general.
The report was released just 2 months after this news organization reported that a podcast hosted by AMA’s top journal was lambasted as racist and out of touch. In the podcast – entitled “Stuctural Racism for Doctors – What Is It?” – one JAMA editor argued that structural racism doesn’t exist. He eventually resigned and the journal’s top editor was placed on administration leave.
The new AMA report’s strategic framework “is driven by the immense need for equity-centered solutions to confront harms produced by systemic racism and other forms of oppression for Black, Latinx, Indigenous, Asian, and other people of color, as well as people who identify as LGBTQ+ and people with disabilities,” the AMA said in a news release. “Its urgency is underscored by ongoing circumstances including inequities exacerbated by the COVID-19 pandemic, ongoing police brutality, and hate crimes targeting Asian, Black, and Brown communities.”
The plan includes five main approaches to addressing inequities in health care and the AMA:
- Implement antiracist equity strategies through AMA practices, programming, policies, and culture.
- Build alliances with marginalized doctors and other stakeholders to elevate the experiences and ideas of historically marginalized and minority health care leaders.
- Strengthen, empower, and equip doctors with the knowledge and tools to dismantle structural and social health inequities.
- Ensure equitable opportunities in innovation.
- Foster truth, racial healing, reconciliation, and transformation for AMA’s past by accounting for how policies and processes excluded, discriminated, and harmed communities.
As the report acknowledges, the AMA has a long history of exclusion of and discrimination against Black physicians, for which the association publicly apologized in 2008. Within the past year, the AMA has reaffirmed its commitment to addressing this legacy and to be proactive on health equity.
Among other things, the association has described racism as a public health crisis, stated that race has nothing to do with biology, said police brutality is a product of structural racism, and called on the federal government to collect and release COVID-19 race/ethnicity data. It also removed the name of AMA founder Nathan Davis, MD, from an annual award and display because of his contribution to explicit racist practices.
Equity-centered solutions
The AMA launched its Center for Health Equity in 2019 with a mandate “to embed health equity across the organization.” Aletha Maybank, MD, was named the AMA’s chief health equity officer to lead the center.
In the report that Dr. Maybank helped write, the AMA discusses the consequences of individual and systemic injustice toward minorities. Among these consequences, the report said, is “segregated and inequitable health care systems.”
The “equity-centered solutions” listed in the report include:
- End segregated health care.
- Establish national health care equity and racial justice standards.
- End the use of race-based clinical decision models.
- Eliminate all forms of discrimination, exclusion and oppression in medical and physician education, training, hiring, and promotion.
- Prevent exclusion of and ensure equal representation of Black, Indigenous and Latinx people in medical school admissions as well as medical school and hospital leadership ranks.
- Ensure equity in innovation, including design, development, implementation along with support for equitable innovation opportunities and entrepreneurship.
- Solidify connections and coordination between health care and public health.
- Acknowledge and repair past harms committed by institutions.
Changing medical education
In an exclusive interview, Gerald E. Harmon, MD, president-elect of the AMA, singled out medical education as an area that is ripe for change. “One of the most threatened phenotypes on the planet is the Black male physician,” he said. “Their numbers among medical school applicants continue to drop. We have increasing numbers of women in medical schools – over 50% of trainees are women – and more Black women are entering medical school, but Black men in medical school are an endangered species.
“We’re trying to get the physician workforce to look like the patient workforce.”
Dr. Harmon cited the “pipeline program” at the Morehouse School of Medicine in Atlanta and the AMA’s “doctors back to school” program as examples of efforts to attract minority high school students to health care careers. Much more needs to be done, he added. “We have to put equity and representation into our medical workforce so we can provide better high quality, more reliable care for underrepresented patients.”
Putting the AMA’s house in order
In its report, the AMA also makes recommendations about how it can improve equity within its own organization. Over the next 3 years, among other things, the association plans to improve the diversity of leadership at the AMA and its journal, JAMA; train all staff on equity requirements; and develop a plan to recruit more racial and ethnic minorities, LGBTQ+ people, and disabled people.
Dr. Maybank, the AMA’s chief health equity officer, said in an interview that she wouldn’t describe these efforts as affirmative action. “This is beyond affirmative action. It’s about intentional activity and action to ensure equity and justice within the AMA.”
The AMA has to thoroughly examine its own processes and determine “how inequity shows up on a day-to-day basis,” she said. “Whether it’s through hiring, innovation, publishing or communications, everybody needs to know how inequity shows up and how their own mental models can exacerbate inequities. People need tools to challenge themselves and ask themselves critical questions about racism in their processes and what they can do to mitigate those.”
A version of this article first appeared on WebMD.com.
The 82-page report, which was created by the association’s Center for Health Equity, argues for both internal changes at the AMA and changes in how the association addresses race-based inequities in general.
The report was released just 2 months after this news organization reported that a podcast hosted by AMA’s top journal was lambasted as racist and out of touch. In the podcast – entitled “Stuctural Racism for Doctors – What Is It?” – one JAMA editor argued that structural racism doesn’t exist. He eventually resigned and the journal’s top editor was placed on administration leave.
The new AMA report’s strategic framework “is driven by the immense need for equity-centered solutions to confront harms produced by systemic racism and other forms of oppression for Black, Latinx, Indigenous, Asian, and other people of color, as well as people who identify as LGBTQ+ and people with disabilities,” the AMA said in a news release. “Its urgency is underscored by ongoing circumstances including inequities exacerbated by the COVID-19 pandemic, ongoing police brutality, and hate crimes targeting Asian, Black, and Brown communities.”
The plan includes five main approaches to addressing inequities in health care and the AMA:
- Implement antiracist equity strategies through AMA practices, programming, policies, and culture.
- Build alliances with marginalized doctors and other stakeholders to elevate the experiences and ideas of historically marginalized and minority health care leaders.
- Strengthen, empower, and equip doctors with the knowledge and tools to dismantle structural and social health inequities.
- Ensure equitable opportunities in innovation.
- Foster truth, racial healing, reconciliation, and transformation for AMA’s past by accounting for how policies and processes excluded, discriminated, and harmed communities.
As the report acknowledges, the AMA has a long history of exclusion of and discrimination against Black physicians, for which the association publicly apologized in 2008. Within the past year, the AMA has reaffirmed its commitment to addressing this legacy and to be proactive on health equity.
Among other things, the association has described racism as a public health crisis, stated that race has nothing to do with biology, said police brutality is a product of structural racism, and called on the federal government to collect and release COVID-19 race/ethnicity data. It also removed the name of AMA founder Nathan Davis, MD, from an annual award and display because of his contribution to explicit racist practices.
Equity-centered solutions
The AMA launched its Center for Health Equity in 2019 with a mandate “to embed health equity across the organization.” Aletha Maybank, MD, was named the AMA’s chief health equity officer to lead the center.
In the report that Dr. Maybank helped write, the AMA discusses the consequences of individual and systemic injustice toward minorities. Among these consequences, the report said, is “segregated and inequitable health care systems.”
The “equity-centered solutions” listed in the report include:
- End segregated health care.
- Establish national health care equity and racial justice standards.
- End the use of race-based clinical decision models.
- Eliminate all forms of discrimination, exclusion and oppression in medical and physician education, training, hiring, and promotion.
- Prevent exclusion of and ensure equal representation of Black, Indigenous and Latinx people in medical school admissions as well as medical school and hospital leadership ranks.
- Ensure equity in innovation, including design, development, implementation along with support for equitable innovation opportunities and entrepreneurship.
- Solidify connections and coordination between health care and public health.
- Acknowledge and repair past harms committed by institutions.
Changing medical education
In an exclusive interview, Gerald E. Harmon, MD, president-elect of the AMA, singled out medical education as an area that is ripe for change. “One of the most threatened phenotypes on the planet is the Black male physician,” he said. “Their numbers among medical school applicants continue to drop. We have increasing numbers of women in medical schools – over 50% of trainees are women – and more Black women are entering medical school, but Black men in medical school are an endangered species.
“We’re trying to get the physician workforce to look like the patient workforce.”
Dr. Harmon cited the “pipeline program” at the Morehouse School of Medicine in Atlanta and the AMA’s “doctors back to school” program as examples of efforts to attract minority high school students to health care careers. Much more needs to be done, he added. “We have to put equity and representation into our medical workforce so we can provide better high quality, more reliable care for underrepresented patients.”
Putting the AMA’s house in order
In its report, the AMA also makes recommendations about how it can improve equity within its own organization. Over the next 3 years, among other things, the association plans to improve the diversity of leadership at the AMA and its journal, JAMA; train all staff on equity requirements; and develop a plan to recruit more racial and ethnic minorities, LGBTQ+ people, and disabled people.
Dr. Maybank, the AMA’s chief health equity officer, said in an interview that she wouldn’t describe these efforts as affirmative action. “This is beyond affirmative action. It’s about intentional activity and action to ensure equity and justice within the AMA.”
The AMA has to thoroughly examine its own processes and determine “how inequity shows up on a day-to-day basis,” she said. “Whether it’s through hiring, innovation, publishing or communications, everybody needs to know how inequity shows up and how their own mental models can exacerbate inequities. People need tools to challenge themselves and ask themselves critical questions about racism in their processes and what they can do to mitigate those.”
A version of this article first appeared on WebMD.com.
Canned diabetes prevention and a haunted COVID castle
Lower blood sugar with sardines
If you’ve ever turned your nose up at someone eating sardines straight from the can, you could be the one missing out on a good way to boost your own health.
New research from Open University of Catalonia (Spain) has found that eating two cans of whole sardines a week can help prevent people from developing type 2 diabetes (T2D). Now you might be thinking: That’s a lot of fish, can’t I just take a supplement pill? Actually, no.
“Nutrients can play an essential role in the prevention and treatment of many different pathologies, but their effect is usually caused by the synergy that exists between them and the food that they are contained in,” study coauthor Diana Rizzolo, PhD, said in a written statement. See, we told you.
In a study of 152 patients with prediabetes, each participant was put on a specific diet to reduce their chances of developing T2D. Among the patients who were not given sardines each week, the proportion considered to be at the highest risk fell from 27% to 22% after 1 year, but for those who did get the sardines, the size of the high-risk group shrank from 37% to just 8%.
Suggesting sardines during checkups could make eating them more widely accepted, Dr. Rizzolo and associates said. Sardines are cheap, easy to find, and also have the benefits of other oily fish, like boosting insulin resistance and increasing good cholesterol.
So why not have a can with a couple of saltine crackers for lunch? Your blood sugar will thank you. Just please avoid indulging on a plane or in your office, where workers are slowly returning – no need to give them another excuse to avoid their cubicle.
Come for the torture, stay for the vaccine
Bran Castle. Home of Dracula and Vlad the Impaler (at least in pop culture’s eyes). A moody Gothic structure atop a hill. You can practically hear the ancient screams of thousands of tortured souls as you wander the grounds and its cursed halls. Naturally, it’s a major tourist destination.
Unfortunately for Romania, the pandemic has rather put a damper on tourism. The restrictions have done their damage, but here’s a quick LOTME theory: Perhaps people don’t want to be reminded of medieval tortures when we’ve got plenty of modern-day ones right now.
The management of Bran Castle has developed a new gimmick to drum up attendance – come to Bran Castle and get your COVID vaccine. Anyone can come and get jabbed with the Pfizer vaccine on all weekends in May, and when they do, they gain free admittance to the castle and the exhibit within, home to 52 medieval torture instruments. “The idea … was to show how people got jabbed 500-600 years ago in Europe,” the castle’s marketing director said.
While it may not be kind of the jabbing ole Vladdy got his name for – fully impaling people on hundreds of wooden stakes while you eat a nice dinner isn’t exactly smiled upon in today’s world – we’re sure he’d approve of this more limited but ultimately beneficial version. Jabbing people while helping them really is the dream.
Fuzzy little COVID detectors
Before we get started, we need a moment to get our deep, movie trailer announcer-type voice ready. Okay, here goes.
“In a world where an organism too tiny to see brings entire economies to a standstill and pits scientists against doofuses, who can humanity turn to for help?”
How about bees? That’s right, we said bees. But not just any bees. Specially trained bees. Specially trained Dutch bees. Bees trained to sniff out our greatest nemesis. No, we’re not talking about Ted Cruz anymore. Let it go, that was just a joke. We’re talking COVID.
We’ll let Wim van der Poel, professor of virology at Wageningen (the Netherlands) University, explain the process: “We collect normal honeybees from a beekeeper, and we put the bees in harnesses.” And you thought their tulips were pretty great – the Dutch are putting harnesses on bees! (Which is much better than our previous story of bees involving a Taiwanese patient.)
The researchers presented the bees with two types of samples: COVID infected and non–COVID infected. The infected samples came with a sugary water reward and the noninfected samples did not, so the bees quickly learned to tell the difference.
The bees, then, could cut the waiting time for test results down to seconds, and at a fraction of the cost, making them an option in countries without a lot of testing infrastructure, the research team suggested.
The plan is not without its flaws, of course, but we’re convinced. More than that, we are true bee-lievers.
A little slice of … well, not heaven
If you’ve been around for the last 2 decades, you’ve seen your share of Internet trends: Remember the ice bucket challenge? Tide pod eating? We know what you’re thinking: Sigh, what could they be doing now?
Well, people are eating old meat, and before you think about the expired ground beef you got on special from the grocery store yesterday, that’s not quite what we mean. We all know expiration dates are “suggestions,” like yield signs and yellow lights. People are eating rotten, decomposing, borderline moldy meat.
They claim that the meat tastes better. We’re not so sure, but don’t worry, because it gets weirder. Some folks, apparently, are getting high from eating this meat, experiencing a feeling of euphoria. Personally, we think that rotten fumes probably knocked these people out and made them hallucinate.
Singaporean dietitian Naras Lapsys says that eating rotten meat can possibly cause a person to go into another state of consciousness, but it’s not a good thing. We don’t think you have to be a dietitian to know that.
It has not been definitively proven that eating rotting meat makes you high, but it’s definitely proven that this is disgusting … and very dangerous.
Lower blood sugar with sardines
If you’ve ever turned your nose up at someone eating sardines straight from the can, you could be the one missing out on a good way to boost your own health.
New research from Open University of Catalonia (Spain) has found that eating two cans of whole sardines a week can help prevent people from developing type 2 diabetes (T2D). Now you might be thinking: That’s a lot of fish, can’t I just take a supplement pill? Actually, no.
“Nutrients can play an essential role in the prevention and treatment of many different pathologies, but their effect is usually caused by the synergy that exists between them and the food that they are contained in,” study coauthor Diana Rizzolo, PhD, said in a written statement. See, we told you.
In a study of 152 patients with prediabetes, each participant was put on a specific diet to reduce their chances of developing T2D. Among the patients who were not given sardines each week, the proportion considered to be at the highest risk fell from 27% to 22% after 1 year, but for those who did get the sardines, the size of the high-risk group shrank from 37% to just 8%.
Suggesting sardines during checkups could make eating them more widely accepted, Dr. Rizzolo and associates said. Sardines are cheap, easy to find, and also have the benefits of other oily fish, like boosting insulin resistance and increasing good cholesterol.
So why not have a can with a couple of saltine crackers for lunch? Your blood sugar will thank you. Just please avoid indulging on a plane or in your office, where workers are slowly returning – no need to give them another excuse to avoid their cubicle.
Come for the torture, stay for the vaccine
Bran Castle. Home of Dracula and Vlad the Impaler (at least in pop culture’s eyes). A moody Gothic structure atop a hill. You can practically hear the ancient screams of thousands of tortured souls as you wander the grounds and its cursed halls. Naturally, it’s a major tourist destination.
Unfortunately for Romania, the pandemic has rather put a damper on tourism. The restrictions have done their damage, but here’s a quick LOTME theory: Perhaps people don’t want to be reminded of medieval tortures when we’ve got plenty of modern-day ones right now.
The management of Bran Castle has developed a new gimmick to drum up attendance – come to Bran Castle and get your COVID vaccine. Anyone can come and get jabbed with the Pfizer vaccine on all weekends in May, and when they do, they gain free admittance to the castle and the exhibit within, home to 52 medieval torture instruments. “The idea … was to show how people got jabbed 500-600 years ago in Europe,” the castle’s marketing director said.
While it may not be kind of the jabbing ole Vladdy got his name for – fully impaling people on hundreds of wooden stakes while you eat a nice dinner isn’t exactly smiled upon in today’s world – we’re sure he’d approve of this more limited but ultimately beneficial version. Jabbing people while helping them really is the dream.
Fuzzy little COVID detectors
Before we get started, we need a moment to get our deep, movie trailer announcer-type voice ready. Okay, here goes.
“In a world where an organism too tiny to see brings entire economies to a standstill and pits scientists against doofuses, who can humanity turn to for help?”
How about bees? That’s right, we said bees. But not just any bees. Specially trained bees. Specially trained Dutch bees. Bees trained to sniff out our greatest nemesis. No, we’re not talking about Ted Cruz anymore. Let it go, that was just a joke. We’re talking COVID.
We’ll let Wim van der Poel, professor of virology at Wageningen (the Netherlands) University, explain the process: “We collect normal honeybees from a beekeeper, and we put the bees in harnesses.” And you thought their tulips were pretty great – the Dutch are putting harnesses on bees! (Which is much better than our previous story of bees involving a Taiwanese patient.)
The researchers presented the bees with two types of samples: COVID infected and non–COVID infected. The infected samples came with a sugary water reward and the noninfected samples did not, so the bees quickly learned to tell the difference.
The bees, then, could cut the waiting time for test results down to seconds, and at a fraction of the cost, making them an option in countries without a lot of testing infrastructure, the research team suggested.
The plan is not without its flaws, of course, but we’re convinced. More than that, we are true bee-lievers.
A little slice of … well, not heaven
If you’ve been around for the last 2 decades, you’ve seen your share of Internet trends: Remember the ice bucket challenge? Tide pod eating? We know what you’re thinking: Sigh, what could they be doing now?
Well, people are eating old meat, and before you think about the expired ground beef you got on special from the grocery store yesterday, that’s not quite what we mean. We all know expiration dates are “suggestions,” like yield signs and yellow lights. People are eating rotten, decomposing, borderline moldy meat.
They claim that the meat tastes better. We’re not so sure, but don’t worry, because it gets weirder. Some folks, apparently, are getting high from eating this meat, experiencing a feeling of euphoria. Personally, we think that rotten fumes probably knocked these people out and made them hallucinate.
Singaporean dietitian Naras Lapsys says that eating rotten meat can possibly cause a person to go into another state of consciousness, but it’s not a good thing. We don’t think you have to be a dietitian to know that.
It has not been definitively proven that eating rotting meat makes you high, but it’s definitely proven that this is disgusting … and very dangerous.
Lower blood sugar with sardines
If you’ve ever turned your nose up at someone eating sardines straight from the can, you could be the one missing out on a good way to boost your own health.
New research from Open University of Catalonia (Spain) has found that eating two cans of whole sardines a week can help prevent people from developing type 2 diabetes (T2D). Now you might be thinking: That’s a lot of fish, can’t I just take a supplement pill? Actually, no.
“Nutrients can play an essential role in the prevention and treatment of many different pathologies, but their effect is usually caused by the synergy that exists between them and the food that they are contained in,” study coauthor Diana Rizzolo, PhD, said in a written statement. See, we told you.
In a study of 152 patients with prediabetes, each participant was put on a specific diet to reduce their chances of developing T2D. Among the patients who were not given sardines each week, the proportion considered to be at the highest risk fell from 27% to 22% after 1 year, but for those who did get the sardines, the size of the high-risk group shrank from 37% to just 8%.
Suggesting sardines during checkups could make eating them more widely accepted, Dr. Rizzolo and associates said. Sardines are cheap, easy to find, and also have the benefits of other oily fish, like boosting insulin resistance and increasing good cholesterol.
So why not have a can with a couple of saltine crackers for lunch? Your blood sugar will thank you. Just please avoid indulging on a plane or in your office, where workers are slowly returning – no need to give them another excuse to avoid their cubicle.
Come for the torture, stay for the vaccine
Bran Castle. Home of Dracula and Vlad the Impaler (at least in pop culture’s eyes). A moody Gothic structure atop a hill. You can practically hear the ancient screams of thousands of tortured souls as you wander the grounds and its cursed halls. Naturally, it’s a major tourist destination.
Unfortunately for Romania, the pandemic has rather put a damper on tourism. The restrictions have done their damage, but here’s a quick LOTME theory: Perhaps people don’t want to be reminded of medieval tortures when we’ve got plenty of modern-day ones right now.
The management of Bran Castle has developed a new gimmick to drum up attendance – come to Bran Castle and get your COVID vaccine. Anyone can come and get jabbed with the Pfizer vaccine on all weekends in May, and when they do, they gain free admittance to the castle and the exhibit within, home to 52 medieval torture instruments. “The idea … was to show how people got jabbed 500-600 years ago in Europe,” the castle’s marketing director said.
While it may not be kind of the jabbing ole Vladdy got his name for – fully impaling people on hundreds of wooden stakes while you eat a nice dinner isn’t exactly smiled upon in today’s world – we’re sure he’d approve of this more limited but ultimately beneficial version. Jabbing people while helping them really is the dream.
Fuzzy little COVID detectors
Before we get started, we need a moment to get our deep, movie trailer announcer-type voice ready. Okay, here goes.
“In a world where an organism too tiny to see brings entire economies to a standstill and pits scientists against doofuses, who can humanity turn to for help?”
How about bees? That’s right, we said bees. But not just any bees. Specially trained bees. Specially trained Dutch bees. Bees trained to sniff out our greatest nemesis. No, we’re not talking about Ted Cruz anymore. Let it go, that was just a joke. We’re talking COVID.
We’ll let Wim van der Poel, professor of virology at Wageningen (the Netherlands) University, explain the process: “We collect normal honeybees from a beekeeper, and we put the bees in harnesses.” And you thought their tulips were pretty great – the Dutch are putting harnesses on bees! (Which is much better than our previous story of bees involving a Taiwanese patient.)
The researchers presented the bees with two types of samples: COVID infected and non–COVID infected. The infected samples came with a sugary water reward and the noninfected samples did not, so the bees quickly learned to tell the difference.
The bees, then, could cut the waiting time for test results down to seconds, and at a fraction of the cost, making them an option in countries without a lot of testing infrastructure, the research team suggested.
The plan is not without its flaws, of course, but we’re convinced. More than that, we are true bee-lievers.
A little slice of … well, not heaven
If you’ve been around for the last 2 decades, you’ve seen your share of Internet trends: Remember the ice bucket challenge? Tide pod eating? We know what you’re thinking: Sigh, what could they be doing now?
Well, people are eating old meat, and before you think about the expired ground beef you got on special from the grocery store yesterday, that’s not quite what we mean. We all know expiration dates are “suggestions,” like yield signs and yellow lights. People are eating rotten, decomposing, borderline moldy meat.
They claim that the meat tastes better. We’re not so sure, but don’t worry, because it gets weirder. Some folks, apparently, are getting high from eating this meat, experiencing a feeling of euphoria. Personally, we think that rotten fumes probably knocked these people out and made them hallucinate.
Singaporean dietitian Naras Lapsys says that eating rotten meat can possibly cause a person to go into another state of consciousness, but it’s not a good thing. We don’t think you have to be a dietitian to know that.
It has not been definitively proven that eating rotting meat makes you high, but it’s definitely proven that this is disgusting … and very dangerous.
CDC recommends use of Pfizer’s COVID vaccine in 12- to 15-year-olds
The Centers for Disease Control and Prevention’s director Rochelle Walensky, MD, signed off on an advisory panel’s recommendation May 12 endorsing the use of the Pfizer-BioNTech COVID-19 vaccine in adolescents aged 12-15 years.
Earlier in the day the CDC’s Advisory Committee on Immunization Practices voted 14-0 in favor of the safety and effectiveness of the vaccine in younger teens.
Dr. Walensky said in an official statement.
The Food and Drug Administration on May 10 issued an emergency use authorization (EUA) for the Pfizer-BioNTech COVID-19 vaccine for the prevention of COVID-19 in individuals 12-15 years old. The FDA first cleared the Pfizer-BioNTech vaccine through an EUA in December 2020 for those ages 16 and older. Pfizer this month also initiated steps with the FDA toward a full approval of its vaccine.
Dr. Walenksy urged parents to seriously consider vaccinating their children.
“Understandably, some parents want more information before their children receive a vaccine,” she said. “I encourage parents with questions to talk to your child’s healthcare provider or your family doctor to learn more about the vaccine.”
Vaccine “safe and effective”
Separately, the American Academy of Pediatrics issued a statement May 12 in support of vaccinating all children ages 12 and older who are eligible for the federally authorized COVID-19 vaccine.
“As a pediatrician and a parent, I have looked forward to getting my own children and patients vaccinated, and I am thrilled that those ages 12 and older can now be protected,” said AAP President Lee Savio Beers, MD, in a statement. “The data continue to show that this vaccine is safe and effective. I urge all parents to call their pediatrician to learn more about how to get their children and teens vaccinated.”
The expanded clearance for the Pfizer vaccine is seen as a critical step for allowing teens to resume activities on which they missed out during the pandemic.
“We’ve seen the harm done to children’s mental and emotional health as they’ve missed out on so many experiences during the pandemic,” Dr. Beers said. “Vaccinating children will protect them and allow them to fully engage in all of the activities – school, sports, socializing with friends and family – that are so important to their health and development.”
A version of this article first appeared on Medscape.com.
The Centers for Disease Control and Prevention’s director Rochelle Walensky, MD, signed off on an advisory panel’s recommendation May 12 endorsing the use of the Pfizer-BioNTech COVID-19 vaccine in adolescents aged 12-15 years.
Earlier in the day the CDC’s Advisory Committee on Immunization Practices voted 14-0 in favor of the safety and effectiveness of the vaccine in younger teens.
Dr. Walensky said in an official statement.
The Food and Drug Administration on May 10 issued an emergency use authorization (EUA) for the Pfizer-BioNTech COVID-19 vaccine for the prevention of COVID-19 in individuals 12-15 years old. The FDA first cleared the Pfizer-BioNTech vaccine through an EUA in December 2020 for those ages 16 and older. Pfizer this month also initiated steps with the FDA toward a full approval of its vaccine.
Dr. Walenksy urged parents to seriously consider vaccinating their children.
“Understandably, some parents want more information before their children receive a vaccine,” she said. “I encourage parents with questions to talk to your child’s healthcare provider or your family doctor to learn more about the vaccine.”
Vaccine “safe and effective”
Separately, the American Academy of Pediatrics issued a statement May 12 in support of vaccinating all children ages 12 and older who are eligible for the federally authorized COVID-19 vaccine.
“As a pediatrician and a parent, I have looked forward to getting my own children and patients vaccinated, and I am thrilled that those ages 12 and older can now be protected,” said AAP President Lee Savio Beers, MD, in a statement. “The data continue to show that this vaccine is safe and effective. I urge all parents to call their pediatrician to learn more about how to get their children and teens vaccinated.”
The expanded clearance for the Pfizer vaccine is seen as a critical step for allowing teens to resume activities on which they missed out during the pandemic.
“We’ve seen the harm done to children’s mental and emotional health as they’ve missed out on so many experiences during the pandemic,” Dr. Beers said. “Vaccinating children will protect them and allow them to fully engage in all of the activities – school, sports, socializing with friends and family – that are so important to their health and development.”
A version of this article first appeared on Medscape.com.
The Centers for Disease Control and Prevention’s director Rochelle Walensky, MD, signed off on an advisory panel’s recommendation May 12 endorsing the use of the Pfizer-BioNTech COVID-19 vaccine in adolescents aged 12-15 years.
Earlier in the day the CDC’s Advisory Committee on Immunization Practices voted 14-0 in favor of the safety and effectiveness of the vaccine in younger teens.
Dr. Walensky said in an official statement.
The Food and Drug Administration on May 10 issued an emergency use authorization (EUA) for the Pfizer-BioNTech COVID-19 vaccine for the prevention of COVID-19 in individuals 12-15 years old. The FDA first cleared the Pfizer-BioNTech vaccine through an EUA in December 2020 for those ages 16 and older. Pfizer this month also initiated steps with the FDA toward a full approval of its vaccine.
Dr. Walenksy urged parents to seriously consider vaccinating their children.
“Understandably, some parents want more information before their children receive a vaccine,” she said. “I encourage parents with questions to talk to your child’s healthcare provider or your family doctor to learn more about the vaccine.”
Vaccine “safe and effective”
Separately, the American Academy of Pediatrics issued a statement May 12 in support of vaccinating all children ages 12 and older who are eligible for the federally authorized COVID-19 vaccine.
“As a pediatrician and a parent, I have looked forward to getting my own children and patients vaccinated, and I am thrilled that those ages 12 and older can now be protected,” said AAP President Lee Savio Beers, MD, in a statement. “The data continue to show that this vaccine is safe and effective. I urge all parents to call their pediatrician to learn more about how to get their children and teens vaccinated.”
The expanded clearance for the Pfizer vaccine is seen as a critical step for allowing teens to resume activities on which they missed out during the pandemic.
“We’ve seen the harm done to children’s mental and emotional health as they’ve missed out on so many experiences during the pandemic,” Dr. Beers said. “Vaccinating children will protect them and allow them to fully engage in all of the activities – school, sports, socializing with friends and family – that are so important to their health and development.”
A version of this article first appeared on Medscape.com.
Multiple sclerosis updates from AAN 2021
Mitzi Joi Williams, MD, Medical Director and CEO of Joi Life Wellness Group in Atlanta, GA, discusses treatment data and other timely updates in multiple sclerosis (MS) coming out of the American Academy of Neurology (AAN) 2021 Annual Meeting.
Dr. Williams introduces the new National African Americans with Multiple Sclerosis Registry, an important initiative aimed at expanding evidence-based knowledge of MS in African American patients, increasing clinical trial participation, and engaging in research that will benefit this community.
In a review of a long-term analysis of evobrutinib, Dr. Williams shares the positive efficacy and safety outcomes that were observed and maintained in patients receiving evobrutinib 75 mg twice daily throughout the duration of the 48-week double-blind, randomized, phase 2 trial and the 60-week open-label extension portions of the study.
Next, Dr. Williams highlights a study of patients taking disease-modifying therapies (DMTs) at the NYU MS Care Center. The analysis found factors such as public insurance status, younger age, and Hispanic ethnicity to be larger predictors of COVID-19 infection in this patient population than any of the DMTs that were studied.
Lastly, Dr. Williams discusses an analysis of racial and ethnic differences in patients receiving ofatumumab in the ASCLEPIOS I/II and APOLITOS trials. The study revealed no clinically relevant differences in annualized relapse rate, pharmacokinetics, B-cell depletion, or safety profile between the study populations.
--
Mitzi Joi Williams, MD, Medical Director, CEO; Department of Neurology, Joi Life Wellness Group, Atlanta, Georgia
Mitzi Joi Williams, MD, has disclosed the following relevant financial relationships:
Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for: Biogen Idec; EMD Serono; Genentech; Novartis; Bristol-Myers Squibb; AbbVie; Alexion; Novartis.
Serve(d) as a speaker or a member of a speakers bureau for: Biogen Idec; EMD Serono; Genentech Roche; Novartis; Bristol-Myers Squibb.
Mitzi Joi Williams, MD, Medical Director and CEO of Joi Life Wellness Group in Atlanta, GA, discusses treatment data and other timely updates in multiple sclerosis (MS) coming out of the American Academy of Neurology (AAN) 2021 Annual Meeting.
Dr. Williams introduces the new National African Americans with Multiple Sclerosis Registry, an important initiative aimed at expanding evidence-based knowledge of MS in African American patients, increasing clinical trial participation, and engaging in research that will benefit this community.
In a review of a long-term analysis of evobrutinib, Dr. Williams shares the positive efficacy and safety outcomes that were observed and maintained in patients receiving evobrutinib 75 mg twice daily throughout the duration of the 48-week double-blind, randomized, phase 2 trial and the 60-week open-label extension portions of the study.
Next, Dr. Williams highlights a study of patients taking disease-modifying therapies (DMTs) at the NYU MS Care Center. The analysis found factors such as public insurance status, younger age, and Hispanic ethnicity to be larger predictors of COVID-19 infection in this patient population than any of the DMTs that were studied.
Lastly, Dr. Williams discusses an analysis of racial and ethnic differences in patients receiving ofatumumab in the ASCLEPIOS I/II and APOLITOS trials. The study revealed no clinically relevant differences in annualized relapse rate, pharmacokinetics, B-cell depletion, or safety profile between the study populations.
--
Mitzi Joi Williams, MD, Medical Director, CEO; Department of Neurology, Joi Life Wellness Group, Atlanta, Georgia
Mitzi Joi Williams, MD, has disclosed the following relevant financial relationships:
Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for: Biogen Idec; EMD Serono; Genentech; Novartis; Bristol-Myers Squibb; AbbVie; Alexion; Novartis.
Serve(d) as a speaker or a member of a speakers bureau for: Biogen Idec; EMD Serono; Genentech Roche; Novartis; Bristol-Myers Squibb.
Mitzi Joi Williams, MD, Medical Director and CEO of Joi Life Wellness Group in Atlanta, GA, discusses treatment data and other timely updates in multiple sclerosis (MS) coming out of the American Academy of Neurology (AAN) 2021 Annual Meeting.
Dr. Williams introduces the new National African Americans with Multiple Sclerosis Registry, an important initiative aimed at expanding evidence-based knowledge of MS in African American patients, increasing clinical trial participation, and engaging in research that will benefit this community.
In a review of a long-term analysis of evobrutinib, Dr. Williams shares the positive efficacy and safety outcomes that were observed and maintained in patients receiving evobrutinib 75 mg twice daily throughout the duration of the 48-week double-blind, randomized, phase 2 trial and the 60-week open-label extension portions of the study.
Next, Dr. Williams highlights a study of patients taking disease-modifying therapies (DMTs) at the NYU MS Care Center. The analysis found factors such as public insurance status, younger age, and Hispanic ethnicity to be larger predictors of COVID-19 infection in this patient population than any of the DMTs that were studied.
Lastly, Dr. Williams discusses an analysis of racial and ethnic differences in patients receiving ofatumumab in the ASCLEPIOS I/II and APOLITOS trials. The study revealed no clinically relevant differences in annualized relapse rate, pharmacokinetics, B-cell depletion, or safety profile between the study populations.
--
Mitzi Joi Williams, MD, Medical Director, CEO; Department of Neurology, Joi Life Wellness Group, Atlanta, Georgia
Mitzi Joi Williams, MD, has disclosed the following relevant financial relationships:
Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for: Biogen Idec; EMD Serono; Genentech; Novartis; Bristol-Myers Squibb; AbbVie; Alexion; Novartis.
Serve(d) as a speaker or a member of a speakers bureau for: Biogen Idec; EMD Serono; Genentech Roche; Novartis; Bristol-Myers Squibb.

Assessing the cognitive nuances between ADHD and autism
Attention-deficit/hyperactivity disorder and autism spectrum disorder (ASD) often coexist in children and adults, but the range of cognitive abilities can vary widely in these patients. Researchers from around the world are leveraging symptom, cognitive assessment, and neurobiological measures to gain insights on how individuals with ADHD/ASD approach and solve problems.
Several experts discussed the progress of their research during the session, “Overlap and differences of ADHD and autism – new findings of functional imaging and cognition studies” at the World Congress on ADHD – Virtual Event.
“The overlap of these two disorders is a critical issue for our field,” said Sarah Karalunas, PhD, assistant professor of clinical psychology at Purdue University, West Lafayette, Ind., who moderated the session. Clinicians are often asked to make differential diagnoses between these two disorders. Only recently has the DSM-5 allowed their codiagnosis. “There’s increasing recognition that there may be shared cognitive and physiological features that reflect their shared risk and account for the high levels of symptom overlap,” said Dr. Karalunas.
Shared cognitive markers
Under the DSM’s change, “it’s now recognized that an estimated 20%-60% of children with ASD have comorbidities with ADHD, and around 20%-40% of children with ADHD have ASD symptoms,” said Beth Johnson, PhD, a research fellow with the Turner Institute for Brain and Mental Health at Monash University, Melbourne.
The shared overlap on genetic traits and comorbidities such as intellectual disability, anxiety, depression, and oppositional defiant disorder, make it difficult for clinicians to predict clinical outcomes, noted Dr. Johnson.
“We’re now understanding that they’re likely to be multiple autisms and ADHDs, that these symptoms exist on a spectrum of severity or ability,” she said. Dr. Johnson discussed a data-driven subtyping approach based on neurocognitive and symptom profiles in children with ADHD. The aim was to better understand how symptoms are managed across ADHD, ASD and comorbid ASD-ADHD.
As part of this research, her team recruited 295 controls and 117 children with ADHD who underwent clinical phenotyping and also completed working memory tasks, stop signal, and sustained attention tasks.
The researchers divided the children into four stable clusters based on the ADHD rating scale and autism questionnaire data: high ASD/ADHD traits, high ADHD/low ASD, low ADHD/moderate ASD, and low ADHD/ASD. Approximately half of the children with ADHD showed moderate to high ASD symptoms. Looking at neurocognition across the tasks, unsurprisingly, performance was lowest among the high-ASD/ADHD children, with performance on the stop signal being the most pronounced. “Notably, performance on the working memory task worsened with increasing ADHD symptoms,” she reported.
Drift model identifies information processing
Dr. Karalunas has also compared subgroups of ADHD and ASD children. “Our analysis examined whether cognitive impairments in ASD reflect a shared risk mechanism or co-occurring ADHD symptoms and why we see an overlap in these types of impairments,” she said.
Her study included 509 children with ADHD, 97 with ASD, and 301 controls (typical development). All three groups underwent a full cognitive assessment battery that measured attention arousal, basic processing speed, and working memory. Those tasks were collapsed into a series of variables as well as a set of tasks measuring response inhibition, switching, interference control, reward discounting, and measure of reaction time variability.
Four cognitive profiles emerged: a typically developing group, an ADHD group, an ASD group with low levels of ADHD symptoms and an ASD group with high levels of ADHD symptoms.
The ADHD group did worse on many of the tasks than the control group, and the ASD group with low ADHD levels also did poorly relative to the typically developing sample. This shows that autism – even in absence of co-occurring ADHD – demonstrates more cognitive impairment than typically developing kids. The ADHD group with high levels of autism did the most poorly across all of the tasks.
The findings also revealed a symptom severity pattern: the group with fewer symptoms did the best and the group with the most symptoms did the worst. “Overall, this reflects severity of impairment,” said Dr. Karalunas.
To identify measures more specific to either ADHD or autism, Dr. Karalunas and colleagues did a follow-up analysis to characterize cognitive performance. To accomplish this, they applied a drift-diffusion model to the same four cognitive profiles. The model assessed three parameters: drift rate, which relates to the speed or efficiency of information processing, boundary separation or speed accuracy trade-offs (impulsivity), and nondecision time such as motor preparation.
Using the same four cognitive profiles, they found that the ADHD group had slower drift rate relative to the control, although the two groups did not differ on boundary separation, which meant there were no differences on waiting to need to respond. The ADHD group had faster nondecision times. “This is a classic pattern, shown in the literature,” said Dr. Karalunas.
In other results, an interesting pattern began to evolve
Both ASD groups, for example, had much wider boundary separations, which meant they were waiting to be sure before they responded than the ADHD or typically developing groups. In contrast, the two ADHD groups had much faster non-decision times, whereas the two non-ADHD groups had similar nondecisions times.
Unlike the previous analysis, which saw a symptom severity pattern develop, “we’re getting two parameters that seem to track much more specifically to specific symptom domains,” observed Dr. Karalunas.
The results suggest there’s a substantial overlap in cognitive impairments in ADHD/ASD. “But we have pretty strong evidence at this point that these similarities are not accounted for by symptom overlap, especially for things like response and inhibition, working memory and processing speed. These seem to be independently related to ADHD and autism, regardless of the level of comorbid ADHD symptoms in the autism group,” said Dr. Karalunas.
The hope is to expand on these types of analyses to address the interaction of cognition-emotion and social cognition, and empirically define groups based on cognitive performance, she said.
Neurocognitive studies
Researchers have also been studying neural networks to assess ASD and ADHD. Roselyne Chauvin, PhD, a postdoctoral associate at Washington University, St. Louis, discussed the concept of “a task generic connectome,” in which researchers look for a common network between targeted task paradigms to get closer to a common alteration across impairments.
In her research, Dr. Chauvin and colleagues looked at connectivity modulations across three tasks: working memory, reward processing tasks, and stop signal tasks, comparing ADHD patients to siblings and controls. The ADHD group showed reduced sensitivity or a smaller number of connections modulated in the tasks compared with the other groups. Researchers wondered where those missed connections were located.
Dividing the cohorts into task generic and task specific groups, Dr. Chauvin and colleagues found that the ADHD group lacked common processing skills. They were also able to identify reproducible missing circuits in the ADHD participants. Among the cohorts, there was a higher modulation of task-specific edges in the ADHD group.
The ADHD patients seemed to be using more task-tailored alternative strategies that were more challenging and suboptimal.
She also previewed her ongoing work with the EU-AIMS Longitudinal European Autism Project (LEAP) database to study ASD-ADHD comorbidity. In this project, she and her colleagues looked at several tasks: probing emotion processing, inhibitory control, theory of mind, and reward anticipation. Comparing ASD groups with or without ADHD comorbidity or a shared connection, she and her team were able to devise a functional profile predictive of ADHD severity. As an example, “for the connection only used by the ASD with ADHD comorbidity, the more they were using those connections of higher amplitude in the modulation, inside this subset of connection, the higher they would have ADHD severity,” said Dr. Chauvin.
Neural correlates of different behavioral and cognitive profiles haven’t been widely studied, according to Charlotte Tye, PhD, who’s based at the Institute of Psychiatry, Psychology & Neuroscience, King’s College, London. Electroencephalography is a useful technique for understanding the neural correlates of cognitive impairments and teasing apart different models of co-occurrence in ASD and ADHD.
Dr. Tye and colleagues tested this approach in a cohort of boys aged 8-13 years diagnosed with ASD and/or ADHD, measuring EEG while the children did various continuous performance tasks to assess changes in brain activity. Examining P3 amplitude (event-related potential components) they found that children with ADHD or ADHD+ASD showed an attenuated amplitude of the P3, compared with typically developing children and those with ASD.
“This suggests children with an ADHD diagnosis exhibited reduced inhibitory control,” said Dr. Tye. In contrast, children with ASD showed reduced conflict monitoring as indexed by altered N2 amplitude across task conditions.
These, and other studies conducted by Dr. Tye and colleagues indicate that children with ADHD show reduced neural responses during attentional processing, whereas autistic children show typical neural responses, supporting specific profiles.
“Autistic children with a diagnosis of ADHD appear to show the unique patterns of neural responses of autism and ADHD, supporting an additive co-occurrence rather than a distinct condition. This contributes to identification of transdiagnostic subgroups within neurodevelopmental conditions for targeting of personalized intervention, and suggests that children with co-occurring autism and ADHD require support for both conditions,” said Dr. Tye.
An important takeaway from all of these findings is “we can’t look just at how someone does overall on a single test,” said Dr. Karalunas in an interview. “There is a tremendous amount of variability between people who have the same diagnosis, and our research really needs to account for this.”
Attention-deficit/hyperactivity disorder and autism spectrum disorder (ASD) often coexist in children and adults, but the range of cognitive abilities can vary widely in these patients. Researchers from around the world are leveraging symptom, cognitive assessment, and neurobiological measures to gain insights on how individuals with ADHD/ASD approach and solve problems.
Several experts discussed the progress of their research during the session, “Overlap and differences of ADHD and autism – new findings of functional imaging and cognition studies” at the World Congress on ADHD – Virtual Event.
“The overlap of these two disorders is a critical issue for our field,” said Sarah Karalunas, PhD, assistant professor of clinical psychology at Purdue University, West Lafayette, Ind., who moderated the session. Clinicians are often asked to make differential diagnoses between these two disorders. Only recently has the DSM-5 allowed their codiagnosis. “There’s increasing recognition that there may be shared cognitive and physiological features that reflect their shared risk and account for the high levels of symptom overlap,” said Dr. Karalunas.
Shared cognitive markers
Under the DSM’s change, “it’s now recognized that an estimated 20%-60% of children with ASD have comorbidities with ADHD, and around 20%-40% of children with ADHD have ASD symptoms,” said Beth Johnson, PhD, a research fellow with the Turner Institute for Brain and Mental Health at Monash University, Melbourne.
The shared overlap on genetic traits and comorbidities such as intellectual disability, anxiety, depression, and oppositional defiant disorder, make it difficult for clinicians to predict clinical outcomes, noted Dr. Johnson.
“We’re now understanding that they’re likely to be multiple autisms and ADHDs, that these symptoms exist on a spectrum of severity or ability,” she said. Dr. Johnson discussed a data-driven subtyping approach based on neurocognitive and symptom profiles in children with ADHD. The aim was to better understand how symptoms are managed across ADHD, ASD and comorbid ASD-ADHD.
As part of this research, her team recruited 295 controls and 117 children with ADHD who underwent clinical phenotyping and also completed working memory tasks, stop signal, and sustained attention tasks.
The researchers divided the children into four stable clusters based on the ADHD rating scale and autism questionnaire data: high ASD/ADHD traits, high ADHD/low ASD, low ADHD/moderate ASD, and low ADHD/ASD. Approximately half of the children with ADHD showed moderate to high ASD symptoms. Looking at neurocognition across the tasks, unsurprisingly, performance was lowest among the high-ASD/ADHD children, with performance on the stop signal being the most pronounced. “Notably, performance on the working memory task worsened with increasing ADHD symptoms,” she reported.
Drift model identifies information processing
Dr. Karalunas has also compared subgroups of ADHD and ASD children. “Our analysis examined whether cognitive impairments in ASD reflect a shared risk mechanism or co-occurring ADHD symptoms and why we see an overlap in these types of impairments,” she said.
Her study included 509 children with ADHD, 97 with ASD, and 301 controls (typical development). All three groups underwent a full cognitive assessment battery that measured attention arousal, basic processing speed, and working memory. Those tasks were collapsed into a series of variables as well as a set of tasks measuring response inhibition, switching, interference control, reward discounting, and measure of reaction time variability.
Four cognitive profiles emerged: a typically developing group, an ADHD group, an ASD group with low levels of ADHD symptoms and an ASD group with high levels of ADHD symptoms.
The ADHD group did worse on many of the tasks than the control group, and the ASD group with low ADHD levels also did poorly relative to the typically developing sample. This shows that autism – even in absence of co-occurring ADHD – demonstrates more cognitive impairment than typically developing kids. The ADHD group with high levels of autism did the most poorly across all of the tasks.
The findings also revealed a symptom severity pattern: the group with fewer symptoms did the best and the group with the most symptoms did the worst. “Overall, this reflects severity of impairment,” said Dr. Karalunas.
To identify measures more specific to either ADHD or autism, Dr. Karalunas and colleagues did a follow-up analysis to characterize cognitive performance. To accomplish this, they applied a drift-diffusion model to the same four cognitive profiles. The model assessed three parameters: drift rate, which relates to the speed or efficiency of information processing, boundary separation or speed accuracy trade-offs (impulsivity), and nondecision time such as motor preparation.
Using the same four cognitive profiles, they found that the ADHD group had slower drift rate relative to the control, although the two groups did not differ on boundary separation, which meant there were no differences on waiting to need to respond. The ADHD group had faster nondecision times. “This is a classic pattern, shown in the literature,” said Dr. Karalunas.
In other results, an interesting pattern began to evolve
Both ASD groups, for example, had much wider boundary separations, which meant they were waiting to be sure before they responded than the ADHD or typically developing groups. In contrast, the two ADHD groups had much faster non-decision times, whereas the two non-ADHD groups had similar nondecisions times.
Unlike the previous analysis, which saw a symptom severity pattern develop, “we’re getting two parameters that seem to track much more specifically to specific symptom domains,” observed Dr. Karalunas.
The results suggest there’s a substantial overlap in cognitive impairments in ADHD/ASD. “But we have pretty strong evidence at this point that these similarities are not accounted for by symptom overlap, especially for things like response and inhibition, working memory and processing speed. These seem to be independently related to ADHD and autism, regardless of the level of comorbid ADHD symptoms in the autism group,” said Dr. Karalunas.
The hope is to expand on these types of analyses to address the interaction of cognition-emotion and social cognition, and empirically define groups based on cognitive performance, she said.
Neurocognitive studies
Researchers have also been studying neural networks to assess ASD and ADHD. Roselyne Chauvin, PhD, a postdoctoral associate at Washington University, St. Louis, discussed the concept of “a task generic connectome,” in which researchers look for a common network between targeted task paradigms to get closer to a common alteration across impairments.
In her research, Dr. Chauvin and colleagues looked at connectivity modulations across three tasks: working memory, reward processing tasks, and stop signal tasks, comparing ADHD patients to siblings and controls. The ADHD group showed reduced sensitivity or a smaller number of connections modulated in the tasks compared with the other groups. Researchers wondered where those missed connections were located.
Dividing the cohorts into task generic and task specific groups, Dr. Chauvin and colleagues found that the ADHD group lacked common processing skills. They were also able to identify reproducible missing circuits in the ADHD participants. Among the cohorts, there was a higher modulation of task-specific edges in the ADHD group.
The ADHD patients seemed to be using more task-tailored alternative strategies that were more challenging and suboptimal.
She also previewed her ongoing work with the EU-AIMS Longitudinal European Autism Project (LEAP) database to study ASD-ADHD comorbidity. In this project, she and her colleagues looked at several tasks: probing emotion processing, inhibitory control, theory of mind, and reward anticipation. Comparing ASD groups with or without ADHD comorbidity or a shared connection, she and her team were able to devise a functional profile predictive of ADHD severity. As an example, “for the connection only used by the ASD with ADHD comorbidity, the more they were using those connections of higher amplitude in the modulation, inside this subset of connection, the higher they would have ADHD severity,” said Dr. Chauvin.
Neural correlates of different behavioral and cognitive profiles haven’t been widely studied, according to Charlotte Tye, PhD, who’s based at the Institute of Psychiatry, Psychology & Neuroscience, King’s College, London. Electroencephalography is a useful technique for understanding the neural correlates of cognitive impairments and teasing apart different models of co-occurrence in ASD and ADHD.
Dr. Tye and colleagues tested this approach in a cohort of boys aged 8-13 years diagnosed with ASD and/or ADHD, measuring EEG while the children did various continuous performance tasks to assess changes in brain activity. Examining P3 amplitude (event-related potential components) they found that children with ADHD or ADHD+ASD showed an attenuated amplitude of the P3, compared with typically developing children and those with ASD.
“This suggests children with an ADHD diagnosis exhibited reduced inhibitory control,” said Dr. Tye. In contrast, children with ASD showed reduced conflict monitoring as indexed by altered N2 amplitude across task conditions.
These, and other studies conducted by Dr. Tye and colleagues indicate that children with ADHD show reduced neural responses during attentional processing, whereas autistic children show typical neural responses, supporting specific profiles.
“Autistic children with a diagnosis of ADHD appear to show the unique patterns of neural responses of autism and ADHD, supporting an additive co-occurrence rather than a distinct condition. This contributes to identification of transdiagnostic subgroups within neurodevelopmental conditions for targeting of personalized intervention, and suggests that children with co-occurring autism and ADHD require support for both conditions,” said Dr. Tye.
An important takeaway from all of these findings is “we can’t look just at how someone does overall on a single test,” said Dr. Karalunas in an interview. “There is a tremendous amount of variability between people who have the same diagnosis, and our research really needs to account for this.”
Attention-deficit/hyperactivity disorder and autism spectrum disorder (ASD) often coexist in children and adults, but the range of cognitive abilities can vary widely in these patients. Researchers from around the world are leveraging symptom, cognitive assessment, and neurobiological measures to gain insights on how individuals with ADHD/ASD approach and solve problems.
Several experts discussed the progress of their research during the session, “Overlap and differences of ADHD and autism – new findings of functional imaging and cognition studies” at the World Congress on ADHD – Virtual Event.
“The overlap of these two disorders is a critical issue for our field,” said Sarah Karalunas, PhD, assistant professor of clinical psychology at Purdue University, West Lafayette, Ind., who moderated the session. Clinicians are often asked to make differential diagnoses between these two disorders. Only recently has the DSM-5 allowed their codiagnosis. “There’s increasing recognition that there may be shared cognitive and physiological features that reflect their shared risk and account for the high levels of symptom overlap,” said Dr. Karalunas.
Shared cognitive markers
Under the DSM’s change, “it’s now recognized that an estimated 20%-60% of children with ASD have comorbidities with ADHD, and around 20%-40% of children with ADHD have ASD symptoms,” said Beth Johnson, PhD, a research fellow with the Turner Institute for Brain and Mental Health at Monash University, Melbourne.
The shared overlap on genetic traits and comorbidities such as intellectual disability, anxiety, depression, and oppositional defiant disorder, make it difficult for clinicians to predict clinical outcomes, noted Dr. Johnson.
“We’re now understanding that they’re likely to be multiple autisms and ADHDs, that these symptoms exist on a spectrum of severity or ability,” she said. Dr. Johnson discussed a data-driven subtyping approach based on neurocognitive and symptom profiles in children with ADHD. The aim was to better understand how symptoms are managed across ADHD, ASD and comorbid ASD-ADHD.
As part of this research, her team recruited 295 controls and 117 children with ADHD who underwent clinical phenotyping and also completed working memory tasks, stop signal, and sustained attention tasks.
The researchers divided the children into four stable clusters based on the ADHD rating scale and autism questionnaire data: high ASD/ADHD traits, high ADHD/low ASD, low ADHD/moderate ASD, and low ADHD/ASD. Approximately half of the children with ADHD showed moderate to high ASD symptoms. Looking at neurocognition across the tasks, unsurprisingly, performance was lowest among the high-ASD/ADHD children, with performance on the stop signal being the most pronounced. “Notably, performance on the working memory task worsened with increasing ADHD symptoms,” she reported.
Drift model identifies information processing
Dr. Karalunas has also compared subgroups of ADHD and ASD children. “Our analysis examined whether cognitive impairments in ASD reflect a shared risk mechanism or co-occurring ADHD symptoms and why we see an overlap in these types of impairments,” she said.
Her study included 509 children with ADHD, 97 with ASD, and 301 controls (typical development). All three groups underwent a full cognitive assessment battery that measured attention arousal, basic processing speed, and working memory. Those tasks were collapsed into a series of variables as well as a set of tasks measuring response inhibition, switching, interference control, reward discounting, and measure of reaction time variability.
Four cognitive profiles emerged: a typically developing group, an ADHD group, an ASD group with low levels of ADHD symptoms and an ASD group with high levels of ADHD symptoms.
The ADHD group did worse on many of the tasks than the control group, and the ASD group with low ADHD levels also did poorly relative to the typically developing sample. This shows that autism – even in absence of co-occurring ADHD – demonstrates more cognitive impairment than typically developing kids. The ADHD group with high levels of autism did the most poorly across all of the tasks.
The findings also revealed a symptom severity pattern: the group with fewer symptoms did the best and the group with the most symptoms did the worst. “Overall, this reflects severity of impairment,” said Dr. Karalunas.
To identify measures more specific to either ADHD or autism, Dr. Karalunas and colleagues did a follow-up analysis to characterize cognitive performance. To accomplish this, they applied a drift-diffusion model to the same four cognitive profiles. The model assessed three parameters: drift rate, which relates to the speed or efficiency of information processing, boundary separation or speed accuracy trade-offs (impulsivity), and nondecision time such as motor preparation.
Using the same four cognitive profiles, they found that the ADHD group had slower drift rate relative to the control, although the two groups did not differ on boundary separation, which meant there were no differences on waiting to need to respond. The ADHD group had faster nondecision times. “This is a classic pattern, shown in the literature,” said Dr. Karalunas.
In other results, an interesting pattern began to evolve
Both ASD groups, for example, had much wider boundary separations, which meant they were waiting to be sure before they responded than the ADHD or typically developing groups. In contrast, the two ADHD groups had much faster non-decision times, whereas the two non-ADHD groups had similar nondecisions times.
Unlike the previous analysis, which saw a symptom severity pattern develop, “we’re getting two parameters that seem to track much more specifically to specific symptom domains,” observed Dr. Karalunas.
The results suggest there’s a substantial overlap in cognitive impairments in ADHD/ASD. “But we have pretty strong evidence at this point that these similarities are not accounted for by symptom overlap, especially for things like response and inhibition, working memory and processing speed. These seem to be independently related to ADHD and autism, regardless of the level of comorbid ADHD symptoms in the autism group,” said Dr. Karalunas.
The hope is to expand on these types of analyses to address the interaction of cognition-emotion and social cognition, and empirically define groups based on cognitive performance, she said.
Neurocognitive studies
Researchers have also been studying neural networks to assess ASD and ADHD. Roselyne Chauvin, PhD, a postdoctoral associate at Washington University, St. Louis, discussed the concept of “a task generic connectome,” in which researchers look for a common network between targeted task paradigms to get closer to a common alteration across impairments.
In her research, Dr. Chauvin and colleagues looked at connectivity modulations across three tasks: working memory, reward processing tasks, and stop signal tasks, comparing ADHD patients to siblings and controls. The ADHD group showed reduced sensitivity or a smaller number of connections modulated in the tasks compared with the other groups. Researchers wondered where those missed connections were located.
Dividing the cohorts into task generic and task specific groups, Dr. Chauvin and colleagues found that the ADHD group lacked common processing skills. They were also able to identify reproducible missing circuits in the ADHD participants. Among the cohorts, there was a higher modulation of task-specific edges in the ADHD group.
The ADHD patients seemed to be using more task-tailored alternative strategies that were more challenging and suboptimal.
She also previewed her ongoing work with the EU-AIMS Longitudinal European Autism Project (LEAP) database to study ASD-ADHD comorbidity. In this project, she and her colleagues looked at several tasks: probing emotion processing, inhibitory control, theory of mind, and reward anticipation. Comparing ASD groups with or without ADHD comorbidity or a shared connection, she and her team were able to devise a functional profile predictive of ADHD severity. As an example, “for the connection only used by the ASD with ADHD comorbidity, the more they were using those connections of higher amplitude in the modulation, inside this subset of connection, the higher they would have ADHD severity,” said Dr. Chauvin.
Neural correlates of different behavioral and cognitive profiles haven’t been widely studied, according to Charlotte Tye, PhD, who’s based at the Institute of Psychiatry, Psychology & Neuroscience, King’s College, London. Electroencephalography is a useful technique for understanding the neural correlates of cognitive impairments and teasing apart different models of co-occurrence in ASD and ADHD.
Dr. Tye and colleagues tested this approach in a cohort of boys aged 8-13 years diagnosed with ASD and/or ADHD, measuring EEG while the children did various continuous performance tasks to assess changes in brain activity. Examining P3 amplitude (event-related potential components) they found that children with ADHD or ADHD+ASD showed an attenuated amplitude of the P3, compared with typically developing children and those with ASD.
“This suggests children with an ADHD diagnosis exhibited reduced inhibitory control,” said Dr. Tye. In contrast, children with ASD showed reduced conflict monitoring as indexed by altered N2 amplitude across task conditions.
These, and other studies conducted by Dr. Tye and colleagues indicate that children with ADHD show reduced neural responses during attentional processing, whereas autistic children show typical neural responses, supporting specific profiles.
“Autistic children with a diagnosis of ADHD appear to show the unique patterns of neural responses of autism and ADHD, supporting an additive co-occurrence rather than a distinct condition. This contributes to identification of transdiagnostic subgroups within neurodevelopmental conditions for targeting of personalized intervention, and suggests that children with co-occurring autism and ADHD require support for both conditions,” said Dr. Tye.
An important takeaway from all of these findings is “we can’t look just at how someone does overall on a single test,” said Dr. Karalunas in an interview. “There is a tremendous amount of variability between people who have the same diagnosis, and our research really needs to account for this.”
FROM ADHD 2021
Reassuring data on impact of mild COVID-19 on the heart
Six months after mild SARS-CoV-2 infection in a representative health care workforce, no long-term cardiovascular sequelae were detected, compared with a matched SARS-CoV-2 seronegative group.
“Mild COVID-19 left no measurable cardiovascular impact on LV structure, function, scar burden, aortic stiffness, or serum biomarkers,” the researchers reported in an article published online May 8 in JACC: Cardiovascular Imaging.
“We provide societal reassurance and support for the position that screening in asymptomatic individuals following mild disease is not indicated,” first author George Joy, MBBS, University College London, said in presenting the results at EuroCMR, the annual CMR congress of the European Association of Cardiovascular Imaging (EACVI).
Briefing comoderator Leyla Elif Sade, MD, University of Baskent, Ankara, Turkey, said, “This is the hot topic of our time because of obvious reasons and I think [this] study is quite important to avoid unnecessary further testing, surveillance testing, and to avoid a significant burden of health care costs.”
‘Alarming’ early data
Early cardiac magnetic resonance (CMR) studies in patients recovered from mild COVID-19 were “alarming,” Dr. Joy said.
As previously reported, one study showed cardiac abnormalities after mild COVID-19 in up to 78% of patients, with evidence of ongoing myocardial inflammation in 60%. The CMR findings correlated with elevations in troponin T by high-sensitivity assay (hs-TnT).
To investigate further, Dr. Joy and colleagues did a nested case-control study within the COVIDsortium, a prospective study of 731 health care workers from three London hospitals who underwent weekly symptom, polymerase chain reaction, and serology assessment over 4 months during the first wave of the pandemic.
A total of 157 (21.5%) participants seroconverted during the study period.
Six months after infection, 74 seropositive (median age, 39; 62% women) and 75 age-, sex-, and ethnicity-matched seronegative controls underwent cardiovascular phenotyping (comprehensive phantom-calibrated CMR and blood biomarkers). The analysis was blinded, using objective artificial intelligence analytics when available.
The results showed no statistically significant differences between seropositive and seronegative participants in cardiac structure (left ventricular volumes, mass, atrial area), function (ejection fraction, global longitudinal shortening, aortic distensibility), tissue characterization (T1, T2, extracellular volume fraction mapping, late gadolinium enhancement) or biomarkers (troponin, N-terminal pro–B-type natriuretic peptide).
Cardiovascular abnormalities were no more common in seropositive than seronegative otherwise healthy health care workers 6 months post mild SARS-CoV-2 infection. Measured abnormalities were “evenly distributed between both groups,” Dr. Joy said.
Therefore, it’s “important to reassure patients with mild SARS-CoV-2 infection regarding its cardiovascular effects,” Dr. Joy and colleagues concluded.
Limitations and caveats
They caution, however, that the study provides insight only into the short- to medium-term sequelae of patients aged 18-69 with mild COVID-19 who did not require hospitalization and had low numbers of comorbidities.
The study does not address the cardiovascular effects after severe COVID-19 infection requiring hospitalization or in those with multiple comorbid conditions, they noted. It also does not prove that apparently mild SARS-CoV-2 never causes chronic myocarditis.
“The study design would not distinguish between people who had sustained completely healed myocarditis and pericarditis and those in whom the heart had never been affected,” the researchers noted.
They pointed to a recent cross-sectional study of athletes 1-month post mild COVID-19 that found significant pericardial involvement (late enhancement and/or pericardial effusion), although no baseline pre-COVID-19 imaging was performed. In the current study at 6 months post infection the pericardium was normal.
The coauthors of a linked editorial say this study provides “welcome, reassuring information that in healthy individuals who experience mild infection with COVID-19, persisting evidence of cardiovascular complications is very uncommon. The results do not support cardiovascular screening in individuals with mild or asymptomatic infection with COVID-19.”
Colin Berry, PhD, and Kenneth Mangion, PhD, both from University of Glasgow, cautioned that the population is restricted to health care workers; therefore, the findings may not necessarily be generalized to a community population .
“Healthcare workers do not reflect the population of individuals most clinically affected by COVID-19 illness. The severity of acute COVID-19 infection is greatest in older individuals and those with preexisting health problems. Healthcare workers are not representative of the wider, unselected, at-risk, community population,” they pointed out.
Cardiovascular risk factors and concomitant health problems (heart and respiratory disease) may be more common in the community than in health care workers, and prior studies have highlighted their potential impact for disease pathogenesis in COVID-19.
Dr. Berry and Dr. Mangion also noted that women made up nearly two-thirds of the seropositive group. This may reflect a selection bias or may naturally reflect the fact that proportionately more women are asymptomatic or have milder forms of illness, whereas severe SARS-CoV-2 infection requiring hospitalization affects men to a greater degree.
COVIDsortium funding was donated by individuals, charitable trusts, and corporations including Goldman Sachs, Citadel and Citadel Securities, The Guy Foundation, GW Pharmaceuticals, Kusuma Trust, and Jagclif Charitable Trust, and enabled by Barts Charity with support from UCLH Charity. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Six months after mild SARS-CoV-2 infection in a representative health care workforce, no long-term cardiovascular sequelae were detected, compared with a matched SARS-CoV-2 seronegative group.
“Mild COVID-19 left no measurable cardiovascular impact on LV structure, function, scar burden, aortic stiffness, or serum biomarkers,” the researchers reported in an article published online May 8 in JACC: Cardiovascular Imaging.
“We provide societal reassurance and support for the position that screening in asymptomatic individuals following mild disease is not indicated,” first author George Joy, MBBS, University College London, said in presenting the results at EuroCMR, the annual CMR congress of the European Association of Cardiovascular Imaging (EACVI).
Briefing comoderator Leyla Elif Sade, MD, University of Baskent, Ankara, Turkey, said, “This is the hot topic of our time because of obvious reasons and I think [this] study is quite important to avoid unnecessary further testing, surveillance testing, and to avoid a significant burden of health care costs.”
‘Alarming’ early data
Early cardiac magnetic resonance (CMR) studies in patients recovered from mild COVID-19 were “alarming,” Dr. Joy said.
As previously reported, one study showed cardiac abnormalities after mild COVID-19 in up to 78% of patients, with evidence of ongoing myocardial inflammation in 60%. The CMR findings correlated with elevations in troponin T by high-sensitivity assay (hs-TnT).
To investigate further, Dr. Joy and colleagues did a nested case-control study within the COVIDsortium, a prospective study of 731 health care workers from three London hospitals who underwent weekly symptom, polymerase chain reaction, and serology assessment over 4 months during the first wave of the pandemic.
A total of 157 (21.5%) participants seroconverted during the study period.
Six months after infection, 74 seropositive (median age, 39; 62% women) and 75 age-, sex-, and ethnicity-matched seronegative controls underwent cardiovascular phenotyping (comprehensive phantom-calibrated CMR and blood biomarkers). The analysis was blinded, using objective artificial intelligence analytics when available.
The results showed no statistically significant differences between seropositive and seronegative participants in cardiac structure (left ventricular volumes, mass, atrial area), function (ejection fraction, global longitudinal shortening, aortic distensibility), tissue characterization (T1, T2, extracellular volume fraction mapping, late gadolinium enhancement) or biomarkers (troponin, N-terminal pro–B-type natriuretic peptide).
Cardiovascular abnormalities were no more common in seropositive than seronegative otherwise healthy health care workers 6 months post mild SARS-CoV-2 infection. Measured abnormalities were “evenly distributed between both groups,” Dr. Joy said.
Therefore, it’s “important to reassure patients with mild SARS-CoV-2 infection regarding its cardiovascular effects,” Dr. Joy and colleagues concluded.
Limitations and caveats
They caution, however, that the study provides insight only into the short- to medium-term sequelae of patients aged 18-69 with mild COVID-19 who did not require hospitalization and had low numbers of comorbidities.
The study does not address the cardiovascular effects after severe COVID-19 infection requiring hospitalization or in those with multiple comorbid conditions, they noted. It also does not prove that apparently mild SARS-CoV-2 never causes chronic myocarditis.
“The study design would not distinguish between people who had sustained completely healed myocarditis and pericarditis and those in whom the heart had never been affected,” the researchers noted.
They pointed to a recent cross-sectional study of athletes 1-month post mild COVID-19 that found significant pericardial involvement (late enhancement and/or pericardial effusion), although no baseline pre-COVID-19 imaging was performed. In the current study at 6 months post infection the pericardium was normal.
The coauthors of a linked editorial say this study provides “welcome, reassuring information that in healthy individuals who experience mild infection with COVID-19, persisting evidence of cardiovascular complications is very uncommon. The results do not support cardiovascular screening in individuals with mild or asymptomatic infection with COVID-19.”
Colin Berry, PhD, and Kenneth Mangion, PhD, both from University of Glasgow, cautioned that the population is restricted to health care workers; therefore, the findings may not necessarily be generalized to a community population .
“Healthcare workers do not reflect the population of individuals most clinically affected by COVID-19 illness. The severity of acute COVID-19 infection is greatest in older individuals and those with preexisting health problems. Healthcare workers are not representative of the wider, unselected, at-risk, community population,” they pointed out.
Cardiovascular risk factors and concomitant health problems (heart and respiratory disease) may be more common in the community than in health care workers, and prior studies have highlighted their potential impact for disease pathogenesis in COVID-19.
Dr. Berry and Dr. Mangion also noted that women made up nearly two-thirds of the seropositive group. This may reflect a selection bias or may naturally reflect the fact that proportionately more women are asymptomatic or have milder forms of illness, whereas severe SARS-CoV-2 infection requiring hospitalization affects men to a greater degree.
COVIDsortium funding was donated by individuals, charitable trusts, and corporations including Goldman Sachs, Citadel and Citadel Securities, The Guy Foundation, GW Pharmaceuticals, Kusuma Trust, and Jagclif Charitable Trust, and enabled by Barts Charity with support from UCLH Charity. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Six months after mild SARS-CoV-2 infection in a representative health care workforce, no long-term cardiovascular sequelae were detected, compared with a matched SARS-CoV-2 seronegative group.
“Mild COVID-19 left no measurable cardiovascular impact on LV structure, function, scar burden, aortic stiffness, or serum biomarkers,” the researchers reported in an article published online May 8 in JACC: Cardiovascular Imaging.
“We provide societal reassurance and support for the position that screening in asymptomatic individuals following mild disease is not indicated,” first author George Joy, MBBS, University College London, said in presenting the results at EuroCMR, the annual CMR congress of the European Association of Cardiovascular Imaging (EACVI).
Briefing comoderator Leyla Elif Sade, MD, University of Baskent, Ankara, Turkey, said, “This is the hot topic of our time because of obvious reasons and I think [this] study is quite important to avoid unnecessary further testing, surveillance testing, and to avoid a significant burden of health care costs.”
‘Alarming’ early data
Early cardiac magnetic resonance (CMR) studies in patients recovered from mild COVID-19 were “alarming,” Dr. Joy said.
As previously reported, one study showed cardiac abnormalities after mild COVID-19 in up to 78% of patients, with evidence of ongoing myocardial inflammation in 60%. The CMR findings correlated with elevations in troponin T by high-sensitivity assay (hs-TnT).
To investigate further, Dr. Joy and colleagues did a nested case-control study within the COVIDsortium, a prospective study of 731 health care workers from three London hospitals who underwent weekly symptom, polymerase chain reaction, and serology assessment over 4 months during the first wave of the pandemic.
A total of 157 (21.5%) participants seroconverted during the study period.
Six months after infection, 74 seropositive (median age, 39; 62% women) and 75 age-, sex-, and ethnicity-matched seronegative controls underwent cardiovascular phenotyping (comprehensive phantom-calibrated CMR and blood biomarkers). The analysis was blinded, using objective artificial intelligence analytics when available.
The results showed no statistically significant differences between seropositive and seronegative participants in cardiac structure (left ventricular volumes, mass, atrial area), function (ejection fraction, global longitudinal shortening, aortic distensibility), tissue characterization (T1, T2, extracellular volume fraction mapping, late gadolinium enhancement) or biomarkers (troponin, N-terminal pro–B-type natriuretic peptide).
Cardiovascular abnormalities were no more common in seropositive than seronegative otherwise healthy health care workers 6 months post mild SARS-CoV-2 infection. Measured abnormalities were “evenly distributed between both groups,” Dr. Joy said.
Therefore, it’s “important to reassure patients with mild SARS-CoV-2 infection regarding its cardiovascular effects,” Dr. Joy and colleagues concluded.
Limitations and caveats
They caution, however, that the study provides insight only into the short- to medium-term sequelae of patients aged 18-69 with mild COVID-19 who did not require hospitalization and had low numbers of comorbidities.
The study does not address the cardiovascular effects after severe COVID-19 infection requiring hospitalization or in those with multiple comorbid conditions, they noted. It also does not prove that apparently mild SARS-CoV-2 never causes chronic myocarditis.
“The study design would not distinguish between people who had sustained completely healed myocarditis and pericarditis and those in whom the heart had never been affected,” the researchers noted.
They pointed to a recent cross-sectional study of athletes 1-month post mild COVID-19 that found significant pericardial involvement (late enhancement and/or pericardial effusion), although no baseline pre-COVID-19 imaging was performed. In the current study at 6 months post infection the pericardium was normal.
The coauthors of a linked editorial say this study provides “welcome, reassuring information that in healthy individuals who experience mild infection with COVID-19, persisting evidence of cardiovascular complications is very uncommon. The results do not support cardiovascular screening in individuals with mild or asymptomatic infection with COVID-19.”
Colin Berry, PhD, and Kenneth Mangion, PhD, both from University of Glasgow, cautioned that the population is restricted to health care workers; therefore, the findings may not necessarily be generalized to a community population .
“Healthcare workers do not reflect the population of individuals most clinically affected by COVID-19 illness. The severity of acute COVID-19 infection is greatest in older individuals and those with preexisting health problems. Healthcare workers are not representative of the wider, unselected, at-risk, community population,” they pointed out.
Cardiovascular risk factors and concomitant health problems (heart and respiratory disease) may be more common in the community than in health care workers, and prior studies have highlighted their potential impact for disease pathogenesis in COVID-19.
Dr. Berry and Dr. Mangion also noted that women made up nearly two-thirds of the seropositive group. This may reflect a selection bias or may naturally reflect the fact that proportionately more women are asymptomatic or have milder forms of illness, whereas severe SARS-CoV-2 infection requiring hospitalization affects men to a greater degree.
COVIDsortium funding was donated by individuals, charitable trusts, and corporations including Goldman Sachs, Citadel and Citadel Securities, The Guy Foundation, GW Pharmaceuticals, Kusuma Trust, and Jagclif Charitable Trust, and enabled by Barts Charity with support from UCLH Charity. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
What to know about COVID-19 vaccines and skin reactions
The good news is that these side effects tend to be minor and vanish within a few days, Esther Freeman, MD, PhD, said in a presentation at the American Academy of Dermatology Virtual Meeting Experience.
“The reality is actually very reassuring,” Dr. Freeman said, especially in light of what is currently known about when the rashes occur and how anaphylaxis is extremely uncommon. Now, she added, dermatologists can tell patients who had reactions to their initial vaccination that “we know you had this big reaction, and we know that it was upsetting and uncomfortable. But it may not happen the second time around. And if it does, [the reaction is] probably going to be smaller.”
Dr. Freeman, associate professor of dermatology at Harvard Medical School, Boston, highlighted a study published in the Journal of the American Academy of Dermatology that she coauthored with dermatologists across the United States. The researchers tracked 414 cutaneous reactions to the Moderna (83%) and Pfizer (17%) COVID-19 vaccines in a group of patients, which was 90% female, 78% White, and mostly from the United States. Their average age was 44 years. The cases were reported to the AAD–International League of Dermatological Societies registry of COVID-19 cutaneous manifestations.
While most were women, “it’s a little hard to know if this is really going to end up being a true finding,” said Dr. Freeman, the registry’s principal investigator and a member of the AAD’s COVID-19 Ad Hoc Task Force. “If you think about who got vaccinated early, it was health care providers, and the American health care workforce is over 70% female. So I think there’s a little bit of bias here. There may also be a bias because women may be slightly more likely to report or go to their health care provider for a rash.”
Delayed large local reactions were the most common, accounting for 66% (175 cases) of the 267 skin reactions reported after the first Moderna vaccine dose and 30% (31 cases) of the 102 reactions reported after the second dose. These reactions represented 15% (5 cases) of the 34 skin reactions reported after the first Pfizer vaccine dose and 18% (7 cases) of the 40 reactions after the second dose.
There are two peaks with that first dose, Dr. Freeman said. “There’s a peak around day 2 or 3. And there’s another peak around day 7 or 8 with some of these reactions. Only 27% who had a reaction with the first dose had the same reaction with the second.” She added that these reactions “are not cellulitis and don’t require antibiotics.”
Other more common reactions included local injection-site reactions (swelling, erythema, and pain), urticaria (after 24 hours in almost all cases, occurring at a higher rate in patients who received the Pfizer vaccine), and morbilliform eruptions.
Dr. Freeman said that patients may experience redness and swelling in the hands and feet that can be “very uncomfortable.” She described one patient “who was having a hard time actually closing his fist, just because of the amount of swelling and redness in his hand. It did resolve, and it’s important to reassure your patients it will go away.”
According to this study, less common reports of other cutaneous findings with both vaccines included 9 reports of swelling at the site of cosmetic fillers, 8 reports of pernio/chilblains, 10 reports of varicella zoster, 4 reports of herpes simplex flares, 4 pityriasis rosea–like reactions, and 4 rashes in infants of vaccinated breastfeeding mothers.
The study noted that “patients responded well to topical corticosteroids, oral antihistamines, and/or pain-relieving medications. These reactions resolved after a median of 3-4 days.”
It’s important to understand that none of the patients developed anaphylaxis after the second dose even if they’d had a reaction to the first dose, Dr. Freeman said. “But I should point out that we’re talking about reactions that have started more than 4 hours after the vaccine. If a rash such as a urticaria specifically starts within 4 hours of vaccination, that’s in a different category. Those are considered more immediate allergic reactions, and those patients need to be seen by allergy before a second dose.”
Dr. Freeman added that “it’s really interesting to think about how our bodies are really reacting to the vaccine in a way that’s mimicking our body’s reactions to COVID-19.” For example, some patients who got vaccinated developed chilblains similar to the “COVID toes” described in infected patients, apparently as part of the body’s immune response to the virus. “We’ve seen this in patients who actually had COVID and had prior COVID toes and then actually got a flare with their vaccine. And then we’ve also seen it in patients who never had COVID.”
In regard to general advice for patients, she said, “I do still encourage my patients who previously had COVID to go ahead and get the vaccine even if they had a skin manifestation with COVID.”
Shari Lipner, MD, PhD, associate professor of clinical dermatology, Weill Cornell Medicine, New York, said she has have seen only a handful of cases of delayed large local reactions and local injection site reactions after COVID-19 vaccination. “I have seen a significant number of cases of acute urticaria following the first and second doses,” she said in an interview. “However, it is important to keep in mind that we cannot determine cause and effect for the cases of acute urticaria. They may or may not be vaccine related.”
Fortunately, none of the adverse effects she’s seen have been severe. “It is important that dermatologists educate the public and their patients that most people do not develop any skin reaction in response to the vaccine,” she said. In the minority who do, “reactions tend to be mild and are not life-threatening. Many of these skin reactions resolve on their own without treatment.”
She added that “patients with pernio/chilblains or herpes zoster following vaccination should be referred by a board-certified dermatologist for prompt treatment and to avoid sequelae.”
‘COVID vaccine arm’
Delayed local reactions to the Moderna vaccine were also described in a report published online on May 12, 2021, in JAMA Dermatology, after the AAD meeting, in 16 patients referred to the Yale New Haven (Conn.) Hospital Dermatology service who experienced delayed localized cutaneous hypersensitivity reactions a median of 7 days after receiving the vaccine (range, 2-12 days), from Jan. 20 to Feb. 12, 2021. No such cases were reported in Pfizer vaccine recipients.
Of the 16 patients, whose median age was 38 years and who were mostly women, 15 developed the reaction after the first dose, described as “pruritic and variably painful erythematous reactions near the injection site,” which lasted a median of 5 days (range, 1-21 days). After the second dose, 12 of the 16 patients developed injection-site reactions (including one patient who had no reaction after dose 1), a median of 2 days after the vaccine was administered (range, 0-5 days). Histologic results of a biopsy in one patient with a reaction to the second dose “ demonstrated mild predominantly perivascular and focal interstitial mixed infiltrate with lymphocytes and eosinophils consistent with a dermal hypersensitivity reaction,” wrote Alicia J. Little, MD, PhD, of the department of dermatology, Yale University, New Haven, and coauthors.
Compared with immediate hypersensitivity reactions, occurring within 4 hours of vaccination, such as anaphylaxis and urticaria, they concluded that “these delayed localized hypersensitivity reactions are not a contraindication to subsequent vaccination,” and they proposed that they be named “COVID vaccine arm.”
Dr. Freeman reported no disclosures. Dr. Lipner also had no relevant disclosures. Dr. Little reported receiving a grant from the National Center for Advancing Translational Science and a Women’s Health Career Development Award from the Dermatology Foundation while the study was conducted; another author reported equity in Johnson & Johnson in his spouse’s retirement fund outside the submitted work.
The good news is that these side effects tend to be minor and vanish within a few days, Esther Freeman, MD, PhD, said in a presentation at the American Academy of Dermatology Virtual Meeting Experience.
“The reality is actually very reassuring,” Dr. Freeman said, especially in light of what is currently known about when the rashes occur and how anaphylaxis is extremely uncommon. Now, she added, dermatologists can tell patients who had reactions to their initial vaccination that “we know you had this big reaction, and we know that it was upsetting and uncomfortable. But it may not happen the second time around. And if it does, [the reaction is] probably going to be smaller.”
Dr. Freeman, associate professor of dermatology at Harvard Medical School, Boston, highlighted a study published in the Journal of the American Academy of Dermatology that she coauthored with dermatologists across the United States. The researchers tracked 414 cutaneous reactions to the Moderna (83%) and Pfizer (17%) COVID-19 vaccines in a group of patients, which was 90% female, 78% White, and mostly from the United States. Their average age was 44 years. The cases were reported to the AAD–International League of Dermatological Societies registry of COVID-19 cutaneous manifestations.
While most were women, “it’s a little hard to know if this is really going to end up being a true finding,” said Dr. Freeman, the registry’s principal investigator and a member of the AAD’s COVID-19 Ad Hoc Task Force. “If you think about who got vaccinated early, it was health care providers, and the American health care workforce is over 70% female. So I think there’s a little bit of bias here. There may also be a bias because women may be slightly more likely to report or go to their health care provider for a rash.”
Delayed large local reactions were the most common, accounting for 66% (175 cases) of the 267 skin reactions reported after the first Moderna vaccine dose and 30% (31 cases) of the 102 reactions reported after the second dose. These reactions represented 15% (5 cases) of the 34 skin reactions reported after the first Pfizer vaccine dose and 18% (7 cases) of the 40 reactions after the second dose.
There are two peaks with that first dose, Dr. Freeman said. “There’s a peak around day 2 or 3. And there’s another peak around day 7 or 8 with some of these reactions. Only 27% who had a reaction with the first dose had the same reaction with the second.” She added that these reactions “are not cellulitis and don’t require antibiotics.”
Other more common reactions included local injection-site reactions (swelling, erythema, and pain), urticaria (after 24 hours in almost all cases, occurring at a higher rate in patients who received the Pfizer vaccine), and morbilliform eruptions.
Dr. Freeman said that patients may experience redness and swelling in the hands and feet that can be “very uncomfortable.” She described one patient “who was having a hard time actually closing his fist, just because of the amount of swelling and redness in his hand. It did resolve, and it’s important to reassure your patients it will go away.”
According to this study, less common reports of other cutaneous findings with both vaccines included 9 reports of swelling at the site of cosmetic fillers, 8 reports of pernio/chilblains, 10 reports of varicella zoster, 4 reports of herpes simplex flares, 4 pityriasis rosea–like reactions, and 4 rashes in infants of vaccinated breastfeeding mothers.
The study noted that “patients responded well to topical corticosteroids, oral antihistamines, and/or pain-relieving medications. These reactions resolved after a median of 3-4 days.”
It’s important to understand that none of the patients developed anaphylaxis after the second dose even if they’d had a reaction to the first dose, Dr. Freeman said. “But I should point out that we’re talking about reactions that have started more than 4 hours after the vaccine. If a rash such as a urticaria specifically starts within 4 hours of vaccination, that’s in a different category. Those are considered more immediate allergic reactions, and those patients need to be seen by allergy before a second dose.”
Dr. Freeman added that “it’s really interesting to think about how our bodies are really reacting to the vaccine in a way that’s mimicking our body’s reactions to COVID-19.” For example, some patients who got vaccinated developed chilblains similar to the “COVID toes” described in infected patients, apparently as part of the body’s immune response to the virus. “We’ve seen this in patients who actually had COVID and had prior COVID toes and then actually got a flare with their vaccine. And then we’ve also seen it in patients who never had COVID.”
In regard to general advice for patients, she said, “I do still encourage my patients who previously had COVID to go ahead and get the vaccine even if they had a skin manifestation with COVID.”
Shari Lipner, MD, PhD, associate professor of clinical dermatology, Weill Cornell Medicine, New York, said she has have seen only a handful of cases of delayed large local reactions and local injection site reactions after COVID-19 vaccination. “I have seen a significant number of cases of acute urticaria following the first and second doses,” she said in an interview. “However, it is important to keep in mind that we cannot determine cause and effect for the cases of acute urticaria. They may or may not be vaccine related.”
Fortunately, none of the adverse effects she’s seen have been severe. “It is important that dermatologists educate the public and their patients that most people do not develop any skin reaction in response to the vaccine,” she said. In the minority who do, “reactions tend to be mild and are not life-threatening. Many of these skin reactions resolve on their own without treatment.”
She added that “patients with pernio/chilblains or herpes zoster following vaccination should be referred by a board-certified dermatologist for prompt treatment and to avoid sequelae.”
‘COVID vaccine arm’
Delayed local reactions to the Moderna vaccine were also described in a report published online on May 12, 2021, in JAMA Dermatology, after the AAD meeting, in 16 patients referred to the Yale New Haven (Conn.) Hospital Dermatology service who experienced delayed localized cutaneous hypersensitivity reactions a median of 7 days after receiving the vaccine (range, 2-12 days), from Jan. 20 to Feb. 12, 2021. No such cases were reported in Pfizer vaccine recipients.
Of the 16 patients, whose median age was 38 years and who were mostly women, 15 developed the reaction after the first dose, described as “pruritic and variably painful erythematous reactions near the injection site,” which lasted a median of 5 days (range, 1-21 days). After the second dose, 12 of the 16 patients developed injection-site reactions (including one patient who had no reaction after dose 1), a median of 2 days after the vaccine was administered (range, 0-5 days). Histologic results of a biopsy in one patient with a reaction to the second dose “ demonstrated mild predominantly perivascular and focal interstitial mixed infiltrate with lymphocytes and eosinophils consistent with a dermal hypersensitivity reaction,” wrote Alicia J. Little, MD, PhD, of the department of dermatology, Yale University, New Haven, and coauthors.
Compared with immediate hypersensitivity reactions, occurring within 4 hours of vaccination, such as anaphylaxis and urticaria, they concluded that “these delayed localized hypersensitivity reactions are not a contraindication to subsequent vaccination,” and they proposed that they be named “COVID vaccine arm.”
Dr. Freeman reported no disclosures. Dr. Lipner also had no relevant disclosures. Dr. Little reported receiving a grant from the National Center for Advancing Translational Science and a Women’s Health Career Development Award from the Dermatology Foundation while the study was conducted; another author reported equity in Johnson & Johnson in his spouse’s retirement fund outside the submitted work.
The good news is that these side effects tend to be minor and vanish within a few days, Esther Freeman, MD, PhD, said in a presentation at the American Academy of Dermatology Virtual Meeting Experience.
“The reality is actually very reassuring,” Dr. Freeman said, especially in light of what is currently known about when the rashes occur and how anaphylaxis is extremely uncommon. Now, she added, dermatologists can tell patients who had reactions to their initial vaccination that “we know you had this big reaction, and we know that it was upsetting and uncomfortable. But it may not happen the second time around. And if it does, [the reaction is] probably going to be smaller.”
Dr. Freeman, associate professor of dermatology at Harvard Medical School, Boston, highlighted a study published in the Journal of the American Academy of Dermatology that she coauthored with dermatologists across the United States. The researchers tracked 414 cutaneous reactions to the Moderna (83%) and Pfizer (17%) COVID-19 vaccines in a group of patients, which was 90% female, 78% White, and mostly from the United States. Their average age was 44 years. The cases were reported to the AAD–International League of Dermatological Societies registry of COVID-19 cutaneous manifestations.
While most were women, “it’s a little hard to know if this is really going to end up being a true finding,” said Dr. Freeman, the registry’s principal investigator and a member of the AAD’s COVID-19 Ad Hoc Task Force. “If you think about who got vaccinated early, it was health care providers, and the American health care workforce is over 70% female. So I think there’s a little bit of bias here. There may also be a bias because women may be slightly more likely to report or go to their health care provider for a rash.”
Delayed large local reactions were the most common, accounting for 66% (175 cases) of the 267 skin reactions reported after the first Moderna vaccine dose and 30% (31 cases) of the 102 reactions reported after the second dose. These reactions represented 15% (5 cases) of the 34 skin reactions reported after the first Pfizer vaccine dose and 18% (7 cases) of the 40 reactions after the second dose.
There are two peaks with that first dose, Dr. Freeman said. “There’s a peak around day 2 or 3. And there’s another peak around day 7 or 8 with some of these reactions. Only 27% who had a reaction with the first dose had the same reaction with the second.” She added that these reactions “are not cellulitis and don’t require antibiotics.”
Other more common reactions included local injection-site reactions (swelling, erythema, and pain), urticaria (after 24 hours in almost all cases, occurring at a higher rate in patients who received the Pfizer vaccine), and morbilliform eruptions.
Dr. Freeman said that patients may experience redness and swelling in the hands and feet that can be “very uncomfortable.” She described one patient “who was having a hard time actually closing his fist, just because of the amount of swelling and redness in his hand. It did resolve, and it’s important to reassure your patients it will go away.”
According to this study, less common reports of other cutaneous findings with both vaccines included 9 reports of swelling at the site of cosmetic fillers, 8 reports of pernio/chilblains, 10 reports of varicella zoster, 4 reports of herpes simplex flares, 4 pityriasis rosea–like reactions, and 4 rashes in infants of vaccinated breastfeeding mothers.
The study noted that “patients responded well to topical corticosteroids, oral antihistamines, and/or pain-relieving medications. These reactions resolved after a median of 3-4 days.”
It’s important to understand that none of the patients developed anaphylaxis after the second dose even if they’d had a reaction to the first dose, Dr. Freeman said. “But I should point out that we’re talking about reactions that have started more than 4 hours after the vaccine. If a rash such as a urticaria specifically starts within 4 hours of vaccination, that’s in a different category. Those are considered more immediate allergic reactions, and those patients need to be seen by allergy before a second dose.”
Dr. Freeman added that “it’s really interesting to think about how our bodies are really reacting to the vaccine in a way that’s mimicking our body’s reactions to COVID-19.” For example, some patients who got vaccinated developed chilblains similar to the “COVID toes” described in infected patients, apparently as part of the body’s immune response to the virus. “We’ve seen this in patients who actually had COVID and had prior COVID toes and then actually got a flare with their vaccine. And then we’ve also seen it in patients who never had COVID.”
In regard to general advice for patients, she said, “I do still encourage my patients who previously had COVID to go ahead and get the vaccine even if they had a skin manifestation with COVID.”
Shari Lipner, MD, PhD, associate professor of clinical dermatology, Weill Cornell Medicine, New York, said she has have seen only a handful of cases of delayed large local reactions and local injection site reactions after COVID-19 vaccination. “I have seen a significant number of cases of acute urticaria following the first and second doses,” she said in an interview. “However, it is important to keep in mind that we cannot determine cause and effect for the cases of acute urticaria. They may or may not be vaccine related.”
Fortunately, none of the adverse effects she’s seen have been severe. “It is important that dermatologists educate the public and their patients that most people do not develop any skin reaction in response to the vaccine,” she said. In the minority who do, “reactions tend to be mild and are not life-threatening. Many of these skin reactions resolve on their own without treatment.”
She added that “patients with pernio/chilblains or herpes zoster following vaccination should be referred by a board-certified dermatologist for prompt treatment and to avoid sequelae.”
‘COVID vaccine arm’
Delayed local reactions to the Moderna vaccine were also described in a report published online on May 12, 2021, in JAMA Dermatology, after the AAD meeting, in 16 patients referred to the Yale New Haven (Conn.) Hospital Dermatology service who experienced delayed localized cutaneous hypersensitivity reactions a median of 7 days after receiving the vaccine (range, 2-12 days), from Jan. 20 to Feb. 12, 2021. No such cases were reported in Pfizer vaccine recipients.
Of the 16 patients, whose median age was 38 years and who were mostly women, 15 developed the reaction after the first dose, described as “pruritic and variably painful erythematous reactions near the injection site,” which lasted a median of 5 days (range, 1-21 days). After the second dose, 12 of the 16 patients developed injection-site reactions (including one patient who had no reaction after dose 1), a median of 2 days after the vaccine was administered (range, 0-5 days). Histologic results of a biopsy in one patient with a reaction to the second dose “ demonstrated mild predominantly perivascular and focal interstitial mixed infiltrate with lymphocytes and eosinophils consistent with a dermal hypersensitivity reaction,” wrote Alicia J. Little, MD, PhD, of the department of dermatology, Yale University, New Haven, and coauthors.
Compared with immediate hypersensitivity reactions, occurring within 4 hours of vaccination, such as anaphylaxis and urticaria, they concluded that “these delayed localized hypersensitivity reactions are not a contraindication to subsequent vaccination,” and they proposed that they be named “COVID vaccine arm.”
Dr. Freeman reported no disclosures. Dr. Lipner also had no relevant disclosures. Dr. Little reported receiving a grant from the National Center for Advancing Translational Science and a Women’s Health Career Development Award from the Dermatology Foundation while the study was conducted; another author reported equity in Johnson & Johnson in his spouse’s retirement fund outside the submitted work.
FROM AAD VMX 2021
Dr. Fauci: Feds may ease indoor mask mandates soon
Federal guidance on indoor mask use may change soon, Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, said on May 9.
He was asked whether it’s time to start relaxing indoor mask requirements.
“I think so, and I think you’re going to probably be seeing that as we go along and as more people get vaccinated,” Dr. Fauci said on ABC News’s This Week.Nearly 150 million adults in the United States – or about 58% of the adult population – have received at least one COVID-19 vaccine dose, according to the latest CDC tally. About 113 million adults, or 44%, are considered fully vaccinated.
“The CDC will be, you know, almost in real time … updating their recommendations and their guidelines,” Dr. Fauci said.
In April, the CDC relaxed its guidance for those who have been vaccinated against COVID-19. Those who have gotten a shot don’t need to wear a mask outdoors or in small indoor gatherings with other vaccinated people, but both vaccinated and unvaccinated people are still advised to wear masks in indoor public spaces.
“We do need to start being more liberal as we get more people vaccinated,” Dr. Fauci said. “As you get more people vaccinated, the number of cases per day will absolutely go down.”
The United States is averaging about 43,000 cases per day, he said, adding that the cases need to be “much, much lower.” When the case numbers drop and vaccination numbers increase, the risk of infection will fall dramatically indoors and outdoors, he said.
Even after the pandemic, though, wearing masks could become a seasonal habit, Dr. Fauci said May 9 on NBC News’s Meet the Press.“I think people have gotten used to the fact that wearing masks, clearly if you look at the data, it diminishes respiratory diseases. We’ve had practically a nonexistent flu season this year,” he said.
“So it is conceivable that as we go on, a year or 2 or more from now, that during certain seasonal periods when you have respiratory-borne viruses like the flu, people might actually elect to wear masks to diminish the likelihood that you’ll spread these respiratory-borne diseases,” he said.
Dr. Fauci was asked about indoor mask guidelines on May 9 after former FDA Commissioner Scott Gottlieb, MD, said face mask requirements should be relaxed.
“Certainly outdoors, we shouldn’t be putting limits on gatherings anymore,” Dr. Gottlieb said on CBS News’s Face the Nation.“The states where prevalence is low, vaccination rates are high, we have good testing in place, and we’re identifying infections, I think we could start lifting these restrictions indoors as well, on a broad basis,” he said.
Lifting pandemic-related restrictions in areas where they’re no longer necessary could also encourage people to implement them again if cases increase during future surges, such as this fall or winter, Dr. Gottlieb said.
At the same time, Americans should continue to follow CDC guidance and wait for new guidelines before changing their indoor mask use, Jeffrey Zients, the White House COVID-19 response coordinator, said on CNN’s State of the Union on May 9.
“We all want to get back to a normal lifestyle,” he said. “I think we’re on the path to do that, but stay disciplined, and let’s take advantage of the new privilege of being vaccinated and not wearing masks outdoors, for example, unless you’re in a crowded place.”
Mr. Zients pointed to President Joe Biden’s goal for 70% of adults to receive at least one vaccine dose by July 4.
“As we all move toward that 70% goal, there will be more and more advantages to being vaccinated,” he said. “And if you’re not vaccinated, you’re not protected.”
A version of this article first appeared on WebMD.com.
Federal guidance on indoor mask use may change soon, Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, said on May 9.
He was asked whether it’s time to start relaxing indoor mask requirements.
“I think so, and I think you’re going to probably be seeing that as we go along and as more people get vaccinated,” Dr. Fauci said on ABC News’s This Week.Nearly 150 million adults in the United States – or about 58% of the adult population – have received at least one COVID-19 vaccine dose, according to the latest CDC tally. About 113 million adults, or 44%, are considered fully vaccinated.
“The CDC will be, you know, almost in real time … updating their recommendations and their guidelines,” Dr. Fauci said.
In April, the CDC relaxed its guidance for those who have been vaccinated against COVID-19. Those who have gotten a shot don’t need to wear a mask outdoors or in small indoor gatherings with other vaccinated people, but both vaccinated and unvaccinated people are still advised to wear masks in indoor public spaces.
“We do need to start being more liberal as we get more people vaccinated,” Dr. Fauci said. “As you get more people vaccinated, the number of cases per day will absolutely go down.”
The United States is averaging about 43,000 cases per day, he said, adding that the cases need to be “much, much lower.” When the case numbers drop and vaccination numbers increase, the risk of infection will fall dramatically indoors and outdoors, he said.
Even after the pandemic, though, wearing masks could become a seasonal habit, Dr. Fauci said May 9 on NBC News’s Meet the Press.“I think people have gotten used to the fact that wearing masks, clearly if you look at the data, it diminishes respiratory diseases. We’ve had practically a nonexistent flu season this year,” he said.
“So it is conceivable that as we go on, a year or 2 or more from now, that during certain seasonal periods when you have respiratory-borne viruses like the flu, people might actually elect to wear masks to diminish the likelihood that you’ll spread these respiratory-borne diseases,” he said.
Dr. Fauci was asked about indoor mask guidelines on May 9 after former FDA Commissioner Scott Gottlieb, MD, said face mask requirements should be relaxed.
“Certainly outdoors, we shouldn’t be putting limits on gatherings anymore,” Dr. Gottlieb said on CBS News’s Face the Nation.“The states where prevalence is low, vaccination rates are high, we have good testing in place, and we’re identifying infections, I think we could start lifting these restrictions indoors as well, on a broad basis,” he said.
Lifting pandemic-related restrictions in areas where they’re no longer necessary could also encourage people to implement them again if cases increase during future surges, such as this fall or winter, Dr. Gottlieb said.
At the same time, Americans should continue to follow CDC guidance and wait for new guidelines before changing their indoor mask use, Jeffrey Zients, the White House COVID-19 response coordinator, said on CNN’s State of the Union on May 9.
“We all want to get back to a normal lifestyle,” he said. “I think we’re on the path to do that, but stay disciplined, and let’s take advantage of the new privilege of being vaccinated and not wearing masks outdoors, for example, unless you’re in a crowded place.”
Mr. Zients pointed to President Joe Biden’s goal for 70% of adults to receive at least one vaccine dose by July 4.
“As we all move toward that 70% goal, there will be more and more advantages to being vaccinated,” he said. “And if you’re not vaccinated, you’re not protected.”
A version of this article first appeared on WebMD.com.
Federal guidance on indoor mask use may change soon, Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, said on May 9.
He was asked whether it’s time to start relaxing indoor mask requirements.
“I think so, and I think you’re going to probably be seeing that as we go along and as more people get vaccinated,” Dr. Fauci said on ABC News’s This Week.Nearly 150 million adults in the United States – or about 58% of the adult population – have received at least one COVID-19 vaccine dose, according to the latest CDC tally. About 113 million adults, or 44%, are considered fully vaccinated.
“The CDC will be, you know, almost in real time … updating their recommendations and their guidelines,” Dr. Fauci said.
In April, the CDC relaxed its guidance for those who have been vaccinated against COVID-19. Those who have gotten a shot don’t need to wear a mask outdoors or in small indoor gatherings with other vaccinated people, but both vaccinated and unvaccinated people are still advised to wear masks in indoor public spaces.
“We do need to start being more liberal as we get more people vaccinated,” Dr. Fauci said. “As you get more people vaccinated, the number of cases per day will absolutely go down.”
The United States is averaging about 43,000 cases per day, he said, adding that the cases need to be “much, much lower.” When the case numbers drop and vaccination numbers increase, the risk of infection will fall dramatically indoors and outdoors, he said.
Even after the pandemic, though, wearing masks could become a seasonal habit, Dr. Fauci said May 9 on NBC News’s Meet the Press.“I think people have gotten used to the fact that wearing masks, clearly if you look at the data, it diminishes respiratory diseases. We’ve had practically a nonexistent flu season this year,” he said.
“So it is conceivable that as we go on, a year or 2 or more from now, that during certain seasonal periods when you have respiratory-borne viruses like the flu, people might actually elect to wear masks to diminish the likelihood that you’ll spread these respiratory-borne diseases,” he said.
Dr. Fauci was asked about indoor mask guidelines on May 9 after former FDA Commissioner Scott Gottlieb, MD, said face mask requirements should be relaxed.
“Certainly outdoors, we shouldn’t be putting limits on gatherings anymore,” Dr. Gottlieb said on CBS News’s Face the Nation.“The states where prevalence is low, vaccination rates are high, we have good testing in place, and we’re identifying infections, I think we could start lifting these restrictions indoors as well, on a broad basis,” he said.
Lifting pandemic-related restrictions in areas where they’re no longer necessary could also encourage people to implement them again if cases increase during future surges, such as this fall or winter, Dr. Gottlieb said.
At the same time, Americans should continue to follow CDC guidance and wait for new guidelines before changing their indoor mask use, Jeffrey Zients, the White House COVID-19 response coordinator, said on CNN’s State of the Union on May 9.
“We all want to get back to a normal lifestyle,” he said. “I think we’re on the path to do that, but stay disciplined, and let’s take advantage of the new privilege of being vaccinated and not wearing masks outdoors, for example, unless you’re in a crowded place.”
Mr. Zients pointed to President Joe Biden’s goal for 70% of adults to receive at least one vaccine dose by July 4.
“As we all move toward that 70% goal, there will be more and more advantages to being vaccinated,” he said. “And if you’re not vaccinated, you’re not protected.”
A version of this article first appeared on WebMD.com.
FDA authorizes Pfizer COVID vaccine for teens 12-15
The Food and Drug Administration on May 10 granted emergency use authorization (EUA) for the Pfizer coronavirus vaccine to be given to children 12-15 years old.
The much-expected decision increases the likelihood that schools in the United States will fully reopen in the fall – a goal of both the Biden and Trump administrations.
Acting FDA Commissioner Janet Woodcock, MD, called the decision “a significant step” in “returning to a sense of normalcy.”
“Today’s action allows for a younger population to be protected from COVID-19, bringing us closer to returning to a sense of normalcy and to ending the pandemic,” she said in a statement. “Parents and guardians can rest assured that the agency undertook a rigorous and thorough review of all available data, as we have with all of our COVID-19 vaccine emergency use authorizations.”
The Pfizer adolescent vaccine is not yet a done deal, though.
Next, the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices will decide on May 12 whether to recommend use of the vaccine in this age group. After that, CDC Director Rochelle Walensky, MD, will decide whether to give the green light for the vaccine to be administered to that age group.
The FDA action on May 10 amends the Dec. 11, 2020, emergency use authorization that allowed the Pfizer vaccine to be given to people 16 and older. Pfizer was the first company to receive an EUA for its adult vaccine and is the first to receive authorization for its adolescent vaccine. Pfizer is conducting clinical trials on much younger children, too.
The Moderna and Johnson & Johnson vaccines are authorized for people 18 and up. Moderna also has launched clinical trials in children.
Most health experts have said the United States needs to vaccinate children before the COVID-19 pandemic can truly be brought under control. The 12- to 15-year-old group represents 17 million people, about 5% of the population. Thus far, 58% of U.S. adults have had at least one dose of a vaccine and 34.8% of all Americans are fully vaccinated.
American Academy of Pediatrics President Lee Savio Beers, MD, praised the agency’s decision, calling it a “critically important step in bringing life-saving vaccines to children and adolescents. Our youngest generations have shouldered heavy burdens over the past year, and the vaccine is a hopeful sign that they will be able to begin to experience all the activities that are so important for their health and development.”
President Joe Biden recently announced a new strategy for expanding vaccinations in which vaccinating 12- to 15-year-olds was a key component. He said the administration was ready to ship the adolescent vaccine directly to pharmacies and pediatricians to speed up the vaccination rate.
In March, Anthony S. Fauci, MD, told a Senate committee, “We don’t really know what that magical point of herd immunity is, but we do know that if we get the overwhelming population vaccinated, we’re going to be in good shape. … We ultimately would like to get and have to get children into that mix.”
Pfizer submitted data to the FDA in late March showing its mRNA vaccine was 100% effective at preventing COVID-19 infection in children ages 12-15 in clinical trials.
Though most children have milder symptoms when infected with the coronavirus, about 1.5 million cases in children aged 11-17 were reported to the CDC between March 1, 2020, and April 30 of this year, the FDA news release said.
Albert Bourla, CEO of Pfizer, tweeted that “today brings very encouraging news for families and adolescents across the United States.
“While this is a meaningful step forward, we are still in a critical period of combating #COVID19 around the world. In the coming weeks, we hope to continue to receive authorizations from global regulators to support worldwide vaccination efforts,” he said.
“It’s essential for children to be vaccinated against COVID-19. According to data compiled by the AAP and Children’s Hospital Association, more than 3.8 million children have tested positive for COVID-19 in the United States since the start of the pandemic,” said Dr. Savio Beers. “While fewer children than adults have suffered the most severe disease, this is not a benign disease in children. Thousands of children have been hospitalized, and hundreds have died. We will soon have a very safe, highly effective vaccine that can prevent so much suffering. I encourage parents to talk with their pediatricians about how to get the vaccine for their adolescents as soon as they are eligible.”
A version of this article first appeared on Medscape.com.
The Food and Drug Administration on May 10 granted emergency use authorization (EUA) for the Pfizer coronavirus vaccine to be given to children 12-15 years old.
The much-expected decision increases the likelihood that schools in the United States will fully reopen in the fall – a goal of both the Biden and Trump administrations.
Acting FDA Commissioner Janet Woodcock, MD, called the decision “a significant step” in “returning to a sense of normalcy.”
“Today’s action allows for a younger population to be protected from COVID-19, bringing us closer to returning to a sense of normalcy and to ending the pandemic,” she said in a statement. “Parents and guardians can rest assured that the agency undertook a rigorous and thorough review of all available data, as we have with all of our COVID-19 vaccine emergency use authorizations.”
The Pfizer adolescent vaccine is not yet a done deal, though.
Next, the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices will decide on May 12 whether to recommend use of the vaccine in this age group. After that, CDC Director Rochelle Walensky, MD, will decide whether to give the green light for the vaccine to be administered to that age group.
The FDA action on May 10 amends the Dec. 11, 2020, emergency use authorization that allowed the Pfizer vaccine to be given to people 16 and older. Pfizer was the first company to receive an EUA for its adult vaccine and is the first to receive authorization for its adolescent vaccine. Pfizer is conducting clinical trials on much younger children, too.
The Moderna and Johnson & Johnson vaccines are authorized for people 18 and up. Moderna also has launched clinical trials in children.
Most health experts have said the United States needs to vaccinate children before the COVID-19 pandemic can truly be brought under control. The 12- to 15-year-old group represents 17 million people, about 5% of the population. Thus far, 58% of U.S. adults have had at least one dose of a vaccine and 34.8% of all Americans are fully vaccinated.
American Academy of Pediatrics President Lee Savio Beers, MD, praised the agency’s decision, calling it a “critically important step in bringing life-saving vaccines to children and adolescents. Our youngest generations have shouldered heavy burdens over the past year, and the vaccine is a hopeful sign that they will be able to begin to experience all the activities that are so important for their health and development.”
President Joe Biden recently announced a new strategy for expanding vaccinations in which vaccinating 12- to 15-year-olds was a key component. He said the administration was ready to ship the adolescent vaccine directly to pharmacies and pediatricians to speed up the vaccination rate.
In March, Anthony S. Fauci, MD, told a Senate committee, “We don’t really know what that magical point of herd immunity is, but we do know that if we get the overwhelming population vaccinated, we’re going to be in good shape. … We ultimately would like to get and have to get children into that mix.”
Pfizer submitted data to the FDA in late March showing its mRNA vaccine was 100% effective at preventing COVID-19 infection in children ages 12-15 in clinical trials.
Though most children have milder symptoms when infected with the coronavirus, about 1.5 million cases in children aged 11-17 were reported to the CDC between March 1, 2020, and April 30 of this year, the FDA news release said.
Albert Bourla, CEO of Pfizer, tweeted that “today brings very encouraging news for families and adolescents across the United States.
“While this is a meaningful step forward, we are still in a critical period of combating #COVID19 around the world. In the coming weeks, we hope to continue to receive authorizations from global regulators to support worldwide vaccination efforts,” he said.
“It’s essential for children to be vaccinated against COVID-19. According to data compiled by the AAP and Children’s Hospital Association, more than 3.8 million children have tested positive for COVID-19 in the United States since the start of the pandemic,” said Dr. Savio Beers. “While fewer children than adults have suffered the most severe disease, this is not a benign disease in children. Thousands of children have been hospitalized, and hundreds have died. We will soon have a very safe, highly effective vaccine that can prevent so much suffering. I encourage parents to talk with their pediatricians about how to get the vaccine for their adolescents as soon as they are eligible.”
A version of this article first appeared on Medscape.com.
The Food and Drug Administration on May 10 granted emergency use authorization (EUA) for the Pfizer coronavirus vaccine to be given to children 12-15 years old.
The much-expected decision increases the likelihood that schools in the United States will fully reopen in the fall – a goal of both the Biden and Trump administrations.
Acting FDA Commissioner Janet Woodcock, MD, called the decision “a significant step” in “returning to a sense of normalcy.”
“Today’s action allows for a younger population to be protected from COVID-19, bringing us closer to returning to a sense of normalcy and to ending the pandemic,” she said in a statement. “Parents and guardians can rest assured that the agency undertook a rigorous and thorough review of all available data, as we have with all of our COVID-19 vaccine emergency use authorizations.”
The Pfizer adolescent vaccine is not yet a done deal, though.
Next, the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices will decide on May 12 whether to recommend use of the vaccine in this age group. After that, CDC Director Rochelle Walensky, MD, will decide whether to give the green light for the vaccine to be administered to that age group.
The FDA action on May 10 amends the Dec. 11, 2020, emergency use authorization that allowed the Pfizer vaccine to be given to people 16 and older. Pfizer was the first company to receive an EUA for its adult vaccine and is the first to receive authorization for its adolescent vaccine. Pfizer is conducting clinical trials on much younger children, too.
The Moderna and Johnson & Johnson vaccines are authorized for people 18 and up. Moderna also has launched clinical trials in children.
Most health experts have said the United States needs to vaccinate children before the COVID-19 pandemic can truly be brought under control. The 12- to 15-year-old group represents 17 million people, about 5% of the population. Thus far, 58% of U.S. adults have had at least one dose of a vaccine and 34.8% of all Americans are fully vaccinated.
American Academy of Pediatrics President Lee Savio Beers, MD, praised the agency’s decision, calling it a “critically important step in bringing life-saving vaccines to children and adolescents. Our youngest generations have shouldered heavy burdens over the past year, and the vaccine is a hopeful sign that they will be able to begin to experience all the activities that are so important for their health and development.”
President Joe Biden recently announced a new strategy for expanding vaccinations in which vaccinating 12- to 15-year-olds was a key component. He said the administration was ready to ship the adolescent vaccine directly to pharmacies and pediatricians to speed up the vaccination rate.
In March, Anthony S. Fauci, MD, told a Senate committee, “We don’t really know what that magical point of herd immunity is, but we do know that if we get the overwhelming population vaccinated, we’re going to be in good shape. … We ultimately would like to get and have to get children into that mix.”
Pfizer submitted data to the FDA in late March showing its mRNA vaccine was 100% effective at preventing COVID-19 infection in children ages 12-15 in clinical trials.
Though most children have milder symptoms when infected with the coronavirus, about 1.5 million cases in children aged 11-17 were reported to the CDC between March 1, 2020, and April 30 of this year, the FDA news release said.
Albert Bourla, CEO of Pfizer, tweeted that “today brings very encouraging news for families and adolescents across the United States.
“While this is a meaningful step forward, we are still in a critical period of combating #COVID19 around the world. In the coming weeks, we hope to continue to receive authorizations from global regulators to support worldwide vaccination efforts,” he said.
“It’s essential for children to be vaccinated against COVID-19. According to data compiled by the AAP and Children’s Hospital Association, more than 3.8 million children have tested positive for COVID-19 in the United States since the start of the pandemic,” said Dr. Savio Beers. “While fewer children than adults have suffered the most severe disease, this is not a benign disease in children. Thousands of children have been hospitalized, and hundreds have died. We will soon have a very safe, highly effective vaccine that can prevent so much suffering. I encourage parents to talk with their pediatricians about how to get the vaccine for their adolescents as soon as they are eligible.”
A version of this article first appeared on Medscape.com.
NSAIDs don’t make COVID-19 worse in hospitalized patients
NSAIDs don’t boost the risk of more severe disease or death in hospitalized patients with COVID-19, a new study finds.
“To our knowledge, our prospective study includes the largest number of patients admitted to hospital with COVID-19 to date, and adds to the literature on the safety of NSAIDs and in-hospital outcomes. NSAIDs do not appear to increase the risk of worse in-hospital outcomes ...” the study authors wrote. “NSAIDs are an important analgesic modality and have a vital opioid-sparing role in pain management. Patients and clinicians should be reassured by these findings that NSAIDs are safe in the context of the pandemic.”
The report was published online May 7 in The Lancet Rheumatology and led by clinical research fellow Thomas M. Drake, MBChB, of the University of Edinburgh’s Usher Institute.
For more than a year, researchers worldwide have debated about whether NSAIDs spell trouble for people at risk of COVID-19. In March 2020, French health officials announced that use of the painkillers such as NSAIDs may increase the severity of the disease, and they recommended that patients take acetaminophen instead. The National Health Service in the United Kingdom made a similar recommendation. But other agencies didn’t believe there was enough evidence to support ditching NSAIDs, and recent research studies published in Annals of the Rheumatic Diseases and PLoS Medicine suggested they may be right.
For the new study, researchers identified 72,179 patients who were treated for COVID-19 in British hospitals during January-August 2020. About 56% were men, 74% were White, and 6% took NSAIDs on a regular basis before they entered the hospital. The average age was 70.
The researchers examined whether the patients in either group were more or less likely to die in the hospital, be admitted into a critical care unit, need oxygen treatment, need a ventilator, or suffer kidney injury.
In terms of outcomes, there weren’t any major gaps between the groups overall. The differences in most comparisons were statistically insignificant. For example, 31% of those who didn’t take NSAIDs died vs. 30% of those who did (P = .227). In both groups, 14% required critical care admission (P = .476).
The researchers then focused on two matched groups of 4,205 patients: One group used NSAIDs regularly, and the other group didn’t. The difference in risk of death in those who took NSAIDs vs. those who didn’t was statistically insignificant (odds ratio, 0.95; 95% confidence interval, 0.84-1.07; P = .35). Other comparisons were also statistically insignificant.
The findings offer insight into whether the use of NSAIDs might actually be helpful for patients who develop COVID-19. Scientists believe that COVID-19 is linked to inflammation in the body, and NSAIDs, of course, reduce inflammation. But the researchers didn’t turn up any sign of a benefit.
The new study has some weaknesses: It doesn’t say anything about whether NSAIDs have an impact on whether people get COVID-19 in the first place. Researchers don’t know if high use of NSAIDs may affect the severity of the disease. And it doesn’t examine the potential effect of acetaminophen, although other research suggests the drug also may not cause harm in patients with COVID-19.
Still, the researchers say the study is the largest of its kind to look at the use of NSAIDs by patients who are admitted to the hospital with COVID-19. “Considering all the evidence, if there was an extreme effect of NSAIDs on COVID-19 outcomes or severity, this would have been observed in one or more of the studies that have been done, including the present study,” they wrote.
In a commentary that accompanied the study, three physicians from hospitals in Denmark, led by Kristian Kragholm, MD, of Aalborg University Hospital, praised the research and wrote that it adds to “a growing body of evidence” that NSAIDs don’t make things worse for patients with COVID-19.
The study was funded by the U.K. National Institute for Health Research and the U.K. Medical Research Council. The study and commentary authors reported no relevant disclosures.
NSAIDs don’t boost the risk of more severe disease or death in hospitalized patients with COVID-19, a new study finds.
“To our knowledge, our prospective study includes the largest number of patients admitted to hospital with COVID-19 to date, and adds to the literature on the safety of NSAIDs and in-hospital outcomes. NSAIDs do not appear to increase the risk of worse in-hospital outcomes ...” the study authors wrote. “NSAIDs are an important analgesic modality and have a vital opioid-sparing role in pain management. Patients and clinicians should be reassured by these findings that NSAIDs are safe in the context of the pandemic.”
The report was published online May 7 in The Lancet Rheumatology and led by clinical research fellow Thomas M. Drake, MBChB, of the University of Edinburgh’s Usher Institute.
For more than a year, researchers worldwide have debated about whether NSAIDs spell trouble for people at risk of COVID-19. In March 2020, French health officials announced that use of the painkillers such as NSAIDs may increase the severity of the disease, and they recommended that patients take acetaminophen instead. The National Health Service in the United Kingdom made a similar recommendation. But other agencies didn’t believe there was enough evidence to support ditching NSAIDs, and recent research studies published in Annals of the Rheumatic Diseases and PLoS Medicine suggested they may be right.
For the new study, researchers identified 72,179 patients who were treated for COVID-19 in British hospitals during January-August 2020. About 56% were men, 74% were White, and 6% took NSAIDs on a regular basis before they entered the hospital. The average age was 70.
The researchers examined whether the patients in either group were more or less likely to die in the hospital, be admitted into a critical care unit, need oxygen treatment, need a ventilator, or suffer kidney injury.
In terms of outcomes, there weren’t any major gaps between the groups overall. The differences in most comparisons were statistically insignificant. For example, 31% of those who didn’t take NSAIDs died vs. 30% of those who did (P = .227). In both groups, 14% required critical care admission (P = .476).
The researchers then focused on two matched groups of 4,205 patients: One group used NSAIDs regularly, and the other group didn’t. The difference in risk of death in those who took NSAIDs vs. those who didn’t was statistically insignificant (odds ratio, 0.95; 95% confidence interval, 0.84-1.07; P = .35). Other comparisons were also statistically insignificant.
The findings offer insight into whether the use of NSAIDs might actually be helpful for patients who develop COVID-19. Scientists believe that COVID-19 is linked to inflammation in the body, and NSAIDs, of course, reduce inflammation. But the researchers didn’t turn up any sign of a benefit.
The new study has some weaknesses: It doesn’t say anything about whether NSAIDs have an impact on whether people get COVID-19 in the first place. Researchers don’t know if high use of NSAIDs may affect the severity of the disease. And it doesn’t examine the potential effect of acetaminophen, although other research suggests the drug also may not cause harm in patients with COVID-19.
Still, the researchers say the study is the largest of its kind to look at the use of NSAIDs by patients who are admitted to the hospital with COVID-19. “Considering all the evidence, if there was an extreme effect of NSAIDs on COVID-19 outcomes or severity, this would have been observed in one or more of the studies that have been done, including the present study,” they wrote.
In a commentary that accompanied the study, three physicians from hospitals in Denmark, led by Kristian Kragholm, MD, of Aalborg University Hospital, praised the research and wrote that it adds to “a growing body of evidence” that NSAIDs don’t make things worse for patients with COVID-19.
The study was funded by the U.K. National Institute for Health Research and the U.K. Medical Research Council. The study and commentary authors reported no relevant disclosures.
NSAIDs don’t boost the risk of more severe disease or death in hospitalized patients with COVID-19, a new study finds.
“To our knowledge, our prospective study includes the largest number of patients admitted to hospital with COVID-19 to date, and adds to the literature on the safety of NSAIDs and in-hospital outcomes. NSAIDs do not appear to increase the risk of worse in-hospital outcomes ...” the study authors wrote. “NSAIDs are an important analgesic modality and have a vital opioid-sparing role in pain management. Patients and clinicians should be reassured by these findings that NSAIDs are safe in the context of the pandemic.”
The report was published online May 7 in The Lancet Rheumatology and led by clinical research fellow Thomas M. Drake, MBChB, of the University of Edinburgh’s Usher Institute.
For more than a year, researchers worldwide have debated about whether NSAIDs spell trouble for people at risk of COVID-19. In March 2020, French health officials announced that use of the painkillers such as NSAIDs may increase the severity of the disease, and they recommended that patients take acetaminophen instead. The National Health Service in the United Kingdom made a similar recommendation. But other agencies didn’t believe there was enough evidence to support ditching NSAIDs, and recent research studies published in Annals of the Rheumatic Diseases and PLoS Medicine suggested they may be right.
For the new study, researchers identified 72,179 patients who were treated for COVID-19 in British hospitals during January-August 2020. About 56% were men, 74% were White, and 6% took NSAIDs on a regular basis before they entered the hospital. The average age was 70.
The researchers examined whether the patients in either group were more or less likely to die in the hospital, be admitted into a critical care unit, need oxygen treatment, need a ventilator, or suffer kidney injury.
In terms of outcomes, there weren’t any major gaps between the groups overall. The differences in most comparisons were statistically insignificant. For example, 31% of those who didn’t take NSAIDs died vs. 30% of those who did (P = .227). In both groups, 14% required critical care admission (P = .476).
The researchers then focused on two matched groups of 4,205 patients: One group used NSAIDs regularly, and the other group didn’t. The difference in risk of death in those who took NSAIDs vs. those who didn’t was statistically insignificant (odds ratio, 0.95; 95% confidence interval, 0.84-1.07; P = .35). Other comparisons were also statistically insignificant.
The findings offer insight into whether the use of NSAIDs might actually be helpful for patients who develop COVID-19. Scientists believe that COVID-19 is linked to inflammation in the body, and NSAIDs, of course, reduce inflammation. But the researchers didn’t turn up any sign of a benefit.
The new study has some weaknesses: It doesn’t say anything about whether NSAIDs have an impact on whether people get COVID-19 in the first place. Researchers don’t know if high use of NSAIDs may affect the severity of the disease. And it doesn’t examine the potential effect of acetaminophen, although other research suggests the drug also may not cause harm in patients with COVID-19.
Still, the researchers say the study is the largest of its kind to look at the use of NSAIDs by patients who are admitted to the hospital with COVID-19. “Considering all the evidence, if there was an extreme effect of NSAIDs on COVID-19 outcomes or severity, this would have been observed in one or more of the studies that have been done, including the present study,” they wrote.
In a commentary that accompanied the study, three physicians from hospitals in Denmark, led by Kristian Kragholm, MD, of Aalborg University Hospital, praised the research and wrote that it adds to “a growing body of evidence” that NSAIDs don’t make things worse for patients with COVID-19.
The study was funded by the U.K. National Institute for Health Research and the U.K. Medical Research Council. The study and commentary authors reported no relevant disclosures.
FROM THE LANCET RHEUMATOLOGY