User login
Commentary: Sex differences, pregnancy, a quicker CRP test, and new drugs in PsA, December 2022
Research published during the past month focused mostly on sex differences, biomarkers, and treatment. Sex differences in psoriatic arthritis (PsA) are a significant focus of current research. One major question is how clinical features differ between men and women. Furer and colleagues investigated differences in musculoskeletal ultrasonographic features between men and women with PsA. In a prospective study including 70 men and 88 women, they demonstrated that although the total synovitis and tenosynovitis scores were similar between the two sexes, compared with women, men had higher total ultrasound and gray scale enthesitis scores (both P = .01) and sonographic active inflammatory score (P = .005). Given the uncertainty associated with the clinical diagnosis of enthesitis, this study emphasizes the importance of careful ultrasonographic evaluation when evaluating enthesitis patients, especially women.
It is important to investigate pregnancy outcomes in women with inflammatory arthritis, including PsA, to appropriately counsel and manage patients in the reproductive-age group. Preeclampsia is an important pregnancy outcome that is less well studied in PsA. Secher and colleagues analyzed data from registries in Sweden and Denmark that included singleton pregnant women with rheumatoid arthritis (n = 1739), axial spondyloarthritis (n = 819), and PsA (n = 489) who were matched with 17,390, 8190, and 4890 control pregnant women, respectively. They found that compared with the control women, the risk for preeclampsia was much higher in women with PsA (adjusted odds ratio [aOR; adjusted for country, maternal age, parity, year of delivery, body mass index (BMI), smoking, and education] 1.85; 95% CI 1.10-3.12), with the risk being primarily driven by the receipt of monotherapy for PsA before pregnancy (aOR 2.72; 95% CI 1.44-5.13), probably reflecting the presence of more severe disease. Women with PsA who tend to have higher BMI and active disease need to be counseled about the risk for preeclampsia and be carefully monitored.
The Disease Activity index for PsA (DAPSA) is a validated instrument used in clinical practice to assess PsA disease activity. One drawback of this instrument is that it requires testing for C-reactive protein (CRP), the results of which may not be available immediately, making it difficult to use DAPSA for implementing treating-to-target strategies during a clinic visit. To alleviate this issue, a quick quantitative CRP (qCRP) assay was developed. In a multicenter, cross-sectional study including 104 patients with PsA and available CRP values (measured by routine laboratory and qCRP assays), Proft and colleagues demonstrated that 98.1% of patients were similarly categorized into disease activity groups (remission and low, moderate, and high disease activity) using DAPSA based on qCRP (Q-DAPSA) and DAPSA. The agreement between the two instruments was excellent (weighted Cohen kappa 0.980; 95% CI 0.952-1.000). Thus, the Q-DAPSA may be used in place of DAPSA when evaluating PsA disease activity.
Regarding treatment, in an exploratory analysis of SELECT-PsA 1, McInnes and colleagues demonstrated that, at week 104, a similar proportion of patients receiving 15/30 mg upadacitinib vs adalimumab achieved ≥ 20% improvement in the American College of Rheumatology (ACR20) criteria (69.0%/69.5% vs 63.4%), whereas significantly more patients receiving 30 mg upadacitinib vs adalimumab achieved minimal disease activity (45.9% vs 37.8%; P < .05). The safety profiles of upadacitinib and adalimumab were comparable. Moreover, analyses of 52-week outcome data from the ongoing phase 3 KEEPsAKE 1 study of risankizumab (IL-23 inhibitor) by Kristensen and colleagues showed that among patients who received risankizumab continuously, the ACR20 response increased from 57.3% at week 24 to 70.0% at week 52. No new safety signals were identified. Thus, upadacitinib and risankizumab are newer, safe, and effective disease-modifying antirheumatic drugs for PsA.
Research published during the past month focused mostly on sex differences, biomarkers, and treatment. Sex differences in psoriatic arthritis (PsA) are a significant focus of current research. One major question is how clinical features differ between men and women. Furer and colleagues investigated differences in musculoskeletal ultrasonographic features between men and women with PsA. In a prospective study including 70 men and 88 women, they demonstrated that although the total synovitis and tenosynovitis scores were similar between the two sexes, compared with women, men had higher total ultrasound and gray scale enthesitis scores (both P = .01) and sonographic active inflammatory score (P = .005). Given the uncertainty associated with the clinical diagnosis of enthesitis, this study emphasizes the importance of careful ultrasonographic evaluation when evaluating enthesitis patients, especially women.
It is important to investigate pregnancy outcomes in women with inflammatory arthritis, including PsA, to appropriately counsel and manage patients in the reproductive-age group. Preeclampsia is an important pregnancy outcome that is less well studied in PsA. Secher and colleagues analyzed data from registries in Sweden and Denmark that included singleton pregnant women with rheumatoid arthritis (n = 1739), axial spondyloarthritis (n = 819), and PsA (n = 489) who were matched with 17,390, 8190, and 4890 control pregnant women, respectively. They found that compared with the control women, the risk for preeclampsia was much higher in women with PsA (adjusted odds ratio [aOR; adjusted for country, maternal age, parity, year of delivery, body mass index (BMI), smoking, and education] 1.85; 95% CI 1.10-3.12), with the risk being primarily driven by the receipt of monotherapy for PsA before pregnancy (aOR 2.72; 95% CI 1.44-5.13), probably reflecting the presence of more severe disease. Women with PsA who tend to have higher BMI and active disease need to be counseled about the risk for preeclampsia and be carefully monitored.
The Disease Activity index for PsA (DAPSA) is a validated instrument used in clinical practice to assess PsA disease activity. One drawback of this instrument is that it requires testing for C-reactive protein (CRP), the results of which may not be available immediately, making it difficult to use DAPSA for implementing treating-to-target strategies during a clinic visit. To alleviate this issue, a quick quantitative CRP (qCRP) assay was developed. In a multicenter, cross-sectional study including 104 patients with PsA and available CRP values (measured by routine laboratory and qCRP assays), Proft and colleagues demonstrated that 98.1% of patients were similarly categorized into disease activity groups (remission and low, moderate, and high disease activity) using DAPSA based on qCRP (Q-DAPSA) and DAPSA. The agreement between the two instruments was excellent (weighted Cohen kappa 0.980; 95% CI 0.952-1.000). Thus, the Q-DAPSA may be used in place of DAPSA when evaluating PsA disease activity.
Regarding treatment, in an exploratory analysis of SELECT-PsA 1, McInnes and colleagues demonstrated that, at week 104, a similar proportion of patients receiving 15/30 mg upadacitinib vs adalimumab achieved ≥ 20% improvement in the American College of Rheumatology (ACR20) criteria (69.0%/69.5% vs 63.4%), whereas significantly more patients receiving 30 mg upadacitinib vs adalimumab achieved minimal disease activity (45.9% vs 37.8%; P < .05). The safety profiles of upadacitinib and adalimumab were comparable. Moreover, analyses of 52-week outcome data from the ongoing phase 3 KEEPsAKE 1 study of risankizumab (IL-23 inhibitor) by Kristensen and colleagues showed that among patients who received risankizumab continuously, the ACR20 response increased from 57.3% at week 24 to 70.0% at week 52. No new safety signals were identified. Thus, upadacitinib and risankizumab are newer, safe, and effective disease-modifying antirheumatic drugs for PsA.
Research published during the past month focused mostly on sex differences, biomarkers, and treatment. Sex differences in psoriatic arthritis (PsA) are a significant focus of current research. One major question is how clinical features differ between men and women. Furer and colleagues investigated differences in musculoskeletal ultrasonographic features between men and women with PsA. In a prospective study including 70 men and 88 women, they demonstrated that although the total synovitis and tenosynovitis scores were similar between the two sexes, compared with women, men had higher total ultrasound and gray scale enthesitis scores (both P = .01) and sonographic active inflammatory score (P = .005). Given the uncertainty associated with the clinical diagnosis of enthesitis, this study emphasizes the importance of careful ultrasonographic evaluation when evaluating enthesitis patients, especially women.
It is important to investigate pregnancy outcomes in women with inflammatory arthritis, including PsA, to appropriately counsel and manage patients in the reproductive-age group. Preeclampsia is an important pregnancy outcome that is less well studied in PsA. Secher and colleagues analyzed data from registries in Sweden and Denmark that included singleton pregnant women with rheumatoid arthritis (n = 1739), axial spondyloarthritis (n = 819), and PsA (n = 489) who were matched with 17,390, 8190, and 4890 control pregnant women, respectively. They found that compared with the control women, the risk for preeclampsia was much higher in women with PsA (adjusted odds ratio [aOR; adjusted for country, maternal age, parity, year of delivery, body mass index (BMI), smoking, and education] 1.85; 95% CI 1.10-3.12), with the risk being primarily driven by the receipt of monotherapy for PsA before pregnancy (aOR 2.72; 95% CI 1.44-5.13), probably reflecting the presence of more severe disease. Women with PsA who tend to have higher BMI and active disease need to be counseled about the risk for preeclampsia and be carefully monitored.
The Disease Activity index for PsA (DAPSA) is a validated instrument used in clinical practice to assess PsA disease activity. One drawback of this instrument is that it requires testing for C-reactive protein (CRP), the results of which may not be available immediately, making it difficult to use DAPSA for implementing treating-to-target strategies during a clinic visit. To alleviate this issue, a quick quantitative CRP (qCRP) assay was developed. In a multicenter, cross-sectional study including 104 patients with PsA and available CRP values (measured by routine laboratory and qCRP assays), Proft and colleagues demonstrated that 98.1% of patients were similarly categorized into disease activity groups (remission and low, moderate, and high disease activity) using DAPSA based on qCRP (Q-DAPSA) and DAPSA. The agreement between the two instruments was excellent (weighted Cohen kappa 0.980; 95% CI 0.952-1.000). Thus, the Q-DAPSA may be used in place of DAPSA when evaluating PsA disease activity.
Regarding treatment, in an exploratory analysis of SELECT-PsA 1, McInnes and colleagues demonstrated that, at week 104, a similar proportion of patients receiving 15/30 mg upadacitinib vs adalimumab achieved ≥ 20% improvement in the American College of Rheumatology (ACR20) criteria (69.0%/69.5% vs 63.4%), whereas significantly more patients receiving 30 mg upadacitinib vs adalimumab achieved minimal disease activity (45.9% vs 37.8%; P < .05). The safety profiles of upadacitinib and adalimumab were comparable. Moreover, analyses of 52-week outcome data from the ongoing phase 3 KEEPsAKE 1 study of risankizumab (IL-23 inhibitor) by Kristensen and colleagues showed that among patients who received risankizumab continuously, the ACR20 response increased from 57.3% at week 24 to 70.0% at week 52. No new safety signals were identified. Thus, upadacitinib and risankizumab are newer, safe, and effective disease-modifying antirheumatic drugs for PsA.
Advancing health equity in neurology is essential to patient care
Black and Latinx older adults are up to three times as likely to develop Alzheimer’s disease than non-Latinx White adults and tend to experience onset at a younger age with more severe symptoms, according to Monica Rivera-Mindt, PhD, a professor of psychology at Fordham University and the Icahn School of Medicine at Mount Sinai, New York. Looking ahead, that means by 2030, nearly 40% of the 8.4 million Americans affected by Alzheimer’s disease will be Black and/or Latinx, she said. These facts were among the stark disparities in health care outcomes Dr. Rivera-Mindt discussed in her presentation on brain health equity at the 2022 annual meeting of the American Neurological Association.
Dr. Rivera-Mindt’s presentation opened the ANA’s plenary session on health disparities and inequities. The plenary, “Advancing Neurologic Equity: Challenges and Paths Forward,” did not simply enumerate racial and ethnic disparities that exist with various neurological conditions. Rather it went beyond the discussion of what disparities exist into understanding the roots of them as well as tips, tools, and resources that can aid clinicians in addressing or ameliorating them.
Roy Hamilton, MD, an associate professor of neurology and physical medicine and rehabilitation at the University of Pennsylvania, Philadelphia, said. “If clinicians are unaware of these disparities or don’t have any sense of how to start to address or think about them, then they’re really missing out on an important component of their education as persons who take care of patients with brain disorders.”
Dr. Hamilton, who organized the plenary, noted that awareness of these disparities is crucial to comprehensively caring for patients.
Missed opportunities
“We’re talking about disadvantages that are structural and large scale, but those disadvantages play themselves out in the individual encounter,” Dr. Hamilton said. “When physicians see patients, they have to treat the whole patient in front of them,” which means being aware of the risks and factors that could affect a patient’s clinical presentation. “Being aware of disparities has practical impacts on physician judgment,” he said.
For example, recent research in multiple sclerosis (MS) has highlighted how clinicians may be missing diagnosis of this condition in non-White populations because the condition has been regarded for so long as a “White person’s” disease, Dr. Hamilton said. In non-White patients exhibiting MS symptoms, then, clinicians may have been less likely to consider MS as a possibility, thereby delaying diagnosis and treatment.
Those patterns may partly explain why the mortality rate for MS is greater in Black patients, who also show more rapid neurodegeneration than White patients with MS, Lilyana Amezcua, MD, an associate professor of neurology at the University of Southern California, Los Angeles, reported in the plenary’s second presentation.
Transgender issues
The third session, presented by Nicole Rosendale, MD, an assistant professor of neurology at the University of California, San Francisco, and director of the San Francisco General Hospital neurology inpatient services, examined disparities in neurology within the LGBTQ+ community through representative case studies and then offered specific ways that neurologists could make their practices more inclusive and equitable for sexual and gender minorities.
Her first case study was a 52-year-old man who presented with new-onset seizures, right hemiparesis, and aphasia. A brain biopsy consistent with adenocarcinoma eventually led his physician to discover he had metastatic breast cancer. It turned out the man was transgender and, despite a family history of breast cancer, hadn’t been advised to get breast cancer screenings.
“Breast cancer was not initially on the differential as no one had identified that the patient was transmasculine,” Dr. Rosendale said. A major challenge to providing care to transgender patients is a dearth of data on risks and screening recommendations. Another barrier is low knowledge of LGBTQ+ health among neurologists, Dr. Rosendale said while sharing findings from her 2019 study on the topic and calling for more research in LGBTQ+ populations.
Dr. Rosendale’s second case study dealt with a nonbinary patient who suffered from debilitating headaches for decades, first because they lacked access to health insurance and then because negative experiences with providers dissuaded them from seeking care. In data from the Center for American Progress she shared, 8% of LGB respondents and 22% of transgender respondents said they had avoided or delayed care because of fear of discrimination or mistreatment.
“So it’s not only access but also what experiences people are having when they go in and whether they’re actually even getting access to care or being taken care of,” Dr. Rosendale said. Other findings from the CAP found that:
- 8% of LGB patients and 29% of transgender patients reported having a clinician refuse to see them.
- 6% of LGB patients and 12% of transgender patients reported that a clinician refused to give them health care.
- 9% of LGB patients and 21% of transgender patients experienced harsh or abusive language during a health care experience.
- 7% of LGB patients and nearly a third (29%) of transgender patients experienced unwanted physical contact, such as fondling or sexual assault.
Reducing the disparities
Adys Mendizabal, MD, an assistant professor of neurology at the Institute of Society and Genetics at the University of California, Los Angeles, who attended the presentation, was grateful to see how the various lectures enriched the discussion beyond stating the fact of racial/ethnic disparities and dug into the nuances on how to think about and address these disparities. She particularly appreciated discussion about the need to go out of the way to recruit diverse patient populations for clinical trials while also providing them care.
“It is definitely complicated, but it’s not impossible for an individual neurologist or an individual department to do something to reduce some of the disparities,” Dr. Mendizabal said. “It starts with just knowing that they exist and being aware of some of the things that may be impacting care for a particular patient.”
Tools to counter disparity
In the final presentation, Amy Kind, MD, PhD, the associate dean for social health sciences and programs at the University of Wisconsin–Madison, rounded out the discussion by exploring social determinants of health and their influence on outcomes.
“Social determinants impact brain health, and brain health is not distributed equally,” Dr. Kind told attendees. “We have known this for decades, yet disparities persist.”
Dr. Kind described the “exposome,” a “measure of all the exposures of an individual in a lifetime and how those exposures relate to health,” according to the CDC, and then introduced a tool clinicians can use to better understand social determinants of health in specific geographic areas. The Neighborhood Atlas, which Dr. Kind described in the New England Journal of Medicine in 2018, measures 17 social determinants across small population-sensitive areas and provides an area deprivation index. A high area deprivation index is linked to a range of negative outcomes, including reshopitalization, later diagnoses, less comprehensive diagnostic evaluation, increased risk of postsurgical complications, and decreased life expectancy.
“One of the things that really stood out to me about Dr. Kind’s discussion of the use of the area deprivation index was the fact that understanding and quantifying these kinds of risks and exposures is the vehicle for creating the kinds of social changes, including policy changes, that will actually lead to addressing and mitigating some of these lifelong risks and exposures,” Dr. Hamilton said. “It is implausible to think that a specific group of people would be genetically more susceptible to basically every disease that we know,” he added. “It makes much more sense to think that groups of individuals have been subjected systematically to conditions that impair health in a variety of ways.”
Not just race, ethnicity, sex, and gender
Following the four presentations from researchers in health inequities was an Emerging Scholar presentation in which Jay B. Lusk, an MD/MBA candidate at Duke University, Durham, N.C., shared new research findings on the role of neighborhood disadvantage in predicting mortality from coma, stroke, and other neurologic conditions. His findings revealed that living in a neighborhood with greater deprivation substantially increased risk of mortality even after accounting for individual wealth and demographics.
Maria Eugenia Diaz-Ortiz, PhD, of the department of neurology, University of Pennsylvania, Philadelphia, said she found the five presentations to be an excellent introduction to people like herself who are in the earlier stages of learning about health equity research.
“I think they introduced various important concepts and frameworks and provided tools for people who don’t know about them,” Dr. Diaz-Ortiz said. “Then they asked important questions and provided some solutions to them.”
Dr. Diaz-Ortiz also appreciated seemingly minor but actually important details in how the speakers presented themselves, such as Dr. Rivera-Mindt opening with a land acknowledgment and her disclosures of “positionality.” The former recognized the traditional Native American custodians of the land on which she lives and works, and the latter revealed details about her as an individual – such as being the Afro-Latinx daughter of immigrants yet being cisgender, able-bodied, and U.S.-born – that show where she falls on the axis of adversity and axis of privilege.
Implications for research
The biggest takeaway for Dr. Diaz-Ortiz, however, came from the first Q&A session when someone asked how to increase underrepresented populations in dementia research. Dr. Rivera-Mindt described her experience engaging these communities by employing “community-based participatory research practices, which involves making yourself a part of the community and making the community active participants in the research,” Dr. Diaz-Ortiz said. “It’s an evidence-based approach that has been shown to increase participation in research not only in her work but in the work of others.”
Preaching to the choir
Dr. Diaz-Ortiz was pleased overall with the plenary but disappointed in its placement at the end of the meeting, when attendance is always lower as attendees head home.
“The people who stayed were people who already know and recognize the value of health equity work, so I think that was a missed opportunity where the session could have been included on day one or two to boost attendance and also to educate like a broader group of neurologists,” Dr. Diaz-Ortiz said in an interview.
Dr. Mendizabal felt similarly, appreciating the plenary but noting it was “definitely overdue” and that it should not be the last session. Instead, sessions on health equity should be as easy as possible to attend to bring in larger audiences. “Perhaps having that session on a Saturday or Sunday would have a higher likelihood of greater attendance than on a Tuesday,” she said. That said, Dr. Mendizabal also noticed that greater attention to health care disparities was woven into many other sessions throughout the conference, which is “the best way of addressing health equity instead of trying to just designate a session,” she said.
Dr. Mendizabal hopes that plenaries like this one and the weaving of health equity issues into presentations throughout neurology conferences continue.
“After the racial reckoning in 2020, there was a big impetus and a big wave of energy in addressing health disparities in the field, and I hope that that momentum is not starting to wane,” Dr. Mendizabal said. “It’s important because not talking about is not going to make this issue go away.”
Dr. Hamilton agreed that it is important that the conversation continue and that physicians recognize the importance of understanding health care disparities and determinants of health, regardless of where they fall on the political spectrum or whether they choose to get involved in policy or advocacy.
“Irrespective of whether you think race or ethnicity or socioeconomic status are political issues or not, it is the case that you’re obligated to have an objective understanding of the factors that contribute to your patient’s health and as points of intervention,” Dr. Hamilton said. “So even if you don’t want to sit down and jot off that email to your senator, you still have to take these factors into account when you’re treating the person who’s sitting right in front of you, and that’s not political. That’s the promise of being a physician.”
Dr. Amezcua has received personal compensation for consulting, speaking, or serving on steering committees or advisory boards for Biogen Idec, Novartis, Genentech, and EMD Serono, and she has received research support from Biogen Idec and Bristol Myers Squibb Foundation. Dr. Kind reported support from the Alzheimer’s Association. Dr. Diaz-Ortiz is coinventor of a provisional patent submitted by the University of Pennsylvania that relates to a potential therapeutic in Parkinson’s disease. Mr. Lusk reported fellowship support from American Heart Association and travel support from the American Neurological Association. No other speakers or sources had relevant disclosures.
Black and Latinx older adults are up to three times as likely to develop Alzheimer’s disease than non-Latinx White adults and tend to experience onset at a younger age with more severe symptoms, according to Monica Rivera-Mindt, PhD, a professor of psychology at Fordham University and the Icahn School of Medicine at Mount Sinai, New York. Looking ahead, that means by 2030, nearly 40% of the 8.4 million Americans affected by Alzheimer’s disease will be Black and/or Latinx, she said. These facts were among the stark disparities in health care outcomes Dr. Rivera-Mindt discussed in her presentation on brain health equity at the 2022 annual meeting of the American Neurological Association.
Dr. Rivera-Mindt’s presentation opened the ANA’s plenary session on health disparities and inequities. The plenary, “Advancing Neurologic Equity: Challenges and Paths Forward,” did not simply enumerate racial and ethnic disparities that exist with various neurological conditions. Rather it went beyond the discussion of what disparities exist into understanding the roots of them as well as tips, tools, and resources that can aid clinicians in addressing or ameliorating them.
Roy Hamilton, MD, an associate professor of neurology and physical medicine and rehabilitation at the University of Pennsylvania, Philadelphia, said. “If clinicians are unaware of these disparities or don’t have any sense of how to start to address or think about them, then they’re really missing out on an important component of their education as persons who take care of patients with brain disorders.”
Dr. Hamilton, who organized the plenary, noted that awareness of these disparities is crucial to comprehensively caring for patients.
Missed opportunities
“We’re talking about disadvantages that are structural and large scale, but those disadvantages play themselves out in the individual encounter,” Dr. Hamilton said. “When physicians see patients, they have to treat the whole patient in front of them,” which means being aware of the risks and factors that could affect a patient’s clinical presentation. “Being aware of disparities has practical impacts on physician judgment,” he said.
For example, recent research in multiple sclerosis (MS) has highlighted how clinicians may be missing diagnosis of this condition in non-White populations because the condition has been regarded for so long as a “White person’s” disease, Dr. Hamilton said. In non-White patients exhibiting MS symptoms, then, clinicians may have been less likely to consider MS as a possibility, thereby delaying diagnosis and treatment.
Those patterns may partly explain why the mortality rate for MS is greater in Black patients, who also show more rapid neurodegeneration than White patients with MS, Lilyana Amezcua, MD, an associate professor of neurology at the University of Southern California, Los Angeles, reported in the plenary’s second presentation.
Transgender issues
The third session, presented by Nicole Rosendale, MD, an assistant professor of neurology at the University of California, San Francisco, and director of the San Francisco General Hospital neurology inpatient services, examined disparities in neurology within the LGBTQ+ community through representative case studies and then offered specific ways that neurologists could make their practices more inclusive and equitable for sexual and gender minorities.
Her first case study was a 52-year-old man who presented with new-onset seizures, right hemiparesis, and aphasia. A brain biopsy consistent with adenocarcinoma eventually led his physician to discover he had metastatic breast cancer. It turned out the man was transgender and, despite a family history of breast cancer, hadn’t been advised to get breast cancer screenings.
“Breast cancer was not initially on the differential as no one had identified that the patient was transmasculine,” Dr. Rosendale said. A major challenge to providing care to transgender patients is a dearth of data on risks and screening recommendations. Another barrier is low knowledge of LGBTQ+ health among neurologists, Dr. Rosendale said while sharing findings from her 2019 study on the topic and calling for more research in LGBTQ+ populations.
Dr. Rosendale’s second case study dealt with a nonbinary patient who suffered from debilitating headaches for decades, first because they lacked access to health insurance and then because negative experiences with providers dissuaded them from seeking care. In data from the Center for American Progress she shared, 8% of LGB respondents and 22% of transgender respondents said they had avoided or delayed care because of fear of discrimination or mistreatment.
“So it’s not only access but also what experiences people are having when they go in and whether they’re actually even getting access to care or being taken care of,” Dr. Rosendale said. Other findings from the CAP found that:
- 8% of LGB patients and 29% of transgender patients reported having a clinician refuse to see them.
- 6% of LGB patients and 12% of transgender patients reported that a clinician refused to give them health care.
- 9% of LGB patients and 21% of transgender patients experienced harsh or abusive language during a health care experience.
- 7% of LGB patients and nearly a third (29%) of transgender patients experienced unwanted physical contact, such as fondling or sexual assault.
Reducing the disparities
Adys Mendizabal, MD, an assistant professor of neurology at the Institute of Society and Genetics at the University of California, Los Angeles, who attended the presentation, was grateful to see how the various lectures enriched the discussion beyond stating the fact of racial/ethnic disparities and dug into the nuances on how to think about and address these disparities. She particularly appreciated discussion about the need to go out of the way to recruit diverse patient populations for clinical trials while also providing them care.
“It is definitely complicated, but it’s not impossible for an individual neurologist or an individual department to do something to reduce some of the disparities,” Dr. Mendizabal said. “It starts with just knowing that they exist and being aware of some of the things that may be impacting care for a particular patient.”
Tools to counter disparity
In the final presentation, Amy Kind, MD, PhD, the associate dean for social health sciences and programs at the University of Wisconsin–Madison, rounded out the discussion by exploring social determinants of health and their influence on outcomes.
“Social determinants impact brain health, and brain health is not distributed equally,” Dr. Kind told attendees. “We have known this for decades, yet disparities persist.”
Dr. Kind described the “exposome,” a “measure of all the exposures of an individual in a lifetime and how those exposures relate to health,” according to the CDC, and then introduced a tool clinicians can use to better understand social determinants of health in specific geographic areas. The Neighborhood Atlas, which Dr. Kind described in the New England Journal of Medicine in 2018, measures 17 social determinants across small population-sensitive areas and provides an area deprivation index. A high area deprivation index is linked to a range of negative outcomes, including reshopitalization, later diagnoses, less comprehensive diagnostic evaluation, increased risk of postsurgical complications, and decreased life expectancy.
“One of the things that really stood out to me about Dr. Kind’s discussion of the use of the area deprivation index was the fact that understanding and quantifying these kinds of risks and exposures is the vehicle for creating the kinds of social changes, including policy changes, that will actually lead to addressing and mitigating some of these lifelong risks and exposures,” Dr. Hamilton said. “It is implausible to think that a specific group of people would be genetically more susceptible to basically every disease that we know,” he added. “It makes much more sense to think that groups of individuals have been subjected systematically to conditions that impair health in a variety of ways.”
Not just race, ethnicity, sex, and gender
Following the four presentations from researchers in health inequities was an Emerging Scholar presentation in which Jay B. Lusk, an MD/MBA candidate at Duke University, Durham, N.C., shared new research findings on the role of neighborhood disadvantage in predicting mortality from coma, stroke, and other neurologic conditions. His findings revealed that living in a neighborhood with greater deprivation substantially increased risk of mortality even after accounting for individual wealth and demographics.
Maria Eugenia Diaz-Ortiz, PhD, of the department of neurology, University of Pennsylvania, Philadelphia, said she found the five presentations to be an excellent introduction to people like herself who are in the earlier stages of learning about health equity research.
“I think they introduced various important concepts and frameworks and provided tools for people who don’t know about them,” Dr. Diaz-Ortiz said. “Then they asked important questions and provided some solutions to them.”
Dr. Diaz-Ortiz also appreciated seemingly minor but actually important details in how the speakers presented themselves, such as Dr. Rivera-Mindt opening with a land acknowledgment and her disclosures of “positionality.” The former recognized the traditional Native American custodians of the land on which she lives and works, and the latter revealed details about her as an individual – such as being the Afro-Latinx daughter of immigrants yet being cisgender, able-bodied, and U.S.-born – that show where she falls on the axis of adversity and axis of privilege.
Implications for research
The biggest takeaway for Dr. Diaz-Ortiz, however, came from the first Q&A session when someone asked how to increase underrepresented populations in dementia research. Dr. Rivera-Mindt described her experience engaging these communities by employing “community-based participatory research practices, which involves making yourself a part of the community and making the community active participants in the research,” Dr. Diaz-Ortiz said. “It’s an evidence-based approach that has been shown to increase participation in research not only in her work but in the work of others.”
Preaching to the choir
Dr. Diaz-Ortiz was pleased overall with the plenary but disappointed in its placement at the end of the meeting, when attendance is always lower as attendees head home.
“The people who stayed were people who already know and recognize the value of health equity work, so I think that was a missed opportunity where the session could have been included on day one or two to boost attendance and also to educate like a broader group of neurologists,” Dr. Diaz-Ortiz said in an interview.
Dr. Mendizabal felt similarly, appreciating the plenary but noting it was “definitely overdue” and that it should not be the last session. Instead, sessions on health equity should be as easy as possible to attend to bring in larger audiences. “Perhaps having that session on a Saturday or Sunday would have a higher likelihood of greater attendance than on a Tuesday,” she said. That said, Dr. Mendizabal also noticed that greater attention to health care disparities was woven into many other sessions throughout the conference, which is “the best way of addressing health equity instead of trying to just designate a session,” she said.
Dr. Mendizabal hopes that plenaries like this one and the weaving of health equity issues into presentations throughout neurology conferences continue.
“After the racial reckoning in 2020, there was a big impetus and a big wave of energy in addressing health disparities in the field, and I hope that that momentum is not starting to wane,” Dr. Mendizabal said. “It’s important because not talking about is not going to make this issue go away.”
Dr. Hamilton agreed that it is important that the conversation continue and that physicians recognize the importance of understanding health care disparities and determinants of health, regardless of where they fall on the political spectrum or whether they choose to get involved in policy or advocacy.
“Irrespective of whether you think race or ethnicity or socioeconomic status are political issues or not, it is the case that you’re obligated to have an objective understanding of the factors that contribute to your patient’s health and as points of intervention,” Dr. Hamilton said. “So even if you don’t want to sit down and jot off that email to your senator, you still have to take these factors into account when you’re treating the person who’s sitting right in front of you, and that’s not political. That’s the promise of being a physician.”
Dr. Amezcua has received personal compensation for consulting, speaking, or serving on steering committees or advisory boards for Biogen Idec, Novartis, Genentech, and EMD Serono, and she has received research support from Biogen Idec and Bristol Myers Squibb Foundation. Dr. Kind reported support from the Alzheimer’s Association. Dr. Diaz-Ortiz is coinventor of a provisional patent submitted by the University of Pennsylvania that relates to a potential therapeutic in Parkinson’s disease. Mr. Lusk reported fellowship support from American Heart Association and travel support from the American Neurological Association. No other speakers or sources had relevant disclosures.
Black and Latinx older adults are up to three times as likely to develop Alzheimer’s disease than non-Latinx White adults and tend to experience onset at a younger age with more severe symptoms, according to Monica Rivera-Mindt, PhD, a professor of psychology at Fordham University and the Icahn School of Medicine at Mount Sinai, New York. Looking ahead, that means by 2030, nearly 40% of the 8.4 million Americans affected by Alzheimer’s disease will be Black and/or Latinx, she said. These facts were among the stark disparities in health care outcomes Dr. Rivera-Mindt discussed in her presentation on brain health equity at the 2022 annual meeting of the American Neurological Association.
Dr. Rivera-Mindt’s presentation opened the ANA’s plenary session on health disparities and inequities. The plenary, “Advancing Neurologic Equity: Challenges and Paths Forward,” did not simply enumerate racial and ethnic disparities that exist with various neurological conditions. Rather it went beyond the discussion of what disparities exist into understanding the roots of them as well as tips, tools, and resources that can aid clinicians in addressing or ameliorating them.
Roy Hamilton, MD, an associate professor of neurology and physical medicine and rehabilitation at the University of Pennsylvania, Philadelphia, said. “If clinicians are unaware of these disparities or don’t have any sense of how to start to address or think about them, then they’re really missing out on an important component of their education as persons who take care of patients with brain disorders.”
Dr. Hamilton, who organized the plenary, noted that awareness of these disparities is crucial to comprehensively caring for patients.
Missed opportunities
“We’re talking about disadvantages that are structural and large scale, but those disadvantages play themselves out in the individual encounter,” Dr. Hamilton said. “When physicians see patients, they have to treat the whole patient in front of them,” which means being aware of the risks and factors that could affect a patient’s clinical presentation. “Being aware of disparities has practical impacts on physician judgment,” he said.
For example, recent research in multiple sclerosis (MS) has highlighted how clinicians may be missing diagnosis of this condition in non-White populations because the condition has been regarded for so long as a “White person’s” disease, Dr. Hamilton said. In non-White patients exhibiting MS symptoms, then, clinicians may have been less likely to consider MS as a possibility, thereby delaying diagnosis and treatment.
Those patterns may partly explain why the mortality rate for MS is greater in Black patients, who also show more rapid neurodegeneration than White patients with MS, Lilyana Amezcua, MD, an associate professor of neurology at the University of Southern California, Los Angeles, reported in the plenary’s second presentation.
Transgender issues
The third session, presented by Nicole Rosendale, MD, an assistant professor of neurology at the University of California, San Francisco, and director of the San Francisco General Hospital neurology inpatient services, examined disparities in neurology within the LGBTQ+ community through representative case studies and then offered specific ways that neurologists could make their practices more inclusive and equitable for sexual and gender minorities.
Her first case study was a 52-year-old man who presented with new-onset seizures, right hemiparesis, and aphasia. A brain biopsy consistent with adenocarcinoma eventually led his physician to discover he had metastatic breast cancer. It turned out the man was transgender and, despite a family history of breast cancer, hadn’t been advised to get breast cancer screenings.
“Breast cancer was not initially on the differential as no one had identified that the patient was transmasculine,” Dr. Rosendale said. A major challenge to providing care to transgender patients is a dearth of data on risks and screening recommendations. Another barrier is low knowledge of LGBTQ+ health among neurologists, Dr. Rosendale said while sharing findings from her 2019 study on the topic and calling for more research in LGBTQ+ populations.
Dr. Rosendale’s second case study dealt with a nonbinary patient who suffered from debilitating headaches for decades, first because they lacked access to health insurance and then because negative experiences with providers dissuaded them from seeking care. In data from the Center for American Progress she shared, 8% of LGB respondents and 22% of transgender respondents said they had avoided or delayed care because of fear of discrimination or mistreatment.
“So it’s not only access but also what experiences people are having when they go in and whether they’re actually even getting access to care or being taken care of,” Dr. Rosendale said. Other findings from the CAP found that:
- 8% of LGB patients and 29% of transgender patients reported having a clinician refuse to see them.
- 6% of LGB patients and 12% of transgender patients reported that a clinician refused to give them health care.
- 9% of LGB patients and 21% of transgender patients experienced harsh or abusive language during a health care experience.
- 7% of LGB patients and nearly a third (29%) of transgender patients experienced unwanted physical contact, such as fondling or sexual assault.
Reducing the disparities
Adys Mendizabal, MD, an assistant professor of neurology at the Institute of Society and Genetics at the University of California, Los Angeles, who attended the presentation, was grateful to see how the various lectures enriched the discussion beyond stating the fact of racial/ethnic disparities and dug into the nuances on how to think about and address these disparities. She particularly appreciated discussion about the need to go out of the way to recruit diverse patient populations for clinical trials while also providing them care.
“It is definitely complicated, but it’s not impossible for an individual neurologist or an individual department to do something to reduce some of the disparities,” Dr. Mendizabal said. “It starts with just knowing that they exist and being aware of some of the things that may be impacting care for a particular patient.”
Tools to counter disparity
In the final presentation, Amy Kind, MD, PhD, the associate dean for social health sciences and programs at the University of Wisconsin–Madison, rounded out the discussion by exploring social determinants of health and their influence on outcomes.
“Social determinants impact brain health, and brain health is not distributed equally,” Dr. Kind told attendees. “We have known this for decades, yet disparities persist.”
Dr. Kind described the “exposome,” a “measure of all the exposures of an individual in a lifetime and how those exposures relate to health,” according to the CDC, and then introduced a tool clinicians can use to better understand social determinants of health in specific geographic areas. The Neighborhood Atlas, which Dr. Kind described in the New England Journal of Medicine in 2018, measures 17 social determinants across small population-sensitive areas and provides an area deprivation index. A high area deprivation index is linked to a range of negative outcomes, including reshopitalization, later diagnoses, less comprehensive diagnostic evaluation, increased risk of postsurgical complications, and decreased life expectancy.
“One of the things that really stood out to me about Dr. Kind’s discussion of the use of the area deprivation index was the fact that understanding and quantifying these kinds of risks and exposures is the vehicle for creating the kinds of social changes, including policy changes, that will actually lead to addressing and mitigating some of these lifelong risks and exposures,” Dr. Hamilton said. “It is implausible to think that a specific group of people would be genetically more susceptible to basically every disease that we know,” he added. “It makes much more sense to think that groups of individuals have been subjected systematically to conditions that impair health in a variety of ways.”
Not just race, ethnicity, sex, and gender
Following the four presentations from researchers in health inequities was an Emerging Scholar presentation in which Jay B. Lusk, an MD/MBA candidate at Duke University, Durham, N.C., shared new research findings on the role of neighborhood disadvantage in predicting mortality from coma, stroke, and other neurologic conditions. His findings revealed that living in a neighborhood with greater deprivation substantially increased risk of mortality even after accounting for individual wealth and demographics.
Maria Eugenia Diaz-Ortiz, PhD, of the department of neurology, University of Pennsylvania, Philadelphia, said she found the five presentations to be an excellent introduction to people like herself who are in the earlier stages of learning about health equity research.
“I think they introduced various important concepts and frameworks and provided tools for people who don’t know about them,” Dr. Diaz-Ortiz said. “Then they asked important questions and provided some solutions to them.”
Dr. Diaz-Ortiz also appreciated seemingly minor but actually important details in how the speakers presented themselves, such as Dr. Rivera-Mindt opening with a land acknowledgment and her disclosures of “positionality.” The former recognized the traditional Native American custodians of the land on which she lives and works, and the latter revealed details about her as an individual – such as being the Afro-Latinx daughter of immigrants yet being cisgender, able-bodied, and U.S.-born – that show where she falls on the axis of adversity and axis of privilege.
Implications for research
The biggest takeaway for Dr. Diaz-Ortiz, however, came from the first Q&A session when someone asked how to increase underrepresented populations in dementia research. Dr. Rivera-Mindt described her experience engaging these communities by employing “community-based participatory research practices, which involves making yourself a part of the community and making the community active participants in the research,” Dr. Diaz-Ortiz said. “It’s an evidence-based approach that has been shown to increase participation in research not only in her work but in the work of others.”
Preaching to the choir
Dr. Diaz-Ortiz was pleased overall with the plenary but disappointed in its placement at the end of the meeting, when attendance is always lower as attendees head home.
“The people who stayed were people who already know and recognize the value of health equity work, so I think that was a missed opportunity where the session could have been included on day one or two to boost attendance and also to educate like a broader group of neurologists,” Dr. Diaz-Ortiz said in an interview.
Dr. Mendizabal felt similarly, appreciating the plenary but noting it was “definitely overdue” and that it should not be the last session. Instead, sessions on health equity should be as easy as possible to attend to bring in larger audiences. “Perhaps having that session on a Saturday or Sunday would have a higher likelihood of greater attendance than on a Tuesday,” she said. That said, Dr. Mendizabal also noticed that greater attention to health care disparities was woven into many other sessions throughout the conference, which is “the best way of addressing health equity instead of trying to just designate a session,” she said.
Dr. Mendizabal hopes that plenaries like this one and the weaving of health equity issues into presentations throughout neurology conferences continue.
“After the racial reckoning in 2020, there was a big impetus and a big wave of energy in addressing health disparities in the field, and I hope that that momentum is not starting to wane,” Dr. Mendizabal said. “It’s important because not talking about is not going to make this issue go away.”
Dr. Hamilton agreed that it is important that the conversation continue and that physicians recognize the importance of understanding health care disparities and determinants of health, regardless of where they fall on the political spectrum or whether they choose to get involved in policy or advocacy.
“Irrespective of whether you think race or ethnicity or socioeconomic status are political issues or not, it is the case that you’re obligated to have an objective understanding of the factors that contribute to your patient’s health and as points of intervention,” Dr. Hamilton said. “So even if you don’t want to sit down and jot off that email to your senator, you still have to take these factors into account when you’re treating the person who’s sitting right in front of you, and that’s not political. That’s the promise of being a physician.”
Dr. Amezcua has received personal compensation for consulting, speaking, or serving on steering committees or advisory boards for Biogen Idec, Novartis, Genentech, and EMD Serono, and she has received research support from Biogen Idec and Bristol Myers Squibb Foundation. Dr. Kind reported support from the Alzheimer’s Association. Dr. Diaz-Ortiz is coinventor of a provisional patent submitted by the University of Pennsylvania that relates to a potential therapeutic in Parkinson’s disease. Mr. Lusk reported fellowship support from American Heart Association and travel support from the American Neurological Association. No other speakers or sources had relevant disclosures.
FROM ANA 2022
Persistent asthma linked to higher carotid plaque burden
Persistent asthma is associated with increased carotid plaque burden and higher levels of inflammation, putting these patients at risk for atherosclerotic cardiovascular disease (ASCVD) events, new research suggests.
Using data from the MESA study, investigators analyzed more than 5,000 individuals, comparing carotid plaque and inflammatory markers in those with and without asthma.
They found that carotid plaque was present in half of participants without asthma and half of those with intermittent asthma but in close to 70% of participants with persistent asthma.
.
“The take-home message is that the current study, paired with prior studies, highlights that individuals with more significant forms of asthma may be at higher cardiovascular risk and makes it imperative to address modifiable risk factors among patients with asthma,” lead author Matthew Tattersall, DO, MS, assistant professor of cardiovascular medicine, University of Wisconsin School of Medicine and Public Health, Madison, told this news organization.
The study was published online in the Journal of the American Heart Association.
Limited data
Asthma and ASCVD are “highly prevalent inflammatory diseases,” the authors write. Carotid artery plaque detected by B-mode ultrasound “represents advanced, typically subclinical atherosclerosis that is a strong independent predictor of incident ASCVD events,” with inflammation playing a “key role” in precipitating these events, they note.
Serum inflammatory markers such as C-reactive protein (CRP) and IL-6 are associated with increased ASCVD events, and in asthma, CRP and other inflammatory biomarkers are elevated and tend to further increase during exacerbations.
Currently, there are limited data looking at the associations of asthma, asthma severity, and atherosclerotic plaque burden, they note, so the researchers turned to the MESA study – a multiethnic population of individuals free of prevalent ASCVD at baseline. They hypothesized that persistent asthma would be associated with higher carotid plaque presence and burden.
They also wanted to explore “whether these associations would be attenuated after adjustment for baseline inflammatory biomarkers.”
Dr. Tattersall said the current study “links our previous work studying the manifestations of asthma,” in which he and his colleagues demonstrated increased cardiovascular events among MESA participants with persistent asthma, as well as late-onset asthma participants in the Wisconsin Sleep Cohort. His group also showed that early arterial injury occurs in adolescents with asthma.
However, there are also few data looking at the association with carotid plaque, “a late manifestation of arterial injury and a strong predictor of future cardiovascular events and asthma,” Dr. Tattersall added.
He and his group therefore “wanted to explore the entire spectrum of arterial injury, from the initial increase in the carotid media thickness to plaque formation to cardiovascular events.”
To do so, they studied participants in MESA, a study of close to 7,000 adults that began in the year 2000 and continues to follow participants today. At the time of enrollment, all were free from CVD.
The current analysis looked at 5,029 MESA participants (mean age 61.6 years, 53% female, 26% Black, 23% Hispanic, 12% Asian), comparing those with persistent asthma, defined as “asthma requiring use of controller medications,” intermittent asthma, defined as “asthma without controller medications,” and no asthma.
Participants underwent B-mode carotid ultrasound to detect carotid plaques, with a total plaque score (TPS) ranging from 0-12. The researchers used multivariable regression modeling to evaluate the association of asthma subtype and carotid plaque burden.
Interpret cautiously
Participants with persistent asthma were more likely to be female, have higher body mass index (BMI), and higher high-density lipoprotein (HDL) cholesterol levels, compared with those without asthma.
Participants with persistent asthma had the highest burden of carotid plaque (P ≤ .003 for comparison of proportions and .002 for comparison of means).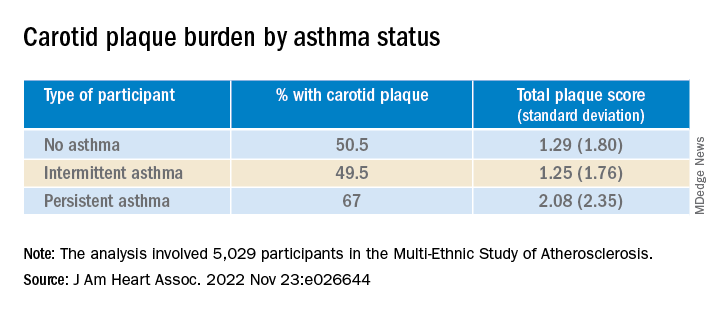
Moreover, participants with persistent asthma also had the highest systemic inflammatory marker levels – both CRP and IL-6 – compared with those without asthma. While participants with intermittent asthma also had higher average CRP, compared with those without asthma, their IL-6 levels were comparable.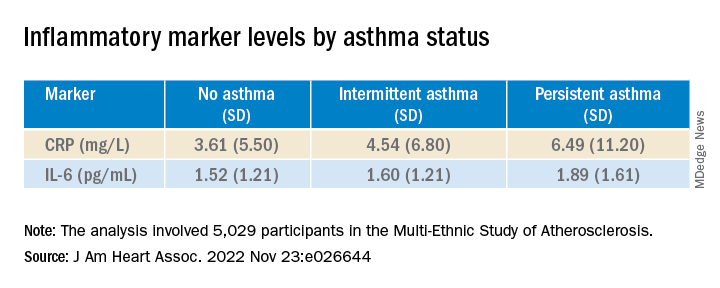
In unadjusted models, persistent asthma was associated with higher odds of carotid plaque presence (odds ratio, 1.97; 95% confidence interval, 1.32-2.95) – an association that persisted even in models that adjusted for biologic confounders (both P < .01). There also was an association between persistent asthma and higher carotid TPS (P < .001).
In further adjusted models, IL-6 was independently associated with presence of carotid plaque (P = .0001 per 1-SD increment of 1.53), as well as TPS (P < .001). CRP was “slightly associated” with carotid TPS (P = .04) but not carotid plaque presence (P = .07).
There was no attenuation after the researchers evaluated the associations of asthma subtype and carotid plaque presence or TPS and fully adjusted for baseline IL-6 or CRP (P = .02 and P = .01, respectively).
“Since this study is observational, we cannot confirm causation, but the study adds to the growing literature exploring the systemic effects of asthma,” Dr. Tattersall commented.
“Our initial hypothesis was that it was driven by inflammation, as both asthma and CVD are inflammatory conditions,” he continued. “We did adjust for inflammatory biomarkers in this analysis, but there was no change in the association.”
Nevertheless, Dr. Tattersall and colleagues are “cautious in the interpretation,” since the inflammatory biomarkers “were only collected at one point, and these measures can be dynamic, thus adjustment may not tell the whole story.”
Heightened awareness
Robert Brook, MD, professor and director of cardiovascular disease prevention, Wayne State University, Detroit, said the “main contribution of this study is the novel demonstration of a significant association between persistent (but not intermittent) asthma with carotid atherosclerosis in the MESA cohort, a large multi-ethnic population.”
These findings “support the biological plausibility of the growing epidemiological evidence that asthma independently increases the risk for cardiovascular morbidity and mortality,” added Dr. Brook, who was not involved with the study.
“The main take-home message for clinicians is that, just like in COPD (which is well-established), asthma is often a systemic condition in that the inflammation and disease process can impact the whole body,” he said.
“Health care providers should have a heightened awareness of the potentially increased cardiovascular risk of their patients with asthma and pay special attention to controlling their heart disease risk factors (for example, hyperlipidemia, hypertension),” Dr. Brook stated.
Dr. Tattersall was supported by an American Heart Association Career Development Award. The Multi-Ethnic Study of Atherosclerosis was supported by the National Heart, Lung, and Blood Institute and the National Center for Research Resources. Dr. Tattersall and co-authors and Dr. Brook declare no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Persistent asthma is associated with increased carotid plaque burden and higher levels of inflammation, putting these patients at risk for atherosclerotic cardiovascular disease (ASCVD) events, new research suggests.
Using data from the MESA study, investigators analyzed more than 5,000 individuals, comparing carotid plaque and inflammatory markers in those with and without asthma.
They found that carotid plaque was present in half of participants without asthma and half of those with intermittent asthma but in close to 70% of participants with persistent asthma.
.
“The take-home message is that the current study, paired with prior studies, highlights that individuals with more significant forms of asthma may be at higher cardiovascular risk and makes it imperative to address modifiable risk factors among patients with asthma,” lead author Matthew Tattersall, DO, MS, assistant professor of cardiovascular medicine, University of Wisconsin School of Medicine and Public Health, Madison, told this news organization.
The study was published online in the Journal of the American Heart Association.
Limited data
Asthma and ASCVD are “highly prevalent inflammatory diseases,” the authors write. Carotid artery plaque detected by B-mode ultrasound “represents advanced, typically subclinical atherosclerosis that is a strong independent predictor of incident ASCVD events,” with inflammation playing a “key role” in precipitating these events, they note.
Serum inflammatory markers such as C-reactive protein (CRP) and IL-6 are associated with increased ASCVD events, and in asthma, CRP and other inflammatory biomarkers are elevated and tend to further increase during exacerbations.
Currently, there are limited data looking at the associations of asthma, asthma severity, and atherosclerotic plaque burden, they note, so the researchers turned to the MESA study – a multiethnic population of individuals free of prevalent ASCVD at baseline. They hypothesized that persistent asthma would be associated with higher carotid plaque presence and burden.
They also wanted to explore “whether these associations would be attenuated after adjustment for baseline inflammatory biomarkers.”
Dr. Tattersall said the current study “links our previous work studying the manifestations of asthma,” in which he and his colleagues demonstrated increased cardiovascular events among MESA participants with persistent asthma, as well as late-onset asthma participants in the Wisconsin Sleep Cohort. His group also showed that early arterial injury occurs in adolescents with asthma.
However, there are also few data looking at the association with carotid plaque, “a late manifestation of arterial injury and a strong predictor of future cardiovascular events and asthma,” Dr. Tattersall added.
He and his group therefore “wanted to explore the entire spectrum of arterial injury, from the initial increase in the carotid media thickness to plaque formation to cardiovascular events.”
To do so, they studied participants in MESA, a study of close to 7,000 adults that began in the year 2000 and continues to follow participants today. At the time of enrollment, all were free from CVD.
The current analysis looked at 5,029 MESA participants (mean age 61.6 years, 53% female, 26% Black, 23% Hispanic, 12% Asian), comparing those with persistent asthma, defined as “asthma requiring use of controller medications,” intermittent asthma, defined as “asthma without controller medications,” and no asthma.
Participants underwent B-mode carotid ultrasound to detect carotid plaques, with a total plaque score (TPS) ranging from 0-12. The researchers used multivariable regression modeling to evaluate the association of asthma subtype and carotid plaque burden.
Interpret cautiously
Participants with persistent asthma were more likely to be female, have higher body mass index (BMI), and higher high-density lipoprotein (HDL) cholesterol levels, compared with those without asthma.
Participants with persistent asthma had the highest burden of carotid plaque (P ≤ .003 for comparison of proportions and .002 for comparison of means).
Moreover, participants with persistent asthma also had the highest systemic inflammatory marker levels – both CRP and IL-6 – compared with those without asthma. While participants with intermittent asthma also had higher average CRP, compared with those without asthma, their IL-6 levels were comparable.
In unadjusted models, persistent asthma was associated with higher odds of carotid plaque presence (odds ratio, 1.97; 95% confidence interval, 1.32-2.95) – an association that persisted even in models that adjusted for biologic confounders (both P < .01). There also was an association between persistent asthma and higher carotid TPS (P < .001).
In further adjusted models, IL-6 was independently associated with presence of carotid plaque (P = .0001 per 1-SD increment of 1.53), as well as TPS (P < .001). CRP was “slightly associated” with carotid TPS (P = .04) but not carotid plaque presence (P = .07).
There was no attenuation after the researchers evaluated the associations of asthma subtype and carotid plaque presence or TPS and fully adjusted for baseline IL-6 or CRP (P = .02 and P = .01, respectively).
“Since this study is observational, we cannot confirm causation, but the study adds to the growing literature exploring the systemic effects of asthma,” Dr. Tattersall commented.
“Our initial hypothesis was that it was driven by inflammation, as both asthma and CVD are inflammatory conditions,” he continued. “We did adjust for inflammatory biomarkers in this analysis, but there was no change in the association.”
Nevertheless, Dr. Tattersall and colleagues are “cautious in the interpretation,” since the inflammatory biomarkers “were only collected at one point, and these measures can be dynamic, thus adjustment may not tell the whole story.”
Heightened awareness
Robert Brook, MD, professor and director of cardiovascular disease prevention, Wayne State University, Detroit, said the “main contribution of this study is the novel demonstration of a significant association between persistent (but not intermittent) asthma with carotid atherosclerosis in the MESA cohort, a large multi-ethnic population.”
These findings “support the biological plausibility of the growing epidemiological evidence that asthma independently increases the risk for cardiovascular morbidity and mortality,” added Dr. Brook, who was not involved with the study.
“The main take-home message for clinicians is that, just like in COPD (which is well-established), asthma is often a systemic condition in that the inflammation and disease process can impact the whole body,” he said.
“Health care providers should have a heightened awareness of the potentially increased cardiovascular risk of their patients with asthma and pay special attention to controlling their heart disease risk factors (for example, hyperlipidemia, hypertension),” Dr. Brook stated.
Dr. Tattersall was supported by an American Heart Association Career Development Award. The Multi-Ethnic Study of Atherosclerosis was supported by the National Heart, Lung, and Blood Institute and the National Center for Research Resources. Dr. Tattersall and co-authors and Dr. Brook declare no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Persistent asthma is associated with increased carotid plaque burden and higher levels of inflammation, putting these patients at risk for atherosclerotic cardiovascular disease (ASCVD) events, new research suggests.
Using data from the MESA study, investigators analyzed more than 5,000 individuals, comparing carotid plaque and inflammatory markers in those with and without asthma.
They found that carotid plaque was present in half of participants without asthma and half of those with intermittent asthma but in close to 70% of participants with persistent asthma.
.
“The take-home message is that the current study, paired with prior studies, highlights that individuals with more significant forms of asthma may be at higher cardiovascular risk and makes it imperative to address modifiable risk factors among patients with asthma,” lead author Matthew Tattersall, DO, MS, assistant professor of cardiovascular medicine, University of Wisconsin School of Medicine and Public Health, Madison, told this news organization.
The study was published online in the Journal of the American Heart Association.
Limited data
Asthma and ASCVD are “highly prevalent inflammatory diseases,” the authors write. Carotid artery plaque detected by B-mode ultrasound “represents advanced, typically subclinical atherosclerosis that is a strong independent predictor of incident ASCVD events,” with inflammation playing a “key role” in precipitating these events, they note.
Serum inflammatory markers such as C-reactive protein (CRP) and IL-6 are associated with increased ASCVD events, and in asthma, CRP and other inflammatory biomarkers are elevated and tend to further increase during exacerbations.
Currently, there are limited data looking at the associations of asthma, asthma severity, and atherosclerotic plaque burden, they note, so the researchers turned to the MESA study – a multiethnic population of individuals free of prevalent ASCVD at baseline. They hypothesized that persistent asthma would be associated with higher carotid plaque presence and burden.
They also wanted to explore “whether these associations would be attenuated after adjustment for baseline inflammatory biomarkers.”
Dr. Tattersall said the current study “links our previous work studying the manifestations of asthma,” in which he and his colleagues demonstrated increased cardiovascular events among MESA participants with persistent asthma, as well as late-onset asthma participants in the Wisconsin Sleep Cohort. His group also showed that early arterial injury occurs in adolescents with asthma.
However, there are also few data looking at the association with carotid plaque, “a late manifestation of arterial injury and a strong predictor of future cardiovascular events and asthma,” Dr. Tattersall added.
He and his group therefore “wanted to explore the entire spectrum of arterial injury, from the initial increase in the carotid media thickness to plaque formation to cardiovascular events.”
To do so, they studied participants in MESA, a study of close to 7,000 adults that began in the year 2000 and continues to follow participants today. At the time of enrollment, all were free from CVD.
The current analysis looked at 5,029 MESA participants (mean age 61.6 years, 53% female, 26% Black, 23% Hispanic, 12% Asian), comparing those with persistent asthma, defined as “asthma requiring use of controller medications,” intermittent asthma, defined as “asthma without controller medications,” and no asthma.
Participants underwent B-mode carotid ultrasound to detect carotid plaques, with a total plaque score (TPS) ranging from 0-12. The researchers used multivariable regression modeling to evaluate the association of asthma subtype and carotid plaque burden.
Interpret cautiously
Participants with persistent asthma were more likely to be female, have higher body mass index (BMI), and higher high-density lipoprotein (HDL) cholesterol levels, compared with those without asthma.
Participants with persistent asthma had the highest burden of carotid plaque (P ≤ .003 for comparison of proportions and .002 for comparison of means).
Moreover, participants with persistent asthma also had the highest systemic inflammatory marker levels – both CRP and IL-6 – compared with those without asthma. While participants with intermittent asthma also had higher average CRP, compared with those without asthma, their IL-6 levels were comparable.
In unadjusted models, persistent asthma was associated with higher odds of carotid plaque presence (odds ratio, 1.97; 95% confidence interval, 1.32-2.95) – an association that persisted even in models that adjusted for biologic confounders (both P < .01). There also was an association between persistent asthma and higher carotid TPS (P < .001).
In further adjusted models, IL-6 was independently associated with presence of carotid plaque (P = .0001 per 1-SD increment of 1.53), as well as TPS (P < .001). CRP was “slightly associated” with carotid TPS (P = .04) but not carotid plaque presence (P = .07).
There was no attenuation after the researchers evaluated the associations of asthma subtype and carotid plaque presence or TPS and fully adjusted for baseline IL-6 or CRP (P = .02 and P = .01, respectively).
“Since this study is observational, we cannot confirm causation, but the study adds to the growing literature exploring the systemic effects of asthma,” Dr. Tattersall commented.
“Our initial hypothesis was that it was driven by inflammation, as both asthma and CVD are inflammatory conditions,” he continued. “We did adjust for inflammatory biomarkers in this analysis, but there was no change in the association.”
Nevertheless, Dr. Tattersall and colleagues are “cautious in the interpretation,” since the inflammatory biomarkers “were only collected at one point, and these measures can be dynamic, thus adjustment may not tell the whole story.”
Heightened awareness
Robert Brook, MD, professor and director of cardiovascular disease prevention, Wayne State University, Detroit, said the “main contribution of this study is the novel demonstration of a significant association between persistent (but not intermittent) asthma with carotid atherosclerosis in the MESA cohort, a large multi-ethnic population.”
These findings “support the biological plausibility of the growing epidemiological evidence that asthma independently increases the risk for cardiovascular morbidity and mortality,” added Dr. Brook, who was not involved with the study.
“The main take-home message for clinicians is that, just like in COPD (which is well-established), asthma is often a systemic condition in that the inflammation and disease process can impact the whole body,” he said.
“Health care providers should have a heightened awareness of the potentially increased cardiovascular risk of their patients with asthma and pay special attention to controlling their heart disease risk factors (for example, hyperlipidemia, hypertension),” Dr. Brook stated.
Dr. Tattersall was supported by an American Heart Association Career Development Award. The Multi-Ethnic Study of Atherosclerosis was supported by the National Heart, Lung, and Blood Institute and the National Center for Research Resources. Dr. Tattersall and co-authors and Dr. Brook declare no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Is it long COVID, or dementia, or both?
In early September, about a week after recovering from COVID-19, Barri Sanders went to the bank to pay a bill. But by mistake, she transferred a large amount of money from the wrong account.
“I’m talking about $20,000,” she said. “I had to go back [later] and fix it.”
Ms. Sanders, 83, had not had confusion like that before. Suddenly, the Albuquerque, N.M., resident found herself looking up from a book and not remembering what she had just read. She would stand up from her chair and forget what she meant to do.
“I kind of thought it was just the aging process,” she said. Combined with sudden balance issues, insomnia, and a nagging postnasal drip, the overall effect was “subtle, but scary,” she said.
After 5 days of this, she went to bed and slept the whole night through. She woke up in the morning to find her balanced restored, her sinuses clear, and the mental fog gone. What she’d had, she realized, wasn’t a rapid start of dementia, but rather a mercifully short form of long COVID.
Somewhere between 22% and 32% of people who recover from COVID-19 get “brain fog,” a nonscientific term used to describe slow or sluggish thinking. While this is disturbing at any age, And some scientists are starting to confirm what doctors, patients, and their families can already see: Older patients who have had COVID-19 have a higher risk of getting dementia or, if they already have mental confusion, the illness may worsen their condition.
British scientists who studied medical records from around the world reported in the journal The Lancet Psychiatry that people who recovered from COVID-19 had a higher risk of problems with their thinking and dementia even after 2 years had passed.
Another 2022 study, published in JAMA Neurology, looked at older COVID-19 patients for a year after they were discharged from hospitals in Wuhan, China. Compared with uninfected people, those who survived a severe case of COVID-19 were at higher risk for early onset, late-onset, and progressive decline in their thinking skills. Those who survived a mild infection were at a higher risk for early onset decline, the study found.
Eran Metzger, MD, assistant professor of psychiatry at Beth Israel Deaconess Medical Center in Boston, said he’s noticed that COVID-19 makes some older patients confused, and their brains don’t regain their former clarity.
“We see a stepwise decline in their cognition during the COVID episode, and then they never get back up to their baseline,” said Dr. Metzger, medical director at Hebrew SeniorLife.
New research is beginning to back up such findings.
People who got COVID-19 were twice as likely to receive a diagnosis of Alzheimer’s disease in the 12 months after infection, compared to those who didn’t get COVID, according to a study published in the journal Nature Medicine , which analyzed the health care databases of the U.S. Department of Veterans Affairs.
Joshua Cahan, MD, a cognitive neurologist at Northwestern University, Chicago, advises caution about applying such a specific label simply from a patient’s medical chart. After all, he noted, few patients get tested to confirm that they have the proteins linked to Alzheimer’s.
“Probably the most appropriate conclusion from that is that there’s an increased risk of dementia after a COVID infection,” he said, “but we don’t know whether it’s truly Alzheimer’s disease or not.”
There could be a number of reasons why COVID-19 triggers a decline in thinking skills, says Michelle Monje, MD, a neuroscientist and neuro-oncologist at Stanford (Calif.) University.
In a paper published in the journal Neuron, Dr. Monje and her coauthor, Akiko Iwasaki, PhD, professor of immunobiology at Yale University, New Haven, Conn., propose possible triggers for brain fog caused by COVID: inflammation in the lungs and respiratory passages that leads to inflammation and dysregulation of the central nervous system; autoimmune reactions that damage the central nervous system; brain infection directly caused by the coronavirus (though, they note, this appears rare); a reactivation of an Epstein-Barr virus, which can lead to neuroinflammation; triggered by the coronavirus; and/or complications from severe cases of COVID-19, possibly involving periods of low blood oxygen and multi-organ failure.
Scientific understanding of brain fog is “part of an emerging picture that inflammation elsewhere in the body can be transmitted to become inflammation in the brain,” Dr. Monje said. “And once there’s inflammation in the brain … that can dysregulate other cell types that normally support healthy cognitive function.”
One issue with the concept of brain fog is that, like the term itself, the condition can be tough to define for doctors and patients alike and difficult, if not impossible, to capture on common cognition tests.
These days, patients often arrive at the Center of Excellence for Alzheimer’s Disease, in Syracuse, N.Y., complaining that they “don’t feel the same” as they did before contracting COVID-19, said Sharon Brangman, MD, the center’s director and the chair of the geriatrics department at Upstate Medical University.
But the evidence of diminished cognition just isn’t there.
“There’s nothing that we can find, objectively, that’s wrong with them,” she said. “They’re not severe enough to score low on mental status testing.”
But specialized, directed testing can find some probable signs, said Dr. Cahan, who evaluates patient cognition in a long COVID clinic at Northwestern University.
He often finds that his long COVID patients score in the low normal range on cognitive testing.
“Patients do have a complaint that something’s changed, and we don’t have prior testing,” he said. “So it’s possible that they were maybe in the high normal range or the superior range, but you just don’t know.”
He said he has seen very high-performing people, such as lawyers, executives, PhDs, and other professionals, who have tests that might be interpreted as normal, but given their level of achievement, “you would expect [higher scores].”
Like Ms. Sanders, many of those who do have muddled thinking after a COVID infection return to their former mental status. A study published in the journal Brain Communications found that people who had recovered from COVID-19, even if they had a mild illness, were significantly more likely to have memory and other cognition issues in the months after infection. But after 9 months, the former COVID patients had returned to their normal level of cognition, the team at Britain’s University of Oxford reported.
Notably, though, the average age of the people in the study was 28.6.
At the Northwestern clinic, Dr. Cahan treats patients who have struggled with COVID-induced cognition issues for months or even years. A rehabilitation program involves working with patients to come up with ways to compensate for cognitive deficits – such as making lists – as well as brain exercises, Dr. Cahan said. Over time, patients may achieve a 75% to 85% improvement, he said.
Dr. Monje hopes that one day, science will come up with ways to fully reverse the decline.
“I think what is likely the most common contributor to brain fog is this neuroinflammation, causing dysfunction of other cell types,” she said. “And, at least in the laboratory, we can rescue that in mouse models of chemotherapy brain fog, which gives me hope that we can rescue that for people.”
A version of this article first appeared on WebMD.com.
In early September, about a week after recovering from COVID-19, Barri Sanders went to the bank to pay a bill. But by mistake, she transferred a large amount of money from the wrong account.
“I’m talking about $20,000,” she said. “I had to go back [later] and fix it.”
Ms. Sanders, 83, had not had confusion like that before. Suddenly, the Albuquerque, N.M., resident found herself looking up from a book and not remembering what she had just read. She would stand up from her chair and forget what she meant to do.
“I kind of thought it was just the aging process,” she said. Combined with sudden balance issues, insomnia, and a nagging postnasal drip, the overall effect was “subtle, but scary,” she said.
After 5 days of this, she went to bed and slept the whole night through. She woke up in the morning to find her balanced restored, her sinuses clear, and the mental fog gone. What she’d had, she realized, wasn’t a rapid start of dementia, but rather a mercifully short form of long COVID.
Somewhere between 22% and 32% of people who recover from COVID-19 get “brain fog,” a nonscientific term used to describe slow or sluggish thinking. While this is disturbing at any age, And some scientists are starting to confirm what doctors, patients, and their families can already see: Older patients who have had COVID-19 have a higher risk of getting dementia or, if they already have mental confusion, the illness may worsen their condition.
British scientists who studied medical records from around the world reported in the journal The Lancet Psychiatry that people who recovered from COVID-19 had a higher risk of problems with their thinking and dementia even after 2 years had passed.
Another 2022 study, published in JAMA Neurology, looked at older COVID-19 patients for a year after they were discharged from hospitals in Wuhan, China. Compared with uninfected people, those who survived a severe case of COVID-19 were at higher risk for early onset, late-onset, and progressive decline in their thinking skills. Those who survived a mild infection were at a higher risk for early onset decline, the study found.
Eran Metzger, MD, assistant professor of psychiatry at Beth Israel Deaconess Medical Center in Boston, said he’s noticed that COVID-19 makes some older patients confused, and their brains don’t regain their former clarity.
“We see a stepwise decline in their cognition during the COVID episode, and then they never get back up to their baseline,” said Dr. Metzger, medical director at Hebrew SeniorLife.
New research is beginning to back up such findings.
People who got COVID-19 were twice as likely to receive a diagnosis of Alzheimer’s disease in the 12 months after infection, compared to those who didn’t get COVID, according to a study published in the journal Nature Medicine , which analyzed the health care databases of the U.S. Department of Veterans Affairs.
Joshua Cahan, MD, a cognitive neurologist at Northwestern University, Chicago, advises caution about applying such a specific label simply from a patient’s medical chart. After all, he noted, few patients get tested to confirm that they have the proteins linked to Alzheimer’s.
“Probably the most appropriate conclusion from that is that there’s an increased risk of dementia after a COVID infection,” he said, “but we don’t know whether it’s truly Alzheimer’s disease or not.”
There could be a number of reasons why COVID-19 triggers a decline in thinking skills, says Michelle Monje, MD, a neuroscientist and neuro-oncologist at Stanford (Calif.) University.
In a paper published in the journal Neuron, Dr. Monje and her coauthor, Akiko Iwasaki, PhD, professor of immunobiology at Yale University, New Haven, Conn., propose possible triggers for brain fog caused by COVID: inflammation in the lungs and respiratory passages that leads to inflammation and dysregulation of the central nervous system; autoimmune reactions that damage the central nervous system; brain infection directly caused by the coronavirus (though, they note, this appears rare); a reactivation of an Epstein-Barr virus, which can lead to neuroinflammation; triggered by the coronavirus; and/or complications from severe cases of COVID-19, possibly involving periods of low blood oxygen and multi-organ failure.
Scientific understanding of brain fog is “part of an emerging picture that inflammation elsewhere in the body can be transmitted to become inflammation in the brain,” Dr. Monje said. “And once there’s inflammation in the brain … that can dysregulate other cell types that normally support healthy cognitive function.”
One issue with the concept of brain fog is that, like the term itself, the condition can be tough to define for doctors and patients alike and difficult, if not impossible, to capture on common cognition tests.
These days, patients often arrive at the Center of Excellence for Alzheimer’s Disease, in Syracuse, N.Y., complaining that they “don’t feel the same” as they did before contracting COVID-19, said Sharon Brangman, MD, the center’s director and the chair of the geriatrics department at Upstate Medical University.
But the evidence of diminished cognition just isn’t there.
“There’s nothing that we can find, objectively, that’s wrong with them,” she said. “They’re not severe enough to score low on mental status testing.”
But specialized, directed testing can find some probable signs, said Dr. Cahan, who evaluates patient cognition in a long COVID clinic at Northwestern University.
He often finds that his long COVID patients score in the low normal range on cognitive testing.
“Patients do have a complaint that something’s changed, and we don’t have prior testing,” he said. “So it’s possible that they were maybe in the high normal range or the superior range, but you just don’t know.”
He said he has seen very high-performing people, such as lawyers, executives, PhDs, and other professionals, who have tests that might be interpreted as normal, but given their level of achievement, “you would expect [higher scores].”
Like Ms. Sanders, many of those who do have muddled thinking after a COVID infection return to their former mental status. A study published in the journal Brain Communications found that people who had recovered from COVID-19, even if they had a mild illness, were significantly more likely to have memory and other cognition issues in the months after infection. But after 9 months, the former COVID patients had returned to their normal level of cognition, the team at Britain’s University of Oxford reported.
Notably, though, the average age of the people in the study was 28.6.
At the Northwestern clinic, Dr. Cahan treats patients who have struggled with COVID-induced cognition issues for months or even years. A rehabilitation program involves working with patients to come up with ways to compensate for cognitive deficits – such as making lists – as well as brain exercises, Dr. Cahan said. Over time, patients may achieve a 75% to 85% improvement, he said.
Dr. Monje hopes that one day, science will come up with ways to fully reverse the decline.
“I think what is likely the most common contributor to brain fog is this neuroinflammation, causing dysfunction of other cell types,” she said. “And, at least in the laboratory, we can rescue that in mouse models of chemotherapy brain fog, which gives me hope that we can rescue that for people.”
A version of this article first appeared on WebMD.com.
In early September, about a week after recovering from COVID-19, Barri Sanders went to the bank to pay a bill. But by mistake, she transferred a large amount of money from the wrong account.
“I’m talking about $20,000,” she said. “I had to go back [later] and fix it.”
Ms. Sanders, 83, had not had confusion like that before. Suddenly, the Albuquerque, N.M., resident found herself looking up from a book and not remembering what she had just read. She would stand up from her chair and forget what she meant to do.
“I kind of thought it was just the aging process,” she said. Combined with sudden balance issues, insomnia, and a nagging postnasal drip, the overall effect was “subtle, but scary,” she said.
After 5 days of this, she went to bed and slept the whole night through. She woke up in the morning to find her balanced restored, her sinuses clear, and the mental fog gone. What she’d had, she realized, wasn’t a rapid start of dementia, but rather a mercifully short form of long COVID.
Somewhere between 22% and 32% of people who recover from COVID-19 get “brain fog,” a nonscientific term used to describe slow or sluggish thinking. While this is disturbing at any age, And some scientists are starting to confirm what doctors, patients, and their families can already see: Older patients who have had COVID-19 have a higher risk of getting dementia or, if they already have mental confusion, the illness may worsen their condition.
British scientists who studied medical records from around the world reported in the journal The Lancet Psychiatry that people who recovered from COVID-19 had a higher risk of problems with their thinking and dementia even after 2 years had passed.
Another 2022 study, published in JAMA Neurology, looked at older COVID-19 patients for a year after they were discharged from hospitals in Wuhan, China. Compared with uninfected people, those who survived a severe case of COVID-19 were at higher risk for early onset, late-onset, and progressive decline in their thinking skills. Those who survived a mild infection were at a higher risk for early onset decline, the study found.
Eran Metzger, MD, assistant professor of psychiatry at Beth Israel Deaconess Medical Center in Boston, said he’s noticed that COVID-19 makes some older patients confused, and their brains don’t regain their former clarity.
“We see a stepwise decline in their cognition during the COVID episode, and then they never get back up to their baseline,” said Dr. Metzger, medical director at Hebrew SeniorLife.
New research is beginning to back up such findings.
People who got COVID-19 were twice as likely to receive a diagnosis of Alzheimer’s disease in the 12 months after infection, compared to those who didn’t get COVID, according to a study published in the journal Nature Medicine , which analyzed the health care databases of the U.S. Department of Veterans Affairs.
Joshua Cahan, MD, a cognitive neurologist at Northwestern University, Chicago, advises caution about applying such a specific label simply from a patient’s medical chart. After all, he noted, few patients get tested to confirm that they have the proteins linked to Alzheimer’s.
“Probably the most appropriate conclusion from that is that there’s an increased risk of dementia after a COVID infection,” he said, “but we don’t know whether it’s truly Alzheimer’s disease or not.”
There could be a number of reasons why COVID-19 triggers a decline in thinking skills, says Michelle Monje, MD, a neuroscientist and neuro-oncologist at Stanford (Calif.) University.
In a paper published in the journal Neuron, Dr. Monje and her coauthor, Akiko Iwasaki, PhD, professor of immunobiology at Yale University, New Haven, Conn., propose possible triggers for brain fog caused by COVID: inflammation in the lungs and respiratory passages that leads to inflammation and dysregulation of the central nervous system; autoimmune reactions that damage the central nervous system; brain infection directly caused by the coronavirus (though, they note, this appears rare); a reactivation of an Epstein-Barr virus, which can lead to neuroinflammation; triggered by the coronavirus; and/or complications from severe cases of COVID-19, possibly involving periods of low blood oxygen and multi-organ failure.
Scientific understanding of brain fog is “part of an emerging picture that inflammation elsewhere in the body can be transmitted to become inflammation in the brain,” Dr. Monje said. “And once there’s inflammation in the brain … that can dysregulate other cell types that normally support healthy cognitive function.”
One issue with the concept of brain fog is that, like the term itself, the condition can be tough to define for doctors and patients alike and difficult, if not impossible, to capture on common cognition tests.
These days, patients often arrive at the Center of Excellence for Alzheimer’s Disease, in Syracuse, N.Y., complaining that they “don’t feel the same” as they did before contracting COVID-19, said Sharon Brangman, MD, the center’s director and the chair of the geriatrics department at Upstate Medical University.
But the evidence of diminished cognition just isn’t there.
“There’s nothing that we can find, objectively, that’s wrong with them,” she said. “They’re not severe enough to score low on mental status testing.”
But specialized, directed testing can find some probable signs, said Dr. Cahan, who evaluates patient cognition in a long COVID clinic at Northwestern University.
He often finds that his long COVID patients score in the low normal range on cognitive testing.
“Patients do have a complaint that something’s changed, and we don’t have prior testing,” he said. “So it’s possible that they were maybe in the high normal range or the superior range, but you just don’t know.”
He said he has seen very high-performing people, such as lawyers, executives, PhDs, and other professionals, who have tests that might be interpreted as normal, but given their level of achievement, “you would expect [higher scores].”
Like Ms. Sanders, many of those who do have muddled thinking after a COVID infection return to their former mental status. A study published in the journal Brain Communications found that people who had recovered from COVID-19, even if they had a mild illness, were significantly more likely to have memory and other cognition issues in the months after infection. But after 9 months, the former COVID patients had returned to their normal level of cognition, the team at Britain’s University of Oxford reported.
Notably, though, the average age of the people in the study was 28.6.
At the Northwestern clinic, Dr. Cahan treats patients who have struggled with COVID-induced cognition issues for months or even years. A rehabilitation program involves working with patients to come up with ways to compensate for cognitive deficits – such as making lists – as well as brain exercises, Dr. Cahan said. Over time, patients may achieve a 75% to 85% improvement, he said.
Dr. Monje hopes that one day, science will come up with ways to fully reverse the decline.
“I think what is likely the most common contributor to brain fog is this neuroinflammation, causing dysfunction of other cell types,” she said. “And, at least in the laboratory, we can rescue that in mouse models of chemotherapy brain fog, which gives me hope that we can rescue that for people.”
A version of this article first appeared on WebMD.com.
More work needed to optimize STI screening in primary care settings
TAMPA – Boosting screening for sexually transmitted infections in primary care settings could help alleviate some of the barriers to optimal testing and treatment, a new quality improvement initiative suggests.
Many primary care doctors are challenged for time and send people to other health care settings, such as a local health department or a clinic that specializes in STI diagnosis and treatment, said Wendy Kays, DNP, APRN, AGNP-BC, AAHIVS, a nurse practitioner and researcher at Care Resource, Miami.
However, for multiple reasons, many patients do not follow up and are not screened or treated, Dr. Kays said at the Association of Nurses in AIDS Care annual meeting. Some people can afford the copay to see a primary care provider, for example, but do not have the resources to pay for a second clinical visit or laboratory testing.
In other instances, transportation can be a problem. “People, especially in the neighborhood where we are located, depend a lot on buses to go to their primary care,” Dr. Kays told this news organization. But “follow-up is very important. It can promote early treatment and prevent the spread of disease.”
Primary care is critical as a gateway into health care that could help address low rates of STI screening, she said. There is also evidence that STIs are on the rise because of the COVID-19 pandemic.
If more primary care doctors tested and treated STIs using standardized Centers for Disease Control and Prevention guidelines, patients would not have to make a trip to another location, Dr. Kays said.
“The primary health setting … is actually the perfect place to get your screening,” said Jimmie Leckliter, MSN-Ed, RN, PHN, in an interview. He was not affiliated with the presentation. “I’m a former ER nurse, and a lot of people are using the ER as primary care, and it’s not really set up to do that screening.”
Mr. Leckliter suggested that primary care doctors incorporate some questions about sexual health during a regular head-to-toe checkup and ask questions in a very clinical, nonjudgmental way.
He also acknowledged that for some physicians it can be uncomfortable to raise the issues. “Unfortunately, I think in our society, talking to people about sex is taboo, and people become uncomfortable. We need to be able to learn to put our biases aside and treat our patients. That’s what our job is, added Mr. Leckliter, an adjunct faculty member at the College of the Desert’s School of Nursing and Allied Health Programs, Palm Springs, Calif.
Clinicians should be aware of the stigma associated with sending a person to an STD clinic for further workup, Mr. Leckliter advised. “You have to look at the stigma in the community in which you’re located. It makes a big difference,” he said. “Is it mainly a Latino or African American community?”
Compliance was a challenge
Dr. Kays and colleague performed a quality improvement project focused on implementing the CDC’s STI treatment guidelines at Care Resource. One goal was to educate a multidisciplinary team on the importance of screening in the primary care setting. The clientele at Care Resource consists primarily of underprivileged minorities, including the Latino, Black, gay, and transgender communities.
Six health care providers participated – two medical doctors and four advanced-practice providers. They evaluated patient charts from the electronic health record system 4 weeks before the intervention and 4 weeks after.
The education had a positive impact, the researchers reported, even though three providers were compliant with the CDC-recommended screening protocol and three others were not.
The quality improvement initiative had some limitations, Dr. Kays noted. “The hope is that the [quality improvement] process will continue moving forward, and early diagnosis and treatment of STIs will be standardized in this primary care practice.”
An evidence-based tool to screen for STIs in primary care is “crucial,” she added. Using a standardized, evidence-based protocol in primary care “can create positive change in patients’ outcomes.”
The study was independently supported. Dr. Kays and Mr. Leckliter report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
TAMPA – Boosting screening for sexually transmitted infections in primary care settings could help alleviate some of the barriers to optimal testing and treatment, a new quality improvement initiative suggests.
Many primary care doctors are challenged for time and send people to other health care settings, such as a local health department or a clinic that specializes in STI diagnosis and treatment, said Wendy Kays, DNP, APRN, AGNP-BC, AAHIVS, a nurse practitioner and researcher at Care Resource, Miami.
However, for multiple reasons, many patients do not follow up and are not screened or treated, Dr. Kays said at the Association of Nurses in AIDS Care annual meeting. Some people can afford the copay to see a primary care provider, for example, but do not have the resources to pay for a second clinical visit or laboratory testing.
In other instances, transportation can be a problem. “People, especially in the neighborhood where we are located, depend a lot on buses to go to their primary care,” Dr. Kays told this news organization. But “follow-up is very important. It can promote early treatment and prevent the spread of disease.”
Primary care is critical as a gateway into health care that could help address low rates of STI screening, she said. There is also evidence that STIs are on the rise because of the COVID-19 pandemic.
If more primary care doctors tested and treated STIs using standardized Centers for Disease Control and Prevention guidelines, patients would not have to make a trip to another location, Dr. Kays said.
“The primary health setting … is actually the perfect place to get your screening,” said Jimmie Leckliter, MSN-Ed, RN, PHN, in an interview. He was not affiliated with the presentation. “I’m a former ER nurse, and a lot of people are using the ER as primary care, and it’s not really set up to do that screening.”
Mr. Leckliter suggested that primary care doctors incorporate some questions about sexual health during a regular head-to-toe checkup and ask questions in a very clinical, nonjudgmental way.
He also acknowledged that for some physicians it can be uncomfortable to raise the issues. “Unfortunately, I think in our society, talking to people about sex is taboo, and people become uncomfortable. We need to be able to learn to put our biases aside and treat our patients. That’s what our job is, added Mr. Leckliter, an adjunct faculty member at the College of the Desert’s School of Nursing and Allied Health Programs, Palm Springs, Calif.
Clinicians should be aware of the stigma associated with sending a person to an STD clinic for further workup, Mr. Leckliter advised. “You have to look at the stigma in the community in which you’re located. It makes a big difference,” he said. “Is it mainly a Latino or African American community?”
Compliance was a challenge
Dr. Kays and colleague performed a quality improvement project focused on implementing the CDC’s STI treatment guidelines at Care Resource. One goal was to educate a multidisciplinary team on the importance of screening in the primary care setting. The clientele at Care Resource consists primarily of underprivileged minorities, including the Latino, Black, gay, and transgender communities.
Six health care providers participated – two medical doctors and four advanced-practice providers. They evaluated patient charts from the electronic health record system 4 weeks before the intervention and 4 weeks after.
The education had a positive impact, the researchers reported, even though three providers were compliant with the CDC-recommended screening protocol and three others were not.
The quality improvement initiative had some limitations, Dr. Kays noted. “The hope is that the [quality improvement] process will continue moving forward, and early diagnosis and treatment of STIs will be standardized in this primary care practice.”
An evidence-based tool to screen for STIs in primary care is “crucial,” she added. Using a standardized, evidence-based protocol in primary care “can create positive change in patients’ outcomes.”
The study was independently supported. Dr. Kays and Mr. Leckliter report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
TAMPA – Boosting screening for sexually transmitted infections in primary care settings could help alleviate some of the barriers to optimal testing and treatment, a new quality improvement initiative suggests.
Many primary care doctors are challenged for time and send people to other health care settings, such as a local health department or a clinic that specializes in STI diagnosis and treatment, said Wendy Kays, DNP, APRN, AGNP-BC, AAHIVS, a nurse practitioner and researcher at Care Resource, Miami.
However, for multiple reasons, many patients do not follow up and are not screened or treated, Dr. Kays said at the Association of Nurses in AIDS Care annual meeting. Some people can afford the copay to see a primary care provider, for example, but do not have the resources to pay for a second clinical visit or laboratory testing.
In other instances, transportation can be a problem. “People, especially in the neighborhood where we are located, depend a lot on buses to go to their primary care,” Dr. Kays told this news organization. But “follow-up is very important. It can promote early treatment and prevent the spread of disease.”
Primary care is critical as a gateway into health care that could help address low rates of STI screening, she said. There is also evidence that STIs are on the rise because of the COVID-19 pandemic.
If more primary care doctors tested and treated STIs using standardized Centers for Disease Control and Prevention guidelines, patients would not have to make a trip to another location, Dr. Kays said.
“The primary health setting … is actually the perfect place to get your screening,” said Jimmie Leckliter, MSN-Ed, RN, PHN, in an interview. He was not affiliated with the presentation. “I’m a former ER nurse, and a lot of people are using the ER as primary care, and it’s not really set up to do that screening.”
Mr. Leckliter suggested that primary care doctors incorporate some questions about sexual health during a regular head-to-toe checkup and ask questions in a very clinical, nonjudgmental way.
He also acknowledged that for some physicians it can be uncomfortable to raise the issues. “Unfortunately, I think in our society, talking to people about sex is taboo, and people become uncomfortable. We need to be able to learn to put our biases aside and treat our patients. That’s what our job is, added Mr. Leckliter, an adjunct faculty member at the College of the Desert’s School of Nursing and Allied Health Programs, Palm Springs, Calif.
Clinicians should be aware of the stigma associated with sending a person to an STD clinic for further workup, Mr. Leckliter advised. “You have to look at the stigma in the community in which you’re located. It makes a big difference,” he said. “Is it mainly a Latino or African American community?”
Compliance was a challenge
Dr. Kays and colleague performed a quality improvement project focused on implementing the CDC’s STI treatment guidelines at Care Resource. One goal was to educate a multidisciplinary team on the importance of screening in the primary care setting. The clientele at Care Resource consists primarily of underprivileged minorities, including the Latino, Black, gay, and transgender communities.
Six health care providers participated – two medical doctors and four advanced-practice providers. They evaluated patient charts from the electronic health record system 4 weeks before the intervention and 4 weeks after.
The education had a positive impact, the researchers reported, even though three providers were compliant with the CDC-recommended screening protocol and three others were not.
The quality improvement initiative had some limitations, Dr. Kays noted. “The hope is that the [quality improvement] process will continue moving forward, and early diagnosis and treatment of STIs will be standardized in this primary care practice.”
An evidence-based tool to screen for STIs in primary care is “crucial,” she added. Using a standardized, evidence-based protocol in primary care “can create positive change in patients’ outcomes.”
The study was independently supported. Dr. Kays and Mr. Leckliter report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Dark spot near ear

Stable slate gray to blue lesions that are asymptomatic raise the possibility of a blue nevus, also known as dermal dendritic melanocytic proliferations. In this case, dermoscopy confirmed a uniform dark color with no signs suggestive of melanoma or pigmented basal cell carcinoma (BCC).
Blue nevi are the result of a benign localized proliferation of dermal dendritic melanocytes. The blue color is due to the increased pigment deep in the dermis that reflects the blue shorter wavelength light while absorbing longer wavelengths.1 In this author’s experience, these “blue” lesions usually appear to be more gray (as was the case with this individual). Dermoscopy shows a steel blue homogenous pigmentation.2
It is helpful to use dermoscopy to screen for an atypical pigment network, regression of pigmentation, or abnormal pigmentation; these are signs of atypical nevi and melanoma. It is also important to look for arborizing blood vessels and leaf-like structures that can be seen in pigmented BCCs. Both melanoma and pigmented BCCs can appear as circumscribed dark lesions.
Reassuring factors for blue nevi are lesions that are stable in size and color over time, asymptomatic, and have not bled nor shown signs of erosion. If the diagnosis is in doubt, excise the lesion in its entirety for definitive pathology. Since the melanocytes are typically deeper in blue nevi than in most other nevi, a deep shave technique may not remove the lesion in its entirety. A deeper than usual shave (or, if feasible, a full-thickness excision) may return better results with quicker healing.
This patient was advised of the benign nature of a blue nevus. He was counseled to watch for any changes in the lesion and to return for reevaluation if symptoms or changes occurred.
Image and text courtesy of Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.
1. Murali R, McCarthy SW, Scolyer RA. Blue nevi and related lesions: a review highlighting atypical and newly described variants, distinguishing features and diagnostic pitfalls. Adv Anat Pathol. 2009;16:365-382. doi: 10.1097/PAP.0b013e3181bb6b53
2. Longo C, Scope A, Lallas A, et al. Blue lesions. Dermatol Clin. 2013;31:637-647, ix. doi: 10.1016/j.det.2013.07.001

Stable slate gray to blue lesions that are asymptomatic raise the possibility of a blue nevus, also known as dermal dendritic melanocytic proliferations. In this case, dermoscopy confirmed a uniform dark color with no signs suggestive of melanoma or pigmented basal cell carcinoma (BCC).
Blue nevi are the result of a benign localized proliferation of dermal dendritic melanocytes. The blue color is due to the increased pigment deep in the dermis that reflects the blue shorter wavelength light while absorbing longer wavelengths.1 In this author’s experience, these “blue” lesions usually appear to be more gray (as was the case with this individual). Dermoscopy shows a steel blue homogenous pigmentation.2
It is helpful to use dermoscopy to screen for an atypical pigment network, regression of pigmentation, or abnormal pigmentation; these are signs of atypical nevi and melanoma. It is also important to look for arborizing blood vessels and leaf-like structures that can be seen in pigmented BCCs. Both melanoma and pigmented BCCs can appear as circumscribed dark lesions.
Reassuring factors for blue nevi are lesions that are stable in size and color over time, asymptomatic, and have not bled nor shown signs of erosion. If the diagnosis is in doubt, excise the lesion in its entirety for definitive pathology. Since the melanocytes are typically deeper in blue nevi than in most other nevi, a deep shave technique may not remove the lesion in its entirety. A deeper than usual shave (or, if feasible, a full-thickness excision) may return better results with quicker healing.
This patient was advised of the benign nature of a blue nevus. He was counseled to watch for any changes in the lesion and to return for reevaluation if symptoms or changes occurred.
Image and text courtesy of Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.

Stable slate gray to blue lesions that are asymptomatic raise the possibility of a blue nevus, also known as dermal dendritic melanocytic proliferations. In this case, dermoscopy confirmed a uniform dark color with no signs suggestive of melanoma or pigmented basal cell carcinoma (BCC).
Blue nevi are the result of a benign localized proliferation of dermal dendritic melanocytes. The blue color is due to the increased pigment deep in the dermis that reflects the blue shorter wavelength light while absorbing longer wavelengths.1 In this author’s experience, these “blue” lesions usually appear to be more gray (as was the case with this individual). Dermoscopy shows a steel blue homogenous pigmentation.2
It is helpful to use dermoscopy to screen for an atypical pigment network, regression of pigmentation, or abnormal pigmentation; these are signs of atypical nevi and melanoma. It is also important to look for arborizing blood vessels and leaf-like structures that can be seen in pigmented BCCs. Both melanoma and pigmented BCCs can appear as circumscribed dark lesions.
Reassuring factors for blue nevi are lesions that are stable in size and color over time, asymptomatic, and have not bled nor shown signs of erosion. If the diagnosis is in doubt, excise the lesion in its entirety for definitive pathology. Since the melanocytes are typically deeper in blue nevi than in most other nevi, a deep shave technique may not remove the lesion in its entirety. A deeper than usual shave (or, if feasible, a full-thickness excision) may return better results with quicker healing.
This patient was advised of the benign nature of a blue nevus. He was counseled to watch for any changes in the lesion and to return for reevaluation if symptoms or changes occurred.
Image and text courtesy of Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.
1. Murali R, McCarthy SW, Scolyer RA. Blue nevi and related lesions: a review highlighting atypical and newly described variants, distinguishing features and diagnostic pitfalls. Adv Anat Pathol. 2009;16:365-382. doi: 10.1097/PAP.0b013e3181bb6b53
2. Longo C, Scope A, Lallas A, et al. Blue lesions. Dermatol Clin. 2013;31:637-647, ix. doi: 10.1016/j.det.2013.07.001
1. Murali R, McCarthy SW, Scolyer RA. Blue nevi and related lesions: a review highlighting atypical and newly described variants, distinguishing features and diagnostic pitfalls. Adv Anat Pathol. 2009;16:365-382. doi: 10.1097/PAP.0b013e3181bb6b53
2. Longo C, Scope A, Lallas A, et al. Blue lesions. Dermatol Clin. 2013;31:637-647, ix. doi: 10.1016/j.det.2013.07.001
Commentary: Combination therapies and immunotherapy in HCC, December 2022
Hatanaka and colleagues investigated whether the etiology of the underlying liver disease affected the efficacy of atezolizumab and bevacizumab (A/B). They reported the results of a retrospective cohort study of 323 patients with Barcelona Clinic Liver Cancer (BCLC) stage B or C hepatocellular carcinoma and Child-Pugh class A cirrhosis who started A/B between September 2020 and December 2021. Patients with viral infection were defined as those who were either serum anti–hepatitis C antibody (anti-HCV Ab)- or hepatitis B surface antigen (HBs-Ag)-positive, while patients with nonviral infection was defined as those who were both serum anti-HCV Ab- and HBs-Ag-negative. After propensity matching, no significant difference in response rate ([RR] 20.6% vs 24.6% in viral and nonviral patients), disease control rate (68.3% vs 69.0%), progression-free survival ([PFS] 7.0 months vs 6.2 months), or 12-month overall survival ([OS] 65.5% vs 71.7%) was seen. The authors concluded that the underlying etiology of liver disease in patients with HCC does not affect the response to treatment with A/B.
Scheiner and colleagues evaluated the efficacy of immunotherapy in patients with HCC who had already received immune checkpoint inhibitors (ICI) in a previous line of therapy. The authors reported the results of an international, retrospective multicenter study of 58 patients with HCC who received at least two lines of ICI-based therapies. The first ICI was discontinued due to disease progression in 90%. Nonetheless, the RR to the second ICI was 26% (compared with 22% for the first ICI), with a time-to-progression (TTP) of 5.4 months (95% CI, 3.0-7.7) for the first ICI and 5.2 months (95% CI, 3.3-7.0) for the second ICI. Grade 3/4 treatment-related adverse events were observed in 16% and 17% of patients with the first and second ICI, respectively. Therefore, the authors believe that ICI rechallenge is safe and results in a treatment benefit for a similar proportion of HCC patients, as is seen with the first ICI treatment. They suggest that ICI-based regimens should be studied in prospective trials of patients who progressed on first-line immunotherapy.
Finally, Kim and colleagues reported outcomes of patients who developed anti-drug antibodies (ADA) against atezolizumab while on A/B. In this prospective cohort study, 174 patients with advanced HCC who were treated with first-line A/B were tested for serum ADA levels prior to treatment and at 3 weeks (cycle 2 day 1 [C2D1]). Clinically, patients with progressive disease exhibited higher ADA levels (median 65.2 [0-520.4] ng/mL) at C2D1 than responders (0-117.5 ng/mL). Patients with high ADA levels at C2D1 had a reduced response rate (29%-34% vs 7-11%) and worse PFS and OS. The investigators found that very high ADA levels (≥ 1000 ng/mL) at 3 weeks were consistently associated with poor clinical outcomes due to reduced systemic exposure to atezolizumab and impaired proliferation and activation of peripheral CD8-positive T cells. They suggested future validation and standardization of ADA assays to optimize treatment with atezolizumab in patients with uHCC.
Hatanaka and colleagues investigated whether the etiology of the underlying liver disease affected the efficacy of atezolizumab and bevacizumab (A/B). They reported the results of a retrospective cohort study of 323 patients with Barcelona Clinic Liver Cancer (BCLC) stage B or C hepatocellular carcinoma and Child-Pugh class A cirrhosis who started A/B between September 2020 and December 2021. Patients with viral infection were defined as those who were either serum anti–hepatitis C antibody (anti-HCV Ab)- or hepatitis B surface antigen (HBs-Ag)-positive, while patients with nonviral infection was defined as those who were both serum anti-HCV Ab- and HBs-Ag-negative. After propensity matching, no significant difference in response rate ([RR] 20.6% vs 24.6% in viral and nonviral patients), disease control rate (68.3% vs 69.0%), progression-free survival ([PFS] 7.0 months vs 6.2 months), or 12-month overall survival ([OS] 65.5% vs 71.7%) was seen. The authors concluded that the underlying etiology of liver disease in patients with HCC does not affect the response to treatment with A/B.
Scheiner and colleagues evaluated the efficacy of immunotherapy in patients with HCC who had already received immune checkpoint inhibitors (ICI) in a previous line of therapy. The authors reported the results of an international, retrospective multicenter study of 58 patients with HCC who received at least two lines of ICI-based therapies. The first ICI was discontinued due to disease progression in 90%. Nonetheless, the RR to the second ICI was 26% (compared with 22% for the first ICI), with a time-to-progression (TTP) of 5.4 months (95% CI, 3.0-7.7) for the first ICI and 5.2 months (95% CI, 3.3-7.0) for the second ICI. Grade 3/4 treatment-related adverse events were observed in 16% and 17% of patients with the first and second ICI, respectively. Therefore, the authors believe that ICI rechallenge is safe and results in a treatment benefit for a similar proportion of HCC patients, as is seen with the first ICI treatment. They suggest that ICI-based regimens should be studied in prospective trials of patients who progressed on first-line immunotherapy.
Finally, Kim and colleagues reported outcomes of patients who developed anti-drug antibodies (ADA) against atezolizumab while on A/B. In this prospective cohort study, 174 patients with advanced HCC who were treated with first-line A/B were tested for serum ADA levels prior to treatment and at 3 weeks (cycle 2 day 1 [C2D1]). Clinically, patients with progressive disease exhibited higher ADA levels (median 65.2 [0-520.4] ng/mL) at C2D1 than responders (0-117.5 ng/mL). Patients with high ADA levels at C2D1 had a reduced response rate (29%-34% vs 7-11%) and worse PFS and OS. The investigators found that very high ADA levels (≥ 1000 ng/mL) at 3 weeks were consistently associated with poor clinical outcomes due to reduced systemic exposure to atezolizumab and impaired proliferation and activation of peripheral CD8-positive T cells. They suggested future validation and standardization of ADA assays to optimize treatment with atezolizumab in patients with uHCC.
Hatanaka and colleagues investigated whether the etiology of the underlying liver disease affected the efficacy of atezolizumab and bevacizumab (A/B). They reported the results of a retrospective cohort study of 323 patients with Barcelona Clinic Liver Cancer (BCLC) stage B or C hepatocellular carcinoma and Child-Pugh class A cirrhosis who started A/B between September 2020 and December 2021. Patients with viral infection were defined as those who were either serum anti–hepatitis C antibody (anti-HCV Ab)- or hepatitis B surface antigen (HBs-Ag)-positive, while patients with nonviral infection was defined as those who were both serum anti-HCV Ab- and HBs-Ag-negative. After propensity matching, no significant difference in response rate ([RR] 20.6% vs 24.6% in viral and nonviral patients), disease control rate (68.3% vs 69.0%), progression-free survival ([PFS] 7.0 months vs 6.2 months), or 12-month overall survival ([OS] 65.5% vs 71.7%) was seen. The authors concluded that the underlying etiology of liver disease in patients with HCC does not affect the response to treatment with A/B.
Scheiner and colleagues evaluated the efficacy of immunotherapy in patients with HCC who had already received immune checkpoint inhibitors (ICI) in a previous line of therapy. The authors reported the results of an international, retrospective multicenter study of 58 patients with HCC who received at least two lines of ICI-based therapies. The first ICI was discontinued due to disease progression in 90%. Nonetheless, the RR to the second ICI was 26% (compared with 22% for the first ICI), with a time-to-progression (TTP) of 5.4 months (95% CI, 3.0-7.7) for the first ICI and 5.2 months (95% CI, 3.3-7.0) for the second ICI. Grade 3/4 treatment-related adverse events were observed in 16% and 17% of patients with the first and second ICI, respectively. Therefore, the authors believe that ICI rechallenge is safe and results in a treatment benefit for a similar proportion of HCC patients, as is seen with the first ICI treatment. They suggest that ICI-based regimens should be studied in prospective trials of patients who progressed on first-line immunotherapy.
Finally, Kim and colleagues reported outcomes of patients who developed anti-drug antibodies (ADA) against atezolizumab while on A/B. In this prospective cohort study, 174 patients with advanced HCC who were treated with first-line A/B were tested for serum ADA levels prior to treatment and at 3 weeks (cycle 2 day 1 [C2D1]). Clinically, patients with progressive disease exhibited higher ADA levels (median 65.2 [0-520.4] ng/mL) at C2D1 than responders (0-117.5 ng/mL). Patients with high ADA levels at C2D1 had a reduced response rate (29%-34% vs 7-11%) and worse PFS and OS. The investigators found that very high ADA levels (≥ 1000 ng/mL) at 3 weeks were consistently associated with poor clinical outcomes due to reduced systemic exposure to atezolizumab and impaired proliferation and activation of peripheral CD8-positive T cells. They suggested future validation and standardization of ADA assays to optimize treatment with atezolizumab in patients with uHCC.
Commentary: Combination therapies and immunotherapy in HCC, December 2022
Hatanaka and colleagues investigated whether the etiology of the underlying liver disease affected the efficacy of atezolizumab and bevacizumab (A/B). They reported the results of a retrospective cohort study of 323 patients with Barcelona Clinic Liver Cancer (BCLC) stage B or C hepatocellular carcinoma and Child-Pugh class A cirrhosis who started A/B between September 2020 and December 2021. Patients with viral infection were defined as those who were either serum anti–hepatitis C antibody (anti-HCV Ab)- or hepatitis B surface antigen (HBs-Ag)-positive, while patients with nonviral infection was defined as those who were both serum anti-HCV Ab- and HBs-Ag-negative. After propensity matching, no significant difference in response rate ([RR] 20.6% vs 24.6% in viral and nonviral patients), disease control rate (68.3% vs 69.0%), progression-free survival ([PFS] 7.0 months vs 6.2 months), or 12-month overall survival ([OS] 65.5% vs 71.7%) was seen. The authors concluded that the underlying etiology of liver disease in patients with HCC does not affect the response to treatment with A/B.
Scheiner and colleagues evaluated the efficacy of immunotherapy in patients with HCC who had already received immune checkpoint inhibitors (ICI) in a previous line of therapy. The authors reported the results of an international, retrospective multicenter study of 58 patients with HCC who received at least two lines of ICI-based therapies. The first ICI was discontinued due to disease progression in 90%. Nonetheless, the RR to the second ICI was 26% (compared with 22% for the first ICI), with a time-to-progression (TTP) of 5.4 months (95% CI, 3.0-7.7) for the first ICI and 5.2 months (95% CI, 3.3-7.0) for the second ICI. Grade 3/4 treatment-related adverse events were observed in 16% and 17% of patients with the first and second ICI, respectively. Therefore, the authors believe that ICI rechallenge is safe and results in a treatment benefit for a similar proportion of HCC patients, as is seen with the first ICI treatment. They suggest that ICI-based regimens should be studied in prospective trials of patients who progressed on first-line immunotherapy.
Finally, Kim and colleagues reported outcomes of patients who developed anti-drug antibodies (ADA) against atezolizumab while on A/B. In this prospective cohort study, 174 patients with advanced HCC who were treated with first-line A/B were tested for serum ADA levels prior to treatment and at 3 weeks (cycle 2 day 1 [C2D1]). Clinically, patients with progressive disease exhibited higher ADA levels (median 65.2 [0-520.4] ng/mL) at C2D1 than responders (0-117.5 ng/mL). Patients with high ADA levels at C2D1 had a reduced response rate (29%-34% vs 7-11%) and worse PFS and OS. The investigators found that very high ADA levels (≥ 1000 ng/mL) at 3 weeks were consistently associated with poor clinical outcomes due to reduced systemic exposure to atezolizumab and impaired proliferation and activation of peripheral CD8-positive T cells. They suggested future validation and standardization of ADA assays to optimize treatment with atezolizumab in patients with uHCC.
Hatanaka and colleagues investigated whether the etiology of the underlying liver disease affected the efficacy of atezolizumab and bevacizumab (A/B). They reported the results of a retrospective cohort study of 323 patients with Barcelona Clinic Liver Cancer (BCLC) stage B or C hepatocellular carcinoma and Child-Pugh class A cirrhosis who started A/B between September 2020 and December 2021. Patients with viral infection were defined as those who were either serum anti–hepatitis C antibody (anti-HCV Ab)- or hepatitis B surface antigen (HBs-Ag)-positive, while patients with nonviral infection was defined as those who were both serum anti-HCV Ab- and HBs-Ag-negative. After propensity matching, no significant difference in response rate ([RR] 20.6% vs 24.6% in viral and nonviral patients), disease control rate (68.3% vs 69.0%), progression-free survival ([PFS] 7.0 months vs 6.2 months), or 12-month overall survival ([OS] 65.5% vs 71.7%) was seen. The authors concluded that the underlying etiology of liver disease in patients with HCC does not affect the response to treatment with A/B.
Scheiner and colleagues evaluated the efficacy of immunotherapy in patients with HCC who had already received immune checkpoint inhibitors (ICI) in a previous line of therapy. The authors reported the results of an international, retrospective multicenter study of 58 patients with HCC who received at least two lines of ICI-based therapies. The first ICI was discontinued due to disease progression in 90%. Nonetheless, the RR to the second ICI was 26% (compared with 22% for the first ICI), with a time-to-progression (TTP) of 5.4 months (95% CI, 3.0-7.7) for the first ICI and 5.2 months (95% CI, 3.3-7.0) for the second ICI. Grade 3/4 treatment-related adverse events were observed in 16% and 17% of patients with the first and second ICI, respectively. Therefore, the authors believe that ICI rechallenge is safe and results in a treatment benefit for a similar proportion of HCC patients, as is seen with the first ICI treatment. They suggest that ICI-based regimens should be studied in prospective trials of patients who progressed on first-line immunotherapy.
Finally, Kim and colleagues reported outcomes of patients who developed anti-drug antibodies (ADA) against atezolizumab while on A/B. In this prospective cohort study, 174 patients with advanced HCC who were treated with first-line A/B were tested for serum ADA levels prior to treatment and at 3 weeks (cycle 2 day 1 [C2D1]). Clinically, patients with progressive disease exhibited higher ADA levels (median 65.2 [0-520.4] ng/mL) at C2D1 than responders (0-117.5 ng/mL). Patients with high ADA levels at C2D1 had a reduced response rate (29%-34% vs 7-11%) and worse PFS and OS. The investigators found that very high ADA levels (≥ 1000 ng/mL) at 3 weeks were consistently associated with poor clinical outcomes due to reduced systemic exposure to atezolizumab and impaired proliferation and activation of peripheral CD8-positive T cells. They suggested future validation and standardization of ADA assays to optimize treatment with atezolizumab in patients with uHCC.
Hatanaka and colleagues investigated whether the etiology of the underlying liver disease affected the efficacy of atezolizumab and bevacizumab (A/B). They reported the results of a retrospective cohort study of 323 patients with Barcelona Clinic Liver Cancer (BCLC) stage B or C hepatocellular carcinoma and Child-Pugh class A cirrhosis who started A/B between September 2020 and December 2021. Patients with viral infection were defined as those who were either serum anti–hepatitis C antibody (anti-HCV Ab)- or hepatitis B surface antigen (HBs-Ag)-positive, while patients with nonviral infection was defined as those who were both serum anti-HCV Ab- and HBs-Ag-negative. After propensity matching, no significant difference in response rate ([RR] 20.6% vs 24.6% in viral and nonviral patients), disease control rate (68.3% vs 69.0%), progression-free survival ([PFS] 7.0 months vs 6.2 months), or 12-month overall survival ([OS] 65.5% vs 71.7%) was seen. The authors concluded that the underlying etiology of liver disease in patients with HCC does not affect the response to treatment with A/B.
Scheiner and colleagues evaluated the efficacy of immunotherapy in patients with HCC who had already received immune checkpoint inhibitors (ICI) in a previous line of therapy. The authors reported the results of an international, retrospective multicenter study of 58 patients with HCC who received at least two lines of ICI-based therapies. The first ICI was discontinued due to disease progression in 90%. Nonetheless, the RR to the second ICI was 26% (compared with 22% for the first ICI), with a time-to-progression (TTP) of 5.4 months (95% CI, 3.0-7.7) for the first ICI and 5.2 months (95% CI, 3.3-7.0) for the second ICI. Grade 3/4 treatment-related adverse events were observed in 16% and 17% of patients with the first and second ICI, respectively. Therefore, the authors believe that ICI rechallenge is safe and results in a treatment benefit for a similar proportion of HCC patients, as is seen with the first ICI treatment. They suggest that ICI-based regimens should be studied in prospective trials of patients who progressed on first-line immunotherapy.
Finally, Kim and colleagues reported outcomes of patients who developed anti-drug antibodies (ADA) against atezolizumab while on A/B. In this prospective cohort study, 174 patients with advanced HCC who were treated with first-line A/B were tested for serum ADA levels prior to treatment and at 3 weeks (cycle 2 day 1 [C2D1]). Clinically, patients with progressive disease exhibited higher ADA levels (median 65.2 [0-520.4] ng/mL) at C2D1 than responders (0-117.5 ng/mL). Patients with high ADA levels at C2D1 had a reduced response rate (29%-34% vs 7-11%) and worse PFS and OS. The investigators found that very high ADA levels (≥ 1000 ng/mL) at 3 weeks were consistently associated with poor clinical outcomes due to reduced systemic exposure to atezolizumab and impaired proliferation and activation of peripheral CD8-positive T cells. They suggested future validation and standardization of ADA assays to optimize treatment with atezolizumab in patients with uHCC.
Commentary: New treatments and management in breast cancer, December 2022
Taxanes are an integral component of various treatment regimens for all stages of breast cancer. As survival outcomes have improved, it has become increasingly important to focus on the long-term quality-of-life impact of treatment. Neurotoxicity is a well-recognized potential side effect of taxane chemotherapy. In a prospective cohort study including 1234 patients diagnosed with breast cancer and receiving taxanes, the risk for patient-reported chemotherapy-induced peripheral neuropathy (CIPN) were lower in the paclitaxel (HR 0.59; P = .008) and docetaxel (HR 0.65; P = .02) groups vs the nab-paclitaxel group. There was less sensory discomfort reported with paclitaxel (HR 0.44; P < .001) and docetaxel (HR 0.52; P < .001) vs nab-paclitaxel; however, reported motor and autonomic symptoms were not significantly lower than in the nab-paclitaxel group (Mo et al). An area of research interest is the identification of biomarkers that may predict a higher likelihood of CIPN development, to aid in early detection and intervention.3
Management strategies for breast cancer diagnosed in older women should take into consideration age and competing medical comorbidities, and hormone receptor–positive histology is the most common subtype in this population. Some older women may be too frail or unfit for surgery, and furthermore, some may prefer to avoid surgery, even if it is considered a safe approach. A retrospective study including 91 older (≥ 70 years) patients with estrogen receptor–positive (ER+) breast cancer who underwent definitive endocrine therapy demonstrated a twofold higher mortality risk than the risk of needing invasive local treatment (surgery or radiation). The 5-year cumulative risks of undergoing invasive local treatment and having uncontrolled disease were 28% and 16%, respectively, whereas the 5-year cumulative overall survival was 42% (Gooijer et al). Although the majority of older women with ER+ early breast cancer will obtain a survival benefit with surgery plus endocrine therapy compared with primary endocrine therapy, there is a selected group with limited life expectancy owing to age, functional status, or medical comorbidities for whom it is appropriate to offer primary endocrine therapy, because breast cancer–specific survival may not be negatively affected.4
Additional References
- Schmid P, Cortes J, Dent R, et al; for the KEYNOTE-522 Investigators. Event-free survival with pembrolizumab in early triple-negative breast cancer. N Engl J Med. 2022;386:556-567. Doi: 10.1056/NEJMoa2112651
- Harbeck N, Rastogi P, Martin M, et al; on behalf of the monarchE Committee Members. Adjuvant abemaciclib combined with endocrine therapy for high-risk early breast cancer: updated efficacy and Ki-67 analysis from the monarchE study. Ann Oncol. 2021;32:1571-1581. Doi: 10.1016/j.annonc.2021.09.015
- Rodwin RL, Siddiq NZ, Ehrlich BE, Lustberg MB. Biomarkers of chemotherapy-induced peripheral neuropathy: current status and future directions. Front Pain Res (Lausanne). 2022;3:864910. Doi: 10.3389/fpain.2022.864910
- Wyld L, Reed MW, Morgan J, et al. Bridging the age gap in breast cancer. Impacts of omission of breast cancer surgery in older women with oestrogen receptor positive early breast cancer. A risk stratified analysis of survival outcomes and quality of life. Eur J Cancer. 2021;142:48-62. Doi: 10.1016/j.ejca.2020.10.015
Taxanes are an integral component of various treatment regimens for all stages of breast cancer. As survival outcomes have improved, it has become increasingly important to focus on the long-term quality-of-life impact of treatment. Neurotoxicity is a well-recognized potential side effect of taxane chemotherapy. In a prospective cohort study including 1234 patients diagnosed with breast cancer and receiving taxanes, the risk for patient-reported chemotherapy-induced peripheral neuropathy (CIPN) were lower in the paclitaxel (HR 0.59; P = .008) and docetaxel (HR 0.65; P = .02) groups vs the nab-paclitaxel group. There was less sensory discomfort reported with paclitaxel (HR 0.44; P < .001) and docetaxel (HR 0.52; P < .001) vs nab-paclitaxel; however, reported motor and autonomic symptoms were not significantly lower than in the nab-paclitaxel group (Mo et al). An area of research interest is the identification of biomarkers that may predict a higher likelihood of CIPN development, to aid in early detection and intervention.3
Management strategies for breast cancer diagnosed in older women should take into consideration age and competing medical comorbidities, and hormone receptor–positive histology is the most common subtype in this population. Some older women may be too frail or unfit for surgery, and furthermore, some may prefer to avoid surgery, even if it is considered a safe approach. A retrospective study including 91 older (≥ 70 years) patients with estrogen receptor–positive (ER+) breast cancer who underwent definitive endocrine therapy demonstrated a twofold higher mortality risk than the risk of needing invasive local treatment (surgery or radiation). The 5-year cumulative risks of undergoing invasive local treatment and having uncontrolled disease were 28% and 16%, respectively, whereas the 5-year cumulative overall survival was 42% (Gooijer et al). Although the majority of older women with ER+ early breast cancer will obtain a survival benefit with surgery plus endocrine therapy compared with primary endocrine therapy, there is a selected group with limited life expectancy owing to age, functional status, or medical comorbidities for whom it is appropriate to offer primary endocrine therapy, because breast cancer–specific survival may not be negatively affected.4
Additional References
- Schmid P, Cortes J, Dent R, et al; for the KEYNOTE-522 Investigators. Event-free survival with pembrolizumab in early triple-negative breast cancer. N Engl J Med. 2022;386:556-567. Doi: 10.1056/NEJMoa2112651
- Harbeck N, Rastogi P, Martin M, et al; on behalf of the monarchE Committee Members. Adjuvant abemaciclib combined with endocrine therapy for high-risk early breast cancer: updated efficacy and Ki-67 analysis from the monarchE study. Ann Oncol. 2021;32:1571-1581. Doi: 10.1016/j.annonc.2021.09.015
- Rodwin RL, Siddiq NZ, Ehrlich BE, Lustberg MB. Biomarkers of chemotherapy-induced peripheral neuropathy: current status and future directions. Front Pain Res (Lausanne). 2022;3:864910. Doi: 10.3389/fpain.2022.864910
- Wyld L, Reed MW, Morgan J, et al. Bridging the age gap in breast cancer. Impacts of omission of breast cancer surgery in older women with oestrogen receptor positive early breast cancer. A risk stratified analysis of survival outcomes and quality of life. Eur J Cancer. 2021;142:48-62. Doi: 10.1016/j.ejca.2020.10.015
Taxanes are an integral component of various treatment regimens for all stages of breast cancer. As survival outcomes have improved, it has become increasingly important to focus on the long-term quality-of-life impact of treatment. Neurotoxicity is a well-recognized potential side effect of taxane chemotherapy. In a prospective cohort study including 1234 patients diagnosed with breast cancer and receiving taxanes, the risk for patient-reported chemotherapy-induced peripheral neuropathy (CIPN) were lower in the paclitaxel (HR 0.59; P = .008) and docetaxel (HR 0.65; P = .02) groups vs the nab-paclitaxel group. There was less sensory discomfort reported with paclitaxel (HR 0.44; P < .001) and docetaxel (HR 0.52; P < .001) vs nab-paclitaxel; however, reported motor and autonomic symptoms were not significantly lower than in the nab-paclitaxel group (Mo et al). An area of research interest is the identification of biomarkers that may predict a higher likelihood of CIPN development, to aid in early detection and intervention.3
Management strategies for breast cancer diagnosed in older women should take into consideration age and competing medical comorbidities, and hormone receptor–positive histology is the most common subtype in this population. Some older women may be too frail or unfit for surgery, and furthermore, some may prefer to avoid surgery, even if it is considered a safe approach. A retrospective study including 91 older (≥ 70 years) patients with estrogen receptor–positive (ER+) breast cancer who underwent definitive endocrine therapy demonstrated a twofold higher mortality risk than the risk of needing invasive local treatment (surgery or radiation). The 5-year cumulative risks of undergoing invasive local treatment and having uncontrolled disease were 28% and 16%, respectively, whereas the 5-year cumulative overall survival was 42% (Gooijer et al). Although the majority of older women with ER+ early breast cancer will obtain a survival benefit with surgery plus endocrine therapy compared with primary endocrine therapy, there is a selected group with limited life expectancy owing to age, functional status, or medical comorbidities for whom it is appropriate to offer primary endocrine therapy, because breast cancer–specific survival may not be negatively affected.4
Additional References
- Schmid P, Cortes J, Dent R, et al; for the KEYNOTE-522 Investigators. Event-free survival with pembrolizumab in early triple-negative breast cancer. N Engl J Med. 2022;386:556-567. Doi: 10.1056/NEJMoa2112651
- Harbeck N, Rastogi P, Martin M, et al; on behalf of the monarchE Committee Members. Adjuvant abemaciclib combined with endocrine therapy for high-risk early breast cancer: updated efficacy and Ki-67 analysis from the monarchE study. Ann Oncol. 2021;32:1571-1581. Doi: 10.1016/j.annonc.2021.09.015
- Rodwin RL, Siddiq NZ, Ehrlich BE, Lustberg MB. Biomarkers of chemotherapy-induced peripheral neuropathy: current status and future directions. Front Pain Res (Lausanne). 2022;3:864910. Doi: 10.3389/fpain.2022.864910
- Wyld L, Reed MW, Morgan J, et al. Bridging the age gap in breast cancer. Impacts of omission of breast cancer surgery in older women with oestrogen receptor positive early breast cancer. A risk stratified analysis of survival outcomes and quality of life. Eur J Cancer. 2021;142:48-62. Doi: 10.1016/j.ejca.2020.10.015
NSAIDs for knee osteoarthritis may worsen pain over time
CHICAGO – Taking NSAIDs for knee osteoarthritis may worsen inflammation and pain over time, suggest new data revealed at the annual meeting of the Radiological Society of North America.
Johanna Luitjens, MD, a postdoctoral scholar in the department of radiology and biomedical Imaging at the University of California, San Francisco, told this news organization that NSAIDs are frequently used to treat OA pain because inflammation is one of the main drivers of OA, but whether they actually help outcomes has been unclear. Her study suggests that they don’t help – and may actually worsen – outcomes.
In particular, this study looked at the impact of NSAIDs on synovitis – the inflammation of the membrane lining the knee joint – by using MRI-based structural biomarkers.
OA, the most common form of arthritis, affects more than 32 million adults in the United States and more than 500 million people worldwide.
No approved therapy to reduce OA progression
Little is known of the long-term effects of NSAIDs on OA progression. Currently, there’s no approved therapy to cure OA or to reduce its advance.
Dr. Luitjens noted, however, that the synovial membrane mediates development and progression of OA and may be a good therapeutic target.
Researchers studied participants from the Osteoarthritis Initiative (OAI) cohort with moderate to severe OA who used NSAIDs regularly for at least 1 year between baseline and 4-year follow-up. All participants had high-quality 3T MRI of the knee at baseline and after 4 years. Images were scored for biomarkers of inflammation, including cartilage thickness and composition.
Dr. Luitjens and associates studied 721 participants who matched the inclusion criteria (129 with and 592 participants without regular NSAID use). The available data did not further specify amounts of NSAIDs used.
At baseline, significantly higher signal intensity in the infrapatellar fat pad (IFP) was seen in patients who used NSAID, compared with controls (adjusted difference in score, 0.26; 95% confidence interval, –0.5 to –0.129; P = .039).
In addition, at the end of the study period, there was a significantly greater increase in signal intensity of IFP (adjusted difference in score, 0.46; 95% CI, 0.2-0.72; P < .001) and higher increase in effusion synovitis (adjusted difference in score, 0.27; 95% CI, 0.06-0.47; P = .01) in NSAID users, compared with controls.
IFP size and synovial proliferation score did not different significantly between groups at the start of the study and showed no significant change over time.
The results showed no long-term benefit of NSAID use. Joint inflammation and cartilage quality were worse at baseline in the participants taking NSAIDs, compared with the control group, and worsened at 4-year follow-up.
Design limits strength
Amanda E. Nelson, MD, associate professor of medicine, division of rheumatology, allergy, and immunology at the University of North Carolina at Chapel Hill, cautioned against assuming causality, pointing out that the OAI is an observational cohort study. (Dr. Nelson was not involved in the OAI or Dr. Luitjens’ analysis.)
“[The OAI is] large and well known, but it wasn’t designed to compare these groups, and this was a small subset,” she said in an interview. Without randomization, it’s hard to judge the results.
“It may be that people on NSAIDs for the duration of the study had more pain and had more disease to begin with, or had more symptoms or had failed other treatments,” she said, adding that the effect sizes were small.
Measures such as the IFP are ranked 0-3, so “the clinical difference of a 0.26 difference on a 0-3 scale is a bit uncertain,” she said.
Dr. Luitjens said that the researchers tried to adjust for potential confounders but agreed that randomized controlled trials are needed to better advise physicians and patients on the benefits or harms of using NSAIDs for OA.
Weighing the risks in older adults
Una Makris, MD, associate professor of internal medicine in the division of rheumatic diseases at the University of Texas Southwestern Medical Center, Dallas, noted that NSAIDs are “not always the safest option.”
“We are still in desperate need of disease-modifying drugs in OA with rigorous randomized trials to show efficacy for outcomes that are most meaningful to patients,” Dr. Makris, who was not involved in the study, told this news organization.
“OA is most common in older adults, those often with multiple comorbidities, so we must always weigh the risks – including known adverse effects which can be amplified in older adults – and benefits with the goal of improved function and less pain,” Dr. Makris said.
NSAID use also should be considered in the context of body mass index, cardiovascular risk, prior trauma or injury, other medication use, and behavioral factors, including physical activity, she said.
Dr. Luitjens, Dr. Nelson, and Dr. Makris reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
CHICAGO – Taking NSAIDs for knee osteoarthritis may worsen inflammation and pain over time, suggest new data revealed at the annual meeting of the Radiological Society of North America.
Johanna Luitjens, MD, a postdoctoral scholar in the department of radiology and biomedical Imaging at the University of California, San Francisco, told this news organization that NSAIDs are frequently used to treat OA pain because inflammation is one of the main drivers of OA, but whether they actually help outcomes has been unclear. Her study suggests that they don’t help – and may actually worsen – outcomes.
In particular, this study looked at the impact of NSAIDs on synovitis – the inflammation of the membrane lining the knee joint – by using MRI-based structural biomarkers.
OA, the most common form of arthritis, affects more than 32 million adults in the United States and more than 500 million people worldwide.
No approved therapy to reduce OA progression
Little is known of the long-term effects of NSAIDs on OA progression. Currently, there’s no approved therapy to cure OA or to reduce its advance.
Dr. Luitjens noted, however, that the synovial membrane mediates development and progression of OA and may be a good therapeutic target.
Researchers studied participants from the Osteoarthritis Initiative (OAI) cohort with moderate to severe OA who used NSAIDs regularly for at least 1 year between baseline and 4-year follow-up. All participants had high-quality 3T MRI of the knee at baseline and after 4 years. Images were scored for biomarkers of inflammation, including cartilage thickness and composition.
Dr. Luitjens and associates studied 721 participants who matched the inclusion criteria (129 with and 592 participants without regular NSAID use). The available data did not further specify amounts of NSAIDs used.
At baseline, significantly higher signal intensity in the infrapatellar fat pad (IFP) was seen in patients who used NSAID, compared with controls (adjusted difference in score, 0.26; 95% confidence interval, –0.5 to –0.129; P = .039).
In addition, at the end of the study period, there was a significantly greater increase in signal intensity of IFP (adjusted difference in score, 0.46; 95% CI, 0.2-0.72; P < .001) and higher increase in effusion synovitis (adjusted difference in score, 0.27; 95% CI, 0.06-0.47; P = .01) in NSAID users, compared with controls.
IFP size and synovial proliferation score did not different significantly between groups at the start of the study and showed no significant change over time.
The results showed no long-term benefit of NSAID use. Joint inflammation and cartilage quality were worse at baseline in the participants taking NSAIDs, compared with the control group, and worsened at 4-year follow-up.
Design limits strength
Amanda E. Nelson, MD, associate professor of medicine, division of rheumatology, allergy, and immunology at the University of North Carolina at Chapel Hill, cautioned against assuming causality, pointing out that the OAI is an observational cohort study. (Dr. Nelson was not involved in the OAI or Dr. Luitjens’ analysis.)
“[The OAI is] large and well known, but it wasn’t designed to compare these groups, and this was a small subset,” she said in an interview. Without randomization, it’s hard to judge the results.
“It may be that people on NSAIDs for the duration of the study had more pain and had more disease to begin with, or had more symptoms or had failed other treatments,” she said, adding that the effect sizes were small.
Measures such as the IFP are ranked 0-3, so “the clinical difference of a 0.26 difference on a 0-3 scale is a bit uncertain,” she said.
Dr. Luitjens said that the researchers tried to adjust for potential confounders but agreed that randomized controlled trials are needed to better advise physicians and patients on the benefits or harms of using NSAIDs for OA.
Weighing the risks in older adults
Una Makris, MD, associate professor of internal medicine in the division of rheumatic diseases at the University of Texas Southwestern Medical Center, Dallas, noted that NSAIDs are “not always the safest option.”
“We are still in desperate need of disease-modifying drugs in OA with rigorous randomized trials to show efficacy for outcomes that are most meaningful to patients,” Dr. Makris, who was not involved in the study, told this news organization.
“OA is most common in older adults, those often with multiple comorbidities, so we must always weigh the risks – including known adverse effects which can be amplified in older adults – and benefits with the goal of improved function and less pain,” Dr. Makris said.
NSAID use also should be considered in the context of body mass index, cardiovascular risk, prior trauma or injury, other medication use, and behavioral factors, including physical activity, she said.
Dr. Luitjens, Dr. Nelson, and Dr. Makris reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
CHICAGO – Taking NSAIDs for knee osteoarthritis may worsen inflammation and pain over time, suggest new data revealed at the annual meeting of the Radiological Society of North America.
Johanna Luitjens, MD, a postdoctoral scholar in the department of radiology and biomedical Imaging at the University of California, San Francisco, told this news organization that NSAIDs are frequently used to treat OA pain because inflammation is one of the main drivers of OA, but whether they actually help outcomes has been unclear. Her study suggests that they don’t help – and may actually worsen – outcomes.
In particular, this study looked at the impact of NSAIDs on synovitis – the inflammation of the membrane lining the knee joint – by using MRI-based structural biomarkers.
OA, the most common form of arthritis, affects more than 32 million adults in the United States and more than 500 million people worldwide.
No approved therapy to reduce OA progression
Little is known of the long-term effects of NSAIDs on OA progression. Currently, there’s no approved therapy to cure OA or to reduce its advance.
Dr. Luitjens noted, however, that the synovial membrane mediates development and progression of OA and may be a good therapeutic target.
Researchers studied participants from the Osteoarthritis Initiative (OAI) cohort with moderate to severe OA who used NSAIDs regularly for at least 1 year between baseline and 4-year follow-up. All participants had high-quality 3T MRI of the knee at baseline and after 4 years. Images were scored for biomarkers of inflammation, including cartilage thickness and composition.
Dr. Luitjens and associates studied 721 participants who matched the inclusion criteria (129 with and 592 participants without regular NSAID use). The available data did not further specify amounts of NSAIDs used.
At baseline, significantly higher signal intensity in the infrapatellar fat pad (IFP) was seen in patients who used NSAID, compared with controls (adjusted difference in score, 0.26; 95% confidence interval, –0.5 to –0.129; P = .039).
In addition, at the end of the study period, there was a significantly greater increase in signal intensity of IFP (adjusted difference in score, 0.46; 95% CI, 0.2-0.72; P < .001) and higher increase in effusion synovitis (adjusted difference in score, 0.27; 95% CI, 0.06-0.47; P = .01) in NSAID users, compared with controls.
IFP size and synovial proliferation score did not different significantly between groups at the start of the study and showed no significant change over time.
The results showed no long-term benefit of NSAID use. Joint inflammation and cartilage quality were worse at baseline in the participants taking NSAIDs, compared with the control group, and worsened at 4-year follow-up.
Design limits strength
Amanda E. Nelson, MD, associate professor of medicine, division of rheumatology, allergy, and immunology at the University of North Carolina at Chapel Hill, cautioned against assuming causality, pointing out that the OAI is an observational cohort study. (Dr. Nelson was not involved in the OAI or Dr. Luitjens’ analysis.)
“[The OAI is] large and well known, but it wasn’t designed to compare these groups, and this was a small subset,” she said in an interview. Without randomization, it’s hard to judge the results.
“It may be that people on NSAIDs for the duration of the study had more pain and had more disease to begin with, or had more symptoms or had failed other treatments,” she said, adding that the effect sizes were small.
Measures such as the IFP are ranked 0-3, so “the clinical difference of a 0.26 difference on a 0-3 scale is a bit uncertain,” she said.
Dr. Luitjens said that the researchers tried to adjust for potential confounders but agreed that randomized controlled trials are needed to better advise physicians and patients on the benefits or harms of using NSAIDs for OA.
Weighing the risks in older adults
Una Makris, MD, associate professor of internal medicine in the division of rheumatic diseases at the University of Texas Southwestern Medical Center, Dallas, noted that NSAIDs are “not always the safest option.”
“We are still in desperate need of disease-modifying drugs in OA with rigorous randomized trials to show efficacy for outcomes that are most meaningful to patients,” Dr. Makris, who was not involved in the study, told this news organization.
“OA is most common in older adults, those often with multiple comorbidities, so we must always weigh the risks – including known adverse effects which can be amplified in older adults – and benefits with the goal of improved function and less pain,” Dr. Makris said.
NSAID use also should be considered in the context of body mass index, cardiovascular risk, prior trauma or injury, other medication use, and behavioral factors, including physical activity, she said.
Dr. Luitjens, Dr. Nelson, and Dr. Makris reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT RSNA 2022









