User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
Variants spur new FDA guidance on COVID vaccines, tests, drugs
The United States is currently facing three main variant threats, according to the Centers for Disease Control and Prevention: B.1.1.7, which originated in the United Kingdom; B.1.351 from South Africa; and the P.1 variant, which originated in Brazil.
Acting FDA Commissioner Janet Woodcock, MD, said on a telephone press briefing call Feb. 22 that the FDA has already been communicating with individual manufacturers as they assess the variants’ effect on their products, but these guidelines are issued for the sake of transparency and to welcome scientific input.
Tailoring may be necessary
Dr. Woodcock emphasized that, “at this time, available data suggest the FDA-authorized vaccines are effective in protecting circulating strains of SARS-CoV-2.” However, in the event the strains start to show resistance, it may be necessary to tailor the vaccine to the variant.
In that case, effectiveness of a modified vaccine should be determined by data from clinical immunogenicity studies, which would compare a recipient’s immune response with virus variants induced by the modified vaccine against the immune response to the authorized vaccine, the guidance states.
Manufacturers should also study the vaccine in both nonvaccinated people and people fully vaccinated with the authorized vaccine, according to the guidance.
Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, said on the call that the clinical immunogenicity data is needed to understand, for instance, whether a new vaccine strain is able to cover the new and old strain or whether it just covers the new strain. Information is also needed to understand whether the modified vaccine, when given to someone fully vaccinated, will still promote a positive response without introducing safety concerns.
Further discussions will be necessary to decide whether future modified vaccines may be authorized without the need for clinical studies.
Variants and testing
The FDA’s updated guidance for test developers, Policy for Evaluating Impact of Viral Mutations on COVID-19 Tests, includes information that test performance can be influenced by the sequence of the variant, prevalence of the variant in the population, or design of the test. For example, molecular tests designed to detect multiple SARS-CoV-2 genetic targets are less susceptible to genetic variants than tests designed to detect a single genetic target.
The FDA already issued a safety alert on Jan. 8 to caution that genetic mutations to the virus in a patient sample can potentially change the performance of a diagnostic test. The FDA identified three tests that had been granted emergency-use authorization (EUA) that are known to be affected.
However, Dr. Woodcock said on the call, “at this time the impact does not appear to be significant.”
Updated guidance for therapeutics
The FDA has issued new guidance on the effect of variants on monoclonal antibody treatments.
“The FDA is aware that some of the monoclonal antibodies that have been authorized are less active against some of the SARS-CoV-2 variants that have emerged,” the FDA noted in its press release. “This guidance provides recommendations on efficient approaches to the generation of ... manufacturing and controls data that could potentially support an EUA for monoclonal antibody products that may be effective against emerging variants.”
While the FDA is monitoring the effects of variants, manufacturers bear a lot of the responsibility as well.
The FDA added: “With these guidances, the FDA is encouraging developers of drugs or biological products targeting SARS-CoV-2 to continuously monitor genomic databases for emerging SARS-CoV-2 variants and evaluate phenotypically any specific variants in the product target that are becoming prevalent or could potentially impact its activity.”
Dr.Woodcock added that “we urge all Americans to continue to get tested, get their vaccines when available, and follow important heath measures such as handwashing, masking, and social distancing.”
A version of this article first appeared on Medscape.com.
The United States is currently facing three main variant threats, according to the Centers for Disease Control and Prevention: B.1.1.7, which originated in the United Kingdom; B.1.351 from South Africa; and the P.1 variant, which originated in Brazil.
Acting FDA Commissioner Janet Woodcock, MD, said on a telephone press briefing call Feb. 22 that the FDA has already been communicating with individual manufacturers as they assess the variants’ effect on their products, but these guidelines are issued for the sake of transparency and to welcome scientific input.
Tailoring may be necessary
Dr. Woodcock emphasized that, “at this time, available data suggest the FDA-authorized vaccines are effective in protecting circulating strains of SARS-CoV-2.” However, in the event the strains start to show resistance, it may be necessary to tailor the vaccine to the variant.
In that case, effectiveness of a modified vaccine should be determined by data from clinical immunogenicity studies, which would compare a recipient’s immune response with virus variants induced by the modified vaccine against the immune response to the authorized vaccine, the guidance states.
Manufacturers should also study the vaccine in both nonvaccinated people and people fully vaccinated with the authorized vaccine, according to the guidance.
Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, said on the call that the clinical immunogenicity data is needed to understand, for instance, whether a new vaccine strain is able to cover the new and old strain or whether it just covers the new strain. Information is also needed to understand whether the modified vaccine, when given to someone fully vaccinated, will still promote a positive response without introducing safety concerns.
Further discussions will be necessary to decide whether future modified vaccines may be authorized without the need for clinical studies.
Variants and testing
The FDA’s updated guidance for test developers, Policy for Evaluating Impact of Viral Mutations on COVID-19 Tests, includes information that test performance can be influenced by the sequence of the variant, prevalence of the variant in the population, or design of the test. For example, molecular tests designed to detect multiple SARS-CoV-2 genetic targets are less susceptible to genetic variants than tests designed to detect a single genetic target.
The FDA already issued a safety alert on Jan. 8 to caution that genetic mutations to the virus in a patient sample can potentially change the performance of a diagnostic test. The FDA identified three tests that had been granted emergency-use authorization (EUA) that are known to be affected.
However, Dr. Woodcock said on the call, “at this time the impact does not appear to be significant.”
Updated guidance for therapeutics
The FDA has issued new guidance on the effect of variants on monoclonal antibody treatments.
“The FDA is aware that some of the monoclonal antibodies that have been authorized are less active against some of the SARS-CoV-2 variants that have emerged,” the FDA noted in its press release. “This guidance provides recommendations on efficient approaches to the generation of ... manufacturing and controls data that could potentially support an EUA for monoclonal antibody products that may be effective against emerging variants.”
While the FDA is monitoring the effects of variants, manufacturers bear a lot of the responsibility as well.
The FDA added: “With these guidances, the FDA is encouraging developers of drugs or biological products targeting SARS-CoV-2 to continuously monitor genomic databases for emerging SARS-CoV-2 variants and evaluate phenotypically any specific variants in the product target that are becoming prevalent or could potentially impact its activity.”
Dr.Woodcock added that “we urge all Americans to continue to get tested, get their vaccines when available, and follow important heath measures such as handwashing, masking, and social distancing.”
A version of this article first appeared on Medscape.com.
The United States is currently facing three main variant threats, according to the Centers for Disease Control and Prevention: B.1.1.7, which originated in the United Kingdom; B.1.351 from South Africa; and the P.1 variant, which originated in Brazil.
Acting FDA Commissioner Janet Woodcock, MD, said on a telephone press briefing call Feb. 22 that the FDA has already been communicating with individual manufacturers as they assess the variants’ effect on their products, but these guidelines are issued for the sake of transparency and to welcome scientific input.
Tailoring may be necessary
Dr. Woodcock emphasized that, “at this time, available data suggest the FDA-authorized vaccines are effective in protecting circulating strains of SARS-CoV-2.” However, in the event the strains start to show resistance, it may be necessary to tailor the vaccine to the variant.
In that case, effectiveness of a modified vaccine should be determined by data from clinical immunogenicity studies, which would compare a recipient’s immune response with virus variants induced by the modified vaccine against the immune response to the authorized vaccine, the guidance states.
Manufacturers should also study the vaccine in both nonvaccinated people and people fully vaccinated with the authorized vaccine, according to the guidance.
Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, said on the call that the clinical immunogenicity data is needed to understand, for instance, whether a new vaccine strain is able to cover the new and old strain or whether it just covers the new strain. Information is also needed to understand whether the modified vaccine, when given to someone fully vaccinated, will still promote a positive response without introducing safety concerns.
Further discussions will be necessary to decide whether future modified vaccines may be authorized without the need for clinical studies.
Variants and testing
The FDA’s updated guidance for test developers, Policy for Evaluating Impact of Viral Mutations on COVID-19 Tests, includes information that test performance can be influenced by the sequence of the variant, prevalence of the variant in the population, or design of the test. For example, molecular tests designed to detect multiple SARS-CoV-2 genetic targets are less susceptible to genetic variants than tests designed to detect a single genetic target.
The FDA already issued a safety alert on Jan. 8 to caution that genetic mutations to the virus in a patient sample can potentially change the performance of a diagnostic test. The FDA identified three tests that had been granted emergency-use authorization (EUA) that are known to be affected.
However, Dr. Woodcock said on the call, “at this time the impact does not appear to be significant.”
Updated guidance for therapeutics
The FDA has issued new guidance on the effect of variants on monoclonal antibody treatments.
“The FDA is aware that some of the monoclonal antibodies that have been authorized are less active against some of the SARS-CoV-2 variants that have emerged,” the FDA noted in its press release. “This guidance provides recommendations on efficient approaches to the generation of ... manufacturing and controls data that could potentially support an EUA for monoclonal antibody products that may be effective against emerging variants.”
While the FDA is monitoring the effects of variants, manufacturers bear a lot of the responsibility as well.
The FDA added: “With these guidances, the FDA is encouraging developers of drugs or biological products targeting SARS-CoV-2 to continuously monitor genomic databases for emerging SARS-CoV-2 variants and evaluate phenotypically any specific variants in the product target that are becoming prevalent or could potentially impact its activity.”
Dr.Woodcock added that “we urge all Americans to continue to get tested, get their vaccines when available, and follow important heath measures such as handwashing, masking, and social distancing.”
A version of this article first appeared on Medscape.com.
Detailed glioblastoma map could lead to better treatment approaches
An integrated analysis of data derived from 99 treatment-naive glioblastomas has identified characteristics that could help stratify patients for more effective treatment, according to the investigators.
The analysis provides a detailed map of genes, proteins, infiltrating cells, and signaling pathways that play key roles in driving glioblastoma, Liang-Bo Wang, MD, of Washington University in St. Louis, and colleagues reported in Cancer Cell.
For example, the team identified key phosphorylation events as potential mediators of oncogenic pathway activation and potential targets for EGFR-, TP53-, and RB1-altered tumors. Specifically, phosphorylated PTPN11 and PLCG1 represent a signaling hub in RTK-altered tumors, they found.
The investigators also identified four immune glioblastoma tumor subtypes characterized by distinct immune cell populations. Type 1 tumors have a high macrophage count and few T cells, type 2 tumors have a moderate macrophage count, type 3 tumors have a high T-cell count and few macrophages, and type 4 tumors have few or no immune cells of any type.
They also found that mesenchymal subtype EMT signature is specific to tumor cells but not to stroma, and histone H2B acetylation is enriched in classical glioblastomas with low macrophage content.
“To improve therapies for this deadly cancer, understanding the tumor cells themselves is important but not enough,” senior author Li Ding, PhD, a professor of medicine and genetics and director of computational biology in the division of oncology at Washington University stated in a press release. “We also must understand the tumor cells’ interactions with the surrounding environment, including immune cells and the connective tissues and blood vessels.”
The investigators, including researchers from Pacific Northwest National Laboratory, Case Western Reserve University, and the National Cancer Institute, performed high-resolution and high-depth analyses on 99 tumors.
“Harnessing new technologies, including proteomics, metabolomics, and single-cell sequencing, this study is an extremely deep dive into glioblastoma tumor biology, revealing new possibilities for therapy,” Dr. Ding said.
The study, which is part of the NCI’s Clinical Proteomic Tumor Analysis Consortium (CPTAC), is the largest and most detailed schematic of glioblastoma tumors to date, according to the press release.
The most immediate implication of the findings is better clinical trial design, study coauthor Milan G. Chheda, MD, stated in the press release.
Stratifying patients by tumor type, as identified in the current analysis, could allow researchers to test targeted therapies in the tumors most likely to respond to those therapies, explained Dr. Chheda, of Siteman Cancer Center at Barnes Jewish Hospital and Washington University.
The findings, particularly of multiple glioblastoma tumor subtypes, may explain the negative findings of trials looking at various immunotherapies for treating glioblastoma. Investigators for those trials haven’t considered the possibility of immune subgroups that may respond differently, the authors note, adding that research is underway to identify the best drugs to assess for the newly identified glioblastoma tumor types.
The study was supported by grants from the National Cancer Institute’s Clinical Proteomic Tumor Analysis Consortium, the National Human Genome Research Institute, and the National Institutes of Health.
Dr. Wang and Dr. Ding reported having no disclosures. Dr. Chheda receives research support from NeoimmuneTech and Orbus Therapeutics, and royalties from UpToDate.
[email protected]
An integrated analysis of data derived from 99 treatment-naive glioblastomas has identified characteristics that could help stratify patients for more effective treatment, according to the investigators.
The analysis provides a detailed map of genes, proteins, infiltrating cells, and signaling pathways that play key roles in driving glioblastoma, Liang-Bo Wang, MD, of Washington University in St. Louis, and colleagues reported in Cancer Cell.
For example, the team identified key phosphorylation events as potential mediators of oncogenic pathway activation and potential targets for EGFR-, TP53-, and RB1-altered tumors. Specifically, phosphorylated PTPN11 and PLCG1 represent a signaling hub in RTK-altered tumors, they found.
The investigators also identified four immune glioblastoma tumor subtypes characterized by distinct immune cell populations. Type 1 tumors have a high macrophage count and few T cells, type 2 tumors have a moderate macrophage count, type 3 tumors have a high T-cell count and few macrophages, and type 4 tumors have few or no immune cells of any type.
They also found that mesenchymal subtype EMT signature is specific to tumor cells but not to stroma, and histone H2B acetylation is enriched in classical glioblastomas with low macrophage content.
“To improve therapies for this deadly cancer, understanding the tumor cells themselves is important but not enough,” senior author Li Ding, PhD, a professor of medicine and genetics and director of computational biology in the division of oncology at Washington University stated in a press release. “We also must understand the tumor cells’ interactions with the surrounding environment, including immune cells and the connective tissues and blood vessels.”
The investigators, including researchers from Pacific Northwest National Laboratory, Case Western Reserve University, and the National Cancer Institute, performed high-resolution and high-depth analyses on 99 tumors.
“Harnessing new technologies, including proteomics, metabolomics, and single-cell sequencing, this study is an extremely deep dive into glioblastoma tumor biology, revealing new possibilities for therapy,” Dr. Ding said.
The study, which is part of the NCI’s Clinical Proteomic Tumor Analysis Consortium (CPTAC), is the largest and most detailed schematic of glioblastoma tumors to date, according to the press release.
The most immediate implication of the findings is better clinical trial design, study coauthor Milan G. Chheda, MD, stated in the press release.
Stratifying patients by tumor type, as identified in the current analysis, could allow researchers to test targeted therapies in the tumors most likely to respond to those therapies, explained Dr. Chheda, of Siteman Cancer Center at Barnes Jewish Hospital and Washington University.
The findings, particularly of multiple glioblastoma tumor subtypes, may explain the negative findings of trials looking at various immunotherapies for treating glioblastoma. Investigators for those trials haven’t considered the possibility of immune subgroups that may respond differently, the authors note, adding that research is underway to identify the best drugs to assess for the newly identified glioblastoma tumor types.
The study was supported by grants from the National Cancer Institute’s Clinical Proteomic Tumor Analysis Consortium, the National Human Genome Research Institute, and the National Institutes of Health.
Dr. Wang and Dr. Ding reported having no disclosures. Dr. Chheda receives research support from NeoimmuneTech and Orbus Therapeutics, and royalties from UpToDate.
[email protected]
An integrated analysis of data derived from 99 treatment-naive glioblastomas has identified characteristics that could help stratify patients for more effective treatment, according to the investigators.
The analysis provides a detailed map of genes, proteins, infiltrating cells, and signaling pathways that play key roles in driving glioblastoma, Liang-Bo Wang, MD, of Washington University in St. Louis, and colleagues reported in Cancer Cell.
For example, the team identified key phosphorylation events as potential mediators of oncogenic pathway activation and potential targets for EGFR-, TP53-, and RB1-altered tumors. Specifically, phosphorylated PTPN11 and PLCG1 represent a signaling hub in RTK-altered tumors, they found.
The investigators also identified four immune glioblastoma tumor subtypes characterized by distinct immune cell populations. Type 1 tumors have a high macrophage count and few T cells, type 2 tumors have a moderate macrophage count, type 3 tumors have a high T-cell count and few macrophages, and type 4 tumors have few or no immune cells of any type.
They also found that mesenchymal subtype EMT signature is specific to tumor cells but not to stroma, and histone H2B acetylation is enriched in classical glioblastomas with low macrophage content.
“To improve therapies for this deadly cancer, understanding the tumor cells themselves is important but not enough,” senior author Li Ding, PhD, a professor of medicine and genetics and director of computational biology in the division of oncology at Washington University stated in a press release. “We also must understand the tumor cells’ interactions with the surrounding environment, including immune cells and the connective tissues and blood vessels.”
The investigators, including researchers from Pacific Northwest National Laboratory, Case Western Reserve University, and the National Cancer Institute, performed high-resolution and high-depth analyses on 99 tumors.
“Harnessing new technologies, including proteomics, metabolomics, and single-cell sequencing, this study is an extremely deep dive into glioblastoma tumor biology, revealing new possibilities for therapy,” Dr. Ding said.
The study, which is part of the NCI’s Clinical Proteomic Tumor Analysis Consortium (CPTAC), is the largest and most detailed schematic of glioblastoma tumors to date, according to the press release.
The most immediate implication of the findings is better clinical trial design, study coauthor Milan G. Chheda, MD, stated in the press release.
Stratifying patients by tumor type, as identified in the current analysis, could allow researchers to test targeted therapies in the tumors most likely to respond to those therapies, explained Dr. Chheda, of Siteman Cancer Center at Barnes Jewish Hospital and Washington University.
The findings, particularly of multiple glioblastoma tumor subtypes, may explain the negative findings of trials looking at various immunotherapies for treating glioblastoma. Investigators for those trials haven’t considered the possibility of immune subgroups that may respond differently, the authors note, adding that research is underway to identify the best drugs to assess for the newly identified glioblastoma tumor types.
The study was supported by grants from the National Cancer Institute’s Clinical Proteomic Tumor Analysis Consortium, the National Human Genome Research Institute, and the National Institutes of Health.
Dr. Wang and Dr. Ding reported having no disclosures. Dr. Chheda receives research support from NeoimmuneTech and Orbus Therapeutics, and royalties from UpToDate.
[email protected]
FROM CANCER CELL
New steroid dosing regimen for myasthenia gravis
. The trial showed that the conventional slow tapering regimen enabled discontinuation of prednisone earlier than previously reported but the new rapid-tapering regimen enabled an even faster discontinuation.
Noting that although both regimens led to a comparable myasthenia gravis status and prednisone dose at 15 months, the authors stated: “We think that the reduction of the cumulative dose over a year (equivalent to 5 mg/day) is a clinically relevant reduction, since the risk of complications is proportional to the daily or cumulative doses of prednisone.
“Our results warrant testing of a more rapid-tapering regimen in a future trial. In the meantime, our trial provides useful information on how prednisone tapering could be managed in patients with generalized myasthenia gravis treated with azathioprine,” they concluded.
The trial was published online Feb. 8 in JAMA Neurology.
Myasthenia gravis is a disorder of neuromuscular transmission, resulting from autoantibodies to components of the neuromuscular junction, most commonly the acetylcholine receptor. The incidence ranges from 0.3 to 2.8 per 100,000, and it is estimated to affect more than 700,000 people worldwide.
The authors of the current paper, led by Tarek Sharshar, MD, PhD, Groupe Hospitalier Universitaire, Paris, explained that many patients whose symptoms are not controlled by cholinesterase inhibitors are treated with corticosteroids and an immunosuppressant, usually azathioprine. No specific dosing protocol for prednisone has been validated, but it is commonly gradually increased to 0.75 mg/kg on alternate days and reduced progressively when minimal manifestation status (MMS; no symptoms or functional limitations) is reached.
They noted that this regimen leads to high and prolonged corticosteroid treatment – often for several years – with the mean daily prednisone dose exceeding 30 mg/day at 15 months and 20 mg/day at 36 months. As long-term use of corticosteroids is often associated with significant complications, reducing or even discontinuing prednisone treatment without destabilizing myasthenia gravis is therefore a therapeutic goal.
Evaluating dosage regimens
To investigate whether different dosage regimens could help wean patients with generalized myasthenia gravis from corticosteroid therapy without compromising efficacy, the researchers conducted this study in which the current recommended regimen was compared with an approach using higher initial corticosteroid doses followed by rapid tapering.
In the conventional slow-tapering group (control group), prednisone was given on alternate days, starting at a dose of 10 mg then increased by increments of 10 mg every 2 days up to 1.5 mg/kg on alternate days without exceeding 100 mg. This dose was maintained until MMS was reached and then reduced by 10 mg every 2 weeks until a dosage of 40 mg was reached, with subsequent slowing of the taper to 5 mg monthly. If MMS was not maintained, the alternate-day prednisone dose was increased by 10 mg every 2 weeks until MMS was restored, and the tapering resumed 4 weeks later.
In the new rapid-tapering group, oral prednisone was immediately started at 0.75 mg/kg per day, and this was followed by an earlier and rapid decrease once improved myasthenia gravis status was attained. Three different tapering schedules were applied dependent on the improvement status of the patient.
First, If the patient reached MMS at 1 month, the dose of prednisone was reduced by 0.1 mg/kg every 10 days up to 0.45 mg/kg per day, then 0.05 mg/kg every 10 days up to 0.25 mg/kg per day, then in decrements of 1 mg by adjusting the duration of the decrements according to the participant’s weight with the aim of achieving complete cessation of corticosteroid therapy within 18-20 weeks for this third stage of tapering.
Second, if the state of MMS was not reached at 1 month but the participant had improved, a slower tapering was conducted, with the dosage reduced in a similar way to the first instance but with each reduction introduced every 20 days. If the participant reached MMS during this tapering process, the tapering of prednisone was similar to the sequence described in the first group.
Third, if MMS was not reached and the participant had not improved, the initial dose was maintained for the first 3 months; beyond that time, a decrease in the prednisone dose was undertaken as in the second group to a minimum dose of 0.25 mg/kg per day, after which the prednisone dose was not reduced further. If the patient improved, the tapering of prednisone followed the sequence described in the second category.
Reductions in prednisone dose could be accelerated in the case of severe prednisone adverse effects, according to the prescriber’s decision.
In the event of a myasthenia gravis exacerbation, the patient was hospitalized and the dose of prednisone was routinely doubled, or for a more moderate aggravation, the dose was increased to the previous dose recommended in the tapering regimen.
Azathioprine, up to a maximum dose of 3 mg/kg per day, was prescribed for all participants. In all, 117 patients were randomly assigned, and 113 completed the study.
The primary outcome was the proportion of participants having reached MMS without prednisone at 12 months and having not relapsed or taken prednisone between months 12 and 15. This was achieved by significantly more patients in the rapid-tapering group (39% vs. 9%; risk ratio, 3.61; P < .001).
Rapid tapering allowed sparing of a mean of 1,898 mg of prednisone over 1 year (5.3 mg/day) per patient.
The rate of myasthenia gravis exacerbation or worsening did not differ significantly between the two groups, nor did the use of plasmapheresis or IVIG or the doses of azathioprine.
The overall number of serious adverse events did not differ significantly between the two groups (slow tapering, 22% vs. rapid-tapering, 36%; P = .15).
The researchers said it is possible that prednisone tapering would differ with another immunosuppressive agent but as azathioprine is the first-line immunosuppressant usually recommended, these results are relevant for a large proportion of patients.
They said the better outcome of the intervention group could have been related to one or more of four differences in prednisone administration: An immediate high dose versus a slow increase of the prednisone dose; daily versus alternate-day dosing; earlier tapering initiation; and faster tapering. However, the structure of the study did not allow identification of which of these factors was responsible.
“Researching the best prednisone-tapering scheme is not only a major issue for patients with myasthenia gravis but also for other autoimmune or inflammatory diseases, because validated prednisone-tapering regimens are scarce,” the authors said.
The rapid tapering of prednisone therapy appears to be feasible, beneficial, and safe in patients with generalized myasthenia gravis and “warrants testing in other autoimmune diseases,” they added.
Particularly relevant to late-onset disease
Commenting on the study, Raffi Topakian, MD, Klinikum Wels-Grieskirchen, Wels, Austria, said the results showed that in patients with moderate to severe generalized myasthenia gravis requiring high-dose prednisone, azathioprine, a widely used immunosuppressant, may have a quicker steroid-sparing effect than previously thought, and that rapid steroid tapering can be achieved safely, resulting in a reduction of the cumulative steroid dose over a year despite higher initial doses.
Dr. Topakian, who was not involved with the research, pointed out that the median age was advanced (around 56 years), and the benefit of a regimen that leads to a reduction of the cumulative steroid dose over a year may be disproportionately larger for older, sicker patients with many comorbidities who are at considerably higher risk for a prednisone-induced increase in cardiovascular complications, osteoporotic fractures, and gastrointestinal bleeding.
“The study findings are particularly relevant for the management of late-onset myasthenia gravis (when first symptoms start after age 45-50 years), which is being encountered more frequently over the past years,” he said.
“But the holy grail of myasthenia gravis treatment has not been found yet,” Dr. Topakian noted. “Disappointingly, rapid tapering of steroids (compared to slow tapering) resulted in a reduction of the cumulative steroid dose only, but was not associated with better myasthenia gravis functional status or lower doses of steroids at 15 months. To my view, this finding points to the limited immunosuppressive efficacy of azathioprine.”
He added that the study findings should not be extrapolated to patients with mild presentations or to those with muscle-specific kinase myasthenia gravis.
Dr. Sharshar disclosed no relevant financial relationships. Disclosures for the study coauthors appear in the original article.
A version of this article first appeared on Medscape.com.
. The trial showed that the conventional slow tapering regimen enabled discontinuation of prednisone earlier than previously reported but the new rapid-tapering regimen enabled an even faster discontinuation.
Noting that although both regimens led to a comparable myasthenia gravis status and prednisone dose at 15 months, the authors stated: “We think that the reduction of the cumulative dose over a year (equivalent to 5 mg/day) is a clinically relevant reduction, since the risk of complications is proportional to the daily or cumulative doses of prednisone.
“Our results warrant testing of a more rapid-tapering regimen in a future trial. In the meantime, our trial provides useful information on how prednisone tapering could be managed in patients with generalized myasthenia gravis treated with azathioprine,” they concluded.
The trial was published online Feb. 8 in JAMA Neurology.
Myasthenia gravis is a disorder of neuromuscular transmission, resulting from autoantibodies to components of the neuromuscular junction, most commonly the acetylcholine receptor. The incidence ranges from 0.3 to 2.8 per 100,000, and it is estimated to affect more than 700,000 people worldwide.
The authors of the current paper, led by Tarek Sharshar, MD, PhD, Groupe Hospitalier Universitaire, Paris, explained that many patients whose symptoms are not controlled by cholinesterase inhibitors are treated with corticosteroids and an immunosuppressant, usually azathioprine. No specific dosing protocol for prednisone has been validated, but it is commonly gradually increased to 0.75 mg/kg on alternate days and reduced progressively when minimal manifestation status (MMS; no symptoms or functional limitations) is reached.
They noted that this regimen leads to high and prolonged corticosteroid treatment – often for several years – with the mean daily prednisone dose exceeding 30 mg/day at 15 months and 20 mg/day at 36 months. As long-term use of corticosteroids is often associated with significant complications, reducing or even discontinuing prednisone treatment without destabilizing myasthenia gravis is therefore a therapeutic goal.
Evaluating dosage regimens
To investigate whether different dosage regimens could help wean patients with generalized myasthenia gravis from corticosteroid therapy without compromising efficacy, the researchers conducted this study in which the current recommended regimen was compared with an approach using higher initial corticosteroid doses followed by rapid tapering.
In the conventional slow-tapering group (control group), prednisone was given on alternate days, starting at a dose of 10 mg then increased by increments of 10 mg every 2 days up to 1.5 mg/kg on alternate days without exceeding 100 mg. This dose was maintained until MMS was reached and then reduced by 10 mg every 2 weeks until a dosage of 40 mg was reached, with subsequent slowing of the taper to 5 mg monthly. If MMS was not maintained, the alternate-day prednisone dose was increased by 10 mg every 2 weeks until MMS was restored, and the tapering resumed 4 weeks later.
In the new rapid-tapering group, oral prednisone was immediately started at 0.75 mg/kg per day, and this was followed by an earlier and rapid decrease once improved myasthenia gravis status was attained. Three different tapering schedules were applied dependent on the improvement status of the patient.
First, If the patient reached MMS at 1 month, the dose of prednisone was reduced by 0.1 mg/kg every 10 days up to 0.45 mg/kg per day, then 0.05 mg/kg every 10 days up to 0.25 mg/kg per day, then in decrements of 1 mg by adjusting the duration of the decrements according to the participant’s weight with the aim of achieving complete cessation of corticosteroid therapy within 18-20 weeks for this third stage of tapering.
Second, if the state of MMS was not reached at 1 month but the participant had improved, a slower tapering was conducted, with the dosage reduced in a similar way to the first instance but with each reduction introduced every 20 days. If the participant reached MMS during this tapering process, the tapering of prednisone was similar to the sequence described in the first group.
Third, if MMS was not reached and the participant had not improved, the initial dose was maintained for the first 3 months; beyond that time, a decrease in the prednisone dose was undertaken as in the second group to a minimum dose of 0.25 mg/kg per day, after which the prednisone dose was not reduced further. If the patient improved, the tapering of prednisone followed the sequence described in the second category.
Reductions in prednisone dose could be accelerated in the case of severe prednisone adverse effects, according to the prescriber’s decision.
In the event of a myasthenia gravis exacerbation, the patient was hospitalized and the dose of prednisone was routinely doubled, or for a more moderate aggravation, the dose was increased to the previous dose recommended in the tapering regimen.
Azathioprine, up to a maximum dose of 3 mg/kg per day, was prescribed for all participants. In all, 117 patients were randomly assigned, and 113 completed the study.
The primary outcome was the proportion of participants having reached MMS without prednisone at 12 months and having not relapsed or taken prednisone between months 12 and 15. This was achieved by significantly more patients in the rapid-tapering group (39% vs. 9%; risk ratio, 3.61; P < .001).
Rapid tapering allowed sparing of a mean of 1,898 mg of prednisone over 1 year (5.3 mg/day) per patient.
The rate of myasthenia gravis exacerbation or worsening did not differ significantly between the two groups, nor did the use of plasmapheresis or IVIG or the doses of azathioprine.
The overall number of serious adverse events did not differ significantly between the two groups (slow tapering, 22% vs. rapid-tapering, 36%; P = .15).
The researchers said it is possible that prednisone tapering would differ with another immunosuppressive agent but as azathioprine is the first-line immunosuppressant usually recommended, these results are relevant for a large proportion of patients.
They said the better outcome of the intervention group could have been related to one or more of four differences in prednisone administration: An immediate high dose versus a slow increase of the prednisone dose; daily versus alternate-day dosing; earlier tapering initiation; and faster tapering. However, the structure of the study did not allow identification of which of these factors was responsible.
“Researching the best prednisone-tapering scheme is not only a major issue for patients with myasthenia gravis but also for other autoimmune or inflammatory diseases, because validated prednisone-tapering regimens are scarce,” the authors said.
The rapid tapering of prednisone therapy appears to be feasible, beneficial, and safe in patients with generalized myasthenia gravis and “warrants testing in other autoimmune diseases,” they added.
Particularly relevant to late-onset disease
Commenting on the study, Raffi Topakian, MD, Klinikum Wels-Grieskirchen, Wels, Austria, said the results showed that in patients with moderate to severe generalized myasthenia gravis requiring high-dose prednisone, azathioprine, a widely used immunosuppressant, may have a quicker steroid-sparing effect than previously thought, and that rapid steroid tapering can be achieved safely, resulting in a reduction of the cumulative steroid dose over a year despite higher initial doses.
Dr. Topakian, who was not involved with the research, pointed out that the median age was advanced (around 56 years), and the benefit of a regimen that leads to a reduction of the cumulative steroid dose over a year may be disproportionately larger for older, sicker patients with many comorbidities who are at considerably higher risk for a prednisone-induced increase in cardiovascular complications, osteoporotic fractures, and gastrointestinal bleeding.
“The study findings are particularly relevant for the management of late-onset myasthenia gravis (when first symptoms start after age 45-50 years), which is being encountered more frequently over the past years,” he said.
“But the holy grail of myasthenia gravis treatment has not been found yet,” Dr. Topakian noted. “Disappointingly, rapid tapering of steroids (compared to slow tapering) resulted in a reduction of the cumulative steroid dose only, but was not associated with better myasthenia gravis functional status or lower doses of steroids at 15 months. To my view, this finding points to the limited immunosuppressive efficacy of azathioprine.”
He added that the study findings should not be extrapolated to patients with mild presentations or to those with muscle-specific kinase myasthenia gravis.
Dr. Sharshar disclosed no relevant financial relationships. Disclosures for the study coauthors appear in the original article.
A version of this article first appeared on Medscape.com.
. The trial showed that the conventional slow tapering regimen enabled discontinuation of prednisone earlier than previously reported but the new rapid-tapering regimen enabled an even faster discontinuation.
Noting that although both regimens led to a comparable myasthenia gravis status and prednisone dose at 15 months, the authors stated: “We think that the reduction of the cumulative dose over a year (equivalent to 5 mg/day) is a clinically relevant reduction, since the risk of complications is proportional to the daily or cumulative doses of prednisone.
“Our results warrant testing of a more rapid-tapering regimen in a future trial. In the meantime, our trial provides useful information on how prednisone tapering could be managed in patients with generalized myasthenia gravis treated with azathioprine,” they concluded.
The trial was published online Feb. 8 in JAMA Neurology.
Myasthenia gravis is a disorder of neuromuscular transmission, resulting from autoantibodies to components of the neuromuscular junction, most commonly the acetylcholine receptor. The incidence ranges from 0.3 to 2.8 per 100,000, and it is estimated to affect more than 700,000 people worldwide.
The authors of the current paper, led by Tarek Sharshar, MD, PhD, Groupe Hospitalier Universitaire, Paris, explained that many patients whose symptoms are not controlled by cholinesterase inhibitors are treated with corticosteroids and an immunosuppressant, usually azathioprine. No specific dosing protocol for prednisone has been validated, but it is commonly gradually increased to 0.75 mg/kg on alternate days and reduced progressively when minimal manifestation status (MMS; no symptoms or functional limitations) is reached.
They noted that this regimen leads to high and prolonged corticosteroid treatment – often for several years – with the mean daily prednisone dose exceeding 30 mg/day at 15 months and 20 mg/day at 36 months. As long-term use of corticosteroids is often associated with significant complications, reducing or even discontinuing prednisone treatment without destabilizing myasthenia gravis is therefore a therapeutic goal.
Evaluating dosage regimens
To investigate whether different dosage regimens could help wean patients with generalized myasthenia gravis from corticosteroid therapy without compromising efficacy, the researchers conducted this study in which the current recommended regimen was compared with an approach using higher initial corticosteroid doses followed by rapid tapering.
In the conventional slow-tapering group (control group), prednisone was given on alternate days, starting at a dose of 10 mg then increased by increments of 10 mg every 2 days up to 1.5 mg/kg on alternate days without exceeding 100 mg. This dose was maintained until MMS was reached and then reduced by 10 mg every 2 weeks until a dosage of 40 mg was reached, with subsequent slowing of the taper to 5 mg monthly. If MMS was not maintained, the alternate-day prednisone dose was increased by 10 mg every 2 weeks until MMS was restored, and the tapering resumed 4 weeks later.
In the new rapid-tapering group, oral prednisone was immediately started at 0.75 mg/kg per day, and this was followed by an earlier and rapid decrease once improved myasthenia gravis status was attained. Three different tapering schedules were applied dependent on the improvement status of the patient.
First, If the patient reached MMS at 1 month, the dose of prednisone was reduced by 0.1 mg/kg every 10 days up to 0.45 mg/kg per day, then 0.05 mg/kg every 10 days up to 0.25 mg/kg per day, then in decrements of 1 mg by adjusting the duration of the decrements according to the participant’s weight with the aim of achieving complete cessation of corticosteroid therapy within 18-20 weeks for this third stage of tapering.
Second, if the state of MMS was not reached at 1 month but the participant had improved, a slower tapering was conducted, with the dosage reduced in a similar way to the first instance but with each reduction introduced every 20 days. If the participant reached MMS during this tapering process, the tapering of prednisone was similar to the sequence described in the first group.
Third, if MMS was not reached and the participant had not improved, the initial dose was maintained for the first 3 months; beyond that time, a decrease in the prednisone dose was undertaken as in the second group to a minimum dose of 0.25 mg/kg per day, after which the prednisone dose was not reduced further. If the patient improved, the tapering of prednisone followed the sequence described in the second category.
Reductions in prednisone dose could be accelerated in the case of severe prednisone adverse effects, according to the prescriber’s decision.
In the event of a myasthenia gravis exacerbation, the patient was hospitalized and the dose of prednisone was routinely doubled, or for a more moderate aggravation, the dose was increased to the previous dose recommended in the tapering regimen.
Azathioprine, up to a maximum dose of 3 mg/kg per day, was prescribed for all participants. In all, 117 patients were randomly assigned, and 113 completed the study.
The primary outcome was the proportion of participants having reached MMS without prednisone at 12 months and having not relapsed or taken prednisone between months 12 and 15. This was achieved by significantly more patients in the rapid-tapering group (39% vs. 9%; risk ratio, 3.61; P < .001).
Rapid tapering allowed sparing of a mean of 1,898 mg of prednisone over 1 year (5.3 mg/day) per patient.
The rate of myasthenia gravis exacerbation or worsening did not differ significantly between the two groups, nor did the use of plasmapheresis or IVIG or the doses of azathioprine.
The overall number of serious adverse events did not differ significantly between the two groups (slow tapering, 22% vs. rapid-tapering, 36%; P = .15).
The researchers said it is possible that prednisone tapering would differ with another immunosuppressive agent but as azathioprine is the first-line immunosuppressant usually recommended, these results are relevant for a large proportion of patients.
They said the better outcome of the intervention group could have been related to one or more of four differences in prednisone administration: An immediate high dose versus a slow increase of the prednisone dose; daily versus alternate-day dosing; earlier tapering initiation; and faster tapering. However, the structure of the study did not allow identification of which of these factors was responsible.
“Researching the best prednisone-tapering scheme is not only a major issue for patients with myasthenia gravis but also for other autoimmune or inflammatory diseases, because validated prednisone-tapering regimens are scarce,” the authors said.
The rapid tapering of prednisone therapy appears to be feasible, beneficial, and safe in patients with generalized myasthenia gravis and “warrants testing in other autoimmune diseases,” they added.
Particularly relevant to late-onset disease
Commenting on the study, Raffi Topakian, MD, Klinikum Wels-Grieskirchen, Wels, Austria, said the results showed that in patients with moderate to severe generalized myasthenia gravis requiring high-dose prednisone, azathioprine, a widely used immunosuppressant, may have a quicker steroid-sparing effect than previously thought, and that rapid steroid tapering can be achieved safely, resulting in a reduction of the cumulative steroid dose over a year despite higher initial doses.
Dr. Topakian, who was not involved with the research, pointed out that the median age was advanced (around 56 years), and the benefit of a regimen that leads to a reduction of the cumulative steroid dose over a year may be disproportionately larger for older, sicker patients with many comorbidities who are at considerably higher risk for a prednisone-induced increase in cardiovascular complications, osteoporotic fractures, and gastrointestinal bleeding.
“The study findings are particularly relevant for the management of late-onset myasthenia gravis (when first symptoms start after age 45-50 years), which is being encountered more frequently over the past years,” he said.
“But the holy grail of myasthenia gravis treatment has not been found yet,” Dr. Topakian noted. “Disappointingly, rapid tapering of steroids (compared to slow tapering) resulted in a reduction of the cumulative steroid dose only, but was not associated with better myasthenia gravis functional status or lower doses of steroids at 15 months. To my view, this finding points to the limited immunosuppressive efficacy of azathioprine.”
He added that the study findings should not be extrapolated to patients with mild presentations or to those with muscle-specific kinase myasthenia gravis.
Dr. Sharshar disclosed no relevant financial relationships. Disclosures for the study coauthors appear in the original article.
A version of this article first appeared on Medscape.com.
FROM JAMA NEUROLOGY
Short sleep predicts incident dementia and all-cause mortality
More evidence has emerged linking sleep deficiency, dementia, and mortality.
“Sleep disturbance and insufficiency have been shown to be associated with both the development and progression of Alzheimer’s disease and with all-cause mortality,” wrote Rebecca S. Robbins, PhD, of Brigham and Women’s Hospital, Boston, and colleagues. However, research on this topic has yielded conflicting results, and “few studies have included a comprehensive set of sleep characteristics in a single examination of incident dementia and all-cause mortality.”
In a study published in Aging, the researchers identified 2,812 adults aged 65 years and older from the National Health and Aging Trends Study (NHATS), a nationally representative longitudinal study of Medicare beneficiaries aged 65 years and older in the United States.
Participants completed surveys about sleep disturbance and duration in 2013 (1,575 individuals) and in 2014 (1,237 individuals), and the researchers examined the relationship between sleep disturbance and deficiency and incident dementia and all-cause mortality over the next 5 years. The average age of the study participants was 76.9 years, 60% were women, and 72% were White.
Overall, approximately 60% of the participants reported never or rarely having problems with alertness, approximately half said that they rarely or never napped, and more than half said they fell asleep in 15 minutes or less. Approximately 70% rated their sleep quality as good or very good, and more than 90% said they rarely or never snored.
The researchers examined the relationships between sleep characteristics and the development of incident dementia over 5 years. In a fully adjusted Cox multivariate analysis, individuals who slept 5 hours or less per night had approximately twice the risk for incident dementia as those who slept longer (hazard ratio, 2.04); risk of dementia also was higher among those who took 30 minutes or longer to fall asleep (HR, 1.45).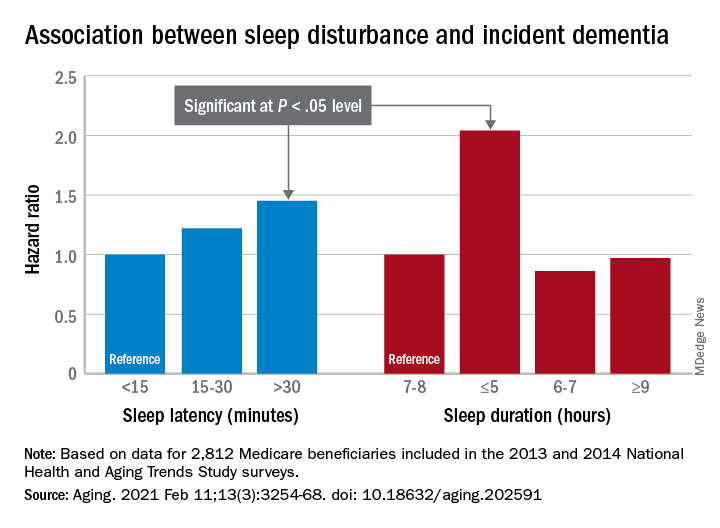
In addition, the risk of all-cause mortality was significantly higher among individuals who reported difficulty maintaining alertness some days or most days/every day (HR, 1.49 and HR, 1.65, respectively), routinely napping some days or most days/every day (HR, 1.38 and HR, 1.73, respectively), poor or very poor sleep quality (HR, 1.75), and sleeping 5 hours or less each night (HR, 2.38).
The study findings were limited by several factors including a population representing only one-quarter of the NHATS cohort, which prevented nationally representative estimates, the availability of only 2 years of sleep data, and small sample size for certain response categories, the researchers noted.
However, “our study offers a contribution to the literature on sleep among aging populations in its assessment of incident dementia and all-cause mortality and a range of sleep characteristics among older adults,” they said. In particular, “short sleep duration was a strong predictor of both incident dementia and all-cause mortality, suggesting this may be a sleep characteristic that is important – over and above the other predictors – of adverse outcomes among older adults,” and future areas for research include the development of novel behavioral interventions to improve sleep in this population.
The study was supported in part by the National Institute for Occupational Safety and Health; the National Heart, Lung, and Blood Institute; the National Institute on Aging; and the Brigham Research Institute Fund to Sustain Research Excellence. Lead author Dr. Robbins disclosed fees from Denihan Hospitality, Rituals Cosmetics, Dagmejan, Asystem, and SleepCycle. Several coauthors disclosed relationships with multiple pharmaceutical companies, and support from various philanthropic organizations.
More evidence has emerged linking sleep deficiency, dementia, and mortality.
“Sleep disturbance and insufficiency have been shown to be associated with both the development and progression of Alzheimer’s disease and with all-cause mortality,” wrote Rebecca S. Robbins, PhD, of Brigham and Women’s Hospital, Boston, and colleagues. However, research on this topic has yielded conflicting results, and “few studies have included a comprehensive set of sleep characteristics in a single examination of incident dementia and all-cause mortality.”
In a study published in Aging, the researchers identified 2,812 adults aged 65 years and older from the National Health and Aging Trends Study (NHATS), a nationally representative longitudinal study of Medicare beneficiaries aged 65 years and older in the United States.
Participants completed surveys about sleep disturbance and duration in 2013 (1,575 individuals) and in 2014 (1,237 individuals), and the researchers examined the relationship between sleep disturbance and deficiency and incident dementia and all-cause mortality over the next 5 years. The average age of the study participants was 76.9 years, 60% were women, and 72% were White.
Overall, approximately 60% of the participants reported never or rarely having problems with alertness, approximately half said that they rarely or never napped, and more than half said they fell asleep in 15 minutes or less. Approximately 70% rated their sleep quality as good or very good, and more than 90% said they rarely or never snored.
The researchers examined the relationships between sleep characteristics and the development of incident dementia over 5 years. In a fully adjusted Cox multivariate analysis, individuals who slept 5 hours or less per night had approximately twice the risk for incident dementia as those who slept longer (hazard ratio, 2.04); risk of dementia also was higher among those who took 30 minutes or longer to fall asleep (HR, 1.45).
In addition, the risk of all-cause mortality was significantly higher among individuals who reported difficulty maintaining alertness some days or most days/every day (HR, 1.49 and HR, 1.65, respectively), routinely napping some days or most days/every day (HR, 1.38 and HR, 1.73, respectively), poor or very poor sleep quality (HR, 1.75), and sleeping 5 hours or less each night (HR, 2.38).
The study findings were limited by several factors including a population representing only one-quarter of the NHATS cohort, which prevented nationally representative estimates, the availability of only 2 years of sleep data, and small sample size for certain response categories, the researchers noted.
However, “our study offers a contribution to the literature on sleep among aging populations in its assessment of incident dementia and all-cause mortality and a range of sleep characteristics among older adults,” they said. In particular, “short sleep duration was a strong predictor of both incident dementia and all-cause mortality, suggesting this may be a sleep characteristic that is important – over and above the other predictors – of adverse outcomes among older adults,” and future areas for research include the development of novel behavioral interventions to improve sleep in this population.
The study was supported in part by the National Institute for Occupational Safety and Health; the National Heart, Lung, and Blood Institute; the National Institute on Aging; and the Brigham Research Institute Fund to Sustain Research Excellence. Lead author Dr. Robbins disclosed fees from Denihan Hospitality, Rituals Cosmetics, Dagmejan, Asystem, and SleepCycle. Several coauthors disclosed relationships with multiple pharmaceutical companies, and support from various philanthropic organizations.
More evidence has emerged linking sleep deficiency, dementia, and mortality.
“Sleep disturbance and insufficiency have been shown to be associated with both the development and progression of Alzheimer’s disease and with all-cause mortality,” wrote Rebecca S. Robbins, PhD, of Brigham and Women’s Hospital, Boston, and colleagues. However, research on this topic has yielded conflicting results, and “few studies have included a comprehensive set of sleep characteristics in a single examination of incident dementia and all-cause mortality.”
In a study published in Aging, the researchers identified 2,812 adults aged 65 years and older from the National Health and Aging Trends Study (NHATS), a nationally representative longitudinal study of Medicare beneficiaries aged 65 years and older in the United States.
Participants completed surveys about sleep disturbance and duration in 2013 (1,575 individuals) and in 2014 (1,237 individuals), and the researchers examined the relationship between sleep disturbance and deficiency and incident dementia and all-cause mortality over the next 5 years. The average age of the study participants was 76.9 years, 60% were women, and 72% were White.
Overall, approximately 60% of the participants reported never or rarely having problems with alertness, approximately half said that they rarely or never napped, and more than half said they fell asleep in 15 minutes or less. Approximately 70% rated their sleep quality as good or very good, and more than 90% said they rarely or never snored.
The researchers examined the relationships between sleep characteristics and the development of incident dementia over 5 years. In a fully adjusted Cox multivariate analysis, individuals who slept 5 hours or less per night had approximately twice the risk for incident dementia as those who slept longer (hazard ratio, 2.04); risk of dementia also was higher among those who took 30 minutes or longer to fall asleep (HR, 1.45).
In addition, the risk of all-cause mortality was significantly higher among individuals who reported difficulty maintaining alertness some days or most days/every day (HR, 1.49 and HR, 1.65, respectively), routinely napping some days or most days/every day (HR, 1.38 and HR, 1.73, respectively), poor or very poor sleep quality (HR, 1.75), and sleeping 5 hours or less each night (HR, 2.38).
The study findings were limited by several factors including a population representing only one-quarter of the NHATS cohort, which prevented nationally representative estimates, the availability of only 2 years of sleep data, and small sample size for certain response categories, the researchers noted.
However, “our study offers a contribution to the literature on sleep among aging populations in its assessment of incident dementia and all-cause mortality and a range of sleep characteristics among older adults,” they said. In particular, “short sleep duration was a strong predictor of both incident dementia and all-cause mortality, suggesting this may be a sleep characteristic that is important – over and above the other predictors – of adverse outcomes among older adults,” and future areas for research include the development of novel behavioral interventions to improve sleep in this population.
The study was supported in part by the National Institute for Occupational Safety and Health; the National Heart, Lung, and Blood Institute; the National Institute on Aging; and the Brigham Research Institute Fund to Sustain Research Excellence. Lead author Dr. Robbins disclosed fees from Denihan Hospitality, Rituals Cosmetics, Dagmejan, Asystem, and SleepCycle. Several coauthors disclosed relationships with multiple pharmaceutical companies, and support from various philanthropic organizations.
FROM AGING
CDC chief lays out attack plan for COVID variants
earlier this week.
As part of JAMA’s Q&A series with JAMA editor in chief Howard Bauchner, MD, Dr. Walensky referenced the blueprint she coathored with Anthony Fauci, MD, the nation’s top infectious disease expert, and Henry T. Walke, MD, MPH, of the CDC, which was published on Feb. 17 in JAMA.
In the viewpoint article, they explain that the Department of Health & Human Services has established the SARS-CoV-2 Interagency Group to improve coordination among the CDC, the National Institutes of Health, the Food and Drug Administration, the Biomedical Advanced Research and Development Authority, the Department of Agriculture, and the Department of Defense.
Dr. Walensky said the first objective is to reinforce vigilance regarding public health mitigation strategies to decrease the amount of virus that’s circulating.
As part of that strategy, she said, the CDC strongly urges against nonessential travel.
In addition, public health leaders are working on a surveillance system to better understand the SARS-CoV-2 variants. That will take ramping up genome sequencing of the SARS-CoV-2 virus and ensuring that sampling is geographically representative.
She said the CDC is partnering with state health labs to obtain about 750 samples every week and is teaming up with commercial labs and academic centers to obtain an interim target of 6,000 samples per week.
She acknowledged the United States “is not where we need to be” with sequencing but has come a long way since January. At that time, they were sequencing 250 samples every week; they are currently sequencing thousands each week.
Data analysis is another concern: “We need to be able to understand at the basic science level what the information means,” Dr. Walensky said.
Researchers aren’t sure how the variants might affect use of convalescent plasma or monoclonal antibody treatments. It is expected that 5% of persons who are vaccinated against COVID-19 will nevertheless contract the disease. Sequencing will help answer whether such persons who have been vaccinated and who subsequently contract the virus are among those 5% or whether have been infected by a variant that evades the vaccine.
Accelerating vaccine administration globally and in the United States is essential, Dr. Walensky said.
As of Feb. 17, 56 million doses had been administered in the United States.
Top three threats
She updated the numbers on the three biggest variant threats.
Regarding B.1.1.7, which originated in the United Kingdom, she said: “So far, we’ve had over 1,200 cases in 41 states.” She noted that the variant is likely to be about 50% more transmissible and 30% to 50% more virulent.
“So far, it looks like that strain doesn’t have any real decrease in susceptibility to our vaccines,” she said.
The strain from South Africa (B.1.351) has been found in 19 cases in the United States.
The P.1. variant, which originated in Brazil, has been identified in two cases in two states.
Outlook for March and April
Dr. Bauchner asked Dr. Walensky what she envisions for March and April. He noted that public optimism is high in light of the continued reductions in COVID-19 case numbers, hospitalizations, and deaths, as well as the fact that warmer weather is coming and that more vaccinations are on the horizon.
“While I really am hopeful for what could happen in March and April,” Dr. Walensky said, “I really do know that this could go bad so fast. We saw it in November. We saw it in December.”
CDC models have projected that, by March, the more transmissible B.1.1.7 strain is likely to be the dominant strain, she reiterated.
“I worry that it will be spring, and we will all have had enough,” Dr. Walensky said. She noted that some states are already relaxing mask mandates.
“Around that time, life will look and feel a little better, and the motivation for those who might be vaccine hesitant may be diminished,” she said.
Dr. Bauchner also asked her to weigh in on whether a third vaccine, from Johnson & Johnson (J&J), may soon gain FDA emergency-use authorization – and whether its lower expected efficacy rate may result in a tiered system of vaccinations, with higher-risk populations receiving the more efficacious vaccines.
Dr. Walensky said more data are needed before that question can be answered.
“It may very well be that the data point us to the best populations in which to use this vaccine,” she said.
In phase 3 data, the J&J vaccine was shown to be 72% effective in the United States for moderate to severe disease.
Dr. Walensky said it’s important to remember that the projected efficacy for that vaccine is higher than that for the flu shot as well as many other vaccines currently in use for other diseases.
She said it also has several advantages. The vaccine has less-stringent storage requirements, requires just one dose, and protects against hospitalization and death, although it’s less efficacious in protecting against contracting the disease.
“I think many people would opt to get that one if they could get it sooner,” she said.
A version of this article first appeared on Medscape.com.
earlier this week.
As part of JAMA’s Q&A series with JAMA editor in chief Howard Bauchner, MD, Dr. Walensky referenced the blueprint she coathored with Anthony Fauci, MD, the nation’s top infectious disease expert, and Henry T. Walke, MD, MPH, of the CDC, which was published on Feb. 17 in JAMA.
In the viewpoint article, they explain that the Department of Health & Human Services has established the SARS-CoV-2 Interagency Group to improve coordination among the CDC, the National Institutes of Health, the Food and Drug Administration, the Biomedical Advanced Research and Development Authority, the Department of Agriculture, and the Department of Defense.
Dr. Walensky said the first objective is to reinforce vigilance regarding public health mitigation strategies to decrease the amount of virus that’s circulating.
As part of that strategy, she said, the CDC strongly urges against nonessential travel.
In addition, public health leaders are working on a surveillance system to better understand the SARS-CoV-2 variants. That will take ramping up genome sequencing of the SARS-CoV-2 virus and ensuring that sampling is geographically representative.
She said the CDC is partnering with state health labs to obtain about 750 samples every week and is teaming up with commercial labs and academic centers to obtain an interim target of 6,000 samples per week.
She acknowledged the United States “is not where we need to be” with sequencing but has come a long way since January. At that time, they were sequencing 250 samples every week; they are currently sequencing thousands each week.
Data analysis is another concern: “We need to be able to understand at the basic science level what the information means,” Dr. Walensky said.
Researchers aren’t sure how the variants might affect use of convalescent plasma or monoclonal antibody treatments. It is expected that 5% of persons who are vaccinated against COVID-19 will nevertheless contract the disease. Sequencing will help answer whether such persons who have been vaccinated and who subsequently contract the virus are among those 5% or whether have been infected by a variant that evades the vaccine.
Accelerating vaccine administration globally and in the United States is essential, Dr. Walensky said.
As of Feb. 17, 56 million doses had been administered in the United States.
Top three threats
She updated the numbers on the three biggest variant threats.
Regarding B.1.1.7, which originated in the United Kingdom, she said: “So far, we’ve had over 1,200 cases in 41 states.” She noted that the variant is likely to be about 50% more transmissible and 30% to 50% more virulent.
“So far, it looks like that strain doesn’t have any real decrease in susceptibility to our vaccines,” she said.
The strain from South Africa (B.1.351) has been found in 19 cases in the United States.
The P.1. variant, which originated in Brazil, has been identified in two cases in two states.
Outlook for March and April
Dr. Bauchner asked Dr. Walensky what she envisions for March and April. He noted that public optimism is high in light of the continued reductions in COVID-19 case numbers, hospitalizations, and deaths, as well as the fact that warmer weather is coming and that more vaccinations are on the horizon.
“While I really am hopeful for what could happen in March and April,” Dr. Walensky said, “I really do know that this could go bad so fast. We saw it in November. We saw it in December.”
CDC models have projected that, by March, the more transmissible B.1.1.7 strain is likely to be the dominant strain, she reiterated.
“I worry that it will be spring, and we will all have had enough,” Dr. Walensky said. She noted that some states are already relaxing mask mandates.
“Around that time, life will look and feel a little better, and the motivation for those who might be vaccine hesitant may be diminished,” she said.
Dr. Bauchner also asked her to weigh in on whether a third vaccine, from Johnson & Johnson (J&J), may soon gain FDA emergency-use authorization – and whether its lower expected efficacy rate may result in a tiered system of vaccinations, with higher-risk populations receiving the more efficacious vaccines.
Dr. Walensky said more data are needed before that question can be answered.
“It may very well be that the data point us to the best populations in which to use this vaccine,” she said.
In phase 3 data, the J&J vaccine was shown to be 72% effective in the United States for moderate to severe disease.
Dr. Walensky said it’s important to remember that the projected efficacy for that vaccine is higher than that for the flu shot as well as many other vaccines currently in use for other diseases.
She said it also has several advantages. The vaccine has less-stringent storage requirements, requires just one dose, and protects against hospitalization and death, although it’s less efficacious in protecting against contracting the disease.
“I think many people would opt to get that one if they could get it sooner,” she said.
A version of this article first appeared on Medscape.com.
earlier this week.
As part of JAMA’s Q&A series with JAMA editor in chief Howard Bauchner, MD, Dr. Walensky referenced the blueprint she coathored with Anthony Fauci, MD, the nation’s top infectious disease expert, and Henry T. Walke, MD, MPH, of the CDC, which was published on Feb. 17 in JAMA.
In the viewpoint article, they explain that the Department of Health & Human Services has established the SARS-CoV-2 Interagency Group to improve coordination among the CDC, the National Institutes of Health, the Food and Drug Administration, the Biomedical Advanced Research and Development Authority, the Department of Agriculture, and the Department of Defense.
Dr. Walensky said the first objective is to reinforce vigilance regarding public health mitigation strategies to decrease the amount of virus that’s circulating.
As part of that strategy, she said, the CDC strongly urges against nonessential travel.
In addition, public health leaders are working on a surveillance system to better understand the SARS-CoV-2 variants. That will take ramping up genome sequencing of the SARS-CoV-2 virus and ensuring that sampling is geographically representative.
She said the CDC is partnering with state health labs to obtain about 750 samples every week and is teaming up with commercial labs and academic centers to obtain an interim target of 6,000 samples per week.
She acknowledged the United States “is not where we need to be” with sequencing but has come a long way since January. At that time, they were sequencing 250 samples every week; they are currently sequencing thousands each week.
Data analysis is another concern: “We need to be able to understand at the basic science level what the information means,” Dr. Walensky said.
Researchers aren’t sure how the variants might affect use of convalescent plasma or monoclonal antibody treatments. It is expected that 5% of persons who are vaccinated against COVID-19 will nevertheless contract the disease. Sequencing will help answer whether such persons who have been vaccinated and who subsequently contract the virus are among those 5% or whether have been infected by a variant that evades the vaccine.
Accelerating vaccine administration globally and in the United States is essential, Dr. Walensky said.
As of Feb. 17, 56 million doses had been administered in the United States.
Top three threats
She updated the numbers on the three biggest variant threats.
Regarding B.1.1.7, which originated in the United Kingdom, she said: “So far, we’ve had over 1,200 cases in 41 states.” She noted that the variant is likely to be about 50% more transmissible and 30% to 50% more virulent.
“So far, it looks like that strain doesn’t have any real decrease in susceptibility to our vaccines,” she said.
The strain from South Africa (B.1.351) has been found in 19 cases in the United States.
The P.1. variant, which originated in Brazil, has been identified in two cases in two states.
Outlook for March and April
Dr. Bauchner asked Dr. Walensky what she envisions for March and April. He noted that public optimism is high in light of the continued reductions in COVID-19 case numbers, hospitalizations, and deaths, as well as the fact that warmer weather is coming and that more vaccinations are on the horizon.
“While I really am hopeful for what could happen in March and April,” Dr. Walensky said, “I really do know that this could go bad so fast. We saw it in November. We saw it in December.”
CDC models have projected that, by March, the more transmissible B.1.1.7 strain is likely to be the dominant strain, she reiterated.
“I worry that it will be spring, and we will all have had enough,” Dr. Walensky said. She noted that some states are already relaxing mask mandates.
“Around that time, life will look and feel a little better, and the motivation for those who might be vaccine hesitant may be diminished,” she said.
Dr. Bauchner also asked her to weigh in on whether a third vaccine, from Johnson & Johnson (J&J), may soon gain FDA emergency-use authorization – and whether its lower expected efficacy rate may result in a tiered system of vaccinations, with higher-risk populations receiving the more efficacious vaccines.
Dr. Walensky said more data are needed before that question can be answered.
“It may very well be that the data point us to the best populations in which to use this vaccine,” she said.
In phase 3 data, the J&J vaccine was shown to be 72% effective in the United States for moderate to severe disease.
Dr. Walensky said it’s important to remember that the projected efficacy for that vaccine is higher than that for the flu shot as well as many other vaccines currently in use for other diseases.
She said it also has several advantages. The vaccine has less-stringent storage requirements, requires just one dose, and protects against hospitalization and death, although it’s less efficacious in protecting against contracting the disease.
“I think many people would opt to get that one if they could get it sooner,” she said.
A version of this article first appeared on Medscape.com.
Alien cells may explain COVID-19 brain fog
, a new report suggests.
The authors report five separate post-mortem cases from patients who died with COVID-19 in which large cells resembling megakaryocytes were identified in cortical capillaries. Immunohistochemistry subsequently confirmed their megakaryocyte identity.
They point out that the finding is of interest as – to their knowledge – megakaryocytes have not been found in the brain before.
The observations are described in a research letter published online Feb. 12 in JAMA Neurology.
Bone marrow cells in the brain
Lead author David Nauen, MD, PhD, a neuropathologist from Johns Hopkins University, Baltimore, reported that he identified these cells in the first analysis of post-mortem brain tissue from a patient who had COVID-19.
“Some other viruses cause changes in the brain such as encephalopathy, and as neurologic symptoms are often reported in COVID-19, I was curious to see if similar effects were seen in brain post-mortem samples from patients who had died with the infection,” Dr. Nauen said.
On his first analysis of the brain tissue of a patient who had COVID-19, Dr. Nauen saw no evidence of viral encephalitis, but he observed some “unusually large” cells in the brain capillaries.
“I was taken aback; I couldn’t figure out what they were. Then I realized these cells were megakaryocytes from the bone marrow. I have never seen these cells in the brain before. I asked several colleagues and none of them had either. After extensive literature searches, I could find no evidence of megakaryocytes being in the brain,” Dr. Nauen noted.
Megakaryocytes, he explained, are “very large cells, and the brain capillaries are very small – just large enough to let red blood cells and lymphocytes pass through. To see these very large cells in such vessels is extremely unusual. It looks like they are causing occlusions.”
By occluding flow through individual capillaries, these large cells could cause ischemic alteration in a distinct pattern, potentially resulting in an atypical form of neurologic impairment, the authors suggest.
“This might alter the hemodynamics and put pressure on other vessels, possibly contributing to the increased risk of stroke that has been reported in COVID-19,” Dr. Nauen said. None of the samples he examined came from patients with COVID-19 who had had a stroke, he reported.
Other than the presence of megakaryocytes in the capillaries, the brain looked normal, he said. He has now examined samples from 15 brains of patients who had COVID-19 and megakaryocytes have been found in the brain capillaries in five cases.
New neurologic complication
Classic encephalitis found with other viruses has not been reported in brain post-mortem examinations from patients who had COVID-19, Dr. Nauen noted. “The cognitive issues such as grogginess associated with COVID-19 would indicate problems with the cortex but that hasn’t been documented. This occlusion of a multitude of tiny vessels by megalokaryocytes may offer some explanation of the cognitive issues. This is a new kind of vascular insult seen on pathology, and suggests a new kind of neurologic complication,” he added.
The big question is what these megakaryocytes are doing in the brain.
“Megakaryocytes are bone marrow cells. They are not immune cells. Their job is to produce platelets to help the blood clot. They are not normally found outside the bone marrow, but they have been reported in other organs in COVID-19 patients.
“But the big puzzle associated with finding them in the brain is how they get through the very fine network of blood vessels in the lungs. The geometry just doesn’t work. We don’t know which part of the COVID inflammatory response makes this happen,” said Dr. Nauen.
The authors suggest one possibility is that altered endothelial or other signaling is recruiting megakaryocytes into the circulation and somehow permitting them to pass through the lungs.
“We need to try and understand if there is anything distinctive about these megakaryocytes – which proteins are they expressing that may explain why they are behaving in such an unusual way,” said Dr. Nauen.
Noting that many patients with severe COVID-19 have problems with clotting, and megakaryocytes are part of the clotting system, he speculated that some sort of aberrant message is being sent to these cells.
“It is notable that we found megakaryocytes in cortical capillaries in 33% of cases examined. Because the standard brain autopsy sections taken sampled at random [are] only a minute portion of the cortical volume, finding these cells suggests the total burden could be considerable,” the authors wrote.
Dr. Nauen added that to his knowledge, this is the first report of such observations, and the next step is to look for similar findings in larger sample sizes.
A version of this article first appeared on Medscape.com.
, a new report suggests.
The authors report five separate post-mortem cases from patients who died with COVID-19 in which large cells resembling megakaryocytes were identified in cortical capillaries. Immunohistochemistry subsequently confirmed their megakaryocyte identity.
They point out that the finding is of interest as – to their knowledge – megakaryocytes have not been found in the brain before.
The observations are described in a research letter published online Feb. 12 in JAMA Neurology.
Bone marrow cells in the brain
Lead author David Nauen, MD, PhD, a neuropathologist from Johns Hopkins University, Baltimore, reported that he identified these cells in the first analysis of post-mortem brain tissue from a patient who had COVID-19.
“Some other viruses cause changes in the brain such as encephalopathy, and as neurologic symptoms are often reported in COVID-19, I was curious to see if similar effects were seen in brain post-mortem samples from patients who had died with the infection,” Dr. Nauen said.
On his first analysis of the brain tissue of a patient who had COVID-19, Dr. Nauen saw no evidence of viral encephalitis, but he observed some “unusually large” cells in the brain capillaries.
“I was taken aback; I couldn’t figure out what they were. Then I realized these cells were megakaryocytes from the bone marrow. I have never seen these cells in the brain before. I asked several colleagues and none of them had either. After extensive literature searches, I could find no evidence of megakaryocytes being in the brain,” Dr. Nauen noted.
Megakaryocytes, he explained, are “very large cells, and the brain capillaries are very small – just large enough to let red blood cells and lymphocytes pass through. To see these very large cells in such vessels is extremely unusual. It looks like they are causing occlusions.”
By occluding flow through individual capillaries, these large cells could cause ischemic alteration in a distinct pattern, potentially resulting in an atypical form of neurologic impairment, the authors suggest.
“This might alter the hemodynamics and put pressure on other vessels, possibly contributing to the increased risk of stroke that has been reported in COVID-19,” Dr. Nauen said. None of the samples he examined came from patients with COVID-19 who had had a stroke, he reported.
Other than the presence of megakaryocytes in the capillaries, the brain looked normal, he said. He has now examined samples from 15 brains of patients who had COVID-19 and megakaryocytes have been found in the brain capillaries in five cases.
New neurologic complication
Classic encephalitis found with other viruses has not been reported in brain post-mortem examinations from patients who had COVID-19, Dr. Nauen noted. “The cognitive issues such as grogginess associated with COVID-19 would indicate problems with the cortex but that hasn’t been documented. This occlusion of a multitude of tiny vessels by megalokaryocytes may offer some explanation of the cognitive issues. This is a new kind of vascular insult seen on pathology, and suggests a new kind of neurologic complication,” he added.
The big question is what these megakaryocytes are doing in the brain.
“Megakaryocytes are bone marrow cells. They are not immune cells. Their job is to produce platelets to help the blood clot. They are not normally found outside the bone marrow, but they have been reported in other organs in COVID-19 patients.
“But the big puzzle associated with finding them in the brain is how they get through the very fine network of blood vessels in the lungs. The geometry just doesn’t work. We don’t know which part of the COVID inflammatory response makes this happen,” said Dr. Nauen.
The authors suggest one possibility is that altered endothelial or other signaling is recruiting megakaryocytes into the circulation and somehow permitting them to pass through the lungs.
“We need to try and understand if there is anything distinctive about these megakaryocytes – which proteins are they expressing that may explain why they are behaving in such an unusual way,” said Dr. Nauen.
Noting that many patients with severe COVID-19 have problems with clotting, and megakaryocytes are part of the clotting system, he speculated that some sort of aberrant message is being sent to these cells.
“It is notable that we found megakaryocytes in cortical capillaries in 33% of cases examined. Because the standard brain autopsy sections taken sampled at random [are] only a minute portion of the cortical volume, finding these cells suggests the total burden could be considerable,” the authors wrote.
Dr. Nauen added that to his knowledge, this is the first report of such observations, and the next step is to look for similar findings in larger sample sizes.
A version of this article first appeared on Medscape.com.
, a new report suggests.
The authors report five separate post-mortem cases from patients who died with COVID-19 in which large cells resembling megakaryocytes were identified in cortical capillaries. Immunohistochemistry subsequently confirmed their megakaryocyte identity.
They point out that the finding is of interest as – to their knowledge – megakaryocytes have not been found in the brain before.
The observations are described in a research letter published online Feb. 12 in JAMA Neurology.
Bone marrow cells in the brain
Lead author David Nauen, MD, PhD, a neuropathologist from Johns Hopkins University, Baltimore, reported that he identified these cells in the first analysis of post-mortem brain tissue from a patient who had COVID-19.
“Some other viruses cause changes in the brain such as encephalopathy, and as neurologic symptoms are often reported in COVID-19, I was curious to see if similar effects were seen in brain post-mortem samples from patients who had died with the infection,” Dr. Nauen said.
On his first analysis of the brain tissue of a patient who had COVID-19, Dr. Nauen saw no evidence of viral encephalitis, but he observed some “unusually large” cells in the brain capillaries.
“I was taken aback; I couldn’t figure out what they were. Then I realized these cells were megakaryocytes from the bone marrow. I have never seen these cells in the brain before. I asked several colleagues and none of them had either. After extensive literature searches, I could find no evidence of megakaryocytes being in the brain,” Dr. Nauen noted.
Megakaryocytes, he explained, are “very large cells, and the brain capillaries are very small – just large enough to let red blood cells and lymphocytes pass through. To see these very large cells in such vessels is extremely unusual. It looks like they are causing occlusions.”
By occluding flow through individual capillaries, these large cells could cause ischemic alteration in a distinct pattern, potentially resulting in an atypical form of neurologic impairment, the authors suggest.
“This might alter the hemodynamics and put pressure on other vessels, possibly contributing to the increased risk of stroke that has been reported in COVID-19,” Dr. Nauen said. None of the samples he examined came from patients with COVID-19 who had had a stroke, he reported.
Other than the presence of megakaryocytes in the capillaries, the brain looked normal, he said. He has now examined samples from 15 brains of patients who had COVID-19 and megakaryocytes have been found in the brain capillaries in five cases.
New neurologic complication
Classic encephalitis found with other viruses has not been reported in brain post-mortem examinations from patients who had COVID-19, Dr. Nauen noted. “The cognitive issues such as grogginess associated with COVID-19 would indicate problems with the cortex but that hasn’t been documented. This occlusion of a multitude of tiny vessels by megalokaryocytes may offer some explanation of the cognitive issues. This is a new kind of vascular insult seen on pathology, and suggests a new kind of neurologic complication,” he added.
The big question is what these megakaryocytes are doing in the brain.
“Megakaryocytes are bone marrow cells. They are not immune cells. Their job is to produce platelets to help the blood clot. They are not normally found outside the bone marrow, but they have been reported in other organs in COVID-19 patients.
“But the big puzzle associated with finding them in the brain is how they get through the very fine network of blood vessels in the lungs. The geometry just doesn’t work. We don’t know which part of the COVID inflammatory response makes this happen,” said Dr. Nauen.
The authors suggest one possibility is that altered endothelial or other signaling is recruiting megakaryocytes into the circulation and somehow permitting them to pass through the lungs.
“We need to try and understand if there is anything distinctive about these megakaryocytes – which proteins are they expressing that may explain why they are behaving in such an unusual way,” said Dr. Nauen.
Noting that many patients with severe COVID-19 have problems with clotting, and megakaryocytes are part of the clotting system, he speculated that some sort of aberrant message is being sent to these cells.
“It is notable that we found megakaryocytes in cortical capillaries in 33% of cases examined. Because the standard brain autopsy sections taken sampled at random [are] only a minute portion of the cortical volume, finding these cells suggests the total burden could be considerable,” the authors wrote.
Dr. Nauen added that to his knowledge, this is the first report of such observations, and the next step is to look for similar findings in larger sample sizes.
A version of this article first appeared on Medscape.com.
FROM JAMA NEUROLOGY
Opioids prescribed for diabetic neuropathy pain, against advice
Prescriptions for opioids as a first-line treatment for painful diabetic peripheral neuropathy (DPN) outnumbered those for other medications between 2014 and 2018, despite the fact that the former is not recommended, new research indicates.
“We know that for any kind of chronic pain, opioids are not ideal. They’re not very effective for chronic pain in general, and they’re definitely not safe,” senior author Rozalina G. McCoy, MD, an endocrinologist and primary care clinician at the Mayo Clinic in Rochester, Minn., told this news organization.
That’s true even for severe DPN pain or painful exacerbations, she added.
“There’s a myth that opioids are the strongest pain meds possible ... For painful neuropathic pain, duloxetine [Cymbalta], pregabalin [Lyrica], and gabapentin [Neurontin] are the most effective pain medications based on multiple studies and extensive experience using them,” she explained. “But I think the public perception is that opioids are the strongest. When a patient comes with severe pain, I think there’s that kind of gut feeling that if the pain is severe, I need to give opioids.”
What’s more, she noted, “evidence is emerging for other harms, not only the potential for dependency and potential overdose, but also the potential for opioid-induced hyperalgesia. Opioids themselves can cause chronic pain. When we think about using opioids for chronic pain, we are really shooting ourselves in the foot. We’re going to harm patients.”
The American Diabetes Association DPN guidelines essentially say as much, advising opioids only as a tertiary option for refractory pain, she observed.
The new findings, from a retrospective study of Mayo Clinic electronic health data, were published online in JAMA Network Open by Jungwei Fan, PhD, also of Mayo Clinic, and colleagues.
Are fewer patients with DPN receiving any treatment now?
The data also reveal that, while opioid prescribing dropped over the study period, there wasn’t a comparable rise in prescriptions of recommended pain medications, suggesting that recent efforts to minimize opioid prescribing may have resulted in less overall treatment of significant pain. (The study had to be stopped in 2018 when Mayo switched to a new electronic health record system, Dr. McCoy explained.)
“The proportion of opioids among new prescriptions has been decreasing. I’m hopeful that the rates are even lower now than they were 2 years ago. What was concerning to me was the proportion of people receiving treatment overall had gone down,” Dr. McCoy noted.
“So, while it’s great that opioids aren’t being used, it’s doubtful that people with DPN are any less symptomatic. So I worry that there’s a proportion of patients who have pain who aren’t getting the treatment they need just because we don’t want to give them opioids. There are other options,” Dr. McCoy said, including nonpharmacologic approaches.
Opioids dominated in new-onset DPN prescribing during 2014-2018
The study involved 3,495 adults with newly diagnosed DPN from all three Mayo Clinic locations in Rochester, Minn.; Phoenix, Ariz.; and Jacksonville, Fla. during the period 2014-2018. Of those, 40.2% (1,406) were prescribed a new pain medication after diagnosis. However, that proportion dropped from 45.6% in 2014 to 35.2% in 2018.
The odds of initiating any treatment were significantly greater among patients with depression (odds ratio, 1.61), arthritis (OR, 1.21), and back pain (OR, 1.34), but decreased over time among all patients.
Among those receiving drug treatment, opioids were prescribed to 43.8%, whereas guideline-recommended medications (gabapentin, pregabalin, and serotonin norepinephrine reuptake inhibitors including duloxetine) were prescribed to 42.9%.
Another 20.6% received medications deemed “acceptable” for treating neuropathic pain, including topical analgesics, tricyclic antidepressants, and other anticonvulsants.
Males were significantly more likely than females to receive opioids (OR, 1.26), while individuals diagnosed with comorbid fibromyalgia were less likely (OR, 0.67). Those with comorbid arthritis were less likely to receive recommended DPN medications (OR, 0.76).
Use of opioids was 29% less likely in 2018, compared with 2014, although this difference did not achieve significance. Similarly, use of recommended medications was 25% more likely in 2018, compared with 2014, also not a significant difference.
Dr. McCoy offers clinical pearls for treating pain in DPN
Clinically, Dr. McCoy said that she individualizes treatment for painful DPN.
“I tend to use duloxetine if the patient also has a mood disorder including depression or anxiety, because it can also help with that. Gabapentin can also be helpful for radiculopathy or for chronic low-back pain. It can even help with degenerative joint disease like arthritis of the knees. So, you maximize benefit if you use one drug to treat multiple things.”
All three recommended medications are generic now, although pregabalin still tends to be more expensive, she noted. Gabapentin can cause drowsiness, which makes it ideal for a patient with insomnia but much less so for a long-haul truck driver. Duloxetine doesn’t cause sleepiness. Pregabalin can, but less so than gabapentin.
“I think that’s why it’s so important to talk to your patient and ask how the neuropathy is affecting them. What other comorbidities do they have? What is their life like? I think you have to figure out what drug works for each individual person.”
Importantly, she advised, if one of the three doesn’t work, stop it and try another. “It doesn’t mean that none of these meds work. All three should be tried to see if they give relief.”
Nonpharmacologic measures such as cognitive behavioral therapy, acupuncture, or physical therapy may help some patients as well.
Supplements such as vitamin B12 – which can also help with metformin-induced B12 deficiency – or alpha-lipoic acid may also be worth a try as long as the patient is made aware of potential risks, she noted.
Dr. McCoy hopes to repeat this study using national data. “I don’t think this is isolated to Mayo ... I think it affects all practices,” she said.
Since the study, “we [Mayo Clinic] have implemented practice changes to limit use of opioids for chronic pain ... so I hope it’s getting better. It’s important to be aware of our patterns in prescribing.”
The study was funded by the National Institute of Diabetes and Digestive and Kidney Diseases. Dr. McCoy reported receiving grants from the AARP Quality Measure Innovation program through a collaboration with OptumLabs and the Mayo Clinic’s Robert D. and Patricia E. Kern Center for the Science of Health Care Delivery.
A version of this article first appeared on Medscape.com.
Prescriptions for opioids as a first-line treatment for painful diabetic peripheral neuropathy (DPN) outnumbered those for other medications between 2014 and 2018, despite the fact that the former is not recommended, new research indicates.
“We know that for any kind of chronic pain, opioids are not ideal. They’re not very effective for chronic pain in general, and they’re definitely not safe,” senior author Rozalina G. McCoy, MD, an endocrinologist and primary care clinician at the Mayo Clinic in Rochester, Minn., told this news organization.
That’s true even for severe DPN pain or painful exacerbations, she added.
“There’s a myth that opioids are the strongest pain meds possible ... For painful neuropathic pain, duloxetine [Cymbalta], pregabalin [Lyrica], and gabapentin [Neurontin] are the most effective pain medications based on multiple studies and extensive experience using them,” she explained. “But I think the public perception is that opioids are the strongest. When a patient comes with severe pain, I think there’s that kind of gut feeling that if the pain is severe, I need to give opioids.”
What’s more, she noted, “evidence is emerging for other harms, not only the potential for dependency and potential overdose, but also the potential for opioid-induced hyperalgesia. Opioids themselves can cause chronic pain. When we think about using opioids for chronic pain, we are really shooting ourselves in the foot. We’re going to harm patients.”
The American Diabetes Association DPN guidelines essentially say as much, advising opioids only as a tertiary option for refractory pain, she observed.
The new findings, from a retrospective study of Mayo Clinic electronic health data, were published online in JAMA Network Open by Jungwei Fan, PhD, also of Mayo Clinic, and colleagues.
Are fewer patients with DPN receiving any treatment now?
The data also reveal that, while opioid prescribing dropped over the study period, there wasn’t a comparable rise in prescriptions of recommended pain medications, suggesting that recent efforts to minimize opioid prescribing may have resulted in less overall treatment of significant pain. (The study had to be stopped in 2018 when Mayo switched to a new electronic health record system, Dr. McCoy explained.)
“The proportion of opioids among new prescriptions has been decreasing. I’m hopeful that the rates are even lower now than they were 2 years ago. What was concerning to me was the proportion of people receiving treatment overall had gone down,” Dr. McCoy noted.
“So, while it’s great that opioids aren’t being used, it’s doubtful that people with DPN are any less symptomatic. So I worry that there’s a proportion of patients who have pain who aren’t getting the treatment they need just because we don’t want to give them opioids. There are other options,” Dr. McCoy said, including nonpharmacologic approaches.
Opioids dominated in new-onset DPN prescribing during 2014-2018
The study involved 3,495 adults with newly diagnosed DPN from all three Mayo Clinic locations in Rochester, Minn.; Phoenix, Ariz.; and Jacksonville, Fla. during the period 2014-2018. Of those, 40.2% (1,406) were prescribed a new pain medication after diagnosis. However, that proportion dropped from 45.6% in 2014 to 35.2% in 2018.
The odds of initiating any treatment were significantly greater among patients with depression (odds ratio, 1.61), arthritis (OR, 1.21), and back pain (OR, 1.34), but decreased over time among all patients.
Among those receiving drug treatment, opioids were prescribed to 43.8%, whereas guideline-recommended medications (gabapentin, pregabalin, and serotonin norepinephrine reuptake inhibitors including duloxetine) were prescribed to 42.9%.
Another 20.6% received medications deemed “acceptable” for treating neuropathic pain, including topical analgesics, tricyclic antidepressants, and other anticonvulsants.
Males were significantly more likely than females to receive opioids (OR, 1.26), while individuals diagnosed with comorbid fibromyalgia were less likely (OR, 0.67). Those with comorbid arthritis were less likely to receive recommended DPN medications (OR, 0.76).
Use of opioids was 29% less likely in 2018, compared with 2014, although this difference did not achieve significance. Similarly, use of recommended medications was 25% more likely in 2018, compared with 2014, also not a significant difference.
Dr. McCoy offers clinical pearls for treating pain in DPN
Clinically, Dr. McCoy said that she individualizes treatment for painful DPN.
“I tend to use duloxetine if the patient also has a mood disorder including depression or anxiety, because it can also help with that. Gabapentin can also be helpful for radiculopathy or for chronic low-back pain. It can even help with degenerative joint disease like arthritis of the knees. So, you maximize benefit if you use one drug to treat multiple things.”
All three recommended medications are generic now, although pregabalin still tends to be more expensive, she noted. Gabapentin can cause drowsiness, which makes it ideal for a patient with insomnia but much less so for a long-haul truck driver. Duloxetine doesn’t cause sleepiness. Pregabalin can, but less so than gabapentin.
“I think that’s why it’s so important to talk to your patient and ask how the neuropathy is affecting them. What other comorbidities do they have? What is their life like? I think you have to figure out what drug works for each individual person.”
Importantly, she advised, if one of the three doesn’t work, stop it and try another. “It doesn’t mean that none of these meds work. All three should be tried to see if they give relief.”
Nonpharmacologic measures such as cognitive behavioral therapy, acupuncture, or physical therapy may help some patients as well.
Supplements such as vitamin B12 – which can also help with metformin-induced B12 deficiency – or alpha-lipoic acid may also be worth a try as long as the patient is made aware of potential risks, she noted.
Dr. McCoy hopes to repeat this study using national data. “I don’t think this is isolated to Mayo ... I think it affects all practices,” she said.
Since the study, “we [Mayo Clinic] have implemented practice changes to limit use of opioids for chronic pain ... so I hope it’s getting better. It’s important to be aware of our patterns in prescribing.”
The study was funded by the National Institute of Diabetes and Digestive and Kidney Diseases. Dr. McCoy reported receiving grants from the AARP Quality Measure Innovation program through a collaboration with OptumLabs and the Mayo Clinic’s Robert D. and Patricia E. Kern Center for the Science of Health Care Delivery.
A version of this article first appeared on Medscape.com.
Prescriptions for opioids as a first-line treatment for painful diabetic peripheral neuropathy (DPN) outnumbered those for other medications between 2014 and 2018, despite the fact that the former is not recommended, new research indicates.
“We know that for any kind of chronic pain, opioids are not ideal. They’re not very effective for chronic pain in general, and they’re definitely not safe,” senior author Rozalina G. McCoy, MD, an endocrinologist and primary care clinician at the Mayo Clinic in Rochester, Minn., told this news organization.
That’s true even for severe DPN pain or painful exacerbations, she added.
“There’s a myth that opioids are the strongest pain meds possible ... For painful neuropathic pain, duloxetine [Cymbalta], pregabalin [Lyrica], and gabapentin [Neurontin] are the most effective pain medications based on multiple studies and extensive experience using them,” she explained. “But I think the public perception is that opioids are the strongest. When a patient comes with severe pain, I think there’s that kind of gut feeling that if the pain is severe, I need to give opioids.”
What’s more, she noted, “evidence is emerging for other harms, not only the potential for dependency and potential overdose, but also the potential for opioid-induced hyperalgesia. Opioids themselves can cause chronic pain. When we think about using opioids for chronic pain, we are really shooting ourselves in the foot. We’re going to harm patients.”
The American Diabetes Association DPN guidelines essentially say as much, advising opioids only as a tertiary option for refractory pain, she observed.
The new findings, from a retrospective study of Mayo Clinic electronic health data, were published online in JAMA Network Open by Jungwei Fan, PhD, also of Mayo Clinic, and colleagues.
Are fewer patients with DPN receiving any treatment now?
The data also reveal that, while opioid prescribing dropped over the study period, there wasn’t a comparable rise in prescriptions of recommended pain medications, suggesting that recent efforts to minimize opioid prescribing may have resulted in less overall treatment of significant pain. (The study had to be stopped in 2018 when Mayo switched to a new electronic health record system, Dr. McCoy explained.)
“The proportion of opioids among new prescriptions has been decreasing. I’m hopeful that the rates are even lower now than they were 2 years ago. What was concerning to me was the proportion of people receiving treatment overall had gone down,” Dr. McCoy noted.
“So, while it’s great that opioids aren’t being used, it’s doubtful that people with DPN are any less symptomatic. So I worry that there’s a proportion of patients who have pain who aren’t getting the treatment they need just because we don’t want to give them opioids. There are other options,” Dr. McCoy said, including nonpharmacologic approaches.
Opioids dominated in new-onset DPN prescribing during 2014-2018
The study involved 3,495 adults with newly diagnosed DPN from all three Mayo Clinic locations in Rochester, Minn.; Phoenix, Ariz.; and Jacksonville, Fla. during the period 2014-2018. Of those, 40.2% (1,406) were prescribed a new pain medication after diagnosis. However, that proportion dropped from 45.6% in 2014 to 35.2% in 2018.
The odds of initiating any treatment were significantly greater among patients with depression (odds ratio, 1.61), arthritis (OR, 1.21), and back pain (OR, 1.34), but decreased over time among all patients.
Among those receiving drug treatment, opioids were prescribed to 43.8%, whereas guideline-recommended medications (gabapentin, pregabalin, and serotonin norepinephrine reuptake inhibitors including duloxetine) were prescribed to 42.9%.
Another 20.6% received medications deemed “acceptable” for treating neuropathic pain, including topical analgesics, tricyclic antidepressants, and other anticonvulsants.
Males were significantly more likely than females to receive opioids (OR, 1.26), while individuals diagnosed with comorbid fibromyalgia were less likely (OR, 0.67). Those with comorbid arthritis were less likely to receive recommended DPN medications (OR, 0.76).
Use of opioids was 29% less likely in 2018, compared with 2014, although this difference did not achieve significance. Similarly, use of recommended medications was 25% more likely in 2018, compared with 2014, also not a significant difference.
Dr. McCoy offers clinical pearls for treating pain in DPN
Clinically, Dr. McCoy said that she individualizes treatment for painful DPN.
“I tend to use duloxetine if the patient also has a mood disorder including depression or anxiety, because it can also help with that. Gabapentin can also be helpful for radiculopathy or for chronic low-back pain. It can even help with degenerative joint disease like arthritis of the knees. So, you maximize benefit if you use one drug to treat multiple things.”
All three recommended medications are generic now, although pregabalin still tends to be more expensive, she noted. Gabapentin can cause drowsiness, which makes it ideal for a patient with insomnia but much less so for a long-haul truck driver. Duloxetine doesn’t cause sleepiness. Pregabalin can, but less so than gabapentin.
“I think that’s why it’s so important to talk to your patient and ask how the neuropathy is affecting them. What other comorbidities do they have? What is their life like? I think you have to figure out what drug works for each individual person.”
Importantly, she advised, if one of the three doesn’t work, stop it and try another. “It doesn’t mean that none of these meds work. All three should be tried to see if they give relief.”
Nonpharmacologic measures such as cognitive behavioral therapy, acupuncture, or physical therapy may help some patients as well.
Supplements such as vitamin B12 – which can also help with metformin-induced B12 deficiency – or alpha-lipoic acid may also be worth a try as long as the patient is made aware of potential risks, she noted.
Dr. McCoy hopes to repeat this study using national data. “I don’t think this is isolated to Mayo ... I think it affects all practices,” she said.
Since the study, “we [Mayo Clinic] have implemented practice changes to limit use of opioids for chronic pain ... so I hope it’s getting better. It’s important to be aware of our patterns in prescribing.”
The study was funded by the National Institute of Diabetes and Digestive and Kidney Diseases. Dr. McCoy reported receiving grants from the AARP Quality Measure Innovation program through a collaboration with OptumLabs and the Mayo Clinic’s Robert D. and Patricia E. Kern Center for the Science of Health Care Delivery.
A version of this article first appeared on Medscape.com.
Don’t fear patients reading their clinical notes: Opinion
Doctors are learning about new rules coming this April that encourage open and transparent communication among patients, families, and clinicians. The rules, putting into effect the bipartisan 21st Century Cures Act, mandate offering patients access to notes (“open notes”) written by clinicians in electronic medical records.
A recent article from this news organization noted that for many doctors this represents both a sudden and troubling change in practice. For others, the rules codify what they have been doing as a matter of routine for a decade. Spurred by the OpenNotes movement, at least 55 million Americans are already offered access to their clinical notes, including, since 2013, more than 9 million veterans with access to the Blue Button function in Veterans Affairs practices and hospitals.
The practice is spreading beyond the United States to other countries, including Canada, Sweden, Norway, Estonia, and the United Kingdom.
In this commentary, we review what patients, clinicians, and policymakers have been learning about open notes.
The patient experience
What do patients experience? In a survey of more than 22,000 patients who read notes in three diverse health systems, more than 90% reported having a good grasp of what their doctors and other clinicians had written, and very few (3%) reported being very confused by what they read. About two-thirds described reading their notes as very important for taking care of their health, remembering details of their visits and their care plans, and understanding why a medication was prescribed.
Indeed, in a clinically exciting finding, 14% of survey respondents reported that reading their notes made them more likely to take their medications as their doctors wished. With about half of Americans with chronic illness failing to take their medicines as prescribed, which sometimes leads to compromised outcomes and associated unnecessary costs (estimated at $300 billion annually), these reports of increased adherence should be taken very seriously.
Some doctors anticipate that open notes will erode patient communication. A growing body of research reveals just the opposite. In multiple surveys, patients describe open notes as “extending the visit,” strengthening collaboration and teamwork with their doctor. Quite possibly, the invitation to read notes may in itself increase trust. Such benefits appear especially pronounced among patients who are older, less educated, are persons of color or Hispanic, or who do not speak English at home.
And in several studies, more than a third of patients also report sharing their notes with others, with older and chronically ill patients in particular sharing access with family and friends who are their care partners.
On the other hand, a small minority of patients (5%) do report being more worried by what they read. It’s unknown whether this is because they are better informed about their care or because baseline anxiety levels increase. Doctors expect also that some patients, particularly those with cancer or serious mental illness, will be upset by their notes. So far, evidence does not support that specific concern.
Conversely, withholding, delaying, or blocking notes may be a source of anxiety or even stigmatization. When clinicians find themselves worried about sharing notes, we suggest that they discuss with their patients the benefits and risks. Recall also that transparency facilitates freedom of choice; patients make their own decision, and quite a few choose to leave notes unread.
Finding mistakes early and preventing harm are important goals for health care, and open notes can make care safer. Inevitably, medical records contain errors, omissions, and inaccuracies. In a large patient survey, 21% reported finding an error in their notes, and 42% perceived the error to be serious.
Moreover, 25% of doctors with more than a year’s experience with open notes reported patients finding errors that they (the doctors) considered “serious.” In 2015, the National Academy of Medicine cited open notes as a mechanism for improving diagnostic accuracy. In regard to possible legal action from patients, most attorneys, patients, and doctors agree that more transparent communication will build trust overall and, if anything, diminish litigation. We know of no instances so far of lawsuits deriving from open notes.
The physician experience
Doctors may worry that open notes will impede workflow, that they will be compelled to “dumb down” their documentation to avoid causing offense or anxiety, and that patients will demand changes to what is written. Here, extensive survey research should allay such fears and expectations. In a survey of more than 1,600 clinicians with at least 1 year of experience with open notes, reports of disruption to workflow were uncommon.
Most doctors (84%) reported that patients contacted them with questions about their notes “less than monthly or never.” Approximately two-thirds (62%) reported spending the same amount of time writing visit notes.
After implementing open notes, many doctors do report being more mindful about their documentation. For example, 41% reported changing how they used language such as “patient denies” or “noncompliant,” and 18% reported changing their use of medical jargon or abbreviations. Might these changes undermine the utility of medical notes? A majority of doctors surveyed (78%) said no, reporting that, after implementing open notes, the value of their documentation was the same or better.
Innovations spotlight difficult and often longstanding challenges. Open notes highlight the complex role of medical records in preserving privacy, especially in the spectrum of abuse, whether domestic or involving elders, children or sexual transgressions. For families with adolescents, issues concerning confidentiality can become a two-way street, and federal and state rules at times provide conflicting and idiosyncratic guidance. It is important to emphasize that the new rules permit information blocking if there is clear evidence that doing so “will substantially reduce the risk of harm” to patients or to other third parties.
Perhaps think of open notes as a new medicine designed to help the vast majority of those who use it but with side effects and even contraindications for a few. Doctors can step in to minimize risks to vulnerable individuals, and imaginative and creative solutions to complex issues may emerge. In a growing number of practices serving adolescents, clinicians can now create two notes, with some elements of care visible on a patient portal and others held privately or visible only to the adolescent.
The shared experience
Overall, when it comes to documenting sensitive social information, open notes may act as a useful catalyst prompting deeper discussion about personal details clinically important to record, as opposed to those perhaps best left unwritten.
The implementation of open notes nationwide calls for exciting explorations. How can transparent systems maximize benefits for targeted populations in diverse settings? For patients with mental illness, can notes become part of the therapy? Given that care partners often report more benefit from reading notes than do patients themselves, how can they be mobilized to maximize their contributions to those acutely ill on hospital floors, or to family members with Alzheimer’s or in long-term care facilities?
How can we harness emerging technologies to translate notes and medical records into other languages or support lower literacy levels, while preserving the clinical detail in the notes? Should patients contribute to their own notes, cogenerating them with their clinicians? Experiments for “OurNotes” interventions are underway, and early reports from both patients and doctors hold considerable promise.
Ownership of medical records is evolving. Once firmly held by clinicians, electronic technologies have rapidly led to what may best be viewed currently as joint ownership by clinicians and patients. As apps evolve further and issues with interoperability of records diminish, it is likely that patients will eventually take control. Then it will be up to patients what to carry in their records. Clinicians will advise, but patients will decide.
The new rules herald clear changes in the fabric of care, and after a decade of study we anticipate that the benefits well outweigh the harms. But in the short run, it’s wrong to predict an avalanche. Two decades ago, when patient portals first revealed laboratory test findings to patients, doctors expected cataclysmic change in their practices. It did not occur. The vast majority of patients who registered on portals benefited and few disturbed their doctors.
Similarly, after notes were first unblinded by the OpenNotes research teams, the question we were asked most commonly by the primary care doctors who volunteered was whether the computers were actually displaying their notes. Even though many patients read them carefully, the doctors heard little from them. Clinicians have now reported the same experience in several subsequent studies.
Patients are resourceful, turning quickly to friends or the Internet for answers to their questions. They know how busy doctors are and don’t want to bother them if at all possible. When notes do trigger questions, the time taken to respond is probably offset by silence from other patients finding answers to their own questions in notes they read.
We believe that clinicians should embrace the spirit of the rules and also view them as HIPAA catching up with a computerized universe. As the new practice takes hold, ambiguities will diminish as further experience and research evolve. Warner V. Slack, MD, the first doctor to ask patients to talk to computers, opined that patients are the “largest and least utilized resource in health care.” Open and transparent communication through electronic medical records may mobilize patients (and their families) far more effectively. Patients will almost certainly benefit. Remembering Dr. Slack’s prophecy, we believe that clinicians will too.
A version of this article first appeared on Medscape.com.
Doctors are learning about new rules coming this April that encourage open and transparent communication among patients, families, and clinicians. The rules, putting into effect the bipartisan 21st Century Cures Act, mandate offering patients access to notes (“open notes”) written by clinicians in electronic medical records.
A recent article from this news organization noted that for many doctors this represents both a sudden and troubling change in practice. For others, the rules codify what they have been doing as a matter of routine for a decade. Spurred by the OpenNotes movement, at least 55 million Americans are already offered access to their clinical notes, including, since 2013, more than 9 million veterans with access to the Blue Button function in Veterans Affairs practices and hospitals.
The practice is spreading beyond the United States to other countries, including Canada, Sweden, Norway, Estonia, and the United Kingdom.
In this commentary, we review what patients, clinicians, and policymakers have been learning about open notes.
The patient experience
What do patients experience? In a survey of more than 22,000 patients who read notes in three diverse health systems, more than 90% reported having a good grasp of what their doctors and other clinicians had written, and very few (3%) reported being very confused by what they read. About two-thirds described reading their notes as very important for taking care of their health, remembering details of their visits and their care plans, and understanding why a medication was prescribed.
Indeed, in a clinically exciting finding, 14% of survey respondents reported that reading their notes made them more likely to take their medications as their doctors wished. With about half of Americans with chronic illness failing to take their medicines as prescribed, which sometimes leads to compromised outcomes and associated unnecessary costs (estimated at $300 billion annually), these reports of increased adherence should be taken very seriously.
Some doctors anticipate that open notes will erode patient communication. A growing body of research reveals just the opposite. In multiple surveys, patients describe open notes as “extending the visit,” strengthening collaboration and teamwork with their doctor. Quite possibly, the invitation to read notes may in itself increase trust. Such benefits appear especially pronounced among patients who are older, less educated, are persons of color or Hispanic, or who do not speak English at home.
And in several studies, more than a third of patients also report sharing their notes with others, with older and chronically ill patients in particular sharing access with family and friends who are their care partners.
On the other hand, a small minority of patients (5%) do report being more worried by what they read. It’s unknown whether this is because they are better informed about their care or because baseline anxiety levels increase. Doctors expect also that some patients, particularly those with cancer or serious mental illness, will be upset by their notes. So far, evidence does not support that specific concern.
Conversely, withholding, delaying, or blocking notes may be a source of anxiety or even stigmatization. When clinicians find themselves worried about sharing notes, we suggest that they discuss with their patients the benefits and risks. Recall also that transparency facilitates freedom of choice; patients make their own decision, and quite a few choose to leave notes unread.
Finding mistakes early and preventing harm are important goals for health care, and open notes can make care safer. Inevitably, medical records contain errors, omissions, and inaccuracies. In a large patient survey, 21% reported finding an error in their notes, and 42% perceived the error to be serious.
Moreover, 25% of doctors with more than a year’s experience with open notes reported patients finding errors that they (the doctors) considered “serious.” In 2015, the National Academy of Medicine cited open notes as a mechanism for improving diagnostic accuracy. In regard to possible legal action from patients, most attorneys, patients, and doctors agree that more transparent communication will build trust overall and, if anything, diminish litigation. We know of no instances so far of lawsuits deriving from open notes.
The physician experience
Doctors may worry that open notes will impede workflow, that they will be compelled to “dumb down” their documentation to avoid causing offense or anxiety, and that patients will demand changes to what is written. Here, extensive survey research should allay such fears and expectations. In a survey of more than 1,600 clinicians with at least 1 year of experience with open notes, reports of disruption to workflow were uncommon.
Most doctors (84%) reported that patients contacted them with questions about their notes “less than monthly or never.” Approximately two-thirds (62%) reported spending the same amount of time writing visit notes.
After implementing open notes, many doctors do report being more mindful about their documentation. For example, 41% reported changing how they used language such as “patient denies” or “noncompliant,” and 18% reported changing their use of medical jargon or abbreviations. Might these changes undermine the utility of medical notes? A majority of doctors surveyed (78%) said no, reporting that, after implementing open notes, the value of their documentation was the same or better.
Innovations spotlight difficult and often longstanding challenges. Open notes highlight the complex role of medical records in preserving privacy, especially in the spectrum of abuse, whether domestic or involving elders, children or sexual transgressions. For families with adolescents, issues concerning confidentiality can become a two-way street, and federal and state rules at times provide conflicting and idiosyncratic guidance. It is important to emphasize that the new rules permit information blocking if there is clear evidence that doing so “will substantially reduce the risk of harm” to patients or to other third parties.
Perhaps think of open notes as a new medicine designed to help the vast majority of those who use it but with side effects and even contraindications for a few. Doctors can step in to minimize risks to vulnerable individuals, and imaginative and creative solutions to complex issues may emerge. In a growing number of practices serving adolescents, clinicians can now create two notes, with some elements of care visible on a patient portal and others held privately or visible only to the adolescent.
The shared experience
Overall, when it comes to documenting sensitive social information, open notes may act as a useful catalyst prompting deeper discussion about personal details clinically important to record, as opposed to those perhaps best left unwritten.
The implementation of open notes nationwide calls for exciting explorations. How can transparent systems maximize benefits for targeted populations in diverse settings? For patients with mental illness, can notes become part of the therapy? Given that care partners often report more benefit from reading notes than do patients themselves, how can they be mobilized to maximize their contributions to those acutely ill on hospital floors, or to family members with Alzheimer’s or in long-term care facilities?
How can we harness emerging technologies to translate notes and medical records into other languages or support lower literacy levels, while preserving the clinical detail in the notes? Should patients contribute to their own notes, cogenerating them with their clinicians? Experiments for “OurNotes” interventions are underway, and early reports from both patients and doctors hold considerable promise.
Ownership of medical records is evolving. Once firmly held by clinicians, electronic technologies have rapidly led to what may best be viewed currently as joint ownership by clinicians and patients. As apps evolve further and issues with interoperability of records diminish, it is likely that patients will eventually take control. Then it will be up to patients what to carry in their records. Clinicians will advise, but patients will decide.
The new rules herald clear changes in the fabric of care, and after a decade of study we anticipate that the benefits well outweigh the harms. But in the short run, it’s wrong to predict an avalanche. Two decades ago, when patient portals first revealed laboratory test findings to patients, doctors expected cataclysmic change in their practices. It did not occur. The vast majority of patients who registered on portals benefited and few disturbed their doctors.
Similarly, after notes were first unblinded by the OpenNotes research teams, the question we were asked most commonly by the primary care doctors who volunteered was whether the computers were actually displaying their notes. Even though many patients read them carefully, the doctors heard little from them. Clinicians have now reported the same experience in several subsequent studies.
Patients are resourceful, turning quickly to friends or the Internet for answers to their questions. They know how busy doctors are and don’t want to bother them if at all possible. When notes do trigger questions, the time taken to respond is probably offset by silence from other patients finding answers to their own questions in notes they read.
We believe that clinicians should embrace the spirit of the rules and also view them as HIPAA catching up with a computerized universe. As the new practice takes hold, ambiguities will diminish as further experience and research evolve. Warner V. Slack, MD, the first doctor to ask patients to talk to computers, opined that patients are the “largest and least utilized resource in health care.” Open and transparent communication through electronic medical records may mobilize patients (and their families) far more effectively. Patients will almost certainly benefit. Remembering Dr. Slack’s prophecy, we believe that clinicians will too.
A version of this article first appeared on Medscape.com.
Doctors are learning about new rules coming this April that encourage open and transparent communication among patients, families, and clinicians. The rules, putting into effect the bipartisan 21st Century Cures Act, mandate offering patients access to notes (“open notes”) written by clinicians in electronic medical records.
A recent article from this news organization noted that for many doctors this represents both a sudden and troubling change in practice. For others, the rules codify what they have been doing as a matter of routine for a decade. Spurred by the OpenNotes movement, at least 55 million Americans are already offered access to their clinical notes, including, since 2013, more than 9 million veterans with access to the Blue Button function in Veterans Affairs practices and hospitals.
The practice is spreading beyond the United States to other countries, including Canada, Sweden, Norway, Estonia, and the United Kingdom.
In this commentary, we review what patients, clinicians, and policymakers have been learning about open notes.
The patient experience
What do patients experience? In a survey of more than 22,000 patients who read notes in three diverse health systems, more than 90% reported having a good grasp of what their doctors and other clinicians had written, and very few (3%) reported being very confused by what they read. About two-thirds described reading their notes as very important for taking care of their health, remembering details of their visits and their care plans, and understanding why a medication was prescribed.
Indeed, in a clinically exciting finding, 14% of survey respondents reported that reading their notes made them more likely to take their medications as their doctors wished. With about half of Americans with chronic illness failing to take their medicines as prescribed, which sometimes leads to compromised outcomes and associated unnecessary costs (estimated at $300 billion annually), these reports of increased adherence should be taken very seriously.
Some doctors anticipate that open notes will erode patient communication. A growing body of research reveals just the opposite. In multiple surveys, patients describe open notes as “extending the visit,” strengthening collaboration and teamwork with their doctor. Quite possibly, the invitation to read notes may in itself increase trust. Such benefits appear especially pronounced among patients who are older, less educated, are persons of color or Hispanic, or who do not speak English at home.
And in several studies, more than a third of patients also report sharing their notes with others, with older and chronically ill patients in particular sharing access with family and friends who are their care partners.
On the other hand, a small minority of patients (5%) do report being more worried by what they read. It’s unknown whether this is because they are better informed about their care or because baseline anxiety levels increase. Doctors expect also that some patients, particularly those with cancer or serious mental illness, will be upset by their notes. So far, evidence does not support that specific concern.
Conversely, withholding, delaying, or blocking notes may be a source of anxiety or even stigmatization. When clinicians find themselves worried about sharing notes, we suggest that they discuss with their patients the benefits and risks. Recall also that transparency facilitates freedom of choice; patients make their own decision, and quite a few choose to leave notes unread.
Finding mistakes early and preventing harm are important goals for health care, and open notes can make care safer. Inevitably, medical records contain errors, omissions, and inaccuracies. In a large patient survey, 21% reported finding an error in their notes, and 42% perceived the error to be serious.
Moreover, 25% of doctors with more than a year’s experience with open notes reported patients finding errors that they (the doctors) considered “serious.” In 2015, the National Academy of Medicine cited open notes as a mechanism for improving diagnostic accuracy. In regard to possible legal action from patients, most attorneys, patients, and doctors agree that more transparent communication will build trust overall and, if anything, diminish litigation. We know of no instances so far of lawsuits deriving from open notes.
The physician experience
Doctors may worry that open notes will impede workflow, that they will be compelled to “dumb down” their documentation to avoid causing offense or anxiety, and that patients will demand changes to what is written. Here, extensive survey research should allay such fears and expectations. In a survey of more than 1,600 clinicians with at least 1 year of experience with open notes, reports of disruption to workflow were uncommon.
Most doctors (84%) reported that patients contacted them with questions about their notes “less than monthly or never.” Approximately two-thirds (62%) reported spending the same amount of time writing visit notes.
After implementing open notes, many doctors do report being more mindful about their documentation. For example, 41% reported changing how they used language such as “patient denies” or “noncompliant,” and 18% reported changing their use of medical jargon or abbreviations. Might these changes undermine the utility of medical notes? A majority of doctors surveyed (78%) said no, reporting that, after implementing open notes, the value of their documentation was the same or better.
Innovations spotlight difficult and often longstanding challenges. Open notes highlight the complex role of medical records in preserving privacy, especially in the spectrum of abuse, whether domestic or involving elders, children or sexual transgressions. For families with adolescents, issues concerning confidentiality can become a two-way street, and federal and state rules at times provide conflicting and idiosyncratic guidance. It is important to emphasize that the new rules permit information blocking if there is clear evidence that doing so “will substantially reduce the risk of harm” to patients or to other third parties.
Perhaps think of open notes as a new medicine designed to help the vast majority of those who use it but with side effects and even contraindications for a few. Doctors can step in to minimize risks to vulnerable individuals, and imaginative and creative solutions to complex issues may emerge. In a growing number of practices serving adolescents, clinicians can now create two notes, with some elements of care visible on a patient portal and others held privately or visible only to the adolescent.
The shared experience
Overall, when it comes to documenting sensitive social information, open notes may act as a useful catalyst prompting deeper discussion about personal details clinically important to record, as opposed to those perhaps best left unwritten.
The implementation of open notes nationwide calls for exciting explorations. How can transparent systems maximize benefits for targeted populations in diverse settings? For patients with mental illness, can notes become part of the therapy? Given that care partners often report more benefit from reading notes than do patients themselves, how can they be mobilized to maximize their contributions to those acutely ill on hospital floors, or to family members with Alzheimer’s or in long-term care facilities?
How can we harness emerging technologies to translate notes and medical records into other languages or support lower literacy levels, while preserving the clinical detail in the notes? Should patients contribute to their own notes, cogenerating them with their clinicians? Experiments for “OurNotes” interventions are underway, and early reports from both patients and doctors hold considerable promise.
Ownership of medical records is evolving. Once firmly held by clinicians, electronic technologies have rapidly led to what may best be viewed currently as joint ownership by clinicians and patients. As apps evolve further and issues with interoperability of records diminish, it is likely that patients will eventually take control. Then it will be up to patients what to carry in their records. Clinicians will advise, but patients will decide.
The new rules herald clear changes in the fabric of care, and after a decade of study we anticipate that the benefits well outweigh the harms. But in the short run, it’s wrong to predict an avalanche. Two decades ago, when patient portals first revealed laboratory test findings to patients, doctors expected cataclysmic change in their practices. It did not occur. The vast majority of patients who registered on portals benefited and few disturbed their doctors.
Similarly, after notes were first unblinded by the OpenNotes research teams, the question we were asked most commonly by the primary care doctors who volunteered was whether the computers were actually displaying their notes. Even though many patients read them carefully, the doctors heard little from them. Clinicians have now reported the same experience in several subsequent studies.
Patients are resourceful, turning quickly to friends or the Internet for answers to their questions. They know how busy doctors are and don’t want to bother them if at all possible. When notes do trigger questions, the time taken to respond is probably offset by silence from other patients finding answers to their own questions in notes they read.
We believe that clinicians should embrace the spirit of the rules and also view them as HIPAA catching up with a computerized universe. As the new practice takes hold, ambiguities will diminish as further experience and research evolve. Warner V. Slack, MD, the first doctor to ask patients to talk to computers, opined that patients are the “largest and least utilized resource in health care.” Open and transparent communication through electronic medical records may mobilize patients (and their families) far more effectively. Patients will almost certainly benefit. Remembering Dr. Slack’s prophecy, we believe that clinicians will too.
A version of this article first appeared on Medscape.com.
What to do if an employee tests positive for COVID-19
An increasingly common question I’m receiving is:
As always, it depends, but here is some general advice: The specifics will vary depending on state/local laws, or your particular situation.
First, you need to determine the level of exposure, and whether it requires action. According to the Centers for Disease Control and Prevention, actionable exposure occurs 2 days prior to the onset of illness, and lasts 10 days after onset.
If action is required, you’ll need to determine who needs to quarantine and who needs to be tested. Vaccinated employees who have been exposed to suspected or confirmed COVID-19 are not required to quarantine or be tested if they are fully vaccinated and have remained asymptomatic since the exposure. Those employees should, however, follow all the usual precautions (masks, social distancing, handwashing, etc.) with increased diligence. Remind them that no vaccine is 100% effective, and suggest they self-monitor for symptoms (fever, cough, shortness of breath, etc.)
All other exposed employees should be tested. A negative test means an individual was not infected at the time the sample was collected, but that does not mean an individual will not get sick later. Some providers are retesting on days 5 and 7 post exposure.
Some experts advise that you monitor exposed employees (vaccinated or not) yourself, with daily temperature readings and inquiries regarding symptoms, and perhaps a daily pulse oximetry check, for 14 days following exposure. Document these screenings in writing. Anyone testing positive or developing a fever or other symptoms should, of course, be sent home and seek medical treatment as necessary.
Employees who develop symptoms or test positive for COVID-19 should remain out of work until all CDC “return-to-work” criteria are met. At this writing, the basic criteria include:
- At least 10 days pass after symptoms first appeared
- At least 24 hours pass after last fever without the use of fever-reducing medications
- Cough, shortness of breath, and any other symptoms improve
Anyone who is significantly immunocompromised may need more time at home, and probably consultation with an infectious disease specialist.
Your facility should be thoroughly cleaned after the exposure. Close off all areas used by the sick individual, and clean and disinfect all areas such as offices, doorknobs, bathrooms, common areas, and shared electronic equipment. Of course, the cleaners should wear gowns, gloves, masks, and goggles. Some practices are hiring cleaning crews to professionally disinfect their offices. Once the area has been disinfected, it can be reopened for use. Workers without close contact with the person who is sick can return to work immediately after disinfection.
If the potential infected area is widespread and cannot be isolated to a room or rooms where doors can be shut, it may be prudent to temporarily close your office, send staff home, and divert patients to other locations if they cannot be rescheduled. Once your facility is cleaned and disinfected and staff have been cleared, your office may reopen.
Use enhanced precautions for any staff or patients who are immunocompromised, or otherwise fall into the high-risk category, to keep them out of the path of potential exposure areas and allow them to self-quarantine if they desire.
You should continue following existing leave policies (paid time off, vacation, sick, short-term disability, leave of absence, Family and Medical Leave Act, and Americans with Disabilities Act). If the employee was exposed at work, contact your workers’ compensation carrier regarding lost wages. Unless your state laws specify otherwise, you are under no obligation to pay beyond your policies, but you may do so if you choose.
Of course, you can take proactive steps to prevent unnecessary exposure and avoid closures in the first place; for example:
- Call patients prior to their visit, or question them upon arrival, regarding fever, shortness of breath, and other COVID-19 symptoms.
- Check employees’ temperatures every morning.
- Check patients’ temperatures as they enter the office.
- Require everyone, patients and employees alike, to wear face coverings.
- Ask patients to leave friends and family members at home.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a long-time monthly columnist for Dermatology News. Write to him at [email protected].
An increasingly common question I’m receiving is:
As always, it depends, but here is some general advice: The specifics will vary depending on state/local laws, or your particular situation.
First, you need to determine the level of exposure, and whether it requires action. According to the Centers for Disease Control and Prevention, actionable exposure occurs 2 days prior to the onset of illness, and lasts 10 days after onset.
If action is required, you’ll need to determine who needs to quarantine and who needs to be tested. Vaccinated employees who have been exposed to suspected or confirmed COVID-19 are not required to quarantine or be tested if they are fully vaccinated and have remained asymptomatic since the exposure. Those employees should, however, follow all the usual precautions (masks, social distancing, handwashing, etc.) with increased diligence. Remind them that no vaccine is 100% effective, and suggest they self-monitor for symptoms (fever, cough, shortness of breath, etc.)
All other exposed employees should be tested. A negative test means an individual was not infected at the time the sample was collected, but that does not mean an individual will not get sick later. Some providers are retesting on days 5 and 7 post exposure.
Some experts advise that you monitor exposed employees (vaccinated or not) yourself, with daily temperature readings and inquiries regarding symptoms, and perhaps a daily pulse oximetry check, for 14 days following exposure. Document these screenings in writing. Anyone testing positive or developing a fever or other symptoms should, of course, be sent home and seek medical treatment as necessary.
Employees who develop symptoms or test positive for COVID-19 should remain out of work until all CDC “return-to-work” criteria are met. At this writing, the basic criteria include:
- At least 10 days pass after symptoms first appeared
- At least 24 hours pass after last fever without the use of fever-reducing medications
- Cough, shortness of breath, and any other symptoms improve
Anyone who is significantly immunocompromised may need more time at home, and probably consultation with an infectious disease specialist.
Your facility should be thoroughly cleaned after the exposure. Close off all areas used by the sick individual, and clean and disinfect all areas such as offices, doorknobs, bathrooms, common areas, and shared electronic equipment. Of course, the cleaners should wear gowns, gloves, masks, and goggles. Some practices are hiring cleaning crews to professionally disinfect their offices. Once the area has been disinfected, it can be reopened for use. Workers without close contact with the person who is sick can return to work immediately after disinfection.
If the potential infected area is widespread and cannot be isolated to a room or rooms where doors can be shut, it may be prudent to temporarily close your office, send staff home, and divert patients to other locations if they cannot be rescheduled. Once your facility is cleaned and disinfected and staff have been cleared, your office may reopen.
Use enhanced precautions for any staff or patients who are immunocompromised, or otherwise fall into the high-risk category, to keep them out of the path of potential exposure areas and allow them to self-quarantine if they desire.
You should continue following existing leave policies (paid time off, vacation, sick, short-term disability, leave of absence, Family and Medical Leave Act, and Americans with Disabilities Act). If the employee was exposed at work, contact your workers’ compensation carrier regarding lost wages. Unless your state laws specify otherwise, you are under no obligation to pay beyond your policies, but you may do so if you choose.
Of course, you can take proactive steps to prevent unnecessary exposure and avoid closures in the first place; for example:
- Call patients prior to their visit, or question them upon arrival, regarding fever, shortness of breath, and other COVID-19 symptoms.
- Check employees’ temperatures every morning.
- Check patients’ temperatures as they enter the office.
- Require everyone, patients and employees alike, to wear face coverings.
- Ask patients to leave friends and family members at home.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a long-time monthly columnist for Dermatology News. Write to him at [email protected].
An increasingly common question I’m receiving is:
As always, it depends, but here is some general advice: The specifics will vary depending on state/local laws, or your particular situation.
First, you need to determine the level of exposure, and whether it requires action. According to the Centers for Disease Control and Prevention, actionable exposure occurs 2 days prior to the onset of illness, and lasts 10 days after onset.
If action is required, you’ll need to determine who needs to quarantine and who needs to be tested. Vaccinated employees who have been exposed to suspected or confirmed COVID-19 are not required to quarantine or be tested if they are fully vaccinated and have remained asymptomatic since the exposure. Those employees should, however, follow all the usual precautions (masks, social distancing, handwashing, etc.) with increased diligence. Remind them that no vaccine is 100% effective, and suggest they self-monitor for symptoms (fever, cough, shortness of breath, etc.)
All other exposed employees should be tested. A negative test means an individual was not infected at the time the sample was collected, but that does not mean an individual will not get sick later. Some providers are retesting on days 5 and 7 post exposure.
Some experts advise that you monitor exposed employees (vaccinated or not) yourself, with daily temperature readings and inquiries regarding symptoms, and perhaps a daily pulse oximetry check, for 14 days following exposure. Document these screenings in writing. Anyone testing positive or developing a fever or other symptoms should, of course, be sent home and seek medical treatment as necessary.
Employees who develop symptoms or test positive for COVID-19 should remain out of work until all CDC “return-to-work” criteria are met. At this writing, the basic criteria include:
- At least 10 days pass after symptoms first appeared
- At least 24 hours pass after last fever without the use of fever-reducing medications
- Cough, shortness of breath, and any other symptoms improve
Anyone who is significantly immunocompromised may need more time at home, and probably consultation with an infectious disease specialist.
Your facility should be thoroughly cleaned after the exposure. Close off all areas used by the sick individual, and clean and disinfect all areas such as offices, doorknobs, bathrooms, common areas, and shared electronic equipment. Of course, the cleaners should wear gowns, gloves, masks, and goggles. Some practices are hiring cleaning crews to professionally disinfect their offices. Once the area has been disinfected, it can be reopened for use. Workers without close contact with the person who is sick can return to work immediately after disinfection.
If the potential infected area is widespread and cannot be isolated to a room or rooms where doors can be shut, it may be prudent to temporarily close your office, send staff home, and divert patients to other locations if they cannot be rescheduled. Once your facility is cleaned and disinfected and staff have been cleared, your office may reopen.
Use enhanced precautions for any staff or patients who are immunocompromised, or otherwise fall into the high-risk category, to keep them out of the path of potential exposure areas and allow them to self-quarantine if they desire.
You should continue following existing leave policies (paid time off, vacation, sick, short-term disability, leave of absence, Family and Medical Leave Act, and Americans with Disabilities Act). If the employee was exposed at work, contact your workers’ compensation carrier regarding lost wages. Unless your state laws specify otherwise, you are under no obligation to pay beyond your policies, but you may do so if you choose.
Of course, you can take proactive steps to prevent unnecessary exposure and avoid closures in the first place; for example:
- Call patients prior to their visit, or question them upon arrival, regarding fever, shortness of breath, and other COVID-19 symptoms.
- Check employees’ temperatures every morning.
- Check patients’ temperatures as they enter the office.
- Require everyone, patients and employees alike, to wear face coverings.
- Ask patients to leave friends and family members at home.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a long-time monthly columnist for Dermatology News. Write to him at [email protected].
The true measure of cluster headache
Patients with cluster headache face a double whammy: Physicians too often fail to recognize it, and their condition is among the most severe and debilitating among headache types. In fact,
The study’s comparison of cluster headaches to other common painful experiences can help nonsufferers relate to the experience, said Larry Schor, PhD, a coauthor of the paper. “Headache is a terrible word. Bee stings sting, burns burn. [A cluster headache] doesn’t ache. It’s a piercing intensity like you just can’t believe,” said Dr. Schor, professor of psychology at the University of West Georgia, Carrollton, and a cluster headache patient since he first experienced an attack at the age of 21.
The study was published in the January 2021 issue of Headache.
Ranking cluster headaches as worse than experiences such as childbirth or kidney stones is “kind of eye opening, and helps to describe the experience in terms that more people can relate to. I think it helps to share the experience of cluster headache more broadly, because we’re in a situation where cluster headache remains underfunded, and we don’t have enough treatments for it. I think one way to overcome that is to spread awareness of what this problem is, and the impact it has on human life,” said Rashmi Halker Singh, MD, associate professor of neurology at the Mayo Clinic in Scottsdale, Ariz., and deputy editor of Headache. She was not involved in the study.
Dr. Schor called for physicians to consider cluster headache an emergency, because of the severity of pain and also the potential for suicidality. Treatments remain comparatively sparse, but high-flow oxygen can help some patients, and intranasal or intravenous triptans can treat acute pain. In 2018, the Food and Drug Administration approved galcanezumab (Eli Lilly) for prevention of episodic cluster headaches.
But cluster headaches are often misdiagnosed. For many patients, it takes more than a year or even as long as 5 years to get an accurate diagnosis, according to Dr. Schor. Women may be particularly vulnerable to misdiagnosis, because migraines are more common in women. It doesn’t help that many neurologists are taught that cluster headache is primarily a male disease. “Because that idea is so ingrained, I think a lot of women who have cluster headache are probably missed and told they have migraine instead. There are a lot of women who have cluster headache, and that gender difference might not be as big a difference as we were initially taught. We need to do a better job of recognizing cluster headache to better understand what the true prevalence is,” said Dr. Halker Singh.
She noted that patients with side-locked headache should be evaluated for cluster headache, and asked how long the pain lasts in the absence of medication. “Also ask about the presence of cranial autonomic symptoms, and if they occur in the context of headache pain, and if they are side-locked to the side of the headache. Those are important questions that can tease out cluster headache from other conditions,” said Dr. Halker Singh.
For the survey, the researchers asked 1,604 patients with cluster headache patients to rate pain on a scale of 1 to 10. Cluster headache ranked highest at 9.7, then labor pain (7.2), pancreatitis (7.0), and nephrolithiasis (6.9). Cluster headache pain was ranked at 10.0 by 72.1% of respondents. Those reporting maximal pain or were more likely to have cranial autonomic features in comparison with patients who reported less pain, including conjunctival injection or lacrimation (91% versus 85%), eyelid edema (77% versus 66%), forehead/facial sweating (60% versus 49%), fullness in the ear (47% versus 35%), and miosis or ptosis (85% versus 75%). They had more frequent attacks (4.0 versus 3.5 per day), higher Hopelessness Depression Symptom Questionnaire scores (24.5 versus 21.1), and reduced effectiveness of calcium channel blockers (2.2 versus 2.5 on a 5-point Likert scale). They were more often female (34% versus 24%). (P < .001 for all).
The study received funding from Autonomic Technologies and Cluster Busters. Dr. Schor and Dr. Halker Singh had no relevant financial disclosures.
Patients with cluster headache face a double whammy: Physicians too often fail to recognize it, and their condition is among the most severe and debilitating among headache types. In fact,
The study’s comparison of cluster headaches to other common painful experiences can help nonsufferers relate to the experience, said Larry Schor, PhD, a coauthor of the paper. “Headache is a terrible word. Bee stings sting, burns burn. [A cluster headache] doesn’t ache. It’s a piercing intensity like you just can’t believe,” said Dr. Schor, professor of psychology at the University of West Georgia, Carrollton, and a cluster headache patient since he first experienced an attack at the age of 21.
The study was published in the January 2021 issue of Headache.
Ranking cluster headaches as worse than experiences such as childbirth or kidney stones is “kind of eye opening, and helps to describe the experience in terms that more people can relate to. I think it helps to share the experience of cluster headache more broadly, because we’re in a situation where cluster headache remains underfunded, and we don’t have enough treatments for it. I think one way to overcome that is to spread awareness of what this problem is, and the impact it has on human life,” said Rashmi Halker Singh, MD, associate professor of neurology at the Mayo Clinic in Scottsdale, Ariz., and deputy editor of Headache. She was not involved in the study.
Dr. Schor called for physicians to consider cluster headache an emergency, because of the severity of pain and also the potential for suicidality. Treatments remain comparatively sparse, but high-flow oxygen can help some patients, and intranasal or intravenous triptans can treat acute pain. In 2018, the Food and Drug Administration approved galcanezumab (Eli Lilly) for prevention of episodic cluster headaches.
But cluster headaches are often misdiagnosed. For many patients, it takes more than a year or even as long as 5 years to get an accurate diagnosis, according to Dr. Schor. Women may be particularly vulnerable to misdiagnosis, because migraines are more common in women. It doesn’t help that many neurologists are taught that cluster headache is primarily a male disease. “Because that idea is so ingrained, I think a lot of women who have cluster headache are probably missed and told they have migraine instead. There are a lot of women who have cluster headache, and that gender difference might not be as big a difference as we were initially taught. We need to do a better job of recognizing cluster headache to better understand what the true prevalence is,” said Dr. Halker Singh.
She noted that patients with side-locked headache should be evaluated for cluster headache, and asked how long the pain lasts in the absence of medication. “Also ask about the presence of cranial autonomic symptoms, and if they occur in the context of headache pain, and if they are side-locked to the side of the headache. Those are important questions that can tease out cluster headache from other conditions,” said Dr. Halker Singh.
For the survey, the researchers asked 1,604 patients with cluster headache patients to rate pain on a scale of 1 to 10. Cluster headache ranked highest at 9.7, then labor pain (7.2), pancreatitis (7.0), and nephrolithiasis (6.9). Cluster headache pain was ranked at 10.0 by 72.1% of respondents. Those reporting maximal pain or were more likely to have cranial autonomic features in comparison with patients who reported less pain, including conjunctival injection or lacrimation (91% versus 85%), eyelid edema (77% versus 66%), forehead/facial sweating (60% versus 49%), fullness in the ear (47% versus 35%), and miosis or ptosis (85% versus 75%). They had more frequent attacks (4.0 versus 3.5 per day), higher Hopelessness Depression Symptom Questionnaire scores (24.5 versus 21.1), and reduced effectiveness of calcium channel blockers (2.2 versus 2.5 on a 5-point Likert scale). They were more often female (34% versus 24%). (P < .001 for all).
The study received funding from Autonomic Technologies and Cluster Busters. Dr. Schor and Dr. Halker Singh had no relevant financial disclosures.
Patients with cluster headache face a double whammy: Physicians too often fail to recognize it, and their condition is among the most severe and debilitating among headache types. In fact,
The study’s comparison of cluster headaches to other common painful experiences can help nonsufferers relate to the experience, said Larry Schor, PhD, a coauthor of the paper. “Headache is a terrible word. Bee stings sting, burns burn. [A cluster headache] doesn’t ache. It’s a piercing intensity like you just can’t believe,” said Dr. Schor, professor of psychology at the University of West Georgia, Carrollton, and a cluster headache patient since he first experienced an attack at the age of 21.
The study was published in the January 2021 issue of Headache.
Ranking cluster headaches as worse than experiences such as childbirth or kidney stones is “kind of eye opening, and helps to describe the experience in terms that more people can relate to. I think it helps to share the experience of cluster headache more broadly, because we’re in a situation where cluster headache remains underfunded, and we don’t have enough treatments for it. I think one way to overcome that is to spread awareness of what this problem is, and the impact it has on human life,” said Rashmi Halker Singh, MD, associate professor of neurology at the Mayo Clinic in Scottsdale, Ariz., and deputy editor of Headache. She was not involved in the study.
Dr. Schor called for physicians to consider cluster headache an emergency, because of the severity of pain and also the potential for suicidality. Treatments remain comparatively sparse, but high-flow oxygen can help some patients, and intranasal or intravenous triptans can treat acute pain. In 2018, the Food and Drug Administration approved galcanezumab (Eli Lilly) for prevention of episodic cluster headaches.
But cluster headaches are often misdiagnosed. For many patients, it takes more than a year or even as long as 5 years to get an accurate diagnosis, according to Dr. Schor. Women may be particularly vulnerable to misdiagnosis, because migraines are more common in women. It doesn’t help that many neurologists are taught that cluster headache is primarily a male disease. “Because that idea is so ingrained, I think a lot of women who have cluster headache are probably missed and told they have migraine instead. There are a lot of women who have cluster headache, and that gender difference might not be as big a difference as we were initially taught. We need to do a better job of recognizing cluster headache to better understand what the true prevalence is,” said Dr. Halker Singh.
She noted that patients with side-locked headache should be evaluated for cluster headache, and asked how long the pain lasts in the absence of medication. “Also ask about the presence of cranial autonomic symptoms, and if they occur in the context of headache pain, and if they are side-locked to the side of the headache. Those are important questions that can tease out cluster headache from other conditions,” said Dr. Halker Singh.
For the survey, the researchers asked 1,604 patients with cluster headache patients to rate pain on a scale of 1 to 10. Cluster headache ranked highest at 9.7, then labor pain (7.2), pancreatitis (7.0), and nephrolithiasis (6.9). Cluster headache pain was ranked at 10.0 by 72.1% of respondents. Those reporting maximal pain or were more likely to have cranial autonomic features in comparison with patients who reported less pain, including conjunctival injection or lacrimation (91% versus 85%), eyelid edema (77% versus 66%), forehead/facial sweating (60% versus 49%), fullness in the ear (47% versus 35%), and miosis or ptosis (85% versus 75%). They had more frequent attacks (4.0 versus 3.5 per day), higher Hopelessness Depression Symptom Questionnaire scores (24.5 versus 21.1), and reduced effectiveness of calcium channel blockers (2.2 versus 2.5 on a 5-point Likert scale). They were more often female (34% versus 24%). (P < .001 for all).
The study received funding from Autonomic Technologies and Cluster Busters. Dr. Schor and Dr. Halker Singh had no relevant financial disclosures.
FROM HEADACHE








