User login
TNF inhibitor use for RA shows beneficial effect in pregnancy
Women with well-controlled rheumatoid arthritis who used a tumor necrosis factor (TNF) inhibitor during pregnancy gave birth to infants with higher birth weight than did other patients, without an increased risk of adverse outcomes, according to findings from a Dutch prospective cohort study published online in Annals of the Rheumatic Diseases.
The study involved 188 patients drawn from the ongoing Preconceptional Counseling in Active RA (PreCARA) study, which followed patients with inflammatory rheumatic diseases before and during pregnancy. Women enrolled in PreCARA were closely monitored and treated with a therapeutic approach that aimed to achieve minimal disease activity, which included the use of TNF inhibitors.
Much research on TNF inhibitors during pregnancy has been limited to the first trimester and focused primarily on congenital malformations. In addition, most previous studies evaluating TNF inhibitors during pregnancy involved patients with different underlying diseases, making it difficult to interpret the results.
Hieronymus T. W. Smeele, MD, and colleagues at Erasmus University Medical Center, Rotterdam, the Netherlands, evaluated participants every 3 months before pregnancy; then again in the first, second, and third trimesters; and at 6, 12, and 26 weeks post partum. At these visits, in addition to undergoing an examination of their joints, patients completed questionnaires and gave blood samples. Disease activity was determined using the Disease Activity Score in 28 joints. Twin births and diagnoses other than RA were excluded.
Bigger babies
The study found that use of TNF inhibitors during pregnancy (n = 92 women) did not increase the risk of birth defects or emergency cesarean sections. While RA is typically associated with small-for-gestational-age (SGA) birth weights, TNF inhibitors were associated with a significant increase in birth weight and fewer infants born SGA, even when the comparison was adjusted for confounders, such as disease activity. At the same time, TNF inhibitors were not associated with high birth weight or with infants who were large for gestational age (LGA).
The results showed that the effects were greatest when TNF inhibitors were used in the third trimester. However, teasing out the effects based on trimester is difficult because participants who used TNF inhibitors during the third trimester were likely to use them in the first and second trimester as well. The study’s authors pointed out that these results need to be replicated.
“The immune system is not only important in the pathogenesis of RA,” the study’s authors wrote, “but also for ensuring and maintaining a normal pregnancy.” They pointed out that many adverse outcomes of pregnancy that are thought to arise from inadequate development of the placenta, such as intrauterine growth restriction, SGA, and hypertensive disorders of pregnancy, can involve an increase in proinflammatory cytokines, such as TNF. “It is tempting to speculate that treatment with [TNF inhibitors] during pregnancy promotes placentation and thereby fetal growth and birth weight by changing the balance between proinflammatory and anti-inflammatory cytokines and by increasing the number and function of [regulatory T cells].” They also hypothesize that treatment with TNF inhibitors induces epigenetic changes in the fetus, which positively influence fetal growth.
Welcomed data
This is a well-done, interesting study that will add to the still-slim body of research on pregnancy in rheumatic diseases, Kevin Byram, MD, assistant professor of medicine in the division of rheumatology and immunology and associate director of the rheumatology training program at Vanderbilt University, Nashville, Tenn., told this news organization.
“Historically, pregnant women have been excluded from clinical trials, not just in rheumatoid arthritis, but in other rheumatic diseases, so we don’t have a lot of great data,” he said, adding that the more interesting part of the study was that it showed there was no increased risk of adverse outcomes. “I’m not sure what to make of the increased birth weight. It will be interesting to see if the hypothesis that there might be a role for this molecule in preventing low birth weight goes anywhere.”
The work was supported by the Dutch Arthritis Foundation. PreCARA is an investigator-initiated study that was financially supported by UCB. The authors declared no competing interests.
A version of this article first appeared on Medscape.com.
Women with well-controlled rheumatoid arthritis who used a tumor necrosis factor (TNF) inhibitor during pregnancy gave birth to infants with higher birth weight than did other patients, without an increased risk of adverse outcomes, according to findings from a Dutch prospective cohort study published online in Annals of the Rheumatic Diseases.
The study involved 188 patients drawn from the ongoing Preconceptional Counseling in Active RA (PreCARA) study, which followed patients with inflammatory rheumatic diseases before and during pregnancy. Women enrolled in PreCARA were closely monitored and treated with a therapeutic approach that aimed to achieve minimal disease activity, which included the use of TNF inhibitors.
Much research on TNF inhibitors during pregnancy has been limited to the first trimester and focused primarily on congenital malformations. In addition, most previous studies evaluating TNF inhibitors during pregnancy involved patients with different underlying diseases, making it difficult to interpret the results.
Hieronymus T. W. Smeele, MD, and colleagues at Erasmus University Medical Center, Rotterdam, the Netherlands, evaluated participants every 3 months before pregnancy; then again in the first, second, and third trimesters; and at 6, 12, and 26 weeks post partum. At these visits, in addition to undergoing an examination of their joints, patients completed questionnaires and gave blood samples. Disease activity was determined using the Disease Activity Score in 28 joints. Twin births and diagnoses other than RA were excluded.
Bigger babies
The study found that use of TNF inhibitors during pregnancy (n = 92 women) did not increase the risk of birth defects or emergency cesarean sections. While RA is typically associated with small-for-gestational-age (SGA) birth weights, TNF inhibitors were associated with a significant increase in birth weight and fewer infants born SGA, even when the comparison was adjusted for confounders, such as disease activity. At the same time, TNF inhibitors were not associated with high birth weight or with infants who were large for gestational age (LGA).
The results showed that the effects were greatest when TNF inhibitors were used in the third trimester. However, teasing out the effects based on trimester is difficult because participants who used TNF inhibitors during the third trimester were likely to use them in the first and second trimester as well. The study’s authors pointed out that these results need to be replicated.
“The immune system is not only important in the pathogenesis of RA,” the study’s authors wrote, “but also for ensuring and maintaining a normal pregnancy.” They pointed out that many adverse outcomes of pregnancy that are thought to arise from inadequate development of the placenta, such as intrauterine growth restriction, SGA, and hypertensive disorders of pregnancy, can involve an increase in proinflammatory cytokines, such as TNF. “It is tempting to speculate that treatment with [TNF inhibitors] during pregnancy promotes placentation and thereby fetal growth and birth weight by changing the balance between proinflammatory and anti-inflammatory cytokines and by increasing the number and function of [regulatory T cells].” They also hypothesize that treatment with TNF inhibitors induces epigenetic changes in the fetus, which positively influence fetal growth.
Welcomed data
This is a well-done, interesting study that will add to the still-slim body of research on pregnancy in rheumatic diseases, Kevin Byram, MD, assistant professor of medicine in the division of rheumatology and immunology and associate director of the rheumatology training program at Vanderbilt University, Nashville, Tenn., told this news organization.
“Historically, pregnant women have been excluded from clinical trials, not just in rheumatoid arthritis, but in other rheumatic diseases, so we don’t have a lot of great data,” he said, adding that the more interesting part of the study was that it showed there was no increased risk of adverse outcomes. “I’m not sure what to make of the increased birth weight. It will be interesting to see if the hypothesis that there might be a role for this molecule in preventing low birth weight goes anywhere.”
The work was supported by the Dutch Arthritis Foundation. PreCARA is an investigator-initiated study that was financially supported by UCB. The authors declared no competing interests.
A version of this article first appeared on Medscape.com.
Women with well-controlled rheumatoid arthritis who used a tumor necrosis factor (TNF) inhibitor during pregnancy gave birth to infants with higher birth weight than did other patients, without an increased risk of adverse outcomes, according to findings from a Dutch prospective cohort study published online in Annals of the Rheumatic Diseases.
The study involved 188 patients drawn from the ongoing Preconceptional Counseling in Active RA (PreCARA) study, which followed patients with inflammatory rheumatic diseases before and during pregnancy. Women enrolled in PreCARA were closely monitored and treated with a therapeutic approach that aimed to achieve minimal disease activity, which included the use of TNF inhibitors.
Much research on TNF inhibitors during pregnancy has been limited to the first trimester and focused primarily on congenital malformations. In addition, most previous studies evaluating TNF inhibitors during pregnancy involved patients with different underlying diseases, making it difficult to interpret the results.
Hieronymus T. W. Smeele, MD, and colleagues at Erasmus University Medical Center, Rotterdam, the Netherlands, evaluated participants every 3 months before pregnancy; then again in the first, second, and third trimesters; and at 6, 12, and 26 weeks post partum. At these visits, in addition to undergoing an examination of their joints, patients completed questionnaires and gave blood samples. Disease activity was determined using the Disease Activity Score in 28 joints. Twin births and diagnoses other than RA were excluded.
Bigger babies
The study found that use of TNF inhibitors during pregnancy (n = 92 women) did not increase the risk of birth defects or emergency cesarean sections. While RA is typically associated with small-for-gestational-age (SGA) birth weights, TNF inhibitors were associated with a significant increase in birth weight and fewer infants born SGA, even when the comparison was adjusted for confounders, such as disease activity. At the same time, TNF inhibitors were not associated with high birth weight or with infants who were large for gestational age (LGA).
The results showed that the effects were greatest when TNF inhibitors were used in the third trimester. However, teasing out the effects based on trimester is difficult because participants who used TNF inhibitors during the third trimester were likely to use them in the first and second trimester as well. The study’s authors pointed out that these results need to be replicated.
“The immune system is not only important in the pathogenesis of RA,” the study’s authors wrote, “but also for ensuring and maintaining a normal pregnancy.” They pointed out that many adverse outcomes of pregnancy that are thought to arise from inadequate development of the placenta, such as intrauterine growth restriction, SGA, and hypertensive disorders of pregnancy, can involve an increase in proinflammatory cytokines, such as TNF. “It is tempting to speculate that treatment with [TNF inhibitors] during pregnancy promotes placentation and thereby fetal growth and birth weight by changing the balance between proinflammatory and anti-inflammatory cytokines and by increasing the number and function of [regulatory T cells].” They also hypothesize that treatment with TNF inhibitors induces epigenetic changes in the fetus, which positively influence fetal growth.
Welcomed data
This is a well-done, interesting study that will add to the still-slim body of research on pregnancy in rheumatic diseases, Kevin Byram, MD, assistant professor of medicine in the division of rheumatology and immunology and associate director of the rheumatology training program at Vanderbilt University, Nashville, Tenn., told this news organization.
“Historically, pregnant women have been excluded from clinical trials, not just in rheumatoid arthritis, but in other rheumatic diseases, so we don’t have a lot of great data,” he said, adding that the more interesting part of the study was that it showed there was no increased risk of adverse outcomes. “I’m not sure what to make of the increased birth weight. It will be interesting to see if the hypothesis that there might be a role for this molecule in preventing low birth weight goes anywhere.”
The work was supported by the Dutch Arthritis Foundation. PreCARA is an investigator-initiated study that was financially supported by UCB. The authors declared no competing interests.
A version of this article first appeared on Medscape.com.
FROM ANNALS OF THE RHEUMATIC DISEASES
Alcohol-related cirrhosis associated with higher risk of fractures, death
Patients with alcohol-related cirrhosis have a higher fracture rate and a higher post-fracture mortality rate, compared with the general population, according to a large new study from Sweden.
Alcohol-related cirrhosis was associated with an almost fourfold increased fracture rate, and the post-fracture mortality rates were higher at both 30 days and 1 year later.
“Half of all fractures were presumably associated with osteoporosis,” write the study authors, who are gastroenterologists and epidemiologists at the Karolinska Institute, Stockholm. “This suggests that existing pharmacotherapy for osteoporosis may reduce the fracture risk in patients with alcohol-related cirrhosis and possibly also reduce mortality rates.”
But, the authors continue, “our data indicate that osteoporosis may not be the only explanatory factor for this increased fracture risk. Removing modifiable risk factors such as smoking, heavy alcohol use, or malnutrition may further reduce the risk of fractures.”
The study was published online in Clinical Gastroenterology and Hepatology.
Analyzing risks
The association between liver cirrhosis and fractures appears strongest in patients with alcohol-related cirrhosis, the most common cause of cirrhosis in many countries, including Sweden, the authors write.
Previous studies have examined mostly relative risk or hip fractures. The authors aimed to determine not only the relative risk but also the absolute risk, which “can better inform clinicians and policymakers of the actual size of the problem,” they write.
In a nationwide population-based cohort study, they analyzed data from the Swedish National Patient Registry between 1969 and 2016, which included 25,090 patients with alcohol-related cirrhosis. Patients were matched for sex, age, and municipality with 239,458 controls from the Swedish Total Population Registry. They calculated the cumulative incidence of fractures and accounted for competing risks, such as death or liver transportation.
Overall, 48,635 fractures occurred during 3.4 million person-years of follow-up, including 3,659 (14.6%) among patients and 44,976 (18.8%) among controls.
Patients with alcohol-related cirrhosis had a 3.8-times higher fracture rate, with 38.7 fractures per 1,000 person-years, compared with 13.3 in controls. Alcohol-related cirrhosis was also associated with a 1.9-times higher fracture rate than nonalcoholic cirrhosis and a 1.3-times higher fracture rate than noncirrhotic alcohol-related liver disease.
The cumulative incidence of fractures was elevated for patients with alcohol-related cirrhosis in the first 19 years of follow-up, with a 5-year risk at nearly 10%, compared with 4.5% for controls, and a 10-year risk of 13.5%, compared with 8.7% for controls.
Among those with a fracture, the median time to death was 2.8 years in patients with alcohol-related cirrhosis and 3.5 years in controls.
Patients with alcohol-related cirrhosis had a 1.6-times higher post-fracture mortality rate at 30 days, as well as a 1.8-times higher post-fracture mortality rate after one year.
“Falls and fractures kill patients with cirrhosis. Data like these are crucial to spread awareness and represent a call to arms,” Elliot Tapper, MD, an assistant professor of gastroenterology at the University of Michigan, Ann Arbor, told this news organization.
Dr. Tapper, who wasn’t involved with this study, researches the health outcomes of patients with cirrhosis. His previous studies have found that falls, injuries, and death are common in patients with cirrhosis, which could be predicted with an algorithm based on a prior history of falls, blood sodium level, mobility, and quality of life.
“The data emphasize that a fall and fracture herald a time of increased risk,” he said. “Research is needed to develop interventions that prevent falls and help patients remain more resilient when they happen.”
Promoting bone health
Osteoporosis was the most common presumed mechanism in both patients with alcohol-related cirrhosis (49.4%) and controls (52.2%), while high-energy trauma from motor vehicle crashes or heights preceded 10.9% of fractures in patients and 13.5% in controls.
The Karolinska Institute study found that patients with alcohol-related cirrhosis had a 4.4-times higher rate of osteoporotic fracture than controls, which remained 3.6-times higher when using a stricter definition of osteoporotic fracture (a diagnosis of osteoporosis before, at, or within 3 months from the date of a fracture of the vertebrae, pelvis, proximal humerus, distal forearm, or hip).
Patients with osteoporosis at baseline had a 2.5-times higher incidence of fractures than controls with baseline osteoporosis. The absolute risk of fractures in patients with alcohol-related cirrhosis and osteoporosis was higher than for controls with osteoporosis during the first 3 years after a cirrhosis diagnosis.
In addition, the post-fracture mortality rate in those with osteoporosis was more than double in patients with cirrhosis in the first 30 days after a fracture and more than tripled after one year.
“Bone health isn’t necessarily prioritized for our patients, even though it is linked to higher mortality and disability,” Arpan Patel, MD, PhD, a hepatologist at the West Los Angeles VA Medical Center and assistant professor at the David Geffen School of Medicine at the University of California, Los Angeles, told this news organization.
Dr. Patel, who wasn’t involved with this study, has researched the associations between osteoporotic fracture risk, hospitalization, and death in patients with cirrhosis.
“Current guidelines support assessing post-liver transplant patients for bone density but do not currently advocate for doing so in patients with cirrhosis or alcohol-associated liver disease, who are a much larger at-risk population,” Dr. Patel said.
“The current paper supports the idea that we should consider the broad ramifications of alcohol use on bone health for our patients and suggests that there should be greater efforts to screen for and manage osteoporosis and osteopenia in our patients earlier,” he added.
The researchers were supported by grants from Region Stockholm and the Syskonen Svensson Foundation, though the funders had no role in the conduct of the study. The study authors reported no other disclosures or conflicts of interest. Dr. Tapper and Dr. Patel report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Patients with alcohol-related cirrhosis have a higher fracture rate and a higher post-fracture mortality rate, compared with the general population, according to a large new study from Sweden.
Alcohol-related cirrhosis was associated with an almost fourfold increased fracture rate, and the post-fracture mortality rates were higher at both 30 days and 1 year later.
“Half of all fractures were presumably associated with osteoporosis,” write the study authors, who are gastroenterologists and epidemiologists at the Karolinska Institute, Stockholm. “This suggests that existing pharmacotherapy for osteoporosis may reduce the fracture risk in patients with alcohol-related cirrhosis and possibly also reduce mortality rates.”
But, the authors continue, “our data indicate that osteoporosis may not be the only explanatory factor for this increased fracture risk. Removing modifiable risk factors such as smoking, heavy alcohol use, or malnutrition may further reduce the risk of fractures.”
The study was published online in Clinical Gastroenterology and Hepatology.
Analyzing risks
The association between liver cirrhosis and fractures appears strongest in patients with alcohol-related cirrhosis, the most common cause of cirrhosis in many countries, including Sweden, the authors write.
Previous studies have examined mostly relative risk or hip fractures. The authors aimed to determine not only the relative risk but also the absolute risk, which “can better inform clinicians and policymakers of the actual size of the problem,” they write.
In a nationwide population-based cohort study, they analyzed data from the Swedish National Patient Registry between 1969 and 2016, which included 25,090 patients with alcohol-related cirrhosis. Patients were matched for sex, age, and municipality with 239,458 controls from the Swedish Total Population Registry. They calculated the cumulative incidence of fractures and accounted for competing risks, such as death or liver transportation.
Overall, 48,635 fractures occurred during 3.4 million person-years of follow-up, including 3,659 (14.6%) among patients and 44,976 (18.8%) among controls.
Patients with alcohol-related cirrhosis had a 3.8-times higher fracture rate, with 38.7 fractures per 1,000 person-years, compared with 13.3 in controls. Alcohol-related cirrhosis was also associated with a 1.9-times higher fracture rate than nonalcoholic cirrhosis and a 1.3-times higher fracture rate than noncirrhotic alcohol-related liver disease.
The cumulative incidence of fractures was elevated for patients with alcohol-related cirrhosis in the first 19 years of follow-up, with a 5-year risk at nearly 10%, compared with 4.5% for controls, and a 10-year risk of 13.5%, compared with 8.7% for controls.
Among those with a fracture, the median time to death was 2.8 years in patients with alcohol-related cirrhosis and 3.5 years in controls.
Patients with alcohol-related cirrhosis had a 1.6-times higher post-fracture mortality rate at 30 days, as well as a 1.8-times higher post-fracture mortality rate after one year.
“Falls and fractures kill patients with cirrhosis. Data like these are crucial to spread awareness and represent a call to arms,” Elliot Tapper, MD, an assistant professor of gastroenterology at the University of Michigan, Ann Arbor, told this news organization.
Dr. Tapper, who wasn’t involved with this study, researches the health outcomes of patients with cirrhosis. His previous studies have found that falls, injuries, and death are common in patients with cirrhosis, which could be predicted with an algorithm based on a prior history of falls, blood sodium level, mobility, and quality of life.
“The data emphasize that a fall and fracture herald a time of increased risk,” he said. “Research is needed to develop interventions that prevent falls and help patients remain more resilient when they happen.”
Promoting bone health
Osteoporosis was the most common presumed mechanism in both patients with alcohol-related cirrhosis (49.4%) and controls (52.2%), while high-energy trauma from motor vehicle crashes or heights preceded 10.9% of fractures in patients and 13.5% in controls.
The Karolinska Institute study found that patients with alcohol-related cirrhosis had a 4.4-times higher rate of osteoporotic fracture than controls, which remained 3.6-times higher when using a stricter definition of osteoporotic fracture (a diagnosis of osteoporosis before, at, or within 3 months from the date of a fracture of the vertebrae, pelvis, proximal humerus, distal forearm, or hip).
Patients with osteoporosis at baseline had a 2.5-times higher incidence of fractures than controls with baseline osteoporosis. The absolute risk of fractures in patients with alcohol-related cirrhosis and osteoporosis was higher than for controls with osteoporosis during the first 3 years after a cirrhosis diagnosis.
In addition, the post-fracture mortality rate in those with osteoporosis was more than double in patients with cirrhosis in the first 30 days after a fracture and more than tripled after one year.
“Bone health isn’t necessarily prioritized for our patients, even though it is linked to higher mortality and disability,” Arpan Patel, MD, PhD, a hepatologist at the West Los Angeles VA Medical Center and assistant professor at the David Geffen School of Medicine at the University of California, Los Angeles, told this news organization.
Dr. Patel, who wasn’t involved with this study, has researched the associations between osteoporotic fracture risk, hospitalization, and death in patients with cirrhosis.
“Current guidelines support assessing post-liver transplant patients for bone density but do not currently advocate for doing so in patients with cirrhosis or alcohol-associated liver disease, who are a much larger at-risk population,” Dr. Patel said.
“The current paper supports the idea that we should consider the broad ramifications of alcohol use on bone health for our patients and suggests that there should be greater efforts to screen for and manage osteoporosis and osteopenia in our patients earlier,” he added.
The researchers were supported by grants from Region Stockholm and the Syskonen Svensson Foundation, though the funders had no role in the conduct of the study. The study authors reported no other disclosures or conflicts of interest. Dr. Tapper and Dr. Patel report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Patients with alcohol-related cirrhosis have a higher fracture rate and a higher post-fracture mortality rate, compared with the general population, according to a large new study from Sweden.
Alcohol-related cirrhosis was associated with an almost fourfold increased fracture rate, and the post-fracture mortality rates were higher at both 30 days and 1 year later.
“Half of all fractures were presumably associated with osteoporosis,” write the study authors, who are gastroenterologists and epidemiologists at the Karolinska Institute, Stockholm. “This suggests that existing pharmacotherapy for osteoporosis may reduce the fracture risk in patients with alcohol-related cirrhosis and possibly also reduce mortality rates.”
But, the authors continue, “our data indicate that osteoporosis may not be the only explanatory factor for this increased fracture risk. Removing modifiable risk factors such as smoking, heavy alcohol use, or malnutrition may further reduce the risk of fractures.”
The study was published online in Clinical Gastroenterology and Hepatology.
Analyzing risks
The association between liver cirrhosis and fractures appears strongest in patients with alcohol-related cirrhosis, the most common cause of cirrhosis in many countries, including Sweden, the authors write.
Previous studies have examined mostly relative risk or hip fractures. The authors aimed to determine not only the relative risk but also the absolute risk, which “can better inform clinicians and policymakers of the actual size of the problem,” they write.
In a nationwide population-based cohort study, they analyzed data from the Swedish National Patient Registry between 1969 and 2016, which included 25,090 patients with alcohol-related cirrhosis. Patients were matched for sex, age, and municipality with 239,458 controls from the Swedish Total Population Registry. They calculated the cumulative incidence of fractures and accounted for competing risks, such as death or liver transportation.
Overall, 48,635 fractures occurred during 3.4 million person-years of follow-up, including 3,659 (14.6%) among patients and 44,976 (18.8%) among controls.
Patients with alcohol-related cirrhosis had a 3.8-times higher fracture rate, with 38.7 fractures per 1,000 person-years, compared with 13.3 in controls. Alcohol-related cirrhosis was also associated with a 1.9-times higher fracture rate than nonalcoholic cirrhosis and a 1.3-times higher fracture rate than noncirrhotic alcohol-related liver disease.
The cumulative incidence of fractures was elevated for patients with alcohol-related cirrhosis in the first 19 years of follow-up, with a 5-year risk at nearly 10%, compared with 4.5% for controls, and a 10-year risk of 13.5%, compared with 8.7% for controls.
Among those with a fracture, the median time to death was 2.8 years in patients with alcohol-related cirrhosis and 3.5 years in controls.
Patients with alcohol-related cirrhosis had a 1.6-times higher post-fracture mortality rate at 30 days, as well as a 1.8-times higher post-fracture mortality rate after one year.
“Falls and fractures kill patients with cirrhosis. Data like these are crucial to spread awareness and represent a call to arms,” Elliot Tapper, MD, an assistant professor of gastroenterology at the University of Michigan, Ann Arbor, told this news organization.
Dr. Tapper, who wasn’t involved with this study, researches the health outcomes of patients with cirrhosis. His previous studies have found that falls, injuries, and death are common in patients with cirrhosis, which could be predicted with an algorithm based on a prior history of falls, blood sodium level, mobility, and quality of life.
“The data emphasize that a fall and fracture herald a time of increased risk,” he said. “Research is needed to develop interventions that prevent falls and help patients remain more resilient when they happen.”
Promoting bone health
Osteoporosis was the most common presumed mechanism in both patients with alcohol-related cirrhosis (49.4%) and controls (52.2%), while high-energy trauma from motor vehicle crashes or heights preceded 10.9% of fractures in patients and 13.5% in controls.
The Karolinska Institute study found that patients with alcohol-related cirrhosis had a 4.4-times higher rate of osteoporotic fracture than controls, which remained 3.6-times higher when using a stricter definition of osteoporotic fracture (a diagnosis of osteoporosis before, at, or within 3 months from the date of a fracture of the vertebrae, pelvis, proximal humerus, distal forearm, or hip).
Patients with osteoporosis at baseline had a 2.5-times higher incidence of fractures than controls with baseline osteoporosis. The absolute risk of fractures in patients with alcohol-related cirrhosis and osteoporosis was higher than for controls with osteoporosis during the first 3 years after a cirrhosis diagnosis.
In addition, the post-fracture mortality rate in those with osteoporosis was more than double in patients with cirrhosis in the first 30 days after a fracture and more than tripled after one year.
“Bone health isn’t necessarily prioritized for our patients, even though it is linked to higher mortality and disability,” Arpan Patel, MD, PhD, a hepatologist at the West Los Angeles VA Medical Center and assistant professor at the David Geffen School of Medicine at the University of California, Los Angeles, told this news organization.
Dr. Patel, who wasn’t involved with this study, has researched the associations between osteoporotic fracture risk, hospitalization, and death in patients with cirrhosis.
“Current guidelines support assessing post-liver transplant patients for bone density but do not currently advocate for doing so in patients with cirrhosis or alcohol-associated liver disease, who are a much larger at-risk population,” Dr. Patel said.
“The current paper supports the idea that we should consider the broad ramifications of alcohol use on bone health for our patients and suggests that there should be greater efforts to screen for and manage osteoporosis and osteopenia in our patients earlier,” he added.
The researchers were supported by grants from Region Stockholm and the Syskonen Svensson Foundation, though the funders had no role in the conduct of the study. The study authors reported no other disclosures or conflicts of interest. Dr. Tapper and Dr. Patel report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
RADIANCE II: Positive signal for ultrasound renal denervation
Top-line results released on July 26 from the RADIANCE II trial show the Paradise ultrasound renal denervation system significantly reduces daytime ambulatory systolic blood pressure, compared with a sham procedure at 2 months in patients with mild to moderate uncontrolled hypertension.
The trial was conducted in 224 patients who were previously treated with up to two medications and were randomized while off medication at more than 60 centers in 8 countries. No further details or results were provided.
The pivotal RADIANCE II trial, required for FDA approval, is the third and largest randomized, sham-controlled study following positive results reported by RADIANCE-HTN SOLO and RADIANCE-HTN TRIO, ReCor Medical and its subsidiary Otsuka Medical Devices noted in the announcement.
The field of renal denervation fell out of favor after the largest trial in 535 patients, SYMPLICITY HTN-3, failed to show a significant reduction in systolic blood pressure at 6 months, compared with sham control in resistant hypertension.
A version of this article first appeared on Medscape.com.
Top-line results released on July 26 from the RADIANCE II trial show the Paradise ultrasound renal denervation system significantly reduces daytime ambulatory systolic blood pressure, compared with a sham procedure at 2 months in patients with mild to moderate uncontrolled hypertension.
The trial was conducted in 224 patients who were previously treated with up to two medications and were randomized while off medication at more than 60 centers in 8 countries. No further details or results were provided.
The pivotal RADIANCE II trial, required for FDA approval, is the third and largest randomized, sham-controlled study following positive results reported by RADIANCE-HTN SOLO and RADIANCE-HTN TRIO, ReCor Medical and its subsidiary Otsuka Medical Devices noted in the announcement.
The field of renal denervation fell out of favor after the largest trial in 535 patients, SYMPLICITY HTN-3, failed to show a significant reduction in systolic blood pressure at 6 months, compared with sham control in resistant hypertension.
A version of this article first appeared on Medscape.com.
Top-line results released on July 26 from the RADIANCE II trial show the Paradise ultrasound renal denervation system significantly reduces daytime ambulatory systolic blood pressure, compared with a sham procedure at 2 months in patients with mild to moderate uncontrolled hypertension.
The trial was conducted in 224 patients who were previously treated with up to two medications and were randomized while off medication at more than 60 centers in 8 countries. No further details or results were provided.
The pivotal RADIANCE II trial, required for FDA approval, is the third and largest randomized, sham-controlled study following positive results reported by RADIANCE-HTN SOLO and RADIANCE-HTN TRIO, ReCor Medical and its subsidiary Otsuka Medical Devices noted in the announcement.
The field of renal denervation fell out of favor after the largest trial in 535 patients, SYMPLICITY HTN-3, failed to show a significant reduction in systolic blood pressure at 6 months, compared with sham control in resistant hypertension.
A version of this article first appeared on Medscape.com.
Vitamin D supplements do not lower risk of fractures
compared with placebo, according to results from an ancillary study of the Vitamin D and Omega-3 Trial (VITAL).
The data showed that taking 2,000 IU of supplemental vitamin D each day without coadministered calcium did not have a significant effect on nonvertebral fractures (hazard ratio, 0.97; P = .50), hip fractures (HR, 1.01; P = .96), or total fractures (HR, 0.98; P = .70), compared with taking placebo, among individuals who did not have osteoporosis, vitamin D deficiency, or low bone mass, report Meryl S. LeBoff, MD, a professor of medicine at Harvard Medical School and chief of the calcium and bone section at Brigham and Women’s Hospital, both in Boston, and colleagues.
The findings were published online in the New England Journal of Medicine.
Prior randomized, controlled trials have presented conflicting findings. Some have shown that there is some benefit to supplemental vitamin D, whereas others have shown no effect or even harm with regard to risk of fractures, Dr. LeBoff noted.
“Because of the conflicting data at the time, we tested this hypothesis in an effort to advance science and understanding of the effects of vitamin D on bone. In a previous study, we did not see an effect of supplemental vitamin D on bone density in a subcohort from the VITAL trial,” Dr. LeBoff said in an interview.
“We previously reported that vitamin D, about 2,000 units per day, did not increase bone density, nor did it affect bone structure, according to PQCT [peripheral quantitative CT]. So that was an indicator that since bone density is a surrogate marker of fractures, there may not be an effect on fractures,” she added.
These results should dispel any idea that vitamin D alone could significantly reduce fracture rates in the general population, noted Steven R. Cummings, MD, of the University of California, San Francisco, and Clifford Rosen, MD, of Maine Medical Center Research Institute, Scarborough, in an accompanying editorial.
“Adding those findings to previous reports from VITAL and other trials showing the lack of an effect for preventing numerous conditions suggests that providers should stop screening for 25-hydroxyvitamin D levels or recommending vitamin D supplements, and people should stop taking vitamin D supplements to prevent major diseases or extend life,” the editorialists wrote.
The researchers assessed 25,871 participants from all 50 states during a median follow-up time of 5.3 years. Participants were randomly assigned in a 1:1 ratio to receive placebo or vitamin D.
The mean age of the participants was 67.1 years; 50.6% of the study cohort were women, and 20.2% of the cohort were Black. Participants did not have low bone mass, vitamin D deficiency, or osteoporosis.
Participants agreed not to supplement their dietary intake with more than 1,200 mg of calcium each day and no more than 800 IU of vitamin D each day.
Participants filled out detailed surveys to evaluate baseline prescription drug use, demographic factors, medical history, and the consumption of supplements, such as fish oil, calcium, and vitamin D, during the run-in stage. Yearly surveys were used to assess side effects, adherence to the investigation protocol, falls, fractures, physical activity, osteoporosis and associated risk factors, onset of major illness, and the use of nontrial prescription drugs and supplements, such as vitamin D and calcium.
The researchers adjudicated incident fracture data using a centralized medical record review. To approximate the therapeutic effect in intention-to-treat analyses, they used proportional-hazard models.
Notably, outcomes were similar for the placebo and vitamin D groups with regard to incident kidney stones and hypercalcemia.
The effect of vitamin D supplementation was not modified by baseline parameters such as race or ethnicity, sex, body mass index, age, or blood 25-hydroxyvitamin D levels.
Dr. Cummings and Dr. Rosen pointed out that these findings, along with other VITAL trial data, show that no subgroups classified on the basis of baseline 25-hydroxyvitamin D levels, including those with levels less than 20 ng/mL, benefited from vitamin supplementation.
“There is no justification for measuring 25-hydroxyvitamin D in the general population or treating to a target serum level. A 25-hydroxyvitamin D level might be a useful diagnostic test for some patients with conditions that may be due to or that may cause severe deficiency,” the editorialists noted.
Except with regard to select patients, such as individuals living in nursing homes who have limited sun exposure, the use of the terms “vitamin D deficiency” and “vitamin D “insufficiency” should now be reevaluated, Dr. Rosen and Dr. Cummings wrote.
The study’s limitations include its assessment of only one dosage of vitamin D supplementation and a lack of adjustment for multiplicity, exploratory, parent trial, or secondary endpoints, the researchers noted.
The number of participants who had vitamin D deficiency was limited, owing to ethical and feasibility concerns regarding these patients. The data are not generalizable to individuals who are older and institutionalized or those who have osteomalacia or osteoporosis, the researchers wrote.
Expert commentary
“The interpretation of this [study] to me is that vitamin D is not for everybody,” said Baha Arafah, MD, professor of medicine at Case Western Reserve University and chief of the division of endocrinology at University Hospital, both in Cleveland, who was not involved in the study.
“This is not the final word; I would suggest that you don’t throw vitamin D at everybody. I would use markers of bone formation as a better measure to determine whether they need vitamin D or not, specifically looking at parathyroid hormone,” Dr. Arafah said in an interview.
Dr. Arafah pointed out that these data do not mean that clinicians should stop thinking about vitamin D altogether. “I think that would be the wrong message to read. If you read through the article, you will find that there are people who do need vitamin D; people who are deficient do need vitamin D. There’s no question that excessive or extreme vitamin D deficiency can lead to other things, specifically, osteomalacia, weak bones, [and] poor mineralization, so we are not totally out of the woods at this time.”
The ancillary study of the VITAL trial was sponsored by the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Pharmavite donated the vitamin D 3 supplements used in the trial. Dr. LeBoff reported that she holds stock in Amgen. Cummings reported receiving personal fees and nonfinancial support from Amgen outside the submitted work. Dr. Rosen is associate editor of the New England Journal of Medicine. Dr. Arafah reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
compared with placebo, according to results from an ancillary study of the Vitamin D and Omega-3 Trial (VITAL).
The data showed that taking 2,000 IU of supplemental vitamin D each day without coadministered calcium did not have a significant effect on nonvertebral fractures (hazard ratio, 0.97; P = .50), hip fractures (HR, 1.01; P = .96), or total fractures (HR, 0.98; P = .70), compared with taking placebo, among individuals who did not have osteoporosis, vitamin D deficiency, or low bone mass, report Meryl S. LeBoff, MD, a professor of medicine at Harvard Medical School and chief of the calcium and bone section at Brigham and Women’s Hospital, both in Boston, and colleagues.
The findings were published online in the New England Journal of Medicine.
Prior randomized, controlled trials have presented conflicting findings. Some have shown that there is some benefit to supplemental vitamin D, whereas others have shown no effect or even harm with regard to risk of fractures, Dr. LeBoff noted.
“Because of the conflicting data at the time, we tested this hypothesis in an effort to advance science and understanding of the effects of vitamin D on bone. In a previous study, we did not see an effect of supplemental vitamin D on bone density in a subcohort from the VITAL trial,” Dr. LeBoff said in an interview.
“We previously reported that vitamin D, about 2,000 units per day, did not increase bone density, nor did it affect bone structure, according to PQCT [peripheral quantitative CT]. So that was an indicator that since bone density is a surrogate marker of fractures, there may not be an effect on fractures,” she added.
These results should dispel any idea that vitamin D alone could significantly reduce fracture rates in the general population, noted Steven R. Cummings, MD, of the University of California, San Francisco, and Clifford Rosen, MD, of Maine Medical Center Research Institute, Scarborough, in an accompanying editorial.
“Adding those findings to previous reports from VITAL and other trials showing the lack of an effect for preventing numerous conditions suggests that providers should stop screening for 25-hydroxyvitamin D levels or recommending vitamin D supplements, and people should stop taking vitamin D supplements to prevent major diseases or extend life,” the editorialists wrote.
The researchers assessed 25,871 participants from all 50 states during a median follow-up time of 5.3 years. Participants were randomly assigned in a 1:1 ratio to receive placebo or vitamin D.
The mean age of the participants was 67.1 years; 50.6% of the study cohort were women, and 20.2% of the cohort were Black. Participants did not have low bone mass, vitamin D deficiency, or osteoporosis.
Participants agreed not to supplement their dietary intake with more than 1,200 mg of calcium each day and no more than 800 IU of vitamin D each day.
Participants filled out detailed surveys to evaluate baseline prescription drug use, demographic factors, medical history, and the consumption of supplements, such as fish oil, calcium, and vitamin D, during the run-in stage. Yearly surveys were used to assess side effects, adherence to the investigation protocol, falls, fractures, physical activity, osteoporosis and associated risk factors, onset of major illness, and the use of nontrial prescription drugs and supplements, such as vitamin D and calcium.
The researchers adjudicated incident fracture data using a centralized medical record review. To approximate the therapeutic effect in intention-to-treat analyses, they used proportional-hazard models.
Notably, outcomes were similar for the placebo and vitamin D groups with regard to incident kidney stones and hypercalcemia.
The effect of vitamin D supplementation was not modified by baseline parameters such as race or ethnicity, sex, body mass index, age, or blood 25-hydroxyvitamin D levels.
Dr. Cummings and Dr. Rosen pointed out that these findings, along with other VITAL trial data, show that no subgroups classified on the basis of baseline 25-hydroxyvitamin D levels, including those with levels less than 20 ng/mL, benefited from vitamin supplementation.
“There is no justification for measuring 25-hydroxyvitamin D in the general population or treating to a target serum level. A 25-hydroxyvitamin D level might be a useful diagnostic test for some patients with conditions that may be due to or that may cause severe deficiency,” the editorialists noted.
Except with regard to select patients, such as individuals living in nursing homes who have limited sun exposure, the use of the terms “vitamin D deficiency” and “vitamin D “insufficiency” should now be reevaluated, Dr. Rosen and Dr. Cummings wrote.
The study’s limitations include its assessment of only one dosage of vitamin D supplementation and a lack of adjustment for multiplicity, exploratory, parent trial, or secondary endpoints, the researchers noted.
The number of participants who had vitamin D deficiency was limited, owing to ethical and feasibility concerns regarding these patients. The data are not generalizable to individuals who are older and institutionalized or those who have osteomalacia or osteoporosis, the researchers wrote.
Expert commentary
“The interpretation of this [study] to me is that vitamin D is not for everybody,” said Baha Arafah, MD, professor of medicine at Case Western Reserve University and chief of the division of endocrinology at University Hospital, both in Cleveland, who was not involved in the study.
“This is not the final word; I would suggest that you don’t throw vitamin D at everybody. I would use markers of bone formation as a better measure to determine whether they need vitamin D or not, specifically looking at parathyroid hormone,” Dr. Arafah said in an interview.
Dr. Arafah pointed out that these data do not mean that clinicians should stop thinking about vitamin D altogether. “I think that would be the wrong message to read. If you read through the article, you will find that there are people who do need vitamin D; people who are deficient do need vitamin D. There’s no question that excessive or extreme vitamin D deficiency can lead to other things, specifically, osteomalacia, weak bones, [and] poor mineralization, so we are not totally out of the woods at this time.”
The ancillary study of the VITAL trial was sponsored by the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Pharmavite donated the vitamin D 3 supplements used in the trial. Dr. LeBoff reported that she holds stock in Amgen. Cummings reported receiving personal fees and nonfinancial support from Amgen outside the submitted work. Dr. Rosen is associate editor of the New England Journal of Medicine. Dr. Arafah reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
compared with placebo, according to results from an ancillary study of the Vitamin D and Omega-3 Trial (VITAL).
The data showed that taking 2,000 IU of supplemental vitamin D each day without coadministered calcium did not have a significant effect on nonvertebral fractures (hazard ratio, 0.97; P = .50), hip fractures (HR, 1.01; P = .96), or total fractures (HR, 0.98; P = .70), compared with taking placebo, among individuals who did not have osteoporosis, vitamin D deficiency, or low bone mass, report Meryl S. LeBoff, MD, a professor of medicine at Harvard Medical School and chief of the calcium and bone section at Brigham and Women’s Hospital, both in Boston, and colleagues.
The findings were published online in the New England Journal of Medicine.
Prior randomized, controlled trials have presented conflicting findings. Some have shown that there is some benefit to supplemental vitamin D, whereas others have shown no effect or even harm with regard to risk of fractures, Dr. LeBoff noted.
“Because of the conflicting data at the time, we tested this hypothesis in an effort to advance science and understanding of the effects of vitamin D on bone. In a previous study, we did not see an effect of supplemental vitamin D on bone density in a subcohort from the VITAL trial,” Dr. LeBoff said in an interview.
“We previously reported that vitamin D, about 2,000 units per day, did not increase bone density, nor did it affect bone structure, according to PQCT [peripheral quantitative CT]. So that was an indicator that since bone density is a surrogate marker of fractures, there may not be an effect on fractures,” she added.
These results should dispel any idea that vitamin D alone could significantly reduce fracture rates in the general population, noted Steven R. Cummings, MD, of the University of California, San Francisco, and Clifford Rosen, MD, of Maine Medical Center Research Institute, Scarborough, in an accompanying editorial.
“Adding those findings to previous reports from VITAL and other trials showing the lack of an effect for preventing numerous conditions suggests that providers should stop screening for 25-hydroxyvitamin D levels or recommending vitamin D supplements, and people should stop taking vitamin D supplements to prevent major diseases or extend life,” the editorialists wrote.
The researchers assessed 25,871 participants from all 50 states during a median follow-up time of 5.3 years. Participants were randomly assigned in a 1:1 ratio to receive placebo or vitamin D.
The mean age of the participants was 67.1 years; 50.6% of the study cohort were women, and 20.2% of the cohort were Black. Participants did not have low bone mass, vitamin D deficiency, or osteoporosis.
Participants agreed not to supplement their dietary intake with more than 1,200 mg of calcium each day and no more than 800 IU of vitamin D each day.
Participants filled out detailed surveys to evaluate baseline prescription drug use, demographic factors, medical history, and the consumption of supplements, such as fish oil, calcium, and vitamin D, during the run-in stage. Yearly surveys were used to assess side effects, adherence to the investigation protocol, falls, fractures, physical activity, osteoporosis and associated risk factors, onset of major illness, and the use of nontrial prescription drugs and supplements, such as vitamin D and calcium.
The researchers adjudicated incident fracture data using a centralized medical record review. To approximate the therapeutic effect in intention-to-treat analyses, they used proportional-hazard models.
Notably, outcomes were similar for the placebo and vitamin D groups with regard to incident kidney stones and hypercalcemia.
The effect of vitamin D supplementation was not modified by baseline parameters such as race or ethnicity, sex, body mass index, age, or blood 25-hydroxyvitamin D levels.
Dr. Cummings and Dr. Rosen pointed out that these findings, along with other VITAL trial data, show that no subgroups classified on the basis of baseline 25-hydroxyvitamin D levels, including those with levels less than 20 ng/mL, benefited from vitamin supplementation.
“There is no justification for measuring 25-hydroxyvitamin D in the general population or treating to a target serum level. A 25-hydroxyvitamin D level might be a useful diagnostic test for some patients with conditions that may be due to or that may cause severe deficiency,” the editorialists noted.
Except with regard to select patients, such as individuals living in nursing homes who have limited sun exposure, the use of the terms “vitamin D deficiency” and “vitamin D “insufficiency” should now be reevaluated, Dr. Rosen and Dr. Cummings wrote.
The study’s limitations include its assessment of only one dosage of vitamin D supplementation and a lack of adjustment for multiplicity, exploratory, parent trial, or secondary endpoints, the researchers noted.
The number of participants who had vitamin D deficiency was limited, owing to ethical and feasibility concerns regarding these patients. The data are not generalizable to individuals who are older and institutionalized or those who have osteomalacia or osteoporosis, the researchers wrote.
Expert commentary
“The interpretation of this [study] to me is that vitamin D is not for everybody,” said Baha Arafah, MD, professor of medicine at Case Western Reserve University and chief of the division of endocrinology at University Hospital, both in Cleveland, who was not involved in the study.
“This is not the final word; I would suggest that you don’t throw vitamin D at everybody. I would use markers of bone formation as a better measure to determine whether they need vitamin D or not, specifically looking at parathyroid hormone,” Dr. Arafah said in an interview.
Dr. Arafah pointed out that these data do not mean that clinicians should stop thinking about vitamin D altogether. “I think that would be the wrong message to read. If you read through the article, you will find that there are people who do need vitamin D; people who are deficient do need vitamin D. There’s no question that excessive or extreme vitamin D deficiency can lead to other things, specifically, osteomalacia, weak bones, [and] poor mineralization, so we are not totally out of the woods at this time.”
The ancillary study of the VITAL trial was sponsored by the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Pharmavite donated the vitamin D 3 supplements used in the trial. Dr. LeBoff reported that she holds stock in Amgen. Cummings reported receiving personal fees and nonfinancial support from Amgen outside the submitted work. Dr. Rosen is associate editor of the New England Journal of Medicine. Dr. Arafah reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Medicare advantage tied to less use of pricey diabetes drugs
U.S. Medicare beneficiaries with type 2 diabetes who had health coverage through a Medicare Advantage (MA) plan received treatment with an sodium-glucose cotransporter 2 inhibitor or glucagonlike peptide–1 receptor agonist significantly less often than patients with traditional fee-for-service (FFS) Medicare coverage in 2014-2019, according to a study of more than 411,000 patients.
concluded Utibe R. Essien, MD, and coauthors in a study published in Diabetes Care.
The report comes as the U.S. Congress is looking closely at the MA program and evidence that insurance companies that provide these policies sometimes impose inappropriate barriers on enrolled beneficiaries by denying or limiting access to treatments and interventions in ways that run counter to Medicare’s coverage policies.
According to Representative Diana DeGette (D-Colo.), who chaired a hearing on MA plans on June 28 by the House of Representatives’ Energy and Commerce Subcommittee on Oversight and Investigations, beneficiaries who are covered through an MA plan “do not always get the care that they are entitled to.”
The study by Dr. Essien and colleagues also documents some positives of care delivered through MA plans for patients with type 2 diabetes, compared with what FFS Medicare beneficiaries generally receive, such as significantly higher rates of screening for nephropathy and ophthalmologic disorders, and foot examinations.
But the apparently dampened use of SGLT2 inhibitors and GLP-1 receptor agonists among MA beneficiaries stand out as notable shortcomings, Dr. Essien maintained.
Cost containment may limit use
“The differences in health outcomes and in treatments in MA plans are important to highlight,” Dr. Essien said in an interview. “We worry that the cost-containment challenges [associated with MA plans] may be limiting use of these newer treatments.”
The study was based on 2014-2019 data from the Diabetes Collaborative Registry, which collects information from more than 5,000 U.S. clinicians whose practices include patients with diabetes, as well as claims data recorded by the Centers for Medicare and Medicaid Services during 2014-2017.
The main analysis focused on 345,911 Medicare beneficiaries with diabetes, which included 34% with MA coverage and 66% with FFS coverage. The two subgroups had similar ages, about 75 years old, and roughly half were women in both subgroups. The rate at which both subgroups received statin treatment was nearly the same: 72% for those with MA coverage and 71% for those with FFS Medicare.
But MA beneficiaries differed from those with FFS coverage in several other ways. MA beneficiaries had a higher prevalence of Medicaid eligibility than the FFS group (20% vs 12%) and lower rates of consultations with cardiologists (41% vs. 45%) or endocrinologists (7% vs. 10%).
Some of the positive differences in the care received by MA beneficiaries, compared with FFS beneficiaries, after adjustment for potential clinical and sociodemographic confounders, included:
- Screening for nephropathy, at a significant 14% higher relative rate.
- Screening for ophthalmologic disorders, at a significant 8% higher relative rate.
- Undergoing a diabetic foot examination, at a significant 13% higher relative rate.
- Receiving smoking-cessation counseling, at a significant 5% higher relative rate.
- Receiving treatment with an ACE inhibitor or angiotensin-receptor blocker (87% vs. 81%).
- More consistently receiving treatment with metformin, with rates of 72% versus 69% in 2017.
However, these positive differences were accompanied by these relative shortcomings for those with MA, compared with FFS coverage:
- Lower rates of treatment with an SGLT2 inhibitor (5.4% vs. 6.7%), a significant 9% relative difference after adjustment.
- Lower rates of treatment with a GLP-1 agonist (6.9% vs. 9.0%), a significant 20% relative difference after adjustment.
- Higher average levels of LDL cholesterol (81.5 vs. 78.9 mg/dL), a significantly higher average hemoglobin A1c level (7.1% vs. 7.0%), and a trend toward a lower prevalence of blood pressure control (70.3% vs. 71.5%).
Researchers also highlight that the lower rate at which people with MA coverage received SGLT2 inhibitors or GLP-1 agonists was consistent in patients with established cardiovascular or kidney disease, for whom these agents are particularly recommended.
In addition, a secondary analysis of data for another 65,000 Medicare beneficiaries in 2018 and 2019 showed the disparity in use of agents from these two drug classes continued.
Low systemic use of SGLT2 inhibitors, GLP-1 agonists
Dr. Essien acknowledged that, even in people with FFS Medicare coverage, use of SGLT2 inhibitors and GLP-1 agonists was low, but the difference between those with MA coverage is “important.”
Researchers offered four factors that might drive reduced prescribing of agents from these two classes for patients with type 2 diabetes with MA coverage: cost-containment strategies put in place by MA plans; the lower rate of consultations with specialists (cardiologists and endocrinologists); possible exclusion of clinicians from MA provider networks who tend to prescribe these higher-price agents; and lower household incomes of people with MA plans, which may lead to cost-related nonadherence.
Most SGLT2 inhibitors have an average retail cost of about $6,000/year, and some GLP-1 agonists cost more than $10,000/year.
In general, MA coverage includes more oversight of care and its cost than occurs with FFS coverage, noted Dr. Essien, an internal medicine physician at the University of Pittsburgh and a researcher at the Center for Health Equity Research and Promotion of the VA Pittsburgh Healthcare System.
“Incentives for using these more expensive treatments may not be there in MA plans,” he explained. Overcoming cost-related barriers is a challenge that will require “bold policy changes,” as well as better education of clinicians so they make correct treatment decisions, and of patients to resolve possible concerns about treatment safety.
Rep. DeGette hinted during her remarks at the June hearing that policy changes may be coming from Congress.
“Our seniors and their doctors should not be required to jump through numerous hoops to get coverage for straightforward and medically necessary procedures,” she said.
The study received no commercial funding. Dr. Essien reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
U.S. Medicare beneficiaries with type 2 diabetes who had health coverage through a Medicare Advantage (MA) plan received treatment with an sodium-glucose cotransporter 2 inhibitor or glucagonlike peptide–1 receptor agonist significantly less often than patients with traditional fee-for-service (FFS) Medicare coverage in 2014-2019, according to a study of more than 411,000 patients.
concluded Utibe R. Essien, MD, and coauthors in a study published in Diabetes Care.
The report comes as the U.S. Congress is looking closely at the MA program and evidence that insurance companies that provide these policies sometimes impose inappropriate barriers on enrolled beneficiaries by denying or limiting access to treatments and interventions in ways that run counter to Medicare’s coverage policies.
According to Representative Diana DeGette (D-Colo.), who chaired a hearing on MA plans on June 28 by the House of Representatives’ Energy and Commerce Subcommittee on Oversight and Investigations, beneficiaries who are covered through an MA plan “do not always get the care that they are entitled to.”
The study by Dr. Essien and colleagues also documents some positives of care delivered through MA plans for patients with type 2 diabetes, compared with what FFS Medicare beneficiaries generally receive, such as significantly higher rates of screening for nephropathy and ophthalmologic disorders, and foot examinations.
But the apparently dampened use of SGLT2 inhibitors and GLP-1 receptor agonists among MA beneficiaries stand out as notable shortcomings, Dr. Essien maintained.
Cost containment may limit use
“The differences in health outcomes and in treatments in MA plans are important to highlight,” Dr. Essien said in an interview. “We worry that the cost-containment challenges [associated with MA plans] may be limiting use of these newer treatments.”
The study was based on 2014-2019 data from the Diabetes Collaborative Registry, which collects information from more than 5,000 U.S. clinicians whose practices include patients with diabetes, as well as claims data recorded by the Centers for Medicare and Medicaid Services during 2014-2017.
The main analysis focused on 345,911 Medicare beneficiaries with diabetes, which included 34% with MA coverage and 66% with FFS coverage. The two subgroups had similar ages, about 75 years old, and roughly half were women in both subgroups. The rate at which both subgroups received statin treatment was nearly the same: 72% for those with MA coverage and 71% for those with FFS Medicare.
But MA beneficiaries differed from those with FFS coverage in several other ways. MA beneficiaries had a higher prevalence of Medicaid eligibility than the FFS group (20% vs 12%) and lower rates of consultations with cardiologists (41% vs. 45%) or endocrinologists (7% vs. 10%).
Some of the positive differences in the care received by MA beneficiaries, compared with FFS beneficiaries, after adjustment for potential clinical and sociodemographic confounders, included:
- Screening for nephropathy, at a significant 14% higher relative rate.
- Screening for ophthalmologic disorders, at a significant 8% higher relative rate.
- Undergoing a diabetic foot examination, at a significant 13% higher relative rate.
- Receiving smoking-cessation counseling, at a significant 5% higher relative rate.
- Receiving treatment with an ACE inhibitor or angiotensin-receptor blocker (87% vs. 81%).
- More consistently receiving treatment with metformin, with rates of 72% versus 69% in 2017.
However, these positive differences were accompanied by these relative shortcomings for those with MA, compared with FFS coverage:
- Lower rates of treatment with an SGLT2 inhibitor (5.4% vs. 6.7%), a significant 9% relative difference after adjustment.
- Lower rates of treatment with a GLP-1 agonist (6.9% vs. 9.0%), a significant 20% relative difference after adjustment.
- Higher average levels of LDL cholesterol (81.5 vs. 78.9 mg/dL), a significantly higher average hemoglobin A1c level (7.1% vs. 7.0%), and a trend toward a lower prevalence of blood pressure control (70.3% vs. 71.5%).
Researchers also highlight that the lower rate at which people with MA coverage received SGLT2 inhibitors or GLP-1 agonists was consistent in patients with established cardiovascular or kidney disease, for whom these agents are particularly recommended.
In addition, a secondary analysis of data for another 65,000 Medicare beneficiaries in 2018 and 2019 showed the disparity in use of agents from these two drug classes continued.
Low systemic use of SGLT2 inhibitors, GLP-1 agonists
Dr. Essien acknowledged that, even in people with FFS Medicare coverage, use of SGLT2 inhibitors and GLP-1 agonists was low, but the difference between those with MA coverage is “important.”
Researchers offered four factors that might drive reduced prescribing of agents from these two classes for patients with type 2 diabetes with MA coverage: cost-containment strategies put in place by MA plans; the lower rate of consultations with specialists (cardiologists and endocrinologists); possible exclusion of clinicians from MA provider networks who tend to prescribe these higher-price agents; and lower household incomes of people with MA plans, which may lead to cost-related nonadherence.
Most SGLT2 inhibitors have an average retail cost of about $6,000/year, and some GLP-1 agonists cost more than $10,000/year.
In general, MA coverage includes more oversight of care and its cost than occurs with FFS coverage, noted Dr. Essien, an internal medicine physician at the University of Pittsburgh and a researcher at the Center for Health Equity Research and Promotion of the VA Pittsburgh Healthcare System.
“Incentives for using these more expensive treatments may not be there in MA plans,” he explained. Overcoming cost-related barriers is a challenge that will require “bold policy changes,” as well as better education of clinicians so they make correct treatment decisions, and of patients to resolve possible concerns about treatment safety.
Rep. DeGette hinted during her remarks at the June hearing that policy changes may be coming from Congress.
“Our seniors and their doctors should not be required to jump through numerous hoops to get coverage for straightforward and medically necessary procedures,” she said.
The study received no commercial funding. Dr. Essien reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
U.S. Medicare beneficiaries with type 2 diabetes who had health coverage through a Medicare Advantage (MA) plan received treatment with an sodium-glucose cotransporter 2 inhibitor or glucagonlike peptide–1 receptor agonist significantly less often than patients with traditional fee-for-service (FFS) Medicare coverage in 2014-2019, according to a study of more than 411,000 patients.
concluded Utibe R. Essien, MD, and coauthors in a study published in Diabetes Care.
The report comes as the U.S. Congress is looking closely at the MA program and evidence that insurance companies that provide these policies sometimes impose inappropriate barriers on enrolled beneficiaries by denying or limiting access to treatments and interventions in ways that run counter to Medicare’s coverage policies.
According to Representative Diana DeGette (D-Colo.), who chaired a hearing on MA plans on June 28 by the House of Representatives’ Energy and Commerce Subcommittee on Oversight and Investigations, beneficiaries who are covered through an MA plan “do not always get the care that they are entitled to.”
The study by Dr. Essien and colleagues also documents some positives of care delivered through MA plans for patients with type 2 diabetes, compared with what FFS Medicare beneficiaries generally receive, such as significantly higher rates of screening for nephropathy and ophthalmologic disorders, and foot examinations.
But the apparently dampened use of SGLT2 inhibitors and GLP-1 receptor agonists among MA beneficiaries stand out as notable shortcomings, Dr. Essien maintained.
Cost containment may limit use
“The differences in health outcomes and in treatments in MA plans are important to highlight,” Dr. Essien said in an interview. “We worry that the cost-containment challenges [associated with MA plans] may be limiting use of these newer treatments.”
The study was based on 2014-2019 data from the Diabetes Collaborative Registry, which collects information from more than 5,000 U.S. clinicians whose practices include patients with diabetes, as well as claims data recorded by the Centers for Medicare and Medicaid Services during 2014-2017.
The main analysis focused on 345,911 Medicare beneficiaries with diabetes, which included 34% with MA coverage and 66% with FFS coverage. The two subgroups had similar ages, about 75 years old, and roughly half were women in both subgroups. The rate at which both subgroups received statin treatment was nearly the same: 72% for those with MA coverage and 71% for those with FFS Medicare.
But MA beneficiaries differed from those with FFS coverage in several other ways. MA beneficiaries had a higher prevalence of Medicaid eligibility than the FFS group (20% vs 12%) and lower rates of consultations with cardiologists (41% vs. 45%) or endocrinologists (7% vs. 10%).
Some of the positive differences in the care received by MA beneficiaries, compared with FFS beneficiaries, after adjustment for potential clinical and sociodemographic confounders, included:
- Screening for nephropathy, at a significant 14% higher relative rate.
- Screening for ophthalmologic disorders, at a significant 8% higher relative rate.
- Undergoing a diabetic foot examination, at a significant 13% higher relative rate.
- Receiving smoking-cessation counseling, at a significant 5% higher relative rate.
- Receiving treatment with an ACE inhibitor or angiotensin-receptor blocker (87% vs. 81%).
- More consistently receiving treatment with metformin, with rates of 72% versus 69% in 2017.
However, these positive differences were accompanied by these relative shortcomings for those with MA, compared with FFS coverage:
- Lower rates of treatment with an SGLT2 inhibitor (5.4% vs. 6.7%), a significant 9% relative difference after adjustment.
- Lower rates of treatment with a GLP-1 agonist (6.9% vs. 9.0%), a significant 20% relative difference after adjustment.
- Higher average levels of LDL cholesterol (81.5 vs. 78.9 mg/dL), a significantly higher average hemoglobin A1c level (7.1% vs. 7.0%), and a trend toward a lower prevalence of blood pressure control (70.3% vs. 71.5%).
Researchers also highlight that the lower rate at which people with MA coverage received SGLT2 inhibitors or GLP-1 agonists was consistent in patients with established cardiovascular or kidney disease, for whom these agents are particularly recommended.
In addition, a secondary analysis of data for another 65,000 Medicare beneficiaries in 2018 and 2019 showed the disparity in use of agents from these two drug classes continued.
Low systemic use of SGLT2 inhibitors, GLP-1 agonists
Dr. Essien acknowledged that, even in people with FFS Medicare coverage, use of SGLT2 inhibitors and GLP-1 agonists was low, but the difference between those with MA coverage is “important.”
Researchers offered four factors that might drive reduced prescribing of agents from these two classes for patients with type 2 diabetes with MA coverage: cost-containment strategies put in place by MA plans; the lower rate of consultations with specialists (cardiologists and endocrinologists); possible exclusion of clinicians from MA provider networks who tend to prescribe these higher-price agents; and lower household incomes of people with MA plans, which may lead to cost-related nonadherence.
Most SGLT2 inhibitors have an average retail cost of about $6,000/year, and some GLP-1 agonists cost more than $10,000/year.
In general, MA coverage includes more oversight of care and its cost than occurs with FFS coverage, noted Dr. Essien, an internal medicine physician at the University of Pittsburgh and a researcher at the Center for Health Equity Research and Promotion of the VA Pittsburgh Healthcare System.
“Incentives for using these more expensive treatments may not be there in MA plans,” he explained. Overcoming cost-related barriers is a challenge that will require “bold policy changes,” as well as better education of clinicians so they make correct treatment decisions, and of patients to resolve possible concerns about treatment safety.
Rep. DeGette hinted during her remarks at the June hearing that policy changes may be coming from Congress.
“Our seniors and their doctors should not be required to jump through numerous hoops to get coverage for straightforward and medically necessary procedures,” she said.
The study received no commercial funding. Dr. Essien reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM DIABETES CARE
Fourth patient cleared of HIV after stem cell transplant for blood cancer
MONTREAL – from a naturally HIV-resistant donor, U.S. researchers announced at a meeting of the International AIDS Society.
The man received the transplant nearly 3.5 years ago. Since discontinuation of antiretroviral therapy (ART) more than 17 months ago, he has shown no evidence of HIV-1 RNA rebound and no detectable HIV-1 DNA, reported lead investigator Jana K. Dickter, MD, associate clinical professor in the division of infectious diseases at City of Hope, a Duarte, Calif.–based stem cell transplantation center for patients with blood cancers and patients with HIV/blood cancer.
Known as the City of Hope (COH) patient, he is different from the three previously reported patients in that “he was the oldest person to successfully undergo a stem cell transplant with HIV and leukemia and then achieve remission from both conditions,” Dr. Dickter said during a press briefing for the meeting. “He has been living with HIV the longest of any of the patients to date – more than 31 years prior to transplant – and he had also received the least immunosuppressive preparative regimen prior to transplant,” she added.
She said that, like the three previous patients, known as the Berlin, London, and New York patients, the COH patient received a transplant from a donor with natural resistance to HIV because of a rare CCR5-delta 32 mutation.
Dr. Dickter and her coinvestigators used the term “remission” but went further, suggesting that an “HIV cure is feasible” after transplant, given this and the previous cases.
“It’s a bit early to say the patient is cured, but they are clearly in remission,” said Sharon Lewin, MD, president-elect of the International AIDS Society, which runs the meeting. Nevertheless, Dr. Lewin, professor of medicine at the University of Melbourne and director of the Peter Doherty Institute for Infection and Immunity, in Melbourne, acknowledged that cure is “very likely.”
“Two of the previously reported patients have been off ART for long periods of time – Berlin, 12 years (until Timothy’s death in 2020); London, 4 years – and both had far more extensive investigations to try and find intact virus, including very large blood draws, tissue biopsies, etc. For the New York and now this COH patient, the duration off ART has been much shorter. ... But given the prior cases, it is very likely that the New York and COH patients are indeed cured. But I think it’s too early to make that call, hence my preference to use the word, ‘remission,’ “ she told this news organization.
“Although a transplant is not an option for most people with HIV, these cases are still interesting, still inspiring, and help illuminate the search for a cure,” she added.
Dr. Dickter acknowledged that the complexity of stem cell transplant procedures and their potential for significant side effects make them unsuitable as treatment options for most people with HIV, although she said the COH case is evidence that some HIV patients with blood cancers may not need such intensive pretransplant conditioning regimens.
The COH patient received a reduced-intensity fludarabine and melphalan regimen that had been designed at Dr. Dickter’s center “for older and less fit patients to make transplantation more tolerable,” she said. In addition, the graft-vs.-host disease prophylaxis that the COH patient received included only tacrolimus and sirolimus, whereas the previous patients received additional immunosuppressive therapies, and some also had undergone total body irradiation.
Dr. Dickter has disclosed no relevant financial relationships. Dr. Lewin has relationships with AbbVie, BMS, Esfam, Genentech, Gilead, Immunocore, Merck, Vaxxinity, and Viiv.
A version of this article first appeared on Medscape.com.
MONTREAL – from a naturally HIV-resistant donor, U.S. researchers announced at a meeting of the International AIDS Society.
The man received the transplant nearly 3.5 years ago. Since discontinuation of antiretroviral therapy (ART) more than 17 months ago, he has shown no evidence of HIV-1 RNA rebound and no detectable HIV-1 DNA, reported lead investigator Jana K. Dickter, MD, associate clinical professor in the division of infectious diseases at City of Hope, a Duarte, Calif.–based stem cell transplantation center for patients with blood cancers and patients with HIV/blood cancer.
Known as the City of Hope (COH) patient, he is different from the three previously reported patients in that “he was the oldest person to successfully undergo a stem cell transplant with HIV and leukemia and then achieve remission from both conditions,” Dr. Dickter said during a press briefing for the meeting. “He has been living with HIV the longest of any of the patients to date – more than 31 years prior to transplant – and he had also received the least immunosuppressive preparative regimen prior to transplant,” she added.
She said that, like the three previous patients, known as the Berlin, London, and New York patients, the COH patient received a transplant from a donor with natural resistance to HIV because of a rare CCR5-delta 32 mutation.
Dr. Dickter and her coinvestigators used the term “remission” but went further, suggesting that an “HIV cure is feasible” after transplant, given this and the previous cases.
“It’s a bit early to say the patient is cured, but they are clearly in remission,” said Sharon Lewin, MD, president-elect of the International AIDS Society, which runs the meeting. Nevertheless, Dr. Lewin, professor of medicine at the University of Melbourne and director of the Peter Doherty Institute for Infection and Immunity, in Melbourne, acknowledged that cure is “very likely.”
“Two of the previously reported patients have been off ART for long periods of time – Berlin, 12 years (until Timothy’s death in 2020); London, 4 years – and both had far more extensive investigations to try and find intact virus, including very large blood draws, tissue biopsies, etc. For the New York and now this COH patient, the duration off ART has been much shorter. ... But given the prior cases, it is very likely that the New York and COH patients are indeed cured. But I think it’s too early to make that call, hence my preference to use the word, ‘remission,’ “ she told this news organization.
“Although a transplant is not an option for most people with HIV, these cases are still interesting, still inspiring, and help illuminate the search for a cure,” she added.
Dr. Dickter acknowledged that the complexity of stem cell transplant procedures and their potential for significant side effects make them unsuitable as treatment options for most people with HIV, although she said the COH case is evidence that some HIV patients with blood cancers may not need such intensive pretransplant conditioning regimens.
The COH patient received a reduced-intensity fludarabine and melphalan regimen that had been designed at Dr. Dickter’s center “for older and less fit patients to make transplantation more tolerable,” she said. In addition, the graft-vs.-host disease prophylaxis that the COH patient received included only tacrolimus and sirolimus, whereas the previous patients received additional immunosuppressive therapies, and some also had undergone total body irradiation.
Dr. Dickter has disclosed no relevant financial relationships. Dr. Lewin has relationships with AbbVie, BMS, Esfam, Genentech, Gilead, Immunocore, Merck, Vaxxinity, and Viiv.
A version of this article first appeared on Medscape.com.
MONTREAL – from a naturally HIV-resistant donor, U.S. researchers announced at a meeting of the International AIDS Society.
The man received the transplant nearly 3.5 years ago. Since discontinuation of antiretroviral therapy (ART) more than 17 months ago, he has shown no evidence of HIV-1 RNA rebound and no detectable HIV-1 DNA, reported lead investigator Jana K. Dickter, MD, associate clinical professor in the division of infectious diseases at City of Hope, a Duarte, Calif.–based stem cell transplantation center for patients with blood cancers and patients with HIV/blood cancer.
Known as the City of Hope (COH) patient, he is different from the three previously reported patients in that “he was the oldest person to successfully undergo a stem cell transplant with HIV and leukemia and then achieve remission from both conditions,” Dr. Dickter said during a press briefing for the meeting. “He has been living with HIV the longest of any of the patients to date – more than 31 years prior to transplant – and he had also received the least immunosuppressive preparative regimen prior to transplant,” she added.
She said that, like the three previous patients, known as the Berlin, London, and New York patients, the COH patient received a transplant from a donor with natural resistance to HIV because of a rare CCR5-delta 32 mutation.
Dr. Dickter and her coinvestigators used the term “remission” but went further, suggesting that an “HIV cure is feasible” after transplant, given this and the previous cases.
“It’s a bit early to say the patient is cured, but they are clearly in remission,” said Sharon Lewin, MD, president-elect of the International AIDS Society, which runs the meeting. Nevertheless, Dr. Lewin, professor of medicine at the University of Melbourne and director of the Peter Doherty Institute for Infection and Immunity, in Melbourne, acknowledged that cure is “very likely.”
“Two of the previously reported patients have been off ART for long periods of time – Berlin, 12 years (until Timothy’s death in 2020); London, 4 years – and both had far more extensive investigations to try and find intact virus, including very large blood draws, tissue biopsies, etc. For the New York and now this COH patient, the duration off ART has been much shorter. ... But given the prior cases, it is very likely that the New York and COH patients are indeed cured. But I think it’s too early to make that call, hence my preference to use the word, ‘remission,’ “ she told this news organization.
“Although a transplant is not an option for most people with HIV, these cases are still interesting, still inspiring, and help illuminate the search for a cure,” she added.
Dr. Dickter acknowledged that the complexity of stem cell transplant procedures and their potential for significant side effects make them unsuitable as treatment options for most people with HIV, although she said the COH case is evidence that some HIV patients with blood cancers may not need such intensive pretransplant conditioning regimens.
The COH patient received a reduced-intensity fludarabine and melphalan regimen that had been designed at Dr. Dickter’s center “for older and less fit patients to make transplantation more tolerable,” she said. In addition, the graft-vs.-host disease prophylaxis that the COH patient received included only tacrolimus and sirolimus, whereas the previous patients received additional immunosuppressive therapies, and some also had undergone total body irradiation.
Dr. Dickter has disclosed no relevant financial relationships. Dr. Lewin has relationships with AbbVie, BMS, Esfam, Genentech, Gilead, Immunocore, Merck, Vaxxinity, and Viiv.
A version of this article first appeared on Medscape.com.
AT AIDS 2022
Calcinosis Cutis Associated With Subcutaneous Glatiramer Acetate
To the Editor:
Calcinosis cutis is a condition characterized by the deposition of insoluble calcium salts in the skin. Dystrophic calcinosis cutis is the most common type, occurring in previously traumatized skin in the absence of abnormal blood calcium levels. It commonly is seen in patients with connective tissue diseases and is thought to be precipitated by chronic inflammation and vascular hypoxia.1 Herein, we describe a case of calcinosis cutis arising after treatment with subcutaneous glatiramer acetate, an agent that is effective for the treatment of relapsing-remitting multiple sclerosis (MS). Diagnostic workup and treatment modalities for calcinosis cutis in this patient population should be considered in the context of minimizing interruption or discontinuation of this disease-modifying agent.
A 53-year-old woman with a history of relapsing-remitting MS and systemic lupus erythematosus (SLE) presented with multiple firm asymptomatic subcutaneous nodules on the thighs of 1 year’s duration that were increasing in number. The involved areas were the injection sites of subcutaneous glatiramer acetate, an immunomodulator for the treatment of MS, which our patient self-administered 3 times weekly. Physical examination revealed multiple flesh-colored to white, firm, and nontender nodules on the thighs (Figure). There was no epidermal change, and she had no other skin involvement. A punch biopsy of one of the nodules revealed calcium deposits in collagen bundles of the deep dermis. Calcium, phosphorus, parathyroid hormone, and vitamin D levels were within reference range. She declined further treatment for the calcinosis cutis and opted to continue treatment with glatiramer acetate, as her MS was well controlled on this medication.
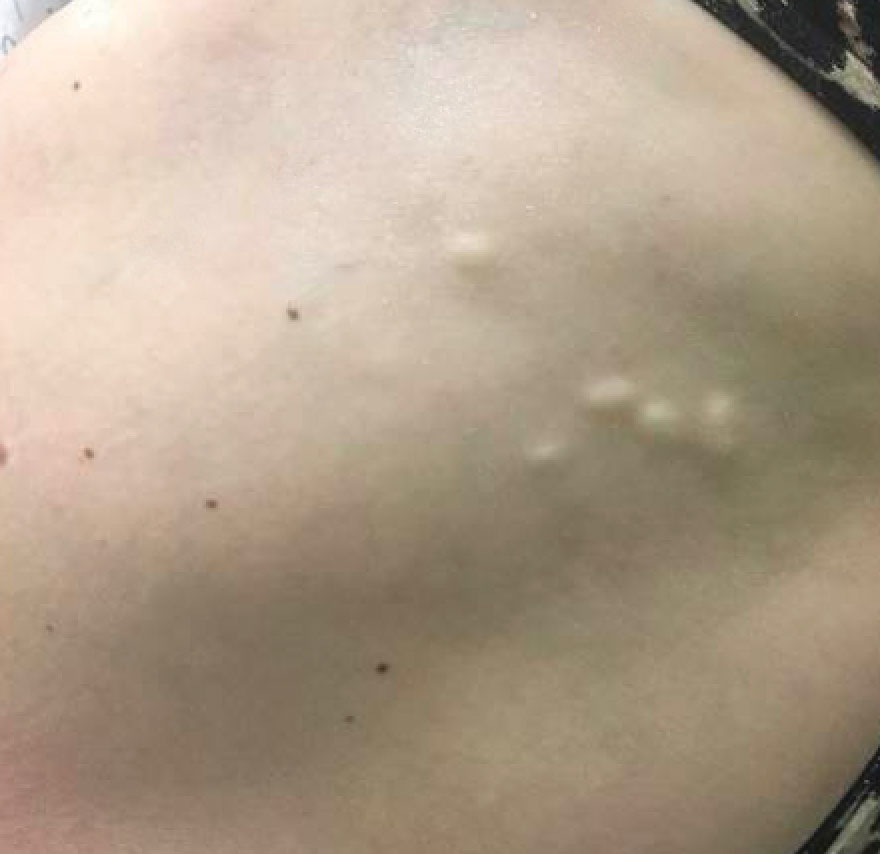
Glatiramer acetate is an immunogenic polypeptide injectable that is approved by the US Food and Drug Administration for the treatment of relapsing-remitting MS.2 It is composed of synthetic polypeptides and contains 4 naturally occurring amino acids. Glatiramer acetate is administered subcutaneously as 20 mg/mL/d or 40 mg/mL 3 times weekly. Transient injection-site reactions are the most common cutaneous adverse events and include localized edema, induration, erythema, pain, and pruritus.3 There have been multiple reports of lobular panniculitis and skin necrosis as well as embolia cutis medicamentosa (Nicolau syndrome).4,5 Our case of calcinosis cutis related to glatiramer acetate is unique. The mechanism of calcinosis cutis in our patient likely was dystrophic due to tissue damage, rather than due to the injection of a calcium-containing substance. Our patient’s history of SLE is a notable risk factor for the development of calcinosis cutis, likely incited by the trauma occurring with subcutaneous injections.6
The mainstay of treatment for localized calcinosis cutis in the setting of connective tissue disease is surgical excision as well as treatment of the underlying disorder. Potential therapies include calcium channel blockers, warfarin, bisphosphonates, intravenous immunoglobulin, minocycline, colchicine, anti–tumor necrosis factor agents, intralesional corticosteroids, intravenous sodium thiosulfate, and CO2 laser.1,6 Our patient was already on intravenous immunoglobulin for MS and hydroxychloroquine for SLE. In select cases where the patient is asymptomatic and prefers not to pursue treatment, no treatment is necessary.
Although calcinosis cutis may occur in SLE alone, it is uncommon and usually is seen in chronic severe SLE, where calcification usually occurs in the setting of pre-existing cutaneous lupus.4 This case report of calcinosis cutis following treatment with glatiramer acetate highlights some of the cutaneous side effects associated with glatiramer acetate injections and should prompt practitioners to consider dystrophic calcinosis cutis in patients requiring subcutaneous medications, particularly in those with pre-existing connective tissue disease.
- Valenzuela A, Chung L. Calcinosis: pathophysiology and management. Curr Opin Rheumatol. 2015;27:542-548.
- Copaxone. Prescribing information. Teva Neuroscience, Inc; 2022. Accessed July 15, 2022. https://www.copaxone.com/globalassets/copaxone/prescribing-information.pdf
- McKeage K. Glatiramer acetate 40 mg/mL in relapsing-remitting multiple sclerosis: a review. CNS Drugs. 2015;29:425-432.
- Balak DMW, Hengstman GJD, Çakmak A, et al. Cutaneous adverse events associated with disease-modifying treatment in multiple sclerosis: a systematic review. Mult Scler. 2012;18:1705-1717.
- Watkins CE, Litchfield J, Youngberg G, et al. Glatiramer acetate-induced lobular panniculitis and skin necrosis. Cutis. 2015;95:E26-E30.
- Reiter N, El-Shabrawi L, Leinweber B, et al. Calcinosis cutis. J Am Acad Dermatol. 2011;65:1-12.
To the Editor:
Calcinosis cutis is a condition characterized by the deposition of insoluble calcium salts in the skin. Dystrophic calcinosis cutis is the most common type, occurring in previously traumatized skin in the absence of abnormal blood calcium levels. It commonly is seen in patients with connective tissue diseases and is thought to be precipitated by chronic inflammation and vascular hypoxia.1 Herein, we describe a case of calcinosis cutis arising after treatment with subcutaneous glatiramer acetate, an agent that is effective for the treatment of relapsing-remitting multiple sclerosis (MS). Diagnostic workup and treatment modalities for calcinosis cutis in this patient population should be considered in the context of minimizing interruption or discontinuation of this disease-modifying agent.
A 53-year-old woman with a history of relapsing-remitting MS and systemic lupus erythematosus (SLE) presented with multiple firm asymptomatic subcutaneous nodules on the thighs of 1 year’s duration that were increasing in number. The involved areas were the injection sites of subcutaneous glatiramer acetate, an immunomodulator for the treatment of MS, which our patient self-administered 3 times weekly. Physical examination revealed multiple flesh-colored to white, firm, and nontender nodules on the thighs (Figure). There was no epidermal change, and she had no other skin involvement. A punch biopsy of one of the nodules revealed calcium deposits in collagen bundles of the deep dermis. Calcium, phosphorus, parathyroid hormone, and vitamin D levels were within reference range. She declined further treatment for the calcinosis cutis and opted to continue treatment with glatiramer acetate, as her MS was well controlled on this medication.

Glatiramer acetate is an immunogenic polypeptide injectable that is approved by the US Food and Drug Administration for the treatment of relapsing-remitting MS.2 It is composed of synthetic polypeptides and contains 4 naturally occurring amino acids. Glatiramer acetate is administered subcutaneously as 20 mg/mL/d or 40 mg/mL 3 times weekly. Transient injection-site reactions are the most common cutaneous adverse events and include localized edema, induration, erythema, pain, and pruritus.3 There have been multiple reports of lobular panniculitis and skin necrosis as well as embolia cutis medicamentosa (Nicolau syndrome).4,5 Our case of calcinosis cutis related to glatiramer acetate is unique. The mechanism of calcinosis cutis in our patient likely was dystrophic due to tissue damage, rather than due to the injection of a calcium-containing substance. Our patient’s history of SLE is a notable risk factor for the development of calcinosis cutis, likely incited by the trauma occurring with subcutaneous injections.6
The mainstay of treatment for localized calcinosis cutis in the setting of connective tissue disease is surgical excision as well as treatment of the underlying disorder. Potential therapies include calcium channel blockers, warfarin, bisphosphonates, intravenous immunoglobulin, minocycline, colchicine, anti–tumor necrosis factor agents, intralesional corticosteroids, intravenous sodium thiosulfate, and CO2 laser.1,6 Our patient was already on intravenous immunoglobulin for MS and hydroxychloroquine for SLE. In select cases where the patient is asymptomatic and prefers not to pursue treatment, no treatment is necessary.
Although calcinosis cutis may occur in SLE alone, it is uncommon and usually is seen in chronic severe SLE, where calcification usually occurs in the setting of pre-existing cutaneous lupus.4 This case report of calcinosis cutis following treatment with glatiramer acetate highlights some of the cutaneous side effects associated with glatiramer acetate injections and should prompt practitioners to consider dystrophic calcinosis cutis in patients requiring subcutaneous medications, particularly in those with pre-existing connective tissue disease.
To the Editor:
Calcinosis cutis is a condition characterized by the deposition of insoluble calcium salts in the skin. Dystrophic calcinosis cutis is the most common type, occurring in previously traumatized skin in the absence of abnormal blood calcium levels. It commonly is seen in patients with connective tissue diseases and is thought to be precipitated by chronic inflammation and vascular hypoxia.1 Herein, we describe a case of calcinosis cutis arising after treatment with subcutaneous glatiramer acetate, an agent that is effective for the treatment of relapsing-remitting multiple sclerosis (MS). Diagnostic workup and treatment modalities for calcinosis cutis in this patient population should be considered in the context of minimizing interruption or discontinuation of this disease-modifying agent.
A 53-year-old woman with a history of relapsing-remitting MS and systemic lupus erythematosus (SLE) presented with multiple firm asymptomatic subcutaneous nodules on the thighs of 1 year’s duration that were increasing in number. The involved areas were the injection sites of subcutaneous glatiramer acetate, an immunomodulator for the treatment of MS, which our patient self-administered 3 times weekly. Physical examination revealed multiple flesh-colored to white, firm, and nontender nodules on the thighs (Figure). There was no epidermal change, and she had no other skin involvement. A punch biopsy of one of the nodules revealed calcium deposits in collagen bundles of the deep dermis. Calcium, phosphorus, parathyroid hormone, and vitamin D levels were within reference range. She declined further treatment for the calcinosis cutis and opted to continue treatment with glatiramer acetate, as her MS was well controlled on this medication.

Glatiramer acetate is an immunogenic polypeptide injectable that is approved by the US Food and Drug Administration for the treatment of relapsing-remitting MS.2 It is composed of synthetic polypeptides and contains 4 naturally occurring amino acids. Glatiramer acetate is administered subcutaneously as 20 mg/mL/d or 40 mg/mL 3 times weekly. Transient injection-site reactions are the most common cutaneous adverse events and include localized edema, induration, erythema, pain, and pruritus.3 There have been multiple reports of lobular panniculitis and skin necrosis as well as embolia cutis medicamentosa (Nicolau syndrome).4,5 Our case of calcinosis cutis related to glatiramer acetate is unique. The mechanism of calcinosis cutis in our patient likely was dystrophic due to tissue damage, rather than due to the injection of a calcium-containing substance. Our patient’s history of SLE is a notable risk factor for the development of calcinosis cutis, likely incited by the trauma occurring with subcutaneous injections.6
The mainstay of treatment for localized calcinosis cutis in the setting of connective tissue disease is surgical excision as well as treatment of the underlying disorder. Potential therapies include calcium channel blockers, warfarin, bisphosphonates, intravenous immunoglobulin, minocycline, colchicine, anti–tumor necrosis factor agents, intralesional corticosteroids, intravenous sodium thiosulfate, and CO2 laser.1,6 Our patient was already on intravenous immunoglobulin for MS and hydroxychloroquine for SLE. In select cases where the patient is asymptomatic and prefers not to pursue treatment, no treatment is necessary.
Although calcinosis cutis may occur in SLE alone, it is uncommon and usually is seen in chronic severe SLE, where calcification usually occurs in the setting of pre-existing cutaneous lupus.4 This case report of calcinosis cutis following treatment with glatiramer acetate highlights some of the cutaneous side effects associated with glatiramer acetate injections and should prompt practitioners to consider dystrophic calcinosis cutis in patients requiring subcutaneous medications, particularly in those with pre-existing connective tissue disease.
- Valenzuela A, Chung L. Calcinosis: pathophysiology and management. Curr Opin Rheumatol. 2015;27:542-548.
- Copaxone. Prescribing information. Teva Neuroscience, Inc; 2022. Accessed July 15, 2022. https://www.copaxone.com/globalassets/copaxone/prescribing-information.pdf
- McKeage K. Glatiramer acetate 40 mg/mL in relapsing-remitting multiple sclerosis: a review. CNS Drugs. 2015;29:425-432.
- Balak DMW, Hengstman GJD, Çakmak A, et al. Cutaneous adverse events associated with disease-modifying treatment in multiple sclerosis: a systematic review. Mult Scler. 2012;18:1705-1717.
- Watkins CE, Litchfield J, Youngberg G, et al. Glatiramer acetate-induced lobular panniculitis and skin necrosis. Cutis. 2015;95:E26-E30.
- Reiter N, El-Shabrawi L, Leinweber B, et al. Calcinosis cutis. J Am Acad Dermatol. 2011;65:1-12.
- Valenzuela A, Chung L. Calcinosis: pathophysiology and management. Curr Opin Rheumatol. 2015;27:542-548.
- Copaxone. Prescribing information. Teva Neuroscience, Inc; 2022. Accessed July 15, 2022. https://www.copaxone.com/globalassets/copaxone/prescribing-information.pdf
- McKeage K. Glatiramer acetate 40 mg/mL in relapsing-remitting multiple sclerosis: a review. CNS Drugs. 2015;29:425-432.
- Balak DMW, Hengstman GJD, Çakmak A, et al. Cutaneous adverse events associated with disease-modifying treatment in multiple sclerosis: a systematic review. Mult Scler. 2012;18:1705-1717.
- Watkins CE, Litchfield J, Youngberg G, et al. Glatiramer acetate-induced lobular panniculitis and skin necrosis. Cutis. 2015;95:E26-E30.
- Reiter N, El-Shabrawi L, Leinweber B, et al. Calcinosis cutis. J Am Acad Dermatol. 2011;65:1-12.
Practice Points
- Glatiramer acetate is a subcutaneous injection utilized for relapsing-remitting multiple sclerosis, and common adverse effects include injection-site reactions such as calcinosis cutis.
- Development of calcinosis cutis in association with glatiramer acetate is not an indication for medication discontinuation.
- Dermatologists should be aware of this potential association, and treatment should be considered in cases of symptomatic calcinosis cutis.
Rituximab for Acquired Hemophilia A in the Setting of Bullous Pemphigoid
To the Editor:
Bullous pemphigoid (BP) is an autoimmune blistering disease characterized by the formation of antihemidesmosomal antibodies, resulting in tense bullae concentrated on the extremities and trunk that often are preceded by a pruritic urticarial phase.1 A rare complication of BP is the subsequent development of acquired hemophilia A. We report a case of BP with associated factor VIII–neutralizing antibodies in a patient who improved with prednisone and rituximab therapy.
A 78-year-old woman presented with red-orange pruritic plaques on the right heel that spread to involve the arms and legs, abdomen, and trunk with new-onset bullae over the course of 2 weeks (Figure 1). Dermatology was consulted, and a diagnosis of BP was confirmed via biopsy and direct immunofluorescence.
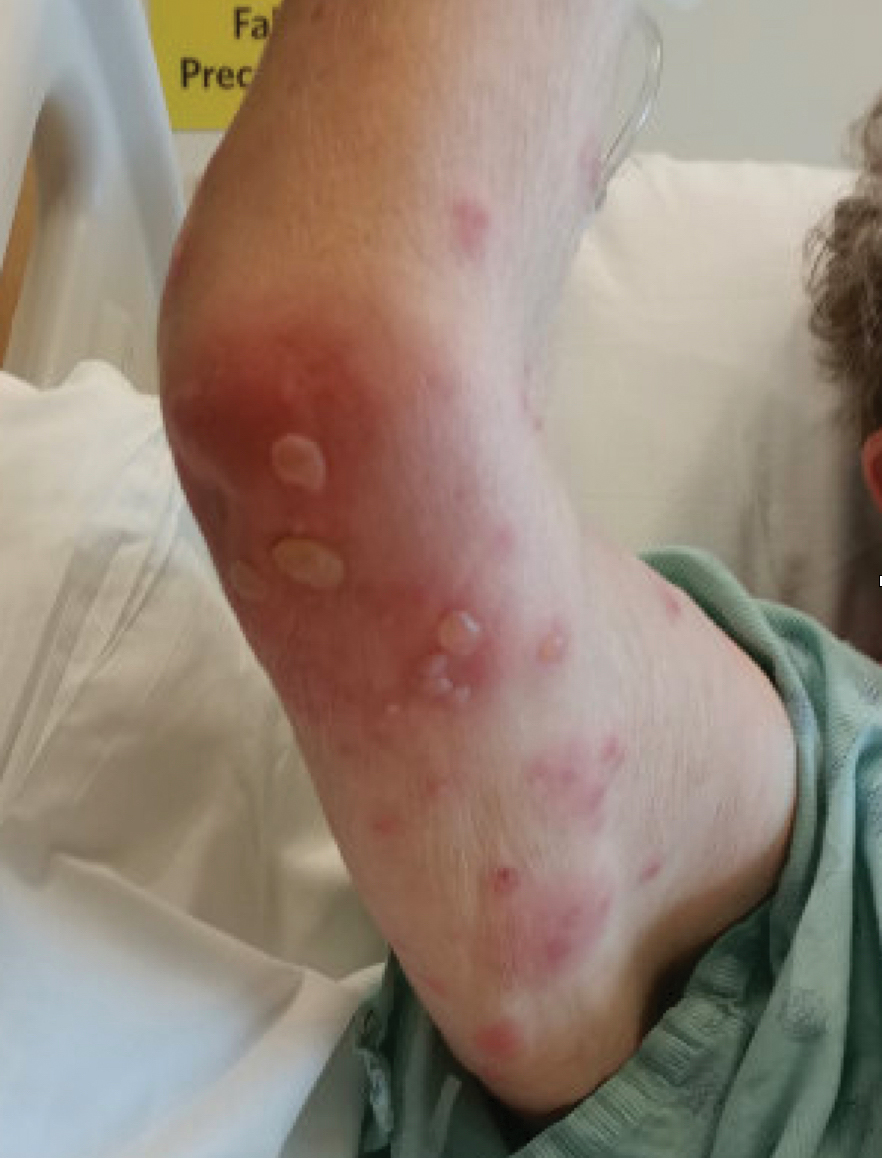
Despite treatment with prednisone 40 mg/d and clobetasol ointment 0.05%, she continued to develop extensive cutaneous bullae and new hemorrhagic bullae on the buccal mucosae (Figure 2), necessitating hospital admission. She clinically improved after prednisone was increased to 60 mg/d and mycophenolate mofetil 500 mg twice daily was added; however, she returned 8 days after discharge from the hospital with altered mental status, new-onset hematomas of the abdomen and right leg, and a hemoglobin level of 5.8 g/dL (reference range, 14.0–17.5 g/dL). Activated prothrombin time was prolonged without correction on mixing studies, raising concern for coagulation factor inhibition. Factor VIII activity was diminished to 9% and then 1% three days later. Mycophenolate mofetil was discontinued, and the patient was acutely stabilized with blood transfusions, intravenous immunoglobulin, tranexamic acid, and aminocaproic acid. Rituximab was initiated at 1000 mg and then administered again 2 weeks later. At 7-week follow-up, coagulation studies normalized, and there was no evidence of blistering dermatosis on examination.
Bullous pemphigoid generally is seen in patients older than 60 years, and the incidence increases with age. The disease course follows formation of IgG antibodies against BP180 or BP230, leading to localized activation of the complement cascade at the basement membrane zone.1 Medications, vaccinations, UV radiation, and burns have been implicated in disease induction.2
Identification of antihemidesmosomal antibodies on lesional biopsy via direct immunofluorescence is the gold standard for diagnosis, though indirect antibodies measured via enzyme-linked immunosorbent assay may provide information regarding disease severity.1 Patients with milder disease may be treated with topical corticosteroids, doxycycline, and nicotinamide; however, severe disease requires treatment with systemic glucocorticoids and steroid-sparing agents.3 Rituximab initially was approved by the US Food and Drug Administration for the treatment of pemphigus vulgaris, and mounting evidence for the use of rituximab in BP is promising. Although data are limited to retrospective studies, rituximab has shown notable remission rates and steroid-sparing effects in those with moderate to severe BP.4
Acquired hemophilia A (AHA) is caused by the production of IgG autoantibodies, which block physiologic interactions between factor VIII and factor IX, phospholipids, and von Willebrand factor.5 Acquired hemophilia A often is diagnosed by prolonged activated prothrombin time and decreased factor VIII activity after a previously unaffected patient develops severe bleeding. Treatment involves re-establishing hemostasis and the use of corticosteroids and immunosuppressive agents to diminish autoantibody production.4
Bullous pemphigoid–associated AHA likely is due to antigenic similarity between BP180 and factor VIII, leading to concomitant neutralization of factor VIII with the production of BP-associated autoantibodies.5 Bullous pemphigoid–associated AHA has been reported with manifestations of bleeding concurrent with or after the development of dermatologic disease. Rituximab use has been reported with clinical efficacy in several cases, including our patient.6 Continued hematologic monitoring is recommended, as recurrences are common within the first 2 years.5
- Bağcı IS, Horváth ON, Ruzicka T, et al. Bullous pemphigoid. Autoimmun Rev. 2017;16:445-455.
- Schiavo AL, Ruocco E, Brancaccio G, et al. Bullous pemphigoid: etiology, pathogenesis, and inducing factors: facts and controversies. Clin Dermatol 2013;31:391-399.
- Schmidt E, Zillikens D. Pemphigoid diseases. Lancet. 2013;381:320-332.
- Cho Y, Chu C, Wang L. First-line combination therapy with rituximab and corticosteroids provides a high complete remission rate in moderate-to-severe bullous pemphigoid. Br J Dermatol. 2015;173:302-304.
- Zdziarska J, Musial J. Acquired hemophilia A: an underdiagnosed severe bleeding disorder. Pol Arch Med Wewn. 2014;124:200-206.
- Binet Q, Lambert C, Sacré L, et al. Successful management of acquired hemophilia associated with bullous pemphigoid: a case report and review of the literature [published online March 28, 2017]. Case Rep Hematol. 2017;2017:2057019.
To the Editor:
Bullous pemphigoid (BP) is an autoimmune blistering disease characterized by the formation of antihemidesmosomal antibodies, resulting in tense bullae concentrated on the extremities and trunk that often are preceded by a pruritic urticarial phase.1 A rare complication of BP is the subsequent development of acquired hemophilia A. We report a case of BP with associated factor VIII–neutralizing antibodies in a patient who improved with prednisone and rituximab therapy.
A 78-year-old woman presented with red-orange pruritic plaques on the right heel that spread to involve the arms and legs, abdomen, and trunk with new-onset bullae over the course of 2 weeks (Figure 1). Dermatology was consulted, and a diagnosis of BP was confirmed via biopsy and direct immunofluorescence.

Despite treatment with prednisone 40 mg/d and clobetasol ointment 0.05%, she continued to develop extensive cutaneous bullae and new hemorrhagic bullae on the buccal mucosae (Figure 2), necessitating hospital admission. She clinically improved after prednisone was increased to 60 mg/d and mycophenolate mofetil 500 mg twice daily was added; however, she returned 8 days after discharge from the hospital with altered mental status, new-onset hematomas of the abdomen and right leg, and a hemoglobin level of 5.8 g/dL (reference range, 14.0–17.5 g/dL). Activated prothrombin time was prolonged without correction on mixing studies, raising concern for coagulation factor inhibition. Factor VIII activity was diminished to 9% and then 1% three days later. Mycophenolate mofetil was discontinued, and the patient was acutely stabilized with blood transfusions, intravenous immunoglobulin, tranexamic acid, and aminocaproic acid. Rituximab was initiated at 1000 mg and then administered again 2 weeks later. At 7-week follow-up, coagulation studies normalized, and there was no evidence of blistering dermatosis on examination.

Bullous pemphigoid generally is seen in patients older than 60 years, and the incidence increases with age. The disease course follows formation of IgG antibodies against BP180 or BP230, leading to localized activation of the complement cascade at the basement membrane zone.1 Medications, vaccinations, UV radiation, and burns have been implicated in disease induction.2
Identification of antihemidesmosomal antibodies on lesional biopsy via direct immunofluorescence is the gold standard for diagnosis, though indirect antibodies measured via enzyme-linked immunosorbent assay may provide information regarding disease severity.1 Patients with milder disease may be treated with topical corticosteroids, doxycycline, and nicotinamide; however, severe disease requires treatment with systemic glucocorticoids and steroid-sparing agents.3 Rituximab initially was approved by the US Food and Drug Administration for the treatment of pemphigus vulgaris, and mounting evidence for the use of rituximab in BP is promising. Although data are limited to retrospective studies, rituximab has shown notable remission rates and steroid-sparing effects in those with moderate to severe BP.4
Acquired hemophilia A (AHA) is caused by the production of IgG autoantibodies, which block physiologic interactions between factor VIII and factor IX, phospholipids, and von Willebrand factor.5 Acquired hemophilia A often is diagnosed by prolonged activated prothrombin time and decreased factor VIII activity after a previously unaffected patient develops severe bleeding. Treatment involves re-establishing hemostasis and the use of corticosteroids and immunosuppressive agents to diminish autoantibody production.4
Bullous pemphigoid–associated AHA likely is due to antigenic similarity between BP180 and factor VIII, leading to concomitant neutralization of factor VIII with the production of BP-associated autoantibodies.5 Bullous pemphigoid–associated AHA has been reported with manifestations of bleeding concurrent with or after the development of dermatologic disease. Rituximab use has been reported with clinical efficacy in several cases, including our patient.6 Continued hematologic monitoring is recommended, as recurrences are common within the first 2 years.5
To the Editor:
Bullous pemphigoid (BP) is an autoimmune blistering disease characterized by the formation of antihemidesmosomal antibodies, resulting in tense bullae concentrated on the extremities and trunk that often are preceded by a pruritic urticarial phase.1 A rare complication of BP is the subsequent development of acquired hemophilia A. We report a case of BP with associated factor VIII–neutralizing antibodies in a patient who improved with prednisone and rituximab therapy.
A 78-year-old woman presented with red-orange pruritic plaques on the right heel that spread to involve the arms and legs, abdomen, and trunk with new-onset bullae over the course of 2 weeks (Figure 1). Dermatology was consulted, and a diagnosis of BP was confirmed via biopsy and direct immunofluorescence.

Despite treatment with prednisone 40 mg/d and clobetasol ointment 0.05%, she continued to develop extensive cutaneous bullae and new hemorrhagic bullae on the buccal mucosae (Figure 2), necessitating hospital admission. She clinically improved after prednisone was increased to 60 mg/d and mycophenolate mofetil 500 mg twice daily was added; however, she returned 8 days after discharge from the hospital with altered mental status, new-onset hematomas of the abdomen and right leg, and a hemoglobin level of 5.8 g/dL (reference range, 14.0–17.5 g/dL). Activated prothrombin time was prolonged without correction on mixing studies, raising concern for coagulation factor inhibition. Factor VIII activity was diminished to 9% and then 1% three days later. Mycophenolate mofetil was discontinued, and the patient was acutely stabilized with blood transfusions, intravenous immunoglobulin, tranexamic acid, and aminocaproic acid. Rituximab was initiated at 1000 mg and then administered again 2 weeks later. At 7-week follow-up, coagulation studies normalized, and there was no evidence of blistering dermatosis on examination.

Bullous pemphigoid generally is seen in patients older than 60 years, and the incidence increases with age. The disease course follows formation of IgG antibodies against BP180 or BP230, leading to localized activation of the complement cascade at the basement membrane zone.1 Medications, vaccinations, UV radiation, and burns have been implicated in disease induction.2
Identification of antihemidesmosomal antibodies on lesional biopsy via direct immunofluorescence is the gold standard for diagnosis, though indirect antibodies measured via enzyme-linked immunosorbent assay may provide information regarding disease severity.1 Patients with milder disease may be treated with topical corticosteroids, doxycycline, and nicotinamide; however, severe disease requires treatment with systemic glucocorticoids and steroid-sparing agents.3 Rituximab initially was approved by the US Food and Drug Administration for the treatment of pemphigus vulgaris, and mounting evidence for the use of rituximab in BP is promising. Although data are limited to retrospective studies, rituximab has shown notable remission rates and steroid-sparing effects in those with moderate to severe BP.4
Acquired hemophilia A (AHA) is caused by the production of IgG autoantibodies, which block physiologic interactions between factor VIII and factor IX, phospholipids, and von Willebrand factor.5 Acquired hemophilia A often is diagnosed by prolonged activated prothrombin time and decreased factor VIII activity after a previously unaffected patient develops severe bleeding. Treatment involves re-establishing hemostasis and the use of corticosteroids and immunosuppressive agents to diminish autoantibody production.4
Bullous pemphigoid–associated AHA likely is due to antigenic similarity between BP180 and factor VIII, leading to concomitant neutralization of factor VIII with the production of BP-associated autoantibodies.5 Bullous pemphigoid–associated AHA has been reported with manifestations of bleeding concurrent with or after the development of dermatologic disease. Rituximab use has been reported with clinical efficacy in several cases, including our patient.6 Continued hematologic monitoring is recommended, as recurrences are common within the first 2 years.5
- Bağcı IS, Horváth ON, Ruzicka T, et al. Bullous pemphigoid. Autoimmun Rev. 2017;16:445-455.
- Schiavo AL, Ruocco E, Brancaccio G, et al. Bullous pemphigoid: etiology, pathogenesis, and inducing factors: facts and controversies. Clin Dermatol 2013;31:391-399.
- Schmidt E, Zillikens D. Pemphigoid diseases. Lancet. 2013;381:320-332.
- Cho Y, Chu C, Wang L. First-line combination therapy with rituximab and corticosteroids provides a high complete remission rate in moderate-to-severe bullous pemphigoid. Br J Dermatol. 2015;173:302-304.
- Zdziarska J, Musial J. Acquired hemophilia A: an underdiagnosed severe bleeding disorder. Pol Arch Med Wewn. 2014;124:200-206.
- Binet Q, Lambert C, Sacré L, et al. Successful management of acquired hemophilia associated with bullous pemphigoid: a case report and review of the literature [published online March 28, 2017]. Case Rep Hematol. 2017;2017:2057019.
- Bağcı IS, Horváth ON, Ruzicka T, et al. Bullous pemphigoid. Autoimmun Rev. 2017;16:445-455.
- Schiavo AL, Ruocco E, Brancaccio G, et al. Bullous pemphigoid: etiology, pathogenesis, and inducing factors: facts and controversies. Clin Dermatol 2013;31:391-399.
- Schmidt E, Zillikens D. Pemphigoid diseases. Lancet. 2013;381:320-332.
- Cho Y, Chu C, Wang L. First-line combination therapy with rituximab and corticosteroids provides a high complete remission rate in moderate-to-severe bullous pemphigoid. Br J Dermatol. 2015;173:302-304.
- Zdziarska J, Musial J. Acquired hemophilia A: an underdiagnosed severe bleeding disorder. Pol Arch Med Wewn. 2014;124:200-206.
- Binet Q, Lambert C, Sacré L, et al. Successful management of acquired hemophilia associated with bullous pemphigoid: a case report and review of the literature [published online March 28, 2017]. Case Rep Hematol. 2017;2017:2057019.
Practice Points
- Physicians must be aware of the potential for acquired hemophilia A in patients with bullous pemphigoid (BP).
- Rituximab is an effective therapy for BP and should be considered for patients in this cohort.