User login
A guide to understanding your anatomy
Effect of Pharmacist Interventions on Hospital Readmissions for Home-Based Primary Care Veterans
Following hospital discharge, patients are often in a vulnerable state due to new medical diagnoses, changes in medications, lack of understanding, and concerns for medical costs. In addition, the discharge process is complex and encompasses decisions regarding the postdischarge site of care, conveying patient instructions, and obtaining supplies and medications. There are several disciplines involved in the transitions of care process that are all essential for ensuring a successful transition and reducing the risk of hospital readmissions. Pharmacists play an integral role in the process.
When pharmacists are provided the opportunity to make therapeutic interventions, medication errors and hospital readmissions decrease and quality of life improves.1 Studies have shown that many older patients return home from the hospital with a limited understanding of their discharge instructions and oftentimes are unable to recall their discharge diagnoses and treatment plan, leaving opportunities for error when patients transition from one level of care to another.2,3 Additionally, high-quality transitional care is especially important for older adults with multiple comorbidities and complex therapeutic regimens as well as for their families and caregivers.4 To prevent hospital readmissions, pharmacists and other health care professionals (HCPs) should work diligently to prevent gaps in care as patients transition between settings. Common factors that lead to increased readmissions include premature discharge, inadequate follow-up, therapeutic errors, and medication-related problems. Furthermore, unintended hospital readmissions are common within the first 30 days following hospital discharge and lead to increased health care costs.2 For these reasons, many health care institutions have developed comprehensive models to improve the discharge process, decrease hospital readmissions, and reduce incidence of adverse events in general medical patients and high-risk populations.5
A study evaluating 693 hospital discharges found that 27.6% of patients were recommended for outpatient workups; however only 9% were actually completed.6 Due to lack of communication regarding discharge summaries, primary care practitioners (PCPs) were unaware of the need for outpatient workups; thus, these patients were lost to follow-up, and appropriate care was not received. Future studies should focus on interventions to improve the quality and dissemination of discharge information to PCPs.6 Fosnight and colleagues assessed a new transitions process focusing on the role of pharmacists. They evaluated medication reconciliations performed and discussed medication adherence barriers, medication recommendations, and time spent performing the interventions.7 After patients received a pharmacy intervention, Fosnight and colleagues reported that readmission rates decreased from 21.0% to 15.3% and mean length of stay decreased from 5.3 to 4.4 days. They also observed greater improvements in patients who received the full pharmacy intervention vs those receiving only parts of the intervention. This study concluded that adding a comprehensive pharmacy intervention to transitions of care resulted in an average of nearly 10 medication recommendations per patient, improved length of stay, and reduced readmission rates. After a review of similar studies, we concluded that a comprehensive discharge model is imperative to improve patient outcomes, along with HCP monitoring of the process to ensure appropriate follow-up.8
At Michael E. DeBakey Veteran Affairs Medical Center (MEDVAMC) in Houston, Texas, 30-day readmissions data were reviewed for veterans 6 months before and 12 months after enrollment in the Home-Based Primary Care (HBPC) service. HBPC is an in-home health care service provided to home-bound veterans with complex health care needs or when routine clinic-based care is not feasible. HBPC programs may differ among various US Department of Veterans Affairs (VA) medical centers. Currently, there are 9 HBPC teams at MEDVAMC and nearly 540 veterans are enrolled in the program. HBPC teams typically consist of PCPs, pharmacists, nurses, psychologists, occupational/physical therapists, social workers, medical support assistants, and dietitians.
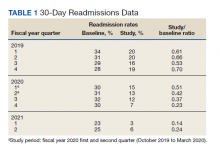
Readmissions data are reviewed quarterly by fiscal year (FY) (Table 1). In FY 2019 quarter (Q) 2, the readmission rate before HBPC enrollment was 31% and decreased to 20% after enrollment. In FY 2019 Q3, the readmission rate was 29% before enrollment and decreased to 16% afterward. In FY 2019 Q4, the readmission rate before HBPC enrollment was 28% and decreased to 19% afterward. Although the readmission rates appeared to be decreasing overall, improvements were needed to decrease these rates further and to ensure readmissions were not rising as there was a slight increase in Q4. After reviewing these data, the HBPC service implemented a streamlined hospital discharge process to lower readmission rates and improve patient outcomes.
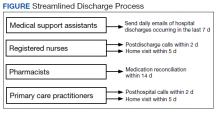
HBPC at MEDVAMC incorporates a team-based approach and the new streamlined discharge process implemented in 2019 highlights the role of each team member (Figure). Medical support assistants send daily emails of hospital discharges occurring in the last 7 days. Registered nurses are responsible for postdischarge calls within 2 days and home visits within 5 days. Pharmacists perform medication reconciliation within 14 days of discharge, review and/or educate on new medications, and change medications. The PCP is responsible for posthospital calls within 2 days and conducts a home visit within 5 days. Because HBPC programs vary among VA medical centers, the streamlined discharge process discussed may be applicable only to MEDVAMC. The primary objective of this quality improvement project was to identify specific pharmacist interventions to improve the HBPC discharge process and improve hospital readmission rates.
Methods
We conducted a Plan-Do-Study-Act quality improvement project. The first step was to conduct a review of veterans enrolled in HBPC at MEDVAMC.9 Patients included were enrolled in HBPC at MEDVAMC from October 2019 to March 2020 (FY 2020 Q1 and Q2). The Computerized Patient Record System was used to access the patients’ electronic health records. Patient information collected included race, age, sex, admission diagnosis, date of discharge, HBPC pharmacist name, PCP notification on the discharge summary, and 30-day readmission rates. Unplanned return to the hospital within 30 days, which was counted as a readmission, was defined as any admission for acute clinical events that required urgent hospital management.10
Next, we identified specific pharmacist interventions, including medication reconciliation completed by an HBPC pharmacist postdischarge; mean time to contact patients postdischarge; correct medications and supplies on discharge; incorrect dose; incorrect medication frequency or route of administration; therapeutic duplications; discontinuation of medications; additional drug therapy recommendations; laboratory test recommendations; maintenance medications not restarted or omitted; new medication education; and medication or formulation changes.
In the third step, we reviewed discharge summaries and clinical pharmacy notes to collect pharmacist intervention data. These data were analyzed to develop a standardized discharge process. Descriptive statistics were used to represent the results of the study.
Results
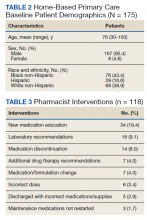
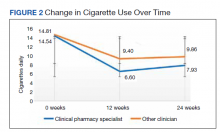
Medication reconciliation was completed postdischarge by an HBPC pharmacist in 118 of 175 study patients (67.4%). The mean age of patients was 76 years, about 95% were male (Table 2). There was a wide variety of admission diagnoses but sepsis, chronic obstructive pulmonary disease, and chronic kidney disease were most common. The PCP was notified on the discharge note for 68 (38.9%) patients. The mean time for HBPC pharmacists to contact patients postdischarge was about 3 days, which was much less than the 14 days allowed in the streamlined discharge process.
Pharmacists made the following interventions during medication reconciliation: New medication education was provided for 34 (19.4%) patients and was the largest intervention completed by HBPC pharmacists. Laboratory tests were recommended for 16 (9.1%) patients, medications were discontinued in 14 (8.0%) patients, and additional drug therapy recommendations were made for 7 (4.0%) patients. Medication or formulation changes were completed in 7 (4.0%) patients, incorrect doses were identified in 6 (3.4%) patients, 5 (2.9%) patients were not discharged with the correct medications or supplies, maintenance medications were not restarted in 3 (1.7%) patients, and there were no therapeutic duplications identified. In total, there were 92 (77.9%) patients with interventions compared with the 118 medication reconciliations completed (Table 3).
Process Improvement
As this was a new streamlined discharge process, it was important to assess the progress of the pharmacist role over time. We evaluated the number of medication reconciliations completed by quarter to determine whether more interventions were completed as the streamlined discharge process was being fully implemented. In FY 2020 Q1, medication reconciliation was completed by an HBPC pharmacist at a rate of 35%, and in FY 2020 Q2, at a rate of 65%.
In addition to assessing interventions completed by an HBPC pharmacist, we noted how many medication reconciliations were completed by an inpatient pharmacist as this may have impacted the results of this study. Of the 175 patients in this study, 49 (28%) received a medication reconciliation by an inpatient clinical pharmacy specialist before discharge. Last, when reviewing the readmissions data for the study period, it was evident that the streamlined discharge process was improving. In FY 2020 Q1, the readmissions rate prior to HBPC enrollment was 30% and decreased to 15% after and in FY 2020 Q2 was 31% before and decreased to 13% after HBPC enrollment. Before the study period in FY 2019 Q4, the readmissions rate after HBPC enrollment was 19%. Therefore, the readmissions rate decreased from 19% before the study period to 13% by the end of the study period.
Discussion
A comparison of the readmissions data from FYs 2019, 2020, and 2021 revealed that the newly implemented discharge process at MEDVAMC had been more effective.
There were 92 interventions made during the study period, which is about 78% of all medication reconciliations completed. Medication doses were changed based on patients’ renal function. Additional laboratory tests were recommended after discharge to ensure safety of therapy. Medications were discontinued if inappropriate or if patients were no longer on them to simplify their medication list and limit polypharmacy. New medication education was provided, including drug name, dose, route of administration, time of administration, frequency, indication, mechanism of action, adverse effect profile, monitoring parameters, and more. The HBPC pharmacists were able to make suitable interventions in a timely fashion as the average time to contact patients postdischarge was 3 days.
Areas for Improvement
The PCP was notified on the discharge note only in 68 (38.9%) patients. This could lead to gaps in care if other mechanisms are not in place to notify the PCP of the patient’s discharge. For this reason, it is imperative not only to implement a streamlined discharge process, but to review it and determine methods for continued improvement.9 The streamlined discharge process implemented by the HBPC team highlights when each team member should contact the patient postdischarge. However, it may be beneficial for each team member to have a list of vital information that should be communicated to the patient postdischarge and to other HCPs. For pharmacists, a standardized discharge note template may aid in the consistency of the medication reconciliation process postdischarge and may also increase interventions from pharmacists. For example, only some HBPC pharmacists inserted a new medication template in their discharge follow-up note. In addition, 23 (13.1%) patients were unreachable, and although a complete medication reconciliation was not feasible, a standardized note to review inpatient and outpatient medications along with the discharge plan may still serve as an asset for HCPs.
As the HBPC team continues to improve the discharge process, it is also important to highlight roles of the inpatient team who may assist with a smoother transition. For example, discharge summaries should be clear, complete, and concise, incorporating key elements from the hospital visit. Methods of communication on discharge should be efficient and understood by both inpatient and outpatient teams. Patients’ health literacy status should be considered when providing discharge instructions. Finally, patients should have a clear understanding of who is included in their primary care team should any questions arise. The potential interventions for HCPs highlighted in this study are critical for preventing adverse outcomes, improving patients’ quality of life, and decreasing hospital readmissions. However, implementing the streamlined discharge process was only step 1. Areas of improvement still exist to provide exceptional patient care.
Our goal is to increase pharmacist-led medication reconciliation after discharge to ≥ 80%. This will be assessed monthly after providing education to the HBPC team regarding the study results. The second goal is to maintain hospital readmission rates to ≤ 10%, which will be assessed with each quarterly review.
Strengths and Limitations
This study was one of the first to evaluate the impact of pharmacist intervention on improving patient outcomes in HBPC veterans. Additionally, only 1 investigator conducted the data collection, which decreased the opportunity for errors.
A notable limitation of this study is that the discharge processes may not be able to be duplicated in other HBPC settings due to variability in programs. Additionally, as this was a new discharge process, there were a few aspects that needed to be worked out in the beginning as it was established. Furthermore, this study did not clarify whether a medication reconciliation was conducted by a physician or nurse after discharge; therefore, this study cannot conclude that the medication interventions were solely attributed to pharmacists. Also this study did not assess readmissions for recurrent events only, which may have impacted the results in a different way from the current results that assessed readmission rates for any hospitalization. Other limitations include the retrospective study design at a single center.
Conclusions
This study outlines several opportunities for interventions to improve patient outcomes and aid in decreasing hospital readmission rates. Using the results from this study, education has been provided for the HBPC Service and its readmission committee. Additionally, the safety concerns identified have been addressed with inpatient and outpatient pharmacy leadership to improve the practices in both settings, prevent delays in patient care, and avoid future adverse outcomes. This project highlights the advantages of having pharmacists involved in transitions of care and demonstrates the benefit of HBPC pharmacists’ role in the streamlined discharge process. This project will be reviewed biannually to further improve the discharge process and quality of care for our veterans.
1. Coleman EA, Chugh A, Williams MV, et al. Understanding and execution of discharge instructions. Am J Med Qual. 2013;28(5):383-391. doi:10.1177/1062860612472931
2. Hume AL, Kirwin J, Bieber HL, et al. Improving care transitions: current practice and future opportunities for pharmacists. Pharmacotherapy. 2012;32(11):e326-e337. doi:10.1002/phar.1215
3. Milfred-LaForest SK, Gee JA, Pugacz AM, et al. Heart failure transitions of care: a pharmacist-led post discharge pilot experience. Prog Cardiovasc Dis. 2017;60(2):249-258. doi:10.1016/j.pcad.2017.08.005
4. Naylor M, Keating SA. Transitional care: moving patients from one care setting to another. Am J Nurs. 2008;108(suppl 9):58-63. doi:10.1097/01.NAJ.0000336420.34946.3a
5. Rennke S, Nguyen OK, Shoeb MH, Magan Y, Wachter RM, Ranji SR. Hospital-initiated transitional care interventions as a patient safety strategy. Ann Intern Med. 2013;158(5, pt 2):433-440. doi:10.7326/0003-4819-158-5-201303051-00011
6. Moore C, McGinn T, Halm E. Tying up loose ends: discharging patients with unresolved medical issues. Arch Intern Med. 2007;167:1305-1311. doi:10.1001/archinte.167.12.1305
7. Fosnight S, King P, Ewald J, et al. Effects of pharmacy interventions at transitions of care on patient outcomes. Am J Health Syst Pharm. 2020;77(12):943-949. doi:10.1093/ajhp/zxaa081
8. Shull MT, Braitman LE, Stites SD, DeLuca A, Hauser D. Effects of a pharmacist-driven intervention program on hospital readmissions. Am J Health Syst Pharm. 2018;75(9):e221-e230. doi:10.2146/ajhp170287
9. US Department of Health and Human Services, Agency for Healthcare Research and Quality. Plan-Do-Study-Act (PDSA) cycle. February 2015. Accessed June 2, 2022. https://www.ahrq.gov/health-literacy/improve/precautions/tool2b.html10. Horwitz L, Partovian C, Lin Z, et al. Yale New Haven Health Services Corporation/Center for Outcomes Research & Evaluation. Hospital-wide (all-condition) 30-day risk-standardized readmission measure. Updated August 20 2011. Accessed June 2, 2022. chrome-extension://efaidnbmnnnibpcajpcglclefindmkaj/https://www.cms.gov/medicare/quality-initiatives-patient-assessment-instruments/mms/downloads/mmshospital-wideall-conditionreadmissionrate.pdf
Following hospital discharge, patients are often in a vulnerable state due to new medical diagnoses, changes in medications, lack of understanding, and concerns for medical costs. In addition, the discharge process is complex and encompasses decisions regarding the postdischarge site of care, conveying patient instructions, and obtaining supplies and medications. There are several disciplines involved in the transitions of care process that are all essential for ensuring a successful transition and reducing the risk of hospital readmissions. Pharmacists play an integral role in the process.
When pharmacists are provided the opportunity to make therapeutic interventions, medication errors and hospital readmissions decrease and quality of life improves.1 Studies have shown that many older patients return home from the hospital with a limited understanding of their discharge instructions and oftentimes are unable to recall their discharge diagnoses and treatment plan, leaving opportunities for error when patients transition from one level of care to another.2,3 Additionally, high-quality transitional care is especially important for older adults with multiple comorbidities and complex therapeutic regimens as well as for their families and caregivers.4 To prevent hospital readmissions, pharmacists and other health care professionals (HCPs) should work diligently to prevent gaps in care as patients transition between settings. Common factors that lead to increased readmissions include premature discharge, inadequate follow-up, therapeutic errors, and medication-related problems. Furthermore, unintended hospital readmissions are common within the first 30 days following hospital discharge and lead to increased health care costs.2 For these reasons, many health care institutions have developed comprehensive models to improve the discharge process, decrease hospital readmissions, and reduce incidence of adverse events in general medical patients and high-risk populations.5
A study evaluating 693 hospital discharges found that 27.6% of patients were recommended for outpatient workups; however only 9% were actually completed.6 Due to lack of communication regarding discharge summaries, primary care practitioners (PCPs) were unaware of the need for outpatient workups; thus, these patients were lost to follow-up, and appropriate care was not received. Future studies should focus on interventions to improve the quality and dissemination of discharge information to PCPs.6 Fosnight and colleagues assessed a new transitions process focusing on the role of pharmacists. They evaluated medication reconciliations performed and discussed medication adherence barriers, medication recommendations, and time spent performing the interventions.7 After patients received a pharmacy intervention, Fosnight and colleagues reported that readmission rates decreased from 21.0% to 15.3% and mean length of stay decreased from 5.3 to 4.4 days. They also observed greater improvements in patients who received the full pharmacy intervention vs those receiving only parts of the intervention. This study concluded that adding a comprehensive pharmacy intervention to transitions of care resulted in an average of nearly 10 medication recommendations per patient, improved length of stay, and reduced readmission rates. After a review of similar studies, we concluded that a comprehensive discharge model is imperative to improve patient outcomes, along with HCP monitoring of the process to ensure appropriate follow-up.8
At Michael E. DeBakey Veteran Affairs Medical Center (MEDVAMC) in Houston, Texas, 30-day readmissions data were reviewed for veterans 6 months before and 12 months after enrollment in the Home-Based Primary Care (HBPC) service. HBPC is an in-home health care service provided to home-bound veterans with complex health care needs or when routine clinic-based care is not feasible. HBPC programs may differ among various US Department of Veterans Affairs (VA) medical centers. Currently, there are 9 HBPC teams at MEDVAMC and nearly 540 veterans are enrolled in the program. HBPC teams typically consist of PCPs, pharmacists, nurses, psychologists, occupational/physical therapists, social workers, medical support assistants, and dietitians.
Readmissions data are reviewed quarterly by fiscal year (FY) (Table 1). In FY 2019 quarter (Q) 2, the readmission rate before HBPC enrollment was 31% and decreased to 20% after enrollment. In FY 2019 Q3, the readmission rate was 29% before enrollment and decreased to 16% afterward. In FY 2019 Q4, the readmission rate before HBPC enrollment was 28% and decreased to 19% afterward. Although the readmission rates appeared to be decreasing overall, improvements were needed to decrease these rates further and to ensure readmissions were not rising as there was a slight increase in Q4. After reviewing these data, the HBPC service implemented a streamlined hospital discharge process to lower readmission rates and improve patient outcomes.
HBPC at MEDVAMC incorporates a team-based approach and the new streamlined discharge process implemented in 2019 highlights the role of each team member (Figure). Medical support assistants send daily emails of hospital discharges occurring in the last 7 days. Registered nurses are responsible for postdischarge calls within 2 days and home visits within 5 days. Pharmacists perform medication reconciliation within 14 days of discharge, review and/or educate on new medications, and change medications. The PCP is responsible for posthospital calls within 2 days and conducts a home visit within 5 days. Because HBPC programs vary among VA medical centers, the streamlined discharge process discussed may be applicable only to MEDVAMC. The primary objective of this quality improvement project was to identify specific pharmacist interventions to improve the HBPC discharge process and improve hospital readmission rates.
Methods
We conducted a Plan-Do-Study-Act quality improvement project. The first step was to conduct a review of veterans enrolled in HBPC at MEDVAMC.9 Patients included were enrolled in HBPC at MEDVAMC from October 2019 to March 2020 (FY 2020 Q1 and Q2). The Computerized Patient Record System was used to access the patients’ electronic health records. Patient information collected included race, age, sex, admission diagnosis, date of discharge, HBPC pharmacist name, PCP notification on the discharge summary, and 30-day readmission rates. Unplanned return to the hospital within 30 days, which was counted as a readmission, was defined as any admission for acute clinical events that required urgent hospital management.10
Next, we identified specific pharmacist interventions, including medication reconciliation completed by an HBPC pharmacist postdischarge; mean time to contact patients postdischarge; correct medications and supplies on discharge; incorrect dose; incorrect medication frequency or route of administration; therapeutic duplications; discontinuation of medications; additional drug therapy recommendations; laboratory test recommendations; maintenance medications not restarted or omitted; new medication education; and medication or formulation changes.
In the third step, we reviewed discharge summaries and clinical pharmacy notes to collect pharmacist intervention data. These data were analyzed to develop a standardized discharge process. Descriptive statistics were used to represent the results of the study.
Results
Medication reconciliation was completed postdischarge by an HBPC pharmacist in 118 of 175 study patients (67.4%). The mean age of patients was 76 years, about 95% were male (Table 2). There was a wide variety of admission diagnoses but sepsis, chronic obstructive pulmonary disease, and chronic kidney disease were most common. The PCP was notified on the discharge note for 68 (38.9%) patients. The mean time for HBPC pharmacists to contact patients postdischarge was about 3 days, which was much less than the 14 days allowed in the streamlined discharge process.
Pharmacists made the following interventions during medication reconciliation: New medication education was provided for 34 (19.4%) patients and was the largest intervention completed by HBPC pharmacists. Laboratory tests were recommended for 16 (9.1%) patients, medications were discontinued in 14 (8.0%) patients, and additional drug therapy recommendations were made for 7 (4.0%) patients. Medication or formulation changes were completed in 7 (4.0%) patients, incorrect doses were identified in 6 (3.4%) patients, 5 (2.9%) patients were not discharged with the correct medications or supplies, maintenance medications were not restarted in 3 (1.7%) patients, and there were no therapeutic duplications identified. In total, there were 92 (77.9%) patients with interventions compared with the 118 medication reconciliations completed (Table 3).
Process Improvement
As this was a new streamlined discharge process, it was important to assess the progress of the pharmacist role over time. We evaluated the number of medication reconciliations completed by quarter to determine whether more interventions were completed as the streamlined discharge process was being fully implemented. In FY 2020 Q1, medication reconciliation was completed by an HBPC pharmacist at a rate of 35%, and in FY 2020 Q2, at a rate of 65%.
In addition to assessing interventions completed by an HBPC pharmacist, we noted how many medication reconciliations were completed by an inpatient pharmacist as this may have impacted the results of this study. Of the 175 patients in this study, 49 (28%) received a medication reconciliation by an inpatient clinical pharmacy specialist before discharge. Last, when reviewing the readmissions data for the study period, it was evident that the streamlined discharge process was improving. In FY 2020 Q1, the readmissions rate prior to HBPC enrollment was 30% and decreased to 15% after and in FY 2020 Q2 was 31% before and decreased to 13% after HBPC enrollment. Before the study period in FY 2019 Q4, the readmissions rate after HBPC enrollment was 19%. Therefore, the readmissions rate decreased from 19% before the study period to 13% by the end of the study period.
Discussion
A comparison of the readmissions data from FYs 2019, 2020, and 2021 revealed that the newly implemented discharge process at MEDVAMC had been more effective.
There were 92 interventions made during the study period, which is about 78% of all medication reconciliations completed. Medication doses were changed based on patients’ renal function. Additional laboratory tests were recommended after discharge to ensure safety of therapy. Medications were discontinued if inappropriate or if patients were no longer on them to simplify their medication list and limit polypharmacy. New medication education was provided, including drug name, dose, route of administration, time of administration, frequency, indication, mechanism of action, adverse effect profile, monitoring parameters, and more. The HBPC pharmacists were able to make suitable interventions in a timely fashion as the average time to contact patients postdischarge was 3 days.
Areas for Improvement
The PCP was notified on the discharge note only in 68 (38.9%) patients. This could lead to gaps in care if other mechanisms are not in place to notify the PCP of the patient’s discharge. For this reason, it is imperative not only to implement a streamlined discharge process, but to review it and determine methods for continued improvement.9 The streamlined discharge process implemented by the HBPC team highlights when each team member should contact the patient postdischarge. However, it may be beneficial for each team member to have a list of vital information that should be communicated to the patient postdischarge and to other HCPs. For pharmacists, a standardized discharge note template may aid in the consistency of the medication reconciliation process postdischarge and may also increase interventions from pharmacists. For example, only some HBPC pharmacists inserted a new medication template in their discharge follow-up note. In addition, 23 (13.1%) patients were unreachable, and although a complete medication reconciliation was not feasible, a standardized note to review inpatient and outpatient medications along with the discharge plan may still serve as an asset for HCPs.
As the HBPC team continues to improve the discharge process, it is also important to highlight roles of the inpatient team who may assist with a smoother transition. For example, discharge summaries should be clear, complete, and concise, incorporating key elements from the hospital visit. Methods of communication on discharge should be efficient and understood by both inpatient and outpatient teams. Patients’ health literacy status should be considered when providing discharge instructions. Finally, patients should have a clear understanding of who is included in their primary care team should any questions arise. The potential interventions for HCPs highlighted in this study are critical for preventing adverse outcomes, improving patients’ quality of life, and decreasing hospital readmissions. However, implementing the streamlined discharge process was only step 1. Areas of improvement still exist to provide exceptional patient care.
Our goal is to increase pharmacist-led medication reconciliation after discharge to ≥ 80%. This will be assessed monthly after providing education to the HBPC team regarding the study results. The second goal is to maintain hospital readmission rates to ≤ 10%, which will be assessed with each quarterly review.
Strengths and Limitations
This study was one of the first to evaluate the impact of pharmacist intervention on improving patient outcomes in HBPC veterans. Additionally, only 1 investigator conducted the data collection, which decreased the opportunity for errors.
A notable limitation of this study is that the discharge processes may not be able to be duplicated in other HBPC settings due to variability in programs. Additionally, as this was a new discharge process, there were a few aspects that needed to be worked out in the beginning as it was established. Furthermore, this study did not clarify whether a medication reconciliation was conducted by a physician or nurse after discharge; therefore, this study cannot conclude that the medication interventions were solely attributed to pharmacists. Also this study did not assess readmissions for recurrent events only, which may have impacted the results in a different way from the current results that assessed readmission rates for any hospitalization. Other limitations include the retrospective study design at a single center.
Conclusions
This study outlines several opportunities for interventions to improve patient outcomes and aid in decreasing hospital readmission rates. Using the results from this study, education has been provided for the HBPC Service and its readmission committee. Additionally, the safety concerns identified have been addressed with inpatient and outpatient pharmacy leadership to improve the practices in both settings, prevent delays in patient care, and avoid future adverse outcomes. This project highlights the advantages of having pharmacists involved in transitions of care and demonstrates the benefit of HBPC pharmacists’ role in the streamlined discharge process. This project will be reviewed biannually to further improve the discharge process and quality of care for our veterans.
Following hospital discharge, patients are often in a vulnerable state due to new medical diagnoses, changes in medications, lack of understanding, and concerns for medical costs. In addition, the discharge process is complex and encompasses decisions regarding the postdischarge site of care, conveying patient instructions, and obtaining supplies and medications. There are several disciplines involved in the transitions of care process that are all essential for ensuring a successful transition and reducing the risk of hospital readmissions. Pharmacists play an integral role in the process.
When pharmacists are provided the opportunity to make therapeutic interventions, medication errors and hospital readmissions decrease and quality of life improves.1 Studies have shown that many older patients return home from the hospital with a limited understanding of their discharge instructions and oftentimes are unable to recall their discharge diagnoses and treatment plan, leaving opportunities for error when patients transition from one level of care to another.2,3 Additionally, high-quality transitional care is especially important for older adults with multiple comorbidities and complex therapeutic regimens as well as for their families and caregivers.4 To prevent hospital readmissions, pharmacists and other health care professionals (HCPs) should work diligently to prevent gaps in care as patients transition between settings. Common factors that lead to increased readmissions include premature discharge, inadequate follow-up, therapeutic errors, and medication-related problems. Furthermore, unintended hospital readmissions are common within the first 30 days following hospital discharge and lead to increased health care costs.2 For these reasons, many health care institutions have developed comprehensive models to improve the discharge process, decrease hospital readmissions, and reduce incidence of adverse events in general medical patients and high-risk populations.5
A study evaluating 693 hospital discharges found that 27.6% of patients were recommended for outpatient workups; however only 9% were actually completed.6 Due to lack of communication regarding discharge summaries, primary care practitioners (PCPs) were unaware of the need for outpatient workups; thus, these patients were lost to follow-up, and appropriate care was not received. Future studies should focus on interventions to improve the quality and dissemination of discharge information to PCPs.6 Fosnight and colleagues assessed a new transitions process focusing on the role of pharmacists. They evaluated medication reconciliations performed and discussed medication adherence barriers, medication recommendations, and time spent performing the interventions.7 After patients received a pharmacy intervention, Fosnight and colleagues reported that readmission rates decreased from 21.0% to 15.3% and mean length of stay decreased from 5.3 to 4.4 days. They also observed greater improvements in patients who received the full pharmacy intervention vs those receiving only parts of the intervention. This study concluded that adding a comprehensive pharmacy intervention to transitions of care resulted in an average of nearly 10 medication recommendations per patient, improved length of stay, and reduced readmission rates. After a review of similar studies, we concluded that a comprehensive discharge model is imperative to improve patient outcomes, along with HCP monitoring of the process to ensure appropriate follow-up.8
At Michael E. DeBakey Veteran Affairs Medical Center (MEDVAMC) in Houston, Texas, 30-day readmissions data were reviewed for veterans 6 months before and 12 months after enrollment in the Home-Based Primary Care (HBPC) service. HBPC is an in-home health care service provided to home-bound veterans with complex health care needs or when routine clinic-based care is not feasible. HBPC programs may differ among various US Department of Veterans Affairs (VA) medical centers. Currently, there are 9 HBPC teams at MEDVAMC and nearly 540 veterans are enrolled in the program. HBPC teams typically consist of PCPs, pharmacists, nurses, psychologists, occupational/physical therapists, social workers, medical support assistants, and dietitians.
Readmissions data are reviewed quarterly by fiscal year (FY) (Table 1). In FY 2019 quarter (Q) 2, the readmission rate before HBPC enrollment was 31% and decreased to 20% after enrollment. In FY 2019 Q3, the readmission rate was 29% before enrollment and decreased to 16% afterward. In FY 2019 Q4, the readmission rate before HBPC enrollment was 28% and decreased to 19% afterward. Although the readmission rates appeared to be decreasing overall, improvements were needed to decrease these rates further and to ensure readmissions were not rising as there was a slight increase in Q4. After reviewing these data, the HBPC service implemented a streamlined hospital discharge process to lower readmission rates and improve patient outcomes.
HBPC at MEDVAMC incorporates a team-based approach and the new streamlined discharge process implemented in 2019 highlights the role of each team member (Figure). Medical support assistants send daily emails of hospital discharges occurring in the last 7 days. Registered nurses are responsible for postdischarge calls within 2 days and home visits within 5 days. Pharmacists perform medication reconciliation within 14 days of discharge, review and/or educate on new medications, and change medications. The PCP is responsible for posthospital calls within 2 days and conducts a home visit within 5 days. Because HBPC programs vary among VA medical centers, the streamlined discharge process discussed may be applicable only to MEDVAMC. The primary objective of this quality improvement project was to identify specific pharmacist interventions to improve the HBPC discharge process and improve hospital readmission rates.
Methods
We conducted a Plan-Do-Study-Act quality improvement project. The first step was to conduct a review of veterans enrolled in HBPC at MEDVAMC.9 Patients included were enrolled in HBPC at MEDVAMC from October 2019 to March 2020 (FY 2020 Q1 and Q2). The Computerized Patient Record System was used to access the patients’ electronic health records. Patient information collected included race, age, sex, admission diagnosis, date of discharge, HBPC pharmacist name, PCP notification on the discharge summary, and 30-day readmission rates. Unplanned return to the hospital within 30 days, which was counted as a readmission, was defined as any admission for acute clinical events that required urgent hospital management.10
Next, we identified specific pharmacist interventions, including medication reconciliation completed by an HBPC pharmacist postdischarge; mean time to contact patients postdischarge; correct medications and supplies on discharge; incorrect dose; incorrect medication frequency or route of administration; therapeutic duplications; discontinuation of medications; additional drug therapy recommendations; laboratory test recommendations; maintenance medications not restarted or omitted; new medication education; and medication or formulation changes.
In the third step, we reviewed discharge summaries and clinical pharmacy notes to collect pharmacist intervention data. These data were analyzed to develop a standardized discharge process. Descriptive statistics were used to represent the results of the study.
Results
Medication reconciliation was completed postdischarge by an HBPC pharmacist in 118 of 175 study patients (67.4%). The mean age of patients was 76 years, about 95% were male (Table 2). There was a wide variety of admission diagnoses but sepsis, chronic obstructive pulmonary disease, and chronic kidney disease were most common. The PCP was notified on the discharge note for 68 (38.9%) patients. The mean time for HBPC pharmacists to contact patients postdischarge was about 3 days, which was much less than the 14 days allowed in the streamlined discharge process.
Pharmacists made the following interventions during medication reconciliation: New medication education was provided for 34 (19.4%) patients and was the largest intervention completed by HBPC pharmacists. Laboratory tests were recommended for 16 (9.1%) patients, medications were discontinued in 14 (8.0%) patients, and additional drug therapy recommendations were made for 7 (4.0%) patients. Medication or formulation changes were completed in 7 (4.0%) patients, incorrect doses were identified in 6 (3.4%) patients, 5 (2.9%) patients were not discharged with the correct medications or supplies, maintenance medications were not restarted in 3 (1.7%) patients, and there were no therapeutic duplications identified. In total, there were 92 (77.9%) patients with interventions compared with the 118 medication reconciliations completed (Table 3).
Process Improvement
As this was a new streamlined discharge process, it was important to assess the progress of the pharmacist role over time. We evaluated the number of medication reconciliations completed by quarter to determine whether more interventions were completed as the streamlined discharge process was being fully implemented. In FY 2020 Q1, medication reconciliation was completed by an HBPC pharmacist at a rate of 35%, and in FY 2020 Q2, at a rate of 65%.
In addition to assessing interventions completed by an HBPC pharmacist, we noted how many medication reconciliations were completed by an inpatient pharmacist as this may have impacted the results of this study. Of the 175 patients in this study, 49 (28%) received a medication reconciliation by an inpatient clinical pharmacy specialist before discharge. Last, when reviewing the readmissions data for the study period, it was evident that the streamlined discharge process was improving. In FY 2020 Q1, the readmissions rate prior to HBPC enrollment was 30% and decreased to 15% after and in FY 2020 Q2 was 31% before and decreased to 13% after HBPC enrollment. Before the study period in FY 2019 Q4, the readmissions rate after HBPC enrollment was 19%. Therefore, the readmissions rate decreased from 19% before the study period to 13% by the end of the study period.
Discussion
A comparison of the readmissions data from FYs 2019, 2020, and 2021 revealed that the newly implemented discharge process at MEDVAMC had been more effective.
There were 92 interventions made during the study period, which is about 78% of all medication reconciliations completed. Medication doses were changed based on patients’ renal function. Additional laboratory tests were recommended after discharge to ensure safety of therapy. Medications were discontinued if inappropriate or if patients were no longer on them to simplify their medication list and limit polypharmacy. New medication education was provided, including drug name, dose, route of administration, time of administration, frequency, indication, mechanism of action, adverse effect profile, monitoring parameters, and more. The HBPC pharmacists were able to make suitable interventions in a timely fashion as the average time to contact patients postdischarge was 3 days.
Areas for Improvement
The PCP was notified on the discharge note only in 68 (38.9%) patients. This could lead to gaps in care if other mechanisms are not in place to notify the PCP of the patient’s discharge. For this reason, it is imperative not only to implement a streamlined discharge process, but to review it and determine methods for continued improvement.9 The streamlined discharge process implemented by the HBPC team highlights when each team member should contact the patient postdischarge. However, it may be beneficial for each team member to have a list of vital information that should be communicated to the patient postdischarge and to other HCPs. For pharmacists, a standardized discharge note template may aid in the consistency of the medication reconciliation process postdischarge and may also increase interventions from pharmacists. For example, only some HBPC pharmacists inserted a new medication template in their discharge follow-up note. In addition, 23 (13.1%) patients were unreachable, and although a complete medication reconciliation was not feasible, a standardized note to review inpatient and outpatient medications along with the discharge plan may still serve as an asset for HCPs.
As the HBPC team continues to improve the discharge process, it is also important to highlight roles of the inpatient team who may assist with a smoother transition. For example, discharge summaries should be clear, complete, and concise, incorporating key elements from the hospital visit. Methods of communication on discharge should be efficient and understood by both inpatient and outpatient teams. Patients’ health literacy status should be considered when providing discharge instructions. Finally, patients should have a clear understanding of who is included in their primary care team should any questions arise. The potential interventions for HCPs highlighted in this study are critical for preventing adverse outcomes, improving patients’ quality of life, and decreasing hospital readmissions. However, implementing the streamlined discharge process was only step 1. Areas of improvement still exist to provide exceptional patient care.
Our goal is to increase pharmacist-led medication reconciliation after discharge to ≥ 80%. This will be assessed monthly after providing education to the HBPC team regarding the study results. The second goal is to maintain hospital readmission rates to ≤ 10%, which will be assessed with each quarterly review.
Strengths and Limitations
This study was one of the first to evaluate the impact of pharmacist intervention on improving patient outcomes in HBPC veterans. Additionally, only 1 investigator conducted the data collection, which decreased the opportunity for errors.
A notable limitation of this study is that the discharge processes may not be able to be duplicated in other HBPC settings due to variability in programs. Additionally, as this was a new discharge process, there were a few aspects that needed to be worked out in the beginning as it was established. Furthermore, this study did not clarify whether a medication reconciliation was conducted by a physician or nurse after discharge; therefore, this study cannot conclude that the medication interventions were solely attributed to pharmacists. Also this study did not assess readmissions for recurrent events only, which may have impacted the results in a different way from the current results that assessed readmission rates for any hospitalization. Other limitations include the retrospective study design at a single center.
Conclusions
This study outlines several opportunities for interventions to improve patient outcomes and aid in decreasing hospital readmission rates. Using the results from this study, education has been provided for the HBPC Service and its readmission committee. Additionally, the safety concerns identified have been addressed with inpatient and outpatient pharmacy leadership to improve the practices in both settings, prevent delays in patient care, and avoid future adverse outcomes. This project highlights the advantages of having pharmacists involved in transitions of care and demonstrates the benefit of HBPC pharmacists’ role in the streamlined discharge process. This project will be reviewed biannually to further improve the discharge process and quality of care for our veterans.
1. Coleman EA, Chugh A, Williams MV, et al. Understanding and execution of discharge instructions. Am J Med Qual. 2013;28(5):383-391. doi:10.1177/1062860612472931
2. Hume AL, Kirwin J, Bieber HL, et al. Improving care transitions: current practice and future opportunities for pharmacists. Pharmacotherapy. 2012;32(11):e326-e337. doi:10.1002/phar.1215
3. Milfred-LaForest SK, Gee JA, Pugacz AM, et al. Heart failure transitions of care: a pharmacist-led post discharge pilot experience. Prog Cardiovasc Dis. 2017;60(2):249-258. doi:10.1016/j.pcad.2017.08.005
4. Naylor M, Keating SA. Transitional care: moving patients from one care setting to another. Am J Nurs. 2008;108(suppl 9):58-63. doi:10.1097/01.NAJ.0000336420.34946.3a
5. Rennke S, Nguyen OK, Shoeb MH, Magan Y, Wachter RM, Ranji SR. Hospital-initiated transitional care interventions as a patient safety strategy. Ann Intern Med. 2013;158(5, pt 2):433-440. doi:10.7326/0003-4819-158-5-201303051-00011
6. Moore C, McGinn T, Halm E. Tying up loose ends: discharging patients with unresolved medical issues. Arch Intern Med. 2007;167:1305-1311. doi:10.1001/archinte.167.12.1305
7. Fosnight S, King P, Ewald J, et al. Effects of pharmacy interventions at transitions of care on patient outcomes. Am J Health Syst Pharm. 2020;77(12):943-949. doi:10.1093/ajhp/zxaa081
8. Shull MT, Braitman LE, Stites SD, DeLuca A, Hauser D. Effects of a pharmacist-driven intervention program on hospital readmissions. Am J Health Syst Pharm. 2018;75(9):e221-e230. doi:10.2146/ajhp170287
9. US Department of Health and Human Services, Agency for Healthcare Research and Quality. Plan-Do-Study-Act (PDSA) cycle. February 2015. Accessed June 2, 2022. https://www.ahrq.gov/health-literacy/improve/precautions/tool2b.html10. Horwitz L, Partovian C, Lin Z, et al. Yale New Haven Health Services Corporation/Center for Outcomes Research & Evaluation. Hospital-wide (all-condition) 30-day risk-standardized readmission measure. Updated August 20 2011. Accessed June 2, 2022. chrome-extension://efaidnbmnnnibpcajpcglclefindmkaj/https://www.cms.gov/medicare/quality-initiatives-patient-assessment-instruments/mms/downloads/mmshospital-wideall-conditionreadmissionrate.pdf
1. Coleman EA, Chugh A, Williams MV, et al. Understanding and execution of discharge instructions. Am J Med Qual. 2013;28(5):383-391. doi:10.1177/1062860612472931
2. Hume AL, Kirwin J, Bieber HL, et al. Improving care transitions: current practice and future opportunities for pharmacists. Pharmacotherapy. 2012;32(11):e326-e337. doi:10.1002/phar.1215
3. Milfred-LaForest SK, Gee JA, Pugacz AM, et al. Heart failure transitions of care: a pharmacist-led post discharge pilot experience. Prog Cardiovasc Dis. 2017;60(2):249-258. doi:10.1016/j.pcad.2017.08.005
4. Naylor M, Keating SA. Transitional care: moving patients from one care setting to another. Am J Nurs. 2008;108(suppl 9):58-63. doi:10.1097/01.NAJ.0000336420.34946.3a
5. Rennke S, Nguyen OK, Shoeb MH, Magan Y, Wachter RM, Ranji SR. Hospital-initiated transitional care interventions as a patient safety strategy. Ann Intern Med. 2013;158(5, pt 2):433-440. doi:10.7326/0003-4819-158-5-201303051-00011
6. Moore C, McGinn T, Halm E. Tying up loose ends: discharging patients with unresolved medical issues. Arch Intern Med. 2007;167:1305-1311. doi:10.1001/archinte.167.12.1305
7. Fosnight S, King P, Ewald J, et al. Effects of pharmacy interventions at transitions of care on patient outcomes. Am J Health Syst Pharm. 2020;77(12):943-949. doi:10.1093/ajhp/zxaa081
8. Shull MT, Braitman LE, Stites SD, DeLuca A, Hauser D. Effects of a pharmacist-driven intervention program on hospital readmissions. Am J Health Syst Pharm. 2018;75(9):e221-e230. doi:10.2146/ajhp170287
9. US Department of Health and Human Services, Agency for Healthcare Research and Quality. Plan-Do-Study-Act (PDSA) cycle. February 2015. Accessed June 2, 2022. https://www.ahrq.gov/health-literacy/improve/precautions/tool2b.html10. Horwitz L, Partovian C, Lin Z, et al. Yale New Haven Health Services Corporation/Center for Outcomes Research & Evaluation. Hospital-wide (all-condition) 30-day risk-standardized readmission measure. Updated August 20 2011. Accessed June 2, 2022. chrome-extension://efaidnbmnnnibpcajpcglclefindmkaj/https://www.cms.gov/medicare/quality-initiatives-patient-assessment-instruments/mms/downloads/mmshospital-wideall-conditionreadmissionrate.pdf
A Learning Health System Approach to Long COVID Care
The Veterans Health Administration (VHA)—along with systems across the world—has spent the past 2 years continuously adapting to meet the emerging needs of persons infected with COVID-19. With the development of effective vaccines and global efforts to mitigate transmission, attention has now shifted to long COVID care as the need for further outpatient health care becomes increasingly apparent.1,2
Background
Multiple terms describe the lingering, multisystem sequelae of COVID-19 that last longer than 4 weeks: long COVID, postacute COVID-19 syndrome, post-COVID condition, postacute sequalae of COVID-19, and COVID long hauler.1,3 Common symptoms include fatigue, shortness of breath, cough, sleep disorders, brain fog or cognitive dysfunction, depression, anxiety, pain, and changes in taste or smell that impact a person’s functioning.4,5 The multisystem nature of the postacute course of COVID-19 necessitates an interdisciplinary approach to devise comprehensive and individualized care plans.6-9 Research is needed to better understand this postacute state (eg, prevalence, underlying effects, characteristics of those who experience long COVID) to establish and evaluate cost-effective treatment approaches.
Many patients who are experiencing symptoms beyond the acute course of COVID-19 have been referred to general outpatient clinics or home health, which may lack the capacity and knowledge of this novel disease to effectively manage complex long COVID cases.2,3 To address this growing need, clinicians and leadership across a variety of disciplines and settings in the VHA created a community of practice (CoP) to create a mechanism for cross-facility communication, identify gaps in long COVID care and research, and cocreate knowledge on best practices for care delivery.
In this spirit, we are embracing a learning health system (LHS) approach that uses rapid-cycle methods to integrate data and real-world experience to iteratively evaluate and adapt models of long COVID care.10 Our clinically identified and data-driven objective is to provide high value health care to patients with long COVID sequalae by creating a framework to learn about this novel condition and develop innovative care models. This article provides an overview of our emerging LHS approach to the study of long COVID care that is fostering innovation and adaptability within the VHA. We describe 3 aspects of our engagement approach central to LHS: the ongoing development of a long COVID CoP dedicated to iteratively informing the bidirectional cycle of data from practice to research, results of a broad environmental scan of VHA long COVID care, and results of a survey administered to CoP members to inform ongoing needs of the community and identify early successful outcomes from participation.
Learning Health System Approach
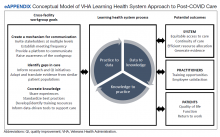
The VHA is one of the largest integrated health care systems in the United States serving more than 9 million veterans.11 Since 2017, the VHA has articulated a vision to become an LHS that informs and improves patient-centered care through practice-based and data-driven research (eAppendix).12 During the early COVID-19 pandemic, an LHS approach in the VHA was critical to rapidly establishing a data infrastructure for disease surveillance, coordinating data-driven solutions, leveraging use of technology, collaborating across the globe to identify best practices, and implementing systematic responses (eg, policies, workforce adjustments).
Our long COVID CoP was developed as clinical observations and ongoing conversations with stakeholders (eg, veterans, health care practitioners [HCPs], leadership) identified a need to effectively identify and treat the growing number of veterans with long COVID. This clinical issue is compounded by the limited but emerging evidence on the clinical presentation of prolonged COVID-19 symptoms, treatment, and subsequent care pathways. The VHA’s efforts and lessons learned within the lens of an LHS are applicable to other systems confronting the complex identification and management of patients with persistent and encumbering long COVID symptoms. The VHA is building upon the LHS approach to proactively prepare for and address future clinical or public health challenges that require cross-system and sector collaborations, expediency, inclusivity, and patient/family centeredness.11
Community of Practice
As of January 25, 2022, our workgroup consisted of 128 VHA employees representing 29 VHA medical centers. Members of the multidisciplinary workgroup have diverse backgrounds with HCPs from primary care (eg, physicians, nurse practitioners), rehabilitation (eg, physical therapists), specialty care (eg, pulmonologists, physiatrists), mental health (eg, psychologists), and complementary and integrated health/Whole Health services (eg, practitoners of services such as yoga, tai chi, mindfulness, acupuncture). Members also include clinical, operations, and research leadership at local, regional, and national VHA levels. Our first objective as a large, diverse group was to establish shared goals, which included: (1) determining efficient communication pathways; (2) identifying gaps in care or research; and (3) cocreating knowledge to provide solutions to identified gaps.
Communication Mechanisms
Our first goal was to create an efficient mechanism for cross-facility communication. The initial CoP was formed in April 2021 and the first virtual meeting focused on reaching a consensus regarding the best way to communicate and proceed. We agreed to convene weekly at a consistent time, created a standard agenda template, and elected a lead facilitator of meeting proceedings. In addition, a member of the CoP recorded and took extensive meeting notes, which were later distributed to the entire CoP to accommodate varying schedules and ability to attend live meetings. Approximately 20 to 30 participants attend the meetings in real-time.
To consolidate working documents, information, and resources in one location, we created a platform to communicate via a Microsoft Teams channel. All CoP members are given access to the folders and allowed to add to the growing library of resources. Resources include clinical assessment and note templates for electronic documentation of care, site-specific process maps, relevant literature on screening and interventions identified by practice members, and meeting notes along with the recordings. A chat feature alerts CoP members to questions posed by other members. Any resources or information shared on the chat discussion are curated by CoP leaders to disseminate to all members. Importantly, this platform allowed us to communicate efficiently within the VHA organization by creating a centralized space for documents and the ability to correspond with all or select members of the CoP. Additional VHA employees can easily be referred and request access.
To increase awareness of the CoP, expand reach, and diversify perspectives, every participant was encouraged to invite colleagues and stakeholders with interest or experience in long COVID care to join. While patients are not included in this CoP, we are working closely with the VHA user experience workgroup (many members overlap) that is gathering patient and caregiver perspectives on their COVID-19 experience and long COVID care. Concurrently, CoP members and leadership facilitate communication and set up formal collaborations with other non-VHA health care systems to create an intersystem network of collaboration for long COVID care. This approach further enhances the speed at which we can work together to share lessons learned and stay up-to-date on emerging evidence surrounding long COVID care.
Identifying Gaps in Care and Research
Our second goal was to identify gaps in care or knowledge to inform future research and quality improvement initiatives, while also creating a foundation to cocreate knowledge about safe, effective care management of the novel long COVID sequelae. To translate knowledge, we must first identify and understand the gaps between the current, best available evidence and current care practices or policies impacting that delivery.13 As such, the structured meeting agenda and facilitated meeting discussions focused on understanding current clinical decision making and the evidence base. We shared VHA evidence synthesis reports and living rapid reviews on complications following COVID-19 illness (ie, major organ damage and posthospitalization health care use) that provided an objective evidence base on common long COVID complications.14,15
Since long COVID is a novel condition, we drew from literature in similar patient populations and translated that information in the context of our current knowledge of this unique syndrome. For example, we discussed the predominant and persistent symptom of fatigue post-COVID.5 In particular, the CoP discussed challenges in identifying and treating post-COVID fatigue, which is often a vague symptom with multiple or interacting etiologies that require a comprehensive, interdisciplinary approach. As such, we reviewed, adapted, and translated identification and treatment strategies from the literature on chronic fatigue syndrome to patients with post-COVID syndrome.16,17 We continue to work collaboratively and engage the appropriate stakeholders to provide input on the gaps to prioritize targeting.
Cocreate Knowledge
Our third goal was to cocreate knowledge regarding the care of patients with long COVID. To accomplish this, our structured meetings and communication pathways invited members to share experiences on the who (delivers and receives care), what (type of care or HCPs), when (identification of post-COVID and access), and how (eg, telehealth) of care to patients post-COVID. As part of the workgroup, we identified and shared resources on standardized, facility-level practices to reduce variability across the VHA system. These resources included intake/assessment forms, care processes, and batteries of tests/measures used for screening and assessment. The knowledge obtained from outside the CoP and cocreated within is being used to inform data-driven tools to support and evaluate care for patients with long COVID. As such, members of the workgroup are in the formative stages of participating in quality improvement innovation pilots to test technologies and processes designed to improve and validate long COVID care pathways. These technologies include screening tools, clinical decision support tools, and population health management technologies. In addition, we are developing a formal collaboration with the VHA Office of Research and Development to create standardized intake forms across VHA long COVID clinics to facilitate both clinical monitoring and research.
Surveys
The US Department of Veterans Affairs Central Office collaborated with our workgroup to draft an initial set of survey questions designed to understand how each VHA facility defines, identifies, and provides care to veterans experiencing post-COVID sequalae. The 41-question survey was distributed through regional directors and chief medical officers at 139 VHA facilities in August 2021. One hundred nineteen responses (86%) were received. Sixteen facilities indicated they had established programs and 26 facilities were considering a program. Our CoP had representation from the 16 facilities with established programs indicating the deep and well-connected nature of our grassroots efforts to bring together stakeholders to learn as part of a CoP.
A separate, follow-up survey generated responses from 18 facilities and identified the need to capture evolving innovations and to develop smaller workstreams (eg, best practices, electronic documentation templates, pathway for referrals, veteran engagement, outcome measures). The survey not only exposed ongoing challenges to providing long COVID care, but importantly, outlined the ways in which CoP members were leveraging community knowledge and resources to inform innovations and processes of care changes at their specific sites. Fourteen of 18 facilities with long COVID programs in place explicitly identified the CoP as a resource they have found most beneficial when employing such innovations. Specific innovations reported included changes in care delivery, engagement in active outreach with veterans and local facility, and infrastructure development to sustain local long COVID clinics (Table).
Future Directions
Our CoP strives to contribute to an evidence base for long COVID care. At the system level, the CoP has the potential to impact access and continuity of care by identifying appropriate processes and ensuring that VHA patients receive outreach and an opportunity for post-COVID care. Comprehensive care requires input from HCP, clinical leadership, and operations levels. In this sense, our CoP provides an opportunity for diverse stakeholders to come together, discuss barriers to screening and delivering post-COVID care, and create an action plan to remove or lessen such barriers.18 Part of the process to remove barriers is to identify and support efficient resource allocation. Our CoP has worked to address issues in resource allocation (eg, space, personnel) for post-COVID care. For example, one facility is currently implementing interdisciplinary virtual post-COVID care. Another facility identified and restructured working assignments for psychologists who served in different capacities throughout the system to fill the need within the long COVID team.
At the HCP level, the CoP is currently developing workshops, media campaigns, written clinical resources, skills training, publications, and webinars/seminars with continuing medical education credits.19 The CoP may also provide learning and growth opportunities, such as clinical or VHA operational fellowships and research grants.
We are still in the formative stages of post-COVID care and future efforts will explore patient-centered outcomes. We are drawing on the Centers for Disease Control and Prevention’s guidance for evaluating patients with long COVID symptoms and examining the feasibility within VHA, as well as patient perspectives on post-COVID sequalae, to ensure we are selecting assessments that measure patient-centered constructs.18
Conclusions
A VHA-wide LHS approach is identifying issues related to the identification, delivery, and evaluation of long COVID care. This long COVID CoP has developed an infrastructure for communication, identified gaps in care, and cocreated knowledge related to best current practices for post-COVID care. This work is contributing to systemwide LHS efforts dedicated to creating a culture of quality care and innovation and is a process that is transferrable to other areas of care in the VHA, as well as other health care systems. The LHS approach continues to be highly relevant as we persist through the COVID-19 pandemic and reimagine a postpandemic world.
Acknowledg
We thank all the members of the Veterans Health Administration long COVID Community of Practice who participate in the meetings and contribute to the sharing and spread of knowledge.
1. Sivan M, Halpin S, Hollingworth L, Snook N, Hickman K, Clifton I. Development of an integrated rehabilitation pathway for individuals recovering from COVID-19 in the community. J Rehabil Med. 2020;52(8):jrm00089. doi:10.2340/16501977-2727
2. Understanding the long-term health effects of COVID-19. EClinicalMedicine. 2020;26:100586. doi:10.1016/j.eclinm.2020.100586
3. Greenhalgh T, Knight M, A’Court C, Buxton M, Husain L. Management of post-acute covid-19 in primary care. BMJ. Published online August 11, 2020:m3026. doi:10.1136/bmj.m3026
4. Iwua CJ, Iwu CD, Wiysonge CS. The occurrence of long COVID: a rapid review. Pan Afr Med J. 2021;38. doi:10.11604/pamj.2021.38.65.27366
5. Carfì A, Bernabei R, Landi F; Gemelli Against COVID-19 Post-Acute Care Study Group. Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324(6):603-605. doi:10.1001/jama.2020.12603
6. Gemelli Against COVID-19 Post-Acute Care Study Group. Post-COVID-19 global health strategies: the need for an interdisciplinary approach. Aging Clin Exp Res. 2020;32(8):1613-1620. doi:10.1007/s40520-020-01616-x
7. Xie Y, Xu E, Bowe B, Al-Aly Z. Long-term cardiovascular outcomes of COVID-19. Nat Med. 2022;28:583-590. doi:10.1038/s41591-022-01689-3
8. Al-Aly Z, Xie Y, Bowe B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature. 2021;594:259-264. doi:10.1038/s41586-021-03553-9
9. Ayoubkhani D, Bermingham C, Pouwels KB, et al. Trajectory of long covid symptoms after covid-19 vaccination: community based cohort study. BMJ. 2022;377:e069676. doi:10.1136/bmj-2021-069676
10. Institute of Medicine (US) Roundtable on Evidence-Based Medicine, Olsen L, Aisner D, McGinnis JM, eds. The Learning Healthcare System: Workshop Summary. Washington (DC): National Academies Press (US); 2007. doi:10.17226/11903
11. Romanelli RJ, Azar KMJ, Sudat S, Hung D, Frosch DL, Pressman AR. Learning health system in crisis: lessons from the COVID-19 pandemic. Mayo Clin Proc Innov Qual Outcomes. 2021;5(1):171-176. doi:10.1016/j.mayocpiqo.2020.10.004
12. Atkins D, Kilbourne AM, Shulkin D. Moving from discovery to system-wide change: the role of research in a learning health care system: experience from three decades of health systems research in the Veterans Health Administration. Annu Rev Public Health. 2017;38:467-487. doi:10.1146/annurev-publhealth-031816-044255
13. Kitson A, Straus SE. The knowledge-to-action cycle: identifying the gaps. CMAJ. 2010;182(2):E73-77. doi:10.1503/cmaj.081231
14. Greer N, Bart B, Billington C, et al. COVID-19 post-acute care major organ damage: a living rapid review. Updated September 2021. Accessed May 31, 2022. https://www.hsrd.research.va.gov/publications/esp/covid-organ-damage.pdf
15. Sharpe JA, Burke C, Gordon AM, et al. COVID-19 post-hospitalization health care utilization: a living review. Updated February 2022. Accessed May 31, 2022. https://www.hsrd.research.va.gov/publications/esp/covid19-post-hosp.pdf
16. Bested AC, Marshall LM. Review of Myalgic Encephalomyelitis/chronic fatigue syndrome: an evidence-based approach to diagnosis and management by clinicians. Rev Environ Health. 2015;30(4):223-249. doi:10.1515/reveh-2015-0026
17. Yancey JR, Thomas SM. Chronic fatigue syndrome: diagnosis and treatment. Am Fam Physician. 2012;86(8):741-746.
18. Kotter JP, Cohen DS. Change Leadership The Kotter Collection. Harvard Business Review Press; 2014.
19. Brownson RC, Eyler AA, Harris JK, Moore JB, Tabak RG. Getting the word out: new approaches for disseminating public health science. J Public Health Manag Pract. 2018;24(2):102-111. doi:10.1097/PHH.0000000000000673
The Veterans Health Administration (VHA)—along with systems across the world—has spent the past 2 years continuously adapting to meet the emerging needs of persons infected with COVID-19. With the development of effective vaccines and global efforts to mitigate transmission, attention has now shifted to long COVID care as the need for further outpatient health care becomes increasingly apparent.1,2
Background
Multiple terms describe the lingering, multisystem sequelae of COVID-19 that last longer than 4 weeks: long COVID, postacute COVID-19 syndrome, post-COVID condition, postacute sequalae of COVID-19, and COVID long hauler.1,3 Common symptoms include fatigue, shortness of breath, cough, sleep disorders, brain fog or cognitive dysfunction, depression, anxiety, pain, and changes in taste or smell that impact a person’s functioning.4,5 The multisystem nature of the postacute course of COVID-19 necessitates an interdisciplinary approach to devise comprehensive and individualized care plans.6-9 Research is needed to better understand this postacute state (eg, prevalence, underlying effects, characteristics of those who experience long COVID) to establish and evaluate cost-effective treatment approaches.
Many patients who are experiencing symptoms beyond the acute course of COVID-19 have been referred to general outpatient clinics or home health, which may lack the capacity and knowledge of this novel disease to effectively manage complex long COVID cases.2,3 To address this growing need, clinicians and leadership across a variety of disciplines and settings in the VHA created a community of practice (CoP) to create a mechanism for cross-facility communication, identify gaps in long COVID care and research, and cocreate knowledge on best practices for care delivery.
In this spirit, we are embracing a learning health system (LHS) approach that uses rapid-cycle methods to integrate data and real-world experience to iteratively evaluate and adapt models of long COVID care.10 Our clinically identified and data-driven objective is to provide high value health care to patients with long COVID sequalae by creating a framework to learn about this novel condition and develop innovative care models. This article provides an overview of our emerging LHS approach to the study of long COVID care that is fostering innovation and adaptability within the VHA. We describe 3 aspects of our engagement approach central to LHS: the ongoing development of a long COVID CoP dedicated to iteratively informing the bidirectional cycle of data from practice to research, results of a broad environmental scan of VHA long COVID care, and results of a survey administered to CoP members to inform ongoing needs of the community and identify early successful outcomes from participation.
Learning Health System Approach
The VHA is one of the largest integrated health care systems in the United States serving more than 9 million veterans.11 Since 2017, the VHA has articulated a vision to become an LHS that informs and improves patient-centered care through practice-based and data-driven research (eAppendix).12 During the early COVID-19 pandemic, an LHS approach in the VHA was critical to rapidly establishing a data infrastructure for disease surveillance, coordinating data-driven solutions, leveraging use of technology, collaborating across the globe to identify best practices, and implementing systematic responses (eg, policies, workforce adjustments).
Our long COVID CoP was developed as clinical observations and ongoing conversations with stakeholders (eg, veterans, health care practitioners [HCPs], leadership) identified a need to effectively identify and treat the growing number of veterans with long COVID. This clinical issue is compounded by the limited but emerging evidence on the clinical presentation of prolonged COVID-19 symptoms, treatment, and subsequent care pathways. The VHA’s efforts and lessons learned within the lens of an LHS are applicable to other systems confronting the complex identification and management of patients with persistent and encumbering long COVID symptoms. The VHA is building upon the LHS approach to proactively prepare for and address future clinical or public health challenges that require cross-system and sector collaborations, expediency, inclusivity, and patient/family centeredness.11
Community of Practice
As of January 25, 2022, our workgroup consisted of 128 VHA employees representing 29 VHA medical centers. Members of the multidisciplinary workgroup have diverse backgrounds with HCPs from primary care (eg, physicians, nurse practitioners), rehabilitation (eg, physical therapists), specialty care (eg, pulmonologists, physiatrists), mental health (eg, psychologists), and complementary and integrated health/Whole Health services (eg, practitoners of services such as yoga, tai chi, mindfulness, acupuncture). Members also include clinical, operations, and research leadership at local, regional, and national VHA levels. Our first objective as a large, diverse group was to establish shared goals, which included: (1) determining efficient communication pathways; (2) identifying gaps in care or research; and (3) cocreating knowledge to provide solutions to identified gaps.
Communication Mechanisms
Our first goal was to create an efficient mechanism for cross-facility communication. The initial CoP was formed in April 2021 and the first virtual meeting focused on reaching a consensus regarding the best way to communicate and proceed. We agreed to convene weekly at a consistent time, created a standard agenda template, and elected a lead facilitator of meeting proceedings. In addition, a member of the CoP recorded and took extensive meeting notes, which were later distributed to the entire CoP to accommodate varying schedules and ability to attend live meetings. Approximately 20 to 30 participants attend the meetings in real-time.
To consolidate working documents, information, and resources in one location, we created a platform to communicate via a Microsoft Teams channel. All CoP members are given access to the folders and allowed to add to the growing library of resources. Resources include clinical assessment and note templates for electronic documentation of care, site-specific process maps, relevant literature on screening and interventions identified by practice members, and meeting notes along with the recordings. A chat feature alerts CoP members to questions posed by other members. Any resources or information shared on the chat discussion are curated by CoP leaders to disseminate to all members. Importantly, this platform allowed us to communicate efficiently within the VHA organization by creating a centralized space for documents and the ability to correspond with all or select members of the CoP. Additional VHA employees can easily be referred and request access.
To increase awareness of the CoP, expand reach, and diversify perspectives, every participant was encouraged to invite colleagues and stakeholders with interest or experience in long COVID care to join. While patients are not included in this CoP, we are working closely with the VHA user experience workgroup (many members overlap) that is gathering patient and caregiver perspectives on their COVID-19 experience and long COVID care. Concurrently, CoP members and leadership facilitate communication and set up formal collaborations with other non-VHA health care systems to create an intersystem network of collaboration for long COVID care. This approach further enhances the speed at which we can work together to share lessons learned and stay up-to-date on emerging evidence surrounding long COVID care.
Identifying Gaps in Care and Research
Our second goal was to identify gaps in care or knowledge to inform future research and quality improvement initiatives, while also creating a foundation to cocreate knowledge about safe, effective care management of the novel long COVID sequelae. To translate knowledge, we must first identify and understand the gaps between the current, best available evidence and current care practices or policies impacting that delivery.13 As such, the structured meeting agenda and facilitated meeting discussions focused on understanding current clinical decision making and the evidence base. We shared VHA evidence synthesis reports and living rapid reviews on complications following COVID-19 illness (ie, major organ damage and posthospitalization health care use) that provided an objective evidence base on common long COVID complications.14,15
Since long COVID is a novel condition, we drew from literature in similar patient populations and translated that information in the context of our current knowledge of this unique syndrome. For example, we discussed the predominant and persistent symptom of fatigue post-COVID.5 In particular, the CoP discussed challenges in identifying and treating post-COVID fatigue, which is often a vague symptom with multiple or interacting etiologies that require a comprehensive, interdisciplinary approach. As such, we reviewed, adapted, and translated identification and treatment strategies from the literature on chronic fatigue syndrome to patients with post-COVID syndrome.16,17 We continue to work collaboratively and engage the appropriate stakeholders to provide input on the gaps to prioritize targeting.
Cocreate Knowledge
Our third goal was to cocreate knowledge regarding the care of patients with long COVID. To accomplish this, our structured meetings and communication pathways invited members to share experiences on the who (delivers and receives care), what (type of care or HCPs), when (identification of post-COVID and access), and how (eg, telehealth) of care to patients post-COVID. As part of the workgroup, we identified and shared resources on standardized, facility-level practices to reduce variability across the VHA system. These resources included intake/assessment forms, care processes, and batteries of tests/measures used for screening and assessment. The knowledge obtained from outside the CoP and cocreated within is being used to inform data-driven tools to support and evaluate care for patients with long COVID. As such, members of the workgroup are in the formative stages of participating in quality improvement innovation pilots to test technologies and processes designed to improve and validate long COVID care pathways. These technologies include screening tools, clinical decision support tools, and population health management technologies. In addition, we are developing a formal collaboration with the VHA Office of Research and Development to create standardized intake forms across VHA long COVID clinics to facilitate both clinical monitoring and research.
Surveys
The US Department of Veterans Affairs Central Office collaborated with our workgroup to draft an initial set of survey questions designed to understand how each VHA facility defines, identifies, and provides care to veterans experiencing post-COVID sequalae. The 41-question survey was distributed through regional directors and chief medical officers at 139 VHA facilities in August 2021. One hundred nineteen responses (86%) were received. Sixteen facilities indicated they had established programs and 26 facilities were considering a program. Our CoP had representation from the 16 facilities with established programs indicating the deep and well-connected nature of our grassroots efforts to bring together stakeholders to learn as part of a CoP.
A separate, follow-up survey generated responses from 18 facilities and identified the need to capture evolving innovations and to develop smaller workstreams (eg, best practices, electronic documentation templates, pathway for referrals, veteran engagement, outcome measures). The survey not only exposed ongoing challenges to providing long COVID care, but importantly, outlined the ways in which CoP members were leveraging community knowledge and resources to inform innovations and processes of care changes at their specific sites. Fourteen of 18 facilities with long COVID programs in place explicitly identified the CoP as a resource they have found most beneficial when employing such innovations. Specific innovations reported included changes in care delivery, engagement in active outreach with veterans and local facility, and infrastructure development to sustain local long COVID clinics (Table).
Future Directions
Our CoP strives to contribute to an evidence base for long COVID care. At the system level, the CoP has the potential to impact access and continuity of care by identifying appropriate processes and ensuring that VHA patients receive outreach and an opportunity for post-COVID care. Comprehensive care requires input from HCP, clinical leadership, and operations levels. In this sense, our CoP provides an opportunity for diverse stakeholders to come together, discuss barriers to screening and delivering post-COVID care, and create an action plan to remove or lessen such barriers.18 Part of the process to remove barriers is to identify and support efficient resource allocation. Our CoP has worked to address issues in resource allocation (eg, space, personnel) for post-COVID care. For example, one facility is currently implementing interdisciplinary virtual post-COVID care. Another facility identified and restructured working assignments for psychologists who served in different capacities throughout the system to fill the need within the long COVID team.
At the HCP level, the CoP is currently developing workshops, media campaigns, written clinical resources, skills training, publications, and webinars/seminars with continuing medical education credits.19 The CoP may also provide learning and growth opportunities, such as clinical or VHA operational fellowships and research grants.
We are still in the formative stages of post-COVID care and future efforts will explore patient-centered outcomes. We are drawing on the Centers for Disease Control and Prevention’s guidance for evaluating patients with long COVID symptoms and examining the feasibility within VHA, as well as patient perspectives on post-COVID sequalae, to ensure we are selecting assessments that measure patient-centered constructs.18
Conclusions
A VHA-wide LHS approach is identifying issues related to the identification, delivery, and evaluation of long COVID care. This long COVID CoP has developed an infrastructure for communication, identified gaps in care, and cocreated knowledge related to best current practices for post-COVID care. This work is contributing to systemwide LHS efforts dedicated to creating a culture of quality care and innovation and is a process that is transferrable to other areas of care in the VHA, as well as other health care systems. The LHS approach continues to be highly relevant as we persist through the COVID-19 pandemic and reimagine a postpandemic world.
Acknowledg
We thank all the members of the Veterans Health Administration long COVID Community of Practice who participate in the meetings and contribute to the sharing and spread of knowledge.
The Veterans Health Administration (VHA)—along with systems across the world—has spent the past 2 years continuously adapting to meet the emerging needs of persons infected with COVID-19. With the development of effective vaccines and global efforts to mitigate transmission, attention has now shifted to long COVID care as the need for further outpatient health care becomes increasingly apparent.1,2
Background
Multiple terms describe the lingering, multisystem sequelae of COVID-19 that last longer than 4 weeks: long COVID, postacute COVID-19 syndrome, post-COVID condition, postacute sequalae of COVID-19, and COVID long hauler.1,3 Common symptoms include fatigue, shortness of breath, cough, sleep disorders, brain fog or cognitive dysfunction, depression, anxiety, pain, and changes in taste or smell that impact a person’s functioning.4,5 The multisystem nature of the postacute course of COVID-19 necessitates an interdisciplinary approach to devise comprehensive and individualized care plans.6-9 Research is needed to better understand this postacute state (eg, prevalence, underlying effects, characteristics of those who experience long COVID) to establish and evaluate cost-effective treatment approaches.
Many patients who are experiencing symptoms beyond the acute course of COVID-19 have been referred to general outpatient clinics or home health, which may lack the capacity and knowledge of this novel disease to effectively manage complex long COVID cases.2,3 To address this growing need, clinicians and leadership across a variety of disciplines and settings in the VHA created a community of practice (CoP) to create a mechanism for cross-facility communication, identify gaps in long COVID care and research, and cocreate knowledge on best practices for care delivery.
In this spirit, we are embracing a learning health system (LHS) approach that uses rapid-cycle methods to integrate data and real-world experience to iteratively evaluate and adapt models of long COVID care.10 Our clinically identified and data-driven objective is to provide high value health care to patients with long COVID sequalae by creating a framework to learn about this novel condition and develop innovative care models. This article provides an overview of our emerging LHS approach to the study of long COVID care that is fostering innovation and adaptability within the VHA. We describe 3 aspects of our engagement approach central to LHS: the ongoing development of a long COVID CoP dedicated to iteratively informing the bidirectional cycle of data from practice to research, results of a broad environmental scan of VHA long COVID care, and results of a survey administered to CoP members to inform ongoing needs of the community and identify early successful outcomes from participation.
Learning Health System Approach
The VHA is one of the largest integrated health care systems in the United States serving more than 9 million veterans.11 Since 2017, the VHA has articulated a vision to become an LHS that informs and improves patient-centered care through practice-based and data-driven research (eAppendix).12 During the early COVID-19 pandemic, an LHS approach in the VHA was critical to rapidly establishing a data infrastructure for disease surveillance, coordinating data-driven solutions, leveraging use of technology, collaborating across the globe to identify best practices, and implementing systematic responses (eg, policies, workforce adjustments).
Our long COVID CoP was developed as clinical observations and ongoing conversations with stakeholders (eg, veterans, health care practitioners [HCPs], leadership) identified a need to effectively identify and treat the growing number of veterans with long COVID. This clinical issue is compounded by the limited but emerging evidence on the clinical presentation of prolonged COVID-19 symptoms, treatment, and subsequent care pathways. The VHA’s efforts and lessons learned within the lens of an LHS are applicable to other systems confronting the complex identification and management of patients with persistent and encumbering long COVID symptoms. The VHA is building upon the LHS approach to proactively prepare for and address future clinical or public health challenges that require cross-system and sector collaborations, expediency, inclusivity, and patient/family centeredness.11
Community of Practice
As of January 25, 2022, our workgroup consisted of 128 VHA employees representing 29 VHA medical centers. Members of the multidisciplinary workgroup have diverse backgrounds with HCPs from primary care (eg, physicians, nurse practitioners), rehabilitation (eg, physical therapists), specialty care (eg, pulmonologists, physiatrists), mental health (eg, psychologists), and complementary and integrated health/Whole Health services (eg, practitoners of services such as yoga, tai chi, mindfulness, acupuncture). Members also include clinical, operations, and research leadership at local, regional, and national VHA levels. Our first objective as a large, diverse group was to establish shared goals, which included: (1) determining efficient communication pathways; (2) identifying gaps in care or research; and (3) cocreating knowledge to provide solutions to identified gaps.
Communication Mechanisms
Our first goal was to create an efficient mechanism for cross-facility communication. The initial CoP was formed in April 2021 and the first virtual meeting focused on reaching a consensus regarding the best way to communicate and proceed. We agreed to convene weekly at a consistent time, created a standard agenda template, and elected a lead facilitator of meeting proceedings. In addition, a member of the CoP recorded and took extensive meeting notes, which were later distributed to the entire CoP to accommodate varying schedules and ability to attend live meetings. Approximately 20 to 30 participants attend the meetings in real-time.
To consolidate working documents, information, and resources in one location, we created a platform to communicate via a Microsoft Teams channel. All CoP members are given access to the folders and allowed to add to the growing library of resources. Resources include clinical assessment and note templates for electronic documentation of care, site-specific process maps, relevant literature on screening and interventions identified by practice members, and meeting notes along with the recordings. A chat feature alerts CoP members to questions posed by other members. Any resources or information shared on the chat discussion are curated by CoP leaders to disseminate to all members. Importantly, this platform allowed us to communicate efficiently within the VHA organization by creating a centralized space for documents and the ability to correspond with all or select members of the CoP. Additional VHA employees can easily be referred and request access.
To increase awareness of the CoP, expand reach, and diversify perspectives, every participant was encouraged to invite colleagues and stakeholders with interest or experience in long COVID care to join. While patients are not included in this CoP, we are working closely with the VHA user experience workgroup (many members overlap) that is gathering patient and caregiver perspectives on their COVID-19 experience and long COVID care. Concurrently, CoP members and leadership facilitate communication and set up formal collaborations with other non-VHA health care systems to create an intersystem network of collaboration for long COVID care. This approach further enhances the speed at which we can work together to share lessons learned and stay up-to-date on emerging evidence surrounding long COVID care.
Identifying Gaps in Care and Research
Our second goal was to identify gaps in care or knowledge to inform future research and quality improvement initiatives, while also creating a foundation to cocreate knowledge about safe, effective care management of the novel long COVID sequelae. To translate knowledge, we must first identify and understand the gaps between the current, best available evidence and current care practices or policies impacting that delivery.13 As such, the structured meeting agenda and facilitated meeting discussions focused on understanding current clinical decision making and the evidence base. We shared VHA evidence synthesis reports and living rapid reviews on complications following COVID-19 illness (ie, major organ damage and posthospitalization health care use) that provided an objective evidence base on common long COVID complications.14,15
Since long COVID is a novel condition, we drew from literature in similar patient populations and translated that information in the context of our current knowledge of this unique syndrome. For example, we discussed the predominant and persistent symptom of fatigue post-COVID.5 In particular, the CoP discussed challenges in identifying and treating post-COVID fatigue, which is often a vague symptom with multiple or interacting etiologies that require a comprehensive, interdisciplinary approach. As such, we reviewed, adapted, and translated identification and treatment strategies from the literature on chronic fatigue syndrome to patients with post-COVID syndrome.16,17 We continue to work collaboratively and engage the appropriate stakeholders to provide input on the gaps to prioritize targeting.
Cocreate Knowledge
Our third goal was to cocreate knowledge regarding the care of patients with long COVID. To accomplish this, our structured meetings and communication pathways invited members to share experiences on the who (delivers and receives care), what (type of care or HCPs), when (identification of post-COVID and access), and how (eg, telehealth) of care to patients post-COVID. As part of the workgroup, we identified and shared resources on standardized, facility-level practices to reduce variability across the VHA system. These resources included intake/assessment forms, care processes, and batteries of tests/measures used for screening and assessment. The knowledge obtained from outside the CoP and cocreated within is being used to inform data-driven tools to support and evaluate care for patients with long COVID. As such, members of the workgroup are in the formative stages of participating in quality improvement innovation pilots to test technologies and processes designed to improve and validate long COVID care pathways. These technologies include screening tools, clinical decision support tools, and population health management technologies. In addition, we are developing a formal collaboration with the VHA Office of Research and Development to create standardized intake forms across VHA long COVID clinics to facilitate both clinical monitoring and research.
Surveys
The US Department of Veterans Affairs Central Office collaborated with our workgroup to draft an initial set of survey questions designed to understand how each VHA facility defines, identifies, and provides care to veterans experiencing post-COVID sequalae. The 41-question survey was distributed through regional directors and chief medical officers at 139 VHA facilities in August 2021. One hundred nineteen responses (86%) were received. Sixteen facilities indicated they had established programs and 26 facilities were considering a program. Our CoP had representation from the 16 facilities with established programs indicating the deep and well-connected nature of our grassroots efforts to bring together stakeholders to learn as part of a CoP.
A separate, follow-up survey generated responses from 18 facilities and identified the need to capture evolving innovations and to develop smaller workstreams (eg, best practices, electronic documentation templates, pathway for referrals, veteran engagement, outcome measures). The survey not only exposed ongoing challenges to providing long COVID care, but importantly, outlined the ways in which CoP members were leveraging community knowledge and resources to inform innovations and processes of care changes at their specific sites. Fourteen of 18 facilities with long COVID programs in place explicitly identified the CoP as a resource they have found most beneficial when employing such innovations. Specific innovations reported included changes in care delivery, engagement in active outreach with veterans and local facility, and infrastructure development to sustain local long COVID clinics (Table).
Future Directions
Our CoP strives to contribute to an evidence base for long COVID care. At the system level, the CoP has the potential to impact access and continuity of care by identifying appropriate processes and ensuring that VHA patients receive outreach and an opportunity for post-COVID care. Comprehensive care requires input from HCP, clinical leadership, and operations levels. In this sense, our CoP provides an opportunity for diverse stakeholders to come together, discuss barriers to screening and delivering post-COVID care, and create an action plan to remove or lessen such barriers.18 Part of the process to remove barriers is to identify and support efficient resource allocation. Our CoP has worked to address issues in resource allocation (eg, space, personnel) for post-COVID care. For example, one facility is currently implementing interdisciplinary virtual post-COVID care. Another facility identified and restructured working assignments for psychologists who served in different capacities throughout the system to fill the need within the long COVID team.
At the HCP level, the CoP is currently developing workshops, media campaigns, written clinical resources, skills training, publications, and webinars/seminars with continuing medical education credits.19 The CoP may also provide learning and growth opportunities, such as clinical or VHA operational fellowships and research grants.
We are still in the formative stages of post-COVID care and future efforts will explore patient-centered outcomes. We are drawing on the Centers for Disease Control and Prevention’s guidance for evaluating patients with long COVID symptoms and examining the feasibility within VHA, as well as patient perspectives on post-COVID sequalae, to ensure we are selecting assessments that measure patient-centered constructs.18
Conclusions
A VHA-wide LHS approach is identifying issues related to the identification, delivery, and evaluation of long COVID care. This long COVID CoP has developed an infrastructure for communication, identified gaps in care, and cocreated knowledge related to best current practices for post-COVID care. This work is contributing to systemwide LHS efforts dedicated to creating a culture of quality care and innovation and is a process that is transferrable to other areas of care in the VHA, as well as other health care systems. The LHS approach continues to be highly relevant as we persist through the COVID-19 pandemic and reimagine a postpandemic world.
Acknowledg
We thank all the members of the Veterans Health Administration long COVID Community of Practice who participate in the meetings and contribute to the sharing and spread of knowledge.
1. Sivan M, Halpin S, Hollingworth L, Snook N, Hickman K, Clifton I. Development of an integrated rehabilitation pathway for individuals recovering from COVID-19 in the community. J Rehabil Med. 2020;52(8):jrm00089. doi:10.2340/16501977-2727
2. Understanding the long-term health effects of COVID-19. EClinicalMedicine. 2020;26:100586. doi:10.1016/j.eclinm.2020.100586
3. Greenhalgh T, Knight M, A’Court C, Buxton M, Husain L. Management of post-acute covid-19 in primary care. BMJ. Published online August 11, 2020:m3026. doi:10.1136/bmj.m3026
4. Iwua CJ, Iwu CD, Wiysonge CS. The occurrence of long COVID: a rapid review. Pan Afr Med J. 2021;38. doi:10.11604/pamj.2021.38.65.27366
5. Carfì A, Bernabei R, Landi F; Gemelli Against COVID-19 Post-Acute Care Study Group. Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324(6):603-605. doi:10.1001/jama.2020.12603
6. Gemelli Against COVID-19 Post-Acute Care Study Group. Post-COVID-19 global health strategies: the need for an interdisciplinary approach. Aging Clin Exp Res. 2020;32(8):1613-1620. doi:10.1007/s40520-020-01616-x
7. Xie Y, Xu E, Bowe B, Al-Aly Z. Long-term cardiovascular outcomes of COVID-19. Nat Med. 2022;28:583-590. doi:10.1038/s41591-022-01689-3
8. Al-Aly Z, Xie Y, Bowe B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature. 2021;594:259-264. doi:10.1038/s41586-021-03553-9
9. Ayoubkhani D, Bermingham C, Pouwels KB, et al. Trajectory of long covid symptoms after covid-19 vaccination: community based cohort study. BMJ. 2022;377:e069676. doi:10.1136/bmj-2021-069676
10. Institute of Medicine (US) Roundtable on Evidence-Based Medicine, Olsen L, Aisner D, McGinnis JM, eds. The Learning Healthcare System: Workshop Summary. Washington (DC): National Academies Press (US); 2007. doi:10.17226/11903
11. Romanelli RJ, Azar KMJ, Sudat S, Hung D, Frosch DL, Pressman AR. Learning health system in crisis: lessons from the COVID-19 pandemic. Mayo Clin Proc Innov Qual Outcomes. 2021;5(1):171-176. doi:10.1016/j.mayocpiqo.2020.10.004
12. Atkins D, Kilbourne AM, Shulkin D. Moving from discovery to system-wide change: the role of research in a learning health care system: experience from three decades of health systems research in the Veterans Health Administration. Annu Rev Public Health. 2017;38:467-487. doi:10.1146/annurev-publhealth-031816-044255
13. Kitson A, Straus SE. The knowledge-to-action cycle: identifying the gaps. CMAJ. 2010;182(2):E73-77. doi:10.1503/cmaj.081231
14. Greer N, Bart B, Billington C, et al. COVID-19 post-acute care major organ damage: a living rapid review. Updated September 2021. Accessed May 31, 2022. https://www.hsrd.research.va.gov/publications/esp/covid-organ-damage.pdf
15. Sharpe JA, Burke C, Gordon AM, et al. COVID-19 post-hospitalization health care utilization: a living review. Updated February 2022. Accessed May 31, 2022. https://www.hsrd.research.va.gov/publications/esp/covid19-post-hosp.pdf
16. Bested AC, Marshall LM. Review of Myalgic Encephalomyelitis/chronic fatigue syndrome: an evidence-based approach to diagnosis and management by clinicians. Rev Environ Health. 2015;30(4):223-249. doi:10.1515/reveh-2015-0026
17. Yancey JR, Thomas SM. Chronic fatigue syndrome: diagnosis and treatment. Am Fam Physician. 2012;86(8):741-746.
18. Kotter JP, Cohen DS. Change Leadership The Kotter Collection. Harvard Business Review Press; 2014.
19. Brownson RC, Eyler AA, Harris JK, Moore JB, Tabak RG. Getting the word out: new approaches for disseminating public health science. J Public Health Manag Pract. 2018;24(2):102-111. doi:10.1097/PHH.0000000000000673
1. Sivan M, Halpin S, Hollingworth L, Snook N, Hickman K, Clifton I. Development of an integrated rehabilitation pathway for individuals recovering from COVID-19 in the community. J Rehabil Med. 2020;52(8):jrm00089. doi:10.2340/16501977-2727
2. Understanding the long-term health effects of COVID-19. EClinicalMedicine. 2020;26:100586. doi:10.1016/j.eclinm.2020.100586
3. Greenhalgh T, Knight M, A’Court C, Buxton M, Husain L. Management of post-acute covid-19 in primary care. BMJ. Published online August 11, 2020:m3026. doi:10.1136/bmj.m3026
4. Iwua CJ, Iwu CD, Wiysonge CS. The occurrence of long COVID: a rapid review. Pan Afr Med J. 2021;38. doi:10.11604/pamj.2021.38.65.27366
5. Carfì A, Bernabei R, Landi F; Gemelli Against COVID-19 Post-Acute Care Study Group. Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324(6):603-605. doi:10.1001/jama.2020.12603
6. Gemelli Against COVID-19 Post-Acute Care Study Group. Post-COVID-19 global health strategies: the need for an interdisciplinary approach. Aging Clin Exp Res. 2020;32(8):1613-1620. doi:10.1007/s40520-020-01616-x
7. Xie Y, Xu E, Bowe B, Al-Aly Z. Long-term cardiovascular outcomes of COVID-19. Nat Med. 2022;28:583-590. doi:10.1038/s41591-022-01689-3
8. Al-Aly Z, Xie Y, Bowe B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature. 2021;594:259-264. doi:10.1038/s41586-021-03553-9
9. Ayoubkhani D, Bermingham C, Pouwels KB, et al. Trajectory of long covid symptoms after covid-19 vaccination: community based cohort study. BMJ. 2022;377:e069676. doi:10.1136/bmj-2021-069676
10. Institute of Medicine (US) Roundtable on Evidence-Based Medicine, Olsen L, Aisner D, McGinnis JM, eds. The Learning Healthcare System: Workshop Summary. Washington (DC): National Academies Press (US); 2007. doi:10.17226/11903
11. Romanelli RJ, Azar KMJ, Sudat S, Hung D, Frosch DL, Pressman AR. Learning health system in crisis: lessons from the COVID-19 pandemic. Mayo Clin Proc Innov Qual Outcomes. 2021;5(1):171-176. doi:10.1016/j.mayocpiqo.2020.10.004
12. Atkins D, Kilbourne AM, Shulkin D. Moving from discovery to system-wide change: the role of research in a learning health care system: experience from three decades of health systems research in the Veterans Health Administration. Annu Rev Public Health. 2017;38:467-487. doi:10.1146/annurev-publhealth-031816-044255
13. Kitson A, Straus SE. The knowledge-to-action cycle: identifying the gaps. CMAJ. 2010;182(2):E73-77. doi:10.1503/cmaj.081231
14. Greer N, Bart B, Billington C, et al. COVID-19 post-acute care major organ damage: a living rapid review. Updated September 2021. Accessed May 31, 2022. https://www.hsrd.research.va.gov/publications/esp/covid-organ-damage.pdf
15. Sharpe JA, Burke C, Gordon AM, et al. COVID-19 post-hospitalization health care utilization: a living review. Updated February 2022. Accessed May 31, 2022. https://www.hsrd.research.va.gov/publications/esp/covid19-post-hosp.pdf
16. Bested AC, Marshall LM. Review of Myalgic Encephalomyelitis/chronic fatigue syndrome: an evidence-based approach to diagnosis and management by clinicians. Rev Environ Health. 2015;30(4):223-249. doi:10.1515/reveh-2015-0026
17. Yancey JR, Thomas SM. Chronic fatigue syndrome: diagnosis and treatment. Am Fam Physician. 2012;86(8):741-746.
18. Kotter JP, Cohen DS. Change Leadership The Kotter Collection. Harvard Business Review Press; 2014.
19. Brownson RC, Eyler AA, Harris JK, Moore JB, Tabak RG. Getting the word out: new approaches for disseminating public health science. J Public Health Manag Pract. 2018;24(2):102-111. doi:10.1097/PHH.0000000000000673
Pharmacist-Assisted Varenicline Tobacco Cessation Treatment for Veterans
Tobacco smoking remains the leading cause of preventable disease and death in the United States, accounting for more than 480,000 deaths annually.1 An estimated 50.6 million US adults (20.8%) identify as tobacco users, with even higher rates among veterans (29.2%).2,3 Tobacco use is estimated to cost the US more than $300 billion annually in direct and indirect medical costs.4 According to a 2015 report, more than two-thirds of adult smokers reported a desire to quit, while only 7.5% reported successfully quitting in the past year.5 According to that same report, only 57.2% of smokers who had seen a health professional in the past year reported receiving advice to quit.5 This statistic is unfortunate, as interventions that combine behavioral and pharmacologic support can drastically increase tobacco cessation rates compared with self-help materials or no treatment.6
Currently, 7 first-line medications (5 nicotine, 2 nonnicotine) have been shown to increase long-term smoking abstinence rates. Varenicline was approved by the US Food and Drug Administration (FDA) in 2006 for use in adults as an aid to smoking cessation treatment. As a partial agonist of the α4β2 nicotinic acetylcholine receptor, varenicline’s mechanism of action is believed to involve reduction of nicotine’s rewarding capacity.7 Varenicline not only aids in complete tobacco cessation but also has been found to be effective for reducing cigarette consumption among smokers not yet willing or able to make a quit attempt.8 Furthermore, varenicline has demonstrated efficacy among users of smokeless tobacco in achieving continuous abstinence.9
Widespread adoption of varenicline into clinical practice was perhaps slowed by early concerns of psychiatric complications, prompting the FDA to issue a boxed warning for risk of serious neuropsychiatric events. This boxed warning was removed in 2016 in response to publication of the Evaluating Adverse Events in a Global Smoking Cessation Study (EAGLES). In this randomized controlled trial of more than 8000 participants, among whom 50.5% had a psychiatric disorder determined to be stable, varenicline significantly increased rates of continuous tobacco cessation compared with bupropion or the nicotine patch without an increased risk of neuropsychiatric events.10 This study underscored not only the safety of varenicline, but also its superiority over other first-line cessation products. The most recently published clinical practice guidelines recommend varenicline as a first-line agent for helping patients achieve long-term smoking cessation.11,12
Pharmacists are uniquely positioned to provide tobacco cessation interventions given their medication expertise and accessibility to the public. Indeed, multiple studies have demonstrated the effectiveness of pharmacist-led interventions on tobacco cessation.13-15 As of 2019, only 12 states had statutes or regulations addressing pharmacist prescribing of tobacco cessation aids without a collaborative practice agreement or local standing order.16 Until recently, most of these states limited pharmacists’ prescriptive authority to
Within the US Department of Veterans Affairs (VA), the clinical pharmacy specialist (CPS) is credentialed as an advanced practitioner with authority to independently manage patient medication therapy for a variety of diseases specified under a scope of practice. Although CPSs have provided tobacco cessation services for years, expansion of their scope to include varenicline did not occur until June 26, 2019, at the Southern Arizona VA Health Care System (SAVAHCS). All VA prescribers must follow the same criteria for prescribing varenicline. Unless previously trialed on varenicline, patients must have failed an appropriate trial of first-line agents (NRT, bupropion, or combination therapy) or have a contraindication to use of these first-line therapies before varenicline can be considered. Exclusions to therapy would include history of serious hypersensitivity to varenicline; suicidal intent, plan, or attempt within the past 12 months; current substance use disorder other than nicotine (unless varenicline recommended or prescribed by mental health professional); or unstable mental health disorder.18
The purpose of this study was to evaluate the efficacy and safety of CPS management of varenicline compared with other clinicians. We hope that this study provides insight regarding how the expansion of CPS scope to include prescriptive authority for varenicline has affected patient outcomes.
Methods
This retrospective chart review was conducted using SAVAHCS electronic health records. This study was granted approval by the institutional review board and the research and development committee at SAVAHCS. Data were obtained through the Computerized Patient Record System from the information provided by the pharmacist informatics department and was recorded electronically on a secure Microsoft Excel spreadsheet.
To be eligible for this study, patients must have been aged ≥ 18 years with a varenicline prescription between July 1, 2019, and July 31, 2020. Patients were excluded if tobacco cessation was managed by community-based (non-VA) clincians or if there was a lack of documentation of tobacco use at baseline and after at least 12 weeks of varenicline therapy. Sample size was not designed to achieve statistical power. Potential patients were queried by a pharmacist specializing in clinical informatics. All patients meeting initial inclusion criteria were then screened individually to evaluate for exclusion criteria.
Data collected included baseline age, sex, race, type of tobacco use (cigarettes, smokeless, both), mean daily tobacco use, prespecified comorbidities (depression, anxiety, or other psychiatric condition), and previous cessation medications prescribed (NRT, bupropion, and previous trials of varenicline).
The primary outcomes were reduction in tobacco use calculated as change at 12 weeks from baseline (and 24 weeks if available), continuous abstinence at 12 weeks (and 24 weeks if available), adherence to varenicline therapy measured by proportion of days covered (days covered by refills during the measurement period divided by days between the first fill and the end of the measurement period), and time to first follow-up in days. For safety evaluation, charts were reviewed for documented adverse events (AEs) in the health record. These AEs were categorized as follows: gastrointestinal, mood disturbance, sleep disturbance, headache, seizures, allergy, or other.
Statistical analyses regarding veteran baseline characteristics were descriptive in nature. χ2 test was used to analyze differences in complete cessation rates and AEs, whereas a Student t test was used to compare reductions of tobacco use, proportion of days covered (ie, adherence), and time to first follow-up. An α of .05 was used to determine significance.
Results
From the initial search, 255 charts met general inclusion criteria. After chart review, only 50 patients from the CPS group and 93 patients from the other clinician group met criteria to be included (Figure 1). The CPS group included pharmacists specializing in ambulatory care and outpatient mental health. The other clinician group was composed primarily of primary care practitioners, psychiatrists, and pulmonologists.
Overall, baseline characteristics were similar between the groups (Table 1). In the overall study population, the mean age was 57.5 years, 90% of patients were male, and 99% of patients were cigarette smokers. Baseline mean (SD) tobacco use was similar between the groups: 14.5 (10.8) vs 14.8 (8.6) cigarettes daily for the CPS and other clinician group, respectively.
While there was a significant reduction in daily cigarette use for both groups at 12 and 24 weeks (Figure 2), there was no mean (SD) between-group difference found among those patients prescribed varenicline by a CPS compared with other clinicians: -7.9 (10.4) vs -5.4 (9.8) cigarettes daily, respectively (P = .15) (Table 2). Change in tobacco use at 24 weeks and rates of complete tobacco abstinence were also not statistically significant between prescriber groups. Adherence (as evidenced by refill data) was higher in the CPS group than in the other clinician group (42% vs 31%, respectively; P = .01). There was also a significant difference in time to first follow-up; patients whose varenicline therapy was managed by a CPS had a mean (SD) follow-up time of 52 (66) vs 163 (110) days when patients were managed by other clinicians (P < .001). AEs were documented in 42% of patients in the CPS group compared with 23% of patients in the other clinician group (Table 3). The most reported AEs were gastrointestinal, as well as mood and sleep disturbances.
Discussion
The results of this single center study suggest that management of varenicline by CPSs is associated with similar reductions in tobacco use and abstinence rates compared with management by other clinicians. These results provide evidence that CPS management of varenicline may be as safe and effective as management by other clinicians.
Adherence rates (reported as proportion of days covered when assessing varenicline refill data) were higher on average among patients managed by a CPS compared with patients managed by other clinicians. However, this outcome may not be as reflective of adherence as initially intended, given delays in follow-up (see limitations section). Time to first follow-up was drastically different between the groups, with much sooner follow-up by CPSs compared with other clinicians. Despite similar tobacco cessation rates between groups, more frequent follow-up by CPSs helps to assess patient barriers to cessation, adherence to therapy, and AEs with varenicline. A higher percentage of AEs were documented within the CPS group that could be attributed to disparities in documentation rather than true rates of AEs. While rates of AEs were initially intended to serve as the primary safety outcome, they may instead reflect pharmacists’ diligence in monitoring and documenting tolerability of medication therapy.
Limitations
Several limitations to this study should be noted. First, the data collected were only as detailed as the extent to which prescribers documented tobacco use, previous cessation trials, and AEs; thus, various data points are likely missing within this study that could impact the results presented. In line with lack of documentation, delays in follow-up (ie, annual primary care visits) sorely undermined proportion of days covered, making these data less indicative of true medication adherence. Furthermore, this study did not account for concurrent therapies, such as combination varenicline and nicotine gum/lozenges, or behavioral treatment strategies like cessation classes.
Another limitation was that some primary care practitioners prescribed varenicline but then referred these patients to a CPS for tobacco cessation follow-up. Per the study’s protocol, these patients were included within the other clinician group, which could have brought results closer to the null. Finally, the timing of this chart review (July 1, 2019, to July 31, 2020) intersects with the start of the COVID-19 pandemic, presenting a possible confounding factor if patients’ quit attempts were hindered by the stress and isolation of the pandemic.19 All pharmacist visits during the pandemic were conducted by telephone, which may have affected results.
Conclusions
In this study of veterans receiving varenicline, management by CPSs resulted in similar reductions of tobacco use and rates of complete abstinence compared with management by other clinicians. Pharmacist management was associated with greater adherence and shorter time to first follow-up compared with other clinicians. Additional research is needed to fully characterize the impact of pharmacist management of varenicline, justify expansion of clinical pharmacist scope of practice, and ultimately enhance patient outcomes regarding tobacco cessation.
It would be interesting to see more studies outside of the VA system to determine the impact of pharmacist management of varenicline for a more heterogenous patient population. At some point, a prospective controlled trial should be conducted to overcome the various confounding factors that limit the results of retrospective chart reviews
Acknowledgments
This article was prepared, and research was conducted with resources and the use of facilities at the Southern Arizona Veterans Affairs Health Care System in Tucson.
1. Centers for Disease Control and Prevention. Current cigarette smoking among adults in the United States. Updated March 17, 2022. Accessed May 31, 2022. https://www.cdc.gov/tobacco/data_statistics/fact_sheets/adult_data/cig_smoking/index.htm 2. Cornelius ME, Wang TW, Jamal A, Loretan CG, Neff LJ. Tobacco product use among adults – United States, 2019. MMWR Morb Mortal Wkly Rep. 2020;69(46):1736-1742. doi:10.15585/mmwr.mm6946a4
3. Odani S, Agaku IT, Graffunder CM, Tynan MA, Armour BS. Tobacco product use among military veterans – United States, 2010-2015. MMWR Morb Mortal Wkly Rep. 2018;67(1):7-12. doi:10.15585/mmwr.mm6701a2
4. Hall W, Doran C. How much can the USA reduce health care costs by reducing smoking? PLoS Med. 2016;13(5):e1002021. doi:10.1371/journal.pmed.1002021.
5. Centers for Disease Control and Prevention. Smoking cessation: fast facts. Updated March 21, 2022. Accessed June 1, 2022. https://www.cdc.gov/tobacco/data_statistics/fact_sheets/cessation/smoking-cessation-fast-facts/index.html
6. US Public Health Service Office of the Surgeon General; National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health. Chapter 6, Interventions for smoking cessation and treatments for nicotine dependence. In: Smoking Cessation: A Report of the Surgeon General [Internet]. Washington, DC: US Department of Health and Human Services; 2020. Accessed June 1, 2022. https://www.ncbi.nlm.nih.gov/books/NBK555596
7. Rollema H, Chambers LK, Coe JW, et al. Pharmacological profile of the α4β2 nicotinic acetylcholine receptor partial agonist varenicline, an effective smoking cessation aid. Neuropharmacology. 2007;52(3):985-994. doi:10.1016/j.neuropharm.2006.10.016
8. Ebbert JO, Hughes JR, West RJ, et al. Effect of varenicline on smoking cessation through smoking reduction: a randomized clinical trial. JAMA. 2015;313(7):687-694. doi:10.1001/jama.2015.280
9. Fagerström K, Gilljam H, Metcalfe M, Tonstad S, Messig M. Stopping smokeless tobacco with varenicline: randomised double blind placebo controlled trial. BMJ. 2010;341:c6549. doi:10.1136/bmj.c6549
10. Anthenelli RM, Benowitz NL, West R, et al. Neuropsychiatric safety and efficacy of varenicline, bupropion, and nicotine patch in smokers with and without psychiatric disorders (EAGLES): a double-blind, randomised, placebo-controlled clinical trial. Lancet. 2016;387(10037):2507-2520. doi:10.1016/S0140-6736(16)30272-0
11. Barua RS, Rigotti NA, Benowitz NL, et al. 2018 ACC expert consensus decision pathway on tobacco cessation treatment: a report of the American College of Cardiology Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol. 2018;72(25):3332-3365. doi:10.1016/j.jacc.2018.10.027
12. Leone FT, Zhang Y, Evers-Casey S, et al. Initiating pharmacologic treatment in tobacco-dependent adults. An official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med. 2020;202(2):e5-e31. doi:10.1164/rccm.202005-1982ST
13. Saba M, Diep J, Saini B, Dhippayom T. Meta-analysis of the effectiveness of smoking cessation interventions in community pharmacy. J Clin Pharm Ther. 2014;39(3):240-247. doi:10.1111/jcpt.12131
14. Augustine JM, Taylor AM, Pelger M, Schiefer D, Warholak TL. Smoking quit rates among patients receiving pharmacist-provided pharmacotherapy and telephonic smoking cessation counseling. J Am Pharm Assoc. 2016;56(2):129-136. doi:10.1016/j.japh.2016.02.001
15. Dent LA, Harris KJ, Noonan CW. Tobacco interventions delivered by pharmacists: a summary and systematic review. Pharmacotherapy. 2007;27(7):1040-1051. doi:10.1592/phco.27.7.1040
16. National Alliance of State Pharmacy Associations. Pharmacist prescribing: tobacco cessation aids. February 10, 2021. Accessed June 1, 2022. https://naspa.us/resource/tobacco-cessation
17. Shen X, Bachyrycz A, Anderson JR, Tinker D, Raisch DW. Quitting patterns and predictors of success among participants in a tobacco cessation program provided by pharmacists in New Mexico. J Manag Care Spec Pharm. 2014;20(6):579-587. doi:10.18553/jmcp.2014.20.6.579
18. VA Center for Medication Safety, Tobacco Use Cessation Technical Advisory Group, Public Health Strategic Healthcare Group, VA Pharmacy Benefits Management Services, VISN Pharmacist Executives, and Medical Advisory Panel. Varenicline criteria for prescribing. 2008. Updated July 2011. Accessed June 9, 2022. https://www.healthquality.va.gov/tuc/VareniclineCriteriaforPrescribing.pdf
19. Jaklevic MC. COVID-19 and the “lost year” for smokers trying to quit. JAMA. 2021;325(19):1929-1930. doi:10.1001/jama.2021.5601
Tobacco smoking remains the leading cause of preventable disease and death in the United States, accounting for more than 480,000 deaths annually.1 An estimated 50.6 million US adults (20.8%) identify as tobacco users, with even higher rates among veterans (29.2%).2,3 Tobacco use is estimated to cost the US more than $300 billion annually in direct and indirect medical costs.4 According to a 2015 report, more than two-thirds of adult smokers reported a desire to quit, while only 7.5% reported successfully quitting in the past year.5 According to that same report, only 57.2% of smokers who had seen a health professional in the past year reported receiving advice to quit.5 This statistic is unfortunate, as interventions that combine behavioral and pharmacologic support can drastically increase tobacco cessation rates compared with self-help materials or no treatment.6
Currently, 7 first-line medications (5 nicotine, 2 nonnicotine) have been shown to increase long-term smoking abstinence rates. Varenicline was approved by the US Food and Drug Administration (FDA) in 2006 for use in adults as an aid to smoking cessation treatment. As a partial agonist of the α4β2 nicotinic acetylcholine receptor, varenicline’s mechanism of action is believed to involve reduction of nicotine’s rewarding capacity.7 Varenicline not only aids in complete tobacco cessation but also has been found to be effective for reducing cigarette consumption among smokers not yet willing or able to make a quit attempt.8 Furthermore, varenicline has demonstrated efficacy among users of smokeless tobacco in achieving continuous abstinence.9
Widespread adoption of varenicline into clinical practice was perhaps slowed by early concerns of psychiatric complications, prompting the FDA to issue a boxed warning for risk of serious neuropsychiatric events. This boxed warning was removed in 2016 in response to publication of the Evaluating Adverse Events in a Global Smoking Cessation Study (EAGLES). In this randomized controlled trial of more than 8000 participants, among whom 50.5% had a psychiatric disorder determined to be stable, varenicline significantly increased rates of continuous tobacco cessation compared with bupropion or the nicotine patch without an increased risk of neuropsychiatric events.10 This study underscored not only the safety of varenicline, but also its superiority over other first-line cessation products. The most recently published clinical practice guidelines recommend varenicline as a first-line agent for helping patients achieve long-term smoking cessation.11,12
Pharmacists are uniquely positioned to provide tobacco cessation interventions given their medication expertise and accessibility to the public. Indeed, multiple studies have demonstrated the effectiveness of pharmacist-led interventions on tobacco cessation.13-15 As of 2019, only 12 states had statutes or regulations addressing pharmacist prescribing of tobacco cessation aids without a collaborative practice agreement or local standing order.16 Until recently, most of these states limited pharmacists’ prescriptive authority to
Within the US Department of Veterans Affairs (VA), the clinical pharmacy specialist (CPS) is credentialed as an advanced practitioner with authority to independently manage patient medication therapy for a variety of diseases specified under a scope of practice. Although CPSs have provided tobacco cessation services for years, expansion of their scope to include varenicline did not occur until June 26, 2019, at the Southern Arizona VA Health Care System (SAVAHCS). All VA prescribers must follow the same criteria for prescribing varenicline. Unless previously trialed on varenicline, patients must have failed an appropriate trial of first-line agents (NRT, bupropion, or combination therapy) or have a contraindication to use of these first-line therapies before varenicline can be considered. Exclusions to therapy would include history of serious hypersensitivity to varenicline; suicidal intent, plan, or attempt within the past 12 months; current substance use disorder other than nicotine (unless varenicline recommended or prescribed by mental health professional); or unstable mental health disorder.18
The purpose of this study was to evaluate the efficacy and safety of CPS management of varenicline compared with other clinicians. We hope that this study provides insight regarding how the expansion of CPS scope to include prescriptive authority for varenicline has affected patient outcomes.
Methods
This retrospective chart review was conducted using SAVAHCS electronic health records. This study was granted approval by the institutional review board and the research and development committee at SAVAHCS. Data were obtained through the Computerized Patient Record System from the information provided by the pharmacist informatics department and was recorded electronically on a secure Microsoft Excel spreadsheet.
To be eligible for this study, patients must have been aged ≥ 18 years with a varenicline prescription between July 1, 2019, and July 31, 2020. Patients were excluded if tobacco cessation was managed by community-based (non-VA) clincians or if there was a lack of documentation of tobacco use at baseline and after at least 12 weeks of varenicline therapy. Sample size was not designed to achieve statistical power. Potential patients were queried by a pharmacist specializing in clinical informatics. All patients meeting initial inclusion criteria were then screened individually to evaluate for exclusion criteria.
Data collected included baseline age, sex, race, type of tobacco use (cigarettes, smokeless, both), mean daily tobacco use, prespecified comorbidities (depression, anxiety, or other psychiatric condition), and previous cessation medications prescribed (NRT, bupropion, and previous trials of varenicline).
The primary outcomes were reduction in tobacco use calculated as change at 12 weeks from baseline (and 24 weeks if available), continuous abstinence at 12 weeks (and 24 weeks if available), adherence to varenicline therapy measured by proportion of days covered (days covered by refills during the measurement period divided by days between the first fill and the end of the measurement period), and time to first follow-up in days. For safety evaluation, charts were reviewed for documented adverse events (AEs) in the health record. These AEs were categorized as follows: gastrointestinal, mood disturbance, sleep disturbance, headache, seizures, allergy, or other.
Statistical analyses regarding veteran baseline characteristics were descriptive in nature. χ2 test was used to analyze differences in complete cessation rates and AEs, whereas a Student t test was used to compare reductions of tobacco use, proportion of days covered (ie, adherence), and time to first follow-up. An α of .05 was used to determine significance.
Results
From the initial search, 255 charts met general inclusion criteria. After chart review, only 50 patients from the CPS group and 93 patients from the other clinician group met criteria to be included (Figure 1). The CPS group included pharmacists specializing in ambulatory care and outpatient mental health. The other clinician group was composed primarily of primary care practitioners, psychiatrists, and pulmonologists.
Overall, baseline characteristics were similar between the groups (Table 1). In the overall study population, the mean age was 57.5 years, 90% of patients were male, and 99% of patients were cigarette smokers. Baseline mean (SD) tobacco use was similar between the groups: 14.5 (10.8) vs 14.8 (8.6) cigarettes daily for the CPS and other clinician group, respectively.
While there was a significant reduction in daily cigarette use for both groups at 12 and 24 weeks (Figure 2), there was no mean (SD) between-group difference found among those patients prescribed varenicline by a CPS compared with other clinicians: -7.9 (10.4) vs -5.4 (9.8) cigarettes daily, respectively (P = .15) (Table 2). Change in tobacco use at 24 weeks and rates of complete tobacco abstinence were also not statistically significant between prescriber groups. Adherence (as evidenced by refill data) was higher in the CPS group than in the other clinician group (42% vs 31%, respectively; P = .01). There was also a significant difference in time to first follow-up; patients whose varenicline therapy was managed by a CPS had a mean (SD) follow-up time of 52 (66) vs 163 (110) days when patients were managed by other clinicians (P < .001). AEs were documented in 42% of patients in the CPS group compared with 23% of patients in the other clinician group (Table 3). The most reported AEs were gastrointestinal, as well as mood and sleep disturbances.
Discussion
The results of this single center study suggest that management of varenicline by CPSs is associated with similar reductions in tobacco use and abstinence rates compared with management by other clinicians. These results provide evidence that CPS management of varenicline may be as safe and effective as management by other clinicians.
Adherence rates (reported as proportion of days covered when assessing varenicline refill data) were higher on average among patients managed by a CPS compared with patients managed by other clinicians. However, this outcome may not be as reflective of adherence as initially intended, given delays in follow-up (see limitations section). Time to first follow-up was drastically different between the groups, with much sooner follow-up by CPSs compared with other clinicians. Despite similar tobacco cessation rates between groups, more frequent follow-up by CPSs helps to assess patient barriers to cessation, adherence to therapy, and AEs with varenicline. A higher percentage of AEs were documented within the CPS group that could be attributed to disparities in documentation rather than true rates of AEs. While rates of AEs were initially intended to serve as the primary safety outcome, they may instead reflect pharmacists’ diligence in monitoring and documenting tolerability of medication therapy.
Limitations
Several limitations to this study should be noted. First, the data collected were only as detailed as the extent to which prescribers documented tobacco use, previous cessation trials, and AEs; thus, various data points are likely missing within this study that could impact the results presented. In line with lack of documentation, delays in follow-up (ie, annual primary care visits) sorely undermined proportion of days covered, making these data less indicative of true medication adherence. Furthermore, this study did not account for concurrent therapies, such as combination varenicline and nicotine gum/lozenges, or behavioral treatment strategies like cessation classes.
Another limitation was that some primary care practitioners prescribed varenicline but then referred these patients to a CPS for tobacco cessation follow-up. Per the study’s protocol, these patients were included within the other clinician group, which could have brought results closer to the null. Finally, the timing of this chart review (July 1, 2019, to July 31, 2020) intersects with the start of the COVID-19 pandemic, presenting a possible confounding factor if patients’ quit attempts were hindered by the stress and isolation of the pandemic.19 All pharmacist visits during the pandemic were conducted by telephone, which may have affected results.
Conclusions
In this study of veterans receiving varenicline, management by CPSs resulted in similar reductions of tobacco use and rates of complete abstinence compared with management by other clinicians. Pharmacist management was associated with greater adherence and shorter time to first follow-up compared with other clinicians. Additional research is needed to fully characterize the impact of pharmacist management of varenicline, justify expansion of clinical pharmacist scope of practice, and ultimately enhance patient outcomes regarding tobacco cessation.
It would be interesting to see more studies outside of the VA system to determine the impact of pharmacist management of varenicline for a more heterogenous patient population. At some point, a prospective controlled trial should be conducted to overcome the various confounding factors that limit the results of retrospective chart reviews
Acknowledgments
This article was prepared, and research was conducted with resources and the use of facilities at the Southern Arizona Veterans Affairs Health Care System in Tucson.
Tobacco smoking remains the leading cause of preventable disease and death in the United States, accounting for more than 480,000 deaths annually.1 An estimated 50.6 million US adults (20.8%) identify as tobacco users, with even higher rates among veterans (29.2%).2,3 Tobacco use is estimated to cost the US more than $300 billion annually in direct and indirect medical costs.4 According to a 2015 report, more than two-thirds of adult smokers reported a desire to quit, while only 7.5% reported successfully quitting in the past year.5 According to that same report, only 57.2% of smokers who had seen a health professional in the past year reported receiving advice to quit.5 This statistic is unfortunate, as interventions that combine behavioral and pharmacologic support can drastically increase tobacco cessation rates compared with self-help materials or no treatment.6
Currently, 7 first-line medications (5 nicotine, 2 nonnicotine) have been shown to increase long-term smoking abstinence rates. Varenicline was approved by the US Food and Drug Administration (FDA) in 2006 for use in adults as an aid to smoking cessation treatment. As a partial agonist of the α4β2 nicotinic acetylcholine receptor, varenicline’s mechanism of action is believed to involve reduction of nicotine’s rewarding capacity.7 Varenicline not only aids in complete tobacco cessation but also has been found to be effective for reducing cigarette consumption among smokers not yet willing or able to make a quit attempt.8 Furthermore, varenicline has demonstrated efficacy among users of smokeless tobacco in achieving continuous abstinence.9
Widespread adoption of varenicline into clinical practice was perhaps slowed by early concerns of psychiatric complications, prompting the FDA to issue a boxed warning for risk of serious neuropsychiatric events. This boxed warning was removed in 2016 in response to publication of the Evaluating Adverse Events in a Global Smoking Cessation Study (EAGLES). In this randomized controlled trial of more than 8000 participants, among whom 50.5% had a psychiatric disorder determined to be stable, varenicline significantly increased rates of continuous tobacco cessation compared with bupropion or the nicotine patch without an increased risk of neuropsychiatric events.10 This study underscored not only the safety of varenicline, but also its superiority over other first-line cessation products. The most recently published clinical practice guidelines recommend varenicline as a first-line agent for helping patients achieve long-term smoking cessation.11,12
Pharmacists are uniquely positioned to provide tobacco cessation interventions given their medication expertise and accessibility to the public. Indeed, multiple studies have demonstrated the effectiveness of pharmacist-led interventions on tobacco cessation.13-15 As of 2019, only 12 states had statutes or regulations addressing pharmacist prescribing of tobacco cessation aids without a collaborative practice agreement or local standing order.16 Until recently, most of these states limited pharmacists’ prescriptive authority to
Within the US Department of Veterans Affairs (VA), the clinical pharmacy specialist (CPS) is credentialed as an advanced practitioner with authority to independently manage patient medication therapy for a variety of diseases specified under a scope of practice. Although CPSs have provided tobacco cessation services for years, expansion of their scope to include varenicline did not occur until June 26, 2019, at the Southern Arizona VA Health Care System (SAVAHCS). All VA prescribers must follow the same criteria for prescribing varenicline. Unless previously trialed on varenicline, patients must have failed an appropriate trial of first-line agents (NRT, bupropion, or combination therapy) or have a contraindication to use of these first-line therapies before varenicline can be considered. Exclusions to therapy would include history of serious hypersensitivity to varenicline; suicidal intent, plan, or attempt within the past 12 months; current substance use disorder other than nicotine (unless varenicline recommended or prescribed by mental health professional); or unstable mental health disorder.18
The purpose of this study was to evaluate the efficacy and safety of CPS management of varenicline compared with other clinicians. We hope that this study provides insight regarding how the expansion of CPS scope to include prescriptive authority for varenicline has affected patient outcomes.
Methods
This retrospective chart review was conducted using SAVAHCS electronic health records. This study was granted approval by the institutional review board and the research and development committee at SAVAHCS. Data were obtained through the Computerized Patient Record System from the information provided by the pharmacist informatics department and was recorded electronically on a secure Microsoft Excel spreadsheet.
To be eligible for this study, patients must have been aged ≥ 18 years with a varenicline prescription between July 1, 2019, and July 31, 2020. Patients were excluded if tobacco cessation was managed by community-based (non-VA) clincians or if there was a lack of documentation of tobacco use at baseline and after at least 12 weeks of varenicline therapy. Sample size was not designed to achieve statistical power. Potential patients were queried by a pharmacist specializing in clinical informatics. All patients meeting initial inclusion criteria were then screened individually to evaluate for exclusion criteria.
Data collected included baseline age, sex, race, type of tobacco use (cigarettes, smokeless, both), mean daily tobacco use, prespecified comorbidities (depression, anxiety, or other psychiatric condition), and previous cessation medications prescribed (NRT, bupropion, and previous trials of varenicline).
The primary outcomes were reduction in tobacco use calculated as change at 12 weeks from baseline (and 24 weeks if available), continuous abstinence at 12 weeks (and 24 weeks if available), adherence to varenicline therapy measured by proportion of days covered (days covered by refills during the measurement period divided by days between the first fill and the end of the measurement period), and time to first follow-up in days. For safety evaluation, charts were reviewed for documented adverse events (AEs) in the health record. These AEs were categorized as follows: gastrointestinal, mood disturbance, sleep disturbance, headache, seizures, allergy, or other.
Statistical analyses regarding veteran baseline characteristics were descriptive in nature. χ2 test was used to analyze differences in complete cessation rates and AEs, whereas a Student t test was used to compare reductions of tobacco use, proportion of days covered (ie, adherence), and time to first follow-up. An α of .05 was used to determine significance.
Results
From the initial search, 255 charts met general inclusion criteria. After chart review, only 50 patients from the CPS group and 93 patients from the other clinician group met criteria to be included (Figure 1). The CPS group included pharmacists specializing in ambulatory care and outpatient mental health. The other clinician group was composed primarily of primary care practitioners, psychiatrists, and pulmonologists.
Overall, baseline characteristics were similar between the groups (Table 1). In the overall study population, the mean age was 57.5 years, 90% of patients were male, and 99% of patients were cigarette smokers. Baseline mean (SD) tobacco use was similar between the groups: 14.5 (10.8) vs 14.8 (8.6) cigarettes daily for the CPS and other clinician group, respectively.
While there was a significant reduction in daily cigarette use for both groups at 12 and 24 weeks (Figure 2), there was no mean (SD) between-group difference found among those patients prescribed varenicline by a CPS compared with other clinicians: -7.9 (10.4) vs -5.4 (9.8) cigarettes daily, respectively (P = .15) (Table 2). Change in tobacco use at 24 weeks and rates of complete tobacco abstinence were also not statistically significant between prescriber groups. Adherence (as evidenced by refill data) was higher in the CPS group than in the other clinician group (42% vs 31%, respectively; P = .01). There was also a significant difference in time to first follow-up; patients whose varenicline therapy was managed by a CPS had a mean (SD) follow-up time of 52 (66) vs 163 (110) days when patients were managed by other clinicians (P < .001). AEs were documented in 42% of patients in the CPS group compared with 23% of patients in the other clinician group (Table 3). The most reported AEs were gastrointestinal, as well as mood and sleep disturbances.
Discussion
The results of this single center study suggest that management of varenicline by CPSs is associated with similar reductions in tobacco use and abstinence rates compared with management by other clinicians. These results provide evidence that CPS management of varenicline may be as safe and effective as management by other clinicians.
Adherence rates (reported as proportion of days covered when assessing varenicline refill data) were higher on average among patients managed by a CPS compared with patients managed by other clinicians. However, this outcome may not be as reflective of adherence as initially intended, given delays in follow-up (see limitations section). Time to first follow-up was drastically different between the groups, with much sooner follow-up by CPSs compared with other clinicians. Despite similar tobacco cessation rates between groups, more frequent follow-up by CPSs helps to assess patient barriers to cessation, adherence to therapy, and AEs with varenicline. A higher percentage of AEs were documented within the CPS group that could be attributed to disparities in documentation rather than true rates of AEs. While rates of AEs were initially intended to serve as the primary safety outcome, they may instead reflect pharmacists’ diligence in monitoring and documenting tolerability of medication therapy.
Limitations
Several limitations to this study should be noted. First, the data collected were only as detailed as the extent to which prescribers documented tobacco use, previous cessation trials, and AEs; thus, various data points are likely missing within this study that could impact the results presented. In line with lack of documentation, delays in follow-up (ie, annual primary care visits) sorely undermined proportion of days covered, making these data less indicative of true medication adherence. Furthermore, this study did not account for concurrent therapies, such as combination varenicline and nicotine gum/lozenges, or behavioral treatment strategies like cessation classes.
Another limitation was that some primary care practitioners prescribed varenicline but then referred these patients to a CPS for tobacco cessation follow-up. Per the study’s protocol, these patients were included within the other clinician group, which could have brought results closer to the null. Finally, the timing of this chart review (July 1, 2019, to July 31, 2020) intersects with the start of the COVID-19 pandemic, presenting a possible confounding factor if patients’ quit attempts were hindered by the stress and isolation of the pandemic.19 All pharmacist visits during the pandemic were conducted by telephone, which may have affected results.
Conclusions
In this study of veterans receiving varenicline, management by CPSs resulted in similar reductions of tobacco use and rates of complete abstinence compared with management by other clinicians. Pharmacist management was associated with greater adherence and shorter time to first follow-up compared with other clinicians. Additional research is needed to fully characterize the impact of pharmacist management of varenicline, justify expansion of clinical pharmacist scope of practice, and ultimately enhance patient outcomes regarding tobacco cessation.
It would be interesting to see more studies outside of the VA system to determine the impact of pharmacist management of varenicline for a more heterogenous patient population. At some point, a prospective controlled trial should be conducted to overcome the various confounding factors that limit the results of retrospective chart reviews
Acknowledgments
This article was prepared, and research was conducted with resources and the use of facilities at the Southern Arizona Veterans Affairs Health Care System in Tucson.
1. Centers for Disease Control and Prevention. Current cigarette smoking among adults in the United States. Updated March 17, 2022. Accessed May 31, 2022. https://www.cdc.gov/tobacco/data_statistics/fact_sheets/adult_data/cig_smoking/index.htm 2. Cornelius ME, Wang TW, Jamal A, Loretan CG, Neff LJ. Tobacco product use among adults – United States, 2019. MMWR Morb Mortal Wkly Rep. 2020;69(46):1736-1742. doi:10.15585/mmwr.mm6946a4
3. Odani S, Agaku IT, Graffunder CM, Tynan MA, Armour BS. Tobacco product use among military veterans – United States, 2010-2015. MMWR Morb Mortal Wkly Rep. 2018;67(1):7-12. doi:10.15585/mmwr.mm6701a2
4. Hall W, Doran C. How much can the USA reduce health care costs by reducing smoking? PLoS Med. 2016;13(5):e1002021. doi:10.1371/journal.pmed.1002021.
5. Centers for Disease Control and Prevention. Smoking cessation: fast facts. Updated March 21, 2022. Accessed June 1, 2022. https://www.cdc.gov/tobacco/data_statistics/fact_sheets/cessation/smoking-cessation-fast-facts/index.html
6. US Public Health Service Office of the Surgeon General; National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health. Chapter 6, Interventions for smoking cessation and treatments for nicotine dependence. In: Smoking Cessation: A Report of the Surgeon General [Internet]. Washington, DC: US Department of Health and Human Services; 2020. Accessed June 1, 2022. https://www.ncbi.nlm.nih.gov/books/NBK555596
7. Rollema H, Chambers LK, Coe JW, et al. Pharmacological profile of the α4β2 nicotinic acetylcholine receptor partial agonist varenicline, an effective smoking cessation aid. Neuropharmacology. 2007;52(3):985-994. doi:10.1016/j.neuropharm.2006.10.016
8. Ebbert JO, Hughes JR, West RJ, et al. Effect of varenicline on smoking cessation through smoking reduction: a randomized clinical trial. JAMA. 2015;313(7):687-694. doi:10.1001/jama.2015.280
9. Fagerström K, Gilljam H, Metcalfe M, Tonstad S, Messig M. Stopping smokeless tobacco with varenicline: randomised double blind placebo controlled trial. BMJ. 2010;341:c6549. doi:10.1136/bmj.c6549
10. Anthenelli RM, Benowitz NL, West R, et al. Neuropsychiatric safety and efficacy of varenicline, bupropion, and nicotine patch in smokers with and without psychiatric disorders (EAGLES): a double-blind, randomised, placebo-controlled clinical trial. Lancet. 2016;387(10037):2507-2520. doi:10.1016/S0140-6736(16)30272-0
11. Barua RS, Rigotti NA, Benowitz NL, et al. 2018 ACC expert consensus decision pathway on tobacco cessation treatment: a report of the American College of Cardiology Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol. 2018;72(25):3332-3365. doi:10.1016/j.jacc.2018.10.027
12. Leone FT, Zhang Y, Evers-Casey S, et al. Initiating pharmacologic treatment in tobacco-dependent adults. An official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med. 2020;202(2):e5-e31. doi:10.1164/rccm.202005-1982ST
13. Saba M, Diep J, Saini B, Dhippayom T. Meta-analysis of the effectiveness of smoking cessation interventions in community pharmacy. J Clin Pharm Ther. 2014;39(3):240-247. doi:10.1111/jcpt.12131
14. Augustine JM, Taylor AM, Pelger M, Schiefer D, Warholak TL. Smoking quit rates among patients receiving pharmacist-provided pharmacotherapy and telephonic smoking cessation counseling. J Am Pharm Assoc. 2016;56(2):129-136. doi:10.1016/j.japh.2016.02.001
15. Dent LA, Harris KJ, Noonan CW. Tobacco interventions delivered by pharmacists: a summary and systematic review. Pharmacotherapy. 2007;27(7):1040-1051. doi:10.1592/phco.27.7.1040
16. National Alliance of State Pharmacy Associations. Pharmacist prescribing: tobacco cessation aids. February 10, 2021. Accessed June 1, 2022. https://naspa.us/resource/tobacco-cessation
17. Shen X, Bachyrycz A, Anderson JR, Tinker D, Raisch DW. Quitting patterns and predictors of success among participants in a tobacco cessation program provided by pharmacists in New Mexico. J Manag Care Spec Pharm. 2014;20(6):579-587. doi:10.18553/jmcp.2014.20.6.579
18. VA Center for Medication Safety, Tobacco Use Cessation Technical Advisory Group, Public Health Strategic Healthcare Group, VA Pharmacy Benefits Management Services, VISN Pharmacist Executives, and Medical Advisory Panel. Varenicline criteria for prescribing. 2008. Updated July 2011. Accessed June 9, 2022. https://www.healthquality.va.gov/tuc/VareniclineCriteriaforPrescribing.pdf
19. Jaklevic MC. COVID-19 and the “lost year” for smokers trying to quit. JAMA. 2021;325(19):1929-1930. doi:10.1001/jama.2021.5601
1. Centers for Disease Control and Prevention. Current cigarette smoking among adults in the United States. Updated March 17, 2022. Accessed May 31, 2022. https://www.cdc.gov/tobacco/data_statistics/fact_sheets/adult_data/cig_smoking/index.htm 2. Cornelius ME, Wang TW, Jamal A, Loretan CG, Neff LJ. Tobacco product use among adults – United States, 2019. MMWR Morb Mortal Wkly Rep. 2020;69(46):1736-1742. doi:10.15585/mmwr.mm6946a4
3. Odani S, Agaku IT, Graffunder CM, Tynan MA, Armour BS. Tobacco product use among military veterans – United States, 2010-2015. MMWR Morb Mortal Wkly Rep. 2018;67(1):7-12. doi:10.15585/mmwr.mm6701a2
4. Hall W, Doran C. How much can the USA reduce health care costs by reducing smoking? PLoS Med. 2016;13(5):e1002021. doi:10.1371/journal.pmed.1002021.
5. Centers for Disease Control and Prevention. Smoking cessation: fast facts. Updated March 21, 2022. Accessed June 1, 2022. https://www.cdc.gov/tobacco/data_statistics/fact_sheets/cessation/smoking-cessation-fast-facts/index.html
6. US Public Health Service Office of the Surgeon General; National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health. Chapter 6, Interventions for smoking cessation and treatments for nicotine dependence. In: Smoking Cessation: A Report of the Surgeon General [Internet]. Washington, DC: US Department of Health and Human Services; 2020. Accessed June 1, 2022. https://www.ncbi.nlm.nih.gov/books/NBK555596
7. Rollema H, Chambers LK, Coe JW, et al. Pharmacological profile of the α4β2 nicotinic acetylcholine receptor partial agonist varenicline, an effective smoking cessation aid. Neuropharmacology. 2007;52(3):985-994. doi:10.1016/j.neuropharm.2006.10.016
8. Ebbert JO, Hughes JR, West RJ, et al. Effect of varenicline on smoking cessation through smoking reduction: a randomized clinical trial. JAMA. 2015;313(7):687-694. doi:10.1001/jama.2015.280
9. Fagerström K, Gilljam H, Metcalfe M, Tonstad S, Messig M. Stopping smokeless tobacco with varenicline: randomised double blind placebo controlled trial. BMJ. 2010;341:c6549. doi:10.1136/bmj.c6549
10. Anthenelli RM, Benowitz NL, West R, et al. Neuropsychiatric safety and efficacy of varenicline, bupropion, and nicotine patch in smokers with and without psychiatric disorders (EAGLES): a double-blind, randomised, placebo-controlled clinical trial. Lancet. 2016;387(10037):2507-2520. doi:10.1016/S0140-6736(16)30272-0
11. Barua RS, Rigotti NA, Benowitz NL, et al. 2018 ACC expert consensus decision pathway on tobacco cessation treatment: a report of the American College of Cardiology Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol. 2018;72(25):3332-3365. doi:10.1016/j.jacc.2018.10.027
12. Leone FT, Zhang Y, Evers-Casey S, et al. Initiating pharmacologic treatment in tobacco-dependent adults. An official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med. 2020;202(2):e5-e31. doi:10.1164/rccm.202005-1982ST
13. Saba M, Diep J, Saini B, Dhippayom T. Meta-analysis of the effectiveness of smoking cessation interventions in community pharmacy. J Clin Pharm Ther. 2014;39(3):240-247. doi:10.1111/jcpt.12131
14. Augustine JM, Taylor AM, Pelger M, Schiefer D, Warholak TL. Smoking quit rates among patients receiving pharmacist-provided pharmacotherapy and telephonic smoking cessation counseling. J Am Pharm Assoc. 2016;56(2):129-136. doi:10.1016/j.japh.2016.02.001
15. Dent LA, Harris KJ, Noonan CW. Tobacco interventions delivered by pharmacists: a summary and systematic review. Pharmacotherapy. 2007;27(7):1040-1051. doi:10.1592/phco.27.7.1040
16. National Alliance of State Pharmacy Associations. Pharmacist prescribing: tobacco cessation aids. February 10, 2021. Accessed June 1, 2022. https://naspa.us/resource/tobacco-cessation
17. Shen X, Bachyrycz A, Anderson JR, Tinker D, Raisch DW. Quitting patterns and predictors of success among participants in a tobacco cessation program provided by pharmacists in New Mexico. J Manag Care Spec Pharm. 2014;20(6):579-587. doi:10.18553/jmcp.2014.20.6.579
18. VA Center for Medication Safety, Tobacco Use Cessation Technical Advisory Group, Public Health Strategic Healthcare Group, VA Pharmacy Benefits Management Services, VISN Pharmacist Executives, and Medical Advisory Panel. Varenicline criteria for prescribing. 2008. Updated July 2011. Accessed June 9, 2022. https://www.healthquality.va.gov/tuc/VareniclineCriteriaforPrescribing.pdf
19. Jaklevic MC. COVID-19 and the “lost year” for smokers trying to quit. JAMA. 2021;325(19):1929-1930. doi:10.1001/jama.2021.5601
A Veteran With Recurrent, Painful Knee Effusion
Case Presentation: A 39-year-old Air Force veteran was admitted to the US Department of Veterans Affairs Boston Healthcare System (VABHS) for evaluation of recurrent, painful right knee effusions. On presentation, his vital signs were stable, and the examination was significant for a right knee with a large effusion and tenderness to palpation without erythema or warmth. His white blood cell count was 12.0 cells/L with an erythrocyte sedimentation rate of 23 mm/h and C-reactive protein of 11.87 mg/L. He was in remission from alcohol use but had relapsed on alcohol in the past day to treat the pain. He had a history of IV drug use but was in remission. He was previously active and enjoyed long hikes. Nine months prior to presentation, he developed his first large right knee effusion associated with pain. He reported no antecedent trauma. At that time, he presented to another hospital and underwent arthrocentesis with orthopedic surgery, but this did not lead to a diagnosis, and the effusion reaccumulated within 24 hours. Four months later, he received a corticosteroid injection that provided only minor, temporary relief. He received 5 additional arthrocenteses over 9 months, all without definitive diagnosis and with rapid reaccumulation of the fluid. His most recent arthrocentesis was 3 weeks before admission.
►Lauren E. Merz, MD, MSc, Chief Medical Resident, VABHS: Dr. Jindal, what is your approach and differential diagnosis for joint effusions in hospitalized patients?
►Shivani Jindal, MD, MPH, Hospitalist, VABHS, Instructor in Medicine, Boston University School of Medicine (BUSM): A thorough history and physical examination are important. I specifically ask about chronicity, pain, and trauma. A medical history of potential infectious exposures and the history of the present illness are also important, such as the risk of sexually transmitted infections, exposure to Lyme disease or other viral illnesses. Gonococcal arthritis is one of the most common causes of nontraumatic monoarthritis in young adults but can also present as a migratory polyarthritis.1
It sounds like he was quite active and liked to hike so a history of tick exposure is important to ascertain. I would also ask about eye inflammation and back pain to assess possible ankylosing spondyarthritis. Other inflammatory etiologies, such as gout are common, but it would be surprising to miss this diagnosis on repeated arthocenteses. A physical examination can confirm monoarthritis over polyarthritis and assess for signs of inflammatory arthritis (eg, warmth and erythema). The most important etiology to assess for and rule out in a person admitted to the hospital is septic arthritis. The severe pain, mild leukocytosis, and mildly elevated inflammatory markers could be consistent with this diagnosis but are nonspecific. However, the chronicity of this patient’s presentation and hemodynamic stability make septic arthritis less likely overall and a more indolent infection or other inflammatory process more likely.
►Dr. Merz: The patient’s medical history included posttraumatic stress disorder (PTSD) and antisocial personality disorder with multiple prior suicide attempts. He also had a history of opioid use disorder (OUD) with prior overdose and alcohol use disorder (AUD). Given his stated preference to avoid opioids and normal liver function and liver chemistry testing, the initial treatment was with acetaminophen. After this failed to provide satisfactory pain control, IV hydromorphone was added.
Dr. Jindal, how do you approach pain control in the hospital for musculoskeletal issues like this?
►Dr. Jindal: Typically, nonsteroidal anti-inflammatory medications (NSAIDs) are most effective for musculoskeletal pain, often in the form of ketorolac or ibuprofen. However, we are often limited in our NSAID use by kidney disease, gastritis, or cardiovascular disease.
►Dr. Merz: On hospital day 1, the patient asked to leave to consume alcohol to ease unremitting pain. He also expressed suicidal ideation and discharge was therefore felt to be unsafe. He was reluctant to engage with psychiatry and became physically combative while attempting to leave the hospital, necessitating the use of sedating medications and physical restraints.
Dr. Shahal, what factors led to the decision to place an involuntary hold, and how do you balance patient autonomy and patient safety?
►Dr. Talya Shahal, MD, Consult-Liaison Psychiatry Service, VABHS, Instructor in Psychiatry, Harvard Medical School: This is a delicate balance that requires constant reassessment. The patient initially presented to the emergency department with suicidal ideation, stating he was not able to tolerate the pain and thus resumed alcohol consumption after a period of nonuse. He had multiple risk factors for suicide, including 9 prior suicide attempts with the latest less than a year before presentation, active substance use with alcohol and other recreational drugs, PTSD, pain, veteran status, male sex, single status, and a history of trauma.3,4 He was also displaying impulsivity and limited insight, did not engage in his psychiatric assessment, and attempted to assault staff. As such, his suicide risk was assessed to be high at the time of the evaluation, which led to the decision to place an involuntary hold. However, we reevaluate this decision at least daily in order to reassess the risk and ensure that the balance between patient safety and autonomy are maintained.
►Dr. Merz: The involuntary hold was removed within 48 hours as the patient remained calm and engaged with the primary and consulting teams. He requested escalating doses of opioids as he felt the short-acting IV medications were not providing sustained relief. However, he was also noted to be walking outside of the hospital without assistance, and he repeatedly declined nonopioid pain modalities as well as buprenorphine/naloxone. The chronic pain service was consulted but was unable to see the patient as he was frequently outside of his room.
Dr. Shahal, how do you address OUD, pain, and stigma in the hospital?
►Dr. Shahal: It is important to remember that patients with substance use disorder (SUD) and mental illness frequently have physical causes for pain and are often undertreated.5 Patients with SUD may also have higher tolerance for opioids and may need higher doses to treat the pain.5 Modalities like buprenorphine/naloxone can be effective to treat OUD and pain, but these usually cannot be initiated while the patient is on short-acting opioids as this would precipitate withdrawal.6 However, withdrawal can be managed while inpatient, and this can be a good time to start these medications as practitioners can aggressively help with symptom control. Proactively addressing mental health concerns, particularly anxiety, AUD, insomnia, PTSD, and depression, can also have a direct impact on the perception of pain and assist with better control.2 In addition, nonpharmacologic options, such as meditation, deep breathing, and even acupuncture and Reiki can be helpful and of course less harmful to treat pain.2
► Dr. Merz: An X-ray of the knee showed no acute fracture or joint space narrowing. Magnetic resonance imaging confirmed a large knee effusion with no evidence of ligament injury. Synovial fluid showed turbid, yellow fluid with 14,110 nucleated cells (84% segmented cells and 4000 RBCs). Gram stain was negative, culture had no growth, and there were no crystals. Anticyclic citrullinated peptide (anti-CCP), rheumatoid factor, HIV testing, and HLA-B27 were negative.
Dr. Serrao, what do these studies tell us about the joint effusion and the possible diagnoses?
► Dr. Richard Serrao, MD, Infectious Disease, VABHS, Clinical Associate Professor in Medicine, BUSM: I would expect the white blood cell (WBC) count to be > 50,000 cells with > 75% polymorphonuclear cells and a positive Gram stain if this was a bacterial infection resulting in septic arthritis.7 This patient’s studies are not consistent with this diagnosis nor is the chronicity of his presentation. There are 2 important bacteria that can present with inflammatory arthritis and less pronounced findings on arthrocentesis: Borrelia burgdorferi (the bacteria causing Lyme arthritis) and Neisseria gonorrhea. Lyme arthritis could be consistent with this relapsing remitting presentation as you expect a WBC count between 3000 and 100,000 cells with a mean value between 10,000 and 25,000 cells, > 50% polymorphonuclear leukocytes, and negative Gram stains.8 Gonococcal infections often do not have marked elevations in the WBC count and the Gram stain can be variable, but you still expect the WBC count to be > 30,000 cells.7 Inflammatory causes such as gout or autoimmune conditions such as lupus often have a WBC count between 2000 and 100,000 with a negative Gram stain, which could be consistent with this patient’s presentation.7 However, the lack of crystals rules out gout and the negative anti-CCP, rheumatoid factor, and HLA-B27 make rheumatologic diseases less likely.
►Dr. Merz: The patient received a phone call from another hospital where an arthrocentesis had been performed 3 weeks before. The results included a positive polymerase chain reaction (PCR) test for Lyme disease in the synovial fluid. A subsequent serum Lyme screen was positive for 1 of 3 immunoglobulin (Ig) M bands and 10 of 10 IgG bands.
Dr. Serrao, how does Lyme arthritis typically present, and are there aspects of this case that make you suspect the diagnosis? Does the serum Lyme test give us any additional information?
►Dr. Serrao: Lyme arthritis is a late manifestation of Lyme disease. Patients typically have persistent or intermittent arthritis, and large joints are more commonly impacted than small joints. Monoarthritis of the knee is the most common, but oligoarthritis is possible as well. The swelling usually begins abruptly, lasts for weeks to months, and effusions typically recur quickly after aspiration. These findings are consistent with the patient’s clinical history.
For diagnostics, the IgG Western blot is positive if 5 of the 10 bands are positive.9 This patient far exceeds the IgG band number to diagnose Lyme disease. All patients with Lyme arthritis will have positive IgG serologies since Lyme arthritis is a late manifestation of the infection. IgM reactivity may be present, but are not necessary to diagnose Lyme arthritis.10 Synovial fluid is often not analyzed for antibody responses as they are susceptible to false positive results, but synovial PCR testing like this patient had detects approximately 70% of patients with untreated Lyme arthritis.11 However, PCR positivity does not necessarily equate with active infection. Serologic testing for Lyme disease by enzyme-linked immunosorbent assay and Western blot as well as careful history and the exclusion of other diagnoses are usually sufficient to make the diagnosis.
► Dr. Merz: On further history the patient reported that 5 years prior he found a tick on his skin with a bull’s-eye rash. He was treated with 28 days of doxycycline at that time. He did not recall any tick bites or rashes in the years since.
Dr. Serrao, is it surprising that he developed Lyme arthritis 5 years after exposure and after being treated appropriately? What is the typical treatment approach for a patient like this?
►Dr. Serrao: It is atypical to develop Lyme arthritis 5 years after reported treatment of what appeared to be early localized disease, namely, erythema migrans. This stage is usually cured with 10 days of treatment alone (he received 28 days) and is generally abortive of subsequent stages, including Lyme arthritis. Furthermore, the patient reported no symptoms of arthritis until recently since that time. Therefore, one can argue that the excessively long span of time from treatment to these first episodes of arthritis suggests the patient could have been reinfected. When available, comparing the types and number of Western blot bands (eg, new and/or more bands on subsequent serologic testing) can support a reinfection diagnosis. A delayed postinfectious inflammatory process from excessive proinflammatory immune responses that block wound repair resulting in proliferative synovitis is also possible.12 This is defined as the postinfectious, postantibiotic, or antibiotic-refractory Lyme arthritis, a diagnosis of exclusion more apparent only after patients receive appropriate antibiotic courses for the possibility of untreated Lyme as an active infection.12
Given the inherent diagnostic uncertainty between an active infection and posttreatment Lyme arthritis syndromes, it is best to approach most cases of Lyme arthritis as an active infection first especially if not yet treated with antibiotics. Diagnosis of postinflammatory processes should be considered if symptoms persist after appropriate antibiotics, and then short-term use of disease-modifying antirheumatic drugs, rather than further courses of antibiotics, is recommended.
► Dr. Merz: The patient was initiated on doxycycline with the plan to transition to ceftriaxone if there was no response. One day after diagnosis and treatment initiation and in the setting of continued pain, the patient again asked to leave the hospital to drink alcohol. After eloping and becoming intoxicated with alcohol, he returned to his room. He remained concerned about his continued pain and lack of adequate pain control. At the time, he was receiving hydromorphone, ketorolac, lorazepam, gabapentin, and quetiapine.
Dr. Serrao, do you expect this degree of pain from Lyme arthritis?
► Dr. Serrao: Lyme arthritis is typically less painful than other forms of infectious or inflammatory arthritis. Pain is usually caused by the pressure from the acute accumulation and reaccumulation of fluid. In this case, the rapid accumulation of fluid that this patient experienced as well as relief with arthrocentesis suggests that the size and acuity of the effusion was causing great discomfort. Repeated arthrocentesis can prove to be a preventative strategy to minimize synovial herniation.
►Dr. Merz: Dr. Shahal, how do you balance the patient subjectively telling you that they are in pain with objective signs that they may be tolerating the pain like walking around unassisted? Is there anything else that could have been done to prevent this adverse outcome?
►Dr. Shahal: This is one of the hardest pieces of pain management. We want to practice beneficence by believing our patients and addressing their discomfort, but we also want to practice nonmaleficence by avoiding inappropriate long-term pain treatments like opioids that have significant harm as well as avoiding exacerbating this patient’s underlying SUD. An agent like buprenorphine/naloxone could have been an excellent fit to treat pain and SUD, but the patient’s lack of interest and the frequent use of short-acting opioids were major barriers. A chronic pain consult early on is helpful in cases like this as well, but they were unable to see him since he was often out of his room. Repeated arthrocentesis may also have helped the pain. Treatment of anxiety and insomnia with medications like hydroxyzine, trazodone, melatonin, gabapentin, or buspirone as well as interventions like sleep hygiene protocols or spiritual care may have helped somewhat as well.
We know that there is a vicious cycle between pain and poorly controlled mood symptoms. Many of our veterans have PTSD, anxiety, and SUD that are exacerbated by hospitalization and pain. Maintaining optimal communication between the patient and the practitioners, using trauma-informed care, understanding the patient’s goals of care, setting expectations and limits, and attempting to address the patient’s needs while attempting to minimize stigma might be helpful. However, despite optimal care, sometimes these events cannot be avoided.
►Dr. Merz: The patient was ultimately transferred to an inpatient psychiatric unit where a taper plan for the short-acting opioids was implemented. He was psychiatrically stabilized and discharged a few days later off opioids and on doxycycline. On follow-up a few weeks later, his pain had markedly improved, and the effusion was significantly reduced in size. His mood and impulsivity had stabilized. He continues to follow-up in the infectious disease clinic.

1. Siva C, Velazquez C, Mody A, Brasington R. Diagnosing acute monoarthritis in adults: a practical approach for the family physician. Am Fam Physician. 2003;68(1):83-90.
2. Qaseem A, McLean RM, O’Gurek D, et al. Nonpharmacologic and pharmacologic management of acute pain from non-low back, musculoskeletal injuries in adults: a clinical guideline from the American College of Physicians and American Academy of Family Physicians. Ann Intern Med. 2020;173(9):739-748. doi:10.7326/M19-3602
3. Silverman MM, Berman AL. Suicide risk assessment and risk formulation part I: a focus on suicide ideation in assessing suicide risk. Suicide Life Threat Behav. 2014;44(4):420-431. doi:10.1111/sltb.12065
4. Berman AL, Silverman MM. Suicide risk assessment and risk formulation part II: Suicide risk formulation and the determination of levels of risk. Suicide Life Threat Behav. 2014;44(4):432-443. doi:10.1111/sltb.12067
5. Quinlan J, Cox F. Acute pain management in patients with drug dependence syndrome. Pain Rep. 2017;2(4):e611. Published 2017 Jul 27. doi:10.1097/PR9.0000000000000611
6. Chou R, Wagner J, Ahmed AY, et al. Treatments for Acute Pain: A Systematic Review. Agency for Healthcare Research and Quality; 2020. https://www.ncbi.nlm.nih.gov/books/NBK566506/
7. Seidman AJ, Limaiem F. Synovial fluid analysis. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022. Updated May 8, 2022. https://www.ncbi.nlm.nih.gov/books/NBK537114
8. Arvikar SL, Steere AC. Diagnosis and treatment of Lyme arthritis. Infect Dis Clin North Am. 2015;29(2):269-280. doi:10.1016/j.idc.2015.02.004
9. Centers for Disease Control and Prevention. Recommendations for test performance and interpretation from the Second National Conference on Serologic Diagnosis of Lyme Disease. JAMA. 1995;274(12):937.
10. Craft JE, Grodzicki RL, Steere AC. Antibody response in Lyme disease: evaluation of diagnostic tests. J Infect Dis. 1984;149(5):789-795. doi:10.1093/infdis/149.5.789
11. Nocton JJ, Dressler F, Rutledge BJ, Rys PN, Persing DH, Steere AC. Detection of Borrelia burgdorferi DNA by polymerase chain reaction in synovial fluid from patients with Lyme arthritis. N Engl J Med. 1994;330(4):229-234. doi:10.1056/NEJM199401273300401
12. Steere AC. Posttreatment Lyme disease syndromes: distinct pathogenesis caused by maladaptive host responses. J Clin Invest. 2020;130(5):2148-2151. doi:10.1172/JCI138062
Case Presentation: A 39-year-old Air Force veteran was admitted to the US Department of Veterans Affairs Boston Healthcare System (VABHS) for evaluation of recurrent, painful right knee effusions. On presentation, his vital signs were stable, and the examination was significant for a right knee with a large effusion and tenderness to palpation without erythema or warmth. His white blood cell count was 12.0 cells/L with an erythrocyte sedimentation rate of 23 mm/h and C-reactive protein of 11.87 mg/L. He was in remission from alcohol use but had relapsed on alcohol in the past day to treat the pain. He had a history of IV drug use but was in remission. He was previously active and enjoyed long hikes. Nine months prior to presentation, he developed his first large right knee effusion associated with pain. He reported no antecedent trauma. At that time, he presented to another hospital and underwent arthrocentesis with orthopedic surgery, but this did not lead to a diagnosis, and the effusion reaccumulated within 24 hours. Four months later, he received a corticosteroid injection that provided only minor, temporary relief. He received 5 additional arthrocenteses over 9 months, all without definitive diagnosis and with rapid reaccumulation of the fluid. His most recent arthrocentesis was 3 weeks before admission.
►Lauren E. Merz, MD, MSc, Chief Medical Resident, VABHS: Dr. Jindal, what is your approach and differential diagnosis for joint effusions in hospitalized patients?
►Shivani Jindal, MD, MPH, Hospitalist, VABHS, Instructor in Medicine, Boston University School of Medicine (BUSM): A thorough history and physical examination are important. I specifically ask about chronicity, pain, and trauma. A medical history of potential infectious exposures and the history of the present illness are also important, such as the risk of sexually transmitted infections, exposure to Lyme disease or other viral illnesses. Gonococcal arthritis is one of the most common causes of nontraumatic monoarthritis in young adults but can also present as a migratory polyarthritis.1
It sounds like he was quite active and liked to hike so a history of tick exposure is important to ascertain. I would also ask about eye inflammation and back pain to assess possible ankylosing spondyarthritis. Other inflammatory etiologies, such as gout are common, but it would be surprising to miss this diagnosis on repeated arthocenteses. A physical examination can confirm monoarthritis over polyarthritis and assess for signs of inflammatory arthritis (eg, warmth and erythema). The most important etiology to assess for and rule out in a person admitted to the hospital is septic arthritis. The severe pain, mild leukocytosis, and mildly elevated inflammatory markers could be consistent with this diagnosis but are nonspecific. However, the chronicity of this patient’s presentation and hemodynamic stability make septic arthritis less likely overall and a more indolent infection or other inflammatory process more likely.
►Dr. Merz: The patient’s medical history included posttraumatic stress disorder (PTSD) and antisocial personality disorder with multiple prior suicide attempts. He also had a history of opioid use disorder (OUD) with prior overdose and alcohol use disorder (AUD). Given his stated preference to avoid opioids and normal liver function and liver chemistry testing, the initial treatment was with acetaminophen. After this failed to provide satisfactory pain control, IV hydromorphone was added.
Dr. Jindal, how do you approach pain control in the hospital for musculoskeletal issues like this?
►Dr. Jindal: Typically, nonsteroidal anti-inflammatory medications (NSAIDs) are most effective for musculoskeletal pain, often in the form of ketorolac or ibuprofen. However, we are often limited in our NSAID use by kidney disease, gastritis, or cardiovascular disease.
►Dr. Merz: On hospital day 1, the patient asked to leave to consume alcohol to ease unremitting pain. He also expressed suicidal ideation and discharge was therefore felt to be unsafe. He was reluctant to engage with psychiatry and became physically combative while attempting to leave the hospital, necessitating the use of sedating medications and physical restraints.
Dr. Shahal, what factors led to the decision to place an involuntary hold, and how do you balance patient autonomy and patient safety?
►Dr. Talya Shahal, MD, Consult-Liaison Psychiatry Service, VABHS, Instructor in Psychiatry, Harvard Medical School: This is a delicate balance that requires constant reassessment. The patient initially presented to the emergency department with suicidal ideation, stating he was not able to tolerate the pain and thus resumed alcohol consumption after a period of nonuse. He had multiple risk factors for suicide, including 9 prior suicide attempts with the latest less than a year before presentation, active substance use with alcohol and other recreational drugs, PTSD, pain, veteran status, male sex, single status, and a history of trauma.3,4 He was also displaying impulsivity and limited insight, did not engage in his psychiatric assessment, and attempted to assault staff. As such, his suicide risk was assessed to be high at the time of the evaluation, which led to the decision to place an involuntary hold. However, we reevaluate this decision at least daily in order to reassess the risk and ensure that the balance between patient safety and autonomy are maintained.
►Dr. Merz: The involuntary hold was removed within 48 hours as the patient remained calm and engaged with the primary and consulting teams. He requested escalating doses of opioids as he felt the short-acting IV medications were not providing sustained relief. However, he was also noted to be walking outside of the hospital without assistance, and he repeatedly declined nonopioid pain modalities as well as buprenorphine/naloxone. The chronic pain service was consulted but was unable to see the patient as he was frequently outside of his room.
Dr. Shahal, how do you address OUD, pain, and stigma in the hospital?
►Dr. Shahal: It is important to remember that patients with substance use disorder (SUD) and mental illness frequently have physical causes for pain and are often undertreated.5 Patients with SUD may also have higher tolerance for opioids and may need higher doses to treat the pain.5 Modalities like buprenorphine/naloxone can be effective to treat OUD and pain, but these usually cannot be initiated while the patient is on short-acting opioids as this would precipitate withdrawal.6 However, withdrawal can be managed while inpatient, and this can be a good time to start these medications as practitioners can aggressively help with symptom control. Proactively addressing mental health concerns, particularly anxiety, AUD, insomnia, PTSD, and depression, can also have a direct impact on the perception of pain and assist with better control.2 In addition, nonpharmacologic options, such as meditation, deep breathing, and even acupuncture and Reiki can be helpful and of course less harmful to treat pain.2
► Dr. Merz: An X-ray of the knee showed no acute fracture or joint space narrowing. Magnetic resonance imaging confirmed a large knee effusion with no evidence of ligament injury. Synovial fluid showed turbid, yellow fluid with 14,110 nucleated cells (84% segmented cells and 4000 RBCs). Gram stain was negative, culture had no growth, and there were no crystals. Anticyclic citrullinated peptide (anti-CCP), rheumatoid factor, HIV testing, and HLA-B27 were negative.
Dr. Serrao, what do these studies tell us about the joint effusion and the possible diagnoses?
► Dr. Richard Serrao, MD, Infectious Disease, VABHS, Clinical Associate Professor in Medicine, BUSM: I would expect the white blood cell (WBC) count to be > 50,000 cells with > 75% polymorphonuclear cells and a positive Gram stain if this was a bacterial infection resulting in septic arthritis.7 This patient’s studies are not consistent with this diagnosis nor is the chronicity of his presentation. There are 2 important bacteria that can present with inflammatory arthritis and less pronounced findings on arthrocentesis: Borrelia burgdorferi (the bacteria causing Lyme arthritis) and Neisseria gonorrhea. Lyme arthritis could be consistent with this relapsing remitting presentation as you expect a WBC count between 3000 and 100,000 cells with a mean value between 10,000 and 25,000 cells, > 50% polymorphonuclear leukocytes, and negative Gram stains.8 Gonococcal infections often do not have marked elevations in the WBC count and the Gram stain can be variable, but you still expect the WBC count to be > 30,000 cells.7 Inflammatory causes such as gout or autoimmune conditions such as lupus often have a WBC count between 2000 and 100,000 with a negative Gram stain, which could be consistent with this patient’s presentation.7 However, the lack of crystals rules out gout and the negative anti-CCP, rheumatoid factor, and HLA-B27 make rheumatologic diseases less likely.
►Dr. Merz: The patient received a phone call from another hospital where an arthrocentesis had been performed 3 weeks before. The results included a positive polymerase chain reaction (PCR) test for Lyme disease in the synovial fluid. A subsequent serum Lyme screen was positive for 1 of 3 immunoglobulin (Ig) M bands and 10 of 10 IgG bands.
Dr. Serrao, how does Lyme arthritis typically present, and are there aspects of this case that make you suspect the diagnosis? Does the serum Lyme test give us any additional information?
►Dr. Serrao: Lyme arthritis is a late manifestation of Lyme disease. Patients typically have persistent or intermittent arthritis, and large joints are more commonly impacted than small joints. Monoarthritis of the knee is the most common, but oligoarthritis is possible as well. The swelling usually begins abruptly, lasts for weeks to months, and effusions typically recur quickly after aspiration. These findings are consistent with the patient’s clinical history.
For diagnostics, the IgG Western blot is positive if 5 of the 10 bands are positive.9 This patient far exceeds the IgG band number to diagnose Lyme disease. All patients with Lyme arthritis will have positive IgG serologies since Lyme arthritis is a late manifestation of the infection. IgM reactivity may be present, but are not necessary to diagnose Lyme arthritis.10 Synovial fluid is often not analyzed for antibody responses as they are susceptible to false positive results, but synovial PCR testing like this patient had detects approximately 70% of patients with untreated Lyme arthritis.11 However, PCR positivity does not necessarily equate with active infection. Serologic testing for Lyme disease by enzyme-linked immunosorbent assay and Western blot as well as careful history and the exclusion of other diagnoses are usually sufficient to make the diagnosis.
► Dr. Merz: On further history the patient reported that 5 years prior he found a tick on his skin with a bull’s-eye rash. He was treated with 28 days of doxycycline at that time. He did not recall any tick bites or rashes in the years since.
Dr. Serrao, is it surprising that he developed Lyme arthritis 5 years after exposure and after being treated appropriately? What is the typical treatment approach for a patient like this?
►Dr. Serrao: It is atypical to develop Lyme arthritis 5 years after reported treatment of what appeared to be early localized disease, namely, erythema migrans. This stage is usually cured with 10 days of treatment alone (he received 28 days) and is generally abortive of subsequent stages, including Lyme arthritis. Furthermore, the patient reported no symptoms of arthritis until recently since that time. Therefore, one can argue that the excessively long span of time from treatment to these first episodes of arthritis suggests the patient could have been reinfected. When available, comparing the types and number of Western blot bands (eg, new and/or more bands on subsequent serologic testing) can support a reinfection diagnosis. A delayed postinfectious inflammatory process from excessive proinflammatory immune responses that block wound repair resulting in proliferative synovitis is also possible.12 This is defined as the postinfectious, postantibiotic, or antibiotic-refractory Lyme arthritis, a diagnosis of exclusion more apparent only after patients receive appropriate antibiotic courses for the possibility of untreated Lyme as an active infection.12
Given the inherent diagnostic uncertainty between an active infection and posttreatment Lyme arthritis syndromes, it is best to approach most cases of Lyme arthritis as an active infection first especially if not yet treated with antibiotics. Diagnosis of postinflammatory processes should be considered if symptoms persist after appropriate antibiotics, and then short-term use of disease-modifying antirheumatic drugs, rather than further courses of antibiotics, is recommended.
► Dr. Merz: The patient was initiated on doxycycline with the plan to transition to ceftriaxone if there was no response. One day after diagnosis and treatment initiation and in the setting of continued pain, the patient again asked to leave the hospital to drink alcohol. After eloping and becoming intoxicated with alcohol, he returned to his room. He remained concerned about his continued pain and lack of adequate pain control. At the time, he was receiving hydromorphone, ketorolac, lorazepam, gabapentin, and quetiapine.
Dr. Serrao, do you expect this degree of pain from Lyme arthritis?
► Dr. Serrao: Lyme arthritis is typically less painful than other forms of infectious or inflammatory arthritis. Pain is usually caused by the pressure from the acute accumulation and reaccumulation of fluid. In this case, the rapid accumulation of fluid that this patient experienced as well as relief with arthrocentesis suggests that the size and acuity of the effusion was causing great discomfort. Repeated arthrocentesis can prove to be a preventative strategy to minimize synovial herniation.
►Dr. Merz: Dr. Shahal, how do you balance the patient subjectively telling you that they are in pain with objective signs that they may be tolerating the pain like walking around unassisted? Is there anything else that could have been done to prevent this adverse outcome?
►Dr. Shahal: This is one of the hardest pieces of pain management. We want to practice beneficence by believing our patients and addressing their discomfort, but we also want to practice nonmaleficence by avoiding inappropriate long-term pain treatments like opioids that have significant harm as well as avoiding exacerbating this patient’s underlying SUD. An agent like buprenorphine/naloxone could have been an excellent fit to treat pain and SUD, but the patient’s lack of interest and the frequent use of short-acting opioids were major barriers. A chronic pain consult early on is helpful in cases like this as well, but they were unable to see him since he was often out of his room. Repeated arthrocentesis may also have helped the pain. Treatment of anxiety and insomnia with medications like hydroxyzine, trazodone, melatonin, gabapentin, or buspirone as well as interventions like sleep hygiene protocols or spiritual care may have helped somewhat as well.
We know that there is a vicious cycle between pain and poorly controlled mood symptoms. Many of our veterans have PTSD, anxiety, and SUD that are exacerbated by hospitalization and pain. Maintaining optimal communication between the patient and the practitioners, using trauma-informed care, understanding the patient’s goals of care, setting expectations and limits, and attempting to address the patient’s needs while attempting to minimize stigma might be helpful. However, despite optimal care, sometimes these events cannot be avoided.
►Dr. Merz: The patient was ultimately transferred to an inpatient psychiatric unit where a taper plan for the short-acting opioids was implemented. He was psychiatrically stabilized and discharged a few days later off opioids and on doxycycline. On follow-up a few weeks later, his pain had markedly improved, and the effusion was significantly reduced in size. His mood and impulsivity had stabilized. He continues to follow-up in the infectious disease clinic.

Case Presentation: A 39-year-old Air Force veteran was admitted to the US Department of Veterans Affairs Boston Healthcare System (VABHS) for evaluation of recurrent, painful right knee effusions. On presentation, his vital signs were stable, and the examination was significant for a right knee with a large effusion and tenderness to palpation without erythema or warmth. His white blood cell count was 12.0 cells/L with an erythrocyte sedimentation rate of 23 mm/h and C-reactive protein of 11.87 mg/L. He was in remission from alcohol use but had relapsed on alcohol in the past day to treat the pain. He had a history of IV drug use but was in remission. He was previously active and enjoyed long hikes. Nine months prior to presentation, he developed his first large right knee effusion associated with pain. He reported no antecedent trauma. At that time, he presented to another hospital and underwent arthrocentesis with orthopedic surgery, but this did not lead to a diagnosis, and the effusion reaccumulated within 24 hours. Four months later, he received a corticosteroid injection that provided only minor, temporary relief. He received 5 additional arthrocenteses over 9 months, all without definitive diagnosis and with rapid reaccumulation of the fluid. His most recent arthrocentesis was 3 weeks before admission.
►Lauren E. Merz, MD, MSc, Chief Medical Resident, VABHS: Dr. Jindal, what is your approach and differential diagnosis for joint effusions in hospitalized patients?
►Shivani Jindal, MD, MPH, Hospitalist, VABHS, Instructor in Medicine, Boston University School of Medicine (BUSM): A thorough history and physical examination are important. I specifically ask about chronicity, pain, and trauma. A medical history of potential infectious exposures and the history of the present illness are also important, such as the risk of sexually transmitted infections, exposure to Lyme disease or other viral illnesses. Gonococcal arthritis is one of the most common causes of nontraumatic monoarthritis in young adults but can also present as a migratory polyarthritis.1
It sounds like he was quite active and liked to hike so a history of tick exposure is important to ascertain. I would also ask about eye inflammation and back pain to assess possible ankylosing spondyarthritis. Other inflammatory etiologies, such as gout are common, but it would be surprising to miss this diagnosis on repeated arthocenteses. A physical examination can confirm monoarthritis over polyarthritis and assess for signs of inflammatory arthritis (eg, warmth and erythema). The most important etiology to assess for and rule out in a person admitted to the hospital is septic arthritis. The severe pain, mild leukocytosis, and mildly elevated inflammatory markers could be consistent with this diagnosis but are nonspecific. However, the chronicity of this patient’s presentation and hemodynamic stability make septic arthritis less likely overall and a more indolent infection or other inflammatory process more likely.
►Dr. Merz: The patient’s medical history included posttraumatic stress disorder (PTSD) and antisocial personality disorder with multiple prior suicide attempts. He also had a history of opioid use disorder (OUD) with prior overdose and alcohol use disorder (AUD). Given his stated preference to avoid opioids and normal liver function and liver chemistry testing, the initial treatment was with acetaminophen. After this failed to provide satisfactory pain control, IV hydromorphone was added.
Dr. Jindal, how do you approach pain control in the hospital for musculoskeletal issues like this?
►Dr. Jindal: Typically, nonsteroidal anti-inflammatory medications (NSAIDs) are most effective for musculoskeletal pain, often in the form of ketorolac or ibuprofen. However, we are often limited in our NSAID use by kidney disease, gastritis, or cardiovascular disease.
►Dr. Merz: On hospital day 1, the patient asked to leave to consume alcohol to ease unremitting pain. He also expressed suicidal ideation and discharge was therefore felt to be unsafe. He was reluctant to engage with psychiatry and became physically combative while attempting to leave the hospital, necessitating the use of sedating medications and physical restraints.
Dr. Shahal, what factors led to the decision to place an involuntary hold, and how do you balance patient autonomy and patient safety?
►Dr. Talya Shahal, MD, Consult-Liaison Psychiatry Service, VABHS, Instructor in Psychiatry, Harvard Medical School: This is a delicate balance that requires constant reassessment. The patient initially presented to the emergency department with suicidal ideation, stating he was not able to tolerate the pain and thus resumed alcohol consumption after a period of nonuse. He had multiple risk factors for suicide, including 9 prior suicide attempts with the latest less than a year before presentation, active substance use with alcohol and other recreational drugs, PTSD, pain, veteran status, male sex, single status, and a history of trauma.3,4 He was also displaying impulsivity and limited insight, did not engage in his psychiatric assessment, and attempted to assault staff. As such, his suicide risk was assessed to be high at the time of the evaluation, which led to the decision to place an involuntary hold. However, we reevaluate this decision at least daily in order to reassess the risk and ensure that the balance between patient safety and autonomy are maintained.
►Dr. Merz: The involuntary hold was removed within 48 hours as the patient remained calm and engaged with the primary and consulting teams. He requested escalating doses of opioids as he felt the short-acting IV medications were not providing sustained relief. However, he was also noted to be walking outside of the hospital without assistance, and he repeatedly declined nonopioid pain modalities as well as buprenorphine/naloxone. The chronic pain service was consulted but was unable to see the patient as he was frequently outside of his room.
Dr. Shahal, how do you address OUD, pain, and stigma in the hospital?
►Dr. Shahal: It is important to remember that patients with substance use disorder (SUD) and mental illness frequently have physical causes for pain and are often undertreated.5 Patients with SUD may also have higher tolerance for opioids and may need higher doses to treat the pain.5 Modalities like buprenorphine/naloxone can be effective to treat OUD and pain, but these usually cannot be initiated while the patient is on short-acting opioids as this would precipitate withdrawal.6 However, withdrawal can be managed while inpatient, and this can be a good time to start these medications as practitioners can aggressively help with symptom control. Proactively addressing mental health concerns, particularly anxiety, AUD, insomnia, PTSD, and depression, can also have a direct impact on the perception of pain and assist with better control.2 In addition, nonpharmacologic options, such as meditation, deep breathing, and even acupuncture and Reiki can be helpful and of course less harmful to treat pain.2
► Dr. Merz: An X-ray of the knee showed no acute fracture or joint space narrowing. Magnetic resonance imaging confirmed a large knee effusion with no evidence of ligament injury. Synovial fluid showed turbid, yellow fluid with 14,110 nucleated cells (84% segmented cells and 4000 RBCs). Gram stain was negative, culture had no growth, and there were no crystals. Anticyclic citrullinated peptide (anti-CCP), rheumatoid factor, HIV testing, and HLA-B27 were negative.
Dr. Serrao, what do these studies tell us about the joint effusion and the possible diagnoses?
► Dr. Richard Serrao, MD, Infectious Disease, VABHS, Clinical Associate Professor in Medicine, BUSM: I would expect the white blood cell (WBC) count to be > 50,000 cells with > 75% polymorphonuclear cells and a positive Gram stain if this was a bacterial infection resulting in septic arthritis.7 This patient’s studies are not consistent with this diagnosis nor is the chronicity of his presentation. There are 2 important bacteria that can present with inflammatory arthritis and less pronounced findings on arthrocentesis: Borrelia burgdorferi (the bacteria causing Lyme arthritis) and Neisseria gonorrhea. Lyme arthritis could be consistent with this relapsing remitting presentation as you expect a WBC count between 3000 and 100,000 cells with a mean value between 10,000 and 25,000 cells, > 50% polymorphonuclear leukocytes, and negative Gram stains.8 Gonococcal infections often do not have marked elevations in the WBC count and the Gram stain can be variable, but you still expect the WBC count to be > 30,000 cells.7 Inflammatory causes such as gout or autoimmune conditions such as lupus often have a WBC count between 2000 and 100,000 with a negative Gram stain, which could be consistent with this patient’s presentation.7 However, the lack of crystals rules out gout and the negative anti-CCP, rheumatoid factor, and HLA-B27 make rheumatologic diseases less likely.
►Dr. Merz: The patient received a phone call from another hospital where an arthrocentesis had been performed 3 weeks before. The results included a positive polymerase chain reaction (PCR) test for Lyme disease in the synovial fluid. A subsequent serum Lyme screen was positive for 1 of 3 immunoglobulin (Ig) M bands and 10 of 10 IgG bands.
Dr. Serrao, how does Lyme arthritis typically present, and are there aspects of this case that make you suspect the diagnosis? Does the serum Lyme test give us any additional information?
►Dr. Serrao: Lyme arthritis is a late manifestation of Lyme disease. Patients typically have persistent or intermittent arthritis, and large joints are more commonly impacted than small joints. Monoarthritis of the knee is the most common, but oligoarthritis is possible as well. The swelling usually begins abruptly, lasts for weeks to months, and effusions typically recur quickly after aspiration. These findings are consistent with the patient’s clinical history.
For diagnostics, the IgG Western blot is positive if 5 of the 10 bands are positive.9 This patient far exceeds the IgG band number to diagnose Lyme disease. All patients with Lyme arthritis will have positive IgG serologies since Lyme arthritis is a late manifestation of the infection. IgM reactivity may be present, but are not necessary to diagnose Lyme arthritis.10 Synovial fluid is often not analyzed for antibody responses as they are susceptible to false positive results, but synovial PCR testing like this patient had detects approximately 70% of patients with untreated Lyme arthritis.11 However, PCR positivity does not necessarily equate with active infection. Serologic testing for Lyme disease by enzyme-linked immunosorbent assay and Western blot as well as careful history and the exclusion of other diagnoses are usually sufficient to make the diagnosis.
► Dr. Merz: On further history the patient reported that 5 years prior he found a tick on his skin with a bull’s-eye rash. He was treated with 28 days of doxycycline at that time. He did not recall any tick bites or rashes in the years since.
Dr. Serrao, is it surprising that he developed Lyme arthritis 5 years after exposure and after being treated appropriately? What is the typical treatment approach for a patient like this?
►Dr. Serrao: It is atypical to develop Lyme arthritis 5 years after reported treatment of what appeared to be early localized disease, namely, erythema migrans. This stage is usually cured with 10 days of treatment alone (he received 28 days) and is generally abortive of subsequent stages, including Lyme arthritis. Furthermore, the patient reported no symptoms of arthritis until recently since that time. Therefore, one can argue that the excessively long span of time from treatment to these first episodes of arthritis suggests the patient could have been reinfected. When available, comparing the types and number of Western blot bands (eg, new and/or more bands on subsequent serologic testing) can support a reinfection diagnosis. A delayed postinfectious inflammatory process from excessive proinflammatory immune responses that block wound repair resulting in proliferative synovitis is also possible.12 This is defined as the postinfectious, postantibiotic, or antibiotic-refractory Lyme arthritis, a diagnosis of exclusion more apparent only after patients receive appropriate antibiotic courses for the possibility of untreated Lyme as an active infection.12
Given the inherent diagnostic uncertainty between an active infection and posttreatment Lyme arthritis syndromes, it is best to approach most cases of Lyme arthritis as an active infection first especially if not yet treated with antibiotics. Diagnosis of postinflammatory processes should be considered if symptoms persist after appropriate antibiotics, and then short-term use of disease-modifying antirheumatic drugs, rather than further courses of antibiotics, is recommended.
► Dr. Merz: The patient was initiated on doxycycline with the plan to transition to ceftriaxone if there was no response. One day after diagnosis and treatment initiation and in the setting of continued pain, the patient again asked to leave the hospital to drink alcohol. After eloping and becoming intoxicated with alcohol, he returned to his room. He remained concerned about his continued pain and lack of adequate pain control. At the time, he was receiving hydromorphone, ketorolac, lorazepam, gabapentin, and quetiapine.
Dr. Serrao, do you expect this degree of pain from Lyme arthritis?
► Dr. Serrao: Lyme arthritis is typically less painful than other forms of infectious or inflammatory arthritis. Pain is usually caused by the pressure from the acute accumulation and reaccumulation of fluid. In this case, the rapid accumulation of fluid that this patient experienced as well as relief with arthrocentesis suggests that the size and acuity of the effusion was causing great discomfort. Repeated arthrocentesis can prove to be a preventative strategy to minimize synovial herniation.
►Dr. Merz: Dr. Shahal, how do you balance the patient subjectively telling you that they are in pain with objective signs that they may be tolerating the pain like walking around unassisted? Is there anything else that could have been done to prevent this adverse outcome?
►Dr. Shahal: This is one of the hardest pieces of pain management. We want to practice beneficence by believing our patients and addressing their discomfort, but we also want to practice nonmaleficence by avoiding inappropriate long-term pain treatments like opioids that have significant harm as well as avoiding exacerbating this patient’s underlying SUD. An agent like buprenorphine/naloxone could have been an excellent fit to treat pain and SUD, but the patient’s lack of interest and the frequent use of short-acting opioids were major barriers. A chronic pain consult early on is helpful in cases like this as well, but they were unable to see him since he was often out of his room. Repeated arthrocentesis may also have helped the pain. Treatment of anxiety and insomnia with medications like hydroxyzine, trazodone, melatonin, gabapentin, or buspirone as well as interventions like sleep hygiene protocols or spiritual care may have helped somewhat as well.
We know that there is a vicious cycle between pain and poorly controlled mood symptoms. Many of our veterans have PTSD, anxiety, and SUD that are exacerbated by hospitalization and pain. Maintaining optimal communication between the patient and the practitioners, using trauma-informed care, understanding the patient’s goals of care, setting expectations and limits, and attempting to address the patient’s needs while attempting to minimize stigma might be helpful. However, despite optimal care, sometimes these events cannot be avoided.
►Dr. Merz: The patient was ultimately transferred to an inpatient psychiatric unit where a taper plan for the short-acting opioids was implemented. He was psychiatrically stabilized and discharged a few days later off opioids and on doxycycline. On follow-up a few weeks later, his pain had markedly improved, and the effusion was significantly reduced in size. His mood and impulsivity had stabilized. He continues to follow-up in the infectious disease clinic.

1. Siva C, Velazquez C, Mody A, Brasington R. Diagnosing acute monoarthritis in adults: a practical approach for the family physician. Am Fam Physician. 2003;68(1):83-90.
2. Qaseem A, McLean RM, O’Gurek D, et al. Nonpharmacologic and pharmacologic management of acute pain from non-low back, musculoskeletal injuries in adults: a clinical guideline from the American College of Physicians and American Academy of Family Physicians. Ann Intern Med. 2020;173(9):739-748. doi:10.7326/M19-3602
3. Silverman MM, Berman AL. Suicide risk assessment and risk formulation part I: a focus on suicide ideation in assessing suicide risk. Suicide Life Threat Behav. 2014;44(4):420-431. doi:10.1111/sltb.12065
4. Berman AL, Silverman MM. Suicide risk assessment and risk formulation part II: Suicide risk formulation and the determination of levels of risk. Suicide Life Threat Behav. 2014;44(4):432-443. doi:10.1111/sltb.12067
5. Quinlan J, Cox F. Acute pain management in patients with drug dependence syndrome. Pain Rep. 2017;2(4):e611. Published 2017 Jul 27. doi:10.1097/PR9.0000000000000611
6. Chou R, Wagner J, Ahmed AY, et al. Treatments for Acute Pain: A Systematic Review. Agency for Healthcare Research and Quality; 2020. https://www.ncbi.nlm.nih.gov/books/NBK566506/
7. Seidman AJ, Limaiem F. Synovial fluid analysis. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022. Updated May 8, 2022. https://www.ncbi.nlm.nih.gov/books/NBK537114
8. Arvikar SL, Steere AC. Diagnosis and treatment of Lyme arthritis. Infect Dis Clin North Am. 2015;29(2):269-280. doi:10.1016/j.idc.2015.02.004
9. Centers for Disease Control and Prevention. Recommendations for test performance and interpretation from the Second National Conference on Serologic Diagnosis of Lyme Disease. JAMA. 1995;274(12):937.
10. Craft JE, Grodzicki RL, Steere AC. Antibody response in Lyme disease: evaluation of diagnostic tests. J Infect Dis. 1984;149(5):789-795. doi:10.1093/infdis/149.5.789
11. Nocton JJ, Dressler F, Rutledge BJ, Rys PN, Persing DH, Steere AC. Detection of Borrelia burgdorferi DNA by polymerase chain reaction in synovial fluid from patients with Lyme arthritis. N Engl J Med. 1994;330(4):229-234. doi:10.1056/NEJM199401273300401
12. Steere AC. Posttreatment Lyme disease syndromes: distinct pathogenesis caused by maladaptive host responses. J Clin Invest. 2020;130(5):2148-2151. doi:10.1172/JCI138062
1. Siva C, Velazquez C, Mody A, Brasington R. Diagnosing acute monoarthritis in adults: a practical approach for the family physician. Am Fam Physician. 2003;68(1):83-90.
2. Qaseem A, McLean RM, O’Gurek D, et al. Nonpharmacologic and pharmacologic management of acute pain from non-low back, musculoskeletal injuries in adults: a clinical guideline from the American College of Physicians and American Academy of Family Physicians. Ann Intern Med. 2020;173(9):739-748. doi:10.7326/M19-3602
3. Silverman MM, Berman AL. Suicide risk assessment and risk formulation part I: a focus on suicide ideation in assessing suicide risk. Suicide Life Threat Behav. 2014;44(4):420-431. doi:10.1111/sltb.12065
4. Berman AL, Silverman MM. Suicide risk assessment and risk formulation part II: Suicide risk formulation and the determination of levels of risk. Suicide Life Threat Behav. 2014;44(4):432-443. doi:10.1111/sltb.12067
5. Quinlan J, Cox F. Acute pain management in patients with drug dependence syndrome. Pain Rep. 2017;2(4):e611. Published 2017 Jul 27. doi:10.1097/PR9.0000000000000611
6. Chou R, Wagner J, Ahmed AY, et al. Treatments for Acute Pain: A Systematic Review. Agency for Healthcare Research and Quality; 2020. https://www.ncbi.nlm.nih.gov/books/NBK566506/
7. Seidman AJ, Limaiem F. Synovial fluid analysis. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022. Updated May 8, 2022. https://www.ncbi.nlm.nih.gov/books/NBK537114
8. Arvikar SL, Steere AC. Diagnosis and treatment of Lyme arthritis. Infect Dis Clin North Am. 2015;29(2):269-280. doi:10.1016/j.idc.2015.02.004
9. Centers for Disease Control and Prevention. Recommendations for test performance and interpretation from the Second National Conference on Serologic Diagnosis of Lyme Disease. JAMA. 1995;274(12):937.
10. Craft JE, Grodzicki RL, Steere AC. Antibody response in Lyme disease: evaluation of diagnostic tests. J Infect Dis. 1984;149(5):789-795. doi:10.1093/infdis/149.5.789
11. Nocton JJ, Dressler F, Rutledge BJ, Rys PN, Persing DH, Steere AC. Detection of Borrelia burgdorferi DNA by polymerase chain reaction in synovial fluid from patients with Lyme arthritis. N Engl J Med. 1994;330(4):229-234. doi:10.1056/NEJM199401273300401
12. Steere AC. Posttreatment Lyme disease syndromes: distinct pathogenesis caused by maladaptive host responses. J Clin Invest. 2020;130(5):2148-2151. doi:10.1172/JCI138062
Appropriateness of Pharmacologic Thromboprophylaxis Prescribing Based on Padua Score Among Inpatient Veterans
Venous thromboembolism (VTE) presents as deep venous thromboembolism (DVT) or pulmonary embolism (PE). VTE is the third most common vascular disease and a leading cardiovascular complication.1,2 Hospitalized patients are at increased risk of developing VTE due to multiple factors such as inflammatory processes from acute illness, recent surgery or trauma leading to hypercoagulable states, and prolonged periods of immobilization.3 Additional risk factors for complications include presence of malignancy, obesity, and prior history of VTE. About half of VTE cases in the community setting occur as a result of a hospital admission for recent or ongoing acute illness or surgery.1 Hospitalized patients are often categorized as high risk for VTE, and this risk may persist postdischarge.4
The risk of hospital-associated VTE may be mitigated with either mechanical or pharmacologic thromboprophylaxis.5 Risk assessment models (RAMs), such as Padua Prediction Score (PPS) and IMPROVEDD, have been developed to assist in evaluating hospitalized patients’ risk of VTE and need for pharmacologic thromboprophylaxis (Table 1).1,5 The PPS is externally validated and can assist clinicians in VTE risk assessment when integrated into clinical decision making.6 Patients with a PPS ≥ 4 are deemed high risk for VTE, and pharmacologic thromboprophylaxis is indicated as long as the patient is not at high risk for bleeding. IMPROVEDD added D-dimer as an additional risk factor to IMPROVE and was validated in 2017 to help predict the risk of symptomatic VTE in acutely ill patients hospitalized for up to 77 days.7 IMPROVEDD scores ≥ 2 identify patients at high risk for symptomatic VTE through 77 days hospitalization, while scores ≥ 4 identify patients who may qualify for extended thromboprophylaxis.7 Despite their utility, RAMs may not be used appropriately within clinical practice, and whether patients should receive extended-duration thromboprophylaxis postdischarge and for how long is debatable.5
VTE events contribute to increased health care spending, morbidity, and mortality, thus it is imperative to evaluate current hospital practices with respect to appropriate prescribing of pharmacologic thromboprophylaxis.8 Appropriately identifying high-risk patients and prescribing pharmacologic thromboprophylaxis to limit preventable VTEs is essential. Conversely, it is important to withhold pharmacologic thromboprophylaxis from those deemed low risk to limit bleeding complications.9 Health care professionals must be good stewards of anticoagulant prescribing when implementing these tools along with clinical knowledge to weigh the risks vs benefits to promote medication safety and prevent further complications.10This quality improvement project aimed to evaluate if VTE thromboprophylaxis was appropriately given or withheld in hospitalized medical patients based on PPS calculated upon admission using a link to an online calculator embedded within an admission order set. Additionally, this study aimed to characterize patients readmitted for VTE within 45 days postdischarge to generate hypotheses for future stu
Methods
This was an observational, retrospective cohort study that took place at the US Department of Veterans Affairs (VA) Tennessee Valley Healthcare System (TVHS). TVHS is a multisite health care system with campuses in Nashville and Murfreesboro. Clinical pharmacists employed at the study site and the primary research investigators designed this study and oversaw its execution. The study was reviewed and deemed exempt as a quality improvement study by the TVHS Institutional Review Board.
This study included adult veterans aged ≥ 18 years admitted to a general medicine floor or the medical intensive care unit between June 1, 2017, and June 30, 2020. Patients were excluded if they were on chronic therapeutic anticoagulation prior to their index hospitalization, required therapeutic anticoagulation on admission for index hospitalization (ie, acute coronary syndrome requiring a heparin drip), or were bedded within the surgical intensive care unit. All patients admitted to the TVHS within the prespecified date range were extracted from the electronic health record. A second subset of patients meeting inclusion criteria and readmitted for VTE within 45 days of index hospitalization with International Classification of Diseases, Tenth Revision (ICD-10) descriptions including thrombosis or embolism were extracted for review of a secondary endpoint. Patients with preexisting clots, history of prior DVT or PE, or history of portal vein thrombosis were not reviewed.
The primary endpoint was the percentage of patients for whom pharmacologic thromboprophylaxis was appropriately initiated or withheld based on a PPS calculated upon admission (Table 2). PPS was chosen for review as it is the only RAM currently used at TVHS. Secondary endpoints were the percentage of patients with documented rationale for ordering thromboprophylaxis when not indicated, based on PPS, or withholding despite indication as well as the number of patients readmitted to TVHS for VTE within 45 days of discharge with IMPROVEDD scores ≥ 4 and < 4 (eAppendix available at doi:10.12788/fp.0291). The primary investigators performed a manual health record review of all patients meeting inclusion criteria. Descriptive statistics were used given this was a quality improvement study, therefore, sample size and power calculations were not necessary. Data were stored in Microsoft Excel spreadsheets that were encrypted and password protected. To maintain security of personal health information, all study files were kept on the TVHS internal network, and access was limited to the research investigators.
Results
Two hundred fifty patients meeting inclusion criteria were randomly selected for review for the primary endpoint. Of the patients reviewed for the primary endpoint, 118 had a PPS < 4 and 132 a PPS ≥ 4 (Figure). Pharmacologic thromboprophylaxis was inappropriately given or withheld based on their PPS for 91 (36.4%) patients. This included 58 (49.2%) patients in the low-risk group (PPS < 4) who had thromboprophylaxis inappropriately given and 33 (25.0%) patients in the high-risk group (PPS ≥ 4) who had thromboprophylaxis inappropriately withheld. Of the 58 patients with a PPS < 4 who were given prophylaxis, only 2 (3.4%) patients had documented rationale as to why anticoagulation was administered. Of the 132 patients with a PPS ≥ 4, 44 patients had thromboprophylaxis withheld. Eleven (8.3%) patients had thromboprophylaxis appropriately withheld due to presence or concern for bleeding. Commonly documented rationale for inappropriately withholding thromboprophylaxis when indicated included use of sequential compression devices (40.9%), pancytopenia (18.2%), dual antiplatelet therapy (9.1%), or patient was ambulatory (4.5%).
A secondary endpoint characterized patients at highest risk for developing a VTE after hospitalization for an acute illness. Seventy patients were readmitted within 45 days of discharge from the index hospitalization with ICD descriptions for embolism or thrombosis. Only 15 of those patients were readmitted with a newly diagnosed VTE not previously identified; 14 (93.3%) had a PPS ≥ 4 upon index admission and 10 (66.7%) appropriately received pharmacologic prophylaxis within 24 hours of admission. Of the 15 patients, 3 (20.0%) did not receive pharmacologic thromboprophylaxis within 24 hours of admission and 1 (6.7%) received thromboprophylaxis despite having a PPS < 4.
Looking at IMPROVEDD scores for the 15 patients at the index hospitalization discharge, 1 (6.7%) patient had an IMPROVEDD score < 2, 11 (73.3%) patients had IMPROVEDD scores ≥ 2, and 3 (20.0%) patients had IMPROVEDD scores ≥ 4. Two of the patients with IMPROVEDD scores ≥ 4 had a history of VTE and were aged > 60 years. Of the 15 patients reviewed, 7 had a diagnosis of cancer, and 3 were actively undergoing chemotherapy.
Discussion
PPS is the RAM embedded in our system’s order set, which identifies hospitalized medical patients at risk for VTE.6 In the original study that validated PPS, the results suggested that implementation of preventive measures during hospitalization in patients labeled as having high thrombotic risk confers longstanding protection against thromboembolic complications in comparison with untreated patients.6 However, PPS must be used consistently and appropriately to realize this benefit. Our results showed that pharmacologic thromboprophylaxis is frequently inappropriately given or withheld despite the incorporation of a RAM in an admission order set, suggesting there is a significant gap between written policy and actual practice. More than one-third of patients had thromboprophylaxis given or withheld inappropriately according to the PPS calculated manually on review. With this, there is concern for over- and underprescribing of thromboprophylaxis, which increases the risk of adverse events. Overprescribing can lead to unnecessary bleeding complications, whereas underprescribing can lead to preventable VTE.
One issue identified during this study was the need for a user-friendly interface. The PPS calculator currently embedded in our admission order set is a hyperlink to an online calculator. This is time consuming and cumbersome for clinicians tending to a high volume of patients, which may cause them to overlook the calculator and estimate risk based on clinician judgement. Noted areas for improvement regarding thromboprophylaxis during inpatient admissions include the failure to implement or adhere to risk stratification protocols, lack of appropriate assessment for thromboprophylaxis, and the overutilization of pharmacologic thromboprophylaxis in low-risk patients.11
Certain patients develop a VTE postdischarge despite efforts at prevention during their index hospitalization, which led us to explore our secondary endpoint looking at readmissions. Regarding thromboprophylaxis postdischarge, the duration of therapy is an area of current debate.5 Extended-duration thromboprophylaxis is defined as anticoagulation prescribed beyond hospitalization for up to 42 days total.1,12 To date, there have been 5 clinical trials to evaluate the utility of extended-duration thromboprophylaxis in hospitalized medically ill patients. While routine use is not recommended by the 2018 American Society of Hematology guidelines for management of VTE, more recent data suggest certain medically ill patients may derive benefit from extended-duration thromboprophylaxis.4 The IMPROVEDD score aimed to address this need, which is why it was calculated on index discharge for our patients readmitted within 45 days. Research is still needed to identify such patients and RAMs for capturing these subpopulations.1,11
Our secondary endpoint sought to characterize patients at highest risk for developing a VTE postdischarge. Of the 15 patients reviewed, 7 had a diagnosis of cancer and 3 were actively undergoing chemotherapy. With that, the Khorana Risk Score may have been a more appropriate RAM for some given the Khorana score is validated in ambulatory patients undergoing chemotherapy. D-dimer was only collected for 1 of the 15 patients, therefore, VTE risk could have been underestimated with the IMPROVEDD scores calculated. More than 75% of patients readmitted for VTE appropriately received thromboprophylaxis on index admission yet still went on to develop a VTE. It is essential to increase clinician awareness about hospital-acquired and postdischarge VTE. In line with guidance from the North American Thrombosis Forum, extended-duration thromboprophylaxis should be thoughtfully considered in high-risk patients.5 Pathways, including follow-up, are needed to implement postdischarge thromboprophylaxis when appropriate
Limitations
There were some inherent limitations to this study with its retrospective nature and small sample size. Data extraction was limited to health records within the VA, so there is a chance relevant history could be missed via incomplete documentation. Thus, our results could be an underestimation of postdischarge VTE prevalence if patients sought medical attention outside of the VA. Given this study was a retrospective chart review, data collection was limited to what was explicitly documented in the chart. Rationale for giving thromboprophylaxis when not indicated or holding when indicated may have been underestimated if clinicians did not document thoroughly in the electronic health record. Last, for the secondary endpoint reviewing the IMPROVEDD score, a D-dimer was not consistently obtained on admission, which could lead to underestimation of risk.
Conclusions
The results of this study showed that more than one-third of patients admitted to our facility within the prespecified timeframe had pharmacologic thromboprophylaxis inappropriately given or withheld according to a PPS manually calculated on admission. The PPS calculator currently embedded within our admission order set is not being utilized appropriately or consistently in clinical practice. Additionally, results from the secondary endpoint looking at IMPROVEDD scores highlight an unmet need for thromboprophylaxis at discharge. Pathways are needed to implement postdischarge thromboprophylaxis when appropriate for patients at highest thromboembolic risk.
1. Schünemann HJ, Cushman M, Burnett AE, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: prophylaxis for hospitalized and nonhospitalized medical patients. Blood Adv. 2018;2(22):3198-3225. doi:10.1182/bloodadvances.2018022954
2. Heit JA. Epidemiology of venous thromboembolism. Nat Rev Cardiol. 2015;12(8):464-474. doi:10.1038/nrcardio.2015.83
3. Turpie AG, Chin BS, Lip GY. Venous thromboembolism: pathophysiology, clinical features, and prevention. BMJ. 2002;325(7369):887-890. doi:10.1136/bmj.325.7369.887
4. Bajaj NS, Vaduganathan M, Qamar A, et al. Extended prophylaxis for venous thromboembolism after hospitalization for medical illness: A trial sequential and cumulative meta-analysis. Cannegieter SC, ed. PLoS Med. 2019;16(4):e1002797. doi:10.1371/journal.pmed.1002797
5. Barkoudah E, Piazza G, Hecht TEH, et al. Extended venous thromboembolism prophylaxis in medically ill patients: an NATF anticoagulation action initiative. Am J Med. 2020;133 (suppl 1):1-27. doi:10.1016/j.amjmed.2019.12.001
6. Barbar S, Noventa F, Rossetto V, et al. A risk assessment model for the identification of hospitalized medical patients at risk for venous thromboembolism: the Padua Prediction Score. J Thromb Haemost. 2010;8(11):2450-7. doi:10.1111/j.1538-7836.2010.04044.x
7. Gibson CM, Spyropoulos AC, Cohen AT, et al. The IMPROVEDD VTE risk score: incorporation of D-dimer into the IMPROVE score to improve venous thromboembolism risk stratification. TH Open. 2017;1(1):e56-e65. doi:10.1055/s-0037-1603929
8. ISTH Steering Committee for World Thrombosis Day. Thrombosis: a major contributor to global disease burden. Thromb Res. 2014;134(5):931-938. doi:10.1016/j.thromres.2014.08.014
9. Pavon JM, Sloane RJ, Pieper CF, et al. Poor adherence to risk stratification guidelines results in overuse of venous thromboembolism prophylaxis in hospitalized older adults. J Hosp Med. 2018;13(6):403-404. doi:10.12788/jhm.2916
10. Core elements of anticoagulation stewardship programs. Anticoagulation Forum. 2019. Accessed June 6, 2022. https://acforum-excellence.org/Resource-Center/resource_files/-2019-09-18-110254.pdf
11. Core elements of anticoagulation stewardship programs administrative oversight gap analysis: hospital and skilled nursing facilities. Anticoagulation Forum. 2019. Accessed June 6, 2022. https://acforum.org/web/downloads/ACF%20Gap%20Analysis%20Report.pdf
12. Falck-Ytter Y, Francis CW, Johanson NA, et al. Prevention of VTE in orthopedic surgery patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(suppl 2):e278S-e325S. doi:10.1378/chest.11-2404
Venous thromboembolism (VTE) presents as deep venous thromboembolism (DVT) or pulmonary embolism (PE). VTE is the third most common vascular disease and a leading cardiovascular complication.1,2 Hospitalized patients are at increased risk of developing VTE due to multiple factors such as inflammatory processes from acute illness, recent surgery or trauma leading to hypercoagulable states, and prolonged periods of immobilization.3 Additional risk factors for complications include presence of malignancy, obesity, and prior history of VTE. About half of VTE cases in the community setting occur as a result of a hospital admission for recent or ongoing acute illness or surgery.1 Hospitalized patients are often categorized as high risk for VTE, and this risk may persist postdischarge.4
The risk of hospital-associated VTE may be mitigated with either mechanical or pharmacologic thromboprophylaxis.5 Risk assessment models (RAMs), such as Padua Prediction Score (PPS) and IMPROVEDD, have been developed to assist in evaluating hospitalized patients’ risk of VTE and need for pharmacologic thromboprophylaxis (Table 1).1,5 The PPS is externally validated and can assist clinicians in VTE risk assessment when integrated into clinical decision making.6 Patients with a PPS ≥ 4 are deemed high risk for VTE, and pharmacologic thromboprophylaxis is indicated as long as the patient is not at high risk for bleeding. IMPROVEDD added D-dimer as an additional risk factor to IMPROVE and was validated in 2017 to help predict the risk of symptomatic VTE in acutely ill patients hospitalized for up to 77 days.7 IMPROVEDD scores ≥ 2 identify patients at high risk for symptomatic VTE through 77 days hospitalization, while scores ≥ 4 identify patients who may qualify for extended thromboprophylaxis.7 Despite their utility, RAMs may not be used appropriately within clinical practice, and whether patients should receive extended-duration thromboprophylaxis postdischarge and for how long is debatable.5
VTE events contribute to increased health care spending, morbidity, and mortality, thus it is imperative to evaluate current hospital practices with respect to appropriate prescribing of pharmacologic thromboprophylaxis.8 Appropriately identifying high-risk patients and prescribing pharmacologic thromboprophylaxis to limit preventable VTEs is essential. Conversely, it is important to withhold pharmacologic thromboprophylaxis from those deemed low risk to limit bleeding complications.9 Health care professionals must be good stewards of anticoagulant prescribing when implementing these tools along with clinical knowledge to weigh the risks vs benefits to promote medication safety and prevent further complications.10This quality improvement project aimed to evaluate if VTE thromboprophylaxis was appropriately given or withheld in hospitalized medical patients based on PPS calculated upon admission using a link to an online calculator embedded within an admission order set. Additionally, this study aimed to characterize patients readmitted for VTE within 45 days postdischarge to generate hypotheses for future stu
Methods
This was an observational, retrospective cohort study that took place at the US Department of Veterans Affairs (VA) Tennessee Valley Healthcare System (TVHS). TVHS is a multisite health care system with campuses in Nashville and Murfreesboro. Clinical pharmacists employed at the study site and the primary research investigators designed this study and oversaw its execution. The study was reviewed and deemed exempt as a quality improvement study by the TVHS Institutional Review Board.
This study included adult veterans aged ≥ 18 years admitted to a general medicine floor or the medical intensive care unit between June 1, 2017, and June 30, 2020. Patients were excluded if they were on chronic therapeutic anticoagulation prior to their index hospitalization, required therapeutic anticoagulation on admission for index hospitalization (ie, acute coronary syndrome requiring a heparin drip), or were bedded within the surgical intensive care unit. All patients admitted to the TVHS within the prespecified date range were extracted from the electronic health record. A second subset of patients meeting inclusion criteria and readmitted for VTE within 45 days of index hospitalization with International Classification of Diseases, Tenth Revision (ICD-10) descriptions including thrombosis or embolism were extracted for review of a secondary endpoint. Patients with preexisting clots, history of prior DVT or PE, or history of portal vein thrombosis were not reviewed.
The primary endpoint was the percentage of patients for whom pharmacologic thromboprophylaxis was appropriately initiated or withheld based on a PPS calculated upon admission (Table 2). PPS was chosen for review as it is the only RAM currently used at TVHS. Secondary endpoints were the percentage of patients with documented rationale for ordering thromboprophylaxis when not indicated, based on PPS, or withholding despite indication as well as the number of patients readmitted to TVHS for VTE within 45 days of discharge with IMPROVEDD scores ≥ 4 and < 4 (eAppendix available at doi:10.12788/fp.0291). The primary investigators performed a manual health record review of all patients meeting inclusion criteria. Descriptive statistics were used given this was a quality improvement study, therefore, sample size and power calculations were not necessary. Data were stored in Microsoft Excel spreadsheets that were encrypted and password protected. To maintain security of personal health information, all study files were kept on the TVHS internal network, and access was limited to the research investigators.
Results
Two hundred fifty patients meeting inclusion criteria were randomly selected for review for the primary endpoint. Of the patients reviewed for the primary endpoint, 118 had a PPS < 4 and 132 a PPS ≥ 4 (Figure). Pharmacologic thromboprophylaxis was inappropriately given or withheld based on their PPS for 91 (36.4%) patients. This included 58 (49.2%) patients in the low-risk group (PPS < 4) who had thromboprophylaxis inappropriately given and 33 (25.0%) patients in the high-risk group (PPS ≥ 4) who had thromboprophylaxis inappropriately withheld. Of the 58 patients with a PPS < 4 who were given prophylaxis, only 2 (3.4%) patients had documented rationale as to why anticoagulation was administered. Of the 132 patients with a PPS ≥ 4, 44 patients had thromboprophylaxis withheld. Eleven (8.3%) patients had thromboprophylaxis appropriately withheld due to presence or concern for bleeding. Commonly documented rationale for inappropriately withholding thromboprophylaxis when indicated included use of sequential compression devices (40.9%), pancytopenia (18.2%), dual antiplatelet therapy (9.1%), or patient was ambulatory (4.5%).
A secondary endpoint characterized patients at highest risk for developing a VTE after hospitalization for an acute illness. Seventy patients were readmitted within 45 days of discharge from the index hospitalization with ICD descriptions for embolism or thrombosis. Only 15 of those patients were readmitted with a newly diagnosed VTE not previously identified; 14 (93.3%) had a PPS ≥ 4 upon index admission and 10 (66.7%) appropriately received pharmacologic prophylaxis within 24 hours of admission. Of the 15 patients, 3 (20.0%) did not receive pharmacologic thromboprophylaxis within 24 hours of admission and 1 (6.7%) received thromboprophylaxis despite having a PPS < 4.
Looking at IMPROVEDD scores for the 15 patients at the index hospitalization discharge, 1 (6.7%) patient had an IMPROVEDD score < 2, 11 (73.3%) patients had IMPROVEDD scores ≥ 2, and 3 (20.0%) patients had IMPROVEDD scores ≥ 4. Two of the patients with IMPROVEDD scores ≥ 4 had a history of VTE and were aged > 60 years. Of the 15 patients reviewed, 7 had a diagnosis of cancer, and 3 were actively undergoing chemotherapy.
Discussion
PPS is the RAM embedded in our system’s order set, which identifies hospitalized medical patients at risk for VTE.6 In the original study that validated PPS, the results suggested that implementation of preventive measures during hospitalization in patients labeled as having high thrombotic risk confers longstanding protection against thromboembolic complications in comparison with untreated patients.6 However, PPS must be used consistently and appropriately to realize this benefit. Our results showed that pharmacologic thromboprophylaxis is frequently inappropriately given or withheld despite the incorporation of a RAM in an admission order set, suggesting there is a significant gap between written policy and actual practice. More than one-third of patients had thromboprophylaxis given or withheld inappropriately according to the PPS calculated manually on review. With this, there is concern for over- and underprescribing of thromboprophylaxis, which increases the risk of adverse events. Overprescribing can lead to unnecessary bleeding complications, whereas underprescribing can lead to preventable VTE.
One issue identified during this study was the need for a user-friendly interface. The PPS calculator currently embedded in our admission order set is a hyperlink to an online calculator. This is time consuming and cumbersome for clinicians tending to a high volume of patients, which may cause them to overlook the calculator and estimate risk based on clinician judgement. Noted areas for improvement regarding thromboprophylaxis during inpatient admissions include the failure to implement or adhere to risk stratification protocols, lack of appropriate assessment for thromboprophylaxis, and the overutilization of pharmacologic thromboprophylaxis in low-risk patients.11
Certain patients develop a VTE postdischarge despite efforts at prevention during their index hospitalization, which led us to explore our secondary endpoint looking at readmissions. Regarding thromboprophylaxis postdischarge, the duration of therapy is an area of current debate.5 Extended-duration thromboprophylaxis is defined as anticoagulation prescribed beyond hospitalization for up to 42 days total.1,12 To date, there have been 5 clinical trials to evaluate the utility of extended-duration thromboprophylaxis in hospitalized medically ill patients. While routine use is not recommended by the 2018 American Society of Hematology guidelines for management of VTE, more recent data suggest certain medically ill patients may derive benefit from extended-duration thromboprophylaxis.4 The IMPROVEDD score aimed to address this need, which is why it was calculated on index discharge for our patients readmitted within 45 days. Research is still needed to identify such patients and RAMs for capturing these subpopulations.1,11
Our secondary endpoint sought to characterize patients at highest risk for developing a VTE postdischarge. Of the 15 patients reviewed, 7 had a diagnosis of cancer and 3 were actively undergoing chemotherapy. With that, the Khorana Risk Score may have been a more appropriate RAM for some given the Khorana score is validated in ambulatory patients undergoing chemotherapy. D-dimer was only collected for 1 of the 15 patients, therefore, VTE risk could have been underestimated with the IMPROVEDD scores calculated. More than 75% of patients readmitted for VTE appropriately received thromboprophylaxis on index admission yet still went on to develop a VTE. It is essential to increase clinician awareness about hospital-acquired and postdischarge VTE. In line with guidance from the North American Thrombosis Forum, extended-duration thromboprophylaxis should be thoughtfully considered in high-risk patients.5 Pathways, including follow-up, are needed to implement postdischarge thromboprophylaxis when appropriate
Limitations
There were some inherent limitations to this study with its retrospective nature and small sample size. Data extraction was limited to health records within the VA, so there is a chance relevant history could be missed via incomplete documentation. Thus, our results could be an underestimation of postdischarge VTE prevalence if patients sought medical attention outside of the VA. Given this study was a retrospective chart review, data collection was limited to what was explicitly documented in the chart. Rationale for giving thromboprophylaxis when not indicated or holding when indicated may have been underestimated if clinicians did not document thoroughly in the electronic health record. Last, for the secondary endpoint reviewing the IMPROVEDD score, a D-dimer was not consistently obtained on admission, which could lead to underestimation of risk.
Conclusions
The results of this study showed that more than one-third of patients admitted to our facility within the prespecified timeframe had pharmacologic thromboprophylaxis inappropriately given or withheld according to a PPS manually calculated on admission. The PPS calculator currently embedded within our admission order set is not being utilized appropriately or consistently in clinical practice. Additionally, results from the secondary endpoint looking at IMPROVEDD scores highlight an unmet need for thromboprophylaxis at discharge. Pathways are needed to implement postdischarge thromboprophylaxis when appropriate for patients at highest thromboembolic risk.
Venous thromboembolism (VTE) presents as deep venous thromboembolism (DVT) or pulmonary embolism (PE). VTE is the third most common vascular disease and a leading cardiovascular complication.1,2 Hospitalized patients are at increased risk of developing VTE due to multiple factors such as inflammatory processes from acute illness, recent surgery or trauma leading to hypercoagulable states, and prolonged periods of immobilization.3 Additional risk factors for complications include presence of malignancy, obesity, and prior history of VTE. About half of VTE cases in the community setting occur as a result of a hospital admission for recent or ongoing acute illness or surgery.1 Hospitalized patients are often categorized as high risk for VTE, and this risk may persist postdischarge.4
The risk of hospital-associated VTE may be mitigated with either mechanical or pharmacologic thromboprophylaxis.5 Risk assessment models (RAMs), such as Padua Prediction Score (PPS) and IMPROVEDD, have been developed to assist in evaluating hospitalized patients’ risk of VTE and need for pharmacologic thromboprophylaxis (Table 1).1,5 The PPS is externally validated and can assist clinicians in VTE risk assessment when integrated into clinical decision making.6 Patients with a PPS ≥ 4 are deemed high risk for VTE, and pharmacologic thromboprophylaxis is indicated as long as the patient is not at high risk for bleeding. IMPROVEDD added D-dimer as an additional risk factor to IMPROVE and was validated in 2017 to help predict the risk of symptomatic VTE in acutely ill patients hospitalized for up to 77 days.7 IMPROVEDD scores ≥ 2 identify patients at high risk for symptomatic VTE through 77 days hospitalization, while scores ≥ 4 identify patients who may qualify for extended thromboprophylaxis.7 Despite their utility, RAMs may not be used appropriately within clinical practice, and whether patients should receive extended-duration thromboprophylaxis postdischarge and for how long is debatable.5
VTE events contribute to increased health care spending, morbidity, and mortality, thus it is imperative to evaluate current hospital practices with respect to appropriate prescribing of pharmacologic thromboprophylaxis.8 Appropriately identifying high-risk patients and prescribing pharmacologic thromboprophylaxis to limit preventable VTEs is essential. Conversely, it is important to withhold pharmacologic thromboprophylaxis from those deemed low risk to limit bleeding complications.9 Health care professionals must be good stewards of anticoagulant prescribing when implementing these tools along with clinical knowledge to weigh the risks vs benefits to promote medication safety and prevent further complications.10This quality improvement project aimed to evaluate if VTE thromboprophylaxis was appropriately given or withheld in hospitalized medical patients based on PPS calculated upon admission using a link to an online calculator embedded within an admission order set. Additionally, this study aimed to characterize patients readmitted for VTE within 45 days postdischarge to generate hypotheses for future stu
Methods
This was an observational, retrospective cohort study that took place at the US Department of Veterans Affairs (VA) Tennessee Valley Healthcare System (TVHS). TVHS is a multisite health care system with campuses in Nashville and Murfreesboro. Clinical pharmacists employed at the study site and the primary research investigators designed this study and oversaw its execution. The study was reviewed and deemed exempt as a quality improvement study by the TVHS Institutional Review Board.
This study included adult veterans aged ≥ 18 years admitted to a general medicine floor or the medical intensive care unit between June 1, 2017, and June 30, 2020. Patients were excluded if they were on chronic therapeutic anticoagulation prior to their index hospitalization, required therapeutic anticoagulation on admission for index hospitalization (ie, acute coronary syndrome requiring a heparin drip), or were bedded within the surgical intensive care unit. All patients admitted to the TVHS within the prespecified date range were extracted from the electronic health record. A second subset of patients meeting inclusion criteria and readmitted for VTE within 45 days of index hospitalization with International Classification of Diseases, Tenth Revision (ICD-10) descriptions including thrombosis or embolism were extracted for review of a secondary endpoint. Patients with preexisting clots, history of prior DVT or PE, or history of portal vein thrombosis were not reviewed.
The primary endpoint was the percentage of patients for whom pharmacologic thromboprophylaxis was appropriately initiated or withheld based on a PPS calculated upon admission (Table 2). PPS was chosen for review as it is the only RAM currently used at TVHS. Secondary endpoints were the percentage of patients with documented rationale for ordering thromboprophylaxis when not indicated, based on PPS, or withholding despite indication as well as the number of patients readmitted to TVHS for VTE within 45 days of discharge with IMPROVEDD scores ≥ 4 and < 4 (eAppendix available at doi:10.12788/fp.0291). The primary investigators performed a manual health record review of all patients meeting inclusion criteria. Descriptive statistics were used given this was a quality improvement study, therefore, sample size and power calculations were not necessary. Data were stored in Microsoft Excel spreadsheets that were encrypted and password protected. To maintain security of personal health information, all study files were kept on the TVHS internal network, and access was limited to the research investigators.
Results
Two hundred fifty patients meeting inclusion criteria were randomly selected for review for the primary endpoint. Of the patients reviewed for the primary endpoint, 118 had a PPS < 4 and 132 a PPS ≥ 4 (Figure). Pharmacologic thromboprophylaxis was inappropriately given or withheld based on their PPS for 91 (36.4%) patients. This included 58 (49.2%) patients in the low-risk group (PPS < 4) who had thromboprophylaxis inappropriately given and 33 (25.0%) patients in the high-risk group (PPS ≥ 4) who had thromboprophylaxis inappropriately withheld. Of the 58 patients with a PPS < 4 who were given prophylaxis, only 2 (3.4%) patients had documented rationale as to why anticoagulation was administered. Of the 132 patients with a PPS ≥ 4, 44 patients had thromboprophylaxis withheld. Eleven (8.3%) patients had thromboprophylaxis appropriately withheld due to presence or concern for bleeding. Commonly documented rationale for inappropriately withholding thromboprophylaxis when indicated included use of sequential compression devices (40.9%), pancytopenia (18.2%), dual antiplatelet therapy (9.1%), or patient was ambulatory (4.5%).
A secondary endpoint characterized patients at highest risk for developing a VTE after hospitalization for an acute illness. Seventy patients were readmitted within 45 days of discharge from the index hospitalization with ICD descriptions for embolism or thrombosis. Only 15 of those patients were readmitted with a newly diagnosed VTE not previously identified; 14 (93.3%) had a PPS ≥ 4 upon index admission and 10 (66.7%) appropriately received pharmacologic prophylaxis within 24 hours of admission. Of the 15 patients, 3 (20.0%) did not receive pharmacologic thromboprophylaxis within 24 hours of admission and 1 (6.7%) received thromboprophylaxis despite having a PPS < 4.
Looking at IMPROVEDD scores for the 15 patients at the index hospitalization discharge, 1 (6.7%) patient had an IMPROVEDD score < 2, 11 (73.3%) patients had IMPROVEDD scores ≥ 2, and 3 (20.0%) patients had IMPROVEDD scores ≥ 4. Two of the patients with IMPROVEDD scores ≥ 4 had a history of VTE and were aged > 60 years. Of the 15 patients reviewed, 7 had a diagnosis of cancer, and 3 were actively undergoing chemotherapy.
Discussion
PPS is the RAM embedded in our system’s order set, which identifies hospitalized medical patients at risk for VTE.6 In the original study that validated PPS, the results suggested that implementation of preventive measures during hospitalization in patients labeled as having high thrombotic risk confers longstanding protection against thromboembolic complications in comparison with untreated patients.6 However, PPS must be used consistently and appropriately to realize this benefit. Our results showed that pharmacologic thromboprophylaxis is frequently inappropriately given or withheld despite the incorporation of a RAM in an admission order set, suggesting there is a significant gap between written policy and actual practice. More than one-third of patients had thromboprophylaxis given or withheld inappropriately according to the PPS calculated manually on review. With this, there is concern for over- and underprescribing of thromboprophylaxis, which increases the risk of adverse events. Overprescribing can lead to unnecessary bleeding complications, whereas underprescribing can lead to preventable VTE.
One issue identified during this study was the need for a user-friendly interface. The PPS calculator currently embedded in our admission order set is a hyperlink to an online calculator. This is time consuming and cumbersome for clinicians tending to a high volume of patients, which may cause them to overlook the calculator and estimate risk based on clinician judgement. Noted areas for improvement regarding thromboprophylaxis during inpatient admissions include the failure to implement or adhere to risk stratification protocols, lack of appropriate assessment for thromboprophylaxis, and the overutilization of pharmacologic thromboprophylaxis in low-risk patients.11
Certain patients develop a VTE postdischarge despite efforts at prevention during their index hospitalization, which led us to explore our secondary endpoint looking at readmissions. Regarding thromboprophylaxis postdischarge, the duration of therapy is an area of current debate.5 Extended-duration thromboprophylaxis is defined as anticoagulation prescribed beyond hospitalization for up to 42 days total.1,12 To date, there have been 5 clinical trials to evaluate the utility of extended-duration thromboprophylaxis in hospitalized medically ill patients. While routine use is not recommended by the 2018 American Society of Hematology guidelines for management of VTE, more recent data suggest certain medically ill patients may derive benefit from extended-duration thromboprophylaxis.4 The IMPROVEDD score aimed to address this need, which is why it was calculated on index discharge for our patients readmitted within 45 days. Research is still needed to identify such patients and RAMs for capturing these subpopulations.1,11
Our secondary endpoint sought to characterize patients at highest risk for developing a VTE postdischarge. Of the 15 patients reviewed, 7 had a diagnosis of cancer and 3 were actively undergoing chemotherapy. With that, the Khorana Risk Score may have been a more appropriate RAM for some given the Khorana score is validated in ambulatory patients undergoing chemotherapy. D-dimer was only collected for 1 of the 15 patients, therefore, VTE risk could have been underestimated with the IMPROVEDD scores calculated. More than 75% of patients readmitted for VTE appropriately received thromboprophylaxis on index admission yet still went on to develop a VTE. It is essential to increase clinician awareness about hospital-acquired and postdischarge VTE. In line with guidance from the North American Thrombosis Forum, extended-duration thromboprophylaxis should be thoughtfully considered in high-risk patients.5 Pathways, including follow-up, are needed to implement postdischarge thromboprophylaxis when appropriate
Limitations
There were some inherent limitations to this study with its retrospective nature and small sample size. Data extraction was limited to health records within the VA, so there is a chance relevant history could be missed via incomplete documentation. Thus, our results could be an underestimation of postdischarge VTE prevalence if patients sought medical attention outside of the VA. Given this study was a retrospective chart review, data collection was limited to what was explicitly documented in the chart. Rationale for giving thromboprophylaxis when not indicated or holding when indicated may have been underestimated if clinicians did not document thoroughly in the electronic health record. Last, for the secondary endpoint reviewing the IMPROVEDD score, a D-dimer was not consistently obtained on admission, which could lead to underestimation of risk.
Conclusions
The results of this study showed that more than one-third of patients admitted to our facility within the prespecified timeframe had pharmacologic thromboprophylaxis inappropriately given or withheld according to a PPS manually calculated on admission. The PPS calculator currently embedded within our admission order set is not being utilized appropriately or consistently in clinical practice. Additionally, results from the secondary endpoint looking at IMPROVEDD scores highlight an unmet need for thromboprophylaxis at discharge. Pathways are needed to implement postdischarge thromboprophylaxis when appropriate for patients at highest thromboembolic risk.
1. Schünemann HJ, Cushman M, Burnett AE, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: prophylaxis for hospitalized and nonhospitalized medical patients. Blood Adv. 2018;2(22):3198-3225. doi:10.1182/bloodadvances.2018022954
2. Heit JA. Epidemiology of venous thromboembolism. Nat Rev Cardiol. 2015;12(8):464-474. doi:10.1038/nrcardio.2015.83
3. Turpie AG, Chin BS, Lip GY. Venous thromboembolism: pathophysiology, clinical features, and prevention. BMJ. 2002;325(7369):887-890. doi:10.1136/bmj.325.7369.887
4. Bajaj NS, Vaduganathan M, Qamar A, et al. Extended prophylaxis for venous thromboembolism after hospitalization for medical illness: A trial sequential and cumulative meta-analysis. Cannegieter SC, ed. PLoS Med. 2019;16(4):e1002797. doi:10.1371/journal.pmed.1002797
5. Barkoudah E, Piazza G, Hecht TEH, et al. Extended venous thromboembolism prophylaxis in medically ill patients: an NATF anticoagulation action initiative. Am J Med. 2020;133 (suppl 1):1-27. doi:10.1016/j.amjmed.2019.12.001
6. Barbar S, Noventa F, Rossetto V, et al. A risk assessment model for the identification of hospitalized medical patients at risk for venous thromboembolism: the Padua Prediction Score. J Thromb Haemost. 2010;8(11):2450-7. doi:10.1111/j.1538-7836.2010.04044.x
7. Gibson CM, Spyropoulos AC, Cohen AT, et al. The IMPROVEDD VTE risk score: incorporation of D-dimer into the IMPROVE score to improve venous thromboembolism risk stratification. TH Open. 2017;1(1):e56-e65. doi:10.1055/s-0037-1603929
8. ISTH Steering Committee for World Thrombosis Day. Thrombosis: a major contributor to global disease burden. Thromb Res. 2014;134(5):931-938. doi:10.1016/j.thromres.2014.08.014
9. Pavon JM, Sloane RJ, Pieper CF, et al. Poor adherence to risk stratification guidelines results in overuse of venous thromboembolism prophylaxis in hospitalized older adults. J Hosp Med. 2018;13(6):403-404. doi:10.12788/jhm.2916
10. Core elements of anticoagulation stewardship programs. Anticoagulation Forum. 2019. Accessed June 6, 2022. https://acforum-excellence.org/Resource-Center/resource_files/-2019-09-18-110254.pdf
11. Core elements of anticoagulation stewardship programs administrative oversight gap analysis: hospital and skilled nursing facilities. Anticoagulation Forum. 2019. Accessed June 6, 2022. https://acforum.org/web/downloads/ACF%20Gap%20Analysis%20Report.pdf
12. Falck-Ytter Y, Francis CW, Johanson NA, et al. Prevention of VTE in orthopedic surgery patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(suppl 2):e278S-e325S. doi:10.1378/chest.11-2404
1. Schünemann HJ, Cushman M, Burnett AE, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: prophylaxis for hospitalized and nonhospitalized medical patients. Blood Adv. 2018;2(22):3198-3225. doi:10.1182/bloodadvances.2018022954
2. Heit JA. Epidemiology of venous thromboembolism. Nat Rev Cardiol. 2015;12(8):464-474. doi:10.1038/nrcardio.2015.83
3. Turpie AG, Chin BS, Lip GY. Venous thromboembolism: pathophysiology, clinical features, and prevention. BMJ. 2002;325(7369):887-890. doi:10.1136/bmj.325.7369.887
4. Bajaj NS, Vaduganathan M, Qamar A, et al. Extended prophylaxis for venous thromboembolism after hospitalization for medical illness: A trial sequential and cumulative meta-analysis. Cannegieter SC, ed. PLoS Med. 2019;16(4):e1002797. doi:10.1371/journal.pmed.1002797
5. Barkoudah E, Piazza G, Hecht TEH, et al. Extended venous thromboembolism prophylaxis in medically ill patients: an NATF anticoagulation action initiative. Am J Med. 2020;133 (suppl 1):1-27. doi:10.1016/j.amjmed.2019.12.001
6. Barbar S, Noventa F, Rossetto V, et al. A risk assessment model for the identification of hospitalized medical patients at risk for venous thromboembolism: the Padua Prediction Score. J Thromb Haemost. 2010;8(11):2450-7. doi:10.1111/j.1538-7836.2010.04044.x
7. Gibson CM, Spyropoulos AC, Cohen AT, et al. The IMPROVEDD VTE risk score: incorporation of D-dimer into the IMPROVE score to improve venous thromboembolism risk stratification. TH Open. 2017;1(1):e56-e65. doi:10.1055/s-0037-1603929
8. ISTH Steering Committee for World Thrombosis Day. Thrombosis: a major contributor to global disease burden. Thromb Res. 2014;134(5):931-938. doi:10.1016/j.thromres.2014.08.014
9. Pavon JM, Sloane RJ, Pieper CF, et al. Poor adherence to risk stratification guidelines results in overuse of venous thromboembolism prophylaxis in hospitalized older adults. J Hosp Med. 2018;13(6):403-404. doi:10.12788/jhm.2916
10. Core elements of anticoagulation stewardship programs. Anticoagulation Forum. 2019. Accessed June 6, 2022. https://acforum-excellence.org/Resource-Center/resource_files/-2019-09-18-110254.pdf
11. Core elements of anticoagulation stewardship programs administrative oversight gap analysis: hospital and skilled nursing facilities. Anticoagulation Forum. 2019. Accessed June 6, 2022. https://acforum.org/web/downloads/ACF%20Gap%20Analysis%20Report.pdf
12. Falck-Ytter Y, Francis CW, Johanson NA, et al. Prevention of VTE in orthopedic surgery patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(suppl 2):e278S-e325S. doi:10.1378/chest.11-2404
Approach to Pancytopenia in a Deployed Service Member
Pancytopenia is a condition in which all 3 hematologic cell lines are lower than expected in the blood, often representing either an increase in cellular destruction or decrease in bone marrow production. Destruction often occurs in the setting of autoimmune conditions (eg, systemic lupus erythematosus, rheumatoid arthritis) or splenic sequestration, often affecting erythrocytes and platelets more than leukocytes. Decreased production represents central etiologies, which are often due to nutritional deficiencies, infections, drug toxicities, or malabsorption.1 Pancytopenia secondary to vitamin B12 deficiency is rare, accounting for about 5% of the hematologic manifestations of symptomatic vitamin B12 deficient patients.2
Pernicious anemia, named for a once lethal disease, is a form of vitamin B12 (cobalamin) deficiency that results from an autoimmune (type II hypersensitivity) reaction to gastric parietal cells or intrinsic factor. Antibodies bind to gastric parietal cells and reduce gastric acid production, leading to atrophic gastritis, or they bind intrinsic factor and block the binding and absorption of vitamin B12 in the gastrointestinal tract. While first described in the 1820s, it was not until a century later when scientists were studying hematopoiesis in response to the heavy casualty burden from battlefield exsanguination in World War I that dogs fed raw liver were noted to have significantly better blood regeneration response than those fed cooked liver. This discovery led physicians Minot and Murphy to use raw liver to treat pernicious anemia and found that jaundice improved, reticulocyte counts increased, and hemoglobin (Hb) concentration improved, resulting in the duo becoming the first American recipients of the Nobel Prize in physiology or medicine.3 It was ultimately determined in 1948 by chemists Folkers and Todd that the active ingredient in raw liver responsible for this phenomenon was vitamin B12.4
Patients with pernicious anemia typically present with macrocytic anemia, low reticulocyte count, hypersegmented neutrophils, as well as mild leukopenia and/or thrombocytopenia, distinguishable from folate deficiency by an elevated serum methylmalonic acid level. World Health Organization cytopenia thresholds are listed in Table 1.5 Treatment consists of lifelong vitamin B12 supplementation, and endoscopic screening is often recommended after diagnosis due to increased risk of gastrointestinal malignancy.6 Pernicious anemia can be difficult to distinguish from thrombotic thrombocytopenia purpura (TTP), a microangiopathic hemolytic anemia that can cause rapid end-organ failure and death if treatment is delayed.7 While pernicious anemia is not typically hemolytic, case reports of hemolysis in severe deficiency have been reported.7 Adequate bone marrow response to hemolysis in TTP results in an elevated reticulocyte count, which can be useful in differentiating from pernicious anemia where there is typically an inadequate bone marrow response and low reticulocyte count.8,9
The approach to working up pancytopenia begins with a detailed history inquiring about medications, exposures (benzenes, pesticides), alcohol use, and infection history. A thorough physical examination may help point the health care practitioner (HCP) toward a certain etiology, as the differential for pancytopenia is broad. In the deployed soldier downrange, resources are often limited, and the history/physical are crucial in preventing an expensive and unnecessary workup.
Case Presentation
A 24-year-old active-duty female patient presented in late December 2020 to a theater hospital in Djibouti after a witnessed syncopal episode. She had a history of Hashimoto thyroiditis and was taking levothyroxine sodium 75 mcg daily. The patient reported gluten intolerance, which was never formally evaluated. The syncopal episode lasted a few seconds and was not associated with any prodromal or postictal symptoms. No seizure activity was observed, and she had no history of syncopal episodes. She reported that she had been feeling ill 24 to 48 hours prior, with nausea, fatigue, decreased oral intake, decreased urine output, and 2 episodes of nonbilious, nonbloody emesis.
When the patient arrived, she was tachycardic with heart rate in the 130s beats per minute (baseline, 100-110 beats per minute), febrile (103 °F), and had systolic blood pressure (SBP) in the low 100s (baseline, SBP 120s-130s). An electrocardiogram and chest radiographs were unremarkable. Her complete blood count (CBC) could not be processed due to Hb and platelet levels too low to detect on assay (Table 2). Lactate dehydrogenase (LDH) was elevated at > 1000 U/L with mild elevation in liver enzymes (aspartate aminotransferase, 98 U/L; alanine aminotransferase, 51 U/L) and prolonged partial thromboplastin time 70 seconds. She did not report any increased bleeding or bruising. The peripheral blood smear demonstrated pancytopenia, without any schistocytes, and she was started on broad-spectrum antibiotics for presumed sepsis from urinary source and possible TTP.
The patient received 5 units of packed red blood cells, transfusion of platelets, and 2 doses of vitamin B12 in Djibouti with clinical improvement and resolution of orthostasis, hypotension, tachycardia, and fever. Her final posttransfusion CBC showed a Hb level of 11.2 g/dL, white blood cell (WBC) count of 1.7 K/µL, and platelet count of 23 K/µL (Table 3). Two days later her Hb level was 9.0 g/dL, WBC count 1.8 K/µL, and platelet count was 12 K/µL. She was evacuated via air to Landstuhl Regional Medical Center (LRMC) in Germany within 48 hours of presentation, given limited testing capabilities and persistent anemia and thrombocytopenia, refractory to transfusion, concerning for aplastic anemia or acute leukemia.
On arrival at LRMC, she was transfused 1 unit of platelets and given 3 doses of intramuscular vitamin B12 for undetectable levels (< 50 pg/mL) at presentation. An extensive infectious workup was obtained, which did not reveal any viral, bacterial, or parasitic causes. The patient also had a bone marrow biopsy performed at a civilian site, which revealed hypocellular bone marrow. She was transferred to Walter Reed National Military Medical Center (WRNMMC) for further workup and evaluation, given the infectious workup, which was negative. Concern for hematologic malignancy remained. At the time of her arrival, the laboratory values had drastically improved with vitamin supplementation. The patient’s absolute reticulocyte count indicated adequate bone marrow response and because of her improvement, a repeat bone marrow biopsy was not performed.
Intrinsic factor antibodies were elevated (34.5 AU/mL; reference range, 0.0-1.1), which confirmed that this patient’s underlying etiology was secondary to pernicious anemia. The patient continued to improve and repeat vitamin B12 and folate levels revealed that she was responding to therapy. At discharge, intramuscular vitamin B12 injections were planned to continue monthly, indefinitely per guidelines. Oral supplementation is typically avoided due to poor absorption.
Of note, during her inpatient admission at WRNMMC, further evaluation of reported gluten intolerance was performed, which revealed a negative celiac disease panel (IgG/IgA tissue transglutaminase antibodies). On discharge, she was to establish care with gastroenterology for further evaluation, likely including endoscopic evaluation, at her next duty station. She was able to resume full travel and duty functions on discharge from WRNMMC.
Discussion
We highlight a complex case of pancytopenia secondary to pernicious anemia in a deployed service member. With limited resources downrange, the workup of pancytopenia can be resource intensive, expensive, and time sensitive, which can have detrimental impacts on medical readiness. Additionally, undiagnosed coagulopathies can have lethal consequences in a deployed service member where bleeding risk may be elevated depending on the mission. The differential for pancytopenia is vast, and given its relative rarity in pernicious anemia, the HCP must use key components of the history and laboratory results to narrow the differential (eAppendix).10
Pernicious anemia commonly presents as an isolated anemia. In a study looking at the hematologic manifestations of 201 cohort patients with well-documented vitamin B12 deficiency, 5% had symptomatic pancytopenia and 1.5% had a hemolytic anemia.2 The majority (> 67%) of hematologic abnormalities were correctable with cobalamin replacement.2 In our case, the solider presented with symptomatic anemia, manifesting as syncope, and was found to have transfusion-resistant pancytopenia.She had a hemolytic anemia with an LDH > 1000 U/L, haptoglobin < 3 mg/dL, and mild transaminitis with hyperbilirubinemia (1.8 mg/dL). No schistocytes were observed on peripheral smear, suggesting intramedullary hemolysis, which is believed to be due to the destruction of megaloblastic cells by macrophages in bone marrow.11 A French study found high LDH levels and low reticulocyte counts to be strongly suggestive of vitamin B12 deficiency and helpful in differentiating pernicious anemia from TTP, given that bone marrow response to anemia in TTP is preserved.8
While vitamin B12 deficiency is not often associated with hemolytic anemia, multiple cases have been reported in the literature.6 Screening for vitamin B12 deficiency may have shortened this patient’s clinical course and limited the need for air evacuation to a stateside quaternary medical center. However, testing for cobalamin levels in overseas deployed environments is difficult, timely, and costly. New technologies, such as optical sensors, can detect vitamin B12 levels in the blood in < 1 minute and offer portable, low-cost options that may be useful in the deployed military setting.12
Diet plays a key role in this case, since the patient had a reported history of gluten intolerance, although it was never documented or evaluated prior to this presentation. Prior to deployment, the patient ate mostly rice, potatoes, and vegetables. While deployed in an austere environment, food options were limited. These conditions forced her to intermittently consume gluten products, which led to gastrointestinal issues, exacerbating her nutritional deficiencies. In the 2 months before her first syncopal episode, she reported worsening fatigue that impacted her ability to exercise. Vitamin B12 stores often take years to deplete, suggesting that she had a chronic nutritional deficiency before deployment. Another possibility was that she developed an autoimmune gastritis that acutely worsened in the setting of poor nutritional intake. Her history of Hashimoto thyroiditis is also important, as up to one-third of patients with autoimmune thyroid disease have been associated with pernicious anemia (range, 3%-32%) with certain shared human leukocyte antigen alleles implicated in autoimmune gastritis.13,14
Conclusions
This rare case of pernicious anemia presenting as pancytopenia illustrates the challenge in working up pancytopenia, especially in austere military environments with limited testing capabilities. Screening for chronic dietary and nutritional deficiency is important in a service member, raising the question of what role predeployment screening may have and what dietary accommodations may be available during overseas deployments, which can potentially dampen inflammation of the gastrointestinal tract, especially for those with preexisting autoimmune gastrointestinal conditions. Also, newer technology allows portable, low-cost testing of cobalamin and may aid in its diagnosis. In patients who are anemic with low vitamin B12, HCPs can begin vitamin B12 supplementation while continuing the workup (eg, antibody testing, endoscopy). If the patient responds appropriately, further workup becomes less urgent, therefore, decreasing resource use and increasing military readiness. When hemolysis is present, a low reticulocyte count can be beneficial to help differentiate this condition from TTP, a life-threatening condition that must also be ruled out or treated. Pernicious anemia should be on the differential in any patients with autoimmune conditions presenting with cytopenias, especially in those with a history of autoimmune thyroid disorders.
1. Takeshima M, Ishikawa H, Kitadate A, et al. Anorexia nervosa-associated pancytopenia mimicking idiopathic aplastic anemia: a case report. BMC Psychiatry. 2018;18(1):150. doi:10.1186/s12888-018-1743-6
2. Andrès E, Affenberger S, Zimmer J, et al. Current hematological findings in cobalamin deficiency. A study of 201 consecutive patients with documented cobalamin deficiency. Clin Lab Haematol. 2006;28(1):50-56. doi:10.1111/j.1365-2257.2006.00755.x
3. Sinclair L. Recognizing, treating and understanding pernicious anaemia. J R Soc Med. 2008;101(5):262-264. doi:10.1258/jrsm.2008.081006
4. Shampo MA, Kyle RA, Steensma DP. William Murphy—Nobel Prize for the treatment of pernicious anemia. Mayo Clin Proc. 2006;81(6):726. doi:10.4065/81.6.726
5. Hong M, He G. The 2016 revision to the World Health Organization classification of myelodysplastic syndromes. J Transl Int Med. 2017;5(3):139-143. doi:10.1515/jtim-2017-0002
6. Tunio NA, Sheriff MZ, Cooper G. Prevalence of gastric cancer in patients with pernicious anemia: a population-based study. Am J Gastroenterol. 2020;115:S665. doi:10.14309/01.ajg.0000707332.16739.72
7. Bailey M, Maestas T, Betancourt R, Mikhael D, Babiker HM. A rare cause of thrombotic thrombocytopenic purpura- (TTP-) like syndrome, vitamin B12 deficiency: interpretation of significant pathological findings. Case Rep Hematol. 2019;2019:1529306. doi:10.1155/2019/1529306
8. Stanley M, Michalski JM. Thrombotic Thrombocytopenic Purpura. StatPearls Publishing LLC; 2021.
9. Noël N, Maigné G, Tertian G, et al. Hemolysis and schistocytosis in the emergency department: consider pseudothrombotic microangiopathy related to vitamin B12 deficiency. QJM. 2013;106(11):1017-1022. doi:10.1093/qjmed/hct142
10. Chiravuri S, De Jesus O. Pancytopenia. StatPearls Publishing LLC; 2021.
11. Gladstone E. Pernicious anemia presenting with pancytopenia and hemolysis: a case report. February 8, 2019. Accessed June 9, 2022. https://www.journalmc.org/index.php/JMC/article/view/3269/2563
12. ScienceDaily. Developing a sensor for vitamin B12 deficiency. October 17, 2016. Accessed June 9, 2022. https://www.sciencedaily.com/releases/2016/10/161017103221.htm
13. Rodriguez NM, Shackelford K. Pernicious Anemia. StatPearls Publishing LLC; 2021.
14. Fernando MM, Stevens CR, Walsh EC, et al. Defining the role of the MHC in autoimmunity: a review and pooled analysis. PLoS Genet. 2008;4(4):e1000024. doi:10.1371/journal.pgen.1000024
Pancytopenia is a condition in which all 3 hematologic cell lines are lower than expected in the blood, often representing either an increase in cellular destruction or decrease in bone marrow production. Destruction often occurs in the setting of autoimmune conditions (eg, systemic lupus erythematosus, rheumatoid arthritis) or splenic sequestration, often affecting erythrocytes and platelets more than leukocytes. Decreased production represents central etiologies, which are often due to nutritional deficiencies, infections, drug toxicities, or malabsorption.1 Pancytopenia secondary to vitamin B12 deficiency is rare, accounting for about 5% of the hematologic manifestations of symptomatic vitamin B12 deficient patients.2
Pernicious anemia, named for a once lethal disease, is a form of vitamin B12 (cobalamin) deficiency that results from an autoimmune (type II hypersensitivity) reaction to gastric parietal cells or intrinsic factor. Antibodies bind to gastric parietal cells and reduce gastric acid production, leading to atrophic gastritis, or they bind intrinsic factor and block the binding and absorption of vitamin B12 in the gastrointestinal tract. While first described in the 1820s, it was not until a century later when scientists were studying hematopoiesis in response to the heavy casualty burden from battlefield exsanguination in World War I that dogs fed raw liver were noted to have significantly better blood regeneration response than those fed cooked liver. This discovery led physicians Minot and Murphy to use raw liver to treat pernicious anemia and found that jaundice improved, reticulocyte counts increased, and hemoglobin (Hb) concentration improved, resulting in the duo becoming the first American recipients of the Nobel Prize in physiology or medicine.3 It was ultimately determined in 1948 by chemists Folkers and Todd that the active ingredient in raw liver responsible for this phenomenon was vitamin B12.4
Patients with pernicious anemia typically present with macrocytic anemia, low reticulocyte count, hypersegmented neutrophils, as well as mild leukopenia and/or thrombocytopenia, distinguishable from folate deficiency by an elevated serum methylmalonic acid level. World Health Organization cytopenia thresholds are listed in Table 1.5 Treatment consists of lifelong vitamin B12 supplementation, and endoscopic screening is often recommended after diagnosis due to increased risk of gastrointestinal malignancy.6 Pernicious anemia can be difficult to distinguish from thrombotic thrombocytopenia purpura (TTP), a microangiopathic hemolytic anemia that can cause rapid end-organ failure and death if treatment is delayed.7 While pernicious anemia is not typically hemolytic, case reports of hemolysis in severe deficiency have been reported.7 Adequate bone marrow response to hemolysis in TTP results in an elevated reticulocyte count, which can be useful in differentiating from pernicious anemia where there is typically an inadequate bone marrow response and low reticulocyte count.8,9
The approach to working up pancytopenia begins with a detailed history inquiring about medications, exposures (benzenes, pesticides), alcohol use, and infection history. A thorough physical examination may help point the health care practitioner (HCP) toward a certain etiology, as the differential for pancytopenia is broad. In the deployed soldier downrange, resources are often limited, and the history/physical are crucial in preventing an expensive and unnecessary workup.
Case Presentation
A 24-year-old active-duty female patient presented in late December 2020 to a theater hospital in Djibouti after a witnessed syncopal episode. She had a history of Hashimoto thyroiditis and was taking levothyroxine sodium 75 mcg daily. The patient reported gluten intolerance, which was never formally evaluated. The syncopal episode lasted a few seconds and was not associated with any prodromal or postictal symptoms. No seizure activity was observed, and she had no history of syncopal episodes. She reported that she had been feeling ill 24 to 48 hours prior, with nausea, fatigue, decreased oral intake, decreased urine output, and 2 episodes of nonbilious, nonbloody emesis.
When the patient arrived, she was tachycardic with heart rate in the 130s beats per minute (baseline, 100-110 beats per minute), febrile (103 °F), and had systolic blood pressure (SBP) in the low 100s (baseline, SBP 120s-130s). An electrocardiogram and chest radiographs were unremarkable. Her complete blood count (CBC) could not be processed due to Hb and platelet levels too low to detect on assay (Table 2). Lactate dehydrogenase (LDH) was elevated at > 1000 U/L with mild elevation in liver enzymes (aspartate aminotransferase, 98 U/L; alanine aminotransferase, 51 U/L) and prolonged partial thromboplastin time 70 seconds. She did not report any increased bleeding or bruising. The peripheral blood smear demonstrated pancytopenia, without any schistocytes, and she was started on broad-spectrum antibiotics for presumed sepsis from urinary source and possible TTP.
The patient received 5 units of packed red blood cells, transfusion of platelets, and 2 doses of vitamin B12 in Djibouti with clinical improvement and resolution of orthostasis, hypotension, tachycardia, and fever. Her final posttransfusion CBC showed a Hb level of 11.2 g/dL, white blood cell (WBC) count of 1.7 K/µL, and platelet count of 23 K/µL (Table 3). Two days later her Hb level was 9.0 g/dL, WBC count 1.8 K/µL, and platelet count was 12 K/µL. She was evacuated via air to Landstuhl Regional Medical Center (LRMC) in Germany within 48 hours of presentation, given limited testing capabilities and persistent anemia and thrombocytopenia, refractory to transfusion, concerning for aplastic anemia or acute leukemia.
On arrival at LRMC, she was transfused 1 unit of platelets and given 3 doses of intramuscular vitamin B12 for undetectable levels (< 50 pg/mL) at presentation. An extensive infectious workup was obtained, which did not reveal any viral, bacterial, or parasitic causes. The patient also had a bone marrow biopsy performed at a civilian site, which revealed hypocellular bone marrow. She was transferred to Walter Reed National Military Medical Center (WRNMMC) for further workup and evaluation, given the infectious workup, which was negative. Concern for hematologic malignancy remained. At the time of her arrival, the laboratory values had drastically improved with vitamin supplementation. The patient’s absolute reticulocyte count indicated adequate bone marrow response and because of her improvement, a repeat bone marrow biopsy was not performed.
Intrinsic factor antibodies were elevated (34.5 AU/mL; reference range, 0.0-1.1), which confirmed that this patient’s underlying etiology was secondary to pernicious anemia. The patient continued to improve and repeat vitamin B12 and folate levels revealed that she was responding to therapy. At discharge, intramuscular vitamin B12 injections were planned to continue monthly, indefinitely per guidelines. Oral supplementation is typically avoided due to poor absorption.
Of note, during her inpatient admission at WRNMMC, further evaluation of reported gluten intolerance was performed, which revealed a negative celiac disease panel (IgG/IgA tissue transglutaminase antibodies). On discharge, she was to establish care with gastroenterology for further evaluation, likely including endoscopic evaluation, at her next duty station. She was able to resume full travel and duty functions on discharge from WRNMMC.
Discussion
We highlight a complex case of pancytopenia secondary to pernicious anemia in a deployed service member. With limited resources downrange, the workup of pancytopenia can be resource intensive, expensive, and time sensitive, which can have detrimental impacts on medical readiness. Additionally, undiagnosed coagulopathies can have lethal consequences in a deployed service member where bleeding risk may be elevated depending on the mission. The differential for pancytopenia is vast, and given its relative rarity in pernicious anemia, the HCP must use key components of the history and laboratory results to narrow the differential (eAppendix).10
Pernicious anemia commonly presents as an isolated anemia. In a study looking at the hematologic manifestations of 201 cohort patients with well-documented vitamin B12 deficiency, 5% had symptomatic pancytopenia and 1.5% had a hemolytic anemia.2 The majority (> 67%) of hematologic abnormalities were correctable with cobalamin replacement.2 In our case, the solider presented with symptomatic anemia, manifesting as syncope, and was found to have transfusion-resistant pancytopenia.She had a hemolytic anemia with an LDH > 1000 U/L, haptoglobin < 3 mg/dL, and mild transaminitis with hyperbilirubinemia (1.8 mg/dL). No schistocytes were observed on peripheral smear, suggesting intramedullary hemolysis, which is believed to be due to the destruction of megaloblastic cells by macrophages in bone marrow.11 A French study found high LDH levels and low reticulocyte counts to be strongly suggestive of vitamin B12 deficiency and helpful in differentiating pernicious anemia from TTP, given that bone marrow response to anemia in TTP is preserved.8
While vitamin B12 deficiency is not often associated with hemolytic anemia, multiple cases have been reported in the literature.6 Screening for vitamin B12 deficiency may have shortened this patient’s clinical course and limited the need for air evacuation to a stateside quaternary medical center. However, testing for cobalamin levels in overseas deployed environments is difficult, timely, and costly. New technologies, such as optical sensors, can detect vitamin B12 levels in the blood in < 1 minute and offer portable, low-cost options that may be useful in the deployed military setting.12
Diet plays a key role in this case, since the patient had a reported history of gluten intolerance, although it was never documented or evaluated prior to this presentation. Prior to deployment, the patient ate mostly rice, potatoes, and vegetables. While deployed in an austere environment, food options were limited. These conditions forced her to intermittently consume gluten products, which led to gastrointestinal issues, exacerbating her nutritional deficiencies. In the 2 months before her first syncopal episode, she reported worsening fatigue that impacted her ability to exercise. Vitamin B12 stores often take years to deplete, suggesting that she had a chronic nutritional deficiency before deployment. Another possibility was that she developed an autoimmune gastritis that acutely worsened in the setting of poor nutritional intake. Her history of Hashimoto thyroiditis is also important, as up to one-third of patients with autoimmune thyroid disease have been associated with pernicious anemia (range, 3%-32%) with certain shared human leukocyte antigen alleles implicated in autoimmune gastritis.13,14
Conclusions
This rare case of pernicious anemia presenting as pancytopenia illustrates the challenge in working up pancytopenia, especially in austere military environments with limited testing capabilities. Screening for chronic dietary and nutritional deficiency is important in a service member, raising the question of what role predeployment screening may have and what dietary accommodations may be available during overseas deployments, which can potentially dampen inflammation of the gastrointestinal tract, especially for those with preexisting autoimmune gastrointestinal conditions. Also, newer technology allows portable, low-cost testing of cobalamin and may aid in its diagnosis. In patients who are anemic with low vitamin B12, HCPs can begin vitamin B12 supplementation while continuing the workup (eg, antibody testing, endoscopy). If the patient responds appropriately, further workup becomes less urgent, therefore, decreasing resource use and increasing military readiness. When hemolysis is present, a low reticulocyte count can be beneficial to help differentiate this condition from TTP, a life-threatening condition that must also be ruled out or treated. Pernicious anemia should be on the differential in any patients with autoimmune conditions presenting with cytopenias, especially in those with a history of autoimmune thyroid disorders.
Pancytopenia is a condition in which all 3 hematologic cell lines are lower than expected in the blood, often representing either an increase in cellular destruction or decrease in bone marrow production. Destruction often occurs in the setting of autoimmune conditions (eg, systemic lupus erythematosus, rheumatoid arthritis) or splenic sequestration, often affecting erythrocytes and platelets more than leukocytes. Decreased production represents central etiologies, which are often due to nutritional deficiencies, infections, drug toxicities, or malabsorption.1 Pancytopenia secondary to vitamin B12 deficiency is rare, accounting for about 5% of the hematologic manifestations of symptomatic vitamin B12 deficient patients.2
Pernicious anemia, named for a once lethal disease, is a form of vitamin B12 (cobalamin) deficiency that results from an autoimmune (type II hypersensitivity) reaction to gastric parietal cells or intrinsic factor. Antibodies bind to gastric parietal cells and reduce gastric acid production, leading to atrophic gastritis, or they bind intrinsic factor and block the binding and absorption of vitamin B12 in the gastrointestinal tract. While first described in the 1820s, it was not until a century later when scientists were studying hematopoiesis in response to the heavy casualty burden from battlefield exsanguination in World War I that dogs fed raw liver were noted to have significantly better blood regeneration response than those fed cooked liver. This discovery led physicians Minot and Murphy to use raw liver to treat pernicious anemia and found that jaundice improved, reticulocyte counts increased, and hemoglobin (Hb) concentration improved, resulting in the duo becoming the first American recipients of the Nobel Prize in physiology or medicine.3 It was ultimately determined in 1948 by chemists Folkers and Todd that the active ingredient in raw liver responsible for this phenomenon was vitamin B12.4
Patients with pernicious anemia typically present with macrocytic anemia, low reticulocyte count, hypersegmented neutrophils, as well as mild leukopenia and/or thrombocytopenia, distinguishable from folate deficiency by an elevated serum methylmalonic acid level. World Health Organization cytopenia thresholds are listed in Table 1.5 Treatment consists of lifelong vitamin B12 supplementation, and endoscopic screening is often recommended after diagnosis due to increased risk of gastrointestinal malignancy.6 Pernicious anemia can be difficult to distinguish from thrombotic thrombocytopenia purpura (TTP), a microangiopathic hemolytic anemia that can cause rapid end-organ failure and death if treatment is delayed.7 While pernicious anemia is not typically hemolytic, case reports of hemolysis in severe deficiency have been reported.7 Adequate bone marrow response to hemolysis in TTP results in an elevated reticulocyte count, which can be useful in differentiating from pernicious anemia where there is typically an inadequate bone marrow response and low reticulocyte count.8,9
The approach to working up pancytopenia begins with a detailed history inquiring about medications, exposures (benzenes, pesticides), alcohol use, and infection history. A thorough physical examination may help point the health care practitioner (HCP) toward a certain etiology, as the differential for pancytopenia is broad. In the deployed soldier downrange, resources are often limited, and the history/physical are crucial in preventing an expensive and unnecessary workup.
Case Presentation
A 24-year-old active-duty female patient presented in late December 2020 to a theater hospital in Djibouti after a witnessed syncopal episode. She had a history of Hashimoto thyroiditis and was taking levothyroxine sodium 75 mcg daily. The patient reported gluten intolerance, which was never formally evaluated. The syncopal episode lasted a few seconds and was not associated with any prodromal or postictal symptoms. No seizure activity was observed, and she had no history of syncopal episodes. She reported that she had been feeling ill 24 to 48 hours prior, with nausea, fatigue, decreased oral intake, decreased urine output, and 2 episodes of nonbilious, nonbloody emesis.
When the patient arrived, she was tachycardic with heart rate in the 130s beats per minute (baseline, 100-110 beats per minute), febrile (103 °F), and had systolic blood pressure (SBP) in the low 100s (baseline, SBP 120s-130s). An electrocardiogram and chest radiographs were unremarkable. Her complete blood count (CBC) could not be processed due to Hb and platelet levels too low to detect on assay (Table 2). Lactate dehydrogenase (LDH) was elevated at > 1000 U/L with mild elevation in liver enzymes (aspartate aminotransferase, 98 U/L; alanine aminotransferase, 51 U/L) and prolonged partial thromboplastin time 70 seconds. She did not report any increased bleeding or bruising. The peripheral blood smear demonstrated pancytopenia, without any schistocytes, and she was started on broad-spectrum antibiotics for presumed sepsis from urinary source and possible TTP.
The patient received 5 units of packed red blood cells, transfusion of platelets, and 2 doses of vitamin B12 in Djibouti with clinical improvement and resolution of orthostasis, hypotension, tachycardia, and fever. Her final posttransfusion CBC showed a Hb level of 11.2 g/dL, white blood cell (WBC) count of 1.7 K/µL, and platelet count of 23 K/µL (Table 3). Two days later her Hb level was 9.0 g/dL, WBC count 1.8 K/µL, and platelet count was 12 K/µL. She was evacuated via air to Landstuhl Regional Medical Center (LRMC) in Germany within 48 hours of presentation, given limited testing capabilities and persistent anemia and thrombocytopenia, refractory to transfusion, concerning for aplastic anemia or acute leukemia.
On arrival at LRMC, she was transfused 1 unit of platelets and given 3 doses of intramuscular vitamin B12 for undetectable levels (< 50 pg/mL) at presentation. An extensive infectious workup was obtained, which did not reveal any viral, bacterial, or parasitic causes. The patient also had a bone marrow biopsy performed at a civilian site, which revealed hypocellular bone marrow. She was transferred to Walter Reed National Military Medical Center (WRNMMC) for further workup and evaluation, given the infectious workup, which was negative. Concern for hematologic malignancy remained. At the time of her arrival, the laboratory values had drastically improved with vitamin supplementation. The patient’s absolute reticulocyte count indicated adequate bone marrow response and because of her improvement, a repeat bone marrow biopsy was not performed.
Intrinsic factor antibodies were elevated (34.5 AU/mL; reference range, 0.0-1.1), which confirmed that this patient’s underlying etiology was secondary to pernicious anemia. The patient continued to improve and repeat vitamin B12 and folate levels revealed that she was responding to therapy. At discharge, intramuscular vitamin B12 injections were planned to continue monthly, indefinitely per guidelines. Oral supplementation is typically avoided due to poor absorption.
Of note, during her inpatient admission at WRNMMC, further evaluation of reported gluten intolerance was performed, which revealed a negative celiac disease panel (IgG/IgA tissue transglutaminase antibodies). On discharge, she was to establish care with gastroenterology for further evaluation, likely including endoscopic evaluation, at her next duty station. She was able to resume full travel and duty functions on discharge from WRNMMC.
Discussion
We highlight a complex case of pancytopenia secondary to pernicious anemia in a deployed service member. With limited resources downrange, the workup of pancytopenia can be resource intensive, expensive, and time sensitive, which can have detrimental impacts on medical readiness. Additionally, undiagnosed coagulopathies can have lethal consequences in a deployed service member where bleeding risk may be elevated depending on the mission. The differential for pancytopenia is vast, and given its relative rarity in pernicious anemia, the HCP must use key components of the history and laboratory results to narrow the differential (eAppendix).10
Pernicious anemia commonly presents as an isolated anemia. In a study looking at the hematologic manifestations of 201 cohort patients with well-documented vitamin B12 deficiency, 5% had symptomatic pancytopenia and 1.5% had a hemolytic anemia.2 The majority (> 67%) of hematologic abnormalities were correctable with cobalamin replacement.2 In our case, the solider presented with symptomatic anemia, manifesting as syncope, and was found to have transfusion-resistant pancytopenia.She had a hemolytic anemia with an LDH > 1000 U/L, haptoglobin < 3 mg/dL, and mild transaminitis with hyperbilirubinemia (1.8 mg/dL). No schistocytes were observed on peripheral smear, suggesting intramedullary hemolysis, which is believed to be due to the destruction of megaloblastic cells by macrophages in bone marrow.11 A French study found high LDH levels and low reticulocyte counts to be strongly suggestive of vitamin B12 deficiency and helpful in differentiating pernicious anemia from TTP, given that bone marrow response to anemia in TTP is preserved.8
While vitamin B12 deficiency is not often associated with hemolytic anemia, multiple cases have been reported in the literature.6 Screening for vitamin B12 deficiency may have shortened this patient’s clinical course and limited the need for air evacuation to a stateside quaternary medical center. However, testing for cobalamin levels in overseas deployed environments is difficult, timely, and costly. New technologies, such as optical sensors, can detect vitamin B12 levels in the blood in < 1 minute and offer portable, low-cost options that may be useful in the deployed military setting.12
Diet plays a key role in this case, since the patient had a reported history of gluten intolerance, although it was never documented or evaluated prior to this presentation. Prior to deployment, the patient ate mostly rice, potatoes, and vegetables. While deployed in an austere environment, food options were limited. These conditions forced her to intermittently consume gluten products, which led to gastrointestinal issues, exacerbating her nutritional deficiencies. In the 2 months before her first syncopal episode, she reported worsening fatigue that impacted her ability to exercise. Vitamin B12 stores often take years to deplete, suggesting that she had a chronic nutritional deficiency before deployment. Another possibility was that she developed an autoimmune gastritis that acutely worsened in the setting of poor nutritional intake. Her history of Hashimoto thyroiditis is also important, as up to one-third of patients with autoimmune thyroid disease have been associated with pernicious anemia (range, 3%-32%) with certain shared human leukocyte antigen alleles implicated in autoimmune gastritis.13,14
Conclusions
This rare case of pernicious anemia presenting as pancytopenia illustrates the challenge in working up pancytopenia, especially in austere military environments with limited testing capabilities. Screening for chronic dietary and nutritional deficiency is important in a service member, raising the question of what role predeployment screening may have and what dietary accommodations may be available during overseas deployments, which can potentially dampen inflammation of the gastrointestinal tract, especially for those with preexisting autoimmune gastrointestinal conditions. Also, newer technology allows portable, low-cost testing of cobalamin and may aid in its diagnosis. In patients who are anemic with low vitamin B12, HCPs can begin vitamin B12 supplementation while continuing the workup (eg, antibody testing, endoscopy). If the patient responds appropriately, further workup becomes less urgent, therefore, decreasing resource use and increasing military readiness. When hemolysis is present, a low reticulocyte count can be beneficial to help differentiate this condition from TTP, a life-threatening condition that must also be ruled out or treated. Pernicious anemia should be on the differential in any patients with autoimmune conditions presenting with cytopenias, especially in those with a history of autoimmune thyroid disorders.
1. Takeshima M, Ishikawa H, Kitadate A, et al. Anorexia nervosa-associated pancytopenia mimicking idiopathic aplastic anemia: a case report. BMC Psychiatry. 2018;18(1):150. doi:10.1186/s12888-018-1743-6
2. Andrès E, Affenberger S, Zimmer J, et al. Current hematological findings in cobalamin deficiency. A study of 201 consecutive patients with documented cobalamin deficiency. Clin Lab Haematol. 2006;28(1):50-56. doi:10.1111/j.1365-2257.2006.00755.x
3. Sinclair L. Recognizing, treating and understanding pernicious anaemia. J R Soc Med. 2008;101(5):262-264. doi:10.1258/jrsm.2008.081006
4. Shampo MA, Kyle RA, Steensma DP. William Murphy—Nobel Prize for the treatment of pernicious anemia. Mayo Clin Proc. 2006;81(6):726. doi:10.4065/81.6.726
5. Hong M, He G. The 2016 revision to the World Health Organization classification of myelodysplastic syndromes. J Transl Int Med. 2017;5(3):139-143. doi:10.1515/jtim-2017-0002
6. Tunio NA, Sheriff MZ, Cooper G. Prevalence of gastric cancer in patients with pernicious anemia: a population-based study. Am J Gastroenterol. 2020;115:S665. doi:10.14309/01.ajg.0000707332.16739.72
7. Bailey M, Maestas T, Betancourt R, Mikhael D, Babiker HM. A rare cause of thrombotic thrombocytopenic purpura- (TTP-) like syndrome, vitamin B12 deficiency: interpretation of significant pathological findings. Case Rep Hematol. 2019;2019:1529306. doi:10.1155/2019/1529306
8. Stanley M, Michalski JM. Thrombotic Thrombocytopenic Purpura. StatPearls Publishing LLC; 2021.
9. Noël N, Maigné G, Tertian G, et al. Hemolysis and schistocytosis in the emergency department: consider pseudothrombotic microangiopathy related to vitamin B12 deficiency. QJM. 2013;106(11):1017-1022. doi:10.1093/qjmed/hct142
10. Chiravuri S, De Jesus O. Pancytopenia. StatPearls Publishing LLC; 2021.
11. Gladstone E. Pernicious anemia presenting with pancytopenia and hemolysis: a case report. February 8, 2019. Accessed June 9, 2022. https://www.journalmc.org/index.php/JMC/article/view/3269/2563
12. ScienceDaily. Developing a sensor for vitamin B12 deficiency. October 17, 2016. Accessed June 9, 2022. https://www.sciencedaily.com/releases/2016/10/161017103221.htm
13. Rodriguez NM, Shackelford K. Pernicious Anemia. StatPearls Publishing LLC; 2021.
14. Fernando MM, Stevens CR, Walsh EC, et al. Defining the role of the MHC in autoimmunity: a review and pooled analysis. PLoS Genet. 2008;4(4):e1000024. doi:10.1371/journal.pgen.1000024
1. Takeshima M, Ishikawa H, Kitadate A, et al. Anorexia nervosa-associated pancytopenia mimicking idiopathic aplastic anemia: a case report. BMC Psychiatry. 2018;18(1):150. doi:10.1186/s12888-018-1743-6
2. Andrès E, Affenberger S, Zimmer J, et al. Current hematological findings in cobalamin deficiency. A study of 201 consecutive patients with documented cobalamin deficiency. Clin Lab Haematol. 2006;28(1):50-56. doi:10.1111/j.1365-2257.2006.00755.x
3. Sinclair L. Recognizing, treating and understanding pernicious anaemia. J R Soc Med. 2008;101(5):262-264. doi:10.1258/jrsm.2008.081006
4. Shampo MA, Kyle RA, Steensma DP. William Murphy—Nobel Prize for the treatment of pernicious anemia. Mayo Clin Proc. 2006;81(6):726. doi:10.4065/81.6.726
5. Hong M, He G. The 2016 revision to the World Health Organization classification of myelodysplastic syndromes. J Transl Int Med. 2017;5(3):139-143. doi:10.1515/jtim-2017-0002
6. Tunio NA, Sheriff MZ, Cooper G. Prevalence of gastric cancer in patients with pernicious anemia: a population-based study. Am J Gastroenterol. 2020;115:S665. doi:10.14309/01.ajg.0000707332.16739.72
7. Bailey M, Maestas T, Betancourt R, Mikhael D, Babiker HM. A rare cause of thrombotic thrombocytopenic purpura- (TTP-) like syndrome, vitamin B12 deficiency: interpretation of significant pathological findings. Case Rep Hematol. 2019;2019:1529306. doi:10.1155/2019/1529306
8. Stanley M, Michalski JM. Thrombotic Thrombocytopenic Purpura. StatPearls Publishing LLC; 2021.
9. Noël N, Maigné G, Tertian G, et al. Hemolysis and schistocytosis in the emergency department: consider pseudothrombotic microangiopathy related to vitamin B12 deficiency. QJM. 2013;106(11):1017-1022. doi:10.1093/qjmed/hct142
10. Chiravuri S, De Jesus O. Pancytopenia. StatPearls Publishing LLC; 2021.
11. Gladstone E. Pernicious anemia presenting with pancytopenia and hemolysis: a case report. February 8, 2019. Accessed June 9, 2022. https://www.journalmc.org/index.php/JMC/article/view/3269/2563
12. ScienceDaily. Developing a sensor for vitamin B12 deficiency. October 17, 2016. Accessed June 9, 2022. https://www.sciencedaily.com/releases/2016/10/161017103221.htm
13. Rodriguez NM, Shackelford K. Pernicious Anemia. StatPearls Publishing LLC; 2021.
14. Fernando MM, Stevens CR, Walsh EC, et al. Defining the role of the MHC in autoimmunity: a review and pooled analysis. PLoS Genet. 2008;4(4):e1000024. doi:10.1371/journal.pgen.1000024
In the Heat of Anger: The Impact of Increasing Temperatures on Veteran and Military Mental Health
In June, an intense heatwave moved across the United States. According to the National Weather Service, about 50 million Americans were warned of excessively hot temperatures. In the Southwest, dozens of cities broke temperature records, and in New Mexico, my home state, terrible wildfires ravaged thousands of acres. Nor will the rest of the country be spared as the dome of heat is expected to move into the Midwest and even the Northeast before it is done.2 The ongoing COVID-19 pandemic and the heatwaves exacerbate each other.
The US Global Change Research Program studies the adverse effects of high temperatures on human health. High ambient heat is associated with cardiovascular, respiratory, renal, metabolic, vector- and food-borne illnesses, and malnutrition.3 Veterans, especially those who are older or homeless, are considered heat-vulnerable populations more susceptible to sunstroke, dehydration, and heat exhaustion. Last July, during record-breaking heat in many large cities, US Department of Veterans Affairs (VA) Secretary McDonough emphasized the availability of community and VA resources for veterans experiencing high temperatures, especially those who were homeless.4
The Veterans Health Administration (VHA) also reached out to service members who were deployed to the baking deserts of Iraq and Afghanistan, advising them that they may experience service-related heat stroke or exhaustion.5 Today’s military confronts many challenges in training troops in hot temperatures at home and in combat overseas.6 The 2019 report from the advocacy and research organization, the Union of Concerned Scientists (UCS) reported the need to ensure the preparedness of the fighting force and that increasing temperatures from climate change will make it harder to balance this mission with protecting the health of service members. The study estimated there were 17 heat-related deaths and a 60% jump in heat-related injuries in the previous decade. More worrisome, UCS predicted that if the temperature trend is not abated, by 2050, military bases will experience an additional month where the heat index will feel like it is 100 °F or more.7
In comparison, less attention has been focused on the adverse effects of high temperatures on mental health. Most of us recognize that too many hot days in the height of summer may cause fatigue, impatience, restlessness, and difficulty concentrating. A 2018 systematic review reported a strong association between high ambient temperature and suicide.8 Suicide has been among the highest public health priorities in the VHA and the military. There is also evidence of the correlation between emergency department (ED) visits for mental health conditions in non-VA hospitals and scorching days and sweltering nights. An analysis of data of > 3 million ED visits involving > 2 million insured patients revealed increased ED visits during periods of extreme heat for stress, substance use, somatoform, anxiety, mood, schizophrenia, schizotypal and delusional disorders, as well as self-harming behaviors. The association is likely even stronger in service members. The largest study ever conducted on the mental health of service members, the Army Study to Assess Risk and Resilience in Servicemembers (ARMY STARRS), found higher rates of mental health disorders than in the civilian population.9
What is the clinical science behind this long-established link between heat and mental health disorders? High temperatures disrupt sleep, interfere with memory and attention, and increase irritability and depression especially in persons with extant mental health disorders. Individuals with schizophrenia may have difficulty with temperature regulation, a problem antipsychotic and antidepressant medications may exacerbate. Extreme heat leads to extreme behavior, including domestic violence, chemical coping, and aggression. Individuals diagnosed with dementia may lack the mental wherewithal and economic resources to prepare for and respond to extreme heat, leading to higher morbidity and mortality.10,11 The so called heat hypothesis holds that extreme high temperatures increase hostile feelings and aggressive thoughts that trigger other directed violence.12
For many, summer is the most enjoyable time of year “when the livin’ is easy,” as the George Gershwin song claims. We can relax in air-conditioned homes or sit by a swimming pool, sipping iced tea. As federal practitioners, and even more as humans, we need to realize that our patients, those on active duty, veterans, and the underserved populations who are the mission of the US Commissioned Corps and the Indian Health Service, are not as fortunate. As temperatures climb, we must redouble our efforts to educate our patients about the dangers of extreme heat and advocate for policies and procedures in our respective federal agencies that will positively impact climate change.
During clinical encounters with patients with mental health conditions, we should be mindful of the potential increase in substance use, suicidal ideation, aggressive impulses, and medication adverse effects when temperatures are high. Most important, we must raise awareness and participate in public health initiatives to ensure that populations whose prior service, current duty, or health disparities place them at greater risk of harm from increasing temperatures have access to shelter, cooling, food, health care, psychosocial services, and mental health treatment.
1. Hanh, TN. Peace Is Every Breath: A Practice for Our Busy Lives. HarperOne; 2012.
2. Jones D. More than 50 million people in the U.S. are under excessive heat warnings. Updated June 12, 2022. Accessed June 20, 2022. https://www.michiganradioorg/2022-06-10/more-than-50-million-people-in-the-u-s-are-under-excessive-heat-warnings
3. US Global Change Research Program. Climate and Health Assessment. Temperature- Related Illness and Death. Accessed June 20, 2022. https://health2016.globalchange.gov/temperature-related-death-and-illness
4. US Department of Veterans Affairs. Extreme heat assistance available for veterans experiencing homelessness. Updated July 14, 2021. Accessed June 20, 2022. https://blogs.va.gov/VAntage/91819/extreme-heat-assistance-available-for-veterans-experiencing-homelessness
5. US Department of Veterans Affairs. Heat injuries. April 10, 2020. Accessed June 20, 2022. https://www.publichealth.va.gov/exposures/heat-injuries/index.asp
6. Hasemeyer D. Rising temperatures put U.S. troops at risk during training, report finds. November 10, 2019. Accessed June 20, 2022. https://www.nbcnews.com/science/environment/rising-temperatures-put-u-s-troops-risk-during-training-report-n1079156
7. Dahl K, Udvardy S. US military on the front lines of extreme heat. November 11, 2019. Updated January 4, 2020. Accessed June 20, 2022. https://www.ucsusa.org/resources/us-military-bases-risk-extreme-heat
8. Thompson R, Horingold R, Page L, Waite T. Associations between high ambient temperatures and heat waves with mental health outcomes: a systematic review. Public Health. 2018;161:171-191. doi:10.1016/j.puhe.2018.06.008
9. Kessler RC, Heeringa SG, Stein MB, et al; Army STARRS Collaborators. Thirty-day prevalence of DSM-IV mental disorders among non-deployed soldiers in the U.S. Army. JAMA Psychiatry. 2014;71(5):504-513. doi:10.1001/jamapsychiatry.2014.28
10. American Psychiatric Association. extreme heat contributes to worsening mental health, especially among vulnerable populations. June 30, 2021. Accessed June 20, 2022. https://psychiatry.org/news-room/news-releases/extreme-heat-contributes-to-worsening-mental-healt
11. Cooper R. The impacts of extreme heat on mental health. Psychiatric Times. July 29, 2019. Accessed June 20, 2022. https://www.psychiatrictimes.com/view/impacts-extreme-heat-mental-health
12. Anderson C. Heat and violence. Current Directions in Psychological Violence. 2011;10(1):38-42. doi.10.1177/0963721410397271
In June, an intense heatwave moved across the United States. According to the National Weather Service, about 50 million Americans were warned of excessively hot temperatures. In the Southwest, dozens of cities broke temperature records, and in New Mexico, my home state, terrible wildfires ravaged thousands of acres. Nor will the rest of the country be spared as the dome of heat is expected to move into the Midwest and even the Northeast before it is done.2 The ongoing COVID-19 pandemic and the heatwaves exacerbate each other.
The US Global Change Research Program studies the adverse effects of high temperatures on human health. High ambient heat is associated with cardiovascular, respiratory, renal, metabolic, vector- and food-borne illnesses, and malnutrition.3 Veterans, especially those who are older or homeless, are considered heat-vulnerable populations more susceptible to sunstroke, dehydration, and heat exhaustion. Last July, during record-breaking heat in many large cities, US Department of Veterans Affairs (VA) Secretary McDonough emphasized the availability of community and VA resources for veterans experiencing high temperatures, especially those who were homeless.4
The Veterans Health Administration (VHA) also reached out to service members who were deployed to the baking deserts of Iraq and Afghanistan, advising them that they may experience service-related heat stroke or exhaustion.5 Today’s military confronts many challenges in training troops in hot temperatures at home and in combat overseas.6 The 2019 report from the advocacy and research organization, the Union of Concerned Scientists (UCS) reported the need to ensure the preparedness of the fighting force and that increasing temperatures from climate change will make it harder to balance this mission with protecting the health of service members. The study estimated there were 17 heat-related deaths and a 60% jump in heat-related injuries in the previous decade. More worrisome, UCS predicted that if the temperature trend is not abated, by 2050, military bases will experience an additional month where the heat index will feel like it is 100 °F or more.7
In comparison, less attention has been focused on the adverse effects of high temperatures on mental health. Most of us recognize that too many hot days in the height of summer may cause fatigue, impatience, restlessness, and difficulty concentrating. A 2018 systematic review reported a strong association between high ambient temperature and suicide.8 Suicide has been among the highest public health priorities in the VHA and the military. There is also evidence of the correlation between emergency department (ED) visits for mental health conditions in non-VA hospitals and scorching days and sweltering nights. An analysis of data of > 3 million ED visits involving > 2 million insured patients revealed increased ED visits during periods of extreme heat for stress, substance use, somatoform, anxiety, mood, schizophrenia, schizotypal and delusional disorders, as well as self-harming behaviors. The association is likely even stronger in service members. The largest study ever conducted on the mental health of service members, the Army Study to Assess Risk and Resilience in Servicemembers (ARMY STARRS), found higher rates of mental health disorders than in the civilian population.9
What is the clinical science behind this long-established link between heat and mental health disorders? High temperatures disrupt sleep, interfere with memory and attention, and increase irritability and depression especially in persons with extant mental health disorders. Individuals with schizophrenia may have difficulty with temperature regulation, a problem antipsychotic and antidepressant medications may exacerbate. Extreme heat leads to extreme behavior, including domestic violence, chemical coping, and aggression. Individuals diagnosed with dementia may lack the mental wherewithal and economic resources to prepare for and respond to extreme heat, leading to higher morbidity and mortality.10,11 The so called heat hypothesis holds that extreme high temperatures increase hostile feelings and aggressive thoughts that trigger other directed violence.12
For many, summer is the most enjoyable time of year “when the livin’ is easy,” as the George Gershwin song claims. We can relax in air-conditioned homes or sit by a swimming pool, sipping iced tea. As federal practitioners, and even more as humans, we need to realize that our patients, those on active duty, veterans, and the underserved populations who are the mission of the US Commissioned Corps and the Indian Health Service, are not as fortunate. As temperatures climb, we must redouble our efforts to educate our patients about the dangers of extreme heat and advocate for policies and procedures in our respective federal agencies that will positively impact climate change.
During clinical encounters with patients with mental health conditions, we should be mindful of the potential increase in substance use, suicidal ideation, aggressive impulses, and medication adverse effects when temperatures are high. Most important, we must raise awareness and participate in public health initiatives to ensure that populations whose prior service, current duty, or health disparities place them at greater risk of harm from increasing temperatures have access to shelter, cooling, food, health care, psychosocial services, and mental health treatment.
In June, an intense heatwave moved across the United States. According to the National Weather Service, about 50 million Americans were warned of excessively hot temperatures. In the Southwest, dozens of cities broke temperature records, and in New Mexico, my home state, terrible wildfires ravaged thousands of acres. Nor will the rest of the country be spared as the dome of heat is expected to move into the Midwest and even the Northeast before it is done.2 The ongoing COVID-19 pandemic and the heatwaves exacerbate each other.
The US Global Change Research Program studies the adverse effects of high temperatures on human health. High ambient heat is associated with cardiovascular, respiratory, renal, metabolic, vector- and food-borne illnesses, and malnutrition.3 Veterans, especially those who are older or homeless, are considered heat-vulnerable populations more susceptible to sunstroke, dehydration, and heat exhaustion. Last July, during record-breaking heat in many large cities, US Department of Veterans Affairs (VA) Secretary McDonough emphasized the availability of community and VA resources for veterans experiencing high temperatures, especially those who were homeless.4
The Veterans Health Administration (VHA) also reached out to service members who were deployed to the baking deserts of Iraq and Afghanistan, advising them that they may experience service-related heat stroke or exhaustion.5 Today’s military confronts many challenges in training troops in hot temperatures at home and in combat overseas.6 The 2019 report from the advocacy and research organization, the Union of Concerned Scientists (UCS) reported the need to ensure the preparedness of the fighting force and that increasing temperatures from climate change will make it harder to balance this mission with protecting the health of service members. The study estimated there were 17 heat-related deaths and a 60% jump in heat-related injuries in the previous decade. More worrisome, UCS predicted that if the temperature trend is not abated, by 2050, military bases will experience an additional month where the heat index will feel like it is 100 °F or more.7
In comparison, less attention has been focused on the adverse effects of high temperatures on mental health. Most of us recognize that too many hot days in the height of summer may cause fatigue, impatience, restlessness, and difficulty concentrating. A 2018 systematic review reported a strong association between high ambient temperature and suicide.8 Suicide has been among the highest public health priorities in the VHA and the military. There is also evidence of the correlation between emergency department (ED) visits for mental health conditions in non-VA hospitals and scorching days and sweltering nights. An analysis of data of > 3 million ED visits involving > 2 million insured patients revealed increased ED visits during periods of extreme heat for stress, substance use, somatoform, anxiety, mood, schizophrenia, schizotypal and delusional disorders, as well as self-harming behaviors. The association is likely even stronger in service members. The largest study ever conducted on the mental health of service members, the Army Study to Assess Risk and Resilience in Servicemembers (ARMY STARRS), found higher rates of mental health disorders than in the civilian population.9
What is the clinical science behind this long-established link between heat and mental health disorders? High temperatures disrupt sleep, interfere with memory and attention, and increase irritability and depression especially in persons with extant mental health disorders. Individuals with schizophrenia may have difficulty with temperature regulation, a problem antipsychotic and antidepressant medications may exacerbate. Extreme heat leads to extreme behavior, including domestic violence, chemical coping, and aggression. Individuals diagnosed with dementia may lack the mental wherewithal and economic resources to prepare for and respond to extreme heat, leading to higher morbidity and mortality.10,11 The so called heat hypothesis holds that extreme high temperatures increase hostile feelings and aggressive thoughts that trigger other directed violence.12
For many, summer is the most enjoyable time of year “when the livin’ is easy,” as the George Gershwin song claims. We can relax in air-conditioned homes or sit by a swimming pool, sipping iced tea. As federal practitioners, and even more as humans, we need to realize that our patients, those on active duty, veterans, and the underserved populations who are the mission of the US Commissioned Corps and the Indian Health Service, are not as fortunate. As temperatures climb, we must redouble our efforts to educate our patients about the dangers of extreme heat and advocate for policies and procedures in our respective federal agencies that will positively impact climate change.
During clinical encounters with patients with mental health conditions, we should be mindful of the potential increase in substance use, suicidal ideation, aggressive impulses, and medication adverse effects when temperatures are high. Most important, we must raise awareness and participate in public health initiatives to ensure that populations whose prior service, current duty, or health disparities place them at greater risk of harm from increasing temperatures have access to shelter, cooling, food, health care, psychosocial services, and mental health treatment.
1. Hanh, TN. Peace Is Every Breath: A Practice for Our Busy Lives. HarperOne; 2012.
2. Jones D. More than 50 million people in the U.S. are under excessive heat warnings. Updated June 12, 2022. Accessed June 20, 2022. https://www.michiganradioorg/2022-06-10/more-than-50-million-people-in-the-u-s-are-under-excessive-heat-warnings
3. US Global Change Research Program. Climate and Health Assessment. Temperature- Related Illness and Death. Accessed June 20, 2022. https://health2016.globalchange.gov/temperature-related-death-and-illness
4. US Department of Veterans Affairs. Extreme heat assistance available for veterans experiencing homelessness. Updated July 14, 2021. Accessed June 20, 2022. https://blogs.va.gov/VAntage/91819/extreme-heat-assistance-available-for-veterans-experiencing-homelessness
5. US Department of Veterans Affairs. Heat injuries. April 10, 2020. Accessed June 20, 2022. https://www.publichealth.va.gov/exposures/heat-injuries/index.asp
6. Hasemeyer D. Rising temperatures put U.S. troops at risk during training, report finds. November 10, 2019. Accessed June 20, 2022. https://www.nbcnews.com/science/environment/rising-temperatures-put-u-s-troops-risk-during-training-report-n1079156
7. Dahl K, Udvardy S. US military on the front lines of extreme heat. November 11, 2019. Updated January 4, 2020. Accessed June 20, 2022. https://www.ucsusa.org/resources/us-military-bases-risk-extreme-heat
8. Thompson R, Horingold R, Page L, Waite T. Associations between high ambient temperatures and heat waves with mental health outcomes: a systematic review. Public Health. 2018;161:171-191. doi:10.1016/j.puhe.2018.06.008
9. Kessler RC, Heeringa SG, Stein MB, et al; Army STARRS Collaborators. Thirty-day prevalence of DSM-IV mental disorders among non-deployed soldiers in the U.S. Army. JAMA Psychiatry. 2014;71(5):504-513. doi:10.1001/jamapsychiatry.2014.28
10. American Psychiatric Association. extreme heat contributes to worsening mental health, especially among vulnerable populations. June 30, 2021. Accessed June 20, 2022. https://psychiatry.org/news-room/news-releases/extreme-heat-contributes-to-worsening-mental-healt
11. Cooper R. The impacts of extreme heat on mental health. Psychiatric Times. July 29, 2019. Accessed June 20, 2022. https://www.psychiatrictimes.com/view/impacts-extreme-heat-mental-health
12. Anderson C. Heat and violence. Current Directions in Psychological Violence. 2011;10(1):38-42. doi.10.1177/0963721410397271
1. Hanh, TN. Peace Is Every Breath: A Practice for Our Busy Lives. HarperOne; 2012.
2. Jones D. More than 50 million people in the U.S. are under excessive heat warnings. Updated June 12, 2022. Accessed June 20, 2022. https://www.michiganradioorg/2022-06-10/more-than-50-million-people-in-the-u-s-are-under-excessive-heat-warnings
3. US Global Change Research Program. Climate and Health Assessment. Temperature- Related Illness and Death. Accessed June 20, 2022. https://health2016.globalchange.gov/temperature-related-death-and-illness
4. US Department of Veterans Affairs. Extreme heat assistance available for veterans experiencing homelessness. Updated July 14, 2021. Accessed June 20, 2022. https://blogs.va.gov/VAntage/91819/extreme-heat-assistance-available-for-veterans-experiencing-homelessness
5. US Department of Veterans Affairs. Heat injuries. April 10, 2020. Accessed June 20, 2022. https://www.publichealth.va.gov/exposures/heat-injuries/index.asp
6. Hasemeyer D. Rising temperatures put U.S. troops at risk during training, report finds. November 10, 2019. Accessed June 20, 2022. https://www.nbcnews.com/science/environment/rising-temperatures-put-u-s-troops-risk-during-training-report-n1079156
7. Dahl K, Udvardy S. US military on the front lines of extreme heat. November 11, 2019. Updated January 4, 2020. Accessed June 20, 2022. https://www.ucsusa.org/resources/us-military-bases-risk-extreme-heat
8. Thompson R, Horingold R, Page L, Waite T. Associations between high ambient temperatures and heat waves with mental health outcomes: a systematic review. Public Health. 2018;161:171-191. doi:10.1016/j.puhe.2018.06.008
9. Kessler RC, Heeringa SG, Stein MB, et al; Army STARRS Collaborators. Thirty-day prevalence of DSM-IV mental disorders among non-deployed soldiers in the U.S. Army. JAMA Psychiatry. 2014;71(5):504-513. doi:10.1001/jamapsychiatry.2014.28
10. American Psychiatric Association. extreme heat contributes to worsening mental health, especially among vulnerable populations. June 30, 2021. Accessed June 20, 2022. https://psychiatry.org/news-room/news-releases/extreme-heat-contributes-to-worsening-mental-healt
11. Cooper R. The impacts of extreme heat on mental health. Psychiatric Times. July 29, 2019. Accessed June 20, 2022. https://www.psychiatrictimes.com/view/impacts-extreme-heat-mental-health
12. Anderson C. Heat and violence. Current Directions in Psychological Violence. 2011;10(1):38-42. doi.10.1177/0963721410397271
Commentary: New Horizons in NSCLC Treatment: Adagrasib, Aumolertinib, and Lorlatinib, July 2022
The Direct KRASG12C Inhibitor Adagrasib in Advanced KRASG12C-Mutant NSCLC: Results From a Registrational Phase 2 Study
KRAS mutations are detected in about one quarter of all lung adenocarcinomas and are the most common oncogene driver in non–small-cell lung cancer (NSCLC). KRASG12C amino acid substitutions are the most common KRAS mutations in NSCLC, comprising just about half of all KRAS mutations in this tumor type. Despite being the most common and first detected oncogene driver in lung cancer, until recently there were no targeted therapies in KRAS mutant NSCLC. The development of direct KRASG12C inhibitors represents an important step forward in targeting KRAS mutations. These inhibitors bind inactive guanosine diphosphate (GDP)–bound RAS and trap it in its inactive state.
Dr Jänne and colleagues recently published a phase 2 registrational trial of the direct KRASG12C inhibitor adagrasib. In this study of 112 patients with measurable disease at baseline treated with adagrasib, 48 (42.9%) had a confirmed objective response. The median duration of response was 8.5 months (95% CI 6.2-13.8), and the median progression-free survival (PFS) was 6.5 months (95% CI 4.7-8.4). The median overall survival (OS) was 12.6 months (95% CI 9.2-19.2). Among 33 patients with previously treated, stable central nervous system (CNS) metastases, the intracranial confirmed objective response rate was 33.3% (95% CI 18.0-51.8). Treatment-related adverse events occurred in 97.4% of the patients: grade 1 or 2 in 52.6% and grade 3 or higher in 44.8% (including two grade 5 events). The most frequent toxicities were fatigue and gastrointestinal-related issues (nausea, vomiting, diarrhea, aspartate transaminase/alanine transaminase elevation). Adagrasib was discontinued in 6.9% of patients.
These results further demonstrate that the KRASG12C mutation is an actionable target in NSCLC. Sotorasib, another direct KRASG12C inhibitor, is currently US Food and Drug Administration approved after initial systemic treatment. The clinical activity of sotorasib and adagrasib are comparable; for sotorasib the rates are an overall response rate (ORR) of 37.1% (95% CI 28.6-46.2), median PFS of 6.8 months (95% CI 5.1-8.2), and median OS of 12.5 months (95% CI 10.0 to nonestimable). Adagrasib also has published evidence of CNS activity that tracks with its systemic activity. Overall, these direct KRASG12C inhibitors represent a major advance in the treatment of KRASG12C-mutant NSCLC.
EGFR-Mutated NSCLC: Aumolertinib vs Gefitinib Extends PFS
Epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKI) have improved clinical outcomes in EGFR-mutant NSCLC.The current standard of care for first-line treatment of advanced NSCLC with the most frequent EGFR activating mutations (EGFR E19del and L858R)is the third-generation EGFR TKI osimertinib. In the FLAURA trial, patients randomly assigned tofirst-lineosimertinib had a substantial PFS benefit (median PFS 18.9 vs 10.2 months) and OS benefit (median OS 38.6 vs 31.8 months)when receivingosimertinib compared with gefitinib or erlotinib.
In the AENEAS trial, published in the Journal of Clinical Oncologyby Dr Lu and colleagues, 420 patients from China with advanced NSCLC harboring EGFR E19del or L858R activating mutations and naive to systemic treatment were enrolled. Patients were randomly assigned to the next-generation EGFR TKI aumolertinib or the first-generation EGFR TKI gefitinib with the primary endpoint of PFS by investigator assessment.Of note, patients with asymptomatic, untreated brain metastases were allowed into the trial. Upon disease progression, patients in the gefitinib group who acquired an EGFR T790M mutation were eligible to crossover to aumolertinib.
The study met its primary endpoint: Patients treated with aumolertinib compared with those treated with gefitinib had a significantly longer median PFS (19.3 vs 9.9 months; hazard ratio [HR] 0.46; P< .0001). This PFS advantage of aumolertinib over gefitinib was also present in the subgroup of patients with CNS metastases (15.3 vs 8.2 months; HR 0.38; P< .0001). The objective response rate was similar inthe aumolertinib and gefitinib groups (objective response rate 73.8% and 72.1%, respectively). The median duration of response was 18.1 months (95% CI 15.2 to not reached) with aumolertinib vs 8.3 months (95% CI 6.9-11.1) with gefitinib. Treatment-emergent adverse events of grade 3 or more were similar in the aumolertinib and gefitinib groups (36.4% vs 35.8%, respectively). There was less rash and diarrhea as well as transaminitis in the aumolertinib arm compared with the gefitinib arm. However, 35.5% of patients developed an elevation in creatinine phosphokinase (CPK), including 7% with grade 3 CPK elevation. However, no rhabdomyolysis was observed.
Overall, the AENEAS study showed comparable median PFS for first-line aumolertinib comparedwith what was observed with osimertinib in the FLAURA study. We still await the OSdata on aumolertinib compared with gefitinib. In the FLAURA study, investigators could choose between erlotinib or gefitinib in the control arm, whereas in AENEAS only gefitinib was allowed, which may have less CNS activity than erlotinib. Moreover, the FLAURA trial was conducted worldwide, whereas the AENEAS trial only enrolled patients in China. This study provides further support for the use of third-generation EGFR TKI over first-generation EGFR TKI as first-line treatment in advanced/metastatic NSCLC harboring EGFR E19del or L858R mutations.
Advanced ALK+ NSCLC With Brain Metastases: Lorlatinib Boosts PFS, Reduces CNS Progression
The CROWN trial was a pivotal randomized phase 3 trial that demonstrated an impressive improvement in PFS in patients treated with the third-generationALK inhibitor lorlatinib compared with the first-generation ALK inhibitor crizotinib as initial treatment for advanced ALK-postive (ALK+) NSCLC (HR for disease progression or death0.28; 95% CI0.19-0.41; P≤ .001). A major driver of this PFS benefit in ALK+ NSCLC in the CROWN study is the superior CNS penetration of lorlatinib compared with crizotinib. Obtaining CNS control in ALK+ lung cancers is important because up to 40% of patients with ALK+ NSCLC have brain metastases at initial evaluation, and CNS progression is often observed in patients with ALK+ lung cancer whether it be intracranial metastases or leptomeningeal carcinomatosis. A potential challenge in treating patients with lorlatinib is a unique side effect profile including neurocognitive side effects from lorlatinib. In a recently published study in the Journal of Clinical Oncology, Dr Solomon and colleagues conducted a post hoc exploratory analysis of intracranial efficacy and safety of lorlatinib in ALK+ NSCLC from a phase 3 trial. PFS by blinded independent central review was improved with lorlatinib vs crizotinib in patients with and without brain metastases at baseline (12-month PFS rates: 78% vs 22% and 78% vs 45%, respectively). Lorlatinib was associated with lower 12-month cumulative incidence of CNS progression compared with crizotinib in patients with (7.4% vs 72%) and without (1% vs 18%) brain metastases at baseline. Complete CNS responses with lorlatinib were seen in 23/38(61%) patients with any brain metastases at baseline compared with 6/40 (15%) with crizotinib. In total, 35% of patients had CNS adverse events with lorlatinib: grade 1 (21%), grade 2 (10%), and grade 3 (3%)in severity. These included cognitive (21%), mood (16%), speech (5%), and psychotic effects (3%), some of which overlapped. Half of all CNS adverse events resolved without intervention or with lorlatinib dose modification. Dose reductions of lorlatinib did not appear to affect PFS on the basis of a landmark analysis. Overall, this study demonstrates the exceptional CNS activity of lorlatinib in ALK+ NSCLC and that the neurocognitive side effects can often be managed. There are several next-generation ALK inhibitors now approved in the first-line setting —alectinib, lorlatinib, and brigatinib — notably all with enhanced CNS penetration and improved PFS compared with crizotinib. This posthoc study further supports the impressive CNS activity of lorlatinib in ALK+ NSCLC and supports the use of lorlatinib as a first-line treatment option in these patients, particularly those with ALK+ NSCLC diagnosed with baseline CNS disease.
The Direct KRASG12C Inhibitor Adagrasib in Advanced KRASG12C-Mutant NSCLC: Results From a Registrational Phase 2 Study
KRAS mutations are detected in about one quarter of all lung adenocarcinomas and are the most common oncogene driver in non–small-cell lung cancer (NSCLC). KRASG12C amino acid substitutions are the most common KRAS mutations in NSCLC, comprising just about half of all KRAS mutations in this tumor type. Despite being the most common and first detected oncogene driver in lung cancer, until recently there were no targeted therapies in KRAS mutant NSCLC. The development of direct KRASG12C inhibitors represents an important step forward in targeting KRAS mutations. These inhibitors bind inactive guanosine diphosphate (GDP)–bound RAS and trap it in its inactive state.
Dr Jänne and colleagues recently published a phase 2 registrational trial of the direct KRASG12C inhibitor adagrasib. In this study of 112 patients with measurable disease at baseline treated with adagrasib, 48 (42.9%) had a confirmed objective response. The median duration of response was 8.5 months (95% CI 6.2-13.8), and the median progression-free survival (PFS) was 6.5 months (95% CI 4.7-8.4). The median overall survival (OS) was 12.6 months (95% CI 9.2-19.2). Among 33 patients with previously treated, stable central nervous system (CNS) metastases, the intracranial confirmed objective response rate was 33.3% (95% CI 18.0-51.8). Treatment-related adverse events occurred in 97.4% of the patients: grade 1 or 2 in 52.6% and grade 3 or higher in 44.8% (including two grade 5 events). The most frequent toxicities were fatigue and gastrointestinal-related issues (nausea, vomiting, diarrhea, aspartate transaminase/alanine transaminase elevation). Adagrasib was discontinued in 6.9% of patients.
These results further demonstrate that the KRASG12C mutation is an actionable target in NSCLC. Sotorasib, another direct KRASG12C inhibitor, is currently US Food and Drug Administration approved after initial systemic treatment. The clinical activity of sotorasib and adagrasib are comparable; for sotorasib the rates are an overall response rate (ORR) of 37.1% (95% CI 28.6-46.2), median PFS of 6.8 months (95% CI 5.1-8.2), and median OS of 12.5 months (95% CI 10.0 to nonestimable). Adagrasib also has published evidence of CNS activity that tracks with its systemic activity. Overall, these direct KRASG12C inhibitors represent a major advance in the treatment of KRASG12C-mutant NSCLC.
EGFR-Mutated NSCLC: Aumolertinib vs Gefitinib Extends PFS
Epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKI) have improved clinical outcomes in EGFR-mutant NSCLC.The current standard of care for first-line treatment of advanced NSCLC with the most frequent EGFR activating mutations (EGFR E19del and L858R)is the third-generation EGFR TKI osimertinib. In the FLAURA trial, patients randomly assigned tofirst-lineosimertinib had a substantial PFS benefit (median PFS 18.9 vs 10.2 months) and OS benefit (median OS 38.6 vs 31.8 months)when receivingosimertinib compared with gefitinib or erlotinib.
In the AENEAS trial, published in the Journal of Clinical Oncologyby Dr Lu and colleagues, 420 patients from China with advanced NSCLC harboring EGFR E19del or L858R activating mutations and naive to systemic treatment were enrolled. Patients were randomly assigned to the next-generation EGFR TKI aumolertinib or the first-generation EGFR TKI gefitinib with the primary endpoint of PFS by investigator assessment.Of note, patients with asymptomatic, untreated brain metastases were allowed into the trial. Upon disease progression, patients in the gefitinib group who acquired an EGFR T790M mutation were eligible to crossover to aumolertinib.
The study met its primary endpoint: Patients treated with aumolertinib compared with those treated with gefitinib had a significantly longer median PFS (19.3 vs 9.9 months; hazard ratio [HR] 0.46; P< .0001). This PFS advantage of aumolertinib over gefitinib was also present in the subgroup of patients with CNS metastases (15.3 vs 8.2 months; HR 0.38; P< .0001). The objective response rate was similar inthe aumolertinib and gefitinib groups (objective response rate 73.8% and 72.1%, respectively). The median duration of response was 18.1 months (95% CI 15.2 to not reached) with aumolertinib vs 8.3 months (95% CI 6.9-11.1) with gefitinib. Treatment-emergent adverse events of grade 3 or more were similar in the aumolertinib and gefitinib groups (36.4% vs 35.8%, respectively). There was less rash and diarrhea as well as transaminitis in the aumolertinib arm compared with the gefitinib arm. However, 35.5% of patients developed an elevation in creatinine phosphokinase (CPK), including 7% with grade 3 CPK elevation. However, no rhabdomyolysis was observed.
Overall, the AENEAS study showed comparable median PFS for first-line aumolertinib comparedwith what was observed with osimertinib in the FLAURA study. We still await the OSdata on aumolertinib compared with gefitinib. In the FLAURA study, investigators could choose between erlotinib or gefitinib in the control arm, whereas in AENEAS only gefitinib was allowed, which may have less CNS activity than erlotinib. Moreover, the FLAURA trial was conducted worldwide, whereas the AENEAS trial only enrolled patients in China. This study provides further support for the use of third-generation EGFR TKI over first-generation EGFR TKI as first-line treatment in advanced/metastatic NSCLC harboring EGFR E19del or L858R mutations.
Advanced ALK+ NSCLC With Brain Metastases: Lorlatinib Boosts PFS, Reduces CNS Progression
The CROWN trial was a pivotal randomized phase 3 trial that demonstrated an impressive improvement in PFS in patients treated with the third-generationALK inhibitor lorlatinib compared with the first-generation ALK inhibitor crizotinib as initial treatment for advanced ALK-postive (ALK+) NSCLC (HR for disease progression or death0.28; 95% CI0.19-0.41; P≤ .001). A major driver of this PFS benefit in ALK+ NSCLC in the CROWN study is the superior CNS penetration of lorlatinib compared with crizotinib. Obtaining CNS control in ALK+ lung cancers is important because up to 40% of patients with ALK+ NSCLC have brain metastases at initial evaluation, and CNS progression is often observed in patients with ALK+ lung cancer whether it be intracranial metastases or leptomeningeal carcinomatosis. A potential challenge in treating patients with lorlatinib is a unique side effect profile including neurocognitive side effects from lorlatinib. In a recently published study in the Journal of Clinical Oncology, Dr Solomon and colleagues conducted a post hoc exploratory analysis of intracranial efficacy and safety of lorlatinib in ALK+ NSCLC from a phase 3 trial. PFS by blinded independent central review was improved with lorlatinib vs crizotinib in patients with and without brain metastases at baseline (12-month PFS rates: 78% vs 22% and 78% vs 45%, respectively). Lorlatinib was associated with lower 12-month cumulative incidence of CNS progression compared with crizotinib in patients with (7.4% vs 72%) and without (1% vs 18%) brain metastases at baseline. Complete CNS responses with lorlatinib were seen in 23/38(61%) patients with any brain metastases at baseline compared with 6/40 (15%) with crizotinib. In total, 35% of patients had CNS adverse events with lorlatinib: grade 1 (21%), grade 2 (10%), and grade 3 (3%)in severity. These included cognitive (21%), mood (16%), speech (5%), and psychotic effects (3%), some of which overlapped. Half of all CNS adverse events resolved without intervention or with lorlatinib dose modification. Dose reductions of lorlatinib did not appear to affect PFS on the basis of a landmark analysis. Overall, this study demonstrates the exceptional CNS activity of lorlatinib in ALK+ NSCLC and that the neurocognitive side effects can often be managed. There are several next-generation ALK inhibitors now approved in the first-line setting —alectinib, lorlatinib, and brigatinib — notably all with enhanced CNS penetration and improved PFS compared with crizotinib. This posthoc study further supports the impressive CNS activity of lorlatinib in ALK+ NSCLC and supports the use of lorlatinib as a first-line treatment option in these patients, particularly those with ALK+ NSCLC diagnosed with baseline CNS disease.
The Direct KRASG12C Inhibitor Adagrasib in Advanced KRASG12C-Mutant NSCLC: Results From a Registrational Phase 2 Study
KRAS mutations are detected in about one quarter of all lung adenocarcinomas and are the most common oncogene driver in non–small-cell lung cancer (NSCLC). KRASG12C amino acid substitutions are the most common KRAS mutations in NSCLC, comprising just about half of all KRAS mutations in this tumor type. Despite being the most common and first detected oncogene driver in lung cancer, until recently there were no targeted therapies in KRAS mutant NSCLC. The development of direct KRASG12C inhibitors represents an important step forward in targeting KRAS mutations. These inhibitors bind inactive guanosine diphosphate (GDP)–bound RAS and trap it in its inactive state.
Dr Jänne and colleagues recently published a phase 2 registrational trial of the direct KRASG12C inhibitor adagrasib. In this study of 112 patients with measurable disease at baseline treated with adagrasib, 48 (42.9%) had a confirmed objective response. The median duration of response was 8.5 months (95% CI 6.2-13.8), and the median progression-free survival (PFS) was 6.5 months (95% CI 4.7-8.4). The median overall survival (OS) was 12.6 months (95% CI 9.2-19.2). Among 33 patients with previously treated, stable central nervous system (CNS) metastases, the intracranial confirmed objective response rate was 33.3% (95% CI 18.0-51.8). Treatment-related adverse events occurred in 97.4% of the patients: grade 1 or 2 in 52.6% and grade 3 or higher in 44.8% (including two grade 5 events). The most frequent toxicities were fatigue and gastrointestinal-related issues (nausea, vomiting, diarrhea, aspartate transaminase/alanine transaminase elevation). Adagrasib was discontinued in 6.9% of patients.
These results further demonstrate that the KRASG12C mutation is an actionable target in NSCLC. Sotorasib, another direct KRASG12C inhibitor, is currently US Food and Drug Administration approved after initial systemic treatment. The clinical activity of sotorasib and adagrasib are comparable; for sotorasib the rates are an overall response rate (ORR) of 37.1% (95% CI 28.6-46.2), median PFS of 6.8 months (95% CI 5.1-8.2), and median OS of 12.5 months (95% CI 10.0 to nonestimable). Adagrasib also has published evidence of CNS activity that tracks with its systemic activity. Overall, these direct KRASG12C inhibitors represent a major advance in the treatment of KRASG12C-mutant NSCLC.
EGFR-Mutated NSCLC: Aumolertinib vs Gefitinib Extends PFS
Epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKI) have improved clinical outcomes in EGFR-mutant NSCLC.The current standard of care for first-line treatment of advanced NSCLC with the most frequent EGFR activating mutations (EGFR E19del and L858R)is the third-generation EGFR TKI osimertinib. In the FLAURA trial, patients randomly assigned tofirst-lineosimertinib had a substantial PFS benefit (median PFS 18.9 vs 10.2 months) and OS benefit (median OS 38.6 vs 31.8 months)when receivingosimertinib compared with gefitinib or erlotinib.
In the AENEAS trial, published in the Journal of Clinical Oncologyby Dr Lu and colleagues, 420 patients from China with advanced NSCLC harboring EGFR E19del or L858R activating mutations and naive to systemic treatment were enrolled. Patients were randomly assigned to the next-generation EGFR TKI aumolertinib or the first-generation EGFR TKI gefitinib with the primary endpoint of PFS by investigator assessment.Of note, patients with asymptomatic, untreated brain metastases were allowed into the trial. Upon disease progression, patients in the gefitinib group who acquired an EGFR T790M mutation were eligible to crossover to aumolertinib.
The study met its primary endpoint: Patients treated with aumolertinib compared with those treated with gefitinib had a significantly longer median PFS (19.3 vs 9.9 months; hazard ratio [HR] 0.46; P< .0001). This PFS advantage of aumolertinib over gefitinib was also present in the subgroup of patients with CNS metastases (15.3 vs 8.2 months; HR 0.38; P< .0001). The objective response rate was similar inthe aumolertinib and gefitinib groups (objective response rate 73.8% and 72.1%, respectively). The median duration of response was 18.1 months (95% CI 15.2 to not reached) with aumolertinib vs 8.3 months (95% CI 6.9-11.1) with gefitinib. Treatment-emergent adverse events of grade 3 or more were similar in the aumolertinib and gefitinib groups (36.4% vs 35.8%, respectively). There was less rash and diarrhea as well as transaminitis in the aumolertinib arm compared with the gefitinib arm. However, 35.5% of patients developed an elevation in creatinine phosphokinase (CPK), including 7% with grade 3 CPK elevation. However, no rhabdomyolysis was observed.
Overall, the AENEAS study showed comparable median PFS for first-line aumolertinib comparedwith what was observed with osimertinib in the FLAURA study. We still await the OSdata on aumolertinib compared with gefitinib. In the FLAURA study, investigators could choose between erlotinib or gefitinib in the control arm, whereas in AENEAS only gefitinib was allowed, which may have less CNS activity than erlotinib. Moreover, the FLAURA trial was conducted worldwide, whereas the AENEAS trial only enrolled patients in China. This study provides further support for the use of third-generation EGFR TKI over first-generation EGFR TKI as first-line treatment in advanced/metastatic NSCLC harboring EGFR E19del or L858R mutations.
Advanced ALK+ NSCLC With Brain Metastases: Lorlatinib Boosts PFS, Reduces CNS Progression
The CROWN trial was a pivotal randomized phase 3 trial that demonstrated an impressive improvement in PFS in patients treated with the third-generationALK inhibitor lorlatinib compared with the first-generation ALK inhibitor crizotinib as initial treatment for advanced ALK-postive (ALK+) NSCLC (HR for disease progression or death0.28; 95% CI0.19-0.41; P≤ .001). A major driver of this PFS benefit in ALK+ NSCLC in the CROWN study is the superior CNS penetration of lorlatinib compared with crizotinib. Obtaining CNS control in ALK+ lung cancers is important because up to 40% of patients with ALK+ NSCLC have brain metastases at initial evaluation, and CNS progression is often observed in patients with ALK+ lung cancer whether it be intracranial metastases or leptomeningeal carcinomatosis. A potential challenge in treating patients with lorlatinib is a unique side effect profile including neurocognitive side effects from lorlatinib. In a recently published study in the Journal of Clinical Oncology, Dr Solomon and colleagues conducted a post hoc exploratory analysis of intracranial efficacy and safety of lorlatinib in ALK+ NSCLC from a phase 3 trial. PFS by blinded independent central review was improved with lorlatinib vs crizotinib in patients with and without brain metastases at baseline (12-month PFS rates: 78% vs 22% and 78% vs 45%, respectively). Lorlatinib was associated with lower 12-month cumulative incidence of CNS progression compared with crizotinib in patients with (7.4% vs 72%) and without (1% vs 18%) brain metastases at baseline. Complete CNS responses with lorlatinib were seen in 23/38(61%) patients with any brain metastases at baseline compared with 6/40 (15%) with crizotinib. In total, 35% of patients had CNS adverse events with lorlatinib: grade 1 (21%), grade 2 (10%), and grade 3 (3%)in severity. These included cognitive (21%), mood (16%), speech (5%), and psychotic effects (3%), some of which overlapped. Half of all CNS adverse events resolved without intervention or with lorlatinib dose modification. Dose reductions of lorlatinib did not appear to affect PFS on the basis of a landmark analysis. Overall, this study demonstrates the exceptional CNS activity of lorlatinib in ALK+ NSCLC and that the neurocognitive side effects can often be managed. There are several next-generation ALK inhibitors now approved in the first-line setting —alectinib, lorlatinib, and brigatinib — notably all with enhanced CNS penetration and improved PFS compared with crizotinib. This posthoc study further supports the impressive CNS activity of lorlatinib in ALK+ NSCLC and supports the use of lorlatinib as a first-line treatment option in these patients, particularly those with ALK+ NSCLC diagnosed with baseline CNS disease.
Commentary: Examining Lower Doses and Effectiveness in RA Treatments, July 2022
In keeping with other studies suggesting that lower-than-standard doses of rituximab (for example, two 500 mg doses every 6 months) are effective in the treatment of rheumatoid arthritis (RA), Bertsias and colleagues evaluated the efficacy of low-dose rituximab (1 g every 6 months) in a cohort of patients with RA. Of 361 patients in the initial registry, 81 achieved sustained low disease activity or remission on standard rituximab regimens and were transitioned to the low-dose regimen; their outcomes were compared with the 280 patients in the registry who received a standard-dose regimen. Only 7.5% experienced flares of RA (compared with 5.9% in the standard-dose group), and patients in the low-dose group had fewer serious adverse events, infections, and hospitalizations. Tapering biologic disease-modifying antirheumatic drugs (DMARD) may thus be a reasonable strategy for some patients with RA, with an eye to their prior results with the medication.
The prospect of achieving sustained DMARD-free remission (SDFR) in people with RA has seemed increasingly feasible in recent studies, although it seems to be affected by anti-citrullinated protein/peptide antibody (ACPA) positivity. That is, people who are ACPA-negative are more likely to reach this target. Verstappen and colleagues examined MRI patterns of joint inflammation in the Leiden Early Arthritis Clinic cohort, as well as another cohort of patients with RA, but only included patients with an RA duration > 1 year to reduce the likelihood of misclassification. Interestingly, ACPA-positive patients who achieved SDFR tended to have lower baseline evidence of joint inflammation (including erosions, synovitis, and osteitis), which was persistent throughout follow-up, whereas ACPA-negative patients who achieved SDFR had similar joint inflammation at baseline to those who did not, but had a better initial response to DMARD therapy. Further study of the potential impact of treatment based on ACPA status would be helpful.
Recent results from the ORAL Surveillance postmarketing safety analysis suggest that tofacitinib is associated with an increased risk for cancer in patients with RA patients who are aged >50 years, compared with anti–tumor necrosis factor (TNF) agents. Khosrow-Khavar and colleagues used insurance claims data to further investigate the risk for cancer with tofacitinib vs anti-TNF agents. They created two cohorts of patients who were new users of anti-TNF agents or tofacitinib: One was a "real-world" cohort including all patients with RA, whereas the other applied the inclusion and exclusion criteria from the ORAL Surveillance trial, including age >50 years, one cardiovascular risk factor, and use of methotrexate. Results from the real-world cohort, including over 10,000 patients who had used tofacitinib, did not show any increase in cancer risk, whereas the trial-simulating conditions did yield higher numbers of cancers in the group exposed to tofacitinib. Though reassuring, these results are limited by the short follow-up duration, and longer-term follow-up studies are necessary for further evaluation.
In another interesting note with respect to medication morbidity and risk, Xu and colleagues performed a meta-analysis looking at the association of methotrexate use and mortality in patients with RA. Only 15 studies were included in the final analysis, though they did encompass a large total number of patients. Overall, methotrexate use in these studies was associated with lower rates of overall mortality as well as mortality due to cardiovascular and interstitial lung disease. However, conclusions as to the direct impact of methotrexate were limited by the heterogeneity of study designs, doses of methotrexate, and additional therapy as well as comparators.
In keeping with other studies suggesting that lower-than-standard doses of rituximab (for example, two 500 mg doses every 6 months) are effective in the treatment of rheumatoid arthritis (RA), Bertsias and colleagues evaluated the efficacy of low-dose rituximab (1 g every 6 months) in a cohort of patients with RA. Of 361 patients in the initial registry, 81 achieved sustained low disease activity or remission on standard rituximab regimens and were transitioned to the low-dose regimen; their outcomes were compared with the 280 patients in the registry who received a standard-dose regimen. Only 7.5% experienced flares of RA (compared with 5.9% in the standard-dose group), and patients in the low-dose group had fewer serious adverse events, infections, and hospitalizations. Tapering biologic disease-modifying antirheumatic drugs (DMARD) may thus be a reasonable strategy for some patients with RA, with an eye to their prior results with the medication.
The prospect of achieving sustained DMARD-free remission (SDFR) in people with RA has seemed increasingly feasible in recent studies, although it seems to be affected by anti-citrullinated protein/peptide antibody (ACPA) positivity. That is, people who are ACPA-negative are more likely to reach this target. Verstappen and colleagues examined MRI patterns of joint inflammation in the Leiden Early Arthritis Clinic cohort, as well as another cohort of patients with RA, but only included patients with an RA duration > 1 year to reduce the likelihood of misclassification. Interestingly, ACPA-positive patients who achieved SDFR tended to have lower baseline evidence of joint inflammation (including erosions, synovitis, and osteitis), which was persistent throughout follow-up, whereas ACPA-negative patients who achieved SDFR had similar joint inflammation at baseline to those who did not, but had a better initial response to DMARD therapy. Further study of the potential impact of treatment based on ACPA status would be helpful.
Recent results from the ORAL Surveillance postmarketing safety analysis suggest that tofacitinib is associated with an increased risk for cancer in patients with RA patients who are aged >50 years, compared with anti–tumor necrosis factor (TNF) agents. Khosrow-Khavar and colleagues used insurance claims data to further investigate the risk for cancer with tofacitinib vs anti-TNF agents. They created two cohorts of patients who were new users of anti-TNF agents or tofacitinib: One was a "real-world" cohort including all patients with RA, whereas the other applied the inclusion and exclusion criteria from the ORAL Surveillance trial, including age >50 years, one cardiovascular risk factor, and use of methotrexate. Results from the real-world cohort, including over 10,000 patients who had used tofacitinib, did not show any increase in cancer risk, whereas the trial-simulating conditions did yield higher numbers of cancers in the group exposed to tofacitinib. Though reassuring, these results are limited by the short follow-up duration, and longer-term follow-up studies are necessary for further evaluation.
In another interesting note with respect to medication morbidity and risk, Xu and colleagues performed a meta-analysis looking at the association of methotrexate use and mortality in patients with RA. Only 15 studies were included in the final analysis, though they did encompass a large total number of patients. Overall, methotrexate use in these studies was associated with lower rates of overall mortality as well as mortality due to cardiovascular and interstitial lung disease. However, conclusions as to the direct impact of methotrexate were limited by the heterogeneity of study designs, doses of methotrexate, and additional therapy as well as comparators.
In keeping with other studies suggesting that lower-than-standard doses of rituximab (for example, two 500 mg doses every 6 months) are effective in the treatment of rheumatoid arthritis (RA), Bertsias and colleagues evaluated the efficacy of low-dose rituximab (1 g every 6 months) in a cohort of patients with RA. Of 361 patients in the initial registry, 81 achieved sustained low disease activity or remission on standard rituximab regimens and were transitioned to the low-dose regimen; their outcomes were compared with the 280 patients in the registry who received a standard-dose regimen. Only 7.5% experienced flares of RA (compared with 5.9% in the standard-dose group), and patients in the low-dose group had fewer serious adverse events, infections, and hospitalizations. Tapering biologic disease-modifying antirheumatic drugs (DMARD) may thus be a reasonable strategy for some patients with RA, with an eye to their prior results with the medication.
The prospect of achieving sustained DMARD-free remission (SDFR) in people with RA has seemed increasingly feasible in recent studies, although it seems to be affected by anti-citrullinated protein/peptide antibody (ACPA) positivity. That is, people who are ACPA-negative are more likely to reach this target. Verstappen and colleagues examined MRI patterns of joint inflammation in the Leiden Early Arthritis Clinic cohort, as well as another cohort of patients with RA, but only included patients with an RA duration > 1 year to reduce the likelihood of misclassification. Interestingly, ACPA-positive patients who achieved SDFR tended to have lower baseline evidence of joint inflammation (including erosions, synovitis, and osteitis), which was persistent throughout follow-up, whereas ACPA-negative patients who achieved SDFR had similar joint inflammation at baseline to those who did not, but had a better initial response to DMARD therapy. Further study of the potential impact of treatment based on ACPA status would be helpful.
Recent results from the ORAL Surveillance postmarketing safety analysis suggest that tofacitinib is associated with an increased risk for cancer in patients with RA patients who are aged >50 years, compared with anti–tumor necrosis factor (TNF) agents. Khosrow-Khavar and colleagues used insurance claims data to further investigate the risk for cancer with tofacitinib vs anti-TNF agents. They created two cohorts of patients who were new users of anti-TNF agents or tofacitinib: One was a "real-world" cohort including all patients with RA, whereas the other applied the inclusion and exclusion criteria from the ORAL Surveillance trial, including age >50 years, one cardiovascular risk factor, and use of methotrexate. Results from the real-world cohort, including over 10,000 patients who had used tofacitinib, did not show any increase in cancer risk, whereas the trial-simulating conditions did yield higher numbers of cancers in the group exposed to tofacitinib. Though reassuring, these results are limited by the short follow-up duration, and longer-term follow-up studies are necessary for further evaluation.
In another interesting note with respect to medication morbidity and risk, Xu and colleagues performed a meta-analysis looking at the association of methotrexate use and mortality in patients with RA. Only 15 studies were included in the final analysis, though they did encompass a large total number of patients. Overall, methotrexate use in these studies was associated with lower rates of overall mortality as well as mortality due to cardiovascular and interstitial lung disease. However, conclusions as to the direct impact of methotrexate were limited by the heterogeneity of study designs, doses of methotrexate, and additional therapy as well as comparators.