User login
Depression biomarkers: Which ones matter most?
Multiple biomarkers of depression involved in several brain circuits are altered in patients with unipolar depression.
because they suggest neuroimmunological alterations, disturbances in the blood-brain-barrier, hyperactivity in the hypothalamic-pituitary-adrenal (HPA) axis, and impaired neuroplasticity as factors in depression pathophysiology.
However, said study investigator Michael E. Benros, MD, PhD, professor and head of research at Mental Health Centre Copenhagen and University of Copenhagen, this is on a group level. “So in order to be relevant in a clinical context, the results need to be validated by further high-quality studies identifying subgroups with different biological underpinnings,” he told this news organization.
Identification of potential subgroups of depression with different biomarkers might help explain the diverse symptomatology and variability in treatment response observed in patients with depression, he noted.
The study was published online in JAMA Psychiatry.
Multiple pathways to depression
The systematic review and meta-analysis included 97 studies investigating 165 CSF biomarkers.
Of the 42 biomarkers investigated in at least two studies, patients with unipolar depression had higher CSF levels of interleukin 6, a marker of chronic inflammation; total protein, which signals blood-brain barrier dysfunction and increased permeability; and cortisol, which is linked to psychological stress, compared with healthy controls.
Depression was also associated with:
- Lower CSF levels of homovanillic acid, the major terminal metabolite of dopamine.
- Gamma-aminobutyric acid (GABA), the major inhibitory neurotransmitter in the CNS thought to play a vital role in the control of stress and depression.
- Somatostatin, a neuropeptide often coexpressed with GABA.
- Brain-derived neurotrophic factor (BDNF), a protein involved in neurogenesis, synaptic plasticity, and neurotransmission.
- Amyloid-β 40, implicated in Alzheimer’s disease.
- Transthyretin, involved in transport of thyroxine across the blood-brain barrier.
Collectively, the findings point toward a “dysregulated dopaminergic system, a compromised inhibitory system, HPA axis hyperactivity, increased neuroinflammation and blood-brain barrier permeability, and impaired neuroplasticity as important factors in depression pathophysiology,” the investigators wrote.
“It is notable that we did not find significant difference in the metabolite levels of serotonin and noradrenalin, which are the most targeted neurotransmitters in modern antidepressant treatment,” said Dr. Benros.
However, this could be explained by substantial heterogeneity between studies and the fact that quantification of total CSF biomarker concentrations does not reflect local alteration within the brain, he explained.
Many of the studies had small cohorts and most quantified only a few biomarkers, making it hard to examine potential interactions between biomarkers or identify specific phenotypes of depression.
“Novel high-quality studies including larger cohorts with an integrative approach and extensive numbers of biomarkers are needed to validate these potential biomarkers of depression and set the stage for the development of more effective and precise treatments,” the researchers noted.
Which ones hold water?
Reached for comment, Dean MacKinnon, MD, associate professor of psychiatry and behavioral sciences at Johns Hopkins University, Baltimore, noted that this analysis “extracts the vast amount of knowledge” gained from different studies on biomarkers in the CSF for depression.
“They were able to identify 97 papers that have enough information in them that they could sort of lump them together and see which ones still hold water. It’s always useful to be able to look at patterns in the research and see if you can find some consistent trends,” he told this news organization.
Dr. MacKinnon, who was not part of the research team, also noted that “nonreplicability” is a problem in psychiatry and psychology research, “so being able to show that at least some studies were sufficiently well done, to get a good result, and that they could be replicated in at least one other good study is useful information.”
When it comes to depression, Dr. MacKinnon said, “We just don’t know enough to really pin down a physiologic pathway to explain it. The fact that some people seem to have high cortisol and some people seem to have high permeability of blood-brain barrier, and others have abnormalities in dopamine, is interesting and suggests that depression is likely not a unitary disease with a single cause.”
He cautioned, however, that the findings don’t have immediate clinical implications for individual patients with depression.
“Theoretically, down the road, if you extrapolate from what they found, and if it’s truly the case that this research maps to something that could suggest a different clinical approach, you might be able to determine whether one patient might respond better to an SSRI or an SNRI or something like that,” Dr. MacKinnon said.
Dr. Benros reported grants from Lundbeck Foundation during the conduct of the study. Dr. MacKinnon has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Multiple biomarkers of depression involved in several brain circuits are altered in patients with unipolar depression.
because they suggest neuroimmunological alterations, disturbances in the blood-brain-barrier, hyperactivity in the hypothalamic-pituitary-adrenal (HPA) axis, and impaired neuroplasticity as factors in depression pathophysiology.
However, said study investigator Michael E. Benros, MD, PhD, professor and head of research at Mental Health Centre Copenhagen and University of Copenhagen, this is on a group level. “So in order to be relevant in a clinical context, the results need to be validated by further high-quality studies identifying subgroups with different biological underpinnings,” he told this news organization.
Identification of potential subgroups of depression with different biomarkers might help explain the diverse symptomatology and variability in treatment response observed in patients with depression, he noted.
The study was published online in JAMA Psychiatry.
Multiple pathways to depression
The systematic review and meta-analysis included 97 studies investigating 165 CSF biomarkers.
Of the 42 biomarkers investigated in at least two studies, patients with unipolar depression had higher CSF levels of interleukin 6, a marker of chronic inflammation; total protein, which signals blood-brain barrier dysfunction and increased permeability; and cortisol, which is linked to psychological stress, compared with healthy controls.
Depression was also associated with:
- Lower CSF levels of homovanillic acid, the major terminal metabolite of dopamine.
- Gamma-aminobutyric acid (GABA), the major inhibitory neurotransmitter in the CNS thought to play a vital role in the control of stress and depression.
- Somatostatin, a neuropeptide often coexpressed with GABA.
- Brain-derived neurotrophic factor (BDNF), a protein involved in neurogenesis, synaptic plasticity, and neurotransmission.
- Amyloid-β 40, implicated in Alzheimer’s disease.
- Transthyretin, involved in transport of thyroxine across the blood-brain barrier.
Collectively, the findings point toward a “dysregulated dopaminergic system, a compromised inhibitory system, HPA axis hyperactivity, increased neuroinflammation and blood-brain barrier permeability, and impaired neuroplasticity as important factors in depression pathophysiology,” the investigators wrote.
“It is notable that we did not find significant difference in the metabolite levels of serotonin and noradrenalin, which are the most targeted neurotransmitters in modern antidepressant treatment,” said Dr. Benros.
However, this could be explained by substantial heterogeneity between studies and the fact that quantification of total CSF biomarker concentrations does not reflect local alteration within the brain, he explained.
Many of the studies had small cohorts and most quantified only a few biomarkers, making it hard to examine potential interactions between biomarkers or identify specific phenotypes of depression.
“Novel high-quality studies including larger cohorts with an integrative approach and extensive numbers of biomarkers are needed to validate these potential biomarkers of depression and set the stage for the development of more effective and precise treatments,” the researchers noted.
Which ones hold water?
Reached for comment, Dean MacKinnon, MD, associate professor of psychiatry and behavioral sciences at Johns Hopkins University, Baltimore, noted that this analysis “extracts the vast amount of knowledge” gained from different studies on biomarkers in the CSF for depression.
“They were able to identify 97 papers that have enough information in them that they could sort of lump them together and see which ones still hold water. It’s always useful to be able to look at patterns in the research and see if you can find some consistent trends,” he told this news organization.
Dr. MacKinnon, who was not part of the research team, also noted that “nonreplicability” is a problem in psychiatry and psychology research, “so being able to show that at least some studies were sufficiently well done, to get a good result, and that they could be replicated in at least one other good study is useful information.”
When it comes to depression, Dr. MacKinnon said, “We just don’t know enough to really pin down a physiologic pathway to explain it. The fact that some people seem to have high cortisol and some people seem to have high permeability of blood-brain barrier, and others have abnormalities in dopamine, is interesting and suggests that depression is likely not a unitary disease with a single cause.”
He cautioned, however, that the findings don’t have immediate clinical implications for individual patients with depression.
“Theoretically, down the road, if you extrapolate from what they found, and if it’s truly the case that this research maps to something that could suggest a different clinical approach, you might be able to determine whether one patient might respond better to an SSRI or an SNRI or something like that,” Dr. MacKinnon said.
Dr. Benros reported grants from Lundbeck Foundation during the conduct of the study. Dr. MacKinnon has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Multiple biomarkers of depression involved in several brain circuits are altered in patients with unipolar depression.
because they suggest neuroimmunological alterations, disturbances in the blood-brain-barrier, hyperactivity in the hypothalamic-pituitary-adrenal (HPA) axis, and impaired neuroplasticity as factors in depression pathophysiology.
However, said study investigator Michael E. Benros, MD, PhD, professor and head of research at Mental Health Centre Copenhagen and University of Copenhagen, this is on a group level. “So in order to be relevant in a clinical context, the results need to be validated by further high-quality studies identifying subgroups with different biological underpinnings,” he told this news organization.
Identification of potential subgroups of depression with different biomarkers might help explain the diverse symptomatology and variability in treatment response observed in patients with depression, he noted.
The study was published online in JAMA Psychiatry.
Multiple pathways to depression
The systematic review and meta-analysis included 97 studies investigating 165 CSF biomarkers.
Of the 42 biomarkers investigated in at least two studies, patients with unipolar depression had higher CSF levels of interleukin 6, a marker of chronic inflammation; total protein, which signals blood-brain barrier dysfunction and increased permeability; and cortisol, which is linked to psychological stress, compared with healthy controls.
Depression was also associated with:
- Lower CSF levels of homovanillic acid, the major terminal metabolite of dopamine.
- Gamma-aminobutyric acid (GABA), the major inhibitory neurotransmitter in the CNS thought to play a vital role in the control of stress and depression.
- Somatostatin, a neuropeptide often coexpressed with GABA.
- Brain-derived neurotrophic factor (BDNF), a protein involved in neurogenesis, synaptic plasticity, and neurotransmission.
- Amyloid-β 40, implicated in Alzheimer’s disease.
- Transthyretin, involved in transport of thyroxine across the blood-brain barrier.
Collectively, the findings point toward a “dysregulated dopaminergic system, a compromised inhibitory system, HPA axis hyperactivity, increased neuroinflammation and blood-brain barrier permeability, and impaired neuroplasticity as important factors in depression pathophysiology,” the investigators wrote.
“It is notable that we did not find significant difference in the metabolite levels of serotonin and noradrenalin, which are the most targeted neurotransmitters in modern antidepressant treatment,” said Dr. Benros.
However, this could be explained by substantial heterogeneity between studies and the fact that quantification of total CSF biomarker concentrations does not reflect local alteration within the brain, he explained.
Many of the studies had small cohorts and most quantified only a few biomarkers, making it hard to examine potential interactions between biomarkers or identify specific phenotypes of depression.
“Novel high-quality studies including larger cohorts with an integrative approach and extensive numbers of biomarkers are needed to validate these potential biomarkers of depression and set the stage for the development of more effective and precise treatments,” the researchers noted.
Which ones hold water?
Reached for comment, Dean MacKinnon, MD, associate professor of psychiatry and behavioral sciences at Johns Hopkins University, Baltimore, noted that this analysis “extracts the vast amount of knowledge” gained from different studies on biomarkers in the CSF for depression.
“They were able to identify 97 papers that have enough information in them that they could sort of lump them together and see which ones still hold water. It’s always useful to be able to look at patterns in the research and see if you can find some consistent trends,” he told this news organization.
Dr. MacKinnon, who was not part of the research team, also noted that “nonreplicability” is a problem in psychiatry and psychology research, “so being able to show that at least some studies were sufficiently well done, to get a good result, and that they could be replicated in at least one other good study is useful information.”
When it comes to depression, Dr. MacKinnon said, “We just don’t know enough to really pin down a physiologic pathway to explain it. The fact that some people seem to have high cortisol and some people seem to have high permeability of blood-brain barrier, and others have abnormalities in dopamine, is interesting and suggests that depression is likely not a unitary disease with a single cause.”
He cautioned, however, that the findings don’t have immediate clinical implications for individual patients with depression.
“Theoretically, down the road, if you extrapolate from what they found, and if it’s truly the case that this research maps to something that could suggest a different clinical approach, you might be able to determine whether one patient might respond better to an SSRI or an SNRI or something like that,” Dr. MacKinnon said.
Dr. Benros reported grants from Lundbeck Foundation during the conduct of the study. Dr. MacKinnon has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA PSYCHIATRY
Burnout ‘highly prevalent’ in psychiatrists across the globe
Burnout in psychiatrists is “highly prevalent” across the globe, new research shows.
In a review and meta-analysis of 36 studies and more than 5,000 psychiatrists in European countries, as well as the United States, Australia, New Zealand, India, Turkey, and Thailand,
“Our review showed that regardless of the identification method of burnout, its prevalence among psychiatrists is high and ranges from 25% to 50%,” lead author Kirill Bykov, MD, a PhD candidate at the Peoples’ Friendship University of Russia (RUDN University), Moscow, Russian Federation, told this news organization.
There was a “high heterogeneity of studies in terms of statistics, screening methods, burnout definitions, and cutoff points in the included studies, which necessitates the unification of future research methodology, but not to the detriment of the development of the theoretical background,” Dr. Bykov said.
The findings were published online in the Journal of Affective Disorders.
‘Unresolved problem’
Although burnout is a serious and prevalent problem among health care workers, little research has focused on burnout in mental health workers compared with other professionals, the investigators noted.
A previous systematic review and meta-analysis that focused specifically on burnout in psychiatrists was limited by methodologic concerns, including that the only burnout screening instrument used in the included studies was the full-length (22-item) MBI.
The current researchers surmised that “the integration of different empirical studies of psychiatrists’ burnout prevalence [remained] an unresolved problem.”
Dr. Bykov noted the current review was “investigator-initiated” and was a part of his PhD dissertation.
“Studying the works devoted to the burnout of psychiatrists, I drew attention to the varying prevalence rates of this phenomenon among them. This prompted me to conduct a systematic review of the literature and summarize the available data,” he said.
Unlike the previous review, the current one “does not contain restrictions regarding the place of research, publication language, covered burnout concepts, definitions, and screening instruments. Thus, its results will be helpful for practitioners and scientists around the world,” Dr. Bykov added.
Among the inclusion criteria was that a study should be empirical and quantitative, contain at least 20 practicing psychiatrists as participants, use a valid and reliable burnout screening instrument, have at least one burnout metric extractable specifically with regard to psychiatrists, and have a national survey or a response rate among psychiatrists of 20% or greater.
Qualitative or review articles or studies consisting of psychiatric trainees (such as medical students or residents) or nonpracticing psychiatrists were excluded.
Pooled prevalence
The researchers included 36 studies that comprised 5,481 participants (51.3% were women; mean age, 46.7 years). All studies had from 20 to 1,157 participants. They were employed in an array of settings in 19 countries.
In 22 studies, survey years ranged from 1996 to 2018; 14 studies did not report the year of data collection.
Most studies (75%) used some version of the MBI, and 19 studies used the full-length 22-item MBI Human Service Survey (MBI-HSS) . The survey rates emotional exhaustion (EE), depersonalization (DP), and low personal achievement (PA) on a 7-point Likert scale from 0 (“never”) to 6 (“almost every day”).
Other instruments included the CBI, the 16-item Oldenburg Burnout Inventory, the 21-item Tedium Measure, the 30-item Professional Quality of Life measure, the Rohland et al. Single-Item Measure of Self-Perceived Burnout, and the 21-item Brief Burnout Questionnaire.
Only three studies were free of methodologic limitations. The remaining 33 studies had some problems, such as not reporting the response rate or comparability between responders and nonresponders.
Results showed that the overall prevalence of burnout, as measured by the MBI and the CBI, was 25.9% (range, 11.1%-40.75%) and 50.3% (range, 30.9%-69.8%), respectively.
The pooled prevalence for burnout components is shown in the table.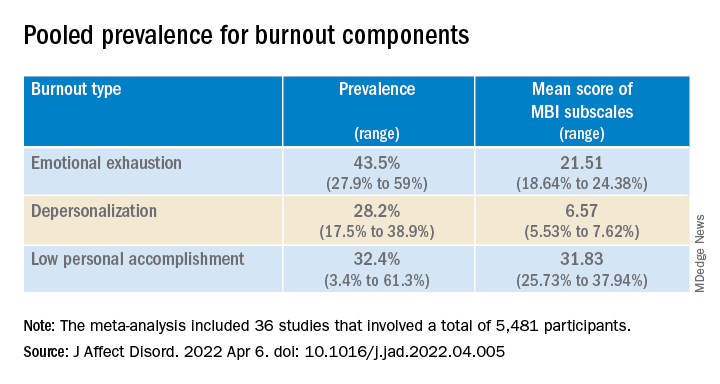
European psychiatrists had lower EE scores (20.82; 95% confidence interval, 7.24-4.41) compared with their non-European counterparts (24.99; 95% CI, 23.05-26.94; P = .045).
‘Carry the hope’
In a comment, Christine Crawford, MD, associate medical director of the National Alliance on Mental Illness (NAMI), said she was surprised the burnout numbers weren’t higher.
Many colleagues she interacts with “have been experiencing pretty significant burnout that has only been exacerbated by the pandemic and ever-growing demand for mental health providers, and there aren’t enough to meet that demand,” said Dr. Crawford, a psychiatrist at Boston Medical Center’s Outpatient Child and Adolescent Psychiatry Clinic and at Codman Square Health Center. She was not involved with the current research.
Dr. Crawford noted that much of the data was from Europeans. Speaking to the experience of U.S.-based psychiatrists, she said there is a “greater appreciation for what we do as mental health providers, due to the growing conversations around mental health and normalizing mental health conditions.”
On the other hand, there is “a lack of parity in reimbursement rates. Although the general public values mental health, the medical system doesn’t value mental health providers in the same way as physicians in other specialties,” Dr. Crawford said. Feeling devalued can contribute to burnout, she added.
One way to counter burnout is to remember “that our role is to carry the hope. We can be hopeful for the patient that the treatment will work or the medications can provide some relief,” Dr. Crawford noted.
Psychiatrists “may need to hold on tightly to that hope because we may not receive that instant gratification from the patient or receive praise or see the change from the patient during that time, which can be challenging,” she said.
“But it’s important for us to keep in mind that, even in that moment when the patient can’t see it, we can work alongside the patient to create the vision of hope and what it will look like in the future,” said Dr. Crawford.
In the 2022 Medscape Psychiatrist Lifestyle, Happiness & Burnout Report, an annual online survey of Medscape member physicians, 47% of respondents reported burnout – which was up from 42% the previous year.
The investigators received no funding for this work. They and Dr. Crawford report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Burnout in psychiatrists is “highly prevalent” across the globe, new research shows.
In a review and meta-analysis of 36 studies and more than 5,000 psychiatrists in European countries, as well as the United States, Australia, New Zealand, India, Turkey, and Thailand,
“Our review showed that regardless of the identification method of burnout, its prevalence among psychiatrists is high and ranges from 25% to 50%,” lead author Kirill Bykov, MD, a PhD candidate at the Peoples’ Friendship University of Russia (RUDN University), Moscow, Russian Federation, told this news organization.
There was a “high heterogeneity of studies in terms of statistics, screening methods, burnout definitions, and cutoff points in the included studies, which necessitates the unification of future research methodology, but not to the detriment of the development of the theoretical background,” Dr. Bykov said.
The findings were published online in the Journal of Affective Disorders.
‘Unresolved problem’
Although burnout is a serious and prevalent problem among health care workers, little research has focused on burnout in mental health workers compared with other professionals, the investigators noted.
A previous systematic review and meta-analysis that focused specifically on burnout in psychiatrists was limited by methodologic concerns, including that the only burnout screening instrument used in the included studies was the full-length (22-item) MBI.
The current researchers surmised that “the integration of different empirical studies of psychiatrists’ burnout prevalence [remained] an unresolved problem.”
Dr. Bykov noted the current review was “investigator-initiated” and was a part of his PhD dissertation.
“Studying the works devoted to the burnout of psychiatrists, I drew attention to the varying prevalence rates of this phenomenon among them. This prompted me to conduct a systematic review of the literature and summarize the available data,” he said.
Unlike the previous review, the current one “does not contain restrictions regarding the place of research, publication language, covered burnout concepts, definitions, and screening instruments. Thus, its results will be helpful for practitioners and scientists around the world,” Dr. Bykov added.
Among the inclusion criteria was that a study should be empirical and quantitative, contain at least 20 practicing psychiatrists as participants, use a valid and reliable burnout screening instrument, have at least one burnout metric extractable specifically with regard to psychiatrists, and have a national survey or a response rate among psychiatrists of 20% or greater.
Qualitative or review articles or studies consisting of psychiatric trainees (such as medical students or residents) or nonpracticing psychiatrists were excluded.
Pooled prevalence
The researchers included 36 studies that comprised 5,481 participants (51.3% were women; mean age, 46.7 years). All studies had from 20 to 1,157 participants. They were employed in an array of settings in 19 countries.
In 22 studies, survey years ranged from 1996 to 2018; 14 studies did not report the year of data collection.
Most studies (75%) used some version of the MBI, and 19 studies used the full-length 22-item MBI Human Service Survey (MBI-HSS) . The survey rates emotional exhaustion (EE), depersonalization (DP), and low personal achievement (PA) on a 7-point Likert scale from 0 (“never”) to 6 (“almost every day”).
Other instruments included the CBI, the 16-item Oldenburg Burnout Inventory, the 21-item Tedium Measure, the 30-item Professional Quality of Life measure, the Rohland et al. Single-Item Measure of Self-Perceived Burnout, and the 21-item Brief Burnout Questionnaire.
Only three studies were free of methodologic limitations. The remaining 33 studies had some problems, such as not reporting the response rate or comparability between responders and nonresponders.
Results showed that the overall prevalence of burnout, as measured by the MBI and the CBI, was 25.9% (range, 11.1%-40.75%) and 50.3% (range, 30.9%-69.8%), respectively.
The pooled prevalence for burnout components is shown in the table.
European psychiatrists had lower EE scores (20.82; 95% confidence interval, 7.24-4.41) compared with their non-European counterparts (24.99; 95% CI, 23.05-26.94; P = .045).
‘Carry the hope’
In a comment, Christine Crawford, MD, associate medical director of the National Alliance on Mental Illness (NAMI), said she was surprised the burnout numbers weren’t higher.
Many colleagues she interacts with “have been experiencing pretty significant burnout that has only been exacerbated by the pandemic and ever-growing demand for mental health providers, and there aren’t enough to meet that demand,” said Dr. Crawford, a psychiatrist at Boston Medical Center’s Outpatient Child and Adolescent Psychiatry Clinic and at Codman Square Health Center. She was not involved with the current research.
Dr. Crawford noted that much of the data was from Europeans. Speaking to the experience of U.S.-based psychiatrists, she said there is a “greater appreciation for what we do as mental health providers, due to the growing conversations around mental health and normalizing mental health conditions.”
On the other hand, there is “a lack of parity in reimbursement rates. Although the general public values mental health, the medical system doesn’t value mental health providers in the same way as physicians in other specialties,” Dr. Crawford said. Feeling devalued can contribute to burnout, she added.
One way to counter burnout is to remember “that our role is to carry the hope. We can be hopeful for the patient that the treatment will work or the medications can provide some relief,” Dr. Crawford noted.
Psychiatrists “may need to hold on tightly to that hope because we may not receive that instant gratification from the patient or receive praise or see the change from the patient during that time, which can be challenging,” she said.
“But it’s important for us to keep in mind that, even in that moment when the patient can’t see it, we can work alongside the patient to create the vision of hope and what it will look like in the future,” said Dr. Crawford.
In the 2022 Medscape Psychiatrist Lifestyle, Happiness & Burnout Report, an annual online survey of Medscape member physicians, 47% of respondents reported burnout – which was up from 42% the previous year.
The investigators received no funding for this work. They and Dr. Crawford report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Burnout in psychiatrists is “highly prevalent” across the globe, new research shows.
In a review and meta-analysis of 36 studies and more than 5,000 psychiatrists in European countries, as well as the United States, Australia, New Zealand, India, Turkey, and Thailand,
“Our review showed that regardless of the identification method of burnout, its prevalence among psychiatrists is high and ranges from 25% to 50%,” lead author Kirill Bykov, MD, a PhD candidate at the Peoples’ Friendship University of Russia (RUDN University), Moscow, Russian Federation, told this news organization.
There was a “high heterogeneity of studies in terms of statistics, screening methods, burnout definitions, and cutoff points in the included studies, which necessitates the unification of future research methodology, but not to the detriment of the development of the theoretical background,” Dr. Bykov said.
The findings were published online in the Journal of Affective Disorders.
‘Unresolved problem’
Although burnout is a serious and prevalent problem among health care workers, little research has focused on burnout in mental health workers compared with other professionals, the investigators noted.
A previous systematic review and meta-analysis that focused specifically on burnout in psychiatrists was limited by methodologic concerns, including that the only burnout screening instrument used in the included studies was the full-length (22-item) MBI.
The current researchers surmised that “the integration of different empirical studies of psychiatrists’ burnout prevalence [remained] an unresolved problem.”
Dr. Bykov noted the current review was “investigator-initiated” and was a part of his PhD dissertation.
“Studying the works devoted to the burnout of psychiatrists, I drew attention to the varying prevalence rates of this phenomenon among them. This prompted me to conduct a systematic review of the literature and summarize the available data,” he said.
Unlike the previous review, the current one “does not contain restrictions regarding the place of research, publication language, covered burnout concepts, definitions, and screening instruments. Thus, its results will be helpful for practitioners and scientists around the world,” Dr. Bykov added.
Among the inclusion criteria was that a study should be empirical and quantitative, contain at least 20 practicing psychiatrists as participants, use a valid and reliable burnout screening instrument, have at least one burnout metric extractable specifically with regard to psychiatrists, and have a national survey or a response rate among psychiatrists of 20% or greater.
Qualitative or review articles or studies consisting of psychiatric trainees (such as medical students or residents) or nonpracticing psychiatrists were excluded.
Pooled prevalence
The researchers included 36 studies that comprised 5,481 participants (51.3% were women; mean age, 46.7 years). All studies had from 20 to 1,157 participants. They were employed in an array of settings in 19 countries.
In 22 studies, survey years ranged from 1996 to 2018; 14 studies did not report the year of data collection.
Most studies (75%) used some version of the MBI, and 19 studies used the full-length 22-item MBI Human Service Survey (MBI-HSS) . The survey rates emotional exhaustion (EE), depersonalization (DP), and low personal achievement (PA) on a 7-point Likert scale from 0 (“never”) to 6 (“almost every day”).
Other instruments included the CBI, the 16-item Oldenburg Burnout Inventory, the 21-item Tedium Measure, the 30-item Professional Quality of Life measure, the Rohland et al. Single-Item Measure of Self-Perceived Burnout, and the 21-item Brief Burnout Questionnaire.
Only three studies were free of methodologic limitations. The remaining 33 studies had some problems, such as not reporting the response rate or comparability between responders and nonresponders.
Results showed that the overall prevalence of burnout, as measured by the MBI and the CBI, was 25.9% (range, 11.1%-40.75%) and 50.3% (range, 30.9%-69.8%), respectively.
The pooled prevalence for burnout components is shown in the table.
European psychiatrists had lower EE scores (20.82; 95% confidence interval, 7.24-4.41) compared with their non-European counterparts (24.99; 95% CI, 23.05-26.94; P = .045).
‘Carry the hope’
In a comment, Christine Crawford, MD, associate medical director of the National Alliance on Mental Illness (NAMI), said she was surprised the burnout numbers weren’t higher.
Many colleagues she interacts with “have been experiencing pretty significant burnout that has only been exacerbated by the pandemic and ever-growing demand for mental health providers, and there aren’t enough to meet that demand,” said Dr. Crawford, a psychiatrist at Boston Medical Center’s Outpatient Child and Adolescent Psychiatry Clinic and at Codman Square Health Center. She was not involved with the current research.
Dr. Crawford noted that much of the data was from Europeans. Speaking to the experience of U.S.-based psychiatrists, she said there is a “greater appreciation for what we do as mental health providers, due to the growing conversations around mental health and normalizing mental health conditions.”
On the other hand, there is “a lack of parity in reimbursement rates. Although the general public values mental health, the medical system doesn’t value mental health providers in the same way as physicians in other specialties,” Dr. Crawford said. Feeling devalued can contribute to burnout, she added.
One way to counter burnout is to remember “that our role is to carry the hope. We can be hopeful for the patient that the treatment will work or the medications can provide some relief,” Dr. Crawford noted.
Psychiatrists “may need to hold on tightly to that hope because we may not receive that instant gratification from the patient or receive praise or see the change from the patient during that time, which can be challenging,” she said.
“But it’s important for us to keep in mind that, even in that moment when the patient can’t see it, we can work alongside the patient to create the vision of hope and what it will look like in the future,” said Dr. Crawford.
In the 2022 Medscape Psychiatrist Lifestyle, Happiness & Burnout Report, an annual online survey of Medscape member physicians, 47% of respondents reported burnout – which was up from 42% the previous year.
The investigators received no funding for this work. They and Dr. Crawford report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF AFFECTIVE DISORDERS
Cellular gene profiling may predict IBD treatment response
Transcriptomic profiling of phagocytes in the lamina propria of patients with inflammatory bowel disease (IBD) may guide future treatment selection, according to investigators.
Mucosal gut biopsies revealed that phagocytic gene expression correlated with inflammatory states, types of IBD, and responses to therapy, lead author Gillian E. Jacobsen a MD/PhD candidate at the University of Miami and colleagues reported.
In an article in Gastro Hep Advances, the investigators wrote that “lamina propria phagocytes along with epithelial cells represent a first line of defense and play a balancing act between tolerance toward commensal microbes and generation of immune responses toward pathogenic microorganisms. ... Inappropriate responses by lamina propria phagocytes have been linked to IBD.”
To better understand these responses, the researchers collected 111 gut mucosal biopsies from 54 patients with IBD, among whom 59% were taking biologics, 72% had inflammation in at least one biopsy site, and 41% had previously used at least one other biologic. Samples were analyzed to determine cell phenotypes, gene expression, and cytokine responses to in vitro Janus kinase (JAK) inhibitor exposure.
Ms. Jacobsen and colleagues noted that most reports that address the function of phagocytes focus on circulating dendritic cells, monocytes, or monocyte-derived macrophages, rather than on resident phagocyte populations located in the lamina propria. However, these circulating cells “do not reflect intestinal inflammation, or whole tissue biopsies.”
Phagocytes based on CD11b expression and phenotyped CD11b+-enriched cells using flow cytometry were identified. In samples with active inflammation, cells were most often granulocytes (45.5%), followed by macrophages (22.6%) and monocytes (9.4%). Uninflamed samples had a slightly lower proportion of granulocytes (33.6%), about the same proportion of macrophages (22.7%), and a higher rate of B cells (15.6% vs. 9.0%).
Ms. Jacobsen and colleagues highlighted the absolute uptick in granulocytes, including neutrophils.
“Neutrophilic infiltration is a major indicator of IBD activity and may be critically linked to ongoing inflammation,” they wrote. “These data demonstrate that CD11b+ enrichment reflects the inflammatory state of the biopsies.”
The investigators also showed that transcriptional profiles of lamina propria CD11b+ cells differed “greatly” between colon and ileum, which suggested that “the location or cellular environment plays a marked role in determining the gene expression of phagocytes.”
CD11b+ cell gene expression profiles also correlated with ulcerative colitis versus Crohn’s disease, although the researchers noted that these patterns were less pronounced than correlations with inflammatory states
“There are pathways common to inflammation regardless of the IBD type that could be used as markers of inflammation or targets for therapy.”
Comparing colon samples from patients who responded to anti–tumor necrosis factor therapy with those who were refractory to anti-TNF therapy revealed significant associations between response type and 52 differentially expressed genes.
“These genes were mostly immunoglobulin genes up-regulated in the anti–TNF-treated inflamed colon, suggesting that CD11b+ B cells may play a role in medication refractoriness.”
Evaluating inflamed colon and anti-TNF refractory ileum revealed differential expression of OSM, a known marker of TNF-resistant disease, as well as TREM1, a proinflammatory marker. In contrast, NTS genes showed high expression in uninflamed samples on anti-TNF therapy. The researchers noted that these findings “may be used to build precision medicine approaches in IBD.”
Further experiments showed that in vitro exposure of anti-TNF refractory samples to JAK inhibitors resulted in significantly reduced secretion of interleukin-8 and TNF-alpha.
“Our study provides functional data that JAK inhibition with tofacitinib (JAK1/JAK3) or ruxolitinib (JAK1/JAK2) inhibits lipopolysaccharide-induced cytokine production even in TNF-refractory samples,” the researchers wrote. “These data inform the response of patients to JAK inhibitors, including those refractory to other treatments.”
The study was supported by Pfizer, the National Institute of Diabetes and Digestive and Kidney Diseases, the Micky & Madeleine Arison Family Foundation Crohn’s & Colitis Discovery Laboratory, and Martin Kalser Chair in Gastroenterology at the University of Miami. The investigators disclosed additional relationships with Takeda, Abbvie, Eli Lilly, and others.
Inflammatory bowel diseases are complex and heterogenous disorders driven by inappropriate immune responses to luminal substances, including diet and microbes, resulting in chronic inflammation of the gastrointestinal tract. Therapies for IBD largely center around suppressing immune responses; however, given the complexity and heterogeneity of these diseases, consensus on which aspect of the immune response to suppress and which cell type to target in a given patient is unclear.
Sreeram Udayan, PhD, and Rodney D. Newberry, MD, are with the division of gastroenterology in the department of medicine at Washington University, St. Louis.
Inflammatory bowel diseases are complex and heterogenous disorders driven by inappropriate immune responses to luminal substances, including diet and microbes, resulting in chronic inflammation of the gastrointestinal tract. Therapies for IBD largely center around suppressing immune responses; however, given the complexity and heterogeneity of these diseases, consensus on which aspect of the immune response to suppress and which cell type to target in a given patient is unclear.
Sreeram Udayan, PhD, and Rodney D. Newberry, MD, are with the division of gastroenterology in the department of medicine at Washington University, St. Louis.
Inflammatory bowel diseases are complex and heterogenous disorders driven by inappropriate immune responses to luminal substances, including diet and microbes, resulting in chronic inflammation of the gastrointestinal tract. Therapies for IBD largely center around suppressing immune responses; however, given the complexity and heterogeneity of these diseases, consensus on which aspect of the immune response to suppress and which cell type to target in a given patient is unclear.
Sreeram Udayan, PhD, and Rodney D. Newberry, MD, are with the division of gastroenterology in the department of medicine at Washington University, St. Louis.
Transcriptomic profiling of phagocytes in the lamina propria of patients with inflammatory bowel disease (IBD) may guide future treatment selection, according to investigators.
Mucosal gut biopsies revealed that phagocytic gene expression correlated with inflammatory states, types of IBD, and responses to therapy, lead author Gillian E. Jacobsen a MD/PhD candidate at the University of Miami and colleagues reported.
In an article in Gastro Hep Advances, the investigators wrote that “lamina propria phagocytes along with epithelial cells represent a first line of defense and play a balancing act between tolerance toward commensal microbes and generation of immune responses toward pathogenic microorganisms. ... Inappropriate responses by lamina propria phagocytes have been linked to IBD.”
To better understand these responses, the researchers collected 111 gut mucosal biopsies from 54 patients with IBD, among whom 59% were taking biologics, 72% had inflammation in at least one biopsy site, and 41% had previously used at least one other biologic. Samples were analyzed to determine cell phenotypes, gene expression, and cytokine responses to in vitro Janus kinase (JAK) inhibitor exposure.
Ms. Jacobsen and colleagues noted that most reports that address the function of phagocytes focus on circulating dendritic cells, monocytes, or monocyte-derived macrophages, rather than on resident phagocyte populations located in the lamina propria. However, these circulating cells “do not reflect intestinal inflammation, or whole tissue biopsies.”
Phagocytes based on CD11b expression and phenotyped CD11b+-enriched cells using flow cytometry were identified. In samples with active inflammation, cells were most often granulocytes (45.5%), followed by macrophages (22.6%) and monocytes (9.4%). Uninflamed samples had a slightly lower proportion of granulocytes (33.6%), about the same proportion of macrophages (22.7%), and a higher rate of B cells (15.6% vs. 9.0%).
Ms. Jacobsen and colleagues highlighted the absolute uptick in granulocytes, including neutrophils.
“Neutrophilic infiltration is a major indicator of IBD activity and may be critically linked to ongoing inflammation,” they wrote. “These data demonstrate that CD11b+ enrichment reflects the inflammatory state of the biopsies.”
The investigators also showed that transcriptional profiles of lamina propria CD11b+ cells differed “greatly” between colon and ileum, which suggested that “the location or cellular environment plays a marked role in determining the gene expression of phagocytes.”
CD11b+ cell gene expression profiles also correlated with ulcerative colitis versus Crohn’s disease, although the researchers noted that these patterns were less pronounced than correlations with inflammatory states
“There are pathways common to inflammation regardless of the IBD type that could be used as markers of inflammation or targets for therapy.”
Comparing colon samples from patients who responded to anti–tumor necrosis factor therapy with those who were refractory to anti-TNF therapy revealed significant associations between response type and 52 differentially expressed genes.
“These genes were mostly immunoglobulin genes up-regulated in the anti–TNF-treated inflamed colon, suggesting that CD11b+ B cells may play a role in medication refractoriness.”
Evaluating inflamed colon and anti-TNF refractory ileum revealed differential expression of OSM, a known marker of TNF-resistant disease, as well as TREM1, a proinflammatory marker. In contrast, NTS genes showed high expression in uninflamed samples on anti-TNF therapy. The researchers noted that these findings “may be used to build precision medicine approaches in IBD.”
Further experiments showed that in vitro exposure of anti-TNF refractory samples to JAK inhibitors resulted in significantly reduced secretion of interleukin-8 and TNF-alpha.
“Our study provides functional data that JAK inhibition with tofacitinib (JAK1/JAK3) or ruxolitinib (JAK1/JAK2) inhibits lipopolysaccharide-induced cytokine production even in TNF-refractory samples,” the researchers wrote. “These data inform the response of patients to JAK inhibitors, including those refractory to other treatments.”
The study was supported by Pfizer, the National Institute of Diabetes and Digestive and Kidney Diseases, the Micky & Madeleine Arison Family Foundation Crohn’s & Colitis Discovery Laboratory, and Martin Kalser Chair in Gastroenterology at the University of Miami. The investigators disclosed additional relationships with Takeda, Abbvie, Eli Lilly, and others.
Transcriptomic profiling of phagocytes in the lamina propria of patients with inflammatory bowel disease (IBD) may guide future treatment selection, according to investigators.
Mucosal gut biopsies revealed that phagocytic gene expression correlated with inflammatory states, types of IBD, and responses to therapy, lead author Gillian E. Jacobsen a MD/PhD candidate at the University of Miami and colleagues reported.
In an article in Gastro Hep Advances, the investigators wrote that “lamina propria phagocytes along with epithelial cells represent a first line of defense and play a balancing act between tolerance toward commensal microbes and generation of immune responses toward pathogenic microorganisms. ... Inappropriate responses by lamina propria phagocytes have been linked to IBD.”
To better understand these responses, the researchers collected 111 gut mucosal biopsies from 54 patients with IBD, among whom 59% were taking biologics, 72% had inflammation in at least one biopsy site, and 41% had previously used at least one other biologic. Samples were analyzed to determine cell phenotypes, gene expression, and cytokine responses to in vitro Janus kinase (JAK) inhibitor exposure.
Ms. Jacobsen and colleagues noted that most reports that address the function of phagocytes focus on circulating dendritic cells, monocytes, or monocyte-derived macrophages, rather than on resident phagocyte populations located in the lamina propria. However, these circulating cells “do not reflect intestinal inflammation, or whole tissue biopsies.”
Phagocytes based on CD11b expression and phenotyped CD11b+-enriched cells using flow cytometry were identified. In samples with active inflammation, cells were most often granulocytes (45.5%), followed by macrophages (22.6%) and monocytes (9.4%). Uninflamed samples had a slightly lower proportion of granulocytes (33.6%), about the same proportion of macrophages (22.7%), and a higher rate of B cells (15.6% vs. 9.0%).
Ms. Jacobsen and colleagues highlighted the absolute uptick in granulocytes, including neutrophils.
“Neutrophilic infiltration is a major indicator of IBD activity and may be critically linked to ongoing inflammation,” they wrote. “These data demonstrate that CD11b+ enrichment reflects the inflammatory state of the biopsies.”
The investigators also showed that transcriptional profiles of lamina propria CD11b+ cells differed “greatly” between colon and ileum, which suggested that “the location or cellular environment plays a marked role in determining the gene expression of phagocytes.”
CD11b+ cell gene expression profiles also correlated with ulcerative colitis versus Crohn’s disease, although the researchers noted that these patterns were less pronounced than correlations with inflammatory states
“There are pathways common to inflammation regardless of the IBD type that could be used as markers of inflammation or targets for therapy.”
Comparing colon samples from patients who responded to anti–tumor necrosis factor therapy with those who were refractory to anti-TNF therapy revealed significant associations between response type and 52 differentially expressed genes.
“These genes were mostly immunoglobulin genes up-regulated in the anti–TNF-treated inflamed colon, suggesting that CD11b+ B cells may play a role in medication refractoriness.”
Evaluating inflamed colon and anti-TNF refractory ileum revealed differential expression of OSM, a known marker of TNF-resistant disease, as well as TREM1, a proinflammatory marker. In contrast, NTS genes showed high expression in uninflamed samples on anti-TNF therapy. The researchers noted that these findings “may be used to build precision medicine approaches in IBD.”
Further experiments showed that in vitro exposure of anti-TNF refractory samples to JAK inhibitors resulted in significantly reduced secretion of interleukin-8 and TNF-alpha.
“Our study provides functional data that JAK inhibition with tofacitinib (JAK1/JAK3) or ruxolitinib (JAK1/JAK2) inhibits lipopolysaccharide-induced cytokine production even in TNF-refractory samples,” the researchers wrote. “These data inform the response of patients to JAK inhibitors, including those refractory to other treatments.”
The study was supported by Pfizer, the National Institute of Diabetes and Digestive and Kidney Diseases, the Micky & Madeleine Arison Family Foundation Crohn’s & Colitis Discovery Laboratory, and Martin Kalser Chair in Gastroenterology at the University of Miami. The investigators disclosed additional relationships with Takeda, Abbvie, Eli Lilly, and others.
FROM GASTRO HEP ADVANCES
Reading Chekhov on the Cancer Ward
Burnout and other forms of psychosocial distress are common among health care professionals necessitating measures to promote well-being and reduce burnout.1 Studies have shown that nonmedical reading is associated with low burnout and that small group study sections can promote wellness.2,3 Narrative medicine, which proposes a model for humane and effective medical practice, advocates for the necessity of narrative competence.
Short Story Club
Narrative competence is the ability to acknowledge, interpret, and act on the stories of others. The narrative skill of close reading also encourages reflective practice, equipping practitioners to better weather the tides of illness.4 In our case, we formed a short story club intervention to closely read, or read and reflect, on literary fiction. We explored how reading and reflecting would result in profound changes in thinking and feeling and noted different ways by which they can cause such well-being. We describe here the 7 ways in which stories led us to increase bonding, improve empathy, and promote meaning in medicine.
Slowing Down
The short story club helped to bond us together and increase our sense of meaning in medicine by slowing us down. One member of the group likened the experience to increasing the pixels in a painting, thereby improving the resolution and seeing more clearly. Another member mentioned the experience as a form of meditation in slowing down the brain, breathing in the story, and breathing out impressions. One story by Anatole Broyard emphasized the importance of slowing down and “brooding” over a patient.5 The author describes his experience as a prostate cancer patient, in which his body was treated but his story was ignored. He begged his doctors to pay more attention to his story to listen and to brood over him. This story was enlightening to us; we saw how desperate our patients are to tell their stories, and for us to hear their stories.
Mirrors and Windows
Another way reading and reflecting on short stories helped was by reflecting our practices to ourselves, as though looking into a mirror to see ourselves and out of a window to see others. We found that stories mirrored our own world and allowed us to discuss issues close to us without the embarrassment or stigma of owning the story. In one session we read “The Doctor’s Visit” by Anton Chekhov.6 Some of the members resonated with the doctor of this story who awkwardly attended to his lady patient whose son was dying of a brain tumor. The doctor was nervous, insecure, and unable to express any empathy. He was also the father of the child who was dying and refused to admit any responsibility. One member of the group stated that he could relate to the doctor’s insecurities and mentioned that he too felt insecure and even sometimes felt like an imposter. This led to a discussion of insecurities, ways to bolster self-confidence, and ways to accept and respect limitations. This was a conversation that may not have taken place without the story as anchor to discuss insecurities that we individually may not have been willing to admit to the group.
In a different session, we discussed the story “Interpreter of Maladies” by Jhumpa Lahiri in which a settled Indian American family returns to India to tour and learn about their heritage from a guide (the interpreter of maladies) who interpreted the culture for them.7 The family professed to be interested in knowing about the culture but could not concentrate: the wife stayed busy flirting with the guide and revealing outrageous secrets to him, the children were engrossed in their squabbles, and the father was essentially absent taking photographs as souvenirs instead of seeing the sites firsthand. Some of the members of the group were Indian American and could relate to the alienation from their home and nostalgia for their country, while others could relate to the same alienation, albeit from other cultures and countries. This allowed us to talk about deeply personal topics, without having to own the topic or reveal personal issues. The discussion led to a deep understanding and empathy for us and our colleagues knowing the pain of alienation that some of them felt but could not discuss.
The stories also served as windows into the world of others which enabled us to see and become the other. For example, in one session we reflected on “Babylon Revisited” by F. Scott Fitzgerald.8 In this story, an American man returns to Paris after the Great Depression and recalls his life as a young artist in the American artist expatriate community of Paris in the 1920s and 1930s. During that time, he partied, drank in excess, lost his wife to pneumonia (for which he was at least partially responsible), lost custody of his daughter, and lost his fortune. As he returned to Paris to try to reclaim his daughter, we feel his pain as he tries but fails to overcome chronic alcoholism, sexual indiscretions, and losses. This gave rise to discussion of losses in general as we became one with the main character. This increased our empathy for others in a way that could not have been possible without this short story as anchor.
In another session we reflected on “Hills Like White Elephants” by Ernest Hemingway, in which a man is waiting for a train while proposing his girlfriend get an abortion.9 She agonizes over her choices and makes no decision in this story. Yet, we the reader could “become” the woman in the story faced with hard choices of having a baby but losing the man she loves, or having an abortion and maybe losing him anyway. In becoming this woman, we could experience the complex emotions and feel an experience of the other.
Exploring the Taboo
A third aspect of the club was enabling discussion of controversial topics. There were topics that arose in the group which never would have arisen in clinical practice discussions. These had to do with the taboo topics such as romantic attachments to patients. We read “The Caves of Lascaux” and reflected on the story of a young doctor who becomes enamored and obsessed with his beautiful but dying patient.10 He becomes so obsessed with her that he almost abandons his wife, family, and stable livelihood to descend with her into the caves. This story gave rise to discussions about romantic attachment to patients and how to handle and extricate one from the situation. The senior doctors explained some of their relevant experiences and how they either transferred care or sought counseling to extricate themselves from a potentially dangerous situation, especially when they too fell under the spell of forbidden romance.
Moral Grounding
These sessions also served to define the moral basis of our own practice. Much of health care psychosocial distress is related to moral injury in which health care professionals do the wrong thing or fail to do the right thing at the right time, due to external pressures related to financial or other gains. Reading and reflecting put us face-to-face with moral dilemmas and let us find our moral grounding. In reading “The Haircut” by Ring Lardner, we explored the disruptive town scoundrel who harassed and tortured his friends and neighbors but in such outrageous ways that he was considered a comedian rather than an abuser.11 Despite his hurtful acts, the townspeople (including the narrator) considered him a clown and laughed at his racist and sexist statements as well as his tricks.He faced no consequences such as confrontation, until the end when fate caught up. This story gave rise to a discussion of how we handle unkind, racist, sexist, or other comments which are disguised as humor, and to what extent we tolerate such controversial behavior. Do we go along with the scoundrel and laugh, or do we confront such people and insist that they respect and honor other people? The story sensitized the group to the ways in which prejudice and racism or sexism can be masked as humor, and to consider our moral responsibilities in society.
In another session we read and reflected on “Three Questions” by Leo Tolstoy.12 In this story, a king travels to another territory but gets distracted by helping a neighbor in need, and thereby inadvertently and fortunately avoids the trap that had been devised to kill him. The author gives us his moral basis by asking and answering 3 questions: Who is the most important person? What is the most important thing to do? What is the most important thing to do now? His answers provided his moral grounding. We discussed our answers and the basis of our moral grounding, whether it be the injunction do no harm, the more complex religious backgrounds of our childhood, or otherwise.
Symbols and Metaphors
The practice of reading and reflecting also taught us symbols and metaphors. Symbols and metaphors are the essence of storytelling, and they provide keys to understanding people. We sought out and studied the metaphors and symbols in each of the stories we read. In “I Stood There Ironing”, a woman is ironing as she is being questioned by a social worker on the upbringing of her first daughter, and its impact on her psychosocial distress.13 The woman remembers the hardships in raising her daughter and her neglect and abuse of the child due to circumstances beyond her control. She keeps ironing back and forth as she recounts the ways in which she neglected her child. The ironing provides a metaphor for attempting to straighten out her life and for recognizing finally at the end of the story that the daughter should not be the dress, under which her iron is pressing. This gave rise to a discussion of metaphors in our lives and the meanings they carry.
Problem-solving Guide
A sixth way the reflections helped was by serving as a guide to solving our problems. Some of the stories we read resonated deeply with members of the group and provided guides to solving problems. In one meeting we discussed “Those Are as Brothers” by Nancy Hale, a story in which a Nazi concentration camp survivor finds refuge in a country home and develops a friendship with a survivor of an abusive marriage.14 Reading and reflecting on this story enabled us to see the impact of trauma on ourselves, our life choices, professions, ways of being, philosophies, and even on our next generation. The story was personal for several members of the group, some of whom were second-generation Holocaust survivors, and for one who admitted to severe trauma as a child. Discussing our backgrounds together, we empathized with each other and helped each other heal. The story also provided a guide to healing from trauma, as its title indicates: sharing stories together can be a way to heal. The solidarity of standing together, as brothers, heals. The concentration camp survivor was mistreated in his job, but the abuse trauma victim rushes to his defense and vows her friendship and support. This soothed his soul and healed his mind. The guidance is clear: we can do the same, find friends, treat them like brothers, support each other and heal.
Bonding Through Shared Experience
The final and possibly most important way in which the club helped was by serving as an adventure to bond group members together through shared experience. We believe that literature can capture imagination in extraordinary ways and provide an opportunity to undertake remarkable journeys. As such, together we traveled to the ends of the earth from the beginning to the end of time and beyond. We traveled through the hills of Africa, meandered in the streets of Russia and Poland, watched the racetracks in Italy, toured the Taj Mahal in India, and descended into the caves of Lascaux, all while working in Little Rock, Arkansas. We shared a wide array of experiences together, which allowed us to know ourselves and others better, to share stories, and to develop a common vision, common ground, and common culture.
Conclusions
Through reading and reflecting on stories, we bonded as a group, increased our empathy for each other and others, and found meaning in medicine. Other studies have shown that participation in small study groups promote physician well-being, improve job satisfaction, and decrease burnout.3 We synergized this effort by reading nonmedical stories on a consistent basis, hoping to gain resilience to psychosocial distress.3 We chose short stories rather than novels to minimize any stress from excess reading. Combining these interventions, small group studies and nonmedical reading, into a single intervention as is typical in the practice of narrative medicine may provide a way to improve team functioning.
This pilot study showed that it is possible to form short story clubs even in a busy oncology program and that such programs benefit participants in a variety of ways with no apparent adverse effects. Further research is needed to study the impact of reading and reflecting on medical work in small study groups in larger numbers of subjects and to evaluate their impact on burnout. Further study is also needed to develop narrative medicine curricula that best address the needs of particular subspecialties and to determine the optimal conditions for implementation.
Acknowledgments
The authors acknowledge Dr. Erick Messias for inspiring and encouraging this project at the University of Arkansas for Medical Sciences where he was Associate Dean for Faculty Affairs. He is presently Chair of Psychiatry and Behavioral Neuroscience at the St. Louis University School of Medicine, St. Louis, Missouri.
1. Messias E, Gathright MM, Freeman ES, et al. Differences in burnout prevalence between clinical professionals and biomedical scientists in an academic medical centre: a cross-sectional survey. BMJ Open. 2019;9(2):e023506. doi:10.1136/bmjopen-2018-023506
2. Marchalik D, Rodriguez A, Namath A, et al. The impact of non-medical reading on clinical burnout: a national survey of palliative care providers. Ann Palliat Med. 2019;8(4):428-435. doi:10.21037/apm.2019.05.02
3. West CP, Dyrbye LN, Rabatin JT, et al. Intervention to promote physician well-being, job satisfaction, and professionalism: a randomized clinical trial. JAMA Intern Med. 2014;174(4):527-533. doi:10.1001/jamainternmed.2013.14387
4. Charon R. Narrative medicine: a model for empathy, reflection, profession, and tust. JAMA. 2001;286(15):1897–1902. doi:10.1001/jama.286.15.1897
5. Broyard A. Doctor Talk to Me. August 26, 1990. Accessed September 2021. https://www.nytimes.com/1990/08/26/magazine/doctor-talk-to-me.html
6. Chekhov A. A Doctor’s Visit,. In: Reynolds R, Stone J, eds. On Doctoring. Simon and Shuster;1995:50-59.
7. Lahiri J. Interpreter of Maladies. In: Lahiri J. Interpreter of Maladies. Mariner Books;2019.
8. Fitzgerald FS. Babylon Revisited. In: Moore L, Pitlor H, eds. 100 Years of the Best American Short Stories. Houghton Mifflin Harcourt;2015:62-81.
9. Hemingway E. Hills like White Elephants. In: Reynolds R, Stone J, eds. On Doctoring. Simon and Shuster;1995:108-111.
10. Karmel M. Caves of Lascaux. In: Ofri D, Staff of the Bellavue Literary Review, eds. The Best of the Bellevue Literary Review. Bellevue Literary Press;2008:168-174.
11. Lardner R. The Haircut. In Moore L, Pitlor H, eds. 100 Years of the Best American Short Stories. Houghton Mifflin Harcourt;2015:48-61.
12. Tolstoy L. The Three Questions. Accessed September 2021. https://www.plough.com/en/topics/culture/short-stories/the-three-questions
13. Olsen T. I Stand Here Ironing. In Moore L, Pitlor H, eds. 100 Years of the Best American Short Stories. Houghton Mifflin Harcourt;2015:173-180.
14. Hale N. Those Are as Brothers. In: Moore L, Pitlor H, eds. 100 Years of the Best American Short Stories. Houghton Mifflin Harcourt;2015:132-141.
Burnout and other forms of psychosocial distress are common among health care professionals necessitating measures to promote well-being and reduce burnout.1 Studies have shown that nonmedical reading is associated with low burnout and that small group study sections can promote wellness.2,3 Narrative medicine, which proposes a model for humane and effective medical practice, advocates for the necessity of narrative competence.
Short Story Club
Narrative competence is the ability to acknowledge, interpret, and act on the stories of others. The narrative skill of close reading also encourages reflective practice, equipping practitioners to better weather the tides of illness.4 In our case, we formed a short story club intervention to closely read, or read and reflect, on literary fiction. We explored how reading and reflecting would result in profound changes in thinking and feeling and noted different ways by which they can cause such well-being. We describe here the 7 ways in which stories led us to increase bonding, improve empathy, and promote meaning in medicine.
Slowing Down
The short story club helped to bond us together and increase our sense of meaning in medicine by slowing us down. One member of the group likened the experience to increasing the pixels in a painting, thereby improving the resolution and seeing more clearly. Another member mentioned the experience as a form of meditation in slowing down the brain, breathing in the story, and breathing out impressions. One story by Anatole Broyard emphasized the importance of slowing down and “brooding” over a patient.5 The author describes his experience as a prostate cancer patient, in which his body was treated but his story was ignored. He begged his doctors to pay more attention to his story to listen and to brood over him. This story was enlightening to us; we saw how desperate our patients are to tell their stories, and for us to hear their stories.
Mirrors and Windows
Another way reading and reflecting on short stories helped was by reflecting our practices to ourselves, as though looking into a mirror to see ourselves and out of a window to see others. We found that stories mirrored our own world and allowed us to discuss issues close to us without the embarrassment or stigma of owning the story. In one session we read “The Doctor’s Visit” by Anton Chekhov.6 Some of the members resonated with the doctor of this story who awkwardly attended to his lady patient whose son was dying of a brain tumor. The doctor was nervous, insecure, and unable to express any empathy. He was also the father of the child who was dying and refused to admit any responsibility. One member of the group stated that he could relate to the doctor’s insecurities and mentioned that he too felt insecure and even sometimes felt like an imposter. This led to a discussion of insecurities, ways to bolster self-confidence, and ways to accept and respect limitations. This was a conversation that may not have taken place without the story as anchor to discuss insecurities that we individually may not have been willing to admit to the group.
In a different session, we discussed the story “Interpreter of Maladies” by Jhumpa Lahiri in which a settled Indian American family returns to India to tour and learn about their heritage from a guide (the interpreter of maladies) who interpreted the culture for them.7 The family professed to be interested in knowing about the culture but could not concentrate: the wife stayed busy flirting with the guide and revealing outrageous secrets to him, the children were engrossed in their squabbles, and the father was essentially absent taking photographs as souvenirs instead of seeing the sites firsthand. Some of the members of the group were Indian American and could relate to the alienation from their home and nostalgia for their country, while others could relate to the same alienation, albeit from other cultures and countries. This allowed us to talk about deeply personal topics, without having to own the topic or reveal personal issues. The discussion led to a deep understanding and empathy for us and our colleagues knowing the pain of alienation that some of them felt but could not discuss.
The stories also served as windows into the world of others which enabled us to see and become the other. For example, in one session we reflected on “Babylon Revisited” by F. Scott Fitzgerald.8 In this story, an American man returns to Paris after the Great Depression and recalls his life as a young artist in the American artist expatriate community of Paris in the 1920s and 1930s. During that time, he partied, drank in excess, lost his wife to pneumonia (for which he was at least partially responsible), lost custody of his daughter, and lost his fortune. As he returned to Paris to try to reclaim his daughter, we feel his pain as he tries but fails to overcome chronic alcoholism, sexual indiscretions, and losses. This gave rise to discussion of losses in general as we became one with the main character. This increased our empathy for others in a way that could not have been possible without this short story as anchor.
In another session we reflected on “Hills Like White Elephants” by Ernest Hemingway, in which a man is waiting for a train while proposing his girlfriend get an abortion.9 She agonizes over her choices and makes no decision in this story. Yet, we the reader could “become” the woman in the story faced with hard choices of having a baby but losing the man she loves, or having an abortion and maybe losing him anyway. In becoming this woman, we could experience the complex emotions and feel an experience of the other.
Exploring the Taboo
A third aspect of the club was enabling discussion of controversial topics. There were topics that arose in the group which never would have arisen in clinical practice discussions. These had to do with the taboo topics such as romantic attachments to patients. We read “The Caves of Lascaux” and reflected on the story of a young doctor who becomes enamored and obsessed with his beautiful but dying patient.10 He becomes so obsessed with her that he almost abandons his wife, family, and stable livelihood to descend with her into the caves. This story gave rise to discussions about romantic attachment to patients and how to handle and extricate one from the situation. The senior doctors explained some of their relevant experiences and how they either transferred care or sought counseling to extricate themselves from a potentially dangerous situation, especially when they too fell under the spell of forbidden romance.
Moral Grounding
These sessions also served to define the moral basis of our own practice. Much of health care psychosocial distress is related to moral injury in which health care professionals do the wrong thing or fail to do the right thing at the right time, due to external pressures related to financial or other gains. Reading and reflecting put us face-to-face with moral dilemmas and let us find our moral grounding. In reading “The Haircut” by Ring Lardner, we explored the disruptive town scoundrel who harassed and tortured his friends and neighbors but in such outrageous ways that he was considered a comedian rather than an abuser.11 Despite his hurtful acts, the townspeople (including the narrator) considered him a clown and laughed at his racist and sexist statements as well as his tricks.He faced no consequences such as confrontation, until the end when fate caught up. This story gave rise to a discussion of how we handle unkind, racist, sexist, or other comments which are disguised as humor, and to what extent we tolerate such controversial behavior. Do we go along with the scoundrel and laugh, or do we confront such people and insist that they respect and honor other people? The story sensitized the group to the ways in which prejudice and racism or sexism can be masked as humor, and to consider our moral responsibilities in society.
In another session we read and reflected on “Three Questions” by Leo Tolstoy.12 In this story, a king travels to another territory but gets distracted by helping a neighbor in need, and thereby inadvertently and fortunately avoids the trap that had been devised to kill him. The author gives us his moral basis by asking and answering 3 questions: Who is the most important person? What is the most important thing to do? What is the most important thing to do now? His answers provided his moral grounding. We discussed our answers and the basis of our moral grounding, whether it be the injunction do no harm, the more complex religious backgrounds of our childhood, or otherwise.
Symbols and Metaphors
The practice of reading and reflecting also taught us symbols and metaphors. Symbols and metaphors are the essence of storytelling, and they provide keys to understanding people. We sought out and studied the metaphors and symbols in each of the stories we read. In “I Stood There Ironing”, a woman is ironing as she is being questioned by a social worker on the upbringing of her first daughter, and its impact on her psychosocial distress.13 The woman remembers the hardships in raising her daughter and her neglect and abuse of the child due to circumstances beyond her control. She keeps ironing back and forth as she recounts the ways in which she neglected her child. The ironing provides a metaphor for attempting to straighten out her life and for recognizing finally at the end of the story that the daughter should not be the dress, under which her iron is pressing. This gave rise to a discussion of metaphors in our lives and the meanings they carry.
Problem-solving Guide
A sixth way the reflections helped was by serving as a guide to solving our problems. Some of the stories we read resonated deeply with members of the group and provided guides to solving problems. In one meeting we discussed “Those Are as Brothers” by Nancy Hale, a story in which a Nazi concentration camp survivor finds refuge in a country home and develops a friendship with a survivor of an abusive marriage.14 Reading and reflecting on this story enabled us to see the impact of trauma on ourselves, our life choices, professions, ways of being, philosophies, and even on our next generation. The story was personal for several members of the group, some of whom were second-generation Holocaust survivors, and for one who admitted to severe trauma as a child. Discussing our backgrounds together, we empathized with each other and helped each other heal. The story also provided a guide to healing from trauma, as its title indicates: sharing stories together can be a way to heal. The solidarity of standing together, as brothers, heals. The concentration camp survivor was mistreated in his job, but the abuse trauma victim rushes to his defense and vows her friendship and support. This soothed his soul and healed his mind. The guidance is clear: we can do the same, find friends, treat them like brothers, support each other and heal.
Bonding Through Shared Experience
The final and possibly most important way in which the club helped was by serving as an adventure to bond group members together through shared experience. We believe that literature can capture imagination in extraordinary ways and provide an opportunity to undertake remarkable journeys. As such, together we traveled to the ends of the earth from the beginning to the end of time and beyond. We traveled through the hills of Africa, meandered in the streets of Russia and Poland, watched the racetracks in Italy, toured the Taj Mahal in India, and descended into the caves of Lascaux, all while working in Little Rock, Arkansas. We shared a wide array of experiences together, which allowed us to know ourselves and others better, to share stories, and to develop a common vision, common ground, and common culture.
Conclusions
Through reading and reflecting on stories, we bonded as a group, increased our empathy for each other and others, and found meaning in medicine. Other studies have shown that participation in small study groups promote physician well-being, improve job satisfaction, and decrease burnout.3 We synergized this effort by reading nonmedical stories on a consistent basis, hoping to gain resilience to psychosocial distress.3 We chose short stories rather than novels to minimize any stress from excess reading. Combining these interventions, small group studies and nonmedical reading, into a single intervention as is typical in the practice of narrative medicine may provide a way to improve team functioning.
This pilot study showed that it is possible to form short story clubs even in a busy oncology program and that such programs benefit participants in a variety of ways with no apparent adverse effects. Further research is needed to study the impact of reading and reflecting on medical work in small study groups in larger numbers of subjects and to evaluate their impact on burnout. Further study is also needed to develop narrative medicine curricula that best address the needs of particular subspecialties and to determine the optimal conditions for implementation.
Acknowledgments
The authors acknowledge Dr. Erick Messias for inspiring and encouraging this project at the University of Arkansas for Medical Sciences where he was Associate Dean for Faculty Affairs. He is presently Chair of Psychiatry and Behavioral Neuroscience at the St. Louis University School of Medicine, St. Louis, Missouri.
Burnout and other forms of psychosocial distress are common among health care professionals necessitating measures to promote well-being and reduce burnout.1 Studies have shown that nonmedical reading is associated with low burnout and that small group study sections can promote wellness.2,3 Narrative medicine, which proposes a model for humane and effective medical practice, advocates for the necessity of narrative competence.
Short Story Club
Narrative competence is the ability to acknowledge, interpret, and act on the stories of others. The narrative skill of close reading also encourages reflective practice, equipping practitioners to better weather the tides of illness.4 In our case, we formed a short story club intervention to closely read, or read and reflect, on literary fiction. We explored how reading and reflecting would result in profound changes in thinking and feeling and noted different ways by which they can cause such well-being. We describe here the 7 ways in which stories led us to increase bonding, improve empathy, and promote meaning in medicine.
Slowing Down
The short story club helped to bond us together and increase our sense of meaning in medicine by slowing us down. One member of the group likened the experience to increasing the pixels in a painting, thereby improving the resolution and seeing more clearly. Another member mentioned the experience as a form of meditation in slowing down the brain, breathing in the story, and breathing out impressions. One story by Anatole Broyard emphasized the importance of slowing down and “brooding” over a patient.5 The author describes his experience as a prostate cancer patient, in which his body was treated but his story was ignored. He begged his doctors to pay more attention to his story to listen and to brood over him. This story was enlightening to us; we saw how desperate our patients are to tell their stories, and for us to hear their stories.
Mirrors and Windows
Another way reading and reflecting on short stories helped was by reflecting our practices to ourselves, as though looking into a mirror to see ourselves and out of a window to see others. We found that stories mirrored our own world and allowed us to discuss issues close to us without the embarrassment or stigma of owning the story. In one session we read “The Doctor’s Visit” by Anton Chekhov.6 Some of the members resonated with the doctor of this story who awkwardly attended to his lady patient whose son was dying of a brain tumor. The doctor was nervous, insecure, and unable to express any empathy. He was also the father of the child who was dying and refused to admit any responsibility. One member of the group stated that he could relate to the doctor’s insecurities and mentioned that he too felt insecure and even sometimes felt like an imposter. This led to a discussion of insecurities, ways to bolster self-confidence, and ways to accept and respect limitations. This was a conversation that may not have taken place without the story as anchor to discuss insecurities that we individually may not have been willing to admit to the group.
In a different session, we discussed the story “Interpreter of Maladies” by Jhumpa Lahiri in which a settled Indian American family returns to India to tour and learn about their heritage from a guide (the interpreter of maladies) who interpreted the culture for them.7 The family professed to be interested in knowing about the culture but could not concentrate: the wife stayed busy flirting with the guide and revealing outrageous secrets to him, the children were engrossed in their squabbles, and the father was essentially absent taking photographs as souvenirs instead of seeing the sites firsthand. Some of the members of the group were Indian American and could relate to the alienation from their home and nostalgia for their country, while others could relate to the same alienation, albeit from other cultures and countries. This allowed us to talk about deeply personal topics, without having to own the topic or reveal personal issues. The discussion led to a deep understanding and empathy for us and our colleagues knowing the pain of alienation that some of them felt but could not discuss.
The stories also served as windows into the world of others which enabled us to see and become the other. For example, in one session we reflected on “Babylon Revisited” by F. Scott Fitzgerald.8 In this story, an American man returns to Paris after the Great Depression and recalls his life as a young artist in the American artist expatriate community of Paris in the 1920s and 1930s. During that time, he partied, drank in excess, lost his wife to pneumonia (for which he was at least partially responsible), lost custody of his daughter, and lost his fortune. As he returned to Paris to try to reclaim his daughter, we feel his pain as he tries but fails to overcome chronic alcoholism, sexual indiscretions, and losses. This gave rise to discussion of losses in general as we became one with the main character. This increased our empathy for others in a way that could not have been possible without this short story as anchor.
In another session we reflected on “Hills Like White Elephants” by Ernest Hemingway, in which a man is waiting for a train while proposing his girlfriend get an abortion.9 She agonizes over her choices and makes no decision in this story. Yet, we the reader could “become” the woman in the story faced with hard choices of having a baby but losing the man she loves, or having an abortion and maybe losing him anyway. In becoming this woman, we could experience the complex emotions and feel an experience of the other.
Exploring the Taboo
A third aspect of the club was enabling discussion of controversial topics. There were topics that arose in the group which never would have arisen in clinical practice discussions. These had to do with the taboo topics such as romantic attachments to patients. We read “The Caves of Lascaux” and reflected on the story of a young doctor who becomes enamored and obsessed with his beautiful but dying patient.10 He becomes so obsessed with her that he almost abandons his wife, family, and stable livelihood to descend with her into the caves. This story gave rise to discussions about romantic attachment to patients and how to handle and extricate one from the situation. The senior doctors explained some of their relevant experiences and how they either transferred care or sought counseling to extricate themselves from a potentially dangerous situation, especially when they too fell under the spell of forbidden romance.
Moral Grounding
These sessions also served to define the moral basis of our own practice. Much of health care psychosocial distress is related to moral injury in which health care professionals do the wrong thing or fail to do the right thing at the right time, due to external pressures related to financial or other gains. Reading and reflecting put us face-to-face with moral dilemmas and let us find our moral grounding. In reading “The Haircut” by Ring Lardner, we explored the disruptive town scoundrel who harassed and tortured his friends and neighbors but in such outrageous ways that he was considered a comedian rather than an abuser.11 Despite his hurtful acts, the townspeople (including the narrator) considered him a clown and laughed at his racist and sexist statements as well as his tricks.He faced no consequences such as confrontation, until the end when fate caught up. This story gave rise to a discussion of how we handle unkind, racist, sexist, or other comments which are disguised as humor, and to what extent we tolerate such controversial behavior. Do we go along with the scoundrel and laugh, or do we confront such people and insist that they respect and honor other people? The story sensitized the group to the ways in which prejudice and racism or sexism can be masked as humor, and to consider our moral responsibilities in society.
In another session we read and reflected on “Three Questions” by Leo Tolstoy.12 In this story, a king travels to another territory but gets distracted by helping a neighbor in need, and thereby inadvertently and fortunately avoids the trap that had been devised to kill him. The author gives us his moral basis by asking and answering 3 questions: Who is the most important person? What is the most important thing to do? What is the most important thing to do now? His answers provided his moral grounding. We discussed our answers and the basis of our moral grounding, whether it be the injunction do no harm, the more complex religious backgrounds of our childhood, or otherwise.
Symbols and Metaphors
The practice of reading and reflecting also taught us symbols and metaphors. Symbols and metaphors are the essence of storytelling, and they provide keys to understanding people. We sought out and studied the metaphors and symbols in each of the stories we read. In “I Stood There Ironing”, a woman is ironing as she is being questioned by a social worker on the upbringing of her first daughter, and its impact on her psychosocial distress.13 The woman remembers the hardships in raising her daughter and her neglect and abuse of the child due to circumstances beyond her control. She keeps ironing back and forth as she recounts the ways in which she neglected her child. The ironing provides a metaphor for attempting to straighten out her life and for recognizing finally at the end of the story that the daughter should not be the dress, under which her iron is pressing. This gave rise to a discussion of metaphors in our lives and the meanings they carry.
Problem-solving Guide
A sixth way the reflections helped was by serving as a guide to solving our problems. Some of the stories we read resonated deeply with members of the group and provided guides to solving problems. In one meeting we discussed “Those Are as Brothers” by Nancy Hale, a story in which a Nazi concentration camp survivor finds refuge in a country home and develops a friendship with a survivor of an abusive marriage.14 Reading and reflecting on this story enabled us to see the impact of trauma on ourselves, our life choices, professions, ways of being, philosophies, and even on our next generation. The story was personal for several members of the group, some of whom were second-generation Holocaust survivors, and for one who admitted to severe trauma as a child. Discussing our backgrounds together, we empathized with each other and helped each other heal. The story also provided a guide to healing from trauma, as its title indicates: sharing stories together can be a way to heal. The solidarity of standing together, as brothers, heals. The concentration camp survivor was mistreated in his job, but the abuse trauma victim rushes to his defense and vows her friendship and support. This soothed his soul and healed his mind. The guidance is clear: we can do the same, find friends, treat them like brothers, support each other and heal.
Bonding Through Shared Experience
The final and possibly most important way in which the club helped was by serving as an adventure to bond group members together through shared experience. We believe that literature can capture imagination in extraordinary ways and provide an opportunity to undertake remarkable journeys. As such, together we traveled to the ends of the earth from the beginning to the end of time and beyond. We traveled through the hills of Africa, meandered in the streets of Russia and Poland, watched the racetracks in Italy, toured the Taj Mahal in India, and descended into the caves of Lascaux, all while working in Little Rock, Arkansas. We shared a wide array of experiences together, which allowed us to know ourselves and others better, to share stories, and to develop a common vision, common ground, and common culture.
Conclusions
Through reading and reflecting on stories, we bonded as a group, increased our empathy for each other and others, and found meaning in medicine. Other studies have shown that participation in small study groups promote physician well-being, improve job satisfaction, and decrease burnout.3 We synergized this effort by reading nonmedical stories on a consistent basis, hoping to gain resilience to psychosocial distress.3 We chose short stories rather than novels to minimize any stress from excess reading. Combining these interventions, small group studies and nonmedical reading, into a single intervention as is typical in the practice of narrative medicine may provide a way to improve team functioning.
This pilot study showed that it is possible to form short story clubs even in a busy oncology program and that such programs benefit participants in a variety of ways with no apparent adverse effects. Further research is needed to study the impact of reading and reflecting on medical work in small study groups in larger numbers of subjects and to evaluate their impact on burnout. Further study is also needed to develop narrative medicine curricula that best address the needs of particular subspecialties and to determine the optimal conditions for implementation.
Acknowledgments
The authors acknowledge Dr. Erick Messias for inspiring and encouraging this project at the University of Arkansas for Medical Sciences where he was Associate Dean for Faculty Affairs. He is presently Chair of Psychiatry and Behavioral Neuroscience at the St. Louis University School of Medicine, St. Louis, Missouri.
1. Messias E, Gathright MM, Freeman ES, et al. Differences in burnout prevalence between clinical professionals and biomedical scientists in an academic medical centre: a cross-sectional survey. BMJ Open. 2019;9(2):e023506. doi:10.1136/bmjopen-2018-023506
2. Marchalik D, Rodriguez A, Namath A, et al. The impact of non-medical reading on clinical burnout: a national survey of palliative care providers. Ann Palliat Med. 2019;8(4):428-435. doi:10.21037/apm.2019.05.02
3. West CP, Dyrbye LN, Rabatin JT, et al. Intervention to promote physician well-being, job satisfaction, and professionalism: a randomized clinical trial. JAMA Intern Med. 2014;174(4):527-533. doi:10.1001/jamainternmed.2013.14387
4. Charon R. Narrative medicine: a model for empathy, reflection, profession, and tust. JAMA. 2001;286(15):1897–1902. doi:10.1001/jama.286.15.1897
5. Broyard A. Doctor Talk to Me. August 26, 1990. Accessed September 2021. https://www.nytimes.com/1990/08/26/magazine/doctor-talk-to-me.html
6. Chekhov A. A Doctor’s Visit,. In: Reynolds R, Stone J, eds. On Doctoring. Simon and Shuster;1995:50-59.
7. Lahiri J. Interpreter of Maladies. In: Lahiri J. Interpreter of Maladies. Mariner Books;2019.
8. Fitzgerald FS. Babylon Revisited. In: Moore L, Pitlor H, eds. 100 Years of the Best American Short Stories. Houghton Mifflin Harcourt;2015:62-81.
9. Hemingway E. Hills like White Elephants. In: Reynolds R, Stone J, eds. On Doctoring. Simon and Shuster;1995:108-111.
10. Karmel M. Caves of Lascaux. In: Ofri D, Staff of the Bellavue Literary Review, eds. The Best of the Bellevue Literary Review. Bellevue Literary Press;2008:168-174.
11. Lardner R. The Haircut. In Moore L, Pitlor H, eds. 100 Years of the Best American Short Stories. Houghton Mifflin Harcourt;2015:48-61.
12. Tolstoy L. The Three Questions. Accessed September 2021. https://www.plough.com/en/topics/culture/short-stories/the-three-questions
13. Olsen T. I Stand Here Ironing. In Moore L, Pitlor H, eds. 100 Years of the Best American Short Stories. Houghton Mifflin Harcourt;2015:173-180.
14. Hale N. Those Are as Brothers. In: Moore L, Pitlor H, eds. 100 Years of the Best American Short Stories. Houghton Mifflin Harcourt;2015:132-141.
1. Messias E, Gathright MM, Freeman ES, et al. Differences in burnout prevalence between clinical professionals and biomedical scientists in an academic medical centre: a cross-sectional survey. BMJ Open. 2019;9(2):e023506. doi:10.1136/bmjopen-2018-023506
2. Marchalik D, Rodriguez A, Namath A, et al. The impact of non-medical reading on clinical burnout: a national survey of palliative care providers. Ann Palliat Med. 2019;8(4):428-435. doi:10.21037/apm.2019.05.02
3. West CP, Dyrbye LN, Rabatin JT, et al. Intervention to promote physician well-being, job satisfaction, and professionalism: a randomized clinical trial. JAMA Intern Med. 2014;174(4):527-533. doi:10.1001/jamainternmed.2013.14387
4. Charon R. Narrative medicine: a model for empathy, reflection, profession, and tust. JAMA. 2001;286(15):1897–1902. doi:10.1001/jama.286.15.1897
5. Broyard A. Doctor Talk to Me. August 26, 1990. Accessed September 2021. https://www.nytimes.com/1990/08/26/magazine/doctor-talk-to-me.html
6. Chekhov A. A Doctor’s Visit,. In: Reynolds R, Stone J, eds. On Doctoring. Simon and Shuster;1995:50-59.
7. Lahiri J. Interpreter of Maladies. In: Lahiri J. Interpreter of Maladies. Mariner Books;2019.
8. Fitzgerald FS. Babylon Revisited. In: Moore L, Pitlor H, eds. 100 Years of the Best American Short Stories. Houghton Mifflin Harcourt;2015:62-81.
9. Hemingway E. Hills like White Elephants. In: Reynolds R, Stone J, eds. On Doctoring. Simon and Shuster;1995:108-111.
10. Karmel M. Caves of Lascaux. In: Ofri D, Staff of the Bellavue Literary Review, eds. The Best of the Bellevue Literary Review. Bellevue Literary Press;2008:168-174.
11. Lardner R. The Haircut. In Moore L, Pitlor H, eds. 100 Years of the Best American Short Stories. Houghton Mifflin Harcourt;2015:48-61.
12. Tolstoy L. The Three Questions. Accessed September 2021. https://www.plough.com/en/topics/culture/short-stories/the-three-questions
13. Olsen T. I Stand Here Ironing. In Moore L, Pitlor H, eds. 100 Years of the Best American Short Stories. Houghton Mifflin Harcourt;2015:173-180.
14. Hale N. Those Are as Brothers. In: Moore L, Pitlor H, eds. 100 Years of the Best American Short Stories. Houghton Mifflin Harcourt;2015:132-141.
‘Forever chemicals’ linked to liver damage
(NAFLD), say the authors of a comprehensive evidence review.
They found “consistent” evidence for PFAS hepatotoxicity from rodent studies. In addition, exposure to PFAS was found to be associated with markers of liver function in observational studies in people.
The review, published online in Environmental Health Perspectives, may be the first systematic analysis of PFAS exposure and liver damage.
Possible contributor to growing NAFLD epidemic
In their analysis, the authors included 85 rodent studies and 24 epidemiologic studies, primarily involving people from the United States and largely focusing on four “legacy” PFAS: perfluorooctanoic acid (PFOA), perfluorooctanesulfonic acid (PFOS), perfluorononanoic acid (PFNA), and perfluorohexanesulfonic acid (PFHxS).
Meta-analyses of human studies found that higher levels of alanine aminotransferase were significantly associated with exposure to three of the older chemicals – PFOA, PFOS, and PFNA.
The “positive” and “convincing” associations between exposure to these synthetic chemicals and higher ALT levels suggest that exposure may contribute to the growing NAFLD epidemic, the researchers write.
Exposure to one of the chemicals, PFOA, was also associated with higher aspartate aminotransferase and gamma-glutamyl transferase levels in people.
In rodents, exposure to these synthetic chemicals consistently resulted in higher ALT levels and steatosis.
“The mechanism is not well understood yet, but there are a few proposed theories,” first author Elizabeth Costello, MPH, PhD student, department of population and public health sciences, University of Southern California, Los Angeles, told this news organization.
“PFAS are similar to fatty acids in chemical structure, so it’s possible that they activate some of the same receptors or otherwise interfere with fat metabolism. This might lead to inflammation or fat accumulation in the liver,” Ms. Costello explained.
People widely exposed
PFAS are ubiquitous in the environment. They have been detected in the blood of most people and have been linked to a variety of health concerns. Possible sources of PFAS exposure run the gamut from nonstick cookware, food wrappers, and waterproof fabrics to cosmetics and even drinking water.
“We are exposed to PFAS in so many ways – through water, food, and products we use. It can be very difficult for individuals to control their own exposure,” Ms. Costello commented.
“At this point, it’s important to look for ways to remove PFAS from the environment and phase them out of our products and carefully consider the safety of any replacement chemicals,” she said.
Although most of the research to date has been limited to the four older PFAS (PFOA, PFOS, PFNA, and PFHxS), there are thousands of different PFAS chemicals.
“We don’t know very much about the effects of exposure to multiple PFAS at the same time or how newer replacement PFAS might affect liver disease or other health conditions,” Ms. Costello said.
Reached for comment, Lisa B. VanWagner, MD, with Northwestern University, Chicago, said this analysis is “very interesting,” but she is also “left wondering how we could do anything since it seems from my reading that these chemicals are ubiquitous and used regularly in the environment.”
Dr. VanWagner, who was not involved in the study, said the major limitation is the small number of human studies and the high heterogeneity between studies, “meaning it is hard to come to a firm conclusion about whether what has been observed in the animal studies does truly apply to humans.
“Overall, this study provides important proof of concept for future work to look more specifically at PFAS exposure, and more specific markers of fatty liver disease and liver damage, like liver biopsy, are needed in humans,” Dr. VanWagner said.
“If data accumulate showing that these chemicals do in fact contribute to fatty liver and worsening inflammation or liver damage as a result of exposure, then public health interventions to remove or reduce use of these chemicals could have wide-ranging public health effects,” Dr. VanWagner added.
Further research needed
The authors of an invited perspective published with the study say it underscores the “urgent need for further research and for immediate and reasonable public health action.”
“This work firmly puts PFAS exposure on the list of persistent pollutants, such as polychlorinated biphenyls, that cause hepatotoxicity and whose mechanism is linked to steatosis,” write Alan Ducatman, MD. MSc, with West Virginia University School of Public Health, Morgantown, and Suzanne Fenton, PhD, MS, with the National Institute of Environmental Health Sciences, Research Triangle Park, N.C.
They say other important questions raised by this review include whether individuals who are overweight or obese and those with diabetes are more susceptible to PFAS hepatoxicity, which “replacement” or emerging PFAS can cause liver damage, and whether high doses cause different kinds of liver toxicity than low doses.
“GenX, a current replacement [chemical] for PFOA, has shown significant hepatotoxicity in several recent experimental studies, suggesting it may not be a safe replacement,” they point out.
“A significant challenge will be deciding which of the multiple metabolic pathways altered by PFAS are most important and predictive for induction of liver damage and for progression of liver disease, so that emerging PFAS may be screened for hepatotoxicity prior to entering the market,” Dr. Ducatman and Dr. Fenton conclude.
Support for this research was provided by the National Institute of Environmental Health Science, part of the National Institutes of Health, and the U.S. Department of Agriculture National Institute of Food and Agriculture. Dr. Costello, Dr. VanWagner, Dr. Ducatman, and Dr. Fenton report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
(NAFLD), say the authors of a comprehensive evidence review.
They found “consistent” evidence for PFAS hepatotoxicity from rodent studies. In addition, exposure to PFAS was found to be associated with markers of liver function in observational studies in people.
The review, published online in Environmental Health Perspectives, may be the first systematic analysis of PFAS exposure and liver damage.
Possible contributor to growing NAFLD epidemic
In their analysis, the authors included 85 rodent studies and 24 epidemiologic studies, primarily involving people from the United States and largely focusing on four “legacy” PFAS: perfluorooctanoic acid (PFOA), perfluorooctanesulfonic acid (PFOS), perfluorononanoic acid (PFNA), and perfluorohexanesulfonic acid (PFHxS).
Meta-analyses of human studies found that higher levels of alanine aminotransferase were significantly associated with exposure to three of the older chemicals – PFOA, PFOS, and PFNA.
The “positive” and “convincing” associations between exposure to these synthetic chemicals and higher ALT levels suggest that exposure may contribute to the growing NAFLD epidemic, the researchers write.
Exposure to one of the chemicals, PFOA, was also associated with higher aspartate aminotransferase and gamma-glutamyl transferase levels in people.
In rodents, exposure to these synthetic chemicals consistently resulted in higher ALT levels and steatosis.
“The mechanism is not well understood yet, but there are a few proposed theories,” first author Elizabeth Costello, MPH, PhD student, department of population and public health sciences, University of Southern California, Los Angeles, told this news organization.
“PFAS are similar to fatty acids in chemical structure, so it’s possible that they activate some of the same receptors or otherwise interfere with fat metabolism. This might lead to inflammation or fat accumulation in the liver,” Ms. Costello explained.
People widely exposed
PFAS are ubiquitous in the environment. They have been detected in the blood of most people and have been linked to a variety of health concerns. Possible sources of PFAS exposure run the gamut from nonstick cookware, food wrappers, and waterproof fabrics to cosmetics and even drinking water.
“We are exposed to PFAS in so many ways – through water, food, and products we use. It can be very difficult for individuals to control their own exposure,” Ms. Costello commented.
“At this point, it’s important to look for ways to remove PFAS from the environment and phase them out of our products and carefully consider the safety of any replacement chemicals,” she said.
Although most of the research to date has been limited to the four older PFAS (PFOA, PFOS, PFNA, and PFHxS), there are thousands of different PFAS chemicals.
“We don’t know very much about the effects of exposure to multiple PFAS at the same time or how newer replacement PFAS might affect liver disease or other health conditions,” Ms. Costello said.
Reached for comment, Lisa B. VanWagner, MD, with Northwestern University, Chicago, said this analysis is “very interesting,” but she is also “left wondering how we could do anything since it seems from my reading that these chemicals are ubiquitous and used regularly in the environment.”
Dr. VanWagner, who was not involved in the study, said the major limitation is the small number of human studies and the high heterogeneity between studies, “meaning it is hard to come to a firm conclusion about whether what has been observed in the animal studies does truly apply to humans.
“Overall, this study provides important proof of concept for future work to look more specifically at PFAS exposure, and more specific markers of fatty liver disease and liver damage, like liver biopsy, are needed in humans,” Dr. VanWagner said.
“If data accumulate showing that these chemicals do in fact contribute to fatty liver and worsening inflammation or liver damage as a result of exposure, then public health interventions to remove or reduce use of these chemicals could have wide-ranging public health effects,” Dr. VanWagner added.
Further research needed
The authors of an invited perspective published with the study say it underscores the “urgent need for further research and for immediate and reasonable public health action.”
“This work firmly puts PFAS exposure on the list of persistent pollutants, such as polychlorinated biphenyls, that cause hepatotoxicity and whose mechanism is linked to steatosis,” write Alan Ducatman, MD. MSc, with West Virginia University School of Public Health, Morgantown, and Suzanne Fenton, PhD, MS, with the National Institute of Environmental Health Sciences, Research Triangle Park, N.C.
They say other important questions raised by this review include whether individuals who are overweight or obese and those with diabetes are more susceptible to PFAS hepatoxicity, which “replacement” or emerging PFAS can cause liver damage, and whether high doses cause different kinds of liver toxicity than low doses.
“GenX, a current replacement [chemical] for PFOA, has shown significant hepatotoxicity in several recent experimental studies, suggesting it may not be a safe replacement,” they point out.
“A significant challenge will be deciding which of the multiple metabolic pathways altered by PFAS are most important and predictive for induction of liver damage and for progression of liver disease, so that emerging PFAS may be screened for hepatotoxicity prior to entering the market,” Dr. Ducatman and Dr. Fenton conclude.
Support for this research was provided by the National Institute of Environmental Health Science, part of the National Institutes of Health, and the U.S. Department of Agriculture National Institute of Food and Agriculture. Dr. Costello, Dr. VanWagner, Dr. Ducatman, and Dr. Fenton report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
(NAFLD), say the authors of a comprehensive evidence review.
They found “consistent” evidence for PFAS hepatotoxicity from rodent studies. In addition, exposure to PFAS was found to be associated with markers of liver function in observational studies in people.
The review, published online in Environmental Health Perspectives, may be the first systematic analysis of PFAS exposure and liver damage.
Possible contributor to growing NAFLD epidemic
In their analysis, the authors included 85 rodent studies and 24 epidemiologic studies, primarily involving people from the United States and largely focusing on four “legacy” PFAS: perfluorooctanoic acid (PFOA), perfluorooctanesulfonic acid (PFOS), perfluorononanoic acid (PFNA), and perfluorohexanesulfonic acid (PFHxS).
Meta-analyses of human studies found that higher levels of alanine aminotransferase were significantly associated with exposure to three of the older chemicals – PFOA, PFOS, and PFNA.
The “positive” and “convincing” associations between exposure to these synthetic chemicals and higher ALT levels suggest that exposure may contribute to the growing NAFLD epidemic, the researchers write.
Exposure to one of the chemicals, PFOA, was also associated with higher aspartate aminotransferase and gamma-glutamyl transferase levels in people.
In rodents, exposure to these synthetic chemicals consistently resulted in higher ALT levels and steatosis.
“The mechanism is not well understood yet, but there are a few proposed theories,” first author Elizabeth Costello, MPH, PhD student, department of population and public health sciences, University of Southern California, Los Angeles, told this news organization.
“PFAS are similar to fatty acids in chemical structure, so it’s possible that they activate some of the same receptors or otherwise interfere with fat metabolism. This might lead to inflammation or fat accumulation in the liver,” Ms. Costello explained.
People widely exposed
PFAS are ubiquitous in the environment. They have been detected in the blood of most people and have been linked to a variety of health concerns. Possible sources of PFAS exposure run the gamut from nonstick cookware, food wrappers, and waterproof fabrics to cosmetics and even drinking water.
“We are exposed to PFAS in so many ways – through water, food, and products we use. It can be very difficult for individuals to control their own exposure,” Ms. Costello commented.
“At this point, it’s important to look for ways to remove PFAS from the environment and phase them out of our products and carefully consider the safety of any replacement chemicals,” she said.
Although most of the research to date has been limited to the four older PFAS (PFOA, PFOS, PFNA, and PFHxS), there are thousands of different PFAS chemicals.
“We don’t know very much about the effects of exposure to multiple PFAS at the same time or how newer replacement PFAS might affect liver disease or other health conditions,” Ms. Costello said.
Reached for comment, Lisa B. VanWagner, MD, with Northwestern University, Chicago, said this analysis is “very interesting,” but she is also “left wondering how we could do anything since it seems from my reading that these chemicals are ubiquitous and used regularly in the environment.”
Dr. VanWagner, who was not involved in the study, said the major limitation is the small number of human studies and the high heterogeneity between studies, “meaning it is hard to come to a firm conclusion about whether what has been observed in the animal studies does truly apply to humans.
“Overall, this study provides important proof of concept for future work to look more specifically at PFAS exposure, and more specific markers of fatty liver disease and liver damage, like liver biopsy, are needed in humans,” Dr. VanWagner said.
“If data accumulate showing that these chemicals do in fact contribute to fatty liver and worsening inflammation or liver damage as a result of exposure, then public health interventions to remove or reduce use of these chemicals could have wide-ranging public health effects,” Dr. VanWagner added.
Further research needed
The authors of an invited perspective published with the study say it underscores the “urgent need for further research and for immediate and reasonable public health action.”
“This work firmly puts PFAS exposure on the list of persistent pollutants, such as polychlorinated biphenyls, that cause hepatotoxicity and whose mechanism is linked to steatosis,” write Alan Ducatman, MD. MSc, with West Virginia University School of Public Health, Morgantown, and Suzanne Fenton, PhD, MS, with the National Institute of Environmental Health Sciences, Research Triangle Park, N.C.
They say other important questions raised by this review include whether individuals who are overweight or obese and those with diabetes are more susceptible to PFAS hepatoxicity, which “replacement” or emerging PFAS can cause liver damage, and whether high doses cause different kinds of liver toxicity than low doses.
“GenX, a current replacement [chemical] for PFOA, has shown significant hepatotoxicity in several recent experimental studies, suggesting it may not be a safe replacement,” they point out.
“A significant challenge will be deciding which of the multiple metabolic pathways altered by PFAS are most important and predictive for induction of liver damage and for progression of liver disease, so that emerging PFAS may be screened for hepatotoxicity prior to entering the market,” Dr. Ducatman and Dr. Fenton conclude.
Support for this research was provided by the National Institute of Environmental Health Science, part of the National Institutes of Health, and the U.S. Department of Agriculture National Institute of Food and Agriculture. Dr. Costello, Dr. VanWagner, Dr. Ducatman, and Dr. Fenton report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ENVIRONMENTAL HEALTH PERSPECTIVES
Deep learning system outmatches pathologists in diagnosing liver lesions
A new deep learning system can classify hepatocellular nodular lesions (HNLs) via whole-slide images, improving risk stratification of patients and diagnostic rate of hepatocellular carcinoma (HCC), according to investigators.
While the model requires further validation, it could eventually be used to optimize accuracy and efficiency of histologic diagnoses, potentially decreasing reliance on pathologists, particularly in areas with limited access to subspecialists.
In an article published in Gastroenterology, Na Cheng, MD, of Sun Yat-sen University, Guangzhou, China, and colleagues wrote that the “diagnostic process [for HNLs] is laborious, time-consuming, and subject to the experience of the pathologists, often with significant interobserver and intraobserver variability. ... Therefore, [an] automated analysis system is highly demanded in the pathology field, which could considerably ease the workload, speed up the diagnosis, and facilitate the in-time treatment.”
To this end, Dr. Cheng and colleagues developed the hepatocellular-nodular artificial intelligence model (HnAIM) that can scan whole-image slides to identify seven types of tissue: well-differentiated HCC, high-grade dysplastic nodules, low-grade dysplastic nodules, hepatocellular adenoma, focal nodular hyperplasia, and background tissue.
Developing and testing HnAIM was a multistep process that began with three subspecialist pathologists, who independently reviewed and classified liver slides from surgical resection. Unanimous agreement was achieved in 649 slides from 462 patients. These slides were then scanned to create whole-slide images, which were divided into sets for training (70%), validation (15%), and internal testing (15%). Accuracy, measured by area under the curve (AUC), was over 99.9% for the internal testing set. The accuracy of HnAIM was independently, externally validated.
First, HnAIM evaluated liver biopsy slides from 30 patients at one center. Results were compared with diagnoses made by nine pathologists classified as either senior, intermediate, or junior. While HnAIM correctly diagnosed 100% of the cases, senior pathologists correctly diagnosed 94.4% of the cases, followed in accuracy by intermediate (86.7%) and junior (73.3%) pathologists.
The researchers noted that the “rate of agreement with subspecialists was higher for HnAIM than for all 9 pathologists at distinguishing 7 liver tissues, with important diagnostic implications for fragmentary or scarce biopsy specimens.”
Next, HnAIM evaluated 234 samples from three hospitals. Accuracy was slightly lower, with an AUC of 93.5%. The researchers highlighted how HnAIM consistently differentiated precancerous lesions and well-defined HCC from benign lesions and background tissues.
A final experiment showed how HnAIM reacted to the most challenging cases. The investigators selected 12 cases without definitive diagnoses and found that, similar to the findings of three subspecialist pathologists, HnAIM did not reach a single diagnostic conclusion.
The researchers reported that “This may be due to a number of potential reasons, such as inherent uncertainty in the 2-dimensional interpretation of a 3-dimensional specimen, the limited number of tissue samples, and cognitive factors such as anchoring.”
However, HnAIM contributed to the diagnostic process by generating multiple diagnostic possibilities with weighted likelihood. After reviewing these results, the expert pathologists reached consensus in 5 out of 12 cases. Moreover, two out of three expert pathologists agreed on all 12 cases, improving agreement rate from 25% to 100%.
The researchers concluded that the model holds the promise to facilitate human HNL diagnoses and improve efficiency and quality. It can also reduce the workload of pathologists, especially where subspecialists are unavailable.
The study was supported by the National Natural Science Foundation of China, the Guangdong Basic and Applied Basic Research Foundation, the Natural Science Foundation of Guangdong Province, and others. The investigators reported no conflicts of interest.
As the prevalence of hepatocellular carcinoma (HCC) continues to rise, the early and accurate detection and diagnosis of HCC remains paramount to improving patient outcomes. In cases of typical or advanced HCC, an accurate diagnosis is made using CT or MR imaging. However, hepatocellular nodular lesions (HNLs) with atypical or inconclusive radiographic appearances are often biopsied to achieve a histopathologic diagnosis. In addition, accurate diagnosis of an HNL following liver resection or transplantation is important to long-term surveillance and management. An accurate histopathologic diagnosis relies on the availability of experienced subspecialty pathologists and remains a costly and labor-intensive process that can lead to delays in diagnosis and care.
Hannah P. Kim, MD, MSCR, is an assistant professor in the division of gastroenterology, hepatology, and nutrition in the department of medicine at Vanderbilt University Medical Center, Nashville, Tenn. She has no conflicts of interest.
As the prevalence of hepatocellular carcinoma (HCC) continues to rise, the early and accurate detection and diagnosis of HCC remains paramount to improving patient outcomes. In cases of typical or advanced HCC, an accurate diagnosis is made using CT or MR imaging. However, hepatocellular nodular lesions (HNLs) with atypical or inconclusive radiographic appearances are often biopsied to achieve a histopathologic diagnosis. In addition, accurate diagnosis of an HNL following liver resection or transplantation is important to long-term surveillance and management. An accurate histopathologic diagnosis relies on the availability of experienced subspecialty pathologists and remains a costly and labor-intensive process that can lead to delays in diagnosis and care.
Hannah P. Kim, MD, MSCR, is an assistant professor in the division of gastroenterology, hepatology, and nutrition in the department of medicine at Vanderbilt University Medical Center, Nashville, Tenn. She has no conflicts of interest.
As the prevalence of hepatocellular carcinoma (HCC) continues to rise, the early and accurate detection and diagnosis of HCC remains paramount to improving patient outcomes. In cases of typical or advanced HCC, an accurate diagnosis is made using CT or MR imaging. However, hepatocellular nodular lesions (HNLs) with atypical or inconclusive radiographic appearances are often biopsied to achieve a histopathologic diagnosis. In addition, accurate diagnosis of an HNL following liver resection or transplantation is important to long-term surveillance and management. An accurate histopathologic diagnosis relies on the availability of experienced subspecialty pathologists and remains a costly and labor-intensive process that can lead to delays in diagnosis and care.
Hannah P. Kim, MD, MSCR, is an assistant professor in the division of gastroenterology, hepatology, and nutrition in the department of medicine at Vanderbilt University Medical Center, Nashville, Tenn. She has no conflicts of interest.
A new deep learning system can classify hepatocellular nodular lesions (HNLs) via whole-slide images, improving risk stratification of patients and diagnostic rate of hepatocellular carcinoma (HCC), according to investigators.
While the model requires further validation, it could eventually be used to optimize accuracy and efficiency of histologic diagnoses, potentially decreasing reliance on pathologists, particularly in areas with limited access to subspecialists.
In an article published in Gastroenterology, Na Cheng, MD, of Sun Yat-sen University, Guangzhou, China, and colleagues wrote that the “diagnostic process [for HNLs] is laborious, time-consuming, and subject to the experience of the pathologists, often with significant interobserver and intraobserver variability. ... Therefore, [an] automated analysis system is highly demanded in the pathology field, which could considerably ease the workload, speed up the diagnosis, and facilitate the in-time treatment.”
To this end, Dr. Cheng and colleagues developed the hepatocellular-nodular artificial intelligence model (HnAIM) that can scan whole-image slides to identify seven types of tissue: well-differentiated HCC, high-grade dysplastic nodules, low-grade dysplastic nodules, hepatocellular adenoma, focal nodular hyperplasia, and background tissue.
Developing and testing HnAIM was a multistep process that began with three subspecialist pathologists, who independently reviewed and classified liver slides from surgical resection. Unanimous agreement was achieved in 649 slides from 462 patients. These slides were then scanned to create whole-slide images, which were divided into sets for training (70%), validation (15%), and internal testing (15%). Accuracy, measured by area under the curve (AUC), was over 99.9% for the internal testing set. The accuracy of HnAIM was independently, externally validated.
First, HnAIM evaluated liver biopsy slides from 30 patients at one center. Results were compared with diagnoses made by nine pathologists classified as either senior, intermediate, or junior. While HnAIM correctly diagnosed 100% of the cases, senior pathologists correctly diagnosed 94.4% of the cases, followed in accuracy by intermediate (86.7%) and junior (73.3%) pathologists.
The researchers noted that the “rate of agreement with subspecialists was higher for HnAIM than for all 9 pathologists at distinguishing 7 liver tissues, with important diagnostic implications for fragmentary or scarce biopsy specimens.”
Next, HnAIM evaluated 234 samples from three hospitals. Accuracy was slightly lower, with an AUC of 93.5%. The researchers highlighted how HnAIM consistently differentiated precancerous lesions and well-defined HCC from benign lesions and background tissues.
A final experiment showed how HnAIM reacted to the most challenging cases. The investigators selected 12 cases without definitive diagnoses and found that, similar to the findings of three subspecialist pathologists, HnAIM did not reach a single diagnostic conclusion.
The researchers reported that “This may be due to a number of potential reasons, such as inherent uncertainty in the 2-dimensional interpretation of a 3-dimensional specimen, the limited number of tissue samples, and cognitive factors such as anchoring.”
However, HnAIM contributed to the diagnostic process by generating multiple diagnostic possibilities with weighted likelihood. After reviewing these results, the expert pathologists reached consensus in 5 out of 12 cases. Moreover, two out of three expert pathologists agreed on all 12 cases, improving agreement rate from 25% to 100%.
The researchers concluded that the model holds the promise to facilitate human HNL diagnoses and improve efficiency and quality. It can also reduce the workload of pathologists, especially where subspecialists are unavailable.
The study was supported by the National Natural Science Foundation of China, the Guangdong Basic and Applied Basic Research Foundation, the Natural Science Foundation of Guangdong Province, and others. The investigators reported no conflicts of interest.
A new deep learning system can classify hepatocellular nodular lesions (HNLs) via whole-slide images, improving risk stratification of patients and diagnostic rate of hepatocellular carcinoma (HCC), according to investigators.
While the model requires further validation, it could eventually be used to optimize accuracy and efficiency of histologic diagnoses, potentially decreasing reliance on pathologists, particularly in areas with limited access to subspecialists.
In an article published in Gastroenterology, Na Cheng, MD, of Sun Yat-sen University, Guangzhou, China, and colleagues wrote that the “diagnostic process [for HNLs] is laborious, time-consuming, and subject to the experience of the pathologists, often with significant interobserver and intraobserver variability. ... Therefore, [an] automated analysis system is highly demanded in the pathology field, which could considerably ease the workload, speed up the diagnosis, and facilitate the in-time treatment.”
To this end, Dr. Cheng and colleagues developed the hepatocellular-nodular artificial intelligence model (HnAIM) that can scan whole-image slides to identify seven types of tissue: well-differentiated HCC, high-grade dysplastic nodules, low-grade dysplastic nodules, hepatocellular adenoma, focal nodular hyperplasia, and background tissue.
Developing and testing HnAIM was a multistep process that began with three subspecialist pathologists, who independently reviewed and classified liver slides from surgical resection. Unanimous agreement was achieved in 649 slides from 462 patients. These slides were then scanned to create whole-slide images, which were divided into sets for training (70%), validation (15%), and internal testing (15%). Accuracy, measured by area under the curve (AUC), was over 99.9% for the internal testing set. The accuracy of HnAIM was independently, externally validated.
First, HnAIM evaluated liver biopsy slides from 30 patients at one center. Results were compared with diagnoses made by nine pathologists classified as either senior, intermediate, or junior. While HnAIM correctly diagnosed 100% of the cases, senior pathologists correctly diagnosed 94.4% of the cases, followed in accuracy by intermediate (86.7%) and junior (73.3%) pathologists.
The researchers noted that the “rate of agreement with subspecialists was higher for HnAIM than for all 9 pathologists at distinguishing 7 liver tissues, with important diagnostic implications for fragmentary or scarce biopsy specimens.”
Next, HnAIM evaluated 234 samples from three hospitals. Accuracy was slightly lower, with an AUC of 93.5%. The researchers highlighted how HnAIM consistently differentiated precancerous lesions and well-defined HCC from benign lesions and background tissues.
A final experiment showed how HnAIM reacted to the most challenging cases. The investigators selected 12 cases without definitive diagnoses and found that, similar to the findings of three subspecialist pathologists, HnAIM did not reach a single diagnostic conclusion.
The researchers reported that “This may be due to a number of potential reasons, such as inherent uncertainty in the 2-dimensional interpretation of a 3-dimensional specimen, the limited number of tissue samples, and cognitive factors such as anchoring.”
However, HnAIM contributed to the diagnostic process by generating multiple diagnostic possibilities with weighted likelihood. After reviewing these results, the expert pathologists reached consensus in 5 out of 12 cases. Moreover, two out of three expert pathologists agreed on all 12 cases, improving agreement rate from 25% to 100%.
The researchers concluded that the model holds the promise to facilitate human HNL diagnoses and improve efficiency and quality. It can also reduce the workload of pathologists, especially where subspecialists are unavailable.
The study was supported by the National Natural Science Foundation of China, the Guangdong Basic and Applied Basic Research Foundation, the Natural Science Foundation of Guangdong Province, and others. The investigators reported no conflicts of interest.
FROM GASTROENTEROLOGY
CDC flags uptick in hypertensive disorders in pregnancy
Hypertensive disorders in pregnancy affect nearly 16% of women who give birth in U.S. hospitals and appear to be increasing, according to an April 29 report from the Centers for Disease Control and Prevention.
Older patients and Black women are substantially more likely to experience hypertension in pregnancy, the analysis found.
“Addressing hypertensive disorders in pregnancy is a key strategy in reducing inequities in pregnancy-related mortality,” study coauthor Wanda Barfield, MD, MPH, director of CDC’s Division of Reproductive Health, said in a statement.
Age, obesity, diabetes
The overall prevalence of hypertensive disorders in pregnancy increased from 13.3% in 2017 to 15.9% in 2019, the researchers reported in the CDC’s Morbidity and Mortality Weekly Report.
The uptick in hypertension coincides with trends toward older maternal age and higher rates of obesity and diabetes, which may explain the increase, they said.
For the study, Dr. Barfield and her colleagues analyzed nationally representative data from the National Inpatient Sample. They identified patients with a diagnosis of chronic hypertension, pregnancy-associated hypertension, or unspecified maternal hypertension during their hospitalization.
Among women aged 45-55 years, the prevalence of hypertension was 31%. Among those aged 35-44 years, it was 18%.
Hypertension diagnoses were more common in women who were Black (20.9%) or American Indian or Alaska Native (16.4%), than in other groups.
Of patients who died during delivery hospitalization, 31.6% had a hypertensive disorder.
The study shows a marked increase in hypertensive disorders over a relatively short time, according to Jane van Dis, MD, of the department of obstetrics and gynecology at the University of Rochester (N.Y.), who was not involved in the research. The phenomenon is consistent with her own experience, she said.
“When I am admitting patients, I’m oftentimes surprised when someone does not have a hypertensive disorder because I feel like the majority of patients these days do,” Dr. van Dis told this news organization.
Dr. Van Dis speculated that factors related to the environment, including air pollution and endocrine disrupters, could contribute to elevated rates of hypertensive disorders.
Natalie Bello, MD, MPH, director of hypertension research at Smidt Heart Institute, Cedars-Sinai Medical Center, Los Angeles, said rates of hypertension today could be even higher than in the study.
The CDC report relied on pre-COVID data, and the pandemic “increased disparities in health outcomes,” Dr. Bello said in an interview. “I’m worried that in actuality these numbers are an underestimation of the current state of hypertension in pregnancy.”
Dr. Bello, who has studied the need for better training in cardio-obstetrics, applauded Vice President Kamala Harris’ efforts to improve maternal health.
“The racial and geographic disparities that we continue to see in the field are disheartening but should be a call to action to redouble our work to improve maternal outcomes,” Dr. Bello told this news organization. “The good news is that a lot of morbidity related to hypertension can be avoided with timely diagnosis and treatment of blood pressure. However, we need to act to provide all pregnant persons with optimal care.”
Janet Wright, MD, director of CDC’s Division for Heart Disease and Stroke Prevention, said blood pressure home monitoring is a “great example” of a strategy clinicians can use to identify and manage patients with hypertension.
But one approach – self-monitoring blood pressure from home during pregnancy – did not significantly improve the health of pregnant women, according to new results from randomized trials in the United Kingdom.
Trial results published in JAMA show that blood pressure home-monitoring coupled to telemonitoring, as compared with usual care, did not significantly improve blood pressure control among patients with chronic or gestational hypertension.
A second trial published in JAMA that included patients at risk for preeclampsia found that self-monitoring with telemonitoring did not lead to significantly earlier diagnoses of hypertension.
“Individuals at risk for a hypertensive disorder of pregnancy, or with gestational or chronic hypertension, cannot be treated with a single approach,” Malavika Prabhu, MD, with Weill Cornell Medicine, New York, and coauthors write in an editorial accompanying the JAMA studies. Although the data suggest that self-monitoring of blood pressure is practical and tolerated, “More research is needed to determine optimal, high-value, equitable approaches to averting adverse perinatal outcomes associated with hypertensive disorders of pregnancy,” they write.
The CDC study authors and Dr. van Dis have disclosed no relevant financial relationships. Dr. Bello is funded by the National Institutes of Health to study blood pressure monitoring in pregnancy. The JAMA editorial authors disclosed university, government, and corporate grants and work with publishing companies.
A version of this article first appeared on Medscape.com.
Hypertensive disorders in pregnancy affect nearly 16% of women who give birth in U.S. hospitals and appear to be increasing, according to an April 29 report from the Centers for Disease Control and Prevention.
Older patients and Black women are substantially more likely to experience hypertension in pregnancy, the analysis found.
“Addressing hypertensive disorders in pregnancy is a key strategy in reducing inequities in pregnancy-related mortality,” study coauthor Wanda Barfield, MD, MPH, director of CDC’s Division of Reproductive Health, said in a statement.
Age, obesity, diabetes
The overall prevalence of hypertensive disorders in pregnancy increased from 13.3% in 2017 to 15.9% in 2019, the researchers reported in the CDC’s Morbidity and Mortality Weekly Report.
The uptick in hypertension coincides with trends toward older maternal age and higher rates of obesity and diabetes, which may explain the increase, they said.
For the study, Dr. Barfield and her colleagues analyzed nationally representative data from the National Inpatient Sample. They identified patients with a diagnosis of chronic hypertension, pregnancy-associated hypertension, or unspecified maternal hypertension during their hospitalization.
Among women aged 45-55 years, the prevalence of hypertension was 31%. Among those aged 35-44 years, it was 18%.
Hypertension diagnoses were more common in women who were Black (20.9%) or American Indian or Alaska Native (16.4%), than in other groups.
Of patients who died during delivery hospitalization, 31.6% had a hypertensive disorder.
The study shows a marked increase in hypertensive disorders over a relatively short time, according to Jane van Dis, MD, of the department of obstetrics and gynecology at the University of Rochester (N.Y.), who was not involved in the research. The phenomenon is consistent with her own experience, she said.
“When I am admitting patients, I’m oftentimes surprised when someone does not have a hypertensive disorder because I feel like the majority of patients these days do,” Dr. van Dis told this news organization.
Dr. Van Dis speculated that factors related to the environment, including air pollution and endocrine disrupters, could contribute to elevated rates of hypertensive disorders.
Natalie Bello, MD, MPH, director of hypertension research at Smidt Heart Institute, Cedars-Sinai Medical Center, Los Angeles, said rates of hypertension today could be even higher than in the study.
The CDC report relied on pre-COVID data, and the pandemic “increased disparities in health outcomes,” Dr. Bello said in an interview. “I’m worried that in actuality these numbers are an underestimation of the current state of hypertension in pregnancy.”
Dr. Bello, who has studied the need for better training in cardio-obstetrics, applauded Vice President Kamala Harris’ efforts to improve maternal health.
“The racial and geographic disparities that we continue to see in the field are disheartening but should be a call to action to redouble our work to improve maternal outcomes,” Dr. Bello told this news organization. “The good news is that a lot of morbidity related to hypertension can be avoided with timely diagnosis and treatment of blood pressure. However, we need to act to provide all pregnant persons with optimal care.”
Janet Wright, MD, director of CDC’s Division for Heart Disease and Stroke Prevention, said blood pressure home monitoring is a “great example” of a strategy clinicians can use to identify and manage patients with hypertension.
But one approach – self-monitoring blood pressure from home during pregnancy – did not significantly improve the health of pregnant women, according to new results from randomized trials in the United Kingdom.
Trial results published in JAMA show that blood pressure home-monitoring coupled to telemonitoring, as compared with usual care, did not significantly improve blood pressure control among patients with chronic or gestational hypertension.
A second trial published in JAMA that included patients at risk for preeclampsia found that self-monitoring with telemonitoring did not lead to significantly earlier diagnoses of hypertension.
“Individuals at risk for a hypertensive disorder of pregnancy, or with gestational or chronic hypertension, cannot be treated with a single approach,” Malavika Prabhu, MD, with Weill Cornell Medicine, New York, and coauthors write in an editorial accompanying the JAMA studies. Although the data suggest that self-monitoring of blood pressure is practical and tolerated, “More research is needed to determine optimal, high-value, equitable approaches to averting adverse perinatal outcomes associated with hypertensive disorders of pregnancy,” they write.
The CDC study authors and Dr. van Dis have disclosed no relevant financial relationships. Dr. Bello is funded by the National Institutes of Health to study blood pressure monitoring in pregnancy. The JAMA editorial authors disclosed university, government, and corporate grants and work with publishing companies.
A version of this article first appeared on Medscape.com.
Hypertensive disorders in pregnancy affect nearly 16% of women who give birth in U.S. hospitals and appear to be increasing, according to an April 29 report from the Centers for Disease Control and Prevention.
Older patients and Black women are substantially more likely to experience hypertension in pregnancy, the analysis found.
“Addressing hypertensive disorders in pregnancy is a key strategy in reducing inequities in pregnancy-related mortality,” study coauthor Wanda Barfield, MD, MPH, director of CDC’s Division of Reproductive Health, said in a statement.
Age, obesity, diabetes
The overall prevalence of hypertensive disorders in pregnancy increased from 13.3% in 2017 to 15.9% in 2019, the researchers reported in the CDC’s Morbidity and Mortality Weekly Report.
The uptick in hypertension coincides with trends toward older maternal age and higher rates of obesity and diabetes, which may explain the increase, they said.
For the study, Dr. Barfield and her colleagues analyzed nationally representative data from the National Inpatient Sample. They identified patients with a diagnosis of chronic hypertension, pregnancy-associated hypertension, or unspecified maternal hypertension during their hospitalization.
Among women aged 45-55 years, the prevalence of hypertension was 31%. Among those aged 35-44 years, it was 18%.
Hypertension diagnoses were more common in women who were Black (20.9%) or American Indian or Alaska Native (16.4%), than in other groups.
Of patients who died during delivery hospitalization, 31.6% had a hypertensive disorder.
The study shows a marked increase in hypertensive disorders over a relatively short time, according to Jane van Dis, MD, of the department of obstetrics and gynecology at the University of Rochester (N.Y.), who was not involved in the research. The phenomenon is consistent with her own experience, she said.
“When I am admitting patients, I’m oftentimes surprised when someone does not have a hypertensive disorder because I feel like the majority of patients these days do,” Dr. van Dis told this news organization.
Dr. Van Dis speculated that factors related to the environment, including air pollution and endocrine disrupters, could contribute to elevated rates of hypertensive disorders.
Natalie Bello, MD, MPH, director of hypertension research at Smidt Heart Institute, Cedars-Sinai Medical Center, Los Angeles, said rates of hypertension today could be even higher than in the study.
The CDC report relied on pre-COVID data, and the pandemic “increased disparities in health outcomes,” Dr. Bello said in an interview. “I’m worried that in actuality these numbers are an underestimation of the current state of hypertension in pregnancy.”
Dr. Bello, who has studied the need for better training in cardio-obstetrics, applauded Vice President Kamala Harris’ efforts to improve maternal health.
“The racial and geographic disparities that we continue to see in the field are disheartening but should be a call to action to redouble our work to improve maternal outcomes,” Dr. Bello told this news organization. “The good news is that a lot of morbidity related to hypertension can be avoided with timely diagnosis and treatment of blood pressure. However, we need to act to provide all pregnant persons with optimal care.”
Janet Wright, MD, director of CDC’s Division for Heart Disease and Stroke Prevention, said blood pressure home monitoring is a “great example” of a strategy clinicians can use to identify and manage patients with hypertension.
But one approach – self-monitoring blood pressure from home during pregnancy – did not significantly improve the health of pregnant women, according to new results from randomized trials in the United Kingdom.
Trial results published in JAMA show that blood pressure home-monitoring coupled to telemonitoring, as compared with usual care, did not significantly improve blood pressure control among patients with chronic or gestational hypertension.
A second trial published in JAMA that included patients at risk for preeclampsia found that self-monitoring with telemonitoring did not lead to significantly earlier diagnoses of hypertension.
“Individuals at risk for a hypertensive disorder of pregnancy, or with gestational or chronic hypertension, cannot be treated with a single approach,” Malavika Prabhu, MD, with Weill Cornell Medicine, New York, and coauthors write in an editorial accompanying the JAMA studies. Although the data suggest that self-monitoring of blood pressure is practical and tolerated, “More research is needed to determine optimal, high-value, equitable approaches to averting adverse perinatal outcomes associated with hypertensive disorders of pregnancy,” they write.
The CDC study authors and Dr. van Dis have disclosed no relevant financial relationships. Dr. Bello is funded by the National Institutes of Health to study blood pressure monitoring in pregnancy. The JAMA editorial authors disclosed university, government, and corporate grants and work with publishing companies.
A version of this article first appeared on Medscape.com.
Seven hours of sleep is ideal for middle aged and older
Sleep disturbances are common in older age, and previous studies have shown associations between too much or too little sleep and increased risk of cognitive decline, but the ideal amount of sleep for preserving mental health has not been well described, according to the authors of the new paper.
In the study published in Nature Aging, the team of researchers from China and the United Kingdom reviewed data from the UK Biobank, a national database of individuals in the United Kingdom that includes cognitive assessments, mental health questionnaires, and brain imaging data, as well as genetic information.
Sleep is important for physical and psychological health, and also serves a neuroprotective function by clearing waste products from the brain, lead author Yuzhu Li of Fudan University, Shanghai, China, and colleagues wrote.
The study population included 498,277 participants, aged 38-73 years, who completed touchscreen questionnaires about sleep duration between 2006 and 2010. The average age at baseline was 56.5 years, 54% were female, and the mean sleep duration was 7.15 hours.
The researchers also reviewed brain imaging data and genetic data from 39,692 participants in 2014 to examine the relationships between sleep duration and brain structure and between sleep duration and genetic risk. In addition, 156,884 participants completed an online follow-up mental health questionnaire in 2016-2017 to assess the longitudinal impact of sleep on mental health.
Both excessive and insufficient sleep was associated with impaired cognitive performance, evidenced by the U-shaped curve found by the researchers in their data analysis, which used quadratic associations.
Specific cognitive functions including pair matching, trail making, prospective memory, and reaction time were significantly impaired with too much or too little sleep, the researchers said. “This demonstrated the positive association of both insufficient and excessive sleep duration with inferior performance on cognitive tasks.”
When the researchers analyzed the association between sleep duration and mental health, sleep duration also showed a U-shaped association with symptoms of anxiety, depression, mental distress, mania, and self-harm, while well-being showed an inverted U-shape. All associations between sleep duration and mental health were statistically significant after controlling for confounding variables (P < .001).
On further analysis (using two-line tests), the researchers determined that consistent sleep duration of approximately 7 hours per night was optimal for cognitive performance and for good mental health.
The researchers also used neuroimaging data to examine the relationship between sleep duration and brain structure. Overall, greater changes were seen in the regions of the brain involved in cognitive processing and memory.
“The most significant cortical volumes nonlinearly associated with sleep duration included the precentral cortex, the superior frontal gyrus, the lateral orbitofrontal cortex, the pars orbitalis, the frontal pole, and the middle temporal cortex,” the researchers wrote (P < .05 for all).
The association between sleep duration and cognitive function diminished among individuals older than 65 years, compared with those aged approximately 40 years, which suggests that optimal sleep duration may be more beneficial in middle age, the researchers noted. However, no similar impact of age was seen for mental health. For brain structure, the nonlinear relationship between sleep duration and cortical volumes was greatest in those aged 44-59 years, and gradually flattened with older age.
Research supports sleep discussions with patients
“Primary care physicians can use this study in their discussions with middle-aged and older patients to recommend optimal sleep duration and measures to achieve this sleep target,” Noel Deep, MD, a general internist in group practice in Antigo, Wisc., who was not involved in the study, said in an interview.
“This study is important because it demonstrated that both inadequate and excessive sleep patterns were associated with cognitive and mental health changes,” said Dr. Deep. “It supported previous observations of cognitive decline and mental health disorders being linked to disturbed sleep. But this study was unique because it provides data supporting an optimal sleep duration of 7 hours and the ill effects of both insufficient and excessive sleep duration.
“The usual thought process has been to assume that older individuals may not require as much sleep as the younger individuals, but this study supports an optimal time duration of sleep of 7 hours that benefits the older individuals. It was also interesting to note the mental health effects caused by the inadequate and excessive sleep durations,” he added.
As for additional research, “I would like to look into the quality of the sleep, in addition to the duration of sleep,” said Dr. Deep. For example, whether the excessive sleep was caused by poor quality sleep or fragmented sleep leading to the structural and subsequent cognitive decline.
Study limitations
“The current study relied on self-reporting of the sleep duration and was not observed and recorded data,” Dr. Deep noted. “It would also be beneficial to not only rely on healthy volunteers reporting the sleep duration, but also obtain sleep data from individuals with known brain disorders.”
The study findings were limited by several other factors, including the use of total sleep duration only, without other measures of sleep hygiene, the researchers noted. More research is needed to investigate the mechanisms driving the association between too much and not enough sleep and poor mental health and cognitive function.
The study was supported by the National Key R&D Program of China, the Shanghai Municipal Science and Technology Major Project, the Shanghai Center for Brain Science and Brain-Inspired Technology, the 111 Project, the National Natural Sciences Foundation of China and the Shanghai Rising Star Program.
The researchers had no financial conflicts to disclose. Dr. Deep had no financial conflicts to disclose, but serves on the editorial advisory board of Internal Medicine News.
Sleep disturbances are common in older age, and previous studies have shown associations between too much or too little sleep and increased risk of cognitive decline, but the ideal amount of sleep for preserving mental health has not been well described, according to the authors of the new paper.
In the study published in Nature Aging, the team of researchers from China and the United Kingdom reviewed data from the UK Biobank, a national database of individuals in the United Kingdom that includes cognitive assessments, mental health questionnaires, and brain imaging data, as well as genetic information.
Sleep is important for physical and psychological health, and also serves a neuroprotective function by clearing waste products from the brain, lead author Yuzhu Li of Fudan University, Shanghai, China, and colleagues wrote.
The study population included 498,277 participants, aged 38-73 years, who completed touchscreen questionnaires about sleep duration between 2006 and 2010. The average age at baseline was 56.5 years, 54% were female, and the mean sleep duration was 7.15 hours.
The researchers also reviewed brain imaging data and genetic data from 39,692 participants in 2014 to examine the relationships between sleep duration and brain structure and between sleep duration and genetic risk. In addition, 156,884 participants completed an online follow-up mental health questionnaire in 2016-2017 to assess the longitudinal impact of sleep on mental health.
Both excessive and insufficient sleep was associated with impaired cognitive performance, evidenced by the U-shaped curve found by the researchers in their data analysis, which used quadratic associations.
Specific cognitive functions including pair matching, trail making, prospective memory, and reaction time were significantly impaired with too much or too little sleep, the researchers said. “This demonstrated the positive association of both insufficient and excessive sleep duration with inferior performance on cognitive tasks.”
When the researchers analyzed the association between sleep duration and mental health, sleep duration also showed a U-shaped association with symptoms of anxiety, depression, mental distress, mania, and self-harm, while well-being showed an inverted U-shape. All associations between sleep duration and mental health were statistically significant after controlling for confounding variables (P < .001).
On further analysis (using two-line tests), the researchers determined that consistent sleep duration of approximately 7 hours per night was optimal for cognitive performance and for good mental health.
The researchers also used neuroimaging data to examine the relationship between sleep duration and brain structure. Overall, greater changes were seen in the regions of the brain involved in cognitive processing and memory.
“The most significant cortical volumes nonlinearly associated with sleep duration included the precentral cortex, the superior frontal gyrus, the lateral orbitofrontal cortex, the pars orbitalis, the frontal pole, and the middle temporal cortex,” the researchers wrote (P < .05 for all).
The association between sleep duration and cognitive function diminished among individuals older than 65 years, compared with those aged approximately 40 years, which suggests that optimal sleep duration may be more beneficial in middle age, the researchers noted. However, no similar impact of age was seen for mental health. For brain structure, the nonlinear relationship between sleep duration and cortical volumes was greatest in those aged 44-59 years, and gradually flattened with older age.
Research supports sleep discussions with patients
“Primary care physicians can use this study in their discussions with middle-aged and older patients to recommend optimal sleep duration and measures to achieve this sleep target,” Noel Deep, MD, a general internist in group practice in Antigo, Wisc., who was not involved in the study, said in an interview.
“This study is important because it demonstrated that both inadequate and excessive sleep patterns were associated with cognitive and mental health changes,” said Dr. Deep. “It supported previous observations of cognitive decline and mental health disorders being linked to disturbed sleep. But this study was unique because it provides data supporting an optimal sleep duration of 7 hours and the ill effects of both insufficient and excessive sleep duration.
“The usual thought process has been to assume that older individuals may not require as much sleep as the younger individuals, but this study supports an optimal time duration of sleep of 7 hours that benefits the older individuals. It was also interesting to note the mental health effects caused by the inadequate and excessive sleep durations,” he added.
As for additional research, “I would like to look into the quality of the sleep, in addition to the duration of sleep,” said Dr. Deep. For example, whether the excessive sleep was caused by poor quality sleep or fragmented sleep leading to the structural and subsequent cognitive decline.
Study limitations
“The current study relied on self-reporting of the sleep duration and was not observed and recorded data,” Dr. Deep noted. “It would also be beneficial to not only rely on healthy volunteers reporting the sleep duration, but also obtain sleep data from individuals with known brain disorders.”
The study findings were limited by several other factors, including the use of total sleep duration only, without other measures of sleep hygiene, the researchers noted. More research is needed to investigate the mechanisms driving the association between too much and not enough sleep and poor mental health and cognitive function.
The study was supported by the National Key R&D Program of China, the Shanghai Municipal Science and Technology Major Project, the Shanghai Center for Brain Science and Brain-Inspired Technology, the 111 Project, the National Natural Sciences Foundation of China and the Shanghai Rising Star Program.
The researchers had no financial conflicts to disclose. Dr. Deep had no financial conflicts to disclose, but serves on the editorial advisory board of Internal Medicine News.
Sleep disturbances are common in older age, and previous studies have shown associations between too much or too little sleep and increased risk of cognitive decline, but the ideal amount of sleep for preserving mental health has not been well described, according to the authors of the new paper.
In the study published in Nature Aging, the team of researchers from China and the United Kingdom reviewed data from the UK Biobank, a national database of individuals in the United Kingdom that includes cognitive assessments, mental health questionnaires, and brain imaging data, as well as genetic information.
Sleep is important for physical and psychological health, and also serves a neuroprotective function by clearing waste products from the brain, lead author Yuzhu Li of Fudan University, Shanghai, China, and colleagues wrote.
The study population included 498,277 participants, aged 38-73 years, who completed touchscreen questionnaires about sleep duration between 2006 and 2010. The average age at baseline was 56.5 years, 54% were female, and the mean sleep duration was 7.15 hours.
The researchers also reviewed brain imaging data and genetic data from 39,692 participants in 2014 to examine the relationships between sleep duration and brain structure and between sleep duration and genetic risk. In addition, 156,884 participants completed an online follow-up mental health questionnaire in 2016-2017 to assess the longitudinal impact of sleep on mental health.
Both excessive and insufficient sleep was associated with impaired cognitive performance, evidenced by the U-shaped curve found by the researchers in their data analysis, which used quadratic associations.
Specific cognitive functions including pair matching, trail making, prospective memory, and reaction time were significantly impaired with too much or too little sleep, the researchers said. “This demonstrated the positive association of both insufficient and excessive sleep duration with inferior performance on cognitive tasks.”
When the researchers analyzed the association between sleep duration and mental health, sleep duration also showed a U-shaped association with symptoms of anxiety, depression, mental distress, mania, and self-harm, while well-being showed an inverted U-shape. All associations between sleep duration and mental health were statistically significant after controlling for confounding variables (P < .001).
On further analysis (using two-line tests), the researchers determined that consistent sleep duration of approximately 7 hours per night was optimal for cognitive performance and for good mental health.
The researchers also used neuroimaging data to examine the relationship between sleep duration and brain structure. Overall, greater changes were seen in the regions of the brain involved in cognitive processing and memory.
“The most significant cortical volumes nonlinearly associated with sleep duration included the precentral cortex, the superior frontal gyrus, the lateral orbitofrontal cortex, the pars orbitalis, the frontal pole, and the middle temporal cortex,” the researchers wrote (P < .05 for all).
The association between sleep duration and cognitive function diminished among individuals older than 65 years, compared with those aged approximately 40 years, which suggests that optimal sleep duration may be more beneficial in middle age, the researchers noted. However, no similar impact of age was seen for mental health. For brain structure, the nonlinear relationship between sleep duration and cortical volumes was greatest in those aged 44-59 years, and gradually flattened with older age.
Research supports sleep discussions with patients
“Primary care physicians can use this study in their discussions with middle-aged and older patients to recommend optimal sleep duration and measures to achieve this sleep target,” Noel Deep, MD, a general internist in group practice in Antigo, Wisc., who was not involved in the study, said in an interview.
“This study is important because it demonstrated that both inadequate and excessive sleep patterns were associated with cognitive and mental health changes,” said Dr. Deep. “It supported previous observations of cognitive decline and mental health disorders being linked to disturbed sleep. But this study was unique because it provides data supporting an optimal sleep duration of 7 hours and the ill effects of both insufficient and excessive sleep duration.
“The usual thought process has been to assume that older individuals may not require as much sleep as the younger individuals, but this study supports an optimal time duration of sleep of 7 hours that benefits the older individuals. It was also interesting to note the mental health effects caused by the inadequate and excessive sleep durations,” he added.
As for additional research, “I would like to look into the quality of the sleep, in addition to the duration of sleep,” said Dr. Deep. For example, whether the excessive sleep was caused by poor quality sleep or fragmented sleep leading to the structural and subsequent cognitive decline.
Study limitations
“The current study relied on self-reporting of the sleep duration and was not observed and recorded data,” Dr. Deep noted. “It would also be beneficial to not only rely on healthy volunteers reporting the sleep duration, but also obtain sleep data from individuals with known brain disorders.”
The study findings were limited by several other factors, including the use of total sleep duration only, without other measures of sleep hygiene, the researchers noted. More research is needed to investigate the mechanisms driving the association between too much and not enough sleep and poor mental health and cognitive function.
The study was supported by the National Key R&D Program of China, the Shanghai Municipal Science and Technology Major Project, the Shanghai Center for Brain Science and Brain-Inspired Technology, the 111 Project, the National Natural Sciences Foundation of China and the Shanghai Rising Star Program.
The researchers had no financial conflicts to disclose. Dr. Deep had no financial conflicts to disclose, but serves on the editorial advisory board of Internal Medicine News.
FROM NATURE AGING
Severe COVID-19 adds 20 years of cognitive aging: Study
adding that the impairment is “equivalent to losing 10 IQ points.”
In their study, published in eClinicalMedicine, a team of scientists from the University of Cambridge and Imperial College London said there is growing evidence that COVID-19 can cause lasting cognitive and mental health problems. Patients report fatigue, “brain fog,” problems recalling words, sleep disturbances, anxiety, and even posttraumatic stress disorder months after infection.
The researchers analyzed data from 46 individuals who received critical care for COVID-19 at Addenbrooke’s Hospital between March and July 2020 (27 females, 19 males, mean age 51 years, 16 of whom had mechanical ventilation) and were recruited to the NIHR COVID-19 BioResource project.
At an average of 6 months after acute COVID-19 illness, the study participants underwent detailed computerized cognitive tests via the Cognitron platform, comprising eight tasks deployed on an iPad measuring mental function such as memory, attention, and reasoning. Also assessed were anxiety, depression, and posttraumatic stress disorder via standard mood, anxiety, and posttraumatic stress scales – specifically the Generalized Anxiety Disorder 7 (GAD-7), the Patient Health Questionnaire 9 (PHQ-9), and the PTSD Checklist for Diagnostic and Statistical Manual of Mental Disorders 5 (PCL-5). Their data were compared against 460 controls – matched for age, sex, education, and first language – and the pattern of deficits across tasks was qualitatively compared with normal age-related decline and early-stage dementia.
Less accurate and slower response times
The authors highlighted how this was the first time a “rigorous assessment and comparison” had been carried out in relation to the after-effects of severe COVID-19.
“Cognitive impairment is common to a wide range of neurological disorders, including dementia, and even routine aging, but the patterns we saw – the cognitive ‘fingerprint’ of COVID-19 – was distinct from all of these,” said David Menon, MD, division of anesthesia at the University of Cambridge, England, and the study’s senior author.
The scientists found that COVID-19 survivors were less accurate and had slower response times than the control population, and added that survivors scored particularly poorly on verbal analogical reasoning and showed slower processing speeds.
Critically, the scale of the cognitive deficits correlated with acute illness severity, but not fatigue or mental health status at the time of cognitive assessment, said the authors.
Recovery ‘at best gradual’
The effects were strongest for those with more severe acute illness, and who required mechanical ventilation, said the authors, who found that acute illness severity was “better at predicting the cognitive deficits.”
The authors pointed out how these deficits were still detectable when patients were followed up 6 months later, and that, although patients’ scores and reaction times began to improve over time, any recovery was “at best gradual” and likely to be influenced by factors such as illness severity and its neurological or psychological impacts.
“We followed some patients up as late as 10 months after their acute infection, so were able to see a very slow improvement,” Dr. Menon said. He explained how, while this improvement was not statistically significant, it was “at least heading in the right direction.”
However, he warned it is very possible that some of these individuals “will never fully recover.”
The cognitive deficits observed may be due to several factors in combination, said the authors, including inadequate oxygen or blood supply to the brain, blockage of large or small blood vessels due to clotting, and microscopic bleeds. They highlighted how the most important mechanism, however, may be “damage caused by the body’s own inflammatory response and immune system.”
Adam Hampshire, PhD, of the department of brain sciences at Imperial College London, one of the study’s authors, described how around 40,000 people have been through intensive care with COVID-19 in England alone, with many more despite having been very sick not admitted to hospital. This means there is a “large number of people out there still experiencing problems with cognition many months later,” he said. “We urgently need to look at what can be done to help these people.”
A version of this article first appeared on Univadis.
adding that the impairment is “equivalent to losing 10 IQ points.”
In their study, published in eClinicalMedicine, a team of scientists from the University of Cambridge and Imperial College London said there is growing evidence that COVID-19 can cause lasting cognitive and mental health problems. Patients report fatigue, “brain fog,” problems recalling words, sleep disturbances, anxiety, and even posttraumatic stress disorder months after infection.
The researchers analyzed data from 46 individuals who received critical care for COVID-19 at Addenbrooke’s Hospital between March and July 2020 (27 females, 19 males, mean age 51 years, 16 of whom had mechanical ventilation) and were recruited to the NIHR COVID-19 BioResource project.
At an average of 6 months after acute COVID-19 illness, the study participants underwent detailed computerized cognitive tests via the Cognitron platform, comprising eight tasks deployed on an iPad measuring mental function such as memory, attention, and reasoning. Also assessed were anxiety, depression, and posttraumatic stress disorder via standard mood, anxiety, and posttraumatic stress scales – specifically the Generalized Anxiety Disorder 7 (GAD-7), the Patient Health Questionnaire 9 (PHQ-9), and the PTSD Checklist for Diagnostic and Statistical Manual of Mental Disorders 5 (PCL-5). Their data were compared against 460 controls – matched for age, sex, education, and first language – and the pattern of deficits across tasks was qualitatively compared with normal age-related decline and early-stage dementia.
Less accurate and slower response times
The authors highlighted how this was the first time a “rigorous assessment and comparison” had been carried out in relation to the after-effects of severe COVID-19.
“Cognitive impairment is common to a wide range of neurological disorders, including dementia, and even routine aging, but the patterns we saw – the cognitive ‘fingerprint’ of COVID-19 – was distinct from all of these,” said David Menon, MD, division of anesthesia at the University of Cambridge, England, and the study’s senior author.
The scientists found that COVID-19 survivors were less accurate and had slower response times than the control population, and added that survivors scored particularly poorly on verbal analogical reasoning and showed slower processing speeds.
Critically, the scale of the cognitive deficits correlated with acute illness severity, but not fatigue or mental health status at the time of cognitive assessment, said the authors.
Recovery ‘at best gradual’
The effects were strongest for those with more severe acute illness, and who required mechanical ventilation, said the authors, who found that acute illness severity was “better at predicting the cognitive deficits.”
The authors pointed out how these deficits were still detectable when patients were followed up 6 months later, and that, although patients’ scores and reaction times began to improve over time, any recovery was “at best gradual” and likely to be influenced by factors such as illness severity and its neurological or psychological impacts.
“We followed some patients up as late as 10 months after their acute infection, so were able to see a very slow improvement,” Dr. Menon said. He explained how, while this improvement was not statistically significant, it was “at least heading in the right direction.”
However, he warned it is very possible that some of these individuals “will never fully recover.”
The cognitive deficits observed may be due to several factors in combination, said the authors, including inadequate oxygen or blood supply to the brain, blockage of large or small blood vessels due to clotting, and microscopic bleeds. They highlighted how the most important mechanism, however, may be “damage caused by the body’s own inflammatory response and immune system.”
Adam Hampshire, PhD, of the department of brain sciences at Imperial College London, one of the study’s authors, described how around 40,000 people have been through intensive care with COVID-19 in England alone, with many more despite having been very sick not admitted to hospital. This means there is a “large number of people out there still experiencing problems with cognition many months later,” he said. “We urgently need to look at what can be done to help these people.”
A version of this article first appeared on Univadis.
adding that the impairment is “equivalent to losing 10 IQ points.”
In their study, published in eClinicalMedicine, a team of scientists from the University of Cambridge and Imperial College London said there is growing evidence that COVID-19 can cause lasting cognitive and mental health problems. Patients report fatigue, “brain fog,” problems recalling words, sleep disturbances, anxiety, and even posttraumatic stress disorder months after infection.
The researchers analyzed data from 46 individuals who received critical care for COVID-19 at Addenbrooke’s Hospital between March and July 2020 (27 females, 19 males, mean age 51 years, 16 of whom had mechanical ventilation) and were recruited to the NIHR COVID-19 BioResource project.
At an average of 6 months after acute COVID-19 illness, the study participants underwent detailed computerized cognitive tests via the Cognitron platform, comprising eight tasks deployed on an iPad measuring mental function such as memory, attention, and reasoning. Also assessed were anxiety, depression, and posttraumatic stress disorder via standard mood, anxiety, and posttraumatic stress scales – specifically the Generalized Anxiety Disorder 7 (GAD-7), the Patient Health Questionnaire 9 (PHQ-9), and the PTSD Checklist for Diagnostic and Statistical Manual of Mental Disorders 5 (PCL-5). Their data were compared against 460 controls – matched for age, sex, education, and first language – and the pattern of deficits across tasks was qualitatively compared with normal age-related decline and early-stage dementia.
Less accurate and slower response times
The authors highlighted how this was the first time a “rigorous assessment and comparison” had been carried out in relation to the after-effects of severe COVID-19.
“Cognitive impairment is common to a wide range of neurological disorders, including dementia, and even routine aging, but the patterns we saw – the cognitive ‘fingerprint’ of COVID-19 – was distinct from all of these,” said David Menon, MD, division of anesthesia at the University of Cambridge, England, and the study’s senior author.
The scientists found that COVID-19 survivors were less accurate and had slower response times than the control population, and added that survivors scored particularly poorly on verbal analogical reasoning and showed slower processing speeds.
Critically, the scale of the cognitive deficits correlated with acute illness severity, but not fatigue or mental health status at the time of cognitive assessment, said the authors.
Recovery ‘at best gradual’
The effects were strongest for those with more severe acute illness, and who required mechanical ventilation, said the authors, who found that acute illness severity was “better at predicting the cognitive deficits.”
The authors pointed out how these deficits were still detectable when patients were followed up 6 months later, and that, although patients’ scores and reaction times began to improve over time, any recovery was “at best gradual” and likely to be influenced by factors such as illness severity and its neurological or psychological impacts.
“We followed some patients up as late as 10 months after their acute infection, so were able to see a very slow improvement,” Dr. Menon said. He explained how, while this improvement was not statistically significant, it was “at least heading in the right direction.”
However, he warned it is very possible that some of these individuals “will never fully recover.”
The cognitive deficits observed may be due to several factors in combination, said the authors, including inadequate oxygen or blood supply to the brain, blockage of large or small blood vessels due to clotting, and microscopic bleeds. They highlighted how the most important mechanism, however, may be “damage caused by the body’s own inflammatory response and immune system.”
Adam Hampshire, PhD, of the department of brain sciences at Imperial College London, one of the study’s authors, described how around 40,000 people have been through intensive care with COVID-19 in England alone, with many more despite having been very sick not admitted to hospital. This means there is a “large number of people out there still experiencing problems with cognition many months later,” he said. “We urgently need to look at what can be done to help these people.”
A version of this article first appeared on Univadis.
FROM ECLINICAL MEDICINE
Few children with early social gender transition change their minds
Approximately 7% of youth who chose gender identity social transition in early childhood had retransitioned 5 years later, based on data from 317 individuals.
“Increasing numbers of children are socially transitioning to live in line with their gender identity, rather than the gender assumed by their sex at birth – a process that typically involves changing a child’s pronouns, first name, hairstyle, and clothing,” wrote Kristina R. Olson, PhD, of Princeton (N.J.) University, and colleagues.
The question of whether early childhood social transitions will result in high rates of retransition continues to be a subject for debate, and long-term data on retransition rates and identity outcomes in children who transition are limited, they said.
To examine retransition in early-transitioning children, the researchers identified 317 binary socially transitioned transgender children to participate in a longitudinal study known as the Trans Youth Project (TYP) between July 2013 and December 2017. The study was published in Pediatrics. The mean age at baseline was 8 years. At study entry, participants had to have made a complete binary social transition, including changing their pronouns from those used at birth. During the 5-year follow-up period, children and parents were asked about use of puberty blockers and/or gender-affirming hormones. At study entry, 37 children had begun some type of puberty blockers. A total of 124 children initially socially transitioned before 6 years of age, and 193 initially socially transitioned at 6 years or older.
The study did not evaluate whether the participants met the DSM-5 criteria for gender dysphoria in childhood, the researchers noted. “Based on data collected at their initial visit, we do know that these participants showed signs of gender identification and gender-typed preferences commonly associated with their gender, not their sex assigned at birth,” they wrote.
Participants were classified as binary transgender, nonbinary, or cisgender based on their pronouns at follow-up. Binary transgender pronouns were associated with the other binary assigned sex, nonbinary pronouns were they/them or a mix of they/them and binary pronouns, and cisgender pronouns were those associated with assigned sex.
Overall, 7.3% of the participants had retransitioned at least once by 5 years after their initial binary social transition. The majority (94%) were living as binary transgender youth, including 1.3% who retransitioned to cisgender or nonbinary and then back to binary transgender during the follow-up period. A total of 2.5% were living as cisgender youth and 3.5% were living as nonbinary youth. These rates were similar across the initial population, as well as the 291 participants who continue to be in contact with the researchers, the 200 who had gone at least 5 years since their initial social transition, and the 280 participants who began the study before starting puberty blockers.
The researchers found no differences in retransition rates related to participant sex at birth. Rates of retransition were slightly higher among participants who made their initial social transition before 6 years of age, but these rates were low, the researchers noted.
The study findings were limited by several factors including the use of a volunteer community sample, with the potential for bias that may not generalize to the population at large, the researchers noted. Other limitations included the use of pronouns as the main criteria for retransition, and the classification of a change from binary transgender to nonbinary as a transition, they said. “Many nonbinary people consider themselves to be transgender,” they noted.
“If we had used a stricter criterion of retransition, more similar to the common use of terms like “detransition” or “desistence,” referring only to youth who are living as cisgender, then our retransition rate would have been lower (2.5%),” the researchers explained. Another limitation was the disproportionate number of trans girls, the researchers said. However, because no significant gender effect appeared in terms of retransition rates, “we do not predict any change in pattern of results if we had a different ratio of participants by sex at birth,” they said.
The researchers stated that they intend to follow the cohort through adolescence and into adulthood.
“As more youth are coming out and being supported in their transitions early in development, it is increasingly critical that clinicians understand the experiences of this cohort and not make assumptions about them as a function of older data from youth who lived under different circumstances,” the researchers emphasized. “Though we can never predict the exact gender trajectory of any child, these data suggest that many youth who identify as transgender early, and are supported through a social transition, will continue to identify as transgender 5 years after initial social transition.” They concluded that more research is needed to determine how best to support initial and later gender transitions in youth.
Study offers support for family discussions
“This study is important to help provide more data regarding the experiences of gender-diverse youth,” M. Brett Cooper, MD, of UT Southwestern Medical Center, Dallas, said in an interview. “The results of a study like this can be used by clinicians to help provide advice and guidance to parents and families as they support their children through their gender journey,” said Dr. Cooper, who was not involved in the study. The current study “also provides evidence to support that persistent, insistent, and consistent youth have an extremely low rate of retransition to a gender that aligns with their sex assigned at birth. This refutes suggestions by politicians and others that those who seek medical care have a high rate of regret or retransition,” Dr. Cooper emphasized.
“I was not surprised at all by their findings,” said Dr. Cooper. “These are very similar to what I have seen in my own panel of gender-diverse patients and what has been seen in other studies,” he noted.
The take-home message of the current study does not suggest any change in clinical practice, Dr. Cooper said. “Guidance already suggests supporting these youth on their gender journey and that for some youth, this may mean retransitioning to identify with their sex assigned at birth,” he explained.
The study was supported in part by grants to the researchers from the National Institutes of Health, the National Science Foundation, the Arcus Foundation, and the MacArthur Foundation. The researchers had no financial conflicts to disclose.
Approximately 7% of youth who chose gender identity social transition in early childhood had retransitioned 5 years later, based on data from 317 individuals.
“Increasing numbers of children are socially transitioning to live in line with their gender identity, rather than the gender assumed by their sex at birth – a process that typically involves changing a child’s pronouns, first name, hairstyle, and clothing,” wrote Kristina R. Olson, PhD, of Princeton (N.J.) University, and colleagues.
The question of whether early childhood social transitions will result in high rates of retransition continues to be a subject for debate, and long-term data on retransition rates and identity outcomes in children who transition are limited, they said.
To examine retransition in early-transitioning children, the researchers identified 317 binary socially transitioned transgender children to participate in a longitudinal study known as the Trans Youth Project (TYP) between July 2013 and December 2017. The study was published in Pediatrics. The mean age at baseline was 8 years. At study entry, participants had to have made a complete binary social transition, including changing their pronouns from those used at birth. During the 5-year follow-up period, children and parents were asked about use of puberty blockers and/or gender-affirming hormones. At study entry, 37 children had begun some type of puberty blockers. A total of 124 children initially socially transitioned before 6 years of age, and 193 initially socially transitioned at 6 years or older.
The study did not evaluate whether the participants met the DSM-5 criteria for gender dysphoria in childhood, the researchers noted. “Based on data collected at their initial visit, we do know that these participants showed signs of gender identification and gender-typed preferences commonly associated with their gender, not their sex assigned at birth,” they wrote.
Participants were classified as binary transgender, nonbinary, or cisgender based on their pronouns at follow-up. Binary transgender pronouns were associated with the other binary assigned sex, nonbinary pronouns were they/them or a mix of they/them and binary pronouns, and cisgender pronouns were those associated with assigned sex.
Overall, 7.3% of the participants had retransitioned at least once by 5 years after their initial binary social transition. The majority (94%) were living as binary transgender youth, including 1.3% who retransitioned to cisgender or nonbinary and then back to binary transgender during the follow-up period. A total of 2.5% were living as cisgender youth and 3.5% were living as nonbinary youth. These rates were similar across the initial population, as well as the 291 participants who continue to be in contact with the researchers, the 200 who had gone at least 5 years since their initial social transition, and the 280 participants who began the study before starting puberty blockers.
The researchers found no differences in retransition rates related to participant sex at birth. Rates of retransition were slightly higher among participants who made their initial social transition before 6 years of age, but these rates were low, the researchers noted.
The study findings were limited by several factors including the use of a volunteer community sample, with the potential for bias that may not generalize to the population at large, the researchers noted. Other limitations included the use of pronouns as the main criteria for retransition, and the classification of a change from binary transgender to nonbinary as a transition, they said. “Many nonbinary people consider themselves to be transgender,” they noted.
“If we had used a stricter criterion of retransition, more similar to the common use of terms like “detransition” or “desistence,” referring only to youth who are living as cisgender, then our retransition rate would have been lower (2.5%),” the researchers explained. Another limitation was the disproportionate number of trans girls, the researchers said. However, because no significant gender effect appeared in terms of retransition rates, “we do not predict any change in pattern of results if we had a different ratio of participants by sex at birth,” they said.
The researchers stated that they intend to follow the cohort through adolescence and into adulthood.
“As more youth are coming out and being supported in their transitions early in development, it is increasingly critical that clinicians understand the experiences of this cohort and not make assumptions about them as a function of older data from youth who lived under different circumstances,” the researchers emphasized. “Though we can never predict the exact gender trajectory of any child, these data suggest that many youth who identify as transgender early, and are supported through a social transition, will continue to identify as transgender 5 years after initial social transition.” They concluded that more research is needed to determine how best to support initial and later gender transitions in youth.
Study offers support for family discussions
“This study is important to help provide more data regarding the experiences of gender-diverse youth,” M. Brett Cooper, MD, of UT Southwestern Medical Center, Dallas, said in an interview. “The results of a study like this can be used by clinicians to help provide advice and guidance to parents and families as they support their children through their gender journey,” said Dr. Cooper, who was not involved in the study. The current study “also provides evidence to support that persistent, insistent, and consistent youth have an extremely low rate of retransition to a gender that aligns with their sex assigned at birth. This refutes suggestions by politicians and others that those who seek medical care have a high rate of regret or retransition,” Dr. Cooper emphasized.
“I was not surprised at all by their findings,” said Dr. Cooper. “These are very similar to what I have seen in my own panel of gender-diverse patients and what has been seen in other studies,” he noted.
The take-home message of the current study does not suggest any change in clinical practice, Dr. Cooper said. “Guidance already suggests supporting these youth on their gender journey and that for some youth, this may mean retransitioning to identify with their sex assigned at birth,” he explained.
The study was supported in part by grants to the researchers from the National Institutes of Health, the National Science Foundation, the Arcus Foundation, and the MacArthur Foundation. The researchers had no financial conflicts to disclose.
Approximately 7% of youth who chose gender identity social transition in early childhood had retransitioned 5 years later, based on data from 317 individuals.
“Increasing numbers of children are socially transitioning to live in line with their gender identity, rather than the gender assumed by their sex at birth – a process that typically involves changing a child’s pronouns, first name, hairstyle, and clothing,” wrote Kristina R. Olson, PhD, of Princeton (N.J.) University, and colleagues.
The question of whether early childhood social transitions will result in high rates of retransition continues to be a subject for debate, and long-term data on retransition rates and identity outcomes in children who transition are limited, they said.
To examine retransition in early-transitioning children, the researchers identified 317 binary socially transitioned transgender children to participate in a longitudinal study known as the Trans Youth Project (TYP) between July 2013 and December 2017. The study was published in Pediatrics. The mean age at baseline was 8 years. At study entry, participants had to have made a complete binary social transition, including changing their pronouns from those used at birth. During the 5-year follow-up period, children and parents were asked about use of puberty blockers and/or gender-affirming hormones. At study entry, 37 children had begun some type of puberty blockers. A total of 124 children initially socially transitioned before 6 years of age, and 193 initially socially transitioned at 6 years or older.
The study did not evaluate whether the participants met the DSM-5 criteria for gender dysphoria in childhood, the researchers noted. “Based on data collected at their initial visit, we do know that these participants showed signs of gender identification and gender-typed preferences commonly associated with their gender, not their sex assigned at birth,” they wrote.
Participants were classified as binary transgender, nonbinary, or cisgender based on their pronouns at follow-up. Binary transgender pronouns were associated with the other binary assigned sex, nonbinary pronouns were they/them or a mix of they/them and binary pronouns, and cisgender pronouns were those associated with assigned sex.
Overall, 7.3% of the participants had retransitioned at least once by 5 years after their initial binary social transition. The majority (94%) were living as binary transgender youth, including 1.3% who retransitioned to cisgender or nonbinary and then back to binary transgender during the follow-up period. A total of 2.5% were living as cisgender youth and 3.5% were living as nonbinary youth. These rates were similar across the initial population, as well as the 291 participants who continue to be in contact with the researchers, the 200 who had gone at least 5 years since their initial social transition, and the 280 participants who began the study before starting puberty blockers.
The researchers found no differences in retransition rates related to participant sex at birth. Rates of retransition were slightly higher among participants who made their initial social transition before 6 years of age, but these rates were low, the researchers noted.
The study findings were limited by several factors including the use of a volunteer community sample, with the potential for bias that may not generalize to the population at large, the researchers noted. Other limitations included the use of pronouns as the main criteria for retransition, and the classification of a change from binary transgender to nonbinary as a transition, they said. “Many nonbinary people consider themselves to be transgender,” they noted.
“If we had used a stricter criterion of retransition, more similar to the common use of terms like “detransition” or “desistence,” referring only to youth who are living as cisgender, then our retransition rate would have been lower (2.5%),” the researchers explained. Another limitation was the disproportionate number of trans girls, the researchers said. However, because no significant gender effect appeared in terms of retransition rates, “we do not predict any change in pattern of results if we had a different ratio of participants by sex at birth,” they said.
The researchers stated that they intend to follow the cohort through adolescence and into adulthood.
“As more youth are coming out and being supported in their transitions early in development, it is increasingly critical that clinicians understand the experiences of this cohort and not make assumptions about them as a function of older data from youth who lived under different circumstances,” the researchers emphasized. “Though we can never predict the exact gender trajectory of any child, these data suggest that many youth who identify as transgender early, and are supported through a social transition, will continue to identify as transgender 5 years after initial social transition.” They concluded that more research is needed to determine how best to support initial and later gender transitions in youth.
Study offers support for family discussions
“This study is important to help provide more data regarding the experiences of gender-diverse youth,” M. Brett Cooper, MD, of UT Southwestern Medical Center, Dallas, said in an interview. “The results of a study like this can be used by clinicians to help provide advice and guidance to parents and families as they support their children through their gender journey,” said Dr. Cooper, who was not involved in the study. The current study “also provides evidence to support that persistent, insistent, and consistent youth have an extremely low rate of retransition to a gender that aligns with their sex assigned at birth. This refutes suggestions by politicians and others that those who seek medical care have a high rate of regret or retransition,” Dr. Cooper emphasized.
“I was not surprised at all by their findings,” said Dr. Cooper. “These are very similar to what I have seen in my own panel of gender-diverse patients and what has been seen in other studies,” he noted.
The take-home message of the current study does not suggest any change in clinical practice, Dr. Cooper said. “Guidance already suggests supporting these youth on their gender journey and that for some youth, this may mean retransitioning to identify with their sex assigned at birth,” he explained.
The study was supported in part by grants to the researchers from the National Institutes of Health, the National Science Foundation, the Arcus Foundation, and the MacArthur Foundation. The researchers had no financial conflicts to disclose.
FROM PEDIATRICS










